User login
The times they are a-changin’ —Bob Dylan, 1964
The beginning of a new year is always associated with changes, accompanied by new challenges and opportunities. This year is no different and, in fact, begins with some significant changes. First, I am incredibly honored, and humbled, to be named your new editor-in-chief. By way of background and introduction, I am residency-trained and board-certified in emergency medicine (EM). I founded the first academic department of EM in Virginia in 1992, and continue to serve in the role of chair. From 1990 to 2010, I served as the program director of our 3-year EM residency program, which I still consider the best job in EM. Most importantly, I continue to see and care for patients in the ED primarily, in addition to supervising and teaching EM residents and fellows in the delivery of care in the clinical arena. I know first-hand the needs of practicing emergency physicians (EPs).
I feel very fortunate to have been associated with Emergency Medicine (EM) since 1988, the year the journal published my very first manuscript. I served on the editorial board from 1999 to 2006, and for the past 11 years, have served as the associate editor-in-chief. I hold a very special regard and respect for this journal, and its role in our specialty. My goal is to continue to publish high-quality content and ensure we consistently provide timely and clinically useful information to the practicing EP. We will invite the very best in our specialty to share their knowledge and clinical tips. We will of course continue some of your favorite sections, like “Emergency Ultrasound,” “Diagnosis at a Glance,” and “Case Studies in Toxicology.” We will also encourage our readers to submit interesting and informative case reports, review articles, and interesting images. While I plan to write a few editorials each year, I will invite thought leaders in EM to write on their area(s) of expertise.
Come writers and critics who prophesize with your pen.
Another major change has to do with the journal itself. This will be the last paper copy of EM (so think about keeping this one for posterity, or eBay). Starting with the February issue, all future issues will be digital and online-only. This decision was not an easy one, and has been in the making for some time. Thanks to the growth in our Web site traffic, it is clear that many of you have already become “digital-first” readers. This fact, combined with the added financial challenge of publishing a large-circulation journal within an environment of declining print advertising, convinced us that this is the right time to make the leap to the digital-only format. While some of you (including myself), will miss physically holding and reading a hard copy of EM, you may simply continue to access the journal as you have for years, on your desktop, laptop, or iPad, and never further away than your cell phone. This change has the advantage of providing opportunities to deliver valuable clinical content in new ways, through increased use of audio and video, as well as text. To ensure that you receive your copy, please e-mail our Editor, Kellie DeSantis ([email protected]) to make sure we have your correct and preferred e-mail address. While our goal is to push each issue out to you via e-mail, you will always be able to access the most recent articles by going to our Web site, www.emed-journal.com.
And don’t criticize
What you can’t understand.
Finally, 2018 promises to be a very interesting year, with the unknown implications of tax reform, the repeal of the individual mandate for health insurance, the opioid crisis, and the curious mergers within the health insurance industry (ie, CVS and Aetna). It is too soon for anyone to say how these changes will affect EM on the national stage. What will not change however, is that EPs will continue to provide outstanding care to any and every patient who presents to the ED. Emergency physicians will ensure that all patients receive the care they need (but not necessarily the care they want) and will do so without regard to gender, religion, national origin, race, age, sexual preference, or insurance status.
I wish each and every one of you a happy and healthy 2018.
The beginning of a new year is always associated with changes, accompanied by new challenges and opportunities. This year is no different and, in fact, begins with some significant changes. First, I am incredibly honored, and humbled, to be named your new editor-in-chief. By way of background and introduction, I am residency-trained and board-certified in emergency medicine (EM). I founded the first academic department of EM in Virginia in 1992, and continue to serve in the role of chair. From 1990 to 2010, I served as the program director of our 3-year EM residency program, which I still consider the best job in EM. Most importantly, I continue to see and care for patients in the ED primarily, in addition to supervising and teaching EM residents and fellows in the delivery of care in the clinical arena. I know first-hand the needs of practicing emergency physicians (EPs).
I feel very fortunate to have been associated with Emergency Medicine (EM) since 1988, the year the journal published my very first manuscript. I served on the editorial board from 1999 to 2006, and for the past 11 years, have served as the associate editor-in-chief. I hold a very special regard and respect for this journal, and its role in our specialty. My goal is to continue to publish high-quality content and ensure we consistently provide timely and clinically useful information to the practicing EP. We will invite the very best in our specialty to share their knowledge and clinical tips. We will of course continue some of your favorite sections, like “Emergency Ultrasound,” “Diagnosis at a Glance,” and “Case Studies in Toxicology.” We will also encourage our readers to submit interesting and informative case reports, review articles, and interesting images. While I plan to write a few editorials each year, I will invite thought leaders in EM to write on their area(s) of expertise.
Come writers and critics who prophesize with your pen.
Another major change has to do with the journal itself. This will be the last paper copy of EM (so think about keeping this one for posterity, or eBay). Starting with the February issue, all future issues will be digital and online-only. This decision was not an easy one, and has been in the making for some time. Thanks to the growth in our Web site traffic, it is clear that many of you have already become “digital-first” readers. This fact, combined with the added financial challenge of publishing a large-circulation journal within an environment of declining print advertising, convinced us that this is the right time to make the leap to the digital-only format. While some of you (including myself), will miss physically holding and reading a hard copy of EM, you may simply continue to access the journal as you have for years, on your desktop, laptop, or iPad, and never further away than your cell phone. This change has the advantage of providing opportunities to deliver valuable clinical content in new ways, through increased use of audio and video, as well as text. To ensure that you receive your copy, please e-mail our Editor, Kellie DeSantis ([email protected]) to make sure we have your correct and preferred e-mail address. While our goal is to push each issue out to you via e-mail, you will always be able to access the most recent articles by going to our Web site, www.emed-journal.com.
And don’t criticize
What you can’t understand.
Finally, 2018 promises to be a very interesting year, with the unknown implications of tax reform, the repeal of the individual mandate for health insurance, the opioid crisis, and the curious mergers within the health insurance industry (ie, CVS and Aetna). It is too soon for anyone to say how these changes will affect EM on the national stage. What will not change however, is that EPs will continue to provide outstanding care to any and every patient who presents to the ED. Emergency physicians will ensure that all patients receive the care they need (but not necessarily the care they want) and will do so without regard to gender, religion, national origin, race, age, sexual preference, or insurance status.
I wish each and every one of you a happy and healthy 2018.
The beginning of a new year is always associated with changes, accompanied by new challenges and opportunities. This year is no different and, in fact, begins with some significant changes. First, I am incredibly honored, and humbled, to be named your new editor-in-chief. By way of background and introduction, I am residency-trained and board-certified in emergency medicine (EM). I founded the first academic department of EM in Virginia in 1992, and continue to serve in the role of chair. From 1990 to 2010, I served as the program director of our 3-year EM residency program, which I still consider the best job in EM. Most importantly, I continue to see and care for patients in the ED primarily, in addition to supervising and teaching EM residents and fellows in the delivery of care in the clinical arena. I know first-hand the needs of practicing emergency physicians (EPs).
I feel very fortunate to have been associated with Emergency Medicine (EM) since 1988, the year the journal published my very first manuscript. I served on the editorial board from 1999 to 2006, and for the past 11 years, have served as the associate editor-in-chief. I hold a very special regard and respect for this journal, and its role in our specialty. My goal is to continue to publish high-quality content and ensure we consistently provide timely and clinically useful information to the practicing EP. We will invite the very best in our specialty to share their knowledge and clinical tips. We will of course continue some of your favorite sections, like “Emergency Ultrasound,” “Diagnosis at a Glance,” and “Case Studies in Toxicology.” We will also encourage our readers to submit interesting and informative case reports, review articles, and interesting images. While I plan to write a few editorials each year, I will invite thought leaders in EM to write on their area(s) of expertise.
Come writers and critics who prophesize with your pen.
Another major change has to do with the journal itself. This will be the last paper copy of EM (so think about keeping this one for posterity, or eBay). Starting with the February issue, all future issues will be digital and online-only. This decision was not an easy one, and has been in the making for some time. Thanks to the growth in our Web site traffic, it is clear that many of you have already become “digital-first” readers. This fact, combined with the added financial challenge of publishing a large-circulation journal within an environment of declining print advertising, convinced us that this is the right time to make the leap to the digital-only format. While some of you (including myself), will miss physically holding and reading a hard copy of EM, you may simply continue to access the journal as you have for years, on your desktop, laptop, or iPad, and never further away than your cell phone. This change has the advantage of providing opportunities to deliver valuable clinical content in new ways, through increased use of audio and video, as well as text. To ensure that you receive your copy, please e-mail our Editor, Kellie DeSantis ([email protected]) to make sure we have your correct and preferred e-mail address. While our goal is to push each issue out to you via e-mail, you will always be able to access the most recent articles by going to our Web site, www.emed-journal.com.
And don’t criticize
What you can’t understand.
Finally, 2018 promises to be a very interesting year, with the unknown implications of tax reform, the repeal of the individual mandate for health insurance, the opioid crisis, and the curious mergers within the health insurance industry (ie, CVS and Aetna). It is too soon for anyone to say how these changes will affect EM on the national stage. What will not change however, is that EPs will continue to provide outstanding care to any and every patient who presents to the ED. Emergency physicians will ensure that all patients receive the care they need (but not necessarily the care they want) and will do so without regard to gender, religion, national origin, race, age, sexual preference, or insurance status.
I wish each and every one of you a happy and healthy 2018.
Complications of Systemic Lupus Erythematosus in the Emergency Department
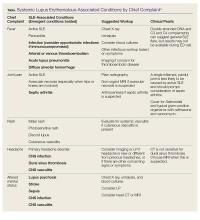
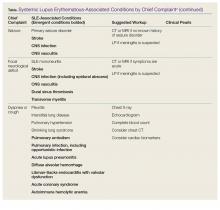
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease characterized by the chronic activation of the immune system, leading to the formation of autoantibodies and multi-organ damage. The prevalence of SLE in the United States is 20 to 150 per 100,000 persons.1 Ninety percent of patients with SLE are women, and the condition is more common and often more severe among patients of black African or of Asian descent.
For patients with known SLE who present to the ED, it can be a challenge to identify whether their symptoms are due to a minor lupus flare that can be managed as an outpatient, a presentation of urgent or emergent conditions caused by SLE, or a condition unrelated to lupus. This article reviews the most common and emergent complications of SLE by organ system to assist emergency physicians (EPs) in better diagnosing and managing this complicated disease.
General Acute-Care Management
While a patient’s presentation could be secondary to a lupus-related complication, consideration must always be given to common conditions that are not related to SLE. Biomarkers such as erythrocyte sedimentation rate, C-reactive protein, C3 and C4 complement, and double-stranded DNA levels can be helpful in assessing lupus disease activity and differentiating a lupus-related complication from an unrelated event. Comparing these biomarkers to the patient’s baseline values can be informative; however, depending on the laboratory facilities, test results may not be available during an ED visit. Lastly, infections should be considered more strongly than usual in the differential diagnosis due to the immunocompromised status of a substantial proportion of these patients, by virtue of their disease or the cytotoxic medications used for treatment.
Musculoskeletal Complications
Common Complications
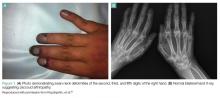
Polyarthralgias and Polymyalgias. More than 90% of SLE patients experience polyarthralgias and polymyalgias. Physical examination findings may be normal, even when joint pain is present, which is often due to mild synovitis. In some cases, Jaccoud arthropathy is seen, which presents as deformities such as swan neck deformities and ulnar deviations that are characteristically reducible on manipulation (Figures 1a and 1b). These deformities are not caused by direct joint damage, but by chronic tenosynovitis and the resulting laxity of tendons and ligaments.1 Classically, plain radiographic imaging reveals nonerosive joint changes. Muscle and joint pains may worsen with disease progression or flare.
Avascular Necrosis. Avascular necrosis affects 5% to 12% of SLE patients.2 Most commonly, this involves the femoral head, but it may also involve the femoral condyle or tibial plateau. Patients may present with acute or subacute onset of pain in the groin or buttocks when the femoral head is involved, or in the knee when the femoral condyle or tibial plateau is involved. Plain radiographs may reveal joint-space narrowing and other evidence of degenerative joint disease. Magnetic resonance imaging (MRI) is more sensitive in diagnosing avascular necrosis, and may be indicated when clinical suspicion is high despite negative plain radiographs, although this would not typically need to be performed urgently in the ED.2 While analgesics and physical therapy may provide some pain relief to patients with avascular necrosis, this condition generally requires nonemergent operative intervention.
Emergent Complications
Septic Arthritis. When a patient with SLE presents with an isolated swollen joint, septic arthritis should be suspected, and diagnosis should be confirmed by arthrocentesis. Synovial fluid samples showing a white blood cell count greater than 50 × 109/Lsuggest infection, which can be confirmed by gram stain and cultures.
For reasons that remain unclear, but may involve primary immune defects and the use of immunosuppressant medications, patients with SLE are predisposed to Salmonella joint infections. In one study, 59% of septic arthritis cases in patients with SLE were due to Salmonella species; therefore, treatment for septic arthritis in this population should include ceftriaxone in addition to vancomycin for typical organisms, such as Staphylococcus and Streptococcus species.3
Cutaneous Manifestations
Common Complications
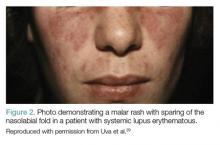
Malar Rash. Eighty percent to 90% of patients with SLE have dermatological involvement,1 the most common finding of which is the malar or butterfly facial rash, which appears as raised erythema over the bridge of the nose and cheeks while sparing the nasolabial folds (Figure 2).
Discoid Lupus. Chronic discoid lupus appears as a scarring rash often found on the face, ears, and scalp. These patients may also exhibit a photosensitive rash, which consists of an erythematous eruption if acute, or annular scaly lesions if subacute.
Oral and Nasal Ulcerations. Common mucous membrane findings include oral or nasal ulcers, which are typically painless.
Worsening of any of these skin findings may be associated with disease flare. Secondary bacterial infection of lupus rashes or ulcerations is uncommon, although cellulitis should be considered when a rash is unilateral, not in a sun-exposed area, or is otherwise different from the patient’s typical lupus rash. Sun avoidance and topical corticosteroids are the mainstays of treatment of dermatological disease in SLE.
Emergent Complications
Systemic Vasculitis. Patients with SLE are susceptible to vasculitis. Although isolated cutaneous vasculitis is not typically an emergent condition, it may portend systemic vasculitis. Any palpable purpura or other evidence of cutaneous vasculitis should prompt a careful review of systems and basic laboratory workup for systemic vasculitis, which can involve the kidneys, lungs, central or peripheral nervous system, or gastrointestinal tract.
Symptoms of systemic vasculitis may include fevers, chills, chest pain, cough, hemoptysis, abdominal pain, and changes in color or amount of urine. Laboratory workup should be tailored to symptoms, and may include basic metabolic panel, liver function tests, complete blood count, and urinalysis.4
Digital Gangrene. Patients with SLE may also develop digital gangrene related to severe Raynaud phenomenon, vasculitis, or thromboembolism. Pharmacological treatment with vasodilators such as sildenafil, endothelin receptor antagonists, or intravenous prostacyclins may be needed.5 To save the involved digit, vascular surgery services should be consulted urgently.6
Renal Complications
Common Complications
Chronic Kidney Disease. Chronic kidney disease (CKD) is common among SLE patients, especially among those with a history of lupus nephritis.7 Patients with CKD may have persistently elevated serum creatinine, chronic hypertension, and/or chronic peripheral edema. Patients presenting with new development of hypertension, peripheral edema, hematuria, or polyuria should be screened for lupus nephritis with urinalysis and serum creatinine. Elevated creatinine or new or worsening proteinuria or hematuria should prompt consultation with nephrology services.
Emergent Complications
Lupus Nephritis. About 50% of SLE patients will develop lupus nephritis during the course of their lives,1 which may present as nephrotic disease with significant proteinuria, peripheral edema, and low serum albumin, or as nephritic disease, with increased serum creatinine and hematuria. Acute kidney injury in SLE patients should generally prompt admission for workup of reversible causes and evaluation for lupus nephritis, which often includes renal biopsy.8
Neuropsychiatric Complications
Common Complications
Neuropsychiatric lupus is a broad category that includes 19 manifestations of SLE in the central and peripheral nervous systems.9 Conditions range from depression or chronic headaches to seizures or psychosis.
Mood and Anxiety Disorders. Anxiety and depression have been observed in up to 75% of SLE patients.1 Mood and anxiety disorders are likely influenced by the psychosocial elements of this chronic disease, as well as by direct effects of SLE on the brain.1
Peripheral Neuropathy. Approximately 10% of SLE patients have a peripheral neuropathy, which generally presents as a mononeuritis (either single or multiplex), rather than the stocking-glove distribution seen in other systemic causes of neuropathy.10
Headache. Headache disorders may also develop in SLE patients, and tend to have similar patterns to primary headache disorders in the general population. In most cases, treatment for headache in SLE patients is similar to that of the general population.11 However, if a patient presents with concerning findings, such as focal neurological deficit, meningismus, or fever, or if the headache is new-onset or different from previous headaches, further investigation should be considered, including a head computed tomography (CT) scan and lumbar puncture (LP).
Emergent Complications
In general, due to the variety of neurological emergencies that may present with SLE, and the subtlety with which true emergencies may present in this population, the threshold to obtain imaging on SLE patients with any new neurological complaints should be low.
Cerebrovascular Accidents. Patients with SLE are susceptible to cerebrovascular accidents (CVAs), typically from occlusive or embolic causes. Etiologies may include primary central nervous system (CNS) vasculitis, embolic disease from antiphospholipid syndrome (APS), or embolic disease from a Libman-Sacks endocarditis.12
Successful thrombolysis has been reported in SLE patients presenting with stroke, but it remains controversial due to risk of hemorrhagic conversion if CNS vasculitis, rather than embolism, is the cause.13 Proper imaging and consultation with a neurologist familiar with the disease is critical for early treatment decisions.
Seizures. Fifteen percent to 35% of SLE patients may develop seizures. These may be focal or generalized, but generalized tonic-clonic seizures tend to be more common in SLE patients.2 Workup and management of seizures in SLE patients is the same as in the general population.
Sinus Thrombosis. Dural sinus thrombosis often presents as a new-onset headache, sometimes with focal neurological deficits. The diagnosis of dural sinus thrombosis can be challenging, as CT imaging studies may be falsely negative. There should be a low threshold for obtaining MRI/magnetic resonance angiography (MRA) in SLE patients presenting with a new-onset headache.14
CNS Vasculitis. Patients with SLE are also susceptible to CNS vasculitis, which can manifest as seizures, psychosis, cognitive decline, altered mental status, or coma. Magnetic resonance imaging/MRA studies may suggest the diagnosis, but if this is equivocal, angiography or even brain biopsy may be needed to make the diagnosis. Unless the patient’s symptoms are very mild (eg, mild cognitive decline), she or he should be admitted for diagnostic workup and consideration of aggressive immunosuppressive therapy.2
Transverse Myelitis and Spinal Artery Thrombosis. Acute loss of lower limb sensation or motor function in SLE patients may be caused by transverse myelitis or spinal artery thrombosis. Epidural abscess should also be considered, especially if the patient is immunocompromised.2
Infection. A CNS infection should be considered in any SLE patient presenting with new neurological complaints. Fever or meningismus, especially in conjunction with headache or focal neurological deficits, should prompt an LP and consideration for imaging. Immunocompromised patients are at increased risk for common organisms as well as atypical organisms, such as fungus or mycobacteria.15
Pulmonary Complications
Common Complications
Pleuritis. Many patients with SLE develop pleuritis, with or without effusion. This may be treated with nonsteroidal anti-inflammatory drugs, or corticosteroids if symptoms are more severe. Pleuritis is the most common respiratory complication of SLE, but due to the number of serious cardiopulmonary complications associated with SLE, pleuritis should be a diagnosis of exclusion.
Interstitial Lung Disease. Interstitial lung disease may be caused by SLE or may be medication-induced. This commonly presents as subacute or chronic dyspnea and/or cough. Patient workup may be done on an outpatient basis with high resolution chest CT and pulmonary function testing.
Pulmonary Hypertension. Patients with SLE may develop pulmonary hypertension, either directly due to SLE or from chronic thromboembolic disease. In general, pulmonary hypertension is managed as an outpatient, but may require emergent inpatient treatment if the condition is rapidly progressive or associated with right heart failure.
Shrinking Lung Syndrome. This condition may cause subacute or chronic dyspnea and pleuritic chest pain. Shrinking lung syndrome is caused by diaphragmatic dysfunction rather than from a primary disease of the lungs, and it is characterized by a restrictive pattern on pulmonary function testing and an elevated hemidiaphragm. Shrinking lung syndrome typically responds well to immunosuppressive therapy.16
Emergent Conditions
Pulmonary Embolism. A pulmonary embolism should be strongly considered in any patient with SLE presenting with the appropri ate clinical picture. Patients with APS are at particularly high risk for thromboembolic disease. However, even SLE patients without this APS are known to be at an increased risk of developing thromboembolism compared to the general public.17 Pulmonary embolism in SLE patients should be diagnosed and treated in the usual manner.
Pneumonia. Immunosuppressed patients are susceptible to opportunistic pulmonary infections as well as typical community pathogens. Fungal or mycobacterial infections may be suspected with a more subacute onset of symptoms.
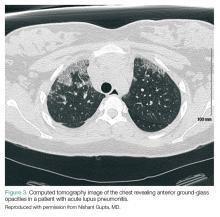
Acute Lupus Pneumonitis. This serious condition may present with severe pneumonia-like signs and symptoms, including fever, cough, dyspnea, hypoxia, and infiltrates on chest radiograph (Figure 3).
Acute lupus pneumonitis is caused by disease flare, and not by infection, although it may not be possible to distinguish it from pneumonia in the ED setting. The mortality rate of acute lupus pneumonitis is as high as 50%, and survivors often progress to chronic interstitial pneumonitis.1
Diffuse Alveolar Hemorrhage. A rare complication with a mortality rate of 50% to 90%, SLE patients who develop diffuse alveolar hemorrhage may present with fever, cough, dyspnea, and hypoxia.18 The condition may be suggested by infiltrates on chest radiograph, a drop in hemoglobin representing bleeding into the lungs, and/or hemoptysis. However, the absence of hemoptysis does not rule out diffuse alveolar hemorrhage, so clinical suspicion should remain high, even in the absence of this symptom.
Because emergent pulmonary conditions often present with similar symptoms, most patients with acute or new-onset symptoms will require admission for diagnostic workup (likely to include chest CT scan and/or bronchoscopy with bronchoalveolar lavage), as well as for close monitoring and initiation of treatment. If hypoxia or respiratory distress is severe, or if diffuse alveolar hemorrhage is suspected, admission to the intensive care unit (ICU) should be considered. We suggest that antibiotics be started in the ED when pneumonia is part of the differential diagnosis. As in the general population, coverage should be chosen based on the patient’s risk factors for antibiotic-resistant organisms. Initiation of corticosteroid therapy or other changes in immune therapy can be delayed until the EP consults with rheumatology and/or pulmonology services.
Cardiac Complications
Common Complications
Pericarditis. Pericarditis with or without pericardial effusion is very common in SLE patients and is usually related to lupus itself, rather than an infectious etiology. Patients may present with substernal, positional chest pain, tachycardia, and diffuse ST-segment elevation on electrocardiogram. Most effusions are small, asymptomatic, and discovered incidentally. However, among patients with symptomatic pericardial effusions, tamponade can be present in 21%.19 Corticosteroid therapy is often required to treat SLE-associated pericarditis, but colchicine is being explored as a possible steroid-sparing agent in this patient population.20,21
Valvular Abnormalities. Approximately 60% of SLE patients have valvular abnormalities detectable by echocardiography. The most common abnormalities in one study were valvular thickening or regurgitation.22 Many of these abnormalities occurred in asymptomatic patients and never progressed to clinical disease in a 5-year follow-up. However, patients with any valvular abnormality were more likely to develop complications, including stroke, peripheral embolism, infective endocarditis, need for valve replacement, congestive heart failure, or death.22
Emergent Complications
Acute Coronary Syndrome. Even in relatively young patients, acute coronary syndrome (ACS) should be considered in SLE patients presenting with chest pain, as this patient population has a 10-fold higher risk of developing coronary artery disease (CAD) than the general population, and SLE patients with CAD often lack traditional risk factors, such as advanced age, family history, or metabolic syndrome.1
A high clinical suspicion should be maintained even in patients who would traditionally be considered low-risk. The EP should have a low-threshold for ECG, cardiac biomarker testing, and stress testing for SLE patients presenting with chest pain. The treatment of ACS in SLE patients is the same as in the general population.
Libman-Sacks Endocarditis. A sterile, fibrinous valvular vegetation, Libman-Sacks endocarditis is unique to patients with SLE. When present, patients usually develop a subacute or chronic onset of dyspnea or chest pain. However, patients may become acutely ill if they develop severe valvular regurgitation. Additionally, the valve damage from Libman-Sacks endocarditis can predispose patients to developing infective endocarditis.20
Hematological Complications
Common Complications
Patients with SLE commonly have mild-to-moderate leukopenia (especially lymphopenia), anemia, and thrombocytopenia. This may be related to the disease process or may be secondary to prescribed medications. A comparison to recent baseline laboratory studies should be sought if there is suspicion for new or worsening cytopenia.
Antiphospholipid Syndrome. Nearly 40% of SLE patients also have APS, which is defined by a clinical history of thrombosis in conjunction with one of the antiphospholipid antibodies (anticardiolipin, anti-beta-2-glycoprotein, lupus anticoagulant). Antiphospholipid syndrome causes both venous and arterial thrombosis and may be associated with recurrent miscarriage. Acute thrombotic events should be treated with heparin or enoxaparin and transitioned to warfarin. The new generation of direct oral anticoagulants have not been well studied in APS, though, multiple small case series suggest a higher thrombotic risk with these drugs than with warfarin.23Patients who have recurrent venous thromboembolism, or who have any arterial thromboembolism should be on lifelong anticoagulation therapy.2
Emergent Complications
Thrombocytopenia. Severe thrombocytopenia or hemolytic anemia can be life-threatening, and often requires inpatient admission for immunosuppressive therapy, monitoring, and supportive care.
Catastrophic Antiphospholipid Syndrome. This condition should be suspected in patients with SLE who present with multiple sites of thrombosis or new multi-organ damage. Catastrophic APS (CAPS) may occur in SLE patients who have no prior history of APS. Since the mortality rate for CAPS approaches 50%, these patients require anticoagulation, immunosuppressant therapy (high-dose corticosteroids, cyclophosphamide, and/or plasma exchange), and admission to the ICU.24
Gastrointestinal Complications
Common Complications
Intestinal Pseudo-obstruction. Dysphagia related to esophageal dysmotility is present in up to 13% of SLE patients.25 Intestinal pseudo-obstruction may be seen in SLE patients, and is characterized by symptoms of intestinal obstruction caused by decreased intestinal motility, rather than from mechanical obstruction. Presenting symptoms may be acute or chronic, and include nausea, vomiting, and abdominal distension. Abdominal CT studies will show dilated bowel loops without evidence of mechanical obstruction. Manometry reveals widespread hypomotility. Intestinal pseudo-obstruction typically responds well to corticosteroids and other immunosuppressant therapies.26
Emergent Conditions
Acute Abdominal Pain. Approximately half of SLE patients who present to the ED with acute abdominal pain are found to have either mesenteric vasculitis or pancreatitis, both of which are thought to be related to SLE disease activity.27 Other causes of acute abdominal pain that are common in the general population remain common in SLE patients, including gallbladder disease, gastroenteritis, appendicitis, and peptic ulcer disease.
Mesenteric Vasculitis. Also known as lupus enteritis, mesenteric vasculitis is a unique cause of acute abdominal pain in SLE patients. The condition presents with acute, diffuse abdominal pain and may be associated with nausea and vomiting, diarrhea, or hematochezia. Abdominal CT findings suggestive of diffuse enteritis support the diagnosis. Medical management with pulse-dose corticosteroids and supportive care is generally sufficient, but if bowel necrosis or intestinal perforation is present or suspected, surgical consultation should be obtained immediately.15
Conclusion
Complications of SLE are diverse and may be difficult to diagnose. Understanding the common and emergent complications of SLE will help the EP to recognize severe illness and make appropriate treatment decisions in this complex patient population.
1. Dall’Era M, Wofsy D. Clinical Features of Systemic Lupus Erythematosus. In: Firestein GS et al, eds. Kelley and Firestein’s Textbook of Rheumatology. 10th ed. Philadelphia, PA: Elsevier; 2017.
2. Dvorkina O, Ginzler EM. Clinical features of systemic lupus erythematosus. In: Hochberg MC, ed. Rheumatology. 6th ed. Philadelphia, PA: Elsevier; 2015.
3. Huang JL, Hung JJ, Wu KC, Lee WI, Chan CK, Ou LS. Septic arthritis in patients with systemic lupus erythematosus: salmonella and nonsalmonella infections compared. Semin Arthritis Rheumatol. 2006;36(1):61-67. doi:10.1016/j.semarthrit.2006.04.003
4. Barile-Fabris L, Hernández-Cabrera MF, Barragan-Garfias JA. Vasculitis in systemic lupus erythematosus. Curr Rheumatol Rep. 2014;16(9):440. doi:10.1007/s11926-014-0440-9.
5. Campion EW, Wigley FM, Flavahan NA. Raynaud’s phenomenon. N Engl J Med. 2016;375(6):556-565. doi:10.1056/NEJMra1507638.
6. Bouaziz JD, Barete S, Le Pelletier F, et al. Cutaneous lesions of the digits in systemic lupus erythematosus: 50 cases. Lupus. 2007;16(3):163-167.
7. Pokroy-Shapira E, Gelernter I, Molad Y. Evolution of chronic kidney disease in patients with systemic lupus erythematosus over a long-period follow-up: a single-center inception cohort study. Clin Rheumatol. 2014;33(5):649-657. doi:10.1007/s10067-014-2527-0.
8. Almaani S, Meara A, Rovin BH. Update on lupus nephritis. Clin J Am Soc Nephrol. 2017;12(5):825-835. doi:10.2215/CJN.05780616.
9. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheumatol. 1999;42(4):599-608.
10. Oomatia A, Fang H, Petri M, et al. Peripheral neuropathies in systemic lupus erythematosus: clinical features, disease associations, and immunologic characteristics evaluated over a twenty-five year study period. Arthritis Rheumatol. 2014;66(4):1000-1009.
11. Mitsikostas DD, Sfikakis PP, Goadsby PJ. A meta-analysis for headache in systemic lupus erythematosus: the evidence and the myth. Brain. 2004;127(pt 5):1200-1209.
12. Timlin H, Petri M. Transient ischemic attack and stroke in systemic lupus erythematosus. Lupus. 2013;22(12):1251-1258. doi:10.1177/0961203313497416.
13. Majdak MR, Vuletić V. Thrombolysis for acute stroke in patient with systemic lupus erythematosus: a case report. J Neurol Sci. 2016;(361):7-8. doi:10.1016/j.jns.2015.12.014.
14. Chen WL, Chang SH, Chen JH, Wu YL. Isolated headache as the sole manifestation of dural sinus thrombosis: a case report with literature review. Am J Emerg Med. 2007;25(2):218-219.
15. Arntfield RT, Hicks CM. Systemic Lupus Erythematosus and the Vasculitides. In: Marx JA, ed. Rosen’s Emergency Medicine. 8th ed. Philadelphia, PA: Elsevier Saunders; 2014.
16. Borrell H, Narváez J, Alegree JJ, et al. Shrinking lung syndrome in systemic lupus erythematosus: a case series and review of the literature. Medicine (Baltimore). 2016;95(33):e4626. doi:10.1097/MD.0000000000004626.
17. Aviña-Zubieta JA, Vostretsova K, De Vera MA, et al. The risk of pulmonary embolism and deep venous thrombosis in systemic lupus erythematosus: a general population-based study. Semin Arthritis Rheum. 2015;45(2):195-201. doi:10.1016/j.semarthrit.2015.05.008.
18. Martínez-Martínez MU, Abud-Mendoza C. Predictors of mortality in diffuse alveolar haemorrhage associated with systemic lupus erythematosus. Lupus. 2011;20(6):568-574. doi:10.1177/0961203310392430.
19. Rosenbaum E, Krebs E, Cohen M, Tiliakos A, Derk CT. The spectrum of clinical manifestations, outcome and treatment of pericardial tamponade in patients with systemic lupus erythematosus: a retrospective study and literature review. Lupus. 2009;18(7):608-612. doi:10.1177/0961203308100659.
20. Miner JJ, Kim AH. Cardiac manifestations of systemic lupus erythematosus. Rheum Dis Clin North Am. 2014;40(1):51-60. doi:10.1016/j.rdc.2013.10.003.
21. Morel N, Bonjour M, Le Guern V, et al. Colchicine: a simple and effective treatment for pericarditis in systemic lupus erythematosus? A report of 10 cases. Lupus. 2015;24(14):1479-1485. doi:10.1177/0961203315593169.
22. Roldan CA, Shively BK, Crawford MH. An echocardiographic study of valvular heart disease associated with systemic lupus erythematosus. N Engl J Med. 1996;335(19):1424-1430.
23. Dufrost V, Risse J, et al. Direct oral anticoagulants use in antiphospholipid syndrome: are these drugs an effective and safe alternative to warfarin? A systematic review of the literature. Curr Rheumatol Rep. 2016;18(12):74. doi:10.1007/s11926-016-0623-7.
24. Cervera R,Rodríguez-Pintó I; G Espinosa on behalf of the Task Force on Catastrophic Antiphospholipid Syndrome. Catastrophic antiphospholipid syndrome: task force report summary. Lupus. 2014;23(12):1283-1285. doi:10.1177/0961203314540764.
25. Sultan SM, Ioannou Y, Isenberg DA. A review of gastrointestinal manifestations of systemic lupus erythematosus. Rheumatology (Oxford). 1999;38(10):917-932.
26. Xu N, Zhao J, Liu J, et al. Clinical analysis of 61 systemic lupus erythematosus patients with intestinal pseudo-obstruction and/or ureterohydronephrosis: a retrospective observational study. Medicine (Baltimore). 2015;94(4):e419.
27. Vergara-Fernandez O, Zeron-Medina J, Mendez-Probst C, et al. Acute abdominal pain in patients with systemic lupus erythematosus. J Gastrointest Surg. 2009;13(7):1351-1357. doi:10.1007/s11605-009-0897-4.
28. Küçükşahin O, Düzgün N, Okoh AK, Kulahçioglu E. Response to rituximab in a case of lupus associated digital ischemia. Case Rep Rheumatol. 2014;2014:763608. doi:10.1155/2014/763608.
29. Uva L, Miguel D, Pinheiro C, Freitas JP, Gomes MM, Filipe P. Cutaneous manifestations of systemic lupus erythematosus. Autoimmune Dis. 2012;2012:834291. doi:10.1155/2012/834291.
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease characterized by the chronic activation of the immune system, leading to the formation of autoantibodies and multi-organ damage. The prevalence of SLE in the United States is 20 to 150 per 100,000 persons.1 Ninety percent of patients with SLE are women, and the condition is more common and often more severe among patients of black African or of Asian descent.
For patients with known SLE who present to the ED, it can be a challenge to identify whether their symptoms are due to a minor lupus flare that can be managed as an outpatient, a presentation of urgent or emergent conditions caused by SLE, or a condition unrelated to lupus. This article reviews the most common and emergent complications of SLE by organ system to assist emergency physicians (EPs) in better diagnosing and managing this complicated disease.
General Acute-Care Management
While a patient’s presentation could be secondary to a lupus-related complication, consideration must always be given to common conditions that are not related to SLE. Biomarkers such as erythrocyte sedimentation rate, C-reactive protein, C3 and C4 complement, and double-stranded DNA levels can be helpful in assessing lupus disease activity and differentiating a lupus-related complication from an unrelated event. Comparing these biomarkers to the patient’s baseline values can be informative; however, depending on the laboratory facilities, test results may not be available during an ED visit. Lastly, infections should be considered more strongly than usual in the differential diagnosis due to the immunocompromised status of a substantial proportion of these patients, by virtue of their disease or the cytotoxic medications used for treatment.
Musculoskeletal Complications
Common Complications
Polyarthralgias and Polymyalgias. More than 90% of SLE patients experience polyarthralgias and polymyalgias. Physical examination findings may be normal, even when joint pain is present, which is often due to mild synovitis. In some cases, Jaccoud arthropathy is seen, which presents as deformities such as swan neck deformities and ulnar deviations that are characteristically reducible on manipulation (Figures 1a and 1b). These deformities are not caused by direct joint damage, but by chronic tenosynovitis and the resulting laxity of tendons and ligaments.1 Classically, plain radiographic imaging reveals nonerosive joint changes. Muscle and joint pains may worsen with disease progression or flare.
Avascular Necrosis. Avascular necrosis affects 5% to 12% of SLE patients.2 Most commonly, this involves the femoral head, but it may also involve the femoral condyle or tibial plateau. Patients may present with acute or subacute onset of pain in the groin or buttocks when the femoral head is involved, or in the knee when the femoral condyle or tibial plateau is involved. Plain radiographs may reveal joint-space narrowing and other evidence of degenerative joint disease. Magnetic resonance imaging (MRI) is more sensitive in diagnosing avascular necrosis, and may be indicated when clinical suspicion is high despite negative plain radiographs, although this would not typically need to be performed urgently in the ED.2 While analgesics and physical therapy may provide some pain relief to patients with avascular necrosis, this condition generally requires nonemergent operative intervention.
Emergent Complications
Septic Arthritis. When a patient with SLE presents with an isolated swollen joint, septic arthritis should be suspected, and diagnosis should be confirmed by arthrocentesis. Synovial fluid samples showing a white blood cell count greater than 50 × 109/Lsuggest infection, which can be confirmed by gram stain and cultures.
For reasons that remain unclear, but may involve primary immune defects and the use of immunosuppressant medications, patients with SLE are predisposed to Salmonella joint infections. In one study, 59% of septic arthritis cases in patients with SLE were due to Salmonella species; therefore, treatment for septic arthritis in this population should include ceftriaxone in addition to vancomycin for typical organisms, such as Staphylococcus and Streptococcus species.3
Cutaneous Manifestations
Common Complications
Malar Rash. Eighty percent to 90% of patients with SLE have dermatological involvement,1 the most common finding of which is the malar or butterfly facial rash, which appears as raised erythema over the bridge of the nose and cheeks while sparing the nasolabial folds (Figure 2).
Discoid Lupus. Chronic discoid lupus appears as a scarring rash often found on the face, ears, and scalp. These patients may also exhibit a photosensitive rash, which consists of an erythematous eruption if acute, or annular scaly lesions if subacute.
Oral and Nasal Ulcerations. Common mucous membrane findings include oral or nasal ulcers, which are typically painless.
Worsening of any of these skin findings may be associated with disease flare. Secondary bacterial infection of lupus rashes or ulcerations is uncommon, although cellulitis should be considered when a rash is unilateral, not in a sun-exposed area, or is otherwise different from the patient’s typical lupus rash. Sun avoidance and topical corticosteroids are the mainstays of treatment of dermatological disease in SLE.
Emergent Complications
Systemic Vasculitis. Patients with SLE are susceptible to vasculitis. Although isolated cutaneous vasculitis is not typically an emergent condition, it may portend systemic vasculitis. Any palpable purpura or other evidence of cutaneous vasculitis should prompt a careful review of systems and basic laboratory workup for systemic vasculitis, which can involve the kidneys, lungs, central or peripheral nervous system, or gastrointestinal tract.
Symptoms of systemic vasculitis may include fevers, chills, chest pain, cough, hemoptysis, abdominal pain, and changes in color or amount of urine. Laboratory workup should be tailored to symptoms, and may include basic metabolic panel, liver function tests, complete blood count, and urinalysis.4
Digital Gangrene. Patients with SLE may also develop digital gangrene related to severe Raynaud phenomenon, vasculitis, or thromboembolism. Pharmacological treatment with vasodilators such as sildenafil, endothelin receptor antagonists, or intravenous prostacyclins may be needed.5 To save the involved digit, vascular surgery services should be consulted urgently.6
Renal Complications
Common Complications
Chronic Kidney Disease. Chronic kidney disease (CKD) is common among SLE patients, especially among those with a history of lupus nephritis.7 Patients with CKD may have persistently elevated serum creatinine, chronic hypertension, and/or chronic peripheral edema. Patients presenting with new development of hypertension, peripheral edema, hematuria, or polyuria should be screened for lupus nephritis with urinalysis and serum creatinine. Elevated creatinine or new or worsening proteinuria or hematuria should prompt consultation with nephrology services.
Emergent Complications
Lupus Nephritis. About 50% of SLE patients will develop lupus nephritis during the course of their lives,1 which may present as nephrotic disease with significant proteinuria, peripheral edema, and low serum albumin, or as nephritic disease, with increased serum creatinine and hematuria. Acute kidney injury in SLE patients should generally prompt admission for workup of reversible causes and evaluation for lupus nephritis, which often includes renal biopsy.8
Neuropsychiatric Complications
Common Complications
Neuropsychiatric lupus is a broad category that includes 19 manifestations of SLE in the central and peripheral nervous systems.9 Conditions range from depression or chronic headaches to seizures or psychosis.
Mood and Anxiety Disorders. Anxiety and depression have been observed in up to 75% of SLE patients.1 Mood and anxiety disorders are likely influenced by the psychosocial elements of this chronic disease, as well as by direct effects of SLE on the brain.1
Peripheral Neuropathy. Approximately 10% of SLE patients have a peripheral neuropathy, which generally presents as a mononeuritis (either single or multiplex), rather than the stocking-glove distribution seen in other systemic causes of neuropathy.10
Headache. Headache disorders may also develop in SLE patients, and tend to have similar patterns to primary headache disorders in the general population. In most cases, treatment for headache in SLE patients is similar to that of the general population.11 However, if a patient presents with concerning findings, such as focal neurological deficit, meningismus, or fever, or if the headache is new-onset or different from previous headaches, further investigation should be considered, including a head computed tomography (CT) scan and lumbar puncture (LP).
Emergent Complications
In general, due to the variety of neurological emergencies that may present with SLE, and the subtlety with which true emergencies may present in this population, the threshold to obtain imaging on SLE patients with any new neurological complaints should be low.
Cerebrovascular Accidents. Patients with SLE are susceptible to cerebrovascular accidents (CVAs), typically from occlusive or embolic causes. Etiologies may include primary central nervous system (CNS) vasculitis, embolic disease from antiphospholipid syndrome (APS), or embolic disease from a Libman-Sacks endocarditis.12
Successful thrombolysis has been reported in SLE patients presenting with stroke, but it remains controversial due to risk of hemorrhagic conversion if CNS vasculitis, rather than embolism, is the cause.13 Proper imaging and consultation with a neurologist familiar with the disease is critical for early treatment decisions.
Seizures. Fifteen percent to 35% of SLE patients may develop seizures. These may be focal or generalized, but generalized tonic-clonic seizures tend to be more common in SLE patients.2 Workup and management of seizures in SLE patients is the same as in the general population.
Sinus Thrombosis. Dural sinus thrombosis often presents as a new-onset headache, sometimes with focal neurological deficits. The diagnosis of dural sinus thrombosis can be challenging, as CT imaging studies may be falsely negative. There should be a low threshold for obtaining MRI/magnetic resonance angiography (MRA) in SLE patients presenting with a new-onset headache.14
CNS Vasculitis. Patients with SLE are also susceptible to CNS vasculitis, which can manifest as seizures, psychosis, cognitive decline, altered mental status, or coma. Magnetic resonance imaging/MRA studies may suggest the diagnosis, but if this is equivocal, angiography or even brain biopsy may be needed to make the diagnosis. Unless the patient’s symptoms are very mild (eg, mild cognitive decline), she or he should be admitted for diagnostic workup and consideration of aggressive immunosuppressive therapy.2
Transverse Myelitis and Spinal Artery Thrombosis. Acute loss of lower limb sensation or motor function in SLE patients may be caused by transverse myelitis or spinal artery thrombosis. Epidural abscess should also be considered, especially if the patient is immunocompromised.2
Infection. A CNS infection should be considered in any SLE patient presenting with new neurological complaints. Fever or meningismus, especially in conjunction with headache or focal neurological deficits, should prompt an LP and consideration for imaging. Immunocompromised patients are at increased risk for common organisms as well as atypical organisms, such as fungus or mycobacteria.15
Pulmonary Complications
Common Complications
Pleuritis. Many patients with SLE develop pleuritis, with or without effusion. This may be treated with nonsteroidal anti-inflammatory drugs, or corticosteroids if symptoms are more severe. Pleuritis is the most common respiratory complication of SLE, but due to the number of serious cardiopulmonary complications associated with SLE, pleuritis should be a diagnosis of exclusion.
Interstitial Lung Disease. Interstitial lung disease may be caused by SLE or may be medication-induced. This commonly presents as subacute or chronic dyspnea and/or cough. Patient workup may be done on an outpatient basis with high resolution chest CT and pulmonary function testing.
Pulmonary Hypertension. Patients with SLE may develop pulmonary hypertension, either directly due to SLE or from chronic thromboembolic disease. In general, pulmonary hypertension is managed as an outpatient, but may require emergent inpatient treatment if the condition is rapidly progressive or associated with right heart failure.
Shrinking Lung Syndrome. This condition may cause subacute or chronic dyspnea and pleuritic chest pain. Shrinking lung syndrome is caused by diaphragmatic dysfunction rather than from a primary disease of the lungs, and it is characterized by a restrictive pattern on pulmonary function testing and an elevated hemidiaphragm. Shrinking lung syndrome typically responds well to immunosuppressive therapy.16
Emergent Conditions
Pulmonary Embolism. A pulmonary embolism should be strongly considered in any patient with SLE presenting with the appropri ate clinical picture. Patients with APS are at particularly high risk for thromboembolic disease. However, even SLE patients without this APS are known to be at an increased risk of developing thromboembolism compared to the general public.17 Pulmonary embolism in SLE patients should be diagnosed and treated in the usual manner.
Pneumonia. Immunosuppressed patients are susceptible to opportunistic pulmonary infections as well as typical community pathogens. Fungal or mycobacterial infections may be suspected with a more subacute onset of symptoms.
Acute Lupus Pneumonitis. This serious condition may present with severe pneumonia-like signs and symptoms, including fever, cough, dyspnea, hypoxia, and infiltrates on chest radiograph (Figure 3).
Acute lupus pneumonitis is caused by disease flare, and not by infection, although it may not be possible to distinguish it from pneumonia in the ED setting. The mortality rate of acute lupus pneumonitis is as high as 50%, and survivors often progress to chronic interstitial pneumonitis.1
Diffuse Alveolar Hemorrhage. A rare complication with a mortality rate of 50% to 90%, SLE patients who develop diffuse alveolar hemorrhage may present with fever, cough, dyspnea, and hypoxia.18 The condition may be suggested by infiltrates on chest radiograph, a drop in hemoglobin representing bleeding into the lungs, and/or hemoptysis. However, the absence of hemoptysis does not rule out diffuse alveolar hemorrhage, so clinical suspicion should remain high, even in the absence of this symptom.
Because emergent pulmonary conditions often present with similar symptoms, most patients with acute or new-onset symptoms will require admission for diagnostic workup (likely to include chest CT scan and/or bronchoscopy with bronchoalveolar lavage), as well as for close monitoring and initiation of treatment. If hypoxia or respiratory distress is severe, or if diffuse alveolar hemorrhage is suspected, admission to the intensive care unit (ICU) should be considered. We suggest that antibiotics be started in the ED when pneumonia is part of the differential diagnosis. As in the general population, coverage should be chosen based on the patient’s risk factors for antibiotic-resistant organisms. Initiation of corticosteroid therapy or other changes in immune therapy can be delayed until the EP consults with rheumatology and/or pulmonology services.
Cardiac Complications
Common Complications
Pericarditis. Pericarditis with or without pericardial effusion is very common in SLE patients and is usually related to lupus itself, rather than an infectious etiology. Patients may present with substernal, positional chest pain, tachycardia, and diffuse ST-segment elevation on electrocardiogram. Most effusions are small, asymptomatic, and discovered incidentally. However, among patients with symptomatic pericardial effusions, tamponade can be present in 21%.19 Corticosteroid therapy is often required to treat SLE-associated pericarditis, but colchicine is being explored as a possible steroid-sparing agent in this patient population.20,21
Valvular Abnormalities. Approximately 60% of SLE patients have valvular abnormalities detectable by echocardiography. The most common abnormalities in one study were valvular thickening or regurgitation.22 Many of these abnormalities occurred in asymptomatic patients and never progressed to clinical disease in a 5-year follow-up. However, patients with any valvular abnormality were more likely to develop complications, including stroke, peripheral embolism, infective endocarditis, need for valve replacement, congestive heart failure, or death.22
Emergent Complications
Acute Coronary Syndrome. Even in relatively young patients, acute coronary syndrome (ACS) should be considered in SLE patients presenting with chest pain, as this patient population has a 10-fold higher risk of developing coronary artery disease (CAD) than the general population, and SLE patients with CAD often lack traditional risk factors, such as advanced age, family history, or metabolic syndrome.1
A high clinical suspicion should be maintained even in patients who would traditionally be considered low-risk. The EP should have a low-threshold for ECG, cardiac biomarker testing, and stress testing for SLE patients presenting with chest pain. The treatment of ACS in SLE patients is the same as in the general population.
Libman-Sacks Endocarditis. A sterile, fibrinous valvular vegetation, Libman-Sacks endocarditis is unique to patients with SLE. When present, patients usually develop a subacute or chronic onset of dyspnea or chest pain. However, patients may become acutely ill if they develop severe valvular regurgitation. Additionally, the valve damage from Libman-Sacks endocarditis can predispose patients to developing infective endocarditis.20
Hematological Complications
Common Complications
Patients with SLE commonly have mild-to-moderate leukopenia (especially lymphopenia), anemia, and thrombocytopenia. This may be related to the disease process or may be secondary to prescribed medications. A comparison to recent baseline laboratory studies should be sought if there is suspicion for new or worsening cytopenia.
Antiphospholipid Syndrome. Nearly 40% of SLE patients also have APS, which is defined by a clinical history of thrombosis in conjunction with one of the antiphospholipid antibodies (anticardiolipin, anti-beta-2-glycoprotein, lupus anticoagulant). Antiphospholipid syndrome causes both venous and arterial thrombosis and may be associated with recurrent miscarriage. Acute thrombotic events should be treated with heparin or enoxaparin and transitioned to warfarin. The new generation of direct oral anticoagulants have not been well studied in APS, though, multiple small case series suggest a higher thrombotic risk with these drugs than with warfarin.23Patients who have recurrent venous thromboembolism, or who have any arterial thromboembolism should be on lifelong anticoagulation therapy.2
Emergent Complications
Thrombocytopenia. Severe thrombocytopenia or hemolytic anemia can be life-threatening, and often requires inpatient admission for immunosuppressive therapy, monitoring, and supportive care.
Catastrophic Antiphospholipid Syndrome. This condition should be suspected in patients with SLE who present with multiple sites of thrombosis or new multi-organ damage. Catastrophic APS (CAPS) may occur in SLE patients who have no prior history of APS. Since the mortality rate for CAPS approaches 50%, these patients require anticoagulation, immunosuppressant therapy (high-dose corticosteroids, cyclophosphamide, and/or plasma exchange), and admission to the ICU.24
Gastrointestinal Complications
Common Complications
Intestinal Pseudo-obstruction. Dysphagia related to esophageal dysmotility is present in up to 13% of SLE patients.25 Intestinal pseudo-obstruction may be seen in SLE patients, and is characterized by symptoms of intestinal obstruction caused by decreased intestinal motility, rather than from mechanical obstruction. Presenting symptoms may be acute or chronic, and include nausea, vomiting, and abdominal distension. Abdominal CT studies will show dilated bowel loops without evidence of mechanical obstruction. Manometry reveals widespread hypomotility. Intestinal pseudo-obstruction typically responds well to corticosteroids and other immunosuppressant therapies.26
Emergent Conditions
Acute Abdominal Pain. Approximately half of SLE patients who present to the ED with acute abdominal pain are found to have either mesenteric vasculitis or pancreatitis, both of which are thought to be related to SLE disease activity.27 Other causes of acute abdominal pain that are common in the general population remain common in SLE patients, including gallbladder disease, gastroenteritis, appendicitis, and peptic ulcer disease.
Mesenteric Vasculitis. Also known as lupus enteritis, mesenteric vasculitis is a unique cause of acute abdominal pain in SLE patients. The condition presents with acute, diffuse abdominal pain and may be associated with nausea and vomiting, diarrhea, or hematochezia. Abdominal CT findings suggestive of diffuse enteritis support the diagnosis. Medical management with pulse-dose corticosteroids and supportive care is generally sufficient, but if bowel necrosis or intestinal perforation is present or suspected, surgical consultation should be obtained immediately.15
Conclusion
Complications of SLE are diverse and may be difficult to diagnose. Understanding the common and emergent complications of SLE will help the EP to recognize severe illness and make appropriate treatment decisions in this complex patient population.
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease characterized by the chronic activation of the immune system, leading to the formation of autoantibodies and multi-organ damage. The prevalence of SLE in the United States is 20 to 150 per 100,000 persons.1 Ninety percent of patients with SLE are women, and the condition is more common and often more severe among patients of black African or of Asian descent.
For patients with known SLE who present to the ED, it can be a challenge to identify whether their symptoms are due to a minor lupus flare that can be managed as an outpatient, a presentation of urgent or emergent conditions caused by SLE, or a condition unrelated to lupus. This article reviews the most common and emergent complications of SLE by organ system to assist emergency physicians (EPs) in better diagnosing and managing this complicated disease.
General Acute-Care Management
While a patient’s presentation could be secondary to a lupus-related complication, consideration must always be given to common conditions that are not related to SLE. Biomarkers such as erythrocyte sedimentation rate, C-reactive protein, C3 and C4 complement, and double-stranded DNA levels can be helpful in assessing lupus disease activity and differentiating a lupus-related complication from an unrelated event. Comparing these biomarkers to the patient’s baseline values can be informative; however, depending on the laboratory facilities, test results may not be available during an ED visit. Lastly, infections should be considered more strongly than usual in the differential diagnosis due to the immunocompromised status of a substantial proportion of these patients, by virtue of their disease or the cytotoxic medications used for treatment.
Musculoskeletal Complications
Common Complications
Polyarthralgias and Polymyalgias. More than 90% of SLE patients experience polyarthralgias and polymyalgias. Physical examination findings may be normal, even when joint pain is present, which is often due to mild synovitis. In some cases, Jaccoud arthropathy is seen, which presents as deformities such as swan neck deformities and ulnar deviations that are characteristically reducible on manipulation (Figures 1a and 1b). These deformities are not caused by direct joint damage, but by chronic tenosynovitis and the resulting laxity of tendons and ligaments.1 Classically, plain radiographic imaging reveals nonerosive joint changes. Muscle and joint pains may worsen with disease progression or flare.
Avascular Necrosis. Avascular necrosis affects 5% to 12% of SLE patients.2 Most commonly, this involves the femoral head, but it may also involve the femoral condyle or tibial plateau. Patients may present with acute or subacute onset of pain in the groin or buttocks when the femoral head is involved, or in the knee when the femoral condyle or tibial plateau is involved. Plain radiographs may reveal joint-space narrowing and other evidence of degenerative joint disease. Magnetic resonance imaging (MRI) is more sensitive in diagnosing avascular necrosis, and may be indicated when clinical suspicion is high despite negative plain radiographs, although this would not typically need to be performed urgently in the ED.2 While analgesics and physical therapy may provide some pain relief to patients with avascular necrosis, this condition generally requires nonemergent operative intervention.
Emergent Complications
Septic Arthritis. When a patient with SLE presents with an isolated swollen joint, septic arthritis should be suspected, and diagnosis should be confirmed by arthrocentesis. Synovial fluid samples showing a white blood cell count greater than 50 × 109/Lsuggest infection, which can be confirmed by gram stain and cultures.
For reasons that remain unclear, but may involve primary immune defects and the use of immunosuppressant medications, patients with SLE are predisposed to Salmonella joint infections. In one study, 59% of septic arthritis cases in patients with SLE were due to Salmonella species; therefore, treatment for septic arthritis in this population should include ceftriaxone in addition to vancomycin for typical organisms, such as Staphylococcus and Streptococcus species.3
Cutaneous Manifestations
Common Complications
Malar Rash. Eighty percent to 90% of patients with SLE have dermatological involvement,1 the most common finding of which is the malar or butterfly facial rash, which appears as raised erythema over the bridge of the nose and cheeks while sparing the nasolabial folds (Figure 2).
Discoid Lupus. Chronic discoid lupus appears as a scarring rash often found on the face, ears, and scalp. These patients may also exhibit a photosensitive rash, which consists of an erythematous eruption if acute, or annular scaly lesions if subacute.
Oral and Nasal Ulcerations. Common mucous membrane findings include oral or nasal ulcers, which are typically painless.
Worsening of any of these skin findings may be associated with disease flare. Secondary bacterial infection of lupus rashes or ulcerations is uncommon, although cellulitis should be considered when a rash is unilateral, not in a sun-exposed area, or is otherwise different from the patient’s typical lupus rash. Sun avoidance and topical corticosteroids are the mainstays of treatment of dermatological disease in SLE.
Emergent Complications
Systemic Vasculitis. Patients with SLE are susceptible to vasculitis. Although isolated cutaneous vasculitis is not typically an emergent condition, it may portend systemic vasculitis. Any palpable purpura or other evidence of cutaneous vasculitis should prompt a careful review of systems and basic laboratory workup for systemic vasculitis, which can involve the kidneys, lungs, central or peripheral nervous system, or gastrointestinal tract.
Symptoms of systemic vasculitis may include fevers, chills, chest pain, cough, hemoptysis, abdominal pain, and changes in color or amount of urine. Laboratory workup should be tailored to symptoms, and may include basic metabolic panel, liver function tests, complete blood count, and urinalysis.4
Digital Gangrene. Patients with SLE may also develop digital gangrene related to severe Raynaud phenomenon, vasculitis, or thromboembolism. Pharmacological treatment with vasodilators such as sildenafil, endothelin receptor antagonists, or intravenous prostacyclins may be needed.5 To save the involved digit, vascular surgery services should be consulted urgently.6
Renal Complications
Common Complications
Chronic Kidney Disease. Chronic kidney disease (CKD) is common among SLE patients, especially among those with a history of lupus nephritis.7 Patients with CKD may have persistently elevated serum creatinine, chronic hypertension, and/or chronic peripheral edema. Patients presenting with new development of hypertension, peripheral edema, hematuria, or polyuria should be screened for lupus nephritis with urinalysis and serum creatinine. Elevated creatinine or new or worsening proteinuria or hematuria should prompt consultation with nephrology services.
Emergent Complications
Lupus Nephritis. About 50% of SLE patients will develop lupus nephritis during the course of their lives,1 which may present as nephrotic disease with significant proteinuria, peripheral edema, and low serum albumin, or as nephritic disease, with increased serum creatinine and hematuria. Acute kidney injury in SLE patients should generally prompt admission for workup of reversible causes and evaluation for lupus nephritis, which often includes renal biopsy.8
Neuropsychiatric Complications
Common Complications
Neuropsychiatric lupus is a broad category that includes 19 manifestations of SLE in the central and peripheral nervous systems.9 Conditions range from depression or chronic headaches to seizures or psychosis.
Mood and Anxiety Disorders. Anxiety and depression have been observed in up to 75% of SLE patients.1 Mood and anxiety disorders are likely influenced by the psychosocial elements of this chronic disease, as well as by direct effects of SLE on the brain.1
Peripheral Neuropathy. Approximately 10% of SLE patients have a peripheral neuropathy, which generally presents as a mononeuritis (either single or multiplex), rather than the stocking-glove distribution seen in other systemic causes of neuropathy.10
Headache. Headache disorders may also develop in SLE patients, and tend to have similar patterns to primary headache disorders in the general population. In most cases, treatment for headache in SLE patients is similar to that of the general population.11 However, if a patient presents with concerning findings, such as focal neurological deficit, meningismus, or fever, or if the headache is new-onset or different from previous headaches, further investigation should be considered, including a head computed tomography (CT) scan and lumbar puncture (LP).
Emergent Complications
In general, due to the variety of neurological emergencies that may present with SLE, and the subtlety with which true emergencies may present in this population, the threshold to obtain imaging on SLE patients with any new neurological complaints should be low.
Cerebrovascular Accidents. Patients with SLE are susceptible to cerebrovascular accidents (CVAs), typically from occlusive or embolic causes. Etiologies may include primary central nervous system (CNS) vasculitis, embolic disease from antiphospholipid syndrome (APS), or embolic disease from a Libman-Sacks endocarditis.12
Successful thrombolysis has been reported in SLE patients presenting with stroke, but it remains controversial due to risk of hemorrhagic conversion if CNS vasculitis, rather than embolism, is the cause.13 Proper imaging and consultation with a neurologist familiar with the disease is critical for early treatment decisions.
Seizures. Fifteen percent to 35% of SLE patients may develop seizures. These may be focal or generalized, but generalized tonic-clonic seizures tend to be more common in SLE patients.2 Workup and management of seizures in SLE patients is the same as in the general population.
Sinus Thrombosis. Dural sinus thrombosis often presents as a new-onset headache, sometimes with focal neurological deficits. The diagnosis of dural sinus thrombosis can be challenging, as CT imaging studies may be falsely negative. There should be a low threshold for obtaining MRI/magnetic resonance angiography (MRA) in SLE patients presenting with a new-onset headache.14
CNS Vasculitis. Patients with SLE are also susceptible to CNS vasculitis, which can manifest as seizures, psychosis, cognitive decline, altered mental status, or coma. Magnetic resonance imaging/MRA studies may suggest the diagnosis, but if this is equivocal, angiography or even brain biopsy may be needed to make the diagnosis. Unless the patient’s symptoms are very mild (eg, mild cognitive decline), she or he should be admitted for diagnostic workup and consideration of aggressive immunosuppressive therapy.2
Transverse Myelitis and Spinal Artery Thrombosis. Acute loss of lower limb sensation or motor function in SLE patients may be caused by transverse myelitis or spinal artery thrombosis. Epidural abscess should also be considered, especially if the patient is immunocompromised.2
Infection. A CNS infection should be considered in any SLE patient presenting with new neurological complaints. Fever or meningismus, especially in conjunction with headache or focal neurological deficits, should prompt an LP and consideration for imaging. Immunocompromised patients are at increased risk for common organisms as well as atypical organisms, such as fungus or mycobacteria.15
Pulmonary Complications
Common Complications
Pleuritis. Many patients with SLE develop pleuritis, with or without effusion. This may be treated with nonsteroidal anti-inflammatory drugs, or corticosteroids if symptoms are more severe. Pleuritis is the most common respiratory complication of SLE, but due to the number of serious cardiopulmonary complications associated with SLE, pleuritis should be a diagnosis of exclusion.
Interstitial Lung Disease. Interstitial lung disease may be caused by SLE or may be medication-induced. This commonly presents as subacute or chronic dyspnea and/or cough. Patient workup may be done on an outpatient basis with high resolution chest CT and pulmonary function testing.
Pulmonary Hypertension. Patients with SLE may develop pulmonary hypertension, either directly due to SLE or from chronic thromboembolic disease. In general, pulmonary hypertension is managed as an outpatient, but may require emergent inpatient treatment if the condition is rapidly progressive or associated with right heart failure.
Shrinking Lung Syndrome. This condition may cause subacute or chronic dyspnea and pleuritic chest pain. Shrinking lung syndrome is caused by diaphragmatic dysfunction rather than from a primary disease of the lungs, and it is characterized by a restrictive pattern on pulmonary function testing and an elevated hemidiaphragm. Shrinking lung syndrome typically responds well to immunosuppressive therapy.16
Emergent Conditions
Pulmonary Embolism. A pulmonary embolism should be strongly considered in any patient with SLE presenting with the appropri ate clinical picture. Patients with APS are at particularly high risk for thromboembolic disease. However, even SLE patients without this APS are known to be at an increased risk of developing thromboembolism compared to the general public.17 Pulmonary embolism in SLE patients should be diagnosed and treated in the usual manner.
Pneumonia. Immunosuppressed patients are susceptible to opportunistic pulmonary infections as well as typical community pathogens. Fungal or mycobacterial infections may be suspected with a more subacute onset of symptoms.
Acute Lupus Pneumonitis. This serious condition may present with severe pneumonia-like signs and symptoms, including fever, cough, dyspnea, hypoxia, and infiltrates on chest radiograph (Figure 3).
Acute lupus pneumonitis is caused by disease flare, and not by infection, although it may not be possible to distinguish it from pneumonia in the ED setting. The mortality rate of acute lupus pneumonitis is as high as 50%, and survivors often progress to chronic interstitial pneumonitis.1
Diffuse Alveolar Hemorrhage. A rare complication with a mortality rate of 50% to 90%, SLE patients who develop diffuse alveolar hemorrhage may present with fever, cough, dyspnea, and hypoxia.18 The condition may be suggested by infiltrates on chest radiograph, a drop in hemoglobin representing bleeding into the lungs, and/or hemoptysis. However, the absence of hemoptysis does not rule out diffuse alveolar hemorrhage, so clinical suspicion should remain high, even in the absence of this symptom.
Because emergent pulmonary conditions often present with similar symptoms, most patients with acute or new-onset symptoms will require admission for diagnostic workup (likely to include chest CT scan and/or bronchoscopy with bronchoalveolar lavage), as well as for close monitoring and initiation of treatment. If hypoxia or respiratory distress is severe, or if diffuse alveolar hemorrhage is suspected, admission to the intensive care unit (ICU) should be considered. We suggest that antibiotics be started in the ED when pneumonia is part of the differential diagnosis. As in the general population, coverage should be chosen based on the patient’s risk factors for antibiotic-resistant organisms. Initiation of corticosteroid therapy or other changes in immune therapy can be delayed until the EP consults with rheumatology and/or pulmonology services.
Cardiac Complications
Common Complications
Pericarditis. Pericarditis with or without pericardial effusion is very common in SLE patients and is usually related to lupus itself, rather than an infectious etiology. Patients may present with substernal, positional chest pain, tachycardia, and diffuse ST-segment elevation on electrocardiogram. Most effusions are small, asymptomatic, and discovered incidentally. However, among patients with symptomatic pericardial effusions, tamponade can be present in 21%.19 Corticosteroid therapy is often required to treat SLE-associated pericarditis, but colchicine is being explored as a possible steroid-sparing agent in this patient population.20,21
Valvular Abnormalities. Approximately 60% of SLE patients have valvular abnormalities detectable by echocardiography. The most common abnormalities in one study were valvular thickening or regurgitation.22 Many of these abnormalities occurred in asymptomatic patients and never progressed to clinical disease in a 5-year follow-up. However, patients with any valvular abnormality were more likely to develop complications, including stroke, peripheral embolism, infective endocarditis, need for valve replacement, congestive heart failure, or death.22
Emergent Complications
Acute Coronary Syndrome. Even in relatively young patients, acute coronary syndrome (ACS) should be considered in SLE patients presenting with chest pain, as this patient population has a 10-fold higher risk of developing coronary artery disease (CAD) than the general population, and SLE patients with CAD often lack traditional risk factors, such as advanced age, family history, or metabolic syndrome.1
A high clinical suspicion should be maintained even in patients who would traditionally be considered low-risk. The EP should have a low-threshold for ECG, cardiac biomarker testing, and stress testing for SLE patients presenting with chest pain. The treatment of ACS in SLE patients is the same as in the general population.
Libman-Sacks Endocarditis. A sterile, fibrinous valvular vegetation, Libman-Sacks endocarditis is unique to patients with SLE. When present, patients usually develop a subacute or chronic onset of dyspnea or chest pain. However, patients may become acutely ill if they develop severe valvular regurgitation. Additionally, the valve damage from Libman-Sacks endocarditis can predispose patients to developing infective endocarditis.20
Hematological Complications
Common Complications
Patients with SLE commonly have mild-to-moderate leukopenia (especially lymphopenia), anemia, and thrombocytopenia. This may be related to the disease process or may be secondary to prescribed medications. A comparison to recent baseline laboratory studies should be sought if there is suspicion for new or worsening cytopenia.
Antiphospholipid Syndrome. Nearly 40% of SLE patients also have APS, which is defined by a clinical history of thrombosis in conjunction with one of the antiphospholipid antibodies (anticardiolipin, anti-beta-2-glycoprotein, lupus anticoagulant). Antiphospholipid syndrome causes both venous and arterial thrombosis and may be associated with recurrent miscarriage. Acute thrombotic events should be treated with heparin or enoxaparin and transitioned to warfarin. The new generation of direct oral anticoagulants have not been well studied in APS, though, multiple small case series suggest a higher thrombotic risk with these drugs than with warfarin.23Patients who have recurrent venous thromboembolism, or who have any arterial thromboembolism should be on lifelong anticoagulation therapy.2
Emergent Complications
Thrombocytopenia. Severe thrombocytopenia or hemolytic anemia can be life-threatening, and often requires inpatient admission for immunosuppressive therapy, monitoring, and supportive care.
Catastrophic Antiphospholipid Syndrome. This condition should be suspected in patients with SLE who present with multiple sites of thrombosis or new multi-organ damage. Catastrophic APS (CAPS) may occur in SLE patients who have no prior history of APS. Since the mortality rate for CAPS approaches 50%, these patients require anticoagulation, immunosuppressant therapy (high-dose corticosteroids, cyclophosphamide, and/or plasma exchange), and admission to the ICU.24
Gastrointestinal Complications
Common Complications
Intestinal Pseudo-obstruction. Dysphagia related to esophageal dysmotility is present in up to 13% of SLE patients.25 Intestinal pseudo-obstruction may be seen in SLE patients, and is characterized by symptoms of intestinal obstruction caused by decreased intestinal motility, rather than from mechanical obstruction. Presenting symptoms may be acute or chronic, and include nausea, vomiting, and abdominal distension. Abdominal CT studies will show dilated bowel loops without evidence of mechanical obstruction. Manometry reveals widespread hypomotility. Intestinal pseudo-obstruction typically responds well to corticosteroids and other immunosuppressant therapies.26
Emergent Conditions
Acute Abdominal Pain. Approximately half of SLE patients who present to the ED with acute abdominal pain are found to have either mesenteric vasculitis or pancreatitis, both of which are thought to be related to SLE disease activity.27 Other causes of acute abdominal pain that are common in the general population remain common in SLE patients, including gallbladder disease, gastroenteritis, appendicitis, and peptic ulcer disease.
Mesenteric Vasculitis. Also known as lupus enteritis, mesenteric vasculitis is a unique cause of acute abdominal pain in SLE patients. The condition presents with acute, diffuse abdominal pain and may be associated with nausea and vomiting, diarrhea, or hematochezia. Abdominal CT findings suggestive of diffuse enteritis support the diagnosis. Medical management with pulse-dose corticosteroids and supportive care is generally sufficient, but if bowel necrosis or intestinal perforation is present or suspected, surgical consultation should be obtained immediately.15
Conclusion
Complications of SLE are diverse and may be difficult to diagnose. Understanding the common and emergent complications of SLE will help the EP to recognize severe illness and make appropriate treatment decisions in this complex patient population.
1. Dall’Era M, Wofsy D. Clinical Features of Systemic Lupus Erythematosus. In: Firestein GS et al, eds. Kelley and Firestein’s Textbook of Rheumatology. 10th ed. Philadelphia, PA: Elsevier; 2017.
2. Dvorkina O, Ginzler EM. Clinical features of systemic lupus erythematosus. In: Hochberg MC, ed. Rheumatology. 6th ed. Philadelphia, PA: Elsevier; 2015.
3. Huang JL, Hung JJ, Wu KC, Lee WI, Chan CK, Ou LS. Septic arthritis in patients with systemic lupus erythematosus: salmonella and nonsalmonella infections compared. Semin Arthritis Rheumatol. 2006;36(1):61-67. doi:10.1016/j.semarthrit.2006.04.003
4. Barile-Fabris L, Hernández-Cabrera MF, Barragan-Garfias JA. Vasculitis in systemic lupus erythematosus. Curr Rheumatol Rep. 2014;16(9):440. doi:10.1007/s11926-014-0440-9.
5. Campion EW, Wigley FM, Flavahan NA. Raynaud’s phenomenon. N Engl J Med. 2016;375(6):556-565. doi:10.1056/NEJMra1507638.
6. Bouaziz JD, Barete S, Le Pelletier F, et al. Cutaneous lesions of the digits in systemic lupus erythematosus: 50 cases. Lupus. 2007;16(3):163-167.
7. Pokroy-Shapira E, Gelernter I, Molad Y. Evolution of chronic kidney disease in patients with systemic lupus erythematosus over a long-period follow-up: a single-center inception cohort study. Clin Rheumatol. 2014;33(5):649-657. doi:10.1007/s10067-014-2527-0.
8. Almaani S, Meara A, Rovin BH. Update on lupus nephritis. Clin J Am Soc Nephrol. 2017;12(5):825-835. doi:10.2215/CJN.05780616.
9. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheumatol. 1999;42(4):599-608.
10. Oomatia A, Fang H, Petri M, et al. Peripheral neuropathies in systemic lupus erythematosus: clinical features, disease associations, and immunologic characteristics evaluated over a twenty-five year study period. Arthritis Rheumatol. 2014;66(4):1000-1009.
11. Mitsikostas DD, Sfikakis PP, Goadsby PJ. A meta-analysis for headache in systemic lupus erythematosus: the evidence and the myth. Brain. 2004;127(pt 5):1200-1209.
12. Timlin H, Petri M. Transient ischemic attack and stroke in systemic lupus erythematosus. Lupus. 2013;22(12):1251-1258. doi:10.1177/0961203313497416.
13. Majdak MR, Vuletić V. Thrombolysis for acute stroke in patient with systemic lupus erythematosus: a case report. J Neurol Sci. 2016;(361):7-8. doi:10.1016/j.jns.2015.12.014.
14. Chen WL, Chang SH, Chen JH, Wu YL. Isolated headache as the sole manifestation of dural sinus thrombosis: a case report with literature review. Am J Emerg Med. 2007;25(2):218-219.
15. Arntfield RT, Hicks CM. Systemic Lupus Erythematosus and the Vasculitides. In: Marx JA, ed. Rosen’s Emergency Medicine. 8th ed. Philadelphia, PA: Elsevier Saunders; 2014.
16. Borrell H, Narváez J, Alegree JJ, et al. Shrinking lung syndrome in systemic lupus erythematosus: a case series and review of the literature. Medicine (Baltimore). 2016;95(33):e4626. doi:10.1097/MD.0000000000004626.
17. Aviña-Zubieta JA, Vostretsova K, De Vera MA, et al. The risk of pulmonary embolism and deep venous thrombosis in systemic lupus erythematosus: a general population-based study. Semin Arthritis Rheum. 2015;45(2):195-201. doi:10.1016/j.semarthrit.2015.05.008.
18. Martínez-Martínez MU, Abud-Mendoza C. Predictors of mortality in diffuse alveolar haemorrhage associated with systemic lupus erythematosus. Lupus. 2011;20(6):568-574. doi:10.1177/0961203310392430.
19. Rosenbaum E, Krebs E, Cohen M, Tiliakos A, Derk CT. The spectrum of clinical manifestations, outcome and treatment of pericardial tamponade in patients with systemic lupus erythematosus: a retrospective study and literature review. Lupus. 2009;18(7):608-612. doi:10.1177/0961203308100659.
20. Miner JJ, Kim AH. Cardiac manifestations of systemic lupus erythematosus. Rheum Dis Clin North Am. 2014;40(1):51-60. doi:10.1016/j.rdc.2013.10.003.
21. Morel N, Bonjour M, Le Guern V, et al. Colchicine: a simple and effective treatment for pericarditis in systemic lupus erythematosus? A report of 10 cases. Lupus. 2015;24(14):1479-1485. doi:10.1177/0961203315593169.
22. Roldan CA, Shively BK, Crawford MH. An echocardiographic study of valvular heart disease associated with systemic lupus erythematosus. N Engl J Med. 1996;335(19):1424-1430.
23. Dufrost V, Risse J, et al. Direct oral anticoagulants use in antiphospholipid syndrome: are these drugs an effective and safe alternative to warfarin? A systematic review of the literature. Curr Rheumatol Rep. 2016;18(12):74. doi:10.1007/s11926-016-0623-7.
24. Cervera R,Rodríguez-Pintó I; G Espinosa on behalf of the Task Force on Catastrophic Antiphospholipid Syndrome. Catastrophic antiphospholipid syndrome: task force report summary. Lupus. 2014;23(12):1283-1285. doi:10.1177/0961203314540764.
25. Sultan SM, Ioannou Y, Isenberg DA. A review of gastrointestinal manifestations of systemic lupus erythematosus. Rheumatology (Oxford). 1999;38(10):917-932.
26. Xu N, Zhao J, Liu J, et al. Clinical analysis of 61 systemic lupus erythematosus patients with intestinal pseudo-obstruction and/or ureterohydronephrosis: a retrospective observational study. Medicine (Baltimore). 2015;94(4):e419.
27. Vergara-Fernandez O, Zeron-Medina J, Mendez-Probst C, et al. Acute abdominal pain in patients with systemic lupus erythematosus. J Gastrointest Surg. 2009;13(7):1351-1357. doi:10.1007/s11605-009-0897-4.
28. Küçükşahin O, Düzgün N, Okoh AK, Kulahçioglu E. Response to rituximab in a case of lupus associated digital ischemia. Case Rep Rheumatol. 2014;2014:763608. doi:10.1155/2014/763608.
29. Uva L, Miguel D, Pinheiro C, Freitas JP, Gomes MM, Filipe P. Cutaneous manifestations of systemic lupus erythematosus. Autoimmune Dis. 2012;2012:834291. doi:10.1155/2012/834291.
1. Dall’Era M, Wofsy D. Clinical Features of Systemic Lupus Erythematosus. In: Firestein GS et al, eds. Kelley and Firestein’s Textbook of Rheumatology. 10th ed. Philadelphia, PA: Elsevier; 2017.
2. Dvorkina O, Ginzler EM. Clinical features of systemic lupus erythematosus. In: Hochberg MC, ed. Rheumatology. 6th ed. Philadelphia, PA: Elsevier; 2015.
3. Huang JL, Hung JJ, Wu KC, Lee WI, Chan CK, Ou LS. Septic arthritis in patients with systemic lupus erythematosus: salmonella and nonsalmonella infections compared. Semin Arthritis Rheumatol. 2006;36(1):61-67. doi:10.1016/j.semarthrit.2006.04.003
4. Barile-Fabris L, Hernández-Cabrera MF, Barragan-Garfias JA. Vasculitis in systemic lupus erythematosus. Curr Rheumatol Rep. 2014;16(9):440. doi:10.1007/s11926-014-0440-9.
5. Campion EW, Wigley FM, Flavahan NA. Raynaud’s phenomenon. N Engl J Med. 2016;375(6):556-565. doi:10.1056/NEJMra1507638.
6. Bouaziz JD, Barete S, Le Pelletier F, et al. Cutaneous lesions of the digits in systemic lupus erythematosus: 50 cases. Lupus. 2007;16(3):163-167.
7. Pokroy-Shapira E, Gelernter I, Molad Y. Evolution of chronic kidney disease in patients with systemic lupus erythematosus over a long-period follow-up: a single-center inception cohort study. Clin Rheumatol. 2014;33(5):649-657. doi:10.1007/s10067-014-2527-0.
8. Almaani S, Meara A, Rovin BH. Update on lupus nephritis. Clin J Am Soc Nephrol. 2017;12(5):825-835. doi:10.2215/CJN.05780616.
9. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheumatol. 1999;42(4):599-608.
10. Oomatia A, Fang H, Petri M, et al. Peripheral neuropathies in systemic lupus erythematosus: clinical features, disease associations, and immunologic characteristics evaluated over a twenty-five year study period. Arthritis Rheumatol. 2014;66(4):1000-1009.
11. Mitsikostas DD, Sfikakis PP, Goadsby PJ. A meta-analysis for headache in systemic lupus erythematosus: the evidence and the myth. Brain. 2004;127(pt 5):1200-1209.
12. Timlin H, Petri M. Transient ischemic attack and stroke in systemic lupus erythematosus. Lupus. 2013;22(12):1251-1258. doi:10.1177/0961203313497416.
13. Majdak MR, Vuletić V. Thrombolysis for acute stroke in patient with systemic lupus erythematosus: a case report. J Neurol Sci. 2016;(361):7-8. doi:10.1016/j.jns.2015.12.014.
14. Chen WL, Chang SH, Chen JH, Wu YL. Isolated headache as the sole manifestation of dural sinus thrombosis: a case report with literature review. Am J Emerg Med. 2007;25(2):218-219.
15. Arntfield RT, Hicks CM. Systemic Lupus Erythematosus and the Vasculitides. In: Marx JA, ed. Rosen’s Emergency Medicine. 8th ed. Philadelphia, PA: Elsevier Saunders; 2014.
16. Borrell H, Narváez J, Alegree JJ, et al. Shrinking lung syndrome in systemic lupus erythematosus: a case series and review of the literature. Medicine (Baltimore). 2016;95(33):e4626. doi:10.1097/MD.0000000000004626.
17. Aviña-Zubieta JA, Vostretsova K, De Vera MA, et al. The risk of pulmonary embolism and deep venous thrombosis in systemic lupus erythematosus: a general population-based study. Semin Arthritis Rheum. 2015;45(2):195-201. doi:10.1016/j.semarthrit.2015.05.008.
18. Martínez-Martínez MU, Abud-Mendoza C. Predictors of mortality in diffuse alveolar haemorrhage associated with systemic lupus erythematosus. Lupus. 2011;20(6):568-574. doi:10.1177/0961203310392430.
19. Rosenbaum E, Krebs E, Cohen M, Tiliakos A, Derk CT. The spectrum of clinical manifestations, outcome and treatment of pericardial tamponade in patients with systemic lupus erythematosus: a retrospective study and literature review. Lupus. 2009;18(7):608-612. doi:10.1177/0961203308100659.
20. Miner JJ, Kim AH. Cardiac manifestations of systemic lupus erythematosus. Rheum Dis Clin North Am. 2014;40(1):51-60. doi:10.1016/j.rdc.2013.10.003.
21. Morel N, Bonjour M, Le Guern V, et al. Colchicine: a simple and effective treatment for pericarditis in systemic lupus erythematosus? A report of 10 cases. Lupus. 2015;24(14):1479-1485. doi:10.1177/0961203315593169.
22. Roldan CA, Shively BK, Crawford MH. An echocardiographic study of valvular heart disease associated with systemic lupus erythematosus. N Engl J Med. 1996;335(19):1424-1430.
23. Dufrost V, Risse J, et al. Direct oral anticoagulants use in antiphospholipid syndrome: are these drugs an effective and safe alternative to warfarin? A systematic review of the literature. Curr Rheumatol Rep. 2016;18(12):74. doi:10.1007/s11926-016-0623-7.
24. Cervera R,Rodríguez-Pintó I; G Espinosa on behalf of the Task Force on Catastrophic Antiphospholipid Syndrome. Catastrophic antiphospholipid syndrome: task force report summary. Lupus. 2014;23(12):1283-1285. doi:10.1177/0961203314540764.
25. Sultan SM, Ioannou Y, Isenberg DA. A review of gastrointestinal manifestations of systemic lupus erythematosus. Rheumatology (Oxford). 1999;38(10):917-932.
26. Xu N, Zhao J, Liu J, et al. Clinical analysis of 61 systemic lupus erythematosus patients with intestinal pseudo-obstruction and/or ureterohydronephrosis: a retrospective observational study. Medicine (Baltimore). 2015;94(4):e419.
27. Vergara-Fernandez O, Zeron-Medina J, Mendez-Probst C, et al. Acute abdominal pain in patients with systemic lupus erythematosus. J Gastrointest Surg. 2009;13(7):1351-1357. doi:10.1007/s11605-009-0897-4.
28. Küçükşahin O, Düzgün N, Okoh AK, Kulahçioglu E. Response to rituximab in a case of lupus associated digital ischemia. Case Rep Rheumatol. 2014;2014:763608. doi:10.1155/2014/763608.
29. Uva L, Miguel D, Pinheiro C, Freitas JP, Gomes MM, Filipe P. Cutaneous manifestations of systemic lupus erythematosus. Autoimmune Dis. 2012;2012:834291. doi:10.1155/2012/834291.
Implementing enhanced recovery protocols for gynecologic surgery
“Enhanced Recovery After Surgery” (ERAS) practices and protocols have been increasingly refined and adopted for the field of gynecology, and there is hope among gynecologic surgeons – and some recent evidence – that, with the ERAS movement, we are improving patient recoveries and outcomes and minimizing the need for opioids.
This applies not only to open surgeries but also to the minimally invasive procedures that already are prized for significant reductions in morbidity and length of stay. The overarching and guiding principle of ERAS is that any surgery – whether open or minimally invasive, major or minor – places stress on the body and is associated with risks and morbidity.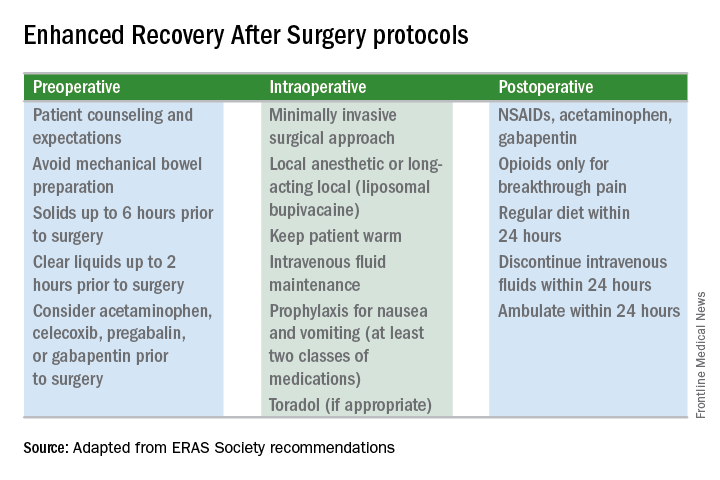
Enhanced recovery protocols are multidisciplinary, perioperative approaches designed to lessen the body’s stress response to surgery. The protocols and pathways offer us a menu of small changes that, in the aggregate, can lead to large and demonstrable benefits – especially when these small changes are chosen across the preoperative, intraoperative, and postoperative arenas and then standardized in one’s practice. Among the major components of ERAS practices and protocols are limiting preoperative fasting, employing multimodal analgesia, encouraging early ambulation and early postsurgical feeding, and creating culture shift that includes greater emphasis on patient expectations.
In our practice, we are incorporating ERAS practices not only in hopes of reducing the stress of all surgeries before, during, and after, but also with the goal of achieving a postoperative opioid-free hysterectomy, myomectomy, and extensive endometriosis surgery. (All of our advanced procedures are performed laparoscopically or robotically.)
Over the past 7 or so years, we have adopted a multimodal approach to pain control that includes a bundle of preoperative analgesics – acetaminophen, pregabalin, and celecoxib (we call it “TLC” for Tylenol, Lyrica, and Celebrex) – and the use of liposomal bupivacaine in our robotic surgeries. We are now turning toward ERAS nutritional changes, most of which run counter to traditional paradigms for surgical care. And in other areas, such as dedicated preoperative counseling, we continue to refine and improve our practices.
Improved Outcomes
The ERAS mindset notably intersected gynecology with the publication in 2016 of a two-part series of guidelines for gynecology/oncology surgery from the ERAS Society. The 8-year-old society has its roots in a study group of European surgeons and others who decided to examine surgical practices and the concept of multimodal surgical care put forth in the 1990s by Henrik Kehlet, MD, PhD, then a professor at the University of Copenhagen.
The first set of recommendations addressed pre- and intraoperative care (Gynecol Oncol. 2016 Feb;140[2]:313-22), and the second set addressed postoperative care (Gynecol Oncol. 2016 Feb;140[2]:323-32). Similar evidence-based recommendations were previously written for colonic resections, rectal and pelvic surgery, and other surgical specialties.
Most of the published outcomes of enhanced recovery protocols come from colorectal surgery. As noted in the ERAS Society gynecology/oncology guidelines, the benefits include an average reduction in length of stay of 2.5 days and a decrease in complications by as much as 50%.
There is growing evidence, however, that ERAS programs are also beneficial for patients undergoing laparoscopic surgery, and outcomes from gynecology – including minimally invasive surgery – are also being reported.
For instance, a retrospective case-control study of 55 consecutive gynecologic oncology patients treated at the University of California, San Francisco, with laparoscopic or robotic surgery and an enhanced recovery pathway – and 110 historical control patients matched on the basis of age and surgery type – found significant improvements in recovery time, decreased pain despite reduced opioid use, and overall lower hospital costs (Obstet Gynecol. 2016 Jul;128[1]:138-44).
The enhanced recovery pathway included patient education, multimodal antiemetics, multimodal analgesia, and balanced fluid administration. Early catheter removal, ambulation, and feeding were also components. Analgesia included routine preoperative gabapentin, diclofenac, and acetaminophen; routine postoperative gabapentin, NSAIDs, and acetaminophen; and transversus abdominis plane blocks in 32 of the ERAS patients.
ERAS patients were significantly more likely to be discharged on day 1 (91%, compared with 60% in the control group). Opioid use decreased by 30%, and pain scores on postoperative day 1 were significantly lower.
Another study looking at the effect of enhanced recovery implementation in gynecologic surgeries at the University of Virginia, Charlottesville, (gynecologic oncology, urogynecology, and general gynecology) similarly reported benefits for vaginal and minimally invasive procedures, as well as for open procedures (Obstet Gynecol. 2016 Sep;128[3]:457-66).
In the minimally invasive group, investigators compared 324 patients before ERAS implementation with 249 patients afterward and found that the median length of stay was unchanged (1 day). However, intraoperative and postoperative opioid consumption decreased significantly and – even though actual pain scores improved only slightly – patient satisfaction scores improved markedly among post-ERAS patients. Patients gave higher marks, for instance, to questions regarding pain control (“how well your pain was controlled”) and teamwork (“staff worked together to care for you”).
Reducing Opioids
New opioid use that persists after a surgical procedure is a postsurgical complication that we all should be working to prevent. It has been estimated that 6% of surgical patients – even those who’ve had relatively minor surgical procedures – will become long-term opioid users, developing a dependence on the drugs prescribed to them for postsurgical pain.
This was shown last year in a national study of insurance claims data from between 2013 and 2014; investigators identified adults without opioid use in the year prior to surgery (including hysterectomy) and found that 5.9%-6.5% were filling opioid prescriptions 90-180 days after their surgical procedure. The incidence in a nonoperative control cohort was 0.4% (JAMA Surg. 2017 Jun 21;152[6]:e170504). Notably, this prolonged use was greatest in patients with prior pain conditions, substance abuse, and mental health disorders – a finding that may have implications for the counseling we provide prior to surgery.
It’s not clear what the optimal analgesic regimen is for minimally invasive or other gynecologic surgeries. What is clearly recommended, however, is that the approach be multifaceted. In our practice, we believe that the preoperative use of acetaminophen, pregabalin, and celecoxib plays an important role in reducing postoperative pain and opioid use. But we also have striven to create a practice-wide culture shift (throughout the operating and recovery rooms), for instance, that encourages using the least amounts of narcotics possible and using the shortest-acting formulations possible.
Transversus abdominis plane (TAP) blocks are also often part of ERAS protocols; they have been shown in at least two randomized controlled trials of abdominal hysterectomy to reduce intraoperative fentanyl requirements and to reduce immediate postoperative pain scores and postoperative morphine requirements (Anesth Analg. 2008 Dec;107[6]:2056-60; J Anaesthesiol Clin Pharmacol. 2014 Jul-Sep;30[3]:391-6).
More recently, liposomal bupivacaine, which is slowly released over several days, has gained traction as a substitute for standard bupivacaine and other agents in TAP blocks. In one recent retrospective study, abdominal incision infiltration with liposomal bupivacaine was associated with less opioid use (with no change in pain scores), compared with bupivacaine hydrochloride after laparotomy for gynecologic malignancies (Obstet Gynecol. 2016 Nov;128[5]:1009-17). It’s significantly more expensive, however, making it likely that the formulation is being used more judiciously in minimally invasive gynecologic surgery than in open surgeries.
Because of costs, we currently are restricted to using liposomal bupivacaine in our robotic surgeries only. In our practice, the single 20 mL vial (266 mg of liposomal bupivacaine) is diluted with 20 mL of normal saline, but it can be further diluted without loss of efficacy. With a 16-gauge needle, the liposomal bupivacaine is distributed across the incisions (usually 20 mL in the umbilicus with a larger incision and 10 mL in each of the two lateral incisions). Patients are counseled that they may have more discomfort after 3 days, but by this point most are mobile and feeling relatively well with a combination of NSAIDs and acetaminophen.
With growing visibility of the problem of narcotic dependence in the United States, patients seem increasingly receptive and even eager to limit or avoid the use of opioids. Patients should be counseled that minimizing or avoiding opioids may also speed recovery. Narcotics cause gut motility to slow down, which may hinder mobilization. Early mobilization (within 24 hours) is among the enhanced recovery elements that the ERAS Society guidelines say is “of particular value” for minimally invasive surgery, along with maintenance of normothermia and normovolemia with maintenance of adequate cardiac output.
Selecting Steps
Our practice is also trying to reduce preoperative bowel preparation and preoperative fasting, both of which have been found to be stressful for the body without evidence of benefit. These practices can lead to insulin resistance and hyperglycemia, which are associated with increased morbidity and length of stay.
It is now recommended that clear fluids be allowed up to 2 hours before surgery and solids up to 6 hours before. Some health systems and practices also recommend presurgical carbohydrate loading (for example, 10 ounces of apple juice 2 hours before surgery) – another small change on the ERAS menu – to further reduce postoperative insulin resistance and help the body cope with its stress response to surgery.
Along with nutritional changes are also various measures aimed at optimizing the body’s functionality before surgery (“prehabilitation”), from walking 30 minutes a day to abstaining from alcohol for patients who drink heavily.
Throughout the country, enhanced recovery protocols are taking shape in gynecologic surgery. ERAS was featured in an aptly titled panel session at the 2017 annual meeting of the American Association of Gynecologic Laparoscopists: “Outpatient Hysterectomy, ERAS, and Same-Day Discharge: The Next Big Thing in Gyn Surgery.” Others are applying ERAS to scheduled cesarean sections. And in our practice, I believe that if we continue making small changes, we will reach our goal of opioid-free recoveries and a better surgical experience for our patients.
Kirsten Sasaki, MD, is an associate of the Advanced Gynecologic Surgery Institute. She reported that she has no disclosures relevant to this Master Class.
“Enhanced Recovery After Surgery” (ERAS) practices and protocols have been increasingly refined and adopted for the field of gynecology, and there is hope among gynecologic surgeons – and some recent evidence – that, with the ERAS movement, we are improving patient recoveries and outcomes and minimizing the need for opioids.
This applies not only to open surgeries but also to the minimally invasive procedures that already are prized for significant reductions in morbidity and length of stay. The overarching and guiding principle of ERAS is that any surgery – whether open or minimally invasive, major or minor – places stress on the body and is associated with risks and morbidity.
Enhanced recovery protocols are multidisciplinary, perioperative approaches designed to lessen the body’s stress response to surgery. The protocols and pathways offer us a menu of small changes that, in the aggregate, can lead to large and demonstrable benefits – especially when these small changes are chosen across the preoperative, intraoperative, and postoperative arenas and then standardized in one’s practice. Among the major components of ERAS practices and protocols are limiting preoperative fasting, employing multimodal analgesia, encouraging early ambulation and early postsurgical feeding, and creating culture shift that includes greater emphasis on patient expectations.
In our practice, we are incorporating ERAS practices not only in hopes of reducing the stress of all surgeries before, during, and after, but also with the goal of achieving a postoperative opioid-free hysterectomy, myomectomy, and extensive endometriosis surgery. (All of our advanced procedures are performed laparoscopically or robotically.)
Over the past 7 or so years, we have adopted a multimodal approach to pain control that includes a bundle of preoperative analgesics – acetaminophen, pregabalin, and celecoxib (we call it “TLC” for Tylenol, Lyrica, and Celebrex) – and the use of liposomal bupivacaine in our robotic surgeries. We are now turning toward ERAS nutritional changes, most of which run counter to traditional paradigms for surgical care. And in other areas, such as dedicated preoperative counseling, we continue to refine and improve our practices.
Improved Outcomes
The ERAS mindset notably intersected gynecology with the publication in 2016 of a two-part series of guidelines for gynecology/oncology surgery from the ERAS Society. The 8-year-old society has its roots in a study group of European surgeons and others who decided to examine surgical practices and the concept of multimodal surgical care put forth in the 1990s by Henrik Kehlet, MD, PhD, then a professor at the University of Copenhagen.
The first set of recommendations addressed pre- and intraoperative care (Gynecol Oncol. 2016 Feb;140[2]:313-22), and the second set addressed postoperative care (Gynecol Oncol. 2016 Feb;140[2]:323-32). Similar evidence-based recommendations were previously written for colonic resections, rectal and pelvic surgery, and other surgical specialties.
Most of the published outcomes of enhanced recovery protocols come from colorectal surgery. As noted in the ERAS Society gynecology/oncology guidelines, the benefits include an average reduction in length of stay of 2.5 days and a decrease in complications by as much as 50%.
There is growing evidence, however, that ERAS programs are also beneficial for patients undergoing laparoscopic surgery, and outcomes from gynecology – including minimally invasive surgery – are also being reported.
For instance, a retrospective case-control study of 55 consecutive gynecologic oncology patients treated at the University of California, San Francisco, with laparoscopic or robotic surgery and an enhanced recovery pathway – and 110 historical control patients matched on the basis of age and surgery type – found significant improvements in recovery time, decreased pain despite reduced opioid use, and overall lower hospital costs (Obstet Gynecol. 2016 Jul;128[1]:138-44).
The enhanced recovery pathway included patient education, multimodal antiemetics, multimodal analgesia, and balanced fluid administration. Early catheter removal, ambulation, and feeding were also components. Analgesia included routine preoperative gabapentin, diclofenac, and acetaminophen; routine postoperative gabapentin, NSAIDs, and acetaminophen; and transversus abdominis plane blocks in 32 of the ERAS patients.
ERAS patients were significantly more likely to be discharged on day 1 (91%, compared with 60% in the control group). Opioid use decreased by 30%, and pain scores on postoperative day 1 were significantly lower.
Another study looking at the effect of enhanced recovery implementation in gynecologic surgeries at the University of Virginia, Charlottesville, (gynecologic oncology, urogynecology, and general gynecology) similarly reported benefits for vaginal and minimally invasive procedures, as well as for open procedures (Obstet Gynecol. 2016 Sep;128[3]:457-66).
In the minimally invasive group, investigators compared 324 patients before ERAS implementation with 249 patients afterward and found that the median length of stay was unchanged (1 day). However, intraoperative and postoperative opioid consumption decreased significantly and – even though actual pain scores improved only slightly – patient satisfaction scores improved markedly among post-ERAS patients. Patients gave higher marks, for instance, to questions regarding pain control (“how well your pain was controlled”) and teamwork (“staff worked together to care for you”).
Reducing Opioids
New opioid use that persists after a surgical procedure is a postsurgical complication that we all should be working to prevent. It has been estimated that 6% of surgical patients – even those who’ve had relatively minor surgical procedures – will become long-term opioid users, developing a dependence on the drugs prescribed to them for postsurgical pain.
This was shown last year in a national study of insurance claims data from between 2013 and 2014; investigators identified adults without opioid use in the year prior to surgery (including hysterectomy) and found that 5.9%-6.5% were filling opioid prescriptions 90-180 days after their surgical procedure. The incidence in a nonoperative control cohort was 0.4% (JAMA Surg. 2017 Jun 21;152[6]:e170504). Notably, this prolonged use was greatest in patients with prior pain conditions, substance abuse, and mental health disorders – a finding that may have implications for the counseling we provide prior to surgery.
It’s not clear what the optimal analgesic regimen is for minimally invasive or other gynecologic surgeries. What is clearly recommended, however, is that the approach be multifaceted. In our practice, we believe that the preoperative use of acetaminophen, pregabalin, and celecoxib plays an important role in reducing postoperative pain and opioid use. But we also have striven to create a practice-wide culture shift (throughout the operating and recovery rooms), for instance, that encourages using the least amounts of narcotics possible and using the shortest-acting formulations possible.
Transversus abdominis plane (TAP) blocks are also often part of ERAS protocols; they have been shown in at least two randomized controlled trials of abdominal hysterectomy to reduce intraoperative fentanyl requirements and to reduce immediate postoperative pain scores and postoperative morphine requirements (Anesth Analg. 2008 Dec;107[6]:2056-60; J Anaesthesiol Clin Pharmacol. 2014 Jul-Sep;30[3]:391-6).
More recently, liposomal bupivacaine, which is slowly released over several days, has gained traction as a substitute for standard bupivacaine and other agents in TAP blocks. In one recent retrospective study, abdominal incision infiltration with liposomal bupivacaine was associated with less opioid use (with no change in pain scores), compared with bupivacaine hydrochloride after laparotomy for gynecologic malignancies (Obstet Gynecol. 2016 Nov;128[5]:1009-17). It’s significantly more expensive, however, making it likely that the formulation is being used more judiciously in minimally invasive gynecologic surgery than in open surgeries.
Because of costs, we currently are restricted to using liposomal bupivacaine in our robotic surgeries only. In our practice, the single 20 mL vial (266 mg of liposomal bupivacaine) is diluted with 20 mL of normal saline, but it can be further diluted without loss of efficacy. With a 16-gauge needle, the liposomal bupivacaine is distributed across the incisions (usually 20 mL in the umbilicus with a larger incision and 10 mL in each of the two lateral incisions). Patients are counseled that they may have more discomfort after 3 days, but by this point most are mobile and feeling relatively well with a combination of NSAIDs and acetaminophen.
With growing visibility of the problem of narcotic dependence in the United States, patients seem increasingly receptive and even eager to limit or avoid the use of opioids. Patients should be counseled that minimizing or avoiding opioids may also speed recovery. Narcotics cause gut motility to slow down, which may hinder mobilization. Early mobilization (within 24 hours) is among the enhanced recovery elements that the ERAS Society guidelines say is “of particular value” for minimally invasive surgery, along with maintenance of normothermia and normovolemia with maintenance of adequate cardiac output.
Selecting Steps
Our practice is also trying to reduce preoperative bowel preparation and preoperative fasting, both of which have been found to be stressful for the body without evidence of benefit. These practices can lead to insulin resistance and hyperglycemia, which are associated with increased morbidity and length of stay.
It is now recommended that clear fluids be allowed up to 2 hours before surgery and solids up to 6 hours before. Some health systems and practices also recommend presurgical carbohydrate loading (for example, 10 ounces of apple juice 2 hours before surgery) – another small change on the ERAS menu – to further reduce postoperative insulin resistance and help the body cope with its stress response to surgery.
Along with nutritional changes are also various measures aimed at optimizing the body’s functionality before surgery (“prehabilitation”), from walking 30 minutes a day to abstaining from alcohol for patients who drink heavily.
Throughout the country, enhanced recovery protocols are taking shape in gynecologic surgery. ERAS was featured in an aptly titled panel session at the 2017 annual meeting of the American Association of Gynecologic Laparoscopists: “Outpatient Hysterectomy, ERAS, and Same-Day Discharge: The Next Big Thing in Gyn Surgery.” Others are applying ERAS to scheduled cesarean sections. And in our practice, I believe that if we continue making small changes, we will reach our goal of opioid-free recoveries and a better surgical experience for our patients.
Kirsten Sasaki, MD, is an associate of the Advanced Gynecologic Surgery Institute. She reported that she has no disclosures relevant to this Master Class.
“Enhanced Recovery After Surgery” (ERAS) practices and protocols have been increasingly refined and adopted for the field of gynecology, and there is hope among gynecologic surgeons – and some recent evidence – that, with the ERAS movement, we are improving patient recoveries and outcomes and minimizing the need for opioids.
This applies not only to open surgeries but also to the minimally invasive procedures that already are prized for significant reductions in morbidity and length of stay. The overarching and guiding principle of ERAS is that any surgery – whether open or minimally invasive, major or minor – places stress on the body and is associated with risks and morbidity.
Enhanced recovery protocols are multidisciplinary, perioperative approaches designed to lessen the body’s stress response to surgery. The protocols and pathways offer us a menu of small changes that, in the aggregate, can lead to large and demonstrable benefits – especially when these small changes are chosen across the preoperative, intraoperative, and postoperative arenas and then standardized in one’s practice. Among the major components of ERAS practices and protocols are limiting preoperative fasting, employing multimodal analgesia, encouraging early ambulation and early postsurgical feeding, and creating culture shift that includes greater emphasis on patient expectations.
In our practice, we are incorporating ERAS practices not only in hopes of reducing the stress of all surgeries before, during, and after, but also with the goal of achieving a postoperative opioid-free hysterectomy, myomectomy, and extensive endometriosis surgery. (All of our advanced procedures are performed laparoscopically or robotically.)
Over the past 7 or so years, we have adopted a multimodal approach to pain control that includes a bundle of preoperative analgesics – acetaminophen, pregabalin, and celecoxib (we call it “TLC” for Tylenol, Lyrica, and Celebrex) – and the use of liposomal bupivacaine in our robotic surgeries. We are now turning toward ERAS nutritional changes, most of which run counter to traditional paradigms for surgical care. And in other areas, such as dedicated preoperative counseling, we continue to refine and improve our practices.
Improved Outcomes
The ERAS mindset notably intersected gynecology with the publication in 2016 of a two-part series of guidelines for gynecology/oncology surgery from the ERAS Society. The 8-year-old society has its roots in a study group of European surgeons and others who decided to examine surgical practices and the concept of multimodal surgical care put forth in the 1990s by Henrik Kehlet, MD, PhD, then a professor at the University of Copenhagen.
The first set of recommendations addressed pre- and intraoperative care (Gynecol Oncol. 2016 Feb;140[2]:313-22), and the second set addressed postoperative care (Gynecol Oncol. 2016 Feb;140[2]:323-32). Similar evidence-based recommendations were previously written for colonic resections, rectal and pelvic surgery, and other surgical specialties.
Most of the published outcomes of enhanced recovery protocols come from colorectal surgery. As noted in the ERAS Society gynecology/oncology guidelines, the benefits include an average reduction in length of stay of 2.5 days and a decrease in complications by as much as 50%.
There is growing evidence, however, that ERAS programs are also beneficial for patients undergoing laparoscopic surgery, and outcomes from gynecology – including minimally invasive surgery – are also being reported.
For instance, a retrospective case-control study of 55 consecutive gynecologic oncology patients treated at the University of California, San Francisco, with laparoscopic or robotic surgery and an enhanced recovery pathway – and 110 historical control patients matched on the basis of age and surgery type – found significant improvements in recovery time, decreased pain despite reduced opioid use, and overall lower hospital costs (Obstet Gynecol. 2016 Jul;128[1]:138-44).
The enhanced recovery pathway included patient education, multimodal antiemetics, multimodal analgesia, and balanced fluid administration. Early catheter removal, ambulation, and feeding were also components. Analgesia included routine preoperative gabapentin, diclofenac, and acetaminophen; routine postoperative gabapentin, NSAIDs, and acetaminophen; and transversus abdominis plane blocks in 32 of the ERAS patients.
ERAS patients were significantly more likely to be discharged on day 1 (91%, compared with 60% in the control group). Opioid use decreased by 30%, and pain scores on postoperative day 1 were significantly lower.
Another study looking at the effect of enhanced recovery implementation in gynecologic surgeries at the University of Virginia, Charlottesville, (gynecologic oncology, urogynecology, and general gynecology) similarly reported benefits for vaginal and minimally invasive procedures, as well as for open procedures (Obstet Gynecol. 2016 Sep;128[3]:457-66).
In the minimally invasive group, investigators compared 324 patients before ERAS implementation with 249 patients afterward and found that the median length of stay was unchanged (1 day). However, intraoperative and postoperative opioid consumption decreased significantly and – even though actual pain scores improved only slightly – patient satisfaction scores improved markedly among post-ERAS patients. Patients gave higher marks, for instance, to questions regarding pain control (“how well your pain was controlled”) and teamwork (“staff worked together to care for you”).
Reducing Opioids
New opioid use that persists after a surgical procedure is a postsurgical complication that we all should be working to prevent. It has been estimated that 6% of surgical patients – even those who’ve had relatively minor surgical procedures – will become long-term opioid users, developing a dependence on the drugs prescribed to them for postsurgical pain.
This was shown last year in a national study of insurance claims data from between 2013 and 2014; investigators identified adults without opioid use in the year prior to surgery (including hysterectomy) and found that 5.9%-6.5% were filling opioid prescriptions 90-180 days after their surgical procedure. The incidence in a nonoperative control cohort was 0.4% (JAMA Surg. 2017 Jun 21;152[6]:e170504). Notably, this prolonged use was greatest in patients with prior pain conditions, substance abuse, and mental health disorders – a finding that may have implications for the counseling we provide prior to surgery.
It’s not clear what the optimal analgesic regimen is for minimally invasive or other gynecologic surgeries. What is clearly recommended, however, is that the approach be multifaceted. In our practice, we believe that the preoperative use of acetaminophen, pregabalin, and celecoxib plays an important role in reducing postoperative pain and opioid use. But we also have striven to create a practice-wide culture shift (throughout the operating and recovery rooms), for instance, that encourages using the least amounts of narcotics possible and using the shortest-acting formulations possible.
Transversus abdominis plane (TAP) blocks are also often part of ERAS protocols; they have been shown in at least two randomized controlled trials of abdominal hysterectomy to reduce intraoperative fentanyl requirements and to reduce immediate postoperative pain scores and postoperative morphine requirements (Anesth Analg. 2008 Dec;107[6]:2056-60; J Anaesthesiol Clin Pharmacol. 2014 Jul-Sep;30[3]:391-6).
More recently, liposomal bupivacaine, which is slowly released over several days, has gained traction as a substitute for standard bupivacaine and other agents in TAP blocks. In one recent retrospective study, abdominal incision infiltration with liposomal bupivacaine was associated with less opioid use (with no change in pain scores), compared with bupivacaine hydrochloride after laparotomy for gynecologic malignancies (Obstet Gynecol. 2016 Nov;128[5]:1009-17). It’s significantly more expensive, however, making it likely that the formulation is being used more judiciously in minimally invasive gynecologic surgery than in open surgeries.
Because of costs, we currently are restricted to using liposomal bupivacaine in our robotic surgeries only. In our practice, the single 20 mL vial (266 mg of liposomal bupivacaine) is diluted with 20 mL of normal saline, but it can be further diluted without loss of efficacy. With a 16-gauge needle, the liposomal bupivacaine is distributed across the incisions (usually 20 mL in the umbilicus with a larger incision and 10 mL in each of the two lateral incisions). Patients are counseled that they may have more discomfort after 3 days, but by this point most are mobile and feeling relatively well with a combination of NSAIDs and acetaminophen.
With growing visibility of the problem of narcotic dependence in the United States, patients seem increasingly receptive and even eager to limit or avoid the use of opioids. Patients should be counseled that minimizing or avoiding opioids may also speed recovery. Narcotics cause gut motility to slow down, which may hinder mobilization. Early mobilization (within 24 hours) is among the enhanced recovery elements that the ERAS Society guidelines say is “of particular value” for minimally invasive surgery, along with maintenance of normothermia and normovolemia with maintenance of adequate cardiac output.
Selecting Steps
Our practice is also trying to reduce preoperative bowel preparation and preoperative fasting, both of which have been found to be stressful for the body without evidence of benefit. These practices can lead to insulin resistance and hyperglycemia, which are associated with increased morbidity and length of stay.
It is now recommended that clear fluids be allowed up to 2 hours before surgery and solids up to 6 hours before. Some health systems and practices also recommend presurgical carbohydrate loading (for example, 10 ounces of apple juice 2 hours before surgery) – another small change on the ERAS menu – to further reduce postoperative insulin resistance and help the body cope with its stress response to surgery.
Along with nutritional changes are also various measures aimed at optimizing the body’s functionality before surgery (“prehabilitation”), from walking 30 minutes a day to abstaining from alcohol for patients who drink heavily.
Throughout the country, enhanced recovery protocols are taking shape in gynecologic surgery. ERAS was featured in an aptly titled panel session at the 2017 annual meeting of the American Association of Gynecologic Laparoscopists: “Outpatient Hysterectomy, ERAS, and Same-Day Discharge: The Next Big Thing in Gyn Surgery.” Others are applying ERAS to scheduled cesarean sections. And in our practice, I believe that if we continue making small changes, we will reach our goal of opioid-free recoveries and a better surgical experience for our patients.
Kirsten Sasaki, MD, is an associate of the Advanced Gynecologic Surgery Institute. She reported that she has no disclosures relevant to this Master Class.
In the Evolving Mystery of BV, an Innovative Oral Treatment Emerges
Click Here to Read the Supplement
Topics include:
- BV terminology and treatment over time
- Current understanding of BV etiology
- BV consequences
- BV treatments
- Future research needs in BV
Author:
Steven E. Chavoustie, MD, FACOG, CCRP
University of Miami
Miller School of Medicine
Miami, Florida
Click Here to Read the Supplement
Topics include:
- BV terminology and treatment over time
- Current understanding of BV etiology
- BV consequences
- BV treatments
- Future research needs in BV
Author:
Steven E. Chavoustie, MD, FACOG, CCRP
University of Miami
Miller School of Medicine
Miami, Florida
Click Here to Read the Supplement
Topics include:
- BV terminology and treatment over time
- Current understanding of BV etiology
- BV consequences
- BV treatments
- Future research needs in BV
Author:
Steven E. Chavoustie, MD, FACOG, CCRP
University of Miami
Miller School of Medicine
Miami, Florida
Best practices address latest trends in PDT, skin cancer treatment
MIAMI – Pearls for providers of photodynamic therapy (PDT) include tips on skin preparation, eye protection, and use of three new codes to maximize reimbursement. Also trending in medical dermatology are best practices for intralesional injections of 5-FU to treat the often challenging isomorphic squamous cell carcinomas (SCCs) or keratoacanthomas on the lower leg, as well as use of neoadjuvant hedgehog inhibitors to shrink large skin cancer lesions, according to Glenn David Goldman, MD.
“This talk is about what you can do medically as a dermatologic surgeon,” Dr. Goldman said at the Orlando Dermatology Aesthetic and Clinical Conference.
Use new billing codes for photodynamic therapy
There are now three new PDT billing codes. “Make sure your coders are using these properly. They are active now, and if you don’t use them, you won’t get paid properly,” said Dr. Goldman, professor and medical director of dermatology at the University of Vermont, Burlington. Specifically, 96567 is for standard PDT applied by staff; 96573 is for PDT applied by a physician; and 96574 is for PDT and curettage performed by a physician.
“Be involved, don’t delegate,” Dr. Goldman added. “If you do, you will get paid half as much as you used to, which means you will lose money on every single patient you treat.”
What type of PDT physicians choose to use in their practice remains controversial. “Do you do short-contact PDT, do you do daylight PDT? We’ve gone back and forth in our practice,” Dr. Goldman said. “I’m not impressed with daylight PDT. I know this is at odds with some of the people here, but at least in Vermont, it doesn’t work very well.”
The way PDT was described in the original trials (a photosensitizer applied in the office followed by PDT) “works the best, with one caveat,” Dr. Goldman said. The caveat is that dermatologists should aim for a PDT clearance that approaches the efficacy of 5-fluorouracil (5-FU). “If you can get to that – which is difficult by the way – I think your patients will really appreciate this.”
An additional PDT pearl Dr. Goldman shared involves skin preparation: the use of acetone to defat the skin, even in patients with very thick lesions. Apply acetone with gauze to the site for 5 minutes and “all of that hyperkeratosis just wipes away,” curette off any residual hyperkeratosis – and consider a ring anesthetic block to control pain for the patient with severe disease, he advised.
Another tip is to forgo the goggles that come with most PDT kits. Instead, purchase smaller, disposable laser eye shields for PDT patients, Dr. Goldman said. “They work better. You can get closer to the eye … and they are more comfortable for the patient.”
Dr. Goldman’s practice is providing more PDT and much less 5-FU for patient convenience. “I believe if someone is willing to go through 3 weeks of 5-FU or 12-16 weeks of imiquimod, they get the best results. However, most people don’t want to do that if they can sit in front of a light for 15 minutes.”
Consider intralesional injections for SCCs and KAs on the legs
An ongoing challenge in medical dermatology is preventing rapid recurrence of SCCs and/or keratoacanthomas (KAs) near sites of previous excision on the legs. “We all see this quite a bit. Often you get lesions on the leg, you cut them out, and they come right back” close to the excision site, Dr. Goldman said.
He does not recommend methotrexate injections for these lesions. “Methotrexate does not work. It doesn’t hurt, but I’ve injected methotrexate into squamous cell carcinomas many times and they’ve never gone away.” In contrast, 5-FU “works incredibly well. They go away, I’ve had tremendous success. This has changed the way we treat these lesions.” 5-FU is inexpensive and can be obtained from oncology pharmacies. One caveat is 5-FU injections can be painful and patients require anesthesia prior to injection.
Using a 25-gauge or 27-gauge needle, Dr. Goldman injects 5-FU “exactly as I would a hypertrophic scar. I inject a squamous cell carcinoma carefully and ‘expand’ the tumor.” He typically injects a lesion every 2 weeks until it resolves completely, which typically takes two or three sessions.
“I want to emphasize that that’s really true about intralesional 5-FU for those KAs and scars on the legs,” said session moderator James Spencer, MD, a dermatologist in private practice in St. Petersburg, Florida. “Otherwise, you’re just chasing your tail trying to cut them out. You’ll do much better with the intralesional 5-FU; it’s easy to get, it’s affordable, it comes as 50 mg/mL … just keep it in the office.”
A recommended role for hedgehog inhibitors
Hedgehog inhibitors work best as neoadjuvant therapy to shrink large skin cancer tumors prior to excision, Dr. Goldman said. “Hedgehog inhibitors don’t cure anything … except for rare cases of small basal cell carcinomas.” For most lesions, however, the strategy is not curative.
“I don’t believe in hedgehog inhibitors for things that are readily resectable. We use them to shrink things down,” he added.
Dr. Goldman recommended treating patients with neoadjuvant hedgehog inhibitors to achieve the maximum tumor shrinking effect. Adverse effects tend to develop slowly over time, typically after a 6-week “grace period.” Nighttime leg muscle cramps, loss of taste, hair loss, and weight loss can occur. Also, electrolyte imbalances can occur, particularly in older patients with renal clearance issues. When the patient can no longer reasonably tolerate the adverse effects, which is usually the case, “then you do the surgery,” Dr. Goldman said.
“The benefits of neoadjuvant hedgehog inhibitors include predictable shrinkage of tumors,” and manufacturers have been helpful with financial issues, he noted.
Dr. Goldman had no relevant financial disclosures. Dr. Spencer has served on the speakers bureau for Genentech and Leo Pharma.
MIAMI – Pearls for providers of photodynamic therapy (PDT) include tips on skin preparation, eye protection, and use of three new codes to maximize reimbursement. Also trending in medical dermatology are best practices for intralesional injections of 5-FU to treat the often challenging isomorphic squamous cell carcinomas (SCCs) or keratoacanthomas on the lower leg, as well as use of neoadjuvant hedgehog inhibitors to shrink large skin cancer lesions, according to Glenn David Goldman, MD.
“This talk is about what you can do medically as a dermatologic surgeon,” Dr. Goldman said at the Orlando Dermatology Aesthetic and Clinical Conference.
Use new billing codes for photodynamic therapy
There are now three new PDT billing codes. “Make sure your coders are using these properly. They are active now, and if you don’t use them, you won’t get paid properly,” said Dr. Goldman, professor and medical director of dermatology at the University of Vermont, Burlington. Specifically, 96567 is for standard PDT applied by staff; 96573 is for PDT applied by a physician; and 96574 is for PDT and curettage performed by a physician.
“Be involved, don’t delegate,” Dr. Goldman added. “If you do, you will get paid half as much as you used to, which means you will lose money on every single patient you treat.”
What type of PDT physicians choose to use in their practice remains controversial. “Do you do short-contact PDT, do you do daylight PDT? We’ve gone back and forth in our practice,” Dr. Goldman said. “I’m not impressed with daylight PDT. I know this is at odds with some of the people here, but at least in Vermont, it doesn’t work very well.”
The way PDT was described in the original trials (a photosensitizer applied in the office followed by PDT) “works the best, with one caveat,” Dr. Goldman said. The caveat is that dermatologists should aim for a PDT clearance that approaches the efficacy of 5-fluorouracil (5-FU). “If you can get to that – which is difficult by the way – I think your patients will really appreciate this.”
An additional PDT pearl Dr. Goldman shared involves skin preparation: the use of acetone to defat the skin, even in patients with very thick lesions. Apply acetone with gauze to the site for 5 minutes and “all of that hyperkeratosis just wipes away,” curette off any residual hyperkeratosis – and consider a ring anesthetic block to control pain for the patient with severe disease, he advised.
Another tip is to forgo the goggles that come with most PDT kits. Instead, purchase smaller, disposable laser eye shields for PDT patients, Dr. Goldman said. “They work better. You can get closer to the eye … and they are more comfortable for the patient.”
Dr. Goldman’s practice is providing more PDT and much less 5-FU for patient convenience. “I believe if someone is willing to go through 3 weeks of 5-FU or 12-16 weeks of imiquimod, they get the best results. However, most people don’t want to do that if they can sit in front of a light for 15 minutes.”
Consider intralesional injections for SCCs and KAs on the legs
An ongoing challenge in medical dermatology is preventing rapid recurrence of SCCs and/or keratoacanthomas (KAs) near sites of previous excision on the legs. “We all see this quite a bit. Often you get lesions on the leg, you cut them out, and they come right back” close to the excision site, Dr. Goldman said.
He does not recommend methotrexate injections for these lesions. “Methotrexate does not work. It doesn’t hurt, but I’ve injected methotrexate into squamous cell carcinomas many times and they’ve never gone away.” In contrast, 5-FU “works incredibly well. They go away, I’ve had tremendous success. This has changed the way we treat these lesions.” 5-FU is inexpensive and can be obtained from oncology pharmacies. One caveat is 5-FU injections can be painful and patients require anesthesia prior to injection.
Using a 25-gauge or 27-gauge needle, Dr. Goldman injects 5-FU “exactly as I would a hypertrophic scar. I inject a squamous cell carcinoma carefully and ‘expand’ the tumor.” He typically injects a lesion every 2 weeks until it resolves completely, which typically takes two or three sessions.
“I want to emphasize that that’s really true about intralesional 5-FU for those KAs and scars on the legs,” said session moderator James Spencer, MD, a dermatologist in private practice in St. Petersburg, Florida. “Otherwise, you’re just chasing your tail trying to cut them out. You’ll do much better with the intralesional 5-FU; it’s easy to get, it’s affordable, it comes as 50 mg/mL … just keep it in the office.”
A recommended role for hedgehog inhibitors
Hedgehog inhibitors work best as neoadjuvant therapy to shrink large skin cancer tumors prior to excision, Dr. Goldman said. “Hedgehog inhibitors don’t cure anything … except for rare cases of small basal cell carcinomas.” For most lesions, however, the strategy is not curative.
“I don’t believe in hedgehog inhibitors for things that are readily resectable. We use them to shrink things down,” he added.
Dr. Goldman recommended treating patients with neoadjuvant hedgehog inhibitors to achieve the maximum tumor shrinking effect. Adverse effects tend to develop slowly over time, typically after a 6-week “grace period.” Nighttime leg muscle cramps, loss of taste, hair loss, and weight loss can occur. Also, electrolyte imbalances can occur, particularly in older patients with renal clearance issues. When the patient can no longer reasonably tolerate the adverse effects, which is usually the case, “then you do the surgery,” Dr. Goldman said.
“The benefits of neoadjuvant hedgehog inhibitors include predictable shrinkage of tumors,” and manufacturers have been helpful with financial issues, he noted.
Dr. Goldman had no relevant financial disclosures. Dr. Spencer has served on the speakers bureau for Genentech and Leo Pharma.
MIAMI – Pearls for providers of photodynamic therapy (PDT) include tips on skin preparation, eye protection, and use of three new codes to maximize reimbursement. Also trending in medical dermatology are best practices for intralesional injections of 5-FU to treat the often challenging isomorphic squamous cell carcinomas (SCCs) or keratoacanthomas on the lower leg, as well as use of neoadjuvant hedgehog inhibitors to shrink large skin cancer lesions, according to Glenn David Goldman, MD.
“This talk is about what you can do medically as a dermatologic surgeon,” Dr. Goldman said at the Orlando Dermatology Aesthetic and Clinical Conference.
Use new billing codes for photodynamic therapy
There are now three new PDT billing codes. “Make sure your coders are using these properly. They are active now, and if you don’t use them, you won’t get paid properly,” said Dr. Goldman, professor and medical director of dermatology at the University of Vermont, Burlington. Specifically, 96567 is for standard PDT applied by staff; 96573 is for PDT applied by a physician; and 96574 is for PDT and curettage performed by a physician.
“Be involved, don’t delegate,” Dr. Goldman added. “If you do, you will get paid half as much as you used to, which means you will lose money on every single patient you treat.”
What type of PDT physicians choose to use in their practice remains controversial. “Do you do short-contact PDT, do you do daylight PDT? We’ve gone back and forth in our practice,” Dr. Goldman said. “I’m not impressed with daylight PDT. I know this is at odds with some of the people here, but at least in Vermont, it doesn’t work very well.”
The way PDT was described in the original trials (a photosensitizer applied in the office followed by PDT) “works the best, with one caveat,” Dr. Goldman said. The caveat is that dermatologists should aim for a PDT clearance that approaches the efficacy of 5-fluorouracil (5-FU). “If you can get to that – which is difficult by the way – I think your patients will really appreciate this.”
An additional PDT pearl Dr. Goldman shared involves skin preparation: the use of acetone to defat the skin, even in patients with very thick lesions. Apply acetone with gauze to the site for 5 minutes and “all of that hyperkeratosis just wipes away,” curette off any residual hyperkeratosis – and consider a ring anesthetic block to control pain for the patient with severe disease, he advised.
Another tip is to forgo the goggles that come with most PDT kits. Instead, purchase smaller, disposable laser eye shields for PDT patients, Dr. Goldman said. “They work better. You can get closer to the eye … and they are more comfortable for the patient.”
Dr. Goldman’s practice is providing more PDT and much less 5-FU for patient convenience. “I believe if someone is willing to go through 3 weeks of 5-FU or 12-16 weeks of imiquimod, they get the best results. However, most people don’t want to do that if they can sit in front of a light for 15 minutes.”
Consider intralesional injections for SCCs and KAs on the legs
An ongoing challenge in medical dermatology is preventing rapid recurrence of SCCs and/or keratoacanthomas (KAs) near sites of previous excision on the legs. “We all see this quite a bit. Often you get lesions on the leg, you cut them out, and they come right back” close to the excision site, Dr. Goldman said.
He does not recommend methotrexate injections for these lesions. “Methotrexate does not work. It doesn’t hurt, but I’ve injected methotrexate into squamous cell carcinomas many times and they’ve never gone away.” In contrast, 5-FU “works incredibly well. They go away, I’ve had tremendous success. This has changed the way we treat these lesions.” 5-FU is inexpensive and can be obtained from oncology pharmacies. One caveat is 5-FU injections can be painful and patients require anesthesia prior to injection.
Using a 25-gauge or 27-gauge needle, Dr. Goldman injects 5-FU “exactly as I would a hypertrophic scar. I inject a squamous cell carcinoma carefully and ‘expand’ the tumor.” He typically injects a lesion every 2 weeks until it resolves completely, which typically takes two or three sessions.
“I want to emphasize that that’s really true about intralesional 5-FU for those KAs and scars on the legs,” said session moderator James Spencer, MD, a dermatologist in private practice in St. Petersburg, Florida. “Otherwise, you’re just chasing your tail trying to cut them out. You’ll do much better with the intralesional 5-FU; it’s easy to get, it’s affordable, it comes as 50 mg/mL … just keep it in the office.”
A recommended role for hedgehog inhibitors
Hedgehog inhibitors work best as neoadjuvant therapy to shrink large skin cancer tumors prior to excision, Dr. Goldman said. “Hedgehog inhibitors don’t cure anything … except for rare cases of small basal cell carcinomas.” For most lesions, however, the strategy is not curative.
“I don’t believe in hedgehog inhibitors for things that are readily resectable. We use them to shrink things down,” he added.
Dr. Goldman recommended treating patients with neoadjuvant hedgehog inhibitors to achieve the maximum tumor shrinking effect. Adverse effects tend to develop slowly over time, typically after a 6-week “grace period.” Nighttime leg muscle cramps, loss of taste, hair loss, and weight loss can occur. Also, electrolyte imbalances can occur, particularly in older patients with renal clearance issues. When the patient can no longer reasonably tolerate the adverse effects, which is usually the case, “then you do the surgery,” Dr. Goldman said.
“The benefits of neoadjuvant hedgehog inhibitors include predictable shrinkage of tumors,” and manufacturers have been helpful with financial issues, he noted.
Dr. Goldman had no relevant financial disclosures. Dr. Spencer has served on the speakers bureau for Genentech and Leo Pharma.
REPORTING FROM ODAC 2018
Clinical trial: Telescopic vs. balloon dissection for hernia
A randomized clinical trial will compare two techniques for achieving extraperitoneal space during hernia surgery.
The in operative times, early postoperative pain scores, surgical complications, and rate of hernia recurrence. The Spacemaker Balloon Dissector will be used for the trial.
The techniques will be timed and measured in minutes from incision to end of procedure. Pain outcomes will be measured using the Numeric Pain Rating Scale (NRS-11) at postoperative days 1, 7, and 30. Intraoperative complications, 30-day infections, and 1-year recurrence will be reported in numbers and percent as appropriate.
The study is sponsored by The Cleveland Clinic.
For more details about the trial, go to www.clinicaltrials.gov.
SOURCE: Clinical Trial NCT03276871.
A randomized clinical trial will compare two techniques for achieving extraperitoneal space during hernia surgery.
The in operative times, early postoperative pain scores, surgical complications, and rate of hernia recurrence. The Spacemaker Balloon Dissector will be used for the trial.
The techniques will be timed and measured in minutes from incision to end of procedure. Pain outcomes will be measured using the Numeric Pain Rating Scale (NRS-11) at postoperative days 1, 7, and 30. Intraoperative complications, 30-day infections, and 1-year recurrence will be reported in numbers and percent as appropriate.
The study is sponsored by The Cleveland Clinic.
For more details about the trial, go to www.clinicaltrials.gov.
SOURCE: Clinical Trial NCT03276871.
A randomized clinical trial will compare two techniques for achieving extraperitoneal space during hernia surgery.
The in operative times, early postoperative pain scores, surgical complications, and rate of hernia recurrence. The Spacemaker Balloon Dissector will be used for the trial.
The techniques will be timed and measured in minutes from incision to end of procedure. Pain outcomes will be measured using the Numeric Pain Rating Scale (NRS-11) at postoperative days 1, 7, and 30. Intraoperative complications, 30-day infections, and 1-year recurrence will be reported in numbers and percent as appropriate.
The study is sponsored by The Cleveland Clinic.
For more details about the trial, go to www.clinicaltrials.gov.
SOURCE: Clinical Trial NCT03276871.
FROM CLINICALTRIALS.GOV
New hematologic, cardiovascular system link may have therapeutic implications
ATLANTA – An intriguing link between the sex steroid hormonal milieu and platelet mitochondria has potential implications for reducing thrombosis risk.
The link, which involves a mitochondrial protein known as optic atrophy 1 (OPA1), appears to play a role in the regulation of thrombosis, and provides a possible explanation for the marked differences in cardiovascular risks between men and women, according to E. Dale Abel, MD, chair of the department of internal medicine, and director of the Fraternal Order of Eagles Diabetes Research Center at the University of Iowa, Iowa City.
The findings could lead to risk-stratification strategies and the identification of therapeutic targets, Dr. Abel said in an interview.
OPA1 is an inner mitochondrial membrane protein involved in mitochondrial fusion, he explained.
“My laboratory, for a very long time, has been interested in the cardiovascular complications of diabetes, and a lot of our work has focused on the heart and on the relationship between changes in metabolism and mitochondrial biology in those complications. We got interested in platelets because of a collaboration that actually started with Dr. [Andrew] Weyrich when my lab was at the University of Utah. There was a request for proposals from the National Heart, Lung, and Blood Institute for projects that would seek to understand the increased risk of thrombosis that occurs in people with diabetes,” he said.
Specifically, Dr. Weyrich had some preliminary data showing a backup of intermediates of glucose metabolism occurring in the platelets of diabetics.
“This suggested either that there was increased import of glucose into those cells or a decreased ability of those cells to metabolize glucose,” Dr. Abel said, adding that a closer look at the expression of certain genes in platelets as they related to the risk of thrombosis showed that a number of mitochondrial genes were involved, including OPA1.
Since Dr. Abel’s lab was already involved with studying glucose metabolism and mitochondrial metabolism, and had created a number of tools for modifying alleles, which would enable the targeting of expression of some of these genes, he and his colleagues began to look closer at the role of OPA1.
“The relationship between OPA1 and platelet biology, at least based on epidemiological studies from Dr. [Jane] Freedman’s analysis of platelet RNA expression in the Framingham cohort, really seemed to suggest that this had more to do with events in females rather than males,” Dr. Abel said.
He and his colleagues then generated a mouse model in which OPA1 levels in platelets could be manipulated. The goal was to determine if such manipulation would affect platelet function or platelet biology, and also to see if the effects differed between males and females.
“Initially, we didn’t have an expectation that we would see a difference between males and females, but in retrospect, it actually fits very nicely with what the epidemiological data in humans would suggest,” he said, referring to the differences in thrombosis risk between men and women.
Mitochondria go through a process of fusion and fission; OPA1 is involved in the fusion of the inner mitochondrial membrane, which has many folds known as cristae.
“These cristae are very important in the ability of mitochondria to generate energy, and OPA1 plays a very important role in maintaining the structure of these cristae,” he explained.
He and his colleagues generated mice that lacked OPA1 specifically in platelets. They then characterized the mitochondria and platelet function in these knockout mice.
“We saw that there was a difference between males and females in terms of how they responded to OPA1 deletion. Specifically, the males appeared to get more overt mitochondrial damage in terms of their structure and function, whereas the mitochondria appeared remarkably normal in females,” he said.
A look at platelet function showed that platelets in males were somewhat hyperactive, while in females they were somewhat underactive.
When the researchers used a model of deep venous thrombosis (DVT), more than 90% of male knockout mice developed a DVT versus 50% of wild-type controls. In contrast, there was no increase in DVT in female knockout mice relative to wild-type controls.
“So they were really opposite phenotypes in terms of platelet activity, and whenever one sees a difference between sexes in any biological variable or phenotype, you wonder if this is because of sex hormones,” he said.
This question led to a number of additional experiments.
In one of those experiments, Dr. Abel and his colleagues used a mouse model in which platelets were depleted and replaced via transfusion with platelets from another animal.
“We took male mice that were wild type and we depleted their platelets, and then we took platelets from an OPA1 deficient female and transfused these back into male mice, and took OPA1 deficient platelets from males and transfused them back into platelet-depleted female hosts. The really interesting thing in those experiments was that the phenotype switched,” he said.
That is, platelets in male mice with OPA1 deficiency, which had increased platelet activation in the male mice, became hypoactive when they were transfused into female mice. Similarly, hypoactive platelets from female mice became hyperactive when transfused into platelet-depleted male mice.
“What this told us then, is that the hormonal milieu interacts with OPA1 deficiency to modulate the function of the platelets,” he said.
Additional hormonal manipulations, involving orchiectomy in male mice to lower testosterone levels and increase estrogen levels, and ovariectomy in female mice to lower estrogen levels, showed that this could also modify platelet response.
“So we have discovered that somehow the amount of OPA1 in platelets interacts with circulating estrogens to modify the activity of platelets. This is not a trivial issue, because, as in the epidemiological study, the relationship is something that seems to be particularly true in females, and it also turned out that the OPA1 tended to track with increased cardiovascular events,” he said.
Preliminary studies involving pregnant women, looking at both the first and third trimester (when estrogen levels spike), also showed a correlation between increased platelet activity in the third trimester and higher levels of OPA1 in their platelets.
“It seems there is a relationship between OPA1 and platelets in women and estrogen levels that may then increase the risk of thrombosis. Maybe our mouse model phenotype is explained by the fact that we did the opposite: We reduced OPA1 in the platelets of females, and we actually saw that this was protective,” he said.
The findings are generating excitement, according to Dr. Weyrich, professor of internal medicine and vice president for research at the University of Utah, Salt Lake City, who was involved in the earlier studies that led Dr. Abel and his team to delve into the OPA1 research.
During a presentation of Dr. Abel’s findings at the annual meeting of the American Society of Hematology, Dr. Weyrich called the work “really, really striking,” and said the gender-specific findings are of particular importance.
“It’s something we often overlook and don’t think about, but I think it’s something that’s probably going to be more and more important as we begin to understand all types of diseases both in benign and malignant hematology,” he said.
Dr. Abel and his team plan to do their part to further that understanding. They are awaiting word on a new National Institutes of Health grant that will allow for expansion of their mouse studies into humans. Specifically, those studies will look at correlations between levels of OPA1 expression in platelets in women and history of/risk for developing a thrombotic event.
“Thrombosis is a significant problem in women who are exposed to estrogens ... and with the exception of a small number of specific genetic disorders of platelets, very little is known about what the risk factors are for this estrogen dependent increase in thrombotic risk,” he said.
What needs to be uncovered, Dr. Abel said, is whether women with the highest levels of OPA1 carry the highest risk of thrombosis.
“If we understand that, we may be in a position to stratify these women based on thrombosis risk in the setting of estrogen exposure. I think the other thing that will come out of the work, as we begin to understand the mechanisms for this relationship, is the identification of targets that we could therapeutically modulate to reduce this risk,” he added.
Eventually, as more is learned about the mechanisms that underlie the relationship between OPA1 and platelet activation, the findings might also lead to new approaches for reducing the risk of thrombosis in men, he noted.
Dr. Abel and Dr. Weyrich reported having no relevant financial disclosures.
ATLANTA – An intriguing link between the sex steroid hormonal milieu and platelet mitochondria has potential implications for reducing thrombosis risk.
The link, which involves a mitochondrial protein known as optic atrophy 1 (OPA1), appears to play a role in the regulation of thrombosis, and provides a possible explanation for the marked differences in cardiovascular risks between men and women, according to E. Dale Abel, MD, chair of the department of internal medicine, and director of the Fraternal Order of Eagles Diabetes Research Center at the University of Iowa, Iowa City.
The findings could lead to risk-stratification strategies and the identification of therapeutic targets, Dr. Abel said in an interview.
OPA1 is an inner mitochondrial membrane protein involved in mitochondrial fusion, he explained.
“My laboratory, for a very long time, has been interested in the cardiovascular complications of diabetes, and a lot of our work has focused on the heart and on the relationship between changes in metabolism and mitochondrial biology in those complications. We got interested in platelets because of a collaboration that actually started with Dr. [Andrew] Weyrich when my lab was at the University of Utah. There was a request for proposals from the National Heart, Lung, and Blood Institute for projects that would seek to understand the increased risk of thrombosis that occurs in people with diabetes,” he said.
Specifically, Dr. Weyrich had some preliminary data showing a backup of intermediates of glucose metabolism occurring in the platelets of diabetics.
“This suggested either that there was increased import of glucose into those cells or a decreased ability of those cells to metabolize glucose,” Dr. Abel said, adding that a closer look at the expression of certain genes in platelets as they related to the risk of thrombosis showed that a number of mitochondrial genes were involved, including OPA1.
Since Dr. Abel’s lab was already involved with studying glucose metabolism and mitochondrial metabolism, and had created a number of tools for modifying alleles, which would enable the targeting of expression of some of these genes, he and his colleagues began to look closer at the role of OPA1.
“The relationship between OPA1 and platelet biology, at least based on epidemiological studies from Dr. [Jane] Freedman’s analysis of platelet RNA expression in the Framingham cohort, really seemed to suggest that this had more to do with events in females rather than males,” Dr. Abel said.
He and his colleagues then generated a mouse model in which OPA1 levels in platelets could be manipulated. The goal was to determine if such manipulation would affect platelet function or platelet biology, and also to see if the effects differed between males and females.
“Initially, we didn’t have an expectation that we would see a difference between males and females, but in retrospect, it actually fits very nicely with what the epidemiological data in humans would suggest,” he said, referring to the differences in thrombosis risk between men and women.
Mitochondria go through a process of fusion and fission; OPA1 is involved in the fusion of the inner mitochondrial membrane, which has many folds known as cristae.
“These cristae are very important in the ability of mitochondria to generate energy, and OPA1 plays a very important role in maintaining the structure of these cristae,” he explained.
He and his colleagues generated mice that lacked OPA1 specifically in platelets. They then characterized the mitochondria and platelet function in these knockout mice.
“We saw that there was a difference between males and females in terms of how they responded to OPA1 deletion. Specifically, the males appeared to get more overt mitochondrial damage in terms of their structure and function, whereas the mitochondria appeared remarkably normal in females,” he said.
A look at platelet function showed that platelets in males were somewhat hyperactive, while in females they were somewhat underactive.
When the researchers used a model of deep venous thrombosis (DVT), more than 90% of male knockout mice developed a DVT versus 50% of wild-type controls. In contrast, there was no increase in DVT in female knockout mice relative to wild-type controls.
“So they were really opposite phenotypes in terms of platelet activity, and whenever one sees a difference between sexes in any biological variable or phenotype, you wonder if this is because of sex hormones,” he said.
This question led to a number of additional experiments.
In one of those experiments, Dr. Abel and his colleagues used a mouse model in which platelets were depleted and replaced via transfusion with platelets from another animal.
“We took male mice that were wild type and we depleted their platelets, and then we took platelets from an OPA1 deficient female and transfused these back into male mice, and took OPA1 deficient platelets from males and transfused them back into platelet-depleted female hosts. The really interesting thing in those experiments was that the phenotype switched,” he said.
That is, platelets in male mice with OPA1 deficiency, which had increased platelet activation in the male mice, became hypoactive when they were transfused into female mice. Similarly, hypoactive platelets from female mice became hyperactive when transfused into platelet-depleted male mice.
“What this told us then, is that the hormonal milieu interacts with OPA1 deficiency to modulate the function of the platelets,” he said.
Additional hormonal manipulations, involving orchiectomy in male mice to lower testosterone levels and increase estrogen levels, and ovariectomy in female mice to lower estrogen levels, showed that this could also modify platelet response.
“So we have discovered that somehow the amount of OPA1 in platelets interacts with circulating estrogens to modify the activity of platelets. This is not a trivial issue, because, as in the epidemiological study, the relationship is something that seems to be particularly true in females, and it also turned out that the OPA1 tended to track with increased cardiovascular events,” he said.
Preliminary studies involving pregnant women, looking at both the first and third trimester (when estrogen levels spike), also showed a correlation between increased platelet activity in the third trimester and higher levels of OPA1 in their platelets.
“It seems there is a relationship between OPA1 and platelets in women and estrogen levels that may then increase the risk of thrombosis. Maybe our mouse model phenotype is explained by the fact that we did the opposite: We reduced OPA1 in the platelets of females, and we actually saw that this was protective,” he said.
The findings are generating excitement, according to Dr. Weyrich, professor of internal medicine and vice president for research at the University of Utah, Salt Lake City, who was involved in the earlier studies that led Dr. Abel and his team to delve into the OPA1 research.
During a presentation of Dr. Abel’s findings at the annual meeting of the American Society of Hematology, Dr. Weyrich called the work “really, really striking,” and said the gender-specific findings are of particular importance.
“It’s something we often overlook and don’t think about, but I think it’s something that’s probably going to be more and more important as we begin to understand all types of diseases both in benign and malignant hematology,” he said.
Dr. Abel and his team plan to do their part to further that understanding. They are awaiting word on a new National Institutes of Health grant that will allow for expansion of their mouse studies into humans. Specifically, those studies will look at correlations between levels of OPA1 expression in platelets in women and history of/risk for developing a thrombotic event.
“Thrombosis is a significant problem in women who are exposed to estrogens ... and with the exception of a small number of specific genetic disorders of platelets, very little is known about what the risk factors are for this estrogen dependent increase in thrombotic risk,” he said.
What needs to be uncovered, Dr. Abel said, is whether women with the highest levels of OPA1 carry the highest risk of thrombosis.
“If we understand that, we may be in a position to stratify these women based on thrombosis risk in the setting of estrogen exposure. I think the other thing that will come out of the work, as we begin to understand the mechanisms for this relationship, is the identification of targets that we could therapeutically modulate to reduce this risk,” he added.
Eventually, as more is learned about the mechanisms that underlie the relationship between OPA1 and platelet activation, the findings might also lead to new approaches for reducing the risk of thrombosis in men, he noted.
Dr. Abel and Dr. Weyrich reported having no relevant financial disclosures.
ATLANTA – An intriguing link between the sex steroid hormonal milieu and platelet mitochondria has potential implications for reducing thrombosis risk.
The link, which involves a mitochondrial protein known as optic atrophy 1 (OPA1), appears to play a role in the regulation of thrombosis, and provides a possible explanation for the marked differences in cardiovascular risks between men and women, according to E. Dale Abel, MD, chair of the department of internal medicine, and director of the Fraternal Order of Eagles Diabetes Research Center at the University of Iowa, Iowa City.
The findings could lead to risk-stratification strategies and the identification of therapeutic targets, Dr. Abel said in an interview.
OPA1 is an inner mitochondrial membrane protein involved in mitochondrial fusion, he explained.
“My laboratory, for a very long time, has been interested in the cardiovascular complications of diabetes, and a lot of our work has focused on the heart and on the relationship between changes in metabolism and mitochondrial biology in those complications. We got interested in platelets because of a collaboration that actually started with Dr. [Andrew] Weyrich when my lab was at the University of Utah. There was a request for proposals from the National Heart, Lung, and Blood Institute for projects that would seek to understand the increased risk of thrombosis that occurs in people with diabetes,” he said.
Specifically, Dr. Weyrich had some preliminary data showing a backup of intermediates of glucose metabolism occurring in the platelets of diabetics.
“This suggested either that there was increased import of glucose into those cells or a decreased ability of those cells to metabolize glucose,” Dr. Abel said, adding that a closer look at the expression of certain genes in platelets as they related to the risk of thrombosis showed that a number of mitochondrial genes were involved, including OPA1.
Since Dr. Abel’s lab was already involved with studying glucose metabolism and mitochondrial metabolism, and had created a number of tools for modifying alleles, which would enable the targeting of expression of some of these genes, he and his colleagues began to look closer at the role of OPA1.
“The relationship between OPA1 and platelet biology, at least based on epidemiological studies from Dr. [Jane] Freedman’s analysis of platelet RNA expression in the Framingham cohort, really seemed to suggest that this had more to do with events in females rather than males,” Dr. Abel said.
He and his colleagues then generated a mouse model in which OPA1 levels in platelets could be manipulated. The goal was to determine if such manipulation would affect platelet function or platelet biology, and also to see if the effects differed between males and females.
“Initially, we didn’t have an expectation that we would see a difference between males and females, but in retrospect, it actually fits very nicely with what the epidemiological data in humans would suggest,” he said, referring to the differences in thrombosis risk between men and women.
Mitochondria go through a process of fusion and fission; OPA1 is involved in the fusion of the inner mitochondrial membrane, which has many folds known as cristae.
“These cristae are very important in the ability of mitochondria to generate energy, and OPA1 plays a very important role in maintaining the structure of these cristae,” he explained.
He and his colleagues generated mice that lacked OPA1 specifically in platelets. They then characterized the mitochondria and platelet function in these knockout mice.
“We saw that there was a difference between males and females in terms of how they responded to OPA1 deletion. Specifically, the males appeared to get more overt mitochondrial damage in terms of their structure and function, whereas the mitochondria appeared remarkably normal in females,” he said.
A look at platelet function showed that platelets in males were somewhat hyperactive, while in females they were somewhat underactive.
When the researchers used a model of deep venous thrombosis (DVT), more than 90% of male knockout mice developed a DVT versus 50% of wild-type controls. In contrast, there was no increase in DVT in female knockout mice relative to wild-type controls.
“So they were really opposite phenotypes in terms of platelet activity, and whenever one sees a difference between sexes in any biological variable or phenotype, you wonder if this is because of sex hormones,” he said.
This question led to a number of additional experiments.
In one of those experiments, Dr. Abel and his colleagues used a mouse model in which platelets were depleted and replaced via transfusion with platelets from another animal.
“We took male mice that were wild type and we depleted their platelets, and then we took platelets from an OPA1 deficient female and transfused these back into male mice, and took OPA1 deficient platelets from males and transfused them back into platelet-depleted female hosts. The really interesting thing in those experiments was that the phenotype switched,” he said.
That is, platelets in male mice with OPA1 deficiency, which had increased platelet activation in the male mice, became hypoactive when they were transfused into female mice. Similarly, hypoactive platelets from female mice became hyperactive when transfused into platelet-depleted male mice.
“What this told us then, is that the hormonal milieu interacts with OPA1 deficiency to modulate the function of the platelets,” he said.
Additional hormonal manipulations, involving orchiectomy in male mice to lower testosterone levels and increase estrogen levels, and ovariectomy in female mice to lower estrogen levels, showed that this could also modify platelet response.
“So we have discovered that somehow the amount of OPA1 in platelets interacts with circulating estrogens to modify the activity of platelets. This is not a trivial issue, because, as in the epidemiological study, the relationship is something that seems to be particularly true in females, and it also turned out that the OPA1 tended to track with increased cardiovascular events,” he said.
Preliminary studies involving pregnant women, looking at both the first and third trimester (when estrogen levels spike), also showed a correlation between increased platelet activity in the third trimester and higher levels of OPA1 in their platelets.
“It seems there is a relationship between OPA1 and platelets in women and estrogen levels that may then increase the risk of thrombosis. Maybe our mouse model phenotype is explained by the fact that we did the opposite: We reduced OPA1 in the platelets of females, and we actually saw that this was protective,” he said.
The findings are generating excitement, according to Dr. Weyrich, professor of internal medicine and vice president for research at the University of Utah, Salt Lake City, who was involved in the earlier studies that led Dr. Abel and his team to delve into the OPA1 research.
During a presentation of Dr. Abel’s findings at the annual meeting of the American Society of Hematology, Dr. Weyrich called the work “really, really striking,” and said the gender-specific findings are of particular importance.
“It’s something we often overlook and don’t think about, but I think it’s something that’s probably going to be more and more important as we begin to understand all types of diseases both in benign and malignant hematology,” he said.
Dr. Abel and his team plan to do their part to further that understanding. They are awaiting word on a new National Institutes of Health grant that will allow for expansion of their mouse studies into humans. Specifically, those studies will look at correlations between levels of OPA1 expression in platelets in women and history of/risk for developing a thrombotic event.
“Thrombosis is a significant problem in women who are exposed to estrogens ... and with the exception of a small number of specific genetic disorders of platelets, very little is known about what the risk factors are for this estrogen dependent increase in thrombotic risk,” he said.
What needs to be uncovered, Dr. Abel said, is whether women with the highest levels of OPA1 carry the highest risk of thrombosis.
“If we understand that, we may be in a position to stratify these women based on thrombosis risk in the setting of estrogen exposure. I think the other thing that will come out of the work, as we begin to understand the mechanisms for this relationship, is the identification of targets that we could therapeutically modulate to reduce this risk,” he added.
Eventually, as more is learned about the mechanisms that underlie the relationship between OPA1 and platelet activation, the findings might also lead to new approaches for reducing the risk of thrombosis in men, he noted.
Dr. Abel and Dr. Weyrich reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM ASH 2017
Study IDs predictors of nonmelanoma skin cancer in IBD
LAS VEGAS – , a large national analysis showed.
“Some studies have shown that patients with IBD may be at increased risk for nonmelanoma skin cancer (NMSC) because of the immunomodulators that they take for the management of their disease,” Zubair Khan, MD, said in an interview at the Crohn’s & Colitis Congress, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association. “But these are mostly small, single-center studies.” In an effort to determine the epidemiology of NMSC in patients hospitalized with IBD, Dr. Khan, associate chief resident in the internal medicine department at the University of Toledo (Ohio) Medical Center and his associates analyzed the National Inpatient Sample (NIS) database for all subjects who had a primary or secondary discharge diagnosis of IBD during 2002-2014. Next, they used ICD-9 codes to identify the rate of NMSC in this population.
Compared with IBD patients without NMSC, most of the IBD patients with NMSC were males (54% vs. 42%; P less than 0.001), covered by Medicare (65% vs. 37%), were white (96% vs. 81%; P less than .001), lived in the Midwest or Western United States (27% and 26% vs. 22% and 17%), were admitted to urban teaching hospitals (57% vs. 51%; P less than .001), were discharged to skilled nursing facilities (16% vs. 10%; P less than .001), required home health care (17% vs. 11%), and were admitted electively (27% vs. 20%). The researchers observed no significant difference in mortality among IBD patients with and without NMSC (1.61% vs. 1.53%; P = .22).
Multivariate analysis revealed that the following factors were predictive of NMSC in IBD: comorbid diagnosis of rheumatoid arthritis, collagen vasculature diseases, male sex, and white race. “Patients with those risk factors should be made more aware of their risk for developing NMSC,” Dr. Khan said. “They shouldn’t be taken lightly.”
Dr. Khan reported having no financial disclosures.
*This story was updated on 3/26.
SOURCE: Khan Z et al. Crohn’s & Colitis Congress, Poster 209.
LAS VEGAS – , a large national analysis showed.
“Some studies have shown that patients with IBD may be at increased risk for nonmelanoma skin cancer (NMSC) because of the immunomodulators that they take for the management of their disease,” Zubair Khan, MD, said in an interview at the Crohn’s & Colitis Congress, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association. “But these are mostly small, single-center studies.” In an effort to determine the epidemiology of NMSC in patients hospitalized with IBD, Dr. Khan, associate chief resident in the internal medicine department at the University of Toledo (Ohio) Medical Center and his associates analyzed the National Inpatient Sample (NIS) database for all subjects who had a primary or secondary discharge diagnosis of IBD during 2002-2014. Next, they used ICD-9 codes to identify the rate of NMSC in this population.
Compared with IBD patients without NMSC, most of the IBD patients with NMSC were males (54% vs. 42%; P less than 0.001), covered by Medicare (65% vs. 37%), were white (96% vs. 81%; P less than .001), lived in the Midwest or Western United States (27% and 26% vs. 22% and 17%), were admitted to urban teaching hospitals (57% vs. 51%; P less than .001), were discharged to skilled nursing facilities (16% vs. 10%; P less than .001), required home health care (17% vs. 11%), and were admitted electively (27% vs. 20%). The researchers observed no significant difference in mortality among IBD patients with and without NMSC (1.61% vs. 1.53%; P = .22).
Multivariate analysis revealed that the following factors were predictive of NMSC in IBD: comorbid diagnosis of rheumatoid arthritis, collagen vasculature diseases, male sex, and white race. “Patients with those risk factors should be made more aware of their risk for developing NMSC,” Dr. Khan said. “They shouldn’t be taken lightly.”
Dr. Khan reported having no financial disclosures.
*This story was updated on 3/26.
SOURCE: Khan Z et al. Crohn’s & Colitis Congress, Poster 209.
LAS VEGAS – , a large national analysis showed.
“Some studies have shown that patients with IBD may be at increased risk for nonmelanoma skin cancer (NMSC) because of the immunomodulators that they take for the management of their disease,” Zubair Khan, MD, said in an interview at the Crohn’s & Colitis Congress, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association. “But these are mostly small, single-center studies.” In an effort to determine the epidemiology of NMSC in patients hospitalized with IBD, Dr. Khan, associate chief resident in the internal medicine department at the University of Toledo (Ohio) Medical Center and his associates analyzed the National Inpatient Sample (NIS) database for all subjects who had a primary or secondary discharge diagnosis of IBD during 2002-2014. Next, they used ICD-9 codes to identify the rate of NMSC in this population.
Compared with IBD patients without NMSC, most of the IBD patients with NMSC were males (54% vs. 42%; P less than 0.001), covered by Medicare (65% vs. 37%), were white (96% vs. 81%; P less than .001), lived in the Midwest or Western United States (27% and 26% vs. 22% and 17%), were admitted to urban teaching hospitals (57% vs. 51%; P less than .001), were discharged to skilled nursing facilities (16% vs. 10%; P less than .001), required home health care (17% vs. 11%), and were admitted electively (27% vs. 20%). The researchers observed no significant difference in mortality among IBD patients with and without NMSC (1.61% vs. 1.53%; P = .22).
Multivariate analysis revealed that the following factors were predictive of NMSC in IBD: comorbid diagnosis of rheumatoid arthritis, collagen vasculature diseases, male sex, and white race. “Patients with those risk factors should be made more aware of their risk for developing NMSC,” Dr. Khan said. “They shouldn’t be taken lightly.”
Dr. Khan reported having no financial disclosures.
*This story was updated on 3/26.
SOURCE: Khan Z et al. Crohn’s & Colitis Congress, Poster 209.
REPORTING FROM THE CROHN’S & COLITIS CONGRESS
Key clinical point: Many factors predict which IBD patients are at risk for developing nonmelanoma skin cancer.
Major finding: Compared with IBD patients without NMSC, most of the IBD patients with NMSC were males (54% vs. 42%; P less than .001) and white (96% vs. 81%; P less than .001).
Study details: An analysis of 22,620 patients who had a primary or secondary discharge diagnosis of IBD during 2002-2014.
Disclosures: Dr. Khan reported having no financial disclosures.
Source: Khan Z et al. Crohn’s & Colitis Congress, Poster 209.
Three in 10 of your diabetic patients may have liver fibrosis
LOS ANGELES – For every 10 of your adult patients with type 2 diabetes, three are likely to have moderate to severe liver fibrosis, according to Kenneth Cusi, MD, FACP, FACE.
“If in the last 10 patients, you didn’t diagnose anybody with fibrosis, you probably missed it,” he said at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease. “The question is, How are we going to tackle this problem? My academic goal is that we incorporate screening for NASH [nonalcoholic steatohepatitis], or for fibrosis more specifically, in the same way we do for retinopathy or nephropathy, because we do have a way to treat it.”
As part of the large population-based Rotterdam study of individuals aged 45 years and older, researchers found that liver stiffness of 8 kPa or more by transient elastography was present in 5.6% of the study participants and was strongly associated with steatosis and diabetes (Hepatology. 2016;63:138-47). According to Dr. Cusi, individuals who have steatosis without diabetes face a 5%-10% risk of fibrosis, while those with steatosis and diabetes face a 15%-20% risk.
“It’s well established in a number of studies that if you have fibrosis, you’re at high risk not only of cirrhosis, but also of hepatocellular carcinoma,” he said. “The key thing is not detecting fat, which is not really the target. The target is if there’s fibrosis or not.” Three ways to assess for fibrosis include MR elastography, transient elastography (which is the most commonly used), and fibrosis marker panels.
Liver fibrosis likely starts with adipose tissue dysfunction, said Dr. Cusi, who authored a review on the pathophysiology of interactions between adipose tissue and target organs in obesity and the resulting clinical implications for the management of nonalcoholic steatohepatitis (Gastroenterology. 2012;142[4]:711-25.e6).
“When you have insulin-resistant, sick adipose tissue, that leads to the accumulation of fat in the liver,” he said. “. Even if you get people who are matched for BMIs [body mass indexes] between 30 and 35 kg/m2, there is a spectrum in which some individuals have very insulin-resistant adipose tissue and others less so. I would say that 1 out of 10 are metabolically healthy, and we don’t understand exactly why.”
In a recent cross-sectional analysis of 352 healthy individuals, Dr. Cusi and his associates found that intrahepatic triglyceride (IHTG) accumulation is strongly associated with adipose tissue insulin resistance, supporting the current theory of lipotoxicity as a driver of IHTG accumulation (Hepatology. 2017;65[4]:1132-44). The researchers observed that once IHTG accumulation reaches about 6%, skeletal muscle insulin resistance, hypertriglyceridemia, and low HDL cholesterol become fully established.
“The next question is, How does this correlate with NASH?” Dr. Cusi said. “Our take is that there is a threshold effect. Once you have a critical amount of triglycerides in your liver, some individuals are going to activate pathways that are harmful. NASH is not something exclusive to individuals who are obese. Lean people can also develop NASH. The key feature is insulin resistance, not metabolic syndrome. Once you develop a fatty liver, your chances of NASH are comparable to that of an obese individual. The paradox is that lean individuals get a fatty liver, but when they get a fatty liver, they are at risk for NASH and for fibrosis.”
Why lean individuals develop NASH is not fully understood, but Dr. Cusi said he suspects that the problem develops at the mitochondrial level.
Results from an unpublished animal model in which mice were fed a high–trans-fat diet for 24 weeks showed that the mice developed steatosis by week 8 and NASH by week 24. The mice had an increase in the tricarboxylic acid (TCA) cycle, which is typical of the NASH period, as well as an increase in ceramides.
“Perhaps a unifying hypothesis would be that the development of NASH is linked to inflammation and to insulin signaling,” Dr. Cusi said. “Not surprisingly, it had a number of effects on the mitochondria, and in this animal model it decreases the TCA.” He noted that the biology of fibrosis remains unknown in humans. “What we have been familiar with is the high-triglyceride, low-HDL pattern,” he said. “If you look at how that correlates with the amount of liver fat, it is basically a threshold effect. Once you have steatosis, you don’t see much worse dyslipidemia, which is typical of these patients.”
Recently published guidance from the American Association for the Study of Liver Diseases on the diagnosis and management of nonalcoholic fatty liver disease (NAFLD) suggests that patients require a weight loss of 3%-5% to improve steatosis, but a loss of 7%-10% to improve most histologic features of NASH, including fibrosis (Hepatology. 2018;67[1]:328-57). Exercise alone may prevent or reduce steatosis, but its ability to improve other aspects of liver histology remains unknown. Bariatric surgery can be considered in otherwise eligible obese individuals with NAFLD or NASH. The procedure’s impact on fibrosis is unknown.
The AASLD practice guideline notes that metformin is not recommended for treating NASH in adult patients, but pioglitazone improves liver histology in patients with and without type 2 diabetes with biopsy-proven NASH.
“Pioglitazone has had the greatest benefit in terms of treatment effect, compared to placebo,” Dr. Cusi said. “It’s a generic drug; at the VA [Veterans Affairs], it costs 8 cents per tablet. I think that pioglitazone will be to NASH what metformin has been to type 2 diabetes. The most common side effect is weight gain, typically between 4 and 9 lb. Risks and benefits should be discussed with each patient. It should not be used for NAFLD without biopsy-proven NASH.”
The guideline goes on to say that it’s currently premature to consider GLP-1 (glucagonlike peptide–1) agonists for treating liver disease in patients with NAFLD or NASH. Meanwhile, vitamin E at 800 IU has been shown to improve liver histology in nondiabetic adults with NASH, but the risks and benefits should be discussed with each patient. Vitamin E is not recommended for NASH in diabetic patients, NAFLD without a liver biopsy, NASH cirrhosis, or cryptogenic cirrhosis.
The AASLD practice guideline also states that the best evidence for using SGLT2 (sodium-glucose cotransporter–2) inhibitors in NAFLD comes from animal studies, which report a reduction in steatosis with and without weight loss. Clinical studies reporting a reduction in steatosis are limited. There are positive observational studies with a reduction in alanine aminotransferase and some studies that have shown a reduction in liver fat. “For me, the best option is to tailor treatment to the pathophysiology of the disease,” Dr. Cusi said. “You reduce fat by weight loss in some way, or you change the biology of fat with a thiazolidinedione.”
Dr. Cusi reported that he has received grant support from the Burroughs Wellcome Fund, the American Diabetes Association, and the National Institutes of Health.
LOS ANGELES – For every 10 of your adult patients with type 2 diabetes, three are likely to have moderate to severe liver fibrosis, according to Kenneth Cusi, MD, FACP, FACE.
“If in the last 10 patients, you didn’t diagnose anybody with fibrosis, you probably missed it,” he said at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease. “The question is, How are we going to tackle this problem? My academic goal is that we incorporate screening for NASH [nonalcoholic steatohepatitis], or for fibrosis more specifically, in the same way we do for retinopathy or nephropathy, because we do have a way to treat it.”
As part of the large population-based Rotterdam study of individuals aged 45 years and older, researchers found that liver stiffness of 8 kPa or more by transient elastography was present in 5.6% of the study participants and was strongly associated with steatosis and diabetes (Hepatology. 2016;63:138-47). According to Dr. Cusi, individuals who have steatosis without diabetes face a 5%-10% risk of fibrosis, while those with steatosis and diabetes face a 15%-20% risk.
“It’s well established in a number of studies that if you have fibrosis, you’re at high risk not only of cirrhosis, but also of hepatocellular carcinoma,” he said. “The key thing is not detecting fat, which is not really the target. The target is if there’s fibrosis or not.” Three ways to assess for fibrosis include MR elastography, transient elastography (which is the most commonly used), and fibrosis marker panels.
Liver fibrosis likely starts with adipose tissue dysfunction, said Dr. Cusi, who authored a review on the pathophysiology of interactions between adipose tissue and target organs in obesity and the resulting clinical implications for the management of nonalcoholic steatohepatitis (Gastroenterology. 2012;142[4]:711-25.e6).
“When you have insulin-resistant, sick adipose tissue, that leads to the accumulation of fat in the liver,” he said. “. Even if you get people who are matched for BMIs [body mass indexes] between 30 and 35 kg/m2, there is a spectrum in which some individuals have very insulin-resistant adipose tissue and others less so. I would say that 1 out of 10 are metabolically healthy, and we don’t understand exactly why.”
In a recent cross-sectional analysis of 352 healthy individuals, Dr. Cusi and his associates found that intrahepatic triglyceride (IHTG) accumulation is strongly associated with adipose tissue insulin resistance, supporting the current theory of lipotoxicity as a driver of IHTG accumulation (Hepatology. 2017;65[4]:1132-44). The researchers observed that once IHTG accumulation reaches about 6%, skeletal muscle insulin resistance, hypertriglyceridemia, and low HDL cholesterol become fully established.
“The next question is, How does this correlate with NASH?” Dr. Cusi said. “Our take is that there is a threshold effect. Once you have a critical amount of triglycerides in your liver, some individuals are going to activate pathways that are harmful. NASH is not something exclusive to individuals who are obese. Lean people can also develop NASH. The key feature is insulin resistance, not metabolic syndrome. Once you develop a fatty liver, your chances of NASH are comparable to that of an obese individual. The paradox is that lean individuals get a fatty liver, but when they get a fatty liver, they are at risk for NASH and for fibrosis.”
Why lean individuals develop NASH is not fully understood, but Dr. Cusi said he suspects that the problem develops at the mitochondrial level.
Results from an unpublished animal model in which mice were fed a high–trans-fat diet for 24 weeks showed that the mice developed steatosis by week 8 and NASH by week 24. The mice had an increase in the tricarboxylic acid (TCA) cycle, which is typical of the NASH period, as well as an increase in ceramides.
“Perhaps a unifying hypothesis would be that the development of NASH is linked to inflammation and to insulin signaling,” Dr. Cusi said. “Not surprisingly, it had a number of effects on the mitochondria, and in this animal model it decreases the TCA.” He noted that the biology of fibrosis remains unknown in humans. “What we have been familiar with is the high-triglyceride, low-HDL pattern,” he said. “If you look at how that correlates with the amount of liver fat, it is basically a threshold effect. Once you have steatosis, you don’t see much worse dyslipidemia, which is typical of these patients.”
Recently published guidance from the American Association for the Study of Liver Diseases on the diagnosis and management of nonalcoholic fatty liver disease (NAFLD) suggests that patients require a weight loss of 3%-5% to improve steatosis, but a loss of 7%-10% to improve most histologic features of NASH, including fibrosis (Hepatology. 2018;67[1]:328-57). Exercise alone may prevent or reduce steatosis, but its ability to improve other aspects of liver histology remains unknown. Bariatric surgery can be considered in otherwise eligible obese individuals with NAFLD or NASH. The procedure’s impact on fibrosis is unknown.
The AASLD practice guideline notes that metformin is not recommended for treating NASH in adult patients, but pioglitazone improves liver histology in patients with and without type 2 diabetes with biopsy-proven NASH.
“Pioglitazone has had the greatest benefit in terms of treatment effect, compared to placebo,” Dr. Cusi said. “It’s a generic drug; at the VA [Veterans Affairs], it costs 8 cents per tablet. I think that pioglitazone will be to NASH what metformin has been to type 2 diabetes. The most common side effect is weight gain, typically between 4 and 9 lb. Risks and benefits should be discussed with each patient. It should not be used for NAFLD without biopsy-proven NASH.”
The guideline goes on to say that it’s currently premature to consider GLP-1 (glucagonlike peptide–1) agonists for treating liver disease in patients with NAFLD or NASH. Meanwhile, vitamin E at 800 IU has been shown to improve liver histology in nondiabetic adults with NASH, but the risks and benefits should be discussed with each patient. Vitamin E is not recommended for NASH in diabetic patients, NAFLD without a liver biopsy, NASH cirrhosis, or cryptogenic cirrhosis.
The AASLD practice guideline also states that the best evidence for using SGLT2 (sodium-glucose cotransporter–2) inhibitors in NAFLD comes from animal studies, which report a reduction in steatosis with and without weight loss. Clinical studies reporting a reduction in steatosis are limited. There are positive observational studies with a reduction in alanine aminotransferase and some studies that have shown a reduction in liver fat. “For me, the best option is to tailor treatment to the pathophysiology of the disease,” Dr. Cusi said. “You reduce fat by weight loss in some way, or you change the biology of fat with a thiazolidinedione.”
Dr. Cusi reported that he has received grant support from the Burroughs Wellcome Fund, the American Diabetes Association, and the National Institutes of Health.
LOS ANGELES – For every 10 of your adult patients with type 2 diabetes, three are likely to have moderate to severe liver fibrosis, according to Kenneth Cusi, MD, FACP, FACE.
“If in the last 10 patients, you didn’t diagnose anybody with fibrosis, you probably missed it,” he said at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease. “The question is, How are we going to tackle this problem? My academic goal is that we incorporate screening for NASH [nonalcoholic steatohepatitis], or for fibrosis more specifically, in the same way we do for retinopathy or nephropathy, because we do have a way to treat it.”
As part of the large population-based Rotterdam study of individuals aged 45 years and older, researchers found that liver stiffness of 8 kPa or more by transient elastography was present in 5.6% of the study participants and was strongly associated with steatosis and diabetes (Hepatology. 2016;63:138-47). According to Dr. Cusi, individuals who have steatosis without diabetes face a 5%-10% risk of fibrosis, while those with steatosis and diabetes face a 15%-20% risk.
“It’s well established in a number of studies that if you have fibrosis, you’re at high risk not only of cirrhosis, but also of hepatocellular carcinoma,” he said. “The key thing is not detecting fat, which is not really the target. The target is if there’s fibrosis or not.” Three ways to assess for fibrosis include MR elastography, transient elastography (which is the most commonly used), and fibrosis marker panels.
Liver fibrosis likely starts with adipose tissue dysfunction, said Dr. Cusi, who authored a review on the pathophysiology of interactions between adipose tissue and target organs in obesity and the resulting clinical implications for the management of nonalcoholic steatohepatitis (Gastroenterology. 2012;142[4]:711-25.e6).
“When you have insulin-resistant, sick adipose tissue, that leads to the accumulation of fat in the liver,” he said. “. Even if you get people who are matched for BMIs [body mass indexes] between 30 and 35 kg/m2, there is a spectrum in which some individuals have very insulin-resistant adipose tissue and others less so. I would say that 1 out of 10 are metabolically healthy, and we don’t understand exactly why.”
In a recent cross-sectional analysis of 352 healthy individuals, Dr. Cusi and his associates found that intrahepatic triglyceride (IHTG) accumulation is strongly associated with adipose tissue insulin resistance, supporting the current theory of lipotoxicity as a driver of IHTG accumulation (Hepatology. 2017;65[4]:1132-44). The researchers observed that once IHTG accumulation reaches about 6%, skeletal muscle insulin resistance, hypertriglyceridemia, and low HDL cholesterol become fully established.
“The next question is, How does this correlate with NASH?” Dr. Cusi said. “Our take is that there is a threshold effect. Once you have a critical amount of triglycerides in your liver, some individuals are going to activate pathways that are harmful. NASH is not something exclusive to individuals who are obese. Lean people can also develop NASH. The key feature is insulin resistance, not metabolic syndrome. Once you develop a fatty liver, your chances of NASH are comparable to that of an obese individual. The paradox is that lean individuals get a fatty liver, but when they get a fatty liver, they are at risk for NASH and for fibrosis.”
Why lean individuals develop NASH is not fully understood, but Dr. Cusi said he suspects that the problem develops at the mitochondrial level.
Results from an unpublished animal model in which mice were fed a high–trans-fat diet for 24 weeks showed that the mice developed steatosis by week 8 and NASH by week 24. The mice had an increase in the tricarboxylic acid (TCA) cycle, which is typical of the NASH period, as well as an increase in ceramides.
“Perhaps a unifying hypothesis would be that the development of NASH is linked to inflammation and to insulin signaling,” Dr. Cusi said. “Not surprisingly, it had a number of effects on the mitochondria, and in this animal model it decreases the TCA.” He noted that the biology of fibrosis remains unknown in humans. “What we have been familiar with is the high-triglyceride, low-HDL pattern,” he said. “If you look at how that correlates with the amount of liver fat, it is basically a threshold effect. Once you have steatosis, you don’t see much worse dyslipidemia, which is typical of these patients.”
Recently published guidance from the American Association for the Study of Liver Diseases on the diagnosis and management of nonalcoholic fatty liver disease (NAFLD) suggests that patients require a weight loss of 3%-5% to improve steatosis, but a loss of 7%-10% to improve most histologic features of NASH, including fibrosis (Hepatology. 2018;67[1]:328-57). Exercise alone may prevent or reduce steatosis, but its ability to improve other aspects of liver histology remains unknown. Bariatric surgery can be considered in otherwise eligible obese individuals with NAFLD or NASH. The procedure’s impact on fibrosis is unknown.
The AASLD practice guideline notes that metformin is not recommended for treating NASH in adult patients, but pioglitazone improves liver histology in patients with and without type 2 diabetes with biopsy-proven NASH.
“Pioglitazone has had the greatest benefit in terms of treatment effect, compared to placebo,” Dr. Cusi said. “It’s a generic drug; at the VA [Veterans Affairs], it costs 8 cents per tablet. I think that pioglitazone will be to NASH what metformin has been to type 2 diabetes. The most common side effect is weight gain, typically between 4 and 9 lb. Risks and benefits should be discussed with each patient. It should not be used for NAFLD without biopsy-proven NASH.”
The guideline goes on to say that it’s currently premature to consider GLP-1 (glucagonlike peptide–1) agonists for treating liver disease in patients with NAFLD or NASH. Meanwhile, vitamin E at 800 IU has been shown to improve liver histology in nondiabetic adults with NASH, but the risks and benefits should be discussed with each patient. Vitamin E is not recommended for NASH in diabetic patients, NAFLD without a liver biopsy, NASH cirrhosis, or cryptogenic cirrhosis.
The AASLD practice guideline also states that the best evidence for using SGLT2 (sodium-glucose cotransporter–2) inhibitors in NAFLD comes from animal studies, which report a reduction in steatosis with and without weight loss. Clinical studies reporting a reduction in steatosis are limited. There are positive observational studies with a reduction in alanine aminotransferase and some studies that have shown a reduction in liver fat. “For me, the best option is to tailor treatment to the pathophysiology of the disease,” Dr. Cusi said. “You reduce fat by weight loss in some way, or you change the biology of fat with a thiazolidinedione.”
Dr. Cusi reported that he has received grant support from the Burroughs Wellcome Fund, the American Diabetes Association, and the National Institutes of Health.
EXPERT ANALYSIS FROM WCIRDC 2017
Daily update: An emerging cardiac disease, CHIP, and more
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
“This is an emerging disease that you’re all going to see in your practices this year. You’ve got to know what to do with these patients. Get to them early,” one expert warns.
To learn about this important development and more, click on the link above for today's four-minute medical news update.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
“This is an emerging disease that you’re all going to see in your practices this year. You’ve got to know what to do with these patients. Get to them early,” one expert warns.
To learn about this important development and more, click on the link above for today's four-minute medical news update.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
“This is an emerging disease that you’re all going to see in your practices this year. You’ve got to know what to do with these patients. Get to them early,” one expert warns.
To learn about this important development and more, click on the link above for today's four-minute medical news update.