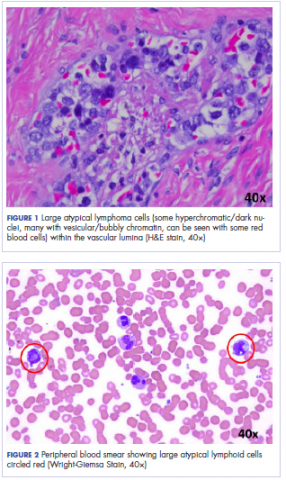
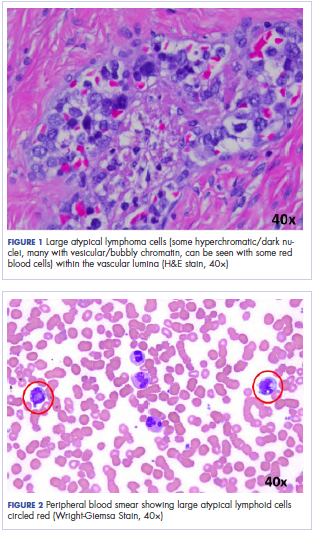
User login
The Gift and the Thought Both Count
It is that time of year when federal compliance officers, clinical ethicists, and staff counsels are flooded with queries about the legal and ethical acceptability of gifts. And no wonder, all the winter holidays often involve giving gifts. The simple and spontaneous acts of giving and receiving gifts become more complicated and deliberative in the federal health care system. Both legal rules and ethical values bear upon who can offer and accept what gift to whom upon what occasion and in what amount. The “Standards of Ethical Conduct for Employees of the Executive Branch” devotes 2 entire subparts to the subject of gifts.1 We will examine a small section of the document that can become a big issue for federal practitioners during that holiday—gifts from patients.
First, veterans (patients) are “prohibited sources” in section 5 CFR §2635.203 (d).1 And since VA employees are subject to restrictions on accepting gifts from sources outside the government, unless an exception applies, federal employees may not accept a gift because of their official position (eg, Federal Practitioner, editor-in-chief) or a gift from a patient (prohibited source; [5 CFR §2635.201]).
It might seem like this is going to be a very short column this month, as gifts from patients are forbidden. Yet, a Christmas card or homemade fudge isn’t really a gift, is it?
5 CFR §2635.203 (b) defines what is and is not a gift: For example, minor items of food or items like a thank-you card are specifically excluded in section (b) 1-10.
Is Christmas an exception or are just types of gifts excluded?
There are exceptions to the general regulation about accepting gifts from prohibited sources. Many staff will recall hearing about the “$20 rule,” which is actually the “$20-50” rule stating that a federal employee may accept a gift from a patient (prohibited source) if the value of said gift is under $20 and the employee does not accept more than $50 from any single source in a calendar year. §2635.204 lists the exceptions. However, starting in 2017, the regulations changed to require that federal employees also consider not just whether they could accept a gift but—ethically—they should take the gift even if it was permitted under the law. The Office of Government Ethics made this change because it wanted to emphasize the importance of considering not just how things are but how things appear to be. The regulations contain detailed descriptions of what employees should think about and stipulates that the decisions will not be further scrutinized (5 CFR §2635.201[b]).1
Based on this new emphasis on appearances, ethically, no doctor or nurse should accept the keys to a new BMW from a patient who owns a luxury car dealership. But what about more prosaic and probable presents: the holiday cookies a single father made with his children for the nurse practitioner who has been his primary care practitioner for years; the birdhouse a Vietnam veteran made in her crafts class for the surgeon who removed her gallbladder; or even the store-bought but no less heartfelt tin of popcorn from an elderly veteran for the hospitalist who saw him through a rough bought of pneumonia?
Related: Happy Federal New Year
The rules about practitioners accepting patient gifts are rational and unambiguous: It is the values conflict surrounding patient gifts that is often emotion driven and muddled; it may be easier and safer to adopt the “just say no” policy. And yet, while this might seem the most unassailable position to avoid a conflict of interest, could this possibly be a more practitioner- than patient-centered standard? Authoritative sources in the ethics literature are equally divided and ambivalent on the question.2,3 The American College of Physicians Ethics Manual states: “In deciding whether to accept a gift from a patient, the physician should consider the nature of the gift and its value to the patient, the potential implications for the patient-physician relationship of accepting or refusing it, and the patient’s probable intention and expectations.”4 A small gift as a token of appreciation is not ethically problematic. Favored treatment as a result of acceptance of any gift is problematic, undermines professionalism, and may interfere with objectivity in the care of the patient.4
Related: Am I My Brother’s/Sister’s Keeper?
Many an ethics commentator have cautioned, “beware of patients bearing gifts.”5 In making an ethical assessment of whether or not to accept the gift, a key question a practitioner needs to ask him or herself is about the patient’s motive. Even the patient may be unaware of the reasons behind their giving, and the wise practitioner will take a mindful moment to think about the context and timing of the gift and the nature of his or her relationship to the patient. Sadly, many of our patients are lonely especially at this family time of year and on some level may hope the gift will help slide the professional relationship toward a more personal one. Some patients may think a gift might earn them preferential treatment. Finally, a few patients may have a romantic or sexual attraction toward a clinician.
Often in the latter 2 cases, a pattern will develop that discloses the patient’s true intent. Very expensive gifts, monetary gifts, excessively personal gifts, or frequent gifts should alert the practitioner that more may be going on. A kind reminder to the patient that providing good care is the only reward needed may be sufficient. For practitioners whose ethical code does not permit them to accept gifts, then a genuine thank you and an explanation of the rules and/or values behind the refusal may be necessary. There are other times when the practitioner or a supervisor/advisor may need to reset the boundaries or even to transfer the patient to another practitioner. The norms in mental health care and psychotherapy are more stringent because of the intimacy of the relationship and the potential vulnerability of patients.6
For gifts that seem genuine and generous or cost a trivial amount, then there is an ethical argument to be made for accepting them with gratitude. In many cultures, hospitality is a tradition, and expressing appreciation a virtue, so when a practitioner refuses to graciously take a small gift, they risk offending the patient. Rejection of a gift can be seen as disrespectful and could cause a rupture in an otherwise sound practitioner-patient relationship. Other patients experience strong feelings of gratitude and admiration for their practitioners, stronger than most of us recognize. The ability for a patient to give their practitioner a holiday gift, particularly one they invested time and energy in creating or choosing, can enhance their sense of self-worth and individual agency. These gifts are not so much an attempt to diminish the professional power differential but to close the gap between 2 human beings in an unequal relationship that is yet one of shared decision making. All practitioners should be aware of the often underappreciated social power of a gift to influence decisions.
Related: Caring Under a Microscope
At the same time, clinicians can strive to be sensitively attuned to the reality that, sometimes the cookie is just a cookie so eat and enjoy, just remember to share with your group. External judgments are often cited as practical rules of thumb for determining the ethical acceptability of a gift: Would you want your mother, newspaper, or colleague to know you took the present? I prefer an internal moral compass that steers always to the true north of is accepting this gift really in the patient’s best interest?
1. Standards of ethical conduct for employees of the executive branch. Fed Regist. 2016;81(223):81641-81657. To be codified at 5 CFR §2635.
2. Spence SA. Patients bearing gifts: are there strings attached. BMJ. 2005;331.
3. American Medical Association. American Medical Association Code of Medical Ethics Opinion 1.2.8. https://www.ama-assn.org/delivering-care/code-medical-ethics-patient-physician-relationships. Accessed November 27, 2018.
4. Snyder L; Ethics, Professionalism, and Human Rights Committee. American College of Physicians Ethics Manual, 6th ed. Ann Intern Med. 2012;156(1, Part 2):73-104.
5. Levine A, Valeriote T. Beware the patient bearing gifts. http://epmonthly.com/article/beware-patient-bearing-gifts. Published December 14, 2016. Accessed November 27, 2018.
6. Brendel DH, Chu J, Radden J, et al. The price of a gift: an approach to receiving gifts from psychiatric patients. Harv Rev Psychiatry. 2007;15(2):43-51.
It is that time of year when federal compliance officers, clinical ethicists, and staff counsels are flooded with queries about the legal and ethical acceptability of gifts. And no wonder, all the winter holidays often involve giving gifts. The simple and spontaneous acts of giving and receiving gifts become more complicated and deliberative in the federal health care system. Both legal rules and ethical values bear upon who can offer and accept what gift to whom upon what occasion and in what amount. The “Standards of Ethical Conduct for Employees of the Executive Branch” devotes 2 entire subparts to the subject of gifts.1 We will examine a small section of the document that can become a big issue for federal practitioners during that holiday—gifts from patients.
First, veterans (patients) are “prohibited sources” in section 5 CFR §2635.203 (d).1 And since VA employees are subject to restrictions on accepting gifts from sources outside the government, unless an exception applies, federal employees may not accept a gift because of their official position (eg, Federal Practitioner, editor-in-chief) or a gift from a patient (prohibited source; [5 CFR §2635.201]).
It might seem like this is going to be a very short column this month, as gifts from patients are forbidden. Yet, a Christmas card or homemade fudge isn’t really a gift, is it?
5 CFR §2635.203 (b) defines what is and is not a gift: For example, minor items of food or items like a thank-you card are specifically excluded in section (b) 1-10.
Is Christmas an exception or are just types of gifts excluded?
There are exceptions to the general regulation about accepting gifts from prohibited sources. Many staff will recall hearing about the “$20 rule,” which is actually the “$20-50” rule stating that a federal employee may accept a gift from a patient (prohibited source) if the value of said gift is under $20 and the employee does not accept more than $50 from any single source in a calendar year. §2635.204 lists the exceptions. However, starting in 2017, the regulations changed to require that federal employees also consider not just whether they could accept a gift but—ethically—they should take the gift even if it was permitted under the law. The Office of Government Ethics made this change because it wanted to emphasize the importance of considering not just how things are but how things appear to be. The regulations contain detailed descriptions of what employees should think about and stipulates that the decisions will not be further scrutinized (5 CFR §2635.201[b]).1
Based on this new emphasis on appearances, ethically, no doctor or nurse should accept the keys to a new BMW from a patient who owns a luxury car dealership. But what about more prosaic and probable presents: the holiday cookies a single father made with his children for the nurse practitioner who has been his primary care practitioner for years; the birdhouse a Vietnam veteran made in her crafts class for the surgeon who removed her gallbladder; or even the store-bought but no less heartfelt tin of popcorn from an elderly veteran for the hospitalist who saw him through a rough bought of pneumonia?
Related: Happy Federal New Year
The rules about practitioners accepting patient gifts are rational and unambiguous: It is the values conflict surrounding patient gifts that is often emotion driven and muddled; it may be easier and safer to adopt the “just say no” policy. And yet, while this might seem the most unassailable position to avoid a conflict of interest, could this possibly be a more practitioner- than patient-centered standard? Authoritative sources in the ethics literature are equally divided and ambivalent on the question.2,3 The American College of Physicians Ethics Manual states: “In deciding whether to accept a gift from a patient, the physician should consider the nature of the gift and its value to the patient, the potential implications for the patient-physician relationship of accepting or refusing it, and the patient’s probable intention and expectations.”4 A small gift as a token of appreciation is not ethically problematic. Favored treatment as a result of acceptance of any gift is problematic, undermines professionalism, and may interfere with objectivity in the care of the patient.4
Related: Am I My Brother’s/Sister’s Keeper?
Many an ethics commentator have cautioned, “beware of patients bearing gifts.”5 In making an ethical assessment of whether or not to accept the gift, a key question a practitioner needs to ask him or herself is about the patient’s motive. Even the patient may be unaware of the reasons behind their giving, and the wise practitioner will take a mindful moment to think about the context and timing of the gift and the nature of his or her relationship to the patient. Sadly, many of our patients are lonely especially at this family time of year and on some level may hope the gift will help slide the professional relationship toward a more personal one. Some patients may think a gift might earn them preferential treatment. Finally, a few patients may have a romantic or sexual attraction toward a clinician.
Often in the latter 2 cases, a pattern will develop that discloses the patient’s true intent. Very expensive gifts, monetary gifts, excessively personal gifts, or frequent gifts should alert the practitioner that more may be going on. A kind reminder to the patient that providing good care is the only reward needed may be sufficient. For practitioners whose ethical code does not permit them to accept gifts, then a genuine thank you and an explanation of the rules and/or values behind the refusal may be necessary. There are other times when the practitioner or a supervisor/advisor may need to reset the boundaries or even to transfer the patient to another practitioner. The norms in mental health care and psychotherapy are more stringent because of the intimacy of the relationship and the potential vulnerability of patients.6
For gifts that seem genuine and generous or cost a trivial amount, then there is an ethical argument to be made for accepting them with gratitude. In many cultures, hospitality is a tradition, and expressing appreciation a virtue, so when a practitioner refuses to graciously take a small gift, they risk offending the patient. Rejection of a gift can be seen as disrespectful and could cause a rupture in an otherwise sound practitioner-patient relationship. Other patients experience strong feelings of gratitude and admiration for their practitioners, stronger than most of us recognize. The ability for a patient to give their practitioner a holiday gift, particularly one they invested time and energy in creating or choosing, can enhance their sense of self-worth and individual agency. These gifts are not so much an attempt to diminish the professional power differential but to close the gap between 2 human beings in an unequal relationship that is yet one of shared decision making. All practitioners should be aware of the often underappreciated social power of a gift to influence decisions.
Related: Caring Under a Microscope
At the same time, clinicians can strive to be sensitively attuned to the reality that, sometimes the cookie is just a cookie so eat and enjoy, just remember to share with your group. External judgments are often cited as practical rules of thumb for determining the ethical acceptability of a gift: Would you want your mother, newspaper, or colleague to know you took the present? I prefer an internal moral compass that steers always to the true north of is accepting this gift really in the patient’s best interest?
It is that time of year when federal compliance officers, clinical ethicists, and staff counsels are flooded with queries about the legal and ethical acceptability of gifts. And no wonder, all the winter holidays often involve giving gifts. The simple and spontaneous acts of giving and receiving gifts become more complicated and deliberative in the federal health care system. Both legal rules and ethical values bear upon who can offer and accept what gift to whom upon what occasion and in what amount. The “Standards of Ethical Conduct for Employees of the Executive Branch” devotes 2 entire subparts to the subject of gifts.1 We will examine a small section of the document that can become a big issue for federal practitioners during that holiday—gifts from patients.
First, veterans (patients) are “prohibited sources” in section 5 CFR §2635.203 (d).1 And since VA employees are subject to restrictions on accepting gifts from sources outside the government, unless an exception applies, federal employees may not accept a gift because of their official position (eg, Federal Practitioner, editor-in-chief) or a gift from a patient (prohibited source; [5 CFR §2635.201]).
It might seem like this is going to be a very short column this month, as gifts from patients are forbidden. Yet, a Christmas card or homemade fudge isn’t really a gift, is it?
5 CFR §2635.203 (b) defines what is and is not a gift: For example, minor items of food or items like a thank-you card are specifically excluded in section (b) 1-10.
Is Christmas an exception or are just types of gifts excluded?
There are exceptions to the general regulation about accepting gifts from prohibited sources. Many staff will recall hearing about the “$20 rule,” which is actually the “$20-50” rule stating that a federal employee may accept a gift from a patient (prohibited source) if the value of said gift is under $20 and the employee does not accept more than $50 from any single source in a calendar year. §2635.204 lists the exceptions. However, starting in 2017, the regulations changed to require that federal employees also consider not just whether they could accept a gift but—ethically—they should take the gift even if it was permitted under the law. The Office of Government Ethics made this change because it wanted to emphasize the importance of considering not just how things are but how things appear to be. The regulations contain detailed descriptions of what employees should think about and stipulates that the decisions will not be further scrutinized (5 CFR §2635.201[b]).1
Based on this new emphasis on appearances, ethically, no doctor or nurse should accept the keys to a new BMW from a patient who owns a luxury car dealership. But what about more prosaic and probable presents: the holiday cookies a single father made with his children for the nurse practitioner who has been his primary care practitioner for years; the birdhouse a Vietnam veteran made in her crafts class for the surgeon who removed her gallbladder; or even the store-bought but no less heartfelt tin of popcorn from an elderly veteran for the hospitalist who saw him through a rough bought of pneumonia?
Related: Happy Federal New Year
The rules about practitioners accepting patient gifts are rational and unambiguous: It is the values conflict surrounding patient gifts that is often emotion driven and muddled; it may be easier and safer to adopt the “just say no” policy. And yet, while this might seem the most unassailable position to avoid a conflict of interest, could this possibly be a more practitioner- than patient-centered standard? Authoritative sources in the ethics literature are equally divided and ambivalent on the question.2,3 The American College of Physicians Ethics Manual states: “In deciding whether to accept a gift from a patient, the physician should consider the nature of the gift and its value to the patient, the potential implications for the patient-physician relationship of accepting or refusing it, and the patient’s probable intention and expectations.”4 A small gift as a token of appreciation is not ethically problematic. Favored treatment as a result of acceptance of any gift is problematic, undermines professionalism, and may interfere with objectivity in the care of the patient.4
Related: Am I My Brother’s/Sister’s Keeper?
Many an ethics commentator have cautioned, “beware of patients bearing gifts.”5 In making an ethical assessment of whether or not to accept the gift, a key question a practitioner needs to ask him or herself is about the patient’s motive. Even the patient may be unaware of the reasons behind their giving, and the wise practitioner will take a mindful moment to think about the context and timing of the gift and the nature of his or her relationship to the patient. Sadly, many of our patients are lonely especially at this family time of year and on some level may hope the gift will help slide the professional relationship toward a more personal one. Some patients may think a gift might earn them preferential treatment. Finally, a few patients may have a romantic or sexual attraction toward a clinician.
Often in the latter 2 cases, a pattern will develop that discloses the patient’s true intent. Very expensive gifts, monetary gifts, excessively personal gifts, or frequent gifts should alert the practitioner that more may be going on. A kind reminder to the patient that providing good care is the only reward needed may be sufficient. For practitioners whose ethical code does not permit them to accept gifts, then a genuine thank you and an explanation of the rules and/or values behind the refusal may be necessary. There are other times when the practitioner or a supervisor/advisor may need to reset the boundaries or even to transfer the patient to another practitioner. The norms in mental health care and psychotherapy are more stringent because of the intimacy of the relationship and the potential vulnerability of patients.6
For gifts that seem genuine and generous or cost a trivial amount, then there is an ethical argument to be made for accepting them with gratitude. In many cultures, hospitality is a tradition, and expressing appreciation a virtue, so when a practitioner refuses to graciously take a small gift, they risk offending the patient. Rejection of a gift can be seen as disrespectful and could cause a rupture in an otherwise sound practitioner-patient relationship. Other patients experience strong feelings of gratitude and admiration for their practitioners, stronger than most of us recognize. The ability for a patient to give their practitioner a holiday gift, particularly one they invested time and energy in creating or choosing, can enhance their sense of self-worth and individual agency. These gifts are not so much an attempt to diminish the professional power differential but to close the gap between 2 human beings in an unequal relationship that is yet one of shared decision making. All practitioners should be aware of the often underappreciated social power of a gift to influence decisions.
Related: Caring Under a Microscope
At the same time, clinicians can strive to be sensitively attuned to the reality that, sometimes the cookie is just a cookie so eat and enjoy, just remember to share with your group. External judgments are often cited as practical rules of thumb for determining the ethical acceptability of a gift: Would you want your mother, newspaper, or colleague to know you took the present? I prefer an internal moral compass that steers always to the true north of is accepting this gift really in the patient’s best interest?
1. Standards of ethical conduct for employees of the executive branch. Fed Regist. 2016;81(223):81641-81657. To be codified at 5 CFR §2635.
2. Spence SA. Patients bearing gifts: are there strings attached. BMJ. 2005;331.
3. American Medical Association. American Medical Association Code of Medical Ethics Opinion 1.2.8. https://www.ama-assn.org/delivering-care/code-medical-ethics-patient-physician-relationships. Accessed November 27, 2018.
4. Snyder L; Ethics, Professionalism, and Human Rights Committee. American College of Physicians Ethics Manual, 6th ed. Ann Intern Med. 2012;156(1, Part 2):73-104.
5. Levine A, Valeriote T. Beware the patient bearing gifts. http://epmonthly.com/article/beware-patient-bearing-gifts. Published December 14, 2016. Accessed November 27, 2018.
6. Brendel DH, Chu J, Radden J, et al. The price of a gift: an approach to receiving gifts from psychiatric patients. Harv Rev Psychiatry. 2007;15(2):43-51.
1. Standards of ethical conduct for employees of the executive branch. Fed Regist. 2016;81(223):81641-81657. To be codified at 5 CFR §2635.
2. Spence SA. Patients bearing gifts: are there strings attached. BMJ. 2005;331.
3. American Medical Association. American Medical Association Code of Medical Ethics Opinion 1.2.8. https://www.ama-assn.org/delivering-care/code-medical-ethics-patient-physician-relationships. Accessed November 27, 2018.
4. Snyder L; Ethics, Professionalism, and Human Rights Committee. American College of Physicians Ethics Manual, 6th ed. Ann Intern Med. 2012;156(1, Part 2):73-104.
5. Levine A, Valeriote T. Beware the patient bearing gifts. http://epmonthly.com/article/beware-patient-bearing-gifts. Published December 14, 2016. Accessed November 27, 2018.
6. Brendel DH, Chu J, Radden J, et al. The price of a gift: an approach to receiving gifts from psychiatric patients. Harv Rev Psychiatry. 2007;15(2):43-51.
Dienogest as an option for endometriosis pain
Dienogest as an option for endometriosis pain
For treatment of endometriosis-related pain, what about the drug dienogest and the cyclic oral contraceptive Qlaira, which contains dienogest?
Chow Kah Kiong, MBBS
Singapore
--
--
Norethindrone’s conversion to ethinyl estradiol
Dr. Barbieri’s editorial on the medical treatment of endometriosis is excellent! Does norethindrone acetate metabolize to ethinyl estradiol in a higher percentage when the dose is higher, or is it still 1%? We were taught that at doses of greater than 15 mg daily, norethindrone can contribute significant amounts of estrogen.
Lauren Barnes, MD
Albuquerque, New Mexico
Endometriosis is a surgical, not a medical, disease
I read with some dismay Dr. Barbieri’s editorial on medical treatment of endometriosis. As a long-time disciple of the eminent Dr. David Redwine, I have dedicated my practice focus over the past 28 years to minimally invasive curative solutions to many gynecologic problems. The data on the histology, qualitative hormonal differences, and inconsistent and poor long-term response of endometriosis to traditional hormonal suppressive therapies falls strongly in favor of complete and thorough laparoscopic excision—not “biopsy”—as the only truly curative treatment, certainly not medical therapy. Endometriosis is a surgical disease. The experience of the dedicated few in our field who have taken the time and effort to become experts in excision (not ablation) of endometriosis bears this out.
The tragedy is that the only Current Procedural Terminology code that is usable for reimbursement is 58662. Sadly, this code was assigned a resource-based relative value scale “value” many years ago, when the operation consisted of putting a scope in the abdomen and taking a sampling biopsy (which took all of 10 minutes). Of course, we know that a prolonged, delicate procedure requiring retroperitoneal dissection, ureterolysis, excision of deeply infiltrating rectovaginal septum endometriosis, and discoid or segmental bowel resection requires the kind of surgical expertise developed only by those who put in the time and effort to get good at this type of surgery. The majority of ObGyns who have a full obstetric practice and low surgical volumes simply are not going to struggle in the operating room over the many cases that it takes to become good, and safe, at this procedure only to receive an insulting reimbursement.
It is emblematic of this travesty that many of the best minimally invasive surgery practitioners do not accept insurance or other thirdparty payment such as Medicaid as they would otherwise not cover their overhead.
Putting premenopausal women into a severely hypoestrogenic state with medication is cruel and, even worse, does not cure the disease.
Balanced information on surgical management should have been presented in the article. And physicians who are not capable of proper laparoscopic excision should refer the patient.
Hugo Ribot, MD
Cartersville, Georgia
Continue to: Dr. Barbieri responds
Dr. Barbieri responds
I thank Drs. Chow, Barnes, and Ribot for their interest in my recent editorial on the medical treatment of endometriosis. I agree with Dr. Chow that dienogest, a synthetic progestin, is effective in the treatment of pelvic pain caused by endometriosis. In one observational study, norethindrone acetate 2.5 mg daily and dienogest 2 mg daily had similar efficacy in the treatment of pelvic pain. Dienogest treatment was associated with fewer side effects but was much more expensive than norethindrone acetate.1 The US Food and Drug Administration has approved a combination estradiol- progestin pill (Natazia, Qlaira) as a contraceptive, and I have occasionally used this medication in my practice for women with pelvic pain caused by endometriosis. Dienogest monotherapy is not available in the United States.
Dr. Barnes reminds us that norethindrone is a substrate for the aromatase enzyme system and can be converted to ethinyl estradiol.2 The conversion occurs at a very low rate, likely less than 0.4%.3 At a norethindrone acetate dose of 5 mg daily, aromatization would result in the production of less than 2 μg of ethinyl estradiol daily.
Dr. Ribot advocates for surgery as the primary treatment of pelvic pain caused by endometriosis. I agree with Dr. Ribot that, for severe pain caused by deep infiltrating endometriosis, surgery is an optimal approach. However, for women with pelvic pain and Stage I endometriosis, hormonal treatment after initial surgical diagnosis and treatment reduces pain recurrence and repetitive surgical procedures.4
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Vercellini P, Bracco B, Mosconi P, et al. Norethindrone acetate or dienogest for the treatment of symptomatic endometriosis: a before and after study. Fertil Steril. 2016;105:734-743.
- Barbieri RL, Petro Z, Canick JA, et al. Aromatization of norethindrone to ethinyl estradiol by human placental microsomes. J Clin Endocrinol Metab. 1983;57:299-303.
- Chu MC, Zhang X, Gentzschein E, et al. Formation of ethinyl estradiol in women during treatment with norethindrone acetate. J Clin Endocrinol Metab. 2007;92:2205-2207.
- Soliman AM, Bonafede M, Farr AM, et al. Analysis of subsequent surgery rates among endometriosis patients who underwent surgery with and without concomitant leuprolide acetate therapy. Curr Med Res Opin. 2016;32:1073-1082.
Dienogest as an option for endometriosis pain
For treatment of endometriosis-related pain, what about the drug dienogest and the cyclic oral contraceptive Qlaira, which contains dienogest?
Chow Kah Kiong, MBBS
Singapore
--
--
Norethindrone’s conversion to ethinyl estradiol
Dr. Barbieri’s editorial on the medical treatment of endometriosis is excellent! Does norethindrone acetate metabolize to ethinyl estradiol in a higher percentage when the dose is higher, or is it still 1%? We were taught that at doses of greater than 15 mg daily, norethindrone can contribute significant amounts of estrogen.
Lauren Barnes, MD
Albuquerque, New Mexico
Endometriosis is a surgical, not a medical, disease
I read with some dismay Dr. Barbieri’s editorial on medical treatment of endometriosis. As a long-time disciple of the eminent Dr. David Redwine, I have dedicated my practice focus over the past 28 years to minimally invasive curative solutions to many gynecologic problems. The data on the histology, qualitative hormonal differences, and inconsistent and poor long-term response of endometriosis to traditional hormonal suppressive therapies falls strongly in favor of complete and thorough laparoscopic excision—not “biopsy”—as the only truly curative treatment, certainly not medical therapy. Endometriosis is a surgical disease. The experience of the dedicated few in our field who have taken the time and effort to become experts in excision (not ablation) of endometriosis bears this out.
The tragedy is that the only Current Procedural Terminology code that is usable for reimbursement is 58662. Sadly, this code was assigned a resource-based relative value scale “value” many years ago, when the operation consisted of putting a scope in the abdomen and taking a sampling biopsy (which took all of 10 minutes). Of course, we know that a prolonged, delicate procedure requiring retroperitoneal dissection, ureterolysis, excision of deeply infiltrating rectovaginal septum endometriosis, and discoid or segmental bowel resection requires the kind of surgical expertise developed only by those who put in the time and effort to get good at this type of surgery. The majority of ObGyns who have a full obstetric practice and low surgical volumes simply are not going to struggle in the operating room over the many cases that it takes to become good, and safe, at this procedure only to receive an insulting reimbursement.
It is emblematic of this travesty that many of the best minimally invasive surgery practitioners do not accept insurance or other thirdparty payment such as Medicaid as they would otherwise not cover their overhead.
Putting premenopausal women into a severely hypoestrogenic state with medication is cruel and, even worse, does not cure the disease.
Balanced information on surgical management should have been presented in the article. And physicians who are not capable of proper laparoscopic excision should refer the patient.
Hugo Ribot, MD
Cartersville, Georgia
Continue to: Dr. Barbieri responds
Dr. Barbieri responds
I thank Drs. Chow, Barnes, and Ribot for their interest in my recent editorial on the medical treatment of endometriosis. I agree with Dr. Chow that dienogest, a synthetic progestin, is effective in the treatment of pelvic pain caused by endometriosis. In one observational study, norethindrone acetate 2.5 mg daily and dienogest 2 mg daily had similar efficacy in the treatment of pelvic pain. Dienogest treatment was associated with fewer side effects but was much more expensive than norethindrone acetate.1 The US Food and Drug Administration has approved a combination estradiol- progestin pill (Natazia, Qlaira) as a contraceptive, and I have occasionally used this medication in my practice for women with pelvic pain caused by endometriosis. Dienogest monotherapy is not available in the United States.
Dr. Barnes reminds us that norethindrone is a substrate for the aromatase enzyme system and can be converted to ethinyl estradiol.2 The conversion occurs at a very low rate, likely less than 0.4%.3 At a norethindrone acetate dose of 5 mg daily, aromatization would result in the production of less than 2 μg of ethinyl estradiol daily.
Dr. Ribot advocates for surgery as the primary treatment of pelvic pain caused by endometriosis. I agree with Dr. Ribot that, for severe pain caused by deep infiltrating endometriosis, surgery is an optimal approach. However, for women with pelvic pain and Stage I endometriosis, hormonal treatment after initial surgical diagnosis and treatment reduces pain recurrence and repetitive surgical procedures.4
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Dienogest as an option for endometriosis pain
For treatment of endometriosis-related pain, what about the drug dienogest and the cyclic oral contraceptive Qlaira, which contains dienogest?
Chow Kah Kiong, MBBS
Singapore
--
--
Norethindrone’s conversion to ethinyl estradiol
Dr. Barbieri’s editorial on the medical treatment of endometriosis is excellent! Does norethindrone acetate metabolize to ethinyl estradiol in a higher percentage when the dose is higher, or is it still 1%? We were taught that at doses of greater than 15 mg daily, norethindrone can contribute significant amounts of estrogen.
Lauren Barnes, MD
Albuquerque, New Mexico
Endometriosis is a surgical, not a medical, disease
I read with some dismay Dr. Barbieri’s editorial on medical treatment of endometriosis. As a long-time disciple of the eminent Dr. David Redwine, I have dedicated my practice focus over the past 28 years to minimally invasive curative solutions to many gynecologic problems. The data on the histology, qualitative hormonal differences, and inconsistent and poor long-term response of endometriosis to traditional hormonal suppressive therapies falls strongly in favor of complete and thorough laparoscopic excision—not “biopsy”—as the only truly curative treatment, certainly not medical therapy. Endometriosis is a surgical disease. The experience of the dedicated few in our field who have taken the time and effort to become experts in excision (not ablation) of endometriosis bears this out.
The tragedy is that the only Current Procedural Terminology code that is usable for reimbursement is 58662. Sadly, this code was assigned a resource-based relative value scale “value” many years ago, when the operation consisted of putting a scope in the abdomen and taking a sampling biopsy (which took all of 10 minutes). Of course, we know that a prolonged, delicate procedure requiring retroperitoneal dissection, ureterolysis, excision of deeply infiltrating rectovaginal septum endometriosis, and discoid or segmental bowel resection requires the kind of surgical expertise developed only by those who put in the time and effort to get good at this type of surgery. The majority of ObGyns who have a full obstetric practice and low surgical volumes simply are not going to struggle in the operating room over the many cases that it takes to become good, and safe, at this procedure only to receive an insulting reimbursement.
It is emblematic of this travesty that many of the best minimally invasive surgery practitioners do not accept insurance or other thirdparty payment such as Medicaid as they would otherwise not cover their overhead.
Putting premenopausal women into a severely hypoestrogenic state with medication is cruel and, even worse, does not cure the disease.
Balanced information on surgical management should have been presented in the article. And physicians who are not capable of proper laparoscopic excision should refer the patient.
Hugo Ribot, MD
Cartersville, Georgia
Continue to: Dr. Barbieri responds
Dr. Barbieri responds
I thank Drs. Chow, Barnes, and Ribot for their interest in my recent editorial on the medical treatment of endometriosis. I agree with Dr. Chow that dienogest, a synthetic progestin, is effective in the treatment of pelvic pain caused by endometriosis. In one observational study, norethindrone acetate 2.5 mg daily and dienogest 2 mg daily had similar efficacy in the treatment of pelvic pain. Dienogest treatment was associated with fewer side effects but was much more expensive than norethindrone acetate.1 The US Food and Drug Administration has approved a combination estradiol- progestin pill (Natazia, Qlaira) as a contraceptive, and I have occasionally used this medication in my practice for women with pelvic pain caused by endometriosis. Dienogest monotherapy is not available in the United States.
Dr. Barnes reminds us that norethindrone is a substrate for the aromatase enzyme system and can be converted to ethinyl estradiol.2 The conversion occurs at a very low rate, likely less than 0.4%.3 At a norethindrone acetate dose of 5 mg daily, aromatization would result in the production of less than 2 μg of ethinyl estradiol daily.
Dr. Ribot advocates for surgery as the primary treatment of pelvic pain caused by endometriosis. I agree with Dr. Ribot that, for severe pain caused by deep infiltrating endometriosis, surgery is an optimal approach. However, for women with pelvic pain and Stage I endometriosis, hormonal treatment after initial surgical diagnosis and treatment reduces pain recurrence and repetitive surgical procedures.4
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Vercellini P, Bracco B, Mosconi P, et al. Norethindrone acetate or dienogest for the treatment of symptomatic endometriosis: a before and after study. Fertil Steril. 2016;105:734-743.
- Barbieri RL, Petro Z, Canick JA, et al. Aromatization of norethindrone to ethinyl estradiol by human placental microsomes. J Clin Endocrinol Metab. 1983;57:299-303.
- Chu MC, Zhang X, Gentzschein E, et al. Formation of ethinyl estradiol in women during treatment with norethindrone acetate. J Clin Endocrinol Metab. 2007;92:2205-2207.
- Soliman AM, Bonafede M, Farr AM, et al. Analysis of subsequent surgery rates among endometriosis patients who underwent surgery with and without concomitant leuprolide acetate therapy. Curr Med Res Opin. 2016;32:1073-1082.
- Vercellini P, Bracco B, Mosconi P, et al. Norethindrone acetate or dienogest for the treatment of symptomatic endometriosis: a before and after study. Fertil Steril. 2016;105:734-743.
- Barbieri RL, Petro Z, Canick JA, et al. Aromatization of norethindrone to ethinyl estradiol by human placental microsomes. J Clin Endocrinol Metab. 1983;57:299-303.
- Chu MC, Zhang X, Gentzschein E, et al. Formation of ethinyl estradiol in women during treatment with norethindrone acetate. J Clin Endocrinol Metab. 2007;92:2205-2207.
- Soliman AM, Bonafede M, Farr AM, et al. Analysis of subsequent surgery rates among endometriosis patients who underwent surgery with and without concomitant leuprolide acetate therapy. Curr Med Res Opin. 2016;32:1073-1082.
Maternal health benefits of breastfeeding
In the past decade, breastfeeding rates have increased substantially. Between 2000 and 2015, the proportion of infants who continued to breastfeed at 12 months increased from 16% to 36%. The proportion of infants who had any breastfeeding increased from 71% to 83%.1 While the infant health benefits of breastfeeding are widely recognized, the maternal health benefits of breastfeeding are many and likely underappreciated.
Infant health benefits of breastfeeding
There are no large-scale, randomized studies of the long-term health benefits of breastfeeding versus formula feeding. The evidence supporting the health benefits of breastfeeding is derived from case-control and cohort studies. Breastfeeding directly benefits newborn and infant nutrition, gastrointestinal function, host defense, and psychological well-being. Compared with formula-fed newborns, breastfed infants have a reduced risk of infectious diseases including otitis media, gastroenteritis, respiratory infections, sudden infant death syndrome, and metabolic disease. These benefits alone strongly support the public health benefit of breastfeeding.2 In addition, breastfeeding greatly benefits maternal health.
Maternal health benefits of breastfeeding
Breastfeeding reduces a woman’s risk for type 2 diabetes, hypertension, and coronary artery disease, myocardial infarction, as well as breast, ovarian, and endometrial cancer. There are few exposures that have such a multitude of positive health benefits.
filler
Type 2 diabetes
In a prospective cohort study of 1,238 women without diabetes in 1985–1986, 182 women developed type 2 diabetes after 30 years of follow-up. Compared with never breastfeeding, breastfeeding for 0 to 6 months, >6 months to <12 months, or ≥12 months reduced the risk of type 2 diabetes by 25%, 48%, and 69% respectively.3 In the prospective Nurses’ Health Study, among parous women, each additional year of breastfeeding decreased the risk of type 2 diabetes by 15% compared with women who did not breastfeed.4

Hypertension

In the Women’s Health Initiative (WHI) study of postmenopausal women, a lifetime history of breastfeeding for 12 months or more was associated with a 12% decrease in the risk of hypertension.5 For parous women, the prevalence of hypertension among breastfeeding (≥12 months) and never breastfeeding women was estimated to be 38.6% versus 42.1%.5 Similar results were observed in the Nurses’ Health Study II.6
Myocardial infarction and coronary heart disease
In the prospective Nurses’ Health Study, during 1,350,965 person-years of follow-up, 2,540 women had a myocardial infarction (MI). Women who had breastfed for ≥ 2 years had a 37% decreased risk of MI compared with women who never breastfed. After adjustment for family history, lifestyle factors, and adiposity, the observed reduction in risk was 23%.7 In the WHI (observational study plus controlled trial), women with a single live birth who breastfed for 7 to 12 months had a lower risk of cardiovascular disease than women with a single live birth who did not breastfeed (hazard ratio, 0.72; 95% confidence interval, 0.53–97).5

Breast cancer

In a systematic review and meta-analysis of 100 publications, breastfeeding >12 months reduced the risk of breast cancer by 26%.8 In a systematic review of 47 studies, the relative risk of breast cancer decreased by 4.7% for every 12 months of breastfeeding.9 In a systematic review and meta-analysis of 3 studies, ever breastfeeding was associated with a 28% reduced risk for triple-negative (ER-, PR-, HER2-) breast cancer among parous women.10 Triple-negative breast cancer generally has a poorer prognosis than receptor-positive breast cancers.
Continue to: Ovarian Cancer
Ovarian cancer
In a systematic review and meta-analysis of 40 publications, ever breastfeeding was associated with a 37% reduction in the risk of ovarian cancer.8 In a prospective study of 1.1 million women in the United Kingdom, 8,719 developed ovarian cancer. Among parous women, ovarian cancer risk was reduced by 10% for every 12 months of breastfeeding.11

Endometrial cancer

In a meta-analysis of 17 publications, including 8,981 cases and 17,241 controls, ever breastfeeding was associated with an 11% reduction in breast cancer risk.12 In a meta-analysis of 15 publications with 6,704 cases, breastfeeding was associated with a 26% reduction in endometrial cancer. After controlling for hormone use and body mass index, the reduced risk was in the range of 35%. A linear relationship between breastfeeding and reduced risk of endometrial cancer was observed, with 1 month of breastfeeding being associated with a 1.2% reduction in the risk of endometrial cancer.13
Let’s support our patients’ health by encouraging successful breastfeeding
Obstetrician-gynecologists play an important role in helping women make informed decisions about breastfeeding. Most professional organizations, including the American College of Obstetricians and Gynecologists, recommend exclusive breastfeeding for the first 6 months of life, with continued breastfeeding and introduction of complementary food from 6 to 12 months.14,15 Birth practices that help to increase successful breastfeeding include:
- inform all pregnant women about the newborn and maternal health benefits and management of breastfeeding
- initiate skin-to-skin contact at birth
- encourage the initiation of breastfeeding within 1 hour of birth
- ensure that breastfeeding newborns do not receive any food or drink other than breast milk, unless medically indicated
- encourage breastfeeding women to not use pacifiers or artificial nipples.15
When women are discharged from the maternity center, providing information about community-based lactation support is helpful in ensuring continuation of successful breastfeeding.16
Most patients know that exercise and maintaining a healthy weight can reduce the risk of developing many prevalent diseases. However, far fewer patients know that breastfeeding can reduce the risk of developing type 2 diabetes, hypertension, and coronary artery disease, as well as breast, ovarian, and endometrial cancers. Educating our patients about these health benefits may help them to more fully commit to breastfeeding.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Centers for Disease Control and Prevention. Breastfeeding Among U.S. Children Born 2009–2015, CDC National Immunization Survey. https://www.cdc.gov/breastfeeding/data/nis_data/results.html. Updated August 2018. Accessed November 19, 2018.
- Ip S, Chung M, Raman G, et al. A summary of the Agency for Healthcare Research and Quality’s evidence report on breastfeeding in developed countries. Breastfeed Med. 2009;4 (suppl 1):S17.
- Gunderson Ep, Lewis CE, Lin Y, et al. Lactation duration and progression to diabetes in women across the childbearing years: the 30-year CARDIA study. JAMA Int Med. 2018;178:328-337.
- Stuebe AM, Rich-Edwards JW, Willett WC, et al. Duration of lactation and incidence of type 2 diabetes. JAMA. 2005;294:2601-2610.
- Schwarz EB, Ray RM, Stuebe AM, et al. Duration of lactation and risk factors for maternal cardiovascular disease. Obstet Gynecol. 2009;113:974-982.
- Stuebe Am, Schwarz EB, Grewen K, et al. Duration of lactation and incidence of maternal hypertension: a longitudinal cohort study. Am J Epidemiol. 2011;174:1147-1158.
- Stuebe AM, Michels KB, Willett WC, et al. Duration of lactation and incidence of myocardial infarction in middle to late adulthood. Am J Obstet Gynecol. 2009;200:138.e1-e8.
- Chowdhury R, Sinha B, Sankar MJ, et al. Breastfeeding and maternal health outcomes: a systematic review and meta-analysis. Acta Paediatr. 2015;104:96-113.
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries including 50,302 women with breast cancer and 96,973 women without the disease. Lancet. 2002;360:187-195.
- Islami F, Liu Y, Jemal A, et al. Breastfeeding and breast cancer risk by receptor status—a systematic review and meta-analysis. Ann Oncol. 2015;26:2398-2407.
- Gaitskell K, Green J, Pirie K, et al. Million Women Study Collaborators. Histological subtypes of ovarian cancer associated with parity and breastfeeding in the Million Women Study. Int J Cancer. 2018;142:281-289.
- Jordan SJ, Na R, Johnatty SE, et al. Breastfeeding and endometrial cancer risk: an analysis from the epidemiology of endometrial cancer consortium. Obstet Gynecol. 2017;129:1059-1067.
- Zhan B, Liu X, Li F, Zhang D, et al. Breastfeeding and the incidence of endometrial cancer: a meta-analysis. Oncotarget. 2015;6:38398-38409.
- Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst Rev. 2012;CD003517.
- ACOG Committee Opinion No. 756. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2018;132:e187-e196.
- McFadden A, Gavine A, Renfrew M, et al. Support for healthy breastfeeding mothers with healthy term babies. Cochrane Database Syst Rev. 2017;CD001141.
In the past decade, breastfeeding rates have increased substantially. Between 2000 and 2015, the proportion of infants who continued to breastfeed at 12 months increased from 16% to 36%. The proportion of infants who had any breastfeeding increased from 71% to 83%.1 While the infant health benefits of breastfeeding are widely recognized, the maternal health benefits of breastfeeding are many and likely underappreciated.
Infant health benefits of breastfeeding
There are no large-scale, randomized studies of the long-term health benefits of breastfeeding versus formula feeding. The evidence supporting the health benefits of breastfeeding is derived from case-control and cohort studies. Breastfeeding directly benefits newborn and infant nutrition, gastrointestinal function, host defense, and psychological well-being. Compared with formula-fed newborns, breastfed infants have a reduced risk of infectious diseases including otitis media, gastroenteritis, respiratory infections, sudden infant death syndrome, and metabolic disease. These benefits alone strongly support the public health benefit of breastfeeding.2 In addition, breastfeeding greatly benefits maternal health.
Maternal health benefits of breastfeeding
Breastfeeding reduces a woman’s risk for type 2 diabetes, hypertension, and coronary artery disease, myocardial infarction, as well as breast, ovarian, and endometrial cancer. There are few exposures that have such a multitude of positive health benefits.
filler
Type 2 diabetes
In a prospective cohort study of 1,238 women without diabetes in 1985–1986, 182 women developed type 2 diabetes after 30 years of follow-up. Compared with never breastfeeding, breastfeeding for 0 to 6 months, >6 months to <12 months, or ≥12 months reduced the risk of type 2 diabetes by 25%, 48%, and 69% respectively.3 In the prospective Nurses’ Health Study, among parous women, each additional year of breastfeeding decreased the risk of type 2 diabetes by 15% compared with women who did not breastfeed.4

Hypertension

In the Women’s Health Initiative (WHI) study of postmenopausal women, a lifetime history of breastfeeding for 12 months or more was associated with a 12% decrease in the risk of hypertension.5 For parous women, the prevalence of hypertension among breastfeeding (≥12 months) and never breastfeeding women was estimated to be 38.6% versus 42.1%.5 Similar results were observed in the Nurses’ Health Study II.6
Myocardial infarction and coronary heart disease
In the prospective Nurses’ Health Study, during 1,350,965 person-years of follow-up, 2,540 women had a myocardial infarction (MI). Women who had breastfed for ≥ 2 years had a 37% decreased risk of MI compared with women who never breastfed. After adjustment for family history, lifestyle factors, and adiposity, the observed reduction in risk was 23%.7 In the WHI (observational study plus controlled trial), women with a single live birth who breastfed for 7 to 12 months had a lower risk of cardiovascular disease than women with a single live birth who did not breastfeed (hazard ratio, 0.72; 95% confidence interval, 0.53–97).5

Breast cancer

In a systematic review and meta-analysis of 100 publications, breastfeeding >12 months reduced the risk of breast cancer by 26%.8 In a systematic review of 47 studies, the relative risk of breast cancer decreased by 4.7% for every 12 months of breastfeeding.9 In a systematic review and meta-analysis of 3 studies, ever breastfeeding was associated with a 28% reduced risk for triple-negative (ER-, PR-, HER2-) breast cancer among parous women.10 Triple-negative breast cancer generally has a poorer prognosis than receptor-positive breast cancers.
Continue to: Ovarian Cancer
Ovarian cancer
In a systematic review and meta-analysis of 40 publications, ever breastfeeding was associated with a 37% reduction in the risk of ovarian cancer.8 In a prospective study of 1.1 million women in the United Kingdom, 8,719 developed ovarian cancer. Among parous women, ovarian cancer risk was reduced by 10% for every 12 months of breastfeeding.11

Endometrial cancer

In a meta-analysis of 17 publications, including 8,981 cases and 17,241 controls, ever breastfeeding was associated with an 11% reduction in breast cancer risk.12 In a meta-analysis of 15 publications with 6,704 cases, breastfeeding was associated with a 26% reduction in endometrial cancer. After controlling for hormone use and body mass index, the reduced risk was in the range of 35%. A linear relationship between breastfeeding and reduced risk of endometrial cancer was observed, with 1 month of breastfeeding being associated with a 1.2% reduction in the risk of endometrial cancer.13
Let’s support our patients’ health by encouraging successful breastfeeding
Obstetrician-gynecologists play an important role in helping women make informed decisions about breastfeeding. Most professional organizations, including the American College of Obstetricians and Gynecologists, recommend exclusive breastfeeding for the first 6 months of life, with continued breastfeeding and introduction of complementary food from 6 to 12 months.14,15 Birth practices that help to increase successful breastfeeding include:
- inform all pregnant women about the newborn and maternal health benefits and management of breastfeeding
- initiate skin-to-skin contact at birth
- encourage the initiation of breastfeeding within 1 hour of birth
- ensure that breastfeeding newborns do not receive any food or drink other than breast milk, unless medically indicated
- encourage breastfeeding women to not use pacifiers or artificial nipples.15
When women are discharged from the maternity center, providing information about community-based lactation support is helpful in ensuring continuation of successful breastfeeding.16
Most patients know that exercise and maintaining a healthy weight can reduce the risk of developing many prevalent diseases. However, far fewer patients know that breastfeeding can reduce the risk of developing type 2 diabetes, hypertension, and coronary artery disease, as well as breast, ovarian, and endometrial cancers. Educating our patients about these health benefits may help them to more fully commit to breastfeeding.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In the past decade, breastfeeding rates have increased substantially. Between 2000 and 2015, the proportion of infants who continued to breastfeed at 12 months increased from 16% to 36%. The proportion of infants who had any breastfeeding increased from 71% to 83%.1 While the infant health benefits of breastfeeding are widely recognized, the maternal health benefits of breastfeeding are many and likely underappreciated.
Infant health benefits of breastfeeding
There are no large-scale, randomized studies of the long-term health benefits of breastfeeding versus formula feeding. The evidence supporting the health benefits of breastfeeding is derived from case-control and cohort studies. Breastfeeding directly benefits newborn and infant nutrition, gastrointestinal function, host defense, and psychological well-being. Compared with formula-fed newborns, breastfed infants have a reduced risk of infectious diseases including otitis media, gastroenteritis, respiratory infections, sudden infant death syndrome, and metabolic disease. These benefits alone strongly support the public health benefit of breastfeeding.2 In addition, breastfeeding greatly benefits maternal health.
Maternal health benefits of breastfeeding
Breastfeeding reduces a woman’s risk for type 2 diabetes, hypertension, and coronary artery disease, myocardial infarction, as well as breast, ovarian, and endometrial cancer. There are few exposures that have such a multitude of positive health benefits.
filler
Type 2 diabetes
In a prospective cohort study of 1,238 women without diabetes in 1985–1986, 182 women developed type 2 diabetes after 30 years of follow-up. Compared with never breastfeeding, breastfeeding for 0 to 6 months, >6 months to <12 months, or ≥12 months reduced the risk of type 2 diabetes by 25%, 48%, and 69% respectively.3 In the prospective Nurses’ Health Study, among parous women, each additional year of breastfeeding decreased the risk of type 2 diabetes by 15% compared with women who did not breastfeed.4

Hypertension

In the Women’s Health Initiative (WHI) study of postmenopausal women, a lifetime history of breastfeeding for 12 months or more was associated with a 12% decrease in the risk of hypertension.5 For parous women, the prevalence of hypertension among breastfeeding (≥12 months) and never breastfeeding women was estimated to be 38.6% versus 42.1%.5 Similar results were observed in the Nurses’ Health Study II.6
Myocardial infarction and coronary heart disease
In the prospective Nurses’ Health Study, during 1,350,965 person-years of follow-up, 2,540 women had a myocardial infarction (MI). Women who had breastfed for ≥ 2 years had a 37% decreased risk of MI compared with women who never breastfed. After adjustment for family history, lifestyle factors, and adiposity, the observed reduction in risk was 23%.7 In the WHI (observational study plus controlled trial), women with a single live birth who breastfed for 7 to 12 months had a lower risk of cardiovascular disease than women with a single live birth who did not breastfeed (hazard ratio, 0.72; 95% confidence interval, 0.53–97).5

Breast cancer

In a systematic review and meta-analysis of 100 publications, breastfeeding >12 months reduced the risk of breast cancer by 26%.8 In a systematic review of 47 studies, the relative risk of breast cancer decreased by 4.7% for every 12 months of breastfeeding.9 In a systematic review and meta-analysis of 3 studies, ever breastfeeding was associated with a 28% reduced risk for triple-negative (ER-, PR-, HER2-) breast cancer among parous women.10 Triple-negative breast cancer generally has a poorer prognosis than receptor-positive breast cancers.
Continue to: Ovarian Cancer
Ovarian cancer
In a systematic review and meta-analysis of 40 publications, ever breastfeeding was associated with a 37% reduction in the risk of ovarian cancer.8 In a prospective study of 1.1 million women in the United Kingdom, 8,719 developed ovarian cancer. Among parous women, ovarian cancer risk was reduced by 10% for every 12 months of breastfeeding.11

Endometrial cancer

In a meta-analysis of 17 publications, including 8,981 cases and 17,241 controls, ever breastfeeding was associated with an 11% reduction in breast cancer risk.12 In a meta-analysis of 15 publications with 6,704 cases, breastfeeding was associated with a 26% reduction in endometrial cancer. After controlling for hormone use and body mass index, the reduced risk was in the range of 35%. A linear relationship between breastfeeding and reduced risk of endometrial cancer was observed, with 1 month of breastfeeding being associated with a 1.2% reduction in the risk of endometrial cancer.13
Let’s support our patients’ health by encouraging successful breastfeeding
Obstetrician-gynecologists play an important role in helping women make informed decisions about breastfeeding. Most professional organizations, including the American College of Obstetricians and Gynecologists, recommend exclusive breastfeeding for the first 6 months of life, with continued breastfeeding and introduction of complementary food from 6 to 12 months.14,15 Birth practices that help to increase successful breastfeeding include:
- inform all pregnant women about the newborn and maternal health benefits and management of breastfeeding
- initiate skin-to-skin contact at birth
- encourage the initiation of breastfeeding within 1 hour of birth
- ensure that breastfeeding newborns do not receive any food or drink other than breast milk, unless medically indicated
- encourage breastfeeding women to not use pacifiers or artificial nipples.15
When women are discharged from the maternity center, providing information about community-based lactation support is helpful in ensuring continuation of successful breastfeeding.16
Most patients know that exercise and maintaining a healthy weight can reduce the risk of developing many prevalent diseases. However, far fewer patients know that breastfeeding can reduce the risk of developing type 2 diabetes, hypertension, and coronary artery disease, as well as breast, ovarian, and endometrial cancers. Educating our patients about these health benefits may help them to more fully commit to breastfeeding.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Centers for Disease Control and Prevention. Breastfeeding Among U.S. Children Born 2009–2015, CDC National Immunization Survey. https://www.cdc.gov/breastfeeding/data/nis_data/results.html. Updated August 2018. Accessed November 19, 2018.
- Ip S, Chung M, Raman G, et al. A summary of the Agency for Healthcare Research and Quality’s evidence report on breastfeeding in developed countries. Breastfeed Med. 2009;4 (suppl 1):S17.
- Gunderson Ep, Lewis CE, Lin Y, et al. Lactation duration and progression to diabetes in women across the childbearing years: the 30-year CARDIA study. JAMA Int Med. 2018;178:328-337.
- Stuebe AM, Rich-Edwards JW, Willett WC, et al. Duration of lactation and incidence of type 2 diabetes. JAMA. 2005;294:2601-2610.
- Schwarz EB, Ray RM, Stuebe AM, et al. Duration of lactation and risk factors for maternal cardiovascular disease. Obstet Gynecol. 2009;113:974-982.
- Stuebe Am, Schwarz EB, Grewen K, et al. Duration of lactation and incidence of maternal hypertension: a longitudinal cohort study. Am J Epidemiol. 2011;174:1147-1158.
- Stuebe AM, Michels KB, Willett WC, et al. Duration of lactation and incidence of myocardial infarction in middle to late adulthood. Am J Obstet Gynecol. 2009;200:138.e1-e8.
- Chowdhury R, Sinha B, Sankar MJ, et al. Breastfeeding and maternal health outcomes: a systematic review and meta-analysis. Acta Paediatr. 2015;104:96-113.
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries including 50,302 women with breast cancer and 96,973 women without the disease. Lancet. 2002;360:187-195.
- Islami F, Liu Y, Jemal A, et al. Breastfeeding and breast cancer risk by receptor status—a systematic review and meta-analysis. Ann Oncol. 2015;26:2398-2407.
- Gaitskell K, Green J, Pirie K, et al. Million Women Study Collaborators. Histological subtypes of ovarian cancer associated with parity and breastfeeding in the Million Women Study. Int J Cancer. 2018;142:281-289.
- Jordan SJ, Na R, Johnatty SE, et al. Breastfeeding and endometrial cancer risk: an analysis from the epidemiology of endometrial cancer consortium. Obstet Gynecol. 2017;129:1059-1067.
- Zhan B, Liu X, Li F, Zhang D, et al. Breastfeeding and the incidence of endometrial cancer: a meta-analysis. Oncotarget. 2015;6:38398-38409.
- Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst Rev. 2012;CD003517.
- ACOG Committee Opinion No. 756. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2018;132:e187-e196.
- McFadden A, Gavine A, Renfrew M, et al. Support for healthy breastfeeding mothers with healthy term babies. Cochrane Database Syst Rev. 2017;CD001141.
- Centers for Disease Control and Prevention. Breastfeeding Among U.S. Children Born 2009–2015, CDC National Immunization Survey. https://www.cdc.gov/breastfeeding/data/nis_data/results.html. Updated August 2018. Accessed November 19, 2018.
- Ip S, Chung M, Raman G, et al. A summary of the Agency for Healthcare Research and Quality’s evidence report on breastfeeding in developed countries. Breastfeed Med. 2009;4 (suppl 1):S17.
- Gunderson Ep, Lewis CE, Lin Y, et al. Lactation duration and progression to diabetes in women across the childbearing years: the 30-year CARDIA study. JAMA Int Med. 2018;178:328-337.
- Stuebe AM, Rich-Edwards JW, Willett WC, et al. Duration of lactation and incidence of type 2 diabetes. JAMA. 2005;294:2601-2610.
- Schwarz EB, Ray RM, Stuebe AM, et al. Duration of lactation and risk factors for maternal cardiovascular disease. Obstet Gynecol. 2009;113:974-982.
- Stuebe Am, Schwarz EB, Grewen K, et al. Duration of lactation and incidence of maternal hypertension: a longitudinal cohort study. Am J Epidemiol. 2011;174:1147-1158.
- Stuebe AM, Michels KB, Willett WC, et al. Duration of lactation and incidence of myocardial infarction in middle to late adulthood. Am J Obstet Gynecol. 2009;200:138.e1-e8.
- Chowdhury R, Sinha B, Sankar MJ, et al. Breastfeeding and maternal health outcomes: a systematic review and meta-analysis. Acta Paediatr. 2015;104:96-113.
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries including 50,302 women with breast cancer and 96,973 women without the disease. Lancet. 2002;360:187-195.
- Islami F, Liu Y, Jemal A, et al. Breastfeeding and breast cancer risk by receptor status—a systematic review and meta-analysis. Ann Oncol. 2015;26:2398-2407.
- Gaitskell K, Green J, Pirie K, et al. Million Women Study Collaborators. Histological subtypes of ovarian cancer associated with parity and breastfeeding in the Million Women Study. Int J Cancer. 2018;142:281-289.
- Jordan SJ, Na R, Johnatty SE, et al. Breastfeeding and endometrial cancer risk: an analysis from the epidemiology of endometrial cancer consortium. Obstet Gynecol. 2017;129:1059-1067.
- Zhan B, Liu X, Li F, Zhang D, et al. Breastfeeding and the incidence of endometrial cancer: a meta-analysis. Oncotarget. 2015;6:38398-38409.
- Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst Rev. 2012;CD003517.
- ACOG Committee Opinion No. 756. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2018;132:e187-e196.
- McFadden A, Gavine A, Renfrew M, et al. Support for healthy breastfeeding mothers with healthy term babies. Cochrane Database Syst Rev. 2017;CD001141.
No difference between PPI prophylaxis, placebo for GI bleeding
There was no significant difference in mortality between critically ill patients who received pantoprazole prophylaxis for gastrointestinal bleeding, and those who received placebo, new findings suggest.
In a multicenter, randomized trial of 3,298 adult patients at risk for gastrointestinal bleeding, 510 patients (31.1%) in the pantoprazole group and 499 (30.4%) in the placebo group had died at 90 days (relative risk, 1.02; 95% confidence interval, 0.91-1.13; P = .76). The results were published in the New England Journal of Medicine.
Patients were aged 18 years or older; had been admitted to the ICU for an acute condition in one of six international centers; and had at least one risk factor for gastrointestinal bleeding including shock, use of anticoagulant agents, renal replacement therapy, mechanical ventilation (expected to last more than 24 hours), any history of liver disease, or any history of or ongoing coagulopathy. A total of 1,645 patients were randomly assigned to receive 40 mg of intravenous pantoprazole once daily and 1,653 received placebo, reported Mette Krag, MD, of the department of intensive care at Rigshospitalet in Copenhagen, and her coauthors.
The primary outcome was 90-day mortality. Secondary outcomes were clinically important events in the ICU, clinically important gastrointestinal bleeding in the ICU, infectious adverse events in the ICU, and days alive without the use of life support within the 90-day period.
One or more clinically important events occurred in 21.9% of patients in the pantoprazole group and in 22.6% in the placebo group (RR, 0.96; 95% CI, 0.83-1.11). In the pantoprazole group, 2.5% of patients had clinically important gastrointestinal bleeding, compared with 4.2% in the placebo group, Dr. Krag and her coauthors wrote.
The findings are similar to other recently published results, which showed “no significant differences ... in the rates of death or infectious complications between patients receiving placebo or no prophylaxis and those receiving proton pump inhibitors,” the authors wrote.
Dr. Krag reported financial support from Innovation Fund Denmark, Ehrenreich’s Foundation, and several other organizations.
SOURCE: Krag M et al. N Engl J Med. 2018 Dec 6. doi: 10.1056/NEJMoa1714919.
This article was updated 12/6/18.
“The take-home message from this trial is that, given the low incidence of clinically important upper gastrointestinal bleeding in the ICU, prophylaxis with a PPI [proton pump inhibitor], if initiated, should be reserved for seriously ill patients who are at high risk for this complication,” wrote Alan Barkun, MD, CM, of McGill University, Montreal, and Marc Bardou, MD, PhD, of the Centre Hospitalier Universitaire Dijon–Bourgogne (France), in an editorial published with the study.
Though 90-day mortality was similar between groups in this trial, “the between-group difference in the rate of important upper gastrointestinal bleeding may still support the recommendation of using a prophylactic PPI” given the absence of a difference in the rate of adverse events between the two groups, they added.
Dr. Barkun reported no disclosures; Dr. Bardou reported support from the French Medicines Agency.
“The take-home message from this trial is that, given the low incidence of clinically important upper gastrointestinal bleeding in the ICU, prophylaxis with a PPI [proton pump inhibitor], if initiated, should be reserved for seriously ill patients who are at high risk for this complication,” wrote Alan Barkun, MD, CM, of McGill University, Montreal, and Marc Bardou, MD, PhD, of the Centre Hospitalier Universitaire Dijon–Bourgogne (France), in an editorial published with the study.
Though 90-day mortality was similar between groups in this trial, “the between-group difference in the rate of important upper gastrointestinal bleeding may still support the recommendation of using a prophylactic PPI” given the absence of a difference in the rate of adverse events between the two groups, they added.
Dr. Barkun reported no disclosures; Dr. Bardou reported support from the French Medicines Agency.
“The take-home message from this trial is that, given the low incidence of clinically important upper gastrointestinal bleeding in the ICU, prophylaxis with a PPI [proton pump inhibitor], if initiated, should be reserved for seriously ill patients who are at high risk for this complication,” wrote Alan Barkun, MD, CM, of McGill University, Montreal, and Marc Bardou, MD, PhD, of the Centre Hospitalier Universitaire Dijon–Bourgogne (France), in an editorial published with the study.
Though 90-day mortality was similar between groups in this trial, “the between-group difference in the rate of important upper gastrointestinal bleeding may still support the recommendation of using a prophylactic PPI” given the absence of a difference in the rate of adverse events between the two groups, they added.
Dr. Barkun reported no disclosures; Dr. Bardou reported support from the French Medicines Agency.
There was no significant difference in mortality between critically ill patients who received pantoprazole prophylaxis for gastrointestinal bleeding, and those who received placebo, new findings suggest.
In a multicenter, randomized trial of 3,298 adult patients at risk for gastrointestinal bleeding, 510 patients (31.1%) in the pantoprazole group and 499 (30.4%) in the placebo group had died at 90 days (relative risk, 1.02; 95% confidence interval, 0.91-1.13; P = .76). The results were published in the New England Journal of Medicine.
Patients were aged 18 years or older; had been admitted to the ICU for an acute condition in one of six international centers; and had at least one risk factor for gastrointestinal bleeding including shock, use of anticoagulant agents, renal replacement therapy, mechanical ventilation (expected to last more than 24 hours), any history of liver disease, or any history of or ongoing coagulopathy. A total of 1,645 patients were randomly assigned to receive 40 mg of intravenous pantoprazole once daily and 1,653 received placebo, reported Mette Krag, MD, of the department of intensive care at Rigshospitalet in Copenhagen, and her coauthors.
The primary outcome was 90-day mortality. Secondary outcomes were clinically important events in the ICU, clinically important gastrointestinal bleeding in the ICU, infectious adverse events in the ICU, and days alive without the use of life support within the 90-day period.
One or more clinically important events occurred in 21.9% of patients in the pantoprazole group and in 22.6% in the placebo group (RR, 0.96; 95% CI, 0.83-1.11). In the pantoprazole group, 2.5% of patients had clinically important gastrointestinal bleeding, compared with 4.2% in the placebo group, Dr. Krag and her coauthors wrote.
The findings are similar to other recently published results, which showed “no significant differences ... in the rates of death or infectious complications between patients receiving placebo or no prophylaxis and those receiving proton pump inhibitors,” the authors wrote.
Dr. Krag reported financial support from Innovation Fund Denmark, Ehrenreich’s Foundation, and several other organizations.
SOURCE: Krag M et al. N Engl J Med. 2018 Dec 6. doi: 10.1056/NEJMoa1714919.
This article was updated 12/6/18.
There was no significant difference in mortality between critically ill patients who received pantoprazole prophylaxis for gastrointestinal bleeding, and those who received placebo, new findings suggest.
In a multicenter, randomized trial of 3,298 adult patients at risk for gastrointestinal bleeding, 510 patients (31.1%) in the pantoprazole group and 499 (30.4%) in the placebo group had died at 90 days (relative risk, 1.02; 95% confidence interval, 0.91-1.13; P = .76). The results were published in the New England Journal of Medicine.
Patients were aged 18 years or older; had been admitted to the ICU for an acute condition in one of six international centers; and had at least one risk factor for gastrointestinal bleeding including shock, use of anticoagulant agents, renal replacement therapy, mechanical ventilation (expected to last more than 24 hours), any history of liver disease, or any history of or ongoing coagulopathy. A total of 1,645 patients were randomly assigned to receive 40 mg of intravenous pantoprazole once daily and 1,653 received placebo, reported Mette Krag, MD, of the department of intensive care at Rigshospitalet in Copenhagen, and her coauthors.
The primary outcome was 90-day mortality. Secondary outcomes were clinically important events in the ICU, clinically important gastrointestinal bleeding in the ICU, infectious adverse events in the ICU, and days alive without the use of life support within the 90-day period.
One or more clinically important events occurred in 21.9% of patients in the pantoprazole group and in 22.6% in the placebo group (RR, 0.96; 95% CI, 0.83-1.11). In the pantoprazole group, 2.5% of patients had clinically important gastrointestinal bleeding, compared with 4.2% in the placebo group, Dr. Krag and her coauthors wrote.
The findings are similar to other recently published results, which showed “no significant differences ... in the rates of death or infectious complications between patients receiving placebo or no prophylaxis and those receiving proton pump inhibitors,” the authors wrote.
Dr. Krag reported financial support from Innovation Fund Denmark, Ehrenreich’s Foundation, and several other organizations.
SOURCE: Krag M et al. N Engl J Med. 2018 Dec 6. doi: 10.1056/NEJMoa1714919.
This article was updated 12/6/18.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: There was no significant difference in mortality between patients who received pantoprazole prophylaxis for gastrointestinal bleeding, and those who received placebo.
Major finding: Just over 31% of patients in the pantoprazole group and 30.4% in the placebo group had died at 90 days (relative risk, 1.02; 95% confidence interval, 0.91-1.13; P = .76).
Study details: A multicenter, randomized trial of 3,298 adult ICU patients at risk for gastrointestinal bleeding.
Disclosures: Dr. Krag reported financial support from Innovation Fund Denmark, Ehrenreich’s Foundation, and several other organizations.
Source: Krag M et al. N Engl J Med. 2018 Dec 6. doi: 10.1056/NEJMoa1714919.
Growths on scalp
The FP recognized the severely sun damaged scalp as a major risk factor for skin cancers. He looked closely at the lesions and realized that the ulcerated areas were at particularly high risk for squamous cell carcinoma (SCC).
He performed broad shave biopsies with sufficient depth to obtain the needed diagnosis. (See the Watch & Learn video on “Shave biopsy.”) The pathology demonstrated that 2 of the 3 biopsy sites were positive for SCC (E and G were SCC, while F was read as actinic keratosis). Cutaneous SCC is a malignant tumor of keratinocytes. Most cutaneous SCCs arise from precursor lesions, often actinic keratoses. SCC usually spreads by local extension, but it is also capable of regional lymph node metastasis and distant metastasis.
Unsure of the margins of the tumors and aware that surgery of the scalp can be challenging, the FP referred the patient for Mohs surgery. The FP also provided counseling about sun avoidance, the consistent use of a hat outdoors, and the use of sunscreens when exposed to the sun.
The Mohs surgeon recommended field treatment with 5% fluorouracil cream twice daily for 4 weeks before surgery to minimize the amount of cutting that would be needed to clear the SCC from this diffusely sun-damaged scalp. After the 5% fluorouracil cream treatment, the surgeon waited 1 month to allow the scalp to heal before performing surgery.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:999-1007.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
The new third edition will be available in January 2019: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/.
You can also get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP recognized the severely sun damaged scalp as a major risk factor for skin cancers. He looked closely at the lesions and realized that the ulcerated areas were at particularly high risk for squamous cell carcinoma (SCC).
He performed broad shave biopsies with sufficient depth to obtain the needed diagnosis. (See the Watch & Learn video on “Shave biopsy.”) The pathology demonstrated that 2 of the 3 biopsy sites were positive for SCC (E and G were SCC, while F was read as actinic keratosis). Cutaneous SCC is a malignant tumor of keratinocytes. Most cutaneous SCCs arise from precursor lesions, often actinic keratoses. SCC usually spreads by local extension, but it is also capable of regional lymph node metastasis and distant metastasis.
Unsure of the margins of the tumors and aware that surgery of the scalp can be challenging, the FP referred the patient for Mohs surgery. The FP also provided counseling about sun avoidance, the consistent use of a hat outdoors, and the use of sunscreens when exposed to the sun.
The Mohs surgeon recommended field treatment with 5% fluorouracil cream twice daily for 4 weeks before surgery to minimize the amount of cutting that would be needed to clear the SCC from this diffusely sun-damaged scalp. After the 5% fluorouracil cream treatment, the surgeon waited 1 month to allow the scalp to heal before performing surgery.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:999-1007.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
The new third edition will be available in January 2019: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/.
You can also get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP recognized the severely sun damaged scalp as a major risk factor for skin cancers. He looked closely at the lesions and realized that the ulcerated areas were at particularly high risk for squamous cell carcinoma (SCC).
He performed broad shave biopsies with sufficient depth to obtain the needed diagnosis. (See the Watch & Learn video on “Shave biopsy.”) The pathology demonstrated that 2 of the 3 biopsy sites were positive for SCC (E and G were SCC, while F was read as actinic keratosis). Cutaneous SCC is a malignant tumor of keratinocytes. Most cutaneous SCCs arise from precursor lesions, often actinic keratoses. SCC usually spreads by local extension, but it is also capable of regional lymph node metastasis and distant metastasis.
Unsure of the margins of the tumors and aware that surgery of the scalp can be challenging, the FP referred the patient for Mohs surgery. The FP also provided counseling about sun avoidance, the consistent use of a hat outdoors, and the use of sunscreens when exposed to the sun.
The Mohs surgeon recommended field treatment with 5% fluorouracil cream twice daily for 4 weeks before surgery to minimize the amount of cutting that would be needed to clear the SCC from this diffusely sun-damaged scalp. After the 5% fluorouracil cream treatment, the surgeon waited 1 month to allow the scalp to heal before performing surgery.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:999-1007.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
The new third edition will be available in January 2019: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/.
You can also get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
Trio of biosimilars have good showing
Biosimilars for three widely used oncology drugs showed efficacy and safety in lung cancer and breast cancer similar to those of the reference products, according to findings reported at the 2018 annual meeting of the American Society of Clinical Oncology in Chicago.
Oncology biosimilars for bevacizumab (Avastin), trastuzumab (Herceptin), and filgrastim (Neupogen and others) have yielded positive results in various patient populations and clinical settings, investigators reported at the annual ASCO meeting. The findings advance the promise of new agents that have no clinically meaningful differences in efficacy and safety when compared with their reference drugs but have substantially lower cost.
“Biosimilars are here,” said Michael A Thompson, MD, PhD, of Aurora Health Care in Milwaukee, Wisconsin, “[although] issues remain, including clinical decision support and pathway adoption, naming differences across the world, competition and lower prices versus the illusion of a free market, and adoption to decrease costs and increase value to our patients.” Dr Thompson was commenting during an invited discussion at the meeting. He is the medical director of the Early Phase Cancer Research Program and the Oncology Precision Medicine Program at Aurora Health (also see Commentary at end of article).
Bevacizumab biosimilar
The REFLECTIONS trial (NCT02364999) was a multinational, first-line, randomized, controlled trial among 719 patients with advanced nonsquamous non–small-cell lung cancer (NSCLC). Patients were randomized to paclitaxel and carboplatin chemotherapy plus either bevacizumab (sourced from the European Union) or the candidate bevacizumab biosimilar PF-06439535 on a double-blind basis, followed by monotherapy with the same assigned agent.
The overall response rate by week 19, confirmed by week 25 – the trial’s primary endpoint – was 45.3% with the biosimilar and 44.6% with bevacizumab, reported lead author Mark A Socinski, MD, executive medical director of the Florida Hospital Cancer Institute in Orlando. The confidence interval (CI) for the risk difference fell within the equivalence margins set by European Union regulators (-13% and +13% for the 95% CI). And the confidence interval for the risk ratio fell within the equivalence margins set by the US Food and Drug Administration (0.73 and 1.37 for the 90% CI) and Japanese regulators (0.729 and 1.371 for the 95% CI).
Median progression-free survival (PFS) was 9.0 months with the biosimilar and 7.7 months with bevacizumab (hazard ratio [HR], 0.974; P = .814), and corresponding 1-year rates were 30.8% and 29.3%, respectively, Dr Socinski reported. Median overall survival was 18.4 months and 17.8 months (HR, 1.001; P = .991), and corresponding 1-year rates were 66.4% and 68.8%.
Rates of grade 3 or higher hypertension, cardiac disorders, and bleeding did not differ significantly with the 2 agents. Patients also had similar rates of grade 3 or higher serious adverse events (AEs) and of fatal (grade 5) serious AEs with the biosimilar and bevacizumab (5.3% and 5.9%, respectively).
“Similarity between PF-06439535 and bevacizumab-EU was demonstrated for the primary efficacy endpoint of overall response rate. ... There were no clinically meaningful differences in safety profile shown in this trial, and similar pharmacokinetic and immunogenicity results were seen across treatment groups,” Dr Socinski summarized. “These results confirm the similarity demonstrated in earlier analytical, nonclinical, and clinical studies of PF-06439535 with bevacizumab-EU.”
Funding Pfizer sponsored the REFLECTIONS trial. Disclosures Dr Socinski disclosed that his institution receives research funding from Pfizer. Source Socinski MA et al. A comparative clinical study of PF-06439535, a candidate bevacizumab biosimilar, and reference bevacizumab, in patients with advanced non-squamous non-small cell lung cancer. ASCO 2018, Abstract 109. https://meetinglibrary.asco.org/record/161702/abstract. Clinical trial registry number NCT02364999 https://clinicaltrials.gov/ct2/show/NCT02364999
Trastuzumab biosimilar
The phase 3 HERITAGE trial was a first-line, randomized, controlled trial that compared biosimilar trastuzumab-dkst (Ogivri) with trastuzumab in combination with taxane chemotherapy and then as maintenance monotherapy in 458 patients with HER2+ advanced breast cancer. The 24-week results, previously reported (JAMA. 2017 Jan 3;317[1]:37-47), showed a similar overall response rate with each agent when combined with chemotherapy. Rates of various AEs were essentially the same.
The 48-week results showed a median PFS of 11.1 months with trastuzumab-dkst and 11.1 months with trastuzumab (HR, 0.95; P = .842), reported senior investigator Hope S Rugo, MD, a clinical professor of medicine and director of the Breast Oncology Clinical Trials Program at the University of California, San Francisco, Helen Diller Family Comprehensive Cancer Center. “The overall survival is immature but is impressive at over 80% at 52 weeks,” she noted.
Presence of overall response at 24 weeks correlated with duration of PFS at 48 weeks (biserial r = .752). “Additional patients achieved a response during the monotherapy portion of the treatment, which is intriguing and clearly emphasizes the importance of monotherapy, as well as the importance of having alternate agents at lower cost available,” Dr Rugo commented.
Common AEs through week 48 were much the same as those seen at week 24, with few additional [events] occurring during monotherapy. “No new safety issues were observed, and in fact, toxicity during monotherapy was quite minor,” she noted. “One thing that’s interesting here is that there was more arthralgia during the first 24 weeks with trastuzumab-dkst than with trastuzumab, but in monotherapy, this fell to a very low number and was identical between the 2 arms. Paclitaxel, which people stayed on for longer [with the biosimilar], may have been the cause of this.”
The 48-week rates of AEs of special interest – respiratory events, cardiac disorders, and infusion-related AEs – and of serious AEs were similar for the 2 agents.
“We didn’t see any additional serious cardiac events during monotherapy,” Dr Rugo noted. Mean and median left ventricular ejection fraction over 48 weeks were similar, as was the rate of LVEF, which dropped below 50% (4.0% with trastuzumab-dkst and 3.3% with trastuzumab). The incidences of antidrug antibody and neutralizing antibody were also comparably low in both groups.
“HERITAGE data, now at week 48, supports trastuzumab-dkst as a biosimilar to trastuzumab in all approved indications,” Dr Rugo said. “Final overall survival will be assessed after 36 months or after 240 deaths, whichever occurs first. Based on current data, this is predicted to conclude by the end of 2018, with final overall survival data available next year.”
Dr Rugo emphasized that trastuzumab-dkst provides “an additional high-quality treatment option for patients with HER2+ breast cancers in any setting. This study shows that biosimilars offer the potential for worldwide cost savings and improved access to life-saving therapies. It’s sobering to think that the patients enrolled in this study would not otherwise have had access to continued trastuzumab therapy, and so many of them are still alive with longer follow-up.”
Funding Mylan sponsored the HERITAGE trial. Disclosures Dr Rugo disclosed that she receives travel, accommodations, and/or expenses from Mylan. Source Manikhas A et al. Biosimilar trastuzumab-dkst monotherapy versus trastuzumab monotherapy after combination therapy: Toxicity, efficacy, and immunogenicity from the phase 3 Heritage trial. ASCO 2018, Abstract 110. https://meetinglibrary.asco.org/record/161572/abstract. Clinical trial registry number NCT02472964 https://clinicaltrials.gov/ct2/show/NCT02472964
Filgrastim biosimilar
Investigators led by Nadia Harbeck, MD, PhD, head of the Breast Center and chair for Conservative Oncology in the department of OB&GYN at the University of Munich (Germany), compared efficacy of filgrastim-sndz (Zarxio), a biosimilar of filgrastim (recombinant granulocyte colony-stimulating factor, or G-CSF), in a trial population with that of a real-world population of women receiving chemotherapy for breast cancer.
Data for the former came from PIONEER, a phase 3, randomized, controlled trial among patients with nonmetastatic breast cancer undergoing docetaxel, doxorubicin, and cyclophosphamide (TAC) chemotherapy in the neoadjuvant or adjuvant setting (Ann Oncol. 2015;26[9]:1948-53). Data for the latter came from MONITOR-GCSF, a postmarketing, open-label, observational cohort study among patients from 12 European countries receiving chemotherapy for various solid and hematologic malignancies (Support Care Cancer. 2016;24[2]:911-25).
Dr Harbeck and her colleagues compared 217 women who had nonmetastatic breast cancer from the trial with 466 women who had any-stage breast cancer (42% metastatic) from the real-world cohort.
Results showed that the 6.2% rate of chemotherapy-induced febrile neutropenia in any cycle seen in the real-world population was much the same as the 5.1% rate seen previously in the trial/biosimilar population. Findings were similar for temperature exceeding 38.5°C in any cycle: 3.4% and 5.6%, respectively. The real-world population had a lower rate of severe neutropenia than did the trial population (19.5% and 74.3%) and higher rates of infection (15.5% and 7.9%) and hospitalization caused by febrile neutropenia (3.9% and 1.8%). Findings were essentially the same in cycle-level analyses.
The real-world cohort had many fewer any-severity safety events of special interest than did the trial cohort, such as musculoskeletal/connective tissue disorders (20 and 261 events, respectively) and skin/subcutaneous tissue disorders (5 and 258 events). “Seeing these data, you have to keep in mind that the patients received totally different chemotherapy. TAC chemotherapy has a lot of chemotherapy-associated side effects,” Dr Harbeck noted. “The other thing is that MONITOR was a real-world database, and one could assume that there is some underreporting of events that are not directly correlated to the events that are of particular interest.”
Additional results available only from the trial showed that no patients developed binding or neutralizing antibodies against G-CSF.
“From a clinician’s point of view, it is very reassuring that we did not see any other safety signals in the real-world data than we saw in the randomized controlled trial and the efficacy was very, very similar,” Dr Harbeck commented. “Having seen the discrepancies in the data, I think it’s important to have randomized controlled trials to assess and monitor AEs for registration purposes and real-world evidence to reflect the daily clinical routine,” she concluded.
Funding Sandoz sponsored the PIONEER and MONITOR-GCSF trials. Disclosures Dr Harbeck disclosed that she has a consulting or advisory role with Sandoz. Source Harbeck N et al. Comparison of efficacy and safety of biosimilar filgrastim in a RCT (PIONEER) and real-world practice (MONITOR-GCSF). ASCO 2018, Abstract 111. https://meetinglibrary.asco.org/record/161688/abstract. Clinical trial registry number NCT01519700 https://clinicaltrials.gov/ct2/show/NCT01519700
Biosimilars for three widely used oncology drugs showed efficacy and safety in lung cancer and breast cancer similar to those of the reference products, according to findings reported at the 2018 annual meeting of the American Society of Clinical Oncology in Chicago.
Oncology biosimilars for bevacizumab (Avastin), trastuzumab (Herceptin), and filgrastim (Neupogen and others) have yielded positive results in various patient populations and clinical settings, investigators reported at the annual ASCO meeting. The findings advance the promise of new agents that have no clinically meaningful differences in efficacy and safety when compared with their reference drugs but have substantially lower cost.
“Biosimilars are here,” said Michael A Thompson, MD, PhD, of Aurora Health Care in Milwaukee, Wisconsin, “[although] issues remain, including clinical decision support and pathway adoption, naming differences across the world, competition and lower prices versus the illusion of a free market, and adoption to decrease costs and increase value to our patients.” Dr Thompson was commenting during an invited discussion at the meeting. He is the medical director of the Early Phase Cancer Research Program and the Oncology Precision Medicine Program at Aurora Health (also see Commentary at end of article).
Bevacizumab biosimilar
The REFLECTIONS trial (NCT02364999) was a multinational, first-line, randomized, controlled trial among 719 patients with advanced nonsquamous non–small-cell lung cancer (NSCLC). Patients were randomized to paclitaxel and carboplatin chemotherapy plus either bevacizumab (sourced from the European Union) or the candidate bevacizumab biosimilar PF-06439535 on a double-blind basis, followed by monotherapy with the same assigned agent.
The overall response rate by week 19, confirmed by week 25 – the trial’s primary endpoint – was 45.3% with the biosimilar and 44.6% with bevacizumab, reported lead author Mark A Socinski, MD, executive medical director of the Florida Hospital Cancer Institute in Orlando. The confidence interval (CI) for the risk difference fell within the equivalence margins set by European Union regulators (-13% and +13% for the 95% CI). And the confidence interval for the risk ratio fell within the equivalence margins set by the US Food and Drug Administration (0.73 and 1.37 for the 90% CI) and Japanese regulators (0.729 and 1.371 for the 95% CI).
Median progression-free survival (PFS) was 9.0 months with the biosimilar and 7.7 months with bevacizumab (hazard ratio [HR], 0.974; P = .814), and corresponding 1-year rates were 30.8% and 29.3%, respectively, Dr Socinski reported. Median overall survival was 18.4 months and 17.8 months (HR, 1.001; P = .991), and corresponding 1-year rates were 66.4% and 68.8%.
Rates of grade 3 or higher hypertension, cardiac disorders, and bleeding did not differ significantly with the 2 agents. Patients also had similar rates of grade 3 or higher serious adverse events (AEs) and of fatal (grade 5) serious AEs with the biosimilar and bevacizumab (5.3% and 5.9%, respectively).
“Similarity between PF-06439535 and bevacizumab-EU was demonstrated for the primary efficacy endpoint of overall response rate. ... There were no clinically meaningful differences in safety profile shown in this trial, and similar pharmacokinetic and immunogenicity results were seen across treatment groups,” Dr Socinski summarized. “These results confirm the similarity demonstrated in earlier analytical, nonclinical, and clinical studies of PF-06439535 with bevacizumab-EU.”
Funding Pfizer sponsored the REFLECTIONS trial. Disclosures Dr Socinski disclosed that his institution receives research funding from Pfizer. Source Socinski MA et al. A comparative clinical study of PF-06439535, a candidate bevacizumab biosimilar, and reference bevacizumab, in patients with advanced non-squamous non-small cell lung cancer. ASCO 2018, Abstract 109. https://meetinglibrary.asco.org/record/161702/abstract. Clinical trial registry number NCT02364999 https://clinicaltrials.gov/ct2/show/NCT02364999
Trastuzumab biosimilar
The phase 3 HERITAGE trial was a first-line, randomized, controlled trial that compared biosimilar trastuzumab-dkst (Ogivri) with trastuzumab in combination with taxane chemotherapy and then as maintenance monotherapy in 458 patients with HER2+ advanced breast cancer. The 24-week results, previously reported (JAMA. 2017 Jan 3;317[1]:37-47), showed a similar overall response rate with each agent when combined with chemotherapy. Rates of various AEs were essentially the same.
The 48-week results showed a median PFS of 11.1 months with trastuzumab-dkst and 11.1 months with trastuzumab (HR, 0.95; P = .842), reported senior investigator Hope S Rugo, MD, a clinical professor of medicine and director of the Breast Oncology Clinical Trials Program at the University of California, San Francisco, Helen Diller Family Comprehensive Cancer Center. “The overall survival is immature but is impressive at over 80% at 52 weeks,” she noted.
Presence of overall response at 24 weeks correlated with duration of PFS at 48 weeks (biserial r = .752). “Additional patients achieved a response during the monotherapy portion of the treatment, which is intriguing and clearly emphasizes the importance of monotherapy, as well as the importance of having alternate agents at lower cost available,” Dr Rugo commented.
Common AEs through week 48 were much the same as those seen at week 24, with few additional [events] occurring during monotherapy. “No new safety issues were observed, and in fact, toxicity during monotherapy was quite minor,” she noted. “One thing that’s interesting here is that there was more arthralgia during the first 24 weeks with trastuzumab-dkst than with trastuzumab, but in monotherapy, this fell to a very low number and was identical between the 2 arms. Paclitaxel, which people stayed on for longer [with the biosimilar], may have been the cause of this.”
The 48-week rates of AEs of special interest – respiratory events, cardiac disorders, and infusion-related AEs – and of serious AEs were similar for the 2 agents.
“We didn’t see any additional serious cardiac events during monotherapy,” Dr Rugo noted. Mean and median left ventricular ejection fraction over 48 weeks were similar, as was the rate of LVEF, which dropped below 50% (4.0% with trastuzumab-dkst and 3.3% with trastuzumab). The incidences of antidrug antibody and neutralizing antibody were also comparably low in both groups.
“HERITAGE data, now at week 48, supports trastuzumab-dkst as a biosimilar to trastuzumab in all approved indications,” Dr Rugo said. “Final overall survival will be assessed after 36 months or after 240 deaths, whichever occurs first. Based on current data, this is predicted to conclude by the end of 2018, with final overall survival data available next year.”
Dr Rugo emphasized that trastuzumab-dkst provides “an additional high-quality treatment option for patients with HER2+ breast cancers in any setting. This study shows that biosimilars offer the potential for worldwide cost savings and improved access to life-saving therapies. It’s sobering to think that the patients enrolled in this study would not otherwise have had access to continued trastuzumab therapy, and so many of them are still alive with longer follow-up.”
Funding Mylan sponsored the HERITAGE trial. Disclosures Dr Rugo disclosed that she receives travel, accommodations, and/or expenses from Mylan. Source Manikhas A et al. Biosimilar trastuzumab-dkst monotherapy versus trastuzumab monotherapy after combination therapy: Toxicity, efficacy, and immunogenicity from the phase 3 Heritage trial. ASCO 2018, Abstract 110. https://meetinglibrary.asco.org/record/161572/abstract. Clinical trial registry number NCT02472964 https://clinicaltrials.gov/ct2/show/NCT02472964
Filgrastim biosimilar
Investigators led by Nadia Harbeck, MD, PhD, head of the Breast Center and chair for Conservative Oncology in the department of OB&GYN at the University of Munich (Germany), compared efficacy of filgrastim-sndz (Zarxio), a biosimilar of filgrastim (recombinant granulocyte colony-stimulating factor, or G-CSF), in a trial population with that of a real-world population of women receiving chemotherapy for breast cancer.
Data for the former came from PIONEER, a phase 3, randomized, controlled trial among patients with nonmetastatic breast cancer undergoing docetaxel, doxorubicin, and cyclophosphamide (TAC) chemotherapy in the neoadjuvant or adjuvant setting (Ann Oncol. 2015;26[9]:1948-53). Data for the latter came from MONITOR-GCSF, a postmarketing, open-label, observational cohort study among patients from 12 European countries receiving chemotherapy for various solid and hematologic malignancies (Support Care Cancer. 2016;24[2]:911-25).
Dr Harbeck and her colleagues compared 217 women who had nonmetastatic breast cancer from the trial with 466 women who had any-stage breast cancer (42% metastatic) from the real-world cohort.
Results showed that the 6.2% rate of chemotherapy-induced febrile neutropenia in any cycle seen in the real-world population was much the same as the 5.1% rate seen previously in the trial/biosimilar population. Findings were similar for temperature exceeding 38.5°C in any cycle: 3.4% and 5.6%, respectively. The real-world population had a lower rate of severe neutropenia than did the trial population (19.5% and 74.3%) and higher rates of infection (15.5% and 7.9%) and hospitalization caused by febrile neutropenia (3.9% and 1.8%). Findings were essentially the same in cycle-level analyses.
The real-world cohort had many fewer any-severity safety events of special interest than did the trial cohort, such as musculoskeletal/connective tissue disorders (20 and 261 events, respectively) and skin/subcutaneous tissue disorders (5 and 258 events). “Seeing these data, you have to keep in mind that the patients received totally different chemotherapy. TAC chemotherapy has a lot of chemotherapy-associated side effects,” Dr Harbeck noted. “The other thing is that MONITOR was a real-world database, and one could assume that there is some underreporting of events that are not directly correlated to the events that are of particular interest.”
Additional results available only from the trial showed that no patients developed binding or neutralizing antibodies against G-CSF.
“From a clinician’s point of view, it is very reassuring that we did not see any other safety signals in the real-world data than we saw in the randomized controlled trial and the efficacy was very, very similar,” Dr Harbeck commented. “Having seen the discrepancies in the data, I think it’s important to have randomized controlled trials to assess and monitor AEs for registration purposes and real-world evidence to reflect the daily clinical routine,” she concluded.
Funding Sandoz sponsored the PIONEER and MONITOR-GCSF trials. Disclosures Dr Harbeck disclosed that she has a consulting or advisory role with Sandoz. Source Harbeck N et al. Comparison of efficacy and safety of biosimilar filgrastim in a RCT (PIONEER) and real-world practice (MONITOR-GCSF). ASCO 2018, Abstract 111. https://meetinglibrary.asco.org/record/161688/abstract. Clinical trial registry number NCT01519700 https://clinicaltrials.gov/ct2/show/NCT01519700
Biosimilars for three widely used oncology drugs showed efficacy and safety in lung cancer and breast cancer similar to those of the reference products, according to findings reported at the 2018 annual meeting of the American Society of Clinical Oncology in Chicago.
Oncology biosimilars for bevacizumab (Avastin), trastuzumab (Herceptin), and filgrastim (Neupogen and others) have yielded positive results in various patient populations and clinical settings, investigators reported at the annual ASCO meeting. The findings advance the promise of new agents that have no clinically meaningful differences in efficacy and safety when compared with their reference drugs but have substantially lower cost.
“Biosimilars are here,” said Michael A Thompson, MD, PhD, of Aurora Health Care in Milwaukee, Wisconsin, “[although] issues remain, including clinical decision support and pathway adoption, naming differences across the world, competition and lower prices versus the illusion of a free market, and adoption to decrease costs and increase value to our patients.” Dr Thompson was commenting during an invited discussion at the meeting. He is the medical director of the Early Phase Cancer Research Program and the Oncology Precision Medicine Program at Aurora Health (also see Commentary at end of article).
Bevacizumab biosimilar
The REFLECTIONS trial (NCT02364999) was a multinational, first-line, randomized, controlled trial among 719 patients with advanced nonsquamous non–small-cell lung cancer (NSCLC). Patients were randomized to paclitaxel and carboplatin chemotherapy plus either bevacizumab (sourced from the European Union) or the candidate bevacizumab biosimilar PF-06439535 on a double-blind basis, followed by monotherapy with the same assigned agent.
The overall response rate by week 19, confirmed by week 25 – the trial’s primary endpoint – was 45.3% with the biosimilar and 44.6% with bevacizumab, reported lead author Mark A Socinski, MD, executive medical director of the Florida Hospital Cancer Institute in Orlando. The confidence interval (CI) for the risk difference fell within the equivalence margins set by European Union regulators (-13% and +13% for the 95% CI). And the confidence interval for the risk ratio fell within the equivalence margins set by the US Food and Drug Administration (0.73 and 1.37 for the 90% CI) and Japanese regulators (0.729 and 1.371 for the 95% CI).
Median progression-free survival (PFS) was 9.0 months with the biosimilar and 7.7 months with bevacizumab (hazard ratio [HR], 0.974; P = .814), and corresponding 1-year rates were 30.8% and 29.3%, respectively, Dr Socinski reported. Median overall survival was 18.4 months and 17.8 months (HR, 1.001; P = .991), and corresponding 1-year rates were 66.4% and 68.8%.
Rates of grade 3 or higher hypertension, cardiac disorders, and bleeding did not differ significantly with the 2 agents. Patients also had similar rates of grade 3 or higher serious adverse events (AEs) and of fatal (grade 5) serious AEs with the biosimilar and bevacizumab (5.3% and 5.9%, respectively).
“Similarity between PF-06439535 and bevacizumab-EU was demonstrated for the primary efficacy endpoint of overall response rate. ... There were no clinically meaningful differences in safety profile shown in this trial, and similar pharmacokinetic and immunogenicity results were seen across treatment groups,” Dr Socinski summarized. “These results confirm the similarity demonstrated in earlier analytical, nonclinical, and clinical studies of PF-06439535 with bevacizumab-EU.”
Funding Pfizer sponsored the REFLECTIONS trial. Disclosures Dr Socinski disclosed that his institution receives research funding from Pfizer. Source Socinski MA et al. A comparative clinical study of PF-06439535, a candidate bevacizumab biosimilar, and reference bevacizumab, in patients with advanced non-squamous non-small cell lung cancer. ASCO 2018, Abstract 109. https://meetinglibrary.asco.org/record/161702/abstract. Clinical trial registry number NCT02364999 https://clinicaltrials.gov/ct2/show/NCT02364999
Trastuzumab biosimilar
The phase 3 HERITAGE trial was a first-line, randomized, controlled trial that compared biosimilar trastuzumab-dkst (Ogivri) with trastuzumab in combination with taxane chemotherapy and then as maintenance monotherapy in 458 patients with HER2+ advanced breast cancer. The 24-week results, previously reported (JAMA. 2017 Jan 3;317[1]:37-47), showed a similar overall response rate with each agent when combined with chemotherapy. Rates of various AEs were essentially the same.
The 48-week results showed a median PFS of 11.1 months with trastuzumab-dkst and 11.1 months with trastuzumab (HR, 0.95; P = .842), reported senior investigator Hope S Rugo, MD, a clinical professor of medicine and director of the Breast Oncology Clinical Trials Program at the University of California, San Francisco, Helen Diller Family Comprehensive Cancer Center. “The overall survival is immature but is impressive at over 80% at 52 weeks,” she noted.
Presence of overall response at 24 weeks correlated with duration of PFS at 48 weeks (biserial r = .752). “Additional patients achieved a response during the monotherapy portion of the treatment, which is intriguing and clearly emphasizes the importance of monotherapy, as well as the importance of having alternate agents at lower cost available,” Dr Rugo commented.
Common AEs through week 48 were much the same as those seen at week 24, with few additional [events] occurring during monotherapy. “No new safety issues were observed, and in fact, toxicity during monotherapy was quite minor,” she noted. “One thing that’s interesting here is that there was more arthralgia during the first 24 weeks with trastuzumab-dkst than with trastuzumab, but in monotherapy, this fell to a very low number and was identical between the 2 arms. Paclitaxel, which people stayed on for longer [with the biosimilar], may have been the cause of this.”
The 48-week rates of AEs of special interest – respiratory events, cardiac disorders, and infusion-related AEs – and of serious AEs were similar for the 2 agents.
“We didn’t see any additional serious cardiac events during monotherapy,” Dr Rugo noted. Mean and median left ventricular ejection fraction over 48 weeks were similar, as was the rate of LVEF, which dropped below 50% (4.0% with trastuzumab-dkst and 3.3% with trastuzumab). The incidences of antidrug antibody and neutralizing antibody were also comparably low in both groups.
“HERITAGE data, now at week 48, supports trastuzumab-dkst as a biosimilar to trastuzumab in all approved indications,” Dr Rugo said. “Final overall survival will be assessed after 36 months or after 240 deaths, whichever occurs first. Based on current data, this is predicted to conclude by the end of 2018, with final overall survival data available next year.”
Dr Rugo emphasized that trastuzumab-dkst provides “an additional high-quality treatment option for patients with HER2+ breast cancers in any setting. This study shows that biosimilars offer the potential for worldwide cost savings and improved access to life-saving therapies. It’s sobering to think that the patients enrolled in this study would not otherwise have had access to continued trastuzumab therapy, and so many of them are still alive with longer follow-up.”
Funding Mylan sponsored the HERITAGE trial. Disclosures Dr Rugo disclosed that she receives travel, accommodations, and/or expenses from Mylan. Source Manikhas A et al. Biosimilar trastuzumab-dkst monotherapy versus trastuzumab monotherapy after combination therapy: Toxicity, efficacy, and immunogenicity from the phase 3 Heritage trial. ASCO 2018, Abstract 110. https://meetinglibrary.asco.org/record/161572/abstract. Clinical trial registry number NCT02472964 https://clinicaltrials.gov/ct2/show/NCT02472964
Filgrastim biosimilar
Investigators led by Nadia Harbeck, MD, PhD, head of the Breast Center and chair for Conservative Oncology in the department of OB&GYN at the University of Munich (Germany), compared efficacy of filgrastim-sndz (Zarxio), a biosimilar of filgrastim (recombinant granulocyte colony-stimulating factor, or G-CSF), in a trial population with that of a real-world population of women receiving chemotherapy for breast cancer.
Data for the former came from PIONEER, a phase 3, randomized, controlled trial among patients with nonmetastatic breast cancer undergoing docetaxel, doxorubicin, and cyclophosphamide (TAC) chemotherapy in the neoadjuvant or adjuvant setting (Ann Oncol. 2015;26[9]:1948-53). Data for the latter came from MONITOR-GCSF, a postmarketing, open-label, observational cohort study among patients from 12 European countries receiving chemotherapy for various solid and hematologic malignancies (Support Care Cancer. 2016;24[2]:911-25).
Dr Harbeck and her colleagues compared 217 women who had nonmetastatic breast cancer from the trial with 466 women who had any-stage breast cancer (42% metastatic) from the real-world cohort.
Results showed that the 6.2% rate of chemotherapy-induced febrile neutropenia in any cycle seen in the real-world population was much the same as the 5.1% rate seen previously in the trial/biosimilar population. Findings were similar for temperature exceeding 38.5°C in any cycle: 3.4% and 5.6%, respectively. The real-world population had a lower rate of severe neutropenia than did the trial population (19.5% and 74.3%) and higher rates of infection (15.5% and 7.9%) and hospitalization caused by febrile neutropenia (3.9% and 1.8%). Findings were essentially the same in cycle-level analyses.
The real-world cohort had many fewer any-severity safety events of special interest than did the trial cohort, such as musculoskeletal/connective tissue disorders (20 and 261 events, respectively) and skin/subcutaneous tissue disorders (5 and 258 events). “Seeing these data, you have to keep in mind that the patients received totally different chemotherapy. TAC chemotherapy has a lot of chemotherapy-associated side effects,” Dr Harbeck noted. “The other thing is that MONITOR was a real-world database, and one could assume that there is some underreporting of events that are not directly correlated to the events that are of particular interest.”
Additional results available only from the trial showed that no patients developed binding or neutralizing antibodies against G-CSF.
“From a clinician’s point of view, it is very reassuring that we did not see any other safety signals in the real-world data than we saw in the randomized controlled trial and the efficacy was very, very similar,” Dr Harbeck commented. “Having seen the discrepancies in the data, I think it’s important to have randomized controlled trials to assess and monitor AEs for registration purposes and real-world evidence to reflect the daily clinical routine,” she concluded.
Funding Sandoz sponsored the PIONEER and MONITOR-GCSF trials. Disclosures Dr Harbeck disclosed that she has a consulting or advisory role with Sandoz. Source Harbeck N et al. Comparison of efficacy and safety of biosimilar filgrastim in a RCT (PIONEER) and real-world practice (MONITOR-GCSF). ASCO 2018, Abstract 111. https://meetinglibrary.asco.org/record/161688/abstract. Clinical trial registry number NCT01519700 https://clinicaltrials.gov/ct2/show/NCT01519700
Key clinical points Biosimilars for bevacizumab, trastuzumab, and filgrastim showed similar efficacy and safety compared with their reference drugs.
Major findings Bevacizumab In patients with advanced nonsquamous NSCLC, the ORR was 45.3% with a candidate bevacizumab biosimilar and 44.6% with bevacizumab. Trastuzumab In patients with HER2+ advanced breast cancer, 48-week median PFS was 11.1 months for both trastuzumab-dkst and trastuzumab. Filgrastim The rate of chemotherapy-induced febrile neutropenia among breast cancer patients given a biosimilar for filgrastim was 5.1% in a trial population and 6.2% in a real-world population.
Study details Randomized, controlled trials of first-line therapy among 719 patients with advanced nonsquamous NSCLC (REFLECTIONS trial with bevacizumab) and among 458 patients with HER2+ advanced breast cancer (HERITAGE trial with trastuzumab). Comparison of outcomes in a randomized, controlled trial among 217 patients with nonmetastatic breast cancer (PIONEER trial with filgrastim) and a real-world cohort study of 466 patients with any-stage breast cancer (MONITOR-GCSF with filgrastim).
Disclosures and sources See article text.
Combined risk factors for coronary events
Also today, a device malfunction muddles results for insulin inhaler for Alzheimer’s, it’s time for universal hepatitis C virus screening in the ED, and there is a date for the 2019 Sickle Cell Disease guidelines.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, a device malfunction muddles results for insulin inhaler for Alzheimer’s, it’s time for universal hepatitis C virus screening in the ED, and there is a date for the 2019 Sickle Cell Disease guidelines.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, a device malfunction muddles results for insulin inhaler for Alzheimer’s, it’s time for universal hepatitis C virus screening in the ED, and there is a date for the 2019 Sickle Cell Disease guidelines.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Emerging biosimilars market presents opportunities and challenges
The development of biologic therapies has led to some of the most significant advances in the treatment of cancer, but these drugs are also very expensive. As patents for the biologics begin to expire, the development of biosimilars has the potential to dramatically cut therapy costs thereby making the therapies more readily accessible to patients. Here, we discuss biosimilar development and the challenges that need to be overcome to create a robust market.
Biosimilar, not generic
Biologic therapies are derived from living organisms and include the targeted monoclonal antibodies (mAbs) and cell-based therapies that have revolutionized the treatment of certain cancer types. Yet, their greater complexity makes them more difficult to manufacture, store, and administer, making them a costly therapeutic option that ultimately drives up health care costs. According to a 2011 drug expenditure analysis, biologic therapies accounted for more than half of the total expenditure on anticancer drugs in the US health care system.1,2
Generally, when drug patents expire, other companies can develop their own identical generic versions to increase competition in the marketplace and drive down costs. However, the paradigm for generic development cannot be applied to biologic therapies because the way in which they are manufactured makes it impossible to generate an identical copy.
Instead, the Biologics Price Competition and Innovation Act, a provision of the Patient Protection and Affordable Care Act, has allowed for submission of an application for “licensure of a biologic product based on its similarity to a licensed biologic product”.3
These “biosimilars” have been positioned as game-changers in oncology, with the potential to reduce costs and improve access to biologic therapies. With the patents on several blockbuster cancer biologics already expired or due to expire by 2020, an increasing number of biosimilars are being developed.4
Totality of evidence
Biosimilars require more rigorous testing than generics, but they don’t require the same type of scientific data that the original biologic products, termed “reference products,” did. Therefore, they are governed by legislation unique to them and approved by different regulatory pathways. The US Food and Drug Administration (FDA) has established a unique shortened regulatory pathway for their approval, known as the 351(k) pathway. So whereas the pathway for reference products is geared toward demonstrating patient benefit, biosimilars are required instead to show equivalence to the reference product.5
Biosimilars are produced through reverse engineering the reference product. Then, through a stepwise process, to generate what the FDA calls a “totality of evidence,” biosimilar manufacturers must demonstrate structural and functional similarities (through comparative quality studies) and comparable pharmacokinetics and pharmacodynamics (through comparative nonclinical and clinical studies) to the reference product. Final approval is based on 1 or more comparative clinical studies performed in the most sensitive patient population(s) (Figure 1).6
The primary endpoint of biosimilar clinical trials is chosen to detect clinically relevant differences and may not be the same as that used in pivotal trials of the reference product. Endpoints such as progression-free survival (PFS) and overall survival (OS) may not be feasible or sensitive enough to demonstrate biosimilarity.
Clinical trials of biosimilars should also be carried out in the most sensitive patient population, so that any potential differences can be attributed to the drug and not the patient population itself. If the reference product is approved across several different indications and there is sufficient scientific evidence to allow it, including the demonstration that the mechanism of action of the drug is the same across all indications, the FDA can extend the approval of the biosimilar to all of these indications without the need for individual clinical trials through a process known as extrapolation.
Biosimilar manufacturers must also provide evidence of the composition of their formulation and of quality control in their manufacturing processes, to ensure that biosimilarity can be maintained from batch to batch. As with the reference product, even small changes in the manufacturing process can have serious ramifications for clinical efficacy and safety.7,8
A flurry of approvals
The first biosimilar approvals in oncology in the United States came in the supportive care niche (Table 1). Filgrastim-sndz (Zarxio), approved in March 2015, is a biosimilar of the granulocyte-macrophage colony stimulating factor (G-CSF) analog filgrastim (Neupogen). Owing to its mechanism of action in stimulating the production of neutrophils in the bone marrow, filgrastim is used to help reduce the risk or severity of neutropenia in patients undergoing myelosuppressive chemotherapy regimens.
Filgrastim-sndz was approved for use across all 5 indications for which the reference product is approved, based on the totality of evidence, which included results from the key phase 3 PIONEER study.9 Market entry was initially delayed by lawsuits filed by Amgen, the maker of the reference product, but the biosimilar was subsequently cleared by the US Court of Appeals for the Federal Circuit. The wholesale acquisition cost (WAC) for a 300µg syringe is $324.30 for filgrastim and $275.66 figrastim-sndz, representing a 15% reduction on the reference product.10
In 2018, the FDA approved a second filgrastim biosimilar, filgrastim-aafi (Nivestym),11 in addition to 2 biosimilars of the pegylated form of filgrastim, pegfilgrastim-jmdb (Fulphila)12 and pegfilgrastim-cbqv (Udenyca)13 – these forms of filgrastim have been modified by the addition of polyethylene glycol polymer chains that help to increase circulation time.
Approval for the 2 pegfilgrastm biosimilars was originally delayed by complete response letters (CRLs) from the FDA. For pegfilgrastim-jmdb, the CRL was reported to be related to a pending update of the Biologic’s License Application (BLA) to include information regarding facility requalification activities that had been taken after the addition of plant modifications. The CRL for pegfilgrastim-cbqv requested that the company provide additional manufacturing information and reanalyze a subset of samples with a revised immunogenicity assay.
Once the CRL concerns were addressed, regulatory approval was awarded and Mylan recently confirmed that pegfilgrastim-jmdb has been launched in the US marketplace at a WAC that reflects a 33% discount over the reference product.14
Approval data for filgrastim-aafi and pegfilgrastim-cbqv have not yet been published, however the respective manufacturers reported that approval was based on totality of evidence demonstrating a high degree of similarity to the reference products. Filgrastim-aafi was approved for all of the indications of the reference product and launched in the US on October 1, 2018 at a 30% discounted WAC.15
Epoetin alfa-epbx (Retacrit), a biosimilar of epoetin alfa, was also approved in 2018. It is a recombinant analog of erythropoietin (EPO), which stimulates the production of blood cells and has proved useful for the treatment of anemia, including in cancer patients receiving myelosuppressive chemotherapy. Approval of the biosimilar followed earlier receipt of a CRL from the FDA citing concerns relating to the manufacturing facility, which the company addressed. Pfizer has said that it expects to launch the biosimilar this year (2018), but a WAC has not been disclosed.16The FDA also recently approved the first biosimilars for the treatment of cancer. Trastuzumab-dkst (Ogivri) and bevacizumab-awwb (Mvasi) were approved in the second half of 2017 for the same indications as their respective reference products, which are mAbs directed at the human epidermal growth factor receptor 2 (HER2) and vascular endothelial growth factor, respectively.17,18
Approval data for bevacizumab-awwb included a comparative clinical trial in patients with advanced/metastatic non–small-cell lung cancer (NSCLC), which was considered the most sensitive patient population. The BLA for trastuzumab-dkst included data from the phase 3 comparative HERiTAge clinical trial, in which the biosimilar was compared with the reference product, both in combination with docetaxel or paclitaxel, in patients with previously untreated HER2-positive metastatic breast cancer. Neither biosimilar has been launched on the US market yet because the patents for their reference products do not expire until 2019, so it is not clear what the price discount will be for these drugs (Table 2).9,19-22
Biosimilars in development
While numerous other biosimilars of filgrastim and pegfilgrastim are in development, the major focus has been on the development of more biosimilars to treat cancer (Table 3). BLAs have been submitted for 4 biosimilars of trastuzumab and 1 bevacizumab biosimilar. Approval for several of the trastuzumab biosimilars has been delayed by CRLs from the FDA, mostly regarding issues with the manufacturing process or facility. Several other trastuzumab and bevacizumab biosimilars are in late-stage clinical trials.
The results of several phase 3 comparative clinical trials were recently published or reported at annual conferences. Pfizer’s PF-05280014 was compared with the European Union (EU)–approved trastuzumab, both in combination with paclitaxel, in patients with previously untreated HER2-positive metastatic breast cancer. Data reported at the European Society for Medical Oncology congress in 2017 demonstrated equivalence between the reference product and biosimilar in overall response rate (ORR).23
Another recently published trial compared this biosimilar to EU-trastuzumab, both in combination with carboplatin and docetaxel, as neoadjuvant treatment for patients with resectable HER2-positive breast cancer. Among 226 patients randomized to receive 8 mg/kg in cycle 1 and 6 mg/kg thereafter of the biosimilar or reference product, every 3 weeks for 6 cycles, the pathologic complete response (pCR) rates were 47% and 50%, respectively.24
The results of a phase 3 study comparing Samsung Bioepis/Merck’s joint offering SB3 were recently published. A total of 875 patients were randomized 1:1 to receive SB3 or reference trastuzumab in combination with chemotherapy (4 cycles docetaxel followed by 4 cycles 5-fluorouracil/epirubicin/cyclophosphamide) prior to surgery, followed by 10 cycles of adjuvant SB3 or trastuzumab reference. Rates of event-free survival (EFS) were comparable between the 2 groups at 12 months (93.7% vs 96.1%, respectively).25
Amgen’s ABP980 was evaluated in the phase 3 LILAC trial, which measured the effect of the biosimilar on pCR in women with HER2-positive early breast cancer compared with reference trastuzumab. After 4 cycles of run-in anthracycline-based chemotherapy, ABP980 or reference trastuzumab were administered in combination with paclitaxel. This was followed by surgery and then ABP980 or reference trastuzumab in the adjuvant setting for up to 1 year, with the option to continue on the same drug as the neoadjuvant setting or to switch to the other. Among 696 assessable patients, the pCR rates were 48% and 42%, respectively.26
Most advanced in clinical testing among the upcoming bevacizumab biosimilars is Pfizer’s PF-06439535, for which the results of a phase 3 comparative trial were presented at the 2018 annual meeting of the American Society for Clinical Oncology. PF-06439535 was compared with the EU-approved bevacizumab, both in combination with paclitaxel and carboplatin, as first-line therapy for patients with advanced non-squamous NSCLC. Among 719 patients, the primary endpoint of ORR was 45.3% and 44.6%, respectively.27
Biosimilars of a third blockbuster cancer drug, the CD20-targeting mAb rituximab (Rituxan) are also in development and FDA approval is pending for 2. The patent for Rituxan expired in 2016, so these drugs could hit the market as soon as they are approved.
In a race to the finish for the first US-approved rituximab biosimilar, Celltrion-Teva’s CT-P10 (Truxima) seems most likely to come first; the Oncologic Drugs Advisory Committee voted unanimously in October 2018 to recommend its approval. Phase 3 comparative data were recently published; patients with newly diagnosed advanced-stage follicular lymphoma were randomized to receive intravenous infusions of 375 mg/m2 CT-P10 or reference rituximab, both in combination with cyclophosphamide, vincristine, and prednisone, on day 1 of 8 21-day cycles. The ORRs were identical (92.6%) for both drugs, pharmacokinetics data also suggested bioequivalence, and the incidence of AEs was also comparable (83% vs 80%).28
Biosimilars of the epidermal growth factor receptor (EGFR)-targeting mAb cetuximab are also listed in the pipeline for several biosimilar developers, but there is no indication of their developmental status as yet and no clinical trials are ongoing in the US.
Sorrento is developing STI-001, a cetuximab biosimilar, and reported that a phase 3 trial had been completed. Instead of a comparison with the reference product, however, the trial compared STI-001 in combination with irinotecan with irinotecan alone. They reported significantly higher ORR, PFS, and OS with the biosimilar compared with irinotecan alone, and a significant increase over historical data with the reference product, as well as fewer side effects and immunogenicity, which they attribute to its manufacture in a different cell line. However, no data has been published and no trials are ongoing in the United States, so the status of its development remains unclear.29
Challenges to a robust market
It is an exciting time for biosimilars, with many approvals and drugs being brought to market in the US in the past several years and more poised to follow suit as patents expire. Yet many challenges remain around the growth of a robust biosimilars market.
Several surveys conducted in recent years have demonstrated suboptimal knowledge of all aspects of biosimilars and highlighted the need for evidence-based education across specialties.30,31 In response, the FDA recently announced that it was launching an educational campaign to further understanding of biosimilars, including naming conventions (Figure 2).32,33 Numerous other medical professional societies have produced or are in the process of producing biosimilar guidelines.
Educational outreach by the FDA forms part of their 4-step plan to aid biosimilar development, which also aims to improve the efficiency of biosimilar development and approval, to provide regulatory clarity for manufacturers, to facilitate public understanding and acceptance, and to support a competitive marketplace.
Among the most critical educational gaps is confusion over the issue of interchangeability. Once approved by the FDA, generic drugs are considered interchangeable with the brand name drug and can be substituted at the pharmacy level without referring to the prescribing physician. This is not the case for biosimilars; owing to their more complex nature, biosimilars require a separate designation for interchangeability and none of those approved so far have been given this designation by the FDA.
There has been some confusion about what will be required to demonstrate interchangeability, and the FDA recently produced draft guidance, saying that essentially it should be proven that switching out the reference product for a biosimilar does not increase risk in terms of diminished efficacy or safety. Several companies are beginning to incorporate a switching component into their clinical trials of biosimilars.
Continued postmarketing and real-world studies will also be particularly important for biosimilars to increase confidence in prescribing them by demonstrating their continued efficacy and safety in the long-term. Several real-world studies are now ongoing, including the MONITOR-GCSF trial of filgrastim biosimilars.
Another major barrier to the development of a thriving biosimilars market that achieves the goals of reduced costs and increased access is the financial burden of their development. They are vastly more costly to develop and produce than generics. Added to litigation costs, this can limit their ability to compete in terms of price, which has been reflected in the lower-than-anticipated cost savings with some approved biosimilars thus far.
Experts have suggested that there might be much to learn from the European market, where biosimilars have been available for more than a decade and over time have reached even higher-than-expected savings. With high financial stakes and an increasingly important role in the treatment of cancer, the need to iron out the kinks is more pressing than ever.7,8,34,35
. Abraham J. Developing oncology biosimilars: an essential approach for the future. Semin Oncol. 2013;40 Suppl 1:S5-24.
2. Doloresco F, Fominaya C, Schumock GT, et al. Projecting future drug expenditures: 2011. Am J Health Syst Pharm. 2011;68(10):921-932.
3. Prepared by the Office of the Legislative Counsel. HHS website. Compilation of the Patient Protection and Affordable Care Act [as amended through May 1, 2010] including Patient Protection and Affordable Care Act health-related portions of the Health Care and Education Reconciliation Act of 2010. https://www.hhs.gov/sites/default/files/ppacacon.pdf. Released June 9, 2010. Accessed November 7, 2018.
4. Mulcahy AW, Hlavka JP, Case SR. Biosimilar cost savings in the United States: initial experience and future potential. Rand Health Q. 2018;7(4):3-3.
5. Hung A, Vu Q, Mostovoy L. A systematic review of US biosimilar approvals: what evidence does the FDA require and how are manufacturers responding? J Manag Care Spec Pharm. 2017;23(12):1234-1244.
6. Uifălean A, Ilieş M, Nicoară R, Rus LM, Hegheş SC, Iuga C-A. Concepts and challenges of biosimilars in breast cancer: the emergence of trastuzumab biosimilars. Pharmaceutics. 2018;10(4):E168.
7. Rugo HS, Linton KM, Cervi P, Rosenberg JA, Jacobs I. A clinician's guide to biosimilars in oncology. Cancer Treat Rev. 2016;46:73-79.
8. Chopra R, Lopes G. Improving access to cancer treatments: the role of biosimilars. J Glob Oncol. 2017;3(5):596-610.
9. Blackwell K, Semiglazov V, Krasnozhon D, et al. Comparison of EP2006, a filgrastim biosimilar, to the reference: a phase III, randomized, double-blind clinical study in the prevention of severe neutropenia in patients with breast cancer receiving myelosuppressive chemotherapy. Ann Oncol. 2015;26(9):1948-1953.
10. FDA News. Sandoz launches Zarxio at 15 percent lower price than Neupogen. https://www.fdanews.com/articles/173036-sandoz-launches-zarxio-at-15-percent-lower-price-than-neupogen. Released September 11, 2015. Accessed November 7, 2018.
11. Pfizer. US FDA approves Pfizer's biosimilar Nivestym (filgrastim-aafi). https://www.pfizer.com/news/press-release/press-release-detail/u_s_fda_approves_pfizer_s_biosimilar_nivestym_filgrastim_aafi-0. Released July 2o, 2018. Accessed November 7, 2018.
12. United States Food and Drug Administration. FDA approves first biosimilar to Neulasta to help reduce the risk of infection during cancer treatment. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm609805.htm. Released on June 4, 2018. Accessed November 7, 2018.
13. Coherus Biosciences. US FDA approves Udenyca (pegfilgrastim-cbqv). http://investors.coherus.com/news-releases/news-release-details/us-fda-approves-udenycatm-pegfilgrastim-cbqv. Released November 2, 2018. Accessed November 7, 2018.
14. The Center for Biosimilars. Mylan confirms that it has launched Fulphila in the United States. https://www.centerforbiosimilars.com/news/mylan-confirms-that-it-has-launched-fulphila-in-the-united-states. Released July 30, 2018. Accessed November 7, 2018.
15. The Center for Biosimilars. Pfizer launches biosimilar filgrastim, Nivestym, at a substantial discount. https://www.centerforbiosimilars.com/news/pfizer-launches-biosimilar-filgrastim-nivestym-at-a-substantial-discount. Released October 3, 2018. Accessed November 7, 2018.
16. The Center for Biosimilars. FDA approves Pfizer's epoetin alfa biosimilar, Retacrit. https://www.centerforbiosimilars.com/news/fda-approves-pfizers-epoetin-alfa-biosimilar-retacrit. Released May 15, 2018. Accessed November 7, 2018.
17. United States Food and Drug Administration. FDA approves Ogivri as a biosimilar to Herceptin. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm587404.htm. Last updated December 1, 2017. Accessed November 7, 2018.
18. United States Food and Drug Administration. FDA approves first biosimilar for the treatment of cancer. 2017; https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm576112.htm. Last updated March 26, 2018. Accessed November 7, 2018.
19. Waller CF, Blakeley C, Pennella E, et al. Phase 3 efficacy and safety trial of proposed pegfilgrastim biosimilar MYL-1401H vs EU-neulasta in the prophylaxis of chemotherapy-induced neutropenia. Ann Oncol. 2016;27(suppl_6):14330.
20. US Food and Drug Administration. 'Epoetin Hospira,' a proposed biosimilar to US-licensed Epogen/Procrit. 2017. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/OncologicDrugsAdvisoryCommittee/UCM559962.pdf. Accessed November 7, 2018.
21. Manikhas A, Pennella EJ, Bondarenko I, et al. Biosimilar trastuzumab-dkst monotherapy versus trastuzumab monotherapy after combination therapy: toxicity, efficacy, and immunogenicity from the phase 3 Heritage trial. J Clin Oncol. 2018;36(15_suppl):110.
22. Thatcher N, Thomas M, Paz-Ares L, et al. Randomized, double-blind, phase 3 study evaluating efficacy and safety of ABP 215 compared with bevacizumab in patients with non-squamous NSCLC. J Clin Oncol. 2016;34(15_suppl):9095.
23. Pegram M, Tan-Chiu E, Freyman A, et al. A randomized, double-blind study of PF-05280014 (a potential trastuzumab biosimilar) vs trastuzumab, both in combination with paclitaxel, as first-line therapy. Ann Oncol. 2017;28(suppl_5):v74-v108.
24. Lammers PE, Dank M, Masetti R, et al. Neoadjuvant PF-05280014 (a potential trastuzumab biosimilar) versus trastuzumab for operable HER2+ breast cancer. Br J Cancer. 2018;119(3):266-273.
25. Pivot X, Bondarenko I, Nowecki Z, et al. A phase III study comparing SB3 (a proposed trastuzumab biosimilar) and trastuzumab reference product in HER2-positive early breast cancer treated with neoadjuvant-adjuvant treatment: final safety, immunogenicity and survival results. Eur J Cancer. 2018;93:19-27.
26. von Minckwitz G, Colleoni M, Kolberg HC, et al. Efficacy and safety of ABP 980 compared with reference trastuzumab in women with HER2-positive early breast cancer (LILAC study): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2018;19(7):987-998.
27. Socinski MA, Pawel JV, Kasahara K, et al. A comparative clinical study of PF-06439535, a candidate bevacizumab biosimilar, and reference bevacizumab, in patients with advanced non-squamous non-small cell lung cancer. J Clin Oncol. 2018;36(15_suppl):109-109.
28. Kim WS, Buske C, Ogura M, et al. Efficacy, pharmacokinetics, and safety of the biosimilar CT-P10 compared with rituximab in patients with previously untreated advanced-stage follicular lymphoma: a randomised, double-blind, parallel-group, non-inferiority phase 3 trial. Lancet Haematol. 2017;4(8):e362-e373.
29. PRNewsire. Sorrento announces positive data from phase 3 studies of biosimilar antibodies, STI-001 and STI-002. https://www.prnewswire.com/news-releases/sorrento-announces-positive-data-from-phase-3-studies-of-biosimilar-antibodies-sti-001-and-sti-002-300202054.html. Released January 11, 2016. Accessed November 7, 2018.
30. Molinari AL, Gewanter HL, Loaiza-Bonilla A, Reilly M, Kennedy B, Charles D. Global survey of physicians' attitudes toward biologic and biosimilar therapies. J Clin Oncol. 2016;34(15_suppl):e18025-e18025.
31. Cohen H, Beydoun D, Chien D, et al. Awareness, knowledge, and perceptions of biosimilars among specialty physicians. Adv Ther. 2017;33(12):2160-2172.
32. Tomaszewski D. Biosimilar naming conventions: pharmacist perceptions and impact on confidence in dispensing biologics. J Manag Care Spec Pharm. 2016;22(8):919-926.
33. US Food and Drug Administration. Nonproprietary naming of biological products: guidance for industry. https://www.fda.gov/downloads/drugs/guidances/ucm459987.pdf. Released January 2017. Accessed November 7, 2018.
34. Lyman GH. Emerging opportunities and challenges of biosimilars in oncology practice. J Clin Oncol Pract. 2017;13(9_suppl):7s-9s.
35. Nabhan C, Parsad S, Mato AR, Feinberg BA. Biosimilars in oncology in the United States: a review. JAMA Oncol. 2018;4(2):241-247.
The development of biologic therapies has led to some of the most significant advances in the treatment of cancer, but these drugs are also very expensive. As patents for the biologics begin to expire, the development of biosimilars has the potential to dramatically cut therapy costs thereby making the therapies more readily accessible to patients. Here, we discuss biosimilar development and the challenges that need to be overcome to create a robust market.
Biosimilar, not generic
Biologic therapies are derived from living organisms and include the targeted monoclonal antibodies (mAbs) and cell-based therapies that have revolutionized the treatment of certain cancer types. Yet, their greater complexity makes them more difficult to manufacture, store, and administer, making them a costly therapeutic option that ultimately drives up health care costs. According to a 2011 drug expenditure analysis, biologic therapies accounted for more than half of the total expenditure on anticancer drugs in the US health care system.1,2
Generally, when drug patents expire, other companies can develop their own identical generic versions to increase competition in the marketplace and drive down costs. However, the paradigm for generic development cannot be applied to biologic therapies because the way in which they are manufactured makes it impossible to generate an identical copy.
Instead, the Biologics Price Competition and Innovation Act, a provision of the Patient Protection and Affordable Care Act, has allowed for submission of an application for “licensure of a biologic product based on its similarity to a licensed biologic product”.3
These “biosimilars” have been positioned as game-changers in oncology, with the potential to reduce costs and improve access to biologic therapies. With the patents on several blockbuster cancer biologics already expired or due to expire by 2020, an increasing number of biosimilars are being developed.4
Totality of evidence
Biosimilars require more rigorous testing than generics, but they don’t require the same type of scientific data that the original biologic products, termed “reference products,” did. Therefore, they are governed by legislation unique to them and approved by different regulatory pathways. The US Food and Drug Administration (FDA) has established a unique shortened regulatory pathway for their approval, known as the 351(k) pathway. So whereas the pathway for reference products is geared toward demonstrating patient benefit, biosimilars are required instead to show equivalence to the reference product.5
Biosimilars are produced through reverse engineering the reference product. Then, through a stepwise process, to generate what the FDA calls a “totality of evidence,” biosimilar manufacturers must demonstrate structural and functional similarities (through comparative quality studies) and comparable pharmacokinetics and pharmacodynamics (through comparative nonclinical and clinical studies) to the reference product. Final approval is based on 1 or more comparative clinical studies performed in the most sensitive patient population(s) (Figure 1).6
The primary endpoint of biosimilar clinical trials is chosen to detect clinically relevant differences and may not be the same as that used in pivotal trials of the reference product. Endpoints such as progression-free survival (PFS) and overall survival (OS) may not be feasible or sensitive enough to demonstrate biosimilarity.
Clinical trials of biosimilars should also be carried out in the most sensitive patient population, so that any potential differences can be attributed to the drug and not the patient population itself. If the reference product is approved across several different indications and there is sufficient scientific evidence to allow it, including the demonstration that the mechanism of action of the drug is the same across all indications, the FDA can extend the approval of the biosimilar to all of these indications without the need for individual clinical trials through a process known as extrapolation.
Biosimilar manufacturers must also provide evidence of the composition of their formulation and of quality control in their manufacturing processes, to ensure that biosimilarity can be maintained from batch to batch. As with the reference product, even small changes in the manufacturing process can have serious ramifications for clinical efficacy and safety.7,8
A flurry of approvals
The first biosimilar approvals in oncology in the United States came in the supportive care niche (Table 1). Filgrastim-sndz (Zarxio), approved in March 2015, is a biosimilar of the granulocyte-macrophage colony stimulating factor (G-CSF) analog filgrastim (Neupogen). Owing to its mechanism of action in stimulating the production of neutrophils in the bone marrow, filgrastim is used to help reduce the risk or severity of neutropenia in patients undergoing myelosuppressive chemotherapy regimens.
Filgrastim-sndz was approved for use across all 5 indications for which the reference product is approved, based on the totality of evidence, which included results from the key phase 3 PIONEER study.9 Market entry was initially delayed by lawsuits filed by Amgen, the maker of the reference product, but the biosimilar was subsequently cleared by the US Court of Appeals for the Federal Circuit. The wholesale acquisition cost (WAC) for a 300µg syringe is $324.30 for filgrastim and $275.66 figrastim-sndz, representing a 15% reduction on the reference product.10
In 2018, the FDA approved a second filgrastim biosimilar, filgrastim-aafi (Nivestym),11 in addition to 2 biosimilars of the pegylated form of filgrastim, pegfilgrastim-jmdb (Fulphila)12 and pegfilgrastim-cbqv (Udenyca)13 – these forms of filgrastim have been modified by the addition of polyethylene glycol polymer chains that help to increase circulation time.
Approval for the 2 pegfilgrastm biosimilars was originally delayed by complete response letters (CRLs) from the FDA. For pegfilgrastim-jmdb, the CRL was reported to be related to a pending update of the Biologic’s License Application (BLA) to include information regarding facility requalification activities that had been taken after the addition of plant modifications. The CRL for pegfilgrastim-cbqv requested that the company provide additional manufacturing information and reanalyze a subset of samples with a revised immunogenicity assay.
Once the CRL concerns were addressed, regulatory approval was awarded and Mylan recently confirmed that pegfilgrastim-jmdb has been launched in the US marketplace at a WAC that reflects a 33% discount over the reference product.14
Approval data for filgrastim-aafi and pegfilgrastim-cbqv have not yet been published, however the respective manufacturers reported that approval was based on totality of evidence demonstrating a high degree of similarity to the reference products. Filgrastim-aafi was approved for all of the indications of the reference product and launched in the US on October 1, 2018 at a 30% discounted WAC.15
Epoetin alfa-epbx (Retacrit), a biosimilar of epoetin alfa, was also approved in 2018. It is a recombinant analog of erythropoietin (EPO), which stimulates the production of blood cells and has proved useful for the treatment of anemia, including in cancer patients receiving myelosuppressive chemotherapy. Approval of the biosimilar followed earlier receipt of a CRL from the FDA citing concerns relating to the manufacturing facility, which the company addressed. Pfizer has said that it expects to launch the biosimilar this year (2018), but a WAC has not been disclosed.16The FDA also recently approved the first biosimilars for the treatment of cancer. Trastuzumab-dkst (Ogivri) and bevacizumab-awwb (Mvasi) were approved in the second half of 2017 for the same indications as their respective reference products, which are mAbs directed at the human epidermal growth factor receptor 2 (HER2) and vascular endothelial growth factor, respectively.17,18
Approval data for bevacizumab-awwb included a comparative clinical trial in patients with advanced/metastatic non–small-cell lung cancer (NSCLC), which was considered the most sensitive patient population. The BLA for trastuzumab-dkst included data from the phase 3 comparative HERiTAge clinical trial, in which the biosimilar was compared with the reference product, both in combination with docetaxel or paclitaxel, in patients with previously untreated HER2-positive metastatic breast cancer. Neither biosimilar has been launched on the US market yet because the patents for their reference products do not expire until 2019, so it is not clear what the price discount will be for these drugs (Table 2).9,19-22
Biosimilars in development
While numerous other biosimilars of filgrastim and pegfilgrastim are in development, the major focus has been on the development of more biosimilars to treat cancer (Table 3). BLAs have been submitted for 4 biosimilars of trastuzumab and 1 bevacizumab biosimilar. Approval for several of the trastuzumab biosimilars has been delayed by CRLs from the FDA, mostly regarding issues with the manufacturing process or facility. Several other trastuzumab and bevacizumab biosimilars are in late-stage clinical trials.
The results of several phase 3 comparative clinical trials were recently published or reported at annual conferences. Pfizer’s PF-05280014 was compared with the European Union (EU)–approved trastuzumab, both in combination with paclitaxel, in patients with previously untreated HER2-positive metastatic breast cancer. Data reported at the European Society for Medical Oncology congress in 2017 demonstrated equivalence between the reference product and biosimilar in overall response rate (ORR).23
Another recently published trial compared this biosimilar to EU-trastuzumab, both in combination with carboplatin and docetaxel, as neoadjuvant treatment for patients with resectable HER2-positive breast cancer. Among 226 patients randomized to receive 8 mg/kg in cycle 1 and 6 mg/kg thereafter of the biosimilar or reference product, every 3 weeks for 6 cycles, the pathologic complete response (pCR) rates were 47% and 50%, respectively.24
The results of a phase 3 study comparing Samsung Bioepis/Merck’s joint offering SB3 were recently published. A total of 875 patients were randomized 1:1 to receive SB3 or reference trastuzumab in combination with chemotherapy (4 cycles docetaxel followed by 4 cycles 5-fluorouracil/epirubicin/cyclophosphamide) prior to surgery, followed by 10 cycles of adjuvant SB3 or trastuzumab reference. Rates of event-free survival (EFS) were comparable between the 2 groups at 12 months (93.7% vs 96.1%, respectively).25
Amgen’s ABP980 was evaluated in the phase 3 LILAC trial, which measured the effect of the biosimilar on pCR in women with HER2-positive early breast cancer compared with reference trastuzumab. After 4 cycles of run-in anthracycline-based chemotherapy, ABP980 or reference trastuzumab were administered in combination with paclitaxel. This was followed by surgery and then ABP980 or reference trastuzumab in the adjuvant setting for up to 1 year, with the option to continue on the same drug as the neoadjuvant setting or to switch to the other. Among 696 assessable patients, the pCR rates were 48% and 42%, respectively.26
Most advanced in clinical testing among the upcoming bevacizumab biosimilars is Pfizer’s PF-06439535, for which the results of a phase 3 comparative trial were presented at the 2018 annual meeting of the American Society for Clinical Oncology. PF-06439535 was compared with the EU-approved bevacizumab, both in combination with paclitaxel and carboplatin, as first-line therapy for patients with advanced non-squamous NSCLC. Among 719 patients, the primary endpoint of ORR was 45.3% and 44.6%, respectively.27
Biosimilars of a third blockbuster cancer drug, the CD20-targeting mAb rituximab (Rituxan) are also in development and FDA approval is pending for 2. The patent for Rituxan expired in 2016, so these drugs could hit the market as soon as they are approved.
In a race to the finish for the first US-approved rituximab biosimilar, Celltrion-Teva’s CT-P10 (Truxima) seems most likely to come first; the Oncologic Drugs Advisory Committee voted unanimously in October 2018 to recommend its approval. Phase 3 comparative data were recently published; patients with newly diagnosed advanced-stage follicular lymphoma were randomized to receive intravenous infusions of 375 mg/m2 CT-P10 or reference rituximab, both in combination with cyclophosphamide, vincristine, and prednisone, on day 1 of 8 21-day cycles. The ORRs were identical (92.6%) for both drugs, pharmacokinetics data also suggested bioequivalence, and the incidence of AEs was also comparable (83% vs 80%).28
Biosimilars of the epidermal growth factor receptor (EGFR)-targeting mAb cetuximab are also listed in the pipeline for several biosimilar developers, but there is no indication of their developmental status as yet and no clinical trials are ongoing in the US.
Sorrento is developing STI-001, a cetuximab biosimilar, and reported that a phase 3 trial had been completed. Instead of a comparison with the reference product, however, the trial compared STI-001 in combination with irinotecan with irinotecan alone. They reported significantly higher ORR, PFS, and OS with the biosimilar compared with irinotecan alone, and a significant increase over historical data with the reference product, as well as fewer side effects and immunogenicity, which they attribute to its manufacture in a different cell line. However, no data has been published and no trials are ongoing in the United States, so the status of its development remains unclear.29
Challenges to a robust market
It is an exciting time for biosimilars, with many approvals and drugs being brought to market in the US in the past several years and more poised to follow suit as patents expire. Yet many challenges remain around the growth of a robust biosimilars market.
Several surveys conducted in recent years have demonstrated suboptimal knowledge of all aspects of biosimilars and highlighted the need for evidence-based education across specialties.30,31 In response, the FDA recently announced that it was launching an educational campaign to further understanding of biosimilars, including naming conventions (Figure 2).32,33 Numerous other medical professional societies have produced or are in the process of producing biosimilar guidelines.
Educational outreach by the FDA forms part of their 4-step plan to aid biosimilar development, which also aims to improve the efficiency of biosimilar development and approval, to provide regulatory clarity for manufacturers, to facilitate public understanding and acceptance, and to support a competitive marketplace.
Among the most critical educational gaps is confusion over the issue of interchangeability. Once approved by the FDA, generic drugs are considered interchangeable with the brand name drug and can be substituted at the pharmacy level without referring to the prescribing physician. This is not the case for biosimilars; owing to their more complex nature, biosimilars require a separate designation for interchangeability and none of those approved so far have been given this designation by the FDA.
There has been some confusion about what will be required to demonstrate interchangeability, and the FDA recently produced draft guidance, saying that essentially it should be proven that switching out the reference product for a biosimilar does not increase risk in terms of diminished efficacy or safety. Several companies are beginning to incorporate a switching component into their clinical trials of biosimilars.
Continued postmarketing and real-world studies will also be particularly important for biosimilars to increase confidence in prescribing them by demonstrating their continued efficacy and safety in the long-term. Several real-world studies are now ongoing, including the MONITOR-GCSF trial of filgrastim biosimilars.
Another major barrier to the development of a thriving biosimilars market that achieves the goals of reduced costs and increased access is the financial burden of their development. They are vastly more costly to develop and produce than generics. Added to litigation costs, this can limit their ability to compete in terms of price, which has been reflected in the lower-than-anticipated cost savings with some approved biosimilars thus far.
Experts have suggested that there might be much to learn from the European market, where biosimilars have been available for more than a decade and over time have reached even higher-than-expected savings. With high financial stakes and an increasingly important role in the treatment of cancer, the need to iron out the kinks is more pressing than ever.7,8,34,35
The development of biologic therapies has led to some of the most significant advances in the treatment of cancer, but these drugs are also very expensive. As patents for the biologics begin to expire, the development of biosimilars has the potential to dramatically cut therapy costs thereby making the therapies more readily accessible to patients. Here, we discuss biosimilar development and the challenges that need to be overcome to create a robust market.
Biosimilar, not generic
Biologic therapies are derived from living organisms and include the targeted monoclonal antibodies (mAbs) and cell-based therapies that have revolutionized the treatment of certain cancer types. Yet, their greater complexity makes them more difficult to manufacture, store, and administer, making them a costly therapeutic option that ultimately drives up health care costs. According to a 2011 drug expenditure analysis, biologic therapies accounted for more than half of the total expenditure on anticancer drugs in the US health care system.1,2
Generally, when drug patents expire, other companies can develop their own identical generic versions to increase competition in the marketplace and drive down costs. However, the paradigm for generic development cannot be applied to biologic therapies because the way in which they are manufactured makes it impossible to generate an identical copy.
Instead, the Biologics Price Competition and Innovation Act, a provision of the Patient Protection and Affordable Care Act, has allowed for submission of an application for “licensure of a biologic product based on its similarity to a licensed biologic product”.3
These “biosimilars” have been positioned as game-changers in oncology, with the potential to reduce costs and improve access to biologic therapies. With the patents on several blockbuster cancer biologics already expired or due to expire by 2020, an increasing number of biosimilars are being developed.4
Totality of evidence
Biosimilars require more rigorous testing than generics, but they don’t require the same type of scientific data that the original biologic products, termed “reference products,” did. Therefore, they are governed by legislation unique to them and approved by different regulatory pathways. The US Food and Drug Administration (FDA) has established a unique shortened regulatory pathway for their approval, known as the 351(k) pathway. So whereas the pathway for reference products is geared toward demonstrating patient benefit, biosimilars are required instead to show equivalence to the reference product.5
Biosimilars are produced through reverse engineering the reference product. Then, through a stepwise process, to generate what the FDA calls a “totality of evidence,” biosimilar manufacturers must demonstrate structural and functional similarities (through comparative quality studies) and comparable pharmacokinetics and pharmacodynamics (through comparative nonclinical and clinical studies) to the reference product. Final approval is based on 1 or more comparative clinical studies performed in the most sensitive patient population(s) (Figure 1).6
The primary endpoint of biosimilar clinical trials is chosen to detect clinically relevant differences and may not be the same as that used in pivotal trials of the reference product. Endpoints such as progression-free survival (PFS) and overall survival (OS) may not be feasible or sensitive enough to demonstrate biosimilarity.
Clinical trials of biosimilars should also be carried out in the most sensitive patient population, so that any potential differences can be attributed to the drug and not the patient population itself. If the reference product is approved across several different indications and there is sufficient scientific evidence to allow it, including the demonstration that the mechanism of action of the drug is the same across all indications, the FDA can extend the approval of the biosimilar to all of these indications without the need for individual clinical trials through a process known as extrapolation.
Biosimilar manufacturers must also provide evidence of the composition of their formulation and of quality control in their manufacturing processes, to ensure that biosimilarity can be maintained from batch to batch. As with the reference product, even small changes in the manufacturing process can have serious ramifications for clinical efficacy and safety.7,8
A flurry of approvals
The first biosimilar approvals in oncology in the United States came in the supportive care niche (Table 1). Filgrastim-sndz (Zarxio), approved in March 2015, is a biosimilar of the granulocyte-macrophage colony stimulating factor (G-CSF) analog filgrastim (Neupogen). Owing to its mechanism of action in stimulating the production of neutrophils in the bone marrow, filgrastim is used to help reduce the risk or severity of neutropenia in patients undergoing myelosuppressive chemotherapy regimens.
Filgrastim-sndz was approved for use across all 5 indications for which the reference product is approved, based on the totality of evidence, which included results from the key phase 3 PIONEER study.9 Market entry was initially delayed by lawsuits filed by Amgen, the maker of the reference product, but the biosimilar was subsequently cleared by the US Court of Appeals for the Federal Circuit. The wholesale acquisition cost (WAC) for a 300µg syringe is $324.30 for filgrastim and $275.66 figrastim-sndz, representing a 15% reduction on the reference product.10
In 2018, the FDA approved a second filgrastim biosimilar, filgrastim-aafi (Nivestym),11 in addition to 2 biosimilars of the pegylated form of filgrastim, pegfilgrastim-jmdb (Fulphila)12 and pegfilgrastim-cbqv (Udenyca)13 – these forms of filgrastim have been modified by the addition of polyethylene glycol polymer chains that help to increase circulation time.
Approval for the 2 pegfilgrastm biosimilars was originally delayed by complete response letters (CRLs) from the FDA. For pegfilgrastim-jmdb, the CRL was reported to be related to a pending update of the Biologic’s License Application (BLA) to include information regarding facility requalification activities that had been taken after the addition of plant modifications. The CRL for pegfilgrastim-cbqv requested that the company provide additional manufacturing information and reanalyze a subset of samples with a revised immunogenicity assay.
Once the CRL concerns were addressed, regulatory approval was awarded and Mylan recently confirmed that pegfilgrastim-jmdb has been launched in the US marketplace at a WAC that reflects a 33% discount over the reference product.14
Approval data for filgrastim-aafi and pegfilgrastim-cbqv have not yet been published, however the respective manufacturers reported that approval was based on totality of evidence demonstrating a high degree of similarity to the reference products. Filgrastim-aafi was approved for all of the indications of the reference product and launched in the US on October 1, 2018 at a 30% discounted WAC.15
Epoetin alfa-epbx (Retacrit), a biosimilar of epoetin alfa, was also approved in 2018. It is a recombinant analog of erythropoietin (EPO), which stimulates the production of blood cells and has proved useful for the treatment of anemia, including in cancer patients receiving myelosuppressive chemotherapy. Approval of the biosimilar followed earlier receipt of a CRL from the FDA citing concerns relating to the manufacturing facility, which the company addressed. Pfizer has said that it expects to launch the biosimilar this year (2018), but a WAC has not been disclosed.16The FDA also recently approved the first biosimilars for the treatment of cancer. Trastuzumab-dkst (Ogivri) and bevacizumab-awwb (Mvasi) were approved in the second half of 2017 for the same indications as their respective reference products, which are mAbs directed at the human epidermal growth factor receptor 2 (HER2) and vascular endothelial growth factor, respectively.17,18
Approval data for bevacizumab-awwb included a comparative clinical trial in patients with advanced/metastatic non–small-cell lung cancer (NSCLC), which was considered the most sensitive patient population. The BLA for trastuzumab-dkst included data from the phase 3 comparative HERiTAge clinical trial, in which the biosimilar was compared with the reference product, both in combination with docetaxel or paclitaxel, in patients with previously untreated HER2-positive metastatic breast cancer. Neither biosimilar has been launched on the US market yet because the patents for their reference products do not expire until 2019, so it is not clear what the price discount will be for these drugs (Table 2).9,19-22
Biosimilars in development
While numerous other biosimilars of filgrastim and pegfilgrastim are in development, the major focus has been on the development of more biosimilars to treat cancer (Table 3). BLAs have been submitted for 4 biosimilars of trastuzumab and 1 bevacizumab biosimilar. Approval for several of the trastuzumab biosimilars has been delayed by CRLs from the FDA, mostly regarding issues with the manufacturing process or facility. Several other trastuzumab and bevacizumab biosimilars are in late-stage clinical trials.
The results of several phase 3 comparative clinical trials were recently published or reported at annual conferences. Pfizer’s PF-05280014 was compared with the European Union (EU)–approved trastuzumab, both in combination with paclitaxel, in patients with previously untreated HER2-positive metastatic breast cancer. Data reported at the European Society for Medical Oncology congress in 2017 demonstrated equivalence between the reference product and biosimilar in overall response rate (ORR).23
Another recently published trial compared this biosimilar to EU-trastuzumab, both in combination with carboplatin and docetaxel, as neoadjuvant treatment for patients with resectable HER2-positive breast cancer. Among 226 patients randomized to receive 8 mg/kg in cycle 1 and 6 mg/kg thereafter of the biosimilar or reference product, every 3 weeks for 6 cycles, the pathologic complete response (pCR) rates were 47% and 50%, respectively.24
The results of a phase 3 study comparing Samsung Bioepis/Merck’s joint offering SB3 were recently published. A total of 875 patients were randomized 1:1 to receive SB3 or reference trastuzumab in combination with chemotherapy (4 cycles docetaxel followed by 4 cycles 5-fluorouracil/epirubicin/cyclophosphamide) prior to surgery, followed by 10 cycles of adjuvant SB3 or trastuzumab reference. Rates of event-free survival (EFS) were comparable between the 2 groups at 12 months (93.7% vs 96.1%, respectively).25
Amgen’s ABP980 was evaluated in the phase 3 LILAC trial, which measured the effect of the biosimilar on pCR in women with HER2-positive early breast cancer compared with reference trastuzumab. After 4 cycles of run-in anthracycline-based chemotherapy, ABP980 or reference trastuzumab were administered in combination with paclitaxel. This was followed by surgery and then ABP980 or reference trastuzumab in the adjuvant setting for up to 1 year, with the option to continue on the same drug as the neoadjuvant setting or to switch to the other. Among 696 assessable patients, the pCR rates were 48% and 42%, respectively.26
Most advanced in clinical testing among the upcoming bevacizumab biosimilars is Pfizer’s PF-06439535, for which the results of a phase 3 comparative trial were presented at the 2018 annual meeting of the American Society for Clinical Oncology. PF-06439535 was compared with the EU-approved bevacizumab, both in combination with paclitaxel and carboplatin, as first-line therapy for patients with advanced non-squamous NSCLC. Among 719 patients, the primary endpoint of ORR was 45.3% and 44.6%, respectively.27
Biosimilars of a third blockbuster cancer drug, the CD20-targeting mAb rituximab (Rituxan) are also in development and FDA approval is pending for 2. The patent for Rituxan expired in 2016, so these drugs could hit the market as soon as they are approved.
In a race to the finish for the first US-approved rituximab biosimilar, Celltrion-Teva’s CT-P10 (Truxima) seems most likely to come first; the Oncologic Drugs Advisory Committee voted unanimously in October 2018 to recommend its approval. Phase 3 comparative data were recently published; patients with newly diagnosed advanced-stage follicular lymphoma were randomized to receive intravenous infusions of 375 mg/m2 CT-P10 or reference rituximab, both in combination with cyclophosphamide, vincristine, and prednisone, on day 1 of 8 21-day cycles. The ORRs were identical (92.6%) for both drugs, pharmacokinetics data also suggested bioequivalence, and the incidence of AEs was also comparable (83% vs 80%).28
Biosimilars of the epidermal growth factor receptor (EGFR)-targeting mAb cetuximab are also listed in the pipeline for several biosimilar developers, but there is no indication of their developmental status as yet and no clinical trials are ongoing in the US.
Sorrento is developing STI-001, a cetuximab biosimilar, and reported that a phase 3 trial had been completed. Instead of a comparison with the reference product, however, the trial compared STI-001 in combination with irinotecan with irinotecan alone. They reported significantly higher ORR, PFS, and OS with the biosimilar compared with irinotecan alone, and a significant increase over historical data with the reference product, as well as fewer side effects and immunogenicity, which they attribute to its manufacture in a different cell line. However, no data has been published and no trials are ongoing in the United States, so the status of its development remains unclear.29
Challenges to a robust market
It is an exciting time for biosimilars, with many approvals and drugs being brought to market in the US in the past several years and more poised to follow suit as patents expire. Yet many challenges remain around the growth of a robust biosimilars market.
Several surveys conducted in recent years have demonstrated suboptimal knowledge of all aspects of biosimilars and highlighted the need for evidence-based education across specialties.30,31 In response, the FDA recently announced that it was launching an educational campaign to further understanding of biosimilars, including naming conventions (Figure 2).32,33 Numerous other medical professional societies have produced or are in the process of producing biosimilar guidelines.
Educational outreach by the FDA forms part of their 4-step plan to aid biosimilar development, which also aims to improve the efficiency of biosimilar development and approval, to provide regulatory clarity for manufacturers, to facilitate public understanding and acceptance, and to support a competitive marketplace.
Among the most critical educational gaps is confusion over the issue of interchangeability. Once approved by the FDA, generic drugs are considered interchangeable with the brand name drug and can be substituted at the pharmacy level without referring to the prescribing physician. This is not the case for biosimilars; owing to their more complex nature, biosimilars require a separate designation for interchangeability and none of those approved so far have been given this designation by the FDA.
There has been some confusion about what will be required to demonstrate interchangeability, and the FDA recently produced draft guidance, saying that essentially it should be proven that switching out the reference product for a biosimilar does not increase risk in terms of diminished efficacy or safety. Several companies are beginning to incorporate a switching component into their clinical trials of biosimilars.
Continued postmarketing and real-world studies will also be particularly important for biosimilars to increase confidence in prescribing them by demonstrating their continued efficacy and safety in the long-term. Several real-world studies are now ongoing, including the MONITOR-GCSF trial of filgrastim biosimilars.
Another major barrier to the development of a thriving biosimilars market that achieves the goals of reduced costs and increased access is the financial burden of their development. They are vastly more costly to develop and produce than generics. Added to litigation costs, this can limit their ability to compete in terms of price, which has been reflected in the lower-than-anticipated cost savings with some approved biosimilars thus far.
Experts have suggested that there might be much to learn from the European market, where biosimilars have been available for more than a decade and over time have reached even higher-than-expected savings. With high financial stakes and an increasingly important role in the treatment of cancer, the need to iron out the kinks is more pressing than ever.7,8,34,35
. Abraham J. Developing oncology biosimilars: an essential approach for the future. Semin Oncol. 2013;40 Suppl 1:S5-24.
2. Doloresco F, Fominaya C, Schumock GT, et al. Projecting future drug expenditures: 2011. Am J Health Syst Pharm. 2011;68(10):921-932.
3. Prepared by the Office of the Legislative Counsel. HHS website. Compilation of the Patient Protection and Affordable Care Act [as amended through May 1, 2010] including Patient Protection and Affordable Care Act health-related portions of the Health Care and Education Reconciliation Act of 2010. https://www.hhs.gov/sites/default/files/ppacacon.pdf. Released June 9, 2010. Accessed November 7, 2018.
4. Mulcahy AW, Hlavka JP, Case SR. Biosimilar cost savings in the United States: initial experience and future potential. Rand Health Q. 2018;7(4):3-3.
5. Hung A, Vu Q, Mostovoy L. A systematic review of US biosimilar approvals: what evidence does the FDA require and how are manufacturers responding? J Manag Care Spec Pharm. 2017;23(12):1234-1244.
6. Uifălean A, Ilieş M, Nicoară R, Rus LM, Hegheş SC, Iuga C-A. Concepts and challenges of biosimilars in breast cancer: the emergence of trastuzumab biosimilars. Pharmaceutics. 2018;10(4):E168.
7. Rugo HS, Linton KM, Cervi P, Rosenberg JA, Jacobs I. A clinician's guide to biosimilars in oncology. Cancer Treat Rev. 2016;46:73-79.
8. Chopra R, Lopes G. Improving access to cancer treatments: the role of biosimilars. J Glob Oncol. 2017;3(5):596-610.
9. Blackwell K, Semiglazov V, Krasnozhon D, et al. Comparison of EP2006, a filgrastim biosimilar, to the reference: a phase III, randomized, double-blind clinical study in the prevention of severe neutropenia in patients with breast cancer receiving myelosuppressive chemotherapy. Ann Oncol. 2015;26(9):1948-1953.
10. FDA News. Sandoz launches Zarxio at 15 percent lower price than Neupogen. https://www.fdanews.com/articles/173036-sandoz-launches-zarxio-at-15-percent-lower-price-than-neupogen. Released September 11, 2015. Accessed November 7, 2018.
11. Pfizer. US FDA approves Pfizer's biosimilar Nivestym (filgrastim-aafi). https://www.pfizer.com/news/press-release/press-release-detail/u_s_fda_approves_pfizer_s_biosimilar_nivestym_filgrastim_aafi-0. Released July 2o, 2018. Accessed November 7, 2018.
12. United States Food and Drug Administration. FDA approves first biosimilar to Neulasta to help reduce the risk of infection during cancer treatment. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm609805.htm. Released on June 4, 2018. Accessed November 7, 2018.
13. Coherus Biosciences. US FDA approves Udenyca (pegfilgrastim-cbqv). http://investors.coherus.com/news-releases/news-release-details/us-fda-approves-udenycatm-pegfilgrastim-cbqv. Released November 2, 2018. Accessed November 7, 2018.
14. The Center for Biosimilars. Mylan confirms that it has launched Fulphila in the United States. https://www.centerforbiosimilars.com/news/mylan-confirms-that-it-has-launched-fulphila-in-the-united-states. Released July 30, 2018. Accessed November 7, 2018.
15. The Center for Biosimilars. Pfizer launches biosimilar filgrastim, Nivestym, at a substantial discount. https://www.centerforbiosimilars.com/news/pfizer-launches-biosimilar-filgrastim-nivestym-at-a-substantial-discount. Released October 3, 2018. Accessed November 7, 2018.
16. The Center for Biosimilars. FDA approves Pfizer's epoetin alfa biosimilar, Retacrit. https://www.centerforbiosimilars.com/news/fda-approves-pfizers-epoetin-alfa-biosimilar-retacrit. Released May 15, 2018. Accessed November 7, 2018.
17. United States Food and Drug Administration. FDA approves Ogivri as a biosimilar to Herceptin. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm587404.htm. Last updated December 1, 2017. Accessed November 7, 2018.
18. United States Food and Drug Administration. FDA approves first biosimilar for the treatment of cancer. 2017; https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm576112.htm. Last updated March 26, 2018. Accessed November 7, 2018.
19. Waller CF, Blakeley C, Pennella E, et al. Phase 3 efficacy and safety trial of proposed pegfilgrastim biosimilar MYL-1401H vs EU-neulasta in the prophylaxis of chemotherapy-induced neutropenia. Ann Oncol. 2016;27(suppl_6):14330.
20. US Food and Drug Administration. 'Epoetin Hospira,' a proposed biosimilar to US-licensed Epogen/Procrit. 2017. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/OncologicDrugsAdvisoryCommittee/UCM559962.pdf. Accessed November 7, 2018.
21. Manikhas A, Pennella EJ, Bondarenko I, et al. Biosimilar trastuzumab-dkst monotherapy versus trastuzumab monotherapy after combination therapy: toxicity, efficacy, and immunogenicity from the phase 3 Heritage trial. J Clin Oncol. 2018;36(15_suppl):110.
22. Thatcher N, Thomas M, Paz-Ares L, et al. Randomized, double-blind, phase 3 study evaluating efficacy and safety of ABP 215 compared with bevacizumab in patients with non-squamous NSCLC. J Clin Oncol. 2016;34(15_suppl):9095.
23. Pegram M, Tan-Chiu E, Freyman A, et al. A randomized, double-blind study of PF-05280014 (a potential trastuzumab biosimilar) vs trastuzumab, both in combination with paclitaxel, as first-line therapy. Ann Oncol. 2017;28(suppl_5):v74-v108.
24. Lammers PE, Dank M, Masetti R, et al. Neoadjuvant PF-05280014 (a potential trastuzumab biosimilar) versus trastuzumab for operable HER2+ breast cancer. Br J Cancer. 2018;119(3):266-273.
25. Pivot X, Bondarenko I, Nowecki Z, et al. A phase III study comparing SB3 (a proposed trastuzumab biosimilar) and trastuzumab reference product in HER2-positive early breast cancer treated with neoadjuvant-adjuvant treatment: final safety, immunogenicity and survival results. Eur J Cancer. 2018;93:19-27.
26. von Minckwitz G, Colleoni M, Kolberg HC, et al. Efficacy and safety of ABP 980 compared with reference trastuzumab in women with HER2-positive early breast cancer (LILAC study): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2018;19(7):987-998.
27. Socinski MA, Pawel JV, Kasahara K, et al. A comparative clinical study of PF-06439535, a candidate bevacizumab biosimilar, and reference bevacizumab, in patients with advanced non-squamous non-small cell lung cancer. J Clin Oncol. 2018;36(15_suppl):109-109.
28. Kim WS, Buske C, Ogura M, et al. Efficacy, pharmacokinetics, and safety of the biosimilar CT-P10 compared with rituximab in patients with previously untreated advanced-stage follicular lymphoma: a randomised, double-blind, parallel-group, non-inferiority phase 3 trial. Lancet Haematol. 2017;4(8):e362-e373.
29. PRNewsire. Sorrento announces positive data from phase 3 studies of biosimilar antibodies, STI-001 and STI-002. https://www.prnewswire.com/news-releases/sorrento-announces-positive-data-from-phase-3-studies-of-biosimilar-antibodies-sti-001-and-sti-002-300202054.html. Released January 11, 2016. Accessed November 7, 2018.
30. Molinari AL, Gewanter HL, Loaiza-Bonilla A, Reilly M, Kennedy B, Charles D. Global survey of physicians' attitudes toward biologic and biosimilar therapies. J Clin Oncol. 2016;34(15_suppl):e18025-e18025.
31. Cohen H, Beydoun D, Chien D, et al. Awareness, knowledge, and perceptions of biosimilars among specialty physicians. Adv Ther. 2017;33(12):2160-2172.
32. Tomaszewski D. Biosimilar naming conventions: pharmacist perceptions and impact on confidence in dispensing biologics. J Manag Care Spec Pharm. 2016;22(8):919-926.
33. US Food and Drug Administration. Nonproprietary naming of biological products: guidance for industry. https://www.fda.gov/downloads/drugs/guidances/ucm459987.pdf. Released January 2017. Accessed November 7, 2018.
34. Lyman GH. Emerging opportunities and challenges of biosimilars in oncology practice. J Clin Oncol Pract. 2017;13(9_suppl):7s-9s.
35. Nabhan C, Parsad S, Mato AR, Feinberg BA. Biosimilars in oncology in the United States: a review. JAMA Oncol. 2018;4(2):241-247.
. Abraham J. Developing oncology biosimilars: an essential approach for the future. Semin Oncol. 2013;40 Suppl 1:S5-24.
2. Doloresco F, Fominaya C, Schumock GT, et al. Projecting future drug expenditures: 2011. Am J Health Syst Pharm. 2011;68(10):921-932.
3. Prepared by the Office of the Legislative Counsel. HHS website. Compilation of the Patient Protection and Affordable Care Act [as amended through May 1, 2010] including Patient Protection and Affordable Care Act health-related portions of the Health Care and Education Reconciliation Act of 2010. https://www.hhs.gov/sites/default/files/ppacacon.pdf. Released June 9, 2010. Accessed November 7, 2018.
4. Mulcahy AW, Hlavka JP, Case SR. Biosimilar cost savings in the United States: initial experience and future potential. Rand Health Q. 2018;7(4):3-3.
5. Hung A, Vu Q, Mostovoy L. A systematic review of US biosimilar approvals: what evidence does the FDA require and how are manufacturers responding? J Manag Care Spec Pharm. 2017;23(12):1234-1244.
6. Uifălean A, Ilieş M, Nicoară R, Rus LM, Hegheş SC, Iuga C-A. Concepts and challenges of biosimilars in breast cancer: the emergence of trastuzumab biosimilars. Pharmaceutics. 2018;10(4):E168.
7. Rugo HS, Linton KM, Cervi P, Rosenberg JA, Jacobs I. A clinician's guide to biosimilars in oncology. Cancer Treat Rev. 2016;46:73-79.
8. Chopra R, Lopes G. Improving access to cancer treatments: the role of biosimilars. J Glob Oncol. 2017;3(5):596-610.
9. Blackwell K, Semiglazov V, Krasnozhon D, et al. Comparison of EP2006, a filgrastim biosimilar, to the reference: a phase III, randomized, double-blind clinical study in the prevention of severe neutropenia in patients with breast cancer receiving myelosuppressive chemotherapy. Ann Oncol. 2015;26(9):1948-1953.
10. FDA News. Sandoz launches Zarxio at 15 percent lower price than Neupogen. https://www.fdanews.com/articles/173036-sandoz-launches-zarxio-at-15-percent-lower-price-than-neupogen. Released September 11, 2015. Accessed November 7, 2018.
11. Pfizer. US FDA approves Pfizer's biosimilar Nivestym (filgrastim-aafi). https://www.pfizer.com/news/press-release/press-release-detail/u_s_fda_approves_pfizer_s_biosimilar_nivestym_filgrastim_aafi-0. Released July 2o, 2018. Accessed November 7, 2018.
12. United States Food and Drug Administration. FDA approves first biosimilar to Neulasta to help reduce the risk of infection during cancer treatment. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm609805.htm. Released on June 4, 2018. Accessed November 7, 2018.
13. Coherus Biosciences. US FDA approves Udenyca (pegfilgrastim-cbqv). http://investors.coherus.com/news-releases/news-release-details/us-fda-approves-udenycatm-pegfilgrastim-cbqv. Released November 2, 2018. Accessed November 7, 2018.
14. The Center for Biosimilars. Mylan confirms that it has launched Fulphila in the United States. https://www.centerforbiosimilars.com/news/mylan-confirms-that-it-has-launched-fulphila-in-the-united-states. Released July 30, 2018. Accessed November 7, 2018.
15. The Center for Biosimilars. Pfizer launches biosimilar filgrastim, Nivestym, at a substantial discount. https://www.centerforbiosimilars.com/news/pfizer-launches-biosimilar-filgrastim-nivestym-at-a-substantial-discount. Released October 3, 2018. Accessed November 7, 2018.
16. The Center for Biosimilars. FDA approves Pfizer's epoetin alfa biosimilar, Retacrit. https://www.centerforbiosimilars.com/news/fda-approves-pfizers-epoetin-alfa-biosimilar-retacrit. Released May 15, 2018. Accessed November 7, 2018.
17. United States Food and Drug Administration. FDA approves Ogivri as a biosimilar to Herceptin. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm587404.htm. Last updated December 1, 2017. Accessed November 7, 2018.
18. United States Food and Drug Administration. FDA approves first biosimilar for the treatment of cancer. 2017; https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm576112.htm. Last updated March 26, 2018. Accessed November 7, 2018.
19. Waller CF, Blakeley C, Pennella E, et al. Phase 3 efficacy and safety trial of proposed pegfilgrastim biosimilar MYL-1401H vs EU-neulasta in the prophylaxis of chemotherapy-induced neutropenia. Ann Oncol. 2016;27(suppl_6):14330.
20. US Food and Drug Administration. 'Epoetin Hospira,' a proposed biosimilar to US-licensed Epogen/Procrit. 2017. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/OncologicDrugsAdvisoryCommittee/UCM559962.pdf. Accessed November 7, 2018.
21. Manikhas A, Pennella EJ, Bondarenko I, et al. Biosimilar trastuzumab-dkst monotherapy versus trastuzumab monotherapy after combination therapy: toxicity, efficacy, and immunogenicity from the phase 3 Heritage trial. J Clin Oncol. 2018;36(15_suppl):110.
22. Thatcher N, Thomas M, Paz-Ares L, et al. Randomized, double-blind, phase 3 study evaluating efficacy and safety of ABP 215 compared with bevacizumab in patients with non-squamous NSCLC. J Clin Oncol. 2016;34(15_suppl):9095.
23. Pegram M, Tan-Chiu E, Freyman A, et al. A randomized, double-blind study of PF-05280014 (a potential trastuzumab biosimilar) vs trastuzumab, both in combination with paclitaxel, as first-line therapy. Ann Oncol. 2017;28(suppl_5):v74-v108.
24. Lammers PE, Dank M, Masetti R, et al. Neoadjuvant PF-05280014 (a potential trastuzumab biosimilar) versus trastuzumab for operable HER2+ breast cancer. Br J Cancer. 2018;119(3):266-273.
25. Pivot X, Bondarenko I, Nowecki Z, et al. A phase III study comparing SB3 (a proposed trastuzumab biosimilar) and trastuzumab reference product in HER2-positive early breast cancer treated with neoadjuvant-adjuvant treatment: final safety, immunogenicity and survival results. Eur J Cancer. 2018;93:19-27.
26. von Minckwitz G, Colleoni M, Kolberg HC, et al. Efficacy and safety of ABP 980 compared with reference trastuzumab in women with HER2-positive early breast cancer (LILAC study): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2018;19(7):987-998.
27. Socinski MA, Pawel JV, Kasahara K, et al. A comparative clinical study of PF-06439535, a candidate bevacizumab biosimilar, and reference bevacizumab, in patients with advanced non-squamous non-small cell lung cancer. J Clin Oncol. 2018;36(15_suppl):109-109.
28. Kim WS, Buske C, Ogura M, et al. Efficacy, pharmacokinetics, and safety of the biosimilar CT-P10 compared with rituximab in patients with previously untreated advanced-stage follicular lymphoma: a randomised, double-blind, parallel-group, non-inferiority phase 3 trial. Lancet Haematol. 2017;4(8):e362-e373.
29. PRNewsire. Sorrento announces positive data from phase 3 studies of biosimilar antibodies, STI-001 and STI-002. https://www.prnewswire.com/news-releases/sorrento-announces-positive-data-from-phase-3-studies-of-biosimilar-antibodies-sti-001-and-sti-002-300202054.html. Released January 11, 2016. Accessed November 7, 2018.
30. Molinari AL, Gewanter HL, Loaiza-Bonilla A, Reilly M, Kennedy B, Charles D. Global survey of physicians' attitudes toward biologic and biosimilar therapies. J Clin Oncol. 2016;34(15_suppl):e18025-e18025.
31. Cohen H, Beydoun D, Chien D, et al. Awareness, knowledge, and perceptions of biosimilars among specialty physicians. Adv Ther. 2017;33(12):2160-2172.
32. Tomaszewski D. Biosimilar naming conventions: pharmacist perceptions and impact on confidence in dispensing biologics. J Manag Care Spec Pharm. 2016;22(8):919-926.
33. US Food and Drug Administration. Nonproprietary naming of biological products: guidance for industry. https://www.fda.gov/downloads/drugs/guidances/ucm459987.pdf. Released January 2017. Accessed November 7, 2018.
34. Lyman GH. Emerging opportunities and challenges of biosimilars in oncology practice. J Clin Oncol Pract. 2017;13(9_suppl):7s-9s.
35. Nabhan C, Parsad S, Mato AR, Feinberg BA. Biosimilars in oncology in the United States: a review. JAMA Oncol. 2018;4(2):241-247.
Intravascular large B-cell lymphoma: an elusive diagnosis with challenging management
Intravascular large B-cell lymphoma (IVBCL) is an aggressive and systemically disseminated disease that affects the elderly, with a median age of diagnosis around 70 years and no gender predilection. It is a rare subtype of extranodal diffuse large B-cell lymphoma (DLBCL) characterized by selective growth of neoplastic cells within blood vessel lumen without any obvious extravascular tumor mass. Hence, an absence of marked lymphadenopathy and heterogeneous clinical presentation make it difficult to diagnose accurately and timely, with roughly half of the cases found postmortem in previous case reports.1,2 The exact incidence of this disease is not known, but more recently, the accuracy of diagnosis of this type of lymphoma has improved with random skin and bone marrow biopsy.1,2 We present here a clinical case of this disease with an atypical presentation followed by a detailed review of its clinical aspects.
Case presentation and summary
A 43-year-old white woman with a history of hypothyroidism and recurrent ovarian cysts presented to clinic with 3 months of loss of appetite, abdominal distension, pelvic pain, and progressive lower-extremity swelling. A physical examination was notable for marked abdominal distension, diffuse lower abdominal tenderness, and pitting lower-extremity edema. No skin rash or any other cutaneous abnormality was noted on exam. Laboratory test results revealed a lactate dehydrogenase (LDH) level of 1652 U/L and a CA-125 level of 50 U/mL (reference range, 0-35 U/mL). No significant beta-human chorionic gonadotropin and alpha-fetoprotein levels were detected. Computed-tomographic (CT) imaging revealed small bilateral pleural effusions and gallbladder wall thickening with abdominal wall edema, but it was otherwise unrevealing. An echocardiogram showed normal cardiac structure and function, with a left ventricular ejection fraction of 60%. No protein was detected in the patient’s urine, and thyroid function tests were unrevealing. Doppler ultrasound studies of her lower extremities and abdomen revealed no thrombosis. Given the patient’s continued pelvic pain, history of ovarian cysts, and elevation in CA-125, she underwent a laparoscopic total abdominal hysterectomy and bilateral salpingoopherectomy.
Histologic examination revealed neoplastic cells involving only the vascular lumina of the cervix, endomyometrium, bilateral fallopian tubes, and bilateral ovaries (Figure 1). Immunohistochemistry stains were positive for CD5, CD20, PAX-5, CD45, BCL-2, and BCL-6 and focally positive for CD10. Peripheral smear showed pseudo-Pelger–Huet cells with 5% atypical lymphoma cells (Figure 2). Complete staging with positron-emission and CT (PET–CT) imaging revealed no metabolic activity, and a bone marrow biopsy showed trilineage hematopoiesis with adequate maturation and less than 5% of the marrow involved with large B-cell lymphoma cells. A diagnosis of IVBCL was made.
Further work-up to rule out involvement of the central nervous system (CNS) included magnetic-resonance imaging (MRI) of the brain and cerebrospinal fluid (CSF) cytology and flow cytometry, which were negative.
Our patient underwent treatment with 6 cycles of infusional, dose-adjusted R-EPOCH (rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, doxorubicin hydrochloride) and 6 doses of prophylactic intrathecal chemotherapy with alternating methotrexate and cytarabine (Ara-C), and initial and subsequent CSF sampling showed no disease involvement. Consolidation with high-dose chemotherapy with R-BEAM (rituximab, carmustine, etoposide, Ara-C [cytarabine], melphalan) followed by rescue autologous stem cell transplantation (ASCT) was performed, and the patient has remained in clinical and hematologic remission for the past 24 months.
Discussion
Clinical presentation
The clinical manifestation of this disease is highly variable, and virtually any organ can be involved. Besides causing constitutional symptoms, including fatigue, B symptoms, and decline in performance status, heterogeneity of the clinical presentation depends on the organ system involved. One of the exceptional features of this disease is the difference in clinical presentation based on the geographical origin of the patient.2-4
Western-variant IVBCL has a higher frequency of CNS and skin involvement, whereas Asian-variant IVBCL shows preferential involvement of bone marrow with hemophagocytosis, hepatosplenomegaly, and thrombocytopenia. However, these 2 clinical variants have no difference in clinical outcome, except with the cutaneous-variant kind.24 A retrospective case series of 38 Western-variant IVBCL cases showed that 55% of patients had B symptoms with poor performance status.3 Brain and skin were the organs that were most frequently involved, with 68% of patients having involvement of at least 1 of those organs. Ten patients in this case series had disease that was exclusively limited to the skin and described as a “cutaneous variant” of IVBCL.3
Similarly, a retrospective case series of 96 cases of Asian-variant IVBCL showed B symptoms in 76% of patients, with predominant bone marrow involvement in 75% of patients, accompanied by hemophagocytosis in 66% and hepatosplenomegaly and anemia/thrombocytopenia in 77% and 84% of the patients, respectively.4 This difference in clinical presentation might have existed as a result of ethnic difference associated with production of inflammatory cytokines, including interferon gamma, tumor necrosis factor-alpha, interlukin-1 beta, and soluble interlukin-2 receptor, with levels of soluble interlukin-2 receptor found to be significantly higher in Asian patients than non-Asian patients.2
Diagnosis
Involved organ biopsy is mandatory for establishing the diagnosis of IVBCL. Laboratory findings are nonspecific, with the most common abnormality being increased serum LDH and beta-2 microglobulin levels observed in 80% to 90% or more of patients. Despite its intravascular growth pattern, IVBCL was associated with peripheral blood involvement in only 5% to 9% of patients.1
Staging
Clinical staging work-up suggested for IVBCL patients by International Extranodal lymphoma study group in 2005 included physical examination (with emphasis on nervous system and skin), routine blood studies, peripheral blood smear, total body CT scan with contrast or PET–CT scan, MRI brain with contrast, CSF cytology, and bone marrow or organ biopsy.1 The role of fluorodeoxyglucose-PET scan is controversial but can be helpful to detect unexpected locations for biopsy and to assess treatment response.5,6
Morphology and immunophenotyping
In general, IVBCL histopathology shows large neoplastic lymphoid cells with large nuclei along with one or more nucleoli and scant cytoplasm within blood vessel lumen. Immunophenotypically, IVBCL cells mostly express nongerminal B-cell–associated markers with CD79a (100%), CD20 (96%), MUM-IRF4 (95%), CD5 (38%), and CD10 (12%) expressions. IVBCL cells have been demonstrated to lack cell surface protein CD29 and CD54 critical to transvascular migration. Similarly, aberrant expression of proteins such as CD11a and CXCR3 allows lymphoma cells to be attracted to endothelial cells, which might explain their intravascular confinement.7
Genetics
No pathognomic cytogenetic abnormalities have been reported in IVBCL to date, and the genetic features of this disease are not yet completely understood.2,7
Management
IVBCL is considered a stage IV disseminated disease with an International Prognostic Index score of high-intermediate to high in most cases. Half of the patients with IVBCL who were treated with anthracycline-based chemotherapy relapsed and died within 18 months of diagnosis. One third of the relapses involved the CNS, thereby highlighting the importance of prophylactic CNS-directed Intrathecal therapy in an induction treatment regimen.2-4 Ferreri and colleagues reported in their case series response rates of about 60%, with an overall survival (OS) of 3 years of 30% in patients who were treated with anthracycline-based chemotherapy. A multivariate analysis of the entire series showed cutaneous variant of the disease to be an independent favorable prognostic factor for OS.3
In the Murase and colleagues case series, the authors reported 67% response rates and a median OS of 13 months with CHOP (cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, prednisone) or CHOP-like regimens. Multivariate analysis showed older age, thrombocytopenia, and absence of anthracycline-based chemotherapy to be an independent negative prognostic factor for OS.4 Another retrospective analysis by Shimada and colleagues of 106 patients with IVBCL showed improved outcome with the addition of rituximab to CHOP-based chemotherapy (R-CHOP). Complete response rate (CR), 2-year progression-free survival, and OS were significantly higher for patients in rituximab-chemotherapy group than for those in the chemotherapy-alone group (CR, 82% vs 51%, respectively, P = .001; PFS, 56% vs 27%; OS, 66% vs 46%, P = .001), thereby establishing rituximab with CHOP-based therapy as induction therapy for IVBCL patients.8
The role of high-dose chemotherapy followed by ASCT could also be used as consolidation therapy to improve clinical outcomes as reported in 7 patients, showing durable remission after transplant in these 2 case series.3,4 Another retrospective analysis of 6 patients with IVBCL who were treated with 6 cycles of R-CHOP as induction therapy and consolidated with ASCT reported all patients to be alive and in complete remission after a median follow-up of 56 months.9 Based on the retrospective case series data by Kato and colleagues and considering that more than 80% of the patients with IVBCL were in the high-risk International Prognostic Index group, ASCT in first remission might be a useful treatment option for durable remission; however, because the median age for the diagnosis of IVBCL is about 70 years, ASCT may not be a realistic option for all patients.
Conclusions
IVBCL is a rare, aggressive, and distinct type of DLBCL with complex constellations of symptoms requiring strong clinical suspicion to establish this challenging diagnosis. Rituximab with anthracycline-based therapy along with prophylactic CNS-directed therapy followed by consolidative ASCT may lead to long-term remission. More research is needed into the genetic features of this disease to better understand its pathogenesis and potential targets for treatment.
1. Ponzoni M, Ferreri AJ, Campo E, et al. Definition, diagnosis, and management of intravascular large B-cell lymphoma: proposals and perspectives from an international consensus meeting. J Clin Oncol. 2007;25(21):3168-3173.
2. Shimada K, Kinoshita T, Naoe T, Nakamura S. Presentation and management of intravascular large B-cell lymphoma. Lancet Oncol. 2009;10(9):895-902.
3. Ferreri AJ, Campo E, Seymour JF, et al. Intravascular lymphoma: clinical presentation, natural history, management and prognostic factors in a series of 38 cases, with special emphasis on the ‘cutaneous variant’. Br J Haematol. 2004;127(2):173-183.
4. Murase T, Yamaguchi M, Suzuki R, et al. Intravascular large B-cell lymphoma (IVLBCL): a clinicopathologic study of 96 cases with special reference to the immunophenotypic heterogeneity of CD5. Blood. 2007;109(2):478-485.
5. Miura Y, Tsudo M. Fluorodeoxyglucose-PET/CT for diagnosis of intravascular large B-cell lymphoma. Mayo Clin Proc. 2010;85(8):e56-e57.
6. Shimada K, Kosugi H, Shimada S, et al. Evaluation of organ involvement in intravascular large B-cell lymphoma by 18F-fluorodeoxyglucose positron emission tomography. Int J Hematol. 2008;88(2):149-153.
7. Orwat DE, Batalis NI. Intravascular large B-cell lymphoma. Arch Pathol Lab Med. 2012;136(3):333-338.
8. Shimada K, Matsue K, Yamamoto K, et al. Retrospective analysis of intravascular large B-cell lymphoma treated with rituximab-containing chemotherapy as reported by the IVL study group in Japan. J Clin Oncol. 2008;26(19):3189-3195.
9. Kato K, Ohno Y, Kamimura T, et al. Long-term remission after high-dose chemotherapy followed by auto-SCT as consolidation for intravascular large B-cell lymphoma. Bone Marrow Transplant. 2014;49(12):1543-1544.
Intravascular large B-cell lymphoma (IVBCL) is an aggressive and systemically disseminated disease that affects the elderly, with a median age of diagnosis around 70 years and no gender predilection. It is a rare subtype of extranodal diffuse large B-cell lymphoma (DLBCL) characterized by selective growth of neoplastic cells within blood vessel lumen without any obvious extravascular tumor mass. Hence, an absence of marked lymphadenopathy and heterogeneous clinical presentation make it difficult to diagnose accurately and timely, with roughly half of the cases found postmortem in previous case reports.1,2 The exact incidence of this disease is not known, but more recently, the accuracy of diagnosis of this type of lymphoma has improved with random skin and bone marrow biopsy.1,2 We present here a clinical case of this disease with an atypical presentation followed by a detailed review of its clinical aspects.
Case presentation and summary
A 43-year-old white woman with a history of hypothyroidism and recurrent ovarian cysts presented to clinic with 3 months of loss of appetite, abdominal distension, pelvic pain, and progressive lower-extremity swelling. A physical examination was notable for marked abdominal distension, diffuse lower abdominal tenderness, and pitting lower-extremity edema. No skin rash or any other cutaneous abnormality was noted on exam. Laboratory test results revealed a lactate dehydrogenase (LDH) level of 1652 U/L and a CA-125 level of 50 U/mL (reference range, 0-35 U/mL). No significant beta-human chorionic gonadotropin and alpha-fetoprotein levels were detected. Computed-tomographic (CT) imaging revealed small bilateral pleural effusions and gallbladder wall thickening with abdominal wall edema, but it was otherwise unrevealing. An echocardiogram showed normal cardiac structure and function, with a left ventricular ejection fraction of 60%. No protein was detected in the patient’s urine, and thyroid function tests were unrevealing. Doppler ultrasound studies of her lower extremities and abdomen revealed no thrombosis. Given the patient’s continued pelvic pain, history of ovarian cysts, and elevation in CA-125, she underwent a laparoscopic total abdominal hysterectomy and bilateral salpingoopherectomy.
Histologic examination revealed neoplastic cells involving only the vascular lumina of the cervix, endomyometrium, bilateral fallopian tubes, and bilateral ovaries (Figure 1). Immunohistochemistry stains were positive for CD5, CD20, PAX-5, CD45, BCL-2, and BCL-6 and focally positive for CD10. Peripheral smear showed pseudo-Pelger–Huet cells with 5% atypical lymphoma cells (Figure 2). Complete staging with positron-emission and CT (PET–CT) imaging revealed no metabolic activity, and a bone marrow biopsy showed trilineage hematopoiesis with adequate maturation and less than 5% of the marrow involved with large B-cell lymphoma cells. A diagnosis of IVBCL was made.
Further work-up to rule out involvement of the central nervous system (CNS) included magnetic-resonance imaging (MRI) of the brain and cerebrospinal fluid (CSF) cytology and flow cytometry, which were negative.
Our patient underwent treatment with 6 cycles of infusional, dose-adjusted R-EPOCH (rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, doxorubicin hydrochloride) and 6 doses of prophylactic intrathecal chemotherapy with alternating methotrexate and cytarabine (Ara-C), and initial and subsequent CSF sampling showed no disease involvement. Consolidation with high-dose chemotherapy with R-BEAM (rituximab, carmustine, etoposide, Ara-C [cytarabine], melphalan) followed by rescue autologous stem cell transplantation (ASCT) was performed, and the patient has remained in clinical and hematologic remission for the past 24 months.
Discussion
Clinical presentation
The clinical manifestation of this disease is highly variable, and virtually any organ can be involved. Besides causing constitutional symptoms, including fatigue, B symptoms, and decline in performance status, heterogeneity of the clinical presentation depends on the organ system involved. One of the exceptional features of this disease is the difference in clinical presentation based on the geographical origin of the patient.2-4
Western-variant IVBCL has a higher frequency of CNS and skin involvement, whereas Asian-variant IVBCL shows preferential involvement of bone marrow with hemophagocytosis, hepatosplenomegaly, and thrombocytopenia. However, these 2 clinical variants have no difference in clinical outcome, except with the cutaneous-variant kind.24 A retrospective case series of 38 Western-variant IVBCL cases showed that 55% of patients had B symptoms with poor performance status.3 Brain and skin were the organs that were most frequently involved, with 68% of patients having involvement of at least 1 of those organs. Ten patients in this case series had disease that was exclusively limited to the skin and described as a “cutaneous variant” of IVBCL.3
Similarly, a retrospective case series of 96 cases of Asian-variant IVBCL showed B symptoms in 76% of patients, with predominant bone marrow involvement in 75% of patients, accompanied by hemophagocytosis in 66% and hepatosplenomegaly and anemia/thrombocytopenia in 77% and 84% of the patients, respectively.4 This difference in clinical presentation might have existed as a result of ethnic difference associated with production of inflammatory cytokines, including interferon gamma, tumor necrosis factor-alpha, interlukin-1 beta, and soluble interlukin-2 receptor, with levels of soluble interlukin-2 receptor found to be significantly higher in Asian patients than non-Asian patients.2
Diagnosis
Involved organ biopsy is mandatory for establishing the diagnosis of IVBCL. Laboratory findings are nonspecific, with the most common abnormality being increased serum LDH and beta-2 microglobulin levels observed in 80% to 90% or more of patients. Despite its intravascular growth pattern, IVBCL was associated with peripheral blood involvement in only 5% to 9% of patients.1
Staging
Clinical staging work-up suggested for IVBCL patients by International Extranodal lymphoma study group in 2005 included physical examination (with emphasis on nervous system and skin), routine blood studies, peripheral blood smear, total body CT scan with contrast or PET–CT scan, MRI brain with contrast, CSF cytology, and bone marrow or organ biopsy.1 The role of fluorodeoxyglucose-PET scan is controversial but can be helpful to detect unexpected locations for biopsy and to assess treatment response.5,6
Morphology and immunophenotyping
In general, IVBCL histopathology shows large neoplastic lymphoid cells with large nuclei along with one or more nucleoli and scant cytoplasm within blood vessel lumen. Immunophenotypically, IVBCL cells mostly express nongerminal B-cell–associated markers with CD79a (100%), CD20 (96%), MUM-IRF4 (95%), CD5 (38%), and CD10 (12%) expressions. IVBCL cells have been demonstrated to lack cell surface protein CD29 and CD54 critical to transvascular migration. Similarly, aberrant expression of proteins such as CD11a and CXCR3 allows lymphoma cells to be attracted to endothelial cells, which might explain their intravascular confinement.7
Genetics
No pathognomic cytogenetic abnormalities have been reported in IVBCL to date, and the genetic features of this disease are not yet completely understood.2,7
Management
IVBCL is considered a stage IV disseminated disease with an International Prognostic Index score of high-intermediate to high in most cases. Half of the patients with IVBCL who were treated with anthracycline-based chemotherapy relapsed and died within 18 months of diagnosis. One third of the relapses involved the CNS, thereby highlighting the importance of prophylactic CNS-directed Intrathecal therapy in an induction treatment regimen.2-4 Ferreri and colleagues reported in their case series response rates of about 60%, with an overall survival (OS) of 3 years of 30% in patients who were treated with anthracycline-based chemotherapy. A multivariate analysis of the entire series showed cutaneous variant of the disease to be an independent favorable prognostic factor for OS.3
In the Murase and colleagues case series, the authors reported 67% response rates and a median OS of 13 months with CHOP (cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, prednisone) or CHOP-like regimens. Multivariate analysis showed older age, thrombocytopenia, and absence of anthracycline-based chemotherapy to be an independent negative prognostic factor for OS.4 Another retrospective analysis by Shimada and colleagues of 106 patients with IVBCL showed improved outcome with the addition of rituximab to CHOP-based chemotherapy (R-CHOP). Complete response rate (CR), 2-year progression-free survival, and OS were significantly higher for patients in rituximab-chemotherapy group than for those in the chemotherapy-alone group (CR, 82% vs 51%, respectively, P = .001; PFS, 56% vs 27%; OS, 66% vs 46%, P = .001), thereby establishing rituximab with CHOP-based therapy as induction therapy for IVBCL patients.8
The role of high-dose chemotherapy followed by ASCT could also be used as consolidation therapy to improve clinical outcomes as reported in 7 patients, showing durable remission after transplant in these 2 case series.3,4 Another retrospective analysis of 6 patients with IVBCL who were treated with 6 cycles of R-CHOP as induction therapy and consolidated with ASCT reported all patients to be alive and in complete remission after a median follow-up of 56 months.9 Based on the retrospective case series data by Kato and colleagues and considering that more than 80% of the patients with IVBCL were in the high-risk International Prognostic Index group, ASCT in first remission might be a useful treatment option for durable remission; however, because the median age for the diagnosis of IVBCL is about 70 years, ASCT may not be a realistic option for all patients.
Conclusions
IVBCL is a rare, aggressive, and distinct type of DLBCL with complex constellations of symptoms requiring strong clinical suspicion to establish this challenging diagnosis. Rituximab with anthracycline-based therapy along with prophylactic CNS-directed therapy followed by consolidative ASCT may lead to long-term remission. More research is needed into the genetic features of this disease to better understand its pathogenesis and potential targets for treatment.
Intravascular large B-cell lymphoma (IVBCL) is an aggressive and systemically disseminated disease that affects the elderly, with a median age of diagnosis around 70 years and no gender predilection. It is a rare subtype of extranodal diffuse large B-cell lymphoma (DLBCL) characterized by selective growth of neoplastic cells within blood vessel lumen without any obvious extravascular tumor mass. Hence, an absence of marked lymphadenopathy and heterogeneous clinical presentation make it difficult to diagnose accurately and timely, with roughly half of the cases found postmortem in previous case reports.1,2 The exact incidence of this disease is not known, but more recently, the accuracy of diagnosis of this type of lymphoma has improved with random skin and bone marrow biopsy.1,2 We present here a clinical case of this disease with an atypical presentation followed by a detailed review of its clinical aspects.
Case presentation and summary
A 43-year-old white woman with a history of hypothyroidism and recurrent ovarian cysts presented to clinic with 3 months of loss of appetite, abdominal distension, pelvic pain, and progressive lower-extremity swelling. A physical examination was notable for marked abdominal distension, diffuse lower abdominal tenderness, and pitting lower-extremity edema. No skin rash or any other cutaneous abnormality was noted on exam. Laboratory test results revealed a lactate dehydrogenase (LDH) level of 1652 U/L and a CA-125 level of 50 U/mL (reference range, 0-35 U/mL). No significant beta-human chorionic gonadotropin and alpha-fetoprotein levels were detected. Computed-tomographic (CT) imaging revealed small bilateral pleural effusions and gallbladder wall thickening with abdominal wall edema, but it was otherwise unrevealing. An echocardiogram showed normal cardiac structure and function, with a left ventricular ejection fraction of 60%. No protein was detected in the patient’s urine, and thyroid function tests were unrevealing. Doppler ultrasound studies of her lower extremities and abdomen revealed no thrombosis. Given the patient’s continued pelvic pain, history of ovarian cysts, and elevation in CA-125, she underwent a laparoscopic total abdominal hysterectomy and bilateral salpingoopherectomy.
Histologic examination revealed neoplastic cells involving only the vascular lumina of the cervix, endomyometrium, bilateral fallopian tubes, and bilateral ovaries (Figure 1). Immunohistochemistry stains were positive for CD5, CD20, PAX-5, CD45, BCL-2, and BCL-6 and focally positive for CD10. Peripheral smear showed pseudo-Pelger–Huet cells with 5% atypical lymphoma cells (Figure 2). Complete staging with positron-emission and CT (PET–CT) imaging revealed no metabolic activity, and a bone marrow biopsy showed trilineage hematopoiesis with adequate maturation and less than 5% of the marrow involved with large B-cell lymphoma cells. A diagnosis of IVBCL was made.
Further work-up to rule out involvement of the central nervous system (CNS) included magnetic-resonance imaging (MRI) of the brain and cerebrospinal fluid (CSF) cytology and flow cytometry, which were negative.
Our patient underwent treatment with 6 cycles of infusional, dose-adjusted R-EPOCH (rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, doxorubicin hydrochloride) and 6 doses of prophylactic intrathecal chemotherapy with alternating methotrexate and cytarabine (Ara-C), and initial and subsequent CSF sampling showed no disease involvement. Consolidation with high-dose chemotherapy with R-BEAM (rituximab, carmustine, etoposide, Ara-C [cytarabine], melphalan) followed by rescue autologous stem cell transplantation (ASCT) was performed, and the patient has remained in clinical and hematologic remission for the past 24 months.
Discussion
Clinical presentation
The clinical manifestation of this disease is highly variable, and virtually any organ can be involved. Besides causing constitutional symptoms, including fatigue, B symptoms, and decline in performance status, heterogeneity of the clinical presentation depends on the organ system involved. One of the exceptional features of this disease is the difference in clinical presentation based on the geographical origin of the patient.2-4
Western-variant IVBCL has a higher frequency of CNS and skin involvement, whereas Asian-variant IVBCL shows preferential involvement of bone marrow with hemophagocytosis, hepatosplenomegaly, and thrombocytopenia. However, these 2 clinical variants have no difference in clinical outcome, except with the cutaneous-variant kind.24 A retrospective case series of 38 Western-variant IVBCL cases showed that 55% of patients had B symptoms with poor performance status.3 Brain and skin were the organs that were most frequently involved, with 68% of patients having involvement of at least 1 of those organs. Ten patients in this case series had disease that was exclusively limited to the skin and described as a “cutaneous variant” of IVBCL.3
Similarly, a retrospective case series of 96 cases of Asian-variant IVBCL showed B symptoms in 76% of patients, with predominant bone marrow involvement in 75% of patients, accompanied by hemophagocytosis in 66% and hepatosplenomegaly and anemia/thrombocytopenia in 77% and 84% of the patients, respectively.4 This difference in clinical presentation might have existed as a result of ethnic difference associated with production of inflammatory cytokines, including interferon gamma, tumor necrosis factor-alpha, interlukin-1 beta, and soluble interlukin-2 receptor, with levels of soluble interlukin-2 receptor found to be significantly higher in Asian patients than non-Asian patients.2
Diagnosis
Involved organ biopsy is mandatory for establishing the diagnosis of IVBCL. Laboratory findings are nonspecific, with the most common abnormality being increased serum LDH and beta-2 microglobulin levels observed in 80% to 90% or more of patients. Despite its intravascular growth pattern, IVBCL was associated with peripheral blood involvement in only 5% to 9% of patients.1
Staging
Clinical staging work-up suggested for IVBCL patients by International Extranodal lymphoma study group in 2005 included physical examination (with emphasis on nervous system and skin), routine blood studies, peripheral blood smear, total body CT scan with contrast or PET–CT scan, MRI brain with contrast, CSF cytology, and bone marrow or organ biopsy.1 The role of fluorodeoxyglucose-PET scan is controversial but can be helpful to detect unexpected locations for biopsy and to assess treatment response.5,6
Morphology and immunophenotyping
In general, IVBCL histopathology shows large neoplastic lymphoid cells with large nuclei along with one or more nucleoli and scant cytoplasm within blood vessel lumen. Immunophenotypically, IVBCL cells mostly express nongerminal B-cell–associated markers with CD79a (100%), CD20 (96%), MUM-IRF4 (95%), CD5 (38%), and CD10 (12%) expressions. IVBCL cells have been demonstrated to lack cell surface protein CD29 and CD54 critical to transvascular migration. Similarly, aberrant expression of proteins such as CD11a and CXCR3 allows lymphoma cells to be attracted to endothelial cells, which might explain their intravascular confinement.7
Genetics
No pathognomic cytogenetic abnormalities have been reported in IVBCL to date, and the genetic features of this disease are not yet completely understood.2,7
Management
IVBCL is considered a stage IV disseminated disease with an International Prognostic Index score of high-intermediate to high in most cases. Half of the patients with IVBCL who were treated with anthracycline-based chemotherapy relapsed and died within 18 months of diagnosis. One third of the relapses involved the CNS, thereby highlighting the importance of prophylactic CNS-directed Intrathecal therapy in an induction treatment regimen.2-4 Ferreri and colleagues reported in their case series response rates of about 60%, with an overall survival (OS) of 3 years of 30% in patients who were treated with anthracycline-based chemotherapy. A multivariate analysis of the entire series showed cutaneous variant of the disease to be an independent favorable prognostic factor for OS.3
In the Murase and colleagues case series, the authors reported 67% response rates and a median OS of 13 months with CHOP (cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, prednisone) or CHOP-like regimens. Multivariate analysis showed older age, thrombocytopenia, and absence of anthracycline-based chemotherapy to be an independent negative prognostic factor for OS.4 Another retrospective analysis by Shimada and colleagues of 106 patients with IVBCL showed improved outcome with the addition of rituximab to CHOP-based chemotherapy (R-CHOP). Complete response rate (CR), 2-year progression-free survival, and OS were significantly higher for patients in rituximab-chemotherapy group than for those in the chemotherapy-alone group (CR, 82% vs 51%, respectively, P = .001; PFS, 56% vs 27%; OS, 66% vs 46%, P = .001), thereby establishing rituximab with CHOP-based therapy as induction therapy for IVBCL patients.8
The role of high-dose chemotherapy followed by ASCT could also be used as consolidation therapy to improve clinical outcomes as reported in 7 patients, showing durable remission after transplant in these 2 case series.3,4 Another retrospective analysis of 6 patients with IVBCL who were treated with 6 cycles of R-CHOP as induction therapy and consolidated with ASCT reported all patients to be alive and in complete remission after a median follow-up of 56 months.9 Based on the retrospective case series data by Kato and colleagues and considering that more than 80% of the patients with IVBCL were in the high-risk International Prognostic Index group, ASCT in first remission might be a useful treatment option for durable remission; however, because the median age for the diagnosis of IVBCL is about 70 years, ASCT may not be a realistic option for all patients.
Conclusions
IVBCL is a rare, aggressive, and distinct type of DLBCL with complex constellations of symptoms requiring strong clinical suspicion to establish this challenging diagnosis. Rituximab with anthracycline-based therapy along with prophylactic CNS-directed therapy followed by consolidative ASCT may lead to long-term remission. More research is needed into the genetic features of this disease to better understand its pathogenesis and potential targets for treatment.
1. Ponzoni M, Ferreri AJ, Campo E, et al. Definition, diagnosis, and management of intravascular large B-cell lymphoma: proposals and perspectives from an international consensus meeting. J Clin Oncol. 2007;25(21):3168-3173.
2. Shimada K, Kinoshita T, Naoe T, Nakamura S. Presentation and management of intravascular large B-cell lymphoma. Lancet Oncol. 2009;10(9):895-902.
3. Ferreri AJ, Campo E, Seymour JF, et al. Intravascular lymphoma: clinical presentation, natural history, management and prognostic factors in a series of 38 cases, with special emphasis on the ‘cutaneous variant’. Br J Haematol. 2004;127(2):173-183.
4. Murase T, Yamaguchi M, Suzuki R, et al. Intravascular large B-cell lymphoma (IVLBCL): a clinicopathologic study of 96 cases with special reference to the immunophenotypic heterogeneity of CD5. Blood. 2007;109(2):478-485.
5. Miura Y, Tsudo M. Fluorodeoxyglucose-PET/CT for diagnosis of intravascular large B-cell lymphoma. Mayo Clin Proc. 2010;85(8):e56-e57.
6. Shimada K, Kosugi H, Shimada S, et al. Evaluation of organ involvement in intravascular large B-cell lymphoma by 18F-fluorodeoxyglucose positron emission tomography. Int J Hematol. 2008;88(2):149-153.
7. Orwat DE, Batalis NI. Intravascular large B-cell lymphoma. Arch Pathol Lab Med. 2012;136(3):333-338.
8. Shimada K, Matsue K, Yamamoto K, et al. Retrospective analysis of intravascular large B-cell lymphoma treated with rituximab-containing chemotherapy as reported by the IVL study group in Japan. J Clin Oncol. 2008;26(19):3189-3195.
9. Kato K, Ohno Y, Kamimura T, et al. Long-term remission after high-dose chemotherapy followed by auto-SCT as consolidation for intravascular large B-cell lymphoma. Bone Marrow Transplant. 2014;49(12):1543-1544.
1. Ponzoni M, Ferreri AJ, Campo E, et al. Definition, diagnosis, and management of intravascular large B-cell lymphoma: proposals and perspectives from an international consensus meeting. J Clin Oncol. 2007;25(21):3168-3173.
2. Shimada K, Kinoshita T, Naoe T, Nakamura S. Presentation and management of intravascular large B-cell lymphoma. Lancet Oncol. 2009;10(9):895-902.
3. Ferreri AJ, Campo E, Seymour JF, et al. Intravascular lymphoma: clinical presentation, natural history, management and prognostic factors in a series of 38 cases, with special emphasis on the ‘cutaneous variant’. Br J Haematol. 2004;127(2):173-183.
4. Murase T, Yamaguchi M, Suzuki R, et al. Intravascular large B-cell lymphoma (IVLBCL): a clinicopathologic study of 96 cases with special reference to the immunophenotypic heterogeneity of CD5. Blood. 2007;109(2):478-485.
5. Miura Y, Tsudo M. Fluorodeoxyglucose-PET/CT for diagnosis of intravascular large B-cell lymphoma. Mayo Clin Proc. 2010;85(8):e56-e57.
6. Shimada K, Kosugi H, Shimada S, et al. Evaluation of organ involvement in intravascular large B-cell lymphoma by 18F-fluorodeoxyglucose positron emission tomography. Int J Hematol. 2008;88(2):149-153.
7. Orwat DE, Batalis NI. Intravascular large B-cell lymphoma. Arch Pathol Lab Med. 2012;136(3):333-338.
8. Shimada K, Matsue K, Yamamoto K, et al. Retrospective analysis of intravascular large B-cell lymphoma treated with rituximab-containing chemotherapy as reported by the IVL study group in Japan. J Clin Oncol. 2008;26(19):3189-3195.
9. Kato K, Ohno Y, Kamimura T, et al. Long-term remission after high-dose chemotherapy followed by auto-SCT as consolidation for intravascular large B-cell lymphoma. Bone Marrow Transplant. 2014;49(12):1543-1544.
Elevated liver function tests in a patient on palbociclib and fulvestrant
About 12.4% of women in the United States will be diagnosed with breast cancer at some point in their lifetime.1 A percentage of these women will develop metastatic disease and are estimated to have a 5-year survival rate of 22%.2 There have been meaningful improvements in su
However, endocrine resistance inevitably occurs, and a great deal of research has been focused on developing strategies to combat resistance. One mechanism of endocrine resistance is though the Cyclin-dependent kinases 4 and 6 (CDK4/6) complexes. Among the most promising of the strategies to prevent resistance are the CDK4/6 inhibitors. There are now 3 approved CDK4/6 inhibitor drugs that can be used in combination with endocrine therapy, 1 of which can also be used as a single agent. When used in combination with endocrine therapy, the use of CDK 4/6 inhibitors has significantly improved progression-free survival (PFS) in patients with hormone-sensitive HER2-negative metastatic breast cancer by inhibiting cellular division and growth.3 In postmenopausal women, endocrine therapy plus CDK4/6 inhibitors are the preferred first-line regimen for metastatic disease.
Since the approval of palbociclib by the US Food and Drug Administration in 2015, the most common hematologic lab abnormalities are anemia, leukopenia, neutropenia, and thrombocytopenia. The most common nonhematologic adverse events (AEs) are fatigue, infection, nausea, and stomatitis. Hepatic toxicity has not been commonly observed. We report here the case of a 57-year-old woman on palbociclib and fulvestrant who developed significant elevation of liver function tests after starting palbociclib, suggesting a possible drug-induced liver injury from palbociclib.
Case presentation and summary
A 57-year-old woman with history of hypothyroidism and hypertension presented in May 2016 with a lump in her right breast and back pain. The lump was biopsied and revealed invasive ductal carcinoma, moderately differentiated, estrogen receptor (ER) positive 100%, progesterone receptor (PR) positive 95%, and HER2 negative. A positron emission tomography (PET)–computed tomography (CT) scan and magnetic resonance imaging showed bone metastasis at several vertebral levels, and the results of a bone biopsy confirmed metastatic adenocarcinoma of breast origin, ER positive 60%, PR positive 40%, and HER2 negative. No liver lesions were seen on imaging, but there was suggestion of fatty liver. She was started on letrozole 2.5 mg daily in July 2016 while undergoing kyphoplasty and subsequent radiation. A restaging PET scan revealed progression of disease on letrozole, with possible new rib lesion and progression in the breast. No liver disease was noted. Therapy was changed to fulvestrant and palbociclib. Fulvestrant was started in March 2017 with standard dosing of 500 mg intramuscular on days 1, 15, and 29, and then once a month thereafter. Her first cycle of palbociclib was started on April 5, dosed at 125 mg by mouth daily for 21 days, followed by 7 days off, repeated every 28 days (all dates hereinafter fell within 2017, unless otherwise stipulated).
Labs checked on April 28 and May 26 were unremarkable. A restaging CT scan of the chest, abdomen, and pelvis was done on June 21 after completion of 3 cycles of fulvestrant and palbociclib. There was no evidence of liver metastases, only the fatty infiltration of the liver that had been seen previously. On June 23, 2017, lab results showed a transaminitis with an alanine aminotransferase (ALT) level of 446 IU/L (reference range 10-33 IU/L) and aspartate aminotransferase (AST) level of 183 IU/L (reference range 0-32 IU/L).
The patient’s liver enzyme levels continued to increase and peaked on July 3 at ALT >700 IU/L and AST at 421 IU/L. Her total bilirubin and alkaline phosphatase levels remained within normal limits. She had received her final dose of fulvestrant on May 31 and had taken her last dose of palbociclib on June 20, 2017. She had no history of elevated liver enzymes or liver disease, although the initial PET scan done at diagnosis had suggested hepatic steatosis. She said she had not recently used antibiotics, alcohol, or over-the-counter medications or supplements. There was no family history of liver problems, inflammatory bowel disease, or gastrointestinal malignancy. The only other medications she had taken recently were denosumab, levothyroxine for hypothyroidism, and amlodipine for hypertension. She was seen by hepatology for evaluation of acute hepatitis. Other etiologies for her elevated liver enzymes were ruled out, and she was diagnosed with a drug-induced liver injury from one of her anticancer medications. Her treatments with fulvestrant and palbociclib were held, and the results of her liver function tests normalized by September 2017.
Fulvestrant was restarted on August 24, and her lab results remained normal through November of that year, when restaging scans showed progression with new axillary adenopathy suspicious for metastasis. Imaging also showed a 1.6-cm hepatic lesion suggestive of a focal area of fat deposition or atypical hemangioma without definitive evidence of metastasis. Follow-up imaging was recommended. She was therefore rechallenged with palbociclib at a reduced dose of 100 mg by mouth daily and received the first dose on November 30. On December 8, repeat labs again showed elevated liver function tests (ALT, 285 IU/L; AST, 112 IU/L). Treatment with palbociclib was discontinued on December 10. Because the patient was not able to tolerate palbociclib, and fulvestrant alone was not controlling the disease, she was started on an alternate endocrine therapy with tamoxifen on December 26. The patient’s liver function tests normalized again by January 2018.
Discussion
The use of targeted therapies has changed the landscape of oncologic treatments. Several studies have evaluated the safety and efficacy of palbociclib in combination with endocrine therapy. The Palbociclib Ongoing Trials in the Management of Breast Cancer (PALOMA)-1 study, an open-label, randomized, phase-2 trial involving patients with newly diagnosed metastatic hormone sensitive HER2-negative breast cancer, demonstrated that palbociclib in combination with letrozole was associated with significantly longer PFS than letrozole alone.4 These results were later confirmed in the larger PALOMA-2 study, a randomized, double-blind, phase-3 trial that evaluated 666 postmenopausal patients with no prior systemic therapy. In that study, median PFS for the palbociclib–letrozole group was 24.8 months, compared with 14.5 months for the letrozole-alone group (hazard ratio [HR] for disease progression or death, 0.58 [0.46–0.72], P < .001).5 The most recent PALOMA-3 study, a phase-3 trial involving 521 patients with advanced hormone receptor–positive, HER2-negative breast cancer that had progressed during initial endocrine therapy, evaluated the efficacy of combined palbociclib and fulvestrant in a randomized, double-blind, placebo-controlled, parallel-group trial. The result was that the palbociclib–fulvestrant combination resulted in longer median PFS of 9.2 months, compared with 3.8 months with fulvestrant alone (P < .001).6
These trials also monitored the number of AEs as secondary aims. The most commonly reported AEs in the PALOMA trials for those patients in the palbociclib group were hematologic, with neutropenia being the most common, followed by leukopenia, anemia, and thrombocytopenia. The most common nonhematologic AEs reported in the palbociclib-fulvestrant group were fatigue, nausea, and headache. Elevated liver function tests were a rare but reported AE in 7.2% of the palbociclib-treated patients in the PALOMA-1 study.7 In the PALOMA-2 study, ALT and AST elevations were reported as AEs (all grades) in 9.9% and 9.7% of palbociclib-treated patients, respectively.5 In the PALOMA-3 study, there was 1 fatal serious AE of hepatic failure with grade 5 disease progression in the palbociclib group; however, the patient’s medical history included progressive liver metastasis and disease progression.6 A pooled safety analysis conducted across all PALOMA studies demonstrated that grade 3/4 AST and ALT elevations occurred in 3.3% and 2.3% of palbociclib-treated patients, respectively, again highlighting a reported but rare occurrence.8
The patient described in the present case report started on combination fulvestrant and palbociclib after her disease showed progression on letrozole. She developed an increase in transaminases after completing 3 cycles of palbociclib. Liver function tests increased nearly 12 weeks after beginning her first cycle of the CDK 4/6 inhibitor. Staging scans of the patient demonstrated fatty liver. It is not known if her fatty liver contributed to her transminitis; however, her baseline labs showed normal liver function tests, and they did not increase until after therapy with fulvestrant–palbociclib was started. It might have been that her fatty liver caused her to be at higher risk of transaminitis with administration of palbociclib, although we cannot be certain. Her lab results remained normal while she was on fulvestrant alone, and the liver function test results increased only after palbociclib was started, making this drug the more likely culprit.
Both events of increased liver enzymes occurred within a week of the last palbociclib dose; however, we note that hepatotoxicity developed at a faster rate when the patient was rechallenged with palbociclib at a lower dose, with elevated liver function tests increasing 1 week after restarting treatment as opposed to the first episode that occurred after 3 cycles of the palbociclib. After discontinuation of the medication, liver function tests again normalized, suggesting that palbociclib was most likely the causative agent. In addition, the degree of elevated liver enzymes was less severe on re-exposure at the lower dose of 100 mg, which raises the possibility that there could be a dose-dependent association between palbociclib and hepatotoxicity. There have been few case reports of increased liver enzymes associated with palbociclib, and it is only recently that this association has been more recognized. A meta-analysis by Zaw and colleagues has demonstrated that CDK 4/6 inhibitor–based regimens are associated with a higher risk of elevated AST and ALT; however, their relation with dose dependence was not described. In particular, they found that CDK 4/6 inhibitors increased the risk of high-grade, elevated ALT with a relative risk of 4.33 (95% confidence interval, 2.15-8.71; P < .0001). The meta-analysis also included other CDK 4/6 inhibitors such as abemaciclib and ribociclib, which have been more commonly associated with liver toxicity than palbociclib has.9 Our case report highlights the specific association between palbociclib and elevated liver enzymes.
In conclusion, this case report illustrates that our patient’s elevated liver enzymes were likely related to palbociclib. This is further supported by the fact that this AE occurred twice, both times after palbociclib exposure. In each instance, liver enzymes normalized after discontinuation of palbociclib. One cannot entirely rule out that fulvestrant might have been the culprit medication, but the patient’s normal hepatic panel for several months after starting fulvestrant suggests that is less likely. This case report is indicative of an uncommon complication in the treatment of metastatic breast cancer, one that is starting to gain more recognition, and we must think of palbociclib as a possible cause of drug-induced liver injury when targeted CDK 4/6–based regimens are used.
1. Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975-2014. Bethesda, MD: National Cancer Institute; 2017. https://seer.cancer.gov/csr/1975_2014/. Accessed April 3, 2018.
2. American Cancer Society. Breast cancer survival rates. https://www.cancer.org/cancer/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-survival-rates.html. Accessed April 3, 2018.
3. Wolff AC. CDK4 and CDK6 inhibition in breast cancer - a new standard. N Engl J Med. 2016; 375(20):1993-1994.
4. Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomized phase 2 study. Lancet Oncol. 2015;16(1):25-35.
5. Finn RS, Martin M, Rugo, HS et. al. Palbociclib and letrozole in advanced breast cancer. New Engl J Med. 2016;375:1925-1936
6. Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomized controlled trial. Lancet Oncol. 2016;17(4):425-439.
7. Turner NC, Ro J, André F, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373(3):209-219.
8. Dieras V, Rugo HS, Schnell P, et al. Long-term pooled safety analysis of palbociclib in combination with endocrine therapy for HR+/HR- advanced breast cancer [published online July 18, 2018]. Natl Cancer Inst. 2018;111.
9. Zaw M, Thein KZ, Tun A, et al. A systematic review and meta-analysis of randomized controlled trials to evaluate the risk of gastrointestinal and hepatic toxicities in patients with hormone receptor positive HER2-negative breast cancer treated with CKD 4/6 inhibitors. J Clin Oncol. 2017;35(suppl 31):209.
About 12.4% of women in the United States will be diagnosed with breast cancer at some point in their lifetime.1 A percentage of these women will develop metastatic disease and are estimated to have a 5-year survival rate of 22%.2 There have been meaningful improvements in su
However, endocrine resistance inevitably occurs, and a great deal of research has been focused on developing strategies to combat resistance. One mechanism of endocrine resistance is though the Cyclin-dependent kinases 4 and 6 (CDK4/6) complexes. Among the most promising of the strategies to prevent resistance are the CDK4/6 inhibitors. There are now 3 approved CDK4/6 inhibitor drugs that can be used in combination with endocrine therapy, 1 of which can also be used as a single agent. When used in combination with endocrine therapy, the use of CDK 4/6 inhibitors has significantly improved progression-free survival (PFS) in patients with hormone-sensitive HER2-negative metastatic breast cancer by inhibiting cellular division and growth.3 In postmenopausal women, endocrine therapy plus CDK4/6 inhibitors are the preferred first-line regimen for metastatic disease.
Since the approval of palbociclib by the US Food and Drug Administration in 2015, the most common hematologic lab abnormalities are anemia, leukopenia, neutropenia, and thrombocytopenia. The most common nonhematologic adverse events (AEs) are fatigue, infection, nausea, and stomatitis. Hepatic toxicity has not been commonly observed. We report here the case of a 57-year-old woman on palbociclib and fulvestrant who developed significant elevation of liver function tests after starting palbociclib, suggesting a possible drug-induced liver injury from palbociclib.
Case presentation and summary
A 57-year-old woman with history of hypothyroidism and hypertension presented in May 2016 with a lump in her right breast and back pain. The lump was biopsied and revealed invasive ductal carcinoma, moderately differentiated, estrogen receptor (ER) positive 100%, progesterone receptor (PR) positive 95%, and HER2 negative. A positron emission tomography (PET)–computed tomography (CT) scan and magnetic resonance imaging showed bone metastasis at several vertebral levels, and the results of a bone biopsy confirmed metastatic adenocarcinoma of breast origin, ER positive 60%, PR positive 40%, and HER2 negative. No liver lesions were seen on imaging, but there was suggestion of fatty liver. She was started on letrozole 2.5 mg daily in July 2016 while undergoing kyphoplasty and subsequent radiation. A restaging PET scan revealed progression of disease on letrozole, with possible new rib lesion and progression in the breast. No liver disease was noted. Therapy was changed to fulvestrant and palbociclib. Fulvestrant was started in March 2017 with standard dosing of 500 mg intramuscular on days 1, 15, and 29, and then once a month thereafter. Her first cycle of palbociclib was started on April 5, dosed at 125 mg by mouth daily for 21 days, followed by 7 days off, repeated every 28 days (all dates hereinafter fell within 2017, unless otherwise stipulated).
Labs checked on April 28 and May 26 were unremarkable. A restaging CT scan of the chest, abdomen, and pelvis was done on June 21 after completion of 3 cycles of fulvestrant and palbociclib. There was no evidence of liver metastases, only the fatty infiltration of the liver that had been seen previously. On June 23, 2017, lab results showed a transaminitis with an alanine aminotransferase (ALT) level of 446 IU/L (reference range 10-33 IU/L) and aspartate aminotransferase (AST) level of 183 IU/L (reference range 0-32 IU/L).
The patient’s liver enzyme levels continued to increase and peaked on July 3 at ALT >700 IU/L and AST at 421 IU/L. Her total bilirubin and alkaline phosphatase levels remained within normal limits. She had received her final dose of fulvestrant on May 31 and had taken her last dose of palbociclib on June 20, 2017. She had no history of elevated liver enzymes or liver disease, although the initial PET scan done at diagnosis had suggested hepatic steatosis. She said she had not recently used antibiotics, alcohol, or over-the-counter medications or supplements. There was no family history of liver problems, inflammatory bowel disease, or gastrointestinal malignancy. The only other medications she had taken recently were denosumab, levothyroxine for hypothyroidism, and amlodipine for hypertension. She was seen by hepatology for evaluation of acute hepatitis. Other etiologies for her elevated liver enzymes were ruled out, and she was diagnosed with a drug-induced liver injury from one of her anticancer medications. Her treatments with fulvestrant and palbociclib were held, and the results of her liver function tests normalized by September 2017.
Fulvestrant was restarted on August 24, and her lab results remained normal through November of that year, when restaging scans showed progression with new axillary adenopathy suspicious for metastasis. Imaging also showed a 1.6-cm hepatic lesion suggestive of a focal area of fat deposition or atypical hemangioma without definitive evidence of metastasis. Follow-up imaging was recommended. She was therefore rechallenged with palbociclib at a reduced dose of 100 mg by mouth daily and received the first dose on November 30. On December 8, repeat labs again showed elevated liver function tests (ALT, 285 IU/L; AST, 112 IU/L). Treatment with palbociclib was discontinued on December 10. Because the patient was not able to tolerate palbociclib, and fulvestrant alone was not controlling the disease, she was started on an alternate endocrine therapy with tamoxifen on December 26. The patient’s liver function tests normalized again by January 2018.
Discussion
The use of targeted therapies has changed the landscape of oncologic treatments. Several studies have evaluated the safety and efficacy of palbociclib in combination with endocrine therapy. The Palbociclib Ongoing Trials in the Management of Breast Cancer (PALOMA)-1 study, an open-label, randomized, phase-2 trial involving patients with newly diagnosed metastatic hormone sensitive HER2-negative breast cancer, demonstrated that palbociclib in combination with letrozole was associated with significantly longer PFS than letrozole alone.4 These results were later confirmed in the larger PALOMA-2 study, a randomized, double-blind, phase-3 trial that evaluated 666 postmenopausal patients with no prior systemic therapy. In that study, median PFS for the palbociclib–letrozole group was 24.8 months, compared with 14.5 months for the letrozole-alone group (hazard ratio [HR] for disease progression or death, 0.58 [0.46–0.72], P < .001).5 The most recent PALOMA-3 study, a phase-3 trial involving 521 patients with advanced hormone receptor–positive, HER2-negative breast cancer that had progressed during initial endocrine therapy, evaluated the efficacy of combined palbociclib and fulvestrant in a randomized, double-blind, placebo-controlled, parallel-group trial. The result was that the palbociclib–fulvestrant combination resulted in longer median PFS of 9.2 months, compared with 3.8 months with fulvestrant alone (P < .001).6
These trials also monitored the number of AEs as secondary aims. The most commonly reported AEs in the PALOMA trials for those patients in the palbociclib group were hematologic, with neutropenia being the most common, followed by leukopenia, anemia, and thrombocytopenia. The most common nonhematologic AEs reported in the palbociclib-fulvestrant group were fatigue, nausea, and headache. Elevated liver function tests were a rare but reported AE in 7.2% of the palbociclib-treated patients in the PALOMA-1 study.7 In the PALOMA-2 study, ALT and AST elevations were reported as AEs (all grades) in 9.9% and 9.7% of palbociclib-treated patients, respectively.5 In the PALOMA-3 study, there was 1 fatal serious AE of hepatic failure with grade 5 disease progression in the palbociclib group; however, the patient’s medical history included progressive liver metastasis and disease progression.6 A pooled safety analysis conducted across all PALOMA studies demonstrated that grade 3/4 AST and ALT elevations occurred in 3.3% and 2.3% of palbociclib-treated patients, respectively, again highlighting a reported but rare occurrence.8
The patient described in the present case report started on combination fulvestrant and palbociclib after her disease showed progression on letrozole. She developed an increase in transaminases after completing 3 cycles of palbociclib. Liver function tests increased nearly 12 weeks after beginning her first cycle of the CDK 4/6 inhibitor. Staging scans of the patient demonstrated fatty liver. It is not known if her fatty liver contributed to her transminitis; however, her baseline labs showed normal liver function tests, and they did not increase until after therapy with fulvestrant–palbociclib was started. It might have been that her fatty liver caused her to be at higher risk of transaminitis with administration of palbociclib, although we cannot be certain. Her lab results remained normal while she was on fulvestrant alone, and the liver function test results increased only after palbociclib was started, making this drug the more likely culprit.
Both events of increased liver enzymes occurred within a week of the last palbociclib dose; however, we note that hepatotoxicity developed at a faster rate when the patient was rechallenged with palbociclib at a lower dose, with elevated liver function tests increasing 1 week after restarting treatment as opposed to the first episode that occurred after 3 cycles of the palbociclib. After discontinuation of the medication, liver function tests again normalized, suggesting that palbociclib was most likely the causative agent. In addition, the degree of elevated liver enzymes was less severe on re-exposure at the lower dose of 100 mg, which raises the possibility that there could be a dose-dependent association between palbociclib and hepatotoxicity. There have been few case reports of increased liver enzymes associated with palbociclib, and it is only recently that this association has been more recognized. A meta-analysis by Zaw and colleagues has demonstrated that CDK 4/6 inhibitor–based regimens are associated with a higher risk of elevated AST and ALT; however, their relation with dose dependence was not described. In particular, they found that CDK 4/6 inhibitors increased the risk of high-grade, elevated ALT with a relative risk of 4.33 (95% confidence interval, 2.15-8.71; P < .0001). The meta-analysis also included other CDK 4/6 inhibitors such as abemaciclib and ribociclib, which have been more commonly associated with liver toxicity than palbociclib has.9 Our case report highlights the specific association between palbociclib and elevated liver enzymes.
In conclusion, this case report illustrates that our patient’s elevated liver enzymes were likely related to palbociclib. This is further supported by the fact that this AE occurred twice, both times after palbociclib exposure. In each instance, liver enzymes normalized after discontinuation of palbociclib. One cannot entirely rule out that fulvestrant might have been the culprit medication, but the patient’s normal hepatic panel for several months after starting fulvestrant suggests that is less likely. This case report is indicative of an uncommon complication in the treatment of metastatic breast cancer, one that is starting to gain more recognition, and we must think of palbociclib as a possible cause of drug-induced liver injury when targeted CDK 4/6–based regimens are used.
About 12.4% of women in the United States will be diagnosed with breast cancer at some point in their lifetime.1 A percentage of these women will develop metastatic disease and are estimated to have a 5-year survival rate of 22%.2 There have been meaningful improvements in su
However, endocrine resistance inevitably occurs, and a great deal of research has been focused on developing strategies to combat resistance. One mechanism of endocrine resistance is though the Cyclin-dependent kinases 4 and 6 (CDK4/6) complexes. Among the most promising of the strategies to prevent resistance are the CDK4/6 inhibitors. There are now 3 approved CDK4/6 inhibitor drugs that can be used in combination with endocrine therapy, 1 of which can also be used as a single agent. When used in combination with endocrine therapy, the use of CDK 4/6 inhibitors has significantly improved progression-free survival (PFS) in patients with hormone-sensitive HER2-negative metastatic breast cancer by inhibiting cellular division and growth.3 In postmenopausal women, endocrine therapy plus CDK4/6 inhibitors are the preferred first-line regimen for metastatic disease.
Since the approval of palbociclib by the US Food and Drug Administration in 2015, the most common hematologic lab abnormalities are anemia, leukopenia, neutropenia, and thrombocytopenia. The most common nonhematologic adverse events (AEs) are fatigue, infection, nausea, and stomatitis. Hepatic toxicity has not been commonly observed. We report here the case of a 57-year-old woman on palbociclib and fulvestrant who developed significant elevation of liver function tests after starting palbociclib, suggesting a possible drug-induced liver injury from palbociclib.
Case presentation and summary
A 57-year-old woman with history of hypothyroidism and hypertension presented in May 2016 with a lump in her right breast and back pain. The lump was biopsied and revealed invasive ductal carcinoma, moderately differentiated, estrogen receptor (ER) positive 100%, progesterone receptor (PR) positive 95%, and HER2 negative. A positron emission tomography (PET)–computed tomography (CT) scan and magnetic resonance imaging showed bone metastasis at several vertebral levels, and the results of a bone biopsy confirmed metastatic adenocarcinoma of breast origin, ER positive 60%, PR positive 40%, and HER2 negative. No liver lesions were seen on imaging, but there was suggestion of fatty liver. She was started on letrozole 2.5 mg daily in July 2016 while undergoing kyphoplasty and subsequent radiation. A restaging PET scan revealed progression of disease on letrozole, with possible new rib lesion and progression in the breast. No liver disease was noted. Therapy was changed to fulvestrant and palbociclib. Fulvestrant was started in March 2017 with standard dosing of 500 mg intramuscular on days 1, 15, and 29, and then once a month thereafter. Her first cycle of palbociclib was started on April 5, dosed at 125 mg by mouth daily for 21 days, followed by 7 days off, repeated every 28 days (all dates hereinafter fell within 2017, unless otherwise stipulated).
Labs checked on April 28 and May 26 were unremarkable. A restaging CT scan of the chest, abdomen, and pelvis was done on June 21 after completion of 3 cycles of fulvestrant and palbociclib. There was no evidence of liver metastases, only the fatty infiltration of the liver that had been seen previously. On June 23, 2017, lab results showed a transaminitis with an alanine aminotransferase (ALT) level of 446 IU/L (reference range 10-33 IU/L) and aspartate aminotransferase (AST) level of 183 IU/L (reference range 0-32 IU/L).
The patient’s liver enzyme levels continued to increase and peaked on July 3 at ALT >700 IU/L and AST at 421 IU/L. Her total bilirubin and alkaline phosphatase levels remained within normal limits. She had received her final dose of fulvestrant on May 31 and had taken her last dose of palbociclib on June 20, 2017. She had no history of elevated liver enzymes or liver disease, although the initial PET scan done at diagnosis had suggested hepatic steatosis. She said she had not recently used antibiotics, alcohol, or over-the-counter medications or supplements. There was no family history of liver problems, inflammatory bowel disease, or gastrointestinal malignancy. The only other medications she had taken recently were denosumab, levothyroxine for hypothyroidism, and amlodipine for hypertension. She was seen by hepatology for evaluation of acute hepatitis. Other etiologies for her elevated liver enzymes were ruled out, and she was diagnosed with a drug-induced liver injury from one of her anticancer medications. Her treatments with fulvestrant and palbociclib were held, and the results of her liver function tests normalized by September 2017.
Fulvestrant was restarted on August 24, and her lab results remained normal through November of that year, when restaging scans showed progression with new axillary adenopathy suspicious for metastasis. Imaging also showed a 1.6-cm hepatic lesion suggestive of a focal area of fat deposition or atypical hemangioma without definitive evidence of metastasis. Follow-up imaging was recommended. She was therefore rechallenged with palbociclib at a reduced dose of 100 mg by mouth daily and received the first dose on November 30. On December 8, repeat labs again showed elevated liver function tests (ALT, 285 IU/L; AST, 112 IU/L). Treatment with palbociclib was discontinued on December 10. Because the patient was not able to tolerate palbociclib, and fulvestrant alone was not controlling the disease, she was started on an alternate endocrine therapy with tamoxifen on December 26. The patient’s liver function tests normalized again by January 2018.
Discussion
The use of targeted therapies has changed the landscape of oncologic treatments. Several studies have evaluated the safety and efficacy of palbociclib in combination with endocrine therapy. The Palbociclib Ongoing Trials in the Management of Breast Cancer (PALOMA)-1 study, an open-label, randomized, phase-2 trial involving patients with newly diagnosed metastatic hormone sensitive HER2-negative breast cancer, demonstrated that palbociclib in combination with letrozole was associated with significantly longer PFS than letrozole alone.4 These results were later confirmed in the larger PALOMA-2 study, a randomized, double-blind, phase-3 trial that evaluated 666 postmenopausal patients with no prior systemic therapy. In that study, median PFS for the palbociclib–letrozole group was 24.8 months, compared with 14.5 months for the letrozole-alone group (hazard ratio [HR] for disease progression or death, 0.58 [0.46–0.72], P < .001).5 The most recent PALOMA-3 study, a phase-3 trial involving 521 patients with advanced hormone receptor–positive, HER2-negative breast cancer that had progressed during initial endocrine therapy, evaluated the efficacy of combined palbociclib and fulvestrant in a randomized, double-blind, placebo-controlled, parallel-group trial. The result was that the palbociclib–fulvestrant combination resulted in longer median PFS of 9.2 months, compared with 3.8 months with fulvestrant alone (P < .001).6
These trials also monitored the number of AEs as secondary aims. The most commonly reported AEs in the PALOMA trials for those patients in the palbociclib group were hematologic, with neutropenia being the most common, followed by leukopenia, anemia, and thrombocytopenia. The most common nonhematologic AEs reported in the palbociclib-fulvestrant group were fatigue, nausea, and headache. Elevated liver function tests were a rare but reported AE in 7.2% of the palbociclib-treated patients in the PALOMA-1 study.7 In the PALOMA-2 study, ALT and AST elevations were reported as AEs (all grades) in 9.9% and 9.7% of palbociclib-treated patients, respectively.5 In the PALOMA-3 study, there was 1 fatal serious AE of hepatic failure with grade 5 disease progression in the palbociclib group; however, the patient’s medical history included progressive liver metastasis and disease progression.6 A pooled safety analysis conducted across all PALOMA studies demonstrated that grade 3/4 AST and ALT elevations occurred in 3.3% and 2.3% of palbociclib-treated patients, respectively, again highlighting a reported but rare occurrence.8
The patient described in the present case report started on combination fulvestrant and palbociclib after her disease showed progression on letrozole. She developed an increase in transaminases after completing 3 cycles of palbociclib. Liver function tests increased nearly 12 weeks after beginning her first cycle of the CDK 4/6 inhibitor. Staging scans of the patient demonstrated fatty liver. It is not known if her fatty liver contributed to her transminitis; however, her baseline labs showed normal liver function tests, and they did not increase until after therapy with fulvestrant–palbociclib was started. It might have been that her fatty liver caused her to be at higher risk of transaminitis with administration of palbociclib, although we cannot be certain. Her lab results remained normal while she was on fulvestrant alone, and the liver function test results increased only after palbociclib was started, making this drug the more likely culprit.
Both events of increased liver enzymes occurred within a week of the last palbociclib dose; however, we note that hepatotoxicity developed at a faster rate when the patient was rechallenged with palbociclib at a lower dose, with elevated liver function tests increasing 1 week after restarting treatment as opposed to the first episode that occurred after 3 cycles of the palbociclib. After discontinuation of the medication, liver function tests again normalized, suggesting that palbociclib was most likely the causative agent. In addition, the degree of elevated liver enzymes was less severe on re-exposure at the lower dose of 100 mg, which raises the possibility that there could be a dose-dependent association between palbociclib and hepatotoxicity. There have been few case reports of increased liver enzymes associated with palbociclib, and it is only recently that this association has been more recognized. A meta-analysis by Zaw and colleagues has demonstrated that CDK 4/6 inhibitor–based regimens are associated with a higher risk of elevated AST and ALT; however, their relation with dose dependence was not described. In particular, they found that CDK 4/6 inhibitors increased the risk of high-grade, elevated ALT with a relative risk of 4.33 (95% confidence interval, 2.15-8.71; P < .0001). The meta-analysis also included other CDK 4/6 inhibitors such as abemaciclib and ribociclib, which have been more commonly associated with liver toxicity than palbociclib has.9 Our case report highlights the specific association between palbociclib and elevated liver enzymes.
In conclusion, this case report illustrates that our patient’s elevated liver enzymes were likely related to palbociclib. This is further supported by the fact that this AE occurred twice, both times after palbociclib exposure. In each instance, liver enzymes normalized after discontinuation of palbociclib. One cannot entirely rule out that fulvestrant might have been the culprit medication, but the patient’s normal hepatic panel for several months after starting fulvestrant suggests that is less likely. This case report is indicative of an uncommon complication in the treatment of metastatic breast cancer, one that is starting to gain more recognition, and we must think of palbociclib as a possible cause of drug-induced liver injury when targeted CDK 4/6–based regimens are used.
1. Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975-2014. Bethesda, MD: National Cancer Institute; 2017. https://seer.cancer.gov/csr/1975_2014/. Accessed April 3, 2018.
2. American Cancer Society. Breast cancer survival rates. https://www.cancer.org/cancer/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-survival-rates.html. Accessed April 3, 2018.
3. Wolff AC. CDK4 and CDK6 inhibition in breast cancer - a new standard. N Engl J Med. 2016; 375(20):1993-1994.
4. Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomized phase 2 study. Lancet Oncol. 2015;16(1):25-35.
5. Finn RS, Martin M, Rugo, HS et. al. Palbociclib and letrozole in advanced breast cancer. New Engl J Med. 2016;375:1925-1936
6. Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomized controlled trial. Lancet Oncol. 2016;17(4):425-439.
7. Turner NC, Ro J, André F, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373(3):209-219.
8. Dieras V, Rugo HS, Schnell P, et al. Long-term pooled safety analysis of palbociclib in combination with endocrine therapy for HR+/HR- advanced breast cancer [published online July 18, 2018]. Natl Cancer Inst. 2018;111.
9. Zaw M, Thein KZ, Tun A, et al. A systematic review and meta-analysis of randomized controlled trials to evaluate the risk of gastrointestinal and hepatic toxicities in patients with hormone receptor positive HER2-negative breast cancer treated with CKD 4/6 inhibitors. J Clin Oncol. 2017;35(suppl 31):209.
1. Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975-2014. Bethesda, MD: National Cancer Institute; 2017. https://seer.cancer.gov/csr/1975_2014/. Accessed April 3, 2018.
2. American Cancer Society. Breast cancer survival rates. https://www.cancer.org/cancer/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-survival-rates.html. Accessed April 3, 2018.
3. Wolff AC. CDK4 and CDK6 inhibition in breast cancer - a new standard. N Engl J Med. 2016; 375(20):1993-1994.
4. Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomized phase 2 study. Lancet Oncol. 2015;16(1):25-35.
5. Finn RS, Martin M, Rugo, HS et. al. Palbociclib and letrozole in advanced breast cancer. New Engl J Med. 2016;375:1925-1936
6. Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomized controlled trial. Lancet Oncol. 2016;17(4):425-439.
7. Turner NC, Ro J, André F, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373(3):209-219.
8. Dieras V, Rugo HS, Schnell P, et al. Long-term pooled safety analysis of palbociclib in combination with endocrine therapy for HR+/HR- advanced breast cancer [published online July 18, 2018]. Natl Cancer Inst. 2018;111.
9. Zaw M, Thein KZ, Tun A, et al. A systematic review and meta-analysis of randomized controlled trials to evaluate the risk of gastrointestinal and hepatic toxicities in patients with hormone receptor positive HER2-negative breast cancer treated with CKD 4/6 inhibitors. J Clin Oncol. 2017;35(suppl 31):209.