User login
Combo could treat double-hit lymphoma
Existing drugs could be combined to treat double-hit lymphoma (DHL), according to preclinical research published in Science Translational Medicine.
The drugs are tigecycline, an antibiotic, and venetoclax, a BCL2 inhibitor.
Researchers observed promising activity with these drugs in combination, both in cell lines and mouse models of DHL.
The team therefore believes the drugs could be repurposed to treat DHL, which currently has a dismal prognosis.
Study author Micol Ravà, PhD, of the European Institute of Oncology in Milan, Italy, and her colleagues noted that DHL is driven by the abnormal activation of MYC and BCL2. However, selective BCL2 inhibitors like venetoclax have failed to halt disease progression in DHL patients.
Seeking a way to sensitize DHL to BCL2 inhibitors, the researchers turned to tigecycline, which interferes with mitochondria to trigger a MYC-dependent cell death pathway.
The team found that tigecycline and venetoclax demonstrated synergy in 5 DHL cell lines. The drugs were synergistic in 3 cell lines—Karpas-422, SU-DHL-6, and DOHH-2—in which neither drug alone showed significant pro-apoptotic activity.
In 2 other cell lines—SU-DHL-4 and OCI-LY8—venetoclax was active when given alone, but its activity was enhanced by the addition of tigecycline.
In mouse models of DHL (using the human cell lines SU-DHL-6, DOHH-2, and OCI-LY8), each of the drugs alone were able to slow tumor progression somewhat.
However, combination tigecycline and venetoclax exhibited “strong antitumoral activity,” according to the researchers. In fact, the combination caused full disease regression in all 8 SU-DHL-6 mice and 3 of 9 OCI-LY8 mice.
Dr Ravà and her colleagues also found the combination produced “rapid and marked tumor regression” in mice with a patient-derived xenograft.
The researchers observed no toxicity when tigecycline and venetoclax were given at low doses. However, mice receiving more aggressive treatment had some inflammation in the liver and spleen. And some mice treated with high doses of tigecycline and venetoclax died within 1 week of treatment initiation.
Finally, Dr Ravà and her colleagues found that tigecycline and venetoclax each synergized with rituximab. The team therefore concluded that tigecycline and venetoclax “have the potential to reinforce rituximab-containing therapies in the clinic.” ![]()
Existing drugs could be combined to treat double-hit lymphoma (DHL), according to preclinical research published in Science Translational Medicine.
The drugs are tigecycline, an antibiotic, and venetoclax, a BCL2 inhibitor.
Researchers observed promising activity with these drugs in combination, both in cell lines and mouse models of DHL.
The team therefore believes the drugs could be repurposed to treat DHL, which currently has a dismal prognosis.
Study author Micol Ravà, PhD, of the European Institute of Oncology in Milan, Italy, and her colleagues noted that DHL is driven by the abnormal activation of MYC and BCL2. However, selective BCL2 inhibitors like venetoclax have failed to halt disease progression in DHL patients.
Seeking a way to sensitize DHL to BCL2 inhibitors, the researchers turned to tigecycline, which interferes with mitochondria to trigger a MYC-dependent cell death pathway.
The team found that tigecycline and venetoclax demonstrated synergy in 5 DHL cell lines. The drugs were synergistic in 3 cell lines—Karpas-422, SU-DHL-6, and DOHH-2—in which neither drug alone showed significant pro-apoptotic activity.
In 2 other cell lines—SU-DHL-4 and OCI-LY8—venetoclax was active when given alone, but its activity was enhanced by the addition of tigecycline.
In mouse models of DHL (using the human cell lines SU-DHL-6, DOHH-2, and OCI-LY8), each of the drugs alone were able to slow tumor progression somewhat.
However, combination tigecycline and venetoclax exhibited “strong antitumoral activity,” according to the researchers. In fact, the combination caused full disease regression in all 8 SU-DHL-6 mice and 3 of 9 OCI-LY8 mice.
Dr Ravà and her colleagues also found the combination produced “rapid and marked tumor regression” in mice with a patient-derived xenograft.
The researchers observed no toxicity when tigecycline and venetoclax were given at low doses. However, mice receiving more aggressive treatment had some inflammation in the liver and spleen. And some mice treated with high doses of tigecycline and venetoclax died within 1 week of treatment initiation.
Finally, Dr Ravà and her colleagues found that tigecycline and venetoclax each synergized with rituximab. The team therefore concluded that tigecycline and venetoclax “have the potential to reinforce rituximab-containing therapies in the clinic.” ![]()
Existing drugs could be combined to treat double-hit lymphoma (DHL), according to preclinical research published in Science Translational Medicine.
The drugs are tigecycline, an antibiotic, and venetoclax, a BCL2 inhibitor.
Researchers observed promising activity with these drugs in combination, both in cell lines and mouse models of DHL.
The team therefore believes the drugs could be repurposed to treat DHL, which currently has a dismal prognosis.
Study author Micol Ravà, PhD, of the European Institute of Oncology in Milan, Italy, and her colleagues noted that DHL is driven by the abnormal activation of MYC and BCL2. However, selective BCL2 inhibitors like venetoclax have failed to halt disease progression in DHL patients.
Seeking a way to sensitize DHL to BCL2 inhibitors, the researchers turned to tigecycline, which interferes with mitochondria to trigger a MYC-dependent cell death pathway.
The team found that tigecycline and venetoclax demonstrated synergy in 5 DHL cell lines. The drugs were synergistic in 3 cell lines—Karpas-422, SU-DHL-6, and DOHH-2—in which neither drug alone showed significant pro-apoptotic activity.
In 2 other cell lines—SU-DHL-4 and OCI-LY8—venetoclax was active when given alone, but its activity was enhanced by the addition of tigecycline.
In mouse models of DHL (using the human cell lines SU-DHL-6, DOHH-2, and OCI-LY8), each of the drugs alone were able to slow tumor progression somewhat.
However, combination tigecycline and venetoclax exhibited “strong antitumoral activity,” according to the researchers. In fact, the combination caused full disease regression in all 8 SU-DHL-6 mice and 3 of 9 OCI-LY8 mice.
Dr Ravà and her colleagues also found the combination produced “rapid and marked tumor regression” in mice with a patient-derived xenograft.
The researchers observed no toxicity when tigecycline and venetoclax were given at low doses. However, mice receiving more aggressive treatment had some inflammation in the liver and spleen. And some mice treated with high doses of tigecycline and venetoclax died within 1 week of treatment initiation.
Finally, Dr Ravà and her colleagues found that tigecycline and venetoclax each synergized with rituximab. The team therefore concluded that tigecycline and venetoclax “have the potential to reinforce rituximab-containing therapies in the clinic.” ![]()
Interim trial results may be misleading
Interim results from randomized trials may be misleading at times, according to research published in JAMA.
Researchers compared interim and final publications on randomized trials and found that, 21% of the time, results changed significantly.
“Changes between interim and final publication matter because clinicians and the public could have been misled about whether an intervention was beneficial, harmful, or ineffective,” said study author Lisa Schwartz, MD, of Dartmouth Institute for Health Policy and Clinical Practice in Lebanon, New Hampshire.
She and her colleagues searched PubMed for randomized trials from 2006 to 2015 with “interim,” “not mature,” or “immature” in the title or abstract.
To identify final publications, they searched PubMed, ClinicalTrials.gov, and Web of Science through 2016. The team emailed authors of interim reports when no final publication was identified.
For interim and final publications reporting the same efficacy and or safety outcome, the researchers compared trial characteristics and prominence. They also categorized abstract conclusions (not different, beneficial, or harmful) and compared changes between interim and final publications.
Findings
Interim results were reported in 613 of 1267 screened publications.
Of those publications, 72% reported on trials stopped early (for benefit, harm, futility, or other problems). The remaining 171 ongoing trials (mostly in oncology, surgery, or cardiology) reported interim efficacy or safety results.
Forty-one percent of the publications stated that the interim analysis was specified in the protocol, but half provided no reason for the interim publication.
Final results were published for 98 of the 160 trials (61%) that were more than 1 year beyond the completion date.
The researchers compared 73 interim and final publications reporting the same efficacy or safety outcome. And they found that interim and final publications had similar prominence.
In most cases (79%), the abstract conclusions did not change from the interim publication to the final publication. However, for 21% of trials, there were significant changes.
For 4 trials, the conclusions changed from “no difference” between randomized to treatments to the study treatment being “beneficial.” In 3 trials, the conclusions changed from “not different” to “harmful or possibly harmful.”
In 6 trials, the conclusions changed from “beneficial” to “not different.” One trial changed from “beneficial” to “harmful,” and another changed from “inconclusive” to “noninferior.”
Dr Schwartz and her colleagues concluded that while most interim and final publications reached similar conclusions, frequent non-publication of final results can lead to confusion or unfounded assumptions with true treatment effects remaining unknown.
To safeguard against any such confusion, the researchers recommended routinely adding the word “interim” in the title and justifying the reason in the publication. (Many interim publications reported analyses without any justification.)
“Most importantly, journals, authors, and funders should commit to making final results accessible by linking interim publications to final reports whenever available,” said study author Steven Woloshin, MD, of Dartmouth Institute for Health Policy and Clinical Practice. ![]()
Interim results from randomized trials may be misleading at times, according to research published in JAMA.
Researchers compared interim and final publications on randomized trials and found that, 21% of the time, results changed significantly.
“Changes between interim and final publication matter because clinicians and the public could have been misled about whether an intervention was beneficial, harmful, or ineffective,” said study author Lisa Schwartz, MD, of Dartmouth Institute for Health Policy and Clinical Practice in Lebanon, New Hampshire.
She and her colleagues searched PubMed for randomized trials from 2006 to 2015 with “interim,” “not mature,” or “immature” in the title or abstract.
To identify final publications, they searched PubMed, ClinicalTrials.gov, and Web of Science through 2016. The team emailed authors of interim reports when no final publication was identified.
For interim and final publications reporting the same efficacy and or safety outcome, the researchers compared trial characteristics and prominence. They also categorized abstract conclusions (not different, beneficial, or harmful) and compared changes between interim and final publications.
Findings
Interim results were reported in 613 of 1267 screened publications.
Of those publications, 72% reported on trials stopped early (for benefit, harm, futility, or other problems). The remaining 171 ongoing trials (mostly in oncology, surgery, or cardiology) reported interim efficacy or safety results.
Forty-one percent of the publications stated that the interim analysis was specified in the protocol, but half provided no reason for the interim publication.
Final results were published for 98 of the 160 trials (61%) that were more than 1 year beyond the completion date.
The researchers compared 73 interim and final publications reporting the same efficacy or safety outcome. And they found that interim and final publications had similar prominence.
In most cases (79%), the abstract conclusions did not change from the interim publication to the final publication. However, for 21% of trials, there were significant changes.
For 4 trials, the conclusions changed from “no difference” between randomized to treatments to the study treatment being “beneficial.” In 3 trials, the conclusions changed from “not different” to “harmful or possibly harmful.”
In 6 trials, the conclusions changed from “beneficial” to “not different.” One trial changed from “beneficial” to “harmful,” and another changed from “inconclusive” to “noninferior.”
Dr Schwartz and her colleagues concluded that while most interim and final publications reached similar conclusions, frequent non-publication of final results can lead to confusion or unfounded assumptions with true treatment effects remaining unknown.
To safeguard against any such confusion, the researchers recommended routinely adding the word “interim” in the title and justifying the reason in the publication. (Many interim publications reported analyses without any justification.)
“Most importantly, journals, authors, and funders should commit to making final results accessible by linking interim publications to final reports whenever available,” said study author Steven Woloshin, MD, of Dartmouth Institute for Health Policy and Clinical Practice. ![]()
Interim results from randomized trials may be misleading at times, according to research published in JAMA.
Researchers compared interim and final publications on randomized trials and found that, 21% of the time, results changed significantly.
“Changes between interim and final publication matter because clinicians and the public could have been misled about whether an intervention was beneficial, harmful, or ineffective,” said study author Lisa Schwartz, MD, of Dartmouth Institute for Health Policy and Clinical Practice in Lebanon, New Hampshire.
She and her colleagues searched PubMed for randomized trials from 2006 to 2015 with “interim,” “not mature,” or “immature” in the title or abstract.
To identify final publications, they searched PubMed, ClinicalTrials.gov, and Web of Science through 2016. The team emailed authors of interim reports when no final publication was identified.
For interim and final publications reporting the same efficacy and or safety outcome, the researchers compared trial characteristics and prominence. They also categorized abstract conclusions (not different, beneficial, or harmful) and compared changes between interim and final publications.
Findings
Interim results were reported in 613 of 1267 screened publications.
Of those publications, 72% reported on trials stopped early (for benefit, harm, futility, or other problems). The remaining 171 ongoing trials (mostly in oncology, surgery, or cardiology) reported interim efficacy or safety results.
Forty-one percent of the publications stated that the interim analysis was specified in the protocol, but half provided no reason for the interim publication.
Final results were published for 98 of the 160 trials (61%) that were more than 1 year beyond the completion date.
The researchers compared 73 interim and final publications reporting the same efficacy or safety outcome. And they found that interim and final publications had similar prominence.
In most cases (79%), the abstract conclusions did not change from the interim publication to the final publication. However, for 21% of trials, there were significant changes.
For 4 trials, the conclusions changed from “no difference” between randomized to treatments to the study treatment being “beneficial.” In 3 trials, the conclusions changed from “not different” to “harmful or possibly harmful.”
In 6 trials, the conclusions changed from “beneficial” to “not different.” One trial changed from “beneficial” to “harmful,” and another changed from “inconclusive” to “noninferior.”
Dr Schwartz and her colleagues concluded that while most interim and final publications reached similar conclusions, frequent non-publication of final results can lead to confusion or unfounded assumptions with true treatment effects remaining unknown.
To safeguard against any such confusion, the researchers recommended routinely adding the word “interim” in the title and justifying the reason in the publication. (Many interim publications reported analyses without any justification.)
“Most importantly, journals, authors, and funders should commit to making final results accessible by linking interim publications to final reports whenever available,” said study author Steven Woloshin, MD, of Dartmouth Institute for Health Policy and Clinical Practice. ![]()
Boy, 9, With Eye Pain, Blurred Vision, and Tearing
IN THIS ARTICLE
- Diagnosis
- Management
- Outcome for the case patient
An otherwise healthy 9-year-old boy is brought to the emergency department (ED) by his father for evaluation of severe pain, blurry vision, and four hours of tearing in his right eye. The patient was in school when he experienced sudden-onset irritation and scratching pain that caused him to rub his eye. He says it “feels like there is something in my eye,” but he denies any known substance or foreign body. He has no medical or surgical history, does not wear contact lenses or eyeglasses, and denies loss of vision. There is no history of recent illness or travel.
On evaluation, the patient is in no acute distress but is holding his right eye closed due to foreign-body sensation and increased photosensitivity and tearing. There is no obvious erythema or swelling in the upper or lower eyelids bilaterally. A visual acuity test with a Snellen eye chart shows 20/20 vision in the left eye and 20/50 in the right, secondary to pain, photophobia, and excessive tearing. The patient’s right sclera is significantly injected. Intraocular pressure, measured with a tonometer, is 12 to 14 mm Hg. A fluorescein stain of the eye yields no significant findings. The globe is intact.
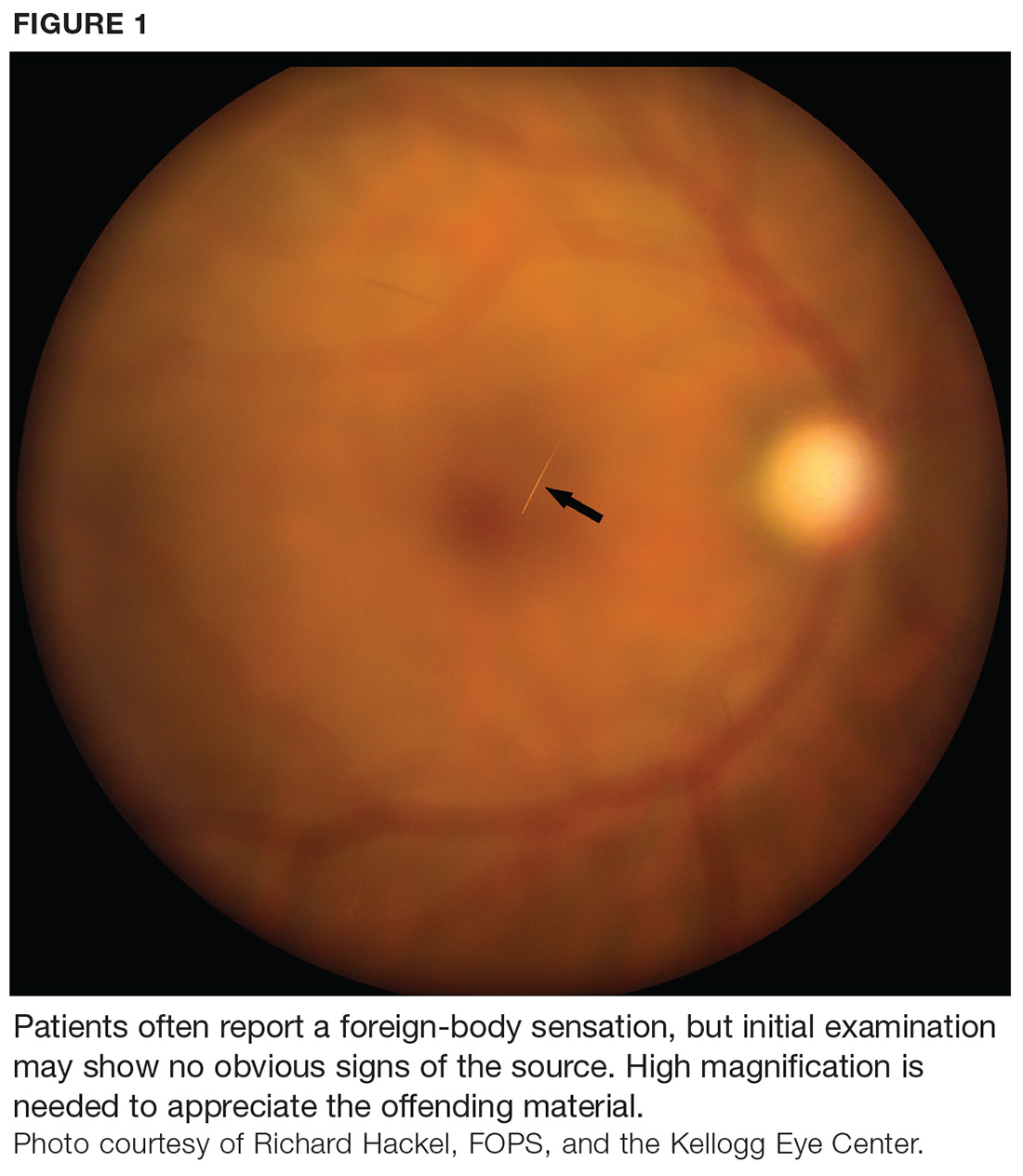
At first glance, a slit-lamp exam shows no obvious signs of a foreign body. But much higher magnification reveals substantial conjunctival injection and numerous intracorneal linear foreign bodies in the right eye (see Figure 1 for example [not the case patient]). The anterior chamber shows no inflammatory reaction, and findings in the posterior segment are unremarkable.
The initial diagnosis is simple conjunctivitis—but closer examination reveals multiple fine, barbed hairs embedded in the patient’s right cornea. Upon further questioning, the patient reports that prior to symptom onset, he had been holding the classroom pet, a Chilean Rose tarantula, in the palm of his hands.
DISCUSSION
Foreign body injury is a common cause of ocular pain and corneal damage, which can lead to challenging complications. Ophthalmic emergencies account for 2% of ED visits in the US annually and are a major cause of visual impairment.1 But when a painful eye is the chief complaint, contact with insects, plants, or spiders is rarely included in the differential. Tarantulas are popular classroom and household pets, however, and ocular injury should be suspected in anyone who has been holding a tarantula prior to onset of pain.
Ophthalmia nodosa
Tarantulas are one of the most common arachnids known to cause ophthalmia nodosa—a granulomatous reaction of the conjunctiva or cornea to an implanted plant, insect, or spider hair that typically manifests with photophobia, irritation, and chemosis.2,3 Tarantulas, when scared or defending their eggs, shoot urticating setae at the threat—a defensive mechanism largely unknown to parents, tarantula owners, and medical professionals.
Urticating setae are found in roughly 90% of tarantula species throughout tropical and subtropical regions.4 Depending on the species, setae can be located on the distal prolateral surface of the palpal femur or the dorsum of the abdomen. They can be released when the tarantula scratches its legs against the abdominal urticating setae patch or scratches the palps against the chelicerae (appendages in front of the mouth), or when direct exterior contact is made with the abdominal setae.4
There are six types of urticating hairs. Each is attached to the spider’s cuticle by either a stalk (which represents the break-off region) or a socket.4 Tarantula hairs range in size from 0.1 mm to 0.3 mm and have a sharp, pointed head and numerous barbs, which help embed them in the target.5 They are long and thin, to facilitate deep tissue penetration, and can enter the eyes, lungs, or other body parts (see Figure 2).
Ocular injury from tarantula hairs commonly involves conjunctival injection, foreign body sensation, periorbital facial rash, photophobia, and tearing.3 When a tarantula’s cloud of barbed hairs is flicked into the eye and pierces the cornea, it can cause infection, irritation, scarring on the cornea, or vision loss. Eye movement or rubbing can cause the hairs—and their toxins—to migrate over time, traveling like an arrow (the tip and barbs resist backward movement) to the anterior chamber, lens, vitreous, and retina.6,7 This can cause corneal scars, cataracts, vitritis, or macular edema, and creates the possibility for acute or chronic conjunctivitis.7
Diagnosis and management
Ophthalmic emergencies can affect the visual system and, if left untreated, can lead to permanent vision loss. Affected patients require immediate medical attention and should be referred to an ophthalmologist for follow-up care.
Diagnosis. A thorough history and physical exam are of utmost importance; tiny setae can be easily overlooked if the examiner is not diligent, and the similar symptomatology can lead to misdiagnosis as simple conjunctivitis.3 A visual acuity test and slit-lamp exam are useful for confirmation.
Treatment. Once the diagnosis is confirmed, treatment should consist of mild topical antibiotics and steroids to effectively control infection and inflammation. While topical steroids may be appropriate, local adverse events associated with their use (eg, glaucoma, cataracts) can be problematic. Gentle eye irrigation has been noted by some researchers as contraindicated, while others find it useful to flush out some of the hairs.5,8,9
Most of the visible protruding tarantula hairs can and should be removed under microscopy during slit-lamp exam. Hairs that are buried in the cornea, however, are nearly impossible to remove and pose a threat of further complications, as described. Conservative management with careful observation is therefore recommended. If the patient develops a granuloma, excision—along with a course of systemic steroids and setae removal via vitrectomy—may be needed.9
The good news is that, in many cases, deeper hairs are absorbed without complication, making their removal unnecessary.5 Factors that encourage leaving the setae untouched include a large number of hairs, deep corneal penetration, lack of patient tolerance for the procedure, and risk for perforation.3
More invasive treatments (eg, laser photocoagulation, intraocular surgery) to remove offending hairs are possible, but literature on the outcome of these interventions is limited. One report to date used argon laser photocoagulation to treat endophthalmitis from vitreous hairs.10 The laser can fragment the hairs so that they lose their barbed characteristic and cannot penetrate deeper.6
Follow-up. Close follow-up is advised, and patients should be educated on the importance of medication compliance and return visits for reevaluation. Given the potential dangers of handling these spiders, tarantula owners should be advised to use protective gloving and goggles.2,5,8,9
OUTCOME FOR THE CASE PATIENT
The case patient was sent to an ophthalmologist on day 1. Proparacaine was placed in his right eye, and all of the superficial tarantula hairs were removed using 25- and 30-gauge needles with jeweler forceps under slit-lamp microscopy. Most of the hairs were removed from the superior cornea; fewer were found in the paracentral and inferior regions of the cornea. Approximately five hairs in the paracentral area of the cornea were embedded in the midstromal depth and could not be removed. One drop of ciprofloxacin was administered.
The patient was sent home with an eye shield and instructions to use tobramycin/dexamethasone eye drops (qid in his right eye) and avoid rubbing the eye. (The eye shield, though not technically necessary, was deemed beneficial to help the patient avoid touching the eye.) He was scheduled to return to the clinic one week later.
On follow-up, a careful exam performed under microscopy showed that the five tarantula hairs were still embedded, and an additional six hairs were found in the deep stroma. Superficial punctate keratitis—an eye disorder caused by epithelial cell death on the surface of the cornea—was noted, but no anterior chamber cells were seen. The patient was instructed to continue using the eye drops as prescribed until finished, then start using loteprednol (tid) and artificial lubricating tears (every 2 h).
He returned to the clinic every two weeks for a total of 10 visits. At the end of the treatment course, the remaining tarantula hairs were unable to be removed. The patient used tapering doses of topical eye steroids and antibiotic drops secondary to flare-up.
CONCLUSION
Determining the etiology of ophthalmic emergencies is essential to timely and appropriate management. In this case, a recognized but often overlooked cause, tarantula hairs, made the diagnosis more complicated than simple conjunctivitis. When ocular injury is suspected, the provider must obtain an accurate and detailed history along with a thorough physical exam. Since patients must comply with medication regimens to prevent acute and chronic infection, a clear treatment and follow-up plan should be established. With these in place, ophthalmia nodosa caused by urticating setae can be effectively managed.
1. Fitzpatrick J, Hickman R, Alfes CM. A Guide to Mastery in Clinical Nursing: The Comprehensive Reference. New York, NY: Springer; 2018:114.
2. Lambert SR, Lyons CJ. Taylor and Hoyt’s Pediatric Ophthalmology and Strabismus. 5th ed. New York, NY: Elsevier; 2017:138.
3. Stagg BC, Ambati BK. Tarantula hairs as corneal foreign bodies. Case Rep Ophthalmol. 2011;2(3):323-326.
4. Bertani R, Guadanucci JPL. Morphology, evolution, and usage of urticating setae by tarantulas (Araneae: Theraphosidae). Zoologia (Curitiba). 2013;30(4):403-418.
5. McAnena L, Murphy C, O’Connor J. Tarantula keratitis: a case report. Ir J Med Sci. 2013;182(3):349-350.
6. Yang Y, Christakis T, Mireskandari K. Acute conjunctivitis and corneal foreign bodies secondary to tarantula hairs. CMAJ. 2016;183(3):212-214.
7. Jain N, Soong HK, Gardner TW. Ophthalmia nodosa. EyeNet Magazine. November 2013. www.aao.org/eyenet/article/blink-mystery-image-17. Accessed January 24, 2018.
8. Choi JTL, Rauf A. Ophthalmia nodosa secondary to tarantula hairs. Eye (Lond). 2003;17(3):433-434.
9. Comez AT, Tufan HA, Gencer B. Ophthalmia nodosa as an occupational disease: is it unusual or is it casual? Ocul Immunol Inflamm. 2013;21(2):144-147.
10. Marti-Huguet T, Pujol O, Cabiro I, et al. Endophthalmos caused by intravitreal caterpillar hairs. Treatment by direct photocoagulation with argon laser [article in French]. J Fr Ophthalmol. 1987;10(10):559-564.
IN THIS ARTICLE
- Diagnosis
- Management
- Outcome for the case patient
An otherwise healthy 9-year-old boy is brought to the emergency department (ED) by his father for evaluation of severe pain, blurry vision, and four hours of tearing in his right eye. The patient was in school when he experienced sudden-onset irritation and scratching pain that caused him to rub his eye. He says it “feels like there is something in my eye,” but he denies any known substance or foreign body. He has no medical or surgical history, does not wear contact lenses or eyeglasses, and denies loss of vision. There is no history of recent illness or travel.
On evaluation, the patient is in no acute distress but is holding his right eye closed due to foreign-body sensation and increased photosensitivity and tearing. There is no obvious erythema or swelling in the upper or lower eyelids bilaterally. A visual acuity test with a Snellen eye chart shows 20/20 vision in the left eye and 20/50 in the right, secondary to pain, photophobia, and excessive tearing. The patient’s right sclera is significantly injected. Intraocular pressure, measured with a tonometer, is 12 to 14 mm Hg. A fluorescein stain of the eye yields no significant findings. The globe is intact.
At first glance, a slit-lamp exam shows no obvious signs of a foreign body. But much higher magnification reveals substantial conjunctival injection and numerous intracorneal linear foreign bodies in the right eye (see Figure 1 for example [not the case patient]). The anterior chamber shows no inflammatory reaction, and findings in the posterior segment are unremarkable.
The initial diagnosis is simple conjunctivitis—but closer examination reveals multiple fine, barbed hairs embedded in the patient’s right cornea. Upon further questioning, the patient reports that prior to symptom onset, he had been holding the classroom pet, a Chilean Rose tarantula, in the palm of his hands.

DISCUSSION
Foreign body injury is a common cause of ocular pain and corneal damage, which can lead to challenging complications. Ophthalmic emergencies account for 2% of ED visits in the US annually and are a major cause of visual impairment.1 But when a painful eye is the chief complaint, contact with insects, plants, or spiders is rarely included in the differential. Tarantulas are popular classroom and household pets, however, and ocular injury should be suspected in anyone who has been holding a tarantula prior to onset of pain.
Ophthalmia nodosa
Tarantulas are one of the most common arachnids known to cause ophthalmia nodosa—a granulomatous reaction of the conjunctiva or cornea to an implanted plant, insect, or spider hair that typically manifests with photophobia, irritation, and chemosis.2,3 Tarantulas, when scared or defending their eggs, shoot urticating setae at the threat—a defensive mechanism largely unknown to parents, tarantula owners, and medical professionals.
Urticating setae are found in roughly 90% of tarantula species throughout tropical and subtropical regions.4 Depending on the species, setae can be located on the distal prolateral surface of the palpal femur or the dorsum of the abdomen. They can be released when the tarantula scratches its legs against the abdominal urticating setae patch or scratches the palps against the chelicerae (appendages in front of the mouth), or when direct exterior contact is made with the abdominal setae.4
There are six types of urticating hairs. Each is attached to the spider’s cuticle by either a stalk (which represents the break-off region) or a socket.4 Tarantula hairs range in size from 0.1 mm to 0.3 mm and have a sharp, pointed head and numerous barbs, which help embed them in the target.5 They are long and thin, to facilitate deep tissue penetration, and can enter the eyes, lungs, or other body parts (see Figure 2).
Ocular injury from tarantula hairs commonly involves conjunctival injection, foreign body sensation, periorbital facial rash, photophobia, and tearing.3 When a tarantula’s cloud of barbed hairs is flicked into the eye and pierces the cornea, it can cause infection, irritation, scarring on the cornea, or vision loss. Eye movement or rubbing can cause the hairs—and their toxins—to migrate over time, traveling like an arrow (the tip and barbs resist backward movement) to the anterior chamber, lens, vitreous, and retina.6,7 This can cause corneal scars, cataracts, vitritis, or macular edema, and creates the possibility for acute or chronic conjunctivitis.7
Diagnosis and management
Ophthalmic emergencies can affect the visual system and, if left untreated, can lead to permanent vision loss. Affected patients require immediate medical attention and should be referred to an ophthalmologist for follow-up care.
Diagnosis. A thorough history and physical exam are of utmost importance; tiny setae can be easily overlooked if the examiner is not diligent, and the similar symptomatology can lead to misdiagnosis as simple conjunctivitis.3 A visual acuity test and slit-lamp exam are useful for confirmation.
Treatment. Once the diagnosis is confirmed, treatment should consist of mild topical antibiotics and steroids to effectively control infection and inflammation. While topical steroids may be appropriate, local adverse events associated with their use (eg, glaucoma, cataracts) can be problematic. Gentle eye irrigation has been noted by some researchers as contraindicated, while others find it useful to flush out some of the hairs.5,8,9
Most of the visible protruding tarantula hairs can and should be removed under microscopy during slit-lamp exam. Hairs that are buried in the cornea, however, are nearly impossible to remove and pose a threat of further complications, as described. Conservative management with careful observation is therefore recommended. If the patient develops a granuloma, excision—along with a course of systemic steroids and setae removal via vitrectomy—may be needed.9
The good news is that, in many cases, deeper hairs are absorbed without complication, making their removal unnecessary.5 Factors that encourage leaving the setae untouched include a large number of hairs, deep corneal penetration, lack of patient tolerance for the procedure, and risk for perforation.3
More invasive treatments (eg, laser photocoagulation, intraocular surgery) to remove offending hairs are possible, but literature on the outcome of these interventions is limited. One report to date used argon laser photocoagulation to treat endophthalmitis from vitreous hairs.10 The laser can fragment the hairs so that they lose their barbed characteristic and cannot penetrate deeper.6
Follow-up. Close follow-up is advised, and patients should be educated on the importance of medication compliance and return visits for reevaluation. Given the potential dangers of handling these spiders, tarantula owners should be advised to use protective gloving and goggles.2,5,8,9
OUTCOME FOR THE CASE PATIENT
The case patient was sent to an ophthalmologist on day 1. Proparacaine was placed in his right eye, and all of the superficial tarantula hairs were removed using 25- and 30-gauge needles with jeweler forceps under slit-lamp microscopy. Most of the hairs were removed from the superior cornea; fewer were found in the paracentral and inferior regions of the cornea. Approximately five hairs in the paracentral area of the cornea were embedded in the midstromal depth and could not be removed. One drop of ciprofloxacin was administered.
The patient was sent home with an eye shield and instructions to use tobramycin/dexamethasone eye drops (qid in his right eye) and avoid rubbing the eye. (The eye shield, though not technically necessary, was deemed beneficial to help the patient avoid touching the eye.) He was scheduled to return to the clinic one week later.
On follow-up, a careful exam performed under microscopy showed that the five tarantula hairs were still embedded, and an additional six hairs were found in the deep stroma. Superficial punctate keratitis—an eye disorder caused by epithelial cell death on the surface of the cornea—was noted, but no anterior chamber cells were seen. The patient was instructed to continue using the eye drops as prescribed until finished, then start using loteprednol (tid) and artificial lubricating tears (every 2 h).
He returned to the clinic every two weeks for a total of 10 visits. At the end of the treatment course, the remaining tarantula hairs were unable to be removed. The patient used tapering doses of topical eye steroids and antibiotic drops secondary to flare-up.
CONCLUSION
Determining the etiology of ophthalmic emergencies is essential to timely and appropriate management. In this case, a recognized but often overlooked cause, tarantula hairs, made the diagnosis more complicated than simple conjunctivitis. When ocular injury is suspected, the provider must obtain an accurate and detailed history along with a thorough physical exam. Since patients must comply with medication regimens to prevent acute and chronic infection, a clear treatment and follow-up plan should be established. With these in place, ophthalmia nodosa caused by urticating setae can be effectively managed.
IN THIS ARTICLE
- Diagnosis
- Management
- Outcome for the case patient
An otherwise healthy 9-year-old boy is brought to the emergency department (ED) by his father for evaluation of severe pain, blurry vision, and four hours of tearing in his right eye. The patient was in school when he experienced sudden-onset irritation and scratching pain that caused him to rub his eye. He says it “feels like there is something in my eye,” but he denies any known substance or foreign body. He has no medical or surgical history, does not wear contact lenses or eyeglasses, and denies loss of vision. There is no history of recent illness or travel.
On evaluation, the patient is in no acute distress but is holding his right eye closed due to foreign-body sensation and increased photosensitivity and tearing. There is no obvious erythema or swelling in the upper or lower eyelids bilaterally. A visual acuity test with a Snellen eye chart shows 20/20 vision in the left eye and 20/50 in the right, secondary to pain, photophobia, and excessive tearing. The patient’s right sclera is significantly injected. Intraocular pressure, measured with a tonometer, is 12 to 14 mm Hg. A fluorescein stain of the eye yields no significant findings. The globe is intact.
At first glance, a slit-lamp exam shows no obvious signs of a foreign body. But much higher magnification reveals substantial conjunctival injection and numerous intracorneal linear foreign bodies in the right eye (see Figure 1 for example [not the case patient]). The anterior chamber shows no inflammatory reaction, and findings in the posterior segment are unremarkable.
The initial diagnosis is simple conjunctivitis—but closer examination reveals multiple fine, barbed hairs embedded in the patient’s right cornea. Upon further questioning, the patient reports that prior to symptom onset, he had been holding the classroom pet, a Chilean Rose tarantula, in the palm of his hands.

DISCUSSION
Foreign body injury is a common cause of ocular pain and corneal damage, which can lead to challenging complications. Ophthalmic emergencies account for 2% of ED visits in the US annually and are a major cause of visual impairment.1 But when a painful eye is the chief complaint, contact with insects, plants, or spiders is rarely included in the differential. Tarantulas are popular classroom and household pets, however, and ocular injury should be suspected in anyone who has been holding a tarantula prior to onset of pain.
Ophthalmia nodosa
Tarantulas are one of the most common arachnids known to cause ophthalmia nodosa—a granulomatous reaction of the conjunctiva or cornea to an implanted plant, insect, or spider hair that typically manifests with photophobia, irritation, and chemosis.2,3 Tarantulas, when scared or defending their eggs, shoot urticating setae at the threat—a defensive mechanism largely unknown to parents, tarantula owners, and medical professionals.
Urticating setae are found in roughly 90% of tarantula species throughout tropical and subtropical regions.4 Depending on the species, setae can be located on the distal prolateral surface of the palpal femur or the dorsum of the abdomen. They can be released when the tarantula scratches its legs against the abdominal urticating setae patch or scratches the palps against the chelicerae (appendages in front of the mouth), or when direct exterior contact is made with the abdominal setae.4
There are six types of urticating hairs. Each is attached to the spider’s cuticle by either a stalk (which represents the break-off region) or a socket.4 Tarantula hairs range in size from 0.1 mm to 0.3 mm and have a sharp, pointed head and numerous barbs, which help embed them in the target.5 They are long and thin, to facilitate deep tissue penetration, and can enter the eyes, lungs, or other body parts (see Figure 2).
Ocular injury from tarantula hairs commonly involves conjunctival injection, foreign body sensation, periorbital facial rash, photophobia, and tearing.3 When a tarantula’s cloud of barbed hairs is flicked into the eye and pierces the cornea, it can cause infection, irritation, scarring on the cornea, or vision loss. Eye movement or rubbing can cause the hairs—and their toxins—to migrate over time, traveling like an arrow (the tip and barbs resist backward movement) to the anterior chamber, lens, vitreous, and retina.6,7 This can cause corneal scars, cataracts, vitritis, or macular edema, and creates the possibility for acute or chronic conjunctivitis.7
Diagnosis and management
Ophthalmic emergencies can affect the visual system and, if left untreated, can lead to permanent vision loss. Affected patients require immediate medical attention and should be referred to an ophthalmologist for follow-up care.
Diagnosis. A thorough history and physical exam are of utmost importance; tiny setae can be easily overlooked if the examiner is not diligent, and the similar symptomatology can lead to misdiagnosis as simple conjunctivitis.3 A visual acuity test and slit-lamp exam are useful for confirmation.
Treatment. Once the diagnosis is confirmed, treatment should consist of mild topical antibiotics and steroids to effectively control infection and inflammation. While topical steroids may be appropriate, local adverse events associated with their use (eg, glaucoma, cataracts) can be problematic. Gentle eye irrigation has been noted by some researchers as contraindicated, while others find it useful to flush out some of the hairs.5,8,9
Most of the visible protruding tarantula hairs can and should be removed under microscopy during slit-lamp exam. Hairs that are buried in the cornea, however, are nearly impossible to remove and pose a threat of further complications, as described. Conservative management with careful observation is therefore recommended. If the patient develops a granuloma, excision—along with a course of systemic steroids and setae removal via vitrectomy—may be needed.9
The good news is that, in many cases, deeper hairs are absorbed without complication, making their removal unnecessary.5 Factors that encourage leaving the setae untouched include a large number of hairs, deep corneal penetration, lack of patient tolerance for the procedure, and risk for perforation.3
More invasive treatments (eg, laser photocoagulation, intraocular surgery) to remove offending hairs are possible, but literature on the outcome of these interventions is limited. One report to date used argon laser photocoagulation to treat endophthalmitis from vitreous hairs.10 The laser can fragment the hairs so that they lose their barbed characteristic and cannot penetrate deeper.6
Follow-up. Close follow-up is advised, and patients should be educated on the importance of medication compliance and return visits for reevaluation. Given the potential dangers of handling these spiders, tarantula owners should be advised to use protective gloving and goggles.2,5,8,9
OUTCOME FOR THE CASE PATIENT
The case patient was sent to an ophthalmologist on day 1. Proparacaine was placed in his right eye, and all of the superficial tarantula hairs were removed using 25- and 30-gauge needles with jeweler forceps under slit-lamp microscopy. Most of the hairs were removed from the superior cornea; fewer were found in the paracentral and inferior regions of the cornea. Approximately five hairs in the paracentral area of the cornea were embedded in the midstromal depth and could not be removed. One drop of ciprofloxacin was administered.
The patient was sent home with an eye shield and instructions to use tobramycin/dexamethasone eye drops (qid in his right eye) and avoid rubbing the eye. (The eye shield, though not technically necessary, was deemed beneficial to help the patient avoid touching the eye.) He was scheduled to return to the clinic one week later.
On follow-up, a careful exam performed under microscopy showed that the five tarantula hairs were still embedded, and an additional six hairs were found in the deep stroma. Superficial punctate keratitis—an eye disorder caused by epithelial cell death on the surface of the cornea—was noted, but no anterior chamber cells were seen. The patient was instructed to continue using the eye drops as prescribed until finished, then start using loteprednol (tid) and artificial lubricating tears (every 2 h).
He returned to the clinic every two weeks for a total of 10 visits. At the end of the treatment course, the remaining tarantula hairs were unable to be removed. The patient used tapering doses of topical eye steroids and antibiotic drops secondary to flare-up.
CONCLUSION
Determining the etiology of ophthalmic emergencies is essential to timely and appropriate management. In this case, a recognized but often overlooked cause, tarantula hairs, made the diagnosis more complicated than simple conjunctivitis. When ocular injury is suspected, the provider must obtain an accurate and detailed history along with a thorough physical exam. Since patients must comply with medication regimens to prevent acute and chronic infection, a clear treatment and follow-up plan should be established. With these in place, ophthalmia nodosa caused by urticating setae can be effectively managed.
1. Fitzpatrick J, Hickman R, Alfes CM. A Guide to Mastery in Clinical Nursing: The Comprehensive Reference. New York, NY: Springer; 2018:114.
2. Lambert SR, Lyons CJ. Taylor and Hoyt’s Pediatric Ophthalmology and Strabismus. 5th ed. New York, NY: Elsevier; 2017:138.
3. Stagg BC, Ambati BK. Tarantula hairs as corneal foreign bodies. Case Rep Ophthalmol. 2011;2(3):323-326.
4. Bertani R, Guadanucci JPL. Morphology, evolution, and usage of urticating setae by tarantulas (Araneae: Theraphosidae). Zoologia (Curitiba). 2013;30(4):403-418.
5. McAnena L, Murphy C, O’Connor J. Tarantula keratitis: a case report. Ir J Med Sci. 2013;182(3):349-350.
6. Yang Y, Christakis T, Mireskandari K. Acute conjunctivitis and corneal foreign bodies secondary to tarantula hairs. CMAJ. 2016;183(3):212-214.
7. Jain N, Soong HK, Gardner TW. Ophthalmia nodosa. EyeNet Magazine. November 2013. www.aao.org/eyenet/article/blink-mystery-image-17. Accessed January 24, 2018.
8. Choi JTL, Rauf A. Ophthalmia nodosa secondary to tarantula hairs. Eye (Lond). 2003;17(3):433-434.
9. Comez AT, Tufan HA, Gencer B. Ophthalmia nodosa as an occupational disease: is it unusual or is it casual? Ocul Immunol Inflamm. 2013;21(2):144-147.
10. Marti-Huguet T, Pujol O, Cabiro I, et al. Endophthalmos caused by intravitreal caterpillar hairs. Treatment by direct photocoagulation with argon laser [article in French]. J Fr Ophthalmol. 1987;10(10):559-564.
1. Fitzpatrick J, Hickman R, Alfes CM. A Guide to Mastery in Clinical Nursing: The Comprehensive Reference. New York, NY: Springer; 2018:114.
2. Lambert SR, Lyons CJ. Taylor and Hoyt’s Pediatric Ophthalmology and Strabismus. 5th ed. New York, NY: Elsevier; 2017:138.
3. Stagg BC, Ambati BK. Tarantula hairs as corneal foreign bodies. Case Rep Ophthalmol. 2011;2(3):323-326.
4. Bertani R, Guadanucci JPL. Morphology, evolution, and usage of urticating setae by tarantulas (Araneae: Theraphosidae). Zoologia (Curitiba). 2013;30(4):403-418.
5. McAnena L, Murphy C, O’Connor J. Tarantula keratitis: a case report. Ir J Med Sci. 2013;182(3):349-350.
6. Yang Y, Christakis T, Mireskandari K. Acute conjunctivitis and corneal foreign bodies secondary to tarantula hairs. CMAJ. 2016;183(3):212-214.
7. Jain N, Soong HK, Gardner TW. Ophthalmia nodosa. EyeNet Magazine. November 2013. www.aao.org/eyenet/article/blink-mystery-image-17. Accessed January 24, 2018.
8. Choi JTL, Rauf A. Ophthalmia nodosa secondary to tarantula hairs. Eye (Lond). 2003;17(3):433-434.
9. Comez AT, Tufan HA, Gencer B. Ophthalmia nodosa as an occupational disease: is it unusual or is it casual? Ocul Immunol Inflamm. 2013;21(2):144-147.
10. Marti-Huguet T, Pujol O, Cabiro I, et al. Endophthalmos caused by intravitreal caterpillar hairs. Treatment by direct photocoagulation with argon laser [article in French]. J Fr Ophthalmol. 1987;10(10):559-564.
Medication pricing: So this is how it works
This is the second part in a series on medication pricing.
In my last column, I looked at the tremendous variation in prices among pharmacies for two psychotropic medications, aripiprazole and modafinil. The cash price variation could be as much as 45 times more from one pharmacy to the next, which I found to be both outrageous and incomprehensible.
To learn more about pharmaceutical pricing, I contacted Doug Hirsch, the cofounder of GoodRx, a firm based in Santa Monica, Calif., that offers deep discounts on some medications. The company sends discount cards to physicians’ offices – call me if you need some, I have many boxes of GoodRx cards – and has a website (www.GoodRx.com) and an app. It advertises that it is about transparency, and if you’ve ever tried the company’s site or app, the service it offers is remarkable and simple to use.
I approached Mr. Hirsch with two simple questions. The company offers “up to 90% discount” on the cash price of medications through its app, website, or discount card – all of which can be gotten for free. I wanted to know 1) Who pays for this difference in the medication cost, and 2) How does the company, with 95 employees, make any money? Mr. Hirsch was gracious enough (and patient enough!) to spend the next hour walking me through the steps of medication pricing. It was a lively conversation, so let me share with you what I have learned.
Medications are made by a pharmaceutical company or, for generics, there may be many manufacturers. The medications are sent to a pharmaceutical distributor, such as McKesson, and it, in turn, sells and delivers the products to pharmacy chains, as well as to smaller, independent pharmacies. The pharmacies pay an acquisition cost for medications then set a price for these medications that are considerably – or even astronomically – higher than the acquisition price. This is the cash retail price, or in medicine, what is called the Usual & Customary (U&C) cost of the medication. The price may be neither usual, customary, nor reasonable, and it’s not the price the pharmacy expects to recoup on sales.
Every major insurance company contracts with a pharmacy benefits manager (for example, Caremark, Express Scripts, and Optum) to negotiate the cost of medications with each major pharmacy chain. Physicians are familiar with PBMs, who intercede by requiring preauthorization procedures for certain medications or by instituting stepwise, fail-first, requirements before they will allow pharmacy benefits toward the purchase of medications. When the PBMs negotiate with the pharmacies, they will negotiate for a discount off the pharmacy’s U&C charge for medication, perhaps a discount as much as 75% or 80%. Mr. Hirsch noted, “The discount is not negotiated on a per-medication basis but as an across-the-board average, so for one medication, the insurance price may be 2% discount from the U&C cost, and on another medicine it may be 95%. There is a dramatic variation, more than you’d ever expect.”
GoodRx gathers prices from many places, including partnerships with a number of PBMs. In addition to providing discounted prices for insured customers, the PBMs also include in their negotiations a slightly less-discounted price for cash-paying patients who present with a GoodRx card or coupon. You might be surprised to learn that discounted prices can often be less than the typical patient copay. For patients with a high deductible, for medications that are not covered at all, or for times when the copay is higher than the cost of the medication, it will often be less expensive for patients to use a GoodRx discount instead of their insurance. And whether patients uses either their insurance or a GoodRx discount, part of the cost of the prescription includes an administrative fee that goes to the PBM. When GoodRx cards are used, the PBM pays GoodRx part of that fee. I hope you are still with me, because this is the part of the conversation where I started telling Mr. Hirsch that I was getting a headache.
I went back to the enormous cost discrepancy that I had discovered a couple of years ago with Provigil (modafinil). Thirty pills cost just under $35 at Costco, while all other pharmacies were charging close to $1,000. Mr. Hirsch explained, “From what I’ve been told, Costco bases their prices on their acquisition costs and then raises them a certain percent. It’s one way to provide a fair price, but that doesn’t mean they always have the lowest price. They are also the only major pharmacy that lists their drug prices on their website.”
I wanted to know what was in it for the PBMs. Why would Express Scripts be motivated to negotiate a discount in price for cash-paying customers outside of the insurance networks, and how did partnering with GoodRx benefit them? The answer, in part, lies with the fact that the website and app allow patients to comparison shop and go to pharmacies with lower prices. If patients use their insurance, the insurance company is paying less; if they don’t use their insurance because they learned the cash cost is less, then the cost burden has shifted from the insurance company entirely to the patient.
What’s in it for the pharmacies? Why would they be willing to accept less money from a patient bearing a discount card? Mr. Hirsch explained, “Pharmacies want to honor their contracts with PBMs, and the U&C prices are set high to enable negotiation so that they still make some profit. Most people couldn’t afford to pay the high U&C, but they can’t lower them for individual cash-pay customers because that would violate their agreements with PBMs, and Medicare and Medicaid, which is a felony. With the GoodRx price, they still make a profit, and people in drugstores buy other items as well.
GoodRx has 95 employees, and I was still left wondering how they generate income. Mr. Hirsch pinned it down to three sources: the portion of the administration fees the PBMs pay GoodRx, a small amount of advertising, and finally, GoodRx provides technology for the PBMs and charges for this service.
“We started asking how we could gather prices in this bizarre marketplace and address the pricing inefficiencies,” Mr. Hirsch said, “and now I get emails every day expressing gratitude.”
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016).
This is the second part in a series on medication pricing.
In my last column, I looked at the tremendous variation in prices among pharmacies for two psychotropic medications, aripiprazole and modafinil. The cash price variation could be as much as 45 times more from one pharmacy to the next, which I found to be both outrageous and incomprehensible.
To learn more about pharmaceutical pricing, I contacted Doug Hirsch, the cofounder of GoodRx, a firm based in Santa Monica, Calif., that offers deep discounts on some medications. The company sends discount cards to physicians’ offices – call me if you need some, I have many boxes of GoodRx cards – and has a website (www.GoodRx.com) and an app. It advertises that it is about transparency, and if you’ve ever tried the company’s site or app, the service it offers is remarkable and simple to use.
I approached Mr. Hirsch with two simple questions. The company offers “up to 90% discount” on the cash price of medications through its app, website, or discount card – all of which can be gotten for free. I wanted to know 1) Who pays for this difference in the medication cost, and 2) How does the company, with 95 employees, make any money? Mr. Hirsch was gracious enough (and patient enough!) to spend the next hour walking me through the steps of medication pricing. It was a lively conversation, so let me share with you what I have learned.
Medications are made by a pharmaceutical company or, for generics, there may be many manufacturers. The medications are sent to a pharmaceutical distributor, such as McKesson, and it, in turn, sells and delivers the products to pharmacy chains, as well as to smaller, independent pharmacies. The pharmacies pay an acquisition cost for medications then set a price for these medications that are considerably – or even astronomically – higher than the acquisition price. This is the cash retail price, or in medicine, what is called the Usual & Customary (U&C) cost of the medication. The price may be neither usual, customary, nor reasonable, and it’s not the price the pharmacy expects to recoup on sales.
Every major insurance company contracts with a pharmacy benefits manager (for example, Caremark, Express Scripts, and Optum) to negotiate the cost of medications with each major pharmacy chain. Physicians are familiar with PBMs, who intercede by requiring preauthorization procedures for certain medications or by instituting stepwise, fail-first, requirements before they will allow pharmacy benefits toward the purchase of medications. When the PBMs negotiate with the pharmacies, they will negotiate for a discount off the pharmacy’s U&C charge for medication, perhaps a discount as much as 75% or 80%. Mr. Hirsch noted, “The discount is not negotiated on a per-medication basis but as an across-the-board average, so for one medication, the insurance price may be 2% discount from the U&C cost, and on another medicine it may be 95%. There is a dramatic variation, more than you’d ever expect.”
GoodRx gathers prices from many places, including partnerships with a number of PBMs. In addition to providing discounted prices for insured customers, the PBMs also include in their negotiations a slightly less-discounted price for cash-paying patients who present with a GoodRx card or coupon. You might be surprised to learn that discounted prices can often be less than the typical patient copay. For patients with a high deductible, for medications that are not covered at all, or for times when the copay is higher than the cost of the medication, it will often be less expensive for patients to use a GoodRx discount instead of their insurance. And whether patients uses either their insurance or a GoodRx discount, part of the cost of the prescription includes an administrative fee that goes to the PBM. When GoodRx cards are used, the PBM pays GoodRx part of that fee. I hope you are still with me, because this is the part of the conversation where I started telling Mr. Hirsch that I was getting a headache.
I went back to the enormous cost discrepancy that I had discovered a couple of years ago with Provigil (modafinil). Thirty pills cost just under $35 at Costco, while all other pharmacies were charging close to $1,000. Mr. Hirsch explained, “From what I’ve been told, Costco bases their prices on their acquisition costs and then raises them a certain percent. It’s one way to provide a fair price, but that doesn’t mean they always have the lowest price. They are also the only major pharmacy that lists their drug prices on their website.”
I wanted to know what was in it for the PBMs. Why would Express Scripts be motivated to negotiate a discount in price for cash-paying customers outside of the insurance networks, and how did partnering with GoodRx benefit them? The answer, in part, lies with the fact that the website and app allow patients to comparison shop and go to pharmacies with lower prices. If patients use their insurance, the insurance company is paying less; if they don’t use their insurance because they learned the cash cost is less, then the cost burden has shifted from the insurance company entirely to the patient.
What’s in it for the pharmacies? Why would they be willing to accept less money from a patient bearing a discount card? Mr. Hirsch explained, “Pharmacies want to honor their contracts with PBMs, and the U&C prices are set high to enable negotiation so that they still make some profit. Most people couldn’t afford to pay the high U&C, but they can’t lower them for individual cash-pay customers because that would violate their agreements with PBMs, and Medicare and Medicaid, which is a felony. With the GoodRx price, they still make a profit, and people in drugstores buy other items as well.
GoodRx has 95 employees, and I was still left wondering how they generate income. Mr. Hirsch pinned it down to three sources: the portion of the administration fees the PBMs pay GoodRx, a small amount of advertising, and finally, GoodRx provides technology for the PBMs and charges for this service.
“We started asking how we could gather prices in this bizarre marketplace and address the pricing inefficiencies,” Mr. Hirsch said, “and now I get emails every day expressing gratitude.”
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016).
This is the second part in a series on medication pricing.
In my last column, I looked at the tremendous variation in prices among pharmacies for two psychotropic medications, aripiprazole and modafinil. The cash price variation could be as much as 45 times more from one pharmacy to the next, which I found to be both outrageous and incomprehensible.
To learn more about pharmaceutical pricing, I contacted Doug Hirsch, the cofounder of GoodRx, a firm based in Santa Monica, Calif., that offers deep discounts on some medications. The company sends discount cards to physicians’ offices – call me if you need some, I have many boxes of GoodRx cards – and has a website (www.GoodRx.com) and an app. It advertises that it is about transparency, and if you’ve ever tried the company’s site or app, the service it offers is remarkable and simple to use.
I approached Mr. Hirsch with two simple questions. The company offers “up to 90% discount” on the cash price of medications through its app, website, or discount card – all of which can be gotten for free. I wanted to know 1) Who pays for this difference in the medication cost, and 2) How does the company, with 95 employees, make any money? Mr. Hirsch was gracious enough (and patient enough!) to spend the next hour walking me through the steps of medication pricing. It was a lively conversation, so let me share with you what I have learned.
Medications are made by a pharmaceutical company or, for generics, there may be many manufacturers. The medications are sent to a pharmaceutical distributor, such as McKesson, and it, in turn, sells and delivers the products to pharmacy chains, as well as to smaller, independent pharmacies. The pharmacies pay an acquisition cost for medications then set a price for these medications that are considerably – or even astronomically – higher than the acquisition price. This is the cash retail price, or in medicine, what is called the Usual & Customary (U&C) cost of the medication. The price may be neither usual, customary, nor reasonable, and it’s not the price the pharmacy expects to recoup on sales.
Every major insurance company contracts with a pharmacy benefits manager (for example, Caremark, Express Scripts, and Optum) to negotiate the cost of medications with each major pharmacy chain. Physicians are familiar with PBMs, who intercede by requiring preauthorization procedures for certain medications or by instituting stepwise, fail-first, requirements before they will allow pharmacy benefits toward the purchase of medications. When the PBMs negotiate with the pharmacies, they will negotiate for a discount off the pharmacy’s U&C charge for medication, perhaps a discount as much as 75% or 80%. Mr. Hirsch noted, “The discount is not negotiated on a per-medication basis but as an across-the-board average, so for one medication, the insurance price may be 2% discount from the U&C cost, and on another medicine it may be 95%. There is a dramatic variation, more than you’d ever expect.”
GoodRx gathers prices from many places, including partnerships with a number of PBMs. In addition to providing discounted prices for insured customers, the PBMs also include in their negotiations a slightly less-discounted price for cash-paying patients who present with a GoodRx card or coupon. You might be surprised to learn that discounted prices can often be less than the typical patient copay. For patients with a high deductible, for medications that are not covered at all, or for times when the copay is higher than the cost of the medication, it will often be less expensive for patients to use a GoodRx discount instead of their insurance. And whether patients uses either their insurance or a GoodRx discount, part of the cost of the prescription includes an administrative fee that goes to the PBM. When GoodRx cards are used, the PBM pays GoodRx part of that fee. I hope you are still with me, because this is the part of the conversation where I started telling Mr. Hirsch that I was getting a headache.
I went back to the enormous cost discrepancy that I had discovered a couple of years ago with Provigil (modafinil). Thirty pills cost just under $35 at Costco, while all other pharmacies were charging close to $1,000. Mr. Hirsch explained, “From what I’ve been told, Costco bases their prices on their acquisition costs and then raises them a certain percent. It’s one way to provide a fair price, but that doesn’t mean they always have the lowest price. They are also the only major pharmacy that lists their drug prices on their website.”
I wanted to know what was in it for the PBMs. Why would Express Scripts be motivated to negotiate a discount in price for cash-paying customers outside of the insurance networks, and how did partnering with GoodRx benefit them? The answer, in part, lies with the fact that the website and app allow patients to comparison shop and go to pharmacies with lower prices. If patients use their insurance, the insurance company is paying less; if they don’t use their insurance because they learned the cash cost is less, then the cost burden has shifted from the insurance company entirely to the patient.
What’s in it for the pharmacies? Why would they be willing to accept less money from a patient bearing a discount card? Mr. Hirsch explained, “Pharmacies want to honor their contracts with PBMs, and the U&C prices are set high to enable negotiation so that they still make some profit. Most people couldn’t afford to pay the high U&C, but they can’t lower them for individual cash-pay customers because that would violate their agreements with PBMs, and Medicare and Medicaid, which is a felony. With the GoodRx price, they still make a profit, and people in drugstores buy other items as well.
GoodRx has 95 employees, and I was still left wondering how they generate income. Mr. Hirsch pinned it down to three sources: the portion of the administration fees the PBMs pay GoodRx, a small amount of advertising, and finally, GoodRx provides technology for the PBMs and charges for this service.
“We started asking how we could gather prices in this bizarre marketplace and address the pricing inefficiencies,” Mr. Hirsch said, “and now I get emails every day expressing gratitude.”
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016).
Mental health stressors still loom for Puerto Ricans after Maria
The physical and mental health needs of the people of Puerto Rico cannot be underestimated. Just think of what they have been through over the last few months.
When Hurricane Maria barreled onto the island on Sept. 20, 2017 – just 2 weeks after Hurricane Irma reportedly left more than 1 million residents without power – it ripped off roofs and left behind massive flooding, roads washed out, and utility poles and transmission lines knocked down. Whole forests were defoliated, a massive loss of flora and fauna occurred, and 80% of the crop value was destroyed, along with massive loss of stray dogs and cats, dairy cows, industrial chicken coops, and tropical birds, including endangered species. Beloved pets were displaced.
The official death toll as a result of Maria was 64 in December, but according to reporting by The New York Times, that number could be as high as 1,052. Most of the people who died reportedly were men and women over age 50 in hospitals and nursing homes suffering from illnesses such as diabetes, Alzheimer’s, kidney disease, hypertension, pneumonia, and other respiratory diseases.
One grassroots organization that mobilized to provide supplies and medical assistance was Doctoras Boricuas, a group of all-female doctors in the United States and Puerto Rico that formed after the hurricanes to coordinate the delivery and distribution of supplies directly to Puerto Rico and the Virgin Islands. Two groups affiliated with the University of Missouri at Columbia joined forces to help: Global First Responder or GFR, a nonprofit, secular international medical relief organization founded in 2011 by Adam Beckett, MD, and the International Center for Psychosocial Trauma, or ICPT, a group established in 1995 by Syed Arshad Husain, MD, to help war-traumatized children in Bosnia. I joined Dr. Husain’s group of professionals – Kathryn Dewein, PhD; Andra Ferguson, PhD; and Cathy Grigg, PsyD, – all of whom have traveled broadly in the field of disaster psychiatry – to see how we could help the people of Puerto Rico in Maria’s aftermath.
What we did
ICPT and GFR were a combined team, but we served different functions. As part of ICPT, I focused on the mental health component and helped to train doctors, psychologists, social workers, and other mental health workers in both San Juan and Ponce. All told, we worked with about 50 people using the model of “Training the Trainers.” Many of our students were participants in the outreach teams. Our hope is that they will be able to train their peers to recognize and alleviate symptoms of acute and chronic stress disorders. Some of the techniques taught include patient education, relaxation training, breath work, visualization techniques, mindfulness training, narrative therapy, art therapy, and other expressive techniques.
What the PMSF did
Before Maria, the Ponce Medical School Foundation was in the process of facilitating the transfer of medical records into an electronic format. After the hurricane hit, however, PMSF’s program director, Antonio Fernandez, led a shift to disaster recovery work. PMSF got involved in airlifting dialysis patients off the island to safety, provided health care, and also collaborated with the Primary Care Psychology Program at Ponce Health Services University to assist in locating patients, identifying their health needs – including mental health – and providing for those needs to the extent possible.
At the time of our visit, Puerto Rico’s network of more than 90 largely rural federally funded primary care clinics mostly had reopened, but nearly half remained on back-up generators. Even with the medical centers open, patients were not coming in for one reason or another. People had medical problems, but the daily reality of survival, obtaining food and water, took precedence. Some patients were not showing up because they had left the country, or they were in shelters without transportation. Some people did not have fuel. Some could not keep track of their appointments without cell phones and electricity allowing them to access electronic planners. Some, having been without their medications since the storms, were too sick to travel. Outreach teams were necessary to locate patients, identify their needs, and provide medical and psychological care.
Community outreach
Nydia M. Cappas, PsyD, director of the Primary Care Psychology Program, told us that the outreach teams – consisting of doctors or other medical professionals, social workers, and psychologists, were being sent out to communities once a week. They visited homes for the elderly, orphans’ homes, and children in foster care, as well as individual patients. A similar service was provided by Vargas Medicine (VARMED) in the San Juan area.
Team members found that many people were suffering symptoms of posttraumatic stress disorder, even people who did not have prior psychiatric symptoms. They were having flashbacks and nightmares. Those flashbacks and nightmares were being triggered by clouds, by rain, by supplies beginning to run out.
Another trend we observed is that terrain changes prompted by Maria triggered PTSD symptoms among many veterans. The defoliated trees and brown earth were causing them to have flashbacks to the deserts of Afghanistan and Iraq. Children were showing regressive behaviors, loss of developmental milestones, and symptoms of separation anxiety such as wanting to sleep with their parents. In severe cases, they were having psychotic symptoms and auditory hallucinations. The children were grieving the loss of their homes, toys, pets, and family members, in some cases. , including medications, food, toiletries, and other household goods.
Puerto Rico’s future
Two and a half months after Maria, we learned from our students that things gradually had begun to improve. For example, the public schools had just reopened, and that change was expected to have a stabilizing effect on the children. We also learned that, of the 80 shelters that had been set up housing about 12,500 people, 40 shelters had closed. The five medical shelters that had been set up and funded by FEMA also were in the process of closing, and private donations were beginning to slow down. People were slowly returning to their tarped or otherwise repaired homes, albeit all too often without power.
During the storm, nearly 500,000* homes were destroyed. FEMA offered to airlift about 3,000 people who had no home 2 months past Maria to the U.S. mainland – either Florida or New York.
According to our students, people living in the mountains, mainly coffee growers and retired people and comprising about one-third of the population, remain in acute crisis. Part of the challenge is being able to reach this population: Some roads are still impassable, and supplies – such as drinking water – can be delivered only by helicopter. Despite current conditions, FEMA reportedly has announced that it would end emergency operations on the island.
Our team is currently involved in applying for grant funding that will enable us to return to provide additional training to physicians’ and teachers’ groups. Over the course of the next year, we would like to make six trips to Puerto Rico and focus each trip on a different region and different group of professionals so that the entire island has resources. In addition, we will offer follow-up consultations to professionals we trained previously. The regions to be trained would be San Juan, Ponce, Utuado, Mayagüez, Guayama, and a sixth to be determined upon need. We also would like to address the needs of any ongoing relief workers so that they will be more effective in their ongoing role. Meanwhile, financial assistance from the mainland remains uneven.
Many months after Maria (and Irma), the physical and mental health needs of the Puerto Rican people remain great. However, as mental health professionals, we have the tools to help them move forward.
Judith R. Milner, MD, MEd, SpecEd, is a general, child, and adolescent psychiatrist in private practice in Everett, Wash. She has traveled with various groups over the years in an effort to alleviate some of the suffering caused by war and natural disaster. Her predominant association has been with the International Center for Psychosocial Trauma. She also has worked with Step Up Rwanda Women and Pygmy Survival Alliance, as well as on the Committee for Women at the American Psychiatric Association and the Consumer Issues Committee and Membership Committee for the American Academy of Child and Adolescent Psychiatry.
*Correction, 2/12/2018: An earlier version of this story misstated the number of homes reportedly destroyed by Hurricane Maria.
The physical and mental health needs of the people of Puerto Rico cannot be underestimated. Just think of what they have been through over the last few months.
When Hurricane Maria barreled onto the island on Sept. 20, 2017 – just 2 weeks after Hurricane Irma reportedly left more than 1 million residents without power – it ripped off roofs and left behind massive flooding, roads washed out, and utility poles and transmission lines knocked down. Whole forests were defoliated, a massive loss of flora and fauna occurred, and 80% of the crop value was destroyed, along with massive loss of stray dogs and cats, dairy cows, industrial chicken coops, and tropical birds, including endangered species. Beloved pets were displaced.
The official death toll as a result of Maria was 64 in December, but according to reporting by The New York Times, that number could be as high as 1,052. Most of the people who died reportedly were men and women over age 50 in hospitals and nursing homes suffering from illnesses such as diabetes, Alzheimer’s, kidney disease, hypertension, pneumonia, and other respiratory diseases.
One grassroots organization that mobilized to provide supplies and medical assistance was Doctoras Boricuas, a group of all-female doctors in the United States and Puerto Rico that formed after the hurricanes to coordinate the delivery and distribution of supplies directly to Puerto Rico and the Virgin Islands. Two groups affiliated with the University of Missouri at Columbia joined forces to help: Global First Responder or GFR, a nonprofit, secular international medical relief organization founded in 2011 by Adam Beckett, MD, and the International Center for Psychosocial Trauma, or ICPT, a group established in 1995 by Syed Arshad Husain, MD, to help war-traumatized children in Bosnia. I joined Dr. Husain’s group of professionals – Kathryn Dewein, PhD; Andra Ferguson, PhD; and Cathy Grigg, PsyD, – all of whom have traveled broadly in the field of disaster psychiatry – to see how we could help the people of Puerto Rico in Maria’s aftermath.
What we did
ICPT and GFR were a combined team, but we served different functions. As part of ICPT, I focused on the mental health component and helped to train doctors, psychologists, social workers, and other mental health workers in both San Juan and Ponce. All told, we worked with about 50 people using the model of “Training the Trainers.” Many of our students were participants in the outreach teams. Our hope is that they will be able to train their peers to recognize and alleviate symptoms of acute and chronic stress disorders. Some of the techniques taught include patient education, relaxation training, breath work, visualization techniques, mindfulness training, narrative therapy, art therapy, and other expressive techniques.
What the PMSF did
Before Maria, the Ponce Medical School Foundation was in the process of facilitating the transfer of medical records into an electronic format. After the hurricane hit, however, PMSF’s program director, Antonio Fernandez, led a shift to disaster recovery work. PMSF got involved in airlifting dialysis patients off the island to safety, provided health care, and also collaborated with the Primary Care Psychology Program at Ponce Health Services University to assist in locating patients, identifying their health needs – including mental health – and providing for those needs to the extent possible.
At the time of our visit, Puerto Rico’s network of more than 90 largely rural federally funded primary care clinics mostly had reopened, but nearly half remained on back-up generators. Even with the medical centers open, patients were not coming in for one reason or another. People had medical problems, but the daily reality of survival, obtaining food and water, took precedence. Some patients were not showing up because they had left the country, or they were in shelters without transportation. Some people did not have fuel. Some could not keep track of their appointments without cell phones and electricity allowing them to access electronic planners. Some, having been without their medications since the storms, were too sick to travel. Outreach teams were necessary to locate patients, identify their needs, and provide medical and psychological care.
Community outreach
Nydia M. Cappas, PsyD, director of the Primary Care Psychology Program, told us that the outreach teams – consisting of doctors or other medical professionals, social workers, and psychologists, were being sent out to communities once a week. They visited homes for the elderly, orphans’ homes, and children in foster care, as well as individual patients. A similar service was provided by Vargas Medicine (VARMED) in the San Juan area.
Team members found that many people were suffering symptoms of posttraumatic stress disorder, even people who did not have prior psychiatric symptoms. They were having flashbacks and nightmares. Those flashbacks and nightmares were being triggered by clouds, by rain, by supplies beginning to run out.
Another trend we observed is that terrain changes prompted by Maria triggered PTSD symptoms among many veterans. The defoliated trees and brown earth were causing them to have flashbacks to the deserts of Afghanistan and Iraq. Children were showing regressive behaviors, loss of developmental milestones, and symptoms of separation anxiety such as wanting to sleep with their parents. In severe cases, they were having psychotic symptoms and auditory hallucinations. The children were grieving the loss of their homes, toys, pets, and family members, in some cases. , including medications, food, toiletries, and other household goods.
Puerto Rico’s future
Two and a half months after Maria, we learned from our students that things gradually had begun to improve. For example, the public schools had just reopened, and that change was expected to have a stabilizing effect on the children. We also learned that, of the 80 shelters that had been set up housing about 12,500 people, 40 shelters had closed. The five medical shelters that had been set up and funded by FEMA also were in the process of closing, and private donations were beginning to slow down. People were slowly returning to their tarped or otherwise repaired homes, albeit all too often without power.
During the storm, nearly 500,000* homes were destroyed. FEMA offered to airlift about 3,000 people who had no home 2 months past Maria to the U.S. mainland – either Florida or New York.
According to our students, people living in the mountains, mainly coffee growers and retired people and comprising about one-third of the population, remain in acute crisis. Part of the challenge is being able to reach this population: Some roads are still impassable, and supplies – such as drinking water – can be delivered only by helicopter. Despite current conditions, FEMA reportedly has announced that it would end emergency operations on the island.
Our team is currently involved in applying for grant funding that will enable us to return to provide additional training to physicians’ and teachers’ groups. Over the course of the next year, we would like to make six trips to Puerto Rico and focus each trip on a different region and different group of professionals so that the entire island has resources. In addition, we will offer follow-up consultations to professionals we trained previously. The regions to be trained would be San Juan, Ponce, Utuado, Mayagüez, Guayama, and a sixth to be determined upon need. We also would like to address the needs of any ongoing relief workers so that they will be more effective in their ongoing role. Meanwhile, financial assistance from the mainland remains uneven.
Many months after Maria (and Irma), the physical and mental health needs of the Puerto Rican people remain great. However, as mental health professionals, we have the tools to help them move forward.
Judith R. Milner, MD, MEd, SpecEd, is a general, child, and adolescent psychiatrist in private practice in Everett, Wash. She has traveled with various groups over the years in an effort to alleviate some of the suffering caused by war and natural disaster. Her predominant association has been with the International Center for Psychosocial Trauma. She also has worked with Step Up Rwanda Women and Pygmy Survival Alliance, as well as on the Committee for Women at the American Psychiatric Association and the Consumer Issues Committee and Membership Committee for the American Academy of Child and Adolescent Psychiatry.
*Correction, 2/12/2018: An earlier version of this story misstated the number of homes reportedly destroyed by Hurricane Maria.
The physical and mental health needs of the people of Puerto Rico cannot be underestimated. Just think of what they have been through over the last few months.
When Hurricane Maria barreled onto the island on Sept. 20, 2017 – just 2 weeks after Hurricane Irma reportedly left more than 1 million residents without power – it ripped off roofs and left behind massive flooding, roads washed out, and utility poles and transmission lines knocked down. Whole forests were defoliated, a massive loss of flora and fauna occurred, and 80% of the crop value was destroyed, along with massive loss of stray dogs and cats, dairy cows, industrial chicken coops, and tropical birds, including endangered species. Beloved pets were displaced.
The official death toll as a result of Maria was 64 in December, but according to reporting by The New York Times, that number could be as high as 1,052. Most of the people who died reportedly were men and women over age 50 in hospitals and nursing homes suffering from illnesses such as diabetes, Alzheimer’s, kidney disease, hypertension, pneumonia, and other respiratory diseases.
One grassroots organization that mobilized to provide supplies and medical assistance was Doctoras Boricuas, a group of all-female doctors in the United States and Puerto Rico that formed after the hurricanes to coordinate the delivery and distribution of supplies directly to Puerto Rico and the Virgin Islands. Two groups affiliated with the University of Missouri at Columbia joined forces to help: Global First Responder or GFR, a nonprofit, secular international medical relief organization founded in 2011 by Adam Beckett, MD, and the International Center for Psychosocial Trauma, or ICPT, a group established in 1995 by Syed Arshad Husain, MD, to help war-traumatized children in Bosnia. I joined Dr. Husain’s group of professionals – Kathryn Dewein, PhD; Andra Ferguson, PhD; and Cathy Grigg, PsyD, – all of whom have traveled broadly in the field of disaster psychiatry – to see how we could help the people of Puerto Rico in Maria’s aftermath.
What we did
ICPT and GFR were a combined team, but we served different functions. As part of ICPT, I focused on the mental health component and helped to train doctors, psychologists, social workers, and other mental health workers in both San Juan and Ponce. All told, we worked with about 50 people using the model of “Training the Trainers.” Many of our students were participants in the outreach teams. Our hope is that they will be able to train their peers to recognize and alleviate symptoms of acute and chronic stress disorders. Some of the techniques taught include patient education, relaxation training, breath work, visualization techniques, mindfulness training, narrative therapy, art therapy, and other expressive techniques.
What the PMSF did
Before Maria, the Ponce Medical School Foundation was in the process of facilitating the transfer of medical records into an electronic format. After the hurricane hit, however, PMSF’s program director, Antonio Fernandez, led a shift to disaster recovery work. PMSF got involved in airlifting dialysis patients off the island to safety, provided health care, and also collaborated with the Primary Care Psychology Program at Ponce Health Services University to assist in locating patients, identifying their health needs – including mental health – and providing for those needs to the extent possible.
At the time of our visit, Puerto Rico’s network of more than 90 largely rural federally funded primary care clinics mostly had reopened, but nearly half remained on back-up generators. Even with the medical centers open, patients were not coming in for one reason or another. People had medical problems, but the daily reality of survival, obtaining food and water, took precedence. Some patients were not showing up because they had left the country, or they were in shelters without transportation. Some people did not have fuel. Some could not keep track of their appointments without cell phones and electricity allowing them to access electronic planners. Some, having been without their medications since the storms, were too sick to travel. Outreach teams were necessary to locate patients, identify their needs, and provide medical and psychological care.
Community outreach
Nydia M. Cappas, PsyD, director of the Primary Care Psychology Program, told us that the outreach teams – consisting of doctors or other medical professionals, social workers, and psychologists, were being sent out to communities once a week. They visited homes for the elderly, orphans’ homes, and children in foster care, as well as individual patients. A similar service was provided by Vargas Medicine (VARMED) in the San Juan area.
Team members found that many people were suffering symptoms of posttraumatic stress disorder, even people who did not have prior psychiatric symptoms. They were having flashbacks and nightmares. Those flashbacks and nightmares were being triggered by clouds, by rain, by supplies beginning to run out.
Another trend we observed is that terrain changes prompted by Maria triggered PTSD symptoms among many veterans. The defoliated trees and brown earth were causing them to have flashbacks to the deserts of Afghanistan and Iraq. Children were showing regressive behaviors, loss of developmental milestones, and symptoms of separation anxiety such as wanting to sleep with their parents. In severe cases, they were having psychotic symptoms and auditory hallucinations. The children were grieving the loss of their homes, toys, pets, and family members, in some cases. , including medications, food, toiletries, and other household goods.
Puerto Rico’s future
Two and a half months after Maria, we learned from our students that things gradually had begun to improve. For example, the public schools had just reopened, and that change was expected to have a stabilizing effect on the children. We also learned that, of the 80 shelters that had been set up housing about 12,500 people, 40 shelters had closed. The five medical shelters that had been set up and funded by FEMA also were in the process of closing, and private donations were beginning to slow down. People were slowly returning to their tarped or otherwise repaired homes, albeit all too often without power.
During the storm, nearly 500,000* homes were destroyed. FEMA offered to airlift about 3,000 people who had no home 2 months past Maria to the U.S. mainland – either Florida or New York.
According to our students, people living in the mountains, mainly coffee growers and retired people and comprising about one-third of the population, remain in acute crisis. Part of the challenge is being able to reach this population: Some roads are still impassable, and supplies – such as drinking water – can be delivered only by helicopter. Despite current conditions, FEMA reportedly has announced that it would end emergency operations on the island.
Our team is currently involved in applying for grant funding that will enable us to return to provide additional training to physicians’ and teachers’ groups. Over the course of the next year, we would like to make six trips to Puerto Rico and focus each trip on a different region and different group of professionals so that the entire island has resources. In addition, we will offer follow-up consultations to professionals we trained previously. The regions to be trained would be San Juan, Ponce, Utuado, Mayagüez, Guayama, and a sixth to be determined upon need. We also would like to address the needs of any ongoing relief workers so that they will be more effective in their ongoing role. Meanwhile, financial assistance from the mainland remains uneven.
Many months after Maria (and Irma), the physical and mental health needs of the Puerto Rican people remain great. However, as mental health professionals, we have the tools to help them move forward.
Judith R. Milner, MD, MEd, SpecEd, is a general, child, and adolescent psychiatrist in private practice in Everett, Wash. She has traveled with various groups over the years in an effort to alleviate some of the suffering caused by war and natural disaster. Her predominant association has been with the International Center for Psychosocial Trauma. She also has worked with Step Up Rwanda Women and Pygmy Survival Alliance, as well as on the Committee for Women at the American Psychiatric Association and the Consumer Issues Committee and Membership Committee for the American Academy of Child and Adolescent Psychiatry.
*Correction, 2/12/2018: An earlier version of this story misstated the number of homes reportedly destroyed by Hurricane Maria.
VIDEO: Cystic fibrosis patients need earlier, more frequent colorectal cancer screening
Adults with cystic fibrosis (CF) should undergo screening colonoscopy for colorectal cancer every 5 years beginning at age 40 years, unless they have had a solid organ transplant – in which case, screening should begin at age 30 years. For both groups, screening intervals should be shortened to 3 years if any adenomatous polyps are recovered.
The new screening recommendation is 1 of 10 set forth by the Cystic Fibrosis Foundation, in conjunction with the American Gastroenterological Association. The document reflects the significantly increased risk of colorectal cancer among adults with the chronic lung disorder, Denis Hadjiliadis, MD, and his colleagues wrote in the February issue of Gastroenterology. ; the risk approaches a 30-fold increase among CF patients who have undergone a lung transplant.
SOURCE: American Gastroenterological Association
In addition to making recommendations on screening intervals and protocols, the document asks clinicians to reframe their thinking of CF as a respiratory-only disease.
“Physicians should recognize that CF is a colon cancer syndrome,” wrote Dr. Hadjiliadis, director of the Adult Cystic Fibrosis Program at the University of Pennsylvania, Philadelphia, and his coauthors.
The increased colorectal cancer risk has become increasingly evident as CF patients live longer, Dr. Hadjiliadis and the panel wrote.
“The current median predicted survival is 41 years, and persons born in 2015 have an estimated average life expectancy of 45 years. The increasing longevity of adults with CF puts them at risk for other diseases, such as gastrointestinal cancer.”
In addition to the normal age-related risk, however, CF patients seem to have an elevated risk profile unique to the disease. The underlying causes have not been fully elucidated but may have to do with mutations in the cystic fibrosis transmembrane conductance regulator (CFTR), which are responsible for the excess thickened mucosal secretions that characterize CF. CFTR also is a tumor-suppressor gene in the intestinal tract of mice, and is important in gastrointestinal epithelial homeostasis. “Absence of CFTR is associated with dysregulation of the immune response, intestinal stem cells, and growth signaling regulators,” the authors noted.
In response to this observed increased risk of colorectal cancers among CF patients, the Cystic Fibrosis Foundation convened an 18-member task force to review the extant literature and compile colorectal cancer screening recommendations for CF patients who show no signs of such malignancies. The team reviewed 1,159 articles and based its findings on the 50 most relevant. The papers comprised observational studies, case-control studies, and case reports; there are no randomized clinical trials of screening for this population.
The American Gastroenterological Association reviewed and approved all of the recommendations:
- Screening decisions should be a collaborative process between the CF patient and clinician, taking into account comorbidities, safety, and quality of life. This should include a discussion of expected lifespan; patients with limited lifespan won’t benefit from screening for a slow-growing cancer. Patients should also consider that the colonoscopy prep for CF patients is somewhat more complex than for non-CF patients. “Given these complexities, the task force agreed that individuals with CF and their providers should … carefully assess the risks and benefits of CRC screening and its impact on the health and quality of life for the adult with CF.”
- The decision team should include an endoscopist. An endoscopist with CF training is preferred, but the panel noted these specialists are rare.
- Colonoscopy is the preferred method of screening for CF patients, since it can both detect and remove polyps. “This is one of the main reasons why colonoscopy is the screening procedure of choice for other high-risk groups,” the panel noted.
- There is insufficient evidence to recommend alternate screening methods in CF patients, including CT scanning, colonography, stool-based tests, or flexible sigmoidoscopy.
- In CF patients without signs of CRC, screening should commence at age 40 years and be repeated every 5 years as long as the results are negative.
- Any CF patient who has had adenomatous polyps on a screening colonoscopy should have a repeat colonoscopy within 3 years, unless clinical findings support more frequent screening.
- For any adult CF patient older than age 30 years who has undergone a solid organ transplant, screening colonoscopy should commence within 2 years of transplantation. “Although the absolute risk of CRC in individuals with CF is extremely low for patients younger than 30 years, the risk … greatly increases after lung transplantation,” to 25-30 times the age-adjusted baseline, the panel wrote. “Increased posttransplantation survival means that many transplant patients will enter older age groups where there is an increased risk of cancer.” Screening should be performed after recovery and within 2 years, unless there was a negative colonoscopy in the 5 years before transplant.
- Thereafter, patients who have had a solid organ transplant should undergo colonoscopy every 5 years, based on their life expectancy. “In cases where the expected survival time is limited (less than 10 years), screening should not be performed. For adults appropriately selected, lung transplantation usually increases survival probability. Therefore, a lung transplantation candidate with a short life expectancy is likely to become a screening candidate before and after transplantation at the appropriate ages described here, because the potential survival increases to approximately 10 years.”
- Colonoscopy should be repeated every 3 years on CF patients with transplants with a history of adenomatous polyps. This interval may be as short as 1 year for patients with high-risk, large, or multiple polyps.
- CF patients should undergo more intense bowel prep for colonoscopy, with three-four washes of a minimum of one liter of purgative per wash; the last wash should occur 4-6 hours before the procedure. Split-prep regimens (several smaller-volume washes) are better than a single larger-volume wash. The panel suggested a sample CF-specific regimen available from the Minnesota Cystic Fibrosis Center.
The new document reflects expert consensus on the currently available data, the panel said. As more data emerge, the recommendations might change.
“It is possible that different subpopulations will need more or less frequent schedules for rescreening and surveillance. Our recommendations are making an effort to balance the risk of missing advanced colorectal cancer and minimizing the burden and risk of too frequent examinations.”
None of the panel members had any financial disclosures.
SOURCE: Hadjiliadis D et al. Gastroenterology. 2017 Dec 28. doi. org/10.1053/j.gastro.2017.12.012
According to the Cystic Fibrosis Foundation Patient Registry, more than 30,000 people are living with cystic fibrosis (CF ) in the United States. More than half of the CF population is over 18 years of age! It is extremely important to talk to patients about preventative medicine which was not a topic of conversation CF healthcare providers were adding to their management plan in the past.
According to the Cystic Fibrosis Foundation Patient Registry, more than 30,000 people are living with cystic fibrosis (CF ) in the United States. More than half of the CF population is over 18 years of age! It is extremely important to talk to patients about preventative medicine which was not a topic of conversation CF healthcare providers were adding to their management plan in the past.
According to the Cystic Fibrosis Foundation Patient Registry, more than 30,000 people are living with cystic fibrosis (CF ) in the United States. More than half of the CF population is over 18 years of age! It is extremely important to talk to patients about preventative medicine which was not a topic of conversation CF healthcare providers were adding to their management plan in the past.
Adults with cystic fibrosis (CF) should undergo screening colonoscopy for colorectal cancer every 5 years beginning at age 40 years, unless they have had a solid organ transplant – in which case, screening should begin at age 30 years. For both groups, screening intervals should be shortened to 3 years if any adenomatous polyps are recovered.
The new screening recommendation is 1 of 10 set forth by the Cystic Fibrosis Foundation, in conjunction with the American Gastroenterological Association. The document reflects the significantly increased risk of colorectal cancer among adults with the chronic lung disorder, Denis Hadjiliadis, MD, and his colleagues wrote in the February issue of Gastroenterology. ; the risk approaches a 30-fold increase among CF patients who have undergone a lung transplant.
SOURCE: American Gastroenterological Association
In addition to making recommendations on screening intervals and protocols, the document asks clinicians to reframe their thinking of CF as a respiratory-only disease.
“Physicians should recognize that CF is a colon cancer syndrome,” wrote Dr. Hadjiliadis, director of the Adult Cystic Fibrosis Program at the University of Pennsylvania, Philadelphia, and his coauthors.
The increased colorectal cancer risk has become increasingly evident as CF patients live longer, Dr. Hadjiliadis and the panel wrote.
“The current median predicted survival is 41 years, and persons born in 2015 have an estimated average life expectancy of 45 years. The increasing longevity of adults with CF puts them at risk for other diseases, such as gastrointestinal cancer.”
In addition to the normal age-related risk, however, CF patients seem to have an elevated risk profile unique to the disease. The underlying causes have not been fully elucidated but may have to do with mutations in the cystic fibrosis transmembrane conductance regulator (CFTR), which are responsible for the excess thickened mucosal secretions that characterize CF. CFTR also is a tumor-suppressor gene in the intestinal tract of mice, and is important in gastrointestinal epithelial homeostasis. “Absence of CFTR is associated with dysregulation of the immune response, intestinal stem cells, and growth signaling regulators,” the authors noted.
In response to this observed increased risk of colorectal cancers among CF patients, the Cystic Fibrosis Foundation convened an 18-member task force to review the extant literature and compile colorectal cancer screening recommendations for CF patients who show no signs of such malignancies. The team reviewed 1,159 articles and based its findings on the 50 most relevant. The papers comprised observational studies, case-control studies, and case reports; there are no randomized clinical trials of screening for this population.
The American Gastroenterological Association reviewed and approved all of the recommendations:
- Screening decisions should be a collaborative process between the CF patient and clinician, taking into account comorbidities, safety, and quality of life. This should include a discussion of expected lifespan; patients with limited lifespan won’t benefit from screening for a slow-growing cancer. Patients should also consider that the colonoscopy prep for CF patients is somewhat more complex than for non-CF patients. “Given these complexities, the task force agreed that individuals with CF and their providers should … carefully assess the risks and benefits of CRC screening and its impact on the health and quality of life for the adult with CF.”
- The decision team should include an endoscopist. An endoscopist with CF training is preferred, but the panel noted these specialists are rare.
- Colonoscopy is the preferred method of screening for CF patients, since it can both detect and remove polyps. “This is one of the main reasons why colonoscopy is the screening procedure of choice for other high-risk groups,” the panel noted.
- There is insufficient evidence to recommend alternate screening methods in CF patients, including CT scanning, colonography, stool-based tests, or flexible sigmoidoscopy.
- In CF patients without signs of CRC, screening should commence at age 40 years and be repeated every 5 years as long as the results are negative.
- Any CF patient who has had adenomatous polyps on a screening colonoscopy should have a repeat colonoscopy within 3 years, unless clinical findings support more frequent screening.
- For any adult CF patient older than age 30 years who has undergone a solid organ transplant, screening colonoscopy should commence within 2 years of transplantation. “Although the absolute risk of CRC in individuals with CF is extremely low for patients younger than 30 years, the risk … greatly increases after lung transplantation,” to 25-30 times the age-adjusted baseline, the panel wrote. “Increased posttransplantation survival means that many transplant patients will enter older age groups where there is an increased risk of cancer.” Screening should be performed after recovery and within 2 years, unless there was a negative colonoscopy in the 5 years before transplant.
- Thereafter, patients who have had a solid organ transplant should undergo colonoscopy every 5 years, based on their life expectancy. “In cases where the expected survival time is limited (less than 10 years), screening should not be performed. For adults appropriately selected, lung transplantation usually increases survival probability. Therefore, a lung transplantation candidate with a short life expectancy is likely to become a screening candidate before and after transplantation at the appropriate ages described here, because the potential survival increases to approximately 10 years.”
- Colonoscopy should be repeated every 3 years on CF patients with transplants with a history of adenomatous polyps. This interval may be as short as 1 year for patients with high-risk, large, or multiple polyps.
- CF patients should undergo more intense bowel prep for colonoscopy, with three-four washes of a minimum of one liter of purgative per wash; the last wash should occur 4-6 hours before the procedure. Split-prep regimens (several smaller-volume washes) are better than a single larger-volume wash. The panel suggested a sample CF-specific regimen available from the Minnesota Cystic Fibrosis Center.
The new document reflects expert consensus on the currently available data, the panel said. As more data emerge, the recommendations might change.
“It is possible that different subpopulations will need more or less frequent schedules for rescreening and surveillance. Our recommendations are making an effort to balance the risk of missing advanced colorectal cancer and minimizing the burden and risk of too frequent examinations.”
None of the panel members had any financial disclosures.
SOURCE: Hadjiliadis D et al. Gastroenterology. 2017 Dec 28. doi. org/10.1053/j.gastro.2017.12.012
Adults with cystic fibrosis (CF) should undergo screening colonoscopy for colorectal cancer every 5 years beginning at age 40 years, unless they have had a solid organ transplant – in which case, screening should begin at age 30 years. For both groups, screening intervals should be shortened to 3 years if any adenomatous polyps are recovered.
The new screening recommendation is 1 of 10 set forth by the Cystic Fibrosis Foundation, in conjunction with the American Gastroenterological Association. The document reflects the significantly increased risk of colorectal cancer among adults with the chronic lung disorder, Denis Hadjiliadis, MD, and his colleagues wrote in the February issue of Gastroenterology. ; the risk approaches a 30-fold increase among CF patients who have undergone a lung transplant.
SOURCE: American Gastroenterological Association
In addition to making recommendations on screening intervals and protocols, the document asks clinicians to reframe their thinking of CF as a respiratory-only disease.
“Physicians should recognize that CF is a colon cancer syndrome,” wrote Dr. Hadjiliadis, director of the Adult Cystic Fibrosis Program at the University of Pennsylvania, Philadelphia, and his coauthors.
The increased colorectal cancer risk has become increasingly evident as CF patients live longer, Dr. Hadjiliadis and the panel wrote.
“The current median predicted survival is 41 years, and persons born in 2015 have an estimated average life expectancy of 45 years. The increasing longevity of adults with CF puts them at risk for other diseases, such as gastrointestinal cancer.”
In addition to the normal age-related risk, however, CF patients seem to have an elevated risk profile unique to the disease. The underlying causes have not been fully elucidated but may have to do with mutations in the cystic fibrosis transmembrane conductance regulator (CFTR), which are responsible for the excess thickened mucosal secretions that characterize CF. CFTR also is a tumor-suppressor gene in the intestinal tract of mice, and is important in gastrointestinal epithelial homeostasis. “Absence of CFTR is associated with dysregulation of the immune response, intestinal stem cells, and growth signaling regulators,” the authors noted.
In response to this observed increased risk of colorectal cancers among CF patients, the Cystic Fibrosis Foundation convened an 18-member task force to review the extant literature and compile colorectal cancer screening recommendations for CF patients who show no signs of such malignancies. The team reviewed 1,159 articles and based its findings on the 50 most relevant. The papers comprised observational studies, case-control studies, and case reports; there are no randomized clinical trials of screening for this population.
The American Gastroenterological Association reviewed and approved all of the recommendations:
- Screening decisions should be a collaborative process between the CF patient and clinician, taking into account comorbidities, safety, and quality of life. This should include a discussion of expected lifespan; patients with limited lifespan won’t benefit from screening for a slow-growing cancer. Patients should also consider that the colonoscopy prep for CF patients is somewhat more complex than for non-CF patients. “Given these complexities, the task force agreed that individuals with CF and their providers should … carefully assess the risks and benefits of CRC screening and its impact on the health and quality of life for the adult with CF.”
- The decision team should include an endoscopist. An endoscopist with CF training is preferred, but the panel noted these specialists are rare.
- Colonoscopy is the preferred method of screening for CF patients, since it can both detect and remove polyps. “This is one of the main reasons why colonoscopy is the screening procedure of choice for other high-risk groups,” the panel noted.
- There is insufficient evidence to recommend alternate screening methods in CF patients, including CT scanning, colonography, stool-based tests, or flexible sigmoidoscopy.
- In CF patients without signs of CRC, screening should commence at age 40 years and be repeated every 5 years as long as the results are negative.
- Any CF patient who has had adenomatous polyps on a screening colonoscopy should have a repeat colonoscopy within 3 years, unless clinical findings support more frequent screening.
- For any adult CF patient older than age 30 years who has undergone a solid organ transplant, screening colonoscopy should commence within 2 years of transplantation. “Although the absolute risk of CRC in individuals with CF is extremely low for patients younger than 30 years, the risk … greatly increases after lung transplantation,” to 25-30 times the age-adjusted baseline, the panel wrote. “Increased posttransplantation survival means that many transplant patients will enter older age groups where there is an increased risk of cancer.” Screening should be performed after recovery and within 2 years, unless there was a negative colonoscopy in the 5 years before transplant.
- Thereafter, patients who have had a solid organ transplant should undergo colonoscopy every 5 years, based on their life expectancy. “In cases where the expected survival time is limited (less than 10 years), screening should not be performed. For adults appropriately selected, lung transplantation usually increases survival probability. Therefore, a lung transplantation candidate with a short life expectancy is likely to become a screening candidate before and after transplantation at the appropriate ages described here, because the potential survival increases to approximately 10 years.”
- Colonoscopy should be repeated every 3 years on CF patients with transplants with a history of adenomatous polyps. This interval may be as short as 1 year for patients with high-risk, large, or multiple polyps.
- CF patients should undergo more intense bowel prep for colonoscopy, with three-four washes of a minimum of one liter of purgative per wash; the last wash should occur 4-6 hours before the procedure. Split-prep regimens (several smaller-volume washes) are better than a single larger-volume wash. The panel suggested a sample CF-specific regimen available from the Minnesota Cystic Fibrosis Center.
The new document reflects expert consensus on the currently available data, the panel said. As more data emerge, the recommendations might change.
“It is possible that different subpopulations will need more or less frequent schedules for rescreening and surveillance. Our recommendations are making an effort to balance the risk of missing advanced colorectal cancer and minimizing the burden and risk of too frequent examinations.”
None of the panel members had any financial disclosures.
SOURCE: Hadjiliadis D et al. Gastroenterology. 2017 Dec 28. doi. org/10.1053/j.gastro.2017.12.012
FROM GASTROENTEROLOGY
VIDEO: Gluten-free diet tied to heavy metal bioaccumulation
A gluten-free diet was associated with significantly increased blood levels of mercury, lead, and cadmium and with significantly increased urinary levels of arsenic in a large cross-sectional population-based survey study.
Source: American Gastroenterological Association
After researchers controlled for demographic characteristics, “levels of all heavy metals remained significantly higher in persons following a gluten-free diet, compared with those not following a gluten-free diet,” Stephanie L. Raehsler, MPH, of Mayo Clinic in Rochester, Minn., wrote with her associates in an article published in the February issue of Clinical Gastroenterology and Hepatology.
The purported (unproven) benefits of a gluten-free diet (GFD) have propelled them into the mainstream outside the settings of celiac disease, dermatitis herpetiformis, and wheat allergy. However, GFDs have been linked to nutritional deficits of iron, ferritin, zinc, and fiber, to increased consumption of sugar, fats, and salt, and to excessive bioaccumulation of mercury, the investigators noted.
High intake of rice, a staple of many GFDs, also has been associated with elevated urinary excretion of arsenic (PLoS One. 2014 Sep 8;9[9]:e104768. doi: 10.1371/journal.pone.0104768). To further characterize these relationships, the researchers analyzed data for 2009 through 2012 from 11,354 participants in the National Health and Nutrition Examination Survey (NHANES). Blood levels of lead, mercury, and cadmium were available from 115 participants who reported following a GFD, and data on urinary arsenic levels were available from 32 such individuals.
In the overall study group, blood mercury levels averaged 1.37 mcg/L (95% confidence interval, 1.02-1.85 mcg/L) among persons on a GFD and 0.93 mcg/L (95% CI, 0.86-1.0 mcg/L) in persons not on a GFD (P = .008). Individuals on a GFD also had significantly higher total blood levels of lead (1.42 vs. 1.13 mcg/L; P = .007 ) and cadmium (0.42 vs. 0.34; P = .03), and they had significantly higher urinary levels of total arsenic (15.2 vs. 8.4 mcg/L; P = .003). These significant differences persisted after researchers controlled for age, sex, race, and smoking status.
Additionally, among 101 individuals on GFDs who had no laboratory or clinical indication of celiac disease, blood levels of total mercury were significantly elevated, compared with individuals not on a GFD (1.40 vs. 0.93 mcg/L; P = .02), as were blood lead concentrations (1.44 vs. 1.13 mcg/L; P = .01) and urinary arsenic levels (14.7 vs. 8.3 mcg/L; P = .01). Blood cadmium levels also were increased (0.42 vs. 0.34 mcg/L), but this difference did not reach statistical significance (P = .06).
Individuals who reported eating fish or shellfish in the past month had higher blood mercury levels than those who did not, regardless of whether they were on a GFD. However, only two individuals in the study exceeded the toxicity threshold for mercury and neither was on a GFD, the researchers said. For most individuals on a GFD, levels of all heavy metals except urinary arsenic stayed under the recognized limits for toxicity, they noted.
The number of respondents following a GFD was small, but the investigators followed NHANES recommendations on sampling weights and sample design variables. Also, although the NHANES included only one question on GFDs, trained interviewers were used to help minimize bias. “Studies are needed to determine the long-term effects of accumulation of these elements in persons on a GFD,” the researchers concluded.
The Centers for Disease Control and Prevention provided partial funding. The researchers reported having no conflicts of interest.
SOURCE: Raehsler S et al. Clin Gastro Hepatol. 2018;(in press).
A gluten-free diet was associated with significantly increased blood levels of mercury, lead, and cadmium and with significantly increased urinary levels of arsenic in a large cross-sectional population-based survey study.
Source: American Gastroenterological Association
After researchers controlled for demographic characteristics, “levels of all heavy metals remained significantly higher in persons following a gluten-free diet, compared with those not following a gluten-free diet,” Stephanie L. Raehsler, MPH, of Mayo Clinic in Rochester, Minn., wrote with her associates in an article published in the February issue of Clinical Gastroenterology and Hepatology.
The purported (unproven) benefits of a gluten-free diet (GFD) have propelled them into the mainstream outside the settings of celiac disease, dermatitis herpetiformis, and wheat allergy. However, GFDs have been linked to nutritional deficits of iron, ferritin, zinc, and fiber, to increased consumption of sugar, fats, and salt, and to excessive bioaccumulation of mercury, the investigators noted.
High intake of rice, a staple of many GFDs, also has been associated with elevated urinary excretion of arsenic (PLoS One. 2014 Sep 8;9[9]:e104768. doi: 10.1371/journal.pone.0104768). To further characterize these relationships, the researchers analyzed data for 2009 through 2012 from 11,354 participants in the National Health and Nutrition Examination Survey (NHANES). Blood levels of lead, mercury, and cadmium were available from 115 participants who reported following a GFD, and data on urinary arsenic levels were available from 32 such individuals.
In the overall study group, blood mercury levels averaged 1.37 mcg/L (95% confidence interval, 1.02-1.85 mcg/L) among persons on a GFD and 0.93 mcg/L (95% CI, 0.86-1.0 mcg/L) in persons not on a GFD (P = .008). Individuals on a GFD also had significantly higher total blood levels of lead (1.42 vs. 1.13 mcg/L; P = .007 ) and cadmium (0.42 vs. 0.34; P = .03), and they had significantly higher urinary levels of total arsenic (15.2 vs. 8.4 mcg/L; P = .003). These significant differences persisted after researchers controlled for age, sex, race, and smoking status.
Additionally, among 101 individuals on GFDs who had no laboratory or clinical indication of celiac disease, blood levels of total mercury were significantly elevated, compared with individuals not on a GFD (1.40 vs. 0.93 mcg/L; P = .02), as were blood lead concentrations (1.44 vs. 1.13 mcg/L; P = .01) and urinary arsenic levels (14.7 vs. 8.3 mcg/L; P = .01). Blood cadmium levels also were increased (0.42 vs. 0.34 mcg/L), but this difference did not reach statistical significance (P = .06).
Individuals who reported eating fish or shellfish in the past month had higher blood mercury levels than those who did not, regardless of whether they were on a GFD. However, only two individuals in the study exceeded the toxicity threshold for mercury and neither was on a GFD, the researchers said. For most individuals on a GFD, levels of all heavy metals except urinary arsenic stayed under the recognized limits for toxicity, they noted.
The number of respondents following a GFD was small, but the investigators followed NHANES recommendations on sampling weights and sample design variables. Also, although the NHANES included only one question on GFDs, trained interviewers were used to help minimize bias. “Studies are needed to determine the long-term effects of accumulation of these elements in persons on a GFD,” the researchers concluded.
The Centers for Disease Control and Prevention provided partial funding. The researchers reported having no conflicts of interest.
SOURCE: Raehsler S et al. Clin Gastro Hepatol. 2018;(in press).
A gluten-free diet was associated with significantly increased blood levels of mercury, lead, and cadmium and with significantly increased urinary levels of arsenic in a large cross-sectional population-based survey study.
Source: American Gastroenterological Association
After researchers controlled for demographic characteristics, “levels of all heavy metals remained significantly higher in persons following a gluten-free diet, compared with those not following a gluten-free diet,” Stephanie L. Raehsler, MPH, of Mayo Clinic in Rochester, Minn., wrote with her associates in an article published in the February issue of Clinical Gastroenterology and Hepatology.
The purported (unproven) benefits of a gluten-free diet (GFD) have propelled them into the mainstream outside the settings of celiac disease, dermatitis herpetiformis, and wheat allergy. However, GFDs have been linked to nutritional deficits of iron, ferritin, zinc, and fiber, to increased consumption of sugar, fats, and salt, and to excessive bioaccumulation of mercury, the investigators noted.
High intake of rice, a staple of many GFDs, also has been associated with elevated urinary excretion of arsenic (PLoS One. 2014 Sep 8;9[9]:e104768. doi: 10.1371/journal.pone.0104768). To further characterize these relationships, the researchers analyzed data for 2009 through 2012 from 11,354 participants in the National Health and Nutrition Examination Survey (NHANES). Blood levels of lead, mercury, and cadmium were available from 115 participants who reported following a GFD, and data on urinary arsenic levels were available from 32 such individuals.
In the overall study group, blood mercury levels averaged 1.37 mcg/L (95% confidence interval, 1.02-1.85 mcg/L) among persons on a GFD and 0.93 mcg/L (95% CI, 0.86-1.0 mcg/L) in persons not on a GFD (P = .008). Individuals on a GFD also had significantly higher total blood levels of lead (1.42 vs. 1.13 mcg/L; P = .007 ) and cadmium (0.42 vs. 0.34; P = .03), and they had significantly higher urinary levels of total arsenic (15.2 vs. 8.4 mcg/L; P = .003). These significant differences persisted after researchers controlled for age, sex, race, and smoking status.
Additionally, among 101 individuals on GFDs who had no laboratory or clinical indication of celiac disease, blood levels of total mercury were significantly elevated, compared with individuals not on a GFD (1.40 vs. 0.93 mcg/L; P = .02), as were blood lead concentrations (1.44 vs. 1.13 mcg/L; P = .01) and urinary arsenic levels (14.7 vs. 8.3 mcg/L; P = .01). Blood cadmium levels also were increased (0.42 vs. 0.34 mcg/L), but this difference did not reach statistical significance (P = .06).
Individuals who reported eating fish or shellfish in the past month had higher blood mercury levels than those who did not, regardless of whether they were on a GFD. However, only two individuals in the study exceeded the toxicity threshold for mercury and neither was on a GFD, the researchers said. For most individuals on a GFD, levels of all heavy metals except urinary arsenic stayed under the recognized limits for toxicity, they noted.
The number of respondents following a GFD was small, but the investigators followed NHANES recommendations on sampling weights and sample design variables. Also, although the NHANES included only one question on GFDs, trained interviewers were used to help minimize bias. “Studies are needed to determine the long-term effects of accumulation of these elements in persons on a GFD,” the researchers concluded.
The Centers for Disease Control and Prevention provided partial funding. The researchers reported having no conflicts of interest.
SOURCE: Raehsler S et al. Clin Gastro Hepatol. 2018;(in press).
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: A gluten-free diet was associated with significantly increased bioaccumulation of several heavy metals.
Major finding: After accounting for demographic factors, blood or urinary levels of lead, cadmium, arsenic, and mercury were significantly higher in persons following a gluten-free diet, compared with those who did not follow a gluten-free diet.
Data source: A population-based, cross-sectional study of 11,354 respondents to NHANES 2009-2012, including 115 persons on a gluten-free diet.
Disclosures: The Centers for Disease Control and Prevention provided partial funding. The researchers reported having no conflicts of interest.
Source: Raehsler S et al. Clin Gastro Hepatol. 2018 (in press).
Using Intraindividual Variability to Evaluate Pediatric Epilepsy
The ability of a patient to focus over time is often impaired among children with epilepsy, and intraindividual variability may be an important way to measure such attentional problems according to a study published in Epilepsy & Behavior.
- Intraindividual variability, a measure of changes in an individual’s transient behavioral performance, has been identified in both pediatric and adult patients with epilepsy.
- Srnka et al evaluated intraindividual variability in 144 patients who had just been diagnosed with epilepsy, using the Connors Continuous Performance Task-II as a metric.
- The children with epilepsy were between the ages of 8 and 18 years and were compared to 82 healthy children.
- The researchers found a large difference in variability between the two groups, with an effect size difference of 0.68.
- They also discovered that intraindividual variability predicted intellectual functioning and academic achievement.
Srnka K, Seidenberg M, Hermann B, Jones J. Intraindividual variability in attentional vigilance in children with epilepsy. Epilepsy Behav. 2018;79:42-45.
The ability of a patient to focus over time is often impaired among children with epilepsy, and intraindividual variability may be an important way to measure such attentional problems according to a study published in Epilepsy & Behavior.
- Intraindividual variability, a measure of changes in an individual’s transient behavioral performance, has been identified in both pediatric and adult patients with epilepsy.
- Srnka et al evaluated intraindividual variability in 144 patients who had just been diagnosed with epilepsy, using the Connors Continuous Performance Task-II as a metric.
- The children with epilepsy were between the ages of 8 and 18 years and were compared to 82 healthy children.
- The researchers found a large difference in variability between the two groups, with an effect size difference of 0.68.
- They also discovered that intraindividual variability predicted intellectual functioning and academic achievement.
Srnka K, Seidenberg M, Hermann B, Jones J. Intraindividual variability in attentional vigilance in children with epilepsy. Epilepsy Behav. 2018;79:42-45.
The ability of a patient to focus over time is often impaired among children with epilepsy, and intraindividual variability may be an important way to measure such attentional problems according to a study published in Epilepsy & Behavior.
- Intraindividual variability, a measure of changes in an individual’s transient behavioral performance, has been identified in both pediatric and adult patients with epilepsy.
- Srnka et al evaluated intraindividual variability in 144 patients who had just been diagnosed with epilepsy, using the Connors Continuous Performance Task-II as a metric.
- The children with epilepsy were between the ages of 8 and 18 years and were compared to 82 healthy children.
- The researchers found a large difference in variability between the two groups, with an effect size difference of 0.68.
- They also discovered that intraindividual variability predicted intellectual functioning and academic achievement.
Srnka K, Seidenberg M, Hermann B, Jones J. Intraindividual variability in attentional vigilance in children with epilepsy. Epilepsy Behav. 2018;79:42-45.
Finding a Presurgical Role for Magnetoencephalography
Magnetoencephalography (MEG) may play an important role in the presurgical workup of patients with nonlesional refractory focal epilepsy suggests a recent observational study.
- Investigators observed 31 patients at an academic epilepsy center to determine if MEG would have had an impact on patient care; (they were unable to analyze the MEG early enough to influence the decision-making process).
- Had the test been integrated into the presurgical workup, 68% of patients would have received different management initially.
- MEG would have reduced the number of patients who received intracranial electrodes.
- MEG would also have led to the position of the electrodes being changed or provided adequate evidence to justify the use of an intracranial electrode.
- The results of the MEG studies would have let surgeons do direct surgery with no need for intracranial electrodes in 2 of 11 patients.
- 6 patients fared poorly after surgery, but MEG would have changed their outcomes in 3 of these patients by modifying the resection margin.
Mohamed IS, Bout hillier A, Bérubé A, et al. The clinical impact of integration of magnetoencephalography in the presurgical workup for refractory nonlesional epilepsy. Epilepsy Behav. 2018;79:34-41.
Magnetoencephalography (MEG) may play an important role in the presurgical workup of patients with nonlesional refractory focal epilepsy suggests a recent observational study.
- Investigators observed 31 patients at an academic epilepsy center to determine if MEG would have had an impact on patient care; (they were unable to analyze the MEG early enough to influence the decision-making process).
- Had the test been integrated into the presurgical workup, 68% of patients would have received different management initially.
- MEG would have reduced the number of patients who received intracranial electrodes.
- MEG would also have led to the position of the electrodes being changed or provided adequate evidence to justify the use of an intracranial electrode.
- The results of the MEG studies would have let surgeons do direct surgery with no need for intracranial electrodes in 2 of 11 patients.
- 6 patients fared poorly after surgery, but MEG would have changed their outcomes in 3 of these patients by modifying the resection margin.
Mohamed IS, Bout hillier A, Bérubé A, et al. The clinical impact of integration of magnetoencephalography in the presurgical workup for refractory nonlesional epilepsy. Epilepsy Behav. 2018;79:34-41.
Magnetoencephalography (MEG) may play an important role in the presurgical workup of patients with nonlesional refractory focal epilepsy suggests a recent observational study.
- Investigators observed 31 patients at an academic epilepsy center to determine if MEG would have had an impact on patient care; (they were unable to analyze the MEG early enough to influence the decision-making process).
- Had the test been integrated into the presurgical workup, 68% of patients would have received different management initially.
- MEG would have reduced the number of patients who received intracranial electrodes.
- MEG would also have led to the position of the electrodes being changed or provided adequate evidence to justify the use of an intracranial electrode.
- The results of the MEG studies would have let surgeons do direct surgery with no need for intracranial electrodes in 2 of 11 patients.
- 6 patients fared poorly after surgery, but MEG would have changed their outcomes in 3 of these patients by modifying the resection margin.
Mohamed IS, Bout hillier A, Bérubé A, et al. The clinical impact of integration of magnetoencephalography in the presurgical workup for refractory nonlesional epilepsy. Epilepsy Behav. 2018;79:34-41.
Comparing PreOp High-Gamma Modulation With Electrical Stimulation
Electrocorticographic (ECoG) high-γ modulation (HGM) can serve as a specific way to localize language preoperatively, when compared with electrical stimulation mapping (ESM), which is considered the gold standard, according to a recent meta-analysis. But the same analysis concluded that it was not sensitive enough when compared with ESM.
- The meta-analysis reviewed several metrics for diagnostic validity, including area under the summary receiver operating characteristic (SROC) curve, diagnostic odds ratio, and pooled estimates of sensitivity and specificity.
- To determine language mapping, the most common task used was overt picture naming.
- ECoG was analyzed at 50 to 400 Hz, with different studies using different bandwidths.
- Among the studies that looked at ESM, there were wide variations in pulse duration, train duration, and maximum current.
- The pooled diagnostic odds ratio was 6.44 and the AUC was 0.77, making HGM a fairly reliable way to ascertain electrodes overlying ESM cortical language sites.
Aryaa R, Horn PS, Crone NE, et al. ECoG high-gamma modulation versus electrical stimulation for presurgical language mapping. Epilepsy Behav. 2018;79:26-33.
Electrocorticographic (ECoG) high-γ modulation (HGM) can serve as a specific way to localize language preoperatively, when compared with electrical stimulation mapping (ESM), which is considered the gold standard, according to a recent meta-analysis. But the same analysis concluded that it was not sensitive enough when compared with ESM.
- The meta-analysis reviewed several metrics for diagnostic validity, including area under the summary receiver operating characteristic (SROC) curve, diagnostic odds ratio, and pooled estimates of sensitivity and specificity.
- To determine language mapping, the most common task used was overt picture naming.
- ECoG was analyzed at 50 to 400 Hz, with different studies using different bandwidths.
- Among the studies that looked at ESM, there were wide variations in pulse duration, train duration, and maximum current.
- The pooled diagnostic odds ratio was 6.44 and the AUC was 0.77, making HGM a fairly reliable way to ascertain electrodes overlying ESM cortical language sites.
Aryaa R, Horn PS, Crone NE, et al. ECoG high-gamma modulation versus electrical stimulation for presurgical language mapping. Epilepsy Behav. 2018;79:26-33.
Electrocorticographic (ECoG) high-γ modulation (HGM) can serve as a specific way to localize language preoperatively, when compared with electrical stimulation mapping (ESM), which is considered the gold standard, according to a recent meta-analysis. But the same analysis concluded that it was not sensitive enough when compared with ESM.
- The meta-analysis reviewed several metrics for diagnostic validity, including area under the summary receiver operating characteristic (SROC) curve, diagnostic odds ratio, and pooled estimates of sensitivity and specificity.
- To determine language mapping, the most common task used was overt picture naming.
- ECoG was analyzed at 50 to 400 Hz, with different studies using different bandwidths.
- Among the studies that looked at ESM, there were wide variations in pulse duration, train duration, and maximum current.
- The pooled diagnostic odds ratio was 6.44 and the AUC was 0.77, making HGM a fairly reliable way to ascertain electrodes overlying ESM cortical language sites.
Aryaa R, Horn PS, Crone NE, et al. ECoG high-gamma modulation versus electrical stimulation for presurgical language mapping. Epilepsy Behav. 2018;79:26-33.