User login
PTSD can persist in cancer survivors
Cancer patients may experience lasting post-traumatic stress disorder (PTSD), according to a study published in the journal Cancer.
Approximately one-fifth of patients involved in the study experienced PTSD several months after their cancer diagnosis, and roughly a third of these patients continued to live with PTSD 4 years later.
Researchers say these findings highlight the need for early identification, careful monitoring, and treatment of PTSD in cancer survivors.
Caryn Mei Hsien Chan, PhD, of the National University of Malaysia in Kuala Lumpur, and her colleagues conducted this research.
The study included 469 adults with various cancers who were within 1 month of cancer diagnosis at enrollment.
Patients who had significant psychological distress (defined as a Hospital Anxiety and Depression Scale total cutoff score of 16 or higher) underwent
testing for PTSD at 6 months of follow-up. All patients were tested for PTSD at 4 years of follow-up (regardless of their Hospital Anxiety and Depression Scale score).
The incidence of PTSD was 21.7% at 6 months and 6.1% at 4 years. Although overall rates of PTSD decreased with time, roughly one-third of patients initially diagnosed with PTSD were found to have persistent or worsening symptoms 4 years later.
“Many cancer patients believe they need to adopt a ‘warrior mentality’ and remain positive and optimistic from diagnosis through treatment to stand a better chance of beating their cancer,” Dr Chan said.
“To these patients, seeking help for the emotional issues they face is akin to admitting weakness. There needs to be greater awareness that there is nothing wrong with getting help to manage the emotional upheaval—particularly depression, anxiety, and PTSD—post-cancer.”
Dr Chan also stressed that many patients live in fear that their cancer may come back, and they may think the cancer has returned with every lump or bump, pain or ache, fatigue or fever.
In addition, cancer survivors might skip visits to their oncologists or other physicians to avoid triggering memories of their past cancer experience. This can lead to delays in seeking help for new symptoms or even refusal of treatment for unrelated conditions.
“We need psychological evaluation and support services for patients with cancer at an initial stage and at continued follows-up because psychological well-being and mental health—and by extension, quality of life—are just as important as physical health,” Dr Chan noted. ![]()
Cancer patients may experience lasting post-traumatic stress disorder (PTSD), according to a study published in the journal Cancer.
Approximately one-fifth of patients involved in the study experienced PTSD several months after their cancer diagnosis, and roughly a third of these patients continued to live with PTSD 4 years later.
Researchers say these findings highlight the need for early identification, careful monitoring, and treatment of PTSD in cancer survivors.
Caryn Mei Hsien Chan, PhD, of the National University of Malaysia in Kuala Lumpur, and her colleagues conducted this research.
The study included 469 adults with various cancers who were within 1 month of cancer diagnosis at enrollment.
Patients who had significant psychological distress (defined as a Hospital Anxiety and Depression Scale total cutoff score of 16 or higher) underwent
testing for PTSD at 6 months of follow-up. All patients were tested for PTSD at 4 years of follow-up (regardless of their Hospital Anxiety and Depression Scale score).
The incidence of PTSD was 21.7% at 6 months and 6.1% at 4 years. Although overall rates of PTSD decreased with time, roughly one-third of patients initially diagnosed with PTSD were found to have persistent or worsening symptoms 4 years later.
“Many cancer patients believe they need to adopt a ‘warrior mentality’ and remain positive and optimistic from diagnosis through treatment to stand a better chance of beating their cancer,” Dr Chan said.
“To these patients, seeking help for the emotional issues they face is akin to admitting weakness. There needs to be greater awareness that there is nothing wrong with getting help to manage the emotional upheaval—particularly depression, anxiety, and PTSD—post-cancer.”
Dr Chan also stressed that many patients live in fear that their cancer may come back, and they may think the cancer has returned with every lump or bump, pain or ache, fatigue or fever.
In addition, cancer survivors might skip visits to their oncologists or other physicians to avoid triggering memories of their past cancer experience. This can lead to delays in seeking help for new symptoms or even refusal of treatment for unrelated conditions.
“We need psychological evaluation and support services for patients with cancer at an initial stage and at continued follows-up because psychological well-being and mental health—and by extension, quality of life—are just as important as physical health,” Dr Chan noted. ![]()
Cancer patients may experience lasting post-traumatic stress disorder (PTSD), according to a study published in the journal Cancer.
Approximately one-fifth of patients involved in the study experienced PTSD several months after their cancer diagnosis, and roughly a third of these patients continued to live with PTSD 4 years later.
Researchers say these findings highlight the need for early identification, careful monitoring, and treatment of PTSD in cancer survivors.
Caryn Mei Hsien Chan, PhD, of the National University of Malaysia in Kuala Lumpur, and her colleagues conducted this research.
The study included 469 adults with various cancers who were within 1 month of cancer diagnosis at enrollment.
Patients who had significant psychological distress (defined as a Hospital Anxiety and Depression Scale total cutoff score of 16 or higher) underwent
testing for PTSD at 6 months of follow-up. All patients were tested for PTSD at 4 years of follow-up (regardless of their Hospital Anxiety and Depression Scale score).
The incidence of PTSD was 21.7% at 6 months and 6.1% at 4 years. Although overall rates of PTSD decreased with time, roughly one-third of patients initially diagnosed with PTSD were found to have persistent or worsening symptoms 4 years later.
“Many cancer patients believe they need to adopt a ‘warrior mentality’ and remain positive and optimistic from diagnosis through treatment to stand a better chance of beating their cancer,” Dr Chan said.
“To these patients, seeking help for the emotional issues they face is akin to admitting weakness. There needs to be greater awareness that there is nothing wrong with getting help to manage the emotional upheaval—particularly depression, anxiety, and PTSD—post-cancer.”
Dr Chan also stressed that many patients live in fear that their cancer may come back, and they may think the cancer has returned with every lump or bump, pain or ache, fatigue or fever.
In addition, cancer survivors might skip visits to their oncologists or other physicians to avoid triggering memories of their past cancer experience. This can lead to delays in seeking help for new symptoms or even refusal of treatment for unrelated conditions.
“We need psychological evaluation and support services for patients with cancer at an initial stage and at continued follows-up because psychological well-being and mental health—and by extension, quality of life—are just as important as physical health,” Dr Chan noted. ![]()
Withdrawn drug receives orphan designation for HA
The US Food and Drug Administration (FDA) has granted orphan drug designation to rofecoxib (TRM-201) as a potential treatment for degenerative joint disease in hemophilia, also known as hemophilic arthropathy (HA).
Rofecoxib is a COX-2 selective non-steroidal anti-inflammatory drug (NSAID) that was previously sold in the US under the name Vioxx.
Vioxx was FDA-approved to relieve the signs and symptoms of osteoarthritis, manage acute pain in adults, and treat primary dysmenorrhea.
Merck & Co. pulled Vioxx from the US market in 2004 due to safety concerns. The drug was shown to increase a person’s risk of cardiovascular events, including heart attack and stroke.
Now, Tremeau Pharmaceuticals, Inc., is working to bring rofecoxib back to market to treat patients with HA.
HA patients should not receive traditional NSAIDs due to their effects on platelet aggregation and the risk of gastrointestinal ulcers associated with these drugs. Therefore, high potency opioids are the current standard of care in HA.
“Being granted an orphan drug designation for rofecoxib by FDA is an important regulatory milestone for Tremeau and affirms our strategy of providing non-opioid pain treatments for rare diseases like hemophilic arthropathy,” said Bradford C. Sippy, chief executive officer of Tremeau.
Sippy is a former Merck employee who helped with the recall of Vioxx and knew the final patent protecting the drug’s monopoly was expiring this fall.
When it stopped making Vioxx, Merck was facing thousands of lawsuits from people claiming the drug caused their heart attacks or strokes.
Merck’s own research showed the drug doubled those risks, but lawyers for patients claimed the company downplayed or concealed that. Merck initially fought the lawsuits but, in 2007, agreed to a $4.85 billion settlement.
If approved to treat HA, rofecoxib would carry a warning about the increased risk of heart attack and stroke associated with the drug.
Although the orphan designation for rofecoxib is a step toward FDA approval, Sippy said Tremeau must still raise $25 million or more to fund trials of the drug in hemophilia patients.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation to rofecoxib (TRM-201) as a potential treatment for degenerative joint disease in hemophilia, also known as hemophilic arthropathy (HA).
Rofecoxib is a COX-2 selective non-steroidal anti-inflammatory drug (NSAID) that was previously sold in the US under the name Vioxx.
Vioxx was FDA-approved to relieve the signs and symptoms of osteoarthritis, manage acute pain in adults, and treat primary dysmenorrhea.
Merck & Co. pulled Vioxx from the US market in 2004 due to safety concerns. The drug was shown to increase a person’s risk of cardiovascular events, including heart attack and stroke.
Now, Tremeau Pharmaceuticals, Inc., is working to bring rofecoxib back to market to treat patients with HA.
HA patients should not receive traditional NSAIDs due to their effects on platelet aggregation and the risk of gastrointestinal ulcers associated with these drugs. Therefore, high potency opioids are the current standard of care in HA.
“Being granted an orphan drug designation for rofecoxib by FDA is an important regulatory milestone for Tremeau and affirms our strategy of providing non-opioid pain treatments for rare diseases like hemophilic arthropathy,” said Bradford C. Sippy, chief executive officer of Tremeau.
Sippy is a former Merck employee who helped with the recall of Vioxx and knew the final patent protecting the drug’s monopoly was expiring this fall.
When it stopped making Vioxx, Merck was facing thousands of lawsuits from people claiming the drug caused their heart attacks or strokes.
Merck’s own research showed the drug doubled those risks, but lawyers for patients claimed the company downplayed or concealed that. Merck initially fought the lawsuits but, in 2007, agreed to a $4.85 billion settlement.
If approved to treat HA, rofecoxib would carry a warning about the increased risk of heart attack and stroke associated with the drug.
Although the orphan designation for rofecoxib is a step toward FDA approval, Sippy said Tremeau must still raise $25 million or more to fund trials of the drug in hemophilia patients.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation to rofecoxib (TRM-201) as a potential treatment for degenerative joint disease in hemophilia, also known as hemophilic arthropathy (HA).
Rofecoxib is a COX-2 selective non-steroidal anti-inflammatory drug (NSAID) that was previously sold in the US under the name Vioxx.
Vioxx was FDA-approved to relieve the signs and symptoms of osteoarthritis, manage acute pain in adults, and treat primary dysmenorrhea.
Merck & Co. pulled Vioxx from the US market in 2004 due to safety concerns. The drug was shown to increase a person’s risk of cardiovascular events, including heart attack and stroke.
Now, Tremeau Pharmaceuticals, Inc., is working to bring rofecoxib back to market to treat patients with HA.
HA patients should not receive traditional NSAIDs due to their effects on platelet aggregation and the risk of gastrointestinal ulcers associated with these drugs. Therefore, high potency opioids are the current standard of care in HA.
“Being granted an orphan drug designation for rofecoxib by FDA is an important regulatory milestone for Tremeau and affirms our strategy of providing non-opioid pain treatments for rare diseases like hemophilic arthropathy,” said Bradford C. Sippy, chief executive officer of Tremeau.
Sippy is a former Merck employee who helped with the recall of Vioxx and knew the final patent protecting the drug’s monopoly was expiring this fall.
When it stopped making Vioxx, Merck was facing thousands of lawsuits from people claiming the drug caused their heart attacks or strokes.
Merck’s own research showed the drug doubled those risks, but lawyers for patients claimed the company downplayed or concealed that. Merck initially fought the lawsuits but, in 2007, agreed to a $4.85 billion settlement.
If approved to treat HA, rofecoxib would carry a warning about the increased risk of heart attack and stroke associated with the drug.
Although the orphan designation for rofecoxib is a step toward FDA approval, Sippy said Tremeau must still raise $25 million or more to fund trials of the drug in hemophilia patients.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
Conjunctivitis and oral inflammation
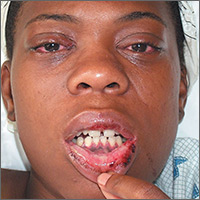
This patient was diagnosed with reactive arthritis, based on her clinical syndrome of conjunctivitis, arthralgias, mucositis, and cervicitis. The widespread distribution of symptoms in this syndrome may be due to activation of the immune system by a viral or bacterial agent. In this case, a complete blood count revealed a white blood cell count of 17,000/mcL (with a left shift of 6% bands). An endocervical DNA probe was positive for Chlamydia trachomatis.
Interestingly, a test for the human leukocyte antigen HLA-B27 came back negative. This did not, however, change the diagnosis. Only 85% of patients with reactive arthritis are positive for HLA-B27, and the test results are frequently negative in African Americans.
Because many sexually transmitted diseases occur concurrently—or are transmitted together—the patient was also tested for human immunodeficiency virus, syphilis, and hepatitis B and C. All of these tests were negative.
The patient was hospitalized and given an injection of ceftriaxone 250 mg, as well as oral azithromycin 1 g for chlamydia (and possible gonorrhea). She also received nonsteroidal anti-inflammatory drugs for pain control. She responded rapidly to therapy as evidenced by decreased arthralgias, normalization of temperature and white blood cell count, and decreased abdominal pain. The patient was discharged after her third day in the hospital with instructions to take doxycycline twice daily, and to finish the 14-day course.
Historical note: “Reiter’s syndrome” is no longer the preferred term for reactive arthritis, as Dr. Reiter was affiliated with the Nazi Party and performed unethical experimentation on human subjects.
Photo courtesy of Joseph Mazziotta, MD. Text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Chumley H, Shedd A, Reddy S, et al. Reactive arthritis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 910-914; Mazziotta JM, Ahmed N. Conjunctivitis and cervicitis. J Fam Pract. 2004;53:121-123.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

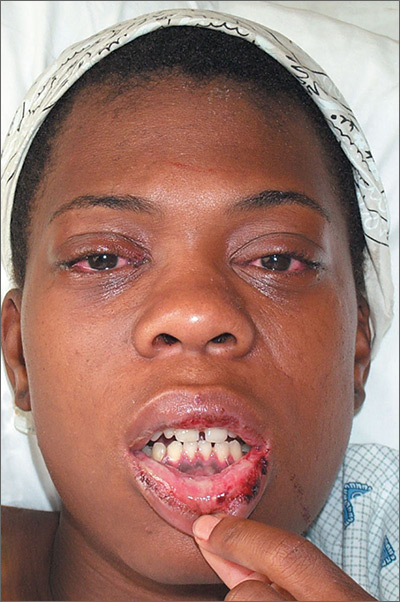
This patient was diagnosed with reactive arthritis, based on her clinical syndrome of conjunctivitis, arthralgias, mucositis, and cervicitis. The widespread distribution of symptoms in this syndrome may be due to activation of the immune system by a viral or bacterial agent. In this case, a complete blood count revealed a white blood cell count of 17,000/mcL (with a left shift of 6% bands). An endocervical DNA probe was positive for Chlamydia trachomatis.
Interestingly, a test for the human leukocyte antigen HLA-B27 came back negative. This did not, however, change the diagnosis. Only 85% of patients with reactive arthritis are positive for HLA-B27, and the test results are frequently negative in African Americans.
Because many sexually transmitted diseases occur concurrently—or are transmitted together—the patient was also tested for human immunodeficiency virus, syphilis, and hepatitis B and C. All of these tests were negative.
The patient was hospitalized and given an injection of ceftriaxone 250 mg, as well as oral azithromycin 1 g for chlamydia (and possible gonorrhea). She also received nonsteroidal anti-inflammatory drugs for pain control. She responded rapidly to therapy as evidenced by decreased arthralgias, normalization of temperature and white blood cell count, and decreased abdominal pain. The patient was discharged after her third day in the hospital with instructions to take doxycycline twice daily, and to finish the 14-day course.
Historical note: “Reiter’s syndrome” is no longer the preferred term for reactive arthritis, as Dr. Reiter was affiliated with the Nazi Party and performed unethical experimentation on human subjects.
Photo courtesy of Joseph Mazziotta, MD. Text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Chumley H, Shedd A, Reddy S, et al. Reactive arthritis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 910-914; Mazziotta JM, Ahmed N. Conjunctivitis and cervicitis. J Fam Pract. 2004;53:121-123.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

This patient was diagnosed with reactive arthritis, based on her clinical syndrome of conjunctivitis, arthralgias, mucositis, and cervicitis. The widespread distribution of symptoms in this syndrome may be due to activation of the immune system by a viral or bacterial agent. In this case, a complete blood count revealed a white blood cell count of 17,000/mcL (with a left shift of 6% bands). An endocervical DNA probe was positive for Chlamydia trachomatis.
Interestingly, a test for the human leukocyte antigen HLA-B27 came back negative. This did not, however, change the diagnosis. Only 85% of patients with reactive arthritis are positive for HLA-B27, and the test results are frequently negative in African Americans.
Because many sexually transmitted diseases occur concurrently—or are transmitted together—the patient was also tested for human immunodeficiency virus, syphilis, and hepatitis B and C. All of these tests were negative.
The patient was hospitalized and given an injection of ceftriaxone 250 mg, as well as oral azithromycin 1 g for chlamydia (and possible gonorrhea). She also received nonsteroidal anti-inflammatory drugs for pain control. She responded rapidly to therapy as evidenced by decreased arthralgias, normalization of temperature and white blood cell count, and decreased abdominal pain. The patient was discharged after her third day in the hospital with instructions to take doxycycline twice daily, and to finish the 14-day course.
Historical note: “Reiter’s syndrome” is no longer the preferred term for reactive arthritis, as Dr. Reiter was affiliated with the Nazi Party and performed unethical experimentation on human subjects.
Photo courtesy of Joseph Mazziotta, MD. Text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Chumley H, Shedd A, Reddy S, et al. Reactive arthritis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 910-914; Mazziotta JM, Ahmed N. Conjunctivitis and cervicitis. J Fam Pract. 2004;53:121-123.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
HTN in CKD: How Should I Be Treating It?
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CMS to enlist Medicare Part D plans to combat opioid abuse
The Centers for Medicare & Medicaid Services aims to enlist the help of Medicare Part D plan sponsors in fighting opioid abuse, under a proposed rule scheduled for publication in the Federal Register on Nov. 28.
The agency is proposing to expand monitoring of patients taking opioids in its current Part D Opioid Drug Utilization Review (DUR) and Overutilization Monitoring System (OMS). To do so, officials would like to extend the use the criteria for overutilizers to identify at-risk beneficiaries. Part D plans then would be allowed to restrict at-risk beneficiaries’ access to opioids to a selected prescriber and/or network pharmacy, according to a fact sheet on proposal, which is part of the proposed 2018 update to regulations governing Medicare Advantage and Medicare Part D.
Under the proposal, plans would not be required to use these tools. However, CMS expects they will.
“While plan sponsors would have the option to implement a drug management program, our proposal codifies a framework that would place requirements upon such programs,” according to the proposal. “We foresee that all plan sponsors will implement such drug management programs based on our experience that all plan sponsors’ are complying with the current policy as laid out in guidance, the fact that our proposal largely incorporates the CARA [Comprehensive Addiction and Recovery Act of 2016] drug management provisions into existing CMS and sponsor operations, and especially, in light of the national opioid epidemic and the declaration that the opioid crisis is a nationwide Public Health Emergency.”
According to the proposal, potentially at-risk and at-risk beneficiaries would be identified based on the type and frequency of prescriptions. Those include the use of opioids with an average daily morphine milligram equivalent (MME) greater than or equal to 90 mg for any duration during the most recent 6 months and either four or more opioid prescribers plus four or more opioid-dispensing pharmacies or six or more opioid prescribers, regardless of pharmacies.
CMS notes in the regulation that, if the proposed 2019 criteria were used against those enrolled in Medicare Part D in 2015, it would have identified as at-risk about 33,000 Part D beneficiaries, or 0.08% of the 42 million people enrolled at that time. The agency said this population should be manageable for oversight by Part D plan sponsors, noting that, in Medicaid in 2015, 0.37% were included in some kind of pharmacy/prescriber lock-in program.
The proposed regulation includes an exemption for patients diagnosed with cancer, those in hospice, and those in a long-term care facility. CMS also plans to limit the availability of the special enrollment period for dual-eligible beneficiaries (those who qualify for both Medicare and Medicaid) and those who receive the low-income subsidy who are identified as at risk or potentially at risk.
Beneficiaries would be allowed to appeal their at-risk/potential at-risk determination under the proposal.
The Centers for Medicare & Medicaid Services aims to enlist the help of Medicare Part D plan sponsors in fighting opioid abuse, under a proposed rule scheduled for publication in the Federal Register on Nov. 28.
The agency is proposing to expand monitoring of patients taking opioids in its current Part D Opioid Drug Utilization Review (DUR) and Overutilization Monitoring System (OMS). To do so, officials would like to extend the use the criteria for overutilizers to identify at-risk beneficiaries. Part D plans then would be allowed to restrict at-risk beneficiaries’ access to opioids to a selected prescriber and/or network pharmacy, according to a fact sheet on proposal, which is part of the proposed 2018 update to regulations governing Medicare Advantage and Medicare Part D.
Under the proposal, plans would not be required to use these tools. However, CMS expects they will.
“While plan sponsors would have the option to implement a drug management program, our proposal codifies a framework that would place requirements upon such programs,” according to the proposal. “We foresee that all plan sponsors will implement such drug management programs based on our experience that all plan sponsors’ are complying with the current policy as laid out in guidance, the fact that our proposal largely incorporates the CARA [Comprehensive Addiction and Recovery Act of 2016] drug management provisions into existing CMS and sponsor operations, and especially, in light of the national opioid epidemic and the declaration that the opioid crisis is a nationwide Public Health Emergency.”
According to the proposal, potentially at-risk and at-risk beneficiaries would be identified based on the type and frequency of prescriptions. Those include the use of opioids with an average daily morphine milligram equivalent (MME) greater than or equal to 90 mg for any duration during the most recent 6 months and either four or more opioid prescribers plus four or more opioid-dispensing pharmacies or six or more opioid prescribers, regardless of pharmacies.
CMS notes in the regulation that, if the proposed 2019 criteria were used against those enrolled in Medicare Part D in 2015, it would have identified as at-risk about 33,000 Part D beneficiaries, or 0.08% of the 42 million people enrolled at that time. The agency said this population should be manageable for oversight by Part D plan sponsors, noting that, in Medicaid in 2015, 0.37% were included in some kind of pharmacy/prescriber lock-in program.
The proposed regulation includes an exemption for patients diagnosed with cancer, those in hospice, and those in a long-term care facility. CMS also plans to limit the availability of the special enrollment period for dual-eligible beneficiaries (those who qualify for both Medicare and Medicaid) and those who receive the low-income subsidy who are identified as at risk or potentially at risk.
Beneficiaries would be allowed to appeal their at-risk/potential at-risk determination under the proposal.
The Centers for Medicare & Medicaid Services aims to enlist the help of Medicare Part D plan sponsors in fighting opioid abuse, under a proposed rule scheduled for publication in the Federal Register on Nov. 28.
The agency is proposing to expand monitoring of patients taking opioids in its current Part D Opioid Drug Utilization Review (DUR) and Overutilization Monitoring System (OMS). To do so, officials would like to extend the use the criteria for overutilizers to identify at-risk beneficiaries. Part D plans then would be allowed to restrict at-risk beneficiaries’ access to opioids to a selected prescriber and/or network pharmacy, according to a fact sheet on proposal, which is part of the proposed 2018 update to regulations governing Medicare Advantage and Medicare Part D.
Under the proposal, plans would not be required to use these tools. However, CMS expects they will.
“While plan sponsors would have the option to implement a drug management program, our proposal codifies a framework that would place requirements upon such programs,” according to the proposal. “We foresee that all plan sponsors will implement such drug management programs based on our experience that all plan sponsors’ are complying with the current policy as laid out in guidance, the fact that our proposal largely incorporates the CARA [Comprehensive Addiction and Recovery Act of 2016] drug management provisions into existing CMS and sponsor operations, and especially, in light of the national opioid epidemic and the declaration that the opioid crisis is a nationwide Public Health Emergency.”
According to the proposal, potentially at-risk and at-risk beneficiaries would be identified based on the type and frequency of prescriptions. Those include the use of opioids with an average daily morphine milligram equivalent (MME) greater than or equal to 90 mg for any duration during the most recent 6 months and either four or more opioid prescribers plus four or more opioid-dispensing pharmacies or six or more opioid prescribers, regardless of pharmacies.
CMS notes in the regulation that, if the proposed 2019 criteria were used against those enrolled in Medicare Part D in 2015, it would have identified as at-risk about 33,000 Part D beneficiaries, or 0.08% of the 42 million people enrolled at that time. The agency said this population should be manageable for oversight by Part D plan sponsors, noting that, in Medicaid in 2015, 0.37% were included in some kind of pharmacy/prescriber lock-in program.
The proposed regulation includes an exemption for patients diagnosed with cancer, those in hospice, and those in a long-term care facility. CMS also plans to limit the availability of the special enrollment period for dual-eligible beneficiaries (those who qualify for both Medicare and Medicaid) and those who receive the low-income subsidy who are identified as at risk or potentially at risk.
Beneficiaries would be allowed to appeal their at-risk/potential at-risk determination under the proposal.
To predict macrosomia, focus on the abdomen
WASHINGTON – , John C. Hobbins, MD, said at the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
“Everything that the estimated fetal weight [EFW] can do, the abdominal circumference can do better,” especially in diabetic mothers, said Dr. Hobbins, who is widely regarded as one of the early pioneers in the development and use of obstetric ultrasound as a diagnostic tool.
Because it reflects liver size and incorporates subcutaneous fat, the abdominal circumference (AC) “concentrates on where the action is,” he said. It also “roughly correlates” with the size of the fetal shoulders and is not affected by genetic factors.
Moreover, “it focuses on one task rather than putting into play four variables, each with its own standard error of the method,” said Dr. Hobbins, referring to the four fetal biometric parameters incorporated into the Hadlock formula for EFW that is provided “upon fire-up of virtually every ultrasound machine.”
These four parameters (biparietal diameter, head circumference, femur length, and AC) each contribute to fetal weight but the AC has been shown to correlate better with weight at birth than the other variables, and it is the only measure that reflects how corpulent the fetus is, he noted.
The “general rule of thumb is that the EFW [as calculated by ultrasound machine–based equations] has a standard error of the method of plus or minus 10%. … which means that an EFW of 4,000 g is associated with a splay of plus or minus 1.2 pounds,” said Dr. Hobbins, professor of obstetrics and gynecology at the University of Colorado at Denver, Aurora.
“But the problem for us is not the failure of the ultrasound – it’s the way we use it,” he said.
An assortment of customized formulas have been developed for macrosomia, including one designed for use in diabetes that incorporates AC, head circumference, femur length, and 3D volumes of the thigh and abdomen. “If one used this and set a cut-off at 4,300 g, you’d pick up 93%. … but at false positive rate of 38%,” he said.
While not perfect, the AC alone is as accurate as more complicated formulas to detect macrosomic fetuses, he emphasized. “It’s a tough [measurement] to get just before the baby is born, but you can do it a little bit earlier,” he said. Research has shown that screening at 30-34 weeks can capture a majority of the fetuses destined to be greater than 4,000 g at birth, with a lower false-positive rate.
He pointed to one “very interesting” recent study in which AC was measured with a handheld ultrasound device at 24-40 weeks’ gestation, prior to formal ultrasound estimation of EFW. Early AC was a better predictor of large-for-gestational age babies at birth than EFW or early fundal height measurement, with sensitivities of 67%, 25%, and 50%, respectively (Am J Obstet Gynecol. 2015 Jun;212[6]:820.e1-8).
“And AC had a false-positive rate of only 10%,” Dr. Hobbins said.
Macrosomia occurs in 20% of cases of gestational diabetes and 25% of pregestational diabetes, he said. “And even if there is adequate glucose control, 17% will be macrosomic.”
The condition correlates with childhood and adulthood metabolic dysfunction and is associated with significantly increased risk of birth injury to the infant and to the mother. The alternative – cesarean delivery – is “not innocuous,” and “[we have] a very low threshold for cesarean if macrosomia is suspected,” Dr. Hobbins said.
Dr. Hobbins reported having no financial disclosures.
WASHINGTON – , John C. Hobbins, MD, said at the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
“Everything that the estimated fetal weight [EFW] can do, the abdominal circumference can do better,” especially in diabetic mothers, said Dr. Hobbins, who is widely regarded as one of the early pioneers in the development and use of obstetric ultrasound as a diagnostic tool.
Because it reflects liver size and incorporates subcutaneous fat, the abdominal circumference (AC) “concentrates on where the action is,” he said. It also “roughly correlates” with the size of the fetal shoulders and is not affected by genetic factors.
Moreover, “it focuses on one task rather than putting into play four variables, each with its own standard error of the method,” said Dr. Hobbins, referring to the four fetal biometric parameters incorporated into the Hadlock formula for EFW that is provided “upon fire-up of virtually every ultrasound machine.”
These four parameters (biparietal diameter, head circumference, femur length, and AC) each contribute to fetal weight but the AC has been shown to correlate better with weight at birth than the other variables, and it is the only measure that reflects how corpulent the fetus is, he noted.
The “general rule of thumb is that the EFW [as calculated by ultrasound machine–based equations] has a standard error of the method of plus or minus 10%. … which means that an EFW of 4,000 g is associated with a splay of plus or minus 1.2 pounds,” said Dr. Hobbins, professor of obstetrics and gynecology at the University of Colorado at Denver, Aurora.
“But the problem for us is not the failure of the ultrasound – it’s the way we use it,” he said.
An assortment of customized formulas have been developed for macrosomia, including one designed for use in diabetes that incorporates AC, head circumference, femur length, and 3D volumes of the thigh and abdomen. “If one used this and set a cut-off at 4,300 g, you’d pick up 93%. … but at false positive rate of 38%,” he said.
While not perfect, the AC alone is as accurate as more complicated formulas to detect macrosomic fetuses, he emphasized. “It’s a tough [measurement] to get just before the baby is born, but you can do it a little bit earlier,” he said. Research has shown that screening at 30-34 weeks can capture a majority of the fetuses destined to be greater than 4,000 g at birth, with a lower false-positive rate.
He pointed to one “very interesting” recent study in which AC was measured with a handheld ultrasound device at 24-40 weeks’ gestation, prior to formal ultrasound estimation of EFW. Early AC was a better predictor of large-for-gestational age babies at birth than EFW or early fundal height measurement, with sensitivities of 67%, 25%, and 50%, respectively (Am J Obstet Gynecol. 2015 Jun;212[6]:820.e1-8).
“And AC had a false-positive rate of only 10%,” Dr. Hobbins said.
Macrosomia occurs in 20% of cases of gestational diabetes and 25% of pregestational diabetes, he said. “And even if there is adequate glucose control, 17% will be macrosomic.”
The condition correlates with childhood and adulthood metabolic dysfunction and is associated with significantly increased risk of birth injury to the infant and to the mother. The alternative – cesarean delivery – is “not innocuous,” and “[we have] a very low threshold for cesarean if macrosomia is suspected,” Dr. Hobbins said.
Dr. Hobbins reported having no financial disclosures.
WASHINGTON – , John C. Hobbins, MD, said at the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
“Everything that the estimated fetal weight [EFW] can do, the abdominal circumference can do better,” especially in diabetic mothers, said Dr. Hobbins, who is widely regarded as one of the early pioneers in the development and use of obstetric ultrasound as a diagnostic tool.
Because it reflects liver size and incorporates subcutaneous fat, the abdominal circumference (AC) “concentrates on where the action is,” he said. It also “roughly correlates” with the size of the fetal shoulders and is not affected by genetic factors.
Moreover, “it focuses on one task rather than putting into play four variables, each with its own standard error of the method,” said Dr. Hobbins, referring to the four fetal biometric parameters incorporated into the Hadlock formula for EFW that is provided “upon fire-up of virtually every ultrasound machine.”
These four parameters (biparietal diameter, head circumference, femur length, and AC) each contribute to fetal weight but the AC has been shown to correlate better with weight at birth than the other variables, and it is the only measure that reflects how corpulent the fetus is, he noted.
The “general rule of thumb is that the EFW [as calculated by ultrasound machine–based equations] has a standard error of the method of plus or minus 10%. … which means that an EFW of 4,000 g is associated with a splay of plus or minus 1.2 pounds,” said Dr. Hobbins, professor of obstetrics and gynecology at the University of Colorado at Denver, Aurora.
“But the problem for us is not the failure of the ultrasound – it’s the way we use it,” he said.
An assortment of customized formulas have been developed for macrosomia, including one designed for use in diabetes that incorporates AC, head circumference, femur length, and 3D volumes of the thigh and abdomen. “If one used this and set a cut-off at 4,300 g, you’d pick up 93%. … but at false positive rate of 38%,” he said.
While not perfect, the AC alone is as accurate as more complicated formulas to detect macrosomic fetuses, he emphasized. “It’s a tough [measurement] to get just before the baby is born, but you can do it a little bit earlier,” he said. Research has shown that screening at 30-34 weeks can capture a majority of the fetuses destined to be greater than 4,000 g at birth, with a lower false-positive rate.
He pointed to one “very interesting” recent study in which AC was measured with a handheld ultrasound device at 24-40 weeks’ gestation, prior to formal ultrasound estimation of EFW. Early AC was a better predictor of large-for-gestational age babies at birth than EFW or early fundal height measurement, with sensitivities of 67%, 25%, and 50%, respectively (Am J Obstet Gynecol. 2015 Jun;212[6]:820.e1-8).
“And AC had a false-positive rate of only 10%,” Dr. Hobbins said.
Macrosomia occurs in 20% of cases of gestational diabetes and 25% of pregestational diabetes, he said. “And even if there is adequate glucose control, 17% will be macrosomic.”
The condition correlates with childhood and adulthood metabolic dysfunction and is associated with significantly increased risk of birth injury to the infant and to the mother. The alternative – cesarean delivery – is “not innocuous,” and “[we have] a very low threshold for cesarean if macrosomia is suspected,” Dr. Hobbins said.
Dr. Hobbins reported having no financial disclosures.
EXPERT ANALYSIS FROM DPSG-NA 2017
Reducing harm: When doing less is enough
Launched in April 2012 – the same year an article in the Journal of the American Medical Association estimated the U.S. health care system was wasting between $600 billion and $1 trillion annually because of issues such as overtreatment – Choosing Wisely continues to change both conversations and practices across the medical field.1
In creating Choosing Wisely, the ABIM Foundation sought to establish a framework for physicians to think about managing resources and to talk to patients about which medical tests and procedures might be unnecessary – or even harmful.
Today, more than 75 medical specialties have their own “five things” lists: procedures that practitioners should question before ordering. Hospitalists have a total of 10 – 5 for adults and 5 for pediatrics – and hospitalists play a pivotal role in Choosing Wisely’s implementation, with crucial control over service lines. “Hospitalists are on the front line of patient care,” said Moises Auron, MD, FAAP, FACP, SFHM, a hospitalist at the Cleveland Clinic. “We are actually the frontline workers in the hospital.”
Choosing Wisely’s successes
In terms of its initial goal – starting a conversation and encouraging physicians to interrogate their habits – Choosing Wisely has been a success.
“It’s brought a lot of awareness about the problem of matching best evidence with the patient you have in front of you,” said John Bulger , DO, MACP, MBA, SFHM, chief medical officer of Geisinger Health Plan. “Some people call that evidence-based medicine, but the problem with calling it that is that you can have a study, but it may not match up with the patient you’re seeing right now. There are many things we do because we did them in the past or because we didn’t have all the information, and I think Choosing Wisely has made people think twice about some of the things they do.”
The message of Choosing Wisely continues to spread, even internationally. It’s now present in 18 countries, Mr. Wolfson said. “We’re also seeing on the horizon many state efforts, such as in Connecticut and Rhode Island; and Delaware is organizing a statewide effort. I see that as the next big thing: statewide efforts that pair delivery systems with multistakeholder groups, regional health collaboratives, and physician organizations, all working to reduce use.”As it spreads, Choosing Wisely is sparking a new generation of related initiatives, such as Costs of Care and Johns Hopkins’ High Value Practice Academic Alliance. There’s a new section in the Journal of Hospital Medicine called “Things We Do for No Reason” highlighting different practices each month, and a nationwide Student High Value Care Initiative introduces value concepts to medical students. “It’s not Choosing Wisely by itself; it’s provided the backbone for all these new efforts,” Dr. Auron said.
Challenges remain
While it has spread, Choosing Wisely also has met some obstacles. Among them is that even with the help of Consumer Reports’ tools, the physician-patient conversations can be difficult. A behavioral economics concept called loss aversion is part of the reason: It’s basic human nature to feel the pain of loss more acutely than the pleasure of gain.
“It’s tough because that conversation requires specific training,” he said. “It’s one thing to tell the clinician, or to have it pop up on an EHR, that provision of an antibiotic for this clinical presentation is not appropriate. However, it’s an entirely different thing to look a patient in the face who comes in expecting a course of antibiotics and tell them that they’re not going to get it.”
Another hurdle is the existing fee-for-service system, which obviously does not promote cost consciousness. Since there’s really no disincentive to a physician ordering an additional test, acceptance of Choosing Wisely can vary widely between institutions. “Choosing Wisely permeated very nicely here at the Cleveland Clinic,” Dr. Auron said. “But other hospitals – especially private hospitals that are not owned by doctors – what they want is just the service line.”
Physicians’ discomfort with uncertainty is another challenge, according to Mr. Mainor. “A lot of it can be by virtue of medical training and how particular residents were taught to always run this panel when you have this presentation,” he said. “Sometimes it’s hard to separate Choosing Wisely from the concept of defensive medicine, but this is more wanting to be able to tell the patient that you did everything that you could before proceeding to a particular next step or treatment.”
Getting patient input from the outset and making sure goals are aligned can help with some of these issues – but can itself be a hurdle.
The road ahead
The time it takes to have these conversations is more than a sticking point for Choosing Wisely, it’s an underlying challenge in our health care system.
“For example, it takes more time to have a discussion about what the alternatives are to alleviate pain – other than taking an opiate,” Dr. Bulger said. “The easiest thing to do is to write the script for the opiate – which is part of the reason why we got where we are with opioids – or to write the script for an antibiotic – which is part of the reason why we got here with drug resistance. We haven’t done a great deal to address those underlying drivers. Without doing that, you can only go so far with a campaign like Choosing Wisely.”
Issues around costs fall into a similar category: an underlying issue that demands a broader conversation. ”It’s just so elusive,” Dr. Cho said. “There are so many different versions of cost, and from a hospital medicine standpoint, that process is so prolonged. We may not touch base with that patient when they get their bill, so for us to have a conversation about exactly how much this would cost can be difficult. It’s so complex; I would love for that to be tackled so that it’s a little more straightforward.”
“There are people involved in career paths in education, quality and safety, research, and administration, but there are very few people actually focused on value – and then finding the resources and the mobilization to do that,” Dr. Cho said. “I think it would really be helpful moving forward to find more people doing this and getting more support from their organizations.”
In one step toward that goal, a value track has been added to the Society of Hospital Medicine annual meeting.
“I think you’re going to see more emphasis on this, especially with younger hospitalists that are really pushing the value theme,” Dr. Bulger said. “I think those are really the lessons learned in what we started with Choosing Wisely.”
References
1. Berwick DM et al. Eliminating waste in US health care. JAMA. 2012;307(14):1513-6.
2. Colla CH et al. Physician perceptions of Choosing Wisely and drivers of overuse. Am J Manag Care. 2016 May;22(5):337-43.
Launched in April 2012 – the same year an article in the Journal of the American Medical Association estimated the U.S. health care system was wasting between $600 billion and $1 trillion annually because of issues such as overtreatment – Choosing Wisely continues to change both conversations and practices across the medical field.1
In creating Choosing Wisely, the ABIM Foundation sought to establish a framework for physicians to think about managing resources and to talk to patients about which medical tests and procedures might be unnecessary – or even harmful.
Today, more than 75 medical specialties have their own “five things” lists: procedures that practitioners should question before ordering. Hospitalists have a total of 10 – 5 for adults and 5 for pediatrics – and hospitalists play a pivotal role in Choosing Wisely’s implementation, with crucial control over service lines. “Hospitalists are on the front line of patient care,” said Moises Auron, MD, FAAP, FACP, SFHM, a hospitalist at the Cleveland Clinic. “We are actually the frontline workers in the hospital.”
Choosing Wisely’s successes
In terms of its initial goal – starting a conversation and encouraging physicians to interrogate their habits – Choosing Wisely has been a success.
“It’s brought a lot of awareness about the problem of matching best evidence with the patient you have in front of you,” said John Bulger , DO, MACP, MBA, SFHM, chief medical officer of Geisinger Health Plan. “Some people call that evidence-based medicine, but the problem with calling it that is that you can have a study, but it may not match up with the patient you’re seeing right now. There are many things we do because we did them in the past or because we didn’t have all the information, and I think Choosing Wisely has made people think twice about some of the things they do.”
The message of Choosing Wisely continues to spread, even internationally. It’s now present in 18 countries, Mr. Wolfson said. “We’re also seeing on the horizon many state efforts, such as in Connecticut and Rhode Island; and Delaware is organizing a statewide effort. I see that as the next big thing: statewide efforts that pair delivery systems with multistakeholder groups, regional health collaboratives, and physician organizations, all working to reduce use.”As it spreads, Choosing Wisely is sparking a new generation of related initiatives, such as Costs of Care and Johns Hopkins’ High Value Practice Academic Alliance. There’s a new section in the Journal of Hospital Medicine called “Things We Do for No Reason” highlighting different practices each month, and a nationwide Student High Value Care Initiative introduces value concepts to medical students. “It’s not Choosing Wisely by itself; it’s provided the backbone for all these new efforts,” Dr. Auron said.
Challenges remain
While it has spread, Choosing Wisely also has met some obstacles. Among them is that even with the help of Consumer Reports’ tools, the physician-patient conversations can be difficult. A behavioral economics concept called loss aversion is part of the reason: It’s basic human nature to feel the pain of loss more acutely than the pleasure of gain.
“It’s tough because that conversation requires specific training,” he said. “It’s one thing to tell the clinician, or to have it pop up on an EHR, that provision of an antibiotic for this clinical presentation is not appropriate. However, it’s an entirely different thing to look a patient in the face who comes in expecting a course of antibiotics and tell them that they’re not going to get it.”
Another hurdle is the existing fee-for-service system, which obviously does not promote cost consciousness. Since there’s really no disincentive to a physician ordering an additional test, acceptance of Choosing Wisely can vary widely between institutions. “Choosing Wisely permeated very nicely here at the Cleveland Clinic,” Dr. Auron said. “But other hospitals – especially private hospitals that are not owned by doctors – what they want is just the service line.”
Physicians’ discomfort with uncertainty is another challenge, according to Mr. Mainor. “A lot of it can be by virtue of medical training and how particular residents were taught to always run this panel when you have this presentation,” he said. “Sometimes it’s hard to separate Choosing Wisely from the concept of defensive medicine, but this is more wanting to be able to tell the patient that you did everything that you could before proceeding to a particular next step or treatment.”
Getting patient input from the outset and making sure goals are aligned can help with some of these issues – but can itself be a hurdle.
The road ahead
The time it takes to have these conversations is more than a sticking point for Choosing Wisely, it’s an underlying challenge in our health care system.
“For example, it takes more time to have a discussion about what the alternatives are to alleviate pain – other than taking an opiate,” Dr. Bulger said. “The easiest thing to do is to write the script for the opiate – which is part of the reason why we got where we are with opioids – or to write the script for an antibiotic – which is part of the reason why we got here with drug resistance. We haven’t done a great deal to address those underlying drivers. Without doing that, you can only go so far with a campaign like Choosing Wisely.”
Issues around costs fall into a similar category: an underlying issue that demands a broader conversation. ”It’s just so elusive,” Dr. Cho said. “There are so many different versions of cost, and from a hospital medicine standpoint, that process is so prolonged. We may not touch base with that patient when they get their bill, so for us to have a conversation about exactly how much this would cost can be difficult. It’s so complex; I would love for that to be tackled so that it’s a little more straightforward.”
“There are people involved in career paths in education, quality and safety, research, and administration, but there are very few people actually focused on value – and then finding the resources and the mobilization to do that,” Dr. Cho said. “I think it would really be helpful moving forward to find more people doing this and getting more support from their organizations.”
In one step toward that goal, a value track has been added to the Society of Hospital Medicine annual meeting.
“I think you’re going to see more emphasis on this, especially with younger hospitalists that are really pushing the value theme,” Dr. Bulger said. “I think those are really the lessons learned in what we started with Choosing Wisely.”
References
1. Berwick DM et al. Eliminating waste in US health care. JAMA. 2012;307(14):1513-6.
2. Colla CH et al. Physician perceptions of Choosing Wisely and drivers of overuse. Am J Manag Care. 2016 May;22(5):337-43.
Launched in April 2012 – the same year an article in the Journal of the American Medical Association estimated the U.S. health care system was wasting between $600 billion and $1 trillion annually because of issues such as overtreatment – Choosing Wisely continues to change both conversations and practices across the medical field.1
In creating Choosing Wisely, the ABIM Foundation sought to establish a framework for physicians to think about managing resources and to talk to patients about which medical tests and procedures might be unnecessary – or even harmful.
Today, more than 75 medical specialties have their own “five things” lists: procedures that practitioners should question before ordering. Hospitalists have a total of 10 – 5 for adults and 5 for pediatrics – and hospitalists play a pivotal role in Choosing Wisely’s implementation, with crucial control over service lines. “Hospitalists are on the front line of patient care,” said Moises Auron, MD, FAAP, FACP, SFHM, a hospitalist at the Cleveland Clinic. “We are actually the frontline workers in the hospital.”
Choosing Wisely’s successes
In terms of its initial goal – starting a conversation and encouraging physicians to interrogate their habits – Choosing Wisely has been a success.
“It’s brought a lot of awareness about the problem of matching best evidence with the patient you have in front of you,” said John Bulger , DO, MACP, MBA, SFHM, chief medical officer of Geisinger Health Plan. “Some people call that evidence-based medicine, but the problem with calling it that is that you can have a study, but it may not match up with the patient you’re seeing right now. There are many things we do because we did them in the past or because we didn’t have all the information, and I think Choosing Wisely has made people think twice about some of the things they do.”
The message of Choosing Wisely continues to spread, even internationally. It’s now present in 18 countries, Mr. Wolfson said. “We’re also seeing on the horizon many state efforts, such as in Connecticut and Rhode Island; and Delaware is organizing a statewide effort. I see that as the next big thing: statewide efforts that pair delivery systems with multistakeholder groups, regional health collaboratives, and physician organizations, all working to reduce use.”As it spreads, Choosing Wisely is sparking a new generation of related initiatives, such as Costs of Care and Johns Hopkins’ High Value Practice Academic Alliance. There’s a new section in the Journal of Hospital Medicine called “Things We Do for No Reason” highlighting different practices each month, and a nationwide Student High Value Care Initiative introduces value concepts to medical students. “It’s not Choosing Wisely by itself; it’s provided the backbone for all these new efforts,” Dr. Auron said.
Challenges remain
While it has spread, Choosing Wisely also has met some obstacles. Among them is that even with the help of Consumer Reports’ tools, the physician-patient conversations can be difficult. A behavioral economics concept called loss aversion is part of the reason: It’s basic human nature to feel the pain of loss more acutely than the pleasure of gain.
“It’s tough because that conversation requires specific training,” he said. “It’s one thing to tell the clinician, or to have it pop up on an EHR, that provision of an antibiotic for this clinical presentation is not appropriate. However, it’s an entirely different thing to look a patient in the face who comes in expecting a course of antibiotics and tell them that they’re not going to get it.”
Another hurdle is the existing fee-for-service system, which obviously does not promote cost consciousness. Since there’s really no disincentive to a physician ordering an additional test, acceptance of Choosing Wisely can vary widely between institutions. “Choosing Wisely permeated very nicely here at the Cleveland Clinic,” Dr. Auron said. “But other hospitals – especially private hospitals that are not owned by doctors – what they want is just the service line.”
Physicians’ discomfort with uncertainty is another challenge, according to Mr. Mainor. “A lot of it can be by virtue of medical training and how particular residents were taught to always run this panel when you have this presentation,” he said. “Sometimes it’s hard to separate Choosing Wisely from the concept of defensive medicine, but this is more wanting to be able to tell the patient that you did everything that you could before proceeding to a particular next step or treatment.”
Getting patient input from the outset and making sure goals are aligned can help with some of these issues – but can itself be a hurdle.
The road ahead
The time it takes to have these conversations is more than a sticking point for Choosing Wisely, it’s an underlying challenge in our health care system.
“For example, it takes more time to have a discussion about what the alternatives are to alleviate pain – other than taking an opiate,” Dr. Bulger said. “The easiest thing to do is to write the script for the opiate – which is part of the reason why we got where we are with opioids – or to write the script for an antibiotic – which is part of the reason why we got here with drug resistance. We haven’t done a great deal to address those underlying drivers. Without doing that, you can only go so far with a campaign like Choosing Wisely.”
Issues around costs fall into a similar category: an underlying issue that demands a broader conversation. ”It’s just so elusive,” Dr. Cho said. “There are so many different versions of cost, and from a hospital medicine standpoint, that process is so prolonged. We may not touch base with that patient when they get their bill, so for us to have a conversation about exactly how much this would cost can be difficult. It’s so complex; I would love for that to be tackled so that it’s a little more straightforward.”
“There are people involved in career paths in education, quality and safety, research, and administration, but there are very few people actually focused on value – and then finding the resources and the mobilization to do that,” Dr. Cho said. “I think it would really be helpful moving forward to find more people doing this and getting more support from their organizations.”
In one step toward that goal, a value track has been added to the Society of Hospital Medicine annual meeting.
“I think you’re going to see more emphasis on this, especially with younger hospitalists that are really pushing the value theme,” Dr. Bulger said. “I think those are really the lessons learned in what we started with Choosing Wisely.”
References
1. Berwick DM et al. Eliminating waste in US health care. JAMA. 2012;307(14):1513-6.
2. Colla CH et al. Physician perceptions of Choosing Wisely and drivers of overuse. Am J Manag Care. 2016 May;22(5):337-43.
CD22 CAR activity in B-ALL highlights promise of multispecific CARs
NATIONAL HARBOR, MD. – A new CD22-targeted chimeric antigen receptor (CAR) demonstrated clinical activity in a phase 1 study of adults and children with relapsed/refractory B-cell acute lymphoblastic leukemia (B-ALL), including several who were previously treated with CD19-directed immunotherapy.
In 21 children and adults with B-ALL who were treated with the CD22 CAR, dose-dependent antileukemic activity was observed; complete remission occurred in 11 of 15 (73%) who received bioactive doses (at least 1 x 106 CD22 CAR T-cells per kg of body weight), including 5 of 5 patients with CD19dim or CD19neg B-ALL, Terry J. Fry, MD, of the National Institutes of Health, Bethesda, Md., and colleagues reported in Nature Medicine (2017 Nov 20. doi: 10.1038.nm.4441).
This study is the first to establish the clinical activity of a CD22 CAR in B-ALL. The investigators developed the CAR in an effort to counter the resistance sometimes seen in patients who receive CD19 CAR T-cell therapy. CD22 is also expressed in most B-ALL cases – and usually is retained following CD19 loss, they explained.
The findings, when considered in light of efficacy demonstrated in leukemia that is resistant to anti-CD19 immunotherapy, highlights the potential for – and importance of – developing multispecific CARs, Crystal L. Mackall, MD, the senior author of the study, said during an update on CAR T-cell research at the annual meeting of the Society for Immunotherapy of Cancer.
“All in all, once we got to the dose that was appropriate ... this CAR had really impressive activity,” said Dr. Mackall, director of the Parker Institute for Cancer Immunotherapy at Stanford (Calif.) University. “In some patients, this was all the patient needed for a prolonged disease-free interval.”
Three patients had ongoing responses, at 21 months, 9 months, and 6 months, she said. There was a high rate of relapse among the study participants, but all patients had previously received at least one bone marrow transplant, and 17 had received CD19-based immunotherapy.
“But nonetheless, the interrogation of these relapses was really essential to understand more about the Achilles heels of these CAR T-cells,” she said. “What we saw is that it was all about the antigen.”
Unlike CD19, which tends to disappear after relapse, CD22-expressing tumors that relapse tend to come back with “simply lower expression of CD22,” she said. The CD22 CAR was unable to control the CD22lo leukemias. This is not unique to the CD22 CAR, she said.
“Every CAR we’ve looked at so far has this exquisite dependence on antigen density for functionality,” she explained, noting that heterogeneity in antigen expression will pose major challenges for the development of therapies, and “maybe has been one of the main reasons we haven’t yet seen the effectiveness of CAR T-cells in solid tumors that we have for hematological malignancies where we’ve typically had targets that are expressed homogenously and at high levels.”
“So we believe very strongly that multispecific CARS are going to be essential for progress, especially as we move into solid tumors,” she added.
Early attempts at developing multispecific CARS suggest that coadministration is not ideal, but two other approaches – coexpression using two vectors or a bicistronic vector, or by creation of a bivalent-bispecific CAR (also known as a tandem CAR) – are both still on the table, she said.
Two clinical first-in-human trials evaluating a CD19/22-bispecific CAR (one in children and one in adults) for relapsed/refractory B-cell malignancies are underway at Stanford.
“We predict that this is going to be the beginning of a wave of bispecific, trispecific, and maybe even quad CARs,” she said. “There’s a lot of work to do, but this is an area that’s going to be very active in the coming years.”
Dr. Mackall has received consulting fees from Adaptimmune, GSK, Roche, Unum Therapeutics, and Vore Pharmaceuticals; has conducted research for Bluebird Bio; and has ownership interest from Juno Therapeutics.
NATIONAL HARBOR, MD. – A new CD22-targeted chimeric antigen receptor (CAR) demonstrated clinical activity in a phase 1 study of adults and children with relapsed/refractory B-cell acute lymphoblastic leukemia (B-ALL), including several who were previously treated with CD19-directed immunotherapy.
In 21 children and adults with B-ALL who were treated with the CD22 CAR, dose-dependent antileukemic activity was observed; complete remission occurred in 11 of 15 (73%) who received bioactive doses (at least 1 x 106 CD22 CAR T-cells per kg of body weight), including 5 of 5 patients with CD19dim or CD19neg B-ALL, Terry J. Fry, MD, of the National Institutes of Health, Bethesda, Md., and colleagues reported in Nature Medicine (2017 Nov 20. doi: 10.1038.nm.4441).
This study is the first to establish the clinical activity of a CD22 CAR in B-ALL. The investigators developed the CAR in an effort to counter the resistance sometimes seen in patients who receive CD19 CAR T-cell therapy. CD22 is also expressed in most B-ALL cases – and usually is retained following CD19 loss, they explained.
The findings, when considered in light of efficacy demonstrated in leukemia that is resistant to anti-CD19 immunotherapy, highlights the potential for – and importance of – developing multispecific CARs, Crystal L. Mackall, MD, the senior author of the study, said during an update on CAR T-cell research at the annual meeting of the Society for Immunotherapy of Cancer.
“All in all, once we got to the dose that was appropriate ... this CAR had really impressive activity,” said Dr. Mackall, director of the Parker Institute for Cancer Immunotherapy at Stanford (Calif.) University. “In some patients, this was all the patient needed for a prolonged disease-free interval.”
Three patients had ongoing responses, at 21 months, 9 months, and 6 months, she said. There was a high rate of relapse among the study participants, but all patients had previously received at least one bone marrow transplant, and 17 had received CD19-based immunotherapy.
“But nonetheless, the interrogation of these relapses was really essential to understand more about the Achilles heels of these CAR T-cells,” she said. “What we saw is that it was all about the antigen.”
Unlike CD19, which tends to disappear after relapse, CD22-expressing tumors that relapse tend to come back with “simply lower expression of CD22,” she said. The CD22 CAR was unable to control the CD22lo leukemias. This is not unique to the CD22 CAR, she said.
“Every CAR we’ve looked at so far has this exquisite dependence on antigen density for functionality,” she explained, noting that heterogeneity in antigen expression will pose major challenges for the development of therapies, and “maybe has been one of the main reasons we haven’t yet seen the effectiveness of CAR T-cells in solid tumors that we have for hematological malignancies where we’ve typically had targets that are expressed homogenously and at high levels.”
“So we believe very strongly that multispecific CARS are going to be essential for progress, especially as we move into solid tumors,” she added.
Early attempts at developing multispecific CARS suggest that coadministration is not ideal, but two other approaches – coexpression using two vectors or a bicistronic vector, or by creation of a bivalent-bispecific CAR (also known as a tandem CAR) – are both still on the table, she said.
Two clinical first-in-human trials evaluating a CD19/22-bispecific CAR (one in children and one in adults) for relapsed/refractory B-cell malignancies are underway at Stanford.
“We predict that this is going to be the beginning of a wave of bispecific, trispecific, and maybe even quad CARs,” she said. “There’s a lot of work to do, but this is an area that’s going to be very active in the coming years.”
Dr. Mackall has received consulting fees from Adaptimmune, GSK, Roche, Unum Therapeutics, and Vore Pharmaceuticals; has conducted research for Bluebird Bio; and has ownership interest from Juno Therapeutics.
NATIONAL HARBOR, MD. – A new CD22-targeted chimeric antigen receptor (CAR) demonstrated clinical activity in a phase 1 study of adults and children with relapsed/refractory B-cell acute lymphoblastic leukemia (B-ALL), including several who were previously treated with CD19-directed immunotherapy.
In 21 children and adults with B-ALL who were treated with the CD22 CAR, dose-dependent antileukemic activity was observed; complete remission occurred in 11 of 15 (73%) who received bioactive doses (at least 1 x 106 CD22 CAR T-cells per kg of body weight), including 5 of 5 patients with CD19dim or CD19neg B-ALL, Terry J. Fry, MD, of the National Institutes of Health, Bethesda, Md., and colleagues reported in Nature Medicine (2017 Nov 20. doi: 10.1038.nm.4441).
This study is the first to establish the clinical activity of a CD22 CAR in B-ALL. The investigators developed the CAR in an effort to counter the resistance sometimes seen in patients who receive CD19 CAR T-cell therapy. CD22 is also expressed in most B-ALL cases – and usually is retained following CD19 loss, they explained.
The findings, when considered in light of efficacy demonstrated in leukemia that is resistant to anti-CD19 immunotherapy, highlights the potential for – and importance of – developing multispecific CARs, Crystal L. Mackall, MD, the senior author of the study, said during an update on CAR T-cell research at the annual meeting of the Society for Immunotherapy of Cancer.
“All in all, once we got to the dose that was appropriate ... this CAR had really impressive activity,” said Dr. Mackall, director of the Parker Institute for Cancer Immunotherapy at Stanford (Calif.) University. “In some patients, this was all the patient needed for a prolonged disease-free interval.”
Three patients had ongoing responses, at 21 months, 9 months, and 6 months, she said. There was a high rate of relapse among the study participants, but all patients had previously received at least one bone marrow transplant, and 17 had received CD19-based immunotherapy.
“But nonetheless, the interrogation of these relapses was really essential to understand more about the Achilles heels of these CAR T-cells,” she said. “What we saw is that it was all about the antigen.”
Unlike CD19, which tends to disappear after relapse, CD22-expressing tumors that relapse tend to come back with “simply lower expression of CD22,” she said. The CD22 CAR was unable to control the CD22lo leukemias. This is not unique to the CD22 CAR, she said.
“Every CAR we’ve looked at so far has this exquisite dependence on antigen density for functionality,” she explained, noting that heterogeneity in antigen expression will pose major challenges for the development of therapies, and “maybe has been one of the main reasons we haven’t yet seen the effectiveness of CAR T-cells in solid tumors that we have for hematological malignancies where we’ve typically had targets that are expressed homogenously and at high levels.”
“So we believe very strongly that multispecific CARS are going to be essential for progress, especially as we move into solid tumors,” she added.
Early attempts at developing multispecific CARS suggest that coadministration is not ideal, but two other approaches – coexpression using two vectors or a bicistronic vector, or by creation of a bivalent-bispecific CAR (also known as a tandem CAR) – are both still on the table, she said.
Two clinical first-in-human trials evaluating a CD19/22-bispecific CAR (one in children and one in adults) for relapsed/refractory B-cell malignancies are underway at Stanford.
“We predict that this is going to be the beginning of a wave of bispecific, trispecific, and maybe even quad CARs,” she said. “There’s a lot of work to do, but this is an area that’s going to be very active in the coming years.”
Dr. Mackall has received consulting fees from Adaptimmune, GSK, Roche, Unum Therapeutics, and Vore Pharmaceuticals; has conducted research for Bluebird Bio; and has ownership interest from Juno Therapeutics.
AT SITC 2017
Key clinical point:
Major finding: Complete remission occurred in 73% of patients who received bioactive doses of the CD22 CAR.
Data source: A phase 1 study of 21 patients.
Disclosures: Dr. Mackall has received consulting fees from Adaptimmune, GSK, Roche, Unum Therapeutics, and Vore Pharmaceuticals; has conducted research for Bluebird Bio; and has ownership interest from Juno Therapeutics.
For women with RA, small-joint surgery rate nearly twice that of men
SAN DIEGO – but the rate of small-joint procedures is declining for both sexes. However, no differences in rates of large-joint procedures between sexes were observed during the same time period.
Those are key findings from a retrospective study which set out to determine if there are sex differences in the incidence and trends of large- versus small-joint surgery rates in rheumatoid arthritis over time. “Why is orthopedic surgery important to rheumatology? The main reason is because it’s a surrogate for failed medical management,” lead study author Michael D. Richter, MD, said at the annual meeting of the American College of Rheumatology.
Dr. Richter, an internal medicine resident at Mayo Clinic, Rochester, Minn., said that women with RA generally present with more severe symptoms and higher rates of disability, while men have a better treatment response and a higher remission rate. For example, results from the multinational Quantitative Standard Monitoring of Patients with RA study found that remission rates were around 30% in men and 17% in women (Arthritis Res Ther. 2009;11[1]:R7). “However, a lot of these studies are criticized because it’s thought that gender can play a role in the disease measures,” he said. “By looking at joint surgery we have an objective outcome, and we can look at differences in treatment efficacy.”
Dr. Richter and his associates drew from the Rochester Epidemiology Project to identify 1,077 patients from Olmstead County, Minn., who fulfilled ACR criteria for RA between 1980 and 2013, and who were followed up until death, migration, or July 1, 2016. They classified surgeries as small joint (wrist, hand, or foot) or large joint (shoulder, elbow, hip, knee, or ankle). A majority of the patients (70%) were women. Compared with women, men were slightly older at diagnosis (a mean of 58 years vs. 55 years, respectively), were more likely to have a history of smoking (67% vs. 46%), and were more likely to have large-joint swelling upon initial presentation (49% vs. 42%). The mean follow-up was 12 years. No differences between men and women were noted in obesity, inflammatory biomarkers, or seropositivity.
During the study period, 112 patients underwent at least one small-joint surgery, 90 of whom were women (80%). The cumulative incidence of small-joint surgery at 15 years was nearly double that of men: 14.4% vs. 7.6%, respectively (P = .008). “Prior to the year 2000 there were no significant trends in the rate of small-joint surgery but it was more common in women,” he said. “After 2000 there was a significant decline for men and women (P = .002), but no significant difference between sexes.”
At the same time, 204 patients underwent at least one large-joint surgery during the time period, 141 of whom were women (69%). The cumulative incidence of large-joint surgery at 15 years was 20.2% for women and 18.8% for men, which was statistically similar (P = .55). “We saw no significant change over time in the rate of large-joint surgery from 1980 to 2016,” Dr. Richter said. “This is in contrast to what we see in the general population, where orthopedic procedures for osteoarthritis are more common.”
He acknowledged certain limitations of the study, including its retrospective design and the fact that the researchers were unable to include specific surgical indications in the analysis. “This becomes particularly important for the large-joint procedures,” he said. “We don’t know if osteoarthritis or chronic inflammatory arthritis is leading to the large-joint procedure.”
The National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Aging funded the study. Dr. Richter reported having no financial disclosures.
SAN DIEGO – but the rate of small-joint procedures is declining for both sexes. However, no differences in rates of large-joint procedures between sexes were observed during the same time period.
Those are key findings from a retrospective study which set out to determine if there are sex differences in the incidence and trends of large- versus small-joint surgery rates in rheumatoid arthritis over time. “Why is orthopedic surgery important to rheumatology? The main reason is because it’s a surrogate for failed medical management,” lead study author Michael D. Richter, MD, said at the annual meeting of the American College of Rheumatology.
Dr. Richter, an internal medicine resident at Mayo Clinic, Rochester, Minn., said that women with RA generally present with more severe symptoms and higher rates of disability, while men have a better treatment response and a higher remission rate. For example, results from the multinational Quantitative Standard Monitoring of Patients with RA study found that remission rates were around 30% in men and 17% in women (Arthritis Res Ther. 2009;11[1]:R7). “However, a lot of these studies are criticized because it’s thought that gender can play a role in the disease measures,” he said. “By looking at joint surgery we have an objective outcome, and we can look at differences in treatment efficacy.”
Dr. Richter and his associates drew from the Rochester Epidemiology Project to identify 1,077 patients from Olmstead County, Minn., who fulfilled ACR criteria for RA between 1980 and 2013, and who were followed up until death, migration, or July 1, 2016. They classified surgeries as small joint (wrist, hand, or foot) or large joint (shoulder, elbow, hip, knee, or ankle). A majority of the patients (70%) were women. Compared with women, men were slightly older at diagnosis (a mean of 58 years vs. 55 years, respectively), were more likely to have a history of smoking (67% vs. 46%), and were more likely to have large-joint swelling upon initial presentation (49% vs. 42%). The mean follow-up was 12 years. No differences between men and women were noted in obesity, inflammatory biomarkers, or seropositivity.
During the study period, 112 patients underwent at least one small-joint surgery, 90 of whom were women (80%). The cumulative incidence of small-joint surgery at 15 years was nearly double that of men: 14.4% vs. 7.6%, respectively (P = .008). “Prior to the year 2000 there were no significant trends in the rate of small-joint surgery but it was more common in women,” he said. “After 2000 there was a significant decline for men and women (P = .002), but no significant difference between sexes.”
At the same time, 204 patients underwent at least one large-joint surgery during the time period, 141 of whom were women (69%). The cumulative incidence of large-joint surgery at 15 years was 20.2% for women and 18.8% for men, which was statistically similar (P = .55). “We saw no significant change over time in the rate of large-joint surgery from 1980 to 2016,” Dr. Richter said. “This is in contrast to what we see in the general population, where orthopedic procedures for osteoarthritis are more common.”
He acknowledged certain limitations of the study, including its retrospective design and the fact that the researchers were unable to include specific surgical indications in the analysis. “This becomes particularly important for the large-joint procedures,” he said. “We don’t know if osteoarthritis or chronic inflammatory arthritis is leading to the large-joint procedure.”
The National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Aging funded the study. Dr. Richter reported having no financial disclosures.
SAN DIEGO – but the rate of small-joint procedures is declining for both sexes. However, no differences in rates of large-joint procedures between sexes were observed during the same time period.
Those are key findings from a retrospective study which set out to determine if there are sex differences in the incidence and trends of large- versus small-joint surgery rates in rheumatoid arthritis over time. “Why is orthopedic surgery important to rheumatology? The main reason is because it’s a surrogate for failed medical management,” lead study author Michael D. Richter, MD, said at the annual meeting of the American College of Rheumatology.
Dr. Richter, an internal medicine resident at Mayo Clinic, Rochester, Minn., said that women with RA generally present with more severe symptoms and higher rates of disability, while men have a better treatment response and a higher remission rate. For example, results from the multinational Quantitative Standard Monitoring of Patients with RA study found that remission rates were around 30% in men and 17% in women (Arthritis Res Ther. 2009;11[1]:R7). “However, a lot of these studies are criticized because it’s thought that gender can play a role in the disease measures,” he said. “By looking at joint surgery we have an objective outcome, and we can look at differences in treatment efficacy.”
Dr. Richter and his associates drew from the Rochester Epidemiology Project to identify 1,077 patients from Olmstead County, Minn., who fulfilled ACR criteria for RA between 1980 and 2013, and who were followed up until death, migration, or July 1, 2016. They classified surgeries as small joint (wrist, hand, or foot) or large joint (shoulder, elbow, hip, knee, or ankle). A majority of the patients (70%) were women. Compared with women, men were slightly older at diagnosis (a mean of 58 years vs. 55 years, respectively), were more likely to have a history of smoking (67% vs. 46%), and were more likely to have large-joint swelling upon initial presentation (49% vs. 42%). The mean follow-up was 12 years. No differences between men and women were noted in obesity, inflammatory biomarkers, or seropositivity.
During the study period, 112 patients underwent at least one small-joint surgery, 90 of whom were women (80%). The cumulative incidence of small-joint surgery at 15 years was nearly double that of men: 14.4% vs. 7.6%, respectively (P = .008). “Prior to the year 2000 there were no significant trends in the rate of small-joint surgery but it was more common in women,” he said. “After 2000 there was a significant decline for men and women (P = .002), but no significant difference between sexes.”
At the same time, 204 patients underwent at least one large-joint surgery during the time period, 141 of whom were women (69%). The cumulative incidence of large-joint surgery at 15 years was 20.2% for women and 18.8% for men, which was statistically similar (P = .55). “We saw no significant change over time in the rate of large-joint surgery from 1980 to 2016,” Dr. Richter said. “This is in contrast to what we see in the general population, where orthopedic procedures for osteoarthritis are more common.”
He acknowledged certain limitations of the study, including its retrospective design and the fact that the researchers were unable to include specific surgical indications in the analysis. “This becomes particularly important for the large-joint procedures,” he said. “We don’t know if osteoarthritis or chronic inflammatory arthritis is leading to the large-joint procedure.”
The National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Aging funded the study. Dr. Richter reported having no financial disclosures.
AT ACR 2017
Key clinical point: Women with RA had a higher rate of small-joint surgery, compared with men.
Major finding: The cumulative incidence of small-joint surgery was significantly higher among women, compared with men (14.4% vs. 7.6%, respectively), but there were no differences between sexes in the rates of large-joint surgery.
Study details: A retrospective, population-based study of 1,077 patients with RA.
Disclosures: The National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Aging funded the study. Dr. Richter reported having no financial disclosures.
Improving the risk assessment of patients with acute pulmonary embolism
Who doesn’t like alphabet soup? Those noodles shaped as letters floating in a delicious hot broth serve as an educational way for young children to play with their food. Of course, this commentary is not about warm food suitable for a cold day but, instead, about another notion of alphabet soup – a metaphor for physicians’ failure to optimally utilize our alphabet soup of venous thromboembolism studies.
The medical literature that has studied the incidence, prevalence, diagnosis, and management of acute pulmonary embolism (PE) is vast. Yes, we have our own PE “alphabet soup” – a “hodgepodge especially of initials,” according to the Merriam-Webster Dictionary. ICOPER (International Cooperative Pulmonary Embolism Registry) and RIETE (Computerized Registry of Patients with Venous Thromboembolism) help estimate prevalence of the disease and indicate high-risk groups. PESI (Pulmonary Embolism Severity Index) and PERC (Pulmonary Embolism Rule-Out Criteria) provide useful predictive information prior to proceeding with diagnostic testing for PE. The PEITHO (Pulmonary Embolism Thrombolysis), MOPETT (Moderate Pulmonary Embolism Treated with Thrombolysis), and PERFECT (Pulmonary Embolism Response to Fragmentation, Embolectomy, and Catheter Thrombolysis) studies help to guide the decision to administer fibrinolytic therapy, particularly in the cases of so-called submassive or intermediate-risk PE.
And yet, in a study published in Hospital Practice (2017 Aug 30. doi: 10.1080/21548331.2017.1372033), my colleagues and I showed that, to a large extent, physicians admitting patients to the hospital between 2011 and 2013 for acute PE failed to order or perform even the most basic noninvasive testing on their patients, despite strong recommendations for such testing from both the 2011 American Heart Association (AHA) and the 2014 European Society of Cardiology as part of the risk assessment of acute PE.
At the time of the patients’ PE admissions, the 2011 AHA statement already had been published, and it was recognized that medical and interventional therapies needed to be appropriate to the characteristics of the patient with PE. Specifically, in order to determine a prognosis, assessment of right heart strain with an echocardiogram, ECG, and brain natriuretic peptide testing was recommended. For similar reasons, a serum troponin test was recommended to evaluate any myocardial necrosis.
While the 2011 AHA statement was based on a preponderance of evidence-based medicine, our research suggests that physicians may be doing a suboptimal job of collecting the necessary data to manage our acute PE patients. In defense of those physicians we evaluated, these data were collected just before the European Society of Cardiology disseminated their recommendations for risk stratification in 2014. The PEITHO, MOPETT, and PERFECT findings – though already presented at medical meetings in part – were not published in scientific journals in full until 2013 or thereafter.
Nevertheless, I believe these findings illustrate the merits of the Pulmonary Embolism Response Team (PERT) concept. As the data we studied suggest, not only might hospital-based physicians inadequately assess the severity of patients’ PE, but they also may fail to ask for consultations from the specialists who could be most useful in assisting in the proper evaluation and management of these patients. PERTs ensure that every patient admitted to the hospital with the diagnosis of PE will be assessed by a team of physicians with the expertise and professional interest in best managing these patients.
The expectation is that, with the institution of a PERT at each hospital, a more complete evaluation of patients’ PE may lead to optimal management and, thereby, to improved short- and long-term outcomes. Also, we should not assume that a greater degree of expertise and imaging capabilities at an academic university hospital translates to a more complete evaluation of PE; our research shows that this is not necessarily the case. PERTs may help mobilize resources that are underutilized even at academic university hospitals. Those interested in learning more about PERTs may contact the National PERT Consortium via email at [email protected] or go online to www.pertconsortium.org.
Rather than wallow in an alphabet soup of acronyms, let us place our acronymic clinical PE trials to good use. Gather the appropriate clinical data, consult experts in pulmonary embolism, and stratify patients admitted to the hospital with acute PE so that we can manage the expectations of short- and long-term outcomes.
When he became president in 1933, Franklin D. Roosevelt sought to lift the United States from the Great Depression, in part with a New Deal “alphabet soup” of programs. Like FDR, let us put our own alphabet soup – of PE studies and position statements – to good use: to work for the good of people hospitalized with acute pulmonary embolism.
Dr. Scharf is medical director of pulmonary outpatient services at the Jane and Leonard Korman Respiratory Institute at Jefferson Medical College, Philadelphia. He is also director of the pulmonary vascular disease program at Jefferson.
Who doesn’t like alphabet soup? Those noodles shaped as letters floating in a delicious hot broth serve as an educational way for young children to play with their food. Of course, this commentary is not about warm food suitable for a cold day but, instead, about another notion of alphabet soup – a metaphor for physicians’ failure to optimally utilize our alphabet soup of venous thromboembolism studies.
The medical literature that has studied the incidence, prevalence, diagnosis, and management of acute pulmonary embolism (PE) is vast. Yes, we have our own PE “alphabet soup” – a “hodgepodge especially of initials,” according to the Merriam-Webster Dictionary. ICOPER (International Cooperative Pulmonary Embolism Registry) and RIETE (Computerized Registry of Patients with Venous Thromboembolism) help estimate prevalence of the disease and indicate high-risk groups. PESI (Pulmonary Embolism Severity Index) and PERC (Pulmonary Embolism Rule-Out Criteria) provide useful predictive information prior to proceeding with diagnostic testing for PE. The PEITHO (Pulmonary Embolism Thrombolysis), MOPETT (Moderate Pulmonary Embolism Treated with Thrombolysis), and PERFECT (Pulmonary Embolism Response to Fragmentation, Embolectomy, and Catheter Thrombolysis) studies help to guide the decision to administer fibrinolytic therapy, particularly in the cases of so-called submassive or intermediate-risk PE.
And yet, in a study published in Hospital Practice (2017 Aug 30. doi: 10.1080/21548331.2017.1372033), my colleagues and I showed that, to a large extent, physicians admitting patients to the hospital between 2011 and 2013 for acute PE failed to order or perform even the most basic noninvasive testing on their patients, despite strong recommendations for such testing from both the 2011 American Heart Association (AHA) and the 2014 European Society of Cardiology as part of the risk assessment of acute PE.
At the time of the patients’ PE admissions, the 2011 AHA statement already had been published, and it was recognized that medical and interventional therapies needed to be appropriate to the characteristics of the patient with PE. Specifically, in order to determine a prognosis, assessment of right heart strain with an echocardiogram, ECG, and brain natriuretic peptide testing was recommended. For similar reasons, a serum troponin test was recommended to evaluate any myocardial necrosis.
While the 2011 AHA statement was based on a preponderance of evidence-based medicine, our research suggests that physicians may be doing a suboptimal job of collecting the necessary data to manage our acute PE patients. In defense of those physicians we evaluated, these data were collected just before the European Society of Cardiology disseminated their recommendations for risk stratification in 2014. The PEITHO, MOPETT, and PERFECT findings – though already presented at medical meetings in part – were not published in scientific journals in full until 2013 or thereafter.
Nevertheless, I believe these findings illustrate the merits of the Pulmonary Embolism Response Team (PERT) concept. As the data we studied suggest, not only might hospital-based physicians inadequately assess the severity of patients’ PE, but they also may fail to ask for consultations from the specialists who could be most useful in assisting in the proper evaluation and management of these patients. PERTs ensure that every patient admitted to the hospital with the diagnosis of PE will be assessed by a team of physicians with the expertise and professional interest in best managing these patients.
The expectation is that, with the institution of a PERT at each hospital, a more complete evaluation of patients’ PE may lead to optimal management and, thereby, to improved short- and long-term outcomes. Also, we should not assume that a greater degree of expertise and imaging capabilities at an academic university hospital translates to a more complete evaluation of PE; our research shows that this is not necessarily the case. PERTs may help mobilize resources that are underutilized even at academic university hospitals. Those interested in learning more about PERTs may contact the National PERT Consortium via email at [email protected] or go online to www.pertconsortium.org.
Rather than wallow in an alphabet soup of acronyms, let us place our acronymic clinical PE trials to good use. Gather the appropriate clinical data, consult experts in pulmonary embolism, and stratify patients admitted to the hospital with acute PE so that we can manage the expectations of short- and long-term outcomes.
When he became president in 1933, Franklin D. Roosevelt sought to lift the United States from the Great Depression, in part with a New Deal “alphabet soup” of programs. Like FDR, let us put our own alphabet soup – of PE studies and position statements – to good use: to work for the good of people hospitalized with acute pulmonary embolism.
Dr. Scharf is medical director of pulmonary outpatient services at the Jane and Leonard Korman Respiratory Institute at Jefferson Medical College, Philadelphia. He is also director of the pulmonary vascular disease program at Jefferson.
Who doesn’t like alphabet soup? Those noodles shaped as letters floating in a delicious hot broth serve as an educational way for young children to play with their food. Of course, this commentary is not about warm food suitable for a cold day but, instead, about another notion of alphabet soup – a metaphor for physicians’ failure to optimally utilize our alphabet soup of venous thromboembolism studies.
The medical literature that has studied the incidence, prevalence, diagnosis, and management of acute pulmonary embolism (PE) is vast. Yes, we have our own PE “alphabet soup” – a “hodgepodge especially of initials,” according to the Merriam-Webster Dictionary. ICOPER (International Cooperative Pulmonary Embolism Registry) and RIETE (Computerized Registry of Patients with Venous Thromboembolism) help estimate prevalence of the disease and indicate high-risk groups. PESI (Pulmonary Embolism Severity Index) and PERC (Pulmonary Embolism Rule-Out Criteria) provide useful predictive information prior to proceeding with diagnostic testing for PE. The PEITHO (Pulmonary Embolism Thrombolysis), MOPETT (Moderate Pulmonary Embolism Treated with Thrombolysis), and PERFECT (Pulmonary Embolism Response to Fragmentation, Embolectomy, and Catheter Thrombolysis) studies help to guide the decision to administer fibrinolytic therapy, particularly in the cases of so-called submassive or intermediate-risk PE.
And yet, in a study published in Hospital Practice (2017 Aug 30. doi: 10.1080/21548331.2017.1372033), my colleagues and I showed that, to a large extent, physicians admitting patients to the hospital between 2011 and 2013 for acute PE failed to order or perform even the most basic noninvasive testing on their patients, despite strong recommendations for such testing from both the 2011 American Heart Association (AHA) and the 2014 European Society of Cardiology as part of the risk assessment of acute PE.
At the time of the patients’ PE admissions, the 2011 AHA statement already had been published, and it was recognized that medical and interventional therapies needed to be appropriate to the characteristics of the patient with PE. Specifically, in order to determine a prognosis, assessment of right heart strain with an echocardiogram, ECG, and brain natriuretic peptide testing was recommended. For similar reasons, a serum troponin test was recommended to evaluate any myocardial necrosis.
While the 2011 AHA statement was based on a preponderance of evidence-based medicine, our research suggests that physicians may be doing a suboptimal job of collecting the necessary data to manage our acute PE patients. In defense of those physicians we evaluated, these data were collected just before the European Society of Cardiology disseminated their recommendations for risk stratification in 2014. The PEITHO, MOPETT, and PERFECT findings – though already presented at medical meetings in part – were not published in scientific journals in full until 2013 or thereafter.
Nevertheless, I believe these findings illustrate the merits of the Pulmonary Embolism Response Team (PERT) concept. As the data we studied suggest, not only might hospital-based physicians inadequately assess the severity of patients’ PE, but they also may fail to ask for consultations from the specialists who could be most useful in assisting in the proper evaluation and management of these patients. PERTs ensure that every patient admitted to the hospital with the diagnosis of PE will be assessed by a team of physicians with the expertise and professional interest in best managing these patients.
The expectation is that, with the institution of a PERT at each hospital, a more complete evaluation of patients’ PE may lead to optimal management and, thereby, to improved short- and long-term outcomes. Also, we should not assume that a greater degree of expertise and imaging capabilities at an academic university hospital translates to a more complete evaluation of PE; our research shows that this is not necessarily the case. PERTs may help mobilize resources that are underutilized even at academic university hospitals. Those interested in learning more about PERTs may contact the National PERT Consortium via email at [email protected] or go online to www.pertconsortium.org.
Rather than wallow in an alphabet soup of acronyms, let us place our acronymic clinical PE trials to good use. Gather the appropriate clinical data, consult experts in pulmonary embolism, and stratify patients admitted to the hospital with acute PE so that we can manage the expectations of short- and long-term outcomes.
When he became president in 1933, Franklin D. Roosevelt sought to lift the United States from the Great Depression, in part with a New Deal “alphabet soup” of programs. Like FDR, let us put our own alphabet soup – of PE studies and position statements – to good use: to work for the good of people hospitalized with acute pulmonary embolism.
Dr. Scharf is medical director of pulmonary outpatient services at the Jane and Leonard Korman Respiratory Institute at Jefferson Medical College, Philadelphia. He is also director of the pulmonary vascular disease program at Jefferson.