User login
Physicians do not trust bone biopsy culture data
Editor’s Note: The Society of Hospital Medicine’s (SHM’s) Physician in Training Committee launched a scholarship program in 2015 for medical students to help transform health care and revolutionize patient care. The program has been expanded for the 2017-18 year, offering two options for students to receive funding and engage in scholarly work during their 1st, 2nd, and 3rd years of medical school. As a part of the program, recipients are required to write about their experience on a biweekly basis.
As I approach the end of my summer research project, my team and I have reflected on what we’ve learned from both the research itself and the experience of working on the project.
As work-life balance is important to me, I would usually I would balk at the idea of sacrificing my personal time, but in this case, I am driven by a sense of ownership and pride over the project that I haven’t felt with past projects. I truly believe the results of this research have the potential to change the way physicians think about and manage patients with osteomyelitis, and I am eager to publish our results and attend conferences where I can present and discuss the findings with the medical community.
We hypothesized that the use of image-guided bone biopsies in patients with non-vertebral osteomyelitis would not have a significant impact on antibiotic management. Our results showed that physicians usually do not trust culture data provided by bone biopsy results. Negative bone cultures almost never lead physicians to discontinue antibiotics due to the low yield and reliability of bone biopsy culture data. Similarly, positive cultures almost never lead physicians to prescribe targeted antibiotics. 75% of the patients in our study had contiguous osteomyelitis caused by an overlying ulcer (e.g., diabetic foot ulcers or sacral decubitus ulcers). Exposure of the wound to the outside world often results in polymicrobial infections, and as such physicians rarely narrowed antibiotic coverage when a single organism was cultured. We also found that empiric antibiotic therapy adequately treated cultured micro-organisms in 95% of cases.
While many questions remained unanswered by this study, our results are an important contribution to the body of evidence that image-guided bone biopsies have low utility in the management of contiguous non-vertebral osteomyelitis. I look forward to seeing how the results of future research will compare with our findings. I am grateful to have had the opportunity to work in such an exciting area of research and I hope to continue participating in research projects throughout my medical career.
Cole Hirschfeld is originally from Phoenix. He received undergraduate degrees in finance and entrepreneurship from the University of Arizona and went on to work in the finance industry for 2 years before deciding to change careers and attend medical school. He is now a 4th year medical student at Cornell University, New York, and plans to apply for residency in internal medicine.
Editor’s Note: The Society of Hospital Medicine’s (SHM’s) Physician in Training Committee launched a scholarship program in 2015 for medical students to help transform health care and revolutionize patient care. The program has been expanded for the 2017-18 year, offering two options for students to receive funding and engage in scholarly work during their 1st, 2nd, and 3rd years of medical school. As a part of the program, recipients are required to write about their experience on a biweekly basis.
As I approach the end of my summer research project, my team and I have reflected on what we’ve learned from both the research itself and the experience of working on the project.
As work-life balance is important to me, I would usually I would balk at the idea of sacrificing my personal time, but in this case, I am driven by a sense of ownership and pride over the project that I haven’t felt with past projects. I truly believe the results of this research have the potential to change the way physicians think about and manage patients with osteomyelitis, and I am eager to publish our results and attend conferences where I can present and discuss the findings with the medical community.
We hypothesized that the use of image-guided bone biopsies in patients with non-vertebral osteomyelitis would not have a significant impact on antibiotic management. Our results showed that physicians usually do not trust culture data provided by bone biopsy results. Negative bone cultures almost never lead physicians to discontinue antibiotics due to the low yield and reliability of bone biopsy culture data. Similarly, positive cultures almost never lead physicians to prescribe targeted antibiotics. 75% of the patients in our study had contiguous osteomyelitis caused by an overlying ulcer (e.g., diabetic foot ulcers or sacral decubitus ulcers). Exposure of the wound to the outside world often results in polymicrobial infections, and as such physicians rarely narrowed antibiotic coverage when a single organism was cultured. We also found that empiric antibiotic therapy adequately treated cultured micro-organisms in 95% of cases.
While many questions remained unanswered by this study, our results are an important contribution to the body of evidence that image-guided bone biopsies have low utility in the management of contiguous non-vertebral osteomyelitis. I look forward to seeing how the results of future research will compare with our findings. I am grateful to have had the opportunity to work in such an exciting area of research and I hope to continue participating in research projects throughout my medical career.
Cole Hirschfeld is originally from Phoenix. He received undergraduate degrees in finance and entrepreneurship from the University of Arizona and went on to work in the finance industry for 2 years before deciding to change careers and attend medical school. He is now a 4th year medical student at Cornell University, New York, and plans to apply for residency in internal medicine.
Editor’s Note: The Society of Hospital Medicine’s (SHM’s) Physician in Training Committee launched a scholarship program in 2015 for medical students to help transform health care and revolutionize patient care. The program has been expanded for the 2017-18 year, offering two options for students to receive funding and engage in scholarly work during their 1st, 2nd, and 3rd years of medical school. As a part of the program, recipients are required to write about their experience on a biweekly basis.
As I approach the end of my summer research project, my team and I have reflected on what we’ve learned from both the research itself and the experience of working on the project.
As work-life balance is important to me, I would usually I would balk at the idea of sacrificing my personal time, but in this case, I am driven by a sense of ownership and pride over the project that I haven’t felt with past projects. I truly believe the results of this research have the potential to change the way physicians think about and manage patients with osteomyelitis, and I am eager to publish our results and attend conferences where I can present and discuss the findings with the medical community.
We hypothesized that the use of image-guided bone biopsies in patients with non-vertebral osteomyelitis would not have a significant impact on antibiotic management. Our results showed that physicians usually do not trust culture data provided by bone biopsy results. Negative bone cultures almost never lead physicians to discontinue antibiotics due to the low yield and reliability of bone biopsy culture data. Similarly, positive cultures almost never lead physicians to prescribe targeted antibiotics. 75% of the patients in our study had contiguous osteomyelitis caused by an overlying ulcer (e.g., diabetic foot ulcers or sacral decubitus ulcers). Exposure of the wound to the outside world often results in polymicrobial infections, and as such physicians rarely narrowed antibiotic coverage when a single organism was cultured. We also found that empiric antibiotic therapy adequately treated cultured micro-organisms in 95% of cases.
While many questions remained unanswered by this study, our results are an important contribution to the body of evidence that image-guided bone biopsies have low utility in the management of contiguous non-vertebral osteomyelitis. I look forward to seeing how the results of future research will compare with our findings. I am grateful to have had the opportunity to work in such an exciting area of research and I hope to continue participating in research projects throughout my medical career.
Cole Hirschfeld is originally from Phoenix. He received undergraduate degrees in finance and entrepreneurship from the University of Arizona and went on to work in the finance industry for 2 years before deciding to change careers and attend medical school. He is now a 4th year medical student at Cornell University, New York, and plans to apply for residency in internal medicine.
Review suggests low incidence of BIA-ALCL in Canada
Results of a safety review suggest there is a low incidence of breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL) in Canada.
Health Canada undertook the review because of an increase in reporting of BIA-ALCL internationally.
The review showed that 5 confirmed cases of BIA-ALCL have been reported by Canadian manufacturers in the last 10 years.
This is equal to 1 case of BIA-ALCL per 77,190 implants sold, or 0.0013%.
However, Health Canada acknowledges that some cases may not have been reported to the manufacturers or Health Canada.
Available data suggest that BIA-ALCL is more frequently reported with textured surface implants than smooth surface implants. Textured surface implants account for a quarter of all breast implants sold in Canada.
Four of the 5 reported Canadian cases of BIA-ALCL involved textured implants. The surface type was not reported in the remaining case.
The rate of occurrence of BIA‑ALCL per textured implant sold in Canada is 1 case per 24,177 or 0.0041%.
As a result of its safety review, Health Canada is working with manufacturers to update the safety information on the product labeling for all breast implants.
The agency is also communicating this safety information to Canadians through the Recalls and Safety Alerts database on the Healthy Canadians website.
Health Canada continues to monitor the safety profile of breast implants through its post-market surveillance program.
The agency will also monitor cases of BIA-ALCL through an annual follow-up with manufacturers of breast implants.
Health Canada is recommending that healthcare professionals learn about the signs, symptoms, and testing steps to recognize and diagnose BIA‑ALCL.
In addition, healthcare professionals in Canada should report incidents of BIA-ALCL to Health Canada. These reports should include specific details, such as symptoms, how BIA-ALCL was discovered, the age of the patient at implantation, prior implant history, the age of the patient at discovery, tests conducted to diagnose BIA-ALCL, staging information, the course of therapy, and clinical outcomes.
Reports can be made by calling Health Canada at 1-866-234-2345. Alternatively, visit Health Canada’s webpage on Adverse Reaction Reporting for information on how to report online, by mail, or by fax. ![]()
Results of a safety review suggest there is a low incidence of breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL) in Canada.
Health Canada undertook the review because of an increase in reporting of BIA-ALCL internationally.
The review showed that 5 confirmed cases of BIA-ALCL have been reported by Canadian manufacturers in the last 10 years.
This is equal to 1 case of BIA-ALCL per 77,190 implants sold, or 0.0013%.
However, Health Canada acknowledges that some cases may not have been reported to the manufacturers or Health Canada.
Available data suggest that BIA-ALCL is more frequently reported with textured surface implants than smooth surface implants. Textured surface implants account for a quarter of all breast implants sold in Canada.
Four of the 5 reported Canadian cases of BIA-ALCL involved textured implants. The surface type was not reported in the remaining case.
The rate of occurrence of BIA‑ALCL per textured implant sold in Canada is 1 case per 24,177 or 0.0041%.
As a result of its safety review, Health Canada is working with manufacturers to update the safety information on the product labeling for all breast implants.
The agency is also communicating this safety information to Canadians through the Recalls and Safety Alerts database on the Healthy Canadians website.
Health Canada continues to monitor the safety profile of breast implants through its post-market surveillance program.
The agency will also monitor cases of BIA-ALCL through an annual follow-up with manufacturers of breast implants.
Health Canada is recommending that healthcare professionals learn about the signs, symptoms, and testing steps to recognize and diagnose BIA‑ALCL.
In addition, healthcare professionals in Canada should report incidents of BIA-ALCL to Health Canada. These reports should include specific details, such as symptoms, how BIA-ALCL was discovered, the age of the patient at implantation, prior implant history, the age of the patient at discovery, tests conducted to diagnose BIA-ALCL, staging information, the course of therapy, and clinical outcomes.
Reports can be made by calling Health Canada at 1-866-234-2345. Alternatively, visit Health Canada’s webpage on Adverse Reaction Reporting for information on how to report online, by mail, or by fax. ![]()
Results of a safety review suggest there is a low incidence of breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL) in Canada.
Health Canada undertook the review because of an increase in reporting of BIA-ALCL internationally.
The review showed that 5 confirmed cases of BIA-ALCL have been reported by Canadian manufacturers in the last 10 years.
This is equal to 1 case of BIA-ALCL per 77,190 implants sold, or 0.0013%.
However, Health Canada acknowledges that some cases may not have been reported to the manufacturers or Health Canada.
Available data suggest that BIA-ALCL is more frequently reported with textured surface implants than smooth surface implants. Textured surface implants account for a quarter of all breast implants sold in Canada.
Four of the 5 reported Canadian cases of BIA-ALCL involved textured implants. The surface type was not reported in the remaining case.
The rate of occurrence of BIA‑ALCL per textured implant sold in Canada is 1 case per 24,177 or 0.0041%.
As a result of its safety review, Health Canada is working with manufacturers to update the safety information on the product labeling for all breast implants.
The agency is also communicating this safety information to Canadians through the Recalls and Safety Alerts database on the Healthy Canadians website.
Health Canada continues to monitor the safety profile of breast implants through its post-market surveillance program.
The agency will also monitor cases of BIA-ALCL through an annual follow-up with manufacturers of breast implants.
Health Canada is recommending that healthcare professionals learn about the signs, symptoms, and testing steps to recognize and diagnose BIA‑ALCL.
In addition, healthcare professionals in Canada should report incidents of BIA-ALCL to Health Canada. These reports should include specific details, such as symptoms, how BIA-ALCL was discovered, the age of the patient at implantation, prior implant history, the age of the patient at discovery, tests conducted to diagnose BIA-ALCL, staging information, the course of therapy, and clinical outcomes.
Reports can be made by calling Health Canada at 1-866-234-2345. Alternatively, visit Health Canada’s webpage on Adverse Reaction Reporting for information on how to report online, by mail, or by fax. ![]()
Responding to the Opioid Crisis: An Indian Health Service Pharmacist-Led Pain Management Clinic
According to the National Academy of Medicine (NAM), about 100 million people live with chronic pain in the U.S.1 There is evidence for the use of opioids to treat acute pain lasting 12 weeks or less. However, high-quality studies that analyze the benefit and safety of long-term opioid therapy are not yet available.2 In 2013, 249 million opioid prescriptions were written, equivalent to about 1 prescription per adult living in the U.S.3
Between 1999 and 2008, nonmedical use of prescription pain killers in the American Indian and Alaska Native populations was 2 to 3 times the frequency found in the white and black populations, respectively.1 These clinically contradictory practices created an environment conducive to opioid abuse and overdose. According to the Centers for Disease Control and Prevention, 1 in 4 people on chronic opioid therapy struggle with addiction.4 In 2014, more than 14,000 people in the U.S. died from overdoses involving prescription opioids.
The Comprehensive Addiction and Recovery Act of 2016 authorized prescription drug monitoring programs (PMPs) and a task force to create optimal pain treatment practices.5 The American Pharmacists Association (APhA), a major proponent of this law, argued that pharmacists are an underutilized resource despite having valuable clinical knowledge in the initiation, monitoring, and discontinuation of opioids. Additionally, pharmacists are able to refer patients to nonpharmacologic forms of pain management and dispense naloxone for emergent opioid overdose reversal.
Former Surgeon General Vivek Murthy developed the Turn the Tide Rx campaign to curb and reverse the opioid crisis in the U.S.3 The turnthetiderx.org website offers a pledge for clinicians who agree to be educated about pain management. It encourages open communication among prescribers and contains guideline-based resources on assessing pain and addiction risk, appropriate opioid prescribing, and how to manage opioid overdose. As required by law for prescribers in most states, there are instructions on how to access and analyze PMP opioid usage. The website also provides fact sheets about opioid treatments, safe disposal of medications, and helpline information.
Local Opioid Misuse Initiatives
New Mexico (NM) has one of the nation’s highest opioid and heroin overdose death rates.6 In January 2015, the U.S. Attorney’s Office and the University of New Mexico Health and Sciences Center partnered to launch the Heroin and Opioid Prevention and Education Initiative. The partners recognized that joint action between medical sciences and law enforcement was crucial to address the consequences of the opioid epidemic on public health and safety.
In 2017, the IHS established the National Committee on Heroin, Opioids, and Pain Efforts. Comprised of a variety of pharmacy and other subject-matter experts, this committee has a multipronged strategy to address the opioid epidemic from training to expanding medication-assisted treatment.7
Pharmacists provide clinical services in a variety of interdisciplinary ambulatory care clinics at Gallup Indian Medical Center (GIMC) in NM. The GIMC is located outside reservation boundaries but is centrally located to serve Navajo, Zuni, and a variety of other native populations. Pharmacist-run clinics include diabetes mellitus, anticoagulation, asthma, anemia, infectious diseases, and chronic pain. At GIMC, pain management pharmacists use a collective approach to curb opioid misuse. This article describes the establishment and impact of a pharmacist-led pain management clinic (PMC) at GIMC.
Pain Management Clinic
The understaffed urgent care clinic (UCC), emergency department (ED), primary care, and specialty practices plus a growing burden of complicated pain patients incentivized the development of the GIMC PMC. Under a collaborative practice agreement, pain management pharmacists were tasked with assessing, treating, and controlling noncancer chronic pain while improving quality of care and patient satisfaction (eAppendix 1, available at www.fedprac.com). The PMC goal was to improve functionality and pain scores and to reduce patient visits to the UCC and ED.
Originally, the PMC only performed medication titration for patients. In 2012, a former pharmacy resident became the PMC coordinator and has since helped to transform and expand its services. Currently, the coordinator dedicates about 20 hours per week managing pain patients in various capacities. Over time, other pain management pharmacists joined the PMC and support activities for 5 to 10 hours per week. There are now 4 pain management pharmacists who rotate through the PMC.
Pain management clinic visits generally are held once weekly for 3 hours and are occasionally expanded to full days based on patient schedule load. The initial 2 PMC appointments for each patient are 1 hour and are held within a 2-week period. Subsequent visits are each 30 minutes at 1 to 2 month intervals, depending on patient pain level and medication titration requirements. This standardized follow-up ensures new patients receive the close monitoring of slow dosage titrations necessary to achieve maximum therapeutic benefit.
Referrals
Patients only are admitted into the PMC via consults from primary care providers (PCPs). The consultations generally involve patients with complicated medical histories or who require complex therapies. The patients are contacted by telephone prior to scheduling. If a patient is not interested in PMC services, the consult is amended and the PCP is notified. This policy has been newly implemented to reduce the no-show rates. Pain management pharmacists then conduct PMC visits. The PMC activities are reported to the Pharmacy and Therapeutics committee annually. The PMC pharmacists also are available for telephone consultations from any internal hospital system department during weekday business hours.
The PMC patient population is fluid. New patients are accepted and clinically stable patients are discharged from PMC on a regular basis. The stable patients are released back to the PCP for further follow-up. Patients are considered stable if they have reached pain-related goals, are on maintenance doses of opioids, and/or are regularly participating in applicable interventional and referred therapies. If a patient requires reentry into PMC care, a new referral can be placed.
Interventional Programs
Since its inception, PMC has been a referral service to interventional programs. Physicians in the family medicine clinic provide twice weekly pain/palliative care clinic visits. These PCPs typically perform trigger point injections with lidocaine to relax muscles, which may disrupt nerve fibers. Ideally, this treatment reduces the use of opioids, non-steroidal anti-inflammatory drugs (NSAIDs), steroids, and epidural medications. Three acupuncturists were hired, which has reduced scheduling bottlenecks and wait times for patients to return for follow-up, especially in a treatment modality requiring frequent visits for effectiveness.
Pain Committee
As a part of an expansion of pain management services, GIMC established a pain committee (PC). The PC includes the GIMC medical director, PMC coordinator, pain management pharmacists, palliative care providers, PCPs, and specialty care providers. The PC created a detailed policy and procedures on management of chronic non-cancer pain for the Gallup Service Unit (eAppendix 2, available at www.fedprac.com). The PC offers guidance and completes consultations, performs internal review of prescribing patterns, and provides an appeals process for patients who have broken pain agreements. Physician and administrative champions have been instrumental to ensure proper pain management at GIMC.
Many VA facilities have deployed pain management clinics. At the VA Boston Healthcare System (VABHS), a pain management center is staffed by a multidisciplinary team that consists of anesthesiologists, neurologists, psychiatrists, nurses, and pharmacists.8 This pain clinic operates multiple days per week to accommodate demand, and patients are followed at least once a month. The VABHS often synchronized clinic visit dates with medication refill dates. Pharmacists offer an e-consult pain service to provide immediate recommendations to PCPs to bridge those patients awaiting appointments with pain clinic specialists at some VA facilities in Florida.9 Insufficient funding has prevented GIMC from increased PMC clinic hours.
Clinic Scope
Currently, the PMC sees the majority of PC cases. Pain management pharmacists are selected to conduct pain management visits based on interest and competency. Qualifications to work as a PMC pharmacist include on-the-job training, at least 6 annual pain management continuing medical education (CME) credits, participation in the NM naloxone training webinar, and completion of the physical assessment portion of the NM pharmacist clinician training course. It is highly recommended that pharmacists attend the PAINWeek conference in Las Vegas, Nevada, to obtain the necessary CME credits. In addition, pharmacists are requested to obtain the IHS National Clinical Pharmacy Specialist (NCPS) qualification within a year of practice.
Pain management pharmacists in the PMC review the indications for pain management, monitor medication therapy and adherence, adjust doses, manage adverse effects, study trends in pain and mood screening tools, and assess changes in functionality. These pharmacists are able to prescribe, discontinue, or titrate noncontrolled substance adjunctive therapies without a PCP cosignature. Adjunctive medication therapies can be NSAIDs, anticonvulsants, neuropathic pain relievers, muscle relaxers, and topical analgesics.
If adjustments to controlled substances are warranted, pain management pharmacists present the case to the PCP via electronic health record (EHR) notification or telephone conversation. These pharmacists ensure hard copies of controlled substance prescriptions are retrieved and provide refill coordination if assistance is requested by the patient. Pharmacists provide 28-day (not 30-day) prescriptions for opioid and controlled substance prescriptions to reduce weekend refill requests. Pain management pharmacists also order a variety of laboratory tests (eg, liver and renal function tests, and complete blood counts) related to the safe use of medications. If a patient is deemed unfit for PMC management, such as due to pain agreement violations, the PMC coordinator formally presents the case during PC meetings.
The PMC often recommends a multitude of nonpharmacologic treatments, including ice, hot rice socks, an anti-inflammatory diet, massage therapy, tennis ball massage for muscle tension and pinched nerves, chair exercises, transcutaneous electrical nerve stimulation therapy, aquatic therapy, and distraction therapy. Pain management pharmacists also can coordinate referrals to specialists and interventional therapies (eg, physical therapy; occupational therapy; acupuncture; trigger point injections; podiatry; orthopedics; ear, nose and throat; and diabetes mellitus).
Pain management pharmacists use a variety of established tools in the pain management clinic. The PMC EHR template and interview process are consistent with the universal precautions approach to unified pain management.10 Many of the questionnaires, tools, and laboratory tests are repeated periodically based on PMC policy and patient-specific need. These tools include a controlled substance pain agreement, consent for chronic opioid therapy, Opioid Risk Tool (ORT), Current Opioid Misuse Measure (COMM) for opioid abuse risk assessment, and the Patient Health Questionnaire (PHQ-9) for concurrent depression (Table and eAppendices 1, 3, and 4, available at www.fedprac.com). The ORT recently was added to the patient assessment packet to provide a stronger assessment of opioid misuse and abuse risk. Patient goals also are discussed with an emphasis on realistic changes, the level of control that would satisfy the patient and is feasible, what activities of daily living or hobbies the patient would like to regain, and what relationships the patient would like to improve.
PMC Patients
New patients are required to complete urine drug tests (UDTs), which can be performed in house or sent out if specific drug levels are required. Federally, marijuana is an illicit substance and is not prescribed or dispensed. Unpredictable effects of traditional medicine on the UDT have been observed. If a UDT shows positive for illicit substances, a discussion with the patient on toxicity and risks is initiated allowing them to choose to continue to use other substances for pain or use only the pain medication(s) appropriately prescribed by GIMC providers. Patients can be deemed ineligible for PMC management if they do not discontinue the use of illicit substances.
Health care providers and pharmacists involved in the prescribing or dispensing of controlled substances also complete a review of the patient’s PMP profile. It is mandatory in NM to complete PMP surveillance prior to prescribing controlled substances in quantities greater than 12 units within a 72-hour period and every 3 months for refills of chronic opioids.11 The PMP Interconnect service allows registered providers in NM to search for controlled substance usage in 25 states. All but 1 state in the U.S. has a PMP in development or in place. Federal providers who do not have a NM professional license also may apply for a NM PMP login to take advantage of the PMP Interconnect service. If the patient’s PMP is negative for prescribed opioids or positive for nonprescribed substances, the providers will conduct more research to reassess the risks and benefits of ongoing opioid therapy.
Random pill counts for tracked patients also have been incorporated into the PMC policy. These pill counts can be requested regardless of clinic appointment dates and can show whether patients are taking too many pills or diverting pills. Pill counts also may show that the patient may not need as many tablets per prescription if they consistently have more than expected based on dosing frequency. If a patient has adhered to prescribing recommendations, he or she may be allowed an early refill of the chronic opioid to cover them during a vacation or other unexpected event. However, if the pill counts are not consistent with instructions, then prescribing may be restricted to a 5-day, 7-day, or 14-day supply only. If patients do not present for pill count within 24 hours of the request, they can have their opioid use privileges at GIMC revoked as consented in the pain agreement. Patients are educated on proper storage and security of opioids medication at home and funding for lock boxes is expected in the near future. Patients may be referred to other services if opioid-use disorders are identified and confirmed.
Other administrative components of the pain management pharmacist responsibilities include clinical chart reviews before appointments or telephone consultations, sending appointment letters, contacting patients about and conducting random pill counts, and documenting PMC visit notes and updating flowsheets (eAppendices 4 and 5, available at www.fedprac.com). Currently under development is an EHR function that can quickly provide a summary of a patient’s pain management without a tedious search through the chart. Pain management pharmacists regularly instruct pharmacists and providers on changes in regulatory requirements of pain management as it pertains to prescription fills or clinical indications.
Ancillary Pharmacy Services
All pain patients on opioids therapies receive an annual pain evaluation. This evaluation provides a holistic description of the patient’s pain, a second opinion, and a collective review of the safety and efficacy of treatment. Components of the pain evaluation include review of medication toxicities and adverse effects, reconciliation of the treatment with current pain diagnosis and intensity, and coordination of opioid tapering schedules. Physical assessments and pill counts also are performed. Issues that have been uncovered include incidences of opioid-induced hyperalgesia and patients filling opioids prescriptions without taking the medications because they did not want to tell the provider the medication did not work.
Naloxone overdose prevention training and dispensing has been critical for ensuring maximum safety and life-saving methods in the realm of opioid therapy. Providers, patients, and rescue buddies are trained in the indication for, the administration of, and directions after naloxone use. Patients then are provided with naloxone nasal spray after the conclusion of the training and with instruction on refill procedures. Naloxone can be administered without legal penalty by anyone in NM. When refills for naloxone are requested, a naloxone-specific refill note is filed in the EHR to collect data on naloxone usage. In collaboration with a local suicide and substance abuse prevention network, 2 pain management pharmacists have created a Pills Can Kill booklet for dispersion to patients in waiting rooms, offices, schools, and throughout the county.
MedSafe receptacles (Houston, Texas) for collection of unused or unwanted medications, including controlled substances, are available in the pharmacy. These receptacles give patients a convenient and free outlet to remove circulating opioids from general access in the community.
Clinic Impact
Previously at GIMC, clinical pain conditions were managed by providers with minimal pain management expertise. Providers in the UCC and ED treated both acute and chronic pain with minimally enforced opioid prescribing limits. Often, patients were prescribed the dangerous drug trio of opioids, benzodiazepines, and muscle relaxers. In addition, there were inconsistent PMP checks, scheduled or random UDTs, or pill counts.
Currently, providers adhere more strongly to their scope of practice, which has resulted in less erratic opioid prescribing, from initiating therapy to prescribing large quantities. Providers in the UCC and ED more often triage chronic opioid pain patients to the PCP rather than obliging patients’ requests for chronic pain medication refills. Since UCC and ED providers now focus on the treatment of acute pain, there have been fewer drug seekers frequenting those departments, which has reduced the drug-seeking burden on PCPs and the outpatient pharmacy.
Increased utilization and study of the PMP profiles at GIMC has helped uncover important information, including incidences of theft and diversion. For example, a patient or caregiver who claimed stolen opioids was discovered to be diverting opioids from patients under her care and acutely drugging her patients in order for their UDTs to appear positive for opioids. Patients have been found drug and doctor shopping under maiden and married names, under multiple birthdays, in other states, or under a different gender altogether. Some patients have been selling immense amounts of opioids and others consuming immense amounts.
Statewide statistics from the NM Board of Pharmacy from 2016 describes a 20.1% increase in PMP usage over the previous year.12 Data from 3 million prescriptions are uploaded to the PMP annually, and more than 100,000 search requests are processed monthly.11 There was a 16% decrease in opioid prescriptions per patient from multiple providers, a 14.2% decline in concurrent opioid and benzodiazepine prescriptions, and a 7.2% reduction in total opioid prescriptions.12 Fortunately, opioid overdose death rates are down 7% from the previous year.
There is an assumption of mutual trust between patient and provider. However, patients are given more responsibility for pain management through the use of a variety of objective tools and labs. Only a few people have requested refills on the naloxone kits other than for replacement of expired product. Based on experience, many patients and rescue buddies have inquired why they did not know of opioid-related risks earlier and stated that if they had naloxone available at home earlier, they could have saved a life. Further research is needed on the true impact of naloxone and its expanding access in the community.
The PMC has shown clinical success beyond simply helping to curb inappropriate opioid prescribing and overdose deaths. Investigative work such as initiating the right drug for the pain level or defining twice daily dosing as every 12 hours and not any 2 times per day has improved pain and function greatly. Many cases of hyperalgesia have been reduced by careful reductions in daily opioid doses.
Conclusion
The PMC and partners will continue to sustain efforts and transform now that the opioid crisis is exposed in the media, the IHS, and the PHS. The ongoing goals of the pain committee are to lift the dependence on opioids as long-term treatment, identify alternative solutions to pain conditions, and execute appropriate coordination of treatment. Optimization of pain management will depend on a variety of administrative and supportive service changes. Expansion of more PMC days is being considered to reduce no-show rates and the delay to first scheduled appointment. Recruitment and maintenance of specialists in behavioral health are crucial.
Securing avenues for medication-assisted therapies with buprenorphine and methadone also will help to reverse the prevalence of the opioid use disorders. The public hospital in Gallup, NM, has begun to mirror GIMC policies for consistent community actions toward improved pain management. Additionally, the GIMC experiences have been shared with area IHS facility pharmacists who are becoming increasingly involved in the national efforts to improve pain care. The pharmacy pain management clinic services at GIMC have emphasized judicious opioid prescribing, reduced overdose risk in the community, and improved patient functionality and quality of care through close pharmacotherapy monitoring.
1. The American Academy of Pain Medicine. AAPM Facts and Figures in Pain. http://www.painmed.org/patientcenter/facts_on_pain.aspx. Accessed September 18, 2017.
2. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain---United States, 2016. JAMA. 2016;315(15):1624-1645.
3. U.S. Department of Health and Human Services. The surgeon general’s call to end the opioid crisis. https://turnthetiderx.org/. Accessed September 18, 2017.
4. Centers for Disease Control and Prevention. Prescription opioid overdose data. http://www.cdc.gov/drugoverdose/data/overdose.html. Updated August 1, 2017. Accessed September 18, 2017.
5. American Pharmacists Association. United States House of Representatives approves opioid bill package. http://www.pharmacist.com/united-states-house-representatives-approves-opioid-bill-package. Published May 13, 2016. Accessed September 18, 2017.
6. U.S. Attorney’s Office, District of New MexicoHeroin and opioid prevention and education (HOPE) initiative. http://www.hopeinitiativenm.org/. Ac cessed May 6, 2017.
7. U.S. Department of Health and Human Services,National Committee on Heroin, Opioids, and Pain Efforts (HOPE). Indian Health Service Circular No. 17-04. https://www.ihs.gov/ihm/index.cfm?module=dsp_ihm_circ_main&circ=ihm_circ_1704. Published March 24, 2017. Accessed May 6, 2017.
8. Rapoport A, Akbik H. Pharmacist-managed pain clinic at a Veterans Affairs Medical Center. Am J Health Syst Pharm. 2004;61(13):1341-1343.
9. Miller DM, Harvey TL. Pharmacist pain e-consults that result in a therapy change. Fed Pract. 2015 July;32(7):14-19.
10. Zacharoff, KL, Menefee Pujol, L, Corsini, E. PainEDU.org Manual. A Pocket Guide to Pain Management. 4th ed. Newton, MA : Inflexxion, Inc; 2010.
11. New Mexico Board of Pharmacy. Prescription monitoring program. http://nmpmp.org/. Accessed May 6, 2017.
12. New Mexico Department of Health Reports on Improved Opioid Prescribing Practices, Reduced Drug Overdose Death Rates. NABP e-News. http://nabp.bmetrack.com/c/v?e=A90B1D&c=8AB9&t= 0&l=193A4F8E. Accessed January 16, 2017.
According to the National Academy of Medicine (NAM), about 100 million people live with chronic pain in the U.S.1 There is evidence for the use of opioids to treat acute pain lasting 12 weeks or less. However, high-quality studies that analyze the benefit and safety of long-term opioid therapy are not yet available.2 In 2013, 249 million opioid prescriptions were written, equivalent to about 1 prescription per adult living in the U.S.3
Between 1999 and 2008, nonmedical use of prescription pain killers in the American Indian and Alaska Native populations was 2 to 3 times the frequency found in the white and black populations, respectively.1 These clinically contradictory practices created an environment conducive to opioid abuse and overdose. According to the Centers for Disease Control and Prevention, 1 in 4 people on chronic opioid therapy struggle with addiction.4 In 2014, more than 14,000 people in the U.S. died from overdoses involving prescription opioids.
The Comprehensive Addiction and Recovery Act of 2016 authorized prescription drug monitoring programs (PMPs) and a task force to create optimal pain treatment practices.5 The American Pharmacists Association (APhA), a major proponent of this law, argued that pharmacists are an underutilized resource despite having valuable clinical knowledge in the initiation, monitoring, and discontinuation of opioids. Additionally, pharmacists are able to refer patients to nonpharmacologic forms of pain management and dispense naloxone for emergent opioid overdose reversal.
Former Surgeon General Vivek Murthy developed the Turn the Tide Rx campaign to curb and reverse the opioid crisis in the U.S.3 The turnthetiderx.org website offers a pledge for clinicians who agree to be educated about pain management. It encourages open communication among prescribers and contains guideline-based resources on assessing pain and addiction risk, appropriate opioid prescribing, and how to manage opioid overdose. As required by law for prescribers in most states, there are instructions on how to access and analyze PMP opioid usage. The website also provides fact sheets about opioid treatments, safe disposal of medications, and helpline information.
Local Opioid Misuse Initiatives
New Mexico (NM) has one of the nation’s highest opioid and heroin overdose death rates.6 In January 2015, the U.S. Attorney’s Office and the University of New Mexico Health and Sciences Center partnered to launch the Heroin and Opioid Prevention and Education Initiative. The partners recognized that joint action between medical sciences and law enforcement was crucial to address the consequences of the opioid epidemic on public health and safety.
In 2017, the IHS established the National Committee on Heroin, Opioids, and Pain Efforts. Comprised of a variety of pharmacy and other subject-matter experts, this committee has a multipronged strategy to address the opioid epidemic from training to expanding medication-assisted treatment.7
Pharmacists provide clinical services in a variety of interdisciplinary ambulatory care clinics at Gallup Indian Medical Center (GIMC) in NM. The GIMC is located outside reservation boundaries but is centrally located to serve Navajo, Zuni, and a variety of other native populations. Pharmacist-run clinics include diabetes mellitus, anticoagulation, asthma, anemia, infectious diseases, and chronic pain. At GIMC, pain management pharmacists use a collective approach to curb opioid misuse. This article describes the establishment and impact of a pharmacist-led pain management clinic (PMC) at GIMC.
Pain Management Clinic
The understaffed urgent care clinic (UCC), emergency department (ED), primary care, and specialty practices plus a growing burden of complicated pain patients incentivized the development of the GIMC PMC. Under a collaborative practice agreement, pain management pharmacists were tasked with assessing, treating, and controlling noncancer chronic pain while improving quality of care and patient satisfaction (eAppendix 1, available at www.fedprac.com). The PMC goal was to improve functionality and pain scores and to reduce patient visits to the UCC and ED.
Originally, the PMC only performed medication titration for patients. In 2012, a former pharmacy resident became the PMC coordinator and has since helped to transform and expand its services. Currently, the coordinator dedicates about 20 hours per week managing pain patients in various capacities. Over time, other pain management pharmacists joined the PMC and support activities for 5 to 10 hours per week. There are now 4 pain management pharmacists who rotate through the PMC.
Pain management clinic visits generally are held once weekly for 3 hours and are occasionally expanded to full days based on patient schedule load. The initial 2 PMC appointments for each patient are 1 hour and are held within a 2-week period. Subsequent visits are each 30 minutes at 1 to 2 month intervals, depending on patient pain level and medication titration requirements. This standardized follow-up ensures new patients receive the close monitoring of slow dosage titrations necessary to achieve maximum therapeutic benefit.
Referrals
Patients only are admitted into the PMC via consults from primary care providers (PCPs). The consultations generally involve patients with complicated medical histories or who require complex therapies. The patients are contacted by telephone prior to scheduling. If a patient is not interested in PMC services, the consult is amended and the PCP is notified. This policy has been newly implemented to reduce the no-show rates. Pain management pharmacists then conduct PMC visits. The PMC activities are reported to the Pharmacy and Therapeutics committee annually. The PMC pharmacists also are available for telephone consultations from any internal hospital system department during weekday business hours.
The PMC patient population is fluid. New patients are accepted and clinically stable patients are discharged from PMC on a regular basis. The stable patients are released back to the PCP for further follow-up. Patients are considered stable if they have reached pain-related goals, are on maintenance doses of opioids, and/or are regularly participating in applicable interventional and referred therapies. If a patient requires reentry into PMC care, a new referral can be placed.
Interventional Programs
Since its inception, PMC has been a referral service to interventional programs. Physicians in the family medicine clinic provide twice weekly pain/palliative care clinic visits. These PCPs typically perform trigger point injections with lidocaine to relax muscles, which may disrupt nerve fibers. Ideally, this treatment reduces the use of opioids, non-steroidal anti-inflammatory drugs (NSAIDs), steroids, and epidural medications. Three acupuncturists were hired, which has reduced scheduling bottlenecks and wait times for patients to return for follow-up, especially in a treatment modality requiring frequent visits for effectiveness.
Pain Committee
As a part of an expansion of pain management services, GIMC established a pain committee (PC). The PC includes the GIMC medical director, PMC coordinator, pain management pharmacists, palliative care providers, PCPs, and specialty care providers. The PC created a detailed policy and procedures on management of chronic non-cancer pain for the Gallup Service Unit (eAppendix 2, available at www.fedprac.com). The PC offers guidance and completes consultations, performs internal review of prescribing patterns, and provides an appeals process for patients who have broken pain agreements. Physician and administrative champions have been instrumental to ensure proper pain management at GIMC.
Many VA facilities have deployed pain management clinics. At the VA Boston Healthcare System (VABHS), a pain management center is staffed by a multidisciplinary team that consists of anesthesiologists, neurologists, psychiatrists, nurses, and pharmacists.8 This pain clinic operates multiple days per week to accommodate demand, and patients are followed at least once a month. The VABHS often synchronized clinic visit dates with medication refill dates. Pharmacists offer an e-consult pain service to provide immediate recommendations to PCPs to bridge those patients awaiting appointments with pain clinic specialists at some VA facilities in Florida.9 Insufficient funding has prevented GIMC from increased PMC clinic hours.
Clinic Scope
Currently, the PMC sees the majority of PC cases. Pain management pharmacists are selected to conduct pain management visits based on interest and competency. Qualifications to work as a PMC pharmacist include on-the-job training, at least 6 annual pain management continuing medical education (CME) credits, participation in the NM naloxone training webinar, and completion of the physical assessment portion of the NM pharmacist clinician training course. It is highly recommended that pharmacists attend the PAINWeek conference in Las Vegas, Nevada, to obtain the necessary CME credits. In addition, pharmacists are requested to obtain the IHS National Clinical Pharmacy Specialist (NCPS) qualification within a year of practice.
Pain management pharmacists in the PMC review the indications for pain management, monitor medication therapy and adherence, adjust doses, manage adverse effects, study trends in pain and mood screening tools, and assess changes in functionality. These pharmacists are able to prescribe, discontinue, or titrate noncontrolled substance adjunctive therapies without a PCP cosignature. Adjunctive medication therapies can be NSAIDs, anticonvulsants, neuropathic pain relievers, muscle relaxers, and topical analgesics.
If adjustments to controlled substances are warranted, pain management pharmacists present the case to the PCP via electronic health record (EHR) notification or telephone conversation. These pharmacists ensure hard copies of controlled substance prescriptions are retrieved and provide refill coordination if assistance is requested by the patient. Pharmacists provide 28-day (not 30-day) prescriptions for opioid and controlled substance prescriptions to reduce weekend refill requests. Pain management pharmacists also order a variety of laboratory tests (eg, liver and renal function tests, and complete blood counts) related to the safe use of medications. If a patient is deemed unfit for PMC management, such as due to pain agreement violations, the PMC coordinator formally presents the case during PC meetings.
The PMC often recommends a multitude of nonpharmacologic treatments, including ice, hot rice socks, an anti-inflammatory diet, massage therapy, tennis ball massage for muscle tension and pinched nerves, chair exercises, transcutaneous electrical nerve stimulation therapy, aquatic therapy, and distraction therapy. Pain management pharmacists also can coordinate referrals to specialists and interventional therapies (eg, physical therapy; occupational therapy; acupuncture; trigger point injections; podiatry; orthopedics; ear, nose and throat; and diabetes mellitus).
Pain management pharmacists use a variety of established tools in the pain management clinic. The PMC EHR template and interview process are consistent with the universal precautions approach to unified pain management.10 Many of the questionnaires, tools, and laboratory tests are repeated periodically based on PMC policy and patient-specific need. These tools include a controlled substance pain agreement, consent for chronic opioid therapy, Opioid Risk Tool (ORT), Current Opioid Misuse Measure (COMM) for opioid abuse risk assessment, and the Patient Health Questionnaire (PHQ-9) for concurrent depression (Table and eAppendices 1, 3, and 4, available at www.fedprac.com). The ORT recently was added to the patient assessment packet to provide a stronger assessment of opioid misuse and abuse risk. Patient goals also are discussed with an emphasis on realistic changes, the level of control that would satisfy the patient and is feasible, what activities of daily living or hobbies the patient would like to regain, and what relationships the patient would like to improve.
PMC Patients
New patients are required to complete urine drug tests (UDTs), which can be performed in house or sent out if specific drug levels are required. Federally, marijuana is an illicit substance and is not prescribed or dispensed. Unpredictable effects of traditional medicine on the UDT have been observed. If a UDT shows positive for illicit substances, a discussion with the patient on toxicity and risks is initiated allowing them to choose to continue to use other substances for pain or use only the pain medication(s) appropriately prescribed by GIMC providers. Patients can be deemed ineligible for PMC management if they do not discontinue the use of illicit substances.
Health care providers and pharmacists involved in the prescribing or dispensing of controlled substances also complete a review of the patient’s PMP profile. It is mandatory in NM to complete PMP surveillance prior to prescribing controlled substances in quantities greater than 12 units within a 72-hour period and every 3 months for refills of chronic opioids.11 The PMP Interconnect service allows registered providers in NM to search for controlled substance usage in 25 states. All but 1 state in the U.S. has a PMP in development or in place. Federal providers who do not have a NM professional license also may apply for a NM PMP login to take advantage of the PMP Interconnect service. If the patient’s PMP is negative for prescribed opioids or positive for nonprescribed substances, the providers will conduct more research to reassess the risks and benefits of ongoing opioid therapy.
Random pill counts for tracked patients also have been incorporated into the PMC policy. These pill counts can be requested regardless of clinic appointment dates and can show whether patients are taking too many pills or diverting pills. Pill counts also may show that the patient may not need as many tablets per prescription if they consistently have more than expected based on dosing frequency. If a patient has adhered to prescribing recommendations, he or she may be allowed an early refill of the chronic opioid to cover them during a vacation or other unexpected event. However, if the pill counts are not consistent with instructions, then prescribing may be restricted to a 5-day, 7-day, or 14-day supply only. If patients do not present for pill count within 24 hours of the request, they can have their opioid use privileges at GIMC revoked as consented in the pain agreement. Patients are educated on proper storage and security of opioids medication at home and funding for lock boxes is expected in the near future. Patients may be referred to other services if opioid-use disorders are identified and confirmed.
Other administrative components of the pain management pharmacist responsibilities include clinical chart reviews before appointments or telephone consultations, sending appointment letters, contacting patients about and conducting random pill counts, and documenting PMC visit notes and updating flowsheets (eAppendices 4 and 5, available at www.fedprac.com). Currently under development is an EHR function that can quickly provide a summary of a patient’s pain management without a tedious search through the chart. Pain management pharmacists regularly instruct pharmacists and providers on changes in regulatory requirements of pain management as it pertains to prescription fills or clinical indications.
Ancillary Pharmacy Services
All pain patients on opioids therapies receive an annual pain evaluation. This evaluation provides a holistic description of the patient’s pain, a second opinion, and a collective review of the safety and efficacy of treatment. Components of the pain evaluation include review of medication toxicities and adverse effects, reconciliation of the treatment with current pain diagnosis and intensity, and coordination of opioid tapering schedules. Physical assessments and pill counts also are performed. Issues that have been uncovered include incidences of opioid-induced hyperalgesia and patients filling opioids prescriptions without taking the medications because they did not want to tell the provider the medication did not work.
Naloxone overdose prevention training and dispensing has been critical for ensuring maximum safety and life-saving methods in the realm of opioid therapy. Providers, patients, and rescue buddies are trained in the indication for, the administration of, and directions after naloxone use. Patients then are provided with naloxone nasal spray after the conclusion of the training and with instruction on refill procedures. Naloxone can be administered without legal penalty by anyone in NM. When refills for naloxone are requested, a naloxone-specific refill note is filed in the EHR to collect data on naloxone usage. In collaboration with a local suicide and substance abuse prevention network, 2 pain management pharmacists have created a Pills Can Kill booklet for dispersion to patients in waiting rooms, offices, schools, and throughout the county.
MedSafe receptacles (Houston, Texas) for collection of unused or unwanted medications, including controlled substances, are available in the pharmacy. These receptacles give patients a convenient and free outlet to remove circulating opioids from general access in the community.
Clinic Impact
Previously at GIMC, clinical pain conditions were managed by providers with minimal pain management expertise. Providers in the UCC and ED treated both acute and chronic pain with minimally enforced opioid prescribing limits. Often, patients were prescribed the dangerous drug trio of opioids, benzodiazepines, and muscle relaxers. In addition, there were inconsistent PMP checks, scheduled or random UDTs, or pill counts.
Currently, providers adhere more strongly to their scope of practice, which has resulted in less erratic opioid prescribing, from initiating therapy to prescribing large quantities. Providers in the UCC and ED more often triage chronic opioid pain patients to the PCP rather than obliging patients’ requests for chronic pain medication refills. Since UCC and ED providers now focus on the treatment of acute pain, there have been fewer drug seekers frequenting those departments, which has reduced the drug-seeking burden on PCPs and the outpatient pharmacy.
Increased utilization and study of the PMP profiles at GIMC has helped uncover important information, including incidences of theft and diversion. For example, a patient or caregiver who claimed stolen opioids was discovered to be diverting opioids from patients under her care and acutely drugging her patients in order for their UDTs to appear positive for opioids. Patients have been found drug and doctor shopping under maiden and married names, under multiple birthdays, in other states, or under a different gender altogether. Some patients have been selling immense amounts of opioids and others consuming immense amounts.
Statewide statistics from the NM Board of Pharmacy from 2016 describes a 20.1% increase in PMP usage over the previous year.12 Data from 3 million prescriptions are uploaded to the PMP annually, and more than 100,000 search requests are processed monthly.11 There was a 16% decrease in opioid prescriptions per patient from multiple providers, a 14.2% decline in concurrent opioid and benzodiazepine prescriptions, and a 7.2% reduction in total opioid prescriptions.12 Fortunately, opioid overdose death rates are down 7% from the previous year.
There is an assumption of mutual trust between patient and provider. However, patients are given more responsibility for pain management through the use of a variety of objective tools and labs. Only a few people have requested refills on the naloxone kits other than for replacement of expired product. Based on experience, many patients and rescue buddies have inquired why they did not know of opioid-related risks earlier and stated that if they had naloxone available at home earlier, they could have saved a life. Further research is needed on the true impact of naloxone and its expanding access in the community.
The PMC has shown clinical success beyond simply helping to curb inappropriate opioid prescribing and overdose deaths. Investigative work such as initiating the right drug for the pain level or defining twice daily dosing as every 12 hours and not any 2 times per day has improved pain and function greatly. Many cases of hyperalgesia have been reduced by careful reductions in daily opioid doses.
Conclusion
The PMC and partners will continue to sustain efforts and transform now that the opioid crisis is exposed in the media, the IHS, and the PHS. The ongoing goals of the pain committee are to lift the dependence on opioids as long-term treatment, identify alternative solutions to pain conditions, and execute appropriate coordination of treatment. Optimization of pain management will depend on a variety of administrative and supportive service changes. Expansion of more PMC days is being considered to reduce no-show rates and the delay to first scheduled appointment. Recruitment and maintenance of specialists in behavioral health are crucial.
Securing avenues for medication-assisted therapies with buprenorphine and methadone also will help to reverse the prevalence of the opioid use disorders. The public hospital in Gallup, NM, has begun to mirror GIMC policies for consistent community actions toward improved pain management. Additionally, the GIMC experiences have been shared with area IHS facility pharmacists who are becoming increasingly involved in the national efforts to improve pain care. The pharmacy pain management clinic services at GIMC have emphasized judicious opioid prescribing, reduced overdose risk in the community, and improved patient functionality and quality of care through close pharmacotherapy monitoring.
According to the National Academy of Medicine (NAM), about 100 million people live with chronic pain in the U.S.1 There is evidence for the use of opioids to treat acute pain lasting 12 weeks or less. However, high-quality studies that analyze the benefit and safety of long-term opioid therapy are not yet available.2 In 2013, 249 million opioid prescriptions were written, equivalent to about 1 prescription per adult living in the U.S.3
Between 1999 and 2008, nonmedical use of prescription pain killers in the American Indian and Alaska Native populations was 2 to 3 times the frequency found in the white and black populations, respectively.1 These clinically contradictory practices created an environment conducive to opioid abuse and overdose. According to the Centers for Disease Control and Prevention, 1 in 4 people on chronic opioid therapy struggle with addiction.4 In 2014, more than 14,000 people in the U.S. died from overdoses involving prescription opioids.
The Comprehensive Addiction and Recovery Act of 2016 authorized prescription drug monitoring programs (PMPs) and a task force to create optimal pain treatment practices.5 The American Pharmacists Association (APhA), a major proponent of this law, argued that pharmacists are an underutilized resource despite having valuable clinical knowledge in the initiation, monitoring, and discontinuation of opioids. Additionally, pharmacists are able to refer patients to nonpharmacologic forms of pain management and dispense naloxone for emergent opioid overdose reversal.
Former Surgeon General Vivek Murthy developed the Turn the Tide Rx campaign to curb and reverse the opioid crisis in the U.S.3 The turnthetiderx.org website offers a pledge for clinicians who agree to be educated about pain management. It encourages open communication among prescribers and contains guideline-based resources on assessing pain and addiction risk, appropriate opioid prescribing, and how to manage opioid overdose. As required by law for prescribers in most states, there are instructions on how to access and analyze PMP opioid usage. The website also provides fact sheets about opioid treatments, safe disposal of medications, and helpline information.
Local Opioid Misuse Initiatives
New Mexico (NM) has one of the nation’s highest opioid and heroin overdose death rates.6 In January 2015, the U.S. Attorney’s Office and the University of New Mexico Health and Sciences Center partnered to launch the Heroin and Opioid Prevention and Education Initiative. The partners recognized that joint action between medical sciences and law enforcement was crucial to address the consequences of the opioid epidemic on public health and safety.
In 2017, the IHS established the National Committee on Heroin, Opioids, and Pain Efforts. Comprised of a variety of pharmacy and other subject-matter experts, this committee has a multipronged strategy to address the opioid epidemic from training to expanding medication-assisted treatment.7
Pharmacists provide clinical services in a variety of interdisciplinary ambulatory care clinics at Gallup Indian Medical Center (GIMC) in NM. The GIMC is located outside reservation boundaries but is centrally located to serve Navajo, Zuni, and a variety of other native populations. Pharmacist-run clinics include diabetes mellitus, anticoagulation, asthma, anemia, infectious diseases, and chronic pain. At GIMC, pain management pharmacists use a collective approach to curb opioid misuse. This article describes the establishment and impact of a pharmacist-led pain management clinic (PMC) at GIMC.
Pain Management Clinic
The understaffed urgent care clinic (UCC), emergency department (ED), primary care, and specialty practices plus a growing burden of complicated pain patients incentivized the development of the GIMC PMC. Under a collaborative practice agreement, pain management pharmacists were tasked with assessing, treating, and controlling noncancer chronic pain while improving quality of care and patient satisfaction (eAppendix 1, available at www.fedprac.com). The PMC goal was to improve functionality and pain scores and to reduce patient visits to the UCC and ED.
Originally, the PMC only performed medication titration for patients. In 2012, a former pharmacy resident became the PMC coordinator and has since helped to transform and expand its services. Currently, the coordinator dedicates about 20 hours per week managing pain patients in various capacities. Over time, other pain management pharmacists joined the PMC and support activities for 5 to 10 hours per week. There are now 4 pain management pharmacists who rotate through the PMC.
Pain management clinic visits generally are held once weekly for 3 hours and are occasionally expanded to full days based on patient schedule load. The initial 2 PMC appointments for each patient are 1 hour and are held within a 2-week period. Subsequent visits are each 30 minutes at 1 to 2 month intervals, depending on patient pain level and medication titration requirements. This standardized follow-up ensures new patients receive the close monitoring of slow dosage titrations necessary to achieve maximum therapeutic benefit.
Referrals
Patients only are admitted into the PMC via consults from primary care providers (PCPs). The consultations generally involve patients with complicated medical histories or who require complex therapies. The patients are contacted by telephone prior to scheduling. If a patient is not interested in PMC services, the consult is amended and the PCP is notified. This policy has been newly implemented to reduce the no-show rates. Pain management pharmacists then conduct PMC visits. The PMC activities are reported to the Pharmacy and Therapeutics committee annually. The PMC pharmacists also are available for telephone consultations from any internal hospital system department during weekday business hours.
The PMC patient population is fluid. New patients are accepted and clinically stable patients are discharged from PMC on a regular basis. The stable patients are released back to the PCP for further follow-up. Patients are considered stable if they have reached pain-related goals, are on maintenance doses of opioids, and/or are regularly participating in applicable interventional and referred therapies. If a patient requires reentry into PMC care, a new referral can be placed.
Interventional Programs
Since its inception, PMC has been a referral service to interventional programs. Physicians in the family medicine clinic provide twice weekly pain/palliative care clinic visits. These PCPs typically perform trigger point injections with lidocaine to relax muscles, which may disrupt nerve fibers. Ideally, this treatment reduces the use of opioids, non-steroidal anti-inflammatory drugs (NSAIDs), steroids, and epidural medications. Three acupuncturists were hired, which has reduced scheduling bottlenecks and wait times for patients to return for follow-up, especially in a treatment modality requiring frequent visits for effectiveness.
Pain Committee
As a part of an expansion of pain management services, GIMC established a pain committee (PC). The PC includes the GIMC medical director, PMC coordinator, pain management pharmacists, palliative care providers, PCPs, and specialty care providers. The PC created a detailed policy and procedures on management of chronic non-cancer pain for the Gallup Service Unit (eAppendix 2, available at www.fedprac.com). The PC offers guidance and completes consultations, performs internal review of prescribing patterns, and provides an appeals process for patients who have broken pain agreements. Physician and administrative champions have been instrumental to ensure proper pain management at GIMC.
Many VA facilities have deployed pain management clinics. At the VA Boston Healthcare System (VABHS), a pain management center is staffed by a multidisciplinary team that consists of anesthesiologists, neurologists, psychiatrists, nurses, and pharmacists.8 This pain clinic operates multiple days per week to accommodate demand, and patients are followed at least once a month. The VABHS often synchronized clinic visit dates with medication refill dates. Pharmacists offer an e-consult pain service to provide immediate recommendations to PCPs to bridge those patients awaiting appointments with pain clinic specialists at some VA facilities in Florida.9 Insufficient funding has prevented GIMC from increased PMC clinic hours.
Clinic Scope
Currently, the PMC sees the majority of PC cases. Pain management pharmacists are selected to conduct pain management visits based on interest and competency. Qualifications to work as a PMC pharmacist include on-the-job training, at least 6 annual pain management continuing medical education (CME) credits, participation in the NM naloxone training webinar, and completion of the physical assessment portion of the NM pharmacist clinician training course. It is highly recommended that pharmacists attend the PAINWeek conference in Las Vegas, Nevada, to obtain the necessary CME credits. In addition, pharmacists are requested to obtain the IHS National Clinical Pharmacy Specialist (NCPS) qualification within a year of practice.
Pain management pharmacists in the PMC review the indications for pain management, monitor medication therapy and adherence, adjust doses, manage adverse effects, study trends in pain and mood screening tools, and assess changes in functionality. These pharmacists are able to prescribe, discontinue, or titrate noncontrolled substance adjunctive therapies without a PCP cosignature. Adjunctive medication therapies can be NSAIDs, anticonvulsants, neuropathic pain relievers, muscle relaxers, and topical analgesics.
If adjustments to controlled substances are warranted, pain management pharmacists present the case to the PCP via electronic health record (EHR) notification or telephone conversation. These pharmacists ensure hard copies of controlled substance prescriptions are retrieved and provide refill coordination if assistance is requested by the patient. Pharmacists provide 28-day (not 30-day) prescriptions for opioid and controlled substance prescriptions to reduce weekend refill requests. Pain management pharmacists also order a variety of laboratory tests (eg, liver and renal function tests, and complete blood counts) related to the safe use of medications. If a patient is deemed unfit for PMC management, such as due to pain agreement violations, the PMC coordinator formally presents the case during PC meetings.
The PMC often recommends a multitude of nonpharmacologic treatments, including ice, hot rice socks, an anti-inflammatory diet, massage therapy, tennis ball massage for muscle tension and pinched nerves, chair exercises, transcutaneous electrical nerve stimulation therapy, aquatic therapy, and distraction therapy. Pain management pharmacists also can coordinate referrals to specialists and interventional therapies (eg, physical therapy; occupational therapy; acupuncture; trigger point injections; podiatry; orthopedics; ear, nose and throat; and diabetes mellitus).
Pain management pharmacists use a variety of established tools in the pain management clinic. The PMC EHR template and interview process are consistent with the universal precautions approach to unified pain management.10 Many of the questionnaires, tools, and laboratory tests are repeated periodically based on PMC policy and patient-specific need. These tools include a controlled substance pain agreement, consent for chronic opioid therapy, Opioid Risk Tool (ORT), Current Opioid Misuse Measure (COMM) for opioid abuse risk assessment, and the Patient Health Questionnaire (PHQ-9) for concurrent depression (Table and eAppendices 1, 3, and 4, available at www.fedprac.com). The ORT recently was added to the patient assessment packet to provide a stronger assessment of opioid misuse and abuse risk. Patient goals also are discussed with an emphasis on realistic changes, the level of control that would satisfy the patient and is feasible, what activities of daily living or hobbies the patient would like to regain, and what relationships the patient would like to improve.
PMC Patients
New patients are required to complete urine drug tests (UDTs), which can be performed in house or sent out if specific drug levels are required. Federally, marijuana is an illicit substance and is not prescribed or dispensed. Unpredictable effects of traditional medicine on the UDT have been observed. If a UDT shows positive for illicit substances, a discussion with the patient on toxicity and risks is initiated allowing them to choose to continue to use other substances for pain or use only the pain medication(s) appropriately prescribed by GIMC providers. Patients can be deemed ineligible for PMC management if they do not discontinue the use of illicit substances.
Health care providers and pharmacists involved in the prescribing or dispensing of controlled substances also complete a review of the patient’s PMP profile. It is mandatory in NM to complete PMP surveillance prior to prescribing controlled substances in quantities greater than 12 units within a 72-hour period and every 3 months for refills of chronic opioids.11 The PMP Interconnect service allows registered providers in NM to search for controlled substance usage in 25 states. All but 1 state in the U.S. has a PMP in development or in place. Federal providers who do not have a NM professional license also may apply for a NM PMP login to take advantage of the PMP Interconnect service. If the patient’s PMP is negative for prescribed opioids or positive for nonprescribed substances, the providers will conduct more research to reassess the risks and benefits of ongoing opioid therapy.
Random pill counts for tracked patients also have been incorporated into the PMC policy. These pill counts can be requested regardless of clinic appointment dates and can show whether patients are taking too many pills or diverting pills. Pill counts also may show that the patient may not need as many tablets per prescription if they consistently have more than expected based on dosing frequency. If a patient has adhered to prescribing recommendations, he or she may be allowed an early refill of the chronic opioid to cover them during a vacation or other unexpected event. However, if the pill counts are not consistent with instructions, then prescribing may be restricted to a 5-day, 7-day, or 14-day supply only. If patients do not present for pill count within 24 hours of the request, they can have their opioid use privileges at GIMC revoked as consented in the pain agreement. Patients are educated on proper storage and security of opioids medication at home and funding for lock boxes is expected in the near future. Patients may be referred to other services if opioid-use disorders are identified and confirmed.
Other administrative components of the pain management pharmacist responsibilities include clinical chart reviews before appointments or telephone consultations, sending appointment letters, contacting patients about and conducting random pill counts, and documenting PMC visit notes and updating flowsheets (eAppendices 4 and 5, available at www.fedprac.com). Currently under development is an EHR function that can quickly provide a summary of a patient’s pain management without a tedious search through the chart. Pain management pharmacists regularly instruct pharmacists and providers on changes in regulatory requirements of pain management as it pertains to prescription fills or clinical indications.
Ancillary Pharmacy Services
All pain patients on opioids therapies receive an annual pain evaluation. This evaluation provides a holistic description of the patient’s pain, a second opinion, and a collective review of the safety and efficacy of treatment. Components of the pain evaluation include review of medication toxicities and adverse effects, reconciliation of the treatment with current pain diagnosis and intensity, and coordination of opioid tapering schedules. Physical assessments and pill counts also are performed. Issues that have been uncovered include incidences of opioid-induced hyperalgesia and patients filling opioids prescriptions without taking the medications because they did not want to tell the provider the medication did not work.
Naloxone overdose prevention training and dispensing has been critical for ensuring maximum safety and life-saving methods in the realm of opioid therapy. Providers, patients, and rescue buddies are trained in the indication for, the administration of, and directions after naloxone use. Patients then are provided with naloxone nasal spray after the conclusion of the training and with instruction on refill procedures. Naloxone can be administered without legal penalty by anyone in NM. When refills for naloxone are requested, a naloxone-specific refill note is filed in the EHR to collect data on naloxone usage. In collaboration with a local suicide and substance abuse prevention network, 2 pain management pharmacists have created a Pills Can Kill booklet for dispersion to patients in waiting rooms, offices, schools, and throughout the county.
MedSafe receptacles (Houston, Texas) for collection of unused or unwanted medications, including controlled substances, are available in the pharmacy. These receptacles give patients a convenient and free outlet to remove circulating opioids from general access in the community.
Clinic Impact
Previously at GIMC, clinical pain conditions were managed by providers with minimal pain management expertise. Providers in the UCC and ED treated both acute and chronic pain with minimally enforced opioid prescribing limits. Often, patients were prescribed the dangerous drug trio of opioids, benzodiazepines, and muscle relaxers. In addition, there were inconsistent PMP checks, scheduled or random UDTs, or pill counts.
Currently, providers adhere more strongly to their scope of practice, which has resulted in less erratic opioid prescribing, from initiating therapy to prescribing large quantities. Providers in the UCC and ED more often triage chronic opioid pain patients to the PCP rather than obliging patients’ requests for chronic pain medication refills. Since UCC and ED providers now focus on the treatment of acute pain, there have been fewer drug seekers frequenting those departments, which has reduced the drug-seeking burden on PCPs and the outpatient pharmacy.
Increased utilization and study of the PMP profiles at GIMC has helped uncover important information, including incidences of theft and diversion. For example, a patient or caregiver who claimed stolen opioids was discovered to be diverting opioids from patients under her care and acutely drugging her patients in order for their UDTs to appear positive for opioids. Patients have been found drug and doctor shopping under maiden and married names, under multiple birthdays, in other states, or under a different gender altogether. Some patients have been selling immense amounts of opioids and others consuming immense amounts.
Statewide statistics from the NM Board of Pharmacy from 2016 describes a 20.1% increase in PMP usage over the previous year.12 Data from 3 million prescriptions are uploaded to the PMP annually, and more than 100,000 search requests are processed monthly.11 There was a 16% decrease in opioid prescriptions per patient from multiple providers, a 14.2% decline in concurrent opioid and benzodiazepine prescriptions, and a 7.2% reduction in total opioid prescriptions.12 Fortunately, opioid overdose death rates are down 7% from the previous year.
There is an assumption of mutual trust between patient and provider. However, patients are given more responsibility for pain management through the use of a variety of objective tools and labs. Only a few people have requested refills on the naloxone kits other than for replacement of expired product. Based on experience, many patients and rescue buddies have inquired why they did not know of opioid-related risks earlier and stated that if they had naloxone available at home earlier, they could have saved a life. Further research is needed on the true impact of naloxone and its expanding access in the community.
The PMC has shown clinical success beyond simply helping to curb inappropriate opioid prescribing and overdose deaths. Investigative work such as initiating the right drug for the pain level or defining twice daily dosing as every 12 hours and not any 2 times per day has improved pain and function greatly. Many cases of hyperalgesia have been reduced by careful reductions in daily opioid doses.
Conclusion
The PMC and partners will continue to sustain efforts and transform now that the opioid crisis is exposed in the media, the IHS, and the PHS. The ongoing goals of the pain committee are to lift the dependence on opioids as long-term treatment, identify alternative solutions to pain conditions, and execute appropriate coordination of treatment. Optimization of pain management will depend on a variety of administrative and supportive service changes. Expansion of more PMC days is being considered to reduce no-show rates and the delay to first scheduled appointment. Recruitment and maintenance of specialists in behavioral health are crucial.
Securing avenues for medication-assisted therapies with buprenorphine and methadone also will help to reverse the prevalence of the opioid use disorders. The public hospital in Gallup, NM, has begun to mirror GIMC policies for consistent community actions toward improved pain management. Additionally, the GIMC experiences have been shared with area IHS facility pharmacists who are becoming increasingly involved in the national efforts to improve pain care. The pharmacy pain management clinic services at GIMC have emphasized judicious opioid prescribing, reduced overdose risk in the community, and improved patient functionality and quality of care through close pharmacotherapy monitoring.
1. The American Academy of Pain Medicine. AAPM Facts and Figures in Pain. http://www.painmed.org/patientcenter/facts_on_pain.aspx. Accessed September 18, 2017.
2. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain---United States, 2016. JAMA. 2016;315(15):1624-1645.
3. U.S. Department of Health and Human Services. The surgeon general’s call to end the opioid crisis. https://turnthetiderx.org/. Accessed September 18, 2017.
4. Centers for Disease Control and Prevention. Prescription opioid overdose data. http://www.cdc.gov/drugoverdose/data/overdose.html. Updated August 1, 2017. Accessed September 18, 2017.
5. American Pharmacists Association. United States House of Representatives approves opioid bill package. http://www.pharmacist.com/united-states-house-representatives-approves-opioid-bill-package. Published May 13, 2016. Accessed September 18, 2017.
6. U.S. Attorney’s Office, District of New MexicoHeroin and opioid prevention and education (HOPE) initiative. http://www.hopeinitiativenm.org/. Ac cessed May 6, 2017.
7. U.S. Department of Health and Human Services,National Committee on Heroin, Opioids, and Pain Efforts (HOPE). Indian Health Service Circular No. 17-04. https://www.ihs.gov/ihm/index.cfm?module=dsp_ihm_circ_main&circ=ihm_circ_1704. Published March 24, 2017. Accessed May 6, 2017.
8. Rapoport A, Akbik H. Pharmacist-managed pain clinic at a Veterans Affairs Medical Center. Am J Health Syst Pharm. 2004;61(13):1341-1343.
9. Miller DM, Harvey TL. Pharmacist pain e-consults that result in a therapy change. Fed Pract. 2015 July;32(7):14-19.
10. Zacharoff, KL, Menefee Pujol, L, Corsini, E. PainEDU.org Manual. A Pocket Guide to Pain Management. 4th ed. Newton, MA : Inflexxion, Inc; 2010.
11. New Mexico Board of Pharmacy. Prescription monitoring program. http://nmpmp.org/. Accessed May 6, 2017.
12. New Mexico Department of Health Reports on Improved Opioid Prescribing Practices, Reduced Drug Overdose Death Rates. NABP e-News. http://nabp.bmetrack.com/c/v?e=A90B1D&c=8AB9&t= 0&l=193A4F8E. Accessed January 16, 2017.
1. The American Academy of Pain Medicine. AAPM Facts and Figures in Pain. http://www.painmed.org/patientcenter/facts_on_pain.aspx. Accessed September 18, 2017.
2. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain---United States, 2016. JAMA. 2016;315(15):1624-1645.
3. U.S. Department of Health and Human Services. The surgeon general’s call to end the opioid crisis. https://turnthetiderx.org/. Accessed September 18, 2017.
4. Centers for Disease Control and Prevention. Prescription opioid overdose data. http://www.cdc.gov/drugoverdose/data/overdose.html. Updated August 1, 2017. Accessed September 18, 2017.
5. American Pharmacists Association. United States House of Representatives approves opioid bill package. http://www.pharmacist.com/united-states-house-representatives-approves-opioid-bill-package. Published May 13, 2016. Accessed September 18, 2017.
6. U.S. Attorney’s Office, District of New MexicoHeroin and opioid prevention and education (HOPE) initiative. http://www.hopeinitiativenm.org/. Ac cessed May 6, 2017.
7. U.S. Department of Health and Human Services,National Committee on Heroin, Opioids, and Pain Efforts (HOPE). Indian Health Service Circular No. 17-04. https://www.ihs.gov/ihm/index.cfm?module=dsp_ihm_circ_main&circ=ihm_circ_1704. Published March 24, 2017. Accessed May 6, 2017.
8. Rapoport A, Akbik H. Pharmacist-managed pain clinic at a Veterans Affairs Medical Center. Am J Health Syst Pharm. 2004;61(13):1341-1343.
9. Miller DM, Harvey TL. Pharmacist pain e-consults that result in a therapy change. Fed Pract. 2015 July;32(7):14-19.
10. Zacharoff, KL, Menefee Pujol, L, Corsini, E. PainEDU.org Manual. A Pocket Guide to Pain Management. 4th ed. Newton, MA : Inflexxion, Inc; 2010.
11. New Mexico Board of Pharmacy. Prescription monitoring program. http://nmpmp.org/. Accessed May 6, 2017.
12. New Mexico Department of Health Reports on Improved Opioid Prescribing Practices, Reduced Drug Overdose Death Rates. NABP e-News. http://nabp.bmetrack.com/c/v?e=A90B1D&c=8AB9&t= 0&l=193A4F8E. Accessed January 16, 2017.
CCSs have increased risk of hypertension
A study of childhood cancer survivors (CCSs) suggests these individuals have an increased risk of developing hypertension as adults.
The CCSs studied had more than double the rate of hypertension observed in the matched general population.
Sex, age, race, and weight were all significantly associated with hypertension among CCSs, but most treatment types were not.
The exception was nephrectomy, which was associated with an increased risk of hypertension.
Todd M. Gibson, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee, and his colleagues conducted this research and reported the results in Cancer Epidemiology, Biomarkers & Prevention.
“High blood pressure is an important modifiable risk factor that increases the risk of heart problems in everyone,” Dr Gibson said. “Research has shown that high blood pressure can have an even greater negative impact on survivors of childhood cancer who were treated with cardiotoxic therapies such as anthracyclines or chest radiation.”
To assess the prevalence of hypertension among CCSs, Dr Gibson and his colleagues examined 3016 adults who were 10-year survivors of childhood cancers. The subjects were enrolled in the St. Jude Lifetime Cohort Study, which provides ongoing medical assessments of CCSs to advance knowledge of their long-term health outcomes.
The subjects’ mean age at the initial study assessment was 32, and 52% were male. Most (83%) were non-Hispanic white, 14% were non-Hispanic black, 2% were Hispanic, and 1% were “other.”
Thirty-seven percent of subjects had leukemia, 12% had Hodgkin lymphoma, and 7% had non-Hodgkin lymphoma.
Eighty-six percent of subjects had received chemotherapy, and 59% received radiation.
Results
Subjects were considered to have hypertension if their systolic blood pressure was 140 or greater, their diastolic blood pressure was 90 or greater, or if they had been previously diagnosed with hypertension and were taking antihypertensive medication.
The prevalence of hypertension was 2.6 times higher among CCSs than expected, based on age-, sex-, race- and body mass index-specific rates in the general population.
In addition, the incidence of hypertension increased for CCSs over time. Thirteen percent of CCSs had hypertension at age 30, 37% had it at age 40, and more than 70% had it at age 50.
Dr Gibson said rates of hypertension in CCSs matched rates in the general population of people about a decade older.
The researchers identified several factors that were significantly associated with hypertension among CCSs, including:
- Male sex (odd ratio [OR], 1.38; 95% CI, 1.14–1.67)
- Non-Hispanic black race (OR, 1.66; 95% CI, 1.28–2.16)
- Older age at assessment (OR per 1 year of age, 1.10; 95% CI, 1.08–1.11)
- Being overweight (OR, 1.58; 95% CI, 1.21–2.07)
- Obesity (OR, 3.02; 95% CI, 2.34–3.88).
Exposure to any type of radiation or chemotherapy was not significantly associated with hypertension, but nephrectomy was (OR, 1.68; 95% CI, 1.11–2.53).
Dr Gibson said the lack of an association between hypertension and radiation/chemotherapy was surprising. It suggests the connection between childhood cancer survival and adult hypertension is multifactorial and worthy of future research.
In the meantime, he said, clinicians should be mindful that CCSs are more likely than the general public to develop hypertension.
“The good news is that, unlike prior cancer therapy, high blood pressure is a modifiable risk factor,” Dr Gibson noted. “Research is needed to identify effective interventions to prevent hypertension in survivors, but our results emphasize the importance of blood pressure surveillance and management.”
Dr Gibson said a limitation of this study is that it was based on blood pressure measurements taken at a single study visit. A clinical diagnosis of hypertension typically requires measurements taken at multiple intervals.
In addition, the St. Jude Lifetime Cohort is a group of CCSs who undergo frequent clinical follow-up, so its participants may have benefited from being monitored and may therefore be in better health than CCSs who have less comprehensive follow-up. ![]()
A study of childhood cancer survivors (CCSs) suggests these individuals have an increased risk of developing hypertension as adults.
The CCSs studied had more than double the rate of hypertension observed in the matched general population.
Sex, age, race, and weight were all significantly associated with hypertension among CCSs, but most treatment types were not.
The exception was nephrectomy, which was associated with an increased risk of hypertension.
Todd M. Gibson, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee, and his colleagues conducted this research and reported the results in Cancer Epidemiology, Biomarkers & Prevention.
“High blood pressure is an important modifiable risk factor that increases the risk of heart problems in everyone,” Dr Gibson said. “Research has shown that high blood pressure can have an even greater negative impact on survivors of childhood cancer who were treated with cardiotoxic therapies such as anthracyclines or chest radiation.”
To assess the prevalence of hypertension among CCSs, Dr Gibson and his colleagues examined 3016 adults who were 10-year survivors of childhood cancers. The subjects were enrolled in the St. Jude Lifetime Cohort Study, which provides ongoing medical assessments of CCSs to advance knowledge of their long-term health outcomes.
The subjects’ mean age at the initial study assessment was 32, and 52% were male. Most (83%) were non-Hispanic white, 14% were non-Hispanic black, 2% were Hispanic, and 1% were “other.”
Thirty-seven percent of subjects had leukemia, 12% had Hodgkin lymphoma, and 7% had non-Hodgkin lymphoma.
Eighty-six percent of subjects had received chemotherapy, and 59% received radiation.
Results
Subjects were considered to have hypertension if their systolic blood pressure was 140 or greater, their diastolic blood pressure was 90 or greater, or if they had been previously diagnosed with hypertension and were taking antihypertensive medication.
The prevalence of hypertension was 2.6 times higher among CCSs than expected, based on age-, sex-, race- and body mass index-specific rates in the general population.
In addition, the incidence of hypertension increased for CCSs over time. Thirteen percent of CCSs had hypertension at age 30, 37% had it at age 40, and more than 70% had it at age 50.
Dr Gibson said rates of hypertension in CCSs matched rates in the general population of people about a decade older.
The researchers identified several factors that were significantly associated with hypertension among CCSs, including:
- Male sex (odd ratio [OR], 1.38; 95% CI, 1.14–1.67)
- Non-Hispanic black race (OR, 1.66; 95% CI, 1.28–2.16)
- Older age at assessment (OR per 1 year of age, 1.10; 95% CI, 1.08–1.11)
- Being overweight (OR, 1.58; 95% CI, 1.21–2.07)
- Obesity (OR, 3.02; 95% CI, 2.34–3.88).
Exposure to any type of radiation or chemotherapy was not significantly associated with hypertension, but nephrectomy was (OR, 1.68; 95% CI, 1.11–2.53).
Dr Gibson said the lack of an association between hypertension and radiation/chemotherapy was surprising. It suggests the connection between childhood cancer survival and adult hypertension is multifactorial and worthy of future research.
In the meantime, he said, clinicians should be mindful that CCSs are more likely than the general public to develop hypertension.
“The good news is that, unlike prior cancer therapy, high blood pressure is a modifiable risk factor,” Dr Gibson noted. “Research is needed to identify effective interventions to prevent hypertension in survivors, but our results emphasize the importance of blood pressure surveillance and management.”
Dr Gibson said a limitation of this study is that it was based on blood pressure measurements taken at a single study visit. A clinical diagnosis of hypertension typically requires measurements taken at multiple intervals.
In addition, the St. Jude Lifetime Cohort is a group of CCSs who undergo frequent clinical follow-up, so its participants may have benefited from being monitored and may therefore be in better health than CCSs who have less comprehensive follow-up. ![]()
A study of childhood cancer survivors (CCSs) suggests these individuals have an increased risk of developing hypertension as adults.
The CCSs studied had more than double the rate of hypertension observed in the matched general population.
Sex, age, race, and weight were all significantly associated with hypertension among CCSs, but most treatment types were not.
The exception was nephrectomy, which was associated with an increased risk of hypertension.
Todd M. Gibson, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee, and his colleagues conducted this research and reported the results in Cancer Epidemiology, Biomarkers & Prevention.
“High blood pressure is an important modifiable risk factor that increases the risk of heart problems in everyone,” Dr Gibson said. “Research has shown that high blood pressure can have an even greater negative impact on survivors of childhood cancer who were treated with cardiotoxic therapies such as anthracyclines or chest radiation.”
To assess the prevalence of hypertension among CCSs, Dr Gibson and his colleagues examined 3016 adults who were 10-year survivors of childhood cancers. The subjects were enrolled in the St. Jude Lifetime Cohort Study, which provides ongoing medical assessments of CCSs to advance knowledge of their long-term health outcomes.
The subjects’ mean age at the initial study assessment was 32, and 52% were male. Most (83%) were non-Hispanic white, 14% were non-Hispanic black, 2% were Hispanic, and 1% were “other.”
Thirty-seven percent of subjects had leukemia, 12% had Hodgkin lymphoma, and 7% had non-Hodgkin lymphoma.
Eighty-six percent of subjects had received chemotherapy, and 59% received radiation.
Results
Subjects were considered to have hypertension if their systolic blood pressure was 140 or greater, their diastolic blood pressure was 90 or greater, or if they had been previously diagnosed with hypertension and were taking antihypertensive medication.
The prevalence of hypertension was 2.6 times higher among CCSs than expected, based on age-, sex-, race- and body mass index-specific rates in the general population.
In addition, the incidence of hypertension increased for CCSs over time. Thirteen percent of CCSs had hypertension at age 30, 37% had it at age 40, and more than 70% had it at age 50.
Dr Gibson said rates of hypertension in CCSs matched rates in the general population of people about a decade older.
The researchers identified several factors that were significantly associated with hypertension among CCSs, including:
- Male sex (odd ratio [OR], 1.38; 95% CI, 1.14–1.67)
- Non-Hispanic black race (OR, 1.66; 95% CI, 1.28–2.16)
- Older age at assessment (OR per 1 year of age, 1.10; 95% CI, 1.08–1.11)
- Being overweight (OR, 1.58; 95% CI, 1.21–2.07)
- Obesity (OR, 3.02; 95% CI, 2.34–3.88).
Exposure to any type of radiation or chemotherapy was not significantly associated with hypertension, but nephrectomy was (OR, 1.68; 95% CI, 1.11–2.53).
Dr Gibson said the lack of an association between hypertension and radiation/chemotherapy was surprising. It suggests the connection between childhood cancer survival and adult hypertension is multifactorial and worthy of future research.
In the meantime, he said, clinicians should be mindful that CCSs are more likely than the general public to develop hypertension.
“The good news is that, unlike prior cancer therapy, high blood pressure is a modifiable risk factor,” Dr Gibson noted. “Research is needed to identify effective interventions to prevent hypertension in survivors, but our results emphasize the importance of blood pressure surveillance and management.”
Dr Gibson said a limitation of this study is that it was based on blood pressure measurements taken at a single study visit. A clinical diagnosis of hypertension typically requires measurements taken at multiple intervals.
In addition, the St. Jude Lifetime Cohort is a group of CCSs who undergo frequent clinical follow-up, so its participants may have benefited from being monitored and may therefore be in better health than CCSs who have less comprehensive follow-up. ![]()
Race may transcend social, geographical parameters in lupus mortality
SAN DIEGO – Blacks with systemic lupus erythematosus (SLE) who share the same social and geographic contexts as whites with the disease were disproportionately more likely to die young and to show severe patterns of mortality, according to a study of death certificate data.
“One of the most salient aspects of the epidemiology of lupus is the predilection of the disease for women and racial minorities,” lead study author Titilola Falasinnu, PhD, said at the annual meeting of the American College of Rheumatology.
Dr. Falasinnu, a postdoctoral fellow in Stanford (Calif.) University’s department of health research and policy, and her associates examined SLE mortality across eight groups of race-county combinations published in 2006 and known as the “Eight Americas” (PLoS Med. 2006 Sep 12;3[9]:e260). This seminal work, which has been validated across multiple disease states, jointly characterized race, socioeconomic status, and geographical location in relation to health disparities in the United States to demonstrate the most important factors accounting for these disparities within the Eight Americas.
For the current analysis, Dr. Falasinnu and her associates were most interested in America 6 (black middle America), America 7 (Southern low-income rural blacks), and America 8 (high-risk urban blacks). “The question we wanted to ask is whether, on average, poorer individuals have more severe SLE mortality experiences, compared with richer individuals in the black community,” she said. “What happens when you hold race constant and you vary socioeconomic indices?” Using death certificate data from the National Center for Health Statistics Multiple Causes of Death database, they identified SLE-related deaths between 2003 and 2014. Next, they compared these data with mortality statistics from each of the Eight Americas.
In all, there were nearly 25,000 SLE-related deaths, of which 85% were female. More than one-third of deaths occurred among those aged 45-64 years, and the mean age at death was 57 years. More than half of deaths (64%) occurred among whites, and 31% among blacks. Among SLE decedents, northern rural whites in America 2 had the lowest mortality rates. Blacks in America 6, 7, and 8 had the highest mortality, yet there were no significant differences in the death rates among those three groups. “We found that in general, blacks were three times more likely to die with SLE, compared with those in America 3 [middle America],” Dr. Falasinnu said.
Asians, Native Americans, and blacks with SLE died at an average age of 48-49 years, regardless of their social context, while Northern whites had the highest life expectancy: an average age of 69 years. They also found that 17% of SLE deaths in America 2 occurred before the age of 50 years, compared with more than 50% in Americas 6, 7, and 8. The most frequently reported associated causes of death were cardiovascular disease (about 50% of all SLE-related deaths) and kidney manifestations (about 20% of all SLE-related deaths). Compared with those in America 3, racial minorities had a 23%-53% higher risk of infections, a 5%-64% higher risk of kidney disease, a 7%-23% lower risk of cardiovascular disease, and a 20%-52% lower risk of cancers.
“Although blacks inhabited three vastly different geographical and social contexts, SLE mortality parameters did not vary among socially advantaged and disadvantaged black Americas,” Dr. Falasinnu concluded. “Blacks sharing the same social and geographical contexts as whites were disproportionately more likely to die young and exhibit patterns of mortality associated with active SLE disease.”
She acknowledged certain limitations of the study, including that differences in the degree of underreporting on death certificates across racial groups could bias the results. “The Eight Americas framework does not allow for evaluation of ethnicity,” she added. “We were also unable to examine causes for the disparities in SLE mortality. One could argue that there are a lot of other social factors that are likely race related that are not necessarily captured by the Eight Americas. Also, as with most epidemiological studies, we cannot rule out the role that ecological fallacy may play where the population average may not be appropriate in estimating an individual’s risk of mortality.”
One of the study coauthors reported receiving partial salary support through the Dr. Elaine Lambert Lupus Foundation via the John and Marcia Goldman Foundation and previously receiving salary support through a lupus-related grant from the Genomics Institute of the Novartis Research Foundation. The other coauthors reported having no relevant disclosures.
[email protected]
SAN DIEGO – Blacks with systemic lupus erythematosus (SLE) who share the same social and geographic contexts as whites with the disease were disproportionately more likely to die young and to show severe patterns of mortality, according to a study of death certificate data.
“One of the most salient aspects of the epidemiology of lupus is the predilection of the disease for women and racial minorities,” lead study author Titilola Falasinnu, PhD, said at the annual meeting of the American College of Rheumatology.
Dr. Falasinnu, a postdoctoral fellow in Stanford (Calif.) University’s department of health research and policy, and her associates examined SLE mortality across eight groups of race-county combinations published in 2006 and known as the “Eight Americas” (PLoS Med. 2006 Sep 12;3[9]:e260). This seminal work, which has been validated across multiple disease states, jointly characterized race, socioeconomic status, and geographical location in relation to health disparities in the United States to demonstrate the most important factors accounting for these disparities within the Eight Americas.
For the current analysis, Dr. Falasinnu and her associates were most interested in America 6 (black middle America), America 7 (Southern low-income rural blacks), and America 8 (high-risk urban blacks). “The question we wanted to ask is whether, on average, poorer individuals have more severe SLE mortality experiences, compared with richer individuals in the black community,” she said. “What happens when you hold race constant and you vary socioeconomic indices?” Using death certificate data from the National Center for Health Statistics Multiple Causes of Death database, they identified SLE-related deaths between 2003 and 2014. Next, they compared these data with mortality statistics from each of the Eight Americas.
In all, there were nearly 25,000 SLE-related deaths, of which 85% were female. More than one-third of deaths occurred among those aged 45-64 years, and the mean age at death was 57 years. More than half of deaths (64%) occurred among whites, and 31% among blacks. Among SLE decedents, northern rural whites in America 2 had the lowest mortality rates. Blacks in America 6, 7, and 8 had the highest mortality, yet there were no significant differences in the death rates among those three groups. “We found that in general, blacks were three times more likely to die with SLE, compared with those in America 3 [middle America],” Dr. Falasinnu said.
Asians, Native Americans, and blacks with SLE died at an average age of 48-49 years, regardless of their social context, while Northern whites had the highest life expectancy: an average age of 69 years. They also found that 17% of SLE deaths in America 2 occurred before the age of 50 years, compared with more than 50% in Americas 6, 7, and 8. The most frequently reported associated causes of death were cardiovascular disease (about 50% of all SLE-related deaths) and kidney manifestations (about 20% of all SLE-related deaths). Compared with those in America 3, racial minorities had a 23%-53% higher risk of infections, a 5%-64% higher risk of kidney disease, a 7%-23% lower risk of cardiovascular disease, and a 20%-52% lower risk of cancers.
“Although blacks inhabited three vastly different geographical and social contexts, SLE mortality parameters did not vary among socially advantaged and disadvantaged black Americas,” Dr. Falasinnu concluded. “Blacks sharing the same social and geographical contexts as whites were disproportionately more likely to die young and exhibit patterns of mortality associated with active SLE disease.”
She acknowledged certain limitations of the study, including that differences in the degree of underreporting on death certificates across racial groups could bias the results. “The Eight Americas framework does not allow for evaluation of ethnicity,” she added. “We were also unable to examine causes for the disparities in SLE mortality. One could argue that there are a lot of other social factors that are likely race related that are not necessarily captured by the Eight Americas. Also, as with most epidemiological studies, we cannot rule out the role that ecological fallacy may play where the population average may not be appropriate in estimating an individual’s risk of mortality.”
One of the study coauthors reported receiving partial salary support through the Dr. Elaine Lambert Lupus Foundation via the John and Marcia Goldman Foundation and previously receiving salary support through a lupus-related grant from the Genomics Institute of the Novartis Research Foundation. The other coauthors reported having no relevant disclosures.
[email protected]
SAN DIEGO – Blacks with systemic lupus erythematosus (SLE) who share the same social and geographic contexts as whites with the disease were disproportionately more likely to die young and to show severe patterns of mortality, according to a study of death certificate data.
“One of the most salient aspects of the epidemiology of lupus is the predilection of the disease for women and racial minorities,” lead study author Titilola Falasinnu, PhD, said at the annual meeting of the American College of Rheumatology.
Dr. Falasinnu, a postdoctoral fellow in Stanford (Calif.) University’s department of health research and policy, and her associates examined SLE mortality across eight groups of race-county combinations published in 2006 and known as the “Eight Americas” (PLoS Med. 2006 Sep 12;3[9]:e260). This seminal work, which has been validated across multiple disease states, jointly characterized race, socioeconomic status, and geographical location in relation to health disparities in the United States to demonstrate the most important factors accounting for these disparities within the Eight Americas.
For the current analysis, Dr. Falasinnu and her associates were most interested in America 6 (black middle America), America 7 (Southern low-income rural blacks), and America 8 (high-risk urban blacks). “The question we wanted to ask is whether, on average, poorer individuals have more severe SLE mortality experiences, compared with richer individuals in the black community,” she said. “What happens when you hold race constant and you vary socioeconomic indices?” Using death certificate data from the National Center for Health Statistics Multiple Causes of Death database, they identified SLE-related deaths between 2003 and 2014. Next, they compared these data with mortality statistics from each of the Eight Americas.
In all, there were nearly 25,000 SLE-related deaths, of which 85% were female. More than one-third of deaths occurred among those aged 45-64 years, and the mean age at death was 57 years. More than half of deaths (64%) occurred among whites, and 31% among blacks. Among SLE decedents, northern rural whites in America 2 had the lowest mortality rates. Blacks in America 6, 7, and 8 had the highest mortality, yet there were no significant differences in the death rates among those three groups. “We found that in general, blacks were three times more likely to die with SLE, compared with those in America 3 [middle America],” Dr. Falasinnu said.
Asians, Native Americans, and blacks with SLE died at an average age of 48-49 years, regardless of their social context, while Northern whites had the highest life expectancy: an average age of 69 years. They also found that 17% of SLE deaths in America 2 occurred before the age of 50 years, compared with more than 50% in Americas 6, 7, and 8. The most frequently reported associated causes of death were cardiovascular disease (about 50% of all SLE-related deaths) and kidney manifestations (about 20% of all SLE-related deaths). Compared with those in America 3, racial minorities had a 23%-53% higher risk of infections, a 5%-64% higher risk of kidney disease, a 7%-23% lower risk of cardiovascular disease, and a 20%-52% lower risk of cancers.
“Although blacks inhabited three vastly different geographical and social contexts, SLE mortality parameters did not vary among socially advantaged and disadvantaged black Americas,” Dr. Falasinnu concluded. “Blacks sharing the same social and geographical contexts as whites were disproportionately more likely to die young and exhibit patterns of mortality associated with active SLE disease.”
She acknowledged certain limitations of the study, including that differences in the degree of underreporting on death certificates across racial groups could bias the results. “The Eight Americas framework does not allow for evaluation of ethnicity,” she added. “We were also unable to examine causes for the disparities in SLE mortality. One could argue that there are a lot of other social factors that are likely race related that are not necessarily captured by the Eight Americas. Also, as with most epidemiological studies, we cannot rule out the role that ecological fallacy may play where the population average may not be appropriate in estimating an individual’s risk of mortality.”
One of the study coauthors reported receiving partial salary support through the Dr. Elaine Lambert Lupus Foundation via the John and Marcia Goldman Foundation and previously receiving salary support through a lupus-related grant from the Genomics Institute of the Novartis Research Foundation. The other coauthors reported having no relevant disclosures.
[email protected]
AT ACR 2017
Key clinical point: Blacks sharing the same social and geographical contexts as whites were more likely to die young and exhibit patterns of mortality associated with active SLE disease.
Major finding: Blacks in three race-geographic contexts were about three times more likely than whites in middle America to die with SLE.
Study details: An analysis of nearly 25,000 SLE-related deaths from the National Center for Health Statistics Multiple Causes of Death database.
Disclosures: One of the study coauthors reported receiving partial salary support through the Dr. Elaine Lambert Lupus Foundation via the John and Marcia Goldman Foundation and previously receiving salary support through a lupus-related grant from the Genomics Institute of the Novartis Research Foundation. The other coauthors reported having no relevant disclosures.
Mutations influence prognostic value of tumor location in stage III colon cancer
Among patients with resected stage III colon cancer, the prognostic value of primary tumor location varied according to patients’ BRAF or RAS mutational status, according to a post hoc analysis of patients in the Pan-European Trials in Alimentary Tract Cancer (PETACC)-8 trial.
Specifically, in patients with BRAF or RAS mutations, disease-free survival (DFS) was better in right-sided vs. left-sided tumors, while in patients with RAS and BRAF double wild type, DFS was worse in right-sided vs. left-sided tumors, authors of the study reported in JAMA Oncology.
Right-sided tumor location has been associated with poor survival in previous studies of patients with metastatic colorectal cancer. However, for patients with stage III disease, such as those in this study, little is known about how tumor sidedness affects outcomes, the investigators wrote (JAMA Oncol. 2017 Nov 22. doi: 10.1001/jamaoncol.2017.3695).
“Although primary tumor location does not seem to be associated with DFS in the whole study population, opposite sidedness prognostic values are observed for RAS and BRAF wild-type and mutant tumors,” wrote Julien Taieb, MD, PhD, Department of Hepatogastroenterology and GI Oncology, Hôpital Européen Georges-Pompidou, Paris, and his coauthors.
The post hoc analysis included 1,869 resected stage III colon cancer patients with full information on primary tumor location in the PETACC-8 phase 3 randomized trial. The primary objective of the trial was to evaluate adjuvant treatment with the FOLFOX regimen with or without cetuximab.
Forty percent of the patients had a right-sided tumor, data show.
Right-sided tumor location was not prognostic for DFS for all patients, though it was associated with shorter survival after relapse (hazard ratio, 1.54; 95% confidence interval, 1.23-1.93; P = .001) and overall survival (HR, 1.25; 95%CI, 1.02-1.54; P = .03).
However, when looking at different molecular subgroups, Dr. Taieb and his colleagues found that DFS was better in right-sided vs. left-sided tumors in patients with RAS mutations (HR, 0.80; 95% CI, 0.64-1.00; P = .046), and similar findings were seen in BRAF-mutated tumors.
Conversely, DFS was worse in right-sided vs. left-sided tumors among those patients with RAS and BRAF double wild type (HR, 1.39; 95% CI, 1.01-1.92; P = .04), the investigators reported.
Investigators also sought to evaluate whether tumor location predicted the benefit of cetuximab.
However, “no benefit of adding cetuximab to FOLFOX was observed in our population of patients with stage III left-sided tumors, nor was any detrimental effect of adding cetuximab observed in right-sided tumors,” Dr. Taieb and his coauthors wrote.
PETACC-8 was sponsored by the Fédération Francophone de Cancérologie Digestive with some support from Merck KGaA and Sanofi-Aventis. Dr. Taieb reported participating in consulting or advisory boards for Merck KGaA, Sanofi, Roche/Genentech, Pfizer, and Amgen.
Among patients with resected stage III colon cancer, the prognostic value of primary tumor location varied according to patients’ BRAF or RAS mutational status, according to a post hoc analysis of patients in the Pan-European Trials in Alimentary Tract Cancer (PETACC)-8 trial.
Specifically, in patients with BRAF or RAS mutations, disease-free survival (DFS) was better in right-sided vs. left-sided tumors, while in patients with RAS and BRAF double wild type, DFS was worse in right-sided vs. left-sided tumors, authors of the study reported in JAMA Oncology.
Right-sided tumor location has been associated with poor survival in previous studies of patients with metastatic colorectal cancer. However, for patients with stage III disease, such as those in this study, little is known about how tumor sidedness affects outcomes, the investigators wrote (JAMA Oncol. 2017 Nov 22. doi: 10.1001/jamaoncol.2017.3695).
“Although primary tumor location does not seem to be associated with DFS in the whole study population, opposite sidedness prognostic values are observed for RAS and BRAF wild-type and mutant tumors,” wrote Julien Taieb, MD, PhD, Department of Hepatogastroenterology and GI Oncology, Hôpital Européen Georges-Pompidou, Paris, and his coauthors.
The post hoc analysis included 1,869 resected stage III colon cancer patients with full information on primary tumor location in the PETACC-8 phase 3 randomized trial. The primary objective of the trial was to evaluate adjuvant treatment with the FOLFOX regimen with or without cetuximab.
Forty percent of the patients had a right-sided tumor, data show.
Right-sided tumor location was not prognostic for DFS for all patients, though it was associated with shorter survival after relapse (hazard ratio, 1.54; 95% confidence interval, 1.23-1.93; P = .001) and overall survival (HR, 1.25; 95%CI, 1.02-1.54; P = .03).
However, when looking at different molecular subgroups, Dr. Taieb and his colleagues found that DFS was better in right-sided vs. left-sided tumors in patients with RAS mutations (HR, 0.80; 95% CI, 0.64-1.00; P = .046), and similar findings were seen in BRAF-mutated tumors.
Conversely, DFS was worse in right-sided vs. left-sided tumors among those patients with RAS and BRAF double wild type (HR, 1.39; 95% CI, 1.01-1.92; P = .04), the investigators reported.
Investigators also sought to evaluate whether tumor location predicted the benefit of cetuximab.
However, “no benefit of adding cetuximab to FOLFOX was observed in our population of patients with stage III left-sided tumors, nor was any detrimental effect of adding cetuximab observed in right-sided tumors,” Dr. Taieb and his coauthors wrote.
PETACC-8 was sponsored by the Fédération Francophone de Cancérologie Digestive with some support from Merck KGaA and Sanofi-Aventis. Dr. Taieb reported participating in consulting or advisory boards for Merck KGaA, Sanofi, Roche/Genentech, Pfizer, and Amgen.
Among patients with resected stage III colon cancer, the prognostic value of primary tumor location varied according to patients’ BRAF or RAS mutational status, according to a post hoc analysis of patients in the Pan-European Trials in Alimentary Tract Cancer (PETACC)-8 trial.
Specifically, in patients with BRAF or RAS mutations, disease-free survival (DFS) was better in right-sided vs. left-sided tumors, while in patients with RAS and BRAF double wild type, DFS was worse in right-sided vs. left-sided tumors, authors of the study reported in JAMA Oncology.
Right-sided tumor location has been associated with poor survival in previous studies of patients with metastatic colorectal cancer. However, for patients with stage III disease, such as those in this study, little is known about how tumor sidedness affects outcomes, the investigators wrote (JAMA Oncol. 2017 Nov 22. doi: 10.1001/jamaoncol.2017.3695).
“Although primary tumor location does not seem to be associated with DFS in the whole study population, opposite sidedness prognostic values are observed for RAS and BRAF wild-type and mutant tumors,” wrote Julien Taieb, MD, PhD, Department of Hepatogastroenterology and GI Oncology, Hôpital Européen Georges-Pompidou, Paris, and his coauthors.
The post hoc analysis included 1,869 resected stage III colon cancer patients with full information on primary tumor location in the PETACC-8 phase 3 randomized trial. The primary objective of the trial was to evaluate adjuvant treatment with the FOLFOX regimen with or without cetuximab.
Forty percent of the patients had a right-sided tumor, data show.
Right-sided tumor location was not prognostic for DFS for all patients, though it was associated with shorter survival after relapse (hazard ratio, 1.54; 95% confidence interval, 1.23-1.93; P = .001) and overall survival (HR, 1.25; 95%CI, 1.02-1.54; P = .03).
However, when looking at different molecular subgroups, Dr. Taieb and his colleagues found that DFS was better in right-sided vs. left-sided tumors in patients with RAS mutations (HR, 0.80; 95% CI, 0.64-1.00; P = .046), and similar findings were seen in BRAF-mutated tumors.
Conversely, DFS was worse in right-sided vs. left-sided tumors among those patients with RAS and BRAF double wild type (HR, 1.39; 95% CI, 1.01-1.92; P = .04), the investigators reported.
Investigators also sought to evaluate whether tumor location predicted the benefit of cetuximab.
However, “no benefit of adding cetuximab to FOLFOX was observed in our population of patients with stage III left-sided tumors, nor was any detrimental effect of adding cetuximab observed in right-sided tumors,” Dr. Taieb and his coauthors wrote.
PETACC-8 was sponsored by the Fédération Francophone de Cancérologie Digestive with some support from Merck KGaA and Sanofi-Aventis. Dr. Taieb reported participating in consulting or advisory boards for Merck KGaA, Sanofi, Roche/Genentech, Pfizer, and Amgen.
FROM JAMA ONCOLOGY
Key clinical point: In patients with resected stage III colon cancer, prognosis associated with right-sided vs. left-sided tumors appears to vary according to RAS and BRAF mutational status.
Major finding: In patients with RAS mutations, DFS was better in right-sided vs. left-sided tumors (HR, 0.80; 95% CI, 0.64-1.00; P = .046), while in patients with RAS and BRAF double wild type, DFS was worse in right-sided vs. left-sided tumors (HR, 1.39; 95% CI, 1.01-1.92; P = .04).
Data source: A post hoc analysis of 1,869 patients in the Pan-European Trials in Alimentary Tract Cancer (PETACC)-8 phase 3 randomized trial with available tumor blocks of resected stage III colon adenocarcinoma.
Disclosures: PETACC-8 was sponsored by the Fédération Francophone de Cancérologie Digestive with some support from Merck KGaA and Sanofi-Aventis. Study authors reported various disclosures; first author Julien Taieb, MD, PhD reported participating in consulting or advisory boards for Merck KGaA, Sanofi, Roche/Genentech, Pfizer, and Amgen.
Does incretin therapy increase pancreatic cancer risk?
Risk of pancreatic cancer doubled among diabetes patients on incretin therapy in a recent retrospective cohort study, although it is unlikely that the drugs actually caused the increase, according to investigators.
, wrote Dr. Mathieu Boniol, vice president of statistics, International Prevention Research Institute, Lyon, France, and colleagues.
The study was sparked by concerns about a possible increased risk of cancer among patients prescribed incretin drugs, which include glucagonlike peptide receptor–1 (GLP-1R) agonists and dipeptidyl peptidase 4 (DPP-4) inhibitors, authors said.
Results of the retrospective cohort analysis are based on two European public health insurance databases, comprising 33,292 diabetes patients treated with incretin drugs and 525,733 treated with other noninsulin antidiabetic drugs.
The risk of pancreatic cancer doubled for patients receiving incretin treatment (adjusted hazard ratio, 2.14; 95% confidence interval, 1.71-2.67), compared with patients receiving other noninsulin drugs, after adjusting for age, sex, and whether or not patients subsequently received an insulin prescription, investigators reported.
However, risk of pancreatic cancer decreased over time in patients on incretin therapy, according to further analyses by Dr. Boniol and colleagues.
That finding of diminishing risk over time argues against concluding that incretin drugs cause pancreatic cancer, according to investigators.
“A direct causal effect could be suspected if a steadily increasing risk of pancreatic cancer was associated with a steadily longer exposure to incretin drugs,” they said.
In the first 3 months after the first incretin prescription, risk of pancreatic cancer was 3.35 times greater (95% CI, 2.32-4.84), which “gradually diminished” to 1.69 (95% CI, 1.12-2.55) a year after the first prescription, the investigators said in the report.
As such, these new data do not help prove or disprove a causal link between incretin use and pancreatic cancer, continued Dr. Hellman, a past president of the American Association of Clinical Endocrinologists.
“I think, right now, we don’t have the data,” he said in an interview. “It’s a question that makes us nervous, because of what we know about the laboratory effects of the drugs, but we don’t have the answer yet. We need longer studies with larger numbers of people to answer that question.”
The study, funded by Sanofi, was conducted at the request of the European Medicines Agency to assess risk of pancreatic cancer associated with lixisenatide. However, the study covers a period prior to use of lixisenatide in the general population because the approval of the GLP-1R agonist was “too recent,” the study authors wrote.
The analyses in the study were conducted in 2015 and 2016 for two cohorts of subjects: one in Belgium, for which data had been collected from 2008 to 2013, and one in Italy, with data from 2000 to 2012.
Further studies are needed to assess how long-term use of incretin drugs may affect risk of pancreatic cancer, the authors said.
The analysis was conducted as part of a postauthorization safety study requested by the European Medicines Agency and funded by Sanofi. One study author reported receiving grants from Roche, Amgen, and Bristol-Myers Squibb outside the submitted work; the remaining investigators had no relevant financial disclosures.
Risk of pancreatic cancer doubled among diabetes patients on incretin therapy in a recent retrospective cohort study, although it is unlikely that the drugs actually caused the increase, according to investigators.
, wrote Dr. Mathieu Boniol, vice president of statistics, International Prevention Research Institute, Lyon, France, and colleagues.
The study was sparked by concerns about a possible increased risk of cancer among patients prescribed incretin drugs, which include glucagonlike peptide receptor–1 (GLP-1R) agonists and dipeptidyl peptidase 4 (DPP-4) inhibitors, authors said.
Results of the retrospective cohort analysis are based on two European public health insurance databases, comprising 33,292 diabetes patients treated with incretin drugs and 525,733 treated with other noninsulin antidiabetic drugs.
The risk of pancreatic cancer doubled for patients receiving incretin treatment (adjusted hazard ratio, 2.14; 95% confidence interval, 1.71-2.67), compared with patients receiving other noninsulin drugs, after adjusting for age, sex, and whether or not patients subsequently received an insulin prescription, investigators reported.
However, risk of pancreatic cancer decreased over time in patients on incretin therapy, according to further analyses by Dr. Boniol and colleagues.
That finding of diminishing risk over time argues against concluding that incretin drugs cause pancreatic cancer, according to investigators.
“A direct causal effect could be suspected if a steadily increasing risk of pancreatic cancer was associated with a steadily longer exposure to incretin drugs,” they said.
In the first 3 months after the first incretin prescription, risk of pancreatic cancer was 3.35 times greater (95% CI, 2.32-4.84), which “gradually diminished” to 1.69 (95% CI, 1.12-2.55) a year after the first prescription, the investigators said in the report.
As such, these new data do not help prove or disprove a causal link between incretin use and pancreatic cancer, continued Dr. Hellman, a past president of the American Association of Clinical Endocrinologists.
“I think, right now, we don’t have the data,” he said in an interview. “It’s a question that makes us nervous, because of what we know about the laboratory effects of the drugs, but we don’t have the answer yet. We need longer studies with larger numbers of people to answer that question.”
The study, funded by Sanofi, was conducted at the request of the European Medicines Agency to assess risk of pancreatic cancer associated with lixisenatide. However, the study covers a period prior to use of lixisenatide in the general population because the approval of the GLP-1R agonist was “too recent,” the study authors wrote.
The analyses in the study were conducted in 2015 and 2016 for two cohorts of subjects: one in Belgium, for which data had been collected from 2008 to 2013, and one in Italy, with data from 2000 to 2012.
Further studies are needed to assess how long-term use of incretin drugs may affect risk of pancreatic cancer, the authors said.
The analysis was conducted as part of a postauthorization safety study requested by the European Medicines Agency and funded by Sanofi. One study author reported receiving grants from Roche, Amgen, and Bristol-Myers Squibb outside the submitted work; the remaining investigators had no relevant financial disclosures.
Risk of pancreatic cancer doubled among diabetes patients on incretin therapy in a recent retrospective cohort study, although it is unlikely that the drugs actually caused the increase, according to investigators.
, wrote Dr. Mathieu Boniol, vice president of statistics, International Prevention Research Institute, Lyon, France, and colleagues.
The study was sparked by concerns about a possible increased risk of cancer among patients prescribed incretin drugs, which include glucagonlike peptide receptor–1 (GLP-1R) agonists and dipeptidyl peptidase 4 (DPP-4) inhibitors, authors said.
Results of the retrospective cohort analysis are based on two European public health insurance databases, comprising 33,292 diabetes patients treated with incretin drugs and 525,733 treated with other noninsulin antidiabetic drugs.
The risk of pancreatic cancer doubled for patients receiving incretin treatment (adjusted hazard ratio, 2.14; 95% confidence interval, 1.71-2.67), compared with patients receiving other noninsulin drugs, after adjusting for age, sex, and whether or not patients subsequently received an insulin prescription, investigators reported.
However, risk of pancreatic cancer decreased over time in patients on incretin therapy, according to further analyses by Dr. Boniol and colleagues.
That finding of diminishing risk over time argues against concluding that incretin drugs cause pancreatic cancer, according to investigators.
“A direct causal effect could be suspected if a steadily increasing risk of pancreatic cancer was associated with a steadily longer exposure to incretin drugs,” they said.
In the first 3 months after the first incretin prescription, risk of pancreatic cancer was 3.35 times greater (95% CI, 2.32-4.84), which “gradually diminished” to 1.69 (95% CI, 1.12-2.55) a year after the first prescription, the investigators said in the report.
As such, these new data do not help prove or disprove a causal link between incretin use and pancreatic cancer, continued Dr. Hellman, a past president of the American Association of Clinical Endocrinologists.
“I think, right now, we don’t have the data,” he said in an interview. “It’s a question that makes us nervous, because of what we know about the laboratory effects of the drugs, but we don’t have the answer yet. We need longer studies with larger numbers of people to answer that question.”
The study, funded by Sanofi, was conducted at the request of the European Medicines Agency to assess risk of pancreatic cancer associated with lixisenatide. However, the study covers a period prior to use of lixisenatide in the general population because the approval of the GLP-1R agonist was “too recent,” the study authors wrote.
The analyses in the study were conducted in 2015 and 2016 for two cohorts of subjects: one in Belgium, for which data had been collected from 2008 to 2013, and one in Italy, with data from 2000 to 2012.
Further studies are needed to assess how long-term use of incretin drugs may affect risk of pancreatic cancer, the authors said.
The analysis was conducted as part of a postauthorization safety study requested by the European Medicines Agency and funded by Sanofi. One study author reported receiving grants from Roche, Amgen, and Bristol-Myers Squibb outside the submitted work; the remaining investigators had no relevant financial disclosures.
FROM DIABETES CARE
Key clinical point: Incretin therapy was associated with increased pancreatic cancer risk, but possibly because of occult pancreatic cancer that aggravates diabetes.
Major finding: Patients receiving incretin drugs had a doubling of pancreatic cancer risk (aHR 2.14 (95% CI, 1.71-2.67).
Data source: Retrospective cohort analysis of public health insurance databases including 33,292 diabetes patients treated with incretin drugs and 525,733 treated with another noninsulin antidiabetic drug.
Disclosures: The analysis was conducted as part of a postauthorization safety study requested by the European Medicines Agency and funded by Sanofi. One study author reported receiving grants from Roche, Amgen, and Bristol-Myers Squibb outside the submitted work; the remaining investigators had no relevant financial disclosures.
Consider calcipotriol contact allergy when psoriasis doesn’t improve
Researchers at the University of Leuven (Belgium) conducted patch tests on six patients between 2004 and 2016 who presented with psoriasis that did not improve with use of topical calcipotriol.
Reports of contact allergy to calcipotriol are rare in the literature, considering its widespread use. However, the patch testing and successful alternative treatment confirmed the diagnosis of allergic contact dermatitis in all six cases.
“The lesions improved following replacement of calcipotriol therapy with topical corticosteroids and/or oral medication,” wrote An Goossens, MD, of the contact allergy unit in the department of dermatology at the university.
Five of the patients were adults ranging in age from 26 to 59 years (two men and three women), and the sixth was a 10-year-old girl. They all had lesions on their feet, scalp, or hands.
The successful patch test consisted of a 2 mcg/mL solution of calcipotriol in citrate-buffered isopropanol.
Patients who are diagnosed with this specific allergy may be able to tolerate treatment with a different topical vitamin D analog such as tacalcitol (Contact Derm. 2017 Nov. doi: 10.1111/cod.12910).
Researchers at the University of Leuven (Belgium) conducted patch tests on six patients between 2004 and 2016 who presented with psoriasis that did not improve with use of topical calcipotriol.
Reports of contact allergy to calcipotriol are rare in the literature, considering its widespread use. However, the patch testing and successful alternative treatment confirmed the diagnosis of allergic contact dermatitis in all six cases.
“The lesions improved following replacement of calcipotriol therapy with topical corticosteroids and/or oral medication,” wrote An Goossens, MD, of the contact allergy unit in the department of dermatology at the university.
Five of the patients were adults ranging in age from 26 to 59 years (two men and three women), and the sixth was a 10-year-old girl. They all had lesions on their feet, scalp, or hands.
The successful patch test consisted of a 2 mcg/mL solution of calcipotriol in citrate-buffered isopropanol.
Patients who are diagnosed with this specific allergy may be able to tolerate treatment with a different topical vitamin D analog such as tacalcitol (Contact Derm. 2017 Nov. doi: 10.1111/cod.12910).
Researchers at the University of Leuven (Belgium) conducted patch tests on six patients between 2004 and 2016 who presented with psoriasis that did not improve with use of topical calcipotriol.
Reports of contact allergy to calcipotriol are rare in the literature, considering its widespread use. However, the patch testing and successful alternative treatment confirmed the diagnosis of allergic contact dermatitis in all six cases.
“The lesions improved following replacement of calcipotriol therapy with topical corticosteroids and/or oral medication,” wrote An Goossens, MD, of the contact allergy unit in the department of dermatology at the university.
Five of the patients were adults ranging in age from 26 to 59 years (two men and three women), and the sixth was a 10-year-old girl. They all had lesions on their feet, scalp, or hands.
The successful patch test consisted of a 2 mcg/mL solution of calcipotriol in citrate-buffered isopropanol.
Patients who are diagnosed with this specific allergy may be able to tolerate treatment with a different topical vitamin D analog such as tacalcitol (Contact Derm. 2017 Nov. doi: 10.1111/cod.12910).
FROM CONTACT DERMATITIS
Tezacaftor-ivacaftor combo shows promise in cystic fibrosis
A new drug that targets the cystic fibrosis transmembrane conductance regulator protein could significantly improve lung function and other symptoms in patients with cystic fibrosis.
In a phase 3 double-blind, placebo controlled crossover trial, researchers examined the effects of tezacaftor – a cystic fibrosis transmembrane conductance regulator (CFTR) corrector – in combination with the CFTR potentiator ivacaftor, compared with ivacaftor alone or placebo.
“The addition of the CFTR corrector tezacaftor was hypothesized to enhance clinical benefit in patients with these mutations by increasing overall CFTR function,” wrote Dr. Steven M. Rowe of the division of pulmonary, allergy, and critical care medicine at the University of Alabama at Birmingham, and his coauthors. “This combination treatment is particularly important for restoring activity to those carrying two copies of the Phe508del CFTR mutation, as shown for the approved corrector-potentiator combination lumacaftor-ivacaftor, and may provide benefit to patients with other CFTR mutations.”
After two 8-week treatment periods in which patients were randomized to two of the three regimens, separated by an 8-week washout period, researchers saw significant improvements in predicted forced expiratory volume in 1 second (FEV1), both with tezacaftor-ivacaftor and ivacaftor alone, compared with placebo (N Engl J Med. 2017 Nov 23;377:2024-35. doi: 10.1056/NEJMoa1709847).
From baseline to the average of week 4 and 8, the combination of tezacaftor-ivacaftor was associated with a 6.8-percentage-point absolute change, and there was a 4.7-percentage-point improvement with ivacaftor alone compared with placebo. The difference between the tezacaftor-ivacaftor combination and ivacaftor monotherapy was also statistically significant in favor of the combination treatment.
Researchers also saw significant improvements in the secondary endpoint of absolute change in the Cystic Fibrosis Questionnaire–Revised score; the combination treatment was associated with an 11.1 point improvement compared with placebo, and monotherapy achieved a 9.7-point improvement. In the combination therapy group, 65% of patients achieved a clinically important difference of 4 points or greater, compared with 58% of patients in the monotherapy group and 33% of patients in the placebo group.
“These findings confirm the benefits of potentiator therapy in patients with residual CFTR function mutations and the added benefit conferred by corrector-potentiator combination therapy in this population,” the authors wrote.
The investigators also saw a lower rate of pulmonary exacerbations in the combined therapy group, but this did not reach statistical significance.
The rate of adverse events was similar across all three groups. Most were considered mild or moderate in severity and were largely clinical manifestations of cystic fibrosis.
Vertex Pharmaceuticals, which manufactures tezacaftor and ivacaftor, funded the study. Twelve of the thirteen authors reported receiving various kinds of support from Vertex, including personal fees, grant support, and nonfinancial support. Several authors reported ties to other industry sources.
This NEJM article came out with a companion article reporting phase 3 tezacaftor-ivacaftor results in homozygous CFTR Phe508del patients. Tezacaftor is a CFTR corrector developed by a company that has another corrector on the market, called lumacaftor. Lumacaftor is formulated with ivacaftor and available in the United States for patients age 6 and above who have two copies of Phe508del. It is exciting that another combination therapy has been developed that appears to be very effective and can be used for a second group of patients. This era of personalized medicine is extremely exciting, and we look forward to future therapies for all CFTR mutations.
This NEJM article came out with a companion article reporting phase 3 tezacaftor-ivacaftor results in homozygous CFTR Phe508del patients. Tezacaftor is a CFTR corrector developed by a company that has another corrector on the market, called lumacaftor. Lumacaftor is formulated with ivacaftor and available in the United States for patients age 6 and above who have two copies of Phe508del. It is exciting that another combination therapy has been developed that appears to be very effective and can be used for a second group of patients. This era of personalized medicine is extremely exciting, and we look forward to future therapies for all CFTR mutations.
This NEJM article came out with a companion article reporting phase 3 tezacaftor-ivacaftor results in homozygous CFTR Phe508del patients. Tezacaftor is a CFTR corrector developed by a company that has another corrector on the market, called lumacaftor. Lumacaftor is formulated with ivacaftor and available in the United States for patients age 6 and above who have two copies of Phe508del. It is exciting that another combination therapy has been developed that appears to be very effective and can be used for a second group of patients. This era of personalized medicine is extremely exciting, and we look forward to future therapies for all CFTR mutations.
A new drug that targets the cystic fibrosis transmembrane conductance regulator protein could significantly improve lung function and other symptoms in patients with cystic fibrosis.
In a phase 3 double-blind, placebo controlled crossover trial, researchers examined the effects of tezacaftor – a cystic fibrosis transmembrane conductance regulator (CFTR) corrector – in combination with the CFTR potentiator ivacaftor, compared with ivacaftor alone or placebo.
“The addition of the CFTR corrector tezacaftor was hypothesized to enhance clinical benefit in patients with these mutations by increasing overall CFTR function,” wrote Dr. Steven M. Rowe of the division of pulmonary, allergy, and critical care medicine at the University of Alabama at Birmingham, and his coauthors. “This combination treatment is particularly important for restoring activity to those carrying two copies of the Phe508del CFTR mutation, as shown for the approved corrector-potentiator combination lumacaftor-ivacaftor, and may provide benefit to patients with other CFTR mutations.”
After two 8-week treatment periods in which patients were randomized to two of the three regimens, separated by an 8-week washout period, researchers saw significant improvements in predicted forced expiratory volume in 1 second (FEV1), both with tezacaftor-ivacaftor and ivacaftor alone, compared with placebo (N Engl J Med. 2017 Nov 23;377:2024-35. doi: 10.1056/NEJMoa1709847).
From baseline to the average of week 4 and 8, the combination of tezacaftor-ivacaftor was associated with a 6.8-percentage-point absolute change, and there was a 4.7-percentage-point improvement with ivacaftor alone compared with placebo. The difference between the tezacaftor-ivacaftor combination and ivacaftor monotherapy was also statistically significant in favor of the combination treatment.
Researchers also saw significant improvements in the secondary endpoint of absolute change in the Cystic Fibrosis Questionnaire–Revised score; the combination treatment was associated with an 11.1 point improvement compared with placebo, and monotherapy achieved a 9.7-point improvement. In the combination therapy group, 65% of patients achieved a clinically important difference of 4 points or greater, compared with 58% of patients in the monotherapy group and 33% of patients in the placebo group.
“These findings confirm the benefits of potentiator therapy in patients with residual CFTR function mutations and the added benefit conferred by corrector-potentiator combination therapy in this population,” the authors wrote.
The investigators also saw a lower rate of pulmonary exacerbations in the combined therapy group, but this did not reach statistical significance.
The rate of adverse events was similar across all three groups. Most were considered mild or moderate in severity and were largely clinical manifestations of cystic fibrosis.
Vertex Pharmaceuticals, which manufactures tezacaftor and ivacaftor, funded the study. Twelve of the thirteen authors reported receiving various kinds of support from Vertex, including personal fees, grant support, and nonfinancial support. Several authors reported ties to other industry sources.
A new drug that targets the cystic fibrosis transmembrane conductance regulator protein could significantly improve lung function and other symptoms in patients with cystic fibrosis.
In a phase 3 double-blind, placebo controlled crossover trial, researchers examined the effects of tezacaftor – a cystic fibrosis transmembrane conductance regulator (CFTR) corrector – in combination with the CFTR potentiator ivacaftor, compared with ivacaftor alone or placebo.
“The addition of the CFTR corrector tezacaftor was hypothesized to enhance clinical benefit in patients with these mutations by increasing overall CFTR function,” wrote Dr. Steven M. Rowe of the division of pulmonary, allergy, and critical care medicine at the University of Alabama at Birmingham, and his coauthors. “This combination treatment is particularly important for restoring activity to those carrying two copies of the Phe508del CFTR mutation, as shown for the approved corrector-potentiator combination lumacaftor-ivacaftor, and may provide benefit to patients with other CFTR mutations.”
After two 8-week treatment periods in which patients were randomized to two of the three regimens, separated by an 8-week washout period, researchers saw significant improvements in predicted forced expiratory volume in 1 second (FEV1), both with tezacaftor-ivacaftor and ivacaftor alone, compared with placebo (N Engl J Med. 2017 Nov 23;377:2024-35. doi: 10.1056/NEJMoa1709847).
From baseline to the average of week 4 and 8, the combination of tezacaftor-ivacaftor was associated with a 6.8-percentage-point absolute change, and there was a 4.7-percentage-point improvement with ivacaftor alone compared with placebo. The difference between the tezacaftor-ivacaftor combination and ivacaftor monotherapy was also statistically significant in favor of the combination treatment.
Researchers also saw significant improvements in the secondary endpoint of absolute change in the Cystic Fibrosis Questionnaire–Revised score; the combination treatment was associated with an 11.1 point improvement compared with placebo, and monotherapy achieved a 9.7-point improvement. In the combination therapy group, 65% of patients achieved a clinically important difference of 4 points or greater, compared with 58% of patients in the monotherapy group and 33% of patients in the placebo group.
“These findings confirm the benefits of potentiator therapy in patients with residual CFTR function mutations and the added benefit conferred by corrector-potentiator combination therapy in this population,” the authors wrote.
The investigators also saw a lower rate of pulmonary exacerbations in the combined therapy group, but this did not reach statistical significance.
The rate of adverse events was similar across all three groups. Most were considered mild or moderate in severity and were largely clinical manifestations of cystic fibrosis.
Vertex Pharmaceuticals, which manufactures tezacaftor and ivacaftor, funded the study. Twelve of the thirteen authors reported receiving various kinds of support from Vertex, including personal fees, grant support, and nonfinancial support. Several authors reported ties to other industry sources.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: The combination of tezacaftor and ivacaftor is associated with significant improvements in lung function in cystic fibrosis, compared with placebo or ivacaftor alone.
Major finding: Combined treatment with tezacaftor-ivacaftor was associated with a 6.8-percentage-point absolute change in FEV1 compared with placebo.
Data source: A phase 3 double-blind, placebo controlled, randomized crossover trial of 248 patients with cystic fibrosis.
Disclosures: Vertex Pharmaceuticals, which manufactures tezacaftor and ivacaftor, funded the study. Twelve of the thirteen authors reported receiving various kinds of support from Vertex, including personal fees, grant support, and nonfinancial support. Several authors reported ties to other industry sources.
Preventing substance use
Substance use disorders are affecting every pediatric practice as they are major contributors to morbidity and mortality in young people. With the ongoing risks of binge drinking, the current epidemic of opioid addiction and overdose deaths in the United States, and the shifting legal status and public perception of the risk of marijuana, how to deal with substance use disorders seems to be the focus of public conversation these days. , such as parent education and early recognition in pediatric practice.
Substance abuse risk
We cannot yet predict who can safely “experiment” with substances or who will develop dependency. However, there is information that we can use to identify those at greater risk. Youth who have a first-degree relative with a substance use disorder are at greater risk for developing such a disorder themselves, and this is especially so if there is a family history of alcoholism. Youth who suffer from a psychiatric illness, particularly from anxiety and mood disorders, have a special vulnerability to abusing substances, particularly when their underlying illness is untreated or incompletely treated. Youth with ADHD are at substantially elevated risk of developing substance use disorders, although there is a complex relationship between these two problems. The evidence currently suggests that for youth who began effective treatment prior to puberty, there is no elevation in risk, but for those who did not, there is a substantially elevated risk of substance use disorders. Finally, there has been research that indicates that children with a combination of sensation-seeking, high impulsivity, anxiety-sensitivity, and hopelessness are at the highest risk for substance use disorders.2
Prevention efforts you can make: To your patients
The first step in your prevention efforts is an open conversation about drugs and alcohol. Ask your middle schoolers about whether they have tried alcohol or any drugs. Have their friends? What are kids saying about alcohol? About marijuana? Vaping? Are there other substances that kids are talking about or trying? Be genuinely curious, warm, and nonjudgmental. Find out what they think the risks of these substances may be. If appropriate, offer them some education about known risks of substances to the developing brain, to school or athletic performance, and so on. You can teach them about other trusted resources, such as the National Institute on Drug Abuse (NIDA), which has a resource specifically for teens (teens.drugabuse.gov).
For your high school students and those heading off to college, provide a safe place to talk about what they have tried and whether they (or you) have any worries about substance use. You have a unique combination of clinical authority and expertise in them as individuals, and can help them meaningfully plan how to handle their choices. You might talk about the specific risks of binge drinking, from sexual assault to alcohol poisoning and permanent cognitive effects on their developing brains. They also can benefit from hearing about the actual risks of frequent marijuana use, including impaired cognitive performance (and permanent IQ decline), and ongoing risks to their still-developing brains. Don’t be surprised if your older adolescent patients want to educate you about risks. Be curious and humble, and don’t be afraid to go together to a third party for information. You should encourage their efforts to think critically, and be empathic to their dilemma as they try to balance risks against their drive to have new experiences, to be independent, and to be strongly connected to their peers.
Adolescents should hear about your concern about their specific risks with drugs and alcohol, such as a history of traumatic brain injury (concussion), a family history of drug or alcohol dependence, or their own diagnosis of anxiety, depression, or ADHD. You might point out that because they have not tried any drugs or alcohol in high school, they may be prone to having too much to drink when they first try it. Or you might observe that because they have an anxiety disorder, they are vulnerable to becoming dependent on alcohol. Hearing about their specific level of risk equips them to make wiser choices in the context of their growing autonomy.
Prevention efforts you can make: To the parents
Your other prevention strategies should include parents. Studies have shown that when parents have clear rules and expectations about drug and alcohol use, and are consistent about enforcing consequences in their home, their children are significantly less likely than their peers to have experimented with drugs or alcohol by their senior year in high school. Parents of children headed to middle school should hear about this fact, alongside accurate information about the risks associated with alcohol and specific drugs for the developing brain.
Finally, parents need to hear that they can be effective disciplinarians, while also making clear to their children that safety comes first, and that their rules should have clear exceptions for safety. If the parents have a rule against any use of alcohol or drugs, there should be an exception if their child is out and feels unsafe. If they are drunk, or their driver has been drinking, they can call for a ride and will not be in (much) trouble. Rules don’t have to be draconian to be effective; they should always support honesty and safety first. This is a lot of territory to cover, and you do not have to be the only resource for parents. Reliable online resources, such as NIDA’s and SAMHSA’s websites, are full of useful information, and others, such as teen-safe.org, have detailed resources for parents in particular.
References
1. Hum Genet. 2012 Jun;131(6):779-89.
2. Alcohol Clin Exp Res. 2013 Jan;37(Suppl 1):E281-90.
Dr. Swick is an attending psychiatrist in the division of child psychiatry at Massachusetts General Hospital, Boston, and director of the Parenting at a Challenging Time (PACT) Program at the Vernon Cancer Center at Newton Wellesley Hospital, also in Boston. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston.
Substance use disorders are affecting every pediatric practice as they are major contributors to morbidity and mortality in young people. With the ongoing risks of binge drinking, the current epidemic of opioid addiction and overdose deaths in the United States, and the shifting legal status and public perception of the risk of marijuana, how to deal with substance use disorders seems to be the focus of public conversation these days. , such as parent education and early recognition in pediatric practice.
Substance abuse risk
We cannot yet predict who can safely “experiment” with substances or who will develop dependency. However, there is information that we can use to identify those at greater risk. Youth who have a first-degree relative with a substance use disorder are at greater risk for developing such a disorder themselves, and this is especially so if there is a family history of alcoholism. Youth who suffer from a psychiatric illness, particularly from anxiety and mood disorders, have a special vulnerability to abusing substances, particularly when their underlying illness is untreated or incompletely treated. Youth with ADHD are at substantially elevated risk of developing substance use disorders, although there is a complex relationship between these two problems. The evidence currently suggests that for youth who began effective treatment prior to puberty, there is no elevation in risk, but for those who did not, there is a substantially elevated risk of substance use disorders. Finally, there has been research that indicates that children with a combination of sensation-seeking, high impulsivity, anxiety-sensitivity, and hopelessness are at the highest risk for substance use disorders.2
Prevention efforts you can make: To your patients
The first step in your prevention efforts is an open conversation about drugs and alcohol. Ask your middle schoolers about whether they have tried alcohol or any drugs. Have their friends? What are kids saying about alcohol? About marijuana? Vaping? Are there other substances that kids are talking about or trying? Be genuinely curious, warm, and nonjudgmental. Find out what they think the risks of these substances may be. If appropriate, offer them some education about known risks of substances to the developing brain, to school or athletic performance, and so on. You can teach them about other trusted resources, such as the National Institute on Drug Abuse (NIDA), which has a resource specifically for teens (teens.drugabuse.gov).
For your high school students and those heading off to college, provide a safe place to talk about what they have tried and whether they (or you) have any worries about substance use. You have a unique combination of clinical authority and expertise in them as individuals, and can help them meaningfully plan how to handle their choices. You might talk about the specific risks of binge drinking, from sexual assault to alcohol poisoning and permanent cognitive effects on their developing brains. They also can benefit from hearing about the actual risks of frequent marijuana use, including impaired cognitive performance (and permanent IQ decline), and ongoing risks to their still-developing brains. Don’t be surprised if your older adolescent patients want to educate you about risks. Be curious and humble, and don’t be afraid to go together to a third party for information. You should encourage their efforts to think critically, and be empathic to their dilemma as they try to balance risks against their drive to have new experiences, to be independent, and to be strongly connected to their peers.
Adolescents should hear about your concern about their specific risks with drugs and alcohol, such as a history of traumatic brain injury (concussion), a family history of drug or alcohol dependence, or their own diagnosis of anxiety, depression, or ADHD. You might point out that because they have not tried any drugs or alcohol in high school, they may be prone to having too much to drink when they first try it. Or you might observe that because they have an anxiety disorder, they are vulnerable to becoming dependent on alcohol. Hearing about their specific level of risk equips them to make wiser choices in the context of their growing autonomy.
Prevention efforts you can make: To the parents
Your other prevention strategies should include parents. Studies have shown that when parents have clear rules and expectations about drug and alcohol use, and are consistent about enforcing consequences in their home, their children are significantly less likely than their peers to have experimented with drugs or alcohol by their senior year in high school. Parents of children headed to middle school should hear about this fact, alongside accurate information about the risks associated with alcohol and specific drugs for the developing brain.
Finally, parents need to hear that they can be effective disciplinarians, while also making clear to their children that safety comes first, and that their rules should have clear exceptions for safety. If the parents have a rule against any use of alcohol or drugs, there should be an exception if their child is out and feels unsafe. If they are drunk, or their driver has been drinking, they can call for a ride and will not be in (much) trouble. Rules don’t have to be draconian to be effective; they should always support honesty and safety first. This is a lot of territory to cover, and you do not have to be the only resource for parents. Reliable online resources, such as NIDA’s and SAMHSA’s websites, are full of useful information, and others, such as teen-safe.org, have detailed resources for parents in particular.
References
1. Hum Genet. 2012 Jun;131(6):779-89.
2. Alcohol Clin Exp Res. 2013 Jan;37(Suppl 1):E281-90.
Dr. Swick is an attending psychiatrist in the division of child psychiatry at Massachusetts General Hospital, Boston, and director of the Parenting at a Challenging Time (PACT) Program at the Vernon Cancer Center at Newton Wellesley Hospital, also in Boston. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston.
Substance use disorders are affecting every pediatric practice as they are major contributors to morbidity and mortality in young people. With the ongoing risks of binge drinking, the current epidemic of opioid addiction and overdose deaths in the United States, and the shifting legal status and public perception of the risk of marijuana, how to deal with substance use disorders seems to be the focus of public conversation these days. , such as parent education and early recognition in pediatric practice.
Substance abuse risk
We cannot yet predict who can safely “experiment” with substances or who will develop dependency. However, there is information that we can use to identify those at greater risk. Youth who have a first-degree relative with a substance use disorder are at greater risk for developing such a disorder themselves, and this is especially so if there is a family history of alcoholism. Youth who suffer from a psychiatric illness, particularly from anxiety and mood disorders, have a special vulnerability to abusing substances, particularly when their underlying illness is untreated or incompletely treated. Youth with ADHD are at substantially elevated risk of developing substance use disorders, although there is a complex relationship between these two problems. The evidence currently suggests that for youth who began effective treatment prior to puberty, there is no elevation in risk, but for those who did not, there is a substantially elevated risk of substance use disorders. Finally, there has been research that indicates that children with a combination of sensation-seeking, high impulsivity, anxiety-sensitivity, and hopelessness are at the highest risk for substance use disorders.2
Prevention efforts you can make: To your patients
The first step in your prevention efforts is an open conversation about drugs and alcohol. Ask your middle schoolers about whether they have tried alcohol or any drugs. Have their friends? What are kids saying about alcohol? About marijuana? Vaping? Are there other substances that kids are talking about or trying? Be genuinely curious, warm, and nonjudgmental. Find out what they think the risks of these substances may be. If appropriate, offer them some education about known risks of substances to the developing brain, to school or athletic performance, and so on. You can teach them about other trusted resources, such as the National Institute on Drug Abuse (NIDA), which has a resource specifically for teens (teens.drugabuse.gov).
For your high school students and those heading off to college, provide a safe place to talk about what they have tried and whether they (or you) have any worries about substance use. You have a unique combination of clinical authority and expertise in them as individuals, and can help them meaningfully plan how to handle their choices. You might talk about the specific risks of binge drinking, from sexual assault to alcohol poisoning and permanent cognitive effects on their developing brains. They also can benefit from hearing about the actual risks of frequent marijuana use, including impaired cognitive performance (and permanent IQ decline), and ongoing risks to their still-developing brains. Don’t be surprised if your older adolescent patients want to educate you about risks. Be curious and humble, and don’t be afraid to go together to a third party for information. You should encourage their efforts to think critically, and be empathic to their dilemma as they try to balance risks against their drive to have new experiences, to be independent, and to be strongly connected to their peers.
Adolescents should hear about your concern about their specific risks with drugs and alcohol, such as a history of traumatic brain injury (concussion), a family history of drug or alcohol dependence, or their own diagnosis of anxiety, depression, or ADHD. You might point out that because they have not tried any drugs or alcohol in high school, they may be prone to having too much to drink when they first try it. Or you might observe that because they have an anxiety disorder, they are vulnerable to becoming dependent on alcohol. Hearing about their specific level of risk equips them to make wiser choices in the context of their growing autonomy.
Prevention efforts you can make: To the parents
Your other prevention strategies should include parents. Studies have shown that when parents have clear rules and expectations about drug and alcohol use, and are consistent about enforcing consequences in their home, their children are significantly less likely than their peers to have experimented with drugs or alcohol by their senior year in high school. Parents of children headed to middle school should hear about this fact, alongside accurate information about the risks associated with alcohol and specific drugs for the developing brain.
Finally, parents need to hear that they can be effective disciplinarians, while also making clear to their children that safety comes first, and that their rules should have clear exceptions for safety. If the parents have a rule against any use of alcohol or drugs, there should be an exception if their child is out and feels unsafe. If they are drunk, or their driver has been drinking, they can call for a ride and will not be in (much) trouble. Rules don’t have to be draconian to be effective; they should always support honesty and safety first. This is a lot of territory to cover, and you do not have to be the only resource for parents. Reliable online resources, such as NIDA’s and SAMHSA’s websites, are full of useful information, and others, such as teen-safe.org, have detailed resources for parents in particular.
References
1. Hum Genet. 2012 Jun;131(6):779-89.
2. Alcohol Clin Exp Res. 2013 Jan;37(Suppl 1):E281-90.
Dr. Swick is an attending psychiatrist in the division of child psychiatry at Massachusetts General Hospital, Boston, and director of the Parenting at a Challenging Time (PACT) Program at the Vernon Cancer Center at Newton Wellesley Hospital, also in Boston. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston.




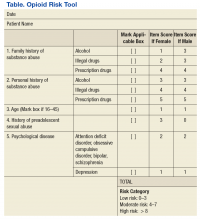
 Disclaimer
Disclaimer