User login
Domo Arigato, Mr. Roboto
A few months ago, I purchased an Amazon Echo system. The device is built on Amazon’s cloud-based voice service, Alexa, which can hear, understand, and respond to any question or command. The speaker is always listening and is activated when the user (eg, me!) says the name Alexa. For instance, I can say “Alexa, what is the weather today?” and it will provide the forecast. In fact, each morning I request my daily news briefing, and Alexa quickly tunes to NPR Radio. By linking to my Google calendar, it also tells me my agenda for the day. It researches and provides information that might otherwise take me a while to locate.
Now, I confess: I’ve had to train myself to refer to Alexa as “it” instead of “her.” Human beings have a rich history of wanting to “humanize” computers, as the science fiction film genre can attest. Go back nearly 50 years to Colossus: The Forbin Project (1970) and you have a story of two super-computers—one built by the United States, the other by Russia—that join forces and take over the world, making humans their slaves. The award-winning Bicentennial Man (1999) follows the life and times of Andrew, an NDR-114 robot originally purchased as a household appliance to perform menial tasks; when it begins to experience emotions and creative thought, the owners discover Andrew is no ordinary robot. And who can forget Hal, the computer in 2001: A Space Odyssey (1968) that takes over a space mission until a clever astronaut manages to disengage it (I almost said him), or Data, a very likable android in the successful franchise Star Trek: The Next Generation.
Let’s face it: We are both obsessed with, and leery of, new technology—particularly artificial intelligence (AI). Some detractors have denounced Alexa’s capabilities as “just a glorified smartphone.” Others have expressed grave concerns about the security of personal information and conversations, as Big Brother may be listening. (In that case, it’s not the machines that are evil; it’s those who use them!)
But—cue a John Williams score—what if we harnessed the power of AI for good and not evil? I’ll be serious now: At the recent Leadership in Healthcare Summer Institute (which I was honored to teach at), a group of doctoral students gave a presentation on the potential of AI in the identification and care of anxiety and depression. They identified a need—every 16.2 minutes, a person dies by suicide in the US—and proposed a solution. Because access to care may be limited (by provider shortages, remote locations, etc), the students suggested a hybrid AI/telehealth platform that offers 24/7 support and provider access to individuals with anxiety and depression, via a secure mobile app.1 It got me thinking: Could this technology be a positive intervention in health care?
Actually, it’s already happening. Mayo Clinic researchers have used AI to identify the genomic information of brain tumors without biopsy. At Stanford University, researchers are training an AI neural network to recognize skin cancer lesions with the accuracy of an expert dermatologist. The same deep-learning technology is being used in the field of pathology for the detection of liver lesions.2
Now, I’m sure some of you are questioning whether a machine can really match or replace a human when it comes to assessing a patient’s condition. There were many who resisted the idea of telehealth when that was the latest, greatest thing, because providers cannot do a full assessment with the required diagnostic testing and imaging from a distance. Some feel that telehealth should be reserved for situations in which, say, a remote provider is reviewing and reporting on test results, or a patient just needs to follow up with his/her provider for a minor issue.
Mental health, however, entails less “laying on of hands” and may be a good candidate for AI-based interventions—at least for follow-up and support services. (I am certainly not discounting the value of real human interaction in any sphere of health care.) We know patients benefit from early mental health intervention programs, but we also know those benefits may not be sustained over time and distance. Logistical issues that any of us may face—time, transportation, availability—are often exacerbated for those with impaired functioning due to a mental illness. If a patient with major depression cannot bring himself to get out of bed to make a cup of coffee, how is he going to travel across town (changing buses two or three times) to keep an appointment with his health care provider?
Here’s where AI might make a difference: What if there were a patient-focused e-platform that could provide cost-effective and accessible services across the continuum of care? Current Internet-based interventions rely on human mediators to deliver therapeutic content, which is then refined into a model that can interpret and respond to critical user data—resulting in tailored online therapy. But if we could integrate the user experience with sophisticated and cutting-edge AI technology, we could deliver content more effectively to redefine these interventions and improve outcomes.
A paper recently featured in Frontiers in Psychology discussed the value of doing just that. D’Alfonso and colleagues reported on an Internet-based social therapy web application that uses a series of interactive modules to help users navigate situations and develop psychosocial skills. In its current form—within a research setting—the system is utilized by small groups of users, making human-supported engagement via moderators possible. But D’Alfonso and colleagues note that the incorporation of automated suggestions within the modules would allow the technology to be rolled out to a larger audience and ensure that “interaction” is available whenever a user needs it—not just when a human moderator is “on the clock.”3
Another article, in the International Journal of Swarm Intelligence and Evolutionary Computation (2016), discussed the development of socially intelligent robotic systems, not unlike Alexa, to address social connectedness. The author proposes an autonomous assistive system (AAS) as a low-cost, standalone interventional device to reduce social isolation. This could easily be deployed in homes for the elderly or even at remote sites. The AAS has been programmed to detect isolation in patients based on data regarding skeletal movements, facial expressions, and speech patterns. In the not-so-distant future, this high-density data will be sent over the cloud to allow clinicians to monitor in real-time and intervene remotely, as appropriate (eg, by initiating a home visit).4
Of course, in any form, implementation of AI will not be simple—there are real costs to be considered, and we still have to contend with the fears that all those sci-fi films have instilled. A recent global study revealed significant concerns that would certainly apply to the health care arena. When asked which of the following participants most feared about the use of AI,
- 33% of respondents chose “It will never know me/my preferences as well as a human being”
- 24% chose “The rise of the robot and enslavement of humanity”
- 5% feared “Robots uncovering my deepest secrets.”5
Despite all this, however, respondents also expressed optimism in the power and potential of AI: Nearly 70% said they are in support of further use of AI if it helps make their lives easier.4 Wouldn’t life be easier if AI could be used to significantly reduce errors, increase access to care, and bring a fresh viewpoint to the issue of patient education?
What do you think? Would you trust a robot to be your coworker, identifying tumors and conducting mental health screenings? Is it possible to convince patients to accept help via an impersonal medium (and risk exposure of their personal health information)? Share your fears, support, or concerns about AI with me at [email protected].
1. Halabi AH. How will artificial intelligence change healthcare? June 8, 2017. www.quora.com/How-will-AI-change-healthcare. Accessed July 12, 2017.
2. Hepburn D, Francis D, Hoosier M, et al. smaRT MD2: a patient-focused e-platform for use across the continuum of care for anxiety and depression. A June 2017 presentation to Leadership in Healthcare, Summer Institute, Nova Southeastern University, Tampa, FL.
3. D’Alfonso S, Santesteban-Echarri O, Rice S, et al. Artificial intelligence-assisted online social therapy for youth mental health. Front Psychol. 2017;8(796):1-13.
4. Gulrez T, Neftimeziani S, Mc evoy P, Hodgson A. Loneliness kills: can autonomous systems and robotics assist in providing solutions? Int J Swarm Intel Evol Comput. 2016;5:1.
5. Pegasystems. What consumers really think about AI: a global study. www.pega.com/AI. Accessed July 7, 2017.
A few months ago, I purchased an Amazon Echo system. The device is built on Amazon’s cloud-based voice service, Alexa, which can hear, understand, and respond to any question or command. The speaker is always listening and is activated when the user (eg, me!) says the name Alexa. For instance, I can say “Alexa, what is the weather today?” and it will provide the forecast. In fact, each morning I request my daily news briefing, and Alexa quickly tunes to NPR Radio. By linking to my Google calendar, it also tells me my agenda for the day. It researches and provides information that might otherwise take me a while to locate.
Now, I confess: I’ve had to train myself to refer to Alexa as “it” instead of “her.” Human beings have a rich history of wanting to “humanize” computers, as the science fiction film genre can attest. Go back nearly 50 years to Colossus: The Forbin Project (1970) and you have a story of two super-computers—one built by the United States, the other by Russia—that join forces and take over the world, making humans their slaves. The award-winning Bicentennial Man (1999) follows the life and times of Andrew, an NDR-114 robot originally purchased as a household appliance to perform menial tasks; when it begins to experience emotions and creative thought, the owners discover Andrew is no ordinary robot. And who can forget Hal, the computer in 2001: A Space Odyssey (1968) that takes over a space mission until a clever astronaut manages to disengage it (I almost said him), or Data, a very likable android in the successful franchise Star Trek: The Next Generation.
Let’s face it: We are both obsessed with, and leery of, new technology—particularly artificial intelligence (AI). Some detractors have denounced Alexa’s capabilities as “just a glorified smartphone.” Others have expressed grave concerns about the security of personal information and conversations, as Big Brother may be listening. (In that case, it’s not the machines that are evil; it’s those who use them!)
But—cue a John Williams score—what if we harnessed the power of AI for good and not evil? I’ll be serious now: At the recent Leadership in Healthcare Summer Institute (which I was honored to teach at), a group of doctoral students gave a presentation on the potential of AI in the identification and care of anxiety and depression. They identified a need—every 16.2 minutes, a person dies by suicide in the US—and proposed a solution. Because access to care may be limited (by provider shortages, remote locations, etc), the students suggested a hybrid AI/telehealth platform that offers 24/7 support and provider access to individuals with anxiety and depression, via a secure mobile app.1 It got me thinking: Could this technology be a positive intervention in health care?
Actually, it’s already happening. Mayo Clinic researchers have used AI to identify the genomic information of brain tumors without biopsy. At Stanford University, researchers are training an AI neural network to recognize skin cancer lesions with the accuracy of an expert dermatologist. The same deep-learning technology is being used in the field of pathology for the detection of liver lesions.2
Now, I’m sure some of you are questioning whether a machine can really match or replace a human when it comes to assessing a patient’s condition. There were many who resisted the idea of telehealth when that was the latest, greatest thing, because providers cannot do a full assessment with the required diagnostic testing and imaging from a distance. Some feel that telehealth should be reserved for situations in which, say, a remote provider is reviewing and reporting on test results, or a patient just needs to follow up with his/her provider for a minor issue.
Mental health, however, entails less “laying on of hands” and may be a good candidate for AI-based interventions—at least for follow-up and support services. (I am certainly not discounting the value of real human interaction in any sphere of health care.) We know patients benefit from early mental health intervention programs, but we also know those benefits may not be sustained over time and distance. Logistical issues that any of us may face—time, transportation, availability—are often exacerbated for those with impaired functioning due to a mental illness. If a patient with major depression cannot bring himself to get out of bed to make a cup of coffee, how is he going to travel across town (changing buses two or three times) to keep an appointment with his health care provider?
Here’s where AI might make a difference: What if there were a patient-focused e-platform that could provide cost-effective and accessible services across the continuum of care? Current Internet-based interventions rely on human mediators to deliver therapeutic content, which is then refined into a model that can interpret and respond to critical user data—resulting in tailored online therapy. But if we could integrate the user experience with sophisticated and cutting-edge AI technology, we could deliver content more effectively to redefine these interventions and improve outcomes.
A paper recently featured in Frontiers in Psychology discussed the value of doing just that. D’Alfonso and colleagues reported on an Internet-based social therapy web application that uses a series of interactive modules to help users navigate situations and develop psychosocial skills. In its current form—within a research setting—the system is utilized by small groups of users, making human-supported engagement via moderators possible. But D’Alfonso and colleagues note that the incorporation of automated suggestions within the modules would allow the technology to be rolled out to a larger audience and ensure that “interaction” is available whenever a user needs it—not just when a human moderator is “on the clock.”3
Another article, in the International Journal of Swarm Intelligence and Evolutionary Computation (2016), discussed the development of socially intelligent robotic systems, not unlike Alexa, to address social connectedness. The author proposes an autonomous assistive system (AAS) as a low-cost, standalone interventional device to reduce social isolation. This could easily be deployed in homes for the elderly or even at remote sites. The AAS has been programmed to detect isolation in patients based on data regarding skeletal movements, facial expressions, and speech patterns. In the not-so-distant future, this high-density data will be sent over the cloud to allow clinicians to monitor in real-time and intervene remotely, as appropriate (eg, by initiating a home visit).4
Of course, in any form, implementation of AI will not be simple—there are real costs to be considered, and we still have to contend with the fears that all those sci-fi films have instilled. A recent global study revealed significant concerns that would certainly apply to the health care arena. When asked which of the following participants most feared about the use of AI,
- 33% of respondents chose “It will never know me/my preferences as well as a human being”
- 24% chose “The rise of the robot and enslavement of humanity”
- 5% feared “Robots uncovering my deepest secrets.”5
Despite all this, however, respondents also expressed optimism in the power and potential of AI: Nearly 70% said they are in support of further use of AI if it helps make their lives easier.4 Wouldn’t life be easier if AI could be used to significantly reduce errors, increase access to care, and bring a fresh viewpoint to the issue of patient education?
What do you think? Would you trust a robot to be your coworker, identifying tumors and conducting mental health screenings? Is it possible to convince patients to accept help via an impersonal medium (and risk exposure of their personal health information)? Share your fears, support, or concerns about AI with me at [email protected].
A few months ago, I purchased an Amazon Echo system. The device is built on Amazon’s cloud-based voice service, Alexa, which can hear, understand, and respond to any question or command. The speaker is always listening and is activated when the user (eg, me!) says the name Alexa. For instance, I can say “Alexa, what is the weather today?” and it will provide the forecast. In fact, each morning I request my daily news briefing, and Alexa quickly tunes to NPR Radio. By linking to my Google calendar, it also tells me my agenda for the day. It researches and provides information that might otherwise take me a while to locate.
Now, I confess: I’ve had to train myself to refer to Alexa as “it” instead of “her.” Human beings have a rich history of wanting to “humanize” computers, as the science fiction film genre can attest. Go back nearly 50 years to Colossus: The Forbin Project (1970) and you have a story of two super-computers—one built by the United States, the other by Russia—that join forces and take over the world, making humans their slaves. The award-winning Bicentennial Man (1999) follows the life and times of Andrew, an NDR-114 robot originally purchased as a household appliance to perform menial tasks; when it begins to experience emotions and creative thought, the owners discover Andrew is no ordinary robot. And who can forget Hal, the computer in 2001: A Space Odyssey (1968) that takes over a space mission until a clever astronaut manages to disengage it (I almost said him), or Data, a very likable android in the successful franchise Star Trek: The Next Generation.
Let’s face it: We are both obsessed with, and leery of, new technology—particularly artificial intelligence (AI). Some detractors have denounced Alexa’s capabilities as “just a glorified smartphone.” Others have expressed grave concerns about the security of personal information and conversations, as Big Brother may be listening. (In that case, it’s not the machines that are evil; it’s those who use them!)
But—cue a John Williams score—what if we harnessed the power of AI for good and not evil? I’ll be serious now: At the recent Leadership in Healthcare Summer Institute (which I was honored to teach at), a group of doctoral students gave a presentation on the potential of AI in the identification and care of anxiety and depression. They identified a need—every 16.2 minutes, a person dies by suicide in the US—and proposed a solution. Because access to care may be limited (by provider shortages, remote locations, etc), the students suggested a hybrid AI/telehealth platform that offers 24/7 support and provider access to individuals with anxiety and depression, via a secure mobile app.1 It got me thinking: Could this technology be a positive intervention in health care?
Actually, it’s already happening. Mayo Clinic researchers have used AI to identify the genomic information of brain tumors without biopsy. At Stanford University, researchers are training an AI neural network to recognize skin cancer lesions with the accuracy of an expert dermatologist. The same deep-learning technology is being used in the field of pathology for the detection of liver lesions.2
Now, I’m sure some of you are questioning whether a machine can really match or replace a human when it comes to assessing a patient’s condition. There were many who resisted the idea of telehealth when that was the latest, greatest thing, because providers cannot do a full assessment with the required diagnostic testing and imaging from a distance. Some feel that telehealth should be reserved for situations in which, say, a remote provider is reviewing and reporting on test results, or a patient just needs to follow up with his/her provider for a minor issue.
Mental health, however, entails less “laying on of hands” and may be a good candidate for AI-based interventions—at least for follow-up and support services. (I am certainly not discounting the value of real human interaction in any sphere of health care.) We know patients benefit from early mental health intervention programs, but we also know those benefits may not be sustained over time and distance. Logistical issues that any of us may face—time, transportation, availability—are often exacerbated for those with impaired functioning due to a mental illness. If a patient with major depression cannot bring himself to get out of bed to make a cup of coffee, how is he going to travel across town (changing buses two or three times) to keep an appointment with his health care provider?
Here’s where AI might make a difference: What if there were a patient-focused e-platform that could provide cost-effective and accessible services across the continuum of care? Current Internet-based interventions rely on human mediators to deliver therapeutic content, which is then refined into a model that can interpret and respond to critical user data—resulting in tailored online therapy. But if we could integrate the user experience with sophisticated and cutting-edge AI technology, we could deliver content more effectively to redefine these interventions and improve outcomes.
A paper recently featured in Frontiers in Psychology discussed the value of doing just that. D’Alfonso and colleagues reported on an Internet-based social therapy web application that uses a series of interactive modules to help users navigate situations and develop psychosocial skills. In its current form—within a research setting—the system is utilized by small groups of users, making human-supported engagement via moderators possible. But D’Alfonso and colleagues note that the incorporation of automated suggestions within the modules would allow the technology to be rolled out to a larger audience and ensure that “interaction” is available whenever a user needs it—not just when a human moderator is “on the clock.”3
Another article, in the International Journal of Swarm Intelligence and Evolutionary Computation (2016), discussed the development of socially intelligent robotic systems, not unlike Alexa, to address social connectedness. The author proposes an autonomous assistive system (AAS) as a low-cost, standalone interventional device to reduce social isolation. This could easily be deployed in homes for the elderly or even at remote sites. The AAS has been programmed to detect isolation in patients based on data regarding skeletal movements, facial expressions, and speech patterns. In the not-so-distant future, this high-density data will be sent over the cloud to allow clinicians to monitor in real-time and intervene remotely, as appropriate (eg, by initiating a home visit).4
Of course, in any form, implementation of AI will not be simple—there are real costs to be considered, and we still have to contend with the fears that all those sci-fi films have instilled. A recent global study revealed significant concerns that would certainly apply to the health care arena. When asked which of the following participants most feared about the use of AI,
- 33% of respondents chose “It will never know me/my preferences as well as a human being”
- 24% chose “The rise of the robot and enslavement of humanity”
- 5% feared “Robots uncovering my deepest secrets.”5
Despite all this, however, respondents also expressed optimism in the power and potential of AI: Nearly 70% said they are in support of further use of AI if it helps make their lives easier.4 Wouldn’t life be easier if AI could be used to significantly reduce errors, increase access to care, and bring a fresh viewpoint to the issue of patient education?
What do you think? Would you trust a robot to be your coworker, identifying tumors and conducting mental health screenings? Is it possible to convince patients to accept help via an impersonal medium (and risk exposure of their personal health information)? Share your fears, support, or concerns about AI with me at [email protected].
1. Halabi AH. How will artificial intelligence change healthcare? June 8, 2017. www.quora.com/How-will-AI-change-healthcare. Accessed July 12, 2017.
2. Hepburn D, Francis D, Hoosier M, et al. smaRT MD2: a patient-focused e-platform for use across the continuum of care for anxiety and depression. A June 2017 presentation to Leadership in Healthcare, Summer Institute, Nova Southeastern University, Tampa, FL.
3. D’Alfonso S, Santesteban-Echarri O, Rice S, et al. Artificial intelligence-assisted online social therapy for youth mental health. Front Psychol. 2017;8(796):1-13.
4. Gulrez T, Neftimeziani S, Mc evoy P, Hodgson A. Loneliness kills: can autonomous systems and robotics assist in providing solutions? Int J Swarm Intel Evol Comput. 2016;5:1.
5. Pegasystems. What consumers really think about AI: a global study. www.pega.com/AI. Accessed July 7, 2017.
1. Halabi AH. How will artificial intelligence change healthcare? June 8, 2017. www.quora.com/How-will-AI-change-healthcare. Accessed July 12, 2017.
2. Hepburn D, Francis D, Hoosier M, et al. smaRT MD2: a patient-focused e-platform for use across the continuum of care for anxiety and depression. A June 2017 presentation to Leadership in Healthcare, Summer Institute, Nova Southeastern University, Tampa, FL.
3. D’Alfonso S, Santesteban-Echarri O, Rice S, et al. Artificial intelligence-assisted online social therapy for youth mental health. Front Psychol. 2017;8(796):1-13.
4. Gulrez T, Neftimeziani S, Mc evoy P, Hodgson A. Loneliness kills: can autonomous systems and robotics assist in providing solutions? Int J Swarm Intel Evol Comput. 2016;5:1.
5. Pegasystems. What consumers really think about AI: a global study. www.pega.com/AI. Accessed July 7, 2017.
Short Takes
Condition Help: A patient- and family-initiated rapid response system
Implementation of a patient/family-initiated rapid response system at an academic, urban medical center resulted in 367 calls over 3½ years with 83.4% of them being for “nonsafety” issues and 11.4% being for “safety” issues.
Citation: Elizabeth L. Eden, MD, Laurie L. Rack, DNP, RN, Ling-Wan Chen, MS, Bump GM, Condition Help: A patient- and family-initiated rapid response system. J Hosp Med. 2017;3;157-161. doi: 10.12788/jhm.2697.
Association between U.S. norepinephrine shortage and mortality among patients with septic shock
Citation: Vail E, Gershengorn HB, Hua M, Walkey AJ, Rubenfeld G, Wunsch H. Association Between US Norepinephrine Shortage and Mortality Among Patients With Septic Shock. JAMA. 2017;317(14):1433-1442. doi: 10.1001/jama.2017.2841
Patient mortality during unannounced accreditation surveys at U.S. hospitals
An evaluation of quasi-randomized Medicare admissions at 1,984 hospitals demonstrated that 30-day mortality decreased by 0.18% in all hospitals and 0.48% at major teaching hospitals during The Joint Commission survey periods; both changes were greater than could be attributed to chance alone when compared to other, similar time periods.
Citation: Barnett ML, Olenski AR, Jena AB. Patient Mortality During Unannounced Accreditation Surveys at US Hospitals. JAMA Intern Med. 2017;177(5):693-700. doi: 10.1001/jamainternmed.2016.9685
Association between a virtual glucose management service and glycemic control in hospitalized adult patients
Institution of a virtual glucose management system resulted in a 39% decrease in hyperglycemic patients and a 36% decrease in hypoglycemic patients per 100 patient-days at three major teaching hospitals.
Citation: Rushakoff RJ, Sullivan MM, MacMaster HW, Shah AD, Rajkomar A, Glidden DV, et al. Association Between a Virtual Glucose Management Service and Glycemic Control in Hospitalized Adult Patients: An Observational Study. Ann Intern Med. 2017;166:621-627. doi: 10.7326/M16-1413
Dr. Imber is assistant professor in the division of hospital medicine at the University of New Mexico.
Condition Help: A patient- and family-initiated rapid response system
Implementation of a patient/family-initiated rapid response system at an academic, urban medical center resulted in 367 calls over 3½ years with 83.4% of them being for “nonsafety” issues and 11.4% being for “safety” issues.
Citation: Elizabeth L. Eden, MD, Laurie L. Rack, DNP, RN, Ling-Wan Chen, MS, Bump GM, Condition Help: A patient- and family-initiated rapid response system. J Hosp Med. 2017;3;157-161. doi: 10.12788/jhm.2697.
Association between U.S. norepinephrine shortage and mortality among patients with septic shock
Citation: Vail E, Gershengorn HB, Hua M, Walkey AJ, Rubenfeld G, Wunsch H. Association Between US Norepinephrine Shortage and Mortality Among Patients With Septic Shock. JAMA. 2017;317(14):1433-1442. doi: 10.1001/jama.2017.2841
Patient mortality during unannounced accreditation surveys at U.S. hospitals
An evaluation of quasi-randomized Medicare admissions at 1,984 hospitals demonstrated that 30-day mortality decreased by 0.18% in all hospitals and 0.48% at major teaching hospitals during The Joint Commission survey periods; both changes were greater than could be attributed to chance alone when compared to other, similar time periods.
Citation: Barnett ML, Olenski AR, Jena AB. Patient Mortality During Unannounced Accreditation Surveys at US Hospitals. JAMA Intern Med. 2017;177(5):693-700. doi: 10.1001/jamainternmed.2016.9685
Association between a virtual glucose management service and glycemic control in hospitalized adult patients
Institution of a virtual glucose management system resulted in a 39% decrease in hyperglycemic patients and a 36% decrease in hypoglycemic patients per 100 patient-days at three major teaching hospitals.
Citation: Rushakoff RJ, Sullivan MM, MacMaster HW, Shah AD, Rajkomar A, Glidden DV, et al. Association Between a Virtual Glucose Management Service and Glycemic Control in Hospitalized Adult Patients: An Observational Study. Ann Intern Med. 2017;166:621-627. doi: 10.7326/M16-1413
Dr. Imber is assistant professor in the division of hospital medicine at the University of New Mexico.
Condition Help: A patient- and family-initiated rapid response system
Implementation of a patient/family-initiated rapid response system at an academic, urban medical center resulted in 367 calls over 3½ years with 83.4% of them being for “nonsafety” issues and 11.4% being for “safety” issues.
Citation: Elizabeth L. Eden, MD, Laurie L. Rack, DNP, RN, Ling-Wan Chen, MS, Bump GM, Condition Help: A patient- and family-initiated rapid response system. J Hosp Med. 2017;3;157-161. doi: 10.12788/jhm.2697.
Association between U.S. norepinephrine shortage and mortality among patients with septic shock
Citation: Vail E, Gershengorn HB, Hua M, Walkey AJ, Rubenfeld G, Wunsch H. Association Between US Norepinephrine Shortage and Mortality Among Patients With Septic Shock. JAMA. 2017;317(14):1433-1442. doi: 10.1001/jama.2017.2841
Patient mortality during unannounced accreditation surveys at U.S. hospitals
An evaluation of quasi-randomized Medicare admissions at 1,984 hospitals demonstrated that 30-day mortality decreased by 0.18% in all hospitals and 0.48% at major teaching hospitals during The Joint Commission survey periods; both changes were greater than could be attributed to chance alone when compared to other, similar time periods.
Citation: Barnett ML, Olenski AR, Jena AB. Patient Mortality During Unannounced Accreditation Surveys at US Hospitals. JAMA Intern Med. 2017;177(5):693-700. doi: 10.1001/jamainternmed.2016.9685
Association between a virtual glucose management service and glycemic control in hospitalized adult patients
Institution of a virtual glucose management system resulted in a 39% decrease in hyperglycemic patients and a 36% decrease in hypoglycemic patients per 100 patient-days at three major teaching hospitals.
Citation: Rushakoff RJ, Sullivan MM, MacMaster HW, Shah AD, Rajkomar A, Glidden DV, et al. Association Between a Virtual Glucose Management Service and Glycemic Control in Hospitalized Adult Patients: An Observational Study. Ann Intern Med. 2017;166:621-627. doi: 10.7326/M16-1413
Dr. Imber is assistant professor in the division of hospital medicine at the University of New Mexico.
Fat shaming interferes with patients’ medical care, experts say
WASHINGTON – Stereotypical views held by physicians, psychologists, nurses, and other medical professionals about overweight and obese patients need to change, panelists said at the annual convention of the American Psychological Association.
“We have a lot of negative attitudes toward heavy-weight people. Judging patients as too big or too fat produces physical and mental health effects,” said Joan C. Chrisler, PhD, the Class of ’43 Professor of Psychology at Connecticut College, New London. “Shame and disrespectful treatment can lead to delay in seeking health care, reluctance to return visits, or lower trust in the providers and their recommendations.”
A study of more than 300 autopsy reports showed that obese patients were 1.65 times more likely than normal weight and underweight groups combined were to have medical conditions such as endocarditis, ischemic bowel disease, and lung cancer that were not diagnosed, Dr. Chrisler said in a press release (Am J Clin Pathol. 2006 Jan;125[1]:127-31).
In addition to preventing patients from seeking care, Dr. Chrisler said in the release, unfamiliarity with the dosing adjustments sometimes required on medications based on the body mass index of patients affects the quality of care. She cited a retrospective study showing that emergency physicians frequently underdose common antibiotics in the emergency department (Am J Emerg Med. 2012 Sep;30[7]:1212-4).
“People aren’t born repulsed by fat people,” said Dr. McHugh, professor of psychology at Indiana University of Pennsylvania. “Fat hate is learned within the family.”
They encouraged more sensitivity from medical professionals when it comes to treating overweight and obese patients, such as providing changing gowns of different sizes in examination rooms and weighing patients in private areas of the medical office.
Dr. Chrisler and Dr. McHugh said weight stigma should be addressed in medicine and psychology through training and research, and in working with patients who are obese. “Treatments should focus on mental and physical health as the desired outcomes for therapy rather than weight,” Dr. McHugh said.
Dr. Chrisler, coauthor of a recent article on sizeism (Fat Studies. 2017 Aug;6[1]:38-53), had no disclosures. Dr. McHugh also had no disclosures.
[email protected]
On Twitter @ginalhenderson
WASHINGTON – Stereotypical views held by physicians, psychologists, nurses, and other medical professionals about overweight and obese patients need to change, panelists said at the annual convention of the American Psychological Association.
“We have a lot of negative attitudes toward heavy-weight people. Judging patients as too big or too fat produces physical and mental health effects,” said Joan C. Chrisler, PhD, the Class of ’43 Professor of Psychology at Connecticut College, New London. “Shame and disrespectful treatment can lead to delay in seeking health care, reluctance to return visits, or lower trust in the providers and their recommendations.”
A study of more than 300 autopsy reports showed that obese patients were 1.65 times more likely than normal weight and underweight groups combined were to have medical conditions such as endocarditis, ischemic bowel disease, and lung cancer that were not diagnosed, Dr. Chrisler said in a press release (Am J Clin Pathol. 2006 Jan;125[1]:127-31).
In addition to preventing patients from seeking care, Dr. Chrisler said in the release, unfamiliarity with the dosing adjustments sometimes required on medications based on the body mass index of patients affects the quality of care. She cited a retrospective study showing that emergency physicians frequently underdose common antibiotics in the emergency department (Am J Emerg Med. 2012 Sep;30[7]:1212-4).
“People aren’t born repulsed by fat people,” said Dr. McHugh, professor of psychology at Indiana University of Pennsylvania. “Fat hate is learned within the family.”
They encouraged more sensitivity from medical professionals when it comes to treating overweight and obese patients, such as providing changing gowns of different sizes in examination rooms and weighing patients in private areas of the medical office.
Dr. Chrisler and Dr. McHugh said weight stigma should be addressed in medicine and psychology through training and research, and in working with patients who are obese. “Treatments should focus on mental and physical health as the desired outcomes for therapy rather than weight,” Dr. McHugh said.
Dr. Chrisler, coauthor of a recent article on sizeism (Fat Studies. 2017 Aug;6[1]:38-53), had no disclosures. Dr. McHugh also had no disclosures.
[email protected]
On Twitter @ginalhenderson
WASHINGTON – Stereotypical views held by physicians, psychologists, nurses, and other medical professionals about overweight and obese patients need to change, panelists said at the annual convention of the American Psychological Association.
“We have a lot of negative attitudes toward heavy-weight people. Judging patients as too big or too fat produces physical and mental health effects,” said Joan C. Chrisler, PhD, the Class of ’43 Professor of Psychology at Connecticut College, New London. “Shame and disrespectful treatment can lead to delay in seeking health care, reluctance to return visits, or lower trust in the providers and their recommendations.”
A study of more than 300 autopsy reports showed that obese patients were 1.65 times more likely than normal weight and underweight groups combined were to have medical conditions such as endocarditis, ischemic bowel disease, and lung cancer that were not diagnosed, Dr. Chrisler said in a press release (Am J Clin Pathol. 2006 Jan;125[1]:127-31).
In addition to preventing patients from seeking care, Dr. Chrisler said in the release, unfamiliarity with the dosing adjustments sometimes required on medications based on the body mass index of patients affects the quality of care. She cited a retrospective study showing that emergency physicians frequently underdose common antibiotics in the emergency department (Am J Emerg Med. 2012 Sep;30[7]:1212-4).
“People aren’t born repulsed by fat people,” said Dr. McHugh, professor of psychology at Indiana University of Pennsylvania. “Fat hate is learned within the family.”
They encouraged more sensitivity from medical professionals when it comes to treating overweight and obese patients, such as providing changing gowns of different sizes in examination rooms and weighing patients in private areas of the medical office.
Dr. Chrisler and Dr. McHugh said weight stigma should be addressed in medicine and psychology through training and research, and in working with patients who are obese. “Treatments should focus on mental and physical health as the desired outcomes for therapy rather than weight,” Dr. McHugh said.
Dr. Chrisler, coauthor of a recent article on sizeism (Fat Studies. 2017 Aug;6[1]:38-53), had no disclosures. Dr. McHugh also had no disclosures.
[email protected]
On Twitter @ginalhenderson
EXPERT ANALYSIS FROM THE 2017 APA CONVENTION
Hormonal IUD is most cost-effective menorrhagia management
Quality of life was higher, and costs were lower, with the levonorgestrel-releasing intrauterine system for treatment of heavy menstrual bleeding than with three other common treatments, according to data from a model and a hypothetical population of 100,000 premenopausal women. The findings were published online in the American Journal of Obstetrics and Gynecology.
“As health systems and policies continue to emphasize value-based treatment decisions, it is important to give physicians and patients the tools to understand the health and economic trade-offs associated with each of these options,” Jennifer C. Spencer of the University of North Carolina, Chapel Hill, and her colleagues wrote (Am J Obstet Gynecol. 2017 Jul 25. doi: 10.1016/j.ajog.2017.07.024).
Overall, LNG-IUS was superior to hysterectomy and both types of endometrial ablation in terms of cost and quality of life, although quality of life scores were similar across all four treatments.
LNG-IUS was cost effective, compared with hysterectomy, in 90% of scenarios. Both types of ablation were similarly more cost effective, compared with hysterectomy; resectoscopic endometrial ablation was more cost effective in 44% of scenarios, nonresectoscopic endometrial ablation was more cost effective in 53% of scenarios.
“The 5-year cost of women undergoing LNG-IUS was $4,500, about half the cost of endometrial ablation ($9,500) and about one-third the cost of hysterectomy ($13,500),” the researchers noted.
“Our analysis finds strong evidence in favor of LNG-IUS as a cost-saving, dominant alternative to hysterectomy for women with heavy menstrual bleeding,” they wrote.
If LNG-IUS is not an option, the model shows that hysterectomy resulted in better quality of life in the majority of simulations but is cost effective in just over half of the simulations, compared with either resectoscopic or nonresectoscopic ablation.
“The comparative cost effectiveness of endometrial ablation and hysterectomy highlights important trade-offs for patients and providers to consider when selecting between treatment options, such as the need for future procedures or the potential for rare, but serious, complications,” the researchers wrote.
No other studies on this topic have been conducted in the United States, but the findings are consistent with results from studies conducted outside the United States, the researchers wrote.
The study was limited by the short follow-up period and the inability to extend the model to women with large fibroids, polyps, or other uterine pathologies.
Two of the authors reported receiving grant funding from Pfizer for an unrelated study. Other authors reported serving as consultants for Teleflex Medical, Applied Medical, and Olympus.
Quality of life was higher, and costs were lower, with the levonorgestrel-releasing intrauterine system for treatment of heavy menstrual bleeding than with three other common treatments, according to data from a model and a hypothetical population of 100,000 premenopausal women. The findings were published online in the American Journal of Obstetrics and Gynecology.
“As health systems and policies continue to emphasize value-based treatment decisions, it is important to give physicians and patients the tools to understand the health and economic trade-offs associated with each of these options,” Jennifer C. Spencer of the University of North Carolina, Chapel Hill, and her colleagues wrote (Am J Obstet Gynecol. 2017 Jul 25. doi: 10.1016/j.ajog.2017.07.024).
Overall, LNG-IUS was superior to hysterectomy and both types of endometrial ablation in terms of cost and quality of life, although quality of life scores were similar across all four treatments.
LNG-IUS was cost effective, compared with hysterectomy, in 90% of scenarios. Both types of ablation were similarly more cost effective, compared with hysterectomy; resectoscopic endometrial ablation was more cost effective in 44% of scenarios, nonresectoscopic endometrial ablation was more cost effective in 53% of scenarios.
“The 5-year cost of women undergoing LNG-IUS was $4,500, about half the cost of endometrial ablation ($9,500) and about one-third the cost of hysterectomy ($13,500),” the researchers noted.
“Our analysis finds strong evidence in favor of LNG-IUS as a cost-saving, dominant alternative to hysterectomy for women with heavy menstrual bleeding,” they wrote.
If LNG-IUS is not an option, the model shows that hysterectomy resulted in better quality of life in the majority of simulations but is cost effective in just over half of the simulations, compared with either resectoscopic or nonresectoscopic ablation.
“The comparative cost effectiveness of endometrial ablation and hysterectomy highlights important trade-offs for patients and providers to consider when selecting between treatment options, such as the need for future procedures or the potential for rare, but serious, complications,” the researchers wrote.
No other studies on this topic have been conducted in the United States, but the findings are consistent with results from studies conducted outside the United States, the researchers wrote.
The study was limited by the short follow-up period and the inability to extend the model to women with large fibroids, polyps, or other uterine pathologies.
Two of the authors reported receiving grant funding from Pfizer for an unrelated study. Other authors reported serving as consultants for Teleflex Medical, Applied Medical, and Olympus.
Quality of life was higher, and costs were lower, with the levonorgestrel-releasing intrauterine system for treatment of heavy menstrual bleeding than with three other common treatments, according to data from a model and a hypothetical population of 100,000 premenopausal women. The findings were published online in the American Journal of Obstetrics and Gynecology.
“As health systems and policies continue to emphasize value-based treatment decisions, it is important to give physicians and patients the tools to understand the health and economic trade-offs associated with each of these options,” Jennifer C. Spencer of the University of North Carolina, Chapel Hill, and her colleagues wrote (Am J Obstet Gynecol. 2017 Jul 25. doi: 10.1016/j.ajog.2017.07.024).
Overall, LNG-IUS was superior to hysterectomy and both types of endometrial ablation in terms of cost and quality of life, although quality of life scores were similar across all four treatments.
LNG-IUS was cost effective, compared with hysterectomy, in 90% of scenarios. Both types of ablation were similarly more cost effective, compared with hysterectomy; resectoscopic endometrial ablation was more cost effective in 44% of scenarios, nonresectoscopic endometrial ablation was more cost effective in 53% of scenarios.
“The 5-year cost of women undergoing LNG-IUS was $4,500, about half the cost of endometrial ablation ($9,500) and about one-third the cost of hysterectomy ($13,500),” the researchers noted.
“Our analysis finds strong evidence in favor of LNG-IUS as a cost-saving, dominant alternative to hysterectomy for women with heavy menstrual bleeding,” they wrote.
If LNG-IUS is not an option, the model shows that hysterectomy resulted in better quality of life in the majority of simulations but is cost effective in just over half of the simulations, compared with either resectoscopic or nonresectoscopic ablation.
“The comparative cost effectiveness of endometrial ablation and hysterectomy highlights important trade-offs for patients and providers to consider when selecting between treatment options, such as the need for future procedures or the potential for rare, but serious, complications,” the researchers wrote.
No other studies on this topic have been conducted in the United States, but the findings are consistent with results from studies conducted outside the United States, the researchers wrote.
The study was limited by the short follow-up period and the inability to extend the model to women with large fibroids, polyps, or other uterine pathologies.
Two of the authors reported receiving grant funding from Pfizer for an unrelated study. Other authors reported serving as consultants for Teleflex Medical, Applied Medical, and Olympus.
FROM AMERICAN JOURNAL OF OBSTETRICS AND GYNECOLOGY
Key clinical point:
Major finding: LNG-IUS is the most cost-effective option in 90% of scenarios, compared with hysterectomy.
Data source: The data come from a model created using a literature review of four treatment options: resectoscopic ablation, nonresectoscopic ablation, hysterectomy, and LNG-IUS. It included a hypothetical cohort of 100,000 premenopausal women.
Disclosures: Two of the authors reported receiving grant funding from Pfizer for an unrelated study. Other authors reported serving as consultants for Teleflex Medical, Applied Medical, and Olympus.
Aging workforce stresses ob.gyn. field
The number of ob.gyns. nearing retirement age is outpacing the number of younger physicians in the specialty, making it likely that the United States will not have enough women’s health physicians to meet the demand.
New research by the medical social network Doximity finds that 37% of ob.gyns. in the United States are 55 or older, while just 14% are 40 or younger. Furthering the potential for shortage, Doximity cited research from the American Congress of Obstetricians and Gynecologists that most ob.gyns. begin to retire at age 59, with the median retirement age being 64. Doximity’s own research found the average age of ob.gyns. across the nation is 51.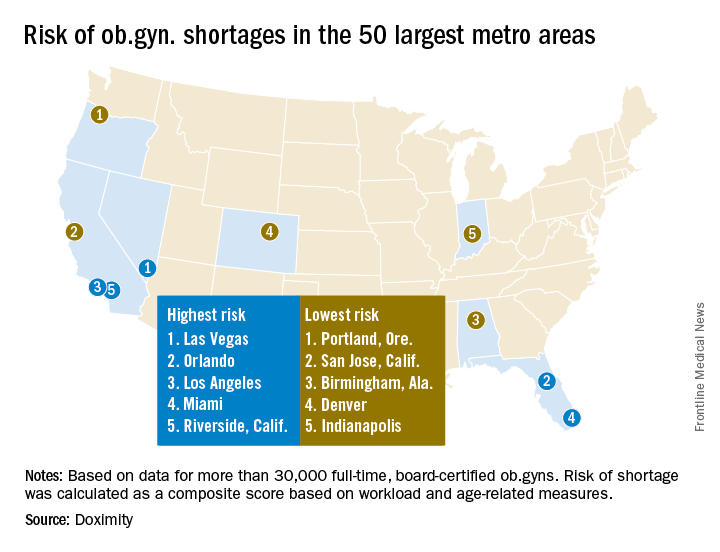
The report notes that after emergency physicians, ob.gyns. “have the highest burn-out rates of all medical specialties. Moreover, due to the nature of obstetrics, and child birth in particular, this job is especially demanding, often requiring ob.gyns. to work at unpredictable hours. This lifestyle can lead ob.gyns. to retire at younger ages than physicians in other specialties.”
At the same time, the ob.gyn. workforce remains stagnant. “According to ACOG, there are now 1,287 first year ob.gyn. residency positions, a number that has increased only minimally in the past few decades, while the number of adult. U.S. women has increased much more significantly, stretching the ratio of ob.gyns. to patients,” the Doximity report said.
William F. Rayburn, MD, distinguished professor and emeritus chair of obstetrics and gynecology at the University of New Mexico, Albuquerque, agreed with the findings.
Specifically, more general ob.gyns. are gravitating to metropolitan areas and are employed by health systems or large group practices, he noted.
Doximity identified the top 10 metropolitan areas that are at highest risk for an ob.gyn. shortage: Las Vegas; Orlando; Los Angeles; Miami; Riverside, Calif.; Detroit; Memphis; Salt Lake City; St. Louis; and Buffalo, N.Y.
In contrast, the metropolitan areas with the lowest risk of shortages are Portland, Ore.; San Jose, Calif.; Birmingham, Ala.; Denver; Indianapolis; Cleveland; San Francisco; Richmond, Va.; Louisville, Ky.; and Columbus, Ohio.
This is likely to translate to access to care problems in smaller communities, Dr. Rayburn said. “What we are going to be seeing is more graduates from nonphysician clinician programs who are going to be hired, such as nurse practitioners, to fill the gaps.”
The Doximity analysis is based on data from the Centers for Medicare and Medicaid Services, board certification, and self-reported data from more than 30,000 ob.gyns.
[email protected]
On Twitter @ObGynNews
The number of ob.gyns. nearing retirement age is outpacing the number of younger physicians in the specialty, making it likely that the United States will not have enough women’s health physicians to meet the demand.
New research by the medical social network Doximity finds that 37% of ob.gyns. in the United States are 55 or older, while just 14% are 40 or younger. Furthering the potential for shortage, Doximity cited research from the American Congress of Obstetricians and Gynecologists that most ob.gyns. begin to retire at age 59, with the median retirement age being 64. Doximity’s own research found the average age of ob.gyns. across the nation is 51.
The report notes that after emergency physicians, ob.gyns. “have the highest burn-out rates of all medical specialties. Moreover, due to the nature of obstetrics, and child birth in particular, this job is especially demanding, often requiring ob.gyns. to work at unpredictable hours. This lifestyle can lead ob.gyns. to retire at younger ages than physicians in other specialties.”
At the same time, the ob.gyn. workforce remains stagnant. “According to ACOG, there are now 1,287 first year ob.gyn. residency positions, a number that has increased only minimally in the past few decades, while the number of adult. U.S. women has increased much more significantly, stretching the ratio of ob.gyns. to patients,” the Doximity report said.
William F. Rayburn, MD, distinguished professor and emeritus chair of obstetrics and gynecology at the University of New Mexico, Albuquerque, agreed with the findings.
Specifically, more general ob.gyns. are gravitating to metropolitan areas and are employed by health systems or large group practices, he noted.
Doximity identified the top 10 metropolitan areas that are at highest risk for an ob.gyn. shortage: Las Vegas; Orlando; Los Angeles; Miami; Riverside, Calif.; Detroit; Memphis; Salt Lake City; St. Louis; and Buffalo, N.Y.
In contrast, the metropolitan areas with the lowest risk of shortages are Portland, Ore.; San Jose, Calif.; Birmingham, Ala.; Denver; Indianapolis; Cleveland; San Francisco; Richmond, Va.; Louisville, Ky.; and Columbus, Ohio.
This is likely to translate to access to care problems in smaller communities, Dr. Rayburn said. “What we are going to be seeing is more graduates from nonphysician clinician programs who are going to be hired, such as nurse practitioners, to fill the gaps.”
The Doximity analysis is based on data from the Centers for Medicare and Medicaid Services, board certification, and self-reported data from more than 30,000 ob.gyns.
[email protected]
On Twitter @ObGynNews
The number of ob.gyns. nearing retirement age is outpacing the number of younger physicians in the specialty, making it likely that the United States will not have enough women’s health physicians to meet the demand.
New research by the medical social network Doximity finds that 37% of ob.gyns. in the United States are 55 or older, while just 14% are 40 or younger. Furthering the potential for shortage, Doximity cited research from the American Congress of Obstetricians and Gynecologists that most ob.gyns. begin to retire at age 59, with the median retirement age being 64. Doximity’s own research found the average age of ob.gyns. across the nation is 51.
The report notes that after emergency physicians, ob.gyns. “have the highest burn-out rates of all medical specialties. Moreover, due to the nature of obstetrics, and child birth in particular, this job is especially demanding, often requiring ob.gyns. to work at unpredictable hours. This lifestyle can lead ob.gyns. to retire at younger ages than physicians in other specialties.”
At the same time, the ob.gyn. workforce remains stagnant. “According to ACOG, there are now 1,287 first year ob.gyn. residency positions, a number that has increased only minimally in the past few decades, while the number of adult. U.S. women has increased much more significantly, stretching the ratio of ob.gyns. to patients,” the Doximity report said.
William F. Rayburn, MD, distinguished professor and emeritus chair of obstetrics and gynecology at the University of New Mexico, Albuquerque, agreed with the findings.
Specifically, more general ob.gyns. are gravitating to metropolitan areas and are employed by health systems or large group practices, he noted.
Doximity identified the top 10 metropolitan areas that are at highest risk for an ob.gyn. shortage: Las Vegas; Orlando; Los Angeles; Miami; Riverside, Calif.; Detroit; Memphis; Salt Lake City; St. Louis; and Buffalo, N.Y.
In contrast, the metropolitan areas with the lowest risk of shortages are Portland, Ore.; San Jose, Calif.; Birmingham, Ala.; Denver; Indianapolis; Cleveland; San Francisco; Richmond, Va.; Louisville, Ky.; and Columbus, Ohio.
This is likely to translate to access to care problems in smaller communities, Dr. Rayburn said. “What we are going to be seeing is more graduates from nonphysician clinician programs who are going to be hired, such as nurse practitioners, to fill the gaps.”
The Doximity analysis is based on data from the Centers for Medicare and Medicaid Services, board certification, and self-reported data from more than 30,000 ob.gyns.
[email protected]
On Twitter @ObGynNews
FDA committee recommends approval of tofacitinib for PsA
Convinced largely by encouraging efficacy data, the Food and Drug Administration’s Arthritis Advisory Committee voted overwhelmingly in favor of approval of tofacitinib for the treatment of adult patients with active psoriatic arthritis.
If approved by the FDA, which usually adheres to advisory committee recommendations, the oral inhibitor of Janus-associated kinases (JAK) would be the first JAK inhibitor approved for the treatment of psoriatic arthritis (PsA). Pfizer submitted supplemental new drug applications (sNDAs) for both tofacitinib tablets (Xeljanz) and tofacitinib extended-release tablets (Xeljanz XR) at a dose of 5 mg twice daily and 11 mg once daily, respectively and, despite some reservations with respect to adverse events and lack of evidence regarding inhibition of radiographic progression, the committee voted 10-1 in favor of approval at an Aug. 3 meeting.
“I voted yes and, although there are safety concerns, I feel like it’s nothing different than what we see with other biologics, and I want to make sure that patients have options,” said Jennifer Horonjeff, PhD, a research fellow and patient advocate with the Center for Immune Disease with Onset in Childhood at Columbia University Medical Center, New York, and a consumer representative on the committee.
Dr. Horonjeff added that she hopes there is continued conversation between the sponsor and the FDA on “what we can do to make patients aware of these risks.”
Similarly, committee member Daniel H. Solomon, MD, a professor of medicine at Harvard Medical School and chief of the section of clinical sciences in the divisions of rheumatology and pharmacoepidemiology at Brigham and Women’s Hospital, both in Boston, said he sees a “great opportunity for risk mitigation that the sponsor and the [FDA] can take together.”
“We have a clear risk, we have a clear strategy for mitigating the risk, and there are going to be a lot more people exposed to this drug with a known risk, so let’s do something about it,” Dr. Solomon said about a plan put forward by Pfizer, and discussed at some length, to mitigate risks through measures such as vaccination against herpes zoster and additional study.
Temporary voting member Steven Meisel, PharmD, system director of patient safety at Fairview Health Services in Minneapolis added: “These are nasty drugs, but I think those who use them understand that, and this is no different than any of the other nasty drugs in these categories.”
Diane Aronson, a patient representative and temporary voting member on the committee, cast the only vote against approval, citing concerns about the lack of inhibition of radiographic progression of the disease and about the infection risks in a vulnerable population.
Tofacitinib was initially approved in 2012 at a dose of 5 mg, twice daily, for the treatment of adults with moderately to severely active rheumatoid arthritis who had an inadequate response or intolerance to methotrexate. The extended-release formulation was approved in 2016 at a dose of 11 mg daily.
With respect to the current sNDAs, Pfizer presented data from two placebo-controlled phase 3 trials in patients with psoriatic arthritis. The FDA deemed these trials to be adequate and well-controlled, providing “corroborating evidence of the efficacy of tofacitinib for reducing signs and symptoms of PsA, based on the proportion of patients experiencing the American College of Rheumatology (ACR) 20% response criteria,” according to a report presented to the committee. The report also noted that both phase 3 trials provided evidence of improvement in physical function, but did not provide sufficient evidence that tofacitinib inhibits radiographic progression in PsA.
The report also stated that the safety profile of tofacitinib in PsA was consistent with that established in RA; risks include serious infections, opportunistic infections, malignancy, gastrointestinal perforation, and laboratory abnormalities, including elevations in low-density lipoprotein and triglycerides.
“No new safety signals were identified in PsA,” the report states.
Of note, the sNDAs do not include an indication for generalized psoriasis; an application for that indication was withdrawn in 2016, and Dr. Meisel cautioned against any “unintentional leakage of the use of this drug for generalized psoriasis.”
He and others also cautioned against any implied endorsement in labeling that the drug inhibits radiographic progression of PsA.
Two individuals who participated in the open public hearing portion of the meeting each urged the committee to recommend approval of the sNDAs, with one, Stephen Marmaras, manager of state and national advocacy for the Global Healthy Living Foundation, noting that the joint pain and stiffness associated with PsA are a primary concern of patients.
“Our members with psoriatic arthritis overwhelmingly prioritize joint pain and stiffness as the most bothersome symptoms they experience,” Mr. Marmaras said. “With that in mind, we were encouraged to read that tofacitinib has particularly notable efficacy in treating the joint symptoms of the disease in clinical trials.”
All voting advisory committee members were screened and cleared with respect to potential conflicts of interest.
Convinced largely by encouraging efficacy data, the Food and Drug Administration’s Arthritis Advisory Committee voted overwhelmingly in favor of approval of tofacitinib for the treatment of adult patients with active psoriatic arthritis.
If approved by the FDA, which usually adheres to advisory committee recommendations, the oral inhibitor of Janus-associated kinases (JAK) would be the first JAK inhibitor approved for the treatment of psoriatic arthritis (PsA). Pfizer submitted supplemental new drug applications (sNDAs) for both tofacitinib tablets (Xeljanz) and tofacitinib extended-release tablets (Xeljanz XR) at a dose of 5 mg twice daily and 11 mg once daily, respectively and, despite some reservations with respect to adverse events and lack of evidence regarding inhibition of radiographic progression, the committee voted 10-1 in favor of approval at an Aug. 3 meeting.
“I voted yes and, although there are safety concerns, I feel like it’s nothing different than what we see with other biologics, and I want to make sure that patients have options,” said Jennifer Horonjeff, PhD, a research fellow and patient advocate with the Center for Immune Disease with Onset in Childhood at Columbia University Medical Center, New York, and a consumer representative on the committee.
Dr. Horonjeff added that she hopes there is continued conversation between the sponsor and the FDA on “what we can do to make patients aware of these risks.”
Similarly, committee member Daniel H. Solomon, MD, a professor of medicine at Harvard Medical School and chief of the section of clinical sciences in the divisions of rheumatology and pharmacoepidemiology at Brigham and Women’s Hospital, both in Boston, said he sees a “great opportunity for risk mitigation that the sponsor and the [FDA] can take together.”
“We have a clear risk, we have a clear strategy for mitigating the risk, and there are going to be a lot more people exposed to this drug with a known risk, so let’s do something about it,” Dr. Solomon said about a plan put forward by Pfizer, and discussed at some length, to mitigate risks through measures such as vaccination against herpes zoster and additional study.
Temporary voting member Steven Meisel, PharmD, system director of patient safety at Fairview Health Services in Minneapolis added: “These are nasty drugs, but I think those who use them understand that, and this is no different than any of the other nasty drugs in these categories.”
Diane Aronson, a patient representative and temporary voting member on the committee, cast the only vote against approval, citing concerns about the lack of inhibition of radiographic progression of the disease and about the infection risks in a vulnerable population.
Tofacitinib was initially approved in 2012 at a dose of 5 mg, twice daily, for the treatment of adults with moderately to severely active rheumatoid arthritis who had an inadequate response or intolerance to methotrexate. The extended-release formulation was approved in 2016 at a dose of 11 mg daily.
With respect to the current sNDAs, Pfizer presented data from two placebo-controlled phase 3 trials in patients with psoriatic arthritis. The FDA deemed these trials to be adequate and well-controlled, providing “corroborating evidence of the efficacy of tofacitinib for reducing signs and symptoms of PsA, based on the proportion of patients experiencing the American College of Rheumatology (ACR) 20% response criteria,” according to a report presented to the committee. The report also noted that both phase 3 trials provided evidence of improvement in physical function, but did not provide sufficient evidence that tofacitinib inhibits radiographic progression in PsA.
The report also stated that the safety profile of tofacitinib in PsA was consistent with that established in RA; risks include serious infections, opportunistic infections, malignancy, gastrointestinal perforation, and laboratory abnormalities, including elevations in low-density lipoprotein and triglycerides.
“No new safety signals were identified in PsA,” the report states.
Of note, the sNDAs do not include an indication for generalized psoriasis; an application for that indication was withdrawn in 2016, and Dr. Meisel cautioned against any “unintentional leakage of the use of this drug for generalized psoriasis.”
He and others also cautioned against any implied endorsement in labeling that the drug inhibits radiographic progression of PsA.
Two individuals who participated in the open public hearing portion of the meeting each urged the committee to recommend approval of the sNDAs, with one, Stephen Marmaras, manager of state and national advocacy for the Global Healthy Living Foundation, noting that the joint pain and stiffness associated with PsA are a primary concern of patients.
“Our members with psoriatic arthritis overwhelmingly prioritize joint pain and stiffness as the most bothersome symptoms they experience,” Mr. Marmaras said. “With that in mind, we were encouraged to read that tofacitinib has particularly notable efficacy in treating the joint symptoms of the disease in clinical trials.”
All voting advisory committee members were screened and cleared with respect to potential conflicts of interest.
Convinced largely by encouraging efficacy data, the Food and Drug Administration’s Arthritis Advisory Committee voted overwhelmingly in favor of approval of tofacitinib for the treatment of adult patients with active psoriatic arthritis.
If approved by the FDA, which usually adheres to advisory committee recommendations, the oral inhibitor of Janus-associated kinases (JAK) would be the first JAK inhibitor approved for the treatment of psoriatic arthritis (PsA). Pfizer submitted supplemental new drug applications (sNDAs) for both tofacitinib tablets (Xeljanz) and tofacitinib extended-release tablets (Xeljanz XR) at a dose of 5 mg twice daily and 11 mg once daily, respectively and, despite some reservations with respect to adverse events and lack of evidence regarding inhibition of radiographic progression, the committee voted 10-1 in favor of approval at an Aug. 3 meeting.
“I voted yes and, although there are safety concerns, I feel like it’s nothing different than what we see with other biologics, and I want to make sure that patients have options,” said Jennifer Horonjeff, PhD, a research fellow and patient advocate with the Center for Immune Disease with Onset in Childhood at Columbia University Medical Center, New York, and a consumer representative on the committee.
Dr. Horonjeff added that she hopes there is continued conversation between the sponsor and the FDA on “what we can do to make patients aware of these risks.”
Similarly, committee member Daniel H. Solomon, MD, a professor of medicine at Harvard Medical School and chief of the section of clinical sciences in the divisions of rheumatology and pharmacoepidemiology at Brigham and Women’s Hospital, both in Boston, said he sees a “great opportunity for risk mitigation that the sponsor and the [FDA] can take together.”
“We have a clear risk, we have a clear strategy for mitigating the risk, and there are going to be a lot more people exposed to this drug with a known risk, so let’s do something about it,” Dr. Solomon said about a plan put forward by Pfizer, and discussed at some length, to mitigate risks through measures such as vaccination against herpes zoster and additional study.
Temporary voting member Steven Meisel, PharmD, system director of patient safety at Fairview Health Services in Minneapolis added: “These are nasty drugs, but I think those who use them understand that, and this is no different than any of the other nasty drugs in these categories.”
Diane Aronson, a patient representative and temporary voting member on the committee, cast the only vote against approval, citing concerns about the lack of inhibition of radiographic progression of the disease and about the infection risks in a vulnerable population.
Tofacitinib was initially approved in 2012 at a dose of 5 mg, twice daily, for the treatment of adults with moderately to severely active rheumatoid arthritis who had an inadequate response or intolerance to methotrexate. The extended-release formulation was approved in 2016 at a dose of 11 mg daily.
With respect to the current sNDAs, Pfizer presented data from two placebo-controlled phase 3 trials in patients with psoriatic arthritis. The FDA deemed these trials to be adequate and well-controlled, providing “corroborating evidence of the efficacy of tofacitinib for reducing signs and symptoms of PsA, based on the proportion of patients experiencing the American College of Rheumatology (ACR) 20% response criteria,” according to a report presented to the committee. The report also noted that both phase 3 trials provided evidence of improvement in physical function, but did not provide sufficient evidence that tofacitinib inhibits radiographic progression in PsA.
The report also stated that the safety profile of tofacitinib in PsA was consistent with that established in RA; risks include serious infections, opportunistic infections, malignancy, gastrointestinal perforation, and laboratory abnormalities, including elevations in low-density lipoprotein and triglycerides.
“No new safety signals were identified in PsA,” the report states.
Of note, the sNDAs do not include an indication for generalized psoriasis; an application for that indication was withdrawn in 2016, and Dr. Meisel cautioned against any “unintentional leakage of the use of this drug for generalized psoriasis.”
He and others also cautioned against any implied endorsement in labeling that the drug inhibits radiographic progression of PsA.
Two individuals who participated in the open public hearing portion of the meeting each urged the committee to recommend approval of the sNDAs, with one, Stephen Marmaras, manager of state and national advocacy for the Global Healthy Living Foundation, noting that the joint pain and stiffness associated with PsA are a primary concern of patients.
“Our members with psoriatic arthritis overwhelmingly prioritize joint pain and stiffness as the most bothersome symptoms they experience,” Mr. Marmaras said. “With that in mind, we were encouraged to read that tofacitinib has particularly notable efficacy in treating the joint symptoms of the disease in clinical trials.”
All voting advisory committee members were screened and cleared with respect to potential conflicts of interest.
Landmark women’s health care remains law of the land
Starting in 2010 with the Patient Protection and Affordable Care Act (ACA), our patients have had insurance that provides maternity care coverage, no-deductible or copay contraceptives, and access to breast cancer screening. They also have been protected from predatory insurance practices—such as preexisting condition exclusions, arbitrary rescission, and annual or lifetime coverage limits—which had previously and regularly been used to deny coverage. These landmark protections apply to all our patients, regardless of where they live, how much they earn, who their employers are, and which insurance plan they use. They have become part of the fabric of our society.
Between 2008 and 2010, members of the American College of Obstetricians and Gynecologists (ACOG) worked hard to define and help enact these provisions, which we considered the women’s piece of the health care reform puzzle. We also worked with a broad community of clinicians to try to make sure reform would benefit them too. That effort did not go as well, and ACOG ultimately did not endorse the ACA.
Early ACA troubles, misguided solutions
Since the ACA was signed into law 7 years ago, insurers have raised premiums and deductibles and narrowed their provider networks—putting needed care out of the reach of many patients. In addition, skyrocketing prescription drug prices have driven health care costs even higher. Against this backdrop, Congress in 2017 started trying to pass bills that would undo the ACA.
ACOG and our medical colleague organizations stepped up. We brought many ideas to House and Senate Republicans and Democrats and sought opportunities to work with them to improve the ACA for our physicians and patients. Unfortunately, the statute was so polarizing that few in Congress wanted to amend or revise it; most wanted it repealed or left as is.
Throughout these proceedings, ACOG remained committed to ensuring that no one with health insurance coverage would lose it and that Congress would not turn back the clock on women’s health. As long as these 2 principles were assured, we would work with anyone on improving health insurance.
Path to a better way
We delivered our message repeatedly. ACOG President Haywood Brown, MD, often accompanied by his American College of Physicians, American Academy of Pediatrics, American Academy of Family Physicians, American Psychiatric Association, and American Osteopathic Association counterparts, attended high-level meetings with Congressional Republicans and Democrats. Dr. Brown also led fly-ins of our members. In addition, ACOG Past President Tom Gellhaus, MD, together with all 600 ObGyns at the 2017 ACOG Congressional Leadership Conference, spoke out.
Somehow, though, the proposed bills kept getting worse—more patients would be losing coverage, and women’s health protections would be stripped away—and Congress was not seeking or including physician input. None at all.
The ACOG teleconference
In response, ACOG set up a member teleconference headed by Dr. Brown, Dr. Gellhaus, Incoming President Lisa Hollier, MD, Past President and ObGyn PAC Chair Mark DeFrancesco, MD, and Executive Vice President and CEO Hal Lawrence, MD. Discussing our concerns, we focused on the Senate’s Better Care Reconciliation Act (BCRA) and its potential impact on maternity care coverage, preexisting condition coverage, Medicaid, Planned Parenthood (PP), and the opioid epidemic.
BCRA
Dr. Brown led off the teleconference with this assessment: “Without a doubt, the BCRA would not result in better care for our patients. This legislation would pull the rug out from under women and families. The nonpartisan Congressional Budget Office estimated that 22 million Americans, more than half of them women, would lose coverage. More than $770 billion would be cut from Medicaid, the program that covers nearly half of all births nationwide as well as primary and preventive care for low-income patients.”
Coverage for maternity care and preexisting conditions
Dr. Gellhaus discussed how the BCRA would gut maternity care coverage and hurt patients with preexisting conditions. Under this bill, states would be able to drop the requirement for such coverage, thereby creating an enormous hole in patient care. He asked an important question: “If your state opted out and allowed private insurers not to offer maternity care or preventive care, what would this mean for your patients?”
His answer: “It would take us back to a time when only 9 states required insurers cover maternity care, and when only 12% of plans included such coverage; a time when patients had to buy expensive riders, sometimes with 12-month waiting periods, if they wanted maternity coverage; a time when expecting families faced thousands of dollars in out-of-pocket costs. Do we want to go back to that time? It is also important to note that roughly half of all pregnancies are unplanned. Pregnancy should not leave patients fearing bankruptcy and unable to afford the full range of prenatal and postnatal care.
“States that opt out of covering preventive care would discontinue no-copay coverage for women’s preventive services, including contraception. Fifty-five million American women currently have this coverage, and as a result the country’s unintended pregnancy rate is at a 30-year low, and its teen pregnancy rate the lowest in recorded history. We cannot go back.”
Medicaid
Dr. Hollier pointed out that the BCRA would cut $772 billion from Medicaid, ending the program as we know it and shifting costs to states. “This section alone would devastate our patients in every state,” she said.
ACOG is a strong supporter of Medicaid expansion, which increased access to primary and preventive care, including contraception, for low-income women who otherwise would not see a physician until they became pregnant. Thirty-two states and the District of Columbia expanded their Medicaid programs, and other states have expressed interest in doing the same.
Medicaid expansion was a major factor in the almost 50% decrease in the rate of uninsured women since 2010. The BCRA would roll back coverage for essential health benefits beginning in 2020 and end federal expansion funding by 2023.
Dr. Hollier continued, “Regular Medicaid would be threatened, too. The Senate bill would limit, for the first time ever, federal funding for Medicaid services per beneficiary. This would jeopardize the ability of the United States to respond to disasters and public health crises and pose a threat to health care coverage and benefits for tens of millions of Americans.”
“Given that nearly half of US births are covered by Medicaid, cutting this program would have a huge impact on our practices and on our patients with high-risk and expensive pregnancies. What happens when a low-income pregnant patient with hypertension, gestational diabetes, or preeclampsia reaches her Medicaid cap? What happens to a patient with an opioid use disorder or a patient who may have been exposed to the Zika virus? In all likelihood, physicians would have to continue providing care, regardless of coverage, or states would have to reduce physician payments to fill the gap in federal funding. I am sure you are as horrified as I am by these scenarios,” said Dr. Hollier.
Planned Parenthood
Dr. DeFrancesco discussed the threat to PP. First, he explained what defunding the organization would mean. “Planned Parenthood does not just receive a check from the government each year. Like other qualified providers, like us, PP health centers receive federal reimbursement for primary and preventive services provided to patients with Medicaid coverage. Fifty-four percent of these centers are located in rural and medically underserved communities.”
The BCRA would exclude PP health centers from the Medicaid program, which means Medicaid patients would be denied primary and preventive care at these centers. Within the first year, up to 1 million women would find their access to care restricted. In addition, about half of all PP centers would have to close, and most would not reopen. Dr. DeFrancesco asked, “How would this move help our patients? It wouldn’t.”
Two examples shed light on the situation. First, when PP was excluded from a Texas program serving low-income patients, the number of women using the most effective birth control methods decreased by 35%, and the number of Medicaid-covered births increased by 27%. Second, when public health funding cuts forced many Indiana clinics to close, rural areas of the state experienced one of the fastest and largest HIV outbreaks ever to occur in the United States.
Dr. DeFrancesco said, “Excluding Planned Parenthood from the Medicaid program interferes with the patient–physician relationship and sets a dangerous precedent of targeting qualified providers for political purposes.”
Opioid epidemic
Dr. Brown indicated that the BCRA would cripple attempts to address our very serious national opioid epidemic. The $2 billion the bill would allocate for opioid use disorder treatment for 1 year would replace funding lost by Medicaid and would pay for only a fraction of what is needed. Dr. Brown called this measure a “token, not a commitment, and a big step back in the progress we have made to address this public health crisis.”
The Hippocratic oath
While preparing for the teleconference, I kept thinking about the Hippocratic oath and our deep obligation to our patients. Every physician I know goes beyond the exam room to care for patients. We lose sleep not only when we get up to deliver babies, but when we worry about the ailing mother of four we saw yesterday, or the scared teenager who missed last week’s appointment. We care for our patients because it is the right thing to do, and it is our calling. Well, this year, our patients needed us more than ever. We had to step up, speak out, do everything we could to stop BCRA from passing. The stakes could not have been higher.
The vote, and the road ahead
The morning after Senators Collins, Murkowski, and McCain joined Senate Democrats to end the bill, Dr. Brown wrote the following to ACOG members and the US Congress:
“This was a battle we simply had to win to protect our patients. Thanks to your tireless advocacy, landmark women’s health care protections remain law, and millions of our patients will continue to get the care they need. And our work continues. The ACA is not perfect and needs major reform. ACOG is ready, willing, and able to work with Republicans and Democrats in the US House and Senate to reform the law, through an open and collaborative process. We hope it is clear to everyone in Congress that physicians must be part of the conversation and the solution.”
Starting in 2010 with the Patient Protection and Affordable Care Act (ACA), our patients have had insurance that provides maternity care coverage, no-deductible or copay contraceptives, and access to breast cancer screening. They also have been protected from predatory insurance practices—such as preexisting condition exclusions, arbitrary rescission, and annual or lifetime coverage limits—which had previously and regularly been used to deny coverage. These landmark protections apply to all our patients, regardless of where they live, how much they earn, who their employers are, and which insurance plan they use. They have become part of the fabric of our society.
Between 2008 and 2010, members of the American College of Obstetricians and Gynecologists (ACOG) worked hard to define and help enact these provisions, which we considered the women’s piece of the health care reform puzzle. We also worked with a broad community of clinicians to try to make sure reform would benefit them too. That effort did not go as well, and ACOG ultimately did not endorse the ACA.
Early ACA troubles, misguided solutions
Since the ACA was signed into law 7 years ago, insurers have raised premiums and deductibles and narrowed their provider networks—putting needed care out of the reach of many patients. In addition, skyrocketing prescription drug prices have driven health care costs even higher. Against this backdrop, Congress in 2017 started trying to pass bills that would undo the ACA.
ACOG and our medical colleague organizations stepped up. We brought many ideas to House and Senate Republicans and Democrats and sought opportunities to work with them to improve the ACA for our physicians and patients. Unfortunately, the statute was so polarizing that few in Congress wanted to amend or revise it; most wanted it repealed or left as is.
Throughout these proceedings, ACOG remained committed to ensuring that no one with health insurance coverage would lose it and that Congress would not turn back the clock on women’s health. As long as these 2 principles were assured, we would work with anyone on improving health insurance.
Path to a better way
We delivered our message repeatedly. ACOG President Haywood Brown, MD, often accompanied by his American College of Physicians, American Academy of Pediatrics, American Academy of Family Physicians, American Psychiatric Association, and American Osteopathic Association counterparts, attended high-level meetings with Congressional Republicans and Democrats. Dr. Brown also led fly-ins of our members. In addition, ACOG Past President Tom Gellhaus, MD, together with all 600 ObGyns at the 2017 ACOG Congressional Leadership Conference, spoke out.
Somehow, though, the proposed bills kept getting worse—more patients would be losing coverage, and women’s health protections would be stripped away—and Congress was not seeking or including physician input. None at all.
The ACOG teleconference
In response, ACOG set up a member teleconference headed by Dr. Brown, Dr. Gellhaus, Incoming President Lisa Hollier, MD, Past President and ObGyn PAC Chair Mark DeFrancesco, MD, and Executive Vice President and CEO Hal Lawrence, MD. Discussing our concerns, we focused on the Senate’s Better Care Reconciliation Act (BCRA) and its potential impact on maternity care coverage, preexisting condition coverage, Medicaid, Planned Parenthood (PP), and the opioid epidemic.
BCRA
Dr. Brown led off the teleconference with this assessment: “Without a doubt, the BCRA would not result in better care for our patients. This legislation would pull the rug out from under women and families. The nonpartisan Congressional Budget Office estimated that 22 million Americans, more than half of them women, would lose coverage. More than $770 billion would be cut from Medicaid, the program that covers nearly half of all births nationwide as well as primary and preventive care for low-income patients.”
Coverage for maternity care and preexisting conditions
Dr. Gellhaus discussed how the BCRA would gut maternity care coverage and hurt patients with preexisting conditions. Under this bill, states would be able to drop the requirement for such coverage, thereby creating an enormous hole in patient care. He asked an important question: “If your state opted out and allowed private insurers not to offer maternity care or preventive care, what would this mean for your patients?”
His answer: “It would take us back to a time when only 9 states required insurers cover maternity care, and when only 12% of plans included such coverage; a time when patients had to buy expensive riders, sometimes with 12-month waiting periods, if they wanted maternity coverage; a time when expecting families faced thousands of dollars in out-of-pocket costs. Do we want to go back to that time? It is also important to note that roughly half of all pregnancies are unplanned. Pregnancy should not leave patients fearing bankruptcy and unable to afford the full range of prenatal and postnatal care.
“States that opt out of covering preventive care would discontinue no-copay coverage for women’s preventive services, including contraception. Fifty-five million American women currently have this coverage, and as a result the country’s unintended pregnancy rate is at a 30-year low, and its teen pregnancy rate the lowest in recorded history. We cannot go back.”
Medicaid
Dr. Hollier pointed out that the BCRA would cut $772 billion from Medicaid, ending the program as we know it and shifting costs to states. “This section alone would devastate our patients in every state,” she said.
ACOG is a strong supporter of Medicaid expansion, which increased access to primary and preventive care, including contraception, for low-income women who otherwise would not see a physician until they became pregnant. Thirty-two states and the District of Columbia expanded their Medicaid programs, and other states have expressed interest in doing the same.
Medicaid expansion was a major factor in the almost 50% decrease in the rate of uninsured women since 2010. The BCRA would roll back coverage for essential health benefits beginning in 2020 and end federal expansion funding by 2023.
Dr. Hollier continued, “Regular Medicaid would be threatened, too. The Senate bill would limit, for the first time ever, federal funding for Medicaid services per beneficiary. This would jeopardize the ability of the United States to respond to disasters and public health crises and pose a threat to health care coverage and benefits for tens of millions of Americans.”
“Given that nearly half of US births are covered by Medicaid, cutting this program would have a huge impact on our practices and on our patients with high-risk and expensive pregnancies. What happens when a low-income pregnant patient with hypertension, gestational diabetes, or preeclampsia reaches her Medicaid cap? What happens to a patient with an opioid use disorder or a patient who may have been exposed to the Zika virus? In all likelihood, physicians would have to continue providing care, regardless of coverage, or states would have to reduce physician payments to fill the gap in federal funding. I am sure you are as horrified as I am by these scenarios,” said Dr. Hollier.
Planned Parenthood
Dr. DeFrancesco discussed the threat to PP. First, he explained what defunding the organization would mean. “Planned Parenthood does not just receive a check from the government each year. Like other qualified providers, like us, PP health centers receive federal reimbursement for primary and preventive services provided to patients with Medicaid coverage. Fifty-four percent of these centers are located in rural and medically underserved communities.”
The BCRA would exclude PP health centers from the Medicaid program, which means Medicaid patients would be denied primary and preventive care at these centers. Within the first year, up to 1 million women would find their access to care restricted. In addition, about half of all PP centers would have to close, and most would not reopen. Dr. DeFrancesco asked, “How would this move help our patients? It wouldn’t.”
Two examples shed light on the situation. First, when PP was excluded from a Texas program serving low-income patients, the number of women using the most effective birth control methods decreased by 35%, and the number of Medicaid-covered births increased by 27%. Second, when public health funding cuts forced many Indiana clinics to close, rural areas of the state experienced one of the fastest and largest HIV outbreaks ever to occur in the United States.
Dr. DeFrancesco said, “Excluding Planned Parenthood from the Medicaid program interferes with the patient–physician relationship and sets a dangerous precedent of targeting qualified providers for political purposes.”
Opioid epidemic
Dr. Brown indicated that the BCRA would cripple attempts to address our very serious national opioid epidemic. The $2 billion the bill would allocate for opioid use disorder treatment for 1 year would replace funding lost by Medicaid and would pay for only a fraction of what is needed. Dr. Brown called this measure a “token, not a commitment, and a big step back in the progress we have made to address this public health crisis.”
The Hippocratic oath
While preparing for the teleconference, I kept thinking about the Hippocratic oath and our deep obligation to our patients. Every physician I know goes beyond the exam room to care for patients. We lose sleep not only when we get up to deliver babies, but when we worry about the ailing mother of four we saw yesterday, or the scared teenager who missed last week’s appointment. We care for our patients because it is the right thing to do, and it is our calling. Well, this year, our patients needed us more than ever. We had to step up, speak out, do everything we could to stop BCRA from passing. The stakes could not have been higher.
The vote, and the road ahead
The morning after Senators Collins, Murkowski, and McCain joined Senate Democrats to end the bill, Dr. Brown wrote the following to ACOG members and the US Congress:
“This was a battle we simply had to win to protect our patients. Thanks to your tireless advocacy, landmark women’s health care protections remain law, and millions of our patients will continue to get the care they need. And our work continues. The ACA is not perfect and needs major reform. ACOG is ready, willing, and able to work with Republicans and Democrats in the US House and Senate to reform the law, through an open and collaborative process. We hope it is clear to everyone in Congress that physicians must be part of the conversation and the solution.”
Starting in 2010 with the Patient Protection and Affordable Care Act (ACA), our patients have had insurance that provides maternity care coverage, no-deductible or copay contraceptives, and access to breast cancer screening. They also have been protected from predatory insurance practices—such as preexisting condition exclusions, arbitrary rescission, and annual or lifetime coverage limits—which had previously and regularly been used to deny coverage. These landmark protections apply to all our patients, regardless of where they live, how much they earn, who their employers are, and which insurance plan they use. They have become part of the fabric of our society.
Between 2008 and 2010, members of the American College of Obstetricians and Gynecologists (ACOG) worked hard to define and help enact these provisions, which we considered the women’s piece of the health care reform puzzle. We also worked with a broad community of clinicians to try to make sure reform would benefit them too. That effort did not go as well, and ACOG ultimately did not endorse the ACA.
Early ACA troubles, misguided solutions
Since the ACA was signed into law 7 years ago, insurers have raised premiums and deductibles and narrowed their provider networks—putting needed care out of the reach of many patients. In addition, skyrocketing prescription drug prices have driven health care costs even higher. Against this backdrop, Congress in 2017 started trying to pass bills that would undo the ACA.
ACOG and our medical colleague organizations stepped up. We brought many ideas to House and Senate Republicans and Democrats and sought opportunities to work with them to improve the ACA for our physicians and patients. Unfortunately, the statute was so polarizing that few in Congress wanted to amend or revise it; most wanted it repealed or left as is.
Throughout these proceedings, ACOG remained committed to ensuring that no one with health insurance coverage would lose it and that Congress would not turn back the clock on women’s health. As long as these 2 principles were assured, we would work with anyone on improving health insurance.
Path to a better way
We delivered our message repeatedly. ACOG President Haywood Brown, MD, often accompanied by his American College of Physicians, American Academy of Pediatrics, American Academy of Family Physicians, American Psychiatric Association, and American Osteopathic Association counterparts, attended high-level meetings with Congressional Republicans and Democrats. Dr. Brown also led fly-ins of our members. In addition, ACOG Past President Tom Gellhaus, MD, together with all 600 ObGyns at the 2017 ACOG Congressional Leadership Conference, spoke out.
Somehow, though, the proposed bills kept getting worse—more patients would be losing coverage, and women’s health protections would be stripped away—and Congress was not seeking or including physician input. None at all.
The ACOG teleconference
In response, ACOG set up a member teleconference headed by Dr. Brown, Dr. Gellhaus, Incoming President Lisa Hollier, MD, Past President and ObGyn PAC Chair Mark DeFrancesco, MD, and Executive Vice President and CEO Hal Lawrence, MD. Discussing our concerns, we focused on the Senate’s Better Care Reconciliation Act (BCRA) and its potential impact on maternity care coverage, preexisting condition coverage, Medicaid, Planned Parenthood (PP), and the opioid epidemic.
BCRA
Dr. Brown led off the teleconference with this assessment: “Without a doubt, the BCRA would not result in better care for our patients. This legislation would pull the rug out from under women and families. The nonpartisan Congressional Budget Office estimated that 22 million Americans, more than half of them women, would lose coverage. More than $770 billion would be cut from Medicaid, the program that covers nearly half of all births nationwide as well as primary and preventive care for low-income patients.”
Coverage for maternity care and preexisting conditions
Dr. Gellhaus discussed how the BCRA would gut maternity care coverage and hurt patients with preexisting conditions. Under this bill, states would be able to drop the requirement for such coverage, thereby creating an enormous hole in patient care. He asked an important question: “If your state opted out and allowed private insurers not to offer maternity care or preventive care, what would this mean for your patients?”
His answer: “It would take us back to a time when only 9 states required insurers cover maternity care, and when only 12% of plans included such coverage; a time when patients had to buy expensive riders, sometimes with 12-month waiting periods, if they wanted maternity coverage; a time when expecting families faced thousands of dollars in out-of-pocket costs. Do we want to go back to that time? It is also important to note that roughly half of all pregnancies are unplanned. Pregnancy should not leave patients fearing bankruptcy and unable to afford the full range of prenatal and postnatal care.
“States that opt out of covering preventive care would discontinue no-copay coverage for women’s preventive services, including contraception. Fifty-five million American women currently have this coverage, and as a result the country’s unintended pregnancy rate is at a 30-year low, and its teen pregnancy rate the lowest in recorded history. We cannot go back.”
Medicaid
Dr. Hollier pointed out that the BCRA would cut $772 billion from Medicaid, ending the program as we know it and shifting costs to states. “This section alone would devastate our patients in every state,” she said.
ACOG is a strong supporter of Medicaid expansion, which increased access to primary and preventive care, including contraception, for low-income women who otherwise would not see a physician until they became pregnant. Thirty-two states and the District of Columbia expanded their Medicaid programs, and other states have expressed interest in doing the same.
Medicaid expansion was a major factor in the almost 50% decrease in the rate of uninsured women since 2010. The BCRA would roll back coverage for essential health benefits beginning in 2020 and end federal expansion funding by 2023.
Dr. Hollier continued, “Regular Medicaid would be threatened, too. The Senate bill would limit, for the first time ever, federal funding for Medicaid services per beneficiary. This would jeopardize the ability of the United States to respond to disasters and public health crises and pose a threat to health care coverage and benefits for tens of millions of Americans.”
“Given that nearly half of US births are covered by Medicaid, cutting this program would have a huge impact on our practices and on our patients with high-risk and expensive pregnancies. What happens when a low-income pregnant patient with hypertension, gestational diabetes, or preeclampsia reaches her Medicaid cap? What happens to a patient with an opioid use disorder or a patient who may have been exposed to the Zika virus? In all likelihood, physicians would have to continue providing care, regardless of coverage, or states would have to reduce physician payments to fill the gap in federal funding. I am sure you are as horrified as I am by these scenarios,” said Dr. Hollier.
Planned Parenthood
Dr. DeFrancesco discussed the threat to PP. First, he explained what defunding the organization would mean. “Planned Parenthood does not just receive a check from the government each year. Like other qualified providers, like us, PP health centers receive federal reimbursement for primary and preventive services provided to patients with Medicaid coverage. Fifty-four percent of these centers are located in rural and medically underserved communities.”
The BCRA would exclude PP health centers from the Medicaid program, which means Medicaid patients would be denied primary and preventive care at these centers. Within the first year, up to 1 million women would find their access to care restricted. In addition, about half of all PP centers would have to close, and most would not reopen. Dr. DeFrancesco asked, “How would this move help our patients? It wouldn’t.”
Two examples shed light on the situation. First, when PP was excluded from a Texas program serving low-income patients, the number of women using the most effective birth control methods decreased by 35%, and the number of Medicaid-covered births increased by 27%. Second, when public health funding cuts forced many Indiana clinics to close, rural areas of the state experienced one of the fastest and largest HIV outbreaks ever to occur in the United States.
Dr. DeFrancesco said, “Excluding Planned Parenthood from the Medicaid program interferes with the patient–physician relationship and sets a dangerous precedent of targeting qualified providers for political purposes.”
Opioid epidemic
Dr. Brown indicated that the BCRA would cripple attempts to address our very serious national opioid epidemic. The $2 billion the bill would allocate for opioid use disorder treatment for 1 year would replace funding lost by Medicaid and would pay for only a fraction of what is needed. Dr. Brown called this measure a “token, not a commitment, and a big step back in the progress we have made to address this public health crisis.”
The Hippocratic oath
While preparing for the teleconference, I kept thinking about the Hippocratic oath and our deep obligation to our patients. Every physician I know goes beyond the exam room to care for patients. We lose sleep not only when we get up to deliver babies, but when we worry about the ailing mother of four we saw yesterday, or the scared teenager who missed last week’s appointment. We care for our patients because it is the right thing to do, and it is our calling. Well, this year, our patients needed us more than ever. We had to step up, speak out, do everything we could to stop BCRA from passing. The stakes could not have been higher.
The vote, and the road ahead
The morning after Senators Collins, Murkowski, and McCain joined Senate Democrats to end the bill, Dr. Brown wrote the following to ACOG members and the US Congress:
“This was a battle we simply had to win to protect our patients. Thanks to your tireless advocacy, landmark women’s health care protections remain law, and millions of our patients will continue to get the care they need. And our work continues. The ACA is not perfect and needs major reform. ACOG is ready, willing, and able to work with Republicans and Democrats in the US House and Senate to reform the law, through an open and collaborative process. We hope it is clear to everyone in Congress that physicians must be part of the conversation and the solution.”
CGRP Monoclonal Antibodies May Be Beneficial for Migraine Prevention
BOSTON—A new class of drugs for the prevention of chronic and episodic migraine demonstrated promising results in recent phase II and phase III trials. Data for four humanized calcitonin gene–related peptide (CGRP) monoclonal antibodies—eptinezumab, erenumab, fremanezumab, and galcanezumab—were presented at the 59th Annual Scientific Meeting of the American Headache Society. Previous research has long implicated CGRP (which is elevated in jugular vein blood during acute migraine and cluster headaches) in disease pathophysiology. Recent advances in molecular neuroscience have helped shed further light on the pathogenic mechanisms and possible targets for treatment.
Eptinezumab
In a phase II study, a single infusion of eptinezumab (which Alder BioPharmaceuticals is developing under the name ALD403) reduced the number of migraine days per month, as well as the number of migraines classified as severe, in patients with chronic migraine. Of the four drugs presented at the meeting, eptinezumab was the only one to have an IV mode of delivery.
“We used International Classification of Headache Disorders 3 criteria to define chronic migraine, which is at least 15 headache days per month, of which eight must be migraine-like,” said Jeffrey T. L. Smith, MD, Senior Vice President of Translational Medicine at Alder BioPharmaceuticals in Bothell, Washington. “Patients also had to have a history of migraine for a year or more.”
On average, the patients included in this multisite study were slightly younger than 40. Most patients were women, and the average number of migraine days was between 16.2 and 16.5. After a one-month run-in period, patients were randomized to receive placebo or one of four doses of eptinezumab (ie, 300 mg, 100 mg, 30 mg, or 10 mg).
Reporting 12-week data from the 48-week trial, Dr. Smith and colleagues found that significantly more patients taking the 300-mg (33%) and 100-mg (31%) doses of eptinezumab reached the primary end point of a 75% reduction from baseline in migraine days, compared with patients who received placebo (21%). Differences in patients taking the 30-mg and 10-mg doses (28% and 27%, respectively) were not statistically significant. “Overall, approximately half of patients treated with eptinezumab experienced a 50% reduction in migraine days versus 41% of patients on placebo,” Dr. Smith added. Eptinezumab was well tolerated, and no serious adverse events were reported.
Post hoc analyses indicated that of the migraines that were not eliminated as a result of treatment, the percentage classified as severe was significantly reduced from baseline in each of the eptinezumab groups, compared with placebo. Also, fewer patients in the eptinezumab groups than in the placebo group experienced a migraine within the first 24 to 48 hours after infusion. “Although the post hoc results are exploratory, these findings are important as well,” said Dr. Smith. “Other things are happening besides the reduction in migraine frequency that may be of benefit.”
Erenumab
A registration, pivotal study on erenumab for chronic migraine prevention was reported at the meeting. “Unlike eptinezumab, fremanezumab, and galcanezumab, which are monoclonal antibodies against the CGRP ligand, erenumab is an anti-CGRP receptor monoclonal antibody,” said Stewart J. Tepper, MD, Professor of Neurology at the Geisel School of Medicine at Dartmouth in Hanover, New Hampshire. Amgen is developing this antibody.
In the 12-week randomized controlled trial, patients were enrolled in the US and around the world, Dr. Tepper noted. He and his colleagues included patients who had 15 or more headache days per month with at least eight days of migraine per month. Patients were excluded if they had experienced treatment failure with more than three classes of preventive agents. Mean age was 40, about 80% of patients were women, and roughly half of patients had previously experienced treatment failure with at least two preventive agents.
A total of 667 patients were randomized 3:2:2 to placebo, erenumab (70 mg), or erenumab (140 mg). Treatment was given monthly via subcutaneous injection. The primary end point was change from baseline in monthly migraine days (MMD). Secondary end points included at least a 50% reduction in MMD, change from baseline in acute migraine-specific medication days, and cumulative headache hours.
In both erenumab groups, patients experienced a mean 6.6-day reduction in MMD from a baseline of 18.0 days, which was significantly different, compared with the 4.2-day reduction seen in the placebo group. Furthermore, a significantly greater proportion of patients in the 70-mg and 140-mg groups (40% and 41%, respectively) experienced at least a 50% reduction in MMD, compared with the placebo group (23%). For the secondary end points, both erenumab groups had significantly greater reductions in acute migraine-specific medication days versus placebo. However, the difference in the reduction of cumulative headache hours did not reach statistical significance. “We speculated that this was because headache hours is a relatively variable end point, and it is collected in differing ways, with wide confidence intervals,” Dr. Tepper said.
The safety profile for both erenumab groups and the placebo group was similar. “No serious adverse events were reported by more than one patient in any treatment arm, and no serious adverse events were considered related to treatment,” he said.
Another erenumab trial enrolled patients with episodic migraine. Randomization was stratified by region and current and prior migraine preventive medication, said Daniel Mikol, MD, PhD, Executive Medical Director of Neuroscience Development at Amgen in Thousand Oaks, California. Patients included in the phase III study could be taking a single concomitant migraine preventive therapy. “Patients could have also failed previous preventive therapies due to poor tolerability or lack of efficacy,” he said. “There was no limit regarding the number of previous failures due to poor tolerability. With regard to lack of efficacy, patients could have no therapeutic response to two or fewer preventive therapies.”
Adults with episodic migraine (n = 955) were randomized 1:1:1 to monthly subcutaneous injections of placebo, erenumab (70 mg), or erenumab (140 mg) for 24 weeks. The primary end point was change from baseline in MMD from weeks 13 to 24. Secondary end points included achievement of 50% or more reduction in MMD, change in mean acute migraine-specific medication days, and changes in mean physical impairment and impact on everyday activity scores, as measured by the Migraine Physical Function Impact Diary, a novel patient-reported instrument, Dr. Mikol explained.
Subjects had a mean of 8.3 MMD at baseline. This measurement was reduced by 3.2, 3.7, and 1.8 days in the 70-mg, 140-mg, and placebo groups, respectively—a statistically significant difference in favor of the erenumab groups. Statistically superior results for erenumab 70 mg and 140 mg over placebo also were seen in the secondary end points of > 50% response (43%, 50%, and 27%, respectively), reduction from baseline in monthly acute migraine-specific medication days (1.1, 1.6, and 0.2), improved physical impairment score from baseline (4.2, 4.8, and 2.5), and improved everyday activity scores from baseline (5.5, 5.9, and 3.3).
“The safety profiles for erenumab were similar to that of placebo,” Dr. Mikol said. “Specifically, 63% of patients on placebo had adverse events, compared with 56% to 57% of patients in the erenumab groups. Serious adverse events and adverse events leading to treatment discontinuation were low in frequency and balanced across the groups.”
Galcanezumab
The efficacy, tolerability, and safety of galcanezumab, an investigational drug for the treatment of episodic migraine, was assessed in a six-month phase III study called EVOLVE-2. In this study, patients with episodic migraine experienced four to 14 migraine headache days per month with or without aura. “Our patient population had a mean age of about 40, and most [participants] were Caucasian women,” said Robert Conley, MD, Distinguished Lilly Scholar of Neuroscience at Eli Lilly and Company (the developer of galcanezumab) in Indianapolis.
“Half of our subjects were from North America, a quarter from Europe, and a quarter from the rest of the world.” Subjects had a 20-year migraine history on average and a mean of nine headache days per month at baseline, he noted. “In addition, two-thirds had been on prior preventive treatment, and they had relatively severe levels of disease.”
After screening 1,700 patients for the study, Dr. Conley and colleagues randomized 914 patients 1:1:2 to subcutaneous injection of galcanezumab (120 mg/month), galcanezumab (240 mg/month), or placebo. “There was about an 87% completion rate for the galcanezumab groups and an 83% completion rate in the placebo group. The higher number of dropouts in the placebo group was due to lack of efficacy,” said Dr. Conley. The primary end point was change from baseline in MMD at the end of six months. Secondary end points were percentages of patients with 50%, 75%, and 100% response rates; reduction in MMD requiring acute migraine medication; change in function score on the Migraine-Specific Quality of Life Questionnaire (MSQ); and change in the Patient Global Impression of Severity (PGI-S) rating.
After adjusting for multiplicity, the investigators found statistically significant differences between placebo and the galcanezumab arms in the primary end point. Mean reductions in headache days were 4.29 in the 120-mg group and 4.18 in the 240-mg group. Patients in the placebo group experienced a reduction of 2.28 days. “We looked at the early effects of treatment and found statistically significant improvements within a few days of injection that persisted over time,” Dr. Conley said.
In addition, statistically significant differences between galcanezumab and placebo were observed for all secondary end points. “In the 120-mg and 240-mg treatment groups, 59.3% and 56.5%, respectively, had at least a 50% reduction in monthly headache days, which was significantly different from placebo, but not different from each other,” said Dr. Conley. “We also saw that approximately one-third of patients in the galcanezumab arms experienced 75% response rates, which is double that seen in the placebo group. The 100% response rate was 11.5% to 13.8% in the treated groups—again, not a significant difference between them, but significantly different from placebo.” The change in the MSQ function score and the PGI-S rating averaged for months 4, 5, and 6 were significantly improved in the galcanezumab arms, compared with the placebo arm.
For most of the safety parameters, there were no significant differences between either dose of galcanezumab and placebo. However, the rates of injection-site reactions and pruritus were significantly higher for both drug doses, compared with placebo. Injection-site erythema was significantly higher in the 240-mg dose only.
Fremanezumab
“The phase III trial for fremanezumab in chronic migraine investigated a flexible dose for a quarterly and a monthly regimen,” said Ernesto Aycardi, MD, Vice President and Therapeutic Area Head for Migraine and Headache at Teva Pharmaceuticals
The primary end point of this multicenter trial was the mean change from baseline in monthly days of headache of at least moderate severity. The four main secondary end points were the proportion of patients with at least a 50% reduction in headache days, mean change in the number of days using acute headache medication at the end of the 12-week period of the trial, mean change from baseline in the days of at least moderate severity in the first four weeks of the trial, and change in disability scale (HIT-6) score from baseline. In this trial, patients treated with fremanezumab experienced significant improvement, compared with placebo on all primary and secondary end points for both monthly and quarterly dosing regimens.
“The average age of the study population was 41, with an average history of migraine of about 20 years,” Dr. Aycardi said. “All demographic characteristics, including weight, height, and ethnicity, were well balanced between the groups.”
Patients on the monthly regimen had a mean reduction of 4.6 headache days of at least moderate severity, and patients on the quarterly regimen had a reduction of 4.3 headache days of at least moderate severity, which was significantly different from the placebo group (2.5 headache days). Researchers also observed significant differences for each of the four secondary end points in favor of the monthly and quarterly regimens.
“Fremanezumab was well tolerated, compared with placebo,” Dr. Aycardi said. “The most commonly reported adverse event in the study was injection-site pain, with similar rates in the placebo and active groups.”
Dr. Aycardi highlighted how phase II results validated that target in chronic migraine. “With these phase III results, this is confirmed, bringing hope for patients with this disabling condition.”
—Adriene Marshall
Suggested Reading
Durham PL. Calcitonin gene-related peptide (CGRP) and migraine. Headache. 2006;46(Suppl 1):S3–S8.
Reuter U. Anti-CGRP antibodies: a new approach to migraine prevention. Lancet Neurol. 2014;13(9):857-859.
Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16(6):425-434.
BOSTON—A new class of drugs for the prevention of chronic and episodic migraine demonstrated promising results in recent phase II and phase III trials. Data for four humanized calcitonin gene–related peptide (CGRP) monoclonal antibodies—eptinezumab, erenumab, fremanezumab, and galcanezumab—were presented at the 59th Annual Scientific Meeting of the American Headache Society. Previous research has long implicated CGRP (which is elevated in jugular vein blood during acute migraine and cluster headaches) in disease pathophysiology. Recent advances in molecular neuroscience have helped shed further light on the pathogenic mechanisms and possible targets for treatment.
Eptinezumab
In a phase II study, a single infusion of eptinezumab (which Alder BioPharmaceuticals is developing under the name ALD403) reduced the number of migraine days per month, as well as the number of migraines classified as severe, in patients with chronic migraine. Of the four drugs presented at the meeting, eptinezumab was the only one to have an IV mode of delivery.
“We used International Classification of Headache Disorders 3 criteria to define chronic migraine, which is at least 15 headache days per month, of which eight must be migraine-like,” said Jeffrey T. L. Smith, MD, Senior Vice President of Translational Medicine at Alder BioPharmaceuticals in Bothell, Washington. “Patients also had to have a history of migraine for a year or more.”
On average, the patients included in this multisite study were slightly younger than 40. Most patients were women, and the average number of migraine days was between 16.2 and 16.5. After a one-month run-in period, patients were randomized to receive placebo or one of four doses of eptinezumab (ie, 300 mg, 100 mg, 30 mg, or 10 mg).
Reporting 12-week data from the 48-week trial, Dr. Smith and colleagues found that significantly more patients taking the 300-mg (33%) and 100-mg (31%) doses of eptinezumab reached the primary end point of a 75% reduction from baseline in migraine days, compared with patients who received placebo (21%). Differences in patients taking the 30-mg and 10-mg doses (28% and 27%, respectively) were not statistically significant. “Overall, approximately half of patients treated with eptinezumab experienced a 50% reduction in migraine days versus 41% of patients on placebo,” Dr. Smith added. Eptinezumab was well tolerated, and no serious adverse events were reported.
Post hoc analyses indicated that of the migraines that were not eliminated as a result of treatment, the percentage classified as severe was significantly reduced from baseline in each of the eptinezumab groups, compared with placebo. Also, fewer patients in the eptinezumab groups than in the placebo group experienced a migraine within the first 24 to 48 hours after infusion. “Although the post hoc results are exploratory, these findings are important as well,” said Dr. Smith. “Other things are happening besides the reduction in migraine frequency that may be of benefit.”
Erenumab
A registration, pivotal study on erenumab for chronic migraine prevention was reported at the meeting. “Unlike eptinezumab, fremanezumab, and galcanezumab, which are monoclonal antibodies against the CGRP ligand, erenumab is an anti-CGRP receptor monoclonal antibody,” said Stewart J. Tepper, MD, Professor of Neurology at the Geisel School of Medicine at Dartmouth in Hanover, New Hampshire. Amgen is developing this antibody.
In the 12-week randomized controlled trial, patients were enrolled in the US and around the world, Dr. Tepper noted. He and his colleagues included patients who had 15 or more headache days per month with at least eight days of migraine per month. Patients were excluded if they had experienced treatment failure with more than three classes of preventive agents. Mean age was 40, about 80% of patients were women, and roughly half of patients had previously experienced treatment failure with at least two preventive agents.
A total of 667 patients were randomized 3:2:2 to placebo, erenumab (70 mg), or erenumab (140 mg). Treatment was given monthly via subcutaneous injection. The primary end point was change from baseline in monthly migraine days (MMD). Secondary end points included at least a 50% reduction in MMD, change from baseline in acute migraine-specific medication days, and cumulative headache hours.
In both erenumab groups, patients experienced a mean 6.6-day reduction in MMD from a baseline of 18.0 days, which was significantly different, compared with the 4.2-day reduction seen in the placebo group. Furthermore, a significantly greater proportion of patients in the 70-mg and 140-mg groups (40% and 41%, respectively) experienced at least a 50% reduction in MMD, compared with the placebo group (23%). For the secondary end points, both erenumab groups had significantly greater reductions in acute migraine-specific medication days versus placebo. However, the difference in the reduction of cumulative headache hours did not reach statistical significance. “We speculated that this was because headache hours is a relatively variable end point, and it is collected in differing ways, with wide confidence intervals,” Dr. Tepper said.
The safety profile for both erenumab groups and the placebo group was similar. “No serious adverse events were reported by more than one patient in any treatment arm, and no serious adverse events were considered related to treatment,” he said.
Another erenumab trial enrolled patients with episodic migraine. Randomization was stratified by region and current and prior migraine preventive medication, said Daniel Mikol, MD, PhD, Executive Medical Director of Neuroscience Development at Amgen in Thousand Oaks, California. Patients included in the phase III study could be taking a single concomitant migraine preventive therapy. “Patients could have also failed previous preventive therapies due to poor tolerability or lack of efficacy,” he said. “There was no limit regarding the number of previous failures due to poor tolerability. With regard to lack of efficacy, patients could have no therapeutic response to two or fewer preventive therapies.”
Adults with episodic migraine (n = 955) were randomized 1:1:1 to monthly subcutaneous injections of placebo, erenumab (70 mg), or erenumab (140 mg) for 24 weeks. The primary end point was change from baseline in MMD from weeks 13 to 24. Secondary end points included achievement of 50% or more reduction in MMD, change in mean acute migraine-specific medication days, and changes in mean physical impairment and impact on everyday activity scores, as measured by the Migraine Physical Function Impact Diary, a novel patient-reported instrument, Dr. Mikol explained.
Subjects had a mean of 8.3 MMD at baseline. This measurement was reduced by 3.2, 3.7, and 1.8 days in the 70-mg, 140-mg, and placebo groups, respectively—a statistically significant difference in favor of the erenumab groups. Statistically superior results for erenumab 70 mg and 140 mg over placebo also were seen in the secondary end points of > 50% response (43%, 50%, and 27%, respectively), reduction from baseline in monthly acute migraine-specific medication days (1.1, 1.6, and 0.2), improved physical impairment score from baseline (4.2, 4.8, and 2.5), and improved everyday activity scores from baseline (5.5, 5.9, and 3.3).
“The safety profiles for erenumab were similar to that of placebo,” Dr. Mikol said. “Specifically, 63% of patients on placebo had adverse events, compared with 56% to 57% of patients in the erenumab groups. Serious adverse events and adverse events leading to treatment discontinuation were low in frequency and balanced across the groups.”
Galcanezumab
The efficacy, tolerability, and safety of galcanezumab, an investigational drug for the treatment of episodic migraine, was assessed in a six-month phase III study called EVOLVE-2. In this study, patients with episodic migraine experienced four to 14 migraine headache days per month with or without aura. “Our patient population had a mean age of about 40, and most [participants] were Caucasian women,” said Robert Conley, MD, Distinguished Lilly Scholar of Neuroscience at Eli Lilly and Company (the developer of galcanezumab) in Indianapolis.
“Half of our subjects were from North America, a quarter from Europe, and a quarter from the rest of the world.” Subjects had a 20-year migraine history on average and a mean of nine headache days per month at baseline, he noted. “In addition, two-thirds had been on prior preventive treatment, and they had relatively severe levels of disease.”
After screening 1,700 patients for the study, Dr. Conley and colleagues randomized 914 patients 1:1:2 to subcutaneous injection of galcanezumab (120 mg/month), galcanezumab (240 mg/month), or placebo. “There was about an 87% completion rate for the galcanezumab groups and an 83% completion rate in the placebo group. The higher number of dropouts in the placebo group was due to lack of efficacy,” said Dr. Conley. The primary end point was change from baseline in MMD at the end of six months. Secondary end points were percentages of patients with 50%, 75%, and 100% response rates; reduction in MMD requiring acute migraine medication; change in function score on the Migraine-Specific Quality of Life Questionnaire (MSQ); and change in the Patient Global Impression of Severity (PGI-S) rating.
After adjusting for multiplicity, the investigators found statistically significant differences between placebo and the galcanezumab arms in the primary end point. Mean reductions in headache days were 4.29 in the 120-mg group and 4.18 in the 240-mg group. Patients in the placebo group experienced a reduction of 2.28 days. “We looked at the early effects of treatment and found statistically significant improvements within a few days of injection that persisted over time,” Dr. Conley said.
In addition, statistically significant differences between galcanezumab and placebo were observed for all secondary end points. “In the 120-mg and 240-mg treatment groups, 59.3% and 56.5%, respectively, had at least a 50% reduction in monthly headache days, which was significantly different from placebo, but not different from each other,” said Dr. Conley. “We also saw that approximately one-third of patients in the galcanezumab arms experienced 75% response rates, which is double that seen in the placebo group. The 100% response rate was 11.5% to 13.8% in the treated groups—again, not a significant difference between them, but significantly different from placebo.” The change in the MSQ function score and the PGI-S rating averaged for months 4, 5, and 6 were significantly improved in the galcanezumab arms, compared with the placebo arm.
For most of the safety parameters, there were no significant differences between either dose of galcanezumab and placebo. However, the rates of injection-site reactions and pruritus were significantly higher for both drug doses, compared with placebo. Injection-site erythema was significantly higher in the 240-mg dose only.
Fremanezumab
“The phase III trial for fremanezumab in chronic migraine investigated a flexible dose for a quarterly and a monthly regimen,” said Ernesto Aycardi, MD, Vice President and Therapeutic Area Head for Migraine and Headache at Teva Pharmaceuticals
The primary end point of this multicenter trial was the mean change from baseline in monthly days of headache of at least moderate severity. The four main secondary end points were the proportion of patients with at least a 50% reduction in headache days, mean change in the number of days using acute headache medication at the end of the 12-week period of the trial, mean change from baseline in the days of at least moderate severity in the first four weeks of the trial, and change in disability scale (HIT-6) score from baseline. In this trial, patients treated with fremanezumab experienced significant improvement, compared with placebo on all primary and secondary end points for both monthly and quarterly dosing regimens.
“The average age of the study population was 41, with an average history of migraine of about 20 years,” Dr. Aycardi said. “All demographic characteristics, including weight, height, and ethnicity, were well balanced between the groups.”
Patients on the monthly regimen had a mean reduction of 4.6 headache days of at least moderate severity, and patients on the quarterly regimen had a reduction of 4.3 headache days of at least moderate severity, which was significantly different from the placebo group (2.5 headache days). Researchers also observed significant differences for each of the four secondary end points in favor of the monthly and quarterly regimens.
“Fremanezumab was well tolerated, compared with placebo,” Dr. Aycardi said. “The most commonly reported adverse event in the study was injection-site pain, with similar rates in the placebo and active groups.”
Dr. Aycardi highlighted how phase II results validated that target in chronic migraine. “With these phase III results, this is confirmed, bringing hope for patients with this disabling condition.”
—Adriene Marshall
Suggested Reading
Durham PL. Calcitonin gene-related peptide (CGRP) and migraine. Headache. 2006;46(Suppl 1):S3–S8.
Reuter U. Anti-CGRP antibodies: a new approach to migraine prevention. Lancet Neurol. 2014;13(9):857-859.
Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16(6):425-434.
BOSTON—A new class of drugs for the prevention of chronic and episodic migraine demonstrated promising results in recent phase II and phase III trials. Data for four humanized calcitonin gene–related peptide (CGRP) monoclonal antibodies—eptinezumab, erenumab, fremanezumab, and galcanezumab—were presented at the 59th Annual Scientific Meeting of the American Headache Society. Previous research has long implicated CGRP (which is elevated in jugular vein blood during acute migraine and cluster headaches) in disease pathophysiology. Recent advances in molecular neuroscience have helped shed further light on the pathogenic mechanisms and possible targets for treatment.
Eptinezumab
In a phase II study, a single infusion of eptinezumab (which Alder BioPharmaceuticals is developing under the name ALD403) reduced the number of migraine days per month, as well as the number of migraines classified as severe, in patients with chronic migraine. Of the four drugs presented at the meeting, eptinezumab was the only one to have an IV mode of delivery.
“We used International Classification of Headache Disorders 3 criteria to define chronic migraine, which is at least 15 headache days per month, of which eight must be migraine-like,” said Jeffrey T. L. Smith, MD, Senior Vice President of Translational Medicine at Alder BioPharmaceuticals in Bothell, Washington. “Patients also had to have a history of migraine for a year or more.”
On average, the patients included in this multisite study were slightly younger than 40. Most patients were women, and the average number of migraine days was between 16.2 and 16.5. After a one-month run-in period, patients were randomized to receive placebo or one of four doses of eptinezumab (ie, 300 mg, 100 mg, 30 mg, or 10 mg).
Reporting 12-week data from the 48-week trial, Dr. Smith and colleagues found that significantly more patients taking the 300-mg (33%) and 100-mg (31%) doses of eptinezumab reached the primary end point of a 75% reduction from baseline in migraine days, compared with patients who received placebo (21%). Differences in patients taking the 30-mg and 10-mg doses (28% and 27%, respectively) were not statistically significant. “Overall, approximately half of patients treated with eptinezumab experienced a 50% reduction in migraine days versus 41% of patients on placebo,” Dr. Smith added. Eptinezumab was well tolerated, and no serious adverse events were reported.
Post hoc analyses indicated that of the migraines that were not eliminated as a result of treatment, the percentage classified as severe was significantly reduced from baseline in each of the eptinezumab groups, compared with placebo. Also, fewer patients in the eptinezumab groups than in the placebo group experienced a migraine within the first 24 to 48 hours after infusion. “Although the post hoc results are exploratory, these findings are important as well,” said Dr. Smith. “Other things are happening besides the reduction in migraine frequency that may be of benefit.”
Erenumab
A registration, pivotal study on erenumab for chronic migraine prevention was reported at the meeting. “Unlike eptinezumab, fremanezumab, and galcanezumab, which are monoclonal antibodies against the CGRP ligand, erenumab is an anti-CGRP receptor monoclonal antibody,” said Stewart J. Tepper, MD, Professor of Neurology at the Geisel School of Medicine at Dartmouth in Hanover, New Hampshire. Amgen is developing this antibody.
In the 12-week randomized controlled trial, patients were enrolled in the US and around the world, Dr. Tepper noted. He and his colleagues included patients who had 15 or more headache days per month with at least eight days of migraine per month. Patients were excluded if they had experienced treatment failure with more than three classes of preventive agents. Mean age was 40, about 80% of patients were women, and roughly half of patients had previously experienced treatment failure with at least two preventive agents.
A total of 667 patients were randomized 3:2:2 to placebo, erenumab (70 mg), or erenumab (140 mg). Treatment was given monthly via subcutaneous injection. The primary end point was change from baseline in monthly migraine days (MMD). Secondary end points included at least a 50% reduction in MMD, change from baseline in acute migraine-specific medication days, and cumulative headache hours.
In both erenumab groups, patients experienced a mean 6.6-day reduction in MMD from a baseline of 18.0 days, which was significantly different, compared with the 4.2-day reduction seen in the placebo group. Furthermore, a significantly greater proportion of patients in the 70-mg and 140-mg groups (40% and 41%, respectively) experienced at least a 50% reduction in MMD, compared with the placebo group (23%). For the secondary end points, both erenumab groups had significantly greater reductions in acute migraine-specific medication days versus placebo. However, the difference in the reduction of cumulative headache hours did not reach statistical significance. “We speculated that this was because headache hours is a relatively variable end point, and it is collected in differing ways, with wide confidence intervals,” Dr. Tepper said.
The safety profile for both erenumab groups and the placebo group was similar. “No serious adverse events were reported by more than one patient in any treatment arm, and no serious adverse events were considered related to treatment,” he said.
Another erenumab trial enrolled patients with episodic migraine. Randomization was stratified by region and current and prior migraine preventive medication, said Daniel Mikol, MD, PhD, Executive Medical Director of Neuroscience Development at Amgen in Thousand Oaks, California. Patients included in the phase III study could be taking a single concomitant migraine preventive therapy. “Patients could have also failed previous preventive therapies due to poor tolerability or lack of efficacy,” he said. “There was no limit regarding the number of previous failures due to poor tolerability. With regard to lack of efficacy, patients could have no therapeutic response to two or fewer preventive therapies.”
Adults with episodic migraine (n = 955) were randomized 1:1:1 to monthly subcutaneous injections of placebo, erenumab (70 mg), or erenumab (140 mg) for 24 weeks. The primary end point was change from baseline in MMD from weeks 13 to 24. Secondary end points included achievement of 50% or more reduction in MMD, change in mean acute migraine-specific medication days, and changes in mean physical impairment and impact on everyday activity scores, as measured by the Migraine Physical Function Impact Diary, a novel patient-reported instrument, Dr. Mikol explained.
Subjects had a mean of 8.3 MMD at baseline. This measurement was reduced by 3.2, 3.7, and 1.8 days in the 70-mg, 140-mg, and placebo groups, respectively—a statistically significant difference in favor of the erenumab groups. Statistically superior results for erenumab 70 mg and 140 mg over placebo also were seen in the secondary end points of > 50% response (43%, 50%, and 27%, respectively), reduction from baseline in monthly acute migraine-specific medication days (1.1, 1.6, and 0.2), improved physical impairment score from baseline (4.2, 4.8, and 2.5), and improved everyday activity scores from baseline (5.5, 5.9, and 3.3).
“The safety profiles for erenumab were similar to that of placebo,” Dr. Mikol said. “Specifically, 63% of patients on placebo had adverse events, compared with 56% to 57% of patients in the erenumab groups. Serious adverse events and adverse events leading to treatment discontinuation were low in frequency and balanced across the groups.”
Galcanezumab
The efficacy, tolerability, and safety of galcanezumab, an investigational drug for the treatment of episodic migraine, was assessed in a six-month phase III study called EVOLVE-2. In this study, patients with episodic migraine experienced four to 14 migraine headache days per month with or without aura. “Our patient population had a mean age of about 40, and most [participants] were Caucasian women,” said Robert Conley, MD, Distinguished Lilly Scholar of Neuroscience at Eli Lilly and Company (the developer of galcanezumab) in Indianapolis.
“Half of our subjects were from North America, a quarter from Europe, and a quarter from the rest of the world.” Subjects had a 20-year migraine history on average and a mean of nine headache days per month at baseline, he noted. “In addition, two-thirds had been on prior preventive treatment, and they had relatively severe levels of disease.”
After screening 1,700 patients for the study, Dr. Conley and colleagues randomized 914 patients 1:1:2 to subcutaneous injection of galcanezumab (120 mg/month), galcanezumab (240 mg/month), or placebo. “There was about an 87% completion rate for the galcanezumab groups and an 83% completion rate in the placebo group. The higher number of dropouts in the placebo group was due to lack of efficacy,” said Dr. Conley. The primary end point was change from baseline in MMD at the end of six months. Secondary end points were percentages of patients with 50%, 75%, and 100% response rates; reduction in MMD requiring acute migraine medication; change in function score on the Migraine-Specific Quality of Life Questionnaire (MSQ); and change in the Patient Global Impression of Severity (PGI-S) rating.
After adjusting for multiplicity, the investigators found statistically significant differences between placebo and the galcanezumab arms in the primary end point. Mean reductions in headache days were 4.29 in the 120-mg group and 4.18 in the 240-mg group. Patients in the placebo group experienced a reduction of 2.28 days. “We looked at the early effects of treatment and found statistically significant improvements within a few days of injection that persisted over time,” Dr. Conley said.
In addition, statistically significant differences between galcanezumab and placebo were observed for all secondary end points. “In the 120-mg and 240-mg treatment groups, 59.3% and 56.5%, respectively, had at least a 50% reduction in monthly headache days, which was significantly different from placebo, but not different from each other,” said Dr. Conley. “We also saw that approximately one-third of patients in the galcanezumab arms experienced 75% response rates, which is double that seen in the placebo group. The 100% response rate was 11.5% to 13.8% in the treated groups—again, not a significant difference between them, but significantly different from placebo.” The change in the MSQ function score and the PGI-S rating averaged for months 4, 5, and 6 were significantly improved in the galcanezumab arms, compared with the placebo arm.
For most of the safety parameters, there were no significant differences between either dose of galcanezumab and placebo. However, the rates of injection-site reactions and pruritus were significantly higher for both drug doses, compared with placebo. Injection-site erythema was significantly higher in the 240-mg dose only.
Fremanezumab
“The phase III trial for fremanezumab in chronic migraine investigated a flexible dose for a quarterly and a monthly regimen,” said Ernesto Aycardi, MD, Vice President and Therapeutic Area Head for Migraine and Headache at Teva Pharmaceuticals
The primary end point of this multicenter trial was the mean change from baseline in monthly days of headache of at least moderate severity. The four main secondary end points were the proportion of patients with at least a 50% reduction in headache days, mean change in the number of days using acute headache medication at the end of the 12-week period of the trial, mean change from baseline in the days of at least moderate severity in the first four weeks of the trial, and change in disability scale (HIT-6) score from baseline. In this trial, patients treated with fremanezumab experienced significant improvement, compared with placebo on all primary and secondary end points for both monthly and quarterly dosing regimens.
“The average age of the study population was 41, with an average history of migraine of about 20 years,” Dr. Aycardi said. “All demographic characteristics, including weight, height, and ethnicity, were well balanced between the groups.”
Patients on the monthly regimen had a mean reduction of 4.6 headache days of at least moderate severity, and patients on the quarterly regimen had a reduction of 4.3 headache days of at least moderate severity, which was significantly different from the placebo group (2.5 headache days). Researchers also observed significant differences for each of the four secondary end points in favor of the monthly and quarterly regimens.
“Fremanezumab was well tolerated, compared with placebo,” Dr. Aycardi said. “The most commonly reported adverse event in the study was injection-site pain, with similar rates in the placebo and active groups.”
Dr. Aycardi highlighted how phase II results validated that target in chronic migraine. “With these phase III results, this is confirmed, bringing hope for patients with this disabling condition.”
—Adriene Marshall
Suggested Reading
Durham PL. Calcitonin gene-related peptide (CGRP) and migraine. Headache. 2006;46(Suppl 1):S3–S8.
Reuter U. Anti-CGRP antibodies: a new approach to migraine prevention. Lancet Neurol. 2014;13(9):857-859.
Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16(6):425-434.
Hidden CABG costs will disrupt bundled payment systems
COLORADO SPRINGS – With bundled payment models for coronary artery bypass graft surgery looming ahead, it’s vital that cardiac surgeons take a hard look at the procedure’s hidden costs – namely, the steep price tag for postoperative complications, James H. Mehaffey, MD, said at the annual meeting of the Western Thoracic Surgical Association.
He presented a retrospective study of the 30-day hospital costs for all 36,588 patients who underwent isolated CABG during 2006-2015 at the 19 Virginia centers where the surgery is performed. This was a typical CABG population, with an average predicted risk of mortality of 1.9%. The actual 30-day mortality was 0.6%, so the surgical performance was better than expected.
“The population of patients experiencing one or more major comorbidities demonstrated a significant and dramatic increase in total hospital costs. It was an exponential increase with each additional major morbidity,” reported Dr. Mehaffey of the University of Virginia, Charlottesville.
Indeed, the average cost jumped from $36,580 for uncomplicated surgery to $64,542 with one major complication, $111,239 with two, and $194,043 with three.
The two most frequent major complications were postoperative atrial fibrillation, which occurred in 18.4% of patients, and prolonged ventilation for longer than 24 hours, which occurred in 9%. Over the course of the decade-long study period, the 19 medical centers in the Virginia Cardiac Surgery Quality Initiative collectively spent roughly $59 million on prolonged ventilation and $27 million for postoperative atrial fibrillation.
The cost of CABG during the study years outpaced the CMS health care–specific inflation rate, and this escalating cost was driven primarily by postoperative complications.
For the Virginia cardiac surgery collaborative, these data on the cost of postoperative complications will be utilized to prioritize quality improvement projects.
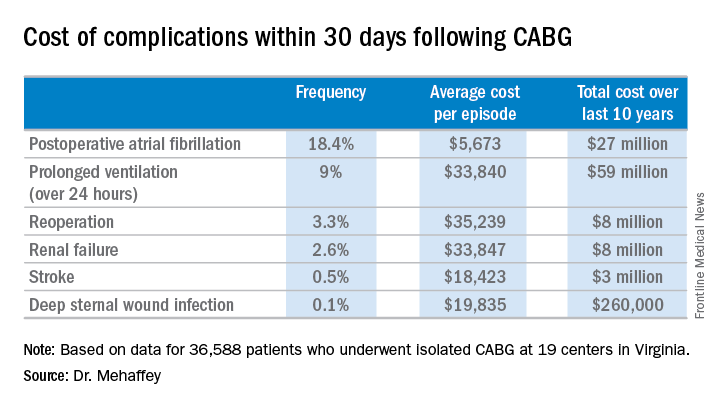
For example, during the past decade, the Virginia collaborative made reduction in the rate of postoperative atrial fibrillation a priority. Toward that end, the collaborative developed a protocol for routine perioperative prophylactic amiodarone therapy.
“At the beginning of the study decade we had postoperative atrial fibrillation rates above 25%. The average for the entire decade was just over 18%, and in the last couple years we’ve been in the 15%-16% range. So I think we are moving the needle on this. We are making a meaningful impact,” Dr. Mehaffey said.
“We’ve already used the complication cost data to do a cost-effectiveness analysis of our prophylactic amiodarone innovation. We showed we saved an average of $250 per patient, even though we’re treating a bunch of patients who’d never get that complication,” he continued.
This sort of data on the cost of adverse events is also critical to accurately risk-adjust bundled payment models.
Discussant Richard J. Shemin, MD, asked if there was much variability in postoperative complication costs between the CABG centers in the Virginia collaborative.
The variability is enormous, Dr. Mehaffey replied. Investigators recently plugged the last 5 years worth of hospital cost and complication rate data into a proposed CABG bundled payment model and extrapolated what that would mean over the next 5 years.
“There were some institutions that would be positive by a couple million dollars from this payment system and some that were losing more than $20 million, just because of the cost variability,” said Dr. Mehaffey.
Dr. Shemin also noted that the Virginia collaborative was able to collect 30-day outcome data only through the STS database, yet the bundled payment programs are based on the 90-day postoperative experience.
“How do we capture the costs in that full 90 days that we’ll be responsible for?” asked Dr. Shemin, professor of surgery and codirector of the UCLA Cardiovascular Center.
Dr. Mehaffey said that’s indeed an important question, since a major complication such as stroke or deep sternal wound infection typically entails considerable long-term costs and repeated hospital admissions beyond the 30-day window. In Virginia, the cardiac surgery collaborative is working with payers to gain access to the 90 days worth of patient data.
He reported having no financial conflicts regarding his study.
COLORADO SPRINGS – With bundled payment models for coronary artery bypass graft surgery looming ahead, it’s vital that cardiac surgeons take a hard look at the procedure’s hidden costs – namely, the steep price tag for postoperative complications, James H. Mehaffey, MD, said at the annual meeting of the Western Thoracic Surgical Association.
He presented a retrospective study of the 30-day hospital costs for all 36,588 patients who underwent isolated CABG during 2006-2015 at the 19 Virginia centers where the surgery is performed. This was a typical CABG population, with an average predicted risk of mortality of 1.9%. The actual 30-day mortality was 0.6%, so the surgical performance was better than expected.
“The population of patients experiencing one or more major comorbidities demonstrated a significant and dramatic increase in total hospital costs. It was an exponential increase with each additional major morbidity,” reported Dr. Mehaffey of the University of Virginia, Charlottesville.
Indeed, the average cost jumped from $36,580 for uncomplicated surgery to $64,542 with one major complication, $111,239 with two, and $194,043 with three.
The two most frequent major complications were postoperative atrial fibrillation, which occurred in 18.4% of patients, and prolonged ventilation for longer than 24 hours, which occurred in 9%. Over the course of the decade-long study period, the 19 medical centers in the Virginia Cardiac Surgery Quality Initiative collectively spent roughly $59 million on prolonged ventilation and $27 million for postoperative atrial fibrillation.
The cost of CABG during the study years outpaced the CMS health care–specific inflation rate, and this escalating cost was driven primarily by postoperative complications.
For the Virginia cardiac surgery collaborative, these data on the cost of postoperative complications will be utilized to prioritize quality improvement projects.

For example, during the past decade, the Virginia collaborative made reduction in the rate of postoperative atrial fibrillation a priority. Toward that end, the collaborative developed a protocol for routine perioperative prophylactic amiodarone therapy.
“At the beginning of the study decade we had postoperative atrial fibrillation rates above 25%. The average for the entire decade was just over 18%, and in the last couple years we’ve been in the 15%-16% range. So I think we are moving the needle on this. We are making a meaningful impact,” Dr. Mehaffey said.
“We’ve already used the complication cost data to do a cost-effectiveness analysis of our prophylactic amiodarone innovation. We showed we saved an average of $250 per patient, even though we’re treating a bunch of patients who’d never get that complication,” he continued.
This sort of data on the cost of adverse events is also critical to accurately risk-adjust bundled payment models.
Discussant Richard J. Shemin, MD, asked if there was much variability in postoperative complication costs between the CABG centers in the Virginia collaborative.
The variability is enormous, Dr. Mehaffey replied. Investigators recently plugged the last 5 years worth of hospital cost and complication rate data into a proposed CABG bundled payment model and extrapolated what that would mean over the next 5 years.
“There were some institutions that would be positive by a couple million dollars from this payment system and some that were losing more than $20 million, just because of the cost variability,” said Dr. Mehaffey.
Dr. Shemin also noted that the Virginia collaborative was able to collect 30-day outcome data only through the STS database, yet the bundled payment programs are based on the 90-day postoperative experience.
“How do we capture the costs in that full 90 days that we’ll be responsible for?” asked Dr. Shemin, professor of surgery and codirector of the UCLA Cardiovascular Center.
Dr. Mehaffey said that’s indeed an important question, since a major complication such as stroke or deep sternal wound infection typically entails considerable long-term costs and repeated hospital admissions beyond the 30-day window. In Virginia, the cardiac surgery collaborative is working with payers to gain access to the 90 days worth of patient data.
He reported having no financial conflicts regarding his study.
COLORADO SPRINGS – With bundled payment models for coronary artery bypass graft surgery looming ahead, it’s vital that cardiac surgeons take a hard look at the procedure’s hidden costs – namely, the steep price tag for postoperative complications, James H. Mehaffey, MD, said at the annual meeting of the Western Thoracic Surgical Association.
He presented a retrospective study of the 30-day hospital costs for all 36,588 patients who underwent isolated CABG during 2006-2015 at the 19 Virginia centers where the surgery is performed. This was a typical CABG population, with an average predicted risk of mortality of 1.9%. The actual 30-day mortality was 0.6%, so the surgical performance was better than expected.
“The population of patients experiencing one or more major comorbidities demonstrated a significant and dramatic increase in total hospital costs. It was an exponential increase with each additional major morbidity,” reported Dr. Mehaffey of the University of Virginia, Charlottesville.
Indeed, the average cost jumped from $36,580 for uncomplicated surgery to $64,542 with one major complication, $111,239 with two, and $194,043 with three.
The two most frequent major complications were postoperative atrial fibrillation, which occurred in 18.4% of patients, and prolonged ventilation for longer than 24 hours, which occurred in 9%. Over the course of the decade-long study period, the 19 medical centers in the Virginia Cardiac Surgery Quality Initiative collectively spent roughly $59 million on prolonged ventilation and $27 million for postoperative atrial fibrillation.
The cost of CABG during the study years outpaced the CMS health care–specific inflation rate, and this escalating cost was driven primarily by postoperative complications.
For the Virginia cardiac surgery collaborative, these data on the cost of postoperative complications will be utilized to prioritize quality improvement projects.

For example, during the past decade, the Virginia collaborative made reduction in the rate of postoperative atrial fibrillation a priority. Toward that end, the collaborative developed a protocol for routine perioperative prophylactic amiodarone therapy.
“At the beginning of the study decade we had postoperative atrial fibrillation rates above 25%. The average for the entire decade was just over 18%, and in the last couple years we’ve been in the 15%-16% range. So I think we are moving the needle on this. We are making a meaningful impact,” Dr. Mehaffey said.
“We’ve already used the complication cost data to do a cost-effectiveness analysis of our prophylactic amiodarone innovation. We showed we saved an average of $250 per patient, even though we’re treating a bunch of patients who’d never get that complication,” he continued.
This sort of data on the cost of adverse events is also critical to accurately risk-adjust bundled payment models.
Discussant Richard J. Shemin, MD, asked if there was much variability in postoperative complication costs between the CABG centers in the Virginia collaborative.
The variability is enormous, Dr. Mehaffey replied. Investigators recently plugged the last 5 years worth of hospital cost and complication rate data into a proposed CABG bundled payment model and extrapolated what that would mean over the next 5 years.
“There were some institutions that would be positive by a couple million dollars from this payment system and some that were losing more than $20 million, just because of the cost variability,” said Dr. Mehaffey.
Dr. Shemin also noted that the Virginia collaborative was able to collect 30-day outcome data only through the STS database, yet the bundled payment programs are based on the 90-day postoperative experience.
“How do we capture the costs in that full 90 days that we’ll be responsible for?” asked Dr. Shemin, professor of surgery and codirector of the UCLA Cardiovascular Center.
Dr. Mehaffey said that’s indeed an important question, since a major complication such as stroke or deep sternal wound infection typically entails considerable long-term costs and repeated hospital admissions beyond the 30-day window. In Virginia, the cardiac surgery collaborative is working with payers to gain access to the 90 days worth of patient data.
He reported having no financial conflicts regarding his study.
AT THE WTSA ANNUAL MEETING
Key clinical point:
Major finding: The average 30-day total hospital cost of an isolated CABG procedure during 2006-2015 in Virginia was $36,580 if there were no postoperative complications, jumping to $64,542 with one major complication and $111,239 with two.
Data source: A retrospective study of the 30-day total hospital costs for all isolated CABG procedures performed in Virginia during 2006-2015.
Disclosures: The study presenter reported having no financial conflicts.
Medicare Part D premiums dip as drug costs continue to rise
As insurance premiums in other sectors of the health care industry continue to soar, seniors enrolled in the Medicare Part D prescription drug plan will see a slight decrease in the average premium for their drug coverage.
The Centers for Medicare & Medicaid Services announced Aug. 2 that the average basic premium for drug coverage is projected to drop to $33.50 per month in 2018, down $1.20 from 2017’s average monthly premium of $34.70.
Average premiums are falling despite the rise in drug spending, according to the 2017 Medicare Trustees report, which details the solvency of the Medicare program.
The report notes, however, that drug costs are lower than previous reports due to higher manufacturer rebates and decreased use of direct acting antiviral therapies for hepatitis C virus. Overall, Medicare Part D expenditures per enrollee are estimated to increase by an average of 4.7% annually from 2017 through 2026.
As insurance premiums in other sectors of the health care industry continue to soar, seniors enrolled in the Medicare Part D prescription drug plan will see a slight decrease in the average premium for their drug coverage.
The Centers for Medicare & Medicaid Services announced Aug. 2 that the average basic premium for drug coverage is projected to drop to $33.50 per month in 2018, down $1.20 from 2017’s average monthly premium of $34.70.
Average premiums are falling despite the rise in drug spending, according to the 2017 Medicare Trustees report, which details the solvency of the Medicare program.
The report notes, however, that drug costs are lower than previous reports due to higher manufacturer rebates and decreased use of direct acting antiviral therapies for hepatitis C virus. Overall, Medicare Part D expenditures per enrollee are estimated to increase by an average of 4.7% annually from 2017 through 2026.
As insurance premiums in other sectors of the health care industry continue to soar, seniors enrolled in the Medicare Part D prescription drug plan will see a slight decrease in the average premium for their drug coverage.
The Centers for Medicare & Medicaid Services announced Aug. 2 that the average basic premium for drug coverage is projected to drop to $33.50 per month in 2018, down $1.20 from 2017’s average monthly premium of $34.70.
Average premiums are falling despite the rise in drug spending, according to the 2017 Medicare Trustees report, which details the solvency of the Medicare program.
The report notes, however, that drug costs are lower than previous reports due to higher manufacturer rebates and decreased use of direct acting antiviral therapies for hepatitis C virus. Overall, Medicare Part D expenditures per enrollee are estimated to increase by an average of 4.7% annually from 2017 through 2026.


















