User login
Antibiotic choice for acute otitis media 2018
It’s a new year and a new respiratory season so my thoughts turn to the most common infection in pediatrics where an antibiotic might appropriately be prescribed – acute otitis media (AOM). The guidelines of the American Academy of Pediatrics were finalized in 2012 and published in 2013 and based on data that the AAP subcommittee considered. A recommendation emerged for amoxicillin to remain the treatment of choice if an antibiotic was to be prescribed at all, leaving the observation option as a continued consideration under defined clinical circumstances. The oral alternative antibiotics recommended were amoxicillin/clavulanate and cefdinir (Pediatrics. 2013. doi: 10.1542/peds.2012-3488).
Since the AAP subcommittee deliberated, changes have occurred in AOM etiology and the frequency of antibiotic resistance among the common bacteria that cause the infection. Our group in Rochester (N.Y.) continues to be the only site in the United States conducting a prospective assessment of AOM; we hope our data are generalizable to the entire country, but that is not certain. In Rochester, we saw an overall drop in AOM incidence after introduction of Prevnar 7 of about 10%-15% overall and that corresponded reasonably well with the frequency of AOM caused by Streptococcus pneumoniae involving the seven serotypes in the PCV7 vaccine. We then had a rebound in AOM infections, largely caused by serotype 19A, such that the overall incidence of AOM returned back to levels nearly the same as before PCV7 by 2010. With the introduction of Prevnar 13, and the dramatic reduction of serotype 19A nasal colonization – a necessary precursor of AOM – the incidence of AOM overall fell again, and compared with the pre-PCV7 era, I estimate that we are seeing about 20%-25% less AOM today.
In late 2017, we published an article describing the epidemiology of AOM in the PCV era (Pediatrics. 2017 Aug. doi: 10.1542/peds.2017-0181), in which we described changes in otopathogen distribution over time from 1996 through 2016. It showed that by end of 2016, the predominant bacteria causing AOM were Haemophilus influenzae, accounting for 60% of all AOM (52% detected by culture from tympanocentesis and another 8% detected by polymerase chain reaction). Among the H. influenzae from middle ear fluid, beta-lactamase production occurred in 45%. Therefore, according to principles of infectious disease antibiotic efficacy predictions, use of amoxicillin in standard dose or high dose would not eradicate about half of the H. influenzae causing AOM. In the table included in this column, I show calculations of predicted outcomes from amoxicillin, amoxicillin/clavulanate, and cefdinir treatment based on the projected otopathogen mix and resistance frequencies of 2016. Added to the data on H. influenzae I have included results of S. pneumoniae high nonsusceptibility at 5% of strains and beta-lactamase production by Moraxella catarrhalis at 100% of strains. 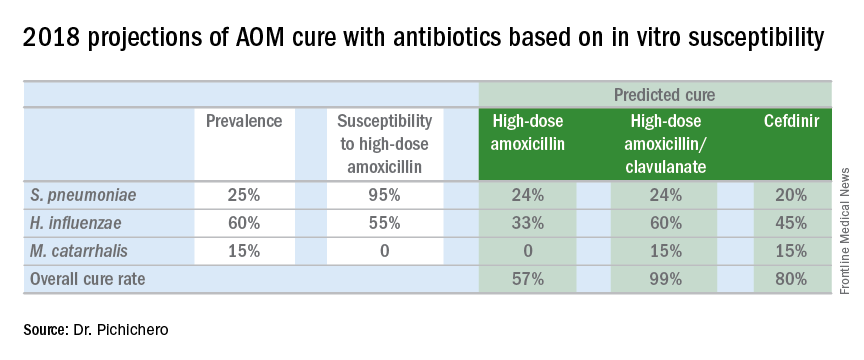
Strictly based on in vitro susceptibility and the known otopathogen mix, the calculations show that amoxicillin could result in a maximum cure of 57%, amoxicillin/clavulanate of 99%, and cefdinir of 80% of treated children.
In vitro susceptibility has its limitations. Pharmacodynamic calculations would drop the predicted success of all three antibiotics because suboptimal absorption after oral dosing occurs with amoxicillin and amoxicillin/clavulanate more so than with cefdinir, thereby resulting in lower than predicted levels of antibiotic at the site of infection within the middle ear, whereas the achievable level of cefdinir with recommended dosing sometimes is below the desired in vitro cut point.
To balance that lowered predicted efficacy, each of the otopathogens has an associated “spontaneous cure rate” that is often quoted as being 20% for S. pneumoniae, 50% for H. influenzae, and 80% for M. catarrhalis. However, to be clear, those rates were derived largely from assessments about 5 days after antibiotic treatment was started with ineffective drugs or with placebos and do not account for the true spontaneous clinical cure rate of AOM if assessed in the first few days after onset (when pain and fever are at their peak) nor if assessed 14-30 days later when almost all children have been cured by their immune systems.
The calculations also do not account for overdiagnosis in clinical practice. Indeed, if the child does not have AOM, then the child will have a cure regardless of which antibiotic is selected. Rates of overdiagnosis of AOM have been assessed with various methods and are subject to limitations. But overall the data and most experts agree that overdiagnosis by pediatricians, family physicians, urgent care physicians, nurse practitioners, and physician assistants is in the range of 30%-50%.
Before the reader leaps to the conclusion that I am endorsing any particular antibiotic strictly based on predicted in vitro efficacy, I would state that many considerations must be given to whether to use an antibiotic for AOM, and which antibiotic to use, at what dose, and for what duration. This column is just pointing out a few key up-to-date facts for your consideration.
It’s a new year and a new respiratory season so my thoughts turn to the most common infection in pediatrics where an antibiotic might appropriately be prescribed – acute otitis media (AOM). The guidelines of the American Academy of Pediatrics were finalized in 2012 and published in 2013 and based on data that the AAP subcommittee considered. A recommendation emerged for amoxicillin to remain the treatment of choice if an antibiotic was to be prescribed at all, leaving the observation option as a continued consideration under defined clinical circumstances. The oral alternative antibiotics recommended were amoxicillin/clavulanate and cefdinir (Pediatrics. 2013. doi: 10.1542/peds.2012-3488).
Since the AAP subcommittee deliberated, changes have occurred in AOM etiology and the frequency of antibiotic resistance among the common bacteria that cause the infection. Our group in Rochester (N.Y.) continues to be the only site in the United States conducting a prospective assessment of AOM; we hope our data are generalizable to the entire country, but that is not certain. In Rochester, we saw an overall drop in AOM incidence after introduction of Prevnar 7 of about 10%-15% overall and that corresponded reasonably well with the frequency of AOM caused by Streptococcus pneumoniae involving the seven serotypes in the PCV7 vaccine. We then had a rebound in AOM infections, largely caused by serotype 19A, such that the overall incidence of AOM returned back to levels nearly the same as before PCV7 by 2010. With the introduction of Prevnar 13, and the dramatic reduction of serotype 19A nasal colonization – a necessary precursor of AOM – the incidence of AOM overall fell again, and compared with the pre-PCV7 era, I estimate that we are seeing about 20%-25% less AOM today.
In late 2017, we published an article describing the epidemiology of AOM in the PCV era (Pediatrics. 2017 Aug. doi: 10.1542/peds.2017-0181), in which we described changes in otopathogen distribution over time from 1996 through 2016. It showed that by end of 2016, the predominant bacteria causing AOM were Haemophilus influenzae, accounting for 60% of all AOM (52% detected by culture from tympanocentesis and another 8% detected by polymerase chain reaction). Among the H. influenzae from middle ear fluid, beta-lactamase production occurred in 45%. Therefore, according to principles of infectious disease antibiotic efficacy predictions, use of amoxicillin in standard dose or high dose would not eradicate about half of the H. influenzae causing AOM. In the table included in this column, I show calculations of predicted outcomes from amoxicillin, amoxicillin/clavulanate, and cefdinir treatment based on the projected otopathogen mix and resistance frequencies of 2016. Added to the data on H. influenzae I have included results of S. pneumoniae high nonsusceptibility at 5% of strains and beta-lactamase production by Moraxella catarrhalis at 100% of strains. 
Strictly based on in vitro susceptibility and the known otopathogen mix, the calculations show that amoxicillin could result in a maximum cure of 57%, amoxicillin/clavulanate of 99%, and cefdinir of 80% of treated children.
In vitro susceptibility has its limitations. Pharmacodynamic calculations would drop the predicted success of all three antibiotics because suboptimal absorption after oral dosing occurs with amoxicillin and amoxicillin/clavulanate more so than with cefdinir, thereby resulting in lower than predicted levels of antibiotic at the site of infection within the middle ear, whereas the achievable level of cefdinir with recommended dosing sometimes is below the desired in vitro cut point.
To balance that lowered predicted efficacy, each of the otopathogens has an associated “spontaneous cure rate” that is often quoted as being 20% for S. pneumoniae, 50% for H. influenzae, and 80% for M. catarrhalis. However, to be clear, those rates were derived largely from assessments about 5 days after antibiotic treatment was started with ineffective drugs or with placebos and do not account for the true spontaneous clinical cure rate of AOM if assessed in the first few days after onset (when pain and fever are at their peak) nor if assessed 14-30 days later when almost all children have been cured by their immune systems.
The calculations also do not account for overdiagnosis in clinical practice. Indeed, if the child does not have AOM, then the child will have a cure regardless of which antibiotic is selected. Rates of overdiagnosis of AOM have been assessed with various methods and are subject to limitations. But overall the data and most experts agree that overdiagnosis by pediatricians, family physicians, urgent care physicians, nurse practitioners, and physician assistants is in the range of 30%-50%.
Before the reader leaps to the conclusion that I am endorsing any particular antibiotic strictly based on predicted in vitro efficacy, I would state that many considerations must be given to whether to use an antibiotic for AOM, and which antibiotic to use, at what dose, and for what duration. This column is just pointing out a few key up-to-date facts for your consideration.
It’s a new year and a new respiratory season so my thoughts turn to the most common infection in pediatrics where an antibiotic might appropriately be prescribed – acute otitis media (AOM). The guidelines of the American Academy of Pediatrics were finalized in 2012 and published in 2013 and based on data that the AAP subcommittee considered. A recommendation emerged for amoxicillin to remain the treatment of choice if an antibiotic was to be prescribed at all, leaving the observation option as a continued consideration under defined clinical circumstances. The oral alternative antibiotics recommended were amoxicillin/clavulanate and cefdinir (Pediatrics. 2013. doi: 10.1542/peds.2012-3488).
Since the AAP subcommittee deliberated, changes have occurred in AOM etiology and the frequency of antibiotic resistance among the common bacteria that cause the infection. Our group in Rochester (N.Y.) continues to be the only site in the United States conducting a prospective assessment of AOM; we hope our data are generalizable to the entire country, but that is not certain. In Rochester, we saw an overall drop in AOM incidence after introduction of Prevnar 7 of about 10%-15% overall and that corresponded reasonably well with the frequency of AOM caused by Streptococcus pneumoniae involving the seven serotypes in the PCV7 vaccine. We then had a rebound in AOM infections, largely caused by serotype 19A, such that the overall incidence of AOM returned back to levels nearly the same as before PCV7 by 2010. With the introduction of Prevnar 13, and the dramatic reduction of serotype 19A nasal colonization – a necessary precursor of AOM – the incidence of AOM overall fell again, and compared with the pre-PCV7 era, I estimate that we are seeing about 20%-25% less AOM today.
In late 2017, we published an article describing the epidemiology of AOM in the PCV era (Pediatrics. 2017 Aug. doi: 10.1542/peds.2017-0181), in which we described changes in otopathogen distribution over time from 1996 through 2016. It showed that by end of 2016, the predominant bacteria causing AOM were Haemophilus influenzae, accounting for 60% of all AOM (52% detected by culture from tympanocentesis and another 8% detected by polymerase chain reaction). Among the H. influenzae from middle ear fluid, beta-lactamase production occurred in 45%. Therefore, according to principles of infectious disease antibiotic efficacy predictions, use of amoxicillin in standard dose or high dose would not eradicate about half of the H. influenzae causing AOM. In the table included in this column, I show calculations of predicted outcomes from amoxicillin, amoxicillin/clavulanate, and cefdinir treatment based on the projected otopathogen mix and resistance frequencies of 2016. Added to the data on H. influenzae I have included results of S. pneumoniae high nonsusceptibility at 5% of strains and beta-lactamase production by Moraxella catarrhalis at 100% of strains. 
Strictly based on in vitro susceptibility and the known otopathogen mix, the calculations show that amoxicillin could result in a maximum cure of 57%, amoxicillin/clavulanate of 99%, and cefdinir of 80% of treated children.
In vitro susceptibility has its limitations. Pharmacodynamic calculations would drop the predicted success of all three antibiotics because suboptimal absorption after oral dosing occurs with amoxicillin and amoxicillin/clavulanate more so than with cefdinir, thereby resulting in lower than predicted levels of antibiotic at the site of infection within the middle ear, whereas the achievable level of cefdinir with recommended dosing sometimes is below the desired in vitro cut point.
To balance that lowered predicted efficacy, each of the otopathogens has an associated “spontaneous cure rate” that is often quoted as being 20% for S. pneumoniae, 50% for H. influenzae, and 80% for M. catarrhalis. However, to be clear, those rates were derived largely from assessments about 5 days after antibiotic treatment was started with ineffective drugs or with placebos and do not account for the true spontaneous clinical cure rate of AOM if assessed in the first few days after onset (when pain and fever are at their peak) nor if assessed 14-30 days later when almost all children have been cured by their immune systems.
The calculations also do not account for overdiagnosis in clinical practice. Indeed, if the child does not have AOM, then the child will have a cure regardless of which antibiotic is selected. Rates of overdiagnosis of AOM have been assessed with various methods and are subject to limitations. But overall the data and most experts agree that overdiagnosis by pediatricians, family physicians, urgent care physicians, nurse practitioners, and physician assistants is in the range of 30%-50%.
Before the reader leaps to the conclusion that I am endorsing any particular antibiotic strictly based on predicted in vitro efficacy, I would state that many considerations must be given to whether to use an antibiotic for AOM, and which antibiotic to use, at what dose, and for what duration. This column is just pointing out a few key up-to-date facts for your consideration.
Checklists to improve patient safety have mixed results
Clinical question: Do checklists improve patient safety among hospitalized patients?
Background: Systematic reviews of nonrandomized studies suggest checklists may reduce adverse events and medical errors. No study has systematically reviewed randomized trials or summarized the quality of evidence on this topic.
Study design: Systematic review of randomized controlled trials (RCTs) with pooled estimates of 30-day mortality.
Setting: RCTs reporting inpatient safety outcomes.
Synopsis: A search among four databases from inception through 2016 yielded nine studies meeting inclusion criteria. Checklists included tools for daily rounding, discharge planning, patient transfer, surgical safety and infection control procedures, pharmaceutical prescribing, and pain control. Three studies examined 30-day mortality, three studied length of stay, and two reported checklist compliance. Five reported patient outcomes and five reported provider-level outcomes related to patient safety. Findings regarding the effectiveness of checklists across studies were mixed. A random-effects model using pooled data from the three studies assessing 30-day mortality showed lower mortality associated with checklist use (odds ratio, 0.6, 95% confidence interval, 0.41-0.89; P = .01). The methodologic quality of studies was assessed as moderate. The review included studies with substantial heterogeneity in checklists employed and outcomes assessed. Though included studies were supposed to have assessed patient outcomes and not the processes of care, several studies cited did not report such outcomes.
Bottom line: Evidence regarding the effectiveness of clinical checklists on patient safety outcomes is mixed, and there is substantial heterogeneity in the types of checklists employed and outcomes assessed.
Citation: Boyd JM et al. The impact of checklists on inpatient safety outcomes: A systematic review of randomized controlled trials. J Hosp Med. 2017 Aug;12:675-82.
Dr. Simonetti is a hospitalist at the University of Colorado School of Medicine.
Clinical question: Do checklists improve patient safety among hospitalized patients?
Background: Systematic reviews of nonrandomized studies suggest checklists may reduce adverse events and medical errors. No study has systematically reviewed randomized trials or summarized the quality of evidence on this topic.
Study design: Systematic review of randomized controlled trials (RCTs) with pooled estimates of 30-day mortality.
Setting: RCTs reporting inpatient safety outcomes.
Synopsis: A search among four databases from inception through 2016 yielded nine studies meeting inclusion criteria. Checklists included tools for daily rounding, discharge planning, patient transfer, surgical safety and infection control procedures, pharmaceutical prescribing, and pain control. Three studies examined 30-day mortality, three studied length of stay, and two reported checklist compliance. Five reported patient outcomes and five reported provider-level outcomes related to patient safety. Findings regarding the effectiveness of checklists across studies were mixed. A random-effects model using pooled data from the three studies assessing 30-day mortality showed lower mortality associated with checklist use (odds ratio, 0.6, 95% confidence interval, 0.41-0.89; P = .01). The methodologic quality of studies was assessed as moderate. The review included studies with substantial heterogeneity in checklists employed and outcomes assessed. Though included studies were supposed to have assessed patient outcomes and not the processes of care, several studies cited did not report such outcomes.
Bottom line: Evidence regarding the effectiveness of clinical checklists on patient safety outcomes is mixed, and there is substantial heterogeneity in the types of checklists employed and outcomes assessed.
Citation: Boyd JM et al. The impact of checklists on inpatient safety outcomes: A systematic review of randomized controlled trials. J Hosp Med. 2017 Aug;12:675-82.
Dr. Simonetti is a hospitalist at the University of Colorado School of Medicine.
Clinical question: Do checklists improve patient safety among hospitalized patients?
Background: Systematic reviews of nonrandomized studies suggest checklists may reduce adverse events and medical errors. No study has systematically reviewed randomized trials or summarized the quality of evidence on this topic.
Study design: Systematic review of randomized controlled trials (RCTs) with pooled estimates of 30-day mortality.
Setting: RCTs reporting inpatient safety outcomes.
Synopsis: A search among four databases from inception through 2016 yielded nine studies meeting inclusion criteria. Checklists included tools for daily rounding, discharge planning, patient transfer, surgical safety and infection control procedures, pharmaceutical prescribing, and pain control. Three studies examined 30-day mortality, three studied length of stay, and two reported checklist compliance. Five reported patient outcomes and five reported provider-level outcomes related to patient safety. Findings regarding the effectiveness of checklists across studies were mixed. A random-effects model using pooled data from the three studies assessing 30-day mortality showed lower mortality associated with checklist use (odds ratio, 0.6, 95% confidence interval, 0.41-0.89; P = .01). The methodologic quality of studies was assessed as moderate. The review included studies with substantial heterogeneity in checklists employed and outcomes assessed. Though included studies were supposed to have assessed patient outcomes and not the processes of care, several studies cited did not report such outcomes.
Bottom line: Evidence regarding the effectiveness of clinical checklists on patient safety outcomes is mixed, and there is substantial heterogeneity in the types of checklists employed and outcomes assessed.
Citation: Boyd JM et al. The impact of checklists on inpatient safety outcomes: A systematic review of randomized controlled trials. J Hosp Med. 2017 Aug;12:675-82.
Dr. Simonetti is a hospitalist at the University of Colorado School of Medicine.
How Do They Test For Epilepsy?
mIDH inhibitors could fill treatment gap in AML
ATLANTA – Enasidenib, a first-in-class oral, selective inhibitor of mutant isocitrate dehydrogenase 2 (mIDH2) protein, shows promise both as monotherapy in older adults with untreated mIDH2 acute myeloid leukemia (AML), and in combination with azacitidine in patients with newly diagnosed AML, according to preliminary data from two phase 1/2 studies.
Of 239 patients aged 60 years and older from the AG221-C-001 phase 1 study of enasidenib monotherapy, 38 had previously untreated mIDH2 AML and were included in the current analysis, Daniel A. Pollyea, MD, reported at the annual meeting of the American Society of Hematology.
The previously untreated patients had a median age of 77 years, and at the Sept. 1, 2017, data cutoff, the median number of enasidenib treatment cycles in these patients was 6.5. Median follow-up was 8.6 months, said Dr. Pollyea of the University of Colorado, Aurora,
Overall, 7 of the 38 patients attained complete remission (CR). The median time to CR was 5.6 months. The overall response rate was 32%, Dr. Pollyea said, noting that the median duration of complete remission was not reached.
The median duration of any response was 12.2 months, he said.
Among all 38 patients, median overall survival was 10.4 months, and among responders and nonresponders it was 19.8 months and 5.4 months, respectively. Median event-free survival was 11.3 months.
Study subjects were adults aged 60 and older with previously untreated AML, who were not candidates for standard treatment. During dose-escalation they received 50-650 mg of enasidenib daily, and all patients in the expansion phase received 100 mg daily in continuous 28-day treatment cycles.
The findings are notable, because older patients with untreated AML, who are not candidates for standard induction therapy because of advanced age or health-related factors, pose a therapeutic challenge.
“We all know that older patients with newly diagnosed AML are often poor candidates for intensive chemotherapy approaches,” Dr. Pollyea said, explaining that this may be due to patient-related factors such as comorbidities that increase the risk of treatment-related mortality, or to adverse biologic features that make them less responsive to intensive chemotherapy. “The majority of older patients in this country are offered no treatment at all.”
In the current analysis, treatment was well tolerated; the rate of treatment-emergent adverse events was low, with only 2 of the 38 patients discontinuing treatment due to such an event. Serious treatment-related adverse events included isocitrate dehydrogenase (IDH) differentiation syndrome in four patients and tumor lysis syndrome in two patients. Grade 3-4 cytopenias were relatively uncommon, occurring in no more than 16% of patients.
The safety profile was similar to that reported for all patients in the phase 1 portions of the study, Dr. Pollyea noted.
These results suggest enasidenib may benefit older adults with mIDH2 AML who are not fit to receive cytotoxic chemotherapy, he said, adding that the encouraging and durable responses have prompted follow-up studies of enasidenib in older patients with previously untreated mIDH2 AML, such as the Beat AML Master Trial, and a study of enasidenib and ivosidenib (a small-molecule inhibitor of mIDH1 protein), each in combination with azacitidine in patients with newly diagnosed AML.
Combination approach
Preliminary findings from the latter trial (AG-221-AML-005) were presented at the ASH meeting by Courtney D. DiNardo, MD, who is also a coauthor on the AG221-C-001 study.
Eleven of 17 patients enrolled remained on study at the Sept. 1, 2017, data cutoff, including 3 of 6 who received enasidenib at doses of either 100 mg or 200 mg, and 8 of 11 who received 500 mg of ivosidenib.
In the enasidenib-treated patients, the overall response rate was 67% at data cutoff. Of those who received 100 mg of enasidenib, two achieved CR, and of those who received 200 mg, one achieved partial remission and one had morphologic leukemia-free state. Another maintained stable disease. One patient in the 100-mg group had progressive disease, said Dr. DiNardo of the University of Texas MD Anderson Cancer Center, Houston.
The patients who received enasidenib had a median age of 68 years, and the median number of treatment cycles overall was nine.
The most common treatment-emergent adverse events were hyperbilirubinemia and nausea, each occurring in four patients. Others, of any grade, included nausea, vomiting, and hyperbilirubinemia. IDH differentiation syndrome occurred in one patient in the 200-mg arm.
In the ivosidenib patients, the overall response rate was 73%; four patients achieved CR, one achieved CR with incomplete neutrophil recovery, one achieved partial remission, and two had morphologic leukemia-free state. Three maintained stable disease.
Patients in this group had a median age of 76 years and the median number of treatment cycles was three.
The most common treatment-emergent adverse events were nausea, constipation, fatigue, and diarrhea.
One patient experienced IDH differentiation syndrome, and two patients developed pneumonia. One of the patients with pneumonia died, but the event was not considered treatment related.
The findings suggest that both enasidenib and ivosidenib in combination with azacitidine are generally well tolerated in patients with newly diagnosed AML, Dr. DiNardo said.
Both agents were shown preclinically to reduce aberrant 2-HG levels and to promote myeloid differentiation. As monotherapies, they induce clinical responses in patients with mIDH relapsed/refractory AML, she said.
Further, azacitidine monotherapy prolongs survival, compared with conventional care, in older patients with newly diagnosed AML, she explained. She said that combinations of mIDH inhibitors and azacitidine in vitro showed synergistic effects on releasing differentiation block in mIDH leukemia models, providing a clinical rationale for combining these agents for the treatment of AML.
The current findings represent the initial results of the phase 1b portion of an ongoing phase 1b/2 study. “Preliminary efficacy results with these combination regimens are encouraging,” Dr. DiNardo said. “Phase 1b confirms the recommended monotherapy doses of enasidenib 100 mg, ivosidenib 500 mg as safe and effective in combination with azacitidine.”
These treatments will move forward for additional study in combination regimens, she said, noting that the evaluation of mIDH inhibitors plus azacitidine continues in two currently enrolling randomized studies, including the expansion phase of the current study and the phase 3 AGILE study of ivosidenib plus azacitidine in newly diagnosed AML patients not suitable for intensive therapy.
Both studies were sponsored by Celgene, the maker of enasidenib. Dr. Pollyea reported ties to Takeda, Ariad, Alexion, Celgene, Pfizer, Pharmacyclics, Gilead, Jazz, Servier, Curis, and Agios. Dr. DiNardo reported ties to Novartis, AbbVie, Celgene, Agios,and Daiichi Sankyo.
SOURCE: Pollyea D et al. ASH 2017 Abstract 638; DiNardo C et al. ASH 2017 Abstract 639
ATLANTA – Enasidenib, a first-in-class oral, selective inhibitor of mutant isocitrate dehydrogenase 2 (mIDH2) protein, shows promise both as monotherapy in older adults with untreated mIDH2 acute myeloid leukemia (AML), and in combination with azacitidine in patients with newly diagnosed AML, according to preliminary data from two phase 1/2 studies.
Of 239 patients aged 60 years and older from the AG221-C-001 phase 1 study of enasidenib monotherapy, 38 had previously untreated mIDH2 AML and were included in the current analysis, Daniel A. Pollyea, MD, reported at the annual meeting of the American Society of Hematology.
The previously untreated patients had a median age of 77 years, and at the Sept. 1, 2017, data cutoff, the median number of enasidenib treatment cycles in these patients was 6.5. Median follow-up was 8.6 months, said Dr. Pollyea of the University of Colorado, Aurora,
Overall, 7 of the 38 patients attained complete remission (CR). The median time to CR was 5.6 months. The overall response rate was 32%, Dr. Pollyea said, noting that the median duration of complete remission was not reached.
The median duration of any response was 12.2 months, he said.
Among all 38 patients, median overall survival was 10.4 months, and among responders and nonresponders it was 19.8 months and 5.4 months, respectively. Median event-free survival was 11.3 months.
Study subjects were adults aged 60 and older with previously untreated AML, who were not candidates for standard treatment. During dose-escalation they received 50-650 mg of enasidenib daily, and all patients in the expansion phase received 100 mg daily in continuous 28-day treatment cycles.
The findings are notable, because older patients with untreated AML, who are not candidates for standard induction therapy because of advanced age or health-related factors, pose a therapeutic challenge.
“We all know that older patients with newly diagnosed AML are often poor candidates for intensive chemotherapy approaches,” Dr. Pollyea said, explaining that this may be due to patient-related factors such as comorbidities that increase the risk of treatment-related mortality, or to adverse biologic features that make them less responsive to intensive chemotherapy. “The majority of older patients in this country are offered no treatment at all.”
In the current analysis, treatment was well tolerated; the rate of treatment-emergent adverse events was low, with only 2 of the 38 patients discontinuing treatment due to such an event. Serious treatment-related adverse events included isocitrate dehydrogenase (IDH) differentiation syndrome in four patients and tumor lysis syndrome in two patients. Grade 3-4 cytopenias were relatively uncommon, occurring in no more than 16% of patients.
The safety profile was similar to that reported for all patients in the phase 1 portions of the study, Dr. Pollyea noted.
These results suggest enasidenib may benefit older adults with mIDH2 AML who are not fit to receive cytotoxic chemotherapy, he said, adding that the encouraging and durable responses have prompted follow-up studies of enasidenib in older patients with previously untreated mIDH2 AML, such as the Beat AML Master Trial, and a study of enasidenib and ivosidenib (a small-molecule inhibitor of mIDH1 protein), each in combination with azacitidine in patients with newly diagnosed AML.
Combination approach
Preliminary findings from the latter trial (AG-221-AML-005) were presented at the ASH meeting by Courtney D. DiNardo, MD, who is also a coauthor on the AG221-C-001 study.
Eleven of 17 patients enrolled remained on study at the Sept. 1, 2017, data cutoff, including 3 of 6 who received enasidenib at doses of either 100 mg or 200 mg, and 8 of 11 who received 500 mg of ivosidenib.
In the enasidenib-treated patients, the overall response rate was 67% at data cutoff. Of those who received 100 mg of enasidenib, two achieved CR, and of those who received 200 mg, one achieved partial remission and one had morphologic leukemia-free state. Another maintained stable disease. One patient in the 100-mg group had progressive disease, said Dr. DiNardo of the University of Texas MD Anderson Cancer Center, Houston.
The patients who received enasidenib had a median age of 68 years, and the median number of treatment cycles overall was nine.
The most common treatment-emergent adverse events were hyperbilirubinemia and nausea, each occurring in four patients. Others, of any grade, included nausea, vomiting, and hyperbilirubinemia. IDH differentiation syndrome occurred in one patient in the 200-mg arm.
In the ivosidenib patients, the overall response rate was 73%; four patients achieved CR, one achieved CR with incomplete neutrophil recovery, one achieved partial remission, and two had morphologic leukemia-free state. Three maintained stable disease.
Patients in this group had a median age of 76 years and the median number of treatment cycles was three.
The most common treatment-emergent adverse events were nausea, constipation, fatigue, and diarrhea.
One patient experienced IDH differentiation syndrome, and two patients developed pneumonia. One of the patients with pneumonia died, but the event was not considered treatment related.
The findings suggest that both enasidenib and ivosidenib in combination with azacitidine are generally well tolerated in patients with newly diagnosed AML, Dr. DiNardo said.
Both agents were shown preclinically to reduce aberrant 2-HG levels and to promote myeloid differentiation. As monotherapies, they induce clinical responses in patients with mIDH relapsed/refractory AML, she said.
Further, azacitidine monotherapy prolongs survival, compared with conventional care, in older patients with newly diagnosed AML, she explained. She said that combinations of mIDH inhibitors and azacitidine in vitro showed synergistic effects on releasing differentiation block in mIDH leukemia models, providing a clinical rationale for combining these agents for the treatment of AML.
The current findings represent the initial results of the phase 1b portion of an ongoing phase 1b/2 study. “Preliminary efficacy results with these combination regimens are encouraging,” Dr. DiNardo said. “Phase 1b confirms the recommended monotherapy doses of enasidenib 100 mg, ivosidenib 500 mg as safe and effective in combination with azacitidine.”
These treatments will move forward for additional study in combination regimens, she said, noting that the evaluation of mIDH inhibitors plus azacitidine continues in two currently enrolling randomized studies, including the expansion phase of the current study and the phase 3 AGILE study of ivosidenib plus azacitidine in newly diagnosed AML patients not suitable for intensive therapy.
Both studies were sponsored by Celgene, the maker of enasidenib. Dr. Pollyea reported ties to Takeda, Ariad, Alexion, Celgene, Pfizer, Pharmacyclics, Gilead, Jazz, Servier, Curis, and Agios. Dr. DiNardo reported ties to Novartis, AbbVie, Celgene, Agios,and Daiichi Sankyo.
SOURCE: Pollyea D et al. ASH 2017 Abstract 638; DiNardo C et al. ASH 2017 Abstract 639
ATLANTA – Enasidenib, a first-in-class oral, selective inhibitor of mutant isocitrate dehydrogenase 2 (mIDH2) protein, shows promise both as monotherapy in older adults with untreated mIDH2 acute myeloid leukemia (AML), and in combination with azacitidine in patients with newly diagnosed AML, according to preliminary data from two phase 1/2 studies.
Of 239 patients aged 60 years and older from the AG221-C-001 phase 1 study of enasidenib monotherapy, 38 had previously untreated mIDH2 AML and were included in the current analysis, Daniel A. Pollyea, MD, reported at the annual meeting of the American Society of Hematology.
The previously untreated patients had a median age of 77 years, and at the Sept. 1, 2017, data cutoff, the median number of enasidenib treatment cycles in these patients was 6.5. Median follow-up was 8.6 months, said Dr. Pollyea of the University of Colorado, Aurora,
Overall, 7 of the 38 patients attained complete remission (CR). The median time to CR was 5.6 months. The overall response rate was 32%, Dr. Pollyea said, noting that the median duration of complete remission was not reached.
The median duration of any response was 12.2 months, he said.
Among all 38 patients, median overall survival was 10.4 months, and among responders and nonresponders it was 19.8 months and 5.4 months, respectively. Median event-free survival was 11.3 months.
Study subjects were adults aged 60 and older with previously untreated AML, who were not candidates for standard treatment. During dose-escalation they received 50-650 mg of enasidenib daily, and all patients in the expansion phase received 100 mg daily in continuous 28-day treatment cycles.
The findings are notable, because older patients with untreated AML, who are not candidates for standard induction therapy because of advanced age or health-related factors, pose a therapeutic challenge.
“We all know that older patients with newly diagnosed AML are often poor candidates for intensive chemotherapy approaches,” Dr. Pollyea said, explaining that this may be due to patient-related factors such as comorbidities that increase the risk of treatment-related mortality, or to adverse biologic features that make them less responsive to intensive chemotherapy. “The majority of older patients in this country are offered no treatment at all.”
In the current analysis, treatment was well tolerated; the rate of treatment-emergent adverse events was low, with only 2 of the 38 patients discontinuing treatment due to such an event. Serious treatment-related adverse events included isocitrate dehydrogenase (IDH) differentiation syndrome in four patients and tumor lysis syndrome in two patients. Grade 3-4 cytopenias were relatively uncommon, occurring in no more than 16% of patients.
The safety profile was similar to that reported for all patients in the phase 1 portions of the study, Dr. Pollyea noted.
These results suggest enasidenib may benefit older adults with mIDH2 AML who are not fit to receive cytotoxic chemotherapy, he said, adding that the encouraging and durable responses have prompted follow-up studies of enasidenib in older patients with previously untreated mIDH2 AML, such as the Beat AML Master Trial, and a study of enasidenib and ivosidenib (a small-molecule inhibitor of mIDH1 protein), each in combination with azacitidine in patients with newly diagnosed AML.
Combination approach
Preliminary findings from the latter trial (AG-221-AML-005) were presented at the ASH meeting by Courtney D. DiNardo, MD, who is also a coauthor on the AG221-C-001 study.
Eleven of 17 patients enrolled remained on study at the Sept. 1, 2017, data cutoff, including 3 of 6 who received enasidenib at doses of either 100 mg or 200 mg, and 8 of 11 who received 500 mg of ivosidenib.
In the enasidenib-treated patients, the overall response rate was 67% at data cutoff. Of those who received 100 mg of enasidenib, two achieved CR, and of those who received 200 mg, one achieved partial remission and one had morphologic leukemia-free state. Another maintained stable disease. One patient in the 100-mg group had progressive disease, said Dr. DiNardo of the University of Texas MD Anderson Cancer Center, Houston.
The patients who received enasidenib had a median age of 68 years, and the median number of treatment cycles overall was nine.
The most common treatment-emergent adverse events were hyperbilirubinemia and nausea, each occurring in four patients. Others, of any grade, included nausea, vomiting, and hyperbilirubinemia. IDH differentiation syndrome occurred in one patient in the 200-mg arm.
In the ivosidenib patients, the overall response rate was 73%; four patients achieved CR, one achieved CR with incomplete neutrophil recovery, one achieved partial remission, and two had morphologic leukemia-free state. Three maintained stable disease.
Patients in this group had a median age of 76 years and the median number of treatment cycles was three.
The most common treatment-emergent adverse events were nausea, constipation, fatigue, and diarrhea.
One patient experienced IDH differentiation syndrome, and two patients developed pneumonia. One of the patients with pneumonia died, but the event was not considered treatment related.
The findings suggest that both enasidenib and ivosidenib in combination with azacitidine are generally well tolerated in patients with newly diagnosed AML, Dr. DiNardo said.
Both agents were shown preclinically to reduce aberrant 2-HG levels and to promote myeloid differentiation. As monotherapies, they induce clinical responses in patients with mIDH relapsed/refractory AML, she said.
Further, azacitidine monotherapy prolongs survival, compared with conventional care, in older patients with newly diagnosed AML, she explained. She said that combinations of mIDH inhibitors and azacitidine in vitro showed synergistic effects on releasing differentiation block in mIDH leukemia models, providing a clinical rationale for combining these agents for the treatment of AML.
The current findings represent the initial results of the phase 1b portion of an ongoing phase 1b/2 study. “Preliminary efficacy results with these combination regimens are encouraging,” Dr. DiNardo said. “Phase 1b confirms the recommended monotherapy doses of enasidenib 100 mg, ivosidenib 500 mg as safe and effective in combination with azacitidine.”
These treatments will move forward for additional study in combination regimens, she said, noting that the evaluation of mIDH inhibitors plus azacitidine continues in two currently enrolling randomized studies, including the expansion phase of the current study and the phase 3 AGILE study of ivosidenib plus azacitidine in newly diagnosed AML patients not suitable for intensive therapy.
Both studies were sponsored by Celgene, the maker of enasidenib. Dr. Pollyea reported ties to Takeda, Ariad, Alexion, Celgene, Pfizer, Pharmacyclics, Gilead, Jazz, Servier, Curis, and Agios. Dr. DiNardo reported ties to Novartis, AbbVie, Celgene, Agios,and Daiichi Sankyo.
SOURCE: Pollyea D et al. ASH 2017 Abstract 638; DiNardo C et al. ASH 2017 Abstract 639
REPORTING FROM ASH 2017
Key clinical point:
Major finding: The overall response rates were 32%, 67%, and 73% with enasidenib, enasidenib plus azacitidine, and ivosidenib plus azacitidine, respectively.
Study details: Two phase 1/2 studies of 38 and 17 patients.
Disclosures: Both studies were sponsored by Celgene. Dr. Pollyea reported ties to Takeda, Ariad, Alexion, Celgene, Pfizer, Pharmacyclics, Gilead, Jazz, Servier, Curis, and Agios. Dr. DiNardo reported ties to Novartis, AbbVie, Celgene, Agios,and Daiichi Sankyo.
Sources: Pollyea D et al. ASH 2017 Abstract 638; DiNardo C et al. ASH 2017 Abstract 639.
MDedge Daily News: Why prior authorization may get easier
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
A mitochondrial protein may help explain gender differences in cardiovascular risk, PPIs may boost the risk of C. difficile infections in hospitals, topical retinoids and benzoyl peroxide are tops for inflammatory acne, and the grueling prior authorization process may get easier.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
A mitochondrial protein may help explain gender differences in cardiovascular risk, PPIs may boost the risk of C. difficile infections in hospitals, topical retinoids and benzoyl peroxide are tops for inflammatory acne, and the grueling prior authorization process may get easier.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
A mitochondrial protein may help explain gender differences in cardiovascular risk, PPIs may boost the risk of C. difficile infections in hospitals, topical retinoids and benzoyl peroxide are tops for inflammatory acne, and the grueling prior authorization process may get easier.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Alex Azar confirmed as HHS Secretary
Alex M. Azar II has been confirmed as secretary of the Department of Health & Human Services following a Jan. 24 vote in the U.S. Senate.
His nomination passed by a vote of 55-43.
Mr. Azar has previously been confirmed to two posts at HHS, first as general counsel and later as deputy HHS secretary during the administration of President George W. Bush between 2001 and 2007. Both appointments were confirmed by unanimous consent in the Senate.
Democrats on the Senate Finance Committee challenged Mr. Azar on drug prices and other issues, but their objections to his pharmaceutical industry past were not enough to stop his confirmation.
“Mr. Azar was part of this broken system [of high drug pricing], and despite the cheerful overtures that he has made to senators on the other side of the aisle over the last few weeks on how he wants to work on the issue, he has not given a single concrete example of how he would actually change the system, change the system that he said is broken,” Senate Finance Committee Ranking Member Ron Wyden (D-Ore.) said on the Senate floor. “He won’t give us an example of how he would change it to make it better.”
In a statement, Senate Health, Education, Labor, and Pensions Chairman Lamar Alexander (R-Tenn.) said that Mr. Azar “has the broad perspective necessary to address the opioid crisis.”
However, Sen. Maggie Hassan (D-N.H.) expressed concern about whether he could effectively address the opioid epidemic.
“I was disappointed that Mr. Azar would not commit to advocating for new funding during his confirmation hearing. Considering his tenure as a top executive at a major pharmaceutical company, I also continue to have serious doubts that Mr. Azar can be a leader in addressing the skyrocketing cost of prescription drugs,” she said.
Alex M. Azar II has been confirmed as secretary of the Department of Health & Human Services following a Jan. 24 vote in the U.S. Senate.
His nomination passed by a vote of 55-43.
Mr. Azar has previously been confirmed to two posts at HHS, first as general counsel and later as deputy HHS secretary during the administration of President George W. Bush between 2001 and 2007. Both appointments were confirmed by unanimous consent in the Senate.
Democrats on the Senate Finance Committee challenged Mr. Azar on drug prices and other issues, but their objections to his pharmaceutical industry past were not enough to stop his confirmation.
“Mr. Azar was part of this broken system [of high drug pricing], and despite the cheerful overtures that he has made to senators on the other side of the aisle over the last few weeks on how he wants to work on the issue, he has not given a single concrete example of how he would actually change the system, change the system that he said is broken,” Senate Finance Committee Ranking Member Ron Wyden (D-Ore.) said on the Senate floor. “He won’t give us an example of how he would change it to make it better.”
In a statement, Senate Health, Education, Labor, and Pensions Chairman Lamar Alexander (R-Tenn.) said that Mr. Azar “has the broad perspective necessary to address the opioid crisis.”
However, Sen. Maggie Hassan (D-N.H.) expressed concern about whether he could effectively address the opioid epidemic.
“I was disappointed that Mr. Azar would not commit to advocating for new funding during his confirmation hearing. Considering his tenure as a top executive at a major pharmaceutical company, I also continue to have serious doubts that Mr. Azar can be a leader in addressing the skyrocketing cost of prescription drugs,” she said.
Alex M. Azar II has been confirmed as secretary of the Department of Health & Human Services following a Jan. 24 vote in the U.S. Senate.
His nomination passed by a vote of 55-43.
Mr. Azar has previously been confirmed to two posts at HHS, first as general counsel and later as deputy HHS secretary during the administration of President George W. Bush between 2001 and 2007. Both appointments were confirmed by unanimous consent in the Senate.
Democrats on the Senate Finance Committee challenged Mr. Azar on drug prices and other issues, but their objections to his pharmaceutical industry past were not enough to stop his confirmation.
“Mr. Azar was part of this broken system [of high drug pricing], and despite the cheerful overtures that he has made to senators on the other side of the aisle over the last few weeks on how he wants to work on the issue, he has not given a single concrete example of how he would actually change the system, change the system that he said is broken,” Senate Finance Committee Ranking Member Ron Wyden (D-Ore.) said on the Senate floor. “He won’t give us an example of how he would change it to make it better.”
In a statement, Senate Health, Education, Labor, and Pensions Chairman Lamar Alexander (R-Tenn.) said that Mr. Azar “has the broad perspective necessary to address the opioid crisis.”
However, Sen. Maggie Hassan (D-N.H.) expressed concern about whether he could effectively address the opioid epidemic.
“I was disappointed that Mr. Azar would not commit to advocating for new funding during his confirmation hearing. Considering his tenure as a top executive at a major pharmaceutical company, I also continue to have serious doubts that Mr. Azar can be a leader in addressing the skyrocketing cost of prescription drugs,” she said.
Gene therapy moves from promise to reality
After decades of hype, dashed hopes, and setbacks, gene therapy has finally arrived and is poised to transform the treatment paradigm for many diseases, according to Cynthia E. Dunbar, MD, senior investigator at the Hematology Branch of the National Heart, Lung, and Blood Institute (NHLBI), part of the National Institutes of Health.
Hematologists can expect more developments that build on current successes with chimeric antigen receptor (CAR) T-cell therapy and gene therapy advances for hemophilia, as well as emerging advances in gene editing techniques including the CRISPR/Cas9 approach, Dr. Dunbar said in an interview.
That’s on top of a small number of regulatory approvals in the United States and Europe, she said. “Along with that, there’s a lot of interest and now involvement from biotechnology companies and even large pharmaceutical companies. I think all those factors really have to come together to create this kind of acceleration, and I’ve never seen anything like this previously.”
Dr. Dunbar – a former editor in chief of the journal Blood – and her colleagues recently published a review of current developments and emerging gene therapy technologies in the journal Science (2018 Jan 12. doi: 10.1126/science.aan4672).
“We really felt it was the right time to write the article,” she said.
Milestones
A new approach to cancer treatment was ushered in on Aug. 30, 2017, with the Food and Drug Administration approval of tisagenlecleucel, the first-ever gene therapy available in the United States. The CD19-directed CAR T-cell therapy is indicated for treatment of certain pediatric or young adult patients with B-cell precursor acute lymphoblastic leukemia that is refractory or in second or later relapse.
Soon afterward, FDA approved another CD19-directed CAR T-cell therapy, axicabtagene ciloleucel, for adult patients with large B-cell lymphoma after two or more lines of systemic therapy.
“It’s a very interesting time for immunotherapies in general,” Dr. Dunbar said. “There’s a huge number of options in terms of PD-1 inhibitors and other pharmacologics or antibodies that allow the patient’s own immune system to attack tumors. CAR T-cell therapy is an obvious step beyond that, in terms of arming your own T cells to very specifically target tumor cells.”
But randomized trials or meta-analyses may be necessary to determine the place of CAR T-cell therapy in the treatment armamentarium for acute lymphoblastic leukemia and large B-cell lymphoma given their cost and the availability of other therapeutic options, Dr. Dunbar suggested.
“Gene therapies have a large upfront cost, but if they’re truly curative and a one-time treatment, then they may in the long run be much cheaper than doing failed multiple transplants or needing monoclonal antibody infusion every 2 weeks for the rest of your life,” she said.
Another major success story still in the works, according to Dr. Dunbar, is the treatment of hemophilia A and B with gene therapy approaches. The positive data include a recent report showing that transgene-derived factor IX coagulant activity allowed for the termination of baseline prophylaxis, and the near elimination of bleeding and factor use, in patients with hemophilia B (N Engl J Med. 2017 Dec 7;377[23]:2215-27).
While gene therapy for hemophilia A has been more challenging, another recent report nevertheless demonstrated sustained normalization of factor VIII activity level with a single intravenous infusion of adeno-associated virus serotype 5 vector encoding a B-domain–deleted human factor VIII (N Engl J Med. 2017 Dec 28;377[26]:2519-30).
“The proof-of-principle was already there in hemophilia B,” Dr. Dunbar said. “It really was just a question of figuring out a way to package and deliver a Factor VIII that would work in the constraints of an AAV [adeno-associated virus] vector.”
Meanwhile, myeloma trials of CAR T-cell therapy seem very promising so far, but the challenge in that disease could be finding a place for gene therapy in a “much more diverse treatment landscape” that includes multiple effective regimens, according to Dr. Dunbar.
Future trends, challenges
Looking forward, she said.
Notably, genome editing approaches to treat sickle cell anemia are likely to move forward in the near future, according to Dr. Dunbar, following reports validating an erythroid enhancer of human BCL11A as a target for reinduction of fetal hemoglobin (Nature. 2015 Nov 12;527[7577]:192-7).
But all of this gene therapy development creates an educational challenge for frontline clinicians, even if the administration of CAR T-cell therapy and other advanced treatments is limited to highly specialized centers.
“There’s a lot of training that needs to go on with hematologists, oncologists, and other doctors about how to care for these patients after these treatments, in terms of what to look for and how to intervene early to prevent, for instance, severe toxicity from cytokine release syndrome,” Dr. Dunbar said.
Dr. Dunbar reported having no relevant financial disclosures.
After decades of hype, dashed hopes, and setbacks, gene therapy has finally arrived and is poised to transform the treatment paradigm for many diseases, according to Cynthia E. Dunbar, MD, senior investigator at the Hematology Branch of the National Heart, Lung, and Blood Institute (NHLBI), part of the National Institutes of Health.
Hematologists can expect more developments that build on current successes with chimeric antigen receptor (CAR) T-cell therapy and gene therapy advances for hemophilia, as well as emerging advances in gene editing techniques including the CRISPR/Cas9 approach, Dr. Dunbar said in an interview.
That’s on top of a small number of regulatory approvals in the United States and Europe, she said. “Along with that, there’s a lot of interest and now involvement from biotechnology companies and even large pharmaceutical companies. I think all those factors really have to come together to create this kind of acceleration, and I’ve never seen anything like this previously.”
Dr. Dunbar – a former editor in chief of the journal Blood – and her colleagues recently published a review of current developments and emerging gene therapy technologies in the journal Science (2018 Jan 12. doi: 10.1126/science.aan4672).
“We really felt it was the right time to write the article,” she said.
Milestones
A new approach to cancer treatment was ushered in on Aug. 30, 2017, with the Food and Drug Administration approval of tisagenlecleucel, the first-ever gene therapy available in the United States. The CD19-directed CAR T-cell therapy is indicated for treatment of certain pediatric or young adult patients with B-cell precursor acute lymphoblastic leukemia that is refractory or in second or later relapse.
Soon afterward, FDA approved another CD19-directed CAR T-cell therapy, axicabtagene ciloleucel, for adult patients with large B-cell lymphoma after two or more lines of systemic therapy.
“It’s a very interesting time for immunotherapies in general,” Dr. Dunbar said. “There’s a huge number of options in terms of PD-1 inhibitors and other pharmacologics or antibodies that allow the patient’s own immune system to attack tumors. CAR T-cell therapy is an obvious step beyond that, in terms of arming your own T cells to very specifically target tumor cells.”
But randomized trials or meta-analyses may be necessary to determine the place of CAR T-cell therapy in the treatment armamentarium for acute lymphoblastic leukemia and large B-cell lymphoma given their cost and the availability of other therapeutic options, Dr. Dunbar suggested.
“Gene therapies have a large upfront cost, but if they’re truly curative and a one-time treatment, then they may in the long run be much cheaper than doing failed multiple transplants or needing monoclonal antibody infusion every 2 weeks for the rest of your life,” she said.
Another major success story still in the works, according to Dr. Dunbar, is the treatment of hemophilia A and B with gene therapy approaches. The positive data include a recent report showing that transgene-derived factor IX coagulant activity allowed for the termination of baseline prophylaxis, and the near elimination of bleeding and factor use, in patients with hemophilia B (N Engl J Med. 2017 Dec 7;377[23]:2215-27).
While gene therapy for hemophilia A has been more challenging, another recent report nevertheless demonstrated sustained normalization of factor VIII activity level with a single intravenous infusion of adeno-associated virus serotype 5 vector encoding a B-domain–deleted human factor VIII (N Engl J Med. 2017 Dec 28;377[26]:2519-30).
“The proof-of-principle was already there in hemophilia B,” Dr. Dunbar said. “It really was just a question of figuring out a way to package and deliver a Factor VIII that would work in the constraints of an AAV [adeno-associated virus] vector.”
Meanwhile, myeloma trials of CAR T-cell therapy seem very promising so far, but the challenge in that disease could be finding a place for gene therapy in a “much more diverse treatment landscape” that includes multiple effective regimens, according to Dr. Dunbar.
Future trends, challenges
Looking forward, she said.
Notably, genome editing approaches to treat sickle cell anemia are likely to move forward in the near future, according to Dr. Dunbar, following reports validating an erythroid enhancer of human BCL11A as a target for reinduction of fetal hemoglobin (Nature. 2015 Nov 12;527[7577]:192-7).
But all of this gene therapy development creates an educational challenge for frontline clinicians, even if the administration of CAR T-cell therapy and other advanced treatments is limited to highly specialized centers.
“There’s a lot of training that needs to go on with hematologists, oncologists, and other doctors about how to care for these patients after these treatments, in terms of what to look for and how to intervene early to prevent, for instance, severe toxicity from cytokine release syndrome,” Dr. Dunbar said.
Dr. Dunbar reported having no relevant financial disclosures.
After decades of hype, dashed hopes, and setbacks, gene therapy has finally arrived and is poised to transform the treatment paradigm for many diseases, according to Cynthia E. Dunbar, MD, senior investigator at the Hematology Branch of the National Heart, Lung, and Blood Institute (NHLBI), part of the National Institutes of Health.
Hematologists can expect more developments that build on current successes with chimeric antigen receptor (CAR) T-cell therapy and gene therapy advances for hemophilia, as well as emerging advances in gene editing techniques including the CRISPR/Cas9 approach, Dr. Dunbar said in an interview.
That’s on top of a small number of regulatory approvals in the United States and Europe, she said. “Along with that, there’s a lot of interest and now involvement from biotechnology companies and even large pharmaceutical companies. I think all those factors really have to come together to create this kind of acceleration, and I’ve never seen anything like this previously.”
Dr. Dunbar – a former editor in chief of the journal Blood – and her colleagues recently published a review of current developments and emerging gene therapy technologies in the journal Science (2018 Jan 12. doi: 10.1126/science.aan4672).
“We really felt it was the right time to write the article,” she said.
Milestones
A new approach to cancer treatment was ushered in on Aug. 30, 2017, with the Food and Drug Administration approval of tisagenlecleucel, the first-ever gene therapy available in the United States. The CD19-directed CAR T-cell therapy is indicated for treatment of certain pediatric or young adult patients with B-cell precursor acute lymphoblastic leukemia that is refractory or in second or later relapse.
Soon afterward, FDA approved another CD19-directed CAR T-cell therapy, axicabtagene ciloleucel, for adult patients with large B-cell lymphoma after two or more lines of systemic therapy.
“It’s a very interesting time for immunotherapies in general,” Dr. Dunbar said. “There’s a huge number of options in terms of PD-1 inhibitors and other pharmacologics or antibodies that allow the patient’s own immune system to attack tumors. CAR T-cell therapy is an obvious step beyond that, in terms of arming your own T cells to very specifically target tumor cells.”
But randomized trials or meta-analyses may be necessary to determine the place of CAR T-cell therapy in the treatment armamentarium for acute lymphoblastic leukemia and large B-cell lymphoma given their cost and the availability of other therapeutic options, Dr. Dunbar suggested.
“Gene therapies have a large upfront cost, but if they’re truly curative and a one-time treatment, then they may in the long run be much cheaper than doing failed multiple transplants or needing monoclonal antibody infusion every 2 weeks for the rest of your life,” she said.
Another major success story still in the works, according to Dr. Dunbar, is the treatment of hemophilia A and B with gene therapy approaches. The positive data include a recent report showing that transgene-derived factor IX coagulant activity allowed for the termination of baseline prophylaxis, and the near elimination of bleeding and factor use, in patients with hemophilia B (N Engl J Med. 2017 Dec 7;377[23]:2215-27).
While gene therapy for hemophilia A has been more challenging, another recent report nevertheless demonstrated sustained normalization of factor VIII activity level with a single intravenous infusion of adeno-associated virus serotype 5 vector encoding a B-domain–deleted human factor VIII (N Engl J Med. 2017 Dec 28;377[26]:2519-30).
“The proof-of-principle was already there in hemophilia B,” Dr. Dunbar said. “It really was just a question of figuring out a way to package and deliver a Factor VIII that would work in the constraints of an AAV [adeno-associated virus] vector.”
Meanwhile, myeloma trials of CAR T-cell therapy seem very promising so far, but the challenge in that disease could be finding a place for gene therapy in a “much more diverse treatment landscape” that includes multiple effective regimens, according to Dr. Dunbar.
Future trends, challenges
Looking forward, she said.
Notably, genome editing approaches to treat sickle cell anemia are likely to move forward in the near future, according to Dr. Dunbar, following reports validating an erythroid enhancer of human BCL11A as a target for reinduction of fetal hemoglobin (Nature. 2015 Nov 12;527[7577]:192-7).
But all of this gene therapy development creates an educational challenge for frontline clinicians, even if the administration of CAR T-cell therapy and other advanced treatments is limited to highly specialized centers.
“There’s a lot of training that needs to go on with hematologists, oncologists, and other doctors about how to care for these patients after these treatments, in terms of what to look for and how to intervene early to prevent, for instance, severe toxicity from cytokine release syndrome,” Dr. Dunbar said.
Dr. Dunbar reported having no relevant financial disclosures.
Uncovering Clues That Explain the Ototoxicity of Cisplatin
Cisplatin and other platinum-based drugs are prescribed to 10% to 20% of patients with cancer. The drugs cause permanent hearing loss in as many as 80% of adult patients and at least half of children. Why is cisplatin so toxic to the inner ear?
Researchers from the National Institute on Deafness and other Communications Disorders may have found the answer. The inner ear readily takes up cisplatin but has little ability to remove the drug. In most areas of the body, cisplatin is eliminated within days or weeks after treatment; in the inner ear it remains much longer.
Using a mouse model, the researchers found cisplatin remained in the inner ear much longer than in most other body tissues and built up with each treatment. They also studied inner ear tissue donated by deceased patients who had been treated with cisplatin and found cisplatin remained in the inner ear many months or even years after treatment. They also examined inner ear tissue from one child, and found cisplatin buildup even higher than that seen in adults.
The highest buildup of cisplatin was in the stria vascularis, which helps maintain the positive electrical charge in inner ear fluid that certain cells need to detect sound, the researchers say. They believe that accumulation contributed to the hearing loss. “If we can prevent cisplatin from entering the stria vascularis in the inner ear during treatment,” Lisa Cunningham, PhD, lead investigator says, “we may be able to protect cancer patients from developing cisplatin-induced hearing loss.”
Cisplatin and other platinum-based drugs are prescribed to 10% to 20% of patients with cancer. The drugs cause permanent hearing loss in as many as 80% of adult patients and at least half of children. Why is cisplatin so toxic to the inner ear?
Researchers from the National Institute on Deafness and other Communications Disorders may have found the answer. The inner ear readily takes up cisplatin but has little ability to remove the drug. In most areas of the body, cisplatin is eliminated within days or weeks after treatment; in the inner ear it remains much longer.
Using a mouse model, the researchers found cisplatin remained in the inner ear much longer than in most other body tissues and built up with each treatment. They also studied inner ear tissue donated by deceased patients who had been treated with cisplatin and found cisplatin remained in the inner ear many months or even years after treatment. They also examined inner ear tissue from one child, and found cisplatin buildup even higher than that seen in adults.
The highest buildup of cisplatin was in the stria vascularis, which helps maintain the positive electrical charge in inner ear fluid that certain cells need to detect sound, the researchers say. They believe that accumulation contributed to the hearing loss. “If we can prevent cisplatin from entering the stria vascularis in the inner ear during treatment,” Lisa Cunningham, PhD, lead investigator says, “we may be able to protect cancer patients from developing cisplatin-induced hearing loss.”
Cisplatin and other platinum-based drugs are prescribed to 10% to 20% of patients with cancer. The drugs cause permanent hearing loss in as many as 80% of adult patients and at least half of children. Why is cisplatin so toxic to the inner ear?
Researchers from the National Institute on Deafness and other Communications Disorders may have found the answer. The inner ear readily takes up cisplatin but has little ability to remove the drug. In most areas of the body, cisplatin is eliminated within days or weeks after treatment; in the inner ear it remains much longer.
Using a mouse model, the researchers found cisplatin remained in the inner ear much longer than in most other body tissues and built up with each treatment. They also studied inner ear tissue donated by deceased patients who had been treated with cisplatin and found cisplatin remained in the inner ear many months or even years after treatment. They also examined inner ear tissue from one child, and found cisplatin buildup even higher than that seen in adults.
The highest buildup of cisplatin was in the stria vascularis, which helps maintain the positive electrical charge in inner ear fluid that certain cells need to detect sound, the researchers say. They believe that accumulation contributed to the hearing loss. “If we can prevent cisplatin from entering the stria vascularis in the inner ear during treatment,” Lisa Cunningham, PhD, lead investigator says, “we may be able to protect cancer patients from developing cisplatin-induced hearing loss.”
Technique could aid treatment of CLL
Researchers say they have developed a new technique for assessing chromosomal abnormalities in chronic lymphocytic leukemia (CLL).
The team believes their method, called immuno-flowFISH, could be used at the time of CLL diagnosis for disease stratification and after treatment to assess residual disease.
Kathryn A. Fuller, PhD, of The University of Western Australia in Crawley, Australia, and her colleagues described immuno-flowFISH in the journal Methods.
The name “immuno-flowFISH” acknowledges what has been incorporated into this technology.
“Immuno” recognizes that immunology testing is used to identify the CLL cells. “Flow” is used because the machine is an imaging flow cytometer. And “FISH” is the test that identifies the chromosomes inside the cells.
The researchers said they found that immuno-flowFISH could detect trisomic chromosomal abnormalities in cells with the phenotype of CLL.
And immuno-flowFISH provided greater specificity and sensitivity than standard FISH.
In particular, the researchers were able to analyze 10,000 to 20,000 cells in each sample, which is 100 to 200 times greater than traditional FISH methods.
“The imaging cytometer can analyze samples at a rate of up to 2000 cells per second, which means we can investigate a large number of cells in a relatively short amount of time, giving us greater sensitivity,” Dr Fuller said.
“This immuno-flowFISH method is an exciting development in personalizing pathology testing for leukemia,” added study author Wendy N. Erber, MD, DPhil, PhD, of The University of Western Australia.
Dr Erber and her colleagues are now expanding immuno-flowFISH so it can be applied to other malignancies as well. ![]()
Researchers say they have developed a new technique for assessing chromosomal abnormalities in chronic lymphocytic leukemia (CLL).
The team believes their method, called immuno-flowFISH, could be used at the time of CLL diagnosis for disease stratification and after treatment to assess residual disease.
Kathryn A. Fuller, PhD, of The University of Western Australia in Crawley, Australia, and her colleagues described immuno-flowFISH in the journal Methods.
The name “immuno-flowFISH” acknowledges what has been incorporated into this technology.
“Immuno” recognizes that immunology testing is used to identify the CLL cells. “Flow” is used because the machine is an imaging flow cytometer. And “FISH” is the test that identifies the chromosomes inside the cells.
The researchers said they found that immuno-flowFISH could detect trisomic chromosomal abnormalities in cells with the phenotype of CLL.
And immuno-flowFISH provided greater specificity and sensitivity than standard FISH.
In particular, the researchers were able to analyze 10,000 to 20,000 cells in each sample, which is 100 to 200 times greater than traditional FISH methods.
“The imaging cytometer can analyze samples at a rate of up to 2000 cells per second, which means we can investigate a large number of cells in a relatively short amount of time, giving us greater sensitivity,” Dr Fuller said.
“This immuno-flowFISH method is an exciting development in personalizing pathology testing for leukemia,” added study author Wendy N. Erber, MD, DPhil, PhD, of The University of Western Australia.
Dr Erber and her colleagues are now expanding immuno-flowFISH so it can be applied to other malignancies as well. ![]()
Researchers say they have developed a new technique for assessing chromosomal abnormalities in chronic lymphocytic leukemia (CLL).
The team believes their method, called immuno-flowFISH, could be used at the time of CLL diagnosis for disease stratification and after treatment to assess residual disease.
Kathryn A. Fuller, PhD, of The University of Western Australia in Crawley, Australia, and her colleagues described immuno-flowFISH in the journal Methods.
The name “immuno-flowFISH” acknowledges what has been incorporated into this technology.
“Immuno” recognizes that immunology testing is used to identify the CLL cells. “Flow” is used because the machine is an imaging flow cytometer. And “FISH” is the test that identifies the chromosomes inside the cells.
The researchers said they found that immuno-flowFISH could detect trisomic chromosomal abnormalities in cells with the phenotype of CLL.
And immuno-flowFISH provided greater specificity and sensitivity than standard FISH.
In particular, the researchers were able to analyze 10,000 to 20,000 cells in each sample, which is 100 to 200 times greater than traditional FISH methods.
“The imaging cytometer can analyze samples at a rate of up to 2000 cells per second, which means we can investigate a large number of cells in a relatively short amount of time, giving us greater sensitivity,” Dr Fuller said.
“This immuno-flowFISH method is an exciting development in personalizing pathology testing for leukemia,” added study author Wendy N. Erber, MD, DPhil, PhD, of The University of Western Australia.
Dr Erber and her colleagues are now expanding immuno-flowFISH so it can be applied to other malignancies as well. ![]()
FDA approves test to diagnose MPNs
The US Food and Drug Administration (FDA) has cleared use of QIAGEN’s ipsogen JAK2 RGQ PCR Kit (ipsogen JAK2 assay) for the diagnosis of all myeloproliferative neoplasms (MPNs).
The ipsogen JAK2 assay is a qualitative, in vitro diagnostic test designed to detect the JAK2 V617F/G1849T allele in genomic DNA extracted from EDTA whole blood.
The assay was previously cleared by the FDA for use in conjunction with other clinicopathological factors to aid the diagnosis of polycythemia vera.
Now, the FDA has cleared use of the assay for 2 additional MPNs—essential thrombocythemia and primary myelofibrosis.
“We are eager to expand the use of our ipsogen JAK2 assay, which is already available in Europe and other markets, for use in a wider range of patients in the US,” said Thierry Bernard, senior vice president and head of QIAGEN’s Molecular Diagnostics Business Area.
“Our JAK2 assay makes it easier for hematologists and oncologists to follow recommended diagnostic testing algorithms and international guidelines for their patients suspected of having MPNs.”
The ipsogen JAK2 assay is a real-time PCR test performed on the QIAGEN Rotor-Gene Q MDx instrument.
The test is intended for use as an adjunct to the evaluation of suspected MPNs, in conjunction with other clinicopathological factors.
The ipsogen JAK2 assay does not detect less common JAK2 mutations associated with MPNs, including mutations in exon 12, and is not intended for stand-alone diagnosis of MPNs. ![]()
The US Food and Drug Administration (FDA) has cleared use of QIAGEN’s ipsogen JAK2 RGQ PCR Kit (ipsogen JAK2 assay) for the diagnosis of all myeloproliferative neoplasms (MPNs).
The ipsogen JAK2 assay is a qualitative, in vitro diagnostic test designed to detect the JAK2 V617F/G1849T allele in genomic DNA extracted from EDTA whole blood.
The assay was previously cleared by the FDA for use in conjunction with other clinicopathological factors to aid the diagnosis of polycythemia vera.
Now, the FDA has cleared use of the assay for 2 additional MPNs—essential thrombocythemia and primary myelofibrosis.
“We are eager to expand the use of our ipsogen JAK2 assay, which is already available in Europe and other markets, for use in a wider range of patients in the US,” said Thierry Bernard, senior vice president and head of QIAGEN’s Molecular Diagnostics Business Area.
“Our JAK2 assay makes it easier for hematologists and oncologists to follow recommended diagnostic testing algorithms and international guidelines for their patients suspected of having MPNs.”
The ipsogen JAK2 assay is a real-time PCR test performed on the QIAGEN Rotor-Gene Q MDx instrument.
The test is intended for use as an adjunct to the evaluation of suspected MPNs, in conjunction with other clinicopathological factors.
The ipsogen JAK2 assay does not detect less common JAK2 mutations associated with MPNs, including mutations in exon 12, and is not intended for stand-alone diagnosis of MPNs. ![]()
The US Food and Drug Administration (FDA) has cleared use of QIAGEN’s ipsogen JAK2 RGQ PCR Kit (ipsogen JAK2 assay) for the diagnosis of all myeloproliferative neoplasms (MPNs).
The ipsogen JAK2 assay is a qualitative, in vitro diagnostic test designed to detect the JAK2 V617F/G1849T allele in genomic DNA extracted from EDTA whole blood.
The assay was previously cleared by the FDA for use in conjunction with other clinicopathological factors to aid the diagnosis of polycythemia vera.
Now, the FDA has cleared use of the assay for 2 additional MPNs—essential thrombocythemia and primary myelofibrosis.
“We are eager to expand the use of our ipsogen JAK2 assay, which is already available in Europe and other markets, for use in a wider range of patients in the US,” said Thierry Bernard, senior vice president and head of QIAGEN’s Molecular Diagnostics Business Area.
“Our JAK2 assay makes it easier for hematologists and oncologists to follow recommended diagnostic testing algorithms and international guidelines for their patients suspected of having MPNs.”
The ipsogen JAK2 assay is a real-time PCR test performed on the QIAGEN Rotor-Gene Q MDx instrument.
The test is intended for use as an adjunct to the evaluation of suspected MPNs, in conjunction with other clinicopathological factors.
The ipsogen JAK2 assay does not detect less common JAK2 mutations associated with MPNs, including mutations in exon 12, and is not intended for stand-alone diagnosis of MPNs. ![]()