User login
Genetic Variations in the CYP2J2 Region May Be Associated With MS Risk
NASHVILLE—Single-nucleotide polymorphisms (SNPs) in the CYP2J2 region of chromosome 1 may be associated with an increased risk of multiple sclerosis (MS) and higher levels of prolactin, according to research presented at the 2018 CMSC Annual Meeting. “To our knowledge, this is the first report to show an association between genetic variants within this region and either MS status or the level of serum prolactin,” said Samantha Jack, Research Coordinator at Saunders Medical Center in Wahoo, Nebraska, and colleagues.
Although the cause of MS is unknown, a combination of genetic, environmental, and infectious risk factors may contribute to its pathogenesis, said Ms. Jack and colleagues. Vitamin D has been suggested as the most attractive environmental factor. In addition, the CYP2J2 gene has been identified as having a role in serum vitamin D levels in cattle, and the CYP2J2-containing region on bovine chromosome 3 is syntenic with that on human chromosome 1, the researchers said.
Evaluating SNPs in the CYP2J2 Region
Ms. Jack and colleagues conducted a study to determine whether associations exist between variations in the genomic region of CYP2J2 and MS status or levels of serum markers associated with vitamin D and calcium metabolism such as prolactin, vitamin D, vitamin D–binding protein, alkaline phosphatase, and calcium.
Participants were recruited from Nebraska, Iowa, and Kansas between October 2014 and December 2016 to participate in a single blood draw.
Ms. Jack and colleagues collected blood samples from 220 patients with MS and 238 age- and sex-matched controls. DNA from blood samples was genotyped for 94 SNPs in a 255,348 base-pair region of chromosome 1 that included CYP2J2 and C1orf87. Researchers analyzed serum samples to quantify concentrations of vitamin D, vitamin D–binding protein, alkaline phosphatase, and prolactin. Almost all participants with MS took supplemental vitamin D.
The 458 participants in the study were predominately Caucasian and had an average age of about 50, said Ms. Jack.
SNPs in the CYP2J2 region were associated with an increased risk of MS. These associations were not significant following Bonferroni correction. Several other SNPs in the CYP2J2 region were associated with prolactin levels, but the associations were not significant following the Bonferroni correction. No SNPs in this region showed significant associations between levels of vitamin D, vitamin D–binding protein, calcium, or alkaline phosphatase, but vitamin D levels may have been skewed since many patients with MS were taking vitamin D supplements, said the researchers.
Overall, SNPs downstream of CYP2J2 in the C1orf87 gene were associated with prolactin levels, and SNPs in the intergenic region between CYP212 and C1orf87 may be associated with MS, said the researchers.
Study Limitations and Future Directions
Study limitations include that the study population was homogeneous (ie, middle-aged and white from the Midwest) and that samples were not always taken in the morning after fasting, which may have affected prolactin levels, said Ms. Jack.
“We are in the process of undertaking a genome-wide association study to continue this work,” Ms. Jack said. “We are currently enrolling more subjects to have a well-powered study, and we hope to have a small subset of people who have not taken vitamin D supplementation so that we will be able to analyze those values.”
—Erica Tricarico
NASHVILLE—Single-nucleotide polymorphisms (SNPs) in the CYP2J2 region of chromosome 1 may be associated with an increased risk of multiple sclerosis (MS) and higher levels of prolactin, according to research presented at the 2018 CMSC Annual Meeting. “To our knowledge, this is the first report to show an association between genetic variants within this region and either MS status or the level of serum prolactin,” said Samantha Jack, Research Coordinator at Saunders Medical Center in Wahoo, Nebraska, and colleagues.
Although the cause of MS is unknown, a combination of genetic, environmental, and infectious risk factors may contribute to its pathogenesis, said Ms. Jack and colleagues. Vitamin D has been suggested as the most attractive environmental factor. In addition, the CYP2J2 gene has been identified as having a role in serum vitamin D levels in cattle, and the CYP2J2-containing region on bovine chromosome 3 is syntenic with that on human chromosome 1, the researchers said.
Evaluating SNPs in the CYP2J2 Region
Ms. Jack and colleagues conducted a study to determine whether associations exist between variations in the genomic region of CYP2J2 and MS status or levels of serum markers associated with vitamin D and calcium metabolism such as prolactin, vitamin D, vitamin D–binding protein, alkaline phosphatase, and calcium.
Participants were recruited from Nebraska, Iowa, and Kansas between October 2014 and December 2016 to participate in a single blood draw.
Ms. Jack and colleagues collected blood samples from 220 patients with MS and 238 age- and sex-matched controls. DNA from blood samples was genotyped for 94 SNPs in a 255,348 base-pair region of chromosome 1 that included CYP2J2 and C1orf87. Researchers analyzed serum samples to quantify concentrations of vitamin D, vitamin D–binding protein, alkaline phosphatase, and prolactin. Almost all participants with MS took supplemental vitamin D.
The 458 participants in the study were predominately Caucasian and had an average age of about 50, said Ms. Jack.
SNPs in the CYP2J2 region were associated with an increased risk of MS. These associations were not significant following Bonferroni correction. Several other SNPs in the CYP2J2 region were associated with prolactin levels, but the associations were not significant following the Bonferroni correction. No SNPs in this region showed significant associations between levels of vitamin D, vitamin D–binding protein, calcium, or alkaline phosphatase, but vitamin D levels may have been skewed since many patients with MS were taking vitamin D supplements, said the researchers.
Overall, SNPs downstream of CYP2J2 in the C1orf87 gene were associated with prolactin levels, and SNPs in the intergenic region between CYP212 and C1orf87 may be associated with MS, said the researchers.
Study Limitations and Future Directions
Study limitations include that the study population was homogeneous (ie, middle-aged and white from the Midwest) and that samples were not always taken in the morning after fasting, which may have affected prolactin levels, said Ms. Jack.
“We are in the process of undertaking a genome-wide association study to continue this work,” Ms. Jack said. “We are currently enrolling more subjects to have a well-powered study, and we hope to have a small subset of people who have not taken vitamin D supplementation so that we will be able to analyze those values.”
—Erica Tricarico
NASHVILLE—Single-nucleotide polymorphisms (SNPs) in the CYP2J2 region of chromosome 1 may be associated with an increased risk of multiple sclerosis (MS) and higher levels of prolactin, according to research presented at the 2018 CMSC Annual Meeting. “To our knowledge, this is the first report to show an association between genetic variants within this region and either MS status or the level of serum prolactin,” said Samantha Jack, Research Coordinator at Saunders Medical Center in Wahoo, Nebraska, and colleagues.
Although the cause of MS is unknown, a combination of genetic, environmental, and infectious risk factors may contribute to its pathogenesis, said Ms. Jack and colleagues. Vitamin D has been suggested as the most attractive environmental factor. In addition, the CYP2J2 gene has been identified as having a role in serum vitamin D levels in cattle, and the CYP2J2-containing region on bovine chromosome 3 is syntenic with that on human chromosome 1, the researchers said.
Evaluating SNPs in the CYP2J2 Region
Ms. Jack and colleagues conducted a study to determine whether associations exist between variations in the genomic region of CYP2J2 and MS status or levels of serum markers associated with vitamin D and calcium metabolism such as prolactin, vitamin D, vitamin D–binding protein, alkaline phosphatase, and calcium.
Participants were recruited from Nebraska, Iowa, and Kansas between October 2014 and December 2016 to participate in a single blood draw.
Ms. Jack and colleagues collected blood samples from 220 patients with MS and 238 age- and sex-matched controls. DNA from blood samples was genotyped for 94 SNPs in a 255,348 base-pair region of chromosome 1 that included CYP2J2 and C1orf87. Researchers analyzed serum samples to quantify concentrations of vitamin D, vitamin D–binding protein, alkaline phosphatase, and prolactin. Almost all participants with MS took supplemental vitamin D.
The 458 participants in the study were predominately Caucasian and had an average age of about 50, said Ms. Jack.
SNPs in the CYP2J2 region were associated with an increased risk of MS. These associations were not significant following Bonferroni correction. Several other SNPs in the CYP2J2 region were associated with prolactin levels, but the associations were not significant following the Bonferroni correction. No SNPs in this region showed significant associations between levels of vitamin D, vitamin D–binding protein, calcium, or alkaline phosphatase, but vitamin D levels may have been skewed since many patients with MS were taking vitamin D supplements, said the researchers.
Overall, SNPs downstream of CYP2J2 in the C1orf87 gene were associated with prolactin levels, and SNPs in the intergenic region between CYP212 and C1orf87 may be associated with MS, said the researchers.
Study Limitations and Future Directions
Study limitations include that the study population was homogeneous (ie, middle-aged and white from the Midwest) and that samples were not always taken in the morning after fasting, which may have affected prolactin levels, said Ms. Jack.
“We are in the process of undertaking a genome-wide association study to continue this work,” Ms. Jack said. “We are currently enrolling more subjects to have a well-powered study, and we hope to have a small subset of people who have not taken vitamin D supplementation so that we will be able to analyze those values.”
—Erica Tricarico
Should breast cancer screening guidelines be tailored to a patient’s race and ethnicity?
EXPERT COMMENTARY
Breast cancer screening is an important aspect of women’s preventative health care, with proven mortality benefits.1,2 Different recommendations have been made for the age at initiation and the frequency of breast cancer screening in an effort to maximize benefit while minimizing unnecessary health care costs and harms of screening.
The American College of Obstetricians and Gynecologists (ACOG) and the National Comprehensive Cancer Network (NCCN) recommend initiating mammography screening at age 40, with annual screening (although ACOG offers deferral of screening to age 50 and biennial screening through shared decision making).3,4 The American Cancer Society (ACS) recommends offering annual mammography at ages 40 to 44 and recommends routinely starting annual mammography from 45 to 54, followed by either annual or biennial screening for women 55 and older.1 Finally, the US Preventive Services Task Force (USPSTF) recommends biennial mammography screening starting at age 50.5 No organization alters screening recommendations based on a woman’s race/ethnicity.
Details of the study
Stapleton and colleagues recently performed a retrospective population-based cohort study using the Surveillance, Epidemiology, and End Results (SEER) Program database to evaluate the age and stage at breast cancer diagnosis across different racial groups in the United States.6 The study (timeframe, January 1, 1973 to December 31, 2010) included 747,763 women, with a racial/ethnic distribution of 77.0% white, 9.3% black, 7.0% Hispanic, and 6.2% Asian.
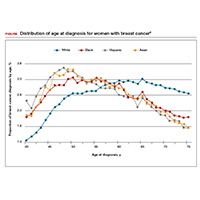
The investigators found 2 distinct age distributions of breast cancer based on race. Among nonwhite women, the highest peak of breast cancer diagnoses occurred between 45 and 50 years (FIGURE). By contrast, breast cancer diagnoses peaked at 60 to 65 years in white women.
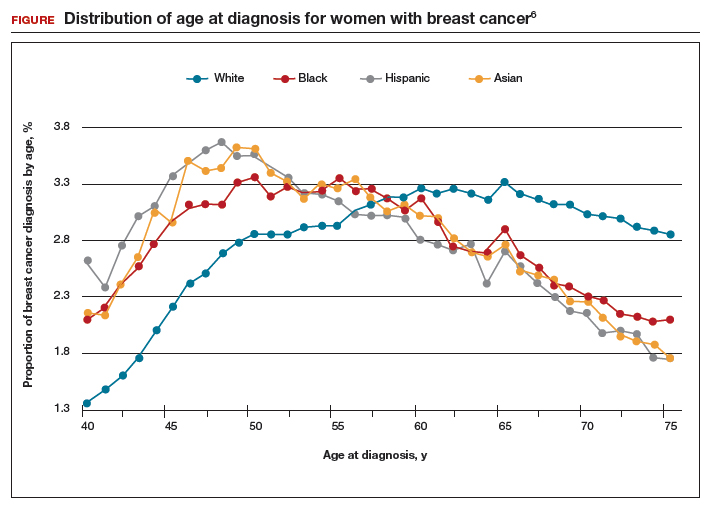
Similarly, a higher proportion of nonwhite women were diagnosed with their breast cancer prior to age 50 compared with white women. While one-quarter of white women with breast cancer develop disease prior to age 50, approximately one-third of black, Asian, and Hispanic women with breast cancer will be diagnosed before age 50 (TABLE).
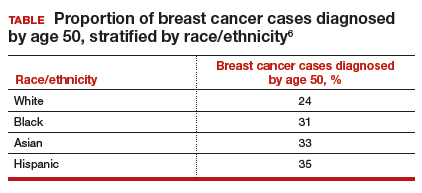
These data suggest that the peak proportion of breast cancer diagnoses in nonwhite women occurs prior to the age of initiation of screening recommended by the USPSTF. Based on these results, Stapleton and colleagues recommend reconsideration of the current USPSTF guidelines to incorporate race/ethnicity–based differences. To diagnose the same proportion of breast cancer cases among nonwhite women as is currently possible among white women at age 50, initiation of breast cancer screening would need to be adjusted to age 47 for black women, age 46 for Hispanic women, and age 47 for Asian women.
Study strengths and weaknesses
This is a unique study that uses the SEER database to capture a large cross section of the American population. The SEER database is a valuable tool because it gathers data from numerous major US metropolitan areas, creating a diverse representative population that minimizes confounding from geographical trends. Nevertheless, any study utilizing a large database is limited by the accuracy and completeness of the data collected at the level of the individual cancer registry. Furthermore, information regarding medical comorbidities and access and adherence to breast cancer screening is lacking in the SEER database; this provides an opportunity for confounding.
Approximately one-third of breast cancer cases in nonwhite women, and one-quarter of cases in white women, occur prior to the age of initiation of screening (50 years) recommended by the USPSTF.
While some screening organizations do recommend that breast cancer screening be initiated prior to age 50, no organizations alter the recommendations for screening based on a woman's race/ethnicity.
Health care providers should be aware that initiation of breast cancer screening at age 50 in nonwhite women misses a disproportionate number of breast cancer cases compared with white women.
Providers should counsel nonwhite women about these differences in age of diagnosis and include that in their consideration of initiating breast cancer screening prior to the age of 50, more in accordance with recommendations of ACOG, NCCN, and ACS.
-- Dana M. Scott, MD, and Mark D. Pearlman, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- Arleo EK, Hendrick RE, Helvie MA, Sickles EA. Comparison of recommendations for screening mammography using CISNET models. Cancer. 2017;123(19):3673–3680.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice Bulletin No. 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1–e16.
- Bevers TB, Anderson BO, Bonaccio E, et al; National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis. J Natl Compr Canc Netw. 2009;7(10):1060–1096.
- US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716–726.
- Stapleton SM, Oseni TO, Bababekov YJ, Hung Y-C, Chang DC. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. Published online March 7, 2018. doi:10.1001/jamasurg.2018.0035.
EXPERT COMMENTARY
Breast cancer screening is an important aspect of women’s preventative health care, with proven mortality benefits.1,2 Different recommendations have been made for the age at initiation and the frequency of breast cancer screening in an effort to maximize benefit while minimizing unnecessary health care costs and harms of screening.
The American College of Obstetricians and Gynecologists (ACOG) and the National Comprehensive Cancer Network (NCCN) recommend initiating mammography screening at age 40, with annual screening (although ACOG offers deferral of screening to age 50 and biennial screening through shared decision making).3,4 The American Cancer Society (ACS) recommends offering annual mammography at ages 40 to 44 and recommends routinely starting annual mammography from 45 to 54, followed by either annual or biennial screening for women 55 and older.1 Finally, the US Preventive Services Task Force (USPSTF) recommends biennial mammography screening starting at age 50.5 No organization alters screening recommendations based on a woman’s race/ethnicity.
Details of the study
Stapleton and colleagues recently performed a retrospective population-based cohort study using the Surveillance, Epidemiology, and End Results (SEER) Program database to evaluate the age and stage at breast cancer diagnosis across different racial groups in the United States.6 The study (timeframe, January 1, 1973 to December 31, 2010) included 747,763 women, with a racial/ethnic distribution of 77.0% white, 9.3% black, 7.0% Hispanic, and 6.2% Asian.
The investigators found 2 distinct age distributions of breast cancer based on race. Among nonwhite women, the highest peak of breast cancer diagnoses occurred between 45 and 50 years (FIGURE). By contrast, breast cancer diagnoses peaked at 60 to 65 years in white women.

Similarly, a higher proportion of nonwhite women were diagnosed with their breast cancer prior to age 50 compared with white women. While one-quarter of white women with breast cancer develop disease prior to age 50, approximately one-third of black, Asian, and Hispanic women with breast cancer will be diagnosed before age 50 (TABLE).

These data suggest that the peak proportion of breast cancer diagnoses in nonwhite women occurs prior to the age of initiation of screening recommended by the USPSTF. Based on these results, Stapleton and colleagues recommend reconsideration of the current USPSTF guidelines to incorporate race/ethnicity–based differences. To diagnose the same proportion of breast cancer cases among nonwhite women as is currently possible among white women at age 50, initiation of breast cancer screening would need to be adjusted to age 47 for black women, age 46 for Hispanic women, and age 47 for Asian women.
Study strengths and weaknesses
This is a unique study that uses the SEER database to capture a large cross section of the American population. The SEER database is a valuable tool because it gathers data from numerous major US metropolitan areas, creating a diverse representative population that minimizes confounding from geographical trends. Nevertheless, any study utilizing a large database is limited by the accuracy and completeness of the data collected at the level of the individual cancer registry. Furthermore, information regarding medical comorbidities and access and adherence to breast cancer screening is lacking in the SEER database; this provides an opportunity for confounding.
Approximately one-third of breast cancer cases in nonwhite women, and one-quarter of cases in white women, occur prior to the age of initiation of screening (50 years) recommended by the USPSTF.
While some screening organizations do recommend that breast cancer screening be initiated prior to age 50, no organizations alter the recommendations for screening based on a woman's race/ethnicity.
Health care providers should be aware that initiation of breast cancer screening at age 50 in nonwhite women misses a disproportionate number of breast cancer cases compared with white women.
Providers should counsel nonwhite women about these differences in age of diagnosis and include that in their consideration of initiating breast cancer screening prior to the age of 50, more in accordance with recommendations of ACOG, NCCN, and ACS.
-- Dana M. Scott, MD, and Mark D. Pearlman, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
Breast cancer screening is an important aspect of women’s preventative health care, with proven mortality benefits.1,2 Different recommendations have been made for the age at initiation and the frequency of breast cancer screening in an effort to maximize benefit while minimizing unnecessary health care costs and harms of screening.
The American College of Obstetricians and Gynecologists (ACOG) and the National Comprehensive Cancer Network (NCCN) recommend initiating mammography screening at age 40, with annual screening (although ACOG offers deferral of screening to age 50 and biennial screening through shared decision making).3,4 The American Cancer Society (ACS) recommends offering annual mammography at ages 40 to 44 and recommends routinely starting annual mammography from 45 to 54, followed by either annual or biennial screening for women 55 and older.1 Finally, the US Preventive Services Task Force (USPSTF) recommends biennial mammography screening starting at age 50.5 No organization alters screening recommendations based on a woman’s race/ethnicity.
Details of the study
Stapleton and colleagues recently performed a retrospective population-based cohort study using the Surveillance, Epidemiology, and End Results (SEER) Program database to evaluate the age and stage at breast cancer diagnosis across different racial groups in the United States.6 The study (timeframe, January 1, 1973 to December 31, 2010) included 747,763 women, with a racial/ethnic distribution of 77.0% white, 9.3% black, 7.0% Hispanic, and 6.2% Asian.
The investigators found 2 distinct age distributions of breast cancer based on race. Among nonwhite women, the highest peak of breast cancer diagnoses occurred between 45 and 50 years (FIGURE). By contrast, breast cancer diagnoses peaked at 60 to 65 years in white women.

Similarly, a higher proportion of nonwhite women were diagnosed with their breast cancer prior to age 50 compared with white women. While one-quarter of white women with breast cancer develop disease prior to age 50, approximately one-third of black, Asian, and Hispanic women with breast cancer will be diagnosed before age 50 (TABLE).

These data suggest that the peak proportion of breast cancer diagnoses in nonwhite women occurs prior to the age of initiation of screening recommended by the USPSTF. Based on these results, Stapleton and colleagues recommend reconsideration of the current USPSTF guidelines to incorporate race/ethnicity–based differences. To diagnose the same proportion of breast cancer cases among nonwhite women as is currently possible among white women at age 50, initiation of breast cancer screening would need to be adjusted to age 47 for black women, age 46 for Hispanic women, and age 47 for Asian women.
Study strengths and weaknesses
This is a unique study that uses the SEER database to capture a large cross section of the American population. The SEER database is a valuable tool because it gathers data from numerous major US metropolitan areas, creating a diverse representative population that minimizes confounding from geographical trends. Nevertheless, any study utilizing a large database is limited by the accuracy and completeness of the data collected at the level of the individual cancer registry. Furthermore, information regarding medical comorbidities and access and adherence to breast cancer screening is lacking in the SEER database; this provides an opportunity for confounding.
Approximately one-third of breast cancer cases in nonwhite women, and one-quarter of cases in white women, occur prior to the age of initiation of screening (50 years) recommended by the USPSTF.
While some screening organizations do recommend that breast cancer screening be initiated prior to age 50, no organizations alter the recommendations for screening based on a woman's race/ethnicity.
Health care providers should be aware that initiation of breast cancer screening at age 50 in nonwhite women misses a disproportionate number of breast cancer cases compared with white women.
Providers should counsel nonwhite women about these differences in age of diagnosis and include that in their consideration of initiating breast cancer screening prior to the age of 50, more in accordance with recommendations of ACOG, NCCN, and ACS.
-- Dana M. Scott, MD, and Mark D. Pearlman, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- Arleo EK, Hendrick RE, Helvie MA, Sickles EA. Comparison of recommendations for screening mammography using CISNET models. Cancer. 2017;123(19):3673–3680.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice Bulletin No. 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1–e16.
- Bevers TB, Anderson BO, Bonaccio E, et al; National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis. J Natl Compr Canc Netw. 2009;7(10):1060–1096.
- US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716–726.
- Stapleton SM, Oseni TO, Bababekov YJ, Hung Y-C, Chang DC. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. Published online March 7, 2018. doi:10.1001/jamasurg.2018.0035.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- Arleo EK, Hendrick RE, Helvie MA, Sickles EA. Comparison of recommendations for screening mammography using CISNET models. Cancer. 2017;123(19):3673–3680.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice Bulletin No. 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1–e16.
- Bevers TB, Anderson BO, Bonaccio E, et al; National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis. J Natl Compr Canc Netw. 2009;7(10):1060–1096.
- US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716–726.
- Stapleton SM, Oseni TO, Bababekov YJ, Hung Y-C, Chang DC. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. Published online March 7, 2018. doi:10.1001/jamasurg.2018.0035.
Inside the complex, surprising world of MS comorbidities
NASHVILLE, Tenn. – Recent research into comorbidities in multiple sclerosis – including head-scratching findings about lower cancer rates – is shedding light on the links between the disease and other illnesses, according to an epidemiologist specializing in MS.
“People should be mindful that if they look at having a positive impact on those comorbidities, they may have the ability to benefit patients in context of their MS,” Helen Tremlett, PhD, said in a video interview at the annual meeting of the Consortium of Multiple Sclerosis Centers. She is the Canada Research Chair in Neuroepidemiology and Multiple Sclerosis at the University of British Columbia, Vancouver.
In recent years, research into comorbidities in MS has risen dramatically. Dr. Tremlett found that the number of papers per year in PubMed that address MS and comorbidity has risen from roughly 30 in 2007 to about 80 in 2015, although the numbers dipped to about 50 and 60, respectively, in 2016 and 2017.
A 2015 systematic review of research into MS and comorbidities reported that while “findings were inconsistent overall,” studies suggested that “meningiomas and possibly urinary system cancers, inflammatory bowel disease, irritable bowel syndrome, epilepsy, depression, anxiety, bipolar disorder, early cataracts, and restless legs syndrome were more common than expected in the MS population.” (Mult Scler. 2015 Mar;21[3]:263-81).
Notably, most cancers are missing from this list. In fact, Dr. Tremlett cowrote a 2012 study that found lower risks of all cancers and several specific types of cancer – breast, lung, colorectal, prostate, and melanoma – in MS patients, compared with age- and gender-matched controls (Brain. 2012 Oct;135[Pt 10]:2973-9).
According to Dr. Tremlett, there are several theories about the apparent lower cancer risk in patients with MS. Perhaps their immune systems are hypervigilant, or maybe MS diagnoses inspire healthier lifestyles.
Researchers have been intrigued by another possibility – that cancer diagnoses are being delayed in patients with MS. Indeed, the 2012 study found that tumor sizes at diagnosis in patients with MS were larger than expected in breast, prostate, lung, and colorectal cancer (P = .04).
“We couldn’t record why that’s the case, but there may be some so-called ‘diagnostic neglect,’ ” she said. “You could imagine a scenario where a typical person with MS goes to see their physician and says, ‘I’m tired. I have fatigue,’ and the physician says, ‘Yes, you have MS, that’s what you should expect.’ Someone in the general population might get additional investigation, get blood work done, and their cancer might be found earlier.”
It’s also possible, she said, that cancer isn’t picked up earlier because it can be difficult to screen people with disabilities. “It’s only recently that physicians can offer the Pap smear to women in a wheelchair.”
On another front, there’s evidence linking comorbidities to worsening MS. A 2018 study coauthored by Dr. Tremlett found that patients with more comorbidities had more disability. Specifically, ischemic heart disease and epilepsy were associated with greater Expanded Disability Status Scale scores (Neurology. 2018 Jan 3. doi: 10.1212/WNL.0000000000004885).
Other research coauthored by Dr. Tremlett has linked comorbidities in MS – specifically, hyperlipidemia, migraine, and three or more comorbidities – to higher risk of MS relapse (Neurology. 2017 Dec 12;89[24]:2455-61).
Dr. Tremlett reported having no relevant disclosures.
NASHVILLE, Tenn. – Recent research into comorbidities in multiple sclerosis – including head-scratching findings about lower cancer rates – is shedding light on the links between the disease and other illnesses, according to an epidemiologist specializing in MS.
“People should be mindful that if they look at having a positive impact on those comorbidities, they may have the ability to benefit patients in context of their MS,” Helen Tremlett, PhD, said in a video interview at the annual meeting of the Consortium of Multiple Sclerosis Centers. She is the Canada Research Chair in Neuroepidemiology and Multiple Sclerosis at the University of British Columbia, Vancouver.
In recent years, research into comorbidities in MS has risen dramatically. Dr. Tremlett found that the number of papers per year in PubMed that address MS and comorbidity has risen from roughly 30 in 2007 to about 80 in 2015, although the numbers dipped to about 50 and 60, respectively, in 2016 and 2017.
A 2015 systematic review of research into MS and comorbidities reported that while “findings were inconsistent overall,” studies suggested that “meningiomas and possibly urinary system cancers, inflammatory bowel disease, irritable bowel syndrome, epilepsy, depression, anxiety, bipolar disorder, early cataracts, and restless legs syndrome were more common than expected in the MS population.” (Mult Scler. 2015 Mar;21[3]:263-81).
Notably, most cancers are missing from this list. In fact, Dr. Tremlett cowrote a 2012 study that found lower risks of all cancers and several specific types of cancer – breast, lung, colorectal, prostate, and melanoma – in MS patients, compared with age- and gender-matched controls (Brain. 2012 Oct;135[Pt 10]:2973-9).
According to Dr. Tremlett, there are several theories about the apparent lower cancer risk in patients with MS. Perhaps their immune systems are hypervigilant, or maybe MS diagnoses inspire healthier lifestyles.
Researchers have been intrigued by another possibility – that cancer diagnoses are being delayed in patients with MS. Indeed, the 2012 study found that tumor sizes at diagnosis in patients with MS were larger than expected in breast, prostate, lung, and colorectal cancer (P = .04).
“We couldn’t record why that’s the case, but there may be some so-called ‘diagnostic neglect,’ ” she said. “You could imagine a scenario where a typical person with MS goes to see their physician and says, ‘I’m tired. I have fatigue,’ and the physician says, ‘Yes, you have MS, that’s what you should expect.’ Someone in the general population might get additional investigation, get blood work done, and their cancer might be found earlier.”
It’s also possible, she said, that cancer isn’t picked up earlier because it can be difficult to screen people with disabilities. “It’s only recently that physicians can offer the Pap smear to women in a wheelchair.”
On another front, there’s evidence linking comorbidities to worsening MS. A 2018 study coauthored by Dr. Tremlett found that patients with more comorbidities had more disability. Specifically, ischemic heart disease and epilepsy were associated with greater Expanded Disability Status Scale scores (Neurology. 2018 Jan 3. doi: 10.1212/WNL.0000000000004885).
Other research coauthored by Dr. Tremlett has linked comorbidities in MS – specifically, hyperlipidemia, migraine, and three or more comorbidities – to higher risk of MS relapse (Neurology. 2017 Dec 12;89[24]:2455-61).
Dr. Tremlett reported having no relevant disclosures.
NASHVILLE, Tenn. – Recent research into comorbidities in multiple sclerosis – including head-scratching findings about lower cancer rates – is shedding light on the links between the disease and other illnesses, according to an epidemiologist specializing in MS.
“People should be mindful that if they look at having a positive impact on those comorbidities, they may have the ability to benefit patients in context of their MS,” Helen Tremlett, PhD, said in a video interview at the annual meeting of the Consortium of Multiple Sclerosis Centers. She is the Canada Research Chair in Neuroepidemiology and Multiple Sclerosis at the University of British Columbia, Vancouver.
In recent years, research into comorbidities in MS has risen dramatically. Dr. Tremlett found that the number of papers per year in PubMed that address MS and comorbidity has risen from roughly 30 in 2007 to about 80 in 2015, although the numbers dipped to about 50 and 60, respectively, in 2016 and 2017.
A 2015 systematic review of research into MS and comorbidities reported that while “findings were inconsistent overall,” studies suggested that “meningiomas and possibly urinary system cancers, inflammatory bowel disease, irritable bowel syndrome, epilepsy, depression, anxiety, bipolar disorder, early cataracts, and restless legs syndrome were more common than expected in the MS population.” (Mult Scler. 2015 Mar;21[3]:263-81).
Notably, most cancers are missing from this list. In fact, Dr. Tremlett cowrote a 2012 study that found lower risks of all cancers and several specific types of cancer – breast, lung, colorectal, prostate, and melanoma – in MS patients, compared with age- and gender-matched controls (Brain. 2012 Oct;135[Pt 10]:2973-9).
According to Dr. Tremlett, there are several theories about the apparent lower cancer risk in patients with MS. Perhaps their immune systems are hypervigilant, or maybe MS diagnoses inspire healthier lifestyles.
Researchers have been intrigued by another possibility – that cancer diagnoses are being delayed in patients with MS. Indeed, the 2012 study found that tumor sizes at diagnosis in patients with MS were larger than expected in breast, prostate, lung, and colorectal cancer (P = .04).
“We couldn’t record why that’s the case, but there may be some so-called ‘diagnostic neglect,’ ” she said. “You could imagine a scenario where a typical person with MS goes to see their physician and says, ‘I’m tired. I have fatigue,’ and the physician says, ‘Yes, you have MS, that’s what you should expect.’ Someone in the general population might get additional investigation, get blood work done, and their cancer might be found earlier.”
It’s also possible, she said, that cancer isn’t picked up earlier because it can be difficult to screen people with disabilities. “It’s only recently that physicians can offer the Pap smear to women in a wheelchair.”
On another front, there’s evidence linking comorbidities to worsening MS. A 2018 study coauthored by Dr. Tremlett found that patients with more comorbidities had more disability. Specifically, ischemic heart disease and epilepsy were associated with greater Expanded Disability Status Scale scores (Neurology. 2018 Jan 3. doi: 10.1212/WNL.0000000000004885).
Other research coauthored by Dr. Tremlett has linked comorbidities in MS – specifically, hyperlipidemia, migraine, and three or more comorbidities – to higher risk of MS relapse (Neurology. 2017 Dec 12;89[24]:2455-61).
Dr. Tremlett reported having no relevant disclosures.
EXPERT ANALYSIS FROM THE CMSC ANNUAL MEETING
Teaching hospitals order more lab testing for certain conditions
Clinical question: Is there a difference in the ordering of laboratory tests between teaching and nonteaching hospitals?
Background: There is a general impression that trainees at teaching hospitals order more unnecessary laboratory testing, compared with those at nonteaching hospitals, but there are not enough data to support this generalization. In addition, there may be factors at teaching hospitals that influence these results.
Study design: Cross-sectional study.
Setting: Teaching and nonteaching hospitals.
Synopsis: Investigators used the Texas Inpatient Public Use Data file to examine hospital discharges from both teaching and nonteaching hospitals with a discharge diagnosis of cellulitis or pneumonia. There were a greater number of laboratory tests ordered at teaching hospitals, compared with nonteaching hospitals. For pneumonia, there were an additional 3.6 tests ordered per day, and for cellulitis, there were an additional 2.6 tests ordered per day. This finding was statistically significant, even when adjusted for illness severity, length of stay, and patient demographics.
This study did not account for confounding diagnoses that may have influenced ordering or the prescribing habits of different practitioner groups (such as residents, attending physicians, or advanced practice providers).
Bottom line: There is an increase in laboratory test orders in teaching versus nonteaching hospitals for pneumonia and cellulitis.
Citation: Valencia V et al. A comparison of laboratory testing in teaching vs nonteaching hospitals for 2 common medical conditions. JAMA Intern Med. 2018 Jan 1;178(1):39-47.
Dr. Shaffie is a hospitalist at Denver Health Medical Center and an assistant professor of medicine at the University of Colorado at Denver, Aurora.
Clinical question: Is there a difference in the ordering of laboratory tests between teaching and nonteaching hospitals?
Background: There is a general impression that trainees at teaching hospitals order more unnecessary laboratory testing, compared with those at nonteaching hospitals, but there are not enough data to support this generalization. In addition, there may be factors at teaching hospitals that influence these results.
Study design: Cross-sectional study.
Setting: Teaching and nonteaching hospitals.
Synopsis: Investigators used the Texas Inpatient Public Use Data file to examine hospital discharges from both teaching and nonteaching hospitals with a discharge diagnosis of cellulitis or pneumonia. There were a greater number of laboratory tests ordered at teaching hospitals, compared with nonteaching hospitals. For pneumonia, there were an additional 3.6 tests ordered per day, and for cellulitis, there were an additional 2.6 tests ordered per day. This finding was statistically significant, even when adjusted for illness severity, length of stay, and patient demographics.
This study did not account for confounding diagnoses that may have influenced ordering or the prescribing habits of different practitioner groups (such as residents, attending physicians, or advanced practice providers).
Bottom line: There is an increase in laboratory test orders in teaching versus nonteaching hospitals for pneumonia and cellulitis.
Citation: Valencia V et al. A comparison of laboratory testing in teaching vs nonteaching hospitals for 2 common medical conditions. JAMA Intern Med. 2018 Jan 1;178(1):39-47.
Dr. Shaffie is a hospitalist at Denver Health Medical Center and an assistant professor of medicine at the University of Colorado at Denver, Aurora.
Clinical question: Is there a difference in the ordering of laboratory tests between teaching and nonteaching hospitals?
Background: There is a general impression that trainees at teaching hospitals order more unnecessary laboratory testing, compared with those at nonteaching hospitals, but there are not enough data to support this generalization. In addition, there may be factors at teaching hospitals that influence these results.
Study design: Cross-sectional study.
Setting: Teaching and nonteaching hospitals.
Synopsis: Investigators used the Texas Inpatient Public Use Data file to examine hospital discharges from both teaching and nonteaching hospitals with a discharge diagnosis of cellulitis or pneumonia. There were a greater number of laboratory tests ordered at teaching hospitals, compared with nonteaching hospitals. For pneumonia, there were an additional 3.6 tests ordered per day, and for cellulitis, there were an additional 2.6 tests ordered per day. This finding was statistically significant, even when adjusted for illness severity, length of stay, and patient demographics.
This study did not account for confounding diagnoses that may have influenced ordering or the prescribing habits of different practitioner groups (such as residents, attending physicians, or advanced practice providers).
Bottom line: There is an increase in laboratory test orders in teaching versus nonteaching hospitals for pneumonia and cellulitis.
Citation: Valencia V et al. A comparison of laboratory testing in teaching vs nonteaching hospitals for 2 common medical conditions. JAMA Intern Med. 2018 Jan 1;178(1):39-47.
Dr. Shaffie is a hospitalist at Denver Health Medical Center and an assistant professor of medicine at the University of Colorado at Denver, Aurora.
Delivering bad news in obstetric practice
Obstetrics is a field filled with joyful experiences highlighted by pregnancy, childbirth, and the growth of healthy families. The field is also filled with many experiences that are sorrowful, including failure to conceive after infertility treatment, miscarriage, ultrasound-detected fetal anomalies, fetuses with genetic problems, fetal and neonatal demise, extremely premature birth, and birth injury. For decades oncologists have evolved their approach to discussing bad news with cancer patients. In the distant past, oncologists often kept a cancer diagnosis from the patient, preferring to spare them the stress of the news. In the modern era of transparency, however, oncologists now uniformly strive to keep patients informed of their situation and have adopted structured approaches to delivering bad news. An adverse pregnancy outcome such as a miscarriage or fetal loss may trigger emotional responses as intense as those experienced by a person hearing about a cancer diagnosis. Women who have recently experienced a miscarriage report emotional responses ranging from “a little disappointed” to “in shock” and “for it to be taken away was crushing.”1 As obstetricians, we can advance our practice by adopting a structured approach to delivering bad news, building on the lessons from cancer medicine. Improving the quality of our communication about adverse pregnancy events will reduce emotional distress and enable patients and families to more effectively cope with challenging situations.

Communicating bad news: The facts, the emotional response, and the impact on identity
Clinicians need to be cognizant that a conversation about bad news is 3 interwoven conversations that involve facts, emotional responses, and an altered self-identity. In addition to communicating the facts of the event in clear language, clinicians need to simultaneously monitor and manage the emotional responses to the adverse event and the impact on the participants’ sense of self.2 Clinicians are steeped in scientific tradition and method, and as experts we are naturally drawn to a discussion of the facts.
However, a discussion about bad news is highly likely to trigger an emotional response in the patient and the clinician. For example, when a clinician tells the patient about delivery events that resulted in an unexpected newborn injury, the patient may become angry and the clinician may be fearful, anxious, and defensive. Managing the emotions of all participants in the conversation is important for an optimal outcome.
An adverse event also may cause those involved to think about their self-identity. A key feature of bad news is that it alters patients’ expectations about their future, juxtaposing the reality of their outcome with the preferable outcome that may have been. Following a stillbirth during her first pregnancy the patient may be wondering, “Will I ever be a mother?”, “Did I cause the loss?”, and “Does all life end in death?” A traumatic event also may impact the self-identity of the clinician. Following a delivery where the newborn was injured, the clinician may be wondering, “Am I a good or bad clinician?”, “Did I do something wrong?”, “Is it time for me to retire from obstetrical practice?”
Following an adverse pregnancy outcome some patients are consumed with intense grief. This may require the patient and her family to move through a series of emotions (similar to those who receive a new diagnosis of cancer), including denial, anger, bargaining, depression, and acceptance.
Responses to grief
Kubler-Ross identified these 5 psychological coping mechanisms that are often used by people experiencing grief: denial, anger, bargaining, depression, and acceptance.3 The goal of the clinician is to help grieving patients move through these stages in an appropriate fashion and not get stuck in the stages of denial, anger, and/or depression. Following a difficult pregnancy some patients and their family members become stuck in a state dominated by anger, rage, and resentment. This is fertile ground for the growth of a professional liability case. Denial and anger are adaptive short-term defenses to protecting self-identity. In time, most people engage in more constructive responses, accept the adverse event, and plan for the future. Kubler-Ross observed that hope helps people survive through a time of great suffering and is present throughout the response to grief. Clinicians can play an important role in ensuring that a flame of hope is kept burning throughout the process of responding to and grieving bad outcomes.
A structured approach to delivering bad news: SPIKES
Dr. Robert Buckman, an oncologist, has proposed using a structured approach, SPIKES, to guide conversations focused on delivering bad news.4–6 SPIKES is focused on trying to deeply understand the patient’s level of knowledge, emotions, and perspective before providing medical information and support. SPIKES consists of 6 key steps.
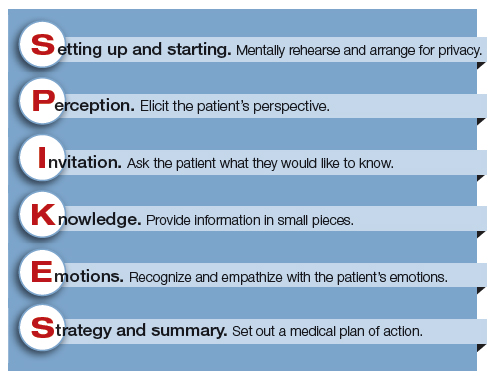
1. Setting up and starting. Mentally rehearse and arrange for privacy. Make sure the patient’s support people are present. Sit down, use open body language, eye contact, and/or touch to make a connection with the patient. Create room for a dialogue by using open-ended questions, silent pauses, listening first, and encouraging the patient to provide their perspective.
2. Perception. Elicit the patient’s perspective. Assess what the patient believes and feels. Assess vocabulary and comprehension.
3. Invitation. Ask the patient what they would like to know. Obtain permission to share knowledge.
4. Knowledge. Provide information in small pieces, always checking back on the patient’s understanding. Use plain language that aligns with the patient’s vocabulary and understanding.
5. Emotions. Explore, explicitly recognize, and empathize with the patient’s emotions.
6. Strategy and summary. Set out a medical plan of action. Express a commitment to be available for the patient as she embarks on the care plan. Arrange for a follow-up conversation.
Some studies have indicated that having a protocol such as SPIKES for delivering bad news helps clinicians to navigate this challenging process, which in turn improves patient satisfaction with disclosure.7 Simulation training focused on communicating bad news could be better utilized to help clinicians practice this skill, similar to the simulation exercises used to practice common clinical problems like hemorrhage and shoulder dystocia.8,9
Physician responses to bad outcomes
Over a career in clinical practice, physicians experience many bad outcomes that expose them to the contagion of sadness and grief. Despite this vicarious trauma, they must always present a professional persona, placing the patient’s needs above their own pain. Due to these experiences, clinicians may become isolated, depressed, and burned out. Drs. Michael and Enid Balint recognized the adverse effect of a lifetime of exposure to suffering and pain. They proposed that physicians could mitigate the trauma of these experiences by participating in small group meetings with a trained leader to discuss their most difficult clinical experiences in a confidential and supportive environment.10,11 By sharing clinical experiences, feelings, and stories with trusted colleagues, physicians can channel painful experiences into a greater understanding of the empathy and compassion needed to care for themselves, their colleagues, and patients. Clinical practice is invariably punctuated by occasional adverse outcomes necessitating that we effectively manage the process of delivering bad news, simultaneously caring for ourselves and our patients.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Flink-Bochacki R, Hamm ME, Borrero S, Chen BA, Achilles SL, Chang JC. Family planning and counseling desires of women who have experienced miscarriage. Obstet Gynecol. 2018;131(4):625-631.
- Stone D, Patton B, Heen S. Difficult conversations. How to discuss what matters most. Penguin Books: New York, NY; 1999:9-10.
- Kubler-Ross E. On death and dying. MacMillan: New York, NY; 1969.
- Buckman R. How to break bad news. A guide for health care professionals. The Johns Hopkins University Press: Baltimore, MD; 1992.
- Buckman R. Practical plans for difficult conversations in medicine. Strategies that work in breaking bad news. The Johns Hopkins University Press: Baltimore, MD; 2010.
- Baile WF, Buckman R, Lenzi R, Glober G, Beale EA, Kudelka AP. SPIKES-a six-step protocol for delivering bad news: application to the patient with cancer. Oncologist. 2000;5(4):302-311.
- Fallowfield L, Jenkins V. Communicating sad, bad, and difficult news in medicine. Lancet. 2004;363(9405):312-319.
- Colletti L, Gruppen L, Barclay M, Stern D. Teaching students to break bad news. Am J Surg. 2001;182(1):20-23.
- Rosenbaum ME, Ferguson KJ, Lobas JG. Teaching medical students and residents skills for delivering bad news: a review of strategies. Acad Med. 2004;79(2):107-117.
- Balint M. The doctor, his patient and the illness. Pitman: London, England; 1957.
- Salinksky J. Balint groups and the Balint method. The Balint Society website. https://balint.co.uk/about/the-balint-method/. Accessed May 17, 2018.
Obstetrics is a field filled with joyful experiences highlighted by pregnancy, childbirth, and the growth of healthy families. The field is also filled with many experiences that are sorrowful, including failure to conceive after infertility treatment, miscarriage, ultrasound-detected fetal anomalies, fetuses with genetic problems, fetal and neonatal demise, extremely premature birth, and birth injury. For decades oncologists have evolved their approach to discussing bad news with cancer patients. In the distant past, oncologists often kept a cancer diagnosis from the patient, preferring to spare them the stress of the news. In the modern era of transparency, however, oncologists now uniformly strive to keep patients informed of their situation and have adopted structured approaches to delivering bad news. An adverse pregnancy outcome such as a miscarriage or fetal loss may trigger emotional responses as intense as those experienced by a person hearing about a cancer diagnosis. Women who have recently experienced a miscarriage report emotional responses ranging from “a little disappointed” to “in shock” and “for it to be taken away was crushing.”1 As obstetricians, we can advance our practice by adopting a structured approach to delivering bad news, building on the lessons from cancer medicine. Improving the quality of our communication about adverse pregnancy events will reduce emotional distress and enable patients and families to more effectively cope with challenging situations.

Communicating bad news: The facts, the emotional response, and the impact on identity
Clinicians need to be cognizant that a conversation about bad news is 3 interwoven conversations that involve facts, emotional responses, and an altered self-identity. In addition to communicating the facts of the event in clear language, clinicians need to simultaneously monitor and manage the emotional responses to the adverse event and the impact on the participants’ sense of self.2 Clinicians are steeped in scientific tradition and method, and as experts we are naturally drawn to a discussion of the facts.
However, a discussion about bad news is highly likely to trigger an emotional response in the patient and the clinician. For example, when a clinician tells the patient about delivery events that resulted in an unexpected newborn injury, the patient may become angry and the clinician may be fearful, anxious, and defensive. Managing the emotions of all participants in the conversation is important for an optimal outcome.
An adverse event also may cause those involved to think about their self-identity. A key feature of bad news is that it alters patients’ expectations about their future, juxtaposing the reality of their outcome with the preferable outcome that may have been. Following a stillbirth during her first pregnancy the patient may be wondering, “Will I ever be a mother?”, “Did I cause the loss?”, and “Does all life end in death?” A traumatic event also may impact the self-identity of the clinician. Following a delivery where the newborn was injured, the clinician may be wondering, “Am I a good or bad clinician?”, “Did I do something wrong?”, “Is it time for me to retire from obstetrical practice?”
Following an adverse pregnancy outcome some patients are consumed with intense grief. This may require the patient and her family to move through a series of emotions (similar to those who receive a new diagnosis of cancer), including denial, anger, bargaining, depression, and acceptance.
Responses to grief
Kubler-Ross identified these 5 psychological coping mechanisms that are often used by people experiencing grief: denial, anger, bargaining, depression, and acceptance.3 The goal of the clinician is to help grieving patients move through these stages in an appropriate fashion and not get stuck in the stages of denial, anger, and/or depression. Following a difficult pregnancy some patients and their family members become stuck in a state dominated by anger, rage, and resentment. This is fertile ground for the growth of a professional liability case. Denial and anger are adaptive short-term defenses to protecting self-identity. In time, most people engage in more constructive responses, accept the adverse event, and plan for the future. Kubler-Ross observed that hope helps people survive through a time of great suffering and is present throughout the response to grief. Clinicians can play an important role in ensuring that a flame of hope is kept burning throughout the process of responding to and grieving bad outcomes.
A structured approach to delivering bad news: SPIKES
Dr. Robert Buckman, an oncologist, has proposed using a structured approach, SPIKES, to guide conversations focused on delivering bad news.4–6 SPIKES is focused on trying to deeply understand the patient’s level of knowledge, emotions, and perspective before providing medical information and support. SPIKES consists of 6 key steps.

1. Setting up and starting. Mentally rehearse and arrange for privacy. Make sure the patient’s support people are present. Sit down, use open body language, eye contact, and/or touch to make a connection with the patient. Create room for a dialogue by using open-ended questions, silent pauses, listening first, and encouraging the patient to provide their perspective.
2. Perception. Elicit the patient’s perspective. Assess what the patient believes and feels. Assess vocabulary and comprehension.
3. Invitation. Ask the patient what they would like to know. Obtain permission to share knowledge.
4. Knowledge. Provide information in small pieces, always checking back on the patient’s understanding. Use plain language that aligns with the patient’s vocabulary and understanding.
5. Emotions. Explore, explicitly recognize, and empathize with the patient’s emotions.
6. Strategy and summary. Set out a medical plan of action. Express a commitment to be available for the patient as she embarks on the care plan. Arrange for a follow-up conversation.
Some studies have indicated that having a protocol such as SPIKES for delivering bad news helps clinicians to navigate this challenging process, which in turn improves patient satisfaction with disclosure.7 Simulation training focused on communicating bad news could be better utilized to help clinicians practice this skill, similar to the simulation exercises used to practice common clinical problems like hemorrhage and shoulder dystocia.8,9
Physician responses to bad outcomes
Over a career in clinical practice, physicians experience many bad outcomes that expose them to the contagion of sadness and grief. Despite this vicarious trauma, they must always present a professional persona, placing the patient’s needs above their own pain. Due to these experiences, clinicians may become isolated, depressed, and burned out. Drs. Michael and Enid Balint recognized the adverse effect of a lifetime of exposure to suffering and pain. They proposed that physicians could mitigate the trauma of these experiences by participating in small group meetings with a trained leader to discuss their most difficult clinical experiences in a confidential and supportive environment.10,11 By sharing clinical experiences, feelings, and stories with trusted colleagues, physicians can channel painful experiences into a greater understanding of the empathy and compassion needed to care for themselves, their colleagues, and patients. Clinical practice is invariably punctuated by occasional adverse outcomes necessitating that we effectively manage the process of delivering bad news, simultaneously caring for ourselves and our patients.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Obstetrics is a field filled with joyful experiences highlighted by pregnancy, childbirth, and the growth of healthy families. The field is also filled with many experiences that are sorrowful, including failure to conceive after infertility treatment, miscarriage, ultrasound-detected fetal anomalies, fetuses with genetic problems, fetal and neonatal demise, extremely premature birth, and birth injury. For decades oncologists have evolved their approach to discussing bad news with cancer patients. In the distant past, oncologists often kept a cancer diagnosis from the patient, preferring to spare them the stress of the news. In the modern era of transparency, however, oncologists now uniformly strive to keep patients informed of their situation and have adopted structured approaches to delivering bad news. An adverse pregnancy outcome such as a miscarriage or fetal loss may trigger emotional responses as intense as those experienced by a person hearing about a cancer diagnosis. Women who have recently experienced a miscarriage report emotional responses ranging from “a little disappointed” to “in shock” and “for it to be taken away was crushing.”1 As obstetricians, we can advance our practice by adopting a structured approach to delivering bad news, building on the lessons from cancer medicine. Improving the quality of our communication about adverse pregnancy events will reduce emotional distress and enable patients and families to more effectively cope with challenging situations.

Communicating bad news: The facts, the emotional response, and the impact on identity
Clinicians need to be cognizant that a conversation about bad news is 3 interwoven conversations that involve facts, emotional responses, and an altered self-identity. In addition to communicating the facts of the event in clear language, clinicians need to simultaneously monitor and manage the emotional responses to the adverse event and the impact on the participants’ sense of self.2 Clinicians are steeped in scientific tradition and method, and as experts we are naturally drawn to a discussion of the facts.
However, a discussion about bad news is highly likely to trigger an emotional response in the patient and the clinician. For example, when a clinician tells the patient about delivery events that resulted in an unexpected newborn injury, the patient may become angry and the clinician may be fearful, anxious, and defensive. Managing the emotions of all participants in the conversation is important for an optimal outcome.
An adverse event also may cause those involved to think about their self-identity. A key feature of bad news is that it alters patients’ expectations about their future, juxtaposing the reality of their outcome with the preferable outcome that may have been. Following a stillbirth during her first pregnancy the patient may be wondering, “Will I ever be a mother?”, “Did I cause the loss?”, and “Does all life end in death?” A traumatic event also may impact the self-identity of the clinician. Following a delivery where the newborn was injured, the clinician may be wondering, “Am I a good or bad clinician?”, “Did I do something wrong?”, “Is it time for me to retire from obstetrical practice?”
Following an adverse pregnancy outcome some patients are consumed with intense grief. This may require the patient and her family to move through a series of emotions (similar to those who receive a new diagnosis of cancer), including denial, anger, bargaining, depression, and acceptance.
Responses to grief
Kubler-Ross identified these 5 psychological coping mechanisms that are often used by people experiencing grief: denial, anger, bargaining, depression, and acceptance.3 The goal of the clinician is to help grieving patients move through these stages in an appropriate fashion and not get stuck in the stages of denial, anger, and/or depression. Following a difficult pregnancy some patients and their family members become stuck in a state dominated by anger, rage, and resentment. This is fertile ground for the growth of a professional liability case. Denial and anger are adaptive short-term defenses to protecting self-identity. In time, most people engage in more constructive responses, accept the adverse event, and plan for the future. Kubler-Ross observed that hope helps people survive through a time of great suffering and is present throughout the response to grief. Clinicians can play an important role in ensuring that a flame of hope is kept burning throughout the process of responding to and grieving bad outcomes.
A structured approach to delivering bad news: SPIKES
Dr. Robert Buckman, an oncologist, has proposed using a structured approach, SPIKES, to guide conversations focused on delivering bad news.4–6 SPIKES is focused on trying to deeply understand the patient’s level of knowledge, emotions, and perspective before providing medical information and support. SPIKES consists of 6 key steps.

1. Setting up and starting. Mentally rehearse and arrange for privacy. Make sure the patient’s support people are present. Sit down, use open body language, eye contact, and/or touch to make a connection with the patient. Create room for a dialogue by using open-ended questions, silent pauses, listening first, and encouraging the patient to provide their perspective.
2. Perception. Elicit the patient’s perspective. Assess what the patient believes and feels. Assess vocabulary and comprehension.
3. Invitation. Ask the patient what they would like to know. Obtain permission to share knowledge.
4. Knowledge. Provide information in small pieces, always checking back on the patient’s understanding. Use plain language that aligns with the patient’s vocabulary and understanding.
5. Emotions. Explore, explicitly recognize, and empathize with the patient’s emotions.
6. Strategy and summary. Set out a medical plan of action. Express a commitment to be available for the patient as she embarks on the care plan. Arrange for a follow-up conversation.
Some studies have indicated that having a protocol such as SPIKES for delivering bad news helps clinicians to navigate this challenging process, which in turn improves patient satisfaction with disclosure.7 Simulation training focused on communicating bad news could be better utilized to help clinicians practice this skill, similar to the simulation exercises used to practice common clinical problems like hemorrhage and shoulder dystocia.8,9
Physician responses to bad outcomes
Over a career in clinical practice, physicians experience many bad outcomes that expose them to the contagion of sadness and grief. Despite this vicarious trauma, they must always present a professional persona, placing the patient’s needs above their own pain. Due to these experiences, clinicians may become isolated, depressed, and burned out. Drs. Michael and Enid Balint recognized the adverse effect of a lifetime of exposure to suffering and pain. They proposed that physicians could mitigate the trauma of these experiences by participating in small group meetings with a trained leader to discuss their most difficult clinical experiences in a confidential and supportive environment.10,11 By sharing clinical experiences, feelings, and stories with trusted colleagues, physicians can channel painful experiences into a greater understanding of the empathy and compassion needed to care for themselves, their colleagues, and patients. Clinical practice is invariably punctuated by occasional adverse outcomes necessitating that we effectively manage the process of delivering bad news, simultaneously caring for ourselves and our patients.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Flink-Bochacki R, Hamm ME, Borrero S, Chen BA, Achilles SL, Chang JC. Family planning and counseling desires of women who have experienced miscarriage. Obstet Gynecol. 2018;131(4):625-631.
- Stone D, Patton B, Heen S. Difficult conversations. How to discuss what matters most. Penguin Books: New York, NY; 1999:9-10.
- Kubler-Ross E. On death and dying. MacMillan: New York, NY; 1969.
- Buckman R. How to break bad news. A guide for health care professionals. The Johns Hopkins University Press: Baltimore, MD; 1992.
- Buckman R. Practical plans for difficult conversations in medicine. Strategies that work in breaking bad news. The Johns Hopkins University Press: Baltimore, MD; 2010.
- Baile WF, Buckman R, Lenzi R, Glober G, Beale EA, Kudelka AP. SPIKES-a six-step protocol for delivering bad news: application to the patient with cancer. Oncologist. 2000;5(4):302-311.
- Fallowfield L, Jenkins V. Communicating sad, bad, and difficult news in medicine. Lancet. 2004;363(9405):312-319.
- Colletti L, Gruppen L, Barclay M, Stern D. Teaching students to break bad news. Am J Surg. 2001;182(1):20-23.
- Rosenbaum ME, Ferguson KJ, Lobas JG. Teaching medical students and residents skills for delivering bad news: a review of strategies. Acad Med. 2004;79(2):107-117.
- Balint M. The doctor, his patient and the illness. Pitman: London, England; 1957.
- Salinksky J. Balint groups and the Balint method. The Balint Society website. https://balint.co.uk/about/the-balint-method/. Accessed May 17, 2018.
- Flink-Bochacki R, Hamm ME, Borrero S, Chen BA, Achilles SL, Chang JC. Family planning and counseling desires of women who have experienced miscarriage. Obstet Gynecol. 2018;131(4):625-631.
- Stone D, Patton B, Heen S. Difficult conversations. How to discuss what matters most. Penguin Books: New York, NY; 1999:9-10.
- Kubler-Ross E. On death and dying. MacMillan: New York, NY; 1969.
- Buckman R. How to break bad news. A guide for health care professionals. The Johns Hopkins University Press: Baltimore, MD; 1992.
- Buckman R. Practical plans for difficult conversations in medicine. Strategies that work in breaking bad news. The Johns Hopkins University Press: Baltimore, MD; 2010.
- Baile WF, Buckman R, Lenzi R, Glober G, Beale EA, Kudelka AP. SPIKES-a six-step protocol for delivering bad news: application to the patient with cancer. Oncologist. 2000;5(4):302-311.
- Fallowfield L, Jenkins V. Communicating sad, bad, and difficult news in medicine. Lancet. 2004;363(9405):312-319.
- Colletti L, Gruppen L, Barclay M, Stern D. Teaching students to break bad news. Am J Surg. 2001;182(1):20-23.
- Rosenbaum ME, Ferguson KJ, Lobas JG. Teaching medical students and residents skills for delivering bad news: a review of strategies. Acad Med. 2004;79(2):107-117.
- Balint M. The doctor, his patient and the illness. Pitman: London, England; 1957.
- Salinksky J. Balint groups and the Balint method. The Balint Society website. https://balint.co.uk/about/the-balint-method/. Accessed May 17, 2018.
Value-based care spawns new challenges for MS physicians
NASHVILLE, TENN. – Jeffrey B. English, MD, of the MS Center of Atlanta, knows which quality measures physicians and their patients with multiple sclerosis think are important. After all, he and his colleagues have surveyed them about that very topic.
But he has little time to monitor these measures since he’s too busy with a more overwhelming task: keeping track of unrelated quality measures as required by the federal government.
“When they developed quality measures under the MACRA law, they were not thinking about MS people in general. They were very primary care based,” Dr. English said in an interview at the annual meeting of the Consortium of Multiple Sclerosis Centers.
In terms of MS, he said, “no one really knows what the correct outcome measures are.”
Dr. English knows more than most about quality measures preferred by neurologists and patients. At the annual CMSC meeting last year, he presented results from a survey of 11 physicians and 423 patients about the measures of care they consider most important. The patient survey asked about several measures recommended by the American Academy of Neurology plus other measures recommended by the physicians.
The two groups – physicians and patients – agreed on the top four measures: change observed via MRI, change observed via exam, quality of life, and fatigue. However, they disagreed on the ranking within the top four spots.
The least important measures for patients were exercise levels, depression, medication compliance, and relapses.
Dr. English wants to “be able to follow what the patients want me to follow.” However, he hasn’t been able to do so since “25% of my time with patients, in between patients and after hours, is spent trying to comply with outcome measures from the new health care system that are of no benefit to the patient,” he said.
He’s referring to the quality measures that many physicians are tracking to get reimbursed by Medicare and Medicaid.
Value-based care posts other challenges for MS physicians, he said, since MS care is especially expensive. Accountable Care Organizations are looking at cost savings in closed systems, he said, and that could spell trouble because patients with MS cost more.
As a 2015 report noted, first-generation disease-modifying therapies (DMTs) for MS cost about $60,000, and “costs for these agents have increased annually at rates 5 to 7 times higher than prescription drug inflation. Newer DMTs commonly entered the market with a cost 25%-60% higher than existing DMTs” (Neurology. 2015 May 26;84[21]:2185-92).
“If I’m in an ACO, and I’m taking care of a lot of MS patients, I’ll already lose money for the accountable care system,” Dr. English said. “They may not necessarily want an MS center inside an ACO.”
What can doctors do? “Advocacy efforts are pretty difficult for physicians,” Dr. English said. “Our hope is that the CMSC will be a clearinghouse for doctors who have ideas and efforts and advocacy, and somehow channel that into the actual provision of care. You have people advocating for medications and for research and for patients, but there’s nobody advocating for the actual care that’s going on, the boots-on-the-ground care. That’s where CMSC should play a big role.”
Dr. English disclosed that he has served as a consultant for multiple pharmaceutical companies.
NASHVILLE, TENN. – Jeffrey B. English, MD, of the MS Center of Atlanta, knows which quality measures physicians and their patients with multiple sclerosis think are important. After all, he and his colleagues have surveyed them about that very topic.
But he has little time to monitor these measures since he’s too busy with a more overwhelming task: keeping track of unrelated quality measures as required by the federal government.
“When they developed quality measures under the MACRA law, they were not thinking about MS people in general. They were very primary care based,” Dr. English said in an interview at the annual meeting of the Consortium of Multiple Sclerosis Centers.
In terms of MS, he said, “no one really knows what the correct outcome measures are.”
Dr. English knows more than most about quality measures preferred by neurologists and patients. At the annual CMSC meeting last year, he presented results from a survey of 11 physicians and 423 patients about the measures of care they consider most important. The patient survey asked about several measures recommended by the American Academy of Neurology plus other measures recommended by the physicians.
The two groups – physicians and patients – agreed on the top four measures: change observed via MRI, change observed via exam, quality of life, and fatigue. However, they disagreed on the ranking within the top four spots.
The least important measures for patients were exercise levels, depression, medication compliance, and relapses.
Dr. English wants to “be able to follow what the patients want me to follow.” However, he hasn’t been able to do so since “25% of my time with patients, in between patients and after hours, is spent trying to comply with outcome measures from the new health care system that are of no benefit to the patient,” he said.
He’s referring to the quality measures that many physicians are tracking to get reimbursed by Medicare and Medicaid.
Value-based care posts other challenges for MS physicians, he said, since MS care is especially expensive. Accountable Care Organizations are looking at cost savings in closed systems, he said, and that could spell trouble because patients with MS cost more.
As a 2015 report noted, first-generation disease-modifying therapies (DMTs) for MS cost about $60,000, and “costs for these agents have increased annually at rates 5 to 7 times higher than prescription drug inflation. Newer DMTs commonly entered the market with a cost 25%-60% higher than existing DMTs” (Neurology. 2015 May 26;84[21]:2185-92).
“If I’m in an ACO, and I’m taking care of a lot of MS patients, I’ll already lose money for the accountable care system,” Dr. English said. “They may not necessarily want an MS center inside an ACO.”
What can doctors do? “Advocacy efforts are pretty difficult for physicians,” Dr. English said. “Our hope is that the CMSC will be a clearinghouse for doctors who have ideas and efforts and advocacy, and somehow channel that into the actual provision of care. You have people advocating for medications and for research and for patients, but there’s nobody advocating for the actual care that’s going on, the boots-on-the-ground care. That’s where CMSC should play a big role.”
Dr. English disclosed that he has served as a consultant for multiple pharmaceutical companies.
NASHVILLE, TENN. – Jeffrey B. English, MD, of the MS Center of Atlanta, knows which quality measures physicians and their patients with multiple sclerosis think are important. After all, he and his colleagues have surveyed them about that very topic.
But he has little time to monitor these measures since he’s too busy with a more overwhelming task: keeping track of unrelated quality measures as required by the federal government.
“When they developed quality measures under the MACRA law, they were not thinking about MS people in general. They were very primary care based,” Dr. English said in an interview at the annual meeting of the Consortium of Multiple Sclerosis Centers.
In terms of MS, he said, “no one really knows what the correct outcome measures are.”
Dr. English knows more than most about quality measures preferred by neurologists and patients. At the annual CMSC meeting last year, he presented results from a survey of 11 physicians and 423 patients about the measures of care they consider most important. The patient survey asked about several measures recommended by the American Academy of Neurology plus other measures recommended by the physicians.
The two groups – physicians and patients – agreed on the top four measures: change observed via MRI, change observed via exam, quality of life, and fatigue. However, they disagreed on the ranking within the top four spots.
The least important measures for patients were exercise levels, depression, medication compliance, and relapses.
Dr. English wants to “be able to follow what the patients want me to follow.” However, he hasn’t been able to do so since “25% of my time with patients, in between patients and after hours, is spent trying to comply with outcome measures from the new health care system that are of no benefit to the patient,” he said.
He’s referring to the quality measures that many physicians are tracking to get reimbursed by Medicare and Medicaid.
Value-based care posts other challenges for MS physicians, he said, since MS care is especially expensive. Accountable Care Organizations are looking at cost savings in closed systems, he said, and that could spell trouble because patients with MS cost more.
As a 2015 report noted, first-generation disease-modifying therapies (DMTs) for MS cost about $60,000, and “costs for these agents have increased annually at rates 5 to 7 times higher than prescription drug inflation. Newer DMTs commonly entered the market with a cost 25%-60% higher than existing DMTs” (Neurology. 2015 May 26;84[21]:2185-92).
“If I’m in an ACO, and I’m taking care of a lot of MS patients, I’ll already lose money for the accountable care system,” Dr. English said. “They may not necessarily want an MS center inside an ACO.”
What can doctors do? “Advocacy efforts are pretty difficult for physicians,” Dr. English said. “Our hope is that the CMSC will be a clearinghouse for doctors who have ideas and efforts and advocacy, and somehow channel that into the actual provision of care. You have people advocating for medications and for research and for patients, but there’s nobody advocating for the actual care that’s going on, the boots-on-the-ground care. That’s where CMSC should play a big role.”
Dr. English disclosed that he has served as a consultant for multiple pharmaceutical companies.
REPORTING FROM THE CMSC ANNUAL MEETING
June 2018 - What's your diagnosis?
By Guilherme Piovezani Ramos, MD, Seth Sweetser, MD, and John B. Kisiel, MD
Recurrent SBO owing to contained perforation after accidental ingestion of a metal bristle of a barbecue grill brush
SBO may be caused by a variety of intrinsic or extrinsic lesions. Postoperative adhesions (60%) and malignant tumors (20%) are responsible for the majority of cases in the United States. CD accounts for approximately 5% of all SBOs.1 The unusual root cause of our patient's SBO was unintentional ingestion of a foreign body.
The CT image in Figure A shows an extraluminal foreign body in the left lower quadrant, surrounded by soft tissue thickening which represents reactive granulation tissue. She underwent surgical exploration, which revealed a single adhesion from a loop of the midjejunum to the neighboring mesentery (Figure B) that, when lysed, uncovered a small metal fragment consistent with a wire bristle from a grill-cleaning brush (Figure C). On further history, she reported frequent outdoor residential food grilling and admitted to using a wire grill cleaning brush. It is likely that she unintentionally ingested a metal bristle from a barbecue grill brush that was embedded in cooked food and penetrated through the small bowel wall, causing an adhesive inflammatory reaction and subsequent recurrent SBO.
Ingested foreign bodies are most frequently encountered in the pediatric population. Injury from inadvertent ingestion of wire grill cleaning brush bristles is being reported with increasing frequency in adults.2 Gastroenterologists should be aware of this type of foreign body injury to help prevent delay in diagnosis and ultimately treatment. This case highlights several additional important points. First, the evaluation of SBO must begin with a broad differential diagnosis, even in a patient with established CD. Second, the bristles are small and difficult to visualize on imaging. After resolution of acute obstruction, diagnostic imaging should be performed without positive oral contrast agent, which can obscure subtle mucosal findings or in this case, a diminutive extraluminal foreign body. Finally, greater public awareness should be raised that bristles might dislodge from wire grill brushes and embed in cooked food.3
References:
1. Hayanga AJ, Bass-Wilkins K, Bulkley GB. Current management of small-bowel obstruction. Adv Surg. 2005;39:1-33.
2. Harlor EJ, Lindemann TL, Kennedy TL. Outdoor grilling hazard: wire bristle esophageal foreign body - a report of six cases. Laryngoscope. 2012;122:2216-8.
3. Centers for Disease Control and Prevention (CDC). Injuries from ingestion of wire bristles from grill-cleaning brush - Providence, Rhode Island, March 2011-June 2012. MMWR Morb Mortal Wkly Rep. 2012;61:490-2.
By Guilherme Piovezani Ramos, MD, Seth Sweetser, MD, and John B. Kisiel, MD
Recurrent SBO owing to contained perforation after accidental ingestion of a metal bristle of a barbecue grill brush
SBO may be caused by a variety of intrinsic or extrinsic lesions. Postoperative adhesions (60%) and malignant tumors (20%) are responsible for the majority of cases in the United States. CD accounts for approximately 5% of all SBOs.1 The unusual root cause of our patient's SBO was unintentional ingestion of a foreign body.
The CT image in Figure A shows an extraluminal foreign body in the left lower quadrant, surrounded by soft tissue thickening which represents reactive granulation tissue. She underwent surgical exploration, which revealed a single adhesion from a loop of the midjejunum to the neighboring mesentery (Figure B) that, when lysed, uncovered a small metal fragment consistent with a wire bristle from a grill-cleaning brush (Figure C). On further history, she reported frequent outdoor residential food grilling and admitted to using a wire grill cleaning brush. It is likely that she unintentionally ingested a metal bristle from a barbecue grill brush that was embedded in cooked food and penetrated through the small bowel wall, causing an adhesive inflammatory reaction and subsequent recurrent SBO.
Ingested foreign bodies are most frequently encountered in the pediatric population. Injury from inadvertent ingestion of wire grill cleaning brush bristles is being reported with increasing frequency in adults.2 Gastroenterologists should be aware of this type of foreign body injury to help prevent delay in diagnosis and ultimately treatment. This case highlights several additional important points. First, the evaluation of SBO must begin with a broad differential diagnosis, even in a patient with established CD. Second, the bristles are small and difficult to visualize on imaging. After resolution of acute obstruction, diagnostic imaging should be performed without positive oral contrast agent, which can obscure subtle mucosal findings or in this case, a diminutive extraluminal foreign body. Finally, greater public awareness should be raised that bristles might dislodge from wire grill brushes and embed in cooked food.3
References:
1. Hayanga AJ, Bass-Wilkins K, Bulkley GB. Current management of small-bowel obstruction. Adv Surg. 2005;39:1-33.
2. Harlor EJ, Lindemann TL, Kennedy TL. Outdoor grilling hazard: wire bristle esophageal foreign body - a report of six cases. Laryngoscope. 2012;122:2216-8.
3. Centers for Disease Control and Prevention (CDC). Injuries from ingestion of wire bristles from grill-cleaning brush - Providence, Rhode Island, March 2011-June 2012. MMWR Morb Mortal Wkly Rep. 2012;61:490-2.
By Guilherme Piovezani Ramos, MD, Seth Sweetser, MD, and John B. Kisiel, MD
Recurrent SBO owing to contained perforation after accidental ingestion of a metal bristle of a barbecue grill brush
SBO may be caused by a variety of intrinsic or extrinsic lesions. Postoperative adhesions (60%) and malignant tumors (20%) are responsible for the majority of cases in the United States. CD accounts for approximately 5% of all SBOs.1 The unusual root cause of our patient's SBO was unintentional ingestion of a foreign body.
The CT image in Figure A shows an extraluminal foreign body in the left lower quadrant, surrounded by soft tissue thickening which represents reactive granulation tissue. She underwent surgical exploration, which revealed a single adhesion from a loop of the midjejunum to the neighboring mesentery (Figure B) that, when lysed, uncovered a small metal fragment consistent with a wire bristle from a grill-cleaning brush (Figure C). On further history, she reported frequent outdoor residential food grilling and admitted to using a wire grill cleaning brush. It is likely that she unintentionally ingested a metal bristle from a barbecue grill brush that was embedded in cooked food and penetrated through the small bowel wall, causing an adhesive inflammatory reaction and subsequent recurrent SBO.
Ingested foreign bodies are most frequently encountered in the pediatric population. Injury from inadvertent ingestion of wire grill cleaning brush bristles is being reported with increasing frequency in adults.2 Gastroenterologists should be aware of this type of foreign body injury to help prevent delay in diagnosis and ultimately treatment. This case highlights several additional important points. First, the evaluation of SBO must begin with a broad differential diagnosis, even in a patient with established CD. Second, the bristles are small and difficult to visualize on imaging. After resolution of acute obstruction, diagnostic imaging should be performed without positive oral contrast agent, which can obscure subtle mucosal findings or in this case, a diminutive extraluminal foreign body. Finally, greater public awareness should be raised that bristles might dislodge from wire grill brushes and embed in cooked food.3
References:
1. Hayanga AJ, Bass-Wilkins K, Bulkley GB. Current management of small-bowel obstruction. Adv Surg. 2005;39:1-33.
2. Harlor EJ, Lindemann TL, Kennedy TL. Outdoor grilling hazard: wire bristle esophageal foreign body - a report of six cases. Laryngoscope. 2012;122:2216-8.
3. Centers for Disease Control and Prevention (CDC). Injuries from ingestion of wire bristles from grill-cleaning brush - Providence, Rhode Island, March 2011-June 2012. MMWR Morb Mortal Wkly Rep. 2012;61:490-2.
A 63-year-old woman with a 6-year history of fibrostenotic ileocolonic Crohn's disease (CD) presented for evaluation after multiple hospitalizations for recurrent small bowel obstruction (SBO). Treatment for CD included adalimumab and azathioprine. Before initiation of these medications, she had undergone a 30-cm ileal resection for partial SBO secondary to a fibroinflammatory ileal stricture. She had no other coexistent medical conditions.
She denied nonsteroidal anti-inflammatory drug use, but did take acetaminophen for intermittent low back pain. Family history was notable for a daughter with CD. The patient denied use of alcohol, tobacco, or illicit drugs.
Over the past year, she has been hospitalized three times for partial SBOs that were treated conservatively. Her most recent hospitalization was 1 month ago, after which she was referred for assessment of inflammatory CD as the cause of recurrent SBO. She continued to complain of intermittent left lower abdominal pain after hospital discharge. Physical examination revealed a mildly distended abdomen with positive bowel sounds, tenderness to deep palpation in the lower quadrants, and no tympany to percussion or peritoneal signs. Laboratory evaluation, including complete blood count and basic metabolic profile, was unrevealing. Inflammatory markers, including erythrocyte sedimentation rate and C-reactive protein, had been within normal limits over the past year. On review of the abdominal computed tomographic (CT) images performed during the episodes of partial SBO, there was a transition point localized on the left lower quadrant; no mural enhancement or bowel wall thickening was seen.
Reevaluation with noncontrast CT of the abdomen showed resolution of previous acute obstruction. An enteroclysis tube was then placed and a second set of images demonstrated a curvilinear density in the left lower quadrant (Figure A, arrow), in the area of prior obstructions, which was not present on previous imaging studies.
What is the most likely cause of her recurrent SBOs?
Under his IDH2: Epigenetic drivers of clonal hematopoiesis
The world has surely changed in recent years, leading television and Hollywood to recreate many former works of postapocalyptic fiction. Watching the recent Hulu drama “The Handmaid’s Tale” leads many to make modern-day comparisons, but to this hematologist the connection is bone deep.
Hematopoietic stem cells face a century of carefully regulated symmetric division. They are tasked with the mission to generate the heterogeneous and environmentally responsive progenitor populations that will undergo coordinated differentiation and maturation.1
Within this epic battle of Gilead, much like hematopoiesis, there is a hierarchy within the Sons of Jacob. At the top there are few who drive leukemogenicity and require no other co-conspirators. MLL fusions and core binding factor are the Commanders that can overthrow the regulated governance of normal marrow homeostasis. Other myeloid disease alleles are soldiers of Gilead (i.e. FLT3-ITD, IDH1, IDH2, NPM1c, Spliceosome/Cohesin). Their individual characteristics can influence the natural history of the disease and define its strengths and weaknesses, particularly as new targeted therapies are developed.
Recently, Jongen-Lavrencic et al. reported that all disease alleles that persist as minimal residual disease after induction have a negative influence on outcome, except for three – DNMT3A, TET2, and ASXL1.2 What do we make of the “DTA” mutations? Are they the “Eye” – spies embedded in the polyclonal background of the marrow – intrinsically wired with malevolent intent? Are they the innocent, abused Handmaids – reprogrammed by inflammatory “Aunts” at the marrow’s Red Center and forced to clonally procreate? Previous works have suggested the latter, that persistence of mutations in genes found in clonal hematopoiesis (CH) do not portend an equivalent risk of relapse.3,4
A more encompassing question remains as to the equivalency of CH in the absence of a hematopoietic tumor versus CH postleukemia therapy. Comparisons to small cell lymphomas after successful treatment of transformed disease are disingenuous; CH is not itself a malignant state.5 Forty months of median follow-up for post-AML CH is simply not enough time. Work presented at the 2017 annual meeting of the American Society of Hematology by Jaiswal et al. showed that the approximately 1% risk of transformation per year was consistent over a 20-year period of follow up in the Swedish Nurse’s Study.
The recent work in the New England Journal of Medicine adds to the growing understanding of CH, but requires the test of time to know if these cells are truly innocent Handmaids or the Eye in a red cloak. I’ll be exploring these issues and taking your questions during a live Twitter Q&A on June 14 at noon ET. Follow me at @TheDoctorIsVin and @HematologyNews1 for more details. Blessed be the fruit.
Dr. Viny is with the Memorial Sloan-Kettering Cancer Center, N.Y., where he is a clinical instructor, is on the staff of the leukemia service, and is a clinical researcher in the Ross Levine Lab.
References
1. Orkin SH and Zon L. Cell. 2008 Feb 22;132(4):631-44.
2. Jongen-Lavrencic M et al. N Engl J Med 2018; 378:1189-99.
3. Bhatnagar B et al. Br J Haematol. 2016 Oct;175(2):226-36.
4. Shlush LI et al. Nature. 2017;547:104-8.
5. Bowman RL et al. Cell Stem Cell. 2018 Feb 1;22(2):157-70.
The world has surely changed in recent years, leading television and Hollywood to recreate many former works of postapocalyptic fiction. Watching the recent Hulu drama “The Handmaid’s Tale” leads many to make modern-day comparisons, but to this hematologist the connection is bone deep.
Hematopoietic stem cells face a century of carefully regulated symmetric division. They are tasked with the mission to generate the heterogeneous and environmentally responsive progenitor populations that will undergo coordinated differentiation and maturation.1
Within this epic battle of Gilead, much like hematopoiesis, there is a hierarchy within the Sons of Jacob. At the top there are few who drive leukemogenicity and require no other co-conspirators. MLL fusions and core binding factor are the Commanders that can overthrow the regulated governance of normal marrow homeostasis. Other myeloid disease alleles are soldiers of Gilead (i.e. FLT3-ITD, IDH1, IDH2, NPM1c, Spliceosome/Cohesin). Their individual characteristics can influence the natural history of the disease and define its strengths and weaknesses, particularly as new targeted therapies are developed.
Recently, Jongen-Lavrencic et al. reported that all disease alleles that persist as minimal residual disease after induction have a negative influence on outcome, except for three – DNMT3A, TET2, and ASXL1.2 What do we make of the “DTA” mutations? Are they the “Eye” – spies embedded in the polyclonal background of the marrow – intrinsically wired with malevolent intent? Are they the innocent, abused Handmaids – reprogrammed by inflammatory “Aunts” at the marrow’s Red Center and forced to clonally procreate? Previous works have suggested the latter, that persistence of mutations in genes found in clonal hematopoiesis (CH) do not portend an equivalent risk of relapse.3,4
A more encompassing question remains as to the equivalency of CH in the absence of a hematopoietic tumor versus CH postleukemia therapy. Comparisons to small cell lymphomas after successful treatment of transformed disease are disingenuous; CH is not itself a malignant state.5 Forty months of median follow-up for post-AML CH is simply not enough time. Work presented at the 2017 annual meeting of the American Society of Hematology by Jaiswal et al. showed that the approximately 1% risk of transformation per year was consistent over a 20-year period of follow up in the Swedish Nurse’s Study.
The recent work in the New England Journal of Medicine adds to the growing understanding of CH, but requires the test of time to know if these cells are truly innocent Handmaids or the Eye in a red cloak. I’ll be exploring these issues and taking your questions during a live Twitter Q&A on June 14 at noon ET. Follow me at @TheDoctorIsVin and @HematologyNews1 for more details. Blessed be the fruit.
Dr. Viny is with the Memorial Sloan-Kettering Cancer Center, N.Y., where he is a clinical instructor, is on the staff of the leukemia service, and is a clinical researcher in the Ross Levine Lab.
References
1. Orkin SH and Zon L. Cell. 2008 Feb 22;132(4):631-44.
2. Jongen-Lavrencic M et al. N Engl J Med 2018; 378:1189-99.
3. Bhatnagar B et al. Br J Haematol. 2016 Oct;175(2):226-36.
4. Shlush LI et al. Nature. 2017;547:104-8.
5. Bowman RL et al. Cell Stem Cell. 2018 Feb 1;22(2):157-70.
The world has surely changed in recent years, leading television and Hollywood to recreate many former works of postapocalyptic fiction. Watching the recent Hulu drama “The Handmaid’s Tale” leads many to make modern-day comparisons, but to this hematologist the connection is bone deep.
Hematopoietic stem cells face a century of carefully regulated symmetric division. They are tasked with the mission to generate the heterogeneous and environmentally responsive progenitor populations that will undergo coordinated differentiation and maturation.1
Within this epic battle of Gilead, much like hematopoiesis, there is a hierarchy within the Sons of Jacob. At the top there are few who drive leukemogenicity and require no other co-conspirators. MLL fusions and core binding factor are the Commanders that can overthrow the regulated governance of normal marrow homeostasis. Other myeloid disease alleles are soldiers of Gilead (i.e. FLT3-ITD, IDH1, IDH2, NPM1c, Spliceosome/Cohesin). Their individual characteristics can influence the natural history of the disease and define its strengths and weaknesses, particularly as new targeted therapies are developed.
Recently, Jongen-Lavrencic et al. reported that all disease alleles that persist as minimal residual disease after induction have a negative influence on outcome, except for three – DNMT3A, TET2, and ASXL1.2 What do we make of the “DTA” mutations? Are they the “Eye” – spies embedded in the polyclonal background of the marrow – intrinsically wired with malevolent intent? Are they the innocent, abused Handmaids – reprogrammed by inflammatory “Aunts” at the marrow’s Red Center and forced to clonally procreate? Previous works have suggested the latter, that persistence of mutations in genes found in clonal hematopoiesis (CH) do not portend an equivalent risk of relapse.3,4
A more encompassing question remains as to the equivalency of CH in the absence of a hematopoietic tumor versus CH postleukemia therapy. Comparisons to small cell lymphomas after successful treatment of transformed disease are disingenuous; CH is not itself a malignant state.5 Forty months of median follow-up for post-AML CH is simply not enough time. Work presented at the 2017 annual meeting of the American Society of Hematology by Jaiswal et al. showed that the approximately 1% risk of transformation per year was consistent over a 20-year period of follow up in the Swedish Nurse’s Study.
The recent work in the New England Journal of Medicine adds to the growing understanding of CH, but requires the test of time to know if these cells are truly innocent Handmaids or the Eye in a red cloak. I’ll be exploring these issues and taking your questions during a live Twitter Q&A on June 14 at noon ET. Follow me at @TheDoctorIsVin and @HematologyNews1 for more details. Blessed be the fruit.
Dr. Viny is with the Memorial Sloan-Kettering Cancer Center, N.Y., where he is a clinical instructor, is on the staff of the leukemia service, and is a clinical researcher in the Ross Levine Lab.
References
1. Orkin SH and Zon L. Cell. 2008 Feb 22;132(4):631-44.
2. Jongen-Lavrencic M et al. N Engl J Med 2018; 378:1189-99.
3. Bhatnagar B et al. Br J Haematol. 2016 Oct;175(2):226-36.
4. Shlush LI et al. Nature. 2017;547:104-8.
5. Bowman RL et al. Cell Stem Cell. 2018 Feb 1;22(2):157-70.
Advances in Hematology and Oncology (May 2018)
Click here to access May 2018 Advances In Hematology and Oncology Digital Edition.
Table of Contents
- Risk of Cancer-Associated Thrombosis and Bleeding in Veterans With Malignancy Who Are Receiving Direct Oral Anticoagulants
- Using Dermoscopy to Identify Melanoma and Improve Diagnostic Discrimination
- Prevalence of Suspicious Ultrasound Features in Hot Thyroid Nodules
- The Effect of Immunonutrition on Veterans Undergoing Major Surgery for Gastrointestinal Cancer
- Protons and Prostate Cancer
- The Use of Immuno-Oncology Therapies in the VHA
Click here to access May 2018 Advances In Hematology and Oncology Digital Edition.
Table of Contents
- Risk of Cancer-Associated Thrombosis and Bleeding in Veterans With Malignancy Who Are Receiving Direct Oral Anticoagulants
- Using Dermoscopy to Identify Melanoma and Improve Diagnostic Discrimination
- Prevalence of Suspicious Ultrasound Features in Hot Thyroid Nodules
- The Effect of Immunonutrition on Veterans Undergoing Major Surgery for Gastrointestinal Cancer
- Protons and Prostate Cancer
- The Use of Immuno-Oncology Therapies in the VHA

Click here to access May 2018 Advances In Hematology and Oncology Digital Edition.
Table of Contents
- Risk of Cancer-Associated Thrombosis and Bleeding in Veterans With Malignancy Who Are Receiving Direct Oral Anticoagulants
- Using Dermoscopy to Identify Melanoma and Improve Diagnostic Discrimination
- Prevalence of Suspicious Ultrasound Features in Hot Thyroid Nodules
- The Effect of Immunonutrition on Veterans Undergoing Major Surgery for Gastrointestinal Cancer
- Protons and Prostate Cancer
- The Use of Immuno-Oncology Therapies in the VHA

VF incidence increases significantly in kids after ALL treatment
A 6-year prospective study from the Canadian STOPP investigators revealed that following treatment for acute lymphoblastic leukemia (ALL), approximately 1 out of 3 children experiences vertebral fractures (VF) and 1 out of 5 children shows non-VF.
Glucocorticoid use and vertebral fractures at diagnosis emerged as significant predictive factors.
Investigators reported a cumulative incidence of 32.5% for vertebral fractures (VF) following treatment and 23.0% for non-VF after 6 years.
Thirty-nine percent of children with VF were asymptomatic.
“The osteotoxicity of leukemia and its treatment are underscored by the observation that the non-VF incidence was more than 2.5 times higher than the general pediatric population,” the study authors wrote, “whereas the VF incidence was increased more than 2000 times.”
The STOPP investigators—the Steroid-Associated Osteoporosis in the Pediatric Population Consortium—published their findings in the Journal of Bone and Mineral Research.
To document the incidence and predictors of fractures and profile who is at greatest risk, the STOPP investigators enrolled 186 children who were diagnosed and treated at 10 children’s hospitals across Canada between 2005 and 2007.
Median age at diagnosis was 5.3 years, median duration of follow-up was 6 years, and 38.1% were boys.
Approximately 4 out of 5 children were treated with Children’s Oncology Group protocols and 1 out of 5 was treated with the Dana-Farber protocols.
In girls, glucocorticoid exposure was 7.0 g/m2 and in boys it was 8.9 g/m2.
The entire cohort received 54.3% of the total cumulative glucocorticoid dose in the first year and 83.1% in the second year.
For VF, incidence at baseline was 15.1%. Skeletal fractures at any site were reported in 36% of the children over the 6-year period, 71% occurring during the first 2 years. No VF was reported in the sixth year.
Of 38 children with VF, 32 sustained at least 1 VF in the first 2 years and 77% occurred while on therapy or within 6 months of the last glucocorticoid dose. None of the children had disease relapse.
Non-VF were reported in 1.6% of children at baseline; incidence peaked at years 2 (5.4%) and 5 (4.8%) and none occurred in the sixth year.
Thirty-one non-VFs were reported in 26 children; 18 occurred in the first 2 years and 23 occurred on therapy or within 6 months of the last glucocorticoid dose.
The STOPP investigators showed that glucocorticoid exposure, VF, and low spine bone mineral density Z-scores at baseline were significant predictors for risk of VF.
“[This] observation suggests the importance of baseline spine phenotype (both VF and low lumbar spine bone mineral density) in signaling the potential for future bone morbidity,” the STOPP investigators note.
“In revealing that vertebral fractures are frequent in children with ALL on chemotherapy, and that older children and those with more severe collapse are at risk for residual vertebral deformities, strategies to prevent vertebral fractures in those at greatest risk for permanent sequelae now merit further study,” said lead author Leanne Ward, MD, of the University of Ottawa, Canada, in a statement.
The study was primarily supported by an operating grant from the Canadian Institutes for Health Research (CIHR) with additional funding from a CIHR New Investigator Award, a Canadian Child Health Clinician Scientist Career Enhancement Award, a University of Ottawa Research Chair Award, a Children’s Hospital of Eastern Ontario (CHEO) Research Institute Capacity Building Award, and the CHEO Departments of Pediatrics and Surgery. This work was also supported by the University of Alberta Women and Children’s Health Research Institute.
A 6-year prospective study from the Canadian STOPP investigators revealed that following treatment for acute lymphoblastic leukemia (ALL), approximately 1 out of 3 children experiences vertebral fractures (VF) and 1 out of 5 children shows non-VF.
Glucocorticoid use and vertebral fractures at diagnosis emerged as significant predictive factors.
Investigators reported a cumulative incidence of 32.5% for vertebral fractures (VF) following treatment and 23.0% for non-VF after 6 years.
Thirty-nine percent of children with VF were asymptomatic.
“The osteotoxicity of leukemia and its treatment are underscored by the observation that the non-VF incidence was more than 2.5 times higher than the general pediatric population,” the study authors wrote, “whereas the VF incidence was increased more than 2000 times.”
The STOPP investigators—the Steroid-Associated Osteoporosis in the Pediatric Population Consortium—published their findings in the Journal of Bone and Mineral Research.
To document the incidence and predictors of fractures and profile who is at greatest risk, the STOPP investigators enrolled 186 children who were diagnosed and treated at 10 children’s hospitals across Canada between 2005 and 2007.
Median age at diagnosis was 5.3 years, median duration of follow-up was 6 years, and 38.1% were boys.
Approximately 4 out of 5 children were treated with Children’s Oncology Group protocols and 1 out of 5 was treated with the Dana-Farber protocols.
In girls, glucocorticoid exposure was 7.0 g/m2 and in boys it was 8.9 g/m2.
The entire cohort received 54.3% of the total cumulative glucocorticoid dose in the first year and 83.1% in the second year.
For VF, incidence at baseline was 15.1%. Skeletal fractures at any site were reported in 36% of the children over the 6-year period, 71% occurring during the first 2 years. No VF was reported in the sixth year.
Of 38 children with VF, 32 sustained at least 1 VF in the first 2 years and 77% occurred while on therapy or within 6 months of the last glucocorticoid dose. None of the children had disease relapse.
Non-VF were reported in 1.6% of children at baseline; incidence peaked at years 2 (5.4%) and 5 (4.8%) and none occurred in the sixth year.
Thirty-one non-VFs were reported in 26 children; 18 occurred in the first 2 years and 23 occurred on therapy or within 6 months of the last glucocorticoid dose.
The STOPP investigators showed that glucocorticoid exposure, VF, and low spine bone mineral density Z-scores at baseline were significant predictors for risk of VF.
“[This] observation suggests the importance of baseline spine phenotype (both VF and low lumbar spine bone mineral density) in signaling the potential for future bone morbidity,” the STOPP investigators note.
“In revealing that vertebral fractures are frequent in children with ALL on chemotherapy, and that older children and those with more severe collapse are at risk for residual vertebral deformities, strategies to prevent vertebral fractures in those at greatest risk for permanent sequelae now merit further study,” said lead author Leanne Ward, MD, of the University of Ottawa, Canada, in a statement.
The study was primarily supported by an operating grant from the Canadian Institutes for Health Research (CIHR) with additional funding from a CIHR New Investigator Award, a Canadian Child Health Clinician Scientist Career Enhancement Award, a University of Ottawa Research Chair Award, a Children’s Hospital of Eastern Ontario (CHEO) Research Institute Capacity Building Award, and the CHEO Departments of Pediatrics and Surgery. This work was also supported by the University of Alberta Women and Children’s Health Research Institute.
A 6-year prospective study from the Canadian STOPP investigators revealed that following treatment for acute lymphoblastic leukemia (ALL), approximately 1 out of 3 children experiences vertebral fractures (VF) and 1 out of 5 children shows non-VF.
Glucocorticoid use and vertebral fractures at diagnosis emerged as significant predictive factors.
Investigators reported a cumulative incidence of 32.5% for vertebral fractures (VF) following treatment and 23.0% for non-VF after 6 years.
Thirty-nine percent of children with VF were asymptomatic.
“The osteotoxicity of leukemia and its treatment are underscored by the observation that the non-VF incidence was more than 2.5 times higher than the general pediatric population,” the study authors wrote, “whereas the VF incidence was increased more than 2000 times.”
The STOPP investigators—the Steroid-Associated Osteoporosis in the Pediatric Population Consortium—published their findings in the Journal of Bone and Mineral Research.
To document the incidence and predictors of fractures and profile who is at greatest risk, the STOPP investigators enrolled 186 children who were diagnosed and treated at 10 children’s hospitals across Canada between 2005 and 2007.
Median age at diagnosis was 5.3 years, median duration of follow-up was 6 years, and 38.1% were boys.
Approximately 4 out of 5 children were treated with Children’s Oncology Group protocols and 1 out of 5 was treated with the Dana-Farber protocols.
In girls, glucocorticoid exposure was 7.0 g/m2 and in boys it was 8.9 g/m2.
The entire cohort received 54.3% of the total cumulative glucocorticoid dose in the first year and 83.1% in the second year.
For VF, incidence at baseline was 15.1%. Skeletal fractures at any site were reported in 36% of the children over the 6-year period, 71% occurring during the first 2 years. No VF was reported in the sixth year.
Of 38 children with VF, 32 sustained at least 1 VF in the first 2 years and 77% occurred while on therapy or within 6 months of the last glucocorticoid dose. None of the children had disease relapse.
Non-VF were reported in 1.6% of children at baseline; incidence peaked at years 2 (5.4%) and 5 (4.8%) and none occurred in the sixth year.
Thirty-one non-VFs were reported in 26 children; 18 occurred in the first 2 years and 23 occurred on therapy or within 6 months of the last glucocorticoid dose.
The STOPP investigators showed that glucocorticoid exposure, VF, and low spine bone mineral density Z-scores at baseline were significant predictors for risk of VF.
“[This] observation suggests the importance of baseline spine phenotype (both VF and low lumbar spine bone mineral density) in signaling the potential for future bone morbidity,” the STOPP investigators note.
“In revealing that vertebral fractures are frequent in children with ALL on chemotherapy, and that older children and those with more severe collapse are at risk for residual vertebral deformities, strategies to prevent vertebral fractures in those at greatest risk for permanent sequelae now merit further study,” said lead author Leanne Ward, MD, of the University of Ottawa, Canada, in a statement.
The study was primarily supported by an operating grant from the Canadian Institutes for Health Research (CIHR) with additional funding from a CIHR New Investigator Award, a Canadian Child Health Clinician Scientist Career Enhancement Award, a University of Ottawa Research Chair Award, a Children’s Hospital of Eastern Ontario (CHEO) Research Institute Capacity Building Award, and the CHEO Departments of Pediatrics and Surgery. This work was also supported by the University of Alberta Women and Children’s Health Research Institute.