User login
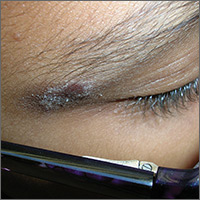
Rash on eyebrows
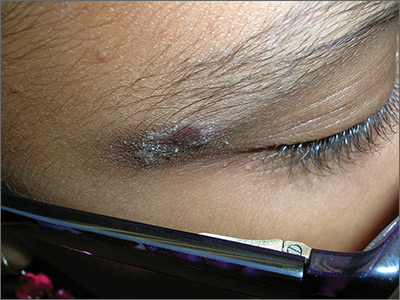
The FP recognized that this was a case of allergic contact dermatitis (ACD), based on the clinical presentation. The distribution of the erythema, scale, and post-inflammatory hyperpigmentation was highly suggestive of an ACD to nickel. In this case, the nickel in the patient’s eyeglasses and the snaps on her pants were the culprit. The plaque near the patient’s eye was actually in the shape of the metal on the inside of her glasses.
ACD is a delayed-type hypersensitivity reaction in which a foreign substance comes into contact with the skin and is linked to skin proteins; this forms an antigen complex, leading to sensitization. When the epidermis is re-exposed to the antigen, the sensitized T cells initiate an inflammatory cascade, leading to the skin changes seen in ACD.
Contact dermatitis can sometimes be diagnosed clinically with a good history and physical exam. However, there are many cases in which patch testing is needed to find the offending allergens or confirm the suspicion regarding a specific allergen. The differential diagnosis includes cutaneous candidiasis, impetigo, plaque psoriasis, and seborrheic dermatitis.
Patients with ACD should avoid the allergen that is causing the reaction. In cases of nickel ACD, the patient may cover the metal tab of his or her jeans with an iron-on patch or a few coats of clear nail polish. Fortunately, some jeans manufacturers now make nickel-free metal tabs (eg, Levis’s).
Cool compresses can soothe the symptoms of acute cases of ACD. Localized acute ACD lesions respond best to mid-potency (eg, 0.1% triamcinolone) to high-potency (eg, 0.05% clobetasol) topical steroids. On areas of thinner skin, lower-potency steroids such as desonide ointment can minimize the risk of skin atrophy.
In this case, the FP recommended that the patient get glasses that were nickel free and explained how to avoid the nickel that still exists in some pants. She was also given desonide 0.05% cream to apply to the affected area for symptomatic relief.
Adapted from: Usatine RP, Jacob SE. Rash on eyebrows and periumbilical region. J Fam Pract. 2017;66:45-47.

The FP recognized that this was a case of allergic contact dermatitis (ACD), based on the clinical presentation. The distribution of the erythema, scale, and post-inflammatory hyperpigmentation was highly suggestive of an ACD to nickel. In this case, the nickel in the patient’s eyeglasses and the snaps on her pants were the culprit. The plaque near the patient’s eye was actually in the shape of the metal on the inside of her glasses.
ACD is a delayed-type hypersensitivity reaction in which a foreign substance comes into contact with the skin and is linked to skin proteins; this forms an antigen complex, leading to sensitization. When the epidermis is re-exposed to the antigen, the sensitized T cells initiate an inflammatory cascade, leading to the skin changes seen in ACD.
Contact dermatitis can sometimes be diagnosed clinically with a good history and physical exam. However, there are many cases in which patch testing is needed to find the offending allergens or confirm the suspicion regarding a specific allergen. The differential diagnosis includes cutaneous candidiasis, impetigo, plaque psoriasis, and seborrheic dermatitis.
Patients with ACD should avoid the allergen that is causing the reaction. In cases of nickel ACD, the patient may cover the metal tab of his or her jeans with an iron-on patch or a few coats of clear nail polish. Fortunately, some jeans manufacturers now make nickel-free metal tabs (eg, Levis’s).
Cool compresses can soothe the symptoms of acute cases of ACD. Localized acute ACD lesions respond best to mid-potency (eg, 0.1% triamcinolone) to high-potency (eg, 0.05% clobetasol) topical steroids. On areas of thinner skin, lower-potency steroids such as desonide ointment can minimize the risk of skin atrophy.
In this case, the FP recommended that the patient get glasses that were nickel free and explained how to avoid the nickel that still exists in some pants. She was also given desonide 0.05% cream to apply to the affected area for symptomatic relief.
Adapted from: Usatine RP, Jacob SE. Rash on eyebrows and periumbilical region. J Fam Pract. 2017;66:45-47.

The FP recognized that this was a case of allergic contact dermatitis (ACD), based on the clinical presentation. The distribution of the erythema, scale, and post-inflammatory hyperpigmentation was highly suggestive of an ACD to nickel. In this case, the nickel in the patient’s eyeglasses and the snaps on her pants were the culprit. The plaque near the patient’s eye was actually in the shape of the metal on the inside of her glasses.
ACD is a delayed-type hypersensitivity reaction in which a foreign substance comes into contact with the skin and is linked to skin proteins; this forms an antigen complex, leading to sensitization. When the epidermis is re-exposed to the antigen, the sensitized T cells initiate an inflammatory cascade, leading to the skin changes seen in ACD.
Contact dermatitis can sometimes be diagnosed clinically with a good history and physical exam. However, there are many cases in which patch testing is needed to find the offending allergens or confirm the suspicion regarding a specific allergen. The differential diagnosis includes cutaneous candidiasis, impetigo, plaque psoriasis, and seborrheic dermatitis.
Patients with ACD should avoid the allergen that is causing the reaction. In cases of nickel ACD, the patient may cover the metal tab of his or her jeans with an iron-on patch or a few coats of clear nail polish. Fortunately, some jeans manufacturers now make nickel-free metal tabs (eg, Levis’s).
Cool compresses can soothe the symptoms of acute cases of ACD. Localized acute ACD lesions respond best to mid-potency (eg, 0.1% triamcinolone) to high-potency (eg, 0.05% clobetasol) topical steroids. On areas of thinner skin, lower-potency steroids such as desonide ointment can minimize the risk of skin atrophy.
In this case, the FP recommended that the patient get glasses that were nickel free and explained how to avoid the nickel that still exists in some pants. She was also given desonide 0.05% cream to apply to the affected area for symptomatic relief.
Adapted from: Usatine RP, Jacob SE. Rash on eyebrows and periumbilical region. J Fam Pract. 2017;66:45-47.
FDA expands Xeljanz approval to certain adults with ulcerative colitis
In two 8-week placebo-controlled trials, 10 mg of Xeljanz given twice daily induced remissions in 17%-18% of patients. In a placebo-controlled trial among the patients who responded by week 8, Xeljanz, at a 5-mg or 10-mg dose given twice daily, was effective in inducing remission by week 52 in 34% and 41% of patients, respectively. Additionally, 35% and 47% of those patients sustained corticosteroid-free remissions when treated with 5-mg and 10-mg doses, respectively.
“New treatments are needed for patients with moderately to severely active ulcerative colitis,” said Julie Beitz, MD, director of the Office of Drug Evaluation III in FDA’s Center for Drug Evaluation and Research in a press release. “Today’s approval provides an alternative therapy for a debilitating disease with limited treatment options.”
Xeljanz is the first oral medication approved for chronic use in moderately to severely active UC. The FDA states that other FDA-approved treatments for the chronic treatment of moderately to severely active ulcerative colitis must be administered through an intravenous infusion or subcutaneous injection.
Xeljanz, made by Pfizer Labs, was previously approved in 2012 for rheumatoid arthritis and in 2017 for psoriatic arthritis.
Find the full press release on the FDA’s website.
In two 8-week placebo-controlled trials, 10 mg of Xeljanz given twice daily induced remissions in 17%-18% of patients. In a placebo-controlled trial among the patients who responded by week 8, Xeljanz, at a 5-mg or 10-mg dose given twice daily, was effective in inducing remission by week 52 in 34% and 41% of patients, respectively. Additionally, 35% and 47% of those patients sustained corticosteroid-free remissions when treated with 5-mg and 10-mg doses, respectively.
“New treatments are needed for patients with moderately to severely active ulcerative colitis,” said Julie Beitz, MD, director of the Office of Drug Evaluation III in FDA’s Center for Drug Evaluation and Research in a press release. “Today’s approval provides an alternative therapy for a debilitating disease with limited treatment options.”
Xeljanz is the first oral medication approved for chronic use in moderately to severely active UC. The FDA states that other FDA-approved treatments for the chronic treatment of moderately to severely active ulcerative colitis must be administered through an intravenous infusion or subcutaneous injection.
Xeljanz, made by Pfizer Labs, was previously approved in 2012 for rheumatoid arthritis and in 2017 for psoriatic arthritis.
Find the full press release on the FDA’s website.
In two 8-week placebo-controlled trials, 10 mg of Xeljanz given twice daily induced remissions in 17%-18% of patients. In a placebo-controlled trial among the patients who responded by week 8, Xeljanz, at a 5-mg or 10-mg dose given twice daily, was effective in inducing remission by week 52 in 34% and 41% of patients, respectively. Additionally, 35% and 47% of those patients sustained corticosteroid-free remissions when treated with 5-mg and 10-mg doses, respectively.
“New treatments are needed for patients with moderately to severely active ulcerative colitis,” said Julie Beitz, MD, director of the Office of Drug Evaluation III in FDA’s Center for Drug Evaluation and Research in a press release. “Today’s approval provides an alternative therapy for a debilitating disease with limited treatment options.”
Xeljanz is the first oral medication approved for chronic use in moderately to severely active UC. The FDA states that other FDA-approved treatments for the chronic treatment of moderately to severely active ulcerative colitis must be administered through an intravenous infusion or subcutaneous injection.
Xeljanz, made by Pfizer Labs, was previously approved in 2012 for rheumatoid arthritis and in 2017 for psoriatic arthritis.
Find the full press release on the FDA’s website.
FDA approves estradiol vaginal inserts for dyspareunia*
, in a 4-mcg and 10-mcg dose. The 4-mcg dose is a lower dose than any currently available.
The hormone treatment is intended for dyspareunia resulting from menopausal vulvar and vaginal atrophy. The patient places a soft gel capsule in the lower part of the vagina, daily for 2 weeks, then at a reduced rate of twice per week. The capsule dissolves and reintroduces estrogen to the tissue.
The manufacturer of the product, TherapeuticsMD, has committed to conduct a postapproval observational study, as a condition of approval.
Estradiol comes with a boxed warning of risks of endometrial cancer, stroke, deep vein thrombosis, pulmonary embolism, myocardial infarction, breast cancer, and “probable dementia.” The most common adverse reaction to the estradiol vaginal inserts was headache.
Editor's Note: This article has been corrected to state that the 4-mcg dose showed significant improvement in severity of dyspareunia.
[email protected]
, in a 4-mcg and 10-mcg dose. The 4-mcg dose is a lower dose than any currently available.
The hormone treatment is intended for dyspareunia resulting from menopausal vulvar and vaginal atrophy. The patient places a soft gel capsule in the lower part of the vagina, daily for 2 weeks, then at a reduced rate of twice per week. The capsule dissolves and reintroduces estrogen to the tissue.
The manufacturer of the product, TherapeuticsMD, has committed to conduct a postapproval observational study, as a condition of approval.
Estradiol comes with a boxed warning of risks of endometrial cancer, stroke, deep vein thrombosis, pulmonary embolism, myocardial infarction, breast cancer, and “probable dementia.” The most common adverse reaction to the estradiol vaginal inserts was headache.
Editor's Note: This article has been corrected to state that the 4-mcg dose showed significant improvement in severity of dyspareunia.
[email protected]
, in a 4-mcg and 10-mcg dose. The 4-mcg dose is a lower dose than any currently available.
The hormone treatment is intended for dyspareunia resulting from menopausal vulvar and vaginal atrophy. The patient places a soft gel capsule in the lower part of the vagina, daily for 2 weeks, then at a reduced rate of twice per week. The capsule dissolves and reintroduces estrogen to the tissue.
The manufacturer of the product, TherapeuticsMD, has committed to conduct a postapproval observational study, as a condition of approval.
Estradiol comes with a boxed warning of risks of endometrial cancer, stroke, deep vein thrombosis, pulmonary embolism, myocardial infarction, breast cancer, and “probable dementia.” The most common adverse reaction to the estradiol vaginal inserts was headache.
Editor's Note: This article has been corrected to state that the 4-mcg dose showed significant improvement in severity of dyspareunia.
[email protected]
iPad app puts cognitive screening in the hands of MS patients
NASHVILLE, TENN. – A new computer tablet application puts cognitive screening literally in the hands of patients with multiple sclerosis.
The Multiple Sclerosis Performance Test (MSPT), created specifically for iPad, presents patients with four assessments that they can complete in a short time before any clinic visit, according to Stephen M. Rao, PhD, who helped develop the tool. After patients complete the test battery, the program translates their results into adjusted normative data and feeds them directly into the individual electronic medical record. When the clinic visit begins, everything is ready for the physician and patient to review together. The program not only provides a solid baseline assessment, but can also, over time, create a longitudinal profile of a patient’s cognitive status, and help to guide management decisions, Dr. Rao said at the annual meeting of the Consortium of Multiple Sclerosis Centers.
“About half of people with MS do have cognitive problems, which, above and beyond the physical problems, can result in major challenges with work, the ability to engage in social activities, and the need for personal assistance,” said Dr. Rao, who is the Ralph and Luci Schey Chair and director of the Schey Center for Cognitive Neuroimaging at the Cleveland Clinic. “But despite that, even comprehensive MS care centers rarely screen for cognitive dysfunction using objective neuropsychological tests.”
Time is the issue for most clinics, he said. Although the paper-and-pencil screening tools out there take only 10 minutes or so, most centers don’t have the luxury of carving out those extra moments or dedicating a staff member to administer the test and handle the data.
The MSPT attempts to sidestep the problem of time and manpower. In Dr. Rao’s center and the other 10 in the United States and Europe that now use the tool, patients simply arrive a bit early for their appointment and complete the three components: a structured patient history; the Neurological Quality of Life assessment; and an electronic adaptation of the MS Functional Composite.
It assesses cognition with a processing speed test based on the Symbol Digit Modalities Test, which has long been validated for MS patients. A contrast sensitivity test assesses visual acuity. A simple manual-dexterity test, in which patients move peg symbols into “holes,” tests upper extremity function, and a video-recorded walking speed test assesses lower extremity function.
The system was validated in 165 patients with MS and 217 healthy controls. It correlated well with the paper-and-pencil Symbol Digits Modalities Test, and correlated more highly than that test with cerebral T2 lesion load (MS Journal. 2017;23:1929-37).
The MSPT is part of a Biogen-sponsored project that Dr. Rao and colleagues unveiled at the American Academy of Neurology annual meeting in April, called the Multiple Sclerosis Partners Advancing Technology and Health Solutions (MS PATHS). It will gather longitudinal data on 11,000 patients using the MSPT program, and correlate it to multiple clinical and socioeconomic outcomes, Dr. Rao said.
The processing speed test portion of MS PATHS isn’t only available to PATHS centers, he added. Any clinician can obtain it by simply registering with Biogen and downloading the standalone version, which is called CogEval.
After downloading, the clinician must register with Biogen, which then will email a code to unlock the program. CogEval can be used on any iPad system that runs iOS 11 or higher. Results don’t get uploaded automatically into an EHR, but they can be entered manually or printed.
Dr. Rao disclosed that he received financial support from Biogen for the research and development of the MSPT program.
SOURCE: Rao SM et al. CMSC 2018. doi: 10.1177/1352458516688955
NASHVILLE, TENN. – A new computer tablet application puts cognitive screening literally in the hands of patients with multiple sclerosis.
The Multiple Sclerosis Performance Test (MSPT), created specifically for iPad, presents patients with four assessments that they can complete in a short time before any clinic visit, according to Stephen M. Rao, PhD, who helped develop the tool. After patients complete the test battery, the program translates their results into adjusted normative data and feeds them directly into the individual electronic medical record. When the clinic visit begins, everything is ready for the physician and patient to review together. The program not only provides a solid baseline assessment, but can also, over time, create a longitudinal profile of a patient’s cognitive status, and help to guide management decisions, Dr. Rao said at the annual meeting of the Consortium of Multiple Sclerosis Centers.
“About half of people with MS do have cognitive problems, which, above and beyond the physical problems, can result in major challenges with work, the ability to engage in social activities, and the need for personal assistance,” said Dr. Rao, who is the Ralph and Luci Schey Chair and director of the Schey Center for Cognitive Neuroimaging at the Cleveland Clinic. “But despite that, even comprehensive MS care centers rarely screen for cognitive dysfunction using objective neuropsychological tests.”
Time is the issue for most clinics, he said. Although the paper-and-pencil screening tools out there take only 10 minutes or so, most centers don’t have the luxury of carving out those extra moments or dedicating a staff member to administer the test and handle the data.
The MSPT attempts to sidestep the problem of time and manpower. In Dr. Rao’s center and the other 10 in the United States and Europe that now use the tool, patients simply arrive a bit early for their appointment and complete the three components: a structured patient history; the Neurological Quality of Life assessment; and an electronic adaptation of the MS Functional Composite.
It assesses cognition with a processing speed test based on the Symbol Digit Modalities Test, which has long been validated for MS patients. A contrast sensitivity test assesses visual acuity. A simple manual-dexterity test, in which patients move peg symbols into “holes,” tests upper extremity function, and a video-recorded walking speed test assesses lower extremity function.
The system was validated in 165 patients with MS and 217 healthy controls. It correlated well with the paper-and-pencil Symbol Digits Modalities Test, and correlated more highly than that test with cerebral T2 lesion load (MS Journal. 2017;23:1929-37).
The MSPT is part of a Biogen-sponsored project that Dr. Rao and colleagues unveiled at the American Academy of Neurology annual meeting in April, called the Multiple Sclerosis Partners Advancing Technology and Health Solutions (MS PATHS). It will gather longitudinal data on 11,000 patients using the MSPT program, and correlate it to multiple clinical and socioeconomic outcomes, Dr. Rao said.
The processing speed test portion of MS PATHS isn’t only available to PATHS centers, he added. Any clinician can obtain it by simply registering with Biogen and downloading the standalone version, which is called CogEval.
After downloading, the clinician must register with Biogen, which then will email a code to unlock the program. CogEval can be used on any iPad system that runs iOS 11 or higher. Results don’t get uploaded automatically into an EHR, but they can be entered manually or printed.
Dr. Rao disclosed that he received financial support from Biogen for the research and development of the MSPT program.
SOURCE: Rao SM et al. CMSC 2018. doi: 10.1177/1352458516688955
NASHVILLE, TENN. – A new computer tablet application puts cognitive screening literally in the hands of patients with multiple sclerosis.
The Multiple Sclerosis Performance Test (MSPT), created specifically for iPad, presents patients with four assessments that they can complete in a short time before any clinic visit, according to Stephen M. Rao, PhD, who helped develop the tool. After patients complete the test battery, the program translates their results into adjusted normative data and feeds them directly into the individual electronic medical record. When the clinic visit begins, everything is ready for the physician and patient to review together. The program not only provides a solid baseline assessment, but can also, over time, create a longitudinal profile of a patient’s cognitive status, and help to guide management decisions, Dr. Rao said at the annual meeting of the Consortium of Multiple Sclerosis Centers.
“About half of people with MS do have cognitive problems, which, above and beyond the physical problems, can result in major challenges with work, the ability to engage in social activities, and the need for personal assistance,” said Dr. Rao, who is the Ralph and Luci Schey Chair and director of the Schey Center for Cognitive Neuroimaging at the Cleveland Clinic. “But despite that, even comprehensive MS care centers rarely screen for cognitive dysfunction using objective neuropsychological tests.”
Time is the issue for most clinics, he said. Although the paper-and-pencil screening tools out there take only 10 minutes or so, most centers don’t have the luxury of carving out those extra moments or dedicating a staff member to administer the test and handle the data.
The MSPT attempts to sidestep the problem of time and manpower. In Dr. Rao’s center and the other 10 in the United States and Europe that now use the tool, patients simply arrive a bit early for their appointment and complete the three components: a structured patient history; the Neurological Quality of Life assessment; and an electronic adaptation of the MS Functional Composite.
It assesses cognition with a processing speed test based on the Symbol Digit Modalities Test, which has long been validated for MS patients. A contrast sensitivity test assesses visual acuity. A simple manual-dexterity test, in which patients move peg symbols into “holes,” tests upper extremity function, and a video-recorded walking speed test assesses lower extremity function.
The system was validated in 165 patients with MS and 217 healthy controls. It correlated well with the paper-and-pencil Symbol Digits Modalities Test, and correlated more highly than that test with cerebral T2 lesion load (MS Journal. 2017;23:1929-37).
The MSPT is part of a Biogen-sponsored project that Dr. Rao and colleagues unveiled at the American Academy of Neurology annual meeting in April, called the Multiple Sclerosis Partners Advancing Technology and Health Solutions (MS PATHS). It will gather longitudinal data on 11,000 patients using the MSPT program, and correlate it to multiple clinical and socioeconomic outcomes, Dr. Rao said.
The processing speed test portion of MS PATHS isn’t only available to PATHS centers, he added. Any clinician can obtain it by simply registering with Biogen and downloading the standalone version, which is called CogEval.
After downloading, the clinician must register with Biogen, which then will email a code to unlock the program. CogEval can be used on any iPad system that runs iOS 11 or higher. Results don’t get uploaded automatically into an EHR, but they can be entered manually or printed.
Dr. Rao disclosed that he received financial support from Biogen for the research and development of the MSPT program.
SOURCE: Rao SM et al. CMSC 2018. doi: 10.1177/1352458516688955
REPORTING FROM THE CMSC ANNUAL MEETING
Tinea Incognito in a Tattoo
To the Editor:
Tinea incognito occurs when superficial fungal infections fail to demonstrate typical clinical features in the setting of immune suppression caused by topical or systemic steroids.1,2 A case of tinea corporis obscured by an allergic tattoo reaction is presented.
A 52-year-old man presented for evaluation of a rash overlying a tattoo on the right calf of 3 weeks’ duration (Figure, A). The tattoo was placed 4 years prior to presentation. Within 6 months of the tattoo’s placement, pruritus, scaling, and edema developed in a 2-mm rim around the outer border and in the eyes of the elephant tattoo but not in the lettering portion of the tattoo, which was added by a different tattoo artist with a different red dye. A diagnosis of red dye tattoo allergic reaction was made. Daily treatment with tacrolimus ointment 0.1% and halobetasol propionate cream 0.05% under occlusion for 18 months provided only partial relief of incessant pruritus. Three months prior to presentation the tattoo reaction appeared to become worse with more pruritus and extension outside the bounds of the original tattoo.

Physical examination revealed the red rim of the tattoo was erythematous, edematous, and crusted. In addition, a 5×4-cm well-demarcated, erythematous, scaling patch was present overlying the elephant tattoo on the right calf and extending superiorly and laterally away from the tattoo. Scaling and maceration also were present in the web spaces between the fourth and fifth toes, and the toenails were yellowed, thickened, and dystrophic with signs of distal onycholysis. A potassium hydroxide preparation performed from the plaque on the right calf demonstrated septate fungal hyphae.
The diagnosis of tinea corporis secondary to tinea pedis overlying a red dye tattoo allergic reaction was made. Tacrolimus and halobetasol propionate were discontinued and treatment with ketoconazole cream 2% twice daily and oral terbinafine 250 mg once daily was started. The erythematous patch beyond the borders of the tattoo cleared within weeks, but the patient reported worsening of cracking, itching, and swelling overlying the red dye in the rim of the tattoo following discontinuation of topical anti-inflammatory drugs (Figure, B).
A potassium hydroxide preparation demonstrated that the expansible rash was tinea corporis disguised in its character by the coloration of the tattoo; the erythematous, edematous, pruritic tattoo allergic reaction at its rim; and suppression of the normal inflammatory response by daily use of a topical steroid and a calcineurin inhibitor. The latter effect (an immunocompromised district) impacts the classic exaggerated scaling, inflamed rim, and central clearing of tinea corporis present in individuals with a normal inflammatory response.2 Although tinea incognito is classically described on the ankles and lower legs of patients with stasis dermatitis chronically treated with topical steroids, it could occur anywhere in the setting of immunosuppression.3
An analysis of this case using Occam’s razor suggests that the association of this tattoo and tinea was not a coincidence. This guiding principle (heuristic) suggests that economy and succinctness in the logic of science is most likely to produce a correct medical diagnosis (eg, associated findings can be explained by identifying one underlying cause).4 The topical anti-inflammatory drugs increase the likelihood that the patient’s interdigital tinea would spread to this precise location symmetrically expanding in the outline of the tattoo.2
- Gathings RM, Abide JM, Brodell RT. An unusual inflammatory rash. JAMA Pediatr. 2014;168:185-186.
- Ruocco V, Brunetti G, Puca RV, et al. The immunocompromised district: a unifying concept for lymphoedematous, herpes-infected and otherwise damaged sites. J Eur Acad Dermatol Venereol. 2009;23:1364-1373.
- Romano C, Maritati E, Gianni C. Tinea incognito in Italy: a 15-year survey. Mycoses. 2006;49:383-387.
- Jefferys WH, Berger JO. Ockham’s razor and Bayesian analysis. American Scientist. 1992;80:64-72.
To the Editor:
Tinea incognito occurs when superficial fungal infections fail to demonstrate typical clinical features in the setting of immune suppression caused by topical or systemic steroids.1,2 A case of tinea corporis obscured by an allergic tattoo reaction is presented.
A 52-year-old man presented for evaluation of a rash overlying a tattoo on the right calf of 3 weeks’ duration (Figure, A). The tattoo was placed 4 years prior to presentation. Within 6 months of the tattoo’s placement, pruritus, scaling, and edema developed in a 2-mm rim around the outer border and in the eyes of the elephant tattoo but not in the lettering portion of the tattoo, which was added by a different tattoo artist with a different red dye. A diagnosis of red dye tattoo allergic reaction was made. Daily treatment with tacrolimus ointment 0.1% and halobetasol propionate cream 0.05% under occlusion for 18 months provided only partial relief of incessant pruritus. Three months prior to presentation the tattoo reaction appeared to become worse with more pruritus and extension outside the bounds of the original tattoo.

Physical examination revealed the red rim of the tattoo was erythematous, edematous, and crusted. In addition, a 5×4-cm well-demarcated, erythematous, scaling patch was present overlying the elephant tattoo on the right calf and extending superiorly and laterally away from the tattoo. Scaling and maceration also were present in the web spaces between the fourth and fifth toes, and the toenails were yellowed, thickened, and dystrophic with signs of distal onycholysis. A potassium hydroxide preparation performed from the plaque on the right calf demonstrated septate fungal hyphae.
The diagnosis of tinea corporis secondary to tinea pedis overlying a red dye tattoo allergic reaction was made. Tacrolimus and halobetasol propionate were discontinued and treatment with ketoconazole cream 2% twice daily and oral terbinafine 250 mg once daily was started. The erythematous patch beyond the borders of the tattoo cleared within weeks, but the patient reported worsening of cracking, itching, and swelling overlying the red dye in the rim of the tattoo following discontinuation of topical anti-inflammatory drugs (Figure, B).
A potassium hydroxide preparation demonstrated that the expansible rash was tinea corporis disguised in its character by the coloration of the tattoo; the erythematous, edematous, pruritic tattoo allergic reaction at its rim; and suppression of the normal inflammatory response by daily use of a topical steroid and a calcineurin inhibitor. The latter effect (an immunocompromised district) impacts the classic exaggerated scaling, inflamed rim, and central clearing of tinea corporis present in individuals with a normal inflammatory response.2 Although tinea incognito is classically described on the ankles and lower legs of patients with stasis dermatitis chronically treated with topical steroids, it could occur anywhere in the setting of immunosuppression.3
An analysis of this case using Occam’s razor suggests that the association of this tattoo and tinea was not a coincidence. This guiding principle (heuristic) suggests that economy and succinctness in the logic of science is most likely to produce a correct medical diagnosis (eg, associated findings can be explained by identifying one underlying cause).4 The topical anti-inflammatory drugs increase the likelihood that the patient’s interdigital tinea would spread to this precise location symmetrically expanding in the outline of the tattoo.2
To the Editor:
Tinea incognito occurs when superficial fungal infections fail to demonstrate typical clinical features in the setting of immune suppression caused by topical or systemic steroids.1,2 A case of tinea corporis obscured by an allergic tattoo reaction is presented.
A 52-year-old man presented for evaluation of a rash overlying a tattoo on the right calf of 3 weeks’ duration (Figure, A). The tattoo was placed 4 years prior to presentation. Within 6 months of the tattoo’s placement, pruritus, scaling, and edema developed in a 2-mm rim around the outer border and in the eyes of the elephant tattoo but not in the lettering portion of the tattoo, which was added by a different tattoo artist with a different red dye. A diagnosis of red dye tattoo allergic reaction was made. Daily treatment with tacrolimus ointment 0.1% and halobetasol propionate cream 0.05% under occlusion for 18 months provided only partial relief of incessant pruritus. Three months prior to presentation the tattoo reaction appeared to become worse with more pruritus and extension outside the bounds of the original tattoo.

Physical examination revealed the red rim of the tattoo was erythematous, edematous, and crusted. In addition, a 5×4-cm well-demarcated, erythematous, scaling patch was present overlying the elephant tattoo on the right calf and extending superiorly and laterally away from the tattoo. Scaling and maceration also were present in the web spaces between the fourth and fifth toes, and the toenails were yellowed, thickened, and dystrophic with signs of distal onycholysis. A potassium hydroxide preparation performed from the plaque on the right calf demonstrated septate fungal hyphae.
The diagnosis of tinea corporis secondary to tinea pedis overlying a red dye tattoo allergic reaction was made. Tacrolimus and halobetasol propionate were discontinued and treatment with ketoconazole cream 2% twice daily and oral terbinafine 250 mg once daily was started. The erythematous patch beyond the borders of the tattoo cleared within weeks, but the patient reported worsening of cracking, itching, and swelling overlying the red dye in the rim of the tattoo following discontinuation of topical anti-inflammatory drugs (Figure, B).
A potassium hydroxide preparation demonstrated that the expansible rash was tinea corporis disguised in its character by the coloration of the tattoo; the erythematous, edematous, pruritic tattoo allergic reaction at its rim; and suppression of the normal inflammatory response by daily use of a topical steroid and a calcineurin inhibitor. The latter effect (an immunocompromised district) impacts the classic exaggerated scaling, inflamed rim, and central clearing of tinea corporis present in individuals with a normal inflammatory response.2 Although tinea incognito is classically described on the ankles and lower legs of patients with stasis dermatitis chronically treated with topical steroids, it could occur anywhere in the setting of immunosuppression.3
An analysis of this case using Occam’s razor suggests that the association of this tattoo and tinea was not a coincidence. This guiding principle (heuristic) suggests that economy and succinctness in the logic of science is most likely to produce a correct medical diagnosis (eg, associated findings can be explained by identifying one underlying cause).4 The topical anti-inflammatory drugs increase the likelihood that the patient’s interdigital tinea would spread to this precise location symmetrically expanding in the outline of the tattoo.2
- Gathings RM, Abide JM, Brodell RT. An unusual inflammatory rash. JAMA Pediatr. 2014;168:185-186.
- Ruocco V, Brunetti G, Puca RV, et al. The immunocompromised district: a unifying concept for lymphoedematous, herpes-infected and otherwise damaged sites. J Eur Acad Dermatol Venereol. 2009;23:1364-1373.
- Romano C, Maritati E, Gianni C. Tinea incognito in Italy: a 15-year survey. Mycoses. 2006;49:383-387.
- Jefferys WH, Berger JO. Ockham’s razor and Bayesian analysis. American Scientist. 1992;80:64-72.
- Gathings RM, Abide JM, Brodell RT. An unusual inflammatory rash. JAMA Pediatr. 2014;168:185-186.
- Ruocco V, Brunetti G, Puca RV, et al. The immunocompromised district: a unifying concept for lymphoedematous, herpes-infected and otherwise damaged sites. J Eur Acad Dermatol Venereol. 2009;23:1364-1373.
- Romano C, Maritati E, Gianni C. Tinea incognito in Italy: a 15-year survey. Mycoses. 2006;49:383-387.
- Jefferys WH, Berger JO. Ockham’s razor and Bayesian analysis. American Scientist. 1992;80:64-72.
Practice Points
- Health care providers should have a low threshold to perform a potassium hydroxide preparation when the possibility of a superficial fungal infection is considered.
- Tinea incognito occurs when a superficial fungal infection has unusual clinical features in the setting of local immune suppression.
Dr Jame Abraham's top ASCO selections in breast cancer
Jame Abraham, MD, FACP, an Editor on The Journal of Community and Supportive Oncology, shares his top selections in breast cancer from this year's annual meeting of the American Society of Clinical Oncology in Chicago.
1001 Efficacy of sacituzumab govitecan (anti-Trop-2-SN-38 antibody-drug conjugate) for treatment-refractory hormone-receptor positive (HR+)/HER2- metastatic breast cancer (mBC) (Aditya Bardia et al). The study drug was well tolerated and produced objective responses in this heavily pretreated population, with an overall response rate of 31% at 6 months and a clinical benefit rate of 48%.
LBA1 TAILORx: Phase III trial of chemoendocrine therapy versus endocrine therapy alone in hormone receptor-positive, HER2-negative, node-negative breast cancer and an intermediate prognosis 21-gene recurrence score (Joseph A Sparano et al)
506 PERSEPHONE: 6 versus 12 months (m) of adjuvant trastuzumab in patients (pts) with HER2 positive (+) early breast cancer (EBC): randomised phase 3 non-inferiority trial with definitive 4-year (yr) disease-free survival (DFS) results (Helena Margaret Earl et al). Six months of trastuzumab was found to be noninferior to 12 months, although cardiac events were reduced in the 6-month group compared with the 12-month group (4% vs 8% of patients, respectively, ended treatment because of cardiotoxicity).
In addition, Dr David Henry, the Editor-in-Chief of JCSO, also selected:
500 Adjuvant denosumab in early breast cancer: disease-free survival analysis of postmenopausal patients in the ABCSG-18 trial (Michael Gnant et al). In this double-blind placebo controlled trial, disease-free survival in the denosumab group was 89% at 5 years and 80% at 8 years, compared with 87% and 77%, respectively, for placebo.
Jame Abraham, MD, FACP, an Editor on The Journal of Community and Supportive Oncology, shares his top selections in breast cancer from this year's annual meeting of the American Society of Clinical Oncology in Chicago.
1001 Efficacy of sacituzumab govitecan (anti-Trop-2-SN-38 antibody-drug conjugate) for treatment-refractory hormone-receptor positive (HR+)/HER2- metastatic breast cancer (mBC) (Aditya Bardia et al). The study drug was well tolerated and produced objective responses in this heavily pretreated population, with an overall response rate of 31% at 6 months and a clinical benefit rate of 48%.
LBA1 TAILORx: Phase III trial of chemoendocrine therapy versus endocrine therapy alone in hormone receptor-positive, HER2-negative, node-negative breast cancer and an intermediate prognosis 21-gene recurrence score (Joseph A Sparano et al)
506 PERSEPHONE: 6 versus 12 months (m) of adjuvant trastuzumab in patients (pts) with HER2 positive (+) early breast cancer (EBC): randomised phase 3 non-inferiority trial with definitive 4-year (yr) disease-free survival (DFS) results (Helena Margaret Earl et al). Six months of trastuzumab was found to be noninferior to 12 months, although cardiac events were reduced in the 6-month group compared with the 12-month group (4% vs 8% of patients, respectively, ended treatment because of cardiotoxicity).
In addition, Dr David Henry, the Editor-in-Chief of JCSO, also selected:
500 Adjuvant denosumab in early breast cancer: disease-free survival analysis of postmenopausal patients in the ABCSG-18 trial (Michael Gnant et al). In this double-blind placebo controlled trial, disease-free survival in the denosumab group was 89% at 5 years and 80% at 8 years, compared with 87% and 77%, respectively, for placebo.
Jame Abraham, MD, FACP, an Editor on The Journal of Community and Supportive Oncology, shares his top selections in breast cancer from this year's annual meeting of the American Society of Clinical Oncology in Chicago.
1001 Efficacy of sacituzumab govitecan (anti-Trop-2-SN-38 antibody-drug conjugate) for treatment-refractory hormone-receptor positive (HR+)/HER2- metastatic breast cancer (mBC) (Aditya Bardia et al). The study drug was well tolerated and produced objective responses in this heavily pretreated population, with an overall response rate of 31% at 6 months and a clinical benefit rate of 48%.
LBA1 TAILORx: Phase III trial of chemoendocrine therapy versus endocrine therapy alone in hormone receptor-positive, HER2-negative, node-negative breast cancer and an intermediate prognosis 21-gene recurrence score (Joseph A Sparano et al)
506 PERSEPHONE: 6 versus 12 months (m) of adjuvant trastuzumab in patients (pts) with HER2 positive (+) early breast cancer (EBC): randomised phase 3 non-inferiority trial with definitive 4-year (yr) disease-free survival (DFS) results (Helena Margaret Earl et al). Six months of trastuzumab was found to be noninferior to 12 months, although cardiac events were reduced in the 6-month group compared with the 12-month group (4% vs 8% of patients, respectively, ended treatment because of cardiotoxicity).
In addition, Dr David Henry, the Editor-in-Chief of JCSO, also selected:
500 Adjuvant denosumab in early breast cancer: disease-free survival analysis of postmenopausal patients in the ABCSG-18 trial (Michael Gnant et al). In this double-blind placebo controlled trial, disease-free survival in the denosumab group was 89% at 5 years and 80% at 8 years, compared with 87% and 77%, respectively, for placebo.
AAN Publishes Practice Guideline on DMTs for MS
LOS ANGELES—Clinicians must engage in an ongoing dialogue with patients with multiple sclerosis (MS) regarding treatment decisions throughout the disease course, according to a new practice guideline from the American Academy of Ne
When considering therapy for a patient with MS, clinicians must take into account the patient’s preferences regarding safety, route of administration, lifestyle, cost, efficacy, common adverse events, and tolerability, the guideline says.
The guideline—which includes 30 recommendations related to starting, switching, and stopping DMTs—“emphasizes that you need to have that discussion with patients, but it does not say for an individual which drug you should take,” said guideline author Ruth Ann Marrie, MD, PhD, Professor of Neurology and Community Health Sciences at the University of Manitoba in Winnipeg. “It provides information about the available therapies so that each clinician–patient dyad can use information specific to that patient to make the best decision for that particular scenario.”
The guideline was presented at the 70th Annual Meeting of the AAN and published in the April 24 issue of Neurology. It was endorsed by the MS Association of America and the National MS Society.
Therapeutic Advances
Neurologists have “little evidence to decide which drug to use at what time,” said lead guideline author Alexander Rae-Grant, MD, Professor of Neurology at the Cleveland Clinic.
The previous AAN clinical practice guideline on DMTs in MS, published in 2002, reviewed the injectable medications that were approved for MS at the time, such as interferon beta-1b, interferon beta-1a, and glatiramer acetate. Since then, “the treatment landscape has changed considerably … with more than 17 medications currently approved and widely prescribed for treating MS in the United States,” the authors said. “As a result, clinicians and people with MS may now choose from several medications with differing mechanisms of action, risk profiles, and monitoring requirements.” Clinicians must balance the potential therapeutic benefits and risks of a medication and the long-term risk of MS-related morbidity.
Since the 2002 guideline was published, studies have evaluated the treatment of patients with a first episode of demyelination, Dr. Rae-Grant said. “We now know that treating earlier is better, in terms of reducing the number of new relapses,” which reduces the risk of further brain injury, he said.
For people with highly active MS, clinicians should prescribe alemtuzumab, fingolimod, or natalizumab (Level B recommendation), according to the guideline. In addition, the guideline discusses pregnancy planning, setting realistic treatment expectations, and managing the risk of progressive multifocal leukoencephalopathy (PML). It mentions off-label options and support programs for people who are unable to afford approved DMTs.
Methods and Limitations
To assess evidence for starting, switching, and stopping DMTs for MS and develop relevant recommendations, the guideline authors surveyed peer-reviewed research articles, systematic reviews, and abstracts that they identified searching MEDLINE, CENTRAL, and EMBASE through November 2016. They selected 20 Cochrane reviews and 73 articles for data extraction and rated the studies using the AAN therapeutic classification of evidence scheme. The authors made recommendations using a modified Delphi process.
The guideline largely is based on trial data, which may limit the generalizability of the results to real-world populations, the authors noted. In addition, they anticipate that new research in the rapidly changing field of MS treatment will necessitate updates to the recommendations.
Future studies of DMTs for MS should address gaps in knowledge about long-term outcomes, safety in patients with comorbidities, predictive markers for patient response to treatment, treatment strategies for the initial management of MS, DMT use in pregnant women, and outcomes after stopping DMTs, according to the guideline. Neurologists also need evidence about the effect of DMTs on measures that are important to patients, but are not standard trial outcomes, such as cognition, fatigue, urinary urgency, pain, and visual function.
Navigating a Complex Landscape
The recommendations and systematic review of the evidence “reflect the complexity of MS management in the current treatment era,” said Tanuja Chitnis, MD, of Partners MS Center at Brigham and Women’s Hospital in Boston, and colleagues, in an accompanying editorial. “These statements serve as guidelines for MS patient care; however, they do not replace the clinician–patient relationship on which the most informed decision rests.”
The summary of more than 50 trials “provides a useful scale that can inform clinical decisions,” Dr. Chitnis and colleagues said. Certain drugs that were found to have limited evidence in the systematic review nevertheless “are in common clinical use … based on scientific principles and clinical acumen,” they said. In addition, the guideline reviews the evidence for combination therapies that rarely are used in clinical practice, which “may confuse the casual reader,” the editorialists noted.
“The experienced MS clinician … is needed to help guide patients through this complex landscape,” Dr. Chitnis and colleagues said. “The revised AAN guidelines are a starting point for the use of the multiple treatments now available for MS; however, further work is needed to further refine the choices appropriate for the individual patient.”
—Jake Remaly
Suggested Reading
Chitnis T, Giovannoni G, Trojano M. Complexity of MS management in the current treatment era. Neurology. 2018;90(17):761-762.
Corboy JR, Weinshenker BG, Wingerchuk DM. Comment on 2018 American Academy of Neurology guidelines on disease-modifying therapies in MS. Neurology. 2018 Apr 23 [Epub ahead of print].
Rae-Grant A, Day GS, Marrie RA, et al. Comprehensive systematic review summary: Disease-modifying therapies for adults with multiple sclerosis. Neurology. 2018;90(17):789-800.
Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: Disease-modifying therapies for adults with multiple sclerosis. Neurology. 2018;90(17):777-788.
LOS ANGELES—Clinicians must engage in an ongoing dialogue with patients with multiple sclerosis (MS) regarding treatment decisions throughout the disease course, according to a new practice guideline from the American Academy of Ne
When considering therapy for a patient with MS, clinicians must take into account the patient’s preferences regarding safety, route of administration, lifestyle, cost, efficacy, common adverse events, and tolerability, the guideline says.
The guideline—which includes 30 recommendations related to starting, switching, and stopping DMTs—“emphasizes that you need to have that discussion with patients, but it does not say for an individual which drug you should take,” said guideline author Ruth Ann Marrie, MD, PhD, Professor of Neurology and Community Health Sciences at the University of Manitoba in Winnipeg. “It provides information about the available therapies so that each clinician–patient dyad can use information specific to that patient to make the best decision for that particular scenario.”
The guideline was presented at the 70th Annual Meeting of the AAN and published in the April 24 issue of Neurology. It was endorsed by the MS Association of America and the National MS Society.
Therapeutic Advances
Neurologists have “little evidence to decide which drug to use at what time,” said lead guideline author Alexander Rae-Grant, MD, Professor of Neurology at the Cleveland Clinic.
The previous AAN clinical practice guideline on DMTs in MS, published in 2002, reviewed the injectable medications that were approved for MS at the time, such as interferon beta-1b, interferon beta-1a, and glatiramer acetate. Since then, “the treatment landscape has changed considerably … with more than 17 medications currently approved and widely prescribed for treating MS in the United States,” the authors said. “As a result, clinicians and people with MS may now choose from several medications with differing mechanisms of action, risk profiles, and monitoring requirements.” Clinicians must balance the potential therapeutic benefits and risks of a medication and the long-term risk of MS-related morbidity.
Since the 2002 guideline was published, studies have evaluated the treatment of patients with a first episode of demyelination, Dr. Rae-Grant said. “We now know that treating earlier is better, in terms of reducing the number of new relapses,” which reduces the risk of further brain injury, he said.
For people with highly active MS, clinicians should prescribe alemtuzumab, fingolimod, or natalizumab (Level B recommendation), according to the guideline. In addition, the guideline discusses pregnancy planning, setting realistic treatment expectations, and managing the risk of progressive multifocal leukoencephalopathy (PML). It mentions off-label options and support programs for people who are unable to afford approved DMTs.
Methods and Limitations
To assess evidence for starting, switching, and stopping DMTs for MS and develop relevant recommendations, the guideline authors surveyed peer-reviewed research articles, systematic reviews, and abstracts that they identified searching MEDLINE, CENTRAL, and EMBASE through November 2016. They selected 20 Cochrane reviews and 73 articles for data extraction and rated the studies using the AAN therapeutic classification of evidence scheme. The authors made recommendations using a modified Delphi process.
The guideline largely is based on trial data, which may limit the generalizability of the results to real-world populations, the authors noted. In addition, they anticipate that new research in the rapidly changing field of MS treatment will necessitate updates to the recommendations.
Future studies of DMTs for MS should address gaps in knowledge about long-term outcomes, safety in patients with comorbidities, predictive markers for patient response to treatment, treatment strategies for the initial management of MS, DMT use in pregnant women, and outcomes after stopping DMTs, according to the guideline. Neurologists also need evidence about the effect of DMTs on measures that are important to patients, but are not standard trial outcomes, such as cognition, fatigue, urinary urgency, pain, and visual function.
Navigating a Complex Landscape
The recommendations and systematic review of the evidence “reflect the complexity of MS management in the current treatment era,” said Tanuja Chitnis, MD, of Partners MS Center at Brigham and Women’s Hospital in Boston, and colleagues, in an accompanying editorial. “These statements serve as guidelines for MS patient care; however, they do not replace the clinician–patient relationship on which the most informed decision rests.”
The summary of more than 50 trials “provides a useful scale that can inform clinical decisions,” Dr. Chitnis and colleagues said. Certain drugs that were found to have limited evidence in the systematic review nevertheless “are in common clinical use … based on scientific principles and clinical acumen,” they said. In addition, the guideline reviews the evidence for combination therapies that rarely are used in clinical practice, which “may confuse the casual reader,” the editorialists noted.
“The experienced MS clinician … is needed to help guide patients through this complex landscape,” Dr. Chitnis and colleagues said. “The revised AAN guidelines are a starting point for the use of the multiple treatments now available for MS; however, further work is needed to further refine the choices appropriate for the individual patient.”
—Jake Remaly
Suggested Reading
Chitnis T, Giovannoni G, Trojano M. Complexity of MS management in the current treatment era. Neurology. 2018;90(17):761-762.
Corboy JR, Weinshenker BG, Wingerchuk DM. Comment on 2018 American Academy of Neurology guidelines on disease-modifying therapies in MS. Neurology. 2018 Apr 23 [Epub ahead of print].
Rae-Grant A, Day GS, Marrie RA, et al. Comprehensive systematic review summary: Disease-modifying therapies for adults with multiple sclerosis. Neurology. 2018;90(17):789-800.
Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: Disease-modifying therapies for adults with multiple sclerosis. Neurology. 2018;90(17):777-788.
LOS ANGELES—Clinicians must engage in an ongoing dialogue with patients with multiple sclerosis (MS) regarding treatment decisions throughout the disease course, according to a new practice guideline from the American Academy of Ne
When considering therapy for a patient with MS, clinicians must take into account the patient’s preferences regarding safety, route of administration, lifestyle, cost, efficacy, common adverse events, and tolerability, the guideline says.
The guideline—which includes 30 recommendations related to starting, switching, and stopping DMTs—“emphasizes that you need to have that discussion with patients, but it does not say for an individual which drug you should take,” said guideline author Ruth Ann Marrie, MD, PhD, Professor of Neurology and Community Health Sciences at the University of Manitoba in Winnipeg. “It provides information about the available therapies so that each clinician–patient dyad can use information specific to that patient to make the best decision for that particular scenario.”
The guideline was presented at the 70th Annual Meeting of the AAN and published in the April 24 issue of Neurology. It was endorsed by the MS Association of America and the National MS Society.
Therapeutic Advances
Neurologists have “little evidence to decide which drug to use at what time,” said lead guideline author Alexander Rae-Grant, MD, Professor of Neurology at the Cleveland Clinic.
The previous AAN clinical practice guideline on DMTs in MS, published in 2002, reviewed the injectable medications that were approved for MS at the time, such as interferon beta-1b, interferon beta-1a, and glatiramer acetate. Since then, “the treatment landscape has changed considerably … with more than 17 medications currently approved and widely prescribed for treating MS in the United States,” the authors said. “As a result, clinicians and people with MS may now choose from several medications with differing mechanisms of action, risk profiles, and monitoring requirements.” Clinicians must balance the potential therapeutic benefits and risks of a medication and the long-term risk of MS-related morbidity.
Since the 2002 guideline was published, studies have evaluated the treatment of patients with a first episode of demyelination, Dr. Rae-Grant said. “We now know that treating earlier is better, in terms of reducing the number of new relapses,” which reduces the risk of further brain injury, he said.
For people with highly active MS, clinicians should prescribe alemtuzumab, fingolimod, or natalizumab (Level B recommendation), according to the guideline. In addition, the guideline discusses pregnancy planning, setting realistic treatment expectations, and managing the risk of progressive multifocal leukoencephalopathy (PML). It mentions off-label options and support programs for people who are unable to afford approved DMTs.
Methods and Limitations
To assess evidence for starting, switching, and stopping DMTs for MS and develop relevant recommendations, the guideline authors surveyed peer-reviewed research articles, systematic reviews, and abstracts that they identified searching MEDLINE, CENTRAL, and EMBASE through November 2016. They selected 20 Cochrane reviews and 73 articles for data extraction and rated the studies using the AAN therapeutic classification of evidence scheme. The authors made recommendations using a modified Delphi process.
The guideline largely is based on trial data, which may limit the generalizability of the results to real-world populations, the authors noted. In addition, they anticipate that new research in the rapidly changing field of MS treatment will necessitate updates to the recommendations.
Future studies of DMTs for MS should address gaps in knowledge about long-term outcomes, safety in patients with comorbidities, predictive markers for patient response to treatment, treatment strategies for the initial management of MS, DMT use in pregnant women, and outcomes after stopping DMTs, according to the guideline. Neurologists also need evidence about the effect of DMTs on measures that are important to patients, but are not standard trial outcomes, such as cognition, fatigue, urinary urgency, pain, and visual function.
Navigating a Complex Landscape
The recommendations and systematic review of the evidence “reflect the complexity of MS management in the current treatment era,” said Tanuja Chitnis, MD, of Partners MS Center at Brigham and Women’s Hospital in Boston, and colleagues, in an accompanying editorial. “These statements serve as guidelines for MS patient care; however, they do not replace the clinician–patient relationship on which the most informed decision rests.”
The summary of more than 50 trials “provides a useful scale that can inform clinical decisions,” Dr. Chitnis and colleagues said. Certain drugs that were found to have limited evidence in the systematic review nevertheless “are in common clinical use … based on scientific principles and clinical acumen,” they said. In addition, the guideline reviews the evidence for combination therapies that rarely are used in clinical practice, which “may confuse the casual reader,” the editorialists noted.
“The experienced MS clinician … is needed to help guide patients through this complex landscape,” Dr. Chitnis and colleagues said. “The revised AAN guidelines are a starting point for the use of the multiple treatments now available for MS; however, further work is needed to further refine the choices appropriate for the individual patient.”
—Jake Remaly
Suggested Reading
Chitnis T, Giovannoni G, Trojano M. Complexity of MS management in the current treatment era. Neurology. 2018;90(17):761-762.
Corboy JR, Weinshenker BG, Wingerchuk DM. Comment on 2018 American Academy of Neurology guidelines on disease-modifying therapies in MS. Neurology. 2018 Apr 23 [Epub ahead of print].
Rae-Grant A, Day GS, Marrie RA, et al. Comprehensive systematic review summary: Disease-modifying therapies for adults with multiple sclerosis. Neurology. 2018;90(17):789-800.
Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: Disease-modifying therapies for adults with multiple sclerosis. Neurology. 2018;90(17):777-788.
Eye-opening findings cast spondyloarthritis in new light, expert says
SANDESTIN, FLA. – Recent findings have led to eye-opening results in the axial spondyloarthritis (SpA) field, including a surprisingly high number of patients with inflammatory back pain who don’t progress to the disease, healthy people who develop SpA-like details on imaging, and significant gender differences in the efficacy of biologic therapy, said Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego.
The findings could lead clinicians to see the disease differently and consult with patients in new ways, he said.
Just over 20% of the patients progressed to SpA within 5 years, and about 30% over 15 years. But after 5 years, the condition resolved in over 30% of patients – and after 15 years, it resolved in almost half.
In about 5% of patients, symptoms persisted but the condition remained unidentified.
“A lot of people with inflammatory back pain, it doesn’t continue to be an issue – this goes out a decade and a half,” Dr. Kavanaugh said. “I was surprised with this. I would have guess that over this many years, more people would have developed ankylosing spondylitis, but they don’t.”
He said that clinicians should cite this information in their discussions with patients. They should review their case and evaluate spinal symptoms, but let them know that the condition might not progress and might not be permanent.
“I would use this information and say, ‘Well you’re having inflammatory back pain, but let’s go review things,’ ” he said. “ ‘If you don’t have the true spondyloarthropathy or ankylosing spondylitis now, there’s a chance that this will go away. It’s almost 50-50, or we still don’t know what it is even if you’re having some symptoms (after 15 years).’ ”
Other important findings underscore the need for a complete clinical picture rather than just findings on imaging for an axial SpA diagnosis, Dr. Kavanaugh said. Researchers examined MRI images of new military recruits who were healthy with no back pain (Rheumatology [Oxford]. 2018 Mar 1;57[3]:508-13). They found that 23% of them at baseline – and 37% after strenuous training – had MRI findings that would qualify as positive for spondyloarthritis by Assessment of Spondyloarthritis international Society criteria. But they wouldn’t meet the definition of disease.
More recent findings showed similar results in imaging of healthy runners and hockey players (Arthritis Rheumatol. 2018 May;70[5]:736-45), with 30%-40% of them having MRI findings that would be considered positive on ASAS, Dr. Kavanaugh said.
“These were just people who were out stressing their joints,” he said. “We were super excited at the start of having MRI because now we can look and evaluate the activity within a joint. But I think, like everything, we have to take it with a little bit of caution. Just in and of itself, without the clinical picture, it does not diagnose axial spondyloarthropathy.”
In another recent study, women with axial SpA were found to have significantly lower responses over time than men with axial SpA (J Rheumatol. 2018 Feb;45[2]:195-201). Dr. Kavanaugh said that there might be some selection bias because of the higher male prevalence of disease but said the findings were noteworthy, especially in light of findings in animal models suggesting gender differences in disease expression and response to treatment.
“I think this is fascinating,” he said. “I think there’s a lot more to come for this.”
Dr. Kavanaugh reported financial relationships with AbbVie, Amgen, AstraZeneca, Bristol-Myers Squibb, Celgene, Gilead, Genentech, Novartis, Pfizer, and other companies.
SANDESTIN, FLA. – Recent findings have led to eye-opening results in the axial spondyloarthritis (SpA) field, including a surprisingly high number of patients with inflammatory back pain who don’t progress to the disease, healthy people who develop SpA-like details on imaging, and significant gender differences in the efficacy of biologic therapy, said Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego.
The findings could lead clinicians to see the disease differently and consult with patients in new ways, he said.
Just over 20% of the patients progressed to SpA within 5 years, and about 30% over 15 years. But after 5 years, the condition resolved in over 30% of patients – and after 15 years, it resolved in almost half.
In about 5% of patients, symptoms persisted but the condition remained unidentified.
“A lot of people with inflammatory back pain, it doesn’t continue to be an issue – this goes out a decade and a half,” Dr. Kavanaugh said. “I was surprised with this. I would have guess that over this many years, more people would have developed ankylosing spondylitis, but they don’t.”
He said that clinicians should cite this information in their discussions with patients. They should review their case and evaluate spinal symptoms, but let them know that the condition might not progress and might not be permanent.
“I would use this information and say, ‘Well you’re having inflammatory back pain, but let’s go review things,’ ” he said. “ ‘If you don’t have the true spondyloarthropathy or ankylosing spondylitis now, there’s a chance that this will go away. It’s almost 50-50, or we still don’t know what it is even if you’re having some symptoms (after 15 years).’ ”
Other important findings underscore the need for a complete clinical picture rather than just findings on imaging for an axial SpA diagnosis, Dr. Kavanaugh said. Researchers examined MRI images of new military recruits who were healthy with no back pain (Rheumatology [Oxford]. 2018 Mar 1;57[3]:508-13). They found that 23% of them at baseline – and 37% after strenuous training – had MRI findings that would qualify as positive for spondyloarthritis by Assessment of Spondyloarthritis international Society criteria. But they wouldn’t meet the definition of disease.
More recent findings showed similar results in imaging of healthy runners and hockey players (Arthritis Rheumatol. 2018 May;70[5]:736-45), with 30%-40% of them having MRI findings that would be considered positive on ASAS, Dr. Kavanaugh said.
“These were just people who were out stressing their joints,” he said. “We were super excited at the start of having MRI because now we can look and evaluate the activity within a joint. But I think, like everything, we have to take it with a little bit of caution. Just in and of itself, without the clinical picture, it does not diagnose axial spondyloarthropathy.”
In another recent study, women with axial SpA were found to have significantly lower responses over time than men with axial SpA (J Rheumatol. 2018 Feb;45[2]:195-201). Dr. Kavanaugh said that there might be some selection bias because of the higher male prevalence of disease but said the findings were noteworthy, especially in light of findings in animal models suggesting gender differences in disease expression and response to treatment.
“I think this is fascinating,” he said. “I think there’s a lot more to come for this.”
Dr. Kavanaugh reported financial relationships with AbbVie, Amgen, AstraZeneca, Bristol-Myers Squibb, Celgene, Gilead, Genentech, Novartis, Pfizer, and other companies.
SANDESTIN, FLA. – Recent findings have led to eye-opening results in the axial spondyloarthritis (SpA) field, including a surprisingly high number of patients with inflammatory back pain who don’t progress to the disease, healthy people who develop SpA-like details on imaging, and significant gender differences in the efficacy of biologic therapy, said Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego.
The findings could lead clinicians to see the disease differently and consult with patients in new ways, he said.
Just over 20% of the patients progressed to SpA within 5 years, and about 30% over 15 years. But after 5 years, the condition resolved in over 30% of patients – and after 15 years, it resolved in almost half.
In about 5% of patients, symptoms persisted but the condition remained unidentified.
“A lot of people with inflammatory back pain, it doesn’t continue to be an issue – this goes out a decade and a half,” Dr. Kavanaugh said. “I was surprised with this. I would have guess that over this many years, more people would have developed ankylosing spondylitis, but they don’t.”
He said that clinicians should cite this information in their discussions with patients. They should review their case and evaluate spinal symptoms, but let them know that the condition might not progress and might not be permanent.
“I would use this information and say, ‘Well you’re having inflammatory back pain, but let’s go review things,’ ” he said. “ ‘If you don’t have the true spondyloarthropathy or ankylosing spondylitis now, there’s a chance that this will go away. It’s almost 50-50, or we still don’t know what it is even if you’re having some symptoms (after 15 years).’ ”
Other important findings underscore the need for a complete clinical picture rather than just findings on imaging for an axial SpA diagnosis, Dr. Kavanaugh said. Researchers examined MRI images of new military recruits who were healthy with no back pain (Rheumatology [Oxford]. 2018 Mar 1;57[3]:508-13). They found that 23% of them at baseline – and 37% after strenuous training – had MRI findings that would qualify as positive for spondyloarthritis by Assessment of Spondyloarthritis international Society criteria. But they wouldn’t meet the definition of disease.
More recent findings showed similar results in imaging of healthy runners and hockey players (Arthritis Rheumatol. 2018 May;70[5]:736-45), with 30%-40% of them having MRI findings that would be considered positive on ASAS, Dr. Kavanaugh said.
“These were just people who were out stressing their joints,” he said. “We were super excited at the start of having MRI because now we can look and evaluate the activity within a joint. But I think, like everything, we have to take it with a little bit of caution. Just in and of itself, without the clinical picture, it does not diagnose axial spondyloarthropathy.”
In another recent study, women with axial SpA were found to have significantly lower responses over time than men with axial SpA (J Rheumatol. 2018 Feb;45[2]:195-201). Dr. Kavanaugh said that there might be some selection bias because of the higher male prevalence of disease but said the findings were noteworthy, especially in light of findings in animal models suggesting gender differences in disease expression and response to treatment.
“I think this is fascinating,” he said. “I think there’s a lot more to come for this.”
Dr. Kavanaugh reported financial relationships with AbbVie, Amgen, AstraZeneca, Bristol-Myers Squibb, Celgene, Gilead, Genentech, Novartis, Pfizer, and other companies.
EXPERT ANALYSIS FROM CCR 18
Can a Finger Displacement Test Help Assess Parkinson’s Disease Dementia?
LOS ANGELES—A simple test of finger displacement may distinguish between Parkinson’s disease dementia and Alzheimer’s disease and help neurologists assess the progression of dementia in Parkinson’s disease, according to a study presented at the 70th Annual Meeting of the American Academy of Neurology.
Parkinson’s disease dementia is one of the most disabling nonmotor complications of Parkinson’s disease, but “there is no simple bedside test available that can measure the progression of dementia,” said Aman Deep, MD, a neurology resident at the University of Tennessee Health Science Center in Memphis, and colleagues.
To study the clinical utility of finger displacement in patients with dementia, Dr. Deep and colleagues examined 56 patients with Parkinson’s disease dementia and 35 patients with Alzheimer’s disease. The patients pointed their index fingers toward a grid ruler. After maintaining the pointing position for 15 seconds, patients were asked to close their eyes for another 15 seconds while maintaining the same position. A positive result was downward index finger displacement of 5 cm or greater while patients had their eyes closed.
The patients with Parkinson’s disease dementia (42 male; mean age, 75) had a mean Parkinson’s disease duration of 9.1 years, a mean dementia duration of 3.1 years, and a mean Unified Parkinson’s Disease Rating Scale score of 37. The group’s mean Mini-Mental State Examination (MMSE) score was 17.5. Fifty-three patients out of 56 (95%) exhibited bilateral downward drift of 5 cm or greater, and three patients exhibited less than 5 cm of downward drift. The mean bilateral downward finger drift was 6.8 cm for the group.
Among patients with Alzheimer’s disease (21 male; mean age, 77.4), the mean dementia duration was 3.9 years. The group’s mean MMSE score was 17.8. In the Alzheimer’s disease group, only one patient had minimal drift, and the group’s mean bilateral downward drift was 0.2 cm.
According to the researchers, the finger displacement test has a sensitivity of 100% and a specificity of 92.1%. “Downward finger displacement, especially bilateral downward displacement, may signal extensive disruption of subcortical–cortical circuits,” Dr. Deep and colleagues said. “The simple and inexpensive bedside test of finger displacement may be used to help distinguish Parkinson’s disease dementia from Alzheimer’s disease.”
—Jake Remaly
Suggested Reading
Lieberman A, Deep A, Shi J, et al. Downward finger displacement distinguishes Parkinson disease dementia from Alzheimer disease. Int J Neurosci. 2018;128(2):151-154.
LOS ANGELES—A simple test of finger displacement may distinguish between Parkinson’s disease dementia and Alzheimer’s disease and help neurologists assess the progression of dementia in Parkinson’s disease, according to a study presented at the 70th Annual Meeting of the American Academy of Neurology.
Parkinson’s disease dementia is one of the most disabling nonmotor complications of Parkinson’s disease, but “there is no simple bedside test available that can measure the progression of dementia,” said Aman Deep, MD, a neurology resident at the University of Tennessee Health Science Center in Memphis, and colleagues.
To study the clinical utility of finger displacement in patients with dementia, Dr. Deep and colleagues examined 56 patients with Parkinson’s disease dementia and 35 patients with Alzheimer’s disease. The patients pointed their index fingers toward a grid ruler. After maintaining the pointing position for 15 seconds, patients were asked to close their eyes for another 15 seconds while maintaining the same position. A positive result was downward index finger displacement of 5 cm or greater while patients had their eyes closed.
The patients with Parkinson’s disease dementia (42 male; mean age, 75) had a mean Parkinson’s disease duration of 9.1 years, a mean dementia duration of 3.1 years, and a mean Unified Parkinson’s Disease Rating Scale score of 37. The group’s mean Mini-Mental State Examination (MMSE) score was 17.5. Fifty-three patients out of 56 (95%) exhibited bilateral downward drift of 5 cm or greater, and three patients exhibited less than 5 cm of downward drift. The mean bilateral downward finger drift was 6.8 cm for the group.
Among patients with Alzheimer’s disease (21 male; mean age, 77.4), the mean dementia duration was 3.9 years. The group’s mean MMSE score was 17.8. In the Alzheimer’s disease group, only one patient had minimal drift, and the group’s mean bilateral downward drift was 0.2 cm.
According to the researchers, the finger displacement test has a sensitivity of 100% and a specificity of 92.1%. “Downward finger displacement, especially bilateral downward displacement, may signal extensive disruption of subcortical–cortical circuits,” Dr. Deep and colleagues said. “The simple and inexpensive bedside test of finger displacement may be used to help distinguish Parkinson’s disease dementia from Alzheimer’s disease.”
—Jake Remaly
Suggested Reading
Lieberman A, Deep A, Shi J, et al. Downward finger displacement distinguishes Parkinson disease dementia from Alzheimer disease. Int J Neurosci. 2018;128(2):151-154.
LOS ANGELES—A simple test of finger displacement may distinguish between Parkinson’s disease dementia and Alzheimer’s disease and help neurologists assess the progression of dementia in Parkinson’s disease, according to a study presented at the 70th Annual Meeting of the American Academy of Neurology.
Parkinson’s disease dementia is one of the most disabling nonmotor complications of Parkinson’s disease, but “there is no simple bedside test available that can measure the progression of dementia,” said Aman Deep, MD, a neurology resident at the University of Tennessee Health Science Center in Memphis, and colleagues.
To study the clinical utility of finger displacement in patients with dementia, Dr. Deep and colleagues examined 56 patients with Parkinson’s disease dementia and 35 patients with Alzheimer’s disease. The patients pointed their index fingers toward a grid ruler. After maintaining the pointing position for 15 seconds, patients were asked to close their eyes for another 15 seconds while maintaining the same position. A positive result was downward index finger displacement of 5 cm or greater while patients had their eyes closed.
The patients with Parkinson’s disease dementia (42 male; mean age, 75) had a mean Parkinson’s disease duration of 9.1 years, a mean dementia duration of 3.1 years, and a mean Unified Parkinson’s Disease Rating Scale score of 37. The group’s mean Mini-Mental State Examination (MMSE) score was 17.5. Fifty-three patients out of 56 (95%) exhibited bilateral downward drift of 5 cm or greater, and three patients exhibited less than 5 cm of downward drift. The mean bilateral downward finger drift was 6.8 cm for the group.
Among patients with Alzheimer’s disease (21 male; mean age, 77.4), the mean dementia duration was 3.9 years. The group’s mean MMSE score was 17.8. In the Alzheimer’s disease group, only one patient had minimal drift, and the group’s mean bilateral downward drift was 0.2 cm.
According to the researchers, the finger displacement test has a sensitivity of 100% and a specificity of 92.1%. “Downward finger displacement, especially bilateral downward displacement, may signal extensive disruption of subcortical–cortical circuits,” Dr. Deep and colleagues said. “The simple and inexpensive bedside test of finger displacement may be used to help distinguish Parkinson’s disease dementia from Alzheimer’s disease.”
—Jake Remaly
Suggested Reading
Lieberman A, Deep A, Shi J, et al. Downward finger displacement distinguishes Parkinson disease dementia from Alzheimer disease. Int J Neurosci. 2018;128(2):151-154.
Kawasaki disease: New info to enhance our index of suspicion
Most U.S. mainland pediatric practitioners will see only one or two cases of Kawasaki disease (KD) in their careers, but no one wants to miss even one case.
Making the diagnosis as early as possible is important to reduce the chance of sequelae, particularly the coronary artery aneurysms that will eventually lead to 5% of overall acute coronary syndromes in adults. And because there is no “KD test,” or sometimes incomplete KD. And there are some new data that complicate this. Despite the recently updated 2017 guideline,1 most cases end up being confirmed and managed by regional “experts.” But nearly all of the approximately 6,000 KD cases/year in U.S. children younger than 5 years old start out with one or more primary care, urgent care, or ED visits.
This means that every clinician in the trenches not only needs a high index of suspicion but also needs to be at least a partial expert, too. What raises our index of suspicion? Classic data tell us we need 5 consecutive days of fever plus four or five other principal clinical findings for a KD diagnosis. The principal findings are:
1. Eyes: Bilateral bulbar nonexudative conjunctival injection.
2. Mouth: Erythema of oral/pharyngeal mucosa or cracked lips or strawberry tongue or oral mucositis.
3. Rash.
4. Hands or feet findings: Swelling/erythema or later periungual desquamation.
5. Cervical adenopathy greater than 1.4 cm, usually unilateral.
Other factors that have classically increased suspicion are winter/early spring presentation in North America, male gender (1.5:1 ratio to females), and Asian (particularly Japanese) ancestry. The importance of genetics was initially based on epidemiology (Japan/Asian risk) but lately has been further associated with six gene polymorphisms. However, molecular genetic testing is not currently a practical tool.
Clinical scenarios that also should raise suspicion include less-than-6-month-old infants with prolonged fever/irritability (may be the only clinical manifestations of KD) and children over 4 years old who more often may have incomplete KD. Both groups have higher prevalence of coronary artery abnormalities. Other high suspicion scenarios include prolonged fever with unexplained/culture-negative shock, or antibiotic treatment failure for cervical adenitis or retro/parapharyngeal phlegmon. Consultation with or referral to a regional KD expert may be needed.
Fuzzy KD math
Current guidelines list an exception to the 5-day fever requirement in that only 4 days of fever are needed with four or more principal clinical features, particularly when hand and feet findings exist. Some call this the “4X4 exception.” Then there is a sub-caveat: “Experienced clinicians who have treated many patients with KD may establish the diagnosis with 3 days of fever in rare cases.”1
Incomplete KD
This is another exception, which seems to be a more frequent diagnosis in the past decade. Incomplete KD requires the 5 days of fever duration plus an elevated C-reactive protein or erythrocyte sedimentation rate. But one needs only two or three of the five principal clinical KD criteria plus three or more of six other laboratory findings (anemia, low albumin, leukocytosis, thrombocytosis, pyuria, or elevated alanine aminotransferase). Incomplete KD can be confirmed by an abnormal echocardiogram – usually not until after 7 days of KD symptoms.1
New KD nuances
In a recent report on 20 years of data from Japan (n = 1,945 KD cases), more granularity on age, seasonal epidemiology, and outcome were seen.2 There was an inverse correlation of male predominance to age, i.e. as age groups got older, there was a gradual shift to female predominance by 7 years of age. The winter/spring predominance (60% of overall cases) did not hold true in younger age groups where summer/fall was the peak season (65% of cases).
With the goal of not missing any KD and diagnosing as early as possible to limit sequelae, we all need to be relative experts and keep alert for clinical scenarios that warrant our raising our index of suspicion. But now the seasonality trends appear blurred in the youngest cases and the male predominance is blurred in the oldest cases. And remember that fever and irritability for longer than 7 days in young infants may be the only clue to KD.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Circulation. 2017 Mar 29. doi: 10.1161/CIR.0000000000000484
2. N Engl J Med. 2018 May 24. doi: 10.1056/NEJMc1804312.
Most U.S. mainland pediatric practitioners will see only one or two cases of Kawasaki disease (KD) in their careers, but no one wants to miss even one case.
Making the diagnosis as early as possible is important to reduce the chance of sequelae, particularly the coronary artery aneurysms that will eventually lead to 5% of overall acute coronary syndromes in adults. And because there is no “KD test,” or sometimes incomplete KD. And there are some new data that complicate this. Despite the recently updated 2017 guideline,1 most cases end up being confirmed and managed by regional “experts.” But nearly all of the approximately 6,000 KD cases/year in U.S. children younger than 5 years old start out with one or more primary care, urgent care, or ED visits.
This means that every clinician in the trenches not only needs a high index of suspicion but also needs to be at least a partial expert, too. What raises our index of suspicion? Classic data tell us we need 5 consecutive days of fever plus four or five other principal clinical findings for a KD diagnosis. The principal findings are:
1. Eyes: Bilateral bulbar nonexudative conjunctival injection.
2. Mouth: Erythema of oral/pharyngeal mucosa or cracked lips or strawberry tongue or oral mucositis.
3. Rash.
4. Hands or feet findings: Swelling/erythema or later periungual desquamation.
5. Cervical adenopathy greater than 1.4 cm, usually unilateral.
Other factors that have classically increased suspicion are winter/early spring presentation in North America, male gender (1.5:1 ratio to females), and Asian (particularly Japanese) ancestry. The importance of genetics was initially based on epidemiology (Japan/Asian risk) but lately has been further associated with six gene polymorphisms. However, molecular genetic testing is not currently a practical tool.
Clinical scenarios that also should raise suspicion include less-than-6-month-old infants with prolonged fever/irritability (may be the only clinical manifestations of KD) and children over 4 years old who more often may have incomplete KD. Both groups have higher prevalence of coronary artery abnormalities. Other high suspicion scenarios include prolonged fever with unexplained/culture-negative shock, or antibiotic treatment failure for cervical adenitis or retro/parapharyngeal phlegmon. Consultation with or referral to a regional KD expert may be needed.
Fuzzy KD math
Current guidelines list an exception to the 5-day fever requirement in that only 4 days of fever are needed with four or more principal clinical features, particularly when hand and feet findings exist. Some call this the “4X4 exception.” Then there is a sub-caveat: “Experienced clinicians who have treated many patients with KD may establish the diagnosis with 3 days of fever in rare cases.”1
Incomplete KD
This is another exception, which seems to be a more frequent diagnosis in the past decade. Incomplete KD requires the 5 days of fever duration plus an elevated C-reactive protein or erythrocyte sedimentation rate. But one needs only two or three of the five principal clinical KD criteria plus three or more of six other laboratory findings (anemia, low albumin, leukocytosis, thrombocytosis, pyuria, or elevated alanine aminotransferase). Incomplete KD can be confirmed by an abnormal echocardiogram – usually not until after 7 days of KD symptoms.1
New KD nuances
In a recent report on 20 years of data from Japan (n = 1,945 KD cases), more granularity on age, seasonal epidemiology, and outcome were seen.2 There was an inverse correlation of male predominance to age, i.e. as age groups got older, there was a gradual shift to female predominance by 7 years of age. The winter/spring predominance (60% of overall cases) did not hold true in younger age groups where summer/fall was the peak season (65% of cases).
With the goal of not missing any KD and diagnosing as early as possible to limit sequelae, we all need to be relative experts and keep alert for clinical scenarios that warrant our raising our index of suspicion. But now the seasonality trends appear blurred in the youngest cases and the male predominance is blurred in the oldest cases. And remember that fever and irritability for longer than 7 days in young infants may be the only clue to KD.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Circulation. 2017 Mar 29. doi: 10.1161/CIR.0000000000000484
2. N Engl J Med. 2018 May 24. doi: 10.1056/NEJMc1804312.
Most U.S. mainland pediatric practitioners will see only one or two cases of Kawasaki disease (KD) in their careers, but no one wants to miss even one case.
Making the diagnosis as early as possible is important to reduce the chance of sequelae, particularly the coronary artery aneurysms that will eventually lead to 5% of overall acute coronary syndromes in adults. And because there is no “KD test,” or sometimes incomplete KD. And there are some new data that complicate this. Despite the recently updated 2017 guideline,1 most cases end up being confirmed and managed by regional “experts.” But nearly all of the approximately 6,000 KD cases/year in U.S. children younger than 5 years old start out with one or more primary care, urgent care, or ED visits.
This means that every clinician in the trenches not only needs a high index of suspicion but also needs to be at least a partial expert, too. What raises our index of suspicion? Classic data tell us we need 5 consecutive days of fever plus four or five other principal clinical findings for a KD diagnosis. The principal findings are:
1. Eyes: Bilateral bulbar nonexudative conjunctival injection.
2. Mouth: Erythema of oral/pharyngeal mucosa or cracked lips or strawberry tongue or oral mucositis.
3. Rash.
4. Hands or feet findings: Swelling/erythema or later periungual desquamation.
5. Cervical adenopathy greater than 1.4 cm, usually unilateral.
Other factors that have classically increased suspicion are winter/early spring presentation in North America, male gender (1.5:1 ratio to females), and Asian (particularly Japanese) ancestry. The importance of genetics was initially based on epidemiology (Japan/Asian risk) but lately has been further associated with six gene polymorphisms. However, molecular genetic testing is not currently a practical tool.
Clinical scenarios that also should raise suspicion include less-than-6-month-old infants with prolonged fever/irritability (may be the only clinical manifestations of KD) and children over 4 years old who more often may have incomplete KD. Both groups have higher prevalence of coronary artery abnormalities. Other high suspicion scenarios include prolonged fever with unexplained/culture-negative shock, or antibiotic treatment failure for cervical adenitis or retro/parapharyngeal phlegmon. Consultation with or referral to a regional KD expert may be needed.
Fuzzy KD math
Current guidelines list an exception to the 5-day fever requirement in that only 4 days of fever are needed with four or more principal clinical features, particularly when hand and feet findings exist. Some call this the “4X4 exception.” Then there is a sub-caveat: “Experienced clinicians who have treated many patients with KD may establish the diagnosis with 3 days of fever in rare cases.”1
Incomplete KD
This is another exception, which seems to be a more frequent diagnosis in the past decade. Incomplete KD requires the 5 days of fever duration plus an elevated C-reactive protein or erythrocyte sedimentation rate. But one needs only two or three of the five principal clinical KD criteria plus three or more of six other laboratory findings (anemia, low albumin, leukocytosis, thrombocytosis, pyuria, or elevated alanine aminotransferase). Incomplete KD can be confirmed by an abnormal echocardiogram – usually not until after 7 days of KD symptoms.1
New KD nuances
In a recent report on 20 years of data from Japan (n = 1,945 KD cases), more granularity on age, seasonal epidemiology, and outcome were seen.2 There was an inverse correlation of male predominance to age, i.e. as age groups got older, there was a gradual shift to female predominance by 7 years of age. The winter/spring predominance (60% of overall cases) did not hold true in younger age groups where summer/fall was the peak season (65% of cases).
With the goal of not missing any KD and diagnosing as early as possible to limit sequelae, we all need to be relative experts and keep alert for clinical scenarios that warrant our raising our index of suspicion. But now the seasonality trends appear blurred in the youngest cases and the male predominance is blurred in the oldest cases. And remember that fever and irritability for longer than 7 days in young infants may be the only clue to KD.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Circulation. 2017 Mar 29. doi: 10.1161/CIR.0000000000000484
2. N Engl J Med. 2018 May 24. doi: 10.1056/NEJMc1804312.