User login
‘Doc in a box’ vs.’tele-teaming’: Contending models of telepsychiatric care
As articulated by Steve Daviss, MD, DFAPA, in the inaugural column of Techiatry, the adoption and diffusion of telepsychiatry (live two-way interactive videoconferencing) have not been as rapid and universal as expected from those of us immersed in the field.
Despite having yet to achieve its full promise, telepsychiatry has reached maturity, and is being widely deployed and used across multiple systems, settings, and applications, albeit at times in an uneven, unsystematic manner. Emerging over the past decade of development are two distinct models/approaches to telepsychiatry, which I refer to as “doc in a box” and “tele-teaming.” Those models coexist, compete, and conflict within and across organizations. The dynamic between those two models highlights a larger emergent dialogue within psychiatry around psychiatrists’ core clinical roles and functions in our evolving health care systems.
The doc in a box model, as captured in early telepsychiatry services in the 1990s and early 2000s, focused on the virtual insertion of a solo psychiatrist into a distant setting. The services involved core psychiatric activities, such as diagnosis and assessment, with a heavy emphasis on pharmacologic management.
Not surprisingly, those services paralleled what was occurring for the rest of psychiatry at the time and were driven by the closer alignment of psychiatry with a biologic framework – and most importantly, reimbursement models that favored pharmacologic interventions and management. The subsequent rise of viable commercial telepsychiatry companies has continued offering this model driven by demands of the marketplace. While there is a legitimate place and need for such services, the phrase “doc in a box” narrows the scope of psychiatric practice, and reinforces current systems of health care structure and funding.
I proffer the phrase “tele-teaming” to denote telepsychiatric care that virtually embeds a psychiatrist as a member of a care team at a distant location. The use of telepsychiatry in integrated care is the clearest example of this. In integrated care, a psychiatrist works as part of a larger behavioral and medical team that may include case managers, social workers, psychologists, nurses, and family physicians to render care to patients in primary care clinics. The psychiatrist performs consultative, direct care, and supervisory roles in the context of the integrated care team, focusing on more holistic and population-based approaches to treatment.
Telepsychiatry, as well as other technologies (for example, electronic medical records, email, and patient registries), enables and enhances integrated care. Telepsychiatry allows smaller primary care practices, which on their own could not support a full-time psychiatrist, to create a full virtual team across multiple sites.
Tele-teaming as a concept is not limited to integrated care. Other notable examples include the use of telepsychiatry in substance rehabilitation facilities, long-term nursing homes, and psychiatric emergency services as a component of ERs. Tele-teaming and doc in a box models are not about the settings or populations to which they provide care, but the structure and philosophy of the psychiatric service.
As an illustration, imagine a rural community mental health center whose long-term psychiatric care provider retires. The center could set up a contract with a psychiatrist to provide medication management services for its patients through telepsychiatry. The psychiatrist, armed with a pen or eprescribing credentials, could provide several days a week of medication management. Treatment planning, therapy, and care coordination could be segmented off to other providers from the center (psychologists, social workers, and case managers). The psychiatrist’s time could be maximized by having the psychiatrist manage prescriptions, with communication between the patients’ various providers through a shared electronic medical record, as in the doc in a box model.
Alternatively, the center could set up a service where the psychiatrist became a virtual team member working to provide complete assessments, treatment planning, supervision, and psychiatric consultation, as well as pharmacologic management. Although in this scenario, the psychiatrist still could devote time to managing prescriptions, she/he also could spend time in team meetings, supervision, and seeing patients, often with her colleagues. In addition, the psychiatrist could work to coordinate care beyond the EMR, for example, through tele-teaming. Either of these scenarios can and do occur with in-person care as well, and the choice of which model to use would not be decided by the technology but by the underlying health care system in which it is being used.
Telepsychiatry begins to become transformative when it’s leveraged to shift, change, or innovate the model of care delivery. Historically, telepsychiatry has been thought of as a solution to patient access issues and as a way to address workforce shortages. The real promise of telepsychiatry in the form of tele-teaming is how it can begin to fundamentally change how care is delivered. The triple aim calls for decreased costs, improved health care for individuals, and a focus on population health.
We know that most Americans with major mental health issues will be seen in primary care, and that providing behavioral health care in primary care settings leads to lower overall health care costs, and improved behavioral and general health outcomes. Telepsychiatry will be essential in the further dissemination and expansion of integrated care, not only in rural and underserved areas, but across large health care systems. Tele-teaming will be essential in other arenas as well, as psychiatry faces the future challenges of an aging and decreasing (per capita) workforce. Team approaches help maximize psychiatrists’ role in the health care system, and help magnify the number of patients their skills and training can support.
Team approaches also help to broaden the scope of psychiatric practice and reclaim psychiatry’s place within the house of medicine. Telepsychiatry, if structured correctly, helps increase flexibility for psychiatrists. It expands the settings available to practice and provides more opportunities to engage in team-based care. Both the doc in a box and tele-teaming models have arisen from forces in the health care marketplace. The differing application of those two models across and within health care systems illustrates overall tensions within psychiatry about the current and future roles of psychiatrists.
Telepsychiatry is a powerful tool that can be leveraged to shape models of psychiatric care delivery or reinforce our existing structures. Which models are embraced will affect how psychiatry continues to evolve and address the challenges before the specialty.
Dr. Shore chairs the American Psychiatric Association’s Committee on Telepsychiatry, and is director of telemedicine at the Helen & Arthur E. Johnson Depression Center and associate professor of psychiatry at the University of Colorado at Denver, Aurora.
As articulated by Steve Daviss, MD, DFAPA, in the inaugural column of Techiatry, the adoption and diffusion of telepsychiatry (live two-way interactive videoconferencing) have not been as rapid and universal as expected from those of us immersed in the field.
Despite having yet to achieve its full promise, telepsychiatry has reached maturity, and is being widely deployed and used across multiple systems, settings, and applications, albeit at times in an uneven, unsystematic manner. Emerging over the past decade of development are two distinct models/approaches to telepsychiatry, which I refer to as “doc in a box” and “tele-teaming.” Those models coexist, compete, and conflict within and across organizations. The dynamic between those two models highlights a larger emergent dialogue within psychiatry around psychiatrists’ core clinical roles and functions in our evolving health care systems.
The doc in a box model, as captured in early telepsychiatry services in the 1990s and early 2000s, focused on the virtual insertion of a solo psychiatrist into a distant setting. The services involved core psychiatric activities, such as diagnosis and assessment, with a heavy emphasis on pharmacologic management.
Not surprisingly, those services paralleled what was occurring for the rest of psychiatry at the time and were driven by the closer alignment of psychiatry with a biologic framework – and most importantly, reimbursement models that favored pharmacologic interventions and management. The subsequent rise of viable commercial telepsychiatry companies has continued offering this model driven by demands of the marketplace. While there is a legitimate place and need for such services, the phrase “doc in a box” narrows the scope of psychiatric practice, and reinforces current systems of health care structure and funding.
I proffer the phrase “tele-teaming” to denote telepsychiatric care that virtually embeds a psychiatrist as a member of a care team at a distant location. The use of telepsychiatry in integrated care is the clearest example of this. In integrated care, a psychiatrist works as part of a larger behavioral and medical team that may include case managers, social workers, psychologists, nurses, and family physicians to render care to patients in primary care clinics. The psychiatrist performs consultative, direct care, and supervisory roles in the context of the integrated care team, focusing on more holistic and population-based approaches to treatment.
Telepsychiatry, as well as other technologies (for example, electronic medical records, email, and patient registries), enables and enhances integrated care. Telepsychiatry allows smaller primary care practices, which on their own could not support a full-time psychiatrist, to create a full virtual team across multiple sites.
Tele-teaming as a concept is not limited to integrated care. Other notable examples include the use of telepsychiatry in substance rehabilitation facilities, long-term nursing homes, and psychiatric emergency services as a component of ERs. Tele-teaming and doc in a box models are not about the settings or populations to which they provide care, but the structure and philosophy of the psychiatric service.
As an illustration, imagine a rural community mental health center whose long-term psychiatric care provider retires. The center could set up a contract with a psychiatrist to provide medication management services for its patients through telepsychiatry. The psychiatrist, armed with a pen or eprescribing credentials, could provide several days a week of medication management. Treatment planning, therapy, and care coordination could be segmented off to other providers from the center (psychologists, social workers, and case managers). The psychiatrist’s time could be maximized by having the psychiatrist manage prescriptions, with communication between the patients’ various providers through a shared electronic medical record, as in the doc in a box model.
Alternatively, the center could set up a service where the psychiatrist became a virtual team member working to provide complete assessments, treatment planning, supervision, and psychiatric consultation, as well as pharmacologic management. Although in this scenario, the psychiatrist still could devote time to managing prescriptions, she/he also could spend time in team meetings, supervision, and seeing patients, often with her colleagues. In addition, the psychiatrist could work to coordinate care beyond the EMR, for example, through tele-teaming. Either of these scenarios can and do occur with in-person care as well, and the choice of which model to use would not be decided by the technology but by the underlying health care system in which it is being used.
Telepsychiatry begins to become transformative when it’s leveraged to shift, change, or innovate the model of care delivery. Historically, telepsychiatry has been thought of as a solution to patient access issues and as a way to address workforce shortages. The real promise of telepsychiatry in the form of tele-teaming is how it can begin to fundamentally change how care is delivered. The triple aim calls for decreased costs, improved health care for individuals, and a focus on population health.
We know that most Americans with major mental health issues will be seen in primary care, and that providing behavioral health care in primary care settings leads to lower overall health care costs, and improved behavioral and general health outcomes. Telepsychiatry will be essential in the further dissemination and expansion of integrated care, not only in rural and underserved areas, but across large health care systems. Tele-teaming will be essential in other arenas as well, as psychiatry faces the future challenges of an aging and decreasing (per capita) workforce. Team approaches help maximize psychiatrists’ role in the health care system, and help magnify the number of patients their skills and training can support.
Team approaches also help to broaden the scope of psychiatric practice and reclaim psychiatry’s place within the house of medicine. Telepsychiatry, if structured correctly, helps increase flexibility for psychiatrists. It expands the settings available to practice and provides more opportunities to engage in team-based care. Both the doc in a box and tele-teaming models have arisen from forces in the health care marketplace. The differing application of those two models across and within health care systems illustrates overall tensions within psychiatry about the current and future roles of psychiatrists.
Telepsychiatry is a powerful tool that can be leveraged to shape models of psychiatric care delivery or reinforce our existing structures. Which models are embraced will affect how psychiatry continues to evolve and address the challenges before the specialty.
Dr. Shore chairs the American Psychiatric Association’s Committee on Telepsychiatry, and is director of telemedicine at the Helen & Arthur E. Johnson Depression Center and associate professor of psychiatry at the University of Colorado at Denver, Aurora.
As articulated by Steve Daviss, MD, DFAPA, in the inaugural column of Techiatry, the adoption and diffusion of telepsychiatry (live two-way interactive videoconferencing) have not been as rapid and universal as expected from those of us immersed in the field.
Despite having yet to achieve its full promise, telepsychiatry has reached maturity, and is being widely deployed and used across multiple systems, settings, and applications, albeit at times in an uneven, unsystematic manner. Emerging over the past decade of development are two distinct models/approaches to telepsychiatry, which I refer to as “doc in a box” and “tele-teaming.” Those models coexist, compete, and conflict within and across organizations. The dynamic between those two models highlights a larger emergent dialogue within psychiatry around psychiatrists’ core clinical roles and functions in our evolving health care systems.
The doc in a box model, as captured in early telepsychiatry services in the 1990s and early 2000s, focused on the virtual insertion of a solo psychiatrist into a distant setting. The services involved core psychiatric activities, such as diagnosis and assessment, with a heavy emphasis on pharmacologic management.
Not surprisingly, those services paralleled what was occurring for the rest of psychiatry at the time and were driven by the closer alignment of psychiatry with a biologic framework – and most importantly, reimbursement models that favored pharmacologic interventions and management. The subsequent rise of viable commercial telepsychiatry companies has continued offering this model driven by demands of the marketplace. While there is a legitimate place and need for such services, the phrase “doc in a box” narrows the scope of psychiatric practice, and reinforces current systems of health care structure and funding.
I proffer the phrase “tele-teaming” to denote telepsychiatric care that virtually embeds a psychiatrist as a member of a care team at a distant location. The use of telepsychiatry in integrated care is the clearest example of this. In integrated care, a psychiatrist works as part of a larger behavioral and medical team that may include case managers, social workers, psychologists, nurses, and family physicians to render care to patients in primary care clinics. The psychiatrist performs consultative, direct care, and supervisory roles in the context of the integrated care team, focusing on more holistic and population-based approaches to treatment.
Telepsychiatry, as well as other technologies (for example, electronic medical records, email, and patient registries), enables and enhances integrated care. Telepsychiatry allows smaller primary care practices, which on their own could not support a full-time psychiatrist, to create a full virtual team across multiple sites.
Tele-teaming as a concept is not limited to integrated care. Other notable examples include the use of telepsychiatry in substance rehabilitation facilities, long-term nursing homes, and psychiatric emergency services as a component of ERs. Tele-teaming and doc in a box models are not about the settings or populations to which they provide care, but the structure and philosophy of the psychiatric service.
As an illustration, imagine a rural community mental health center whose long-term psychiatric care provider retires. The center could set up a contract with a psychiatrist to provide medication management services for its patients through telepsychiatry. The psychiatrist, armed with a pen or eprescribing credentials, could provide several days a week of medication management. Treatment planning, therapy, and care coordination could be segmented off to other providers from the center (psychologists, social workers, and case managers). The psychiatrist’s time could be maximized by having the psychiatrist manage prescriptions, with communication between the patients’ various providers through a shared electronic medical record, as in the doc in a box model.
Alternatively, the center could set up a service where the psychiatrist became a virtual team member working to provide complete assessments, treatment planning, supervision, and psychiatric consultation, as well as pharmacologic management. Although in this scenario, the psychiatrist still could devote time to managing prescriptions, she/he also could spend time in team meetings, supervision, and seeing patients, often with her colleagues. In addition, the psychiatrist could work to coordinate care beyond the EMR, for example, through tele-teaming. Either of these scenarios can and do occur with in-person care as well, and the choice of which model to use would not be decided by the technology but by the underlying health care system in which it is being used.
Telepsychiatry begins to become transformative when it’s leveraged to shift, change, or innovate the model of care delivery. Historically, telepsychiatry has been thought of as a solution to patient access issues and as a way to address workforce shortages. The real promise of telepsychiatry in the form of tele-teaming is how it can begin to fundamentally change how care is delivered. The triple aim calls for decreased costs, improved health care for individuals, and a focus on population health.
We know that most Americans with major mental health issues will be seen in primary care, and that providing behavioral health care in primary care settings leads to lower overall health care costs, and improved behavioral and general health outcomes. Telepsychiatry will be essential in the further dissemination and expansion of integrated care, not only in rural and underserved areas, but across large health care systems. Tele-teaming will be essential in other arenas as well, as psychiatry faces the future challenges of an aging and decreasing (per capita) workforce. Team approaches help maximize psychiatrists’ role in the health care system, and help magnify the number of patients their skills and training can support.
Team approaches also help to broaden the scope of psychiatric practice and reclaim psychiatry’s place within the house of medicine. Telepsychiatry, if structured correctly, helps increase flexibility for psychiatrists. It expands the settings available to practice and provides more opportunities to engage in team-based care. Both the doc in a box and tele-teaming models have arisen from forces in the health care marketplace. The differing application of those two models across and within health care systems illustrates overall tensions within psychiatry about the current and future roles of psychiatrists.
Telepsychiatry is a powerful tool that can be leveraged to shape models of psychiatric care delivery or reinforce our existing structures. Which models are embraced will affect how psychiatry continues to evolve and address the challenges before the specialty.
Dr. Shore chairs the American Psychiatric Association’s Committee on Telepsychiatry, and is director of telemedicine at the Helen & Arthur E. Johnson Depression Center and associate professor of psychiatry at the University of Colorado at Denver, Aurora.
Inhaled antibiotic for bronchiectasis shows promise
AT CHEST 2016
LOS ANGELES – Long-term inhaled ciprofloxacin therapy appears to be a safe and effective treatment option in patients with bronchiectasis, results from an international phase III trial showed.
“This is really exciting; it’s the first large study of an inhaled antibiotic to show a benefit in this population,” study investigator Kevin Winthrop, MD, said in an interview prior to the annual meeting of the American College of Chest Physicians. “There’s a tremendous unmet need and a lot of these patients have daily struggles and their quality of life is low. To have something that would improve that would be a benefit for patients and physicians alike.”
Compared with patients in the placebo arm, those in the ciprofloxacin dry powder for inhalation (DPI) 14-day on/off arm experienced a significantly prolonged time to first exacerbation (a mean of 336 days versus 186 days, respectively; adjusted hazard ratio, 0.53; P = .0005) and a significantly reduced exacerbation frequency over 48 weeks (a mean of 0.78 vs. 1.42; adjusted incident rate of 0.61; P = .0061). A nonsignificant trend in favor of ciprofloxacin DPI was observed for both primary endpoints among patients in the 28-day on/off arm (time to first exacerbation: HR, 0.73; P = .065; frequency of exacerbations: adjusted incidence rate ratio, 0.98; P = .89).
Treatment-emergent adverse events and adverse events leading to discontinuation were similar across treatment groups (82% in the ciprofloxacin DPI 14-day on/off arm, 83% in the ciprofloxacin DPI 28-day on/off arm, and 83% in the pooled placebo arm. The rates of serious adverse events were also similar in the three treatment groups (17%, 20%, and 23%, respectively). “Tolerability markers like hoarseness, bronchospasm, shortness of breath, or increased cough were similar between the treatment arms,” said Dr. Winthrop, who is an infectious diseases specialist at Oregon Health and Science University, Portland.“The safety profile looks really good. There were no typical fluoroquinolone types of problems such as tendinopathy reported.”
A follow-up trial known as RESPIRE 2 is ongoing. RESPIRE 1 was funded by Bayer. Dr. Winthrop disclosed that he is a consultant for the company.
This article was updated on 10/25/2016 at 9:51 AM Est
AT CHEST 2016
LOS ANGELES – Long-term inhaled ciprofloxacin therapy appears to be a safe and effective treatment option in patients with bronchiectasis, results from an international phase III trial showed.
“This is really exciting; it’s the first large study of an inhaled antibiotic to show a benefit in this population,” study investigator Kevin Winthrop, MD, said in an interview prior to the annual meeting of the American College of Chest Physicians. “There’s a tremendous unmet need and a lot of these patients have daily struggles and their quality of life is low. To have something that would improve that would be a benefit for patients and physicians alike.”
Compared with patients in the placebo arm, those in the ciprofloxacin dry powder for inhalation (DPI) 14-day on/off arm experienced a significantly prolonged time to first exacerbation (a mean of 336 days versus 186 days, respectively; adjusted hazard ratio, 0.53; P = .0005) and a significantly reduced exacerbation frequency over 48 weeks (a mean of 0.78 vs. 1.42; adjusted incident rate of 0.61; P = .0061). A nonsignificant trend in favor of ciprofloxacin DPI was observed for both primary endpoints among patients in the 28-day on/off arm (time to first exacerbation: HR, 0.73; P = .065; frequency of exacerbations: adjusted incidence rate ratio, 0.98; P = .89).
Treatment-emergent adverse events and adverse events leading to discontinuation were similar across treatment groups (82% in the ciprofloxacin DPI 14-day on/off arm, 83% in the ciprofloxacin DPI 28-day on/off arm, and 83% in the pooled placebo arm. The rates of serious adverse events were also similar in the three treatment groups (17%, 20%, and 23%, respectively). “Tolerability markers like hoarseness, bronchospasm, shortness of breath, or increased cough were similar between the treatment arms,” said Dr. Winthrop, who is an infectious diseases specialist at Oregon Health and Science University, Portland.“The safety profile looks really good. There were no typical fluoroquinolone types of problems such as tendinopathy reported.”
A follow-up trial known as RESPIRE 2 is ongoing. RESPIRE 1 was funded by Bayer. Dr. Winthrop disclosed that he is a consultant for the company.
This article was updated on 10/25/2016 at 9:51 AM Est
AT CHEST 2016
LOS ANGELES – Long-term inhaled ciprofloxacin therapy appears to be a safe and effective treatment option in patients with bronchiectasis, results from an international phase III trial showed.
“This is really exciting; it’s the first large study of an inhaled antibiotic to show a benefit in this population,” study investigator Kevin Winthrop, MD, said in an interview prior to the annual meeting of the American College of Chest Physicians. “There’s a tremendous unmet need and a lot of these patients have daily struggles and their quality of life is low. To have something that would improve that would be a benefit for patients and physicians alike.”
Compared with patients in the placebo arm, those in the ciprofloxacin dry powder for inhalation (DPI) 14-day on/off arm experienced a significantly prolonged time to first exacerbation (a mean of 336 days versus 186 days, respectively; adjusted hazard ratio, 0.53; P = .0005) and a significantly reduced exacerbation frequency over 48 weeks (a mean of 0.78 vs. 1.42; adjusted incident rate of 0.61; P = .0061). A nonsignificant trend in favor of ciprofloxacin DPI was observed for both primary endpoints among patients in the 28-day on/off arm (time to first exacerbation: HR, 0.73; P = .065; frequency of exacerbations: adjusted incidence rate ratio, 0.98; P = .89).
Treatment-emergent adverse events and adverse events leading to discontinuation were similar across treatment groups (82% in the ciprofloxacin DPI 14-day on/off arm, 83% in the ciprofloxacin DPI 28-day on/off arm, and 83% in the pooled placebo arm. The rates of serious adverse events were also similar in the three treatment groups (17%, 20%, and 23%, respectively). “Tolerability markers like hoarseness, bronchospasm, shortness of breath, or increased cough were similar between the treatment arms,” said Dr. Winthrop, who is an infectious diseases specialist at Oregon Health and Science University, Portland.“The safety profile looks really good. There were no typical fluoroquinolone types of problems such as tendinopathy reported.”
A follow-up trial known as RESPIRE 2 is ongoing. RESPIRE 1 was funded by Bayer. Dr. Winthrop disclosed that he is a consultant for the company.
This article was updated on 10/25/2016 at 9:51 AM Est
Key clinical point:
Major finding: Compared with patients in the placebo arm, those in the ciprofloxacin 14-day on/off arm experienced a significantly prolonged time to first exacerbation (a mean of 336 days vs. 186 days, respectively; adjusted hazard ratio, 0.53; P = .0005).
Data source: A multicenter study of 416 patients who were randomized 2:1 to ciprofloxacin 32.5 mg or placebo administered twice per day using a pocket-sized inhaler as a cyclical regimen of either 14 days on/off drug or 28 days on/off drug, for 48 weeks.
Disclosures: RESPIRE 1 was funded by Bayer. Dr. Winthrop disclosed that he is a consultant for the company.
Guideline: Supplemental, dietary calcium both heart safe
Both dietary and supplemental calcium should be considered safe for the cardiovascular system as long as total intake doesn’t exceed 2,000-2,500 mg/day – the maximal tolerable level defined by the National Academy of Medicine, according to an updated Clinical Practice Guideline published online October 24 in Annals of Internal Medicine.
For generally healthy patients who don’t consume adequate calcium and take supplements, either alone or in combination with vitamin D, to prevent osteoporosis and related fractures, “discontinuation of supplemental calcium for safety reasons is not necessary and may be harmful to bone health,” said Stephen L. Kopecky, MD, of the Mayo Clinic, Rochester Minn., and his associates on the expert panel that wrote the new guideline.
The National Osteoporosis Foundation (NOF) and the American Society for Preventive Cardiology (ASPC) commissioned an independent review of the current evidence to update the Evidence Report and assembled the expert panel to write the guideline based on the new findings (Ann Intern Med. 2016 Oct 24. doi: 10.7326/M16-1743).
Separately, Mei Chung, PhD, of the department of public health and community medicine, and her associates at Tufts University, Boston, reviewed 4 recent randomized clinical trials, 1 nested case-control study, and 26 cohort studies that assessed the effects of calcium intake on 17 health outcomes in generally healthy adults of all ages. None of the studies evaluated cardiovascular disease risk as a primary outcome. “We conclude that calcium intake (from either food or supplement sources) at levels within the recommended tolerable upper intake range (2,000-2,500 mg/d) are not associated with CVD risks in generally healthy adults,” they said.
“Although a few trials and cohort studies reported increased risks with higher calcium intake, risk estimates in most of those studies were small (10% relative risk) and not considered clinically important, even if they were statistically significant,” Dr. Chung and her associates added (Ann Int Med. 2016 Oct 24. doi: 10.7326/M16-1165).
According to the guideline, “The NOF and the ASPC now adopt the position that there is moderate-quality evidence that calcium with or without vitamin D intake from food or supplements has no relationship (beneficial or harmful) with the risk for cardiovascular or cerebrovascular disease, mortality, or all-cause mortality in generally healthy adults at this time.”
In addition, “Currently, no established biological mechanism supports and association between calcium and cardiovascular disease,” Dr. Kopecky and his associates on the expert panel noted.
The volume of literature on the subject of calcium’s potential harmful cardiovascular disease effects appears to be robust, with the largest meta-analysis to date including 18 studies with 64,000 participants. But this evidence base has some limitations, chief among them the fact that none of the studies was designed to evaluate CVD as a primary outcome.
In addition, concerns about harmful cardiovascular effects arose after most of the trials had already been initiated, so unpublished data on those outcomes were collected and adjudicated retrospectively. In addition, many of the participants showed poor long-term treatment adherence, making it difficult to interpret the data.
Karen L. Margolis, MD, of HealthPartners Institute in Minneapolis and JoAnn E. Manson, MD, DrPH, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston, made these remarks in an editorial accompanying the new Clinical Practice Guideline (Ann Intern Med. 2016 Oct 24. doi: 10.7326/M16-2193). Their financial disclosures are available at www.acponline.org.
The volume of literature on the subject of calcium’s potential harmful cardiovascular disease effects appears to be robust, with the largest meta-analysis to date including 18 studies with 64,000 participants. But this evidence base has some limitations, chief among them the fact that none of the studies was designed to evaluate CVD as a primary outcome.
In addition, concerns about harmful cardiovascular effects arose after most of the trials had already been initiated, so unpublished data on those outcomes were collected and adjudicated retrospectively. In addition, many of the participants showed poor long-term treatment adherence, making it difficult to interpret the data.
Karen L. Margolis, MD, of HealthPartners Institute in Minneapolis and JoAnn E. Manson, MD, DrPH, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston, made these remarks in an editorial accompanying the new Clinical Practice Guideline (Ann Intern Med. 2016 Oct 24. doi: 10.7326/M16-2193). Their financial disclosures are available at www.acponline.org.
The volume of literature on the subject of calcium’s potential harmful cardiovascular disease effects appears to be robust, with the largest meta-analysis to date including 18 studies with 64,000 participants. But this evidence base has some limitations, chief among them the fact that none of the studies was designed to evaluate CVD as a primary outcome.
In addition, concerns about harmful cardiovascular effects arose after most of the trials had already been initiated, so unpublished data on those outcomes were collected and adjudicated retrospectively. In addition, many of the participants showed poor long-term treatment adherence, making it difficult to interpret the data.
Karen L. Margolis, MD, of HealthPartners Institute in Minneapolis and JoAnn E. Manson, MD, DrPH, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston, made these remarks in an editorial accompanying the new Clinical Practice Guideline (Ann Intern Med. 2016 Oct 24. doi: 10.7326/M16-2193). Their financial disclosures are available at www.acponline.org.
Both dietary and supplemental calcium should be considered safe for the cardiovascular system as long as total intake doesn’t exceed 2,000-2,500 mg/day – the maximal tolerable level defined by the National Academy of Medicine, according to an updated Clinical Practice Guideline published online October 24 in Annals of Internal Medicine.
For generally healthy patients who don’t consume adequate calcium and take supplements, either alone or in combination with vitamin D, to prevent osteoporosis and related fractures, “discontinuation of supplemental calcium for safety reasons is not necessary and may be harmful to bone health,” said Stephen L. Kopecky, MD, of the Mayo Clinic, Rochester Minn., and his associates on the expert panel that wrote the new guideline.
The National Osteoporosis Foundation (NOF) and the American Society for Preventive Cardiology (ASPC) commissioned an independent review of the current evidence to update the Evidence Report and assembled the expert panel to write the guideline based on the new findings (Ann Intern Med. 2016 Oct 24. doi: 10.7326/M16-1743).
Separately, Mei Chung, PhD, of the department of public health and community medicine, and her associates at Tufts University, Boston, reviewed 4 recent randomized clinical trials, 1 nested case-control study, and 26 cohort studies that assessed the effects of calcium intake on 17 health outcomes in generally healthy adults of all ages. None of the studies evaluated cardiovascular disease risk as a primary outcome. “We conclude that calcium intake (from either food or supplement sources) at levels within the recommended tolerable upper intake range (2,000-2,500 mg/d) are not associated with CVD risks in generally healthy adults,” they said.
“Although a few trials and cohort studies reported increased risks with higher calcium intake, risk estimates in most of those studies were small (10% relative risk) and not considered clinically important, even if they were statistically significant,” Dr. Chung and her associates added (Ann Int Med. 2016 Oct 24. doi: 10.7326/M16-1165).
According to the guideline, “The NOF and the ASPC now adopt the position that there is moderate-quality evidence that calcium with or without vitamin D intake from food or supplements has no relationship (beneficial or harmful) with the risk for cardiovascular or cerebrovascular disease, mortality, or all-cause mortality in generally healthy adults at this time.”
In addition, “Currently, no established biological mechanism supports and association between calcium and cardiovascular disease,” Dr. Kopecky and his associates on the expert panel noted.
Both dietary and supplemental calcium should be considered safe for the cardiovascular system as long as total intake doesn’t exceed 2,000-2,500 mg/day – the maximal tolerable level defined by the National Academy of Medicine, according to an updated Clinical Practice Guideline published online October 24 in Annals of Internal Medicine.
For generally healthy patients who don’t consume adequate calcium and take supplements, either alone or in combination with vitamin D, to prevent osteoporosis and related fractures, “discontinuation of supplemental calcium for safety reasons is not necessary and may be harmful to bone health,” said Stephen L. Kopecky, MD, of the Mayo Clinic, Rochester Minn., and his associates on the expert panel that wrote the new guideline.
The National Osteoporosis Foundation (NOF) and the American Society for Preventive Cardiology (ASPC) commissioned an independent review of the current evidence to update the Evidence Report and assembled the expert panel to write the guideline based on the new findings (Ann Intern Med. 2016 Oct 24. doi: 10.7326/M16-1743).
Separately, Mei Chung, PhD, of the department of public health and community medicine, and her associates at Tufts University, Boston, reviewed 4 recent randomized clinical trials, 1 nested case-control study, and 26 cohort studies that assessed the effects of calcium intake on 17 health outcomes in generally healthy adults of all ages. None of the studies evaluated cardiovascular disease risk as a primary outcome. “We conclude that calcium intake (from either food or supplement sources) at levels within the recommended tolerable upper intake range (2,000-2,500 mg/d) are not associated with CVD risks in generally healthy adults,” they said.
“Although a few trials and cohort studies reported increased risks with higher calcium intake, risk estimates in most of those studies were small (10% relative risk) and not considered clinically important, even if they were statistically significant,” Dr. Chung and her associates added (Ann Int Med. 2016 Oct 24. doi: 10.7326/M16-1165).
According to the guideline, “The NOF and the ASPC now adopt the position that there is moderate-quality evidence that calcium with or without vitamin D intake from food or supplements has no relationship (beneficial or harmful) with the risk for cardiovascular or cerebrovascular disease, mortality, or all-cause mortality in generally healthy adults at this time.”
In addition, “Currently, no established biological mechanism supports and association between calcium and cardiovascular disease,” Dr. Kopecky and his associates on the expert panel noted.
COPD spirometry use suboptimal in primary care
LOS ANGELES – Spirometry is the standard for diagnosing chronic obstructive pulmonary disease, but it’s underused and misused in primary care, according to investigators from the Corpus Christi (Tex.) Medical Center.
The conclusion is based on a review of just 65 patients from internal medicine and family practice clinics near the medical center, but “I do think this [pattern] is representative of what we are seeing in every primary care office. This has been a problem [documented] in the literature for a decade, and it remains a problem,” said lead investigator Stephen Eikermann, DO, an internal medicine resident at the center.
Meanwhile, of those diagnosed by spirometry, 32% didn’t meet the gold-standard Global Initiative for Chronic Obstructive Lung Disease (GOLD) diagnostic criteria by having a postbronchodilator forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) of less than 70%. Clinicians might not have known that postbronchodilator values are the ones that matter. “People who have asthma are being tagged as having COPD,” and once that diagnosis is in the chart, it’s hard to remove, even when patients improve. “With the COPD readmission penalty in place, an erroneous diagnosis of COPD [has] significant financial risks,” Dr. Eikermann said.
“Misdiagnosis leads to significant financial consequences and puts patients at risk for osteoporotic fractures and malignant arrhythmias. It’s a problem of education.” Busy practitioners might not have had time to catch the latest 2015 GOLD standards, he said.
The guidelines state that COPD should be considered in any patient who has dyspnea, chronic cough, or sputum production, plus smoking or other risks. “Spirometry is required to make the diagnosis.”
To help, Dr. Eikermann and his colleagues plan lectures and a quick reference handout, and maybe a smartphone app. They also plan to remind practitioners that Medicare pays at a reasonable rate for spirometry.
The 65 patients in the study were about evenly split between men and women, and were 70 years old, on average. They had about 34 pack-years of smoking, and some were still smoking despite being on home oxygen.
Men were less likely to have spirometry than women; older subjects and current smokers – as opposed to former smokers – were, too. The risk of COPD increases with age and smoking, so the finding was puzzling. For unknown reasons, “there appears to be a bias against ordering spirometry” for some patients, Dr. Eikermann said.
There was no outside funding for the work, and the investigators had no disclosures.
LOS ANGELES – Spirometry is the standard for diagnosing chronic obstructive pulmonary disease, but it’s underused and misused in primary care, according to investigators from the Corpus Christi (Tex.) Medical Center.
The conclusion is based on a review of just 65 patients from internal medicine and family practice clinics near the medical center, but “I do think this [pattern] is representative of what we are seeing in every primary care office. This has been a problem [documented] in the literature for a decade, and it remains a problem,” said lead investigator Stephen Eikermann, DO, an internal medicine resident at the center.
Meanwhile, of those diagnosed by spirometry, 32% didn’t meet the gold-standard Global Initiative for Chronic Obstructive Lung Disease (GOLD) diagnostic criteria by having a postbronchodilator forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) of less than 70%. Clinicians might not have known that postbronchodilator values are the ones that matter. “People who have asthma are being tagged as having COPD,” and once that diagnosis is in the chart, it’s hard to remove, even when patients improve. “With the COPD readmission penalty in place, an erroneous diagnosis of COPD [has] significant financial risks,” Dr. Eikermann said.
“Misdiagnosis leads to significant financial consequences and puts patients at risk for osteoporotic fractures and malignant arrhythmias. It’s a problem of education.” Busy practitioners might not have had time to catch the latest 2015 GOLD standards, he said.
The guidelines state that COPD should be considered in any patient who has dyspnea, chronic cough, or sputum production, plus smoking or other risks. “Spirometry is required to make the diagnosis.”
To help, Dr. Eikermann and his colleagues plan lectures and a quick reference handout, and maybe a smartphone app. They also plan to remind practitioners that Medicare pays at a reasonable rate for spirometry.
The 65 patients in the study were about evenly split between men and women, and were 70 years old, on average. They had about 34 pack-years of smoking, and some were still smoking despite being on home oxygen.
Men were less likely to have spirometry than women; older subjects and current smokers – as opposed to former smokers – were, too. The risk of COPD increases with age and smoking, so the finding was puzzling. For unknown reasons, “there appears to be a bias against ordering spirometry” for some patients, Dr. Eikermann said.
There was no outside funding for the work, and the investigators had no disclosures.
LOS ANGELES – Spirometry is the standard for diagnosing chronic obstructive pulmonary disease, but it’s underused and misused in primary care, according to investigators from the Corpus Christi (Tex.) Medical Center.
The conclusion is based on a review of just 65 patients from internal medicine and family practice clinics near the medical center, but “I do think this [pattern] is representative of what we are seeing in every primary care office. This has been a problem [documented] in the literature for a decade, and it remains a problem,” said lead investigator Stephen Eikermann, DO, an internal medicine resident at the center.
Meanwhile, of those diagnosed by spirometry, 32% didn’t meet the gold-standard Global Initiative for Chronic Obstructive Lung Disease (GOLD) diagnostic criteria by having a postbronchodilator forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) of less than 70%. Clinicians might not have known that postbronchodilator values are the ones that matter. “People who have asthma are being tagged as having COPD,” and once that diagnosis is in the chart, it’s hard to remove, even when patients improve. “With the COPD readmission penalty in place, an erroneous diagnosis of COPD [has] significant financial risks,” Dr. Eikermann said.
“Misdiagnosis leads to significant financial consequences and puts patients at risk for osteoporotic fractures and malignant arrhythmias. It’s a problem of education.” Busy practitioners might not have had time to catch the latest 2015 GOLD standards, he said.
The guidelines state that COPD should be considered in any patient who has dyspnea, chronic cough, or sputum production, plus smoking or other risks. “Spirometry is required to make the diagnosis.”
To help, Dr. Eikermann and his colleagues plan lectures and a quick reference handout, and maybe a smartphone app. They also plan to remind practitioners that Medicare pays at a reasonable rate for spirometry.
The 65 patients in the study were about evenly split between men and women, and were 70 years old, on average. They had about 34 pack-years of smoking, and some were still smoking despite being on home oxygen.
Men were less likely to have spirometry than women; older subjects and current smokers – as opposed to former smokers – were, too. The risk of COPD increases with age and smoking, so the finding was puzzling. For unknown reasons, “there appears to be a bias against ordering spirometry” for some patients, Dr. Eikermann said.
There was no outside funding for the work, and the investigators had no disclosures.
Key clinical point:
Major finding: Only 29% of patients diagnosed with chronic obstructive pulmonary disease (COPD) at two clinics underwent spirometry. Of those diagnosed by spirometry, 32% didn’t meet the gold-standard Global Initiative for Chronic Obstructive Lung Disease (GOLD) diagnostic criteria by having a postbronchodilator FEV1/FVC of less than 70%.
Data source: Review of 65 COPD cases treated at two primary care clinics.
Disclosures: There was no outside funding for the work, and the investigators had no disclosures.
MOC debate heats up
The American Board of Internal Medicine (ABIM) recently unveiled its first attempt to revitalize the maintenance of certification (MOC) program with pathway alternatives to the 10-year exam. While AGA has advocated for MOC reform and welcomed the efforts of the ABIM in responding to demands for change, we object to the pathways proposed by ABIM, which fall short of our principles of individualization, lifelong education, and low-stakes testing.
ABIM proposes to replace the 10-year exam with either a 2- (or 5-) year alternative MOC pathway. Every 2 (or 5) years, diplomates can take a single, 2- (or 5-) hour open-book exam, from their office or home. The exam would provide granular feedback on learning objectives in need of remediation. The exam is promoted as “low-stakes” since failure does not result in immediate loss of certification. Rather, the diplomate has the opportunity to take the next exam and, if they pass, continue on the pathway. Failure of two exams in a row means the diplomate must pass the traditional 10-year exam before continuing the MOC pathway.
AGA joined unanimously with other GI societies and the majority of other internal medicine societies in rejecting both proposed pathways as unacceptable in their present format.
AGA’s liaison to the ABIM Liaison Committee on Certification and Recertification (LCCR), Art DeCross, MD, outlined AGA’s issues with the proposal on the AGA Community in late September and kicked off a lively discussion about the future of MOC. Visit the AGA Community at community.gasto.org to read or join the conversation.
Additionally, here are two key things to know:
1. Regarding state legislation, AGA President Tim Wang, MD, notes that “We’re monitoring legislative actions in various states and note that there would need to be commitment to the same approach by all 50 states in order to have the necessary impact to completely unlink MOC certification from hospital and insurance credentialing for AGA members. So instead, AGA is focusing our work on national advocacy, which is where we can have the most impact.”
2. While there is a lot of activity related to reforming MOC, the time horizon for changes is long. Dr. DeCross notes that “members really should plan on continuing their current, standard MOC pathway process, including taking their recertification examinations, if they want to retain that credential at this time.”
This conversation is the latest in a long advocacy campaign lead by AGA to push ABIM to reform MOC. Learn more at www.gastro.org/career-center/maintenance-of-certification.
The American Board of Internal Medicine (ABIM) recently unveiled its first attempt to revitalize the maintenance of certification (MOC) program with pathway alternatives to the 10-year exam. While AGA has advocated for MOC reform and welcomed the efforts of the ABIM in responding to demands for change, we object to the pathways proposed by ABIM, which fall short of our principles of individualization, lifelong education, and low-stakes testing.
ABIM proposes to replace the 10-year exam with either a 2- (or 5-) year alternative MOC pathway. Every 2 (or 5) years, diplomates can take a single, 2- (or 5-) hour open-book exam, from their office or home. The exam would provide granular feedback on learning objectives in need of remediation. The exam is promoted as “low-stakes” since failure does not result in immediate loss of certification. Rather, the diplomate has the opportunity to take the next exam and, if they pass, continue on the pathway. Failure of two exams in a row means the diplomate must pass the traditional 10-year exam before continuing the MOC pathway.
AGA joined unanimously with other GI societies and the majority of other internal medicine societies in rejecting both proposed pathways as unacceptable in their present format.
AGA’s liaison to the ABIM Liaison Committee on Certification and Recertification (LCCR), Art DeCross, MD, outlined AGA’s issues with the proposal on the AGA Community in late September and kicked off a lively discussion about the future of MOC. Visit the AGA Community at community.gasto.org to read or join the conversation.
Additionally, here are two key things to know:
1. Regarding state legislation, AGA President Tim Wang, MD, notes that “We’re monitoring legislative actions in various states and note that there would need to be commitment to the same approach by all 50 states in order to have the necessary impact to completely unlink MOC certification from hospital and insurance credentialing for AGA members. So instead, AGA is focusing our work on national advocacy, which is where we can have the most impact.”
2. While there is a lot of activity related to reforming MOC, the time horizon for changes is long. Dr. DeCross notes that “members really should plan on continuing their current, standard MOC pathway process, including taking their recertification examinations, if they want to retain that credential at this time.”
This conversation is the latest in a long advocacy campaign lead by AGA to push ABIM to reform MOC. Learn more at www.gastro.org/career-center/maintenance-of-certification.
The American Board of Internal Medicine (ABIM) recently unveiled its first attempt to revitalize the maintenance of certification (MOC) program with pathway alternatives to the 10-year exam. While AGA has advocated for MOC reform and welcomed the efforts of the ABIM in responding to demands for change, we object to the pathways proposed by ABIM, which fall short of our principles of individualization, lifelong education, and low-stakes testing.
ABIM proposes to replace the 10-year exam with either a 2- (or 5-) year alternative MOC pathway. Every 2 (or 5) years, diplomates can take a single, 2- (or 5-) hour open-book exam, from their office or home. The exam would provide granular feedback on learning objectives in need of remediation. The exam is promoted as “low-stakes” since failure does not result in immediate loss of certification. Rather, the diplomate has the opportunity to take the next exam and, if they pass, continue on the pathway. Failure of two exams in a row means the diplomate must pass the traditional 10-year exam before continuing the MOC pathway.
AGA joined unanimously with other GI societies and the majority of other internal medicine societies in rejecting both proposed pathways as unacceptable in their present format.
AGA’s liaison to the ABIM Liaison Committee on Certification and Recertification (LCCR), Art DeCross, MD, outlined AGA’s issues with the proposal on the AGA Community in late September and kicked off a lively discussion about the future of MOC. Visit the AGA Community at community.gasto.org to read or join the conversation.
Additionally, here are two key things to know:
1. Regarding state legislation, AGA President Tim Wang, MD, notes that “We’re monitoring legislative actions in various states and note that there would need to be commitment to the same approach by all 50 states in order to have the necessary impact to completely unlink MOC certification from hospital and insurance credentialing for AGA members. So instead, AGA is focusing our work on national advocacy, which is where we can have the most impact.”
2. While there is a lot of activity related to reforming MOC, the time horizon for changes is long. Dr. DeCross notes that “members really should plan on continuing their current, standard MOC pathway process, including taking their recertification examinations, if they want to retain that credential at this time.”
This conversation is the latest in a long advocacy campaign lead by AGA to push ABIM to reform MOC. Learn more at www.gastro.org/career-center/maintenance-of-certification.
Lung cancer screening found effective in a community hospital
LOS ANGELES – Lung cancer screening with low-dose CT scans in a community hospital setting replicates results from international and multicenter trials when it comes to diagnosing early-stage lung cancer, findings from a single-center study showed.
“It’s too early in our experience to say that we’re saving lives, but the fact that we’re detecting early lung cancers in the predicted percentages is good for community hospitals that are wondering, ‘Is it worth it to screen for lung cancer? Can we do it?’ ” Richard P. Salzano Jr., MD, said in an interview in advance of the annual meeting of the American College of Chest Physicians.
In July 2013, the 130-bed Griffin Hospital launched a lung cancer screening program codirected by a pulmonologist and a cardiothoracic surgeon. All low-dose CT scans were read by two designated radiologists. Dr. Salzano reported results from 514 patients enrolled in the program between July 2013 and December 2015. A total of nine lung cancers were detected. Seven (78%) were stage I or II lung cancers, and the remaining two (22%) were stage II or IV, results that are in line with data from the I-ELCAP and NLST trials.
In another component of the study, the researchers randomly selected 101 patients from the lung cancer screening program to answer questions by telephone intended to quantify their anxiety about lung cancer before and after participating in the program, attitudes about smoking behaviors, and general impressions of the screening process. On a scale of 0-10, with 10 being “very anxious,” Dr. Salzano reported that the mean anxiety level about lung cancer fell from a level of 4.69 before screening to 3.87 afterward, a difference that reached statistical significance, with a P value of .014. “None of the patients reported negative impacts of the program,” he added. “They reported a general improvement in their well-being as a result of participating in the program.” In addition, of the 53 respondents who were current smokers upon enrolling in the screening program, five quit after intake, and the remaining 48 indicated that they were “more likely to quit” as a result of being enrolled.
“Community hospitals need to embrace lung screening,” Dr. Salzano concluded. “The findings from the large studies are transferable. It’s helping your patients in terms of their attitudes about lung cancer, about smoking cessation, and about improving their wellness.”
He reported having no relevant financial disclosures.
LOS ANGELES – Lung cancer screening with low-dose CT scans in a community hospital setting replicates results from international and multicenter trials when it comes to diagnosing early-stage lung cancer, findings from a single-center study showed.
“It’s too early in our experience to say that we’re saving lives, but the fact that we’re detecting early lung cancers in the predicted percentages is good for community hospitals that are wondering, ‘Is it worth it to screen for lung cancer? Can we do it?’ ” Richard P. Salzano Jr., MD, said in an interview in advance of the annual meeting of the American College of Chest Physicians.
In July 2013, the 130-bed Griffin Hospital launched a lung cancer screening program codirected by a pulmonologist and a cardiothoracic surgeon. All low-dose CT scans were read by two designated radiologists. Dr. Salzano reported results from 514 patients enrolled in the program between July 2013 and December 2015. A total of nine lung cancers were detected. Seven (78%) were stage I or II lung cancers, and the remaining two (22%) were stage II or IV, results that are in line with data from the I-ELCAP and NLST trials.
In another component of the study, the researchers randomly selected 101 patients from the lung cancer screening program to answer questions by telephone intended to quantify their anxiety about lung cancer before and after participating in the program, attitudes about smoking behaviors, and general impressions of the screening process. On a scale of 0-10, with 10 being “very anxious,” Dr. Salzano reported that the mean anxiety level about lung cancer fell from a level of 4.69 before screening to 3.87 afterward, a difference that reached statistical significance, with a P value of .014. “None of the patients reported negative impacts of the program,” he added. “They reported a general improvement in their well-being as a result of participating in the program.” In addition, of the 53 respondents who were current smokers upon enrolling in the screening program, five quit after intake, and the remaining 48 indicated that they were “more likely to quit” as a result of being enrolled.
“Community hospitals need to embrace lung screening,” Dr. Salzano concluded. “The findings from the large studies are transferable. It’s helping your patients in terms of their attitudes about lung cancer, about smoking cessation, and about improving their wellness.”
He reported having no relevant financial disclosures.
LOS ANGELES – Lung cancer screening with low-dose CT scans in a community hospital setting replicates results from international and multicenter trials when it comes to diagnosing early-stage lung cancer, findings from a single-center study showed.
“It’s too early in our experience to say that we’re saving lives, but the fact that we’re detecting early lung cancers in the predicted percentages is good for community hospitals that are wondering, ‘Is it worth it to screen for lung cancer? Can we do it?’ ” Richard P. Salzano Jr., MD, said in an interview in advance of the annual meeting of the American College of Chest Physicians.
In July 2013, the 130-bed Griffin Hospital launched a lung cancer screening program codirected by a pulmonologist and a cardiothoracic surgeon. All low-dose CT scans were read by two designated radiologists. Dr. Salzano reported results from 514 patients enrolled in the program between July 2013 and December 2015. A total of nine lung cancers were detected. Seven (78%) were stage I or II lung cancers, and the remaining two (22%) were stage II or IV, results that are in line with data from the I-ELCAP and NLST trials.
In another component of the study, the researchers randomly selected 101 patients from the lung cancer screening program to answer questions by telephone intended to quantify their anxiety about lung cancer before and after participating in the program, attitudes about smoking behaviors, and general impressions of the screening process. On a scale of 0-10, with 10 being “very anxious,” Dr. Salzano reported that the mean anxiety level about lung cancer fell from a level of 4.69 before screening to 3.87 afterward, a difference that reached statistical significance, with a P value of .014. “None of the patients reported negative impacts of the program,” he added. “They reported a general improvement in their well-being as a result of participating in the program.” In addition, of the 53 respondents who were current smokers upon enrolling in the screening program, five quit after intake, and the remaining 48 indicated that they were “more likely to quit” as a result of being enrolled.
“Community hospitals need to embrace lung screening,” Dr. Salzano concluded. “The findings from the large studies are transferable. It’s helping your patients in terms of their attitudes about lung cancer, about smoking cessation, and about improving their wellness.”
He reported having no relevant financial disclosures.
AT CHEST 2016
Key clinical point:
Major finding: Of nine lung cancers detected, seven (78%) were stage I or II lung cancers and the remaining two (22%) were stage II or IV.
Data source: Results from 514 patients enrolled in a community hospital–based lung cancer screening program between July 2013 and December 2015.
Disclosures: Dr. Salzano reported having no relevant financial disclosures.
Smoking-attributable cancer mortality highest in Kentucky
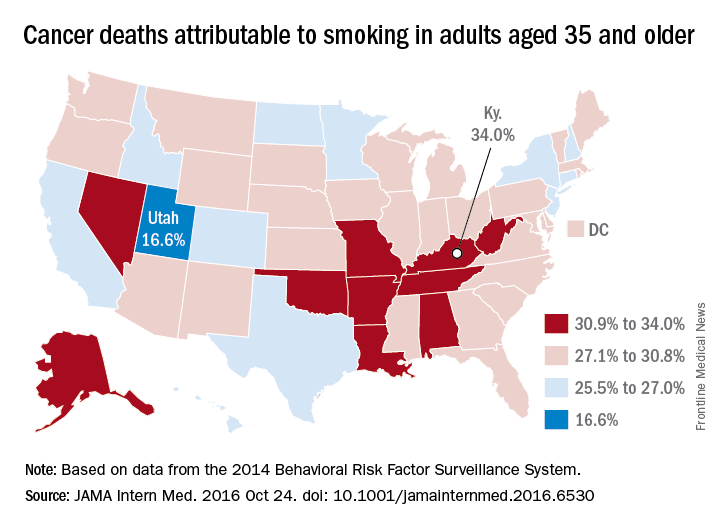
Almost 29% of cancer deaths among U.S. adults aged 35 years and older were attributable to cigarette smoking in 2014, according to investigators from the American Cancer Society.
Of the 585,000 cancer deaths among those 35 years and older that year, more than 167,000 (28.6%) were estimated to be the result of cigarette smoking, reported Joannie Lortet-Tieulent, MSc, and her associates at the ACS in Atlanta (JAMA Intern Med. 2016 Oct 24. doi: 10.1001/jamainternmed.2016.6530).
Among men aged 35 years and older, 33.7% of U.S. cancer deaths were attributable to cigarette smoking, compared with 22.9% for women. Arkansas had the highest rate (39.5%) for men and Kentucky had the highest rate (29.0%) for women. Not surprisingly, Utah had the lowest rate for both men (21.8%) and women (11.1%), according to the analysis of data from the 2014 Behavioral Risk Factor Surveillance System. For men, Utah was the only state with a rate below 30%.
The investigators did not report any conflicts of interest. The study was supported by the intramural research department of the American Cancer Society.
Almost 29% of cancer deaths among U.S. adults aged 35 years and older were attributable to cigarette smoking in 2014, according to investigators from the American Cancer Society.
Of the 585,000 cancer deaths among those 35 years and older that year, more than 167,000 (28.6%) were estimated to be the result of cigarette smoking, reported Joannie Lortet-Tieulent, MSc, and her associates at the ACS in Atlanta (JAMA Intern Med. 2016 Oct 24. doi: 10.1001/jamainternmed.2016.6530).
Among men aged 35 years and older, 33.7% of U.S. cancer deaths were attributable to cigarette smoking, compared with 22.9% for women. Arkansas had the highest rate (39.5%) for men and Kentucky had the highest rate (29.0%) for women. Not surprisingly, Utah had the lowest rate for both men (21.8%) and women (11.1%), according to the analysis of data from the 2014 Behavioral Risk Factor Surveillance System. For men, Utah was the only state with a rate below 30%.
The investigators did not report any conflicts of interest. The study was supported by the intramural research department of the American Cancer Society.
Almost 29% of cancer deaths among U.S. adults aged 35 years and older were attributable to cigarette smoking in 2014, according to investigators from the American Cancer Society.
Of the 585,000 cancer deaths among those 35 years and older that year, more than 167,000 (28.6%) were estimated to be the result of cigarette smoking, reported Joannie Lortet-Tieulent, MSc, and her associates at the ACS in Atlanta (JAMA Intern Med. 2016 Oct 24. doi: 10.1001/jamainternmed.2016.6530).
Among men aged 35 years and older, 33.7% of U.S. cancer deaths were attributable to cigarette smoking, compared with 22.9% for women. Arkansas had the highest rate (39.5%) for men and Kentucky had the highest rate (29.0%) for women. Not surprisingly, Utah had the lowest rate for both men (21.8%) and women (11.1%), according to the analysis of data from the 2014 Behavioral Risk Factor Surveillance System. For men, Utah was the only state with a rate below 30%.
The investigators did not report any conflicts of interest. The study was supported by the intramural research department of the American Cancer Society.
Joint European atrial fibrillation guidelines break new ground
ROME – The 2016 joint European guidelines on management of atrial fibrillation break new ground by declaring as a strong Class IA recommendation that the novel oral anticoagulants are now the drugs of choice – preferred over warfarin – for stroke prevention.
The joint guidelines from the European Society of Cardiology and the European Association for Cardio-Thoracic Surgery recommend that warfarin’s use be reserved for the relatively small proportion of atrial fibrillation (AF) patients who are ineligible for the four commercially available novel oral anticoagulants (NOACs). That’s mainly patients with mechanical heart valves, moderate to severe mitral stenosis, or severe chronic kidney disease.
The ESC/EACTS guidelines, taken together with the American College of Chest Physicians guidelines on antithrombotic therapy for venous thromboembolic disease released earlier in the year, suggest that the old war horse warfarin is being eased out to pasture. The ACCP guidelines recommend any of the four NOACS – apixaban, dabigatran, edoxaban, or rivaroxaban – be used preferentially over warfarin in the treatment of venous thromboembolism (Chest 2016 Feb;149[2]:315-52). Both sets of guidelines cite compelling evidence that the NOACs are significantly safer than warfarin yet equally effective.
The ESC/EACTS guidelines are a full rewrite containing numerous departures from the previous 2012 AF management guidelines as well as from current ACC/AHA guidelines. The report includes more than 1,000 references. Eighty percent of the 154 recommendations provide Class I or IIa guidance. Two-thirds of the recommendations are Level of Evidence A or B, task force chairperson Paulus Kirchhof, MD, said at the annual congress of the European Society of Cardiology.
He and co-chairperson Stefano Benussi, MD, presented some of the highlights.
The guidelines issue a strong call for greater use of targeted ECG screening in populations at risk for silent AF, including stroke survivors and the elderly. And AF should always be documented before starting treatment, given that all of the treatments carry risk, said Dr. Kirchhof, professor of cardiovascular medicine at the University of Birmingham (England).
Once the diagnosis is established, it’s essential to address in a structured way five domains of management: acute rate and rhythm control; management of precipitating factors, including underlying cardiovascular conditions such as hypertension or valvular heart disease; assessment of stroke risk using the CHA2DS2-VASc scoring system; assessment of heart rate; and evaluation of the impact of AF symptoms on the patient’s life, including fatigue and breathlessness, using a structured instrument such as the modified European Heart Rhythm Association symptom scale.
Men with a CHA2DS2-VASc score of 1 and women with a score of 2 should be considered for anticoagulation. And the treatment should be recommended – not merely considered – for men with a score of 2 or more and women with a score of 3; that’s a Class Ia recommendation, Dr. Kirchhof continued.
The use of a specific bleeding risk score is no longer recommended in AF patients on oral anticoagulation. The emphasis has shifted to reduction of modifiable bleeding risk factors, including limiting alcohol intake to fewer than 8 drinks per week, control of hypertension, and discontinuing antiplatelet and anti-inflammatory agents.
Consideration of left atrial appendage occlusion devices should be reserved for the small percentage of patients who have clear contraindications to all forms of oral anticoagulation.
The task force concluded that patients who have bleeding on oral anticoagulation can often be managed with local therapy and discontinuation of anticoagulation therapy for a day or two before resumption. However, decisions regarding resumption of a NOAC or warfarin after an intracranial bleed should be handled by an interdisciplinary panel composed of a stroke neurologist, a cardiologist, a neuroradiologist, and a neurosurgeon.
Evidence-based treatment options in patients with symptomatic AF after failed catheter ablation include minimally invasive surgery with epicardial pulmonary vein isolation, more extensive catheter ablation, and hybrid procedures, according to Dr. Benussi, who is codirector of clinical cardiovascular surgery at University Hospital in Zurich.
The guidelines state that the data supporting catheter ablation to achieve long-term rhythm control are now sufficiently strong that this intervention should be considered as a first-line option alongside antiarrhythmic drugs as a matter of patient preference in the setting of symptomatic paroxysmal AF regardless of whether the patient has CAD, heart failure, valvular heart disease, or no structural heart disease.
Catheter ablation using radiofrequency energy or cryoablation should target complete isolation of the pulmonary veins.
“Additional ablation lines do not provide demonstrable clinical benefit and increase the risk of postablation left atrial arrhythmias,” the surgeon said.
Maze surgery, preferably biatrial, received a favorable Class IIa, Level of Evidence A recommendation as worthy of consideration in patients with symptomatic AF who are already undergoing cardiac surgery. This recommendation was based upon an external review by the Cochrane group which was commissioned by the guidelines task force. The Cochrane review of eight published studies concluded that Maze surgery under such circumstances was associated with a twofold increased freedom from AF, atrial flutter, and atrial tachycardia (Cochrane Database of Systematic Reviews 2016;8: CD012088. doi: 10.1002/14651858.CD012088.pub2).
The AF management guidelines are supported by the ESC Pocket Guidelines app, which includes an overall AF treatment manager developed by the European Union–funded CATCH ME (Characterizing Atrial Fibrillation by Translating its Causes Into Health Modifiers in the Elderly) project.
The multidisciplinary 17-member AF management task force was drawn from cardiology, stroke neurology, cardiac surgery, and specialist nursing. Dr. Kirchhof stressed that only recommendations supported by at least 75% of task force members made it into the guidelines (Eur Heart J. 2016 Aug 27. pii: ehw210. [Epub ahead of print] doi: 10.1093/eurheartj/ehw210).
ROME – The 2016 joint European guidelines on management of atrial fibrillation break new ground by declaring as a strong Class IA recommendation that the novel oral anticoagulants are now the drugs of choice – preferred over warfarin – for stroke prevention.
The joint guidelines from the European Society of Cardiology and the European Association for Cardio-Thoracic Surgery recommend that warfarin’s use be reserved for the relatively small proportion of atrial fibrillation (AF) patients who are ineligible for the four commercially available novel oral anticoagulants (NOACs). That’s mainly patients with mechanical heart valves, moderate to severe mitral stenosis, or severe chronic kidney disease.
The ESC/EACTS guidelines, taken together with the American College of Chest Physicians guidelines on antithrombotic therapy for venous thromboembolic disease released earlier in the year, suggest that the old war horse warfarin is being eased out to pasture. The ACCP guidelines recommend any of the four NOACS – apixaban, dabigatran, edoxaban, or rivaroxaban – be used preferentially over warfarin in the treatment of venous thromboembolism (Chest 2016 Feb;149[2]:315-52). Both sets of guidelines cite compelling evidence that the NOACs are significantly safer than warfarin yet equally effective.
The ESC/EACTS guidelines are a full rewrite containing numerous departures from the previous 2012 AF management guidelines as well as from current ACC/AHA guidelines. The report includes more than 1,000 references. Eighty percent of the 154 recommendations provide Class I or IIa guidance. Two-thirds of the recommendations are Level of Evidence A or B, task force chairperson Paulus Kirchhof, MD, said at the annual congress of the European Society of Cardiology.
He and co-chairperson Stefano Benussi, MD, presented some of the highlights.
The guidelines issue a strong call for greater use of targeted ECG screening in populations at risk for silent AF, including stroke survivors and the elderly. And AF should always be documented before starting treatment, given that all of the treatments carry risk, said Dr. Kirchhof, professor of cardiovascular medicine at the University of Birmingham (England).
Once the diagnosis is established, it’s essential to address in a structured way five domains of management: acute rate and rhythm control; management of precipitating factors, including underlying cardiovascular conditions such as hypertension or valvular heart disease; assessment of stroke risk using the CHA2DS2-VASc scoring system; assessment of heart rate; and evaluation of the impact of AF symptoms on the patient’s life, including fatigue and breathlessness, using a structured instrument such as the modified European Heart Rhythm Association symptom scale.
Men with a CHA2DS2-VASc score of 1 and women with a score of 2 should be considered for anticoagulation. And the treatment should be recommended – not merely considered – for men with a score of 2 or more and women with a score of 3; that’s a Class Ia recommendation, Dr. Kirchhof continued.
The use of a specific bleeding risk score is no longer recommended in AF patients on oral anticoagulation. The emphasis has shifted to reduction of modifiable bleeding risk factors, including limiting alcohol intake to fewer than 8 drinks per week, control of hypertension, and discontinuing antiplatelet and anti-inflammatory agents.
Consideration of left atrial appendage occlusion devices should be reserved for the small percentage of patients who have clear contraindications to all forms of oral anticoagulation.
The task force concluded that patients who have bleeding on oral anticoagulation can often be managed with local therapy and discontinuation of anticoagulation therapy for a day or two before resumption. However, decisions regarding resumption of a NOAC or warfarin after an intracranial bleed should be handled by an interdisciplinary panel composed of a stroke neurologist, a cardiologist, a neuroradiologist, and a neurosurgeon.
Evidence-based treatment options in patients with symptomatic AF after failed catheter ablation include minimally invasive surgery with epicardial pulmonary vein isolation, more extensive catheter ablation, and hybrid procedures, according to Dr. Benussi, who is codirector of clinical cardiovascular surgery at University Hospital in Zurich.
The guidelines state that the data supporting catheter ablation to achieve long-term rhythm control are now sufficiently strong that this intervention should be considered as a first-line option alongside antiarrhythmic drugs as a matter of patient preference in the setting of symptomatic paroxysmal AF regardless of whether the patient has CAD, heart failure, valvular heart disease, or no structural heart disease.
Catheter ablation using radiofrequency energy or cryoablation should target complete isolation of the pulmonary veins.
“Additional ablation lines do not provide demonstrable clinical benefit and increase the risk of postablation left atrial arrhythmias,” the surgeon said.
Maze surgery, preferably biatrial, received a favorable Class IIa, Level of Evidence A recommendation as worthy of consideration in patients with symptomatic AF who are already undergoing cardiac surgery. This recommendation was based upon an external review by the Cochrane group which was commissioned by the guidelines task force. The Cochrane review of eight published studies concluded that Maze surgery under such circumstances was associated with a twofold increased freedom from AF, atrial flutter, and atrial tachycardia (Cochrane Database of Systematic Reviews 2016;8: CD012088. doi: 10.1002/14651858.CD012088.pub2).
The AF management guidelines are supported by the ESC Pocket Guidelines app, which includes an overall AF treatment manager developed by the European Union–funded CATCH ME (Characterizing Atrial Fibrillation by Translating its Causes Into Health Modifiers in the Elderly) project.
The multidisciplinary 17-member AF management task force was drawn from cardiology, stroke neurology, cardiac surgery, and specialist nursing. Dr. Kirchhof stressed that only recommendations supported by at least 75% of task force members made it into the guidelines (Eur Heart J. 2016 Aug 27. pii: ehw210. [Epub ahead of print] doi: 10.1093/eurheartj/ehw210).
ROME – The 2016 joint European guidelines on management of atrial fibrillation break new ground by declaring as a strong Class IA recommendation that the novel oral anticoagulants are now the drugs of choice – preferred over warfarin – for stroke prevention.
The joint guidelines from the European Society of Cardiology and the European Association for Cardio-Thoracic Surgery recommend that warfarin’s use be reserved for the relatively small proportion of atrial fibrillation (AF) patients who are ineligible for the four commercially available novel oral anticoagulants (NOACs). That’s mainly patients with mechanical heart valves, moderate to severe mitral stenosis, or severe chronic kidney disease.
The ESC/EACTS guidelines, taken together with the American College of Chest Physicians guidelines on antithrombotic therapy for venous thromboembolic disease released earlier in the year, suggest that the old war horse warfarin is being eased out to pasture. The ACCP guidelines recommend any of the four NOACS – apixaban, dabigatran, edoxaban, or rivaroxaban – be used preferentially over warfarin in the treatment of venous thromboembolism (Chest 2016 Feb;149[2]:315-52). Both sets of guidelines cite compelling evidence that the NOACs are significantly safer than warfarin yet equally effective.
The ESC/EACTS guidelines are a full rewrite containing numerous departures from the previous 2012 AF management guidelines as well as from current ACC/AHA guidelines. The report includes more than 1,000 references. Eighty percent of the 154 recommendations provide Class I or IIa guidance. Two-thirds of the recommendations are Level of Evidence A or B, task force chairperson Paulus Kirchhof, MD, said at the annual congress of the European Society of Cardiology.
He and co-chairperson Stefano Benussi, MD, presented some of the highlights.
The guidelines issue a strong call for greater use of targeted ECG screening in populations at risk for silent AF, including stroke survivors and the elderly. And AF should always be documented before starting treatment, given that all of the treatments carry risk, said Dr. Kirchhof, professor of cardiovascular medicine at the University of Birmingham (England).
Once the diagnosis is established, it’s essential to address in a structured way five domains of management: acute rate and rhythm control; management of precipitating factors, including underlying cardiovascular conditions such as hypertension or valvular heart disease; assessment of stroke risk using the CHA2DS2-VASc scoring system; assessment of heart rate; and evaluation of the impact of AF symptoms on the patient’s life, including fatigue and breathlessness, using a structured instrument such as the modified European Heart Rhythm Association symptom scale.
Men with a CHA2DS2-VASc score of 1 and women with a score of 2 should be considered for anticoagulation. And the treatment should be recommended – not merely considered – for men with a score of 2 or more and women with a score of 3; that’s a Class Ia recommendation, Dr. Kirchhof continued.
The use of a specific bleeding risk score is no longer recommended in AF patients on oral anticoagulation. The emphasis has shifted to reduction of modifiable bleeding risk factors, including limiting alcohol intake to fewer than 8 drinks per week, control of hypertension, and discontinuing antiplatelet and anti-inflammatory agents.
Consideration of left atrial appendage occlusion devices should be reserved for the small percentage of patients who have clear contraindications to all forms of oral anticoagulation.
The task force concluded that patients who have bleeding on oral anticoagulation can often be managed with local therapy and discontinuation of anticoagulation therapy for a day or two before resumption. However, decisions regarding resumption of a NOAC or warfarin after an intracranial bleed should be handled by an interdisciplinary panel composed of a stroke neurologist, a cardiologist, a neuroradiologist, and a neurosurgeon.
Evidence-based treatment options in patients with symptomatic AF after failed catheter ablation include minimally invasive surgery with epicardial pulmonary vein isolation, more extensive catheter ablation, and hybrid procedures, according to Dr. Benussi, who is codirector of clinical cardiovascular surgery at University Hospital in Zurich.
The guidelines state that the data supporting catheter ablation to achieve long-term rhythm control are now sufficiently strong that this intervention should be considered as a first-line option alongside antiarrhythmic drugs as a matter of patient preference in the setting of symptomatic paroxysmal AF regardless of whether the patient has CAD, heart failure, valvular heart disease, or no structural heart disease.
Catheter ablation using radiofrequency energy or cryoablation should target complete isolation of the pulmonary veins.
“Additional ablation lines do not provide demonstrable clinical benefit and increase the risk of postablation left atrial arrhythmias,” the surgeon said.
Maze surgery, preferably biatrial, received a favorable Class IIa, Level of Evidence A recommendation as worthy of consideration in patients with symptomatic AF who are already undergoing cardiac surgery. This recommendation was based upon an external review by the Cochrane group which was commissioned by the guidelines task force. The Cochrane review of eight published studies concluded that Maze surgery under such circumstances was associated with a twofold increased freedom from AF, atrial flutter, and atrial tachycardia (Cochrane Database of Systematic Reviews 2016;8: CD012088. doi: 10.1002/14651858.CD012088.pub2).
The AF management guidelines are supported by the ESC Pocket Guidelines app, which includes an overall AF treatment manager developed by the European Union–funded CATCH ME (Characterizing Atrial Fibrillation by Translating its Causes Into Health Modifiers in the Elderly) project.
The multidisciplinary 17-member AF management task force was drawn from cardiology, stroke neurology, cardiac surgery, and specialist nursing. Dr. Kirchhof stressed that only recommendations supported by at least 75% of task force members made it into the guidelines (Eur Heart J. 2016 Aug 27. pii: ehw210. [Epub ahead of print] doi: 10.1093/eurheartj/ehw210).
Advisor’s Viewpoint: State legislative update
In mid-September, the American Congress of Obstetricians and Gynecologists’ state legislative chairs and lobbyists from 41 states met to discuss top state legislative issues. This group meets annually in the early fall in order to share legislative issues from the previous legislative session and discuss goals for the upcoming session. The attendees sit state by state around a large, rectangular table, where they can share challenges experienced and challenges expected with current legislation and emerging issues.
The discussion included recent legal decisions and new issues. One hot topic was the June 2016 Supreme Court ruling related to TRAP (Targeted Regulation of Abortion Providers) laws. Some states are still encumbered by TRAP laws, despite the recent ruling against the Texas abortion regulations. There are actions underway in some states to get these regulations reversed to be in compliance with the Supreme Court decision.
Another topic discussed was the push to create a Maternal Mortality Committee in each state. Some states have had this committee for many years and shared pearls for how to get the legislation passed. Collaboration with advocates in the medical community and with the state departments of health was considered to be critical in the passage of legislation to support the creation of these committees.
There was prolonged discussion in the general session, as well as in breakout sessions, about the different types of midwives and the goal to establish a collaborative working relationship with them. Questions discussed included the educational goals for each group, as well as their level of responsibility. The licensing of midwives varies between states, ranging from the State Board of Midwifery to the State Board of Nursing to the State Board of Medicine. Maine was recognized as a state that has successfully created a collaborative relationship with the midwives who practice there. ACOG continues to work with the American College of Nurse-Midwives to create collaborative practice relationships.
Another breakout session focused on reproductive health. This group discussed legislation that interferes with the patient-physician relationship, including mandating an outdated protocol for administering medication abortion and even threatening physicians who provide abortion services with jail time. State legislation to allow pharmacists to prescribe hormonal contraception was also reviewed.
There was consensus among the meeting participants that communication with legislators about evidence-based medicine and sound science, along with ob.gyns.’ advocacy in state legislatures, is critical to counterbalancing inappropriate political interference in the patient-physician relationship. The discussion also touched on the importance of continued collaboration with partners across the medical community, since attempts to legislate the practice of medicine can negatively impact a wide range of clinical practices.
Dr. Bohon is an ob.gyn. in private practice in Washington, D.C. She is an ACOG state legislative chair from the District of Columbia and a member of the Ob.Gyn. News Editorial Advisory Board. She reported having no relevant financial disclosures.
In mid-September, the American Congress of Obstetricians and Gynecologists’ state legislative chairs and lobbyists from 41 states met to discuss top state legislative issues. This group meets annually in the early fall in order to share legislative issues from the previous legislative session and discuss goals for the upcoming session. The attendees sit state by state around a large, rectangular table, where they can share challenges experienced and challenges expected with current legislation and emerging issues.
The discussion included recent legal decisions and new issues. One hot topic was the June 2016 Supreme Court ruling related to TRAP (Targeted Regulation of Abortion Providers) laws. Some states are still encumbered by TRAP laws, despite the recent ruling against the Texas abortion regulations. There are actions underway in some states to get these regulations reversed to be in compliance with the Supreme Court decision.
Another topic discussed was the push to create a Maternal Mortality Committee in each state. Some states have had this committee for many years and shared pearls for how to get the legislation passed. Collaboration with advocates in the medical community and with the state departments of health was considered to be critical in the passage of legislation to support the creation of these committees.
There was prolonged discussion in the general session, as well as in breakout sessions, about the different types of midwives and the goal to establish a collaborative working relationship with them. Questions discussed included the educational goals for each group, as well as their level of responsibility. The licensing of midwives varies between states, ranging from the State Board of Midwifery to the State Board of Nursing to the State Board of Medicine. Maine was recognized as a state that has successfully created a collaborative relationship with the midwives who practice there. ACOG continues to work with the American College of Nurse-Midwives to create collaborative practice relationships.
Another breakout session focused on reproductive health. This group discussed legislation that interferes with the patient-physician relationship, including mandating an outdated protocol for administering medication abortion and even threatening physicians who provide abortion services with jail time. State legislation to allow pharmacists to prescribe hormonal contraception was also reviewed.
There was consensus among the meeting participants that communication with legislators about evidence-based medicine and sound science, along with ob.gyns.’ advocacy in state legislatures, is critical to counterbalancing inappropriate political interference in the patient-physician relationship. The discussion also touched on the importance of continued collaboration with partners across the medical community, since attempts to legislate the practice of medicine can negatively impact a wide range of clinical practices.
Dr. Bohon is an ob.gyn. in private practice in Washington, D.C. She is an ACOG state legislative chair from the District of Columbia and a member of the Ob.Gyn. News Editorial Advisory Board. She reported having no relevant financial disclosures.
In mid-September, the American Congress of Obstetricians and Gynecologists’ state legislative chairs and lobbyists from 41 states met to discuss top state legislative issues. This group meets annually in the early fall in order to share legislative issues from the previous legislative session and discuss goals for the upcoming session. The attendees sit state by state around a large, rectangular table, where they can share challenges experienced and challenges expected with current legislation and emerging issues.
The discussion included recent legal decisions and new issues. One hot topic was the June 2016 Supreme Court ruling related to TRAP (Targeted Regulation of Abortion Providers) laws. Some states are still encumbered by TRAP laws, despite the recent ruling against the Texas abortion regulations. There are actions underway in some states to get these regulations reversed to be in compliance with the Supreme Court decision.
Another topic discussed was the push to create a Maternal Mortality Committee in each state. Some states have had this committee for many years and shared pearls for how to get the legislation passed. Collaboration with advocates in the medical community and with the state departments of health was considered to be critical in the passage of legislation to support the creation of these committees.
There was prolonged discussion in the general session, as well as in breakout sessions, about the different types of midwives and the goal to establish a collaborative working relationship with them. Questions discussed included the educational goals for each group, as well as their level of responsibility. The licensing of midwives varies between states, ranging from the State Board of Midwifery to the State Board of Nursing to the State Board of Medicine. Maine was recognized as a state that has successfully created a collaborative relationship with the midwives who practice there. ACOG continues to work with the American College of Nurse-Midwives to create collaborative practice relationships.
Another breakout session focused on reproductive health. This group discussed legislation that interferes with the patient-physician relationship, including mandating an outdated protocol for administering medication abortion and even threatening physicians who provide abortion services with jail time. State legislation to allow pharmacists to prescribe hormonal contraception was also reviewed.
There was consensus among the meeting participants that communication with legislators about evidence-based medicine and sound science, along with ob.gyns.’ advocacy in state legislatures, is critical to counterbalancing inappropriate political interference in the patient-physician relationship. The discussion also touched on the importance of continued collaboration with partners across the medical community, since attempts to legislate the practice of medicine can negatively impact a wide range of clinical practices.
Dr. Bohon is an ob.gyn. in private practice in Washington, D.C. She is an ACOG state legislative chair from the District of Columbia and a member of the Ob.Gyn. News Editorial Advisory Board. She reported having no relevant financial disclosures.
Automated colonoscopy risk classification for veterans
LAS VEGAS – A new scoring system for military veterans estimates risk of advanced colorectal neoplasia based on a list of lifestyle and clinical factors, and could help prioritize patients for screening colonoscopy. The study used natural-language processing to analyze patient medical records from the VA health care system.
“Those who were at very low risk had no colon cancer, and their risk of advanced polyps was pretty low, while those in the high-risk group should probably give thought to having a colonoscopy,” Thomas F. Imperiale, MD, a gastroenterologist and professor of medicine at the Indiana University School of Medicine, Indianapolis, said in an interview. Dr. Imperiale presented the results at the annual meeting of the American College of Gastroenterology.
When asked during the Q&A session if he could identify a subset of patients who could skip colonoscopies altogether, Dr. Imperiale said that he could not. “Prediction is possible, and if those predictions stand up in validation, they may be used to at least provide veterans with a choice, and perhaps provide the health care system a choice about how they screen their patients,” he said.
The researchers identified 66,725 veterans who underwent a diagnostic or screening colonoscopy between 2002 and 2009 at one of 14 VA medical centers. They used natural language processing to identify the most advanced finding and the location within the colorectum.
The rate of advanced neoplasia was 8.8%, and the rate of colorectal cancer (CRC) was 1.2%. Independent risk factors included age, sex, tobacco use, and exposure to COX-1 and COX-2 nonsteroidal anti-inflammatory drugs. The researchers defined four risk categories, low to high, with advanced neoplasia risks of 2.9% (CRC risk, 0%), 5.3% (CRC risk, 0.5%), 8.5% (CRC risk, 0.9%), and 11.4% (CRC risk, 2.1%). For advanced neoplasia, the goodness-of-fit P value was 1.00 and the c statistic was 0.58. For CRC, these values were 1.00 and 0.64, respectively.
Dr. Imperiale noted that the results remain relatively crude, and that the field of natural-language processing continues to evolve. In fact, software available today outperforms that used in the study. “It’s a field that’s moving quickly,” said Dr. Imperiale.
The results could help prioritize patients for colonoscopies, according to Victor Levy, MD, a senior attending physician at Mary Imogene Bassett Healthcare, Cooperstown, N.Y., who attended the presentation. And the study represents good news for the field overall. “Big data analytics has been accepted in other industries, and it’s now finally beginning to exert its effects in health care,” Dr. Levy said in an interview.
The results don’t generalize to other groups, because veterans have unique covariables and confounding factors. A new model would have to be developed for other populations.
Dr. Imperiale and Dr. Levy have no disclosures.
*Updated on 10/27/16
LAS VEGAS – A new scoring system for military veterans estimates risk of advanced colorectal neoplasia based on a list of lifestyle and clinical factors, and could help prioritize patients for screening colonoscopy. The study used natural-language processing to analyze patient medical records from the VA health care system.
“Those who were at very low risk had no colon cancer, and their risk of advanced polyps was pretty low, while those in the high-risk group should probably give thought to having a colonoscopy,” Thomas F. Imperiale, MD, a gastroenterologist and professor of medicine at the Indiana University School of Medicine, Indianapolis, said in an interview. Dr. Imperiale presented the results at the annual meeting of the American College of Gastroenterology.
When asked during the Q&A session if he could identify a subset of patients who could skip colonoscopies altogether, Dr. Imperiale said that he could not. “Prediction is possible, and if those predictions stand up in validation, they may be used to at least provide veterans with a choice, and perhaps provide the health care system a choice about how they screen their patients,” he said.
The researchers identified 66,725 veterans who underwent a diagnostic or screening colonoscopy between 2002 and 2009 at one of 14 VA medical centers. They used natural language processing to identify the most advanced finding and the location within the colorectum.
The rate of advanced neoplasia was 8.8%, and the rate of colorectal cancer (CRC) was 1.2%. Independent risk factors included age, sex, tobacco use, and exposure to COX-1 and COX-2 nonsteroidal anti-inflammatory drugs. The researchers defined four risk categories, low to high, with advanced neoplasia risks of 2.9% (CRC risk, 0%), 5.3% (CRC risk, 0.5%), 8.5% (CRC risk, 0.9%), and 11.4% (CRC risk, 2.1%). For advanced neoplasia, the goodness-of-fit P value was 1.00 and the c statistic was 0.58. For CRC, these values were 1.00 and 0.64, respectively.
Dr. Imperiale noted that the results remain relatively crude, and that the field of natural-language processing continues to evolve. In fact, software available today outperforms that used in the study. “It’s a field that’s moving quickly,” said Dr. Imperiale.
The results could help prioritize patients for colonoscopies, according to Victor Levy, MD, a senior attending physician at Mary Imogene Bassett Healthcare, Cooperstown, N.Y., who attended the presentation. And the study represents good news for the field overall. “Big data analytics has been accepted in other industries, and it’s now finally beginning to exert its effects in health care,” Dr. Levy said in an interview.
The results don’t generalize to other groups, because veterans have unique covariables and confounding factors. A new model would have to be developed for other populations.
Dr. Imperiale and Dr. Levy have no disclosures.
*Updated on 10/27/16
LAS VEGAS – A new scoring system for military veterans estimates risk of advanced colorectal neoplasia based on a list of lifestyle and clinical factors, and could help prioritize patients for screening colonoscopy. The study used natural-language processing to analyze patient medical records from the VA health care system.
“Those who were at very low risk had no colon cancer, and their risk of advanced polyps was pretty low, while those in the high-risk group should probably give thought to having a colonoscopy,” Thomas F. Imperiale, MD, a gastroenterologist and professor of medicine at the Indiana University School of Medicine, Indianapolis, said in an interview. Dr. Imperiale presented the results at the annual meeting of the American College of Gastroenterology.
When asked during the Q&A session if he could identify a subset of patients who could skip colonoscopies altogether, Dr. Imperiale said that he could not. “Prediction is possible, and if those predictions stand up in validation, they may be used to at least provide veterans with a choice, and perhaps provide the health care system a choice about how they screen their patients,” he said.
The researchers identified 66,725 veterans who underwent a diagnostic or screening colonoscopy between 2002 and 2009 at one of 14 VA medical centers. They used natural language processing to identify the most advanced finding and the location within the colorectum.
The rate of advanced neoplasia was 8.8%, and the rate of colorectal cancer (CRC) was 1.2%. Independent risk factors included age, sex, tobacco use, and exposure to COX-1 and COX-2 nonsteroidal anti-inflammatory drugs. The researchers defined four risk categories, low to high, with advanced neoplasia risks of 2.9% (CRC risk, 0%), 5.3% (CRC risk, 0.5%), 8.5% (CRC risk, 0.9%), and 11.4% (CRC risk, 2.1%). For advanced neoplasia, the goodness-of-fit P value was 1.00 and the c statistic was 0.58. For CRC, these values were 1.00 and 0.64, respectively.
Dr. Imperiale noted that the results remain relatively crude, and that the field of natural-language processing continues to evolve. In fact, software available today outperforms that used in the study. “It’s a field that’s moving quickly,” said Dr. Imperiale.
The results could help prioritize patients for colonoscopies, according to Victor Levy, MD, a senior attending physician at Mary Imogene Bassett Healthcare, Cooperstown, N.Y., who attended the presentation. And the study represents good news for the field overall. “Big data analytics has been accepted in other industries, and it’s now finally beginning to exert its effects in health care,” Dr. Levy said in an interview.
The results don’t generalize to other groups, because veterans have unique covariables and confounding factors. A new model would have to be developed for other populations.
Dr. Imperiale and Dr. Levy have no disclosures.
*Updated on 10/27/16
AT ACG 2016
Key clinical point:
Major finding: Patients in the lowest-risk group were predicted to have no colorectal cancers and to be at low risk for other advanced neoplasia. The high-risk patient group had higher predicted rates of advanced neoplasia and may be candidates for more aggressive screening.
Data source: Retrospective analysis of electronic medical records.
Disclosures: Dr. Imperiale and Dr. Levy have no disclosures.