User login
Elastrographic ultrasound could guide adenomyosis treatment
VANCOUVER – Transvaginal elastrographic (TVEG) ultrasound appears to be a better way to diagnose adenomyosis, outperforming transvaginal ultrasound in identifying lesions, according to new findings.
Researchers at Fudan University in Shanghai compared TVEG results in 152 women with adenomyosis, 89 women with fibroids, and 136 healthy controls. None of the women had received hormone therapy in the previous 6 months. Imaging was performed with both TVEG and transvaginal ultrasound, and tissue samples were taken to test for estrogen receptor (ER)-beta, progesterone receptor (PR), epithelial cadherin, and alpha–smooth muscle actin (SMA).
Image analysis showed that TVEG readily distinguished adenomyosis from fibroids or normal uterine tissue. The elastic value, representing stiffness, was highest in adenomyosis patients (3.74 plus or minus 1.01, P less than .001), followed by fibrosis (2.87 plus or minus 0.74; P less than .001), and normal tissue (1.43 plus or minus 0.59).
Elastic values correlated positively to the extent of fibrosis (r = 0.91; P less than .001), and staining levels of alpha-SMA and ER-beta (r = 0.84; P less than .001). Elasticity correlated negatively with epithelial cadherin and PR (r = –0.86; P less than .001).
The researchers concluded that TVEG outperforms transvaginal ultrasound in diagnosing adenomyosis, and that the close correlation between measurements of stiffness and fibrosis and hormone response markers suggests that it could one day help physicians choose between hormone therapy and hysterectomy.
“If we find more elastic values, maybe that means there is more fibrosis in the lesion, and it may be not as sensitive to hormone treatment, so maybe we should move on to hysterectomy,” Ding Ding, MD, PhD, associate professor of gynecology at Fudan University, said at the World Congress on Endometriosis.
But the current research does not provide those answers yet, since the elastic values weren’t linked to a clinical outcome. “We want to verify in the next step, in women who have higher elastic values, whether they are sensitive to progesterone treatment,” Dr. Ding said.
The study was sponsored by the Chinese government. Dr. Ding reported having no financial disclosures.
VANCOUVER – Transvaginal elastrographic (TVEG) ultrasound appears to be a better way to diagnose adenomyosis, outperforming transvaginal ultrasound in identifying lesions, according to new findings.
Researchers at Fudan University in Shanghai compared TVEG results in 152 women with adenomyosis, 89 women with fibroids, and 136 healthy controls. None of the women had received hormone therapy in the previous 6 months. Imaging was performed with both TVEG and transvaginal ultrasound, and tissue samples were taken to test for estrogen receptor (ER)-beta, progesterone receptor (PR), epithelial cadherin, and alpha–smooth muscle actin (SMA).
Image analysis showed that TVEG readily distinguished adenomyosis from fibroids or normal uterine tissue. The elastic value, representing stiffness, was highest in adenomyosis patients (3.74 plus or minus 1.01, P less than .001), followed by fibrosis (2.87 plus or minus 0.74; P less than .001), and normal tissue (1.43 plus or minus 0.59).
Elastic values correlated positively to the extent of fibrosis (r = 0.91; P less than .001), and staining levels of alpha-SMA and ER-beta (r = 0.84; P less than .001). Elasticity correlated negatively with epithelial cadherin and PR (r = –0.86; P less than .001).
The researchers concluded that TVEG outperforms transvaginal ultrasound in diagnosing adenomyosis, and that the close correlation between measurements of stiffness and fibrosis and hormone response markers suggests that it could one day help physicians choose between hormone therapy and hysterectomy.
“If we find more elastic values, maybe that means there is more fibrosis in the lesion, and it may be not as sensitive to hormone treatment, so maybe we should move on to hysterectomy,” Ding Ding, MD, PhD, associate professor of gynecology at Fudan University, said at the World Congress on Endometriosis.
But the current research does not provide those answers yet, since the elastic values weren’t linked to a clinical outcome. “We want to verify in the next step, in women who have higher elastic values, whether they are sensitive to progesterone treatment,” Dr. Ding said.
The study was sponsored by the Chinese government. Dr. Ding reported having no financial disclosures.
VANCOUVER – Transvaginal elastrographic (TVEG) ultrasound appears to be a better way to diagnose adenomyosis, outperforming transvaginal ultrasound in identifying lesions, according to new findings.
Researchers at Fudan University in Shanghai compared TVEG results in 152 women with adenomyosis, 89 women with fibroids, and 136 healthy controls. None of the women had received hormone therapy in the previous 6 months. Imaging was performed with both TVEG and transvaginal ultrasound, and tissue samples were taken to test for estrogen receptor (ER)-beta, progesterone receptor (PR), epithelial cadherin, and alpha–smooth muscle actin (SMA).
Image analysis showed that TVEG readily distinguished adenomyosis from fibroids or normal uterine tissue. The elastic value, representing stiffness, was highest in adenomyosis patients (3.74 plus or minus 1.01, P less than .001), followed by fibrosis (2.87 plus or minus 0.74; P less than .001), and normal tissue (1.43 plus or minus 0.59).
Elastic values correlated positively to the extent of fibrosis (r = 0.91; P less than .001), and staining levels of alpha-SMA and ER-beta (r = 0.84; P less than .001). Elasticity correlated negatively with epithelial cadherin and PR (r = –0.86; P less than .001).
The researchers concluded that TVEG outperforms transvaginal ultrasound in diagnosing adenomyosis, and that the close correlation between measurements of stiffness and fibrosis and hormone response markers suggests that it could one day help physicians choose between hormone therapy and hysterectomy.
“If we find more elastic values, maybe that means there is more fibrosis in the lesion, and it may be not as sensitive to hormone treatment, so maybe we should move on to hysterectomy,” Ding Ding, MD, PhD, associate professor of gynecology at Fudan University, said at the World Congress on Endometriosis.
But the current research does not provide those answers yet, since the elastic values weren’t linked to a clinical outcome. “We want to verify in the next step, in women who have higher elastic values, whether they are sensitive to progesterone treatment,” Dr. Ding said.
The study was sponsored by the Chinese government. Dr. Ding reported having no financial disclosures.
AT WCE 2017
Key clinical point:
Major finding: Elastic values correlated with fibrosis (r = 0.91), alpha-SMA and ER-beta (r = 0.84), and epithelial cadherin and PR (r = –0.86).
Data source: Prospective case-controlled study of 152 women with adenomyosis, 89 with fibroids, and 136 controls.
Disclosures: The study was sponsored by the Chinese government. Dr. Ding reported having no financial disclosures.
This month in CHEST: Editor’s picks
Giants in Chest MedicineKarlman Wasserman, MD, PhD, FCCP. By Dr. T. Kisaka, et al.
Original ResearchHydrocortisone, Vitamin C, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Before-After Study. By Dr. P. Marik, et al.
Quantitative CT Measures of Bronchiectasis in Smokers. By Dr. A. A. Diaz, et al.
Giants in Chest MedicineKarlman Wasserman, MD, PhD, FCCP. By Dr. T. Kisaka, et al.
Original ResearchHydrocortisone, Vitamin C, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Before-After Study. By Dr. P. Marik, et al.
Quantitative CT Measures of Bronchiectasis in Smokers. By Dr. A. A. Diaz, et al.
Giants in Chest MedicineKarlman Wasserman, MD, PhD, FCCP. By Dr. T. Kisaka, et al.
Original ResearchHydrocortisone, Vitamin C, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Before-After Study. By Dr. P. Marik, et al.
Quantitative CT Measures of Bronchiectasis in Smokers. By Dr. A. A. Diaz, et al.
ABIM Internal Medicine Summit
On April 7, four members of CHEST staff and leadership, along with staff and leadership from other medical specialty societies, participated in the Internal Medicine Summit, hosted by the American Board of Internal Medicine, in Philadelphia. The meeting covered an array of topics related to certification and maintenance of certification (MOC), including the alternative assessment model announced in December 2016, quality improvement (QI) as part of MOC, and practicing medicine in an ever-changing political landscape.
The meeting began with Dr. Richard Baron, President and CEO of the ABIM, explaining how the notion of certification has changed over the years. According to Dr. Baron, the concept of lifetime certification no longer makes sense in the rapidly changing field of medicine. As part of the evolution of certification, the ABIM has moved away from “rules to follow” toward something, co-created with societies, that is more relevant and less burdensome. This shift includes aligning certification and MOC requirements with things physicians are already required to do by their states and institutions. Dr. Baron also stressed that in today’s cultural and political landscape, along with the prevalence of “fake news,” the need for trust in the doctor-patient relationship is increasing; trust is no longer a “given.” Therefore, in an age when credentials can be purchased online, there’s an increasing need for an external certification to build trust and boost credibility.
Dr. Marianne Green, member of the ABIM Board of Directors and the ABIM Council, gave an update on the recertification assessment options. While currently, only an every 2-year assessment option will be offered as an alternative to a 10-year higher stakes exam, the ABIM is looking to partner with societies to deliver education, based on the needs identified via the assessment. Furthermore, in addition to partnering with societies to address the identified knowledge gaps, the ABIM plans to collaborate with societies in future alternatives to both the 2-year and 10-year assessments, with the shared goal of “maintenance and support of a community of life-long learners who hold ourselves accountable to peer-defined standards.” Initially, the 2-year lower stakes assessment will cover the breadth of the knowledge in the specialty/subspecialty, but the ABIM is committed to taking a more modular approach in the future. When asked about the fee structure for the new assessment options, Dr. Green communicated that details regarding fees would be announced in fall 2017.
While the first part of the meeting focused on MOC Part 2, the conversation turned toward quality improvement, or QI, later part of the meeting. The practice improvement, or MOC Part 4, requirement is on hold through the end of 2018. Both the ABIM and represented societies value the importance of quality measures. Dr. Graham McMahon, president and CEO of Accreditation Council for Continuing Medical Education (ACCME), laid the framework for QI as being “activities that address a quality or safety gap with interventions intended to result in improvement and with specific, measurable goals. QI activities are learner-driven, as learner engagement is a key target of ACCME’s standard. Representatives from the Heart Rhythm Society, the Society of Hospital Medicine, the Arthritis Foundation, and the American College of Rheumatology shared their organization’s initiatives related to QI.
Apart from the focus on certification and MOC, the meeting also focused on the needs arising from a changing political world, including what is at stake with the repeal of the Affordable Care Act (ACA) and the challenges arising with the wide dissemination of questionable news and the general disregard of science. Stephen Welch, CHEST EVP/CEO, participated in a panel entitled “Practicing Medicine in a Fact-Free World.” He, along with other media professionals, discussed the challenges that physicians, patients, and physician educators encounter in a time when false facts are published as truth and information is sensationalized to attract more attention.
Since the meeting, CHEST leadership sent a letter to the ABIM leadership noting a desire to be one of the societies with whom the ABIM collaborates for both alternative assessment methods and the open-book resources selected. Additionally, CHEST expressed interest in receiving the data that are culled from the assessments, an interest aligned with CHEST’s current data analytics initiatives. CHEST will continue to collaborate with the ABIM to ensure CHEST members’ needs are represented and prioritized in future discussions.
On April 7, four members of CHEST staff and leadership, along with staff and leadership from other medical specialty societies, participated in the Internal Medicine Summit, hosted by the American Board of Internal Medicine, in Philadelphia. The meeting covered an array of topics related to certification and maintenance of certification (MOC), including the alternative assessment model announced in December 2016, quality improvement (QI) as part of MOC, and practicing medicine in an ever-changing political landscape.
The meeting began with Dr. Richard Baron, President and CEO of the ABIM, explaining how the notion of certification has changed over the years. According to Dr. Baron, the concept of lifetime certification no longer makes sense in the rapidly changing field of medicine. As part of the evolution of certification, the ABIM has moved away from “rules to follow” toward something, co-created with societies, that is more relevant and less burdensome. This shift includes aligning certification and MOC requirements with things physicians are already required to do by their states and institutions. Dr. Baron also stressed that in today’s cultural and political landscape, along with the prevalence of “fake news,” the need for trust in the doctor-patient relationship is increasing; trust is no longer a “given.” Therefore, in an age when credentials can be purchased online, there’s an increasing need for an external certification to build trust and boost credibility.
Dr. Marianne Green, member of the ABIM Board of Directors and the ABIM Council, gave an update on the recertification assessment options. While currently, only an every 2-year assessment option will be offered as an alternative to a 10-year higher stakes exam, the ABIM is looking to partner with societies to deliver education, based on the needs identified via the assessment. Furthermore, in addition to partnering with societies to address the identified knowledge gaps, the ABIM plans to collaborate with societies in future alternatives to both the 2-year and 10-year assessments, with the shared goal of “maintenance and support of a community of life-long learners who hold ourselves accountable to peer-defined standards.” Initially, the 2-year lower stakes assessment will cover the breadth of the knowledge in the specialty/subspecialty, but the ABIM is committed to taking a more modular approach in the future. When asked about the fee structure for the new assessment options, Dr. Green communicated that details regarding fees would be announced in fall 2017.
While the first part of the meeting focused on MOC Part 2, the conversation turned toward quality improvement, or QI, later part of the meeting. The practice improvement, or MOC Part 4, requirement is on hold through the end of 2018. Both the ABIM and represented societies value the importance of quality measures. Dr. Graham McMahon, president and CEO of Accreditation Council for Continuing Medical Education (ACCME), laid the framework for QI as being “activities that address a quality or safety gap with interventions intended to result in improvement and with specific, measurable goals. QI activities are learner-driven, as learner engagement is a key target of ACCME’s standard. Representatives from the Heart Rhythm Society, the Society of Hospital Medicine, the Arthritis Foundation, and the American College of Rheumatology shared their organization’s initiatives related to QI.
Apart from the focus on certification and MOC, the meeting also focused on the needs arising from a changing political world, including what is at stake with the repeal of the Affordable Care Act (ACA) and the challenges arising with the wide dissemination of questionable news and the general disregard of science. Stephen Welch, CHEST EVP/CEO, participated in a panel entitled “Practicing Medicine in a Fact-Free World.” He, along with other media professionals, discussed the challenges that physicians, patients, and physician educators encounter in a time when false facts are published as truth and information is sensationalized to attract more attention.
Since the meeting, CHEST leadership sent a letter to the ABIM leadership noting a desire to be one of the societies with whom the ABIM collaborates for both alternative assessment methods and the open-book resources selected. Additionally, CHEST expressed interest in receiving the data that are culled from the assessments, an interest aligned with CHEST’s current data analytics initiatives. CHEST will continue to collaborate with the ABIM to ensure CHEST members’ needs are represented and prioritized in future discussions.
On April 7, four members of CHEST staff and leadership, along with staff and leadership from other medical specialty societies, participated in the Internal Medicine Summit, hosted by the American Board of Internal Medicine, in Philadelphia. The meeting covered an array of topics related to certification and maintenance of certification (MOC), including the alternative assessment model announced in December 2016, quality improvement (QI) as part of MOC, and practicing medicine in an ever-changing political landscape.
The meeting began with Dr. Richard Baron, President and CEO of the ABIM, explaining how the notion of certification has changed over the years. According to Dr. Baron, the concept of lifetime certification no longer makes sense in the rapidly changing field of medicine. As part of the evolution of certification, the ABIM has moved away from “rules to follow” toward something, co-created with societies, that is more relevant and less burdensome. This shift includes aligning certification and MOC requirements with things physicians are already required to do by their states and institutions. Dr. Baron also stressed that in today’s cultural and political landscape, along with the prevalence of “fake news,” the need for trust in the doctor-patient relationship is increasing; trust is no longer a “given.” Therefore, in an age when credentials can be purchased online, there’s an increasing need for an external certification to build trust and boost credibility.
Dr. Marianne Green, member of the ABIM Board of Directors and the ABIM Council, gave an update on the recertification assessment options. While currently, only an every 2-year assessment option will be offered as an alternative to a 10-year higher stakes exam, the ABIM is looking to partner with societies to deliver education, based on the needs identified via the assessment. Furthermore, in addition to partnering with societies to address the identified knowledge gaps, the ABIM plans to collaborate with societies in future alternatives to both the 2-year and 10-year assessments, with the shared goal of “maintenance and support of a community of life-long learners who hold ourselves accountable to peer-defined standards.” Initially, the 2-year lower stakes assessment will cover the breadth of the knowledge in the specialty/subspecialty, but the ABIM is committed to taking a more modular approach in the future. When asked about the fee structure for the new assessment options, Dr. Green communicated that details regarding fees would be announced in fall 2017.
While the first part of the meeting focused on MOC Part 2, the conversation turned toward quality improvement, or QI, later part of the meeting. The practice improvement, or MOC Part 4, requirement is on hold through the end of 2018. Both the ABIM and represented societies value the importance of quality measures. Dr. Graham McMahon, president and CEO of Accreditation Council for Continuing Medical Education (ACCME), laid the framework for QI as being “activities that address a quality or safety gap with interventions intended to result in improvement and with specific, measurable goals. QI activities are learner-driven, as learner engagement is a key target of ACCME’s standard. Representatives from the Heart Rhythm Society, the Society of Hospital Medicine, the Arthritis Foundation, and the American College of Rheumatology shared their organization’s initiatives related to QI.
Apart from the focus on certification and MOC, the meeting also focused on the needs arising from a changing political world, including what is at stake with the repeal of the Affordable Care Act (ACA) and the challenges arising with the wide dissemination of questionable news and the general disregard of science. Stephen Welch, CHEST EVP/CEO, participated in a panel entitled “Practicing Medicine in a Fact-Free World.” He, along with other media professionals, discussed the challenges that physicians, patients, and physician educators encounter in a time when false facts are published as truth and information is sensationalized to attract more attention.
Since the meeting, CHEST leadership sent a letter to the ABIM leadership noting a desire to be one of the societies with whom the ABIM collaborates for both alternative assessment methods and the open-book resources selected. Additionally, CHEST expressed interest in receiving the data that are culled from the assessments, an interest aligned with CHEST’s current data analytics initiatives. CHEST will continue to collaborate with the ABIM to ensure CHEST members’ needs are represented and prioritized in future discussions.
Learn What’s New at CHEST Annual Meeting 2017
We’ve listened and considered all of your feedback to enhance your experience at CHEST 2017, Oct 28-Nov 1, Toronto, Canada. This year, we have changed the format of our postgraduate courses, updated our interdisciplinary sessions, and added new ways to register. Take a look at what’s new.
Postgraduate courses
New this year at CHEST 2017 is the option to attend a half-day or full-day course for a more flexible experience. There are nine, half-day sessions that include lunch, and the afternoon sessions allow people to fly in that morning to avoid an extra hotel night and missing work.
Interdisciplinary sessions
These sessions are free but require a ticket.
Monday, October 30
- The State of PAH in 2017: An Update on the Science, New Therapies, and the Changing Treatment Algorithm
- Critical Skills for ICU Directors and Their Leadership Team
- Interstitial Lung Disease: 2017 Update on Patient-Centered Management
- Lung Cancer: 2017 Update in Diagnosis and Management
Tuesday, October 31
- Challenges in ICU Management
Wednesday, November 1
- Enhancing Quality of Pulmonary Rehabilitation Programs and Integrated COPD Disease Management
Don’t forget to register for CHEST 2017!
You can now register as a group! Ten or more health-care professionals from your team can register as a group for discounted tuition rates. Group registration is open through October 22 and will not be offered on-site. Learn more about CHEST 2017 updates and how to register at chestmeeting.chestnet.org.
We’ve listened and considered all of your feedback to enhance your experience at CHEST 2017, Oct 28-Nov 1, Toronto, Canada. This year, we have changed the format of our postgraduate courses, updated our interdisciplinary sessions, and added new ways to register. Take a look at what’s new.
Postgraduate courses
New this year at CHEST 2017 is the option to attend a half-day or full-day course for a more flexible experience. There are nine, half-day sessions that include lunch, and the afternoon sessions allow people to fly in that morning to avoid an extra hotel night and missing work.
Interdisciplinary sessions
These sessions are free but require a ticket.
Monday, October 30
- The State of PAH in 2017: An Update on the Science, New Therapies, and the Changing Treatment Algorithm
- Critical Skills for ICU Directors and Their Leadership Team
- Interstitial Lung Disease: 2017 Update on Patient-Centered Management
- Lung Cancer: 2017 Update in Diagnosis and Management
Tuesday, October 31
- Challenges in ICU Management
Wednesday, November 1
- Enhancing Quality of Pulmonary Rehabilitation Programs and Integrated COPD Disease Management
Don’t forget to register for CHEST 2017!
You can now register as a group! Ten or more health-care professionals from your team can register as a group for discounted tuition rates. Group registration is open through October 22 and will not be offered on-site. Learn more about CHEST 2017 updates and how to register at chestmeeting.chestnet.org.
We’ve listened and considered all of your feedback to enhance your experience at CHEST 2017, Oct 28-Nov 1, Toronto, Canada. This year, we have changed the format of our postgraduate courses, updated our interdisciplinary sessions, and added new ways to register. Take a look at what’s new.
Postgraduate courses
New this year at CHEST 2017 is the option to attend a half-day or full-day course for a more flexible experience. There are nine, half-day sessions that include lunch, and the afternoon sessions allow people to fly in that morning to avoid an extra hotel night and missing work.
Interdisciplinary sessions
These sessions are free but require a ticket.
Monday, October 30
- The State of PAH in 2017: An Update on the Science, New Therapies, and the Changing Treatment Algorithm
- Critical Skills for ICU Directors and Their Leadership Team
- Interstitial Lung Disease: 2017 Update on Patient-Centered Management
- Lung Cancer: 2017 Update in Diagnosis and Management
Tuesday, October 31
- Challenges in ICU Management
Wednesday, November 1
- Enhancing Quality of Pulmonary Rehabilitation Programs and Integrated COPD Disease Management
Don’t forget to register for CHEST 2017!
You can now register as a group! Ten or more health-care professionals from your team can register as a group for discounted tuition rates. Group registration is open through October 22 and will not be offered on-site. Learn more about CHEST 2017 updates and how to register at chestmeeting.chestnet.org.
What’s the evidence for stopping DMTs in MS patients?
NEW ORLEANS – There appear to be four clinical situations “when it might be reasonable to open up conversations with patients about discontinuation of DMTs,” Devyn Parsons said at the annual meeting of the Consortium of Multiple Sclerosis Centers.
While disease-modifying treatments (DMTs) are well established in their ability to decrease relapse rates and slow the progression of disability early in the course of relapsing-remitting MS, it remains unknown whether they maintain their efficacy late in the course of disease after many years of treatment or after progression to secondary progressive MS, said Ms. Parsons, a medical student at the University of British Columbia, Vancouver. Scientific evidence related to when disease-modifying treatments should be discontinued in patients with multiple sclerosis is generally poor, she said.
• Patients with secondary progressive MS who have ongoing progression and no new brain or spinal MRI lesions during the prior 12-24 months.
• Patients with stable relapsing-remitting MS aged 65 or older who have had no new brain or spinal MRI lesions during the prior 12-24 months.
• Patients with stable relapsing-remitting MS aged 55-65 years with no new brain or spinal MRI lesions within the prior 5 years.
• Patients who are pregnant or trying to conceive, or breastfeeding.
“Upon discontinuation of DMTs patients should continue to undergo annual assessments and an annual brain MRI for at least 2-5 years,” Ms. Parsons said. “Reuse of DMTs should be considered if there’s any evidence of relapse or new MRI lesion.”
The investigators conducted a systematic review of medical literature from MEDLINE, EMBASE, and the Cochrane Database of Systematic Reviews through June of 2016. They used the keywords “multiple sclerosis” and “disease-modifying treatments” and “treatment withdrawal” or “stopping medication” or “medication withdrawal.” Articles were reviewed in full and classified according to the American Academy of Neurology’s classification of evidence guidelines.
The review yielded what Ms. Parsons described as “a paucity of information” in the existing literature on MS course following discontinuation of DMTs. “There have been no randomized, controlled trials on the subject, and relatively few observational studies,” she said. “Of the observational studies that do exist, several have suggested a return to baseline disease activity following discontinuation of DMTs. In particular these studies examined natalizumab and interferon beta-1a discontinuation. At first glance these studies seem to suggest that discontinuation of DMTs is generally not appropriate, as there is likely to be a return to baseline disease activity. But it’s important to consider that many of these were retrospective, cross-sectional studies with small patient populations and aren’t the best quality data. Furthermore, these studies had relatively short follow-up periods, they didn’t include older patients, and they examined the discontinuation of DMTs after less than 2 years of continuous treatment. These results may not apply to older patients, and they might not apply to patients who have been continuously treated with DMTs for many years. At this point there is sufficient evidence in the literature to allow a randomized, controlled trial in a low-risk patient population of discontinuations of DMTs.”
Ms. Parsons discussed three observational studies from the review. One was a prospective study of 40 patients who discontinued DMTs after a minimum 5 years’ continuous use of a single DMT without new disease activity (Arquivos de Neuro-Psiquiatria 2013;71:516-20). At 46-month follow-up, the investigators found that 90% of patients remained free of clinical attack, and 85% had stable MRIs. “However, this was a really small trial, and the specific DMTs were not reported,” she said.
A larger, separate study evaluated 303 patients aged 40 and older who discontinued DMTs after a minimum of 3 years’ continuous use of a single DMT and who had no clinical relapse in the past 5 years (ECTRIMS Online Library. 2015 Oct 8. 116635). The majority of patients resumed DMT use because of an increase in disease activity following discontinuation. However, for every 10-year increase in patient age, there was a 25% decrease in the rate of resuming DMT. “This might suggest a greater feasibility of discontinuation of DMTs in older patients,” Ms. Parsons said.
The third observational study she discussed included 485 patients, mean age of 45 years, who discontinued DMTs after a minimum of 3 years of treatment with a single DMT and had no clinical relapses in the previous 5 years (J Neurol Neurosurg Psychiatry. 2016 Oct;87[10]:1133-7). These were compared with 854 propensity score–matched individuals who continued DMT. The mean annualized relapse rates and time to first relapse were similar for those who discontinued DMTs and those who continued DMTs. However, survival time to confirmed disability progression was shorter among those who discontinued DMTs (adjusted hazard ratio of 1.47; P = .001). Younger age was found to be a significant predictor of relapse risk among the DMT discontinuation group, with a 25% reduction in relapse risk ratio for every 10-year increase in age.
“DMTs cannot be said with certainty to be effective in older patients, given that patients over the age of 55 have rarely been included in clinical trials of these agents, Ms. Parsons said. Many patients with relapsing-remitting MS are continuously administered DMTs for many years. This long-term use of DMTs is not without cost. It is important to consider things like medication burden of the patient, the potential for adverse effects, as well as the possibility of unnecessary health care costs if these agents are no longer effective in some cases.”
Sanofi Genzyme supported the study. Ms. Parsons reported having no financial disclosures.
NEW ORLEANS – There appear to be four clinical situations “when it might be reasonable to open up conversations with patients about discontinuation of DMTs,” Devyn Parsons said at the annual meeting of the Consortium of Multiple Sclerosis Centers.
While disease-modifying treatments (DMTs) are well established in their ability to decrease relapse rates and slow the progression of disability early in the course of relapsing-remitting MS, it remains unknown whether they maintain their efficacy late in the course of disease after many years of treatment or after progression to secondary progressive MS, said Ms. Parsons, a medical student at the University of British Columbia, Vancouver. Scientific evidence related to when disease-modifying treatments should be discontinued in patients with multiple sclerosis is generally poor, she said.
• Patients with secondary progressive MS who have ongoing progression and no new brain or spinal MRI lesions during the prior 12-24 months.
• Patients with stable relapsing-remitting MS aged 65 or older who have had no new brain or spinal MRI lesions during the prior 12-24 months.
• Patients with stable relapsing-remitting MS aged 55-65 years with no new brain or spinal MRI lesions within the prior 5 years.
• Patients who are pregnant or trying to conceive, or breastfeeding.
“Upon discontinuation of DMTs patients should continue to undergo annual assessments and an annual brain MRI for at least 2-5 years,” Ms. Parsons said. “Reuse of DMTs should be considered if there’s any evidence of relapse or new MRI lesion.”
The investigators conducted a systematic review of medical literature from MEDLINE, EMBASE, and the Cochrane Database of Systematic Reviews through June of 2016. They used the keywords “multiple sclerosis” and “disease-modifying treatments” and “treatment withdrawal” or “stopping medication” or “medication withdrawal.” Articles were reviewed in full and classified according to the American Academy of Neurology’s classification of evidence guidelines.
The review yielded what Ms. Parsons described as “a paucity of information” in the existing literature on MS course following discontinuation of DMTs. “There have been no randomized, controlled trials on the subject, and relatively few observational studies,” she said. “Of the observational studies that do exist, several have suggested a return to baseline disease activity following discontinuation of DMTs. In particular these studies examined natalizumab and interferon beta-1a discontinuation. At first glance these studies seem to suggest that discontinuation of DMTs is generally not appropriate, as there is likely to be a return to baseline disease activity. But it’s important to consider that many of these were retrospective, cross-sectional studies with small patient populations and aren’t the best quality data. Furthermore, these studies had relatively short follow-up periods, they didn’t include older patients, and they examined the discontinuation of DMTs after less than 2 years of continuous treatment. These results may not apply to older patients, and they might not apply to patients who have been continuously treated with DMTs for many years. At this point there is sufficient evidence in the literature to allow a randomized, controlled trial in a low-risk patient population of discontinuations of DMTs.”
Ms. Parsons discussed three observational studies from the review. One was a prospective study of 40 patients who discontinued DMTs after a minimum 5 years’ continuous use of a single DMT without new disease activity (Arquivos de Neuro-Psiquiatria 2013;71:516-20). At 46-month follow-up, the investigators found that 90% of patients remained free of clinical attack, and 85% had stable MRIs. “However, this was a really small trial, and the specific DMTs were not reported,” she said.
A larger, separate study evaluated 303 patients aged 40 and older who discontinued DMTs after a minimum of 3 years’ continuous use of a single DMT and who had no clinical relapse in the past 5 years (ECTRIMS Online Library. 2015 Oct 8. 116635). The majority of patients resumed DMT use because of an increase in disease activity following discontinuation. However, for every 10-year increase in patient age, there was a 25% decrease in the rate of resuming DMT. “This might suggest a greater feasibility of discontinuation of DMTs in older patients,” Ms. Parsons said.
The third observational study she discussed included 485 patients, mean age of 45 years, who discontinued DMTs after a minimum of 3 years of treatment with a single DMT and had no clinical relapses in the previous 5 years (J Neurol Neurosurg Psychiatry. 2016 Oct;87[10]:1133-7). These were compared with 854 propensity score–matched individuals who continued DMT. The mean annualized relapse rates and time to first relapse were similar for those who discontinued DMTs and those who continued DMTs. However, survival time to confirmed disability progression was shorter among those who discontinued DMTs (adjusted hazard ratio of 1.47; P = .001). Younger age was found to be a significant predictor of relapse risk among the DMT discontinuation group, with a 25% reduction in relapse risk ratio for every 10-year increase in age.
“DMTs cannot be said with certainty to be effective in older patients, given that patients over the age of 55 have rarely been included in clinical trials of these agents, Ms. Parsons said. Many patients with relapsing-remitting MS are continuously administered DMTs for many years. This long-term use of DMTs is not without cost. It is important to consider things like medication burden of the patient, the potential for adverse effects, as well as the possibility of unnecessary health care costs if these agents are no longer effective in some cases.”
Sanofi Genzyme supported the study. Ms. Parsons reported having no financial disclosures.
NEW ORLEANS – There appear to be four clinical situations “when it might be reasonable to open up conversations with patients about discontinuation of DMTs,” Devyn Parsons said at the annual meeting of the Consortium of Multiple Sclerosis Centers.
While disease-modifying treatments (DMTs) are well established in their ability to decrease relapse rates and slow the progression of disability early in the course of relapsing-remitting MS, it remains unknown whether they maintain their efficacy late in the course of disease after many years of treatment or after progression to secondary progressive MS, said Ms. Parsons, a medical student at the University of British Columbia, Vancouver. Scientific evidence related to when disease-modifying treatments should be discontinued in patients with multiple sclerosis is generally poor, she said.
• Patients with secondary progressive MS who have ongoing progression and no new brain or spinal MRI lesions during the prior 12-24 months.
• Patients with stable relapsing-remitting MS aged 65 or older who have had no new brain or spinal MRI lesions during the prior 12-24 months.
• Patients with stable relapsing-remitting MS aged 55-65 years with no new brain or spinal MRI lesions within the prior 5 years.
• Patients who are pregnant or trying to conceive, or breastfeeding.
“Upon discontinuation of DMTs patients should continue to undergo annual assessments and an annual brain MRI for at least 2-5 years,” Ms. Parsons said. “Reuse of DMTs should be considered if there’s any evidence of relapse or new MRI lesion.”
The investigators conducted a systematic review of medical literature from MEDLINE, EMBASE, and the Cochrane Database of Systematic Reviews through June of 2016. They used the keywords “multiple sclerosis” and “disease-modifying treatments” and “treatment withdrawal” or “stopping medication” or “medication withdrawal.” Articles were reviewed in full and classified according to the American Academy of Neurology’s classification of evidence guidelines.
The review yielded what Ms. Parsons described as “a paucity of information” in the existing literature on MS course following discontinuation of DMTs. “There have been no randomized, controlled trials on the subject, and relatively few observational studies,” she said. “Of the observational studies that do exist, several have suggested a return to baseline disease activity following discontinuation of DMTs. In particular these studies examined natalizumab and interferon beta-1a discontinuation. At first glance these studies seem to suggest that discontinuation of DMTs is generally not appropriate, as there is likely to be a return to baseline disease activity. But it’s important to consider that many of these were retrospective, cross-sectional studies with small patient populations and aren’t the best quality data. Furthermore, these studies had relatively short follow-up periods, they didn’t include older patients, and they examined the discontinuation of DMTs after less than 2 years of continuous treatment. These results may not apply to older patients, and they might not apply to patients who have been continuously treated with DMTs for many years. At this point there is sufficient evidence in the literature to allow a randomized, controlled trial in a low-risk patient population of discontinuations of DMTs.”
Ms. Parsons discussed three observational studies from the review. One was a prospective study of 40 patients who discontinued DMTs after a minimum 5 years’ continuous use of a single DMT without new disease activity (Arquivos de Neuro-Psiquiatria 2013;71:516-20). At 46-month follow-up, the investigators found that 90% of patients remained free of clinical attack, and 85% had stable MRIs. “However, this was a really small trial, and the specific DMTs were not reported,” she said.
A larger, separate study evaluated 303 patients aged 40 and older who discontinued DMTs after a minimum of 3 years’ continuous use of a single DMT and who had no clinical relapse in the past 5 years (ECTRIMS Online Library. 2015 Oct 8. 116635). The majority of patients resumed DMT use because of an increase in disease activity following discontinuation. However, for every 10-year increase in patient age, there was a 25% decrease in the rate of resuming DMT. “This might suggest a greater feasibility of discontinuation of DMTs in older patients,” Ms. Parsons said.
The third observational study she discussed included 485 patients, mean age of 45 years, who discontinued DMTs after a minimum of 3 years of treatment with a single DMT and had no clinical relapses in the previous 5 years (J Neurol Neurosurg Psychiatry. 2016 Oct;87[10]:1133-7). These were compared with 854 propensity score–matched individuals who continued DMT. The mean annualized relapse rates and time to first relapse were similar for those who discontinued DMTs and those who continued DMTs. However, survival time to confirmed disability progression was shorter among those who discontinued DMTs (adjusted hazard ratio of 1.47; P = .001). Younger age was found to be a significant predictor of relapse risk among the DMT discontinuation group, with a 25% reduction in relapse risk ratio for every 10-year increase in age.
“DMTs cannot be said with certainty to be effective in older patients, given that patients over the age of 55 have rarely been included in clinical trials of these agents, Ms. Parsons said. Many patients with relapsing-remitting MS are continuously administered DMTs for many years. This long-term use of DMTs is not without cost. It is important to consider things like medication burden of the patient, the potential for adverse effects, as well as the possibility of unnecessary health care costs if these agents are no longer effective in some cases.”
Sanofi Genzyme supported the study. Ms. Parsons reported having no financial disclosures.
AT THE CMSC ANNUAL MEETING
Key clinical point:
Major finding: Meaningful clinical data on discontinuation of DMTs in patients with MS are limited.
Data source: A systematic review of the medical literature using the keywords “multiple sclerosis” and “disease-modifying treatments” and “treatment withdrawal” or “stopping medication” or “medication withdrawal.”
Disclosures: Sanofi Genzyme supported the study. Ms. Parsons reported having no financial disclosures.
Cardiovascular risks vary by race/ethnicity in lupus patients
Black patients with systemic lupus erythematosus (SLE) are more likely than whites to have a cardiovascular event, but Hispanics, Asians, and American Indians/Alaska natives with SLE are less likely than whites to experience a CV event, according to a study involving almost 66,000 Medicaid patients.
In a comparison of incidence rate ratios with whites as the reference group (IRR, 1.0), black patients with SLE had an IRR of 1.18 for cardiovascular events (defined as a first acute myocardial infarction or stroke), Hispanic patients had an IRR of 0.84, Asians had an IRR of 0.75, and American Indians/Alaska natives had an IRR of 1.06, reported Medha Barbhaiya, MD, MPH, of Brigham and Women’s Hospital, Boston, and her associates (Arthritis Rheumatol. 2017. doi: 10.1002/art.40174).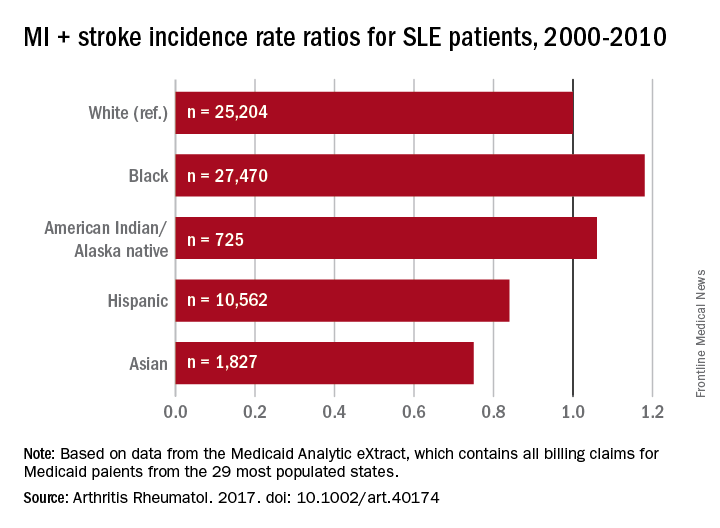
The substantial reduction in MI risk among Hispanics and Asians, compared with white SLE patients, suggests an “Hispanic and Asian paradox” since “SLE has been reported to be more prevalent, more severe, and to result in more end-organ damage in Hispanics and Asians compared to white patients,” Dr. Barbhaiya and her associates wrote.
The data for 65,788 cases of SLE from Jan. 1, 2000, to Dec. 31, 2010, were taken from the Medicaid Analytic eXtract, which contains all Medicaid billing claims from the 29 most populous states. Over a mean follow-up of 3.8 years, those SLE patients had 2,259 first-CV events, which works out to an overall incidence rate of 9.31 per 1,000 person-years.
The study was supported by awards from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Barbhaiya and one of her associates are supported by awards from the Rheumatology Research Foundation. The investigators did not disclose any conflicts.
In the 1,000 Faces of Lupus study, which showed a comparable racial and ethnic paradox, “lower annual income was associated with more health care barriers” and “difficulty in obtaining medications was associated with higher disease activity,” Janet E. Pope, MD, and her associates said in an accompanying editorial.
“The observed ‘paradox’ from the Barbhaiya study may be the result of complex interactions between environmental, cultural, socioeconomic, psychosocial, genetic, and other clinical factors.
Dr. Pope is with the University of Western Ontario in London. Her coauthors were Michael H. Weisman, MD, who is with Cedars-Sinai Medical Center in Los Angeles and Christopher Sjöwall, MD, of Linköping (Sweden) University.
In the 1,000 Faces of Lupus study, which showed a comparable racial and ethnic paradox, “lower annual income was associated with more health care barriers” and “difficulty in obtaining medications was associated with higher disease activity,” Janet E. Pope, MD, and her associates said in an accompanying editorial.
“The observed ‘paradox’ from the Barbhaiya study may be the result of complex interactions between environmental, cultural, socioeconomic, psychosocial, genetic, and other clinical factors.
Dr. Pope is with the University of Western Ontario in London. Her coauthors were Michael H. Weisman, MD, who is with Cedars-Sinai Medical Center in Los Angeles and Christopher Sjöwall, MD, of Linköping (Sweden) University.
In the 1,000 Faces of Lupus study, which showed a comparable racial and ethnic paradox, “lower annual income was associated with more health care barriers” and “difficulty in obtaining medications was associated with higher disease activity,” Janet E. Pope, MD, and her associates said in an accompanying editorial.
“The observed ‘paradox’ from the Barbhaiya study may be the result of complex interactions between environmental, cultural, socioeconomic, psychosocial, genetic, and other clinical factors.
Dr. Pope is with the University of Western Ontario in London. Her coauthors were Michael H. Weisman, MD, who is with Cedars-Sinai Medical Center in Los Angeles and Christopher Sjöwall, MD, of Linköping (Sweden) University.
Black patients with systemic lupus erythematosus (SLE) are more likely than whites to have a cardiovascular event, but Hispanics, Asians, and American Indians/Alaska natives with SLE are less likely than whites to experience a CV event, according to a study involving almost 66,000 Medicaid patients.
In a comparison of incidence rate ratios with whites as the reference group (IRR, 1.0), black patients with SLE had an IRR of 1.18 for cardiovascular events (defined as a first acute myocardial infarction or stroke), Hispanic patients had an IRR of 0.84, Asians had an IRR of 0.75, and American Indians/Alaska natives had an IRR of 1.06, reported Medha Barbhaiya, MD, MPH, of Brigham and Women’s Hospital, Boston, and her associates (Arthritis Rheumatol. 2017. doi: 10.1002/art.40174).
The substantial reduction in MI risk among Hispanics and Asians, compared with white SLE patients, suggests an “Hispanic and Asian paradox” since “SLE has been reported to be more prevalent, more severe, and to result in more end-organ damage in Hispanics and Asians compared to white patients,” Dr. Barbhaiya and her associates wrote.
The data for 65,788 cases of SLE from Jan. 1, 2000, to Dec. 31, 2010, were taken from the Medicaid Analytic eXtract, which contains all Medicaid billing claims from the 29 most populous states. Over a mean follow-up of 3.8 years, those SLE patients had 2,259 first-CV events, which works out to an overall incidence rate of 9.31 per 1,000 person-years.
The study was supported by awards from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Barbhaiya and one of her associates are supported by awards from the Rheumatology Research Foundation. The investigators did not disclose any conflicts.
Black patients with systemic lupus erythematosus (SLE) are more likely than whites to have a cardiovascular event, but Hispanics, Asians, and American Indians/Alaska natives with SLE are less likely than whites to experience a CV event, according to a study involving almost 66,000 Medicaid patients.
In a comparison of incidence rate ratios with whites as the reference group (IRR, 1.0), black patients with SLE had an IRR of 1.18 for cardiovascular events (defined as a first acute myocardial infarction or stroke), Hispanic patients had an IRR of 0.84, Asians had an IRR of 0.75, and American Indians/Alaska natives had an IRR of 1.06, reported Medha Barbhaiya, MD, MPH, of Brigham and Women’s Hospital, Boston, and her associates (Arthritis Rheumatol. 2017. doi: 10.1002/art.40174).
The substantial reduction in MI risk among Hispanics and Asians, compared with white SLE patients, suggests an “Hispanic and Asian paradox” since “SLE has been reported to be more prevalent, more severe, and to result in more end-organ damage in Hispanics and Asians compared to white patients,” Dr. Barbhaiya and her associates wrote.
The data for 65,788 cases of SLE from Jan. 1, 2000, to Dec. 31, 2010, were taken from the Medicaid Analytic eXtract, which contains all Medicaid billing claims from the 29 most populous states. Over a mean follow-up of 3.8 years, those SLE patients had 2,259 first-CV events, which works out to an overall incidence rate of 9.31 per 1,000 person-years.
The study was supported by awards from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Barbhaiya and one of her associates are supported by awards from the Rheumatology Research Foundation. The investigators did not disclose any conflicts.
FROM ARTHRITIS AND RHEUMATOLOGY
Key clinical point:
Major finding: With whites as the reference group, incidence rate ratios of cardiovascular disease were 1.18 for blacks, 1.06 for American Indians/Alaska natives, 0.84 for Hispanics, and 0.75 for Asians.
Data source: 65,788 cases of SLE from Jan. 1, 2000, to Dec. 31, 2010, were taken from the Medicaid Analytic eXtract.
Disclosures: The study was supported by awards from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Barbhaiya and one of her associates are supported by awards from the Rheumatology Research Foundation. The investigators did not disclose any conflicts.
Outpatient appendectomy success depends on patient selection, communication
Outpatient laparoscopic surgery for uncomplicated appendicitis can be safely implemented in a large county hospital that serves a poor, underserved population, findings from a prospective, observational trial have shown.
Outpatient appendectomy has gradually gained acceptance in the United States, and numerous studies support the practice. David R. Rosen, MD, of the University of Southern California, Los Angeles, and his colleagues considered the possible advantages of outpatient laparoscopic appendectomy for their institution, such as decreased length of stay, decreased costs, and fewer admissions.
The research team hypothesized that with a “well-defined protocol consisting of strict inclusion and exclusion criteria, clear patient instructions, and close observation to identify patients who would not succeed with the outpatient appendectomy treatment strategy, outpatient appendectomy would be feasible without worsening patient outcomes or satisfaction.”
The findings were published in the Journal of the American College of Surgeons (2017 May;224[5]:862-7).
The investigators conducted a study of patients presenting at a safety-net county hospital and diagnosed with acute appendicitis. A year-long observation period produced a control group of 178 admitted patients.
The outpatient protocol was then introduced. Patients were counseled on the possibility of their being discharged from the postanesthesia care unit (PACU), depending on intraoperative findings and their capacity to arrange a ride home and willingness to participate in postoperative follow-up. Patient education was a key element of the protocol. In all, 173 patients were identified for the outpatient program.
The intraoperative criteria for discharge from the PACU included no evidence of perforation or gangrene, and no surgical complications or adverse events. Patients were cleared for discharge if they met the following criteria: heart rate less than 100 beats/min; systolic blood pressure greater than 110 mm Hg; pain well controlled (less than 4 on a 1-10 scale); ambulatory; urinated since surgery; oral intake; and dressings dry without evidence of bleeding.
The patients had been thoroughly briefed on what to expect and problems that would necessitate a return to the emergency department. The physician assessed each patient’s readiness to be discharged, wrote a discharge order, and confirmed the pain medication prescription and follow-up appointment.
Of the 173 patients selected for the outpatient program, 113 (65%) ended up being discharged from the PACU. The reasons for these admissions included interoperative findings, failure to pass the discharge criteria, homelessness, and no transportation to get home.
The control and outpatient groups were similar demographically, except that the latter were on average significantly older (mean age 32.4 years vs. 36.6 years, respectively). The outpatient group had a significantly shorter operative time (69 minutes vs. 83 minutes), a significantly longer stay in the PACU (242 minutes vs.141 minutes), and a significantly shorter total postoperative length of stay (9 hours vs.19 hours).
There were no differences between the groups in terms of complications, postdischarge ED visits, or readmissions. Those who were discharged from the PACU had no postoperative complications and no readmissions.
The length of stay in the PACU gradually decreased for the outpatient group. “This can be attributed to the adoption of a new protocol,” the researchers noted. However, “we purposely did not want to rush the discharge process to ensure our patients and families had all questions answered and were comfortable leaving the hospital.”
A key component of the protocol was the follow-up appointment for all appendectomy patients; about one-third of both groups did not return for their follow-up appointments. Those missed follow-ups could mean some patients returned to another hospital, but the investigators suggested that this was unlikely.
“Because our hospital serves a patient population of low socioeconomic status and often without health insurance, our public hospital is often the only hospital to which they would present,” the investigators wrote.
Most of those who did return completed a questionnaire on their level of satisfaction. Survey results showed no differences in satisfaction between the groups and a generally positive view of the protocol among the outpatient group.
The study did not account for actual cost savings, but reduced hospital admissions and readmissions were achieved. Investigators assert that other studies have shown that each day of hospitalization avoided saves about $1,900.
“It is challenging to deliver high-quality, efficient care to an underserved population in a public hospital,” Dr. Rosen said in an interview. “In this setting, communication and patient education are vital components for success. By setting clear expectations and empowering patients to participate in their care, we can maximize our patients’ outcomes.”
The investigators had no disclosures.
Outpatient laparoscopic surgery for uncomplicated appendicitis can be safely implemented in a large county hospital that serves a poor, underserved population, findings from a prospective, observational trial have shown.
Outpatient appendectomy has gradually gained acceptance in the United States, and numerous studies support the practice. David R. Rosen, MD, of the University of Southern California, Los Angeles, and his colleagues considered the possible advantages of outpatient laparoscopic appendectomy for their institution, such as decreased length of stay, decreased costs, and fewer admissions.
The research team hypothesized that with a “well-defined protocol consisting of strict inclusion and exclusion criteria, clear patient instructions, and close observation to identify patients who would not succeed with the outpatient appendectomy treatment strategy, outpatient appendectomy would be feasible without worsening patient outcomes or satisfaction.”
The findings were published in the Journal of the American College of Surgeons (2017 May;224[5]:862-7).
The investigators conducted a study of patients presenting at a safety-net county hospital and diagnosed with acute appendicitis. A year-long observation period produced a control group of 178 admitted patients.
The outpatient protocol was then introduced. Patients were counseled on the possibility of their being discharged from the postanesthesia care unit (PACU), depending on intraoperative findings and their capacity to arrange a ride home and willingness to participate in postoperative follow-up. Patient education was a key element of the protocol. In all, 173 patients were identified for the outpatient program.
The intraoperative criteria for discharge from the PACU included no evidence of perforation or gangrene, and no surgical complications or adverse events. Patients were cleared for discharge if they met the following criteria: heart rate less than 100 beats/min; systolic blood pressure greater than 110 mm Hg; pain well controlled (less than 4 on a 1-10 scale); ambulatory; urinated since surgery; oral intake; and dressings dry without evidence of bleeding.
The patients had been thoroughly briefed on what to expect and problems that would necessitate a return to the emergency department. The physician assessed each patient’s readiness to be discharged, wrote a discharge order, and confirmed the pain medication prescription and follow-up appointment.
Of the 173 patients selected for the outpatient program, 113 (65%) ended up being discharged from the PACU. The reasons for these admissions included interoperative findings, failure to pass the discharge criteria, homelessness, and no transportation to get home.
The control and outpatient groups were similar demographically, except that the latter were on average significantly older (mean age 32.4 years vs. 36.6 years, respectively). The outpatient group had a significantly shorter operative time (69 minutes vs. 83 minutes), a significantly longer stay in the PACU (242 minutes vs.141 minutes), and a significantly shorter total postoperative length of stay (9 hours vs.19 hours).
There were no differences between the groups in terms of complications, postdischarge ED visits, or readmissions. Those who were discharged from the PACU had no postoperative complications and no readmissions.
The length of stay in the PACU gradually decreased for the outpatient group. “This can be attributed to the adoption of a new protocol,” the researchers noted. However, “we purposely did not want to rush the discharge process to ensure our patients and families had all questions answered and were comfortable leaving the hospital.”
A key component of the protocol was the follow-up appointment for all appendectomy patients; about one-third of both groups did not return for their follow-up appointments. Those missed follow-ups could mean some patients returned to another hospital, but the investigators suggested that this was unlikely.
“Because our hospital serves a patient population of low socioeconomic status and often without health insurance, our public hospital is often the only hospital to which they would present,” the investigators wrote.
Most of those who did return completed a questionnaire on their level of satisfaction. Survey results showed no differences in satisfaction between the groups and a generally positive view of the protocol among the outpatient group.
The study did not account for actual cost savings, but reduced hospital admissions and readmissions were achieved. Investigators assert that other studies have shown that each day of hospitalization avoided saves about $1,900.
“It is challenging to deliver high-quality, efficient care to an underserved population in a public hospital,” Dr. Rosen said in an interview. “In this setting, communication and patient education are vital components for success. By setting clear expectations and empowering patients to participate in their care, we can maximize our patients’ outcomes.”
The investigators had no disclosures.
Outpatient laparoscopic surgery for uncomplicated appendicitis can be safely implemented in a large county hospital that serves a poor, underserved population, findings from a prospective, observational trial have shown.
Outpatient appendectomy has gradually gained acceptance in the United States, and numerous studies support the practice. David R. Rosen, MD, of the University of Southern California, Los Angeles, and his colleagues considered the possible advantages of outpatient laparoscopic appendectomy for their institution, such as decreased length of stay, decreased costs, and fewer admissions.
The research team hypothesized that with a “well-defined protocol consisting of strict inclusion and exclusion criteria, clear patient instructions, and close observation to identify patients who would not succeed with the outpatient appendectomy treatment strategy, outpatient appendectomy would be feasible without worsening patient outcomes or satisfaction.”
The findings were published in the Journal of the American College of Surgeons (2017 May;224[5]:862-7).
The investigators conducted a study of patients presenting at a safety-net county hospital and diagnosed with acute appendicitis. A year-long observation period produced a control group of 178 admitted patients.
The outpatient protocol was then introduced. Patients were counseled on the possibility of their being discharged from the postanesthesia care unit (PACU), depending on intraoperative findings and their capacity to arrange a ride home and willingness to participate in postoperative follow-up. Patient education was a key element of the protocol. In all, 173 patients were identified for the outpatient program.
The intraoperative criteria for discharge from the PACU included no evidence of perforation or gangrene, and no surgical complications or adverse events. Patients were cleared for discharge if they met the following criteria: heart rate less than 100 beats/min; systolic blood pressure greater than 110 mm Hg; pain well controlled (less than 4 on a 1-10 scale); ambulatory; urinated since surgery; oral intake; and dressings dry without evidence of bleeding.
The patients had been thoroughly briefed on what to expect and problems that would necessitate a return to the emergency department. The physician assessed each patient’s readiness to be discharged, wrote a discharge order, and confirmed the pain medication prescription and follow-up appointment.
Of the 173 patients selected for the outpatient program, 113 (65%) ended up being discharged from the PACU. The reasons for these admissions included interoperative findings, failure to pass the discharge criteria, homelessness, and no transportation to get home.
The control and outpatient groups were similar demographically, except that the latter were on average significantly older (mean age 32.4 years vs. 36.6 years, respectively). The outpatient group had a significantly shorter operative time (69 minutes vs. 83 minutes), a significantly longer stay in the PACU (242 minutes vs.141 minutes), and a significantly shorter total postoperative length of stay (9 hours vs.19 hours).
There were no differences between the groups in terms of complications, postdischarge ED visits, or readmissions. Those who were discharged from the PACU had no postoperative complications and no readmissions.
The length of stay in the PACU gradually decreased for the outpatient group. “This can be attributed to the adoption of a new protocol,” the researchers noted. However, “we purposely did not want to rush the discharge process to ensure our patients and families had all questions answered and were comfortable leaving the hospital.”
A key component of the protocol was the follow-up appointment for all appendectomy patients; about one-third of both groups did not return for their follow-up appointments. Those missed follow-ups could mean some patients returned to another hospital, but the investigators suggested that this was unlikely.
“Because our hospital serves a patient population of low socioeconomic status and often without health insurance, our public hospital is often the only hospital to which they would present,” the investigators wrote.
Most of those who did return completed a questionnaire on their level of satisfaction. Survey results showed no differences in satisfaction between the groups and a generally positive view of the protocol among the outpatient group.
The study did not account for actual cost savings, but reduced hospital admissions and readmissions were achieved. Investigators assert that other studies have shown that each day of hospitalization avoided saves about $1,900.
“It is challenging to deliver high-quality, efficient care to an underserved population in a public hospital,” Dr. Rosen said in an interview. “In this setting, communication and patient education are vital components for success. By setting clear expectations and empowering patients to participate in their care, we can maximize our patients’ outcomes.”
The investigators had no disclosures.
FROM JOURNAL OF THE AMERICAN COLLEGE OF SURGEONS
Key clinical point: .
Major finding: The outpatient group had a shorter postoperative hospital length of stay (9 hours vs. 19 hours).
Data source: A prospective, observational study of 351 patients with a diagnosis of acute appendicitis at a public safety-net hospital that serves poor and mostly uninsured patients.
Disclosures: The authors had no disclosures.
Personalized snoring video boosts CPAP adherence
BOSTON – Showing patients videos of themselves having apneic episodes may convince them to use continuous positive airway pressure (CPAP), suggests the first results of an ongoing randomized clinical trial.
The investigators based their research project design on a previous pilot study that showed improved adherence to CPAP in patients who were shown videos of themselves sleeping while participating in a sleep study, Mark S. Aloia, PhD, said in a presentation at the annual meeting of the Associated Professional Sleep Societies.
In the new study, patients who had been recently diagnosed with sleep apnea were randomly assigned to participate in one of the three treatment groups. All three groups received sleep apnea and CPAP education prior to the use of CPAP. One group also watched videos of themselves sleeping, snoring, and gasping for air, and another group watched videos of a stranger sleeping and having apneic events.
After adjustment for age, educational level, and baseline sleep apnea severity, those who watched videos of themselves still used their CPAP devices more than 2 hours per night longer than did patients in each of the groups receiving the other two interventions (P = .02).
Both video interventions involved watching 30 minutes of sleep footage shown to each patient once before starting CPAP therapy. CPAP adherence was measured by downloaded data from PAP devices over the first 90 days of use.
The average age of the patients was 50 years, and they had moderate or severe sleep apnea, with mean apnea hypopnea indices ranging from 26.5 to 33.3 in the three study arms. The majority of patients had body mass indexes over 30.
Adherence to CPAP treatment is often poor, with many patients failing to use the device for even 4 hours per night, said Dr. Aloia, a psychologist at National Jewish Health in Denver. Many patients prescribed CPAP for OSA will undergo an educational component that may include watching a video of someone with OSA sleeping and having apneic events, he added. They often have “dramatic responses” to these videos, but then fail to positively change their own behavior.
“Many times we think that if our patient just knew what we know, he or she would use CPAP more, but there is evidence that doctors don’t take their medications any more than patients do, so it is not just a matter of education, it is a little bit deeper than that and it has to be personalized,” he said.
“The use of a personalized video is promising … we hope to present more data next year,” said Dr. Aloia, who has board certification in behavioral sleep medicine,
He noted that the video technique used may be jeopardized as more and more patients partake in home-based rather than lab-based sleep studies. That said, he also reported that the research team had to exclude several patients from the study because they had already viewed videos of themselves sleeping and snoring that had been recorded by their partners.
If the intervention proves effective, Dr. Aloia said he thinks it can be modified for use in home testing.
The study is supported by a grant from the National Heart, Lung, and Blood Institute. Dr. Aloia disclosed that he is a paid employee of Phillips, but that the study used both Phillips and ResMed CPAP devices.
BOSTON – Showing patients videos of themselves having apneic episodes may convince them to use continuous positive airway pressure (CPAP), suggests the first results of an ongoing randomized clinical trial.
The investigators based their research project design on a previous pilot study that showed improved adherence to CPAP in patients who were shown videos of themselves sleeping while participating in a sleep study, Mark S. Aloia, PhD, said in a presentation at the annual meeting of the Associated Professional Sleep Societies.
In the new study, patients who had been recently diagnosed with sleep apnea were randomly assigned to participate in one of the three treatment groups. All three groups received sleep apnea and CPAP education prior to the use of CPAP. One group also watched videos of themselves sleeping, snoring, and gasping for air, and another group watched videos of a stranger sleeping and having apneic events.
After adjustment for age, educational level, and baseline sleep apnea severity, those who watched videos of themselves still used their CPAP devices more than 2 hours per night longer than did patients in each of the groups receiving the other two interventions (P = .02).
Both video interventions involved watching 30 minutes of sleep footage shown to each patient once before starting CPAP therapy. CPAP adherence was measured by downloaded data from PAP devices over the first 90 days of use.
The average age of the patients was 50 years, and they had moderate or severe sleep apnea, with mean apnea hypopnea indices ranging from 26.5 to 33.3 in the three study arms. The majority of patients had body mass indexes over 30.
Adherence to CPAP treatment is often poor, with many patients failing to use the device for even 4 hours per night, said Dr. Aloia, a psychologist at National Jewish Health in Denver. Many patients prescribed CPAP for OSA will undergo an educational component that may include watching a video of someone with OSA sleeping and having apneic events, he added. They often have “dramatic responses” to these videos, but then fail to positively change their own behavior.
“Many times we think that if our patient just knew what we know, he or she would use CPAP more, but there is evidence that doctors don’t take their medications any more than patients do, so it is not just a matter of education, it is a little bit deeper than that and it has to be personalized,” he said.
“The use of a personalized video is promising … we hope to present more data next year,” said Dr. Aloia, who has board certification in behavioral sleep medicine,
He noted that the video technique used may be jeopardized as more and more patients partake in home-based rather than lab-based sleep studies. That said, he also reported that the research team had to exclude several patients from the study because they had already viewed videos of themselves sleeping and snoring that had been recorded by their partners.
If the intervention proves effective, Dr. Aloia said he thinks it can be modified for use in home testing.
The study is supported by a grant from the National Heart, Lung, and Blood Institute. Dr. Aloia disclosed that he is a paid employee of Phillips, but that the study used both Phillips and ResMed CPAP devices.
BOSTON – Showing patients videos of themselves having apneic episodes may convince them to use continuous positive airway pressure (CPAP), suggests the first results of an ongoing randomized clinical trial.
The investigators based their research project design on a previous pilot study that showed improved adherence to CPAP in patients who were shown videos of themselves sleeping while participating in a sleep study, Mark S. Aloia, PhD, said in a presentation at the annual meeting of the Associated Professional Sleep Societies.
In the new study, patients who had been recently diagnosed with sleep apnea were randomly assigned to participate in one of the three treatment groups. All three groups received sleep apnea and CPAP education prior to the use of CPAP. One group also watched videos of themselves sleeping, snoring, and gasping for air, and another group watched videos of a stranger sleeping and having apneic events.
After adjustment for age, educational level, and baseline sleep apnea severity, those who watched videos of themselves still used their CPAP devices more than 2 hours per night longer than did patients in each of the groups receiving the other two interventions (P = .02).
Both video interventions involved watching 30 minutes of sleep footage shown to each patient once before starting CPAP therapy. CPAP adherence was measured by downloaded data from PAP devices over the first 90 days of use.
The average age of the patients was 50 years, and they had moderate or severe sleep apnea, with mean apnea hypopnea indices ranging from 26.5 to 33.3 in the three study arms. The majority of patients had body mass indexes over 30.
Adherence to CPAP treatment is often poor, with many patients failing to use the device for even 4 hours per night, said Dr. Aloia, a psychologist at National Jewish Health in Denver. Many patients prescribed CPAP for OSA will undergo an educational component that may include watching a video of someone with OSA sleeping and having apneic events, he added. They often have “dramatic responses” to these videos, but then fail to positively change their own behavior.
“Many times we think that if our patient just knew what we know, he or she would use CPAP more, but there is evidence that doctors don’t take their medications any more than patients do, so it is not just a matter of education, it is a little bit deeper than that and it has to be personalized,” he said.
“The use of a personalized video is promising … we hope to present more data next year,” said Dr. Aloia, who has board certification in behavioral sleep medicine,
He noted that the video technique used may be jeopardized as more and more patients partake in home-based rather than lab-based sleep studies. That said, he also reported that the research team had to exclude several patients from the study because they had already viewed videos of themselves sleeping and snoring that had been recorded by their partners.
If the intervention proves effective, Dr. Aloia said he thinks it can be modified for use in home testing.
The study is supported by a grant from the National Heart, Lung, and Blood Institute. Dr. Aloia disclosed that he is a paid employee of Phillips, but that the study used both Phillips and ResMed CPAP devices.
AT SLEEP 2017
Key clinical point: Showing patients videos of themselves having an apneic event during CPAP initiation resulted in greater treatment adherence over 3 months, compared to standard ways of initiating CPAP therapy.
Major finding: Patients who watched personalized sleep videos used their CPAP devices more than 2 hours per night longer, compared with those who were not shown a personalized video.
Data source: Randomized controlled trial with 3-month data on 24 individuals out of a planned enrollment of 300 patients.
Disclosures: This ongoing study is supported by a grant from the National Heart, Lung, and Blood Institute. Dr. Aloia disclosed that he is a paid employee of Phillips, but that the study used both Phillips and ResMed CPAP devices.
Algorithm aims to tackle clozapine resistance
SAN DIEGO – The atypical antipsychotic drug clozapine is the standard of care for patients with treatment-resistant schizophrenia, but about half do not see an adequate response.
At the annual meeting of the American Psychiatric Association, Randall F. White, MD, presented an algorithm for clozapine-resistant patients intended to simplify and clarify a path forward. He also presented outcome data from a cohort of patients managed using this approach.
The algorithm recommends electroconvulsive therapy if there is inadequate response to clozapine alone or with fluvoxamine, which sometimes is used to boost clozapine serum level, especially in patients who are heavy smokers. If ECT fails to reduce symptoms or the patient refuses it, topiramate, aripiprazole, or sulpiride may be added to clozapine treatment, said Dr. White of the University of British Columbia, Vancouver.
And finally, if psychosis persists, certain patients should be offered cognitive-behavioral therapy with a psychologist trained in helping people with psychosis, Dr. White and his colleagues advise.
Dr. White said in an interview that the idea for a systematic approach to clozapine resistance came from clinical experience of the British Columbia Psychosis Program, which specializes in patients with severe schizophrenia and treatment resistance.
“About half of our patients come to us already on clozapine and aren’t getting better, so we needed to figure out a coherent approach to help them,” he said, noting that there is no current standard of care and that many “come to us already on four or five medications, and it can seem a bit random.”
When these patients are admitted, “we try to simplify their treatment and figure out what’s going on, and then offer ECT if appropriate,” he said.
Dr. White presented data from a cohort of patients assessed at the program between 2012 and 2017, of which 114 were taking clozapine at admission and had a diagnosis of schizophrenia or schizoaffective disorder.
To be considered clozapine-resistant, patients had to be taking 500 mg or more for at least 60 days, yet have persistent positive symptoms and moderate to severe impairment. Dr. White and his colleagues identified 20 patients with clozapine resistance. Of these, eight were offered ECT, and three accepted. At the time of discharge, 16 patients remained on clozapine with or without fluvoxamine. Four patients were treated with the recommended adjunctive agents aripiprazole or sulpiride and five with other agents.
Dr. White said the three agents recommended in the algorithm were determined by literature reviews, including meta-analyses of randomized trials. Several commonly used adjunctive agents to clozapine, including risperidone, were ruled out, for lack of evidence in this patient group.
Sulpiride, one of the drugs recommended in the algorithm, is not marketed in North America, and in Canada is accessible only by special arrangement. The evidence for aripiprazole, meanwhile, “is not stupendous,” Dr. White said, “but there’s a signal.”
The topiramate recommendation is based on results from a 2016 meta-analysis of randomized controlled trials. “Topiramate has a possible advantage of ameliorating metabolic problems,” Dr. White said, and the meta-analysis showed a significant effect size in improving positive and negative symptoms. However, he noted, on rare occasions, it has been seen to exacerbate psychosis.
Dr. White said few of the treatment-resistant patients seen in his program have had a course of ECT despite evidence of benefit. “Instead of ECT, they usually are put on multiple medications,” he said. “Admittedly, getting some of these patients to accept ECT isn’t easy.”
Because the program is designed to keep patients for 6 or more months as needed, “we have the luxury of time,” Dr. White said, to allow for the discontinuation of extraneous medicines and for an ECT trial if indicated.
“I know that in other hospitals, and other health care settings, they don’t have that – they have to figure out what to do quickly. And ECT is not the quickest treatment.”
Offering cognitive-behavioral therapy to people with psychosis is possible, he stressed, and supported by evidence from at least one study. “In this population, it’s challenging,” he acknowledged. “I don’t think I’d do it concurrently with ECT but as an alternative to or after ECT.”
Dr. White disclosed no conflicts of interest related to his research.
SAN DIEGO – The atypical antipsychotic drug clozapine is the standard of care for patients with treatment-resistant schizophrenia, but about half do not see an adequate response.
At the annual meeting of the American Psychiatric Association, Randall F. White, MD, presented an algorithm for clozapine-resistant patients intended to simplify and clarify a path forward. He also presented outcome data from a cohort of patients managed using this approach.
The algorithm recommends electroconvulsive therapy if there is inadequate response to clozapine alone or with fluvoxamine, which sometimes is used to boost clozapine serum level, especially in patients who are heavy smokers. If ECT fails to reduce symptoms or the patient refuses it, topiramate, aripiprazole, or sulpiride may be added to clozapine treatment, said Dr. White of the University of British Columbia, Vancouver.
And finally, if psychosis persists, certain patients should be offered cognitive-behavioral therapy with a psychologist trained in helping people with psychosis, Dr. White and his colleagues advise.
Dr. White said in an interview that the idea for a systematic approach to clozapine resistance came from clinical experience of the British Columbia Psychosis Program, which specializes in patients with severe schizophrenia and treatment resistance.
“About half of our patients come to us already on clozapine and aren’t getting better, so we needed to figure out a coherent approach to help them,” he said, noting that there is no current standard of care and that many “come to us already on four or five medications, and it can seem a bit random.”
When these patients are admitted, “we try to simplify their treatment and figure out what’s going on, and then offer ECT if appropriate,” he said.
Dr. White presented data from a cohort of patients assessed at the program between 2012 and 2017, of which 114 were taking clozapine at admission and had a diagnosis of schizophrenia or schizoaffective disorder.
To be considered clozapine-resistant, patients had to be taking 500 mg or more for at least 60 days, yet have persistent positive symptoms and moderate to severe impairment. Dr. White and his colleagues identified 20 patients with clozapine resistance. Of these, eight were offered ECT, and three accepted. At the time of discharge, 16 patients remained on clozapine with or without fluvoxamine. Four patients were treated with the recommended adjunctive agents aripiprazole or sulpiride and five with other agents.
Dr. White said the three agents recommended in the algorithm were determined by literature reviews, including meta-analyses of randomized trials. Several commonly used adjunctive agents to clozapine, including risperidone, were ruled out, for lack of evidence in this patient group.
Sulpiride, one of the drugs recommended in the algorithm, is not marketed in North America, and in Canada is accessible only by special arrangement. The evidence for aripiprazole, meanwhile, “is not stupendous,” Dr. White said, “but there’s a signal.”
The topiramate recommendation is based on results from a 2016 meta-analysis of randomized controlled trials. “Topiramate has a possible advantage of ameliorating metabolic problems,” Dr. White said, and the meta-analysis showed a significant effect size in improving positive and negative symptoms. However, he noted, on rare occasions, it has been seen to exacerbate psychosis.
Dr. White said few of the treatment-resistant patients seen in his program have had a course of ECT despite evidence of benefit. “Instead of ECT, they usually are put on multiple medications,” he said. “Admittedly, getting some of these patients to accept ECT isn’t easy.”
Because the program is designed to keep patients for 6 or more months as needed, “we have the luxury of time,” Dr. White said, to allow for the discontinuation of extraneous medicines and for an ECT trial if indicated.
“I know that in other hospitals, and other health care settings, they don’t have that – they have to figure out what to do quickly. And ECT is not the quickest treatment.”
Offering cognitive-behavioral therapy to people with psychosis is possible, he stressed, and supported by evidence from at least one study. “In this population, it’s challenging,” he acknowledged. “I don’t think I’d do it concurrently with ECT but as an alternative to or after ECT.”
Dr. White disclosed no conflicts of interest related to his research.
SAN DIEGO – The atypical antipsychotic drug clozapine is the standard of care for patients with treatment-resistant schizophrenia, but about half do not see an adequate response.
At the annual meeting of the American Psychiatric Association, Randall F. White, MD, presented an algorithm for clozapine-resistant patients intended to simplify and clarify a path forward. He also presented outcome data from a cohort of patients managed using this approach.
The algorithm recommends electroconvulsive therapy if there is inadequate response to clozapine alone or with fluvoxamine, which sometimes is used to boost clozapine serum level, especially in patients who are heavy smokers. If ECT fails to reduce symptoms or the patient refuses it, topiramate, aripiprazole, or sulpiride may be added to clozapine treatment, said Dr. White of the University of British Columbia, Vancouver.
And finally, if psychosis persists, certain patients should be offered cognitive-behavioral therapy with a psychologist trained in helping people with psychosis, Dr. White and his colleagues advise.
Dr. White said in an interview that the idea for a systematic approach to clozapine resistance came from clinical experience of the British Columbia Psychosis Program, which specializes in patients with severe schizophrenia and treatment resistance.
“About half of our patients come to us already on clozapine and aren’t getting better, so we needed to figure out a coherent approach to help them,” he said, noting that there is no current standard of care and that many “come to us already on four or five medications, and it can seem a bit random.”
When these patients are admitted, “we try to simplify their treatment and figure out what’s going on, and then offer ECT if appropriate,” he said.
Dr. White presented data from a cohort of patients assessed at the program between 2012 and 2017, of which 114 were taking clozapine at admission and had a diagnosis of schizophrenia or schizoaffective disorder.
To be considered clozapine-resistant, patients had to be taking 500 mg or more for at least 60 days, yet have persistent positive symptoms and moderate to severe impairment. Dr. White and his colleagues identified 20 patients with clozapine resistance. Of these, eight were offered ECT, and three accepted. At the time of discharge, 16 patients remained on clozapine with or without fluvoxamine. Four patients were treated with the recommended adjunctive agents aripiprazole or sulpiride and five with other agents.
Dr. White said the three agents recommended in the algorithm were determined by literature reviews, including meta-analyses of randomized trials. Several commonly used adjunctive agents to clozapine, including risperidone, were ruled out, for lack of evidence in this patient group.
Sulpiride, one of the drugs recommended in the algorithm, is not marketed in North America, and in Canada is accessible only by special arrangement. The evidence for aripiprazole, meanwhile, “is not stupendous,” Dr. White said, “but there’s a signal.”
The topiramate recommendation is based on results from a 2016 meta-analysis of randomized controlled trials. “Topiramate has a possible advantage of ameliorating metabolic problems,” Dr. White said, and the meta-analysis showed a significant effect size in improving positive and negative symptoms. However, he noted, on rare occasions, it has been seen to exacerbate psychosis.
Dr. White said few of the treatment-resistant patients seen in his program have had a course of ECT despite evidence of benefit. “Instead of ECT, they usually are put on multiple medications,” he said. “Admittedly, getting some of these patients to accept ECT isn’t easy.”
Because the program is designed to keep patients for 6 or more months as needed, “we have the luxury of time,” Dr. White said, to allow for the discontinuation of extraneous medicines and for an ECT trial if indicated.
“I know that in other hospitals, and other health care settings, they don’t have that – they have to figure out what to do quickly. And ECT is not the quickest treatment.”
Offering cognitive-behavioral therapy to people with psychosis is possible, he stressed, and supported by evidence from at least one study. “In this population, it’s challenging,” he acknowledged. “I don’t think I’d do it concurrently with ECT but as an alternative to or after ECT.”
Dr. White disclosed no conflicts of interest related to his research.
EXPERT ANALYSIS FROM APA
Brain amyloid may herald decline in cognitively normal seniors
Cognitively normal seniors with brain amyloid experienced accelerated cognitive decline over as few as 3-4 years, compared with those who had no brain amyloid at baseline.
At 4 years’ follow-up, prodromal Alzheimer’s disease (AD) had developed in 32% of amyloid-positive people and 15% of the amyloid-normal people – a 16.9% difference. Extrapolating the limited 10-year data they collected, the authors projected that after a decade, 88% of the amyloid-positive cohort would have progressed, compared with 29% of the amyloid-normal cohort.
“This suggests that elevated brain amyloid may indicate a presymptomatic stage of disease rather than a risk factor for disease,” wrote Dr. Donohue of the University of Southern California, San Diego. “AD is a gradually progressive disorder that can be identified at the asymptomatic phase using biomarkers of amyloid.”
The study is a project of the Alzheimer’s Disease Neuroimaging Initiative, an ongoing natural history study assessing the clinical, imaging, genetics, and biomarkers of aging, from normal aging to early and late mild cognitive impairment, dementia, or AD.
This particular substudy tracked the association of baseline amyloid-beta 42 (AB42) and cognition among 445 patients, 202 of whom had elevated brain amyloid as measured by cerebrospinal fluid or brain imaging with florbetapir. They were followed for a mean of 3 years, although a limited number of patients had up to 10 years’ worth of data.
The primary endpoints were score changes on four cognitive batteries: the Preclinical Alzheimer Cognitive Composite, the Mini-Mental State Examination; the Clinical Dementia Rating Scale Sum of Boxes, and the Logical Memory Delayed Recall.
At baseline, mean patient age was 74 years, although amyloid-positive patients were slightly older (74.7 years vs. 73.4 years). The mean educational attainment was 16.4 years. Slightly more patients in the amyloid-positive group reported subjective memory complaints (25% vs. 22%). Significantly more had at least one APOE-e4 allele of the apolipoprotein E gene (42% vs. 16%). All were cognitively normal as measured by the four tests. A fifth measure, the Alzheimer’s Disease Assessment Scale 13-item cognitive subscale, was also within normal limits for both groups, but that was not included in the follow-up analysis.
In addition to showing AB42 deposits in the brain and/or decreased AB42 in cerebrospinal fluid (indicating increased binding in the brain), amyloid-positive patients also had significantly different levels of tau. CSF total tau was 76.8 pg/mL in the amyloid-positive group, compared with 59.4 pg/mL in the amyloid-normal group. CSF phosphorylated tau also was elevated in the amyloid-positive group (38.1 pg/mL vs. 26.5 pg/mL). Increased CSF tau is an indicator of neuronal injury.
The mean follow-up time was 3.9 years. At last contact, only four patients had died; 36% were lost to follow-up. Significantly more in the amyloid-positive group had progressed to Alzheimer’s dementia (13 vs. 7). Twenty in the positive group and nine in the negative group had started an antidementia medication.
At 4 years, the amyloid-positive group had declined significantly from baseline on all four cognitive measures, performing significantly worse than the amyloid-negative group on each. The amyloid-negative group, conversely, had either stayed steady or experienced nonsignificant change on the tests.
“The [Preclinical Alzheimer Cognitive Composite] assessment captures performance on tasks of episodic memory, orientation, and executive function – the prominent domains of AD-related cognitive decline,” the authors wrote. “This finding strengthens the link between elevated amyloid and the primary manifestations of AD. Individuals with normal amyloid remain free of AD-pattern impairment for many years.
“The longitudinal curves demonstrated that those participants with elevated amyloid were more likely to develop early symptoms consistent with prodromal amyloid within 6 years,” the researchers added.
The study also tracked biomarker changes in AB42, tau, and ventricular and hippocampal volume. These markers changed in both groups – although in each case, the changes associated with amyloid positivity were more pronounced.
Nevertheless, the biomarker data offered a more complex picture. “Measures of CSF tau, phosphorylated tau, and AB42 showed marked differences between groups at baseline,” the investigators noted. “Longitudinal change … was similar across groups, suggesting that they are sensitive to elevated amyloid but do not reflect cognitive and clinical decline once amyloidosis is established. Brain atrophy, as reflected by enlargement of ventricular size, showed change in participants with elevated amyloid. This trend is consistent with brain atrophy with normal aging that is aggravated in the presence of amyloid.”
The finding that AB42 can identify patients on the path to cognitive decline suggests that the protein is more than a risk factor for AD – it is, rather, a marker of presymptomatic disease.
“AD is a gradually progressive disorder that can be identified at the asymptomatic phase using biomarkers of amyloid,” Dr. Donohue and his colleagues said. If that view is accepted, it would change the way potential therapeutics are viewed, from agents that reduce the risk of disease to agents that actively manage disease, even if given presymptomatically.
“This view is important in weighing therapeutic options from a regulatory perspective and for an individual,” the investigators noted. “Accurate characterization of the potential progression of earliest symptoms may inform discussion of the utility of screening people for amyloid abnormalities, with or without effective therapeutic options.”
The Alzheimer’s Disease Neuroimaging Initiative is funded by grants from the Alzheimer’s Association, the National Institutes of Health, and numerous pharmaceutical companies. Dr. Donohue reported financial relationships with Eli Lilly and Neurotrack.
[email protected]
On Twitter @Alz_gal
Despite a large, well-established body of evidence that cognitively normal older people can have brain amyloid without suffering memory loss, the protein does appear to be a harbinger of cognitive decline, Pieter Jelle Visser, MD, PhD, and Betty Tijms, PhD, said in an accompanying editorial (JAMA. 2017 Jun 13;317[22]:2285-7).
The natural history study of 445 elderly people showed consistent steady changes in cognition, as well as brain structure and function, over 4-10 years in those who were amyloid positive at baseline. This finding supports once again the idea that Alzheimer’s dementia is a slowly progressive disease with an extended preclinical phase but that amyloid and progression seem inextricably linked, wrote the two coauthors.
The study “provides further support for the hypothesis that amyloid pathology in cognitively normal individuals should be considered part of a progressive neurodegenerative disease. The results substantiate that amyloid aggregation in cognitively normal individuals is a harbinger of future cognitive decline,” which becomes evident only after extensive damage has occurred.
The work highlights Alzheimer’s long preclinical phase and may present a regulatory conundrum if any antiamyloid therapeutics are ever approved, Dr. Visser and Dr. Tijms said. Although the study demonstrates that amyloid pathology is most certainly not benign, many amyloid-positive elders never develop dementia, probably because they die of something else before becoming symptomatic. Thus, drugs that slow cognitive decline, effectively preventing dementia, may not be cost effective on a population basis.
“However, the other scenario of postponing treatment until an individual has developed cognitive impairments also has disadvantages, because at that stage, irreversible brain damage is so extensive that treatment becomes less effective,” Dr. Visser and Dr. Tijms noted. “This dilemma will require careful evaluation, because optimal patient management strategies will depend on the effectiveness, mechanism, cost, and adverse effects of treatment, as well as patient characteristics and preferences.”
Dr. Visser and Dr. Tijms are associated with the Alzheimer Center at the VU University Medical Center of Amsterdam. Dr. Visser reported financial relationships with Biogen, Eli Lilly, GE Healthcare, and Janssen.
Despite a large, well-established body of evidence that cognitively normal older people can have brain amyloid without suffering memory loss, the protein does appear to be a harbinger of cognitive decline, Pieter Jelle Visser, MD, PhD, and Betty Tijms, PhD, said in an accompanying editorial (JAMA. 2017 Jun 13;317[22]:2285-7).
The natural history study of 445 elderly people showed consistent steady changes in cognition, as well as brain structure and function, over 4-10 years in those who were amyloid positive at baseline. This finding supports once again the idea that Alzheimer’s dementia is a slowly progressive disease with an extended preclinical phase but that amyloid and progression seem inextricably linked, wrote the two coauthors.
The study “provides further support for the hypothesis that amyloid pathology in cognitively normal individuals should be considered part of a progressive neurodegenerative disease. The results substantiate that amyloid aggregation in cognitively normal individuals is a harbinger of future cognitive decline,” which becomes evident only after extensive damage has occurred.
The work highlights Alzheimer’s long preclinical phase and may present a regulatory conundrum if any antiamyloid therapeutics are ever approved, Dr. Visser and Dr. Tijms said. Although the study demonstrates that amyloid pathology is most certainly not benign, many amyloid-positive elders never develop dementia, probably because they die of something else before becoming symptomatic. Thus, drugs that slow cognitive decline, effectively preventing dementia, may not be cost effective on a population basis.
“However, the other scenario of postponing treatment until an individual has developed cognitive impairments also has disadvantages, because at that stage, irreversible brain damage is so extensive that treatment becomes less effective,” Dr. Visser and Dr. Tijms noted. “This dilemma will require careful evaluation, because optimal patient management strategies will depend on the effectiveness, mechanism, cost, and adverse effects of treatment, as well as patient characteristics and preferences.”
Dr. Visser and Dr. Tijms are associated with the Alzheimer Center at the VU University Medical Center of Amsterdam. Dr. Visser reported financial relationships with Biogen, Eli Lilly, GE Healthcare, and Janssen.
Despite a large, well-established body of evidence that cognitively normal older people can have brain amyloid without suffering memory loss, the protein does appear to be a harbinger of cognitive decline, Pieter Jelle Visser, MD, PhD, and Betty Tijms, PhD, said in an accompanying editorial (JAMA. 2017 Jun 13;317[22]:2285-7).
The natural history study of 445 elderly people showed consistent steady changes in cognition, as well as brain structure and function, over 4-10 years in those who were amyloid positive at baseline. This finding supports once again the idea that Alzheimer’s dementia is a slowly progressive disease with an extended preclinical phase but that amyloid and progression seem inextricably linked, wrote the two coauthors.
The study “provides further support for the hypothesis that amyloid pathology in cognitively normal individuals should be considered part of a progressive neurodegenerative disease. The results substantiate that amyloid aggregation in cognitively normal individuals is a harbinger of future cognitive decline,” which becomes evident only after extensive damage has occurred.
The work highlights Alzheimer’s long preclinical phase and may present a regulatory conundrum if any antiamyloid therapeutics are ever approved, Dr. Visser and Dr. Tijms said. Although the study demonstrates that amyloid pathology is most certainly not benign, many amyloid-positive elders never develop dementia, probably because they die of something else before becoming symptomatic. Thus, drugs that slow cognitive decline, effectively preventing dementia, may not be cost effective on a population basis.
“However, the other scenario of postponing treatment until an individual has developed cognitive impairments also has disadvantages, because at that stage, irreversible brain damage is so extensive that treatment becomes less effective,” Dr. Visser and Dr. Tijms noted. “This dilemma will require careful evaluation, because optimal patient management strategies will depend on the effectiveness, mechanism, cost, and adverse effects of treatment, as well as patient characteristics and preferences.”
Dr. Visser and Dr. Tijms are associated with the Alzheimer Center at the VU University Medical Center of Amsterdam. Dr. Visser reported financial relationships with Biogen, Eli Lilly, GE Healthcare, and Janssen.
Cognitively normal seniors with brain amyloid experienced accelerated cognitive decline over as few as 3-4 years, compared with those who had no brain amyloid at baseline.
At 4 years’ follow-up, prodromal Alzheimer’s disease (AD) had developed in 32% of amyloid-positive people and 15% of the amyloid-normal people – a 16.9% difference. Extrapolating the limited 10-year data they collected, the authors projected that after a decade, 88% of the amyloid-positive cohort would have progressed, compared with 29% of the amyloid-normal cohort.
“This suggests that elevated brain amyloid may indicate a presymptomatic stage of disease rather than a risk factor for disease,” wrote Dr. Donohue of the University of Southern California, San Diego. “AD is a gradually progressive disorder that can be identified at the asymptomatic phase using biomarkers of amyloid.”
The study is a project of the Alzheimer’s Disease Neuroimaging Initiative, an ongoing natural history study assessing the clinical, imaging, genetics, and biomarkers of aging, from normal aging to early and late mild cognitive impairment, dementia, or AD.
This particular substudy tracked the association of baseline amyloid-beta 42 (AB42) and cognition among 445 patients, 202 of whom had elevated brain amyloid as measured by cerebrospinal fluid or brain imaging with florbetapir. They were followed for a mean of 3 years, although a limited number of patients had up to 10 years’ worth of data.
The primary endpoints were score changes on four cognitive batteries: the Preclinical Alzheimer Cognitive Composite, the Mini-Mental State Examination; the Clinical Dementia Rating Scale Sum of Boxes, and the Logical Memory Delayed Recall.
At baseline, mean patient age was 74 years, although amyloid-positive patients were slightly older (74.7 years vs. 73.4 years). The mean educational attainment was 16.4 years. Slightly more patients in the amyloid-positive group reported subjective memory complaints (25% vs. 22%). Significantly more had at least one APOE-e4 allele of the apolipoprotein E gene (42% vs. 16%). All were cognitively normal as measured by the four tests. A fifth measure, the Alzheimer’s Disease Assessment Scale 13-item cognitive subscale, was also within normal limits for both groups, but that was not included in the follow-up analysis.
In addition to showing AB42 deposits in the brain and/or decreased AB42 in cerebrospinal fluid (indicating increased binding in the brain), amyloid-positive patients also had significantly different levels of tau. CSF total tau was 76.8 pg/mL in the amyloid-positive group, compared with 59.4 pg/mL in the amyloid-normal group. CSF phosphorylated tau also was elevated in the amyloid-positive group (38.1 pg/mL vs. 26.5 pg/mL). Increased CSF tau is an indicator of neuronal injury.
The mean follow-up time was 3.9 years. At last contact, only four patients had died; 36% were lost to follow-up. Significantly more in the amyloid-positive group had progressed to Alzheimer’s dementia (13 vs. 7). Twenty in the positive group and nine in the negative group had started an antidementia medication.
At 4 years, the amyloid-positive group had declined significantly from baseline on all four cognitive measures, performing significantly worse than the amyloid-negative group on each. The amyloid-negative group, conversely, had either stayed steady or experienced nonsignificant change on the tests.
“The [Preclinical Alzheimer Cognitive Composite] assessment captures performance on tasks of episodic memory, orientation, and executive function – the prominent domains of AD-related cognitive decline,” the authors wrote. “This finding strengthens the link between elevated amyloid and the primary manifestations of AD. Individuals with normal amyloid remain free of AD-pattern impairment for many years.
“The longitudinal curves demonstrated that those participants with elevated amyloid were more likely to develop early symptoms consistent with prodromal amyloid within 6 years,” the researchers added.
The study also tracked biomarker changes in AB42, tau, and ventricular and hippocampal volume. These markers changed in both groups – although in each case, the changes associated with amyloid positivity were more pronounced.
Nevertheless, the biomarker data offered a more complex picture. “Measures of CSF tau, phosphorylated tau, and AB42 showed marked differences between groups at baseline,” the investigators noted. “Longitudinal change … was similar across groups, suggesting that they are sensitive to elevated amyloid but do not reflect cognitive and clinical decline once amyloidosis is established. Brain atrophy, as reflected by enlargement of ventricular size, showed change in participants with elevated amyloid. This trend is consistent with brain atrophy with normal aging that is aggravated in the presence of amyloid.”
The finding that AB42 can identify patients on the path to cognitive decline suggests that the protein is more than a risk factor for AD – it is, rather, a marker of presymptomatic disease.
“AD is a gradually progressive disorder that can be identified at the asymptomatic phase using biomarkers of amyloid,” Dr. Donohue and his colleagues said. If that view is accepted, it would change the way potential therapeutics are viewed, from agents that reduce the risk of disease to agents that actively manage disease, even if given presymptomatically.
“This view is important in weighing therapeutic options from a regulatory perspective and for an individual,” the investigators noted. “Accurate characterization of the potential progression of earliest symptoms may inform discussion of the utility of screening people for amyloid abnormalities, with or without effective therapeutic options.”
The Alzheimer’s Disease Neuroimaging Initiative is funded by grants from the Alzheimer’s Association, the National Institutes of Health, and numerous pharmaceutical companies. Dr. Donohue reported financial relationships with Eli Lilly and Neurotrack.
[email protected]
On Twitter @Alz_gal
Cognitively normal seniors with brain amyloid experienced accelerated cognitive decline over as few as 3-4 years, compared with those who had no brain amyloid at baseline.
At 4 years’ follow-up, prodromal Alzheimer’s disease (AD) had developed in 32% of amyloid-positive people and 15% of the amyloid-normal people – a 16.9% difference. Extrapolating the limited 10-year data they collected, the authors projected that after a decade, 88% of the amyloid-positive cohort would have progressed, compared with 29% of the amyloid-normal cohort.
“This suggests that elevated brain amyloid may indicate a presymptomatic stage of disease rather than a risk factor for disease,” wrote Dr. Donohue of the University of Southern California, San Diego. “AD is a gradually progressive disorder that can be identified at the asymptomatic phase using biomarkers of amyloid.”
The study is a project of the Alzheimer’s Disease Neuroimaging Initiative, an ongoing natural history study assessing the clinical, imaging, genetics, and biomarkers of aging, from normal aging to early and late mild cognitive impairment, dementia, or AD.
This particular substudy tracked the association of baseline amyloid-beta 42 (AB42) and cognition among 445 patients, 202 of whom had elevated brain amyloid as measured by cerebrospinal fluid or brain imaging with florbetapir. They were followed for a mean of 3 years, although a limited number of patients had up to 10 years’ worth of data.
The primary endpoints were score changes on four cognitive batteries: the Preclinical Alzheimer Cognitive Composite, the Mini-Mental State Examination; the Clinical Dementia Rating Scale Sum of Boxes, and the Logical Memory Delayed Recall.
At baseline, mean patient age was 74 years, although amyloid-positive patients were slightly older (74.7 years vs. 73.4 years). The mean educational attainment was 16.4 years. Slightly more patients in the amyloid-positive group reported subjective memory complaints (25% vs. 22%). Significantly more had at least one APOE-e4 allele of the apolipoprotein E gene (42% vs. 16%). All were cognitively normal as measured by the four tests. A fifth measure, the Alzheimer’s Disease Assessment Scale 13-item cognitive subscale, was also within normal limits for both groups, but that was not included in the follow-up analysis.
In addition to showing AB42 deposits in the brain and/or decreased AB42 in cerebrospinal fluid (indicating increased binding in the brain), amyloid-positive patients also had significantly different levels of tau. CSF total tau was 76.8 pg/mL in the amyloid-positive group, compared with 59.4 pg/mL in the amyloid-normal group. CSF phosphorylated tau also was elevated in the amyloid-positive group (38.1 pg/mL vs. 26.5 pg/mL). Increased CSF tau is an indicator of neuronal injury.
The mean follow-up time was 3.9 years. At last contact, only four patients had died; 36% were lost to follow-up. Significantly more in the amyloid-positive group had progressed to Alzheimer’s dementia (13 vs. 7). Twenty in the positive group and nine in the negative group had started an antidementia medication.
At 4 years, the amyloid-positive group had declined significantly from baseline on all four cognitive measures, performing significantly worse than the amyloid-negative group on each. The amyloid-negative group, conversely, had either stayed steady or experienced nonsignificant change on the tests.
“The [Preclinical Alzheimer Cognitive Composite] assessment captures performance on tasks of episodic memory, orientation, and executive function – the prominent domains of AD-related cognitive decline,” the authors wrote. “This finding strengthens the link between elevated amyloid and the primary manifestations of AD. Individuals with normal amyloid remain free of AD-pattern impairment for many years.
“The longitudinal curves demonstrated that those participants with elevated amyloid were more likely to develop early symptoms consistent with prodromal amyloid within 6 years,” the researchers added.
The study also tracked biomarker changes in AB42, tau, and ventricular and hippocampal volume. These markers changed in both groups – although in each case, the changes associated with amyloid positivity were more pronounced.
Nevertheless, the biomarker data offered a more complex picture. “Measures of CSF tau, phosphorylated tau, and AB42 showed marked differences between groups at baseline,” the investigators noted. “Longitudinal change … was similar across groups, suggesting that they are sensitive to elevated amyloid but do not reflect cognitive and clinical decline once amyloidosis is established. Brain atrophy, as reflected by enlargement of ventricular size, showed change in participants with elevated amyloid. This trend is consistent with brain atrophy with normal aging that is aggravated in the presence of amyloid.”
The finding that AB42 can identify patients on the path to cognitive decline suggests that the protein is more than a risk factor for AD – it is, rather, a marker of presymptomatic disease.
“AD is a gradually progressive disorder that can be identified at the asymptomatic phase using biomarkers of amyloid,” Dr. Donohue and his colleagues said. If that view is accepted, it would change the way potential therapeutics are viewed, from agents that reduce the risk of disease to agents that actively manage disease, even if given presymptomatically.
“This view is important in weighing therapeutic options from a regulatory perspective and for an individual,” the investigators noted. “Accurate characterization of the potential progression of earliest symptoms may inform discussion of the utility of screening people for amyloid abnormalities, with or without effective therapeutic options.”
The Alzheimer’s Disease Neuroimaging Initiative is funded by grants from the Alzheimer’s Association, the National Institutes of Health, and numerous pharmaceutical companies. Dr. Donohue reported financial relationships with Eli Lilly and Neurotrack.
[email protected]
On Twitter @Alz_gal
FROM JAMA
Key clinical point:
Major finding: Over 4 years, when compared with seniors without amyloid, amyloid-positive seniors performed significantly worse on four cognitive tests and experienced larger declines in brain volume.
Data source: The study followed 445 cognitively normal older people, mean age 74 years, for a mean of 4 years.
Disclosures: The Alzheimer’s Disease Neuroimaging Initiative is funded by grants from the Alzheimer’s Association, the National Institutes of Health, and numerous pharmaceutical companies. Dr. Donahue reported financial relationships with Eli Lilly and Neurotrack.













