User login
Everything We Say and Do: Setting discharge goals and visit expectations
Editor’s note: “Everything We Say and Do” is an informational series developed by SHM’s Patient Experience Committee to provide readers with thoughtful and actionable communication tactics that have great potential to positively impact patients’ experience of care. Each article will focus on how the contributor applies one or more of the “key communication” tactics in practice to maintain provider accountability for “everything we say and do that affects our patients’ thoughts, feelings, and well-being.”
What I say and do
I always ensure at the end of my visit with a patient and their family that they know when to expect me to return to see their child again.
Why I do it
One of the biggest frustrations I hear from families pertains to the discharge process. In talking with families, they want to know the approximate time for discharge. Often, during morning rounds, we mention that the patient may be able to go home later in the day and we say that we will come in again later to check on them. However, unless we give families a time frame for when we will come back and do that check, they are left waiting without any clear expectations.
How I do it
One of our goals during morning family-centered rounds is to discuss discharge for every patient, every day. Along with discussing the possibility of going home, we try to give the family goals that they can work on throughout the day that are tied to discharge – for example, the approximate by-mouth intake for a toddler admitted for gastroenteritis and dehydration.
I also give the family an approximate time when either I or the resident team will come back to see if they have achieved this goal. This may be either late afternoon or first thing in the morning if we are planning an early-morning discharge before rounds. The families seem to find this helpful because they are not tied to the room all day waiting for the doctor to come back.
I also make sure that the families know they can contact their nurse any time if they need to see any of the doctors sooner than we planned. I let them know that a physician is here on the floor 24 hours a day and that the nurses can easily reach us at any time if they have further concerns. In my experience, this is reassuring to our families.
Christine Hrach is a pediatric hospitalist at Washington University School of Medicine in St. Louis.
Editor’s note: “Everything We Say and Do” is an informational series developed by SHM’s Patient Experience Committee to provide readers with thoughtful and actionable communication tactics that have great potential to positively impact patients’ experience of care. Each article will focus on how the contributor applies one or more of the “key communication” tactics in practice to maintain provider accountability for “everything we say and do that affects our patients’ thoughts, feelings, and well-being.”
What I say and do
I always ensure at the end of my visit with a patient and their family that they know when to expect me to return to see their child again.
Why I do it
One of the biggest frustrations I hear from families pertains to the discharge process. In talking with families, they want to know the approximate time for discharge. Often, during morning rounds, we mention that the patient may be able to go home later in the day and we say that we will come in again later to check on them. However, unless we give families a time frame for when we will come back and do that check, they are left waiting without any clear expectations.
How I do it
One of our goals during morning family-centered rounds is to discuss discharge for every patient, every day. Along with discussing the possibility of going home, we try to give the family goals that they can work on throughout the day that are tied to discharge – for example, the approximate by-mouth intake for a toddler admitted for gastroenteritis and dehydration.
I also give the family an approximate time when either I or the resident team will come back to see if they have achieved this goal. This may be either late afternoon or first thing in the morning if we are planning an early-morning discharge before rounds. The families seem to find this helpful because they are not tied to the room all day waiting for the doctor to come back.
I also make sure that the families know they can contact their nurse any time if they need to see any of the doctors sooner than we planned. I let them know that a physician is here on the floor 24 hours a day and that the nurses can easily reach us at any time if they have further concerns. In my experience, this is reassuring to our families.
Christine Hrach is a pediatric hospitalist at Washington University School of Medicine in St. Louis.
Editor’s note: “Everything We Say and Do” is an informational series developed by SHM’s Patient Experience Committee to provide readers with thoughtful and actionable communication tactics that have great potential to positively impact patients’ experience of care. Each article will focus on how the contributor applies one or more of the “key communication” tactics in practice to maintain provider accountability for “everything we say and do that affects our patients’ thoughts, feelings, and well-being.”
What I say and do
I always ensure at the end of my visit with a patient and their family that they know when to expect me to return to see their child again.
Why I do it
One of the biggest frustrations I hear from families pertains to the discharge process. In talking with families, they want to know the approximate time for discharge. Often, during morning rounds, we mention that the patient may be able to go home later in the day and we say that we will come in again later to check on them. However, unless we give families a time frame for when we will come back and do that check, they are left waiting without any clear expectations.
How I do it
One of our goals during morning family-centered rounds is to discuss discharge for every patient, every day. Along with discussing the possibility of going home, we try to give the family goals that they can work on throughout the day that are tied to discharge – for example, the approximate by-mouth intake for a toddler admitted for gastroenteritis and dehydration.
I also give the family an approximate time when either I or the resident team will come back to see if they have achieved this goal. This may be either late afternoon or first thing in the morning if we are planning an early-morning discharge before rounds. The families seem to find this helpful because they are not tied to the room all day waiting for the doctor to come back.
I also make sure that the families know they can contact their nurse any time if they need to see any of the doctors sooner than we planned. I let them know that a physician is here on the floor 24 hours a day and that the nurses can easily reach us at any time if they have further concerns. In my experience, this is reassuring to our families.
Christine Hrach is a pediatric hospitalist at Washington University School of Medicine in St. Louis.
ADHD – not OCD – called the key comorbidity in pediatric hoarding
SAN FRANCISCO – Most of the pediatric hoarding literature focuses on hoarding accompanied by obsessive-compulsive disorder. But, “I want to highlight that [attention-deficit/hyperactivity disorder] is across the board something that seems to come up in child hoarding behaviors quite a bit, mirroring the adult literature, which is that hoarding behavior may be much more strongly associated with ADHD than it is with OCD,” Jennifer M. Park, PhD, said at the annual conference of the Anxiety and Depression Association of America.
Multiple studies have established that the prevalence of child hoarding is 2%-3.7%. Onset is typically at age 11-15 years. The course is chronic, and it’s a condition that typically exacerbates over time.
“A lot of the adult literature has shown that hoarding behavior actually starts in childhood. In many retrospective reports, adults say, ‘I’ve had these problems ever since I was a kid,’ ” according to Dr. Park, a psychologist affiliated with Stanford (Calif.) University.
Yet childhood hoarding is not widely perceived as problematic. Indeed, many parents and clinicians view it as developmentally appropriate. That’s to a great extent because the presentation of child hoarding behavior often is very different from and less disturbing than adult hoarding for the obvious reason that parents can limit the amount of clutter in the home.
“I have a bunch of kids who have quite significant hoarding behavior, but the parents are really on top of making sure all of that is left in the closet or within the child’s playroom, or maybe a certain section of the house,” Dr. Park said. “They’re able to keep it contained.”
Also, children and young adolescents lack the resources to accumulate massive clutter. They can’t drive, and have little or no money, so they can’t go on compulsive shopping sprees. “What I have seen in the kids that I work with is they make up for that by collecting things like paper and sticks, rocks, wrappers – anything that might be free, or knickknacks they can pick up along the way,” she said.
The cognitive-behavioral model of hoarding was first described 2 decades ago. It names three main factors as key to maintaining hoarding behaviors: emotional attachment and beliefs associated with one’s possessions, often including anthropomorphization; avoidance behaviors due to severe distress at the prospect of discarding stuff; and information-processing deficits.
“The idea here is that deficits in executive function – things like planning, organization, and inhibition – these are known in an extensive literature to be really strongly associated with ADHD, and executive function deficits link well with hoarding disorder as well,” Dr. Park continued.
Dr. Park was the first author of a recent multicenter study of 431 youths aged 6-17 diagnosed with OCD. They were participants in the OCD Collaborative Genetics Study and the OCD Collaborative Genetics Association Study, during which they completed the Behavior Rating Inventory of Executive Functioning (BRIEF) and the Hoarding Rating Scale–Interview. Clinically significant levels of hoarding compulsions were identified in 113 subjects. Compared with the group with OCD but not hoarding, the OCD/hoarding group had significantly lower scores – meaning problematic deficits – on nearly all of the executive function subscales on the BRIEF, including working memory, emotional control, and planning/organization.
The two groups did not differ significantly in the prevalence of full DSM-IV ADHD. But the hoarding group had significantly more inattention and hyperactivity symptoms, and in a multivariate analysis adjusted for age, sex, and ADHD symptoms, deficits in executive function as measured on the BRIEF instrument were the strongest predictor of hoarding severity in the study population (J Psychiatr Res. 2016 Nov;82:141-8).
In another study by Dr. Park and her coinvestigators involving 99 youth diagnosed with ADHD, the severity of inattention and hyperactivity/impulsivity predicted clinically significant hoarding, whereas nonhoarding OCD symptoms did not (J Atten Disord. 2016 Jul;20[7]:617-26).
In an earlier report by other investigators on 109 children seeking treatment for an anxiety disorder, 22% of the study population proved to have elevated levels of hoarding symptoms. They scored significantly higher than the nonhoarding group on measures of obsession-compulsion, anxiety, inattention, thought problems, rule breaking, aggression, social problems, major depression, and overall functional impairment. But of note, attention problems were a significantly stronger predictor of hoarding symptoms than were OCD or anxiety symptoms (J Anxiety Disord. 2015 Dec;36:9-14).
Discussant Eric Storch, PhD, said that it’s important for clinicians and parents to start taking child hoarding seriously as a legitimate treatment target.
“We know that if you start treatment early, you’re more likely to be successful versus when you start at age 57 and the clutter is 9 or 10 on a scale of 10,” said Dr. Storch, professor of pediatrics and director of clinical research for developmental pediatrics at the University of South Florida, Tampa.
Dr. Park reported having no financial conflicts of interest regarding her presentation.
SAN FRANCISCO – Most of the pediatric hoarding literature focuses on hoarding accompanied by obsessive-compulsive disorder. But, “I want to highlight that [attention-deficit/hyperactivity disorder] is across the board something that seems to come up in child hoarding behaviors quite a bit, mirroring the adult literature, which is that hoarding behavior may be much more strongly associated with ADHD than it is with OCD,” Jennifer M. Park, PhD, said at the annual conference of the Anxiety and Depression Association of America.
Multiple studies have established that the prevalence of child hoarding is 2%-3.7%. Onset is typically at age 11-15 years. The course is chronic, and it’s a condition that typically exacerbates over time.
“A lot of the adult literature has shown that hoarding behavior actually starts in childhood. In many retrospective reports, adults say, ‘I’ve had these problems ever since I was a kid,’ ” according to Dr. Park, a psychologist affiliated with Stanford (Calif.) University.
Yet childhood hoarding is not widely perceived as problematic. Indeed, many parents and clinicians view it as developmentally appropriate. That’s to a great extent because the presentation of child hoarding behavior often is very different from and less disturbing than adult hoarding for the obvious reason that parents can limit the amount of clutter in the home.
“I have a bunch of kids who have quite significant hoarding behavior, but the parents are really on top of making sure all of that is left in the closet or within the child’s playroom, or maybe a certain section of the house,” Dr. Park said. “They’re able to keep it contained.”
Also, children and young adolescents lack the resources to accumulate massive clutter. They can’t drive, and have little or no money, so they can’t go on compulsive shopping sprees. “What I have seen in the kids that I work with is they make up for that by collecting things like paper and sticks, rocks, wrappers – anything that might be free, or knickknacks they can pick up along the way,” she said.
The cognitive-behavioral model of hoarding was first described 2 decades ago. It names three main factors as key to maintaining hoarding behaviors: emotional attachment and beliefs associated with one’s possessions, often including anthropomorphization; avoidance behaviors due to severe distress at the prospect of discarding stuff; and information-processing deficits.
“The idea here is that deficits in executive function – things like planning, organization, and inhibition – these are known in an extensive literature to be really strongly associated with ADHD, and executive function deficits link well with hoarding disorder as well,” Dr. Park continued.
Dr. Park was the first author of a recent multicenter study of 431 youths aged 6-17 diagnosed with OCD. They were participants in the OCD Collaborative Genetics Study and the OCD Collaborative Genetics Association Study, during which they completed the Behavior Rating Inventory of Executive Functioning (BRIEF) and the Hoarding Rating Scale–Interview. Clinically significant levels of hoarding compulsions were identified in 113 subjects. Compared with the group with OCD but not hoarding, the OCD/hoarding group had significantly lower scores – meaning problematic deficits – on nearly all of the executive function subscales on the BRIEF, including working memory, emotional control, and planning/organization.
The two groups did not differ significantly in the prevalence of full DSM-IV ADHD. But the hoarding group had significantly more inattention and hyperactivity symptoms, and in a multivariate analysis adjusted for age, sex, and ADHD symptoms, deficits in executive function as measured on the BRIEF instrument were the strongest predictor of hoarding severity in the study population (J Psychiatr Res. 2016 Nov;82:141-8).
In another study by Dr. Park and her coinvestigators involving 99 youth diagnosed with ADHD, the severity of inattention and hyperactivity/impulsivity predicted clinically significant hoarding, whereas nonhoarding OCD symptoms did not (J Atten Disord. 2016 Jul;20[7]:617-26).
In an earlier report by other investigators on 109 children seeking treatment for an anxiety disorder, 22% of the study population proved to have elevated levels of hoarding symptoms. They scored significantly higher than the nonhoarding group on measures of obsession-compulsion, anxiety, inattention, thought problems, rule breaking, aggression, social problems, major depression, and overall functional impairment. But of note, attention problems were a significantly stronger predictor of hoarding symptoms than were OCD or anxiety symptoms (J Anxiety Disord. 2015 Dec;36:9-14).
Discussant Eric Storch, PhD, said that it’s important for clinicians and parents to start taking child hoarding seriously as a legitimate treatment target.
“We know that if you start treatment early, you’re more likely to be successful versus when you start at age 57 and the clutter is 9 or 10 on a scale of 10,” said Dr. Storch, professor of pediatrics and director of clinical research for developmental pediatrics at the University of South Florida, Tampa.
Dr. Park reported having no financial conflicts of interest regarding her presentation.
SAN FRANCISCO – Most of the pediatric hoarding literature focuses on hoarding accompanied by obsessive-compulsive disorder. But, “I want to highlight that [attention-deficit/hyperactivity disorder] is across the board something that seems to come up in child hoarding behaviors quite a bit, mirroring the adult literature, which is that hoarding behavior may be much more strongly associated with ADHD than it is with OCD,” Jennifer M. Park, PhD, said at the annual conference of the Anxiety and Depression Association of America.
Multiple studies have established that the prevalence of child hoarding is 2%-3.7%. Onset is typically at age 11-15 years. The course is chronic, and it’s a condition that typically exacerbates over time.
“A lot of the adult literature has shown that hoarding behavior actually starts in childhood. In many retrospective reports, adults say, ‘I’ve had these problems ever since I was a kid,’ ” according to Dr. Park, a psychologist affiliated with Stanford (Calif.) University.
Yet childhood hoarding is not widely perceived as problematic. Indeed, many parents and clinicians view it as developmentally appropriate. That’s to a great extent because the presentation of child hoarding behavior often is very different from and less disturbing than adult hoarding for the obvious reason that parents can limit the amount of clutter in the home.
“I have a bunch of kids who have quite significant hoarding behavior, but the parents are really on top of making sure all of that is left in the closet or within the child’s playroom, or maybe a certain section of the house,” Dr. Park said. “They’re able to keep it contained.”
Also, children and young adolescents lack the resources to accumulate massive clutter. They can’t drive, and have little or no money, so they can’t go on compulsive shopping sprees. “What I have seen in the kids that I work with is they make up for that by collecting things like paper and sticks, rocks, wrappers – anything that might be free, or knickknacks they can pick up along the way,” she said.
The cognitive-behavioral model of hoarding was first described 2 decades ago. It names three main factors as key to maintaining hoarding behaviors: emotional attachment and beliefs associated with one’s possessions, often including anthropomorphization; avoidance behaviors due to severe distress at the prospect of discarding stuff; and information-processing deficits.
“The idea here is that deficits in executive function – things like planning, organization, and inhibition – these are known in an extensive literature to be really strongly associated with ADHD, and executive function deficits link well with hoarding disorder as well,” Dr. Park continued.
Dr. Park was the first author of a recent multicenter study of 431 youths aged 6-17 diagnosed with OCD. They were participants in the OCD Collaborative Genetics Study and the OCD Collaborative Genetics Association Study, during which they completed the Behavior Rating Inventory of Executive Functioning (BRIEF) and the Hoarding Rating Scale–Interview. Clinically significant levels of hoarding compulsions were identified in 113 subjects. Compared with the group with OCD but not hoarding, the OCD/hoarding group had significantly lower scores – meaning problematic deficits – on nearly all of the executive function subscales on the BRIEF, including working memory, emotional control, and planning/organization.
The two groups did not differ significantly in the prevalence of full DSM-IV ADHD. But the hoarding group had significantly more inattention and hyperactivity symptoms, and in a multivariate analysis adjusted for age, sex, and ADHD symptoms, deficits in executive function as measured on the BRIEF instrument were the strongest predictor of hoarding severity in the study population (J Psychiatr Res. 2016 Nov;82:141-8).
In another study by Dr. Park and her coinvestigators involving 99 youth diagnosed with ADHD, the severity of inattention and hyperactivity/impulsivity predicted clinically significant hoarding, whereas nonhoarding OCD symptoms did not (J Atten Disord. 2016 Jul;20[7]:617-26).
In an earlier report by other investigators on 109 children seeking treatment for an anxiety disorder, 22% of the study population proved to have elevated levels of hoarding symptoms. They scored significantly higher than the nonhoarding group on measures of obsession-compulsion, anxiety, inattention, thought problems, rule breaking, aggression, social problems, major depression, and overall functional impairment. But of note, attention problems were a significantly stronger predictor of hoarding symptoms than were OCD or anxiety symptoms (J Anxiety Disord. 2015 Dec;36:9-14).
Discussant Eric Storch, PhD, said that it’s important for clinicians and parents to start taking child hoarding seriously as a legitimate treatment target.
“We know that if you start treatment early, you’re more likely to be successful versus when you start at age 57 and the clutter is 9 or 10 on a scale of 10,” said Dr. Storch, professor of pediatrics and director of clinical research for developmental pediatrics at the University of South Florida, Tampa.
Dr. Park reported having no financial conflicts of interest regarding her presentation.
EXPERT ANALYSIS FROM ANXIETY AND DEPRESSION CONFERENCE 2017
Metastasized Renal Cell Cancer in Remission With Sunitinib
Sunitinib, a multityrosine kinase inhibitor approved in 2006, has been shown to significantly prolong progression-free survival and overall survival in patients with metastatic renal cell cancer (mRCC). But while some patients with metastasized RCC have reportedly achieved complete remission with sunitinib, clinical and pathologic remission has seldom been described in reports, say researchers from Jinan University in China. They discuss a patient whose colonic metastasis of RCC resolved with microwave ablation and sunitinib.
Related: Incident Chronic Kidney Disease Following Kidney Cancer Surgery
The patient, who was diagnosed with right RCC, underwent a right radical nephrectomy. A pathologic report indicated clear-cell carcinoma of the kidney. Three years later, the patient presented with recurrent upper abdominal pain. An ultrasound scan revealed cholecystitis and gall bladder stones, and she was referred for treatment to the researchers’ hospital. During exploratory laparotomy, surgeons found a hepatic flexure colonic mass “firmly adhering” to the surrounding tissue; the mass perforated the intestine and could not be radically resected. The surgeons performed an ileostomy. The patient was started on sunitinib at 50 mg/d, on a schedule of 4 weeks of therapy followed by 2 weeks off therapy. During a follow-up, they found that the cancer had spread to the liver. Metastasectomy was not possible, so the patient’s doctors chose microwave ablation. The researchers noted that there is currently little experience with microwave ablation in treatment of liver metastasis of RCC.
A follow-up computerized tomography (CT) scan showed that the treatment was shrinking the mass in the colon. Positron emission tomography-CT imaging showed no local signs of relapse. Three years after the microwave ablation, the patient underwent ileostomy reversal, hysterectomy, right hemicolectomy, and partial ileostomy; all pathologic results were benign.
Related: Major Cancer Death Rates Are Down
In about 25% of patients, RCC metastasizes postoperatively—usually to the lungs and liver, the researchers say. Colonic metastases are rare. On the basis of the patient’s clinical features, imaging data, and pathology results, the researchers concluded that sunitinib, with microwave ablation, can prevent unresectable hepatic metastases of RCC from evolving, and sunitinib alone can achieve clinical and pathologic remission of colonic metastases of RCC.
Source:
Peng B, Gong J. Urol Case Rep. 2017;12:78-80.
doi: 10.1016/j.eucr.2016.11.024.
Sunitinib, a multityrosine kinase inhibitor approved in 2006, has been shown to significantly prolong progression-free survival and overall survival in patients with metastatic renal cell cancer (mRCC). But while some patients with metastasized RCC have reportedly achieved complete remission with sunitinib, clinical and pathologic remission has seldom been described in reports, say researchers from Jinan University in China. They discuss a patient whose colonic metastasis of RCC resolved with microwave ablation and sunitinib.
Related: Incident Chronic Kidney Disease Following Kidney Cancer Surgery
The patient, who was diagnosed with right RCC, underwent a right radical nephrectomy. A pathologic report indicated clear-cell carcinoma of the kidney. Three years later, the patient presented with recurrent upper abdominal pain. An ultrasound scan revealed cholecystitis and gall bladder stones, and she was referred for treatment to the researchers’ hospital. During exploratory laparotomy, surgeons found a hepatic flexure colonic mass “firmly adhering” to the surrounding tissue; the mass perforated the intestine and could not be radically resected. The surgeons performed an ileostomy. The patient was started on sunitinib at 50 mg/d, on a schedule of 4 weeks of therapy followed by 2 weeks off therapy. During a follow-up, they found that the cancer had spread to the liver. Metastasectomy was not possible, so the patient’s doctors chose microwave ablation. The researchers noted that there is currently little experience with microwave ablation in treatment of liver metastasis of RCC.
A follow-up computerized tomography (CT) scan showed that the treatment was shrinking the mass in the colon. Positron emission tomography-CT imaging showed no local signs of relapse. Three years after the microwave ablation, the patient underwent ileostomy reversal, hysterectomy, right hemicolectomy, and partial ileostomy; all pathologic results were benign.
Related: Major Cancer Death Rates Are Down
In about 25% of patients, RCC metastasizes postoperatively—usually to the lungs and liver, the researchers say. Colonic metastases are rare. On the basis of the patient’s clinical features, imaging data, and pathology results, the researchers concluded that sunitinib, with microwave ablation, can prevent unresectable hepatic metastases of RCC from evolving, and sunitinib alone can achieve clinical and pathologic remission of colonic metastases of RCC.
Source:
Peng B, Gong J. Urol Case Rep. 2017;12:78-80.
doi: 10.1016/j.eucr.2016.11.024.
Sunitinib, a multityrosine kinase inhibitor approved in 2006, has been shown to significantly prolong progression-free survival and overall survival in patients with metastatic renal cell cancer (mRCC). But while some patients with metastasized RCC have reportedly achieved complete remission with sunitinib, clinical and pathologic remission has seldom been described in reports, say researchers from Jinan University in China. They discuss a patient whose colonic metastasis of RCC resolved with microwave ablation and sunitinib.
Related: Incident Chronic Kidney Disease Following Kidney Cancer Surgery
The patient, who was diagnosed with right RCC, underwent a right radical nephrectomy. A pathologic report indicated clear-cell carcinoma of the kidney. Three years later, the patient presented with recurrent upper abdominal pain. An ultrasound scan revealed cholecystitis and gall bladder stones, and she was referred for treatment to the researchers’ hospital. During exploratory laparotomy, surgeons found a hepatic flexure colonic mass “firmly adhering” to the surrounding tissue; the mass perforated the intestine and could not be radically resected. The surgeons performed an ileostomy. The patient was started on sunitinib at 50 mg/d, on a schedule of 4 weeks of therapy followed by 2 weeks off therapy. During a follow-up, they found that the cancer had spread to the liver. Metastasectomy was not possible, so the patient’s doctors chose microwave ablation. The researchers noted that there is currently little experience with microwave ablation in treatment of liver metastasis of RCC.
A follow-up computerized tomography (CT) scan showed that the treatment was shrinking the mass in the colon. Positron emission tomography-CT imaging showed no local signs of relapse. Three years after the microwave ablation, the patient underwent ileostomy reversal, hysterectomy, right hemicolectomy, and partial ileostomy; all pathologic results were benign.
Related: Major Cancer Death Rates Are Down
In about 25% of patients, RCC metastasizes postoperatively—usually to the lungs and liver, the researchers say. Colonic metastases are rare. On the basis of the patient’s clinical features, imaging data, and pathology results, the researchers concluded that sunitinib, with microwave ablation, can prevent unresectable hepatic metastases of RCC from evolving, and sunitinib alone can achieve clinical and pathologic remission of colonic metastases of RCC.
Source:
Peng B, Gong J. Urol Case Rep. 2017;12:78-80.
doi: 10.1016/j.eucr.2016.11.024.
Avastin and Eylea: Comparison Shows Similar Success
A head-to-head comparison of 2 widely used drugs for macular edema due to central retinal vein occlusion—bevacizumab (Avastin) and aflibercept (Eylea)—found that both improved visual acuity (VA) similarly. But Eylea does it for $1,850 per dose, versus $60 per dose for Avastin.
In the Study of COmparative Treatments for REtinal Vein Occlusion 2 (SCORE2), National Eye Institute (NEI) researchers randomly assigned 362 patients to either Eylea or Avastin by eye injection every 4 weeks for 6 months. At 6 months, the researchers assessed VA, retinal thickness, and side effects.
On average, VA improved about 4 lines on an eye chart, from 20/100 to 20/40, more than doubling the ability to resolve fine detail, said Frederick Ferris, MD, director of the Division of Epidemiology and Clinical Applications at NEI. “For some patients it restores their ability to drive.”
Both drugs prevent the release of vascular endothelial growth factor, which causes swelling. The researchers found that macular edema declined significantly in both groups. The rates of adverse events, such as elevated intraocular pressure, were low and similar in both groups.
Retinal vein occlusion is associated with diabetes mellitus and hypertension. It is the second most common retinal vascular disease after diabetic retinopathy, according to NIH, and affects more than 16 million adults worldwide.
A head-to-head comparison of 2 widely used drugs for macular edema due to central retinal vein occlusion—bevacizumab (Avastin) and aflibercept (Eylea)—found that both improved visual acuity (VA) similarly. But Eylea does it for $1,850 per dose, versus $60 per dose for Avastin.
In the Study of COmparative Treatments for REtinal Vein Occlusion 2 (SCORE2), National Eye Institute (NEI) researchers randomly assigned 362 patients to either Eylea or Avastin by eye injection every 4 weeks for 6 months. At 6 months, the researchers assessed VA, retinal thickness, and side effects.
On average, VA improved about 4 lines on an eye chart, from 20/100 to 20/40, more than doubling the ability to resolve fine detail, said Frederick Ferris, MD, director of the Division of Epidemiology and Clinical Applications at NEI. “For some patients it restores their ability to drive.”
Both drugs prevent the release of vascular endothelial growth factor, which causes swelling. The researchers found that macular edema declined significantly in both groups. The rates of adverse events, such as elevated intraocular pressure, were low and similar in both groups.
Retinal vein occlusion is associated with diabetes mellitus and hypertension. It is the second most common retinal vascular disease after diabetic retinopathy, according to NIH, and affects more than 16 million adults worldwide.
A head-to-head comparison of 2 widely used drugs for macular edema due to central retinal vein occlusion—bevacizumab (Avastin) and aflibercept (Eylea)—found that both improved visual acuity (VA) similarly. But Eylea does it for $1,850 per dose, versus $60 per dose for Avastin.
In the Study of COmparative Treatments for REtinal Vein Occlusion 2 (SCORE2), National Eye Institute (NEI) researchers randomly assigned 362 patients to either Eylea or Avastin by eye injection every 4 weeks for 6 months. At 6 months, the researchers assessed VA, retinal thickness, and side effects.
On average, VA improved about 4 lines on an eye chart, from 20/100 to 20/40, more than doubling the ability to resolve fine detail, said Frederick Ferris, MD, director of the Division of Epidemiology and Clinical Applications at NEI. “For some patients it restores their ability to drive.”
Both drugs prevent the release of vascular endothelial growth factor, which causes swelling. The researchers found that macular edema declined significantly in both groups. The rates of adverse events, such as elevated intraocular pressure, were low and similar in both groups.
Retinal vein occlusion is associated with diabetes mellitus and hypertension. It is the second most common retinal vascular disease after diabetic retinopathy, according to NIH, and affects more than 16 million adults worldwide.
Team finds inappropriate dosing of blood thinners
Patients with atrial fibrillation (AF) and renal impairment require dose reductions of non-vitamin K antagonist oral anticoagulants (NOACs).
But researchers found that 43% of these patients were potentially overdosed, and as many as 1 in 6 (13%) without renal impairment are potentially under dosed.
Failing to reduce the dose for patients with kidney disease increases their risk of bleeding, while under dosing patients without kidney disease puts them at greater risk of stroke. These inappropriate prescribing patterns may impact patient safety without providing benefit in effectiveness.
Using a large US database of de-identified, linked clinical and administrative claims information, the research team found 14,865 patients with AF who were prescribed apixaban, dabigatran, or rivaroxaban between October 1, 2020, and September 30, 2015. Of these, 1,473 had renal impairment.
All three drugs have a standard dose for most patients and a lower dose for patients with kidney issues. And their analysis revealed that 16% percent of all patients received a dose inconsistent with US Food and Drug Administration labeling.
The research team published its findings in the Journal of the American College of Cardiology.
“We conducted this study to highlight the prevalence of inappropriate dosing in routine clinical practice and the associated adverse outcomes,” said Peter Noseworthy, MD, of the Mayo Clinic in Rochester, Minnesota, and senior author of the paper.
“This study underscores the importance for physicians to be vigilant of kidney function when selecting or adjusting dose.”
Dr Noseworthy explained that overdosing is a fairly straightforward problem that can be avoided by regularly monitoring kidney function.
In the kidney-impaired patients who were potentially overdosed, the hazard ratio for the risk of major bleeding was 2.19 (95% confidence interval: 1.07 to 4.46). There was no statistically significant difference in stroke with the 3 NOACs pooled.
However, under dosing is more complex because a balance needs to be established between stroke reduction and bleeding risk, Dr Noseworthy pointed out.
Among the 13,392 patients without renal impairment and no indication for dose reduction, the hazard ratio for a higher risk of stroke was 4.87 (95% confidence interval: 1.30 to 18.26). There was no statistically significant difference in major bleeding in apixaban-treated patients nor statistically significant relationships in dabigatran- or rivaroxaban-treated patients without renal impairment.
“I think physicians often choose to reduce the dose,” Dr Noseworthy explained, “when they anticipate their patients are at a particularly high bleeding risk—independent of kidney function.”
“Dosing errors of these blood-thinning medications in patients with atrial fibrillation are common and have concerning adverse outcomes,” said Xiaoxi Yao, PhD, also of the Mayo Clinic in Rochester, Minnesota, and lead author of the paper.
Dr Yao noted that the number of patients using these drugs has increased since their introduction in 2010. Before that, the standard blood-thinning drug was warfarin, which requires constant monitoring and doctor visits. ![]()
Patients with atrial fibrillation (AF) and renal impairment require dose reductions of non-vitamin K antagonist oral anticoagulants (NOACs).
But researchers found that 43% of these patients were potentially overdosed, and as many as 1 in 6 (13%) without renal impairment are potentially under dosed.
Failing to reduce the dose for patients with kidney disease increases their risk of bleeding, while under dosing patients without kidney disease puts them at greater risk of stroke. These inappropriate prescribing patterns may impact patient safety without providing benefit in effectiveness.
Using a large US database of de-identified, linked clinical and administrative claims information, the research team found 14,865 patients with AF who were prescribed apixaban, dabigatran, or rivaroxaban between October 1, 2020, and September 30, 2015. Of these, 1,473 had renal impairment.
All three drugs have a standard dose for most patients and a lower dose for patients with kidney issues. And their analysis revealed that 16% percent of all patients received a dose inconsistent with US Food and Drug Administration labeling.
The research team published its findings in the Journal of the American College of Cardiology.
“We conducted this study to highlight the prevalence of inappropriate dosing in routine clinical practice and the associated adverse outcomes,” said Peter Noseworthy, MD, of the Mayo Clinic in Rochester, Minnesota, and senior author of the paper.
“This study underscores the importance for physicians to be vigilant of kidney function when selecting or adjusting dose.”
Dr Noseworthy explained that overdosing is a fairly straightforward problem that can be avoided by regularly monitoring kidney function.
In the kidney-impaired patients who were potentially overdosed, the hazard ratio for the risk of major bleeding was 2.19 (95% confidence interval: 1.07 to 4.46). There was no statistically significant difference in stroke with the 3 NOACs pooled.
However, under dosing is more complex because a balance needs to be established between stroke reduction and bleeding risk, Dr Noseworthy pointed out.
Among the 13,392 patients without renal impairment and no indication for dose reduction, the hazard ratio for a higher risk of stroke was 4.87 (95% confidence interval: 1.30 to 18.26). There was no statistically significant difference in major bleeding in apixaban-treated patients nor statistically significant relationships in dabigatran- or rivaroxaban-treated patients without renal impairment.
“I think physicians often choose to reduce the dose,” Dr Noseworthy explained, “when they anticipate their patients are at a particularly high bleeding risk—independent of kidney function.”
“Dosing errors of these blood-thinning medications in patients with atrial fibrillation are common and have concerning adverse outcomes,” said Xiaoxi Yao, PhD, also of the Mayo Clinic in Rochester, Minnesota, and lead author of the paper.
Dr Yao noted that the number of patients using these drugs has increased since their introduction in 2010. Before that, the standard blood-thinning drug was warfarin, which requires constant monitoring and doctor visits. ![]()
Patients with atrial fibrillation (AF) and renal impairment require dose reductions of non-vitamin K antagonist oral anticoagulants (NOACs).
But researchers found that 43% of these patients were potentially overdosed, and as many as 1 in 6 (13%) without renal impairment are potentially under dosed.
Failing to reduce the dose for patients with kidney disease increases their risk of bleeding, while under dosing patients without kidney disease puts them at greater risk of stroke. These inappropriate prescribing patterns may impact patient safety without providing benefit in effectiveness.
Using a large US database of de-identified, linked clinical and administrative claims information, the research team found 14,865 patients with AF who were prescribed apixaban, dabigatran, or rivaroxaban between October 1, 2020, and September 30, 2015. Of these, 1,473 had renal impairment.
All three drugs have a standard dose for most patients and a lower dose for patients with kidney issues. And their analysis revealed that 16% percent of all patients received a dose inconsistent with US Food and Drug Administration labeling.
The research team published its findings in the Journal of the American College of Cardiology.
“We conducted this study to highlight the prevalence of inappropriate dosing in routine clinical practice and the associated adverse outcomes,” said Peter Noseworthy, MD, of the Mayo Clinic in Rochester, Minnesota, and senior author of the paper.
“This study underscores the importance for physicians to be vigilant of kidney function when selecting or adjusting dose.”
Dr Noseworthy explained that overdosing is a fairly straightforward problem that can be avoided by regularly monitoring kidney function.
In the kidney-impaired patients who were potentially overdosed, the hazard ratio for the risk of major bleeding was 2.19 (95% confidence interval: 1.07 to 4.46). There was no statistically significant difference in stroke with the 3 NOACs pooled.
However, under dosing is more complex because a balance needs to be established between stroke reduction and bleeding risk, Dr Noseworthy pointed out.
Among the 13,392 patients without renal impairment and no indication for dose reduction, the hazard ratio for a higher risk of stroke was 4.87 (95% confidence interval: 1.30 to 18.26). There was no statistically significant difference in major bleeding in apixaban-treated patients nor statistically significant relationships in dabigatran- or rivaroxaban-treated patients without renal impairment.
“I think physicians often choose to reduce the dose,” Dr Noseworthy explained, “when they anticipate their patients are at a particularly high bleeding risk—independent of kidney function.”
“Dosing errors of these blood-thinning medications in patients with atrial fibrillation are common and have concerning adverse outcomes,” said Xiaoxi Yao, PhD, also of the Mayo Clinic in Rochester, Minnesota, and lead author of the paper.
Dr Yao noted that the number of patients using these drugs has increased since their introduction in 2010. Before that, the standard blood-thinning drug was warfarin, which requires constant monitoring and doctor visits. ![]()
Pembrolizumab enhances CAR T-cell persistence in relapsed ALL
CHICAGO—Three of 6 pediatric patients with relapsed or refractory acute lymphoblastic leukemia (ALL) whose CD19 chimeric antigen receptor (CAR) T cells did not persist even after reinfusion demonstrated persistence when the PD-1 checkpoint inhibitor pembrolizumab was added to the regimen.
CAR T cells can persist for months or even years and have the potential to mediate long-term disease control.
But some patients recover their normal B cells, which is a marker of loss of functional CAR T cells. These patients are at a higher risk of disease relapse.
Investigators, therefore, undertook a pilot study to determine whether PD-1 checkpoint pathway inhibition can improve CAR T persistence in these patients.
Shannon L. Maude, MD, of the Children’s Hospital of Philadelphia and the University of Pennsylvania Perelman School of Medicine, shared some of the patient cases in this pilot study at the ASCO 2017 Annual Meeting (Abstract 103*).
The investigators hypothesized that possible anti-murine immunogenicity could be causing poor CAR T-cell persistence, since the first CAR T developed used scFv domains of murine origin.
If T-cell exhaustion caused poor CAR T-cell persistence, immune checkpoints might play a role. In this case, combination with PD-1 checkpoint blockade could improve persistence, they hypothesized.
The investigators proposed to administer a repeat CAR T-cell infusion for relapsed or refractory ALL patients with poor persistence and add pembrolizumab after retreatment if the patients still had poor persistence. Patients were offered the option of another reinfusion prior to treatment with pembrolizumab if their CAR T-cell persistence continued to be poor.
Investigators added pembrolizumab no earlier than 14 days after infusion and only after patients recovered from cytokine release syndrome (CRS).
The infusions in the pilot study were humanized CART19 (huCART19, CTL119), unless otherwise specified.
Patient 1 – Pembrolizumab for partial response
This patient had no response to the prior murine CD19 CAR infusion, but responded well to infusion with huCART19 with good CAR T-cell proliferation.
By day 28, the patient had achieved a complete response (CR) in bone marrow but had a minimal residual disease (MRD) level of 1.2%.
At 7 weeks, the patient had a CD19+ relapse with low levels of huCART19.
Investigators added pembrolizumb on day 52 after infusion, and the patient had a modest increase in huCART19.
The patient had a temporary clearance of peripheral blasts followed by disease progression.
Patient 2 – Pembrolizumab for no response
This patient had a CD19+ relapse at 12 months after prior murine CD19 CAR infusion.
The patient was treated with huCART19, had good proliferation, but a rapid drop of CART19, and no response of the disease with a CD19+ relapse.
The patient had a reinfusion of huCART19 at 6 weeks and investigators added pembrolizumab on day 14 after reinfusion.
The patient experienced good huCART19 proliferation and prolonged persistence, but by day 28 had persistent disease, this time with decreased expression of CD19+ cells.
Patient 3 – Pembrolizumab for poor persistence
This patient had a CR with a prior murine CD19 CAR, but had poor persistence and early B cell recovery at 2 months.
The patient had a CD19+ relapse, was treated with huCART19, and had good proliferation again.
The patient achieved an MRD-negative CR, but because of short persistence and early B-cell recovery at 2 months, relapsed at 15 months.
The patient was reinfused at 17 months and pembrolizumab was added on day 14. The patient entered CR with prolonged persistence, but ultimately B-cell recovery occurred.
The patient was reinfused a third time, and pembrolizumab was added to the regimen every 3 weeks. The patient is experiencing prolonged persistence and continued B-cell aplasia.
Patient 4 – Pembrolizumab for poor persistence
This patient had achieved a CR with murine CAR19, but had a CD19+ relapse at 9 months. The patient then received huCART19, had good proliferation and achieved a CR, but had short persistence and B-cell recovery at 2 months.
The patient relapsed at 12 months and was reinfused with huCART19 at 14 months.
Pembrolizumab was added on day 14 after reinfusion, but the patient had no huCART19 proliferation, no response, and CD19+ MRD.
Patient 5 – Pembrolizumab for poor persistence
The patient responded to a prior murine CART19, but had a CD19+ relapse at 12 months.
The patient was infused with huCART19 and had good proliferation but short persistence. The patient was reinfused at 6 months, but again had short persistence.
The patient was reinfused again at 8 months because of B-cell recovery, and pembrolizumab was added on day 14 and administered every 3 weeks thereafter.
The patient is experiencing prolonged persistence and continued B-cell aplasia.
Patient 6 – Pembrolizumab for lymphomatous disease
This patient, who had widespread lymphadenopathy and M3 bone marrow, had not received prior CAR T cells and was treated for the first time with a murine CART19 for r/r ALL.
The patient had good expansion of CART19, but by day 28, PET scan showed widespread lymph node disease despite CR in the bone marrow.
The patient was given pembrolizumab on day 32 after infusion and every 2-3 weeks thereafter. After the addition of pembrolizumab, the patient had increased CART19 cells in blood and a significant decrease in PET-avid disease.
Summary
Three of six patients achieved objective clinical responses with pembrolizumab: 2 had prolonged B-cell aplasia, and another had a decrease in PET-avid lymphomatous disease.
The addition of pembrolizumab was also well tolerated by the patients, with minimal side effects of fever in 2 patients, cytopenias in 2 patients, and no instances of severe CRS.
The investigators believe the addition of checkpoint pathway inhibitors has the potential to prolong CAR T-cell persistence and warrants further investigation. ![]()
CHICAGO—Three of 6 pediatric patients with relapsed or refractory acute lymphoblastic leukemia (ALL) whose CD19 chimeric antigen receptor (CAR) T cells did not persist even after reinfusion demonstrated persistence when the PD-1 checkpoint inhibitor pembrolizumab was added to the regimen.
CAR T cells can persist for months or even years and have the potential to mediate long-term disease control.
But some patients recover their normal B cells, which is a marker of loss of functional CAR T cells. These patients are at a higher risk of disease relapse.
Investigators, therefore, undertook a pilot study to determine whether PD-1 checkpoint pathway inhibition can improve CAR T persistence in these patients.
Shannon L. Maude, MD, of the Children’s Hospital of Philadelphia and the University of Pennsylvania Perelman School of Medicine, shared some of the patient cases in this pilot study at the ASCO 2017 Annual Meeting (Abstract 103*).
The investigators hypothesized that possible anti-murine immunogenicity could be causing poor CAR T-cell persistence, since the first CAR T developed used scFv domains of murine origin.
If T-cell exhaustion caused poor CAR T-cell persistence, immune checkpoints might play a role. In this case, combination with PD-1 checkpoint blockade could improve persistence, they hypothesized.
The investigators proposed to administer a repeat CAR T-cell infusion for relapsed or refractory ALL patients with poor persistence and add pembrolizumab after retreatment if the patients still had poor persistence. Patients were offered the option of another reinfusion prior to treatment with pembrolizumab if their CAR T-cell persistence continued to be poor.
Investigators added pembrolizumab no earlier than 14 days after infusion and only after patients recovered from cytokine release syndrome (CRS).
The infusions in the pilot study were humanized CART19 (huCART19, CTL119), unless otherwise specified.
Patient 1 – Pembrolizumab for partial response
This patient had no response to the prior murine CD19 CAR infusion, but responded well to infusion with huCART19 with good CAR T-cell proliferation.
By day 28, the patient had achieved a complete response (CR) in bone marrow but had a minimal residual disease (MRD) level of 1.2%.
At 7 weeks, the patient had a CD19+ relapse with low levels of huCART19.
Investigators added pembrolizumb on day 52 after infusion, and the patient had a modest increase in huCART19.
The patient had a temporary clearance of peripheral blasts followed by disease progression.
Patient 2 – Pembrolizumab for no response
This patient had a CD19+ relapse at 12 months after prior murine CD19 CAR infusion.
The patient was treated with huCART19, had good proliferation, but a rapid drop of CART19, and no response of the disease with a CD19+ relapse.
The patient had a reinfusion of huCART19 at 6 weeks and investigators added pembrolizumab on day 14 after reinfusion.
The patient experienced good huCART19 proliferation and prolonged persistence, but by day 28 had persistent disease, this time with decreased expression of CD19+ cells.
Patient 3 – Pembrolizumab for poor persistence
This patient had a CR with a prior murine CD19 CAR, but had poor persistence and early B cell recovery at 2 months.
The patient had a CD19+ relapse, was treated with huCART19, and had good proliferation again.
The patient achieved an MRD-negative CR, but because of short persistence and early B-cell recovery at 2 months, relapsed at 15 months.
The patient was reinfused at 17 months and pembrolizumab was added on day 14. The patient entered CR with prolonged persistence, but ultimately B-cell recovery occurred.
The patient was reinfused a third time, and pembrolizumab was added to the regimen every 3 weeks. The patient is experiencing prolonged persistence and continued B-cell aplasia.
Patient 4 – Pembrolizumab for poor persistence
This patient had achieved a CR with murine CAR19, but had a CD19+ relapse at 9 months. The patient then received huCART19, had good proliferation and achieved a CR, but had short persistence and B-cell recovery at 2 months.
The patient relapsed at 12 months and was reinfused with huCART19 at 14 months.
Pembrolizumab was added on day 14 after reinfusion, but the patient had no huCART19 proliferation, no response, and CD19+ MRD.
Patient 5 – Pembrolizumab for poor persistence
The patient responded to a prior murine CART19, but had a CD19+ relapse at 12 months.
The patient was infused with huCART19 and had good proliferation but short persistence. The patient was reinfused at 6 months, but again had short persistence.
The patient was reinfused again at 8 months because of B-cell recovery, and pembrolizumab was added on day 14 and administered every 3 weeks thereafter.
The patient is experiencing prolonged persistence and continued B-cell aplasia.
Patient 6 – Pembrolizumab for lymphomatous disease
This patient, who had widespread lymphadenopathy and M3 bone marrow, had not received prior CAR T cells and was treated for the first time with a murine CART19 for r/r ALL.
The patient had good expansion of CART19, but by day 28, PET scan showed widespread lymph node disease despite CR in the bone marrow.
The patient was given pembrolizumab on day 32 after infusion and every 2-3 weeks thereafter. After the addition of pembrolizumab, the patient had increased CART19 cells in blood and a significant decrease in PET-avid disease.
Summary
Three of six patients achieved objective clinical responses with pembrolizumab: 2 had prolonged B-cell aplasia, and another had a decrease in PET-avid lymphomatous disease.
The addition of pembrolizumab was also well tolerated by the patients, with minimal side effects of fever in 2 patients, cytopenias in 2 patients, and no instances of severe CRS.
The investigators believe the addition of checkpoint pathway inhibitors has the potential to prolong CAR T-cell persistence and warrants further investigation. ![]()
CHICAGO—Three of 6 pediatric patients with relapsed or refractory acute lymphoblastic leukemia (ALL) whose CD19 chimeric antigen receptor (CAR) T cells did not persist even after reinfusion demonstrated persistence when the PD-1 checkpoint inhibitor pembrolizumab was added to the regimen.
CAR T cells can persist for months or even years and have the potential to mediate long-term disease control.
But some patients recover their normal B cells, which is a marker of loss of functional CAR T cells. These patients are at a higher risk of disease relapse.
Investigators, therefore, undertook a pilot study to determine whether PD-1 checkpoint pathway inhibition can improve CAR T persistence in these patients.
Shannon L. Maude, MD, of the Children’s Hospital of Philadelphia and the University of Pennsylvania Perelman School of Medicine, shared some of the patient cases in this pilot study at the ASCO 2017 Annual Meeting (Abstract 103*).
The investigators hypothesized that possible anti-murine immunogenicity could be causing poor CAR T-cell persistence, since the first CAR T developed used scFv domains of murine origin.
If T-cell exhaustion caused poor CAR T-cell persistence, immune checkpoints might play a role. In this case, combination with PD-1 checkpoint blockade could improve persistence, they hypothesized.
The investigators proposed to administer a repeat CAR T-cell infusion for relapsed or refractory ALL patients with poor persistence and add pembrolizumab after retreatment if the patients still had poor persistence. Patients were offered the option of another reinfusion prior to treatment with pembrolizumab if their CAR T-cell persistence continued to be poor.
Investigators added pembrolizumab no earlier than 14 days after infusion and only after patients recovered from cytokine release syndrome (CRS).
The infusions in the pilot study were humanized CART19 (huCART19, CTL119), unless otherwise specified.
Patient 1 – Pembrolizumab for partial response
This patient had no response to the prior murine CD19 CAR infusion, but responded well to infusion with huCART19 with good CAR T-cell proliferation.
By day 28, the patient had achieved a complete response (CR) in bone marrow but had a minimal residual disease (MRD) level of 1.2%.
At 7 weeks, the patient had a CD19+ relapse with low levels of huCART19.
Investigators added pembrolizumb on day 52 after infusion, and the patient had a modest increase in huCART19.
The patient had a temporary clearance of peripheral blasts followed by disease progression.
Patient 2 – Pembrolizumab for no response
This patient had a CD19+ relapse at 12 months after prior murine CD19 CAR infusion.
The patient was treated with huCART19, had good proliferation, but a rapid drop of CART19, and no response of the disease with a CD19+ relapse.
The patient had a reinfusion of huCART19 at 6 weeks and investigators added pembrolizumab on day 14 after reinfusion.
The patient experienced good huCART19 proliferation and prolonged persistence, but by day 28 had persistent disease, this time with decreased expression of CD19+ cells.
Patient 3 – Pembrolizumab for poor persistence
This patient had a CR with a prior murine CD19 CAR, but had poor persistence and early B cell recovery at 2 months.
The patient had a CD19+ relapse, was treated with huCART19, and had good proliferation again.
The patient achieved an MRD-negative CR, but because of short persistence and early B-cell recovery at 2 months, relapsed at 15 months.
The patient was reinfused at 17 months and pembrolizumab was added on day 14. The patient entered CR with prolonged persistence, but ultimately B-cell recovery occurred.
The patient was reinfused a third time, and pembrolizumab was added to the regimen every 3 weeks. The patient is experiencing prolonged persistence and continued B-cell aplasia.
Patient 4 – Pembrolizumab for poor persistence
This patient had achieved a CR with murine CAR19, but had a CD19+ relapse at 9 months. The patient then received huCART19, had good proliferation and achieved a CR, but had short persistence and B-cell recovery at 2 months.
The patient relapsed at 12 months and was reinfused with huCART19 at 14 months.
Pembrolizumab was added on day 14 after reinfusion, but the patient had no huCART19 proliferation, no response, and CD19+ MRD.
Patient 5 – Pembrolizumab for poor persistence
The patient responded to a prior murine CART19, but had a CD19+ relapse at 12 months.
The patient was infused with huCART19 and had good proliferation but short persistence. The patient was reinfused at 6 months, but again had short persistence.
The patient was reinfused again at 8 months because of B-cell recovery, and pembrolizumab was added on day 14 and administered every 3 weeks thereafter.
The patient is experiencing prolonged persistence and continued B-cell aplasia.
Patient 6 – Pembrolizumab for lymphomatous disease
This patient, who had widespread lymphadenopathy and M3 bone marrow, had not received prior CAR T cells and was treated for the first time with a murine CART19 for r/r ALL.
The patient had good expansion of CART19, but by day 28, PET scan showed widespread lymph node disease despite CR in the bone marrow.
The patient was given pembrolizumab on day 32 after infusion and every 2-3 weeks thereafter. After the addition of pembrolizumab, the patient had increased CART19 cells in blood and a significant decrease in PET-avid disease.
Summary
Three of six patients achieved objective clinical responses with pembrolizumab: 2 had prolonged B-cell aplasia, and another had a decrease in PET-avid lymphomatous disease.
The addition of pembrolizumab was also well tolerated by the patients, with minimal side effects of fever in 2 patients, cytopenias in 2 patients, and no instances of severe CRS.
The investigators believe the addition of checkpoint pathway inhibitors has the potential to prolong CAR T-cell persistence and warrants further investigation. ![]()
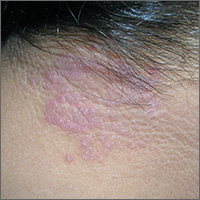
Itchy rash on neck
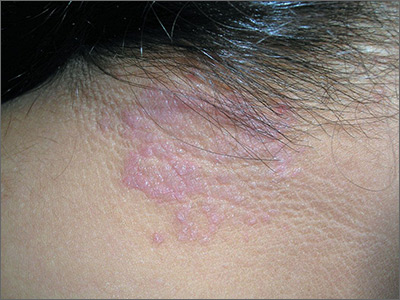
The FP diagnosed lichen simplex chronicus (LSC) in this patient, based on the lesion’s clinical appearance and location, as well as the patient’s history of repeated daily scratching. LSC is more common in women than in men, and occurs mostly in mid- to late-adulthood, with the highest prevalence in people who are 30 to 50 years of age.
A very common location for LSC in women is the back of the neck. In this case the LSC was coexisting with acanthosis nigricans. Fortunately, this patient did not have diabetes, but her obesity and family history predisposed her to acanthosis nigricans.
The treatment for LSC is topical mid- to high-potency corticosteroids. Oral sedating antihistamines can be added at night if pruritus is bad during the evening. If the patient acknowledges that stress is involved, obtain a good psychosocial history and offer the patient treatment for any problems you uncover.
Patients need to minimize touching, scratching, and rubbing of the affected areas. Explain to patients that they are unintentionally hurting their own skin. Suggest that they gently apply their medication or a moisturizer instead of scratching the pruritic areas.
In this case, the FP prescribed topical triamcinolone ointment and stressed the importance of not rubbing or scratching the area. The patient’s LSC healed well.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Johnson A. Self-inflicted dermatoses. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 856-862.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP diagnosed lichen simplex chronicus (LSC) in this patient, based on the lesion’s clinical appearance and location, as well as the patient’s history of repeated daily scratching. LSC is more common in women than in men, and occurs mostly in mid- to late-adulthood, with the highest prevalence in people who are 30 to 50 years of age.
A very common location for LSC in women is the back of the neck. In this case the LSC was coexisting with acanthosis nigricans. Fortunately, this patient did not have diabetes, but her obesity and family history predisposed her to acanthosis nigricans.
The treatment for LSC is topical mid- to high-potency corticosteroids. Oral sedating antihistamines can be added at night if pruritus is bad during the evening. If the patient acknowledges that stress is involved, obtain a good psychosocial history and offer the patient treatment for any problems you uncover.
Patients need to minimize touching, scratching, and rubbing of the affected areas. Explain to patients that they are unintentionally hurting their own skin. Suggest that they gently apply their medication or a moisturizer instead of scratching the pruritic areas.
In this case, the FP prescribed topical triamcinolone ointment and stressed the importance of not rubbing or scratching the area. The patient’s LSC healed well.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Johnson A. Self-inflicted dermatoses. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 856-862.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP diagnosed lichen simplex chronicus (LSC) in this patient, based on the lesion’s clinical appearance and location, as well as the patient’s history of repeated daily scratching. LSC is more common in women than in men, and occurs mostly in mid- to late-adulthood, with the highest prevalence in people who are 30 to 50 years of age.
A very common location for LSC in women is the back of the neck. In this case the LSC was coexisting with acanthosis nigricans. Fortunately, this patient did not have diabetes, but her obesity and family history predisposed her to acanthosis nigricans.
The treatment for LSC is topical mid- to high-potency corticosteroids. Oral sedating antihistamines can be added at night if pruritus is bad during the evening. If the patient acknowledges that stress is involved, obtain a good psychosocial history and offer the patient treatment for any problems you uncover.
Patients need to minimize touching, scratching, and rubbing of the affected areas. Explain to patients that they are unintentionally hurting their own skin. Suggest that they gently apply their medication or a moisturizer instead of scratching the pruritic areas.
In this case, the FP prescribed topical triamcinolone ointment and stressed the importance of not rubbing or scratching the area. The patient’s LSC healed well.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Johnson A. Self-inflicted dermatoses. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 856-862.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
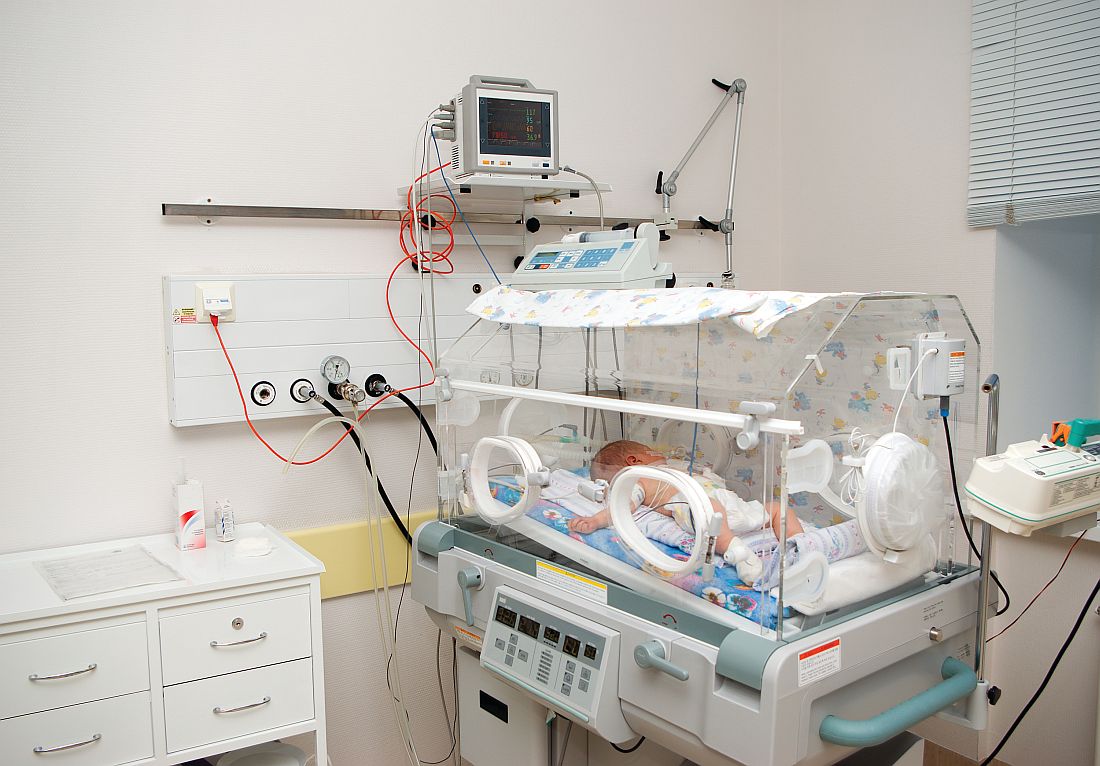
Management of asymptomatic chorioamnionitis-exposed neonates needs revamping
Clinical observation and laboratory evaluation without immediate antibiotic use in asymptomatic chorioamnionitis-exposed neonates prevented neonatal intensive care unit (NICU) admission in two-thirds of these infants, Amanda I. Jan, MD, of the University of Southern California, Los Angeles, and her associates reported in a study.
Since maternal intrapartum antibiotic prophylaxis was introduced, neonatal early-onset sepsis (EOS) rates have dropped considerably, and rates remain low even in chorioamnionitis-exposed infants. Despite these low risks, current American Academy of Pediatrics and Centers for Disease Control and Prevention recommendations still call for a limited laboratory evaluation and immediate empirical antibiotic therapy in all infants exposed to chorioamnionitis, often necessitating NICU admission for IV antibiotics, the researchers noted.
Of the 78 infants admitted to the NICU and put on antibiotics, 76% were treated with antibiotics for more than 72 hours, with a median 7 days of treatment, compared with a median 2 days for nonadmitted infants (P less than .001). Only 85% of admitted infants received any breast milk, compared with 94% of infants in the mother-infant unit (P = .032), and none of the admitted infants were exclusively breastfed.
“When the overall risks of EOS are low, exposure of large numbers of well-appearing infants to even short courses of antibiotics is no longer justified,” Dr. Jan and her associates stated. “The [difference in] cost of a stay in the mother-infant unit for 2 days, compared with a NICU stay, which averaged a week, is substantial. The charge for our NICU is $12,612 per day in contrast to $5,300 per day in the mother-infant unit. The cost savings for the 162 infants who were cared for 2 days in the mother-infant unit, compared with an EOS evaluation and antibiotic therapy in the NICU, totals $2,369,088, or $359,861 per year.
“There were no deaths or morbidities identified in any infant during the study period,” they reported. No infant was readmitted to the study hospital for sepsis after discharge.
Dr. Jan and her associates recommend their alternative management of asymptomatic chorioamnionitis-exposed neonates involving lab evaluations and close clinical observation without immediate antibiotic administration in a mother-infant unit. They believe this prevents unnecessary antibiotic exposure, unnecessarily high hospitalization costs, and disruption of maternal-neonatal bonding and breastfeeding. Additional studies are needed to determine the safety of this approach.
This study received no external funding, and Dr. Jan and her associates reported no relevant financial disclosures.
Dr. Jan and her associates have taken steps in the right direction in altering management of asymptomatic term and near-term newborns with a maternal history of chorioamnionitis to avoid administering empirical antibiotics to all these babies, which is sorely needed as the current American Academy of Pediatrics and Centers for Disease Control and Prevention guidelines are outdated.
However, their alternative plan needs some tweaking. The positive predictive value of abnormal complete blood count or C-reactive protein results is too low to be of use in diagnosing sepsis. “We believe a better approach would be to forgo routine laboratory evaluations among this population altogether and manage them using clinical signs alone.”
They said it was important to state two key caveats. “First, in the immediate postpartum period, mild respiratory distress among term or near-term newborns may be attributable to the physiologic transition, which occurs in all newborn infants. It is not necessary to draw laboratories or start antibiotics on these patients as long as their symptoms improve and resolve within the first 6 hours of life. Second, if newborns with a maternal history of chorioamnionitis are to be monitored for signs of sepsis outside the NICU setting, observations must be frequent (at least hourly for the first 6 hours of life and then every 3 hours for the next 18 hours) and performed by adequately trained medical staff. In the absence of frequent, reliable observation, there is a possibility that the early signs of sepsis will be missed and go untreated with potentially severe consequences.”
This approach, as with any other, needs additional study.
Thomas A. Hooven, MD, and Richard A. Polin, MD, pediatricians at the Columbia University, New York, discussed the study by Jan et al. in a commentary, which is summarized here (Pediatrics. 2017;140[1]:e20171155). They reported that they received no external funding and had no relevant financial disclosures.
Dr. Jan and her associates have taken steps in the right direction in altering management of asymptomatic term and near-term newborns with a maternal history of chorioamnionitis to avoid administering empirical antibiotics to all these babies, which is sorely needed as the current American Academy of Pediatrics and Centers for Disease Control and Prevention guidelines are outdated.
However, their alternative plan needs some tweaking. The positive predictive value of abnormal complete blood count or C-reactive protein results is too low to be of use in diagnosing sepsis. “We believe a better approach would be to forgo routine laboratory evaluations among this population altogether and manage them using clinical signs alone.”
They said it was important to state two key caveats. “First, in the immediate postpartum period, mild respiratory distress among term or near-term newborns may be attributable to the physiologic transition, which occurs in all newborn infants. It is not necessary to draw laboratories or start antibiotics on these patients as long as their symptoms improve and resolve within the first 6 hours of life. Second, if newborns with a maternal history of chorioamnionitis are to be monitored for signs of sepsis outside the NICU setting, observations must be frequent (at least hourly for the first 6 hours of life and then every 3 hours for the next 18 hours) and performed by adequately trained medical staff. In the absence of frequent, reliable observation, there is a possibility that the early signs of sepsis will be missed and go untreated with potentially severe consequences.”
This approach, as with any other, needs additional study.
Thomas A. Hooven, MD, and Richard A. Polin, MD, pediatricians at the Columbia University, New York, discussed the study by Jan et al. in a commentary, which is summarized here (Pediatrics. 2017;140[1]:e20171155). They reported that they received no external funding and had no relevant financial disclosures.
Dr. Jan and her associates have taken steps in the right direction in altering management of asymptomatic term and near-term newborns with a maternal history of chorioamnionitis to avoid administering empirical antibiotics to all these babies, which is sorely needed as the current American Academy of Pediatrics and Centers for Disease Control and Prevention guidelines are outdated.
However, their alternative plan needs some tweaking. The positive predictive value of abnormal complete blood count or C-reactive protein results is too low to be of use in diagnosing sepsis. “We believe a better approach would be to forgo routine laboratory evaluations among this population altogether and manage them using clinical signs alone.”
They said it was important to state two key caveats. “First, in the immediate postpartum period, mild respiratory distress among term or near-term newborns may be attributable to the physiologic transition, which occurs in all newborn infants. It is not necessary to draw laboratories or start antibiotics on these patients as long as their symptoms improve and resolve within the first 6 hours of life. Second, if newborns with a maternal history of chorioamnionitis are to be monitored for signs of sepsis outside the NICU setting, observations must be frequent (at least hourly for the first 6 hours of life and then every 3 hours for the next 18 hours) and performed by adequately trained medical staff. In the absence of frequent, reliable observation, there is a possibility that the early signs of sepsis will be missed and go untreated with potentially severe consequences.”
This approach, as with any other, needs additional study.
Thomas A. Hooven, MD, and Richard A. Polin, MD, pediatricians at the Columbia University, New York, discussed the study by Jan et al. in a commentary, which is summarized here (Pediatrics. 2017;140[1]:e20171155). They reported that they received no external funding and had no relevant financial disclosures.
Clinical observation and laboratory evaluation without immediate antibiotic use in asymptomatic chorioamnionitis-exposed neonates prevented neonatal intensive care unit (NICU) admission in two-thirds of these infants, Amanda I. Jan, MD, of the University of Southern California, Los Angeles, and her associates reported in a study.
Since maternal intrapartum antibiotic prophylaxis was introduced, neonatal early-onset sepsis (EOS) rates have dropped considerably, and rates remain low even in chorioamnionitis-exposed infants. Despite these low risks, current American Academy of Pediatrics and Centers for Disease Control and Prevention recommendations still call for a limited laboratory evaluation and immediate empirical antibiotic therapy in all infants exposed to chorioamnionitis, often necessitating NICU admission for IV antibiotics, the researchers noted.
Of the 78 infants admitted to the NICU and put on antibiotics, 76% were treated with antibiotics for more than 72 hours, with a median 7 days of treatment, compared with a median 2 days for nonadmitted infants (P less than .001). Only 85% of admitted infants received any breast milk, compared with 94% of infants in the mother-infant unit (P = .032), and none of the admitted infants were exclusively breastfed.
“When the overall risks of EOS are low, exposure of large numbers of well-appearing infants to even short courses of antibiotics is no longer justified,” Dr. Jan and her associates stated. “The [difference in] cost of a stay in the mother-infant unit for 2 days, compared with a NICU stay, which averaged a week, is substantial. The charge for our NICU is $12,612 per day in contrast to $5,300 per day in the mother-infant unit. The cost savings for the 162 infants who were cared for 2 days in the mother-infant unit, compared with an EOS evaluation and antibiotic therapy in the NICU, totals $2,369,088, or $359,861 per year.
“There were no deaths or morbidities identified in any infant during the study period,” they reported. No infant was readmitted to the study hospital for sepsis after discharge.
Dr. Jan and her associates recommend their alternative management of asymptomatic chorioamnionitis-exposed neonates involving lab evaluations and close clinical observation without immediate antibiotic administration in a mother-infant unit. They believe this prevents unnecessary antibiotic exposure, unnecessarily high hospitalization costs, and disruption of maternal-neonatal bonding and breastfeeding. Additional studies are needed to determine the safety of this approach.
This study received no external funding, and Dr. Jan and her associates reported no relevant financial disclosures.
Clinical observation and laboratory evaluation without immediate antibiotic use in asymptomatic chorioamnionitis-exposed neonates prevented neonatal intensive care unit (NICU) admission in two-thirds of these infants, Amanda I. Jan, MD, of the University of Southern California, Los Angeles, and her associates reported in a study.
Since maternal intrapartum antibiotic prophylaxis was introduced, neonatal early-onset sepsis (EOS) rates have dropped considerably, and rates remain low even in chorioamnionitis-exposed infants. Despite these low risks, current American Academy of Pediatrics and Centers for Disease Control and Prevention recommendations still call for a limited laboratory evaluation and immediate empirical antibiotic therapy in all infants exposed to chorioamnionitis, often necessitating NICU admission for IV antibiotics, the researchers noted.
Of the 78 infants admitted to the NICU and put on antibiotics, 76% were treated with antibiotics for more than 72 hours, with a median 7 days of treatment, compared with a median 2 days for nonadmitted infants (P less than .001). Only 85% of admitted infants received any breast milk, compared with 94% of infants in the mother-infant unit (P = .032), and none of the admitted infants were exclusively breastfed.
“When the overall risks of EOS are low, exposure of large numbers of well-appearing infants to even short courses of antibiotics is no longer justified,” Dr. Jan and her associates stated. “The [difference in] cost of a stay in the mother-infant unit for 2 days, compared with a NICU stay, which averaged a week, is substantial. The charge for our NICU is $12,612 per day in contrast to $5,300 per day in the mother-infant unit. The cost savings for the 162 infants who were cared for 2 days in the mother-infant unit, compared with an EOS evaluation and antibiotic therapy in the NICU, totals $2,369,088, or $359,861 per year.
“There were no deaths or morbidities identified in any infant during the study period,” they reported. No infant was readmitted to the study hospital for sepsis after discharge.
Dr. Jan and her associates recommend their alternative management of asymptomatic chorioamnionitis-exposed neonates involving lab evaluations and close clinical observation without immediate antibiotic administration in a mother-infant unit. They believe this prevents unnecessary antibiotic exposure, unnecessarily high hospitalization costs, and disruption of maternal-neonatal bonding and breastfeeding. Additional studies are needed to determine the safety of this approach.
This study received no external funding, and Dr. Jan and her associates reported no relevant financial disclosures.
FROM PEDIATRICS
Key clinical point: Alternative management of asymptomatic chorioamnionitis-exposed neonates will prevent unnecessary antibiotic exposure, unnecessarily high hospitalization costs, and disruption of maternal-neonatal bonding and breastfeeding.
Major finding: Of the 240 infants, 67.5% remained well with a routine newborn course in the mother-infant unit and 32.5% subsequently were admitted to the NICU because of abnormal laboratory data, a positive blood culture, or the onset of clinical signs of sepsis.
Data source: A retrospective cohort study of 240 asymptomatic chorioamnionitis-exposed neonates.
Disclosures: This study received no external funding, and Dr. Jan and her associates reported no relevant financial disclosures.
All Is Not Swell
ANSWER
The correct answer is elephantiasis nostras verrucosa (ENV; choice “d”). Cellulitis (choice “a”), venous insufficiency (choice “b”), and lymphedema (choice “c”) are all factors in the broader diagnosis of ENV.
DISCUSSION
ENV is an unusual condition that represents hypertrophic fibrosis secondary to repeated episodes of lymphangitis. This begins with venous insufficiency, which is made worse by increasing obesity (which impedes venous return) and repeated bouts of cellulitis. With ENV, fibroblasts are increased due to extravasation of high-molecular-weight protein (lymphorrhea), which leads to a buildup of keratinocytes, ultimately expressing as extreme hyperkeratosis.
In this patient’s case, his sedentary lifestyle and constant seated position contribute to the problem. Many of his past treatments were reasonable, but—as in many ENV cases—his condition is beyond the point of treatment.
Typically, in-home treatment includes compression and elevation of the legs. Topical application of urea creams is often used to soften the rough skin, but in this patient’s case, the cream burned so badly that it was of no use. Alas, the very things he needs to do are those he cannot: walk, burn calories, and avoid long periods of inactivity.
ANSWER
The correct answer is elephantiasis nostras verrucosa (ENV; choice “d”). Cellulitis (choice “a”), venous insufficiency (choice “b”), and lymphedema (choice “c”) are all factors in the broader diagnosis of ENV.
DISCUSSION
ENV is an unusual condition that represents hypertrophic fibrosis secondary to repeated episodes of lymphangitis. This begins with venous insufficiency, which is made worse by increasing obesity (which impedes venous return) and repeated bouts of cellulitis. With ENV, fibroblasts are increased due to extravasation of high-molecular-weight protein (lymphorrhea), which leads to a buildup of keratinocytes, ultimately expressing as extreme hyperkeratosis.
In this patient’s case, his sedentary lifestyle and constant seated position contribute to the problem. Many of his past treatments were reasonable, but—as in many ENV cases—his condition is beyond the point of treatment.
Typically, in-home treatment includes compression and elevation of the legs. Topical application of urea creams is often used to soften the rough skin, but in this patient’s case, the cream burned so badly that it was of no use. Alas, the very things he needs to do are those he cannot: walk, burn calories, and avoid long periods of inactivity.
ANSWER
The correct answer is elephantiasis nostras verrucosa (ENV; choice “d”). Cellulitis (choice “a”), venous insufficiency (choice “b”), and lymphedema (choice “c”) are all factors in the broader diagnosis of ENV.
DISCUSSION
ENV is an unusual condition that represents hypertrophic fibrosis secondary to repeated episodes of lymphangitis. This begins with venous insufficiency, which is made worse by increasing obesity (which impedes venous return) and repeated bouts of cellulitis. With ENV, fibroblasts are increased due to extravasation of high-molecular-weight protein (lymphorrhea), which leads to a buildup of keratinocytes, ultimately expressing as extreme hyperkeratosis.
In this patient’s case, his sedentary lifestyle and constant seated position contribute to the problem. Many of his past treatments were reasonable, but—as in many ENV cases—his condition is beyond the point of treatment.
Typically, in-home treatment includes compression and elevation of the legs. Topical application of urea creams is often used to soften the rough skin, but in this patient’s case, the cream burned so badly that it was of no use. Alas, the very things he needs to do are those he cannot: walk, burn calories, and avoid long periods of inactivity.
A 70-year-old man is referred to dermatology after trying “everything else” for problems he has had for at least 15 years. In that time, he has been hospitalized repeatedly for swelling and pain in his legs, with odoriferous drainage.
Despite extensive treatment attempts—multiple antibiotics, oral and topical steroids, and OTC creams—the condition is worsening. In ho
The patient denies having cancer or deep vein thrombosis (both of which he has been thoroughly checked for), as well as congestive heart failure. He states that almost every morning, upon rising, the swelling in his legs is considerably lessened.
Both legs are swollen, red, and edematous from just below the knees down. Advanced, pebbly, hyperkeratotic plaques cover the lower two-thirds of both legs, favoring the anterior over the posterior portions. Pitting edema is elicited with minimal digital pressure but does not cause any pain.The patient is in no acute distress but is clearly uncomfortable. He has been confined to a wheelchair for years due to back problems; he can barely stand when asked to do so. He is extremely obese.
First trimester lithium exposure ups risk of cardiac malformations
Cardiac malformations are three times more likely to occur in infants exposed to lithium during the first trimester of gestation than in unexposed infants.
The increased risk could account for one additional cardiac malformation per 100 live births, Elisabetta Patorno, MD, and her colleagues wrote in the June 8 issue of the New England Journal of Medicine (2017;376:2245-54).
Dr. Patorno’s study is the largest conducted since then. It comprised more than 1.3 million pregnancies included in the U.S. Medicaid Analytic eXtract database during 2000-2010. Of these, 663 had first trimester lithium exposure. These were compared with 1,945 pregnancies with first trimester exposure to lamotrigine, another mood stabilizer, and to the remaining 1.3 million pregnancies unexposed to either drug.
There were 16 cardiac malformations in the lithium group (2.41%); 27 in the lamotrigine group (1.39%); and 15,251 in the unexposed group (1.15%). Lithium conferred a 65% increased risk of cardiac defect, compared with unexposed pregnancies. It more than doubled the risk when compared with lamotrigine-exposed pregnancies (risk ratio, 2.25).
The risk was dose dependent, however, with an 11% increase associated with 600 mg/day or less and a 60% increase associated with 601-900 mg/day. Infants exposed to more than 900 mg per day in the first trimester, however, were more than 300% more likely to have a cardiac malformation (RR, 3.22).
The investigators also examined the association of lithium with cardiac defects consistent with Ebstein’s anomaly. Lithium more than doubled the risk, compared with unexposed infants (RR, 2.66). This risk was also dose dependent; all of the right ventricular outflow defects occurred in infants exposed to more than 600 mg/day.
Dr. Patorno reported grant support from National Institute of Mental Health during the study and grant support from Boehringer Ingelheim and GlaxoSmithKline outside of the study. Other authors reported receiving grants or personal fees from various pharmaceutical companies.
[email protected]
On Twitter @Alz_gal
Cardiac malformations are three times more likely to occur in infants exposed to lithium during the first trimester of gestation than in unexposed infants.
The increased risk could account for one additional cardiac malformation per 100 live births, Elisabetta Patorno, MD, and her colleagues wrote in the June 8 issue of the New England Journal of Medicine (2017;376:2245-54).
Dr. Patorno’s study is the largest conducted since then. It comprised more than 1.3 million pregnancies included in the U.S. Medicaid Analytic eXtract database during 2000-2010. Of these, 663 had first trimester lithium exposure. These were compared with 1,945 pregnancies with first trimester exposure to lamotrigine, another mood stabilizer, and to the remaining 1.3 million pregnancies unexposed to either drug.
There were 16 cardiac malformations in the lithium group (2.41%); 27 in the lamotrigine group (1.39%); and 15,251 in the unexposed group (1.15%). Lithium conferred a 65% increased risk of cardiac defect, compared with unexposed pregnancies. It more than doubled the risk when compared with lamotrigine-exposed pregnancies (risk ratio, 2.25).
The risk was dose dependent, however, with an 11% increase associated with 600 mg/day or less and a 60% increase associated with 601-900 mg/day. Infants exposed to more than 900 mg per day in the first trimester, however, were more than 300% more likely to have a cardiac malformation (RR, 3.22).
The investigators also examined the association of lithium with cardiac defects consistent with Ebstein’s anomaly. Lithium more than doubled the risk, compared with unexposed infants (RR, 2.66). This risk was also dose dependent; all of the right ventricular outflow defects occurred in infants exposed to more than 600 mg/day.
Dr. Patorno reported grant support from National Institute of Mental Health during the study and grant support from Boehringer Ingelheim and GlaxoSmithKline outside of the study. Other authors reported receiving grants or personal fees from various pharmaceutical companies.
[email protected]
On Twitter @Alz_gal
Cardiac malformations are three times more likely to occur in infants exposed to lithium during the first trimester of gestation than in unexposed infants.
The increased risk could account for one additional cardiac malformation per 100 live births, Elisabetta Patorno, MD, and her colleagues wrote in the June 8 issue of the New England Journal of Medicine (2017;376:2245-54).
Dr. Patorno’s study is the largest conducted since then. It comprised more than 1.3 million pregnancies included in the U.S. Medicaid Analytic eXtract database during 2000-2010. Of these, 663 had first trimester lithium exposure. These were compared with 1,945 pregnancies with first trimester exposure to lamotrigine, another mood stabilizer, and to the remaining 1.3 million pregnancies unexposed to either drug.
There were 16 cardiac malformations in the lithium group (2.41%); 27 in the lamotrigine group (1.39%); and 15,251 in the unexposed group (1.15%). Lithium conferred a 65% increased risk of cardiac defect, compared with unexposed pregnancies. It more than doubled the risk when compared with lamotrigine-exposed pregnancies (risk ratio, 2.25).
The risk was dose dependent, however, with an 11% increase associated with 600 mg/day or less and a 60% increase associated with 601-900 mg/day. Infants exposed to more than 900 mg per day in the first trimester, however, were more than 300% more likely to have a cardiac malformation (RR, 3.22).
The investigators also examined the association of lithium with cardiac defects consistent with Ebstein’s anomaly. Lithium more than doubled the risk, compared with unexposed infants (RR, 2.66). This risk was also dose dependent; all of the right ventricular outflow defects occurred in infants exposed to more than 600 mg/day.
Dr. Patorno reported grant support from National Institute of Mental Health during the study and grant support from Boehringer Ingelheim and GlaxoSmithKline outside of the study. Other authors reported receiving grants or personal fees from various pharmaceutical companies.
[email protected]
On Twitter @Alz_gal
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: The dose-dependent increased risks ranged from 11% to more than 300%, compared with unexposed pregnancies.
Data source: The Medicaid database review comprised more than 1.3 million pregnancies.
Disclosures: Dr. Patorno reported grant support from National Institute of Mental Health during the study and grant support from Boehringer Ingelheim and GlaxoSmithKline outside of the study. Other authors reported receiving grants or personal fees from various pharmaceutical companies.