User login
Anesthesia Care Practice Models in the Veterans Health Administration
Although the VHA primarily relies on teams for anesthesia care, unsupervised certified registered nurse anesthetists also are used to meet veterans’ surgical care needs.
Anesthesia care is provided by physician anesthesiologists, certified registered nurse anesthetists (CRNAs), anesthesiology residents, and anesthesiologist assistants. These providers may practice alone (anesthesiologists or CRNAs) or in various combinations of supervised roles and teams. Previous studies reveal mixed findings regarding whether patient outcomes differ by anesthesia practice models.1-7However, little is known about the prevalence of various anesthesia models in the US.
Background
In recent years, anesthesiology has undergone substantial expansion in its scope of services provided, the settings in which it is provided, and the diversity of its workforce.8As the field continues to evolve, especially within the context of value-based health care reform, it is imperative to evaluate how anesthesia care models are used in health systems and how these models may optimize care delivery.
The Veterans Health Administration (VHA) is the largest integrated health care system in the US, providing surgical care in 110 inpatient medical centers and 27 ambulatory surgery centers. Despite national integration, anesthesia practices vary widely among facilities. The question of which model of anesthesia care is associated with the best outcomes and offers the most value is widely debated.1,5,7,9 As an important first step in understanding anesthesia care delivery, a baseline assessment of the practice patterns of anesthesia providers is necessary and may benefit future studies of the impact of these care models on outcomes. Thus, the aim of this work was to understand and describe the previously unassessed landscape of anesthesia care delivery within the VHA.
Methods
As part of a larger evaluation of anesthesia care delivery in the VHA, an observational assessment of anesthesia provider practice patterns was conducted using retrospective surgical data. This project complies with VHA policy pertaining to nonresearch operational activities and did not require institutional review board approval and adheres to the EQUATOR Network guidelines described in Strengthening the Reporting of Observational Studies in Epidemiology (STROBE).10
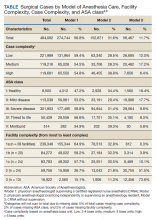
Data were obtained from the VHA Managerial Cost Accounting National Data Extract for Surgery package for all surgical procedures (n = 726,706) between October 1, 2013 and March 31, 2015. There were 420 facilities represented in these surgical data. The VHA facility records were used to specifically identify inpatient and ambulatory surgery facilities for inclusion. Additionally, to ensure facilities were valid surgical sites with sufficient surgical volume, those with 100 or fewer cases during the period were excluded. In total, 288 facilities with 9,434 surgical cases (representing 1% of cases) were excluded. These excluded facilities included nursing homes (38%), domiciliaries (26%), outpatient clinics (11%), rehabilitation programs (9%), other nonsurgical facilities (8%), and medical centers (8%). The majority (80%) of excluded medical centers had 30 or fewer surgical cases.
In 6 instances, data from subfacilities were combined with their organizationally affiliated main facilities. The final sample included 125 facilities. The VHA assigns a complexity level designation to facilities, defined as follows: 1a (most complex), 1b, 1c, 2, and 3 (least complex).11 Facilities with 1a designation perform the most complex surgical cases, such as cardiovascular surgery or neurosurgery and have more staff and resource support, whereas levels 2 and 3 facilities perform fewer and less complex cases.
Surgical records were excluded when the primary Current Procedural Terminology (CPT) code was missing (n = 85,748, or 12% of cases). This resulted in 631,524 remaining cases. The surgical CPT codes were mapped to anesthesia CPT codes to obtain the associated base unit (BU) values via a published crosswalk by the American Society of Anesthesiologists (ASA).12 A higher number of associated BUs indicates a more complex procedure. For example, procedures such as biopsies, arthroscopies, and laparoscopies receive 3 to 4 BUs, whereas a venous thrombectomy of the leg and a transurethral resection of the prostate are both 5 BUs, a total knee arthroplasty is 7 BUs, a craniotomy is 10 BUs, and a coronary artery bypass receives 18 BUs. Surgical case complexity was defined as low (3 or 4 BUs), medium (5 BUs), and high (≥ 6 BUs). Although the VHA has an existing case complexity assignment process based on CPT codes, it defines complexity differently for inpatient facilities and ambulatory surgery centers. Thus, the BU-defined complexity permitted a standardized complexity categorization across all facilities. Categorization of BUs similar to this has previously been used in the literature as a proxy for case complexity.13,14
Patient-level information included the ASA physical status classification, a measure of overall health status determined by an anesthesia provider preoperatively.15 These classifications included ASA I (healthy), ASA II (mild systemic disease), ASA III (severe systemic disease), ASA IV (severe systemic disease that is a constant threat to life), and ASA V (moribund patient who is not expected to survive without surgery). The last classification, ASA VI: brain-dead with planned organ donation, was excluded. The “E” subcategory denoting “emergency” was subsumed within the corresponding ASA category (eg, ASA V-E was combined with ASA V).
Provider data identified the principal and supervising (if present) anesthetists involved in the case. The provision of anesthesia care was categorized into 3 models: Model 1—a physician anesthesiologist supervising a CRNA; Model 2—a physician anesthesiologist practicing independently or supervising an anesthesiology resident; and Model 3—a CRNA without supervision. Surgical cases were excluded when there was no anesthesia provider (n = 95,795, or 15% of remaining cases), or a nonanesthesia provider (n = 51,647, or 8% of remaining cases) on record. The final sample was 484,082 surgical cases conducted at 125 facilities.
Related: Improving Care and Reducing Length of Stay in Patients Undergoing Total Knee Replacement
Statistical Analysis
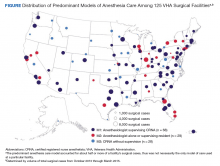
The percentage of surgical cases in each anesthesia care model was calculated overall and by the following characteristics: surgical case complexity, ASA classification, and facility complexity. The anesthesia model was determined for each case and summed at the facility level, yielding a total number of cases attributed to each model for each facility, thus identifying the predominant anesthesia model for each facility. The facilities were geographically displayed by their predominant anesthesia model and total number of surgical cases during the period. Because the aim was to present a descriptive representation of anesthesia care models, rather than infer significance, statistical testing was not included.
Results
A total of 484,082 surgical cases met inclusion criteria (Table). These cases were from 109 inpatient facilities and 16 ambulatory surgery facilities.
The percentage of cases in Model 1 was similar across the levels of surgical case complexity. However, a higher proportion of highly complex cases had a physician anesthesiologist (Model 2, 38.8%) than a CRNA (Model 3, 6.4%) as the primary anesthesia provider. Patients in each ASA classification were most likely to receive anesthesia care via Model 1. As ASA level increased, fewer patients had their anesthesia managed by a CRNA without supervision (Model 3: 18.4% of ASA 1 patients vs 8.3% of ASA 4 patients).
Facility complexity demonstrated notable differences in the proportions of surgical cases within each model. More than half of surgical cases in the largest, most complex facilities used Model 1 (64.9%, 58.2%, and 57.7% of cases in 1a, 1b, and 1c facilities, respectively). In comparison, Model 3 was found almost exclusively among surgical cases in smaller facilities with lower complexity (52% and 74% of cases in level 2 and 3 facilities, respectively).
The Figure displays the 125 facilities by their predominant model of anesthesia care. The diameter of the dots is relative to the facility’s total number of surgical cases. For each facility, the predominant model accounted for about half or more of cases but was not necessarily the only model of care used at a particular facility.
Related: Initiative to Minimize Pharmaceutical Risk in Older Veterans (IMPROVE) Polypharmacy Clinic
Discussion
Anesthesia care in more than half of surgical cases in VHA facilities was delivered by physician anesthesiologists supervising CRNAs. This model of anesthesia care was the dominant model in 54% of the facilities included in the sample. Consistent with a study of non-VHA facilities, this assessment found that the type of facility may influence the model of anesthesia care, with smaller, less complex facilities more often using a CRNA without supervision model.4 In these data, it was noted that among the 28 facilities that predominantly used Model 3, half had 12% or fewer cases that indicated a physician anesthesiologist model of care, and 6 had no cases with physician anesthesiologist involvement. These findings may reflect the limited scope of surgical services offered at lower complexity facilities and/or the reduced availability and/or utilization of physician anesthesiologists in these facilities.
Limitations
We recognize limitations in our assessment of anesthesia care. The documented presence or absence of a supervising anesthesia provider on the surgical record may not adequately characterize the model of anesthesia care in use at a facility, thus limiting an understanding of care delivery relationships among anesthesia providers. In addition, the patterns of anesthesia care delivery are likely influenced by factors not accounted for in this assessment, including the labor market share and economic forces.16,17 The veteran population tends to be older, male, and with substantial chronic disease burden, thus may have differing surgical needs and experiences than that of the general public.18,19 The surgical services offered in VHA facilities as well as the policies and practice environment surrounding anesthesia care also may vary from those found in nongovernmental facilities. However, as the largest health care system in the US, the VHA provides a diverse and robust surgical program. Many VHA facilities are large teaching hospitals with academic affiliations that would parallel some in the public sector. For example, studies have demonstrated similar surgical outcomes for patients in VHA vs non-VHA facilities.20 Therefore, the findings regarding anesthesia care models in VHA are likely relevant to non-VHA surgical sites.
Related: Improving Team-Based Care Coordination Delivery and Documentation in the Health Record
Conclusion
This preliminary assessment of the different models of anesthesia care demonstrates that although primarily relying on teams of anesthesiologists and CRNAs, the VA also uses unsupervised CRNAs to meet veterans’ surgical care needs. Although CRNA practice without supervision represented only 12% of surgical cases in our data, we identified 28 facilities (22%) that predominantly used CRNAs without supervision. Thus, CRNAs with and without supervision deliver a substantial portion of anesthesia care in the VA. The prevalence of CRNAs in documented VA surgical records and among surgical facilities nationwide highlights the importance of further examining their supervised and unsupervised roles in anesthesia care delivery.21 As the practice of anesthesiology continues to evolve, it is imperative that research efforts further investigate ways anesthesia care models may optimize care delivery, benefit anesthesia providers, and improve health outcomes for patients.
1. Dulisse B, Cromwell J. No harm found when nurse anesthetists work without supervision by physicians. Health Aff (Millwood). 2010;29(8):1469-1475
2. Simonson DC, Ahern MM, Hendryx MS. Anesthesia staffing and anesthetic complications during cesarean delivery: a retrospective analysis. Nurs Res. 2007;56(1):9-17.
3. Smith AF, Kane M, Milne R. Comparative effectiveness and safety of physician and nurse anaesthetists: a narrative systematic review. Br J Anaesth. 2004;93(4):540-545.
4. Needleman J, Minnick AF. Anesthesia provider model, hospital resources, and maternal outcomes. Health Serv Res. 2009;44(2, pt 1):464-482.
5. Lewis SR, Nicholson A, Smith AF, Alderson P. Physician anaesthetists versus non-physician providers of anaesthesia for surgical patients. Cochrane Database Syst Rev. 2014(7):CD010357.
6. Silber JH, Kennedy SK, Even-Shoshan O, et al. Anesthesiologist direction and patient outcomes. Anesthesiology. 2000;93(1):152-163.
7. Negrusa B, Hogan PF, Warner JT, Schroeder CH, Pang B. Scope of practice laws and anesthesia complications: no measurable impact of certified registered nurse anesthetist expanded scope of practice on anesthesia-related complications. Med Care. 2016;54(10):913-920.
8. Prielipp RC, Cohen NH. The future of anesthesiology: implications of the changing healthcare environment. Curr Opin Anaesthesiol. 2016;29(2):198-205.
9. Memtsoudis SG, Ma Y, Swamidoss CP, Edwards AM, Mazumdar M, Liguori GA. Factors influencing unexpected disposition after orthopedic ambulatory surgery. J Clin Anesth. 2012;24(2):89-95.
10. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epid. 2008;61:344-349.
11. US Department of Veterans Affairs, Veterans Health Administration, Office of Productivity Efficiency & Staffing. Facility Complexity Levels. http://opes.vssc.med.va.gov/FacilityComplexityLevels/Pages/default.aspx. [Nonpublic document; source not verified.
13. Mathis MR, Sathishkumar S, Kheterpal S, et al. Complications, risk factors, and staffing patterns for noncardiac surgery in patients with left ventricular assist devices. Anesthesiology. 2017;126(3):450-460.
14. Chen Y, Gabriel RA, Kodali BS, Urman RD. Effect of anesthesia staffing ratio on first-case surgical start time. J Med Syst. 2016;40(5):115.
15. American Society of Anesthesiologists. Standards, guidelines and related resources. https://www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system. Published October 15, 2014. Accessed November 5, 2018.
16. Kalist DE, Molinari NA, Spurr SJ. Cooperation and conflict between very similar occupations: the case of anesthesia. Health Econ Policy Law. 2011;6(2):237-264.
17. Daugherty L, Fonseca R, Kumar KB, Michaud PC. An analysis of the labor markets for anesthesiology. Rand Health Q. 2011;1(3):18.
18. Yu W, Ravelo A, Wagner TH, et al. Prevalence and costs of chronic conditions in the VA health care system. Med Care Res Rev. 2003;60(suppl 3):146S-167S.
19. Yoon J, Scott JY, Phibbs CS, Wagner TH. Recent trends in Veterans Affairs chronic condition spending. Popul Health Manag. 2011;14(6):293-298.
20. Shekelle PG, Asch S, Glassman P, Matula S, Trivedi A, Miake-Lye I. Comparison of Quality of Care in VA and Non-VA Settings: A Systematic Review. VA Evidence-based Synthesis Program. Washington, DC: Department of Veterans Affairs; 2010.
21. Baird M, Daugherty L, Kumar KB, Arifkhanova A. Regional and gender differences and trends in the anesthesiologist workforce. Anesthesiology. 2015;123(5):997-1012.
Although the VHA primarily relies on teams for anesthesia care, unsupervised certified registered nurse anesthetists also are used to meet veterans’ surgical care needs.
Although the VHA primarily relies on teams for anesthesia care, unsupervised certified registered nurse anesthetists also are used to meet veterans’ surgical care needs.
Anesthesia care is provided by physician anesthesiologists, certified registered nurse anesthetists (CRNAs), anesthesiology residents, and anesthesiologist assistants. These providers may practice alone (anesthesiologists or CRNAs) or in various combinations of supervised roles and teams. Previous studies reveal mixed findings regarding whether patient outcomes differ by anesthesia practice models.1-7However, little is known about the prevalence of various anesthesia models in the US.
Background
In recent years, anesthesiology has undergone substantial expansion in its scope of services provided, the settings in which it is provided, and the diversity of its workforce.8As the field continues to evolve, especially within the context of value-based health care reform, it is imperative to evaluate how anesthesia care models are used in health systems and how these models may optimize care delivery.
The Veterans Health Administration (VHA) is the largest integrated health care system in the US, providing surgical care in 110 inpatient medical centers and 27 ambulatory surgery centers. Despite national integration, anesthesia practices vary widely among facilities. The question of which model of anesthesia care is associated with the best outcomes and offers the most value is widely debated.1,5,7,9 As an important first step in understanding anesthesia care delivery, a baseline assessment of the practice patterns of anesthesia providers is necessary and may benefit future studies of the impact of these care models on outcomes. Thus, the aim of this work was to understand and describe the previously unassessed landscape of anesthesia care delivery within the VHA.
Methods
As part of a larger evaluation of anesthesia care delivery in the VHA, an observational assessment of anesthesia provider practice patterns was conducted using retrospective surgical data. This project complies with VHA policy pertaining to nonresearch operational activities and did not require institutional review board approval and adheres to the EQUATOR Network guidelines described in Strengthening the Reporting of Observational Studies in Epidemiology (STROBE).10
Data were obtained from the VHA Managerial Cost Accounting National Data Extract for Surgery package for all surgical procedures (n = 726,706) between October 1, 2013 and March 31, 2015. There were 420 facilities represented in these surgical data. The VHA facility records were used to specifically identify inpatient and ambulatory surgery facilities for inclusion. Additionally, to ensure facilities were valid surgical sites with sufficient surgical volume, those with 100 or fewer cases during the period were excluded. In total, 288 facilities with 9,434 surgical cases (representing 1% of cases) were excluded. These excluded facilities included nursing homes (38%), domiciliaries (26%), outpatient clinics (11%), rehabilitation programs (9%), other nonsurgical facilities (8%), and medical centers (8%). The majority (80%) of excluded medical centers had 30 or fewer surgical cases.
In 6 instances, data from subfacilities were combined with their organizationally affiliated main facilities. The final sample included 125 facilities. The VHA assigns a complexity level designation to facilities, defined as follows: 1a (most complex), 1b, 1c, 2, and 3 (least complex).11 Facilities with 1a designation perform the most complex surgical cases, such as cardiovascular surgery or neurosurgery and have more staff and resource support, whereas levels 2 and 3 facilities perform fewer and less complex cases.
Surgical records were excluded when the primary Current Procedural Terminology (CPT) code was missing (n = 85,748, or 12% of cases). This resulted in 631,524 remaining cases. The surgical CPT codes were mapped to anesthesia CPT codes to obtain the associated base unit (BU) values via a published crosswalk by the American Society of Anesthesiologists (ASA).12 A higher number of associated BUs indicates a more complex procedure. For example, procedures such as biopsies, arthroscopies, and laparoscopies receive 3 to 4 BUs, whereas a venous thrombectomy of the leg and a transurethral resection of the prostate are both 5 BUs, a total knee arthroplasty is 7 BUs, a craniotomy is 10 BUs, and a coronary artery bypass receives 18 BUs. Surgical case complexity was defined as low (3 or 4 BUs), medium (5 BUs), and high (≥ 6 BUs). Although the VHA has an existing case complexity assignment process based on CPT codes, it defines complexity differently for inpatient facilities and ambulatory surgery centers. Thus, the BU-defined complexity permitted a standardized complexity categorization across all facilities. Categorization of BUs similar to this has previously been used in the literature as a proxy for case complexity.13,14
Patient-level information included the ASA physical status classification, a measure of overall health status determined by an anesthesia provider preoperatively.15 These classifications included ASA I (healthy), ASA II (mild systemic disease), ASA III (severe systemic disease), ASA IV (severe systemic disease that is a constant threat to life), and ASA V (moribund patient who is not expected to survive without surgery). The last classification, ASA VI: brain-dead with planned organ donation, was excluded. The “E” subcategory denoting “emergency” was subsumed within the corresponding ASA category (eg, ASA V-E was combined with ASA V).
Provider data identified the principal and supervising (if present) anesthetists involved in the case. The provision of anesthesia care was categorized into 3 models: Model 1—a physician anesthesiologist supervising a CRNA; Model 2—a physician anesthesiologist practicing independently or supervising an anesthesiology resident; and Model 3—a CRNA without supervision. Surgical cases were excluded when there was no anesthesia provider (n = 95,795, or 15% of remaining cases), or a nonanesthesia provider (n = 51,647, or 8% of remaining cases) on record. The final sample was 484,082 surgical cases conducted at 125 facilities.
Related: Improving Care and Reducing Length of Stay in Patients Undergoing Total Knee Replacement
Statistical Analysis
The percentage of surgical cases in each anesthesia care model was calculated overall and by the following characteristics: surgical case complexity, ASA classification, and facility complexity. The anesthesia model was determined for each case and summed at the facility level, yielding a total number of cases attributed to each model for each facility, thus identifying the predominant anesthesia model for each facility. The facilities were geographically displayed by their predominant anesthesia model and total number of surgical cases during the period. Because the aim was to present a descriptive representation of anesthesia care models, rather than infer significance, statistical testing was not included.
Results
A total of 484,082 surgical cases met inclusion criteria (Table). These cases were from 109 inpatient facilities and 16 ambulatory surgery facilities.
The percentage of cases in Model 1 was similar across the levels of surgical case complexity. However, a higher proportion of highly complex cases had a physician anesthesiologist (Model 2, 38.8%) than a CRNA (Model 3, 6.4%) as the primary anesthesia provider. Patients in each ASA classification were most likely to receive anesthesia care via Model 1. As ASA level increased, fewer patients had their anesthesia managed by a CRNA without supervision (Model 3: 18.4% of ASA 1 patients vs 8.3% of ASA 4 patients).
Facility complexity demonstrated notable differences in the proportions of surgical cases within each model. More than half of surgical cases in the largest, most complex facilities used Model 1 (64.9%, 58.2%, and 57.7% of cases in 1a, 1b, and 1c facilities, respectively). In comparison, Model 3 was found almost exclusively among surgical cases in smaller facilities with lower complexity (52% and 74% of cases in level 2 and 3 facilities, respectively).
The Figure displays the 125 facilities by their predominant model of anesthesia care. The diameter of the dots is relative to the facility’s total number of surgical cases. For each facility, the predominant model accounted for about half or more of cases but was not necessarily the only model of care used at a particular facility.
Related: Initiative to Minimize Pharmaceutical Risk in Older Veterans (IMPROVE) Polypharmacy Clinic
Discussion
Anesthesia care in more than half of surgical cases in VHA facilities was delivered by physician anesthesiologists supervising CRNAs. This model of anesthesia care was the dominant model in 54% of the facilities included in the sample. Consistent with a study of non-VHA facilities, this assessment found that the type of facility may influence the model of anesthesia care, with smaller, less complex facilities more often using a CRNA without supervision model.4 In these data, it was noted that among the 28 facilities that predominantly used Model 3, half had 12% or fewer cases that indicated a physician anesthesiologist model of care, and 6 had no cases with physician anesthesiologist involvement. These findings may reflect the limited scope of surgical services offered at lower complexity facilities and/or the reduced availability and/or utilization of physician anesthesiologists in these facilities.
Limitations
We recognize limitations in our assessment of anesthesia care. The documented presence or absence of a supervising anesthesia provider on the surgical record may not adequately characterize the model of anesthesia care in use at a facility, thus limiting an understanding of care delivery relationships among anesthesia providers. In addition, the patterns of anesthesia care delivery are likely influenced by factors not accounted for in this assessment, including the labor market share and economic forces.16,17 The veteran population tends to be older, male, and with substantial chronic disease burden, thus may have differing surgical needs and experiences than that of the general public.18,19 The surgical services offered in VHA facilities as well as the policies and practice environment surrounding anesthesia care also may vary from those found in nongovernmental facilities. However, as the largest health care system in the US, the VHA provides a diverse and robust surgical program. Many VHA facilities are large teaching hospitals with academic affiliations that would parallel some in the public sector. For example, studies have demonstrated similar surgical outcomes for patients in VHA vs non-VHA facilities.20 Therefore, the findings regarding anesthesia care models in VHA are likely relevant to non-VHA surgical sites.
Related: Improving Team-Based Care Coordination Delivery and Documentation in the Health Record
Conclusion
This preliminary assessment of the different models of anesthesia care demonstrates that although primarily relying on teams of anesthesiologists and CRNAs, the VA also uses unsupervised CRNAs to meet veterans’ surgical care needs. Although CRNA practice without supervision represented only 12% of surgical cases in our data, we identified 28 facilities (22%) that predominantly used CRNAs without supervision. Thus, CRNAs with and without supervision deliver a substantial portion of anesthesia care in the VA. The prevalence of CRNAs in documented VA surgical records and among surgical facilities nationwide highlights the importance of further examining their supervised and unsupervised roles in anesthesia care delivery.21 As the practice of anesthesiology continues to evolve, it is imperative that research efforts further investigate ways anesthesia care models may optimize care delivery, benefit anesthesia providers, and improve health outcomes for patients.
Anesthesia care is provided by physician anesthesiologists, certified registered nurse anesthetists (CRNAs), anesthesiology residents, and anesthesiologist assistants. These providers may practice alone (anesthesiologists or CRNAs) or in various combinations of supervised roles and teams. Previous studies reveal mixed findings regarding whether patient outcomes differ by anesthesia practice models.1-7However, little is known about the prevalence of various anesthesia models in the US.
Background
In recent years, anesthesiology has undergone substantial expansion in its scope of services provided, the settings in which it is provided, and the diversity of its workforce.8As the field continues to evolve, especially within the context of value-based health care reform, it is imperative to evaluate how anesthesia care models are used in health systems and how these models may optimize care delivery.
The Veterans Health Administration (VHA) is the largest integrated health care system in the US, providing surgical care in 110 inpatient medical centers and 27 ambulatory surgery centers. Despite national integration, anesthesia practices vary widely among facilities. The question of which model of anesthesia care is associated with the best outcomes and offers the most value is widely debated.1,5,7,9 As an important first step in understanding anesthesia care delivery, a baseline assessment of the practice patterns of anesthesia providers is necessary and may benefit future studies of the impact of these care models on outcomes. Thus, the aim of this work was to understand and describe the previously unassessed landscape of anesthesia care delivery within the VHA.
Methods
As part of a larger evaluation of anesthesia care delivery in the VHA, an observational assessment of anesthesia provider practice patterns was conducted using retrospective surgical data. This project complies with VHA policy pertaining to nonresearch operational activities and did not require institutional review board approval and adheres to the EQUATOR Network guidelines described in Strengthening the Reporting of Observational Studies in Epidemiology (STROBE).10
Data were obtained from the VHA Managerial Cost Accounting National Data Extract for Surgery package for all surgical procedures (n = 726,706) between October 1, 2013 and March 31, 2015. There were 420 facilities represented in these surgical data. The VHA facility records were used to specifically identify inpatient and ambulatory surgery facilities for inclusion. Additionally, to ensure facilities were valid surgical sites with sufficient surgical volume, those with 100 or fewer cases during the period were excluded. In total, 288 facilities with 9,434 surgical cases (representing 1% of cases) were excluded. These excluded facilities included nursing homes (38%), domiciliaries (26%), outpatient clinics (11%), rehabilitation programs (9%), other nonsurgical facilities (8%), and medical centers (8%). The majority (80%) of excluded medical centers had 30 or fewer surgical cases.
In 6 instances, data from subfacilities were combined with their organizationally affiliated main facilities. The final sample included 125 facilities. The VHA assigns a complexity level designation to facilities, defined as follows: 1a (most complex), 1b, 1c, 2, and 3 (least complex).11 Facilities with 1a designation perform the most complex surgical cases, such as cardiovascular surgery or neurosurgery and have more staff and resource support, whereas levels 2 and 3 facilities perform fewer and less complex cases.
Surgical records were excluded when the primary Current Procedural Terminology (CPT) code was missing (n = 85,748, or 12% of cases). This resulted in 631,524 remaining cases. The surgical CPT codes were mapped to anesthesia CPT codes to obtain the associated base unit (BU) values via a published crosswalk by the American Society of Anesthesiologists (ASA).12 A higher number of associated BUs indicates a more complex procedure. For example, procedures such as biopsies, arthroscopies, and laparoscopies receive 3 to 4 BUs, whereas a venous thrombectomy of the leg and a transurethral resection of the prostate are both 5 BUs, a total knee arthroplasty is 7 BUs, a craniotomy is 10 BUs, and a coronary artery bypass receives 18 BUs. Surgical case complexity was defined as low (3 or 4 BUs), medium (5 BUs), and high (≥ 6 BUs). Although the VHA has an existing case complexity assignment process based on CPT codes, it defines complexity differently for inpatient facilities and ambulatory surgery centers. Thus, the BU-defined complexity permitted a standardized complexity categorization across all facilities. Categorization of BUs similar to this has previously been used in the literature as a proxy for case complexity.13,14
Patient-level information included the ASA physical status classification, a measure of overall health status determined by an anesthesia provider preoperatively.15 These classifications included ASA I (healthy), ASA II (mild systemic disease), ASA III (severe systemic disease), ASA IV (severe systemic disease that is a constant threat to life), and ASA V (moribund patient who is not expected to survive without surgery). The last classification, ASA VI: brain-dead with planned organ donation, was excluded. The “E” subcategory denoting “emergency” was subsumed within the corresponding ASA category (eg, ASA V-E was combined with ASA V).
Provider data identified the principal and supervising (if present) anesthetists involved in the case. The provision of anesthesia care was categorized into 3 models: Model 1—a physician anesthesiologist supervising a CRNA; Model 2—a physician anesthesiologist practicing independently or supervising an anesthesiology resident; and Model 3—a CRNA without supervision. Surgical cases were excluded when there was no anesthesia provider (n = 95,795, or 15% of remaining cases), or a nonanesthesia provider (n = 51,647, or 8% of remaining cases) on record. The final sample was 484,082 surgical cases conducted at 125 facilities.
Related: Improving Care and Reducing Length of Stay in Patients Undergoing Total Knee Replacement
Statistical Analysis
The percentage of surgical cases in each anesthesia care model was calculated overall and by the following characteristics: surgical case complexity, ASA classification, and facility complexity. The anesthesia model was determined for each case and summed at the facility level, yielding a total number of cases attributed to each model for each facility, thus identifying the predominant anesthesia model for each facility. The facilities were geographically displayed by their predominant anesthesia model and total number of surgical cases during the period. Because the aim was to present a descriptive representation of anesthesia care models, rather than infer significance, statistical testing was not included.
Results
A total of 484,082 surgical cases met inclusion criteria (Table). These cases were from 109 inpatient facilities and 16 ambulatory surgery facilities.
The percentage of cases in Model 1 was similar across the levels of surgical case complexity. However, a higher proportion of highly complex cases had a physician anesthesiologist (Model 2, 38.8%) than a CRNA (Model 3, 6.4%) as the primary anesthesia provider. Patients in each ASA classification were most likely to receive anesthesia care via Model 1. As ASA level increased, fewer patients had their anesthesia managed by a CRNA without supervision (Model 3: 18.4% of ASA 1 patients vs 8.3% of ASA 4 patients).
Facility complexity demonstrated notable differences in the proportions of surgical cases within each model. More than half of surgical cases in the largest, most complex facilities used Model 1 (64.9%, 58.2%, and 57.7% of cases in 1a, 1b, and 1c facilities, respectively). In comparison, Model 3 was found almost exclusively among surgical cases in smaller facilities with lower complexity (52% and 74% of cases in level 2 and 3 facilities, respectively).
The Figure displays the 125 facilities by their predominant model of anesthesia care. The diameter of the dots is relative to the facility’s total number of surgical cases. For each facility, the predominant model accounted for about half or more of cases but was not necessarily the only model of care used at a particular facility.
Related: Initiative to Minimize Pharmaceutical Risk in Older Veterans (IMPROVE) Polypharmacy Clinic
Discussion
Anesthesia care in more than half of surgical cases in VHA facilities was delivered by physician anesthesiologists supervising CRNAs. This model of anesthesia care was the dominant model in 54% of the facilities included in the sample. Consistent with a study of non-VHA facilities, this assessment found that the type of facility may influence the model of anesthesia care, with smaller, less complex facilities more often using a CRNA without supervision model.4 In these data, it was noted that among the 28 facilities that predominantly used Model 3, half had 12% or fewer cases that indicated a physician anesthesiologist model of care, and 6 had no cases with physician anesthesiologist involvement. These findings may reflect the limited scope of surgical services offered at lower complexity facilities and/or the reduced availability and/or utilization of physician anesthesiologists in these facilities.
Limitations
We recognize limitations in our assessment of anesthesia care. The documented presence or absence of a supervising anesthesia provider on the surgical record may not adequately characterize the model of anesthesia care in use at a facility, thus limiting an understanding of care delivery relationships among anesthesia providers. In addition, the patterns of anesthesia care delivery are likely influenced by factors not accounted for in this assessment, including the labor market share and economic forces.16,17 The veteran population tends to be older, male, and with substantial chronic disease burden, thus may have differing surgical needs and experiences than that of the general public.18,19 The surgical services offered in VHA facilities as well as the policies and practice environment surrounding anesthesia care also may vary from those found in nongovernmental facilities. However, as the largest health care system in the US, the VHA provides a diverse and robust surgical program. Many VHA facilities are large teaching hospitals with academic affiliations that would parallel some in the public sector. For example, studies have demonstrated similar surgical outcomes for patients in VHA vs non-VHA facilities.20 Therefore, the findings regarding anesthesia care models in VHA are likely relevant to non-VHA surgical sites.
Related: Improving Team-Based Care Coordination Delivery and Documentation in the Health Record
Conclusion
This preliminary assessment of the different models of anesthesia care demonstrates that although primarily relying on teams of anesthesiologists and CRNAs, the VA also uses unsupervised CRNAs to meet veterans’ surgical care needs. Although CRNA practice without supervision represented only 12% of surgical cases in our data, we identified 28 facilities (22%) that predominantly used CRNAs without supervision. Thus, CRNAs with and without supervision deliver a substantial portion of anesthesia care in the VA. The prevalence of CRNAs in documented VA surgical records and among surgical facilities nationwide highlights the importance of further examining their supervised and unsupervised roles in anesthesia care delivery.21 As the practice of anesthesiology continues to evolve, it is imperative that research efforts further investigate ways anesthesia care models may optimize care delivery, benefit anesthesia providers, and improve health outcomes for patients.
1. Dulisse B, Cromwell J. No harm found when nurse anesthetists work without supervision by physicians. Health Aff (Millwood). 2010;29(8):1469-1475
2. Simonson DC, Ahern MM, Hendryx MS. Anesthesia staffing and anesthetic complications during cesarean delivery: a retrospective analysis. Nurs Res. 2007;56(1):9-17.
3. Smith AF, Kane M, Milne R. Comparative effectiveness and safety of physician and nurse anaesthetists: a narrative systematic review. Br J Anaesth. 2004;93(4):540-545.
4. Needleman J, Minnick AF. Anesthesia provider model, hospital resources, and maternal outcomes. Health Serv Res. 2009;44(2, pt 1):464-482.
5. Lewis SR, Nicholson A, Smith AF, Alderson P. Physician anaesthetists versus non-physician providers of anaesthesia for surgical patients. Cochrane Database Syst Rev. 2014(7):CD010357.
6. Silber JH, Kennedy SK, Even-Shoshan O, et al. Anesthesiologist direction and patient outcomes. Anesthesiology. 2000;93(1):152-163.
7. Negrusa B, Hogan PF, Warner JT, Schroeder CH, Pang B. Scope of practice laws and anesthesia complications: no measurable impact of certified registered nurse anesthetist expanded scope of practice on anesthesia-related complications. Med Care. 2016;54(10):913-920.
8. Prielipp RC, Cohen NH. The future of anesthesiology: implications of the changing healthcare environment. Curr Opin Anaesthesiol. 2016;29(2):198-205.
9. Memtsoudis SG, Ma Y, Swamidoss CP, Edwards AM, Mazumdar M, Liguori GA. Factors influencing unexpected disposition after orthopedic ambulatory surgery. J Clin Anesth. 2012;24(2):89-95.
10. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epid. 2008;61:344-349.
11. US Department of Veterans Affairs, Veterans Health Administration, Office of Productivity Efficiency & Staffing. Facility Complexity Levels. http://opes.vssc.med.va.gov/FacilityComplexityLevels/Pages/default.aspx. [Nonpublic document; source not verified.
13. Mathis MR, Sathishkumar S, Kheterpal S, et al. Complications, risk factors, and staffing patterns for noncardiac surgery in patients with left ventricular assist devices. Anesthesiology. 2017;126(3):450-460.
14. Chen Y, Gabriel RA, Kodali BS, Urman RD. Effect of anesthesia staffing ratio on first-case surgical start time. J Med Syst. 2016;40(5):115.
15. American Society of Anesthesiologists. Standards, guidelines and related resources. https://www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system. Published October 15, 2014. Accessed November 5, 2018.
16. Kalist DE, Molinari NA, Spurr SJ. Cooperation and conflict between very similar occupations: the case of anesthesia. Health Econ Policy Law. 2011;6(2):237-264.
17. Daugherty L, Fonseca R, Kumar KB, Michaud PC. An analysis of the labor markets for anesthesiology. Rand Health Q. 2011;1(3):18.
18. Yu W, Ravelo A, Wagner TH, et al. Prevalence and costs of chronic conditions in the VA health care system. Med Care Res Rev. 2003;60(suppl 3):146S-167S.
19. Yoon J, Scott JY, Phibbs CS, Wagner TH. Recent trends in Veterans Affairs chronic condition spending. Popul Health Manag. 2011;14(6):293-298.
20. Shekelle PG, Asch S, Glassman P, Matula S, Trivedi A, Miake-Lye I. Comparison of Quality of Care in VA and Non-VA Settings: A Systematic Review. VA Evidence-based Synthesis Program. Washington, DC: Department of Veterans Affairs; 2010.
21. Baird M, Daugherty L, Kumar KB, Arifkhanova A. Regional and gender differences and trends in the anesthesiologist workforce. Anesthesiology. 2015;123(5):997-1012.
1. Dulisse B, Cromwell J. No harm found when nurse anesthetists work without supervision by physicians. Health Aff (Millwood). 2010;29(8):1469-1475
2. Simonson DC, Ahern MM, Hendryx MS. Anesthesia staffing and anesthetic complications during cesarean delivery: a retrospective analysis. Nurs Res. 2007;56(1):9-17.
3. Smith AF, Kane M, Milne R. Comparative effectiveness and safety of physician and nurse anaesthetists: a narrative systematic review. Br J Anaesth. 2004;93(4):540-545.
4. Needleman J, Minnick AF. Anesthesia provider model, hospital resources, and maternal outcomes. Health Serv Res. 2009;44(2, pt 1):464-482.
5. Lewis SR, Nicholson A, Smith AF, Alderson P. Physician anaesthetists versus non-physician providers of anaesthesia for surgical patients. Cochrane Database Syst Rev. 2014(7):CD010357.
6. Silber JH, Kennedy SK, Even-Shoshan O, et al. Anesthesiologist direction and patient outcomes. Anesthesiology. 2000;93(1):152-163.
7. Negrusa B, Hogan PF, Warner JT, Schroeder CH, Pang B. Scope of practice laws and anesthesia complications: no measurable impact of certified registered nurse anesthetist expanded scope of practice on anesthesia-related complications. Med Care. 2016;54(10):913-920.
8. Prielipp RC, Cohen NH. The future of anesthesiology: implications of the changing healthcare environment. Curr Opin Anaesthesiol. 2016;29(2):198-205.
9. Memtsoudis SG, Ma Y, Swamidoss CP, Edwards AM, Mazumdar M, Liguori GA. Factors influencing unexpected disposition after orthopedic ambulatory surgery. J Clin Anesth. 2012;24(2):89-95.
10. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epid. 2008;61:344-349.
11. US Department of Veterans Affairs, Veterans Health Administration, Office of Productivity Efficiency & Staffing. Facility Complexity Levels. http://opes.vssc.med.va.gov/FacilityComplexityLevels/Pages/default.aspx. [Nonpublic document; source not verified.
13. Mathis MR, Sathishkumar S, Kheterpal S, et al. Complications, risk factors, and staffing patterns for noncardiac surgery in patients with left ventricular assist devices. Anesthesiology. 2017;126(3):450-460.
14. Chen Y, Gabriel RA, Kodali BS, Urman RD. Effect of anesthesia staffing ratio on first-case surgical start time. J Med Syst. 2016;40(5):115.
15. American Society of Anesthesiologists. Standards, guidelines and related resources. https://www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system. Published October 15, 2014. Accessed November 5, 2018.
16. Kalist DE, Molinari NA, Spurr SJ. Cooperation and conflict between very similar occupations: the case of anesthesia. Health Econ Policy Law. 2011;6(2):237-264.
17. Daugherty L, Fonseca R, Kumar KB, Michaud PC. An analysis of the labor markets for anesthesiology. Rand Health Q. 2011;1(3):18.
18. Yu W, Ravelo A, Wagner TH, et al. Prevalence and costs of chronic conditions in the VA health care system. Med Care Res Rev. 2003;60(suppl 3):146S-167S.
19. Yoon J, Scott JY, Phibbs CS, Wagner TH. Recent trends in Veterans Affairs chronic condition spending. Popul Health Manag. 2011;14(6):293-298.
20. Shekelle PG, Asch S, Glassman P, Matula S, Trivedi A, Miake-Lye I. Comparison of Quality of Care in VA and Non-VA Settings: A Systematic Review. VA Evidence-based Synthesis Program. Washington, DC: Department of Veterans Affairs; 2010.
21. Baird M, Daugherty L, Kumar KB, Arifkhanova A. Regional and gender differences and trends in the anesthesiologist workforce. Anesthesiology. 2015;123(5):997-1012.
What constitutes a clinically meaningful reduction in seizure frequency?
NEW ORLEANS – according to a study described at the annual meeting of the American Epilepsy Society. A reduction in seizure frequency of between 60% and 68% is associated with Clinical Global Impression of Improvement (CGI-I) ratings of “very much improved,” as assessed by caregivers and investigators.
“Further analyses from other phase III studies in Dravet syndrome and other patient populations should be performed to confirm these findings and explore other potential factors that contribute to caregiver and investigator CGI-I ratings, such as nonseizure outcomes and tolerability,” said Arnold Gammaitoni, PharmD, vice president of medical and scientific affairs at Zogenix in San Diego, and his colleagues.
A 50% reduction in seizure frequency is conventionally considered to be the cutoff for a clinically meaningful change. To develop an evidence-based definition of clinically meaningful seizure reduction, Dr. Gammaitoni and colleagues examined data from a phase III, randomized, double-blind, placebo-controlled trial of fenfluramine HCl oral solution for the adjunctive treatment of seizures associated with Dravet syndrome. The investigators took an anchor-based approach and examined the percentage change in seizure frequency, along with caregiver and investigator CGI-I ratings.
A total of 119 patients with Dravet syndrome were enrolled and randomized in equal groups to placebo, 0.2 mg/kg per day of fenfluramine HCl, or 0.8 mg/kg per day of fenfluramine HCl. After a 2-week titration period, patients entered a 12-week maintenance period. Patients in the 0.8-mg/kg per day group had a 63.9% greater reduction in seizure frequency than controls did.
After the 14-week titration and maintenance period, caregivers and investigators rated the change in participants’ clinical status from baseline, using the CGI-I scale, on which responses range from 1 (very much improved) to 7 (very much worse). The investigators considered patients with CGI-I scores of 1 or 2 (much improved) to have achieved a clinically meaningful response. A score of 3 (minimally improved) was not considered meaningful. The researchers pooled the results of the three treatment groups for this analysis. They estimated the clinically meaningful percentage change in seizure frequency using receiver operating characteristic analysis of binary CGI-I score, compared with percentage change in seizure frequency, and defined it as the cut-point for which specificity and sensitivity were equal or most similar.
Caregivers and investigators provided CGI-I assessments for 112 patients and 114 patients, respectively. The receiver operating characteristic analysis identified a 44% reduction in seizure frequency as a clinically meaningful cutoff point for caregiver and investigator assessments. Using this threshold, 75%, 46%, and 12.5% of patients in the 0.8-mg/kg per day, 0.2-mg/kg per day, and placebo groups, respectively, achieved a clinically meaningful reduction from baseline in seizure frequency in the phase III study.
“The use of external anchors is one method to define a clinically meaningful change in seizure frequency,” said Dr. Gammaitoni. “Having a defined minimum clinically important difference like this allows clinicians to assess impacts of treatments on an individual patient basis.... This is a chance for others to do similar types of analyses to confirm the findings that we have had in this first study with bigger data sets, in terms of using external anchors and data to define what a clinically meaningful change is.”
Zogenix, which is developing the fenfluramine formulation examined in this study, provided funding for this research.
SOURCE: Nabbout R et al. AES 2018, Abstract 3.202.
NEW ORLEANS – according to a study described at the annual meeting of the American Epilepsy Society. A reduction in seizure frequency of between 60% and 68% is associated with Clinical Global Impression of Improvement (CGI-I) ratings of “very much improved,” as assessed by caregivers and investigators.
“Further analyses from other phase III studies in Dravet syndrome and other patient populations should be performed to confirm these findings and explore other potential factors that contribute to caregiver and investigator CGI-I ratings, such as nonseizure outcomes and tolerability,” said Arnold Gammaitoni, PharmD, vice president of medical and scientific affairs at Zogenix in San Diego, and his colleagues.
A 50% reduction in seizure frequency is conventionally considered to be the cutoff for a clinically meaningful change. To develop an evidence-based definition of clinically meaningful seizure reduction, Dr. Gammaitoni and colleagues examined data from a phase III, randomized, double-blind, placebo-controlled trial of fenfluramine HCl oral solution for the adjunctive treatment of seizures associated with Dravet syndrome. The investigators took an anchor-based approach and examined the percentage change in seizure frequency, along with caregiver and investigator CGI-I ratings.
A total of 119 patients with Dravet syndrome were enrolled and randomized in equal groups to placebo, 0.2 mg/kg per day of fenfluramine HCl, or 0.8 mg/kg per day of fenfluramine HCl. After a 2-week titration period, patients entered a 12-week maintenance period. Patients in the 0.8-mg/kg per day group had a 63.9% greater reduction in seizure frequency than controls did.
After the 14-week titration and maintenance period, caregivers and investigators rated the change in participants’ clinical status from baseline, using the CGI-I scale, on which responses range from 1 (very much improved) to 7 (very much worse). The investigators considered patients with CGI-I scores of 1 or 2 (much improved) to have achieved a clinically meaningful response. A score of 3 (minimally improved) was not considered meaningful. The researchers pooled the results of the three treatment groups for this analysis. They estimated the clinically meaningful percentage change in seizure frequency using receiver operating characteristic analysis of binary CGI-I score, compared with percentage change in seizure frequency, and defined it as the cut-point for which specificity and sensitivity were equal or most similar.
Caregivers and investigators provided CGI-I assessments for 112 patients and 114 patients, respectively. The receiver operating characteristic analysis identified a 44% reduction in seizure frequency as a clinically meaningful cutoff point for caregiver and investigator assessments. Using this threshold, 75%, 46%, and 12.5% of patients in the 0.8-mg/kg per day, 0.2-mg/kg per day, and placebo groups, respectively, achieved a clinically meaningful reduction from baseline in seizure frequency in the phase III study.
“The use of external anchors is one method to define a clinically meaningful change in seizure frequency,” said Dr. Gammaitoni. “Having a defined minimum clinically important difference like this allows clinicians to assess impacts of treatments on an individual patient basis.... This is a chance for others to do similar types of analyses to confirm the findings that we have had in this first study with bigger data sets, in terms of using external anchors and data to define what a clinically meaningful change is.”
Zogenix, which is developing the fenfluramine formulation examined in this study, provided funding for this research.
SOURCE: Nabbout R et al. AES 2018, Abstract 3.202.
NEW ORLEANS – according to a study described at the annual meeting of the American Epilepsy Society. A reduction in seizure frequency of between 60% and 68% is associated with Clinical Global Impression of Improvement (CGI-I) ratings of “very much improved,” as assessed by caregivers and investigators.
“Further analyses from other phase III studies in Dravet syndrome and other patient populations should be performed to confirm these findings and explore other potential factors that contribute to caregiver and investigator CGI-I ratings, such as nonseizure outcomes and tolerability,” said Arnold Gammaitoni, PharmD, vice president of medical and scientific affairs at Zogenix in San Diego, and his colleagues.
A 50% reduction in seizure frequency is conventionally considered to be the cutoff for a clinically meaningful change. To develop an evidence-based definition of clinically meaningful seizure reduction, Dr. Gammaitoni and colleagues examined data from a phase III, randomized, double-blind, placebo-controlled trial of fenfluramine HCl oral solution for the adjunctive treatment of seizures associated with Dravet syndrome. The investigators took an anchor-based approach and examined the percentage change in seizure frequency, along with caregiver and investigator CGI-I ratings.
A total of 119 patients with Dravet syndrome were enrolled and randomized in equal groups to placebo, 0.2 mg/kg per day of fenfluramine HCl, or 0.8 mg/kg per day of fenfluramine HCl. After a 2-week titration period, patients entered a 12-week maintenance period. Patients in the 0.8-mg/kg per day group had a 63.9% greater reduction in seizure frequency than controls did.
After the 14-week titration and maintenance period, caregivers and investigators rated the change in participants’ clinical status from baseline, using the CGI-I scale, on which responses range from 1 (very much improved) to 7 (very much worse). The investigators considered patients with CGI-I scores of 1 or 2 (much improved) to have achieved a clinically meaningful response. A score of 3 (minimally improved) was not considered meaningful. The researchers pooled the results of the three treatment groups for this analysis. They estimated the clinically meaningful percentage change in seizure frequency using receiver operating characteristic analysis of binary CGI-I score, compared with percentage change in seizure frequency, and defined it as the cut-point for which specificity and sensitivity were equal or most similar.
Caregivers and investigators provided CGI-I assessments for 112 patients and 114 patients, respectively. The receiver operating characteristic analysis identified a 44% reduction in seizure frequency as a clinically meaningful cutoff point for caregiver and investigator assessments. Using this threshold, 75%, 46%, and 12.5% of patients in the 0.8-mg/kg per day, 0.2-mg/kg per day, and placebo groups, respectively, achieved a clinically meaningful reduction from baseline in seizure frequency in the phase III study.
“The use of external anchors is one method to define a clinically meaningful change in seizure frequency,” said Dr. Gammaitoni. “Having a defined minimum clinically important difference like this allows clinicians to assess impacts of treatments on an individual patient basis.... This is a chance for others to do similar types of analyses to confirm the findings that we have had in this first study with bigger data sets, in terms of using external anchors and data to define what a clinically meaningful change is.”
Zogenix, which is developing the fenfluramine formulation examined in this study, provided funding for this research.
SOURCE: Nabbout R et al. AES 2018, Abstract 3.202.
REPORTING FROM AES 2018
Key clinical point: Data support the convention of considering a 50% reduction in seizure frequency as the cutoff for a clinically meaningful change.
Major finding: Statistical analysis indicates that a 44% reduction in seizure frequency is clinically meaningful.
Study details: A phase III, randomized, double-blind, placebo-controlled clinical trial of fenfluramine HCl that included 119 patients.
Disclosures: Zogenix provided funding for the study.
Source: Nabbout R et al. Abstract 3.202.
Older CLL Patients See Better PFS With Ibrutinib
SAN DIEGO – In the phase 3 Alliance A041202 trial of older patients with previously untreated chronic lymphocytic leukemia (CLL), ibrutinib showed superior progression-free survival (PFS). Results of the trial were reported by Jennifer A. Woyach, MD, of the Ohio State University in Columbus during a press briefing at the recently concluded American Society of Hematology 2018 meeting. The briefing was based on an abstract from the meeting.
“There was no difference in progression-free survival between ibrutinib and ibrutinib plus rituximab,” said Dr. Woyach. “We undertook this study to determine the most effective therapy for older patients with CLL.” She noted that the findings justify the use of ibrutinib as a standard-of-care treatment for CLL patients aged 65 years and older.
Median age of patients in the study was 71 years and 67% of the patient were men, a profile that is similar, to those of patients with CLL seen at the US Department of Veterans Affairs.
The 2-year PFS was 74% in 183 patients randomized to receive standard chemoimmunotherapy with bendamustine and rituximab (BR), compared with 87% in 182 patients randomized to receive ibrutinib alone (hazard ratio, 0.39 vs. BR), and 88% in 182 patients who received ibrutinib and rituximab (IR; HR, 0.38 vs. BR). Median PFS in this study was 43 months in the BR arm, and was not reached in either of the ibrutinib-containing arms, she said. No significant differences in overall survival (OS) were seen among the treatment arms, which may have been because of short follow-up and the fact that patients in the BR arm were allowed to cross over to ibrutinib if they progressed on treatment.
The results suggest that the additional of rituximab provided little benefit to the patients though it does add to both the costs and the chair time in an infusion center, according to former Association of VA Hematology/Oncology Mary Thomas, MS, CNS, AOCN.
“I think this really does indicate that ibrutinib as front-line therapy, which many clinicians have been doing, is a very reasonable practice,” said David P. Steensma, MD, of Dana-Farber Cancer Institute in Boston, who moderated the press briefing.
Dr. Woyach added, however, that while ibrutinib represents a major therapeutic advance, its cost and its toxicities in older patients are a concern that warrant close monitoring and development of strategies to reduce the need for long-term continuous treatment.
Thomas agreed noting that health care providers needs to be aware of the risk of atrial fib and bleeding when using ibrutinib and to ensure that patient will be able to adhere to daily dosing.
Additional phase 3 studies set to open soon will compare ibrutinib in combination with venetoclax and obinutuzumab with standard ibrutinib.
Dr. Woyach and Ms. Thomas reported having no disclosures. Dr. Steensma reported receiving research funding from, and/or serving as a consultant, board member, or adviser for Takeda Pharmaceutical, Syros Pharmaceuticals, Otsuka Pharmaceutical, Onconova Therapeutics, Novartis, Kura Oncology, Janssen, H3 Biosciences, Celgene, Amphivena Therapeutics, and Acceleron Pharma.
SAN DIEGO – In the phase 3 Alliance A041202 trial of older patients with previously untreated chronic lymphocytic leukemia (CLL), ibrutinib showed superior progression-free survival (PFS). Results of the trial were reported by Jennifer A. Woyach, MD, of the Ohio State University in Columbus during a press briefing at the recently concluded American Society of Hematology 2018 meeting. The briefing was based on an abstract from the meeting.
“There was no difference in progression-free survival between ibrutinib and ibrutinib plus rituximab,” said Dr. Woyach. “We undertook this study to determine the most effective therapy for older patients with CLL.” She noted that the findings justify the use of ibrutinib as a standard-of-care treatment for CLL patients aged 65 years and older.
Median age of patients in the study was 71 years and 67% of the patient were men, a profile that is similar, to those of patients with CLL seen at the US Department of Veterans Affairs.
The 2-year PFS was 74% in 183 patients randomized to receive standard chemoimmunotherapy with bendamustine and rituximab (BR), compared with 87% in 182 patients randomized to receive ibrutinib alone (hazard ratio, 0.39 vs. BR), and 88% in 182 patients who received ibrutinib and rituximab (IR; HR, 0.38 vs. BR). Median PFS in this study was 43 months in the BR arm, and was not reached in either of the ibrutinib-containing arms, she said. No significant differences in overall survival (OS) were seen among the treatment arms, which may have been because of short follow-up and the fact that patients in the BR arm were allowed to cross over to ibrutinib if they progressed on treatment.
The results suggest that the additional of rituximab provided little benefit to the patients though it does add to both the costs and the chair time in an infusion center, according to former Association of VA Hematology/Oncology Mary Thomas, MS, CNS, AOCN.
“I think this really does indicate that ibrutinib as front-line therapy, which many clinicians have been doing, is a very reasonable practice,” said David P. Steensma, MD, of Dana-Farber Cancer Institute in Boston, who moderated the press briefing.
Dr. Woyach added, however, that while ibrutinib represents a major therapeutic advance, its cost and its toxicities in older patients are a concern that warrant close monitoring and development of strategies to reduce the need for long-term continuous treatment.
Thomas agreed noting that health care providers needs to be aware of the risk of atrial fib and bleeding when using ibrutinib and to ensure that patient will be able to adhere to daily dosing.
Additional phase 3 studies set to open soon will compare ibrutinib in combination with venetoclax and obinutuzumab with standard ibrutinib.
Dr. Woyach and Ms. Thomas reported having no disclosures. Dr. Steensma reported receiving research funding from, and/or serving as a consultant, board member, or adviser for Takeda Pharmaceutical, Syros Pharmaceuticals, Otsuka Pharmaceutical, Onconova Therapeutics, Novartis, Kura Oncology, Janssen, H3 Biosciences, Celgene, Amphivena Therapeutics, and Acceleron Pharma.
SAN DIEGO – In the phase 3 Alliance A041202 trial of older patients with previously untreated chronic lymphocytic leukemia (CLL), ibrutinib showed superior progression-free survival (PFS). Results of the trial were reported by Jennifer A. Woyach, MD, of the Ohio State University in Columbus during a press briefing at the recently concluded American Society of Hematology 2018 meeting. The briefing was based on an abstract from the meeting.
“There was no difference in progression-free survival between ibrutinib and ibrutinib plus rituximab,” said Dr. Woyach. “We undertook this study to determine the most effective therapy for older patients with CLL.” She noted that the findings justify the use of ibrutinib as a standard-of-care treatment for CLL patients aged 65 years and older.
Median age of patients in the study was 71 years and 67% of the patient were men, a profile that is similar, to those of patients with CLL seen at the US Department of Veterans Affairs.
The 2-year PFS was 74% in 183 patients randomized to receive standard chemoimmunotherapy with bendamustine and rituximab (BR), compared with 87% in 182 patients randomized to receive ibrutinib alone (hazard ratio, 0.39 vs. BR), and 88% in 182 patients who received ibrutinib and rituximab (IR; HR, 0.38 vs. BR). Median PFS in this study was 43 months in the BR arm, and was not reached in either of the ibrutinib-containing arms, she said. No significant differences in overall survival (OS) were seen among the treatment arms, which may have been because of short follow-up and the fact that patients in the BR arm were allowed to cross over to ibrutinib if they progressed on treatment.
The results suggest that the additional of rituximab provided little benefit to the patients though it does add to both the costs and the chair time in an infusion center, according to former Association of VA Hematology/Oncology Mary Thomas, MS, CNS, AOCN.
“I think this really does indicate that ibrutinib as front-line therapy, which many clinicians have been doing, is a very reasonable practice,” said David P. Steensma, MD, of Dana-Farber Cancer Institute in Boston, who moderated the press briefing.
Dr. Woyach added, however, that while ibrutinib represents a major therapeutic advance, its cost and its toxicities in older patients are a concern that warrant close monitoring and development of strategies to reduce the need for long-term continuous treatment.
Thomas agreed noting that health care providers needs to be aware of the risk of atrial fib and bleeding when using ibrutinib and to ensure that patient will be able to adhere to daily dosing.
Additional phase 3 studies set to open soon will compare ibrutinib in combination with venetoclax and obinutuzumab with standard ibrutinib.
Dr. Woyach and Ms. Thomas reported having no disclosures. Dr. Steensma reported receiving research funding from, and/or serving as a consultant, board member, or adviser for Takeda Pharmaceutical, Syros Pharmaceuticals, Otsuka Pharmaceutical, Onconova Therapeutics, Novartis, Kura Oncology, Janssen, H3 Biosciences, Celgene, Amphivena Therapeutics, and Acceleron Pharma.
TNBC survival appears better when adjuvant chemotherapy is delivered within 30 days
SAN ANTONIO – The longer the delay in initiating adjuvant chemotherapy, the worse the survival in patients with triple-negative breast cancer (TNBC), findings from a review of nearly 700 cases suggest.
Delays of more than 30 days between surgery and initiation of chemotherapy were associated with lower disease-free survival (DFS), distant recurrence–free survival (DRFS), and overall survival (OS), Zaida Morante, MD, reported at the San Antonio Breast Cancer Symposium.
In 687 women with clinical stage I, II, or III TNBC who were diagnosed at the Instituto Nacional de Enfermedades Neoplasicas in Lima, Peru, during 2000-2014 and followed for a median of 8.5 years, time to chemotherapy was less than 30 days in 189 patients (27.5%), 31-60 days in 329 patients (47.9%), 61-90 days in 115 patients (16.7%), and more than 91 days in 54 patients (7.9%), said Dr. Morante, a medical oncologist at the institute.
Overall survival at 10 years was 82% in those who received chemotherapy within 30 days of surgery, compared with 67.4%, 67.1%, and 65.1% in those treated at 31-60, 61-90, and more than 91 days after surgery, respectively, she said.
“The difference was consistent across the different periods of the evaluation,” she said during a press briefing at the symposium. “Additionally, the benefit of receiving chemotherapy within 30 days exists and is statistically significant for [nodal status] N0 and N1 (hazard ratios, 1.701 and 2.498).”
In those with N2 and N3 nodal status, there was a numerical difference, but it didn’t reach statistical significance.
DFS was also significantly worse if treated later than 30 days after surgery; those treated within 30 days had 10-year DFS of 81.4%, compared with 68.8%, 70.8%, and 68.1% in the other groups, respectively. The difference was even more pronounced for 10-year DRFS, which was 80.2%, 64.9%, 67.5%, and 58.6% in the groups, respectively.
Multivariate analyses confirmed that time to adjuvant chemotherapy was an independent prognostic factor for survival, she said, noting that compared with patients treated within 30 days of surgery, those treated at 31-60 days had 1.9-fold increased risk of death, and those treated at 61-90 days had a 2.4-fold increased risk of death.
“The difference in 10-year overall survival rates between receiving chemotherapy within 30 days after surgery and after 30 days was more than 10%,” she said. “These results represent a feasible opportunity for improving outcomes in triple-negative breast cancer patients.”
Although only 28% of patients in this review received adjuvant chemotherapy within 30 days, most patients in the United States “will fall within the 30 days and under” category, said press briefing moderator Carlos Arteaga, MD, professor and director of the Harold C. Simmons Comprehensive Cancer Center at UT Southwestern Medical Center in Dallas.
However, the findings might suggest a greater role for neoadjuvant chemotherapy in these patients.
“Because this is systemic therapy ... it’s treating the systemic disease. I wonder if this is arguing ... that we need to have an impetus to deliver the systemic therapy as soon as we can – early, even before the operation,” he said.
Indeed, while timing isn’t everything, Dr. Morante’s findings and others presented at the meeting “highlight the possibility that perhaps it is more important than we previously suspected,” discussant Joseph A. Sparano, MD, said at the meeting, adding that the findings raise questions about current paradigms for management of breast cancer.
“We now have substantial data suggesting that the timing of adjuvant chemotherapy matters in triple-negative breast cancer, and that 30 days may be optimal,” said Dr. Sparano, professor at the Albert Einstein College of Medicine, New York.
“This doesn’t mean that patients who may not be ready for the chemotherapy because of complications related to the surgery should be forced into a situation where they are at higher risk from receiving the chemotherapy, but nevertheless, the results are important,” he said.
Dr. Morante and Dr. Arteaga each reported having no relevant conflicts of interest to declare. Dr. Sparano has received consulting fees from Roche, Eli Lilly, Novartis, Celldex, AstraZeneca, Pfizer, and Adgero. He also has ownership interests with MetaStat.
SOURCE: Morante Z et al. SABCS 2018, Abstract GS2-05.
SAN ANTONIO – The longer the delay in initiating adjuvant chemotherapy, the worse the survival in patients with triple-negative breast cancer (TNBC), findings from a review of nearly 700 cases suggest.
Delays of more than 30 days between surgery and initiation of chemotherapy were associated with lower disease-free survival (DFS), distant recurrence–free survival (DRFS), and overall survival (OS), Zaida Morante, MD, reported at the San Antonio Breast Cancer Symposium.
In 687 women with clinical stage I, II, or III TNBC who were diagnosed at the Instituto Nacional de Enfermedades Neoplasicas in Lima, Peru, during 2000-2014 and followed for a median of 8.5 years, time to chemotherapy was less than 30 days in 189 patients (27.5%), 31-60 days in 329 patients (47.9%), 61-90 days in 115 patients (16.7%), and more than 91 days in 54 patients (7.9%), said Dr. Morante, a medical oncologist at the institute.
Overall survival at 10 years was 82% in those who received chemotherapy within 30 days of surgery, compared with 67.4%, 67.1%, and 65.1% in those treated at 31-60, 61-90, and more than 91 days after surgery, respectively, she said.
“The difference was consistent across the different periods of the evaluation,” she said during a press briefing at the symposium. “Additionally, the benefit of receiving chemotherapy within 30 days exists and is statistically significant for [nodal status] N0 and N1 (hazard ratios, 1.701 and 2.498).”
In those with N2 and N3 nodal status, there was a numerical difference, but it didn’t reach statistical significance.
DFS was also significantly worse if treated later than 30 days after surgery; those treated within 30 days had 10-year DFS of 81.4%, compared with 68.8%, 70.8%, and 68.1% in the other groups, respectively. The difference was even more pronounced for 10-year DRFS, which was 80.2%, 64.9%, 67.5%, and 58.6% in the groups, respectively.
Multivariate analyses confirmed that time to adjuvant chemotherapy was an independent prognostic factor for survival, she said, noting that compared with patients treated within 30 days of surgery, those treated at 31-60 days had 1.9-fold increased risk of death, and those treated at 61-90 days had a 2.4-fold increased risk of death.
“The difference in 10-year overall survival rates between receiving chemotherapy within 30 days after surgery and after 30 days was more than 10%,” she said. “These results represent a feasible opportunity for improving outcomes in triple-negative breast cancer patients.”
Although only 28% of patients in this review received adjuvant chemotherapy within 30 days, most patients in the United States “will fall within the 30 days and under” category, said press briefing moderator Carlos Arteaga, MD, professor and director of the Harold C. Simmons Comprehensive Cancer Center at UT Southwestern Medical Center in Dallas.
However, the findings might suggest a greater role for neoadjuvant chemotherapy in these patients.
“Because this is systemic therapy ... it’s treating the systemic disease. I wonder if this is arguing ... that we need to have an impetus to deliver the systemic therapy as soon as we can – early, even before the operation,” he said.
Indeed, while timing isn’t everything, Dr. Morante’s findings and others presented at the meeting “highlight the possibility that perhaps it is more important than we previously suspected,” discussant Joseph A. Sparano, MD, said at the meeting, adding that the findings raise questions about current paradigms for management of breast cancer.
“We now have substantial data suggesting that the timing of adjuvant chemotherapy matters in triple-negative breast cancer, and that 30 days may be optimal,” said Dr. Sparano, professor at the Albert Einstein College of Medicine, New York.
“This doesn’t mean that patients who may not be ready for the chemotherapy because of complications related to the surgery should be forced into a situation where they are at higher risk from receiving the chemotherapy, but nevertheless, the results are important,” he said.
Dr. Morante and Dr. Arteaga each reported having no relevant conflicts of interest to declare. Dr. Sparano has received consulting fees from Roche, Eli Lilly, Novartis, Celldex, AstraZeneca, Pfizer, and Adgero. He also has ownership interests with MetaStat.
SOURCE: Morante Z et al. SABCS 2018, Abstract GS2-05.
SAN ANTONIO – The longer the delay in initiating adjuvant chemotherapy, the worse the survival in patients with triple-negative breast cancer (TNBC), findings from a review of nearly 700 cases suggest.
Delays of more than 30 days between surgery and initiation of chemotherapy were associated with lower disease-free survival (DFS), distant recurrence–free survival (DRFS), and overall survival (OS), Zaida Morante, MD, reported at the San Antonio Breast Cancer Symposium.
In 687 women with clinical stage I, II, or III TNBC who were diagnosed at the Instituto Nacional de Enfermedades Neoplasicas in Lima, Peru, during 2000-2014 and followed for a median of 8.5 years, time to chemotherapy was less than 30 days in 189 patients (27.5%), 31-60 days in 329 patients (47.9%), 61-90 days in 115 patients (16.7%), and more than 91 days in 54 patients (7.9%), said Dr. Morante, a medical oncologist at the institute.
Overall survival at 10 years was 82% in those who received chemotherapy within 30 days of surgery, compared with 67.4%, 67.1%, and 65.1% in those treated at 31-60, 61-90, and more than 91 days after surgery, respectively, she said.
“The difference was consistent across the different periods of the evaluation,” she said during a press briefing at the symposium. “Additionally, the benefit of receiving chemotherapy within 30 days exists and is statistically significant for [nodal status] N0 and N1 (hazard ratios, 1.701 and 2.498).”
In those with N2 and N3 nodal status, there was a numerical difference, but it didn’t reach statistical significance.
DFS was also significantly worse if treated later than 30 days after surgery; those treated within 30 days had 10-year DFS of 81.4%, compared with 68.8%, 70.8%, and 68.1% in the other groups, respectively. The difference was even more pronounced for 10-year DRFS, which was 80.2%, 64.9%, 67.5%, and 58.6% in the groups, respectively.
Multivariate analyses confirmed that time to adjuvant chemotherapy was an independent prognostic factor for survival, she said, noting that compared with patients treated within 30 days of surgery, those treated at 31-60 days had 1.9-fold increased risk of death, and those treated at 61-90 days had a 2.4-fold increased risk of death.
“The difference in 10-year overall survival rates between receiving chemotherapy within 30 days after surgery and after 30 days was more than 10%,” she said. “These results represent a feasible opportunity for improving outcomes in triple-negative breast cancer patients.”
Although only 28% of patients in this review received adjuvant chemotherapy within 30 days, most patients in the United States “will fall within the 30 days and under” category, said press briefing moderator Carlos Arteaga, MD, professor and director of the Harold C. Simmons Comprehensive Cancer Center at UT Southwestern Medical Center in Dallas.
However, the findings might suggest a greater role for neoadjuvant chemotherapy in these patients.
“Because this is systemic therapy ... it’s treating the systemic disease. I wonder if this is arguing ... that we need to have an impetus to deliver the systemic therapy as soon as we can – early, even before the operation,” he said.
Indeed, while timing isn’t everything, Dr. Morante’s findings and others presented at the meeting “highlight the possibility that perhaps it is more important than we previously suspected,” discussant Joseph A. Sparano, MD, said at the meeting, adding that the findings raise questions about current paradigms for management of breast cancer.
“We now have substantial data suggesting that the timing of adjuvant chemotherapy matters in triple-negative breast cancer, and that 30 days may be optimal,” said Dr. Sparano, professor at the Albert Einstein College of Medicine, New York.
“This doesn’t mean that patients who may not be ready for the chemotherapy because of complications related to the surgery should be forced into a situation where they are at higher risk from receiving the chemotherapy, but nevertheless, the results are important,” he said.
Dr. Morante and Dr. Arteaga each reported having no relevant conflicts of interest to declare. Dr. Sparano has received consulting fees from Roche, Eli Lilly, Novartis, Celldex, AstraZeneca, Pfizer, and Adgero. He also has ownership interests with MetaStat.
SOURCE: Morante Z et al. SABCS 2018, Abstract GS2-05.
REPORTING FROM SABCS 2018
Key clinical point: Outcomes are improved with adjuvant chemotherapy within 30 days of surgery, compared with beyond 30 days, in triple-negative breast cancer.
Major finding: 10-year overall survival was 82% with chemotherapy within 30 days of surgery versus 67.4%, 67.1%, and 65.1% with chemotherapy at 31-60, 61-90, and more than 91 days after surgery, respectively.
Study details: A retrospective review of 687 cases of TNBC.
Disclosures: Dr. Morante and Dr. Arteaga each reported having no relevant conflicts of interest to declare. Dr. Sparano has received consulting fees from Roche, Eli Lilly, Novartis, Celldex, AstraZeneca, Pfizer, and Adgero. He also has ownership interests with MetaStat.
Source: Morante Z et al., SABCS 2018 Abstract GS2-05.
ICU-acquired pneumonia mortality risk may be underestimated
In a large prospectively collected database, the risk of death at 30 days in ICU patients was far greater in those with hospital-acquired pneumonia (HAP) than in those with ventilator-associated pneumonia (VAP) even after adjustment for prognostic factors, according to a large study that compared mortality risk for these complications.
The data for this newly published study were drawn from an evaluation of 14,212 patients treated at 23 ICUs participating in a collaborative French network OUTCOMEREA and published Critical Care Medicine.
HAP in ICU patients “was associated with an 82% increase in the risk of death at day 30,” reported a team of investigators led by Wafa Ibn Saied, MD, of the Université Paris Diderot. Although VAP and HAP were independent risk factors (P both less than .0001) for death at 30 days, VAP increased risk by 38%, less than half of HAP, which increased risk by 82%.
From an observational but prospective database initiated in 1997, this study evaluated 7,735 ICU patients at risk for VAP and 9,747 at risk for HAP. Of those at risk, defined by several factors including an ICU stay of more than 48 hours, HAP developed in 8% and VAP developed in 1%.
The 30-day mortality rates at 30 days after pneumonia were 23.9% for HAP and 28.4% for VAP. The greater risk of death by HR was identified after an analysis that adjusted for mortality risk factors, the adequacy of initial treatment, and other factors, such as prior history of pneumonia.
In HAP patients, the rate of mortality at 30 days was 32% in the 75 who were reintubated but only 16% in the 101 who were not. Adequate empirical therapy within the first 24 hours for HAP was not associated with a reduction in the risk of death.
As in the HAP patients, mortality was not significantly higher in VAP patients who received inadequate empirical therapy, compared with those who did, according to the authors.
Previous studies have suggested that both HAP and VAP increase risk of death in ICU patients, but the authors of this study believe that the relative risk of HAP “is underappreciated.” They asserted, based on these most recent data as well as on previously published analyses, that nonventilated HAP results in “significant increases in cost, length of stay, and mortality.”
The researchers had no disclosures.
SOURCE: Saied WI et al. Crit Care Med. 2018 Nov 7. doi: 10.1097/CCM.0000000000003553.
In a large prospectively collected database, the risk of death at 30 days in ICU patients was far greater in those with hospital-acquired pneumonia (HAP) than in those with ventilator-associated pneumonia (VAP) even after adjustment for prognostic factors, according to a large study that compared mortality risk for these complications.
The data for this newly published study were drawn from an evaluation of 14,212 patients treated at 23 ICUs participating in a collaborative French network OUTCOMEREA and published Critical Care Medicine.
HAP in ICU patients “was associated with an 82% increase in the risk of death at day 30,” reported a team of investigators led by Wafa Ibn Saied, MD, of the Université Paris Diderot. Although VAP and HAP were independent risk factors (P both less than .0001) for death at 30 days, VAP increased risk by 38%, less than half of HAP, which increased risk by 82%.
From an observational but prospective database initiated in 1997, this study evaluated 7,735 ICU patients at risk for VAP and 9,747 at risk for HAP. Of those at risk, defined by several factors including an ICU stay of more than 48 hours, HAP developed in 8% and VAP developed in 1%.
The 30-day mortality rates at 30 days after pneumonia were 23.9% for HAP and 28.4% for VAP. The greater risk of death by HR was identified after an analysis that adjusted for mortality risk factors, the adequacy of initial treatment, and other factors, such as prior history of pneumonia.
In HAP patients, the rate of mortality at 30 days was 32% in the 75 who were reintubated but only 16% in the 101 who were not. Adequate empirical therapy within the first 24 hours for HAP was not associated with a reduction in the risk of death.
As in the HAP patients, mortality was not significantly higher in VAP patients who received inadequate empirical therapy, compared with those who did, according to the authors.
Previous studies have suggested that both HAP and VAP increase risk of death in ICU patients, but the authors of this study believe that the relative risk of HAP “is underappreciated.” They asserted, based on these most recent data as well as on previously published analyses, that nonventilated HAP results in “significant increases in cost, length of stay, and mortality.”
The researchers had no disclosures.
SOURCE: Saied WI et al. Crit Care Med. 2018 Nov 7. doi: 10.1097/CCM.0000000000003553.
In a large prospectively collected database, the risk of death at 30 days in ICU patients was far greater in those with hospital-acquired pneumonia (HAP) than in those with ventilator-associated pneumonia (VAP) even after adjustment for prognostic factors, according to a large study that compared mortality risk for these complications.
The data for this newly published study were drawn from an evaluation of 14,212 patients treated at 23 ICUs participating in a collaborative French network OUTCOMEREA and published Critical Care Medicine.
HAP in ICU patients “was associated with an 82% increase in the risk of death at day 30,” reported a team of investigators led by Wafa Ibn Saied, MD, of the Université Paris Diderot. Although VAP and HAP were independent risk factors (P both less than .0001) for death at 30 days, VAP increased risk by 38%, less than half of HAP, which increased risk by 82%.
From an observational but prospective database initiated in 1997, this study evaluated 7,735 ICU patients at risk for VAP and 9,747 at risk for HAP. Of those at risk, defined by several factors including an ICU stay of more than 48 hours, HAP developed in 8% and VAP developed in 1%.
The 30-day mortality rates at 30 days after pneumonia were 23.9% for HAP and 28.4% for VAP. The greater risk of death by HR was identified after an analysis that adjusted for mortality risk factors, the adequacy of initial treatment, and other factors, such as prior history of pneumonia.
In HAP patients, the rate of mortality at 30 days was 32% in the 75 who were reintubated but only 16% in the 101 who were not. Adequate empirical therapy within the first 24 hours for HAP was not associated with a reduction in the risk of death.
As in the HAP patients, mortality was not significantly higher in VAP patients who received inadequate empirical therapy, compared with those who did, according to the authors.
Previous studies have suggested that both HAP and VAP increase risk of death in ICU patients, but the authors of this study believe that the relative risk of HAP “is underappreciated.” They asserted, based on these most recent data as well as on previously published analyses, that nonventilated HAP results in “significant increases in cost, length of stay, and mortality.”
The researchers had no disclosures.
SOURCE: Saied WI et al. Crit Care Med. 2018 Nov 7. doi: 10.1097/CCM.0000000000003553.
FROM CRITICAL CARE MEDICINE
Key clinical point: Hospital-acquired pneumonia poses a greater risk of death in the ICU than ventilator-associated pneumonia.
Major finding: After prognostic adjustment, the mortality hazard ratios were 1.82 and 1.38 for HAP and VAP, respectively.
Study details: Observational cohort study.
Disclosures: The researchers had no disclosures.
Source: Saied WI et al. Crit Care Med. 2018 Nov 7; doi: 10.1097/CCM.0000000000003553.
CTC matches MD judgment for mBC therapeutic choice
SAN ANTONIO – For patients with estrogen-receptor positive, HER2-negative metastatic breast cancer, the use of circulating tumor cell (CTC) counts can help clinicians decide with confidence between ordering first-line hormonal therapy or chemotherapy, investigators say.
In the phase 3 STIC CTC trial, patients were randomly assigned to receive therapy based on either the clinician’s judgment of the best course of therapy for each patient; or on the CTC count with a cutoff of less than 5 CT/7.5 mL, indicating hormonal therapy; and 5 CTC/7.5 mL or above, indicating higher-risk disease requiring chemotherapy. In the clinician’s choice arm, the CTC reading was recorded but not implemented, and in the CTC arm, the clinician’s choice was dismissed.
The trial met its primary noninferiority endpoint, indicating that, in the overall population, CTC counts can provide clinician’s with confidence in the therapeutic choice, said Francois-Clement Bidard, MD, PhD, from Institut Curie in Paris.
In a video interview, Dr. Bidard discussed the trial findings, including the provocative exploratory analysis suggesting that, in patients in whom there is discordance between CTC and clinician choice, chemotherapy may be a better therapeutic option.
The study was funded by the National Cancer Institute of France, Institut Curie, and Menarini Silicon Biosystems. Dr. Bidard disclosed research funding and travel grants from Menarini.
SAN ANTONIO – For patients with estrogen-receptor positive, HER2-negative metastatic breast cancer, the use of circulating tumor cell (CTC) counts can help clinicians decide with confidence between ordering first-line hormonal therapy or chemotherapy, investigators say.
In the phase 3 STIC CTC trial, patients were randomly assigned to receive therapy based on either the clinician’s judgment of the best course of therapy for each patient; or on the CTC count with a cutoff of less than 5 CT/7.5 mL, indicating hormonal therapy; and 5 CTC/7.5 mL or above, indicating higher-risk disease requiring chemotherapy. In the clinician’s choice arm, the CTC reading was recorded but not implemented, and in the CTC arm, the clinician’s choice was dismissed.
The trial met its primary noninferiority endpoint, indicating that, in the overall population, CTC counts can provide clinician’s with confidence in the therapeutic choice, said Francois-Clement Bidard, MD, PhD, from Institut Curie in Paris.
In a video interview, Dr. Bidard discussed the trial findings, including the provocative exploratory analysis suggesting that, in patients in whom there is discordance between CTC and clinician choice, chemotherapy may be a better therapeutic option.
The study was funded by the National Cancer Institute of France, Institut Curie, and Menarini Silicon Biosystems. Dr. Bidard disclosed research funding and travel grants from Menarini.
SAN ANTONIO – For patients with estrogen-receptor positive, HER2-negative metastatic breast cancer, the use of circulating tumor cell (CTC) counts can help clinicians decide with confidence between ordering first-line hormonal therapy or chemotherapy, investigators say.
In the phase 3 STIC CTC trial, patients were randomly assigned to receive therapy based on either the clinician’s judgment of the best course of therapy for each patient; or on the CTC count with a cutoff of less than 5 CT/7.5 mL, indicating hormonal therapy; and 5 CTC/7.5 mL or above, indicating higher-risk disease requiring chemotherapy. In the clinician’s choice arm, the CTC reading was recorded but not implemented, and in the CTC arm, the clinician’s choice was dismissed.
The trial met its primary noninferiority endpoint, indicating that, in the overall population, CTC counts can provide clinician’s with confidence in the therapeutic choice, said Francois-Clement Bidard, MD, PhD, from Institut Curie in Paris.
In a video interview, Dr. Bidard discussed the trial findings, including the provocative exploratory analysis suggesting that, in patients in whom there is discordance between CTC and clinician choice, chemotherapy may be a better therapeutic option.
The study was funded by the National Cancer Institute of France, Institut Curie, and Menarini Silicon Biosystems. Dr. Bidard disclosed research funding and travel grants from Menarini.
REPORTING FROM SABCS 2018
Proposed neuroblastoma classification scheme hinges on telomere maintenance mechanisms
Telomere maintenance mechanisms, RAS mutations, and p53 mutations can be used to mechanistically classify clinical phenotypes of neuroblastoma, according to investigators.
Genomic analysis of neuroblastomas showed that the aforementioned markers were strongly associated with outcome and other disease characteristics, reported Sandra Ackermann, MD, of the department of experimental pediatric oncology at the University Children’s Hospital of Cologne (Germany), and her colleagues.
Although previous studies have shown relationships between genetic alterations and behavior of neuroblastomas, “to date, these genomic data have not produced a coherent model of pathogenesis that can explain the extremely divergent clinical phenotypes of neuroblastoma,” the investigators wrote in Science.
The present study involved genomic sequencing of 416 pretreatment neuroblastomas, with tests for telomere maintenance mechanisms, RAS-pathway mutations, and p53-pathway mutations.
Based on existing data, the investigators first devised a panel based on 17 genes related to the RAS pathway (11 genes included ALK) and 6 related to the p53 pathway. In 198 cases, 28 tested positive for RAS- or p53-pathway abnormalities (17.8%). Positivity was more common in high-risk tumors than non–high-risk tumors (21.3% vs. 13.3%; P = .048), and in both risk groups, positivity was associated with poor outcome (hazard ratio, 2.056; P = .001).
However, because clinical courses varied widely among non–high-risk patients with RAS/p53 mutations, the investigators recognized that a piece of the puzzle was missing. They hypothesized that telomere maintenance mechanisms could also be playing a role. Following several intervening experiments, the investigators devised telomere maintenance mechanism testing, defined by MYCN amplification or TERT rearrangements, elevated TERT expression if negative for these abnormalities, or presence of ALT-associated promyelocytic leukemia nuclear bodies. Subsequent testing revealed that positivity for these parameters was associated with a HR of 5.184 (P less than .001), thereby confirming that telomere maintenance mechanisms could independently predict survival.
“Together, our findings demonstrate that the divergent clinical phenotypes of human neuroblastoma are driven by molecular alterations affecting telomere maintenance and RAS or p53 pathways, suggesting a mechanistic classification of this malignancy,” the authors concluded.
The proposed classification scheme also includes associations with other genetic features (tumor cell ploidy, segmental copy number alterations, MYCN/TERT/ATRX alterations, and gene expression favorability) and clinical characteristics (stage of disease and age at diagnosis).
The study was funded by the German Cancer Aid, the German Ministry of Science and Education, the MYC-NET, the Deutsche Forschungsgemeinschaft, the Berlin Institute of Health, the European Union, and others. One coauthor reported financial relationships with Biogazelle and pxlence, and another reported consulting fees from NEO New Oncology.
SOURCE: Ackermann S et al. Science. 2018 Dec 7. doi: 10.1126/science.aat6768.
Telomere maintenance mechanisms, RAS mutations, and p53 mutations can be used to mechanistically classify clinical phenotypes of neuroblastoma, according to investigators.
Genomic analysis of neuroblastomas showed that the aforementioned markers were strongly associated with outcome and other disease characteristics, reported Sandra Ackermann, MD, of the department of experimental pediatric oncology at the University Children’s Hospital of Cologne (Germany), and her colleagues.
Although previous studies have shown relationships between genetic alterations and behavior of neuroblastomas, “to date, these genomic data have not produced a coherent model of pathogenesis that can explain the extremely divergent clinical phenotypes of neuroblastoma,” the investigators wrote in Science.
The present study involved genomic sequencing of 416 pretreatment neuroblastomas, with tests for telomere maintenance mechanisms, RAS-pathway mutations, and p53-pathway mutations.
Based on existing data, the investigators first devised a panel based on 17 genes related to the RAS pathway (11 genes included ALK) and 6 related to the p53 pathway. In 198 cases, 28 tested positive for RAS- or p53-pathway abnormalities (17.8%). Positivity was more common in high-risk tumors than non–high-risk tumors (21.3% vs. 13.3%; P = .048), and in both risk groups, positivity was associated with poor outcome (hazard ratio, 2.056; P = .001).
However, because clinical courses varied widely among non–high-risk patients with RAS/p53 mutations, the investigators recognized that a piece of the puzzle was missing. They hypothesized that telomere maintenance mechanisms could also be playing a role. Following several intervening experiments, the investigators devised telomere maintenance mechanism testing, defined by MYCN amplification or TERT rearrangements, elevated TERT expression if negative for these abnormalities, or presence of ALT-associated promyelocytic leukemia nuclear bodies. Subsequent testing revealed that positivity for these parameters was associated with a HR of 5.184 (P less than .001), thereby confirming that telomere maintenance mechanisms could independently predict survival.
“Together, our findings demonstrate that the divergent clinical phenotypes of human neuroblastoma are driven by molecular alterations affecting telomere maintenance and RAS or p53 pathways, suggesting a mechanistic classification of this malignancy,” the authors concluded.
The proposed classification scheme also includes associations with other genetic features (tumor cell ploidy, segmental copy number alterations, MYCN/TERT/ATRX alterations, and gene expression favorability) and clinical characteristics (stage of disease and age at diagnosis).
The study was funded by the German Cancer Aid, the German Ministry of Science and Education, the MYC-NET, the Deutsche Forschungsgemeinschaft, the Berlin Institute of Health, the European Union, and others. One coauthor reported financial relationships with Biogazelle and pxlence, and another reported consulting fees from NEO New Oncology.
SOURCE: Ackermann S et al. Science. 2018 Dec 7. doi: 10.1126/science.aat6768.
Telomere maintenance mechanisms, RAS mutations, and p53 mutations can be used to mechanistically classify clinical phenotypes of neuroblastoma, according to investigators.
Genomic analysis of neuroblastomas showed that the aforementioned markers were strongly associated with outcome and other disease characteristics, reported Sandra Ackermann, MD, of the department of experimental pediatric oncology at the University Children’s Hospital of Cologne (Germany), and her colleagues.
Although previous studies have shown relationships between genetic alterations and behavior of neuroblastomas, “to date, these genomic data have not produced a coherent model of pathogenesis that can explain the extremely divergent clinical phenotypes of neuroblastoma,” the investigators wrote in Science.
The present study involved genomic sequencing of 416 pretreatment neuroblastomas, with tests for telomere maintenance mechanisms, RAS-pathway mutations, and p53-pathway mutations.
Based on existing data, the investigators first devised a panel based on 17 genes related to the RAS pathway (11 genes included ALK) and 6 related to the p53 pathway. In 198 cases, 28 tested positive for RAS- or p53-pathway abnormalities (17.8%). Positivity was more common in high-risk tumors than non–high-risk tumors (21.3% vs. 13.3%; P = .048), and in both risk groups, positivity was associated with poor outcome (hazard ratio, 2.056; P = .001).
However, because clinical courses varied widely among non–high-risk patients with RAS/p53 mutations, the investigators recognized that a piece of the puzzle was missing. They hypothesized that telomere maintenance mechanisms could also be playing a role. Following several intervening experiments, the investigators devised telomere maintenance mechanism testing, defined by MYCN amplification or TERT rearrangements, elevated TERT expression if negative for these abnormalities, or presence of ALT-associated promyelocytic leukemia nuclear bodies. Subsequent testing revealed that positivity for these parameters was associated with a HR of 5.184 (P less than .001), thereby confirming that telomere maintenance mechanisms could independently predict survival.
“Together, our findings demonstrate that the divergent clinical phenotypes of human neuroblastoma are driven by molecular alterations affecting telomere maintenance and RAS or p53 pathways, suggesting a mechanistic classification of this malignancy,” the authors concluded.
The proposed classification scheme also includes associations with other genetic features (tumor cell ploidy, segmental copy number alterations, MYCN/TERT/ATRX alterations, and gene expression favorability) and clinical characteristics (stage of disease and age at diagnosis).
The study was funded by the German Cancer Aid, the German Ministry of Science and Education, the MYC-NET, the Deutsche Forschungsgemeinschaft, the Berlin Institute of Health, the European Union, and others. One coauthor reported financial relationships with Biogazelle and pxlence, and another reported consulting fees from NEO New Oncology.
SOURCE: Ackermann S et al. Science. 2018 Dec 7. doi: 10.1126/science.aat6768.
FROM SCIENCE
Key clinical point: A proposed mechanistic classification of clinical phenotypes in neuroblastoma is based on presence of telomere maintenance mechanisms, along with RAS and p53 mutations.
Major finding: The presence of telomere maintenance mechanisms was associated with a hazard ratio of 5.184 (P less than .001).
Study details: A genome sequencing of 416 pretreatment neuroblastomas, with tests for telomere maintenance mechanisms, RAS-pathway mutations, and p53-pathway mutations.
Disclosures: The study was funded by the German Cancer Aid, the German Ministry of Science and Education, the MYC-NET, the Deutsche Forschungsgemeinschaft, the Berlin Institute of Health, the European Union, and others. One coauthor reported financial relationships with Biogazelle and pxlence, and another reported consulting fees from NEO New Oncology.
Source: Ackermann S et al. Science. 2018 Dec 7. doi: 10.1126/science.aat6768.
Potty pathogens in space, fundus photos, and ethnic microbiomes
The earth is not enough
Earthly competitors have proved to be unworthy, so this week, Bacteria vs. the World visits the International Space Station, which – and we double-checked this – is in space. It’s a pretty exclusive location, and admission is by invitation only. Unless, of, course, you happen to be the ultimate hitchhiker. Four samples taken from the toilet of the ISS (and one from a piece of exercise equipment) were found to contain unknown strains of antibiotic-resistant Enterobacter bugandensis, investigators reported (BMC Microbiol. 2018 Nov 23;18[1]:175).
These bacterial stowaways were not virulent, lead author Nitin Singh, PhD, of the Jet Propulsion Laboratory said in a separate statement. But an analysis conducted by the team “reveals that the ISS isolates have a 79% probability of being a human pathogen.”
So, what does this mean for future space exploration? Cue the “Star Trek” music: “Space … the final frontier. These are the voyages of the bacterial transport ship Enterprise.”
Putting the FUN in fundus photos
You just got even more dependent on your phone: The American Academy of Opthalmology has published guidelines on how to use smartphones to take fundus photography, a.k.a. photographs of the back of the eye.
Advancement in smartphone optical quality has turned them into an important clinical tool, especially for specialists in low-funded or rural areas who don’t have access to imaging systems. Doctors can purchase special lenses and phone software to take these photos and then can easily upload the images to their Instagram accounts. (Even doctors need likes.)
An eye hospital in India has taken fundus accessibility a step further and posted a video on YouTube showing how to make a functional fundus camera that costs only 100 rupees. All you need in some cardboard, a water bottle, and a lens. “MacGyver: Chennai Edition.”
I feel it in my gut
Whoever said “inside, we’re all the same” clearly wasn’t considering the gut. A study from Vanderbilt University comprising 1,700 American subjects found that differences in gut microbiomes are most consistently linked with ethnicity. Vanderbilt biologist Seth Bordenstein emphasized how changing the gut microbiome can lead to curing illness but that it’s imperative that medical professionals understand how the gut differs across ethnicities.
Researchers found 12 types of bacteria that vary in abundancy by ethnicity. No comment on whether this was linked to differences in cuisine, but this writer fervently hopes new research arrives proving that tacos produce the healthiest gut microbiome.
F-bombing blood cancer
Call it a tale of two Toms.
TV newsman Tom Brokaw, who has multiple myeloma, says he’s become the “poster boy” for blood cancer. At first, though, he kept his diagnosis secret from just about everyone. But occasionally he let his emotions get the best of him. Especially when he’d see a Manhattan bus stop ad spotlighting the chiseled body of another Tom: the quarterback named Brady.
As he explained in a presentation at the annual meeting of the American Society of Hematology, he found it harder to get around because of back problems, which are common in multiple myeloma. As a result, he couldn’t manage to get to the office.
Still, “every day I’d force myself to leave the walker at home,” he recalled. “In that cold and sleety fall, I’d walk half a block to the coffee shop to get a bagel. There was this enormous new bus stop, with an animated advertisement board. Looking right at me was Tom Brady, advertising Ugg boots. I’d look down 79th Street at every inch of Tom Brady, and all the little old ladies were mooning over him as they were getting on the bus.”
Brokaw knew just what to do to make himself feel better. “I’d hobble over and look at him and drop the F-bomb on him every morning. Frankly, it was therapeutic for me.”
Later, he met the New England Patriots quarterback and told him the story, replacing “F-bomb” with the real word. “He had this little posse with him, and they roared. They said nobody talks to Tom like that.”
Brokaw still resists pleas to slow down from concerned loved ones, such as his emergency physician daughter. “My birth certificate says I’m 78 years old,” he said, “but I still think I’m 38 anchoring the news.” And still tossing tight-spiral F-bombs at cancer and gridiron G.O.A.T.s alike.
The earth is not enough
Earthly competitors have proved to be unworthy, so this week, Bacteria vs. the World visits the International Space Station, which – and we double-checked this – is in space. It’s a pretty exclusive location, and admission is by invitation only. Unless, of, course, you happen to be the ultimate hitchhiker. Four samples taken from the toilet of the ISS (and one from a piece of exercise equipment) were found to contain unknown strains of antibiotic-resistant Enterobacter bugandensis, investigators reported (BMC Microbiol. 2018 Nov 23;18[1]:175).
These bacterial stowaways were not virulent, lead author Nitin Singh, PhD, of the Jet Propulsion Laboratory said in a separate statement. But an analysis conducted by the team “reveals that the ISS isolates have a 79% probability of being a human pathogen.”
So, what does this mean for future space exploration? Cue the “Star Trek” music: “Space … the final frontier. These are the voyages of the bacterial transport ship Enterprise.”
Putting the FUN in fundus photos
You just got even more dependent on your phone: The American Academy of Opthalmology has published guidelines on how to use smartphones to take fundus photography, a.k.a. photographs of the back of the eye.
Advancement in smartphone optical quality has turned them into an important clinical tool, especially for specialists in low-funded or rural areas who don’t have access to imaging systems. Doctors can purchase special lenses and phone software to take these photos and then can easily upload the images to their Instagram accounts. (Even doctors need likes.)
An eye hospital in India has taken fundus accessibility a step further and posted a video on YouTube showing how to make a functional fundus camera that costs only 100 rupees. All you need in some cardboard, a water bottle, and a lens. “MacGyver: Chennai Edition.”
I feel it in my gut
Whoever said “inside, we’re all the same” clearly wasn’t considering the gut. A study from Vanderbilt University comprising 1,700 American subjects found that differences in gut microbiomes are most consistently linked with ethnicity. Vanderbilt biologist Seth Bordenstein emphasized how changing the gut microbiome can lead to curing illness but that it’s imperative that medical professionals understand how the gut differs across ethnicities.
Researchers found 12 types of bacteria that vary in abundancy by ethnicity. No comment on whether this was linked to differences in cuisine, but this writer fervently hopes new research arrives proving that tacos produce the healthiest gut microbiome.
F-bombing blood cancer
Call it a tale of two Toms.
TV newsman Tom Brokaw, who has multiple myeloma, says he’s become the “poster boy” for blood cancer. At first, though, he kept his diagnosis secret from just about everyone. But occasionally he let his emotions get the best of him. Especially when he’d see a Manhattan bus stop ad spotlighting the chiseled body of another Tom: the quarterback named Brady.
As he explained in a presentation at the annual meeting of the American Society of Hematology, he found it harder to get around because of back problems, which are common in multiple myeloma. As a result, he couldn’t manage to get to the office.
Still, “every day I’d force myself to leave the walker at home,” he recalled. “In that cold and sleety fall, I’d walk half a block to the coffee shop to get a bagel. There was this enormous new bus stop, with an animated advertisement board. Looking right at me was Tom Brady, advertising Ugg boots. I’d look down 79th Street at every inch of Tom Brady, and all the little old ladies were mooning over him as they were getting on the bus.”
Brokaw knew just what to do to make himself feel better. “I’d hobble over and look at him and drop the F-bomb on him every morning. Frankly, it was therapeutic for me.”
Later, he met the New England Patriots quarterback and told him the story, replacing “F-bomb” with the real word. “He had this little posse with him, and they roared. They said nobody talks to Tom like that.”
Brokaw still resists pleas to slow down from concerned loved ones, such as his emergency physician daughter. “My birth certificate says I’m 78 years old,” he said, “but I still think I’m 38 anchoring the news.” And still tossing tight-spiral F-bombs at cancer and gridiron G.O.A.T.s alike.
The earth is not enough
Earthly competitors have proved to be unworthy, so this week, Bacteria vs. the World visits the International Space Station, which – and we double-checked this – is in space. It’s a pretty exclusive location, and admission is by invitation only. Unless, of, course, you happen to be the ultimate hitchhiker. Four samples taken from the toilet of the ISS (and one from a piece of exercise equipment) were found to contain unknown strains of antibiotic-resistant Enterobacter bugandensis, investigators reported (BMC Microbiol. 2018 Nov 23;18[1]:175).
These bacterial stowaways were not virulent, lead author Nitin Singh, PhD, of the Jet Propulsion Laboratory said in a separate statement. But an analysis conducted by the team “reveals that the ISS isolates have a 79% probability of being a human pathogen.”
So, what does this mean for future space exploration? Cue the “Star Trek” music: “Space … the final frontier. These are the voyages of the bacterial transport ship Enterprise.”
Putting the FUN in fundus photos
You just got even more dependent on your phone: The American Academy of Opthalmology has published guidelines on how to use smartphones to take fundus photography, a.k.a. photographs of the back of the eye.
Advancement in smartphone optical quality has turned them into an important clinical tool, especially for specialists in low-funded or rural areas who don’t have access to imaging systems. Doctors can purchase special lenses and phone software to take these photos and then can easily upload the images to their Instagram accounts. (Even doctors need likes.)
An eye hospital in India has taken fundus accessibility a step further and posted a video on YouTube showing how to make a functional fundus camera that costs only 100 rupees. All you need in some cardboard, a water bottle, and a lens. “MacGyver: Chennai Edition.”
I feel it in my gut
Whoever said “inside, we’re all the same” clearly wasn’t considering the gut. A study from Vanderbilt University comprising 1,700 American subjects found that differences in gut microbiomes are most consistently linked with ethnicity. Vanderbilt biologist Seth Bordenstein emphasized how changing the gut microbiome can lead to curing illness but that it’s imperative that medical professionals understand how the gut differs across ethnicities.
Researchers found 12 types of bacteria that vary in abundancy by ethnicity. No comment on whether this was linked to differences in cuisine, but this writer fervently hopes new research arrives proving that tacos produce the healthiest gut microbiome.
F-bombing blood cancer
Call it a tale of two Toms.
TV newsman Tom Brokaw, who has multiple myeloma, says he’s become the “poster boy” for blood cancer. At first, though, he kept his diagnosis secret from just about everyone. But occasionally he let his emotions get the best of him. Especially when he’d see a Manhattan bus stop ad spotlighting the chiseled body of another Tom: the quarterback named Brady.
As he explained in a presentation at the annual meeting of the American Society of Hematology, he found it harder to get around because of back problems, which are common in multiple myeloma. As a result, he couldn’t manage to get to the office.
Still, “every day I’d force myself to leave the walker at home,” he recalled. “In that cold and sleety fall, I’d walk half a block to the coffee shop to get a bagel. There was this enormous new bus stop, with an animated advertisement board. Looking right at me was Tom Brady, advertising Ugg boots. I’d look down 79th Street at every inch of Tom Brady, and all the little old ladies were mooning over him as they were getting on the bus.”
Brokaw knew just what to do to make himself feel better. “I’d hobble over and look at him and drop the F-bomb on him every morning. Frankly, it was therapeutic for me.”
Later, he met the New England Patriots quarterback and told him the story, replacing “F-bomb” with the real word. “He had this little posse with him, and they roared. They said nobody talks to Tom like that.”
Brokaw still resists pleas to slow down from concerned loved ones, such as his emergency physician daughter. “My birth certificate says I’m 78 years old,” he said, “but I still think I’m 38 anchoring the news.” And still tossing tight-spiral F-bombs at cancer and gridiron G.O.A.T.s alike.
RT of lymph nodes as good as dissection for the long-term
SAN ANTONIO – Both axillary radiation therapy and axillary lymph node dissection provide excellent, comparable locoregional control in patients with early-stage breast cancer who have a positive sentinel node, according to updated results of the European Organisation for Research and Treatment of Cancer’s AMAROS trial.
The 10-year cumulative incidence rate of axillary recurrence was 1.82% with radiation and 0.93% with lymph node dissection, a nonsignificant difference (hazard ratio, 1.71; P = .365). Distant metastasis–free survival and overall survival also were statistically on par. The findings reinforce the trial’s 5-year results, which additionally showed a markedly lower incidence of lymphedema with axillary radiation therapy. Lead investigator Emiel J. T. Rutgers, MD, PhD, reflected on hesitation in the uptake of axillary radiation therapy among oncologists and discussed the AMAROS results in the context of the ACOSOG Z11 trial. Dr. Rutgers, the principal investigator of the AMAROS trial and a surgical oncologist at the Netherlands Cancer Institute in Amsterdam, also described how the trial’s findings have altered practice at his institution.
Dr. Rutgers disclosed that he had no relevant conflicts of interest. The study was supported by the EORTC Charitable Trust.
SAN ANTONIO – Both axillary radiation therapy and axillary lymph node dissection provide excellent, comparable locoregional control in patients with early-stage breast cancer who have a positive sentinel node, according to updated results of the European Organisation for Research and Treatment of Cancer’s AMAROS trial.
The 10-year cumulative incidence rate of axillary recurrence was 1.82% with radiation and 0.93% with lymph node dissection, a nonsignificant difference (hazard ratio, 1.71; P = .365). Distant metastasis–free survival and overall survival also were statistically on par. The findings reinforce the trial’s 5-year results, which additionally showed a markedly lower incidence of lymphedema with axillary radiation therapy. Lead investigator Emiel J. T. Rutgers, MD, PhD, reflected on hesitation in the uptake of axillary radiation therapy among oncologists and discussed the AMAROS results in the context of the ACOSOG Z11 trial. Dr. Rutgers, the principal investigator of the AMAROS trial and a surgical oncologist at the Netherlands Cancer Institute in Amsterdam, also described how the trial’s findings have altered practice at his institution.
Dr. Rutgers disclosed that he had no relevant conflicts of interest. The study was supported by the EORTC Charitable Trust.
SAN ANTONIO – Both axillary radiation therapy and axillary lymph node dissection provide excellent, comparable locoregional control in patients with early-stage breast cancer who have a positive sentinel node, according to updated results of the European Organisation for Research and Treatment of Cancer’s AMAROS trial.
The 10-year cumulative incidence rate of axillary recurrence was 1.82% with radiation and 0.93% with lymph node dissection, a nonsignificant difference (hazard ratio, 1.71; P = .365). Distant metastasis–free survival and overall survival also were statistically on par. The findings reinforce the trial’s 5-year results, which additionally showed a markedly lower incidence of lymphedema with axillary radiation therapy. Lead investigator Emiel J. T. Rutgers, MD, PhD, reflected on hesitation in the uptake of axillary radiation therapy among oncologists and discussed the AMAROS results in the context of the ACOSOG Z11 trial. Dr. Rutgers, the principal investigator of the AMAROS trial and a surgical oncologist at the Netherlands Cancer Institute in Amsterdam, also described how the trial’s findings have altered practice at his institution.
Dr. Rutgers disclosed that he had no relevant conflicts of interest. The study was supported by the EORTC Charitable Trust.
REPORTING FROM SABCS 2018
Oral Bowenoid Papulosis
To the Editor:
A 22-year-old Somali woman presented to our institution with oral lesions of 2 years’ duration. The lesions started as small papules in the corners of the mouth that gradually continued to spread to the mucosal lips and gums. The lesions did not drain any material. The patient reported that they were not painful and had not regressed. She was concerned about the cosmetic appearance of the lesions. The patient believed the lesions had developed from working in a chicken factory and was concerned that they appeared possibly due to contact with a substance in the factory. Additionally, she noted that her voice had become hoarse. She was otherwise healthy and denied any sexual contact or ever having a blood transfusion.
Physical examination revealed 10 to 15 flesh-colored papules measuring 2 to 3 mm in diameter on the vermilion, mucosal surfaces of the lips, and upper and lower gingivae (Figure 1). No lesions were seen on the hard and soft palate, tongue, buccal mucosa, or posterior pharynx.
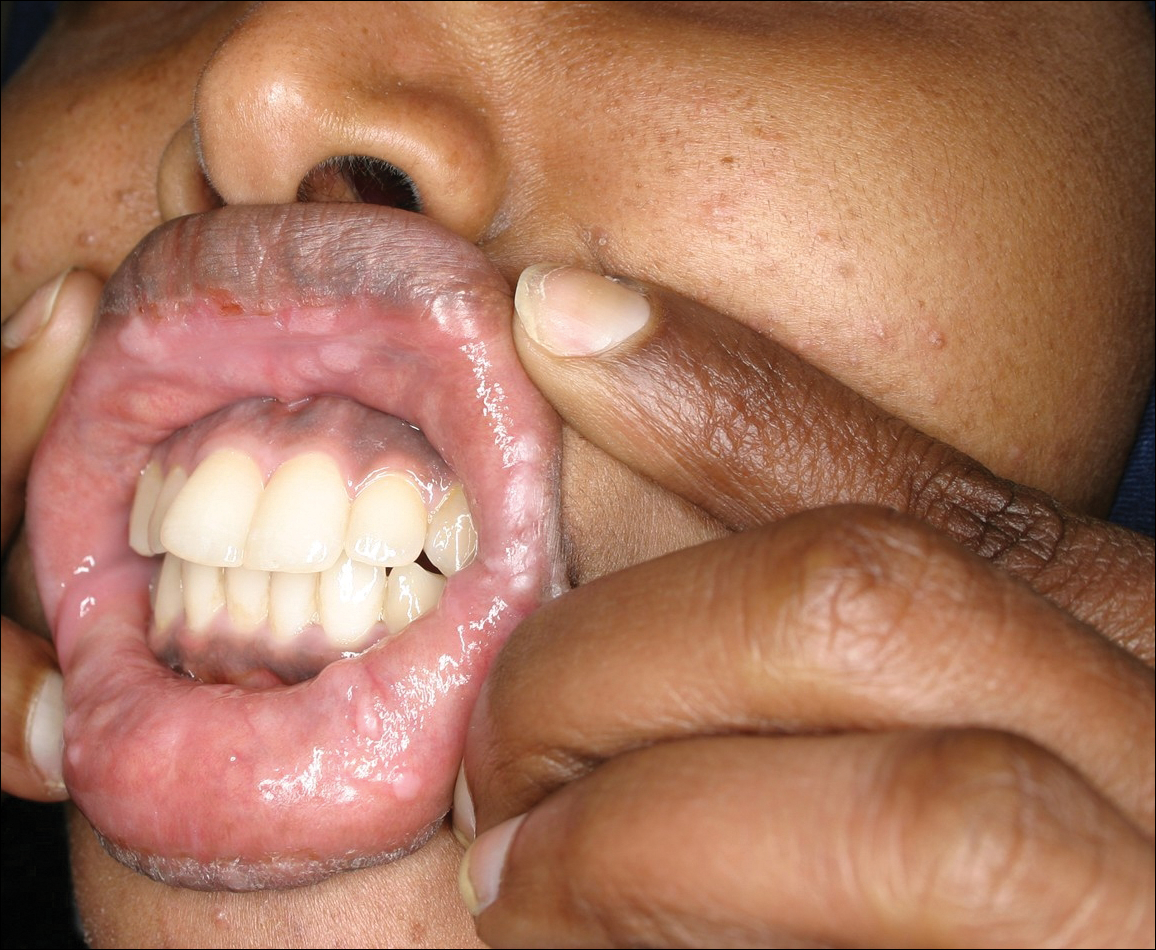
Skin biopsy of the left lower mucosal lip revealed parakeratosis, acanthosis, superficial koilocytes, and atypical keratinocytes with frequent mitoses (Figures 2A–2C). In situ hybridization testing for human papillomavirus (HPV) was negative for low-risk types 6 and 11 but positive for high-risk types 16 and 18 (Figure 2D). Laboratory investigations including complete blood cell count, electrolyte panel, and liver function studies were normal, and serum was negative for syphilis and human immunodeficiency virus antibodies.
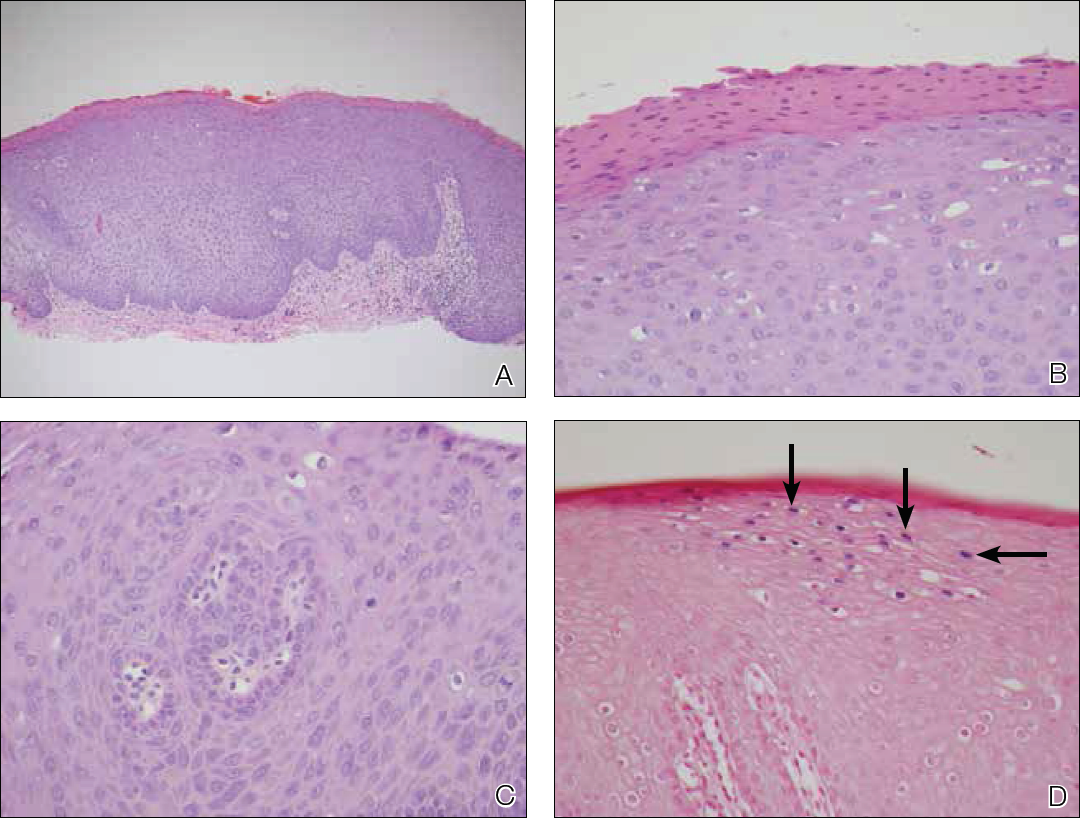
The combined clinical and histologic findings were diagnostic of oral bowenoid papulosis. Gynecologic evaluation showed that the patient had undergone female circumcision, and she had a normal Papanicolaou test. The patient was referred to both the ear, nose, and throat clinic as well as the dermatologic surgery department to discuss treatment options, but she was lost to follow-up.
Bowenoid papulosis is triggered by HPV infection and manifests clinically as solitary or multiple verrucous papules and plaques that are usually located on the genitalia.1 Only a few cases of bowenoid papulosis have been reported in the oral cavity.1-5 Because this disease is sexually transmitted, the mean age of onset of bowenoid papulosis is 31 years.2 There is a small risk (2%–3%) of developing invasive carcinoma in bowenoid papulosis.1-3,6 Most lesions are associated with HPV type 16; however, bowenoid papulosis also has been associated with HPV types 18, 31, 32, 35, and 39.2
Some investigators consider bowenoid papulosis and Bowen disease (a type of squamous cell carcinoma [SCC] in situ) to be histologically identical1,6; however, some histologic differences have been reported.1-3,6 Bowenoid papulosis has more dilated and tortuous dermal capillaries and less atypia and dyskeratosis than Bowen disease.1,6 In contrast to bowenoid papulosis, Bowen disease is characterized clinically as well-defined scaly plaques on sun-exposed areas of the skin in older adults. Invasive SCC can be seen in 5% of skin lesions and 30% of penile lesions associated with Bowen disease.2 Risk factors for Bowen disease include sun exposure; arsenic poisoning; and infection with HPV types 2, 16, 18, 31, 33, 52, and 67.1,6
Oral bowenoid papulosis is rare. A PubMed search of articles indexed for MEDLINE using the term oral bowenoid papulosis yielded 7 additional cases, which are summarized in the Table. In 1987 Lookingbill et al2 described one of the first reported cases of oral disease in a 33-year-old immunosuppressed man receiving prednisone therapy for systemic lupus erythematosus who had both mouth and genital lesions. All lesions were positive for HPV type 16. The patient subsequently developed SCC of the tongue.2

The risk for progression of oral bowenoid papulosis to invasive SCC is not known. Our search yielded only 1 case of this occurrence.2
Two of 3 cases of solitary lip lesions in oral bowenoid papulosis were treated with surgical excision.1 Other treatment options include CO2 laser therapy, cryotherapy, 5-fluorouracil, bleomycin, intralesional interferon alfa, and imiquimod.1-3,5,6
Our case represents a rare report of oral bowenoid papulosis. Recognition of this unusual presentation is important for the diagnosis and management of this disease.
- Daley T, Birek C, Wysocki GP. Oral bowenoid lesions: differential diagnosis and pathogenetic insights. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:466-473.
- Lookingbill DP, Kreider JW, Howett MK, et al. Human papillomavirus type 16 in bowenoid papulosis, intraoral papillomas, and squamous cell carcinoma of the tongue. Arch Dermatol. 1987;123:363-368.
- Kratochvil FJ, Cioffi GA, Auclair PL, et al. Virus-associated dysplasia (bowenoid papulosis?) of the oral cavity. Oral Surg Oral Med Oral Pathol. 1989;68:312-316.
- Degener AM, Latino L, Pierangeli A, et al. Human papilloma virus-32-positive extragenital bowenoid papulosis in a HIV patient with typical genital bowenoid papulosis localization. Sex Transm Dis. 2004;31:619-622.
- Rinaggio J, Glick M, Lambert WC. Oral bowenoid papulosis in an HIV-positive male [published online October 14, 2005]. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:328-332.
- Regezi JA, Dekker NP, Ramos DM, et al. Proliferation and invasion factors in HIV-associated dysplastic and nondysplastic oral warts and in oral squamous cell carcinoma: an immunohistochemical and RT-PCR evaluation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:724-731.
To the Editor:
A 22-year-old Somali woman presented to our institution with oral lesions of 2 years’ duration. The lesions started as small papules in the corners of the mouth that gradually continued to spread to the mucosal lips and gums. The lesions did not drain any material. The patient reported that they were not painful and had not regressed. She was concerned about the cosmetic appearance of the lesions. The patient believed the lesions had developed from working in a chicken factory and was concerned that they appeared possibly due to contact with a substance in the factory. Additionally, she noted that her voice had become hoarse. She was otherwise healthy and denied any sexual contact or ever having a blood transfusion.
Physical examination revealed 10 to 15 flesh-colored papules measuring 2 to 3 mm in diameter on the vermilion, mucosal surfaces of the lips, and upper and lower gingivae (Figure 1). No lesions were seen on the hard and soft palate, tongue, buccal mucosa, or posterior pharynx.

Skin biopsy of the left lower mucosal lip revealed parakeratosis, acanthosis, superficial koilocytes, and atypical keratinocytes with frequent mitoses (Figures 2A–2C). In situ hybridization testing for human papillomavirus (HPV) was negative for low-risk types 6 and 11 but positive for high-risk types 16 and 18 (Figure 2D). Laboratory investigations including complete blood cell count, electrolyte panel, and liver function studies were normal, and serum was negative for syphilis and human immunodeficiency virus antibodies.

The combined clinical and histologic findings were diagnostic of oral bowenoid papulosis. Gynecologic evaluation showed that the patient had undergone female circumcision, and she had a normal Papanicolaou test. The patient was referred to both the ear, nose, and throat clinic as well as the dermatologic surgery department to discuss treatment options, but she was lost to follow-up.
Bowenoid papulosis is triggered by HPV infection and manifests clinically as solitary or multiple verrucous papules and plaques that are usually located on the genitalia.1 Only a few cases of bowenoid papulosis have been reported in the oral cavity.1-5 Because this disease is sexually transmitted, the mean age of onset of bowenoid papulosis is 31 years.2 There is a small risk (2%–3%) of developing invasive carcinoma in bowenoid papulosis.1-3,6 Most lesions are associated with HPV type 16; however, bowenoid papulosis also has been associated with HPV types 18, 31, 32, 35, and 39.2
Some investigators consider bowenoid papulosis and Bowen disease (a type of squamous cell carcinoma [SCC] in situ) to be histologically identical1,6; however, some histologic differences have been reported.1-3,6 Bowenoid papulosis has more dilated and tortuous dermal capillaries and less atypia and dyskeratosis than Bowen disease.1,6 In contrast to bowenoid papulosis, Bowen disease is characterized clinically as well-defined scaly plaques on sun-exposed areas of the skin in older adults. Invasive SCC can be seen in 5% of skin lesions and 30% of penile lesions associated with Bowen disease.2 Risk factors for Bowen disease include sun exposure; arsenic poisoning; and infection with HPV types 2, 16, 18, 31, 33, 52, and 67.1,6
Oral bowenoid papulosis is rare. A PubMed search of articles indexed for MEDLINE using the term oral bowenoid papulosis yielded 7 additional cases, which are summarized in the Table. In 1987 Lookingbill et al2 described one of the first reported cases of oral disease in a 33-year-old immunosuppressed man receiving prednisone therapy for systemic lupus erythematosus who had both mouth and genital lesions. All lesions were positive for HPV type 16. The patient subsequently developed SCC of the tongue.2

The risk for progression of oral bowenoid papulosis to invasive SCC is not known. Our search yielded only 1 case of this occurrence.2
Two of 3 cases of solitary lip lesions in oral bowenoid papulosis were treated with surgical excision.1 Other treatment options include CO2 laser therapy, cryotherapy, 5-fluorouracil, bleomycin, intralesional interferon alfa, and imiquimod.1-3,5,6
Our case represents a rare report of oral bowenoid papulosis. Recognition of this unusual presentation is important for the diagnosis and management of this disease.
To the Editor:
A 22-year-old Somali woman presented to our institution with oral lesions of 2 years’ duration. The lesions started as small papules in the corners of the mouth that gradually continued to spread to the mucosal lips and gums. The lesions did not drain any material. The patient reported that they were not painful and had not regressed. She was concerned about the cosmetic appearance of the lesions. The patient believed the lesions had developed from working in a chicken factory and was concerned that they appeared possibly due to contact with a substance in the factory. Additionally, she noted that her voice had become hoarse. She was otherwise healthy and denied any sexual contact or ever having a blood transfusion.
Physical examination revealed 10 to 15 flesh-colored papules measuring 2 to 3 mm in diameter on the vermilion, mucosal surfaces of the lips, and upper and lower gingivae (Figure 1). No lesions were seen on the hard and soft palate, tongue, buccal mucosa, or posterior pharynx.

Skin biopsy of the left lower mucosal lip revealed parakeratosis, acanthosis, superficial koilocytes, and atypical keratinocytes with frequent mitoses (Figures 2A–2C). In situ hybridization testing for human papillomavirus (HPV) was negative for low-risk types 6 and 11 but positive for high-risk types 16 and 18 (Figure 2D). Laboratory investigations including complete blood cell count, electrolyte panel, and liver function studies were normal, and serum was negative for syphilis and human immunodeficiency virus antibodies.

The combined clinical and histologic findings were diagnostic of oral bowenoid papulosis. Gynecologic evaluation showed that the patient had undergone female circumcision, and she had a normal Papanicolaou test. The patient was referred to both the ear, nose, and throat clinic as well as the dermatologic surgery department to discuss treatment options, but she was lost to follow-up.
Bowenoid papulosis is triggered by HPV infection and manifests clinically as solitary or multiple verrucous papules and plaques that are usually located on the genitalia.1 Only a few cases of bowenoid papulosis have been reported in the oral cavity.1-5 Because this disease is sexually transmitted, the mean age of onset of bowenoid papulosis is 31 years.2 There is a small risk (2%–3%) of developing invasive carcinoma in bowenoid papulosis.1-3,6 Most lesions are associated with HPV type 16; however, bowenoid papulosis also has been associated with HPV types 18, 31, 32, 35, and 39.2
Some investigators consider bowenoid papulosis and Bowen disease (a type of squamous cell carcinoma [SCC] in situ) to be histologically identical1,6; however, some histologic differences have been reported.1-3,6 Bowenoid papulosis has more dilated and tortuous dermal capillaries and less atypia and dyskeratosis than Bowen disease.1,6 In contrast to bowenoid papulosis, Bowen disease is characterized clinically as well-defined scaly plaques on sun-exposed areas of the skin in older adults. Invasive SCC can be seen in 5% of skin lesions and 30% of penile lesions associated with Bowen disease.2 Risk factors for Bowen disease include sun exposure; arsenic poisoning; and infection with HPV types 2, 16, 18, 31, 33, 52, and 67.1,6
Oral bowenoid papulosis is rare. A PubMed search of articles indexed for MEDLINE using the term oral bowenoid papulosis yielded 7 additional cases, which are summarized in the Table. In 1987 Lookingbill et al2 described one of the first reported cases of oral disease in a 33-year-old immunosuppressed man receiving prednisone therapy for systemic lupus erythematosus who had both mouth and genital lesions. All lesions were positive for HPV type 16. The patient subsequently developed SCC of the tongue.2

The risk for progression of oral bowenoid papulosis to invasive SCC is not known. Our search yielded only 1 case of this occurrence.2
Two of 3 cases of solitary lip lesions in oral bowenoid papulosis were treated with surgical excision.1 Other treatment options include CO2 laser therapy, cryotherapy, 5-fluorouracil, bleomycin, intralesional interferon alfa, and imiquimod.1-3,5,6
Our case represents a rare report of oral bowenoid papulosis. Recognition of this unusual presentation is important for the diagnosis and management of this disease.
- Daley T, Birek C, Wysocki GP. Oral bowenoid lesions: differential diagnosis and pathogenetic insights. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:466-473.
- Lookingbill DP, Kreider JW, Howett MK, et al. Human papillomavirus type 16 in bowenoid papulosis, intraoral papillomas, and squamous cell carcinoma of the tongue. Arch Dermatol. 1987;123:363-368.
- Kratochvil FJ, Cioffi GA, Auclair PL, et al. Virus-associated dysplasia (bowenoid papulosis?) of the oral cavity. Oral Surg Oral Med Oral Pathol. 1989;68:312-316.
- Degener AM, Latino L, Pierangeli A, et al. Human papilloma virus-32-positive extragenital bowenoid papulosis in a HIV patient with typical genital bowenoid papulosis localization. Sex Transm Dis. 2004;31:619-622.
- Rinaggio J, Glick M, Lambert WC. Oral bowenoid papulosis in an HIV-positive male [published online October 14, 2005]. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:328-332.
- Regezi JA, Dekker NP, Ramos DM, et al. Proliferation and invasion factors in HIV-associated dysplastic and nondysplastic oral warts and in oral squamous cell carcinoma: an immunohistochemical and RT-PCR evaluation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:724-731.
- Daley T, Birek C, Wysocki GP. Oral bowenoid lesions: differential diagnosis and pathogenetic insights. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:466-473.
- Lookingbill DP, Kreider JW, Howett MK, et al. Human papillomavirus type 16 in bowenoid papulosis, intraoral papillomas, and squamous cell carcinoma of the tongue. Arch Dermatol. 1987;123:363-368.
- Kratochvil FJ, Cioffi GA, Auclair PL, et al. Virus-associated dysplasia (bowenoid papulosis?) of the oral cavity. Oral Surg Oral Med Oral Pathol. 1989;68:312-316.
- Degener AM, Latino L, Pierangeli A, et al. Human papilloma virus-32-positive extragenital bowenoid papulosis in a HIV patient with typical genital bowenoid papulosis localization. Sex Transm Dis. 2004;31:619-622.
- Rinaggio J, Glick M, Lambert WC. Oral bowenoid papulosis in an HIV-positive male [published online October 14, 2005]. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:328-332.
- Regezi JA, Dekker NP, Ramos DM, et al. Proliferation and invasion factors in HIV-associated dysplastic and nondysplastic oral warts and in oral squamous cell carcinoma: an immunohistochemical and RT-PCR evaluation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:724-731.
Practice Points
- Bowenoid papulosis is triggered by human papillomavirus infection and manifests clinically as solitary or multiple verrucous papules and plaques that usually are located on the genitalia.
- Oral bowenoid papulosis is rare, and recognition of this unusual presentation is important for the diagnosis and management of this disease.