User login
Switch From Fingolimod to Alemtuzumab Might Trigger MS Relapse
Nine patients with relapsing multiple sclerosis had significant and unexpected disease activity within 12 months of switching from fingolimod to alemtuzumab, according to a report from six European neuroscience centers. The report was published January 10 in Neurology: Neuroimmunology & Neuroinflammation.
The centers treated 174 patients with alemtuzumab (Lemtrada); 36 had been on fingolimod (Gilenya) beforehand. “Therefore, these nine patients ... represent 25% of the fingolimod-alemtuzumab cohort,” said Mark Willis, MBBCh, Clinical Research Fellow at Cardiff University, Wales, and colleagues.
The researchers speculated that prolonged sequestration of autoreactive lymphocytes following fingolimod withdrawal allowed “these cells to be concealed from the usual biological effect of alemtuzumab. Subsequent lymphocyte egress then provoke[d] disease reactivation ... This may have important implications for sequential drug selection and washout periods in a subset of patients who switch from fingolimod or drugs with similar biological mechanisms,” they said.
The nine patients were on fingolimod for between five and 33 months, but it was not effective. As a result, they were started on alemtuzumab following a median fingolimod washout period of six weeks. Eight patients had at least one clinical relapse within 12 months of the first alemtuzumab infusion cycle; the median time to relapse following alemtuzumab induction was 4.5 months. All nine patients had radiologic evidence of new disease activity.
Five patients had lymphocyte counts below normal when started on alemtuzumab. It has “been suggested that patients continue on an alternative [disease-modifying treatment] after fingolimod discontinuation, preferably until peripheral lymphocyte counts have normalized.” However, “there is currently no consensus as to which subsequent therapeutic agent is optimal,” the investigators said.
All nine patients went on to the second planned infusion of alemtuzumab; eight were relapse free during a mean follow-up of six months after the second treatment cycle. Of seven patients who had further imaging, four were radiologically stable, three had new T2 lesions, and one had a new gadolinium-enhancing lesion.
—M. Alexander Otto
Suggested Reading
Willis M, Pearson O, Illes Z, et al. An observational study of alemtuzumab following fingolimod for multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2017;4(2):e320.
Nine patients with relapsing multiple sclerosis had significant and unexpected disease activity within 12 months of switching from fingolimod to alemtuzumab, according to a report from six European neuroscience centers. The report was published January 10 in Neurology: Neuroimmunology & Neuroinflammation.
The centers treated 174 patients with alemtuzumab (Lemtrada); 36 had been on fingolimod (Gilenya) beforehand. “Therefore, these nine patients ... represent 25% of the fingolimod-alemtuzumab cohort,” said Mark Willis, MBBCh, Clinical Research Fellow at Cardiff University, Wales, and colleagues.
The researchers speculated that prolonged sequestration of autoreactive lymphocytes following fingolimod withdrawal allowed “these cells to be concealed from the usual biological effect of alemtuzumab. Subsequent lymphocyte egress then provoke[d] disease reactivation ... This may have important implications for sequential drug selection and washout periods in a subset of patients who switch from fingolimod or drugs with similar biological mechanisms,” they said.
The nine patients were on fingolimod for between five and 33 months, but it was not effective. As a result, they were started on alemtuzumab following a median fingolimod washout period of six weeks. Eight patients had at least one clinical relapse within 12 months of the first alemtuzumab infusion cycle; the median time to relapse following alemtuzumab induction was 4.5 months. All nine patients had radiologic evidence of new disease activity.
Five patients had lymphocyte counts below normal when started on alemtuzumab. It has “been suggested that patients continue on an alternative [disease-modifying treatment] after fingolimod discontinuation, preferably until peripheral lymphocyte counts have normalized.” However, “there is currently no consensus as to which subsequent therapeutic agent is optimal,” the investigators said.
All nine patients went on to the second planned infusion of alemtuzumab; eight were relapse free during a mean follow-up of six months after the second treatment cycle. Of seven patients who had further imaging, four were radiologically stable, three had new T2 lesions, and one had a new gadolinium-enhancing lesion.
—M. Alexander Otto
Suggested Reading
Willis M, Pearson O, Illes Z, et al. An observational study of alemtuzumab following fingolimod for multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2017;4(2):e320.
Nine patients with relapsing multiple sclerosis had significant and unexpected disease activity within 12 months of switching from fingolimod to alemtuzumab, according to a report from six European neuroscience centers. The report was published January 10 in Neurology: Neuroimmunology & Neuroinflammation.
The centers treated 174 patients with alemtuzumab (Lemtrada); 36 had been on fingolimod (Gilenya) beforehand. “Therefore, these nine patients ... represent 25% of the fingolimod-alemtuzumab cohort,” said Mark Willis, MBBCh, Clinical Research Fellow at Cardiff University, Wales, and colleagues.
The researchers speculated that prolonged sequestration of autoreactive lymphocytes following fingolimod withdrawal allowed “these cells to be concealed from the usual biological effect of alemtuzumab. Subsequent lymphocyte egress then provoke[d] disease reactivation ... This may have important implications for sequential drug selection and washout periods in a subset of patients who switch from fingolimod or drugs with similar biological mechanisms,” they said.
The nine patients were on fingolimod for between five and 33 months, but it was not effective. As a result, they were started on alemtuzumab following a median fingolimod washout period of six weeks. Eight patients had at least one clinical relapse within 12 months of the first alemtuzumab infusion cycle; the median time to relapse following alemtuzumab induction was 4.5 months. All nine patients had radiologic evidence of new disease activity.
Five patients had lymphocyte counts below normal when started on alemtuzumab. It has “been suggested that patients continue on an alternative [disease-modifying treatment] after fingolimod discontinuation, preferably until peripheral lymphocyte counts have normalized.” However, “there is currently no consensus as to which subsequent therapeutic agent is optimal,” the investigators said.
All nine patients went on to the second planned infusion of alemtuzumab; eight were relapse free during a mean follow-up of six months after the second treatment cycle. Of seven patients who had further imaging, four were radiologically stable, three had new T2 lesions, and one had a new gadolinium-enhancing lesion.
—M. Alexander Otto
Suggested Reading
Willis M, Pearson O, Illes Z, et al. An observational study of alemtuzumab following fingolimod for multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2017;4(2):e320.
Relatives of Patients With MS Show Early Signs of Disease
“Our results further point to a possible sequence of events leading to MS, in which changes in vibration sensitivity may precede the appearance of demyelinating lesions in the brain,” said the researchers.
Evaluating First-Degree Relatives
Dr. Xia and colleagues conducted the Genes and Environment in MS (GEMS) project, the first prospective study of populations at risk for MS and the first detailed cross-sectional examination of higher-risk and lower-risk family members of patients with MS. The study involved 100 neurologically asymptomatic adults (ages 18 to 50) who were first-degree relatives of patients with MS and participated in the GEMS project from August 2012 to July 2015.
Forty-one of the participants were high-risk patients who scored in the top 10% of a Genetic and Environmental Risk Score (GERS), and 59 participants were low-risk and scored in the bottom 10% of the GERS. The GERS included genetic risk factors (ie, HLA alleles and several MS-associated non-HLA genetic variants) and environmental factors, such as smoking status, BMI, history of infectious mononucleosis and migraine, and vitamin D levels.
Since 40 of the 41 high-risk individuals were female, and 25 of the 59 low-risk individuals were female, the investigators limited the study to the 65 female participants to avoid “attributing any potential difference primarily to the role of sex,” the researchers said.
Testing Neurologic Function
To help identify early signs of MS, the investigators used brain MRI, optical coherence tomography, and other measures of neurologic function, including the Expanded Disability Status Scale, Timed 25-Foot Walk, Nine-Hole Peg Test, Paced Auditory Serial Addition Test, Symbol Digit Modalities Test, Timed Up and Go, and high-contrast and low-contrast visual acuity.
Overall, women at high risk showed more subclinical signs of MS than women at low risk, based on an omnibus test that globally assessed the burden of neurologic dysfunction by comparing the overall differences between the two groups. Impaired vibration perception yielded a stronger result; of 47 women (27 at high risk and 20 at low risk) tested in this manner, women at high risk showed significantly reduced vibration perception in the distal lower extremities.
One patient in the high-risk group converted to clinically definite MS during the study. Four of the women at high risk had T2-weighted hyperintense lesions that met the 2010 McDonald MRI criteria for dissemination in space, compared with one woman at low risk. Two women at high risk and one at low risk met the 2016 proposed consensus MRI criteria for MS diagnosis. In addition, radiologic isolated syndrome occurred in one woman from each group. Also, there was a single focus of leptomeningeal enhancement in three women at high risk and one woman at low risk.
Some limitations of this study include the small size, the lack of male participants, the cross-sectional design, and the fact that the vibration sensitivity thresholds were in the normal range for individuals at high risk and low risk. Researchers “plan to confirm the finding of change in vibration sensitivity with a follow-up study.” They added that the “study highlights the importantneed to develop and test more sensitive measures, particularly with biometric devices, to detect subtle subclinical changes early in the disease process.”
Identifying High-Risk Individuals
“The GEMS study represents the most ambitious effort yet to identify presymptomatic individuals who are at increased risk for MS, and it is a valuable first step toward targeted screening,” said Fredrik Piehl, MD, PhD, Professor of Neuroimmunology at Karolinska Institutet and Karolinska University Hospital in Stockholm, in an accompanying editorial. “Even if we cannot yet intervene therapeutically using currently available disease-modifying treatments in presymptomatic stages of MS, the ability to better define high-risk individuals is likely to make active surveillance programs more cost effective. It also provides important information to counsel individuals about lifestyle changes, such as quitting smoking. The GERS also can likely be further refined with more up-to-date data on the interaction between specific genetic and environmental factors,” he added. Dr. Piehl disclosed research support, travel grants, and other relationships with Biogen, Genzyme, Novartis, Merck, Roche, Serono, and Teva.
—Heidi Splete
Suggested Reading
Xia Z, Steele SU, Bakshi A, et al. Assessment of early evidence of multiple sclerosis in a prospective study of asymptomatic high-risk family members. JAMA Neurol. 2017 Jan 17 [Epub ahead of print].
Piehl F. Multiple sclerosis-a tuning fork still required. JAMA Neurol. 2017 Jan 17 [Epub ahead of print].
“Our results further point to a possible sequence of events leading to MS, in which changes in vibration sensitivity may precede the appearance of demyelinating lesions in the brain,” said the researchers.
Evaluating First-Degree Relatives
Dr. Xia and colleagues conducted the Genes and Environment in MS (GEMS) project, the first prospective study of populations at risk for MS and the first detailed cross-sectional examination of higher-risk and lower-risk family members of patients with MS. The study involved 100 neurologically asymptomatic adults (ages 18 to 50) who were first-degree relatives of patients with MS and participated in the GEMS project from August 2012 to July 2015.
Forty-one of the participants were high-risk patients who scored in the top 10% of a Genetic and Environmental Risk Score (GERS), and 59 participants were low-risk and scored in the bottom 10% of the GERS. The GERS included genetic risk factors (ie, HLA alleles and several MS-associated non-HLA genetic variants) and environmental factors, such as smoking status, BMI, history of infectious mononucleosis and migraine, and vitamin D levels.
Since 40 of the 41 high-risk individuals were female, and 25 of the 59 low-risk individuals were female, the investigators limited the study to the 65 female participants to avoid “attributing any potential difference primarily to the role of sex,” the researchers said.
Testing Neurologic Function
To help identify early signs of MS, the investigators used brain MRI, optical coherence tomography, and other measures of neurologic function, including the Expanded Disability Status Scale, Timed 25-Foot Walk, Nine-Hole Peg Test, Paced Auditory Serial Addition Test, Symbol Digit Modalities Test, Timed Up and Go, and high-contrast and low-contrast visual acuity.
Overall, women at high risk showed more subclinical signs of MS than women at low risk, based on an omnibus test that globally assessed the burden of neurologic dysfunction by comparing the overall differences between the two groups. Impaired vibration perception yielded a stronger result; of 47 women (27 at high risk and 20 at low risk) tested in this manner, women at high risk showed significantly reduced vibration perception in the distal lower extremities.
One patient in the high-risk group converted to clinically definite MS during the study. Four of the women at high risk had T2-weighted hyperintense lesions that met the 2010 McDonald MRI criteria for dissemination in space, compared with one woman at low risk. Two women at high risk and one at low risk met the 2016 proposed consensus MRI criteria for MS diagnosis. In addition, radiologic isolated syndrome occurred in one woman from each group. Also, there was a single focus of leptomeningeal enhancement in three women at high risk and one woman at low risk.
Some limitations of this study include the small size, the lack of male participants, the cross-sectional design, and the fact that the vibration sensitivity thresholds were in the normal range for individuals at high risk and low risk. Researchers “plan to confirm the finding of change in vibration sensitivity with a follow-up study.” They added that the “study highlights the importantneed to develop and test more sensitive measures, particularly with biometric devices, to detect subtle subclinical changes early in the disease process.”
Identifying High-Risk Individuals
“The GEMS study represents the most ambitious effort yet to identify presymptomatic individuals who are at increased risk for MS, and it is a valuable first step toward targeted screening,” said Fredrik Piehl, MD, PhD, Professor of Neuroimmunology at Karolinska Institutet and Karolinska University Hospital in Stockholm, in an accompanying editorial. “Even if we cannot yet intervene therapeutically using currently available disease-modifying treatments in presymptomatic stages of MS, the ability to better define high-risk individuals is likely to make active surveillance programs more cost effective. It also provides important information to counsel individuals about lifestyle changes, such as quitting smoking. The GERS also can likely be further refined with more up-to-date data on the interaction between specific genetic and environmental factors,” he added. Dr. Piehl disclosed research support, travel grants, and other relationships with Biogen, Genzyme, Novartis, Merck, Roche, Serono, and Teva.
—Heidi Splete
Suggested Reading
Xia Z, Steele SU, Bakshi A, et al. Assessment of early evidence of multiple sclerosis in a prospective study of asymptomatic high-risk family members. JAMA Neurol. 2017 Jan 17 [Epub ahead of print].
Piehl F. Multiple sclerosis-a tuning fork still required. JAMA Neurol. 2017 Jan 17 [Epub ahead of print].
“Our results further point to a possible sequence of events leading to MS, in which changes in vibration sensitivity may precede the appearance of demyelinating lesions in the brain,” said the researchers.
Evaluating First-Degree Relatives
Dr. Xia and colleagues conducted the Genes and Environment in MS (GEMS) project, the first prospective study of populations at risk for MS and the first detailed cross-sectional examination of higher-risk and lower-risk family members of patients with MS. The study involved 100 neurologically asymptomatic adults (ages 18 to 50) who were first-degree relatives of patients with MS and participated in the GEMS project from August 2012 to July 2015.
Forty-one of the participants were high-risk patients who scored in the top 10% of a Genetic and Environmental Risk Score (GERS), and 59 participants were low-risk and scored in the bottom 10% of the GERS. The GERS included genetic risk factors (ie, HLA alleles and several MS-associated non-HLA genetic variants) and environmental factors, such as smoking status, BMI, history of infectious mononucleosis and migraine, and vitamin D levels.
Since 40 of the 41 high-risk individuals were female, and 25 of the 59 low-risk individuals were female, the investigators limited the study to the 65 female participants to avoid “attributing any potential difference primarily to the role of sex,” the researchers said.
Testing Neurologic Function
To help identify early signs of MS, the investigators used brain MRI, optical coherence tomography, and other measures of neurologic function, including the Expanded Disability Status Scale, Timed 25-Foot Walk, Nine-Hole Peg Test, Paced Auditory Serial Addition Test, Symbol Digit Modalities Test, Timed Up and Go, and high-contrast and low-contrast visual acuity.
Overall, women at high risk showed more subclinical signs of MS than women at low risk, based on an omnibus test that globally assessed the burden of neurologic dysfunction by comparing the overall differences between the two groups. Impaired vibration perception yielded a stronger result; of 47 women (27 at high risk and 20 at low risk) tested in this manner, women at high risk showed significantly reduced vibration perception in the distal lower extremities.
One patient in the high-risk group converted to clinically definite MS during the study. Four of the women at high risk had T2-weighted hyperintense lesions that met the 2010 McDonald MRI criteria for dissemination in space, compared with one woman at low risk. Two women at high risk and one at low risk met the 2016 proposed consensus MRI criteria for MS diagnosis. In addition, radiologic isolated syndrome occurred in one woman from each group. Also, there was a single focus of leptomeningeal enhancement in three women at high risk and one woman at low risk.
Some limitations of this study include the small size, the lack of male participants, the cross-sectional design, and the fact that the vibration sensitivity thresholds were in the normal range for individuals at high risk and low risk. Researchers “plan to confirm the finding of change in vibration sensitivity with a follow-up study.” They added that the “study highlights the importantneed to develop and test more sensitive measures, particularly with biometric devices, to detect subtle subclinical changes early in the disease process.”
Identifying High-Risk Individuals
“The GEMS study represents the most ambitious effort yet to identify presymptomatic individuals who are at increased risk for MS, and it is a valuable first step toward targeted screening,” said Fredrik Piehl, MD, PhD, Professor of Neuroimmunology at Karolinska Institutet and Karolinska University Hospital in Stockholm, in an accompanying editorial. “Even if we cannot yet intervene therapeutically using currently available disease-modifying treatments in presymptomatic stages of MS, the ability to better define high-risk individuals is likely to make active surveillance programs more cost effective. It also provides important information to counsel individuals about lifestyle changes, such as quitting smoking. The GERS also can likely be further refined with more up-to-date data on the interaction between specific genetic and environmental factors,” he added. Dr. Piehl disclosed research support, travel grants, and other relationships with Biogen, Genzyme, Novartis, Merck, Roche, Serono, and Teva.
—Heidi Splete
Suggested Reading
Xia Z, Steele SU, Bakshi A, et al. Assessment of early evidence of multiple sclerosis in a prospective study of asymptomatic high-risk family members. JAMA Neurol. 2017 Jan 17 [Epub ahead of print].
Piehl F. Multiple sclerosis-a tuning fork still required. JAMA Neurol. 2017 Jan 17 [Epub ahead of print].
DTaP5-IPV noninferior to DTaP5 plus IPV for fifth dose
The stand-alone diphtheria, tetanus, acellular, pertussis and inactivated poliovirus combination vaccine – DTaP5-IPV – is equivalent as a fifth dose to the separate DTaP5 plus IPV vaccines in children aged 4-6 years, according to a noninferiority study.
In a phase III, controlled, open-label study, 3,372 children who had completed the 4-dose infant/toddler vaccination were randomized to DTaP5-IPV plus MMR and varicella virus (VZV) vaccines, DTaP5+IPV with MMR and VZV, DTaP5-IPV with/without MMR/VZV, or DTaP5+IPV with/without MMR/VZV.
Michael J. Smith, MD, MSCE, of the University of Louisville (Ky.) and coauthors saw significantly higher pertussis antibody levels for all antigens in the group who received the DTaP5-IPV plus MMR and VZV vaccines than in the group who received the DTaP5+IPV with MMR and VZV. Twenty-eight days after the vaccine was given, booster responses ranged from 95% to 97% for the DTaP5-IPV group and from 87% to 93% in the DTaP5+IPV group.
Similarly, the DTaP5-IPV vaccine showed noninferiority in the booster response for antitetanus, antidiphtheria, and antipoliovirus antibody levels (Pediatr Infect Dis J. 2017 Mar;36[3]:319-25).
“Overall, the levels of immune responses described in both treatment groups in the current study are above the levels described in the Swedish infant efficacy study, which demonstrated 85% protective efficacy against World Health Organization–defined pertussis disease,” the authors wrote. “Thus, it is reasonable to conclude that protective efficacy against pertussis will be achieved when either DTaP5-IPV or DTaP5+IPV is given as a booster dose to children 4-6 years of age.”
The two vaccines showed a similar safety profile. The rate of immediate, unsolicited, adverse systemic events was 0.9% in the DTaP5-IPV group and 1% in the DTaP5+IPV group, while the rate of immediate, unsolicited, adverse reactions was 0.1% in the DTaP5-IPV group and 0.2% in the DTaP5+IPV group.
Solicited reactions also were similar between the two groups: 93% of participants who received DTaP5-IPV and 92% of those who received DTaP5+IPV reported reactions such as myalgia, malaise, pain, erythema, and change in limb circumference.
“This is consistent with the established safety profile of DTaP5+IPV vaccine, based on 16 years of postmarketing surveillance and more than 7 million doses distributed,” the authors wrote.
There were also three serious adverse events in the DTaP5-IPV group within 28 days of the vaccination – lobular pneumonia, asthma, and new-onset type 1 diabetes mellitus – but the investigator decided these were unrelated to vaccination.
The study was sponsored by Sanofi Pasteur, which manufactures both vaccines. Three authors were employees of Sanofi Pasteur, and one author declared funding from Sanofi Pasteur to present the study results at a meeting.
The stand-alone diphtheria, tetanus, acellular, pertussis and inactivated poliovirus combination vaccine – DTaP5-IPV – is equivalent as a fifth dose to the separate DTaP5 plus IPV vaccines in children aged 4-6 years, according to a noninferiority study.
In a phase III, controlled, open-label study, 3,372 children who had completed the 4-dose infant/toddler vaccination were randomized to DTaP5-IPV plus MMR and varicella virus (VZV) vaccines, DTaP5+IPV with MMR and VZV, DTaP5-IPV with/without MMR/VZV, or DTaP5+IPV with/without MMR/VZV.
Michael J. Smith, MD, MSCE, of the University of Louisville (Ky.) and coauthors saw significantly higher pertussis antibody levels for all antigens in the group who received the DTaP5-IPV plus MMR and VZV vaccines than in the group who received the DTaP5+IPV with MMR and VZV. Twenty-eight days after the vaccine was given, booster responses ranged from 95% to 97% for the DTaP5-IPV group and from 87% to 93% in the DTaP5+IPV group.
Similarly, the DTaP5-IPV vaccine showed noninferiority in the booster response for antitetanus, antidiphtheria, and antipoliovirus antibody levels (Pediatr Infect Dis J. 2017 Mar;36[3]:319-25).
“Overall, the levels of immune responses described in both treatment groups in the current study are above the levels described in the Swedish infant efficacy study, which demonstrated 85% protective efficacy against World Health Organization–defined pertussis disease,” the authors wrote. “Thus, it is reasonable to conclude that protective efficacy against pertussis will be achieved when either DTaP5-IPV or DTaP5+IPV is given as a booster dose to children 4-6 years of age.”
The two vaccines showed a similar safety profile. The rate of immediate, unsolicited, adverse systemic events was 0.9% in the DTaP5-IPV group and 1% in the DTaP5+IPV group, while the rate of immediate, unsolicited, adverse reactions was 0.1% in the DTaP5-IPV group and 0.2% in the DTaP5+IPV group.
Solicited reactions also were similar between the two groups: 93% of participants who received DTaP5-IPV and 92% of those who received DTaP5+IPV reported reactions such as myalgia, malaise, pain, erythema, and change in limb circumference.
“This is consistent with the established safety profile of DTaP5+IPV vaccine, based on 16 years of postmarketing surveillance and more than 7 million doses distributed,” the authors wrote.
There were also three serious adverse events in the DTaP5-IPV group within 28 days of the vaccination – lobular pneumonia, asthma, and new-onset type 1 diabetes mellitus – but the investigator decided these were unrelated to vaccination.
The study was sponsored by Sanofi Pasteur, which manufactures both vaccines. Three authors were employees of Sanofi Pasteur, and one author declared funding from Sanofi Pasteur to present the study results at a meeting.
The stand-alone diphtheria, tetanus, acellular, pertussis and inactivated poliovirus combination vaccine – DTaP5-IPV – is equivalent as a fifth dose to the separate DTaP5 plus IPV vaccines in children aged 4-6 years, according to a noninferiority study.
In a phase III, controlled, open-label study, 3,372 children who had completed the 4-dose infant/toddler vaccination were randomized to DTaP5-IPV plus MMR and varicella virus (VZV) vaccines, DTaP5+IPV with MMR and VZV, DTaP5-IPV with/without MMR/VZV, or DTaP5+IPV with/without MMR/VZV.
Michael J. Smith, MD, MSCE, of the University of Louisville (Ky.) and coauthors saw significantly higher pertussis antibody levels for all antigens in the group who received the DTaP5-IPV plus MMR and VZV vaccines than in the group who received the DTaP5+IPV with MMR and VZV. Twenty-eight days after the vaccine was given, booster responses ranged from 95% to 97% for the DTaP5-IPV group and from 87% to 93% in the DTaP5+IPV group.
Similarly, the DTaP5-IPV vaccine showed noninferiority in the booster response for antitetanus, antidiphtheria, and antipoliovirus antibody levels (Pediatr Infect Dis J. 2017 Mar;36[3]:319-25).
“Overall, the levels of immune responses described in both treatment groups in the current study are above the levels described in the Swedish infant efficacy study, which demonstrated 85% protective efficacy against World Health Organization–defined pertussis disease,” the authors wrote. “Thus, it is reasonable to conclude that protective efficacy against pertussis will be achieved when either DTaP5-IPV or DTaP5+IPV is given as a booster dose to children 4-6 years of age.”
The two vaccines showed a similar safety profile. The rate of immediate, unsolicited, adverse systemic events was 0.9% in the DTaP5-IPV group and 1% in the DTaP5+IPV group, while the rate of immediate, unsolicited, adverse reactions was 0.1% in the DTaP5-IPV group and 0.2% in the DTaP5+IPV group.
Solicited reactions also were similar between the two groups: 93% of participants who received DTaP5-IPV and 92% of those who received DTaP5+IPV reported reactions such as myalgia, malaise, pain, erythema, and change in limb circumference.
“This is consistent with the established safety profile of DTaP5+IPV vaccine, based on 16 years of postmarketing surveillance and more than 7 million doses distributed,” the authors wrote.
There were also three serious adverse events in the DTaP5-IPV group within 28 days of the vaccination – lobular pneumonia, asthma, and new-onset type 1 diabetes mellitus – but the investigator decided these were unrelated to vaccination.
The study was sponsored by Sanofi Pasteur, which manufactures both vaccines. Three authors were employees of Sanofi Pasteur, and one author declared funding from Sanofi Pasteur to present the study results at a meeting.
Key clinical point: The stand-alone DTaP5-IPV combination vaccine is equivalent as a fifth dose to the separate DTaP5 plus IPV vaccines in children aged 4-6 years.
Major finding:
Data source: A phase III, controlled, randomized open-label study in 3,372 children.
Disclosures: The study was sponsored by Sanofi Pasteur, which manufactures both vaccines. Three authors were employees of Sanofi Pasteur, and one author declared funding from Sanofi Pasteur to present the study results at a meeting.
Is Closed-Loop DBS Ready for Clinical Application?
The Advantages of Closed-Loop DBS
Closed-loop DBS has several advantages over conventional DBS, said Dr. Foote. It reduces the amount of labor-intensive programming required, which ordinarily is based on frequent symptom assessment. Furthermore, closed-loop DBS can adapt to the fluctuating symptoms and interpatient variability that often characterize movement disorders. The technique may reduce the frequency of stimulation-related adverse events, decrease the likelihood of habituation, and extend the stimulator’s battery life, thus reducing the number of replacement surgeries required. “It is the ultimate in patient-tailored treatment,” said Dr. Foote.
In the past 15 years, electrocorticography, the measurement of local field potentials, and mathematical decoding have increased understanding of the brain greatly and enabled researchers to identify biomarkers of disease. Investigators have observed that when a person moves, high-frequency band activity in the motor cortex increases, and low-frequency band (ie, beta band) activity decreases. Beta activity appears to be a suppressive mechanism that gates motor function. These observations have been the basis for recent research in closed-loop DBS for Parkinson’s disease.
Tourette Syndrome
Dr. Foote and his colleagues are studying closed-loop DBS in patients with Tourette syndrome. They believed that the episodic nature of the syndrome’s symptoms would make closed-loop DBS a potentially beneficial treatment. The group hypothesized that if they could find a signal for the premonitory urge that patients generally have before a tic, they could deliver therapeutic stimulation as needed. They decided to target the centromedian (CM) nucleus of the thalamus for stimulation.
When Dr. Foote and colleagues failed to find a biomarker to predict tic onset, they decided to study intermittent stimulation using the NeuroPace system. Scheduled stimulation yielded statistically significant improvements in the Yale Global Tic Severity Scale total score, although they did not reach the prespecified outcome of a 50% improvement. The participant who received the most stimulation had the least improvement.
After this study was completed, the Medtronic PC+S system became available. This system has a longer battery life and improved hardware, compared with the NeuroPace system, said Dr. Foote. Using the PC+S system, he and his colleagues implanted two 24-year-old women with severe, intractable Tourette syndrome with 16 bilateral DBS electrodes on the CM thalamus. The patients also received cortical strips on both sides of the premotor cortex and motor cortex. The investigators found high levels of activity in the CM thalamus during tics, but no activity in that region during voluntary movement. The finding provides “strong evidence that the CM thalamus is participating in that pathologic network,” said Dr. Foote.
Engineers collaborating with Dr. Foote’s group used measurements of local field potentials in the CM thalamus and motor cortex to create a device that detects tics. The detector has a sensitivity of approximately 90% and a precision of 96%. When the investigators implanted the tic detector in one of the patients with Tourette syndrome who had received implantation of the NeuroPace device, it successfully initiated and terminated responsive DBS and reduced the patient’s tics.
Parkinson’s Disease
Investigators have found exaggerated phase amplitude coupling and increased beta activity in the subthalamic nucleus (STN), globus pallidus internus, and primary motor cortex of patients with Parkinson’s disease. In addition, data suggest that STN beta power correlates with the severity of bradykinesia and rigidity. Originally, researchers hypothesized that DBS provided benefit to patients with Parkinson’s disease by decreasing beta activity. A 2015 study by de Hemptinne et al, however, indicated that the main mechanism of action of DBS is disruption of phase amplitude coupling.
Patients with Tourette syndrome have the opposite problem, compared with patients with Parkinson’s disease. Therapeutic DBS reduces excessive movement in Tourette syndrome by increasing phase amplitude coupling, which is low at baseline. “We are helping [patients with Tourette syndrome] apply the brakes, because … they are failing to suppress these extra movements,” said Dr. Foote.
The tic detector has enabled the first chronic closed-loop DBS treatment for movement disorders, but many more such applications will emerge in the near future, he added. Research has suggested that phase amplitude coupling is a better biomarker in movement disorders than beta activity is. Dr. Foote and his colleagues are studying closed-loop DBS in essential tremor, and other researchers around the world are examining the treatment for Parkinson’s disease, Tourette syndrome, and obsessive–compulsive disorder.
“All DBS will be adaptive in the relatively near future,” concluded Dr. Foote. “It is just more intelligent to do it this way.”
—Erik Greb
Suggested Reading
Air EL, Ryapolova-Webb E, de Hemptinne C, et al. Acute effects of thalamic deep brain stimulation and thalamotomy on sensorimotor cortex local field potentials in essential tremor. Clin Neurophysiol. 2012;123(11):2232-2238.
de Hemptinne C, Swann NC, Ostrem JL, et al. Therapeutic deep brain stimulation reduces cortical phase-amplitude coupling in Parkinson’s disease. Nat Neurosci. 2015;18(5): 779-786.
Miller KJ, Hermes D, Honey CJ, et al. Human motor cortical activity is selectively phase-entrained on underlying rhythms. PLoS Comput Biol. 2012;8(9):e1002655.
Okun MS, Foote KD, Wu SS, et al. A trial of scheduled deep brain stimulation for Tourette syndrome: moving away from continuous deep brain stimulation paradigms. JAMA Neurol. 2013;70(1):85-94.
The Advantages of Closed-Loop DBS
Closed-loop DBS has several advantages over conventional DBS, said Dr. Foote. It reduces the amount of labor-intensive programming required, which ordinarily is based on frequent symptom assessment. Furthermore, closed-loop DBS can adapt to the fluctuating symptoms and interpatient variability that often characterize movement disorders. The technique may reduce the frequency of stimulation-related adverse events, decrease the likelihood of habituation, and extend the stimulator’s battery life, thus reducing the number of replacement surgeries required. “It is the ultimate in patient-tailored treatment,” said Dr. Foote.
In the past 15 years, electrocorticography, the measurement of local field potentials, and mathematical decoding have increased understanding of the brain greatly and enabled researchers to identify biomarkers of disease. Investigators have observed that when a person moves, high-frequency band activity in the motor cortex increases, and low-frequency band (ie, beta band) activity decreases. Beta activity appears to be a suppressive mechanism that gates motor function. These observations have been the basis for recent research in closed-loop DBS for Parkinson’s disease.
Tourette Syndrome
Dr. Foote and his colleagues are studying closed-loop DBS in patients with Tourette syndrome. They believed that the episodic nature of the syndrome’s symptoms would make closed-loop DBS a potentially beneficial treatment. The group hypothesized that if they could find a signal for the premonitory urge that patients generally have before a tic, they could deliver therapeutic stimulation as needed. They decided to target the centromedian (CM) nucleus of the thalamus for stimulation.
When Dr. Foote and colleagues failed to find a biomarker to predict tic onset, they decided to study intermittent stimulation using the NeuroPace system. Scheduled stimulation yielded statistically significant improvements in the Yale Global Tic Severity Scale total score, although they did not reach the prespecified outcome of a 50% improvement. The participant who received the most stimulation had the least improvement.
After this study was completed, the Medtronic PC+S system became available. This system has a longer battery life and improved hardware, compared with the NeuroPace system, said Dr. Foote. Using the PC+S system, he and his colleagues implanted two 24-year-old women with severe, intractable Tourette syndrome with 16 bilateral DBS electrodes on the CM thalamus. The patients also received cortical strips on both sides of the premotor cortex and motor cortex. The investigators found high levels of activity in the CM thalamus during tics, but no activity in that region during voluntary movement. The finding provides “strong evidence that the CM thalamus is participating in that pathologic network,” said Dr. Foote.
Engineers collaborating with Dr. Foote’s group used measurements of local field potentials in the CM thalamus and motor cortex to create a device that detects tics. The detector has a sensitivity of approximately 90% and a precision of 96%. When the investigators implanted the tic detector in one of the patients with Tourette syndrome who had received implantation of the NeuroPace device, it successfully initiated and terminated responsive DBS and reduced the patient’s tics.
Parkinson’s Disease
Investigators have found exaggerated phase amplitude coupling and increased beta activity in the subthalamic nucleus (STN), globus pallidus internus, and primary motor cortex of patients with Parkinson’s disease. In addition, data suggest that STN beta power correlates with the severity of bradykinesia and rigidity. Originally, researchers hypothesized that DBS provided benefit to patients with Parkinson’s disease by decreasing beta activity. A 2015 study by de Hemptinne et al, however, indicated that the main mechanism of action of DBS is disruption of phase amplitude coupling.
Patients with Tourette syndrome have the opposite problem, compared with patients with Parkinson’s disease. Therapeutic DBS reduces excessive movement in Tourette syndrome by increasing phase amplitude coupling, which is low at baseline. “We are helping [patients with Tourette syndrome] apply the brakes, because … they are failing to suppress these extra movements,” said Dr. Foote.
The tic detector has enabled the first chronic closed-loop DBS treatment for movement disorders, but many more such applications will emerge in the near future, he added. Research has suggested that phase amplitude coupling is a better biomarker in movement disorders than beta activity is. Dr. Foote and his colleagues are studying closed-loop DBS in essential tremor, and other researchers around the world are examining the treatment for Parkinson’s disease, Tourette syndrome, and obsessive–compulsive disorder.
“All DBS will be adaptive in the relatively near future,” concluded Dr. Foote. “It is just more intelligent to do it this way.”
—Erik Greb
Suggested Reading
Air EL, Ryapolova-Webb E, de Hemptinne C, et al. Acute effects of thalamic deep brain stimulation and thalamotomy on sensorimotor cortex local field potentials in essential tremor. Clin Neurophysiol. 2012;123(11):2232-2238.
de Hemptinne C, Swann NC, Ostrem JL, et al. Therapeutic deep brain stimulation reduces cortical phase-amplitude coupling in Parkinson’s disease. Nat Neurosci. 2015;18(5): 779-786.
Miller KJ, Hermes D, Honey CJ, et al. Human motor cortical activity is selectively phase-entrained on underlying rhythms. PLoS Comput Biol. 2012;8(9):e1002655.
Okun MS, Foote KD, Wu SS, et al. A trial of scheduled deep brain stimulation for Tourette syndrome: moving away from continuous deep brain stimulation paradigms. JAMA Neurol. 2013;70(1):85-94.
The Advantages of Closed-Loop DBS
Closed-loop DBS has several advantages over conventional DBS, said Dr. Foote. It reduces the amount of labor-intensive programming required, which ordinarily is based on frequent symptom assessment. Furthermore, closed-loop DBS can adapt to the fluctuating symptoms and interpatient variability that often characterize movement disorders. The technique may reduce the frequency of stimulation-related adverse events, decrease the likelihood of habituation, and extend the stimulator’s battery life, thus reducing the number of replacement surgeries required. “It is the ultimate in patient-tailored treatment,” said Dr. Foote.
In the past 15 years, electrocorticography, the measurement of local field potentials, and mathematical decoding have increased understanding of the brain greatly and enabled researchers to identify biomarkers of disease. Investigators have observed that when a person moves, high-frequency band activity in the motor cortex increases, and low-frequency band (ie, beta band) activity decreases. Beta activity appears to be a suppressive mechanism that gates motor function. These observations have been the basis for recent research in closed-loop DBS for Parkinson’s disease.
Tourette Syndrome
Dr. Foote and his colleagues are studying closed-loop DBS in patients with Tourette syndrome. They believed that the episodic nature of the syndrome’s symptoms would make closed-loop DBS a potentially beneficial treatment. The group hypothesized that if they could find a signal for the premonitory urge that patients generally have before a tic, they could deliver therapeutic stimulation as needed. They decided to target the centromedian (CM) nucleus of the thalamus for stimulation.
When Dr. Foote and colleagues failed to find a biomarker to predict tic onset, they decided to study intermittent stimulation using the NeuroPace system. Scheduled stimulation yielded statistically significant improvements in the Yale Global Tic Severity Scale total score, although they did not reach the prespecified outcome of a 50% improvement. The participant who received the most stimulation had the least improvement.
After this study was completed, the Medtronic PC+S system became available. This system has a longer battery life and improved hardware, compared with the NeuroPace system, said Dr. Foote. Using the PC+S system, he and his colleagues implanted two 24-year-old women with severe, intractable Tourette syndrome with 16 bilateral DBS electrodes on the CM thalamus. The patients also received cortical strips on both sides of the premotor cortex and motor cortex. The investigators found high levels of activity in the CM thalamus during tics, but no activity in that region during voluntary movement. The finding provides “strong evidence that the CM thalamus is participating in that pathologic network,” said Dr. Foote.
Engineers collaborating with Dr. Foote’s group used measurements of local field potentials in the CM thalamus and motor cortex to create a device that detects tics. The detector has a sensitivity of approximately 90% and a precision of 96%. When the investigators implanted the tic detector in one of the patients with Tourette syndrome who had received implantation of the NeuroPace device, it successfully initiated and terminated responsive DBS and reduced the patient’s tics.
Parkinson’s Disease
Investigators have found exaggerated phase amplitude coupling and increased beta activity in the subthalamic nucleus (STN), globus pallidus internus, and primary motor cortex of patients with Parkinson’s disease. In addition, data suggest that STN beta power correlates with the severity of bradykinesia and rigidity. Originally, researchers hypothesized that DBS provided benefit to patients with Parkinson’s disease by decreasing beta activity. A 2015 study by de Hemptinne et al, however, indicated that the main mechanism of action of DBS is disruption of phase amplitude coupling.
Patients with Tourette syndrome have the opposite problem, compared with patients with Parkinson’s disease. Therapeutic DBS reduces excessive movement in Tourette syndrome by increasing phase amplitude coupling, which is low at baseline. “We are helping [patients with Tourette syndrome] apply the brakes, because … they are failing to suppress these extra movements,” said Dr. Foote.
The tic detector has enabled the first chronic closed-loop DBS treatment for movement disorders, but many more such applications will emerge in the near future, he added. Research has suggested that phase amplitude coupling is a better biomarker in movement disorders than beta activity is. Dr. Foote and his colleagues are studying closed-loop DBS in essential tremor, and other researchers around the world are examining the treatment for Parkinson’s disease, Tourette syndrome, and obsessive–compulsive disorder.
“All DBS will be adaptive in the relatively near future,” concluded Dr. Foote. “It is just more intelligent to do it this way.”
—Erik Greb
Suggested Reading
Air EL, Ryapolova-Webb E, de Hemptinne C, et al. Acute effects of thalamic deep brain stimulation and thalamotomy on sensorimotor cortex local field potentials in essential tremor. Clin Neurophysiol. 2012;123(11):2232-2238.
de Hemptinne C, Swann NC, Ostrem JL, et al. Therapeutic deep brain stimulation reduces cortical phase-amplitude coupling in Parkinson’s disease. Nat Neurosci. 2015;18(5): 779-786.
Miller KJ, Hermes D, Honey CJ, et al. Human motor cortical activity is selectively phase-entrained on underlying rhythms. PLoS Comput Biol. 2012;8(9):e1002655.
Okun MS, Foote KD, Wu SS, et al. A trial of scheduled deep brain stimulation for Tourette syndrome: moving away from continuous deep brain stimulation paradigms. JAMA Neurol. 2013;70(1):85-94.
Diagnosing and Treating Patients With Migraine and Vertigo
Vertigo is a normal response to certain stimuli, such as looking down from heights and abnormal head movements. In addition, any dysfunction along the pathway that processes balance and gravity information (eg, the semicircular canals, acoustic nerve, and brainstem vestibular centers) can cause vertigo.
Migraine is significantly more common in patients with vertigo, and vertigo is significantly more common in migraineurs than in the general population. In addition, migraineurs are predisposed to motion sickness, which often includes vertigo. Migraineurs’ vestibular systems are more sensitive to stimuli than those of nonmigraine controls, and migraineurs experience vestibular stimulation as more unpleasant and more likely to cause emesis, compared with nonmigraine controls, Dr. Levin said.
The reasons for these correlations are unknown. It may be that heightened vestibular sensitivity in migraine is due to migraineurs perceiving all stimuli more intensely, or migraineurs may be more keenly aware of early signs of vestibulopathy. Vertigo may be a migraine trigger, or a subset of patients may have a type of migraine that includes vertigo as a key symptom, he said. This last possibility is the so-called vestibular migraine.
Recognizing Vestibular Migraine
Vestibular migraine, which also has been known as migraine-associated vertigo, migraine-associated dizziness, and migraine-associated vestibulopathy, has been difficult to define. The current generally accepted definition requires two basic diagnostic criteria: current or previous history of migraine and migraine features (eg, headache, photophobia, phonophobia, or visual aura) with at least half of the spells of vertigo.
Vestibular migraine is estimated to affect about 1% of the general population, 7% of patients at dizziness clinics, and 9% of patients at headache centers.
The duration of vertigo in vestibular migraine varies. About a third of the episodes last for minutes, a third for hours, and a third for days. Vertigo can occur between migraine attacks, prior to them, during, or after, and it tends to be spontaneous. Vestibular migraine is common in children and more common in women than in men. It generally arises years after migraines begin.
Unsteadiness and balance problems are common in vestibular migraine, and audiologic disturbances occur in a minority of patients. Migraine with brainstem aura (formerly called basilar migraine) can include vertigo, but the diagnosis also requires at least one other brainstem symptom (eg, tinnitus or dysarthria).
Evaluating Patients
When seeing patients, neurologists’ first step might be to try to distinguish between vertigo and other similar symptoms, such as presyncope, disorientation, or disequilibrium. “A sense of motion is the best indication of vertigo, though even that might be lacking,” Dr. Levin said.
Neurologists can determine whether position triggers vertigo and identify evidence of peripheral biologic problems (eg, tinnitus, changes in vision, or other focal neurologic signs and symptoms). Family history of migraine in people with episodic vertigo may be a clue that the patient has vestibular migraine versus other causes of vertigo, Dr. Levin said. A history of syncope or other signs may suggest that a patient’s symptoms are related to light-headedness instead of vertigo. Psychiatric illness, time course, drug exposure, and stroke or stroke risk factors also should be considered.
Diagnostic tests may help neurologists distinguish between vestibular migraine and other causes of vertigo. Audiograms can assess for hearing loss, and MRIs may rule out masses or other lesions. Brainstem auditory evoked responses, electronystagmography (ENG), and videonystagmography (VNG), which typically includes saccade, tracking, positional, and caloric testing, also can be useful.
Similar Conditions
One diagnostic entity that can be mistaken for vestibular migraine is mal de debarquement, which is marked by a persistent feeling of vertigo after a cruise or other motion experience. Patients with this condition also may experience symptoms such as blurred vision, inability to focus, cognitive changes, headaches, nausea, feelings of pressure, and trouble sleeping. “It can actually start sounding like migraine,” Dr. Levin said. “Strangely enough, patients may not mention their disembarkation from a trip. …You have to sometimes draw it out.”
Vestibular testing is normal in these patients, and oddly, they often feel better when they ride in a car or otherwise experience motion. Migraine treatment does not work for these patients. Benzodiazepines may help, but patients may become tolerant. Mal de debarquement tends to dampen and resolve in many patients.
Other causes of vertigo include Meniere’s disease, benign paroxysmal positional vertigo, meningeal infection or inflammation, labyrinthine or brainstem ischemia, perilymph fistula, and benign positional vertigo of childhood.
In the end, some diagnostic entities may be part of a spectrum, Dr. Levin said. Thirty-eight percent of vestibular migraines have auditory symptoms as in Meniere’s disease, and the prevalence of migraine in patients with Meniere’s disease is twice that of the general population. Many patients fit diagnostic criteria for vestibular migraine and Meniere’s disease.
The pathophysiology of vestibular migraine is unknown. Connections between vestibular nuclei in the brainstem and the trigeminal nuclei may underlie the condition. Vestibular and trigeminal nociceptive pathways may be activated in parallel. Alternatively, structural brain lesions in the temporal lobes or elsewhere may cause vestibular migraine.
Like other migraine auras, vestibular migraine may be a manifestation of focal or generalized cortical spreading depression. “There are cortical centers for vertigo,” Dr. Levin said. When these cortical centers are affected in patients with epilepsy, patients may experience “tornado seizures,” he said.
Treatment Approaches
Some studies suggest that migraine treatments might help patients with vestibular migraine. Zolmitriptan and rizatriptan at the time of vertigo have been tried, with some suggestion that they may provide benefit.
The best evidence for pharmacologic prevention exists for flunarizine, propranolol, and lamotrigine. Other trials suggest that vestibular rehabilitation and combined caffeine cessation, nortriptyline, and topiramate may be effective.
Limitations of trials in vestibular migraine have included small numbers of patients, noncontrolled designs, and inconsistent definitions of vestibular migraine. In addition, case reports have suggested that benzodiazepines, cinnarizine, selective serotonin reuptake inhibitors, pizotifen, dothiepin, acetazolamide, and behavioral modification may benefit patients. Investigators are enrolling patients in a double-blind, placebo-controlled trial that will evaluate the use of metoprolol for the preventive treatment of vestibular migraine.
If occurrences of vertigo are infrequent, symptomatic vertigo treatments are Dr. Levin’s first choice. “I have had good luck with scopolamine, for example,” he said. Dopamine antagonists, neuroleptics, sedatives, and benzodiazepines are also useful symptomatic treatments for vertigo. The Epley maneuver and other canalith repositioning maneuvers may benefit some patients. For acute treatment, it makes sense to try a triptan, Dr. Levin said. “Sometimes it does work. Other times it does not, and you have to resort to symptomatic medication,” he said.
—Jake Remaly
Suggested Reading
Akdal G, Ozge A, Ergör G. The prevalence of vestibular symptoms in migraine or tension-type headache. J Vestib Res. 2013;23(2):101-106.
Dieterich M, Brandt T. Episodic vertigo related to migraine (90 cases): vestibular migraine? J Neurol. 1999;246(10):883-892.
Dieterich M, Obermann M, Celebisoy N. Vestibular migraine: the most frequent entity of episodic vertigo. J Neurol. 2016;263 Suppl 1:S82-89.
Furman JM, Marcus DA, Balaban CD. Vestibular migraine: clinical aspects and pathophysiology. Lancet Neurol. 2013;12(7):706-715.
Lepcha A, Amalanathan S, Augustine AM, et al. Flunarizine in the prophylaxis of migrainous vertigo: a randomized controlled trial. Eur Arch Otorhinolaryngol. 2014;271(11):2931-2936.
Mikulec AA, Faraji F, Kinsella LJ. Evaluation of the efficacy of caffeine cessation, nortriptyline, and topiramate therapy in vestibular migraine and complex dizziness of unknown etiology. Am J Otolaryngol. 2012;33(1):121-127.
Murdin L, Davies RA, Bronstein AM. Vertigo as a migraine trigger. Neurology. 2009;73(8):638-642.
Neuhauser HK, Radtke A, von Brevern M, et al. Migrainous vertigo: prevalence and impact on quality of life. Neurology. 2006;67(6):1028-1033.
Salviz M, Yuce T, Acar H, et al. Propranolol and venlafaxine for vestibular migraine prophylaxis: A randomized controlled trial. Laryngoscope. 2016;126(1):169-174.
Van Ombergen A, Van Rompaey V, Van de Heyning P, Wuyts F. Vestibular migraine in an otolaryngology clinic: prevalence, associated symptoms, and prophylactic medication effectiveness. Otol Neurotol. 2015;36(1):133-138.
Vitkovic J, Winoto A, Rance G, et al. Vestibular rehabilitation outcomes in patients with and without vestibular migraine. J Neurol. 2013;260(12):3039-3048.
Vertigo is a normal response to certain stimuli, such as looking down from heights and abnormal head movements. In addition, any dysfunction along the pathway that processes balance and gravity information (eg, the semicircular canals, acoustic nerve, and brainstem vestibular centers) can cause vertigo.
Migraine is significantly more common in patients with vertigo, and vertigo is significantly more common in migraineurs than in the general population. In addition, migraineurs are predisposed to motion sickness, which often includes vertigo. Migraineurs’ vestibular systems are more sensitive to stimuli than those of nonmigraine controls, and migraineurs experience vestibular stimulation as more unpleasant and more likely to cause emesis, compared with nonmigraine controls, Dr. Levin said.
The reasons for these correlations are unknown. It may be that heightened vestibular sensitivity in migraine is due to migraineurs perceiving all stimuli more intensely, or migraineurs may be more keenly aware of early signs of vestibulopathy. Vertigo may be a migraine trigger, or a subset of patients may have a type of migraine that includes vertigo as a key symptom, he said. This last possibility is the so-called vestibular migraine.
Recognizing Vestibular Migraine
Vestibular migraine, which also has been known as migraine-associated vertigo, migraine-associated dizziness, and migraine-associated vestibulopathy, has been difficult to define. The current generally accepted definition requires two basic diagnostic criteria: current or previous history of migraine and migraine features (eg, headache, photophobia, phonophobia, or visual aura) with at least half of the spells of vertigo.
Vestibular migraine is estimated to affect about 1% of the general population, 7% of patients at dizziness clinics, and 9% of patients at headache centers.
The duration of vertigo in vestibular migraine varies. About a third of the episodes last for minutes, a third for hours, and a third for days. Vertigo can occur between migraine attacks, prior to them, during, or after, and it tends to be spontaneous. Vestibular migraine is common in children and more common in women than in men. It generally arises years after migraines begin.
Unsteadiness and balance problems are common in vestibular migraine, and audiologic disturbances occur in a minority of patients. Migraine with brainstem aura (formerly called basilar migraine) can include vertigo, but the diagnosis also requires at least one other brainstem symptom (eg, tinnitus or dysarthria).
Evaluating Patients
When seeing patients, neurologists’ first step might be to try to distinguish between vertigo and other similar symptoms, such as presyncope, disorientation, or disequilibrium. “A sense of motion is the best indication of vertigo, though even that might be lacking,” Dr. Levin said.
Neurologists can determine whether position triggers vertigo and identify evidence of peripheral biologic problems (eg, tinnitus, changes in vision, or other focal neurologic signs and symptoms). Family history of migraine in people with episodic vertigo may be a clue that the patient has vestibular migraine versus other causes of vertigo, Dr. Levin said. A history of syncope or other signs may suggest that a patient’s symptoms are related to light-headedness instead of vertigo. Psychiatric illness, time course, drug exposure, and stroke or stroke risk factors also should be considered.
Diagnostic tests may help neurologists distinguish between vestibular migraine and other causes of vertigo. Audiograms can assess for hearing loss, and MRIs may rule out masses or other lesions. Brainstem auditory evoked responses, electronystagmography (ENG), and videonystagmography (VNG), which typically includes saccade, tracking, positional, and caloric testing, also can be useful.
Similar Conditions
One diagnostic entity that can be mistaken for vestibular migraine is mal de debarquement, which is marked by a persistent feeling of vertigo after a cruise or other motion experience. Patients with this condition also may experience symptoms such as blurred vision, inability to focus, cognitive changes, headaches, nausea, feelings of pressure, and trouble sleeping. “It can actually start sounding like migraine,” Dr. Levin said. “Strangely enough, patients may not mention their disembarkation from a trip. …You have to sometimes draw it out.”
Vestibular testing is normal in these patients, and oddly, they often feel better when they ride in a car or otherwise experience motion. Migraine treatment does not work for these patients. Benzodiazepines may help, but patients may become tolerant. Mal de debarquement tends to dampen and resolve in many patients.
Other causes of vertigo include Meniere’s disease, benign paroxysmal positional vertigo, meningeal infection or inflammation, labyrinthine or brainstem ischemia, perilymph fistula, and benign positional vertigo of childhood.
In the end, some diagnostic entities may be part of a spectrum, Dr. Levin said. Thirty-eight percent of vestibular migraines have auditory symptoms as in Meniere’s disease, and the prevalence of migraine in patients with Meniere’s disease is twice that of the general population. Many patients fit diagnostic criteria for vestibular migraine and Meniere’s disease.
The pathophysiology of vestibular migraine is unknown. Connections between vestibular nuclei in the brainstem and the trigeminal nuclei may underlie the condition. Vestibular and trigeminal nociceptive pathways may be activated in parallel. Alternatively, structural brain lesions in the temporal lobes or elsewhere may cause vestibular migraine.
Like other migraine auras, vestibular migraine may be a manifestation of focal or generalized cortical spreading depression. “There are cortical centers for vertigo,” Dr. Levin said. When these cortical centers are affected in patients with epilepsy, patients may experience “tornado seizures,” he said.
Treatment Approaches
Some studies suggest that migraine treatments might help patients with vestibular migraine. Zolmitriptan and rizatriptan at the time of vertigo have been tried, with some suggestion that they may provide benefit.
The best evidence for pharmacologic prevention exists for flunarizine, propranolol, and lamotrigine. Other trials suggest that vestibular rehabilitation and combined caffeine cessation, nortriptyline, and topiramate may be effective.
Limitations of trials in vestibular migraine have included small numbers of patients, noncontrolled designs, and inconsistent definitions of vestibular migraine. In addition, case reports have suggested that benzodiazepines, cinnarizine, selective serotonin reuptake inhibitors, pizotifen, dothiepin, acetazolamide, and behavioral modification may benefit patients. Investigators are enrolling patients in a double-blind, placebo-controlled trial that will evaluate the use of metoprolol for the preventive treatment of vestibular migraine.
If occurrences of vertigo are infrequent, symptomatic vertigo treatments are Dr. Levin’s first choice. “I have had good luck with scopolamine, for example,” he said. Dopamine antagonists, neuroleptics, sedatives, and benzodiazepines are also useful symptomatic treatments for vertigo. The Epley maneuver and other canalith repositioning maneuvers may benefit some patients. For acute treatment, it makes sense to try a triptan, Dr. Levin said. “Sometimes it does work. Other times it does not, and you have to resort to symptomatic medication,” he said.
—Jake Remaly
Suggested Reading
Akdal G, Ozge A, Ergör G. The prevalence of vestibular symptoms in migraine or tension-type headache. J Vestib Res. 2013;23(2):101-106.
Dieterich M, Brandt T. Episodic vertigo related to migraine (90 cases): vestibular migraine? J Neurol. 1999;246(10):883-892.
Dieterich M, Obermann M, Celebisoy N. Vestibular migraine: the most frequent entity of episodic vertigo. J Neurol. 2016;263 Suppl 1:S82-89.
Furman JM, Marcus DA, Balaban CD. Vestibular migraine: clinical aspects and pathophysiology. Lancet Neurol. 2013;12(7):706-715.
Lepcha A, Amalanathan S, Augustine AM, et al. Flunarizine in the prophylaxis of migrainous vertigo: a randomized controlled trial. Eur Arch Otorhinolaryngol. 2014;271(11):2931-2936.
Mikulec AA, Faraji F, Kinsella LJ. Evaluation of the efficacy of caffeine cessation, nortriptyline, and topiramate therapy in vestibular migraine and complex dizziness of unknown etiology. Am J Otolaryngol. 2012;33(1):121-127.
Murdin L, Davies RA, Bronstein AM. Vertigo as a migraine trigger. Neurology. 2009;73(8):638-642.
Neuhauser HK, Radtke A, von Brevern M, et al. Migrainous vertigo: prevalence and impact on quality of life. Neurology. 2006;67(6):1028-1033.
Salviz M, Yuce T, Acar H, et al. Propranolol and venlafaxine for vestibular migraine prophylaxis: A randomized controlled trial. Laryngoscope. 2016;126(1):169-174.
Van Ombergen A, Van Rompaey V, Van de Heyning P, Wuyts F. Vestibular migraine in an otolaryngology clinic: prevalence, associated symptoms, and prophylactic medication effectiveness. Otol Neurotol. 2015;36(1):133-138.
Vitkovic J, Winoto A, Rance G, et al. Vestibular rehabilitation outcomes in patients with and without vestibular migraine. J Neurol. 2013;260(12):3039-3048.
Vertigo is a normal response to certain stimuli, such as looking down from heights and abnormal head movements. In addition, any dysfunction along the pathway that processes balance and gravity information (eg, the semicircular canals, acoustic nerve, and brainstem vestibular centers) can cause vertigo.
Migraine is significantly more common in patients with vertigo, and vertigo is significantly more common in migraineurs than in the general population. In addition, migraineurs are predisposed to motion sickness, which often includes vertigo. Migraineurs’ vestibular systems are more sensitive to stimuli than those of nonmigraine controls, and migraineurs experience vestibular stimulation as more unpleasant and more likely to cause emesis, compared with nonmigraine controls, Dr. Levin said.
The reasons for these correlations are unknown. It may be that heightened vestibular sensitivity in migraine is due to migraineurs perceiving all stimuli more intensely, or migraineurs may be more keenly aware of early signs of vestibulopathy. Vertigo may be a migraine trigger, or a subset of patients may have a type of migraine that includes vertigo as a key symptom, he said. This last possibility is the so-called vestibular migraine.
Recognizing Vestibular Migraine
Vestibular migraine, which also has been known as migraine-associated vertigo, migraine-associated dizziness, and migraine-associated vestibulopathy, has been difficult to define. The current generally accepted definition requires two basic diagnostic criteria: current or previous history of migraine and migraine features (eg, headache, photophobia, phonophobia, or visual aura) with at least half of the spells of vertigo.
Vestibular migraine is estimated to affect about 1% of the general population, 7% of patients at dizziness clinics, and 9% of patients at headache centers.
The duration of vertigo in vestibular migraine varies. About a third of the episodes last for minutes, a third for hours, and a third for days. Vertigo can occur between migraine attacks, prior to them, during, or after, and it tends to be spontaneous. Vestibular migraine is common in children and more common in women than in men. It generally arises years after migraines begin.
Unsteadiness and balance problems are common in vestibular migraine, and audiologic disturbances occur in a minority of patients. Migraine with brainstem aura (formerly called basilar migraine) can include vertigo, but the diagnosis also requires at least one other brainstem symptom (eg, tinnitus or dysarthria).
Evaluating Patients
When seeing patients, neurologists’ first step might be to try to distinguish between vertigo and other similar symptoms, such as presyncope, disorientation, or disequilibrium. “A sense of motion is the best indication of vertigo, though even that might be lacking,” Dr. Levin said.
Neurologists can determine whether position triggers vertigo and identify evidence of peripheral biologic problems (eg, tinnitus, changes in vision, or other focal neurologic signs and symptoms). Family history of migraine in people with episodic vertigo may be a clue that the patient has vestibular migraine versus other causes of vertigo, Dr. Levin said. A history of syncope or other signs may suggest that a patient’s symptoms are related to light-headedness instead of vertigo. Psychiatric illness, time course, drug exposure, and stroke or stroke risk factors also should be considered.
Diagnostic tests may help neurologists distinguish between vestibular migraine and other causes of vertigo. Audiograms can assess for hearing loss, and MRIs may rule out masses or other lesions. Brainstem auditory evoked responses, electronystagmography (ENG), and videonystagmography (VNG), which typically includes saccade, tracking, positional, and caloric testing, also can be useful.
Similar Conditions
One diagnostic entity that can be mistaken for vestibular migraine is mal de debarquement, which is marked by a persistent feeling of vertigo after a cruise or other motion experience. Patients with this condition also may experience symptoms such as blurred vision, inability to focus, cognitive changes, headaches, nausea, feelings of pressure, and trouble sleeping. “It can actually start sounding like migraine,” Dr. Levin said. “Strangely enough, patients may not mention their disembarkation from a trip. …You have to sometimes draw it out.”
Vestibular testing is normal in these patients, and oddly, they often feel better when they ride in a car or otherwise experience motion. Migraine treatment does not work for these patients. Benzodiazepines may help, but patients may become tolerant. Mal de debarquement tends to dampen and resolve in many patients.
Other causes of vertigo include Meniere’s disease, benign paroxysmal positional vertigo, meningeal infection or inflammation, labyrinthine or brainstem ischemia, perilymph fistula, and benign positional vertigo of childhood.
In the end, some diagnostic entities may be part of a spectrum, Dr. Levin said. Thirty-eight percent of vestibular migraines have auditory symptoms as in Meniere’s disease, and the prevalence of migraine in patients with Meniere’s disease is twice that of the general population. Many patients fit diagnostic criteria for vestibular migraine and Meniere’s disease.
The pathophysiology of vestibular migraine is unknown. Connections between vestibular nuclei in the brainstem and the trigeminal nuclei may underlie the condition. Vestibular and trigeminal nociceptive pathways may be activated in parallel. Alternatively, structural brain lesions in the temporal lobes or elsewhere may cause vestibular migraine.
Like other migraine auras, vestibular migraine may be a manifestation of focal or generalized cortical spreading depression. “There are cortical centers for vertigo,” Dr. Levin said. When these cortical centers are affected in patients with epilepsy, patients may experience “tornado seizures,” he said.
Treatment Approaches
Some studies suggest that migraine treatments might help patients with vestibular migraine. Zolmitriptan and rizatriptan at the time of vertigo have been tried, with some suggestion that they may provide benefit.
The best evidence for pharmacologic prevention exists for flunarizine, propranolol, and lamotrigine. Other trials suggest that vestibular rehabilitation and combined caffeine cessation, nortriptyline, and topiramate may be effective.
Limitations of trials in vestibular migraine have included small numbers of patients, noncontrolled designs, and inconsistent definitions of vestibular migraine. In addition, case reports have suggested that benzodiazepines, cinnarizine, selective serotonin reuptake inhibitors, pizotifen, dothiepin, acetazolamide, and behavioral modification may benefit patients. Investigators are enrolling patients in a double-blind, placebo-controlled trial that will evaluate the use of metoprolol for the preventive treatment of vestibular migraine.
If occurrences of vertigo are infrequent, symptomatic vertigo treatments are Dr. Levin’s first choice. “I have had good luck with scopolamine, for example,” he said. Dopamine antagonists, neuroleptics, sedatives, and benzodiazepines are also useful symptomatic treatments for vertigo. The Epley maneuver and other canalith repositioning maneuvers may benefit some patients. For acute treatment, it makes sense to try a triptan, Dr. Levin said. “Sometimes it does work. Other times it does not, and you have to resort to symptomatic medication,” he said.
—Jake Remaly
Suggested Reading
Akdal G, Ozge A, Ergör G. The prevalence of vestibular symptoms in migraine or tension-type headache. J Vestib Res. 2013;23(2):101-106.
Dieterich M, Brandt T. Episodic vertigo related to migraine (90 cases): vestibular migraine? J Neurol. 1999;246(10):883-892.
Dieterich M, Obermann M, Celebisoy N. Vestibular migraine: the most frequent entity of episodic vertigo. J Neurol. 2016;263 Suppl 1:S82-89.
Furman JM, Marcus DA, Balaban CD. Vestibular migraine: clinical aspects and pathophysiology. Lancet Neurol. 2013;12(7):706-715.
Lepcha A, Amalanathan S, Augustine AM, et al. Flunarizine in the prophylaxis of migrainous vertigo: a randomized controlled trial. Eur Arch Otorhinolaryngol. 2014;271(11):2931-2936.
Mikulec AA, Faraji F, Kinsella LJ. Evaluation of the efficacy of caffeine cessation, nortriptyline, and topiramate therapy in vestibular migraine and complex dizziness of unknown etiology. Am J Otolaryngol. 2012;33(1):121-127.
Murdin L, Davies RA, Bronstein AM. Vertigo as a migraine trigger. Neurology. 2009;73(8):638-642.
Neuhauser HK, Radtke A, von Brevern M, et al. Migrainous vertigo: prevalence and impact on quality of life. Neurology. 2006;67(6):1028-1033.
Salviz M, Yuce T, Acar H, et al. Propranolol and venlafaxine for vestibular migraine prophylaxis: A randomized controlled trial. Laryngoscope. 2016;126(1):169-174.
Van Ombergen A, Van Rompaey V, Van de Heyning P, Wuyts F. Vestibular migraine in an otolaryngology clinic: prevalence, associated symptoms, and prophylactic medication effectiveness. Otol Neurotol. 2015;36(1):133-138.
Vitkovic J, Winoto A, Rance G, et al. Vestibular rehabilitation outcomes in patients with and without vestibular migraine. J Neurol. 2013;260(12):3039-3048.
Teen indoor tanning drops, but schools fall short on sun safety
Indoor tanning among adolescents in the United States has dropped significantly, but fewer than half of schools in the United States reported sun safety practices to help minimize students’ UV exposure in the school setting, based on data from two studies presented at the annual meeting of the American Academy of Dermatology and published simultaneously in JAMA Dermatology.
“Data suggest that intermittent, recreational exposure (vs. chronic exposure, as with outdoor workers) more often leads to sunburn,” wrote Sherry Everett Jones, PhD, MPH, and Gery P. Guy Jr, PhD, MPH, of the Centers for Disease Control and Prevention. “Although a small proportion of school districts and schools have adopted policies to address sun safety, most have not, even though it is common for students to be outside during the midday hours or after school when the sun is still at peak intensity.”
To characterize sun safety practices at schools, the researchers reviewed data from the 2014 School Health Policies and Practices Study Healthy and Safe School Environment questionnaire including 577 elementary, middle, and high schools (JAMA Dermatol. 2017. doi: 10.1001/jamadermatol.2016.6274).
Overall, 48% of schools reported that teachers allowed students time to apply sunscreen at school (the most frequent sun safety practice). However, only 13% made sunscreen available, 16% asked parents to ensure sunscreen application before school, and 15% made an effort to avoid scheduling outdoor activities during times of peak sun intensity. High schools were less likely than elementary or middle schools to follow sun safety practices.
“None of the sun safety policies or practices were statistically significantly associated with metropolitan status,” the researchers noted. However, the findings were limited by the cross-sectional nature of the study and lack of data about natural shade and man made shade structures in outdoor areas of the schools.
“Interventions driven by the public health and medical community educating school leadership and policy makers about the importance of sun safety are needed regardless of level, location, size, and poverty concentration of the school. These efforts could be instrumental in increasing the adoption of sun safety practices among schools,” Dr. Jones and Dr. Guy emphasized.
However, data from another study showed a significant reduction in the prevalence of indoor tanning among adolescents.
In particular, indoor tanning among non-Hispanic white females (the group at highest risk for skin cancer) dropped from 37% in 2009 to 15% in 2015. CDC researchers led by Dr. Guy pooled data from the 2009, 2011, 2013, and 2015 national Youth Risk Behavior Surveillance System Surveys (JAMA Dermatol. 2017. doi:10.1001/jamadermatol.2016.6273). Overall, the prevalence of indoor tanning among U.S. high school students decreased from 16% in 2009 to 7% in 2015.
“Despite declines in indoor tanning, continued efforts are needed,” the researchers wrote. “Public health efforts could help address the misconception that indoor tanning protects against sunburn. The medical community also can play a key role in counseling adolescents and young adults in accordance with the U.S. Preventive Services Task Force guidelines.”
The findings were limited by several factors including the use of self-reports and the inability to control for skin type, the researchers wrote. However, “Reducing the proportion of youth who engage in indoor tanning and experience sunburns presents an important cancer prevention opportunity.”
None of the researchers on either study had relevant financial conflicts to disclose.
Explore best practices for public education campaigns “For more than 10 years, much effort has been made to educate the public on sun-safety practices, including warnings about the harmful effects of indoor tanning on those at higher risk, such as young adults and children. In this issue of JAMA Dermatology, 2 important articles describe the progress made toward sun safety,” wrote Henry W. Lim, MD, and Samantha L. Schneider, MD, in the accompanying editorial.
Data from a study of indoor tanning showed a significant decrease in prevalence of indoor tanning among adolescents, from 16% in 2009 to 7% in 2015. Although these results are encouraging, public education is needed for further improvement, they said. “One myth is that UV radiation prevents vitamin D deficiency; however, oral vitamin D supplementation is known to be a safer alternative. Another myth is that obtaining a baseline tan before the summer or a vacation reduces the risk of sunburn. However, as Guy and colleagues observed, those who tanned indoors were more likely to develop sunburn than students who did not engage in indoor tanning.”
“Clearly, both the dermatology and medical communities need to continue public awareness campaigns regarding photoprotection, including sun-safety practices such as seeking shade when outdoors and wearing photoprotective clothing, wide-brimmed hats, and sunglasses,” they emphasized.
In addition, “A highly effective means of public education may be to identify a campaign, such as Portugal’s sugar packet initiative, that makes sun-safety awareness and practice a part of everyone’s daily routine,” they said (JAMA Dermatol. 2017. doi: 10.1001/jamadermatol.2016.6272).
Dr. Lim and Dr. Schneider are affiliated with the department of dermatology at Henry Ford Hospital in Detroit. Dr Lim disclosed serving as an investigator or coinvestigator on clinical research projects for Ferndale Pharma, Estée Lauder, and Allergan. Dr. Schneider had no relevant conflicts to disclose.
Explore best practices for public education campaigns “For more than 10 years, much effort has been made to educate the public on sun-safety practices, including warnings about the harmful effects of indoor tanning on those at higher risk, such as young adults and children. In this issue of JAMA Dermatology, 2 important articles describe the progress made toward sun safety,” wrote Henry W. Lim, MD, and Samantha L. Schneider, MD, in the accompanying editorial.
Data from a study of indoor tanning showed a significant decrease in prevalence of indoor tanning among adolescents, from 16% in 2009 to 7% in 2015. Although these results are encouraging, public education is needed for further improvement, they said. “One myth is that UV radiation prevents vitamin D deficiency; however, oral vitamin D supplementation is known to be a safer alternative. Another myth is that obtaining a baseline tan before the summer or a vacation reduces the risk of sunburn. However, as Guy and colleagues observed, those who tanned indoors were more likely to develop sunburn than students who did not engage in indoor tanning.”
“Clearly, both the dermatology and medical communities need to continue public awareness campaigns regarding photoprotection, including sun-safety practices such as seeking shade when outdoors and wearing photoprotective clothing, wide-brimmed hats, and sunglasses,” they emphasized.
In addition, “A highly effective means of public education may be to identify a campaign, such as Portugal’s sugar packet initiative, that makes sun-safety awareness and practice a part of everyone’s daily routine,” they said (JAMA Dermatol. 2017. doi: 10.1001/jamadermatol.2016.6272).
Dr. Lim and Dr. Schneider are affiliated with the department of dermatology at Henry Ford Hospital in Detroit. Dr Lim disclosed serving as an investigator or coinvestigator on clinical research projects for Ferndale Pharma, Estée Lauder, and Allergan. Dr. Schneider had no relevant conflicts to disclose.
Explore best practices for public education campaigns “For more than 10 years, much effort has been made to educate the public on sun-safety practices, including warnings about the harmful effects of indoor tanning on those at higher risk, such as young adults and children. In this issue of JAMA Dermatology, 2 important articles describe the progress made toward sun safety,” wrote Henry W. Lim, MD, and Samantha L. Schneider, MD, in the accompanying editorial.
Data from a study of indoor tanning showed a significant decrease in prevalence of indoor tanning among adolescents, from 16% in 2009 to 7% in 2015. Although these results are encouraging, public education is needed for further improvement, they said. “One myth is that UV radiation prevents vitamin D deficiency; however, oral vitamin D supplementation is known to be a safer alternative. Another myth is that obtaining a baseline tan before the summer or a vacation reduces the risk of sunburn. However, as Guy and colleagues observed, those who tanned indoors were more likely to develop sunburn than students who did not engage in indoor tanning.”
“Clearly, both the dermatology and medical communities need to continue public awareness campaigns regarding photoprotection, including sun-safety practices such as seeking shade when outdoors and wearing photoprotective clothing, wide-brimmed hats, and sunglasses,” they emphasized.
In addition, “A highly effective means of public education may be to identify a campaign, such as Portugal’s sugar packet initiative, that makes sun-safety awareness and practice a part of everyone’s daily routine,” they said (JAMA Dermatol. 2017. doi: 10.1001/jamadermatol.2016.6272).
Dr. Lim and Dr. Schneider are affiliated with the department of dermatology at Henry Ford Hospital in Detroit. Dr Lim disclosed serving as an investigator or coinvestigator on clinical research projects for Ferndale Pharma, Estée Lauder, and Allergan. Dr. Schneider had no relevant conflicts to disclose.
Indoor tanning among adolescents in the United States has dropped significantly, but fewer than half of schools in the United States reported sun safety practices to help minimize students’ UV exposure in the school setting, based on data from two studies presented at the annual meeting of the American Academy of Dermatology and published simultaneously in JAMA Dermatology.
“Data suggest that intermittent, recreational exposure (vs. chronic exposure, as with outdoor workers) more often leads to sunburn,” wrote Sherry Everett Jones, PhD, MPH, and Gery P. Guy Jr, PhD, MPH, of the Centers for Disease Control and Prevention. “Although a small proportion of school districts and schools have adopted policies to address sun safety, most have not, even though it is common for students to be outside during the midday hours or after school when the sun is still at peak intensity.”
To characterize sun safety practices at schools, the researchers reviewed data from the 2014 School Health Policies and Practices Study Healthy and Safe School Environment questionnaire including 577 elementary, middle, and high schools (JAMA Dermatol. 2017. doi: 10.1001/jamadermatol.2016.6274).
Overall, 48% of schools reported that teachers allowed students time to apply sunscreen at school (the most frequent sun safety practice). However, only 13% made sunscreen available, 16% asked parents to ensure sunscreen application before school, and 15% made an effort to avoid scheduling outdoor activities during times of peak sun intensity. High schools were less likely than elementary or middle schools to follow sun safety practices.
“None of the sun safety policies or practices were statistically significantly associated with metropolitan status,” the researchers noted. However, the findings were limited by the cross-sectional nature of the study and lack of data about natural shade and man made shade structures in outdoor areas of the schools.
“Interventions driven by the public health and medical community educating school leadership and policy makers about the importance of sun safety are needed regardless of level, location, size, and poverty concentration of the school. These efforts could be instrumental in increasing the adoption of sun safety practices among schools,” Dr. Jones and Dr. Guy emphasized.
However, data from another study showed a significant reduction in the prevalence of indoor tanning among adolescents.
In particular, indoor tanning among non-Hispanic white females (the group at highest risk for skin cancer) dropped from 37% in 2009 to 15% in 2015. CDC researchers led by Dr. Guy pooled data from the 2009, 2011, 2013, and 2015 national Youth Risk Behavior Surveillance System Surveys (JAMA Dermatol. 2017. doi:10.1001/jamadermatol.2016.6273). Overall, the prevalence of indoor tanning among U.S. high school students decreased from 16% in 2009 to 7% in 2015.
“Despite declines in indoor tanning, continued efforts are needed,” the researchers wrote. “Public health efforts could help address the misconception that indoor tanning protects against sunburn. The medical community also can play a key role in counseling adolescents and young adults in accordance with the U.S. Preventive Services Task Force guidelines.”
The findings were limited by several factors including the use of self-reports and the inability to control for skin type, the researchers wrote. However, “Reducing the proportion of youth who engage in indoor tanning and experience sunburns presents an important cancer prevention opportunity.”
None of the researchers on either study had relevant financial conflicts to disclose.
Indoor tanning among adolescents in the United States has dropped significantly, but fewer than half of schools in the United States reported sun safety practices to help minimize students’ UV exposure in the school setting, based on data from two studies presented at the annual meeting of the American Academy of Dermatology and published simultaneously in JAMA Dermatology.
“Data suggest that intermittent, recreational exposure (vs. chronic exposure, as with outdoor workers) more often leads to sunburn,” wrote Sherry Everett Jones, PhD, MPH, and Gery P. Guy Jr, PhD, MPH, of the Centers for Disease Control and Prevention. “Although a small proportion of school districts and schools have adopted policies to address sun safety, most have not, even though it is common for students to be outside during the midday hours or after school when the sun is still at peak intensity.”
To characterize sun safety practices at schools, the researchers reviewed data from the 2014 School Health Policies and Practices Study Healthy and Safe School Environment questionnaire including 577 elementary, middle, and high schools (JAMA Dermatol. 2017. doi: 10.1001/jamadermatol.2016.6274).
Overall, 48% of schools reported that teachers allowed students time to apply sunscreen at school (the most frequent sun safety practice). However, only 13% made sunscreen available, 16% asked parents to ensure sunscreen application before school, and 15% made an effort to avoid scheduling outdoor activities during times of peak sun intensity. High schools were less likely than elementary or middle schools to follow sun safety practices.
“None of the sun safety policies or practices were statistically significantly associated with metropolitan status,” the researchers noted. However, the findings were limited by the cross-sectional nature of the study and lack of data about natural shade and man made shade structures in outdoor areas of the schools.
“Interventions driven by the public health and medical community educating school leadership and policy makers about the importance of sun safety are needed regardless of level, location, size, and poverty concentration of the school. These efforts could be instrumental in increasing the adoption of sun safety practices among schools,” Dr. Jones and Dr. Guy emphasized.
However, data from another study showed a significant reduction in the prevalence of indoor tanning among adolescents.
In particular, indoor tanning among non-Hispanic white females (the group at highest risk for skin cancer) dropped from 37% in 2009 to 15% in 2015. CDC researchers led by Dr. Guy pooled data from the 2009, 2011, 2013, and 2015 national Youth Risk Behavior Surveillance System Surveys (JAMA Dermatol. 2017. doi:10.1001/jamadermatol.2016.6273). Overall, the prevalence of indoor tanning among U.S. high school students decreased from 16% in 2009 to 7% in 2015.
“Despite declines in indoor tanning, continued efforts are needed,” the researchers wrote. “Public health efforts could help address the misconception that indoor tanning protects against sunburn. The medical community also can play a key role in counseling adolescents and young adults in accordance with the U.S. Preventive Services Task Force guidelines.”
The findings were limited by several factors including the use of self-reports and the inability to control for skin type, the researchers wrote. However, “Reducing the proportion of youth who engage in indoor tanning and experience sunburns presents an important cancer prevention opportunity.”
None of the researchers on either study had relevant financial conflicts to disclose.
FROM AAD 2017
Key clinical point:
Major finding: Fewer than half (48%) of schools in the United States allowed time for sunscreen application, and fewer than 15% provided sunscreen. However, overall prevalence of indoor tanning among U.S. adolescents dropped from 16% in 2009 to 7% in 2015.
Data source: Data were taken from the 2014 School Health Policies and Practices Study in the first study and from the 2009, 2011, 2013, and 2015 national Youth Risk Behavior Surveys in the second.
Disclosures: The researchers had no financial conflicts to disclose.
Zika-infected pregnancies continue to rise in U.S.
Reports of new cases of Zika infection in pregnant women held steady during the 2 weeks ending Feb. 21 as the number of new cases dropped in the territories and rose in the 50 states and D.C., according to the Centers for Disease Control and Prevention.
Compared with the previous 2-week period (Jan. 25-Feb.7), reports of new cases of pregnant women with laboratory evidence of Zika virus infection were up from 146 to 148, an increase from 61 to 79 in the states/D.C. and a decrease from 85 to 69 in the territories. The total number of Zika cases among pregnant women in the United States for 2016-2017 is 4,759, with 1,534 occurring in the states/D.C. and 3,225 in the territories, the CDC reported March 2.
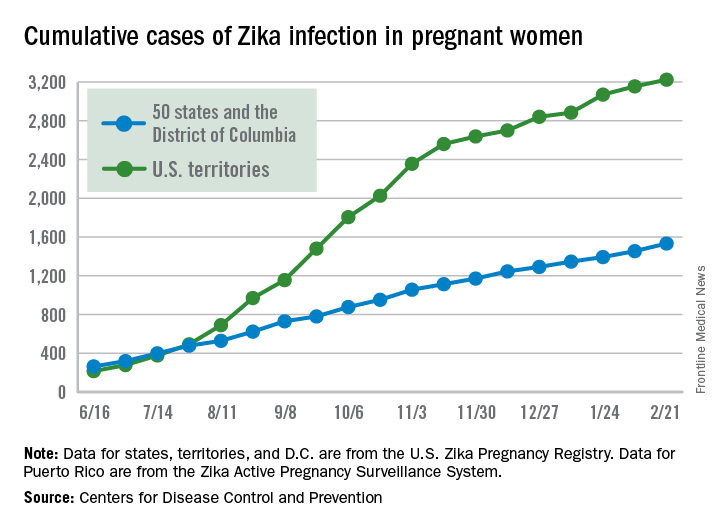
Among all Americans, the number of Zika cases reported is now up to 43,380 since Jan. 1, 2015, with 38,306 occurring in the territories and 5,074 in the states and D.C. The state with the most cases is Florida at 1,107, followed by New York at 1,007 and California with 431. Puerto Rico has reported 37,515 cases so far, and the U.S. Virgin Islands have reported 989, the CDC said.
The figures for states, territories, and the District of Columbia are reported to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System. These are not real-time data and reflect only pregnancy outcomes for women with any laboratory evidence of possible Zika virus infection, although it is not known if Zika virus was the cause of the poor outcomes.
Zika-related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, or termination with evidence of birth defects.
Reports of new cases of Zika infection in pregnant women held steady during the 2 weeks ending Feb. 21 as the number of new cases dropped in the territories and rose in the 50 states and D.C., according to the Centers for Disease Control and Prevention.
Compared with the previous 2-week period (Jan. 25-Feb.7), reports of new cases of pregnant women with laboratory evidence of Zika virus infection were up from 146 to 148, an increase from 61 to 79 in the states/D.C. and a decrease from 85 to 69 in the territories. The total number of Zika cases among pregnant women in the United States for 2016-2017 is 4,759, with 1,534 occurring in the states/D.C. and 3,225 in the territories, the CDC reported March 2.

Among all Americans, the number of Zika cases reported is now up to 43,380 since Jan. 1, 2015, with 38,306 occurring in the territories and 5,074 in the states and D.C. The state with the most cases is Florida at 1,107, followed by New York at 1,007 and California with 431. Puerto Rico has reported 37,515 cases so far, and the U.S. Virgin Islands have reported 989, the CDC said.
The figures for states, territories, and the District of Columbia are reported to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System. These are not real-time data and reflect only pregnancy outcomes for women with any laboratory evidence of possible Zika virus infection, although it is not known if Zika virus was the cause of the poor outcomes.
Zika-related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, or termination with evidence of birth defects.
Reports of new cases of Zika infection in pregnant women held steady during the 2 weeks ending Feb. 21 as the number of new cases dropped in the territories and rose in the 50 states and D.C., according to the Centers for Disease Control and Prevention.
Compared with the previous 2-week period (Jan. 25-Feb.7), reports of new cases of pregnant women with laboratory evidence of Zika virus infection were up from 146 to 148, an increase from 61 to 79 in the states/D.C. and a decrease from 85 to 69 in the territories. The total number of Zika cases among pregnant women in the United States for 2016-2017 is 4,759, with 1,534 occurring in the states/D.C. and 3,225 in the territories, the CDC reported March 2.

Among all Americans, the number of Zika cases reported is now up to 43,380 since Jan. 1, 2015, with 38,306 occurring in the territories and 5,074 in the states and D.C. The state with the most cases is Florida at 1,107, followed by New York at 1,007 and California with 431. Puerto Rico has reported 37,515 cases so far, and the U.S. Virgin Islands have reported 989, the CDC said.
The figures for states, territories, and the District of Columbia are reported to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System. These are not real-time data and reflect only pregnancy outcomes for women with any laboratory evidence of possible Zika virus infection, although it is not known if Zika virus was the cause of the poor outcomes.
Zika-related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, or termination with evidence of birth defects.
Birth defects in United States up 20-fold since Zika outbreak began
Birth defects potentially linked to cases of Zika virus in the United States have increased by a factor of nearly 20 since the virus first made its way into the country, according to new findings by the Centers for Disease Control and Prevention.
“The higher proportion of these defects among pregnancies with laboratory evidence of Zika infection in USZPR [U.S. Zika Pregnancy Registry] supports the relationship between congenital Zika virus infection and these birth defects,” wrote the authors of a new report led by Janet D. Cragan, MD, of the National Center on Birth Defects and Developmental Disabilities at the CDC (MMWR Morb Mortal Wkly Rep. 2017;66:219-22).
[[{"attributes":{},"fields":{}}]]
Dr. Cragan and her coauthors retrospectively examined data on birth defects in three regions of the country: Massachusetts during 2013, North Carolina during 2013, and Atlanta during 2013-2014. The investigators focused on birth defects associated with prenatal Zika virus infections, mainly brain abnormalities and microcephaly.
The rate of total birth defects across the three regions was 2.86 per 1,000 live births, with 747 infants and fetuses identified as having one or more defects. Microcephaly and brain abnormalities alone occurred at a rate of 1.50 per 1,000 live births, with eye abnormalities and central nervous system dysfunction also occurring.
These numbers are relatively low when compared with data from Jan. 15 through Sept. 22, 2016. The birth defect rate jumped up to 58.8 per 1,000 live births, according to data from the USZPR, which found evidence of 26 infants and fetuses with brain or cranial defects in 442 completed pregnancies. These infants were all born to mothers with laboratory-confirmed Zika virus infections.
“Among 410 (55%) infants or fetuses with information on the earliest age a birth defect was recorded, 371 (90%) had evidence of a birth defect meeting the Zika definition before age 3 months,” the authors explained. “More than half of those with brain abnormalities or microcephaly or with neural tube defects and other early brain malformations had evidence of these defects noted prenatally (55% and 89%, respectively).”
Dr. Cragan and her colleagues hope that this evidence will further solidify the link between Zika virus and birth defects and pave the way for more population-based studies.
“These data demonstrate the critical contribution of population-based birth defects surveillance to understanding the impact of Zika virus infection during pregnancy,” the authors concluded. “In 2016, CDC provided funding for 45 local, state, and territorial health departments to conduct rapid population-based surveillance for defects potentially related to Zika virus infection, which will provide essential data to monitor the impact of Zika virus infection in the United States.”
Birth defects potentially linked to cases of Zika virus in the United States have increased by a factor of nearly 20 since the virus first made its way into the country, according to new findings by the Centers for Disease Control and Prevention.
“The higher proportion of these defects among pregnancies with laboratory evidence of Zika infection in USZPR [U.S. Zika Pregnancy Registry] supports the relationship between congenital Zika virus infection and these birth defects,” wrote the authors of a new report led by Janet D. Cragan, MD, of the National Center on Birth Defects and Developmental Disabilities at the CDC (MMWR Morb Mortal Wkly Rep. 2017;66:219-22).
[[{"attributes":{},"fields":{}}]]
Dr. Cragan and her coauthors retrospectively examined data on birth defects in three regions of the country: Massachusetts during 2013, North Carolina during 2013, and Atlanta during 2013-2014. The investigators focused on birth defects associated with prenatal Zika virus infections, mainly brain abnormalities and microcephaly.
The rate of total birth defects across the three regions was 2.86 per 1,000 live births, with 747 infants and fetuses identified as having one or more defects. Microcephaly and brain abnormalities alone occurred at a rate of 1.50 per 1,000 live births, with eye abnormalities and central nervous system dysfunction also occurring.
These numbers are relatively low when compared with data from Jan. 15 through Sept. 22, 2016. The birth defect rate jumped up to 58.8 per 1,000 live births, according to data from the USZPR, which found evidence of 26 infants and fetuses with brain or cranial defects in 442 completed pregnancies. These infants were all born to mothers with laboratory-confirmed Zika virus infections.
“Among 410 (55%) infants or fetuses with information on the earliest age a birth defect was recorded, 371 (90%) had evidence of a birth defect meeting the Zika definition before age 3 months,” the authors explained. “More than half of those with brain abnormalities or microcephaly or with neural tube defects and other early brain malformations had evidence of these defects noted prenatally (55% and 89%, respectively).”
Dr. Cragan and her colleagues hope that this evidence will further solidify the link between Zika virus and birth defects and pave the way for more population-based studies.
“These data demonstrate the critical contribution of population-based birth defects surveillance to understanding the impact of Zika virus infection during pregnancy,” the authors concluded. “In 2016, CDC provided funding for 45 local, state, and territorial health departments to conduct rapid population-based surveillance for defects potentially related to Zika virus infection, which will provide essential data to monitor the impact of Zika virus infection in the United States.”
Birth defects potentially linked to cases of Zika virus in the United States have increased by a factor of nearly 20 since the virus first made its way into the country, according to new findings by the Centers for Disease Control and Prevention.
“The higher proportion of these defects among pregnancies with laboratory evidence of Zika infection in USZPR [U.S. Zika Pregnancy Registry] supports the relationship between congenital Zika virus infection and these birth defects,” wrote the authors of a new report led by Janet D. Cragan, MD, of the National Center on Birth Defects and Developmental Disabilities at the CDC (MMWR Morb Mortal Wkly Rep. 2017;66:219-22).
[[{"attributes":{},"fields":{}}]]
Dr. Cragan and her coauthors retrospectively examined data on birth defects in three regions of the country: Massachusetts during 2013, North Carolina during 2013, and Atlanta during 2013-2014. The investigators focused on birth defects associated with prenatal Zika virus infections, mainly brain abnormalities and microcephaly.
The rate of total birth defects across the three regions was 2.86 per 1,000 live births, with 747 infants and fetuses identified as having one or more defects. Microcephaly and brain abnormalities alone occurred at a rate of 1.50 per 1,000 live births, with eye abnormalities and central nervous system dysfunction also occurring.
These numbers are relatively low when compared with data from Jan. 15 through Sept. 22, 2016. The birth defect rate jumped up to 58.8 per 1,000 live births, according to data from the USZPR, which found evidence of 26 infants and fetuses with brain or cranial defects in 442 completed pregnancies. These infants were all born to mothers with laboratory-confirmed Zika virus infections.
“Among 410 (55%) infants or fetuses with information on the earliest age a birth defect was recorded, 371 (90%) had evidence of a birth defect meeting the Zika definition before age 3 months,” the authors explained. “More than half of those with brain abnormalities or microcephaly or with neural tube defects and other early brain malformations had evidence of these defects noted prenatally (55% and 89%, respectively).”
Dr. Cragan and her colleagues hope that this evidence will further solidify the link between Zika virus and birth defects and pave the way for more population-based studies.
“These data demonstrate the critical contribution of population-based birth defects surveillance to understanding the impact of Zika virus infection during pregnancy,” the authors concluded. “In 2016, CDC provided funding for 45 local, state, and territorial health departments to conduct rapid population-based surveillance for defects potentially related to Zika virus infection, which will provide essential data to monitor the impact of Zika virus infection in the United States.”
Chikungunya implicated in long-term joint disease
A majority of Chikungunya infections can cause arthritis and arthralgia months or years after the initial infection, based on data from a prospective study of 307 patients.
“The most common symptoms of Chikungunya virus infection are fever associated with rheumatic manifestations,” wrote rheumatologist Eric Bouquillard, MD, of Saint-Pierre, Reunion, France, and his colleagues.
Overall, 83% of the patients showed persistent joint pain after an average of 32 months. In addition, synovitis occurred in 64% of the patients who experienced chronic joint pain, mainly in the wrists, fingers, and ankles.
At baseline, the average number of painful joints was 6.5. At follow-up, the average number of painful joints was 3.3, and 43% of patients reported persistence of one or more swollen joints.
However, the patients reported little functional impairment; the average Health Assessment Questionnaire score was 0.44.
“RT-PCR [reverse transcription–polymerase chain reaction] was used in an attempt to detect the viral genome in synovial fluid samples from 10 patients, including 2 patients in the viremic phase, but the results were always negative,” the researchers noted.
Dr. Bouquillard and his colleagues enrolled the patients during April 2005-December 2006. Rheumatologic exams were conducted at baseline, and follow-up data were collected by phone surveys at 1 and 2 years after the onset of Chikungunya infection. Phone surveys were conducted by the Reunion Island Clinical Investigation Centre for Clinical Epidemiology, and interviewers also assessed patients for signs of anxiety, depression, and weakness.
The study was not designed to address treatment, but data from previous studies suggest that combination disease-modifying antirheumatic drug therapy may be more effective than hydroxychloroquine monotherapy for chronic joint pain post Chikungunya, the researchers noted.
The researchers had no financial conflicts to disclose. The study was supported in part by the Union Régionale des Médecins Libéraux de La Réunion.
A majority of Chikungunya infections can cause arthritis and arthralgia months or years after the initial infection, based on data from a prospective study of 307 patients.
“The most common symptoms of Chikungunya virus infection are fever associated with rheumatic manifestations,” wrote rheumatologist Eric Bouquillard, MD, of Saint-Pierre, Reunion, France, and his colleagues.
Overall, 83% of the patients showed persistent joint pain after an average of 32 months. In addition, synovitis occurred in 64% of the patients who experienced chronic joint pain, mainly in the wrists, fingers, and ankles.
At baseline, the average number of painful joints was 6.5. At follow-up, the average number of painful joints was 3.3, and 43% of patients reported persistence of one or more swollen joints.
However, the patients reported little functional impairment; the average Health Assessment Questionnaire score was 0.44.
“RT-PCR [reverse transcription–polymerase chain reaction] was used in an attempt to detect the viral genome in synovial fluid samples from 10 patients, including 2 patients in the viremic phase, but the results were always negative,” the researchers noted.
Dr. Bouquillard and his colleagues enrolled the patients during April 2005-December 2006. Rheumatologic exams were conducted at baseline, and follow-up data were collected by phone surveys at 1 and 2 years after the onset of Chikungunya infection. Phone surveys were conducted by the Reunion Island Clinical Investigation Centre for Clinical Epidemiology, and interviewers also assessed patients for signs of anxiety, depression, and weakness.
The study was not designed to address treatment, but data from previous studies suggest that combination disease-modifying antirheumatic drug therapy may be more effective than hydroxychloroquine monotherapy for chronic joint pain post Chikungunya, the researchers noted.
The researchers had no financial conflicts to disclose. The study was supported in part by the Union Régionale des Médecins Libéraux de La Réunion.
A majority of Chikungunya infections can cause arthritis and arthralgia months or years after the initial infection, based on data from a prospective study of 307 patients.
“The most common symptoms of Chikungunya virus infection are fever associated with rheumatic manifestations,” wrote rheumatologist Eric Bouquillard, MD, of Saint-Pierre, Reunion, France, and his colleagues.
Overall, 83% of the patients showed persistent joint pain after an average of 32 months. In addition, synovitis occurred in 64% of the patients who experienced chronic joint pain, mainly in the wrists, fingers, and ankles.
At baseline, the average number of painful joints was 6.5. At follow-up, the average number of painful joints was 3.3, and 43% of patients reported persistence of one or more swollen joints.
However, the patients reported little functional impairment; the average Health Assessment Questionnaire score was 0.44.
“RT-PCR [reverse transcription–polymerase chain reaction] was used in an attempt to detect the viral genome in synovial fluid samples from 10 patients, including 2 patients in the viremic phase, but the results were always negative,” the researchers noted.
Dr. Bouquillard and his colleagues enrolled the patients during April 2005-December 2006. Rheumatologic exams were conducted at baseline, and follow-up data were collected by phone surveys at 1 and 2 years after the onset of Chikungunya infection. Phone surveys were conducted by the Reunion Island Clinical Investigation Centre for Clinical Epidemiology, and interviewers also assessed patients for signs of anxiety, depression, and weakness.
The study was not designed to address treatment, but data from previous studies suggest that combination disease-modifying antirheumatic drug therapy may be more effective than hydroxychloroquine monotherapy for chronic joint pain post Chikungunya, the researchers noted.
The researchers had no financial conflicts to disclose. The study was supported in part by the Union Régionale des Médecins Libéraux de La Réunion.
FROM JOINT BONE SPINE
Key clinical point:
Major finding: Approximately 83% of adults with Chikungunya virus infections reported persistent joint pain after an average of 32 months.
Data source: A prospective, multicenter study of 307 adults with a history of Chikungunya virus infections.
Disclosures: The researchers had no financial conflicts to disclose. The study was supported in part by the Union Régionale des Médecins Libéraux de La Réunion.
Japan could benefit from hospital medicine expansion, leadership
The need for hospitalists continues to increase in Japan. There are approximately 9,000 hospitals in Japan, and approximately 80% of these hospitals are small- to medium-sized hospitals (<300 beds) where the need for hospital medicine is greatest. Historically, internal medicine subspecialists from nearly all subspecialties served as the primary attending physicians of hospitalized patients because inpatient internal medicine physicians, or hospitalists, did not exist.
Most subspecialists caring for hospitalized patients learned to practice internal medicine “on the fly” because they were not required to complete training in internal medicine before pursuing a subspecialty. After medical school, all graduates are required to complete a 2-year internship known as the National Obligatory Initial Postgraduate Clinical Training Program (NOIPCTP). The level of training during the NOIPCTP is similar to the third and fourth years of medical school in the United States.

The aging population and increasing complexity of hospitalized patients are the two main drivers of hospital medicine in Japan. Recently, the number of patients who have had adverse events because of inpatient medical errors has risen, and the transparency of these adverse events is making the need for hospitalists more apparent. In addition to improving the day-to-day medical management of hospitalized patients, hospitalists are needed to serve as champions of quality improvement, patient safety, and hospital throughput.
Leaders of the Japanese health care system recognize the need to improve the quality of inpatient care. The first step is to establish internal medicine as a specialty with dedicated internal medicine residency training programs. The Japanese Board of Medical Specialties approved establishing standardized, 3-year internal medicine residency training programs starting this spring, but that decision has been met with resistance for various reasons, namely concern for creating a disparity due to the shortage of internists in rural areas. Therefore, launch of this initiative has been postponed until April 2018.
In the meantime, the concept of hospital-based internists has been gradually gaining the support of subspecialists in Japan. Hospitalists are anticipated to work as the primary medical team leaders, directing and coordinating care among subspecialists in the future.
Despite its gradual spread, there are several challenges to growth. First, there are many terms for hospitalists, such as “hospital general practitioners” and “general internal medicine physicians.” A unified term for hospitalists would foster acceptance among Japanese physicians.
Additionally, some physicians, namely subspecialists, still question whether hospitalists are needed in Japan (even though potential loss of clinical revenue is not a significant concern among subspecialists).
Another challenge is lack of standardized training programs that define the skillset of hospitalists. Standardization of internal medicine training will also improve efficiency of communication between hospitalists and subspecialists.
An important milestone in the Japanese hospital medicine movement was the establishment of a society of hospitalists, known as the Japanese Hospitalist Network (JHN). The JHN has a quarterly publication (Hospitalist) targeted at junior faculty and residents that reviews topics in hospital medicine.
The JHN is affiliated with a larger society, the Japanese Society of Hospital General Medicine (JSHGM), which holds meetings twice a year. A unique offering at the next JSHGM meeting in March is a point-of-care ultrasound training workshop. Although this is the first such workshop for hospitalists in Japan, there are many training courses designed for the country’s hospitalists.
The emergence of such courses in Japan has paralleled the increasing need for hospitalists in Japan. We hope these courses for hospitalists will pave the road for the continued growth of hospital medicine in Japan.
Toru Yamada, MD
Dr. Yamada is an internist in the department of general medicine/family and community medicine at Nagoya (Japan) University and practices at Tokyo Bay Urayasu Ichikawa Medical Center in Chiba.
Taro Minami, MD
Dr. Minami is assistant professor of medicine in the division of pulmonary, critical care, and sleep medicine at Brown University in Providence, R.I., and director of ultrasound and simulation training at Memorial Hospital of Rhode Island.
Nilam J. Soni, MD, MS, FHM
Dr. Soni is associate professor of medicine in the division of hospital medicine at the University of Texas, San Antonio, and a hospitalist with the South Texas Veterans Health Care System in San Antonio.
The need for hospitalists continues to increase in Japan. There are approximately 9,000 hospitals in Japan, and approximately 80% of these hospitals are small- to medium-sized hospitals (<300 beds) where the need for hospital medicine is greatest. Historically, internal medicine subspecialists from nearly all subspecialties served as the primary attending physicians of hospitalized patients because inpatient internal medicine physicians, or hospitalists, did not exist.
Most subspecialists caring for hospitalized patients learned to practice internal medicine “on the fly” because they were not required to complete training in internal medicine before pursuing a subspecialty. After medical school, all graduates are required to complete a 2-year internship known as the National Obligatory Initial Postgraduate Clinical Training Program (NOIPCTP). The level of training during the NOIPCTP is similar to the third and fourth years of medical school in the United States.

The aging population and increasing complexity of hospitalized patients are the two main drivers of hospital medicine in Japan. Recently, the number of patients who have had adverse events because of inpatient medical errors has risen, and the transparency of these adverse events is making the need for hospitalists more apparent. In addition to improving the day-to-day medical management of hospitalized patients, hospitalists are needed to serve as champions of quality improvement, patient safety, and hospital throughput.
Leaders of the Japanese health care system recognize the need to improve the quality of inpatient care. The first step is to establish internal medicine as a specialty with dedicated internal medicine residency training programs. The Japanese Board of Medical Specialties approved establishing standardized, 3-year internal medicine residency training programs starting this spring, but that decision has been met with resistance for various reasons, namely concern for creating a disparity due to the shortage of internists in rural areas. Therefore, launch of this initiative has been postponed until April 2018.
In the meantime, the concept of hospital-based internists has been gradually gaining the support of subspecialists in Japan. Hospitalists are anticipated to work as the primary medical team leaders, directing and coordinating care among subspecialists in the future.
Despite its gradual spread, there are several challenges to growth. First, there are many terms for hospitalists, such as “hospital general practitioners” and “general internal medicine physicians.” A unified term for hospitalists would foster acceptance among Japanese physicians.
Additionally, some physicians, namely subspecialists, still question whether hospitalists are needed in Japan (even though potential loss of clinical revenue is not a significant concern among subspecialists).
Another challenge is lack of standardized training programs that define the skillset of hospitalists. Standardization of internal medicine training will also improve efficiency of communication between hospitalists and subspecialists.
An important milestone in the Japanese hospital medicine movement was the establishment of a society of hospitalists, known as the Japanese Hospitalist Network (JHN). The JHN has a quarterly publication (Hospitalist) targeted at junior faculty and residents that reviews topics in hospital medicine.
The JHN is affiliated with a larger society, the Japanese Society of Hospital General Medicine (JSHGM), which holds meetings twice a year. A unique offering at the next JSHGM meeting in March is a point-of-care ultrasound training workshop. Although this is the first such workshop for hospitalists in Japan, there are many training courses designed for the country’s hospitalists.
The emergence of such courses in Japan has paralleled the increasing need for hospitalists in Japan. We hope these courses for hospitalists will pave the road for the continued growth of hospital medicine in Japan.
Toru Yamada, MD
Dr. Yamada is an internist in the department of general medicine/family and community medicine at Nagoya (Japan) University and practices at Tokyo Bay Urayasu Ichikawa Medical Center in Chiba.
Taro Minami, MD
Dr. Minami is assistant professor of medicine in the division of pulmonary, critical care, and sleep medicine at Brown University in Providence, R.I., and director of ultrasound and simulation training at Memorial Hospital of Rhode Island.
Nilam J. Soni, MD, MS, FHM
Dr. Soni is associate professor of medicine in the division of hospital medicine at the University of Texas, San Antonio, and a hospitalist with the South Texas Veterans Health Care System in San Antonio.
The need for hospitalists continues to increase in Japan. There are approximately 9,000 hospitals in Japan, and approximately 80% of these hospitals are small- to medium-sized hospitals (<300 beds) where the need for hospital medicine is greatest. Historically, internal medicine subspecialists from nearly all subspecialties served as the primary attending physicians of hospitalized patients because inpatient internal medicine physicians, or hospitalists, did not exist.
Most subspecialists caring for hospitalized patients learned to practice internal medicine “on the fly” because they were not required to complete training in internal medicine before pursuing a subspecialty. After medical school, all graduates are required to complete a 2-year internship known as the National Obligatory Initial Postgraduate Clinical Training Program (NOIPCTP). The level of training during the NOIPCTP is similar to the third and fourth years of medical school in the United States.

The aging population and increasing complexity of hospitalized patients are the two main drivers of hospital medicine in Japan. Recently, the number of patients who have had adverse events because of inpatient medical errors has risen, and the transparency of these adverse events is making the need for hospitalists more apparent. In addition to improving the day-to-day medical management of hospitalized patients, hospitalists are needed to serve as champions of quality improvement, patient safety, and hospital throughput.
Leaders of the Japanese health care system recognize the need to improve the quality of inpatient care. The first step is to establish internal medicine as a specialty with dedicated internal medicine residency training programs. The Japanese Board of Medical Specialties approved establishing standardized, 3-year internal medicine residency training programs starting this spring, but that decision has been met with resistance for various reasons, namely concern for creating a disparity due to the shortage of internists in rural areas. Therefore, launch of this initiative has been postponed until April 2018.
In the meantime, the concept of hospital-based internists has been gradually gaining the support of subspecialists in Japan. Hospitalists are anticipated to work as the primary medical team leaders, directing and coordinating care among subspecialists in the future.
Despite its gradual spread, there are several challenges to growth. First, there are many terms for hospitalists, such as “hospital general practitioners” and “general internal medicine physicians.” A unified term for hospitalists would foster acceptance among Japanese physicians.
Additionally, some physicians, namely subspecialists, still question whether hospitalists are needed in Japan (even though potential loss of clinical revenue is not a significant concern among subspecialists).
Another challenge is lack of standardized training programs that define the skillset of hospitalists. Standardization of internal medicine training will also improve efficiency of communication between hospitalists and subspecialists.
An important milestone in the Japanese hospital medicine movement was the establishment of a society of hospitalists, known as the Japanese Hospitalist Network (JHN). The JHN has a quarterly publication (Hospitalist) targeted at junior faculty and residents that reviews topics in hospital medicine.
The JHN is affiliated with a larger society, the Japanese Society of Hospital General Medicine (JSHGM), which holds meetings twice a year. A unique offering at the next JSHGM meeting in March is a point-of-care ultrasound training workshop. Although this is the first such workshop for hospitalists in Japan, there are many training courses designed for the country’s hospitalists.
The emergence of such courses in Japan has paralleled the increasing need for hospitalists in Japan. We hope these courses for hospitalists will pave the road for the continued growth of hospital medicine in Japan.
Toru Yamada, MD
Dr. Yamada is an internist in the department of general medicine/family and community medicine at Nagoya (Japan) University and practices at Tokyo Bay Urayasu Ichikawa Medical Center in Chiba.
Taro Minami, MD
Dr. Minami is assistant professor of medicine in the division of pulmonary, critical care, and sleep medicine at Brown University in Providence, R.I., and director of ultrasound and simulation training at Memorial Hospital of Rhode Island.
Nilam J. Soni, MD, MS, FHM
Dr. Soni is associate professor of medicine in the division of hospital medicine at the University of Texas, San Antonio, and a hospitalist with the South Texas Veterans Health Care System in San Antonio.