User login
Xenon imaging could detect lung involvement after HSCT
SALT LAKE CITY – Hyperpolarized xenon-129 magnetic resonance imaging, or 129Xe MRI, showed strong promise for revealing early lung ventilation deficits in pediatric hematopoietic stem cell transplant (HSCT) patients in a proof-of-concept study.
The use of hyperpolarized xenon gas in this setting remains investigational, but is emerging as a safe non-ionizing approach for mapping and quantifying regional airway obstruction in the pediatric population. It has been shown to be more sensitive to early disease than the current clinical gold standard of measuring forced expiratory volume in 1 second (FEV1) by spirometry, Laura L. Walkup, PhD, said at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
The 129Xe MRI provides regional information that spirometry cannot, allowing for a targeted approach to planned procedures such as bronchoscopy, said Dr. Walkup of Cincinnati Children’s Hospital Medical Center.
“We hypothesized that hyperpolarized 129Xe MRI would be sensitive to lung abnormalities in the pediatric HSCT population,” she said.
Of 13 patients aged 6-13 years (mean, 10 years) who were enrolled in the study and underwent 129XeMRI, 9 also completed spirometry successfully, and the average FEV1 in those patients was 83% of the predicted value.
Ventilation deficits were apparent on the 129Xe MRI imaging in 8 of the 13 subjects and varied in regional distribution. The whole-lung 129Xe ventilation defect percentage for the HSCT group was 14%, which was significantly greater than the approximately 6% ventilation defect percentage in a cohort of age-matched controls, Dr. Walkup said, noting that ventilation deficits were seen in three of four subjects who were unable to complete reliable spirometry.
“So those are lung abnormalities that may have otherwise gone undetected,” she said, adding that hyperpolarized xenon gas also highlighted the wide individual variation in ventilation, even among cases with similar FEV1 percentages.
The findings are notable, because pulmonary complications such as bronchiolitis obliterans are a major source of morbidity and mortality in the pediatric HSCT population, and an accurate and early diagnostic tool identifying the location and severity of suspected obstructive lung pathology following HSCT is desperately needed, she said.
The HSCT patients in the current study included four boys and nine girls. Isotopically-enriched xenon gas (86% 129Xe) was hyperpolarized using a commercial polarizer and images were acquired during a breath hold of up to 16 seconds and up to 1 L of xenon gas. Conventional anatomic MR images also were acquired.
The 129Xe ventilation was quantified using a less than 60% mean whole-lung 129Xe signal threshold, and was compared to FEV1 percentage predicted as measured via spirometry.
The procedure was well tolerated by all patients, Dr. Walkup said, noting that no patients withdrew from the study, and all were able to maintain the required breath hold.
Drops in blood oxygen saturation level did occur, but were transient and resolved within 10-30 seconds of normal breathing. Further, there were no changes in heart rate during imaging, and any side effects related to xenon, such as tingling in extremities, dizziness, or euphoria, were also quickly resolved with normal breathing, she said.
“There were no serious adverse events related to the study ... these results are in good agreement with previously published safety assessments of xenon in kids and in adults, and at our institution we routinely perform xenon imaging in children as young as age 6,” she added.
The findings, which are consistent with those seen in studies of other conditions such as cystic fibrosis, asthma, and chronic obstructive pulmonary disease, suggest that 129Xe MRI is an emerging modality with strong translational potential for detecting early pulmonary involvement following HSCT, she said.
“The real power of the xenon MRI is the spatial information that it provides; we can use that information to plan targeted procedures like bronchoscopy and biopsies ... and since it is non-ionizing, it may be used serially to assess disease progression or response to an intervention,” Dr. Walkup said.
She noted, however, that because it is not yet approved by the Food and Drug Administration, and because it requires specialized expertise and hardware, it is available at only a handful of centers worldwide.
There is a long way to go before the technology will be widely clinically implemented, but work is ongoing at Cincinnati Children’s Hospital to determine how xenon MRI may play a role in pulmonary screening of patients, she said.
Dr. Walkup reported having no financial disclosures.
SOURCE: Walkup L et al. 2018 BMT Tandem Meetings Abstract 56.
SALT LAKE CITY – Hyperpolarized xenon-129 magnetic resonance imaging, or 129Xe MRI, showed strong promise for revealing early lung ventilation deficits in pediatric hematopoietic stem cell transplant (HSCT) patients in a proof-of-concept study.
The use of hyperpolarized xenon gas in this setting remains investigational, but is emerging as a safe non-ionizing approach for mapping and quantifying regional airway obstruction in the pediatric population. It has been shown to be more sensitive to early disease than the current clinical gold standard of measuring forced expiratory volume in 1 second (FEV1) by spirometry, Laura L. Walkup, PhD, said at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
The 129Xe MRI provides regional information that spirometry cannot, allowing for a targeted approach to planned procedures such as bronchoscopy, said Dr. Walkup of Cincinnati Children’s Hospital Medical Center.
“We hypothesized that hyperpolarized 129Xe MRI would be sensitive to lung abnormalities in the pediatric HSCT population,” she said.
Of 13 patients aged 6-13 years (mean, 10 years) who were enrolled in the study and underwent 129XeMRI, 9 also completed spirometry successfully, and the average FEV1 in those patients was 83% of the predicted value.
Ventilation deficits were apparent on the 129Xe MRI imaging in 8 of the 13 subjects and varied in regional distribution. The whole-lung 129Xe ventilation defect percentage for the HSCT group was 14%, which was significantly greater than the approximately 6% ventilation defect percentage in a cohort of age-matched controls, Dr. Walkup said, noting that ventilation deficits were seen in three of four subjects who were unable to complete reliable spirometry.
“So those are lung abnormalities that may have otherwise gone undetected,” she said, adding that hyperpolarized xenon gas also highlighted the wide individual variation in ventilation, even among cases with similar FEV1 percentages.
The findings are notable, because pulmonary complications such as bronchiolitis obliterans are a major source of morbidity and mortality in the pediatric HSCT population, and an accurate and early diagnostic tool identifying the location and severity of suspected obstructive lung pathology following HSCT is desperately needed, she said.
The HSCT patients in the current study included four boys and nine girls. Isotopically-enriched xenon gas (86% 129Xe) was hyperpolarized using a commercial polarizer and images were acquired during a breath hold of up to 16 seconds and up to 1 L of xenon gas. Conventional anatomic MR images also were acquired.
The 129Xe ventilation was quantified using a less than 60% mean whole-lung 129Xe signal threshold, and was compared to FEV1 percentage predicted as measured via spirometry.
The procedure was well tolerated by all patients, Dr. Walkup said, noting that no patients withdrew from the study, and all were able to maintain the required breath hold.
Drops in blood oxygen saturation level did occur, but were transient and resolved within 10-30 seconds of normal breathing. Further, there were no changes in heart rate during imaging, and any side effects related to xenon, such as tingling in extremities, dizziness, or euphoria, were also quickly resolved with normal breathing, she said.
“There were no serious adverse events related to the study ... these results are in good agreement with previously published safety assessments of xenon in kids and in adults, and at our institution we routinely perform xenon imaging in children as young as age 6,” she added.
The findings, which are consistent with those seen in studies of other conditions such as cystic fibrosis, asthma, and chronic obstructive pulmonary disease, suggest that 129Xe MRI is an emerging modality with strong translational potential for detecting early pulmonary involvement following HSCT, she said.
“The real power of the xenon MRI is the spatial information that it provides; we can use that information to plan targeted procedures like bronchoscopy and biopsies ... and since it is non-ionizing, it may be used serially to assess disease progression or response to an intervention,” Dr. Walkup said.
She noted, however, that because it is not yet approved by the Food and Drug Administration, and because it requires specialized expertise and hardware, it is available at only a handful of centers worldwide.
There is a long way to go before the technology will be widely clinically implemented, but work is ongoing at Cincinnati Children’s Hospital to determine how xenon MRI may play a role in pulmonary screening of patients, she said.
Dr. Walkup reported having no financial disclosures.
SOURCE: Walkup L et al. 2018 BMT Tandem Meetings Abstract 56.
SALT LAKE CITY – Hyperpolarized xenon-129 magnetic resonance imaging, or 129Xe MRI, showed strong promise for revealing early lung ventilation deficits in pediatric hematopoietic stem cell transplant (HSCT) patients in a proof-of-concept study.
The use of hyperpolarized xenon gas in this setting remains investigational, but is emerging as a safe non-ionizing approach for mapping and quantifying regional airway obstruction in the pediatric population. It has been shown to be more sensitive to early disease than the current clinical gold standard of measuring forced expiratory volume in 1 second (FEV1) by spirometry, Laura L. Walkup, PhD, said at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
The 129Xe MRI provides regional information that spirometry cannot, allowing for a targeted approach to planned procedures such as bronchoscopy, said Dr. Walkup of Cincinnati Children’s Hospital Medical Center.
“We hypothesized that hyperpolarized 129Xe MRI would be sensitive to lung abnormalities in the pediatric HSCT population,” she said.
Of 13 patients aged 6-13 years (mean, 10 years) who were enrolled in the study and underwent 129XeMRI, 9 also completed spirometry successfully, and the average FEV1 in those patients was 83% of the predicted value.
Ventilation deficits were apparent on the 129Xe MRI imaging in 8 of the 13 subjects and varied in regional distribution. The whole-lung 129Xe ventilation defect percentage for the HSCT group was 14%, which was significantly greater than the approximately 6% ventilation defect percentage in a cohort of age-matched controls, Dr. Walkup said, noting that ventilation deficits were seen in three of four subjects who were unable to complete reliable spirometry.
“So those are lung abnormalities that may have otherwise gone undetected,” she said, adding that hyperpolarized xenon gas also highlighted the wide individual variation in ventilation, even among cases with similar FEV1 percentages.
The findings are notable, because pulmonary complications such as bronchiolitis obliterans are a major source of morbidity and mortality in the pediatric HSCT population, and an accurate and early diagnostic tool identifying the location and severity of suspected obstructive lung pathology following HSCT is desperately needed, she said.
The HSCT patients in the current study included four boys and nine girls. Isotopically-enriched xenon gas (86% 129Xe) was hyperpolarized using a commercial polarizer and images were acquired during a breath hold of up to 16 seconds and up to 1 L of xenon gas. Conventional anatomic MR images also were acquired.
The 129Xe ventilation was quantified using a less than 60% mean whole-lung 129Xe signal threshold, and was compared to FEV1 percentage predicted as measured via spirometry.
The procedure was well tolerated by all patients, Dr. Walkup said, noting that no patients withdrew from the study, and all were able to maintain the required breath hold.
Drops in blood oxygen saturation level did occur, but were transient and resolved within 10-30 seconds of normal breathing. Further, there were no changes in heart rate during imaging, and any side effects related to xenon, such as tingling in extremities, dizziness, or euphoria, were also quickly resolved with normal breathing, she said.
“There were no serious adverse events related to the study ... these results are in good agreement with previously published safety assessments of xenon in kids and in adults, and at our institution we routinely perform xenon imaging in children as young as age 6,” she added.
The findings, which are consistent with those seen in studies of other conditions such as cystic fibrosis, asthma, and chronic obstructive pulmonary disease, suggest that 129Xe MRI is an emerging modality with strong translational potential for detecting early pulmonary involvement following HSCT, she said.
“The real power of the xenon MRI is the spatial information that it provides; we can use that information to plan targeted procedures like bronchoscopy and biopsies ... and since it is non-ionizing, it may be used serially to assess disease progression or response to an intervention,” Dr. Walkup said.
She noted, however, that because it is not yet approved by the Food and Drug Administration, and because it requires specialized expertise and hardware, it is available at only a handful of centers worldwide.
There is a long way to go before the technology will be widely clinically implemented, but work is ongoing at Cincinnati Children’s Hospital to determine how xenon MRI may play a role in pulmonary screening of patients, she said.
Dr. Walkup reported having no financial disclosures.
SOURCE: Walkup L et al. 2018 BMT Tandem Meetings Abstract 56.
REPORTING FROM THE 2018 BMT TANDEM MEETINGS
Key clinical point: Hyperpolarized 129Xe MRI shows promise for revealing lung ventilation deficits in pediatric HSCT patients.
Major finding: The whole-lung 129Xe ventilation defect percentage was 14% for HSCT group versus 6% for controls; deficits were seen in three of four subjects who were who were unable to complete reliable spirometry.
Study details: A proof-of-concept study involving 13 children.
Disclosures: Dr. Walkup reported having no financial disclosures.
Source: Walkup L et al. 2018 BMT Tandem Meetings. Abstract 56.
Podcasts
you need no effort to enjoy them. Indeed, you can be actively engaged elsewhere: running, cycling, commuting, or simply loafing. The detail and richness of the sound also creates an intimate connection with the host in a way other mediums cannot. It feels like they are talking only to you.
Yet, there is a problem with podcasts: There are too many of them. If I listened continuously to the episodes in my queue it would take 6 months. I suppose I could see patients and listen at the same time. (Yes, I have a problem.) I also own far more books than I’ll ever read. Aspirational, I call it.
If, like me, you’re unable to dedicate your life to consuming podcasts, you might appreciate a few recommendations. Here’s a charcuterie board of tasty bits, carefully curated to avoid political allergies and Dunning-Kruger references.
1. Physicians Practice. It’s one of the oldest podcasts running and addresses a wide range of issues affecting health care professionals and the industry at large. Episodes are short (typically under 10 minutes) and address a range of issues relevant to both young and seasoned physicians With scores of podcasts from which to choose, I suggest just selecting one and jumping in. With episode titles such as “The Patient Empathy Problem Physicians Must Face” and “EHRs Not Designed with Real People in Mind, Expert Opines,” it’s easy to do.
2. UpToDate. If you’re looking for straight clinical talk buttressed with scientific evidence, then download UpToDate. Episodes typically feature interviews with one or two respected physician leaders who share their clinical findings. You can select episode topics based upon clinical specialty or simply start listening. Here is a sampling of topics: sentinel lymph node metastasis in melanoma; dexamethasone and acute pharyngitis pain in adults; management of anticoagulation for patients with nonvalvular atrial fibrillation. UpToDate states that it is entirely funded by user subscriptions and does not accept advertising or funding unrelated to subscriptions.
3. Bedside Rounds. The tagline for the podcast Bedside Rounds is “Because medicine is awesome.” This is not meant to be ironic. Creator and host, Adam Rodman, MD, a global health hospitalist who divides his time between Boston (at the Beth Israel Deaconess Medical Center) and Botswana, is that eager kid in the classroom who sits in the front row just because he’s so excited to be there. Unlike UpToDate, which focuses on current advances in clinical medicine, Bedside Rounds explores both the science and art of medicine through captivating stories heavily rooted in the history of medicine. Instead of brushing the dust off of your old medical books, tune in to Bedside Rounds to hear stories such as “Bone Portraits,” which explores the history of radiation, and “Curse of the Ninth,” which explores whether or not composer Gustav Mahler, worked his heart murmur into his famous Ninth Symphony.
4. The Adventures of Memento Mori. Creator and host, D.S. Moss, has created a podcast about death, or, to be more upbeat, the quest for the meaning of life. A screenwriter/producer, Mr. Moss deep dives into all things death. But it’s not as depressing as it sounds. “Memento mori,” he explains, is Latin for being mindful that you will die. As a result, Mr. Moss has created his podcast with the goal of encouraging listeners to live a more engaged, mindful, and meaningful life. We can apply many of these lessons to our own professional and personal lives and perhaps learn some ways to help our patients cope with terminal illness and mortality. Topics range from the emotional (“Thoughts in Passing,” which features several hospice patients) to the technological (“Digital Afterlife,” which explores what our digital legacies say about us), to the scientific (“The Science of Immortality,” which explores venture capital’s movement in the science of living forever).
5. Invisibilia. Invisibilia – Latin for invisible things – is an exploration of the “unseeable forces” that shape human behavior – our beliefs, thoughts, and assumptions. Hosts Alix Spiegel and Hanna Rosin, both of National Public Radio, seamlessly weave storytelling, interviewing, and scientific data to tackle a wide range of topics such as prejudice and implicit bias in “The Culture Inside” to people’s desire for radical change in “Bubble Hopping.” Part behavioral economics, part neuroscience, part sociology, part pop culture, fully fascinating.
6. Jocko Podcast. Jocko Willink, a retired Navy SEAL and motivational author and speaker, along with Echo Charles, conduct compelling interviews with leaders from various fields including the military, sports, medicine, and the arts. Mr. Willink’s style of motivation is refreshingly honest and direct. I have taken tips from his podcasts that have helped me become a more efficient and energetic physician and leader. Two fundamental themes that run through his podcast are the value of treating people well and of living your life with discipline. It gets you a long way as a Navy SEAL, as well as a doctor. One of my personal favorites is Episode 69: “The Real Top Gun. Battlefield, Work, and Life are Identical” with Elite Marine Fighter Pilot, David Berke. If that doesn’t pique your interest, no worries; there are over 100 episodes from which to choose.
There are many, many more podcasts I’d like to recommend, but I’ll show some discipline (thanks, Jocko) and save them for next time.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
you need no effort to enjoy them. Indeed, you can be actively engaged elsewhere: running, cycling, commuting, or simply loafing. The detail and richness of the sound also creates an intimate connection with the host in a way other mediums cannot. It feels like they are talking only to you.
Yet, there is a problem with podcasts: There are too many of them. If I listened continuously to the episodes in my queue it would take 6 months. I suppose I could see patients and listen at the same time. (Yes, I have a problem.) I also own far more books than I’ll ever read. Aspirational, I call it.
If, like me, you’re unable to dedicate your life to consuming podcasts, you might appreciate a few recommendations. Here’s a charcuterie board of tasty bits, carefully curated to avoid political allergies and Dunning-Kruger references.
1. Physicians Practice. It’s one of the oldest podcasts running and addresses a wide range of issues affecting health care professionals and the industry at large. Episodes are short (typically under 10 minutes) and address a range of issues relevant to both young and seasoned physicians With scores of podcasts from which to choose, I suggest just selecting one and jumping in. With episode titles such as “The Patient Empathy Problem Physicians Must Face” and “EHRs Not Designed with Real People in Mind, Expert Opines,” it’s easy to do.
2. UpToDate. If you’re looking for straight clinical talk buttressed with scientific evidence, then download UpToDate. Episodes typically feature interviews with one or two respected physician leaders who share their clinical findings. You can select episode topics based upon clinical specialty or simply start listening. Here is a sampling of topics: sentinel lymph node metastasis in melanoma; dexamethasone and acute pharyngitis pain in adults; management of anticoagulation for patients with nonvalvular atrial fibrillation. UpToDate states that it is entirely funded by user subscriptions and does not accept advertising or funding unrelated to subscriptions.
3. Bedside Rounds. The tagline for the podcast Bedside Rounds is “Because medicine is awesome.” This is not meant to be ironic. Creator and host, Adam Rodman, MD, a global health hospitalist who divides his time between Boston (at the Beth Israel Deaconess Medical Center) and Botswana, is that eager kid in the classroom who sits in the front row just because he’s so excited to be there. Unlike UpToDate, which focuses on current advances in clinical medicine, Bedside Rounds explores both the science and art of medicine through captivating stories heavily rooted in the history of medicine. Instead of brushing the dust off of your old medical books, tune in to Bedside Rounds to hear stories such as “Bone Portraits,” which explores the history of radiation, and “Curse of the Ninth,” which explores whether or not composer Gustav Mahler, worked his heart murmur into his famous Ninth Symphony.
4. The Adventures of Memento Mori. Creator and host, D.S. Moss, has created a podcast about death, or, to be more upbeat, the quest for the meaning of life. A screenwriter/producer, Mr. Moss deep dives into all things death. But it’s not as depressing as it sounds. “Memento mori,” he explains, is Latin for being mindful that you will die. As a result, Mr. Moss has created his podcast with the goal of encouraging listeners to live a more engaged, mindful, and meaningful life. We can apply many of these lessons to our own professional and personal lives and perhaps learn some ways to help our patients cope with terminal illness and mortality. Topics range from the emotional (“Thoughts in Passing,” which features several hospice patients) to the technological (“Digital Afterlife,” which explores what our digital legacies say about us), to the scientific (“The Science of Immortality,” which explores venture capital’s movement in the science of living forever).
5. Invisibilia. Invisibilia – Latin for invisible things – is an exploration of the “unseeable forces” that shape human behavior – our beliefs, thoughts, and assumptions. Hosts Alix Spiegel and Hanna Rosin, both of National Public Radio, seamlessly weave storytelling, interviewing, and scientific data to tackle a wide range of topics such as prejudice and implicit bias in “The Culture Inside” to people’s desire for radical change in “Bubble Hopping.” Part behavioral economics, part neuroscience, part sociology, part pop culture, fully fascinating.
6. Jocko Podcast. Jocko Willink, a retired Navy SEAL and motivational author and speaker, along with Echo Charles, conduct compelling interviews with leaders from various fields including the military, sports, medicine, and the arts. Mr. Willink’s style of motivation is refreshingly honest and direct. I have taken tips from his podcasts that have helped me become a more efficient and energetic physician and leader. Two fundamental themes that run through his podcast are the value of treating people well and of living your life with discipline. It gets you a long way as a Navy SEAL, as well as a doctor. One of my personal favorites is Episode 69: “The Real Top Gun. Battlefield, Work, and Life are Identical” with Elite Marine Fighter Pilot, David Berke. If that doesn’t pique your interest, no worries; there are over 100 episodes from which to choose.
There are many, many more podcasts I’d like to recommend, but I’ll show some discipline (thanks, Jocko) and save them for next time.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
you need no effort to enjoy them. Indeed, you can be actively engaged elsewhere: running, cycling, commuting, or simply loafing. The detail and richness of the sound also creates an intimate connection with the host in a way other mediums cannot. It feels like they are talking only to you.
Yet, there is a problem with podcasts: There are too many of them. If I listened continuously to the episodes in my queue it would take 6 months. I suppose I could see patients and listen at the same time. (Yes, I have a problem.) I also own far more books than I’ll ever read. Aspirational, I call it.
If, like me, you’re unable to dedicate your life to consuming podcasts, you might appreciate a few recommendations. Here’s a charcuterie board of tasty bits, carefully curated to avoid political allergies and Dunning-Kruger references.
1. Physicians Practice. It’s one of the oldest podcasts running and addresses a wide range of issues affecting health care professionals and the industry at large. Episodes are short (typically under 10 minutes) and address a range of issues relevant to both young and seasoned physicians With scores of podcasts from which to choose, I suggest just selecting one and jumping in. With episode titles such as “The Patient Empathy Problem Physicians Must Face” and “EHRs Not Designed with Real People in Mind, Expert Opines,” it’s easy to do.
2. UpToDate. If you’re looking for straight clinical talk buttressed with scientific evidence, then download UpToDate. Episodes typically feature interviews with one or two respected physician leaders who share their clinical findings. You can select episode topics based upon clinical specialty or simply start listening. Here is a sampling of topics: sentinel lymph node metastasis in melanoma; dexamethasone and acute pharyngitis pain in adults; management of anticoagulation for patients with nonvalvular atrial fibrillation. UpToDate states that it is entirely funded by user subscriptions and does not accept advertising or funding unrelated to subscriptions.
3. Bedside Rounds. The tagline for the podcast Bedside Rounds is “Because medicine is awesome.” This is not meant to be ironic. Creator and host, Adam Rodman, MD, a global health hospitalist who divides his time between Boston (at the Beth Israel Deaconess Medical Center) and Botswana, is that eager kid in the classroom who sits in the front row just because he’s so excited to be there. Unlike UpToDate, which focuses on current advances in clinical medicine, Bedside Rounds explores both the science and art of medicine through captivating stories heavily rooted in the history of medicine. Instead of brushing the dust off of your old medical books, tune in to Bedside Rounds to hear stories such as “Bone Portraits,” which explores the history of radiation, and “Curse of the Ninth,” which explores whether or not composer Gustav Mahler, worked his heart murmur into his famous Ninth Symphony.
4. The Adventures of Memento Mori. Creator and host, D.S. Moss, has created a podcast about death, or, to be more upbeat, the quest for the meaning of life. A screenwriter/producer, Mr. Moss deep dives into all things death. But it’s not as depressing as it sounds. “Memento mori,” he explains, is Latin for being mindful that you will die. As a result, Mr. Moss has created his podcast with the goal of encouraging listeners to live a more engaged, mindful, and meaningful life. We can apply many of these lessons to our own professional and personal lives and perhaps learn some ways to help our patients cope with terminal illness and mortality. Topics range from the emotional (“Thoughts in Passing,” which features several hospice patients) to the technological (“Digital Afterlife,” which explores what our digital legacies say about us), to the scientific (“The Science of Immortality,” which explores venture capital’s movement in the science of living forever).
5. Invisibilia. Invisibilia – Latin for invisible things – is an exploration of the “unseeable forces” that shape human behavior – our beliefs, thoughts, and assumptions. Hosts Alix Spiegel and Hanna Rosin, both of National Public Radio, seamlessly weave storytelling, interviewing, and scientific data to tackle a wide range of topics such as prejudice and implicit bias in “The Culture Inside” to people’s desire for radical change in “Bubble Hopping.” Part behavioral economics, part neuroscience, part sociology, part pop culture, fully fascinating.
6. Jocko Podcast. Jocko Willink, a retired Navy SEAL and motivational author and speaker, along with Echo Charles, conduct compelling interviews with leaders from various fields including the military, sports, medicine, and the arts. Mr. Willink’s style of motivation is refreshingly honest and direct. I have taken tips from his podcasts that have helped me become a more efficient and energetic physician and leader. Two fundamental themes that run through his podcast are the value of treating people well and of living your life with discipline. It gets you a long way as a Navy SEAL, as well as a doctor. One of my personal favorites is Episode 69: “The Real Top Gun. Battlefield, Work, and Life are Identical” with Elite Marine Fighter Pilot, David Berke. If that doesn’t pique your interest, no worries; there are over 100 episodes from which to choose.
There are many, many more podcasts I’d like to recommend, but I’ll show some discipline (thanks, Jocko) and save them for next time.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
MDedge Daily News: Avoid warfarin’s polypharmacy perils
Which non-AIDS conditions hint at HIV infection? How San Francisco delivers faster HIV treatment. And red meat may fuel the rise of fatty livers.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Which non-AIDS conditions hint at HIV infection? How San Francisco delivers faster HIV treatment. And red meat may fuel the rise of fatty livers.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Which non-AIDS conditions hint at HIV infection? How San Francisco delivers faster HIV treatment. And red meat may fuel the rise of fatty livers.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Unprecedented Study Gathers Data on Adolescent Development
An “unparalleled dataset” is providing high-quality information on the many factors that influence brain, cognitive, social, and emotional development in children.
The Adolescent Brain Cognitive Development (ABCD) study, involving > 7,500 children aged between 9 and 10 years and their families, is the largest long-term study of brain development and child health in the US. Interim results on about 30 terabytes of data (3 times the size of the Library of Congress collection) obtained from the first 4,500 participants will allow scientists to begin analyzing and publishing novel research, according to Nora Volkow, MD, director of the National Institute on Drug Abuse.
Researchers will be able to examine many aspects of growth and development, such as the impact of sports injuries on developmental outcomes, the relationship between screen time and brain and social development, and which brain pathways are associated with the onset and progression of mental health disorders.
The study aims to enroll 11,500 children by the end of 2018. Participants will be followed for 10 years, with data collected every 6 months through interviews and behavioral testing. Neuroimaging data, including high resolution MRI, are collected every 2 years.
An “unparalleled dataset” is providing high-quality information on the many factors that influence brain, cognitive, social, and emotional development in children.
The Adolescent Brain Cognitive Development (ABCD) study, involving > 7,500 children aged between 9 and 10 years and their families, is the largest long-term study of brain development and child health in the US. Interim results on about 30 terabytes of data (3 times the size of the Library of Congress collection) obtained from the first 4,500 participants will allow scientists to begin analyzing and publishing novel research, according to Nora Volkow, MD, director of the National Institute on Drug Abuse.
Researchers will be able to examine many aspects of growth and development, such as the impact of sports injuries on developmental outcomes, the relationship between screen time and brain and social development, and which brain pathways are associated with the onset and progression of mental health disorders.
The study aims to enroll 11,500 children by the end of 2018. Participants will be followed for 10 years, with data collected every 6 months through interviews and behavioral testing. Neuroimaging data, including high resolution MRI, are collected every 2 years.
An “unparalleled dataset” is providing high-quality information on the many factors that influence brain, cognitive, social, and emotional development in children.
The Adolescent Brain Cognitive Development (ABCD) study, involving > 7,500 children aged between 9 and 10 years and their families, is the largest long-term study of brain development and child health in the US. Interim results on about 30 terabytes of data (3 times the size of the Library of Congress collection) obtained from the first 4,500 participants will allow scientists to begin analyzing and publishing novel research, according to Nora Volkow, MD, director of the National Institute on Drug Abuse.
Researchers will be able to examine many aspects of growth and development, such as the impact of sports injuries on developmental outcomes, the relationship between screen time and brain and social development, and which brain pathways are associated with the onset and progression of mental health disorders.
The study aims to enroll 11,500 children by the end of 2018. Participants will be followed for 10 years, with data collected every 6 months through interviews and behavioral testing. Neuroimaging data, including high resolution MRI, are collected every 2 years.
Kids may have higher rate of AdV infection after HSCT
LISBON—Results of a large study revealed a higher incidence of adenovirus (AdV) infection after allogeneic hematopoietic stem cell transplant (allo-HSCT) in children than adults.
The rate of AdV infection in the 6 months after allo-HSCT was 32% in children and 6% in adults.
However, researchers believe this data may be influenced by a lack of routine AdV screening in adults.
These findings—from the AdVance study—were reported at the 44th Annual Meeting of the EBMT as abstracts OS9-7 and B043.*
Other results from AdVance were also presented at the meeting, with mortality data reported as abstract OS9-8 and hospitalization data reported as abstract B073. The AdVance study was sponsored by Chimerix, Inc.
“The robust findings of the AdVance study are extremely important for transplant clinicians, as we seek to better understand the rates and clinical outcomes of adenovirus infection and assess ways to evaluate antiviral therapies,” said study investigator Marco Zecca, MD, of Fondazione IRCCS Policlinico San Matteo in Pavia, Italy.
AdVance is a multi-center, retrospective study of 4276 allo-HSCT recipients—1738 pediatric patients and 2538 adults. The patients underwent transplants at 50 centers in Europe from January 2013 to September 2015.
Abstract OS9-7: Incidence
Thirty-two percent (n=558) of pediatric patients developed an AdV infection in the first 6 months after allo-HSCT, and 23% (n=395) developed detectable AdV viremia. Fourteen percent (n=241) had AdV viremia ≥ 1000 copies/mL, a level previously associated with negative clinical outcomes.
Meanwhile, 6% (n=141) of adults had an AdV infection in the 6 months after transplant, 3% (n=77) developed AdV viremia, and 2% (n=39) had ≥ 1000 copies /mL.
Further analysis revealed that age, donor type, and use of T-cell depletion were independent predictors of AdV viremia ≥ 1000 copies/mL.
Older age was associated with a lower risk of AdV viremia ≥ 1000 copies/mL. There was a stepwise reduction in risk with increasing age (compared to ages 0-2, P=0.104 for ages 2-12, P=0.001 for ages 13-17, and P<0.0001 for ages 18-65+).
T-cell depletion was associated with an increased risk of AdV viremia ≥ 1000 copies/mL. Compared to no T-cell depletion, there was an increased risk for depletion with antithymocyte globulin (P=0.036) or alemtuzumab (P<0.0001) and for ex vivo depletion (P=0.002).
Compared to patients who received a matched related transplant, recipients of other transplants had an increased risk of AdV viremia ≥ 1000 copies/mL. This includes matched unrelated (P=0.016), cord blood (P=0.011), haploidentical (P=0.007), and mismatched (P<0.001) transplants.
Abstract B043: Screening
Dr Zecca and his colleagues believe the difference in AdV incidence between children and adults may have been affected by a lack of routine screening in adults.
To assess the use of screening, the investigators analyzed practice surveys from physicians at centers included in the AdVance study. There were 28 survey respondents who treat pediatric patients and 14 who treat adults.
All 28 physicians treating pediatric patients said there is routine AdV screening at their center. Ninety-three percent of these doctors said they routinely screen all pediatric allo-HSCT recipients for AdV infection. The remaining 7% said they screen patients considered to be at high-risk of AdV infection.
Thirty-six percent of the physicians treating adults (5/14) said they routinely screen for AdV, and 21% (3/14) said they routinely screen all of their adult allo-HSCT recipients for AdV infection.
Of the 11 doctors who said they did not routinely screen adult allo-HSCT recipients, some said they would screen recipients with a high-risk of AdV infection, such as those with graft-versus-host disease (4/11) or those who received haploidentical, mismatched, or cord blood transplants (3/11).
*Data in the abstracts differ from the presentations.
LISBON—Results of a large study revealed a higher incidence of adenovirus (AdV) infection after allogeneic hematopoietic stem cell transplant (allo-HSCT) in children than adults.
The rate of AdV infection in the 6 months after allo-HSCT was 32% in children and 6% in adults.
However, researchers believe this data may be influenced by a lack of routine AdV screening in adults.
These findings—from the AdVance study—were reported at the 44th Annual Meeting of the EBMT as abstracts OS9-7 and B043.*
Other results from AdVance were also presented at the meeting, with mortality data reported as abstract OS9-8 and hospitalization data reported as abstract B073. The AdVance study was sponsored by Chimerix, Inc.
“The robust findings of the AdVance study are extremely important for transplant clinicians, as we seek to better understand the rates and clinical outcomes of adenovirus infection and assess ways to evaluate antiviral therapies,” said study investigator Marco Zecca, MD, of Fondazione IRCCS Policlinico San Matteo in Pavia, Italy.
AdVance is a multi-center, retrospective study of 4276 allo-HSCT recipients—1738 pediatric patients and 2538 adults. The patients underwent transplants at 50 centers in Europe from January 2013 to September 2015.
Abstract OS9-7: Incidence
Thirty-two percent (n=558) of pediatric patients developed an AdV infection in the first 6 months after allo-HSCT, and 23% (n=395) developed detectable AdV viremia. Fourteen percent (n=241) had AdV viremia ≥ 1000 copies/mL, a level previously associated with negative clinical outcomes.
Meanwhile, 6% (n=141) of adults had an AdV infection in the 6 months after transplant, 3% (n=77) developed AdV viremia, and 2% (n=39) had ≥ 1000 copies /mL.
Further analysis revealed that age, donor type, and use of T-cell depletion were independent predictors of AdV viremia ≥ 1000 copies/mL.
Older age was associated with a lower risk of AdV viremia ≥ 1000 copies/mL. There was a stepwise reduction in risk with increasing age (compared to ages 0-2, P=0.104 for ages 2-12, P=0.001 for ages 13-17, and P<0.0001 for ages 18-65+).
T-cell depletion was associated with an increased risk of AdV viremia ≥ 1000 copies/mL. Compared to no T-cell depletion, there was an increased risk for depletion with antithymocyte globulin (P=0.036) or alemtuzumab (P<0.0001) and for ex vivo depletion (P=0.002).
Compared to patients who received a matched related transplant, recipients of other transplants had an increased risk of AdV viremia ≥ 1000 copies/mL. This includes matched unrelated (P=0.016), cord blood (P=0.011), haploidentical (P=0.007), and mismatched (P<0.001) transplants.
Abstract B043: Screening
Dr Zecca and his colleagues believe the difference in AdV incidence between children and adults may have been affected by a lack of routine screening in adults.
To assess the use of screening, the investigators analyzed practice surveys from physicians at centers included in the AdVance study. There were 28 survey respondents who treat pediatric patients and 14 who treat adults.
All 28 physicians treating pediatric patients said there is routine AdV screening at their center. Ninety-three percent of these doctors said they routinely screen all pediatric allo-HSCT recipients for AdV infection. The remaining 7% said they screen patients considered to be at high-risk of AdV infection.
Thirty-six percent of the physicians treating adults (5/14) said they routinely screen for AdV, and 21% (3/14) said they routinely screen all of their adult allo-HSCT recipients for AdV infection.
Of the 11 doctors who said they did not routinely screen adult allo-HSCT recipients, some said they would screen recipients with a high-risk of AdV infection, such as those with graft-versus-host disease (4/11) or those who received haploidentical, mismatched, or cord blood transplants (3/11).
*Data in the abstracts differ from the presentations.
LISBON—Results of a large study revealed a higher incidence of adenovirus (AdV) infection after allogeneic hematopoietic stem cell transplant (allo-HSCT) in children than adults.
The rate of AdV infection in the 6 months after allo-HSCT was 32% in children and 6% in adults.
However, researchers believe this data may be influenced by a lack of routine AdV screening in adults.
These findings—from the AdVance study—were reported at the 44th Annual Meeting of the EBMT as abstracts OS9-7 and B043.*
Other results from AdVance were also presented at the meeting, with mortality data reported as abstract OS9-8 and hospitalization data reported as abstract B073. The AdVance study was sponsored by Chimerix, Inc.
“The robust findings of the AdVance study are extremely important for transplant clinicians, as we seek to better understand the rates and clinical outcomes of adenovirus infection and assess ways to evaluate antiviral therapies,” said study investigator Marco Zecca, MD, of Fondazione IRCCS Policlinico San Matteo in Pavia, Italy.
AdVance is a multi-center, retrospective study of 4276 allo-HSCT recipients—1738 pediatric patients and 2538 adults. The patients underwent transplants at 50 centers in Europe from January 2013 to September 2015.
Abstract OS9-7: Incidence
Thirty-two percent (n=558) of pediatric patients developed an AdV infection in the first 6 months after allo-HSCT, and 23% (n=395) developed detectable AdV viremia. Fourteen percent (n=241) had AdV viremia ≥ 1000 copies/mL, a level previously associated with negative clinical outcomes.
Meanwhile, 6% (n=141) of adults had an AdV infection in the 6 months after transplant, 3% (n=77) developed AdV viremia, and 2% (n=39) had ≥ 1000 copies /mL.
Further analysis revealed that age, donor type, and use of T-cell depletion were independent predictors of AdV viremia ≥ 1000 copies/mL.
Older age was associated with a lower risk of AdV viremia ≥ 1000 copies/mL. There was a stepwise reduction in risk with increasing age (compared to ages 0-2, P=0.104 for ages 2-12, P=0.001 for ages 13-17, and P<0.0001 for ages 18-65+).
T-cell depletion was associated with an increased risk of AdV viremia ≥ 1000 copies/mL. Compared to no T-cell depletion, there was an increased risk for depletion with antithymocyte globulin (P=0.036) or alemtuzumab (P<0.0001) and for ex vivo depletion (P=0.002).
Compared to patients who received a matched related transplant, recipients of other transplants had an increased risk of AdV viremia ≥ 1000 copies/mL. This includes matched unrelated (P=0.016), cord blood (P=0.011), haploidentical (P=0.007), and mismatched (P<0.001) transplants.
Abstract B043: Screening
Dr Zecca and his colleagues believe the difference in AdV incidence between children and adults may have been affected by a lack of routine screening in adults.
To assess the use of screening, the investigators analyzed practice surveys from physicians at centers included in the AdVance study. There were 28 survey respondents who treat pediatric patients and 14 who treat adults.
All 28 physicians treating pediatric patients said there is routine AdV screening at their center. Ninety-three percent of these doctors said they routinely screen all pediatric allo-HSCT recipients for AdV infection. The remaining 7% said they screen patients considered to be at high-risk of AdV infection.
Thirty-six percent of the physicians treating adults (5/14) said they routinely screen for AdV, and 21% (3/14) said they routinely screen all of their adult allo-HSCT recipients for AdV infection.
Of the 11 doctors who said they did not routinely screen adult allo-HSCT recipients, some said they would screen recipients with a high-risk of AdV infection, such as those with graft-versus-host disease (4/11) or those who received haploidentical, mismatched, or cord blood transplants (3/11).
*Data in the abstracts differ from the presentations.
AdV viral burden linked to mortality in kids
LISBON—Data from the AdVance study revealed a “strong correlation” between adenovirus (AdV) viral load and mortality in pediatric recipients of allogeneic hematopoietic stem cell transplant (allo-HSCT), according to researchers.
The team found that patients with the highest AdV viral burden had roughly 12 times the risk of all-cause mortality as patients with the lowest viral burden.
These findings were presented at the 44th Annual Meeting of the EBMT (abstract OS9-8).*
Other findings from AdVance were also reported at the meeting (abstracts OS9-7 and B043 as well as B073). The study was sponsored by Chimerix, Inc.
AdVance is a multi-center, retrospective study of 4276 allo-HSCT recipients, including 1738 pediatric patients. The patients underwent transplants at 50 centers in Europe from January 2013 to September 2015.
Thirty-two percent (n=558) of pediatric patients developed an AdV infection in the first 6 months after allo-HSCT. Twenty-three percent (n=395) developed detectable AdV viremia, and 14% (n=241) had AdV viremia ≥ 1000 copies/mL.
The researchers assessed AdV plasma viral burden, measured by time-averaged area under the curve (AAUC) over 16 weeks from the time of viremia ≥ 1000 copies/mL, and its correlation with all-cause mortality.
“[T]he highest mortality was observed in those with the greatest adenovirus burden,” said AdVance investigator Marco Zecca, MD, of Fondazione IRCCS Policlinico San Matteo in Pavia, Italy.
He and his colleagues divided patients into 4 quartiles according to AdV AAUC, with the third and fourth quartiles reflecting higher, more prolonged AdV viremia.
Rates of all-cause mortality at 6 months (from the first viremia ≥ 1000 copies/mL) were 52% in the fourth quartile, 10% in the third, 7% in the second, and 3% in the first quartile.
Forty percent of patients in the fourth quartile died within 2 months of transplant.
The hazard ratio for mortality was 11.7 in the fourth quartile, 2.7 in the third, and 1.5 in the second, with the first quartile as the reference.
“These data suggest that AdV AAUC is an appropriate endpoint to assess the potential benefits of antiviral therapies for the treatment of adenovirus,” Dr Zecca concluded.
*Data in the abstract differ from the presentation.
LISBON—Data from the AdVance study revealed a “strong correlation” between adenovirus (AdV) viral load and mortality in pediatric recipients of allogeneic hematopoietic stem cell transplant (allo-HSCT), according to researchers.
The team found that patients with the highest AdV viral burden had roughly 12 times the risk of all-cause mortality as patients with the lowest viral burden.
These findings were presented at the 44th Annual Meeting of the EBMT (abstract OS9-8).*
Other findings from AdVance were also reported at the meeting (abstracts OS9-7 and B043 as well as B073). The study was sponsored by Chimerix, Inc.
AdVance is a multi-center, retrospective study of 4276 allo-HSCT recipients, including 1738 pediatric patients. The patients underwent transplants at 50 centers in Europe from January 2013 to September 2015.
Thirty-two percent (n=558) of pediatric patients developed an AdV infection in the first 6 months after allo-HSCT. Twenty-three percent (n=395) developed detectable AdV viremia, and 14% (n=241) had AdV viremia ≥ 1000 copies/mL.
The researchers assessed AdV plasma viral burden, measured by time-averaged area under the curve (AAUC) over 16 weeks from the time of viremia ≥ 1000 copies/mL, and its correlation with all-cause mortality.
“[T]he highest mortality was observed in those with the greatest adenovirus burden,” said AdVance investigator Marco Zecca, MD, of Fondazione IRCCS Policlinico San Matteo in Pavia, Italy.
He and his colleagues divided patients into 4 quartiles according to AdV AAUC, with the third and fourth quartiles reflecting higher, more prolonged AdV viremia.
Rates of all-cause mortality at 6 months (from the first viremia ≥ 1000 copies/mL) were 52% in the fourth quartile, 10% in the third, 7% in the second, and 3% in the first quartile.
Forty percent of patients in the fourth quartile died within 2 months of transplant.
The hazard ratio for mortality was 11.7 in the fourth quartile, 2.7 in the third, and 1.5 in the second, with the first quartile as the reference.
“These data suggest that AdV AAUC is an appropriate endpoint to assess the potential benefits of antiviral therapies for the treatment of adenovirus,” Dr Zecca concluded.
*Data in the abstract differ from the presentation.
LISBON—Data from the AdVance study revealed a “strong correlation” between adenovirus (AdV) viral load and mortality in pediatric recipients of allogeneic hematopoietic stem cell transplant (allo-HSCT), according to researchers.
The team found that patients with the highest AdV viral burden had roughly 12 times the risk of all-cause mortality as patients with the lowest viral burden.
These findings were presented at the 44th Annual Meeting of the EBMT (abstract OS9-8).*
Other findings from AdVance were also reported at the meeting (abstracts OS9-7 and B043 as well as B073). The study was sponsored by Chimerix, Inc.
AdVance is a multi-center, retrospective study of 4276 allo-HSCT recipients, including 1738 pediatric patients. The patients underwent transplants at 50 centers in Europe from January 2013 to September 2015.
Thirty-two percent (n=558) of pediatric patients developed an AdV infection in the first 6 months after allo-HSCT. Twenty-three percent (n=395) developed detectable AdV viremia, and 14% (n=241) had AdV viremia ≥ 1000 copies/mL.
The researchers assessed AdV plasma viral burden, measured by time-averaged area under the curve (AAUC) over 16 weeks from the time of viremia ≥ 1000 copies/mL, and its correlation with all-cause mortality.
“[T]he highest mortality was observed in those with the greatest adenovirus burden,” said AdVance investigator Marco Zecca, MD, of Fondazione IRCCS Policlinico San Matteo in Pavia, Italy.
He and his colleagues divided patients into 4 quartiles according to AdV AAUC, with the third and fourth quartiles reflecting higher, more prolonged AdV viremia.
Rates of all-cause mortality at 6 months (from the first viremia ≥ 1000 copies/mL) were 52% in the fourth quartile, 10% in the third, 7% in the second, and 3% in the first quartile.
Forty percent of patients in the fourth quartile died within 2 months of transplant.
The hazard ratio for mortality was 11.7 in the fourth quartile, 2.7 in the third, and 1.5 in the second, with the first quartile as the reference.
“These data suggest that AdV AAUC is an appropriate endpoint to assess the potential benefits of antiviral therapies for the treatment of adenovirus,” Dr Zecca concluded.
*Data in the abstract differ from the presentation.
FDA releases updates on BIA-ALCL
The US Food and Drug Administration (FDA) has released new information on breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL).
The agency said it is now aware of 414 cases of BIA-ALCL, which includes 9 patients who died.
In addition, the medical literature suggests that patients with textured breast implants have a lifetime risk of developing BIA-ALCL that ranges from 1 in 3817 to 1 in 30,000.
“The FDA has been closely tracking the relationship between breast implants and a rare type of non-Hodgkin’s lymphoma since we first identified this possible association,” said Binita Ashar, MD, director of the division of surgical devices in the FDA’s Center for Devices and Radiological Health.
“We’ve been working to gather additional information to better characterize and quantify the risk so that patients and providers can have more informed discussions about breast implants. As part of that effort, we are working to update and enhance the information we have on this association, including updating the total number of known cases of BIA-ALCL and the lifetime risk of developing BIA-ALCL as reported in medical literature.”
Reports to FDA
Most of the BIA-ALCL cases reported to the FDA occurred in patients with textured implants (n=242), but 30 occurred in patients with smooth implants. In the remaining 173 cases, the implant surface was not specified.
There were more silicone implants (n=234) than saline implants (n=179), and there was 1 case in which the implant filling was not specified.
The patients’ median age was 53 (range, 24-90), and the median time from last implant to BIA-ALCL diagnosis was 8 years (range, 0-44).
Cases of BIA-ALCL were ALK-negative (n=124) or did not have ALK status specified (n=290). And they were CD30-positive (n=126) or did not have CD30 status specified (n=288).
The most common clinical presentation was seroma (n=203), followed by breast swelling/pain (n=101), peri-implant mass/lump (n=45), and capsular contracture (n=42). In some cases, more than one clinical presentation was listed, and there were 141 cases where clinical presentation was unspecified/uncertain.
Medical literature
The FDA said a “significant body of medical literature” on BIA-ALCL has been published since the agency’s 2011 report on this malignancy.
For the aforementioned lifetime risk estimates—1 case of BIA-ALCL in 3817 to 30,000 individuals with textured implants—the FDA cited 3 sources:
- BIA-ALCL Resources : By the numbers, and what they mean
- Breast Implant–Associated Anaplastic Large Cell Lymphoma in Australia and New Zealand: High-Surface-Area Textured Implants Are Associated with Increased Risk
- Breast Implants and the Risk of Anaplastic Large-Cell Lymphoma in the Breast.
Recommendations, more updates
The FDA said this updated information does not change its recommendations regarding breast implants. The agency said the decision to obtain breast implants should be made based on individual needs and with the most complete information about risks and benefits.
“We hope that this information prompts providers and patients to have important, informed conversations about breast implants and the risk of BIA-ALCL,” Dr Ashar said. “At the same time, we remain committed to working in partnership with all stakeholders to continue to study, understand, and provide updates about this important public health issue.”
The FDA is also updating the content and format of the webpage for the agency’s breast implant post-approval studies to make current information about these studies easier for patients to read and understand.
The US Food and Drug Administration (FDA) has released new information on breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL).
The agency said it is now aware of 414 cases of BIA-ALCL, which includes 9 patients who died.
In addition, the medical literature suggests that patients with textured breast implants have a lifetime risk of developing BIA-ALCL that ranges from 1 in 3817 to 1 in 30,000.
“The FDA has been closely tracking the relationship between breast implants and a rare type of non-Hodgkin’s lymphoma since we first identified this possible association,” said Binita Ashar, MD, director of the division of surgical devices in the FDA’s Center for Devices and Radiological Health.
“We’ve been working to gather additional information to better characterize and quantify the risk so that patients and providers can have more informed discussions about breast implants. As part of that effort, we are working to update and enhance the information we have on this association, including updating the total number of known cases of BIA-ALCL and the lifetime risk of developing BIA-ALCL as reported in medical literature.”
Reports to FDA
Most of the BIA-ALCL cases reported to the FDA occurred in patients with textured implants (n=242), but 30 occurred in patients with smooth implants. In the remaining 173 cases, the implant surface was not specified.
There were more silicone implants (n=234) than saline implants (n=179), and there was 1 case in which the implant filling was not specified.
The patients’ median age was 53 (range, 24-90), and the median time from last implant to BIA-ALCL diagnosis was 8 years (range, 0-44).
Cases of BIA-ALCL were ALK-negative (n=124) or did not have ALK status specified (n=290). And they were CD30-positive (n=126) or did not have CD30 status specified (n=288).
The most common clinical presentation was seroma (n=203), followed by breast swelling/pain (n=101), peri-implant mass/lump (n=45), and capsular contracture (n=42). In some cases, more than one clinical presentation was listed, and there were 141 cases where clinical presentation was unspecified/uncertain.
Medical literature
The FDA said a “significant body of medical literature” on BIA-ALCL has been published since the agency’s 2011 report on this malignancy.
For the aforementioned lifetime risk estimates—1 case of BIA-ALCL in 3817 to 30,000 individuals with textured implants—the FDA cited 3 sources:
- BIA-ALCL Resources : By the numbers, and what they mean
- Breast Implant–Associated Anaplastic Large Cell Lymphoma in Australia and New Zealand: High-Surface-Area Textured Implants Are Associated with Increased Risk
- Breast Implants and the Risk of Anaplastic Large-Cell Lymphoma in the Breast.
Recommendations, more updates
The FDA said this updated information does not change its recommendations regarding breast implants. The agency said the decision to obtain breast implants should be made based on individual needs and with the most complete information about risks and benefits.
“We hope that this information prompts providers and patients to have important, informed conversations about breast implants and the risk of BIA-ALCL,” Dr Ashar said. “At the same time, we remain committed to working in partnership with all stakeholders to continue to study, understand, and provide updates about this important public health issue.”
The FDA is also updating the content and format of the webpage for the agency’s breast implant post-approval studies to make current information about these studies easier for patients to read and understand.
The US Food and Drug Administration (FDA) has released new information on breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL).
The agency said it is now aware of 414 cases of BIA-ALCL, which includes 9 patients who died.
In addition, the medical literature suggests that patients with textured breast implants have a lifetime risk of developing BIA-ALCL that ranges from 1 in 3817 to 1 in 30,000.
“The FDA has been closely tracking the relationship between breast implants and a rare type of non-Hodgkin’s lymphoma since we first identified this possible association,” said Binita Ashar, MD, director of the division of surgical devices in the FDA’s Center for Devices and Radiological Health.
“We’ve been working to gather additional information to better characterize and quantify the risk so that patients and providers can have more informed discussions about breast implants. As part of that effort, we are working to update and enhance the information we have on this association, including updating the total number of known cases of BIA-ALCL and the lifetime risk of developing BIA-ALCL as reported in medical literature.”
Reports to FDA
Most of the BIA-ALCL cases reported to the FDA occurred in patients with textured implants (n=242), but 30 occurred in patients with smooth implants. In the remaining 173 cases, the implant surface was not specified.
There were more silicone implants (n=234) than saline implants (n=179), and there was 1 case in which the implant filling was not specified.
The patients’ median age was 53 (range, 24-90), and the median time from last implant to BIA-ALCL diagnosis was 8 years (range, 0-44).
Cases of BIA-ALCL were ALK-negative (n=124) or did not have ALK status specified (n=290). And they were CD30-positive (n=126) or did not have CD30 status specified (n=288).
The most common clinical presentation was seroma (n=203), followed by breast swelling/pain (n=101), peri-implant mass/lump (n=45), and capsular contracture (n=42). In some cases, more than one clinical presentation was listed, and there were 141 cases where clinical presentation was unspecified/uncertain.
Medical literature
The FDA said a “significant body of medical literature” on BIA-ALCL has been published since the agency’s 2011 report on this malignancy.
For the aforementioned lifetime risk estimates—1 case of BIA-ALCL in 3817 to 30,000 individuals with textured implants—the FDA cited 3 sources:
- BIA-ALCL Resources : By the numbers, and what they mean
- Breast Implant–Associated Anaplastic Large Cell Lymphoma in Australia and New Zealand: High-Surface-Area Textured Implants Are Associated with Increased Risk
- Breast Implants and the Risk of Anaplastic Large-Cell Lymphoma in the Breast.
Recommendations, more updates
The FDA said this updated information does not change its recommendations regarding breast implants. The agency said the decision to obtain breast implants should be made based on individual needs and with the most complete information about risks and benefits.
“We hope that this information prompts providers and patients to have important, informed conversations about breast implants and the risk of BIA-ALCL,” Dr Ashar said. “At the same time, we remain committed to working in partnership with all stakeholders to continue to study, understand, and provide updates about this important public health issue.”
The FDA is also updating the content and format of the webpage for the agency’s breast implant post-approval studies to make current information about these studies easier for patients to read and understand.
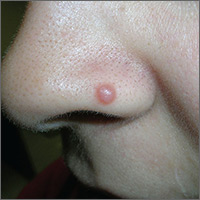
Growth on nose
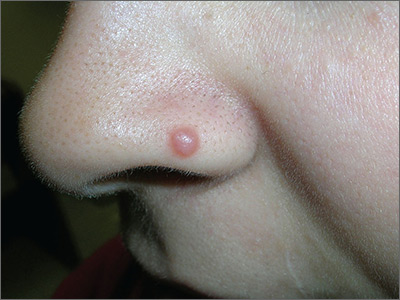
The FP considered several possibilities as part of the differential diagnosis: compound nevus, Spitz nevus, dysplastic nevus, fibrous papule, angiokeratoma, and amelanotic melanoma. A shave biopsy identified the lesion as a Spitz nevus.
Spitz nevi are uncommon solitary pink to black colored dome-shaped papules that usually appear in the first 2 decades of life. They have features that are histologically similar to melanoma and some may, in fact, be Spitzoid melanomas. When a Spitz nevus is suspected, the lesion should be biopsied for histopathologic diagnosis. If the diagnosis is confirmed, the typical treatment is to perform a complete excision with clear margins.
In this case, the pathologist noted that the deep margin was positive and recommended a conservative re-excision. The patient was sent to a dermatologist who fully excised the lesion with no complications.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M, Usatine R. Benign nevi. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:945-952.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP considered several possibilities as part of the differential diagnosis: compound nevus, Spitz nevus, dysplastic nevus, fibrous papule, angiokeratoma, and amelanotic melanoma. A shave biopsy identified the lesion as a Spitz nevus.
Spitz nevi are uncommon solitary pink to black colored dome-shaped papules that usually appear in the first 2 decades of life. They have features that are histologically similar to melanoma and some may, in fact, be Spitzoid melanomas. When a Spitz nevus is suspected, the lesion should be biopsied for histopathologic diagnosis. If the diagnosis is confirmed, the typical treatment is to perform a complete excision with clear margins.
In this case, the pathologist noted that the deep margin was positive and recommended a conservative re-excision. The patient was sent to a dermatologist who fully excised the lesion with no complications.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M, Usatine R. Benign nevi. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:945-952.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP considered several possibilities as part of the differential diagnosis: compound nevus, Spitz nevus, dysplastic nevus, fibrous papule, angiokeratoma, and amelanotic melanoma. A shave biopsy identified the lesion as a Spitz nevus.
Spitz nevi are uncommon solitary pink to black colored dome-shaped papules that usually appear in the first 2 decades of life. They have features that are histologically similar to melanoma and some may, in fact, be Spitzoid melanomas. When a Spitz nevus is suspected, the lesion should be biopsied for histopathologic diagnosis. If the diagnosis is confirmed, the typical treatment is to perform a complete excision with clear margins.
In this case, the pathologist noted that the deep margin was positive and recommended a conservative re-excision. The patient was sent to a dermatologist who fully excised the lesion with no complications.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M, Usatine R. Benign nevi. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:945-952.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
DPP-4 inhibitors increase IBD risk in diabetes
a study has found.
Researchers reported the results of an observational cohort study of 141,170 patients with type 2 diabetes newly treated with noninsulin antidiabetic drugs, with 552,413 person years of follow-up. Of these, 30,488 patients (21.6%) received at least one prescription for a dipeptidyl peptidase-4 inhibitor, and median duration of use was 1.6 years.
The report was published March 21 in the BMJ.
The researchers found that dipeptidyl peptidase-4 (DPP-4) inhibitors were associated with a 75% increased risk of IBD, compared with other antidiabetic drugs (53.4 vs. 34.5 per 100,000 per year, 95% confidence interval, 1.22-2.49).
The risk increased with longer duration of use, peaking at a nearly threefold increase in the risk of IBD after 3-4 years of taking DPP-4 inhibitors (hazard ratio 2.9, 95% CI, 1.31-6.41), and declining to a 45% increase in risk with 4 years of use.
“Although the absolute risk is low, physicians should be aware of this possible association and perhaps refrain from prescribing dipeptidyl peptidase-4 inhibitors for people at high risk (that is, those with a family history of disease or with known autoimmune conditions),” wrote Devin Abrahami of McGill University, Montreal, and coauthors. “Moreover, patients presenting with persistent gastrointestinal symptoms such as abdominal pain or diarrhoea should be closely monitored for worsening of symptoms.”
The same pattern was seen with years since initiation of medication, with a peak in the risk of IBD seen at 3-4 years after initiation followed by a decline.
“This gradual increase in the risk is consistent with the hypothesis of a possible delayed effect of the use of dipeptidyl peptidase-4 inhibitors on the incidence of inflammatory bowel disease,” the authors wrote.
When compared directly with insulin, the use of DPP-4 inhibitors was associated with an over twofold increase in the risk of IBD (HR, 2.28, 95% CI, 1.07-4.85).
The use of DPP-4 inhibitors was also associated with a greater than twofold increase in the risk of ulcerative colitis but no significant effect was seen for Crohn’s disease. However, the authors noted that this result was based on relatively few events and should be interpreted with caution.
The research did not find any difference in risk across different DPP-4 inhibitor drugs.
The DPP-4 enzyme is known to be expressed on the surface of cell types involved in immune response, and patients with IBD have been found to have lower serum DPP-4 enzyme concentrations than healthy controls.
Yet the authors said this was the first study to their knowledge that specifically investigated the effect of DPP-4 inhibitor use on the incidence of IBD.
One previous observational study actually found a decreased risk of a composite outcome of several autoimmune disorders – including IBD – with the use of DPP-4 inhibitors, but it did not report on IBD specifically. The authors also noted that DPP-4 may have a different biological function in IBD.
The Canadian Institutes of Health Research funded the study. No conflicts of interest were declared.
SOURCE: Abrahami D et al. BMJ. 2018;360:k872.
a study has found.
Researchers reported the results of an observational cohort study of 141,170 patients with type 2 diabetes newly treated with noninsulin antidiabetic drugs, with 552,413 person years of follow-up. Of these, 30,488 patients (21.6%) received at least one prescription for a dipeptidyl peptidase-4 inhibitor, and median duration of use was 1.6 years.
The report was published March 21 in the BMJ.
The researchers found that dipeptidyl peptidase-4 (DPP-4) inhibitors were associated with a 75% increased risk of IBD, compared with other antidiabetic drugs (53.4 vs. 34.5 per 100,000 per year, 95% confidence interval, 1.22-2.49).
The risk increased with longer duration of use, peaking at a nearly threefold increase in the risk of IBD after 3-4 years of taking DPP-4 inhibitors (hazard ratio 2.9, 95% CI, 1.31-6.41), and declining to a 45% increase in risk with 4 years of use.
“Although the absolute risk is low, physicians should be aware of this possible association and perhaps refrain from prescribing dipeptidyl peptidase-4 inhibitors for people at high risk (that is, those with a family history of disease or with known autoimmune conditions),” wrote Devin Abrahami of McGill University, Montreal, and coauthors. “Moreover, patients presenting with persistent gastrointestinal symptoms such as abdominal pain or diarrhoea should be closely monitored for worsening of symptoms.”
The same pattern was seen with years since initiation of medication, with a peak in the risk of IBD seen at 3-4 years after initiation followed by a decline.
“This gradual increase in the risk is consistent with the hypothesis of a possible delayed effect of the use of dipeptidyl peptidase-4 inhibitors on the incidence of inflammatory bowel disease,” the authors wrote.
When compared directly with insulin, the use of DPP-4 inhibitors was associated with an over twofold increase in the risk of IBD (HR, 2.28, 95% CI, 1.07-4.85).
The use of DPP-4 inhibitors was also associated with a greater than twofold increase in the risk of ulcerative colitis but no significant effect was seen for Crohn’s disease. However, the authors noted that this result was based on relatively few events and should be interpreted with caution.
The research did not find any difference in risk across different DPP-4 inhibitor drugs.
The DPP-4 enzyme is known to be expressed on the surface of cell types involved in immune response, and patients with IBD have been found to have lower serum DPP-4 enzyme concentrations than healthy controls.
Yet the authors said this was the first study to their knowledge that specifically investigated the effect of DPP-4 inhibitor use on the incidence of IBD.
One previous observational study actually found a decreased risk of a composite outcome of several autoimmune disorders – including IBD – with the use of DPP-4 inhibitors, but it did not report on IBD specifically. The authors also noted that DPP-4 may have a different biological function in IBD.
The Canadian Institutes of Health Research funded the study. No conflicts of interest were declared.
SOURCE: Abrahami D et al. BMJ. 2018;360:k872.
a study has found.
Researchers reported the results of an observational cohort study of 141,170 patients with type 2 diabetes newly treated with noninsulin antidiabetic drugs, with 552,413 person years of follow-up. Of these, 30,488 patients (21.6%) received at least one prescription for a dipeptidyl peptidase-4 inhibitor, and median duration of use was 1.6 years.
The report was published March 21 in the BMJ.
The researchers found that dipeptidyl peptidase-4 (DPP-4) inhibitors were associated with a 75% increased risk of IBD, compared with other antidiabetic drugs (53.4 vs. 34.5 per 100,000 per year, 95% confidence interval, 1.22-2.49).
The risk increased with longer duration of use, peaking at a nearly threefold increase in the risk of IBD after 3-4 years of taking DPP-4 inhibitors (hazard ratio 2.9, 95% CI, 1.31-6.41), and declining to a 45% increase in risk with 4 years of use.
“Although the absolute risk is low, physicians should be aware of this possible association and perhaps refrain from prescribing dipeptidyl peptidase-4 inhibitors for people at high risk (that is, those with a family history of disease or with known autoimmune conditions),” wrote Devin Abrahami of McGill University, Montreal, and coauthors. “Moreover, patients presenting with persistent gastrointestinal symptoms such as abdominal pain or diarrhoea should be closely monitored for worsening of symptoms.”
The same pattern was seen with years since initiation of medication, with a peak in the risk of IBD seen at 3-4 years after initiation followed by a decline.
“This gradual increase in the risk is consistent with the hypothesis of a possible delayed effect of the use of dipeptidyl peptidase-4 inhibitors on the incidence of inflammatory bowel disease,” the authors wrote.
When compared directly with insulin, the use of DPP-4 inhibitors was associated with an over twofold increase in the risk of IBD (HR, 2.28, 95% CI, 1.07-4.85).
The use of DPP-4 inhibitors was also associated with a greater than twofold increase in the risk of ulcerative colitis but no significant effect was seen for Crohn’s disease. However, the authors noted that this result was based on relatively few events and should be interpreted with caution.
The research did not find any difference in risk across different DPP-4 inhibitor drugs.
The DPP-4 enzyme is known to be expressed on the surface of cell types involved in immune response, and patients with IBD have been found to have lower serum DPP-4 enzyme concentrations than healthy controls.
Yet the authors said this was the first study to their knowledge that specifically investigated the effect of DPP-4 inhibitor use on the incidence of IBD.
One previous observational study actually found a decreased risk of a composite outcome of several autoimmune disorders – including IBD – with the use of DPP-4 inhibitors, but it did not report on IBD specifically. The authors also noted that DPP-4 may have a different biological function in IBD.
The Canadian Institutes of Health Research funded the study. No conflicts of interest were declared.
SOURCE: Abrahami D et al. BMJ. 2018;360:k872.
FROM THE BMJ
Key clinical point: Use of DPP-4 inhibitors may put patients with type 2 diabetes at increased risk of developing IBD.
Major finding: Use of DPP-4 inhibitors linked to 75% increase in IBD risk in type 2 diabetes.
Study details: An observational cohort study of 141,170 patients with type 2 diabetes.
Disclosures: The Canadian Institutes of Health Research funded the study. No conflicts of interest were declared.
Source: Abrahami D et al. BMJ. 2018;360:k872.
VIDEO: Case for rivaroxaban & aspirin for PAD gets stronger
ORLANDO – Combination treatment with aspirin and a low dosage of the anticoagulant rivaroxaban had a broader benefit for reducing adverse events in patients with peripheral artery disease than initially reported from the COMPASS trial, which included more than 7,000 patients whose primary diagnosis at study entry was stable PAD.
Secondary analysis of data from the PAD patients enrolled in the COMPASS (Rivaroxaban for the Prevention of Major Cardiovascular Events in Coronary or Peripheral Artery Disease) trial showed that, compared with aspirin alone, treatment with 100 mg aspirin daily plus a low dosage of the anticoagulant rivaroxaban (2.5 mg b.i.d.) resulted in a statistically significant, 24% relative cut in the combined rate of vascular interventions: acute or chronic limb ischemia, vascular amputations, peripheral vascular bypass or percutaneous intervention, and vascular hospitalizations, Sonia S. Anand, MD, said at the annual meeting of the American College of Cardiology.
This finding plus the results in a prior report from COMPASS that rivaroxaban plus aspirin cut major adverse limb events (MALE) by 33%, compared with aspirin alone (Lancet. 2018 Jan 20;391:219-9), together show that rivaroxaban plus aspirin represent a new, effective regimen for a majority of PAD patients (in addition to treating with a statin and an ACE inhibitor) to cut adverse outcomes in a high-risk but historically undertreated patient population, Dr. Anand said in a video interview.
Additional analysis that Dr. Anand reported in her talk showed that patients with PAD who had an index MALE during follow-up had a very high rate of subsequent events. Among the 128 PAD patients in COMPASS who had an index MALE (major vascular amputation or severe limb ischemia that resulted in an intervention) and, compared with the PAD patients who did not have a MALE, the rate of subsequent death was more than three times higher, the rate of hospitalization increased more than 7-fold, and the rate of amputation increased nearly 200-fold.
Concurrently with Dr. Anand’s report at the meeting the results appeared in an article online in the Journal of the American College of Cardiology.
The analysis of post-MALE outcomes, as well as the expanded vascular-outcomes analysis, focused on 6,391 of the COMPASS patients who had lower-extremity PAD and were randomized to either 100 mg aspirin daily or the low-dosage rivaroxaban plus aspirin regimen. COMPASS also randomized patients to a higher-dosage rivaroxaban-only regimen administered at 5 mg b.i.d., but this arm did not perform as well as the lower-dosage regimen, with an efficacy that equaled aspirin only and with more bleeding. The primary efficacy and safety data from COMPASS for all 27,395 enrolled patients, which included many patients with stable coronary artery disease and without diagnosed PAD, appeared in 2017 (N Engl J Med. Oct 5;377[14]:1319-30).
Dr. Anand also reported on a comparison of the clinical and demographic profiles of the 128 PAD patients who developed MALE during follow-up and the 6,263 who did not, a 2% incidence during almost 2 years.
Multivariate analysis identified four significant factors that closely linked with MALE incidence: a history of peripheral surgery or angioplasty, prior limb amputation, baseline Fontaine classification of stage III or IV, and treatment in COMPASS with aspirin alone and not with rivaroxaban plus aspirin.
The new, additional analyses Dr. Anand reported also showed total peripheral vascular outcomes during follow-up in COMPASS in 8.0% of patients on aspirin only and 6.2% of patients on aspirin plus low-dosage rivaroxaban, a 24% relative risk reduction, and vascular interventions in 7.1% of aspirin-only patients and in 5.5% of the combined-regimen patients, also a 24% relative risk reduction. MALE occurred in 2.6% of the aspirin-only patients and in 1.5% of patients on both drugs, a 33% relative risk reduction. All three of these relative risk reductions were statistically significant, said Dr. Anand, a cardiologist and professor of medicine at McMaster University in Hamilton, Ont.
She estimated that about two-thirds of the PAD patients she sees in routine practice would qualify for treatment with aspirin plus low-dosage rivaroxaban once the 2.5 mg formulation becomes approved by regulators. The companies that jointly market rivaroxaban (Xarelto)have an application pending with the Food and Drug Administration to market a 2.5-mg pill based on the COMPASS results. Patients with stable PAD who are not good candidates for the COMPASS regimen are those with a history of a major bleed, those who require full-dose anticoagulation for a comorbidity such as atrial fibrillation or a mechanical heart valve, and patients with newly diagnosed, stable PAD without concurrent coronary artery disease who might receive adequate protection from aspirin alone, Dr. Anand said.
COMPASS was sponsored by Bayer, the company that along with Janssen markets rivaroxaban. Dr. Anand has been a consultant to Bayer and Novartis. Dr. Beckman has been a consultant to Janssen and other pharmaceutical companies.
SOURCE: Anand SS et al. J Amer Coll Cardiol. 2018 Mar 11. doi: 10.1016/j.jacc.2018.03.008.
ORLANDO – Combination treatment with aspirin and a low dosage of the anticoagulant rivaroxaban had a broader benefit for reducing adverse events in patients with peripheral artery disease than initially reported from the COMPASS trial, which included more than 7,000 patients whose primary diagnosis at study entry was stable PAD.
Secondary analysis of data from the PAD patients enrolled in the COMPASS (Rivaroxaban for the Prevention of Major Cardiovascular Events in Coronary or Peripheral Artery Disease) trial showed that, compared with aspirin alone, treatment with 100 mg aspirin daily plus a low dosage of the anticoagulant rivaroxaban (2.5 mg b.i.d.) resulted in a statistically significant, 24% relative cut in the combined rate of vascular interventions: acute or chronic limb ischemia, vascular amputations, peripheral vascular bypass or percutaneous intervention, and vascular hospitalizations, Sonia S. Anand, MD, said at the annual meeting of the American College of Cardiology.
This finding plus the results in a prior report from COMPASS that rivaroxaban plus aspirin cut major adverse limb events (MALE) by 33%, compared with aspirin alone (Lancet. 2018 Jan 20;391:219-9), together show that rivaroxaban plus aspirin represent a new, effective regimen for a majority of PAD patients (in addition to treating with a statin and an ACE inhibitor) to cut adverse outcomes in a high-risk but historically undertreated patient population, Dr. Anand said in a video interview.
Additional analysis that Dr. Anand reported in her talk showed that patients with PAD who had an index MALE during follow-up had a very high rate of subsequent events. Among the 128 PAD patients in COMPASS who had an index MALE (major vascular amputation or severe limb ischemia that resulted in an intervention) and, compared with the PAD patients who did not have a MALE, the rate of subsequent death was more than three times higher, the rate of hospitalization increased more than 7-fold, and the rate of amputation increased nearly 200-fold.
Concurrently with Dr. Anand’s report at the meeting the results appeared in an article online in the Journal of the American College of Cardiology.
The analysis of post-MALE outcomes, as well as the expanded vascular-outcomes analysis, focused on 6,391 of the COMPASS patients who had lower-extremity PAD and were randomized to either 100 mg aspirin daily or the low-dosage rivaroxaban plus aspirin regimen. COMPASS also randomized patients to a higher-dosage rivaroxaban-only regimen administered at 5 mg b.i.d., but this arm did not perform as well as the lower-dosage regimen, with an efficacy that equaled aspirin only and with more bleeding. The primary efficacy and safety data from COMPASS for all 27,395 enrolled patients, which included many patients with stable coronary artery disease and without diagnosed PAD, appeared in 2017 (N Engl J Med. Oct 5;377[14]:1319-30).
Dr. Anand also reported on a comparison of the clinical and demographic profiles of the 128 PAD patients who developed MALE during follow-up and the 6,263 who did not, a 2% incidence during almost 2 years.
Multivariate analysis identified four significant factors that closely linked with MALE incidence: a history of peripheral surgery or angioplasty, prior limb amputation, baseline Fontaine classification of stage III or IV, and treatment in COMPASS with aspirin alone and not with rivaroxaban plus aspirin.
The new, additional analyses Dr. Anand reported also showed total peripheral vascular outcomes during follow-up in COMPASS in 8.0% of patients on aspirin only and 6.2% of patients on aspirin plus low-dosage rivaroxaban, a 24% relative risk reduction, and vascular interventions in 7.1% of aspirin-only patients and in 5.5% of the combined-regimen patients, also a 24% relative risk reduction. MALE occurred in 2.6% of the aspirin-only patients and in 1.5% of patients on both drugs, a 33% relative risk reduction. All three of these relative risk reductions were statistically significant, said Dr. Anand, a cardiologist and professor of medicine at McMaster University in Hamilton, Ont.
She estimated that about two-thirds of the PAD patients she sees in routine practice would qualify for treatment with aspirin plus low-dosage rivaroxaban once the 2.5 mg formulation becomes approved by regulators. The companies that jointly market rivaroxaban (Xarelto)have an application pending with the Food and Drug Administration to market a 2.5-mg pill based on the COMPASS results. Patients with stable PAD who are not good candidates for the COMPASS regimen are those with a history of a major bleed, those who require full-dose anticoagulation for a comorbidity such as atrial fibrillation or a mechanical heart valve, and patients with newly diagnosed, stable PAD without concurrent coronary artery disease who might receive adequate protection from aspirin alone, Dr. Anand said.
COMPASS was sponsored by Bayer, the company that along with Janssen markets rivaroxaban. Dr. Anand has been a consultant to Bayer and Novartis. Dr. Beckman has been a consultant to Janssen and other pharmaceutical companies.
SOURCE: Anand SS et al. J Amer Coll Cardiol. 2018 Mar 11. doi: 10.1016/j.jacc.2018.03.008.
ORLANDO – Combination treatment with aspirin and a low dosage of the anticoagulant rivaroxaban had a broader benefit for reducing adverse events in patients with peripheral artery disease than initially reported from the COMPASS trial, which included more than 7,000 patients whose primary diagnosis at study entry was stable PAD.
Secondary analysis of data from the PAD patients enrolled in the COMPASS (Rivaroxaban for the Prevention of Major Cardiovascular Events in Coronary or Peripheral Artery Disease) trial showed that, compared with aspirin alone, treatment with 100 mg aspirin daily plus a low dosage of the anticoagulant rivaroxaban (2.5 mg b.i.d.) resulted in a statistically significant, 24% relative cut in the combined rate of vascular interventions: acute or chronic limb ischemia, vascular amputations, peripheral vascular bypass or percutaneous intervention, and vascular hospitalizations, Sonia S. Anand, MD, said at the annual meeting of the American College of Cardiology.
This finding plus the results in a prior report from COMPASS that rivaroxaban plus aspirin cut major adverse limb events (MALE) by 33%, compared with aspirin alone (Lancet. 2018 Jan 20;391:219-9), together show that rivaroxaban plus aspirin represent a new, effective regimen for a majority of PAD patients (in addition to treating with a statin and an ACE inhibitor) to cut adverse outcomes in a high-risk but historically undertreated patient population, Dr. Anand said in a video interview.
Additional analysis that Dr. Anand reported in her talk showed that patients with PAD who had an index MALE during follow-up had a very high rate of subsequent events. Among the 128 PAD patients in COMPASS who had an index MALE (major vascular amputation or severe limb ischemia that resulted in an intervention) and, compared with the PAD patients who did not have a MALE, the rate of subsequent death was more than three times higher, the rate of hospitalization increased more than 7-fold, and the rate of amputation increased nearly 200-fold.
Concurrently with Dr. Anand’s report at the meeting the results appeared in an article online in the Journal of the American College of Cardiology.
The analysis of post-MALE outcomes, as well as the expanded vascular-outcomes analysis, focused on 6,391 of the COMPASS patients who had lower-extremity PAD and were randomized to either 100 mg aspirin daily or the low-dosage rivaroxaban plus aspirin regimen. COMPASS also randomized patients to a higher-dosage rivaroxaban-only regimen administered at 5 mg b.i.d., but this arm did not perform as well as the lower-dosage regimen, with an efficacy that equaled aspirin only and with more bleeding. The primary efficacy and safety data from COMPASS for all 27,395 enrolled patients, which included many patients with stable coronary artery disease and without diagnosed PAD, appeared in 2017 (N Engl J Med. Oct 5;377[14]:1319-30).
Dr. Anand also reported on a comparison of the clinical and demographic profiles of the 128 PAD patients who developed MALE during follow-up and the 6,263 who did not, a 2% incidence during almost 2 years.
Multivariate analysis identified four significant factors that closely linked with MALE incidence: a history of peripheral surgery or angioplasty, prior limb amputation, baseline Fontaine classification of stage III or IV, and treatment in COMPASS with aspirin alone and not with rivaroxaban plus aspirin.
The new, additional analyses Dr. Anand reported also showed total peripheral vascular outcomes during follow-up in COMPASS in 8.0% of patients on aspirin only and 6.2% of patients on aspirin plus low-dosage rivaroxaban, a 24% relative risk reduction, and vascular interventions in 7.1% of aspirin-only patients and in 5.5% of the combined-regimen patients, also a 24% relative risk reduction. MALE occurred in 2.6% of the aspirin-only patients and in 1.5% of patients on both drugs, a 33% relative risk reduction. All three of these relative risk reductions were statistically significant, said Dr. Anand, a cardiologist and professor of medicine at McMaster University in Hamilton, Ont.
She estimated that about two-thirds of the PAD patients she sees in routine practice would qualify for treatment with aspirin plus low-dosage rivaroxaban once the 2.5 mg formulation becomes approved by regulators. The companies that jointly market rivaroxaban (Xarelto)have an application pending with the Food and Drug Administration to market a 2.5-mg pill based on the COMPASS results. Patients with stable PAD who are not good candidates for the COMPASS regimen are those with a history of a major bleed, those who require full-dose anticoagulation for a comorbidity such as atrial fibrillation or a mechanical heart valve, and patients with newly diagnosed, stable PAD without concurrent coronary artery disease who might receive adequate protection from aspirin alone, Dr. Anand said.
COMPASS was sponsored by Bayer, the company that along with Janssen markets rivaroxaban. Dr. Anand has been a consultant to Bayer and Novartis. Dr. Beckman has been a consultant to Janssen and other pharmaceutical companies.
SOURCE: Anand SS et al. J Amer Coll Cardiol. 2018 Mar 11. doi: 10.1016/j.jacc.2018.03.008.
REPORTING FROM ACC 18
Key clinical point: PAD patients on rivaroxaban plus aspirin had significantly fewer adverse peripheral vascular outcomes.
Major finding: Dual therapy cut total adverse peripheral vascular outcomes by a relative 24%, compared with aspirin alone, during 23-month follow-up.
Study details: Secondary analysis of data from COMPASS, a multicenter, randomized trial with 6,391 patients in the new analysis.
Disclosures: COMPASS was sponsored by Bayer, the company that, along with Janssen markets rivaroxaban. Dr. Anand has been a consultant to Bayer and Novartis. Dr. Beckman has been a consultant to Janssen and other pharmaceutical companies.
Source: Anand SS et al. J Amer Coll Cardiol. 2018 Mar 11. doi: 10.1016/j.jacc.2018.03.008.