User login
Cost-effective wound healing described with fetal bovine collagen matrix
CHICAGO – A novel, commercially available fetal bovine collagen matrix provides “an ideal wound healing environment” for outpatient treatment of partial and full thickness wounds, ulcers, burns, and surgical wounds, Katarina R. Kesty, MD, declared at the annual meeting of the American College of Mohs Surgery.
“. We applied this product to 46 patients over 10 months and have observed favorable healing times and good cosmesis,” said Dr. Kesty, a dermatology resident at Wake Forest University, Winston-Salem, N.C.
She shared the clinical experience she and her colleagues have accrued with this product, which is called PriMatrix and is manufactured by Integra LifeSciences. She also explained how to successfully code and bill for its use.
“In-office application of this product is cost-effective when compared to similar products applied in the operating room by plastic surgeons and other specialists,” Dr. Kesty noted.
How cost-effective? She provided one example of a patient with a 12.6-cm2 defect on the scalp repaired with fetal bovine collagen matrix. Upon application of the appropriate billing codes, this repair was reimbursed by Medicare to the tune of $1,208. In contrast, another patient at Wake Forest had a 16.6-cm2 Mohs defect on the scalp repaired in the operating room by an oculoplastic surgeon who used split thickness skin grafts. For this procedure, Medicare was billed $30,805.11, and the medical center received $9,241.53 in reimbursement.
“An office repair using this fetal bovine collagen matrix is much more cost-effective,” she observed. “It also saves the patient from the risks of general anesthesia or conscious sedation.”
PriMatrix is a porous acellular collagen matrix derived from fetal bovine dermis. It contains type I and type III collagen, with the latter being particularly effective at attracting growth factors, blood, and angiogenic cytokines in support of dermal regeneration and revascularization. The product is available in solid sheets, mesh, and fenestrated forms in a variety of sizes. It needs to be rehydrated for 1 minute in room temperature saline. It can then be cut to the size of the wound and secured to the wound bed, periosteum, fascia, or cartilage with sutures or staples. The site is then covered with a thick layer of petrolatum and a tie-over bolster.
Dr. Kesty and her dermatology colleagues have applied the matrix to surgical defects ranging in size from 0.2 cm2 to 70 cm2, with an average area of 19 cm2. They have utilized the mesh format most often in order to allow drainage. They found the average healing time when the matrix was applied to exposed bone, periosteum, or perichondrium was 13.8 weeks, compared with 10.8 weeks for subcutaneous wounds.
With the use of the fetal bovine collagen matrix, wounds less than 10 cm2 in size healed in an average of 9.3 weeks, those from 10 cm2 to 25 cm2 in size healed in an average of 10.4 weeks, and wounds larger than 25 cm2 healed in an average of 15.7 weeks.
Coding and reimbursement
PriMatrix has been available for outpatient office use and reimbursement by Medicare since January 2017. Successful reimbursement requires completion of a preauthorization form, which is typically approved on the same day by Medicare and other payers. The proper CPT codes are 1527x, signifying a skin substitute graft less than 100 cm2 in size; Q4110 times the number of 1-cm2 units of PriMatrix utilized; and, when appropriate, ICD10 code Z85.828, for personal history of nonmelanoma skin cancer.
Dr. Kesty reported no financial conflicts of interest.
CHICAGO – A novel, commercially available fetal bovine collagen matrix provides “an ideal wound healing environment” for outpatient treatment of partial and full thickness wounds, ulcers, burns, and surgical wounds, Katarina R. Kesty, MD, declared at the annual meeting of the American College of Mohs Surgery.
“. We applied this product to 46 patients over 10 months and have observed favorable healing times and good cosmesis,” said Dr. Kesty, a dermatology resident at Wake Forest University, Winston-Salem, N.C.
She shared the clinical experience she and her colleagues have accrued with this product, which is called PriMatrix and is manufactured by Integra LifeSciences. She also explained how to successfully code and bill for its use.
“In-office application of this product is cost-effective when compared to similar products applied in the operating room by plastic surgeons and other specialists,” Dr. Kesty noted.
How cost-effective? She provided one example of a patient with a 12.6-cm2 defect on the scalp repaired with fetal bovine collagen matrix. Upon application of the appropriate billing codes, this repair was reimbursed by Medicare to the tune of $1,208. In contrast, another patient at Wake Forest had a 16.6-cm2 Mohs defect on the scalp repaired in the operating room by an oculoplastic surgeon who used split thickness skin grafts. For this procedure, Medicare was billed $30,805.11, and the medical center received $9,241.53 in reimbursement.
“An office repair using this fetal bovine collagen matrix is much more cost-effective,” she observed. “It also saves the patient from the risks of general anesthesia or conscious sedation.”
PriMatrix is a porous acellular collagen matrix derived from fetal bovine dermis. It contains type I and type III collagen, with the latter being particularly effective at attracting growth factors, blood, and angiogenic cytokines in support of dermal regeneration and revascularization. The product is available in solid sheets, mesh, and fenestrated forms in a variety of sizes. It needs to be rehydrated for 1 minute in room temperature saline. It can then be cut to the size of the wound and secured to the wound bed, periosteum, fascia, or cartilage with sutures or staples. The site is then covered with a thick layer of petrolatum and a tie-over bolster.
Dr. Kesty and her dermatology colleagues have applied the matrix to surgical defects ranging in size from 0.2 cm2 to 70 cm2, with an average area of 19 cm2. They have utilized the mesh format most often in order to allow drainage. They found the average healing time when the matrix was applied to exposed bone, periosteum, or perichondrium was 13.8 weeks, compared with 10.8 weeks for subcutaneous wounds.
With the use of the fetal bovine collagen matrix, wounds less than 10 cm2 in size healed in an average of 9.3 weeks, those from 10 cm2 to 25 cm2 in size healed in an average of 10.4 weeks, and wounds larger than 25 cm2 healed in an average of 15.7 weeks.
Coding and reimbursement
PriMatrix has been available for outpatient office use and reimbursement by Medicare since January 2017. Successful reimbursement requires completion of a preauthorization form, which is typically approved on the same day by Medicare and other payers. The proper CPT codes are 1527x, signifying a skin substitute graft less than 100 cm2 in size; Q4110 times the number of 1-cm2 units of PriMatrix utilized; and, when appropriate, ICD10 code Z85.828, for personal history of nonmelanoma skin cancer.
Dr. Kesty reported no financial conflicts of interest.
CHICAGO – A novel, commercially available fetal bovine collagen matrix provides “an ideal wound healing environment” for outpatient treatment of partial and full thickness wounds, ulcers, burns, and surgical wounds, Katarina R. Kesty, MD, declared at the annual meeting of the American College of Mohs Surgery.
“. We applied this product to 46 patients over 10 months and have observed favorable healing times and good cosmesis,” said Dr. Kesty, a dermatology resident at Wake Forest University, Winston-Salem, N.C.
She shared the clinical experience she and her colleagues have accrued with this product, which is called PriMatrix and is manufactured by Integra LifeSciences. She also explained how to successfully code and bill for its use.
“In-office application of this product is cost-effective when compared to similar products applied in the operating room by plastic surgeons and other specialists,” Dr. Kesty noted.
How cost-effective? She provided one example of a patient with a 12.6-cm2 defect on the scalp repaired with fetal bovine collagen matrix. Upon application of the appropriate billing codes, this repair was reimbursed by Medicare to the tune of $1,208. In contrast, another patient at Wake Forest had a 16.6-cm2 Mohs defect on the scalp repaired in the operating room by an oculoplastic surgeon who used split thickness skin grafts. For this procedure, Medicare was billed $30,805.11, and the medical center received $9,241.53 in reimbursement.
“An office repair using this fetal bovine collagen matrix is much more cost-effective,” she observed. “It also saves the patient from the risks of general anesthesia or conscious sedation.”
PriMatrix is a porous acellular collagen matrix derived from fetal bovine dermis. It contains type I and type III collagen, with the latter being particularly effective at attracting growth factors, blood, and angiogenic cytokines in support of dermal regeneration and revascularization. The product is available in solid sheets, mesh, and fenestrated forms in a variety of sizes. It needs to be rehydrated for 1 minute in room temperature saline. It can then be cut to the size of the wound and secured to the wound bed, periosteum, fascia, or cartilage with sutures or staples. The site is then covered with a thick layer of petrolatum and a tie-over bolster.
Dr. Kesty and her dermatology colleagues have applied the matrix to surgical defects ranging in size from 0.2 cm2 to 70 cm2, with an average area of 19 cm2. They have utilized the mesh format most often in order to allow drainage. They found the average healing time when the matrix was applied to exposed bone, periosteum, or perichondrium was 13.8 weeks, compared with 10.8 weeks for subcutaneous wounds.
With the use of the fetal bovine collagen matrix, wounds less than 10 cm2 in size healed in an average of 9.3 weeks, those from 10 cm2 to 25 cm2 in size healed in an average of 10.4 weeks, and wounds larger than 25 cm2 healed in an average of 15.7 weeks.
Coding and reimbursement
PriMatrix has been available for outpatient office use and reimbursement by Medicare since January 2017. Successful reimbursement requires completion of a preauthorization form, which is typically approved on the same day by Medicare and other payers. The proper CPT codes are 1527x, signifying a skin substitute graft less than 100 cm2 in size; Q4110 times the number of 1-cm2 units of PriMatrix utilized; and, when appropriate, ICD10 code Z85.828, for personal history of nonmelanoma skin cancer.
Dr. Kesty reported no financial conflicts of interest.
EXPERT ANALYSIS FROM THE ACMS ANNUAL MEETING
CMS targets Part B drug policy in 2019 regulatory updates
Doctors could see changes in how they are paid by the Centers for Medicare & Medicaid Services for the drugs they administer in their office, depending on the outcome of two recent regulatory actions proposed by the agency.
The more immediate action could see an alteration to payment rates for newly launched drugs. The more long-term action could be the relaunch of the Competitive Acquisition Program, although there is much more uncertainty surrounding that change.
CMS is seeking to lower the Part B add-on payment for drugs that are new to market and do not yet have an average sales price (ASP) established. The proposal calls for these drugs to be reimbursed at the wholesale acquisition cost (WAC) plus 3%, rather than the current rate of WAC plus 6%. The change is part of the proposed physician fee schedule for 2019.
The add-on payment has no statutory definition as to what it is intended to cover, but CMS noted in the proposed rule that it “is widely believed to include services associated with drug acquisition that are not separately paid for, such as handling and storage, as well as additional mark-ups in drug distribution channels.”
Agency officials said that the add-on payment has raised concerns in recent years “because more revenue can be generated from percentage-based add-on payments for expensive drugs, and an opportunity to generate more revenue may create an incentive for the use of more expensive drugs.”
CMS also noted that once an ASP has been established – generally after a drug has been available for several months – the price for that drug is generally lower than the WAC price and, citing a 2014 HHS Office of Inspector General report, noted that “WACs often do not reflect the actual market price for drugs.”
The move to lower payments to WAC plus 3% for new drugs is consistent with a recent recommendation from the Medicare Payment Advisory Commission (MedPAC).
CMS added that the reduction would reduce beneficiary out-of-pocket costs, since copayments are a percentage of the total cost of the drug, including the add-on payment amount.
“The proposed approach would help Medicare beneficiaries afford to pay for new drugs by reducing out-of-pocket expenses and would help counteract the effects of increasing launch prices for newly approved drugs and biologicals,” CMS said in the proposed regulation.
But the American College of Rheumatology raised concerns about the proposal. Specifically, ACR is concerned that plans to cut add-on payments for new drugs “could slow market uptake of biosimilars and thwart the Administration’s efforts to reduce drug prices,” the group said in a statement.
The Community Oncology Alliance (COA) also took issue with the proposal. “This is a payment cut from the current rate of Wholesale Acquisition Cost (WAC) plus 6%, or what is really plus 4.3% when factoring in the sequester,” the COA said in a statement. “COA believes that this payment cut for new cancer therapies will result in drug manufacturers actually increasing WAC list prices so that their new products will not be at a competitive disadvantage to existing products, which are reimbursed at average sales price (ASP) plus 6%.”
The second proposal, which could take longer to materialize, revolves around the potential relaunch of the failed competitive acquisition program (CAP) for Part B drugs. CMS is currently requesting information, with questions on what a revamped program could look like if the agency were to move forward with it. The request for information is part of the proposed rule updating the Outpatient Prospective Payment System for 2019.
Under the original CAP, physicians who participated in the program would order drugs from an approved vendor, who would then bill Medicare and collect cost-sharing payments from the beneficiary. The original program was in operation for 18 months, ending on Dec. 31, 2008, after it had little participation and faced other concerns.
More recently, MedPAC recommended a revised version of the program, which they dubbed the Part B Drug Value Program (DVP). Under this construct, private vendors would acquire drugs at lower prices using various negotiation tools, and physicians would be encouraged to make more value-based use decisions based on opportunities for shared savings though their Medicare billing for the use of Part B drugs.
CMS is asking for feedback on a wide range of questions on how the revamped CAP program should be designed, including program design, which suppliers and drugs to include, how to incentivize participation, how to structure outcomes-based arrangements, and whether indication-based pricing should be used.
Doctors could see changes in how they are paid by the Centers for Medicare & Medicaid Services for the drugs they administer in their office, depending on the outcome of two recent regulatory actions proposed by the agency.
The more immediate action could see an alteration to payment rates for newly launched drugs. The more long-term action could be the relaunch of the Competitive Acquisition Program, although there is much more uncertainty surrounding that change.
CMS is seeking to lower the Part B add-on payment for drugs that are new to market and do not yet have an average sales price (ASP) established. The proposal calls for these drugs to be reimbursed at the wholesale acquisition cost (WAC) plus 3%, rather than the current rate of WAC plus 6%. The change is part of the proposed physician fee schedule for 2019.
The add-on payment has no statutory definition as to what it is intended to cover, but CMS noted in the proposed rule that it “is widely believed to include services associated with drug acquisition that are not separately paid for, such as handling and storage, as well as additional mark-ups in drug distribution channels.”
Agency officials said that the add-on payment has raised concerns in recent years “because more revenue can be generated from percentage-based add-on payments for expensive drugs, and an opportunity to generate more revenue may create an incentive for the use of more expensive drugs.”
CMS also noted that once an ASP has been established – generally after a drug has been available for several months – the price for that drug is generally lower than the WAC price and, citing a 2014 HHS Office of Inspector General report, noted that “WACs often do not reflect the actual market price for drugs.”
The move to lower payments to WAC plus 3% for new drugs is consistent with a recent recommendation from the Medicare Payment Advisory Commission (MedPAC).
CMS added that the reduction would reduce beneficiary out-of-pocket costs, since copayments are a percentage of the total cost of the drug, including the add-on payment amount.
“The proposed approach would help Medicare beneficiaries afford to pay for new drugs by reducing out-of-pocket expenses and would help counteract the effects of increasing launch prices for newly approved drugs and biologicals,” CMS said in the proposed regulation.
But the American College of Rheumatology raised concerns about the proposal. Specifically, ACR is concerned that plans to cut add-on payments for new drugs “could slow market uptake of biosimilars and thwart the Administration’s efforts to reduce drug prices,” the group said in a statement.
The Community Oncology Alliance (COA) also took issue with the proposal. “This is a payment cut from the current rate of Wholesale Acquisition Cost (WAC) plus 6%, or what is really plus 4.3% when factoring in the sequester,” the COA said in a statement. “COA believes that this payment cut for new cancer therapies will result in drug manufacturers actually increasing WAC list prices so that their new products will not be at a competitive disadvantage to existing products, which are reimbursed at average sales price (ASP) plus 6%.”
The second proposal, which could take longer to materialize, revolves around the potential relaunch of the failed competitive acquisition program (CAP) for Part B drugs. CMS is currently requesting information, with questions on what a revamped program could look like if the agency were to move forward with it. The request for information is part of the proposed rule updating the Outpatient Prospective Payment System for 2019.
Under the original CAP, physicians who participated in the program would order drugs from an approved vendor, who would then bill Medicare and collect cost-sharing payments from the beneficiary. The original program was in operation for 18 months, ending on Dec. 31, 2008, after it had little participation and faced other concerns.
More recently, MedPAC recommended a revised version of the program, which they dubbed the Part B Drug Value Program (DVP). Under this construct, private vendors would acquire drugs at lower prices using various negotiation tools, and physicians would be encouraged to make more value-based use decisions based on opportunities for shared savings though their Medicare billing for the use of Part B drugs.
CMS is asking for feedback on a wide range of questions on how the revamped CAP program should be designed, including program design, which suppliers and drugs to include, how to incentivize participation, how to structure outcomes-based arrangements, and whether indication-based pricing should be used.
Doctors could see changes in how they are paid by the Centers for Medicare & Medicaid Services for the drugs they administer in their office, depending on the outcome of two recent regulatory actions proposed by the agency.
The more immediate action could see an alteration to payment rates for newly launched drugs. The more long-term action could be the relaunch of the Competitive Acquisition Program, although there is much more uncertainty surrounding that change.
CMS is seeking to lower the Part B add-on payment for drugs that are new to market and do not yet have an average sales price (ASP) established. The proposal calls for these drugs to be reimbursed at the wholesale acquisition cost (WAC) plus 3%, rather than the current rate of WAC plus 6%. The change is part of the proposed physician fee schedule for 2019.
The add-on payment has no statutory definition as to what it is intended to cover, but CMS noted in the proposed rule that it “is widely believed to include services associated with drug acquisition that are not separately paid for, such as handling and storage, as well as additional mark-ups in drug distribution channels.”
Agency officials said that the add-on payment has raised concerns in recent years “because more revenue can be generated from percentage-based add-on payments for expensive drugs, and an opportunity to generate more revenue may create an incentive for the use of more expensive drugs.”
CMS also noted that once an ASP has been established – generally after a drug has been available for several months – the price for that drug is generally lower than the WAC price and, citing a 2014 HHS Office of Inspector General report, noted that “WACs often do not reflect the actual market price for drugs.”
The move to lower payments to WAC plus 3% for new drugs is consistent with a recent recommendation from the Medicare Payment Advisory Commission (MedPAC).
CMS added that the reduction would reduce beneficiary out-of-pocket costs, since copayments are a percentage of the total cost of the drug, including the add-on payment amount.
“The proposed approach would help Medicare beneficiaries afford to pay for new drugs by reducing out-of-pocket expenses and would help counteract the effects of increasing launch prices for newly approved drugs and biologicals,” CMS said in the proposed regulation.
But the American College of Rheumatology raised concerns about the proposal. Specifically, ACR is concerned that plans to cut add-on payments for new drugs “could slow market uptake of biosimilars and thwart the Administration’s efforts to reduce drug prices,” the group said in a statement.
The Community Oncology Alliance (COA) also took issue with the proposal. “This is a payment cut from the current rate of Wholesale Acquisition Cost (WAC) plus 6%, or what is really plus 4.3% when factoring in the sequester,” the COA said in a statement. “COA believes that this payment cut for new cancer therapies will result in drug manufacturers actually increasing WAC list prices so that their new products will not be at a competitive disadvantage to existing products, which are reimbursed at average sales price (ASP) plus 6%.”
The second proposal, which could take longer to materialize, revolves around the potential relaunch of the failed competitive acquisition program (CAP) for Part B drugs. CMS is currently requesting information, with questions on what a revamped program could look like if the agency were to move forward with it. The request for information is part of the proposed rule updating the Outpatient Prospective Payment System for 2019.
Under the original CAP, physicians who participated in the program would order drugs from an approved vendor, who would then bill Medicare and collect cost-sharing payments from the beneficiary. The original program was in operation for 18 months, ending on Dec. 31, 2008, after it had little participation and faced other concerns.
More recently, MedPAC recommended a revised version of the program, which they dubbed the Part B Drug Value Program (DVP). Under this construct, private vendors would acquire drugs at lower prices using various negotiation tools, and physicians would be encouraged to make more value-based use decisions based on opportunities for shared savings though their Medicare billing for the use of Part B drugs.
CMS is asking for feedback on a wide range of questions on how the revamped CAP program should be designed, including program design, which suppliers and drugs to include, how to incentivize participation, how to structure outcomes-based arrangements, and whether indication-based pricing should be used.
Five common pitfalls of retailing skin care
Others believe that providing patients with the correct skin care product recommendations for their skin’s needs is a crucial step to improving outcomes and educating patients.
There is a wide range of challenges related to skin care retail that many physicians face. I will be running a course on Skin Care Retail at the American Society for Dermatologic Surgery meeting in October in Scottsdale, Ariz., if you want to learn more or share your opinions. I have surveyed plastic surgeons and dermatologists via LinkedIn about what they believe are some of the biggest pitfalls to retailing skin care. Here, I will share some of their insights and suggestions for overcoming these obstacles.
1. Patients are more knowledgeable about skin care than ever before
Facing an increasing number of over-the-counter skin care products available, as well as buzzwords like “organic ingredients” and “vegan,” patients are now bombarded with information from a variety of different sources. Because of this, patients come to the doctor with preconceived ideas that can affect compliance if their specific needs and beliefs are not properly addressed.
For New York plastic surgeon Sonita M. Sadio, MD, this is one of the reasons why she chooses not to sell skin care in her office.
“My practice is highly consultative, and ongoing skin care recommendations are a significant part of what I do to optimize patient outcomes,” Dr. Sadio said. “Patients are well-educated about skin care today. They know their ingredients and insist on clean formulations, free of certain ingredients, such as ‘cruelty-free’ and ‘vegan.’ Others feel deprived if they are not using an expensive product in elegant packaging. Still, others insist on drugstore favorites or ‘eco’ offerings and have their own sense of what that means. My job is to optimize the clinical outcome while also meeting these patients needs to ensure compliance.”
Not all doctors have the time, knowledge or desire to personally design each patient’s skin care regimen. Many delegate this to the staff. However, it is impossible to ensure that your staff matches patients to the proper products unless they have had extensive training on both skin care products and how to match them to the patient’s skin issues.
2. Patients are wary when the doctors sells only one product brand
Many studies have shown that, although consumers desire a choice when making purchases, they get overwhelmed if they are presented with too many options. One study showed that it is optimal to carry at least 3 brands of products. For this reason, limiting the skin care you sell to one brand or doing your own private label is not optimal.
New York dermatologist Rebecca Tamez, MD, pointed out the same problem when selling practice-specific skin care. “At my previous job, we sold skin care products directly to patients. I had no issues selling products that were readily available in drugstores or online (such as Vanicream and EltaMD). We usually sold these around the same cost as the drugstore or Amazon. However, it was harder to sell the practice-specific skin care line. I feel patients were more wary of these products.”
3. Doctors do not want to feel like salespeople
If you have read my Dermatology News columns in the past, you may know that I think it is unethical for dermatologists to not offer specific skin care advice to their patients. If patients do not get ethical and scientific recommendations from us, they will follow the advice of a friend or salesperson or purchase based on often inflated marketing claims.
Dermatologists often tell me: “I am not a cosmetic dermatologist so I do not sell skin care.” I feel strongly that general dermatologists should be giving specific written skin care recommendations for their patients too. Acne, rosacea, melasma, eczema, psoriasis, keratosis pilaris, and many other conditions will improve faster with an efficacious skin care regimen, assuming the patient is compliant with the instructions. Retailing skin care improves compliance by eliminating a few barriers to beginning the skin care regimen. I believe that the mindset of dermatologists needs to change: It is not about selling products to patients, it is about educating them on what to use and offering the products out of convenience and the desire to improve compliance.
Meadowbrook, Pa., dermatologist Michael A. Tomeo, MD, explained an obstacle faced by many dermatologists:
“I suspect, like many of my colleagues,” said Dr. Tomeo, “that I am held back in terms of salesmanship, having been trained in the traditional way. Physicians of my generation were taught to be ethical and professional and to focus on academic and clinical excellence, and salesmanship and advertising one’s services were frowned upon. It takes time to reset one’s former proclivities. Cosmeceuticals and nutraceuticals are revolutionizing the skin care world, and as experts in all things skin, we need to be well informed and offer our patients safe, effective, and cutting-edge treatments.”
4. Providers are concerned about product costs and time constraints
Providing excellent patient care and improving outcomes is at the forefront of our business, but financial concerns and time constraints prevent some doctors from offering skin care to their patients.
Rochester Hills, Mich., plastic surgeon Richard Hainer, MD, has found that “skin care is often too complex with too many products and is not very profitable.” For those reasons, Dr. Hainer has chosen not to retail skin care in his practice.
Nampa, Idaho, dermatologist Ryan S. Owsley, MD, explained that “the required minimum purchases by some of the product lines can leave the practice with expired product if it is not selling a particular line well. Cost can also be an issue for some patients in the area we are located.”
As a burn survivor and burn surgeon, Mark McDonough, MD, from Orlando “has a long history with skin care and rejuvenation. I did have a private label skin care line, including a moisturizer, a hydroquinone product, a retinol cream, and a sunscreen,” Dr. McDonough said. “However, and regrettably, I have not kept up with marketing and promotion, with most of my energy invested in trauma and disease survivors through a book, a blog, and my platform through my website.”
Doing your own product line is costly and spending the time and resources to promote it is not always possible. Buying the minimum order of products is often expensive, and you will not be able to sell them without a proven methodology in place. New products enter the market frequently, and it is expensive to always carry the latest technologies because new minimum orders must be met with each new brand that you add.
5. Selling skin care requires ongoing education
Properly recommending and retailing skin care involves physician, staff, and patient education. Unfortunately, most practices rely on training from the cosmeceutical sales reps who obviously have a brand bias. There is minimal unbiased “brand agnostic” skin care training for dermatologists and their staff. In fact, the AAD meeting has only a few skin care lectures in the program. Plastic surgeon Gaurav Bharti, MD, of Charlotte, N.C., explained that “motivating staff to help with retail skin care can be challenging. The first step is to get the staff familiar with the products with open discussions with the representatives. The next step has been to have the staff actually use the products and believe in them. Once they believe in the product, we have used an incentivization model that’s simple, transparent, and predictable.”
We are all too busy to spend adequate time with our patients, so it is critical that our staff be able to properly recommended skin care for us. We have to ensure that our staff is taking an ethical and scientific approach to skin care retail rather than a financial one. Rigorous staff training on how to match skin care products to skin type is the key to improving outcomes with skin care recommendations.
In a similar sense, Cincinnati plastic surgeon Richard Williams, MD, commented that “aestheticians often succumb to the desires of our patients to carry too many products in inventory, for which they do not have enough knowledge of the product’s benefits. This can be a very frustrating challenge.”
Conclusion
Although there are many obstacles to retailing skin care in your medical practice, the benefits that it provides to both your patients (improved outcomes) and your practice (increased profitability) far outweigh the challenges. I solved these pitfalls in my own practice by developing a standardized staff training program and skin care diagnostic software that is now used by over 100 medical practices. If you want to start retaining skin care, my advice is develop a training plan and a methodology for the recommendation and patient education process before you spend a lot of money on the required minimum product order. Feel free to contact me for advice. Alternatively, if you already do a great job of retailing skin care and want to provide tips to include in my American Society for Dermatologic Surgery course, contact me on LinkedIn or [email protected]. You can also find blogs I have written on skin care retail advice at STSFranchise.com.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014); she also wrote a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems.
Others believe that providing patients with the correct skin care product recommendations for their skin’s needs is a crucial step to improving outcomes and educating patients.
There is a wide range of challenges related to skin care retail that many physicians face. I will be running a course on Skin Care Retail at the American Society for Dermatologic Surgery meeting in October in Scottsdale, Ariz., if you want to learn more or share your opinions. I have surveyed plastic surgeons and dermatologists via LinkedIn about what they believe are some of the biggest pitfalls to retailing skin care. Here, I will share some of their insights and suggestions for overcoming these obstacles.
1. Patients are more knowledgeable about skin care than ever before
Facing an increasing number of over-the-counter skin care products available, as well as buzzwords like “organic ingredients” and “vegan,” patients are now bombarded with information from a variety of different sources. Because of this, patients come to the doctor with preconceived ideas that can affect compliance if their specific needs and beliefs are not properly addressed.
For New York plastic surgeon Sonita M. Sadio, MD, this is one of the reasons why she chooses not to sell skin care in her office.
“My practice is highly consultative, and ongoing skin care recommendations are a significant part of what I do to optimize patient outcomes,” Dr. Sadio said. “Patients are well-educated about skin care today. They know their ingredients and insist on clean formulations, free of certain ingredients, such as ‘cruelty-free’ and ‘vegan.’ Others feel deprived if they are not using an expensive product in elegant packaging. Still, others insist on drugstore favorites or ‘eco’ offerings and have their own sense of what that means. My job is to optimize the clinical outcome while also meeting these patients needs to ensure compliance.”
Not all doctors have the time, knowledge or desire to personally design each patient’s skin care regimen. Many delegate this to the staff. However, it is impossible to ensure that your staff matches patients to the proper products unless they have had extensive training on both skin care products and how to match them to the patient’s skin issues.
2. Patients are wary when the doctors sells only one product brand
Many studies have shown that, although consumers desire a choice when making purchases, they get overwhelmed if they are presented with too many options. One study showed that it is optimal to carry at least 3 brands of products. For this reason, limiting the skin care you sell to one brand or doing your own private label is not optimal.
New York dermatologist Rebecca Tamez, MD, pointed out the same problem when selling practice-specific skin care. “At my previous job, we sold skin care products directly to patients. I had no issues selling products that were readily available in drugstores or online (such as Vanicream and EltaMD). We usually sold these around the same cost as the drugstore or Amazon. However, it was harder to sell the practice-specific skin care line. I feel patients were more wary of these products.”
3. Doctors do not want to feel like salespeople
If you have read my Dermatology News columns in the past, you may know that I think it is unethical for dermatologists to not offer specific skin care advice to their patients. If patients do not get ethical and scientific recommendations from us, they will follow the advice of a friend or salesperson or purchase based on often inflated marketing claims.
Dermatologists often tell me: “I am not a cosmetic dermatologist so I do not sell skin care.” I feel strongly that general dermatologists should be giving specific written skin care recommendations for their patients too. Acne, rosacea, melasma, eczema, psoriasis, keratosis pilaris, and many other conditions will improve faster with an efficacious skin care regimen, assuming the patient is compliant with the instructions. Retailing skin care improves compliance by eliminating a few barriers to beginning the skin care regimen. I believe that the mindset of dermatologists needs to change: It is not about selling products to patients, it is about educating them on what to use and offering the products out of convenience and the desire to improve compliance.
Meadowbrook, Pa., dermatologist Michael A. Tomeo, MD, explained an obstacle faced by many dermatologists:
“I suspect, like many of my colleagues,” said Dr. Tomeo, “that I am held back in terms of salesmanship, having been trained in the traditional way. Physicians of my generation were taught to be ethical and professional and to focus on academic and clinical excellence, and salesmanship and advertising one’s services were frowned upon. It takes time to reset one’s former proclivities. Cosmeceuticals and nutraceuticals are revolutionizing the skin care world, and as experts in all things skin, we need to be well informed and offer our patients safe, effective, and cutting-edge treatments.”
4. Providers are concerned about product costs and time constraints
Providing excellent patient care and improving outcomes is at the forefront of our business, but financial concerns and time constraints prevent some doctors from offering skin care to their patients.
Rochester Hills, Mich., plastic surgeon Richard Hainer, MD, has found that “skin care is often too complex with too many products and is not very profitable.” For those reasons, Dr. Hainer has chosen not to retail skin care in his practice.
Nampa, Idaho, dermatologist Ryan S. Owsley, MD, explained that “the required minimum purchases by some of the product lines can leave the practice with expired product if it is not selling a particular line well. Cost can also be an issue for some patients in the area we are located.”
As a burn survivor and burn surgeon, Mark McDonough, MD, from Orlando “has a long history with skin care and rejuvenation. I did have a private label skin care line, including a moisturizer, a hydroquinone product, a retinol cream, and a sunscreen,” Dr. McDonough said. “However, and regrettably, I have not kept up with marketing and promotion, with most of my energy invested in trauma and disease survivors through a book, a blog, and my platform through my website.”
Doing your own product line is costly and spending the time and resources to promote it is not always possible. Buying the minimum order of products is often expensive, and you will not be able to sell them without a proven methodology in place. New products enter the market frequently, and it is expensive to always carry the latest technologies because new minimum orders must be met with each new brand that you add.
5. Selling skin care requires ongoing education
Properly recommending and retailing skin care involves physician, staff, and patient education. Unfortunately, most practices rely on training from the cosmeceutical sales reps who obviously have a brand bias. There is minimal unbiased “brand agnostic” skin care training for dermatologists and their staff. In fact, the AAD meeting has only a few skin care lectures in the program. Plastic surgeon Gaurav Bharti, MD, of Charlotte, N.C., explained that “motivating staff to help with retail skin care can be challenging. The first step is to get the staff familiar with the products with open discussions with the representatives. The next step has been to have the staff actually use the products and believe in them. Once they believe in the product, we have used an incentivization model that’s simple, transparent, and predictable.”
We are all too busy to spend adequate time with our patients, so it is critical that our staff be able to properly recommended skin care for us. We have to ensure that our staff is taking an ethical and scientific approach to skin care retail rather than a financial one. Rigorous staff training on how to match skin care products to skin type is the key to improving outcomes with skin care recommendations.
In a similar sense, Cincinnati plastic surgeon Richard Williams, MD, commented that “aestheticians often succumb to the desires of our patients to carry too many products in inventory, for which they do not have enough knowledge of the product’s benefits. This can be a very frustrating challenge.”
Conclusion
Although there are many obstacles to retailing skin care in your medical practice, the benefits that it provides to both your patients (improved outcomes) and your practice (increased profitability) far outweigh the challenges. I solved these pitfalls in my own practice by developing a standardized staff training program and skin care diagnostic software that is now used by over 100 medical practices. If you want to start retaining skin care, my advice is develop a training plan and a methodology for the recommendation and patient education process before you spend a lot of money on the required minimum product order. Feel free to contact me for advice. Alternatively, if you already do a great job of retailing skin care and want to provide tips to include in my American Society for Dermatologic Surgery course, contact me on LinkedIn or [email protected]. You can also find blogs I have written on skin care retail advice at STSFranchise.com.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014); she also wrote a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems.
Others believe that providing patients with the correct skin care product recommendations for their skin’s needs is a crucial step to improving outcomes and educating patients.
There is a wide range of challenges related to skin care retail that many physicians face. I will be running a course on Skin Care Retail at the American Society for Dermatologic Surgery meeting in October in Scottsdale, Ariz., if you want to learn more or share your opinions. I have surveyed plastic surgeons and dermatologists via LinkedIn about what they believe are some of the biggest pitfalls to retailing skin care. Here, I will share some of their insights and suggestions for overcoming these obstacles.
1. Patients are more knowledgeable about skin care than ever before
Facing an increasing number of over-the-counter skin care products available, as well as buzzwords like “organic ingredients” and “vegan,” patients are now bombarded with information from a variety of different sources. Because of this, patients come to the doctor with preconceived ideas that can affect compliance if their specific needs and beliefs are not properly addressed.
For New York plastic surgeon Sonita M. Sadio, MD, this is one of the reasons why she chooses not to sell skin care in her office.
“My practice is highly consultative, and ongoing skin care recommendations are a significant part of what I do to optimize patient outcomes,” Dr. Sadio said. “Patients are well-educated about skin care today. They know their ingredients and insist on clean formulations, free of certain ingredients, such as ‘cruelty-free’ and ‘vegan.’ Others feel deprived if they are not using an expensive product in elegant packaging. Still, others insist on drugstore favorites or ‘eco’ offerings and have their own sense of what that means. My job is to optimize the clinical outcome while also meeting these patients needs to ensure compliance.”
Not all doctors have the time, knowledge or desire to personally design each patient’s skin care regimen. Many delegate this to the staff. However, it is impossible to ensure that your staff matches patients to the proper products unless they have had extensive training on both skin care products and how to match them to the patient’s skin issues.
2. Patients are wary when the doctors sells only one product brand
Many studies have shown that, although consumers desire a choice when making purchases, they get overwhelmed if they are presented with too many options. One study showed that it is optimal to carry at least 3 brands of products. For this reason, limiting the skin care you sell to one brand or doing your own private label is not optimal.
New York dermatologist Rebecca Tamez, MD, pointed out the same problem when selling practice-specific skin care. “At my previous job, we sold skin care products directly to patients. I had no issues selling products that were readily available in drugstores or online (such as Vanicream and EltaMD). We usually sold these around the same cost as the drugstore or Amazon. However, it was harder to sell the practice-specific skin care line. I feel patients were more wary of these products.”
3. Doctors do not want to feel like salespeople
If you have read my Dermatology News columns in the past, you may know that I think it is unethical for dermatologists to not offer specific skin care advice to their patients. If patients do not get ethical and scientific recommendations from us, they will follow the advice of a friend or salesperson or purchase based on often inflated marketing claims.
Dermatologists often tell me: “I am not a cosmetic dermatologist so I do not sell skin care.” I feel strongly that general dermatologists should be giving specific written skin care recommendations for their patients too. Acne, rosacea, melasma, eczema, psoriasis, keratosis pilaris, and many other conditions will improve faster with an efficacious skin care regimen, assuming the patient is compliant with the instructions. Retailing skin care improves compliance by eliminating a few barriers to beginning the skin care regimen. I believe that the mindset of dermatologists needs to change: It is not about selling products to patients, it is about educating them on what to use and offering the products out of convenience and the desire to improve compliance.
Meadowbrook, Pa., dermatologist Michael A. Tomeo, MD, explained an obstacle faced by many dermatologists:
“I suspect, like many of my colleagues,” said Dr. Tomeo, “that I am held back in terms of salesmanship, having been trained in the traditional way. Physicians of my generation were taught to be ethical and professional and to focus on academic and clinical excellence, and salesmanship and advertising one’s services were frowned upon. It takes time to reset one’s former proclivities. Cosmeceuticals and nutraceuticals are revolutionizing the skin care world, and as experts in all things skin, we need to be well informed and offer our patients safe, effective, and cutting-edge treatments.”
4. Providers are concerned about product costs and time constraints
Providing excellent patient care and improving outcomes is at the forefront of our business, but financial concerns and time constraints prevent some doctors from offering skin care to their patients.
Rochester Hills, Mich., plastic surgeon Richard Hainer, MD, has found that “skin care is often too complex with too many products and is not very profitable.” For those reasons, Dr. Hainer has chosen not to retail skin care in his practice.
Nampa, Idaho, dermatologist Ryan S. Owsley, MD, explained that “the required minimum purchases by some of the product lines can leave the practice with expired product if it is not selling a particular line well. Cost can also be an issue for some patients in the area we are located.”
As a burn survivor and burn surgeon, Mark McDonough, MD, from Orlando “has a long history with skin care and rejuvenation. I did have a private label skin care line, including a moisturizer, a hydroquinone product, a retinol cream, and a sunscreen,” Dr. McDonough said. “However, and regrettably, I have not kept up with marketing and promotion, with most of my energy invested in trauma and disease survivors through a book, a blog, and my platform through my website.”
Doing your own product line is costly and spending the time and resources to promote it is not always possible. Buying the minimum order of products is often expensive, and you will not be able to sell them without a proven methodology in place. New products enter the market frequently, and it is expensive to always carry the latest technologies because new minimum orders must be met with each new brand that you add.
5. Selling skin care requires ongoing education
Properly recommending and retailing skin care involves physician, staff, and patient education. Unfortunately, most practices rely on training from the cosmeceutical sales reps who obviously have a brand bias. There is minimal unbiased “brand agnostic” skin care training for dermatologists and their staff. In fact, the AAD meeting has only a few skin care lectures in the program. Plastic surgeon Gaurav Bharti, MD, of Charlotte, N.C., explained that “motivating staff to help with retail skin care can be challenging. The first step is to get the staff familiar with the products with open discussions with the representatives. The next step has been to have the staff actually use the products and believe in them. Once they believe in the product, we have used an incentivization model that’s simple, transparent, and predictable.”
We are all too busy to spend adequate time with our patients, so it is critical that our staff be able to properly recommended skin care for us. We have to ensure that our staff is taking an ethical and scientific approach to skin care retail rather than a financial one. Rigorous staff training on how to match skin care products to skin type is the key to improving outcomes with skin care recommendations.
In a similar sense, Cincinnati plastic surgeon Richard Williams, MD, commented that “aestheticians often succumb to the desires of our patients to carry too many products in inventory, for which they do not have enough knowledge of the product’s benefits. This can be a very frustrating challenge.”
Conclusion
Although there are many obstacles to retailing skin care in your medical practice, the benefits that it provides to both your patients (improved outcomes) and your practice (increased profitability) far outweigh the challenges. I solved these pitfalls in my own practice by developing a standardized staff training program and skin care diagnostic software that is now used by over 100 medical practices. If you want to start retaining skin care, my advice is develop a training plan and a methodology for the recommendation and patient education process before you spend a lot of money on the required minimum product order. Feel free to contact me for advice. Alternatively, if you already do a great job of retailing skin care and want to provide tips to include in my American Society for Dermatologic Surgery course, contact me on LinkedIn or [email protected]. You can also find blogs I have written on skin care retail advice at STSFranchise.com.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014); she also wrote a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems.
MOC: ACOG’s role in developing a solution to the heated controversy

The American Board of Medical Specialties (ABMS) has decided to trade the phrase “maintenance of certification” (MOC) for “continuing board certification,” a seemingly minor change that has an important backstory. This is the story of how the physician community flexed its collective muscle and how the American College of Obstetricians and Gynecologists (ACOG) helped broker an important détente and pathway in a highly contentious issue.
Founded in 1933 as a nonprofit organization dedicated to maintaining high uniform standards among physicians, the ABMS and many of its specialty boards have found themselves, for more than a decade, under heavy fire from physicians (especially family physicians, internists, and surgeons), their 24 subspecialties, and the state medical societies representing them.
The ObGyn experience with the American Board of Obstetrics and Gynecology (ABOG), however, is better for a number of reasons. Historically, ABOG and ACOG have worked closely together, which is an anomaly among boards as many boards have an arms-length or even an antagonistic relationship with their specialty society.
The discussion below outlines physician concerns with the ABMS and related boards and describes efforts to address and rebuild the continuing board certification process.
Direct and indirect costs
Physicians are very concerned with the costs involved in MOC. Measurable costs include testing fees, while indirect costs include time, stress, travel to test centers, and threats to livelihood for failing a high-stakes examination. Physicians want the high-stakes exam eliminated.
Relevance to practice
Physicians often feel that the MOC has little relevance to their practice, which fuels a sense of resentment toward boards that they believe are dominated by physicians who no longer practice. Subspecialists feel farther away from general practice and the base exams. Generalists feel that the exams miss the points of their daily practice.
Lack of data to show improved quality of care
Physicians want to know that the MOC is worth their time, effort, and money because it improves patient care. To date, however, empirical or clinical data on patient outcomes are absent or ambiguous; most studies lack high-level data or do not investigate the MOC requirements. Physicians want to know what the best MOC practices are, what improves care, and that practices that make no difference will be discarded. In addition, they want timely knowledge alerts when evidence changes.
Relationship to licensing, employment, privileging, credentialing, and reimbursement
Hospitals, insurers, and states increasingly—and inappropriately—use board certification as the primary (sometimes only) default measure of a physician’s fitness for patient care. Physicians without board certification often are denied hospital privileges, inclusion in insurance panels, and even medical licenses. This changes certification from a voluntary physician self-improvement exercise into a can’t-earn-a-living-without-it cudgel.
Variation
Boards vary significantly in their MOC requirements and costs. The importance of an equal standard across all boards is a clear theme among physician concerns.
Role and authority of the ABMS and related boards
Many physicians are frustrated with the perceived autocratic nature of their boards—boards that lack transparency, do not solicit or allow input from practicing physicians, and are unresponsive to physician concerns.
According to Susan Ramin, MD, ABOG Associate Executive Director, ABOG is leading in a number of these areas, including:
- rapidly disseminating clinical information on emerging topics, such as Zika virus infection and opioid misuse
- offering physician choice of testing categories
- exempting high scorers from the secured written exam, which saved physicians a total of $881,000 in exam fees
- crediting physicians for what they already are doing, including serving on maternal mortality review committees, participating in registries, and participating in the Alliance for Innovation on Maternal Health (AIM)
- providing Lifelong Learning and Self-Assessment (LLSA) articles that, according to 90% of diplomates surveyed, are beneficial to their clinical practice (FIGURE).1,2
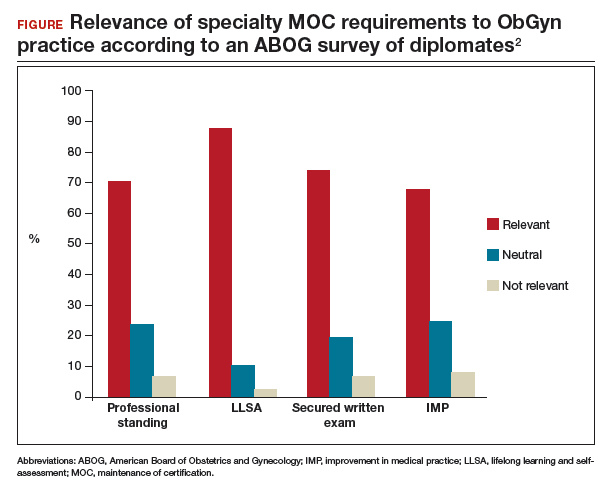
Our colleague physicians are not so lucky. In a 2015 New England Journal of Medicine Perspective, one physician called out the American Board of Internal Medicine as “a private, self-appointed certifying organization,” a not-for-profit organization that has “grown into a $55-million-per-year business.”3 He concluded that “many physicians are waking up to the fact that our profession is increasingly controlled by people not directly involved in patient care who have lost contact with the realities of day-to-day clinical practice.”3
State and society responses to MOC requirements
Frustration with an inability to resolve these concerns has grown steadily, bubbling over into state governments. The American Medical Association developed “model state legislation intended to prohibit hospitals, health care insurers, and state boards of medicine and osteopathic medicine from requiring participation in MOC processes as a condition of credentialing, privileging, insurance panel participation, licensure, or licensure renewal.”4
Some states are proposing or have enacted legislation that prohibits the use of MOC as a criterion for licensure, privileging, employment, reimbursement, and/or insurance panel participation. Eight states (Arizona, Georgia, Kentucky, Maryland, Maine, Missouri, Oklahoma, Tennessee) have enacted laws to prohibit the use of MOC for initial and renewal licensure decisions. Many states are actively considering MOC-related legislation, including Alaska, Florida, Iowa, Indiana, Maryland, Massachusetts, Michigan, Missouri, New Hampshire, New York, Ohio, Oklahoma, Rhode Island, South Carolina, Tennessee, Utah, Washington, and Wisconsin.
Legislation is not the only outlet for physician frustration. Some medical specialty societies are considering dropping board certification as a membership requirement; physicians are exploring developing alternative boards; and some physicians are defying the board certification requirement altogether, with thousands signing anti-MOC petitions.
ACOG asserts importance of maintaining self-regulation
While other specialties are actively advocating state legislation, ACOG and ABOG have worked together to oppose state legislation, believing that physician self-regulation is paramount. In fact, in 2017, ACOG and ABOG issued a joint statement urging state lawmakers to “not interfere with our decades of successful self-regulation and to realize that each medical society has its own experience with its MOC program.”5
Negotiations lead to new initiative
This brings us to an interesting situation. ACOG’s Executive Vice President and CEO Hal Lawrence III, MD, was tapped (in his position as Chair of the Specialty Society CEO Consortium) to represent physician specialties in negotiations and discussions with the boards, which were represented by Lois Nora, MD, JD, President and CEO of the ABMS, and state medical societies, represented by Donald Palmisano Jr, JD, Executive Director and CEO of the Medical Association of Georgia. Many state medical societies, boards, and physician specialty organizations participated in these meetings.
Throughout months of debate, Dr. Lawrence urged his colleagues to stay at the table and do the hard work of reaching an agreement, rather than ask politicians to solve medicine’s problems. This approach was leveraged by the serious efforts and threats of state legislation, which brought the boards to the table. In August 2017, 41 state medical societies and 33 national medical specialty societies wrote to Dr. Nora expressing their concerns that “professional self-regulation is under attack. Concerns regarding the usefulness of the high-stakes exam, the exorbitant costs of the MOC process, and the lack of transparent communication from the certifying boards have led to damaging the MOC brand, and creating state-based attacks on the MOC process.”6
In December 2017, Dr. Lawrence and Mr. Palmisano led a meeting of principals from the national medical specialty societies and state medical societies with leaders of ABMS and 8 specialty boards, including ABOG, an opportunity to secure meaningful change. Dr. Lawrence began by stressing that the interests of physicians and patients would be best served by all parties coming together and collaborating on a meaningful solution, to repair trust and preserve physician self-regulation.
Dr. Ramin presented ABOG’s approach to continuous certification, lifelong learning, and self-assessment. The American Board of Urology and the American Board of Psychiatry and Neurology indicated that they were basing important changes in their MOC process on ABOG’s work, including using 5 modules (1 general and 4 specific to the physician’s practice) and multiple open-book mini-exams based on selected journal articles as an alternative to the 10-year MOC exam.
The Vision Initiative. At that meeting and others, the ABMS and other boards heard physicians’ candid and sometimes blunt concerns. Dr. Nora spoke to the recently announced Continuing Board Certification: Vision for the Future program, also known as the “Vision Initiative,” a process designed to fundamentally rebuild the continuing certification process with input and guidance from practicing physicians. Physician response seemed uniform: Seeing is believing.
Importantly, all participants at the December meeting agreed to work together to rebuild trust and ensure professionalism and professional self-regulation, reflected in this Statement of Shared Purpose:
ABMS certifying boards and national medical specialty societies will collaborate to resolve differences in the process of ongoing certification and to fulfill the principles of professional self-regulation, achieving appropriate standardization, and assuring that ongoing certification is relevant to the practices of physicians without undue burden. Furthermore, the boards and societies, and their organizations (ABMS and CMSS [Council of Medical Specialty Societies]), will undertake necessary changes in a timely manner, and will commit to ongoing communication with state medical associations to solicit their input.4
Two ObGyns participating in the Vision Initiative are Haywood Brown, MD, ACOG’s Immediate Past President, and George Wendel, MD, ABOG’s Executive Director. The Vision Initiative is composed of 3 parts. Part 1, Organization, is complete. The committee is currently working on part 2, Envisioning the Future, an information-gathering component that includes physician surveys, hearings, open solicited input, and identifying new and better approaches. After the final report is delivered to the ABMS in February 2019, part 3, Implementation, will begin.
The Vision Initiative offers physicians an important opportunity to help shape the future of continuing education and certification. ObGyns and other physicians should consider reviewing and commenting on the draft report, due in November, during the public comment period. Visit https://visioninitiative.org for more information and to sign up for email updates.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- American Board of Obstetrics and Gynecology. From pilot to permanent: ABOG's program offering an innovative pathway integrating lifelong learning and self-assessment and external assessment is approved. https://www.abog.org/new/ABOG_PilotToPermanent.aspx. Accessed July 6, 2018.
- Ramin S. American Board of Obstetrics and Gynecology MOC program. PowerPoint presentation; December 4, 2017.
- Teirstein PS. Boarded to death--why maintenance of certification is bad for doctors and patients. N Engl J Med. 2015;372(2):106-108.
- AMA Council on Medical Education. Executive summary. 2017. https://www.ama-assn.org/sites/default/files/media-browser/public/council-on-med-ed/a18-cme-02.pdf. Accessed July 6, 2018.
- American College of Obstetricians and Gynecologists. ACOG-ABOG joint statement: political interference in physician maintenance of skills threatens women's health care. https://www.acog.org/-/media/Departments/State-Legislative-Activities/2017ACOG-ABMS-MOC-Statement.pdf?dmc=1&ts=20180706T1615538746. Accessed July 6, 2018.
- Letter to Lois Nora, MD, JD. August 18, 2017. https://www.mainemed.com/sites/default/files/content/MOC%20Letter%20082117.pdf. Accessed July 6, 2018.

The American Board of Medical Specialties (ABMS) has decided to trade the phrase “maintenance of certification” (MOC) for “continuing board certification,” a seemingly minor change that has an important backstory. This is the story of how the physician community flexed its collective muscle and how the American College of Obstetricians and Gynecologists (ACOG) helped broker an important détente and pathway in a highly contentious issue.
Founded in 1933 as a nonprofit organization dedicated to maintaining high uniform standards among physicians, the ABMS and many of its specialty boards have found themselves, for more than a decade, under heavy fire from physicians (especially family physicians, internists, and surgeons), their 24 subspecialties, and the state medical societies representing them.
The ObGyn experience with the American Board of Obstetrics and Gynecology (ABOG), however, is better for a number of reasons. Historically, ABOG and ACOG have worked closely together, which is an anomaly among boards as many boards have an arms-length or even an antagonistic relationship with their specialty society.
The discussion below outlines physician concerns with the ABMS and related boards and describes efforts to address and rebuild the continuing board certification process.
Direct and indirect costs
Physicians are very concerned with the costs involved in MOC. Measurable costs include testing fees, while indirect costs include time, stress, travel to test centers, and threats to livelihood for failing a high-stakes examination. Physicians want the high-stakes exam eliminated.
Relevance to practice
Physicians often feel that the MOC has little relevance to their practice, which fuels a sense of resentment toward boards that they believe are dominated by physicians who no longer practice. Subspecialists feel farther away from general practice and the base exams. Generalists feel that the exams miss the points of their daily practice.
Lack of data to show improved quality of care
Physicians want to know that the MOC is worth their time, effort, and money because it improves patient care. To date, however, empirical or clinical data on patient outcomes are absent or ambiguous; most studies lack high-level data or do not investigate the MOC requirements. Physicians want to know what the best MOC practices are, what improves care, and that practices that make no difference will be discarded. In addition, they want timely knowledge alerts when evidence changes.
Relationship to licensing, employment, privileging, credentialing, and reimbursement
Hospitals, insurers, and states increasingly—and inappropriately—use board certification as the primary (sometimes only) default measure of a physician’s fitness for patient care. Physicians without board certification often are denied hospital privileges, inclusion in insurance panels, and even medical licenses. This changes certification from a voluntary physician self-improvement exercise into a can’t-earn-a-living-without-it cudgel.
Variation
Boards vary significantly in their MOC requirements and costs. The importance of an equal standard across all boards is a clear theme among physician concerns.
Role and authority of the ABMS and related boards
Many physicians are frustrated with the perceived autocratic nature of their boards—boards that lack transparency, do not solicit or allow input from practicing physicians, and are unresponsive to physician concerns.
According to Susan Ramin, MD, ABOG Associate Executive Director, ABOG is leading in a number of these areas, including:
- rapidly disseminating clinical information on emerging topics, such as Zika virus infection and opioid misuse
- offering physician choice of testing categories
- exempting high scorers from the secured written exam, which saved physicians a total of $881,000 in exam fees
- crediting physicians for what they already are doing, including serving on maternal mortality review committees, participating in registries, and participating in the Alliance for Innovation on Maternal Health (AIM)
- providing Lifelong Learning and Self-Assessment (LLSA) articles that, according to 90% of diplomates surveyed, are beneficial to their clinical practice (FIGURE).1,2

Our colleague physicians are not so lucky. In a 2015 New England Journal of Medicine Perspective, one physician called out the American Board of Internal Medicine as “a private, self-appointed certifying organization,” a not-for-profit organization that has “grown into a $55-million-per-year business.”3 He concluded that “many physicians are waking up to the fact that our profession is increasingly controlled by people not directly involved in patient care who have lost contact with the realities of day-to-day clinical practice.”3
State and society responses to MOC requirements
Frustration with an inability to resolve these concerns has grown steadily, bubbling over into state governments. The American Medical Association developed “model state legislation intended to prohibit hospitals, health care insurers, and state boards of medicine and osteopathic medicine from requiring participation in MOC processes as a condition of credentialing, privileging, insurance panel participation, licensure, or licensure renewal.”4
Some states are proposing or have enacted legislation that prohibits the use of MOC as a criterion for licensure, privileging, employment, reimbursement, and/or insurance panel participation. Eight states (Arizona, Georgia, Kentucky, Maryland, Maine, Missouri, Oklahoma, Tennessee) have enacted laws to prohibit the use of MOC for initial and renewal licensure decisions. Many states are actively considering MOC-related legislation, including Alaska, Florida, Iowa, Indiana, Maryland, Massachusetts, Michigan, Missouri, New Hampshire, New York, Ohio, Oklahoma, Rhode Island, South Carolina, Tennessee, Utah, Washington, and Wisconsin.
Legislation is not the only outlet for physician frustration. Some medical specialty societies are considering dropping board certification as a membership requirement; physicians are exploring developing alternative boards; and some physicians are defying the board certification requirement altogether, with thousands signing anti-MOC petitions.
ACOG asserts importance of maintaining self-regulation
While other specialties are actively advocating state legislation, ACOG and ABOG have worked together to oppose state legislation, believing that physician self-regulation is paramount. In fact, in 2017, ACOG and ABOG issued a joint statement urging state lawmakers to “not interfere with our decades of successful self-regulation and to realize that each medical society has its own experience with its MOC program.”5
Negotiations lead to new initiative
This brings us to an interesting situation. ACOG’s Executive Vice President and CEO Hal Lawrence III, MD, was tapped (in his position as Chair of the Specialty Society CEO Consortium) to represent physician specialties in negotiations and discussions with the boards, which were represented by Lois Nora, MD, JD, President and CEO of the ABMS, and state medical societies, represented by Donald Palmisano Jr, JD, Executive Director and CEO of the Medical Association of Georgia. Many state medical societies, boards, and physician specialty organizations participated in these meetings.
Throughout months of debate, Dr. Lawrence urged his colleagues to stay at the table and do the hard work of reaching an agreement, rather than ask politicians to solve medicine’s problems. This approach was leveraged by the serious efforts and threats of state legislation, which brought the boards to the table. In August 2017, 41 state medical societies and 33 national medical specialty societies wrote to Dr. Nora expressing their concerns that “professional self-regulation is under attack. Concerns regarding the usefulness of the high-stakes exam, the exorbitant costs of the MOC process, and the lack of transparent communication from the certifying boards have led to damaging the MOC brand, and creating state-based attacks on the MOC process.”6
In December 2017, Dr. Lawrence and Mr. Palmisano led a meeting of principals from the national medical specialty societies and state medical societies with leaders of ABMS and 8 specialty boards, including ABOG, an opportunity to secure meaningful change. Dr. Lawrence began by stressing that the interests of physicians and patients would be best served by all parties coming together and collaborating on a meaningful solution, to repair trust and preserve physician self-regulation.
Dr. Ramin presented ABOG’s approach to continuous certification, lifelong learning, and self-assessment. The American Board of Urology and the American Board of Psychiatry and Neurology indicated that they were basing important changes in their MOC process on ABOG’s work, including using 5 modules (1 general and 4 specific to the physician’s practice) and multiple open-book mini-exams based on selected journal articles as an alternative to the 10-year MOC exam.
The Vision Initiative. At that meeting and others, the ABMS and other boards heard physicians’ candid and sometimes blunt concerns. Dr. Nora spoke to the recently announced Continuing Board Certification: Vision for the Future program, also known as the “Vision Initiative,” a process designed to fundamentally rebuild the continuing certification process with input and guidance from practicing physicians. Physician response seemed uniform: Seeing is believing.
Importantly, all participants at the December meeting agreed to work together to rebuild trust and ensure professionalism and professional self-regulation, reflected in this Statement of Shared Purpose:
ABMS certifying boards and national medical specialty societies will collaborate to resolve differences in the process of ongoing certification and to fulfill the principles of professional self-regulation, achieving appropriate standardization, and assuring that ongoing certification is relevant to the practices of physicians without undue burden. Furthermore, the boards and societies, and their organizations (ABMS and CMSS [Council of Medical Specialty Societies]), will undertake necessary changes in a timely manner, and will commit to ongoing communication with state medical associations to solicit their input.4
Two ObGyns participating in the Vision Initiative are Haywood Brown, MD, ACOG’s Immediate Past President, and George Wendel, MD, ABOG’s Executive Director. The Vision Initiative is composed of 3 parts. Part 1, Organization, is complete. The committee is currently working on part 2, Envisioning the Future, an information-gathering component that includes physician surveys, hearings, open solicited input, and identifying new and better approaches. After the final report is delivered to the ABMS in February 2019, part 3, Implementation, will begin.
The Vision Initiative offers physicians an important opportunity to help shape the future of continuing education and certification. ObGyns and other physicians should consider reviewing and commenting on the draft report, due in November, during the public comment period. Visit https://visioninitiative.org for more information and to sign up for email updates.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.

The American Board of Medical Specialties (ABMS) has decided to trade the phrase “maintenance of certification” (MOC) for “continuing board certification,” a seemingly minor change that has an important backstory. This is the story of how the physician community flexed its collective muscle and how the American College of Obstetricians and Gynecologists (ACOG) helped broker an important détente and pathway in a highly contentious issue.
Founded in 1933 as a nonprofit organization dedicated to maintaining high uniform standards among physicians, the ABMS and many of its specialty boards have found themselves, for more than a decade, under heavy fire from physicians (especially family physicians, internists, and surgeons), their 24 subspecialties, and the state medical societies representing them.
The ObGyn experience with the American Board of Obstetrics and Gynecology (ABOG), however, is better for a number of reasons. Historically, ABOG and ACOG have worked closely together, which is an anomaly among boards as many boards have an arms-length or even an antagonistic relationship with their specialty society.
The discussion below outlines physician concerns with the ABMS and related boards and describes efforts to address and rebuild the continuing board certification process.
Direct and indirect costs
Physicians are very concerned with the costs involved in MOC. Measurable costs include testing fees, while indirect costs include time, stress, travel to test centers, and threats to livelihood for failing a high-stakes examination. Physicians want the high-stakes exam eliminated.
Relevance to practice
Physicians often feel that the MOC has little relevance to their practice, which fuels a sense of resentment toward boards that they believe are dominated by physicians who no longer practice. Subspecialists feel farther away from general practice and the base exams. Generalists feel that the exams miss the points of their daily practice.
Lack of data to show improved quality of care
Physicians want to know that the MOC is worth their time, effort, and money because it improves patient care. To date, however, empirical or clinical data on patient outcomes are absent or ambiguous; most studies lack high-level data or do not investigate the MOC requirements. Physicians want to know what the best MOC practices are, what improves care, and that practices that make no difference will be discarded. In addition, they want timely knowledge alerts when evidence changes.
Relationship to licensing, employment, privileging, credentialing, and reimbursement
Hospitals, insurers, and states increasingly—and inappropriately—use board certification as the primary (sometimes only) default measure of a physician’s fitness for patient care. Physicians without board certification often are denied hospital privileges, inclusion in insurance panels, and even medical licenses. This changes certification from a voluntary physician self-improvement exercise into a can’t-earn-a-living-without-it cudgel.
Variation
Boards vary significantly in their MOC requirements and costs. The importance of an equal standard across all boards is a clear theme among physician concerns.
Role and authority of the ABMS and related boards
Many physicians are frustrated with the perceived autocratic nature of their boards—boards that lack transparency, do not solicit or allow input from practicing physicians, and are unresponsive to physician concerns.
According to Susan Ramin, MD, ABOG Associate Executive Director, ABOG is leading in a number of these areas, including:
- rapidly disseminating clinical information on emerging topics, such as Zika virus infection and opioid misuse
- offering physician choice of testing categories
- exempting high scorers from the secured written exam, which saved physicians a total of $881,000 in exam fees
- crediting physicians for what they already are doing, including serving on maternal mortality review committees, participating in registries, and participating in the Alliance for Innovation on Maternal Health (AIM)
- providing Lifelong Learning and Self-Assessment (LLSA) articles that, according to 90% of diplomates surveyed, are beneficial to their clinical practice (FIGURE).1,2

Our colleague physicians are not so lucky. In a 2015 New England Journal of Medicine Perspective, one physician called out the American Board of Internal Medicine as “a private, self-appointed certifying organization,” a not-for-profit organization that has “grown into a $55-million-per-year business.”3 He concluded that “many physicians are waking up to the fact that our profession is increasingly controlled by people not directly involved in patient care who have lost contact with the realities of day-to-day clinical practice.”3
State and society responses to MOC requirements
Frustration with an inability to resolve these concerns has grown steadily, bubbling over into state governments. The American Medical Association developed “model state legislation intended to prohibit hospitals, health care insurers, and state boards of medicine and osteopathic medicine from requiring participation in MOC processes as a condition of credentialing, privileging, insurance panel participation, licensure, or licensure renewal.”4
Some states are proposing or have enacted legislation that prohibits the use of MOC as a criterion for licensure, privileging, employment, reimbursement, and/or insurance panel participation. Eight states (Arizona, Georgia, Kentucky, Maryland, Maine, Missouri, Oklahoma, Tennessee) have enacted laws to prohibit the use of MOC for initial and renewal licensure decisions. Many states are actively considering MOC-related legislation, including Alaska, Florida, Iowa, Indiana, Maryland, Massachusetts, Michigan, Missouri, New Hampshire, New York, Ohio, Oklahoma, Rhode Island, South Carolina, Tennessee, Utah, Washington, and Wisconsin.
Legislation is not the only outlet for physician frustration. Some medical specialty societies are considering dropping board certification as a membership requirement; physicians are exploring developing alternative boards; and some physicians are defying the board certification requirement altogether, with thousands signing anti-MOC petitions.
ACOG asserts importance of maintaining self-regulation
While other specialties are actively advocating state legislation, ACOG and ABOG have worked together to oppose state legislation, believing that physician self-regulation is paramount. In fact, in 2017, ACOG and ABOG issued a joint statement urging state lawmakers to “not interfere with our decades of successful self-regulation and to realize that each medical society has its own experience with its MOC program.”5
Negotiations lead to new initiative
This brings us to an interesting situation. ACOG’s Executive Vice President and CEO Hal Lawrence III, MD, was tapped (in his position as Chair of the Specialty Society CEO Consortium) to represent physician specialties in negotiations and discussions with the boards, which were represented by Lois Nora, MD, JD, President and CEO of the ABMS, and state medical societies, represented by Donald Palmisano Jr, JD, Executive Director and CEO of the Medical Association of Georgia. Many state medical societies, boards, and physician specialty organizations participated in these meetings.
Throughout months of debate, Dr. Lawrence urged his colleagues to stay at the table and do the hard work of reaching an agreement, rather than ask politicians to solve medicine’s problems. This approach was leveraged by the serious efforts and threats of state legislation, which brought the boards to the table. In August 2017, 41 state medical societies and 33 national medical specialty societies wrote to Dr. Nora expressing their concerns that “professional self-regulation is under attack. Concerns regarding the usefulness of the high-stakes exam, the exorbitant costs of the MOC process, and the lack of transparent communication from the certifying boards have led to damaging the MOC brand, and creating state-based attacks on the MOC process.”6
In December 2017, Dr. Lawrence and Mr. Palmisano led a meeting of principals from the national medical specialty societies and state medical societies with leaders of ABMS and 8 specialty boards, including ABOG, an opportunity to secure meaningful change. Dr. Lawrence began by stressing that the interests of physicians and patients would be best served by all parties coming together and collaborating on a meaningful solution, to repair trust and preserve physician self-regulation.
Dr. Ramin presented ABOG’s approach to continuous certification, lifelong learning, and self-assessment. The American Board of Urology and the American Board of Psychiatry and Neurology indicated that they were basing important changes in their MOC process on ABOG’s work, including using 5 modules (1 general and 4 specific to the physician’s practice) and multiple open-book mini-exams based on selected journal articles as an alternative to the 10-year MOC exam.
The Vision Initiative. At that meeting and others, the ABMS and other boards heard physicians’ candid and sometimes blunt concerns. Dr. Nora spoke to the recently announced Continuing Board Certification: Vision for the Future program, also known as the “Vision Initiative,” a process designed to fundamentally rebuild the continuing certification process with input and guidance from practicing physicians. Physician response seemed uniform: Seeing is believing.
Importantly, all participants at the December meeting agreed to work together to rebuild trust and ensure professionalism and professional self-regulation, reflected in this Statement of Shared Purpose:
ABMS certifying boards and national medical specialty societies will collaborate to resolve differences in the process of ongoing certification and to fulfill the principles of professional self-regulation, achieving appropriate standardization, and assuring that ongoing certification is relevant to the practices of physicians without undue burden. Furthermore, the boards and societies, and their organizations (ABMS and CMSS [Council of Medical Specialty Societies]), will undertake necessary changes in a timely manner, and will commit to ongoing communication with state medical associations to solicit their input.4
Two ObGyns participating in the Vision Initiative are Haywood Brown, MD, ACOG’s Immediate Past President, and George Wendel, MD, ABOG’s Executive Director. The Vision Initiative is composed of 3 parts. Part 1, Organization, is complete. The committee is currently working on part 2, Envisioning the Future, an information-gathering component that includes physician surveys, hearings, open solicited input, and identifying new and better approaches. After the final report is delivered to the ABMS in February 2019, part 3, Implementation, will begin.
The Vision Initiative offers physicians an important opportunity to help shape the future of continuing education and certification. ObGyns and other physicians should consider reviewing and commenting on the draft report, due in November, during the public comment period. Visit https://visioninitiative.org for more information and to sign up for email updates.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- American Board of Obstetrics and Gynecology. From pilot to permanent: ABOG's program offering an innovative pathway integrating lifelong learning and self-assessment and external assessment is approved. https://www.abog.org/new/ABOG_PilotToPermanent.aspx. Accessed July 6, 2018.
- Ramin S. American Board of Obstetrics and Gynecology MOC program. PowerPoint presentation; December 4, 2017.
- Teirstein PS. Boarded to death--why maintenance of certification is bad for doctors and patients. N Engl J Med. 2015;372(2):106-108.
- AMA Council on Medical Education. Executive summary. 2017. https://www.ama-assn.org/sites/default/files/media-browser/public/council-on-med-ed/a18-cme-02.pdf. Accessed July 6, 2018.
- American College of Obstetricians and Gynecologists. ACOG-ABOG joint statement: political interference in physician maintenance of skills threatens women's health care. https://www.acog.org/-/media/Departments/State-Legislative-Activities/2017ACOG-ABMS-MOC-Statement.pdf?dmc=1&ts=20180706T1615538746. Accessed July 6, 2018.
- Letter to Lois Nora, MD, JD. August 18, 2017. https://www.mainemed.com/sites/default/files/content/MOC%20Letter%20082117.pdf. Accessed July 6, 2018.
- American Board of Obstetrics and Gynecology. From pilot to permanent: ABOG's program offering an innovative pathway integrating lifelong learning and self-assessment and external assessment is approved. https://www.abog.org/new/ABOG_PilotToPermanent.aspx. Accessed July 6, 2018.
- Ramin S. American Board of Obstetrics and Gynecology MOC program. PowerPoint presentation; December 4, 2017.
- Teirstein PS. Boarded to death--why maintenance of certification is bad for doctors and patients. N Engl J Med. 2015;372(2):106-108.
- AMA Council on Medical Education. Executive summary. 2017. https://www.ama-assn.org/sites/default/files/media-browser/public/council-on-med-ed/a18-cme-02.pdf. Accessed July 6, 2018.
- American College of Obstetricians and Gynecologists. ACOG-ABOG joint statement: political interference in physician maintenance of skills threatens women's health care. https://www.acog.org/-/media/Departments/State-Legislative-Activities/2017ACOG-ABMS-MOC-Statement.pdf?dmc=1&ts=20180706T1615538746. Accessed July 6, 2018.
- Letter to Lois Nora, MD, JD. August 18, 2017. https://www.mainemed.com/sites/default/files/content/MOC%20Letter%20082117.pdf. Accessed July 6, 2018.
CDC apps specific for ObGyns
The Centers for Disease Control and Prevention (CDC) is a US federal agency under the Department of Health and Human Services. It is the nation’s leading public health institute. Its main goal is to save lives and protect people from health, safety, and security threats. The CDC website lists 25 no-cost applications that the agency has developed: https://www.cdc.gov/mobile/mobileapp.html.
This review will focus on 3 CDC apps (Table) that I feel are useful to ObGyn health care providers: Prevent Group B Strep (GBS), STD Tx Guide, and US Medical Eligibility Criteria for Contraceptive Use. In fact, in an evaluation of contraception apps for providers of family planning services, US Medical Eligibility Criteria for Contraceptive Use was one of the highest scoring apps.1 I will evaluate each app by a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, and important special features).2 I commend the CDC for developing these useful tools to assist health care providers.
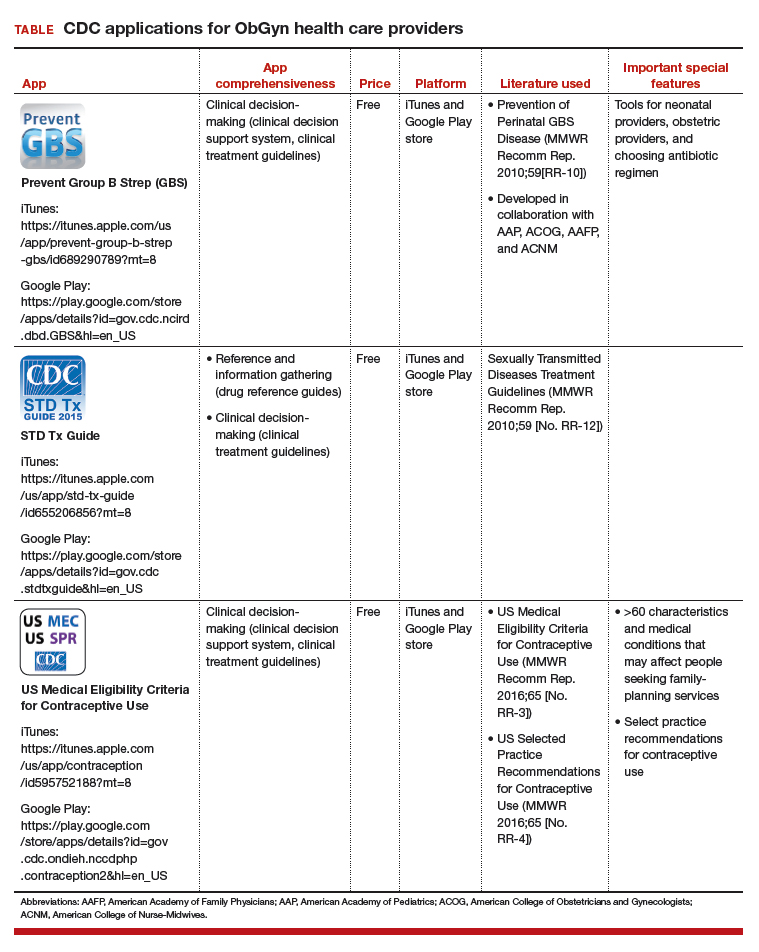
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Perry R, Lunde B, Chen KT. An evaluation of contraception mobile applications for providers of family planning services. Contraception. 2016;93(6):539-544.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125(6):1478-1483.
The Centers for Disease Control and Prevention (CDC) is a US federal agency under the Department of Health and Human Services. It is the nation’s leading public health institute. Its main goal is to save lives and protect people from health, safety, and security threats. The CDC website lists 25 no-cost applications that the agency has developed: https://www.cdc.gov/mobile/mobileapp.html.
This review will focus on 3 CDC apps (Table) that I feel are useful to ObGyn health care providers: Prevent Group B Strep (GBS), STD Tx Guide, and US Medical Eligibility Criteria for Contraceptive Use. In fact, in an evaluation of contraception apps for providers of family planning services, US Medical Eligibility Criteria for Contraceptive Use was one of the highest scoring apps.1 I will evaluate each app by a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, and important special features).2 I commend the CDC for developing these useful tools to assist health care providers.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The Centers for Disease Control and Prevention (CDC) is a US federal agency under the Department of Health and Human Services. It is the nation’s leading public health institute. Its main goal is to save lives and protect people from health, safety, and security threats. The CDC website lists 25 no-cost applications that the agency has developed: https://www.cdc.gov/mobile/mobileapp.html.
This review will focus on 3 CDC apps (Table) that I feel are useful to ObGyn health care providers: Prevent Group B Strep (GBS), STD Tx Guide, and US Medical Eligibility Criteria for Contraceptive Use. In fact, in an evaluation of contraception apps for providers of family planning services, US Medical Eligibility Criteria for Contraceptive Use was one of the highest scoring apps.1 I will evaluate each app by a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, and important special features).2 I commend the CDC for developing these useful tools to assist health care providers.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Perry R, Lunde B, Chen KT. An evaluation of contraception mobile applications for providers of family planning services. Contraception. 2016;93(6):539-544.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125(6):1478-1483.
- Perry R, Lunde B, Chen KT. An evaluation of contraception mobile applications for providers of family planning services. Contraception. 2016;93(6):539-544.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125(6):1478-1483.
IN THIS ARTICLE
- Details on recommended apps
Liver enzymes: No trivial elevations, even if asymptomatic
Elevated levels of circulating enzymes that are frequently of hepatic origin (aminotransferases and alkaline phosphatase) and bilirubin in the absence of symptoms are common in clinical practice. A dogmatic but true statement holds that there are no trivial elevations in these substances. All persistent elevations of liver enzymes need a methodical evaluation and an appropriate working diagnosis.1
Here, we outline a framework for the workup and treatment of common causes of liver enzyme elevations.
PATTERN OF ELEVATION: CHOLESTATIC OR HEPATOCELLULAR
Based on the pattern of elevation, causes of elevated liver enzymes can be sorted into disorders of cholestasis and disorders of hepatocellular injury (Table 1).1
Cholestatic disorders tend to cause elevations in alkaline phosphatase, bilirubin, and gamma-glutamyl transferase (GGT).
Hepatocellular injury raises levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST).
HOW SHOULD ABNORMAL RESULTS BE EVALUATED?
When approaching liver enzyme elevations, the clinician should develop a working differential diagnosis based on the medical and social history and physical examination.
Think about alcohol, drugs, and fat
The most common causes of liver enzyme elevation are alcohol toxicity, medication overdose, and fatty liver disease.
Alcohol intake should be ascertained. “Significant” consumption is defined as more than 21 drinks per week in men or more than 14 drinks per week in women, over a period of at least 2 years.2
The exact pathogenesis of alcoholic hepatitis is incompletely understood, but alcohol is primarily metabolized by the liver, and damage likely occurs during metabolism of the ingested alcohol. AST elevations tend to be higher than ALT elevations; the reason is ascribed to hepatic deficiency of pyridoxal 5´-phosphate, a cofactor of the enzymatic activity of ALT, which leads to a lesser increase in ALT than in AST.
Alcoholic liver disease can be difficult to diagnose, as many people are initially reluctant to fully disclose how much they drink, but it should be suspected when the ratio of AST to ALT is 2 or greater.
In a classic study, a ratio greater than 2 was found in 70% of patients with alcoholic hepatitis and cirrhosis, compared with 26% of patients with postnecrotic cirrhosis, 8% with chronic hepatitis, 4% with viral hepatitis, and none with obstructive jaundice.3 Importantly, the disorder is often correctable if the patient is able to remain abstinent from alcohol over time.
A detailed medication history is important and should focus especially on recently added medications, dosage changes, medication overuse, and use of nonprescription drugs and herbal supplements. Common medications that affect liver enzyme levels include statins, which cause hepatic dysfunction primarily during the first 3 months of therapy, nonsteroidal anti-inflammatory drugs, antiepileptic drugs, antibiotics, anabolic steroids, and acetaminophen (Table 2).1 Use of illicit drugs and herbal remedies should be discussed, as they may cause toxin-mediated hepatitis.
Although inflammation from drug toxicity will resolve if the offending agent is discontinued, complete recovery may take weeks to months.4
A pertinent social history includes exposure to environmental hepatotoxins such as amatoxin (contained in some wild mushrooms) and occupational hazards (eg, vinyl chloride). Risk factors for viral hepatitis should be evaluated, including intravenous drug use, blood transfusions, unprotected sexual contact, organ transplant, perinatal transmission, and a history of work in healthcare facilities or travel to regions in which hepatitis A or E is endemic.
The medical and family history should include details of associated conditions, such as:
- Right heart failure (a cause of congestive hepatopathy)
- Metabolic syndrome (associated with fatty liver disease)
- Inflammatory bowel disease and primary sclerosing cholangitis
- Early-onset emphysema and alpha-1 antitrypsin deficiency.
The physical examination should be thorough, with emphasis on the abdomen, and search for stigmata of advanced liver disease such as hepatomegaly, splenomegaly, ascites, edema, spider angiomata, jaundice, and asterixis. Any patient with evidence of chronic liver disease should be referred to a subspecialist for further evaluation.
Further diagnostic workup
Abnormal liver enzyme findings or physical examination findings should direct the subsequent diagnostic workup with laboratory testing and imaging.5
For cholestasis. If laboratory data are consistent with cholestasis or abnormal bile flow, it should be further characterized as extrahepatic or intrahepatic. Common causes of extrahepatic cholestasis include biliary tree obstruction due to stones or malignancy, often visualized as intraductal biliary dilation on ultrasonography of the right upper quadrant. Common causes of intrahepatic cholestasis include viral and alcoholic hepatitis, nonalcoholic steatohepatitis, certain drugs and toxins such as alkylated steroids and herbal medications, infiltrative diseases such as amyloid, sarcoid, lymphoma, and tuberculosis, and primary biliary cholangitis.
Abnormal findings on ultrasonography should be further pursued with advanced imaging, ie, computed tomography or magnetic resonance cholangiopancreatography (MRCP). The confirmation of a lesion on imaging is often followed by endoscopic retrograde cholangiopancreatography (ERCP) in an attempt to obtain biopsy samples, remove obstructions, and place therapeutic stents. In instances when endoscopic attempts fail to relieve the obstruction, surgical referral may be appropriate.
For nonhepatobiliary problems. Depending on clinical presentation, it may also be important to consider nonhepatobiliary causes of elevated liver enzymes.
Alkaline phosphatase is found in many other tissue types, including bone, kidney, and the placenta, and can be elevated during pregnancy, adolescence, and even after fatty meals due to intestinal release.6 After screening for the aforementioned physiologic conditions, isolated elevated alkaline phosphatase should be further evaluated by obtaining GGT or 5-nucleotidase levels, which are more specifically of hepatic origin. If these levels are within normal limits, further evaluation for conditions of bone growth and cellular turnover such as Paget disease, hyperparathyroidism, and malignancy should be considered. Specifically, Stauffer syndrome should be considered when there is a paraneoplastic rise in the alkaline phosphatase level in the setting of renal cell carcinoma without liver metastases.
AST and ALT levels may also be elevated in clinical situations and syndromes unrelated to liver disease. Rhabdomyolysis, for instance, may be associated with elevations of AST in more than 90% of cases, and ALT in more than 75%.7 Markers of muscle injury including serum creatine kinase should be obtained in the setting of heat stroke, muscle weakness, strenuous activity, or seizures, as related elevations in AST and ALT may not always be clinically indicative of liver injury.
Given the many conditions that may cause elevated liver enzymes, evaluation and treatment should focus on identifying and removing offending agents and targeting the underlying process with appropriate medical therapy.
FATTY LIVER
With rates of obesity and type 2 diabetes on the rise in the general population, identifying and treating nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) require increased awareness and close coordination between primary care providers and subspecialists.
According to current estimates, up to one-third of the US population (100 million people) may have NAFLD, and 1% to 3% of the population (4–6 million people) likely have NASH, defined as steatosis with inflammation. Development of NASH places patients at a significantly higher risk of fibrosis, hepatocellular injury, and cancer.8
NAFLD is more common in men than in women. It is present in around 80% to 90% of obese adults, two-thirds of adults with type 2 diabetes, and many people with hyperlipidemia. It is also becoming more common in children, with 40% to 70% of obese children likely having some element of NAFLD.
Diagnosis of fatty liver
Although liver enzymes are more likely to be abnormal in individuals with NAFLD, many individuals with underlying NAFLD may have normal laboratory evaluations. ALT may be elevated in only up to 20% of cases and does not likely correlate with the level of underlying liver damage, although increasing GGT may serve as a marker of fibrosis over time.9–11 In contrast to alcohol injury, however, the AST-ALT ratio is usually less than 1.0.
Noninvasive tools for diagnosing NAFLD include the NAFLD fibrosis score, which incorporates age, hyperglycemia, body mass index, platelet count, albumin level, and AST-ALT ratio. This and related scoring algorithms may be useful in differentiating patients with minimal fibrosis from those with advanced fibrosis.12,13
Ultrasonography is a first-line diagnostic test for steatosis, although it may demonstrate fatty infiltration only around 60% of the time. Computed tomography and magnetic resonance imaging are more sensitive, but costlier. Transient elastography (FibroScan; Echosens, Paris, France) has become more popular and has been shown to correlate with findings on liver biopsy in diagnosing or excluding advanced liver fibrosis.14,15
The gold standard for diagnosing NAFLD and NASH is identifying fat-laden hepatocytes or portal inflammation on biopsy; however, biopsy is generally reserved for cases in which the diagnosis remains uncertain.
Behavioral treatment
The primary treatment for NAFLD consists of behavioral modification including weight loss, exercise, and adherence to a low-fat diet, in addition to tight glycemic control and treatment of any underlying lipid abnormalities. Studies have shown that a reduction of 7% to 10% of body weight is associated with a decrease in the inflammation of NAFLD, though no strict guidelines have been established.16
Given the prevalence of NAFLD and the need for longitudinal treatment, primary care physicians will play a significant role in long-term monitoring and management of patients with fatty liver disease.
OTHER DISORDERS OF LIVER FUNCTION
Hereditary hemochromatosis
Hereditary hemochromatosis is the most common inherited liver disorder in adults of European descent,17 and can be effectively treated if discovered early. But its clinical diagnosis can be challenging, as many patients have no symptoms at presentation despite abnormal liver enzyme levels. Early symptoms may include severe fatigue, arthralgias, and, in men, impotence, before the appearance of the classic triad of “bronze diabetes” with cirrhosis, diabetes, and darkening of the skin.18
If hemochromatosis is suspected, laboratory tests should include a calculation of percent transferrin saturation, with saturation greater than 45% warranting serum ferritin measurement to evaluate for iron overload (ferritin > 200–300 ng/mL in men, > 150–200 ng/mL in women).19 If iron overload is confirmed, referral to a gastroenterologist is recommended.
Genetic evaluation is often pursued, but patients may ultimately require liver biopsy regardless of the findings, as some patients homozygous for the HFE mutation C282Y may not have clinical hemochromatosis, whereas others with hereditary hemochromatosis may not have the HFE mutation.
Therapeutic phlebotomy is the treatment of choice, and most patients tolerate it well.
Chronic hepatitis B virus and hepatitis C virus infections
Chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are common in the United States, with HBV affecting more than 1 million people and HCV affecting an estimated 3.5 million.
Chronic HCV infection. Direct-acting antiviral drugs have revolutionized HCV treatment and have led to a sustained viral response and presumed cure at 12 weeks in more than 95% of cases across all HCV genotypes.20 Given the recent development of effective and well-tolerated treatments, primary care physicians have assumed a pivotal role in screening for HCV.
The American Association for the Study of Liver Diseases and the Infectious Diseases Society of America21 recommend screening for HCV in people who have risk factors for it, ie:
- HCV exposure
- HIV infection
- Behavioral or environmental risks for contracting the virus such as intravenous drug use or incarceration
- Birth between 1945 and 1965 (one-time testing).
If HCV antibody screening is positive, HCV RNA should be obtained to quantify the viral load and confirm active infection, and genotype testing should be performed to guide treatment. Among the 6 most common HCV genotypes, genotype 1 is the most common in North America, accounting for over 70% of cases in the United States.
Although recommendations and therapies are constantly evolving, the selection of a treatment regimen and the duration of therapy are determined by viral genotype, history of prior treatment, stage of liver fibrosis, potential drug interactions, and frequently, medication cost and insurance coverage.
HBV infection. The treatment for acute HBV infection is generally supportive, though viral suppression with tenofovir or entecavir may be required for those who develop coagulopathy, bilirubinemia, or liver failure. Treatment of chronic HBV infection may not be required and is generally considered for those with elevated ALT, high viral load, or evidence of liver fibrosis on noninvasive measurements such as transient elastography.
Autoimmune hepatitis
Autoimmune causes of liver enzyme elevations should also be considered during initial screening. Positive antinuclear antibody and positive antismooth muscle antibody tests are common in cases of autoimmune hepatitis.22 Autoimmune hepatitis affects women more often than men, with a ratio of 4:1. The peaks of incidence occur during adolescence and between ages 30 and 45.23
Primary biliary cholangitis
Additionally, an elevated alkaline phosphatase level should raise concern for underlying primary biliary cholangitis (formerly called primary biliary cirrhosis), an autoimmune disorder that affects the small and medium intrahepatic bile ducts. Diagnosis of primary biliary cholangitis can be assisted by a positive test for antimitochondrial antibody, present in almost 90% of patients.24
Primary sclerosing cholangitis
Elevated alkaline phosphatase is also the hallmark of primary sclerosing cholangitis, which is associated with inflammatory bowel disease.25 Primary sclerosing cholangitis is characterized by inflammation and fibrosis of the intrahepatic and extrahepatic bile ducts, which are visualized on MRCP and confirmed by biopsy if needed.
REFERRAL
Subspecialty referral should be considered if the cause remains ambiguous or unknown, if there is concern for a rare hepatic disorder such as an autoimmune condition, Wilson disease, or alpha-1 antitrypsin deficiency, or if there is evidence of advanced or chronic liver disease.
Primary care physicians are at the forefront of detecting and diagnosing liver disease, and close coordination with subspecialists will remain crucial in delivering patient care.
- Aragon G, Younossi ZM. When and how to evaluate mildly elevated liver enzymes in apparently healthy patients. Cleve Clin J Med 2010; 77(3):195–204. doi:10.3949/ccjm.77a.09064
- Chalasani N, Younossi Z, Lavine JE, et al; American Gastroenterological Association; American Association for the Study of Liver Diseases; American College of Gastroenterology. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 2012; 142(7):1592–1609. doi:10.1053/j.gastro.2012.04.001
- Cohen JA, Kaplan MM. The SGOT/SGPT ratio—an indicator of alcoholic liver disease. Dig Dis Sci 1979; 24(11):835–838. pmid:520102
- Kaplan MM. Alanine aminotransferase levels: what’s normal? Ann Intern Med 2002; 137(1):49-51. pmid:12093245
- Pratt DS, Kaplan MM. Evaluation of abnormal liver enzyme results in asymptomatic patients. N Engl J Med 2000; 342(17):1266–1271. doi:10.1056/NEJM200004273421707
- Sharma U, Pal D, Prasad R. Alkaline phosphatase: an overview. Indian J Clin Biochem 2014; 29(3):269–278. doi:10.1007/s12291-013-0408-y
- Weibrecht K, Dayno M, Darling C, Bird SB. Liver aminotransferases are elevated with rhabdomyolysis in the absence of significant liver injury. J Med Toxicol 2010; 6(3):294–300. doi:10.1007/s13181-010-0075-9
- Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis 2010; 28(1):155–161. doi:10.1159/000282080
- Adams LA, Feldstein AE. Non-invasive diagnosis of nonalcoholic fatty liver and nonalcoholic steatohepatitis. J Dig Dis 2011; 12(1):10–16. doi:10.1111/j.1751-2980.2010.00471.x
- Fracanzani AL, Valenti L, Bugianesi E, et al. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology 2008; 48(3):792–798. doi:10.1002/hep.22429
- Tahan V, Canbakan B, Balci H, et al. Serum gamma-glutamyltranspeptidase distinguishes non-alcoholic fatty liver disease at high risk. Hepatogastroenterolgoy 2008; 55(85):1433-1438. pmid:18795706
- McPherson S, Stewart S, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010; 59(9):1265–1269. doi:10.1136/gut.2010.216077
- Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007; 45(4):846–854. doi:10.1002/hep.21496
- Petta S, Vanni E, Bugianesi E, et al. The combination of liver stiffness measurement and NAFLD fibrosis score improves the noninvasive diagnostic accuracy for severe liver fibrosis in patients with nonalcoholic fatty liver disease. Liver Int 2015; 35(5):1566–1573. doi:10.1111/liv.12584
- Hashemi SA, Alavian SM, Gholami-Fesharaki M. Assessment of transient elastography (FibroScan) for diagnosis of fibrosis in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Caspian J Intern Med 2016; 7(4):242–252. pmid:27999641
- Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010; 51(1):121–129. doi:10.1002/hep.23276
- Adams PH, Reboussin DM, Barton JC, et al. Hemochromatosis and iron-overload screening in a racially diverse population. N Engl J Med 2005; 352(17):1769-1778. doi:10.1056/NEJMoa041534
- Brissot P, de Bels F. Current approaches to the management of hemochromatosis. Hematology Am Soc Hematol Educ Program 2006; 2006(1):36–41. doi:10.1182/asheducation-2006.1.36
- Bacon BR, Adams PC, Kowdley KV, Powell LW, Tavill AS; American Association for the Study of Liver Diseases. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology 2011; 54(1):328–343. doi:10.1002/hep.24330
- Weiler N, Zeuzem S, Welker MW. Concise review: interferon-free treatment of hepatitis C virus-associated cirrhosis and liver graft infection. World J Gastroenterol 2016; 22(41):9044–9056. doi:10.3748/wjg.v22.i41.9044
- American Association for the Study of Liver Disease, Infectious Diseases Society of America. HCV guidance: recommendations for testing, managing, and treating hepatitis C. www.hcvguidelines.org. Accessed July 16, 2018.
- Manns MP, Czaja AJ, Gorham JD, et al; American Association for the Study of Liver Diseases. Diagnosis and management of autoimmune hepatitis. Hepatology 2010; 51(6):2193–2213. doi:10.1002/hep.23584
- Liberal R, Krawitt EL, Vierling JM, Manns MP, Mieli-Vergani G, Vergani D. Cutting edge issues in autoimmune hepatitis. J Autoimmun 2016; 75:6–19. doi:10.1016/j.jaut.2016.07.005
- Mousa HS, Carbone M, Malinverno F, Ronca V, Gershwin ME, Invernizzi P. Novel therapeutics for primary biliary cholangitis: Toward a disease-stage-based approach. Autoimmun Rev 2016; 15(9):870–876. doi:10.1016/j.autrev.2016.07.003
- de Vries AB, Janse M, Blokzijl H, Weersma RK. Distinctive inflammatory bowel disease phenotype in primary sclerosing cholangitis. World J Gastroenterol 2015; 21(6):1956–1971. doi:10.3748/wjg.v21.i6.1956
Elevated levels of circulating enzymes that are frequently of hepatic origin (aminotransferases and alkaline phosphatase) and bilirubin in the absence of symptoms are common in clinical practice. A dogmatic but true statement holds that there are no trivial elevations in these substances. All persistent elevations of liver enzymes need a methodical evaluation and an appropriate working diagnosis.1
Here, we outline a framework for the workup and treatment of common causes of liver enzyme elevations.
PATTERN OF ELEVATION: CHOLESTATIC OR HEPATOCELLULAR
Based on the pattern of elevation, causes of elevated liver enzymes can be sorted into disorders of cholestasis and disorders of hepatocellular injury (Table 1).1
Cholestatic disorders tend to cause elevations in alkaline phosphatase, bilirubin, and gamma-glutamyl transferase (GGT).
Hepatocellular injury raises levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST).
HOW SHOULD ABNORMAL RESULTS BE EVALUATED?
When approaching liver enzyme elevations, the clinician should develop a working differential diagnosis based on the medical and social history and physical examination.
Think about alcohol, drugs, and fat
The most common causes of liver enzyme elevation are alcohol toxicity, medication overdose, and fatty liver disease.
Alcohol intake should be ascertained. “Significant” consumption is defined as more than 21 drinks per week in men or more than 14 drinks per week in women, over a period of at least 2 years.2
The exact pathogenesis of alcoholic hepatitis is incompletely understood, but alcohol is primarily metabolized by the liver, and damage likely occurs during metabolism of the ingested alcohol. AST elevations tend to be higher than ALT elevations; the reason is ascribed to hepatic deficiency of pyridoxal 5´-phosphate, a cofactor of the enzymatic activity of ALT, which leads to a lesser increase in ALT than in AST.
Alcoholic liver disease can be difficult to diagnose, as many people are initially reluctant to fully disclose how much they drink, but it should be suspected when the ratio of AST to ALT is 2 or greater.
In a classic study, a ratio greater than 2 was found in 70% of patients with alcoholic hepatitis and cirrhosis, compared with 26% of patients with postnecrotic cirrhosis, 8% with chronic hepatitis, 4% with viral hepatitis, and none with obstructive jaundice.3 Importantly, the disorder is often correctable if the patient is able to remain abstinent from alcohol over time.
A detailed medication history is important and should focus especially on recently added medications, dosage changes, medication overuse, and use of nonprescription drugs and herbal supplements. Common medications that affect liver enzyme levels include statins, which cause hepatic dysfunction primarily during the first 3 months of therapy, nonsteroidal anti-inflammatory drugs, antiepileptic drugs, antibiotics, anabolic steroids, and acetaminophen (Table 2).1 Use of illicit drugs and herbal remedies should be discussed, as they may cause toxin-mediated hepatitis.
Although inflammation from drug toxicity will resolve if the offending agent is discontinued, complete recovery may take weeks to months.4
A pertinent social history includes exposure to environmental hepatotoxins such as amatoxin (contained in some wild mushrooms) and occupational hazards (eg, vinyl chloride). Risk factors for viral hepatitis should be evaluated, including intravenous drug use, blood transfusions, unprotected sexual contact, organ transplant, perinatal transmission, and a history of work in healthcare facilities or travel to regions in which hepatitis A or E is endemic.
The medical and family history should include details of associated conditions, such as:
- Right heart failure (a cause of congestive hepatopathy)
- Metabolic syndrome (associated with fatty liver disease)
- Inflammatory bowel disease and primary sclerosing cholangitis
- Early-onset emphysema and alpha-1 antitrypsin deficiency.
The physical examination should be thorough, with emphasis on the abdomen, and search for stigmata of advanced liver disease such as hepatomegaly, splenomegaly, ascites, edema, spider angiomata, jaundice, and asterixis. Any patient with evidence of chronic liver disease should be referred to a subspecialist for further evaluation.
Further diagnostic workup
Abnormal liver enzyme findings or physical examination findings should direct the subsequent diagnostic workup with laboratory testing and imaging.5
For cholestasis. If laboratory data are consistent with cholestasis or abnormal bile flow, it should be further characterized as extrahepatic or intrahepatic. Common causes of extrahepatic cholestasis include biliary tree obstruction due to stones or malignancy, often visualized as intraductal biliary dilation on ultrasonography of the right upper quadrant. Common causes of intrahepatic cholestasis include viral and alcoholic hepatitis, nonalcoholic steatohepatitis, certain drugs and toxins such as alkylated steroids and herbal medications, infiltrative diseases such as amyloid, sarcoid, lymphoma, and tuberculosis, and primary biliary cholangitis.
Abnormal findings on ultrasonography should be further pursued with advanced imaging, ie, computed tomography or magnetic resonance cholangiopancreatography (MRCP). The confirmation of a lesion on imaging is often followed by endoscopic retrograde cholangiopancreatography (ERCP) in an attempt to obtain biopsy samples, remove obstructions, and place therapeutic stents. In instances when endoscopic attempts fail to relieve the obstruction, surgical referral may be appropriate.
For nonhepatobiliary problems. Depending on clinical presentation, it may also be important to consider nonhepatobiliary causes of elevated liver enzymes.
Alkaline phosphatase is found in many other tissue types, including bone, kidney, and the placenta, and can be elevated during pregnancy, adolescence, and even after fatty meals due to intestinal release.6 After screening for the aforementioned physiologic conditions, isolated elevated alkaline phosphatase should be further evaluated by obtaining GGT or 5-nucleotidase levels, which are more specifically of hepatic origin. If these levels are within normal limits, further evaluation for conditions of bone growth and cellular turnover such as Paget disease, hyperparathyroidism, and malignancy should be considered. Specifically, Stauffer syndrome should be considered when there is a paraneoplastic rise in the alkaline phosphatase level in the setting of renal cell carcinoma without liver metastases.
AST and ALT levels may also be elevated in clinical situations and syndromes unrelated to liver disease. Rhabdomyolysis, for instance, may be associated with elevations of AST in more than 90% of cases, and ALT in more than 75%.7 Markers of muscle injury including serum creatine kinase should be obtained in the setting of heat stroke, muscle weakness, strenuous activity, or seizures, as related elevations in AST and ALT may not always be clinically indicative of liver injury.
Given the many conditions that may cause elevated liver enzymes, evaluation and treatment should focus on identifying and removing offending agents and targeting the underlying process with appropriate medical therapy.
FATTY LIVER
With rates of obesity and type 2 diabetes on the rise in the general population, identifying and treating nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) require increased awareness and close coordination between primary care providers and subspecialists.
According to current estimates, up to one-third of the US population (100 million people) may have NAFLD, and 1% to 3% of the population (4–6 million people) likely have NASH, defined as steatosis with inflammation. Development of NASH places patients at a significantly higher risk of fibrosis, hepatocellular injury, and cancer.8
NAFLD is more common in men than in women. It is present in around 80% to 90% of obese adults, two-thirds of adults with type 2 diabetes, and many people with hyperlipidemia. It is also becoming more common in children, with 40% to 70% of obese children likely having some element of NAFLD.
Diagnosis of fatty liver
Although liver enzymes are more likely to be abnormal in individuals with NAFLD, many individuals with underlying NAFLD may have normal laboratory evaluations. ALT may be elevated in only up to 20% of cases and does not likely correlate with the level of underlying liver damage, although increasing GGT may serve as a marker of fibrosis over time.9–11 In contrast to alcohol injury, however, the AST-ALT ratio is usually less than 1.0.
Noninvasive tools for diagnosing NAFLD include the NAFLD fibrosis score, which incorporates age, hyperglycemia, body mass index, platelet count, albumin level, and AST-ALT ratio. This and related scoring algorithms may be useful in differentiating patients with minimal fibrosis from those with advanced fibrosis.12,13
Ultrasonography is a first-line diagnostic test for steatosis, although it may demonstrate fatty infiltration only around 60% of the time. Computed tomography and magnetic resonance imaging are more sensitive, but costlier. Transient elastography (FibroScan; Echosens, Paris, France) has become more popular and has been shown to correlate with findings on liver biopsy in diagnosing or excluding advanced liver fibrosis.14,15
The gold standard for diagnosing NAFLD and NASH is identifying fat-laden hepatocytes or portal inflammation on biopsy; however, biopsy is generally reserved for cases in which the diagnosis remains uncertain.
Behavioral treatment
The primary treatment for NAFLD consists of behavioral modification including weight loss, exercise, and adherence to a low-fat diet, in addition to tight glycemic control and treatment of any underlying lipid abnormalities. Studies have shown that a reduction of 7% to 10% of body weight is associated with a decrease in the inflammation of NAFLD, though no strict guidelines have been established.16
Given the prevalence of NAFLD and the need for longitudinal treatment, primary care physicians will play a significant role in long-term monitoring and management of patients with fatty liver disease.
OTHER DISORDERS OF LIVER FUNCTION
Hereditary hemochromatosis
Hereditary hemochromatosis is the most common inherited liver disorder in adults of European descent,17 and can be effectively treated if discovered early. But its clinical diagnosis can be challenging, as many patients have no symptoms at presentation despite abnormal liver enzyme levels. Early symptoms may include severe fatigue, arthralgias, and, in men, impotence, before the appearance of the classic triad of “bronze diabetes” with cirrhosis, diabetes, and darkening of the skin.18
If hemochromatosis is suspected, laboratory tests should include a calculation of percent transferrin saturation, with saturation greater than 45% warranting serum ferritin measurement to evaluate for iron overload (ferritin > 200–300 ng/mL in men, > 150–200 ng/mL in women).19 If iron overload is confirmed, referral to a gastroenterologist is recommended.
Genetic evaluation is often pursued, but patients may ultimately require liver biopsy regardless of the findings, as some patients homozygous for the HFE mutation C282Y may not have clinical hemochromatosis, whereas others with hereditary hemochromatosis may not have the HFE mutation.
Therapeutic phlebotomy is the treatment of choice, and most patients tolerate it well.
Chronic hepatitis B virus and hepatitis C virus infections
Chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are common in the United States, with HBV affecting more than 1 million people and HCV affecting an estimated 3.5 million.
Chronic HCV infection. Direct-acting antiviral drugs have revolutionized HCV treatment and have led to a sustained viral response and presumed cure at 12 weeks in more than 95% of cases across all HCV genotypes.20 Given the recent development of effective and well-tolerated treatments, primary care physicians have assumed a pivotal role in screening for HCV.
The American Association for the Study of Liver Diseases and the Infectious Diseases Society of America21 recommend screening for HCV in people who have risk factors for it, ie:
- HCV exposure
- HIV infection
- Behavioral or environmental risks for contracting the virus such as intravenous drug use or incarceration
- Birth between 1945 and 1965 (one-time testing).
If HCV antibody screening is positive, HCV RNA should be obtained to quantify the viral load and confirm active infection, and genotype testing should be performed to guide treatment. Among the 6 most common HCV genotypes, genotype 1 is the most common in North America, accounting for over 70% of cases in the United States.
Although recommendations and therapies are constantly evolving, the selection of a treatment regimen and the duration of therapy are determined by viral genotype, history of prior treatment, stage of liver fibrosis, potential drug interactions, and frequently, medication cost and insurance coverage.
HBV infection. The treatment for acute HBV infection is generally supportive, though viral suppression with tenofovir or entecavir may be required for those who develop coagulopathy, bilirubinemia, or liver failure. Treatment of chronic HBV infection may not be required and is generally considered for those with elevated ALT, high viral load, or evidence of liver fibrosis on noninvasive measurements such as transient elastography.
Autoimmune hepatitis
Autoimmune causes of liver enzyme elevations should also be considered during initial screening. Positive antinuclear antibody and positive antismooth muscle antibody tests are common in cases of autoimmune hepatitis.22 Autoimmune hepatitis affects women more often than men, with a ratio of 4:1. The peaks of incidence occur during adolescence and between ages 30 and 45.23
Primary biliary cholangitis
Additionally, an elevated alkaline phosphatase level should raise concern for underlying primary biliary cholangitis (formerly called primary biliary cirrhosis), an autoimmune disorder that affects the small and medium intrahepatic bile ducts. Diagnosis of primary biliary cholangitis can be assisted by a positive test for antimitochondrial antibody, present in almost 90% of patients.24
Primary sclerosing cholangitis
Elevated alkaline phosphatase is also the hallmark of primary sclerosing cholangitis, which is associated with inflammatory bowel disease.25 Primary sclerosing cholangitis is characterized by inflammation and fibrosis of the intrahepatic and extrahepatic bile ducts, which are visualized on MRCP and confirmed by biopsy if needed.
REFERRAL
Subspecialty referral should be considered if the cause remains ambiguous or unknown, if there is concern for a rare hepatic disorder such as an autoimmune condition, Wilson disease, or alpha-1 antitrypsin deficiency, or if there is evidence of advanced or chronic liver disease.
Primary care physicians are at the forefront of detecting and diagnosing liver disease, and close coordination with subspecialists will remain crucial in delivering patient care.
Elevated levels of circulating enzymes that are frequently of hepatic origin (aminotransferases and alkaline phosphatase) and bilirubin in the absence of symptoms are common in clinical practice. A dogmatic but true statement holds that there are no trivial elevations in these substances. All persistent elevations of liver enzymes need a methodical evaluation and an appropriate working diagnosis.1
Here, we outline a framework for the workup and treatment of common causes of liver enzyme elevations.
PATTERN OF ELEVATION: CHOLESTATIC OR HEPATOCELLULAR
Based on the pattern of elevation, causes of elevated liver enzymes can be sorted into disorders of cholestasis and disorders of hepatocellular injury (Table 1).1
Cholestatic disorders tend to cause elevations in alkaline phosphatase, bilirubin, and gamma-glutamyl transferase (GGT).
Hepatocellular injury raises levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST).
HOW SHOULD ABNORMAL RESULTS BE EVALUATED?
When approaching liver enzyme elevations, the clinician should develop a working differential diagnosis based on the medical and social history and physical examination.
Think about alcohol, drugs, and fat
The most common causes of liver enzyme elevation are alcohol toxicity, medication overdose, and fatty liver disease.
Alcohol intake should be ascertained. “Significant” consumption is defined as more than 21 drinks per week in men or more than 14 drinks per week in women, over a period of at least 2 years.2
The exact pathogenesis of alcoholic hepatitis is incompletely understood, but alcohol is primarily metabolized by the liver, and damage likely occurs during metabolism of the ingested alcohol. AST elevations tend to be higher than ALT elevations; the reason is ascribed to hepatic deficiency of pyridoxal 5´-phosphate, a cofactor of the enzymatic activity of ALT, which leads to a lesser increase in ALT than in AST.
Alcoholic liver disease can be difficult to diagnose, as many people are initially reluctant to fully disclose how much they drink, but it should be suspected when the ratio of AST to ALT is 2 or greater.
In a classic study, a ratio greater than 2 was found in 70% of patients with alcoholic hepatitis and cirrhosis, compared with 26% of patients with postnecrotic cirrhosis, 8% with chronic hepatitis, 4% with viral hepatitis, and none with obstructive jaundice.3 Importantly, the disorder is often correctable if the patient is able to remain abstinent from alcohol over time.
A detailed medication history is important and should focus especially on recently added medications, dosage changes, medication overuse, and use of nonprescription drugs and herbal supplements. Common medications that affect liver enzyme levels include statins, which cause hepatic dysfunction primarily during the first 3 months of therapy, nonsteroidal anti-inflammatory drugs, antiepileptic drugs, antibiotics, anabolic steroids, and acetaminophen (Table 2).1 Use of illicit drugs and herbal remedies should be discussed, as they may cause toxin-mediated hepatitis.
Although inflammation from drug toxicity will resolve if the offending agent is discontinued, complete recovery may take weeks to months.4
A pertinent social history includes exposure to environmental hepatotoxins such as amatoxin (contained in some wild mushrooms) and occupational hazards (eg, vinyl chloride). Risk factors for viral hepatitis should be evaluated, including intravenous drug use, blood transfusions, unprotected sexual contact, organ transplant, perinatal transmission, and a history of work in healthcare facilities or travel to regions in which hepatitis A or E is endemic.
The medical and family history should include details of associated conditions, such as:
- Right heart failure (a cause of congestive hepatopathy)
- Metabolic syndrome (associated with fatty liver disease)
- Inflammatory bowel disease and primary sclerosing cholangitis
- Early-onset emphysema and alpha-1 antitrypsin deficiency.
The physical examination should be thorough, with emphasis on the abdomen, and search for stigmata of advanced liver disease such as hepatomegaly, splenomegaly, ascites, edema, spider angiomata, jaundice, and asterixis. Any patient with evidence of chronic liver disease should be referred to a subspecialist for further evaluation.
Further diagnostic workup
Abnormal liver enzyme findings or physical examination findings should direct the subsequent diagnostic workup with laboratory testing and imaging.5
For cholestasis. If laboratory data are consistent with cholestasis or abnormal bile flow, it should be further characterized as extrahepatic or intrahepatic. Common causes of extrahepatic cholestasis include biliary tree obstruction due to stones or malignancy, often visualized as intraductal biliary dilation on ultrasonography of the right upper quadrant. Common causes of intrahepatic cholestasis include viral and alcoholic hepatitis, nonalcoholic steatohepatitis, certain drugs and toxins such as alkylated steroids and herbal medications, infiltrative diseases such as amyloid, sarcoid, lymphoma, and tuberculosis, and primary biliary cholangitis.
Abnormal findings on ultrasonography should be further pursued with advanced imaging, ie, computed tomography or magnetic resonance cholangiopancreatography (MRCP). The confirmation of a lesion on imaging is often followed by endoscopic retrograde cholangiopancreatography (ERCP) in an attempt to obtain biopsy samples, remove obstructions, and place therapeutic stents. In instances when endoscopic attempts fail to relieve the obstruction, surgical referral may be appropriate.
For nonhepatobiliary problems. Depending on clinical presentation, it may also be important to consider nonhepatobiliary causes of elevated liver enzymes.
Alkaline phosphatase is found in many other tissue types, including bone, kidney, and the placenta, and can be elevated during pregnancy, adolescence, and even after fatty meals due to intestinal release.6 After screening for the aforementioned physiologic conditions, isolated elevated alkaline phosphatase should be further evaluated by obtaining GGT or 5-nucleotidase levels, which are more specifically of hepatic origin. If these levels are within normal limits, further evaluation for conditions of bone growth and cellular turnover such as Paget disease, hyperparathyroidism, and malignancy should be considered. Specifically, Stauffer syndrome should be considered when there is a paraneoplastic rise in the alkaline phosphatase level in the setting of renal cell carcinoma without liver metastases.
AST and ALT levels may also be elevated in clinical situations and syndromes unrelated to liver disease. Rhabdomyolysis, for instance, may be associated with elevations of AST in more than 90% of cases, and ALT in more than 75%.7 Markers of muscle injury including serum creatine kinase should be obtained in the setting of heat stroke, muscle weakness, strenuous activity, or seizures, as related elevations in AST and ALT may not always be clinically indicative of liver injury.
Given the many conditions that may cause elevated liver enzymes, evaluation and treatment should focus on identifying and removing offending agents and targeting the underlying process with appropriate medical therapy.
FATTY LIVER
With rates of obesity and type 2 diabetes on the rise in the general population, identifying and treating nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) require increased awareness and close coordination between primary care providers and subspecialists.
According to current estimates, up to one-third of the US population (100 million people) may have NAFLD, and 1% to 3% of the population (4–6 million people) likely have NASH, defined as steatosis with inflammation. Development of NASH places patients at a significantly higher risk of fibrosis, hepatocellular injury, and cancer.8
NAFLD is more common in men than in women. It is present in around 80% to 90% of obese adults, two-thirds of adults with type 2 diabetes, and many people with hyperlipidemia. It is also becoming more common in children, with 40% to 70% of obese children likely having some element of NAFLD.
Diagnosis of fatty liver
Although liver enzymes are more likely to be abnormal in individuals with NAFLD, many individuals with underlying NAFLD may have normal laboratory evaluations. ALT may be elevated in only up to 20% of cases and does not likely correlate with the level of underlying liver damage, although increasing GGT may serve as a marker of fibrosis over time.9–11 In contrast to alcohol injury, however, the AST-ALT ratio is usually less than 1.0.
Noninvasive tools for diagnosing NAFLD include the NAFLD fibrosis score, which incorporates age, hyperglycemia, body mass index, platelet count, albumin level, and AST-ALT ratio. This and related scoring algorithms may be useful in differentiating patients with minimal fibrosis from those with advanced fibrosis.12,13
Ultrasonography is a first-line diagnostic test for steatosis, although it may demonstrate fatty infiltration only around 60% of the time. Computed tomography and magnetic resonance imaging are more sensitive, but costlier. Transient elastography (FibroScan; Echosens, Paris, France) has become more popular and has been shown to correlate with findings on liver biopsy in diagnosing or excluding advanced liver fibrosis.14,15
The gold standard for diagnosing NAFLD and NASH is identifying fat-laden hepatocytes or portal inflammation on biopsy; however, biopsy is generally reserved for cases in which the diagnosis remains uncertain.
Behavioral treatment
The primary treatment for NAFLD consists of behavioral modification including weight loss, exercise, and adherence to a low-fat diet, in addition to tight glycemic control and treatment of any underlying lipid abnormalities. Studies have shown that a reduction of 7% to 10% of body weight is associated with a decrease in the inflammation of NAFLD, though no strict guidelines have been established.16
Given the prevalence of NAFLD and the need for longitudinal treatment, primary care physicians will play a significant role in long-term monitoring and management of patients with fatty liver disease.
OTHER DISORDERS OF LIVER FUNCTION
Hereditary hemochromatosis
Hereditary hemochromatosis is the most common inherited liver disorder in adults of European descent,17 and can be effectively treated if discovered early. But its clinical diagnosis can be challenging, as many patients have no symptoms at presentation despite abnormal liver enzyme levels. Early symptoms may include severe fatigue, arthralgias, and, in men, impotence, before the appearance of the classic triad of “bronze diabetes” with cirrhosis, diabetes, and darkening of the skin.18
If hemochromatosis is suspected, laboratory tests should include a calculation of percent transferrin saturation, with saturation greater than 45% warranting serum ferritin measurement to evaluate for iron overload (ferritin > 200–300 ng/mL in men, > 150–200 ng/mL in women).19 If iron overload is confirmed, referral to a gastroenterologist is recommended.
Genetic evaluation is often pursued, but patients may ultimately require liver biopsy regardless of the findings, as some patients homozygous for the HFE mutation C282Y may not have clinical hemochromatosis, whereas others with hereditary hemochromatosis may not have the HFE mutation.
Therapeutic phlebotomy is the treatment of choice, and most patients tolerate it well.
Chronic hepatitis B virus and hepatitis C virus infections
Chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are common in the United States, with HBV affecting more than 1 million people and HCV affecting an estimated 3.5 million.
Chronic HCV infection. Direct-acting antiviral drugs have revolutionized HCV treatment and have led to a sustained viral response and presumed cure at 12 weeks in more than 95% of cases across all HCV genotypes.20 Given the recent development of effective and well-tolerated treatments, primary care physicians have assumed a pivotal role in screening for HCV.
The American Association for the Study of Liver Diseases and the Infectious Diseases Society of America21 recommend screening for HCV in people who have risk factors for it, ie:
- HCV exposure
- HIV infection
- Behavioral or environmental risks for contracting the virus such as intravenous drug use or incarceration
- Birth between 1945 and 1965 (one-time testing).
If HCV antibody screening is positive, HCV RNA should be obtained to quantify the viral load and confirm active infection, and genotype testing should be performed to guide treatment. Among the 6 most common HCV genotypes, genotype 1 is the most common in North America, accounting for over 70% of cases in the United States.
Although recommendations and therapies are constantly evolving, the selection of a treatment regimen and the duration of therapy are determined by viral genotype, history of prior treatment, stage of liver fibrosis, potential drug interactions, and frequently, medication cost and insurance coverage.
HBV infection. The treatment for acute HBV infection is generally supportive, though viral suppression with tenofovir or entecavir may be required for those who develop coagulopathy, bilirubinemia, or liver failure. Treatment of chronic HBV infection may not be required and is generally considered for those with elevated ALT, high viral load, or evidence of liver fibrosis on noninvasive measurements such as transient elastography.
Autoimmune hepatitis
Autoimmune causes of liver enzyme elevations should also be considered during initial screening. Positive antinuclear antibody and positive antismooth muscle antibody tests are common in cases of autoimmune hepatitis.22 Autoimmune hepatitis affects women more often than men, with a ratio of 4:1. The peaks of incidence occur during adolescence and between ages 30 and 45.23
Primary biliary cholangitis
Additionally, an elevated alkaline phosphatase level should raise concern for underlying primary biliary cholangitis (formerly called primary biliary cirrhosis), an autoimmune disorder that affects the small and medium intrahepatic bile ducts. Diagnosis of primary biliary cholangitis can be assisted by a positive test for antimitochondrial antibody, present in almost 90% of patients.24
Primary sclerosing cholangitis
Elevated alkaline phosphatase is also the hallmark of primary sclerosing cholangitis, which is associated with inflammatory bowel disease.25 Primary sclerosing cholangitis is characterized by inflammation and fibrosis of the intrahepatic and extrahepatic bile ducts, which are visualized on MRCP and confirmed by biopsy if needed.
REFERRAL
Subspecialty referral should be considered if the cause remains ambiguous or unknown, if there is concern for a rare hepatic disorder such as an autoimmune condition, Wilson disease, or alpha-1 antitrypsin deficiency, or if there is evidence of advanced or chronic liver disease.
Primary care physicians are at the forefront of detecting and diagnosing liver disease, and close coordination with subspecialists will remain crucial in delivering patient care.
- Aragon G, Younossi ZM. When and how to evaluate mildly elevated liver enzymes in apparently healthy patients. Cleve Clin J Med 2010; 77(3):195–204. doi:10.3949/ccjm.77a.09064
- Chalasani N, Younossi Z, Lavine JE, et al; American Gastroenterological Association; American Association for the Study of Liver Diseases; American College of Gastroenterology. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 2012; 142(7):1592–1609. doi:10.1053/j.gastro.2012.04.001
- Cohen JA, Kaplan MM. The SGOT/SGPT ratio—an indicator of alcoholic liver disease. Dig Dis Sci 1979; 24(11):835–838. pmid:520102
- Kaplan MM. Alanine aminotransferase levels: what’s normal? Ann Intern Med 2002; 137(1):49-51. pmid:12093245
- Pratt DS, Kaplan MM. Evaluation of abnormal liver enzyme results in asymptomatic patients. N Engl J Med 2000; 342(17):1266–1271. doi:10.1056/NEJM200004273421707
- Sharma U, Pal D, Prasad R. Alkaline phosphatase: an overview. Indian J Clin Biochem 2014; 29(3):269–278. doi:10.1007/s12291-013-0408-y
- Weibrecht K, Dayno M, Darling C, Bird SB. Liver aminotransferases are elevated with rhabdomyolysis in the absence of significant liver injury. J Med Toxicol 2010; 6(3):294–300. doi:10.1007/s13181-010-0075-9
- Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis 2010; 28(1):155–161. doi:10.1159/000282080
- Adams LA, Feldstein AE. Non-invasive diagnosis of nonalcoholic fatty liver and nonalcoholic steatohepatitis. J Dig Dis 2011; 12(1):10–16. doi:10.1111/j.1751-2980.2010.00471.x
- Fracanzani AL, Valenti L, Bugianesi E, et al. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology 2008; 48(3):792–798. doi:10.1002/hep.22429
- Tahan V, Canbakan B, Balci H, et al. Serum gamma-glutamyltranspeptidase distinguishes non-alcoholic fatty liver disease at high risk. Hepatogastroenterolgoy 2008; 55(85):1433-1438. pmid:18795706
- McPherson S, Stewart S, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010; 59(9):1265–1269. doi:10.1136/gut.2010.216077
- Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007; 45(4):846–854. doi:10.1002/hep.21496
- Petta S, Vanni E, Bugianesi E, et al. The combination of liver stiffness measurement and NAFLD fibrosis score improves the noninvasive diagnostic accuracy for severe liver fibrosis in patients with nonalcoholic fatty liver disease. Liver Int 2015; 35(5):1566–1573. doi:10.1111/liv.12584
- Hashemi SA, Alavian SM, Gholami-Fesharaki M. Assessment of transient elastography (FibroScan) for diagnosis of fibrosis in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Caspian J Intern Med 2016; 7(4):242–252. pmid:27999641
- Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010; 51(1):121–129. doi:10.1002/hep.23276
- Adams PH, Reboussin DM, Barton JC, et al. Hemochromatosis and iron-overload screening in a racially diverse population. N Engl J Med 2005; 352(17):1769-1778. doi:10.1056/NEJMoa041534
- Brissot P, de Bels F. Current approaches to the management of hemochromatosis. Hematology Am Soc Hematol Educ Program 2006; 2006(1):36–41. doi:10.1182/asheducation-2006.1.36
- Bacon BR, Adams PC, Kowdley KV, Powell LW, Tavill AS; American Association for the Study of Liver Diseases. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology 2011; 54(1):328–343. doi:10.1002/hep.24330
- Weiler N, Zeuzem S, Welker MW. Concise review: interferon-free treatment of hepatitis C virus-associated cirrhosis and liver graft infection. World J Gastroenterol 2016; 22(41):9044–9056. doi:10.3748/wjg.v22.i41.9044
- American Association for the Study of Liver Disease, Infectious Diseases Society of America. HCV guidance: recommendations for testing, managing, and treating hepatitis C. www.hcvguidelines.org. Accessed July 16, 2018.
- Manns MP, Czaja AJ, Gorham JD, et al; American Association for the Study of Liver Diseases. Diagnosis and management of autoimmune hepatitis. Hepatology 2010; 51(6):2193–2213. doi:10.1002/hep.23584
- Liberal R, Krawitt EL, Vierling JM, Manns MP, Mieli-Vergani G, Vergani D. Cutting edge issues in autoimmune hepatitis. J Autoimmun 2016; 75:6–19. doi:10.1016/j.jaut.2016.07.005
- Mousa HS, Carbone M, Malinverno F, Ronca V, Gershwin ME, Invernizzi P. Novel therapeutics for primary biliary cholangitis: Toward a disease-stage-based approach. Autoimmun Rev 2016; 15(9):870–876. doi:10.1016/j.autrev.2016.07.003
- de Vries AB, Janse M, Blokzijl H, Weersma RK. Distinctive inflammatory bowel disease phenotype in primary sclerosing cholangitis. World J Gastroenterol 2015; 21(6):1956–1971. doi:10.3748/wjg.v21.i6.1956
- Aragon G, Younossi ZM. When and how to evaluate mildly elevated liver enzymes in apparently healthy patients. Cleve Clin J Med 2010; 77(3):195–204. doi:10.3949/ccjm.77a.09064
- Chalasani N, Younossi Z, Lavine JE, et al; American Gastroenterological Association; American Association for the Study of Liver Diseases; American College of Gastroenterology. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 2012; 142(7):1592–1609. doi:10.1053/j.gastro.2012.04.001
- Cohen JA, Kaplan MM. The SGOT/SGPT ratio—an indicator of alcoholic liver disease. Dig Dis Sci 1979; 24(11):835–838. pmid:520102
- Kaplan MM. Alanine aminotransferase levels: what’s normal? Ann Intern Med 2002; 137(1):49-51. pmid:12093245
- Pratt DS, Kaplan MM. Evaluation of abnormal liver enzyme results in asymptomatic patients. N Engl J Med 2000; 342(17):1266–1271. doi:10.1056/NEJM200004273421707
- Sharma U, Pal D, Prasad R. Alkaline phosphatase: an overview. Indian J Clin Biochem 2014; 29(3):269–278. doi:10.1007/s12291-013-0408-y
- Weibrecht K, Dayno M, Darling C, Bird SB. Liver aminotransferases are elevated with rhabdomyolysis in the absence of significant liver injury. J Med Toxicol 2010; 6(3):294–300. doi:10.1007/s13181-010-0075-9
- Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis 2010; 28(1):155–161. doi:10.1159/000282080
- Adams LA, Feldstein AE. Non-invasive diagnosis of nonalcoholic fatty liver and nonalcoholic steatohepatitis. J Dig Dis 2011; 12(1):10–16. doi:10.1111/j.1751-2980.2010.00471.x
- Fracanzani AL, Valenti L, Bugianesi E, et al. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology 2008; 48(3):792–798. doi:10.1002/hep.22429
- Tahan V, Canbakan B, Balci H, et al. Serum gamma-glutamyltranspeptidase distinguishes non-alcoholic fatty liver disease at high risk. Hepatogastroenterolgoy 2008; 55(85):1433-1438. pmid:18795706
- McPherson S, Stewart S, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010; 59(9):1265–1269. doi:10.1136/gut.2010.216077
- Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007; 45(4):846–854. doi:10.1002/hep.21496
- Petta S, Vanni E, Bugianesi E, et al. The combination of liver stiffness measurement and NAFLD fibrosis score improves the noninvasive diagnostic accuracy for severe liver fibrosis in patients with nonalcoholic fatty liver disease. Liver Int 2015; 35(5):1566–1573. doi:10.1111/liv.12584
- Hashemi SA, Alavian SM, Gholami-Fesharaki M. Assessment of transient elastography (FibroScan) for diagnosis of fibrosis in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Caspian J Intern Med 2016; 7(4):242–252. pmid:27999641
- Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010; 51(1):121–129. doi:10.1002/hep.23276
- Adams PH, Reboussin DM, Barton JC, et al. Hemochromatosis and iron-overload screening in a racially diverse population. N Engl J Med 2005; 352(17):1769-1778. doi:10.1056/NEJMoa041534
- Brissot P, de Bels F. Current approaches to the management of hemochromatosis. Hematology Am Soc Hematol Educ Program 2006; 2006(1):36–41. doi:10.1182/asheducation-2006.1.36
- Bacon BR, Adams PC, Kowdley KV, Powell LW, Tavill AS; American Association for the Study of Liver Diseases. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology 2011; 54(1):328–343. doi:10.1002/hep.24330
- Weiler N, Zeuzem S, Welker MW. Concise review: interferon-free treatment of hepatitis C virus-associated cirrhosis and liver graft infection. World J Gastroenterol 2016; 22(41):9044–9056. doi:10.3748/wjg.v22.i41.9044
- American Association for the Study of Liver Disease, Infectious Diseases Society of America. HCV guidance: recommendations for testing, managing, and treating hepatitis C. www.hcvguidelines.org. Accessed July 16, 2018.
- Manns MP, Czaja AJ, Gorham JD, et al; American Association for the Study of Liver Diseases. Diagnosis and management of autoimmune hepatitis. Hepatology 2010; 51(6):2193–2213. doi:10.1002/hep.23584
- Liberal R, Krawitt EL, Vierling JM, Manns MP, Mieli-Vergani G, Vergani D. Cutting edge issues in autoimmune hepatitis. J Autoimmun 2016; 75:6–19. doi:10.1016/j.jaut.2016.07.005
- Mousa HS, Carbone M, Malinverno F, Ronca V, Gershwin ME, Invernizzi P. Novel therapeutics for primary biliary cholangitis: Toward a disease-stage-based approach. Autoimmun Rev 2016; 15(9):870–876. doi:10.1016/j.autrev.2016.07.003
- de Vries AB, Janse M, Blokzijl H, Weersma RK. Distinctive inflammatory bowel disease phenotype in primary sclerosing cholangitis. World J Gastroenterol 2015; 21(6):1956–1971. doi:10.3748/wjg.v21.i6.1956
KEY POINTS
- Disorders of hepatocellular injury tend to elevate levels of aminotransferases, whereas cholestatic disorders cause elevations of alkaline phosphatase and bilirubin.
- The three most common causes of liver enzyme elevation are alcohol toxicity, medication overdose, and fatty liver disease.
- Other disorders of liver dysfunction include hereditary hemochromatosis, viral hepatitis, autoimmune hepatitis, primary biliary cholangitis, primary sclerosing cholangitis, and alpha-1 antitrypsin disease.
- Nonhepatic causes of elevated “liver enzymes” also need to be considered. For instance, rhabdomyolysis causes elevations in aminotransferase levels.
The bias of word choice and the interpretation of laboratory tests
In the current sociopolitical environment in the United States, the slogan “words matter” has become a battle cry for several groups and causes, emphasizing that our choice of words can influence the way we assess a specific person or situation. We are not immune to the subliminal bias of words, even as we evaluate such seemingly objective components of clinical management as laboratory test results.
Several years ago, I was supervising teaching rounds on a general medicine service. It was the first rounds of the month, and the patients were relatively new to the residents and totally unknown to me. One patient was an elderly man with weight loss, fatigue, weakness, and a history of excessive alcohol ingestion. His family had corroborated the last detail, but he had stopped drinking a long time before his admission. He had normal creatinine, minimal anemia, and markedly elevated and unexplained “liver function tests.” Liver biopsy was planned.
As we entered his room, we saw a gaunt man struggle to rise from the bedside chair to get back into bed. He rocked several times and then pushed himself up from the chair using his arms. Then, after a few short steps, he plopped back into bed and greeted us. His breakfast tray was untouched at the bedside. I introduced myself, we chatted for a short while as I examined him in front of our team, and we left.
In the hallway I asked, “Who would like to get an additional blood test before we do a liver biopsy?” Without waiting for a response I asked a second question, “What exactly are liver function tests?”
Words do matter, and they influence the way we analyze clinical scenarios. It could be argued that a complete and careful history would have established that our patient’s fatigue and weakness were due to proximal muscle weakness and not general asthenia, and that detailed questioning would have revealed that his weight loss was mainly from difficulty in swallowing without a sense of choking and coughing. But faced with an elderly man, a likely explanation for liver disease, and markedly elevated aspartate and alanine aminotransferase (AST and ALT) levels, there was premature closure of the diagnosis, and the decision was made to obtain a liver biopsy—which our hepatology consultants surely would not have done. I believe that a major contributor to the premature diagnosis was the choice of the words “liver function tests” and the default assumption that elevated serum levels of these enzymes always reflect liver disease.
Aminotransferases are fairly ubiquitous, likely present in various concentrations in all cells in our body. AST exists in mitochondrial and cytosolic forms, and ALT in the cytosol. The concentration of ALT is higher in the liver than in other organs, and its enzymatic activity is suppressed by hepatic exposure to alcohol. Both enzymes are present in muscle, and although AST is more abundant in cells other than hepatocytes, the longer serum half-life of ALT may result in roughly equal serum levels in the setting of chronic muscle injury such as myositis (the true diagnosis in our weak patient).
While a meticulous history and examination would indeed have led to the diagnosis of muscle disease in this man, they alone could not have determined whether he had coexistent liver and muscle disease. And this is a real challenge when acute muscle toxicity and liver toxicity are equally possible (eg, statin or immune checkpoint autoimmune tissue damage, or after significant trauma).
There are many nuances in the interpretation of even the most common laboratory tests. In this issue of the Journal, Agganis et al discuss liver enzymes (a term slightly more acceptable to me than liver function tests). In future issues, we will address the interpretation of other laboratory tests.
In the current sociopolitical environment in the United States, the slogan “words matter” has become a battle cry for several groups and causes, emphasizing that our choice of words can influence the way we assess a specific person or situation. We are not immune to the subliminal bias of words, even as we evaluate such seemingly objective components of clinical management as laboratory test results.
Several years ago, I was supervising teaching rounds on a general medicine service. It was the first rounds of the month, and the patients were relatively new to the residents and totally unknown to me. One patient was an elderly man with weight loss, fatigue, weakness, and a history of excessive alcohol ingestion. His family had corroborated the last detail, but he had stopped drinking a long time before his admission. He had normal creatinine, minimal anemia, and markedly elevated and unexplained “liver function tests.” Liver biopsy was planned.
As we entered his room, we saw a gaunt man struggle to rise from the bedside chair to get back into bed. He rocked several times and then pushed himself up from the chair using his arms. Then, after a few short steps, he plopped back into bed and greeted us. His breakfast tray was untouched at the bedside. I introduced myself, we chatted for a short while as I examined him in front of our team, and we left.
In the hallway I asked, “Who would like to get an additional blood test before we do a liver biopsy?” Without waiting for a response I asked a second question, “What exactly are liver function tests?”
Words do matter, and they influence the way we analyze clinical scenarios. It could be argued that a complete and careful history would have established that our patient’s fatigue and weakness were due to proximal muscle weakness and not general asthenia, and that detailed questioning would have revealed that his weight loss was mainly from difficulty in swallowing without a sense of choking and coughing. But faced with an elderly man, a likely explanation for liver disease, and markedly elevated aspartate and alanine aminotransferase (AST and ALT) levels, there was premature closure of the diagnosis, and the decision was made to obtain a liver biopsy—which our hepatology consultants surely would not have done. I believe that a major contributor to the premature diagnosis was the choice of the words “liver function tests” and the default assumption that elevated serum levels of these enzymes always reflect liver disease.
Aminotransferases are fairly ubiquitous, likely present in various concentrations in all cells in our body. AST exists in mitochondrial and cytosolic forms, and ALT in the cytosol. The concentration of ALT is higher in the liver than in other organs, and its enzymatic activity is suppressed by hepatic exposure to alcohol. Both enzymes are present in muscle, and although AST is more abundant in cells other than hepatocytes, the longer serum half-life of ALT may result in roughly equal serum levels in the setting of chronic muscle injury such as myositis (the true diagnosis in our weak patient).
While a meticulous history and examination would indeed have led to the diagnosis of muscle disease in this man, they alone could not have determined whether he had coexistent liver and muscle disease. And this is a real challenge when acute muscle toxicity and liver toxicity are equally possible (eg, statin or immune checkpoint autoimmune tissue damage, or after significant trauma).
There are many nuances in the interpretation of even the most common laboratory tests. In this issue of the Journal, Agganis et al discuss liver enzymes (a term slightly more acceptable to me than liver function tests). In future issues, we will address the interpretation of other laboratory tests.
In the current sociopolitical environment in the United States, the slogan “words matter” has become a battle cry for several groups and causes, emphasizing that our choice of words can influence the way we assess a specific person or situation. We are not immune to the subliminal bias of words, even as we evaluate such seemingly objective components of clinical management as laboratory test results.
Several years ago, I was supervising teaching rounds on a general medicine service. It was the first rounds of the month, and the patients were relatively new to the residents and totally unknown to me. One patient was an elderly man with weight loss, fatigue, weakness, and a history of excessive alcohol ingestion. His family had corroborated the last detail, but he had stopped drinking a long time before his admission. He had normal creatinine, minimal anemia, and markedly elevated and unexplained “liver function tests.” Liver biopsy was planned.
As we entered his room, we saw a gaunt man struggle to rise from the bedside chair to get back into bed. He rocked several times and then pushed himself up from the chair using his arms. Then, after a few short steps, he plopped back into bed and greeted us. His breakfast tray was untouched at the bedside. I introduced myself, we chatted for a short while as I examined him in front of our team, and we left.
In the hallway I asked, “Who would like to get an additional blood test before we do a liver biopsy?” Without waiting for a response I asked a second question, “What exactly are liver function tests?”
Words do matter, and they influence the way we analyze clinical scenarios. It could be argued that a complete and careful history would have established that our patient’s fatigue and weakness were due to proximal muscle weakness and not general asthenia, and that detailed questioning would have revealed that his weight loss was mainly from difficulty in swallowing without a sense of choking and coughing. But faced with an elderly man, a likely explanation for liver disease, and markedly elevated aspartate and alanine aminotransferase (AST and ALT) levels, there was premature closure of the diagnosis, and the decision was made to obtain a liver biopsy—which our hepatology consultants surely would not have done. I believe that a major contributor to the premature diagnosis was the choice of the words “liver function tests” and the default assumption that elevated serum levels of these enzymes always reflect liver disease.
Aminotransferases are fairly ubiquitous, likely present in various concentrations in all cells in our body. AST exists in mitochondrial and cytosolic forms, and ALT in the cytosol. The concentration of ALT is higher in the liver than in other organs, and its enzymatic activity is suppressed by hepatic exposure to alcohol. Both enzymes are present in muscle, and although AST is more abundant in cells other than hepatocytes, the longer serum half-life of ALT may result in roughly equal serum levels in the setting of chronic muscle injury such as myositis (the true diagnosis in our weak patient).
While a meticulous history and examination would indeed have led to the diagnosis of muscle disease in this man, they alone could not have determined whether he had coexistent liver and muscle disease. And this is a real challenge when acute muscle toxicity and liver toxicity are equally possible (eg, statin or immune checkpoint autoimmune tissue damage, or after significant trauma).
There are many nuances in the interpretation of even the most common laboratory tests. In this issue of the Journal, Agganis et al discuss liver enzymes (a term slightly more acceptable to me than liver function tests). In future issues, we will address the interpretation of other laboratory tests.
Phosphorus binders: The new and the old, and how to choose
The balance between dietary intake and excretion of phosphorus can be impaired in patients with decreased renal function, leading to hyperphosphatemia. Many patients with end-stage renal disease on dialysis require phosphorus-binding drugs to control their serum phosphorus levels.
See related editorial and article
In this review, we discuss the pathophysiology of hyperphosphatemia in kidney disease, its consequences, and how to control it, focusing on the different classes of phosphorus binders.
ROLE OF THE INTERNIST
With kidney disease common and on the increase,1 nephrologists and internists need to work together to provide optimal care.
Further, many internists in managed care plans and accountable care organizations now handle many tasks previously left to specialists—including prescribing and managing phosphorus binders in patients with kidney disease.
PATHOPHYSIOLOGY OF HYPERPHOSPHATEMIA
The pathophysiology of bone mineral disorders in kidney disease is complex. To simplify the discussion, we will address it in 3 parts:
- Phosphorus balance
- The interplay of hormones, including fibroblast growth factor 23 (FGF23)
- The mechanism of hyperphosphatemia in kidney disease.
Phosphorus balance
Phosphorus is a macronutrient essential for a range of cellular functions that include structure, energy production, metabolism, and cell signaling. It exists primarily in the form of inorganic phosphate.
An average Western diet provides 20 mg of phosphorus per kilogram of body weight per day. Of this, 13 mg/kg is absorbed, and the rest is excreted in the feces.2
Absorption of dietary phosphorus occurs mainly in the jejunum. It is mediated by both a paracellular sodium-independent pathway (driven by high intraluminal phosphorus content) and by active sodium-dependent cotransporters. It is also influenced by diet and promoted by active vitamin D (1,25 dihydroxyvitamin D3, also called calcitriol).3
Absorbed phosphorus enters the extracellular fluid and shifts in and out of the skeleton under the influence of parathyroid hormone.
Phosphorus excretion is handled almost entirely by the kidneys. Phosphorus is freely filtered at the glomerulus and reabsorbed mainly in the proximal tubule by sodium-phosphate cotransporters.
Normally, when phosphorus intake is adequate, most of the filtered phosphorus is reabsorbed and only 10% to 20% is excreted in the urine. However, the threshold for phosphorus reabsorption in the proximal tubule is influenced by parathyroid hormone, FGF23, and dietary phosphorus intake: low serum phosphate levels lead to an increase in the synthesis of sodium-phosphorus cotransporters, resulting in increased (nearly complete) proximal reabsorption and an increase in the serum phosphorus concentration.4 Conversely, both parathyroid hormone and FGF23 are phosphaturic and decrease the number of phosphorus transporters, which in turn leads to increased phosphorus excretion and a decrease in serum phosphorus concentration.5
Interplay of hormones
FGF23 is a phosphaturic glycoprotein secreted by osteoblasts and osteocytes. It acts by binding to fibroblastic growth receptor 1 in the presence of its coreceptor, the Klotho protein.6
FGF23 is regulated by serum phosphorus levels and plays a major role in the response to elevated serum phosphorus. It causes a direct increase in urinary phosphorus excretion, a decrease in intestinal phosphorus absorption (indirectly via inhibition of calcitriol), and decreased bone resorption via a decrease in parathyroid hormone production.7
Mechanism of hyperphosphatemia in kidney disease
In chronic kidney disease, phosphorus retention can trigger secondary hyperparathyroidism, as rising phosphorus levels stimulate FGF23. In the early stages of chronic kidney disease, this response can correct the phosphorus levels, but with several consequences:
- Decreased calcitriol due to its inhibition by FGF239
- Hypocalcemia due to decreased calcitriol (leading to decreased intestinal calcium absorption) and calcium binding of retained phosphorus
- Elevated parathyroid hormone due to low calcitriol levels (lack of inhibitory feedback by calcitriol), hyperphosphatemia, and hypocalcemia (direct parathyroid hormone stimulation).
As the elevated phosphorus level is likely to be the triggering event behind secondary renal hyperparathyroidism, it needs to be controlled. This is accomplished by restricting dietary phosphorus and using phosphorus binders.
HYPERPHOSPHATEMIA MAY LEAD TO VASCULAR CALCIFICATION
Elevated serum phosphorus levels (normal range 2.48–4.65 mg/dL in adults11) are associated with cardiovascular calcification and subsequent increases in mortality and morbidity rates. Elevations in serum phosphorus and calcium levels are associated with progression in vascular calcification12 and likely account for the accelerated vascular calcification that is seen in kidney disease.13
Hyperphosphatemia has been identified as an independent risk factor for death in patients with end-stage renal disease,14 but that relationship is less clear in patients with chronic kidney disease. A study in patients with chronic kidney disease and not on dialysis found a lower mortality rate in those who were prescribed phosphorus binders,15 but the study was criticized for limitations in its design.
Hyperphosphatemia can also lead to adverse effects on bone health due to complications such as renal osteodystrophy.
However, in its 2017 update, the Kidney Disease: Improving Global Outcomes (KDIGO) program only “suggests” lowering elevated phosphorus levels “toward” the normal range in patients with chronic kidney disease stages G3a through G5D, ie, those with glomerular filtration rates less than 60 mL/min/1.73 m2, including those on dialysis. The recommendation is graded 2C, ie, weak, based on low-quality evidence (https://kdigo.org/guidelines/ckd-mbd).
DIETARY RESTRICTION OF PHOSPHORUS
Diet is the major source of phosphorus intake. The average daily phosphorus consumption is 20 mg/kg, or 1,400 mg, and protein is the major source of dietary phosphorus.
In patients with stage 4 or 5 chronic kidney disease, the Kidney Disease Outcomes Quality Initiative recommends limiting protein intake to 0.6 mg/kg/day.16 However, in patients on hemodialysis, they recommend increasing protein intake to 1.1 mg/kg/day while limiting phosphorus intake to about 800 to 1,000 mg/day. This poses a challenge, as limiting phosphorus intake can reduce protein intake.
Sources of protein can be broadly classified as plant-based or animal-based. Animal protein contains organic phosphorus, which is easily absorbed.18 Plant protein may not be absorbed as easily.
Moe et al19 studied the importance of the protein source of phosphorus after 7 days of controlled diets. Despite equivalent protein and phosphorus concentrations in the vegetarian and meat-based diets, participants on the vegetarian diet had lower serum phosphorus levels, a trend toward lower 24-hour urinary phosphorus excretion, and significantly lower FGF23 levels than those on the meat-based diet. This suggests that a vegetarian diet may have advantages in terms of preventing hyperphosphatemia.
Another measure to reduce phosphorus absorption from meat is to boil it, which reduces the phosphorus content by 50%.20
Processed foods containing additives and preservatives are very high in phosphorus21 and should be avoided, particularly as there is no mandate to label phosphorus content in food.
PHOSPHORUS AND DIALYSIS
Although hemodialysis removes phosphorus, it does not remove enough to keep levels within normal limits. Indeed, even when patients adhere to a daily phosphorus limit of 1,000 mg, phosphorus accumulates. If 70% of the phosphorus in the diet is absorbed, this is 4,500 to 5,000 mg in a week. A 4-hour hemodialysis session will remove only 1,000 mg of phosphorus, which equals about 3,000 mg for patients undergoing dialysis 3 times a week,22 far less than phosphorus absorption.
In patients on continuous ambulatory peritoneal dialysis, a daily regimen of 4 exchanges of 2 L per exchange removes about 200 mg of phosphorus per day. In a 2012 study, patients on nocturnal dialysis or home dialysis involving longer session length had greater lowering of phosphorus levels than patients undergoing routine hemodialysis.23
Hence, phosphorus binders are often necessary in patients on routine hemodialysis or peritoneal dialysis.
PHOSPHORUS BINDERS
Phosphorus binders reduce serum phosphorus levels by binding with ingested phosphorus in the gastrointestinal tract and forming insoluble complexes that are not absorbed. For this reason they are much more effective when taken with meals. Phosphorus binders come in different formulations: pills, capsules, chewable tablets, liquids, and even powders that can be sprinkled on food.
The potency of each binder is quantified by its “phosphorus binder equivalent dose,” ie, its binding capacity compared with that of calcium carbonate as a reference.24
Phosphorus binders are broadly divided into those that contain calcium and those that do not.
Calcium-containing binders
The 2 most commonly used preparations are calcium carbonate (eg, Tums) and calcium acetate (eg, Phoslo). While these are relatively safe, some studies suggest that their use can lead to accelerated vascular calcification.25
According to KDIGO,26 calcium-containing binders should be avoided in hypercalcemia and adynamic bone disease. Additionally, the daily elemental calcium intake from binders should be limited to 1,500 mg, with a total daily intake that does not exceed 2,000 mg.
The elemental calcium content of calcium carbonate is about 40% of its weight (eg, 200 mg of elemental calcium in a 500-mg tablet of Tums), while the elemental calcium content of calcium acetate is about 25%. Therefore, a patient who needs 6 g of calcium carbonate for efficacy will be ingesting 2.4 g of elemental calcium per day, and that exceeds the recommended daily maximum. The main advantage of calcium carbonate is its low cost and easy availability. Commonly reported side effects include nausea and constipation.
A less commonly used calcium-based binder is calcium citrate (eg, Calcitrate). It should, however, be avoided in chronic kidney disease because of the risk of aluminum accumulation. Calcium citrate can enhance intestinal absorption of aluminum from dietary sources, as aluminum can form complexes with citrate.27
Calcium-free binders
There are several calcium-free binders. Some are based on metals such as aluminum, magnesium, iron, and lanthanum; others, such as sevelamer, are resin-based.
Aluminum- and magnesium-based binders are generally not used long-term in kidney disease because of the toxicity associated with aluminum and magnesium accumulation. However, aluminum hydroxide has an off-label use as a phosphorus binder in the acute setting, particularly when serum phosphorus levels are above 7 mg/dL.28 The dose is 300 to 600 mg 3 times daily with meals for a maximum of 4 weeks.
Sevelamer. Approved by the US Food and Drug Administration (FDA) in 1998, sevelamer acts by trapping phosphorus through ion exchange and hydrogen binding. It has the advantage of being calcium-free, which makes it particularly desirable in patients with hypercalcemia.
The Renagel in New Dialysis25 and Treat-To-Goal29 studies were randomized controlled trials that looked at the effects of sevelamer vs calcium-based binders on the risk of vascular calcification. The primary end points were serum phosphorus and calcium levels, while the secondary end points were coronary artery calcification on computed tomography and thoracic vertebral bone density. Both studies demonstrated a higher risk of vascular calcification with the calcium-based binders.
Another possible benefit of sevelamer is an improvement in lipid profile. Sevelamer lowers total cholesterol and low-density lipoprotein cholesterol levels without affecting high-density lipoprotein cholesterol or triglyceride levels.30 This is likely due to its bile acid-binding effect.31 Sevelamer has also been shown to lower C-reactive protein levels.32 While the cardiovascular profile appears to be improved with the treatment, there are no convincing data to confirm that those properties translate to a proven independent survival benefit.
The Calcium Acetate Renagel Evaluation33 was a randomized controlled study comparing sevelamer and calcium acetate. The authors attempted to control for the lipid-lowering effects of sevelamer by giving atorvastatin to all patients in both groups who had a low-density lipoprotein level greater than 70 mg/dL. The study found sevelamer to be not inferior to calcium acetate in terms of mortality and coronary calcification.
Further studies such as the Brazilian Renagel and Calcium trial34 and the Dialysis Clinical Outcomes Revisited trial failed to show a significant long-term benefit of sevelamer over calcium-based binders. However, a secondary statistical analysis of the latter study showed possible benefit of sevelamer over calcium acetate among those age 65 and older.35
To understand how sevelamer could affect vascular calcification, Yilmaz et al36 compared the effects of sevelamer vs calcium acetate on FGF23 and fetuin A levels. Fetuin A is an important inhibitor of vascular calcification and is progressively diminished in kidney disease, leading to accelerated calcification.37 Patients on sevelamer had higher levels of fetuin A than their counterparts on calcium acetate.37 The authors proposed increased fetuin A levels as a mechanism for decreased vascular calcification.
In summary, some studies suggest that sevelamer may offer the advantage of decreasing vascular calcification, but the data are mixed and do not provide a solid answer. The main disadvantages of sevelamer are a high pill burden and side effects of nausea and dyspepsia.
Lanthanum, a metallic element, was approved as a phosphorus binder by the FDA in 2008. It comes as a chewable tablet and offers the advantage of requiring the patient to take fewer pills than sevelamer and calcium-based binders.
Sucroferric oxyhydroxide comes as a chewable tablet. It was approved by the FDA in 2013. Although each tablet contains 500 mg of iron, it has not been shown to improve iron markers. In terms of phosphorus-lowering ability, it has been shown to be noninferior to sevelamer.39 Advantages include a significantly lower pill burden. Disadvantages include gastrointestinal side effects such as diarrhea and nausea and the drug’s high cost.
Ferric citrate was approved by the FDA in 2014, and 1 g delivers 210 mg of elemental iron. The main advantage of ferric citrate is its ability to increase iron markers. The phase 3 trial that demonstrated its efficacy as a binder showed an increase in ferritin compared with the active control.40 The study also showed a decrease in the need to use intravenous iron and erythropoesis-stimulating agents. This was thought to be due to improved iron stores, leading to decreased erythropoietin resistance.41
The mean number of ferric citrate tablets needed to achieve the desired phosphorus-lowering effect was 8 per day, containing 1,680 mg of iron. In comparison, oral ferrous sulfate typically provides 210 mg of iron per day.42
Disadvantages of ferric citrate include high pill burden, high cost, and gastrointestinal side effects such as nausea and constipation.
Chitosan binds salivary phosphorus. It can potentially be used, but it is not approved, and its efficacy in lowering serum phosphorus remains unclear.43
CHOOSING THE APPROPRIATE PHOSPHORUS BINDER
The choice of phosphorus binder is based on the patient’s serum calcium level and iron stores and on the drug’s side effect profile, iron pill burden, and cost. Involving patients in the choice after discussing potential side effects, pill burden, and cost is important for shared decision-making and could play a role in improving adherence.
Phosphorus binders are a major portion of the pill burden in patients with end-stage renal disease, possibly affecting patient adherence. The cost of phosphorus binders is estimated at half a billion dollars annually, underlining the significant economic impact of phosphorus control.11
Calcium-based binders should be the first choice when there is secondary hyperparathyroidism without hypercalcemia. There is no clear evidence regarding the benefit of correcting hypocalcemia, but KDIGO recommends keeping the serum calcium level within the reference range. KDIGO also recommends restricting calcium-based binders in persistent hypercalcemia, arterial calcification, and adynamic bone disease. This recommendation is largely based on expert opinion.
Noncalcium-based binders, which in theory might prevent vascular calcification, should be considered for patients with at least 1 of the following44:
- Complicated diabetes mellitus
- Vascular or valvular calcification
- Persistent inflammation.
Noncalcium-based binders are also preferred in low bone-turnover states such as adynamic bone disease, as elevated calcium can inhibit parathyroid hormone.
However, the advantage of noncalcium-based binders regarding vascular calcification is largely theoretical and has not been proven clinically. Indeed, there are data comparing long-term outcomes of the different classes of phosphorus binders, but studies were limited by short follow-up, and individual studies have lacked power to detect statistical significance between two classes of binders on long-term outcomes. Meta-analyses have provided conflicting data, with some suggesting better outcomes with sevelamer than with calcium-based binders, and with others failing to show any difference.45
Because iron deficiency is common in kidney disease, ferric citrate, which can improve iron markers, may be a suitable option, provided its cost is covered by insurance.
SPECIAL CIRCUMSTANCES FOR THE USE OF PHOSPHORUS BINDERS
Tumor lysis syndrome
Tumor lysis syndrome occurs when tumor cells release their contents into the bloodstream, either spontaneously or in response to therapy, leading to the characteristic findings of hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia.46 Phosphorus binders in conjunction with intravenous hydration are used to treat hyperphosphatemia, but evidence about their efficacy in this setting is limited.
Hypocalcemia in tumor lysis syndrome is usually not treated unless symptomatic, as the calcium-phosphorus product can increase, leading to calcium phosphate crystallization. When the calcium-phosphorus product is greater than 60, there is a higher risk of calcium phosphate deposition in the renal tubules that can lead to acute renal failure in tumor lysis syndrome.47 To lower the risk of calcium phosphate crystallization, calcium-based binders should be avoided in tumor lysis syndrome.
Total parenteral nutrition
Since patients on total parenteral nutrition do not eat, phosphorus binders are considered ineffective; there are no concrete data showing that phosphorus binders are effective in these patients.48 In patients with kidney disease, the phosphorus content in the parenteral nutrition formulation must be reduced.
Pregnancy
Data on phosphorus binders in pregnancy are limited. Calcium can cross the placenta. Calcium carbonate can be used in pregnancy, and fetal harm is not expected if calcium concentrations are within normal limits.49 Calcium acetate, sevelamer, and lanthanum are considered pregnancy category C drugs. Patients with advanced chronic kidney disease and end-stage renal disease who become pregnant must receive specialized obstetric care for high-risk pregnancy.
FUTURE DIRECTIONS
Future therapies may target FGF23 and other inflammatory markers that are up-regulated in renal hyperparathyroidism. However, trials studying these markers are needed to provide a better understanding of their role in bone mineral and cardiovascular health and in overall long-term outcomes. Additionally, randomized controlled trials are needed to study long-term nonsurrogate outcomes such as reduction in cardiovascular disease and rates of overall mortality.
- Collins AJ, Foley RN, Herzog C, et al. US renal data system 2012 annual data report. Am J Kidney Dis 2013; 61(1 suppl 1):A7,e1–476. doi:10.1053/j.ajkd.2012.11.031
- Tenenhouse HS. Regulation of phosphorus homeostasis by the type iia Na/phosphate cotransporter. Annu Rev Nutr 2005; 25:197–214. doi:10.1146/annurev.nutr.25.050304.092642
- Lederer E. Regulation of serum phosphate. J Physiol 2014; 592(18):3985–3995. doi:10.1113/jphysiol.2014.273979
- Lederer E. Renal phosphate transporters. Curr Opin Nephrol Hypertens 2014; 23(5):502–506. doi:10.1097/MNH.0000000000000053
- Weinman EJ, Lederer ED. NHERF-1 and the regulation of renal phosphate reabsoption: a tale of three hormones. Am J Physiol Renal Physiol 2012; 303(3):F321–F327. doi:10.1152/ajprenal.00093.2012
- Block GA, Ix JH, Ketteler M, et al. Phosphate homeostasis in CKD: report of a scientific symposium sponsored by the National Kidney Foundation. Am J Kidney Dis 2013; 62(3):457–473. doi:10.1053/j.ajkd.2013.03.042
- Martin A, David V, Quarles LD. Regulation and function of the FGF23/klotho endocrine pathways. Physiol Rev 2012; 92(1):131–155. doi:10.1152/physrev.00002.2011
- Nissenson RA, Juppner H. Parathyroid hormone. In: Rosen CJ, ed. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 8th ed. Ames, IA: Wiley-Blackwell; 2013:208–214.
- Chauhan V, Kelepouris E, Chauhan N, Vaid M. Current concepts and management strategies in chronic kidney disease-mineral and bone disorder. South Med J 2012; 105(9):479–485. doi:10.1097/SMJ.0b013e318261f7fe
- Slatopolsky E, Robson AM, Elkan I, Bricker NS. Control of phosphate excretion in uremic man. J Clin Invest 1968; 47(8):1865–1874. doi:10.1172/JCI105877
- Ritter CS, Slatopolsky E. Phosphate toxicity in CKD: the killer among us. Clin J Am Soc Nephrol 2016; 11(6):1088–1100. doi:10.2215/CJN.11901115
- Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 2004; 15(8):2208–2218. doi:10.1097/01.ASN.0000133041.27682.A2
- Shroff RC, McNair R, Skepper JN, et al. Chronic mineral dysregulation promotes vascular smooth muscle cell adaptation and extracellular matrix calcification. J Am Soc Nephrol 2010; 21(1):103–112. doi:10.1681/ASN.2009060640
- Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis 1998; 31(4):607–617. pmid:9531176
- Bhandari SK, Liu IA, Kujubu DA, et al. Use of phosphorus binders among non-dialysis chronic kidney disease patients and mortality outcomes. Am J Nephrol 2017; 45(5):431–441. doi:10.1159/000474959
- Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis 2000; 35(6 suppl 2):S1–S140. pmid:10895784
- Streja E, Lau WL, Goldstein L, et al. Hyperphosphatemia is a combined function of high serum PTH and high dietary protein intake in dialysis patients. Kidney Int Suppl (2011) 2013; 3(5):462–468. doi:10.1038/kisup.2013.96
- Kalantar-Zadeh K, Gutekunst L, Mehrotra R, et al. Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clin J Am Soc Nephrol 2010; 5(3):519–530. doi:10.2215/CJN.06080809
- Moe SM, Zidehsarai MP, Chambers MA, et al. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol 2011; 6(2):257–264. doi:10.2215/CJN.05040610
- Cupisti A, Comar F, Benini O, et al. Effect of boiling on dietary phosphate and nitrogen intake. J Ren Nutr 2006; 16(1):36–40. doi:10.1053/j.jrn.2005.10.005
- Uribarri J, Calvo MS. Hidden sources of phosphorus in the typical American diet: does it matter in nephrology? Semin Dial 2003; 16(3):186–188. pmid:12753675
- Hou SH, Zhao J, Ellman CF, et al. Calcium and phosphorus fluxes during hemodialysis with low calcium dialysate. Am J Kidney Dis 1991; 18(2):217–224. pmid:1867178
- Daugirdas JT, Chertow GM, Larive B, et al; Frequent Hemodialysis Network (FHN) Trial Group. Effects of frequent hemodialysis on measures of CKD mineral and bone disorder. J Am Soc Nephrol 2012; 23(4):727–738. doi:10.1681/ASN.2011070688
- Daugirdas JT, Finn WF, Emmett M, Chertow GM; Frequent Hemodialysis Network Trial Group. The phosphate binder equivalent dose. Semin Dial 2011; 24(1):41–49. doi:10.1111/j.1525-139X.2011.00849.x
- Block GA, Spiegel DM, Ehrlich J, et al. Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int 2005; 68(4):1815–1824. doi:10.1111/j.1523-1755.2005.00600.x
- National Kidney Foundation. KDOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003; 42(4 suppl 3):S1–S201. pmid:14520607
- Nolan CR, Califano JR, Butzin CA. Influence of calcium acetate or calcium citrate on intestinal aluminum absorption. Kidney Int 1990; 38(5):937–941. pmid:2266679
- Schucker JJ, Ward KE. Hyperphosphatemia and phosphate binders. Am J Health Syst Pharm 2005; 62(22):2355–2361. doi:10.2146/ajhp050198
- Chertow GM, Burke SK, Raggi P; Treat to Goal Working Group. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int 2002; 62(1):245–252. doi:10.1046/j.1523-1755.2002.00434.x
- Chertow GM, Burke SK, Dillon MA, Slatopolsky E. Long-term effects of sevelamer hydrochloride on the calcium x phosphate product and lipid profile of haemodialysis patients. Nephrol Dial Transplant 1999; 14(12):2907–2914. pmid:10570096
- Braunlin W, Zhorov E, Guo A, et al. Bile acid binding to sevelamer HCl. Kidney Int 2002; 62(2):611–619. doi:10.1046/j.1523-1755.2002.00459.x
- Yamada K, Fujimoto S, Tokura T, et al. Effect of sevelamer on dyslipidemia and chronic inflammation in maintenance hemodialysis patients. Ren Fail 2005; 27(4):361–365. pmid:16060120
- Qunibi W, Moustafa M, Muenz LR, et al; CARE-2 Investigators. A 1-year randomized trial of calcium acetate versus sevelamer on progression of coronary artery calcification in hemodialysis patients with comparable lipid control: the Calcium Acetate Renagel Evaluation-2 (CARE-2) study. Am J Kidney Dis 2008; 51(6):952–965. doi:10.1053/j.ajkd.2008.02.298
- Barreto DV, Barreto Fde C, de Carvalho AB, et al. Phosphate binder impact on bone remodeling and coronary calcification—results from the BRIC study. Nephron Clin Pract 2008; 110(4):c273–c283. doi:10.1159/000170783
- Cozzolino M, Mazzaferro S, Brandenburg V. The treatment of hyperphosphataemia in CKD: calcium-based or calcium-free phosphate binders? Nephrol Dial Transplant 2011; 26(2):402–407. doi:10.1093/ndt/gfq691
- Yilmaz MI, Sonmez A, Saglam M, et al. Comparison of calcium acetate and sevelamer on vascular function and fibroblast growth factor 23 in CKD patients: a randomized clinical trial. Am J Kidney Dis 2012; 59(2):177–185. doi:10.1053/j.ajkd.2011.11.007
- Shroff RC, McNair R, Skepper JN, et al. Chronic mineral dysregulation promotes vascular smooth muscle cell adaptation and extracellular matrix calcification. J Am Soc Nephrol 2010; 21(1):103–112. doi:10.1681/ASN.2009060640
- Hutchison AJ, Wilson RJ, Garafola S, Copley JB. Lanthanum carbonate: safety data after 10 years. Nephrology (Carlton) 2016; 21(12):987–994. doi:10.1111/nep.12864
- Floege J, Covic AC, Ketteler M, et al; PA21 Study Group. A phase III study of the efficacy and safety of a novel iron-based phosphate binder in dialysis patients. Kidney Int 2014; 86(3):638–647. doi:10.1038/ki.2014.58
- Lewis JB, Sika M, Koury MJ, et al; Collaborative Study Group. Ferric citrate controls phosphorus and delivers iron in patients on dialysis. J Am Soc Nephrol 2015; 26(2):493–503. doi:10.1681/ASN.2014020212
- Liu K, Kaffes AJ. Iron deficiency anemia: a review of diagnosis, investigation and management. Eur J Gastroenterol Hepatol 2012; 24(2):109–116. doi:10.1097/MEG.0b013e32834f3140
- Shah HH, Hazzan AD, Fishbane S. Novel iron-based phosphate binders in patients with chronic kidney disease. Curr Opin Nephrol Hypertens 2015; 24(4):330–335. doi:10.1097/MNH.0000000000000128
- Eknoyan G. Salivary phosphorus binding: a novel approach to control hyperphosphatemia. J Am Soc Nephrol 2009; 20(3):460–462. doi:10.1681/ASN.2009010067
- Raggi P, Vukicevic S, Moysés RM, Wesseling K, Spiegel DM. Ten-year experience with sevelamer and calcium salts as phosphate binders. Clin J Am Soc Nephrol 2010; 5(suppl 1):S31–S40. doi:10.2215/CJN.05880809
- Airy M, Winkelmayer WC, Navaneethan SD. Phosphate binders: the evidence gap persists. Am J Kidney Dis 2016; 68(5):667–670. doi:10.1053/j.ajkd.2016.08.008
- Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med 2011; 364(19):1844–1854. doi:10.1056/NEJMra0904569
- Van den Berg H, Reintsema AM. Renal tubular damage in rasburicase: risks of alkalinisation. Ann Oncol 2004; 15(1):175–176. pmid:14679140
- Suzuki NT. Hyperphosphatemia in nondialyzed TPN patients. JPEN J Parenter Enteral Nutr 1987; 11(5):512. doi:10.1177/0148607187011005512
- Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011; 96(1):53–58. doi:10.1210/jc.2010-2704
The balance between dietary intake and excretion of phosphorus can be impaired in patients with decreased renal function, leading to hyperphosphatemia. Many patients with end-stage renal disease on dialysis require phosphorus-binding drugs to control their serum phosphorus levels.
See related editorial and article
In this review, we discuss the pathophysiology of hyperphosphatemia in kidney disease, its consequences, and how to control it, focusing on the different classes of phosphorus binders.
ROLE OF THE INTERNIST
With kidney disease common and on the increase,1 nephrologists and internists need to work together to provide optimal care.
Further, many internists in managed care plans and accountable care organizations now handle many tasks previously left to specialists—including prescribing and managing phosphorus binders in patients with kidney disease.
PATHOPHYSIOLOGY OF HYPERPHOSPHATEMIA
The pathophysiology of bone mineral disorders in kidney disease is complex. To simplify the discussion, we will address it in 3 parts:
- Phosphorus balance
- The interplay of hormones, including fibroblast growth factor 23 (FGF23)
- The mechanism of hyperphosphatemia in kidney disease.
Phosphorus balance
Phosphorus is a macronutrient essential for a range of cellular functions that include structure, energy production, metabolism, and cell signaling. It exists primarily in the form of inorganic phosphate.
An average Western diet provides 20 mg of phosphorus per kilogram of body weight per day. Of this, 13 mg/kg is absorbed, and the rest is excreted in the feces.2
Absorption of dietary phosphorus occurs mainly in the jejunum. It is mediated by both a paracellular sodium-independent pathway (driven by high intraluminal phosphorus content) and by active sodium-dependent cotransporters. It is also influenced by diet and promoted by active vitamin D (1,25 dihydroxyvitamin D3, also called calcitriol).3
Absorbed phosphorus enters the extracellular fluid and shifts in and out of the skeleton under the influence of parathyroid hormone.
Phosphorus excretion is handled almost entirely by the kidneys. Phosphorus is freely filtered at the glomerulus and reabsorbed mainly in the proximal tubule by sodium-phosphate cotransporters.
Normally, when phosphorus intake is adequate, most of the filtered phosphorus is reabsorbed and only 10% to 20% is excreted in the urine. However, the threshold for phosphorus reabsorption in the proximal tubule is influenced by parathyroid hormone, FGF23, and dietary phosphorus intake: low serum phosphate levels lead to an increase in the synthesis of sodium-phosphorus cotransporters, resulting in increased (nearly complete) proximal reabsorption and an increase in the serum phosphorus concentration.4 Conversely, both parathyroid hormone and FGF23 are phosphaturic and decrease the number of phosphorus transporters, which in turn leads to increased phosphorus excretion and a decrease in serum phosphorus concentration.5
Interplay of hormones
FGF23 is a phosphaturic glycoprotein secreted by osteoblasts and osteocytes. It acts by binding to fibroblastic growth receptor 1 in the presence of its coreceptor, the Klotho protein.6
FGF23 is regulated by serum phosphorus levels and plays a major role in the response to elevated serum phosphorus. It causes a direct increase in urinary phosphorus excretion, a decrease in intestinal phosphorus absorption (indirectly via inhibition of calcitriol), and decreased bone resorption via a decrease in parathyroid hormone production.7
Mechanism of hyperphosphatemia in kidney disease
In chronic kidney disease, phosphorus retention can trigger secondary hyperparathyroidism, as rising phosphorus levels stimulate FGF23. In the early stages of chronic kidney disease, this response can correct the phosphorus levels, but with several consequences:
- Decreased calcitriol due to its inhibition by FGF239
- Hypocalcemia due to decreased calcitriol (leading to decreased intestinal calcium absorption) and calcium binding of retained phosphorus
- Elevated parathyroid hormone due to low calcitriol levels (lack of inhibitory feedback by calcitriol), hyperphosphatemia, and hypocalcemia (direct parathyroid hormone stimulation).
As the elevated phosphorus level is likely to be the triggering event behind secondary renal hyperparathyroidism, it needs to be controlled. This is accomplished by restricting dietary phosphorus and using phosphorus binders.
HYPERPHOSPHATEMIA MAY LEAD TO VASCULAR CALCIFICATION
Elevated serum phosphorus levels (normal range 2.48–4.65 mg/dL in adults11) are associated with cardiovascular calcification and subsequent increases in mortality and morbidity rates. Elevations in serum phosphorus and calcium levels are associated with progression in vascular calcification12 and likely account for the accelerated vascular calcification that is seen in kidney disease.13
Hyperphosphatemia has been identified as an independent risk factor for death in patients with end-stage renal disease,14 but that relationship is less clear in patients with chronic kidney disease. A study in patients with chronic kidney disease and not on dialysis found a lower mortality rate in those who were prescribed phosphorus binders,15 but the study was criticized for limitations in its design.
Hyperphosphatemia can also lead to adverse effects on bone health due to complications such as renal osteodystrophy.
However, in its 2017 update, the Kidney Disease: Improving Global Outcomes (KDIGO) program only “suggests” lowering elevated phosphorus levels “toward” the normal range in patients with chronic kidney disease stages G3a through G5D, ie, those with glomerular filtration rates less than 60 mL/min/1.73 m2, including those on dialysis. The recommendation is graded 2C, ie, weak, based on low-quality evidence (https://kdigo.org/guidelines/ckd-mbd).
DIETARY RESTRICTION OF PHOSPHORUS
Diet is the major source of phosphorus intake. The average daily phosphorus consumption is 20 mg/kg, or 1,400 mg, and protein is the major source of dietary phosphorus.
In patients with stage 4 or 5 chronic kidney disease, the Kidney Disease Outcomes Quality Initiative recommends limiting protein intake to 0.6 mg/kg/day.16 However, in patients on hemodialysis, they recommend increasing protein intake to 1.1 mg/kg/day while limiting phosphorus intake to about 800 to 1,000 mg/day. This poses a challenge, as limiting phosphorus intake can reduce protein intake.
Sources of protein can be broadly classified as plant-based or animal-based. Animal protein contains organic phosphorus, which is easily absorbed.18 Plant protein may not be absorbed as easily.
Moe et al19 studied the importance of the protein source of phosphorus after 7 days of controlled diets. Despite equivalent protein and phosphorus concentrations in the vegetarian and meat-based diets, participants on the vegetarian diet had lower serum phosphorus levels, a trend toward lower 24-hour urinary phosphorus excretion, and significantly lower FGF23 levels than those on the meat-based diet. This suggests that a vegetarian diet may have advantages in terms of preventing hyperphosphatemia.
Another measure to reduce phosphorus absorption from meat is to boil it, which reduces the phosphorus content by 50%.20
Processed foods containing additives and preservatives are very high in phosphorus21 and should be avoided, particularly as there is no mandate to label phosphorus content in food.
PHOSPHORUS AND DIALYSIS
Although hemodialysis removes phosphorus, it does not remove enough to keep levels within normal limits. Indeed, even when patients adhere to a daily phosphorus limit of 1,000 mg, phosphorus accumulates. If 70% of the phosphorus in the diet is absorbed, this is 4,500 to 5,000 mg in a week. A 4-hour hemodialysis session will remove only 1,000 mg of phosphorus, which equals about 3,000 mg for patients undergoing dialysis 3 times a week,22 far less than phosphorus absorption.
In patients on continuous ambulatory peritoneal dialysis, a daily regimen of 4 exchanges of 2 L per exchange removes about 200 mg of phosphorus per day. In a 2012 study, patients on nocturnal dialysis or home dialysis involving longer session length had greater lowering of phosphorus levels than patients undergoing routine hemodialysis.23
Hence, phosphorus binders are often necessary in patients on routine hemodialysis or peritoneal dialysis.
PHOSPHORUS BINDERS
Phosphorus binders reduce serum phosphorus levels by binding with ingested phosphorus in the gastrointestinal tract and forming insoluble complexes that are not absorbed. For this reason they are much more effective when taken with meals. Phosphorus binders come in different formulations: pills, capsules, chewable tablets, liquids, and even powders that can be sprinkled on food.
The potency of each binder is quantified by its “phosphorus binder equivalent dose,” ie, its binding capacity compared with that of calcium carbonate as a reference.24
Phosphorus binders are broadly divided into those that contain calcium and those that do not.
Calcium-containing binders
The 2 most commonly used preparations are calcium carbonate (eg, Tums) and calcium acetate (eg, Phoslo). While these are relatively safe, some studies suggest that their use can lead to accelerated vascular calcification.25
According to KDIGO,26 calcium-containing binders should be avoided in hypercalcemia and adynamic bone disease. Additionally, the daily elemental calcium intake from binders should be limited to 1,500 mg, with a total daily intake that does not exceed 2,000 mg.
The elemental calcium content of calcium carbonate is about 40% of its weight (eg, 200 mg of elemental calcium in a 500-mg tablet of Tums), while the elemental calcium content of calcium acetate is about 25%. Therefore, a patient who needs 6 g of calcium carbonate for efficacy will be ingesting 2.4 g of elemental calcium per day, and that exceeds the recommended daily maximum. The main advantage of calcium carbonate is its low cost and easy availability. Commonly reported side effects include nausea and constipation.
A less commonly used calcium-based binder is calcium citrate (eg, Calcitrate). It should, however, be avoided in chronic kidney disease because of the risk of aluminum accumulation. Calcium citrate can enhance intestinal absorption of aluminum from dietary sources, as aluminum can form complexes with citrate.27
Calcium-free binders
There are several calcium-free binders. Some are based on metals such as aluminum, magnesium, iron, and lanthanum; others, such as sevelamer, are resin-based.
Aluminum- and magnesium-based binders are generally not used long-term in kidney disease because of the toxicity associated with aluminum and magnesium accumulation. However, aluminum hydroxide has an off-label use as a phosphorus binder in the acute setting, particularly when serum phosphorus levels are above 7 mg/dL.28 The dose is 300 to 600 mg 3 times daily with meals for a maximum of 4 weeks.
Sevelamer. Approved by the US Food and Drug Administration (FDA) in 1998, sevelamer acts by trapping phosphorus through ion exchange and hydrogen binding. It has the advantage of being calcium-free, which makes it particularly desirable in patients with hypercalcemia.
The Renagel in New Dialysis25 and Treat-To-Goal29 studies were randomized controlled trials that looked at the effects of sevelamer vs calcium-based binders on the risk of vascular calcification. The primary end points were serum phosphorus and calcium levels, while the secondary end points were coronary artery calcification on computed tomography and thoracic vertebral bone density. Both studies demonstrated a higher risk of vascular calcification with the calcium-based binders.
Another possible benefit of sevelamer is an improvement in lipid profile. Sevelamer lowers total cholesterol and low-density lipoprotein cholesterol levels without affecting high-density lipoprotein cholesterol or triglyceride levels.30 This is likely due to its bile acid-binding effect.31 Sevelamer has also been shown to lower C-reactive protein levels.32 While the cardiovascular profile appears to be improved with the treatment, there are no convincing data to confirm that those properties translate to a proven independent survival benefit.
The Calcium Acetate Renagel Evaluation33 was a randomized controlled study comparing sevelamer and calcium acetate. The authors attempted to control for the lipid-lowering effects of sevelamer by giving atorvastatin to all patients in both groups who had a low-density lipoprotein level greater than 70 mg/dL. The study found sevelamer to be not inferior to calcium acetate in terms of mortality and coronary calcification.
Further studies such as the Brazilian Renagel and Calcium trial34 and the Dialysis Clinical Outcomes Revisited trial failed to show a significant long-term benefit of sevelamer over calcium-based binders. However, a secondary statistical analysis of the latter study showed possible benefit of sevelamer over calcium acetate among those age 65 and older.35
To understand how sevelamer could affect vascular calcification, Yilmaz et al36 compared the effects of sevelamer vs calcium acetate on FGF23 and fetuin A levels. Fetuin A is an important inhibitor of vascular calcification and is progressively diminished in kidney disease, leading to accelerated calcification.37 Patients on sevelamer had higher levels of fetuin A than their counterparts on calcium acetate.37 The authors proposed increased fetuin A levels as a mechanism for decreased vascular calcification.
In summary, some studies suggest that sevelamer may offer the advantage of decreasing vascular calcification, but the data are mixed and do not provide a solid answer. The main disadvantages of sevelamer are a high pill burden and side effects of nausea and dyspepsia.
Lanthanum, a metallic element, was approved as a phosphorus binder by the FDA in 2008. It comes as a chewable tablet and offers the advantage of requiring the patient to take fewer pills than sevelamer and calcium-based binders.
Sucroferric oxyhydroxide comes as a chewable tablet. It was approved by the FDA in 2013. Although each tablet contains 500 mg of iron, it has not been shown to improve iron markers. In terms of phosphorus-lowering ability, it has been shown to be noninferior to sevelamer.39 Advantages include a significantly lower pill burden. Disadvantages include gastrointestinal side effects such as diarrhea and nausea and the drug’s high cost.
Ferric citrate was approved by the FDA in 2014, and 1 g delivers 210 mg of elemental iron. The main advantage of ferric citrate is its ability to increase iron markers. The phase 3 trial that demonstrated its efficacy as a binder showed an increase in ferritin compared with the active control.40 The study also showed a decrease in the need to use intravenous iron and erythropoesis-stimulating agents. This was thought to be due to improved iron stores, leading to decreased erythropoietin resistance.41
The mean number of ferric citrate tablets needed to achieve the desired phosphorus-lowering effect was 8 per day, containing 1,680 mg of iron. In comparison, oral ferrous sulfate typically provides 210 mg of iron per day.42
Disadvantages of ferric citrate include high pill burden, high cost, and gastrointestinal side effects such as nausea and constipation.
Chitosan binds salivary phosphorus. It can potentially be used, but it is not approved, and its efficacy in lowering serum phosphorus remains unclear.43
CHOOSING THE APPROPRIATE PHOSPHORUS BINDER
The choice of phosphorus binder is based on the patient’s serum calcium level and iron stores and on the drug’s side effect profile, iron pill burden, and cost. Involving patients in the choice after discussing potential side effects, pill burden, and cost is important for shared decision-making and could play a role in improving adherence.
Phosphorus binders are a major portion of the pill burden in patients with end-stage renal disease, possibly affecting patient adherence. The cost of phosphorus binders is estimated at half a billion dollars annually, underlining the significant economic impact of phosphorus control.11
Calcium-based binders should be the first choice when there is secondary hyperparathyroidism without hypercalcemia. There is no clear evidence regarding the benefit of correcting hypocalcemia, but KDIGO recommends keeping the serum calcium level within the reference range. KDIGO also recommends restricting calcium-based binders in persistent hypercalcemia, arterial calcification, and adynamic bone disease. This recommendation is largely based on expert opinion.
Noncalcium-based binders, which in theory might prevent vascular calcification, should be considered for patients with at least 1 of the following44:
- Complicated diabetes mellitus
- Vascular or valvular calcification
- Persistent inflammation.
Noncalcium-based binders are also preferred in low bone-turnover states such as adynamic bone disease, as elevated calcium can inhibit parathyroid hormone.
However, the advantage of noncalcium-based binders regarding vascular calcification is largely theoretical and has not been proven clinically. Indeed, there are data comparing long-term outcomes of the different classes of phosphorus binders, but studies were limited by short follow-up, and individual studies have lacked power to detect statistical significance between two classes of binders on long-term outcomes. Meta-analyses have provided conflicting data, with some suggesting better outcomes with sevelamer than with calcium-based binders, and with others failing to show any difference.45
Because iron deficiency is common in kidney disease, ferric citrate, which can improve iron markers, may be a suitable option, provided its cost is covered by insurance.
SPECIAL CIRCUMSTANCES FOR THE USE OF PHOSPHORUS BINDERS
Tumor lysis syndrome
Tumor lysis syndrome occurs when tumor cells release their contents into the bloodstream, either spontaneously or in response to therapy, leading to the characteristic findings of hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia.46 Phosphorus binders in conjunction with intravenous hydration are used to treat hyperphosphatemia, but evidence about their efficacy in this setting is limited.
Hypocalcemia in tumor lysis syndrome is usually not treated unless symptomatic, as the calcium-phosphorus product can increase, leading to calcium phosphate crystallization. When the calcium-phosphorus product is greater than 60, there is a higher risk of calcium phosphate deposition in the renal tubules that can lead to acute renal failure in tumor lysis syndrome.47 To lower the risk of calcium phosphate crystallization, calcium-based binders should be avoided in tumor lysis syndrome.
Total parenteral nutrition
Since patients on total parenteral nutrition do not eat, phosphorus binders are considered ineffective; there are no concrete data showing that phosphorus binders are effective in these patients.48 In patients with kidney disease, the phosphorus content in the parenteral nutrition formulation must be reduced.
Pregnancy
Data on phosphorus binders in pregnancy are limited. Calcium can cross the placenta. Calcium carbonate can be used in pregnancy, and fetal harm is not expected if calcium concentrations are within normal limits.49 Calcium acetate, sevelamer, and lanthanum are considered pregnancy category C drugs. Patients with advanced chronic kidney disease and end-stage renal disease who become pregnant must receive specialized obstetric care for high-risk pregnancy.
FUTURE DIRECTIONS
Future therapies may target FGF23 and other inflammatory markers that are up-regulated in renal hyperparathyroidism. However, trials studying these markers are needed to provide a better understanding of their role in bone mineral and cardiovascular health and in overall long-term outcomes. Additionally, randomized controlled trials are needed to study long-term nonsurrogate outcomes such as reduction in cardiovascular disease and rates of overall mortality.
The balance between dietary intake and excretion of phosphorus can be impaired in patients with decreased renal function, leading to hyperphosphatemia. Many patients with end-stage renal disease on dialysis require phosphorus-binding drugs to control their serum phosphorus levels.
See related editorial and article
In this review, we discuss the pathophysiology of hyperphosphatemia in kidney disease, its consequences, and how to control it, focusing on the different classes of phosphorus binders.
ROLE OF THE INTERNIST
With kidney disease common and on the increase,1 nephrologists and internists need to work together to provide optimal care.
Further, many internists in managed care plans and accountable care organizations now handle many tasks previously left to specialists—including prescribing and managing phosphorus binders in patients with kidney disease.
PATHOPHYSIOLOGY OF HYPERPHOSPHATEMIA
The pathophysiology of bone mineral disorders in kidney disease is complex. To simplify the discussion, we will address it in 3 parts:
- Phosphorus balance
- The interplay of hormones, including fibroblast growth factor 23 (FGF23)
- The mechanism of hyperphosphatemia in kidney disease.
Phosphorus balance
Phosphorus is a macronutrient essential for a range of cellular functions that include structure, energy production, metabolism, and cell signaling. It exists primarily in the form of inorganic phosphate.
An average Western diet provides 20 mg of phosphorus per kilogram of body weight per day. Of this, 13 mg/kg is absorbed, and the rest is excreted in the feces.2
Absorption of dietary phosphorus occurs mainly in the jejunum. It is mediated by both a paracellular sodium-independent pathway (driven by high intraluminal phosphorus content) and by active sodium-dependent cotransporters. It is also influenced by diet and promoted by active vitamin D (1,25 dihydroxyvitamin D3, also called calcitriol).3
Absorbed phosphorus enters the extracellular fluid and shifts in and out of the skeleton under the influence of parathyroid hormone.
Phosphorus excretion is handled almost entirely by the kidneys. Phosphorus is freely filtered at the glomerulus and reabsorbed mainly in the proximal tubule by sodium-phosphate cotransporters.
Normally, when phosphorus intake is adequate, most of the filtered phosphorus is reabsorbed and only 10% to 20% is excreted in the urine. However, the threshold for phosphorus reabsorption in the proximal tubule is influenced by parathyroid hormone, FGF23, and dietary phosphorus intake: low serum phosphate levels lead to an increase in the synthesis of sodium-phosphorus cotransporters, resulting in increased (nearly complete) proximal reabsorption and an increase in the serum phosphorus concentration.4 Conversely, both parathyroid hormone and FGF23 are phosphaturic and decrease the number of phosphorus transporters, which in turn leads to increased phosphorus excretion and a decrease in serum phosphorus concentration.5
Interplay of hormones
FGF23 is a phosphaturic glycoprotein secreted by osteoblasts and osteocytes. It acts by binding to fibroblastic growth receptor 1 in the presence of its coreceptor, the Klotho protein.6
FGF23 is regulated by serum phosphorus levels and plays a major role in the response to elevated serum phosphorus. It causes a direct increase in urinary phosphorus excretion, a decrease in intestinal phosphorus absorption (indirectly via inhibition of calcitriol), and decreased bone resorption via a decrease in parathyroid hormone production.7
Mechanism of hyperphosphatemia in kidney disease
In chronic kidney disease, phosphorus retention can trigger secondary hyperparathyroidism, as rising phosphorus levels stimulate FGF23. In the early stages of chronic kidney disease, this response can correct the phosphorus levels, but with several consequences:
- Decreased calcitriol due to its inhibition by FGF239
- Hypocalcemia due to decreased calcitriol (leading to decreased intestinal calcium absorption) and calcium binding of retained phosphorus
- Elevated parathyroid hormone due to low calcitriol levels (lack of inhibitory feedback by calcitriol), hyperphosphatemia, and hypocalcemia (direct parathyroid hormone stimulation).
As the elevated phosphorus level is likely to be the triggering event behind secondary renal hyperparathyroidism, it needs to be controlled. This is accomplished by restricting dietary phosphorus and using phosphorus binders.
HYPERPHOSPHATEMIA MAY LEAD TO VASCULAR CALCIFICATION
Elevated serum phosphorus levels (normal range 2.48–4.65 mg/dL in adults11) are associated with cardiovascular calcification and subsequent increases in mortality and morbidity rates. Elevations in serum phosphorus and calcium levels are associated with progression in vascular calcification12 and likely account for the accelerated vascular calcification that is seen in kidney disease.13
Hyperphosphatemia has been identified as an independent risk factor for death in patients with end-stage renal disease,14 but that relationship is less clear in patients with chronic kidney disease. A study in patients with chronic kidney disease and not on dialysis found a lower mortality rate in those who were prescribed phosphorus binders,15 but the study was criticized for limitations in its design.
Hyperphosphatemia can also lead to adverse effects on bone health due to complications such as renal osteodystrophy.
However, in its 2017 update, the Kidney Disease: Improving Global Outcomes (KDIGO) program only “suggests” lowering elevated phosphorus levels “toward” the normal range in patients with chronic kidney disease stages G3a through G5D, ie, those with glomerular filtration rates less than 60 mL/min/1.73 m2, including those on dialysis. The recommendation is graded 2C, ie, weak, based on low-quality evidence (https://kdigo.org/guidelines/ckd-mbd).
DIETARY RESTRICTION OF PHOSPHORUS
Diet is the major source of phosphorus intake. The average daily phosphorus consumption is 20 mg/kg, or 1,400 mg, and protein is the major source of dietary phosphorus.
In patients with stage 4 or 5 chronic kidney disease, the Kidney Disease Outcomes Quality Initiative recommends limiting protein intake to 0.6 mg/kg/day.16 However, in patients on hemodialysis, they recommend increasing protein intake to 1.1 mg/kg/day while limiting phosphorus intake to about 800 to 1,000 mg/day. This poses a challenge, as limiting phosphorus intake can reduce protein intake.
Sources of protein can be broadly classified as plant-based or animal-based. Animal protein contains organic phosphorus, which is easily absorbed.18 Plant protein may not be absorbed as easily.
Moe et al19 studied the importance of the protein source of phosphorus after 7 days of controlled diets. Despite equivalent protein and phosphorus concentrations in the vegetarian and meat-based diets, participants on the vegetarian diet had lower serum phosphorus levels, a trend toward lower 24-hour urinary phosphorus excretion, and significantly lower FGF23 levels than those on the meat-based diet. This suggests that a vegetarian diet may have advantages in terms of preventing hyperphosphatemia.
Another measure to reduce phosphorus absorption from meat is to boil it, which reduces the phosphorus content by 50%.20
Processed foods containing additives and preservatives are very high in phosphorus21 and should be avoided, particularly as there is no mandate to label phosphorus content in food.
PHOSPHORUS AND DIALYSIS
Although hemodialysis removes phosphorus, it does not remove enough to keep levels within normal limits. Indeed, even when patients adhere to a daily phosphorus limit of 1,000 mg, phosphorus accumulates. If 70% of the phosphorus in the diet is absorbed, this is 4,500 to 5,000 mg in a week. A 4-hour hemodialysis session will remove only 1,000 mg of phosphorus, which equals about 3,000 mg for patients undergoing dialysis 3 times a week,22 far less than phosphorus absorption.
In patients on continuous ambulatory peritoneal dialysis, a daily regimen of 4 exchanges of 2 L per exchange removes about 200 mg of phosphorus per day. In a 2012 study, patients on nocturnal dialysis or home dialysis involving longer session length had greater lowering of phosphorus levels than patients undergoing routine hemodialysis.23
Hence, phosphorus binders are often necessary in patients on routine hemodialysis or peritoneal dialysis.
PHOSPHORUS BINDERS
Phosphorus binders reduce serum phosphorus levels by binding with ingested phosphorus in the gastrointestinal tract and forming insoluble complexes that are not absorbed. For this reason they are much more effective when taken with meals. Phosphorus binders come in different formulations: pills, capsules, chewable tablets, liquids, and even powders that can be sprinkled on food.
The potency of each binder is quantified by its “phosphorus binder equivalent dose,” ie, its binding capacity compared with that of calcium carbonate as a reference.24
Phosphorus binders are broadly divided into those that contain calcium and those that do not.
Calcium-containing binders
The 2 most commonly used preparations are calcium carbonate (eg, Tums) and calcium acetate (eg, Phoslo). While these are relatively safe, some studies suggest that their use can lead to accelerated vascular calcification.25
According to KDIGO,26 calcium-containing binders should be avoided in hypercalcemia and adynamic bone disease. Additionally, the daily elemental calcium intake from binders should be limited to 1,500 mg, with a total daily intake that does not exceed 2,000 mg.
The elemental calcium content of calcium carbonate is about 40% of its weight (eg, 200 mg of elemental calcium in a 500-mg tablet of Tums), while the elemental calcium content of calcium acetate is about 25%. Therefore, a patient who needs 6 g of calcium carbonate for efficacy will be ingesting 2.4 g of elemental calcium per day, and that exceeds the recommended daily maximum. The main advantage of calcium carbonate is its low cost and easy availability. Commonly reported side effects include nausea and constipation.
A less commonly used calcium-based binder is calcium citrate (eg, Calcitrate). It should, however, be avoided in chronic kidney disease because of the risk of aluminum accumulation. Calcium citrate can enhance intestinal absorption of aluminum from dietary sources, as aluminum can form complexes with citrate.27
Calcium-free binders
There are several calcium-free binders. Some are based on metals such as aluminum, magnesium, iron, and lanthanum; others, such as sevelamer, are resin-based.
Aluminum- and magnesium-based binders are generally not used long-term in kidney disease because of the toxicity associated with aluminum and magnesium accumulation. However, aluminum hydroxide has an off-label use as a phosphorus binder in the acute setting, particularly when serum phosphorus levels are above 7 mg/dL.28 The dose is 300 to 600 mg 3 times daily with meals for a maximum of 4 weeks.
Sevelamer. Approved by the US Food and Drug Administration (FDA) in 1998, sevelamer acts by trapping phosphorus through ion exchange and hydrogen binding. It has the advantage of being calcium-free, which makes it particularly desirable in patients with hypercalcemia.
The Renagel in New Dialysis25 and Treat-To-Goal29 studies were randomized controlled trials that looked at the effects of sevelamer vs calcium-based binders on the risk of vascular calcification. The primary end points were serum phosphorus and calcium levels, while the secondary end points were coronary artery calcification on computed tomography and thoracic vertebral bone density. Both studies demonstrated a higher risk of vascular calcification with the calcium-based binders.
Another possible benefit of sevelamer is an improvement in lipid profile. Sevelamer lowers total cholesterol and low-density lipoprotein cholesterol levels without affecting high-density lipoprotein cholesterol or triglyceride levels.30 This is likely due to its bile acid-binding effect.31 Sevelamer has also been shown to lower C-reactive protein levels.32 While the cardiovascular profile appears to be improved with the treatment, there are no convincing data to confirm that those properties translate to a proven independent survival benefit.
The Calcium Acetate Renagel Evaluation33 was a randomized controlled study comparing sevelamer and calcium acetate. The authors attempted to control for the lipid-lowering effects of sevelamer by giving atorvastatin to all patients in both groups who had a low-density lipoprotein level greater than 70 mg/dL. The study found sevelamer to be not inferior to calcium acetate in terms of mortality and coronary calcification.
Further studies such as the Brazilian Renagel and Calcium trial34 and the Dialysis Clinical Outcomes Revisited trial failed to show a significant long-term benefit of sevelamer over calcium-based binders. However, a secondary statistical analysis of the latter study showed possible benefit of sevelamer over calcium acetate among those age 65 and older.35
To understand how sevelamer could affect vascular calcification, Yilmaz et al36 compared the effects of sevelamer vs calcium acetate on FGF23 and fetuin A levels. Fetuin A is an important inhibitor of vascular calcification and is progressively diminished in kidney disease, leading to accelerated calcification.37 Patients on sevelamer had higher levels of fetuin A than their counterparts on calcium acetate.37 The authors proposed increased fetuin A levels as a mechanism for decreased vascular calcification.
In summary, some studies suggest that sevelamer may offer the advantage of decreasing vascular calcification, but the data are mixed and do not provide a solid answer. The main disadvantages of sevelamer are a high pill burden and side effects of nausea and dyspepsia.
Lanthanum, a metallic element, was approved as a phosphorus binder by the FDA in 2008. It comes as a chewable tablet and offers the advantage of requiring the patient to take fewer pills than sevelamer and calcium-based binders.
Sucroferric oxyhydroxide comes as a chewable tablet. It was approved by the FDA in 2013. Although each tablet contains 500 mg of iron, it has not been shown to improve iron markers. In terms of phosphorus-lowering ability, it has been shown to be noninferior to sevelamer.39 Advantages include a significantly lower pill burden. Disadvantages include gastrointestinal side effects such as diarrhea and nausea and the drug’s high cost.
Ferric citrate was approved by the FDA in 2014, and 1 g delivers 210 mg of elemental iron. The main advantage of ferric citrate is its ability to increase iron markers. The phase 3 trial that demonstrated its efficacy as a binder showed an increase in ferritin compared with the active control.40 The study also showed a decrease in the need to use intravenous iron and erythropoesis-stimulating agents. This was thought to be due to improved iron stores, leading to decreased erythropoietin resistance.41
The mean number of ferric citrate tablets needed to achieve the desired phosphorus-lowering effect was 8 per day, containing 1,680 mg of iron. In comparison, oral ferrous sulfate typically provides 210 mg of iron per day.42
Disadvantages of ferric citrate include high pill burden, high cost, and gastrointestinal side effects such as nausea and constipation.
Chitosan binds salivary phosphorus. It can potentially be used, but it is not approved, and its efficacy in lowering serum phosphorus remains unclear.43
CHOOSING THE APPROPRIATE PHOSPHORUS BINDER
The choice of phosphorus binder is based on the patient’s serum calcium level and iron stores and on the drug’s side effect profile, iron pill burden, and cost. Involving patients in the choice after discussing potential side effects, pill burden, and cost is important for shared decision-making and could play a role in improving adherence.
Phosphorus binders are a major portion of the pill burden in patients with end-stage renal disease, possibly affecting patient adherence. The cost of phosphorus binders is estimated at half a billion dollars annually, underlining the significant economic impact of phosphorus control.11
Calcium-based binders should be the first choice when there is secondary hyperparathyroidism without hypercalcemia. There is no clear evidence regarding the benefit of correcting hypocalcemia, but KDIGO recommends keeping the serum calcium level within the reference range. KDIGO also recommends restricting calcium-based binders in persistent hypercalcemia, arterial calcification, and adynamic bone disease. This recommendation is largely based on expert opinion.
Noncalcium-based binders, which in theory might prevent vascular calcification, should be considered for patients with at least 1 of the following44:
- Complicated diabetes mellitus
- Vascular or valvular calcification
- Persistent inflammation.
Noncalcium-based binders are also preferred in low bone-turnover states such as adynamic bone disease, as elevated calcium can inhibit parathyroid hormone.
However, the advantage of noncalcium-based binders regarding vascular calcification is largely theoretical and has not been proven clinically. Indeed, there are data comparing long-term outcomes of the different classes of phosphorus binders, but studies were limited by short follow-up, and individual studies have lacked power to detect statistical significance between two classes of binders on long-term outcomes. Meta-analyses have provided conflicting data, with some suggesting better outcomes with sevelamer than with calcium-based binders, and with others failing to show any difference.45
Because iron deficiency is common in kidney disease, ferric citrate, which can improve iron markers, may be a suitable option, provided its cost is covered by insurance.
SPECIAL CIRCUMSTANCES FOR THE USE OF PHOSPHORUS BINDERS
Tumor lysis syndrome
Tumor lysis syndrome occurs when tumor cells release their contents into the bloodstream, either spontaneously or in response to therapy, leading to the characteristic findings of hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia.46 Phosphorus binders in conjunction with intravenous hydration are used to treat hyperphosphatemia, but evidence about their efficacy in this setting is limited.
Hypocalcemia in tumor lysis syndrome is usually not treated unless symptomatic, as the calcium-phosphorus product can increase, leading to calcium phosphate crystallization. When the calcium-phosphorus product is greater than 60, there is a higher risk of calcium phosphate deposition in the renal tubules that can lead to acute renal failure in tumor lysis syndrome.47 To lower the risk of calcium phosphate crystallization, calcium-based binders should be avoided in tumor lysis syndrome.
Total parenteral nutrition
Since patients on total parenteral nutrition do not eat, phosphorus binders are considered ineffective; there are no concrete data showing that phosphorus binders are effective in these patients.48 In patients with kidney disease, the phosphorus content in the parenteral nutrition formulation must be reduced.
Pregnancy
Data on phosphorus binders in pregnancy are limited. Calcium can cross the placenta. Calcium carbonate can be used in pregnancy, and fetal harm is not expected if calcium concentrations are within normal limits.49 Calcium acetate, sevelamer, and lanthanum are considered pregnancy category C drugs. Patients with advanced chronic kidney disease and end-stage renal disease who become pregnant must receive specialized obstetric care for high-risk pregnancy.
FUTURE DIRECTIONS
Future therapies may target FGF23 and other inflammatory markers that are up-regulated in renal hyperparathyroidism. However, trials studying these markers are needed to provide a better understanding of their role in bone mineral and cardiovascular health and in overall long-term outcomes. Additionally, randomized controlled trials are needed to study long-term nonsurrogate outcomes such as reduction in cardiovascular disease and rates of overall mortality.
- Collins AJ, Foley RN, Herzog C, et al. US renal data system 2012 annual data report. Am J Kidney Dis 2013; 61(1 suppl 1):A7,e1–476. doi:10.1053/j.ajkd.2012.11.031
- Tenenhouse HS. Regulation of phosphorus homeostasis by the type iia Na/phosphate cotransporter. Annu Rev Nutr 2005; 25:197–214. doi:10.1146/annurev.nutr.25.050304.092642
- Lederer E. Regulation of serum phosphate. J Physiol 2014; 592(18):3985–3995. doi:10.1113/jphysiol.2014.273979
- Lederer E. Renal phosphate transporters. Curr Opin Nephrol Hypertens 2014; 23(5):502–506. doi:10.1097/MNH.0000000000000053
- Weinman EJ, Lederer ED. NHERF-1 and the regulation of renal phosphate reabsoption: a tale of three hormones. Am J Physiol Renal Physiol 2012; 303(3):F321–F327. doi:10.1152/ajprenal.00093.2012
- Block GA, Ix JH, Ketteler M, et al. Phosphate homeostasis in CKD: report of a scientific symposium sponsored by the National Kidney Foundation. Am J Kidney Dis 2013; 62(3):457–473. doi:10.1053/j.ajkd.2013.03.042
- Martin A, David V, Quarles LD. Regulation and function of the FGF23/klotho endocrine pathways. Physiol Rev 2012; 92(1):131–155. doi:10.1152/physrev.00002.2011
- Nissenson RA, Juppner H. Parathyroid hormone. In: Rosen CJ, ed. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 8th ed. Ames, IA: Wiley-Blackwell; 2013:208–214.
- Chauhan V, Kelepouris E, Chauhan N, Vaid M. Current concepts and management strategies in chronic kidney disease-mineral and bone disorder. South Med J 2012; 105(9):479–485. doi:10.1097/SMJ.0b013e318261f7fe
- Slatopolsky E, Robson AM, Elkan I, Bricker NS. Control of phosphate excretion in uremic man. J Clin Invest 1968; 47(8):1865–1874. doi:10.1172/JCI105877
- Ritter CS, Slatopolsky E. Phosphate toxicity in CKD: the killer among us. Clin J Am Soc Nephrol 2016; 11(6):1088–1100. doi:10.2215/CJN.11901115
- Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 2004; 15(8):2208–2218. doi:10.1097/01.ASN.0000133041.27682.A2
- Shroff RC, McNair R, Skepper JN, et al. Chronic mineral dysregulation promotes vascular smooth muscle cell adaptation and extracellular matrix calcification. J Am Soc Nephrol 2010; 21(1):103–112. doi:10.1681/ASN.2009060640
- Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis 1998; 31(4):607–617. pmid:9531176
- Bhandari SK, Liu IA, Kujubu DA, et al. Use of phosphorus binders among non-dialysis chronic kidney disease patients and mortality outcomes. Am J Nephrol 2017; 45(5):431–441. doi:10.1159/000474959
- Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis 2000; 35(6 suppl 2):S1–S140. pmid:10895784
- Streja E, Lau WL, Goldstein L, et al. Hyperphosphatemia is a combined function of high serum PTH and high dietary protein intake in dialysis patients. Kidney Int Suppl (2011) 2013; 3(5):462–468. doi:10.1038/kisup.2013.96
- Kalantar-Zadeh K, Gutekunst L, Mehrotra R, et al. Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clin J Am Soc Nephrol 2010; 5(3):519–530. doi:10.2215/CJN.06080809
- Moe SM, Zidehsarai MP, Chambers MA, et al. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol 2011; 6(2):257–264. doi:10.2215/CJN.05040610
- Cupisti A, Comar F, Benini O, et al. Effect of boiling on dietary phosphate and nitrogen intake. J Ren Nutr 2006; 16(1):36–40. doi:10.1053/j.jrn.2005.10.005
- Uribarri J, Calvo MS. Hidden sources of phosphorus in the typical American diet: does it matter in nephrology? Semin Dial 2003; 16(3):186–188. pmid:12753675
- Hou SH, Zhao J, Ellman CF, et al. Calcium and phosphorus fluxes during hemodialysis with low calcium dialysate. Am J Kidney Dis 1991; 18(2):217–224. pmid:1867178
- Daugirdas JT, Chertow GM, Larive B, et al; Frequent Hemodialysis Network (FHN) Trial Group. Effects of frequent hemodialysis on measures of CKD mineral and bone disorder. J Am Soc Nephrol 2012; 23(4):727–738. doi:10.1681/ASN.2011070688
- Daugirdas JT, Finn WF, Emmett M, Chertow GM; Frequent Hemodialysis Network Trial Group. The phosphate binder equivalent dose. Semin Dial 2011; 24(1):41–49. doi:10.1111/j.1525-139X.2011.00849.x
- Block GA, Spiegel DM, Ehrlich J, et al. Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int 2005; 68(4):1815–1824. doi:10.1111/j.1523-1755.2005.00600.x
- National Kidney Foundation. KDOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003; 42(4 suppl 3):S1–S201. pmid:14520607
- Nolan CR, Califano JR, Butzin CA. Influence of calcium acetate or calcium citrate on intestinal aluminum absorption. Kidney Int 1990; 38(5):937–941. pmid:2266679
- Schucker JJ, Ward KE. Hyperphosphatemia and phosphate binders. Am J Health Syst Pharm 2005; 62(22):2355–2361. doi:10.2146/ajhp050198
- Chertow GM, Burke SK, Raggi P; Treat to Goal Working Group. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int 2002; 62(1):245–252. doi:10.1046/j.1523-1755.2002.00434.x
- Chertow GM, Burke SK, Dillon MA, Slatopolsky E. Long-term effects of sevelamer hydrochloride on the calcium x phosphate product and lipid profile of haemodialysis patients. Nephrol Dial Transplant 1999; 14(12):2907–2914. pmid:10570096
- Braunlin W, Zhorov E, Guo A, et al. Bile acid binding to sevelamer HCl. Kidney Int 2002; 62(2):611–619. doi:10.1046/j.1523-1755.2002.00459.x
- Yamada K, Fujimoto S, Tokura T, et al. Effect of sevelamer on dyslipidemia and chronic inflammation in maintenance hemodialysis patients. Ren Fail 2005; 27(4):361–365. pmid:16060120
- Qunibi W, Moustafa M, Muenz LR, et al; CARE-2 Investigators. A 1-year randomized trial of calcium acetate versus sevelamer on progression of coronary artery calcification in hemodialysis patients with comparable lipid control: the Calcium Acetate Renagel Evaluation-2 (CARE-2) study. Am J Kidney Dis 2008; 51(6):952–965. doi:10.1053/j.ajkd.2008.02.298
- Barreto DV, Barreto Fde C, de Carvalho AB, et al. Phosphate binder impact on bone remodeling and coronary calcification—results from the BRIC study. Nephron Clin Pract 2008; 110(4):c273–c283. doi:10.1159/000170783
- Cozzolino M, Mazzaferro S, Brandenburg V. The treatment of hyperphosphataemia in CKD: calcium-based or calcium-free phosphate binders? Nephrol Dial Transplant 2011; 26(2):402–407. doi:10.1093/ndt/gfq691
- Yilmaz MI, Sonmez A, Saglam M, et al. Comparison of calcium acetate and sevelamer on vascular function and fibroblast growth factor 23 in CKD patients: a randomized clinical trial. Am J Kidney Dis 2012; 59(2):177–185. doi:10.1053/j.ajkd.2011.11.007
- Shroff RC, McNair R, Skepper JN, et al. Chronic mineral dysregulation promotes vascular smooth muscle cell adaptation and extracellular matrix calcification. J Am Soc Nephrol 2010; 21(1):103–112. doi:10.1681/ASN.2009060640
- Hutchison AJ, Wilson RJ, Garafola S, Copley JB. Lanthanum carbonate: safety data after 10 years. Nephrology (Carlton) 2016; 21(12):987–994. doi:10.1111/nep.12864
- Floege J, Covic AC, Ketteler M, et al; PA21 Study Group. A phase III study of the efficacy and safety of a novel iron-based phosphate binder in dialysis patients. Kidney Int 2014; 86(3):638–647. doi:10.1038/ki.2014.58
- Lewis JB, Sika M, Koury MJ, et al; Collaborative Study Group. Ferric citrate controls phosphorus and delivers iron in patients on dialysis. J Am Soc Nephrol 2015; 26(2):493–503. doi:10.1681/ASN.2014020212
- Liu K, Kaffes AJ. Iron deficiency anemia: a review of diagnosis, investigation and management. Eur J Gastroenterol Hepatol 2012; 24(2):109–116. doi:10.1097/MEG.0b013e32834f3140
- Shah HH, Hazzan AD, Fishbane S. Novel iron-based phosphate binders in patients with chronic kidney disease. Curr Opin Nephrol Hypertens 2015; 24(4):330–335. doi:10.1097/MNH.0000000000000128
- Eknoyan G. Salivary phosphorus binding: a novel approach to control hyperphosphatemia. J Am Soc Nephrol 2009; 20(3):460–462. doi:10.1681/ASN.2009010067
- Raggi P, Vukicevic S, Moysés RM, Wesseling K, Spiegel DM. Ten-year experience with sevelamer and calcium salts as phosphate binders. Clin J Am Soc Nephrol 2010; 5(suppl 1):S31–S40. doi:10.2215/CJN.05880809
- Airy M, Winkelmayer WC, Navaneethan SD. Phosphate binders: the evidence gap persists. Am J Kidney Dis 2016; 68(5):667–670. doi:10.1053/j.ajkd.2016.08.008
- Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med 2011; 364(19):1844–1854. doi:10.1056/NEJMra0904569
- Van den Berg H, Reintsema AM. Renal tubular damage in rasburicase: risks of alkalinisation. Ann Oncol 2004; 15(1):175–176. pmid:14679140
- Suzuki NT. Hyperphosphatemia in nondialyzed TPN patients. JPEN J Parenter Enteral Nutr 1987; 11(5):512. doi:10.1177/0148607187011005512
- Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011; 96(1):53–58. doi:10.1210/jc.2010-2704
- Collins AJ, Foley RN, Herzog C, et al. US renal data system 2012 annual data report. Am J Kidney Dis 2013; 61(1 suppl 1):A7,e1–476. doi:10.1053/j.ajkd.2012.11.031
- Tenenhouse HS. Regulation of phosphorus homeostasis by the type iia Na/phosphate cotransporter. Annu Rev Nutr 2005; 25:197–214. doi:10.1146/annurev.nutr.25.050304.092642
- Lederer E. Regulation of serum phosphate. J Physiol 2014; 592(18):3985–3995. doi:10.1113/jphysiol.2014.273979
- Lederer E. Renal phosphate transporters. Curr Opin Nephrol Hypertens 2014; 23(5):502–506. doi:10.1097/MNH.0000000000000053
- Weinman EJ, Lederer ED. NHERF-1 and the regulation of renal phosphate reabsoption: a tale of three hormones. Am J Physiol Renal Physiol 2012; 303(3):F321–F327. doi:10.1152/ajprenal.00093.2012
- Block GA, Ix JH, Ketteler M, et al. Phosphate homeostasis in CKD: report of a scientific symposium sponsored by the National Kidney Foundation. Am J Kidney Dis 2013; 62(3):457–473. doi:10.1053/j.ajkd.2013.03.042
- Martin A, David V, Quarles LD. Regulation and function of the FGF23/klotho endocrine pathways. Physiol Rev 2012; 92(1):131–155. doi:10.1152/physrev.00002.2011
- Nissenson RA, Juppner H. Parathyroid hormone. In: Rosen CJ, ed. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 8th ed. Ames, IA: Wiley-Blackwell; 2013:208–214.
- Chauhan V, Kelepouris E, Chauhan N, Vaid M. Current concepts and management strategies in chronic kidney disease-mineral and bone disorder. South Med J 2012; 105(9):479–485. doi:10.1097/SMJ.0b013e318261f7fe
- Slatopolsky E, Robson AM, Elkan I, Bricker NS. Control of phosphate excretion in uremic man. J Clin Invest 1968; 47(8):1865–1874. doi:10.1172/JCI105877
- Ritter CS, Slatopolsky E. Phosphate toxicity in CKD: the killer among us. Clin J Am Soc Nephrol 2016; 11(6):1088–1100. doi:10.2215/CJN.11901115
- Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 2004; 15(8):2208–2218. doi:10.1097/01.ASN.0000133041.27682.A2
- Shroff RC, McNair R, Skepper JN, et al. Chronic mineral dysregulation promotes vascular smooth muscle cell adaptation and extracellular matrix calcification. J Am Soc Nephrol 2010; 21(1):103–112. doi:10.1681/ASN.2009060640
- Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis 1998; 31(4):607–617. pmid:9531176
- Bhandari SK, Liu IA, Kujubu DA, et al. Use of phosphorus binders among non-dialysis chronic kidney disease patients and mortality outcomes. Am J Nephrol 2017; 45(5):431–441. doi:10.1159/000474959
- Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis 2000; 35(6 suppl 2):S1–S140. pmid:10895784
- Streja E, Lau WL, Goldstein L, et al. Hyperphosphatemia is a combined function of high serum PTH and high dietary protein intake in dialysis patients. Kidney Int Suppl (2011) 2013; 3(5):462–468. doi:10.1038/kisup.2013.96
- Kalantar-Zadeh K, Gutekunst L, Mehrotra R, et al. Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clin J Am Soc Nephrol 2010; 5(3):519–530. doi:10.2215/CJN.06080809
- Moe SM, Zidehsarai MP, Chambers MA, et al. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol 2011; 6(2):257–264. doi:10.2215/CJN.05040610
- Cupisti A, Comar F, Benini O, et al. Effect of boiling on dietary phosphate and nitrogen intake. J Ren Nutr 2006; 16(1):36–40. doi:10.1053/j.jrn.2005.10.005
- Uribarri J, Calvo MS. Hidden sources of phosphorus in the typical American diet: does it matter in nephrology? Semin Dial 2003; 16(3):186–188. pmid:12753675
- Hou SH, Zhao J, Ellman CF, et al. Calcium and phosphorus fluxes during hemodialysis with low calcium dialysate. Am J Kidney Dis 1991; 18(2):217–224. pmid:1867178
- Daugirdas JT, Chertow GM, Larive B, et al; Frequent Hemodialysis Network (FHN) Trial Group. Effects of frequent hemodialysis on measures of CKD mineral and bone disorder. J Am Soc Nephrol 2012; 23(4):727–738. doi:10.1681/ASN.2011070688
- Daugirdas JT, Finn WF, Emmett M, Chertow GM; Frequent Hemodialysis Network Trial Group. The phosphate binder equivalent dose. Semin Dial 2011; 24(1):41–49. doi:10.1111/j.1525-139X.2011.00849.x
- Block GA, Spiegel DM, Ehrlich J, et al. Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int 2005; 68(4):1815–1824. doi:10.1111/j.1523-1755.2005.00600.x
- National Kidney Foundation. KDOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003; 42(4 suppl 3):S1–S201. pmid:14520607
- Nolan CR, Califano JR, Butzin CA. Influence of calcium acetate or calcium citrate on intestinal aluminum absorption. Kidney Int 1990; 38(5):937–941. pmid:2266679
- Schucker JJ, Ward KE. Hyperphosphatemia and phosphate binders. Am J Health Syst Pharm 2005; 62(22):2355–2361. doi:10.2146/ajhp050198
- Chertow GM, Burke SK, Raggi P; Treat to Goal Working Group. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int 2002; 62(1):245–252. doi:10.1046/j.1523-1755.2002.00434.x
- Chertow GM, Burke SK, Dillon MA, Slatopolsky E. Long-term effects of sevelamer hydrochloride on the calcium x phosphate product and lipid profile of haemodialysis patients. Nephrol Dial Transplant 1999; 14(12):2907–2914. pmid:10570096
- Braunlin W, Zhorov E, Guo A, et al. Bile acid binding to sevelamer HCl. Kidney Int 2002; 62(2):611–619. doi:10.1046/j.1523-1755.2002.00459.x
- Yamada K, Fujimoto S, Tokura T, et al. Effect of sevelamer on dyslipidemia and chronic inflammation in maintenance hemodialysis patients. Ren Fail 2005; 27(4):361–365. pmid:16060120
- Qunibi W, Moustafa M, Muenz LR, et al; CARE-2 Investigators. A 1-year randomized trial of calcium acetate versus sevelamer on progression of coronary artery calcification in hemodialysis patients with comparable lipid control: the Calcium Acetate Renagel Evaluation-2 (CARE-2) study. Am J Kidney Dis 2008; 51(6):952–965. doi:10.1053/j.ajkd.2008.02.298
- Barreto DV, Barreto Fde C, de Carvalho AB, et al. Phosphate binder impact on bone remodeling and coronary calcification—results from the BRIC study. Nephron Clin Pract 2008; 110(4):c273–c283. doi:10.1159/000170783
- Cozzolino M, Mazzaferro S, Brandenburg V. The treatment of hyperphosphataemia in CKD: calcium-based or calcium-free phosphate binders? Nephrol Dial Transplant 2011; 26(2):402–407. doi:10.1093/ndt/gfq691
- Yilmaz MI, Sonmez A, Saglam M, et al. Comparison of calcium acetate and sevelamer on vascular function and fibroblast growth factor 23 in CKD patients: a randomized clinical trial. Am J Kidney Dis 2012; 59(2):177–185. doi:10.1053/j.ajkd.2011.11.007
- Shroff RC, McNair R, Skepper JN, et al. Chronic mineral dysregulation promotes vascular smooth muscle cell adaptation and extracellular matrix calcification. J Am Soc Nephrol 2010; 21(1):103–112. doi:10.1681/ASN.2009060640
- Hutchison AJ, Wilson RJ, Garafola S, Copley JB. Lanthanum carbonate: safety data after 10 years. Nephrology (Carlton) 2016; 21(12):987–994. doi:10.1111/nep.12864
- Floege J, Covic AC, Ketteler M, et al; PA21 Study Group. A phase III study of the efficacy and safety of a novel iron-based phosphate binder in dialysis patients. Kidney Int 2014; 86(3):638–647. doi:10.1038/ki.2014.58
- Lewis JB, Sika M, Koury MJ, et al; Collaborative Study Group. Ferric citrate controls phosphorus and delivers iron in patients on dialysis. J Am Soc Nephrol 2015; 26(2):493–503. doi:10.1681/ASN.2014020212
- Liu K, Kaffes AJ. Iron deficiency anemia: a review of diagnosis, investigation and management. Eur J Gastroenterol Hepatol 2012; 24(2):109–116. doi:10.1097/MEG.0b013e32834f3140
- Shah HH, Hazzan AD, Fishbane S. Novel iron-based phosphate binders in patients with chronic kidney disease. Curr Opin Nephrol Hypertens 2015; 24(4):330–335. doi:10.1097/MNH.0000000000000128
- Eknoyan G. Salivary phosphorus binding: a novel approach to control hyperphosphatemia. J Am Soc Nephrol 2009; 20(3):460–462. doi:10.1681/ASN.2009010067
- Raggi P, Vukicevic S, Moysés RM, Wesseling K, Spiegel DM. Ten-year experience with sevelamer and calcium salts as phosphate binders. Clin J Am Soc Nephrol 2010; 5(suppl 1):S31–S40. doi:10.2215/CJN.05880809
- Airy M, Winkelmayer WC, Navaneethan SD. Phosphate binders: the evidence gap persists. Am J Kidney Dis 2016; 68(5):667–670. doi:10.1053/j.ajkd.2016.08.008
- Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med 2011; 364(19):1844–1854. doi:10.1056/NEJMra0904569
- Van den Berg H, Reintsema AM. Renal tubular damage in rasburicase: risks of alkalinisation. Ann Oncol 2004; 15(1):175–176. pmid:14679140
- Suzuki NT. Hyperphosphatemia in nondialyzed TPN patients. JPEN J Parenter Enteral Nutr 1987; 11(5):512. doi:10.1177/0148607187011005512
- Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011; 96(1):53–58. doi:10.1210/jc.2010-2704
KEY POINTS
- Serum phosphorus is maintained within normal levels in a tightly regulated system involving interplay between organs, hormones, diet, and other factors.
- Dietary phosphorus comes mainly from protein, so restricting phosphorus without introducing protein deficiency is difficult. Food with a low phosphorus-to-protein ratio and plant-based sources of protein may be preferable.
- Although dialysis removes phosphorus, it usually does not remove enough, and many patients require phosphorus-binding drugs.
- Selection of an appropriate binder should consider serum calcium levels, pill burden, serum iron stores, and cost.
Phosphorus in kidney disease: Culprit or bystander?
Phosphorus is essential for life. However, both low and high levels of phosphorus in the body have consequences, and its concentration in the blood is tightly regulated through dietary absorption, bone flux, and renal excretion and is influenced by calcitriol (1,25 hydroxyvitamin D3), parathyroid hormone, and fibroblast growth factor 23 (FGF23).
See related articles by M. Shetty and A. Sekar
Sekar et al,1 in this issue of the Journal, provide an extensive review of the pathophysiology of phosphorus metabolism and strategies to control phosphorus levels in patients with hyperphosphatemia and end-stage kidney disease.
PHOSPHORUS OR PHOSPHATE?
What's in a name? That which we call a rose
By any other word would smell as sweet.
—Shakespeare, Romeo and Juliet
The terms phosphate and phosphorus are often used interchangeably, though most writers still prefer phosphate over phosphorus.
The serum concentrations of phosphate and phosphorus are the same when expressed in millimoles per liter, as every mole of phosphate contains 1 mole of phosphorus, but not the same when expressed in milligrams per deciliter.2 The molecular weight of phosphorus is 30.97, whereas the molecular weight of the phosphate ion (PO43–) is 94.97—more than 3 times higher. Therefore, using these terms interchangeably in this context can lead to numerical error.3
Phosphorus, being highly reactive, does not exist by itself in nature and is typically present as phosphates in biologic systems. When describing phosphorus metabolism, the term phosphates should ideally be used because phosphates are the actual participants in the bodily processes. But in the clinical laboratory, all methods that measure serum phosphorus in fact measure inorganic phosphate and are expressed in terms of milligrams of phosphorus per deciliter rather than milligrams of phosphate per deciliter, and using these 2 terms interchangeably in clinical practice should not be of concern.4
THE PROBLEM
US adults typically ingest 1,200 mg of phosphorus each day, and about 60% to 70% of the ingested phosphorus is absorbed both by passive paracellular diffusion via tight junctions and by active transcellular transport via sodium-phosphate cotransport. The kidneys must excrete the same amount daily to maintain a steady state. As kidney function declines, phosphorus accumulates in the blood, leading to hyperphosphatemia.
Hyperphosphatemia is often asymptomatic, but it can cause generalized itching, red eyes, and adverse effects on the bone and parathyroid glands. Higher serum phosphorus levels have been shown to be associated with vascular calcification,5 cardiovascular events, and higher all-cause mortality rates in the general population,6 in patients with diabetes,7 and in those with chronic kidney disease.8 This association between higher serum phosphorus levels and the all-cause mortality rate led to the assumption that lowering serum phosphorus levels in these patients could reduce the rates of cardiovascular events and death, and to efforts to correct hyperphosphatemia.
Research into FGF23 continues, especially its role in cardiovascular complications of chronic kidney disease, as both phosphorus and FGF23 levels are elevated in chronic kidney disease and are implicated in poor clinical outcomes in these patients. However, both FGF23 and parathyroid hormone levels rise early in the course of kidney disease, long before overt hyperphosphatemia develops. Further, FGF23 rises earlier than parathyroid hormone and has been found to be an independent risk factor for cardiovascular events and death from any cause in end-stage kidney disease.9
Whether hyperphosphatemia is the culprit or merely an epiphenomenon of metabolic complications of chronic kidney disease is still unclear, as more molecules are being identified in the complex process of cardiovascular calcification.10
However, one thing is clear: vascular calcification is not just a simple precipitation of calcium and phosphorus. Instead, it is an active process that involves many regulators of mineral metabolism.10 The complex nature of this process is likely one of the reasons that evidence is conflicting11 about the benefits of phosphorus binders in terms of cardiovascular events or all-cause mortality in these patients.
STRATEGIES TO CONTROL HYPERPHOSPHATEMIA
Reducing intake
Dietary phosphorus restriction is the first step in controlling serum phosphorus. But reducing phosphorus intake while otherwise trying to optimize the nutritional status can be challenging.
The recommended daily protein intake is 1.0 to 1.2 g/kg. But phosphorus is typically found in foods rich in proteins, and restricting protein severely can compromise nutritional status and may be as bad as elevated phosphate levels in terms of outcomes.
Although plant-based foods contain more phosphate per gram of protein (ie, they have a higher ratio of phosphorus to protein) than animal-based foods, the bioavailability of phosphorus from plant foods is lower. Phosphorus in plant-based foods is mainly in the form of phytate. Humans cannot hydrolyze phytate because we lack the phytase enzyme; hence, the phosphorus in plant-based foods is not well absorbed. Therefore, a vegetarian diet may be preferable and beneficial in patients with chronic kidney disease. A small study in humans showed that a vegetarian diet resulted in lower serum phosphorus and FGF23 levels, but the study was limited by its small sample size.12
Patients should be advised to avoid foods that have a high phosphate content, such as processed foods, fast foods, and cola beverages, which often have phosphate-based food additives.
Further, one should be cautious about using supplements with healthy-sounding names. A case in point is “vitamin water”: 12 oz of this fruit punch-flavored beverage contains 392 mg of phosphorus,13 and this alone would require 12 to 15 phosphate binder tablets to bind its phosphorus content.
In addition, many prescription drugs have significant amounts of phosphorus, and this is often unrecognized.
Sherman et al14 reviewed 200 of the most commonly prescribed drugs in dialysis patients and found that 23 (11.5%) of the drug labels listed phosphorus-containing ingredients, but the actual amount of phosphorus was not listed. The phosphorus content ranged from 1.4 mg (clonidine 0.2 mg, Blue Point Laboratories, Dublin, Ireland) to 111.5 mg (paroxetine 40 mg, GlaxoSmith Kline, Philadelphia, PA). The phosphorus content was inconsistent and varied with the dose of the agent, type of formulation (tablet or syrup), branded or generic formulation, and manufacturer.
Branded lisinopril (Merck, Kenilworth, NJ) had 21.4 mg of phosphorus per 10-mg dose, while a generic product (Blue Point Laboratories, Dublin, Ireland) had 32.6 mg. Different brands of generic amlodipine 10 mg varied in their phosphorus content from 8.6 mg (Lupin Pharmaceuticals, Mumbai, India) to 27.8 mg (Greenstone LLC, Peapack, NJ) to 40.1 mg (Qualitest Pharmaceuticals, Huntsville, AL. Rena-Vite (Cypress Pharmaceuticals, Madison, MS), a multivitamin marketed to patients with kidney disease, had 37.7 mg of phosphorus per tablet. Thus, just to bind the phosphorus content of these 3 tablets (lisinopril, amlodipine, and Rena-Vite), a patient could need at least 3 to 4 extra doses of phosphate binder.
The phosphate content of medications should be considered when prescribing. For example, Reno Caps (Nnodum Pharmaceuticals, Cincinnati, OH), another vitamin supplement, has only 1.7 mg of phosphorus per tablet and should be considered, especially in patients with poorly controlled serum phosphorus levels. However, the challenge is that medication labels do not provide the phosphorus content.
Reducing phosphorus absorption
Although these agents reduce serum phosphorus and help reduce symptoms, an important quality-of-life measure, it is uncertain whether they improve clinical outcomes.11 To date, no specific phosphorus binder offers a survival benefit over placebo.11
Based on the limited and conflicting evidence, the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines, recently updated, suggest that oral phosphorus binders should be used in patients with hyperphosphatemia to lower serum phosphorus levels toward the normal range.15 They further recommend not exceeding 1,500 mg of elemental calcium per day if a calcium-based binder is used, and they recommend avoiding calcium-based binders in patients with hypercalcemia, adynamic bone disease, or vascular calcification.
Phosphorus binders may account for up to 50% of the daily pill burden and may contribute to poor medication adherence.16 Dialysis patients need to take a lot of these drugs: by weight, 5 to 6 pounds per year.
These drugs can bind and interfere with the absorption of other vital medications and so should be taken with meals and separately from other medications.
Removing phosphorus
Removal of phosphorus by adequate dialysis or kidney transplant is the final strategy.
New agents under study
To improve phosphorus control, other agents that inhibit absorption of phosphate are being investigated.
Nicotinamide reduces expression of the sodium-phosphorus cotransporter NTP2b. Its use in combination with a low-phosphorus diet and phosphorus binders may maximize reductions in phosphorus absorption and is being studied in the CKD Optimal Management With Binders and Nicotinamide (COMBINE) study.
Tenapanor, an inhibitor of the sodium-hydrogen transporter NHE3, has been shown in animal studies to increase fecal phosphate excretion and decrease urinary phosphate excretion17 but requires further evaluation.
- Sekar A, Kaur T, Nally JV Jr, Rincon-Choles H, Jolly S, Nakhoul G. Phosphorus binders: the new and the old, and how to choose. Cleve Clin J Med 2018; 85(8):629–638. doi:10.3949/ccjm.85a.17054
- Young DS. "Phosphorus" or "phosphate." Ann Intern Med 1980; 93(4):631. pmid:7436198
- Bartter FC. Reporting of phosphate and phosphorus plasma values. Am J Med 1981; 71(5):848. pmid:7304659.
- Iheagwara OS, Ing TS, Kjellstrand CM, Lew SQ. Phosphorus, phosphorous, and phosphate. Hemodial Int 2013; 17(4):479–482. doi:10.1111/hdi.12010
- Adeney KL, Siscovick DS, Ix JH, et al. Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol 2009; 20(2):381–387. doi:10.1681/ASN.2008040349
- Dhingra R, Sullivan LM, Fox CS, et al. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med 2007; 167(9):879–885. doi:10.1001/archinte.167.9.879
- Chonchol M, Dale R, Schrier RW, Estacio R. Serum phosphorus and cardiovascular mortality in type 2 diabetes. Am J Med 2009; 122(4):380–386. doi:10.1016/j.amjmed.2008.09.039
- Covic A, Kothawala P, Bernal M, Robbins S, Chalian A, Goldsmith D. Systematic review of the evidence underlying the association between mineral metabolism disturbances and risk of all-cause mortality, cardiovascular mortality and cardiovascular events in chronic kidney disease. Nephrol Dial Transplant 2009; 24(5):1506–1523. doi:10.1093/ndt/gfn613
- Gutiérrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 2008; 359(6):584–592. doi:10.1056/NEJMoa0706130
- Lullo LD, Barbera V, Bellasi A, et al. Vascular and valvular calcifications in chronic kidney disease: an update. EMJ Nephrol 2016; 4(1):84–91. https://pdfs.semanticscholar.org/150f/c7b5dfe671c9b61e4c76d54b7d713b60ba6a.pdf. Accesssed June 5, 2018.
- Palmer SC, Gardner S, Tonelli M, et al. Phosphate-binding agents in adults with CKD: a network meta-analysis of randomized trials. Am J Kidney Dis 2016; 68(5):691–702. doi:10.1053/j.ajkd.2016.05.015
- Moe SM, Zidehsarai MP, Chambers MA, et al. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol 2011; 6(2):257–264. doi:10.2215/CJN.05040610
- Moser M, White K, Henry B, et al. Phosphorus content of popular beverages. Am J Kidney Dis 2015; 65(6):969–971. doi:10.1053/j.ajkd.2015.02.330
- Sherman RA, Ravella S, Kapoian T. A dearth of data: the problem of phosphorus in prescription medications. Kidney Int 2015; 87(6):1097–1099. doi:10.1038/ki.2015.67
- KDIGO 2017 clinical practice guideline update for diagnosis, evaluation, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Supplements 2017; 7(1 suppl): 1–59. www.kisupplements.org/article/S2157-1716(17)30001-1/pdf. Accessed June 5, 2018.
- Fissell RB, Karaboyas A, Bieber BA, et al. Phosphate binder pill burden, patient-reported non-adherence, and mineral bone disorder markers: findings from the DOPPS. Hemodial Int 2016; 20(1):38–49. doi:10.1111/hdi.12315
- Labonté ED, Carreras CW, Leadbetter MR, et al. Gastrointestinal inhibition of sodium-hydrogen exchanger 3 reduces phosphorus absorption and protects against vascular calcification in CKD. J Am Soc Nephrol 2015; 26(5):1138–1149. doi:10.1681/ASN.2014030317
Phosphorus is essential for life. However, both low and high levels of phosphorus in the body have consequences, and its concentration in the blood is tightly regulated through dietary absorption, bone flux, and renal excretion and is influenced by calcitriol (1,25 hydroxyvitamin D3), parathyroid hormone, and fibroblast growth factor 23 (FGF23).
See related articles by M. Shetty and A. Sekar
Sekar et al,1 in this issue of the Journal, provide an extensive review of the pathophysiology of phosphorus metabolism and strategies to control phosphorus levels in patients with hyperphosphatemia and end-stage kidney disease.
PHOSPHORUS OR PHOSPHATE?
What's in a name? That which we call a rose
By any other word would smell as sweet.
—Shakespeare, Romeo and Juliet
The terms phosphate and phosphorus are often used interchangeably, though most writers still prefer phosphate over phosphorus.
The serum concentrations of phosphate and phosphorus are the same when expressed in millimoles per liter, as every mole of phosphate contains 1 mole of phosphorus, but not the same when expressed in milligrams per deciliter.2 The molecular weight of phosphorus is 30.97, whereas the molecular weight of the phosphate ion (PO43–) is 94.97—more than 3 times higher. Therefore, using these terms interchangeably in this context can lead to numerical error.3
Phosphorus, being highly reactive, does not exist by itself in nature and is typically present as phosphates in biologic systems. When describing phosphorus metabolism, the term phosphates should ideally be used because phosphates are the actual participants in the bodily processes. But in the clinical laboratory, all methods that measure serum phosphorus in fact measure inorganic phosphate and are expressed in terms of milligrams of phosphorus per deciliter rather than milligrams of phosphate per deciliter, and using these 2 terms interchangeably in clinical practice should not be of concern.4
THE PROBLEM
US adults typically ingest 1,200 mg of phosphorus each day, and about 60% to 70% of the ingested phosphorus is absorbed both by passive paracellular diffusion via tight junctions and by active transcellular transport via sodium-phosphate cotransport. The kidneys must excrete the same amount daily to maintain a steady state. As kidney function declines, phosphorus accumulates in the blood, leading to hyperphosphatemia.
Hyperphosphatemia is often asymptomatic, but it can cause generalized itching, red eyes, and adverse effects on the bone and parathyroid glands. Higher serum phosphorus levels have been shown to be associated with vascular calcification,5 cardiovascular events, and higher all-cause mortality rates in the general population,6 in patients with diabetes,7 and in those with chronic kidney disease.8 This association between higher serum phosphorus levels and the all-cause mortality rate led to the assumption that lowering serum phosphorus levels in these patients could reduce the rates of cardiovascular events and death, and to efforts to correct hyperphosphatemia.
Research into FGF23 continues, especially its role in cardiovascular complications of chronic kidney disease, as both phosphorus and FGF23 levels are elevated in chronic kidney disease and are implicated in poor clinical outcomes in these patients. However, both FGF23 and parathyroid hormone levels rise early in the course of kidney disease, long before overt hyperphosphatemia develops. Further, FGF23 rises earlier than parathyroid hormone and has been found to be an independent risk factor for cardiovascular events and death from any cause in end-stage kidney disease.9
Whether hyperphosphatemia is the culprit or merely an epiphenomenon of metabolic complications of chronic kidney disease is still unclear, as more molecules are being identified in the complex process of cardiovascular calcification.10
However, one thing is clear: vascular calcification is not just a simple precipitation of calcium and phosphorus. Instead, it is an active process that involves many regulators of mineral metabolism.10 The complex nature of this process is likely one of the reasons that evidence is conflicting11 about the benefits of phosphorus binders in terms of cardiovascular events or all-cause mortality in these patients.
STRATEGIES TO CONTROL HYPERPHOSPHATEMIA
Reducing intake
Dietary phosphorus restriction is the first step in controlling serum phosphorus. But reducing phosphorus intake while otherwise trying to optimize the nutritional status can be challenging.
The recommended daily protein intake is 1.0 to 1.2 g/kg. But phosphorus is typically found in foods rich in proteins, and restricting protein severely can compromise nutritional status and may be as bad as elevated phosphate levels in terms of outcomes.
Although plant-based foods contain more phosphate per gram of protein (ie, they have a higher ratio of phosphorus to protein) than animal-based foods, the bioavailability of phosphorus from plant foods is lower. Phosphorus in plant-based foods is mainly in the form of phytate. Humans cannot hydrolyze phytate because we lack the phytase enzyme; hence, the phosphorus in plant-based foods is not well absorbed. Therefore, a vegetarian diet may be preferable and beneficial in patients with chronic kidney disease. A small study in humans showed that a vegetarian diet resulted in lower serum phosphorus and FGF23 levels, but the study was limited by its small sample size.12
Patients should be advised to avoid foods that have a high phosphate content, such as processed foods, fast foods, and cola beverages, which often have phosphate-based food additives.
Further, one should be cautious about using supplements with healthy-sounding names. A case in point is “vitamin water”: 12 oz of this fruit punch-flavored beverage contains 392 mg of phosphorus,13 and this alone would require 12 to 15 phosphate binder tablets to bind its phosphorus content.
In addition, many prescription drugs have significant amounts of phosphorus, and this is often unrecognized.
Sherman et al14 reviewed 200 of the most commonly prescribed drugs in dialysis patients and found that 23 (11.5%) of the drug labels listed phosphorus-containing ingredients, but the actual amount of phosphorus was not listed. The phosphorus content ranged from 1.4 mg (clonidine 0.2 mg, Blue Point Laboratories, Dublin, Ireland) to 111.5 mg (paroxetine 40 mg, GlaxoSmith Kline, Philadelphia, PA). The phosphorus content was inconsistent and varied with the dose of the agent, type of formulation (tablet or syrup), branded or generic formulation, and manufacturer.
Branded lisinopril (Merck, Kenilworth, NJ) had 21.4 mg of phosphorus per 10-mg dose, while a generic product (Blue Point Laboratories, Dublin, Ireland) had 32.6 mg. Different brands of generic amlodipine 10 mg varied in their phosphorus content from 8.6 mg (Lupin Pharmaceuticals, Mumbai, India) to 27.8 mg (Greenstone LLC, Peapack, NJ) to 40.1 mg (Qualitest Pharmaceuticals, Huntsville, AL. Rena-Vite (Cypress Pharmaceuticals, Madison, MS), a multivitamin marketed to patients with kidney disease, had 37.7 mg of phosphorus per tablet. Thus, just to bind the phosphorus content of these 3 tablets (lisinopril, amlodipine, and Rena-Vite), a patient could need at least 3 to 4 extra doses of phosphate binder.
The phosphate content of medications should be considered when prescribing. For example, Reno Caps (Nnodum Pharmaceuticals, Cincinnati, OH), another vitamin supplement, has only 1.7 mg of phosphorus per tablet and should be considered, especially in patients with poorly controlled serum phosphorus levels. However, the challenge is that medication labels do not provide the phosphorus content.
Reducing phosphorus absorption
Although these agents reduce serum phosphorus and help reduce symptoms, an important quality-of-life measure, it is uncertain whether they improve clinical outcomes.11 To date, no specific phosphorus binder offers a survival benefit over placebo.11
Based on the limited and conflicting evidence, the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines, recently updated, suggest that oral phosphorus binders should be used in patients with hyperphosphatemia to lower serum phosphorus levels toward the normal range.15 They further recommend not exceeding 1,500 mg of elemental calcium per day if a calcium-based binder is used, and they recommend avoiding calcium-based binders in patients with hypercalcemia, adynamic bone disease, or vascular calcification.
Phosphorus binders may account for up to 50% of the daily pill burden and may contribute to poor medication adherence.16 Dialysis patients need to take a lot of these drugs: by weight, 5 to 6 pounds per year.
These drugs can bind and interfere with the absorption of other vital medications and so should be taken with meals and separately from other medications.
Removing phosphorus
Removal of phosphorus by adequate dialysis or kidney transplant is the final strategy.
New agents under study
To improve phosphorus control, other agents that inhibit absorption of phosphate are being investigated.
Nicotinamide reduces expression of the sodium-phosphorus cotransporter NTP2b. Its use in combination with a low-phosphorus diet and phosphorus binders may maximize reductions in phosphorus absorption and is being studied in the CKD Optimal Management With Binders and Nicotinamide (COMBINE) study.
Tenapanor, an inhibitor of the sodium-hydrogen transporter NHE3, has been shown in animal studies to increase fecal phosphate excretion and decrease urinary phosphate excretion17 but requires further evaluation.
Phosphorus is essential for life. However, both low and high levels of phosphorus in the body have consequences, and its concentration in the blood is tightly regulated through dietary absorption, bone flux, and renal excretion and is influenced by calcitriol (1,25 hydroxyvitamin D3), parathyroid hormone, and fibroblast growth factor 23 (FGF23).
See related articles by M. Shetty and A. Sekar
Sekar et al,1 in this issue of the Journal, provide an extensive review of the pathophysiology of phosphorus metabolism and strategies to control phosphorus levels in patients with hyperphosphatemia and end-stage kidney disease.
PHOSPHORUS OR PHOSPHATE?
What's in a name? That which we call a rose
By any other word would smell as sweet.
—Shakespeare, Romeo and Juliet
The terms phosphate and phosphorus are often used interchangeably, though most writers still prefer phosphate over phosphorus.
The serum concentrations of phosphate and phosphorus are the same when expressed in millimoles per liter, as every mole of phosphate contains 1 mole of phosphorus, but not the same when expressed in milligrams per deciliter.2 The molecular weight of phosphorus is 30.97, whereas the molecular weight of the phosphate ion (PO43–) is 94.97—more than 3 times higher. Therefore, using these terms interchangeably in this context can lead to numerical error.3
Phosphorus, being highly reactive, does not exist by itself in nature and is typically present as phosphates in biologic systems. When describing phosphorus metabolism, the term phosphates should ideally be used because phosphates are the actual participants in the bodily processes. But in the clinical laboratory, all methods that measure serum phosphorus in fact measure inorganic phosphate and are expressed in terms of milligrams of phosphorus per deciliter rather than milligrams of phosphate per deciliter, and using these 2 terms interchangeably in clinical practice should not be of concern.4
THE PROBLEM
US adults typically ingest 1,200 mg of phosphorus each day, and about 60% to 70% of the ingested phosphorus is absorbed both by passive paracellular diffusion via tight junctions and by active transcellular transport via sodium-phosphate cotransport. The kidneys must excrete the same amount daily to maintain a steady state. As kidney function declines, phosphorus accumulates in the blood, leading to hyperphosphatemia.
Hyperphosphatemia is often asymptomatic, but it can cause generalized itching, red eyes, and adverse effects on the bone and parathyroid glands. Higher serum phosphorus levels have been shown to be associated with vascular calcification,5 cardiovascular events, and higher all-cause mortality rates in the general population,6 in patients with diabetes,7 and in those with chronic kidney disease.8 This association between higher serum phosphorus levels and the all-cause mortality rate led to the assumption that lowering serum phosphorus levels in these patients could reduce the rates of cardiovascular events and death, and to efforts to correct hyperphosphatemia.
Research into FGF23 continues, especially its role in cardiovascular complications of chronic kidney disease, as both phosphorus and FGF23 levels are elevated in chronic kidney disease and are implicated in poor clinical outcomes in these patients. However, both FGF23 and parathyroid hormone levels rise early in the course of kidney disease, long before overt hyperphosphatemia develops. Further, FGF23 rises earlier than parathyroid hormone and has been found to be an independent risk factor for cardiovascular events and death from any cause in end-stage kidney disease.9
Whether hyperphosphatemia is the culprit or merely an epiphenomenon of metabolic complications of chronic kidney disease is still unclear, as more molecules are being identified in the complex process of cardiovascular calcification.10
However, one thing is clear: vascular calcification is not just a simple precipitation of calcium and phosphorus. Instead, it is an active process that involves many regulators of mineral metabolism.10 The complex nature of this process is likely one of the reasons that evidence is conflicting11 about the benefits of phosphorus binders in terms of cardiovascular events or all-cause mortality in these patients.
STRATEGIES TO CONTROL HYPERPHOSPHATEMIA
Reducing intake
Dietary phosphorus restriction is the first step in controlling serum phosphorus. But reducing phosphorus intake while otherwise trying to optimize the nutritional status can be challenging.
The recommended daily protein intake is 1.0 to 1.2 g/kg. But phosphorus is typically found in foods rich in proteins, and restricting protein severely can compromise nutritional status and may be as bad as elevated phosphate levels in terms of outcomes.
Although plant-based foods contain more phosphate per gram of protein (ie, they have a higher ratio of phosphorus to protein) than animal-based foods, the bioavailability of phosphorus from plant foods is lower. Phosphorus in plant-based foods is mainly in the form of phytate. Humans cannot hydrolyze phytate because we lack the phytase enzyme; hence, the phosphorus in plant-based foods is not well absorbed. Therefore, a vegetarian diet may be preferable and beneficial in patients with chronic kidney disease. A small study in humans showed that a vegetarian diet resulted in lower serum phosphorus and FGF23 levels, but the study was limited by its small sample size.12
Patients should be advised to avoid foods that have a high phosphate content, such as processed foods, fast foods, and cola beverages, which often have phosphate-based food additives.
Further, one should be cautious about using supplements with healthy-sounding names. A case in point is “vitamin water”: 12 oz of this fruit punch-flavored beverage contains 392 mg of phosphorus,13 and this alone would require 12 to 15 phosphate binder tablets to bind its phosphorus content.
In addition, many prescription drugs have significant amounts of phosphorus, and this is often unrecognized.
Sherman et al14 reviewed 200 of the most commonly prescribed drugs in dialysis patients and found that 23 (11.5%) of the drug labels listed phosphorus-containing ingredients, but the actual amount of phosphorus was not listed. The phosphorus content ranged from 1.4 mg (clonidine 0.2 mg, Blue Point Laboratories, Dublin, Ireland) to 111.5 mg (paroxetine 40 mg, GlaxoSmith Kline, Philadelphia, PA). The phosphorus content was inconsistent and varied with the dose of the agent, type of formulation (tablet or syrup), branded or generic formulation, and manufacturer.
Branded lisinopril (Merck, Kenilworth, NJ) had 21.4 mg of phosphorus per 10-mg dose, while a generic product (Blue Point Laboratories, Dublin, Ireland) had 32.6 mg. Different brands of generic amlodipine 10 mg varied in their phosphorus content from 8.6 mg (Lupin Pharmaceuticals, Mumbai, India) to 27.8 mg (Greenstone LLC, Peapack, NJ) to 40.1 mg (Qualitest Pharmaceuticals, Huntsville, AL. Rena-Vite (Cypress Pharmaceuticals, Madison, MS), a multivitamin marketed to patients with kidney disease, had 37.7 mg of phosphorus per tablet. Thus, just to bind the phosphorus content of these 3 tablets (lisinopril, amlodipine, and Rena-Vite), a patient could need at least 3 to 4 extra doses of phosphate binder.
The phosphate content of medications should be considered when prescribing. For example, Reno Caps (Nnodum Pharmaceuticals, Cincinnati, OH), another vitamin supplement, has only 1.7 mg of phosphorus per tablet and should be considered, especially in patients with poorly controlled serum phosphorus levels. However, the challenge is that medication labels do not provide the phosphorus content.
Reducing phosphorus absorption
Although these agents reduce serum phosphorus and help reduce symptoms, an important quality-of-life measure, it is uncertain whether they improve clinical outcomes.11 To date, no specific phosphorus binder offers a survival benefit over placebo.11
Based on the limited and conflicting evidence, the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines, recently updated, suggest that oral phosphorus binders should be used in patients with hyperphosphatemia to lower serum phosphorus levels toward the normal range.15 They further recommend not exceeding 1,500 mg of elemental calcium per day if a calcium-based binder is used, and they recommend avoiding calcium-based binders in patients with hypercalcemia, adynamic bone disease, or vascular calcification.
Phosphorus binders may account for up to 50% of the daily pill burden and may contribute to poor medication adherence.16 Dialysis patients need to take a lot of these drugs: by weight, 5 to 6 pounds per year.
These drugs can bind and interfere with the absorption of other vital medications and so should be taken with meals and separately from other medications.
Removing phosphorus
Removal of phosphorus by adequate dialysis or kidney transplant is the final strategy.
New agents under study
To improve phosphorus control, other agents that inhibit absorption of phosphate are being investigated.
Nicotinamide reduces expression of the sodium-phosphorus cotransporter NTP2b. Its use in combination with a low-phosphorus diet and phosphorus binders may maximize reductions in phosphorus absorption and is being studied in the CKD Optimal Management With Binders and Nicotinamide (COMBINE) study.
Tenapanor, an inhibitor of the sodium-hydrogen transporter NHE3, has been shown in animal studies to increase fecal phosphate excretion and decrease urinary phosphate excretion17 but requires further evaluation.
- Sekar A, Kaur T, Nally JV Jr, Rincon-Choles H, Jolly S, Nakhoul G. Phosphorus binders: the new and the old, and how to choose. Cleve Clin J Med 2018; 85(8):629–638. doi:10.3949/ccjm.85a.17054
- Young DS. "Phosphorus" or "phosphate." Ann Intern Med 1980; 93(4):631. pmid:7436198
- Bartter FC. Reporting of phosphate and phosphorus plasma values. Am J Med 1981; 71(5):848. pmid:7304659.
- Iheagwara OS, Ing TS, Kjellstrand CM, Lew SQ. Phosphorus, phosphorous, and phosphate. Hemodial Int 2013; 17(4):479–482. doi:10.1111/hdi.12010
- Adeney KL, Siscovick DS, Ix JH, et al. Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol 2009; 20(2):381–387. doi:10.1681/ASN.2008040349
- Dhingra R, Sullivan LM, Fox CS, et al. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med 2007; 167(9):879–885. doi:10.1001/archinte.167.9.879
- Chonchol M, Dale R, Schrier RW, Estacio R. Serum phosphorus and cardiovascular mortality in type 2 diabetes. Am J Med 2009; 122(4):380–386. doi:10.1016/j.amjmed.2008.09.039
- Covic A, Kothawala P, Bernal M, Robbins S, Chalian A, Goldsmith D. Systematic review of the evidence underlying the association between mineral metabolism disturbances and risk of all-cause mortality, cardiovascular mortality and cardiovascular events in chronic kidney disease. Nephrol Dial Transplant 2009; 24(5):1506–1523. doi:10.1093/ndt/gfn613
- Gutiérrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 2008; 359(6):584–592. doi:10.1056/NEJMoa0706130
- Lullo LD, Barbera V, Bellasi A, et al. Vascular and valvular calcifications in chronic kidney disease: an update. EMJ Nephrol 2016; 4(1):84–91. https://pdfs.semanticscholar.org/150f/c7b5dfe671c9b61e4c76d54b7d713b60ba6a.pdf. Accesssed June 5, 2018.
- Palmer SC, Gardner S, Tonelli M, et al. Phosphate-binding agents in adults with CKD: a network meta-analysis of randomized trials. Am J Kidney Dis 2016; 68(5):691–702. doi:10.1053/j.ajkd.2016.05.015
- Moe SM, Zidehsarai MP, Chambers MA, et al. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol 2011; 6(2):257–264. doi:10.2215/CJN.05040610
- Moser M, White K, Henry B, et al. Phosphorus content of popular beverages. Am J Kidney Dis 2015; 65(6):969–971. doi:10.1053/j.ajkd.2015.02.330
- Sherman RA, Ravella S, Kapoian T. A dearth of data: the problem of phosphorus in prescription medications. Kidney Int 2015; 87(6):1097–1099. doi:10.1038/ki.2015.67
- KDIGO 2017 clinical practice guideline update for diagnosis, evaluation, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Supplements 2017; 7(1 suppl): 1–59. www.kisupplements.org/article/S2157-1716(17)30001-1/pdf. Accessed June 5, 2018.
- Fissell RB, Karaboyas A, Bieber BA, et al. Phosphate binder pill burden, patient-reported non-adherence, and mineral bone disorder markers: findings from the DOPPS. Hemodial Int 2016; 20(1):38–49. doi:10.1111/hdi.12315
- Labonté ED, Carreras CW, Leadbetter MR, et al. Gastrointestinal inhibition of sodium-hydrogen exchanger 3 reduces phosphorus absorption and protects against vascular calcification in CKD. J Am Soc Nephrol 2015; 26(5):1138–1149. doi:10.1681/ASN.2014030317
- Sekar A, Kaur T, Nally JV Jr, Rincon-Choles H, Jolly S, Nakhoul G. Phosphorus binders: the new and the old, and how to choose. Cleve Clin J Med 2018; 85(8):629–638. doi:10.3949/ccjm.85a.17054
- Young DS. "Phosphorus" or "phosphate." Ann Intern Med 1980; 93(4):631. pmid:7436198
- Bartter FC. Reporting of phosphate and phosphorus plasma values. Am J Med 1981; 71(5):848. pmid:7304659.
- Iheagwara OS, Ing TS, Kjellstrand CM, Lew SQ. Phosphorus, phosphorous, and phosphate. Hemodial Int 2013; 17(4):479–482. doi:10.1111/hdi.12010
- Adeney KL, Siscovick DS, Ix JH, et al. Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol 2009; 20(2):381–387. doi:10.1681/ASN.2008040349
- Dhingra R, Sullivan LM, Fox CS, et al. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med 2007; 167(9):879–885. doi:10.1001/archinte.167.9.879
- Chonchol M, Dale R, Schrier RW, Estacio R. Serum phosphorus and cardiovascular mortality in type 2 diabetes. Am J Med 2009; 122(4):380–386. doi:10.1016/j.amjmed.2008.09.039
- Covic A, Kothawala P, Bernal M, Robbins S, Chalian A, Goldsmith D. Systematic review of the evidence underlying the association between mineral metabolism disturbances and risk of all-cause mortality, cardiovascular mortality and cardiovascular events in chronic kidney disease. Nephrol Dial Transplant 2009; 24(5):1506–1523. doi:10.1093/ndt/gfn613
- Gutiérrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 2008; 359(6):584–592. doi:10.1056/NEJMoa0706130
- Lullo LD, Barbera V, Bellasi A, et al. Vascular and valvular calcifications in chronic kidney disease: an update. EMJ Nephrol 2016; 4(1):84–91. https://pdfs.semanticscholar.org/150f/c7b5dfe671c9b61e4c76d54b7d713b60ba6a.pdf. Accesssed June 5, 2018.
- Palmer SC, Gardner S, Tonelli M, et al. Phosphate-binding agents in adults with CKD: a network meta-analysis of randomized trials. Am J Kidney Dis 2016; 68(5):691–702. doi:10.1053/j.ajkd.2016.05.015
- Moe SM, Zidehsarai MP, Chambers MA, et al. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol 2011; 6(2):257–264. doi:10.2215/CJN.05040610
- Moser M, White K, Henry B, et al. Phosphorus content of popular beverages. Am J Kidney Dis 2015; 65(6):969–971. doi:10.1053/j.ajkd.2015.02.330
- Sherman RA, Ravella S, Kapoian T. A dearth of data: the problem of phosphorus in prescription medications. Kidney Int 2015; 87(6):1097–1099. doi:10.1038/ki.2015.67
- KDIGO 2017 clinical practice guideline update for diagnosis, evaluation, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Supplements 2017; 7(1 suppl): 1–59. www.kisupplements.org/article/S2157-1716(17)30001-1/pdf. Accessed June 5, 2018.
- Fissell RB, Karaboyas A, Bieber BA, et al. Phosphate binder pill burden, patient-reported non-adherence, and mineral bone disorder markers: findings from the DOPPS. Hemodial Int 2016; 20(1):38–49. doi:10.1111/hdi.12315
- Labonté ED, Carreras CW, Leadbetter MR, et al. Gastrointestinal inhibition of sodium-hydrogen exchanger 3 reduces phosphorus absorption and protects against vascular calcification in CKD. J Am Soc Nephrol 2015; 26(5):1138–1149. doi:10.1681/ASN.2014030317
Calcific uremic arteriolopathy
A 51-year-old man with end-stage renal disease, on peritoneal dialysis for the past 4 years, presented to the emergency department with severe pain in both legs. The pain had started 2 months previously and had progressively worsened. After multiple admissions in the past for hyperkalemia and volume overload due to noncompliance, he had been advised to switch to hemodialysis.
See related article and editorial
Laboratory analysis revealed the following values:
- Serum creatinine 12.62 mg/dL (reference range 0.73–1.22)
- Blood urea nitrogen 159 mg/dL (9–24)
- Serum calcium corrected for serum albumin 8.1 mg/dL (8.4–10.0)
- Serum phosphorus 10.6 mg/dL (2.7–4.8).
His history of end-stage renal disease, failure of peritoneal dialysis, high calcium-phosphorus product (8.1 mg/dL × 10.6 mg/dL = 85.9 mg2/dL2, reference range ≤ 55), and characteristic physical findings led to the diagnosis of calcific uremic arteriolopathy.
CALCIFIC UREMIC ARTERIOLOPATHY
Calcific uremic arteriolopathy or “calciphylaxis,” seen most often in patients with end-stage renal disease, is caused by calcium deposition in the media of the dermo-hypodermic arterioles, leading to infarction of adjacent tissue.1–3 A high calcium-phosphorus product (> 55) has been implicated in its development; however, the calcium-phosphorus product can be normal despite hyperphosphatemia, which itself may promote ectopic calcification.
Early ischemic manifestations include livedo reticularis and painful retiform purpura on the thighs and other areas of high adiposity. Lesions evolve into violaceous plaquelike subcutaneous nodules that can infarct, become necrotic, ulcerate, and become infected. Punch biopsy demonstrating arteriolar calcification, subintimal fibrosis, and thrombosis confirms the diagnosis.
Differential diagnosis
Warfarin necrosis can cause large, irregular, bloody bullae that ulcerate and turn into eschar that may resemble lesions of calcific uremic arteriolopathy. Our patient, however, had no exposure to warfarin.
Pemphigus foliaceus, an immunoglobulin G4-mediated autoimmune disorder targeted against desmoglein-1, leads to the formation of fragile blisters that easily rupture when rubbed (Nikolsky sign). Lesions evolve into scaling, crusty erosions on an erythematous base. With tender blisters and lack of mucous membrane involvement, pemphigus foliaceus shares similarities with calcific uremic arteriolopathy, but the presence of necrotic eschar surrounded by violaceous plaques in our patient made it an unlikely diagnosis.
Cryofibrinogenemia. In the right clinical scenario, ie, in a patient with vasculitis, malignancy, infection, cryoglobulinemia, or collagen diseases, cryofibrinogen-mediated cold-induced occlusive lesions may mimic calcific uremic arteriolopathy, with painful or pruritic erythema, purpura, livedo reticularis, necrosis, and ulceration.4 Our patient had no color changes with exposure to cold, nor any history of Raynaud phenomenon or joint pain, making the diagnosis of cryofibrinogenemia less likely.
Nephrogenic systemic fibrosis. Gadolinium contrast medium in magnetic resonance imaging can cause nephrogenic systemic fibrosis, characterized by erythematous papules that coalesce into brawny plaques with surrounding woody induration, which may resemble lesions of calcific uremic arteriolopathy.5 However, our patient had not been exposed to gadolinium.
Management
Management is multidisciplinary and includes the following1:
- Hemodialysis, modified to optimize calcium balance2
- Intravenous sodium thiosulfate: the exact mechanism of action remains unclear, but it is thought to play a role in chelating calcium from tissue deposits, thus decreasing pain and promoting regression of skin lesions3
- Wound care, including chemical debridement agents, negative-pressure wound therapy, and surgical debridement for infected wounds6
- Pain management with opioid analgesics.
The patient was treated with all these measures. However, he died of sudden cardiac arrest during the same admission.
- Weenig RH, Sewell LD, Davis MD, McCarthy JT, Pittelkow MR. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol 2007; 56(4):569–579. doi:10.1016/j.jaad.2006.08.065
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis 2015; 66(1):133–146. doi:10.1053/j.ajkd.2015.01.034
- Janigan DT, Hirsch DJ, Klassen GA, MacDonald AS. Calcified subcutaneous arterioles with infarcts of the subcutis and skin (“calciphylaxis”) in chronic renal failure. Am J Kidney Dis 2000; 35(4):588–597. pmid:10739777
- Michaud M, Pourrat J. Cryofibrinogenemia. J Clin Rheumatol 2013; 19(3):142–148. doi:10.1097/RHU.0b013e318289e06e
- Galan A, Cowper SE, Bucala R. Nephrogenic systemic fibrosis (nephrogenic fibrosing dermopathy). Curr Opin Rheumatol 2006; 18(6):614–617. doi:10.1097/01.bor.0000245725.94887.8d
- Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, eds. Fitzpatrick’s Dermatology in General Medicine. 6th ed. New York, NY: McGraw-Hill Professional; 2003:558–562.
A 51-year-old man with end-stage renal disease, on peritoneal dialysis for the past 4 years, presented to the emergency department with severe pain in both legs. The pain had started 2 months previously and had progressively worsened. After multiple admissions in the past for hyperkalemia and volume overload due to noncompliance, he had been advised to switch to hemodialysis.
See related article and editorial
Laboratory analysis revealed the following values:
- Serum creatinine 12.62 mg/dL (reference range 0.73–1.22)
- Blood urea nitrogen 159 mg/dL (9–24)
- Serum calcium corrected for serum albumin 8.1 mg/dL (8.4–10.0)
- Serum phosphorus 10.6 mg/dL (2.7–4.8).
His history of end-stage renal disease, failure of peritoneal dialysis, high calcium-phosphorus product (8.1 mg/dL × 10.6 mg/dL = 85.9 mg2/dL2, reference range ≤ 55), and characteristic physical findings led to the diagnosis of calcific uremic arteriolopathy.
CALCIFIC UREMIC ARTERIOLOPATHY
Calcific uremic arteriolopathy or “calciphylaxis,” seen most often in patients with end-stage renal disease, is caused by calcium deposition in the media of the dermo-hypodermic arterioles, leading to infarction of adjacent tissue.1–3 A high calcium-phosphorus product (> 55) has been implicated in its development; however, the calcium-phosphorus product can be normal despite hyperphosphatemia, which itself may promote ectopic calcification.
Early ischemic manifestations include livedo reticularis and painful retiform purpura on the thighs and other areas of high adiposity. Lesions evolve into violaceous plaquelike subcutaneous nodules that can infarct, become necrotic, ulcerate, and become infected. Punch biopsy demonstrating arteriolar calcification, subintimal fibrosis, and thrombosis confirms the diagnosis.
Differential diagnosis
Warfarin necrosis can cause large, irregular, bloody bullae that ulcerate and turn into eschar that may resemble lesions of calcific uremic arteriolopathy. Our patient, however, had no exposure to warfarin.
Pemphigus foliaceus, an immunoglobulin G4-mediated autoimmune disorder targeted against desmoglein-1, leads to the formation of fragile blisters that easily rupture when rubbed (Nikolsky sign). Lesions evolve into scaling, crusty erosions on an erythematous base. With tender blisters and lack of mucous membrane involvement, pemphigus foliaceus shares similarities with calcific uremic arteriolopathy, but the presence of necrotic eschar surrounded by violaceous plaques in our patient made it an unlikely diagnosis.
Cryofibrinogenemia. In the right clinical scenario, ie, in a patient with vasculitis, malignancy, infection, cryoglobulinemia, or collagen diseases, cryofibrinogen-mediated cold-induced occlusive lesions may mimic calcific uremic arteriolopathy, with painful or pruritic erythema, purpura, livedo reticularis, necrosis, and ulceration.4 Our patient had no color changes with exposure to cold, nor any history of Raynaud phenomenon or joint pain, making the diagnosis of cryofibrinogenemia less likely.
Nephrogenic systemic fibrosis. Gadolinium contrast medium in magnetic resonance imaging can cause nephrogenic systemic fibrosis, characterized by erythematous papules that coalesce into brawny plaques with surrounding woody induration, which may resemble lesions of calcific uremic arteriolopathy.5 However, our patient had not been exposed to gadolinium.
Management
Management is multidisciplinary and includes the following1:
- Hemodialysis, modified to optimize calcium balance2
- Intravenous sodium thiosulfate: the exact mechanism of action remains unclear, but it is thought to play a role in chelating calcium from tissue deposits, thus decreasing pain and promoting regression of skin lesions3
- Wound care, including chemical debridement agents, negative-pressure wound therapy, and surgical debridement for infected wounds6
- Pain management with opioid analgesics.
The patient was treated with all these measures. However, he died of sudden cardiac arrest during the same admission.
A 51-year-old man with end-stage renal disease, on peritoneal dialysis for the past 4 years, presented to the emergency department with severe pain in both legs. The pain had started 2 months previously and had progressively worsened. After multiple admissions in the past for hyperkalemia and volume overload due to noncompliance, he had been advised to switch to hemodialysis.
See related article and editorial
Laboratory analysis revealed the following values:
- Serum creatinine 12.62 mg/dL (reference range 0.73–1.22)
- Blood urea nitrogen 159 mg/dL (9–24)
- Serum calcium corrected for serum albumin 8.1 mg/dL (8.4–10.0)
- Serum phosphorus 10.6 mg/dL (2.7–4.8).
His history of end-stage renal disease, failure of peritoneal dialysis, high calcium-phosphorus product (8.1 mg/dL × 10.6 mg/dL = 85.9 mg2/dL2, reference range ≤ 55), and characteristic physical findings led to the diagnosis of calcific uremic arteriolopathy.
CALCIFIC UREMIC ARTERIOLOPATHY
Calcific uremic arteriolopathy or “calciphylaxis,” seen most often in patients with end-stage renal disease, is caused by calcium deposition in the media of the dermo-hypodermic arterioles, leading to infarction of adjacent tissue.1–3 A high calcium-phosphorus product (> 55) has been implicated in its development; however, the calcium-phosphorus product can be normal despite hyperphosphatemia, which itself may promote ectopic calcification.
Early ischemic manifestations include livedo reticularis and painful retiform purpura on the thighs and other areas of high adiposity. Lesions evolve into violaceous plaquelike subcutaneous nodules that can infarct, become necrotic, ulcerate, and become infected. Punch biopsy demonstrating arteriolar calcification, subintimal fibrosis, and thrombosis confirms the diagnosis.
Differential diagnosis
Warfarin necrosis can cause large, irregular, bloody bullae that ulcerate and turn into eschar that may resemble lesions of calcific uremic arteriolopathy. Our patient, however, had no exposure to warfarin.
Pemphigus foliaceus, an immunoglobulin G4-mediated autoimmune disorder targeted against desmoglein-1, leads to the formation of fragile blisters that easily rupture when rubbed (Nikolsky sign). Lesions evolve into scaling, crusty erosions on an erythematous base. With tender blisters and lack of mucous membrane involvement, pemphigus foliaceus shares similarities with calcific uremic arteriolopathy, but the presence of necrotic eschar surrounded by violaceous plaques in our patient made it an unlikely diagnosis.
Cryofibrinogenemia. In the right clinical scenario, ie, in a patient with vasculitis, malignancy, infection, cryoglobulinemia, or collagen diseases, cryofibrinogen-mediated cold-induced occlusive lesions may mimic calcific uremic arteriolopathy, with painful or pruritic erythema, purpura, livedo reticularis, necrosis, and ulceration.4 Our patient had no color changes with exposure to cold, nor any history of Raynaud phenomenon or joint pain, making the diagnosis of cryofibrinogenemia less likely.
Nephrogenic systemic fibrosis. Gadolinium contrast medium in magnetic resonance imaging can cause nephrogenic systemic fibrosis, characterized by erythematous papules that coalesce into brawny plaques with surrounding woody induration, which may resemble lesions of calcific uremic arteriolopathy.5 However, our patient had not been exposed to gadolinium.
Management
Management is multidisciplinary and includes the following1:
- Hemodialysis, modified to optimize calcium balance2
- Intravenous sodium thiosulfate: the exact mechanism of action remains unclear, but it is thought to play a role in chelating calcium from tissue deposits, thus decreasing pain and promoting regression of skin lesions3
- Wound care, including chemical debridement agents, negative-pressure wound therapy, and surgical debridement for infected wounds6
- Pain management with opioid analgesics.
The patient was treated with all these measures. However, he died of sudden cardiac arrest during the same admission.
- Weenig RH, Sewell LD, Davis MD, McCarthy JT, Pittelkow MR. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol 2007; 56(4):569–579. doi:10.1016/j.jaad.2006.08.065
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis 2015; 66(1):133–146. doi:10.1053/j.ajkd.2015.01.034
- Janigan DT, Hirsch DJ, Klassen GA, MacDonald AS. Calcified subcutaneous arterioles with infarcts of the subcutis and skin (“calciphylaxis”) in chronic renal failure. Am J Kidney Dis 2000; 35(4):588–597. pmid:10739777
- Michaud M, Pourrat J. Cryofibrinogenemia. J Clin Rheumatol 2013; 19(3):142–148. doi:10.1097/RHU.0b013e318289e06e
- Galan A, Cowper SE, Bucala R. Nephrogenic systemic fibrosis (nephrogenic fibrosing dermopathy). Curr Opin Rheumatol 2006; 18(6):614–617. doi:10.1097/01.bor.0000245725.94887.8d
- Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, eds. Fitzpatrick’s Dermatology in General Medicine. 6th ed. New York, NY: McGraw-Hill Professional; 2003:558–562.
- Weenig RH, Sewell LD, Davis MD, McCarthy JT, Pittelkow MR. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol 2007; 56(4):569–579. doi:10.1016/j.jaad.2006.08.065
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis 2015; 66(1):133–146. doi:10.1053/j.ajkd.2015.01.034
- Janigan DT, Hirsch DJ, Klassen GA, MacDonald AS. Calcified subcutaneous arterioles with infarcts of the subcutis and skin (“calciphylaxis”) in chronic renal failure. Am J Kidney Dis 2000; 35(4):588–597. pmid:10739777
- Michaud M, Pourrat J. Cryofibrinogenemia. J Clin Rheumatol 2013; 19(3):142–148. doi:10.1097/RHU.0b013e318289e06e
- Galan A, Cowper SE, Bucala R. Nephrogenic systemic fibrosis (nephrogenic fibrosing dermopathy). Curr Opin Rheumatol 2006; 18(6):614–617. doi:10.1097/01.bor.0000245725.94887.8d
- Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, eds. Fitzpatrick’s Dermatology in General Medicine. 6th ed. New York, NY: McGraw-Hill Professional; 2003:558–562.