User login
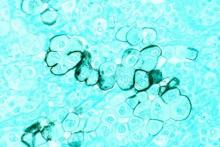
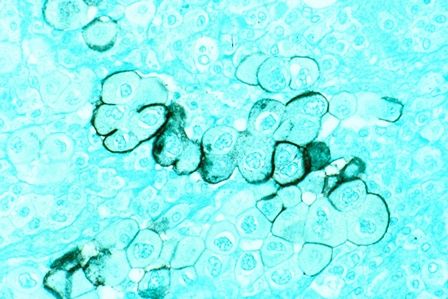
Biomarker identifies precancerous pancreatic cysts
LAS VEGAS – In fluid derived from pancreatic cysts, methylated DNA markers predict the presence of high-grade dysplasia (HGD) or cancer, and could help physicians decide whether to surgically remove cysts – a procedure that often has serious complications.
If validated in larger studies, the biomarkers have the potential to supplant the Fukuoka criteria that is currently used. “The markers could cause a paradigm shift in how we approach these lesions in our clinical practice,” Shounak Majumder, MD, a fellow at the Mayo Clinic in Rochester, Minn., said in an interview.
Less than 50% of cysts that are surgically resected turn out to be HGD or cancerous. “Having a cyst fluid marker could identify the patients that would benefit the most from surgery. If you’re going to go through a pancreatic resection, we’d rather give you the best chance of saying that we removed something that either has early cancer in it or will turn into cancer in the near future,” said Dr. Majumder.
The study looked at pancreatic cyst fluid from 83 cysts that had been surgically resected. The DNA samples were taken from the cyst fluid. Dr. Majumder believes that the cells shed from the cyst wall into the fluid. As a result, DNA from the fluid captures heterogeneity in the cyst more effectively than a biopsied sample.
The researchers found five methylated DNA markers that distinguished cancer or HGD from controls with areas under the ROC curve of 0.90 or higher. The top two (BMP3, EMX1) detected 93% of cases (95% CI, 66%-100%) at a specificity of 90% (95% CI, 80%-96%). Applied to eight cysts with intermediate-grade dysplasia, the biomarkers would have identified three at 95% specificity.
By comparison, the Fukuoka guidelines have 56% sensitivity and 73% specificity.
A limitation to the technique is that DNA cannot be extracted from all samples. About 5%-10% of pancreatic fluid samples are unusable, according to Somashekar Krishna, MD, MPH, assistant professor of medicine at the Ohio State University Medical Center, who attended the session. Dr. Krishna is conducting research combining endomicroscopy with molecular markers.
“We should have a foolproof system where if one fails, the other kicks in, and we have an answer for every patient. My opinion is that endomicroscopy has to be combined with molecular studies. I think combined we’ll have an excellent diagnostic yield,” Dr. Krishna said in an interview.
Dr. Majumder and Dr. Krishna have declared no conflicts of interest.
LAS VEGAS – In fluid derived from pancreatic cysts, methylated DNA markers predict the presence of high-grade dysplasia (HGD) or cancer, and could help physicians decide whether to surgically remove cysts – a procedure that often has serious complications.
If validated in larger studies, the biomarkers have the potential to supplant the Fukuoka criteria that is currently used. “The markers could cause a paradigm shift in how we approach these lesions in our clinical practice,” Shounak Majumder, MD, a fellow at the Mayo Clinic in Rochester, Minn., said in an interview.
Less than 50% of cysts that are surgically resected turn out to be HGD or cancerous. “Having a cyst fluid marker could identify the patients that would benefit the most from surgery. If you’re going to go through a pancreatic resection, we’d rather give you the best chance of saying that we removed something that either has early cancer in it or will turn into cancer in the near future,” said Dr. Majumder.
The study looked at pancreatic cyst fluid from 83 cysts that had been surgically resected. The DNA samples were taken from the cyst fluid. Dr. Majumder believes that the cells shed from the cyst wall into the fluid. As a result, DNA from the fluid captures heterogeneity in the cyst more effectively than a biopsied sample.
The researchers found five methylated DNA markers that distinguished cancer or HGD from controls with areas under the ROC curve of 0.90 or higher. The top two (BMP3, EMX1) detected 93% of cases (95% CI, 66%-100%) at a specificity of 90% (95% CI, 80%-96%). Applied to eight cysts with intermediate-grade dysplasia, the biomarkers would have identified three at 95% specificity.
By comparison, the Fukuoka guidelines have 56% sensitivity and 73% specificity.
A limitation to the technique is that DNA cannot be extracted from all samples. About 5%-10% of pancreatic fluid samples are unusable, according to Somashekar Krishna, MD, MPH, assistant professor of medicine at the Ohio State University Medical Center, who attended the session. Dr. Krishna is conducting research combining endomicroscopy with molecular markers.
“We should have a foolproof system where if one fails, the other kicks in, and we have an answer for every patient. My opinion is that endomicroscopy has to be combined with molecular studies. I think combined we’ll have an excellent diagnostic yield,” Dr. Krishna said in an interview.
Dr. Majumder and Dr. Krishna have declared no conflicts of interest.
LAS VEGAS – In fluid derived from pancreatic cysts, methylated DNA markers predict the presence of high-grade dysplasia (HGD) or cancer, and could help physicians decide whether to surgically remove cysts – a procedure that often has serious complications.
If validated in larger studies, the biomarkers have the potential to supplant the Fukuoka criteria that is currently used. “The markers could cause a paradigm shift in how we approach these lesions in our clinical practice,” Shounak Majumder, MD, a fellow at the Mayo Clinic in Rochester, Minn., said in an interview.
Less than 50% of cysts that are surgically resected turn out to be HGD or cancerous. “Having a cyst fluid marker could identify the patients that would benefit the most from surgery. If you’re going to go through a pancreatic resection, we’d rather give you the best chance of saying that we removed something that either has early cancer in it or will turn into cancer in the near future,” said Dr. Majumder.
The study looked at pancreatic cyst fluid from 83 cysts that had been surgically resected. The DNA samples were taken from the cyst fluid. Dr. Majumder believes that the cells shed from the cyst wall into the fluid. As a result, DNA from the fluid captures heterogeneity in the cyst more effectively than a biopsied sample.
The researchers found five methylated DNA markers that distinguished cancer or HGD from controls with areas under the ROC curve of 0.90 or higher. The top two (BMP3, EMX1) detected 93% of cases (95% CI, 66%-100%) at a specificity of 90% (95% CI, 80%-96%). Applied to eight cysts with intermediate-grade dysplasia, the biomarkers would have identified three at 95% specificity.
By comparison, the Fukuoka guidelines have 56% sensitivity and 73% specificity.
A limitation to the technique is that DNA cannot be extracted from all samples. About 5%-10% of pancreatic fluid samples are unusable, according to Somashekar Krishna, MD, MPH, assistant professor of medicine at the Ohio State University Medical Center, who attended the session. Dr. Krishna is conducting research combining endomicroscopy with molecular markers.
“We should have a foolproof system where if one fails, the other kicks in, and we have an answer for every patient. My opinion is that endomicroscopy has to be combined with molecular studies. I think combined we’ll have an excellent diagnostic yield,” Dr. Krishna said in an interview.
Dr. Majumder and Dr. Krishna have declared no conflicts of interest.
AT ACG 2016
Key clinical point:
Major finding: DNA markers isolated from pancreatic fluid predicted cancer or high-grade dysplasia with 90% specificity and 93% sensitivity.
Data source: Pilot study, retrospective analysis.
Disclosures: Dr. Majumder and Dr. Krishna have declared no conflicts of interest.
Study provides best support to date for using ultrasound to detect gout
Ultrasound examination of patients with at least one swollen joint or a subcutaneous nodule detected gout with a sensitivity of 76.9% and specificity of 84.3% in an international, multicenter study.
“Ultrasound is a good test for gout,” said study lead author Alexis R. Ogdie-Beatty, MD, of the University of Pennsylvania, Philadelphia. “It has high specificity, even among patients with early disease. The sensitivity was lower so it doesn’t pick up all cases of gout, although it was higher in people with a longer duration of disease.”
But gout remains difficult to diagnose because of the many similar types of inflammatory arthritis. A 2016 report from the Agency for Healthcare Research and Quality says proper diagnosis is a “major challenge,” especially in primary care and urgent/emergency settings.
Monosodium urate (MSU) crystal analysis via joint aspiration is considered the “gold standard” of gout diagnostic tools, but the agency report notes that it “can be technically difficult to perform and painful to the patient.”
There are other challenges. “Sometimes we see people between flares, or it’s already started to improve so there’s not enough fluid to perform an arthrocentesis,” Dr. Ogdie-Beatty said in an interview. “Additionally, while rheumatologists are generally very good at joint aspirations, many primary care physicians are not specifically trained for this. Thus, an imaging study can be helpful in these cases.”
The new study (Arthritis Rheumatol. 2016 Oct 16. doi: 10.1002/art.39959) follows up on findings from the multicenter, cross-sectional Study for Updated Gout Classification Criteria, which independently linked ultrasound analysis to diagnosis of gout with an odds ratio of 7.2 (Arthritis Care Res. 2015 Sep;67[9]:1304-15). One goal of the new study is to determine specificity and sensitivity of ultrasound exams.
The researchers examined data from the previous study, which enrolled consecutive patients in clinical practice settings across 25 countries who had at least one swollen joint or a subcutaneous nodule in whom gout was on the differential diagnosis. They underwent ultrasound examinations (most commonly of the knees, metatarsophalangeal joints, and ankles) and were deemed to have a “positive test” if at least one of three signs appeared: a “double contour” sign, tophus, or a “snowstorm” appearance. However, the study did not require a specific ultrasound scanning protocol or training.
MSU crystal examinations confirmed which of the subjects actually had gout. In total, ultrasound examinations were performed on 824 patients, of whom 416 were confirmed to have gout (mean age, 60, and 87% male), and the other 408 did not have gout (mean age, 60, and 54% male).
The researchers found that the sensitivity of the ultrasound exams was 76.9%, meaning they correctly identified patients with gout just over three-quarters of the time. Dr. Ogdie-Beatty referred to this as “moderate” sensitivity. The researchers found that the sensitivity was highest in patients who’d had disease for 2 or more years and in those who didn’t show clinical signs of tophi.
The specificity for these signs – the percentage of the time that ultrasound exams correctly identified patients without gout – was 84.3%. The positive and negative predictive values were 83.3% and 78.1%, respectively.
Dr. Ogdie-Beatty said the cost of ultrasound examinations is variable, although insurance covers it for trained providers. It’s unclear how commonly ultrasound examinations are used to detect gout, she said, but “many rheumatologists now have ultrasound as a part of their practice, and ultrasound training during fellowship has become important.”
Dr. Ogdie-Beatty reported receiving consulting fees from Novartis, and her coauthors reported various financial relationships with industry. The study was supported by various National Institutes of Health grants to several authors, as well as the American College of Rheumatology, the European League Against Rheumatism, and various arthritis-related organizations.
The new study provides evidence that both answers and raises questions about the technical standards by which musculoskeletal ultrasound examination is used to detect gout and about what we mean when we make a clinical diagnosis of gout.
The study’s results showed that ultrasound provided excellent specificity for gout in the setting of acute calcium pyrophosphate deposition disease (CPPD), also known as pseudogout, particularly in distinguishing the deposition of monosodium urate on the surface of cartilage (double contour sign), and calcium pyrophosphate within the matrix of the cartilage (interface sign). Ultrasound’s specificity for gout apart from CPPD was particularly high when more than one feature of gout was present because one feature of gout was more often present in patients with CPPD than in non-CPPD controls, but this was not the case when two features of gout were present. However, this leaves open the possibility that some patients may concurrently have both CPPD and gout, which the study does not fully address. Apart from the level of operator experience in using ultrasound, diagnostic bias did not seem to play a role in the diagnostic utility of ultrasound because clinical appearance of scanned joints or presence of tophi did not greatly affect its sensitivity when considering that patients with tophi have more advanced disease and probably have more obvious ultrasound findings.
The study’s demonstration of ultrasound’s ability to identify tophi is important because rheumatologists consider the presence of tophi as marking the transition from hyperuricemia to gout, which often involves of inflammation and tissue damage. Many other studies have noted how people with hyperuricemia can have the double contour sign without meeting clinical criteria for gout.
It still remains important to note that, even though the test characteristics of ultrasound in the study should be generalizable, its ability to support a diagnosis of acute gout does not rule out infection in the same joint.
Eugene Y. Kissin, MD, is with the division of rheumatology at Boston University, and Michael H. Pillinger, MD, is with the division of rheumatology at New York University, New York. Their comments are derived from their editorial accompanying the study by Dr. Ogdie-Beatty and her colleagues (Arthritis Rheumatol. 2016 Oct 16. doi: 10.1002/art.39958). Dr. Kissin reported no disclosures. Dr. Pillinger reported consulting for AstraZeneca, Crealta/Horizon, and Sobi, and he has served as an investigator for a trial sponsored by Takeda.
The new study provides evidence that both answers and raises questions about the technical standards by which musculoskeletal ultrasound examination is used to detect gout and about what we mean when we make a clinical diagnosis of gout.
The study’s results showed that ultrasound provided excellent specificity for gout in the setting of acute calcium pyrophosphate deposition disease (CPPD), also known as pseudogout, particularly in distinguishing the deposition of monosodium urate on the surface of cartilage (double contour sign), and calcium pyrophosphate within the matrix of the cartilage (interface sign). Ultrasound’s specificity for gout apart from CPPD was particularly high when more than one feature of gout was present because one feature of gout was more often present in patients with CPPD than in non-CPPD controls, but this was not the case when two features of gout were present. However, this leaves open the possibility that some patients may concurrently have both CPPD and gout, which the study does not fully address. Apart from the level of operator experience in using ultrasound, diagnostic bias did not seem to play a role in the diagnostic utility of ultrasound because clinical appearance of scanned joints or presence of tophi did not greatly affect its sensitivity when considering that patients with tophi have more advanced disease and probably have more obvious ultrasound findings.
The study’s demonstration of ultrasound’s ability to identify tophi is important because rheumatologists consider the presence of tophi as marking the transition from hyperuricemia to gout, which often involves of inflammation and tissue damage. Many other studies have noted how people with hyperuricemia can have the double contour sign without meeting clinical criteria for gout.
It still remains important to note that, even though the test characteristics of ultrasound in the study should be generalizable, its ability to support a diagnosis of acute gout does not rule out infection in the same joint.
Eugene Y. Kissin, MD, is with the division of rheumatology at Boston University, and Michael H. Pillinger, MD, is with the division of rheumatology at New York University, New York. Their comments are derived from their editorial accompanying the study by Dr. Ogdie-Beatty and her colleagues (Arthritis Rheumatol. 2016 Oct 16. doi: 10.1002/art.39958). Dr. Kissin reported no disclosures. Dr. Pillinger reported consulting for AstraZeneca, Crealta/Horizon, and Sobi, and he has served as an investigator for a trial sponsored by Takeda.
The new study provides evidence that both answers and raises questions about the technical standards by which musculoskeletal ultrasound examination is used to detect gout and about what we mean when we make a clinical diagnosis of gout.
The study’s results showed that ultrasound provided excellent specificity for gout in the setting of acute calcium pyrophosphate deposition disease (CPPD), also known as pseudogout, particularly in distinguishing the deposition of monosodium urate on the surface of cartilage (double contour sign), and calcium pyrophosphate within the matrix of the cartilage (interface sign). Ultrasound’s specificity for gout apart from CPPD was particularly high when more than one feature of gout was present because one feature of gout was more often present in patients with CPPD than in non-CPPD controls, but this was not the case when two features of gout were present. However, this leaves open the possibility that some patients may concurrently have both CPPD and gout, which the study does not fully address. Apart from the level of operator experience in using ultrasound, diagnostic bias did not seem to play a role in the diagnostic utility of ultrasound because clinical appearance of scanned joints or presence of tophi did not greatly affect its sensitivity when considering that patients with tophi have more advanced disease and probably have more obvious ultrasound findings.
The study’s demonstration of ultrasound’s ability to identify tophi is important because rheumatologists consider the presence of tophi as marking the transition from hyperuricemia to gout, which often involves of inflammation and tissue damage. Many other studies have noted how people with hyperuricemia can have the double contour sign without meeting clinical criteria for gout.
It still remains important to note that, even though the test characteristics of ultrasound in the study should be generalizable, its ability to support a diagnosis of acute gout does not rule out infection in the same joint.
Eugene Y. Kissin, MD, is with the division of rheumatology at Boston University, and Michael H. Pillinger, MD, is with the division of rheumatology at New York University, New York. Their comments are derived from their editorial accompanying the study by Dr. Ogdie-Beatty and her colleagues (Arthritis Rheumatol. 2016 Oct 16. doi: 10.1002/art.39958). Dr. Kissin reported no disclosures. Dr. Pillinger reported consulting for AstraZeneca, Crealta/Horizon, and Sobi, and he has served as an investigator for a trial sponsored by Takeda.
Ultrasound examination of patients with at least one swollen joint or a subcutaneous nodule detected gout with a sensitivity of 76.9% and specificity of 84.3% in an international, multicenter study.
“Ultrasound is a good test for gout,” said study lead author Alexis R. Ogdie-Beatty, MD, of the University of Pennsylvania, Philadelphia. “It has high specificity, even among patients with early disease. The sensitivity was lower so it doesn’t pick up all cases of gout, although it was higher in people with a longer duration of disease.”
But gout remains difficult to diagnose because of the many similar types of inflammatory arthritis. A 2016 report from the Agency for Healthcare Research and Quality says proper diagnosis is a “major challenge,” especially in primary care and urgent/emergency settings.
Monosodium urate (MSU) crystal analysis via joint aspiration is considered the “gold standard” of gout diagnostic tools, but the agency report notes that it “can be technically difficult to perform and painful to the patient.”
There are other challenges. “Sometimes we see people between flares, or it’s already started to improve so there’s not enough fluid to perform an arthrocentesis,” Dr. Ogdie-Beatty said in an interview. “Additionally, while rheumatologists are generally very good at joint aspirations, many primary care physicians are not specifically trained for this. Thus, an imaging study can be helpful in these cases.”
The new study (Arthritis Rheumatol. 2016 Oct 16. doi: 10.1002/art.39959) follows up on findings from the multicenter, cross-sectional Study for Updated Gout Classification Criteria, which independently linked ultrasound analysis to diagnosis of gout with an odds ratio of 7.2 (Arthritis Care Res. 2015 Sep;67[9]:1304-15). One goal of the new study is to determine specificity and sensitivity of ultrasound exams.
The researchers examined data from the previous study, which enrolled consecutive patients in clinical practice settings across 25 countries who had at least one swollen joint or a subcutaneous nodule in whom gout was on the differential diagnosis. They underwent ultrasound examinations (most commonly of the knees, metatarsophalangeal joints, and ankles) and were deemed to have a “positive test” if at least one of three signs appeared: a “double contour” sign, tophus, or a “snowstorm” appearance. However, the study did not require a specific ultrasound scanning protocol or training.
MSU crystal examinations confirmed which of the subjects actually had gout. In total, ultrasound examinations were performed on 824 patients, of whom 416 were confirmed to have gout (mean age, 60, and 87% male), and the other 408 did not have gout (mean age, 60, and 54% male).
The researchers found that the sensitivity of the ultrasound exams was 76.9%, meaning they correctly identified patients with gout just over three-quarters of the time. Dr. Ogdie-Beatty referred to this as “moderate” sensitivity. The researchers found that the sensitivity was highest in patients who’d had disease for 2 or more years and in those who didn’t show clinical signs of tophi.
The specificity for these signs – the percentage of the time that ultrasound exams correctly identified patients without gout – was 84.3%. The positive and negative predictive values were 83.3% and 78.1%, respectively.
Dr. Ogdie-Beatty said the cost of ultrasound examinations is variable, although insurance covers it for trained providers. It’s unclear how commonly ultrasound examinations are used to detect gout, she said, but “many rheumatologists now have ultrasound as a part of their practice, and ultrasound training during fellowship has become important.”
Dr. Ogdie-Beatty reported receiving consulting fees from Novartis, and her coauthors reported various financial relationships with industry. The study was supported by various National Institutes of Health grants to several authors, as well as the American College of Rheumatology, the European League Against Rheumatism, and various arthritis-related organizations.
Ultrasound examination of patients with at least one swollen joint or a subcutaneous nodule detected gout with a sensitivity of 76.9% and specificity of 84.3% in an international, multicenter study.
“Ultrasound is a good test for gout,” said study lead author Alexis R. Ogdie-Beatty, MD, of the University of Pennsylvania, Philadelphia. “It has high specificity, even among patients with early disease. The sensitivity was lower so it doesn’t pick up all cases of gout, although it was higher in people with a longer duration of disease.”
But gout remains difficult to diagnose because of the many similar types of inflammatory arthritis. A 2016 report from the Agency for Healthcare Research and Quality says proper diagnosis is a “major challenge,” especially in primary care and urgent/emergency settings.
Monosodium urate (MSU) crystal analysis via joint aspiration is considered the “gold standard” of gout diagnostic tools, but the agency report notes that it “can be technically difficult to perform and painful to the patient.”
There are other challenges. “Sometimes we see people between flares, or it’s already started to improve so there’s not enough fluid to perform an arthrocentesis,” Dr. Ogdie-Beatty said in an interview. “Additionally, while rheumatologists are generally very good at joint aspirations, many primary care physicians are not specifically trained for this. Thus, an imaging study can be helpful in these cases.”
The new study (Arthritis Rheumatol. 2016 Oct 16. doi: 10.1002/art.39959) follows up on findings from the multicenter, cross-sectional Study for Updated Gout Classification Criteria, which independently linked ultrasound analysis to diagnosis of gout with an odds ratio of 7.2 (Arthritis Care Res. 2015 Sep;67[9]:1304-15). One goal of the new study is to determine specificity and sensitivity of ultrasound exams.
The researchers examined data from the previous study, which enrolled consecutive patients in clinical practice settings across 25 countries who had at least one swollen joint or a subcutaneous nodule in whom gout was on the differential diagnosis. They underwent ultrasound examinations (most commonly of the knees, metatarsophalangeal joints, and ankles) and were deemed to have a “positive test” if at least one of three signs appeared: a “double contour” sign, tophus, or a “snowstorm” appearance. However, the study did not require a specific ultrasound scanning protocol or training.
MSU crystal examinations confirmed which of the subjects actually had gout. In total, ultrasound examinations were performed on 824 patients, of whom 416 were confirmed to have gout (mean age, 60, and 87% male), and the other 408 did not have gout (mean age, 60, and 54% male).
The researchers found that the sensitivity of the ultrasound exams was 76.9%, meaning they correctly identified patients with gout just over three-quarters of the time. Dr. Ogdie-Beatty referred to this as “moderate” sensitivity. The researchers found that the sensitivity was highest in patients who’d had disease for 2 or more years and in those who didn’t show clinical signs of tophi.
The specificity for these signs – the percentage of the time that ultrasound exams correctly identified patients without gout – was 84.3%. The positive and negative predictive values were 83.3% and 78.1%, respectively.
Dr. Ogdie-Beatty said the cost of ultrasound examinations is variable, although insurance covers it for trained providers. It’s unclear how commonly ultrasound examinations are used to detect gout, she said, but “many rheumatologists now have ultrasound as a part of their practice, and ultrasound training during fellowship has become important.”
Dr. Ogdie-Beatty reported receiving consulting fees from Novartis, and her coauthors reported various financial relationships with industry. The study was supported by various National Institutes of Health grants to several authors, as well as the American College of Rheumatology, the European League Against Rheumatism, and various arthritis-related organizations.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point:
Major finding: Examiners looked for at least one of three potential signs of gout. The sensitivity for gout was 76.9%, and the specificity was 84.3%.
Data source: 824 patients with potential signs of gout who underwent ultrasound examination in an international, multicenter, observational cross-sectional study
Disclosures: Dr. Ogdie-Beatty reported receiving consulting fees from Novartis, and her coauthors reported various financial relationships with industry. The study was supported by various National Institutes of Health grants to several authors, as well as the American College of Rheumatology, the European League Against Rheumatism, and various arthritis-related organizations.
FDA expands indication for pembrolizumab in NSCLC
The Food and Drug Administration has approved pembrolizumab for the treatment of patients with metastatic non–small cell lung cancer (NSCLC) whose tumors express PD-L1 as determined by an FDA-approved test. This is the first approval of a checkpoint inhibitor for first-line treatment of the disease.
Pembrolizumab (Keytruda) is now approved to treat patients with metastatic NSCLC whose tumors have high PD-L1 expression (Tumor Proportion Score [TPS] greater than or equal to 50%), with no EGFR or ALK genomic tumor aberrations, and no prior systemic chemotherapy treatment for metastatic NSCLC, the FDA said in a written statement.
The FDA based its approval on improvement in overall survival in two trials comparing treatment with pembrolizumab to treatment from chemotherapy. In one trial of 305 patients who had no prior treatment for metastatic NSCLC and TPS greater than or equal to 50%, those who received pembrolizumab (200 mg every 3 weeks) had a statistically significant improvement in overall survival, compared with patients randomized to receive chemotherapy (hazard ratio, 0.60; 95% confidence interval, 0.41-0.89; P less than .005). There was also significant improvement in progression-free survival for those receiving the checkpoint inhibitor (HR, 0.50; 95% CI, 0.37-0.68; P less than .001).
In the second trial, a three-arm trial of 1,033 patients who were previously treated for metastatic NSCLC with a TPS greater than or equal to 1%, those randomized to pembrolizumab 2 mg/kg every 3 weeks (HR, 0.71; 95% CI, 0.58-0.88; P less than .001) or pembrolizumab 10 mg/kg every 3 weeks (HR, 0.61; 95% CI, 0.49-0.75; P less than .001) had an improved overall survival, compared with patients receiving docetaxel. The median survival was 10.4 months in the pembrolizumab 2 mg/kg arm, 12.7 months in the pembrolizumab 10 mg/kg arm, and 8.5 months in the docetaxel arm.
The most common side effects of treatment with pembrolizumab included decreased appetite, fatigue, nausea, dyspnea, cough, and constipation. Rare but serious adverse events included immune-mediated pneumonitis, colitis, hepatitis, endocrinopathies, and nephritis, the FDA said.
The recommended dose and schedule of pembrolizumab for NSCLC is 200 mg intravenously every 3 weeks. Full prescribing information is available here.
[email protected]
On Twitter @nikolaideslaura
The Food and Drug Administration has approved pembrolizumab for the treatment of patients with metastatic non–small cell lung cancer (NSCLC) whose tumors express PD-L1 as determined by an FDA-approved test. This is the first approval of a checkpoint inhibitor for first-line treatment of the disease.
Pembrolizumab (Keytruda) is now approved to treat patients with metastatic NSCLC whose tumors have high PD-L1 expression (Tumor Proportion Score [TPS] greater than or equal to 50%), with no EGFR or ALK genomic tumor aberrations, and no prior systemic chemotherapy treatment for metastatic NSCLC, the FDA said in a written statement.
The FDA based its approval on improvement in overall survival in two trials comparing treatment with pembrolizumab to treatment from chemotherapy. In one trial of 305 patients who had no prior treatment for metastatic NSCLC and TPS greater than or equal to 50%, those who received pembrolizumab (200 mg every 3 weeks) had a statistically significant improvement in overall survival, compared with patients randomized to receive chemotherapy (hazard ratio, 0.60; 95% confidence interval, 0.41-0.89; P less than .005). There was also significant improvement in progression-free survival for those receiving the checkpoint inhibitor (HR, 0.50; 95% CI, 0.37-0.68; P less than .001).
In the second trial, a three-arm trial of 1,033 patients who were previously treated for metastatic NSCLC with a TPS greater than or equal to 1%, those randomized to pembrolizumab 2 mg/kg every 3 weeks (HR, 0.71; 95% CI, 0.58-0.88; P less than .001) or pembrolizumab 10 mg/kg every 3 weeks (HR, 0.61; 95% CI, 0.49-0.75; P less than .001) had an improved overall survival, compared with patients receiving docetaxel. The median survival was 10.4 months in the pembrolizumab 2 mg/kg arm, 12.7 months in the pembrolizumab 10 mg/kg arm, and 8.5 months in the docetaxel arm.
The most common side effects of treatment with pembrolizumab included decreased appetite, fatigue, nausea, dyspnea, cough, and constipation. Rare but serious adverse events included immune-mediated pneumonitis, colitis, hepatitis, endocrinopathies, and nephritis, the FDA said.
The recommended dose and schedule of pembrolizumab for NSCLC is 200 mg intravenously every 3 weeks. Full prescribing information is available here.
[email protected]
On Twitter @nikolaideslaura
The Food and Drug Administration has approved pembrolizumab for the treatment of patients with metastatic non–small cell lung cancer (NSCLC) whose tumors express PD-L1 as determined by an FDA-approved test. This is the first approval of a checkpoint inhibitor for first-line treatment of the disease.
Pembrolizumab (Keytruda) is now approved to treat patients with metastatic NSCLC whose tumors have high PD-L1 expression (Tumor Proportion Score [TPS] greater than or equal to 50%), with no EGFR or ALK genomic tumor aberrations, and no prior systemic chemotherapy treatment for metastatic NSCLC, the FDA said in a written statement.
The FDA based its approval on improvement in overall survival in two trials comparing treatment with pembrolizumab to treatment from chemotherapy. In one trial of 305 patients who had no prior treatment for metastatic NSCLC and TPS greater than or equal to 50%, those who received pembrolizumab (200 mg every 3 weeks) had a statistically significant improvement in overall survival, compared with patients randomized to receive chemotherapy (hazard ratio, 0.60; 95% confidence interval, 0.41-0.89; P less than .005). There was also significant improvement in progression-free survival for those receiving the checkpoint inhibitor (HR, 0.50; 95% CI, 0.37-0.68; P less than .001).
In the second trial, a three-arm trial of 1,033 patients who were previously treated for metastatic NSCLC with a TPS greater than or equal to 1%, those randomized to pembrolizumab 2 mg/kg every 3 weeks (HR, 0.71; 95% CI, 0.58-0.88; P less than .001) or pembrolizumab 10 mg/kg every 3 weeks (HR, 0.61; 95% CI, 0.49-0.75; P less than .001) had an improved overall survival, compared with patients receiving docetaxel. The median survival was 10.4 months in the pembrolizumab 2 mg/kg arm, 12.7 months in the pembrolizumab 10 mg/kg arm, and 8.5 months in the docetaxel arm.
The most common side effects of treatment with pembrolizumab included decreased appetite, fatigue, nausea, dyspnea, cough, and constipation. Rare but serious adverse events included immune-mediated pneumonitis, colitis, hepatitis, endocrinopathies, and nephritis, the FDA said.
The recommended dose and schedule of pembrolizumab for NSCLC is 200 mg intravenously every 3 weeks. Full prescribing information is available here.
[email protected]
On Twitter @nikolaideslaura
Ketamine curbs suicidal ideation independent of antidepressant effect
VIENNA – Repeated infusions of ketamine appear to have a direct effect in quelling suicidal ideation independent of the drug’s impact on depressive symptoms, Pierre Blier, MD, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
“Our preliminary results indicate that ketamine reduced suicidal ideation even in patients who failed to show a reduction in depression severity with repeated infusions,” observed Dr. Blier, professor of psychiatry at the University of Ottawa.
If the findings from this proof-of-concept study are confirmed, it would be very good news indeed.
“Suicidal ideation often requires rapid intervention, but there are very few treatments that are effective in reducing suicidal ideation, and none are fast acting,” the psychiatrist noted.
Therapeutic interest in ketamine is running high because subanesthetic doses of the drug have been shown to reduce depressive symptoms within a matter of hours, albeit transiently, in patients with treatment-resistant depression. If the drug acts as quickly in decreasing suicidal ideation as it does for depression, it would have major implications for clinical practice. An estimated 1 million people die each year worldwide from suicide, and two-thirds of them have major depressive disorder.
Dr. Blier reported on 27 patients with treatment-resistant depression, defined as failure to respond to at least two antidepressant medications from different pharmacologic classes at adequate dosages for at least 6 weeks each, along with two augmentation strategies. Their mean baseline score on the Montgomery-Asberg Depression Rating Scale (MADRS) was roughly 35. Five of the 27 had made one or more prior suicide attempts. Twenty-six of the 27 had suicidal ideation at baseline as defined by a MADRS suicidal ideation score (MADRS-SI) of more than 0. The MADRS-SI is based upon item 10 on the MADRS and is scored 0-6. Eight patients reported marked suicidal ideation as defined by a baseline MADRS-SI score of 4 or higher.
Study participants received a total of seven intravenous infusions of ketamine over roughly 3 weeks. Patients were retested 2-3 days after the seventh infusion, at which time 16 patients were categorized as treatment responders on the basis of at least a 50% reduction in their total MADRS score. Indeed, they averaged a 20.7-point reduction in the non–suicide-related MADRS score – that is, items 1-9, which reflect symptoms of depression – compared with a mere 3.9-point decrease in the 11 ketamine nonresponders. This 59% response rate for depressive symptoms is consistent with reported results of other studies of ketamine in patients with treatment-resistant depression, according to Dr. Blier, also professor of cellular/molecular medicines at the university.
The baseline MADRS-SI score averaged 2.9 in treatment responders and was similar at 2.5 in the nonresponders. But after seven infusions of ketamine, 21 of 27 patients had a MADRS-SI score of 0 or 1. The key study finding was that both the ketamine responders and nonresponders in terms of depressive symptoms experienced significant reductions in suicidal ideation. The 16 responders averaged a 2.4-point decrease from baseline in MADRS-SI scores, while the 11 nonresponders averaged a 1.5-point reduction. The magnitude of the reduction in MADRS-SI was significantly greater in the antidepressant responders than the nonresponders, but the improvement in suicidal ideation was statistically significant and clinically meaningful in both groups.
Seven of the 8 patients with baseline marked suicidal ideation and a MADRS-SI of 4 or greater improved to a score of 2 or less after their seven ketamine infusions, meaning they were having at most only fleeting suicidal thoughts.
Dr. Blier and coinvestigators are now expanding the ketamine study, enrolling up to 40 additional patients with treatment-resistant depression and suicidality in order to confirm the preliminary findings.
Dr. Blier reported having no financial conflicts of interest regarding this study, which was funded by the Canadian Institutes of Health Research. He noted that he receives research grants from and/or serves on advisory boards for more than a dozen pharmaceutical companies.
VIENNA – Repeated infusions of ketamine appear to have a direct effect in quelling suicidal ideation independent of the drug’s impact on depressive symptoms, Pierre Blier, MD, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
“Our preliminary results indicate that ketamine reduced suicidal ideation even in patients who failed to show a reduction in depression severity with repeated infusions,” observed Dr. Blier, professor of psychiatry at the University of Ottawa.
If the findings from this proof-of-concept study are confirmed, it would be very good news indeed.
“Suicidal ideation often requires rapid intervention, but there are very few treatments that are effective in reducing suicidal ideation, and none are fast acting,” the psychiatrist noted.
Therapeutic interest in ketamine is running high because subanesthetic doses of the drug have been shown to reduce depressive symptoms within a matter of hours, albeit transiently, in patients with treatment-resistant depression. If the drug acts as quickly in decreasing suicidal ideation as it does for depression, it would have major implications for clinical practice. An estimated 1 million people die each year worldwide from suicide, and two-thirds of them have major depressive disorder.
Dr. Blier reported on 27 patients with treatment-resistant depression, defined as failure to respond to at least two antidepressant medications from different pharmacologic classes at adequate dosages for at least 6 weeks each, along with two augmentation strategies. Their mean baseline score on the Montgomery-Asberg Depression Rating Scale (MADRS) was roughly 35. Five of the 27 had made one or more prior suicide attempts. Twenty-six of the 27 had suicidal ideation at baseline as defined by a MADRS suicidal ideation score (MADRS-SI) of more than 0. The MADRS-SI is based upon item 10 on the MADRS and is scored 0-6. Eight patients reported marked suicidal ideation as defined by a baseline MADRS-SI score of 4 or higher.
Study participants received a total of seven intravenous infusions of ketamine over roughly 3 weeks. Patients were retested 2-3 days after the seventh infusion, at which time 16 patients were categorized as treatment responders on the basis of at least a 50% reduction in their total MADRS score. Indeed, they averaged a 20.7-point reduction in the non–suicide-related MADRS score – that is, items 1-9, which reflect symptoms of depression – compared with a mere 3.9-point decrease in the 11 ketamine nonresponders. This 59% response rate for depressive symptoms is consistent with reported results of other studies of ketamine in patients with treatment-resistant depression, according to Dr. Blier, also professor of cellular/molecular medicines at the university.
The baseline MADRS-SI score averaged 2.9 in treatment responders and was similar at 2.5 in the nonresponders. But after seven infusions of ketamine, 21 of 27 patients had a MADRS-SI score of 0 or 1. The key study finding was that both the ketamine responders and nonresponders in terms of depressive symptoms experienced significant reductions in suicidal ideation. The 16 responders averaged a 2.4-point decrease from baseline in MADRS-SI scores, while the 11 nonresponders averaged a 1.5-point reduction. The magnitude of the reduction in MADRS-SI was significantly greater in the antidepressant responders than the nonresponders, but the improvement in suicidal ideation was statistically significant and clinically meaningful in both groups.
Seven of the 8 patients with baseline marked suicidal ideation and a MADRS-SI of 4 or greater improved to a score of 2 or less after their seven ketamine infusions, meaning they were having at most only fleeting suicidal thoughts.
Dr. Blier and coinvestigators are now expanding the ketamine study, enrolling up to 40 additional patients with treatment-resistant depression and suicidality in order to confirm the preliminary findings.
Dr. Blier reported having no financial conflicts of interest regarding this study, which was funded by the Canadian Institutes of Health Research. He noted that he receives research grants from and/or serves on advisory boards for more than a dozen pharmaceutical companies.
VIENNA – Repeated infusions of ketamine appear to have a direct effect in quelling suicidal ideation independent of the drug’s impact on depressive symptoms, Pierre Blier, MD, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
“Our preliminary results indicate that ketamine reduced suicidal ideation even in patients who failed to show a reduction in depression severity with repeated infusions,” observed Dr. Blier, professor of psychiatry at the University of Ottawa.
If the findings from this proof-of-concept study are confirmed, it would be very good news indeed.
“Suicidal ideation often requires rapid intervention, but there are very few treatments that are effective in reducing suicidal ideation, and none are fast acting,” the psychiatrist noted.
Therapeutic interest in ketamine is running high because subanesthetic doses of the drug have been shown to reduce depressive symptoms within a matter of hours, albeit transiently, in patients with treatment-resistant depression. If the drug acts as quickly in decreasing suicidal ideation as it does for depression, it would have major implications for clinical practice. An estimated 1 million people die each year worldwide from suicide, and two-thirds of them have major depressive disorder.
Dr. Blier reported on 27 patients with treatment-resistant depression, defined as failure to respond to at least two antidepressant medications from different pharmacologic classes at adequate dosages for at least 6 weeks each, along with two augmentation strategies. Their mean baseline score on the Montgomery-Asberg Depression Rating Scale (MADRS) was roughly 35. Five of the 27 had made one or more prior suicide attempts. Twenty-six of the 27 had suicidal ideation at baseline as defined by a MADRS suicidal ideation score (MADRS-SI) of more than 0. The MADRS-SI is based upon item 10 on the MADRS and is scored 0-6. Eight patients reported marked suicidal ideation as defined by a baseline MADRS-SI score of 4 or higher.
Study participants received a total of seven intravenous infusions of ketamine over roughly 3 weeks. Patients were retested 2-3 days after the seventh infusion, at which time 16 patients were categorized as treatment responders on the basis of at least a 50% reduction in their total MADRS score. Indeed, they averaged a 20.7-point reduction in the non–suicide-related MADRS score – that is, items 1-9, which reflect symptoms of depression – compared with a mere 3.9-point decrease in the 11 ketamine nonresponders. This 59% response rate for depressive symptoms is consistent with reported results of other studies of ketamine in patients with treatment-resistant depression, according to Dr. Blier, also professor of cellular/molecular medicines at the university.
The baseline MADRS-SI score averaged 2.9 in treatment responders and was similar at 2.5 in the nonresponders. But after seven infusions of ketamine, 21 of 27 patients had a MADRS-SI score of 0 or 1. The key study finding was that both the ketamine responders and nonresponders in terms of depressive symptoms experienced significant reductions in suicidal ideation. The 16 responders averaged a 2.4-point decrease from baseline in MADRS-SI scores, while the 11 nonresponders averaged a 1.5-point reduction. The magnitude of the reduction in MADRS-SI was significantly greater in the antidepressant responders than the nonresponders, but the improvement in suicidal ideation was statistically significant and clinically meaningful in both groups.
Seven of the 8 patients with baseline marked suicidal ideation and a MADRS-SI of 4 or greater improved to a score of 2 or less after their seven ketamine infusions, meaning they were having at most only fleeting suicidal thoughts.
Dr. Blier and coinvestigators are now expanding the ketamine study, enrolling up to 40 additional patients with treatment-resistant depression and suicidality in order to confirm the preliminary findings.
Dr. Blier reported having no financial conflicts of interest regarding this study, which was funded by the Canadian Institutes of Health Research. He noted that he receives research grants from and/or serves on advisory boards for more than a dozen pharmaceutical companies.
AT THE ECNP CONGRESS
Key clinical point:
Major finding: Ketamine infusions significantly reduced suicidal ideation even in patients whose treatment-resistant depression did not respond to the drug.
Data source: This ongoing observational study included 27 patients with treatment-resistant depression who received seven intravenous infusions of ketamine over roughly a 3-week period.
Disclosures: The presenter reported having no financial conflicts of interest regarding this study, which was funded by the Canadian Institutes of Health Research.
Telementoring expands PCPs’ role in managing pediatric chronic disease
SAN FRANCISCO – Telementoring empowers primary care pediatric providers (PCPs) to take on a greater role in managing their patients’ chronic diseases, new data suggest. Leaders in this emerging field gave a snapshot of early experience with the model at the annual meeting of the American Academy of Pediatrics.
“About a quarter of children live with chronic health conditions, and there is an increasing need for specialty care. But many children don’t have access to the quality specialty care that they really need, particularly in rural and medically underserved areas,” explained Dr. Sucheta M. Joshi, a pediatric neurologist and epileptologist at the University of Michigan, Ann Arbor. “The goal of telementoring is to build the capacity of primary care doctors.”
“We need to empower primary care providers to work ‘at the top of their license’ because we don’t have enough specialists,” agreed Dr. David
L. Wood, a general pediatrician and chair of the department of pediatrics at East Tennessee State University in Johnson City. “We as primary care [physicians] have to shoulder more of the care of kids with chronic disease. But we need backup, we need support to do that because the science is growing rapidly, we can’t keep up.”
Telementoring first gained recognition through the University of New Mexico’s Project Extension for Community Healthcare Outcomes (ECHO), which links specialists at an academic “hub” with PCPs in local communities, or “spokes,” in a learning network.
Through regular, interactive, multisite telementoring sessions, ECHO provides training to increase PCPs’ knowledge, self-efficacy, and comfort in managing chronic diseases not typically considered within their scope of practice. Sessions combine short didactic presentations and case-based learning.
The model was initially tested in pediatrics as ECHO for Epilepsy, a partnership of the AAP and the University of New Mexico, Albuquerque. Topics covered ranged from first seizures to work-up to treatment, including when to refer to a neurologist, according to Dr. Joshi, who helped develop the curriculum. Encouraging findings among the 49 clinics participating in the first year led to expansion of the program to five more states.
Preliminary data from the full cohort show reductions from baseline to end of the program in the proportions of participants who felt not at all or not very knowledgeable about pharmacologic management of pediatric epilepsy (from 69% to 45%), related school and education issues (from 51% to 18%), pertinent state driving laws (from 69% to 45%), and when to refer to a specialist (from 34% to 0%), Dr. Joshi reported.
There were also reductions in the proportions of participants who felt not at all or not very confident about aspects of care such as medical testing in this population (from 52% to 45%), communicating with patients about the transition to adult care (from 52% to 27%), and communicating with families and caregivers about the impact of epilepsy on everyday life (from 59% to 36%).
“This has been a good demonstration to say that telementoring does improve provider knowledge and confidence,” Dr. Joshi maintained. “Everybody felt quite uniformly that the case discussions were useful, and it really fostered a sense of a community of learners and was very much an iterative process.”
The AAP has since been designated as a “superhub” for Project ECHO that can train others to start programs in specialty areas. Additional programs have been developed in pediatric endocrinology, sickle cell disease, and surgery.
The main costs of ECHO are the personnel and physician time, and financing has yet to be worked out. “Unlike traditional telemedicine, where it’s one patient and one physician, and you can actually bill for it, this is not a billable service,” Dr. Joshi noted. “What you can get is more of a downstream effect, which can take some time to become obvious.”
The key attraction for providers is obtaining CME credits, where offered. And a draw for institutions is the potential impact of ECHO in reducing provider turnover and improving value-based reimbursement.
“This is really an innovative program that can help us achieve the triple aim” of improving the patient’s experience, reducing costs, and achieving better health outcomes, Dr. Wood said. “I think this is a great enhancement to the medical home.”
Both Dr. Joshi and Dr. Wood disclosed that they had no relevant conflicts of interest.
SAN FRANCISCO – Telementoring empowers primary care pediatric providers (PCPs) to take on a greater role in managing their patients’ chronic diseases, new data suggest. Leaders in this emerging field gave a snapshot of early experience with the model at the annual meeting of the American Academy of Pediatrics.
“About a quarter of children live with chronic health conditions, and there is an increasing need for specialty care. But many children don’t have access to the quality specialty care that they really need, particularly in rural and medically underserved areas,” explained Dr. Sucheta M. Joshi, a pediatric neurologist and epileptologist at the University of Michigan, Ann Arbor. “The goal of telementoring is to build the capacity of primary care doctors.”
“We need to empower primary care providers to work ‘at the top of their license’ because we don’t have enough specialists,” agreed Dr. David
L. Wood, a general pediatrician and chair of the department of pediatrics at East Tennessee State University in Johnson City. “We as primary care [physicians] have to shoulder more of the care of kids with chronic disease. But we need backup, we need support to do that because the science is growing rapidly, we can’t keep up.”
Telementoring first gained recognition through the University of New Mexico’s Project Extension for Community Healthcare Outcomes (ECHO), which links specialists at an academic “hub” with PCPs in local communities, or “spokes,” in a learning network.
Through regular, interactive, multisite telementoring sessions, ECHO provides training to increase PCPs’ knowledge, self-efficacy, and comfort in managing chronic diseases not typically considered within their scope of practice. Sessions combine short didactic presentations and case-based learning.
The model was initially tested in pediatrics as ECHO for Epilepsy, a partnership of the AAP and the University of New Mexico, Albuquerque. Topics covered ranged from first seizures to work-up to treatment, including when to refer to a neurologist, according to Dr. Joshi, who helped develop the curriculum. Encouraging findings among the 49 clinics participating in the first year led to expansion of the program to five more states.
Preliminary data from the full cohort show reductions from baseline to end of the program in the proportions of participants who felt not at all or not very knowledgeable about pharmacologic management of pediatric epilepsy (from 69% to 45%), related school and education issues (from 51% to 18%), pertinent state driving laws (from 69% to 45%), and when to refer to a specialist (from 34% to 0%), Dr. Joshi reported.
There were also reductions in the proportions of participants who felt not at all or not very confident about aspects of care such as medical testing in this population (from 52% to 45%), communicating with patients about the transition to adult care (from 52% to 27%), and communicating with families and caregivers about the impact of epilepsy on everyday life (from 59% to 36%).
“This has been a good demonstration to say that telementoring does improve provider knowledge and confidence,” Dr. Joshi maintained. “Everybody felt quite uniformly that the case discussions were useful, and it really fostered a sense of a community of learners and was very much an iterative process.”
The AAP has since been designated as a “superhub” for Project ECHO that can train others to start programs in specialty areas. Additional programs have been developed in pediatric endocrinology, sickle cell disease, and surgery.
The main costs of ECHO are the personnel and physician time, and financing has yet to be worked out. “Unlike traditional telemedicine, where it’s one patient and one physician, and you can actually bill for it, this is not a billable service,” Dr. Joshi noted. “What you can get is more of a downstream effect, which can take some time to become obvious.”
The key attraction for providers is obtaining CME credits, where offered. And a draw for institutions is the potential impact of ECHO in reducing provider turnover and improving value-based reimbursement.
“This is really an innovative program that can help us achieve the triple aim” of improving the patient’s experience, reducing costs, and achieving better health outcomes, Dr. Wood said. “I think this is a great enhancement to the medical home.”
Both Dr. Joshi and Dr. Wood disclosed that they had no relevant conflicts of interest.
SAN FRANCISCO – Telementoring empowers primary care pediatric providers (PCPs) to take on a greater role in managing their patients’ chronic diseases, new data suggest. Leaders in this emerging field gave a snapshot of early experience with the model at the annual meeting of the American Academy of Pediatrics.
“About a quarter of children live with chronic health conditions, and there is an increasing need for specialty care. But many children don’t have access to the quality specialty care that they really need, particularly in rural and medically underserved areas,” explained Dr. Sucheta M. Joshi, a pediatric neurologist and epileptologist at the University of Michigan, Ann Arbor. “The goal of telementoring is to build the capacity of primary care doctors.”
“We need to empower primary care providers to work ‘at the top of their license’ because we don’t have enough specialists,” agreed Dr. David
L. Wood, a general pediatrician and chair of the department of pediatrics at East Tennessee State University in Johnson City. “We as primary care [physicians] have to shoulder more of the care of kids with chronic disease. But we need backup, we need support to do that because the science is growing rapidly, we can’t keep up.”
Telementoring first gained recognition through the University of New Mexico’s Project Extension for Community Healthcare Outcomes (ECHO), which links specialists at an academic “hub” with PCPs in local communities, or “spokes,” in a learning network.
Through regular, interactive, multisite telementoring sessions, ECHO provides training to increase PCPs’ knowledge, self-efficacy, and comfort in managing chronic diseases not typically considered within their scope of practice. Sessions combine short didactic presentations and case-based learning.
The model was initially tested in pediatrics as ECHO for Epilepsy, a partnership of the AAP and the University of New Mexico, Albuquerque. Topics covered ranged from first seizures to work-up to treatment, including when to refer to a neurologist, according to Dr. Joshi, who helped develop the curriculum. Encouraging findings among the 49 clinics participating in the first year led to expansion of the program to five more states.
Preliminary data from the full cohort show reductions from baseline to end of the program in the proportions of participants who felt not at all or not very knowledgeable about pharmacologic management of pediatric epilepsy (from 69% to 45%), related school and education issues (from 51% to 18%), pertinent state driving laws (from 69% to 45%), and when to refer to a specialist (from 34% to 0%), Dr. Joshi reported.
There were also reductions in the proportions of participants who felt not at all or not very confident about aspects of care such as medical testing in this population (from 52% to 45%), communicating with patients about the transition to adult care (from 52% to 27%), and communicating with families and caregivers about the impact of epilepsy on everyday life (from 59% to 36%).
“This has been a good demonstration to say that telementoring does improve provider knowledge and confidence,” Dr. Joshi maintained. “Everybody felt quite uniformly that the case discussions were useful, and it really fostered a sense of a community of learners and was very much an iterative process.”
The AAP has since been designated as a “superhub” for Project ECHO that can train others to start programs in specialty areas. Additional programs have been developed in pediatric endocrinology, sickle cell disease, and surgery.
The main costs of ECHO are the personnel and physician time, and financing has yet to be worked out. “Unlike traditional telemedicine, where it’s one patient and one physician, and you can actually bill for it, this is not a billable service,” Dr. Joshi noted. “What you can get is more of a downstream effect, which can take some time to become obvious.”
The key attraction for providers is obtaining CME credits, where offered. And a draw for institutions is the potential impact of ECHO in reducing provider turnover and improving value-based reimbursement.
“This is really an innovative program that can help us achieve the triple aim” of improving the patient’s experience, reducing costs, and achieving better health outcomes, Dr. Wood said. “I think this is a great enhancement to the medical home.”
Both Dr. Joshi and Dr. Wood disclosed that they had no relevant conflicts of interest.
EXPERT ANALYSIS FROM AAP 16
Chemical Peels
Review the PDF of the fact sheet on Chemical Peels with board-relevant, easy-to-review material. This fact sheet will review the use of chemical peels for dermatologic indications.
Practice Questions
1. Which peel requires neutralization?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
2. Which peel contains resorcinol?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
3. Which peel would be the best treatment of severe actinic photodamage?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
4. Which peel would not be indicated for treatment of melasma in a patient with Fitzpatrick skin type IV?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
5. Which peel is a β-hydroxy acid?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
Answers to practice questions provided on next page
Practice Question Answers
1. Which peel requires neutralization?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
2. Which peel contains resorcinol?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
3. Which peel would be the best treatment of severe actinic photodamage?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
4. Which peel would not be indicated for treatment of melasma in a patient with Fitzpatrick skin type IV?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
5. Which peel is a β-hydroxy acid?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
Review the PDF of the fact sheet on Chemical Peels with board-relevant, easy-to-review material. This fact sheet will review the use of chemical peels for dermatologic indications.
Practice Questions
1. Which peel requires neutralization?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
2. Which peel contains resorcinol?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
3. Which peel would be the best treatment of severe actinic photodamage?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
4. Which peel would not be indicated for treatment of melasma in a patient with Fitzpatrick skin type IV?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
5. Which peel is a β-hydroxy acid?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
Answers to practice questions provided on next page
Practice Question Answers
1. Which peel requires neutralization?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
2. Which peel contains resorcinol?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
3. Which peel would be the best treatment of severe actinic photodamage?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
4. Which peel would not be indicated for treatment of melasma in a patient with Fitzpatrick skin type IV?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
5. Which peel is a β-hydroxy acid?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
Review the PDF of the fact sheet on Chemical Peels with board-relevant, easy-to-review material. This fact sheet will review the use of chemical peels for dermatologic indications.
Practice Questions
1. Which peel requires neutralization?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
2. Which peel contains resorcinol?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
3. Which peel would be the best treatment of severe actinic photodamage?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
4. Which peel would not be indicated for treatment of melasma in a patient with Fitzpatrick skin type IV?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
5. Which peel is a β-hydroxy acid?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
Answers to practice questions provided on next page
Practice Question Answers
1. Which peel requires neutralization?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
2. Which peel contains resorcinol?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
3. Which peel would be the best treatment of severe actinic photodamage?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
4. Which peel would not be indicated for treatment of melasma in a patient with Fitzpatrick skin type IV?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
5. Which peel is a β-hydroxy acid?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
‘Committed’ takes a nonpatronizing approach to involuntary care
Psychiatrists are trained to view involuntary treatment as an unpleasant means to a desirable end, a necessary evil. And we make the assumption that patients who are helped by the care they receive involuntarily will ultimately be grateful for that care.
Dinah Miller, MD, and Annette Hanson, MD, were inspired to write “Committed: The Battle Over Involuntary Psychiatric Care,” when they discovered that this assumption is false, that there are no clear data about the long-term effects of involuntary care, and that many patients whose mental illnesses improved as the result of involuntary care were terribly traumatized by their experiences of being forced into treatment. In writing “Committed,” Dr. Miller and Dr. Hanson set out to understand those experiences, and the contexts, both psychiatric and legal, in which they occurred.
There are two main cases that help tell the “Committed” “story,” Eleanor and Lily. Both were hospitalized involuntarily for psychotic episodes; both improved psychiatrically because of hospitalization; and both currently are functioning well. But years later, Eleanor is still resentful and traumatized, and Lily is grateful.
One of the many strengths of “Committed” is in the open-minded, nonpatronizing way it approaches differences in perspective. Numerous patients were interviewed for the book, and their complaints are taken seriously, not simply dismissed as manifestations of psychosis or “borderline traits.” At the same time, Dr. Miller and Dr. Hanson are well aware of distortions and errors in memory, as well as misperceptions about care, and they share their questioning of patients’ stories.
Another strength of “Committed” is that it does not shy away from controversy. It takes an honest look at the gamut of positions with regard to involuntary treatment, from Dr. E. Fuller Torrey’s Treatment Advocacy Center, which takes the view that it is a disservice not to force patients who are unaware that they are ill into treatment, to the Church of Scientology’s Citizens Commission on Human Rights, which doesn’t believe in the existence of mental illness, and therefore views involuntary treatment as unacceptable under any circumstance. The authors genuinely try to understand each group’s rationale, but they are also courageous enough to state their own position: that involuntary care should be avoided if at all possible but is sometimes necessary as a last resort. They also make the invaluable point that simply placing someone in a locked ward, or assigning him to involuntary outpatient care, will accomplish nothing if there are no adequate services to support his long-term care plan, and that those services require funding.
The book’s one drawback for me is the authors’ choice to break up Eleanor’s and Lily’s cases into segments. I found it a little difficult to pick up where the case had been left off several chapters earlier. But the reason for this choice is clearly that it allows the reader to consider an individual topic in the context of that topic’s application to the main cases.
“Committed” is easy to read and well-written, even waxing poetic at times. In describing Lily, Dr. Miller writes, “Her right cheek was punctuated by the best of dimples.” It is written in such a way that it is easily understandable by a lay person but still has a plethora of information that will be new and useful to mental health professionals. In fact, I am flabbergasted by the amount of research that went into writing it.
Committed bravely addresses the complex question of what it means to take away someone’s rights, not because she committed a crime, but because her mind is not working “normally.” It is an excellent book that should be required reading for anyone interested in the concept of autonomy, which is to say, everyone.
Dr. Twersky-Kengmana is a psychiatrist and psychoanalyst in private practice in New York City.
Psychiatrists are trained to view involuntary treatment as an unpleasant means to a desirable end, a necessary evil. And we make the assumption that patients who are helped by the care they receive involuntarily will ultimately be grateful for that care.
Dinah Miller, MD, and Annette Hanson, MD, were inspired to write “Committed: The Battle Over Involuntary Psychiatric Care,” when they discovered that this assumption is false, that there are no clear data about the long-term effects of involuntary care, and that many patients whose mental illnesses improved as the result of involuntary care were terribly traumatized by their experiences of being forced into treatment. In writing “Committed,” Dr. Miller and Dr. Hanson set out to understand those experiences, and the contexts, both psychiatric and legal, in which they occurred.
There are two main cases that help tell the “Committed” “story,” Eleanor and Lily. Both were hospitalized involuntarily for psychotic episodes; both improved psychiatrically because of hospitalization; and both currently are functioning well. But years later, Eleanor is still resentful and traumatized, and Lily is grateful.
One of the many strengths of “Committed” is in the open-minded, nonpatronizing way it approaches differences in perspective. Numerous patients were interviewed for the book, and their complaints are taken seriously, not simply dismissed as manifestations of psychosis or “borderline traits.” At the same time, Dr. Miller and Dr. Hanson are well aware of distortions and errors in memory, as well as misperceptions about care, and they share their questioning of patients’ stories.
Another strength of “Committed” is that it does not shy away from controversy. It takes an honest look at the gamut of positions with regard to involuntary treatment, from Dr. E. Fuller Torrey’s Treatment Advocacy Center, which takes the view that it is a disservice not to force patients who are unaware that they are ill into treatment, to the Church of Scientology’s Citizens Commission on Human Rights, which doesn’t believe in the existence of mental illness, and therefore views involuntary treatment as unacceptable under any circumstance. The authors genuinely try to understand each group’s rationale, but they are also courageous enough to state their own position: that involuntary care should be avoided if at all possible but is sometimes necessary as a last resort. They also make the invaluable point that simply placing someone in a locked ward, or assigning him to involuntary outpatient care, will accomplish nothing if there are no adequate services to support his long-term care plan, and that those services require funding.
The book’s one drawback for me is the authors’ choice to break up Eleanor’s and Lily’s cases into segments. I found it a little difficult to pick up where the case had been left off several chapters earlier. But the reason for this choice is clearly that it allows the reader to consider an individual topic in the context of that topic’s application to the main cases.
“Committed” is easy to read and well-written, even waxing poetic at times. In describing Lily, Dr. Miller writes, “Her right cheek was punctuated by the best of dimples.” It is written in such a way that it is easily understandable by a lay person but still has a plethora of information that will be new and useful to mental health professionals. In fact, I am flabbergasted by the amount of research that went into writing it.
Committed bravely addresses the complex question of what it means to take away someone’s rights, not because she committed a crime, but because her mind is not working “normally.” It is an excellent book that should be required reading for anyone interested in the concept of autonomy, which is to say, everyone.
Dr. Twersky-Kengmana is a psychiatrist and psychoanalyst in private practice in New York City.
Psychiatrists are trained to view involuntary treatment as an unpleasant means to a desirable end, a necessary evil. And we make the assumption that patients who are helped by the care they receive involuntarily will ultimately be grateful for that care.
Dinah Miller, MD, and Annette Hanson, MD, were inspired to write “Committed: The Battle Over Involuntary Psychiatric Care,” when they discovered that this assumption is false, that there are no clear data about the long-term effects of involuntary care, and that many patients whose mental illnesses improved as the result of involuntary care were terribly traumatized by their experiences of being forced into treatment. In writing “Committed,” Dr. Miller and Dr. Hanson set out to understand those experiences, and the contexts, both psychiatric and legal, in which they occurred.
There are two main cases that help tell the “Committed” “story,” Eleanor and Lily. Both were hospitalized involuntarily for psychotic episodes; both improved psychiatrically because of hospitalization; and both currently are functioning well. But years later, Eleanor is still resentful and traumatized, and Lily is grateful.
One of the many strengths of “Committed” is in the open-minded, nonpatronizing way it approaches differences in perspective. Numerous patients were interviewed for the book, and their complaints are taken seriously, not simply dismissed as manifestations of psychosis or “borderline traits.” At the same time, Dr. Miller and Dr. Hanson are well aware of distortions and errors in memory, as well as misperceptions about care, and they share their questioning of patients’ stories.
Another strength of “Committed” is that it does not shy away from controversy. It takes an honest look at the gamut of positions with regard to involuntary treatment, from Dr. E. Fuller Torrey’s Treatment Advocacy Center, which takes the view that it is a disservice not to force patients who are unaware that they are ill into treatment, to the Church of Scientology’s Citizens Commission on Human Rights, which doesn’t believe in the existence of mental illness, and therefore views involuntary treatment as unacceptable under any circumstance. The authors genuinely try to understand each group’s rationale, but they are also courageous enough to state their own position: that involuntary care should be avoided if at all possible but is sometimes necessary as a last resort. They also make the invaluable point that simply placing someone in a locked ward, or assigning him to involuntary outpatient care, will accomplish nothing if there are no adequate services to support his long-term care plan, and that those services require funding.
The book’s one drawback for me is the authors’ choice to break up Eleanor’s and Lily’s cases into segments. I found it a little difficult to pick up where the case had been left off several chapters earlier. But the reason for this choice is clearly that it allows the reader to consider an individual topic in the context of that topic’s application to the main cases.
“Committed” is easy to read and well-written, even waxing poetic at times. In describing Lily, Dr. Miller writes, “Her right cheek was punctuated by the best of dimples.” It is written in such a way that it is easily understandable by a lay person but still has a plethora of information that will be new and useful to mental health professionals. In fact, I am flabbergasted by the amount of research that went into writing it.
Committed bravely addresses the complex question of what it means to take away someone’s rights, not because she committed a crime, but because her mind is not working “normally.” It is an excellent book that should be required reading for anyone interested in the concept of autonomy, which is to say, everyone.
Dr. Twersky-Kengmana is a psychiatrist and psychoanalyst in private practice in New York City.
Riociguat shows benefit in PAH with poor response to PDE inhibitors
LOS ANGELES – Pulmonary artery hypertension patients who have an insufficient response to phosphodiesterase inhibitors may benefit from a switch to riociguat (Adempas), based on results from a 24-week, open-label investigation from the drug’s maker, Bayer.
The 61 patients – mean age 54 years, 74% of whom were women – had World Health Organization functional class 3 disease, with 6-minute walking distances of 165-440 meters, and cardiac indices below 3 L/min/m2 despite being on a phosphodiesterase inhibitor (PDEi) for 90 days or more, 40 (66%) on sildenafil (Viagra) and 21 (34%) on tadalafil (Cialis).
After PDEi washout, the researchers swapped them for riociguat titrated to a maximum oral dose of 2.5 mg t.i.d. The 50 patients (82%) on concomitant endothelin receptor antagonists (ERA) were allowed to stay on them. At week 24, 16 patients (34% of the 47 evaluable patients) achieved the combined endpoint of no clinical worsening, functional class 1 or 2, and 30 meters or more improvement on their walk test.
“The key point is that it was safe to switch people from a [PDEi] to riociguat, and it seemed to have improved efficacy, but we hesitate on these conclusions because it was an open-label trial, and the results need to be confirmed,” lead investigator James Klinger, MD, professor of medicine at Brown University, Providence, R.I., said at the annual meeting of the American College of Chest Physicians. A randomized, controlled trial is underway, he added.
The usual approach to PAH is to start patients on a PDEi, often with an ERA. If that doesn’t work, a prostacyclin is added. The cost can exceed $200,000 a year.
“This is a study that says, wait a minute, before you add a third drug to the first two, maybe it would be worthwhile to” switch out the PDEi. “You would save the person the addition of a third drug,” and the cost would be comparable or less than a three-drug regimen, Dr. Klinger said.
The researchers also found that after 24 weeks on riociguat, mean plasma cyclic guanosine monophosphate (cGMP) increased by 4.6 pmol/mL, urinary cGMP by 1,005 pmol/mL, and asymmetric dimethylarginine (ADMA) by 0.004 micromol/L.
The biomarkers were important because the team is trying to find a way to identify patients who would benefit most from riociguat. “As a field, we still don’t understand the disease well enough to know” how to best match patients and drugs. “We are trying to find a biomarker that would identify patients who are more likely to respond to one medication versus another,” Dr. Klinger said.
ADMA inhibits the production of nitric oxide, and is a bad player in PAH. The study results were reassuring because riociguat didn’t have much of an effect on ADMA levels, although the “slight difference was not enough to identify people who might do better with” it, Dr. Klinger said.
As for cGMP, “when we raise [levels] with riociguat, patients get better, so we think riociguat is raising cGMP in the right place, which we think is the lungs. Unfortunately, we don’t’ have a biomarker that can distinguish lung cGMP from total body cGMP,” he said.
The work was funded by Bayer, maker of riociguat. Dr. Klinger is an unpaid consultant, and also receives research support from the company.
LOS ANGELES – Pulmonary artery hypertension patients who have an insufficient response to phosphodiesterase inhibitors may benefit from a switch to riociguat (Adempas), based on results from a 24-week, open-label investigation from the drug’s maker, Bayer.
The 61 patients – mean age 54 years, 74% of whom were women – had World Health Organization functional class 3 disease, with 6-minute walking distances of 165-440 meters, and cardiac indices below 3 L/min/m2 despite being on a phosphodiesterase inhibitor (PDEi) for 90 days or more, 40 (66%) on sildenafil (Viagra) and 21 (34%) on tadalafil (Cialis).
After PDEi washout, the researchers swapped them for riociguat titrated to a maximum oral dose of 2.5 mg t.i.d. The 50 patients (82%) on concomitant endothelin receptor antagonists (ERA) were allowed to stay on them. At week 24, 16 patients (34% of the 47 evaluable patients) achieved the combined endpoint of no clinical worsening, functional class 1 or 2, and 30 meters or more improvement on their walk test.
“The key point is that it was safe to switch people from a [PDEi] to riociguat, and it seemed to have improved efficacy, but we hesitate on these conclusions because it was an open-label trial, and the results need to be confirmed,” lead investigator James Klinger, MD, professor of medicine at Brown University, Providence, R.I., said at the annual meeting of the American College of Chest Physicians. A randomized, controlled trial is underway, he added.
The usual approach to PAH is to start patients on a PDEi, often with an ERA. If that doesn’t work, a prostacyclin is added. The cost can exceed $200,000 a year.
“This is a study that says, wait a minute, before you add a third drug to the first two, maybe it would be worthwhile to” switch out the PDEi. “You would save the person the addition of a third drug,” and the cost would be comparable or less than a three-drug regimen, Dr. Klinger said.
The researchers also found that after 24 weeks on riociguat, mean plasma cyclic guanosine monophosphate (cGMP) increased by 4.6 pmol/mL, urinary cGMP by 1,005 pmol/mL, and asymmetric dimethylarginine (ADMA) by 0.004 micromol/L.
The biomarkers were important because the team is trying to find a way to identify patients who would benefit most from riociguat. “As a field, we still don’t understand the disease well enough to know” how to best match patients and drugs. “We are trying to find a biomarker that would identify patients who are more likely to respond to one medication versus another,” Dr. Klinger said.
ADMA inhibits the production of nitric oxide, and is a bad player in PAH. The study results were reassuring because riociguat didn’t have much of an effect on ADMA levels, although the “slight difference was not enough to identify people who might do better with” it, Dr. Klinger said.
As for cGMP, “when we raise [levels] with riociguat, patients get better, so we think riociguat is raising cGMP in the right place, which we think is the lungs. Unfortunately, we don’t’ have a biomarker that can distinguish lung cGMP from total body cGMP,” he said.
The work was funded by Bayer, maker of riociguat. Dr. Klinger is an unpaid consultant, and also receives research support from the company.
LOS ANGELES – Pulmonary artery hypertension patients who have an insufficient response to phosphodiesterase inhibitors may benefit from a switch to riociguat (Adempas), based on results from a 24-week, open-label investigation from the drug’s maker, Bayer.
The 61 patients – mean age 54 years, 74% of whom were women – had World Health Organization functional class 3 disease, with 6-minute walking distances of 165-440 meters, and cardiac indices below 3 L/min/m2 despite being on a phosphodiesterase inhibitor (PDEi) for 90 days or more, 40 (66%) on sildenafil (Viagra) and 21 (34%) on tadalafil (Cialis).
After PDEi washout, the researchers swapped them for riociguat titrated to a maximum oral dose of 2.5 mg t.i.d. The 50 patients (82%) on concomitant endothelin receptor antagonists (ERA) were allowed to stay on them. At week 24, 16 patients (34% of the 47 evaluable patients) achieved the combined endpoint of no clinical worsening, functional class 1 or 2, and 30 meters or more improvement on their walk test.
“The key point is that it was safe to switch people from a [PDEi] to riociguat, and it seemed to have improved efficacy, but we hesitate on these conclusions because it was an open-label trial, and the results need to be confirmed,” lead investigator James Klinger, MD, professor of medicine at Brown University, Providence, R.I., said at the annual meeting of the American College of Chest Physicians. A randomized, controlled trial is underway, he added.
The usual approach to PAH is to start patients on a PDEi, often with an ERA. If that doesn’t work, a prostacyclin is added. The cost can exceed $200,000 a year.
“This is a study that says, wait a minute, before you add a third drug to the first two, maybe it would be worthwhile to” switch out the PDEi. “You would save the person the addition of a third drug,” and the cost would be comparable or less than a three-drug regimen, Dr. Klinger said.
The researchers also found that after 24 weeks on riociguat, mean plasma cyclic guanosine monophosphate (cGMP) increased by 4.6 pmol/mL, urinary cGMP by 1,005 pmol/mL, and asymmetric dimethylarginine (ADMA) by 0.004 micromol/L.
The biomarkers were important because the team is trying to find a way to identify patients who would benefit most from riociguat. “As a field, we still don’t understand the disease well enough to know” how to best match patients and drugs. “We are trying to find a biomarker that would identify patients who are more likely to respond to one medication versus another,” Dr. Klinger said.
ADMA inhibits the production of nitric oxide, and is a bad player in PAH. The study results were reassuring because riociguat didn’t have much of an effect on ADMA levels, although the “slight difference was not enough to identify people who might do better with” it, Dr. Klinger said.
As for cGMP, “when we raise [levels] with riociguat, patients get better, so we think riociguat is raising cGMP in the right place, which we think is the lungs. Unfortunately, we don’t’ have a biomarker that can distinguish lung cGMP from total body cGMP,” he said.
The work was funded by Bayer, maker of riociguat. Dr. Klinger is an unpaid consultant, and also receives research support from the company.
AT CHEST 2016
Key clinical point:
Major finding: At week 24, 34% of 47 evaluable patients (16%) achieved the combined endpoint of no clinical worsening, functional class 1 or 2, and a 30-meter or more improvement on their walk test.
Data source: Open-label, nonrandomized 24-week study of 61 patients
Disclosures: The work was funded by Bayer, maker of riociguat. The lead investigator is an unpaid consultant, and also receives research support from the company.
Cigna drops preauthorization for buprenorphine in treatment for opioid dependency
Health insurer Cigna will no longer require preauthorization for the opioid partial agonist buprenorphine in patients with opioid use disorder.
Although the change in policy applies to plans nationwide, the announcement came not long after New York State Attorney General Eric T. Schneiderman requested information from the insurer about its medication-assisted treatment policies as part of an investigation into barriers to treatment.
“Under our commercial plans, we have agreed to voluntarily remove prior authorization from all medications used in medication-assisted treatment for opioid use disorder. This will help make it easier for our customers to access coverage for the medications they need,” she said.
Medication-assisted treatment (MAT) employs a combination of psychosocial therapies and full or partial opioid agonists such as methadone, naltrexone, or buprenorphine to treat opioid dependency. The Substance Abuse and Mental Health Services Administration and the Centers for Disease Control and Prevention both endorse the use of MAT, as numerous studies have shown it is significantly more effective than either medication or psychosocial support alone.
Because providers of MAT are federally required to specialize in addiction medicine and obtain a license from the Drug Enforcement Agency to prescribe buprenorphine, the new policy will not affect most clinical practices. For those who treat people with opioid use disorder, however, the impact could be powerful.
“I can tell you in my 10 years or so of prescribing buprenorphine, I have never had a preauthorization turned down,” Margaret Chaplin, MD, a psychiatrist and addiction medicine specialist in New Britain, Conn., said in an interview. “It was really just a hoop we had to jump through that seemed designed to discourage treatment more than to insure appropriate treatment.
“At our clinic, we are thrilled to see this barrier coming down,” said Dr. Chaplin, who also serves as director of Community Mental Health Affiliates in New Britain.
Cheers also went up from others who treat this patient population. American Society of Addiction Medicine President-Elect Kelly J. Clark, MD, MBA, said in a statement: “There is often a small window of opportunity for a person seeking help to engage in treatment. Waiting for days to obtain medication to treat this condition represents an unnecessary risk for a deadly outcome. Hopefully, other payers will follow Cigna’s lead.”
Dr. Chaplin did not have any relevant disclosures.
[email protected]
On Twitter @whitneymcknight
Health insurer Cigna will no longer require preauthorization for the opioid partial agonist buprenorphine in patients with opioid use disorder.
Although the change in policy applies to plans nationwide, the announcement came not long after New York State Attorney General Eric T. Schneiderman requested information from the insurer about its medication-assisted treatment policies as part of an investigation into barriers to treatment.
“Under our commercial plans, we have agreed to voluntarily remove prior authorization from all medications used in medication-assisted treatment for opioid use disorder. This will help make it easier for our customers to access coverage for the medications they need,” she said.
Medication-assisted treatment (MAT) employs a combination of psychosocial therapies and full or partial opioid agonists such as methadone, naltrexone, or buprenorphine to treat opioid dependency. The Substance Abuse and Mental Health Services Administration and the Centers for Disease Control and Prevention both endorse the use of MAT, as numerous studies have shown it is significantly more effective than either medication or psychosocial support alone.
Because providers of MAT are federally required to specialize in addiction medicine and obtain a license from the Drug Enforcement Agency to prescribe buprenorphine, the new policy will not affect most clinical practices. For those who treat people with opioid use disorder, however, the impact could be powerful.
“I can tell you in my 10 years or so of prescribing buprenorphine, I have never had a preauthorization turned down,” Margaret Chaplin, MD, a psychiatrist and addiction medicine specialist in New Britain, Conn., said in an interview. “It was really just a hoop we had to jump through that seemed designed to discourage treatment more than to insure appropriate treatment.
“At our clinic, we are thrilled to see this barrier coming down,” said Dr. Chaplin, who also serves as director of Community Mental Health Affiliates in New Britain.
Cheers also went up from others who treat this patient population. American Society of Addiction Medicine President-Elect Kelly J. Clark, MD, MBA, said in a statement: “There is often a small window of opportunity for a person seeking help to engage in treatment. Waiting for days to obtain medication to treat this condition represents an unnecessary risk for a deadly outcome. Hopefully, other payers will follow Cigna’s lead.”
Dr. Chaplin did not have any relevant disclosures.
[email protected]
On Twitter @whitneymcknight
Health insurer Cigna will no longer require preauthorization for the opioid partial agonist buprenorphine in patients with opioid use disorder.
Although the change in policy applies to plans nationwide, the announcement came not long after New York State Attorney General Eric T. Schneiderman requested information from the insurer about its medication-assisted treatment policies as part of an investigation into barriers to treatment.
“Under our commercial plans, we have agreed to voluntarily remove prior authorization from all medications used in medication-assisted treatment for opioid use disorder. This will help make it easier for our customers to access coverage for the medications they need,” she said.
Medication-assisted treatment (MAT) employs a combination of psychosocial therapies and full or partial opioid agonists such as methadone, naltrexone, or buprenorphine to treat opioid dependency. The Substance Abuse and Mental Health Services Administration and the Centers for Disease Control and Prevention both endorse the use of MAT, as numerous studies have shown it is significantly more effective than either medication or psychosocial support alone.
Because providers of MAT are federally required to specialize in addiction medicine and obtain a license from the Drug Enforcement Agency to prescribe buprenorphine, the new policy will not affect most clinical practices. For those who treat people with opioid use disorder, however, the impact could be powerful.
“I can tell you in my 10 years or so of prescribing buprenorphine, I have never had a preauthorization turned down,” Margaret Chaplin, MD, a psychiatrist and addiction medicine specialist in New Britain, Conn., said in an interview. “It was really just a hoop we had to jump through that seemed designed to discourage treatment more than to insure appropriate treatment.
“At our clinic, we are thrilled to see this barrier coming down,” said Dr. Chaplin, who also serves as director of Community Mental Health Affiliates in New Britain.
Cheers also went up from others who treat this patient population. American Society of Addiction Medicine President-Elect Kelly J. Clark, MD, MBA, said in a statement: “There is often a small window of opportunity for a person seeking help to engage in treatment. Waiting for days to obtain medication to treat this condition represents an unnecessary risk for a deadly outcome. Hopefully, other payers will follow Cigna’s lead.”
Dr. Chaplin did not have any relevant disclosures.
[email protected]
On Twitter @whitneymcknight
Are we OK?
Lately, I’ve been thinking a lot about you and me. No, not being creepy. I meant all of us, vascular surgeons. Something might be wrong. I credit my concern to Dawn Coleman. Last spring, I was attending a session at the SVS and Dr. Coleman’s presentation was running over. The red light signal began to flash and she quickly covered the content of her last few slides. As she finished her talk I thought I heard her say, almost under her breath, that vascular surgeons lead all medical specialists in suicidal ideation. “Did she just say suicidal ideation?”, I asked the person next to me. It was an odd statement and certainly a fact I had never heard.
For the next few weeks, the thought stuck with me. Why us? It turns out the study she was quoting from 1 was an American College of Surgeons survey that found that suicidal ideation (SI) was 6.4% among American surgeons, compared with 3.3% of the general population. And yes, the highest incidence of suicidal ideation was found in vascular surgeons (7.7%). The study also found that 50% of those with SI will make an attempt but only 26% will seek psychiatric treatment. The most commonly stated deterrent to seeking professional help was fear of a negative impact on their licensure. While SI and suicide rates are disproportionately higher in physicians, clinical depression is not. Therefore there are other factors in play. So we must look at burnout and quality of life (QOL) issues.
Unfortunately, there are other markers of a problem in our field. A study3 based on the 2004-2005 Community Tracking Survey (CTS) looked at 6,381 physicians who reported working between 20 and 100 hours per week. The respondents comprised 41 medical specialties. Mean annual hours worked were 2,524, and accounting for a 48-week work-year, this would extrapolate to 52.6 hours per week among all specialties. The authors devised an interesting (arbitrary?) “hours above or below family practice” metric and here vascular surgery is once again the unfortunate winner. Our adjusted mean work hours were 888 above family practice, worst of any specialty.
Recently, Samuel Money, MD, former president of the Society for Clinical Vascular Surgery, sent a survey on physical discomfort to vascular surgeons on behalf of the SCVS. I wonder about the power of suggestion with these types of studies. While filling it out, I found myself thinking, “Now that you mentioned it, my back DOES kind of hurt, Sam, thanks for asking!” I am reminded of the pain scale questions our clinic patients fill out each visit. If these were accurate our waiting room would look like the aftermath of the Battle of Gettysburg. I realize our periodical selection is suboptimal but I don’t think it should cause true agony.
None of this is to disparage the validity of surveys. Vascular surgeons face real health risks related to radiation exposure, repetitive movements, and long surgical times. Spine issues and cataracts have been closely correlated to our vocation, and I do look forward to the results of this study from the SCVS.
So what do we do? Michael Sosin, MD, and his colleagues recently published an extensive review of quality of life and burnout rates across surgical specialties.4
I’ll paraphrase some of their recommendations here:
• Streamline specialty training.
• Optimize reimbursement.
• Reevaluate training paradigms.
• Examine department cultures.
• Look carefully at tort reform.
• Examine the medical education dept.
The good news is that the ACS survey was performed 8 years ago and the work hours report from the Community Tracking Survey is nearly 12 years old. Looking at the recommendations from Dr. Sosin, we have made a massive move to streamline our training with the advent of the 0+5 integrated vascular residencies. Work hours correlate closely to caring for acutely ill patients who require intensive monitoring. The widespread adoption of endovascular options has likely had a positive impact on our work hours with shorter lengths of stay and fewer ICU patients.
Nevertheless, being the lead specialty in several of these surveys should be eye opening, to say the least. We need to take a closer look at burnout, depression, and suicidal ideation in our field. We need to fight with the SVS for serious reform in reimbursements and malpractice legislation. We need to identify and transform malignant department cultures where they exist. Finally, removing the stigma of psychiatric assistance may be the most important goal of all.
References
1. Arch Surg. 2011;146(1):54-62.
2. Ann Surg. 2011;254:558-68.
3. Arch Intern Med. 2011;171(13):1211-3.
4. JAMA Surg. 2016;151(10):970-8.
Dr. Malachi Sheahan III, from the Louisiana State University Health Sciences Center, New Orleans, is the Associate Medical Editor of Vascular Specialist.
Lately, I’ve been thinking a lot about you and me. No, not being creepy. I meant all of us, vascular surgeons. Something might be wrong. I credit my concern to Dawn Coleman. Last spring, I was attending a session at the SVS and Dr. Coleman’s presentation was running over. The red light signal began to flash and she quickly covered the content of her last few slides. As she finished her talk I thought I heard her say, almost under her breath, that vascular surgeons lead all medical specialists in suicidal ideation. “Did she just say suicidal ideation?”, I asked the person next to me. It was an odd statement and certainly a fact I had never heard.
For the next few weeks, the thought stuck with me. Why us? It turns out the study she was quoting from 1 was an American College of Surgeons survey that found that suicidal ideation (SI) was 6.4% among American surgeons, compared with 3.3% of the general population. And yes, the highest incidence of suicidal ideation was found in vascular surgeons (7.7%). The study also found that 50% of those with SI will make an attempt but only 26% will seek psychiatric treatment. The most commonly stated deterrent to seeking professional help was fear of a negative impact on their licensure. While SI and suicide rates are disproportionately higher in physicians, clinical depression is not. Therefore there are other factors in play. So we must look at burnout and quality of life (QOL) issues.
Unfortunately, there are other markers of a problem in our field. A study3 based on the 2004-2005 Community Tracking Survey (CTS) looked at 6,381 physicians who reported working between 20 and 100 hours per week. The respondents comprised 41 medical specialties. Mean annual hours worked were 2,524, and accounting for a 48-week work-year, this would extrapolate to 52.6 hours per week among all specialties. The authors devised an interesting (arbitrary?) “hours above or below family practice” metric and here vascular surgery is once again the unfortunate winner. Our adjusted mean work hours were 888 above family practice, worst of any specialty.
Recently, Samuel Money, MD, former president of the Society for Clinical Vascular Surgery, sent a survey on physical discomfort to vascular surgeons on behalf of the SCVS. I wonder about the power of suggestion with these types of studies. While filling it out, I found myself thinking, “Now that you mentioned it, my back DOES kind of hurt, Sam, thanks for asking!” I am reminded of the pain scale questions our clinic patients fill out each visit. If these were accurate our waiting room would look like the aftermath of the Battle of Gettysburg. I realize our periodical selection is suboptimal but I don’t think it should cause true agony.
None of this is to disparage the validity of surveys. Vascular surgeons face real health risks related to radiation exposure, repetitive movements, and long surgical times. Spine issues and cataracts have been closely correlated to our vocation, and I do look forward to the results of this study from the SCVS.
So what do we do? Michael Sosin, MD, and his colleagues recently published an extensive review of quality of life and burnout rates across surgical specialties.4
I’ll paraphrase some of their recommendations here:
• Streamline specialty training.
• Optimize reimbursement.
• Reevaluate training paradigms.
• Examine department cultures.
• Look carefully at tort reform.
• Examine the medical education dept.
The good news is that the ACS survey was performed 8 years ago and the work hours report from the Community Tracking Survey is nearly 12 years old. Looking at the recommendations from Dr. Sosin, we have made a massive move to streamline our training with the advent of the 0+5 integrated vascular residencies. Work hours correlate closely to caring for acutely ill patients who require intensive monitoring. The widespread adoption of endovascular options has likely had a positive impact on our work hours with shorter lengths of stay and fewer ICU patients.
Nevertheless, being the lead specialty in several of these surveys should be eye opening, to say the least. We need to take a closer look at burnout, depression, and suicidal ideation in our field. We need to fight with the SVS for serious reform in reimbursements and malpractice legislation. We need to identify and transform malignant department cultures where they exist. Finally, removing the stigma of psychiatric assistance may be the most important goal of all.
References
1. Arch Surg. 2011;146(1):54-62.
2. Ann Surg. 2011;254:558-68.
3. Arch Intern Med. 2011;171(13):1211-3.
4. JAMA Surg. 2016;151(10):970-8.
Dr. Malachi Sheahan III, from the Louisiana State University Health Sciences Center, New Orleans, is the Associate Medical Editor of Vascular Specialist.
Lately, I’ve been thinking a lot about you and me. No, not being creepy. I meant all of us, vascular surgeons. Something might be wrong. I credit my concern to Dawn Coleman. Last spring, I was attending a session at the SVS and Dr. Coleman’s presentation was running over. The red light signal began to flash and she quickly covered the content of her last few slides. As she finished her talk I thought I heard her say, almost under her breath, that vascular surgeons lead all medical specialists in suicidal ideation. “Did she just say suicidal ideation?”, I asked the person next to me. It was an odd statement and certainly a fact I had never heard.
For the next few weeks, the thought stuck with me. Why us? It turns out the study she was quoting from 1 was an American College of Surgeons survey that found that suicidal ideation (SI) was 6.4% among American surgeons, compared with 3.3% of the general population. And yes, the highest incidence of suicidal ideation was found in vascular surgeons (7.7%). The study also found that 50% of those with SI will make an attempt but only 26% will seek psychiatric treatment. The most commonly stated deterrent to seeking professional help was fear of a negative impact on their licensure. While SI and suicide rates are disproportionately higher in physicians, clinical depression is not. Therefore there are other factors in play. So we must look at burnout and quality of life (QOL) issues.
Unfortunately, there are other markers of a problem in our field. A study3 based on the 2004-2005 Community Tracking Survey (CTS) looked at 6,381 physicians who reported working between 20 and 100 hours per week. The respondents comprised 41 medical specialties. Mean annual hours worked were 2,524, and accounting for a 48-week work-year, this would extrapolate to 52.6 hours per week among all specialties. The authors devised an interesting (arbitrary?) “hours above or below family practice” metric and here vascular surgery is once again the unfortunate winner. Our adjusted mean work hours were 888 above family practice, worst of any specialty.
Recently, Samuel Money, MD, former president of the Society for Clinical Vascular Surgery, sent a survey on physical discomfort to vascular surgeons on behalf of the SCVS. I wonder about the power of suggestion with these types of studies. While filling it out, I found myself thinking, “Now that you mentioned it, my back DOES kind of hurt, Sam, thanks for asking!” I am reminded of the pain scale questions our clinic patients fill out each visit. If these were accurate our waiting room would look like the aftermath of the Battle of Gettysburg. I realize our periodical selection is suboptimal but I don’t think it should cause true agony.
None of this is to disparage the validity of surveys. Vascular surgeons face real health risks related to radiation exposure, repetitive movements, and long surgical times. Spine issues and cataracts have been closely correlated to our vocation, and I do look forward to the results of this study from the SCVS.
So what do we do? Michael Sosin, MD, and his colleagues recently published an extensive review of quality of life and burnout rates across surgical specialties.4
I’ll paraphrase some of their recommendations here:
• Streamline specialty training.
• Optimize reimbursement.
• Reevaluate training paradigms.
• Examine department cultures.
• Look carefully at tort reform.
• Examine the medical education dept.
The good news is that the ACS survey was performed 8 years ago and the work hours report from the Community Tracking Survey is nearly 12 years old. Looking at the recommendations from Dr. Sosin, we have made a massive move to streamline our training with the advent of the 0+5 integrated vascular residencies. Work hours correlate closely to caring for acutely ill patients who require intensive monitoring. The widespread adoption of endovascular options has likely had a positive impact on our work hours with shorter lengths of stay and fewer ICU patients.
Nevertheless, being the lead specialty in several of these surveys should be eye opening, to say the least. We need to take a closer look at burnout, depression, and suicidal ideation in our field. We need to fight with the SVS for serious reform in reimbursements and malpractice legislation. We need to identify and transform malignant department cultures where they exist. Finally, removing the stigma of psychiatric assistance may be the most important goal of all.
References
1. Arch Surg. 2011;146(1):54-62.
2. Ann Surg. 2011;254:558-68.
3. Arch Intern Med. 2011;171(13):1211-3.
4. JAMA Surg. 2016;151(10):970-8.
Dr. Malachi Sheahan III, from the Louisiana State University Health Sciences Center, New Orleans, is the Associate Medical Editor of Vascular Specialist.