User login
Public speaking fundamentals. The program: Key elements in capturing and holding audience attention
In the first part of this article series (“Preparation: Tips that lead to a solid, engaging presentation,” OBG Manag. 2016;28[7]:31–36.), we offered tips on preparing for a group presentation. In this article, part 2, we discuss the presentation itself and what you can do to capture and hold your audience’s attention.

How to connect with the audience
Let’s assume the meeting host has just introduced you to the audience using, as we suggested in the previous article, an autobiographical profile you provided. You now have the audience’s undivided attention. What you do and say in the next 30 to 60 seconds will set the stage for your program. Following the requisite “thank you” to the host and meeting sponsor, use this time to establish your expertise as a spokesperson on the chosen topic. Or, if the introductory remarks made your expertise plain, you may choose to connect with the audience on an informal, personal level. If you are from out of town, for instance, you could remark on an interesting aspect of the city or region you are visiting that you learned on the Internet before arriving.
Underscore the topic’s importance. On the other hand, you might want to begin with an insightful statistic germane to your talk. For example, a talk on breast cancer might begin with, “According to the American Cancer Society, there are nearly 250,000 new cases of breast cancer each year, and breast cancer accounts for more than 40,000 deaths per year. That means more women die from breast cancer than die in auto accidents each year. So this emphasizes the importance of appropriately screening women for breast cancer annually after age 40.”
An opening story about a patient can be powerful. Better yet, a personal experience reflecting your topic is a great way to connect with your audience members and get their attention. For example, one of us (NHB) gives talks on practice management and practice efficiency. I might talk about when I was called from an exam room 3 times to answer “emergency” phone calls from a patient who wanted only to request her medical records. To ensure that this embarrassment would never happen again, I put in place a system that I then describe for the audience.
Alternatively, an opening that addresses the audience’s unspoken question, “What’s in this for me?” is sure to grab their attention. For instance, a talk on office productivity might begin by promising to share a way to increase annual collections by $250,000 per physician through scheduling adjustments that can increase the number of examined patients by one per hour.
Steer clear of these openings. In general, avoid “I’m delighted to be here” and other clichés. One exception would be if you can make that cliché humorous. For example, if a speaker from the deep South is visiting the northern part of the country in summer, she might say, “Most speakers say they’re delighted to be here, and you may well question their sincerity. However, I’m from New Orleans where the temperature is approaching 105 degrees with 95% humidity. You know I’m really delighted to be here!”
Importantly, avoid starting with an apology. Do not mention problems with the audiovisual equipment or why you arrived late. The audience does not care, and you will immediately lose their attention. They want to be educated and entertained. There is no better way to do this than by offering a compelling and captivating opening that begins the moment after you are introduced.
Finally, avoid use of the “royal I,” as in “I am here to talk about XYZ.” It places you in a position superior to the audience, and that is a turnoff. Instead, you could say to the audience, “The reason you are here is to learn about XYZ.” This places the audience on an equal level with you, and they know there will be something in the presentation for them.
Housekeeping notes
The audience will appreciate knowing how long you plan to speak and whether you will take questions during or after the presentation. Based on our experience, if there are fewer than 20 attendees, we often encourage questions during the program instead of waiting until the end. This makes the program more conversational and usually generates more questions. With a dinner presentation, we prefer to speak while the audience is eating. We usually start after the waiters have taken the orders and the attendees have had their appetizers. We might say we will finish the program by the time they are ready for dessert. We also mention that we will distribute a handout after the presentation so they do not have to worry about following the handout, taking notes, and watching the speaker while trying to eat.
The main body of the program
As for structuring your talk, we suggest you follow this time-honored advice often attributed to Aristotle: Tell the audience what you are going to say, say it, and tell them what you said.
So we begin a presentation by stating the objectives of our program, usually limited to 3 and no more than 4. For example, a talk on hormone therapy (HT) for treating vasomotor symptoms of menopause might mention 1) the history of HT use, 2) which women are appropriate candidates for HT, and 3) how to monitor women who receive HT.
Enhance the talk’s relevance. We like to begin a clinical program with a case scenario wherein we describe how one of our patients had the specific problem and how we used a particular drug, treatment, or device to manage the case. We try to select a patient similar to ones who would be seen by members of the audience.
Simplify as much as possible. We then present the slides exactly as they have been provided by the pharmaceutical company. Most company slides contain too many words as well as diagrams that are too complex for the audience to grasp easily. We try to find one salient point on each slide and focus attention on that single word, phrase, or sentence. We can do this in a small audience by walking over to the screen and pointing it out, or we can use the laser pointer from a distance.
Change things up to keep the message fresh. Let’s be honest, most medical talks are dry and boring. Try to inject some energy and enthusiasm in the middle of the presentation. Every few minutes we tell a story or ask the audience a question. For example, during a program on practice management, one of us (NHB) will relate a story about an unhappy patient and then ask a physician in the audience how he or she might handle the disgruntled patient. This is a nice break from the main content of the presentation, re-engaging the audience in an interactive exchange.
Should you use humor?
Although many physicians attempt to use humor during a presentation, few are talented at stand-up comedy. However, used judiciously humor, like seasoning in fine cuisine, can do great things for a presentation. It can break the ice, drive home a point, and enhance your likeability. It can, though, also backfire. One of us (NHB) once gave a talk to a large audience of pharmaceutical representatives. As part of my wrap-up I displayed a slide from the cover of Economics that showed 2 camels in the mating position. My closing line was that reps need to “hump to it” and get involved with their physicians and be value-added in their product detailing. Afterward, the meeting planner told me that he would never hire me again. He said I had a great program, great material, and a good connection with the audience. But my closing was over the top. I learned my lesson. Never use material that has the potential to offend. If you want to use humor, the self-deprecatingkind is always safest.
Try using visual aids
Our observation is that few physician speakers use visual aids other than their slides. We have learned that audience attention will stay focused on you if you make use of visual aids. For example, if we are speaking to a lay audience about urinary incontinence, we might use a balloon to demonstrate the bladder and the urethra.
Studies have shown that there are more nerve endings from the eye to the brain than from the ear to the brain. Humans purportedly receive 25 times as much stimulus from visual cues than from auditory ones. To paraphrase an old proverb, “One seeing is better than 100 times hearing about”!
A few suggestions regarding the use of visual aids:
- Keep the visual aid out of sight until you are ready to use it. You do not want the audience staring at it when they should be focusing on you or your slide material. We usually keep our visual aids under the table that supports the computer and projector.
- Make certain the visual aid is large enough to be seen by everyone in the audience.
- Do not hand out the aid to the audience during your program. Doing so will divert their attention from you and your material.
- When you have finished using the aid, put it away.
Closing out the program
After we have covered the program’s 3 objectives, we let the audience know we are approaching the end of the presentation. For a dinner program, we try to time the ending just as plates are being cleared and before dessert is served. We then restate the 3 objectives as they might pertain to the attendees’ patients and practices. At this time, we take questions from the audience, even if some were asked during the presentation. We repeat each question when it is asked so that everyone can hear it. (This also gives us a few seconds to think about it and frame our answer.) If it appears that many questions will be asked, we assure everyone that we plan to finish on time and will remain after the program is over to answer additional questions.
Tips on fielding questions. When responding to a question, direct your attention initially to the person who asked it. After that, spend about 20% of the time focused on that person and 80% of the time on the rest of the audience. If you focus only on the questioner, it becomes a one-on-one conversation. You want to end your response with your eyes on the group and not on the questioner. Looking at the group will also act as a bridge to the next question. Although we used to reply to an inquiry with, “That’s a great question,” we now suggest avoiding this comment. Why? Because it is unlikely that you’ll keep using that line, and the next questioner who does not receive the same compliment might feel slighted.
Wrap up. When you announce, “I would like to conclude my program with…,” this is the magical time when you hold the complete attention of the audience. Often, the speaker’s last words are the ones the audience remembers the longest. So this is the time to offer your take-home message. For example, a talk on how to motivate your staff might conclude, “Remember, your staff members are the people that patients encounter first and the ones they see last as they leave the office. Every patient can have a positive experience with you and your practice if you ensure that your personnel are highly motivated. This happens in part by your effort to recognize their accomplishments.” Then hold up your hands and spread out your arms as you end with “Thank you.” The audience likely will applaud and, if your speech is truly exceptional, you might receive a gratifying standing ovation!
Be seated
Renowned for his speeches, Franklin Delano Roosevelt summarized the art of effective speaking when he said, “Be sincere. Be brief. Be seated.” When your time is up, turn the program back over to the meeting host and take a seat.
In the final article in this public speaking series, we will discuss the follow-up steps to take once the program is over, including the call to action or what you want the audience to do after you have left the podium or the speaking venue.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In the first part of this article series (“Preparation: Tips that lead to a solid, engaging presentation,” OBG Manag. 2016;28[7]:31–36.), we offered tips on preparing for a group presentation. In this article, part 2, we discuss the presentation itself and what you can do to capture and hold your audience’s attention.

How to connect with the audience
Let’s assume the meeting host has just introduced you to the audience using, as we suggested in the previous article, an autobiographical profile you provided. You now have the audience’s undivided attention. What you do and say in the next 30 to 60 seconds will set the stage for your program. Following the requisite “thank you” to the host and meeting sponsor, use this time to establish your expertise as a spokesperson on the chosen topic. Or, if the introductory remarks made your expertise plain, you may choose to connect with the audience on an informal, personal level. If you are from out of town, for instance, you could remark on an interesting aspect of the city or region you are visiting that you learned on the Internet before arriving.
Underscore the topic’s importance. On the other hand, you might want to begin with an insightful statistic germane to your talk. For example, a talk on breast cancer might begin with, “According to the American Cancer Society, there are nearly 250,000 new cases of breast cancer each year, and breast cancer accounts for more than 40,000 deaths per year. That means more women die from breast cancer than die in auto accidents each year. So this emphasizes the importance of appropriately screening women for breast cancer annually after age 40.”
An opening story about a patient can be powerful. Better yet, a personal experience reflecting your topic is a great way to connect with your audience members and get their attention. For example, one of us (NHB) gives talks on practice management and practice efficiency. I might talk about when I was called from an exam room 3 times to answer “emergency” phone calls from a patient who wanted only to request her medical records. To ensure that this embarrassment would never happen again, I put in place a system that I then describe for the audience.
Alternatively, an opening that addresses the audience’s unspoken question, “What’s in this for me?” is sure to grab their attention. For instance, a talk on office productivity might begin by promising to share a way to increase annual collections by $250,000 per physician through scheduling adjustments that can increase the number of examined patients by one per hour.
Steer clear of these openings. In general, avoid “I’m delighted to be here” and other clichés. One exception would be if you can make that cliché humorous. For example, if a speaker from the deep South is visiting the northern part of the country in summer, she might say, “Most speakers say they’re delighted to be here, and you may well question their sincerity. However, I’m from New Orleans where the temperature is approaching 105 degrees with 95% humidity. You know I’m really delighted to be here!”
Importantly, avoid starting with an apology. Do not mention problems with the audiovisual equipment or why you arrived late. The audience does not care, and you will immediately lose their attention. They want to be educated and entertained. There is no better way to do this than by offering a compelling and captivating opening that begins the moment after you are introduced.
Finally, avoid use of the “royal I,” as in “I am here to talk about XYZ.” It places you in a position superior to the audience, and that is a turnoff. Instead, you could say to the audience, “The reason you are here is to learn about XYZ.” This places the audience on an equal level with you, and they know there will be something in the presentation for them.
Housekeeping notes
The audience will appreciate knowing how long you plan to speak and whether you will take questions during or after the presentation. Based on our experience, if there are fewer than 20 attendees, we often encourage questions during the program instead of waiting until the end. This makes the program more conversational and usually generates more questions. With a dinner presentation, we prefer to speak while the audience is eating. We usually start after the waiters have taken the orders and the attendees have had their appetizers. We might say we will finish the program by the time they are ready for dessert. We also mention that we will distribute a handout after the presentation so they do not have to worry about following the handout, taking notes, and watching the speaker while trying to eat.
The main body of the program
As for structuring your talk, we suggest you follow this time-honored advice often attributed to Aristotle: Tell the audience what you are going to say, say it, and tell them what you said.
So we begin a presentation by stating the objectives of our program, usually limited to 3 and no more than 4. For example, a talk on hormone therapy (HT) for treating vasomotor symptoms of menopause might mention 1) the history of HT use, 2) which women are appropriate candidates for HT, and 3) how to monitor women who receive HT.
Enhance the talk’s relevance. We like to begin a clinical program with a case scenario wherein we describe how one of our patients had the specific problem and how we used a particular drug, treatment, or device to manage the case. We try to select a patient similar to ones who would be seen by members of the audience.
Simplify as much as possible. We then present the slides exactly as they have been provided by the pharmaceutical company. Most company slides contain too many words as well as diagrams that are too complex for the audience to grasp easily. We try to find one salient point on each slide and focus attention on that single word, phrase, or sentence. We can do this in a small audience by walking over to the screen and pointing it out, or we can use the laser pointer from a distance.
Change things up to keep the message fresh. Let’s be honest, most medical talks are dry and boring. Try to inject some energy and enthusiasm in the middle of the presentation. Every few minutes we tell a story or ask the audience a question. For example, during a program on practice management, one of us (NHB) will relate a story about an unhappy patient and then ask a physician in the audience how he or she might handle the disgruntled patient. This is a nice break from the main content of the presentation, re-engaging the audience in an interactive exchange.
Should you use humor?
Although many physicians attempt to use humor during a presentation, few are talented at stand-up comedy. However, used judiciously humor, like seasoning in fine cuisine, can do great things for a presentation. It can break the ice, drive home a point, and enhance your likeability. It can, though, also backfire. One of us (NHB) once gave a talk to a large audience of pharmaceutical representatives. As part of my wrap-up I displayed a slide from the cover of Economics that showed 2 camels in the mating position. My closing line was that reps need to “hump to it” and get involved with their physicians and be value-added in their product detailing. Afterward, the meeting planner told me that he would never hire me again. He said I had a great program, great material, and a good connection with the audience. But my closing was over the top. I learned my lesson. Never use material that has the potential to offend. If you want to use humor, the self-deprecatingkind is always safest.
Try using visual aids
Our observation is that few physician speakers use visual aids other than their slides. We have learned that audience attention will stay focused on you if you make use of visual aids. For example, if we are speaking to a lay audience about urinary incontinence, we might use a balloon to demonstrate the bladder and the urethra.
Studies have shown that there are more nerve endings from the eye to the brain than from the ear to the brain. Humans purportedly receive 25 times as much stimulus from visual cues than from auditory ones. To paraphrase an old proverb, “One seeing is better than 100 times hearing about”!
A few suggestions regarding the use of visual aids:
- Keep the visual aid out of sight until you are ready to use it. You do not want the audience staring at it when they should be focusing on you or your slide material. We usually keep our visual aids under the table that supports the computer and projector.
- Make certain the visual aid is large enough to be seen by everyone in the audience.
- Do not hand out the aid to the audience during your program. Doing so will divert their attention from you and your material.
- When you have finished using the aid, put it away.
Closing out the program
After we have covered the program’s 3 objectives, we let the audience know we are approaching the end of the presentation. For a dinner program, we try to time the ending just as plates are being cleared and before dessert is served. We then restate the 3 objectives as they might pertain to the attendees’ patients and practices. At this time, we take questions from the audience, even if some were asked during the presentation. We repeat each question when it is asked so that everyone can hear it. (This also gives us a few seconds to think about it and frame our answer.) If it appears that many questions will be asked, we assure everyone that we plan to finish on time and will remain after the program is over to answer additional questions.
Tips on fielding questions. When responding to a question, direct your attention initially to the person who asked it. After that, spend about 20% of the time focused on that person and 80% of the time on the rest of the audience. If you focus only on the questioner, it becomes a one-on-one conversation. You want to end your response with your eyes on the group and not on the questioner. Looking at the group will also act as a bridge to the next question. Although we used to reply to an inquiry with, “That’s a great question,” we now suggest avoiding this comment. Why? Because it is unlikely that you’ll keep using that line, and the next questioner who does not receive the same compliment might feel slighted.
Wrap up. When you announce, “I would like to conclude my program with…,” this is the magical time when you hold the complete attention of the audience. Often, the speaker’s last words are the ones the audience remembers the longest. So this is the time to offer your take-home message. For example, a talk on how to motivate your staff might conclude, “Remember, your staff members are the people that patients encounter first and the ones they see last as they leave the office. Every patient can have a positive experience with you and your practice if you ensure that your personnel are highly motivated. This happens in part by your effort to recognize their accomplishments.” Then hold up your hands and spread out your arms as you end with “Thank you.” The audience likely will applaud and, if your speech is truly exceptional, you might receive a gratifying standing ovation!
Be seated
Renowned for his speeches, Franklin Delano Roosevelt summarized the art of effective speaking when he said, “Be sincere. Be brief. Be seated.” When your time is up, turn the program back over to the meeting host and take a seat.
In the final article in this public speaking series, we will discuss the follow-up steps to take once the program is over, including the call to action or what you want the audience to do after you have left the podium or the speaking venue.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In the first part of this article series (“Preparation: Tips that lead to a solid, engaging presentation,” OBG Manag. 2016;28[7]:31–36.), we offered tips on preparing for a group presentation. In this article, part 2, we discuss the presentation itself and what you can do to capture and hold your audience’s attention.

How to connect with the audience
Let’s assume the meeting host has just introduced you to the audience using, as we suggested in the previous article, an autobiographical profile you provided. You now have the audience’s undivided attention. What you do and say in the next 30 to 60 seconds will set the stage for your program. Following the requisite “thank you” to the host and meeting sponsor, use this time to establish your expertise as a spokesperson on the chosen topic. Or, if the introductory remarks made your expertise plain, you may choose to connect with the audience on an informal, personal level. If you are from out of town, for instance, you could remark on an interesting aspect of the city or region you are visiting that you learned on the Internet before arriving.
Underscore the topic’s importance. On the other hand, you might want to begin with an insightful statistic germane to your talk. For example, a talk on breast cancer might begin with, “According to the American Cancer Society, there are nearly 250,000 new cases of breast cancer each year, and breast cancer accounts for more than 40,000 deaths per year. That means more women die from breast cancer than die in auto accidents each year. So this emphasizes the importance of appropriately screening women for breast cancer annually after age 40.”
An opening story about a patient can be powerful. Better yet, a personal experience reflecting your topic is a great way to connect with your audience members and get their attention. For example, one of us (NHB) gives talks on practice management and practice efficiency. I might talk about when I was called from an exam room 3 times to answer “emergency” phone calls from a patient who wanted only to request her medical records. To ensure that this embarrassment would never happen again, I put in place a system that I then describe for the audience.
Alternatively, an opening that addresses the audience’s unspoken question, “What’s in this for me?” is sure to grab their attention. For instance, a talk on office productivity might begin by promising to share a way to increase annual collections by $250,000 per physician through scheduling adjustments that can increase the number of examined patients by one per hour.
Steer clear of these openings. In general, avoid “I’m delighted to be here” and other clichés. One exception would be if you can make that cliché humorous. For example, if a speaker from the deep South is visiting the northern part of the country in summer, she might say, “Most speakers say they’re delighted to be here, and you may well question their sincerity. However, I’m from New Orleans where the temperature is approaching 105 degrees with 95% humidity. You know I’m really delighted to be here!”
Importantly, avoid starting with an apology. Do not mention problems with the audiovisual equipment or why you arrived late. The audience does not care, and you will immediately lose their attention. They want to be educated and entertained. There is no better way to do this than by offering a compelling and captivating opening that begins the moment after you are introduced.
Finally, avoid use of the “royal I,” as in “I am here to talk about XYZ.” It places you in a position superior to the audience, and that is a turnoff. Instead, you could say to the audience, “The reason you are here is to learn about XYZ.” This places the audience on an equal level with you, and they know there will be something in the presentation for them.
Housekeeping notes
The audience will appreciate knowing how long you plan to speak and whether you will take questions during or after the presentation. Based on our experience, if there are fewer than 20 attendees, we often encourage questions during the program instead of waiting until the end. This makes the program more conversational and usually generates more questions. With a dinner presentation, we prefer to speak while the audience is eating. We usually start after the waiters have taken the orders and the attendees have had their appetizers. We might say we will finish the program by the time they are ready for dessert. We also mention that we will distribute a handout after the presentation so they do not have to worry about following the handout, taking notes, and watching the speaker while trying to eat.
The main body of the program
As for structuring your talk, we suggest you follow this time-honored advice often attributed to Aristotle: Tell the audience what you are going to say, say it, and tell them what you said.
So we begin a presentation by stating the objectives of our program, usually limited to 3 and no more than 4. For example, a talk on hormone therapy (HT) for treating vasomotor symptoms of menopause might mention 1) the history of HT use, 2) which women are appropriate candidates for HT, and 3) how to monitor women who receive HT.
Enhance the talk’s relevance. We like to begin a clinical program with a case scenario wherein we describe how one of our patients had the specific problem and how we used a particular drug, treatment, or device to manage the case. We try to select a patient similar to ones who would be seen by members of the audience.
Simplify as much as possible. We then present the slides exactly as they have been provided by the pharmaceutical company. Most company slides contain too many words as well as diagrams that are too complex for the audience to grasp easily. We try to find one salient point on each slide and focus attention on that single word, phrase, or sentence. We can do this in a small audience by walking over to the screen and pointing it out, or we can use the laser pointer from a distance.
Change things up to keep the message fresh. Let’s be honest, most medical talks are dry and boring. Try to inject some energy and enthusiasm in the middle of the presentation. Every few minutes we tell a story or ask the audience a question. For example, during a program on practice management, one of us (NHB) will relate a story about an unhappy patient and then ask a physician in the audience how he or she might handle the disgruntled patient. This is a nice break from the main content of the presentation, re-engaging the audience in an interactive exchange.
Should you use humor?
Although many physicians attempt to use humor during a presentation, few are talented at stand-up comedy. However, used judiciously humor, like seasoning in fine cuisine, can do great things for a presentation. It can break the ice, drive home a point, and enhance your likeability. It can, though, also backfire. One of us (NHB) once gave a talk to a large audience of pharmaceutical representatives. As part of my wrap-up I displayed a slide from the cover of Economics that showed 2 camels in the mating position. My closing line was that reps need to “hump to it” and get involved with their physicians and be value-added in their product detailing. Afterward, the meeting planner told me that he would never hire me again. He said I had a great program, great material, and a good connection with the audience. But my closing was over the top. I learned my lesson. Never use material that has the potential to offend. If you want to use humor, the self-deprecatingkind is always safest.
Try using visual aids
Our observation is that few physician speakers use visual aids other than their slides. We have learned that audience attention will stay focused on you if you make use of visual aids. For example, if we are speaking to a lay audience about urinary incontinence, we might use a balloon to demonstrate the bladder and the urethra.
Studies have shown that there are more nerve endings from the eye to the brain than from the ear to the brain. Humans purportedly receive 25 times as much stimulus from visual cues than from auditory ones. To paraphrase an old proverb, “One seeing is better than 100 times hearing about”!
A few suggestions regarding the use of visual aids:
- Keep the visual aid out of sight until you are ready to use it. You do not want the audience staring at it when they should be focusing on you or your slide material. We usually keep our visual aids under the table that supports the computer and projector.
- Make certain the visual aid is large enough to be seen by everyone in the audience.
- Do not hand out the aid to the audience during your program. Doing so will divert their attention from you and your material.
- When you have finished using the aid, put it away.
Closing out the program
After we have covered the program’s 3 objectives, we let the audience know we are approaching the end of the presentation. For a dinner program, we try to time the ending just as plates are being cleared and before dessert is served. We then restate the 3 objectives as they might pertain to the attendees’ patients and practices. At this time, we take questions from the audience, even if some were asked during the presentation. We repeat each question when it is asked so that everyone can hear it. (This also gives us a few seconds to think about it and frame our answer.) If it appears that many questions will be asked, we assure everyone that we plan to finish on time and will remain after the program is over to answer additional questions.
Tips on fielding questions. When responding to a question, direct your attention initially to the person who asked it. After that, spend about 20% of the time focused on that person and 80% of the time on the rest of the audience. If you focus only on the questioner, it becomes a one-on-one conversation. You want to end your response with your eyes on the group and not on the questioner. Looking at the group will also act as a bridge to the next question. Although we used to reply to an inquiry with, “That’s a great question,” we now suggest avoiding this comment. Why? Because it is unlikely that you’ll keep using that line, and the next questioner who does not receive the same compliment might feel slighted.
Wrap up. When you announce, “I would like to conclude my program with…,” this is the magical time when you hold the complete attention of the audience. Often, the speaker’s last words are the ones the audience remembers the longest. So this is the time to offer your take-home message. For example, a talk on how to motivate your staff might conclude, “Remember, your staff members are the people that patients encounter first and the ones they see last as they leave the office. Every patient can have a positive experience with you and your practice if you ensure that your personnel are highly motivated. This happens in part by your effort to recognize their accomplishments.” Then hold up your hands and spread out your arms as you end with “Thank you.” The audience likely will applaud and, if your speech is truly exceptional, you might receive a gratifying standing ovation!
Be seated
Renowned for his speeches, Franklin Delano Roosevelt summarized the art of effective speaking when he said, “Be sincere. Be brief. Be seated.” When your time is up, turn the program back over to the meeting host and take a seat.
In the final article in this public speaking series, we will discuss the follow-up steps to take once the program is over, including the call to action or what you want the audience to do after you have left the podium or the speaking venue.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Benefit of Anti-Tau Therapy in Alzheimer’s Disease Is in Question
TORONTO—LMTM, an investigational tau-aggregation inhibitor, may not benefit patients with Alzheimer’s disease who are receiving standard of care, according to research presented at the Alzheimer’s Association International Conference. As monotherapy, however, the drug may stabilize cognition and reduce brain atrophy, according to Serge Gauthier, MD, Director of the Alzheimer’s Disease Research Unit at McGill University in Montreal.
The aggregation of tau protein is one of the hallmarks of Alzheimer’s disease, but most therapies to date have been designed to reduce the number of amyloid plaques. LMTM is a stabilized, reduced form of the methylthionium moiety. As the oxidized chloride salt (methylthionium chloride or MTC), it is commonly known as methylene blue. LMTM is better absorbed, better tolerated, and can be administered in a broader dose range than MTC. Phase II studies of MTC provided evidence for the treatment’s efficacy as monotherapy, but the drug was poorly absorbed at higher doses. MTC and LMTM block tau aggregation in vitro and in tau transgenic animal models.
An International Trial
Dr. Gauthier and colleagues conducted a 15-month phase III study of LMTM in patients with mild to moderate Alzheimer’s disease. Researchers in 16 countries randomized 891 patients to 8 mg/day, 150 mg/day, or 250 mg/day of LMTM. Like methylene blue, LMTM discolors the urine. Because no dye achieves the same effect, researchers administered 8 mg/day of LMTM as a control dose. This dose is enough to discolor the urine, and thus avoid breaking the blind, but is believed to be pharmacologically inactive.
The primary end point was a composite of cognition, as measured by the Alzheimer’s Disease Assessment Scale-Cognition (ADAS-Cog), and activities of daily living, as measured by the Alzheimer’s Disease Cooperative Study-Activities of Daily Living (ADCS-ADL). The secondary end points were brain volume (ie, lateral ventricular volume) as measured by MRI, Clinical Global Impression of Change, and the Mini-Mental State Examination (MMSE).
The investigators examined the data using a standard, mixed-model, repeated-measure analysis with no imputation for missing data. They included patients’ Alzheimer’s disease treatment status as an additive term in their primary analysis. In addition, they planned a second analysis that used this treatment status as a covariate.
LMTM Failed Primary End Point
Approximately 62% of the population was female, and the population’s mean age was 70.6. About 85% of participants were taking approved treatments for Alzheimer’s disease at baseline. Baseline MMSE score was 18.6, and dementia was of moderate severity in 62% of patients. The study’s retention rate was 69%.
At 15 months, Dr. Gauthier and colleagues found no difference in ADAS-Cog score or ADCS-ADL score between the three treatment arms. Patients who took LMTM as monotherapy, however, had stable scores during the study period, compared with patients who took LMTM and standard treatments for Alzheimer’s disease. ADAS-Cog scores were 5.8 and 6.3 points higher, respectively, in patients taking the two higher doses of LMTM as monotherapy, compared with patients taking LMTM in combination with standard treatments. In addition, the rate of brain atrophy was 33% lower among patients taking LMTM as monotherapy, compared with patients taking LMTM and standard treatments.
Diarrhea was the most common side effect of the 250-mg/day dose of LMTM. This side effect may lead to a reduction of dose or treatment cessation. Dysuria was another adverse event and is explained by the drug effect in the urinary tract, according to the researchers.
What Is the Future of Tau Therapy?
The study results are disappointing, according to David Knopman, MD, Professor of Neurology at the Mayo Clinic College of Medicine in Rochester, Minnesota, who was not involved in the study. “The only thing that really counts is the primary outcome that was prespecified…. It was the group as a whole, and there weren’t any benefits.” Although the secondary results are interesting, secondary analyses are “fraught with interpretive difficulties because of hidden biases,” Dr. Knopman added. “When we’re only looking at a small subset of participants, and 15% were on no drug, I think it becomes difficult to interpret.” In addition, the results may have limited relevance for North American populations, where few people with Alzheimer’s disease are not receiving standard care.
A second, predominantly North American, study of LMTM in 800 patients with mild Alzheimer’s disease will be reported in the fall. In light of LMTM’s lack of efficacy as add-on to existing treatments, the researchers modified the primary analyses of the second study before unblinding to focus on monotherapy. “Tau therapy is a way to go. It’s just maybe more complicated than we thought,” said Dr. Gauthier, who is a member of the scientific advisory board of TauRx, the company that sponsored the study.
TORONTO—LMTM, an investigational tau-aggregation inhibitor, may not benefit patients with Alzheimer’s disease who are receiving standard of care, according to research presented at the Alzheimer’s Association International Conference. As monotherapy, however, the drug may stabilize cognition and reduce brain atrophy, according to Serge Gauthier, MD, Director of the Alzheimer’s Disease Research Unit at McGill University in Montreal.
The aggregation of tau protein is one of the hallmarks of Alzheimer’s disease, but most therapies to date have been designed to reduce the number of amyloid plaques. LMTM is a stabilized, reduced form of the methylthionium moiety. As the oxidized chloride salt (methylthionium chloride or MTC), it is commonly known as methylene blue. LMTM is better absorbed, better tolerated, and can be administered in a broader dose range than MTC. Phase II studies of MTC provided evidence for the treatment’s efficacy as monotherapy, but the drug was poorly absorbed at higher doses. MTC and LMTM block tau aggregation in vitro and in tau transgenic animal models.
An International Trial
Dr. Gauthier and colleagues conducted a 15-month phase III study of LMTM in patients with mild to moderate Alzheimer’s disease. Researchers in 16 countries randomized 891 patients to 8 mg/day, 150 mg/day, or 250 mg/day of LMTM. Like methylene blue, LMTM discolors the urine. Because no dye achieves the same effect, researchers administered 8 mg/day of LMTM as a control dose. This dose is enough to discolor the urine, and thus avoid breaking the blind, but is believed to be pharmacologically inactive.
The primary end point was a composite of cognition, as measured by the Alzheimer’s Disease Assessment Scale-Cognition (ADAS-Cog), and activities of daily living, as measured by the Alzheimer’s Disease Cooperative Study-Activities of Daily Living (ADCS-ADL). The secondary end points were brain volume (ie, lateral ventricular volume) as measured by MRI, Clinical Global Impression of Change, and the Mini-Mental State Examination (MMSE).
The investigators examined the data using a standard, mixed-model, repeated-measure analysis with no imputation for missing data. They included patients’ Alzheimer’s disease treatment status as an additive term in their primary analysis. In addition, they planned a second analysis that used this treatment status as a covariate.
LMTM Failed Primary End Point
Approximately 62% of the population was female, and the population’s mean age was 70.6. About 85% of participants were taking approved treatments for Alzheimer’s disease at baseline. Baseline MMSE score was 18.6, and dementia was of moderate severity in 62% of patients. The study’s retention rate was 69%.
At 15 months, Dr. Gauthier and colleagues found no difference in ADAS-Cog score or ADCS-ADL score between the three treatment arms. Patients who took LMTM as monotherapy, however, had stable scores during the study period, compared with patients who took LMTM and standard treatments for Alzheimer’s disease. ADAS-Cog scores were 5.8 and 6.3 points higher, respectively, in patients taking the two higher doses of LMTM as monotherapy, compared with patients taking LMTM in combination with standard treatments. In addition, the rate of brain atrophy was 33% lower among patients taking LMTM as monotherapy, compared with patients taking LMTM and standard treatments.
Diarrhea was the most common side effect of the 250-mg/day dose of LMTM. This side effect may lead to a reduction of dose or treatment cessation. Dysuria was another adverse event and is explained by the drug effect in the urinary tract, according to the researchers.
What Is the Future of Tau Therapy?
The study results are disappointing, according to David Knopman, MD, Professor of Neurology at the Mayo Clinic College of Medicine in Rochester, Minnesota, who was not involved in the study. “The only thing that really counts is the primary outcome that was prespecified…. It was the group as a whole, and there weren’t any benefits.” Although the secondary results are interesting, secondary analyses are “fraught with interpretive difficulties because of hidden biases,” Dr. Knopman added. “When we’re only looking at a small subset of participants, and 15% were on no drug, I think it becomes difficult to interpret.” In addition, the results may have limited relevance for North American populations, where few people with Alzheimer’s disease are not receiving standard care.
A second, predominantly North American, study of LMTM in 800 patients with mild Alzheimer’s disease will be reported in the fall. In light of LMTM’s lack of efficacy as add-on to existing treatments, the researchers modified the primary analyses of the second study before unblinding to focus on monotherapy. “Tau therapy is a way to go. It’s just maybe more complicated than we thought,” said Dr. Gauthier, who is a member of the scientific advisory board of TauRx, the company that sponsored the study.
TORONTO—LMTM, an investigational tau-aggregation inhibitor, may not benefit patients with Alzheimer’s disease who are receiving standard of care, according to research presented at the Alzheimer’s Association International Conference. As monotherapy, however, the drug may stabilize cognition and reduce brain atrophy, according to Serge Gauthier, MD, Director of the Alzheimer’s Disease Research Unit at McGill University in Montreal.
The aggregation of tau protein is one of the hallmarks of Alzheimer’s disease, but most therapies to date have been designed to reduce the number of amyloid plaques. LMTM is a stabilized, reduced form of the methylthionium moiety. As the oxidized chloride salt (methylthionium chloride or MTC), it is commonly known as methylene blue. LMTM is better absorbed, better tolerated, and can be administered in a broader dose range than MTC. Phase II studies of MTC provided evidence for the treatment’s efficacy as monotherapy, but the drug was poorly absorbed at higher doses. MTC and LMTM block tau aggregation in vitro and in tau transgenic animal models.
An International Trial
Dr. Gauthier and colleagues conducted a 15-month phase III study of LMTM in patients with mild to moderate Alzheimer’s disease. Researchers in 16 countries randomized 891 patients to 8 mg/day, 150 mg/day, or 250 mg/day of LMTM. Like methylene blue, LMTM discolors the urine. Because no dye achieves the same effect, researchers administered 8 mg/day of LMTM as a control dose. This dose is enough to discolor the urine, and thus avoid breaking the blind, but is believed to be pharmacologically inactive.
The primary end point was a composite of cognition, as measured by the Alzheimer’s Disease Assessment Scale-Cognition (ADAS-Cog), and activities of daily living, as measured by the Alzheimer’s Disease Cooperative Study-Activities of Daily Living (ADCS-ADL). The secondary end points were brain volume (ie, lateral ventricular volume) as measured by MRI, Clinical Global Impression of Change, and the Mini-Mental State Examination (MMSE).
The investigators examined the data using a standard, mixed-model, repeated-measure analysis with no imputation for missing data. They included patients’ Alzheimer’s disease treatment status as an additive term in their primary analysis. In addition, they planned a second analysis that used this treatment status as a covariate.
LMTM Failed Primary End Point
Approximately 62% of the population was female, and the population’s mean age was 70.6. About 85% of participants were taking approved treatments for Alzheimer’s disease at baseline. Baseline MMSE score was 18.6, and dementia was of moderate severity in 62% of patients. The study’s retention rate was 69%.
At 15 months, Dr. Gauthier and colleagues found no difference in ADAS-Cog score or ADCS-ADL score between the three treatment arms. Patients who took LMTM as monotherapy, however, had stable scores during the study period, compared with patients who took LMTM and standard treatments for Alzheimer’s disease. ADAS-Cog scores were 5.8 and 6.3 points higher, respectively, in patients taking the two higher doses of LMTM as monotherapy, compared with patients taking LMTM in combination with standard treatments. In addition, the rate of brain atrophy was 33% lower among patients taking LMTM as monotherapy, compared with patients taking LMTM and standard treatments.
Diarrhea was the most common side effect of the 250-mg/day dose of LMTM. This side effect may lead to a reduction of dose or treatment cessation. Dysuria was another adverse event and is explained by the drug effect in the urinary tract, according to the researchers.
What Is the Future of Tau Therapy?
The study results are disappointing, according to David Knopman, MD, Professor of Neurology at the Mayo Clinic College of Medicine in Rochester, Minnesota, who was not involved in the study. “The only thing that really counts is the primary outcome that was prespecified…. It was the group as a whole, and there weren’t any benefits.” Although the secondary results are interesting, secondary analyses are “fraught with interpretive difficulties because of hidden biases,” Dr. Knopman added. “When we’re only looking at a small subset of participants, and 15% were on no drug, I think it becomes difficult to interpret.” In addition, the results may have limited relevance for North American populations, where few people with Alzheimer’s disease are not receiving standard care.
A second, predominantly North American, study of LMTM in 800 patients with mild Alzheimer’s disease will be reported in the fall. In light of LMTM’s lack of efficacy as add-on to existing treatments, the researchers modified the primary analyses of the second study before unblinding to focus on monotherapy. “Tau therapy is a way to go. It’s just maybe more complicated than we thought,” said Dr. Gauthier, who is a member of the scientific advisory board of TauRx, the company that sponsored the study.
Should lower uterine segment thickness measurement be included in the TOLAC decision-making process?
EXPERT COMMENTARY
After having a previous cesarean delivery (CD), women who subsequently become pregnant inevitably face the decision to undergo a repeat CD or attempt a trial of labor after cesarean (TOLAC). Currently in the United States, 83% of women with a prior uterine scar are delivered by repeat CD.1 According to the Consortium on Safe Labor, more than half of all CD indications are attributed to having a prior uterine scar.1 Furthermore, only 28% of women attempt a TOLAC, with a successful vaginal birth after cesarean (VBAC) rate of approximately 57%.1
The reason for the low TOLAC rate is multifactorial, but a primary concern may be the safety risk of a TOLAC as it relates to uterine rupture, a rare but potentially catastrophic complication. In a large, multicenter prospective observational trial of more than 17,800 women attempting a TOLAC, the symptomatic uterine rupture rate was 0.7%.2 As such, efforts to identify women at highest risk for uterine rupture and those with characteristics predictive of a successful VBAC have remained ongoing. Jastrow and colleagues have expanded this body of knowledge with their prospective cohort study.
Details of the study
The researchers assessed lower uterine segment thickness via vaginal and abdominal ultrasound at 34 to 38 weeks’ gestation in more than 1,850 women with a previous CD. Women enrolled in the trial were classified into 3 risk categories based on lower uterine segment thickness: high risk (<2.0 mm), intermediate risk (2.0 to 2.4 mm), and low risk (≥2.5 mm). The investigators’ objective was to estimate the occurrence of uterine rupture when this measurement was included in the decision-making process on mode of delivery.
An important aspect of this study involved how the provider discussed the mode of delivery with the patient after the lower uterine segment measurement was obtained. Both the provider and the patient were informed of the risk category, and further counseling included the following:
- average overall uterine rupture risk, 0.5% to 1%
- if <2.0 mm, uterine rupture risk likely >1%
- if ≥2.5 mm, uterine rupture risk likely <0.5%
- uterine rupture risks (including perinatal asphyxia and death)
- maternal and neonatal complications of cesarean
- estimation of likelihood for successful VBAC.
How did risk-stratified women fare?
In approximately 1,000 cases, the authors reported no symptomatic uterine ruptures. Of particular interest, however, is the rate of women attempting a TOLAC in each category:
- 194 women with high risk
- 9% underwent a TOLAC
- 82% had a successful vaginal birth
- 217 women with intermediate risk
- 42% underwent a TOLAC
- 78% had a successful vaginal birth
- 1,438 women with low risk
- 61% underwent a TOLAC
- 66% had a successful vaginal birth.
Considering cesarean scar defect
Finally, uterine scar defects at CD in those who underwent a TOLAC were 0/3 (0%), 5/21 (25%), and 20/276 (7%) in the high-, intermediate-, and low-risk groups, respectively. Given the observational nature of the study, the authors suggest that uterine scar dehiscence may be predictive of labor dystocia, but it remains unclear if it predicts or is a prerequisite for subsequent uterine rupture if labor occurs.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Zhang J, Troendle J, Reddy UM, et al; Consortium on Safe Labor. Am J Obstet Gynecol. 2010;203(4):326.e1–326.e10.
- Landon MB, Hauth JC, Leveno KJ, et al; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Maternal and perinatal outcomes associated with a trial of labor after prior cesarean delivery. N Engl J Med. 2004;16;351(25):2581–2589.
EXPERT COMMENTARY
After having a previous cesarean delivery (CD), women who subsequently become pregnant inevitably face the decision to undergo a repeat CD or attempt a trial of labor after cesarean (TOLAC). Currently in the United States, 83% of women with a prior uterine scar are delivered by repeat CD.1 According to the Consortium on Safe Labor, more than half of all CD indications are attributed to having a prior uterine scar.1 Furthermore, only 28% of women attempt a TOLAC, with a successful vaginal birth after cesarean (VBAC) rate of approximately 57%.1
The reason for the low TOLAC rate is multifactorial, but a primary concern may be the safety risk of a TOLAC as it relates to uterine rupture, a rare but potentially catastrophic complication. In a large, multicenter prospective observational trial of more than 17,800 women attempting a TOLAC, the symptomatic uterine rupture rate was 0.7%.2 As such, efforts to identify women at highest risk for uterine rupture and those with characteristics predictive of a successful VBAC have remained ongoing. Jastrow and colleagues have expanded this body of knowledge with their prospective cohort study.
Details of the study
The researchers assessed lower uterine segment thickness via vaginal and abdominal ultrasound at 34 to 38 weeks’ gestation in more than 1,850 women with a previous CD. Women enrolled in the trial were classified into 3 risk categories based on lower uterine segment thickness: high risk (<2.0 mm), intermediate risk (2.0 to 2.4 mm), and low risk (≥2.5 mm). The investigators’ objective was to estimate the occurrence of uterine rupture when this measurement was included in the decision-making process on mode of delivery.
An important aspect of this study involved how the provider discussed the mode of delivery with the patient after the lower uterine segment measurement was obtained. Both the provider and the patient were informed of the risk category, and further counseling included the following:
- average overall uterine rupture risk, 0.5% to 1%
- if <2.0 mm, uterine rupture risk likely >1%
- if ≥2.5 mm, uterine rupture risk likely <0.5%
- uterine rupture risks (including perinatal asphyxia and death)
- maternal and neonatal complications of cesarean
- estimation of likelihood for successful VBAC.
How did risk-stratified women fare?
In approximately 1,000 cases, the authors reported no symptomatic uterine ruptures. Of particular interest, however, is the rate of women attempting a TOLAC in each category:
- 194 women with high risk
- 9% underwent a TOLAC
- 82% had a successful vaginal birth
- 217 women with intermediate risk
- 42% underwent a TOLAC
- 78% had a successful vaginal birth
- 1,438 women with low risk
- 61% underwent a TOLAC
- 66% had a successful vaginal birth.
Considering cesarean scar defect
Finally, uterine scar defects at CD in those who underwent a TOLAC were 0/3 (0%), 5/21 (25%), and 20/276 (7%) in the high-, intermediate-, and low-risk groups, respectively. Given the observational nature of the study, the authors suggest that uterine scar dehiscence may be predictive of labor dystocia, but it remains unclear if it predicts or is a prerequisite for subsequent uterine rupture if labor occurs.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
After having a previous cesarean delivery (CD), women who subsequently become pregnant inevitably face the decision to undergo a repeat CD or attempt a trial of labor after cesarean (TOLAC). Currently in the United States, 83% of women with a prior uterine scar are delivered by repeat CD.1 According to the Consortium on Safe Labor, more than half of all CD indications are attributed to having a prior uterine scar.1 Furthermore, only 28% of women attempt a TOLAC, with a successful vaginal birth after cesarean (VBAC) rate of approximately 57%.1
The reason for the low TOLAC rate is multifactorial, but a primary concern may be the safety risk of a TOLAC as it relates to uterine rupture, a rare but potentially catastrophic complication. In a large, multicenter prospective observational trial of more than 17,800 women attempting a TOLAC, the symptomatic uterine rupture rate was 0.7%.2 As such, efforts to identify women at highest risk for uterine rupture and those with characteristics predictive of a successful VBAC have remained ongoing. Jastrow and colleagues have expanded this body of knowledge with their prospective cohort study.
Details of the study
The researchers assessed lower uterine segment thickness via vaginal and abdominal ultrasound at 34 to 38 weeks’ gestation in more than 1,850 women with a previous CD. Women enrolled in the trial were classified into 3 risk categories based on lower uterine segment thickness: high risk (<2.0 mm), intermediate risk (2.0 to 2.4 mm), and low risk (≥2.5 mm). The investigators’ objective was to estimate the occurrence of uterine rupture when this measurement was included in the decision-making process on mode of delivery.
An important aspect of this study involved how the provider discussed the mode of delivery with the patient after the lower uterine segment measurement was obtained. Both the provider and the patient were informed of the risk category, and further counseling included the following:
- average overall uterine rupture risk, 0.5% to 1%
- if <2.0 mm, uterine rupture risk likely >1%
- if ≥2.5 mm, uterine rupture risk likely <0.5%
- uterine rupture risks (including perinatal asphyxia and death)
- maternal and neonatal complications of cesarean
- estimation of likelihood for successful VBAC.
How did risk-stratified women fare?
In approximately 1,000 cases, the authors reported no symptomatic uterine ruptures. Of particular interest, however, is the rate of women attempting a TOLAC in each category:
- 194 women with high risk
- 9% underwent a TOLAC
- 82% had a successful vaginal birth
- 217 women with intermediate risk
- 42% underwent a TOLAC
- 78% had a successful vaginal birth
- 1,438 women with low risk
- 61% underwent a TOLAC
- 66% had a successful vaginal birth.
Considering cesarean scar defect
Finally, uterine scar defects at CD in those who underwent a TOLAC were 0/3 (0%), 5/21 (25%), and 20/276 (7%) in the high-, intermediate-, and low-risk groups, respectively. Given the observational nature of the study, the authors suggest that uterine scar dehiscence may be predictive of labor dystocia, but it remains unclear if it predicts or is a prerequisite for subsequent uterine rupture if labor occurs.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Zhang J, Troendle J, Reddy UM, et al; Consortium on Safe Labor. Am J Obstet Gynecol. 2010;203(4):326.e1–326.e10.
- Landon MB, Hauth JC, Leveno KJ, et al; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Maternal and perinatal outcomes associated with a trial of labor after prior cesarean delivery. N Engl J Med. 2004;16;351(25):2581–2589.
- Zhang J, Troendle J, Reddy UM, et al; Consortium on Safe Labor. Am J Obstet Gynecol. 2010;203(4):326.e1–326.e10.
- Landon MB, Hauth JC, Leveno KJ, et al; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Maternal and perinatal outcomes associated with a trial of labor after prior cesarean delivery. N Engl J Med. 2004;16;351(25):2581–2589.
VIDEO: Apheresis shows promise for refractory angina with high Lp(a)
ROME – Weekly lipoprotein apheresis in patients with highly refractory angina accompanied by high plasma lipoprotein(a) without elevated LDL cholesterol led to significantly improved myocardial blood flow in a randomized, blinded, sham-controlled clinical trial, Tina Khan, MD, reported at the annual congress of the European Society of Cardiology.
Participants also experienced clinically meaningful improvements in the secondary endpoints of quality of life, angina symptoms, exercise capacity, and atheroma burden, added Dr. Khan of Imperial College London.
Angina pectoris that is refractory to maximal pharmacologic, percutaneous, and surgical interventions is a major and growing problem. More than 100,000 new cases occur per year in the United States.
“We have a desperate need to develop new therapeutic options,” she said.
Lipoprotein(a), or Lp(a), is a potent independent cardiovascular risk factor. And it figures prominently in refractory angina. Indeed, 60% of the patients with refractory angina screened by Dr. Khan for her clinical trial had an isolated plasma Lp(a) level of 50 mg/dL or more, the threshold at which cardiovascular risk sharply increases. Statins have no effect on Lp(a) levels.
While a couple of observational cohort studies have suggested that reducing elevated Lp(a) in patients with cardiovascular disease is associated with a decrease in major adverse cardiovascular events, until now there have been no randomized controlled trials of apheresis as Lp(a)-lowering therapy in patients with refractory angina, according to Dr. Khan.
She presented a randomized, crossover design study in 20 patients with severe refractory angina and an Lp(a) in excess of 50 mg/dL but no elevation in LDL. They underwent 3 months of blinded weekly extracorporeal lipoprotein apheresis using a dextran sulfate filtration system or sham apheresis, followed by a month-long washout period. Participants were then crossed over to the other study arm to increase the statistical power of this small study.
The primary study endpoint was change in myocardial perfusion reserve as measured by cardiovascular magnetic resonance imaging at baseline and after 3 months of true or sham apheresis. The myocardial perfusion reserve index improved by a net of 0.63 after apheresis from a baseline of 1.45. This effect was strongly driven by a substantial increase in stress myocardial perfusion, with very little change in perfusion at rest.
Significant improvements were also recorded after apheresis in the secondary endpoints of change in carotid atheroma as reflected in total carotid wall volume, improvement on various domains of the Seattle Angina Questionnaire, physical limitations as scored on the SF-36, and exercise capacity as measured on the 6-minute walk test.
Discussant Peter Libby, MD, was effusive in his praise for Dr. Khan’s study.
“This is a wonderful example of how we may be able to offer new hope for patients and families with high Lp(a),” declared Dr. Libby, chief of cardiovascular medicine at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, Boston.
He approved of the methodology and embraced what he called “the intriguing preliminary picture of benefit for lowering Lp(a).”
Most of all, he was pleased that Dr. Khan’s well conducted albeit small study has thrown a spotlight on Lp(a), which he characterized as a greatly underappreciated causal risk factor for a range of cardiovascular diseases.
“Lp(a) stands out like a Manhattan skyscraper as the major driver of calcific aortic stenosis, an epidemic that we see in our aging population,” he observed.
He added that topics worthy of further research with regard to Lp(a)-lowering apheresis as a treatment for refractory angina include the question of whether the mechanism of benefit involves structural changes in atherosclerosis or functional changes. And if it’s the latter, is it a matter of changes in microvascular function, macrovascular function, or a combination of the two?
Apheresis is extremely costly, inconvenient, and invasive, and there are only several dozen apheresis centers in the United States. So the future of Lp(a)-lowering to treat refractory angina, calcific aortic stenosis, and other cardiovascular conditions where elevated Lp(a) is an important player may lie in pharmacotherapy with the PCSK9 inhibitors, Dr. Libby predicted.
He cited “very promising” data showing that evolocumab (Repatha) and alirocumab (Praluent) lower Lp(a) in dose-dependent fashion. Ongoing very large clinical trials with hard clinical endpoints should eventually provide key information regarding the cardiovascular benefits of lowering Lp(a).
“We may be entering an era where we may be able to offer our patients and families – because this is often a familial problem – a non-apheresis approach to controlling what we are learning is a very important causal risk factor for atherosclerosis,” Dr. Libby said.
Patrick M. Moriarty, MD, said in a video interview that he was very intrigued by the results, and “not personally surprised.” They should stimulate interest in cardiologists to start measuring Lp(a) in their patients like those in this study, in whom the disease severity doesn’t match up with the clinical risk factors, said Dr. Moriarty of the University of Kansas, Kansas City.
Dr. Khan’s study was funded by the UK National Institute for Health Research. She reported having no financial conflicts of interest.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ROME – Weekly lipoprotein apheresis in patients with highly refractory angina accompanied by high plasma lipoprotein(a) without elevated LDL cholesterol led to significantly improved myocardial blood flow in a randomized, blinded, sham-controlled clinical trial, Tina Khan, MD, reported at the annual congress of the European Society of Cardiology.
Participants also experienced clinically meaningful improvements in the secondary endpoints of quality of life, angina symptoms, exercise capacity, and atheroma burden, added Dr. Khan of Imperial College London.
Angina pectoris that is refractory to maximal pharmacologic, percutaneous, and surgical interventions is a major and growing problem. More than 100,000 new cases occur per year in the United States.
“We have a desperate need to develop new therapeutic options,” she said.
Lipoprotein(a), or Lp(a), is a potent independent cardiovascular risk factor. And it figures prominently in refractory angina. Indeed, 60% of the patients with refractory angina screened by Dr. Khan for her clinical trial had an isolated plasma Lp(a) level of 50 mg/dL or more, the threshold at which cardiovascular risk sharply increases. Statins have no effect on Lp(a) levels.
While a couple of observational cohort studies have suggested that reducing elevated Lp(a) in patients with cardiovascular disease is associated with a decrease in major adverse cardiovascular events, until now there have been no randomized controlled trials of apheresis as Lp(a)-lowering therapy in patients with refractory angina, according to Dr. Khan.
She presented a randomized, crossover design study in 20 patients with severe refractory angina and an Lp(a) in excess of 50 mg/dL but no elevation in LDL. They underwent 3 months of blinded weekly extracorporeal lipoprotein apheresis using a dextran sulfate filtration system or sham apheresis, followed by a month-long washout period. Participants were then crossed over to the other study arm to increase the statistical power of this small study.
The primary study endpoint was change in myocardial perfusion reserve as measured by cardiovascular magnetic resonance imaging at baseline and after 3 months of true or sham apheresis. The myocardial perfusion reserve index improved by a net of 0.63 after apheresis from a baseline of 1.45. This effect was strongly driven by a substantial increase in stress myocardial perfusion, with very little change in perfusion at rest.
Significant improvements were also recorded after apheresis in the secondary endpoints of change in carotid atheroma as reflected in total carotid wall volume, improvement on various domains of the Seattle Angina Questionnaire, physical limitations as scored on the SF-36, and exercise capacity as measured on the 6-minute walk test.
Discussant Peter Libby, MD, was effusive in his praise for Dr. Khan’s study.
“This is a wonderful example of how we may be able to offer new hope for patients and families with high Lp(a),” declared Dr. Libby, chief of cardiovascular medicine at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, Boston.
He approved of the methodology and embraced what he called “the intriguing preliminary picture of benefit for lowering Lp(a).”
Most of all, he was pleased that Dr. Khan’s well conducted albeit small study has thrown a spotlight on Lp(a), which he characterized as a greatly underappreciated causal risk factor for a range of cardiovascular diseases.
“Lp(a) stands out like a Manhattan skyscraper as the major driver of calcific aortic stenosis, an epidemic that we see in our aging population,” he observed.
He added that topics worthy of further research with regard to Lp(a)-lowering apheresis as a treatment for refractory angina include the question of whether the mechanism of benefit involves structural changes in atherosclerosis or functional changes. And if it’s the latter, is it a matter of changes in microvascular function, macrovascular function, or a combination of the two?
Apheresis is extremely costly, inconvenient, and invasive, and there are only several dozen apheresis centers in the United States. So the future of Lp(a)-lowering to treat refractory angina, calcific aortic stenosis, and other cardiovascular conditions where elevated Lp(a) is an important player may lie in pharmacotherapy with the PCSK9 inhibitors, Dr. Libby predicted.
He cited “very promising” data showing that evolocumab (Repatha) and alirocumab (Praluent) lower Lp(a) in dose-dependent fashion. Ongoing very large clinical trials with hard clinical endpoints should eventually provide key information regarding the cardiovascular benefits of lowering Lp(a).
“We may be entering an era where we may be able to offer our patients and families – because this is often a familial problem – a non-apheresis approach to controlling what we are learning is a very important causal risk factor for atherosclerosis,” Dr. Libby said.
Patrick M. Moriarty, MD, said in a video interview that he was very intrigued by the results, and “not personally surprised.” They should stimulate interest in cardiologists to start measuring Lp(a) in their patients like those in this study, in whom the disease severity doesn’t match up with the clinical risk factors, said Dr. Moriarty of the University of Kansas, Kansas City.
Dr. Khan’s study was funded by the UK National Institute for Health Research. She reported having no financial conflicts of interest.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ROME – Weekly lipoprotein apheresis in patients with highly refractory angina accompanied by high plasma lipoprotein(a) without elevated LDL cholesterol led to significantly improved myocardial blood flow in a randomized, blinded, sham-controlled clinical trial, Tina Khan, MD, reported at the annual congress of the European Society of Cardiology.
Participants also experienced clinically meaningful improvements in the secondary endpoints of quality of life, angina symptoms, exercise capacity, and atheroma burden, added Dr. Khan of Imperial College London.
Angina pectoris that is refractory to maximal pharmacologic, percutaneous, and surgical interventions is a major and growing problem. More than 100,000 new cases occur per year in the United States.
“We have a desperate need to develop new therapeutic options,” she said.
Lipoprotein(a), or Lp(a), is a potent independent cardiovascular risk factor. And it figures prominently in refractory angina. Indeed, 60% of the patients with refractory angina screened by Dr. Khan for her clinical trial had an isolated plasma Lp(a) level of 50 mg/dL or more, the threshold at which cardiovascular risk sharply increases. Statins have no effect on Lp(a) levels.
While a couple of observational cohort studies have suggested that reducing elevated Lp(a) in patients with cardiovascular disease is associated with a decrease in major adverse cardiovascular events, until now there have been no randomized controlled trials of apheresis as Lp(a)-lowering therapy in patients with refractory angina, according to Dr. Khan.
She presented a randomized, crossover design study in 20 patients with severe refractory angina and an Lp(a) in excess of 50 mg/dL but no elevation in LDL. They underwent 3 months of blinded weekly extracorporeal lipoprotein apheresis using a dextran sulfate filtration system or sham apheresis, followed by a month-long washout period. Participants were then crossed over to the other study arm to increase the statistical power of this small study.
The primary study endpoint was change in myocardial perfusion reserve as measured by cardiovascular magnetic resonance imaging at baseline and after 3 months of true or sham apheresis. The myocardial perfusion reserve index improved by a net of 0.63 after apheresis from a baseline of 1.45. This effect was strongly driven by a substantial increase in stress myocardial perfusion, with very little change in perfusion at rest.
Significant improvements were also recorded after apheresis in the secondary endpoints of change in carotid atheroma as reflected in total carotid wall volume, improvement on various domains of the Seattle Angina Questionnaire, physical limitations as scored on the SF-36, and exercise capacity as measured on the 6-minute walk test.
Discussant Peter Libby, MD, was effusive in his praise for Dr. Khan’s study.
“This is a wonderful example of how we may be able to offer new hope for patients and families with high Lp(a),” declared Dr. Libby, chief of cardiovascular medicine at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, Boston.
He approved of the methodology and embraced what he called “the intriguing preliminary picture of benefit for lowering Lp(a).”
Most of all, he was pleased that Dr. Khan’s well conducted albeit small study has thrown a spotlight on Lp(a), which he characterized as a greatly underappreciated causal risk factor for a range of cardiovascular diseases.
“Lp(a) stands out like a Manhattan skyscraper as the major driver of calcific aortic stenosis, an epidemic that we see in our aging population,” he observed.
He added that topics worthy of further research with regard to Lp(a)-lowering apheresis as a treatment for refractory angina include the question of whether the mechanism of benefit involves structural changes in atherosclerosis or functional changes. And if it’s the latter, is it a matter of changes in microvascular function, macrovascular function, or a combination of the two?
Apheresis is extremely costly, inconvenient, and invasive, and there are only several dozen apheresis centers in the United States. So the future of Lp(a)-lowering to treat refractory angina, calcific aortic stenosis, and other cardiovascular conditions where elevated Lp(a) is an important player may lie in pharmacotherapy with the PCSK9 inhibitors, Dr. Libby predicted.
He cited “very promising” data showing that evolocumab (Repatha) and alirocumab (Praluent) lower Lp(a) in dose-dependent fashion. Ongoing very large clinical trials with hard clinical endpoints should eventually provide key information regarding the cardiovascular benefits of lowering Lp(a).
“We may be entering an era where we may be able to offer our patients and families – because this is often a familial problem – a non-apheresis approach to controlling what we are learning is a very important causal risk factor for atherosclerosis,” Dr. Libby said.
Patrick M. Moriarty, MD, said in a video interview that he was very intrigued by the results, and “not personally surprised.” They should stimulate interest in cardiologists to start measuring Lp(a) in their patients like those in this study, in whom the disease severity doesn’t match up with the clinical risk factors, said Dr. Moriarty of the University of Kansas, Kansas City.
Dr. Khan’s study was funded by the UK National Institute for Health Research. She reported having no financial conflicts of interest.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE ESC CONGRESS 2016
Key clinical point: Lipoprotein apheresis may provide a needed novel treatment for many patients with refractory angina.
Major finding: Myocardial perfusion reserve improved by 43% after apheresis, compared with no significant change after sham apheresis in patients with refractory angina and elevated lipoprotein(a).
Data source: A randomized, blinded, sham-controlled, crossover trial in 20 patients with refractory angina and elevated Lp(a) in the absence of high LDL cholesterol.
Disclosures: The UK National Institute for Health Research funded the study. The presenter reported having no financial conflicts of interest.
Study Identifies Two Biomarkers That Contribute to Spine Osteoarthritis
Researchers have discovered a pair of tissue biomarkers that directly contribute to the joint degeneration associated with spine osteoarthritis, according to a study published in the Journal of Clinical Investigation Insight.
The study evaluated tissue biopsies from 55 patients undergoing decompression or discectomy. Investigators screened 2,100 microRNAs and found that microRNA-181a-5p and microRNA-4454 biomarkers are involved in destroying cartilage and increase inflammation, and that measuring these two biomarkers can help clinicians determine the stage to which spine osteoarthritis has progressed, and provide a tool for determining the degree of cartilage destruction.
Suggested Reading
Nakamura A, Rampersaud R. Y., Sharma A. Identification of microRNA-181a-5p and microRNA-4454 as mediators of facet cartilage degeneration. JCI Insight. 2016;1(12):e86820.
Researchers have discovered a pair of tissue biomarkers that directly contribute to the joint degeneration associated with spine osteoarthritis, according to a study published in the Journal of Clinical Investigation Insight.
The study evaluated tissue biopsies from 55 patients undergoing decompression or discectomy. Investigators screened 2,100 microRNAs and found that microRNA-181a-5p and microRNA-4454 biomarkers are involved in destroying cartilage and increase inflammation, and that measuring these two biomarkers can help clinicians determine the stage to which spine osteoarthritis has progressed, and provide a tool for determining the degree of cartilage destruction.
Researchers have discovered a pair of tissue biomarkers that directly contribute to the joint degeneration associated with spine osteoarthritis, according to a study published in the Journal of Clinical Investigation Insight.
The study evaluated tissue biopsies from 55 patients undergoing decompression or discectomy. Investigators screened 2,100 microRNAs and found that microRNA-181a-5p and microRNA-4454 biomarkers are involved in destroying cartilage and increase inflammation, and that measuring these two biomarkers can help clinicians determine the stage to which spine osteoarthritis has progressed, and provide a tool for determining the degree of cartilage destruction.
Suggested Reading
Nakamura A, Rampersaud R. Y., Sharma A. Identification of microRNA-181a-5p and microRNA-4454 as mediators of facet cartilage degeneration. JCI Insight. 2016;1(12):e86820.
Suggested Reading
Nakamura A, Rampersaud R. Y., Sharma A. Identification of microRNA-181a-5p and microRNA-4454 as mediators of facet cartilage degeneration. JCI Insight. 2016;1(12):e86820.
In which clinical situations can the use of the 52-mg levonorgestrel-releasing IUD (Mirena) and the TCu380A copper-IUD (ParaGard) be extended?
One of the most important medical interventions to improve maternal-child health is providing effective contraception to men and women of reproductive age. The 52-mg levonorgestrel-intrauterine device (LNG-IUD; Mirena) is one of the most effective forms of reversible contraception available to women, with a failure rate of 1.1% over 5 years of use.1 The TCu380A copper-IUD (ParaGard), another highly effective reversible contraceptive, is reported to have failure rates of approximately 1.4% and 2.2%, over 5 and 10 years of use.2
An interesting question is whether—in certain clinical situations—a single IUD can be used for longer than the currently recommended 5 and 10 years for a Mirena IUD and a ParaGard IUD, respectively.
The LNG-IUD containing 52 mg LNG may be effective up to 7 years
The US Food and Drug Administration (FDA) package insert for the Mirena 52-mg LNG-IUD states that the device is “indicated for contraception for up to 5 years. Thereafter if continued contraception is desired, the system should be replaced.”1 The FDA package insert for the levonorgestrel-releasing intrauterine system, Liletta 52-mg LNG-IUD, states that it is “indicated for prevention of pregnancy up to 3 years.”3 The FDA guidance is based on data submitted to the agency by the manufacturers to support the approval process. Completing large-scale clinical trials that extend past 5 years or more is challenging, because of the cost and the loss of study participants to follow-up. Hence, few clinical trials of contraceptive IUDs continue for more than 5 to 10 years.
Although the FDA-approved indication for Mirena and Liletta is 5 and 3 years, respectively, evidence suggests that the 52-mg LNG-IUD is an effective contraceptive beyond 5 years. In fact, multiple studies report that this IUD is an effective contraceptive for at least 6 or 7 years (TABLE 1).4–9 Among 895 women using the 52-mg LNG-IUD for 6 to 7 years, only 1 pregnancy was reported in the last year of use. In that case, the IUD was in the cervix and partially expelled from the uterus.8 These data indicate that the 52-mg LNG-IUD is likely an effective contraceptive for up to 7 years, with pregnancy rates below 1% in the last year of use.
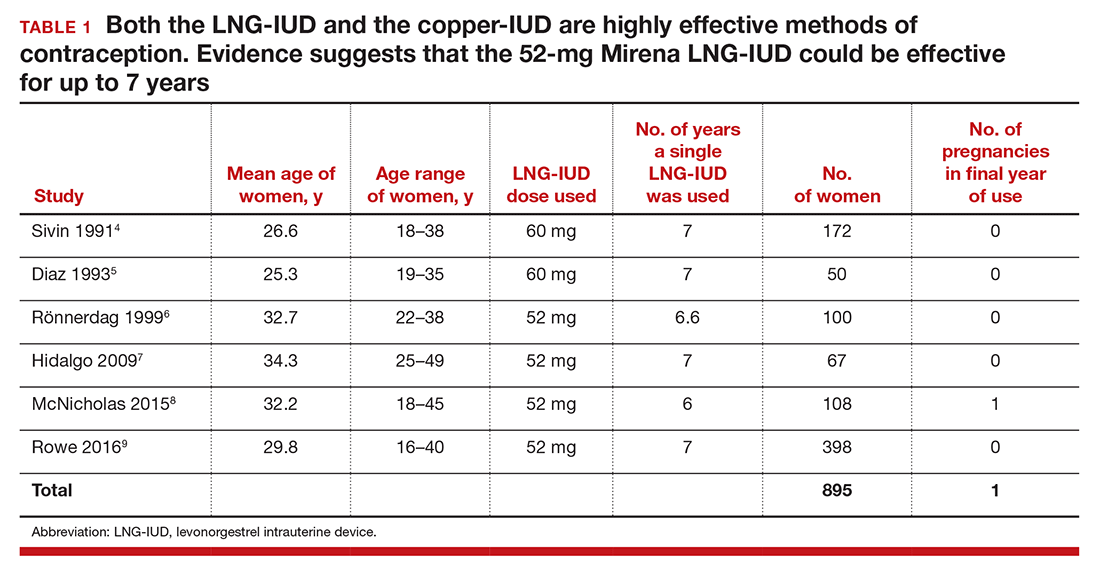
The TCu380A copper-IUD is effective up to 12 years
The currently available TCu380A copper-IUD (ParaGard) is FDA approved for 10 years.2 Studies evaluating the efficacy of this copper-IUD are limited, but those that have been published reported that it is effective for at least 12 years and possibly up to 20 years (TABLE 2).10−13
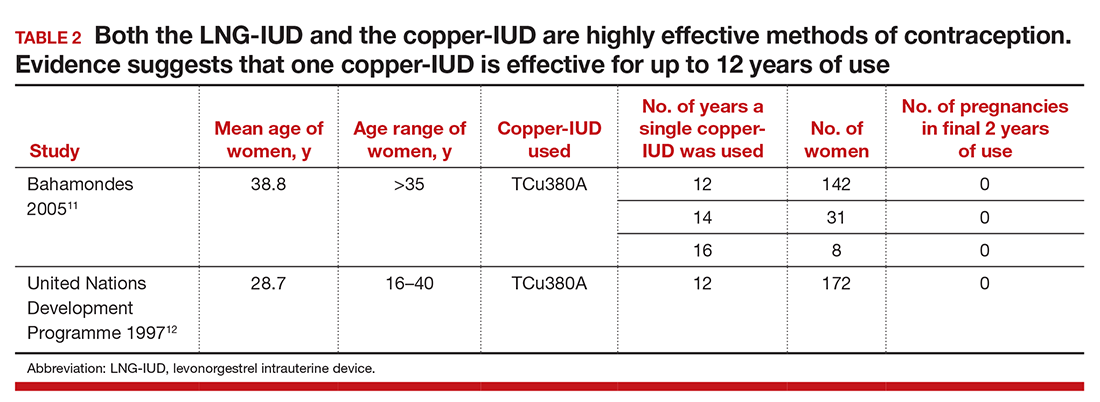
Recently I saw a patient who had a copper-IUD (ParaGard, TCu380A) inserted as a teen after a birth, and had successfully used the same device for 17 years. She presented for removal of the IUD so that she could attempt conception. After removal of the IUD, copper wire was visible on the device. Long-term studies of the TCu220 copper-IUD, which contains less copper than the ParaGard, report pregnancies with the use of the device beyond 10 years.12 These devices, which are not available in the United States, should not be used past their recommended interval.
- Mirena [package insert]. Wayne, NJ: Bayer HealthCare Pharmaceuticals; July 2008. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021225s019lbl.pdf. Accessed July 28, 2016.
- ParaGard [package insert]. N. Tonawanda, NY: FEI Women’s Health LLC; revised September 2005. http://www.accessdata.fda.gov/drugsatfda_docs/label/2005/018680s060lbl.pdf. Accessed July 28, 2016.
- Liletta [package insert]. Parsippany, NJ: Actavis Pharma, Inc; February 2015. http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206229s000lbl.pdf. Accessed July 28, 2016.
- Sivin I, Stern J, Coutinho E, et al. Prolonged intrauterine contraception: a seven-year randomized study of the levonorgestrel 20 mcg/day (LNg 20) and the copper T380 Ag IUDS. Contraception. 1991;44(5):473–480.
- Díaz J, Faúndes A, Díaz M, Marchi N. Evaluation of the clinical performance of a levonorgestrel-releasing IUD, up to seven years of use, in Campinas, Brazil. Contraception. 1993;47(2):169–175.
- Rönnerdag M, Odlind V. Health effects of long-term use of the intrauterine levonorgestrel-releasing system. A follow-up study over 12 years of continuous use. Acta Obstet Gynecol Scand. 1999;78(8):716–721.
- Hidalgo MM, Hidalgo-Regina C, Bahamondes MV, Monteiro I, Petta CA, Bahamondes L. Serum levonorgestrel levels and endometrial thickness during extended use of the levonorgestrel-releasing intrauterine system. Contraception. 2009;80(1):84–89.
- McNicholas C, Maddipati R, Zhao Q, Swor E, Peipert JF. Use of the etonogestrel implant and levonorgestrel intrauterine device beyond the U.S. Food and Drug Administration-approved duration. Obstet Gynecol. 2015;125(3):599–604.
- Rowe P, Farley T, Peregoudov A, et al. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93(6):498–506.
- Wu JP, Pickle S. Extended use of the intrauterine device: a literature review and recommendations for clinical practice. Contraception. 2014;89(6):495–503.
- Bahamondes L, Faundes A, Sobreira-Lima B, Liu-Filho JF, Pecci P, Matera S. TCu 380A IUD: a reversible permanent contraceptive method in women over 35 years of age. Contraception. 2005;72(5):337–341.
- United Nations Development Programme. Long-term reversible contraception. Twelve years of experience with the TCu380A and TCu220C. Contraception. 1997;56(6):341–352.
- Sivin I. Utility and drawbacks of continuous use of a copper T IUD for 20 years. Contraception. 2007;75(6 suppl):S70–S75.
One of the most important medical interventions to improve maternal-child health is providing effective contraception to men and women of reproductive age. The 52-mg levonorgestrel-intrauterine device (LNG-IUD; Mirena) is one of the most effective forms of reversible contraception available to women, with a failure rate of 1.1% over 5 years of use.1 The TCu380A copper-IUD (ParaGard), another highly effective reversible contraceptive, is reported to have failure rates of approximately 1.4% and 2.2%, over 5 and 10 years of use.2
An interesting question is whether—in certain clinical situations—a single IUD can be used for longer than the currently recommended 5 and 10 years for a Mirena IUD and a ParaGard IUD, respectively.
The LNG-IUD containing 52 mg LNG may be effective up to 7 years
The US Food and Drug Administration (FDA) package insert for the Mirena 52-mg LNG-IUD states that the device is “indicated for contraception for up to 5 years. Thereafter if continued contraception is desired, the system should be replaced.”1 The FDA package insert for the levonorgestrel-releasing intrauterine system, Liletta 52-mg LNG-IUD, states that it is “indicated for prevention of pregnancy up to 3 years.”3 The FDA guidance is based on data submitted to the agency by the manufacturers to support the approval process. Completing large-scale clinical trials that extend past 5 years or more is challenging, because of the cost and the loss of study participants to follow-up. Hence, few clinical trials of contraceptive IUDs continue for more than 5 to 10 years.
Although the FDA-approved indication for Mirena and Liletta is 5 and 3 years, respectively, evidence suggests that the 52-mg LNG-IUD is an effective contraceptive beyond 5 years. In fact, multiple studies report that this IUD is an effective contraceptive for at least 6 or 7 years (TABLE 1).4–9 Among 895 women using the 52-mg LNG-IUD for 6 to 7 years, only 1 pregnancy was reported in the last year of use. In that case, the IUD was in the cervix and partially expelled from the uterus.8 These data indicate that the 52-mg LNG-IUD is likely an effective contraceptive for up to 7 years, with pregnancy rates below 1% in the last year of use.

The TCu380A copper-IUD is effective up to 12 years
The currently available TCu380A copper-IUD (ParaGard) is FDA approved for 10 years.2 Studies evaluating the efficacy of this copper-IUD are limited, but those that have been published reported that it is effective for at least 12 years and possibly up to 20 years (TABLE 2).10−13

Recently I saw a patient who had a copper-IUD (ParaGard, TCu380A) inserted as a teen after a birth, and had successfully used the same device for 17 years. She presented for removal of the IUD so that she could attempt conception. After removal of the IUD, copper wire was visible on the device. Long-term studies of the TCu220 copper-IUD, which contains less copper than the ParaGard, report pregnancies with the use of the device beyond 10 years.12 These devices, which are not available in the United States, should not be used past their recommended interval.
One of the most important medical interventions to improve maternal-child health is providing effective contraception to men and women of reproductive age. The 52-mg levonorgestrel-intrauterine device (LNG-IUD; Mirena) is one of the most effective forms of reversible contraception available to women, with a failure rate of 1.1% over 5 years of use.1 The TCu380A copper-IUD (ParaGard), another highly effective reversible contraceptive, is reported to have failure rates of approximately 1.4% and 2.2%, over 5 and 10 years of use.2
An interesting question is whether—in certain clinical situations—a single IUD can be used for longer than the currently recommended 5 and 10 years for a Mirena IUD and a ParaGard IUD, respectively.
The LNG-IUD containing 52 mg LNG may be effective up to 7 years
The US Food and Drug Administration (FDA) package insert for the Mirena 52-mg LNG-IUD states that the device is “indicated for contraception for up to 5 years. Thereafter if continued contraception is desired, the system should be replaced.”1 The FDA package insert for the levonorgestrel-releasing intrauterine system, Liletta 52-mg LNG-IUD, states that it is “indicated for prevention of pregnancy up to 3 years.”3 The FDA guidance is based on data submitted to the agency by the manufacturers to support the approval process. Completing large-scale clinical trials that extend past 5 years or more is challenging, because of the cost and the loss of study participants to follow-up. Hence, few clinical trials of contraceptive IUDs continue for more than 5 to 10 years.
Although the FDA-approved indication for Mirena and Liletta is 5 and 3 years, respectively, evidence suggests that the 52-mg LNG-IUD is an effective contraceptive beyond 5 years. In fact, multiple studies report that this IUD is an effective contraceptive for at least 6 or 7 years (TABLE 1).4–9 Among 895 women using the 52-mg LNG-IUD for 6 to 7 years, only 1 pregnancy was reported in the last year of use. In that case, the IUD was in the cervix and partially expelled from the uterus.8 These data indicate that the 52-mg LNG-IUD is likely an effective contraceptive for up to 7 years, with pregnancy rates below 1% in the last year of use.

The TCu380A copper-IUD is effective up to 12 years
The currently available TCu380A copper-IUD (ParaGard) is FDA approved for 10 years.2 Studies evaluating the efficacy of this copper-IUD are limited, but those that have been published reported that it is effective for at least 12 years and possibly up to 20 years (TABLE 2).10−13

Recently I saw a patient who had a copper-IUD (ParaGard, TCu380A) inserted as a teen after a birth, and had successfully used the same device for 17 years. She presented for removal of the IUD so that she could attempt conception. After removal of the IUD, copper wire was visible on the device. Long-term studies of the TCu220 copper-IUD, which contains less copper than the ParaGard, report pregnancies with the use of the device beyond 10 years.12 These devices, which are not available in the United States, should not be used past their recommended interval.
- Mirena [package insert]. Wayne, NJ: Bayer HealthCare Pharmaceuticals; July 2008. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021225s019lbl.pdf. Accessed July 28, 2016.
- ParaGard [package insert]. N. Tonawanda, NY: FEI Women’s Health LLC; revised September 2005. http://www.accessdata.fda.gov/drugsatfda_docs/label/2005/018680s060lbl.pdf. Accessed July 28, 2016.
- Liletta [package insert]. Parsippany, NJ: Actavis Pharma, Inc; February 2015. http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206229s000lbl.pdf. Accessed July 28, 2016.
- Sivin I, Stern J, Coutinho E, et al. Prolonged intrauterine contraception: a seven-year randomized study of the levonorgestrel 20 mcg/day (LNg 20) and the copper T380 Ag IUDS. Contraception. 1991;44(5):473–480.
- Díaz J, Faúndes A, Díaz M, Marchi N. Evaluation of the clinical performance of a levonorgestrel-releasing IUD, up to seven years of use, in Campinas, Brazil. Contraception. 1993;47(2):169–175.
- Rönnerdag M, Odlind V. Health effects of long-term use of the intrauterine levonorgestrel-releasing system. A follow-up study over 12 years of continuous use. Acta Obstet Gynecol Scand. 1999;78(8):716–721.
- Hidalgo MM, Hidalgo-Regina C, Bahamondes MV, Monteiro I, Petta CA, Bahamondes L. Serum levonorgestrel levels and endometrial thickness during extended use of the levonorgestrel-releasing intrauterine system. Contraception. 2009;80(1):84–89.
- McNicholas C, Maddipati R, Zhao Q, Swor E, Peipert JF. Use of the etonogestrel implant and levonorgestrel intrauterine device beyond the U.S. Food and Drug Administration-approved duration. Obstet Gynecol. 2015;125(3):599–604.
- Rowe P, Farley T, Peregoudov A, et al. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93(6):498–506.
- Wu JP, Pickle S. Extended use of the intrauterine device: a literature review and recommendations for clinical practice. Contraception. 2014;89(6):495–503.
- Bahamondes L, Faundes A, Sobreira-Lima B, Liu-Filho JF, Pecci P, Matera S. TCu 380A IUD: a reversible permanent contraceptive method in women over 35 years of age. Contraception. 2005;72(5):337–341.
- United Nations Development Programme. Long-term reversible contraception. Twelve years of experience with the TCu380A and TCu220C. Contraception. 1997;56(6):341–352.
- Sivin I. Utility and drawbacks of continuous use of a copper T IUD for 20 years. Contraception. 2007;75(6 suppl):S70–S75.
- Mirena [package insert]. Wayne, NJ: Bayer HealthCare Pharmaceuticals; July 2008. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021225s019lbl.pdf. Accessed July 28, 2016.
- ParaGard [package insert]. N. Tonawanda, NY: FEI Women’s Health LLC; revised September 2005. http://www.accessdata.fda.gov/drugsatfda_docs/label/2005/018680s060lbl.pdf. Accessed July 28, 2016.
- Liletta [package insert]. Parsippany, NJ: Actavis Pharma, Inc; February 2015. http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206229s000lbl.pdf. Accessed July 28, 2016.
- Sivin I, Stern J, Coutinho E, et al. Prolonged intrauterine contraception: a seven-year randomized study of the levonorgestrel 20 mcg/day (LNg 20) and the copper T380 Ag IUDS. Contraception. 1991;44(5):473–480.
- Díaz J, Faúndes A, Díaz M, Marchi N. Evaluation of the clinical performance of a levonorgestrel-releasing IUD, up to seven years of use, in Campinas, Brazil. Contraception. 1993;47(2):169–175.
- Rönnerdag M, Odlind V. Health effects of long-term use of the intrauterine levonorgestrel-releasing system. A follow-up study over 12 years of continuous use. Acta Obstet Gynecol Scand. 1999;78(8):716–721.
- Hidalgo MM, Hidalgo-Regina C, Bahamondes MV, Monteiro I, Petta CA, Bahamondes L. Serum levonorgestrel levels and endometrial thickness during extended use of the levonorgestrel-releasing intrauterine system. Contraception. 2009;80(1):84–89.
- McNicholas C, Maddipati R, Zhao Q, Swor E, Peipert JF. Use of the etonogestrel implant and levonorgestrel intrauterine device beyond the U.S. Food and Drug Administration-approved duration. Obstet Gynecol. 2015;125(3):599–604.
- Rowe P, Farley T, Peregoudov A, et al. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93(6):498–506.
- Wu JP, Pickle S. Extended use of the intrauterine device: a literature review and recommendations for clinical practice. Contraception. 2014;89(6):495–503.
- Bahamondes L, Faundes A, Sobreira-Lima B, Liu-Filho JF, Pecci P, Matera S. TCu 380A IUD: a reversible permanent contraceptive method in women over 35 years of age. Contraception. 2005;72(5):337–341.
- United Nations Development Programme. Long-term reversible contraception. Twelve years of experience with the TCu380A and TCu220C. Contraception. 1997;56(6):341–352.
- Sivin I. Utility and drawbacks of continuous use of a copper T IUD for 20 years. Contraception. 2007;75(6 suppl):S70–S75.
Minorities Have Fewer Knee Replacement Surgeries, But Are More Likely to Experience Complications
Compared to white patients, minority patients have lower rates of total knee replacement (TKR), but higher rates of adverse health outcomes associated with this procedure, according to a study in the Journal of Bone and Joint Surgery.
The study analyzed data on 547,380 patients from 8 racially diverse states who underwent TKR from 2001 to 2008. Race was categorized as white, black, Hispanic, Asian, Native American, and mixed race.
In comparison to the white patients, minorities had lower rates of TKR. Minorities also were less likely to undergo TKR in a high-volume hospital. In addition, the risk for in-hospital mortality and the rate of complications following TKR were significantly higher for patients who were black, Native American, or mixed race.
Suggested Reading
Zhang W, Lyman S, Boutin-Foster C, et al. Racial and ethnic disparities in utilization rate, hospital volume, and perioperative outcomes after total knee arthroplasty. J Bone Joint Surg Am. 2016 Aug 3;98(15):1243-1252.
Compared to white patients, minority patients have lower rates of total knee replacement (TKR), but higher rates of adverse health outcomes associated with this procedure, according to a study in the Journal of Bone and Joint Surgery.
The study analyzed data on 547,380 patients from 8 racially diverse states who underwent TKR from 2001 to 2008. Race was categorized as white, black, Hispanic, Asian, Native American, and mixed race.
In comparison to the white patients, minorities had lower rates of TKR. Minorities also were less likely to undergo TKR in a high-volume hospital. In addition, the risk for in-hospital mortality and the rate of complications following TKR were significantly higher for patients who were black, Native American, or mixed race.
Compared to white patients, minority patients have lower rates of total knee replacement (TKR), but higher rates of adverse health outcomes associated with this procedure, according to a study in the Journal of Bone and Joint Surgery.
The study analyzed data on 547,380 patients from 8 racially diverse states who underwent TKR from 2001 to 2008. Race was categorized as white, black, Hispanic, Asian, Native American, and mixed race.
In comparison to the white patients, minorities had lower rates of TKR. Minorities also were less likely to undergo TKR in a high-volume hospital. In addition, the risk for in-hospital mortality and the rate of complications following TKR were significantly higher for patients who were black, Native American, or mixed race.
Suggested Reading
Zhang W, Lyman S, Boutin-Foster C, et al. Racial and ethnic disparities in utilization rate, hospital volume, and perioperative outcomes after total knee arthroplasty. J Bone Joint Surg Am. 2016 Aug 3;98(15):1243-1252.
Suggested Reading
Zhang W, Lyman S, Boutin-Foster C, et al. Racial and ethnic disparities in utilization rate, hospital volume, and perioperative outcomes after total knee arthroplasty. J Bone Joint Surg Am. 2016 Aug 3;98(15):1243-1252.
Letters to the Editor: Treating uterine atony
“STOP USING RECTAL MISOPROSTOL FOR THE TREATMENT OF POSTPARTUM HEMORRHAGE CAUSED BY UTERINE ATONY”Robert L. Barbieri, MD (Editorial; July 2016)
The BEPCOP strategy for uterine atony
I appreciated Dr. Barbieri’s editorial about oxytocics for postpartum uterine atony and have personally noted the poor effectiveness of rectal misoprostol. I was reminded of his previous editorial that recommended administering intravenous (IV) oxytocin to postcesarean delivery patients for about 6 to 8 hours to reduce the risk of postoperative hemorrhage.
At my current hospital we usually use postpartum oxytocin, 30 units in 500 mL of 5% dextrose in water (D5W) for vaginal deliveries, and that infusion typically is administered for only 1 to 2 hours. Cesarean delivery patients receive oxytocin, 20 units in 1,000 mL of Ringer’s lactate, over the first 1 to 2 hours postoperatively. As an OB hospitalist I have been summoned occasionally to the bedside of patients who have uterine atony and hemorrhage, which usually occurs several hours after their oxytocin infusion has finished.
With this in mind I developed a proactive protocol that I call BEPCOP, an acronym for “Barnes’ Excellent Post Cesarean Oxytocin Protocol.” This involves simply running a 500-mL bag of oxytocin (30 units in 500 mL of D5W) at a constant rate of 50 mL/hour, which provides 50 mU/min oxytocin over the first 10 hours postdelivery.
I recommend BEPCOP for every cesarean delivery patient, as well as for any vaginally delivered patients who are at increased risk for atony, such as those with prolonged labor, large babies, polyhydramnios, multifetal gestation, chorioamnio‑nitis, and history of hemorrhage after a previous delivery, and for patients who are Jehovah’s Witnesses. It is important to reduce the rate of the mainline IV bag while the oxytocin is infusing to reduce the risk of fluid overload.
Since starting this routine I have seen a noticeable decrease in postpartum and postcesarean uterine atony.
E. Darryl Barnes, MD
Mechanicsville, Virginia
Nondissolving misoprostol is ineffective
There is something about misoprostol that is not mentioned in Dr. Barbieri’s editorial. There are 2 types of misoprostol: the proprietary formulation (Cytotec, Pfizer) and the generic form (probably the one used in most hospitals, and possibly also the one used in the randomized studies alluded to).
The generic form, manufactured overseas, is literally insoluble. In my experience, these undissolved tabletsare expelled intact from the rectum5 hours after insertion and they therefore do nothing. The proprietary brand of misoprostol dissolves instantly in the rectum, and the results are dramatic to say the least.
Helio Zapata, MD
Skokie, Illinois
Bundles of care protocols useful in critical events
We often assume the etiology of the postpartum hemorrhage (PPH) is purely and exclusively uterine atony. A frequent clinical scenario is as follows: A hospital birth is conducted by a trained attendant, in a US learning hospital, on a parturient assessed as being at low risk; the single circulating nurse is busy at the keyboard complying with the data entry requirements; the just-delivered patient is enjoying skin-to-skin contact as recommended; and the new father is obtaining all the appropriate pictorial material when a massive vaginal bleed ensues, diagnosed as due to uterine atony. There is little time to remember the results of the randomized controlled trials condemning the use of misoprostol, or the effectiveness of the individual components of the AMTSL (active management of the third stage of labor). The IV oxytocin at the prescribed dose is running wide open, extra personnel are summoned to help, the first doses of methylergonovine are given, and the misoprostol tablets are stored in the nearby drawer as prescribed by the institution’s protocol.
Currently, a multi-state, multi-institutional initiative spearheaded by the American College of Obstetricians and Gynecologists and known as AIM (Alliance for Innovation in Maternity Care) supports the use of “bundles of care” to standardize obstetric care as recommended by the Joint Commission and the Society for Maternal-Fetal Medicine. One of the “bundles” addresses PPH. It is understood that each institution may adjust the steps in accord with its individual capabilities. Included in the algorithm is the use of rectal misoprostol 800 to 1,000 μg.1−5
The International Federation of Gynecology and Obstetrics, in referencing the Blum trial,6 states that the results indicated misoprostol was noninferior to oxytocin at controlling bleeding (90% vs 89%) and preventing additional blood loss (31% vs 34%).7 Misoprostol’s contraindications and side effects are recognized by all investigators. In the field of obstetrics, changes are slow in permeating into daily practice.8 Dr. Barbieri’s recommendation, originating from an influential academic institution, opens the door to continue the dialog on a critical clinical event.
Federico G. Mariona, MD
Dearborn, Michigan
References
- Shields LE, Wiesner S, Fulton J, Pelletreau B. Comprehensive maternal hemorrhage protocols reduce the use of blood products and improve patient safety. Am J Obstet Gynecol. 2015;212(3):272–280.
- Obstetric hemorrhage checklist. Beaumont Health, Michigan. October 2015.
- California Maternal Quality Care Collaborative, California Department of Public Health. OB hemorrhage toolkit, Version 2.0. https://www.cmqcc.org/resources-tool-kits/toolkits/ob-hemorrhage-toolkit. Published March 24, 2015. Accessed August 24, 2015.
- Main EK, Goffman D, Scavone BM, et al; National Partnership for Maternal Safety Council on Patient Safety in Women’s Health Care. Consensus bundle on obstetric hemorrhage. Obstet Gynecol. 2015;126(1):155–162.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 76: postpartum hemorrhage. Obstet Gynecol. 2006;108(4):1039–1047.
- Blum J, Winikoff B, Raghavan S, et al. Treatment of post-partum haemorrhage with sublingual misoprostol versus oxytocin in women receiving prophylactic oxytocin: a double blind randomised, non-inferiority trial. Lancet. 2010;375(9710):217–223.
- International Federation of Gynecology and Obstetrics. Treatment of post-partum haemorrhage with misoprostol. FIGO guideline, annotated version. http://www.figo.org/sites/default/files/uploads/project-publications/Miso/PPH%20treatment/Annotated%20versions/Treatment%20of%20PPH%20with%20Misoprostol_Annotated_2012_English.pdf. Published May 2012. Accessed August 24, 2015.
- Mariona FG, Roura LC. The role of placental alpha macroglobulin-1 amnisure in determining the status of the fetal membranes; its associations with preterm birth. Traditions, traditions. J Matern Fetal Neonatal Med. 2016;29(6):1016–1020.
Rectal misoprostol has merit
It is well established in medicine that IV medications have a rapid onset of action. Therefore, IV uterotonics would be the first choice to control PPH. Most likely they will control the majority of uterine atony.
However, the causes of uterine atony are numerous, and they most commonly include prolonged labor and/or infection. Like any fatigued muscle, there is rebound relaxation. Intravenous uterotonics have a very short half-life and have a maximum total dose. Repeating oxytocin 40 U in a 1,000-mL infusion over 15 minutes carries the risk of water intoxication due to the antidiuretic effect.
Misoprostol 800 to 1,000 mg when used rectally will have a longer effect—up to 4 hours—and fewer side effects. It should be used in combination with other parenteral uterotonics to act in synergistic way. This way the more serious cases of PPH can be reduced or even prevented.
Raymond Michael, MD
Marshall, Minnesota
Dr. Barbieri responds
I deeply appreciate the perspectives provided by Drs. Barnes, Zapata, Mariona, and Michael. The obstetricians and gynecologists who read OBG
As a hospitalist, Dr. Barnes is privileged to care for women at the highest-risk time of their pregnancy. I think his BEPCOP proactive protocol to reduce the rate of PPH is superb and urge him to publish his experience. I appreciate Dr. Zapata’s insight that misoprostol tablets from different manufacturers have markedly different rates of dissolution. I agree with him that I have seen entire, undissolved misoprostol tablets expelled from the rectum many hours after they were administered for the treatment of PPH. If the tablet does not dissolve, it certainly cannot work. Dr. Mariona’s guidance to adhere to protocol bundles and continuously improve and update the bundles is absolutely critical to advancing health care for pregnant women. Dr. Michael rightly points out that one advantage of misoprostol is that it has a longer half-life than many parenteral uterotonics. However, in my practice I prefer Dr. Barnes’ BEPCOP protocol involving the multi-hour administration of oxytocin to prevent and treat a PPH.
“STOP USING RECTAL MISOPROSTOL FOR THE TREATMENT OF POSTPARTUM HEMORRHAGE CAUSED BY UTERINE ATONY”Robert L. Barbieri, MD (Editorial; July 2016)
The BEPCOP strategy for uterine atony
I appreciated Dr. Barbieri’s editorial about oxytocics for postpartum uterine atony and have personally noted the poor effectiveness of rectal misoprostol. I was reminded of his previous editorial that recommended administering intravenous (IV) oxytocin to postcesarean delivery patients for about 6 to 8 hours to reduce the risk of postoperative hemorrhage.
At my current hospital we usually use postpartum oxytocin, 30 units in 500 mL of 5% dextrose in water (D5W) for vaginal deliveries, and that infusion typically is administered for only 1 to 2 hours. Cesarean delivery patients receive oxytocin, 20 units in 1,000 mL of Ringer’s lactate, over the first 1 to 2 hours postoperatively. As an OB hospitalist I have been summoned occasionally to the bedside of patients who have uterine atony and hemorrhage, which usually occurs several hours after their oxytocin infusion has finished.
With this in mind I developed a proactive protocol that I call BEPCOP, an acronym for “Barnes’ Excellent Post Cesarean Oxytocin Protocol.” This involves simply running a 500-mL bag of oxytocin (30 units in 500 mL of D5W) at a constant rate of 50 mL/hour, which provides 50 mU/min oxytocin over the first 10 hours postdelivery.
I recommend BEPCOP for every cesarean delivery patient, as well as for any vaginally delivered patients who are at increased risk for atony, such as those with prolonged labor, large babies, polyhydramnios, multifetal gestation, chorioamnio‑nitis, and history of hemorrhage after a previous delivery, and for patients who are Jehovah’s Witnesses. It is important to reduce the rate of the mainline IV bag while the oxytocin is infusing to reduce the risk of fluid overload.
Since starting this routine I have seen a noticeable decrease in postpartum and postcesarean uterine atony.
E. Darryl Barnes, MD
Mechanicsville, Virginia
Nondissolving misoprostol is ineffective
There is something about misoprostol that is not mentioned in Dr. Barbieri’s editorial. There are 2 types of misoprostol: the proprietary formulation (Cytotec, Pfizer) and the generic form (probably the one used in most hospitals, and possibly also the one used in the randomized studies alluded to).
The generic form, manufactured overseas, is literally insoluble. In my experience, these undissolved tabletsare expelled intact from the rectum5 hours after insertion and they therefore do nothing. The proprietary brand of misoprostol dissolves instantly in the rectum, and the results are dramatic to say the least.
Helio Zapata, MD
Skokie, Illinois
Bundles of care protocols useful in critical events
We often assume the etiology of the postpartum hemorrhage (PPH) is purely and exclusively uterine atony. A frequent clinical scenario is as follows: A hospital birth is conducted by a trained attendant, in a US learning hospital, on a parturient assessed as being at low risk; the single circulating nurse is busy at the keyboard complying with the data entry requirements; the just-delivered patient is enjoying skin-to-skin contact as recommended; and the new father is obtaining all the appropriate pictorial material when a massive vaginal bleed ensues, diagnosed as due to uterine atony. There is little time to remember the results of the randomized controlled trials condemning the use of misoprostol, or the effectiveness of the individual components of the AMTSL (active management of the third stage of labor). The IV oxytocin at the prescribed dose is running wide open, extra personnel are summoned to help, the first doses of methylergonovine are given, and the misoprostol tablets are stored in the nearby drawer as prescribed by the institution’s protocol.
Currently, a multi-state, multi-institutional initiative spearheaded by the American College of Obstetricians and Gynecologists and known as AIM (Alliance for Innovation in Maternity Care) supports the use of “bundles of care” to standardize obstetric care as recommended by the Joint Commission and the Society for Maternal-Fetal Medicine. One of the “bundles” addresses PPH. It is understood that each institution may adjust the steps in accord with its individual capabilities. Included in the algorithm is the use of rectal misoprostol 800 to 1,000 μg.1−5
The International Federation of Gynecology and Obstetrics, in referencing the Blum trial,6 states that the results indicated misoprostol was noninferior to oxytocin at controlling bleeding (90% vs 89%) and preventing additional blood loss (31% vs 34%).7 Misoprostol’s contraindications and side effects are recognized by all investigators. In the field of obstetrics, changes are slow in permeating into daily practice.8 Dr. Barbieri’s recommendation, originating from an influential academic institution, opens the door to continue the dialog on a critical clinical event.
Federico G. Mariona, MD
Dearborn, Michigan
References
- Shields LE, Wiesner S, Fulton J, Pelletreau B. Comprehensive maternal hemorrhage protocols reduce the use of blood products and improve patient safety. Am J Obstet Gynecol. 2015;212(3):272–280.
- Obstetric hemorrhage checklist. Beaumont Health, Michigan. October 2015.
- California Maternal Quality Care Collaborative, California Department of Public Health. OB hemorrhage toolkit, Version 2.0. https://www.cmqcc.org/resources-tool-kits/toolkits/ob-hemorrhage-toolkit. Published March 24, 2015. Accessed August 24, 2015.
- Main EK, Goffman D, Scavone BM, et al; National Partnership for Maternal Safety Council on Patient Safety in Women’s Health Care. Consensus bundle on obstetric hemorrhage. Obstet Gynecol. 2015;126(1):155–162.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 76: postpartum hemorrhage. Obstet Gynecol. 2006;108(4):1039–1047.
- Blum J, Winikoff B, Raghavan S, et al. Treatment of post-partum haemorrhage with sublingual misoprostol versus oxytocin in women receiving prophylactic oxytocin: a double blind randomised, non-inferiority trial. Lancet. 2010;375(9710):217–223.
- International Federation of Gynecology and Obstetrics. Treatment of post-partum haemorrhage with misoprostol. FIGO guideline, annotated version. http://www.figo.org/sites/default/files/uploads/project-publications/Miso/PPH%20treatment/Annotated%20versions/Treatment%20of%20PPH%20with%20Misoprostol_Annotated_2012_English.pdf. Published May 2012. Accessed August 24, 2015.
- Mariona FG, Roura LC. The role of placental alpha macroglobulin-1 amnisure in determining the status of the fetal membranes; its associations with preterm birth. Traditions, traditions. J Matern Fetal Neonatal Med. 2016;29(6):1016–1020.
Rectal misoprostol has merit
It is well established in medicine that IV medications have a rapid onset of action. Therefore, IV uterotonics would be the first choice to control PPH. Most likely they will control the majority of uterine atony.
However, the causes of uterine atony are numerous, and they most commonly include prolonged labor and/or infection. Like any fatigued muscle, there is rebound relaxation. Intravenous uterotonics have a very short half-life and have a maximum total dose. Repeating oxytocin 40 U in a 1,000-mL infusion over 15 minutes carries the risk of water intoxication due to the antidiuretic effect.
Misoprostol 800 to 1,000 mg when used rectally will have a longer effect—up to 4 hours—and fewer side effects. It should be used in combination with other parenteral uterotonics to act in synergistic way. This way the more serious cases of PPH can be reduced or even prevented.
Raymond Michael, MD
Marshall, Minnesota
Dr. Barbieri responds
I deeply appreciate the perspectives provided by Drs. Barnes, Zapata, Mariona, and Michael. The obstetricians and gynecologists who read OBG
As a hospitalist, Dr. Barnes is privileged to care for women at the highest-risk time of their pregnancy. I think his BEPCOP proactive protocol to reduce the rate of PPH is superb and urge him to publish his experience. I appreciate Dr. Zapata’s insight that misoprostol tablets from different manufacturers have markedly different rates of dissolution. I agree with him that I have seen entire, undissolved misoprostol tablets expelled from the rectum many hours after they were administered for the treatment of PPH. If the tablet does not dissolve, it certainly cannot work. Dr. Mariona’s guidance to adhere to protocol bundles and continuously improve and update the bundles is absolutely critical to advancing health care for pregnant women. Dr. Michael rightly points out that one advantage of misoprostol is that it has a longer half-life than many parenteral uterotonics. However, in my practice I prefer Dr. Barnes’ BEPCOP protocol involving the multi-hour administration of oxytocin to prevent and treat a PPH.
“STOP USING RECTAL MISOPROSTOL FOR THE TREATMENT OF POSTPARTUM HEMORRHAGE CAUSED BY UTERINE ATONY”Robert L. Barbieri, MD (Editorial; July 2016)
The BEPCOP strategy for uterine atony
I appreciated Dr. Barbieri’s editorial about oxytocics for postpartum uterine atony and have personally noted the poor effectiveness of rectal misoprostol. I was reminded of his previous editorial that recommended administering intravenous (IV) oxytocin to postcesarean delivery patients for about 6 to 8 hours to reduce the risk of postoperative hemorrhage.
At my current hospital we usually use postpartum oxytocin, 30 units in 500 mL of 5% dextrose in water (D5W) for vaginal deliveries, and that infusion typically is administered for only 1 to 2 hours. Cesarean delivery patients receive oxytocin, 20 units in 1,000 mL of Ringer’s lactate, over the first 1 to 2 hours postoperatively. As an OB hospitalist I have been summoned occasionally to the bedside of patients who have uterine atony and hemorrhage, which usually occurs several hours after their oxytocin infusion has finished.
With this in mind I developed a proactive protocol that I call BEPCOP, an acronym for “Barnes’ Excellent Post Cesarean Oxytocin Protocol.” This involves simply running a 500-mL bag of oxytocin (30 units in 500 mL of D5W) at a constant rate of 50 mL/hour, which provides 50 mU/min oxytocin over the first 10 hours postdelivery.
I recommend BEPCOP for every cesarean delivery patient, as well as for any vaginally delivered patients who are at increased risk for atony, such as those with prolonged labor, large babies, polyhydramnios, multifetal gestation, chorioamnio‑nitis, and history of hemorrhage after a previous delivery, and for patients who are Jehovah’s Witnesses. It is important to reduce the rate of the mainline IV bag while the oxytocin is infusing to reduce the risk of fluid overload.
Since starting this routine I have seen a noticeable decrease in postpartum and postcesarean uterine atony.
E. Darryl Barnes, MD
Mechanicsville, Virginia
Nondissolving misoprostol is ineffective
There is something about misoprostol that is not mentioned in Dr. Barbieri’s editorial. There are 2 types of misoprostol: the proprietary formulation (Cytotec, Pfizer) and the generic form (probably the one used in most hospitals, and possibly also the one used in the randomized studies alluded to).
The generic form, manufactured overseas, is literally insoluble. In my experience, these undissolved tabletsare expelled intact from the rectum5 hours after insertion and they therefore do nothing. The proprietary brand of misoprostol dissolves instantly in the rectum, and the results are dramatic to say the least.
Helio Zapata, MD
Skokie, Illinois
Bundles of care protocols useful in critical events
We often assume the etiology of the postpartum hemorrhage (PPH) is purely and exclusively uterine atony. A frequent clinical scenario is as follows: A hospital birth is conducted by a trained attendant, in a US learning hospital, on a parturient assessed as being at low risk; the single circulating nurse is busy at the keyboard complying with the data entry requirements; the just-delivered patient is enjoying skin-to-skin contact as recommended; and the new father is obtaining all the appropriate pictorial material when a massive vaginal bleed ensues, diagnosed as due to uterine atony. There is little time to remember the results of the randomized controlled trials condemning the use of misoprostol, or the effectiveness of the individual components of the AMTSL (active management of the third stage of labor). The IV oxytocin at the prescribed dose is running wide open, extra personnel are summoned to help, the first doses of methylergonovine are given, and the misoprostol tablets are stored in the nearby drawer as prescribed by the institution’s protocol.
Currently, a multi-state, multi-institutional initiative spearheaded by the American College of Obstetricians and Gynecologists and known as AIM (Alliance for Innovation in Maternity Care) supports the use of “bundles of care” to standardize obstetric care as recommended by the Joint Commission and the Society for Maternal-Fetal Medicine. One of the “bundles” addresses PPH. It is understood that each institution may adjust the steps in accord with its individual capabilities. Included in the algorithm is the use of rectal misoprostol 800 to 1,000 μg.1−5
The International Federation of Gynecology and Obstetrics, in referencing the Blum trial,6 states that the results indicated misoprostol was noninferior to oxytocin at controlling bleeding (90% vs 89%) and preventing additional blood loss (31% vs 34%).7 Misoprostol’s contraindications and side effects are recognized by all investigators. In the field of obstetrics, changes are slow in permeating into daily practice.8 Dr. Barbieri’s recommendation, originating from an influential academic institution, opens the door to continue the dialog on a critical clinical event.
Federico G. Mariona, MD
Dearborn, Michigan
References
- Shields LE, Wiesner S, Fulton J, Pelletreau B. Comprehensive maternal hemorrhage protocols reduce the use of blood products and improve patient safety. Am J Obstet Gynecol. 2015;212(3):272–280.
- Obstetric hemorrhage checklist. Beaumont Health, Michigan. October 2015.
- California Maternal Quality Care Collaborative, California Department of Public Health. OB hemorrhage toolkit, Version 2.0. https://www.cmqcc.org/resources-tool-kits/toolkits/ob-hemorrhage-toolkit. Published March 24, 2015. Accessed August 24, 2015.
- Main EK, Goffman D, Scavone BM, et al; National Partnership for Maternal Safety Council on Patient Safety in Women’s Health Care. Consensus bundle on obstetric hemorrhage. Obstet Gynecol. 2015;126(1):155–162.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 76: postpartum hemorrhage. Obstet Gynecol. 2006;108(4):1039–1047.
- Blum J, Winikoff B, Raghavan S, et al. Treatment of post-partum haemorrhage with sublingual misoprostol versus oxytocin in women receiving prophylactic oxytocin: a double blind randomised, non-inferiority trial. Lancet. 2010;375(9710):217–223.
- International Federation of Gynecology and Obstetrics. Treatment of post-partum haemorrhage with misoprostol. FIGO guideline, annotated version. http://www.figo.org/sites/default/files/uploads/project-publications/Miso/PPH%20treatment/Annotated%20versions/Treatment%20of%20PPH%20with%20Misoprostol_Annotated_2012_English.pdf. Published May 2012. Accessed August 24, 2015.
- Mariona FG, Roura LC. The role of placental alpha macroglobulin-1 amnisure in determining the status of the fetal membranes; its associations with preterm birth. Traditions, traditions. J Matern Fetal Neonatal Med. 2016;29(6):1016–1020.
Rectal misoprostol has merit
It is well established in medicine that IV medications have a rapid onset of action. Therefore, IV uterotonics would be the first choice to control PPH. Most likely they will control the majority of uterine atony.
However, the causes of uterine atony are numerous, and they most commonly include prolonged labor and/or infection. Like any fatigued muscle, there is rebound relaxation. Intravenous uterotonics have a very short half-life and have a maximum total dose. Repeating oxytocin 40 U in a 1,000-mL infusion over 15 minutes carries the risk of water intoxication due to the antidiuretic effect.
Misoprostol 800 to 1,000 mg when used rectally will have a longer effect—up to 4 hours—and fewer side effects. It should be used in combination with other parenteral uterotonics to act in synergistic way. This way the more serious cases of PPH can be reduced or even prevented.
Raymond Michael, MD
Marshall, Minnesota
Dr. Barbieri responds
I deeply appreciate the perspectives provided by Drs. Barnes, Zapata, Mariona, and Michael. The obstetricians and gynecologists who read OBG
As a hospitalist, Dr. Barnes is privileged to care for women at the highest-risk time of their pregnancy. I think his BEPCOP proactive protocol to reduce the rate of PPH is superb and urge him to publish his experience. I appreciate Dr. Zapata’s insight that misoprostol tablets from different manufacturers have markedly different rates of dissolution. I agree with him that I have seen entire, undissolved misoprostol tablets expelled from the rectum many hours after they were administered for the treatment of PPH. If the tablet does not dissolve, it certainly cannot work. Dr. Mariona’s guidance to adhere to protocol bundles and continuously improve and update the bundles is absolutely critical to advancing health care for pregnant women. Dr. Michael rightly points out that one advantage of misoprostol is that it has a longer half-life than many parenteral uterotonics. However, in my practice I prefer Dr. Barnes’ BEPCOP protocol involving the multi-hour administration of oxytocin to prevent and treat a PPH.
Product Update: AVYCAZ, Electro Lube, Methergine Oral Tablets, ChartLogic
TREATING GRAM-NEGATIVE BACTERIAL INFECTIONS

Allergan reports an updated label approved by the US Food and Drug Administration (FDA) for AVYCAZ® (ceftazidime and avibactam) in combination with metronidazole for the treatment of complicated intraabdominal infections caused by designated susceptible microorganisms. The label change was made after completion of a Phase 3 trial evaluating the safety and efficacy of AVYCAZ in combination with metronidazole. Results of the trial also included data from subsets of patients with infections due to ceftazidime- nonsusceptible pathogens and with pathogens producing certain extended-spectrum beta-lactamases (ESBLs).
First FDA-approved in February 2015, AVYCAZ is an antibiotic developed to treat certain Gram-negative bacterial infections. It consists of ceftazidime, a third- generation cephalosporin, and avibactam, a non-ß lactam ß-lactamase inhibitor.
Allergan says that AVYCAZ has demonstrated in vitro activity against Enterobacteriaceae in the presence of some beta-lactamases and ESBLs of the following groups: TEM, SHV, CTX-M, Klebsiella pneumoniae carbapenemase, AmpC, and certain oxacillinases. AVYCAZ also demonstrated in vitro activity against Pseudomonas aeruginosa in the presence of some AmpC beta-lactamases, and certain strains lacking outer membrane porin.
FOR MORE INFORMATION, VISIT: www.avycaz.com
ANTI-STICK SOLUTION FOR ELECTROSURGICAL INSTRUMENTS
Electro Lube is a mixture of natural, nonsynthetic, nonflammable, nonallergenic biocompatible phospholipids with no known side effects associated with patient use. It can be used on a variety of monopolar and bipolar instrumentation including: cutting forceps, Kleppingers, bipolar forceps, Bovie pencils, suction coagulators, robots, curved hot scissors, spatula electrodes, and l-hook electrodes.
FOR MORE INFORMATION, VISIT: www.electrolubesurgical.com
ORAL TREATMENT FOR PPH
A semi-synthetic ergot alkaloid, Methergine is indicated for routine management of uterine atony, hemorrhage, and subinvolution of the uterus following delivery of the placenta, and for control of uterine hemorrhage in the second stage of labor following delivery of the anterior shoulder. Methergine provides specific and rapid (onset of action under 10 minutes) uterotonic action on the smooth muscle of the uterus to increase contractions, resulting in restricting blood loss.
The Methergine dosing schedule is one tablet (0.2 mg) 3 or 4 times daily in the puerperium for a maximum of 1 week. The most common adverse event is hypertension, associated in several cases with seizure and/or headache. Methylergonovine maleate is also available as an intramuscular injection.
FOR MORE INFORMATION, VISIT: www.methergine.com
FREE MEDICAL BILLING CALCULATOR

ChartLogic, Inc. has launched its Medical Billing Analysis Calculator, a free, online, interactive tool that provides key billing metrics and compares those results to Medical Group Management Association (MGMA) benchmarks for a practice’s specialty. The calculator consists of an input section where practice specialty, annual charges, annual collections, outstanding accounts receivable (A/R), number of annual encounters, and contractual adjustments are entered. The tool, accessible on desktop or mobile devices, then calculates the practice’s billing analysis, including average reimbursement per encounter, net collection percentages, average monthly collections, and A/R data and displays the results beside specialty benchmarks. After submission, the user can alter the inputs or request more information from a ChartLogic billing expert.
ChartLogic’s goal as a medical billing service is to increase a practice’s performance. Services include a full ambulatory EHR suite with electronic medical records, practice management tools, e-prescribing, a patient portal, and more.
FOR MORE INFORMATION, VISIT: www.chartlogic.com
TREATING GRAM-NEGATIVE BACTERIAL INFECTIONS

Allergan reports an updated label approved by the US Food and Drug Administration (FDA) for AVYCAZ® (ceftazidime and avibactam) in combination with metronidazole for the treatment of complicated intraabdominal infections caused by designated susceptible microorganisms. The label change was made after completion of a Phase 3 trial evaluating the safety and efficacy of AVYCAZ in combination with metronidazole. Results of the trial also included data from subsets of patients with infections due to ceftazidime- nonsusceptible pathogens and with pathogens producing certain extended-spectrum beta-lactamases (ESBLs).
First FDA-approved in February 2015, AVYCAZ is an antibiotic developed to treat certain Gram-negative bacterial infections. It consists of ceftazidime, a third- generation cephalosporin, and avibactam, a non-ß lactam ß-lactamase inhibitor.
Allergan says that AVYCAZ has demonstrated in vitro activity against Enterobacteriaceae in the presence of some beta-lactamases and ESBLs of the following groups: TEM, SHV, CTX-M, Klebsiella pneumoniae carbapenemase, AmpC, and certain oxacillinases. AVYCAZ also demonstrated in vitro activity against Pseudomonas aeruginosa in the presence of some AmpC beta-lactamases, and certain strains lacking outer membrane porin.
FOR MORE INFORMATION, VISIT: www.avycaz.com
ANTI-STICK SOLUTION FOR ELECTROSURGICAL INSTRUMENTS
Electro Lube is a mixture of natural, nonsynthetic, nonflammable, nonallergenic biocompatible phospholipids with no known side effects associated with patient use. It can be used on a variety of monopolar and bipolar instrumentation including: cutting forceps, Kleppingers, bipolar forceps, Bovie pencils, suction coagulators, robots, curved hot scissors, spatula electrodes, and l-hook electrodes.
FOR MORE INFORMATION, VISIT: www.electrolubesurgical.com
ORAL TREATMENT FOR PPH
A semi-synthetic ergot alkaloid, Methergine is indicated for routine management of uterine atony, hemorrhage, and subinvolution of the uterus following delivery of the placenta, and for control of uterine hemorrhage in the second stage of labor following delivery of the anterior shoulder. Methergine provides specific and rapid (onset of action under 10 minutes) uterotonic action on the smooth muscle of the uterus to increase contractions, resulting in restricting blood loss.
The Methergine dosing schedule is one tablet (0.2 mg) 3 or 4 times daily in the puerperium for a maximum of 1 week. The most common adverse event is hypertension, associated in several cases with seizure and/or headache. Methylergonovine maleate is also available as an intramuscular injection.
FOR MORE INFORMATION, VISIT: www.methergine.com
FREE MEDICAL BILLING CALCULATOR

ChartLogic, Inc. has launched its Medical Billing Analysis Calculator, a free, online, interactive tool that provides key billing metrics and compares those results to Medical Group Management Association (MGMA) benchmarks for a practice’s specialty. The calculator consists of an input section where practice specialty, annual charges, annual collections, outstanding accounts receivable (A/R), number of annual encounters, and contractual adjustments are entered. The tool, accessible on desktop or mobile devices, then calculates the practice’s billing analysis, including average reimbursement per encounter, net collection percentages, average monthly collections, and A/R data and displays the results beside specialty benchmarks. After submission, the user can alter the inputs or request more information from a ChartLogic billing expert.
ChartLogic’s goal as a medical billing service is to increase a practice’s performance. Services include a full ambulatory EHR suite with electronic medical records, practice management tools, e-prescribing, a patient portal, and more.
FOR MORE INFORMATION, VISIT: www.chartlogic.com
TREATING GRAM-NEGATIVE BACTERIAL INFECTIONS

Allergan reports an updated label approved by the US Food and Drug Administration (FDA) for AVYCAZ® (ceftazidime and avibactam) in combination with metronidazole for the treatment of complicated intraabdominal infections caused by designated susceptible microorganisms. The label change was made after completion of a Phase 3 trial evaluating the safety and efficacy of AVYCAZ in combination with metronidazole. Results of the trial also included data from subsets of patients with infections due to ceftazidime- nonsusceptible pathogens and with pathogens producing certain extended-spectrum beta-lactamases (ESBLs).
First FDA-approved in February 2015, AVYCAZ is an antibiotic developed to treat certain Gram-negative bacterial infections. It consists of ceftazidime, a third- generation cephalosporin, and avibactam, a non-ß lactam ß-lactamase inhibitor.
Allergan says that AVYCAZ has demonstrated in vitro activity against Enterobacteriaceae in the presence of some beta-lactamases and ESBLs of the following groups: TEM, SHV, CTX-M, Klebsiella pneumoniae carbapenemase, AmpC, and certain oxacillinases. AVYCAZ also demonstrated in vitro activity against Pseudomonas aeruginosa in the presence of some AmpC beta-lactamases, and certain strains lacking outer membrane porin.
FOR MORE INFORMATION, VISIT: www.avycaz.com
ANTI-STICK SOLUTION FOR ELECTROSURGICAL INSTRUMENTS
Electro Lube is a mixture of natural, nonsynthetic, nonflammable, nonallergenic biocompatible phospholipids with no known side effects associated with patient use. It can be used on a variety of monopolar and bipolar instrumentation including: cutting forceps, Kleppingers, bipolar forceps, Bovie pencils, suction coagulators, robots, curved hot scissors, spatula electrodes, and l-hook electrodes.
FOR MORE INFORMATION, VISIT: www.electrolubesurgical.com
ORAL TREATMENT FOR PPH
A semi-synthetic ergot alkaloid, Methergine is indicated for routine management of uterine atony, hemorrhage, and subinvolution of the uterus following delivery of the placenta, and for control of uterine hemorrhage in the second stage of labor following delivery of the anterior shoulder. Methergine provides specific and rapid (onset of action under 10 minutes) uterotonic action on the smooth muscle of the uterus to increase contractions, resulting in restricting blood loss.
The Methergine dosing schedule is one tablet (0.2 mg) 3 or 4 times daily in the puerperium for a maximum of 1 week. The most common adverse event is hypertension, associated in several cases with seizure and/or headache. Methylergonovine maleate is also available as an intramuscular injection.
FOR MORE INFORMATION, VISIT: www.methergine.com
FREE MEDICAL BILLING CALCULATOR

ChartLogic, Inc. has launched its Medical Billing Analysis Calculator, a free, online, interactive tool that provides key billing metrics and compares those results to Medical Group Management Association (MGMA) benchmarks for a practice’s specialty. The calculator consists of an input section where practice specialty, annual charges, annual collections, outstanding accounts receivable (A/R), number of annual encounters, and contractual adjustments are entered. The tool, accessible on desktop or mobile devices, then calculates the practice’s billing analysis, including average reimbursement per encounter, net collection percentages, average monthly collections, and A/R data and displays the results beside specialty benchmarks. After submission, the user can alter the inputs or request more information from a ChartLogic billing expert.
ChartLogic’s goal as a medical billing service is to increase a practice’s performance. Services include a full ambulatory EHR suite with electronic medical records, practice management tools, e-prescribing, a patient portal, and more.
FOR MORE INFORMATION, VISIT: www.chartlogic.com
AAP speaks out on dismissal of vaccine-refusing patients, vaccine hesitancy
For years, pediatricians have sought a blessing from the American Academy of Pediatrics that acknowledged it was valid for members to dismiss families from their practice if they refused to vaccinate despite all attempts to persuade them. Now, a new clinical report has essentially delivered just that.
The report does not represent an official policy change from the AAP, but it does for the first time acknowledge that “firing” patients who persistently refuse vaccination is “an acceptable option” (Pediatrics. 2016 Aug. doi: 10.1542/peds.2016-2146).
“A number of pediatricians feel so strongly that if they don’t agree on vaccines, which are so basic to the delivery of care and have made such a big difference in children’s lives, how will they agree on a number of other things they’ll need to discuss?” Kathryn M. Edwards, MD, director of the Vanderbilt Vaccine Research Program, Nashville, Tenn., and a coauthor of the report, explained in an interview.
The AAP has received pressure from its members over recent years as increasing numbers of pediatricians choose to dismiss some or all of their patients whose parents were resolved not vaccinate, coauthor Jesse M. Hackell, MD, a practicing pediatrician and managing partner at Pomona Pediatrics, an affiliate of Boston Children’s Health Physicians, said in an interview.
In fact, a new study has revealed that 12% of pediatricians reported dismissing vaccine-refusing families in 2013, up from 6% in 2006. At the same time, the proportion of families refusing vaccines has nearly doubled in the same time.
“There was a groundswell of opinion that enough is enough and we can’t provide quality care if we can’t provide something we know is so important,” Dr. Hackell said. “We felt the Academy needed to stop being so adamantly opposed to the possibility of dismissal – not to recommend dismissal but simply to state it is an acceptable option.”
The AAP responds to fellows’ concerns
While the AAP continues to recommend doctors attempt to persuade families as long as possible to vaccinate, the new report discusses dismissal as a viable option as long as it adheres to relevant state laws that prohibit abandonment of patients.
“The decision to dismiss a family who continues to refuse immunization is not one that should be made lightly, nor should it be made without considering and respecting the reasons for the parents’ point of view,” the report states. “Nevertheless, the individual pediatrician may consider dismissal of families who refuse vaccination as an acceptable option.”
The report does note that some practice settings, such as hospitals or large health care organizations, may not allow dismissal of patients, and that pediatricians “should carefully evaluate the availability of other qualified providers for the family” if they live in an area with limited access to pediatric care.
But the report finally acknowledges those pediatricians who are “just philosophically wired to not accept vaccine refusals,” Stuart A. Cohen, MD, an assistant professor of pediatrics at the University of California, San Diego, and chair of AAP District 9 in California, said in an interview.
“It really interferes with your physician-patient relationship,” Dr. Cohen said, who was not a coauthor of the report.
Now, if pediatricians feel it necessary to dismiss nonvaccinating patients, “then the Academy understands because of concerns for other patients, but it must be done in a way that’s respectful and tries to ensure patients understand the safety and necessity of vaccines,” Dr. Edwards said.
The report still includes the AAP recommendation that “pediatricians continue to engage with vaccine-hesitant parents, provide other health care services to their children, and attempt to modify their opposition to vaccines.” And a number of members of the AAP’s infectious diseases and bioethics committees were uncomfortable with dismissing patients, Dr. Edwards said, but “there were certain people who needed this, who needed some blessing that this was not inappropriate after all the other things the pediatrician had done.”
Vaccines undergo thorough testing for safety and effectiveness
But the report also aims to provide pediatricians with strategies for doing everything possible first.
“We needed to address enabling the clinician to have some very specific talking points to use and not get involved in a philosophical discussion that can take an hour,” Dr. Hackell said. “They need to make a clear statement that vaccines are important, and if you don’t get them, bad things like death can happen.”
The report therefore provides a comprehensive overview of vaccine development, from the initial identification of the need for a vaccine through the various phases of clinical testing and ongoing postlicensure monitoring. This background information can arm pediatricians with foundational knowledge that’s helpful in talking with patients.
“Vaccine development is a long and arduous process, often lasting many years and involving a combination of public and private partnerships,” the report states. “The current system for developing, testing and regulating vaccines requires that the vaccines demonstrate both safety and efficacy before licensure and that long-term safety is monitored.”
The report briefly explains the multiple mechanisms for continuing to track and study adverse events and other safety concerns:
• Vaccine Adverse Events Reporting System (VAERS). A voluntary passive reporting system used to identify potential safety signals.
• Vaccine Safety Datalink (VSD). A network of linked databases from health care systems across the United States involving millions of individuals
• Post-Licensure Rapid Immunization Safety Monitoring system (PRISM). A system which monitors vaccine safety using health insurance claims data from 107 million individuals .
• Clinical Immunization Safety Assessment Project (CISA). A system that answers individual health care providers’ specific questions on vaccine safety.
Vaccine hesitancy and vaccine exemptions
Opposition to vaccination is not new, the report states, describing it as dating back to Edward Jenner’s smallpox vaccine in the early 1800s.
“Although vaccine hesitancy is not a new phenomenon, it may have a greater effect on public health today,” the report states. “With the ease of global travel, vaccine-preventable diseases are spread more quickly and may unexpectedly appear in areas where health care professionals are unfamiliar with their clinical presentation.”
The historical presence of vaccination opposition has led to circumstances in the United States today in which parents can seek nonmedical exemptions from vaccines in 47 states, and their use has increased with their availability. Yet, the increase in use of exemptions and of “alternative” immunization schedules runs the risk of eroding the herd immunity that protects the community, the report notes.
“For these reasons, we believe the better approach is to work to eliminate all nonmedical exemptions for childhood vaccines,” the authors write. The American Medical Association and the Infectious Diseases Society of America espouse this position, and the AAP is developing a similar statement.
“Families should not have to fear going to school or the grocery store or a house of worship and worry about their kids getting sick,” said Dr. Cohen. “We now strongly say that we need to work with legislators, families, and other advocates for children at the state level to spread more laws like California’s SB 277 that would abolish philosophical exemptions.”
Dr. Cohen also emphasized the importance of communicating to parents that there are no valid “alternative schedules” for vaccination. There is the Centers for Disease Control and Prevention recommended schedule and anything else is a “nonrecommended vaccine schedule because it hasn’t been studied.” That change in terminology drives home the point that the CDC schedule is the only one fully tested for safety and effectiveness.
Meanwhile, however, pediatricians need the tools to address vaccine hesitancy, starting with understanding it. The report describes the pattern of disease incidence, vaccine uptake, disease reduction, adverse event increase and resulting vaccine hesitancy, punctuated by periodic outbreaks that restore eroded confidence in vaccines.
“When diseases are present, parents are worried and want a vaccine, and when they’re gone, they don’t,” Dr. Edwards said. “We need to remember that there is a dependence on the maintenance of herd immunity by immunization by your neighbors.”
Specific strategies to counter vaccine hesitancy
Just over half of physicians spend 10-19 minutes discussing vaccines with concerned parents, and 8% spend at least 20 minutes with such parents, found a study cited in the report. Other research has found these discussions take a toll on doctors’ job satisfaction.
Yet pediatricians remain the single biggest influence on parents’ vaccination decisions, cited by nearly 80% of parents in one large study.
“The pediatrician should appreciate that vaccine-hesitant parents are a heterogeneous group and that specific parental vaccine concerns should be individually identified and addressed,” the report states. “Although many techniques for working with vaccine-hesitant parents have been suggested, scant data are available to determine the efficacy of these methods.”
It goes on to recommend that physicians should discuss the development and safety testing of vaccines “in a nonconfrontational dialogue with the parents while listening to and acknowledging their concerns.”
Pediatricians should not, however, delay vaccines or limit the number per visit – thereby deviating from the CDC recommended and AAP-endorsed schedule – unless it’s the only way a parent agrees to vaccinate.
Another strategy is the presumptive approach: Present all vaccine recommendations as required immunizations that the provider expects a parent to agree to, although pediatricians should consider their experience and relationship with a family since this approach may not work well for some parents.
The report also emphasizes the potential effectiveness of personalizing vaccine conversations by having doctors share their own experience, such as the fact that they vaccinated themselves, their children, and/or their grandchildren.
“Parents often are more likely to be persuaded by stories and anecdotes about the successes of vaccines,” the authors write. “Personal examples of children who were sick with vaccine-preventable illnesses can be much more effective than simply reading the numbers of children infected with a disease each year.”
The report also offers several suggestions for reducing the pain from administering vaccinations: administering vaccines quickly without aspirating; saving the most painful injection for last; holding the child upright; providing tactile stimulation; breastfeeding or providing sweet solutions and topical anesthetics after administration; and using distraction, such as deep breathing, pinwheels, or toys to decrease children’s pain and anxiety.
But the bottom line is that pediatricians have one key message they must communicate to parents, the report states: “The clear message parents should hear is that vaccines are safe and effective, and serious disease can occur if your child and family are not immunized.”
For years, pediatricians have sought a blessing from the American Academy of Pediatrics that acknowledged it was valid for members to dismiss families from their practice if they refused to vaccinate despite all attempts to persuade them. Now, a new clinical report has essentially delivered just that.
The report does not represent an official policy change from the AAP, but it does for the first time acknowledge that “firing” patients who persistently refuse vaccination is “an acceptable option” (Pediatrics. 2016 Aug. doi: 10.1542/peds.2016-2146).
“A number of pediatricians feel so strongly that if they don’t agree on vaccines, which are so basic to the delivery of care and have made such a big difference in children’s lives, how will they agree on a number of other things they’ll need to discuss?” Kathryn M. Edwards, MD, director of the Vanderbilt Vaccine Research Program, Nashville, Tenn., and a coauthor of the report, explained in an interview.
The AAP has received pressure from its members over recent years as increasing numbers of pediatricians choose to dismiss some or all of their patients whose parents were resolved not vaccinate, coauthor Jesse M. Hackell, MD, a practicing pediatrician and managing partner at Pomona Pediatrics, an affiliate of Boston Children’s Health Physicians, said in an interview.
In fact, a new study has revealed that 12% of pediatricians reported dismissing vaccine-refusing families in 2013, up from 6% in 2006. At the same time, the proportion of families refusing vaccines has nearly doubled in the same time.
“There was a groundswell of opinion that enough is enough and we can’t provide quality care if we can’t provide something we know is so important,” Dr. Hackell said. “We felt the Academy needed to stop being so adamantly opposed to the possibility of dismissal – not to recommend dismissal but simply to state it is an acceptable option.”
The AAP responds to fellows’ concerns
While the AAP continues to recommend doctors attempt to persuade families as long as possible to vaccinate, the new report discusses dismissal as a viable option as long as it adheres to relevant state laws that prohibit abandonment of patients.
“The decision to dismiss a family who continues to refuse immunization is not one that should be made lightly, nor should it be made without considering and respecting the reasons for the parents’ point of view,” the report states. “Nevertheless, the individual pediatrician may consider dismissal of families who refuse vaccination as an acceptable option.”
The report does note that some practice settings, such as hospitals or large health care organizations, may not allow dismissal of patients, and that pediatricians “should carefully evaluate the availability of other qualified providers for the family” if they live in an area with limited access to pediatric care.
But the report finally acknowledges those pediatricians who are “just philosophically wired to not accept vaccine refusals,” Stuart A. Cohen, MD, an assistant professor of pediatrics at the University of California, San Diego, and chair of AAP District 9 in California, said in an interview.
“It really interferes with your physician-patient relationship,” Dr. Cohen said, who was not a coauthor of the report.
Now, if pediatricians feel it necessary to dismiss nonvaccinating patients, “then the Academy understands because of concerns for other patients, but it must be done in a way that’s respectful and tries to ensure patients understand the safety and necessity of vaccines,” Dr. Edwards said.
The report still includes the AAP recommendation that “pediatricians continue to engage with vaccine-hesitant parents, provide other health care services to their children, and attempt to modify their opposition to vaccines.” And a number of members of the AAP’s infectious diseases and bioethics committees were uncomfortable with dismissing patients, Dr. Edwards said, but “there were certain people who needed this, who needed some blessing that this was not inappropriate after all the other things the pediatrician had done.”
Vaccines undergo thorough testing for safety and effectiveness
But the report also aims to provide pediatricians with strategies for doing everything possible first.
“We needed to address enabling the clinician to have some very specific talking points to use and not get involved in a philosophical discussion that can take an hour,” Dr. Hackell said. “They need to make a clear statement that vaccines are important, and if you don’t get them, bad things like death can happen.”
The report therefore provides a comprehensive overview of vaccine development, from the initial identification of the need for a vaccine through the various phases of clinical testing and ongoing postlicensure monitoring. This background information can arm pediatricians with foundational knowledge that’s helpful in talking with patients.
“Vaccine development is a long and arduous process, often lasting many years and involving a combination of public and private partnerships,” the report states. “The current system for developing, testing and regulating vaccines requires that the vaccines demonstrate both safety and efficacy before licensure and that long-term safety is monitored.”
The report briefly explains the multiple mechanisms for continuing to track and study adverse events and other safety concerns:
• Vaccine Adverse Events Reporting System (VAERS). A voluntary passive reporting system used to identify potential safety signals.
• Vaccine Safety Datalink (VSD). A network of linked databases from health care systems across the United States involving millions of individuals
• Post-Licensure Rapid Immunization Safety Monitoring system (PRISM). A system which monitors vaccine safety using health insurance claims data from 107 million individuals .
• Clinical Immunization Safety Assessment Project (CISA). A system that answers individual health care providers’ specific questions on vaccine safety.
Vaccine hesitancy and vaccine exemptions
Opposition to vaccination is not new, the report states, describing it as dating back to Edward Jenner’s smallpox vaccine in the early 1800s.
“Although vaccine hesitancy is not a new phenomenon, it may have a greater effect on public health today,” the report states. “With the ease of global travel, vaccine-preventable diseases are spread more quickly and may unexpectedly appear in areas where health care professionals are unfamiliar with their clinical presentation.”
The historical presence of vaccination opposition has led to circumstances in the United States today in which parents can seek nonmedical exemptions from vaccines in 47 states, and their use has increased with their availability. Yet, the increase in use of exemptions and of “alternative” immunization schedules runs the risk of eroding the herd immunity that protects the community, the report notes.
“For these reasons, we believe the better approach is to work to eliminate all nonmedical exemptions for childhood vaccines,” the authors write. The American Medical Association and the Infectious Diseases Society of America espouse this position, and the AAP is developing a similar statement.
“Families should not have to fear going to school or the grocery store or a house of worship and worry about their kids getting sick,” said Dr. Cohen. “We now strongly say that we need to work with legislators, families, and other advocates for children at the state level to spread more laws like California’s SB 277 that would abolish philosophical exemptions.”
Dr. Cohen also emphasized the importance of communicating to parents that there are no valid “alternative schedules” for vaccination. There is the Centers for Disease Control and Prevention recommended schedule and anything else is a “nonrecommended vaccine schedule because it hasn’t been studied.” That change in terminology drives home the point that the CDC schedule is the only one fully tested for safety and effectiveness.
Meanwhile, however, pediatricians need the tools to address vaccine hesitancy, starting with understanding it. The report describes the pattern of disease incidence, vaccine uptake, disease reduction, adverse event increase and resulting vaccine hesitancy, punctuated by periodic outbreaks that restore eroded confidence in vaccines.
“When diseases are present, parents are worried and want a vaccine, and when they’re gone, they don’t,” Dr. Edwards said. “We need to remember that there is a dependence on the maintenance of herd immunity by immunization by your neighbors.”
Specific strategies to counter vaccine hesitancy
Just over half of physicians spend 10-19 minutes discussing vaccines with concerned parents, and 8% spend at least 20 minutes with such parents, found a study cited in the report. Other research has found these discussions take a toll on doctors’ job satisfaction.
Yet pediatricians remain the single biggest influence on parents’ vaccination decisions, cited by nearly 80% of parents in one large study.
“The pediatrician should appreciate that vaccine-hesitant parents are a heterogeneous group and that specific parental vaccine concerns should be individually identified and addressed,” the report states. “Although many techniques for working with vaccine-hesitant parents have been suggested, scant data are available to determine the efficacy of these methods.”
It goes on to recommend that physicians should discuss the development and safety testing of vaccines “in a nonconfrontational dialogue with the parents while listening to and acknowledging their concerns.”
Pediatricians should not, however, delay vaccines or limit the number per visit – thereby deviating from the CDC recommended and AAP-endorsed schedule – unless it’s the only way a parent agrees to vaccinate.
Another strategy is the presumptive approach: Present all vaccine recommendations as required immunizations that the provider expects a parent to agree to, although pediatricians should consider their experience and relationship with a family since this approach may not work well for some parents.
The report also emphasizes the potential effectiveness of personalizing vaccine conversations by having doctors share their own experience, such as the fact that they vaccinated themselves, their children, and/or their grandchildren.
“Parents often are more likely to be persuaded by stories and anecdotes about the successes of vaccines,” the authors write. “Personal examples of children who were sick with vaccine-preventable illnesses can be much more effective than simply reading the numbers of children infected with a disease each year.”
The report also offers several suggestions for reducing the pain from administering vaccinations: administering vaccines quickly without aspirating; saving the most painful injection for last; holding the child upright; providing tactile stimulation; breastfeeding or providing sweet solutions and topical anesthetics after administration; and using distraction, such as deep breathing, pinwheels, or toys to decrease children’s pain and anxiety.
But the bottom line is that pediatricians have one key message they must communicate to parents, the report states: “The clear message parents should hear is that vaccines are safe and effective, and serious disease can occur if your child and family are not immunized.”
For years, pediatricians have sought a blessing from the American Academy of Pediatrics that acknowledged it was valid for members to dismiss families from their practice if they refused to vaccinate despite all attempts to persuade them. Now, a new clinical report has essentially delivered just that.
The report does not represent an official policy change from the AAP, but it does for the first time acknowledge that “firing” patients who persistently refuse vaccination is “an acceptable option” (Pediatrics. 2016 Aug. doi: 10.1542/peds.2016-2146).
“A number of pediatricians feel so strongly that if they don’t agree on vaccines, which are so basic to the delivery of care and have made such a big difference in children’s lives, how will they agree on a number of other things they’ll need to discuss?” Kathryn M. Edwards, MD, director of the Vanderbilt Vaccine Research Program, Nashville, Tenn., and a coauthor of the report, explained in an interview.
The AAP has received pressure from its members over recent years as increasing numbers of pediatricians choose to dismiss some or all of their patients whose parents were resolved not vaccinate, coauthor Jesse M. Hackell, MD, a practicing pediatrician and managing partner at Pomona Pediatrics, an affiliate of Boston Children’s Health Physicians, said in an interview.
In fact, a new study has revealed that 12% of pediatricians reported dismissing vaccine-refusing families in 2013, up from 6% in 2006. At the same time, the proportion of families refusing vaccines has nearly doubled in the same time.
“There was a groundswell of opinion that enough is enough and we can’t provide quality care if we can’t provide something we know is so important,” Dr. Hackell said. “We felt the Academy needed to stop being so adamantly opposed to the possibility of dismissal – not to recommend dismissal but simply to state it is an acceptable option.”
The AAP responds to fellows’ concerns
While the AAP continues to recommend doctors attempt to persuade families as long as possible to vaccinate, the new report discusses dismissal as a viable option as long as it adheres to relevant state laws that prohibit abandonment of patients.
“The decision to dismiss a family who continues to refuse immunization is not one that should be made lightly, nor should it be made without considering and respecting the reasons for the parents’ point of view,” the report states. “Nevertheless, the individual pediatrician may consider dismissal of families who refuse vaccination as an acceptable option.”
The report does note that some practice settings, such as hospitals or large health care organizations, may not allow dismissal of patients, and that pediatricians “should carefully evaluate the availability of other qualified providers for the family” if they live in an area with limited access to pediatric care.
But the report finally acknowledges those pediatricians who are “just philosophically wired to not accept vaccine refusals,” Stuart A. Cohen, MD, an assistant professor of pediatrics at the University of California, San Diego, and chair of AAP District 9 in California, said in an interview.
“It really interferes with your physician-patient relationship,” Dr. Cohen said, who was not a coauthor of the report.
Now, if pediatricians feel it necessary to dismiss nonvaccinating patients, “then the Academy understands because of concerns for other patients, but it must be done in a way that’s respectful and tries to ensure patients understand the safety and necessity of vaccines,” Dr. Edwards said.
The report still includes the AAP recommendation that “pediatricians continue to engage with vaccine-hesitant parents, provide other health care services to their children, and attempt to modify their opposition to vaccines.” And a number of members of the AAP’s infectious diseases and bioethics committees were uncomfortable with dismissing patients, Dr. Edwards said, but “there were certain people who needed this, who needed some blessing that this was not inappropriate after all the other things the pediatrician had done.”
Vaccines undergo thorough testing for safety and effectiveness
But the report also aims to provide pediatricians with strategies for doing everything possible first.
“We needed to address enabling the clinician to have some very specific talking points to use and not get involved in a philosophical discussion that can take an hour,” Dr. Hackell said. “They need to make a clear statement that vaccines are important, and if you don’t get them, bad things like death can happen.”
The report therefore provides a comprehensive overview of vaccine development, from the initial identification of the need for a vaccine through the various phases of clinical testing and ongoing postlicensure monitoring. This background information can arm pediatricians with foundational knowledge that’s helpful in talking with patients.
“Vaccine development is a long and arduous process, often lasting many years and involving a combination of public and private partnerships,” the report states. “The current system for developing, testing and regulating vaccines requires that the vaccines demonstrate both safety and efficacy before licensure and that long-term safety is monitored.”
The report briefly explains the multiple mechanisms for continuing to track and study adverse events and other safety concerns:
• Vaccine Adverse Events Reporting System (VAERS). A voluntary passive reporting system used to identify potential safety signals.
• Vaccine Safety Datalink (VSD). A network of linked databases from health care systems across the United States involving millions of individuals
• Post-Licensure Rapid Immunization Safety Monitoring system (PRISM). A system which monitors vaccine safety using health insurance claims data from 107 million individuals .
• Clinical Immunization Safety Assessment Project (CISA). A system that answers individual health care providers’ specific questions on vaccine safety.
Vaccine hesitancy and vaccine exemptions
Opposition to vaccination is not new, the report states, describing it as dating back to Edward Jenner’s smallpox vaccine in the early 1800s.
“Although vaccine hesitancy is not a new phenomenon, it may have a greater effect on public health today,” the report states. “With the ease of global travel, vaccine-preventable diseases are spread more quickly and may unexpectedly appear in areas where health care professionals are unfamiliar with their clinical presentation.”
The historical presence of vaccination opposition has led to circumstances in the United States today in which parents can seek nonmedical exemptions from vaccines in 47 states, and their use has increased with their availability. Yet, the increase in use of exemptions and of “alternative” immunization schedules runs the risk of eroding the herd immunity that protects the community, the report notes.
“For these reasons, we believe the better approach is to work to eliminate all nonmedical exemptions for childhood vaccines,” the authors write. The American Medical Association and the Infectious Diseases Society of America espouse this position, and the AAP is developing a similar statement.
“Families should not have to fear going to school or the grocery store or a house of worship and worry about their kids getting sick,” said Dr. Cohen. “We now strongly say that we need to work with legislators, families, and other advocates for children at the state level to spread more laws like California’s SB 277 that would abolish philosophical exemptions.”
Dr. Cohen also emphasized the importance of communicating to parents that there are no valid “alternative schedules” for vaccination. There is the Centers for Disease Control and Prevention recommended schedule and anything else is a “nonrecommended vaccine schedule because it hasn’t been studied.” That change in terminology drives home the point that the CDC schedule is the only one fully tested for safety and effectiveness.
Meanwhile, however, pediatricians need the tools to address vaccine hesitancy, starting with understanding it. The report describes the pattern of disease incidence, vaccine uptake, disease reduction, adverse event increase and resulting vaccine hesitancy, punctuated by periodic outbreaks that restore eroded confidence in vaccines.
“When diseases are present, parents are worried and want a vaccine, and when they’re gone, they don’t,” Dr. Edwards said. “We need to remember that there is a dependence on the maintenance of herd immunity by immunization by your neighbors.”
Specific strategies to counter vaccine hesitancy
Just over half of physicians spend 10-19 minutes discussing vaccines with concerned parents, and 8% spend at least 20 minutes with such parents, found a study cited in the report. Other research has found these discussions take a toll on doctors’ job satisfaction.
Yet pediatricians remain the single biggest influence on parents’ vaccination decisions, cited by nearly 80% of parents in one large study.
“The pediatrician should appreciate that vaccine-hesitant parents are a heterogeneous group and that specific parental vaccine concerns should be individually identified and addressed,” the report states. “Although many techniques for working with vaccine-hesitant parents have been suggested, scant data are available to determine the efficacy of these methods.”
It goes on to recommend that physicians should discuss the development and safety testing of vaccines “in a nonconfrontational dialogue with the parents while listening to and acknowledging their concerns.”
Pediatricians should not, however, delay vaccines or limit the number per visit – thereby deviating from the CDC recommended and AAP-endorsed schedule – unless it’s the only way a parent agrees to vaccinate.
Another strategy is the presumptive approach: Present all vaccine recommendations as required immunizations that the provider expects a parent to agree to, although pediatricians should consider their experience and relationship with a family since this approach may not work well for some parents.
The report also emphasizes the potential effectiveness of personalizing vaccine conversations by having doctors share their own experience, such as the fact that they vaccinated themselves, their children, and/or their grandchildren.
“Parents often are more likely to be persuaded by stories and anecdotes about the successes of vaccines,” the authors write. “Personal examples of children who were sick with vaccine-preventable illnesses can be much more effective than simply reading the numbers of children infected with a disease each year.”
The report also offers several suggestions for reducing the pain from administering vaccinations: administering vaccines quickly without aspirating; saving the most painful injection for last; holding the child upright; providing tactile stimulation; breastfeeding or providing sweet solutions and topical anesthetics after administration; and using distraction, such as deep breathing, pinwheels, or toys to decrease children’s pain and anxiety.
But the bottom line is that pediatricians have one key message they must communicate to parents, the report states: “The clear message parents should hear is that vaccines are safe and effective, and serious disease can occur if your child and family are not immunized.”
FROM PEDIATRICS