User login
Endovenous thermal ablation and thrombotic complications
“CLINICAL CORRELATION OF SUCCESS AND ACUTE THROMBOTIC COMPLICATIONS OF LOWER EXTREMITY ENDOVENOUS THERMAL ABLATION.” Journal of Vascular Surgery Venous and Lymphatic Disorders, January 2018
A large single center experience with endovenous thermal ablation reveals risk factors for thrombotic complications.
Minimally invasive techniques for treating reflux disease in the saphenous system have greatly improved the quality of life and comfort of those suffering with chronic venous disease and more advanced venous insufficiency. Painful procedures of the past, sometimes including hospital stays, have largely been replaced by safe and efficacious office procedures (lasting often less than an hour) with minimal subsequent activity restrictions.
Despite these obvious advantages, these therapies do have a very low but definite risk of thrombotic complications, including endovenous heat-induced thrombosis (EHIT) superficial venous thrombosis (SVT) and deep vein thrombosis (DVT). EHIT includes development of a blood clot at the junction of one of the treated saphenous veins and the femoral or the popliteal vein.
While major DVT and pulmonary embolism are extremely rare, the diagnosis of EHIT may require a period of anticoagulation as well as follow-up visits and studies. Further, acute SVT can be painful for several weeks following the procedure. As such, further understanding the risk factors for these complications will allow therapists to better inform patients as to their specific risks for developing them.
As reported in the January 2018 edition of the Journal of Vascular Surgery: Venous and Lymphatic Disorders, researchers from Total Vascular Care and NYU Lutheran Medical Center led by Afsha Aurshina, MBBS, evaluated their large single center experience treating multiple vein types using both radiofrequency (RFA) and endovenous laser (EVLA) ablation techniques. They retrospectively studied the outcomes of 1811 procedures performed on 808 patients from 2012-2014. The aim of the study was to define better the success and thrombotic complications of these procedures with respect to technique and vein type.
Overall success (defined as absence of reflux in the targeted vein by post-operative duplex) rates included:
- RFA 98.4% (excluding perforating vein)
- EVLA 98.1%
- Great saphenous (GSV) 98.5%
- Lesser saphenous (LSV) 98.2%
- Accessory saphenous (ASV) 97.2%
- Perforator (PV) 82.4%
With regards to thrombotic complications, the authors reported EHIT rates of:
- Class 1-4 5.9%
- Class 2-4 1.16%
Acute superficial thrombosis rates included:
- Overall 4.6%
- RFA 7.7%
- EVLA 11.4% (no difference in multi-factor analysis)
- GSV 11.8%
- LSV 5.5%
- ASV 6.5%
- PV 2.4%
“Our study demonstrates that there is no significant difference in the success rate of RFA and EVLA in the treatment of venous reflux for GSV, SSV, and ASV,” notes first author Aurshina. “We found an acceptably low incidence of clinically significant thrombotic complication rates for EHIT and acute superficial thrombosis, with only a 1.16% risk of Class 2-4 EHIT, that may require short term anticoagulation. We noted risk factors for these complications, after multi-factor analysis, include higher vein diameter and type of vein, with the latter being the most important.”
Large experiences such as these are important to understand the true incidence of these complications and how practitioners might tailor their consent process with their patients.
To download the complete article (link available free from 12/14/2017 through 2/28/2018),
click: http://vsweb.org/JVSVL-EVTA
For information your patients may be interested in, click:
Regarding Varicose Veins:
https://vascular.org/patient-resources/vascular-conditions/varicose-veins
Regarding Deep Venous Thrombosis:
https://vascular.org/patient-resources/vascular-conditions/deep-vein-thrombosis
“CLINICAL CORRELATION OF SUCCESS AND ACUTE THROMBOTIC COMPLICATIONS OF LOWER EXTREMITY ENDOVENOUS THERMAL ABLATION.” Journal of Vascular Surgery Venous and Lymphatic Disorders, January 2018
A large single center experience with endovenous thermal ablation reveals risk factors for thrombotic complications.
Minimally invasive techniques for treating reflux disease in the saphenous system have greatly improved the quality of life and comfort of those suffering with chronic venous disease and more advanced venous insufficiency. Painful procedures of the past, sometimes including hospital stays, have largely been replaced by safe and efficacious office procedures (lasting often less than an hour) with minimal subsequent activity restrictions.
Despite these obvious advantages, these therapies do have a very low but definite risk of thrombotic complications, including endovenous heat-induced thrombosis (EHIT) superficial venous thrombosis (SVT) and deep vein thrombosis (DVT). EHIT includes development of a blood clot at the junction of one of the treated saphenous veins and the femoral or the popliteal vein.
While major DVT and pulmonary embolism are extremely rare, the diagnosis of EHIT may require a period of anticoagulation as well as follow-up visits and studies. Further, acute SVT can be painful for several weeks following the procedure. As such, further understanding the risk factors for these complications will allow therapists to better inform patients as to their specific risks for developing them.
As reported in the January 2018 edition of the Journal of Vascular Surgery: Venous and Lymphatic Disorders, researchers from Total Vascular Care and NYU Lutheran Medical Center led by Afsha Aurshina, MBBS, evaluated their large single center experience treating multiple vein types using both radiofrequency (RFA) and endovenous laser (EVLA) ablation techniques. They retrospectively studied the outcomes of 1811 procedures performed on 808 patients from 2012-2014. The aim of the study was to define better the success and thrombotic complications of these procedures with respect to technique and vein type.
Overall success (defined as absence of reflux in the targeted vein by post-operative duplex) rates included:
- RFA 98.4% (excluding perforating vein)
- EVLA 98.1%
- Great saphenous (GSV) 98.5%
- Lesser saphenous (LSV) 98.2%
- Accessory saphenous (ASV) 97.2%
- Perforator (PV) 82.4%
With regards to thrombotic complications, the authors reported EHIT rates of:
- Class 1-4 5.9%
- Class 2-4 1.16%
Acute superficial thrombosis rates included:
- Overall 4.6%
- RFA 7.7%
- EVLA 11.4% (no difference in multi-factor analysis)
- GSV 11.8%
- LSV 5.5%
- ASV 6.5%
- PV 2.4%
“Our study demonstrates that there is no significant difference in the success rate of RFA and EVLA in the treatment of venous reflux for GSV, SSV, and ASV,” notes first author Aurshina. “We found an acceptably low incidence of clinically significant thrombotic complication rates for EHIT and acute superficial thrombosis, with only a 1.16% risk of Class 2-4 EHIT, that may require short term anticoagulation. We noted risk factors for these complications, after multi-factor analysis, include higher vein diameter and type of vein, with the latter being the most important.”
Large experiences such as these are important to understand the true incidence of these complications and how practitioners might tailor their consent process with their patients.
To download the complete article (link available free from 12/14/2017 through 2/28/2018),
click: http://vsweb.org/JVSVL-EVTA
For information your patients may be interested in, click:
Regarding Varicose Veins:
https://vascular.org/patient-resources/vascular-conditions/varicose-veins
Regarding Deep Venous Thrombosis:
https://vascular.org/patient-resources/vascular-conditions/deep-vein-thrombosis
“CLINICAL CORRELATION OF SUCCESS AND ACUTE THROMBOTIC COMPLICATIONS OF LOWER EXTREMITY ENDOVENOUS THERMAL ABLATION.” Journal of Vascular Surgery Venous and Lymphatic Disorders, January 2018
A large single center experience with endovenous thermal ablation reveals risk factors for thrombotic complications.
Minimally invasive techniques for treating reflux disease in the saphenous system have greatly improved the quality of life and comfort of those suffering with chronic venous disease and more advanced venous insufficiency. Painful procedures of the past, sometimes including hospital stays, have largely been replaced by safe and efficacious office procedures (lasting often less than an hour) with minimal subsequent activity restrictions.
Despite these obvious advantages, these therapies do have a very low but definite risk of thrombotic complications, including endovenous heat-induced thrombosis (EHIT) superficial venous thrombosis (SVT) and deep vein thrombosis (DVT). EHIT includes development of a blood clot at the junction of one of the treated saphenous veins and the femoral or the popliteal vein.
While major DVT and pulmonary embolism are extremely rare, the diagnosis of EHIT may require a period of anticoagulation as well as follow-up visits and studies. Further, acute SVT can be painful for several weeks following the procedure. As such, further understanding the risk factors for these complications will allow therapists to better inform patients as to their specific risks for developing them.
As reported in the January 2018 edition of the Journal of Vascular Surgery: Venous and Lymphatic Disorders, researchers from Total Vascular Care and NYU Lutheran Medical Center led by Afsha Aurshina, MBBS, evaluated their large single center experience treating multiple vein types using both radiofrequency (RFA) and endovenous laser (EVLA) ablation techniques. They retrospectively studied the outcomes of 1811 procedures performed on 808 patients from 2012-2014. The aim of the study was to define better the success and thrombotic complications of these procedures with respect to technique and vein type.
Overall success (defined as absence of reflux in the targeted vein by post-operative duplex) rates included:
- RFA 98.4% (excluding perforating vein)
- EVLA 98.1%
- Great saphenous (GSV) 98.5%
- Lesser saphenous (LSV) 98.2%
- Accessory saphenous (ASV) 97.2%
- Perforator (PV) 82.4%
With regards to thrombotic complications, the authors reported EHIT rates of:
- Class 1-4 5.9%
- Class 2-4 1.16%
Acute superficial thrombosis rates included:
- Overall 4.6%
- RFA 7.7%
- EVLA 11.4% (no difference in multi-factor analysis)
- GSV 11.8%
- LSV 5.5%
- ASV 6.5%
- PV 2.4%
“Our study demonstrates that there is no significant difference in the success rate of RFA and EVLA in the treatment of venous reflux for GSV, SSV, and ASV,” notes first author Aurshina. “We found an acceptably low incidence of clinically significant thrombotic complication rates for EHIT and acute superficial thrombosis, with only a 1.16% risk of Class 2-4 EHIT, that may require short term anticoagulation. We noted risk factors for these complications, after multi-factor analysis, include higher vein diameter and type of vein, with the latter being the most important.”
Large experiences such as these are important to understand the true incidence of these complications and how practitioners might tailor their consent process with their patients.
To download the complete article (link available free from 12/14/2017 through 2/28/2018),
click: http://vsweb.org/JVSVL-EVTA
For information your patients may be interested in, click:
Regarding Varicose Veins:
https://vascular.org/patient-resources/vascular-conditions/varicose-veins
Regarding Deep Venous Thrombosis:
https://vascular.org/patient-resources/vascular-conditions/deep-vein-thrombosis
Register for VRIC; Abstracts due Jan. 10
Registration is now open for the Vascular Research Initiatives Conference, to be held Thursday, May 9, in San Francisco. Abstracts for VRIC are due Wednesday, Jan. 10. Learn more about VRIC, and submit your abstracts here.
Registration is now open for the Vascular Research Initiatives Conference, to be held Thursday, May 9, in San Francisco. Abstracts for VRIC are due Wednesday, Jan. 10. Learn more about VRIC, and submit your abstracts here.
Registration is now open for the Vascular Research Initiatives Conference, to be held Thursday, May 9, in San Francisco. Abstracts for VRIC are due Wednesday, Jan. 10. Learn more about VRIC, and submit your abstracts here.
JVS Access Expires Jan. 15 for Those Who Haven’t Paid Dues
Have you put off paying your 2018 SVS membership dues? Don’t wait too much longer! Access to the Journal of Vascular Surgery suite of publications expires on Jan. 15 for those who have not yet paid their 2018 dues. Renew today.
Have you put off paying your 2018 SVS membership dues? Don’t wait too much longer! Access to the Journal of Vascular Surgery suite of publications expires on Jan. 15 for those who have not yet paid their 2018 dues. Renew today.
Have you put off paying your 2018 SVS membership dues? Don’t wait too much longer! Access to the Journal of Vascular Surgery suite of publications expires on Jan. 15 for those who have not yet paid their 2018 dues. Renew today.
VAM Abstract Deadline Approaches
Abstracts are due Wednesday, Jan. 17, for the 2018 Vascular Annual Meeting, set for June 20-23 in Boston. Guidelines, submission policies and general information on VAM are available online. VAM plenaries are June 21-23 and exhibits are June 21-22. Registration and housing will open in early March.
Abstracts are due Wednesday, Jan. 17, for the 2018 Vascular Annual Meeting, set for June 20-23 in Boston. Guidelines, submission policies and general information on VAM are available online. VAM plenaries are June 21-23 and exhibits are June 21-22. Registration and housing will open in early March.
Abstracts are due Wednesday, Jan. 17, for the 2018 Vascular Annual Meeting, set for June 20-23 in Boston. Guidelines, submission policies and general information on VAM are available online. VAM plenaries are June 21-23 and exhibits are June 21-22. Registration and housing will open in early March.
Regenerative Medicine in Cosmetic Dermatology
Regenerative medicine encompasses innovative therapies that allow the body to repair or regenerate aging cells, tissues, and organs. The skin is a particularly attractive organ for the application of novel regenerative therapies due to its easy accessibility. Among these therapies, stem cells and platelet-rich plasma (PRP) have garnered interest based on their therapeutic potential in scar reduction, antiaging effects, and treatment of alopecia.
Stem cells possess the cardinal features of self-renewal and plasticity. Self-renewal refers to symmetric cell division generating daughter cells identical to the parent cell.1 Plasticity is the ability to generate cell types other than the germ line or tissue lineage from which stem cells derive.2 Stem cells can be categorized according to their differentiation potential. Totipotent stem cells may develop into any primary germ cell layer (ectoderm, mesoderm, endoderm) of the embryo, as well as extraembryonic tissue such as the trophoblast, which gives rise to the placenta. Pluripotent stem cells such as embryonic stem cells have the capacity to differentiate into any derivative of the 3 germ cell layers but have lost their ability to differentiate into the trophoblast.3 Adults lack totipotent or pluripotent cells; they have multipotent or unipotent cells. Multipotent stem cells are able to differentiate into multiple cell types from similar lineages; mesenchymal stem cells (MSCs), for example, can differentiate into adipogenic, osteogenic, chondrogenic, and myogenic cells.4 Unipotent stem cells have the lowest differentiation potential and can only self-regenerate. Herein, we review stem cell sources and their therapeutic potential in aesthetic dermatology.
Multipotent Stem Cells
Multipotent stem cells derived from the bone marrow, umbilical cord, adipose tissue, dermis, or hair follicle bulge have various clinical applications in dermatology. Stem cells from these sources are primarily utilized in an autologous manner in which they are processed outside the body and reintroduced into the donor. Autologous multipotent hematopoietic bone marrow cells were first successfully used for the treatment of chronic wounds and show promise for the treatment of atrophic scars.5,6 However, due to the invasive nature of extracting bone marrow stem cells and their declining number with age, other sources of multipotent stem cells have fallen into favor.
Umbilical cord blood is a source of multipotent hematopoietic stem cells for which surgical intervention is not necessary because they are retrieved after umbilical cord clamping.7 Advantages of sourcing stem cells from umbilical cord blood includes high regenerative power compared to a newborn’s skin and low immunogenicity given that the newborn is immunologically immature.8
Another popular source for autologous stem cells is adipose tissue due to its ease of accessibility and relative abundance. Given that adipose tissue–derived stem cells (ASCs) are capable of differentiating into adipocytes that help maintain volume over time, they are being used for midface contouring, lip augmentation, facial rejuvenation, facial scarring, lipodystrophy, penile girth enhancement, and vaginal augmentation. Adipose tissue–derived stem cells also are capable of differentiating into other types of tissue, including cartilage and bone. Thus, they have been successfully harnessed in the treatment of patients affected by systemic sclerosis and Parry-Romberg syndrome as well in the functional and aesthetic reconstruction of various military combat–related deformities.9,10
Adipose tissue–derived stem cells are commonly harvested from lipoaspirate of the abdomen and are combined with supportive mechanical scaffolds such as hydrogels. Lipoaspirate itself can serve as a scaffold for ASCs. Accordingly, ASCs also are being utilized as a scaffold for autologous fat transfer procedures in an effort to increase the viability of transplanted donor tissue, a process known as cell-assisted lipotransfer (CAL). In CAL, a fraction of the aspirated fat is processed for isolation of ASCs, which are then recombined with the remainder of the aspirated fat prior to grafting.11 However, there is conflicting evidence as to whether CAL leads to improved graft success relative to conventional autologous fat transfer.12,13
The skin also serves as an easily accessible and abundant autologous source of stem cells. A subtype of dermal fibroblasts has been proven to have multipotent potential.14,15 These dermal fibroblasts are harvested from one area of the skin using punch biopsy and are processed and reinjected into another desired area of the skin.16 Autologous human fibroblasts have proven to be effective for the treatment of wrinkles, rhytides, and acne scars.17 In June 2011, the US Food and Drug Administration approved azficel-T, an autologous cellular product created by harvesting fibroblasts from a patient’s own postauricular skin, culture-expanding them in vitro for 3 months, and reinjecting the cells into the desired area of dermis in a series of treatments. This product was the first personalized cell therapy approved by the US Food and Drug Administration for aesthetic uses, specifically for the improvement of nasolabial fold wrinkles.18
In adults, hair follicles contain an area known as the bulge, which is a site rich in epithelial and melanocytic stem cells. Bulge stem cells have the ability to reproduce the interfollicular epidermis, hair follicle structures, and sebaceous glands, and they have been used to construct entirely new hair follicles in an artificial in vivo system.19 Sugiyama-Nakagiri et al20 demonstrated that an entire hair follicle epithelium and interfollicular epidermis can be regenerated using cultured bulge stem cells. The cultured bulge stem cells were mixed with dermal papilla cells from neonatal rat vibrissae and engrafted into a silicone chamber implanted on the backs of severe combined immune deficient (SCID) mice. The grafts exhibited tufts of hair as well as a complete interfollicular epidermis at 4 weeks after transplantation.20 Thus, these bulge stem cells have the potential to treat male androgenic alopecia and female pattern hair loss. Bulge stem cells also have been shown to accelerate wound healing.21 Additionally, autologous melanocytic stem cells located at the hair follicle bulge are effective for treating vitiligo and are being investigated for the treatment of hair graying.22
Induced Pluripotent Stem Cells
Given the ethical concerns that surround the procurement and use of embryonic stem cells, efforts have been made to retrieve pluripotent stem cells from adults. A major breakthrough occurred in 2006 when researchers altered the genes of specialized adult mouse cells to cause dedifferentiation and the return to an embryoniclike stem cell state.23 Mouse somatic cells were reprogrammed through the activation of a combination of transcription factors. The resulting cells were termed induced pluripotent stem cells (iPSCs) and have since been recreated in human cell lines. The discovery of iPSCs precipitated a translational science revolution. Physician-scientists sought ways to apply the reprogrammed cells to the pathophysiology of obscure diseases, examination of drug targets, and regeneration of human tissue.24 Tissue regeneration via induced naïve somatic cells has shown promise as a future method to treat neurologic, cardiovascular, and ophthalmologic diseases.25
As the technology of cultivating and identifying optimal sources of iPSCs continues to advance, stem cell–based treatments have evolved as leading prospects in the field of biogerontology.26-29 Although much of the research in antiaging medicine has utilized iPSCs to reprogram cell senescence, the altering of iPSCs at a cellular level also allows for the stimulation of collagen synthesis. This potential for collagen generation may have direct applicability in dermatologic practice, particularly for aesthetic treatments.
Much of the research into iPSC-derived collagen has focused on genodermatoses. Itoh et al30 examined the creation of collagen through iPSCs to identify possible treatments for recessive dystrophic epidermolysis bullosa (DEB). Recessive DEB is characterized by mutations in the COL7A1 gene, which encodes type VII collagen, a basement membrane protein and component of the anchoring fibrils essential for skin integrity.31 Itoh et al30 began with source cells obtained from a skin biopsy. The cells were dedifferentiated to iPSCs and then induced into dermal fibroblasts according to the methods established in prior studies of embryonic stem cells, namely with the use of ascorbic acid and transforming growth factor b. The newly formed fibroblasts were determined to be functional based on their ability to synthesize mature type VII collagen.30 Once the viability of the iPSC-derived fibroblasts was confirmed in vitro, the cells were further tested through combination with human keratinocytes on SCID mice. The human keratinocytes grew together with the iPSC-derived fibroblasts, producing type VII collagen in the basement membrane zone and creating an epidermis with the normal markers.30 Similarly, Robbins et al32 utilized SCID mice to successfully demonstrate that the transfection of keratinocytes from patients with junctional epidermolysis bullosa into SCID mice produced phenotypically normal skin.
Sebastiano et al33 combined the concepts of iPSCs and genome editing in another study of recessive DEB. The investigators first cultured iPSCs from biopsies of affected patients. After deriving iPSCs and correcting their mutation via adenovirus-associated viral gene editing, the COL7A1 mutation-free cells were differentiated into keratinocytes. These iPSC-derived keratinocytes were subsequently grafted onto mice, which led to the production of wild-type collagen VII and a stratified epidermis. Despite this successful outcome, the grafts of iPSC-derived epidermis did not survive longer than 1 month.33
One of the many obstacles facing the practical use of stem cells is their successful incorporation into human tissue. A possible solution was uncovered by Zhang et al34 who examined iPSC-derived MSCs. Mesenchymal stem cells communicate via paracrine mechanisms, whereby exosomes containing RNA and proteins are released to potentiate a regenerative effect.35 Zhang et al34 found that injecting exosomes from human iPSC-derived MSCs into the wound sites of rats stimulated the production of type I collagen, type III collagen, and elastin. The wound sites demonstrated accelerated closure, narrower scar widths, and increased collagen maturity.
Understanding the role that local environment plays in stem cell differentiation, Xu et al36 aimed to create an extracellular scaffold to induce fibroblast behavior from iPSCs. The authors engineered a framework similar to the normal extracellular membrane using proteoglycans, glycosaminoglycans, fibrinogen, and connective tissue growth factor. The iPSCs were then applied to the scaffolding, which led to successful fibroblast differentiation and type I collagen synthesis.36 This use of local biosignaling cues holds important ramifications for controlling the fate of stem cells that have been introduced into a new environment.
Although the application of iPSCs in clinical dermatology has yet to be achieved, progress in the field is moving at a rapid pace. Several logistical elements require further mastery before therapeutics can be delivered. These areas include the optimal environment for iPSC differentiation, methods for maximization of graft survival, and different modes of transplanting iPSC-derived cells into patients. In cosmetic practice, success will depend on intradermal injections of collagen-producing iPSC-derived cells that possess long-term proliferative potential. Current research in mice models has demonstrated viability up to 16 weeks after intradermal injection of such cells.37
Plant Stem Cells
In discussing the dermatologic applications of stem cell technology, clinicians should be aware of the plant stem cell products that have become a popular cosmeceutical trend. Companies advertise plant cells as a natural source of regenerative cells that can induce rejuvenation in human skin; however, there are no significant data to indicate that plant stem cells encourage or activate cellular growth in humans. Indeed, for stem cells to differentiate and produce viable components, the cells must first be incorporated as living components in the host tissue. Because plant stem cells do not survive in human tissue and plant cell cytokines fail to interact with the receptors on human cells, their current value in cosmeceuticals may be overstated.
Platelet-Rich Plasma
Platelet-rich plasma also is commonly associated with stem cell therapy, as PRP is known to potentiate stem cell proliferation, migration, and differentiation. However, PRP does not contain stem cells and is instead autologous plasma concentrated with platelets. In fact, platelets cannot even be classified as cells given that they lack a nucleus; platelets are considered cell fragments. The regenerative potential of PRP can be attributed to the growth factors released from platelets, which play an important role in tissue regeneration and repair. Platelet-rich plasma currently is being used in dermatology for skin rejuvenation (reduction of wrinkles and furrows) and treatment of acne scars.38 There also is evidence supporting the effectiveness of PRP for alopecia and wound therapy, as growth factors play a vital role in hair growth and wound healing.38 Apart from the use of PRP on its own, it can be used as a supplement to enhance the effects of antiaging procedures such as microneedling.39
Future Directions
Multipotent stem cells and iPSCs discussed herein provide much promise in the field of regenerative dermatology. They are increasingly accessible and circumvent the use of ethically questionable embryonic stem cells. Although there is a general consensus on the great potential of stem cells for treating aesthetic skin conditions, high-quality randomized controlled trials remain scarce within the literature. Recognizing and optimizing these opportunities remains the next step in the future delivery of evidence-based care in regenerative dermatology.
- Thomas ED, Lochte HL, Lu WC, et al. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957;257:491-496.
- Ogliari KS, Marinowic D, Brum DE, et al. Stem cells in dermatology. An Bras Dermatol. 2014;89:286-291.
- Xu C, Inokuma MS, Denham J, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971-974.
- Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211-228.
- Badiavas EV, Falanga V. Treatment of chronic wounds with bone marrow-derived cells. Arch Dermatol. 2003;139:510-516.
- Ibrahim ZA, Eltatawy RA, Ghaly NR, et al. Autologous bone marrow stem cells in atrophic acne scars: a pilot study. J Dermatolog Treat. 2015;26:260-265.
- Broxmeyer HE, Douglas GW, Hangoc G, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci U S A. 1989;86:3828-3832.
- Gluckman E, Rocha V, Boyer-Chammard A, et al. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. N Engl J Med. 1997;337:373-381.
- Valerio IL, Sabino JM, Dearth CL. Plastic surgery challenges in war wounded II: regenerative medicine. Adv Wound Care (New Rochelle). 2016;5:412-419.
- Vescarelli E, D’Amici S, Onesti MG, et al. Adipose-derived stem cell: an innovative therapeutic approach in systemic sclerosis and Parry-Romberg syndrome. CellR4. 2014;2:E791-E797.
- Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008;32:48-55.
- Grabin S, Antes G, Stark GB, et al. Cell-assisted lipotransfer: a critical appraisal of the evidence. Dtsch Arztebl Int. 2015;112:255.
- Zhou Y, Wang J, Li H, et al. Efficacy and safety of cell-assisted lipotransfer: a systematic review and meta-analysis. Plast Reconstr Surg. 2016;137:E44-E57.
- Toma JG, Akhavan M, Fernandes KJL, et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778-784.
- Toma JG, McKenzie IA, Bagli D, et al. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005;23:727-737.
- Homicz MR, Watson D. Review of injectable materials for soft tissue augmentation. Facial Plast Surg. 2004;20:21-29.
- Kumar S, Mahajan BB, Kaur S, et al. Autologous therapies in dermatology. J Clin Aesthet Dermatol. 2014;7:38-45.
- Schmidt C. FDA approves first cell therapy for wrinkle-free visage. Nat Biotech. 2011;29:674-675.
- Gentile P, Scioli MG, Bielli A, et al. Stem cells from human hair follicles: first mechanical isolation for immediate autologous clinical use in androgenetic alopecia and hair loss. Stem Cell Investig. 2017;4:58.
- Sugiyama-Nakagiri Y, Akiyama M, Shimizu H. Hair follicle stem cell-targeted gene transfer and reconstitution system. Gene Ther. 2006;13:732-737.
- Heidari F, Yari A, Rasoolijazi H, et al. Bulge hair follicle stem cells accelerate cutaneous wound healing in rats. Wounds. 2016;28:132-141.
- Lee JH, Fisher DE. Melanocyte stem cells as potential therapeutics in skin disorders. Expert Opin Biol Ther. 2014;14:1-11.
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676.
- Singh VK, Kalsan M, Kumar N, et al. Induced pluripotent stem cells: applications in regenerative medicine, disease modeling, and drug discovery. Front Cell Dev Biol. 2015;3:2.
- Aoi T. 10th anniversary of iPS cells: The challenges that lie ahead. J Biochem. 2016;160:121-129.
- Lowry WE, Plath K. The many ways to make an iPS cell. Nat Biotechnol. 2008;26:1246-1248.
- Kim K, Doi A, Wen B, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285-290.
- Gafni O, Weinberger L, Mansour AA, et al. Derivation of novel human ground state naive pluripotent stem cells. Nature. 2013;504:282-286.
- Pareja-Galeano H, Sanchis-Gomar F, Pérez LM, et al. IPSCs-based anti-aging therapies: Recent discoveries and future challenges. Ageing Res Rev. 2016;27:37-41.
- Itoh M, Umegaki-Arao N, Guo Z, et al. Generation of 3D skin equivalents fully reconstituted from human induced pluripotent stem cells (iPSCs). PLoS One. 2013;8:e77673.
- Nyström A, Velati D, Mittapalli VR, et al. Collagen VII plays a dual role in wound healing. J Clin Invest. 2013;123:3498-3509.
- Robbins PB, Lin Q, Goodnough JB, et al. In vivo restoration of laminin 5 β3 expression and function in junctional epidermolysis bullosa. Proc Natl Acad Sci. 2001;98:5193-5198.
- Sebastiano V, Zhen HH, Haddad B, et al. Human COL7A1-corrected induced pluripotent stem cells for the treatment of recessive dystrophic epidermolysis bullosa. Sci Transl Med. 2014;6:264ra163.
- Zhang J, Guan J, Niu X, et al. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13:49.
- Pap E, Pállinger É, Pásztói M, et al. Highlights of a new type of intercellular communication: microvesicle-based information transfer. Inflamm Res. 2009;58:1-8.
- Xu R, Taskin MB, Rubert M, et al. hiPS-MSCs differentiation towards fibroblasts on a 3D ECM mimicking scaffold. Sci Rep. 2015;5:8480.
- Wenzel D, Bayerl J, Nyström A, et al. Genetically corrected iPSCs as cell therapy for recessive dystrophic epidermolysis bullosa. Sci Transl Med. 2014;6:264ra165.
- Bednarska K, Kieszek R, Domagała P, et al. The use of platelet-rich-plasma in aesthetic and regenerative medicine. MEDtube Science. 2015;2:8-15.
- Hashim PW, Levy Z, Cohen JL, et al. Microneedling therapy with and without platelet-rich plasma. Cutis. 2017;99:239-242.
Regenerative medicine encompasses innovative therapies that allow the body to repair or regenerate aging cells, tissues, and organs. The skin is a particularly attractive organ for the application of novel regenerative therapies due to its easy accessibility. Among these therapies, stem cells and platelet-rich plasma (PRP) have garnered interest based on their therapeutic potential in scar reduction, antiaging effects, and treatment of alopecia.
Stem cells possess the cardinal features of self-renewal and plasticity. Self-renewal refers to symmetric cell division generating daughter cells identical to the parent cell.1 Plasticity is the ability to generate cell types other than the germ line or tissue lineage from which stem cells derive.2 Stem cells can be categorized according to their differentiation potential. Totipotent stem cells may develop into any primary germ cell layer (ectoderm, mesoderm, endoderm) of the embryo, as well as extraembryonic tissue such as the trophoblast, which gives rise to the placenta. Pluripotent stem cells such as embryonic stem cells have the capacity to differentiate into any derivative of the 3 germ cell layers but have lost their ability to differentiate into the trophoblast.3 Adults lack totipotent or pluripotent cells; they have multipotent or unipotent cells. Multipotent stem cells are able to differentiate into multiple cell types from similar lineages; mesenchymal stem cells (MSCs), for example, can differentiate into adipogenic, osteogenic, chondrogenic, and myogenic cells.4 Unipotent stem cells have the lowest differentiation potential and can only self-regenerate. Herein, we review stem cell sources and their therapeutic potential in aesthetic dermatology.
Multipotent Stem Cells
Multipotent stem cells derived from the bone marrow, umbilical cord, adipose tissue, dermis, or hair follicle bulge have various clinical applications in dermatology. Stem cells from these sources are primarily utilized in an autologous manner in which they are processed outside the body and reintroduced into the donor. Autologous multipotent hematopoietic bone marrow cells were first successfully used for the treatment of chronic wounds and show promise for the treatment of atrophic scars.5,6 However, due to the invasive nature of extracting bone marrow stem cells and their declining number with age, other sources of multipotent stem cells have fallen into favor.
Umbilical cord blood is a source of multipotent hematopoietic stem cells for which surgical intervention is not necessary because they are retrieved after umbilical cord clamping.7 Advantages of sourcing stem cells from umbilical cord blood includes high regenerative power compared to a newborn’s skin and low immunogenicity given that the newborn is immunologically immature.8
Another popular source for autologous stem cells is adipose tissue due to its ease of accessibility and relative abundance. Given that adipose tissue–derived stem cells (ASCs) are capable of differentiating into adipocytes that help maintain volume over time, they are being used for midface contouring, lip augmentation, facial rejuvenation, facial scarring, lipodystrophy, penile girth enhancement, and vaginal augmentation. Adipose tissue–derived stem cells also are capable of differentiating into other types of tissue, including cartilage and bone. Thus, they have been successfully harnessed in the treatment of patients affected by systemic sclerosis and Parry-Romberg syndrome as well in the functional and aesthetic reconstruction of various military combat–related deformities.9,10
Adipose tissue–derived stem cells are commonly harvested from lipoaspirate of the abdomen and are combined with supportive mechanical scaffolds such as hydrogels. Lipoaspirate itself can serve as a scaffold for ASCs. Accordingly, ASCs also are being utilized as a scaffold for autologous fat transfer procedures in an effort to increase the viability of transplanted donor tissue, a process known as cell-assisted lipotransfer (CAL). In CAL, a fraction of the aspirated fat is processed for isolation of ASCs, which are then recombined with the remainder of the aspirated fat prior to grafting.11 However, there is conflicting evidence as to whether CAL leads to improved graft success relative to conventional autologous fat transfer.12,13
The skin also serves as an easily accessible and abundant autologous source of stem cells. A subtype of dermal fibroblasts has been proven to have multipotent potential.14,15 These dermal fibroblasts are harvested from one area of the skin using punch biopsy and are processed and reinjected into another desired area of the skin.16 Autologous human fibroblasts have proven to be effective for the treatment of wrinkles, rhytides, and acne scars.17 In June 2011, the US Food and Drug Administration approved azficel-T, an autologous cellular product created by harvesting fibroblasts from a patient’s own postauricular skin, culture-expanding them in vitro for 3 months, and reinjecting the cells into the desired area of dermis in a series of treatments. This product was the first personalized cell therapy approved by the US Food and Drug Administration for aesthetic uses, specifically for the improvement of nasolabial fold wrinkles.18
In adults, hair follicles contain an area known as the bulge, which is a site rich in epithelial and melanocytic stem cells. Bulge stem cells have the ability to reproduce the interfollicular epidermis, hair follicle structures, and sebaceous glands, and they have been used to construct entirely new hair follicles in an artificial in vivo system.19 Sugiyama-Nakagiri et al20 demonstrated that an entire hair follicle epithelium and interfollicular epidermis can be regenerated using cultured bulge stem cells. The cultured bulge stem cells were mixed with dermal papilla cells from neonatal rat vibrissae and engrafted into a silicone chamber implanted on the backs of severe combined immune deficient (SCID) mice. The grafts exhibited tufts of hair as well as a complete interfollicular epidermis at 4 weeks after transplantation.20 Thus, these bulge stem cells have the potential to treat male androgenic alopecia and female pattern hair loss. Bulge stem cells also have been shown to accelerate wound healing.21 Additionally, autologous melanocytic stem cells located at the hair follicle bulge are effective for treating vitiligo and are being investigated for the treatment of hair graying.22
Induced Pluripotent Stem Cells
Given the ethical concerns that surround the procurement and use of embryonic stem cells, efforts have been made to retrieve pluripotent stem cells from adults. A major breakthrough occurred in 2006 when researchers altered the genes of specialized adult mouse cells to cause dedifferentiation and the return to an embryoniclike stem cell state.23 Mouse somatic cells were reprogrammed through the activation of a combination of transcription factors. The resulting cells were termed induced pluripotent stem cells (iPSCs) and have since been recreated in human cell lines. The discovery of iPSCs precipitated a translational science revolution. Physician-scientists sought ways to apply the reprogrammed cells to the pathophysiology of obscure diseases, examination of drug targets, and regeneration of human tissue.24 Tissue regeneration via induced naïve somatic cells has shown promise as a future method to treat neurologic, cardiovascular, and ophthalmologic diseases.25
As the technology of cultivating and identifying optimal sources of iPSCs continues to advance, stem cell–based treatments have evolved as leading prospects in the field of biogerontology.26-29 Although much of the research in antiaging medicine has utilized iPSCs to reprogram cell senescence, the altering of iPSCs at a cellular level also allows for the stimulation of collagen synthesis. This potential for collagen generation may have direct applicability in dermatologic practice, particularly for aesthetic treatments.
Much of the research into iPSC-derived collagen has focused on genodermatoses. Itoh et al30 examined the creation of collagen through iPSCs to identify possible treatments for recessive dystrophic epidermolysis bullosa (DEB). Recessive DEB is characterized by mutations in the COL7A1 gene, which encodes type VII collagen, a basement membrane protein and component of the anchoring fibrils essential for skin integrity.31 Itoh et al30 began with source cells obtained from a skin biopsy. The cells were dedifferentiated to iPSCs and then induced into dermal fibroblasts according to the methods established in prior studies of embryonic stem cells, namely with the use of ascorbic acid and transforming growth factor b. The newly formed fibroblasts were determined to be functional based on their ability to synthesize mature type VII collagen.30 Once the viability of the iPSC-derived fibroblasts was confirmed in vitro, the cells were further tested through combination with human keratinocytes on SCID mice. The human keratinocytes grew together with the iPSC-derived fibroblasts, producing type VII collagen in the basement membrane zone and creating an epidermis with the normal markers.30 Similarly, Robbins et al32 utilized SCID mice to successfully demonstrate that the transfection of keratinocytes from patients with junctional epidermolysis bullosa into SCID mice produced phenotypically normal skin.
Sebastiano et al33 combined the concepts of iPSCs and genome editing in another study of recessive DEB. The investigators first cultured iPSCs from biopsies of affected patients. After deriving iPSCs and correcting their mutation via adenovirus-associated viral gene editing, the COL7A1 mutation-free cells were differentiated into keratinocytes. These iPSC-derived keratinocytes were subsequently grafted onto mice, which led to the production of wild-type collagen VII and a stratified epidermis. Despite this successful outcome, the grafts of iPSC-derived epidermis did not survive longer than 1 month.33
One of the many obstacles facing the practical use of stem cells is their successful incorporation into human tissue. A possible solution was uncovered by Zhang et al34 who examined iPSC-derived MSCs. Mesenchymal stem cells communicate via paracrine mechanisms, whereby exosomes containing RNA and proteins are released to potentiate a regenerative effect.35 Zhang et al34 found that injecting exosomes from human iPSC-derived MSCs into the wound sites of rats stimulated the production of type I collagen, type III collagen, and elastin. The wound sites demonstrated accelerated closure, narrower scar widths, and increased collagen maturity.
Understanding the role that local environment plays in stem cell differentiation, Xu et al36 aimed to create an extracellular scaffold to induce fibroblast behavior from iPSCs. The authors engineered a framework similar to the normal extracellular membrane using proteoglycans, glycosaminoglycans, fibrinogen, and connective tissue growth factor. The iPSCs were then applied to the scaffolding, which led to successful fibroblast differentiation and type I collagen synthesis.36 This use of local biosignaling cues holds important ramifications for controlling the fate of stem cells that have been introduced into a new environment.
Although the application of iPSCs in clinical dermatology has yet to be achieved, progress in the field is moving at a rapid pace. Several logistical elements require further mastery before therapeutics can be delivered. These areas include the optimal environment for iPSC differentiation, methods for maximization of graft survival, and different modes of transplanting iPSC-derived cells into patients. In cosmetic practice, success will depend on intradermal injections of collagen-producing iPSC-derived cells that possess long-term proliferative potential. Current research in mice models has demonstrated viability up to 16 weeks after intradermal injection of such cells.37
Plant Stem Cells
In discussing the dermatologic applications of stem cell technology, clinicians should be aware of the plant stem cell products that have become a popular cosmeceutical trend. Companies advertise plant cells as a natural source of regenerative cells that can induce rejuvenation in human skin; however, there are no significant data to indicate that plant stem cells encourage or activate cellular growth in humans. Indeed, for stem cells to differentiate and produce viable components, the cells must first be incorporated as living components in the host tissue. Because plant stem cells do not survive in human tissue and plant cell cytokines fail to interact with the receptors on human cells, their current value in cosmeceuticals may be overstated.
Platelet-Rich Plasma
Platelet-rich plasma also is commonly associated with stem cell therapy, as PRP is known to potentiate stem cell proliferation, migration, and differentiation. However, PRP does not contain stem cells and is instead autologous plasma concentrated with platelets. In fact, platelets cannot even be classified as cells given that they lack a nucleus; platelets are considered cell fragments. The regenerative potential of PRP can be attributed to the growth factors released from platelets, which play an important role in tissue regeneration and repair. Platelet-rich plasma currently is being used in dermatology for skin rejuvenation (reduction of wrinkles and furrows) and treatment of acne scars.38 There also is evidence supporting the effectiveness of PRP for alopecia and wound therapy, as growth factors play a vital role in hair growth and wound healing.38 Apart from the use of PRP on its own, it can be used as a supplement to enhance the effects of antiaging procedures such as microneedling.39
Future Directions
Multipotent stem cells and iPSCs discussed herein provide much promise in the field of regenerative dermatology. They are increasingly accessible and circumvent the use of ethically questionable embryonic stem cells. Although there is a general consensus on the great potential of stem cells for treating aesthetic skin conditions, high-quality randomized controlled trials remain scarce within the literature. Recognizing and optimizing these opportunities remains the next step in the future delivery of evidence-based care in regenerative dermatology.
Regenerative medicine encompasses innovative therapies that allow the body to repair or regenerate aging cells, tissues, and organs. The skin is a particularly attractive organ for the application of novel regenerative therapies due to its easy accessibility. Among these therapies, stem cells and platelet-rich plasma (PRP) have garnered interest based on their therapeutic potential in scar reduction, antiaging effects, and treatment of alopecia.
Stem cells possess the cardinal features of self-renewal and plasticity. Self-renewal refers to symmetric cell division generating daughter cells identical to the parent cell.1 Plasticity is the ability to generate cell types other than the germ line or tissue lineage from which stem cells derive.2 Stem cells can be categorized according to their differentiation potential. Totipotent stem cells may develop into any primary germ cell layer (ectoderm, mesoderm, endoderm) of the embryo, as well as extraembryonic tissue such as the trophoblast, which gives rise to the placenta. Pluripotent stem cells such as embryonic stem cells have the capacity to differentiate into any derivative of the 3 germ cell layers but have lost their ability to differentiate into the trophoblast.3 Adults lack totipotent or pluripotent cells; they have multipotent or unipotent cells. Multipotent stem cells are able to differentiate into multiple cell types from similar lineages; mesenchymal stem cells (MSCs), for example, can differentiate into adipogenic, osteogenic, chondrogenic, and myogenic cells.4 Unipotent stem cells have the lowest differentiation potential and can only self-regenerate. Herein, we review stem cell sources and their therapeutic potential in aesthetic dermatology.
Multipotent Stem Cells
Multipotent stem cells derived from the bone marrow, umbilical cord, adipose tissue, dermis, or hair follicle bulge have various clinical applications in dermatology. Stem cells from these sources are primarily utilized in an autologous manner in which they are processed outside the body and reintroduced into the donor. Autologous multipotent hematopoietic bone marrow cells were first successfully used for the treatment of chronic wounds and show promise for the treatment of atrophic scars.5,6 However, due to the invasive nature of extracting bone marrow stem cells and their declining number with age, other sources of multipotent stem cells have fallen into favor.
Umbilical cord blood is a source of multipotent hematopoietic stem cells for which surgical intervention is not necessary because they are retrieved after umbilical cord clamping.7 Advantages of sourcing stem cells from umbilical cord blood includes high regenerative power compared to a newborn’s skin and low immunogenicity given that the newborn is immunologically immature.8
Another popular source for autologous stem cells is adipose tissue due to its ease of accessibility and relative abundance. Given that adipose tissue–derived stem cells (ASCs) are capable of differentiating into adipocytes that help maintain volume over time, they are being used for midface contouring, lip augmentation, facial rejuvenation, facial scarring, lipodystrophy, penile girth enhancement, and vaginal augmentation. Adipose tissue–derived stem cells also are capable of differentiating into other types of tissue, including cartilage and bone. Thus, they have been successfully harnessed in the treatment of patients affected by systemic sclerosis and Parry-Romberg syndrome as well in the functional and aesthetic reconstruction of various military combat–related deformities.9,10
Adipose tissue–derived stem cells are commonly harvested from lipoaspirate of the abdomen and are combined with supportive mechanical scaffolds such as hydrogels. Lipoaspirate itself can serve as a scaffold for ASCs. Accordingly, ASCs also are being utilized as a scaffold for autologous fat transfer procedures in an effort to increase the viability of transplanted donor tissue, a process known as cell-assisted lipotransfer (CAL). In CAL, a fraction of the aspirated fat is processed for isolation of ASCs, which are then recombined with the remainder of the aspirated fat prior to grafting.11 However, there is conflicting evidence as to whether CAL leads to improved graft success relative to conventional autologous fat transfer.12,13
The skin also serves as an easily accessible and abundant autologous source of stem cells. A subtype of dermal fibroblasts has been proven to have multipotent potential.14,15 These dermal fibroblasts are harvested from one area of the skin using punch biopsy and are processed and reinjected into another desired area of the skin.16 Autologous human fibroblasts have proven to be effective for the treatment of wrinkles, rhytides, and acne scars.17 In June 2011, the US Food and Drug Administration approved azficel-T, an autologous cellular product created by harvesting fibroblasts from a patient’s own postauricular skin, culture-expanding them in vitro for 3 months, and reinjecting the cells into the desired area of dermis in a series of treatments. This product was the first personalized cell therapy approved by the US Food and Drug Administration for aesthetic uses, specifically for the improvement of nasolabial fold wrinkles.18
In adults, hair follicles contain an area known as the bulge, which is a site rich in epithelial and melanocytic stem cells. Bulge stem cells have the ability to reproduce the interfollicular epidermis, hair follicle structures, and sebaceous glands, and they have been used to construct entirely new hair follicles in an artificial in vivo system.19 Sugiyama-Nakagiri et al20 demonstrated that an entire hair follicle epithelium and interfollicular epidermis can be regenerated using cultured bulge stem cells. The cultured bulge stem cells were mixed with dermal papilla cells from neonatal rat vibrissae and engrafted into a silicone chamber implanted on the backs of severe combined immune deficient (SCID) mice. The grafts exhibited tufts of hair as well as a complete interfollicular epidermis at 4 weeks after transplantation.20 Thus, these bulge stem cells have the potential to treat male androgenic alopecia and female pattern hair loss. Bulge stem cells also have been shown to accelerate wound healing.21 Additionally, autologous melanocytic stem cells located at the hair follicle bulge are effective for treating vitiligo and are being investigated for the treatment of hair graying.22
Induced Pluripotent Stem Cells
Given the ethical concerns that surround the procurement and use of embryonic stem cells, efforts have been made to retrieve pluripotent stem cells from adults. A major breakthrough occurred in 2006 when researchers altered the genes of specialized adult mouse cells to cause dedifferentiation and the return to an embryoniclike stem cell state.23 Mouse somatic cells were reprogrammed through the activation of a combination of transcription factors. The resulting cells were termed induced pluripotent stem cells (iPSCs) and have since been recreated in human cell lines. The discovery of iPSCs precipitated a translational science revolution. Physician-scientists sought ways to apply the reprogrammed cells to the pathophysiology of obscure diseases, examination of drug targets, and regeneration of human tissue.24 Tissue regeneration via induced naïve somatic cells has shown promise as a future method to treat neurologic, cardiovascular, and ophthalmologic diseases.25
As the technology of cultivating and identifying optimal sources of iPSCs continues to advance, stem cell–based treatments have evolved as leading prospects in the field of biogerontology.26-29 Although much of the research in antiaging medicine has utilized iPSCs to reprogram cell senescence, the altering of iPSCs at a cellular level also allows for the stimulation of collagen synthesis. This potential for collagen generation may have direct applicability in dermatologic practice, particularly for aesthetic treatments.
Much of the research into iPSC-derived collagen has focused on genodermatoses. Itoh et al30 examined the creation of collagen through iPSCs to identify possible treatments for recessive dystrophic epidermolysis bullosa (DEB). Recessive DEB is characterized by mutations in the COL7A1 gene, which encodes type VII collagen, a basement membrane protein and component of the anchoring fibrils essential for skin integrity.31 Itoh et al30 began with source cells obtained from a skin biopsy. The cells were dedifferentiated to iPSCs and then induced into dermal fibroblasts according to the methods established in prior studies of embryonic stem cells, namely with the use of ascorbic acid and transforming growth factor b. The newly formed fibroblasts were determined to be functional based on their ability to synthesize mature type VII collagen.30 Once the viability of the iPSC-derived fibroblasts was confirmed in vitro, the cells were further tested through combination with human keratinocytes on SCID mice. The human keratinocytes grew together with the iPSC-derived fibroblasts, producing type VII collagen in the basement membrane zone and creating an epidermis with the normal markers.30 Similarly, Robbins et al32 utilized SCID mice to successfully demonstrate that the transfection of keratinocytes from patients with junctional epidermolysis bullosa into SCID mice produced phenotypically normal skin.
Sebastiano et al33 combined the concepts of iPSCs and genome editing in another study of recessive DEB. The investigators first cultured iPSCs from biopsies of affected patients. After deriving iPSCs and correcting their mutation via adenovirus-associated viral gene editing, the COL7A1 mutation-free cells were differentiated into keratinocytes. These iPSC-derived keratinocytes were subsequently grafted onto mice, which led to the production of wild-type collagen VII and a stratified epidermis. Despite this successful outcome, the grafts of iPSC-derived epidermis did not survive longer than 1 month.33
One of the many obstacles facing the practical use of stem cells is their successful incorporation into human tissue. A possible solution was uncovered by Zhang et al34 who examined iPSC-derived MSCs. Mesenchymal stem cells communicate via paracrine mechanisms, whereby exosomes containing RNA and proteins are released to potentiate a regenerative effect.35 Zhang et al34 found that injecting exosomes from human iPSC-derived MSCs into the wound sites of rats stimulated the production of type I collagen, type III collagen, and elastin. The wound sites demonstrated accelerated closure, narrower scar widths, and increased collagen maturity.
Understanding the role that local environment plays in stem cell differentiation, Xu et al36 aimed to create an extracellular scaffold to induce fibroblast behavior from iPSCs. The authors engineered a framework similar to the normal extracellular membrane using proteoglycans, glycosaminoglycans, fibrinogen, and connective tissue growth factor. The iPSCs were then applied to the scaffolding, which led to successful fibroblast differentiation and type I collagen synthesis.36 This use of local biosignaling cues holds important ramifications for controlling the fate of stem cells that have been introduced into a new environment.
Although the application of iPSCs in clinical dermatology has yet to be achieved, progress in the field is moving at a rapid pace. Several logistical elements require further mastery before therapeutics can be delivered. These areas include the optimal environment for iPSC differentiation, methods for maximization of graft survival, and different modes of transplanting iPSC-derived cells into patients. In cosmetic practice, success will depend on intradermal injections of collagen-producing iPSC-derived cells that possess long-term proliferative potential. Current research in mice models has demonstrated viability up to 16 weeks after intradermal injection of such cells.37
Plant Stem Cells
In discussing the dermatologic applications of stem cell technology, clinicians should be aware of the plant stem cell products that have become a popular cosmeceutical trend. Companies advertise plant cells as a natural source of regenerative cells that can induce rejuvenation in human skin; however, there are no significant data to indicate that plant stem cells encourage or activate cellular growth in humans. Indeed, for stem cells to differentiate and produce viable components, the cells must first be incorporated as living components in the host tissue. Because plant stem cells do not survive in human tissue and plant cell cytokines fail to interact with the receptors on human cells, their current value in cosmeceuticals may be overstated.
Platelet-Rich Plasma
Platelet-rich plasma also is commonly associated with stem cell therapy, as PRP is known to potentiate stem cell proliferation, migration, and differentiation. However, PRP does not contain stem cells and is instead autologous plasma concentrated with platelets. In fact, platelets cannot even be classified as cells given that they lack a nucleus; platelets are considered cell fragments. The regenerative potential of PRP can be attributed to the growth factors released from platelets, which play an important role in tissue regeneration and repair. Platelet-rich plasma currently is being used in dermatology for skin rejuvenation (reduction of wrinkles and furrows) and treatment of acne scars.38 There also is evidence supporting the effectiveness of PRP for alopecia and wound therapy, as growth factors play a vital role in hair growth and wound healing.38 Apart from the use of PRP on its own, it can be used as a supplement to enhance the effects of antiaging procedures such as microneedling.39
Future Directions
Multipotent stem cells and iPSCs discussed herein provide much promise in the field of regenerative dermatology. They are increasingly accessible and circumvent the use of ethically questionable embryonic stem cells. Although there is a general consensus on the great potential of stem cells for treating aesthetic skin conditions, high-quality randomized controlled trials remain scarce within the literature. Recognizing and optimizing these opportunities remains the next step in the future delivery of evidence-based care in regenerative dermatology.
- Thomas ED, Lochte HL, Lu WC, et al. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957;257:491-496.
- Ogliari KS, Marinowic D, Brum DE, et al. Stem cells in dermatology. An Bras Dermatol. 2014;89:286-291.
- Xu C, Inokuma MS, Denham J, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971-974.
- Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211-228.
- Badiavas EV, Falanga V. Treatment of chronic wounds with bone marrow-derived cells. Arch Dermatol. 2003;139:510-516.
- Ibrahim ZA, Eltatawy RA, Ghaly NR, et al. Autologous bone marrow stem cells in atrophic acne scars: a pilot study. J Dermatolog Treat. 2015;26:260-265.
- Broxmeyer HE, Douglas GW, Hangoc G, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci U S A. 1989;86:3828-3832.
- Gluckman E, Rocha V, Boyer-Chammard A, et al. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. N Engl J Med. 1997;337:373-381.
- Valerio IL, Sabino JM, Dearth CL. Plastic surgery challenges in war wounded II: regenerative medicine. Adv Wound Care (New Rochelle). 2016;5:412-419.
- Vescarelli E, D’Amici S, Onesti MG, et al. Adipose-derived stem cell: an innovative therapeutic approach in systemic sclerosis and Parry-Romberg syndrome. CellR4. 2014;2:E791-E797.
- Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008;32:48-55.
- Grabin S, Antes G, Stark GB, et al. Cell-assisted lipotransfer: a critical appraisal of the evidence. Dtsch Arztebl Int. 2015;112:255.
- Zhou Y, Wang J, Li H, et al. Efficacy and safety of cell-assisted lipotransfer: a systematic review and meta-analysis. Plast Reconstr Surg. 2016;137:E44-E57.
- Toma JG, Akhavan M, Fernandes KJL, et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778-784.
- Toma JG, McKenzie IA, Bagli D, et al. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005;23:727-737.
- Homicz MR, Watson D. Review of injectable materials for soft tissue augmentation. Facial Plast Surg. 2004;20:21-29.
- Kumar S, Mahajan BB, Kaur S, et al. Autologous therapies in dermatology. J Clin Aesthet Dermatol. 2014;7:38-45.
- Schmidt C. FDA approves first cell therapy for wrinkle-free visage. Nat Biotech. 2011;29:674-675.
- Gentile P, Scioli MG, Bielli A, et al. Stem cells from human hair follicles: first mechanical isolation for immediate autologous clinical use in androgenetic alopecia and hair loss. Stem Cell Investig. 2017;4:58.
- Sugiyama-Nakagiri Y, Akiyama M, Shimizu H. Hair follicle stem cell-targeted gene transfer and reconstitution system. Gene Ther. 2006;13:732-737.
- Heidari F, Yari A, Rasoolijazi H, et al. Bulge hair follicle stem cells accelerate cutaneous wound healing in rats. Wounds. 2016;28:132-141.
- Lee JH, Fisher DE. Melanocyte stem cells as potential therapeutics in skin disorders. Expert Opin Biol Ther. 2014;14:1-11.
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676.
- Singh VK, Kalsan M, Kumar N, et al. Induced pluripotent stem cells: applications in regenerative medicine, disease modeling, and drug discovery. Front Cell Dev Biol. 2015;3:2.
- Aoi T. 10th anniversary of iPS cells: The challenges that lie ahead. J Biochem. 2016;160:121-129.
- Lowry WE, Plath K. The many ways to make an iPS cell. Nat Biotechnol. 2008;26:1246-1248.
- Kim K, Doi A, Wen B, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285-290.
- Gafni O, Weinberger L, Mansour AA, et al. Derivation of novel human ground state naive pluripotent stem cells. Nature. 2013;504:282-286.
- Pareja-Galeano H, Sanchis-Gomar F, Pérez LM, et al. IPSCs-based anti-aging therapies: Recent discoveries and future challenges. Ageing Res Rev. 2016;27:37-41.
- Itoh M, Umegaki-Arao N, Guo Z, et al. Generation of 3D skin equivalents fully reconstituted from human induced pluripotent stem cells (iPSCs). PLoS One. 2013;8:e77673.
- Nyström A, Velati D, Mittapalli VR, et al. Collagen VII plays a dual role in wound healing. J Clin Invest. 2013;123:3498-3509.
- Robbins PB, Lin Q, Goodnough JB, et al. In vivo restoration of laminin 5 β3 expression and function in junctional epidermolysis bullosa. Proc Natl Acad Sci. 2001;98:5193-5198.
- Sebastiano V, Zhen HH, Haddad B, et al. Human COL7A1-corrected induced pluripotent stem cells for the treatment of recessive dystrophic epidermolysis bullosa. Sci Transl Med. 2014;6:264ra163.
- Zhang J, Guan J, Niu X, et al. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13:49.
- Pap E, Pállinger É, Pásztói M, et al. Highlights of a new type of intercellular communication: microvesicle-based information transfer. Inflamm Res. 2009;58:1-8.
- Xu R, Taskin MB, Rubert M, et al. hiPS-MSCs differentiation towards fibroblasts on a 3D ECM mimicking scaffold. Sci Rep. 2015;5:8480.
- Wenzel D, Bayerl J, Nyström A, et al. Genetically corrected iPSCs as cell therapy for recessive dystrophic epidermolysis bullosa. Sci Transl Med. 2014;6:264ra165.
- Bednarska K, Kieszek R, Domagała P, et al. The use of platelet-rich-plasma in aesthetic and regenerative medicine. MEDtube Science. 2015;2:8-15.
- Hashim PW, Levy Z, Cohen JL, et al. Microneedling therapy with and without platelet-rich plasma. Cutis. 2017;99:239-242.
- Thomas ED, Lochte HL, Lu WC, et al. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957;257:491-496.
- Ogliari KS, Marinowic D, Brum DE, et al. Stem cells in dermatology. An Bras Dermatol. 2014;89:286-291.
- Xu C, Inokuma MS, Denham J, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971-974.
- Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211-228.
- Badiavas EV, Falanga V. Treatment of chronic wounds with bone marrow-derived cells. Arch Dermatol. 2003;139:510-516.
- Ibrahim ZA, Eltatawy RA, Ghaly NR, et al. Autologous bone marrow stem cells in atrophic acne scars: a pilot study. J Dermatolog Treat. 2015;26:260-265.
- Broxmeyer HE, Douglas GW, Hangoc G, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci U S A. 1989;86:3828-3832.
- Gluckman E, Rocha V, Boyer-Chammard A, et al. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. N Engl J Med. 1997;337:373-381.
- Valerio IL, Sabino JM, Dearth CL. Plastic surgery challenges in war wounded II: regenerative medicine. Adv Wound Care (New Rochelle). 2016;5:412-419.
- Vescarelli E, D’Amici S, Onesti MG, et al. Adipose-derived stem cell: an innovative therapeutic approach in systemic sclerosis and Parry-Romberg syndrome. CellR4. 2014;2:E791-E797.
- Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008;32:48-55.
- Grabin S, Antes G, Stark GB, et al. Cell-assisted lipotransfer: a critical appraisal of the evidence. Dtsch Arztebl Int. 2015;112:255.
- Zhou Y, Wang J, Li H, et al. Efficacy and safety of cell-assisted lipotransfer: a systematic review and meta-analysis. Plast Reconstr Surg. 2016;137:E44-E57.
- Toma JG, Akhavan M, Fernandes KJL, et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778-784.
- Toma JG, McKenzie IA, Bagli D, et al. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005;23:727-737.
- Homicz MR, Watson D. Review of injectable materials for soft tissue augmentation. Facial Plast Surg. 2004;20:21-29.
- Kumar S, Mahajan BB, Kaur S, et al. Autologous therapies in dermatology. J Clin Aesthet Dermatol. 2014;7:38-45.
- Schmidt C. FDA approves first cell therapy for wrinkle-free visage. Nat Biotech. 2011;29:674-675.
- Gentile P, Scioli MG, Bielli A, et al. Stem cells from human hair follicles: first mechanical isolation for immediate autologous clinical use in androgenetic alopecia and hair loss. Stem Cell Investig. 2017;4:58.
- Sugiyama-Nakagiri Y, Akiyama M, Shimizu H. Hair follicle stem cell-targeted gene transfer and reconstitution system. Gene Ther. 2006;13:732-737.
- Heidari F, Yari A, Rasoolijazi H, et al. Bulge hair follicle stem cells accelerate cutaneous wound healing in rats. Wounds. 2016;28:132-141.
- Lee JH, Fisher DE. Melanocyte stem cells as potential therapeutics in skin disorders. Expert Opin Biol Ther. 2014;14:1-11.
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676.
- Singh VK, Kalsan M, Kumar N, et al. Induced pluripotent stem cells: applications in regenerative medicine, disease modeling, and drug discovery. Front Cell Dev Biol. 2015;3:2.
- Aoi T. 10th anniversary of iPS cells: The challenges that lie ahead. J Biochem. 2016;160:121-129.
- Lowry WE, Plath K. The many ways to make an iPS cell. Nat Biotechnol. 2008;26:1246-1248.
- Kim K, Doi A, Wen B, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285-290.
- Gafni O, Weinberger L, Mansour AA, et al. Derivation of novel human ground state naive pluripotent stem cells. Nature. 2013;504:282-286.
- Pareja-Galeano H, Sanchis-Gomar F, Pérez LM, et al. IPSCs-based anti-aging therapies: Recent discoveries and future challenges. Ageing Res Rev. 2016;27:37-41.
- Itoh M, Umegaki-Arao N, Guo Z, et al. Generation of 3D skin equivalents fully reconstituted from human induced pluripotent stem cells (iPSCs). PLoS One. 2013;8:e77673.
- Nyström A, Velati D, Mittapalli VR, et al. Collagen VII plays a dual role in wound healing. J Clin Invest. 2013;123:3498-3509.
- Robbins PB, Lin Q, Goodnough JB, et al. In vivo restoration of laminin 5 β3 expression and function in junctional epidermolysis bullosa. Proc Natl Acad Sci. 2001;98:5193-5198.
- Sebastiano V, Zhen HH, Haddad B, et al. Human COL7A1-corrected induced pluripotent stem cells for the treatment of recessive dystrophic epidermolysis bullosa. Sci Transl Med. 2014;6:264ra163.
- Zhang J, Guan J, Niu X, et al. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13:49.
- Pap E, Pállinger É, Pásztói M, et al. Highlights of a new type of intercellular communication: microvesicle-based information transfer. Inflamm Res. 2009;58:1-8.
- Xu R, Taskin MB, Rubert M, et al. hiPS-MSCs differentiation towards fibroblasts on a 3D ECM mimicking scaffold. Sci Rep. 2015;5:8480.
- Wenzel D, Bayerl J, Nyström A, et al. Genetically corrected iPSCs as cell therapy for recessive dystrophic epidermolysis bullosa. Sci Transl Med. 2014;6:264ra165.
- Bednarska K, Kieszek R, Domagała P, et al. The use of platelet-rich-plasma in aesthetic and regenerative medicine. MEDtube Science. 2015;2:8-15.
- Hashim PW, Levy Z, Cohen JL, et al. Microneedling therapy with and without platelet-rich plasma. Cutis. 2017;99:239-242.
Practice Points
- Multipotent stem cells derived from the bone marrow, umbilical cord, adipose tissue, dermis, and hair follicle bulge show promise in tissue regeneration for various dermatologic conditions and aesthetic applications.
- Induced pluripotent stem cells, progenitor cells that result from the dedifferentiation of specialized adult cells, have potential for collagen generation.
What’s Eating You? Clinical Manifestations of Dermacentor Tick Bites
Background and Distribution
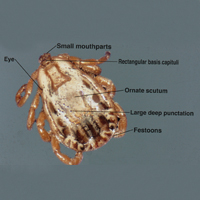
The Dermacentor ticks belong to the family Ixodidae (hard ticks). The 2 best-known ticks of the genus are Dermacentor andersoni (Rocky Mountain wood tick)(Figure, A) and Dermacentor variabilis (American dog tick)(Figure, B). The Dermacentor ticks are large ticks with small anterior mouthparts that attach to a rectangular basis capituli (Figure, A). Both ticks exhibit widely spaced eyes and posterior festoons as well as bifid coxa 1 (the attachment site for the first pair of legs) and enlarged coxa 4. As adults, these ticks display an ornate hard dorsal plate, or scutum, with numerous pits. Female ticks have a much smaller scutum, allowing for abdominal engorgement during feeding.1 Although D andersoni tends to have a brown to yellow hue, the specimens of D variabilis display a somewhat silver color pattern.
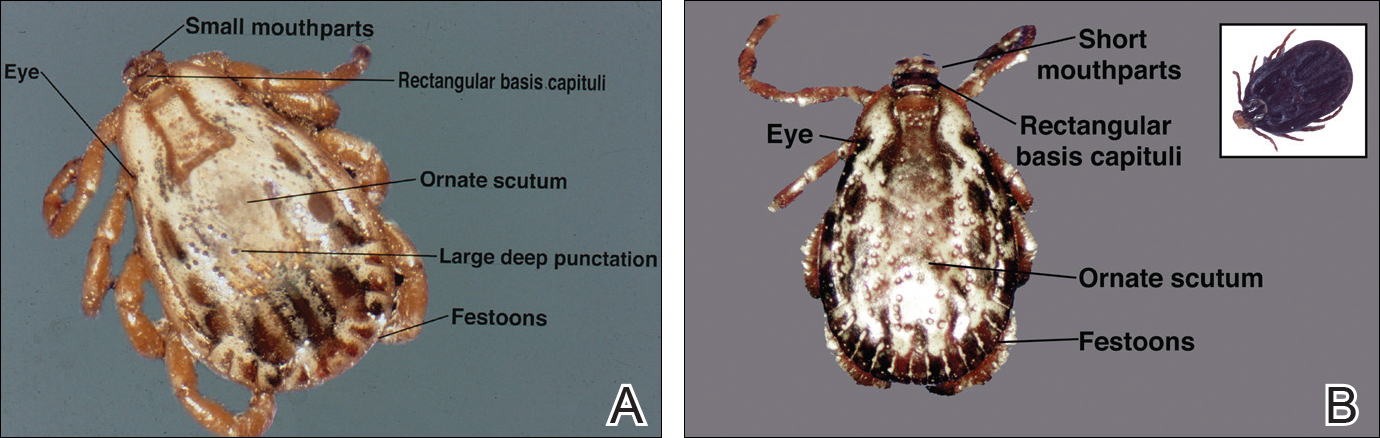
Dermacentor ticks can be found throughout most of North America, with the northern distribution limits of both species previously occurring in the province of Saskatchewan, Canada. Although the range of D andersoni has remained relatively stable within this distribution, the distribution of D variabilis recently has expanded westward and northward of these limits.2 The ranges of the 2 species overlap in certain areas, though D andersoni primarily is found in the Rocky Mountain and northwestern states as well as southwestern Canada, whereas D variabilis can be found throughout most parts of the United States, except in the Rocky Mountain states.3 Within these regions the ticks can be found in heavily wooded areas, but they most commonly inhabit fields with tall grass, crops, bushes, and shrubbery, often clustering where these types of vegetation form clearly defined edges.4 The diseases transmitted by the Dermacentor ticks include Rocky Mountain spotted fever (RMSF), Colorado tick fever, tularemia, tick paralysis, and even human monocytic erlichiosis, though Amblyomma americanum is the major vector for human monocytic erlichiosis.
Rocky Mountain Spotted Fever
Both species of ticks are known to serve as vectors for RMSF, but D variabilis is the major vector in the United States, especially in the eastern and southeastern parts of the United States. Overall, the majority of cases occur in North Carolina, South Carolina, Tennessee, and Oklahoma,5 with North Carolina having the highest incidence. In endemic areas, RMSF should be suspected in any patient with fever and headache, and empiric treatment with antibiotics should be started while awaiting the results of diagnostic tests. Serologic testing with indirect fluorescent antibodies is widely available and is considered the best method for detection; although the sensitivity is poor during the first 10 to 12 days of infection, it increases to 94% during days 14 to 21.6 Therapeutic decisions should be influenced by clinical suspicion and epidemiologic data. Treatment should be started promptly and should never be delayed until confirmatory tests are available. Doxycycline is considered the gold standard therapy in both adults and children, with a typical treatment duration of 10 days. The only other recommended agent for pregnant women in the first or second trimesters or patients with severe hypersensitivity reactions to tetracyclines is chloramphenicol.7
Colorado Tick Fever
Colorado tick fever, also known as mountain fever, is an arboviral infection transmitted by D andersoni. Its distribution coincides with the tick’s natural geographic range in the western United States and Rocky Mountains. Colorado tick fever causes an acute febrile illness consisting of chills, headaches, myalgia, retro-orbital pain, and malaise, which tend to occur within 3 to 5 days of the tick bite. Some cases may be accompanied by a nonspecific rash that may be morbilliform or petechial in appearance. Notably, approximately half of all patients will experience transient resolution of symptoms for 24 to 48 hours followed by a recurrence of fever, a phenomenon that has been referred to as saddleback fever. Routine laboratory findings may include leukopenia, thrombocytopenia, and a peripheral smear with atypical lymphocytes. Reverse transcription polymerase chain reaction is both sensitive and specific for detecting viral loads in the blood during the first week of infection, though testing does not alter management, which is largely supportive.8
Tularemia
Tularemia is a relatively rare disease but has been documented in every US state except Hawaii.9 The disease is caused by Francisella tularensis, a small, aerobic, gram-negative coccobacillus transmitted via inhalation, bitingflies, or tick bites; the most common ticks to transmit the disease include D andersoni, D variabilis, and A americanum.10 Clinical manifestations depend on the form of exposure, with tick bites most often resulting in an ulcerated skin lesion at the site of the vector bite accompanied by regional lymphadenopathy and systemic symptoms such as fever, chills, myalgia, and headache.11 Mucosal manifestations such as pharyngitis, conjunctivitis, and other ocular lesions also are commonly seen. Diagnosis most frequently is made using serology because F tularensis is both challenging and dangerous to culture; in fact, because of the high risk of contagion, F tularensis should only be cultured in biosafety level 3 laboratories. Polymerase chain reaction assays can be used on tissue samples with decent sensitivity (78%) and specificity (96%); however, these assays cannot distinguish between Francisella subspecies and are not readily available to most clinicians.12 First-line therapy for the treatment of tularemia is streptomycin given as twice-daily intramuscular injections over the course of 7 to 10 days. Alternative agents include gentamicin, ciprofloxacin, imipenem, doxycycline, and chloramphenicol.10 Because tularemia is relatively rare, a high index of suspicion is necessary to reduce the morbidity and mortality associated with the disease.
Tick Paralysis
More than 40 different species of ticks have been implicated worldwide as causes of tick paralysis, though D andersoni has been the most common in North America. Female patients account for most cases, possibly because long hair conceals ticks on the scalp or neck, the preferred attachment locations for Dermacentor ticks.13 The classic presentation of tick paralysis is an acute, flaccid, ascending paralysis that occurs from a neurotoxin in the tick saliva that impairs afferent nerve signal propagation.14,15 The paralysis progresses over hours to days and typically occurs 5 to 6 days after attachment of the tick. Notably, there is no associated fever with tick paralysis, and without intervention, patients may die of respiratory failure. Overall, the condition carries a fatality rate of nearly 10%16 but reverses rapidly if the tick is identified and removed.
Protection against tick bites and tick-borne illnesses includes avoidance of infested areas, treatment of populated habitats with insecticide sprays, use of topical repellants prior to outdoor activities, and diligent full-body tick checks upon return from tick-heavy areas. Permethrin can be used to treat clothing and remains protective through multiple washings. Ticks typically survive washing of untreated clothing but are killed by prolonged drying in a dryer. Pets may be treated with oral, intramuscular, or topical agents prescribed by a veterinarian to prevent tick attachments.
Conclusion
Accurate identification of Dermacentor ticks allows for appropriate surveillance for associated diseases and can improve patient outcomes. Patients who engage in outdoor activities in endemic areas should take steps to avoid exposure, use appropriate acaricides and repellents, and perform tick checks after returning indoors.
- Bowman DD. Georgis’ Parasitology for Veterinarians. 8th ed. New York, NY: Saunders; 2002.
- Dergousoff SJ, Galloway TD, Lindsay LR, et al. Range expansion of Dermacentor variabilis and Dermacentor andersoni near their northern distributional limits. J Med Entomol. 2013;50:510-520.
- Centers for Disease Control and Prevention. Geographic distribution of ticks that bite humans. Center for Disease Control and Prevention website. http://www.cdc.gov/ticks/geographic_distribution.html. Updated August 11, 2017. Accessed December 15
, 2017. - Trout Fryxell RT, Moore JE, Collins MD, et al. Habitat and vegetation variables are not enough when predicting tick populations in the southeastern United States. PLoS One. 2015;10:e0144092.
- Chapman AS, Bakken JS, Folk SM, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, erlichiosis, and anaplasmosis—United States: a practical guide for physicians and other health-care and public health professionals. MMWR Recomm Rep. 2006;55:1-27.
- Nathavitharana RR, Mitty JA. Diseases from North America: focus on tick-borne infections. Clin Med. 2015;15:74-77.
- Chen LF, Sexton DJ. What’s new in Rocky Mountain spotted fever? Infect Dis Clin North Am. 2008;22:415-432.
- Lambert AJ, Kosoy O, Velez JO, et al. Detection of Colorado tick fever viral RNA in acute human serum samples by a quantitative real-time RT-PCR assay. J Virol Methods. 2007;140:43-48.
- Centers for Disease Control and Prevention (CDC). Tularemia—United States, 1990-2000. MMWR Morb Mortal Wkly Rep. 2002;51:182-184.
- Nigrovic LE, Wingerter SL. Tularemia. Infect Dis Clin North Am. 2008;22:489-504.
- Evans ME, Gregory DW, Schaffner W, et al. Tularemia: a 30-year experience with 88 cases. Medicine (Baltimore). 1985;64:251-269.
- Eliasson H, Sjöstedt A, Bäck E. Clinical use of diagnostic PCR for Francisella tularensis in patients with suspected ulceroglandular tularaemia. Scand J Infect Dis. 2005;37:833-837.
- Edlow JA, McGillicuddy DC. Tick paralysis. Infect Dis Clin North Am. 2008;22:397-413.
- Felz MW, Smith CD, Swift TR. A six-year-old girl with tick paralysis. N Engl J Med. 2000;342:90-94.
- Rose I. A review of tick paralysis. Can Med Assoc J. 1954;70:175-176.
- Dworkin MS, Shoemaker PC, Anderson DE. Tick paralysis: 33 human cases in Washington State, 1946-1996. Clin Infect Dis. 1999;29:1435-1439.
Background and Distribution
The Dermacentor ticks belong to the family Ixodidae (hard ticks). The 2 best-known ticks of the genus are Dermacentor andersoni (Rocky Mountain wood tick)(Figure, A) and Dermacentor variabilis (American dog tick)(Figure, B). The Dermacentor ticks are large ticks with small anterior mouthparts that attach to a rectangular basis capituli (Figure, A). Both ticks exhibit widely spaced eyes and posterior festoons as well as bifid coxa 1 (the attachment site for the first pair of legs) and enlarged coxa 4. As adults, these ticks display an ornate hard dorsal plate, or scutum, with numerous pits. Female ticks have a much smaller scutum, allowing for abdominal engorgement during feeding.1 Although D andersoni tends to have a brown to yellow hue, the specimens of D variabilis display a somewhat silver color pattern.

Dermacentor ticks can be found throughout most of North America, with the northern distribution limits of both species previously occurring in the province of Saskatchewan, Canada. Although the range of D andersoni has remained relatively stable within this distribution, the distribution of D variabilis recently has expanded westward and northward of these limits.2 The ranges of the 2 species overlap in certain areas, though D andersoni primarily is found in the Rocky Mountain and northwestern states as well as southwestern Canada, whereas D variabilis can be found throughout most parts of the United States, except in the Rocky Mountain states.3 Within these regions the ticks can be found in heavily wooded areas, but they most commonly inhabit fields with tall grass, crops, bushes, and shrubbery, often clustering where these types of vegetation form clearly defined edges.4 The diseases transmitted by the Dermacentor ticks include Rocky Mountain spotted fever (RMSF), Colorado tick fever, tularemia, tick paralysis, and even human monocytic erlichiosis, though Amblyomma americanum is the major vector for human monocytic erlichiosis.
Rocky Mountain Spotted Fever
Both species of ticks are known to serve as vectors for RMSF, but D variabilis is the major vector in the United States, especially in the eastern and southeastern parts of the United States. Overall, the majority of cases occur in North Carolina, South Carolina, Tennessee, and Oklahoma,5 with North Carolina having the highest incidence. In endemic areas, RMSF should be suspected in any patient with fever and headache, and empiric treatment with antibiotics should be started while awaiting the results of diagnostic tests. Serologic testing with indirect fluorescent antibodies is widely available and is considered the best method for detection; although the sensitivity is poor during the first 10 to 12 days of infection, it increases to 94% during days 14 to 21.6 Therapeutic decisions should be influenced by clinical suspicion and epidemiologic data. Treatment should be started promptly and should never be delayed until confirmatory tests are available. Doxycycline is considered the gold standard therapy in both adults and children, with a typical treatment duration of 10 days. The only other recommended agent for pregnant women in the first or second trimesters or patients with severe hypersensitivity reactions to tetracyclines is chloramphenicol.7
Colorado Tick Fever
Colorado tick fever, also known as mountain fever, is an arboviral infection transmitted by D andersoni. Its distribution coincides with the tick’s natural geographic range in the western United States and Rocky Mountains. Colorado tick fever causes an acute febrile illness consisting of chills, headaches, myalgia, retro-orbital pain, and malaise, which tend to occur within 3 to 5 days of the tick bite. Some cases may be accompanied by a nonspecific rash that may be morbilliform or petechial in appearance. Notably, approximately half of all patients will experience transient resolution of symptoms for 24 to 48 hours followed by a recurrence of fever, a phenomenon that has been referred to as saddleback fever. Routine laboratory findings may include leukopenia, thrombocytopenia, and a peripheral smear with atypical lymphocytes. Reverse transcription polymerase chain reaction is both sensitive and specific for detecting viral loads in the blood during the first week of infection, though testing does not alter management, which is largely supportive.8
Tularemia
Tularemia is a relatively rare disease but has been documented in every US state except Hawaii.9 The disease is caused by Francisella tularensis, a small, aerobic, gram-negative coccobacillus transmitted via inhalation, bitingflies, or tick bites; the most common ticks to transmit the disease include D andersoni, D variabilis, and A americanum.10 Clinical manifestations depend on the form of exposure, with tick bites most often resulting in an ulcerated skin lesion at the site of the vector bite accompanied by regional lymphadenopathy and systemic symptoms such as fever, chills, myalgia, and headache.11 Mucosal manifestations such as pharyngitis, conjunctivitis, and other ocular lesions also are commonly seen. Diagnosis most frequently is made using serology because F tularensis is both challenging and dangerous to culture; in fact, because of the high risk of contagion, F tularensis should only be cultured in biosafety level 3 laboratories. Polymerase chain reaction assays can be used on tissue samples with decent sensitivity (78%) and specificity (96%); however, these assays cannot distinguish between Francisella subspecies and are not readily available to most clinicians.12 First-line therapy for the treatment of tularemia is streptomycin given as twice-daily intramuscular injections over the course of 7 to 10 days. Alternative agents include gentamicin, ciprofloxacin, imipenem, doxycycline, and chloramphenicol.10 Because tularemia is relatively rare, a high index of suspicion is necessary to reduce the morbidity and mortality associated with the disease.
Tick Paralysis
More than 40 different species of ticks have been implicated worldwide as causes of tick paralysis, though D andersoni has been the most common in North America. Female patients account for most cases, possibly because long hair conceals ticks on the scalp or neck, the preferred attachment locations for Dermacentor ticks.13 The classic presentation of tick paralysis is an acute, flaccid, ascending paralysis that occurs from a neurotoxin in the tick saliva that impairs afferent nerve signal propagation.14,15 The paralysis progresses over hours to days and typically occurs 5 to 6 days after attachment of the tick. Notably, there is no associated fever with tick paralysis, and without intervention, patients may die of respiratory failure. Overall, the condition carries a fatality rate of nearly 10%16 but reverses rapidly if the tick is identified and removed.
Protection against tick bites and tick-borne illnesses includes avoidance of infested areas, treatment of populated habitats with insecticide sprays, use of topical repellants prior to outdoor activities, and diligent full-body tick checks upon return from tick-heavy areas. Permethrin can be used to treat clothing and remains protective through multiple washings. Ticks typically survive washing of untreated clothing but are killed by prolonged drying in a dryer. Pets may be treated with oral, intramuscular, or topical agents prescribed by a veterinarian to prevent tick attachments.
Conclusion
Accurate identification of Dermacentor ticks allows for appropriate surveillance for associated diseases and can improve patient outcomes. Patients who engage in outdoor activities in endemic areas should take steps to avoid exposure, use appropriate acaricides and repellents, and perform tick checks after returning indoors.
Background and Distribution
The Dermacentor ticks belong to the family Ixodidae (hard ticks). The 2 best-known ticks of the genus are Dermacentor andersoni (Rocky Mountain wood tick)(Figure, A) and Dermacentor variabilis (American dog tick)(Figure, B). The Dermacentor ticks are large ticks with small anterior mouthparts that attach to a rectangular basis capituli (Figure, A). Both ticks exhibit widely spaced eyes and posterior festoons as well as bifid coxa 1 (the attachment site for the first pair of legs) and enlarged coxa 4. As adults, these ticks display an ornate hard dorsal plate, or scutum, with numerous pits. Female ticks have a much smaller scutum, allowing for abdominal engorgement during feeding.1 Although D andersoni tends to have a brown to yellow hue, the specimens of D variabilis display a somewhat silver color pattern.

Dermacentor ticks can be found throughout most of North America, with the northern distribution limits of both species previously occurring in the province of Saskatchewan, Canada. Although the range of D andersoni has remained relatively stable within this distribution, the distribution of D variabilis recently has expanded westward and northward of these limits.2 The ranges of the 2 species overlap in certain areas, though D andersoni primarily is found in the Rocky Mountain and northwestern states as well as southwestern Canada, whereas D variabilis can be found throughout most parts of the United States, except in the Rocky Mountain states.3 Within these regions the ticks can be found in heavily wooded areas, but they most commonly inhabit fields with tall grass, crops, bushes, and shrubbery, often clustering where these types of vegetation form clearly defined edges.4 The diseases transmitted by the Dermacentor ticks include Rocky Mountain spotted fever (RMSF), Colorado tick fever, tularemia, tick paralysis, and even human monocytic erlichiosis, though Amblyomma americanum is the major vector for human monocytic erlichiosis.
Rocky Mountain Spotted Fever
Both species of ticks are known to serve as vectors for RMSF, but D variabilis is the major vector in the United States, especially in the eastern and southeastern parts of the United States. Overall, the majority of cases occur in North Carolina, South Carolina, Tennessee, and Oklahoma,5 with North Carolina having the highest incidence. In endemic areas, RMSF should be suspected in any patient with fever and headache, and empiric treatment with antibiotics should be started while awaiting the results of diagnostic tests. Serologic testing with indirect fluorescent antibodies is widely available and is considered the best method for detection; although the sensitivity is poor during the first 10 to 12 days of infection, it increases to 94% during days 14 to 21.6 Therapeutic decisions should be influenced by clinical suspicion and epidemiologic data. Treatment should be started promptly and should never be delayed until confirmatory tests are available. Doxycycline is considered the gold standard therapy in both adults and children, with a typical treatment duration of 10 days. The only other recommended agent for pregnant women in the first or second trimesters or patients with severe hypersensitivity reactions to tetracyclines is chloramphenicol.7
Colorado Tick Fever
Colorado tick fever, also known as mountain fever, is an arboviral infection transmitted by D andersoni. Its distribution coincides with the tick’s natural geographic range in the western United States and Rocky Mountains. Colorado tick fever causes an acute febrile illness consisting of chills, headaches, myalgia, retro-orbital pain, and malaise, which tend to occur within 3 to 5 days of the tick bite. Some cases may be accompanied by a nonspecific rash that may be morbilliform or petechial in appearance. Notably, approximately half of all patients will experience transient resolution of symptoms for 24 to 48 hours followed by a recurrence of fever, a phenomenon that has been referred to as saddleback fever. Routine laboratory findings may include leukopenia, thrombocytopenia, and a peripheral smear with atypical lymphocytes. Reverse transcription polymerase chain reaction is both sensitive and specific for detecting viral loads in the blood during the first week of infection, though testing does not alter management, which is largely supportive.8
Tularemia
Tularemia is a relatively rare disease but has been documented in every US state except Hawaii.9 The disease is caused by Francisella tularensis, a small, aerobic, gram-negative coccobacillus transmitted via inhalation, bitingflies, or tick bites; the most common ticks to transmit the disease include D andersoni, D variabilis, and A americanum.10 Clinical manifestations depend on the form of exposure, with tick bites most often resulting in an ulcerated skin lesion at the site of the vector bite accompanied by regional lymphadenopathy and systemic symptoms such as fever, chills, myalgia, and headache.11 Mucosal manifestations such as pharyngitis, conjunctivitis, and other ocular lesions also are commonly seen. Diagnosis most frequently is made using serology because F tularensis is both challenging and dangerous to culture; in fact, because of the high risk of contagion, F tularensis should only be cultured in biosafety level 3 laboratories. Polymerase chain reaction assays can be used on tissue samples with decent sensitivity (78%) and specificity (96%); however, these assays cannot distinguish between Francisella subspecies and are not readily available to most clinicians.12 First-line therapy for the treatment of tularemia is streptomycin given as twice-daily intramuscular injections over the course of 7 to 10 days. Alternative agents include gentamicin, ciprofloxacin, imipenem, doxycycline, and chloramphenicol.10 Because tularemia is relatively rare, a high index of suspicion is necessary to reduce the morbidity and mortality associated with the disease.
Tick Paralysis
More than 40 different species of ticks have been implicated worldwide as causes of tick paralysis, though D andersoni has been the most common in North America. Female patients account for most cases, possibly because long hair conceals ticks on the scalp or neck, the preferred attachment locations for Dermacentor ticks.13 The classic presentation of tick paralysis is an acute, flaccid, ascending paralysis that occurs from a neurotoxin in the tick saliva that impairs afferent nerve signal propagation.14,15 The paralysis progresses over hours to days and typically occurs 5 to 6 days after attachment of the tick. Notably, there is no associated fever with tick paralysis, and without intervention, patients may die of respiratory failure. Overall, the condition carries a fatality rate of nearly 10%16 but reverses rapidly if the tick is identified and removed.
Protection against tick bites and tick-borne illnesses includes avoidance of infested areas, treatment of populated habitats with insecticide sprays, use of topical repellants prior to outdoor activities, and diligent full-body tick checks upon return from tick-heavy areas. Permethrin can be used to treat clothing and remains protective through multiple washings. Ticks typically survive washing of untreated clothing but are killed by prolonged drying in a dryer. Pets may be treated with oral, intramuscular, or topical agents prescribed by a veterinarian to prevent tick attachments.
Conclusion
Accurate identification of Dermacentor ticks allows for appropriate surveillance for associated diseases and can improve patient outcomes. Patients who engage in outdoor activities in endemic areas should take steps to avoid exposure, use appropriate acaricides and repellents, and perform tick checks after returning indoors.
- Bowman DD. Georgis’ Parasitology for Veterinarians. 8th ed. New York, NY: Saunders; 2002.
- Dergousoff SJ, Galloway TD, Lindsay LR, et al. Range expansion of Dermacentor variabilis and Dermacentor andersoni near their northern distributional limits. J Med Entomol. 2013;50:510-520.
- Centers for Disease Control and Prevention. Geographic distribution of ticks that bite humans. Center for Disease Control and Prevention website. http://www.cdc.gov/ticks/geographic_distribution.html. Updated August 11, 2017. Accessed December 15
, 2017. - Trout Fryxell RT, Moore JE, Collins MD, et al. Habitat and vegetation variables are not enough when predicting tick populations in the southeastern United States. PLoS One. 2015;10:e0144092.
- Chapman AS, Bakken JS, Folk SM, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, erlichiosis, and anaplasmosis—United States: a practical guide for physicians and other health-care and public health professionals. MMWR Recomm Rep. 2006;55:1-27.
- Nathavitharana RR, Mitty JA. Diseases from North America: focus on tick-borne infections. Clin Med. 2015;15:74-77.
- Chen LF, Sexton DJ. What’s new in Rocky Mountain spotted fever? Infect Dis Clin North Am. 2008;22:415-432.
- Lambert AJ, Kosoy O, Velez JO, et al. Detection of Colorado tick fever viral RNA in acute human serum samples by a quantitative real-time RT-PCR assay. J Virol Methods. 2007;140:43-48.
- Centers for Disease Control and Prevention (CDC). Tularemia—United States, 1990-2000. MMWR Morb Mortal Wkly Rep. 2002;51:182-184.
- Nigrovic LE, Wingerter SL. Tularemia. Infect Dis Clin North Am. 2008;22:489-504.
- Evans ME, Gregory DW, Schaffner W, et al. Tularemia: a 30-year experience with 88 cases. Medicine (Baltimore). 1985;64:251-269.
- Eliasson H, Sjöstedt A, Bäck E. Clinical use of diagnostic PCR for Francisella tularensis in patients with suspected ulceroglandular tularaemia. Scand J Infect Dis. 2005;37:833-837.
- Edlow JA, McGillicuddy DC. Tick paralysis. Infect Dis Clin North Am. 2008;22:397-413.
- Felz MW, Smith CD, Swift TR. A six-year-old girl with tick paralysis. N Engl J Med. 2000;342:90-94.
- Rose I. A review of tick paralysis. Can Med Assoc J. 1954;70:175-176.
- Dworkin MS, Shoemaker PC, Anderson DE. Tick paralysis: 33 human cases in Washington State, 1946-1996. Clin Infect Dis. 1999;29:1435-1439.
- Bowman DD. Georgis’ Parasitology for Veterinarians. 8th ed. New York, NY: Saunders; 2002.
- Dergousoff SJ, Galloway TD, Lindsay LR, et al. Range expansion of Dermacentor variabilis and Dermacentor andersoni near their northern distributional limits. J Med Entomol. 2013;50:510-520.
- Centers for Disease Control and Prevention. Geographic distribution of ticks that bite humans. Center for Disease Control and Prevention website. http://www.cdc.gov/ticks/geographic_distribution.html. Updated August 11, 2017. Accessed December 15
, 2017. - Trout Fryxell RT, Moore JE, Collins MD, et al. Habitat and vegetation variables are not enough when predicting tick populations in the southeastern United States. PLoS One. 2015;10:e0144092.
- Chapman AS, Bakken JS, Folk SM, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, erlichiosis, and anaplasmosis—United States: a practical guide for physicians and other health-care and public health professionals. MMWR Recomm Rep. 2006;55:1-27.
- Nathavitharana RR, Mitty JA. Diseases from North America: focus on tick-borne infections. Clin Med. 2015;15:74-77.
- Chen LF, Sexton DJ. What’s new in Rocky Mountain spotted fever? Infect Dis Clin North Am. 2008;22:415-432.
- Lambert AJ, Kosoy O, Velez JO, et al. Detection of Colorado tick fever viral RNA in acute human serum samples by a quantitative real-time RT-PCR assay. J Virol Methods. 2007;140:43-48.
- Centers for Disease Control and Prevention (CDC). Tularemia—United States, 1990-2000. MMWR Morb Mortal Wkly Rep. 2002;51:182-184.
- Nigrovic LE, Wingerter SL. Tularemia. Infect Dis Clin North Am. 2008;22:489-504.
- Evans ME, Gregory DW, Schaffner W, et al. Tularemia: a 30-year experience with 88 cases. Medicine (Baltimore). 1985;64:251-269.
- Eliasson H, Sjöstedt A, Bäck E. Clinical use of diagnostic PCR for Francisella tularensis in patients with suspected ulceroglandular tularaemia. Scand J Infect Dis. 2005;37:833-837.
- Edlow JA, McGillicuddy DC. Tick paralysis. Infect Dis Clin North Am. 2008;22:397-413.
- Felz MW, Smith CD, Swift TR. A six-year-old girl with tick paralysis. N Engl J Med. 2000;342:90-94.
- Rose I. A review of tick paralysis. Can Med Assoc J. 1954;70:175-176.
- Dworkin MS, Shoemaker PC, Anderson DE. Tick paralysis: 33 human cases in Washington State, 1946-1996. Clin Infect Dis. 1999;29:1435-1439.
Psoriasis Treatment in HIV-Positive Patients: A Systematic Review of Systemic Immunosuppressive Therapies
The prevalence of psoriasis among human immunodeficiency virus (HIV)–positive patients in the United States is reported to be approximately 1% to 3%, which is similar to the rates reported for the general population.1 Recalcitrant cases of psoriasis in patients with no history of the condition can be the initial manifestation of HIV infection. In patients with preexisting psoriasis, a flare of their disease can be seen following infection, and progression of HIV correlates with worsening psoriasis.2 Psoriatic arthropathy also affects 23% to 50% of HIV-positive patients with psoriasis worldwide, which may be higher than the general population,1 with more severe joint disease.
The management of psoriatic disease in the HIV-positive population is challenging. The current first-line recommendations for treatment include topical therapies, phototherapy, and highly active antiretroviral therapy (HAART), followed by oral retinoids as second-line agents.3 However, the clinical course of psoriasis in HIV-positive patients often is progressive and refractory2; therefore, these therapies often are inadequate to control both skin and joint manifestations. Most other currently available systemic therapies for psoriatic disease are immunosuppressive, which poses a distinct clinical challenge because HIV-positive patients are already immunocompromised.
There currently are many systemic immunosuppressive agents used for the treatment of psoriatic disease, including oral agents (eg, methotrexate, hydroxyurea, cyclosporine), as well as newer biologic medications, including tumor necrosis factor (TNF) α inhibitors etanercept, adalimumab, infliximab, golimumab, and certolizumab pegol. Golimumab and certolizumab pegol currently are indicated for psoriatic arthritis only. Other newer biologic therapies include ustekinumab, which inhibits IL-12 and IL-23, and secukinumab, which inhibits IL-17A. The purpose of this systematic review is to evaluate the most current literature to explore the efficacy and safety data as they pertain to systemic immunosuppressive therapies for the treatment of psoriatic disease in HIV-positive individuals.
Methods
To investigate the efficacy and safety of systemic immunosuppressive therapies for psoriatic disease in HIV-positive individuals, a PubMed search of articles indexed for MEDLINE (1985-2015) was conducted using the terms psoriasis and HIV and psoriatic arthritis and HIV combined with each of the following systemic immunosuppressive agents: methotrexate, hydroxyurea, cyclosporine, etanercept, adalimumab, infliximab, golimumab, certolizumab pegol, ustekinumab, and secukinumab. Pediatric cases and articles that were not available in the English language were excluded.
For each case, patient demographic information (ie, age, sex), prior failed psoriasis treatments, and history of HAART were documented. The dosing regimen of the systemic agent was noted when different from the US Food and Drug administration–approved dosage for psoriasis or psoriatic arthritis. The duration of immunosuppressive therapy as well as pretreatment and posttreatment CD4 and viral counts (when available) were collected. The response to treatment and adverse effects were summarized.
Results
Our review of the literature yielded a total of 25 reported cases of systemic immunosuppressive therapies used to treat psoriatic disease in HIV-positive patients, including methotrexate, cyclosporine, etanercept, adalimumab, in-fliximab, and ustekinumab (Table). There were no reports of the use of hydroxyurea, golimumab, certolizumab pegol, or secukinumab to treat psoriatic disease in this patient population.
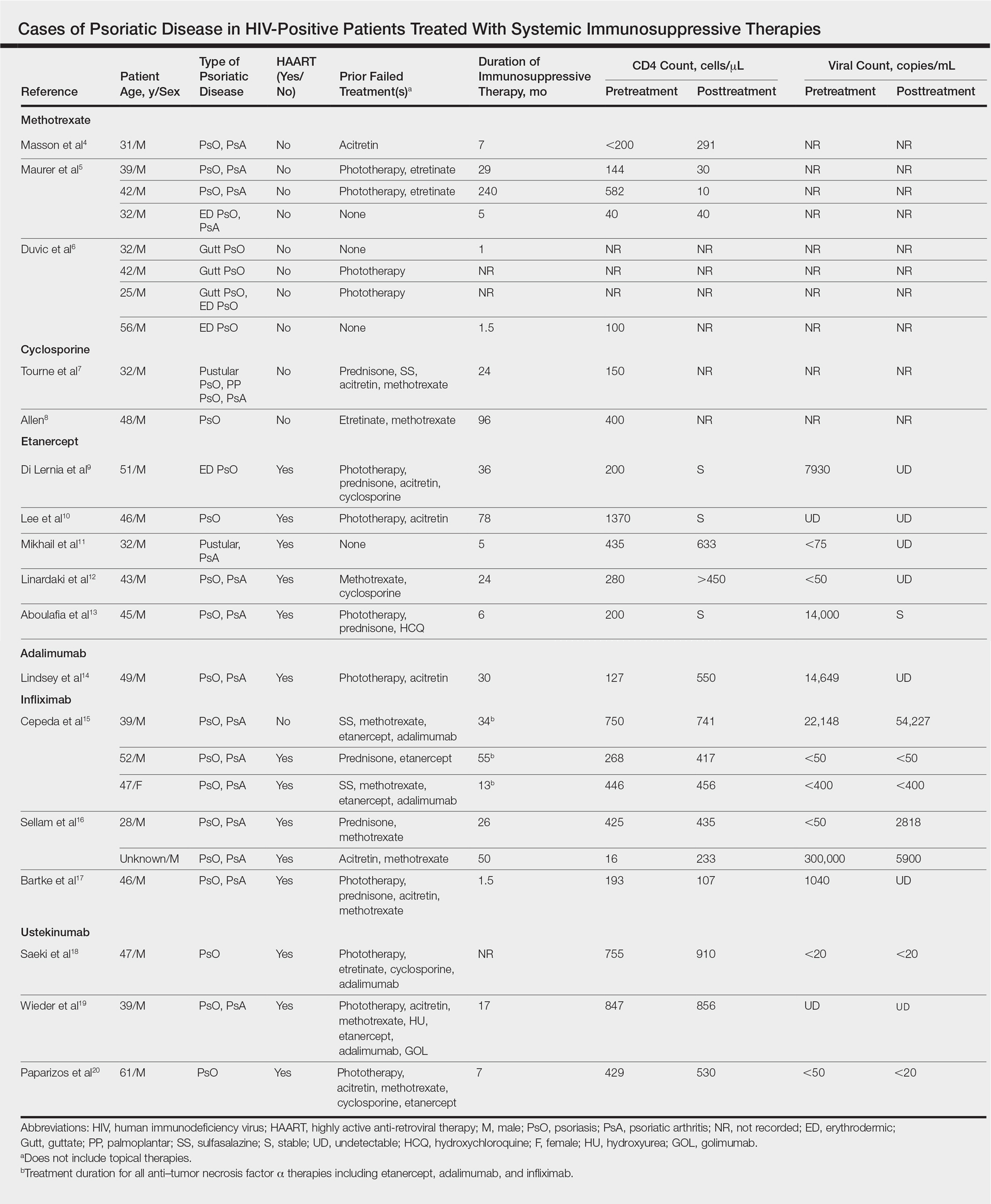
Methotrexate
Eight individual cases of methotrexate used to treat psoriasis and/or psoriatic arthritis in HIV-positive patients were reported.4-6 Duvic et al6 described 4 patients with psoriatic disease that was treated with methotrexate with varying efficacy. One patient developed toxic encephalopathy, which improved after discontinuation of methotrexate; however, he died 5 months later from pneumocystis pneumonia. In this early study, none of the 4 patients were on antiretroviral therapy for HIV.6
In the cases reported by Masson et al4 and Maurer et al,5 4 patients were treated with a single antiretroviral agent and received appropriate prophylaxis against opportunistic infections. In 1 case, methotrexate was given at a chemotherapeutic dose of 525 mg once weekly for Kaposi sarcoma.4 In 2 of 4 cases, the patients developed pneumocystis pneumonia.4,5
Cyclosporine
There were 2 case reports of successful treatment of psoriatic disease with cyclosporine in HIV-positive patients.7,8 Skin and joint manifestations improved rapidly without reports of infection for 27 and 8 years.8 Both patients were treated with one antiretroviral agent.7,8
Etanercept
There were 5 case reports of successful treatment of psoriatic disease with etanercept. In all 5 cases the patients were on HAART, and the CD4 count increased or remained stable and viral count became undetectable or remained stable following treatment.9-13 In 2 cases, the patient also had hepatitis C virus, which remained stable throughout the treatment period.9,12 The maximum duration of treatment was 6 years, with only 1 reported adverse event.13 In this case reported by Aboulafia et al,13 the patient experienced recurrent polymicrobial infections, including enterococcal cellulitis, cystitis, and bacteremia, as well as pseudomonas pneumonia and septic arthritis. Therapy was discontinued at 6 months. Four months after discontinuation of etanercept, the patient died from infectious causes.13
Adalimumab
There was 1 case of successful treatment of psoriatic disease with adalimumab in an HIV-positive patient. In this case, the patient was on HAART, and CD4 and viral counts improved substantially after 30 months of treatment.14
Infliximab
Six individual cases of successful treatment of psoriatic disease with infliximab were reported.15-17 In a report by Cepeda et al,15 HIV-positive patients with various rheumatologic diseases were chosen to receive etanercept followed by adalimumab and/or infliximab if clinical improvement was not observed on etanercept. In 3 patients with psoriasis and psoriatic arthritis, inadequate response was observed on etanercept. Two of these 3 patients received adalimumab with only partial response. All 3 were treated with infliximab in the end and showed excellent response. One of the patients experienced facial abscess responsive to antibiotics and was continued on infliximab therapy without further complications. In all 6 cases of infliximab therapy, the patients were on HAART, and CD4 and viral counts improved or remained stable.15
Ustekinumab
There were 3 case reports of successful treatment of psoriatic disease with ustekinumab in HIV-positive patients on HAART. CD4 and viral counts improved or remained stable.18-20
Comment
Currently, all of the systemic immunosuppressive therapies approved for psoriatic disease have a warning by the US Food and Drug Administration for increased risk of serious infection. Given such labels, these therapies are not routinely prescribed for HIV-positive patients who are already immunocompromised; however, many HIV-positive patients have severe psoriatic disease that cannot be adequately treated with first- and second-line therapies including topical agents, phototherapy, or oral retinoids.
Our comprehensive review yielded a total of 25 reported cases of systemic immunosuppressive therapies used to treat psoriatic disease in HIV-positive patients including methotrexate, cyclosporine, etanercept, adalimumab, in-fliximab, and ustekinumab. Although data are limited to case reports and case series, some trends were observed.
Efficacy
In most of the cases reviewed, the patients had inadequate improvement of psoriatic disease with first- and second-line therapies, which included antiretrovirals alone, topical agents, phototherapy, and oral retinoids. Some cases reported poor response to methotrexate and cyclosporine.4-8 Biologic agents were effective in many such cases.
Safety
Overall, there were 11 cases in which the patient was not on adequate HAART while being treated with systemic immunosuppressive therapy for psoriatic disease.4-8,15 Of them, 3 were associated with serious infection while on methotrexate.5,6 There was only 1 report of serious infection13 of 14 cases in which the patient was on concomitant HAART. In this case, which reported polymicrobial infections and subsequent death of the patient, the infections continued after discontinuing etanercept; thus, the association is unclear. Interestingly, despite multiple infections, the CD4 and viral counts were stable throughout treatment with etanercept.13
From reviewing the 4 total cases5,6,13 of serious infection, HAART appears to be a valuable concomitant treatment during systemic immunosuppressive therapy for HIV-positive patients; however, it does not necessarily prevent serious infections from occurring, and thus the clinician’s diligence in monitoring for signs and symptoms of infection remains important.
CD4 and Viral Counts
Although reports of CD4 and viral counts were not available in earlier studies,4-8 there were 15 cases that reported consistent pretreatment and posttreatment CD4 and viral counts during treatment with etanercept, adalimumab, infliximab, and ustekinumab.9-20 In all cases, the CD4 count was stable or increased. Similarly, the viral count was stable or decreased. All patients, except 1 by Cepeda et al,15 were on concomitant HAART.9-14,16-20
Although data are limited, treatment of psoriatic disease with biologic agents when used in combination with HAART may have beneficial effects on CD4 and viral counts. Tumor necrosis factor has a role in HIV expression through the action of nuclear factor κβ.21 An increase in TNF levels is shown to be associated with increased viral count, decreased CD4 count, and increased symptoms of HIV progression, such as fever, fatigue, cachexia, and dementia.22 Although more studies are necessary, TNF-α inhibitors may have a positive effect on HIV while simultaneously treating psoriatic disease. Other cytokines (eg, IL-12, IL-23, IL-17) involved in the mechanism of action of other biologic agents (ustekinumab and secukinumab) have not been shown to be directly associated with HIV activity; however, studies have shown that IL-10 has a role in inhibiting HIV-1 replication and inhibits secretion of proinflammatory cytokines such as IL-12 and TNF-α.21 It may be speculated that the inhibition of IL-12 and TNF-α may create a positive feedback effect to increase IL-10, which in turn inhibits HIV replication.
Conclusion
Although there are limited data on the efficacy and safety of systemic immunosuppressive therapies for the treatment of psoriatic disease in HIV-positive patients, a review of 25 individual cases suggest that these treatments are not only required but also are sufficient to treat some of the most resistant cases. It is possible that with adequate concomitant HAART and monitoring for signs and symptoms of infection, the likelihood of serious infection may be low. Furthermore, biologic agents may have a positive effect over other systemic immunosuppressive agents, such as methotrexate and cyclosporine, in improving CD4 and viral counts when used in combination with HAART. Although randomized controlled trials are necessary, current biologic therapies such as etanercept, adalimumab, infliximab, and ustekinumab may be safe viable options as third-line treatment of severe psoriasis in the HIV-positive population.
- Mallon
E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDS. 2000;14:239-246. - Montaz
eri A, Kanitakis J, Bazex J. Psoriasis and HIV infection. Int J Dermatol. 1996;35:475-479. - Menon
K, Van Vorhees AS, Bebo BF, et al; National Psoriasis Foundation. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299. - Masso
n C, Chennebault JM, Leclech C. Is HIV infection contraindication to the use of methotrexate in psoriatic arthritis? J Rheumatol. 1995;22:2191. - Maurer
TA, Zackheim HS, Tuffanelli L, et al. The use of methotrexate for treatment of psoriasis in patients with HIV infection. J Am Acad Dermatol. 1994;31:372-375. - Duvic
M, Johnson TM, Rapini RP, et al. Acquired immunodeficiency syndrome-associated psoriasis and Reiter’s syndrome. Arch Dermatol. 1987;123:1622-1632. - Tourne
L, Durez P, Van Vooren JP, et al. Alleviation of HIV-associated psoriasis and psoriatic arthritis with cyclosporine. J Am Acad Dermatol. 1997;37:501-502. - Allen
BR. Use of cyclosporine for psoriasis in HIV-positive patient. Lancet. 1992;339:686. - Di Ler
nia V, Zoboli G, Ficarelli E. Long-term management of HIV/hepatitis C virus associated psoriasis with etanercept. Indian J Dermatol Venereol Leprol. 2013;79:444. - Lee E
S, Heller MM, Kamangar F, et al. Long-term etanercept use for severe generalized psoriasis in an HIV-infected individual: a case study. J Drugs Dermatol. 2012;11:413-414. - Mikha
il M, Weinberg JM, Smith BL. Successful treatment with etanercept of von Zumbusch pustular psoriasis in a patient with human immunodeficiency virus. Arch Dermatol. 2008;144:453-456. - Linar
daki G, Katsarou O, Ioannidou P, et al. Effective etanercept treatment for psoriatic arthritis complicating concomitant human immunodeficiency virus and hepatitis C virus infection. J Rheumatol. 2007;34:1353-1355. - Aboul
afia DM, Bundow D, Wilske K, et al. Etanercept for the treatment of human immunodeficiency virus-associated psoriatic arthritis. Mayo Clin Proc. 2000;75:1093-1098. - Linds
ey SF, Weiss J, Lee ES, et al. Treatment of severe psoriasis and psoriatic arthritis with adalimumab in an HIV-positive patient. J Drugs Dermatol. 2014;13:869-871. - Ceped
a EJ, Williams FM, Ishimori ML, et al. The use of anti-tumor necrosis factor therapy in HIV-positive individuals with rheumatic disease. Ann Rheum Dis. 2008;67:710-712. - Sella
m J, Bouvard B, Masson C, et al. Use of infliximab to treat psoriatic arthritis in HIV-positive patients. Joint Bone Spine. 2007;74:197-200. - Bartk
e U, Venten I, Kreuter A, et al. Human immunodeficiency virus-associated psoriasis and psoriatic arthritis treated with infliximab. Br J Dermatol. 2004;150:784-786. - Saeki
H, Ito T, Hayashi M, et al. Successful treatment of ustekinumab in a severe psoriasis patient with human immunodeficiency virus infection. J Eur Acad Dermatol Venereol. 2015;29:1653-1655. - Wiede
r S, Routt E, Levitt J, et al. Treatment of refractory psoriasis with ustekinumab in an HIV-positive patient: a case presentation and review of the biologic literature. Psoriasis Forum. 2014;20:96-102. - Papar
izos V, Rallis E, Kirsten L, et al. Ustekinumab for the treatment of HIV psoriasis. J Dermatol Treat. 2012;23:398-399. - Kedzierska K, Crowe SM, Turville S, et al. The influence of cytokines, chemokines, and their receptors on HIV-1 replication in monocytes and macrophages. Rev Med Virol. 2003;13:39-56.
- Emer JJ. Is there a potential role for anti-tumor necrosis factor therapy in patients with human immunodeficiency virus? J Clin Aesthet Dermatol. 2009;2:29-35.
The prevalence of psoriasis among human immunodeficiency virus (HIV)–positive patients in the United States is reported to be approximately 1% to 3%, which is similar to the rates reported for the general population.1 Recalcitrant cases of psoriasis in patients with no history of the condition can be the initial manifestation of HIV infection. In patients with preexisting psoriasis, a flare of their disease can be seen following infection, and progression of HIV correlates with worsening psoriasis.2 Psoriatic arthropathy also affects 23% to 50% of HIV-positive patients with psoriasis worldwide, which may be higher than the general population,1 with more severe joint disease.
The management of psoriatic disease in the HIV-positive population is challenging. The current first-line recommendations for treatment include topical therapies, phototherapy, and highly active antiretroviral therapy (HAART), followed by oral retinoids as second-line agents.3 However, the clinical course of psoriasis in HIV-positive patients often is progressive and refractory2; therefore, these therapies often are inadequate to control both skin and joint manifestations. Most other currently available systemic therapies for psoriatic disease are immunosuppressive, which poses a distinct clinical challenge because HIV-positive patients are already immunocompromised.
There currently are many systemic immunosuppressive agents used for the treatment of psoriatic disease, including oral agents (eg, methotrexate, hydroxyurea, cyclosporine), as well as newer biologic medications, including tumor necrosis factor (TNF) α inhibitors etanercept, adalimumab, infliximab, golimumab, and certolizumab pegol. Golimumab and certolizumab pegol currently are indicated for psoriatic arthritis only. Other newer biologic therapies include ustekinumab, which inhibits IL-12 and IL-23, and secukinumab, which inhibits IL-17A. The purpose of this systematic review is to evaluate the most current literature to explore the efficacy and safety data as they pertain to systemic immunosuppressive therapies for the treatment of psoriatic disease in HIV-positive individuals.
Methods
To investigate the efficacy and safety of systemic immunosuppressive therapies for psoriatic disease in HIV-positive individuals, a PubMed search of articles indexed for MEDLINE (1985-2015) was conducted using the terms psoriasis and HIV and psoriatic arthritis and HIV combined with each of the following systemic immunosuppressive agents: methotrexate, hydroxyurea, cyclosporine, etanercept, adalimumab, infliximab, golimumab, certolizumab pegol, ustekinumab, and secukinumab. Pediatric cases and articles that were not available in the English language were excluded.
For each case, patient demographic information (ie, age, sex), prior failed psoriasis treatments, and history of HAART were documented. The dosing regimen of the systemic agent was noted when different from the US Food and Drug administration–approved dosage for psoriasis or psoriatic arthritis. The duration of immunosuppressive therapy as well as pretreatment and posttreatment CD4 and viral counts (when available) were collected. The response to treatment and adverse effects were summarized.
Results
Our review of the literature yielded a total of 25 reported cases of systemic immunosuppressive therapies used to treat psoriatic disease in HIV-positive patients, including methotrexate, cyclosporine, etanercept, adalimumab, in-fliximab, and ustekinumab (Table). There were no reports of the use of hydroxyurea, golimumab, certolizumab pegol, or secukinumab to treat psoriatic disease in this patient population.

Methotrexate
Eight individual cases of methotrexate used to treat psoriasis and/or psoriatic arthritis in HIV-positive patients were reported.4-6 Duvic et al6 described 4 patients with psoriatic disease that was treated with methotrexate with varying efficacy. One patient developed toxic encephalopathy, which improved after discontinuation of methotrexate; however, he died 5 months later from pneumocystis pneumonia. In this early study, none of the 4 patients were on antiretroviral therapy for HIV.6
In the cases reported by Masson et al4 and Maurer et al,5 4 patients were treated with a single antiretroviral agent and received appropriate prophylaxis against opportunistic infections. In 1 case, methotrexate was given at a chemotherapeutic dose of 525 mg once weekly for Kaposi sarcoma.4 In 2 of 4 cases, the patients developed pneumocystis pneumonia.4,5
Cyclosporine
There were 2 case reports of successful treatment of psoriatic disease with cyclosporine in HIV-positive patients.7,8 Skin and joint manifestations improved rapidly without reports of infection for 27 and 8 years.8 Both patients were treated with one antiretroviral agent.7,8
Etanercept
There were 5 case reports of successful treatment of psoriatic disease with etanercept. In all 5 cases the patients were on HAART, and the CD4 count increased or remained stable and viral count became undetectable or remained stable following treatment.9-13 In 2 cases, the patient also had hepatitis C virus, which remained stable throughout the treatment period.9,12 The maximum duration of treatment was 6 years, with only 1 reported adverse event.13 In this case reported by Aboulafia et al,13 the patient experienced recurrent polymicrobial infections, including enterococcal cellulitis, cystitis, and bacteremia, as well as pseudomonas pneumonia and septic arthritis. Therapy was discontinued at 6 months. Four months after discontinuation of etanercept, the patient died from infectious causes.13
Adalimumab
There was 1 case of successful treatment of psoriatic disease with adalimumab in an HIV-positive patient. In this case, the patient was on HAART, and CD4 and viral counts improved substantially after 30 months of treatment.14
Infliximab
Six individual cases of successful treatment of psoriatic disease with infliximab were reported.15-17 In a report by Cepeda et al,15 HIV-positive patients with various rheumatologic diseases were chosen to receive etanercept followed by adalimumab and/or infliximab if clinical improvement was not observed on etanercept. In 3 patients with psoriasis and psoriatic arthritis, inadequate response was observed on etanercept. Two of these 3 patients received adalimumab with only partial response. All 3 were treated with infliximab in the end and showed excellent response. One of the patients experienced facial abscess responsive to antibiotics and was continued on infliximab therapy without further complications. In all 6 cases of infliximab therapy, the patients were on HAART, and CD4 and viral counts improved or remained stable.15
Ustekinumab
There were 3 case reports of successful treatment of psoriatic disease with ustekinumab in HIV-positive patients on HAART. CD4 and viral counts improved or remained stable.18-20
Comment
Currently, all of the systemic immunosuppressive therapies approved for psoriatic disease have a warning by the US Food and Drug Administration for increased risk of serious infection. Given such labels, these therapies are not routinely prescribed for HIV-positive patients who are already immunocompromised; however, many HIV-positive patients have severe psoriatic disease that cannot be adequately treated with first- and second-line therapies including topical agents, phototherapy, or oral retinoids.
Our comprehensive review yielded a total of 25 reported cases of systemic immunosuppressive therapies used to treat psoriatic disease in HIV-positive patients including methotrexate, cyclosporine, etanercept, adalimumab, in-fliximab, and ustekinumab. Although data are limited to case reports and case series, some trends were observed.
Efficacy
In most of the cases reviewed, the patients had inadequate improvement of psoriatic disease with first- and second-line therapies, which included antiretrovirals alone, topical agents, phototherapy, and oral retinoids. Some cases reported poor response to methotrexate and cyclosporine.4-8 Biologic agents were effective in many such cases.
Safety
Overall, there were 11 cases in which the patient was not on adequate HAART while being treated with systemic immunosuppressive therapy for psoriatic disease.4-8,15 Of them, 3 were associated with serious infection while on methotrexate.5,6 There was only 1 report of serious infection13 of 14 cases in which the patient was on concomitant HAART. In this case, which reported polymicrobial infections and subsequent death of the patient, the infections continued after discontinuing etanercept; thus, the association is unclear. Interestingly, despite multiple infections, the CD4 and viral counts were stable throughout treatment with etanercept.13
From reviewing the 4 total cases5,6,13 of serious infection, HAART appears to be a valuable concomitant treatment during systemic immunosuppressive therapy for HIV-positive patients; however, it does not necessarily prevent serious infections from occurring, and thus the clinician’s diligence in monitoring for signs and symptoms of infection remains important.
CD4 and Viral Counts
Although reports of CD4 and viral counts were not available in earlier studies,4-8 there were 15 cases that reported consistent pretreatment and posttreatment CD4 and viral counts during treatment with etanercept, adalimumab, infliximab, and ustekinumab.9-20 In all cases, the CD4 count was stable or increased. Similarly, the viral count was stable or decreased. All patients, except 1 by Cepeda et al,15 were on concomitant HAART.9-14,16-20
Although data are limited, treatment of psoriatic disease with biologic agents when used in combination with HAART may have beneficial effects on CD4 and viral counts. Tumor necrosis factor has a role in HIV expression through the action of nuclear factor κβ.21 An increase in TNF levels is shown to be associated with increased viral count, decreased CD4 count, and increased symptoms of HIV progression, such as fever, fatigue, cachexia, and dementia.22 Although more studies are necessary, TNF-α inhibitors may have a positive effect on HIV while simultaneously treating psoriatic disease. Other cytokines (eg, IL-12, IL-23, IL-17) involved in the mechanism of action of other biologic agents (ustekinumab and secukinumab) have not been shown to be directly associated with HIV activity; however, studies have shown that IL-10 has a role in inhibiting HIV-1 replication and inhibits secretion of proinflammatory cytokines such as IL-12 and TNF-α.21 It may be speculated that the inhibition of IL-12 and TNF-α may create a positive feedback effect to increase IL-10, which in turn inhibits HIV replication.
Conclusion
Although there are limited data on the efficacy and safety of systemic immunosuppressive therapies for the treatment of psoriatic disease in HIV-positive patients, a review of 25 individual cases suggest that these treatments are not only required but also are sufficient to treat some of the most resistant cases. It is possible that with adequate concomitant HAART and monitoring for signs and symptoms of infection, the likelihood of serious infection may be low. Furthermore, biologic agents may have a positive effect over other systemic immunosuppressive agents, such as methotrexate and cyclosporine, in improving CD4 and viral counts when used in combination with HAART. Although randomized controlled trials are necessary, current biologic therapies such as etanercept, adalimumab, infliximab, and ustekinumab may be safe viable options as third-line treatment of severe psoriasis in the HIV-positive population.
The prevalence of psoriasis among human immunodeficiency virus (HIV)–positive patients in the United States is reported to be approximately 1% to 3%, which is similar to the rates reported for the general population.1 Recalcitrant cases of psoriasis in patients with no history of the condition can be the initial manifestation of HIV infection. In patients with preexisting psoriasis, a flare of their disease can be seen following infection, and progression of HIV correlates with worsening psoriasis.2 Psoriatic arthropathy also affects 23% to 50% of HIV-positive patients with psoriasis worldwide, which may be higher than the general population,1 with more severe joint disease.
The management of psoriatic disease in the HIV-positive population is challenging. The current first-line recommendations for treatment include topical therapies, phototherapy, and highly active antiretroviral therapy (HAART), followed by oral retinoids as second-line agents.3 However, the clinical course of psoriasis in HIV-positive patients often is progressive and refractory2; therefore, these therapies often are inadequate to control both skin and joint manifestations. Most other currently available systemic therapies for psoriatic disease are immunosuppressive, which poses a distinct clinical challenge because HIV-positive patients are already immunocompromised.
There currently are many systemic immunosuppressive agents used for the treatment of psoriatic disease, including oral agents (eg, methotrexate, hydroxyurea, cyclosporine), as well as newer biologic medications, including tumor necrosis factor (TNF) α inhibitors etanercept, adalimumab, infliximab, golimumab, and certolizumab pegol. Golimumab and certolizumab pegol currently are indicated for psoriatic arthritis only. Other newer biologic therapies include ustekinumab, which inhibits IL-12 and IL-23, and secukinumab, which inhibits IL-17A. The purpose of this systematic review is to evaluate the most current literature to explore the efficacy and safety data as they pertain to systemic immunosuppressive therapies for the treatment of psoriatic disease in HIV-positive individuals.
Methods
To investigate the efficacy and safety of systemic immunosuppressive therapies for psoriatic disease in HIV-positive individuals, a PubMed search of articles indexed for MEDLINE (1985-2015) was conducted using the terms psoriasis and HIV and psoriatic arthritis and HIV combined with each of the following systemic immunosuppressive agents: methotrexate, hydroxyurea, cyclosporine, etanercept, adalimumab, infliximab, golimumab, certolizumab pegol, ustekinumab, and secukinumab. Pediatric cases and articles that were not available in the English language were excluded.
For each case, patient demographic information (ie, age, sex), prior failed psoriasis treatments, and history of HAART were documented. The dosing regimen of the systemic agent was noted when different from the US Food and Drug administration–approved dosage for psoriasis or psoriatic arthritis. The duration of immunosuppressive therapy as well as pretreatment and posttreatment CD4 and viral counts (when available) were collected. The response to treatment and adverse effects were summarized.
Results
Our review of the literature yielded a total of 25 reported cases of systemic immunosuppressive therapies used to treat psoriatic disease in HIV-positive patients, including methotrexate, cyclosporine, etanercept, adalimumab, in-fliximab, and ustekinumab (Table). There were no reports of the use of hydroxyurea, golimumab, certolizumab pegol, or secukinumab to treat psoriatic disease in this patient population.

Methotrexate
Eight individual cases of methotrexate used to treat psoriasis and/or psoriatic arthritis in HIV-positive patients were reported.4-6 Duvic et al6 described 4 patients with psoriatic disease that was treated with methotrexate with varying efficacy. One patient developed toxic encephalopathy, which improved after discontinuation of methotrexate; however, he died 5 months later from pneumocystis pneumonia. In this early study, none of the 4 patients were on antiretroviral therapy for HIV.6
In the cases reported by Masson et al4 and Maurer et al,5 4 patients were treated with a single antiretroviral agent and received appropriate prophylaxis against opportunistic infections. In 1 case, methotrexate was given at a chemotherapeutic dose of 525 mg once weekly for Kaposi sarcoma.4 In 2 of 4 cases, the patients developed pneumocystis pneumonia.4,5
Cyclosporine
There were 2 case reports of successful treatment of psoriatic disease with cyclosporine in HIV-positive patients.7,8 Skin and joint manifestations improved rapidly without reports of infection for 27 and 8 years.8 Both patients were treated with one antiretroviral agent.7,8
Etanercept
There were 5 case reports of successful treatment of psoriatic disease with etanercept. In all 5 cases the patients were on HAART, and the CD4 count increased or remained stable and viral count became undetectable or remained stable following treatment.9-13 In 2 cases, the patient also had hepatitis C virus, which remained stable throughout the treatment period.9,12 The maximum duration of treatment was 6 years, with only 1 reported adverse event.13 In this case reported by Aboulafia et al,13 the patient experienced recurrent polymicrobial infections, including enterococcal cellulitis, cystitis, and bacteremia, as well as pseudomonas pneumonia and septic arthritis. Therapy was discontinued at 6 months. Four months after discontinuation of etanercept, the patient died from infectious causes.13
Adalimumab
There was 1 case of successful treatment of psoriatic disease with adalimumab in an HIV-positive patient. In this case, the patient was on HAART, and CD4 and viral counts improved substantially after 30 months of treatment.14
Infliximab
Six individual cases of successful treatment of psoriatic disease with infliximab were reported.15-17 In a report by Cepeda et al,15 HIV-positive patients with various rheumatologic diseases were chosen to receive etanercept followed by adalimumab and/or infliximab if clinical improvement was not observed on etanercept. In 3 patients with psoriasis and psoriatic arthritis, inadequate response was observed on etanercept. Two of these 3 patients received adalimumab with only partial response. All 3 were treated with infliximab in the end and showed excellent response. One of the patients experienced facial abscess responsive to antibiotics and was continued on infliximab therapy without further complications. In all 6 cases of infliximab therapy, the patients were on HAART, and CD4 and viral counts improved or remained stable.15
Ustekinumab
There were 3 case reports of successful treatment of psoriatic disease with ustekinumab in HIV-positive patients on HAART. CD4 and viral counts improved or remained stable.18-20
Comment
Currently, all of the systemic immunosuppressive therapies approved for psoriatic disease have a warning by the US Food and Drug Administration for increased risk of serious infection. Given such labels, these therapies are not routinely prescribed for HIV-positive patients who are already immunocompromised; however, many HIV-positive patients have severe psoriatic disease that cannot be adequately treated with first- and second-line therapies including topical agents, phototherapy, or oral retinoids.
Our comprehensive review yielded a total of 25 reported cases of systemic immunosuppressive therapies used to treat psoriatic disease in HIV-positive patients including methotrexate, cyclosporine, etanercept, adalimumab, in-fliximab, and ustekinumab. Although data are limited to case reports and case series, some trends were observed.
Efficacy
In most of the cases reviewed, the patients had inadequate improvement of psoriatic disease with first- and second-line therapies, which included antiretrovirals alone, topical agents, phototherapy, and oral retinoids. Some cases reported poor response to methotrexate and cyclosporine.4-8 Biologic agents were effective in many such cases.
Safety
Overall, there were 11 cases in which the patient was not on adequate HAART while being treated with systemic immunosuppressive therapy for psoriatic disease.4-8,15 Of them, 3 were associated with serious infection while on methotrexate.5,6 There was only 1 report of serious infection13 of 14 cases in which the patient was on concomitant HAART. In this case, which reported polymicrobial infections and subsequent death of the patient, the infections continued after discontinuing etanercept; thus, the association is unclear. Interestingly, despite multiple infections, the CD4 and viral counts were stable throughout treatment with etanercept.13
From reviewing the 4 total cases5,6,13 of serious infection, HAART appears to be a valuable concomitant treatment during systemic immunosuppressive therapy for HIV-positive patients; however, it does not necessarily prevent serious infections from occurring, and thus the clinician’s diligence in monitoring for signs and symptoms of infection remains important.
CD4 and Viral Counts
Although reports of CD4 and viral counts were not available in earlier studies,4-8 there were 15 cases that reported consistent pretreatment and posttreatment CD4 and viral counts during treatment with etanercept, adalimumab, infliximab, and ustekinumab.9-20 In all cases, the CD4 count was stable or increased. Similarly, the viral count was stable or decreased. All patients, except 1 by Cepeda et al,15 were on concomitant HAART.9-14,16-20
Although data are limited, treatment of psoriatic disease with biologic agents when used in combination with HAART may have beneficial effects on CD4 and viral counts. Tumor necrosis factor has a role in HIV expression through the action of nuclear factor κβ.21 An increase in TNF levels is shown to be associated with increased viral count, decreased CD4 count, and increased symptoms of HIV progression, such as fever, fatigue, cachexia, and dementia.22 Although more studies are necessary, TNF-α inhibitors may have a positive effect on HIV while simultaneously treating psoriatic disease. Other cytokines (eg, IL-12, IL-23, IL-17) involved in the mechanism of action of other biologic agents (ustekinumab and secukinumab) have not been shown to be directly associated with HIV activity; however, studies have shown that IL-10 has a role in inhibiting HIV-1 replication and inhibits secretion of proinflammatory cytokines such as IL-12 and TNF-α.21 It may be speculated that the inhibition of IL-12 and TNF-α may create a positive feedback effect to increase IL-10, which in turn inhibits HIV replication.
Conclusion
Although there are limited data on the efficacy and safety of systemic immunosuppressive therapies for the treatment of psoriatic disease in HIV-positive patients, a review of 25 individual cases suggest that these treatments are not only required but also are sufficient to treat some of the most resistant cases. It is possible that with adequate concomitant HAART and monitoring for signs and symptoms of infection, the likelihood of serious infection may be low. Furthermore, biologic agents may have a positive effect over other systemic immunosuppressive agents, such as methotrexate and cyclosporine, in improving CD4 and viral counts when used in combination with HAART. Although randomized controlled trials are necessary, current biologic therapies such as etanercept, adalimumab, infliximab, and ustekinumab may be safe viable options as third-line treatment of severe psoriasis in the HIV-positive population.
- Mallon
E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDS. 2000;14:239-246. - Montaz
eri A, Kanitakis J, Bazex J. Psoriasis and HIV infection. Int J Dermatol. 1996;35:475-479. - Menon
K, Van Vorhees AS, Bebo BF, et al; National Psoriasis Foundation. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299. - Masso
n C, Chennebault JM, Leclech C. Is HIV infection contraindication to the use of methotrexate in psoriatic arthritis? J Rheumatol. 1995;22:2191. - Maurer
TA, Zackheim HS, Tuffanelli L, et al. The use of methotrexate for treatment of psoriasis in patients with HIV infection. J Am Acad Dermatol. 1994;31:372-375. - Duvic
M, Johnson TM, Rapini RP, et al. Acquired immunodeficiency syndrome-associated psoriasis and Reiter’s syndrome. Arch Dermatol. 1987;123:1622-1632. - Tourne
L, Durez P, Van Vooren JP, et al. Alleviation of HIV-associated psoriasis and psoriatic arthritis with cyclosporine. J Am Acad Dermatol. 1997;37:501-502. - Allen
BR. Use of cyclosporine for psoriasis in HIV-positive patient. Lancet. 1992;339:686. - Di Ler
nia V, Zoboli G, Ficarelli E. Long-term management of HIV/hepatitis C virus associated psoriasis with etanercept. Indian J Dermatol Venereol Leprol. 2013;79:444. - Lee E
S, Heller MM, Kamangar F, et al. Long-term etanercept use for severe generalized psoriasis in an HIV-infected individual: a case study. J Drugs Dermatol. 2012;11:413-414. - Mikha
il M, Weinberg JM, Smith BL. Successful treatment with etanercept of von Zumbusch pustular psoriasis in a patient with human immunodeficiency virus. Arch Dermatol. 2008;144:453-456. - Linar
daki G, Katsarou O, Ioannidou P, et al. Effective etanercept treatment for psoriatic arthritis complicating concomitant human immunodeficiency virus and hepatitis C virus infection. J Rheumatol. 2007;34:1353-1355. - Aboul
afia DM, Bundow D, Wilske K, et al. Etanercept for the treatment of human immunodeficiency virus-associated psoriatic arthritis. Mayo Clin Proc. 2000;75:1093-1098. - Linds
ey SF, Weiss J, Lee ES, et al. Treatment of severe psoriasis and psoriatic arthritis with adalimumab in an HIV-positive patient. J Drugs Dermatol. 2014;13:869-871. - Ceped
a EJ, Williams FM, Ishimori ML, et al. The use of anti-tumor necrosis factor therapy in HIV-positive individuals with rheumatic disease. Ann Rheum Dis. 2008;67:710-712. - Sella
m J, Bouvard B, Masson C, et al. Use of infliximab to treat psoriatic arthritis in HIV-positive patients. Joint Bone Spine. 2007;74:197-200. - Bartk
e U, Venten I, Kreuter A, et al. Human immunodeficiency virus-associated psoriasis and psoriatic arthritis treated with infliximab. Br J Dermatol. 2004;150:784-786. - Saeki
H, Ito T, Hayashi M, et al. Successful treatment of ustekinumab in a severe psoriasis patient with human immunodeficiency virus infection. J Eur Acad Dermatol Venereol. 2015;29:1653-1655. - Wiede
r S, Routt E, Levitt J, et al. Treatment of refractory psoriasis with ustekinumab in an HIV-positive patient: a case presentation and review of the biologic literature. Psoriasis Forum. 2014;20:96-102. - Papar
izos V, Rallis E, Kirsten L, et al. Ustekinumab for the treatment of HIV psoriasis. J Dermatol Treat. 2012;23:398-399. - Kedzierska K, Crowe SM, Turville S, et al. The influence of cytokines, chemokines, and their receptors on HIV-1 replication in monocytes and macrophages. Rev Med Virol. 2003;13:39-56.
- Emer JJ. Is there a potential role for anti-tumor necrosis factor therapy in patients with human immunodeficiency virus? J Clin Aesthet Dermatol. 2009;2:29-35.
- Mallon
E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDS. 2000;14:239-246. - Montaz
eri A, Kanitakis J, Bazex J. Psoriasis and HIV infection. Int J Dermatol. 1996;35:475-479. - Menon
K, Van Vorhees AS, Bebo BF, et al; National Psoriasis Foundation. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299. - Masso
n C, Chennebault JM, Leclech C. Is HIV infection contraindication to the use of methotrexate in psoriatic arthritis? J Rheumatol. 1995;22:2191. - Maurer
TA, Zackheim HS, Tuffanelli L, et al. The use of methotrexate for treatment of psoriasis in patients with HIV infection. J Am Acad Dermatol. 1994;31:372-375. - Duvic
M, Johnson TM, Rapini RP, et al. Acquired immunodeficiency syndrome-associated psoriasis and Reiter’s syndrome. Arch Dermatol. 1987;123:1622-1632. - Tourne
L, Durez P, Van Vooren JP, et al. Alleviation of HIV-associated psoriasis and psoriatic arthritis with cyclosporine. J Am Acad Dermatol. 1997;37:501-502. - Allen
BR. Use of cyclosporine for psoriasis in HIV-positive patient. Lancet. 1992;339:686. - Di Ler
nia V, Zoboli G, Ficarelli E. Long-term management of HIV/hepatitis C virus associated psoriasis with etanercept. Indian J Dermatol Venereol Leprol. 2013;79:444. - Lee E
S, Heller MM, Kamangar F, et al. Long-term etanercept use for severe generalized psoriasis in an HIV-infected individual: a case study. J Drugs Dermatol. 2012;11:413-414. - Mikha
il M, Weinberg JM, Smith BL. Successful treatment with etanercept of von Zumbusch pustular psoriasis in a patient with human immunodeficiency virus. Arch Dermatol. 2008;144:453-456. - Linar
daki G, Katsarou O, Ioannidou P, et al. Effective etanercept treatment for psoriatic arthritis complicating concomitant human immunodeficiency virus and hepatitis C virus infection. J Rheumatol. 2007;34:1353-1355. - Aboul
afia DM, Bundow D, Wilske K, et al. Etanercept for the treatment of human immunodeficiency virus-associated psoriatic arthritis. Mayo Clin Proc. 2000;75:1093-1098. - Linds
ey SF, Weiss J, Lee ES, et al. Treatment of severe psoriasis and psoriatic arthritis with adalimumab in an HIV-positive patient. J Drugs Dermatol. 2014;13:869-871. - Ceped
a EJ, Williams FM, Ishimori ML, et al. The use of anti-tumor necrosis factor therapy in HIV-positive individuals with rheumatic disease. Ann Rheum Dis. 2008;67:710-712. - Sella
m J, Bouvard B, Masson C, et al. Use of infliximab to treat psoriatic arthritis in HIV-positive patients. Joint Bone Spine. 2007;74:197-200. - Bartk
e U, Venten I, Kreuter A, et al. Human immunodeficiency virus-associated psoriasis and psoriatic arthritis treated with infliximab. Br J Dermatol. 2004;150:784-786. - Saeki
H, Ito T, Hayashi M, et al. Successful treatment of ustekinumab in a severe psoriasis patient with human immunodeficiency virus infection. J Eur Acad Dermatol Venereol. 2015;29:1653-1655. - Wiede
r S, Routt E, Levitt J, et al. Treatment of refractory psoriasis with ustekinumab in an HIV-positive patient: a case presentation and review of the biologic literature. Psoriasis Forum. 2014;20:96-102. - Papar
izos V, Rallis E, Kirsten L, et al. Ustekinumab for the treatment of HIV psoriasis. J Dermatol Treat. 2012;23:398-399. - Kedzierska K, Crowe SM, Turville S, et al. The influence of cytokines, chemokines, and their receptors on HIV-1 replication in monocytes and macrophages. Rev Med Virol. 2003;13:39-56.
- Emer JJ. Is there a potential role for anti-tumor necrosis factor therapy in patients with human immunodeficiency virus? J Clin Aesthet Dermatol. 2009;2:29-35.
Practice Points
- There are limited data on the use of systemic immunosuppressive therapies for the treatment of psoriatic disease in human immunodeficiency virus–positive patients.
- The limited data suggest that biologic therapies may be effective for cases of psoriasis recalcitrant to other systemic agents and may have a positive effect on CD4 and viral counts when used in combination with highly active antiretroviral therapy.
- Further research is needed.
The Clock Is Ticking
Over the last decade we have come to understand the nature of psoriasis as a systemic inflammatory condition rather than as simply a skin disease. With this concept, we have continued to identify systemic comorbidities associated with psoriasis, including cardiovascular risk, diabetes mellitus, and metabolic syndrome. As dermatologists, we must serve as the gatekeeper for our patients with psoriasis and help to screen for comorbidities as well as provide appropriate counseling and referral.
Of the potential benefits of novel systemic therapies for psoriasis, the potential for addressing comorbid conditions with these treatments is critically important. Therefore, when I discuss psoriasis treatments, I always review and emphasize the anti-inflammatory effects of these agents. Although we know that psoriasis increases the risk for vascular inflammation and major adverse cardiovascular events (MACEs), it has been unclear if psoriasis duration affects these risks.
Egeberg et al1 utilized 2 resources to understand the effect of psoriasis duration on vascular disease and cardiovascular events: a human imaging study and a population-based study of cardiovascular disease events. In the first part of the study, patients with psoriasis (N=190) underwent fludeoxyglucose F 18 positron emission tomography/computed tomography. Next, MACE risk was examined using nationwide registries (adjusted hazard ratio in patients with psoriasis [n=87,161] vs the general population [n=4,234,793]). In the imaging study, participants had low cardiovascular risk by traditional risk scores. The authors found that vascular inflammation as demonstrated by the imaging system was significantly associated with disease duration (β=.171; P=.002). In the population-based study, psoriasis duration had a strong relationship with MACE risk (1.0% per additional year of psoriasis duration [hazard ratio, 1.010; 95% confidence interval, 1.007-1.013]). The researchers reported that every standard deviation increase in disease duration increased the target-to-background ratio by 2.5%, which translated into an absolute increase of approximately 10% in future adverse events.1
Therefore, the authors concluded that there were negative effects of psoriasis duration on vascular inflammation and MACEs,1 which suggests that the cumulative duration of low-grade chronic inflammation may accelerate vascular disease development and MACEs. The authors therefore noted that providers should consider inquiring about duration of disease to counsel for heightened cardiovascular disease risk in psoriasis patients.1
We have some evidence that therapeutic intervention may be useful. Wu et al2 compared MACE risk in psoriasis patients receiving methotrexate or tumor necrosis factor α (TNF-α) inhibitors.
The findings of these studies are poignant and help to further emphasize the importance of proper identification and treatment of psoriasis and its comorbidities. This information also adds an element of urgency to the way we look at this disease and demonstrates that we must intervene as soon as possible in this process.
- Egeberg A, Skov L, Joshi AA, et al. The relationship between duration of psoriasis, vascular inflammation, and cardiovascular events [published online August 18, 2017]. J Am Acad Dermatol. 2017;77:650.e3-656.e3.
- Wu JJ, Guerin AD, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-α inhibitors versus methotrexate [published online October 26, 2016]. J Am Acad Dermatol. 2017;76:81-90.
Over the last decade we have come to understand the nature of psoriasis as a systemic inflammatory condition rather than as simply a skin disease. With this concept, we have continued to identify systemic comorbidities associated with psoriasis, including cardiovascular risk, diabetes mellitus, and metabolic syndrome. As dermatologists, we must serve as the gatekeeper for our patients with psoriasis and help to screen for comorbidities as well as provide appropriate counseling and referral.
Of the potential benefits of novel systemic therapies for psoriasis, the potential for addressing comorbid conditions with these treatments is critically important. Therefore, when I discuss psoriasis treatments, I always review and emphasize the anti-inflammatory effects of these agents. Although we know that psoriasis increases the risk for vascular inflammation and major adverse cardiovascular events (MACEs), it has been unclear if psoriasis duration affects these risks.
Egeberg et al1 utilized 2 resources to understand the effect of psoriasis duration on vascular disease and cardiovascular events: a human imaging study and a population-based study of cardiovascular disease events. In the first part of the study, patients with psoriasis (N=190) underwent fludeoxyglucose F 18 positron emission tomography/computed tomography. Next, MACE risk was examined using nationwide registries (adjusted hazard ratio in patients with psoriasis [n=87,161] vs the general population [n=4,234,793]). In the imaging study, participants had low cardiovascular risk by traditional risk scores. The authors found that vascular inflammation as demonstrated by the imaging system was significantly associated with disease duration (β=.171; P=.002). In the population-based study, psoriasis duration had a strong relationship with MACE risk (1.0% per additional year of psoriasis duration [hazard ratio, 1.010; 95% confidence interval, 1.007-1.013]). The researchers reported that every standard deviation increase in disease duration increased the target-to-background ratio by 2.5%, which translated into an absolute increase of approximately 10% in future adverse events.1
Therefore, the authors concluded that there were negative effects of psoriasis duration on vascular inflammation and MACEs,1 which suggests that the cumulative duration of low-grade chronic inflammation may accelerate vascular disease development and MACEs. The authors therefore noted that providers should consider inquiring about duration of disease to counsel for heightened cardiovascular disease risk in psoriasis patients.1
We have some evidence that therapeutic intervention may be useful. Wu et al2 compared MACE risk in psoriasis patients receiving methotrexate or tumor necrosis factor α (TNF-α) inhibitors.
The findings of these studies are poignant and help to further emphasize the importance of proper identification and treatment of psoriasis and its comorbidities. This information also adds an element of urgency to the way we look at this disease and demonstrates that we must intervene as soon as possible in this process.
Over the last decade we have come to understand the nature of psoriasis as a systemic inflammatory condition rather than as simply a skin disease. With this concept, we have continued to identify systemic comorbidities associated with psoriasis, including cardiovascular risk, diabetes mellitus, and metabolic syndrome. As dermatologists, we must serve as the gatekeeper for our patients with psoriasis and help to screen for comorbidities as well as provide appropriate counseling and referral.
Of the potential benefits of novel systemic therapies for psoriasis, the potential for addressing comorbid conditions with these treatments is critically important. Therefore, when I discuss psoriasis treatments, I always review and emphasize the anti-inflammatory effects of these agents. Although we know that psoriasis increases the risk for vascular inflammation and major adverse cardiovascular events (MACEs), it has been unclear if psoriasis duration affects these risks.
Egeberg et al1 utilized 2 resources to understand the effect of psoriasis duration on vascular disease and cardiovascular events: a human imaging study and a population-based study of cardiovascular disease events. In the first part of the study, patients with psoriasis (N=190) underwent fludeoxyglucose F 18 positron emission tomography/computed tomography. Next, MACE risk was examined using nationwide registries (adjusted hazard ratio in patients with psoriasis [n=87,161] vs the general population [n=4,234,793]). In the imaging study, participants had low cardiovascular risk by traditional risk scores. The authors found that vascular inflammation as demonstrated by the imaging system was significantly associated with disease duration (β=.171; P=.002). In the population-based study, psoriasis duration had a strong relationship with MACE risk (1.0% per additional year of psoriasis duration [hazard ratio, 1.010; 95% confidence interval, 1.007-1.013]). The researchers reported that every standard deviation increase in disease duration increased the target-to-background ratio by 2.5%, which translated into an absolute increase of approximately 10% in future adverse events.1
Therefore, the authors concluded that there were negative effects of psoriasis duration on vascular inflammation and MACEs,1 which suggests that the cumulative duration of low-grade chronic inflammation may accelerate vascular disease development and MACEs. The authors therefore noted that providers should consider inquiring about duration of disease to counsel for heightened cardiovascular disease risk in psoriasis patients.1
We have some evidence that therapeutic intervention may be useful. Wu et al2 compared MACE risk in psoriasis patients receiving methotrexate or tumor necrosis factor α (TNF-α) inhibitors.
The findings of these studies are poignant and help to further emphasize the importance of proper identification and treatment of psoriasis and its comorbidities. This information also adds an element of urgency to the way we look at this disease and demonstrates that we must intervene as soon as possible in this process.
- Egeberg A, Skov L, Joshi AA, et al. The relationship between duration of psoriasis, vascular inflammation, and cardiovascular events [published online August 18, 2017]. J Am Acad Dermatol. 2017;77:650.e3-656.e3.
- Wu JJ, Guerin AD, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-α inhibitors versus methotrexate [published online October 26, 2016]. J Am Acad Dermatol. 2017;76:81-90.
- Egeberg A, Skov L, Joshi AA, et al. The relationship between duration of psoriasis, vascular inflammation, and cardiovascular events [published online August 18, 2017]. J Am Acad Dermatol. 2017;77:650.e3-656.e3.
- Wu JJ, Guerin AD, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-α inhibitors versus methotrexate [published online October 26, 2016]. J Am Acad Dermatol. 2017;76:81-90.
Related Content Online
The Role of Biologic Therapy for Psoriasis in Cardiovascular Risk Reduction
A Review of Neurologic Complications of Biologic Therapy in Plaque Psoriasis
Biologic agents have provided patients with moderate to severe psoriasis with treatment alternatives that have improved systemic safety profiles and disease control1; however, case reports of associated neurologic complications have been emerging. Tumor necrosis factor α (TNF-α) inhibitors have been associated with central and peripheral demyelinating disorders. Notably, efalizumab was withdrawn from the market for its association with fatal cases of progressive multifocal leukoencephalopathy (PML).2,3 It is imperative for dermatologists to be familiar with the clinical presentation, evaluation, and diagnostic criteria of neurologic complications of biologic agents used in the treatment of psoriasis.
Leukoencephalopathy
Progressive multifocal leukoencephalopathy is a fatal demyelinating neurodegenerative disease caused by reactivation of the ubiquitous John Cunningham virus. Primary asymptomatic infection is thought to occur during childhood, then the virus remains latent. Reactivation usually occurs during severe immunosuppression and is classically described in human immunodeficiency virus infection, lymphoproliferative disorders, and other forms of cancer.4 A summary of PML and its association with biologics is found in Table 1.5-13 Few case reports of TNF-α inhibitor–associated PML exist, mostly in the presence of confounding factors such as immunosuppression or underlying autoimmune disease.10-13 Presenting symptoms of PML often are subacute, rapidly progressive, and can be focal or multifocal and include motor, cognitive, and visual deficits. Of note, there are 2 reported cases of ustekinumab associated with reversible posterior leukoencephalopathy syndrome, which is a hypertensive encephalopathy characterized by headache, altered mental status, vision abnormalities, and seizures.14,15 Fortunately, this disease is reversible with blood pressure control and removal of the immunosuppressive agent.16
Demyelinating Disorders
Clinical presentation of demyelinating events associated with biologic agents are varied but include optic neuritis, multiple sclerosis, transverse myelitis, and Guillain-Barré syndrome, among others.17-28 These demyelinating disorders with their salient features and associated biologics are summarized in Table 2.17-20,22-28 Patients on biologic agents, especially TNF-α inhibitors, with new-onset visual, motor, or sensory changes warrant closer inspection. Currently, there are no data on any neurologic side effects occurring with the new biologic secukinumab.29
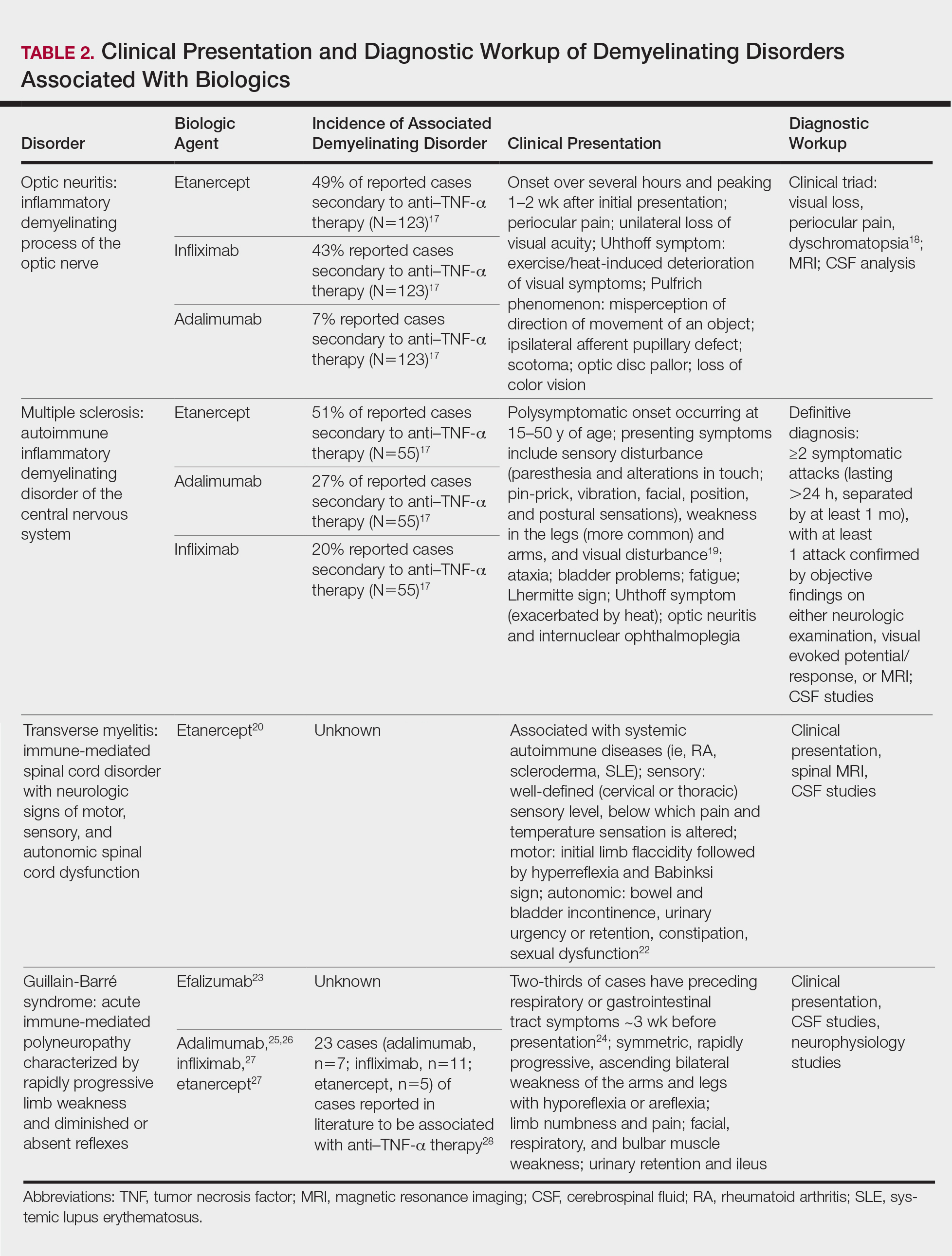
Conclusion
Biologic agents are effective in treating moderate to severe plaque psoriasis, but awareness of associated neurological adverse effects, though rare, is important to consider. Physicians need to be able to counsel patients concerning these risks and promote informed decision-making prior to initiating biologics. Patients with a personal or strong family history of demyelinating disease should be considered for alternative treatment options before initiating anti–TNF-α therapy. Since the withdrawal of efalizumab, no new cases of PML have been reported in patients who were previously on a long-term course. Dermatologists should be vigilant in detecting signs of neurological complications so that an expedited evaluation and neurology referral may prevent progression of disease.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- FDA Statement on the Voluntary Withdrawal of Raptiva From the U.S. Market. US Food and Drug Administration website. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrug-SafetyInformationforPatientsandProviders/ucm143347.htm. Published April 8, 2009. Accessed December 21, 2017.
- Kothary N, Diak IL, Brinker A, et al. Progressive multifocal leukoencephalopathy associated with efalizumab use in psoriasis patients. J Am Acad Dermatol. 2011;65:546-551.
- Tavazzi E, Ferrante P, Khalili K. Progressive multifocal leukoencephalopathy: an unexpected complication of modern therapeutic monoclonal antibody therapies. Clin Microbiol Infect. 2011;17:1776-1780.
- Korman BD, Tyler KL, Korman NJ. Progressive multifocal leukoencephalopathy, efalizumab, and immunosuppression: a cautionary tale for dermatologists. Arch Dermatol. 2009;145:937-942.
- Sudhakar P, Bachman DM, Mark AS, et al. Progressive multifocal leukoencephalopathy: recent advances and a neuro-ophthalmological review. J Neuroophthalmol. 2015;35:296-305.
- Berger JR, Aksamit AJ, Clifford DB, et al. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology. 2013;80:1430-1438.
- Koralnik IJ, Boden D, Mai VX, et al. JC virus DNA load in patients with and without progressive multifocal leukoencephalopathy. Neurology. 1999;52:253-260.
- Clifford DB, Ances B, Costello C, et al. Rituximab-associated progressive multifocal leukoencephalopathy in rheumatoid arthritis. Arch Neurol. 2011;68:1156-1164.
- Babi MA, Pendlebury W, Braff S, et al. JC virus PCR detection is not infallible: a fulminant case of progressive multifocal leukoencephalopathy with false-negative cerebrospinal fluid studies despite progressive clinical course and radiological findings [published online March 12, 2015]. Case Rep Neurol Med. 2015;2015:643216.
- Ray M, Curtis JR, Baddley JW. A case report of progressive multifocal leucoencephalopathy (PML) associated with adalimumab. Ann Rheum Dis. 2014;73:1429-1430.
- Kumar D, Bouldin TW, Berger RG. A case of progressive multifocal leukoencephalopathy in a patient treated with infliximab. Arthritis Rheum. 2010;62:3191-3195.
- Graff-Radford J, Robinson MT, Warsame RM, et al. Progressive multifocal leukoencephalopathy in a patient treated with etanercept. Neurologist. 2012;18:85-87.
- Dickson L, Menter A. Reversible posterior leukoencephalopathy syndrome (RPLS) in a psoriasis patient treated with ustekinumab. J Drugs Dermatol. 2017;16:177-179.
- Gratton D, Szapary P, Goyal K, et al. Reversible posterior leukoencephalopathy syndrome in a patient treated with ustekinumab: case report and review of the literature. Arch Dermatol. 2011;147:1197-1202.
- Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494-500.
- Ramos-Casals M, Roberto-Perez A, Diaz-Lagares C, et al. Autoimmune diseases induced by biological agents: a double-edged sword? Autoimmun Rev. 2010;9:188-193.
- Hoorbakht H, Bagherkashi F. Optic neuritis, its differential diagnosis and management. Open Ophthalmol J. 2012;6:65-72.
- Richards RG, Sampson FC, Beard SM, et al. A review of the natural history and epidemiology of multiple sclerosis: implications for resource allocation and health economic models. Health Technol Assess. 2002;6:1-73.
- Caracseghi F, Izquierdo-Blasco J, Sanchez-Montanez A, et al. Etanercept-induced myelopathy in a pediatric case of blau syndrome [published online January 15, 2012]. Case Rep Rheumatol. 2011;2011:134106.
- Fromont A, De Seze J, Fleury MC, et al. Inflammatory demyelinating events following treatment with anti-tumor necrosis factor. Cytokine. 2009;45:55-57.
- Sellner J, Lüthi N, Schüpbach WM, et al. Diagnostic workup of patients with acute transverse myelitis: spectrum of clinical presentation, neuroimaging and laboratory findings. Spinal Cord. 2009;47:312-317.
- Turatti M, Tamburin S, Idone D, et al. Guillain-Barré syndrome after short-course efalizumab treatment. J Neurol. 2010;257:1404-1405.
- Koga M, Yuki N, Hirata K. Antecedent symptoms in Guillain-Barré syndrome: an important indicator for clinical and serological subgroups. Acta Neurol Scand. 2001;103:278-287.
- Cesarini M, Angelucci E, Foglietta T, et al. Guillain-Barré syndrome after treatment with human anti-tumor necrosis factor alpha (adalimumab) in a Crohn’s disease patient: case report and literature review [published online July 28, 2011]. J Crohns Colitis. 2011;5:619-622.
- Soto-Cabrera E, Hernández-Martínez A, Yañez H, et al. Guillain-Barré syndrome. Its association with alpha tumor necrosis factor [in Spanish]. Rev Med Inst Mex Seguro Soc. 2012;50:565-567.
- Shin IS, Baer AN, Kwon HJ, et al. Guillain-Barré and Miller Fisher syndromes occurring with tumor necrosis factor alpha antagonist therapy. Arthritis Rheum. 2006;54:1429-1434.
- Alvarez-Lario B, Prieto-Tejedo R, Colazo-Burlato M, et al. Severe Guillain-Barré syndrome in a patient receiving anti-TNF therapy. consequence or coincidence. a case-based review. Clin Rheumatol. 2013;32:1407-1412.
- Garnock-Jones KP. Secukinumab: a review in moderate to severe plaque psoriasis. Am J Clin Dermatol. 2015;16:323-330.
Biologic agents have provided patients with moderate to severe psoriasis with treatment alternatives that have improved systemic safety profiles and disease control1; however, case reports of associated neurologic complications have been emerging. Tumor necrosis factor α (TNF-α) inhibitors have been associated with central and peripheral demyelinating disorders. Notably, efalizumab was withdrawn from the market for its association with fatal cases of progressive multifocal leukoencephalopathy (PML).2,3 It is imperative for dermatologists to be familiar with the clinical presentation, evaluation, and diagnostic criteria of neurologic complications of biologic agents used in the treatment of psoriasis.
Leukoencephalopathy
Progressive multifocal leukoencephalopathy is a fatal demyelinating neurodegenerative disease caused by reactivation of the ubiquitous John Cunningham virus. Primary asymptomatic infection is thought to occur during childhood, then the virus remains latent. Reactivation usually occurs during severe immunosuppression and is classically described in human immunodeficiency virus infection, lymphoproliferative disorders, and other forms of cancer.4 A summary of PML and its association with biologics is found in Table 1.5-13 Few case reports of TNF-α inhibitor–associated PML exist, mostly in the presence of confounding factors such as immunosuppression or underlying autoimmune disease.10-13 Presenting symptoms of PML often are subacute, rapidly progressive, and can be focal or multifocal and include motor, cognitive, and visual deficits. Of note, there are 2 reported cases of ustekinumab associated with reversible posterior leukoencephalopathy syndrome, which is a hypertensive encephalopathy characterized by headache, altered mental status, vision abnormalities, and seizures.14,15 Fortunately, this disease is reversible with blood pressure control and removal of the immunosuppressive agent.16

Demyelinating Disorders
Clinical presentation of demyelinating events associated with biologic agents are varied but include optic neuritis, multiple sclerosis, transverse myelitis, and Guillain-Barré syndrome, among others.17-28 These demyelinating disorders with their salient features and associated biologics are summarized in Table 2.17-20,22-28 Patients on biologic agents, especially TNF-α inhibitors, with new-onset visual, motor, or sensory changes warrant closer inspection. Currently, there are no data on any neurologic side effects occurring with the new biologic secukinumab.29

Conclusion
Biologic agents are effective in treating moderate to severe plaque psoriasis, but awareness of associated neurological adverse effects, though rare, is important to consider. Physicians need to be able to counsel patients concerning these risks and promote informed decision-making prior to initiating biologics. Patients with a personal or strong family history of demyelinating disease should be considered for alternative treatment options before initiating anti–TNF-α therapy. Since the withdrawal of efalizumab, no new cases of PML have been reported in patients who were previously on a long-term course. Dermatologists should be vigilant in detecting signs of neurological complications so that an expedited evaluation and neurology referral may prevent progression of disease.
Biologic agents have provided patients with moderate to severe psoriasis with treatment alternatives that have improved systemic safety profiles and disease control1; however, case reports of associated neurologic complications have been emerging. Tumor necrosis factor α (TNF-α) inhibitors have been associated with central and peripheral demyelinating disorders. Notably, efalizumab was withdrawn from the market for its association with fatal cases of progressive multifocal leukoencephalopathy (PML).2,3 It is imperative for dermatologists to be familiar with the clinical presentation, evaluation, and diagnostic criteria of neurologic complications of biologic agents used in the treatment of psoriasis.
Leukoencephalopathy
Progressive multifocal leukoencephalopathy is a fatal demyelinating neurodegenerative disease caused by reactivation of the ubiquitous John Cunningham virus. Primary asymptomatic infection is thought to occur during childhood, then the virus remains latent. Reactivation usually occurs during severe immunosuppression and is classically described in human immunodeficiency virus infection, lymphoproliferative disorders, and other forms of cancer.4 A summary of PML and its association with biologics is found in Table 1.5-13 Few case reports of TNF-α inhibitor–associated PML exist, mostly in the presence of confounding factors such as immunosuppression or underlying autoimmune disease.10-13 Presenting symptoms of PML often are subacute, rapidly progressive, and can be focal or multifocal and include motor, cognitive, and visual deficits. Of note, there are 2 reported cases of ustekinumab associated with reversible posterior leukoencephalopathy syndrome, which is a hypertensive encephalopathy characterized by headache, altered mental status, vision abnormalities, and seizures.14,15 Fortunately, this disease is reversible with blood pressure control and removal of the immunosuppressive agent.16

Demyelinating Disorders
Clinical presentation of demyelinating events associated with biologic agents are varied but include optic neuritis, multiple sclerosis, transverse myelitis, and Guillain-Barré syndrome, among others.17-28 These demyelinating disorders with their salient features and associated biologics are summarized in Table 2.17-20,22-28 Patients on biologic agents, especially TNF-α inhibitors, with new-onset visual, motor, or sensory changes warrant closer inspection. Currently, there are no data on any neurologic side effects occurring with the new biologic secukinumab.29

Conclusion
Biologic agents are effective in treating moderate to severe plaque psoriasis, but awareness of associated neurological adverse effects, though rare, is important to consider. Physicians need to be able to counsel patients concerning these risks and promote informed decision-making prior to initiating biologics. Patients with a personal or strong family history of demyelinating disease should be considered for alternative treatment options before initiating anti–TNF-α therapy. Since the withdrawal of efalizumab, no new cases of PML have been reported in patients who were previously on a long-term course. Dermatologists should be vigilant in detecting signs of neurological complications so that an expedited evaluation and neurology referral may prevent progression of disease.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- FDA Statement on the Voluntary Withdrawal of Raptiva From the U.S. Market. US Food and Drug Administration website. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrug-SafetyInformationforPatientsandProviders/ucm143347.htm. Published April 8, 2009. Accessed December 21, 2017.
- Kothary N, Diak IL, Brinker A, et al. Progressive multifocal leukoencephalopathy associated with efalizumab use in psoriasis patients. J Am Acad Dermatol. 2011;65:546-551.
- Tavazzi E, Ferrante P, Khalili K. Progressive multifocal leukoencephalopathy: an unexpected complication of modern therapeutic monoclonal antibody therapies. Clin Microbiol Infect. 2011;17:1776-1780.
- Korman BD, Tyler KL, Korman NJ. Progressive multifocal leukoencephalopathy, efalizumab, and immunosuppression: a cautionary tale for dermatologists. Arch Dermatol. 2009;145:937-942.
- Sudhakar P, Bachman DM, Mark AS, et al. Progressive multifocal leukoencephalopathy: recent advances and a neuro-ophthalmological review. J Neuroophthalmol. 2015;35:296-305.
- Berger JR, Aksamit AJ, Clifford DB, et al. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology. 2013;80:1430-1438.
- Koralnik IJ, Boden D, Mai VX, et al. JC virus DNA load in patients with and without progressive multifocal leukoencephalopathy. Neurology. 1999;52:253-260.
- Clifford DB, Ances B, Costello C, et al. Rituximab-associated progressive multifocal leukoencephalopathy in rheumatoid arthritis. Arch Neurol. 2011;68:1156-1164.
- Babi MA, Pendlebury W, Braff S, et al. JC virus PCR detection is not infallible: a fulminant case of progressive multifocal leukoencephalopathy with false-negative cerebrospinal fluid studies despite progressive clinical course and radiological findings [published online March 12, 2015]. Case Rep Neurol Med. 2015;2015:643216.
- Ray M, Curtis JR, Baddley JW. A case report of progressive multifocal leucoencephalopathy (PML) associated with adalimumab. Ann Rheum Dis. 2014;73:1429-1430.
- Kumar D, Bouldin TW, Berger RG. A case of progressive multifocal leukoencephalopathy in a patient treated with infliximab. Arthritis Rheum. 2010;62:3191-3195.
- Graff-Radford J, Robinson MT, Warsame RM, et al. Progressive multifocal leukoencephalopathy in a patient treated with etanercept. Neurologist. 2012;18:85-87.
- Dickson L, Menter A. Reversible posterior leukoencephalopathy syndrome (RPLS) in a psoriasis patient treated with ustekinumab. J Drugs Dermatol. 2017;16:177-179.
- Gratton D, Szapary P, Goyal K, et al. Reversible posterior leukoencephalopathy syndrome in a patient treated with ustekinumab: case report and review of the literature. Arch Dermatol. 2011;147:1197-1202.
- Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494-500.
- Ramos-Casals M, Roberto-Perez A, Diaz-Lagares C, et al. Autoimmune diseases induced by biological agents: a double-edged sword? Autoimmun Rev. 2010;9:188-193.
- Hoorbakht H, Bagherkashi F. Optic neuritis, its differential diagnosis and management. Open Ophthalmol J. 2012;6:65-72.
- Richards RG, Sampson FC, Beard SM, et al. A review of the natural history and epidemiology of multiple sclerosis: implications for resource allocation and health economic models. Health Technol Assess. 2002;6:1-73.
- Caracseghi F, Izquierdo-Blasco J, Sanchez-Montanez A, et al. Etanercept-induced myelopathy in a pediatric case of blau syndrome [published online January 15, 2012]. Case Rep Rheumatol. 2011;2011:134106.
- Fromont A, De Seze J, Fleury MC, et al. Inflammatory demyelinating events following treatment with anti-tumor necrosis factor. Cytokine. 2009;45:55-57.
- Sellner J, Lüthi N, Schüpbach WM, et al. Diagnostic workup of patients with acute transverse myelitis: spectrum of clinical presentation, neuroimaging and laboratory findings. Spinal Cord. 2009;47:312-317.
- Turatti M, Tamburin S, Idone D, et al. Guillain-Barré syndrome after short-course efalizumab treatment. J Neurol. 2010;257:1404-1405.
- Koga M, Yuki N, Hirata K. Antecedent symptoms in Guillain-Barré syndrome: an important indicator for clinical and serological subgroups. Acta Neurol Scand. 2001;103:278-287.
- Cesarini M, Angelucci E, Foglietta T, et al. Guillain-Barré syndrome after treatment with human anti-tumor necrosis factor alpha (adalimumab) in a Crohn’s disease patient: case report and literature review [published online July 28, 2011]. J Crohns Colitis. 2011;5:619-622.
- Soto-Cabrera E, Hernández-Martínez A, Yañez H, et al. Guillain-Barré syndrome. Its association with alpha tumor necrosis factor [in Spanish]. Rev Med Inst Mex Seguro Soc. 2012;50:565-567.
- Shin IS, Baer AN, Kwon HJ, et al. Guillain-Barré and Miller Fisher syndromes occurring with tumor necrosis factor alpha antagonist therapy. Arthritis Rheum. 2006;54:1429-1434.
- Alvarez-Lario B, Prieto-Tejedo R, Colazo-Burlato M, et al. Severe Guillain-Barré syndrome in a patient receiving anti-TNF therapy. consequence or coincidence. a case-based review. Clin Rheumatol. 2013;32:1407-1412.
- Garnock-Jones KP. Secukinumab: a review in moderate to severe plaque psoriasis. Am J Clin Dermatol. 2015;16:323-330.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- FDA Statement on the Voluntary Withdrawal of Raptiva From the U.S. Market. US Food and Drug Administration website. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrug-SafetyInformationforPatientsandProviders/ucm143347.htm. Published April 8, 2009. Accessed December 21, 2017.
- Kothary N, Diak IL, Brinker A, et al. Progressive multifocal leukoencephalopathy associated with efalizumab use in psoriasis patients. J Am Acad Dermatol. 2011;65:546-551.
- Tavazzi E, Ferrante P, Khalili K. Progressive multifocal leukoencephalopathy: an unexpected complication of modern therapeutic monoclonal antibody therapies. Clin Microbiol Infect. 2011;17:1776-1780.
- Korman BD, Tyler KL, Korman NJ. Progressive multifocal leukoencephalopathy, efalizumab, and immunosuppression: a cautionary tale for dermatologists. Arch Dermatol. 2009;145:937-942.
- Sudhakar P, Bachman DM, Mark AS, et al. Progressive multifocal leukoencephalopathy: recent advances and a neuro-ophthalmological review. J Neuroophthalmol. 2015;35:296-305.
- Berger JR, Aksamit AJ, Clifford DB, et al. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology. 2013;80:1430-1438.
- Koralnik IJ, Boden D, Mai VX, et al. JC virus DNA load in patients with and without progressive multifocal leukoencephalopathy. Neurology. 1999;52:253-260.
- Clifford DB, Ances B, Costello C, et al. Rituximab-associated progressive multifocal leukoencephalopathy in rheumatoid arthritis. Arch Neurol. 2011;68:1156-1164.
- Babi MA, Pendlebury W, Braff S, et al. JC virus PCR detection is not infallible: a fulminant case of progressive multifocal leukoencephalopathy with false-negative cerebrospinal fluid studies despite progressive clinical course and radiological findings [published online March 12, 2015]. Case Rep Neurol Med. 2015;2015:643216.
- Ray M, Curtis JR, Baddley JW. A case report of progressive multifocal leucoencephalopathy (PML) associated with adalimumab. Ann Rheum Dis. 2014;73:1429-1430.
- Kumar D, Bouldin TW, Berger RG. A case of progressive multifocal leukoencephalopathy in a patient treated with infliximab. Arthritis Rheum. 2010;62:3191-3195.
- Graff-Radford J, Robinson MT, Warsame RM, et al. Progressive multifocal leukoencephalopathy in a patient treated with etanercept. Neurologist. 2012;18:85-87.
- Dickson L, Menter A. Reversible posterior leukoencephalopathy syndrome (RPLS) in a psoriasis patient treated with ustekinumab. J Drugs Dermatol. 2017;16:177-179.
- Gratton D, Szapary P, Goyal K, et al. Reversible posterior leukoencephalopathy syndrome in a patient treated with ustekinumab: case report and review of the literature. Arch Dermatol. 2011;147:1197-1202.
- Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494-500.
- Ramos-Casals M, Roberto-Perez A, Diaz-Lagares C, et al. Autoimmune diseases induced by biological agents: a double-edged sword? Autoimmun Rev. 2010;9:188-193.
- Hoorbakht H, Bagherkashi F. Optic neuritis, its differential diagnosis and management. Open Ophthalmol J. 2012;6:65-72.
- Richards RG, Sampson FC, Beard SM, et al. A review of the natural history and epidemiology of multiple sclerosis: implications for resource allocation and health economic models. Health Technol Assess. 2002;6:1-73.
- Caracseghi F, Izquierdo-Blasco J, Sanchez-Montanez A, et al. Etanercept-induced myelopathy in a pediatric case of blau syndrome [published online January 15, 2012]. Case Rep Rheumatol. 2011;2011:134106.
- Fromont A, De Seze J, Fleury MC, et al. Inflammatory demyelinating events following treatment with anti-tumor necrosis factor. Cytokine. 2009;45:55-57.
- Sellner J, Lüthi N, Schüpbach WM, et al. Diagnostic workup of patients with acute transverse myelitis: spectrum of clinical presentation, neuroimaging and laboratory findings. Spinal Cord. 2009;47:312-317.
- Turatti M, Tamburin S, Idone D, et al. Guillain-Barré syndrome after short-course efalizumab treatment. J Neurol. 2010;257:1404-1405.
- Koga M, Yuki N, Hirata K. Antecedent symptoms in Guillain-Barré syndrome: an important indicator for clinical and serological subgroups. Acta Neurol Scand. 2001;103:278-287.
- Cesarini M, Angelucci E, Foglietta T, et al. Guillain-Barré syndrome after treatment with human anti-tumor necrosis factor alpha (adalimumab) in a Crohn’s disease patient: case report and literature review [published online July 28, 2011]. J Crohns Colitis. 2011;5:619-622.
- Soto-Cabrera E, Hernández-Martínez A, Yañez H, et al. Guillain-Barré syndrome. Its association with alpha tumor necrosis factor [in Spanish]. Rev Med Inst Mex Seguro Soc. 2012;50:565-567.
- Shin IS, Baer AN, Kwon HJ, et al. Guillain-Barré and Miller Fisher syndromes occurring with tumor necrosis factor alpha antagonist therapy. Arthritis Rheum. 2006;54:1429-1434.
- Alvarez-Lario B, Prieto-Tejedo R, Colazo-Burlato M, et al. Severe Guillain-Barré syndrome in a patient receiving anti-TNF therapy. consequence or coincidence. a case-based review. Clin Rheumatol. 2013;32:1407-1412.
- Garnock-Jones KP. Secukinumab: a review in moderate to severe plaque psoriasis. Am J Clin Dermatol. 2015;16:323-330.
Practice Points
- Patients with a personal or strong family history of demyelinating disease should be considered for alternative treatment options before initiating anti–tumor necrosis factor (TNF) α therapy.
- Patients on biologic agents, especially TNF-α inhibitors, with subacute or rapidly progressive visual, motor, or sensory changes or a single neurologic deficit may warrant referral to neurology and/or neuroimaging.
Perceptions of Tanning Risk Among Melanoma Patients With a History of Indoor Tanning
The incidence of melanoma is increasing at a rate greater than any other cancer,1 possibly due to the increasing use of indoor tanning devices. These devices emit unnaturally high levels of UVA and low levels of UVA and UVB rays.2 The risks of using these devices include increased incidence of melanoma (3438 cases attributed to indoor tanning in 2008) and keratinocytes cancer (increased risk of squamous cell carcinoma by 67% and basal cell carcinoma by 29%), severe sunburns (61.1% of female users and 44.6% of male users have reported sunburns), and aggravation of underlying disorders such as systemic lupus erythematosus.3-5
The literature varies in its explanation of how indoor tanning increases the risk of developing melanoma. Some authors suggest it is due to increased frequency of use, duration of sessions, and years of using tanning devices.1,6 Others suggest the increased cancer risk is the result of starting to tan at an earlier age.2,3,6-10 There is conflicting literature on the level of increased risk of melanoma in those who tan indoors at a young age (<35 years). Although the estimated rate of increased skin cancer risk varies, with rates up to 75% compared to nonusers, nearly all sources support an increased rate.6 Despite the growing body of knowledge that indoor tanning is dangerous, as well as the academic publication of these risks (eg, carcinogenesis, short-term and long-term eye injury, burns, UV sensitivity when combined with certain medications), teenagers in the United States and affluent countries appear to disregard the risks of tanning.11
Tanning companies have promoted the misconception that only UVB rays cause cell damage and UVA rays, which the devices emit, result in “damage-free” or “safe” tans.2,3 Until 2013, indoor tanning devices were classified by the US Food and Drug Administration (FDA) as class I, indicating that they are safe in terms of electrical shock. Many indoor tanning facilities have promoted the FDA “safe” label without clarifying that the safety indications only referred to electrical-shock potential. Nonetheless, it is known now that these devices, which emit high UVA and low UVB rays, promote melanoma, nonmelanoma skin cancers, and severe sunburns, as well as aggravate existing conditions (eg, systemic lupus erythematosus).4 As a result of an unacceptably high incidence of these disease complications, a 2014 FDA regulation categorized tanning beds as class II, requiring that tanning bed users be informed of the risk of skin cancer in an effort to reverse the growing trend of indoor tanning.12 Despite these regulatory interventions, it is not clear if this knowledge of cancer risk deters patients from indoor tanning.
The purpose of this study was to investigate the patients’ perspective on indoor tanning behaviors as associated with the severity of their melanoma and the time frame in which they were diagnosed as well as their perceived views on the safety of indoor tanning and the frequency in which they continue to tan indoors. This information is highly relevant in helping to determine if requiring a warning of the risk of skin cancer will deter patients from this unhealthy habit, especially given recent reclassification of sunbeds as class II by the FDA. Additional insights from these data may clarify if indoor tanning decreases the time frame in which melanoma is diagnosed or increases the severity of the resulting melanoma. Moreover, it will help elucidate whether or not the age at which indoor tanning is initiated affects the time frame to melanoma onset and corresponding severity.
Methods
An original unvalidated online survey was conducted worldwide via a link distributed to the following supporting institutions: Advanced Dermatology & Cosmetic Surgery, Ameriderm Research, Melanoma Research Foundation (a melanoma patient advocacy group), Florida State University Department of Dermatology, Moffitt Cancer Center Cutaneous Oncology Program, Cleveland Clinic, Ohio State University Division of Medical Oncology, Harvard Medical School Department of Dermatology, The University of Texas MD Anderson Cancer Center Department of Dermatology, University of Colorado Department of Dermatology, and Northwestern University Department of Dermatology. However, there was not confirmation that all of these institutions promoted the survey. Additionally, respondents were recruited through patient advocacy groups and social media sites including Facebook, Twitter, LinkedIn, Tumblr, and Instagram. The patient advocacy groups and social media sites invited participation through recruitment announcements, including DermNetNZ (a global dermatology patient information site), with additional help from the International Federation of Dermatology Clinical Trial Network.
The survey was restricted to those who were self-identified as 18 years or older and who self-reported a diagnosis of melanoma following the use of indoor tanning devices. The survey was hosted by SurveyMonkey, which allowed consent to be obtained and responses to remain anonymous. Access to the survey was sponsored by the Basal Cell Carcinoma Nevus Syndrome Life Support Network. The University of Central Florida (Orlando, Florida) institutional review board reviewed and approved this study as exempt human research.
Survey responses collected from January 2014 to June 2015 were analyzed herein. The survey contained 58 questions and was divided into different topics including indoor tanning background (eg, states/countries in which participants tanned indoors, age when they first tanned, frequency of tanning), consenting process (eg, length, did someone review the consent with participants, what was contained in the consent), indoor tanning and melanoma (eg, how long after tanning did melanoma develop, age at development, location of melanoma), indoor tanning postmelanoma (eg, did participants tan after diagnosis and why), and other risk factors (eg, did participants smoke or drink pre- or postmelanoma).
Statistical Analysis
The data consist of both categorical and continuous variables. The categorical variables included age (<35 years or ≥35 years), frequency of indoor tanning (≤1 time weekly or >1 time weekly), and onset of melanoma diagnosis (within or after 5 years
Difference in proportions among groups, age, frequency of tanning, onset of melanoma diagnosis within or after 5 years of starting indoor tanning, and knowledge of cancer risks was tested for significance using the χ² test. Reported P values were 2-tailed, corresponding with a significance level of P<.05. All data were analyzed using SPSS (version 21.0). All statistical analyses were conducted independent of the participants’ sex.
Results
Of the 454 participants who accessed the survey, 448 were analyzed in this study; 6 participants did not complete the questionnaire. Both males and females were analyzed: 289 females, 12 males, and 153 who did not report gender. The age range of participants was 18 to 69 years. The age at start of indoor tanning ranged from 8 to 54 years, with a mean of 22 years. Additional participant characteristics are described in Table 1. The mean frequency of indoor tanning was reported as 2 times weekly. When participants were asked if they were warned of the risk of skin cancer, 21.5% reported yes while 78.4% reported not being told of the risk. This knowledge was compared to their frequency of indoor tanning. Having the knowledge of the risk of skin cancer had no influence on their frequency of indoor tanning (Table 2).
Among responders, those who perceived indoor tanning as safer than outdoor tanning tanned indoors more frequently than those who do not (Spearman r=−0.224; P<.05)(Table 3). The frequency of indoor tanning was divided into those who tanned indoors more than once weekly and those who tanned indoors once a week or less. This study showed that the frequency of indoor tanning had no effect on the latency time between the commencement of indoor tanning and diagnosis of melanoma (Table 4). The time frame from the onset of melanoma diagnosis also was compared to the age at which the participants started to tan indoors. Age was divided into those younger than 35 years and those 35 years and older. There was no correlation between the age when indoor tanning began and the time frame in which the melanoma was diagnosed (eTable).
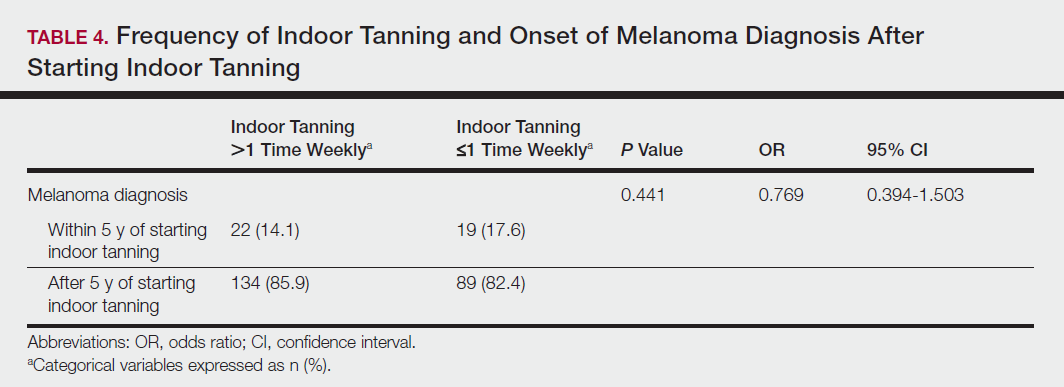
Table 5 shows the correlations between indoor tanning behaviors and melanoma characteristics. Those who started indoor tanning at an earlier age were diagnosed with melanoma at an earlier age compared to those who started indoor tanning later in life (r=0.549; P<.01). Moreover, those who started indoor tanning at a later age reported being diagnosed with a melanoma of greater Breslow depth (r=0.173; P<.01). Those who reported being diagnosed with a greater Breslow depth also reported a higher Clark level (r=0.608; P<.01). Among responders, those who more frequently tanned indoors also reported greater frequency of outdoor tanning (r=0.197; P<.01). This study showed no correlation between the age at melanoma diagnosis and the frequency of indoor (r=0.004; P>.05 not significant) or outdoor (r=0.093; P>.05 not significant) tanning. Having the knowledge of the risk of skin cancer had no relationship on the frequency of indoor tanning (r=−0.04; P>.05 not significant).
Comment
Thirty million Americans utilize indoor tanning devices at least once a year.13 UVA light comprises the majority of the spectrum used by indoor tanning devices, with a fraction (<5%) being UVB light. Until recently, UVB light was the only solar spectrum considered carcinogenic. In 2009, the International Agency for Research on Cancer classified the whole spectrum as carcinogenic to humans.5,11 Despite this evidence, indoor tanning facilities have promoted indoor tanning as damage free.3 The goal of this study was to collect the patient perspective on the safety of indoor tanning, indoor tanning behaviors, time frame of onset of melanoma, and the severity (ie, Breslow depth) of those melanomas.
Melanoma is the most prevalent cancer in females aged 25 to 29 years.3 The median age of diagnosis of melanoma (with and without the use of indoor tanning devices) is approximately 60 years14 versus our study, which found the average age at diagnosis was 37.6 years. Our findings are consistent with other literature in that those who start indoor tanning earlier (<35 years of age) develop melanoma at an earlier age.14,15 Cust et al14 also promoted the idea that patients develop melanoma earlier because younger individuals are more biologically susceptible to the carcinogenic effects of artificial UV light. However, our study found that those who started indoor tanning at an older age reported being diagnosed with a melanoma of greater Breslow depth, seemingly incongruent with the aforementioned hypothesis. One limitation is the age range for this research sample (18–69 years). The young age range may be attributable to the recruitment through social media, which is geared toward a younger population. Additionally, indoor tanning is a relatively new phenomenon practiced since the 1980s,2 which may contribute to the younger sample size. However, 2.7 billion individuals use social media worldwide with 40% of those older than 65 years on social media.16
Prior research has shown that those who start indoor tanning before the age of 35 years have a 75% increased risk of developing melanoma.14 Another study also has suggested that UVA-rich sunlamps may shorten the latency period for induction of melanoma and nonmelanoma skin cancers.3 Our study used similar age cutoffs in concluding that there was no earlier onset of melanoma diagnosis between those who started indoor tanning before the age of 35 years and those who started at the age of 35 years or older. Limitations include that our study is cross-sectional, and therefore time course cannot be established. Also, survey responses were self-reported, allowing the possibility of recall bias.
A plethora of research has been conducted to determine if there is a connection between the use of indoor tanning devices and developing melanoma. Cust et al14 suggested the risk of melanoma was 41% higher for those who had ever used a sunbed in comparison to those who had not. Other studies describe the difficulty in making the connection between indoor tanning and melanoma, as those who more frequently tan indoors also more frequently tan outdoors,11 as suggested by this study. However, there is a paucity of literature on the patients’ perspectives on the safety of indoor tanning. This study determined that those who more frequently tan indoors believed that indoor tanning is safer than outdoor tanning. With this altered perception promoted by the indoor tanning industry, the FDA has added a warning label to all indoor tanning devices about the risk of skin cancer. Our study revealed that having the knowledge of the risk of skin cancer had no influence on the frequency of indoor tanning. This concerning finding highlights a pressing need for an alternative approach to increase awareness of the harmful consequences that accompany indoor tanning. Further studies may elaborate on potential effective methods and messages to relate to an indoor tanning population comprised mostly of young females.
Acknowledgments
Supported and funded by the Basal Cell Carcinoma Nevus Syndrome Life Support Network. This research project was completed as part of the FIRE Module at the University of Central Florida, College of Medicine. We thank the FIRE Module faculty and staff for their assistance with this project.
- Fisher DE, James WD. Indoor tanning—science, behavior, and policy. N Engl J Med. 2010;363:901-903.
- Boniol M, Autier P, Boyle P, et al. Cutaneous melanoma attributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:e4757.
- Coelho SG, Hearing VJ. UVA tanning is involved in the increased incidence of skin cancers in fair-skinned young women. Pigment Cell Melanoma Res. 2010;23:57-63.
- Klein RS, Sayre RM, Dowdy JC, et al. The risk of ultraviolet radiation exposure from indoor lamps in lupus erythematosus. Autoimmun Rev. 2009;8:320-324.
- O’Sullivan NA, Tait CP. Tanning bed and nail lamp use and the risk of cutaneous malignancy: a review of the literature. Australas J Dermatol. 2014;55:99-106.
- Schmidt CW. UV radiation and skin cancer: the science behind age restrictions for tanning beds. Environ Health Perspect. 2012;120:a308-a313.
- Lazovich D, Vogel RI, Berwick M, et al. Indoor tanning and risk of melanoma: a case-control study in a highly exposed population. Cancer Epidemiol Biomarkers Prev. 2010;19:1557-1568.
- Centers for Disease Control and Prevention (CDC). Use of indoor tanning devices by adults—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:323-326.
- Nielsen K, Masback A, Olsson H, et al. A prospective, population-based study of 40,000 women regarding host factors, UV exposure and sunbed use in relation to risk and anatomic site of cutaneous melanoma. Int J Cancer. 2012;131:706-715.
- Gandini S, Autier P, Boniol M. Reviews on sun exposure and artificial light and melanoma. Prog Biophys Mol Biol. 2011;107:362-366.
- Indoor tanning: the risks of ultraviolet rays. US Food and Drug Administration website. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm186687.htm. Updated September 11, 2017. Accessed November 2, 2017.
- Food and Drug Administration, HHS. General and plastic surgery devices: reclassification of ultraviolet lamps for tanning, henceforth to be known as sunlamp products and ultraviolet lamps intended for use in sunlamp products. Fed Regist. 2014;79:31205-31214.
- Brady MS. Public health and the tanning bed controversy. J Clin Oncol. 2012;30:1571-1573.
- Cust AE, Armstrong BK, Goumas C, et al. Sunbed use during adolescence and early adulthood is associated with increased risk of early-onset melanoma. Int J Cancer. 2011;128:2425-2435.
- International Agency for Research on Cancer Working Group on artificial ultraviolet (UV) light and skin cancer. The association of use of sunbeds with cutaneous malignant melanoma and other skin cancers: a systematic review. Int J Cancer. 2007;120:1116-1122.
- Greenwood S, Perrin A, Duggan M. Social media update 2016. Pew Research Center website. http://www.pewinternet.org/2016/11/11/social-media-update-2016/. Published November 11, 2016. Accessed December 12, 2017.
The incidence of melanoma is increasing at a rate greater than any other cancer,1 possibly due to the increasing use of indoor tanning devices. These devices emit unnaturally high levels of UVA and low levels of UVA and UVB rays.2 The risks of using these devices include increased incidence of melanoma (3438 cases attributed to indoor tanning in 2008) and keratinocytes cancer (increased risk of squamous cell carcinoma by 67% and basal cell carcinoma by 29%), severe sunburns (61.1% of female users and 44.6% of male users have reported sunburns), and aggravation of underlying disorders such as systemic lupus erythematosus.3-5
The literature varies in its explanation of how indoor tanning increases the risk of developing melanoma. Some authors suggest it is due to increased frequency of use, duration of sessions, and years of using tanning devices.1,6 Others suggest the increased cancer risk is the result of starting to tan at an earlier age.2,3,6-10 There is conflicting literature on the level of increased risk of melanoma in those who tan indoors at a young age (<35 years). Although the estimated rate of increased skin cancer risk varies, with rates up to 75% compared to nonusers, nearly all sources support an increased rate.6 Despite the growing body of knowledge that indoor tanning is dangerous, as well as the academic publication of these risks (eg, carcinogenesis, short-term and long-term eye injury, burns, UV sensitivity when combined with certain medications), teenagers in the United States and affluent countries appear to disregard the risks of tanning.11
Tanning companies have promoted the misconception that only UVB rays cause cell damage and UVA rays, which the devices emit, result in “damage-free” or “safe” tans.2,3 Until 2013, indoor tanning devices were classified by the US Food and Drug Administration (FDA) as class I, indicating that they are safe in terms of electrical shock. Many indoor tanning facilities have promoted the FDA “safe” label without clarifying that the safety indications only referred to electrical-shock potential. Nonetheless, it is known now that these devices, which emit high UVA and low UVB rays, promote melanoma, nonmelanoma skin cancers, and severe sunburns, as well as aggravate existing conditions (eg, systemic lupus erythematosus).4 As a result of an unacceptably high incidence of these disease complications, a 2014 FDA regulation categorized tanning beds as class II, requiring that tanning bed users be informed of the risk of skin cancer in an effort to reverse the growing trend of indoor tanning.12 Despite these regulatory interventions, it is not clear if this knowledge of cancer risk deters patients from indoor tanning.
The purpose of this study was to investigate the patients’ perspective on indoor tanning behaviors as associated with the severity of their melanoma and the time frame in which they were diagnosed as well as their perceived views on the safety of indoor tanning and the frequency in which they continue to tan indoors. This information is highly relevant in helping to determine if requiring a warning of the risk of skin cancer will deter patients from this unhealthy habit, especially given recent reclassification of sunbeds as class II by the FDA. Additional insights from these data may clarify if indoor tanning decreases the time frame in which melanoma is diagnosed or increases the severity of the resulting melanoma. Moreover, it will help elucidate whether or not the age at which indoor tanning is initiated affects the time frame to melanoma onset and corresponding severity.
Methods
An original unvalidated online survey was conducted worldwide via a link distributed to the following supporting institutions: Advanced Dermatology & Cosmetic Surgery, Ameriderm Research, Melanoma Research Foundation (a melanoma patient advocacy group), Florida State University Department of Dermatology, Moffitt Cancer Center Cutaneous Oncology Program, Cleveland Clinic, Ohio State University Division of Medical Oncology, Harvard Medical School Department of Dermatology, The University of Texas MD Anderson Cancer Center Department of Dermatology, University of Colorado Department of Dermatology, and Northwestern University Department of Dermatology. However, there was not confirmation that all of these institutions promoted the survey. Additionally, respondents were recruited through patient advocacy groups and social media sites including Facebook, Twitter, LinkedIn, Tumblr, and Instagram. The patient advocacy groups and social media sites invited participation through recruitment announcements, including DermNetNZ (a global dermatology patient information site), with additional help from the International Federation of Dermatology Clinical Trial Network.
The survey was restricted to those who were self-identified as 18 years or older and who self-reported a diagnosis of melanoma following the use of indoor tanning devices. The survey was hosted by SurveyMonkey, which allowed consent to be obtained and responses to remain anonymous. Access to the survey was sponsored by the Basal Cell Carcinoma Nevus Syndrome Life Support Network. The University of Central Florida (Orlando, Florida) institutional review board reviewed and approved this study as exempt human research.
Survey responses collected from January 2014 to June 2015 were analyzed herein. The survey contained 58 questions and was divided into different topics including indoor tanning background (eg, states/countries in which participants tanned indoors, age when they first tanned, frequency of tanning), consenting process (eg, length, did someone review the consent with participants, what was contained in the consent), indoor tanning and melanoma (eg, how long after tanning did melanoma develop, age at development, location of melanoma), indoor tanning postmelanoma (eg, did participants tan after diagnosis and why), and other risk factors (eg, did participants smoke or drink pre- or postmelanoma).
Statistical Analysis
The data consist of both categorical and continuous variables. The categorical variables included age (<35 years or ≥35 years), frequency of indoor tanning (≤1 time weekly or >1 time weekly), and onset of melanoma diagnosis (within or after 5 years
Difference in proportions among groups, age, frequency of tanning, onset of melanoma diagnosis within or after 5 years of starting indoor tanning, and knowledge of cancer risks was tested for significance using the χ² test. Reported P values were 2-tailed, corresponding with a significance level of P<.05. All data were analyzed using SPSS (version 21.0). All statistical analyses were conducted independent of the participants’ sex.
Results
Of the 454 participants who accessed the survey, 448 were analyzed in this study; 6 participants did not complete the questionnaire. Both males and females were analyzed: 289 females, 12 males, and 153 who did not report gender. The age range of participants was 18 to 69 years. The age at start of indoor tanning ranged from 8 to 54 years, with a mean of 22 years. Additional participant characteristics are described in Table 1. The mean frequency of indoor tanning was reported as 2 times weekly. When participants were asked if they were warned of the risk of skin cancer, 21.5% reported yes while 78.4% reported not being told of the risk. This knowledge was compared to their frequency of indoor tanning. Having the knowledge of the risk of skin cancer had no influence on their frequency of indoor tanning (Table 2).


Among responders, those who perceived indoor tanning as safer than outdoor tanning tanned indoors more frequently than those who do not (Spearman r=−0.224; P<.05)(Table 3). The frequency of indoor tanning was divided into those who tanned indoors more than once weekly and those who tanned indoors once a week or less. This study showed that the frequency of indoor tanning had no effect on the latency time between the commencement of indoor tanning and diagnosis of melanoma (Table 4). The time frame from the onset of melanoma diagnosis also was compared to the age at which the participants started to tan indoors. Age was divided into those younger than 35 years and those 35 years and older. There was no correlation between the age when indoor tanning began and the time frame in which the melanoma was diagnosed (eTable).



Table 5 shows the correlations between indoor tanning behaviors and melanoma characteristics. Those who started indoor tanning at an earlier age were diagnosed with melanoma at an earlier age compared to those who started indoor tanning later in life (r=0.549; P<.01). Moreover, those who started indoor tanning at a later age reported being diagnosed with a melanoma of greater Breslow depth (r=0.173; P<.01). Those who reported being diagnosed with a greater Breslow depth also reported a higher Clark level (r=0.608; P<.01). Among responders, those who more frequently tanned indoors also reported greater frequency of outdoor tanning (r=0.197; P<.01). This study showed no correlation between the age at melanoma diagnosis and the frequency of indoor (r=0.004; P>.05 not significant) or outdoor (r=0.093; P>.05 not significant) tanning. Having the knowledge of the risk of skin cancer had no relationship on the frequency of indoor tanning (r=−0.04; P>.05 not significant).

Comment
Thirty million Americans utilize indoor tanning devices at least once a year.13 UVA light comprises the majority of the spectrum used by indoor tanning devices, with a fraction (<5%) being UVB light. Until recently, UVB light was the only solar spectrum considered carcinogenic. In 2009, the International Agency for Research on Cancer classified the whole spectrum as carcinogenic to humans.5,11 Despite this evidence, indoor tanning facilities have promoted indoor tanning as damage free.3 The goal of this study was to collect the patient perspective on the safety of indoor tanning, indoor tanning behaviors, time frame of onset of melanoma, and the severity (ie, Breslow depth) of those melanomas.
Melanoma is the most prevalent cancer in females aged 25 to 29 years.3 The median age of diagnosis of melanoma (with and without the use of indoor tanning devices) is approximately 60 years14 versus our study, which found the average age at diagnosis was 37.6 years. Our findings are consistent with other literature in that those who start indoor tanning earlier (<35 years of age) develop melanoma at an earlier age.14,15 Cust et al14 also promoted the idea that patients develop melanoma earlier because younger individuals are more biologically susceptible to the carcinogenic effects of artificial UV light. However, our study found that those who started indoor tanning at an older age reported being diagnosed with a melanoma of greater Breslow depth, seemingly incongruent with the aforementioned hypothesis. One limitation is the age range for this research sample (18–69 years). The young age range may be attributable to the recruitment through social media, which is geared toward a younger population. Additionally, indoor tanning is a relatively new phenomenon practiced since the 1980s,2 which may contribute to the younger sample size. However, 2.7 billion individuals use social media worldwide with 40% of those older than 65 years on social media.16
Prior research has shown that those who start indoor tanning before the age of 35 years have a 75% increased risk of developing melanoma.14 Another study also has suggested that UVA-rich sunlamps may shorten the latency period for induction of melanoma and nonmelanoma skin cancers.3 Our study used similar age cutoffs in concluding that there was no earlier onset of melanoma diagnosis between those who started indoor tanning before the age of 35 years and those who started at the age of 35 years or older. Limitations include that our study is cross-sectional, and therefore time course cannot be established. Also, survey responses were self-reported, allowing the possibility of recall bias.
A plethora of research has been conducted to determine if there is a connection between the use of indoor tanning devices and developing melanoma. Cust et al14 suggested the risk of melanoma was 41% higher for those who had ever used a sunbed in comparison to those who had not. Other studies describe the difficulty in making the connection between indoor tanning and melanoma, as those who more frequently tan indoors also more frequently tan outdoors,11 as suggested by this study. However, there is a paucity of literature on the patients’ perspectives on the safety of indoor tanning. This study determined that those who more frequently tan indoors believed that indoor tanning is safer than outdoor tanning. With this altered perception promoted by the indoor tanning industry, the FDA has added a warning label to all indoor tanning devices about the risk of skin cancer. Our study revealed that having the knowledge of the risk of skin cancer had no influence on the frequency of indoor tanning. This concerning finding highlights a pressing need for an alternative approach to increase awareness of the harmful consequences that accompany indoor tanning. Further studies may elaborate on potential effective methods and messages to relate to an indoor tanning population comprised mostly of young females.
Acknowledgments
Supported and funded by the Basal Cell Carcinoma Nevus Syndrome Life Support Network. This research project was completed as part of the FIRE Module at the University of Central Florida, College of Medicine. We thank the FIRE Module faculty and staff for their assistance with this project.
The incidence of melanoma is increasing at a rate greater than any other cancer,1 possibly due to the increasing use of indoor tanning devices. These devices emit unnaturally high levels of UVA and low levels of UVA and UVB rays.2 The risks of using these devices include increased incidence of melanoma (3438 cases attributed to indoor tanning in 2008) and keratinocytes cancer (increased risk of squamous cell carcinoma by 67% and basal cell carcinoma by 29%), severe sunburns (61.1% of female users and 44.6% of male users have reported sunburns), and aggravation of underlying disorders such as systemic lupus erythematosus.3-5
The literature varies in its explanation of how indoor tanning increases the risk of developing melanoma. Some authors suggest it is due to increased frequency of use, duration of sessions, and years of using tanning devices.1,6 Others suggest the increased cancer risk is the result of starting to tan at an earlier age.2,3,6-10 There is conflicting literature on the level of increased risk of melanoma in those who tan indoors at a young age (<35 years). Although the estimated rate of increased skin cancer risk varies, with rates up to 75% compared to nonusers, nearly all sources support an increased rate.6 Despite the growing body of knowledge that indoor tanning is dangerous, as well as the academic publication of these risks (eg, carcinogenesis, short-term and long-term eye injury, burns, UV sensitivity when combined with certain medications), teenagers in the United States and affluent countries appear to disregard the risks of tanning.11
Tanning companies have promoted the misconception that only UVB rays cause cell damage and UVA rays, which the devices emit, result in “damage-free” or “safe” tans.2,3 Until 2013, indoor tanning devices were classified by the US Food and Drug Administration (FDA) as class I, indicating that they are safe in terms of electrical shock. Many indoor tanning facilities have promoted the FDA “safe” label without clarifying that the safety indications only referred to electrical-shock potential. Nonetheless, it is known now that these devices, which emit high UVA and low UVB rays, promote melanoma, nonmelanoma skin cancers, and severe sunburns, as well as aggravate existing conditions (eg, systemic lupus erythematosus).4 As a result of an unacceptably high incidence of these disease complications, a 2014 FDA regulation categorized tanning beds as class II, requiring that tanning bed users be informed of the risk of skin cancer in an effort to reverse the growing trend of indoor tanning.12 Despite these regulatory interventions, it is not clear if this knowledge of cancer risk deters patients from indoor tanning.
The purpose of this study was to investigate the patients’ perspective on indoor tanning behaviors as associated with the severity of their melanoma and the time frame in which they were diagnosed as well as their perceived views on the safety of indoor tanning and the frequency in which they continue to tan indoors. This information is highly relevant in helping to determine if requiring a warning of the risk of skin cancer will deter patients from this unhealthy habit, especially given recent reclassification of sunbeds as class II by the FDA. Additional insights from these data may clarify if indoor tanning decreases the time frame in which melanoma is diagnosed or increases the severity of the resulting melanoma. Moreover, it will help elucidate whether or not the age at which indoor tanning is initiated affects the time frame to melanoma onset and corresponding severity.
Methods
An original unvalidated online survey was conducted worldwide via a link distributed to the following supporting institutions: Advanced Dermatology & Cosmetic Surgery, Ameriderm Research, Melanoma Research Foundation (a melanoma patient advocacy group), Florida State University Department of Dermatology, Moffitt Cancer Center Cutaneous Oncology Program, Cleveland Clinic, Ohio State University Division of Medical Oncology, Harvard Medical School Department of Dermatology, The University of Texas MD Anderson Cancer Center Department of Dermatology, University of Colorado Department of Dermatology, and Northwestern University Department of Dermatology. However, there was not confirmation that all of these institutions promoted the survey. Additionally, respondents were recruited through patient advocacy groups and social media sites including Facebook, Twitter, LinkedIn, Tumblr, and Instagram. The patient advocacy groups and social media sites invited participation through recruitment announcements, including DermNetNZ (a global dermatology patient information site), with additional help from the International Federation of Dermatology Clinical Trial Network.
The survey was restricted to those who were self-identified as 18 years or older and who self-reported a diagnosis of melanoma following the use of indoor tanning devices. The survey was hosted by SurveyMonkey, which allowed consent to be obtained and responses to remain anonymous. Access to the survey was sponsored by the Basal Cell Carcinoma Nevus Syndrome Life Support Network. The University of Central Florida (Orlando, Florida) institutional review board reviewed and approved this study as exempt human research.
Survey responses collected from January 2014 to June 2015 were analyzed herein. The survey contained 58 questions and was divided into different topics including indoor tanning background (eg, states/countries in which participants tanned indoors, age when they first tanned, frequency of tanning), consenting process (eg, length, did someone review the consent with participants, what was contained in the consent), indoor tanning and melanoma (eg, how long after tanning did melanoma develop, age at development, location of melanoma), indoor tanning postmelanoma (eg, did participants tan after diagnosis and why), and other risk factors (eg, did participants smoke or drink pre- or postmelanoma).
Statistical Analysis
The data consist of both categorical and continuous variables. The categorical variables included age (<35 years or ≥35 years), frequency of indoor tanning (≤1 time weekly or >1 time weekly), and onset of melanoma diagnosis (within or after 5 years
Difference in proportions among groups, age, frequency of tanning, onset of melanoma diagnosis within or after 5 years of starting indoor tanning, and knowledge of cancer risks was tested for significance using the χ² test. Reported P values were 2-tailed, corresponding with a significance level of P<.05. All data were analyzed using SPSS (version 21.0). All statistical analyses were conducted independent of the participants’ sex.
Results
Of the 454 participants who accessed the survey, 448 were analyzed in this study; 6 participants did not complete the questionnaire. Both males and females were analyzed: 289 females, 12 males, and 153 who did not report gender. The age range of participants was 18 to 69 years. The age at start of indoor tanning ranged from 8 to 54 years, with a mean of 22 years. Additional participant characteristics are described in Table 1. The mean frequency of indoor tanning was reported as 2 times weekly. When participants were asked if they were warned of the risk of skin cancer, 21.5% reported yes while 78.4% reported not being told of the risk. This knowledge was compared to their frequency of indoor tanning. Having the knowledge of the risk of skin cancer had no influence on their frequency of indoor tanning (Table 2).


Among responders, those who perceived indoor tanning as safer than outdoor tanning tanned indoors more frequently than those who do not (Spearman r=−0.224; P<.05)(Table 3). The frequency of indoor tanning was divided into those who tanned indoors more than once weekly and those who tanned indoors once a week or less. This study showed that the frequency of indoor tanning had no effect on the latency time between the commencement of indoor tanning and diagnosis of melanoma (Table 4). The time frame from the onset of melanoma diagnosis also was compared to the age at which the participants started to tan indoors. Age was divided into those younger than 35 years and those 35 years and older. There was no correlation between the age when indoor tanning began and the time frame in which the melanoma was diagnosed (eTable).



Table 5 shows the correlations between indoor tanning behaviors and melanoma characteristics. Those who started indoor tanning at an earlier age were diagnosed with melanoma at an earlier age compared to those who started indoor tanning later in life (r=0.549; P<.01). Moreover, those who started indoor tanning at a later age reported being diagnosed with a melanoma of greater Breslow depth (r=0.173; P<.01). Those who reported being diagnosed with a greater Breslow depth also reported a higher Clark level (r=0.608; P<.01). Among responders, those who more frequently tanned indoors also reported greater frequency of outdoor tanning (r=0.197; P<.01). This study showed no correlation between the age at melanoma diagnosis and the frequency of indoor (r=0.004; P>.05 not significant) or outdoor (r=0.093; P>.05 not significant) tanning. Having the knowledge of the risk of skin cancer had no relationship on the frequency of indoor tanning (r=−0.04; P>.05 not significant).

Comment
Thirty million Americans utilize indoor tanning devices at least once a year.13 UVA light comprises the majority of the spectrum used by indoor tanning devices, with a fraction (<5%) being UVB light. Until recently, UVB light was the only solar spectrum considered carcinogenic. In 2009, the International Agency for Research on Cancer classified the whole spectrum as carcinogenic to humans.5,11 Despite this evidence, indoor tanning facilities have promoted indoor tanning as damage free.3 The goal of this study was to collect the patient perspective on the safety of indoor tanning, indoor tanning behaviors, time frame of onset of melanoma, and the severity (ie, Breslow depth) of those melanomas.
Melanoma is the most prevalent cancer in females aged 25 to 29 years.3 The median age of diagnosis of melanoma (with and without the use of indoor tanning devices) is approximately 60 years14 versus our study, which found the average age at diagnosis was 37.6 years. Our findings are consistent with other literature in that those who start indoor tanning earlier (<35 years of age) develop melanoma at an earlier age.14,15 Cust et al14 also promoted the idea that patients develop melanoma earlier because younger individuals are more biologically susceptible to the carcinogenic effects of artificial UV light. However, our study found that those who started indoor tanning at an older age reported being diagnosed with a melanoma of greater Breslow depth, seemingly incongruent with the aforementioned hypothesis. One limitation is the age range for this research sample (18–69 years). The young age range may be attributable to the recruitment through social media, which is geared toward a younger population. Additionally, indoor tanning is a relatively new phenomenon practiced since the 1980s,2 which may contribute to the younger sample size. However, 2.7 billion individuals use social media worldwide with 40% of those older than 65 years on social media.16
Prior research has shown that those who start indoor tanning before the age of 35 years have a 75% increased risk of developing melanoma.14 Another study also has suggested that UVA-rich sunlamps may shorten the latency period for induction of melanoma and nonmelanoma skin cancers.3 Our study used similar age cutoffs in concluding that there was no earlier onset of melanoma diagnosis between those who started indoor tanning before the age of 35 years and those who started at the age of 35 years or older. Limitations include that our study is cross-sectional, and therefore time course cannot be established. Also, survey responses were self-reported, allowing the possibility of recall bias.
A plethora of research has been conducted to determine if there is a connection between the use of indoor tanning devices and developing melanoma. Cust et al14 suggested the risk of melanoma was 41% higher for those who had ever used a sunbed in comparison to those who had not. Other studies describe the difficulty in making the connection between indoor tanning and melanoma, as those who more frequently tan indoors also more frequently tan outdoors,11 as suggested by this study. However, there is a paucity of literature on the patients’ perspectives on the safety of indoor tanning. This study determined that those who more frequently tan indoors believed that indoor tanning is safer than outdoor tanning. With this altered perception promoted by the indoor tanning industry, the FDA has added a warning label to all indoor tanning devices about the risk of skin cancer. Our study revealed that having the knowledge of the risk of skin cancer had no influence on the frequency of indoor tanning. This concerning finding highlights a pressing need for an alternative approach to increase awareness of the harmful consequences that accompany indoor tanning. Further studies may elaborate on potential effective methods and messages to relate to an indoor tanning population comprised mostly of young females.
Acknowledgments
Supported and funded by the Basal Cell Carcinoma Nevus Syndrome Life Support Network. This research project was completed as part of the FIRE Module at the University of Central Florida, College of Medicine. We thank the FIRE Module faculty and staff for their assistance with this project.
- Fisher DE, James WD. Indoor tanning—science, behavior, and policy. N Engl J Med. 2010;363:901-903.
- Boniol M, Autier P, Boyle P, et al. Cutaneous melanoma attributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:e4757.
- Coelho SG, Hearing VJ. UVA tanning is involved in the increased incidence of skin cancers in fair-skinned young women. Pigment Cell Melanoma Res. 2010;23:57-63.
- Klein RS, Sayre RM, Dowdy JC, et al. The risk of ultraviolet radiation exposure from indoor lamps in lupus erythematosus. Autoimmun Rev. 2009;8:320-324.
- O’Sullivan NA, Tait CP. Tanning bed and nail lamp use and the risk of cutaneous malignancy: a review of the literature. Australas J Dermatol. 2014;55:99-106.
- Schmidt CW. UV radiation and skin cancer: the science behind age restrictions for tanning beds. Environ Health Perspect. 2012;120:a308-a313.
- Lazovich D, Vogel RI, Berwick M, et al. Indoor tanning and risk of melanoma: a case-control study in a highly exposed population. Cancer Epidemiol Biomarkers Prev. 2010;19:1557-1568.
- Centers for Disease Control and Prevention (CDC). Use of indoor tanning devices by adults—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:323-326.
- Nielsen K, Masback A, Olsson H, et al. A prospective, population-based study of 40,000 women regarding host factors, UV exposure and sunbed use in relation to risk and anatomic site of cutaneous melanoma. Int J Cancer. 2012;131:706-715.
- Gandini S, Autier P, Boniol M. Reviews on sun exposure and artificial light and melanoma. Prog Biophys Mol Biol. 2011;107:362-366.
- Indoor tanning: the risks of ultraviolet rays. US Food and Drug Administration website. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm186687.htm. Updated September 11, 2017. Accessed November 2, 2017.
- Food and Drug Administration, HHS. General and plastic surgery devices: reclassification of ultraviolet lamps for tanning, henceforth to be known as sunlamp products and ultraviolet lamps intended for use in sunlamp products. Fed Regist. 2014;79:31205-31214.
- Brady MS. Public health and the tanning bed controversy. J Clin Oncol. 2012;30:1571-1573.
- Cust AE, Armstrong BK, Goumas C, et al. Sunbed use during adolescence and early adulthood is associated with increased risk of early-onset melanoma. Int J Cancer. 2011;128:2425-2435.
- International Agency for Research on Cancer Working Group on artificial ultraviolet (UV) light and skin cancer. The association of use of sunbeds with cutaneous malignant melanoma and other skin cancers: a systematic review. Int J Cancer. 2007;120:1116-1122.
- Greenwood S, Perrin A, Duggan M. Social media update 2016. Pew Research Center website. http://www.pewinternet.org/2016/11/11/social-media-update-2016/. Published November 11, 2016. Accessed December 12, 2017.
- Fisher DE, James WD. Indoor tanning—science, behavior, and policy. N Engl J Med. 2010;363:901-903.
- Boniol M, Autier P, Boyle P, et al. Cutaneous melanoma attributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:e4757.
- Coelho SG, Hearing VJ. UVA tanning is involved in the increased incidence of skin cancers in fair-skinned young women. Pigment Cell Melanoma Res. 2010;23:57-63.
- Klein RS, Sayre RM, Dowdy JC, et al. The risk of ultraviolet radiation exposure from indoor lamps in lupus erythematosus. Autoimmun Rev. 2009;8:320-324.
- O’Sullivan NA, Tait CP. Tanning bed and nail lamp use and the risk of cutaneous malignancy: a review of the literature. Australas J Dermatol. 2014;55:99-106.
- Schmidt CW. UV radiation and skin cancer: the science behind age restrictions for tanning beds. Environ Health Perspect. 2012;120:a308-a313.
- Lazovich D, Vogel RI, Berwick M, et al. Indoor tanning and risk of melanoma: a case-control study in a highly exposed population. Cancer Epidemiol Biomarkers Prev. 2010;19:1557-1568.
- Centers for Disease Control and Prevention (CDC). Use of indoor tanning devices by adults—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:323-326.
- Nielsen K, Masback A, Olsson H, et al. A prospective, population-based study of 40,000 women regarding host factors, UV exposure and sunbed use in relation to risk and anatomic site of cutaneous melanoma. Int J Cancer. 2012;131:706-715.
- Gandini S, Autier P, Boniol M. Reviews on sun exposure and artificial light and melanoma. Prog Biophys Mol Biol. 2011;107:362-366.
- Indoor tanning: the risks of ultraviolet rays. US Food and Drug Administration website. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm186687.htm. Updated September 11, 2017. Accessed November 2, 2017.
- Food and Drug Administration, HHS. General and plastic surgery devices: reclassification of ultraviolet lamps for tanning, henceforth to be known as sunlamp products and ultraviolet lamps intended for use in sunlamp products. Fed Regist. 2014;79:31205-31214.
- Brady MS. Public health and the tanning bed controversy. J Clin Oncol. 2012;30:1571-1573.
- Cust AE, Armstrong BK, Goumas C, et al. Sunbed use during adolescence and early adulthood is associated with increased risk of early-onset melanoma. Int J Cancer. 2011;128:2425-2435.
- International Agency for Research on Cancer Working Group on artificial ultraviolet (UV) light and skin cancer. The association of use of sunbeds with cutaneous malignant melanoma and other skin cancers: a systematic review. Int J Cancer. 2007;120:1116-1122.
- Greenwood S, Perrin A, Duggan M. Social media update 2016. Pew Research Center website. http://www.pewinternet.org/2016/11/11/social-media-update-2016/. Published November 11, 2016. Accessed December 12, 2017.
Practice Points
- Despite US Food and Drug Administration reclassification and publicity of the risks of skin cancer, many patients continue to use sunbeds.
- It is important to assess how patients are obtaining information regarding sunbed safety, as indoor tanning companies are promoting sunbeds as “safe” tans.
- The increased combination of sunbed use and outdoor tanning is putting people at greater risk for the development of melanoma and nonmelanoma skin cancer.