User login
Adding rituximab to reduced intensity conditioning boosts PFS
ORLANDO – Rituximab conferred a significant progression-free survival benefit in reduced intensity conditioning regimens for patients with B-cell non-Hodgkin lymphoma who underwent allogeneic hematopoietic cell transplantation, based on data from the Center for International Blood & Marrow Transplant Research.
Further, higher cumulative rituximab doses appeared to confer a benefit in overall survival.
Rituximab is frequently a component of reduced intensity conditioning (RIC) regimens in allogeneic hematopoietic cell transplantation (HCT), but there has been a “paucity of comparative data” for rituximab-containing (R-RIC) versus non–R-RIC conditioning regimens for allogeneic transplant patients, Narendranath Epperla, MD, of the Medical College of Wisconsin, Milwaukee, said during the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society of Blood and Marrow Transplantation.
Using data from the Center for International Blood & Marrow Transplant Research, Dr. Epperla and his colleagues identified 1,022 patients who received rituximab and 379 patients who did not with diffuse large B-cell lymphoma, follicular lymphoma, mantle cell lymphoma, and marginal zone lymphoma. The patients received their first RIC or non-myeloablative allogeneic HCT between 2008 and 2014. The donors were matched, and either related or 8x8 allele-matched unrelated; the graft source could be bone marrow or peripheral blood. Graft versus host disease (GVHD) suppression was calcineurin inhibitor based.
Patients who had received myeloablative conditioning, or who had received radioimmunotherapy or alemtuzumab were excluded, as were those who received alternative donor allografts.
Dr. Epperla and his colleagues factored in patient and disease characteristics, as well as differences in transplant regimen, in determining the adjusted cumulative incidence of relapse or progression, as well as the incidence of nonrelapse mortality.
In the multivariable analysis, overall survival did not differ between the R-RIC and the non–R-RIC cohorts (relative risk [RR] of all-cause mortality, R-RIC = 0.83, 95% CI 0.67-1.03, P = .09).
Based on the cumulative dose of rituximab that patients had received, though, “we noted that patients who got higher doses of rituximab had lower risk of nonrelapse mortality,” Dr. Epperla said. “Higher cumulative doses of rituximab seem to confer overall survival benefit.” This was true even though the higher rituximab doses had no significant effect on the risk of therapy failure, nonrelapse mortality, or the risk of progression/relapse.
When the cumulative rituximab dose was 2,000 to 3,375 mg/m2, the hazard ratio for all-cause mortality fell to 0.43 compared to a cumulative rituximab dose of less than 1,000 mg/m2 (95% confidence interval [CI] 0.21-0.90, P = .02).
Among the R-RIC group, there was a nonsignificant trend toward reduced risk of progression or relapse (relative risk of progression/relapse, R-RIC = 0.79, 95% CI 0.63-1.01, P = .055). However, the R-RIC group fared significantly better in terms of progression-free survival (RR of PFS, R-RIC = 0.76, 95% CI 0.62-0.92, P = .006).
After transplant, patients in the R-RIC group were no more likely than those in the non–R-RIC group to experience chronic GVHD (RR of GVHD, R-RIC = 1.15, 95% CI 0.96-1.39, P = .13). There was no difference in the adjusted curves of nonrelapse mortality between the groups (RR of nonrelapse mortality, R-RIC = 0.90, 95% CI 0.67-1.22, P = .51).
Also, there were no fatal cytopenias in the R-RIC arm, although the literature warrants some concern for increased risk of infection with rituximab, Dr. Epperla said.
At baseline, there were no significant differences in demographic characteristics between the nonrituximab and rituximab arms of the study population. More than 90% of patients were white, and 65% were male; the median age was 57 years (range, 18-74).
Patients had been diagnosed about 3 years before receiving HCT; about 60% of patients had a baseline Karnofsky performance score greater than 90, and the HCT comorbidity index was 2. About 86% of patients were chemosensitive, and patients in both study arms had received a median of three prior lines of therapy.
There were some differences in conditioning regimens between the two groups. “There were a significantly higher number of patients in the nonrituximab group who received fludarabine/busulfan, while there were a significantly high number in the rituximab group who received a fludarabine/cyclophosphamide-based conditioning regimen,” Dr. Epperla said. Follicular lymphomas were more common in the R-RIC arm, while diffuse large B-cell lymphomas were seen more in the non–R-RIC arm.
Given the survival benefit and similar rates of chronic GVHD seen in the retrospective analysis, a prospective, randomized head-to-head trial of R-RIC versus non–R-RIC is warranted, Dr. Epperla concluded.
During the postpresentation discussion, Dr. Epperla acknowledged the variability of the lymphomas in the study, but that there was no significant statistical effect of specific histologies on the findings in a subgroup analysis. Dr. Epperla added that the chemosensitivity status at transplant was checked to account for patient exposure to rituximab before RIC, and that there was no effect of prior rituximab exposure on the outcomes examined.
Dr. Epperla reported no conflicts of interest.
[email protected]
On Twitter @karioakes
ORLANDO – Rituximab conferred a significant progression-free survival benefit in reduced intensity conditioning regimens for patients with B-cell non-Hodgkin lymphoma who underwent allogeneic hematopoietic cell transplantation, based on data from the Center for International Blood & Marrow Transplant Research.
Further, higher cumulative rituximab doses appeared to confer a benefit in overall survival.
Rituximab is frequently a component of reduced intensity conditioning (RIC) regimens in allogeneic hematopoietic cell transplantation (HCT), but there has been a “paucity of comparative data” for rituximab-containing (R-RIC) versus non–R-RIC conditioning regimens for allogeneic transplant patients, Narendranath Epperla, MD, of the Medical College of Wisconsin, Milwaukee, said during the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society of Blood and Marrow Transplantation.
Using data from the Center for International Blood & Marrow Transplant Research, Dr. Epperla and his colleagues identified 1,022 patients who received rituximab and 379 patients who did not with diffuse large B-cell lymphoma, follicular lymphoma, mantle cell lymphoma, and marginal zone lymphoma. The patients received their first RIC or non-myeloablative allogeneic HCT between 2008 and 2014. The donors were matched, and either related or 8x8 allele-matched unrelated; the graft source could be bone marrow or peripheral blood. Graft versus host disease (GVHD) suppression was calcineurin inhibitor based.
Patients who had received myeloablative conditioning, or who had received radioimmunotherapy or alemtuzumab were excluded, as were those who received alternative donor allografts.
Dr. Epperla and his colleagues factored in patient and disease characteristics, as well as differences in transplant regimen, in determining the adjusted cumulative incidence of relapse or progression, as well as the incidence of nonrelapse mortality.
In the multivariable analysis, overall survival did not differ between the R-RIC and the non–R-RIC cohorts (relative risk [RR] of all-cause mortality, R-RIC = 0.83, 95% CI 0.67-1.03, P = .09).
Based on the cumulative dose of rituximab that patients had received, though, “we noted that patients who got higher doses of rituximab had lower risk of nonrelapse mortality,” Dr. Epperla said. “Higher cumulative doses of rituximab seem to confer overall survival benefit.” This was true even though the higher rituximab doses had no significant effect on the risk of therapy failure, nonrelapse mortality, or the risk of progression/relapse.
When the cumulative rituximab dose was 2,000 to 3,375 mg/m2, the hazard ratio for all-cause mortality fell to 0.43 compared to a cumulative rituximab dose of less than 1,000 mg/m2 (95% confidence interval [CI] 0.21-0.90, P = .02).
Among the R-RIC group, there was a nonsignificant trend toward reduced risk of progression or relapse (relative risk of progression/relapse, R-RIC = 0.79, 95% CI 0.63-1.01, P = .055). However, the R-RIC group fared significantly better in terms of progression-free survival (RR of PFS, R-RIC = 0.76, 95% CI 0.62-0.92, P = .006).
After transplant, patients in the R-RIC group were no more likely than those in the non–R-RIC group to experience chronic GVHD (RR of GVHD, R-RIC = 1.15, 95% CI 0.96-1.39, P = .13). There was no difference in the adjusted curves of nonrelapse mortality between the groups (RR of nonrelapse mortality, R-RIC = 0.90, 95% CI 0.67-1.22, P = .51).
Also, there were no fatal cytopenias in the R-RIC arm, although the literature warrants some concern for increased risk of infection with rituximab, Dr. Epperla said.
At baseline, there were no significant differences in demographic characteristics between the nonrituximab and rituximab arms of the study population. More than 90% of patients were white, and 65% were male; the median age was 57 years (range, 18-74).
Patients had been diagnosed about 3 years before receiving HCT; about 60% of patients had a baseline Karnofsky performance score greater than 90, and the HCT comorbidity index was 2. About 86% of patients were chemosensitive, and patients in both study arms had received a median of three prior lines of therapy.
There were some differences in conditioning regimens between the two groups. “There were a significantly higher number of patients in the nonrituximab group who received fludarabine/busulfan, while there were a significantly high number in the rituximab group who received a fludarabine/cyclophosphamide-based conditioning regimen,” Dr. Epperla said. Follicular lymphomas were more common in the R-RIC arm, while diffuse large B-cell lymphomas were seen more in the non–R-RIC arm.
Given the survival benefit and similar rates of chronic GVHD seen in the retrospective analysis, a prospective, randomized head-to-head trial of R-RIC versus non–R-RIC is warranted, Dr. Epperla concluded.
During the postpresentation discussion, Dr. Epperla acknowledged the variability of the lymphomas in the study, but that there was no significant statistical effect of specific histologies on the findings in a subgroup analysis. Dr. Epperla added that the chemosensitivity status at transplant was checked to account for patient exposure to rituximab before RIC, and that there was no effect of prior rituximab exposure on the outcomes examined.
Dr. Epperla reported no conflicts of interest.
[email protected]
On Twitter @karioakes
ORLANDO – Rituximab conferred a significant progression-free survival benefit in reduced intensity conditioning regimens for patients with B-cell non-Hodgkin lymphoma who underwent allogeneic hematopoietic cell transplantation, based on data from the Center for International Blood & Marrow Transplant Research.
Further, higher cumulative rituximab doses appeared to confer a benefit in overall survival.
Rituximab is frequently a component of reduced intensity conditioning (RIC) regimens in allogeneic hematopoietic cell transplantation (HCT), but there has been a “paucity of comparative data” for rituximab-containing (R-RIC) versus non–R-RIC conditioning regimens for allogeneic transplant patients, Narendranath Epperla, MD, of the Medical College of Wisconsin, Milwaukee, said during the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society of Blood and Marrow Transplantation.
Using data from the Center for International Blood & Marrow Transplant Research, Dr. Epperla and his colleagues identified 1,022 patients who received rituximab and 379 patients who did not with diffuse large B-cell lymphoma, follicular lymphoma, mantle cell lymphoma, and marginal zone lymphoma. The patients received their first RIC or non-myeloablative allogeneic HCT between 2008 and 2014. The donors were matched, and either related or 8x8 allele-matched unrelated; the graft source could be bone marrow or peripheral blood. Graft versus host disease (GVHD) suppression was calcineurin inhibitor based.
Patients who had received myeloablative conditioning, or who had received radioimmunotherapy or alemtuzumab were excluded, as were those who received alternative donor allografts.
Dr. Epperla and his colleagues factored in patient and disease characteristics, as well as differences in transplant regimen, in determining the adjusted cumulative incidence of relapse or progression, as well as the incidence of nonrelapse mortality.
In the multivariable analysis, overall survival did not differ between the R-RIC and the non–R-RIC cohorts (relative risk [RR] of all-cause mortality, R-RIC = 0.83, 95% CI 0.67-1.03, P = .09).
Based on the cumulative dose of rituximab that patients had received, though, “we noted that patients who got higher doses of rituximab had lower risk of nonrelapse mortality,” Dr. Epperla said. “Higher cumulative doses of rituximab seem to confer overall survival benefit.” This was true even though the higher rituximab doses had no significant effect on the risk of therapy failure, nonrelapse mortality, or the risk of progression/relapse.
When the cumulative rituximab dose was 2,000 to 3,375 mg/m2, the hazard ratio for all-cause mortality fell to 0.43 compared to a cumulative rituximab dose of less than 1,000 mg/m2 (95% confidence interval [CI] 0.21-0.90, P = .02).
Among the R-RIC group, there was a nonsignificant trend toward reduced risk of progression or relapse (relative risk of progression/relapse, R-RIC = 0.79, 95% CI 0.63-1.01, P = .055). However, the R-RIC group fared significantly better in terms of progression-free survival (RR of PFS, R-RIC = 0.76, 95% CI 0.62-0.92, P = .006).
After transplant, patients in the R-RIC group were no more likely than those in the non–R-RIC group to experience chronic GVHD (RR of GVHD, R-RIC = 1.15, 95% CI 0.96-1.39, P = .13). There was no difference in the adjusted curves of nonrelapse mortality between the groups (RR of nonrelapse mortality, R-RIC = 0.90, 95% CI 0.67-1.22, P = .51).
Also, there were no fatal cytopenias in the R-RIC arm, although the literature warrants some concern for increased risk of infection with rituximab, Dr. Epperla said.
At baseline, there were no significant differences in demographic characteristics between the nonrituximab and rituximab arms of the study population. More than 90% of patients were white, and 65% were male; the median age was 57 years (range, 18-74).
Patients had been diagnosed about 3 years before receiving HCT; about 60% of patients had a baseline Karnofsky performance score greater than 90, and the HCT comorbidity index was 2. About 86% of patients were chemosensitive, and patients in both study arms had received a median of three prior lines of therapy.
There were some differences in conditioning regimens between the two groups. “There were a significantly higher number of patients in the nonrituximab group who received fludarabine/busulfan, while there were a significantly high number in the rituximab group who received a fludarabine/cyclophosphamide-based conditioning regimen,” Dr. Epperla said. Follicular lymphomas were more common in the R-RIC arm, while diffuse large B-cell lymphomas were seen more in the non–R-RIC arm.
Given the survival benefit and similar rates of chronic GVHD seen in the retrospective analysis, a prospective, randomized head-to-head trial of R-RIC versus non–R-RIC is warranted, Dr. Epperla concluded.
During the postpresentation discussion, Dr. Epperla acknowledged the variability of the lymphomas in the study, but that there was no significant statistical effect of specific histologies on the findings in a subgroup analysis. Dr. Epperla added that the chemosensitivity status at transplant was checked to account for patient exposure to rituximab before RIC, and that there was no effect of prior rituximab exposure on the outcomes examined.
Dr. Epperla reported no conflicts of interest.
[email protected]
On Twitter @karioakes
Key clinical point:
Major finding: Patients with rituximab-containing RIC regimens had better progression-free survival (PFS; relative risk of PFS, non–R-RIC=1, R-RIC=076, 95% CI 0.62-092, P = .006).
Data source: Retrospective review of 1,022 allogeneic HCT B-cell non-Hodgkin lymphoma patients who received rituximab and 379 who did not.
Disclosures: The data were obtained from the Center for International Blood & Marrow Transplant Research. Dr. Epperla reported no disclosures.
Hemorrhagic stroke increases risk of depression and subsequent dementia
HOUSTON – Hemorrhagic stroke sharply increases the risk of new-onset depression which, in turn, is associated with a 30% increased risk of dementia within 5 years.
New-onset depression developed in 40% of intracerebral hemorrhage survivors in a large prospective study, Alessandro Biffi, MD, said at the International Stroke Conference sponsored by the American Heart Association. By the end of 5 years, 80% of these patients had developed some form of dementia.
“This is of great importance from a research and clinical standpoint, as it may represent a marker of ongoing cognitive deterioration,” said Dr. Biffi of Massachusetts General Hospital, Boston.
A number of studies have found that survivors of intracerebral hemorrhage (ICH) face a significantly increased risk of mood disorders and cognitive decline. “There is probably a link between mood disorders and cognition after ICH, as is the case for a number of other neurological conditions,” Dr. Biffi said. “Cerebrovascular small-vessel disease is likely to be involved in the underlying pathogenesis for these disorders, as it is also a risk factor for late-life depression in the general population. Therefore, depression and dementia after ICH may share some etiological connections.”
He and his colleagues enrolled 695 patients who had experienced an ICH and followed them for a mean of 5 years. None of the subjects had ever been diagnosed with a mood disorder or cognitive decline. The subjects were interviewed by telephone every 6 months.
At baseline, investigators collected CT and MRI imaging data, epidemiologic exposure data, and apolipoprotein E4 genotype. The outcomes were new-onset depression and incident dementia.
At baseline, subjects were a mean of 74 years old. About 70% had hypertension and 15% had heart disease. Less than 1% of the cohort was positive for the APOE e4 gene. Imaging-confirmed white matter disease was present in 65%.
Over the follow-up period, new-onset depression developed in 278 (40%). The temporal incidence of this was consistent, at about 7% per year.
Two baseline characteristics were significantly associated with new-onset depression. These included having more than a single copy of the APOE e4 allele (hazard ratio, 1.7) and the presence of white matter disease (HR, 1.82), Dr. Biffi reported.
Having had at least 10 years of school was protective against depression (HR, 0.75), as was functional independence (HR, 0.52).
By the end of the follow-up period, dementia had developed in 80% of those with depression (220). In 81% of cases, depression preceded dementia, with an average time lag of 1.5 years, he noted.
In a multivariate analysis, several factors were significantly associated with incident dementia. Higher education reduced the risk by 40% (HR, 0.60). Factors that increased the risk of dementia were black race (HR, 1.48), carrying the APOE e4 gene (HR, 2.12), presence of white matter disease (HR, 1.7), and poststroke new-onset depression (HR, 1.29).
The study shows only association, Dr. Biffi cautioned. “No causal relationship can be inferred by this study. We also can’t capture the severity of the mood symptoms, and we are unable to examine the relationship between cognition and apathy, which is another highly relevant neuropsychiatric manifestation of small-vessel disease.”
He had no financial disclosures.
[email protected]
On Twitter @alz_gal
HOUSTON – Hemorrhagic stroke sharply increases the risk of new-onset depression which, in turn, is associated with a 30% increased risk of dementia within 5 years.
New-onset depression developed in 40% of intracerebral hemorrhage survivors in a large prospective study, Alessandro Biffi, MD, said at the International Stroke Conference sponsored by the American Heart Association. By the end of 5 years, 80% of these patients had developed some form of dementia.
“This is of great importance from a research and clinical standpoint, as it may represent a marker of ongoing cognitive deterioration,” said Dr. Biffi of Massachusetts General Hospital, Boston.
A number of studies have found that survivors of intracerebral hemorrhage (ICH) face a significantly increased risk of mood disorders and cognitive decline. “There is probably a link between mood disorders and cognition after ICH, as is the case for a number of other neurological conditions,” Dr. Biffi said. “Cerebrovascular small-vessel disease is likely to be involved in the underlying pathogenesis for these disorders, as it is also a risk factor for late-life depression in the general population. Therefore, depression and dementia after ICH may share some etiological connections.”
He and his colleagues enrolled 695 patients who had experienced an ICH and followed them for a mean of 5 years. None of the subjects had ever been diagnosed with a mood disorder or cognitive decline. The subjects were interviewed by telephone every 6 months.
At baseline, investigators collected CT and MRI imaging data, epidemiologic exposure data, and apolipoprotein E4 genotype. The outcomes were new-onset depression and incident dementia.
At baseline, subjects were a mean of 74 years old. About 70% had hypertension and 15% had heart disease. Less than 1% of the cohort was positive for the APOE e4 gene. Imaging-confirmed white matter disease was present in 65%.
Over the follow-up period, new-onset depression developed in 278 (40%). The temporal incidence of this was consistent, at about 7% per year.
Two baseline characteristics were significantly associated with new-onset depression. These included having more than a single copy of the APOE e4 allele (hazard ratio, 1.7) and the presence of white matter disease (HR, 1.82), Dr. Biffi reported.
Having had at least 10 years of school was protective against depression (HR, 0.75), as was functional independence (HR, 0.52).
By the end of the follow-up period, dementia had developed in 80% of those with depression (220). In 81% of cases, depression preceded dementia, with an average time lag of 1.5 years, he noted.
In a multivariate analysis, several factors were significantly associated with incident dementia. Higher education reduced the risk by 40% (HR, 0.60). Factors that increased the risk of dementia were black race (HR, 1.48), carrying the APOE e4 gene (HR, 2.12), presence of white matter disease (HR, 1.7), and poststroke new-onset depression (HR, 1.29).
The study shows only association, Dr. Biffi cautioned. “No causal relationship can be inferred by this study. We also can’t capture the severity of the mood symptoms, and we are unable to examine the relationship between cognition and apathy, which is another highly relevant neuropsychiatric manifestation of small-vessel disease.”
He had no financial disclosures.
[email protected]
On Twitter @alz_gal
HOUSTON – Hemorrhagic stroke sharply increases the risk of new-onset depression which, in turn, is associated with a 30% increased risk of dementia within 5 years.
New-onset depression developed in 40% of intracerebral hemorrhage survivors in a large prospective study, Alessandro Biffi, MD, said at the International Stroke Conference sponsored by the American Heart Association. By the end of 5 years, 80% of these patients had developed some form of dementia.
“This is of great importance from a research and clinical standpoint, as it may represent a marker of ongoing cognitive deterioration,” said Dr. Biffi of Massachusetts General Hospital, Boston.
A number of studies have found that survivors of intracerebral hemorrhage (ICH) face a significantly increased risk of mood disorders and cognitive decline. “There is probably a link between mood disorders and cognition after ICH, as is the case for a number of other neurological conditions,” Dr. Biffi said. “Cerebrovascular small-vessel disease is likely to be involved in the underlying pathogenesis for these disorders, as it is also a risk factor for late-life depression in the general population. Therefore, depression and dementia after ICH may share some etiological connections.”
He and his colleagues enrolled 695 patients who had experienced an ICH and followed them for a mean of 5 years. None of the subjects had ever been diagnosed with a mood disorder or cognitive decline. The subjects were interviewed by telephone every 6 months.
At baseline, investigators collected CT and MRI imaging data, epidemiologic exposure data, and apolipoprotein E4 genotype. The outcomes were new-onset depression and incident dementia.
At baseline, subjects were a mean of 74 years old. About 70% had hypertension and 15% had heart disease. Less than 1% of the cohort was positive for the APOE e4 gene. Imaging-confirmed white matter disease was present in 65%.
Over the follow-up period, new-onset depression developed in 278 (40%). The temporal incidence of this was consistent, at about 7% per year.
Two baseline characteristics were significantly associated with new-onset depression. These included having more than a single copy of the APOE e4 allele (hazard ratio, 1.7) and the presence of white matter disease (HR, 1.82), Dr. Biffi reported.
Having had at least 10 years of school was protective against depression (HR, 0.75), as was functional independence (HR, 0.52).
By the end of the follow-up period, dementia had developed in 80% of those with depression (220). In 81% of cases, depression preceded dementia, with an average time lag of 1.5 years, he noted.
In a multivariate analysis, several factors were significantly associated with incident dementia. Higher education reduced the risk by 40% (HR, 0.60). Factors that increased the risk of dementia were black race (HR, 1.48), carrying the APOE e4 gene (HR, 2.12), presence of white matter disease (HR, 1.7), and poststroke new-onset depression (HR, 1.29).
The study shows only association, Dr. Biffi cautioned. “No causal relationship can be inferred by this study. We also can’t capture the severity of the mood symptoms, and we are unable to examine the relationship between cognition and apathy, which is another highly relevant neuropsychiatric manifestation of small-vessel disease.”
He had no financial disclosures.
[email protected]
On Twitter @alz_gal
AT THE INTERNATIONAL STROKE CONFERENCE
Key clinical point:
Major finding: Depression developed in 40% of survivors, with dementia developing in 80% of that group.
Data source: The prospective longitudinal study involved 695 patients.
Disclosures: Dr. Biffi had no financial disclosures.
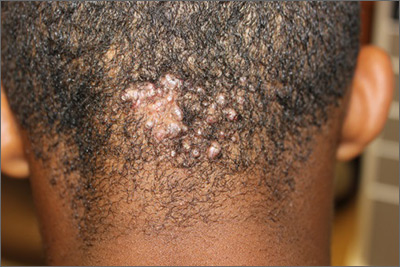
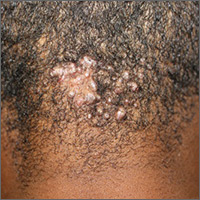
Scalp papules in a teenage boy
The patient was given a diagnosis of acne keloidalis nuchae (AKN), a chronic folliculitis that is characterized by smooth, dome-shaped papules on the posterior scalp and neck that become confluent and form firm papules and hairless, keloid-like plaques. Seen almost exclusively in young, postpubescent African American males, the condition is often asymptomatic, although some patients complain of itching at the affected area.
The cause of AKN may be associated with an acute pseudofolliculitis secondary to close-shaved curly hair reentering the skin; this leads to a foreign body reaction to hair protein and subsequent fibrosis. AKN is diagnosed based on the appearance and location of the papules, as well as the patient’s history.
Treatment of AKN is often difficult, but early treatment decreases the potential of developing larger lesions and long-term disfigurement. Topical steroid therapy is indicated for mild to moderate AKN. Application of tretinoin 0.01% gel once or twice daily for several months has an anti-inflammatory effect and alters keratinocyte differentiation, which may discharge ingrown hairs. Topical and systemic antibiotics minimize infection associated with pseudofolliculitis and have anti-inflammatory effects. Intralesional steroid injections (triamcinolone acetonide 2.5-5 mg/cc) with 0.1 cc injected into each lesion every 2 to 3 weeks for 3 to 6 injections can reduce inflammation and pruritus and reduce the thickness of keloidal scars. (For a how-to video that illustrates intralesional injections, go to http://www.mdedge.com/jfponline/article/88050/dermatology/intralesional-injections.)
Surgical management is generally reserved for large lesions that do not respond to medical management. The use of CO2 laser ablation can be considered for advanced cases.
Patients with AKN can prevent further irritation of the affected area by not wearing anything on their head that rubs on the involved area. Patients should also refrain from shaving the posterior scalp and neck to prevent the pseudofolliculitis that may be causing this condition. Electric barber trimmers that leave a short stubble (but do not cleanly shave the skin) are OK.
In this case, the patient’s papules flattened and became asymptomatic over several months of treatment with tretinoin 0.01% gel, doxycycline 100 mg/d, and a series of biweekly intralesional steroid injections. A flat-scarred patch remained.
Adapted from: Rafferty E, Brodell R. Occipital scalp papules in a teenage boy. J Fam Pract. 2014;63:739-740.
The patient was given a diagnosis of acne keloidalis nuchae (AKN), a chronic folliculitis that is characterized by smooth, dome-shaped papules on the posterior scalp and neck that become confluent and form firm papules and hairless, keloid-like plaques. Seen almost exclusively in young, postpubescent African American males, the condition is often asymptomatic, although some patients complain of itching at the affected area.
The cause of AKN may be associated with an acute pseudofolliculitis secondary to close-shaved curly hair reentering the skin; this leads to a foreign body reaction to hair protein and subsequent fibrosis. AKN is diagnosed based on the appearance and location of the papules, as well as the patient’s history.
Treatment of AKN is often difficult, but early treatment decreases the potential of developing larger lesions and long-term disfigurement. Topical steroid therapy is indicated for mild to moderate AKN. Application of tretinoin 0.01% gel once or twice daily for several months has an anti-inflammatory effect and alters keratinocyte differentiation, which may discharge ingrown hairs. Topical and systemic antibiotics minimize infection associated with pseudofolliculitis and have anti-inflammatory effects. Intralesional steroid injections (triamcinolone acetonide 2.5-5 mg/cc) with 0.1 cc injected into each lesion every 2 to 3 weeks for 3 to 6 injections can reduce inflammation and pruritus and reduce the thickness of keloidal scars. (For a how-to video that illustrates intralesional injections, go to http://www.mdedge.com/jfponline/article/88050/dermatology/intralesional-injections.)
Surgical management is generally reserved for large lesions that do not respond to medical management. The use of CO2 laser ablation can be considered for advanced cases.
Patients with AKN can prevent further irritation of the affected area by not wearing anything on their head that rubs on the involved area. Patients should also refrain from shaving the posterior scalp and neck to prevent the pseudofolliculitis that may be causing this condition. Electric barber trimmers that leave a short stubble (but do not cleanly shave the skin) are OK.
In this case, the patient’s papules flattened and became asymptomatic over several months of treatment with tretinoin 0.01% gel, doxycycline 100 mg/d, and a series of biweekly intralesional steroid injections. A flat-scarred patch remained.
Adapted from: Rafferty E, Brodell R. Occipital scalp papules in a teenage boy. J Fam Pract. 2014;63:739-740.
The patient was given a diagnosis of acne keloidalis nuchae (AKN), a chronic folliculitis that is characterized by smooth, dome-shaped papules on the posterior scalp and neck that become confluent and form firm papules and hairless, keloid-like plaques. Seen almost exclusively in young, postpubescent African American males, the condition is often asymptomatic, although some patients complain of itching at the affected area.
The cause of AKN may be associated with an acute pseudofolliculitis secondary to close-shaved curly hair reentering the skin; this leads to a foreign body reaction to hair protein and subsequent fibrosis. AKN is diagnosed based on the appearance and location of the papules, as well as the patient’s history.
Treatment of AKN is often difficult, but early treatment decreases the potential of developing larger lesions and long-term disfigurement. Topical steroid therapy is indicated for mild to moderate AKN. Application of tretinoin 0.01% gel once or twice daily for several months has an anti-inflammatory effect and alters keratinocyte differentiation, which may discharge ingrown hairs. Topical and systemic antibiotics minimize infection associated with pseudofolliculitis and have anti-inflammatory effects. Intralesional steroid injections (triamcinolone acetonide 2.5-5 mg/cc) with 0.1 cc injected into each lesion every 2 to 3 weeks for 3 to 6 injections can reduce inflammation and pruritus and reduce the thickness of keloidal scars. (For a how-to video that illustrates intralesional injections, go to http://www.mdedge.com/jfponline/article/88050/dermatology/intralesional-injections.)
Surgical management is generally reserved for large lesions that do not respond to medical management. The use of CO2 laser ablation can be considered for advanced cases.
Patients with AKN can prevent further irritation of the affected area by not wearing anything on their head that rubs on the involved area. Patients should also refrain from shaving the posterior scalp and neck to prevent the pseudofolliculitis that may be causing this condition. Electric barber trimmers that leave a short stubble (but do not cleanly shave the skin) are OK.
In this case, the patient’s papules flattened and became asymptomatic over several months of treatment with tretinoin 0.01% gel, doxycycline 100 mg/d, and a series of biweekly intralesional steroid injections. A flat-scarred patch remained.
Adapted from: Rafferty E, Brodell R. Occipital scalp papules in a teenage boy. J Fam Pract. 2014;63:739-740.
Potential therapeutic strategy for BL, DLBCL
Preclinical research has revealed a potential strategy for treating Burkitt lymphoma (BL) and diffuse large B-cell lymphoma (DLBCL).
Investigators discovered that miR-28 inhibits the growth of B-cell lymphomas, but this microRNA is often lost in these lymphomas.
Re-expressing miR-28 in mouse models of BL and DLBCL inhibited tumor growth, which supports the potential of synthetic miR-28 analogs for the treatment of these lymphomas.
In fact, the investigators believe their work could lead to the development of the first miRNA analog therapy for the treatment of B-cell lymphoma and provide the basis for clinical trials.
Almudena Ramiro, PhD, of Centro Nacional de Investigaciones Cardiovasculares in Madrid, Spain, and her colleagues described the work in Blood.
The team characterized the function of miR-28 in the biology of mature B lymphocytes and in the development of lymphomas associated with this cell type.
The investigators found that miR-28 regulates the terminal differentiation of B lymphocytes, a fundamental process in the biology of these cells that generates memory B lymphocytes and highly specific plasma cells.
But the team found that miR-28 expression is lost in several germinal center-derived lymphoma subtypes, including BL, DLBCL, follicular lymphoma, and chronic lymphocytic leukemia.
In vitro experiments showed that miR-28 expression dampens B-cell receptor signaling and diminishes the proliferation and survival of primary B cells and lymphoma cells.
And in vivo experiments showed that re-establishing miR-28 expression slows tumor growth in DLBCL and BL.
The investigators re-expressed miR-28 in xenograft models of BL and DLBCL via the use of viral vectors or synthetic molecules and found that both methods blocked tumor growth. The same effect was observed in mice with established BL tumors.
Dr Ramiro and her colleagues said these results reveal the therapeutic potential of miR-28 and provide ample justification for the initiation of clinical trials of miR-28-based therapies to treat B-cell lymphomas. ![]()
Preclinical research has revealed a potential strategy for treating Burkitt lymphoma (BL) and diffuse large B-cell lymphoma (DLBCL).
Investigators discovered that miR-28 inhibits the growth of B-cell lymphomas, but this microRNA is often lost in these lymphomas.
Re-expressing miR-28 in mouse models of BL and DLBCL inhibited tumor growth, which supports the potential of synthetic miR-28 analogs for the treatment of these lymphomas.
In fact, the investigators believe their work could lead to the development of the first miRNA analog therapy for the treatment of B-cell lymphoma and provide the basis for clinical trials.
Almudena Ramiro, PhD, of Centro Nacional de Investigaciones Cardiovasculares in Madrid, Spain, and her colleagues described the work in Blood.
The team characterized the function of miR-28 in the biology of mature B lymphocytes and in the development of lymphomas associated with this cell type.
The investigators found that miR-28 regulates the terminal differentiation of B lymphocytes, a fundamental process in the biology of these cells that generates memory B lymphocytes and highly specific plasma cells.
But the team found that miR-28 expression is lost in several germinal center-derived lymphoma subtypes, including BL, DLBCL, follicular lymphoma, and chronic lymphocytic leukemia.
In vitro experiments showed that miR-28 expression dampens B-cell receptor signaling and diminishes the proliferation and survival of primary B cells and lymphoma cells.
And in vivo experiments showed that re-establishing miR-28 expression slows tumor growth in DLBCL and BL.
The investigators re-expressed miR-28 in xenograft models of BL and DLBCL via the use of viral vectors or synthetic molecules and found that both methods blocked tumor growth. The same effect was observed in mice with established BL tumors.
Dr Ramiro and her colleagues said these results reveal the therapeutic potential of miR-28 and provide ample justification for the initiation of clinical trials of miR-28-based therapies to treat B-cell lymphomas. ![]()
Preclinical research has revealed a potential strategy for treating Burkitt lymphoma (BL) and diffuse large B-cell lymphoma (DLBCL).
Investigators discovered that miR-28 inhibits the growth of B-cell lymphomas, but this microRNA is often lost in these lymphomas.
Re-expressing miR-28 in mouse models of BL and DLBCL inhibited tumor growth, which supports the potential of synthetic miR-28 analogs for the treatment of these lymphomas.
In fact, the investigators believe their work could lead to the development of the first miRNA analog therapy for the treatment of B-cell lymphoma and provide the basis for clinical trials.
Almudena Ramiro, PhD, of Centro Nacional de Investigaciones Cardiovasculares in Madrid, Spain, and her colleagues described the work in Blood.
The team characterized the function of miR-28 in the biology of mature B lymphocytes and in the development of lymphomas associated with this cell type.
The investigators found that miR-28 regulates the terminal differentiation of B lymphocytes, a fundamental process in the biology of these cells that generates memory B lymphocytes and highly specific plasma cells.
But the team found that miR-28 expression is lost in several germinal center-derived lymphoma subtypes, including BL, DLBCL, follicular lymphoma, and chronic lymphocytic leukemia.
In vitro experiments showed that miR-28 expression dampens B-cell receptor signaling and diminishes the proliferation and survival of primary B cells and lymphoma cells.
And in vivo experiments showed that re-establishing miR-28 expression slows tumor growth in DLBCL and BL.
The investigators re-expressed miR-28 in xenograft models of BL and DLBCL via the use of viral vectors or synthetic molecules and found that both methods blocked tumor growth. The same effect was observed in mice with established BL tumors.
Dr Ramiro and her colleagues said these results reveal the therapeutic potential of miR-28 and provide ample justification for the initiation of clinical trials of miR-28-based therapies to treat B-cell lymphomas. ![]()
Drug granted priority review for relapsed/refractory AML
The US Food and Drug Administration (FDA) has granted priority review for the new drug application (NDA) for enasidenib (AG-221), an inhibitor of mutant IDH2.
The drug is under review for the treatment of patients with relapsed or refractory acute myeloid leukemia (AML) with an IDH2 mutation.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10-month period.
The NDA for enasidenib has been given a Prescription Drug User Fee Act action date of August 30, 2017.
Enasidenib is being developed by Celgene Corporation and Agios Pharmaceuticals.
Phase 1/2 trial
The NDA submission for enasidenib is based on results from AG221-C-001, a single-arm, phase 1/2 study of the drug in patients with advanced hematologic malignancies with an IDH2 mutation.
Early data from the relapsed or refractory AML patients in this study were presented at the 2015 ASH Annual Meeting. (The presentation included updated data that differ from the data in the abstract.)
The trial included a dose-escalation phase and 5 expansion cohorts. The first 4 expansion cohorts had completed enrollment as of the presentation.
- Arm 1: 25 patients with IDH2-mutant-positive relapsed or refractory AML age ≥60 years, or any patient with AML regardless of age who relapsed after a bone marrow transplant (BMT)
- Arm 2: 25 patients with IDH2-mutant-positive relapsed or refractory AML age <60 years, excluding patients with AML who relapsed after a BMT
- Arm 3: 25 patients with IDH2-mutant-positive untreated AML age ≥60 years who decline standard of care chemotherapy
- Arm 4: 25 patients with IDH2-mutant-positive advanced hematologic malignancies not eligible for arms 1 to 3
- Arm 5: The phase 2 portion of the trial included 125 patients with IDH2-mutant-positive AML who were in second or later relapse, refractory to second-line induction or reinduction treatment, or relapsed after allogeneic transplant.
The data reported at ASH were from patients receiving enasidenib administered from 50-mg to 650-mg total daily doses in the dose-escalation arm and 100 mg once daily in the first 4 expansion arms, as of September 1, 2015.
The median age of these patients was 69 (range, 19-100). Patients with relapsed or refractory AML received a median of 2 prior lines of therapy (range, 1-6).
Safety data
A safety analysis was conducted for all 231 treated patients. As of the ASH presentation, a maximum tolerated dose of enasidenib had not been reached.
The majority of adverse events were mild to moderate, with the most common being nausea, diarrhea, fatigue, and febrile neutropenia.
Twenty-three percent of patients had treatment-related serious adverse events—notably, differentiation syndrome (4%), leukocytosis (4%), and nausea (2%).
Drug-related grade 5 serious adverse events include atrial flutter (n=1), cardiac tamponade (n=1), pericardial effusion (n=1), and respiratory failure (n=1).
Efficacy Data
Seventy-nine of the 209 response-evaluable patients achieved investigator-assessed objective responses, for an overall response rate of 38%.
There were 37 (18%) complete remissions (CR), 3 CRs with incomplete platelet recovery (CRp), 14 marrow CRs (mCR), 3 CRs with incomplete hematologic recovery (CRi), and 22 partial remissions (PR).
Of the 159 patients with relapsed or refractory AML, 59 (37%) achieved an objective response, including 29 (18%) CRs, 1 CRp, 9 mCRs, 3 CRis, and 17 PRs.
Of the 24 patients with AML who declined standard of care chemotherapy, 10 achieved an objective response, including 4 CRs, 1 CRp, 1 mCR, and 4 PRs.
The median duration of response was 6.9 months in patients with relapsed or refractory AML.
Responding relapsed/refractory AML patients were on study treatment for up to 18 months. The median duration of treatment was 6.8 months (range, 1.8 to 18 months). ![]()
The US Food and Drug Administration (FDA) has granted priority review for the new drug application (NDA) for enasidenib (AG-221), an inhibitor of mutant IDH2.
The drug is under review for the treatment of patients with relapsed or refractory acute myeloid leukemia (AML) with an IDH2 mutation.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10-month period.
The NDA for enasidenib has been given a Prescription Drug User Fee Act action date of August 30, 2017.
Enasidenib is being developed by Celgene Corporation and Agios Pharmaceuticals.
Phase 1/2 trial
The NDA submission for enasidenib is based on results from AG221-C-001, a single-arm, phase 1/2 study of the drug in patients with advanced hematologic malignancies with an IDH2 mutation.
Early data from the relapsed or refractory AML patients in this study were presented at the 2015 ASH Annual Meeting. (The presentation included updated data that differ from the data in the abstract.)
The trial included a dose-escalation phase and 5 expansion cohorts. The first 4 expansion cohorts had completed enrollment as of the presentation.
- Arm 1: 25 patients with IDH2-mutant-positive relapsed or refractory AML age ≥60 years, or any patient with AML regardless of age who relapsed after a bone marrow transplant (BMT)
- Arm 2: 25 patients with IDH2-mutant-positive relapsed or refractory AML age <60 years, excluding patients with AML who relapsed after a BMT
- Arm 3: 25 patients with IDH2-mutant-positive untreated AML age ≥60 years who decline standard of care chemotherapy
- Arm 4: 25 patients with IDH2-mutant-positive advanced hematologic malignancies not eligible for arms 1 to 3
- Arm 5: The phase 2 portion of the trial included 125 patients with IDH2-mutant-positive AML who were in second or later relapse, refractory to second-line induction or reinduction treatment, or relapsed after allogeneic transplant.
The data reported at ASH were from patients receiving enasidenib administered from 50-mg to 650-mg total daily doses in the dose-escalation arm and 100 mg once daily in the first 4 expansion arms, as of September 1, 2015.
The median age of these patients was 69 (range, 19-100). Patients with relapsed or refractory AML received a median of 2 prior lines of therapy (range, 1-6).
Safety data
A safety analysis was conducted for all 231 treated patients. As of the ASH presentation, a maximum tolerated dose of enasidenib had not been reached.
The majority of adverse events were mild to moderate, with the most common being nausea, diarrhea, fatigue, and febrile neutropenia.
Twenty-three percent of patients had treatment-related serious adverse events—notably, differentiation syndrome (4%), leukocytosis (4%), and nausea (2%).
Drug-related grade 5 serious adverse events include atrial flutter (n=1), cardiac tamponade (n=1), pericardial effusion (n=1), and respiratory failure (n=1).
Efficacy Data
Seventy-nine of the 209 response-evaluable patients achieved investigator-assessed objective responses, for an overall response rate of 38%.
There were 37 (18%) complete remissions (CR), 3 CRs with incomplete platelet recovery (CRp), 14 marrow CRs (mCR), 3 CRs with incomplete hematologic recovery (CRi), and 22 partial remissions (PR).
Of the 159 patients with relapsed or refractory AML, 59 (37%) achieved an objective response, including 29 (18%) CRs, 1 CRp, 9 mCRs, 3 CRis, and 17 PRs.
Of the 24 patients with AML who declined standard of care chemotherapy, 10 achieved an objective response, including 4 CRs, 1 CRp, 1 mCR, and 4 PRs.
The median duration of response was 6.9 months in patients with relapsed or refractory AML.
Responding relapsed/refractory AML patients were on study treatment for up to 18 months. The median duration of treatment was 6.8 months (range, 1.8 to 18 months). ![]()
The US Food and Drug Administration (FDA) has granted priority review for the new drug application (NDA) for enasidenib (AG-221), an inhibitor of mutant IDH2.
The drug is under review for the treatment of patients with relapsed or refractory acute myeloid leukemia (AML) with an IDH2 mutation.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10-month period.
The NDA for enasidenib has been given a Prescription Drug User Fee Act action date of August 30, 2017.
Enasidenib is being developed by Celgene Corporation and Agios Pharmaceuticals.
Phase 1/2 trial
The NDA submission for enasidenib is based on results from AG221-C-001, a single-arm, phase 1/2 study of the drug in patients with advanced hematologic malignancies with an IDH2 mutation.
Early data from the relapsed or refractory AML patients in this study were presented at the 2015 ASH Annual Meeting. (The presentation included updated data that differ from the data in the abstract.)
The trial included a dose-escalation phase and 5 expansion cohorts. The first 4 expansion cohorts had completed enrollment as of the presentation.
- Arm 1: 25 patients with IDH2-mutant-positive relapsed or refractory AML age ≥60 years, or any patient with AML regardless of age who relapsed after a bone marrow transplant (BMT)
- Arm 2: 25 patients with IDH2-mutant-positive relapsed or refractory AML age <60 years, excluding patients with AML who relapsed after a BMT
- Arm 3: 25 patients with IDH2-mutant-positive untreated AML age ≥60 years who decline standard of care chemotherapy
- Arm 4: 25 patients with IDH2-mutant-positive advanced hematologic malignancies not eligible for arms 1 to 3
- Arm 5: The phase 2 portion of the trial included 125 patients with IDH2-mutant-positive AML who were in second or later relapse, refractory to second-line induction or reinduction treatment, or relapsed after allogeneic transplant.
The data reported at ASH were from patients receiving enasidenib administered from 50-mg to 650-mg total daily doses in the dose-escalation arm and 100 mg once daily in the first 4 expansion arms, as of September 1, 2015.
The median age of these patients was 69 (range, 19-100). Patients with relapsed or refractory AML received a median of 2 prior lines of therapy (range, 1-6).
Safety data
A safety analysis was conducted for all 231 treated patients. As of the ASH presentation, a maximum tolerated dose of enasidenib had not been reached.
The majority of adverse events were mild to moderate, with the most common being nausea, diarrhea, fatigue, and febrile neutropenia.
Twenty-three percent of patients had treatment-related serious adverse events—notably, differentiation syndrome (4%), leukocytosis (4%), and nausea (2%).
Drug-related grade 5 serious adverse events include atrial flutter (n=1), cardiac tamponade (n=1), pericardial effusion (n=1), and respiratory failure (n=1).
Efficacy Data
Seventy-nine of the 209 response-evaluable patients achieved investigator-assessed objective responses, for an overall response rate of 38%.
There were 37 (18%) complete remissions (CR), 3 CRs with incomplete platelet recovery (CRp), 14 marrow CRs (mCR), 3 CRs with incomplete hematologic recovery (CRi), and 22 partial remissions (PR).
Of the 159 patients with relapsed or refractory AML, 59 (37%) achieved an objective response, including 29 (18%) CRs, 1 CRp, 9 mCRs, 3 CRis, and 17 PRs.
Of the 24 patients with AML who declined standard of care chemotherapy, 10 achieved an objective response, including 4 CRs, 1 CRp, 1 mCR, and 4 PRs.
The median duration of response was 6.9 months in patients with relapsed or refractory AML.
Responding relapsed/refractory AML patients were on study treatment for up to 18 months. The median duration of treatment was 6.8 months (range, 1.8 to 18 months). ![]()
Hospital floors pose infection risk, team says
Hospital room floors may be an overlooked source of infection, according to a study published in the American Journal of Infection Control.
Researchers surveyed 5 hospitals and found that floors in patient rooms were often contaminated with pathogens.
Certain objects, such as personal items and medical devices and supplies, were in contact with the floor, and touching these objects resulted in the transfer of pathogens to bare and gloved hands.
Abhishek Deshpande, MD, PhD, of Case Western Reserve University School of Medicine in Cleveland, Ohio, and his colleagues conducted this research.
The team cultured 318 floor sites from 159 patient rooms (2 sites per room) in 5 hospitals in the Cleveland area. The rooms included both Clostridium difficile infection (CDI) isolation rooms and non-CDI rooms.
The researchers also cultured hands (gloved and bare) as well as other “high-touch” surfaces such as clothing and medical devices/supplies.
The team found that floors in patient rooms were often contaminated with Methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and C difficile.
C difficile was recovered in 55% of CDI rooms and 47% of non-CDI rooms. MRSA was recovered in 32% of CDI rooms and 8% of non-CDI rooms. VRE was recovered in 30% of CDI rooms and 13% of non-CDI rooms.
The researchers said the frequency of contamination was similar for each of the 5 hospitals and from room and bathroom floor sites.
Of the 100 occupied rooms surveyed, 41% had one or more high-touch objects that were in contact with the floor. These included personal items (eg, clothing, canes, and cellular phone chargers), medical devices and supplies (eg, pulse oximeter, call button, heating pad, urinal, blood pressure cuff, wash basin, and heel protector), and bed linens or towels.
The findings indicate that handling such items resulted in the transfer of pathogens. All 3 pathogens were recovered from bare or gloved hand cultures—MRSA in 6 (18%), VRE in 2 (6%), and C difficile in 1 (3%).
The researchers said these results suggest hospital floors could be an underappreciated source for dissemination of pathogens and are an important area for additional research.
“Understanding gaps in infection prevention is critically important for institutions seeking to improve the quality of care offered to patients,” said Linda Greene, RN, current president of the Association for Professionals in Infection Control and Epidemiology.
“Even though most facilities believe they are taking the proper precautions, this study points out the importance of ensuring cleanliness of the hospital environment and the need for education of both staff and patients on this issue.” ![]()
Hospital room floors may be an overlooked source of infection, according to a study published in the American Journal of Infection Control.
Researchers surveyed 5 hospitals and found that floors in patient rooms were often contaminated with pathogens.
Certain objects, such as personal items and medical devices and supplies, were in contact with the floor, and touching these objects resulted in the transfer of pathogens to bare and gloved hands.
Abhishek Deshpande, MD, PhD, of Case Western Reserve University School of Medicine in Cleveland, Ohio, and his colleagues conducted this research.
The team cultured 318 floor sites from 159 patient rooms (2 sites per room) in 5 hospitals in the Cleveland area. The rooms included both Clostridium difficile infection (CDI) isolation rooms and non-CDI rooms.
The researchers also cultured hands (gloved and bare) as well as other “high-touch” surfaces such as clothing and medical devices/supplies.
The team found that floors in patient rooms were often contaminated with Methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and C difficile.
C difficile was recovered in 55% of CDI rooms and 47% of non-CDI rooms. MRSA was recovered in 32% of CDI rooms and 8% of non-CDI rooms. VRE was recovered in 30% of CDI rooms and 13% of non-CDI rooms.
The researchers said the frequency of contamination was similar for each of the 5 hospitals and from room and bathroom floor sites.
Of the 100 occupied rooms surveyed, 41% had one or more high-touch objects that were in contact with the floor. These included personal items (eg, clothing, canes, and cellular phone chargers), medical devices and supplies (eg, pulse oximeter, call button, heating pad, urinal, blood pressure cuff, wash basin, and heel protector), and bed linens or towels.
The findings indicate that handling such items resulted in the transfer of pathogens. All 3 pathogens were recovered from bare or gloved hand cultures—MRSA in 6 (18%), VRE in 2 (6%), and C difficile in 1 (3%).
The researchers said these results suggest hospital floors could be an underappreciated source for dissemination of pathogens and are an important area for additional research.
“Understanding gaps in infection prevention is critically important for institutions seeking to improve the quality of care offered to patients,” said Linda Greene, RN, current president of the Association for Professionals in Infection Control and Epidemiology.
“Even though most facilities believe they are taking the proper precautions, this study points out the importance of ensuring cleanliness of the hospital environment and the need for education of both staff and patients on this issue.” ![]()
Hospital room floors may be an overlooked source of infection, according to a study published in the American Journal of Infection Control.
Researchers surveyed 5 hospitals and found that floors in patient rooms were often contaminated with pathogens.
Certain objects, such as personal items and medical devices and supplies, were in contact with the floor, and touching these objects resulted in the transfer of pathogens to bare and gloved hands.
Abhishek Deshpande, MD, PhD, of Case Western Reserve University School of Medicine in Cleveland, Ohio, and his colleagues conducted this research.
The team cultured 318 floor sites from 159 patient rooms (2 sites per room) in 5 hospitals in the Cleveland area. The rooms included both Clostridium difficile infection (CDI) isolation rooms and non-CDI rooms.
The researchers also cultured hands (gloved and bare) as well as other “high-touch” surfaces such as clothing and medical devices/supplies.
The team found that floors in patient rooms were often contaminated with Methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and C difficile.
C difficile was recovered in 55% of CDI rooms and 47% of non-CDI rooms. MRSA was recovered in 32% of CDI rooms and 8% of non-CDI rooms. VRE was recovered in 30% of CDI rooms and 13% of non-CDI rooms.
The researchers said the frequency of contamination was similar for each of the 5 hospitals and from room and bathroom floor sites.
Of the 100 occupied rooms surveyed, 41% had one or more high-touch objects that were in contact with the floor. These included personal items (eg, clothing, canes, and cellular phone chargers), medical devices and supplies (eg, pulse oximeter, call button, heating pad, urinal, blood pressure cuff, wash basin, and heel protector), and bed linens or towels.
The findings indicate that handling such items resulted in the transfer of pathogens. All 3 pathogens were recovered from bare or gloved hand cultures—MRSA in 6 (18%), VRE in 2 (6%), and C difficile in 1 (3%).
The researchers said these results suggest hospital floors could be an underappreciated source for dissemination of pathogens and are an important area for additional research.
“Understanding gaps in infection prevention is critically important for institutions seeking to improve the quality of care offered to patients,” said Linda Greene, RN, current president of the Association for Professionals in Infection Control and Epidemiology.
“Even though most facilities believe they are taking the proper precautions, this study points out the importance of ensuring cleanliness of the hospital environment and the need for education of both staff and patients on this issue.” ![]()
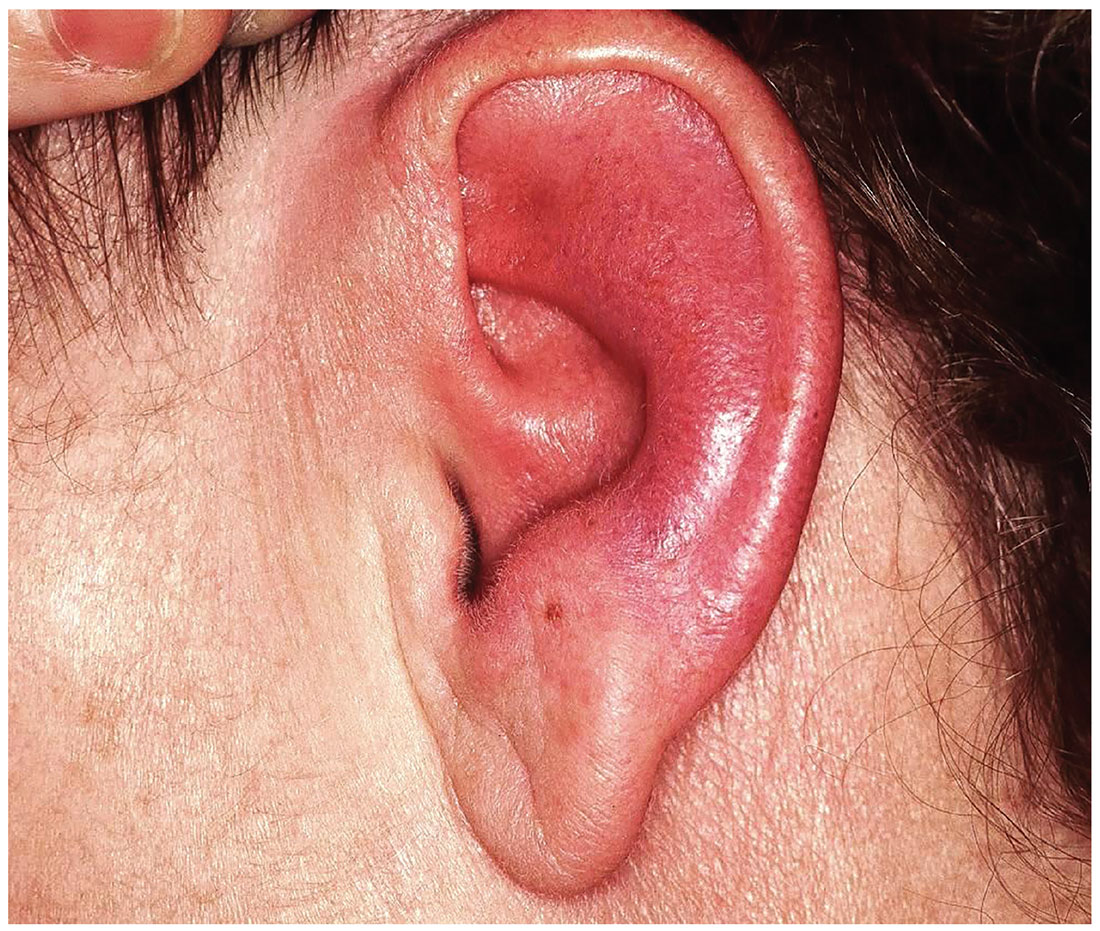
Seeing Redness and Ear-itation
ANSWER
The correct diagnosis is relapsing polychondritis (RP; choice “a”). The lack of surface changes in the affected skin rules out contact dermatitis, while the lack of a positive response to antibiotics and absence of an entrance wound eliminate the possibility of an infectious etiology.
DISCUSSION
There are no tests to confirm the diagnosis of RP. It is a rare autoimmune condition that usually manifests in the later decades of life and equally affects men and women.
RP’s ability to appear in cartilage anywhere in the body and in a variety of forms makes timely diagnosis almost impossible. But this case illustrates some diagnostically useful signs to watch for.
The unexplained erythema in the ear, which very obviously spared the cartilage-free lobe, prompted a biopsy of the cartilage; this showed changes consistent with RP. A subsequent review of the patient’s ophthalmology records indicated a chronic episcleritis, most likely due to inflammation of eyelid cartilage.
Further testing was performed to rule out other explanations, such as gout, or autoimmune diseases, such as lupus. Results were negative.
The patient was then referred to a pulmonologist, who found no respiratory involvement, and a rheumatologist, for further evaluation (including blood work) to rule out other conditions and end-organ (eg, renal) involvement.
On follow-up, the patient was responding well to prednisone prescribed by her rheumatologist. Given her limited disease, her prognosis is fairly good.
ANSWER
The correct diagnosis is relapsing polychondritis (RP; choice “a”). The lack of surface changes in the affected skin rules out contact dermatitis, while the lack of a positive response to antibiotics and absence of an entrance wound eliminate the possibility of an infectious etiology.
DISCUSSION
There are no tests to confirm the diagnosis of RP. It is a rare autoimmune condition that usually manifests in the later decades of life and equally affects men and women.
RP’s ability to appear in cartilage anywhere in the body and in a variety of forms makes timely diagnosis almost impossible. But this case illustrates some diagnostically useful signs to watch for.
The unexplained erythema in the ear, which very obviously spared the cartilage-free lobe, prompted a biopsy of the cartilage; this showed changes consistent with RP. A subsequent review of the patient’s ophthalmology records indicated a chronic episcleritis, most likely due to inflammation of eyelid cartilage.
Further testing was performed to rule out other explanations, such as gout, or autoimmune diseases, such as lupus. Results were negative.
The patient was then referred to a pulmonologist, who found no respiratory involvement, and a rheumatologist, for further evaluation (including blood work) to rule out other conditions and end-organ (eg, renal) involvement.
On follow-up, the patient was responding well to prednisone prescribed by her rheumatologist. Given her limited disease, her prognosis is fairly good.
ANSWER
The correct diagnosis is relapsing polychondritis (RP; choice “a”). The lack of surface changes in the affected skin rules out contact dermatitis, while the lack of a positive response to antibiotics and absence of an entrance wound eliminate the possibility of an infectious etiology.
DISCUSSION
There are no tests to confirm the diagnosis of RP. It is a rare autoimmune condition that usually manifests in the later decades of life and equally affects men and women.
RP’s ability to appear in cartilage anywhere in the body and in a variety of forms makes timely diagnosis almost impossible. But this case illustrates some diagnostically useful signs to watch for.
The unexplained erythema in the ear, which very obviously spared the cartilage-free lobe, prompted a biopsy of the cartilage; this showed changes consistent with RP. A subsequent review of the patient’s ophthalmology records indicated a chronic episcleritis, most likely due to inflammation of eyelid cartilage.
Further testing was performed to rule out other explanations, such as gout, or autoimmune diseases, such as lupus. Results were negative.
The patient was then referred to a pulmonologist, who found no respiratory involvement, and a rheumatologist, for further evaluation (including blood work) to rule out other conditions and end-organ (eg, renal) involvement.
On follow-up, the patient was responding well to prednisone prescribed by her rheumatologist. Given her limited disease, her prognosis is fairly good.
Several months ago, family members pointed out that this 60-year-old woman’s left ear was red. She consulted her primary care provider, who prescribed antibiotics. But when these failed to clear the problem, she was referred to dermatology.
Today, the patient complains of some discomfort in the ear but denies actual pain; she is, for example, able to sleep despite the problem. She reports that the redness manifested slowly but has spread over time to encompass most of her ear.
Uniformly distributed, bright red erythema on the left ear spares only the lobe. No wound or epidermal component (eg, scaling or blistering) is noted. However, there is increased warmth and tenderness on palpation of the erythematous portion. No nodes can be felt in the vicinity, nor are any abnormalities observed in the other ear.
The patient denies other skin problems, joint pain, and breathing difficulty. But she does have an ongoing history of irritation in both eyes. She has been seeing an ophthalmologist for months without relief. On examination, both eyes appear injected, with slightly swollen eyelids. Inspection and palpation of the nose reveal no abnormalities.
HIV update: Legalizing sex work, novel one-drug therapy, concomitant opioid use
Here’s your weekly quick take on recent notable news and journal articles related to HIV and AIDS research:
Novel therapy tested. An unboosted HIV-1 integrase strand-transfer inhibitor produced led to rapid declines in HIV-1 RNA after 10 days of monotherapy in a phase 1B study reported in JAIDS. The drug (bictegravir) was well tolerated, and displayed rapid absorption and a half-life supportive of once-daily therapy in HIV-infected subjects.
New diagnoses declining in black women. Based on three different measures, the disparity in HIV diagnoses in black women decreased in 2014, compared with 2010, according to a CDC report.
Legalizing sex work is linked to lower HIV rates. Rates of HIV infection in female sex workers are lower in European countries that have legalized of some aspects of sex work, according to a study in the Lancet HIV.
Flu vaccine likely safe in HIV-positive women on therapy. H1N1 influenza vaccination in HIV-infected women on effective antiretroviral treatment did not induce measurable antigen-driven proliferation of the HIV-1 proviral reservoir, according to a study in AIDS Research and Therapy.
Could vitamin supplements lower subsequent TB risk? At the start of ART, vitamin A and D deficiencies were more common in adults who went on to develop tuberculosis, according to a study in JAIDS.
Cerebrospinal fluid CD8+ T cells beneficial in acute HIV infection. CD8+ T cells expand in the central nervous system during acute HIV infection and are functional and directed against HIV antigens. If combination antiretroviral therapy is initiated early, the cells could protect against injury seen in chronic HIV infection based on a study in JAIDS.
Opioid use declined with HIV care. Opioid prescription use decreased substantially with longer time in HIV care among both episodic and chronic opioid users infected with HIV, according to a study in JAIDS.
HIV treatment goals need to improve. Meeting the National HIV/AIDS Strategy goals will require more efforts to link and retain black patients to care. Among blacks living with diagnosed HIV at year-end 2013, 53.5% were retained in care and 48.5% achieved viral suppression. The lowest levels of care and viral suppression were among persons with infection attributed to injection drug use and males with infection attributed to heterosexual contact, according to a report in MMWR. The strategy goals are 85% linkage to care, 90% retention in care, and 80% viral load suppression by 2020.
[email protected]
On Twitter @richpizzi
Here’s your weekly quick take on recent notable news and journal articles related to HIV and AIDS research:
Novel therapy tested. An unboosted HIV-1 integrase strand-transfer inhibitor produced led to rapid declines in HIV-1 RNA after 10 days of monotherapy in a phase 1B study reported in JAIDS. The drug (bictegravir) was well tolerated, and displayed rapid absorption and a half-life supportive of once-daily therapy in HIV-infected subjects.
New diagnoses declining in black women. Based on three different measures, the disparity in HIV diagnoses in black women decreased in 2014, compared with 2010, according to a CDC report.
Legalizing sex work is linked to lower HIV rates. Rates of HIV infection in female sex workers are lower in European countries that have legalized of some aspects of sex work, according to a study in the Lancet HIV.
Flu vaccine likely safe in HIV-positive women on therapy. H1N1 influenza vaccination in HIV-infected women on effective antiretroviral treatment did not induce measurable antigen-driven proliferation of the HIV-1 proviral reservoir, according to a study in AIDS Research and Therapy.
Could vitamin supplements lower subsequent TB risk? At the start of ART, vitamin A and D deficiencies were more common in adults who went on to develop tuberculosis, according to a study in JAIDS.
Cerebrospinal fluid CD8+ T cells beneficial in acute HIV infection. CD8+ T cells expand in the central nervous system during acute HIV infection and are functional and directed against HIV antigens. If combination antiretroviral therapy is initiated early, the cells could protect against injury seen in chronic HIV infection based on a study in JAIDS.
Opioid use declined with HIV care. Opioid prescription use decreased substantially with longer time in HIV care among both episodic and chronic opioid users infected with HIV, according to a study in JAIDS.
HIV treatment goals need to improve. Meeting the National HIV/AIDS Strategy goals will require more efforts to link and retain black patients to care. Among blacks living with diagnosed HIV at year-end 2013, 53.5% were retained in care and 48.5% achieved viral suppression. The lowest levels of care and viral suppression were among persons with infection attributed to injection drug use and males with infection attributed to heterosexual contact, according to a report in MMWR. The strategy goals are 85% linkage to care, 90% retention in care, and 80% viral load suppression by 2020.
[email protected]
On Twitter @richpizzi
Here’s your weekly quick take on recent notable news and journal articles related to HIV and AIDS research:
Novel therapy tested. An unboosted HIV-1 integrase strand-transfer inhibitor produced led to rapid declines in HIV-1 RNA after 10 days of monotherapy in a phase 1B study reported in JAIDS. The drug (bictegravir) was well tolerated, and displayed rapid absorption and a half-life supportive of once-daily therapy in HIV-infected subjects.
New diagnoses declining in black women. Based on three different measures, the disparity in HIV diagnoses in black women decreased in 2014, compared with 2010, according to a CDC report.
Legalizing sex work is linked to lower HIV rates. Rates of HIV infection in female sex workers are lower in European countries that have legalized of some aspects of sex work, according to a study in the Lancet HIV.
Flu vaccine likely safe in HIV-positive women on therapy. H1N1 influenza vaccination in HIV-infected women on effective antiretroviral treatment did not induce measurable antigen-driven proliferation of the HIV-1 proviral reservoir, according to a study in AIDS Research and Therapy.
Could vitamin supplements lower subsequent TB risk? At the start of ART, vitamin A and D deficiencies were more common in adults who went on to develop tuberculosis, according to a study in JAIDS.
Cerebrospinal fluid CD8+ T cells beneficial in acute HIV infection. CD8+ T cells expand in the central nervous system during acute HIV infection and are functional and directed against HIV antigens. If combination antiretroviral therapy is initiated early, the cells could protect against injury seen in chronic HIV infection based on a study in JAIDS.
Opioid use declined with HIV care. Opioid prescription use decreased substantially with longer time in HIV care among both episodic and chronic opioid users infected with HIV, according to a study in JAIDS.
HIV treatment goals need to improve. Meeting the National HIV/AIDS Strategy goals will require more efforts to link and retain black patients to care. Among blacks living with diagnosed HIV at year-end 2013, 53.5% were retained in care and 48.5% achieved viral suppression. The lowest levels of care and viral suppression were among persons with infection attributed to injection drug use and males with infection attributed to heterosexual contact, according to a report in MMWR. The strategy goals are 85% linkage to care, 90% retention in care, and 80% viral load suppression by 2020.
[email protected]
On Twitter @richpizzi
Rotavirus vaccine and PCV reduce hospital burden of young children
Vaccination programs targeting rotavirus and pneumonia in children younger than 2 years both contributed to a “rapid and considerable” decline in the hospital burden of pediatric patients, both in relation to those diseases and overall, according to an observational study.
Three vaccines were added to the National Immunization Plan in Israel within a 1.5-year interval, between July 2009 and January 2011: rotavirus vaccine and the 7-valent and 13-valent pneumococcal conjugate vaccines (PCV). Researchers studied the population at the Soroka University Medical Center in Beer Sheva, Israel, which was split roughly 50/50 between Jewish children and Bedouin Muslim children.
The rates of rotavirus gastroenteritis, nonrotavirus gastroenteritis, alveolar pneumonia, and nonalveolar lower respiratory tract infections in the 37,591 hospitalized children younger than 2 years declined by 78%, 21%, 46%, and 7%, respectively, over the course of the study period. Outpatient ED visits for the same diseases declined 80%, 16%, 67%, and 13%, respectively.
The results are more evidence that rotavirus vaccine can help prevent diarrhea not caused by rotavirus and, similarly, that PCV can help prevent lower respiratory tract infections not caused by pneumococci.
Overall, hospitalizations and outpatient ED visits also declined significantly, by 11% and 12%, respectively.
“The impact of [rotavirus vaccine] and PCV may not be limited to prevention of diarrhea and respiratory disease, respectively. In one study, it was suggested that diarrhea may increase the risk of subsequent pneumonia in young children, pointing to potential synergistic benefits” of the vaccines, the authors wrote (Am J Epidemiol. 2005;162[10]:999-1007).
The study was supported by Merck Sharp & Dohme and Pfizer. Authors received speaker fees, research support, and consulting fees from those companies and from GlaxoSmithKline.
Vaccination programs targeting rotavirus and pneumonia in children younger than 2 years both contributed to a “rapid and considerable” decline in the hospital burden of pediatric patients, both in relation to those diseases and overall, according to an observational study.
Three vaccines were added to the National Immunization Plan in Israel within a 1.5-year interval, between July 2009 and January 2011: rotavirus vaccine and the 7-valent and 13-valent pneumococcal conjugate vaccines (PCV). Researchers studied the population at the Soroka University Medical Center in Beer Sheva, Israel, which was split roughly 50/50 between Jewish children and Bedouin Muslim children.
The rates of rotavirus gastroenteritis, nonrotavirus gastroenteritis, alveolar pneumonia, and nonalveolar lower respiratory tract infections in the 37,591 hospitalized children younger than 2 years declined by 78%, 21%, 46%, and 7%, respectively, over the course of the study period. Outpatient ED visits for the same diseases declined 80%, 16%, 67%, and 13%, respectively.
The results are more evidence that rotavirus vaccine can help prevent diarrhea not caused by rotavirus and, similarly, that PCV can help prevent lower respiratory tract infections not caused by pneumococci.
Overall, hospitalizations and outpatient ED visits also declined significantly, by 11% and 12%, respectively.
“The impact of [rotavirus vaccine] and PCV may not be limited to prevention of diarrhea and respiratory disease, respectively. In one study, it was suggested that diarrhea may increase the risk of subsequent pneumonia in young children, pointing to potential synergistic benefits” of the vaccines, the authors wrote (Am J Epidemiol. 2005;162[10]:999-1007).
The study was supported by Merck Sharp & Dohme and Pfizer. Authors received speaker fees, research support, and consulting fees from those companies and from GlaxoSmithKline.
Vaccination programs targeting rotavirus and pneumonia in children younger than 2 years both contributed to a “rapid and considerable” decline in the hospital burden of pediatric patients, both in relation to those diseases and overall, according to an observational study.
Three vaccines were added to the National Immunization Plan in Israel within a 1.5-year interval, between July 2009 and January 2011: rotavirus vaccine and the 7-valent and 13-valent pneumococcal conjugate vaccines (PCV). Researchers studied the population at the Soroka University Medical Center in Beer Sheva, Israel, which was split roughly 50/50 between Jewish children and Bedouin Muslim children.
The rates of rotavirus gastroenteritis, nonrotavirus gastroenteritis, alveolar pneumonia, and nonalveolar lower respiratory tract infections in the 37,591 hospitalized children younger than 2 years declined by 78%, 21%, 46%, and 7%, respectively, over the course of the study period. Outpatient ED visits for the same diseases declined 80%, 16%, 67%, and 13%, respectively.
The results are more evidence that rotavirus vaccine can help prevent diarrhea not caused by rotavirus and, similarly, that PCV can help prevent lower respiratory tract infections not caused by pneumococci.
Overall, hospitalizations and outpatient ED visits also declined significantly, by 11% and 12%, respectively.
“The impact of [rotavirus vaccine] and PCV may not be limited to prevention of diarrhea and respiratory disease, respectively. In one study, it was suggested that diarrhea may increase the risk of subsequent pneumonia in young children, pointing to potential synergistic benefits” of the vaccines, the authors wrote (Am J Epidemiol. 2005;162[10]:999-1007).
The study was supported by Merck Sharp & Dohme and Pfizer. Authors received speaker fees, research support, and consulting fees from those companies and from GlaxoSmithKline.
FROM THE JOURNAL OF PEDIATRICS
Key clinical point:
Major finding: The rates of rotavirus gastroenteritis, nonrotavirus gastroenteritis, alveolar pneumonia, and nonalveolar lower respiratory tract infections in hospitalized children younger than 2 years declined by 78%, 21%, 46%, and 7%, respectively, over the course of the study period.
Data source: A prospective, population-based observational study of one hospital in southern Israel.
Disclosures: The study was supported by Merck Sharp & Dohme and Pfizer. Authors received speaker fees, research support, and consulting fees from those companies and from GlaxoSmithKline.