User login
FDA approves second drug for thrombocytopenia in liver disease
The Food and Drug Administration has approved lusutrombopag (Mulpleta) for the treatment of thrombocytopenia in patients with chronic liver disease who are scheduled to undergo medical or dental procedures. The drug is expected to be available in the United States in September 2018.
In May 2018, the FDA approved avatrombopag (Doptelet) as the first treatment for thrombocytopenia in this patient population.
The approval is based on a pair of phase 3, double-blind, placebo-controlled clinical trials – L-PLUS 1 and L-PLUS 2 (NCT02389621) – that included 312 patients who have chronic liver disease with severe thrombocytopenia. In L-PLUS 1, the primary endpoint was the percentage of patients who didn’t need platelet transfusions before the primary procedure was performed; this was met by 78% in the lusutrombopag group versus just 13% in the control group. In L-PLUS 2, the primary endpoint was the percentage of patients who didn’t need platelet transfusions before the procedure and didn’t require rescue therapy from randomization through 7 days post procedure; this was met by 65% of patients the lusutrombopag group versus 29% of patients the control group.
The most common adverse event was headache, and the recommended dosage for lusutrombopag is 3 mg orally once daily with or without food for 7 days. Lusutrombopag is not indicated for general platelet normalization in patients with chronic liver disease and thrombocytopenia. Full prescribing information and further information about the approval can be found on the FDA website.
Lusutrombopag was approved in Japan in 2015 for this indication and is slated to be evaluated by the European Medicines Agency in 2019. The drug is marketed by Shionogi.
The Food and Drug Administration has approved lusutrombopag (Mulpleta) for the treatment of thrombocytopenia in patients with chronic liver disease who are scheduled to undergo medical or dental procedures. The drug is expected to be available in the United States in September 2018.
In May 2018, the FDA approved avatrombopag (Doptelet) as the first treatment for thrombocytopenia in this patient population.
The approval is based on a pair of phase 3, double-blind, placebo-controlled clinical trials – L-PLUS 1 and L-PLUS 2 (NCT02389621) – that included 312 patients who have chronic liver disease with severe thrombocytopenia. In L-PLUS 1, the primary endpoint was the percentage of patients who didn’t need platelet transfusions before the primary procedure was performed; this was met by 78% in the lusutrombopag group versus just 13% in the control group. In L-PLUS 2, the primary endpoint was the percentage of patients who didn’t need platelet transfusions before the procedure and didn’t require rescue therapy from randomization through 7 days post procedure; this was met by 65% of patients the lusutrombopag group versus 29% of patients the control group.
The most common adverse event was headache, and the recommended dosage for lusutrombopag is 3 mg orally once daily with or without food for 7 days. Lusutrombopag is not indicated for general platelet normalization in patients with chronic liver disease and thrombocytopenia. Full prescribing information and further information about the approval can be found on the FDA website.
Lusutrombopag was approved in Japan in 2015 for this indication and is slated to be evaluated by the European Medicines Agency in 2019. The drug is marketed by Shionogi.
The Food and Drug Administration has approved lusutrombopag (Mulpleta) for the treatment of thrombocytopenia in patients with chronic liver disease who are scheduled to undergo medical or dental procedures. The drug is expected to be available in the United States in September 2018.
In May 2018, the FDA approved avatrombopag (Doptelet) as the first treatment for thrombocytopenia in this patient population.
The approval is based on a pair of phase 3, double-blind, placebo-controlled clinical trials – L-PLUS 1 and L-PLUS 2 (NCT02389621) – that included 312 patients who have chronic liver disease with severe thrombocytopenia. In L-PLUS 1, the primary endpoint was the percentage of patients who didn’t need platelet transfusions before the primary procedure was performed; this was met by 78% in the lusutrombopag group versus just 13% in the control group. In L-PLUS 2, the primary endpoint was the percentage of patients who didn’t need platelet transfusions before the procedure and didn’t require rescue therapy from randomization through 7 days post procedure; this was met by 65% of patients the lusutrombopag group versus 29% of patients the control group.
The most common adverse event was headache, and the recommended dosage for lusutrombopag is 3 mg orally once daily with or without food for 7 days. Lusutrombopag is not indicated for general platelet normalization in patients with chronic liver disease and thrombocytopenia. Full prescribing information and further information about the approval can be found on the FDA website.
Lusutrombopag was approved in Japan in 2015 for this indication and is slated to be evaluated by the European Medicines Agency in 2019. The drug is marketed by Shionogi.
Peer-comparison letters reduce physician quetiapine prescribing
A behavioral “nudge” intervention, targeting primary care prescribers who have particularly high off-label prescription rates of the antipsychotic quetiapine fumarate to older and disabled adults, has shown significant and long-lasting reductions in prescriptions.
A study, published Aug. 1 online by JAMA Psychiatry, looked at the effect of a “peer-comparison” letter, compared with a placebo letter, sent to 5,055 high quetiapine-prescribing primary care physicians in the Medicare program.
The letters said that the physicians’ quetiapine prescribing was extremely high, compared with their peers’ prescribing in the same state. Furthermore, the letters said the high-volume prescribers’ practices were under review because of concerns over medically unjustified use. They also encouraged the doctors to review their prescribing habits, while the placebo letter simply discussed an unrelated Medicare enrollment regulation.
Over the 9-month study, researchers saw a significant 11.1% reduction in the total number days of quetiapine prescribing among physicians who received the intervention letter, compared with those who received the control letter (95% confidence interval, –13.1 to –9.2 days; P less than .001; adjusted difference, –319 days; 95% CI, –374 to –263 days; P less than .001). At 2 years, the cumulative reduction was 15.6% fewer days in the intervention group (95% CI, –18.1 to –13.0; P less than .001), compared with the control group.
The study also used Medicare data to look at the impact on patients and found that individuals whose physicians were in the intervention arm had 3.9% fewer days of quetiapine usage over the 9 months (95% CI, –5.0 to –2.9; P less than 0.11), compared with those in the control arm. The reduction was even greater among patients whose indications for quetiapine were deemed to be of “low value,” as opposed to those who were prescribed for guideline-concordant indications, reported Adam Sacarny, PhD, of Columbia University, New York, and his coauthors.
When researchers looked in more detail at the reductions in prescriptions for guideline-concordant patients, they found that much of this was offset by prescriptions from other prescribers; in particular, physicians with psychiatric specialization or other study prescribers who the patient had not previously received a quetiapine prescription from.
The authors noted that the reductions for guideline-concordant patients could have negative effects if prescribers were reducing their quetiapine prescriptions indiscriminately.
“If this represented a harmful change for patients, we may have expected to see higher rates of adverse outcomes in the guideline-concordant patient group as prescribing rates decreased,” wrote Dr. Sacarny, and his coauthors. “However, if anything, guideline-concordant patients experienced lower rates of hospital encounters after the intervention.”
The study did not see any evidence of substitution to other antipsychotics, nor was any significant difference found in hospital use or mortality between the two arms of the study.
Dr. Sacarny and his coauthors cited several limitations. One is that the analysis looked at prescribing covered by Medicare Part D only. Nevertheless, they said, the results show the impact that peer comparison letters can have on prescribing patterns.
“These results provide encouraging evidence that high prescribers of antipsychotics can decrease quetiapine prescribing, without adverse clinical consequences, in response to a letter highlighting their overall high rates of prescribing,” the authors wrote.
The study was supported by the Robert Wood Johnson Foundation, Abdul Latif Jameel Poverty Action Lab North America, and the Laura and John Arnold Foundation. No conflicts of interest were reported.
SOURCE: Sacarny A et al. JAMA Psychiatry. 2018 Aug 1. doi: 10.1001/jamapsychiatry.2018.1867.
A behavioral “nudge” intervention, targeting primary care prescribers who have particularly high off-label prescription rates of the antipsychotic quetiapine fumarate to older and disabled adults, has shown significant and long-lasting reductions in prescriptions.
A study, published Aug. 1 online by JAMA Psychiatry, looked at the effect of a “peer-comparison” letter, compared with a placebo letter, sent to 5,055 high quetiapine-prescribing primary care physicians in the Medicare program.
The letters said that the physicians’ quetiapine prescribing was extremely high, compared with their peers’ prescribing in the same state. Furthermore, the letters said the high-volume prescribers’ practices were under review because of concerns over medically unjustified use. They also encouraged the doctors to review their prescribing habits, while the placebo letter simply discussed an unrelated Medicare enrollment regulation.
Over the 9-month study, researchers saw a significant 11.1% reduction in the total number days of quetiapine prescribing among physicians who received the intervention letter, compared with those who received the control letter (95% confidence interval, –13.1 to –9.2 days; P less than .001; adjusted difference, –319 days; 95% CI, –374 to –263 days; P less than .001). At 2 years, the cumulative reduction was 15.6% fewer days in the intervention group (95% CI, –18.1 to –13.0; P less than .001), compared with the control group.
The study also used Medicare data to look at the impact on patients and found that individuals whose physicians were in the intervention arm had 3.9% fewer days of quetiapine usage over the 9 months (95% CI, –5.0 to –2.9; P less than 0.11), compared with those in the control arm. The reduction was even greater among patients whose indications for quetiapine were deemed to be of “low value,” as opposed to those who were prescribed for guideline-concordant indications, reported Adam Sacarny, PhD, of Columbia University, New York, and his coauthors.
When researchers looked in more detail at the reductions in prescriptions for guideline-concordant patients, they found that much of this was offset by prescriptions from other prescribers; in particular, physicians with psychiatric specialization or other study prescribers who the patient had not previously received a quetiapine prescription from.
The authors noted that the reductions for guideline-concordant patients could have negative effects if prescribers were reducing their quetiapine prescriptions indiscriminately.
“If this represented a harmful change for patients, we may have expected to see higher rates of adverse outcomes in the guideline-concordant patient group as prescribing rates decreased,” wrote Dr. Sacarny, and his coauthors. “However, if anything, guideline-concordant patients experienced lower rates of hospital encounters after the intervention.”
The study did not see any evidence of substitution to other antipsychotics, nor was any significant difference found in hospital use or mortality between the two arms of the study.
Dr. Sacarny and his coauthors cited several limitations. One is that the analysis looked at prescribing covered by Medicare Part D only. Nevertheless, they said, the results show the impact that peer comparison letters can have on prescribing patterns.
“These results provide encouraging evidence that high prescribers of antipsychotics can decrease quetiapine prescribing, without adverse clinical consequences, in response to a letter highlighting their overall high rates of prescribing,” the authors wrote.
The study was supported by the Robert Wood Johnson Foundation, Abdul Latif Jameel Poverty Action Lab North America, and the Laura and John Arnold Foundation. No conflicts of interest were reported.
SOURCE: Sacarny A et al. JAMA Psychiatry. 2018 Aug 1. doi: 10.1001/jamapsychiatry.2018.1867.
A behavioral “nudge” intervention, targeting primary care prescribers who have particularly high off-label prescription rates of the antipsychotic quetiapine fumarate to older and disabled adults, has shown significant and long-lasting reductions in prescriptions.
A study, published Aug. 1 online by JAMA Psychiatry, looked at the effect of a “peer-comparison” letter, compared with a placebo letter, sent to 5,055 high quetiapine-prescribing primary care physicians in the Medicare program.
The letters said that the physicians’ quetiapine prescribing was extremely high, compared with their peers’ prescribing in the same state. Furthermore, the letters said the high-volume prescribers’ practices were under review because of concerns over medically unjustified use. They also encouraged the doctors to review their prescribing habits, while the placebo letter simply discussed an unrelated Medicare enrollment regulation.
Over the 9-month study, researchers saw a significant 11.1% reduction in the total number days of quetiapine prescribing among physicians who received the intervention letter, compared with those who received the control letter (95% confidence interval, –13.1 to –9.2 days; P less than .001; adjusted difference, –319 days; 95% CI, –374 to –263 days; P less than .001). At 2 years, the cumulative reduction was 15.6% fewer days in the intervention group (95% CI, –18.1 to –13.0; P less than .001), compared with the control group.
The study also used Medicare data to look at the impact on patients and found that individuals whose physicians were in the intervention arm had 3.9% fewer days of quetiapine usage over the 9 months (95% CI, –5.0 to –2.9; P less than 0.11), compared with those in the control arm. The reduction was even greater among patients whose indications for quetiapine were deemed to be of “low value,” as opposed to those who were prescribed for guideline-concordant indications, reported Adam Sacarny, PhD, of Columbia University, New York, and his coauthors.
When researchers looked in more detail at the reductions in prescriptions for guideline-concordant patients, they found that much of this was offset by prescriptions from other prescribers; in particular, physicians with psychiatric specialization or other study prescribers who the patient had not previously received a quetiapine prescription from.
The authors noted that the reductions for guideline-concordant patients could have negative effects if prescribers were reducing their quetiapine prescriptions indiscriminately.
“If this represented a harmful change for patients, we may have expected to see higher rates of adverse outcomes in the guideline-concordant patient group as prescribing rates decreased,” wrote Dr. Sacarny, and his coauthors. “However, if anything, guideline-concordant patients experienced lower rates of hospital encounters after the intervention.”
The study did not see any evidence of substitution to other antipsychotics, nor was any significant difference found in hospital use or mortality between the two arms of the study.
Dr. Sacarny and his coauthors cited several limitations. One is that the analysis looked at prescribing covered by Medicare Part D only. Nevertheless, they said, the results show the impact that peer comparison letters can have on prescribing patterns.
“These results provide encouraging evidence that high prescribers of antipsychotics can decrease quetiapine prescribing, without adverse clinical consequences, in response to a letter highlighting their overall high rates of prescribing,” the authors wrote.
The study was supported by the Robert Wood Johnson Foundation, Abdul Latif Jameel Poverty Action Lab North America, and the Laura and John Arnold Foundation. No conflicts of interest were reported.
SOURCE: Sacarny A et al. JAMA Psychiatry. 2018 Aug 1. doi: 10.1001/jamapsychiatry.2018.1867.
FROM JAMA PSYCHIATRY
Key clinical point: Letter intervention significantly reduces quetiapine prescription rates by physicians.
Major finding: Peer-comparison letters achieved an 11.1% reduction in days of quetiapine prescribed (95% confidence interval, –13.1 to –9.2 days; P less than .001; adjusted difference, –319 days; 95% CI, –374 to –263 days; P less than .001).
Study details: Randomized controlled trial in 5,055 high quetiapine-prescribing rates by primary care physicians.
Disclosures: The study was supported by the Robert Wood Johnson Foundation, Abdul Latif Jameel Poverty Action Lab North America, and the Laura and John Arnold Foundation. No conflicts of interest were declared.
Source: Sacarny A et al. JAMA Psychiatry. 2018 Aug 1. doi: 10.1001/jamapsychiatry.2018.1867.
Study models surveillance interval after ablation of Barrett’s esophagus
Surveillance endoscopy should be spaced at 1 and 3 years after complete eradication of low-grade intestinal metaplasia and at 3 months, 6 months, 1 year, and then annually in cases of high-grade dysplasia, researchers wrote in Gastroenterology.
This “much-attenuated schedule of surveillance endoscopy would provide protection from invasive adenocarcinoma,” wrote Cary C. Cotton, MD, of the department of medicine at the University of North Carolina at Chapel Hill, with his associates. Following this schedule could prevent unnecessary endoscopies while still detecting unresectable cancers at rates under 1 in 1,000 endoscopies, they added.
Barrett’s esophagus recurs in at least one in four cases after successful radiofrequency ablation, the researchers noted. Therefore, surveillance endoscopy is recommended after complete eradication of intestinal metaplasia, but only expert opinion informs the frequency of surveillance. This study modeled and validated rates of neoplastic recurrence by using data from the United States Radiofrequency Ablation Registry during 2004-2013 and from the United Kingdom National Halo Registry during 2007-2015.
In line with prior research, predictors of neoplastic recurrence included baseline histologic grade, age, sex, endoscopic mucosal resection, and baseline length of Barrett’s esophagus, the researchers said. The strongest predictor of recurrence was the most severe histologic grade identified before complete eradication of intestinal metaplasia. After controlling for covariates, a model based only on most-severe baseline histology predicted neoplastic recurrence with a C statistic of 0.892 (95% confidence interval, 0.86-0.92) in the United States Radiofrequency Ablation Registry.
Dysplasia recurred at similar rates regardless of whether patients had nondysplastic Barrett’s esophagus or indeterminate dysplasia. Recurrence rates also were similar between patients with high-grade dysplasia and patients with intramucosal carcinoma. Thus, the researchers identified three risk groups based on most-severe baseline histology: dysplastic Barrett’s esophagus or indefinite for dysplasia, low-grade dysplasia, and high-grade lesions or intramucosal adenocarcinoma.
The annual rate of any-grade neoplastic recurrence was 0.19% in the lowest-risk group, 1.98% in the intermediate-risk group, and 5.93% in the highest-risk group. “In the higher-risk groups, neoplastic recurrence occurred at a higher rate in the first year, but at a constant estimated rate thereafter,” the investigators wrote. Among 114 initial cases of neoplastic recurrence, 1.8% were esophageal adenocarcinoma and another 1.8% of patients developed esophageal adenocarcinoma within 6 months.
The researchers then modeled surveillance intervals by assuming a 2.9% rate of neoplastic recurrence per visit, which yielded a 0.1% rate of invasive adenocarcinoma. “This level of risk tolerance was chosen so that the risk of complications from surveillance endoscopy – approximately one in 1,000 in this patient population – would roughly approximate the risk of invasive carcinoma discovered at the exam,” they wrote. For patients at higher risk of endoscopic complications, they set the rate of neoplastic recurrence at 5.7%, which yielded a 0.2% rate of invasive cancer. “As would be expected, the higher the risk tolerance, the longer the period between endoscopic surveillance intervals.”
Based on the model, the researchers proposed surveillance intervals of 1 year, followed by 3 years, followed by more than 5 years for patients with completely eradicated low-grade dysplasia. For cases of high-grade dysplasia or carcinoma in situ, the proposed surveillance intervals were 3 months, 6 months, 1 year, and then annually. The model also performed well when applied to data from the United Kingdom National Halo Registry, the investigators said, noting that their approach was the first to directly establish an evidence base for surveillance practices in Barrett’s esophagus.
The National Institutes of Health and Barrx/Covidien/Medtronic provided funding. Dr. Cotton reported having no relevant disclosures. Three coinvestigators disclosed ties to Pentax Europe, Medtronic, Beamline Diagnostics, C2 Therapeutics, Boston Scientific, and CDx Medical.
SOURCE: Cotton CC et al. Gastroenterology. 2018 Apr 12. doi: 10.1053/j.gastro.2018.04.011.
Surveillance endoscopy should be spaced at 1 and 3 years after complete eradication of low-grade intestinal metaplasia and at 3 months, 6 months, 1 year, and then annually in cases of high-grade dysplasia, researchers wrote in Gastroenterology.
This “much-attenuated schedule of surveillance endoscopy would provide protection from invasive adenocarcinoma,” wrote Cary C. Cotton, MD, of the department of medicine at the University of North Carolina at Chapel Hill, with his associates. Following this schedule could prevent unnecessary endoscopies while still detecting unresectable cancers at rates under 1 in 1,000 endoscopies, they added.
Barrett’s esophagus recurs in at least one in four cases after successful radiofrequency ablation, the researchers noted. Therefore, surveillance endoscopy is recommended after complete eradication of intestinal metaplasia, but only expert opinion informs the frequency of surveillance. This study modeled and validated rates of neoplastic recurrence by using data from the United States Radiofrequency Ablation Registry during 2004-2013 and from the United Kingdom National Halo Registry during 2007-2015.
In line with prior research, predictors of neoplastic recurrence included baseline histologic grade, age, sex, endoscopic mucosal resection, and baseline length of Barrett’s esophagus, the researchers said. The strongest predictor of recurrence was the most severe histologic grade identified before complete eradication of intestinal metaplasia. After controlling for covariates, a model based only on most-severe baseline histology predicted neoplastic recurrence with a C statistic of 0.892 (95% confidence interval, 0.86-0.92) in the United States Radiofrequency Ablation Registry.
Dysplasia recurred at similar rates regardless of whether patients had nondysplastic Barrett’s esophagus or indeterminate dysplasia. Recurrence rates also were similar between patients with high-grade dysplasia and patients with intramucosal carcinoma. Thus, the researchers identified three risk groups based on most-severe baseline histology: dysplastic Barrett’s esophagus or indefinite for dysplasia, low-grade dysplasia, and high-grade lesions or intramucosal adenocarcinoma.
The annual rate of any-grade neoplastic recurrence was 0.19% in the lowest-risk group, 1.98% in the intermediate-risk group, and 5.93% in the highest-risk group. “In the higher-risk groups, neoplastic recurrence occurred at a higher rate in the first year, but at a constant estimated rate thereafter,” the investigators wrote. Among 114 initial cases of neoplastic recurrence, 1.8% were esophageal adenocarcinoma and another 1.8% of patients developed esophageal adenocarcinoma within 6 months.
The researchers then modeled surveillance intervals by assuming a 2.9% rate of neoplastic recurrence per visit, which yielded a 0.1% rate of invasive adenocarcinoma. “This level of risk tolerance was chosen so that the risk of complications from surveillance endoscopy – approximately one in 1,000 in this patient population – would roughly approximate the risk of invasive carcinoma discovered at the exam,” they wrote. For patients at higher risk of endoscopic complications, they set the rate of neoplastic recurrence at 5.7%, which yielded a 0.2% rate of invasive cancer. “As would be expected, the higher the risk tolerance, the longer the period between endoscopic surveillance intervals.”
Based on the model, the researchers proposed surveillance intervals of 1 year, followed by 3 years, followed by more than 5 years for patients with completely eradicated low-grade dysplasia. For cases of high-grade dysplasia or carcinoma in situ, the proposed surveillance intervals were 3 months, 6 months, 1 year, and then annually. The model also performed well when applied to data from the United Kingdom National Halo Registry, the investigators said, noting that their approach was the first to directly establish an evidence base for surveillance practices in Barrett’s esophagus.
The National Institutes of Health and Barrx/Covidien/Medtronic provided funding. Dr. Cotton reported having no relevant disclosures. Three coinvestigators disclosed ties to Pentax Europe, Medtronic, Beamline Diagnostics, C2 Therapeutics, Boston Scientific, and CDx Medical.
SOURCE: Cotton CC et al. Gastroenterology. 2018 Apr 12. doi: 10.1053/j.gastro.2018.04.011.
Surveillance endoscopy should be spaced at 1 and 3 years after complete eradication of low-grade intestinal metaplasia and at 3 months, 6 months, 1 year, and then annually in cases of high-grade dysplasia, researchers wrote in Gastroenterology.
This “much-attenuated schedule of surveillance endoscopy would provide protection from invasive adenocarcinoma,” wrote Cary C. Cotton, MD, of the department of medicine at the University of North Carolina at Chapel Hill, with his associates. Following this schedule could prevent unnecessary endoscopies while still detecting unresectable cancers at rates under 1 in 1,000 endoscopies, they added.
Barrett’s esophagus recurs in at least one in four cases after successful radiofrequency ablation, the researchers noted. Therefore, surveillance endoscopy is recommended after complete eradication of intestinal metaplasia, but only expert opinion informs the frequency of surveillance. This study modeled and validated rates of neoplastic recurrence by using data from the United States Radiofrequency Ablation Registry during 2004-2013 and from the United Kingdom National Halo Registry during 2007-2015.
In line with prior research, predictors of neoplastic recurrence included baseline histologic grade, age, sex, endoscopic mucosal resection, and baseline length of Barrett’s esophagus, the researchers said. The strongest predictor of recurrence was the most severe histologic grade identified before complete eradication of intestinal metaplasia. After controlling for covariates, a model based only on most-severe baseline histology predicted neoplastic recurrence with a C statistic of 0.892 (95% confidence interval, 0.86-0.92) in the United States Radiofrequency Ablation Registry.
Dysplasia recurred at similar rates regardless of whether patients had nondysplastic Barrett’s esophagus or indeterminate dysplasia. Recurrence rates also were similar between patients with high-grade dysplasia and patients with intramucosal carcinoma. Thus, the researchers identified three risk groups based on most-severe baseline histology: dysplastic Barrett’s esophagus or indefinite for dysplasia, low-grade dysplasia, and high-grade lesions or intramucosal adenocarcinoma.
The annual rate of any-grade neoplastic recurrence was 0.19% in the lowest-risk group, 1.98% in the intermediate-risk group, and 5.93% in the highest-risk group. “In the higher-risk groups, neoplastic recurrence occurred at a higher rate in the first year, but at a constant estimated rate thereafter,” the investigators wrote. Among 114 initial cases of neoplastic recurrence, 1.8% were esophageal adenocarcinoma and another 1.8% of patients developed esophageal adenocarcinoma within 6 months.
The researchers then modeled surveillance intervals by assuming a 2.9% rate of neoplastic recurrence per visit, which yielded a 0.1% rate of invasive adenocarcinoma. “This level of risk tolerance was chosen so that the risk of complications from surveillance endoscopy – approximately one in 1,000 in this patient population – would roughly approximate the risk of invasive carcinoma discovered at the exam,” they wrote. For patients at higher risk of endoscopic complications, they set the rate of neoplastic recurrence at 5.7%, which yielded a 0.2% rate of invasive cancer. “As would be expected, the higher the risk tolerance, the longer the period between endoscopic surveillance intervals.”
Based on the model, the researchers proposed surveillance intervals of 1 year, followed by 3 years, followed by more than 5 years for patients with completely eradicated low-grade dysplasia. For cases of high-grade dysplasia or carcinoma in situ, the proposed surveillance intervals were 3 months, 6 months, 1 year, and then annually. The model also performed well when applied to data from the United Kingdom National Halo Registry, the investigators said, noting that their approach was the first to directly establish an evidence base for surveillance practices in Barrett’s esophagus.
The National Institutes of Health and Barrx/Covidien/Medtronic provided funding. Dr. Cotton reported having no relevant disclosures. Three coinvestigators disclosed ties to Pentax Europe, Medtronic, Beamline Diagnostics, C2 Therapeutics, Boston Scientific, and CDx Medical.
SOURCE: Cotton CC et al. Gastroenterology. 2018 Apr 12. doi: 10.1053/j.gastro.2018.04.011.
FROM GASTROENTEROLOGY
Key clinical point: Baseline histologic grade was the most important predictor of recurrence after radiofrequency ablation of Barrett’s esophagus.
Major finding: The proposed surveillance intervals were 1 year, followed by 3 years, followed by more than 5 years for patients with completely eradicated low-grade dysplasia. For cases of high-grade dysplasia or carcinoma in situ, the proposed surveillance intervals were 3 months, 6 months, 1 year, and then annually.
Study details: An analysis of data from the United States Radiofrequency Ablation Registry and the United Kingdom National Halo Registry.
Disclosures: The National Institutes of Health and Barrx/Covidien/Medtronic provided funding. Dr. Cotton reported having no relevant disclosures. Three coinvestigators disclosed ties to Pentax Europe, Medtronic, Beamline Diagnostics, C2 Therapeutics, Boston Scientific, and CDx Medical.
Source: Cotton CC et al. Gastroenterology. 2018 Apr 12. doi: 10.1053/j.gastro.2018.04.011.
Laterality Predicts Endovascular Treatment in Mild to Moderate Strokes
The NIH Stroke Scale score is biased toward dominant-hemisphere strokes, a researcher says.
LOS ANGELES—Laterality is an independent predictor of endovascular thrombectomy in patients with an NIH Stroke Scale (NIHSS) score of 12 or lower, according to research described at the 70th Annual Meeting of the American Academy of Neurology. “There may be a need for a laterality-based NIHSS score or at least a conscious effort to look for right-hemisphere strokes with a large-vessel occlusion,” said Shashvat Desai, MD, a neurology trainee at the University of Pittsburgh.
Endovascular thrombectomy is the standard of care for acute ischemic stroke resulting from a large-vessel occlusion. Among the eligibility criteria for thrombectomy is an NIHSS score of 6 or higher.
“The NIHSS score is biased and will allow a higher score for dominant-hemisphere strokes, as compared with nondominant-hemisphere strokes,” said Dr. Desai. “Seven points on this scale are directly related to language, which is a dominant-hemisphere function, and only two points are for neglect, which occurs during nondominant-hemisphere strokes.”
A Retrospective Data Analysis
To test their hypothesis that NIHSS score hemispheric lateralization bias affects stroke patient selection for acute endovascular thrombectomy, Dr. Desai and colleagues analyzed data from consecutive patients with acute ischemic stroke resulting from large-vessel occlusion who were admitted to a comprehensive stroke center between June 2015 and December 2016. Eligible patients were identified within 24 hours of when they were last known to be well, were functionally independent at baseline, had an Alberta Stroke Program Early CT (ASPECT) score of 6 or higher, and had an NIHSS score of 6 or higher. The investigators included 211 patients in their study. They examined variables such as age, NIHSS score, occlusion location, baseline modified Rankin Scale (mRS) score, time to presentation, and treatment received. When they separated patients with left-hemisphere strokes from those with right-hemisphere strokes, they found that the two groups were well matched in terms of age, gender, and site of occlusion. The median admission NIHSS score was 19 for left-hemisphere strokes and 15 for right-hemisphere strokes, and the difference between groups was statistically significant.
Thrombectomy Was More Common for Left-Hemisphere Strokes
Endovascular thrombectomy was performed for 87% of left-hemisphere strokes, compared with 78% of right-hemisphere strokes, and the difference between groups was statistically significant. When the researchers examined only participants with a low NIHSS score (ie, 12 or lower), they found that thrombectomy was performed in 81% of left-hemisphere strokes, compared with 52% of right-hemisphere strokes. The difference in the rate of treatment was statistically significant. Among patients with a high NIHSS score (ie, higher than 12), endovascular therapy was performed in 88% of participants, regardless of laterality.
A regression analysis that included variables such as age, sex, occlusion location, time to presentation, ASPECT score, IV t-PA, and laterality indicated that laterality was the sole and independent predictor of receiving endovascular thrombectomy in patients with low NIHSS score. “The preservation of language in right-hemisphere strokes with low NIHSS score reduces the chances of receiving endovascular thrombectomy,” said Dr. Desai.
Among patients with low NIHSS scores who received thrombectomy, the rate of good outcome (ie, an mRS score of 0 to 2) at three months was 88% for left-hemisphere strokes and 71% for right-hemisphere strokes. This difference was not statistically significant. Among all patients with low NIHSS scores, irrespective of treatment, 86% of left-hemisphere strokes had a good outcome, compared with 48% of right-hemisphere strokes. This difference was statistically significant.
—Erik Greb
The NIH Stroke Scale score is biased toward dominant-hemisphere strokes, a researcher says.
The NIH Stroke Scale score is biased toward dominant-hemisphere strokes, a researcher says.
LOS ANGELES—Laterality is an independent predictor of endovascular thrombectomy in patients with an NIH Stroke Scale (NIHSS) score of 12 or lower, according to research described at the 70th Annual Meeting of the American Academy of Neurology. “There may be a need for a laterality-based NIHSS score or at least a conscious effort to look for right-hemisphere strokes with a large-vessel occlusion,” said Shashvat Desai, MD, a neurology trainee at the University of Pittsburgh.
Endovascular thrombectomy is the standard of care for acute ischemic stroke resulting from a large-vessel occlusion. Among the eligibility criteria for thrombectomy is an NIHSS score of 6 or higher.
“The NIHSS score is biased and will allow a higher score for dominant-hemisphere strokes, as compared with nondominant-hemisphere strokes,” said Dr. Desai. “Seven points on this scale are directly related to language, which is a dominant-hemisphere function, and only two points are for neglect, which occurs during nondominant-hemisphere strokes.”
A Retrospective Data Analysis
To test their hypothesis that NIHSS score hemispheric lateralization bias affects stroke patient selection for acute endovascular thrombectomy, Dr. Desai and colleagues analyzed data from consecutive patients with acute ischemic stroke resulting from large-vessel occlusion who were admitted to a comprehensive stroke center between June 2015 and December 2016. Eligible patients were identified within 24 hours of when they were last known to be well, were functionally independent at baseline, had an Alberta Stroke Program Early CT (ASPECT) score of 6 or higher, and had an NIHSS score of 6 or higher. The investigators included 211 patients in their study. They examined variables such as age, NIHSS score, occlusion location, baseline modified Rankin Scale (mRS) score, time to presentation, and treatment received. When they separated patients with left-hemisphere strokes from those with right-hemisphere strokes, they found that the two groups were well matched in terms of age, gender, and site of occlusion. The median admission NIHSS score was 19 for left-hemisphere strokes and 15 for right-hemisphere strokes, and the difference between groups was statistically significant.
Thrombectomy Was More Common for Left-Hemisphere Strokes
Endovascular thrombectomy was performed for 87% of left-hemisphere strokes, compared with 78% of right-hemisphere strokes, and the difference between groups was statistically significant. When the researchers examined only participants with a low NIHSS score (ie, 12 or lower), they found that thrombectomy was performed in 81% of left-hemisphere strokes, compared with 52% of right-hemisphere strokes. The difference in the rate of treatment was statistically significant. Among patients with a high NIHSS score (ie, higher than 12), endovascular therapy was performed in 88% of participants, regardless of laterality.
A regression analysis that included variables such as age, sex, occlusion location, time to presentation, ASPECT score, IV t-PA, and laterality indicated that laterality was the sole and independent predictor of receiving endovascular thrombectomy in patients with low NIHSS score. “The preservation of language in right-hemisphere strokes with low NIHSS score reduces the chances of receiving endovascular thrombectomy,” said Dr. Desai.
Among patients with low NIHSS scores who received thrombectomy, the rate of good outcome (ie, an mRS score of 0 to 2) at three months was 88% for left-hemisphere strokes and 71% for right-hemisphere strokes. This difference was not statistically significant. Among all patients with low NIHSS scores, irrespective of treatment, 86% of left-hemisphere strokes had a good outcome, compared with 48% of right-hemisphere strokes. This difference was statistically significant.
—Erik Greb
LOS ANGELES—Laterality is an independent predictor of endovascular thrombectomy in patients with an NIH Stroke Scale (NIHSS) score of 12 or lower, according to research described at the 70th Annual Meeting of the American Academy of Neurology. “There may be a need for a laterality-based NIHSS score or at least a conscious effort to look for right-hemisphere strokes with a large-vessel occlusion,” said Shashvat Desai, MD, a neurology trainee at the University of Pittsburgh.
Endovascular thrombectomy is the standard of care for acute ischemic stroke resulting from a large-vessel occlusion. Among the eligibility criteria for thrombectomy is an NIHSS score of 6 or higher.
“The NIHSS score is biased and will allow a higher score for dominant-hemisphere strokes, as compared with nondominant-hemisphere strokes,” said Dr. Desai. “Seven points on this scale are directly related to language, which is a dominant-hemisphere function, and only two points are for neglect, which occurs during nondominant-hemisphere strokes.”
A Retrospective Data Analysis
To test their hypothesis that NIHSS score hemispheric lateralization bias affects stroke patient selection for acute endovascular thrombectomy, Dr. Desai and colleagues analyzed data from consecutive patients with acute ischemic stroke resulting from large-vessel occlusion who were admitted to a comprehensive stroke center between June 2015 and December 2016. Eligible patients were identified within 24 hours of when they were last known to be well, were functionally independent at baseline, had an Alberta Stroke Program Early CT (ASPECT) score of 6 or higher, and had an NIHSS score of 6 or higher. The investigators included 211 patients in their study. They examined variables such as age, NIHSS score, occlusion location, baseline modified Rankin Scale (mRS) score, time to presentation, and treatment received. When they separated patients with left-hemisphere strokes from those with right-hemisphere strokes, they found that the two groups were well matched in terms of age, gender, and site of occlusion. The median admission NIHSS score was 19 for left-hemisphere strokes and 15 for right-hemisphere strokes, and the difference between groups was statistically significant.
Thrombectomy Was More Common for Left-Hemisphere Strokes
Endovascular thrombectomy was performed for 87% of left-hemisphere strokes, compared with 78% of right-hemisphere strokes, and the difference between groups was statistically significant. When the researchers examined only participants with a low NIHSS score (ie, 12 or lower), they found that thrombectomy was performed in 81% of left-hemisphere strokes, compared with 52% of right-hemisphere strokes. The difference in the rate of treatment was statistically significant. Among patients with a high NIHSS score (ie, higher than 12), endovascular therapy was performed in 88% of participants, regardless of laterality.
A regression analysis that included variables such as age, sex, occlusion location, time to presentation, ASPECT score, IV t-PA, and laterality indicated that laterality was the sole and independent predictor of receiving endovascular thrombectomy in patients with low NIHSS score. “The preservation of language in right-hemisphere strokes with low NIHSS score reduces the chances of receiving endovascular thrombectomy,” said Dr. Desai.
Among patients with low NIHSS scores who received thrombectomy, the rate of good outcome (ie, an mRS score of 0 to 2) at three months was 88% for left-hemisphere strokes and 71% for right-hemisphere strokes. This difference was not statistically significant. Among all patients with low NIHSS scores, irrespective of treatment, 86% of left-hemisphere strokes had a good outcome, compared with 48% of right-hemisphere strokes. This difference was statistically significant.
—Erik Greb
Single Dose of Rimegepant Shows Durable Effects in Acute Migraine
Rimegepant may be a novel approach to the treatment of acute migraine.
SAN FRANCISCO—Among patients with acute migraine, significant and durable clinical effects were seen with a single dose of rimegepant across multiple outcome measures, including pain freedom, freedom from most bothersome symptom, pain relief, and recovery of normal function, according to data presented at the 60th Annual Scientific Meeting of the American Headache Society. Rimegepant (75 mg oral tablet) demonstrated favorable tolerability and safety, including a liver safety profile, similar to placebo. “These clinically meaningful results complement the benefits seen in an identical phase III study and a previous phase IIb study,” said Richard B. Lipton, MD, Edwin S. Lowe Chair in Neurology at Albert Einstein College of Medicine in New York, and colleagues. “Rimegepant may ultimately offer patients a novel approach for the acute treatment of migraine.”
Dr. Lipton and colleagues conducted a double-blind, randomized, placebo-controlled trial to compare the efficacy, safety, and tolerability of the calcitonin gene-related peptide (CGRP) receptor antagonist rimegepant (75 mg oral tablet) with placebo in the acute treatment of migraine in adults.
The study included adults 18 or older with at least a one-year history of migraine according to ICHD 3-beta criteria. Following a three- to 28-day screening period, subjects were randomized to receive 75 mg of rimegepant or placebo and instructed to treat a single migraine attack with one dose of the blinded study drug (ie, rimegepant or placebo) when headache pain reached moderate or severe intensity. The coprimary end points were pain freedom at two hours postdose and freedom from the most bothersome symptom at two hours postdose. Safety assessments included adverse events, ECGs, vital signs, physical measurements, and routine laboratory tests, including assessment of liver function.
In total, 1,162 subjects were randomized to receive rimegepant (n = 582) or placebo (n = 580), and 1,084 were evaluated for efficacy (rimegepant [n = 543], placebo [n = 541]). Subjects had a mean age of 41.6, 85.5% were female, and participants by history averaged 4.7 attacks per month. At two hours postdose, rimegepant-treated patients had higher pain-free rates than placebo-treated patients did (19.2% vs 14.2%, respectively), were more likely to be free of their most bothersome symptom (36.6% vs 27.7%, respectively), and had higher rates of pain relief (56.0% vs 45.7%, respectively).
A single dose of rimegepant, without the use of rescue medication, demonstrated superiority versus placebo for sustained pain freedom and pain relief from two through 48 hours postdose. On a measure of functional disability, a greater proportion of rimegepant-treated patients achieved normal function at two hours. The safety and tolerability profiles of rimegepant were similar to those of placebo. The most common adverse events in the rimegepant and placebo groups were nausea (0.9% [5 of 546] vs 1.1% [6 of 549], respectively) and dizziness (0.7% [4 of 546] vs 0.4% [2 of 549], respectively).
Rimegepant may be a novel approach to the treatment of acute migraine.
Rimegepant may be a novel approach to the treatment of acute migraine.
SAN FRANCISCO—Among patients with acute migraine, significant and durable clinical effects were seen with a single dose of rimegepant across multiple outcome measures, including pain freedom, freedom from most bothersome symptom, pain relief, and recovery of normal function, according to data presented at the 60th Annual Scientific Meeting of the American Headache Society. Rimegepant (75 mg oral tablet) demonstrated favorable tolerability and safety, including a liver safety profile, similar to placebo. “These clinically meaningful results complement the benefits seen in an identical phase III study and a previous phase IIb study,” said Richard B. Lipton, MD, Edwin S. Lowe Chair in Neurology at Albert Einstein College of Medicine in New York, and colleagues. “Rimegepant may ultimately offer patients a novel approach for the acute treatment of migraine.”
Dr. Lipton and colleagues conducted a double-blind, randomized, placebo-controlled trial to compare the efficacy, safety, and tolerability of the calcitonin gene-related peptide (CGRP) receptor antagonist rimegepant (75 mg oral tablet) with placebo in the acute treatment of migraine in adults.
The study included adults 18 or older with at least a one-year history of migraine according to ICHD 3-beta criteria. Following a three- to 28-day screening period, subjects were randomized to receive 75 mg of rimegepant or placebo and instructed to treat a single migraine attack with one dose of the blinded study drug (ie, rimegepant or placebo) when headache pain reached moderate or severe intensity. The coprimary end points were pain freedom at two hours postdose and freedom from the most bothersome symptom at two hours postdose. Safety assessments included adverse events, ECGs, vital signs, physical measurements, and routine laboratory tests, including assessment of liver function.
In total, 1,162 subjects were randomized to receive rimegepant (n = 582) or placebo (n = 580), and 1,084 were evaluated for efficacy (rimegepant [n = 543], placebo [n = 541]). Subjects had a mean age of 41.6, 85.5% were female, and participants by history averaged 4.7 attacks per month. At two hours postdose, rimegepant-treated patients had higher pain-free rates than placebo-treated patients did (19.2% vs 14.2%, respectively), were more likely to be free of their most bothersome symptom (36.6% vs 27.7%, respectively), and had higher rates of pain relief (56.0% vs 45.7%, respectively).
A single dose of rimegepant, without the use of rescue medication, demonstrated superiority versus placebo for sustained pain freedom and pain relief from two through 48 hours postdose. On a measure of functional disability, a greater proportion of rimegepant-treated patients achieved normal function at two hours. The safety and tolerability profiles of rimegepant were similar to those of placebo. The most common adverse events in the rimegepant and placebo groups were nausea (0.9% [5 of 546] vs 1.1% [6 of 549], respectively) and dizziness (0.7% [4 of 546] vs 0.4% [2 of 549], respectively).
SAN FRANCISCO—Among patients with acute migraine, significant and durable clinical effects were seen with a single dose of rimegepant across multiple outcome measures, including pain freedom, freedom from most bothersome symptom, pain relief, and recovery of normal function, according to data presented at the 60th Annual Scientific Meeting of the American Headache Society. Rimegepant (75 mg oral tablet) demonstrated favorable tolerability and safety, including a liver safety profile, similar to placebo. “These clinically meaningful results complement the benefits seen in an identical phase III study and a previous phase IIb study,” said Richard B. Lipton, MD, Edwin S. Lowe Chair in Neurology at Albert Einstein College of Medicine in New York, and colleagues. “Rimegepant may ultimately offer patients a novel approach for the acute treatment of migraine.”
Dr. Lipton and colleagues conducted a double-blind, randomized, placebo-controlled trial to compare the efficacy, safety, and tolerability of the calcitonin gene-related peptide (CGRP) receptor antagonist rimegepant (75 mg oral tablet) with placebo in the acute treatment of migraine in adults.
The study included adults 18 or older with at least a one-year history of migraine according to ICHD 3-beta criteria. Following a three- to 28-day screening period, subjects were randomized to receive 75 mg of rimegepant or placebo and instructed to treat a single migraine attack with one dose of the blinded study drug (ie, rimegepant or placebo) when headache pain reached moderate or severe intensity. The coprimary end points were pain freedom at two hours postdose and freedom from the most bothersome symptom at two hours postdose. Safety assessments included adverse events, ECGs, vital signs, physical measurements, and routine laboratory tests, including assessment of liver function.
In total, 1,162 subjects were randomized to receive rimegepant (n = 582) or placebo (n = 580), and 1,084 were evaluated for efficacy (rimegepant [n = 543], placebo [n = 541]). Subjects had a mean age of 41.6, 85.5% were female, and participants by history averaged 4.7 attacks per month. At two hours postdose, rimegepant-treated patients had higher pain-free rates than placebo-treated patients did (19.2% vs 14.2%, respectively), were more likely to be free of their most bothersome symptom (36.6% vs 27.7%, respectively), and had higher rates of pain relief (56.0% vs 45.7%, respectively).
A single dose of rimegepant, without the use of rescue medication, demonstrated superiority versus placebo for sustained pain freedom and pain relief from two through 48 hours postdose. On a measure of functional disability, a greater proportion of rimegepant-treated patients achieved normal function at two hours. The safety and tolerability profiles of rimegepant were similar to those of placebo. The most common adverse events in the rimegepant and placebo groups were nausea (0.9% [5 of 546] vs 1.1% [6 of 549], respectively) and dizziness (0.7% [4 of 546] vs 0.4% [2 of 549], respectively).
Triptan Use and Discontinuation: Results From the MAST Study
Migraine continues to be associated with significant unmet acute treatment need.
SAN FRANCISCO—Although triptans are considered the gold standard for acute migraine therapy, only 37% of migraineurs had ever used a triptan and just 15.9% were current triptan users, according to the results of a study presented at the 60th Annual Scientific Meeting of the American Headache Society. Aftab Alam, MBBS, MBA, from Medical Affairs at Dr. Reddy’s Laboratories in Princeton, New Jersey, and colleagues from the Migraine in America Symptoms and Treatment (MAST) study determined that while oral treatment was the most common route of administration, only 11.5% of their total sample (31.1% of ever triptan users and 40.4% of current triptan users) had ever used a non-oral formulation.
The MAST Study collected detailed information regarding patterns of medication use in a sample of patients with migraine. The objectives of the analysis were to understand past and current usage patterns for triptans by route of administration and the rates and reasons for discontinuation.
Study respondents were recruited from a nationwide online research panel. Stratified random sampling identified a representative cohort of individuals 18 and older. A validated migraine symptom screen based on modified ICHD-3 beta criteria identified those with migraine. Study inclusion required an average of at least one headache day per month over the previous three months. Qualified respondents provided sociodemographic data (age, gender, and race) as well as patterns of past and current medication use. For past triptan users, Dr. Alam and his MAST study collaborators assessed reasons for discontinuation from a pre-coded list of side effects and triptan sensation symptoms. Other responses were allowed and coded. The researchers examined descriptive results for each route of administration, but “the groups are not mutually exclusive,” Dr. Alam noted.
Triptan Usage Results
Among 15,133 respondents with migraine, the mean age was 43.1; 73% were women, and 81% were Caucasian. Median monthly headache frequency was 3.3 days per month. A total of 5,596 (37%) had ever used a triptan. Among this subgroup, 81.8% had used oral, 21.3% had used a nasal spray, and 19.0% had used injectable forms; 22.2% had used more than one route of administration. Among current triptan users (2,421, 15.9%), 84.7% use oral, 16.5% use nasal spray, and 8.1% use injectable; 9.3% currently use more than one route of administration. Discontinuation rates were highest for injectable triptans (81.5%), followed by nasal sprays (66.5%) and oral medications (55.2%).
Reasons for Discontinuation
The most common reason for discontinuation was perceived lack of efficacy (38.4% oral, 39.8% nasal spray, 25.7% injectable), followed by side effects (22.8% oral, 17% nasal spray, 20.6% injectable). The most commonly reported side effects were dizziness (37.4% oral, 29.4% nasal spray, 33.5% injectable) followed by nausea (30.7% oral, 32.4% nasal spray, 24.6% injectable) and fatigue (26.2% oral, 24.3% nasal spray, 21.2% injectable). One or more triptan sensation symptom was reported among 60.3% of injection users, 46.5% of oral users, and 39.7% of nasal spray users.
—Glenn S. Williams
Suggested Reading
Wells RE, Markowitz SY, Baron EP, et al. Identifying factors underlying discontinuation of triptans. Headache. 2014;54(2):278-289.
Migraine continues to be associated with significant unmet acute treatment need.
Migraine continues to be associated with significant unmet acute treatment need.
SAN FRANCISCO—Although triptans are considered the gold standard for acute migraine therapy, only 37% of migraineurs had ever used a triptan and just 15.9% were current triptan users, according to the results of a study presented at the 60th Annual Scientific Meeting of the American Headache Society. Aftab Alam, MBBS, MBA, from Medical Affairs at Dr. Reddy’s Laboratories in Princeton, New Jersey, and colleagues from the Migraine in America Symptoms and Treatment (MAST) study determined that while oral treatment was the most common route of administration, only 11.5% of their total sample (31.1% of ever triptan users and 40.4% of current triptan users) had ever used a non-oral formulation.
The MAST Study collected detailed information regarding patterns of medication use in a sample of patients with migraine. The objectives of the analysis were to understand past and current usage patterns for triptans by route of administration and the rates and reasons for discontinuation.
Study respondents were recruited from a nationwide online research panel. Stratified random sampling identified a representative cohort of individuals 18 and older. A validated migraine symptom screen based on modified ICHD-3 beta criteria identified those with migraine. Study inclusion required an average of at least one headache day per month over the previous three months. Qualified respondents provided sociodemographic data (age, gender, and race) as well as patterns of past and current medication use. For past triptan users, Dr. Alam and his MAST study collaborators assessed reasons for discontinuation from a pre-coded list of side effects and triptan sensation symptoms. Other responses were allowed and coded. The researchers examined descriptive results for each route of administration, but “the groups are not mutually exclusive,” Dr. Alam noted.
Triptan Usage Results
Among 15,133 respondents with migraine, the mean age was 43.1; 73% were women, and 81% were Caucasian. Median monthly headache frequency was 3.3 days per month. A total of 5,596 (37%) had ever used a triptan. Among this subgroup, 81.8% had used oral, 21.3% had used a nasal spray, and 19.0% had used injectable forms; 22.2% had used more than one route of administration. Among current triptan users (2,421, 15.9%), 84.7% use oral, 16.5% use nasal spray, and 8.1% use injectable; 9.3% currently use more than one route of administration. Discontinuation rates were highest for injectable triptans (81.5%), followed by nasal sprays (66.5%) and oral medications (55.2%).
Reasons for Discontinuation
The most common reason for discontinuation was perceived lack of efficacy (38.4% oral, 39.8% nasal spray, 25.7% injectable), followed by side effects (22.8% oral, 17% nasal spray, 20.6% injectable). The most commonly reported side effects were dizziness (37.4% oral, 29.4% nasal spray, 33.5% injectable) followed by nausea (30.7% oral, 32.4% nasal spray, 24.6% injectable) and fatigue (26.2% oral, 24.3% nasal spray, 21.2% injectable). One or more triptan sensation symptom was reported among 60.3% of injection users, 46.5% of oral users, and 39.7% of nasal spray users.
—Glenn S. Williams
Suggested Reading
Wells RE, Markowitz SY, Baron EP, et al. Identifying factors underlying discontinuation of triptans. Headache. 2014;54(2):278-289.
SAN FRANCISCO—Although triptans are considered the gold standard for acute migraine therapy, only 37% of migraineurs had ever used a triptan and just 15.9% were current triptan users, according to the results of a study presented at the 60th Annual Scientific Meeting of the American Headache Society. Aftab Alam, MBBS, MBA, from Medical Affairs at Dr. Reddy’s Laboratories in Princeton, New Jersey, and colleagues from the Migraine in America Symptoms and Treatment (MAST) study determined that while oral treatment was the most common route of administration, only 11.5% of their total sample (31.1% of ever triptan users and 40.4% of current triptan users) had ever used a non-oral formulation.
The MAST Study collected detailed information regarding patterns of medication use in a sample of patients with migraine. The objectives of the analysis were to understand past and current usage patterns for triptans by route of administration and the rates and reasons for discontinuation.
Study respondents were recruited from a nationwide online research panel. Stratified random sampling identified a representative cohort of individuals 18 and older. A validated migraine symptom screen based on modified ICHD-3 beta criteria identified those with migraine. Study inclusion required an average of at least one headache day per month over the previous three months. Qualified respondents provided sociodemographic data (age, gender, and race) as well as patterns of past and current medication use. For past triptan users, Dr. Alam and his MAST study collaborators assessed reasons for discontinuation from a pre-coded list of side effects and triptan sensation symptoms. Other responses were allowed and coded. The researchers examined descriptive results for each route of administration, but “the groups are not mutually exclusive,” Dr. Alam noted.
Triptan Usage Results
Among 15,133 respondents with migraine, the mean age was 43.1; 73% were women, and 81% were Caucasian. Median monthly headache frequency was 3.3 days per month. A total of 5,596 (37%) had ever used a triptan. Among this subgroup, 81.8% had used oral, 21.3% had used a nasal spray, and 19.0% had used injectable forms; 22.2% had used more than one route of administration. Among current triptan users (2,421, 15.9%), 84.7% use oral, 16.5% use nasal spray, and 8.1% use injectable; 9.3% currently use more than one route of administration. Discontinuation rates were highest for injectable triptans (81.5%), followed by nasal sprays (66.5%) and oral medications (55.2%).
Reasons for Discontinuation
The most common reason for discontinuation was perceived lack of efficacy (38.4% oral, 39.8% nasal spray, 25.7% injectable), followed by side effects (22.8% oral, 17% nasal spray, 20.6% injectable). The most commonly reported side effects were dizziness (37.4% oral, 29.4% nasal spray, 33.5% injectable) followed by nausea (30.7% oral, 32.4% nasal spray, 24.6% injectable) and fatigue (26.2% oral, 24.3% nasal spray, 21.2% injectable). One or more triptan sensation symptom was reported among 60.3% of injection users, 46.5% of oral users, and 39.7% of nasal spray users.
—Glenn S. Williams
Suggested Reading
Wells RE, Markowitz SY, Baron EP, et al. Identifying factors underlying discontinuation of triptans. Headache. 2014;54(2):278-289.
2018 Update on abnormal uterine bleeding
Over the past year, a few gems have been published to help us manage and treat abnormal uterine bleeding (AUB). One study suggests an order of performing hysteroscopy and endometrial biopsy, another emphasizes the continued cost-effectiveness of the levonorgestrel-releasing intrauterine system (LNG-IUS), while a third provides more evidence that ulipristal acetate is effective in the management of leiomyomas.
Optimal order of office hysteroscopy and endometrial biopsy?
Sarkar P, Mikhail E, Schickler R, Plosker S, Imudia AN. Optimal order of successive office hysteroscopy and endometrial biopsy for the evaluation of abnormal uterine bleeding: a randomized controlled trial. Obstet Gynecol. 2017;130(3):565-572.
Office hysteroscopy and endometrial biopsy are frequently used in the evaluation of women presenting with AUB. Sarkar and colleagues conducted a study aimed at estimating the optimal order of office hysteroscopy and endometrial biopsy when performed successively among premenopausal women.
Pain perception, procedure duration, and other outcomes
This prospective single-blind randomized trial included 78 consecutive patients. The primary outcome was detection of any difference in patients' global pain perception based on the order of the procedures. Secondary outcome measures included determining whether the procedure order affected the duration of the procedures, the adequacy of the endometrial biopsy sample, the number of attempts to obtain an adequate tissue sample, and optimal visualization of the endometrial cavity during office hysteroscopy.
Order not important, but other factors may be
Not surprisingly, the results showed that the order in which the procedures were performed had no effect on patients' pain perception or on the overall procedure duration. Assessed using a visual analog scale scored from 1 to 10, global pain perception in the hysteroscopy-first patients (group A, n = 40) compared with the biopsy-first patients (group B, n = 38) was similar (7 vs 7, P = .57; 95% confidence interval [CI], 5.8-7.1). Procedure duration also was similar in group A and group B (3 vs 3, P = .32; 95% CI, 3.3-4.1).
However, when hysteroscopy was performed first, the quality of endometrial cavity images was superior compared with images from patients in whom biopsy was performed first. The number of endometrial biopsy curette passes required to obtain an adequate tissue sample was lower in the biopsy-first patients. The endometrial biopsy specimen was adequate for histologic evaluation regardless of whether hysteroscopy or biopsy was performed first.
Sarkar and colleagues suggested that their study findings emphasize the importance of individualizing the order of successive procedures to achieve the most clinically relevant result with maximum ease and comfort. They proposed that patients who have a high index of suspicion for occult malignancy or endometrial hyperplasia should have a biopsy procedure first so that adequate tissue samples can be obtained with fewer attempts. In patients with underlying uterine anatomic defects, performing hysteroscopy first would be clinically relevant to obtain the best images for optimal surgical planning.
Read next: Which treatment for AUB is most cost-effective?
Which treatment for AUB is most cost-effective?
Spencer JC, Louie M, Moulder JK, et al. Cost-effectiveness of treatments for heavy menstrual bleeding. Am J Obstet Gynecol. 2017;217(5):574.e1-574e.9.
The costs associated with heavy menstrual bleeding are significant. Spencer and colleagues sought to evaluate the relative cost-effectiveness of 4 treatment options for heavy menstrual bleeding: hysterectomy, resectoscopic endometrial ablation, nonresectoscopic endometrial ablation, and the LNG-IUS in a hypothetical cohort of 100,000 premenopausal women. No previous studies have examined the cost-effectiveness of these options in the context of the US health care setting.
Decision tree used for analysis
The authors formulated a decision tree to evaluate private payer costs and quality-adjusted life-years over a 5-year time horizon for premenopausal women with heavy menstrual bleeding and no suspected malignancy. For each treatment option, the authors used probabilities to estimate frequencies of complications and treatment failure leading to additional therapies. They compared the treatments in terms of total average costs, quality-adjusted life years, and incremental cost-effectiveness ratios.
Comparing costs, quality of life, and complications
Quality of life was fairly high for all treatment options; however, the estimated costs and the complications of each treatment were markedly different between treatment options. The LNG-IUS was superior to all alternatives in terms of both cost and quality, making it the dominant strategy. The 5-year cost for the LNG-IUS was $4,500, about half the cost of endometrial ablation ($9,500) and about one-third the cost of hysterectomy ($13,500). When examined over a range of possible values, the LNG-IUS was cost-effective compared with hysterectomy in the large majority of scenarios (90%).
If the LNG-IUS is removed from consideration because of either patient preference or clinical judgment, the decision between hysterectomy and ablation is more complex. Hysterectomy results in better quality of life in the majority of simulations, but it is cost-effective in just more than half of the simulations compared with either resectoscopic or nonresectoscopic ablation. Therefore, consideration of cost, procedure-specific complications, and patient preferences may guide the therapeutic decision between hysterectomy and endometrial ablation.
The 52-mg LNG-IUS was superior to all treatment alternatives in both cost and quality, making it the dominant strategy for the treatment of heavy menstrual bleeding.
Ulipristal may be useful for managing AUB associated with uterine leiomyomas
Simon JA, Catherino W, Segars JH, et al. Ulipristal acetate for treatment of symptomatic uterine leiomyomas: a randomized controlled trial. Obstet Gynecol. 2018;131(3):431-439.
Managing uterine leiomyomas is a common issue for gynecologists, as up to 70% of white women and more than 80% of black women of reproductive age in the United States have leiomyomas.
Ulipristal acetate is an orally administered selective progesterone-receptor modulator that decreases bleeding and reduces leiomyoma size. Although trials conducted in Europe found ulipristal to be superior to placebo and noninferior to leuprolide acetate in controlling bleeding and reducing leiomyoma size, those initial trials were conducted in a predominantly white population.
Study assessed efficacy and safety
Simon and colleagues recently conducted a randomized double-blind, placebo-controlled trial designed to assess the safety and efficacy of ulipristal in a more diverse population, such as patients in the United States. The 148 participants included in the study were randomly assigned on a 1:1:1 basis to once-daily oral ulipristal 5 mg, ulipristal 10 mg, or placebo for 12 weeks, with a 12-week drug-free follow-up.
Amenorrhea achieved and quality of life improved
The investigators found that ulipristal in 5-mg and 10-mg doses was well tolerated and superior to placebo in both the rate of and the time to amenorrhea (the coprimary end points) in women with symptomatic leiomyomas. In women treated with ulipristal 5 mg, amenorrhea was achieved in 25 of 53 (47.2%; 97.5% CI, 31.6-63.2), and of those treated with the 10-mg dose, 28 of 48 (58.3%; 97.5% CI, 41.2-74.1) achieved amenorrhea (P<.001 for both groups), compared with 1 of 56 (1.8%; 97.5% CI, 0.0-10.9) in the placebo group.
AUB continues to be a significant issue for many women. As women's health care providers, it is important that we deliver care with high value (Quality ÷ Cost). Therefore, consider these takeaway points:
- The LNG-IUS consistently delivers high value by affecting both sides of this equation. We should use it more.
- Although we do not yet know what ulipristal acetate will cost in the United States, effective medical treatments usually affect both sides of the Quality ÷ Cost equation, and new medications on the horizon are worth knowing about.
- Last, efficiency with office-based hysteroscopy is also an opportunity to increase value by improving biopsy and visualization quality.
Ulipristal treatment also was shown to improve health-related quality of life, including physical and social activities. No patient discontinued ulipristal because of lack of efficacy, and 1 patient in the placebo group stopped taking the drug because of an adverse event. Estradiol levels were maintained at midfollicular levels during ulipristal treatment, and endometrial biopsies did not show any atypical or malignant changes. These results are consistent with those of the studies conducted in Europe in a predominantly white, nonobese population.
Results of this study help to define a niche for ulipristal when hysterectomy is not an option for women who wish to preserve fertility. Further, although leuprolide is used for preoperative hematologic improvement of anemia, its use results in hypoestrogenic adverse effects.
The findings from this and other studies suggest that ulipristal may be useful for the medical management of AUB associated with uterine leiomyomas, especially for patients desiring uterine- and fertility-sparing treatment. Hopefully, this treatment will be available soon in the United States.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Over the past year, a few gems have been published to help us manage and treat abnormal uterine bleeding (AUB). One study suggests an order of performing hysteroscopy and endometrial biopsy, another emphasizes the continued cost-effectiveness of the levonorgestrel-releasing intrauterine system (LNG-IUS), while a third provides more evidence that ulipristal acetate is effective in the management of leiomyomas.
Optimal order of office hysteroscopy and endometrial biopsy?
Sarkar P, Mikhail E, Schickler R, Plosker S, Imudia AN. Optimal order of successive office hysteroscopy and endometrial biopsy for the evaluation of abnormal uterine bleeding: a randomized controlled trial. Obstet Gynecol. 2017;130(3):565-572.
Office hysteroscopy and endometrial biopsy are frequently used in the evaluation of women presenting with AUB. Sarkar and colleagues conducted a study aimed at estimating the optimal order of office hysteroscopy and endometrial biopsy when performed successively among premenopausal women.
Pain perception, procedure duration, and other outcomes
This prospective single-blind randomized trial included 78 consecutive patients. The primary outcome was detection of any difference in patients' global pain perception based on the order of the procedures. Secondary outcome measures included determining whether the procedure order affected the duration of the procedures, the adequacy of the endometrial biopsy sample, the number of attempts to obtain an adequate tissue sample, and optimal visualization of the endometrial cavity during office hysteroscopy.

Order not important, but other factors may be
Not surprisingly, the results showed that the order in which the procedures were performed had no effect on patients' pain perception or on the overall procedure duration. Assessed using a visual analog scale scored from 1 to 10, global pain perception in the hysteroscopy-first patients (group A, n = 40) compared with the biopsy-first patients (group B, n = 38) was similar (7 vs 7, P = .57; 95% confidence interval [CI], 5.8-7.1). Procedure duration also was similar in group A and group B (3 vs 3, P = .32; 95% CI, 3.3-4.1).
However, when hysteroscopy was performed first, the quality of endometrial cavity images was superior compared with images from patients in whom biopsy was performed first. The number of endometrial biopsy curette passes required to obtain an adequate tissue sample was lower in the biopsy-first patients. The endometrial biopsy specimen was adequate for histologic evaluation regardless of whether hysteroscopy or biopsy was performed first.
Sarkar and colleagues suggested that their study findings emphasize the importance of individualizing the order of successive procedures to achieve the most clinically relevant result with maximum ease and comfort. They proposed that patients who have a high index of suspicion for occult malignancy or endometrial hyperplasia should have a biopsy procedure first so that adequate tissue samples can be obtained with fewer attempts. In patients with underlying uterine anatomic defects, performing hysteroscopy first would be clinically relevant to obtain the best images for optimal surgical planning.
Read next: Which treatment for AUB is most cost-effective?
Which treatment for AUB is most cost-effective?
Spencer JC, Louie M, Moulder JK, et al. Cost-effectiveness of treatments for heavy menstrual bleeding. Am J Obstet Gynecol. 2017;217(5):574.e1-574e.9.
The costs associated with heavy menstrual bleeding are significant. Spencer and colleagues sought to evaluate the relative cost-effectiveness of 4 treatment options for heavy menstrual bleeding: hysterectomy, resectoscopic endometrial ablation, nonresectoscopic endometrial ablation, and the LNG-IUS in a hypothetical cohort of 100,000 premenopausal women. No previous studies have examined the cost-effectiveness of these options in the context of the US health care setting.
Decision tree used for analysis
The authors formulated a decision tree to evaluate private payer costs and quality-adjusted life-years over a 5-year time horizon for premenopausal women with heavy menstrual bleeding and no suspected malignancy. For each treatment option, the authors used probabilities to estimate frequencies of complications and treatment failure leading to additional therapies. They compared the treatments in terms of total average costs, quality-adjusted life years, and incremental cost-effectiveness ratios.
Comparing costs, quality of life, and complications
Quality of life was fairly high for all treatment options; however, the estimated costs and the complications of each treatment were markedly different between treatment options. The LNG-IUS was superior to all alternatives in terms of both cost and quality, making it the dominant strategy. The 5-year cost for the LNG-IUS was $4,500, about half the cost of endometrial ablation ($9,500) and about one-third the cost of hysterectomy ($13,500). When examined over a range of possible values, the LNG-IUS was cost-effective compared with hysterectomy in the large majority of scenarios (90%).
If the LNG-IUS is removed from consideration because of either patient preference or clinical judgment, the decision between hysterectomy and ablation is more complex. Hysterectomy results in better quality of life in the majority of simulations, but it is cost-effective in just more than half of the simulations compared with either resectoscopic or nonresectoscopic ablation. Therefore, consideration of cost, procedure-specific complications, and patient preferences may guide the therapeutic decision between hysterectomy and endometrial ablation.
The 52-mg LNG-IUS was superior to all treatment alternatives in both cost and quality, making it the dominant strategy for the treatment of heavy menstrual bleeding.
Ulipristal may be useful for managing AUB associated with uterine leiomyomas
Simon JA, Catherino W, Segars JH, et al. Ulipristal acetate for treatment of symptomatic uterine leiomyomas: a randomized controlled trial. Obstet Gynecol. 2018;131(3):431-439.
Managing uterine leiomyomas is a common issue for gynecologists, as up to 70% of white women and more than 80% of black women of reproductive age in the United States have leiomyomas.
Ulipristal acetate is an orally administered selective progesterone-receptor modulator that decreases bleeding and reduces leiomyoma size. Although trials conducted in Europe found ulipristal to be superior to placebo and noninferior to leuprolide acetate in controlling bleeding and reducing leiomyoma size, those initial trials were conducted in a predominantly white population.
Study assessed efficacy and safety
Simon and colleagues recently conducted a randomized double-blind, placebo-controlled trial designed to assess the safety and efficacy of ulipristal in a more diverse population, such as patients in the United States. The 148 participants included in the study were randomly assigned on a 1:1:1 basis to once-daily oral ulipristal 5 mg, ulipristal 10 mg, or placebo for 12 weeks, with a 12-week drug-free follow-up.
Amenorrhea achieved and quality of life improved
The investigators found that ulipristal in 5-mg and 10-mg doses was well tolerated and superior to placebo in both the rate of and the time to amenorrhea (the coprimary end points) in women with symptomatic leiomyomas. In women treated with ulipristal 5 mg, amenorrhea was achieved in 25 of 53 (47.2%; 97.5% CI, 31.6-63.2), and of those treated with the 10-mg dose, 28 of 48 (58.3%; 97.5% CI, 41.2-74.1) achieved amenorrhea (P<.001 for both groups), compared with 1 of 56 (1.8%; 97.5% CI, 0.0-10.9) in the placebo group.
AUB continues to be a significant issue for many women. As women's health care providers, it is important that we deliver care with high value (Quality ÷ Cost). Therefore, consider these takeaway points:
- The LNG-IUS consistently delivers high value by affecting both sides of this equation. We should use it more.
- Although we do not yet know what ulipristal acetate will cost in the United States, effective medical treatments usually affect both sides of the Quality ÷ Cost equation, and new medications on the horizon are worth knowing about.
- Last, efficiency with office-based hysteroscopy is also an opportunity to increase value by improving biopsy and visualization quality.
Ulipristal treatment also was shown to improve health-related quality of life, including physical and social activities. No patient discontinued ulipristal because of lack of efficacy, and 1 patient in the placebo group stopped taking the drug because of an adverse event. Estradiol levels were maintained at midfollicular levels during ulipristal treatment, and endometrial biopsies did not show any atypical or malignant changes. These results are consistent with those of the studies conducted in Europe in a predominantly white, nonobese population.
Results of this study help to define a niche for ulipristal when hysterectomy is not an option for women who wish to preserve fertility. Further, although leuprolide is used for preoperative hematologic improvement of anemia, its use results in hypoestrogenic adverse effects.
The findings from this and other studies suggest that ulipristal may be useful for the medical management of AUB associated with uterine leiomyomas, especially for patients desiring uterine- and fertility-sparing treatment. Hopefully, this treatment will be available soon in the United States.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Over the past year, a few gems have been published to help us manage and treat abnormal uterine bleeding (AUB). One study suggests an order of performing hysteroscopy and endometrial biopsy, another emphasizes the continued cost-effectiveness of the levonorgestrel-releasing intrauterine system (LNG-IUS), while a third provides more evidence that ulipristal acetate is effective in the management of leiomyomas.
Optimal order of office hysteroscopy and endometrial biopsy?
Sarkar P, Mikhail E, Schickler R, Plosker S, Imudia AN. Optimal order of successive office hysteroscopy and endometrial biopsy for the evaluation of abnormal uterine bleeding: a randomized controlled trial. Obstet Gynecol. 2017;130(3):565-572.
Office hysteroscopy and endometrial biopsy are frequently used in the evaluation of women presenting with AUB. Sarkar and colleagues conducted a study aimed at estimating the optimal order of office hysteroscopy and endometrial biopsy when performed successively among premenopausal women.
Pain perception, procedure duration, and other outcomes
This prospective single-blind randomized trial included 78 consecutive patients. The primary outcome was detection of any difference in patients' global pain perception based on the order of the procedures. Secondary outcome measures included determining whether the procedure order affected the duration of the procedures, the adequacy of the endometrial biopsy sample, the number of attempts to obtain an adequate tissue sample, and optimal visualization of the endometrial cavity during office hysteroscopy.

Order not important, but other factors may be
Not surprisingly, the results showed that the order in which the procedures were performed had no effect on patients' pain perception or on the overall procedure duration. Assessed using a visual analog scale scored from 1 to 10, global pain perception in the hysteroscopy-first patients (group A, n = 40) compared with the biopsy-first patients (group B, n = 38) was similar (7 vs 7, P = .57; 95% confidence interval [CI], 5.8-7.1). Procedure duration also was similar in group A and group B (3 vs 3, P = .32; 95% CI, 3.3-4.1).
However, when hysteroscopy was performed first, the quality of endometrial cavity images was superior compared with images from patients in whom biopsy was performed first. The number of endometrial biopsy curette passes required to obtain an adequate tissue sample was lower in the biopsy-first patients. The endometrial biopsy specimen was adequate for histologic evaluation regardless of whether hysteroscopy or biopsy was performed first.
Sarkar and colleagues suggested that their study findings emphasize the importance of individualizing the order of successive procedures to achieve the most clinically relevant result with maximum ease and comfort. They proposed that patients who have a high index of suspicion for occult malignancy or endometrial hyperplasia should have a biopsy procedure first so that adequate tissue samples can be obtained with fewer attempts. In patients with underlying uterine anatomic defects, performing hysteroscopy first would be clinically relevant to obtain the best images for optimal surgical planning.
Read next: Which treatment for AUB is most cost-effective?
Which treatment for AUB is most cost-effective?
Spencer JC, Louie M, Moulder JK, et al. Cost-effectiveness of treatments for heavy menstrual bleeding. Am J Obstet Gynecol. 2017;217(5):574.e1-574e.9.
The costs associated with heavy menstrual bleeding are significant. Spencer and colleagues sought to evaluate the relative cost-effectiveness of 4 treatment options for heavy menstrual bleeding: hysterectomy, resectoscopic endometrial ablation, nonresectoscopic endometrial ablation, and the LNG-IUS in a hypothetical cohort of 100,000 premenopausal women. No previous studies have examined the cost-effectiveness of these options in the context of the US health care setting.
Decision tree used for analysis
The authors formulated a decision tree to evaluate private payer costs and quality-adjusted life-years over a 5-year time horizon for premenopausal women with heavy menstrual bleeding and no suspected malignancy. For each treatment option, the authors used probabilities to estimate frequencies of complications and treatment failure leading to additional therapies. They compared the treatments in terms of total average costs, quality-adjusted life years, and incremental cost-effectiveness ratios.
Comparing costs, quality of life, and complications
Quality of life was fairly high for all treatment options; however, the estimated costs and the complications of each treatment were markedly different between treatment options. The LNG-IUS was superior to all alternatives in terms of both cost and quality, making it the dominant strategy. The 5-year cost for the LNG-IUS was $4,500, about half the cost of endometrial ablation ($9,500) and about one-third the cost of hysterectomy ($13,500). When examined over a range of possible values, the LNG-IUS was cost-effective compared with hysterectomy in the large majority of scenarios (90%).
If the LNG-IUS is removed from consideration because of either patient preference or clinical judgment, the decision between hysterectomy and ablation is more complex. Hysterectomy results in better quality of life in the majority of simulations, but it is cost-effective in just more than half of the simulations compared with either resectoscopic or nonresectoscopic ablation. Therefore, consideration of cost, procedure-specific complications, and patient preferences may guide the therapeutic decision between hysterectomy and endometrial ablation.
The 52-mg LNG-IUS was superior to all treatment alternatives in both cost and quality, making it the dominant strategy for the treatment of heavy menstrual bleeding.
Ulipristal may be useful for managing AUB associated with uterine leiomyomas
Simon JA, Catherino W, Segars JH, et al. Ulipristal acetate for treatment of symptomatic uterine leiomyomas: a randomized controlled trial. Obstet Gynecol. 2018;131(3):431-439.
Managing uterine leiomyomas is a common issue for gynecologists, as up to 70% of white women and more than 80% of black women of reproductive age in the United States have leiomyomas.
Ulipristal acetate is an orally administered selective progesterone-receptor modulator that decreases bleeding and reduces leiomyoma size. Although trials conducted in Europe found ulipristal to be superior to placebo and noninferior to leuprolide acetate in controlling bleeding and reducing leiomyoma size, those initial trials were conducted in a predominantly white population.
Study assessed efficacy and safety
Simon and colleagues recently conducted a randomized double-blind, placebo-controlled trial designed to assess the safety and efficacy of ulipristal in a more diverse population, such as patients in the United States. The 148 participants included in the study were randomly assigned on a 1:1:1 basis to once-daily oral ulipristal 5 mg, ulipristal 10 mg, or placebo for 12 weeks, with a 12-week drug-free follow-up.
Amenorrhea achieved and quality of life improved
The investigators found that ulipristal in 5-mg and 10-mg doses was well tolerated and superior to placebo in both the rate of and the time to amenorrhea (the coprimary end points) in women with symptomatic leiomyomas. In women treated with ulipristal 5 mg, amenorrhea was achieved in 25 of 53 (47.2%; 97.5% CI, 31.6-63.2), and of those treated with the 10-mg dose, 28 of 48 (58.3%; 97.5% CI, 41.2-74.1) achieved amenorrhea (P<.001 for both groups), compared with 1 of 56 (1.8%; 97.5% CI, 0.0-10.9) in the placebo group.
AUB continues to be a significant issue for many women. As women's health care providers, it is important that we deliver care with high value (Quality ÷ Cost). Therefore, consider these takeaway points:
- The LNG-IUS consistently delivers high value by affecting both sides of this equation. We should use it more.
- Although we do not yet know what ulipristal acetate will cost in the United States, effective medical treatments usually affect both sides of the Quality ÷ Cost equation, and new medications on the horizon are worth knowing about.
- Last, efficiency with office-based hysteroscopy is also an opportunity to increase value by improving biopsy and visualization quality.
Ulipristal treatment also was shown to improve health-related quality of life, including physical and social activities. No patient discontinued ulipristal because of lack of efficacy, and 1 patient in the placebo group stopped taking the drug because of an adverse event. Estradiol levels were maintained at midfollicular levels during ulipristal treatment, and endometrial biopsies did not show any atypical or malignant changes. These results are consistent with those of the studies conducted in Europe in a predominantly white, nonobese population.
Results of this study help to define a niche for ulipristal when hysterectomy is not an option for women who wish to preserve fertility. Further, although leuprolide is used for preoperative hematologic improvement of anemia, its use results in hypoestrogenic adverse effects.
The findings from this and other studies suggest that ulipristal may be useful for the medical management of AUB associated with uterine leiomyomas, especially for patients desiring uterine- and fertility-sparing treatment. Hopefully, this treatment will be available soon in the United States.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Endoscopic screening tied to significantly lower risk of death from gastric cancer
Endoscopic screening was associated with an approximately 40% reduction in risk of death from gastric cancer in a systematic review and meta-analysis of studies from Asian countries.
The study is the first systematic review and meta-analysis of gastric cancer mortality and incidence after endoscopic screening, wrote Xing Zhang, MD, of the China Academy of Chinese Medical Sciences in Beijing with his associates. “Population-based prospective cohort studies are warranted to confirm our findings,” the reviewers wrote in the August issue of Gastroenterology.
In general, the rates of gastric cancer and related mortality in East Asian countries are significantly higher than global averages. As a result, countries in this region have implemented a variety of national and opportunistic screening programs that vary from country to country. Japan, for example, has a national screening program based on photofluorography. “Although data are inconsistent, most studies have shown a 40%-60% decrease in the mortality of gastric cancer in those who have been screened using photofluorography,” the reviewers noted. When findings are positive, follow-up endoscopy is recommended. However, debates persist about whether population-level endoscopy significantly improves hard endpoints in gastric cancer, such as incidence and mortality.
To help clarify the population-level benefits of endoscopic screening, Dr. Zhang and his associates searched PubMed and EMBASE; they identified six cohort studies and four nested case-control studies that included approximately 342,000 adults from Asia who did not have baseline gastric cancer but did undergo surveillance endoscopy at least once. Studies of both mass and opportunistic screening were included. Each study included a comparator; reported incidence, mortality, or both; and was published by March 8, 2018.
Endoscopic screening was tied to a 40% reduction in the relative risk of death from gastric cancer (risk ratio, 0.60; 95% confidence interval, 0.49-0.73). There also was a slight trend toward increased incidence of gastric cancer, which was not statistically significant (RR, 1.14; 95% CI, 0.93-1.40). However, only two studies examined the incidence of gastric cancer, so this outcome “should be interpreted with caution,” the reviewers wrote. Endoscopic screening also was associated with a significantly lower risk of death from gastric cancer, compared with radiographic screening (RR, 0.33; 95% CI, 0.12-0.91).
Endoscopic screening did not significantly reduce mortality, compared with expected deaths (RR, 0.67; 95% CI, 0.38-1.16), the reviewers reported. This might be because the reviewers included an outlier study conducted in Linqu County, China, which has some of the highest rates of gastric cancer death in the world, they noted. Endoscopic surveillance did not reduce mortality in the Linqu County study, but screenings were spaced by 4.5 years, which was probably too long to show an effect, especially in a high-risk region, they added. The study in Linqu County accounted for most of the heterogeneity among studies, and removing it from the pooled analysis produced a “slightly more pronounced reduction in gastric cancer mortality,” with an RR of 0.56, they noted.
Funders included the National Natural Science Foundation and the National Twelfth Five-Year Plan for Science and Technology Support Program of China. The reviewers reported having no relevant conflicts of interest.
SOURCE: Zhang X, et al. Gastroenterology. 2018 Apr 30. doi: 10.1053/j.gastro.2018.04.026.
Gastric cancer remains the third most common cause of cancer death worldwide, although incidence and mortality rates have been declining for several years. For populations in eastern Asia – a region that carries the unenviable tag of having the highest gastric cancer mortality rates in the world – finding ways to reduce the burden of this disease remains a key priority.
New findings reported by Zhang and his colleagues have highlighted a strong evidence base for one mode of gastric cancer control in eastern Asia. The systematic review team demonstrated a significant 40% reduction in the risk of death from gastric cancer when screening was conducted in general populations. The magnitude of this benefit likely reflects the appropriateness of screening in such high-incidence areas, although this finding might not necessarily be extrapolated to other regions.
The authors cautioned that screening did not reduce gastric cancer incidence, although only two studies were included. However, reduced incidence is often not an aim of screening programs; indeed, to detect more gastric cancers at an earlier stage can be an intentional outcome.
The observed benefits might be somewhat attributed to lead time bias (whereby individuals are diagnosed at a younger age than they would have presented symptomatically but still die at the same age) or length time bias (detecting only the slower-growing biological tumors). Further refinement of optimal screening intervals is also required. Nevertheless, public health policy makers in Japan and Korea, where national screening programs already exist, should be reassured by these review findings.
Helen Coleman, PhD, BSc(Hons), is a senior lecturer and lead of the Cancer Epidemiology Research Group at the Centre for Public Health and Centre for Cancer Research and Cell Biology at Queen’s University Belfast. She has no conflicts of interest.
Gastric cancer remains the third most common cause of cancer death worldwide, although incidence and mortality rates have been declining for several years. For populations in eastern Asia – a region that carries the unenviable tag of having the highest gastric cancer mortality rates in the world – finding ways to reduce the burden of this disease remains a key priority.
New findings reported by Zhang and his colleagues have highlighted a strong evidence base for one mode of gastric cancer control in eastern Asia. The systematic review team demonstrated a significant 40% reduction in the risk of death from gastric cancer when screening was conducted in general populations. The magnitude of this benefit likely reflects the appropriateness of screening in such high-incidence areas, although this finding might not necessarily be extrapolated to other regions.
The authors cautioned that screening did not reduce gastric cancer incidence, although only two studies were included. However, reduced incidence is often not an aim of screening programs; indeed, to detect more gastric cancers at an earlier stage can be an intentional outcome.
The observed benefits might be somewhat attributed to lead time bias (whereby individuals are diagnosed at a younger age than they would have presented symptomatically but still die at the same age) or length time bias (detecting only the slower-growing biological tumors). Further refinement of optimal screening intervals is also required. Nevertheless, public health policy makers in Japan and Korea, where national screening programs already exist, should be reassured by these review findings.
Helen Coleman, PhD, BSc(Hons), is a senior lecturer and lead of the Cancer Epidemiology Research Group at the Centre for Public Health and Centre for Cancer Research and Cell Biology at Queen’s University Belfast. She has no conflicts of interest.
Gastric cancer remains the third most common cause of cancer death worldwide, although incidence and mortality rates have been declining for several years. For populations in eastern Asia – a region that carries the unenviable tag of having the highest gastric cancer mortality rates in the world – finding ways to reduce the burden of this disease remains a key priority.
New findings reported by Zhang and his colleagues have highlighted a strong evidence base for one mode of gastric cancer control in eastern Asia. The systematic review team demonstrated a significant 40% reduction in the risk of death from gastric cancer when screening was conducted in general populations. The magnitude of this benefit likely reflects the appropriateness of screening in such high-incidence areas, although this finding might not necessarily be extrapolated to other regions.
The authors cautioned that screening did not reduce gastric cancer incidence, although only two studies were included. However, reduced incidence is often not an aim of screening programs; indeed, to detect more gastric cancers at an earlier stage can be an intentional outcome.
The observed benefits might be somewhat attributed to lead time bias (whereby individuals are diagnosed at a younger age than they would have presented symptomatically but still die at the same age) or length time bias (detecting only the slower-growing biological tumors). Further refinement of optimal screening intervals is also required. Nevertheless, public health policy makers in Japan and Korea, where national screening programs already exist, should be reassured by these review findings.
Helen Coleman, PhD, BSc(Hons), is a senior lecturer and lead of the Cancer Epidemiology Research Group at the Centre for Public Health and Centre for Cancer Research and Cell Biology at Queen’s University Belfast. She has no conflicts of interest.
Endoscopic screening was associated with an approximately 40% reduction in risk of death from gastric cancer in a systematic review and meta-analysis of studies from Asian countries.
The study is the first systematic review and meta-analysis of gastric cancer mortality and incidence after endoscopic screening, wrote Xing Zhang, MD, of the China Academy of Chinese Medical Sciences in Beijing with his associates. “Population-based prospective cohort studies are warranted to confirm our findings,” the reviewers wrote in the August issue of Gastroenterology.
In general, the rates of gastric cancer and related mortality in East Asian countries are significantly higher than global averages. As a result, countries in this region have implemented a variety of national and opportunistic screening programs that vary from country to country. Japan, for example, has a national screening program based on photofluorography. “Although data are inconsistent, most studies have shown a 40%-60% decrease in the mortality of gastric cancer in those who have been screened using photofluorography,” the reviewers noted. When findings are positive, follow-up endoscopy is recommended. However, debates persist about whether population-level endoscopy significantly improves hard endpoints in gastric cancer, such as incidence and mortality.
To help clarify the population-level benefits of endoscopic screening, Dr. Zhang and his associates searched PubMed and EMBASE; they identified six cohort studies and four nested case-control studies that included approximately 342,000 adults from Asia who did not have baseline gastric cancer but did undergo surveillance endoscopy at least once. Studies of both mass and opportunistic screening were included. Each study included a comparator; reported incidence, mortality, or both; and was published by March 8, 2018.
Endoscopic screening was tied to a 40% reduction in the relative risk of death from gastric cancer (risk ratio, 0.60; 95% confidence interval, 0.49-0.73). There also was a slight trend toward increased incidence of gastric cancer, which was not statistically significant (RR, 1.14; 95% CI, 0.93-1.40). However, only two studies examined the incidence of gastric cancer, so this outcome “should be interpreted with caution,” the reviewers wrote. Endoscopic screening also was associated with a significantly lower risk of death from gastric cancer, compared with radiographic screening (RR, 0.33; 95% CI, 0.12-0.91).
Endoscopic screening did not significantly reduce mortality, compared with expected deaths (RR, 0.67; 95% CI, 0.38-1.16), the reviewers reported. This might be because the reviewers included an outlier study conducted in Linqu County, China, which has some of the highest rates of gastric cancer death in the world, they noted. Endoscopic surveillance did not reduce mortality in the Linqu County study, but screenings were spaced by 4.5 years, which was probably too long to show an effect, especially in a high-risk region, they added. The study in Linqu County accounted for most of the heterogeneity among studies, and removing it from the pooled analysis produced a “slightly more pronounced reduction in gastric cancer mortality,” with an RR of 0.56, they noted.
Funders included the National Natural Science Foundation and the National Twelfth Five-Year Plan for Science and Technology Support Program of China. The reviewers reported having no relevant conflicts of interest.
SOURCE: Zhang X, et al. Gastroenterology. 2018 Apr 30. doi: 10.1053/j.gastro.2018.04.026.
Endoscopic screening was associated with an approximately 40% reduction in risk of death from gastric cancer in a systematic review and meta-analysis of studies from Asian countries.
The study is the first systematic review and meta-analysis of gastric cancer mortality and incidence after endoscopic screening, wrote Xing Zhang, MD, of the China Academy of Chinese Medical Sciences in Beijing with his associates. “Population-based prospective cohort studies are warranted to confirm our findings,” the reviewers wrote in the August issue of Gastroenterology.
In general, the rates of gastric cancer and related mortality in East Asian countries are significantly higher than global averages. As a result, countries in this region have implemented a variety of national and opportunistic screening programs that vary from country to country. Japan, for example, has a national screening program based on photofluorography. “Although data are inconsistent, most studies have shown a 40%-60% decrease in the mortality of gastric cancer in those who have been screened using photofluorography,” the reviewers noted. When findings are positive, follow-up endoscopy is recommended. However, debates persist about whether population-level endoscopy significantly improves hard endpoints in gastric cancer, such as incidence and mortality.
To help clarify the population-level benefits of endoscopic screening, Dr. Zhang and his associates searched PubMed and EMBASE; they identified six cohort studies and four nested case-control studies that included approximately 342,000 adults from Asia who did not have baseline gastric cancer but did undergo surveillance endoscopy at least once. Studies of both mass and opportunistic screening were included. Each study included a comparator; reported incidence, mortality, or both; and was published by March 8, 2018.
Endoscopic screening was tied to a 40% reduction in the relative risk of death from gastric cancer (risk ratio, 0.60; 95% confidence interval, 0.49-0.73). There also was a slight trend toward increased incidence of gastric cancer, which was not statistically significant (RR, 1.14; 95% CI, 0.93-1.40). However, only two studies examined the incidence of gastric cancer, so this outcome “should be interpreted with caution,” the reviewers wrote. Endoscopic screening also was associated with a significantly lower risk of death from gastric cancer, compared with radiographic screening (RR, 0.33; 95% CI, 0.12-0.91).
Endoscopic screening did not significantly reduce mortality, compared with expected deaths (RR, 0.67; 95% CI, 0.38-1.16), the reviewers reported. This might be because the reviewers included an outlier study conducted in Linqu County, China, which has some of the highest rates of gastric cancer death in the world, they noted. Endoscopic surveillance did not reduce mortality in the Linqu County study, but screenings were spaced by 4.5 years, which was probably too long to show an effect, especially in a high-risk region, they added. The study in Linqu County accounted for most of the heterogeneity among studies, and removing it from the pooled analysis produced a “slightly more pronounced reduction in gastric cancer mortality,” with an RR of 0.56, they noted.
Funders included the National Natural Science Foundation and the National Twelfth Five-Year Plan for Science and Technology Support Program of China. The reviewers reported having no relevant conflicts of interest.
SOURCE: Zhang X, et al. Gastroenterology. 2018 Apr 30. doi: 10.1053/j.gastro.2018.04.026.
FROM GASTROENTEROLOGY
Key clinical point: Endoscopic screening was associated with a significant decrease in risk of death from gastric cancer.
Major finding: Compared with no screening, the reduction in risk was 40% (risk ratio, 0.60; 95% confidence interval, 0.49-0.73).
Study details: Systematic review and meta-analysis of six cohort studies and four nested case-control studies of approximately 342,000 adults.
Disclosures: Funders included the National Natural Science Foundation and the National Twelfth Five-Year Plan for Science and Technology Support Program of China. The reviewers reported having no relevant conflicts of interest.
Source: Zhang X et al. Gastroenterology. 2018 Apr 30. doi: 10.1053/j.gastro.2018.04.026.
Investigators Describe the MS Prodrome
Patients who later develop MS are more likely than others to consult physicians for nervous system and genitourinary symptoms.
The prodrome of multiple sclerosis (MS) may include an increased risk of nervous system, sensory, and musculoskeletal disorders, according to research published online ahead of print July 1 in Multiple Sclerosis Journal. Patients who later develop MS also may be more likely to have genitourinary and psychiatric symptoms in the five years before diagnosis.
“The existence of such warning signs is well-accepted for Alzheimer’s disease and Parkinson’s disease, but there has been little investigation into a similar pattern for MS,” said Helen Tremlett, PhD, Professor in the Division of Neurology at the University of British Columbia in Canada. “We now need to delve deeper into this phenomenon, perhaps using data-mining techniques. We want to see if there are discernible patterns related to sex, age, or the type of MS they eventually develop.”
Clinical and Administrative Matched Cohorts
Dr. Tremlett and colleagues analyzed data from a matched-cohort record-linkage study to examine the MS prodrome. The investigators used population-based health administrative data and clinical data from the Canadian provinces of British Columbia, Saskatchewan, Manitoba, and Nova Scotia. The information included demographics, hospital visits, physician encounters, and prescriptions filled. Clinical data were for patients diagnosed by a neurologist at an MS clinic and included first clinical visit (or date of diagnosis) and date of symptom onset. Data were collected from April 1984 to April 2014.
Using the data, Dr. Tremlett and colleagues created a health-administrative cohort and a clinical cohort. The clinical cohort did not include data from Saskatchewan. To create the cohorts, the investigators identified patients with MS and matched them by sex, year of birth, and postal code with as many as five controls. The index date was the earliest recorded claim for a demyelinating disease for the health-administrative cohort and the date of MS symptom onset for the clinical cohort. Study outcomes were the number of physician and hospital encounters per ICD-10 chapter, the number of physician encounters per physician specialty, and the percentage of people with one or more prescriptions per drug class in the five years before the index date.
Clinical Cohort Results May Be More Accurate
The administrative cohort included 13,951 cases and 66,940 controls. The clinical cohort included 3,202 cases and 16,006 controls. Compared with controls, people with MS had more physician and hospital encounters for the nervous (rate ratio [RR], 2.31 to 4.75), sensory (RR, 1.40 to 2.28), musculoskeletal (RR, 1.19 to 1.70), and genitourinary systems (RR, 1.17 to 1.59) in the five years before the first demyelinating claim or symptom onset. Cases had more visits with psychiatrists and urologists (RR, 1.48 to 1.80) and higher proportions of musculoskeletal, genitourinary, or hormonal-related prescriptions (1.1–1.5 times higher), compared with controls. People with MS had fewer pregnancy-related encounters than controls, however (RR, 0.78 to 0.88).
The “more conservative” results for the clinical cohort are more likely to reflect the MS prodrome accurately because they are “unlikely to be influenced by a physician’s suspicion or consideration of MS,” said Dr. Tremlett and colleagues. “Although not all individuals with MS attend an MS specialty clinic, the clinical cohort represents a subgroup of the population that may differ with respect to demographic and clinical characteristics from nonclinic attendees (eg, have fewer comorbidities),” they added. NR
—Erik Greb
Suggested Reading
Wijnands JM, Zhu F, Kingwell E, et al. Five years before multiple sclerosis onset: Phenotyping the prodrome. Mult Scler. 2018 Jul 1 [Epub ahead of print].
Patients who later develop MS are more likely than others to consult physicians for nervous system and genitourinary symptoms.
Patients who later develop MS are more likely than others to consult physicians for nervous system and genitourinary symptoms.
The prodrome of multiple sclerosis (MS) may include an increased risk of nervous system, sensory, and musculoskeletal disorders, according to research published online ahead of print July 1 in Multiple Sclerosis Journal. Patients who later develop MS also may be more likely to have genitourinary and psychiatric symptoms in the five years before diagnosis.
“The existence of such warning signs is well-accepted for Alzheimer’s disease and Parkinson’s disease, but there has been little investigation into a similar pattern for MS,” said Helen Tremlett, PhD, Professor in the Division of Neurology at the University of British Columbia in Canada. “We now need to delve deeper into this phenomenon, perhaps using data-mining techniques. We want to see if there are discernible patterns related to sex, age, or the type of MS they eventually develop.”
Clinical and Administrative Matched Cohorts
Dr. Tremlett and colleagues analyzed data from a matched-cohort record-linkage study to examine the MS prodrome. The investigators used population-based health administrative data and clinical data from the Canadian provinces of British Columbia, Saskatchewan, Manitoba, and Nova Scotia. The information included demographics, hospital visits, physician encounters, and prescriptions filled. Clinical data were for patients diagnosed by a neurologist at an MS clinic and included first clinical visit (or date of diagnosis) and date of symptom onset. Data were collected from April 1984 to April 2014.
Using the data, Dr. Tremlett and colleagues created a health-administrative cohort and a clinical cohort. The clinical cohort did not include data from Saskatchewan. To create the cohorts, the investigators identified patients with MS and matched them by sex, year of birth, and postal code with as many as five controls. The index date was the earliest recorded claim for a demyelinating disease for the health-administrative cohort and the date of MS symptom onset for the clinical cohort. Study outcomes were the number of physician and hospital encounters per ICD-10 chapter, the number of physician encounters per physician specialty, and the percentage of people with one or more prescriptions per drug class in the five years before the index date.
Clinical Cohort Results May Be More Accurate
The administrative cohort included 13,951 cases and 66,940 controls. The clinical cohort included 3,202 cases and 16,006 controls. Compared with controls, people with MS had more physician and hospital encounters for the nervous (rate ratio [RR], 2.31 to 4.75), sensory (RR, 1.40 to 2.28), musculoskeletal (RR, 1.19 to 1.70), and genitourinary systems (RR, 1.17 to 1.59) in the five years before the first demyelinating claim or symptom onset. Cases had more visits with psychiatrists and urologists (RR, 1.48 to 1.80) and higher proportions of musculoskeletal, genitourinary, or hormonal-related prescriptions (1.1–1.5 times higher), compared with controls. People with MS had fewer pregnancy-related encounters than controls, however (RR, 0.78 to 0.88).
The “more conservative” results for the clinical cohort are more likely to reflect the MS prodrome accurately because they are “unlikely to be influenced by a physician’s suspicion or consideration of MS,” said Dr. Tremlett and colleagues. “Although not all individuals with MS attend an MS specialty clinic, the clinical cohort represents a subgroup of the population that may differ with respect to demographic and clinical characteristics from nonclinic attendees (eg, have fewer comorbidities),” they added. NR
—Erik Greb
Suggested Reading
Wijnands JM, Zhu F, Kingwell E, et al. Five years before multiple sclerosis onset: Phenotyping the prodrome. Mult Scler. 2018 Jul 1 [Epub ahead of print].
The prodrome of multiple sclerosis (MS) may include an increased risk of nervous system, sensory, and musculoskeletal disorders, according to research published online ahead of print July 1 in Multiple Sclerosis Journal. Patients who later develop MS also may be more likely to have genitourinary and psychiatric symptoms in the five years before diagnosis.
“The existence of such warning signs is well-accepted for Alzheimer’s disease and Parkinson’s disease, but there has been little investigation into a similar pattern for MS,” said Helen Tremlett, PhD, Professor in the Division of Neurology at the University of British Columbia in Canada. “We now need to delve deeper into this phenomenon, perhaps using data-mining techniques. We want to see if there are discernible patterns related to sex, age, or the type of MS they eventually develop.”
Clinical and Administrative Matched Cohorts
Dr. Tremlett and colleagues analyzed data from a matched-cohort record-linkage study to examine the MS prodrome. The investigators used population-based health administrative data and clinical data from the Canadian provinces of British Columbia, Saskatchewan, Manitoba, and Nova Scotia. The information included demographics, hospital visits, physician encounters, and prescriptions filled. Clinical data were for patients diagnosed by a neurologist at an MS clinic and included first clinical visit (or date of diagnosis) and date of symptom onset. Data were collected from April 1984 to April 2014.
Using the data, Dr. Tremlett and colleagues created a health-administrative cohort and a clinical cohort. The clinical cohort did not include data from Saskatchewan. To create the cohorts, the investigators identified patients with MS and matched them by sex, year of birth, and postal code with as many as five controls. The index date was the earliest recorded claim for a demyelinating disease for the health-administrative cohort and the date of MS symptom onset for the clinical cohort. Study outcomes were the number of physician and hospital encounters per ICD-10 chapter, the number of physician encounters per physician specialty, and the percentage of people with one or more prescriptions per drug class in the five years before the index date.
Clinical Cohort Results May Be More Accurate
The administrative cohort included 13,951 cases and 66,940 controls. The clinical cohort included 3,202 cases and 16,006 controls. Compared with controls, people with MS had more physician and hospital encounters for the nervous (rate ratio [RR], 2.31 to 4.75), sensory (RR, 1.40 to 2.28), musculoskeletal (RR, 1.19 to 1.70), and genitourinary systems (RR, 1.17 to 1.59) in the five years before the first demyelinating claim or symptom onset. Cases had more visits with psychiatrists and urologists (RR, 1.48 to 1.80) and higher proportions of musculoskeletal, genitourinary, or hormonal-related prescriptions (1.1–1.5 times higher), compared with controls. People with MS had fewer pregnancy-related encounters than controls, however (RR, 0.78 to 0.88).
The “more conservative” results for the clinical cohort are more likely to reflect the MS prodrome accurately because they are “unlikely to be influenced by a physician’s suspicion or consideration of MS,” said Dr. Tremlett and colleagues. “Although not all individuals with MS attend an MS specialty clinic, the clinical cohort represents a subgroup of the population that may differ with respect to demographic and clinical characteristics from nonclinic attendees (eg, have fewer comorbidities),” they added. NR
—Erik Greb
Suggested Reading
Wijnands JM, Zhu F, Kingwell E, et al. Five years before multiple sclerosis onset: Phenotyping the prodrome. Mult Scler. 2018 Jul 1 [Epub ahead of print].
Contraceptive considerations for women with headache and migraine
The use of hormonal contraception in women with headaches, especially migraine headaches, is an important topic. Approximately 43% of women in the United States report migraines.1 Roughly the same percentage of reproductive-aged women use hormonal contraception.2 Data suggest that all migraineurs have some increased risk of stroke. Therefore, can women with migraine headaches use combination hormonal contraception? And can women with severe headaches that are nonmigrainous use combination hormonal contraception? Let’s examine available data to help us answer these questions.
Risk factors for stroke
Migraine without aura is the most common subset, but migraine with aura is more problematic relative to the increased incidence of stroke.1
A migraine aura is visual 90% of the time.1 Symptoms can include flickering lights, spots, zigzag lines, a sense of pins and needles, or dysphasic speech. Aura precedes the headache and usually resolves within 1 hour after the aura begins.
In addition to migraine headaches, risk factors for stroke include increasing age, hypertension, the use of combination oral contraceptives (COCs), the contraceptive patch and ring, and smoking.1
Data indicate that the risk for ischemic stroke is increased in women with migraines even without the presence of other risk factors. In a meta-analysis of 14 observational studies, the risk of ischemic stroke among all migraineurs was about 2-fold (relative risk [RR], 2.2; 95% confidence interval [CI], 1.9–2.5) compared with the risk of ischemic stroke in women of the same age group who did not have migraine headaches. When there is migraine without aura, it was slightly less than 2-fold (RR, 1.8; 95% CI, 1.1–3.2). The risk of ischemic stroke among migraineurs with aura is increased more than 2 times compared with women without migraine (RR, 2.27; 95% CI, 1.61–3.19).3 However, the absolute risk of ischemic stroke among reproductive-aged women is 11 per 100,000 women years.4
Two observational studies show how additional risk factors increase that risk (TABLE).5,6 There are similar trends in terms of overall risk of stroke among women with all types of migraine. However, when you add smoking as an additional risk factor for women with migraine headaches, there is a substantial increase in the risk of stroke. When a woman who has migraines uses COCs, there is increased risk varying from 2-fold to almost 4-fold. When you combine migraine, smoking, and COCs, a very, very large risk factor (odds ratio [OR], 34.4; 95% CI, 3.27–3.61) was reported by Chang and colleagues.6
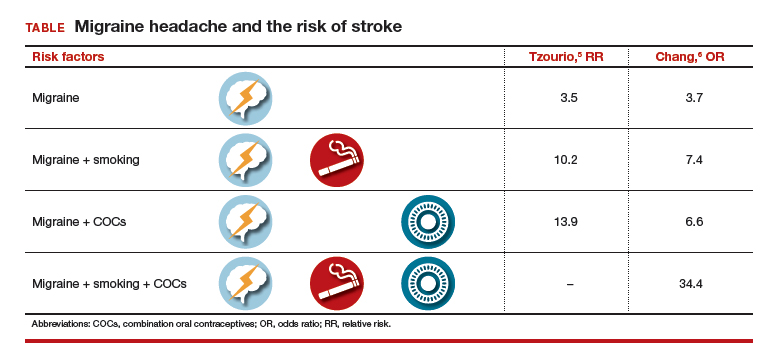
Although these risks are impressive, it is important to keep in mind that even with a 10-fold increase, we are only talking about 1 case per 1,000 migraineurs.4 Unfortunately, stroke often leads to major disability and even death, such that any reduction in risk is still important.
Preventing estrogen withdrawal or menstrual migraines
How should we treat a woman who uses hormonal contraception and reports estrogen withdrawal or menstrual migraines? Based on clinical evidence, there are 2 ways to reduce her symptoms:
- COCs. Reduce the hormone-free interval by having her take COCs for 3 to 4 days instead of 7 days, or eliminate the hormone-free interval altogether by continuous use of COCs, usually 3 months at a time.7
- NSAIDs. For those who do not want to alter how they take their hormonal product, use nonsteroidal anti-inflammatory drugs (NSAIDs) starting 7 days before the onset of menses and continuing for 13 days. In a clinical trial by Sances and colleagues, this plan reduced the frequency, duration, and severity of menstrual migraines.8
Probably altering how she takes the COC would make the most sense for most individuals instead of taking NSAIDs for 75% of each month.
Recommendations from the US MEC
The US Medical Eligibility Criteria (US MEC) from the Centers for Disease Control and Prevention (CDC) offers recommendations for contraceptive use9:
- For nonmigrainous headache, the CDC suggests that the benefits of using COCs outweigh the risks unless the headaches persist after 3 months of COC use.
- For migraine without aura, the benefits outweigh the risks in starting women who are younger than age 35 years on oral contraceptives. However, the risks of COCs outweigh the benefits in women who are age 35 years and older who develop migraine headache while on COCs, or who have risk factors for stroke.
- For migraine with aura, COCs are contraindicated.
- Progestin-only contraceptives. The CDC considers that the benefits of COC use outweigh any theoretical risk of stroke, even in women with risk factors or in women who have migraine with aura. Progestin-only contraceptives do not alter one’s risk of stroke, unlike contraceptives that contain estrogen.
My bottom line
Can women with migraine headaches begin the use of combination hormonal methods? Yes, if there is no aura in their migraines and they are not older than age 35.
Can women with severe headaches that are nonmigrainous use combination hormonal methods? Possibly, but you should discontinue COCs if headache severity persists or worsens, using a 3-month time period for evaluation.
How do you manage women with migraines during the hormone-free interval? Consider the continuous method or shorten the hormone-free interval.
Recommendations for complicated patients. Consulting the CDC’s US MEC database7 can provide assistance in your care of more complicated patients requesting contraception. I also recommend the book, “Contraception for the Medically Challenging Patient,” edited by Rebecca Allen and Carrie Cwiak.10 It links nicely with the CDC guidelines and presents more detail on each subject.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Stewart WF, Wood C, Reed MD, et al. Cumulative lifetime migraine incidence in women and men. Cephalalgia. 2008;28(11):1170–1178.
- Finer LB, Frohwirth LF, Dauphinee LA, Singh S, Moore AM. Reasons U.S. women have abortions: quantitative and qualitative perspectives. Perspect Sex Reprod Health. 2005;37(3):110–118.
- Etminan M, Takkouche B, Isorna FC, Samii A. Risk of ischemic stroke in people with migraine: systematic review and meta-analysis of observational studies. BMJ. 2005;330(7482):63–66.
- Petitti DB, Sydney S, Bernstein A, Wolf S, Quesenberry C, Ziel HK. Stoke in users of low-dose oral contraceptives. N Engl J Med. 1996;335(1):8–15.
- Tzourio C, Tehindrazanarivelo A, Iglesias S, et al. Case-control study of migraine and risk of ischemic stroke in young women. BMJ. 1995;310:830–833.
- Chang CL, Donaghy M, Poulter N. Migraine and stroke in young women: case-control study. The World Health Organisation Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. BMJ. 1999;318(7175):13–18.
- Edelman A, Gallo MF, Nichols MD, Jensen JT, Schulz KF, Grimes DA. Continuous versus cyclic use of combined oral contraceptives for contraception: systematic Cochrane review of randomized controlled trials. Hum Reprod. 2006;21(3):573–578.
- Sances G, Martignoni E, Fioroni L, Blandini F, Facchinetti F, Nappi G. Naproxen sodium in menstrual migraine prophylaxis: a double-blind placebo controlled study. Headache. 1990;30(11):705–709.
- US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1–86. https://www.cdc .gov/mmwr/pdf/rr/rr59e0528.pdf. Accessed October 4, 2016.
- Allen RH, Cwiak CA, eds. Contraception for the medically challenging patient. New York, New York: Springer New York; 2014.
The use of hormonal contraception in women with headaches, especially migraine headaches, is an important topic. Approximately 43% of women in the United States report migraines.1 Roughly the same percentage of reproductive-aged women use hormonal contraception.2 Data suggest that all migraineurs have some increased risk of stroke. Therefore, can women with migraine headaches use combination hormonal contraception? And can women with severe headaches that are nonmigrainous use combination hormonal contraception? Let’s examine available data to help us answer these questions.
Risk factors for stroke
Migraine without aura is the most common subset, but migraine with aura is more problematic relative to the increased incidence of stroke.1
A migraine aura is visual 90% of the time.1 Symptoms can include flickering lights, spots, zigzag lines, a sense of pins and needles, or dysphasic speech. Aura precedes the headache and usually resolves within 1 hour after the aura begins.
In addition to migraine headaches, risk factors for stroke include increasing age, hypertension, the use of combination oral contraceptives (COCs), the contraceptive patch and ring, and smoking.1
Data indicate that the risk for ischemic stroke is increased in women with migraines even without the presence of other risk factors. In a meta-analysis of 14 observational studies, the risk of ischemic stroke among all migraineurs was about 2-fold (relative risk [RR], 2.2; 95% confidence interval [CI], 1.9–2.5) compared with the risk of ischemic stroke in women of the same age group who did not have migraine headaches. When there is migraine without aura, it was slightly less than 2-fold (RR, 1.8; 95% CI, 1.1–3.2). The risk of ischemic stroke among migraineurs with aura is increased more than 2 times compared with women without migraine (RR, 2.27; 95% CI, 1.61–3.19).3 However, the absolute risk of ischemic stroke among reproductive-aged women is 11 per 100,000 women years.4
Two observational studies show how additional risk factors increase that risk (TABLE).5,6 There are similar trends in terms of overall risk of stroke among women with all types of migraine. However, when you add smoking as an additional risk factor for women with migraine headaches, there is a substantial increase in the risk of stroke. When a woman who has migraines uses COCs, there is increased risk varying from 2-fold to almost 4-fold. When you combine migraine, smoking, and COCs, a very, very large risk factor (odds ratio [OR], 34.4; 95% CI, 3.27–3.61) was reported by Chang and colleagues.6

Although these risks are impressive, it is important to keep in mind that even with a 10-fold increase, we are only talking about 1 case per 1,000 migraineurs.4 Unfortunately, stroke often leads to major disability and even death, such that any reduction in risk is still important.
Preventing estrogen withdrawal or menstrual migraines
How should we treat a woman who uses hormonal contraception and reports estrogen withdrawal or menstrual migraines? Based on clinical evidence, there are 2 ways to reduce her symptoms:
- COCs. Reduce the hormone-free interval by having her take COCs for 3 to 4 days instead of 7 days, or eliminate the hormone-free interval altogether by continuous use of COCs, usually 3 months at a time.7
- NSAIDs. For those who do not want to alter how they take their hormonal product, use nonsteroidal anti-inflammatory drugs (NSAIDs) starting 7 days before the onset of menses and continuing for 13 days. In a clinical trial by Sances and colleagues, this plan reduced the frequency, duration, and severity of menstrual migraines.8
Probably altering how she takes the COC would make the most sense for most individuals instead of taking NSAIDs for 75% of each month.
Recommendations from the US MEC
The US Medical Eligibility Criteria (US MEC) from the Centers for Disease Control and Prevention (CDC) offers recommendations for contraceptive use9:
- For nonmigrainous headache, the CDC suggests that the benefits of using COCs outweigh the risks unless the headaches persist after 3 months of COC use.
- For migraine without aura, the benefits outweigh the risks in starting women who are younger than age 35 years on oral contraceptives. However, the risks of COCs outweigh the benefits in women who are age 35 years and older who develop migraine headache while on COCs, or who have risk factors for stroke.
- For migraine with aura, COCs are contraindicated.
- Progestin-only contraceptives. The CDC considers that the benefits of COC use outweigh any theoretical risk of stroke, even in women with risk factors or in women who have migraine with aura. Progestin-only contraceptives do not alter one’s risk of stroke, unlike contraceptives that contain estrogen.
My bottom line
Can women with migraine headaches begin the use of combination hormonal methods? Yes, if there is no aura in their migraines and they are not older than age 35.
Can women with severe headaches that are nonmigrainous use combination hormonal methods? Possibly, but you should discontinue COCs if headache severity persists or worsens, using a 3-month time period for evaluation.
How do you manage women with migraines during the hormone-free interval? Consider the continuous method or shorten the hormone-free interval.
Recommendations for complicated patients. Consulting the CDC’s US MEC database7 can provide assistance in your care of more complicated patients requesting contraception. I also recommend the book, “Contraception for the Medically Challenging Patient,” edited by Rebecca Allen and Carrie Cwiak.10 It links nicely with the CDC guidelines and presents more detail on each subject.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The use of hormonal contraception in women with headaches, especially migraine headaches, is an important topic. Approximately 43% of women in the United States report migraines.1 Roughly the same percentage of reproductive-aged women use hormonal contraception.2 Data suggest that all migraineurs have some increased risk of stroke. Therefore, can women with migraine headaches use combination hormonal contraception? And can women with severe headaches that are nonmigrainous use combination hormonal contraception? Let’s examine available data to help us answer these questions.
Risk factors for stroke
Migraine without aura is the most common subset, but migraine with aura is more problematic relative to the increased incidence of stroke.1
A migraine aura is visual 90% of the time.1 Symptoms can include flickering lights, spots, zigzag lines, a sense of pins and needles, or dysphasic speech. Aura precedes the headache and usually resolves within 1 hour after the aura begins.
In addition to migraine headaches, risk factors for stroke include increasing age, hypertension, the use of combination oral contraceptives (COCs), the contraceptive patch and ring, and smoking.1
Data indicate that the risk for ischemic stroke is increased in women with migraines even without the presence of other risk factors. In a meta-analysis of 14 observational studies, the risk of ischemic stroke among all migraineurs was about 2-fold (relative risk [RR], 2.2; 95% confidence interval [CI], 1.9–2.5) compared with the risk of ischemic stroke in women of the same age group who did not have migraine headaches. When there is migraine without aura, it was slightly less than 2-fold (RR, 1.8; 95% CI, 1.1–3.2). The risk of ischemic stroke among migraineurs with aura is increased more than 2 times compared with women without migraine (RR, 2.27; 95% CI, 1.61–3.19).3 However, the absolute risk of ischemic stroke among reproductive-aged women is 11 per 100,000 women years.4
Two observational studies show how additional risk factors increase that risk (TABLE).5,6 There are similar trends in terms of overall risk of stroke among women with all types of migraine. However, when you add smoking as an additional risk factor for women with migraine headaches, there is a substantial increase in the risk of stroke. When a woman who has migraines uses COCs, there is increased risk varying from 2-fold to almost 4-fold. When you combine migraine, smoking, and COCs, a very, very large risk factor (odds ratio [OR], 34.4; 95% CI, 3.27–3.61) was reported by Chang and colleagues.6

Although these risks are impressive, it is important to keep in mind that even with a 10-fold increase, we are only talking about 1 case per 1,000 migraineurs.4 Unfortunately, stroke often leads to major disability and even death, such that any reduction in risk is still important.
Preventing estrogen withdrawal or menstrual migraines
How should we treat a woman who uses hormonal contraception and reports estrogen withdrawal or menstrual migraines? Based on clinical evidence, there are 2 ways to reduce her symptoms:
- COCs. Reduce the hormone-free interval by having her take COCs for 3 to 4 days instead of 7 days, or eliminate the hormone-free interval altogether by continuous use of COCs, usually 3 months at a time.7
- NSAIDs. For those who do not want to alter how they take their hormonal product, use nonsteroidal anti-inflammatory drugs (NSAIDs) starting 7 days before the onset of menses and continuing for 13 days. In a clinical trial by Sances and colleagues, this plan reduced the frequency, duration, and severity of menstrual migraines.8
Probably altering how she takes the COC would make the most sense for most individuals instead of taking NSAIDs for 75% of each month.
Recommendations from the US MEC
The US Medical Eligibility Criteria (US MEC) from the Centers for Disease Control and Prevention (CDC) offers recommendations for contraceptive use9:
- For nonmigrainous headache, the CDC suggests that the benefits of using COCs outweigh the risks unless the headaches persist after 3 months of COC use.
- For migraine without aura, the benefits outweigh the risks in starting women who are younger than age 35 years on oral contraceptives. However, the risks of COCs outweigh the benefits in women who are age 35 years and older who develop migraine headache while on COCs, or who have risk factors for stroke.
- For migraine with aura, COCs are contraindicated.
- Progestin-only contraceptives. The CDC considers that the benefits of COC use outweigh any theoretical risk of stroke, even in women with risk factors or in women who have migraine with aura. Progestin-only contraceptives do not alter one’s risk of stroke, unlike contraceptives that contain estrogen.
My bottom line
Can women with migraine headaches begin the use of combination hormonal methods? Yes, if there is no aura in their migraines and they are not older than age 35.
Can women with severe headaches that are nonmigrainous use combination hormonal methods? Possibly, but you should discontinue COCs if headache severity persists or worsens, using a 3-month time period for evaluation.
How do you manage women with migraines during the hormone-free interval? Consider the continuous method or shorten the hormone-free interval.
Recommendations for complicated patients. Consulting the CDC’s US MEC database7 can provide assistance in your care of more complicated patients requesting contraception. I also recommend the book, “Contraception for the Medically Challenging Patient,” edited by Rebecca Allen and Carrie Cwiak.10 It links nicely with the CDC guidelines and presents more detail on each subject.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Stewart WF, Wood C, Reed MD, et al. Cumulative lifetime migraine incidence in women and men. Cephalalgia. 2008;28(11):1170–1178.
- Finer LB, Frohwirth LF, Dauphinee LA, Singh S, Moore AM. Reasons U.S. women have abortions: quantitative and qualitative perspectives. Perspect Sex Reprod Health. 2005;37(3):110–118.
- Etminan M, Takkouche B, Isorna FC, Samii A. Risk of ischemic stroke in people with migraine: systematic review and meta-analysis of observational studies. BMJ. 2005;330(7482):63–66.
- Petitti DB, Sydney S, Bernstein A, Wolf S, Quesenberry C, Ziel HK. Stoke in users of low-dose oral contraceptives. N Engl J Med. 1996;335(1):8–15.
- Tzourio C, Tehindrazanarivelo A, Iglesias S, et al. Case-control study of migraine and risk of ischemic stroke in young women. BMJ. 1995;310:830–833.
- Chang CL, Donaghy M, Poulter N. Migraine and stroke in young women: case-control study. The World Health Organisation Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. BMJ. 1999;318(7175):13–18.
- Edelman A, Gallo MF, Nichols MD, Jensen JT, Schulz KF, Grimes DA. Continuous versus cyclic use of combined oral contraceptives for contraception: systematic Cochrane review of randomized controlled trials. Hum Reprod. 2006;21(3):573–578.
- Sances G, Martignoni E, Fioroni L, Blandini F, Facchinetti F, Nappi G. Naproxen sodium in menstrual migraine prophylaxis: a double-blind placebo controlled study. Headache. 1990;30(11):705–709.
- US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1–86. https://www.cdc .gov/mmwr/pdf/rr/rr59e0528.pdf. Accessed October 4, 2016.
- Allen RH, Cwiak CA, eds. Contraception for the medically challenging patient. New York, New York: Springer New York; 2014.
- Stewart WF, Wood C, Reed MD, et al. Cumulative lifetime migraine incidence in women and men. Cephalalgia. 2008;28(11):1170–1178.
- Finer LB, Frohwirth LF, Dauphinee LA, Singh S, Moore AM. Reasons U.S. women have abortions: quantitative and qualitative perspectives. Perspect Sex Reprod Health. 2005;37(3):110–118.
- Etminan M, Takkouche B, Isorna FC, Samii A. Risk of ischemic stroke in people with migraine: systematic review and meta-analysis of observational studies. BMJ. 2005;330(7482):63–66.
- Petitti DB, Sydney S, Bernstein A, Wolf S, Quesenberry C, Ziel HK. Stoke in users of low-dose oral contraceptives. N Engl J Med. 1996;335(1):8–15.
- Tzourio C, Tehindrazanarivelo A, Iglesias S, et al. Case-control study of migraine and risk of ischemic stroke in young women. BMJ. 1995;310:830–833.
- Chang CL, Donaghy M, Poulter N. Migraine and stroke in young women: case-control study. The World Health Organisation Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. BMJ. 1999;318(7175):13–18.
- Edelman A, Gallo MF, Nichols MD, Jensen JT, Schulz KF, Grimes DA. Continuous versus cyclic use of combined oral contraceptives for contraception: systematic Cochrane review of randomized controlled trials. Hum Reprod. 2006;21(3):573–578.
- Sances G, Martignoni E, Fioroni L, Blandini F, Facchinetti F, Nappi G. Naproxen sodium in menstrual migraine prophylaxis: a double-blind placebo controlled study. Headache. 1990;30(11):705–709.
- US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1–86. https://www.cdc .gov/mmwr/pdf/rr/rr59e0528.pdf. Accessed October 4, 2016.
- Allen RH, Cwiak CA, eds. Contraception for the medically challenging patient. New York, New York: Springer New York; 2014.