User login
Teens with PID underscreened for HIV, syphilis
CHICAGO – Adolescents with pelvic inflammatory disease (PID) were unlikely to be screened for HIV or syphilis, and many didn’t receive an appropriate antibiotic regimen, according to a recent study reported at the annual meeting of the American Academy of Pediatrics.
Patients who were sent home rather than admitted were especially likely to miss screening, as were Hispanic patients and those with private insurance.
The Centers for Disease Control and Prevention strongly recommends that all women diagnosed with PID be tested for HIV, and that high-risk individuals also be tested for syphilis, wrote Amanda Jichlinski, MD, and her coauthors at Children’s National Health System, Washington.
The study, presented during a poster session, used data from the national Pediatric Health Information System database from 2010 to 2015. A total of 10,698 records with a diagnostic code for PID were included; patients were females aged 12-21 years seen in a pediatric emergency department.
In addition to the primary outcome of syphilis and HIV testing, the authors also looked at whether antibiotic administration for PID was in line with CDC recommendations – and it wasn’t. “Fewer than half of patients in the ED received antibiotic regimens adherent to CDC guidelines,” wrote Dr. Jichlinski and her coauthors.
Forty-six percent of patients received ceftriaxone and doxycycline, 21% received ceftriaxone and azithromycin, and 6% received ceftriaxone and metronidazole. Ceftriaxone monotherapy was given to 15% of patients. One in 10 patients with a PID diagnosis received no antibiotic at all; 2% of patients received some other regimen.
The researchers used multivariable analysis to examine separately which patient and hospital characteristics were associated with an increased likelihood of testing for both HIV and syphilis. With white, non-Hispanic adolescents used as the referent, Hispanic females with PID were less likely to receive screening for either HIV or syphilis (adjusted odds ratio, 0.8 for both; 95% confidence interval, 0.7-1.0 for both).
In contrast, black non-Hispanic females were screened more often; the aOR for HIV screening was 1.4 (95% CI, 1.2-1.6), and the aOR for syphilis screening was 1.8 (95% CI, 1.6-2.0) for this group of adolescents.
Patients were dichotomized into older (17-21 years of age; n = 4,737, 44%) and younger (12-16 years of age; n = 5,961, 56%) age groups; younger patients were slightly more likely to receive HIV (aOR, 1.2) and syphilis (aOR, 1.1) screening.
Just under a third of patients in the study were seen in a hospital with fewer than 300 beds, and these facilities were more likely to screen for HIV (aOR, 1.4) and syphilis (aOR, 1.1) than the larger hospitals.
By far the largest predictor of whether HIV and syphilis screening was done, though, was a hospital admission. Patients who were admitted (n = 4,043, 38%) were 7 times more likely to be screened for HIV and 4.6 times more likely to be screened for syphilis than those who were sent home from the emergency department.
Although the large, nationally representative study had many strengths, Dr. Jichlinski and her coauthors acknowledged that the data they were provided couldn’t account for medication that was prescribed, rather than administered in the emergency department. Also, the results may not be generalizable to adolescents treated in nonpediatric emergency departments or other facilities, such as urgent care centers.
“Adolescents with PID are underscreened for HIV and syphilis,” wrote Dr. Jichlinski and her coauthors. They called for pediatricians to receive more education about management of PID in adolescents. From a practical perspective, the investigators also suggested incorporating order sets for sexually transmitted infection testing and antibiotic administration into electronic medical records; in this way, a PID diagnosis code would trigger simplified testing and treatment choices.
Dr. Jichlinski reported no conflicts of interest. Dr. Monika Goyal, MD, senior author on the study, reported funding support by the National Institute of Child Health and Human Development. Dr. Goyal also holds an appointment at the George Washington University, Washington.
SOURCE: Jichlinski A et al. AAP 2017 Abstract 5, AAP Section on Emergency Medicine.
CHICAGO – Adolescents with pelvic inflammatory disease (PID) were unlikely to be screened for HIV or syphilis, and many didn’t receive an appropriate antibiotic regimen, according to a recent study reported at the annual meeting of the American Academy of Pediatrics.
Patients who were sent home rather than admitted were especially likely to miss screening, as were Hispanic patients and those with private insurance.
The Centers for Disease Control and Prevention strongly recommends that all women diagnosed with PID be tested for HIV, and that high-risk individuals also be tested for syphilis, wrote Amanda Jichlinski, MD, and her coauthors at Children’s National Health System, Washington.
The study, presented during a poster session, used data from the national Pediatric Health Information System database from 2010 to 2015. A total of 10,698 records with a diagnostic code for PID were included; patients were females aged 12-21 years seen in a pediatric emergency department.
In addition to the primary outcome of syphilis and HIV testing, the authors also looked at whether antibiotic administration for PID was in line with CDC recommendations – and it wasn’t. “Fewer than half of patients in the ED received antibiotic regimens adherent to CDC guidelines,” wrote Dr. Jichlinski and her coauthors.
Forty-six percent of patients received ceftriaxone and doxycycline, 21% received ceftriaxone and azithromycin, and 6% received ceftriaxone and metronidazole. Ceftriaxone monotherapy was given to 15% of patients. One in 10 patients with a PID diagnosis received no antibiotic at all; 2% of patients received some other regimen.
The researchers used multivariable analysis to examine separately which patient and hospital characteristics were associated with an increased likelihood of testing for both HIV and syphilis. With white, non-Hispanic adolescents used as the referent, Hispanic females with PID were less likely to receive screening for either HIV or syphilis (adjusted odds ratio, 0.8 for both; 95% confidence interval, 0.7-1.0 for both).
In contrast, black non-Hispanic females were screened more often; the aOR for HIV screening was 1.4 (95% CI, 1.2-1.6), and the aOR for syphilis screening was 1.8 (95% CI, 1.6-2.0) for this group of adolescents.
Patients were dichotomized into older (17-21 years of age; n = 4,737, 44%) and younger (12-16 years of age; n = 5,961, 56%) age groups; younger patients were slightly more likely to receive HIV (aOR, 1.2) and syphilis (aOR, 1.1) screening.
Just under a third of patients in the study were seen in a hospital with fewer than 300 beds, and these facilities were more likely to screen for HIV (aOR, 1.4) and syphilis (aOR, 1.1) than the larger hospitals.
By far the largest predictor of whether HIV and syphilis screening was done, though, was a hospital admission. Patients who were admitted (n = 4,043, 38%) were 7 times more likely to be screened for HIV and 4.6 times more likely to be screened for syphilis than those who were sent home from the emergency department.
Although the large, nationally representative study had many strengths, Dr. Jichlinski and her coauthors acknowledged that the data they were provided couldn’t account for medication that was prescribed, rather than administered in the emergency department. Also, the results may not be generalizable to adolescents treated in nonpediatric emergency departments or other facilities, such as urgent care centers.
“Adolescents with PID are underscreened for HIV and syphilis,” wrote Dr. Jichlinski and her coauthors. They called for pediatricians to receive more education about management of PID in adolescents. From a practical perspective, the investigators also suggested incorporating order sets for sexually transmitted infection testing and antibiotic administration into electronic medical records; in this way, a PID diagnosis code would trigger simplified testing and treatment choices.
Dr. Jichlinski reported no conflicts of interest. Dr. Monika Goyal, MD, senior author on the study, reported funding support by the National Institute of Child Health and Human Development. Dr. Goyal also holds an appointment at the George Washington University, Washington.
SOURCE: Jichlinski A et al. AAP 2017 Abstract 5, AAP Section on Emergency Medicine.
CHICAGO – Adolescents with pelvic inflammatory disease (PID) were unlikely to be screened for HIV or syphilis, and many didn’t receive an appropriate antibiotic regimen, according to a recent study reported at the annual meeting of the American Academy of Pediatrics.
Patients who were sent home rather than admitted were especially likely to miss screening, as were Hispanic patients and those with private insurance.
The Centers for Disease Control and Prevention strongly recommends that all women diagnosed with PID be tested for HIV, and that high-risk individuals also be tested for syphilis, wrote Amanda Jichlinski, MD, and her coauthors at Children’s National Health System, Washington.
The study, presented during a poster session, used data from the national Pediatric Health Information System database from 2010 to 2015. A total of 10,698 records with a diagnostic code for PID were included; patients were females aged 12-21 years seen in a pediatric emergency department.
In addition to the primary outcome of syphilis and HIV testing, the authors also looked at whether antibiotic administration for PID was in line with CDC recommendations – and it wasn’t. “Fewer than half of patients in the ED received antibiotic regimens adherent to CDC guidelines,” wrote Dr. Jichlinski and her coauthors.
Forty-six percent of patients received ceftriaxone and doxycycline, 21% received ceftriaxone and azithromycin, and 6% received ceftriaxone and metronidazole. Ceftriaxone monotherapy was given to 15% of patients. One in 10 patients with a PID diagnosis received no antibiotic at all; 2% of patients received some other regimen.
The researchers used multivariable analysis to examine separately which patient and hospital characteristics were associated with an increased likelihood of testing for both HIV and syphilis. With white, non-Hispanic adolescents used as the referent, Hispanic females with PID were less likely to receive screening for either HIV or syphilis (adjusted odds ratio, 0.8 for both; 95% confidence interval, 0.7-1.0 for both).
In contrast, black non-Hispanic females were screened more often; the aOR for HIV screening was 1.4 (95% CI, 1.2-1.6), and the aOR for syphilis screening was 1.8 (95% CI, 1.6-2.0) for this group of adolescents.
Patients were dichotomized into older (17-21 years of age; n = 4,737, 44%) and younger (12-16 years of age; n = 5,961, 56%) age groups; younger patients were slightly more likely to receive HIV (aOR, 1.2) and syphilis (aOR, 1.1) screening.
Just under a third of patients in the study were seen in a hospital with fewer than 300 beds, and these facilities were more likely to screen for HIV (aOR, 1.4) and syphilis (aOR, 1.1) than the larger hospitals.
By far the largest predictor of whether HIV and syphilis screening was done, though, was a hospital admission. Patients who were admitted (n = 4,043, 38%) were 7 times more likely to be screened for HIV and 4.6 times more likely to be screened for syphilis than those who were sent home from the emergency department.
Although the large, nationally representative study had many strengths, Dr. Jichlinski and her coauthors acknowledged that the data they were provided couldn’t account for medication that was prescribed, rather than administered in the emergency department. Also, the results may not be generalizable to adolescents treated in nonpediatric emergency departments or other facilities, such as urgent care centers.
“Adolescents with PID are underscreened for HIV and syphilis,” wrote Dr. Jichlinski and her coauthors. They called for pediatricians to receive more education about management of PID in adolescents. From a practical perspective, the investigators also suggested incorporating order sets for sexually transmitted infection testing and antibiotic administration into electronic medical records; in this way, a PID diagnosis code would trigger simplified testing and treatment choices.
Dr. Jichlinski reported no conflicts of interest. Dr. Monika Goyal, MD, senior author on the study, reported funding support by the National Institute of Child Health and Human Development. Dr. Goyal also holds an appointment at the George Washington University, Washington.
SOURCE: Jichlinski A et al. AAP 2017 Abstract 5, AAP Section on Emergency Medicine.
REPORTING FROM AAP 2017
Key clinical point:
Major finding: Hispanic females were least likely to be screened (adjusted OR, 0.8), compared with non-Hispanic white females.
Study details: Retrospective study of 10,698 adolescent patients with PID from a national database.
Disclosures: The study was funded in part by the National Institute of Child Health and Development. The authors had no relevant financial disclosures.
Source: Jichlinski A et al. AAP 2017 Abstract 5, AAP Section on Emergency Medicine
Simple Patient Care Instructions Translate Best: Safety Guidelines for Physician Use of Google Translate
From the University of Arizona College of Medicine – Tucson, Tucson, AZ.
Abstract
- Objective: To determine predictors of quality and safety of machine translation (Google Translate) of patient care instructions (PCIs), and to determine if machine back translation is useful in quality assessment.
- Methods: 100 sample English PCIs were contributed by 88 clinical faculty. Each example PCI was up to 3 sentences of typical patient instruction that might be included in an after visit summary. Google Translate was used to first translate the English to Spanish, then back to English. A panel of 6 English/Spanish translators assessed the Spanish translations for safety and quality. A panel of 6 English-speaking health care workers assessed the back translation. A 5-point scale was used to assess quality. Safety was assessed as safe or unsafe.
- Results: Google Translate was usually (> 90%) capable of safe and comprehensible translation from English to Spanish. Instructions with incresed complexity, especially regarding medications, were prone to unsafe translation. Back translation was not reliable in detecting unsafe Spanish.
- Conclusion: Google Translate is a continuously evolving resource for clinicians that offers the promise of improved physician-patient communication. Simple declarative sentences are most reliably translated with high quality and safety.
Keywords: translation; machine translation; electronic health record; after-visit summary; patient safety; physician-patient communication.
Acore measure of the meaningful use of electronic health records incentive program is the generation and provision of the after visit summary (AVS), a mechanism for physicians to provide patients with a written summary of the patient encounter [1,2]. Although not a required element for meaningful use, free text patient care instructions (PCIs) provide the physician an opportunity to improve patient engagement either at the time of service or through the patient portal [3] by providing a short written summary of the key points of the office visit based upon the visit’s clinical discussion. For patients who do not speak English, a verbal translation service is required [4], but seldom are specific patient instructions provided in writing in the patient’s preferred language. A mechanism to improve communication might be through translation of the PCI into the patient’s preferred language. Spanish is the most common language, other than English, spoken at home in the United States [5,6]. For this reason, we chose to investigate if it is feasible to use machine translation (Google Translate) to safely and reliably translate a variety of PCIs from English to Spanish, and to assess the types of translation errors and ambiguities that might result in unsafe communication. We further investigate if machine back translation might allow the author of patient care instructions to evaluate the quality of the Spanish machine translation.
There is evidence to suggest that patient communication and satisfaction will improve if portions of the AVS are communicated in Spanish to primarily Spanish-speaking patients. Pavlik et al conducted a randomized controlled trial on the association of patient recall, satisfaction, and adherence to the information communicated in an AVS, in a largely Hispanic (61%) primary care clinic setting [7]. The AVS was provided in English. They noted that Spanish speakers wished to receive information in Spanish, although most had access to translation by a family member. They also noted that a lack of ability to provide an AVS in Spanish was a concern among providers. There was no difference in recall or satisfaction between English and Spanish speakers with respect to medications and allergies, suggesting that not all portions of the AVS might need to be translated.
Machine translation refers to the automated process of translating one language to another. The most recent methods of machine translation, as exemplified by Google Translate (Google Inc., Mountain View, CA), do not use rules of grammar and dictionaries to perform translations but instead use artificial neural networks to learn from “millions of examples” of translation [8]. However, unsupervised machine translation can result in serious errors [9]. Patil gives as an example of a serious error of translation from English (“Your child is fitting”) to Swahili (“Your child is dead”). In British parlance, “fitting” is a term for “having a seizure” and represents an example of a term that is context sensitive. However, others note that there is reason to be optimistic about the state of machine translation for biomedical text [10].
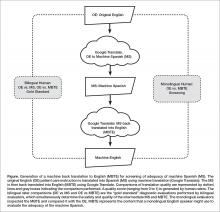
One method of assessing translation quality is through back translation, where one translator takes the author’s work into the desired target language, and then a different translator takes the target language back to the language of the author. Like the children’s game Chinese Whispers (Telephone in the United States) [11], where a “secret message” is whispered from one child to the next and spoken aloud at the end of the line of children, back translation can test to see if a message “gets through.” In this analogy, when information is machine translated from English to Spanish, and then machine translated from Spanish to English (Figure), we can compare the initial message to the final translation to see if the message “gets through.” We further investigate if machine back translation might allow a non-Spanish speaking author of PCIs to evaluate the quality of the Spanish translation.
Our intention was to determine if machine back translation [12] could be used by an English-only author to assess the quality of an intermediate Spanish translation. If poorly worded Spanish translated back into poorly worded English, the author might choose to either refine their original message until an acceptable machine back translation was achieved or to not release the Spanish translation to the patient. We were also concerned that there might be instances where the intermediate Spanish was unacceptable, but when translated back into English by machine translation, relatively acceptable English might result. If this were the case, then back translation would fail to detect a relatively poor intermediate Spanish translation.
Methods
Patient Care Instructions
Original English PCIs
Example original English PCIs were solicited from the clinical faculty and resident staff of the University of Arizona College of Medicine by an email-based survey tool (Qualtrics, Inc, Provo UT). The solicitation stated the following:
We are conducting a study to assess how well Google Translate might perform in translating patient instructions from English to Spanish. Would you please take the time to type three sentences that might comprise a typical “nugget” of patient instruction using language that you would typically include in an After Visit Summary for a patient? An example might be: “Take two Tylenol 325 mg tablets every four hours while awake for the next two days. If you have a sudden increase in pain or fever, or begin vomiting, call our office. Drink plenty of fluids.”
A total of 100 PCIs were collected. The breadth of the clinical practice and writing styles of a College of Medicine faculty are represented: not all were completely clear or were well-formed sentences, but did represent examples provided by busy clinicians of typical language that they would provide in an AVS PCI.
Machine Translation into Spanish
The 100 original English (OE) PCIs were submitted to the Google Translate web interface (https://translate.google.com/) by cutting and pasting and selecting “Spanish,” resulting in machine Spanish. The translations were performed in January 2016. No specific version number is provided by Google on their web page, and the service is described to be constantly evolving (https://translate.google.com/about/intl/en_ALL/contribute.html).
Machine Back Translation into English (MBTE)
Google Translate was then used to translate the machine Spanish back into into English. MBTE represents the content that a monolingual English speaker might use to evaluate the machine Spanish.
Ratings of Translation Quality and Safety
Two panels of 6 raters evaluated machine Spanish and MBTE quality and safety. A bilingual English/Spanish speaking panel simultaneously evaluated the machine Spanish and MBTE compared to OE, with the goal of inferring where in the process an undesirable back translation error occurred. Bilingual raters were experienced bilingual clinicians or certified translators. A monolingual English speaking panel also evaluated the MBTE (compared to OE). They could only infer the quality and safety of the machine Spanish indirectly through inspection of MBTE, and their assessment was free of the potential bias of knowledge of the intermediate Spanish translation.
The raters used Likert scales to rate grammar similarity and content similarity (scale from 1 to 5: 1 = very dissimilar, 5 = identical). For each PCI, grammar and content scores for each rater were summed and then divided by 10 to yield a within-rater quality score ranging from 0 to 1. A panel-level (bilingual or monolingual) quality score was calculated by averaging the quality scores across raters.
Safety of translation was rated as 0 or Safe (“While the translation may be awkward, it is not dangerous” or 1 or Unsafe (“A dangerous translation error is present that might cause harm to the patient if instructions were followed”). If any panel member considered an item to be unsafe, the item as a whole was scored as unsafe.
Data Analysis
Descriptive Summary of PCI Contributions
The 100 PCIs were summarized in terms of volume (word count), complexity (Flesch-Kincaid Grade Level index [13]), and content (medication names, references, formatting) (Table 1). Word count and grade level were calculated using Microsoft Word (Microsoft Corp, Redmond WA).
Safety Analysis
Concordance analysis. A safety translation concern as defined in this study (“might cause harm”) is very subjective. To reduce some of the variation in assessment of safety, we identified 4 members of the bilingual panel whose safety assessments of MBTE were most similar to the most concordant 4 monolingual raters’ assessment of MBTE safety. The goal was to select the bilingual panel of 4 that was most “typical” of the behavior of a “typical” monolingual individual with respect to assessing the safety of an individual MBTE translation. We then used this bilingual panel to identify 2 sets of “unsafe” machine Spanish and MBTE PCI translations: PCIs where ANY of the 4 bilingual raters identified a safety concern in machine Spanish or MBTE, and PCIs where MOST (at least 3) of the 4 bilingual raters agree that PCI translation was “unsafe”.
An expansion of Cohen’s kappa was used to identify the most concordant pairing of 4 bilingual panel members and 4 monolingual panel members [14]. All pairwise comparisons of monolingual and bilingual panel members were coded as follows: +1 was scored when 2 raters were concordant (both scored safe or unsafe) and –1 was scored for discordant pairs. For the 225 possible pairings of 4 panel members (15 combinations of 4 of 6 bilingual, 15 combinations of 4 of 6 monolingual raters), the 100 PCI items scores ranged from +16 (absolute agreement of the 2 panels of 4) to –16 (absolute discordance). For each pairing, we summed the scores for the 100 PCIs to determine the most concordant 4 monolingual and 4 bilingual raters (highest summed scores), which were then used for all subsequent analyses of safety and quality.
Original English characteristics of unsafe translation.
A logistic regression was performed with safety as the dependent variable (safe/unsafe defined by bilingual raters) with explanatory variables of word count, grade level, and reference to medication in OE.
Quality Assessment
Bilingual and monolingual raters assessments of translation quality. We assessed the correlation between the bilingual quality ratings of machine Spanish vs. MBTE and conducted paired t tests comparing mean bilingual machine Spanish and MBTE ratings. High correlation and absence of a significant difference in means would support the notion that MBTE could be used to reliably assess machine Spanish quality.
We also assessed the correlation between bilingual quality assessments of MS vs. monolingual raters’ assessments of MBTE, and conducted paired comparison t tests comparing bilingual machine Spanish and monolingual MBTE quality ratings. These analyses assess the ability of an English-only reader of MBTE to predict the quality of machine Spanish, as determined by a bilingual rater. High correlation and absence of a significant difference in means would support the notion that MBTE could be used by an English-only speaker to reliably assess machine Spanish quality.
Associations between original English content and translation quality. Objective measures of original English were correlated via stepwise linear regression with bilingual assessment of machine Spanish quality.
Results
PCI Contributions
Example PCIs were contributed by 88 individuals and are summarized in Table 1. The 100 original English PCIs and the machine Spanish and MBTE translations obtained via Google Translate are available from the authors upon request.
Safety
Concordance Analysis
The 6 monolingual and bilingual raters agreed on the safety of 73 MBTE PCIs. The most concordant pairings of 4 agreed on 81 items. The least and most concordant pairings had concordance values of 0.68 and 0.84, respectively. Subsequent analyses include data from only the 4 most concordant monolingual and bilingual raters.
Bilingual and Monolingual Safety Ratings
Both bilingual and monolingual raters assessed MBTE. On average, bilingual ratings of MBTE of safety were higher (0.987) than monolingual ratings (0.925) (t = –3.897, P = 0.0002).
Identification of Unsafe Translations in Machine Spanish and MBTE
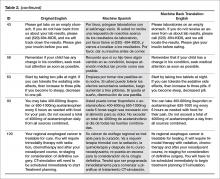
The bilingual panel identified 11 translations (either machine Spanish or MBTE) as unsafe: MS translation was unsafe for 9 items, MBTE unsafe for 5 items, with some items identified as unsafe in terms of both machine Spanish and MBTE. The original English, machine Spanish, and MBTE for these PCIs are listed in Table 2. One item (#93) revealed a machine Spanish drug dosing ambiguity that was not present in the MBTE, with safety concern expressed by 3 of 4 bilingual raters.
Original English c haracteristics of Unsafe Translation
A stepwise logistic regression was performed to evaluate whether characteristics of the original English text predicted the PCI being judged as having a safe or unsafe machine Spanish translation. The explanatory variables (listed in Table 1) evaluated were word count, reading grade level, inclusion of reference to a specific medication, inclusion of numbers (as in "take 2 tablets"), and inclusion of numbered statements (as in "1. Call if your cough worsens"). The stepwise selection procedure dropped number references and numbered sentences, although post hoc analysis showed that number references and medication references occurred so commonly together that they were essentially interchangeable. The final regression model included word count, reading grade level, and medication reference. The significant factors of reading grade level and medication reference had odds ratio (95% confidence interval) of 1.12 (1.01 to 1.41) and 4.91 (1.07 to 22.7) respectively (P = 0.042 each). As reading grade level includes word count per sentence and syllable count per word as linear predictors, the inclusion of word count in the model is likely to increase the discrimination of complex words of many syllables in predicting the occurrence of unsafe machine Spanish.
Quality
Bilingual and Monolingual Raters Assessments of Quality
The bilingual evaluators found similar mean quality for machine Spanish (mean 0.855, SD 0.0859) and MBTE (0.857, SD 0.0755) (P = 0.811). However, the correlation of R2=0.355 (P = 0.000) suggests that despite similarity in mean ratings, a good forward translation from original English to machine Spanish did not assure a good back translation from machine Spanish to MBTE. No difference in mean MBTE quality was identified between bilingual (0.857, SD 0.0754) and monolingual (0.852, SD 0.126) raters (P = 0.598), with correlation R2=0.565 (P = 0.000).
Discussion
In this article, we have collected a corpus of example PCIs across a large number of authors, and investigated how well Google Translate was able to translate the example instructions first to Spanish, and then back again to English. We learned that one can not always spot a problem in the intermediate Spanish by inspection of the back-translated English. We also learned that simple sentences were least likely to be associated with troublesome translations, and that specific instructions about medication usage should probably be approached with great care.
We learned that some authors readily use simple language (eg: “Have your blood work drawn in the lab in the next two weeks,” reading level 1.2) while others gravitate to very complex language (“If you develop headache, chest pain, abdominal pain or back pain, or if you have any spontaneous bleeding please go to the emergency department, advise them that you were recently treated for rattlesnake envenomation and have them call the poison center,” reading level 20.2).
The development in confidence in machine translation can be compared to development of self-driving cars. At early stages of development, the self-driving cars had drivers with a foot near the brake and hands near the steering wheel, ready to take over at any instant. Now, after much data has been collected, there is evidence that the machine may operate more predictably and safely than some human drivers [15,16]. Should the self-driving cars always have an operator behind the wheel, supervising the function of the software, and ready to take over at any instant, or is the purpose of the self-driving car to allow non-drivers to be transported in an automobile that they either cannot operate or choose not to operate at that time?
The benefit of using professional interpreters in communicating clinically significant data is unquestioned, especially when compared to ad-hoc interpreters who lack professional understanding of context [4]. Like a good human driver (as compared to a self-driving car that is operated by a program that is still learning), a qualified human translator will outperform machine translation in complex tasks. Similarly, for relatively simple translations that are meant to be generated by human speakers to be understood by individuals with a grammar school education and vocabulary, is the state of machine translation such that less human translation is now required?
Our use of 2 teams of evaluators allowed us to use the game of Telephone analogy to provide insight into how well the machine translation proceeded, first to Spanish, then back to English. Mostly (90 times in 100), an acceptable Spanish translation resulted in an acceptable English back translation. In 2 instances (Samples 7 and 32), the first translation into Spanish was unacceptable, and a subsequent translation back to English was also unacceptable, as might be expected. In 2 instances (Samples 60 and 92), the Spanish translation was acceptable, but the translation back to English was unacceptable. The rules of Telephone worked 94 times in 100.
Still, 6 times in 100, the unexpected occurred, where a relatively poor Spanish translation returned a relatively acceptable English back translation. The rules of Telephone were not followed. The Spanish in the middle was garbled, but became acceptable when translated back to English. A fluent Spanish speaker found the intermediate Spanish to be of concern, and the back translation did not identify the concern. This argues against widespread adoption of machine back translation for quality assessment, at least until better understanding of the limitations of machine back translation are better understood. Looking at examples where back translation “worked” is useful. In the 6 instances where the intermediate Spanish was judged to be unacceptable, but the English back translation acceptable, complex sentence structures were found, along with medication instructions.
Not tested was if the raters found the original English instructions to be unclear or unsafe as a starting point. Here is where we find the potential benefit of the present study, as it provides insight into the type of content that seems to translate well in this set of data. where the machine Spanish error was not present in MBTE. Overall, ratings of translation quality by bilingual and monolingual raters was high, suggesting that there may be some utility in the machine translation with safeguards other than, or in addition to, inspection of machine back translation of machine Spanish. We found there was an astonishing range in reading difficulty across the contributed samples. While the average estimated grade level for comprehension of the original English contributions was the 8th grade, the maximum was 22, indicating extreme complexity of both words used and sentence length.
In gathering the example PCIs, we did not give any additional instructions to the authors to limit complexity, we only asked for their “typical” language, and if the examples received are indeed typical, the instructions we provide are often quite complex. Wu [17] explored the readability of medical information intended for the public and found that on average, 18 years of education would be required to read and understand the clinical trial descriptions available at ClinicalTrials.gov. It seems apparent that the first step to improving the safety of machine translation is to simplify the task of the translator, by making the language that is used for translation as unambiguous and straightforward as possible. The article by Patil and Davies on the use of Google Translate in the clinic [9] generated a considerable number of rapid responses (similar to letters to the editor) [18]. The responses emphasized the need to keep the language used simple, the sentences short, and the communication direct.
A simple and straightforward suggestion to improve all patient care instructions (not just those anticipated to be translated) would be to display the Flesch-Kincaid reading level in real time as the content is generated. The computer resources required to perform reading level analysis are nearly identical to those required for real-time spell checking: a dictionary that breaks words into syllables. Showing authors the reading level in real time would provide a tool to improve all instructions, not just those intended for translation. Limiting the dictionary to specifically exclude potentially dangerous, complex, or confusing words as well as forbidden abbreviations would further identify troublesome language to the author, and would improve communication overall. Implementing such real-time feedback to authors of patient instructions is a logical next step in adding utility to the electronic health record.
It is important that culture and contextual understanding is taken into consideration while organizations use interpretation services. In the United States, federal law requires that language interpreters employed by health care organization receiving federal funds are not only bilingual but also bicultural [16]. We did not find examples of dangerous synonyms being misapplied in translation, but we cannot rule out the possibility that such errors can occur. This is beyond the scope of typical machine translation software.
Our data suggest that use of medication names and dosing frequencies should not be repeated in the PCI where confusion can arise from imprecise language translation. Translation ambiguities that generate safety concerns in PCI might be mitigated by moving such content into structured areas of the AVS.
Conclusion
This study suggests that 9 times out of 10, the quality of machine translation using Google Translate is acceptable in terms of quality and safety. Currently, machine back translation may fail to reveal a relatively poor translation from English to Spanish. This study showed that increasing sentence complexity, as measured by the reading level index, was associated with a significant (P < 0.05) increase in unsafe machine translation. Similarly, including medication instructions in machine translations were associated with increased risk (P < 0.05) of machine translation safety error in this study.
A simple way to improve communication now would be to display the reading level to authors of patient communication content in real time, and limit the dictionary of acceptable words to forbid the use of known ambiguous terms or forbidden abbreviations. This would teach authors to use simple language, and increase the chance that translation (either human or machine) would be effective. This preliminary study suggests that keeping medication dosing instructions in a structured format is advisable, as is keeping sentences simple. As with spoken language [4], starting with clear, simple to understand English instructions provides the best machine translations into Spanish.
The Clinical Machine Translation Study Group: Todd W. Altenbernd, Steven Bedrick, Mark D. Berg, Nerida Berrios, Mark A. Brown, Colleen K. Cagno, Charles B. Cairns, Elizabeth Calhoun, Raymond Carmody, Tara F. Carr, Clara Choo, Melissa L. Cox, Janiel Cragun, Rachel E.M. Cramton, Paola Davis, Archita Desai, Sarah M. Desoky, Sean Elliot, Mindi J. Fain, Albert Fiorello, Hillary Franke, Kimberly Gerhart, Victor Jose Gonzalez, Aaron John Goshinska, Lynn M. Gries, Erin M. Harvey, Karen Herbst, Elizabeth Juneman, Lauren Marie Imbornoni, Anita Koshy, Lisa Laughlin, Christina M. Laukaitis, Kwan Lee, Hong Lei, Joseph M. Miller, Prashanthinie Mohan, Wayne J. Morgan, Jarrod Mosier, Leigh A. Neumayer, Valentine Nfonsam, Vivienne Ng, Terence O'Keeffe, Merri Pendergrass, Jessie M. Pettit, John Leander Po, Claudia Marie Prospero Ponce, Sydney Rice, Marie Anoushka Ricker, Arielle E. Rubin, Robert J. Segal, Aurora A.G. Selpides, Whitney A. Smith, Jordana M. Smith, William Stevenson, Amy N. Sussman, Ole J. Thienhaus, Patrick Tsai, J. Daniel Twelker, Richard Wahl, Jillian Wang, Mingwu Wang, Samuel C. Werner, Mark D. Wheeler, Jason Wild, Sun Kun Yi, Karl Andrew Yousef, Le Yu.
Corresponding author: Joseph M. Miller, MD, MPH, Department of Ophthalmology and Vision Science, University of Arizona, 655 North Alvernon Way, Suite 108, Tucson AZ 85711, [email protected].
Financial disclosures: None.
1. Hummel J, Evans P. Providing clinical summaries to patients after each office visit: a technical guide. Qualis Health 2012. Accessed 14 Mar 2016 at http://hit.qualishealth.org/sites/default/files/hit.qualishealth.org/Providing-Clinical-Summaries-0712.pdf.
2. Neuberger M, Dontje K, Holzman G, et al. Examination of office visit patient preferences for the after-visit summary (AVS). Persp Health Infor Manage 2014;11:1d.
3. Kruse CS, Bolton K, Freriks G. The effect of patient portals on quality outcomes and its implications to meaningful use: a systematic review. J Med Internet Res 2015;17:e44.
4. Schoonover, K. Using a medical interpreter with persons of limited English proficiency. J Clin Outcomes Manage 2016;23:567–75.
5. Shin HB, Bruno R. Language use and English-speaking ability: 2000. Census 2000 Brief. Accessed 9 Nov 2017 at https://census.gov/content/dam/Census/library/publications/2013/acs/acs-22.pdf.
6. Lewis MP, Simons GF, Fennig CD, editors. Ethnologue: languages of the Americas and the Pacific. 19th ed. Dallas: Sil International; 2016.
7. Pavlik V, Brown AE, Nash S, et al. Association of patient recall, satisfaction, and adherence to content of an electronic health record (EHR)-generated after visit summary: a randomized clinical trial. J Am Board Fam Med 2014;27:209–18.
8. Johnson M, Schuster M, Le QV, et al. Google’s multilingual neural machine translation system: enabling zero-shot translation. Accessed 9 Nov 2017 at https://arxiv.org/pdf/1611.04558.pdf.
9. Patil S, Davies P. Use of Google Translate in medical communication: evaluation of accuracy. BMJ 2014;349:g7392.
10. Kaliyadan F, Gopinathan Pillai S. The use of Google language tools as an interpretation aid in cross-cultural doctor-patient interaction: a pilot study. Inform Prim Care 2010;18:141–3.
11. Zhang Y, Zhou S, Zhang Z, et al. Rumor evolution in social networks. Physical Review E 2013;87.
12. Shingenobu T. Evaluation and usability of back translation for intercultural communication. In: N. Aykin, editor. Usability and internationalization. Global and local user interfaces. UI-HCII 2007, Lecture Notes in Computer Science, vol 4560. Springer, Berlin, Heidelberg.
13. Kincaid JP, Fishburne Jr RP, Rogers RL, et al. Derivation of new readability formulas (automated readability index, fog count and Flesch reading ease formula) for Navy enlisted personnel. Naval Technical Training Command Millington TN Research Branch. 1975. Accessed 7 May 2016 at http://www.dtic.mil/dtic/tr/fulltext/u2/a006655.pdf.
14. Kwiecien R, Kopp-Schneider A, Blettner M. Concordance analysis—part 16 of a series on evaluation of scientific publications. Dtsch Arztebl Int 2011;108:515–21.
15. Goodall N. Ethical decision making during automated vehicle crashes. Transportation Research Record: Journal of the Transportation Research Board 2014;2424:58–65.
16. Kalra N, Groves D. The enemy of good: estimating the cost of waiting for nearly perfect automated vehicles. Santa Monica, CA: RAND Corporation, 2017.
17. Wu DT, Hanauer DA., Mei Q, et al. Assessing the readability of ClinicalTrials.gov. J Am Med Inform Assoc 2016;23:269–75.
18. Responses to: Use of Google Translate in medical communication: evaluation of accuracy. BMJ 2014;349:g7392 Accessed 10 Dec 2017 at www.bmj.com/content/349/bmj.g7392/rapid-responses.
19. Nápoles AM, Santoyo-Olsson J, Karliner LS, et al. Inaccurate language interpretation and its clinical significance in the medical encounters of Spanish-speaking Latinos. Med Care 2015;53:940–7.
From the University of Arizona College of Medicine – Tucson, Tucson, AZ.
Abstract
- Objective: To determine predictors of quality and safety of machine translation (Google Translate) of patient care instructions (PCIs), and to determine if machine back translation is useful in quality assessment.
- Methods: 100 sample English PCIs were contributed by 88 clinical faculty. Each example PCI was up to 3 sentences of typical patient instruction that might be included in an after visit summary. Google Translate was used to first translate the English to Spanish, then back to English. A panel of 6 English/Spanish translators assessed the Spanish translations for safety and quality. A panel of 6 English-speaking health care workers assessed the back translation. A 5-point scale was used to assess quality. Safety was assessed as safe or unsafe.
- Results: Google Translate was usually (> 90%) capable of safe and comprehensible translation from English to Spanish. Instructions with incresed complexity, especially regarding medications, were prone to unsafe translation. Back translation was not reliable in detecting unsafe Spanish.
- Conclusion: Google Translate is a continuously evolving resource for clinicians that offers the promise of improved physician-patient communication. Simple declarative sentences are most reliably translated with high quality and safety.
Keywords: translation; machine translation; electronic health record; after-visit summary; patient safety; physician-patient communication.
Acore measure of the meaningful use of electronic health records incentive program is the generation and provision of the after visit summary (AVS), a mechanism for physicians to provide patients with a written summary of the patient encounter [1,2]. Although not a required element for meaningful use, free text patient care instructions (PCIs) provide the physician an opportunity to improve patient engagement either at the time of service or through the patient portal [3] by providing a short written summary of the key points of the office visit based upon the visit’s clinical discussion. For patients who do not speak English, a verbal translation service is required [4], but seldom are specific patient instructions provided in writing in the patient’s preferred language. A mechanism to improve communication might be through translation of the PCI into the patient’s preferred language. Spanish is the most common language, other than English, spoken at home in the United States [5,6]. For this reason, we chose to investigate if it is feasible to use machine translation (Google Translate) to safely and reliably translate a variety of PCIs from English to Spanish, and to assess the types of translation errors and ambiguities that might result in unsafe communication. We further investigate if machine back translation might allow the author of patient care instructions to evaluate the quality of the Spanish machine translation.
There is evidence to suggest that patient communication and satisfaction will improve if portions of the AVS are communicated in Spanish to primarily Spanish-speaking patients. Pavlik et al conducted a randomized controlled trial on the association of patient recall, satisfaction, and adherence to the information communicated in an AVS, in a largely Hispanic (61%) primary care clinic setting [7]. The AVS was provided in English. They noted that Spanish speakers wished to receive information in Spanish, although most had access to translation by a family member. They also noted that a lack of ability to provide an AVS in Spanish was a concern among providers. There was no difference in recall or satisfaction between English and Spanish speakers with respect to medications and allergies, suggesting that not all portions of the AVS might need to be translated.
Machine translation refers to the automated process of translating one language to another. The most recent methods of machine translation, as exemplified by Google Translate (Google Inc., Mountain View, CA), do not use rules of grammar and dictionaries to perform translations but instead use artificial neural networks to learn from “millions of examples” of translation [8]. However, unsupervised machine translation can result in serious errors [9]. Patil gives as an example of a serious error of translation from English (“Your child is fitting”) to Swahili (“Your child is dead”). In British parlance, “fitting” is a term for “having a seizure” and represents an example of a term that is context sensitive. However, others note that there is reason to be optimistic about the state of machine translation for biomedical text [10].
One method of assessing translation quality is through back translation, where one translator takes the author’s work into the desired target language, and then a different translator takes the target language back to the language of the author. Like the children’s game Chinese Whispers (Telephone in the United States) [11], where a “secret message” is whispered from one child to the next and spoken aloud at the end of the line of children, back translation can test to see if a message “gets through.” In this analogy, when information is machine translated from English to Spanish, and then machine translated from Spanish to English (Figure), we can compare the initial message to the final translation to see if the message “gets through.” We further investigate if machine back translation might allow a non-Spanish speaking author of PCIs to evaluate the quality of the Spanish translation.
Our intention was to determine if machine back translation [12] could be used by an English-only author to assess the quality of an intermediate Spanish translation. If poorly worded Spanish translated back into poorly worded English, the author might choose to either refine their original message until an acceptable machine back translation was achieved or to not release the Spanish translation to the patient. We were also concerned that there might be instances where the intermediate Spanish was unacceptable, but when translated back into English by machine translation, relatively acceptable English might result. If this were the case, then back translation would fail to detect a relatively poor intermediate Spanish translation.
Methods
Patient Care Instructions
Original English PCIs
Example original English PCIs were solicited from the clinical faculty and resident staff of the University of Arizona College of Medicine by an email-based survey tool (Qualtrics, Inc, Provo UT). The solicitation stated the following:
We are conducting a study to assess how well Google Translate might perform in translating patient instructions from English to Spanish. Would you please take the time to type three sentences that might comprise a typical “nugget” of patient instruction using language that you would typically include in an After Visit Summary for a patient? An example might be: “Take two Tylenol 325 mg tablets every four hours while awake for the next two days. If you have a sudden increase in pain or fever, or begin vomiting, call our office. Drink plenty of fluids.”
A total of 100 PCIs were collected. The breadth of the clinical practice and writing styles of a College of Medicine faculty are represented: not all were completely clear or were well-formed sentences, but did represent examples provided by busy clinicians of typical language that they would provide in an AVS PCI.
Machine Translation into Spanish
The 100 original English (OE) PCIs were submitted to the Google Translate web interface (https://translate.google.com/) by cutting and pasting and selecting “Spanish,” resulting in machine Spanish. The translations were performed in January 2016. No specific version number is provided by Google on their web page, and the service is described to be constantly evolving (https://translate.google.com/about/intl/en_ALL/contribute.html).
Machine Back Translation into English (MBTE)
Google Translate was then used to translate the machine Spanish back into into English. MBTE represents the content that a monolingual English speaker might use to evaluate the machine Spanish.
Ratings of Translation Quality and Safety
Two panels of 6 raters evaluated machine Spanish and MBTE quality and safety. A bilingual English/Spanish speaking panel simultaneously evaluated the machine Spanish and MBTE compared to OE, with the goal of inferring where in the process an undesirable back translation error occurred. Bilingual raters were experienced bilingual clinicians or certified translators. A monolingual English speaking panel also evaluated the MBTE (compared to OE). They could only infer the quality and safety of the machine Spanish indirectly through inspection of MBTE, and their assessment was free of the potential bias of knowledge of the intermediate Spanish translation.
The raters used Likert scales to rate grammar similarity and content similarity (scale from 1 to 5: 1 = very dissimilar, 5 = identical). For each PCI, grammar and content scores for each rater were summed and then divided by 10 to yield a within-rater quality score ranging from 0 to 1. A panel-level (bilingual or monolingual) quality score was calculated by averaging the quality scores across raters.
Safety of translation was rated as 0 or Safe (“While the translation may be awkward, it is not dangerous” or 1 or Unsafe (“A dangerous translation error is present that might cause harm to the patient if instructions were followed”). If any panel member considered an item to be unsafe, the item as a whole was scored as unsafe.
Data Analysis
Descriptive Summary of PCI Contributions
The 100 PCIs were summarized in terms of volume (word count), complexity (Flesch-Kincaid Grade Level index [13]), and content (medication names, references, formatting) (Table 1). Word count and grade level were calculated using Microsoft Word (Microsoft Corp, Redmond WA).
Safety Analysis
Concordance analysis. A safety translation concern as defined in this study (“might cause harm”) is very subjective. To reduce some of the variation in assessment of safety, we identified 4 members of the bilingual panel whose safety assessments of MBTE were most similar to the most concordant 4 monolingual raters’ assessment of MBTE safety. The goal was to select the bilingual panel of 4 that was most “typical” of the behavior of a “typical” monolingual individual with respect to assessing the safety of an individual MBTE translation. We then used this bilingual panel to identify 2 sets of “unsafe” machine Spanish and MBTE PCI translations: PCIs where ANY of the 4 bilingual raters identified a safety concern in machine Spanish or MBTE, and PCIs where MOST (at least 3) of the 4 bilingual raters agree that PCI translation was “unsafe”.
An expansion of Cohen’s kappa was used to identify the most concordant pairing of 4 bilingual panel members and 4 monolingual panel members [14]. All pairwise comparisons of monolingual and bilingual panel members were coded as follows: +1 was scored when 2 raters were concordant (both scored safe or unsafe) and –1 was scored for discordant pairs. For the 225 possible pairings of 4 panel members (15 combinations of 4 of 6 bilingual, 15 combinations of 4 of 6 monolingual raters), the 100 PCI items scores ranged from +16 (absolute agreement of the 2 panels of 4) to –16 (absolute discordance). For each pairing, we summed the scores for the 100 PCIs to determine the most concordant 4 monolingual and 4 bilingual raters (highest summed scores), which were then used for all subsequent analyses of safety and quality.
Original English characteristics of unsafe translation.
A logistic regression was performed with safety as the dependent variable (safe/unsafe defined by bilingual raters) with explanatory variables of word count, grade level, and reference to medication in OE.
Quality Assessment
Bilingual and monolingual raters assessments of translation quality. We assessed the correlation between the bilingual quality ratings of machine Spanish vs. MBTE and conducted paired t tests comparing mean bilingual machine Spanish and MBTE ratings. High correlation and absence of a significant difference in means would support the notion that MBTE could be used to reliably assess machine Spanish quality.
We also assessed the correlation between bilingual quality assessments of MS vs. monolingual raters’ assessments of MBTE, and conducted paired comparison t tests comparing bilingual machine Spanish and monolingual MBTE quality ratings. These analyses assess the ability of an English-only reader of MBTE to predict the quality of machine Spanish, as determined by a bilingual rater. High correlation and absence of a significant difference in means would support the notion that MBTE could be used by an English-only speaker to reliably assess machine Spanish quality.
Associations between original English content and translation quality. Objective measures of original English were correlated via stepwise linear regression with bilingual assessment of machine Spanish quality.
Results
PCI Contributions
Example PCIs were contributed by 88 individuals and are summarized in Table 1. The 100 original English PCIs and the machine Spanish and MBTE translations obtained via Google Translate are available from the authors upon request.
Safety
Concordance Analysis
The 6 monolingual and bilingual raters agreed on the safety of 73 MBTE PCIs. The most concordant pairings of 4 agreed on 81 items. The least and most concordant pairings had concordance values of 0.68 and 0.84, respectively. Subsequent analyses include data from only the 4 most concordant monolingual and bilingual raters.
Bilingual and Monolingual Safety Ratings
Both bilingual and monolingual raters assessed MBTE. On average, bilingual ratings of MBTE of safety were higher (0.987) than monolingual ratings (0.925) (t = –3.897, P = 0.0002).
Identification of Unsafe Translations in Machine Spanish and MBTE
The bilingual panel identified 11 translations (either machine Spanish or MBTE) as unsafe: MS translation was unsafe for 9 items, MBTE unsafe for 5 items, with some items identified as unsafe in terms of both machine Spanish and MBTE. The original English, machine Spanish, and MBTE for these PCIs are listed in Table 2. One item (#93) revealed a machine Spanish drug dosing ambiguity that was not present in the MBTE, with safety concern expressed by 3 of 4 bilingual raters.
Original English c haracteristics of Unsafe Translation
A stepwise logistic regression was performed to evaluate whether characteristics of the original English text predicted the PCI being judged as having a safe or unsafe machine Spanish translation. The explanatory variables (listed in Table 1) evaluated were word count, reading grade level, inclusion of reference to a specific medication, inclusion of numbers (as in "take 2 tablets"), and inclusion of numbered statements (as in "1. Call if your cough worsens"). The stepwise selection procedure dropped number references and numbered sentences, although post hoc analysis showed that number references and medication references occurred so commonly together that they were essentially interchangeable. The final regression model included word count, reading grade level, and medication reference. The significant factors of reading grade level and medication reference had odds ratio (95% confidence interval) of 1.12 (1.01 to 1.41) and 4.91 (1.07 to 22.7) respectively (P = 0.042 each). As reading grade level includes word count per sentence and syllable count per word as linear predictors, the inclusion of word count in the model is likely to increase the discrimination of complex words of many syllables in predicting the occurrence of unsafe machine Spanish.
Quality
Bilingual and Monolingual Raters Assessments of Quality
The bilingual evaluators found similar mean quality for machine Spanish (mean 0.855, SD 0.0859) and MBTE (0.857, SD 0.0755) (P = 0.811). However, the correlation of R2=0.355 (P = 0.000) suggests that despite similarity in mean ratings, a good forward translation from original English to machine Spanish did not assure a good back translation from machine Spanish to MBTE. No difference in mean MBTE quality was identified between bilingual (0.857, SD 0.0754) and monolingual (0.852, SD 0.126) raters (P = 0.598), with correlation R2=0.565 (P = 0.000).
Discussion
In this article, we have collected a corpus of example PCIs across a large number of authors, and investigated how well Google Translate was able to translate the example instructions first to Spanish, and then back again to English. We learned that one can not always spot a problem in the intermediate Spanish by inspection of the back-translated English. We also learned that simple sentences were least likely to be associated with troublesome translations, and that specific instructions about medication usage should probably be approached with great care.
We learned that some authors readily use simple language (eg: “Have your blood work drawn in the lab in the next two weeks,” reading level 1.2) while others gravitate to very complex language (“If you develop headache, chest pain, abdominal pain or back pain, or if you have any spontaneous bleeding please go to the emergency department, advise them that you were recently treated for rattlesnake envenomation and have them call the poison center,” reading level 20.2).
The development in confidence in machine translation can be compared to development of self-driving cars. At early stages of development, the self-driving cars had drivers with a foot near the brake and hands near the steering wheel, ready to take over at any instant. Now, after much data has been collected, there is evidence that the machine may operate more predictably and safely than some human drivers [15,16]. Should the self-driving cars always have an operator behind the wheel, supervising the function of the software, and ready to take over at any instant, or is the purpose of the self-driving car to allow non-drivers to be transported in an automobile that they either cannot operate or choose not to operate at that time?
The benefit of using professional interpreters in communicating clinically significant data is unquestioned, especially when compared to ad-hoc interpreters who lack professional understanding of context [4]. Like a good human driver (as compared to a self-driving car that is operated by a program that is still learning), a qualified human translator will outperform machine translation in complex tasks. Similarly, for relatively simple translations that are meant to be generated by human speakers to be understood by individuals with a grammar school education and vocabulary, is the state of machine translation such that less human translation is now required?
Our use of 2 teams of evaluators allowed us to use the game of Telephone analogy to provide insight into how well the machine translation proceeded, first to Spanish, then back to English. Mostly (90 times in 100), an acceptable Spanish translation resulted in an acceptable English back translation. In 2 instances (Samples 7 and 32), the first translation into Spanish was unacceptable, and a subsequent translation back to English was also unacceptable, as might be expected. In 2 instances (Samples 60 and 92), the Spanish translation was acceptable, but the translation back to English was unacceptable. The rules of Telephone worked 94 times in 100.
Still, 6 times in 100, the unexpected occurred, where a relatively poor Spanish translation returned a relatively acceptable English back translation. The rules of Telephone were not followed. The Spanish in the middle was garbled, but became acceptable when translated back to English. A fluent Spanish speaker found the intermediate Spanish to be of concern, and the back translation did not identify the concern. This argues against widespread adoption of machine back translation for quality assessment, at least until better understanding of the limitations of machine back translation are better understood. Looking at examples where back translation “worked” is useful. In the 6 instances where the intermediate Spanish was judged to be unacceptable, but the English back translation acceptable, complex sentence structures were found, along with medication instructions.
Not tested was if the raters found the original English instructions to be unclear or unsafe as a starting point. Here is where we find the potential benefit of the present study, as it provides insight into the type of content that seems to translate well in this set of data. where the machine Spanish error was not present in MBTE. Overall, ratings of translation quality by bilingual and monolingual raters was high, suggesting that there may be some utility in the machine translation with safeguards other than, or in addition to, inspection of machine back translation of machine Spanish. We found there was an astonishing range in reading difficulty across the contributed samples. While the average estimated grade level for comprehension of the original English contributions was the 8th grade, the maximum was 22, indicating extreme complexity of both words used and sentence length.
In gathering the example PCIs, we did not give any additional instructions to the authors to limit complexity, we only asked for their “typical” language, and if the examples received are indeed typical, the instructions we provide are often quite complex. Wu [17] explored the readability of medical information intended for the public and found that on average, 18 years of education would be required to read and understand the clinical trial descriptions available at ClinicalTrials.gov. It seems apparent that the first step to improving the safety of machine translation is to simplify the task of the translator, by making the language that is used for translation as unambiguous and straightforward as possible. The article by Patil and Davies on the use of Google Translate in the clinic [9] generated a considerable number of rapid responses (similar to letters to the editor) [18]. The responses emphasized the need to keep the language used simple, the sentences short, and the communication direct.
A simple and straightforward suggestion to improve all patient care instructions (not just those anticipated to be translated) would be to display the Flesch-Kincaid reading level in real time as the content is generated. The computer resources required to perform reading level analysis are nearly identical to those required for real-time spell checking: a dictionary that breaks words into syllables. Showing authors the reading level in real time would provide a tool to improve all instructions, not just those intended for translation. Limiting the dictionary to specifically exclude potentially dangerous, complex, or confusing words as well as forbidden abbreviations would further identify troublesome language to the author, and would improve communication overall. Implementing such real-time feedback to authors of patient instructions is a logical next step in adding utility to the electronic health record.
It is important that culture and contextual understanding is taken into consideration while organizations use interpretation services. In the United States, federal law requires that language interpreters employed by health care organization receiving federal funds are not only bilingual but also bicultural [16]. We did not find examples of dangerous synonyms being misapplied in translation, but we cannot rule out the possibility that such errors can occur. This is beyond the scope of typical machine translation software.
Our data suggest that use of medication names and dosing frequencies should not be repeated in the PCI where confusion can arise from imprecise language translation. Translation ambiguities that generate safety concerns in PCI might be mitigated by moving such content into structured areas of the AVS.
Conclusion
This study suggests that 9 times out of 10, the quality of machine translation using Google Translate is acceptable in terms of quality and safety. Currently, machine back translation may fail to reveal a relatively poor translation from English to Spanish. This study showed that increasing sentence complexity, as measured by the reading level index, was associated with a significant (P < 0.05) increase in unsafe machine translation. Similarly, including medication instructions in machine translations were associated with increased risk (P < 0.05) of machine translation safety error in this study.
A simple way to improve communication now would be to display the reading level to authors of patient communication content in real time, and limit the dictionary of acceptable words to forbid the use of known ambiguous terms or forbidden abbreviations. This would teach authors to use simple language, and increase the chance that translation (either human or machine) would be effective. This preliminary study suggests that keeping medication dosing instructions in a structured format is advisable, as is keeping sentences simple. As with spoken language [4], starting with clear, simple to understand English instructions provides the best machine translations into Spanish.
The Clinical Machine Translation Study Group: Todd W. Altenbernd, Steven Bedrick, Mark D. Berg, Nerida Berrios, Mark A. Brown, Colleen K. Cagno, Charles B. Cairns, Elizabeth Calhoun, Raymond Carmody, Tara F. Carr, Clara Choo, Melissa L. Cox, Janiel Cragun, Rachel E.M. Cramton, Paola Davis, Archita Desai, Sarah M. Desoky, Sean Elliot, Mindi J. Fain, Albert Fiorello, Hillary Franke, Kimberly Gerhart, Victor Jose Gonzalez, Aaron John Goshinska, Lynn M. Gries, Erin M. Harvey, Karen Herbst, Elizabeth Juneman, Lauren Marie Imbornoni, Anita Koshy, Lisa Laughlin, Christina M. Laukaitis, Kwan Lee, Hong Lei, Joseph M. Miller, Prashanthinie Mohan, Wayne J. Morgan, Jarrod Mosier, Leigh A. Neumayer, Valentine Nfonsam, Vivienne Ng, Terence O'Keeffe, Merri Pendergrass, Jessie M. Pettit, John Leander Po, Claudia Marie Prospero Ponce, Sydney Rice, Marie Anoushka Ricker, Arielle E. Rubin, Robert J. Segal, Aurora A.G. Selpides, Whitney A. Smith, Jordana M. Smith, William Stevenson, Amy N. Sussman, Ole J. Thienhaus, Patrick Tsai, J. Daniel Twelker, Richard Wahl, Jillian Wang, Mingwu Wang, Samuel C. Werner, Mark D. Wheeler, Jason Wild, Sun Kun Yi, Karl Andrew Yousef, Le Yu.
Corresponding author: Joseph M. Miller, MD, MPH, Department of Ophthalmology and Vision Science, University of Arizona, 655 North Alvernon Way, Suite 108, Tucson AZ 85711, [email protected].
Financial disclosures: None.
From the University of Arizona College of Medicine – Tucson, Tucson, AZ.
Abstract
- Objective: To determine predictors of quality and safety of machine translation (Google Translate) of patient care instructions (PCIs), and to determine if machine back translation is useful in quality assessment.
- Methods: 100 sample English PCIs were contributed by 88 clinical faculty. Each example PCI was up to 3 sentences of typical patient instruction that might be included in an after visit summary. Google Translate was used to first translate the English to Spanish, then back to English. A panel of 6 English/Spanish translators assessed the Spanish translations for safety and quality. A panel of 6 English-speaking health care workers assessed the back translation. A 5-point scale was used to assess quality. Safety was assessed as safe or unsafe.
- Results: Google Translate was usually (> 90%) capable of safe and comprehensible translation from English to Spanish. Instructions with incresed complexity, especially regarding medications, were prone to unsafe translation. Back translation was not reliable in detecting unsafe Spanish.
- Conclusion: Google Translate is a continuously evolving resource for clinicians that offers the promise of improved physician-patient communication. Simple declarative sentences are most reliably translated with high quality and safety.
Keywords: translation; machine translation; electronic health record; after-visit summary; patient safety; physician-patient communication.
Acore measure of the meaningful use of electronic health records incentive program is the generation and provision of the after visit summary (AVS), a mechanism for physicians to provide patients with a written summary of the patient encounter [1,2]. Although not a required element for meaningful use, free text patient care instructions (PCIs) provide the physician an opportunity to improve patient engagement either at the time of service or through the patient portal [3] by providing a short written summary of the key points of the office visit based upon the visit’s clinical discussion. For patients who do not speak English, a verbal translation service is required [4], but seldom are specific patient instructions provided in writing in the patient’s preferred language. A mechanism to improve communication might be through translation of the PCI into the patient’s preferred language. Spanish is the most common language, other than English, spoken at home in the United States [5,6]. For this reason, we chose to investigate if it is feasible to use machine translation (Google Translate) to safely and reliably translate a variety of PCIs from English to Spanish, and to assess the types of translation errors and ambiguities that might result in unsafe communication. We further investigate if machine back translation might allow the author of patient care instructions to evaluate the quality of the Spanish machine translation.
There is evidence to suggest that patient communication and satisfaction will improve if portions of the AVS are communicated in Spanish to primarily Spanish-speaking patients. Pavlik et al conducted a randomized controlled trial on the association of patient recall, satisfaction, and adherence to the information communicated in an AVS, in a largely Hispanic (61%) primary care clinic setting [7]. The AVS was provided in English. They noted that Spanish speakers wished to receive information in Spanish, although most had access to translation by a family member. They also noted that a lack of ability to provide an AVS in Spanish was a concern among providers. There was no difference in recall or satisfaction between English and Spanish speakers with respect to medications and allergies, suggesting that not all portions of the AVS might need to be translated.
Machine translation refers to the automated process of translating one language to another. The most recent methods of machine translation, as exemplified by Google Translate (Google Inc., Mountain View, CA), do not use rules of grammar and dictionaries to perform translations but instead use artificial neural networks to learn from “millions of examples” of translation [8]. However, unsupervised machine translation can result in serious errors [9]. Patil gives as an example of a serious error of translation from English (“Your child is fitting”) to Swahili (“Your child is dead”). In British parlance, “fitting” is a term for “having a seizure” and represents an example of a term that is context sensitive. However, others note that there is reason to be optimistic about the state of machine translation for biomedical text [10].
One method of assessing translation quality is through back translation, where one translator takes the author’s work into the desired target language, and then a different translator takes the target language back to the language of the author. Like the children’s game Chinese Whispers (Telephone in the United States) [11], where a “secret message” is whispered from one child to the next and spoken aloud at the end of the line of children, back translation can test to see if a message “gets through.” In this analogy, when information is machine translated from English to Spanish, and then machine translated from Spanish to English (Figure), we can compare the initial message to the final translation to see if the message “gets through.” We further investigate if machine back translation might allow a non-Spanish speaking author of PCIs to evaluate the quality of the Spanish translation.
Our intention was to determine if machine back translation [12] could be used by an English-only author to assess the quality of an intermediate Spanish translation. If poorly worded Spanish translated back into poorly worded English, the author might choose to either refine their original message until an acceptable machine back translation was achieved or to not release the Spanish translation to the patient. We were also concerned that there might be instances where the intermediate Spanish was unacceptable, but when translated back into English by machine translation, relatively acceptable English might result. If this were the case, then back translation would fail to detect a relatively poor intermediate Spanish translation.
Methods
Patient Care Instructions
Original English PCIs
Example original English PCIs were solicited from the clinical faculty and resident staff of the University of Arizona College of Medicine by an email-based survey tool (Qualtrics, Inc, Provo UT). The solicitation stated the following:
We are conducting a study to assess how well Google Translate might perform in translating patient instructions from English to Spanish. Would you please take the time to type three sentences that might comprise a typical “nugget” of patient instruction using language that you would typically include in an After Visit Summary for a patient? An example might be: “Take two Tylenol 325 mg tablets every four hours while awake for the next two days. If you have a sudden increase in pain or fever, or begin vomiting, call our office. Drink plenty of fluids.”
A total of 100 PCIs were collected. The breadth of the clinical practice and writing styles of a College of Medicine faculty are represented: not all were completely clear or were well-formed sentences, but did represent examples provided by busy clinicians of typical language that they would provide in an AVS PCI.
Machine Translation into Spanish
The 100 original English (OE) PCIs were submitted to the Google Translate web interface (https://translate.google.com/) by cutting and pasting and selecting “Spanish,” resulting in machine Spanish. The translations were performed in January 2016. No specific version number is provided by Google on their web page, and the service is described to be constantly evolving (https://translate.google.com/about/intl/en_ALL/contribute.html).
Machine Back Translation into English (MBTE)
Google Translate was then used to translate the machine Spanish back into into English. MBTE represents the content that a monolingual English speaker might use to evaluate the machine Spanish.
Ratings of Translation Quality and Safety
Two panels of 6 raters evaluated machine Spanish and MBTE quality and safety. A bilingual English/Spanish speaking panel simultaneously evaluated the machine Spanish and MBTE compared to OE, with the goal of inferring where in the process an undesirable back translation error occurred. Bilingual raters were experienced bilingual clinicians or certified translators. A monolingual English speaking panel also evaluated the MBTE (compared to OE). They could only infer the quality and safety of the machine Spanish indirectly through inspection of MBTE, and their assessment was free of the potential bias of knowledge of the intermediate Spanish translation.
The raters used Likert scales to rate grammar similarity and content similarity (scale from 1 to 5: 1 = very dissimilar, 5 = identical). For each PCI, grammar and content scores for each rater were summed and then divided by 10 to yield a within-rater quality score ranging from 0 to 1. A panel-level (bilingual or monolingual) quality score was calculated by averaging the quality scores across raters.
Safety of translation was rated as 0 or Safe (“While the translation may be awkward, it is not dangerous” or 1 or Unsafe (“A dangerous translation error is present that might cause harm to the patient if instructions were followed”). If any panel member considered an item to be unsafe, the item as a whole was scored as unsafe.
Data Analysis
Descriptive Summary of PCI Contributions
The 100 PCIs were summarized in terms of volume (word count), complexity (Flesch-Kincaid Grade Level index [13]), and content (medication names, references, formatting) (Table 1). Word count and grade level were calculated using Microsoft Word (Microsoft Corp, Redmond WA).
Safety Analysis
Concordance analysis. A safety translation concern as defined in this study (“might cause harm”) is very subjective. To reduce some of the variation in assessment of safety, we identified 4 members of the bilingual panel whose safety assessments of MBTE were most similar to the most concordant 4 monolingual raters’ assessment of MBTE safety. The goal was to select the bilingual panel of 4 that was most “typical” of the behavior of a “typical” monolingual individual with respect to assessing the safety of an individual MBTE translation. We then used this bilingual panel to identify 2 sets of “unsafe” machine Spanish and MBTE PCI translations: PCIs where ANY of the 4 bilingual raters identified a safety concern in machine Spanish or MBTE, and PCIs where MOST (at least 3) of the 4 bilingual raters agree that PCI translation was “unsafe”.
An expansion of Cohen’s kappa was used to identify the most concordant pairing of 4 bilingual panel members and 4 monolingual panel members [14]. All pairwise comparisons of monolingual and bilingual panel members were coded as follows: +1 was scored when 2 raters were concordant (both scored safe or unsafe) and –1 was scored for discordant pairs. For the 225 possible pairings of 4 panel members (15 combinations of 4 of 6 bilingual, 15 combinations of 4 of 6 monolingual raters), the 100 PCI items scores ranged from +16 (absolute agreement of the 2 panels of 4) to –16 (absolute discordance). For each pairing, we summed the scores for the 100 PCIs to determine the most concordant 4 monolingual and 4 bilingual raters (highest summed scores), which were then used for all subsequent analyses of safety and quality.
Original English characteristics of unsafe translation.
A logistic regression was performed with safety as the dependent variable (safe/unsafe defined by bilingual raters) with explanatory variables of word count, grade level, and reference to medication in OE.
Quality Assessment
Bilingual and monolingual raters assessments of translation quality. We assessed the correlation between the bilingual quality ratings of machine Spanish vs. MBTE and conducted paired t tests comparing mean bilingual machine Spanish and MBTE ratings. High correlation and absence of a significant difference in means would support the notion that MBTE could be used to reliably assess machine Spanish quality.
We also assessed the correlation between bilingual quality assessments of MS vs. monolingual raters’ assessments of MBTE, and conducted paired comparison t tests comparing bilingual machine Spanish and monolingual MBTE quality ratings. These analyses assess the ability of an English-only reader of MBTE to predict the quality of machine Spanish, as determined by a bilingual rater. High correlation and absence of a significant difference in means would support the notion that MBTE could be used by an English-only speaker to reliably assess machine Spanish quality.
Associations between original English content and translation quality. Objective measures of original English were correlated via stepwise linear regression with bilingual assessment of machine Spanish quality.
Results
PCI Contributions
Example PCIs were contributed by 88 individuals and are summarized in Table 1. The 100 original English PCIs and the machine Spanish and MBTE translations obtained via Google Translate are available from the authors upon request.
Safety
Concordance Analysis
The 6 monolingual and bilingual raters agreed on the safety of 73 MBTE PCIs. The most concordant pairings of 4 agreed on 81 items. The least and most concordant pairings had concordance values of 0.68 and 0.84, respectively. Subsequent analyses include data from only the 4 most concordant monolingual and bilingual raters.
Bilingual and Monolingual Safety Ratings
Both bilingual and monolingual raters assessed MBTE. On average, bilingual ratings of MBTE of safety were higher (0.987) than monolingual ratings (0.925) (t = –3.897, P = 0.0002).
Identification of Unsafe Translations in Machine Spanish and MBTE
The bilingual panel identified 11 translations (either machine Spanish or MBTE) as unsafe: MS translation was unsafe for 9 items, MBTE unsafe for 5 items, with some items identified as unsafe in terms of both machine Spanish and MBTE. The original English, machine Spanish, and MBTE for these PCIs are listed in Table 2. One item (#93) revealed a machine Spanish drug dosing ambiguity that was not present in the MBTE, with safety concern expressed by 3 of 4 bilingual raters.
Original English c haracteristics of Unsafe Translation
A stepwise logistic regression was performed to evaluate whether characteristics of the original English text predicted the PCI being judged as having a safe or unsafe machine Spanish translation. The explanatory variables (listed in Table 1) evaluated were word count, reading grade level, inclusion of reference to a specific medication, inclusion of numbers (as in "take 2 tablets"), and inclusion of numbered statements (as in "1. Call if your cough worsens"). The stepwise selection procedure dropped number references and numbered sentences, although post hoc analysis showed that number references and medication references occurred so commonly together that they were essentially interchangeable. The final regression model included word count, reading grade level, and medication reference. The significant factors of reading grade level and medication reference had odds ratio (95% confidence interval) of 1.12 (1.01 to 1.41) and 4.91 (1.07 to 22.7) respectively (P = 0.042 each). As reading grade level includes word count per sentence and syllable count per word as linear predictors, the inclusion of word count in the model is likely to increase the discrimination of complex words of many syllables in predicting the occurrence of unsafe machine Spanish.
Quality
Bilingual and Monolingual Raters Assessments of Quality
The bilingual evaluators found similar mean quality for machine Spanish (mean 0.855, SD 0.0859) and MBTE (0.857, SD 0.0755) (P = 0.811). However, the correlation of R2=0.355 (P = 0.000) suggests that despite similarity in mean ratings, a good forward translation from original English to machine Spanish did not assure a good back translation from machine Spanish to MBTE. No difference in mean MBTE quality was identified between bilingual (0.857, SD 0.0754) and monolingual (0.852, SD 0.126) raters (P = 0.598), with correlation R2=0.565 (P = 0.000).
Discussion
In this article, we have collected a corpus of example PCIs across a large number of authors, and investigated how well Google Translate was able to translate the example instructions first to Spanish, and then back again to English. We learned that one can not always spot a problem in the intermediate Spanish by inspection of the back-translated English. We also learned that simple sentences were least likely to be associated with troublesome translations, and that specific instructions about medication usage should probably be approached with great care.
We learned that some authors readily use simple language (eg: “Have your blood work drawn in the lab in the next two weeks,” reading level 1.2) while others gravitate to very complex language (“If you develop headache, chest pain, abdominal pain or back pain, or if you have any spontaneous bleeding please go to the emergency department, advise them that you were recently treated for rattlesnake envenomation and have them call the poison center,” reading level 20.2).
The development in confidence in machine translation can be compared to development of self-driving cars. At early stages of development, the self-driving cars had drivers with a foot near the brake and hands near the steering wheel, ready to take over at any instant. Now, after much data has been collected, there is evidence that the machine may operate more predictably and safely than some human drivers [15,16]. Should the self-driving cars always have an operator behind the wheel, supervising the function of the software, and ready to take over at any instant, or is the purpose of the self-driving car to allow non-drivers to be transported in an automobile that they either cannot operate or choose not to operate at that time?
The benefit of using professional interpreters in communicating clinically significant data is unquestioned, especially when compared to ad-hoc interpreters who lack professional understanding of context [4]. Like a good human driver (as compared to a self-driving car that is operated by a program that is still learning), a qualified human translator will outperform machine translation in complex tasks. Similarly, for relatively simple translations that are meant to be generated by human speakers to be understood by individuals with a grammar school education and vocabulary, is the state of machine translation such that less human translation is now required?
Our use of 2 teams of evaluators allowed us to use the game of Telephone analogy to provide insight into how well the machine translation proceeded, first to Spanish, then back to English. Mostly (90 times in 100), an acceptable Spanish translation resulted in an acceptable English back translation. In 2 instances (Samples 7 and 32), the first translation into Spanish was unacceptable, and a subsequent translation back to English was also unacceptable, as might be expected. In 2 instances (Samples 60 and 92), the Spanish translation was acceptable, but the translation back to English was unacceptable. The rules of Telephone worked 94 times in 100.
Still, 6 times in 100, the unexpected occurred, where a relatively poor Spanish translation returned a relatively acceptable English back translation. The rules of Telephone were not followed. The Spanish in the middle was garbled, but became acceptable when translated back to English. A fluent Spanish speaker found the intermediate Spanish to be of concern, and the back translation did not identify the concern. This argues against widespread adoption of machine back translation for quality assessment, at least until better understanding of the limitations of machine back translation are better understood. Looking at examples where back translation “worked” is useful. In the 6 instances where the intermediate Spanish was judged to be unacceptable, but the English back translation acceptable, complex sentence structures were found, along with medication instructions.
Not tested was if the raters found the original English instructions to be unclear or unsafe as a starting point. Here is where we find the potential benefit of the present study, as it provides insight into the type of content that seems to translate well in this set of data. where the machine Spanish error was not present in MBTE. Overall, ratings of translation quality by bilingual and monolingual raters was high, suggesting that there may be some utility in the machine translation with safeguards other than, or in addition to, inspection of machine back translation of machine Spanish. We found there was an astonishing range in reading difficulty across the contributed samples. While the average estimated grade level for comprehension of the original English contributions was the 8th grade, the maximum was 22, indicating extreme complexity of both words used and sentence length.
In gathering the example PCIs, we did not give any additional instructions to the authors to limit complexity, we only asked for their “typical” language, and if the examples received are indeed typical, the instructions we provide are often quite complex. Wu [17] explored the readability of medical information intended for the public and found that on average, 18 years of education would be required to read and understand the clinical trial descriptions available at ClinicalTrials.gov. It seems apparent that the first step to improving the safety of machine translation is to simplify the task of the translator, by making the language that is used for translation as unambiguous and straightforward as possible. The article by Patil and Davies on the use of Google Translate in the clinic [9] generated a considerable number of rapid responses (similar to letters to the editor) [18]. The responses emphasized the need to keep the language used simple, the sentences short, and the communication direct.
A simple and straightforward suggestion to improve all patient care instructions (not just those anticipated to be translated) would be to display the Flesch-Kincaid reading level in real time as the content is generated. The computer resources required to perform reading level analysis are nearly identical to those required for real-time spell checking: a dictionary that breaks words into syllables. Showing authors the reading level in real time would provide a tool to improve all instructions, not just those intended for translation. Limiting the dictionary to specifically exclude potentially dangerous, complex, or confusing words as well as forbidden abbreviations would further identify troublesome language to the author, and would improve communication overall. Implementing such real-time feedback to authors of patient instructions is a logical next step in adding utility to the electronic health record.
It is important that culture and contextual understanding is taken into consideration while organizations use interpretation services. In the United States, federal law requires that language interpreters employed by health care organization receiving federal funds are not only bilingual but also bicultural [16]. We did not find examples of dangerous synonyms being misapplied in translation, but we cannot rule out the possibility that such errors can occur. This is beyond the scope of typical machine translation software.
Our data suggest that use of medication names and dosing frequencies should not be repeated in the PCI where confusion can arise from imprecise language translation. Translation ambiguities that generate safety concerns in PCI might be mitigated by moving such content into structured areas of the AVS.
Conclusion
This study suggests that 9 times out of 10, the quality of machine translation using Google Translate is acceptable in terms of quality and safety. Currently, machine back translation may fail to reveal a relatively poor translation from English to Spanish. This study showed that increasing sentence complexity, as measured by the reading level index, was associated with a significant (P < 0.05) increase in unsafe machine translation. Similarly, including medication instructions in machine translations were associated with increased risk (P < 0.05) of machine translation safety error in this study.
A simple way to improve communication now would be to display the reading level to authors of patient communication content in real time, and limit the dictionary of acceptable words to forbid the use of known ambiguous terms or forbidden abbreviations. This would teach authors to use simple language, and increase the chance that translation (either human or machine) would be effective. This preliminary study suggests that keeping medication dosing instructions in a structured format is advisable, as is keeping sentences simple. As with spoken language [4], starting with clear, simple to understand English instructions provides the best machine translations into Spanish.
The Clinical Machine Translation Study Group: Todd W. Altenbernd, Steven Bedrick, Mark D. Berg, Nerida Berrios, Mark A. Brown, Colleen K. Cagno, Charles B. Cairns, Elizabeth Calhoun, Raymond Carmody, Tara F. Carr, Clara Choo, Melissa L. Cox, Janiel Cragun, Rachel E.M. Cramton, Paola Davis, Archita Desai, Sarah M. Desoky, Sean Elliot, Mindi J. Fain, Albert Fiorello, Hillary Franke, Kimberly Gerhart, Victor Jose Gonzalez, Aaron John Goshinska, Lynn M. Gries, Erin M. Harvey, Karen Herbst, Elizabeth Juneman, Lauren Marie Imbornoni, Anita Koshy, Lisa Laughlin, Christina M. Laukaitis, Kwan Lee, Hong Lei, Joseph M. Miller, Prashanthinie Mohan, Wayne J. Morgan, Jarrod Mosier, Leigh A. Neumayer, Valentine Nfonsam, Vivienne Ng, Terence O'Keeffe, Merri Pendergrass, Jessie M. Pettit, John Leander Po, Claudia Marie Prospero Ponce, Sydney Rice, Marie Anoushka Ricker, Arielle E. Rubin, Robert J. Segal, Aurora A.G. Selpides, Whitney A. Smith, Jordana M. Smith, William Stevenson, Amy N. Sussman, Ole J. Thienhaus, Patrick Tsai, J. Daniel Twelker, Richard Wahl, Jillian Wang, Mingwu Wang, Samuel C. Werner, Mark D. Wheeler, Jason Wild, Sun Kun Yi, Karl Andrew Yousef, Le Yu.
Corresponding author: Joseph M. Miller, MD, MPH, Department of Ophthalmology and Vision Science, University of Arizona, 655 North Alvernon Way, Suite 108, Tucson AZ 85711, [email protected].
Financial disclosures: None.
1. Hummel J, Evans P. Providing clinical summaries to patients after each office visit: a technical guide. Qualis Health 2012. Accessed 14 Mar 2016 at http://hit.qualishealth.org/sites/default/files/hit.qualishealth.org/Providing-Clinical-Summaries-0712.pdf.
2. Neuberger M, Dontje K, Holzman G, et al. Examination of office visit patient preferences for the after-visit summary (AVS). Persp Health Infor Manage 2014;11:1d.
3. Kruse CS, Bolton K, Freriks G. The effect of patient portals on quality outcomes and its implications to meaningful use: a systematic review. J Med Internet Res 2015;17:e44.
4. Schoonover, K. Using a medical interpreter with persons of limited English proficiency. J Clin Outcomes Manage 2016;23:567–75.
5. Shin HB, Bruno R. Language use and English-speaking ability: 2000. Census 2000 Brief. Accessed 9 Nov 2017 at https://census.gov/content/dam/Census/library/publications/2013/acs/acs-22.pdf.
6. Lewis MP, Simons GF, Fennig CD, editors. Ethnologue: languages of the Americas and the Pacific. 19th ed. Dallas: Sil International; 2016.
7. Pavlik V, Brown AE, Nash S, et al. Association of patient recall, satisfaction, and adherence to content of an electronic health record (EHR)-generated after visit summary: a randomized clinical trial. J Am Board Fam Med 2014;27:209–18.
8. Johnson M, Schuster M, Le QV, et al. Google’s multilingual neural machine translation system: enabling zero-shot translation. Accessed 9 Nov 2017 at https://arxiv.org/pdf/1611.04558.pdf.
9. Patil S, Davies P. Use of Google Translate in medical communication: evaluation of accuracy. BMJ 2014;349:g7392.
10. Kaliyadan F, Gopinathan Pillai S. The use of Google language tools as an interpretation aid in cross-cultural doctor-patient interaction: a pilot study. Inform Prim Care 2010;18:141–3.
11. Zhang Y, Zhou S, Zhang Z, et al. Rumor evolution in social networks. Physical Review E 2013;87.
12. Shingenobu T. Evaluation and usability of back translation for intercultural communication. In: N. Aykin, editor. Usability and internationalization. Global and local user interfaces. UI-HCII 2007, Lecture Notes in Computer Science, vol 4560. Springer, Berlin, Heidelberg.
13. Kincaid JP, Fishburne Jr RP, Rogers RL, et al. Derivation of new readability formulas (automated readability index, fog count and Flesch reading ease formula) for Navy enlisted personnel. Naval Technical Training Command Millington TN Research Branch. 1975. Accessed 7 May 2016 at http://www.dtic.mil/dtic/tr/fulltext/u2/a006655.pdf.
14. Kwiecien R, Kopp-Schneider A, Blettner M. Concordance analysis—part 16 of a series on evaluation of scientific publications. Dtsch Arztebl Int 2011;108:515–21.
15. Goodall N. Ethical decision making during automated vehicle crashes. Transportation Research Record: Journal of the Transportation Research Board 2014;2424:58–65.
16. Kalra N, Groves D. The enemy of good: estimating the cost of waiting for nearly perfect automated vehicles. Santa Monica, CA: RAND Corporation, 2017.
17. Wu DT, Hanauer DA., Mei Q, et al. Assessing the readability of ClinicalTrials.gov. J Am Med Inform Assoc 2016;23:269–75.
18. Responses to: Use of Google Translate in medical communication: evaluation of accuracy. BMJ 2014;349:g7392 Accessed 10 Dec 2017 at www.bmj.com/content/349/bmj.g7392/rapid-responses.
19. Nápoles AM, Santoyo-Olsson J, Karliner LS, et al. Inaccurate language interpretation and its clinical significance in the medical encounters of Spanish-speaking Latinos. Med Care 2015;53:940–7.
1. Hummel J, Evans P. Providing clinical summaries to patients after each office visit: a technical guide. Qualis Health 2012. Accessed 14 Mar 2016 at http://hit.qualishealth.org/sites/default/files/hit.qualishealth.org/Providing-Clinical-Summaries-0712.pdf.
2. Neuberger M, Dontje K, Holzman G, et al. Examination of office visit patient preferences for the after-visit summary (AVS). Persp Health Infor Manage 2014;11:1d.
3. Kruse CS, Bolton K, Freriks G. The effect of patient portals on quality outcomes and its implications to meaningful use: a systematic review. J Med Internet Res 2015;17:e44.
4. Schoonover, K. Using a medical interpreter with persons of limited English proficiency. J Clin Outcomes Manage 2016;23:567–75.
5. Shin HB, Bruno R. Language use and English-speaking ability: 2000. Census 2000 Brief. Accessed 9 Nov 2017 at https://census.gov/content/dam/Census/library/publications/2013/acs/acs-22.pdf.
6. Lewis MP, Simons GF, Fennig CD, editors. Ethnologue: languages of the Americas and the Pacific. 19th ed. Dallas: Sil International; 2016.
7. Pavlik V, Brown AE, Nash S, et al. Association of patient recall, satisfaction, and adherence to content of an electronic health record (EHR)-generated after visit summary: a randomized clinical trial. J Am Board Fam Med 2014;27:209–18.
8. Johnson M, Schuster M, Le QV, et al. Google’s multilingual neural machine translation system: enabling zero-shot translation. Accessed 9 Nov 2017 at https://arxiv.org/pdf/1611.04558.pdf.
9. Patil S, Davies P. Use of Google Translate in medical communication: evaluation of accuracy. BMJ 2014;349:g7392.
10. Kaliyadan F, Gopinathan Pillai S. The use of Google language tools as an interpretation aid in cross-cultural doctor-patient interaction: a pilot study. Inform Prim Care 2010;18:141–3.
11. Zhang Y, Zhou S, Zhang Z, et al. Rumor evolution in social networks. Physical Review E 2013;87.
12. Shingenobu T. Evaluation and usability of back translation for intercultural communication. In: N. Aykin, editor. Usability and internationalization. Global and local user interfaces. UI-HCII 2007, Lecture Notes in Computer Science, vol 4560. Springer, Berlin, Heidelberg.
13. Kincaid JP, Fishburne Jr RP, Rogers RL, et al. Derivation of new readability formulas (automated readability index, fog count and Flesch reading ease formula) for Navy enlisted personnel. Naval Technical Training Command Millington TN Research Branch. 1975. Accessed 7 May 2016 at http://www.dtic.mil/dtic/tr/fulltext/u2/a006655.pdf.
14. Kwiecien R, Kopp-Schneider A, Blettner M. Concordance analysis—part 16 of a series on evaluation of scientific publications. Dtsch Arztebl Int 2011;108:515–21.
15. Goodall N. Ethical decision making during automated vehicle crashes. Transportation Research Record: Journal of the Transportation Research Board 2014;2424:58–65.
16. Kalra N, Groves D. The enemy of good: estimating the cost of waiting for nearly perfect automated vehicles. Santa Monica, CA: RAND Corporation, 2017.
17. Wu DT, Hanauer DA., Mei Q, et al. Assessing the readability of ClinicalTrials.gov. J Am Med Inform Assoc 2016;23:269–75.
18. Responses to: Use of Google Translate in medical communication: evaluation of accuracy. BMJ 2014;349:g7392 Accessed 10 Dec 2017 at www.bmj.com/content/349/bmj.g7392/rapid-responses.
19. Nápoles AM, Santoyo-Olsson J, Karliner LS, et al. Inaccurate language interpretation and its clinical significance in the medical encounters of Spanish-speaking Latinos. Med Care 2015;53:940–7.
Nondrug Treatments May Benefit Patients With Epilepsy
WASHINGTON, DC—Many patients with pharmacoresistant epilepsy may benefit from nondrug treatments, including vagus nerve stimulation (VNS), the ketogenic diet, and corpus callosotomy, according to a study presented at the 71st Annual Meeting of the American Epilepsy Society. The treatments may reduce generalized and focal seizures, and most parents whose children underwent these procedures would opt for the same treatment under similar circumstances, the researchers said.
About 20% to 30% of patients have pharmacoresistant epilepsy. The ketogenic diet, corpus callosotomy, and VNS have been studied as alternatives to antiepileptic drugs (AEDs) for these patients, but few studies have compared the modalities.
Dave F. Clarke, MD, MBBS, Professor of Pediatric Neurology at the Baylor College of Medicine and Clinical Director of Epilepsy at Texas Children’s Hospital in Houston, and colleagues compared seizure control, cognitive and behavioral factors, quality of life, and parent satisfaction among patients who received VNS, underwent corpus callosotomy, or initiated the ketogenic diet. They identified 336 patients who had received one of these treatments at Dell Children’s Medical Center of Central Texas in Austin between January 2010 and November 2015. Parents of 210 of the patients completed a nine-item telephone survey.
Of the 210 patients whose parents completed the survey, 98 (33.6%) had initiated the ketogenic diet, 150 (51.4%) had received VNS, and 44 (15.1%) had undergone corpus callosotomy. Patients were between the ages of 8 months and 20 years. Patients who had initiated the ketogenic diet had a mean age of about 7, and patients who received VNS or underwent corpus callosotomy had a mean age of about 10. Patients had failed more than three AEDs on average (range, two to 13).
Parents reported a 50% or greater reduction in generalized seizures in 63% of patients who went on the ketogenic diet, 54% of patients who underwent corpus callosotomy, and 52% of patients who received VNS. Parents reported a 50% or greater reduction in focal seizures in 56% of children who went on the ketogenic diet, 56% of patients who had corpus callosotomy, and 53% of patients who received VNS.
In addition, parents reported improved quality of life in 48% of patients on the ketogenic diet, 63% of patients who had corpus callosotomy, and 44% of patients who received VNS. Overall, 80% of parents whose children were on the ketogenic diet or received VNS and 75% of parents whose children underwent corpus callosotomy reported that they were satisfied with the treatment that their child had received.
“Higher health-related quality of life after intervention was predicted by improved behavior, increased engagement, diminished frequency of atonic or generalized tonic-clonic seizures, and reduction in epilepsy-related injuries,” the researchers concluded. Parents were more likely to say that they would repeat the procedure if, after the treatment, “their child was more engaged, had diminished frequency of atonic or generalized tonic-clonic seizures, and had a reduction in epilepsy-related injuries.”
“Unfortunately, many doctors keep trying medications without considering alternatives,” said Dr. Clarke. “Based on the parents’ feedback, I would suggest doctors introduce the concept of alternatives after two AEDs fail to control seizures.” If surgery to ablate or remove the area of the brain where seizures originate is not an option, neurologists should talk to parents about the ketogenic diet, VNS, or corpus callosotomy. “If parents think the diet can be tolerated, trying it first may not be a bad option,” he said.
—Jake Remaly
WASHINGTON, DC—Many patients with pharmacoresistant epilepsy may benefit from nondrug treatments, including vagus nerve stimulation (VNS), the ketogenic diet, and corpus callosotomy, according to a study presented at the 71st Annual Meeting of the American Epilepsy Society. The treatments may reduce generalized and focal seizures, and most parents whose children underwent these procedures would opt for the same treatment under similar circumstances, the researchers said.
About 20% to 30% of patients have pharmacoresistant epilepsy. The ketogenic diet, corpus callosotomy, and VNS have been studied as alternatives to antiepileptic drugs (AEDs) for these patients, but few studies have compared the modalities.
Dave F. Clarke, MD, MBBS, Professor of Pediatric Neurology at the Baylor College of Medicine and Clinical Director of Epilepsy at Texas Children’s Hospital in Houston, and colleagues compared seizure control, cognitive and behavioral factors, quality of life, and parent satisfaction among patients who received VNS, underwent corpus callosotomy, or initiated the ketogenic diet. They identified 336 patients who had received one of these treatments at Dell Children’s Medical Center of Central Texas in Austin between January 2010 and November 2015. Parents of 210 of the patients completed a nine-item telephone survey.
Of the 210 patients whose parents completed the survey, 98 (33.6%) had initiated the ketogenic diet, 150 (51.4%) had received VNS, and 44 (15.1%) had undergone corpus callosotomy. Patients were between the ages of 8 months and 20 years. Patients who had initiated the ketogenic diet had a mean age of about 7, and patients who received VNS or underwent corpus callosotomy had a mean age of about 10. Patients had failed more than three AEDs on average (range, two to 13).
Parents reported a 50% or greater reduction in generalized seizures in 63% of patients who went on the ketogenic diet, 54% of patients who underwent corpus callosotomy, and 52% of patients who received VNS. Parents reported a 50% or greater reduction in focal seizures in 56% of children who went on the ketogenic diet, 56% of patients who had corpus callosotomy, and 53% of patients who received VNS.
In addition, parents reported improved quality of life in 48% of patients on the ketogenic diet, 63% of patients who had corpus callosotomy, and 44% of patients who received VNS. Overall, 80% of parents whose children were on the ketogenic diet or received VNS and 75% of parents whose children underwent corpus callosotomy reported that they were satisfied with the treatment that their child had received.
“Higher health-related quality of life after intervention was predicted by improved behavior, increased engagement, diminished frequency of atonic or generalized tonic-clonic seizures, and reduction in epilepsy-related injuries,” the researchers concluded. Parents were more likely to say that they would repeat the procedure if, after the treatment, “their child was more engaged, had diminished frequency of atonic or generalized tonic-clonic seizures, and had a reduction in epilepsy-related injuries.”
“Unfortunately, many doctors keep trying medications without considering alternatives,” said Dr. Clarke. “Based on the parents’ feedback, I would suggest doctors introduce the concept of alternatives after two AEDs fail to control seizures.” If surgery to ablate or remove the area of the brain where seizures originate is not an option, neurologists should talk to parents about the ketogenic diet, VNS, or corpus callosotomy. “If parents think the diet can be tolerated, trying it first may not be a bad option,” he said.
—Jake Remaly
WASHINGTON, DC—Many patients with pharmacoresistant epilepsy may benefit from nondrug treatments, including vagus nerve stimulation (VNS), the ketogenic diet, and corpus callosotomy, according to a study presented at the 71st Annual Meeting of the American Epilepsy Society. The treatments may reduce generalized and focal seizures, and most parents whose children underwent these procedures would opt for the same treatment under similar circumstances, the researchers said.
About 20% to 30% of patients have pharmacoresistant epilepsy. The ketogenic diet, corpus callosotomy, and VNS have been studied as alternatives to antiepileptic drugs (AEDs) for these patients, but few studies have compared the modalities.
Dave F. Clarke, MD, MBBS, Professor of Pediatric Neurology at the Baylor College of Medicine and Clinical Director of Epilepsy at Texas Children’s Hospital in Houston, and colleagues compared seizure control, cognitive and behavioral factors, quality of life, and parent satisfaction among patients who received VNS, underwent corpus callosotomy, or initiated the ketogenic diet. They identified 336 patients who had received one of these treatments at Dell Children’s Medical Center of Central Texas in Austin between January 2010 and November 2015. Parents of 210 of the patients completed a nine-item telephone survey.
Of the 210 patients whose parents completed the survey, 98 (33.6%) had initiated the ketogenic diet, 150 (51.4%) had received VNS, and 44 (15.1%) had undergone corpus callosotomy. Patients were between the ages of 8 months and 20 years. Patients who had initiated the ketogenic diet had a mean age of about 7, and patients who received VNS or underwent corpus callosotomy had a mean age of about 10. Patients had failed more than three AEDs on average (range, two to 13).
Parents reported a 50% or greater reduction in generalized seizures in 63% of patients who went on the ketogenic diet, 54% of patients who underwent corpus callosotomy, and 52% of patients who received VNS. Parents reported a 50% or greater reduction in focal seizures in 56% of children who went on the ketogenic diet, 56% of patients who had corpus callosotomy, and 53% of patients who received VNS.
In addition, parents reported improved quality of life in 48% of patients on the ketogenic diet, 63% of patients who had corpus callosotomy, and 44% of patients who received VNS. Overall, 80% of parents whose children were on the ketogenic diet or received VNS and 75% of parents whose children underwent corpus callosotomy reported that they were satisfied with the treatment that their child had received.
“Higher health-related quality of life after intervention was predicted by improved behavior, increased engagement, diminished frequency of atonic or generalized tonic-clonic seizures, and reduction in epilepsy-related injuries,” the researchers concluded. Parents were more likely to say that they would repeat the procedure if, after the treatment, “their child was more engaged, had diminished frequency of atonic or generalized tonic-clonic seizures, and had a reduction in epilepsy-related injuries.”
“Unfortunately, many doctors keep trying medications without considering alternatives,” said Dr. Clarke. “Based on the parents’ feedback, I would suggest doctors introduce the concept of alternatives after two AEDs fail to control seizures.” If surgery to ablate or remove the area of the brain where seizures originate is not an option, neurologists should talk to parents about the ketogenic diet, VNS, or corpus callosotomy. “If parents think the diet can be tolerated, trying it first may not be a bad option,” he said.
—Jake Remaly
Homelessness: Whose job is it?
Despite programs to end homelessness, it remains a substantial and growing problem in many cities in the United States.1,2 In 2016, there were an estimated 10,550 homeless people living in my home state of Colorado, a 6% increase from the prior year.2 A recent point-estimate study found that there were more than 5,000 homeless individuals in the Denver metropolitan area on a single night in January 2017.3 Because of the relative scarcity of housing, a growing number of cities like Denver now utilize a practice known as vulnerability indexing to prioritize homeless persons at high risk of mortality from medical conditions for placement in permanent supportive housing.4
Although hospitalists like myself frequently care for vulnerable homeless patients in the hospital, most have little formal training in how best to care for and advocate for these individuals beyond treating their acute medical need, and little direct contact with community organizations with expertise in doing so. Instead, we have learned informally through experience. Hospital providers are often frustrated by the perceived lack of services and support available to these patients, and there is substantial variability in the extent to which providers engage patients and community partners during and after hospitalization. Despite the growing practice of vulnerability indexing in the community, hospital-based providers do not routinely assess vulnerability with respect to housing. Previous research indicates that housing status is assessed in only a minority of homeless patients during their hospital stay.12 Thus, hospitalization often represents a missed opportunity to identify vulnerability and utilize it to connect patients with housing and other resources.
Addressing the significant known health disparities faced by homeless persons is one of the greatest health equity challenges of our time.13 We need better ways of understanding, identifying, and addressing vulnerability among homeless patients who are hospitalized, paired with improved integration with local community organizations. This will require moving beyond the idea that homelessness is the social worker’s job to one of shared responsibility and advocacy.
Collaborative research and other partnerships that engage both community organizations and individuals affected by homelessness are crucial to further understand the specific needs, barriers, challenges, and opportunities for improving hospital care and care transitions in this population. As well-respected community members and systems thinkers who witness these inequities on a daily basis, hospitalists are well positioned to help lead this work.
Dr. Stella is a hospitalist at Denver Health and Hospital Authority, and an associate professor of medicine at the University of Colorado. She is a member of The Hospitalist editorial advisory board.
References
1. Ending Chronic Homelessness. (Aug 2017). U.S. Interagency Council on Homelessness. Available at: https://www.usich.gov/goals/chronicsness. Accessed: Oct 21, 2017.
2. 2016 Annual Homeless Assessment Report (AHAR) to Congress. (Nov 2016). U.S. Department of Housing and Urban Development Office of Community Planning and Development, Part 1. Available at: https://www.hudexchange.info/resources/documents/2016-AHAR-Part-1.pdf. Accessed: Oct 21, 2017.
3. 2017 Point-In-Time Report, Seven-County Metro Denver Region. Metro Denver Homeless Initiative. Available at: http://www.mdhi.org/2017_pit. Accessed Oct 22, 2017.
4. Henwood BF et al. Examining mortality among formerly homeless adults enrolled in Housing First: An observational study. BMC Public Health. 2015;15:1209.
5. Weinstein LC et al. Moving from street to home: Health status of entrants to a Housing First program. J Prim Care Community Health. 2011;2:11–5.
6. Kushel MB et al. Factors associated with the health care utilization of homeless persons. JAMA. 2001;285(2):200-6.
7. Kushel MB et al. Emergency department use among the homeless and marginally housed: Results from a community-based study. Am J Public Health. 2002;92(5):778-84.
8. Baggett TP et al. Mortality among homeless adults in Boston: Shifts in causes of death over a 15-year period. JAMA Intern Med. 2013 Feb 11;173(3):189–95.
9. Johnson et al. For many patients who use large amounts of health care services, the need is intense yet temporary. Health Aff (Millwood). 2015 Aug;34(8):1312-9.
10. Durfee J et al. The impact of tailored intervention services on charges and mortality for adult super-utilizers. Healthc (Amst). 2017 Aug 25. pii: S2213-0764(17)30057-X. doi: 10.1016/j.hjdsi.2017.08.004. [Epub ahead of print]
11. Rinehart DJ et al. Identifying subgroups of adult super utilizers in an urban safety-net system using latent class analysis: Implications for clinical practice. Med Care. 2016 Sep 14. doi: 10.1097/MLR.0000000000000628. [Epub ahead of print]
12. Greysen RS et al. Understanding transitions of care from hospital to homeless shelter: A mixed-methods, community-based participatory approach. J Gen Intern Med. 2012;27(11):1484-91.
13. National Health Care for the Homeless Council. (Oct 2012). Improving Care Transitions for People Experiencing Homelessness. (Lead author: Sabrina Edgington, policy and program specialist.) Available at: www.nhchc.org/wp-content/uploads/2012/12/Policy_Brief_Care_Transitions.pdf. Accessed Oct 21, 2017.
14. Koh HK et al. Improving healthcare for homeless people. JAMA. 2016;316(24):2586-7.
Despite programs to end homelessness, it remains a substantial and growing problem in many cities in the United States.1,2 In 2016, there were an estimated 10,550 homeless people living in my home state of Colorado, a 6% increase from the prior year.2 A recent point-estimate study found that there were more than 5,000 homeless individuals in the Denver metropolitan area on a single night in January 2017.3 Because of the relative scarcity of housing, a growing number of cities like Denver now utilize a practice known as vulnerability indexing to prioritize homeless persons at high risk of mortality from medical conditions for placement in permanent supportive housing.4
Although hospitalists like myself frequently care for vulnerable homeless patients in the hospital, most have little formal training in how best to care for and advocate for these individuals beyond treating their acute medical need, and little direct contact with community organizations with expertise in doing so. Instead, we have learned informally through experience. Hospital providers are often frustrated by the perceived lack of services and support available to these patients, and there is substantial variability in the extent to which providers engage patients and community partners during and after hospitalization. Despite the growing practice of vulnerability indexing in the community, hospital-based providers do not routinely assess vulnerability with respect to housing. Previous research indicates that housing status is assessed in only a minority of homeless patients during their hospital stay.12 Thus, hospitalization often represents a missed opportunity to identify vulnerability and utilize it to connect patients with housing and other resources.
Addressing the significant known health disparities faced by homeless persons is one of the greatest health equity challenges of our time.13 We need better ways of understanding, identifying, and addressing vulnerability among homeless patients who are hospitalized, paired with improved integration with local community organizations. This will require moving beyond the idea that homelessness is the social worker’s job to one of shared responsibility and advocacy.
Collaborative research and other partnerships that engage both community organizations and individuals affected by homelessness are crucial to further understand the specific needs, barriers, challenges, and opportunities for improving hospital care and care transitions in this population. As well-respected community members and systems thinkers who witness these inequities on a daily basis, hospitalists are well positioned to help lead this work.
Dr. Stella is a hospitalist at Denver Health and Hospital Authority, and an associate professor of medicine at the University of Colorado. She is a member of The Hospitalist editorial advisory board.
References
1. Ending Chronic Homelessness. (Aug 2017). U.S. Interagency Council on Homelessness. Available at: https://www.usich.gov/goals/chronicsness. Accessed: Oct 21, 2017.
2. 2016 Annual Homeless Assessment Report (AHAR) to Congress. (Nov 2016). U.S. Department of Housing and Urban Development Office of Community Planning and Development, Part 1. Available at: https://www.hudexchange.info/resources/documents/2016-AHAR-Part-1.pdf. Accessed: Oct 21, 2017.
3. 2017 Point-In-Time Report, Seven-County Metro Denver Region. Metro Denver Homeless Initiative. Available at: http://www.mdhi.org/2017_pit. Accessed Oct 22, 2017.
4. Henwood BF et al. Examining mortality among formerly homeless adults enrolled in Housing First: An observational study. BMC Public Health. 2015;15:1209.
5. Weinstein LC et al. Moving from street to home: Health status of entrants to a Housing First program. J Prim Care Community Health. 2011;2:11–5.
6. Kushel MB et al. Factors associated with the health care utilization of homeless persons. JAMA. 2001;285(2):200-6.
7. Kushel MB et al. Emergency department use among the homeless and marginally housed: Results from a community-based study. Am J Public Health. 2002;92(5):778-84.
8. Baggett TP et al. Mortality among homeless adults in Boston: Shifts in causes of death over a 15-year period. JAMA Intern Med. 2013 Feb 11;173(3):189–95.
9. Johnson et al. For many patients who use large amounts of health care services, the need is intense yet temporary. Health Aff (Millwood). 2015 Aug;34(8):1312-9.
10. Durfee J et al. The impact of tailored intervention services on charges and mortality for adult super-utilizers. Healthc (Amst). 2017 Aug 25. pii: S2213-0764(17)30057-X. doi: 10.1016/j.hjdsi.2017.08.004. [Epub ahead of print]
11. Rinehart DJ et al. Identifying subgroups of adult super utilizers in an urban safety-net system using latent class analysis: Implications for clinical practice. Med Care. 2016 Sep 14. doi: 10.1097/MLR.0000000000000628. [Epub ahead of print]
12. Greysen RS et al. Understanding transitions of care from hospital to homeless shelter: A mixed-methods, community-based participatory approach. J Gen Intern Med. 2012;27(11):1484-91.
13. National Health Care for the Homeless Council. (Oct 2012). Improving Care Transitions for People Experiencing Homelessness. (Lead author: Sabrina Edgington, policy and program specialist.) Available at: www.nhchc.org/wp-content/uploads/2012/12/Policy_Brief_Care_Transitions.pdf. Accessed Oct 21, 2017.
14. Koh HK et al. Improving healthcare for homeless people. JAMA. 2016;316(24):2586-7.
Despite programs to end homelessness, it remains a substantial and growing problem in many cities in the United States.1,2 In 2016, there were an estimated 10,550 homeless people living in my home state of Colorado, a 6% increase from the prior year.2 A recent point-estimate study found that there were more than 5,000 homeless individuals in the Denver metropolitan area on a single night in January 2017.3 Because of the relative scarcity of housing, a growing number of cities like Denver now utilize a practice known as vulnerability indexing to prioritize homeless persons at high risk of mortality from medical conditions for placement in permanent supportive housing.4
Although hospitalists like myself frequently care for vulnerable homeless patients in the hospital, most have little formal training in how best to care for and advocate for these individuals beyond treating their acute medical need, and little direct contact with community organizations with expertise in doing so. Instead, we have learned informally through experience. Hospital providers are often frustrated by the perceived lack of services and support available to these patients, and there is substantial variability in the extent to which providers engage patients and community partners during and after hospitalization. Despite the growing practice of vulnerability indexing in the community, hospital-based providers do not routinely assess vulnerability with respect to housing. Previous research indicates that housing status is assessed in only a minority of homeless patients during their hospital stay.12 Thus, hospitalization often represents a missed opportunity to identify vulnerability and utilize it to connect patients with housing and other resources.
Addressing the significant known health disparities faced by homeless persons is one of the greatest health equity challenges of our time.13 We need better ways of understanding, identifying, and addressing vulnerability among homeless patients who are hospitalized, paired with improved integration with local community organizations. This will require moving beyond the idea that homelessness is the social worker’s job to one of shared responsibility and advocacy.
Collaborative research and other partnerships that engage both community organizations and individuals affected by homelessness are crucial to further understand the specific needs, barriers, challenges, and opportunities for improving hospital care and care transitions in this population. As well-respected community members and systems thinkers who witness these inequities on a daily basis, hospitalists are well positioned to help lead this work.
Dr. Stella is a hospitalist at Denver Health and Hospital Authority, and an associate professor of medicine at the University of Colorado. She is a member of The Hospitalist editorial advisory board.
References
1. Ending Chronic Homelessness. (Aug 2017). U.S. Interagency Council on Homelessness. Available at: https://www.usich.gov/goals/chronicsness. Accessed: Oct 21, 2017.
2. 2016 Annual Homeless Assessment Report (AHAR) to Congress. (Nov 2016). U.S. Department of Housing and Urban Development Office of Community Planning and Development, Part 1. Available at: https://www.hudexchange.info/resources/documents/2016-AHAR-Part-1.pdf. Accessed: Oct 21, 2017.
3. 2017 Point-In-Time Report, Seven-County Metro Denver Region. Metro Denver Homeless Initiative. Available at: http://www.mdhi.org/2017_pit. Accessed Oct 22, 2017.
4. Henwood BF et al. Examining mortality among formerly homeless adults enrolled in Housing First: An observational study. BMC Public Health. 2015;15:1209.
5. Weinstein LC et al. Moving from street to home: Health status of entrants to a Housing First program. J Prim Care Community Health. 2011;2:11–5.
6. Kushel MB et al. Factors associated with the health care utilization of homeless persons. JAMA. 2001;285(2):200-6.
7. Kushel MB et al. Emergency department use among the homeless and marginally housed: Results from a community-based study. Am J Public Health. 2002;92(5):778-84.
8. Baggett TP et al. Mortality among homeless adults in Boston: Shifts in causes of death over a 15-year period. JAMA Intern Med. 2013 Feb 11;173(3):189–95.
9. Johnson et al. For many patients who use large amounts of health care services, the need is intense yet temporary. Health Aff (Millwood). 2015 Aug;34(8):1312-9.
10. Durfee J et al. The impact of tailored intervention services on charges and mortality for adult super-utilizers. Healthc (Amst). 2017 Aug 25. pii: S2213-0764(17)30057-X. doi: 10.1016/j.hjdsi.2017.08.004. [Epub ahead of print]
11. Rinehart DJ et al. Identifying subgroups of adult super utilizers in an urban safety-net system using latent class analysis: Implications for clinical practice. Med Care. 2016 Sep 14. doi: 10.1097/MLR.0000000000000628. [Epub ahead of print]
12. Greysen RS et al. Understanding transitions of care from hospital to homeless shelter: A mixed-methods, community-based participatory approach. J Gen Intern Med. 2012;27(11):1484-91.
13. National Health Care for the Homeless Council. (Oct 2012). Improving Care Transitions for People Experiencing Homelessness. (Lead author: Sabrina Edgington, policy and program specialist.) Available at: www.nhchc.org/wp-content/uploads/2012/12/Policy_Brief_Care_Transitions.pdf. Accessed Oct 21, 2017.
14. Koh HK et al. Improving healthcare for homeless people. JAMA. 2016;316(24):2586-7.
Continue to opt for HDT/ASCT for multiple myeloma
High-dose therapy with melphalan followed by autologous stem cell transplant (HDT/ASCT) is still the best option for multiple myeloma even after almost 2 decades with newer and highly effective induction agents, according to a recent systematic review and two meta-analyses.
Given the “unprecedented efficacy” of “modern induction therapy with immunomodulatory drugs and proteasome inhibitors (also called ‘novel agents’),” investigators “have sought to reevaluate the role of HDT/ASCT,” wrote Binod Dhakal, MD, of the Medical College of Wisconsin, and his colleagues. The report is in JAMA Oncology.
To solve the issue, they analyzed five randomized controlled trials conducted since 2000 and concluded that HDT/ASCT is still the preferred treatment approach.
Despite a lack of demonstrable overall survival benefit, there is a significant progression-free survival (PFS) benefit, low treatment-related mortality, and potential high minimal residual disease-negative rates conferred by HDT/ASCT in newly-diagnosed multiple myeloma, the researchers noted.
The combined odds for complete response were 1.27 (95% confidence interval, 0.97-1.65, P = .07) with HDT/ASCT, compared with standard-dose therapy (SDT). The combined hazard ratio (HR) for PFS was 0.55 (95% CI, 0.41-0.7, P less than .001) and 0.76 for overall survival (95% CI, 0.42-1.36, P = .20) in favor of HDT.
PFS was best with tandem HDT/ASCT (HR, 0.49, 95% CI, 0.37-0.65) followed by single HDT/ASCT with bortezomib, lenalidomide, and dexamethasone consolidation (HR, 0.53, 95% CI, 0.37-0.76) and single HDT/ASCT alone (HR, 0.68, 95% CI, 0.53-0.87), compared with SDT. However, none of the HDT/ASCT approaches had a significant impact on overall survival.
Meanwhile, treatment-related mortality with HDT/ASCT was minimal, at less than 1%.
“The achievement of high [minimal residual disease] rates with HDT/ASCT may render this approach the ideal platform for testing novel approaches (e.g., immunotherapy) aiming at disease eradication and cures,” the researchers wrote.
The researchers reported relationships with a number of companies, including Takeda, Celgene, and Amgen, that make novel induction agents.
SOURCE: Dhakal B et al. JAMA Oncol. 2018 Jan 4. doi: 10.1001/jamaoncol.2017.4600.
High-dose therapy with melphalan followed by autologous stem cell transplant (HDT/ASCT) is still the best option for multiple myeloma even after almost 2 decades with newer and highly effective induction agents, according to a recent systematic review and two meta-analyses.
Given the “unprecedented efficacy” of “modern induction therapy with immunomodulatory drugs and proteasome inhibitors (also called ‘novel agents’),” investigators “have sought to reevaluate the role of HDT/ASCT,” wrote Binod Dhakal, MD, of the Medical College of Wisconsin, and his colleagues. The report is in JAMA Oncology.
To solve the issue, they analyzed five randomized controlled trials conducted since 2000 and concluded that HDT/ASCT is still the preferred treatment approach.
Despite a lack of demonstrable overall survival benefit, there is a significant progression-free survival (PFS) benefit, low treatment-related mortality, and potential high minimal residual disease-negative rates conferred by HDT/ASCT in newly-diagnosed multiple myeloma, the researchers noted.
The combined odds for complete response were 1.27 (95% confidence interval, 0.97-1.65, P = .07) with HDT/ASCT, compared with standard-dose therapy (SDT). The combined hazard ratio (HR) for PFS was 0.55 (95% CI, 0.41-0.7, P less than .001) and 0.76 for overall survival (95% CI, 0.42-1.36, P = .20) in favor of HDT.
PFS was best with tandem HDT/ASCT (HR, 0.49, 95% CI, 0.37-0.65) followed by single HDT/ASCT with bortezomib, lenalidomide, and dexamethasone consolidation (HR, 0.53, 95% CI, 0.37-0.76) and single HDT/ASCT alone (HR, 0.68, 95% CI, 0.53-0.87), compared with SDT. However, none of the HDT/ASCT approaches had a significant impact on overall survival.
Meanwhile, treatment-related mortality with HDT/ASCT was minimal, at less than 1%.
“The achievement of high [minimal residual disease] rates with HDT/ASCT may render this approach the ideal platform for testing novel approaches (e.g., immunotherapy) aiming at disease eradication and cures,” the researchers wrote.
The researchers reported relationships with a number of companies, including Takeda, Celgene, and Amgen, that make novel induction agents.
SOURCE: Dhakal B et al. JAMA Oncol. 2018 Jan 4. doi: 10.1001/jamaoncol.2017.4600.
High-dose therapy with melphalan followed by autologous stem cell transplant (HDT/ASCT) is still the best option for multiple myeloma even after almost 2 decades with newer and highly effective induction agents, according to a recent systematic review and two meta-analyses.
Given the “unprecedented efficacy” of “modern induction therapy with immunomodulatory drugs and proteasome inhibitors (also called ‘novel agents’),” investigators “have sought to reevaluate the role of HDT/ASCT,” wrote Binod Dhakal, MD, of the Medical College of Wisconsin, and his colleagues. The report is in JAMA Oncology.
To solve the issue, they analyzed five randomized controlled trials conducted since 2000 and concluded that HDT/ASCT is still the preferred treatment approach.
Despite a lack of demonstrable overall survival benefit, there is a significant progression-free survival (PFS) benefit, low treatment-related mortality, and potential high minimal residual disease-negative rates conferred by HDT/ASCT in newly-diagnosed multiple myeloma, the researchers noted.
The combined odds for complete response were 1.27 (95% confidence interval, 0.97-1.65, P = .07) with HDT/ASCT, compared with standard-dose therapy (SDT). The combined hazard ratio (HR) for PFS was 0.55 (95% CI, 0.41-0.7, P less than .001) and 0.76 for overall survival (95% CI, 0.42-1.36, P = .20) in favor of HDT.
PFS was best with tandem HDT/ASCT (HR, 0.49, 95% CI, 0.37-0.65) followed by single HDT/ASCT with bortezomib, lenalidomide, and dexamethasone consolidation (HR, 0.53, 95% CI, 0.37-0.76) and single HDT/ASCT alone (HR, 0.68, 95% CI, 0.53-0.87), compared with SDT. However, none of the HDT/ASCT approaches had a significant impact on overall survival.
Meanwhile, treatment-related mortality with HDT/ASCT was minimal, at less than 1%.
“The achievement of high [minimal residual disease] rates with HDT/ASCT may render this approach the ideal platform for testing novel approaches (e.g., immunotherapy) aiming at disease eradication and cures,” the researchers wrote.
The researchers reported relationships with a number of companies, including Takeda, Celgene, and Amgen, that make novel induction agents.
SOURCE: Dhakal B et al. JAMA Oncol. 2018 Jan 4. doi: 10.1001/jamaoncol.2017.4600.
FROM JAMA ONCOLOGY
Key clinical point:
Major finding: The combined odds for complete response were 1.27 (95% CI 0.97-1.65, P = .07) with HDT/ASCT, compared with standard-dose therapy (SDT).
Study details: A systematic review and two meta-analyses examining five phase 3 clinical trials reported since 2000.
Disclosures: The researchers reported relationships with a number of companies, including Takeda, Celgene, and Amgen, that make novel induction agents.
Source: Dhakal B et al. JAMA Oncol. 2018 Jan 4. doi: 10.1001/jamaoncol.2017.4600.
Folic acid and multivitamin supplements associated with reduced autism risk
Taking folic acid and/or multivitamin supplements preceding and during pregnancy is associated with a lower risk of offspring developing autism spectrum disorder (ASD), an observational epidemiologic study published Jan. 3 showed.
The findings could have important public health implications, reported Stephen Z. Levine, PhD, and his associates.
The investigators found that 572 children, or 1.3%, received an ASD diagnosis. Dr. Levine and his associates found that children whose mothers took folic acid and multivitamin supplements during pregnancy had a lower risk of developing ASD (relative risk, 0.27; 95% confidence interval, 0.22-0.33; P less than .001), compared with those whose mothers took no supplements. Similarly, there was reduced risk among those whose mothers took only folic acid during pregnancy (RR, 0.32; CI, 0.26-0.41; P less than .001) or only multivitamins (RR, 0.35; CI, 0.28-0.44; P less than .001). Likewise, lower risks were seen among offspring whose mothers took supplements before pregnancy: Compared with no supplements, the RR was 0.39 for folic acid and/or multivitamins (CI, 0.30-0.50; P less than .001), 0.56 for just folic acid (95%CI, 0.42-0.74; P = .001), and 0.36 for just multivitamins (95%CI, 0.24-0.52; P less than .001). Similar associations were found among male and female offspring.
“This finding may reflect noncompliance, higher rates of vitamin deficiency, or poor diet among persons with psychiatric conditions,” wrote Dr. Levine, of the department of community mental health at the University of Haifa, Israel, and his associates in JAMA Psychiatry.
Another important finding is that maternal exposure to folic acid and multivitamin supplements 2 years before pregnancy is tied to a lower ASD risk.
The investigators acknowledged that the study was limited by their inability to determine possible confounding factors, such as the vehicle of vitamin dispensations, use of over-the-counter supplements, false-positive classifications from noncompliance, and absence of information on gestational age. In addition, they said, “causality cannot be inferred from observational studies such as this one.” In light of those limitations, investigators said, additional studies replicating these findings are needed.
The study was funded by several entities, including the National Institutes of Health, the Fredrik and Ingrid Thuring Foundation, and the Swedish Society of Medicine. Dr. Levine reported receiving support from Shire Pharmaceuticals, and coauthor Arad Kodesh, MD, is an employee of Meuhedet Health Services. No other relevant financial disclosures were reported.
SOURCE: Levine SZ et al. JAMA Psychiatry. 2018 Jan 3. doi: 10.1001/jamapsychiatry.2017.4050.
Taking folic acid and/or multivitamin supplements preceding and during pregnancy is associated with a lower risk of offspring developing autism spectrum disorder (ASD), an observational epidemiologic study published Jan. 3 showed.
The findings could have important public health implications, reported Stephen Z. Levine, PhD, and his associates.
The investigators found that 572 children, or 1.3%, received an ASD diagnosis. Dr. Levine and his associates found that children whose mothers took folic acid and multivitamin supplements during pregnancy had a lower risk of developing ASD (relative risk, 0.27; 95% confidence interval, 0.22-0.33; P less than .001), compared with those whose mothers took no supplements. Similarly, there was reduced risk among those whose mothers took only folic acid during pregnancy (RR, 0.32; CI, 0.26-0.41; P less than .001) or only multivitamins (RR, 0.35; CI, 0.28-0.44; P less than .001). Likewise, lower risks were seen among offspring whose mothers took supplements before pregnancy: Compared with no supplements, the RR was 0.39 for folic acid and/or multivitamins (CI, 0.30-0.50; P less than .001), 0.56 for just folic acid (95%CI, 0.42-0.74; P = .001), and 0.36 for just multivitamins (95%CI, 0.24-0.52; P less than .001). Similar associations were found among male and female offspring.
“This finding may reflect noncompliance, higher rates of vitamin deficiency, or poor diet among persons with psychiatric conditions,” wrote Dr. Levine, of the department of community mental health at the University of Haifa, Israel, and his associates in JAMA Psychiatry.
Another important finding is that maternal exposure to folic acid and multivitamin supplements 2 years before pregnancy is tied to a lower ASD risk.
The investigators acknowledged that the study was limited by their inability to determine possible confounding factors, such as the vehicle of vitamin dispensations, use of over-the-counter supplements, false-positive classifications from noncompliance, and absence of information on gestational age. In addition, they said, “causality cannot be inferred from observational studies such as this one.” In light of those limitations, investigators said, additional studies replicating these findings are needed.
The study was funded by several entities, including the National Institutes of Health, the Fredrik and Ingrid Thuring Foundation, and the Swedish Society of Medicine. Dr. Levine reported receiving support from Shire Pharmaceuticals, and coauthor Arad Kodesh, MD, is an employee of Meuhedet Health Services. No other relevant financial disclosures were reported.
SOURCE: Levine SZ et al. JAMA Psychiatry. 2018 Jan 3. doi: 10.1001/jamapsychiatry.2017.4050.
Taking folic acid and/or multivitamin supplements preceding and during pregnancy is associated with a lower risk of offspring developing autism spectrum disorder (ASD), an observational epidemiologic study published Jan. 3 showed.
The findings could have important public health implications, reported Stephen Z. Levine, PhD, and his associates.
The investigators found that 572 children, or 1.3%, received an ASD diagnosis. Dr. Levine and his associates found that children whose mothers took folic acid and multivitamin supplements during pregnancy had a lower risk of developing ASD (relative risk, 0.27; 95% confidence interval, 0.22-0.33; P less than .001), compared with those whose mothers took no supplements. Similarly, there was reduced risk among those whose mothers took only folic acid during pregnancy (RR, 0.32; CI, 0.26-0.41; P less than .001) or only multivitamins (RR, 0.35; CI, 0.28-0.44; P less than .001). Likewise, lower risks were seen among offspring whose mothers took supplements before pregnancy: Compared with no supplements, the RR was 0.39 for folic acid and/or multivitamins (CI, 0.30-0.50; P less than .001), 0.56 for just folic acid (95%CI, 0.42-0.74; P = .001), and 0.36 for just multivitamins (95%CI, 0.24-0.52; P less than .001). Similar associations were found among male and female offspring.
“This finding may reflect noncompliance, higher rates of vitamin deficiency, or poor diet among persons with psychiatric conditions,” wrote Dr. Levine, of the department of community mental health at the University of Haifa, Israel, and his associates in JAMA Psychiatry.
Another important finding is that maternal exposure to folic acid and multivitamin supplements 2 years before pregnancy is tied to a lower ASD risk.
The investigators acknowledged that the study was limited by their inability to determine possible confounding factors, such as the vehicle of vitamin dispensations, use of over-the-counter supplements, false-positive classifications from noncompliance, and absence of information on gestational age. In addition, they said, “causality cannot be inferred from observational studies such as this one.” In light of those limitations, investigators said, additional studies replicating these findings are needed.
The study was funded by several entities, including the National Institutes of Health, the Fredrik and Ingrid Thuring Foundation, and the Swedish Society of Medicine. Dr. Levine reported receiving support from Shire Pharmaceuticals, and coauthor Arad Kodesh, MD, is an employee of Meuhedet Health Services. No other relevant financial disclosures were reported.
SOURCE: Levine SZ et al. JAMA Psychiatry. 2018 Jan 3. doi: 10.1001/jamapsychiatry.2017.4050.
Key clinical point: Taking folic acid and multivitamin supplements before and during pregnancy can reduce risk of autism in children.
Major finding: Children whose mothers took folic acid and/or multivitamin supplements during pregnancy had a decreased risk of developing ASD, compared with those whose mothers did not (relative risk, 0.27; 95% confidence interval, 0.22-0.33; P less than .001).
Study details: Observational epidemiologic study of 45,300 Israeli children born between January 2003 and December 2007 and followed until January 2015.
Disclosures: The study was funded by several entities, including the National Institutes of Health, the Fredrik and Ingrid Thuring Foundation, and the Swedish Society of Medicine. Dr. Levine reported receiving support from Shire Pharmaceuticals, and coauthor Arad Kodesh, MD, is an employee of Meuhedet Health Services. No other relevant financial disclosures were reported.
Source: Levine SZ et al. JAMA Psychiatry. 2018 Jan 3. doi: 10.1001/jamapsychiatry.2017.4050.
Sacred cows
Within the academic pediatric community, there is little argument that the concepts “evidence based” and “early intervention” are gold standards against which we must measure our efforts.
It should be obvious to everyone that if we can intervene early in a child’s developmental trajectory, our chances of affecting his/her outcome are improved. And the earlier the better. If we aren’t supremely committed to prevention, then what sets pediatrics apart from the other specialties?
Likewise, if we aren’t willing to systematically measure our efforts at improving the health of our patients, we run the risk of simply spinning our wheels and even worse, squandering our patients’ time and their parents’ energies. However, a recent article in Pediatrics and a companion commentary suggest that we need to be more careful as we interpret the buzz that surrounds the terms “early intervention” and “evidence based.”
In their one-sentence conclusion of a paper reviewing 48 studies of early intervention in early childhood development, the authors observe, “Although several interventions resulted in improved child development outcomes age 0 to 3 years, comparison across studies and interventions is limited by the use of different outcome measures, time of evaluation, and variability of results” (“Primary Care Interventions for Early Childhood Development: A Systematic Review,” Peacock-Chambers et al. Pediatrics. 2017, Nov 14. doi: 10.1542/peds.2017-1661). Unless you are looking for another reason to slip further into an abyss of despair, I urge that you skip reading the details of the Peacock-Chambers paper and turn instead to Dr. Jack P. Shonkoff’s excellent commentary (“Rethinking the Definition of Evidence-Based Interventions to Promote Early Child Development,” Pediatrics. 2017, Dec. doi: 10.1542/peds.2017-3136).
Dr. Shonkoff observes that there is ample evidence to support the general concept of early intervention as it relates to childhood development. However, he acknowledges that the improvements observed generally have been small. And there has been little success in scaling these few successes to larger populations. It would seem that the sacred cow of early intervention remains standing, albeit on somewhat shaky legs.
Dr. Shonkoff points out that an obsession with statistical significance often has blinded some of us to the importance of the magnitude of (or the lack of) impact when interpreting studies of early intervention. As a result, we may have failed to realize how far research in early childhood development has fallen behind the other fields of biomedical research such as cancer, HIV, and AIDS. His plea is that we begin to leverage our successes in fields such as molecular biology, epigenetics, and neuroscience when designing future studies of early childhood development. He asserts that this kind of basic science – in concert with “on-the-ground experience” (that’s you and me) and “authentic parental engagement” – is more likely to result in greater scalable impact for our patients threatened by developmental delays.
It is refreshing and encouraging reading a critical consideration of the evidence-based sacred cow. Evidence can be viewed from a variety of perspectives. If we continue to filter all of our observations through a statistical significance filter, we run the risk of missing both the forest and the trees.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Within the academic pediatric community, there is little argument that the concepts “evidence based” and “early intervention” are gold standards against which we must measure our efforts.
It should be obvious to everyone that if we can intervene early in a child’s developmental trajectory, our chances of affecting his/her outcome are improved. And the earlier the better. If we aren’t supremely committed to prevention, then what sets pediatrics apart from the other specialties?
Likewise, if we aren’t willing to systematically measure our efforts at improving the health of our patients, we run the risk of simply spinning our wheels and even worse, squandering our patients’ time and their parents’ energies. However, a recent article in Pediatrics and a companion commentary suggest that we need to be more careful as we interpret the buzz that surrounds the terms “early intervention” and “evidence based.”
In their one-sentence conclusion of a paper reviewing 48 studies of early intervention in early childhood development, the authors observe, “Although several interventions resulted in improved child development outcomes age 0 to 3 years, comparison across studies and interventions is limited by the use of different outcome measures, time of evaluation, and variability of results” (“Primary Care Interventions for Early Childhood Development: A Systematic Review,” Peacock-Chambers et al. Pediatrics. 2017, Nov 14. doi: 10.1542/peds.2017-1661). Unless you are looking for another reason to slip further into an abyss of despair, I urge that you skip reading the details of the Peacock-Chambers paper and turn instead to Dr. Jack P. Shonkoff’s excellent commentary (“Rethinking the Definition of Evidence-Based Interventions to Promote Early Child Development,” Pediatrics. 2017, Dec. doi: 10.1542/peds.2017-3136).
Dr. Shonkoff observes that there is ample evidence to support the general concept of early intervention as it relates to childhood development. However, he acknowledges that the improvements observed generally have been small. And there has been little success in scaling these few successes to larger populations. It would seem that the sacred cow of early intervention remains standing, albeit on somewhat shaky legs.
Dr. Shonkoff points out that an obsession with statistical significance often has blinded some of us to the importance of the magnitude of (or the lack of) impact when interpreting studies of early intervention. As a result, we may have failed to realize how far research in early childhood development has fallen behind the other fields of biomedical research such as cancer, HIV, and AIDS. His plea is that we begin to leverage our successes in fields such as molecular biology, epigenetics, and neuroscience when designing future studies of early childhood development. He asserts that this kind of basic science – in concert with “on-the-ground experience” (that’s you and me) and “authentic parental engagement” – is more likely to result in greater scalable impact for our patients threatened by developmental delays.
It is refreshing and encouraging reading a critical consideration of the evidence-based sacred cow. Evidence can be viewed from a variety of perspectives. If we continue to filter all of our observations through a statistical significance filter, we run the risk of missing both the forest and the trees.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Within the academic pediatric community, there is little argument that the concepts “evidence based” and “early intervention” are gold standards against which we must measure our efforts.
It should be obvious to everyone that if we can intervene early in a child’s developmental trajectory, our chances of affecting his/her outcome are improved. And the earlier the better. If we aren’t supremely committed to prevention, then what sets pediatrics apart from the other specialties?
Likewise, if we aren’t willing to systematically measure our efforts at improving the health of our patients, we run the risk of simply spinning our wheels and even worse, squandering our patients’ time and their parents’ energies. However, a recent article in Pediatrics and a companion commentary suggest that we need to be more careful as we interpret the buzz that surrounds the terms “early intervention” and “evidence based.”
In their one-sentence conclusion of a paper reviewing 48 studies of early intervention in early childhood development, the authors observe, “Although several interventions resulted in improved child development outcomes age 0 to 3 years, comparison across studies and interventions is limited by the use of different outcome measures, time of evaluation, and variability of results” (“Primary Care Interventions for Early Childhood Development: A Systematic Review,” Peacock-Chambers et al. Pediatrics. 2017, Nov 14. doi: 10.1542/peds.2017-1661). Unless you are looking for another reason to slip further into an abyss of despair, I urge that you skip reading the details of the Peacock-Chambers paper and turn instead to Dr. Jack P. Shonkoff’s excellent commentary (“Rethinking the Definition of Evidence-Based Interventions to Promote Early Child Development,” Pediatrics. 2017, Dec. doi: 10.1542/peds.2017-3136).
Dr. Shonkoff observes that there is ample evidence to support the general concept of early intervention as it relates to childhood development. However, he acknowledges that the improvements observed generally have been small. And there has been little success in scaling these few successes to larger populations. It would seem that the sacred cow of early intervention remains standing, albeit on somewhat shaky legs.
Dr. Shonkoff points out that an obsession with statistical significance often has blinded some of us to the importance of the magnitude of (or the lack of) impact when interpreting studies of early intervention. As a result, we may have failed to realize how far research in early childhood development has fallen behind the other fields of biomedical research such as cancer, HIV, and AIDS. His plea is that we begin to leverage our successes in fields such as molecular biology, epigenetics, and neuroscience when designing future studies of early childhood development. He asserts that this kind of basic science – in concert with “on-the-ground experience” (that’s you and me) and “authentic parental engagement” – is more likely to result in greater scalable impact for our patients threatened by developmental delays.
It is refreshing and encouraging reading a critical consideration of the evidence-based sacred cow. Evidence can be viewed from a variety of perspectives. If we continue to filter all of our observations through a statistical significance filter, we run the risk of missing both the forest and the trees.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Minorities less likely to seek treatment for psoriasis
Black, Asian, and other non-Hispanic Americans are less likely than are whites to seek treatment for psoriasis, according to data on 842 patients, reported Alexander H. Fischer, MD, of the University of Pennsylvania, Philadelphia, and his colleagues.
Data from previous studies have shown that racial and ethnic minorities have more severe psoriasis and a lower quality of life as a result of the disease, compared with white patients, the researchers noted in a study published as a research letter in the Journal of the American Academy of Dermatology.
A total of 51% of non-Hispanic whites with psoriasis sought treatment from a dermatologist, compared with 47% of Hispanic whites and 38% of non-Hispanic minorities (blacks, Asians, native Hawaiians, Pacific Islanders, and others). In addition, non-Hispanic minorities had significantly fewer ambulatory visits for psoriasis per year than did whites (a mean of 1.30 visits vs. 2.69 visits). Black, Asian, and other non-Hispanic minorities were about 40% less likely than were non-Hispanic whites to seek care for psoriasis.
The number of psoriasis prescriptions obtained was not significantly different among the racial/ethnic groups, the researchers reported.
The study is important because of the lack of data on psoriasis in nonwhite populations, senior author Junko Takeshita, MD, PhD, also of the University of Pennsylvania, said in an interview.
“Based on a few existing studies, we know that psoriasis is less common among minorities, but minorities, particularly blacks, may have more severe disease,” she said. “Also, minorities report poorer quality of life due to psoriasis than whites, independent of psoriasis severity. Furthermore, we previously published a study among Medicare beneficiaries with psoriasis that revealed that blacks are about 70% less likely to receive biologic therapies than whites, independent of socioeconomic status and access to medical care,” she added.
“The take-home message for clinicians is that while psoriasis is less common among minorities than whites, minorities may suffer from a larger burden of disease, yet have fewer visits and are less likely to see a dermatologist for their psoriasis,” Dr. Takeshita said. “This disparity in health care utilization for psoriasis does not seem to be entirely explained by racial/ethnic differences in socioeconomic status and health insurance. It is yet unknown why this disparity exists, and I’m not sure that minority patients being ‘hesitant to pursue care’ is the entire answer, though it may be a contributing factor,” she noted.
The study findings were limited by several factors including the relatively small sample size and the use of self-reports.
Many factors could be contributing to the disparity, including patient, physician/other health care provider, and health care system factors, but “once we identify the major causes of the disparity, we can develop methods to address the causes and reduce the disparity,” said Dr. Takeshita, who is a dermatologist and an epidemiologist. In the meantime, she added, “some things I think that are important to ensure equitable care for psoriasis are making sure that clinicians/dermatologists are comfortable diagnosing and treating psoriasis in nonwhite individuals, and encouraging clinicians to help increase awareness of psoriasis by educating their minority patients that psoriasis is still a common skin disease among nonwhite individuals.”
The study was supported in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Takeshita has received a research grant from Pfizer; she and another author, Joel Gelfand, MD, have received payment for psoriasis-related continuing medical education work supported indirectly by Eli Lilly; Dr. Gelfand’s other disclosures included serving as a consultant for, and having received research grants from, several other pharmaceutical companies. Dr. Fischer, a medical student at Johns Hopkins University, Baltimore, at the time of the research, and a fourth author had no financial disclosures.
SOURCE: Fischer AH et al. J Am Acad Dermatol. 2018 Jan;78[1]:200-3. doi: 10.1016/j.jaad.2017.07.052.
Black, Asian, and other non-Hispanic Americans are less likely than are whites to seek treatment for psoriasis, according to data on 842 patients, reported Alexander H. Fischer, MD, of the University of Pennsylvania, Philadelphia, and his colleagues.
Data from previous studies have shown that racial and ethnic minorities have more severe psoriasis and a lower quality of life as a result of the disease, compared with white patients, the researchers noted in a study published as a research letter in the Journal of the American Academy of Dermatology.
A total of 51% of non-Hispanic whites with psoriasis sought treatment from a dermatologist, compared with 47% of Hispanic whites and 38% of non-Hispanic minorities (blacks, Asians, native Hawaiians, Pacific Islanders, and others). In addition, non-Hispanic minorities had significantly fewer ambulatory visits for psoriasis per year than did whites (a mean of 1.30 visits vs. 2.69 visits). Black, Asian, and other non-Hispanic minorities were about 40% less likely than were non-Hispanic whites to seek care for psoriasis.
The number of psoriasis prescriptions obtained was not significantly different among the racial/ethnic groups, the researchers reported.
The study is important because of the lack of data on psoriasis in nonwhite populations, senior author Junko Takeshita, MD, PhD, also of the University of Pennsylvania, said in an interview.
“Based on a few existing studies, we know that psoriasis is less common among minorities, but minorities, particularly blacks, may have more severe disease,” she said. “Also, minorities report poorer quality of life due to psoriasis than whites, independent of psoriasis severity. Furthermore, we previously published a study among Medicare beneficiaries with psoriasis that revealed that blacks are about 70% less likely to receive biologic therapies than whites, independent of socioeconomic status and access to medical care,” she added.
“The take-home message for clinicians is that while psoriasis is less common among minorities than whites, minorities may suffer from a larger burden of disease, yet have fewer visits and are less likely to see a dermatologist for their psoriasis,” Dr. Takeshita said. “This disparity in health care utilization for psoriasis does not seem to be entirely explained by racial/ethnic differences in socioeconomic status and health insurance. It is yet unknown why this disparity exists, and I’m not sure that minority patients being ‘hesitant to pursue care’ is the entire answer, though it may be a contributing factor,” she noted.
The study findings were limited by several factors including the relatively small sample size and the use of self-reports.
Many factors could be contributing to the disparity, including patient, physician/other health care provider, and health care system factors, but “once we identify the major causes of the disparity, we can develop methods to address the causes and reduce the disparity,” said Dr. Takeshita, who is a dermatologist and an epidemiologist. In the meantime, she added, “some things I think that are important to ensure equitable care for psoriasis are making sure that clinicians/dermatologists are comfortable diagnosing and treating psoriasis in nonwhite individuals, and encouraging clinicians to help increase awareness of psoriasis by educating their minority patients that psoriasis is still a common skin disease among nonwhite individuals.”
The study was supported in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Takeshita has received a research grant from Pfizer; she and another author, Joel Gelfand, MD, have received payment for psoriasis-related continuing medical education work supported indirectly by Eli Lilly; Dr. Gelfand’s other disclosures included serving as a consultant for, and having received research grants from, several other pharmaceutical companies. Dr. Fischer, a medical student at Johns Hopkins University, Baltimore, at the time of the research, and a fourth author had no financial disclosures.
SOURCE: Fischer AH et al. J Am Acad Dermatol. 2018 Jan;78[1]:200-3. doi: 10.1016/j.jaad.2017.07.052.
Black, Asian, and other non-Hispanic Americans are less likely than are whites to seek treatment for psoriasis, according to data on 842 patients, reported Alexander H. Fischer, MD, of the University of Pennsylvania, Philadelphia, and his colleagues.
Data from previous studies have shown that racial and ethnic minorities have more severe psoriasis and a lower quality of life as a result of the disease, compared with white patients, the researchers noted in a study published as a research letter in the Journal of the American Academy of Dermatology.
A total of 51% of non-Hispanic whites with psoriasis sought treatment from a dermatologist, compared with 47% of Hispanic whites and 38% of non-Hispanic minorities (blacks, Asians, native Hawaiians, Pacific Islanders, and others). In addition, non-Hispanic minorities had significantly fewer ambulatory visits for psoriasis per year than did whites (a mean of 1.30 visits vs. 2.69 visits). Black, Asian, and other non-Hispanic minorities were about 40% less likely than were non-Hispanic whites to seek care for psoriasis.
The number of psoriasis prescriptions obtained was not significantly different among the racial/ethnic groups, the researchers reported.
The study is important because of the lack of data on psoriasis in nonwhite populations, senior author Junko Takeshita, MD, PhD, also of the University of Pennsylvania, said in an interview.
“Based on a few existing studies, we know that psoriasis is less common among minorities, but minorities, particularly blacks, may have more severe disease,” she said. “Also, minorities report poorer quality of life due to psoriasis than whites, independent of psoriasis severity. Furthermore, we previously published a study among Medicare beneficiaries with psoriasis that revealed that blacks are about 70% less likely to receive biologic therapies than whites, independent of socioeconomic status and access to medical care,” she added.
“The take-home message for clinicians is that while psoriasis is less common among minorities than whites, minorities may suffer from a larger burden of disease, yet have fewer visits and are less likely to see a dermatologist for their psoriasis,” Dr. Takeshita said. “This disparity in health care utilization for psoriasis does not seem to be entirely explained by racial/ethnic differences in socioeconomic status and health insurance. It is yet unknown why this disparity exists, and I’m not sure that minority patients being ‘hesitant to pursue care’ is the entire answer, though it may be a contributing factor,” she noted.
The study findings were limited by several factors including the relatively small sample size and the use of self-reports.
Many factors could be contributing to the disparity, including patient, physician/other health care provider, and health care system factors, but “once we identify the major causes of the disparity, we can develop methods to address the causes and reduce the disparity,” said Dr. Takeshita, who is a dermatologist and an epidemiologist. In the meantime, she added, “some things I think that are important to ensure equitable care for psoriasis are making sure that clinicians/dermatologists are comfortable diagnosing and treating psoriasis in nonwhite individuals, and encouraging clinicians to help increase awareness of psoriasis by educating their minority patients that psoriasis is still a common skin disease among nonwhite individuals.”
The study was supported in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Takeshita has received a research grant from Pfizer; she and another author, Joel Gelfand, MD, have received payment for psoriasis-related continuing medical education work supported indirectly by Eli Lilly; Dr. Gelfand’s other disclosures included serving as a consultant for, and having received research grants from, several other pharmaceutical companies. Dr. Fischer, a medical student at Johns Hopkins University, Baltimore, at the time of the research, and a fourth author had no financial disclosures.
SOURCE: Fischer AH et al. J Am Acad Dermatol. 2018 Jan;78[1]:200-3. doi: 10.1016/j.jaad.2017.07.052.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Key clinical point: Black, Asian, and non-Hispanic patients with psoriasis often have more severe disease than do white patients but are significantly less likely to seek care.
Major finding:
Data source: A cohort study of data from the Medical Expenditure Panel Survey on 842 psoriasis patients in the United States.
Disclosures: The study was supported in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Two of the four authors had no financial disclosures. One author has received a research grant from Pfizer and payment for psoriasis-related continuing medical education work supported indirectly by Eli Lilly; another author’s disclosures included the latter, as well serving as a consultant for, and having received research grants from, several other pharmaceutical companies.
Source: Fischer AH et al. J Am Acad Dermatol. 2018 Jan;78[1]:200-3. doi: 10.1016/j.jaad.2017.07.05
Caplacizumab improves outcomes in aTTP
ATLANTA—Caplacizumab can improve outcomes in patients with acquired thrombotic thrombocytopenic purpura (aTTP), according to research presented at the 2017 ASH Annual Meeting.
In the phase 3 HERCULES trial, researchers compared caplacizumab, an anti-von Willebrand factor nanobody, plus standard care (plasma exchange and immunosuppression) to placebo plus standard care in patients with aTTP.
Patients who received caplacizumab were significantly more likely to achieve platelet normalization and significantly less likely to experience aTTP-related death, aTTP recurrence, and major thromboembolic events.
Patients in the caplacizumab arm also required plasma exchange less frequently and spent less time in the hospital and intensive care unit (ICU).
Bleeding-related adverse events (AEs) were more common among patients who received caplacizumab than those who received placebo.
Marie Scully, MD, of the University College London Hospitals in London, UK, presented these results from HERCULES as a late-breaking abstract at the ASH Annual Meeting (abstract LBA-1). HERCULES was supported by Ablynx.
Patients and treatment
The study enrolled patients with an acute episode of aTTP. They were randomized to receive either caplacizumab (n=72) or placebo (n=73) in addition to standard care, which consisted of plasma exchange and immunosuppression.
Patients received a single intravenous bolus of 10 mg of caplacizumab or placebo followed by a daily subcutaneous dose of 10 mg of caplacizumab or placebo until 30 days after the last daily plasma exchange. If patients had a recurrence during the 30-day treatment period, they could go on to receive open-label caplacizumab.
If, at the end of the 30-day treatment period, there was evidence of persistent underlying disease activity indicative of an imminent risk for recurrence, caplacizumab or placebo could be extended for additional 7-day periods up to a maximum of 28 days. Patients were followed for a further 28 days after discontinuation of treatment.
In all, 71 patients received caplacizumab, and 58 (80.6%) of them completed the treatment. Seventy-three patients received placebo, and 50 of these patients (68.5%) completed treatment. Twenty-six patients in the placebo arm and 2 patients in the caplacizumab arm received open-label caplacizumab.
“If we look at the demographics, they’re relatively comparable to any data we normally see in patients with immune-mediated TTP,” Dr Scully said.
At baseline, the mean age was 44.9 in the caplacizumab arm and 47.3 in the placebo arm. Most patients in both arms were female—68.1% and 69.9%, respectively.
The proportion of patients with an initial aTTP episode was 66.7% in the caplacizumab arm and 46.6% in the placebo arm. The proportion with a recurrent episode was 33.3% and 53.4%, respectively.
Most patients in both arms had ADAMTS13 activity below 10% at baseline—81.7% in the caplacizumab arm and 90.3% in the placebo arm.
The mean platelet count at baseline was 32.0 x 109/L in the caplacizumab arm and 39.1 x 109/L in the placebo arm.
Efficacy
The study’s primary endpoint was the time to normalization of platelet count response, which was defined as initial platelet count of at least 150 x 109/L with subsequent stop of daily plasma exchange within 5 days.
There was a significant reduction in time to platelet count response in the caplacizumab arm compared to the placebo arm. The platelet normalization rate ratio was 1.55 (P<0.01).
“Patients were 55% more likely to achieve normalization of their platelet count at any time in the caplacizumab group, and this was highly significant,” Dr Scully said.
A secondary endpoint was the combination of aTTP-related death, aTTP recurrence, and at least 1 major thromboembolic event during study treatment. The incidence of this combined endpoint was 12.7% (n=9) in the caplacizumab arm and 49.3% (n=36) in the placebo arm (P<0.0001).
The incidence of aTTP-related death was 0% (n=0) in the caplacizumab arm and 4.1% (n=3) in the placebo arm. The incidence of aTTP recurrence was 4.2% (n=3) and 38.4% (n=28), respectively. And the incidence of at least 1 major thromboembolic event was 8.5% (n=6) and 8.2% (n=6), respectively.
The researchers also assessed aTTP recurrence during the overall study period, which occurred in 12.7% (n=9) of patients in the caplacizumab arm and 38.4% (n=28) in the placebo arm (P<0.001).
During the follow-up period, there were 6 relapses (9.1%) in the caplacizumab arm but none in the placebo arm.
“This tells us something about the pathophysiology of TTP and the role of caplacizumab,” Dr Scully said. “All of these patients, on stopping caplacizumab, had ADAMTS13 levels less than 5%. Therefore, it was important that their treatment was continued to ensure removal of antibody.”
According to the International TTP Working Group consensus definition, none of the patients in the caplacizumab arm and 7.0% (n=5) of patients in the placebo arm had refractory aTTP (P=0.018).
The mean number of days of plasma exchange during the overall treatment period was 5.8 days in the caplacizumab arm and 9.4 days in the placebo arm (a 38% relative reduction). The mean volume of plasma used was 21.3L and 35.9L, respectively (a 41% relative reduction).
The mean duration of hospital stay was 9.9 days in the caplacizumab arm and 14.4 days in the placebo arm (a 31% relative reduction).
For patients admitted to the ICU (28 in the caplacizumab arm and 27 in the placebo arm), the mean number of days in the ICU was 3.4 days in the caplacizumab arm and 9.7 days in the placebo arm (a 65% relative reduction).
Safety
“The safety profile [of caplacizumab] was comparable to previous results and in keeping with the mechanism of action,” Dr Scully said.
The proportion of patients with at least 1 treatment-emergent AE was 97.2% in the caplacizumab arm and 97.3% in the placebo arm.
The proportion of patients with at least 1 study-drug-related AE was 57.7% in the caplacizumab arm and 43.8% in the placebo arm. The rate of discontinuation due to at least 1 AE was 7.0% and 12.3%, respectively.
The incidence of bleeding-related AEs was higher in the caplacizumab arm (45.6%) than the placebo arm (23.3%).
Bleeding-related AEs (in the caplacizumab and placebo arms, respectively) included epistaxis (23.9% and 1.4%), gingival bleeding (11.3% and 0%), bruising (7.0% and 4.1%), hematuria (5.6% and 1.4%), vaginal hemorrhage (4.2% and 1.4%), menorrhagia (2.8% and 1.4%), catheter site hemorrhage (2.8% and 4.1%), injection site bruising (2.8% and 2.7%), hematochezia (2.8% and 0%), and hematoma (2.8% and 0%).
The proportion of patients with at least 1 serious AE was 39.4% (n=28) in the caplacizumab arm and 53.4% (n=39) in the placebo arm. The proportion of patients with at least 1 study-drug-related serious AE was 14.1% (n=10) and 5.5% (n=4), respectively.
During the treatment period, there were no deaths in the caplacizumab arm and 3 deaths in the placebo arm. There was 1 death in the caplacizumab arm during the follow-up period, but it was considered unrelated to caplacizumab. ![]()
ATLANTA—Caplacizumab can improve outcomes in patients with acquired thrombotic thrombocytopenic purpura (aTTP), according to research presented at the 2017 ASH Annual Meeting.
In the phase 3 HERCULES trial, researchers compared caplacizumab, an anti-von Willebrand factor nanobody, plus standard care (plasma exchange and immunosuppression) to placebo plus standard care in patients with aTTP.
Patients who received caplacizumab were significantly more likely to achieve platelet normalization and significantly less likely to experience aTTP-related death, aTTP recurrence, and major thromboembolic events.
Patients in the caplacizumab arm also required plasma exchange less frequently and spent less time in the hospital and intensive care unit (ICU).
Bleeding-related adverse events (AEs) were more common among patients who received caplacizumab than those who received placebo.
Marie Scully, MD, of the University College London Hospitals in London, UK, presented these results from HERCULES as a late-breaking abstract at the ASH Annual Meeting (abstract LBA-1). HERCULES was supported by Ablynx.
Patients and treatment
The study enrolled patients with an acute episode of aTTP. They were randomized to receive either caplacizumab (n=72) or placebo (n=73) in addition to standard care, which consisted of plasma exchange and immunosuppression.
Patients received a single intravenous bolus of 10 mg of caplacizumab or placebo followed by a daily subcutaneous dose of 10 mg of caplacizumab or placebo until 30 days after the last daily plasma exchange. If patients had a recurrence during the 30-day treatment period, they could go on to receive open-label caplacizumab.
If, at the end of the 30-day treatment period, there was evidence of persistent underlying disease activity indicative of an imminent risk for recurrence, caplacizumab or placebo could be extended for additional 7-day periods up to a maximum of 28 days. Patients were followed for a further 28 days after discontinuation of treatment.
In all, 71 patients received caplacizumab, and 58 (80.6%) of them completed the treatment. Seventy-three patients received placebo, and 50 of these patients (68.5%) completed treatment. Twenty-six patients in the placebo arm and 2 patients in the caplacizumab arm received open-label caplacizumab.
“If we look at the demographics, they’re relatively comparable to any data we normally see in patients with immune-mediated TTP,” Dr Scully said.
At baseline, the mean age was 44.9 in the caplacizumab arm and 47.3 in the placebo arm. Most patients in both arms were female—68.1% and 69.9%, respectively.
The proportion of patients with an initial aTTP episode was 66.7% in the caplacizumab arm and 46.6% in the placebo arm. The proportion with a recurrent episode was 33.3% and 53.4%, respectively.
Most patients in both arms had ADAMTS13 activity below 10% at baseline—81.7% in the caplacizumab arm and 90.3% in the placebo arm.
The mean platelet count at baseline was 32.0 x 109/L in the caplacizumab arm and 39.1 x 109/L in the placebo arm.
Efficacy
The study’s primary endpoint was the time to normalization of platelet count response, which was defined as initial platelet count of at least 150 x 109/L with subsequent stop of daily plasma exchange within 5 days.
There was a significant reduction in time to platelet count response in the caplacizumab arm compared to the placebo arm. The platelet normalization rate ratio was 1.55 (P<0.01).
“Patients were 55% more likely to achieve normalization of their platelet count at any time in the caplacizumab group, and this was highly significant,” Dr Scully said.
A secondary endpoint was the combination of aTTP-related death, aTTP recurrence, and at least 1 major thromboembolic event during study treatment. The incidence of this combined endpoint was 12.7% (n=9) in the caplacizumab arm and 49.3% (n=36) in the placebo arm (P<0.0001).
The incidence of aTTP-related death was 0% (n=0) in the caplacizumab arm and 4.1% (n=3) in the placebo arm. The incidence of aTTP recurrence was 4.2% (n=3) and 38.4% (n=28), respectively. And the incidence of at least 1 major thromboembolic event was 8.5% (n=6) and 8.2% (n=6), respectively.
The researchers also assessed aTTP recurrence during the overall study period, which occurred in 12.7% (n=9) of patients in the caplacizumab arm and 38.4% (n=28) in the placebo arm (P<0.001).
During the follow-up period, there were 6 relapses (9.1%) in the caplacizumab arm but none in the placebo arm.
“This tells us something about the pathophysiology of TTP and the role of caplacizumab,” Dr Scully said. “All of these patients, on stopping caplacizumab, had ADAMTS13 levels less than 5%. Therefore, it was important that their treatment was continued to ensure removal of antibody.”
According to the International TTP Working Group consensus definition, none of the patients in the caplacizumab arm and 7.0% (n=5) of patients in the placebo arm had refractory aTTP (P=0.018).
The mean number of days of plasma exchange during the overall treatment period was 5.8 days in the caplacizumab arm and 9.4 days in the placebo arm (a 38% relative reduction). The mean volume of plasma used was 21.3L and 35.9L, respectively (a 41% relative reduction).
The mean duration of hospital stay was 9.9 days in the caplacizumab arm and 14.4 days in the placebo arm (a 31% relative reduction).
For patients admitted to the ICU (28 in the caplacizumab arm and 27 in the placebo arm), the mean number of days in the ICU was 3.4 days in the caplacizumab arm and 9.7 days in the placebo arm (a 65% relative reduction).
Safety
“The safety profile [of caplacizumab] was comparable to previous results and in keeping with the mechanism of action,” Dr Scully said.
The proportion of patients with at least 1 treatment-emergent AE was 97.2% in the caplacizumab arm and 97.3% in the placebo arm.
The proportion of patients with at least 1 study-drug-related AE was 57.7% in the caplacizumab arm and 43.8% in the placebo arm. The rate of discontinuation due to at least 1 AE was 7.0% and 12.3%, respectively.
The incidence of bleeding-related AEs was higher in the caplacizumab arm (45.6%) than the placebo arm (23.3%).
Bleeding-related AEs (in the caplacizumab and placebo arms, respectively) included epistaxis (23.9% and 1.4%), gingival bleeding (11.3% and 0%), bruising (7.0% and 4.1%), hematuria (5.6% and 1.4%), vaginal hemorrhage (4.2% and 1.4%), menorrhagia (2.8% and 1.4%), catheter site hemorrhage (2.8% and 4.1%), injection site bruising (2.8% and 2.7%), hematochezia (2.8% and 0%), and hematoma (2.8% and 0%).
The proportion of patients with at least 1 serious AE was 39.4% (n=28) in the caplacizumab arm and 53.4% (n=39) in the placebo arm. The proportion of patients with at least 1 study-drug-related serious AE was 14.1% (n=10) and 5.5% (n=4), respectively.
During the treatment period, there were no deaths in the caplacizumab arm and 3 deaths in the placebo arm. There was 1 death in the caplacizumab arm during the follow-up period, but it was considered unrelated to caplacizumab. ![]()
ATLANTA—Caplacizumab can improve outcomes in patients with acquired thrombotic thrombocytopenic purpura (aTTP), according to research presented at the 2017 ASH Annual Meeting.
In the phase 3 HERCULES trial, researchers compared caplacizumab, an anti-von Willebrand factor nanobody, plus standard care (plasma exchange and immunosuppression) to placebo plus standard care in patients with aTTP.
Patients who received caplacizumab were significantly more likely to achieve platelet normalization and significantly less likely to experience aTTP-related death, aTTP recurrence, and major thromboembolic events.
Patients in the caplacizumab arm also required plasma exchange less frequently and spent less time in the hospital and intensive care unit (ICU).
Bleeding-related adverse events (AEs) were more common among patients who received caplacizumab than those who received placebo.
Marie Scully, MD, of the University College London Hospitals in London, UK, presented these results from HERCULES as a late-breaking abstract at the ASH Annual Meeting (abstract LBA-1). HERCULES was supported by Ablynx.
Patients and treatment
The study enrolled patients with an acute episode of aTTP. They were randomized to receive either caplacizumab (n=72) or placebo (n=73) in addition to standard care, which consisted of plasma exchange and immunosuppression.
Patients received a single intravenous bolus of 10 mg of caplacizumab or placebo followed by a daily subcutaneous dose of 10 mg of caplacizumab or placebo until 30 days after the last daily plasma exchange. If patients had a recurrence during the 30-day treatment period, they could go on to receive open-label caplacizumab.
If, at the end of the 30-day treatment period, there was evidence of persistent underlying disease activity indicative of an imminent risk for recurrence, caplacizumab or placebo could be extended for additional 7-day periods up to a maximum of 28 days. Patients were followed for a further 28 days after discontinuation of treatment.
In all, 71 patients received caplacizumab, and 58 (80.6%) of them completed the treatment. Seventy-three patients received placebo, and 50 of these patients (68.5%) completed treatment. Twenty-six patients in the placebo arm and 2 patients in the caplacizumab arm received open-label caplacizumab.
“If we look at the demographics, they’re relatively comparable to any data we normally see in patients with immune-mediated TTP,” Dr Scully said.
At baseline, the mean age was 44.9 in the caplacizumab arm and 47.3 in the placebo arm. Most patients in both arms were female—68.1% and 69.9%, respectively.
The proportion of patients with an initial aTTP episode was 66.7% in the caplacizumab arm and 46.6% in the placebo arm. The proportion with a recurrent episode was 33.3% and 53.4%, respectively.
Most patients in both arms had ADAMTS13 activity below 10% at baseline—81.7% in the caplacizumab arm and 90.3% in the placebo arm.
The mean platelet count at baseline was 32.0 x 109/L in the caplacizumab arm and 39.1 x 109/L in the placebo arm.
Efficacy
The study’s primary endpoint was the time to normalization of platelet count response, which was defined as initial platelet count of at least 150 x 109/L with subsequent stop of daily plasma exchange within 5 days.
There was a significant reduction in time to platelet count response in the caplacizumab arm compared to the placebo arm. The platelet normalization rate ratio was 1.55 (P<0.01).
“Patients were 55% more likely to achieve normalization of their platelet count at any time in the caplacizumab group, and this was highly significant,” Dr Scully said.
A secondary endpoint was the combination of aTTP-related death, aTTP recurrence, and at least 1 major thromboembolic event during study treatment. The incidence of this combined endpoint was 12.7% (n=9) in the caplacizumab arm and 49.3% (n=36) in the placebo arm (P<0.0001).
The incidence of aTTP-related death was 0% (n=0) in the caplacizumab arm and 4.1% (n=3) in the placebo arm. The incidence of aTTP recurrence was 4.2% (n=3) and 38.4% (n=28), respectively. And the incidence of at least 1 major thromboembolic event was 8.5% (n=6) and 8.2% (n=6), respectively.
The researchers also assessed aTTP recurrence during the overall study period, which occurred in 12.7% (n=9) of patients in the caplacizumab arm and 38.4% (n=28) in the placebo arm (P<0.001).
During the follow-up period, there were 6 relapses (9.1%) in the caplacizumab arm but none in the placebo arm.
“This tells us something about the pathophysiology of TTP and the role of caplacizumab,” Dr Scully said. “All of these patients, on stopping caplacizumab, had ADAMTS13 levels less than 5%. Therefore, it was important that their treatment was continued to ensure removal of antibody.”
According to the International TTP Working Group consensus definition, none of the patients in the caplacizumab arm and 7.0% (n=5) of patients in the placebo arm had refractory aTTP (P=0.018).
The mean number of days of plasma exchange during the overall treatment period was 5.8 days in the caplacizumab arm and 9.4 days in the placebo arm (a 38% relative reduction). The mean volume of plasma used was 21.3L and 35.9L, respectively (a 41% relative reduction).
The mean duration of hospital stay was 9.9 days in the caplacizumab arm and 14.4 days in the placebo arm (a 31% relative reduction).
For patients admitted to the ICU (28 in the caplacizumab arm and 27 in the placebo arm), the mean number of days in the ICU was 3.4 days in the caplacizumab arm and 9.7 days in the placebo arm (a 65% relative reduction).
Safety
“The safety profile [of caplacizumab] was comparable to previous results and in keeping with the mechanism of action,” Dr Scully said.
The proportion of patients with at least 1 treatment-emergent AE was 97.2% in the caplacizumab arm and 97.3% in the placebo arm.
The proportion of patients with at least 1 study-drug-related AE was 57.7% in the caplacizumab arm and 43.8% in the placebo arm. The rate of discontinuation due to at least 1 AE was 7.0% and 12.3%, respectively.
The incidence of bleeding-related AEs was higher in the caplacizumab arm (45.6%) than the placebo arm (23.3%).
Bleeding-related AEs (in the caplacizumab and placebo arms, respectively) included epistaxis (23.9% and 1.4%), gingival bleeding (11.3% and 0%), bruising (7.0% and 4.1%), hematuria (5.6% and 1.4%), vaginal hemorrhage (4.2% and 1.4%), menorrhagia (2.8% and 1.4%), catheter site hemorrhage (2.8% and 4.1%), injection site bruising (2.8% and 2.7%), hematochezia (2.8% and 0%), and hematoma (2.8% and 0%).
The proportion of patients with at least 1 serious AE was 39.4% (n=28) in the caplacizumab arm and 53.4% (n=39) in the placebo arm. The proportion of patients with at least 1 study-drug-related serious AE was 14.1% (n=10) and 5.5% (n=4), respectively.
During the treatment period, there were no deaths in the caplacizumab arm and 3 deaths in the placebo arm. There was 1 death in the caplacizumab arm during the follow-up period, but it was considered unrelated to caplacizumab. ![]()
Drug granted breakthrough status as first-line SAA therapy
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation to eltrombopag (Promacta®) for use in combination with standard immunosuppressive therapy as first-line treatment for patients with severe aplastic anemia (SAA).
Novartis plans to submit an application to the FDA for this indication later this year.
Eltrombopag is already FDA-approved to treat SAA patients who have had an insufficient response to immunosuppressive therapy.
The drug is also approved to treat patients with chronic immune thrombocytopenia who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy.
And eltrombopag is approved to treat thrombocytopenia in patients with chronic hepatitis C to allow for the initiation and maintenance of interferon-based therapy.
Trial data
The breakthrough designation for eltrombopag as first-line treatment in SAA is supported by data from a phase 1/2 trial, which were published in NEJM in April 2017.
The trial included 92 patients with previously untreated SAA. They received immunosuppressive therapy and eltrombopag in 3 different cohorts.
Patients in cohort 1 received eltrombopag from day 14 to 6 months. Patients in cohort 2 received the drug from day 14 to 3 months. And patients in cohort 3 received eltrombopag from day 1 to 6 months.
At 6 months, the overall response rate was 80% in cohort 1, 87% in cohort 2, and 94% in cohort 3. The rate of complete response at 6 months was 33%, 26%, and 58%, respectively.
At a median follow-up of 2 years, the overall survival rate was 97%.
Seven patients briefly stopped taking eltrombopag during the first 2 weeks due to transient elevations in liver-enzyme levels.
There were 2 severe adverse events that were related to eltrombopag and resulted in patients stopping the drug. These were a grade 2 and grade 3 cutaneous eruption.
About breakthrough designation
The FDA’s breakthrough designation is intended to expedite the development and review of new treatments for serious or life-threatening conditions.
The designation entitles the company developing a therapy to more intensive FDA guidance on an efficient and accelerated development program, as well as eligibility for other actions to expedite FDA review, such as rolling submission and priority review.
To earn breakthrough designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need. ![]()
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation to eltrombopag (Promacta®) for use in combination with standard immunosuppressive therapy as first-line treatment for patients with severe aplastic anemia (SAA).
Novartis plans to submit an application to the FDA for this indication later this year.
Eltrombopag is already FDA-approved to treat SAA patients who have had an insufficient response to immunosuppressive therapy.
The drug is also approved to treat patients with chronic immune thrombocytopenia who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy.
And eltrombopag is approved to treat thrombocytopenia in patients with chronic hepatitis C to allow for the initiation and maintenance of interferon-based therapy.
Trial data
The breakthrough designation for eltrombopag as first-line treatment in SAA is supported by data from a phase 1/2 trial, which were published in NEJM in April 2017.
The trial included 92 patients with previously untreated SAA. They received immunosuppressive therapy and eltrombopag in 3 different cohorts.
Patients in cohort 1 received eltrombopag from day 14 to 6 months. Patients in cohort 2 received the drug from day 14 to 3 months. And patients in cohort 3 received eltrombopag from day 1 to 6 months.
At 6 months, the overall response rate was 80% in cohort 1, 87% in cohort 2, and 94% in cohort 3. The rate of complete response at 6 months was 33%, 26%, and 58%, respectively.
At a median follow-up of 2 years, the overall survival rate was 97%.
Seven patients briefly stopped taking eltrombopag during the first 2 weeks due to transient elevations in liver-enzyme levels.
There were 2 severe adverse events that were related to eltrombopag and resulted in patients stopping the drug. These were a grade 2 and grade 3 cutaneous eruption.
About breakthrough designation
The FDA’s breakthrough designation is intended to expedite the development and review of new treatments for serious or life-threatening conditions.
The designation entitles the company developing a therapy to more intensive FDA guidance on an efficient and accelerated development program, as well as eligibility for other actions to expedite FDA review, such as rolling submission and priority review.
To earn breakthrough designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need. ![]()
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation to eltrombopag (Promacta®) for use in combination with standard immunosuppressive therapy as first-line treatment for patients with severe aplastic anemia (SAA).
Novartis plans to submit an application to the FDA for this indication later this year.
Eltrombopag is already FDA-approved to treat SAA patients who have had an insufficient response to immunosuppressive therapy.
The drug is also approved to treat patients with chronic immune thrombocytopenia who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy.
And eltrombopag is approved to treat thrombocytopenia in patients with chronic hepatitis C to allow for the initiation and maintenance of interferon-based therapy.
Trial data
The breakthrough designation for eltrombopag as first-line treatment in SAA is supported by data from a phase 1/2 trial, which were published in NEJM in April 2017.
The trial included 92 patients with previously untreated SAA. They received immunosuppressive therapy and eltrombopag in 3 different cohorts.
Patients in cohort 1 received eltrombopag from day 14 to 6 months. Patients in cohort 2 received the drug from day 14 to 3 months. And patients in cohort 3 received eltrombopag from day 1 to 6 months.
At 6 months, the overall response rate was 80% in cohort 1, 87% in cohort 2, and 94% in cohort 3. The rate of complete response at 6 months was 33%, 26%, and 58%, respectively.
At a median follow-up of 2 years, the overall survival rate was 97%.
Seven patients briefly stopped taking eltrombopag during the first 2 weeks due to transient elevations in liver-enzyme levels.
There were 2 severe adverse events that were related to eltrombopag and resulted in patients stopping the drug. These were a grade 2 and grade 3 cutaneous eruption.
About breakthrough designation
The FDA’s breakthrough designation is intended to expedite the development and review of new treatments for serious or life-threatening conditions.
The designation entitles the company developing a therapy to more intensive FDA guidance on an efficient and accelerated development program, as well as eligibility for other actions to expedite FDA review, such as rolling submission and priority review.
To earn breakthrough designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need. ![]()