User login
Adjunct to HSCT receives orphan designation
The European Commission (EC) has granted orphan designation to NLA101 as an adjunct to hematopoietic stem cell transplant (HSCT).
NLA101 is a universal, off-the-shelf, stem and progenitor cell therapy intended to provide a short-term bridge for hematopoietic recovery while also providing long-term immunologic and clinical benefits in HSCT recipients.
NLA101 is a product of Nohla Therapeutics Inc.
The company says more than 125 infusions of NLA101 have been administered since 2009. The therapy is under investigation in patients receiving intensive chemotherapy as well as in HSCT recipients.
Results from a pilot study of NLA101 in HSCT recipients were presented at the 2014 ASH Annual Meeting.
In this study, 15 patients with hematologic malignancies underwent myeloablative cord blood transplant, with or without NLA101.
Patients who received NLA101 had a significantly reduced median time to platelet and neutrophil recovery, compared to controls. At 5 years, disease-free survival was 86% in the NLA101 group and 67% in the control group.
The rate of grade 3-4 acute graft-versus-host disease was 0% in the NLA101 group and 29% in the control group. The rate of transplant-related mortality was 0% and 22%, respectively.
Phase 2 studies of NLA101 in chemotherapy and HSCT recipients are ongoing.
About orphan designation
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides a 10-year period of marketing exclusivity if the drug receives regulatory approval.
The designation also provides incentives for companies seeking protocol assistance from the European Medicines Agency during the product development phase and direct access to the centralized authorization procedure. ![]()
The European Commission (EC) has granted orphan designation to NLA101 as an adjunct to hematopoietic stem cell transplant (HSCT).
NLA101 is a universal, off-the-shelf, stem and progenitor cell therapy intended to provide a short-term bridge for hematopoietic recovery while also providing long-term immunologic and clinical benefits in HSCT recipients.
NLA101 is a product of Nohla Therapeutics Inc.
The company says more than 125 infusions of NLA101 have been administered since 2009. The therapy is under investigation in patients receiving intensive chemotherapy as well as in HSCT recipients.
Results from a pilot study of NLA101 in HSCT recipients were presented at the 2014 ASH Annual Meeting.
In this study, 15 patients with hematologic malignancies underwent myeloablative cord blood transplant, with or without NLA101.
Patients who received NLA101 had a significantly reduced median time to platelet and neutrophil recovery, compared to controls. At 5 years, disease-free survival was 86% in the NLA101 group and 67% in the control group.
The rate of grade 3-4 acute graft-versus-host disease was 0% in the NLA101 group and 29% in the control group. The rate of transplant-related mortality was 0% and 22%, respectively.
Phase 2 studies of NLA101 in chemotherapy and HSCT recipients are ongoing.
About orphan designation
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides a 10-year period of marketing exclusivity if the drug receives regulatory approval.
The designation also provides incentives for companies seeking protocol assistance from the European Medicines Agency during the product development phase and direct access to the centralized authorization procedure. ![]()
The European Commission (EC) has granted orphan designation to NLA101 as an adjunct to hematopoietic stem cell transplant (HSCT).
NLA101 is a universal, off-the-shelf, stem and progenitor cell therapy intended to provide a short-term bridge for hematopoietic recovery while also providing long-term immunologic and clinical benefits in HSCT recipients.
NLA101 is a product of Nohla Therapeutics Inc.
The company says more than 125 infusions of NLA101 have been administered since 2009. The therapy is under investigation in patients receiving intensive chemotherapy as well as in HSCT recipients.
Results from a pilot study of NLA101 in HSCT recipients were presented at the 2014 ASH Annual Meeting.
In this study, 15 patients with hematologic malignancies underwent myeloablative cord blood transplant, with or without NLA101.
Patients who received NLA101 had a significantly reduced median time to platelet and neutrophil recovery, compared to controls. At 5 years, disease-free survival was 86% in the NLA101 group and 67% in the control group.
The rate of grade 3-4 acute graft-versus-host disease was 0% in the NLA101 group and 29% in the control group. The rate of transplant-related mortality was 0% and 22%, respectively.
Phase 2 studies of NLA101 in chemotherapy and HSCT recipients are ongoing.
About orphan designation
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides a 10-year period of marketing exclusivity if the drug receives regulatory approval.
The designation also provides incentives for companies seeking protocol assistance from the European Medicines Agency during the product development phase and direct access to the centralized authorization procedure. ![]()
EHR application doubles hypertension recognition rate
Hypertension discovery in pediatric patients more than doubled for physicians using a clinical decision support (CDS) tool connected to the EHR, results of a study found.
Elyse O. Kharbanda, MD, MPH, a researcher at the HealthPartners Institute, Minneapolis, and her fellow investigators assert that using such a tool will help rectify the trend of underreported hypertension in adolescents, which remains a serious concern despite providers’ routinely taking blood pressure measurements during outpatient visits.
“Among patients with multiple visits, electronic health records should contain sufficient information to diagnose hypertension,” Dr. Kharbanda and her associates reported in their article published in Pediatrics. “However, even when EHRs are configured to display BP percentiles, information on the patterns of BP percentiles over time, previous diagnoses, and medications is not presented in a format that is useful for clinicians.”
With TeenBP, providers are first prompted to take an initial BP reading, as well as height and weight measurements.
If the first measure is above the 95th percentile, the CDS requests an additional reading, which is then averaged with the first. If average of the two is above or within the 95th percentile, the provider is notified and sent a list of recommendations, including a diagnosis of hypertension, lipid screening, and nutrition referral.
The 2-year trial included 522 pediatric patients with incident hypertension; the data were gathered from 20 primary care clinics within one health system between April 2014 and April 2016.
The rate of clinical recognition of patients’ hypertension in the clinics utilizing the CDS tool was more than double the rate seen in the clinics that weren’t (55% and 21%, respectively; P less than .001).
More of the children seen in CDS clinics were referred to dietitians or weight loss programs, compared with those seen in the control clinics (17% and 4%, respectively; P = .001).
Those who used the tool reported high levels of satisfaction, which is likely partly because investigators consulted physicians to help design the application.
“The CDS tool was based on the guidelines for BP management in children and adolescents in effect at the time of the study with local input from clinical and operational leaders within the medical group, and thus it contained the so-called right information,” according to Dr. Kharbanda and her fellow investigators.
Of the 55 physicians who remembered using the tool, 92% thought is was useful in identifying hypertension, 94% considered the CDS a good use of time, and 95% believed is was a useful shared-decision making tool.
When designing TeenBP, investigators tailored the application to the work flow and culture of the health system used for the study, which may limit the generalizability of the findings.
The study was funded by the National Institutes of Health. Dr. Kharbanda and her associates reported no relevant financial disclosures.
Source: Kharbanda EO et al. Pediatrics. 2018. doi: 10.1542/peds.2017- 2954.
The rate of children and adolescents with elevated blood pressure going unrecognized is an increasingly concerning issue – one that is complicated because physicians lack a simple, single BP value system. Some have tried to fill the gap, creating simplified tables of BP values or automated displays of BP values in EHRs, but having a table that only takes age and sex into consideration for screening does not cover the complexities needed to identify hypertension.
Previous studies have looked into utilizing a clinical decision support (CDS) application, which has great potential as a digital multitool to improve quality of care, increase efficiency, and reduce medical errors. However, to be an effective CDS, it must fill the “CDS Five Rights” framework. This guideline states that a CDS tool needs to provide: “the right information, to the right people, through the right channels, in the right intervention formats, at the right points in the work flow.”
The TeenBP CDS developed by Kharbanda et al. fulfills these requirements and goes beyond any CDS previously designed. Even so, 45% of children with elevated BP or hypertension were not recognized, emphasizing the need for additional strategies outside of relying on new technology.
Visit summaries should be given to parents with BP readings so that they can monitor their children’s levels, for example.
Recognition of abnormal BP in teens is the first step toward preventing cardiovascular disease as an adult, and hopefully, the development of new tools, including this CDS, will help physicians find those children who have been overlooked.
Ari H. Pollack, MD, MSIM, is a pediatric nephrologist at the Seattle Children’s Hospital and an assistant professor of pediatrics at the University of Washington, Seattle. Joseph T. Flynn, MD, MS, is the division chief of nephrology in prenatal diagnosis and treatment at the Seattle Children’s Hospital and a professor of pediatrics at the same university. Dr. Pollack and Dr. Flynn reported no relevant financial disclosures in their commentary in Pediatrics (2018. doi: 10.1542/peds.2017-3756).
The rate of children and adolescents with elevated blood pressure going unrecognized is an increasingly concerning issue – one that is complicated because physicians lack a simple, single BP value system. Some have tried to fill the gap, creating simplified tables of BP values or automated displays of BP values in EHRs, but having a table that only takes age and sex into consideration for screening does not cover the complexities needed to identify hypertension.
Previous studies have looked into utilizing a clinical decision support (CDS) application, which has great potential as a digital multitool to improve quality of care, increase efficiency, and reduce medical errors. However, to be an effective CDS, it must fill the “CDS Five Rights” framework. This guideline states that a CDS tool needs to provide: “the right information, to the right people, through the right channels, in the right intervention formats, at the right points in the work flow.”
The TeenBP CDS developed by Kharbanda et al. fulfills these requirements and goes beyond any CDS previously designed. Even so, 45% of children with elevated BP or hypertension were not recognized, emphasizing the need for additional strategies outside of relying on new technology.
Visit summaries should be given to parents with BP readings so that they can monitor their children’s levels, for example.
Recognition of abnormal BP in teens is the first step toward preventing cardiovascular disease as an adult, and hopefully, the development of new tools, including this CDS, will help physicians find those children who have been overlooked.
Ari H. Pollack, MD, MSIM, is a pediatric nephrologist at the Seattle Children’s Hospital and an assistant professor of pediatrics at the University of Washington, Seattle. Joseph T. Flynn, MD, MS, is the division chief of nephrology in prenatal diagnosis and treatment at the Seattle Children’s Hospital and a professor of pediatrics at the same university. Dr. Pollack and Dr. Flynn reported no relevant financial disclosures in their commentary in Pediatrics (2018. doi: 10.1542/peds.2017-3756).
The rate of children and adolescents with elevated blood pressure going unrecognized is an increasingly concerning issue – one that is complicated because physicians lack a simple, single BP value system. Some have tried to fill the gap, creating simplified tables of BP values or automated displays of BP values in EHRs, but having a table that only takes age and sex into consideration for screening does not cover the complexities needed to identify hypertension.
Previous studies have looked into utilizing a clinical decision support (CDS) application, which has great potential as a digital multitool to improve quality of care, increase efficiency, and reduce medical errors. However, to be an effective CDS, it must fill the “CDS Five Rights” framework. This guideline states that a CDS tool needs to provide: “the right information, to the right people, through the right channels, in the right intervention formats, at the right points in the work flow.”
The TeenBP CDS developed by Kharbanda et al. fulfills these requirements and goes beyond any CDS previously designed. Even so, 45% of children with elevated BP or hypertension were not recognized, emphasizing the need for additional strategies outside of relying on new technology.
Visit summaries should be given to parents with BP readings so that they can monitor their children’s levels, for example.
Recognition of abnormal BP in teens is the first step toward preventing cardiovascular disease as an adult, and hopefully, the development of new tools, including this CDS, will help physicians find those children who have been overlooked.
Ari H. Pollack, MD, MSIM, is a pediatric nephrologist at the Seattle Children’s Hospital and an assistant professor of pediatrics at the University of Washington, Seattle. Joseph T. Flynn, MD, MS, is the division chief of nephrology in prenatal diagnosis and treatment at the Seattle Children’s Hospital and a professor of pediatrics at the same university. Dr. Pollack and Dr. Flynn reported no relevant financial disclosures in their commentary in Pediatrics (2018. doi: 10.1542/peds.2017-3756).
Hypertension discovery in pediatric patients more than doubled for physicians using a clinical decision support (CDS) tool connected to the EHR, results of a study found.
Elyse O. Kharbanda, MD, MPH, a researcher at the HealthPartners Institute, Minneapolis, and her fellow investigators assert that using such a tool will help rectify the trend of underreported hypertension in adolescents, which remains a serious concern despite providers’ routinely taking blood pressure measurements during outpatient visits.
“Among patients with multiple visits, electronic health records should contain sufficient information to diagnose hypertension,” Dr. Kharbanda and her associates reported in their article published in Pediatrics. “However, even when EHRs are configured to display BP percentiles, information on the patterns of BP percentiles over time, previous diagnoses, and medications is not presented in a format that is useful for clinicians.”
With TeenBP, providers are first prompted to take an initial BP reading, as well as height and weight measurements.
If the first measure is above the 95th percentile, the CDS requests an additional reading, which is then averaged with the first. If average of the two is above or within the 95th percentile, the provider is notified and sent a list of recommendations, including a diagnosis of hypertension, lipid screening, and nutrition referral.
The 2-year trial included 522 pediatric patients with incident hypertension; the data were gathered from 20 primary care clinics within one health system between April 2014 and April 2016.
The rate of clinical recognition of patients’ hypertension in the clinics utilizing the CDS tool was more than double the rate seen in the clinics that weren’t (55% and 21%, respectively; P less than .001).
More of the children seen in CDS clinics were referred to dietitians or weight loss programs, compared with those seen in the control clinics (17% and 4%, respectively; P = .001).
Those who used the tool reported high levels of satisfaction, which is likely partly because investigators consulted physicians to help design the application.
“The CDS tool was based on the guidelines for BP management in children and adolescents in effect at the time of the study with local input from clinical and operational leaders within the medical group, and thus it contained the so-called right information,” according to Dr. Kharbanda and her fellow investigators.
Of the 55 physicians who remembered using the tool, 92% thought is was useful in identifying hypertension, 94% considered the CDS a good use of time, and 95% believed is was a useful shared-decision making tool.
When designing TeenBP, investigators tailored the application to the work flow and culture of the health system used for the study, which may limit the generalizability of the findings.
The study was funded by the National Institutes of Health. Dr. Kharbanda and her associates reported no relevant financial disclosures.
Source: Kharbanda EO et al. Pediatrics. 2018. doi: 10.1542/peds.2017- 2954.
Hypertension discovery in pediatric patients more than doubled for physicians using a clinical decision support (CDS) tool connected to the EHR, results of a study found.
Elyse O. Kharbanda, MD, MPH, a researcher at the HealthPartners Institute, Minneapolis, and her fellow investigators assert that using such a tool will help rectify the trend of underreported hypertension in adolescents, which remains a serious concern despite providers’ routinely taking blood pressure measurements during outpatient visits.
“Among patients with multiple visits, electronic health records should contain sufficient information to diagnose hypertension,” Dr. Kharbanda and her associates reported in their article published in Pediatrics. “However, even when EHRs are configured to display BP percentiles, information on the patterns of BP percentiles over time, previous diagnoses, and medications is not presented in a format that is useful for clinicians.”
With TeenBP, providers are first prompted to take an initial BP reading, as well as height and weight measurements.
If the first measure is above the 95th percentile, the CDS requests an additional reading, which is then averaged with the first. If average of the two is above or within the 95th percentile, the provider is notified and sent a list of recommendations, including a diagnosis of hypertension, lipid screening, and nutrition referral.
The 2-year trial included 522 pediatric patients with incident hypertension; the data were gathered from 20 primary care clinics within one health system between April 2014 and April 2016.
The rate of clinical recognition of patients’ hypertension in the clinics utilizing the CDS tool was more than double the rate seen in the clinics that weren’t (55% and 21%, respectively; P less than .001).
More of the children seen in CDS clinics were referred to dietitians or weight loss programs, compared with those seen in the control clinics (17% and 4%, respectively; P = .001).
Those who used the tool reported high levels of satisfaction, which is likely partly because investigators consulted physicians to help design the application.
“The CDS tool was based on the guidelines for BP management in children and adolescents in effect at the time of the study with local input from clinical and operational leaders within the medical group, and thus it contained the so-called right information,” according to Dr. Kharbanda and her fellow investigators.
Of the 55 physicians who remembered using the tool, 92% thought is was useful in identifying hypertension, 94% considered the CDS a good use of time, and 95% believed is was a useful shared-decision making tool.
When designing TeenBP, investigators tailored the application to the work flow and culture of the health system used for the study, which may limit the generalizability of the findings.
The study was funded by the National Institutes of Health. Dr. Kharbanda and her associates reported no relevant financial disclosures.
Source: Kharbanda EO et al. Pediatrics. 2018. doi: 10.1542/peds.2017- 2954.
FROM PEDIATRICS
Key clinical point: Using a clinical decision support (CDS) tool doubles the rate of hypertension detection in children.
Major finding: Providers in clinics that used CDS recognized hypertension in 55% of patients, compared with 21% of patients in usual care (P less than .001).
Study details: Cluster-randomized trial of 522 pediatric patients across 20 primary care clinics who received care between April 2014 and April 2016.
Disclosures: The study was funded by the National Institutes of Health. The investigators reported no relevant financial disclosures.
Source: Kharbanda EO et al. Pediatrics. 2018. doi: 10.1542/peds.2017- 2954.
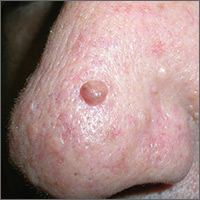
Hyperpigmented growth on nose
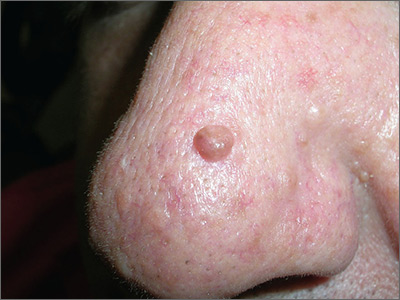
At follow-up 2 weeks later, pathology revealed a diagnosis of sebaceous hyperplasia (SH). On close examination of the face and nose, the FP noted other nonpigmented SHs and telangiectasias, which confirmed an overall diagnosis of rosacea.
The FP explained to the patient that SH is benign and that there are treatments for rosacea. In this case, the FP prescribed 1% metronidazole gel for the rosacea. At follow-up one month later, the shave biopsy had effectively removed the SH and the site had healed well. The metronidazole gel was also improving the appearance of the patient’s face. The patient indicated that he’d been avoiding the sun and drinking less alcohol, which was helping the rosacea and was, of course, good for his overall health.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Sebaceous hyperplasia. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 931-934.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

At follow-up 2 weeks later, pathology revealed a diagnosis of sebaceous hyperplasia (SH). On close examination of the face and nose, the FP noted other nonpigmented SHs and telangiectasias, which confirmed an overall diagnosis of rosacea.
The FP explained to the patient that SH is benign and that there are treatments for rosacea. In this case, the FP prescribed 1% metronidazole gel for the rosacea. At follow-up one month later, the shave biopsy had effectively removed the SH and the site had healed well. The metronidazole gel was also improving the appearance of the patient’s face. The patient indicated that he’d been avoiding the sun and drinking less alcohol, which was helping the rosacea and was, of course, good for his overall health.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Sebaceous hyperplasia. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 931-934.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

At follow-up 2 weeks later, pathology revealed a diagnosis of sebaceous hyperplasia (SH). On close examination of the face and nose, the FP noted other nonpigmented SHs and telangiectasias, which confirmed an overall diagnosis of rosacea.
The FP explained to the patient that SH is benign and that there are treatments for rosacea. In this case, the FP prescribed 1% metronidazole gel for the rosacea. At follow-up one month later, the shave biopsy had effectively removed the SH and the site had healed well. The metronidazole gel was also improving the appearance of the patient’s face. The patient indicated that he’d been avoiding the sun and drinking less alcohol, which was helping the rosacea and was, of course, good for his overall health.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Sebaceous hyperplasia. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 931-934.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Three in 10 diabetic patients may have liver fibrosis
LOS ANGELES – For every 10 adult patients with type 2 diabetes, three are likely to have moderate to severe liver fibrosis, according to Kenneth Cusi, MD, FACP, FACE.
“The question is, How are we going to tackle this problem? My academic goal is that we incorporate screening for NASH [nonalcoholic steatohepatitis], or for fibrosis more specifically, in the same way we do for retinopathy or nephropathy [in diabetes], because we do have a way to treat it,” he said at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease.
Dr. Cusi, chief of the division of endocrinology, diabetes, and metabolism at the University of Florida, Gainesville, predicted that obesity will become the No. 1 cause of liver transplantation. “It’s a real epidemic; you’re not seeing it because the inflexion of obesity happened just 2 decades ago,” he said. “Patients with diabetes face the greatest risk of fatty liver and of fibrosis. Untreated, it’s the equivalent of having macroalbuminuria. If you do nothing and they don’t die of cardiovascular disease, they’re going to have a good chance of getting fibrosis.”
As part of the large population-based Rotterdam study of individuals aged 45 years and older, researchers found that liver stiffness of 8 kPa or more by transient elastography was present in 5.6% of the study participants and was strongly associated with steatosis and diabetes (Hepatology. 2016;63:138-47). According to Dr. Cusi, individuals who have steatosis without diabetes face a 5%-10% risk of fibrosis, while those with steatosis and diabetes face a 15%-20% risk. “It’s well established in a number of studies that if you have fibrosis, you’re at high risk not only of cirrhosis, but also of hepatocellular carcinoma,” he said. “The key thing is not detecting fat, which is not really the target. The target is if there’s fibrosis or not.” Three ways to assess for fibrosis include MR elastography, transient elastography (which is the most commonly used), and fibrosis marker panels.
Liver fibrosis likely starts with adipose tissue dysfunction, said Dr. Cusi, who authored a review on the pathophysiology of interactions between adipose tissue and target organs in obesity and the resulting clinical implications for the management of nonalcoholic steatohepatitis (Gastroenterology. 2012;142[4]:711-25.e6). “When you have insulin-resistant, sick adipose tissue, that leads to the accumulation of fat in the liver,” he said. “. Even if you get people who are matched for BMIs [body mass indexes] between 30 and 35 kg/m2, there is a spectrum in which some individuals have very insulin-resistant adipose tissue and others less so. I would say that 1 out of 10 are metabolically healthy, and we don’t understand exactly why.”
In a recent cross-sectional analysis of 352 healthy individuals, Dr. Cusi and his associates found that intrahepatic triglyceride (IHTG) accumulation is strongly associated with adipose tissue insulin resistance, supporting the current theory of lipotoxicity as a driver of IHTG accumulation (Hepatology. 2017;65[4]:1132-44). The researchers observed that once IHTG accumulation reaches about 6%, skeletal muscle insulin resistance, hypertriglyceridemia, and low HDL cholesterol become fully established. “The next question is, How does this correlate with NASH?” Dr. Cusi said. “Our take is that there is a threshold effect. Once you have a critical amount of triglycerides in your liver, some individuals are going to activate pathways that are harmful. NASH is not something exclusive to individuals who are obese. Lean people can also develop NASH. The key feature is insulin resistance, not metabolic syndrome. Once you develop a fatty liver, your chances of NASH are comparable to that of an obese individual. The paradox is that lean individuals get a fatty liver, but when they get a fatty liver, they are at risk for NASH and for fibrosis.”
Why lean individuals develop NASH is not fully understood, but Dr. Cusi said he suspects that the problem develops at the mitochondrial level. Results from an unpublished animal model in which mice were fed a high–trans-fat diet for 24 weeks showed that the mice developed steatosis by week 8 and NASH by week 24. The mice had an increase in the tricarboxylic acid (TCA) cycle, which is typical of the NASH period, as well as an increase in ceramides. “Perhaps a unifying hypothesis would be that the development of NASH is linked to inflammation and to insulin signaling,” Dr. Cusi said. “Not surprisingly, it had a number of effects on the mitochondria, and in this animal model it decreases the TCA.” He noted that the biology of fibrosis remains unknown in humans. “What we have been familiar with is the high-triglyceride, low-HDL pattern,” he said. “If you look at how that correlates with the amount of liver fat, it is basically a threshold effect. Once you have steatosis, you don’t see much worse dyslipidemia, which is typical of these patients.”
Recently published guidance from the American Association for the Study of Liver Diseases on the diagnosis and management of nonalcoholic fatty liver disease (NAFLD) suggests that patients require a weight loss of 3%-5% to improve steatosis, but a loss of 7%-10% to improve most histologic features of NASH, including fibrosis (Hepatology. 2018;67[1]:328-57). Exercise alone may prevent or reduce steatosis, but its ability to improve other aspects of liver histology remains unknown. Bariatric surgery can be considered in otherwise eligible obese individuals with NAFLD or NASH. The procedure’s impact on fibrosis is unknown.
The AASLD practice guideline notes that metformin is not recommended for treating NASH in adult patients, but pioglitazone improves liver histology in patients with and without type 2 diabetes with biopsy-proven NASH. “Pioglitazone has had the greatest benefit in terms of treatment effect, compared to placebo,” Dr. Cusi said. “It’s a generic drug; at the VA [Veterans Affairs], it costs 8 cents per tablet. I think that pioglitazone will be to NASH what metformin has been to type 2 diabetes. The most common side effect is weight gain, typically between 4 and 9 lb. Risks and benefits should be discussed with each patient. It should not be used for NAFLD without biopsy-proven NASH.” The guideline goes on to say that it’s currently premature to consider GLP-1 (glucagonlike peptide–1) agonists for treating liver disease in patients with NAFLD or NASH. Meanwhile, vitamin E at 800 IU has been shown to improve liver histology in nondiabetic adults with NASH, but the risks and benefits should be discussed with each patient. Vitamin E is not recommended for NASH in diabetic patients, NAFLD without a liver biopsy, NASH cirrhosis, or cryptogenic cirrhosis.
The AASLD practice guideline also states that the best evidence for using SGLT2 (sodium-glucose cotransporter–2) inhibitors in NAFLD comes from animal studies, which report a reduction in steatosis with and without weight loss. Clinical studies reporting a reduction in steatosis are limited. There are positive observational studies with a reduction in alanine aminotransferase and some studies that have shown a reduction in liver fat. “For me, the best option is to tailor treatment to the pathophysiology of the disease,” Dr. Cusi said. “You reduce fat by weight loss in some way, or you change the biology of fat with a thiazolidinedione.”
Dr. Cusi reported that he has received grant support from the Burroughs Wellcome Fund, the American Diabetes Association, and the National Institutes of Health.
LOS ANGELES – For every 10 adult patients with type 2 diabetes, three are likely to have moderate to severe liver fibrosis, according to Kenneth Cusi, MD, FACP, FACE.
“The question is, How are we going to tackle this problem? My academic goal is that we incorporate screening for NASH [nonalcoholic steatohepatitis], or for fibrosis more specifically, in the same way we do for retinopathy or nephropathy [in diabetes], because we do have a way to treat it,” he said at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease.
Dr. Cusi, chief of the division of endocrinology, diabetes, and metabolism at the University of Florida, Gainesville, predicted that obesity will become the No. 1 cause of liver transplantation. “It’s a real epidemic; you’re not seeing it because the inflexion of obesity happened just 2 decades ago,” he said. “Patients with diabetes face the greatest risk of fatty liver and of fibrosis. Untreated, it’s the equivalent of having macroalbuminuria. If you do nothing and they don’t die of cardiovascular disease, they’re going to have a good chance of getting fibrosis.”
As part of the large population-based Rotterdam study of individuals aged 45 years and older, researchers found that liver stiffness of 8 kPa or more by transient elastography was present in 5.6% of the study participants and was strongly associated with steatosis and diabetes (Hepatology. 2016;63:138-47). According to Dr. Cusi, individuals who have steatosis without diabetes face a 5%-10% risk of fibrosis, while those with steatosis and diabetes face a 15%-20% risk. “It’s well established in a number of studies that if you have fibrosis, you’re at high risk not only of cirrhosis, but also of hepatocellular carcinoma,” he said. “The key thing is not detecting fat, which is not really the target. The target is if there’s fibrosis or not.” Three ways to assess for fibrosis include MR elastography, transient elastography (which is the most commonly used), and fibrosis marker panels.
Liver fibrosis likely starts with adipose tissue dysfunction, said Dr. Cusi, who authored a review on the pathophysiology of interactions between adipose tissue and target organs in obesity and the resulting clinical implications for the management of nonalcoholic steatohepatitis (Gastroenterology. 2012;142[4]:711-25.e6). “When you have insulin-resistant, sick adipose tissue, that leads to the accumulation of fat in the liver,” he said. “. Even if you get people who are matched for BMIs [body mass indexes] between 30 and 35 kg/m2, there is a spectrum in which some individuals have very insulin-resistant adipose tissue and others less so. I would say that 1 out of 10 are metabolically healthy, and we don’t understand exactly why.”
In a recent cross-sectional analysis of 352 healthy individuals, Dr. Cusi and his associates found that intrahepatic triglyceride (IHTG) accumulation is strongly associated with adipose tissue insulin resistance, supporting the current theory of lipotoxicity as a driver of IHTG accumulation (Hepatology. 2017;65[4]:1132-44). The researchers observed that once IHTG accumulation reaches about 6%, skeletal muscle insulin resistance, hypertriglyceridemia, and low HDL cholesterol become fully established. “The next question is, How does this correlate with NASH?” Dr. Cusi said. “Our take is that there is a threshold effect. Once you have a critical amount of triglycerides in your liver, some individuals are going to activate pathways that are harmful. NASH is not something exclusive to individuals who are obese. Lean people can also develop NASH. The key feature is insulin resistance, not metabolic syndrome. Once you develop a fatty liver, your chances of NASH are comparable to that of an obese individual. The paradox is that lean individuals get a fatty liver, but when they get a fatty liver, they are at risk for NASH and for fibrosis.”
Why lean individuals develop NASH is not fully understood, but Dr. Cusi said he suspects that the problem develops at the mitochondrial level. Results from an unpublished animal model in which mice were fed a high–trans-fat diet for 24 weeks showed that the mice developed steatosis by week 8 and NASH by week 24. The mice had an increase in the tricarboxylic acid (TCA) cycle, which is typical of the NASH period, as well as an increase in ceramides. “Perhaps a unifying hypothesis would be that the development of NASH is linked to inflammation and to insulin signaling,” Dr. Cusi said. “Not surprisingly, it had a number of effects on the mitochondria, and in this animal model it decreases the TCA.” He noted that the biology of fibrosis remains unknown in humans. “What we have been familiar with is the high-triglyceride, low-HDL pattern,” he said. “If you look at how that correlates with the amount of liver fat, it is basically a threshold effect. Once you have steatosis, you don’t see much worse dyslipidemia, which is typical of these patients.”
Recently published guidance from the American Association for the Study of Liver Diseases on the diagnosis and management of nonalcoholic fatty liver disease (NAFLD) suggests that patients require a weight loss of 3%-5% to improve steatosis, but a loss of 7%-10% to improve most histologic features of NASH, including fibrosis (Hepatology. 2018;67[1]:328-57). Exercise alone may prevent or reduce steatosis, but its ability to improve other aspects of liver histology remains unknown. Bariatric surgery can be considered in otherwise eligible obese individuals with NAFLD or NASH. The procedure’s impact on fibrosis is unknown.
The AASLD practice guideline notes that metformin is not recommended for treating NASH in adult patients, but pioglitazone improves liver histology in patients with and without type 2 diabetes with biopsy-proven NASH. “Pioglitazone has had the greatest benefit in terms of treatment effect, compared to placebo,” Dr. Cusi said. “It’s a generic drug; at the VA [Veterans Affairs], it costs 8 cents per tablet. I think that pioglitazone will be to NASH what metformin has been to type 2 diabetes. The most common side effect is weight gain, typically between 4 and 9 lb. Risks and benefits should be discussed with each patient. It should not be used for NAFLD without biopsy-proven NASH.” The guideline goes on to say that it’s currently premature to consider GLP-1 (glucagonlike peptide–1) agonists for treating liver disease in patients with NAFLD or NASH. Meanwhile, vitamin E at 800 IU has been shown to improve liver histology in nondiabetic adults with NASH, but the risks and benefits should be discussed with each patient. Vitamin E is not recommended for NASH in diabetic patients, NAFLD without a liver biopsy, NASH cirrhosis, or cryptogenic cirrhosis.
The AASLD practice guideline also states that the best evidence for using SGLT2 (sodium-glucose cotransporter–2) inhibitors in NAFLD comes from animal studies, which report a reduction in steatosis with and without weight loss. Clinical studies reporting a reduction in steatosis are limited. There are positive observational studies with a reduction in alanine aminotransferase and some studies that have shown a reduction in liver fat. “For me, the best option is to tailor treatment to the pathophysiology of the disease,” Dr. Cusi said. “You reduce fat by weight loss in some way, or you change the biology of fat with a thiazolidinedione.”
Dr. Cusi reported that he has received grant support from the Burroughs Wellcome Fund, the American Diabetes Association, and the National Institutes of Health.
LOS ANGELES – For every 10 adult patients with type 2 diabetes, three are likely to have moderate to severe liver fibrosis, according to Kenneth Cusi, MD, FACP, FACE.
“The question is, How are we going to tackle this problem? My academic goal is that we incorporate screening for NASH [nonalcoholic steatohepatitis], or for fibrosis more specifically, in the same way we do for retinopathy or nephropathy [in diabetes], because we do have a way to treat it,” he said at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease.
Dr. Cusi, chief of the division of endocrinology, diabetes, and metabolism at the University of Florida, Gainesville, predicted that obesity will become the No. 1 cause of liver transplantation. “It’s a real epidemic; you’re not seeing it because the inflexion of obesity happened just 2 decades ago,” he said. “Patients with diabetes face the greatest risk of fatty liver and of fibrosis. Untreated, it’s the equivalent of having macroalbuminuria. If you do nothing and they don’t die of cardiovascular disease, they’re going to have a good chance of getting fibrosis.”
As part of the large population-based Rotterdam study of individuals aged 45 years and older, researchers found that liver stiffness of 8 kPa or more by transient elastography was present in 5.6% of the study participants and was strongly associated with steatosis and diabetes (Hepatology. 2016;63:138-47). According to Dr. Cusi, individuals who have steatosis without diabetes face a 5%-10% risk of fibrosis, while those with steatosis and diabetes face a 15%-20% risk. “It’s well established in a number of studies that if you have fibrosis, you’re at high risk not only of cirrhosis, but also of hepatocellular carcinoma,” he said. “The key thing is not detecting fat, which is not really the target. The target is if there’s fibrosis or not.” Three ways to assess for fibrosis include MR elastography, transient elastography (which is the most commonly used), and fibrosis marker panels.
Liver fibrosis likely starts with adipose tissue dysfunction, said Dr. Cusi, who authored a review on the pathophysiology of interactions between adipose tissue and target organs in obesity and the resulting clinical implications for the management of nonalcoholic steatohepatitis (Gastroenterology. 2012;142[4]:711-25.e6). “When you have insulin-resistant, sick adipose tissue, that leads to the accumulation of fat in the liver,” he said. “. Even if you get people who are matched for BMIs [body mass indexes] between 30 and 35 kg/m2, there is a spectrum in which some individuals have very insulin-resistant adipose tissue and others less so. I would say that 1 out of 10 are metabolically healthy, and we don’t understand exactly why.”
In a recent cross-sectional analysis of 352 healthy individuals, Dr. Cusi and his associates found that intrahepatic triglyceride (IHTG) accumulation is strongly associated with adipose tissue insulin resistance, supporting the current theory of lipotoxicity as a driver of IHTG accumulation (Hepatology. 2017;65[4]:1132-44). The researchers observed that once IHTG accumulation reaches about 6%, skeletal muscle insulin resistance, hypertriglyceridemia, and low HDL cholesterol become fully established. “The next question is, How does this correlate with NASH?” Dr. Cusi said. “Our take is that there is a threshold effect. Once you have a critical amount of triglycerides in your liver, some individuals are going to activate pathways that are harmful. NASH is not something exclusive to individuals who are obese. Lean people can also develop NASH. The key feature is insulin resistance, not metabolic syndrome. Once you develop a fatty liver, your chances of NASH are comparable to that of an obese individual. The paradox is that lean individuals get a fatty liver, but when they get a fatty liver, they are at risk for NASH and for fibrosis.”
Why lean individuals develop NASH is not fully understood, but Dr. Cusi said he suspects that the problem develops at the mitochondrial level. Results from an unpublished animal model in which mice were fed a high–trans-fat diet for 24 weeks showed that the mice developed steatosis by week 8 and NASH by week 24. The mice had an increase in the tricarboxylic acid (TCA) cycle, which is typical of the NASH period, as well as an increase in ceramides. “Perhaps a unifying hypothesis would be that the development of NASH is linked to inflammation and to insulin signaling,” Dr. Cusi said. “Not surprisingly, it had a number of effects on the mitochondria, and in this animal model it decreases the TCA.” He noted that the biology of fibrosis remains unknown in humans. “What we have been familiar with is the high-triglyceride, low-HDL pattern,” he said. “If you look at how that correlates with the amount of liver fat, it is basically a threshold effect. Once you have steatosis, you don’t see much worse dyslipidemia, which is typical of these patients.”
Recently published guidance from the American Association for the Study of Liver Diseases on the diagnosis and management of nonalcoholic fatty liver disease (NAFLD) suggests that patients require a weight loss of 3%-5% to improve steatosis, but a loss of 7%-10% to improve most histologic features of NASH, including fibrosis (Hepatology. 2018;67[1]:328-57). Exercise alone may prevent or reduce steatosis, but its ability to improve other aspects of liver histology remains unknown. Bariatric surgery can be considered in otherwise eligible obese individuals with NAFLD or NASH. The procedure’s impact on fibrosis is unknown.
The AASLD practice guideline notes that metformin is not recommended for treating NASH in adult patients, but pioglitazone improves liver histology in patients with and without type 2 diabetes with biopsy-proven NASH. “Pioglitazone has had the greatest benefit in terms of treatment effect, compared to placebo,” Dr. Cusi said. “It’s a generic drug; at the VA [Veterans Affairs], it costs 8 cents per tablet. I think that pioglitazone will be to NASH what metformin has been to type 2 diabetes. The most common side effect is weight gain, typically between 4 and 9 lb. Risks and benefits should be discussed with each patient. It should not be used for NAFLD without biopsy-proven NASH.” The guideline goes on to say that it’s currently premature to consider GLP-1 (glucagonlike peptide–1) agonists for treating liver disease in patients with NAFLD or NASH. Meanwhile, vitamin E at 800 IU has been shown to improve liver histology in nondiabetic adults with NASH, but the risks and benefits should be discussed with each patient. Vitamin E is not recommended for NASH in diabetic patients, NAFLD without a liver biopsy, NASH cirrhosis, or cryptogenic cirrhosis.
The AASLD practice guideline also states that the best evidence for using SGLT2 (sodium-glucose cotransporter–2) inhibitors in NAFLD comes from animal studies, which report a reduction in steatosis with and without weight loss. Clinical studies reporting a reduction in steatosis are limited. There are positive observational studies with a reduction in alanine aminotransferase and some studies that have shown a reduction in liver fat. “For me, the best option is to tailor treatment to the pathophysiology of the disease,” Dr. Cusi said. “You reduce fat by weight loss in some way, or you change the biology of fat with a thiazolidinedione.”
Dr. Cusi reported that he has received grant support from the Burroughs Wellcome Fund, the American Diabetes Association, and the National Institutes of Health.
EXPERT ANALYSIS FROM WCIRDC 2017
Influenza: All that and MI too
Myocardial infarction admissions were six times more likely to occur in the week after a positive test for influenza than in the year before or the 51 weeks after the infection, according to analysis of a Canadian cohort that links laboratories with administrative databases.
The investigators used this cohort data to define definitions of “risk interval” – the first 7 days after flu detection – and a combined “control interval” – 52 weeks before the flu detection and 51 weeks after the end of the risk interval.
Among the total of 364 hospital admissions for MI in patients with confirmed influenza, 20 occurred during the defined 1-week risk interval (20 admissions/week) and 344 occurred during the control interval (3.3 admissions/week), giving an incidence ratio (IR) of 6.05, Jeffrey C. Kwong, MD, of the University of Toronto and his associates reported in the New England Journal of Medicine.
There was little difference between days 1 and 3 after flu confirmation (IR, 6.3) and days 4-7 (IR, 5.8), but risk dropped off quickly after that, with IRs of 0.6 at days 8-14 and 0.75 at days 15-28. Risk was increased for older adults, those with influenza B infection, and those who had their first MI, the investigators said.
MI incidence also was elevated after infection with noninfluenza respiratory viruses, although to a lesser extent than with influenza, which suggests that “influenza is illustrative of the role that acute respiratory infections have in precipitating acute myocardial infarction,” Dr. Kwong and his associates wrote.
The study was supported by the Canadian Institutes of Health Research, by Public Health Ontario, and by the Institute for Clinical Evaluative Sciences. Dr. Kwong reported grants from Canadian Institutes of Health Research during the conduct of the study, as well as grants from Canadian Institutes of Health Research and University of Toronto.
SOURCE: Kwong JC et al. N Engl J Med. 2018. 378(4):345-53. doi: 10.1056/NEJMoa1702090.
Myocardial infarction admissions were six times more likely to occur in the week after a positive test for influenza than in the year before or the 51 weeks after the infection, according to analysis of a Canadian cohort that links laboratories with administrative databases.
The investigators used this cohort data to define definitions of “risk interval” – the first 7 days after flu detection – and a combined “control interval” – 52 weeks before the flu detection and 51 weeks after the end of the risk interval.
Among the total of 364 hospital admissions for MI in patients with confirmed influenza, 20 occurred during the defined 1-week risk interval (20 admissions/week) and 344 occurred during the control interval (3.3 admissions/week), giving an incidence ratio (IR) of 6.05, Jeffrey C. Kwong, MD, of the University of Toronto and his associates reported in the New England Journal of Medicine.
There was little difference between days 1 and 3 after flu confirmation (IR, 6.3) and days 4-7 (IR, 5.8), but risk dropped off quickly after that, with IRs of 0.6 at days 8-14 and 0.75 at days 15-28. Risk was increased for older adults, those with influenza B infection, and those who had their first MI, the investigators said.
MI incidence also was elevated after infection with noninfluenza respiratory viruses, although to a lesser extent than with influenza, which suggests that “influenza is illustrative of the role that acute respiratory infections have in precipitating acute myocardial infarction,” Dr. Kwong and his associates wrote.
The study was supported by the Canadian Institutes of Health Research, by Public Health Ontario, and by the Institute for Clinical Evaluative Sciences. Dr. Kwong reported grants from Canadian Institutes of Health Research during the conduct of the study, as well as grants from Canadian Institutes of Health Research and University of Toronto.
SOURCE: Kwong JC et al. N Engl J Med. 2018. 378(4):345-53. doi: 10.1056/NEJMoa1702090.
Myocardial infarction admissions were six times more likely to occur in the week after a positive test for influenza than in the year before or the 51 weeks after the infection, according to analysis of a Canadian cohort that links laboratories with administrative databases.
The investigators used this cohort data to define definitions of “risk interval” – the first 7 days after flu detection – and a combined “control interval” – 52 weeks before the flu detection and 51 weeks after the end of the risk interval.
Among the total of 364 hospital admissions for MI in patients with confirmed influenza, 20 occurred during the defined 1-week risk interval (20 admissions/week) and 344 occurred during the control interval (3.3 admissions/week), giving an incidence ratio (IR) of 6.05, Jeffrey C. Kwong, MD, of the University of Toronto and his associates reported in the New England Journal of Medicine.
There was little difference between days 1 and 3 after flu confirmation (IR, 6.3) and days 4-7 (IR, 5.8), but risk dropped off quickly after that, with IRs of 0.6 at days 8-14 and 0.75 at days 15-28. Risk was increased for older adults, those with influenza B infection, and those who had their first MI, the investigators said.
MI incidence also was elevated after infection with noninfluenza respiratory viruses, although to a lesser extent than with influenza, which suggests that “influenza is illustrative of the role that acute respiratory infections have in precipitating acute myocardial infarction,” Dr. Kwong and his associates wrote.
The study was supported by the Canadian Institutes of Health Research, by Public Health Ontario, and by the Institute for Clinical Evaluative Sciences. Dr. Kwong reported grants from Canadian Institutes of Health Research during the conduct of the study, as well as grants from Canadian Institutes of Health Research and University of Toronto.
SOURCE: Kwong JC et al. N Engl J Med. 2018. 378(4):345-53. doi: 10.1056/NEJMoa1702090.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Persistent opioid use a risk after surgery in teens and young adults
For a subset of opioid-naive adolescents and young adults who received perioperative opioid scripts, those prescriptions were filled for months after the surgery, raising concerns about long-term risk for substance use disorder.
To get an idea of the teen opioid problem, from 1997 to 2012 for adolescents aged 15-19 years, the incidence of hospitalizations for opioid poisonings per 100,000 teens increased from 3.69 to 10.17, an increase of 176%, according to a study in JAMA Pediatrics (2016;170[12]:1195-201). Adolescents are at a three to five time higher risk for serious medical outcomes when hospitalized with opioid poisoning, such as life-threatening symptoms or death, compared with younger children, according to a study reporting prescription drug exposures among children (Pediatrics. 2017;139[4]:e20163382).
These figures are concerning in part because “a significant association between medical use of prescription opioids alone in adolescence and subsequent nonmedical use of prescription opioids was observed at age 35 years” in a national longitudinal study reported in the journal Pain (2016 Oct;157[10]:2173-8), said Calista M. Harbaugh, MD, of the University of Michigan, Ann Arbor, and her study coauthors.
The study in Pediatrics, which drew from a large national insurance claims database, found some patient characteristics had independent associations with increased risk of persistent opioid use. These included being female or older, as well as having a prior history of substance use disorder, chronic pain, or filling an opioid prescription preoperatively.
Dr. Harbaugh and her collaborators used a large national research database to select opioid-naive patients aged 13-21 years who received 1 of 13 surgical procedures. A total of 88,637 opioid-naive surgical patients were included in the study, with 110,432 control nonsurgical patients. The control group consisted of 3% of the database’s nonsurgical patients who met age and opioid-naivete criteria. Patients in both groups also had to have continuous insurance for the prior 12 months, not have had an opioid prescription filled within the prior year, and not have received any subsequent surgical procedures during the study period.
To be able to compare medication use among patients receiving different types of opioids, the opioid component of all prescriptions was converted to milligrams, and then used to calculate oral morphine equivalents (OMEs) for each prescription.
Although the most common procedures were tonsillectomy and/or adenoidectomy (35.9% of patients), arthroscopic knee repair (25.3%), and appendectomy, (18.6%), these were not the procedures that were most associated with persistent opioid use.
Overall, 7.1% of patients had an initial daily dosage greater than 100 OMEs for their first postoperative prescription. These high opioid doses were likely to be seen in patients undergoing three procedures known to have considerable postoperative pain: pectus repair, posterior arthrodesis, and supracondylar fracture fixation. However, patients undergoing these procedures weren’t more likely to have persistent opioid use than other surgical patients in the study, the researchers said.
Rather, cholecystectomy and colectomy had the highest risk for persistent opioid use, with adjusted odds ratios of 1.13 and 2.33, respectively. Dr. Harbaugh and her collaborators, in discussing the study’s findings, noted that these two conditions involve high levels of preoperative inflammation and are characterized by visceral pain. This scenario, they said, may set these patients up for visceral and central sensitization and present an increased risk for chronic pain.
Dr. Harbaugh and her colleagues called for preoperative screening for risk factors for persistent opioid use, so that at-risk patients can receive closer monitoring and attention. “We are not suggesting that … pain should be underappreciated or undertreated,” or that at-risk patients should not be prescribed opioids.
The investigators said that their work “points toward the multifactorial etiology of postoperative pain and its complex nature in both the short and long term.” They called for more work to “elucidate the mechanism that underlies new persistent opioid use after certain procedures,” as well as more efforts to better understand how best to use multimodal pharmacologic and nonpharmacologic pain control measures in the adolescent and young adult population.
The study was funded by the Michigan Department of Health and Human Services. Dr. Harbaugh reported that she had no relevant financial disclosures. Some of the other investigators received grants from various agencies.
SOURCE: Harbaugh CM et al. Pediatrics 2018 Jan 1;141(1):e20172439
For a subset of opioid-naive adolescents and young adults who received perioperative opioid scripts, those prescriptions were filled for months after the surgery, raising concerns about long-term risk for substance use disorder.
To get an idea of the teen opioid problem, from 1997 to 2012 for adolescents aged 15-19 years, the incidence of hospitalizations for opioid poisonings per 100,000 teens increased from 3.69 to 10.17, an increase of 176%, according to a study in JAMA Pediatrics (2016;170[12]:1195-201). Adolescents are at a three to five time higher risk for serious medical outcomes when hospitalized with opioid poisoning, such as life-threatening symptoms or death, compared with younger children, according to a study reporting prescription drug exposures among children (Pediatrics. 2017;139[4]:e20163382).
These figures are concerning in part because “a significant association between medical use of prescription opioids alone in adolescence and subsequent nonmedical use of prescription opioids was observed at age 35 years” in a national longitudinal study reported in the journal Pain (2016 Oct;157[10]:2173-8), said Calista M. Harbaugh, MD, of the University of Michigan, Ann Arbor, and her study coauthors.
The study in Pediatrics, which drew from a large national insurance claims database, found some patient characteristics had independent associations with increased risk of persistent opioid use. These included being female or older, as well as having a prior history of substance use disorder, chronic pain, or filling an opioid prescription preoperatively.
Dr. Harbaugh and her collaborators used a large national research database to select opioid-naive patients aged 13-21 years who received 1 of 13 surgical procedures. A total of 88,637 opioid-naive surgical patients were included in the study, with 110,432 control nonsurgical patients. The control group consisted of 3% of the database’s nonsurgical patients who met age and opioid-naivete criteria. Patients in both groups also had to have continuous insurance for the prior 12 months, not have had an opioid prescription filled within the prior year, and not have received any subsequent surgical procedures during the study period.
To be able to compare medication use among patients receiving different types of opioids, the opioid component of all prescriptions was converted to milligrams, and then used to calculate oral morphine equivalents (OMEs) for each prescription.
Although the most common procedures were tonsillectomy and/or adenoidectomy (35.9% of patients), arthroscopic knee repair (25.3%), and appendectomy, (18.6%), these were not the procedures that were most associated with persistent opioid use.
Overall, 7.1% of patients had an initial daily dosage greater than 100 OMEs for their first postoperative prescription. These high opioid doses were likely to be seen in patients undergoing three procedures known to have considerable postoperative pain: pectus repair, posterior arthrodesis, and supracondylar fracture fixation. However, patients undergoing these procedures weren’t more likely to have persistent opioid use than other surgical patients in the study, the researchers said.
Rather, cholecystectomy and colectomy had the highest risk for persistent opioid use, with adjusted odds ratios of 1.13 and 2.33, respectively. Dr. Harbaugh and her collaborators, in discussing the study’s findings, noted that these two conditions involve high levels of preoperative inflammation and are characterized by visceral pain. This scenario, they said, may set these patients up for visceral and central sensitization and present an increased risk for chronic pain.
Dr. Harbaugh and her colleagues called for preoperative screening for risk factors for persistent opioid use, so that at-risk patients can receive closer monitoring and attention. “We are not suggesting that … pain should be underappreciated or undertreated,” or that at-risk patients should not be prescribed opioids.
The investigators said that their work “points toward the multifactorial etiology of postoperative pain and its complex nature in both the short and long term.” They called for more work to “elucidate the mechanism that underlies new persistent opioid use after certain procedures,” as well as more efforts to better understand how best to use multimodal pharmacologic and nonpharmacologic pain control measures in the adolescent and young adult population.
The study was funded by the Michigan Department of Health and Human Services. Dr. Harbaugh reported that she had no relevant financial disclosures. Some of the other investigators received grants from various agencies.
SOURCE: Harbaugh CM et al. Pediatrics 2018 Jan 1;141(1):e20172439
For a subset of opioid-naive adolescents and young adults who received perioperative opioid scripts, those prescriptions were filled for months after the surgery, raising concerns about long-term risk for substance use disorder.
To get an idea of the teen opioid problem, from 1997 to 2012 for adolescents aged 15-19 years, the incidence of hospitalizations for opioid poisonings per 100,000 teens increased from 3.69 to 10.17, an increase of 176%, according to a study in JAMA Pediatrics (2016;170[12]:1195-201). Adolescents are at a three to five time higher risk for serious medical outcomes when hospitalized with opioid poisoning, such as life-threatening symptoms or death, compared with younger children, according to a study reporting prescription drug exposures among children (Pediatrics. 2017;139[4]:e20163382).
These figures are concerning in part because “a significant association between medical use of prescription opioids alone in adolescence and subsequent nonmedical use of prescription opioids was observed at age 35 years” in a national longitudinal study reported in the journal Pain (2016 Oct;157[10]:2173-8), said Calista M. Harbaugh, MD, of the University of Michigan, Ann Arbor, and her study coauthors.
The study in Pediatrics, which drew from a large national insurance claims database, found some patient characteristics had independent associations with increased risk of persistent opioid use. These included being female or older, as well as having a prior history of substance use disorder, chronic pain, or filling an opioid prescription preoperatively.
Dr. Harbaugh and her collaborators used a large national research database to select opioid-naive patients aged 13-21 years who received 1 of 13 surgical procedures. A total of 88,637 opioid-naive surgical patients were included in the study, with 110,432 control nonsurgical patients. The control group consisted of 3% of the database’s nonsurgical patients who met age and opioid-naivete criteria. Patients in both groups also had to have continuous insurance for the prior 12 months, not have had an opioid prescription filled within the prior year, and not have received any subsequent surgical procedures during the study period.
To be able to compare medication use among patients receiving different types of opioids, the opioid component of all prescriptions was converted to milligrams, and then used to calculate oral morphine equivalents (OMEs) for each prescription.
Although the most common procedures were tonsillectomy and/or adenoidectomy (35.9% of patients), arthroscopic knee repair (25.3%), and appendectomy, (18.6%), these were not the procedures that were most associated with persistent opioid use.
Overall, 7.1% of patients had an initial daily dosage greater than 100 OMEs for their first postoperative prescription. These high opioid doses were likely to be seen in patients undergoing three procedures known to have considerable postoperative pain: pectus repair, posterior arthrodesis, and supracondylar fracture fixation. However, patients undergoing these procedures weren’t more likely to have persistent opioid use than other surgical patients in the study, the researchers said.
Rather, cholecystectomy and colectomy had the highest risk for persistent opioid use, with adjusted odds ratios of 1.13 and 2.33, respectively. Dr. Harbaugh and her collaborators, in discussing the study’s findings, noted that these two conditions involve high levels of preoperative inflammation and are characterized by visceral pain. This scenario, they said, may set these patients up for visceral and central sensitization and present an increased risk for chronic pain.
Dr. Harbaugh and her colleagues called for preoperative screening for risk factors for persistent opioid use, so that at-risk patients can receive closer monitoring and attention. “We are not suggesting that … pain should be underappreciated or undertreated,” or that at-risk patients should not be prescribed opioids.
The investigators said that their work “points toward the multifactorial etiology of postoperative pain and its complex nature in both the short and long term.” They called for more work to “elucidate the mechanism that underlies new persistent opioid use after certain procedures,” as well as more efforts to better understand how best to use multimodal pharmacologic and nonpharmacologic pain control measures in the adolescent and young adult population.
The study was funded by the Michigan Department of Health and Human Services. Dr. Harbaugh reported that she had no relevant financial disclosures. Some of the other investigators received grants from various agencies.
SOURCE: Harbaugh CM et al. Pediatrics 2018 Jan 1;141(1):e20172439
FROM PEDIATRICS
Key clinical point: Especially for females, older teens, and young adults, there’s a risk for persistent postsurgical opioid use.
Major finding: Opioid use persisted for 4.8% of patients undergoing surgery, compared with 0.1% of patients who did not have surgery.
Study details: Retrospective review of claims database including 88,637 adolescent and young adult patients undergoing surgery, and 110,432 controls who did not have surgery.
Disclosures: The study was funded by the Michigan Department of Health and Human Services. Dr. Harbaugh reported that she had no conflicts of interest. Some of the other investigators received grants from various agencies.
Source: Harbaugh CM et al. Pediatrics. 2018 Jan 1;141(1):e20172439
Hyaluronic Acid for Lip Rejuvenation



Circulating tumor cell assay shows promise for colorectal cancer screening
SAN FRANCISCO – A new blood-based assay that measures circulating tumor cells (CTCs) shows good performance in detecting colorectal cancer and precancer, investigators reported at the 2018 GI Cancers Symposium.
Although colorectal cancer screening is a grade A recommendation of the U.S. Preventive Services Task Force, poor uptake remains problematic and contributes to more advanced disease at diagnosis, noted lead investigator Wen-Sy Tsai, MD, assistant professor at Linkou Chang Gung Memorial Hospital, Taoyuan, Taiwan.
“Currently, one-third of Americans have never been screened for colorectal cancer,” he said, due in part to reluctance to undergo colonoscopy and poor compliance with stool tests. Further complicating matters, the fecal immunochemical test (FIT) has a high false-positive rate.
He and his coinvestigators tested the new assay, called CMx (CTCs in Maximum), among 620 individuals in Taiwan who underwent colonoscopy – some with colorectal cancer, some with precancerous lesions, and some healthy. Results showed the assay’s sensitivity was nearly 87% for cancer and 77% for precancerous lesions. Specificity exceeded 97%.
“The CMx assay is capable of detecting … early-stage cancer with a low false-positive rate. As it is a blood test, higher compliance will lead to better outcomes,” Dr. Tsai proposed. “Because the mechanism of CTC dissemination is similar, the CTC assay can be applied in other cancer types such as prostate, breast, and lung cancer.”
The investigators are also identifying collaborators for testing the new assay among U.S. populations, he noted.
“Screening tests offer some of the greatest potential for getting to zero colorectal cancer deaths,” said invited discussant Douglas A. Corley, MD, PhD, clinical professor and gastroenterologist at the University of California, San Francisco, and a research scientist at Kaiser Permanente, San Francisco.
CTCs are especially attractive for screening because they could be both sensitive and specific, he said. “Unlike something such as fecal immunotesting, which is not testing specifically for cancer, or colonoscopy, which is quite invasive and detects a lot of things that may never progress to cancer, CTCs offer this potential,” said Dr. Corley.
Challenges of interpreting new screening tests include their heavy dependence on the population being tested and the need for replication, according to Dr. Corley. For example, initial results for the septin 9 methylated DNA blood test looked very good, with sensitivity of about 90% (BMC Med. 2011;9:133), but after its testing in 14 populations, a meta-analysis showed that pooled sensitivity was just 67% (Biomed Rep. 2017;7[4]:353-60).
“Circulating tumor markers are an incredibly interesting target for screening, particularly because of their potential for being very specific for what you are looking for, and potentially markedly decreasing the subsequent follow-up that would need to be done for invasive tests such as colonoscopy,” Dr. Corley said. “However, this [CTC assay] really requires confirmation in screening populations, especially given some of the information we have from prior tests.”
Study details
Dr. Tsai’s team studied 327 patients with colorectal cancer of all stages, 111 patients with precancerous lesions (adenomas, advanced adenomas, carcinoma in situ/stage 0), and 182 healthy controls. All had blood drawn for the CTC assay before undergoing colonoscopy. Results of each test were ascertained with blinding to the results of the other.
CTCs are rarely shed into the circulation from precancerous lesions, with approximate density of only 1 per billion blood cells, Dr. Tsai said. The CMx assay (manufactured by CellMax Life) is able to detect these cells with high sensitivity through use of advanced technologies such as affinity-based microfluidics and a biomimetic surface coating.
The assay is performed with just 2 mL of whole blood. CTCs are defined as intact nucleated cells staining positive for CD20 and negative for CD45; they were combined with patient age in an algorithm, ultimately producing a risk score.
Study results showed that the CTC assay had an accuracy of 87.9% in the entire cohort, reported Dr. Tsai. The false-positive rate was just 3.3%, and the false-negative rate was 15.8%.
Sensitivity was 84.0% overall (76.6% for precancer and 86.9% for cancer), specificity was 97.3% overall (97.3% and 97.3%), and area under the receiver operating characteristic curve was 0.87 overall (0.84 and 0.88).
Dr. Tsai noted that the CTC assay’s sensitivity of nearly 77% for precancer compares favorably with that of a variety of other screening tests, such as the stool guaiac test for fecal occult blood (2%-10%), FIT alone (23.8%), and a stool DNA test combined with FIT (42%), and, in fact, falls within the range reported for colonoscopy (76%-94%).
The symposium was sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
SOURCE: Tsai W et al., ASCO GI Abstract 556
SAN FRANCISCO – A new blood-based assay that measures circulating tumor cells (CTCs) shows good performance in detecting colorectal cancer and precancer, investigators reported at the 2018 GI Cancers Symposium.
Although colorectal cancer screening is a grade A recommendation of the U.S. Preventive Services Task Force, poor uptake remains problematic and contributes to more advanced disease at diagnosis, noted lead investigator Wen-Sy Tsai, MD, assistant professor at Linkou Chang Gung Memorial Hospital, Taoyuan, Taiwan.
“Currently, one-third of Americans have never been screened for colorectal cancer,” he said, due in part to reluctance to undergo colonoscopy and poor compliance with stool tests. Further complicating matters, the fecal immunochemical test (FIT) has a high false-positive rate.
He and his coinvestigators tested the new assay, called CMx (CTCs in Maximum), among 620 individuals in Taiwan who underwent colonoscopy – some with colorectal cancer, some with precancerous lesions, and some healthy. Results showed the assay’s sensitivity was nearly 87% for cancer and 77% for precancerous lesions. Specificity exceeded 97%.
“The CMx assay is capable of detecting … early-stage cancer with a low false-positive rate. As it is a blood test, higher compliance will lead to better outcomes,” Dr. Tsai proposed. “Because the mechanism of CTC dissemination is similar, the CTC assay can be applied in other cancer types such as prostate, breast, and lung cancer.”
The investigators are also identifying collaborators for testing the new assay among U.S. populations, he noted.
“Screening tests offer some of the greatest potential for getting to zero colorectal cancer deaths,” said invited discussant Douglas A. Corley, MD, PhD, clinical professor and gastroenterologist at the University of California, San Francisco, and a research scientist at Kaiser Permanente, San Francisco.
CTCs are especially attractive for screening because they could be both sensitive and specific, he said. “Unlike something such as fecal immunotesting, which is not testing specifically for cancer, or colonoscopy, which is quite invasive and detects a lot of things that may never progress to cancer, CTCs offer this potential,” said Dr. Corley.
Challenges of interpreting new screening tests include their heavy dependence on the population being tested and the need for replication, according to Dr. Corley. For example, initial results for the septin 9 methylated DNA blood test looked very good, with sensitivity of about 90% (BMC Med. 2011;9:133), but after its testing in 14 populations, a meta-analysis showed that pooled sensitivity was just 67% (Biomed Rep. 2017;7[4]:353-60).
“Circulating tumor markers are an incredibly interesting target for screening, particularly because of their potential for being very specific for what you are looking for, and potentially markedly decreasing the subsequent follow-up that would need to be done for invasive tests such as colonoscopy,” Dr. Corley said. “However, this [CTC assay] really requires confirmation in screening populations, especially given some of the information we have from prior tests.”
Study details
Dr. Tsai’s team studied 327 patients with colorectal cancer of all stages, 111 patients with precancerous lesions (adenomas, advanced adenomas, carcinoma in situ/stage 0), and 182 healthy controls. All had blood drawn for the CTC assay before undergoing colonoscopy. Results of each test were ascertained with blinding to the results of the other.
CTCs are rarely shed into the circulation from precancerous lesions, with approximate density of only 1 per billion blood cells, Dr. Tsai said. The CMx assay (manufactured by CellMax Life) is able to detect these cells with high sensitivity through use of advanced technologies such as affinity-based microfluidics and a biomimetic surface coating.
The assay is performed with just 2 mL of whole blood. CTCs are defined as intact nucleated cells staining positive for CD20 and negative for CD45; they were combined with patient age in an algorithm, ultimately producing a risk score.
Study results showed that the CTC assay had an accuracy of 87.9% in the entire cohort, reported Dr. Tsai. The false-positive rate was just 3.3%, and the false-negative rate was 15.8%.
Sensitivity was 84.0% overall (76.6% for precancer and 86.9% for cancer), specificity was 97.3% overall (97.3% and 97.3%), and area under the receiver operating characteristic curve was 0.87 overall (0.84 and 0.88).
Dr. Tsai noted that the CTC assay’s sensitivity of nearly 77% for precancer compares favorably with that of a variety of other screening tests, such as the stool guaiac test for fecal occult blood (2%-10%), FIT alone (23.8%), and a stool DNA test combined with FIT (42%), and, in fact, falls within the range reported for colonoscopy (76%-94%).
The symposium was sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
SOURCE: Tsai W et al., ASCO GI Abstract 556
SAN FRANCISCO – A new blood-based assay that measures circulating tumor cells (CTCs) shows good performance in detecting colorectal cancer and precancer, investigators reported at the 2018 GI Cancers Symposium.
Although colorectal cancer screening is a grade A recommendation of the U.S. Preventive Services Task Force, poor uptake remains problematic and contributes to more advanced disease at diagnosis, noted lead investigator Wen-Sy Tsai, MD, assistant professor at Linkou Chang Gung Memorial Hospital, Taoyuan, Taiwan.
“Currently, one-third of Americans have never been screened for colorectal cancer,” he said, due in part to reluctance to undergo colonoscopy and poor compliance with stool tests. Further complicating matters, the fecal immunochemical test (FIT) has a high false-positive rate.
He and his coinvestigators tested the new assay, called CMx (CTCs in Maximum), among 620 individuals in Taiwan who underwent colonoscopy – some with colorectal cancer, some with precancerous lesions, and some healthy. Results showed the assay’s sensitivity was nearly 87% for cancer and 77% for precancerous lesions. Specificity exceeded 97%.
“The CMx assay is capable of detecting … early-stage cancer with a low false-positive rate. As it is a blood test, higher compliance will lead to better outcomes,” Dr. Tsai proposed. “Because the mechanism of CTC dissemination is similar, the CTC assay can be applied in other cancer types such as prostate, breast, and lung cancer.”
The investigators are also identifying collaborators for testing the new assay among U.S. populations, he noted.
“Screening tests offer some of the greatest potential for getting to zero colorectal cancer deaths,” said invited discussant Douglas A. Corley, MD, PhD, clinical professor and gastroenterologist at the University of California, San Francisco, and a research scientist at Kaiser Permanente, San Francisco.
CTCs are especially attractive for screening because they could be both sensitive and specific, he said. “Unlike something such as fecal immunotesting, which is not testing specifically for cancer, or colonoscopy, which is quite invasive and detects a lot of things that may never progress to cancer, CTCs offer this potential,” said Dr. Corley.
Challenges of interpreting new screening tests include their heavy dependence on the population being tested and the need for replication, according to Dr. Corley. For example, initial results for the septin 9 methylated DNA blood test looked very good, with sensitivity of about 90% (BMC Med. 2011;9:133), but after its testing in 14 populations, a meta-analysis showed that pooled sensitivity was just 67% (Biomed Rep. 2017;7[4]:353-60).
“Circulating tumor markers are an incredibly interesting target for screening, particularly because of their potential for being very specific for what you are looking for, and potentially markedly decreasing the subsequent follow-up that would need to be done for invasive tests such as colonoscopy,” Dr. Corley said. “However, this [CTC assay] really requires confirmation in screening populations, especially given some of the information we have from prior tests.”
Study details
Dr. Tsai’s team studied 327 patients with colorectal cancer of all stages, 111 patients with precancerous lesions (adenomas, advanced adenomas, carcinoma in situ/stage 0), and 182 healthy controls. All had blood drawn for the CTC assay before undergoing colonoscopy. Results of each test were ascertained with blinding to the results of the other.
CTCs are rarely shed into the circulation from precancerous lesions, with approximate density of only 1 per billion blood cells, Dr. Tsai said. The CMx assay (manufactured by CellMax Life) is able to detect these cells with high sensitivity through use of advanced technologies such as affinity-based microfluidics and a biomimetic surface coating.
The assay is performed with just 2 mL of whole blood. CTCs are defined as intact nucleated cells staining positive for CD20 and negative for CD45; they were combined with patient age in an algorithm, ultimately producing a risk score.
Study results showed that the CTC assay had an accuracy of 87.9% in the entire cohort, reported Dr. Tsai. The false-positive rate was just 3.3%, and the false-negative rate was 15.8%.
Sensitivity was 84.0% overall (76.6% for precancer and 86.9% for cancer), specificity was 97.3% overall (97.3% and 97.3%), and area under the receiver operating characteristic curve was 0.87 overall (0.84 and 0.88).
Dr. Tsai noted that the CTC assay’s sensitivity of nearly 77% for precancer compares favorably with that of a variety of other screening tests, such as the stool guaiac test for fecal occult blood (2%-10%), FIT alone (23.8%), and a stool DNA test combined with FIT (42%), and, in fact, falls within the range reported for colonoscopy (76%-94%).
The symposium was sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
SOURCE: Tsai W et al., ASCO GI Abstract 556
REPORTING FROM 2018 GI CANCERS SYMPOSIUM
Key clinical point:
Major finding: The CMx assay had a sensitivity of nearly 87% for colorectal cancer and nearly 77% for precancerous lesions.
Data source: A single-center, blinded, prospective cohort study among 327 patients with colorectal cancer of various stages, 111 patients with precancerous lesions, and 182 healthy individuals.
Disclosures: Dr. Tsai disclosed that he had no relevant relationships.
Source: Tsai W et al. ASCO GI Abstract 556.
FDA grants priority review to multiple myeloma treatment
, a monoclonal antibody treatment for newly diagnosed multiple myeloma patients who are ineligible for autologous stem cell transplant.
The current application is based on the randomized, multicenter, phase 3 ALCYONE study of daratumumab in combination with bortezomib (Velcade), melphalan, and prednisone (VMP) in de novo multiple myeloma patients.
Priority review is an FDA designation for drugs that treat a serious condition and may provide a significant improvement in safety or efficacy. The agency has assigned the drug a Prescription Drug User Fee Act date of May 21, which is a target date for an approval decision.
Daratumumab is being developed by Janssen Biotech, in partnership with Genmab.
, a monoclonal antibody treatment for newly diagnosed multiple myeloma patients who are ineligible for autologous stem cell transplant.
The current application is based on the randomized, multicenter, phase 3 ALCYONE study of daratumumab in combination with bortezomib (Velcade), melphalan, and prednisone (VMP) in de novo multiple myeloma patients.
Priority review is an FDA designation for drugs that treat a serious condition and may provide a significant improvement in safety or efficacy. The agency has assigned the drug a Prescription Drug User Fee Act date of May 21, which is a target date for an approval decision.
Daratumumab is being developed by Janssen Biotech, in partnership with Genmab.
, a monoclonal antibody treatment for newly diagnosed multiple myeloma patients who are ineligible for autologous stem cell transplant.
The current application is based on the randomized, multicenter, phase 3 ALCYONE study of daratumumab in combination with bortezomib (Velcade), melphalan, and prednisone (VMP) in de novo multiple myeloma patients.
Priority review is an FDA designation for drugs that treat a serious condition and may provide a significant improvement in safety or efficacy. The agency has assigned the drug a Prescription Drug User Fee Act date of May 21, which is a target date for an approval decision.
Daratumumab is being developed by Janssen Biotech, in partnership with Genmab.
Slowed growth in prescription drug spending driven by HCV drugs
National health spending amounted to $3.3 trillion or $10,348/person, a 4.3% increase in 2016, according to the Centers for Medicare & Medicaid Services. This was a slower rate of growth than the 5.8% growth in 2015 because of slower insurance enrollment and declines in spending for services and retail prescription drugs.
The growth in spending on prescription drugs in 2016 declined significantly – 1.3% in 2016 vs. 8.9% in 2015. Strong growth in spending for drugs used to treat hepatitis C contributed to high overall spending growth in 2014 and 2015, which changed in 2016. Prescription drug spending in 2016 grew more slowly partially because spending for drugs used to treat hepatitis C decreased, as fewer patients received treatment and net prices for these drugs declined, the report stated.
"The 2016 rate of prescription drug spending growth is more in line with the lower average annual growth during the period 2010–13 of 1.2 percent—a rate that was driven by the shift to more consumption of generic drugs, which was partly influenced by the loss of patent protection of major brand-name drugs," the authors noted.
National health spending amounted to $3.3 trillion or $10,348/person, a 4.3% increase in 2016, according to the Centers for Medicare & Medicaid Services. This was a slower rate of growth than the 5.8% growth in 2015 because of slower insurance enrollment and declines in spending for services and retail prescription drugs.
The growth in spending on prescription drugs in 2016 declined significantly – 1.3% in 2016 vs. 8.9% in 2015. Strong growth in spending for drugs used to treat hepatitis C contributed to high overall spending growth in 2014 and 2015, which changed in 2016. Prescription drug spending in 2016 grew more slowly partially because spending for drugs used to treat hepatitis C decreased, as fewer patients received treatment and net prices for these drugs declined, the report stated.
"The 2016 rate of prescription drug spending growth is more in line with the lower average annual growth during the period 2010–13 of 1.2 percent—a rate that was driven by the shift to more consumption of generic drugs, which was partly influenced by the loss of patent protection of major brand-name drugs," the authors noted.
National health spending amounted to $3.3 trillion or $10,348/person, a 4.3% increase in 2016, according to the Centers for Medicare & Medicaid Services. This was a slower rate of growth than the 5.8% growth in 2015 because of slower insurance enrollment and declines in spending for services and retail prescription drugs.
The growth in spending on prescription drugs in 2016 declined significantly – 1.3% in 2016 vs. 8.9% in 2015. Strong growth in spending for drugs used to treat hepatitis C contributed to high overall spending growth in 2014 and 2015, which changed in 2016. Prescription drug spending in 2016 grew more slowly partially because spending for drugs used to treat hepatitis C decreased, as fewer patients received treatment and net prices for these drugs declined, the report stated.
"The 2016 rate of prescription drug spending growth is more in line with the lower average annual growth during the period 2010–13 of 1.2 percent—a rate that was driven by the shift to more consumption of generic drugs, which was partly influenced by the loss of patent protection of major brand-name drugs," the authors noted.