User login
An Update on Neurotoxin Products and Administration Methods
The first botulinum neurotoxin (BoNT) approved by the US Food and Drug Administration (FDA) was onabotulinumtoxinA in 1989 for the treatment of strabismus and blepharospasm. It was not until 1992, however, that the aesthetic benefits of BoNT were first reported in the medical literature by Carruthers and Carruthers,1 and a cosmetic indication was not approved by the FDA until 2002. Since that time, the popularity of BoNT products has grown rapidly with a nearly 6500% increase in popularity from 1997 to 2015 in addition to the introduction of a variety of new BoNT formulations to the market.2 It is estimated by the American Society for Aesthetic Plastic Surgery that there were at least 4,000,000 BoNT injections performed in 2015 alone, making it the most popular nonsurgical aesthetic procedure available.2 As the demand for minimally invasive cosmetic procedures continues to increase, we will continue to see the introduction of additional formulations of BoNT products as well as novel administration techniques and delivery devices. In this article, we provide an update on current and upcoming BoNT products and also review the literature on novel administration methods based on studies published from January 1, 2014, to December 31, 2015.
Current Products
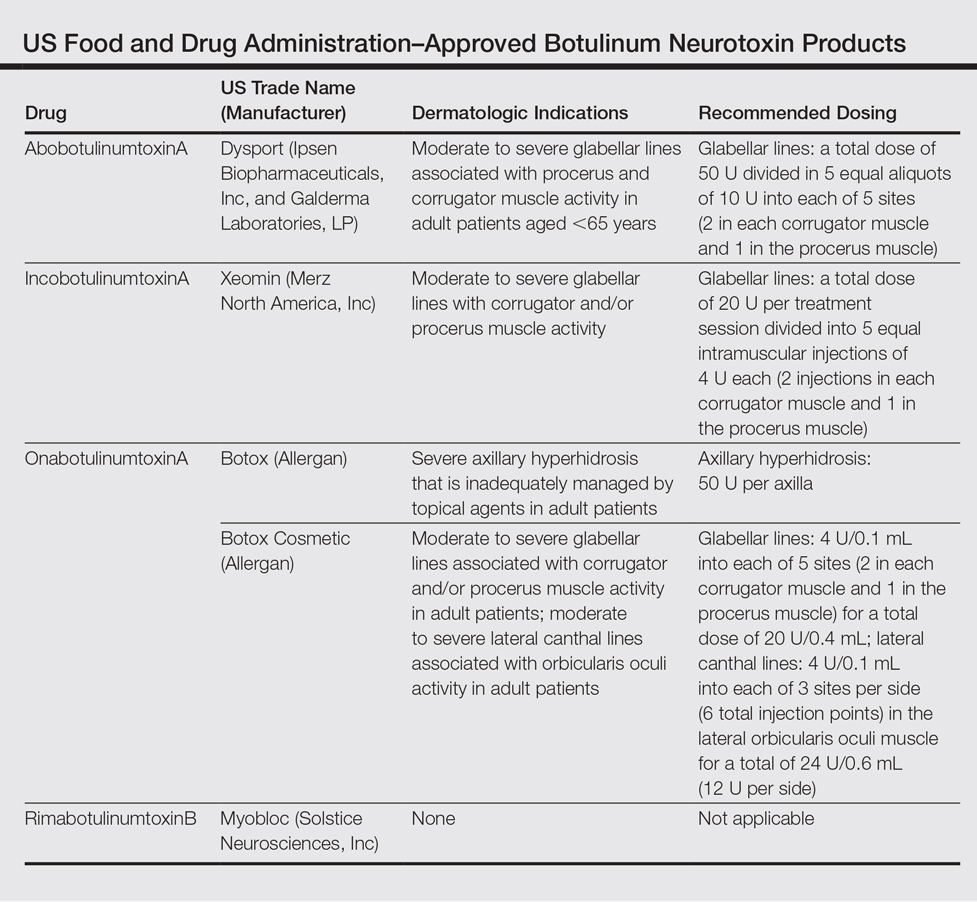
To date, there are only 4 FDA-approved formulations of BoNT available for clinical use (eg, cervical dystonia, strabismus, blepharospasm, headache, urinary incontinence) in the United States: abobotulinumtoxinA, incobotulinumtoxinA, onabotulinumtoxinA, and rimabotulinumtoxinB.The FDA-approved dermatologic indications (eg, moderate to severe glabellar or canthal lines, severe axillary hyperhidrosis) for these products are provided in the Table. On a global scale, there are several other commonly utilized formulations of BoNT, including a Korean serotype resembling onabotulinumtoxinA and a Chinese botulinum toxin type A.3 Although there is some evidence to demonstrate comparable efficacy and safety of these latter products, the literature is relatively lacking in comparison to the FDA-approved products.4,5
Upcoming Products
Currently, there are several new BoNT formulations being studied for clinical use. RT 002 (Revance Therapeutics, Inc) is a novel injectable formulation of onabotulinumtoxinA that consists of the purified neurotoxin in combination with patented TransMTS peptides that have been shown to provide high-binding avidity for the neurotoxin, and thus the product is designed to reduce diffusion to adjacent muscles and diminish unwanted effects. With a reduced level of neurotoxin dissemination, it is theorized that a higher administration of targeted doses can be injected, which may lead to a longer duration of desired effects.6 A clinical pilot study done to establish the safety and efficacy of RT 002 for treatment of moderate to severe glabellar lines evaluated 4 equally sized cohorts of 12 participants, each receiving single-dose administration of RT 002 ranging in potency equivalent to 25 U, 50 U, 75 U, and 100 U of abobotulinumtoxinA as determined by the gelatin phosphate method.6 It was concluded that RT 002 is both safe and efficacious with an extended duration of action, with a median duration of effect of 7 months observed in the highest dose group (dose equivalent to 100 U of abobotulinumtoxinA). Notably, 80% of all 48 participants maintained a minimum 1-point improvement in investigator-determined glabellar line severity scores at the 6-month time point and 60% achieved wrinkle scores of none or mild at 6 months posttreatment.6
DWP 450 (Daewoong Pharmaceutical Co, Ltd) is derived from the wild-type Clostridium botulinum and is reported to be of higher purity than standard onabotulinumtoxinA. An initial 16-week pilot study demonstrated that 20 U of DWP 450 is noninferior and of comparable efficacy and safety to 20 U of onabotulinumtoxinA in the treatment of glabellar lines.7
NTC (Botulax [Hugel, Inc]) is the name of the toxin derived from the C botulinum strain CBFC26, which has already been approved in many Asian, European, and Latin American countries for the treatment of blepharospasm. This formulation has demonstrated noninferiority to onabotulinumtoxinA at equivalent 20-U doses for the treatment of moderate to severe glabellar lines in a double-blind, randomized, multicenter, phase 3 trial of 272 participants with a 16-week follow-up.8
MT 10109L (Medytox Inc) is a unique product in that it is distributed as a liquid type A botulinum toxin rather than the standard freeze-dried formulation; thus, a major advantage of this product is its convenience, as it does not need reconstitution or dilution prior to administration. In a double-blind, randomized, active drug–controlled, phase 3 study of 168 participants, it was determined that MT 10109L (20 U) is comparable in efficacy to onabotulinumtoxinA (20 U) for the treatment of moderate to severe glabellar lines. No significant difference was seen between the 2 treatment groups when glabellar lines were assessed at rest at 4 and 16 weeks after treatment, but a significantly greater improvement in glabellar lines was seen at maximum frown in the MT 10109L group at the 16-week follow-up (P=.0064).9
Administration Techniques
With regard to safe and effective BoNT product administration techniques, a variety of studies have provided insight into optimal practice methods. A 2015 expert consensus statement formed by an American Society for Dermatologic Surgery task force reviewed data from 42 papers and unanimously determined that for all current type A BoNT products available in the United States, a vial of BoNT reconstituted appropriately for the purpose of facial injections can be reconstituted at least 4 weeks prior to administration without contamination risk or decrease in efficacy and that multiple patients can be treated with the same vial.Although the statement was not explicit on whether or not preserved or unpreserved saline is to be used, it is considered routine practice to use preservative-containing saline to reconstitute BoNT, as it has been shown to reduce patient discomfort and is not associated with adverse effects.10
Pain Minimization
With respect to minimizing the pain associated with BoNT injections, several studies have assessed administration techniques to minimize patient discomfort. A split-face, double-blind study of 20 participants demonstrated that the use of a 32-gauge needle has a significantly greater chance of reducing clinically significant levels of pain as compared to a 30-gauge needle when performing facial injections (P=.04). Overall, however, injections of the face and arms were on average only nominally and not significantly more painful with 30-gauge needles compared to 32-gauge needles.11
Another technique that has been found to reduce patient discomfort is the application of cold packs prior to injection. A study of patients with chronic facial palsy observed a significant reduction in pain with the administration of a cold (3°C–5°C) gel pack for 1 minute compared to a room temperature (20°C) gel pack prior to the administration of onabotulinumtoxinA into the platysma (P<.001).12 In the matter of injection with rimabotulinumtoxinB, which has been shown to be considerably more painful to receive than its more popularly administered counterpart onabotulinumtoxinA, a split-face pilot study examined the effect of increasing the pH of rimabotulinumtoxinB to 7.5 with sodium bicarbonate from the usual pH of 5.6.13,14 Pain was reported to be considerably less in the higher pH group and no reduction of efficacy was seen over the 10-week follow-up period.14
Delivery Methods
Several preliminary studies also have examined novel delivery techniques to identify minimally painful yet effective methods for administering BoNT. It has been reported that standard BoNT formulations are not effective as topical agents in a comparison study in which onabotulinumtoxinA injection was compared to topically applied onabotulinumtoxinA.15 However, a follow-up prospective study by the same authors has demonstrated efficacy of topical onabotulinumtoxinA following pretreatment with a fractional ablative CO2 laser for treatment of crow’s-feet. In this randomized, split-face, controlled trial (N=10), participants were first pretreated with topical lidocaine 30% before receiving a single pass of fractional ablative CO2 laser with no overlap and a pulse energy of 100 mJ. Within 60 seconds of laser treatment, participants then received either 100 U of abobotulinumtoxinA diluted in 0.1 mL of saline or simple normal saline applied topically. A clinically significant improvement in periorbital wrinkles was seen both at 1-week and 1-month posttreatment in the laser and onabotulinumtoxinA–treated group compared to the laser and topical saline–treated group (P<.02).15
Another unique administration method studied, albeit with less successful results, involves the use of iontophoresis to deliver BoNT painlessly in a transdermal fashion with the assistance of an electrical current.16 This prospective, randomized, assessor-blinded, split-axilla, controlled trial of 11 participants compared the effectiveness of administering onabotulinumtoxinA via iontophoresis to traditional injection with onabotulinumtoxinA (250 U). Iontophoresis was accomplished with a single electrode pad soaked with 250 U of onabotulinumtoxinA applied directly to the axilla and a second electrode pad soaked in 0.9% saline applied to the hand to complete the circuit. An alternating electrical current was slowly increased for 30 minutes to a maximum current of 15 mA with a voltage of 12 V. Among the 11 participants recruited, the side treated with traditional injection showed a significantly greater percentage reduction in baseline sweating at the 1-week, 1-month, and 6-month posttreatment evaluations compared to iontophoresis (84%, 76%, and 50%, respectively vs 73%, 22%, and 32%, respectively)(P<.05). Despite being less efficacious than standard injection therapy, participants reported that iontophoresis delivery was significantly less painful (P<.05).16
A high-pressure oxygen delivery device, which utilizes a powerful jet of microdroplets containing water, the drug, air, and oxygen to deliver medication onto the skin surface, also has been studied as a means of delivery of BoNT in a minimally painful manner. In this study, the device was used to assess the efficacy of transdermal delivery of BoNT via jet nebulization in the treatment of primary palmar, plantar, and axillary hyperhidrosis.17 The 20 participants included in the study were randomized to receive either a combination of lidocaine and onabotulinumtoxinA (50 U) administered through the device or lidocaine delivered through the device followed by multiple transcutaneous injections of onabotulinumtoxinA (100 U). Both treatments significantly reduced sweating compared to baseline as measured by a visual analogue scale at 3-month follow-up (P<.001), but the combination delivery of lidocaine and onabotulinumtoxinA via the device resulted in significantly less procedure-related pain and sweating (P<.001) as well as significantly greater patient satisfaction (P<.001).17
Optimizing Aesthetic Outcomes
A frequent concern of patients receiving BoNT for cosmetic purposes is a desire to avoid a “frozen” or expressionless look. As such, many clinicians have attempted a variety of techniques to achieve more natural aesthetic results. One such method is known as the multipoint and multilevel injection technique, which consists of utilizing multiple injection sites at varying depths (intramuscular, subcutaneous, or intradermal) and doses (2–6 U) depending on the degree of contractility of the targeted muscle. In a preliminary study of 223 participants using this technique with a total dose of 125 U of abobotulinumtoxinA, good and natural results were reported with perseveration of facial emotion in all participants in addition to a mean overall satisfaction rate of 6.4 of 7 on the Facial Line Treatment Satisfaction Questionnaire with the maximum satisfaction rating possible reported in 66% of cases.18 It also has been postulated that injection depth of BoNT can affect brow elevation whereupon deeper injection depths can result in inactivation of the brow depressors and allow for increased elevation of the eyebrows. This technique has been applied in attempts to correct brow height asymmetry. However, a prospective, split-face study of 23 women suggested that this method is not effective.19 Participants received 64 U of onabotulinumtoxinA via 16 injection sites in the glabella, forehead, and lateral canthal area with either all deep or all shallow injections depending on the side treated and whether brow-lift was desired. Results at 4 weeks posttreatment showed no significant difference in brow height, and it was concluded that eyebrow depressor muscles cannot be accurately targeted with deep injection into the muscle belly for correction of eyebrow height discrepancies.19 Conversely, a 5-year retrospective, nonrandomized study of 227 patients with 563 treatments utilizing a “microdroplet” technique reported success at selectively targeting the eyebrow depressors while leaving the brow elevators unaffected.20 Here, a total dose of 33 U of onabotulinumtoxinA was administered via microdroplets of 10 to 20 μL, each with more than 60 to 100 injections into the brow, glabella, and crow’s-feet area. This method of injection resulted in a statistically significant improvement of forehead lines and brow ptosis and furrowing at follow-up between 10 and 45 days after treatment (P<.0001). Additionally, average brow height was significantly increased from 24.6 mm to 25 mm after treatment (P=.02).20
Conclusion
The use of BoNT products for both on- and off-label cosmetic and medical indications has rapidly grown over the past 2 decades. As demonstrated in this review, a variety of promising new products and delivery techniques are being developed. Given the rise in popularity of BoNT products among both physicians and consumers, clinicians should be aware of the current data and ongoing research.
- Carruthers JD, Carruthers JA. Treatment of glabellar frown lines with C. botulinum-A exotoxin. J Dermatol Surg Oncol. 1992;18:17-21.
- American Society for Aesthetic Plastic Surgery. Cosmetic Surgery National Data Bank statistics. American Society for Aesthetic Plastic Surgery website. http://www.surgery.org/sites/default/files/ASAPS-Stats2015.pdf. Accessed June 12, 2016.
- Walker TJ, Dayan SH. Comparison and overview of currently available neurotoxins. J Clin Aesthet Dermatol. 2014;7:31-39.
- Feng Z, Sun Q, He L, et al. Optimal dosage of botulinum toxin type A for treatment of glabellar frown lines: efficacy and safety in a clinical trial. Dermatol Surg. 2015;41(suppl 1):S56-S63.
- Jiang HY, Chen S, Zhou J, et al. Diffusion of two botulinum toxins type A on the forehead: double-blinded, randomized, controlled study. Dermatol Surg. 2014;40:184-192.
- Garcia-Murray E, Velasco Villasenor ML, Acevedo B, et al. Safety and efficacy of RT002, an injectable botulinum toxin type A, for treating glabellar lines: results of a phase 1/2, open-label, sequential dose-escalation study. Dermatol Surg. 2015;41(suppl 1):S47-S55.
- Won CH, Kim HK, Kim BJ, et al. Comparative trial of a novel botulinum neurotoxin type A versus onabotulinumtoxinA in the treatment of glabellar lines: a multicenter, randomized, double-blind, active-controlled study. Int J Dermatol. 2015;54:227-234.
- Kim BJ, Kwon HH, Park SY, et al. Double-blind, randomized non-inferiority trial of a novel botulinum toxin A processed from the strain CBFC26, compared with onabotulinumtoxin A in the treatment of glabellar lines. J Eur Acad Dermatol Venereol. 2014;28:1761-1767.
- Kim JE, Song EJ, Choi GS, et al. The efficacy and safety of liquid-type botulinum toxin type A for the management of moderate to severe glabellar frown lines. Plast Reconstr Surg. 2015;135:732-741.
- Alam M, Bolotin D, Carruthers J, et al. Consensus statement regarding storage and reuse of previously reconstituted neuromodulators. Dermatol Surg. 2015;41:321-326.
- Alam M, Geisler A, Sadhwani D, et al. Effect of needle size on pain perception in patients treated with botulinum toxin type A injections: a randomized clinical trial. JAMA Dermatol. 2015;151:1194-1199.
- Pucks N, Thomas A, Hallam MJ, et al. Cutaneous cooling to manage botulinum toxin injection-associated pain in patients with facial palsy: a randomised controlled trial. J Plast Reconstr Aesthet Surg. 2015;68:1701-1705.
- Kranz G, Sycha T, Voller B, et al. Pain sensation during intradermal injections of three different botulinum toxin preparations in different doses and dilutions. Dermatol Surg. 2006;32:886-890.
- Lowe PL, Lowe NJ. Botulinum toxin type B: pH change reduces injection pain, retains efficacy. Dermatol Surg. 2014;40:1328-1333.
- Mahmoud BH, Burnett C, Ozog D. Prospective randomized controlled study to determine the effect of topical application of botulinum toxin A for crow’s feet after treatment with ablative fractional CO2 laser. Dermatol Surg. 2015;41(suppl 1):S75-S81.
- Montaser-Kouhsari L, Zartab H, Fanian F, et al. Comparison of intradermal injection with iontophoresis of abo-botulinum toxin A for the treatment of primary axillary hyperhidrosis: a randomized, controlled trial. J Dermatolog Treat. 2014;25:337-341.
- Iannitti T, Palmieri B, Aspiro A, et al. A preliminary study of painless and effective transdermal botulinum toxin A delivery by jet nebulization for treatment of primary hyperhidrosis. Drug Des Devel Ther. 2014;8:931-935.
- Iozzo I, Tengattini V, Antonucci VA. Multipoint and multilevel injection technique of botulinum toxin A in facial aesthetics. J Cosmet Dermatol. 2014;13:135-142.
- Sneath J, Humphrey S, Carruthers A, et al. Injecting botulinum toxin at different depths is not effective for the correction of eyebrow asymmetry. Dermatol Surg. 2015;41(suppl 1):S82-S87.
- Steinsapir KD, Rootman D, Wulc A, et al. Cosmetic microdroplet botulinum toxin A forehead lift: a new treatment paradigm. Ophthal Plast Reconstr Surg. 2015;31:263-268.
The first botulinum neurotoxin (BoNT) approved by the US Food and Drug Administration (FDA) was onabotulinumtoxinA in 1989 for the treatment of strabismus and blepharospasm. It was not until 1992, however, that the aesthetic benefits of BoNT were first reported in the medical literature by Carruthers and Carruthers,1 and a cosmetic indication was not approved by the FDA until 2002. Since that time, the popularity of BoNT products has grown rapidly with a nearly 6500% increase in popularity from 1997 to 2015 in addition to the introduction of a variety of new BoNT formulations to the market.2 It is estimated by the American Society for Aesthetic Plastic Surgery that there were at least 4,000,000 BoNT injections performed in 2015 alone, making it the most popular nonsurgical aesthetic procedure available.2 As the demand for minimally invasive cosmetic procedures continues to increase, we will continue to see the introduction of additional formulations of BoNT products as well as novel administration techniques and delivery devices. In this article, we provide an update on current and upcoming BoNT products and also review the literature on novel administration methods based on studies published from January 1, 2014, to December 31, 2015.
Current Products
To date, there are only 4 FDA-approved formulations of BoNT available for clinical use (eg, cervical dystonia, strabismus, blepharospasm, headache, urinary incontinence) in the United States: abobotulinumtoxinA, incobotulinumtoxinA, onabotulinumtoxinA, and rimabotulinumtoxinB.The FDA-approved dermatologic indications (eg, moderate to severe glabellar or canthal lines, severe axillary hyperhidrosis) for these products are provided in the Table. On a global scale, there are several other commonly utilized formulations of BoNT, including a Korean serotype resembling onabotulinumtoxinA and a Chinese botulinum toxin type A.3 Although there is some evidence to demonstrate comparable efficacy and safety of these latter products, the literature is relatively lacking in comparison to the FDA-approved products.4,5

Upcoming Products
Currently, there are several new BoNT formulations being studied for clinical use. RT 002 (Revance Therapeutics, Inc) is a novel injectable formulation of onabotulinumtoxinA that consists of the purified neurotoxin in combination with patented TransMTS peptides that have been shown to provide high-binding avidity for the neurotoxin, and thus the product is designed to reduce diffusion to adjacent muscles and diminish unwanted effects. With a reduced level of neurotoxin dissemination, it is theorized that a higher administration of targeted doses can be injected, which may lead to a longer duration of desired effects.6 A clinical pilot study done to establish the safety and efficacy of RT 002 for treatment of moderate to severe glabellar lines evaluated 4 equally sized cohorts of 12 participants, each receiving single-dose administration of RT 002 ranging in potency equivalent to 25 U, 50 U, 75 U, and 100 U of abobotulinumtoxinA as determined by the gelatin phosphate method.6 It was concluded that RT 002 is both safe and efficacious with an extended duration of action, with a median duration of effect of 7 months observed in the highest dose group (dose equivalent to 100 U of abobotulinumtoxinA). Notably, 80% of all 48 participants maintained a minimum 1-point improvement in investigator-determined glabellar line severity scores at the 6-month time point and 60% achieved wrinkle scores of none or mild at 6 months posttreatment.6
DWP 450 (Daewoong Pharmaceutical Co, Ltd) is derived from the wild-type Clostridium botulinum and is reported to be of higher purity than standard onabotulinumtoxinA. An initial 16-week pilot study demonstrated that 20 U of DWP 450 is noninferior and of comparable efficacy and safety to 20 U of onabotulinumtoxinA in the treatment of glabellar lines.7
NTC (Botulax [Hugel, Inc]) is the name of the toxin derived from the C botulinum strain CBFC26, which has already been approved in many Asian, European, and Latin American countries for the treatment of blepharospasm. This formulation has demonstrated noninferiority to onabotulinumtoxinA at equivalent 20-U doses for the treatment of moderate to severe glabellar lines in a double-blind, randomized, multicenter, phase 3 trial of 272 participants with a 16-week follow-up.8
MT 10109L (Medytox Inc) is a unique product in that it is distributed as a liquid type A botulinum toxin rather than the standard freeze-dried formulation; thus, a major advantage of this product is its convenience, as it does not need reconstitution or dilution prior to administration. In a double-blind, randomized, active drug–controlled, phase 3 study of 168 participants, it was determined that MT 10109L (20 U) is comparable in efficacy to onabotulinumtoxinA (20 U) for the treatment of moderate to severe glabellar lines. No significant difference was seen between the 2 treatment groups when glabellar lines were assessed at rest at 4 and 16 weeks after treatment, but a significantly greater improvement in glabellar lines was seen at maximum frown in the MT 10109L group at the 16-week follow-up (P=.0064).9
Administration Techniques
With regard to safe and effective BoNT product administration techniques, a variety of studies have provided insight into optimal practice methods. A 2015 expert consensus statement formed by an American Society for Dermatologic Surgery task force reviewed data from 42 papers and unanimously determined that for all current type A BoNT products available in the United States, a vial of BoNT reconstituted appropriately for the purpose of facial injections can be reconstituted at least 4 weeks prior to administration without contamination risk or decrease in efficacy and that multiple patients can be treated with the same vial.Although the statement was not explicit on whether or not preserved or unpreserved saline is to be used, it is considered routine practice to use preservative-containing saline to reconstitute BoNT, as it has been shown to reduce patient discomfort and is not associated with adverse effects.10
Pain Minimization
With respect to minimizing the pain associated with BoNT injections, several studies have assessed administration techniques to minimize patient discomfort. A split-face, double-blind study of 20 participants demonstrated that the use of a 32-gauge needle has a significantly greater chance of reducing clinically significant levels of pain as compared to a 30-gauge needle when performing facial injections (P=.04). Overall, however, injections of the face and arms were on average only nominally and not significantly more painful with 30-gauge needles compared to 32-gauge needles.11
Another technique that has been found to reduce patient discomfort is the application of cold packs prior to injection. A study of patients with chronic facial palsy observed a significant reduction in pain with the administration of a cold (3°C–5°C) gel pack for 1 minute compared to a room temperature (20°C) gel pack prior to the administration of onabotulinumtoxinA into the platysma (P<.001).12 In the matter of injection with rimabotulinumtoxinB, which has been shown to be considerably more painful to receive than its more popularly administered counterpart onabotulinumtoxinA, a split-face pilot study examined the effect of increasing the pH of rimabotulinumtoxinB to 7.5 with sodium bicarbonate from the usual pH of 5.6.13,14 Pain was reported to be considerably less in the higher pH group and no reduction of efficacy was seen over the 10-week follow-up period.14
Delivery Methods
Several preliminary studies also have examined novel delivery techniques to identify minimally painful yet effective methods for administering BoNT. It has been reported that standard BoNT formulations are not effective as topical agents in a comparison study in which onabotulinumtoxinA injection was compared to topically applied onabotulinumtoxinA.15 However, a follow-up prospective study by the same authors has demonstrated efficacy of topical onabotulinumtoxinA following pretreatment with a fractional ablative CO2 laser for treatment of crow’s-feet. In this randomized, split-face, controlled trial (N=10), participants were first pretreated with topical lidocaine 30% before receiving a single pass of fractional ablative CO2 laser with no overlap and a pulse energy of 100 mJ. Within 60 seconds of laser treatment, participants then received either 100 U of abobotulinumtoxinA diluted in 0.1 mL of saline or simple normal saline applied topically. A clinically significant improvement in periorbital wrinkles was seen both at 1-week and 1-month posttreatment in the laser and onabotulinumtoxinA–treated group compared to the laser and topical saline–treated group (P<.02).15
Another unique administration method studied, albeit with less successful results, involves the use of iontophoresis to deliver BoNT painlessly in a transdermal fashion with the assistance of an electrical current.16 This prospective, randomized, assessor-blinded, split-axilla, controlled trial of 11 participants compared the effectiveness of administering onabotulinumtoxinA via iontophoresis to traditional injection with onabotulinumtoxinA (250 U). Iontophoresis was accomplished with a single electrode pad soaked with 250 U of onabotulinumtoxinA applied directly to the axilla and a second electrode pad soaked in 0.9% saline applied to the hand to complete the circuit. An alternating electrical current was slowly increased for 30 minutes to a maximum current of 15 mA with a voltage of 12 V. Among the 11 participants recruited, the side treated with traditional injection showed a significantly greater percentage reduction in baseline sweating at the 1-week, 1-month, and 6-month posttreatment evaluations compared to iontophoresis (84%, 76%, and 50%, respectively vs 73%, 22%, and 32%, respectively)(P<.05). Despite being less efficacious than standard injection therapy, participants reported that iontophoresis delivery was significantly less painful (P<.05).16
A high-pressure oxygen delivery device, which utilizes a powerful jet of microdroplets containing water, the drug, air, and oxygen to deliver medication onto the skin surface, also has been studied as a means of delivery of BoNT in a minimally painful manner. In this study, the device was used to assess the efficacy of transdermal delivery of BoNT via jet nebulization in the treatment of primary palmar, plantar, and axillary hyperhidrosis.17 The 20 participants included in the study were randomized to receive either a combination of lidocaine and onabotulinumtoxinA (50 U) administered through the device or lidocaine delivered through the device followed by multiple transcutaneous injections of onabotulinumtoxinA (100 U). Both treatments significantly reduced sweating compared to baseline as measured by a visual analogue scale at 3-month follow-up (P<.001), but the combination delivery of lidocaine and onabotulinumtoxinA via the device resulted in significantly less procedure-related pain and sweating (P<.001) as well as significantly greater patient satisfaction (P<.001).17
Optimizing Aesthetic Outcomes
A frequent concern of patients receiving BoNT for cosmetic purposes is a desire to avoid a “frozen” or expressionless look. As such, many clinicians have attempted a variety of techniques to achieve more natural aesthetic results. One such method is known as the multipoint and multilevel injection technique, which consists of utilizing multiple injection sites at varying depths (intramuscular, subcutaneous, or intradermal) and doses (2–6 U) depending on the degree of contractility of the targeted muscle. In a preliminary study of 223 participants using this technique with a total dose of 125 U of abobotulinumtoxinA, good and natural results were reported with perseveration of facial emotion in all participants in addition to a mean overall satisfaction rate of 6.4 of 7 on the Facial Line Treatment Satisfaction Questionnaire with the maximum satisfaction rating possible reported in 66% of cases.18 It also has been postulated that injection depth of BoNT can affect brow elevation whereupon deeper injection depths can result in inactivation of the brow depressors and allow for increased elevation of the eyebrows. This technique has been applied in attempts to correct brow height asymmetry. However, a prospective, split-face study of 23 women suggested that this method is not effective.19 Participants received 64 U of onabotulinumtoxinA via 16 injection sites in the glabella, forehead, and lateral canthal area with either all deep or all shallow injections depending on the side treated and whether brow-lift was desired. Results at 4 weeks posttreatment showed no significant difference in brow height, and it was concluded that eyebrow depressor muscles cannot be accurately targeted with deep injection into the muscle belly for correction of eyebrow height discrepancies.19 Conversely, a 5-year retrospective, nonrandomized study of 227 patients with 563 treatments utilizing a “microdroplet” technique reported success at selectively targeting the eyebrow depressors while leaving the brow elevators unaffected.20 Here, a total dose of 33 U of onabotulinumtoxinA was administered via microdroplets of 10 to 20 μL, each with more than 60 to 100 injections into the brow, glabella, and crow’s-feet area. This method of injection resulted in a statistically significant improvement of forehead lines and brow ptosis and furrowing at follow-up between 10 and 45 days after treatment (P<.0001). Additionally, average brow height was significantly increased from 24.6 mm to 25 mm after treatment (P=.02).20
Conclusion
The use of BoNT products for both on- and off-label cosmetic and medical indications has rapidly grown over the past 2 decades. As demonstrated in this review, a variety of promising new products and delivery techniques are being developed. Given the rise in popularity of BoNT products among both physicians and consumers, clinicians should be aware of the current data and ongoing research.
The first botulinum neurotoxin (BoNT) approved by the US Food and Drug Administration (FDA) was onabotulinumtoxinA in 1989 for the treatment of strabismus and blepharospasm. It was not until 1992, however, that the aesthetic benefits of BoNT were first reported in the medical literature by Carruthers and Carruthers,1 and a cosmetic indication was not approved by the FDA until 2002. Since that time, the popularity of BoNT products has grown rapidly with a nearly 6500% increase in popularity from 1997 to 2015 in addition to the introduction of a variety of new BoNT formulations to the market.2 It is estimated by the American Society for Aesthetic Plastic Surgery that there were at least 4,000,000 BoNT injections performed in 2015 alone, making it the most popular nonsurgical aesthetic procedure available.2 As the demand for minimally invasive cosmetic procedures continues to increase, we will continue to see the introduction of additional formulations of BoNT products as well as novel administration techniques and delivery devices. In this article, we provide an update on current and upcoming BoNT products and also review the literature on novel administration methods based on studies published from January 1, 2014, to December 31, 2015.
Current Products
To date, there are only 4 FDA-approved formulations of BoNT available for clinical use (eg, cervical dystonia, strabismus, blepharospasm, headache, urinary incontinence) in the United States: abobotulinumtoxinA, incobotulinumtoxinA, onabotulinumtoxinA, and rimabotulinumtoxinB.The FDA-approved dermatologic indications (eg, moderate to severe glabellar or canthal lines, severe axillary hyperhidrosis) for these products are provided in the Table. On a global scale, there are several other commonly utilized formulations of BoNT, including a Korean serotype resembling onabotulinumtoxinA and a Chinese botulinum toxin type A.3 Although there is some evidence to demonstrate comparable efficacy and safety of these latter products, the literature is relatively lacking in comparison to the FDA-approved products.4,5

Upcoming Products
Currently, there are several new BoNT formulations being studied for clinical use. RT 002 (Revance Therapeutics, Inc) is a novel injectable formulation of onabotulinumtoxinA that consists of the purified neurotoxin in combination with patented TransMTS peptides that have been shown to provide high-binding avidity for the neurotoxin, and thus the product is designed to reduce diffusion to adjacent muscles and diminish unwanted effects. With a reduced level of neurotoxin dissemination, it is theorized that a higher administration of targeted doses can be injected, which may lead to a longer duration of desired effects.6 A clinical pilot study done to establish the safety and efficacy of RT 002 for treatment of moderate to severe glabellar lines evaluated 4 equally sized cohorts of 12 participants, each receiving single-dose administration of RT 002 ranging in potency equivalent to 25 U, 50 U, 75 U, and 100 U of abobotulinumtoxinA as determined by the gelatin phosphate method.6 It was concluded that RT 002 is both safe and efficacious with an extended duration of action, with a median duration of effect of 7 months observed in the highest dose group (dose equivalent to 100 U of abobotulinumtoxinA). Notably, 80% of all 48 participants maintained a minimum 1-point improvement in investigator-determined glabellar line severity scores at the 6-month time point and 60% achieved wrinkle scores of none or mild at 6 months posttreatment.6
DWP 450 (Daewoong Pharmaceutical Co, Ltd) is derived from the wild-type Clostridium botulinum and is reported to be of higher purity than standard onabotulinumtoxinA. An initial 16-week pilot study demonstrated that 20 U of DWP 450 is noninferior and of comparable efficacy and safety to 20 U of onabotulinumtoxinA in the treatment of glabellar lines.7
NTC (Botulax [Hugel, Inc]) is the name of the toxin derived from the C botulinum strain CBFC26, which has already been approved in many Asian, European, and Latin American countries for the treatment of blepharospasm. This formulation has demonstrated noninferiority to onabotulinumtoxinA at equivalent 20-U doses for the treatment of moderate to severe glabellar lines in a double-blind, randomized, multicenter, phase 3 trial of 272 participants with a 16-week follow-up.8
MT 10109L (Medytox Inc) is a unique product in that it is distributed as a liquid type A botulinum toxin rather than the standard freeze-dried formulation; thus, a major advantage of this product is its convenience, as it does not need reconstitution or dilution prior to administration. In a double-blind, randomized, active drug–controlled, phase 3 study of 168 participants, it was determined that MT 10109L (20 U) is comparable in efficacy to onabotulinumtoxinA (20 U) for the treatment of moderate to severe glabellar lines. No significant difference was seen between the 2 treatment groups when glabellar lines were assessed at rest at 4 and 16 weeks after treatment, but a significantly greater improvement in glabellar lines was seen at maximum frown in the MT 10109L group at the 16-week follow-up (P=.0064).9
Administration Techniques
With regard to safe and effective BoNT product administration techniques, a variety of studies have provided insight into optimal practice methods. A 2015 expert consensus statement formed by an American Society for Dermatologic Surgery task force reviewed data from 42 papers and unanimously determined that for all current type A BoNT products available in the United States, a vial of BoNT reconstituted appropriately for the purpose of facial injections can be reconstituted at least 4 weeks prior to administration without contamination risk or decrease in efficacy and that multiple patients can be treated with the same vial.Although the statement was not explicit on whether or not preserved or unpreserved saline is to be used, it is considered routine practice to use preservative-containing saline to reconstitute BoNT, as it has been shown to reduce patient discomfort and is not associated with adverse effects.10
Pain Minimization
With respect to minimizing the pain associated with BoNT injections, several studies have assessed administration techniques to minimize patient discomfort. A split-face, double-blind study of 20 participants demonstrated that the use of a 32-gauge needle has a significantly greater chance of reducing clinically significant levels of pain as compared to a 30-gauge needle when performing facial injections (P=.04). Overall, however, injections of the face and arms were on average only nominally and not significantly more painful with 30-gauge needles compared to 32-gauge needles.11
Another technique that has been found to reduce patient discomfort is the application of cold packs prior to injection. A study of patients with chronic facial palsy observed a significant reduction in pain with the administration of a cold (3°C–5°C) gel pack for 1 minute compared to a room temperature (20°C) gel pack prior to the administration of onabotulinumtoxinA into the platysma (P<.001).12 In the matter of injection with rimabotulinumtoxinB, which has been shown to be considerably more painful to receive than its more popularly administered counterpart onabotulinumtoxinA, a split-face pilot study examined the effect of increasing the pH of rimabotulinumtoxinB to 7.5 with sodium bicarbonate from the usual pH of 5.6.13,14 Pain was reported to be considerably less in the higher pH group and no reduction of efficacy was seen over the 10-week follow-up period.14
Delivery Methods
Several preliminary studies also have examined novel delivery techniques to identify minimally painful yet effective methods for administering BoNT. It has been reported that standard BoNT formulations are not effective as topical agents in a comparison study in which onabotulinumtoxinA injection was compared to topically applied onabotulinumtoxinA.15 However, a follow-up prospective study by the same authors has demonstrated efficacy of topical onabotulinumtoxinA following pretreatment with a fractional ablative CO2 laser for treatment of crow’s-feet. In this randomized, split-face, controlled trial (N=10), participants were first pretreated with topical lidocaine 30% before receiving a single pass of fractional ablative CO2 laser with no overlap and a pulse energy of 100 mJ. Within 60 seconds of laser treatment, participants then received either 100 U of abobotulinumtoxinA diluted in 0.1 mL of saline or simple normal saline applied topically. A clinically significant improvement in periorbital wrinkles was seen both at 1-week and 1-month posttreatment in the laser and onabotulinumtoxinA–treated group compared to the laser and topical saline–treated group (P<.02).15
Another unique administration method studied, albeit with less successful results, involves the use of iontophoresis to deliver BoNT painlessly in a transdermal fashion with the assistance of an electrical current.16 This prospective, randomized, assessor-blinded, split-axilla, controlled trial of 11 participants compared the effectiveness of administering onabotulinumtoxinA via iontophoresis to traditional injection with onabotulinumtoxinA (250 U). Iontophoresis was accomplished with a single electrode pad soaked with 250 U of onabotulinumtoxinA applied directly to the axilla and a second electrode pad soaked in 0.9% saline applied to the hand to complete the circuit. An alternating electrical current was slowly increased for 30 minutes to a maximum current of 15 mA with a voltage of 12 V. Among the 11 participants recruited, the side treated with traditional injection showed a significantly greater percentage reduction in baseline sweating at the 1-week, 1-month, and 6-month posttreatment evaluations compared to iontophoresis (84%, 76%, and 50%, respectively vs 73%, 22%, and 32%, respectively)(P<.05). Despite being less efficacious than standard injection therapy, participants reported that iontophoresis delivery was significantly less painful (P<.05).16
A high-pressure oxygen delivery device, which utilizes a powerful jet of microdroplets containing water, the drug, air, and oxygen to deliver medication onto the skin surface, also has been studied as a means of delivery of BoNT in a minimally painful manner. In this study, the device was used to assess the efficacy of transdermal delivery of BoNT via jet nebulization in the treatment of primary palmar, plantar, and axillary hyperhidrosis.17 The 20 participants included in the study were randomized to receive either a combination of lidocaine and onabotulinumtoxinA (50 U) administered through the device or lidocaine delivered through the device followed by multiple transcutaneous injections of onabotulinumtoxinA (100 U). Both treatments significantly reduced sweating compared to baseline as measured by a visual analogue scale at 3-month follow-up (P<.001), but the combination delivery of lidocaine and onabotulinumtoxinA via the device resulted in significantly less procedure-related pain and sweating (P<.001) as well as significantly greater patient satisfaction (P<.001).17
Optimizing Aesthetic Outcomes
A frequent concern of patients receiving BoNT for cosmetic purposes is a desire to avoid a “frozen” or expressionless look. As such, many clinicians have attempted a variety of techniques to achieve more natural aesthetic results. One such method is known as the multipoint and multilevel injection technique, which consists of utilizing multiple injection sites at varying depths (intramuscular, subcutaneous, or intradermal) and doses (2–6 U) depending on the degree of contractility of the targeted muscle. In a preliminary study of 223 participants using this technique with a total dose of 125 U of abobotulinumtoxinA, good and natural results were reported with perseveration of facial emotion in all participants in addition to a mean overall satisfaction rate of 6.4 of 7 on the Facial Line Treatment Satisfaction Questionnaire with the maximum satisfaction rating possible reported in 66% of cases.18 It also has been postulated that injection depth of BoNT can affect brow elevation whereupon deeper injection depths can result in inactivation of the brow depressors and allow for increased elevation of the eyebrows. This technique has been applied in attempts to correct brow height asymmetry. However, a prospective, split-face study of 23 women suggested that this method is not effective.19 Participants received 64 U of onabotulinumtoxinA via 16 injection sites in the glabella, forehead, and lateral canthal area with either all deep or all shallow injections depending on the side treated and whether brow-lift was desired. Results at 4 weeks posttreatment showed no significant difference in brow height, and it was concluded that eyebrow depressor muscles cannot be accurately targeted with deep injection into the muscle belly for correction of eyebrow height discrepancies.19 Conversely, a 5-year retrospective, nonrandomized study of 227 patients with 563 treatments utilizing a “microdroplet” technique reported success at selectively targeting the eyebrow depressors while leaving the brow elevators unaffected.20 Here, a total dose of 33 U of onabotulinumtoxinA was administered via microdroplets of 10 to 20 μL, each with more than 60 to 100 injections into the brow, glabella, and crow’s-feet area. This method of injection resulted in a statistically significant improvement of forehead lines and brow ptosis and furrowing at follow-up between 10 and 45 days after treatment (P<.0001). Additionally, average brow height was significantly increased from 24.6 mm to 25 mm after treatment (P=.02).20
Conclusion
The use of BoNT products for both on- and off-label cosmetic and medical indications has rapidly grown over the past 2 decades. As demonstrated in this review, a variety of promising new products and delivery techniques are being developed. Given the rise in popularity of BoNT products among both physicians and consumers, clinicians should be aware of the current data and ongoing research.
- Carruthers JD, Carruthers JA. Treatment of glabellar frown lines with C. botulinum-A exotoxin. J Dermatol Surg Oncol. 1992;18:17-21.
- American Society for Aesthetic Plastic Surgery. Cosmetic Surgery National Data Bank statistics. American Society for Aesthetic Plastic Surgery website. http://www.surgery.org/sites/default/files/ASAPS-Stats2015.pdf. Accessed June 12, 2016.
- Walker TJ, Dayan SH. Comparison and overview of currently available neurotoxins. J Clin Aesthet Dermatol. 2014;7:31-39.
- Feng Z, Sun Q, He L, et al. Optimal dosage of botulinum toxin type A for treatment of glabellar frown lines: efficacy and safety in a clinical trial. Dermatol Surg. 2015;41(suppl 1):S56-S63.
- Jiang HY, Chen S, Zhou J, et al. Diffusion of two botulinum toxins type A on the forehead: double-blinded, randomized, controlled study. Dermatol Surg. 2014;40:184-192.
- Garcia-Murray E, Velasco Villasenor ML, Acevedo B, et al. Safety and efficacy of RT002, an injectable botulinum toxin type A, for treating glabellar lines: results of a phase 1/2, open-label, sequential dose-escalation study. Dermatol Surg. 2015;41(suppl 1):S47-S55.
- Won CH, Kim HK, Kim BJ, et al. Comparative trial of a novel botulinum neurotoxin type A versus onabotulinumtoxinA in the treatment of glabellar lines: a multicenter, randomized, double-blind, active-controlled study. Int J Dermatol. 2015;54:227-234.
- Kim BJ, Kwon HH, Park SY, et al. Double-blind, randomized non-inferiority trial of a novel botulinum toxin A processed from the strain CBFC26, compared with onabotulinumtoxin A in the treatment of glabellar lines. J Eur Acad Dermatol Venereol. 2014;28:1761-1767.
- Kim JE, Song EJ, Choi GS, et al. The efficacy and safety of liquid-type botulinum toxin type A for the management of moderate to severe glabellar frown lines. Plast Reconstr Surg. 2015;135:732-741.
- Alam M, Bolotin D, Carruthers J, et al. Consensus statement regarding storage and reuse of previously reconstituted neuromodulators. Dermatol Surg. 2015;41:321-326.
- Alam M, Geisler A, Sadhwani D, et al. Effect of needle size on pain perception in patients treated with botulinum toxin type A injections: a randomized clinical trial. JAMA Dermatol. 2015;151:1194-1199.
- Pucks N, Thomas A, Hallam MJ, et al. Cutaneous cooling to manage botulinum toxin injection-associated pain in patients with facial palsy: a randomised controlled trial. J Plast Reconstr Aesthet Surg. 2015;68:1701-1705.
- Kranz G, Sycha T, Voller B, et al. Pain sensation during intradermal injections of three different botulinum toxin preparations in different doses and dilutions. Dermatol Surg. 2006;32:886-890.
- Lowe PL, Lowe NJ. Botulinum toxin type B: pH change reduces injection pain, retains efficacy. Dermatol Surg. 2014;40:1328-1333.
- Mahmoud BH, Burnett C, Ozog D. Prospective randomized controlled study to determine the effect of topical application of botulinum toxin A for crow’s feet after treatment with ablative fractional CO2 laser. Dermatol Surg. 2015;41(suppl 1):S75-S81.
- Montaser-Kouhsari L, Zartab H, Fanian F, et al. Comparison of intradermal injection with iontophoresis of abo-botulinum toxin A for the treatment of primary axillary hyperhidrosis: a randomized, controlled trial. J Dermatolog Treat. 2014;25:337-341.
- Iannitti T, Palmieri B, Aspiro A, et al. A preliminary study of painless and effective transdermal botulinum toxin A delivery by jet nebulization for treatment of primary hyperhidrosis. Drug Des Devel Ther. 2014;8:931-935.
- Iozzo I, Tengattini V, Antonucci VA. Multipoint and multilevel injection technique of botulinum toxin A in facial aesthetics. J Cosmet Dermatol. 2014;13:135-142.
- Sneath J, Humphrey S, Carruthers A, et al. Injecting botulinum toxin at different depths is not effective for the correction of eyebrow asymmetry. Dermatol Surg. 2015;41(suppl 1):S82-S87.
- Steinsapir KD, Rootman D, Wulc A, et al. Cosmetic microdroplet botulinum toxin A forehead lift: a new treatment paradigm. Ophthal Plast Reconstr Surg. 2015;31:263-268.
- Carruthers JD, Carruthers JA. Treatment of glabellar frown lines with C. botulinum-A exotoxin. J Dermatol Surg Oncol. 1992;18:17-21.
- American Society for Aesthetic Plastic Surgery. Cosmetic Surgery National Data Bank statistics. American Society for Aesthetic Plastic Surgery website. http://www.surgery.org/sites/default/files/ASAPS-Stats2015.pdf. Accessed June 12, 2016.
- Walker TJ, Dayan SH. Comparison and overview of currently available neurotoxins. J Clin Aesthet Dermatol. 2014;7:31-39.
- Feng Z, Sun Q, He L, et al. Optimal dosage of botulinum toxin type A for treatment of glabellar frown lines: efficacy and safety in a clinical trial. Dermatol Surg. 2015;41(suppl 1):S56-S63.
- Jiang HY, Chen S, Zhou J, et al. Diffusion of two botulinum toxins type A on the forehead: double-blinded, randomized, controlled study. Dermatol Surg. 2014;40:184-192.
- Garcia-Murray E, Velasco Villasenor ML, Acevedo B, et al. Safety and efficacy of RT002, an injectable botulinum toxin type A, for treating glabellar lines: results of a phase 1/2, open-label, sequential dose-escalation study. Dermatol Surg. 2015;41(suppl 1):S47-S55.
- Won CH, Kim HK, Kim BJ, et al. Comparative trial of a novel botulinum neurotoxin type A versus onabotulinumtoxinA in the treatment of glabellar lines: a multicenter, randomized, double-blind, active-controlled study. Int J Dermatol. 2015;54:227-234.
- Kim BJ, Kwon HH, Park SY, et al. Double-blind, randomized non-inferiority trial of a novel botulinum toxin A processed from the strain CBFC26, compared with onabotulinumtoxin A in the treatment of glabellar lines. J Eur Acad Dermatol Venereol. 2014;28:1761-1767.
- Kim JE, Song EJ, Choi GS, et al. The efficacy and safety of liquid-type botulinum toxin type A for the management of moderate to severe glabellar frown lines. Plast Reconstr Surg. 2015;135:732-741.
- Alam M, Bolotin D, Carruthers J, et al. Consensus statement regarding storage and reuse of previously reconstituted neuromodulators. Dermatol Surg. 2015;41:321-326.
- Alam M, Geisler A, Sadhwani D, et al. Effect of needle size on pain perception in patients treated with botulinum toxin type A injections: a randomized clinical trial. JAMA Dermatol. 2015;151:1194-1199.
- Pucks N, Thomas A, Hallam MJ, et al. Cutaneous cooling to manage botulinum toxin injection-associated pain in patients with facial palsy: a randomised controlled trial. J Plast Reconstr Aesthet Surg. 2015;68:1701-1705.
- Kranz G, Sycha T, Voller B, et al. Pain sensation during intradermal injections of three different botulinum toxin preparations in different doses and dilutions. Dermatol Surg. 2006;32:886-890.
- Lowe PL, Lowe NJ. Botulinum toxin type B: pH change reduces injection pain, retains efficacy. Dermatol Surg. 2014;40:1328-1333.
- Mahmoud BH, Burnett C, Ozog D. Prospective randomized controlled study to determine the effect of topical application of botulinum toxin A for crow’s feet after treatment with ablative fractional CO2 laser. Dermatol Surg. 2015;41(suppl 1):S75-S81.
- Montaser-Kouhsari L, Zartab H, Fanian F, et al. Comparison of intradermal injection with iontophoresis of abo-botulinum toxin A for the treatment of primary axillary hyperhidrosis: a randomized, controlled trial. J Dermatolog Treat. 2014;25:337-341.
- Iannitti T, Palmieri B, Aspiro A, et al. A preliminary study of painless and effective transdermal botulinum toxin A delivery by jet nebulization for treatment of primary hyperhidrosis. Drug Des Devel Ther. 2014;8:931-935.
- Iozzo I, Tengattini V, Antonucci VA. Multipoint and multilevel injection technique of botulinum toxin A in facial aesthetics. J Cosmet Dermatol. 2014;13:135-142.
- Sneath J, Humphrey S, Carruthers A, et al. Injecting botulinum toxin at different depths is not effective for the correction of eyebrow asymmetry. Dermatol Surg. 2015;41(suppl 1):S82-S87.
- Steinsapir KD, Rootman D, Wulc A, et al. Cosmetic microdroplet botulinum toxin A forehead lift: a new treatment paradigm. Ophthal Plast Reconstr Surg. 2015;31:263-268.
Practice Points
- Botulinum neurotoxin (BoNT) injection is the most popular nonsurgical aesthetic procedure available of which there are currently 4 products approved by the US Food and Drug Administration.
- A variety of new BoNT products with unique properties and formulations are currently being studied, some of which are already available for clinical use in foreign markets.
- Administration technique and novel product delivery methods also can be utilized to minimize pain and maximize aesthetic outcomes.
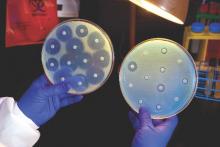
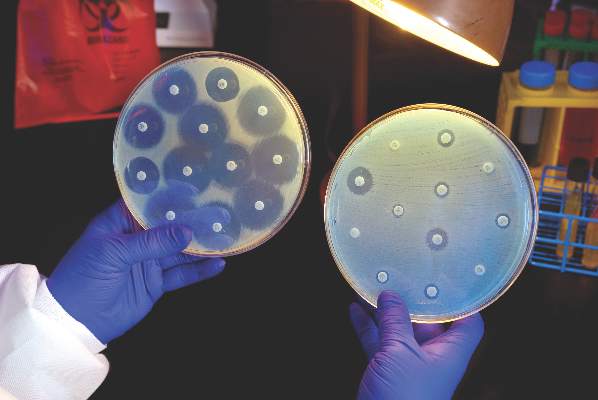
Hospitals increase CRE risk when they share patients
The more hospitals share patients, the more likely they are to have a problem with carbapenem-resistant Enterobacteriaceae (CRE), especially if long-term acute care hospitals (LTACHs) are in the mix, according to a state-wide investigation from Illinois.
Greater hospital centrality was independently associated with higher rates overall, and sharing four or more patients with a long-term acute care hospital (LTACH) in the 3-month study window doubled the rate of CRE cases.
Although it’s possible that was because of chance (P = 0.11), the link between LTACHs and CRE “is consistent with prior analyses that have shown the central role LTACHs have in” spreading the organism, said the researchers, led by Michael Ray of the Illinois Department of Public Health (Clin Infect Dis. 2016 Aug 2. pii: ciw461).
Patients often spend weeks in LTACH facilities for ongoing, serious health problems. The severity of illness, long stay, and sometimes chronic antibiotic use increase the risk of CRE exposure, and the team found that many LTACH patients are colonized.
“These findings have immediate public health implications. … Early interventions should be focused on the most connected facilities, as well as those with strong connections to LTACHs.” When one hospital has an outbreak, facilities that share its patients need to swing into action screening new admissions and taking other steps to prevent regional spread, the team said.
Meanwhile, “state-wide patient-sharing data, which are now increasingly available through sources like the Healthcare Cost and Utilization Project, provide an important way to assess hospital risk of CRE exposure based on its position in regional patient-sharing networks,” they noted. “Public health can play a critical role in identifying tightly connected hospitals and educating personnel at such facilities about their risk and need for enhanced infection control interventions.”
The team came to their conclusions after linking Illinois’ drug-resistant organisms registry with admissions data for 185 hospitals. About half reported at least one CRE case over 3 months, with a mean of 3.5 cases per hospital.
There was an average of 64 patient-sharing connections per facility, with a minimum of one connection and a maximum of 145 connections. Each additional patient two hospitals shared corresponded to a 3% increase in the CRE rate in urban facilities and a 6% increase in rural ones. The investigators didn’t explain the discrepancy, except to note that rural areas don’t have LTACHs.
Almost two-thirds of hospitals reporting CRE were in Chicago-area counties; almost half had shared at least one patient with an LTACH, and 21% had shared four or more.
CRE cases were an average of 64 years old, and equally distributed between men and women and black and white patients.
The Centers for Disease Control and Prevention funded the work. The authors had no disclosures.
The more hospitals share patients, the more likely they are to have a problem with carbapenem-resistant Enterobacteriaceae (CRE), especially if long-term acute care hospitals (LTACHs) are in the mix, according to a state-wide investigation from Illinois.
Greater hospital centrality was independently associated with higher rates overall, and sharing four or more patients with a long-term acute care hospital (LTACH) in the 3-month study window doubled the rate of CRE cases.
Although it’s possible that was because of chance (P = 0.11), the link between LTACHs and CRE “is consistent with prior analyses that have shown the central role LTACHs have in” spreading the organism, said the researchers, led by Michael Ray of the Illinois Department of Public Health (Clin Infect Dis. 2016 Aug 2. pii: ciw461).
Patients often spend weeks in LTACH facilities for ongoing, serious health problems. The severity of illness, long stay, and sometimes chronic antibiotic use increase the risk of CRE exposure, and the team found that many LTACH patients are colonized.
“These findings have immediate public health implications. … Early interventions should be focused on the most connected facilities, as well as those with strong connections to LTACHs.” When one hospital has an outbreak, facilities that share its patients need to swing into action screening new admissions and taking other steps to prevent regional spread, the team said.
Meanwhile, “state-wide patient-sharing data, which are now increasingly available through sources like the Healthcare Cost and Utilization Project, provide an important way to assess hospital risk of CRE exposure based on its position in regional patient-sharing networks,” they noted. “Public health can play a critical role in identifying tightly connected hospitals and educating personnel at such facilities about their risk and need for enhanced infection control interventions.”
The team came to their conclusions after linking Illinois’ drug-resistant organisms registry with admissions data for 185 hospitals. About half reported at least one CRE case over 3 months, with a mean of 3.5 cases per hospital.
There was an average of 64 patient-sharing connections per facility, with a minimum of one connection and a maximum of 145 connections. Each additional patient two hospitals shared corresponded to a 3% increase in the CRE rate in urban facilities and a 6% increase in rural ones. The investigators didn’t explain the discrepancy, except to note that rural areas don’t have LTACHs.
Almost two-thirds of hospitals reporting CRE were in Chicago-area counties; almost half had shared at least one patient with an LTACH, and 21% had shared four or more.
CRE cases were an average of 64 years old, and equally distributed between men and women and black and white patients.
The Centers for Disease Control and Prevention funded the work. The authors had no disclosures.
The more hospitals share patients, the more likely they are to have a problem with carbapenem-resistant Enterobacteriaceae (CRE), especially if long-term acute care hospitals (LTACHs) are in the mix, according to a state-wide investigation from Illinois.
Greater hospital centrality was independently associated with higher rates overall, and sharing four or more patients with a long-term acute care hospital (LTACH) in the 3-month study window doubled the rate of CRE cases.
Although it’s possible that was because of chance (P = 0.11), the link between LTACHs and CRE “is consistent with prior analyses that have shown the central role LTACHs have in” spreading the organism, said the researchers, led by Michael Ray of the Illinois Department of Public Health (Clin Infect Dis. 2016 Aug 2. pii: ciw461).
Patients often spend weeks in LTACH facilities for ongoing, serious health problems. The severity of illness, long stay, and sometimes chronic antibiotic use increase the risk of CRE exposure, and the team found that many LTACH patients are colonized.
“These findings have immediate public health implications. … Early interventions should be focused on the most connected facilities, as well as those with strong connections to LTACHs.” When one hospital has an outbreak, facilities that share its patients need to swing into action screening new admissions and taking other steps to prevent regional spread, the team said.
Meanwhile, “state-wide patient-sharing data, which are now increasingly available through sources like the Healthcare Cost and Utilization Project, provide an important way to assess hospital risk of CRE exposure based on its position in regional patient-sharing networks,” they noted. “Public health can play a critical role in identifying tightly connected hospitals and educating personnel at such facilities about their risk and need for enhanced infection control interventions.”
The team came to their conclusions after linking Illinois’ drug-resistant organisms registry with admissions data for 185 hospitals. About half reported at least one CRE case over 3 months, with a mean of 3.5 cases per hospital.
There was an average of 64 patient-sharing connections per facility, with a minimum of one connection and a maximum of 145 connections. Each additional patient two hospitals shared corresponded to a 3% increase in the CRE rate in urban facilities and a 6% increase in rural ones. The investigators didn’t explain the discrepancy, except to note that rural areas don’t have LTACHs.
Almost two-thirds of hospitals reporting CRE were in Chicago-area counties; almost half had shared at least one patient with an LTACH, and 21% had shared four or more.
CRE cases were an average of 64 years old, and equally distributed between men and women and black and white patients.
The Centers for Disease Control and Prevention funded the work. The authors had no disclosures.
FROM CLINICAL INFECTIOUS DISEASES
Key clinical point: The more hospitals share patients, the more likely they are to have a problem with CRE, especially if long-term acute care hospitals are in the mix.
Major finding: Sharing four or more patients with a long-term acute care hospital in the 3-month study window doubled the rate of CRE cases (P = 0.11).
Data source: 185 Illinois hospitals.
Disclosures: The Centers for Disease Control and Prevention funded the work. The authors had no disclosures.
Proper Wound Management: How to Work With Patients
What does your patient need to know at the first visit?
A thorough patient history is imperative for proper diagnosis of wounds, thus detailed information on the onset, duration, temporality, modifying factors, symptoms, and attempted treatments should be provided. Associated comorbidities that may influence wound healing, such as diabetes mellitus or connective tissue diseases, must be considered when formulating a treatment regimen. Patients should disclose current medications, as certain medications (eg, vascular endothelial growth factor inhibitors) may decrease vascularization or soft tissue matrix regeneration, further complicating the wound healing process. All patients should have a basic understanding of the cause of their wound to have realistic expectations of the prognosis.
What are your go-to treatments?
Treatment ultimately depends on the cause of the wound. In general, proper healing requires a wound bed that is well vascularized and moistened without devitalized tissue or bacterial colonization. Wound dressings should be utilized to reduce dead space, control exudate, prevent bacterial overgrowth, and ensure proper fluid balance. Maintaining good overall health promotes proper healing. Thus, any relevant underlying medical conditions should be properly managed (eg, glycemic control for diabetic patients, management of fluid overload in patients with congestive heart failure).
When treating wounds, it is important to consider several factors. Although all wounds are colonized with microbes, not all wounds are infected. Thus, antibiotic therapy is not necessary for all wounds and should only be used to treat wounds that are clinically infected. Rule out pyoderma gangrenosum prior to wound debridement, as the associated pathergic response will notably worsen the ulcer. Wound dressings have an impact on the speed of wound healing, strength of repaired skin, and cosmetic appearance. Because no single dressing is perfect for all wounds, physicians should use their discretion when determining the type of wound dressing necessary.
Certain wounds require specific treatments. Off-loading and compression dressings/garments are the main components involved in the treatment of pressure ulcers. Protective wound care in conjunction with glycemic control is imperative for diabetic ulcers. Often, the causes of wounds are multifactorial and may complicate treatment. For instance, it is important to confirm that there is no associated arterial insufficiency before treating venous insufficiency with compression. Furthermore, patients with diabetic ulcers in association with venous insufficiency often have minimal response to hyperbaric oxygen treatment.
Several agents have been implicated to improve wound healing. Timolol, a topically applied beta-blocker, may promote keratinocyte migration and epithelialization of chronic refractory wounds. Recombinant human growth factors, most notably becaplermin (a platelet-derived growth factor), have been developed to promote cellular proliferation and angiogenesis, thereby improving healing of chronic wounds. Wounds that have devitalized tissue or contamination require debridement prior to further management.
How do you keep patients compliant with treatment?
Because recurrence is a common complication of chronic wounds, it is imperative that patients understand the importance of preventive care and follow-up appointments. Additionally, an open patient-physician dialogue may help address potential lifestyle limitations that may complicate wound care treatment. For instance, home care arrangement may be necessary to assist certain patient populations with wound care management.
What do you do if they refuse treatment?
Ultimately, it is hard to enforce treatment if the patient refuses. However, in my experience practicing dermatology, I have found it to be uncommon for patients to refuse treatment without a particular reason. If a patient refuses treatment, try to understand why and then try to alleviate any concerns by clarifying misconceptions and/or recommending alternative therapies.
What resources do you recommend to patients for more information?
Consult the American Academy of Dermatology website (https://www.aad.org/File%20Library/Unassigned/Wound-Dressings_Online-BF-DIR-Summer-2016--FINAL.pdf) for more information.
Additional resources include:
- Diabetic Wound Care (Source: American Podiatric Medical Association)(http://www.apma.org/Learn/FootHealth.cfm?ItemNumber=981)
- Pyoderma Gangrenosum (Source: Wound Care Centers)(http://www.woundcarecenters.org/article/wound-types/pyoderma-gangrenosum)
- Take the Pressure Off: A Patient Guide for Preventing and Treating Pressure Ulcers (Source: Association for the Advancement of Wound Care)(http://aawconline.org/wp-content/uploads/2012/04/Take-the-Pressure-Off.pdf)
- Wound Healing Society (http://woundheal.org/home.aspx)
What does your patient need to know at the first visit?
A thorough patient history is imperative for proper diagnosis of wounds, thus detailed information on the onset, duration, temporality, modifying factors, symptoms, and attempted treatments should be provided. Associated comorbidities that may influence wound healing, such as diabetes mellitus or connective tissue diseases, must be considered when formulating a treatment regimen. Patients should disclose current medications, as certain medications (eg, vascular endothelial growth factor inhibitors) may decrease vascularization or soft tissue matrix regeneration, further complicating the wound healing process. All patients should have a basic understanding of the cause of their wound to have realistic expectations of the prognosis.
What are your go-to treatments?
Treatment ultimately depends on the cause of the wound. In general, proper healing requires a wound bed that is well vascularized and moistened without devitalized tissue or bacterial colonization. Wound dressings should be utilized to reduce dead space, control exudate, prevent bacterial overgrowth, and ensure proper fluid balance. Maintaining good overall health promotes proper healing. Thus, any relevant underlying medical conditions should be properly managed (eg, glycemic control for diabetic patients, management of fluid overload in patients with congestive heart failure).
When treating wounds, it is important to consider several factors. Although all wounds are colonized with microbes, not all wounds are infected. Thus, antibiotic therapy is not necessary for all wounds and should only be used to treat wounds that are clinically infected. Rule out pyoderma gangrenosum prior to wound debridement, as the associated pathergic response will notably worsen the ulcer. Wound dressings have an impact on the speed of wound healing, strength of repaired skin, and cosmetic appearance. Because no single dressing is perfect for all wounds, physicians should use their discretion when determining the type of wound dressing necessary.
Certain wounds require specific treatments. Off-loading and compression dressings/garments are the main components involved in the treatment of pressure ulcers. Protective wound care in conjunction with glycemic control is imperative for diabetic ulcers. Often, the causes of wounds are multifactorial and may complicate treatment. For instance, it is important to confirm that there is no associated arterial insufficiency before treating venous insufficiency with compression. Furthermore, patients with diabetic ulcers in association with venous insufficiency often have minimal response to hyperbaric oxygen treatment.
Several agents have been implicated to improve wound healing. Timolol, a topically applied beta-blocker, may promote keratinocyte migration and epithelialization of chronic refractory wounds. Recombinant human growth factors, most notably becaplermin (a platelet-derived growth factor), have been developed to promote cellular proliferation and angiogenesis, thereby improving healing of chronic wounds. Wounds that have devitalized tissue or contamination require debridement prior to further management.
How do you keep patients compliant with treatment?
Because recurrence is a common complication of chronic wounds, it is imperative that patients understand the importance of preventive care and follow-up appointments. Additionally, an open patient-physician dialogue may help address potential lifestyle limitations that may complicate wound care treatment. For instance, home care arrangement may be necessary to assist certain patient populations with wound care management.
What do you do if they refuse treatment?
Ultimately, it is hard to enforce treatment if the patient refuses. However, in my experience practicing dermatology, I have found it to be uncommon for patients to refuse treatment without a particular reason. If a patient refuses treatment, try to understand why and then try to alleviate any concerns by clarifying misconceptions and/or recommending alternative therapies.
What resources do you recommend to patients for more information?
Consult the American Academy of Dermatology website (https://www.aad.org/File%20Library/Unassigned/Wound-Dressings_Online-BF-DIR-Summer-2016--FINAL.pdf) for more information.
Additional resources include:
- Diabetic Wound Care (Source: American Podiatric Medical Association)(http://www.apma.org/Learn/FootHealth.cfm?ItemNumber=981)
- Pyoderma Gangrenosum (Source: Wound Care Centers)(http://www.woundcarecenters.org/article/wound-types/pyoderma-gangrenosum)
- Take the Pressure Off: A Patient Guide for Preventing and Treating Pressure Ulcers (Source: Association for the Advancement of Wound Care)(http://aawconline.org/wp-content/uploads/2012/04/Take-the-Pressure-Off.pdf)
- Wound Healing Society (http://woundheal.org/home.aspx)
What does your patient need to know at the first visit?
A thorough patient history is imperative for proper diagnosis of wounds, thus detailed information on the onset, duration, temporality, modifying factors, symptoms, and attempted treatments should be provided. Associated comorbidities that may influence wound healing, such as diabetes mellitus or connective tissue diseases, must be considered when formulating a treatment regimen. Patients should disclose current medications, as certain medications (eg, vascular endothelial growth factor inhibitors) may decrease vascularization or soft tissue matrix regeneration, further complicating the wound healing process. All patients should have a basic understanding of the cause of their wound to have realistic expectations of the prognosis.
What are your go-to treatments?
Treatment ultimately depends on the cause of the wound. In general, proper healing requires a wound bed that is well vascularized and moistened without devitalized tissue or bacterial colonization. Wound dressings should be utilized to reduce dead space, control exudate, prevent bacterial overgrowth, and ensure proper fluid balance. Maintaining good overall health promotes proper healing. Thus, any relevant underlying medical conditions should be properly managed (eg, glycemic control for diabetic patients, management of fluid overload in patients with congestive heart failure).
When treating wounds, it is important to consider several factors. Although all wounds are colonized with microbes, not all wounds are infected. Thus, antibiotic therapy is not necessary for all wounds and should only be used to treat wounds that are clinically infected. Rule out pyoderma gangrenosum prior to wound debridement, as the associated pathergic response will notably worsen the ulcer. Wound dressings have an impact on the speed of wound healing, strength of repaired skin, and cosmetic appearance. Because no single dressing is perfect for all wounds, physicians should use their discretion when determining the type of wound dressing necessary.
Certain wounds require specific treatments. Off-loading and compression dressings/garments are the main components involved in the treatment of pressure ulcers. Protective wound care in conjunction with glycemic control is imperative for diabetic ulcers. Often, the causes of wounds are multifactorial and may complicate treatment. For instance, it is important to confirm that there is no associated arterial insufficiency before treating venous insufficiency with compression. Furthermore, patients with diabetic ulcers in association with venous insufficiency often have minimal response to hyperbaric oxygen treatment.
Several agents have been implicated to improve wound healing. Timolol, a topically applied beta-blocker, may promote keratinocyte migration and epithelialization of chronic refractory wounds. Recombinant human growth factors, most notably becaplermin (a platelet-derived growth factor), have been developed to promote cellular proliferation and angiogenesis, thereby improving healing of chronic wounds. Wounds that have devitalized tissue or contamination require debridement prior to further management.
How do you keep patients compliant with treatment?
Because recurrence is a common complication of chronic wounds, it is imperative that patients understand the importance of preventive care and follow-up appointments. Additionally, an open patient-physician dialogue may help address potential lifestyle limitations that may complicate wound care treatment. For instance, home care arrangement may be necessary to assist certain patient populations with wound care management.
What do you do if they refuse treatment?
Ultimately, it is hard to enforce treatment if the patient refuses. However, in my experience practicing dermatology, I have found it to be uncommon for patients to refuse treatment without a particular reason. If a patient refuses treatment, try to understand why and then try to alleviate any concerns by clarifying misconceptions and/or recommending alternative therapies.
What resources do you recommend to patients for more information?
Consult the American Academy of Dermatology website (https://www.aad.org/File%20Library/Unassigned/Wound-Dressings_Online-BF-DIR-Summer-2016--FINAL.pdf) for more information.
Additional resources include:
- Diabetic Wound Care (Source: American Podiatric Medical Association)(http://www.apma.org/Learn/FootHealth.cfm?ItemNumber=981)
- Pyoderma Gangrenosum (Source: Wound Care Centers)(http://www.woundcarecenters.org/article/wound-types/pyoderma-gangrenosum)
- Take the Pressure Off: A Patient Guide for Preventing and Treating Pressure Ulcers (Source: Association for the Advancement of Wound Care)(http://aawconline.org/wp-content/uploads/2012/04/Take-the-Pressure-Off.pdf)
- Wound Healing Society (http://woundheal.org/home.aspx)
Combined OCs remain a good choice for teen acne
MINNEAPOLIS – Whether a young female patient has a refractory flare of inflammatory acne, or has a condition that can predispose to androgen excess, using a hormonal approach can be an effective management tool for controlling adolescent acne.
During a presentation at the annual meeting of the Society for Pediatric Dermatology, Dr. Diane Thiboutot outlined tips and tricks for optimizing hormonal therapy for acne in teens, and referred to the new acne treatment guidelines from the American Academy of Dermatology, which clarify when to treat with hormones, which to choose, and when further testing might be indicated.
The full range of hormonal therapy options for acne can include oral contraceptives, which block ovarian hormone production; antiandrogens such as spironolactone, and the less commonly used flutamide, which blocks the effects of androgen on the skin; and glucocorticoids, which block adrenal production.
The 2016 guidelines recommend oral contraceptives as an effective treatment for inflammatory acne in females (J Am Acad Dermatol. 2016 May;74[5]; 945-973.e33). Combined oral contraceptives (COCs) reduce serum androgens, and reduce free testosterone by increasing sex hormone binding globulin production, thus reducing sebum production. “The only things that really decrease sebum are oral contraceptives in women, and isotretinoin,” said Dr. Thiboutot, professor of dermatology at Penn State University, Hershey.
For most female adolescents with acne, hormonal testing is not indicated. The AAD guidelines recommend laboratory evaluation for younger patients with acne who have clinical signs of androgen excess, such as early onset body odor and axillary and/or pubic hair, accelerated growth, advanced bone age, or early genital maturation. Just obtaining a hand film for bone age and mapping growth against a growth chart can be a good initial screening tool when considering whether to perform hormonal testing, she noted.
For postpubertal females in whom polycystic ovary syndrome (PCOS) or other hyperandrogenic states are suspected, hormonal testing is indicated in the presence of the clinical signs of infrequent menses and infertility, hirsutism, truncal obesity, androgenetic alopecia, polycystic ovaries, or clitoromegaly.
In searching for an endocrine disorder, Dr. Thiboutot recommends checking total and free testosterone, luteinizing hormone/follicle stimulating hormone ratio, 17-hydroxyprogesterone levels, and dehydroepiandrosterone (DHEA-S) levels. These tests should be performed at least 6 weeks after the patient has been off hormonal contraception, and should be done during the menstrual period, or during the week prior to menses, in order to avoid ovulation-related hormonal changes.
Lab findings consistent with congenital adrenal hyperplasia include elevated serum DHEA-S, together with elevated 17-hydroxyprogesterone or testosterone. A PCOS diagnosis can be made in adolescent females if there is clinical or laboratory evidence of hyperandrogenism with concomitant persistent oligomenorrhea.
Acne related to hyperandrogenism may respond well to oral contraceptives, but COCs can also be an effective alternative to repeated courses of isotretinoin and antibiotics, as well as an effective adjunct to topical therapy, Dr. Thiboutot said.
When beginning a patient on oral contraceptives, it’s not necessary to perform a pelvic exam or obtain a Pap smear before initiating the COC, but it is important to obtain a thorough medical history and an accurate blood pressure measurement at the outset, she noted. The World Health Organization (WHO) has established recommendations outlining contraindications to COC use, also identifying populations in whom COCs should be used with caution, and who should be monitored.
Headaches are a condition frequently seen among healthy teens and young women, and one for which the WHO advises caution. There are concerns that women with migraines may be at increased risk of stroke if they take COCs, but the overall risk is low, and the American College of Obstetricians and Gynecologists (ACOG) advises that COCs can be considered for women younger than 35 with migraines if they have no focal neurologic signs, are nonsmokers, and are otherwise healthy, Dr. Thiboutot added.
A large Food and Drug Administration–sponsored retrospective cohort study examined the risk of venous thromboembolism in contraceptive users. In April 2012, the FDA concluded that though the risk of blood clots may be higher for those on hormonal contraception methods than for those who are not using them, the risk of blood clots during pregnancy and the postpartum period is higher than the thromboembolism risk for contraceptive users.
Regarding the potential for antibiotics to reduce contraceptive efficacy, Dr. Thiboutot said,“it’s okay to use oral contraceptives with antibiotics. There’s a lot of misunderstanding about antibiotics and combined oral contraceptives.” She cited an ACOG practice bulletin that reported that only rifampin has been shown to reduce serum steroid levels when taken with oral contraceptives (Obstet Gynecol. 2006 Jun;107[6]:1453-72).
According to the 2016 AAD guidelines, the use of oral glucocorticoids may be appropriate over the short term when initiating therapy for severe inflammatory acne. “Pharmacokinetic studies have not demonstrated decreased oral contraceptive levels with common antibiotics,” Dr. Thiboutot said.
Spironolactone, according to the new guidelines, is useful for acne in select females. Spironolactone is an androgen receptor and 5a-reductase blocker, and its antiandrogen effects can improve acne. Many patients do well with 25-50 mg twice daily, though breast tenderness and menstrual irregularities are commonly seen side effects, she noted. If a woman taking spironolactone becomes pregnant, there’s a risk of hypospadias for a male fetus.
Though spironolactone carries a boxed warning because of tumorigenicity observed in animal studies, Dr. Thiboutot said that a large Danish study searched for any association between breast, uterine, or ovarian cancers and spironolactone use. Among the 2.3 million women studied, no increased association was seen (Cancer Epidemiol. 2013 Dec;37:870-5).
She also noted that there’s “low usefulness in monitoring potassium levels in young healthy women on spironolactone.” She cited a study that compared 974 healthy young women taking spironolactone with 1,165 women who were not on spironolactone, which found that the hyperkalemia rate of 0.72% among those on spironolactone was equivalent to the 0.76% baseline rate of hyperkalemia in the young, healthy female population (JAMA Dermatol. 2015;151[9];941-944).
Oral corticosteroids for acne, Dr. Thiboutot said, should be reserved to quiet a severe bout of inflammatory acne while standard therapies are being initiated.
She reported being an investigator or a consultant for a number of pharmaceutical companies.
On Twitter @karioakes
MINNEAPOLIS – Whether a young female patient has a refractory flare of inflammatory acne, or has a condition that can predispose to androgen excess, using a hormonal approach can be an effective management tool for controlling adolescent acne.
During a presentation at the annual meeting of the Society for Pediatric Dermatology, Dr. Diane Thiboutot outlined tips and tricks for optimizing hormonal therapy for acne in teens, and referred to the new acne treatment guidelines from the American Academy of Dermatology, which clarify when to treat with hormones, which to choose, and when further testing might be indicated.
The full range of hormonal therapy options for acne can include oral contraceptives, which block ovarian hormone production; antiandrogens such as spironolactone, and the less commonly used flutamide, which blocks the effects of androgen on the skin; and glucocorticoids, which block adrenal production.
The 2016 guidelines recommend oral contraceptives as an effective treatment for inflammatory acne in females (J Am Acad Dermatol. 2016 May;74[5]; 945-973.e33). Combined oral contraceptives (COCs) reduce serum androgens, and reduce free testosterone by increasing sex hormone binding globulin production, thus reducing sebum production. “The only things that really decrease sebum are oral contraceptives in women, and isotretinoin,” said Dr. Thiboutot, professor of dermatology at Penn State University, Hershey.
For most female adolescents with acne, hormonal testing is not indicated. The AAD guidelines recommend laboratory evaluation for younger patients with acne who have clinical signs of androgen excess, such as early onset body odor and axillary and/or pubic hair, accelerated growth, advanced bone age, or early genital maturation. Just obtaining a hand film for bone age and mapping growth against a growth chart can be a good initial screening tool when considering whether to perform hormonal testing, she noted.
For postpubertal females in whom polycystic ovary syndrome (PCOS) or other hyperandrogenic states are suspected, hormonal testing is indicated in the presence of the clinical signs of infrequent menses and infertility, hirsutism, truncal obesity, androgenetic alopecia, polycystic ovaries, or clitoromegaly.
In searching for an endocrine disorder, Dr. Thiboutot recommends checking total and free testosterone, luteinizing hormone/follicle stimulating hormone ratio, 17-hydroxyprogesterone levels, and dehydroepiandrosterone (DHEA-S) levels. These tests should be performed at least 6 weeks after the patient has been off hormonal contraception, and should be done during the menstrual period, or during the week prior to menses, in order to avoid ovulation-related hormonal changes.
Lab findings consistent with congenital adrenal hyperplasia include elevated serum DHEA-S, together with elevated 17-hydroxyprogesterone or testosterone. A PCOS diagnosis can be made in adolescent females if there is clinical or laboratory evidence of hyperandrogenism with concomitant persistent oligomenorrhea.
Acne related to hyperandrogenism may respond well to oral contraceptives, but COCs can also be an effective alternative to repeated courses of isotretinoin and antibiotics, as well as an effective adjunct to topical therapy, Dr. Thiboutot said.
When beginning a patient on oral contraceptives, it’s not necessary to perform a pelvic exam or obtain a Pap smear before initiating the COC, but it is important to obtain a thorough medical history and an accurate blood pressure measurement at the outset, she noted. The World Health Organization (WHO) has established recommendations outlining contraindications to COC use, also identifying populations in whom COCs should be used with caution, and who should be monitored.
Headaches are a condition frequently seen among healthy teens and young women, and one for which the WHO advises caution. There are concerns that women with migraines may be at increased risk of stroke if they take COCs, but the overall risk is low, and the American College of Obstetricians and Gynecologists (ACOG) advises that COCs can be considered for women younger than 35 with migraines if they have no focal neurologic signs, are nonsmokers, and are otherwise healthy, Dr. Thiboutot added.
A large Food and Drug Administration–sponsored retrospective cohort study examined the risk of venous thromboembolism in contraceptive users. In April 2012, the FDA concluded that though the risk of blood clots may be higher for those on hormonal contraception methods than for those who are not using them, the risk of blood clots during pregnancy and the postpartum period is higher than the thromboembolism risk for contraceptive users.
Regarding the potential for antibiotics to reduce contraceptive efficacy, Dr. Thiboutot said,“it’s okay to use oral contraceptives with antibiotics. There’s a lot of misunderstanding about antibiotics and combined oral contraceptives.” She cited an ACOG practice bulletin that reported that only rifampin has been shown to reduce serum steroid levels when taken with oral contraceptives (Obstet Gynecol. 2006 Jun;107[6]:1453-72).
According to the 2016 AAD guidelines, the use of oral glucocorticoids may be appropriate over the short term when initiating therapy for severe inflammatory acne. “Pharmacokinetic studies have not demonstrated decreased oral contraceptive levels with common antibiotics,” Dr. Thiboutot said.
Spironolactone, according to the new guidelines, is useful for acne in select females. Spironolactone is an androgen receptor and 5a-reductase blocker, and its antiandrogen effects can improve acne. Many patients do well with 25-50 mg twice daily, though breast tenderness and menstrual irregularities are commonly seen side effects, she noted. If a woman taking spironolactone becomes pregnant, there’s a risk of hypospadias for a male fetus.
Though spironolactone carries a boxed warning because of tumorigenicity observed in animal studies, Dr. Thiboutot said that a large Danish study searched for any association between breast, uterine, or ovarian cancers and spironolactone use. Among the 2.3 million women studied, no increased association was seen (Cancer Epidemiol. 2013 Dec;37:870-5).
She also noted that there’s “low usefulness in monitoring potassium levels in young healthy women on spironolactone.” She cited a study that compared 974 healthy young women taking spironolactone with 1,165 women who were not on spironolactone, which found that the hyperkalemia rate of 0.72% among those on spironolactone was equivalent to the 0.76% baseline rate of hyperkalemia in the young, healthy female population (JAMA Dermatol. 2015;151[9];941-944).
Oral corticosteroids for acne, Dr. Thiboutot said, should be reserved to quiet a severe bout of inflammatory acne while standard therapies are being initiated.
She reported being an investigator or a consultant for a number of pharmaceutical companies.
On Twitter @karioakes
MINNEAPOLIS – Whether a young female patient has a refractory flare of inflammatory acne, or has a condition that can predispose to androgen excess, using a hormonal approach can be an effective management tool for controlling adolescent acne.
During a presentation at the annual meeting of the Society for Pediatric Dermatology, Dr. Diane Thiboutot outlined tips and tricks for optimizing hormonal therapy for acne in teens, and referred to the new acne treatment guidelines from the American Academy of Dermatology, which clarify when to treat with hormones, which to choose, and when further testing might be indicated.
The full range of hormonal therapy options for acne can include oral contraceptives, which block ovarian hormone production; antiandrogens such as spironolactone, and the less commonly used flutamide, which blocks the effects of androgen on the skin; and glucocorticoids, which block adrenal production.
The 2016 guidelines recommend oral contraceptives as an effective treatment for inflammatory acne in females (J Am Acad Dermatol. 2016 May;74[5]; 945-973.e33). Combined oral contraceptives (COCs) reduce serum androgens, and reduce free testosterone by increasing sex hormone binding globulin production, thus reducing sebum production. “The only things that really decrease sebum are oral contraceptives in women, and isotretinoin,” said Dr. Thiboutot, professor of dermatology at Penn State University, Hershey.
For most female adolescents with acne, hormonal testing is not indicated. The AAD guidelines recommend laboratory evaluation for younger patients with acne who have clinical signs of androgen excess, such as early onset body odor and axillary and/or pubic hair, accelerated growth, advanced bone age, or early genital maturation. Just obtaining a hand film for bone age and mapping growth against a growth chart can be a good initial screening tool when considering whether to perform hormonal testing, she noted.
For postpubertal females in whom polycystic ovary syndrome (PCOS) or other hyperandrogenic states are suspected, hormonal testing is indicated in the presence of the clinical signs of infrequent menses and infertility, hirsutism, truncal obesity, androgenetic alopecia, polycystic ovaries, or clitoromegaly.
In searching for an endocrine disorder, Dr. Thiboutot recommends checking total and free testosterone, luteinizing hormone/follicle stimulating hormone ratio, 17-hydroxyprogesterone levels, and dehydroepiandrosterone (DHEA-S) levels. These tests should be performed at least 6 weeks after the patient has been off hormonal contraception, and should be done during the menstrual period, or during the week prior to menses, in order to avoid ovulation-related hormonal changes.
Lab findings consistent with congenital adrenal hyperplasia include elevated serum DHEA-S, together with elevated 17-hydroxyprogesterone or testosterone. A PCOS diagnosis can be made in adolescent females if there is clinical or laboratory evidence of hyperandrogenism with concomitant persistent oligomenorrhea.
Acne related to hyperandrogenism may respond well to oral contraceptives, but COCs can also be an effective alternative to repeated courses of isotretinoin and antibiotics, as well as an effective adjunct to topical therapy, Dr. Thiboutot said.
When beginning a patient on oral contraceptives, it’s not necessary to perform a pelvic exam or obtain a Pap smear before initiating the COC, but it is important to obtain a thorough medical history and an accurate blood pressure measurement at the outset, she noted. The World Health Organization (WHO) has established recommendations outlining contraindications to COC use, also identifying populations in whom COCs should be used with caution, and who should be monitored.
Headaches are a condition frequently seen among healthy teens and young women, and one for which the WHO advises caution. There are concerns that women with migraines may be at increased risk of stroke if they take COCs, but the overall risk is low, and the American College of Obstetricians and Gynecologists (ACOG) advises that COCs can be considered for women younger than 35 with migraines if they have no focal neurologic signs, are nonsmokers, and are otherwise healthy, Dr. Thiboutot added.
A large Food and Drug Administration–sponsored retrospective cohort study examined the risk of venous thromboembolism in contraceptive users. In April 2012, the FDA concluded that though the risk of blood clots may be higher for those on hormonal contraception methods than for those who are not using them, the risk of blood clots during pregnancy and the postpartum period is higher than the thromboembolism risk for contraceptive users.
Regarding the potential for antibiotics to reduce contraceptive efficacy, Dr. Thiboutot said,“it’s okay to use oral contraceptives with antibiotics. There’s a lot of misunderstanding about antibiotics and combined oral contraceptives.” She cited an ACOG practice bulletin that reported that only rifampin has been shown to reduce serum steroid levels when taken with oral contraceptives (Obstet Gynecol. 2006 Jun;107[6]:1453-72).
According to the 2016 AAD guidelines, the use of oral glucocorticoids may be appropriate over the short term when initiating therapy for severe inflammatory acne. “Pharmacokinetic studies have not demonstrated decreased oral contraceptive levels with common antibiotics,” Dr. Thiboutot said.
Spironolactone, according to the new guidelines, is useful for acne in select females. Spironolactone is an androgen receptor and 5a-reductase blocker, and its antiandrogen effects can improve acne. Many patients do well with 25-50 mg twice daily, though breast tenderness and menstrual irregularities are commonly seen side effects, she noted. If a woman taking spironolactone becomes pregnant, there’s a risk of hypospadias for a male fetus.
Though spironolactone carries a boxed warning because of tumorigenicity observed in animal studies, Dr. Thiboutot said that a large Danish study searched for any association between breast, uterine, or ovarian cancers and spironolactone use. Among the 2.3 million women studied, no increased association was seen (Cancer Epidemiol. 2013 Dec;37:870-5).
She also noted that there’s “low usefulness in monitoring potassium levels in young healthy women on spironolactone.” She cited a study that compared 974 healthy young women taking spironolactone with 1,165 women who were not on spironolactone, which found that the hyperkalemia rate of 0.72% among those on spironolactone was equivalent to the 0.76% baseline rate of hyperkalemia in the young, healthy female population (JAMA Dermatol. 2015;151[9];941-944).
Oral corticosteroids for acne, Dr. Thiboutot said, should be reserved to quiet a severe bout of inflammatory acne while standard therapies are being initiated.
She reported being an investigator or a consultant for a number of pharmaceutical companies.
On Twitter @karioakes
EXPERT ANALYSIS FROM THE SPD ANNUAL MEETING
Hat-Wearing Patterns in Spectators Attending Baseball Games: A 10-Year Retrospective Comparison
Spectators at baseball games may be exposed to excess solar UV radiation (UVR), which has been linked to the development of both melanoma and nonmelanoma skin cancers.1,2 Although baseball hats traditionally are worn to demonstrate team support, they also may provide some sun protection for the head and face where skin cancers are commonly found.
The importance of protecting the skin from solar UVR has led to sun-protection programs and community education as well as efforts to evaluate the impact of these programs. Major League Baseball (MLB) has partnered with the American Academy of Dermatology since 1999 to promote the importance of sun protection and raise skin cancer awareness through its Play Sun Smart program.3 A study conducted 10 years ago (N=2030) evaluated hat use in spectators at MLB games and noted that less than half of all spectators in seating sections exposed to direct sunlight wore hats.4 The purpose of the current study was to evaluate how public education about sun protection has impacted the use of hats by spectators at MLB games in 2015 compared to the prior study in 2006.
Methods
Data were collected during a 3-game series (2 day games, 1 night game) in August 2015 in New York, New York. During one of the day games, 18,000 fans received a free wide-brimmed hat. High-resolution digital photographs of seating sections were obtained using a camera with a 300-mm lens. Using the same methodology as the prior study,4 sunny and shaded seating sections were photographed during all 3 games (Figure). Photographs of each section were analyzed by an independent reviewer using a high-resolution computer screen. Spectators wearing head coverings—baseball hats, visors, or hats with circumferential brims—were defined as using hats. The number of spectators wearing hats versus not wearing hats was recorded for all identical sections of interest. Bleacher seating was analyzed separately, as spectators presumably knew in advance of the continuous direct sun exposure during day games, and a subset of young children in the bleachers (<10 years of age) also was assessed. A continuously sunny section also was evaluated at the second and sixth innings to see if hats were presumably purchased during exposure. Statistical significance was determined using χ2 tests with P<.05 indicating statistical significance.

Results
This analysis consisted of 3539 spectators. In both the sunny and shaded sections of a day game, there were more spectators wearing hats (49% and 37%, respectively)(P<.001) than in the same sections at night games (35% and 29%, respectively)(Table 1). During the day game, more spectators wore hats in the sunny section than in the adjacent shaded section (49% vs 37%; P<.001). Analysis of the same 2 sections during the night game revealed no significant differences.
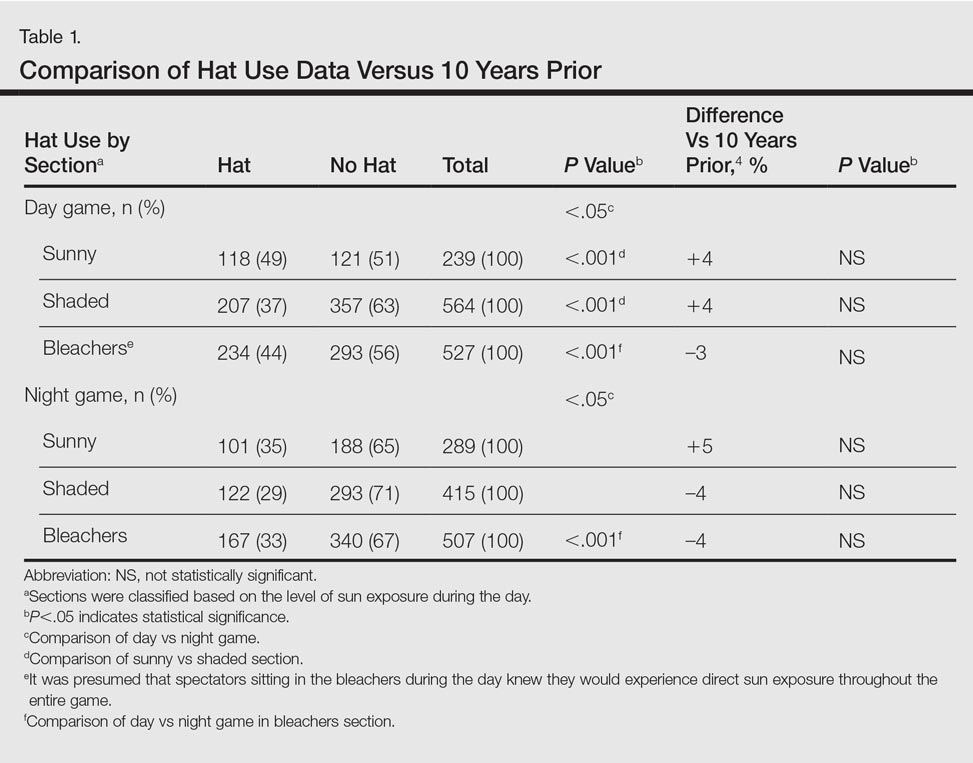
Spectators sitting in the bleachers during a day game who presumably knew to anticipate direct sun exposure showed no significant differences in hat-wearing patterns versus the sunny section (44% vs 49%) but were more likely to wear hats compared to those sitting in the bleachers at the night game (44% vs 33%)(P<.001)(Table 1). There was no significant difference in the number of hats worn by spectators in the sunny section in the second inning (43%) versus the same section after continuous sun exposure at the sixth inning (44%)(Table 2). Significantly more children seated in the bleachers during the day game wore hats compared to adults in the same section (64% vs 42%; P<.001)(Table 3). During the hat giveaway day, significantly more spectators wore hats (the majority of which were the free giveaway hats) across all sections studied (P<.001)(Table 4).

Comment
More than 23 million spectators attended daytime MLB games in 2015, with millions more attending minor league and amateur events.5Although sun-protection messages tend to be well understood and received by society, many choose to ignore them.6
In partnership with the American Academy of Dermatology, the MLB’s Play Sun Smart program has promoted UVR risk awareness at sporting events since 1999.3 Those affiliated with MLB teams also receive annual skin cancer screenings in conjunction with a public education effort in May of each season. However, despite the years of sun-protection education, our study found that less than half of attendees wore hats for UVR protection. In fact, there were no significant differences noted across all of the hat-wearing parameters studied (day vs night game, sunny vs shaded section, sunny section over course of game) between the current study compared to the results from 10 years prior4 (Tables 1 and 2). For spectators in the bleacher section, even presumably knowing in advance that seating would be in the sun did not significantly increase hat-wearing behavior. Although skin cancer rates continue to rise, hat-wearing trends remain stable, revealing a concerning trend.
Increased availability of sunscreen has led to improved sun-protective behaviors in many populations.7 In our study, the free hat giveaway had the greatest impact on hat wearing, which suggests that improved availability and access to hats can lead to an important opportunity for sun-protection programs to partner with hat manufacturers to augment their use and protective impact.
Sun avoidance during childhood and adolescence has been shown to decrease the risk for melanoma.1 Young children had the highest rate of hat usage in the current study, possibly due to parental example or dictates. Research has shown the importance of role models in promoting sun safety to young children,8,9 so perhaps use of hats by parents or MLB players contributed to the hat-wearing behavior observed in this subpopulation.
Given the limited change observed in hat-wearing behaviors over the last decade, a knowledge and behavioral gap appears to exist that may be able to be exploited to enhance future sun protection. Also, based on our findings, the MLB and other sun-protection education campaigns may wish to augment their UVR protective messages by offering hat giveaways, which appear to have a notable impact.
Acknowledgment
The authors thank Jessie Skapik, BS (New York, New York), for her independent review of the spectator photographs.
References
1. Rigel DS. Cutaneous ultraviolet exposure and its relationship to the development of skin cancer. J Am Acad Dermatol. 2008;58(5, suppl 2):S129-S132.
2. Lim HW, James WD, Rigel DS, et al. Adverse effects of ultraviolet radiation from the use of indoor tanning equipment: time to ban the tan. J Am Acad Dermatol. 2011;64:893-902.
3. Play Sun Smart. American Academy of Dermatology website. https://www.aad.org/public/spot-skin-cancer/programs/play-sun-smart. Accessed August 25, 2016.
4. Rigel AS, Lebwohl MG. Hat-wearing patterns in persons attending baseball games. J Am Acad Dermatol. 2006;54:918-919.
5. MLB attendance report - 2016. ESPN website. www.espn.go.com/mlb/attendance. Accessed May 20, 2016.
6. Turner D, Harrison SL, Buettner P, et al. Does being a “SunSmart School” influence hat-wearing compliance? an ecological study of hat-wearing rates at Australian primary schools in a region of high sun exposure [published online December 29, 2013]. Prev Med. 2014;60:107-114.
7. Dubas LE, Adams BB. Sunscreen use and availability among female collegiate athletes [published online February 3, 2012]. J Am Acad Dermatol. 2012;67:876.e1-876.e6.
8. O’Riodran DL, Geller AC, Brooks DR, et al. Sunburn reduction through parental role modeling and sunscreen vigilance. J Pediatr. 2003;142:67-72.
9. Turrisi R, Hillhouse J, Heavin S, et al. Examination of the short-term efficacy of a parent-based intervention to prevent skin cancer. J Behav Med. 2004;27:393-412.
Spectators at baseball games may be exposed to excess solar UV radiation (UVR), which has been linked to the development of both melanoma and nonmelanoma skin cancers.1,2 Although baseball hats traditionally are worn to demonstrate team support, they also may provide some sun protection for the head and face where skin cancers are commonly found.
The importance of protecting the skin from solar UVR has led to sun-protection programs and community education as well as efforts to evaluate the impact of these programs. Major League Baseball (MLB) has partnered with the American Academy of Dermatology since 1999 to promote the importance of sun protection and raise skin cancer awareness through its Play Sun Smart program.3 A study conducted 10 years ago (N=2030) evaluated hat use in spectators at MLB games and noted that less than half of all spectators in seating sections exposed to direct sunlight wore hats.4 The purpose of the current study was to evaluate how public education about sun protection has impacted the use of hats by spectators at MLB games in 2015 compared to the prior study in 2006.
Methods
Data were collected during a 3-game series (2 day games, 1 night game) in August 2015 in New York, New York. During one of the day games, 18,000 fans received a free wide-brimmed hat. High-resolution digital photographs of seating sections were obtained using a camera with a 300-mm lens. Using the same methodology as the prior study,4 sunny and shaded seating sections were photographed during all 3 games (Figure). Photographs of each section were analyzed by an independent reviewer using a high-resolution computer screen. Spectators wearing head coverings—baseball hats, visors, or hats with circumferential brims—were defined as using hats. The number of spectators wearing hats versus not wearing hats was recorded for all identical sections of interest. Bleacher seating was analyzed separately, as spectators presumably knew in advance of the continuous direct sun exposure during day games, and a subset of young children in the bleachers (<10 years of age) also was assessed. A continuously sunny section also was evaluated at the second and sixth innings to see if hats were presumably purchased during exposure. Statistical significance was determined using χ2 tests with P<.05 indicating statistical significance.

Results
This analysis consisted of 3539 spectators. In both the sunny and shaded sections of a day game, there were more spectators wearing hats (49% and 37%, respectively)(P<.001) than in the same sections at night games (35% and 29%, respectively)(Table 1). During the day game, more spectators wore hats in the sunny section than in the adjacent shaded section (49% vs 37%; P<.001). Analysis of the same 2 sections during the night game revealed no significant differences.

Spectators sitting in the bleachers during a day game who presumably knew to anticipate direct sun exposure showed no significant differences in hat-wearing patterns versus the sunny section (44% vs 49%) but were more likely to wear hats compared to those sitting in the bleachers at the night game (44% vs 33%)(P<.001)(Table 1). There was no significant difference in the number of hats worn by spectators in the sunny section in the second inning (43%) versus the same section after continuous sun exposure at the sixth inning (44%)(Table 2). Significantly more children seated in the bleachers during the day game wore hats compared to adults in the same section (64% vs 42%; P<.001)(Table 3). During the hat giveaway day, significantly more spectators wore hats (the majority of which were the free giveaway hats) across all sections studied (P<.001)(Table 4).



Comment
More than 23 million spectators attended daytime MLB games in 2015, with millions more attending minor league and amateur events.5Although sun-protection messages tend to be well understood and received by society, many choose to ignore them.6
In partnership with the American Academy of Dermatology, the MLB’s Play Sun Smart program has promoted UVR risk awareness at sporting events since 1999.3 Those affiliated with MLB teams also receive annual skin cancer screenings in conjunction with a public education effort in May of each season. However, despite the years of sun-protection education, our study found that less than half of attendees wore hats for UVR protection. In fact, there were no significant differences noted across all of the hat-wearing parameters studied (day vs night game, sunny vs shaded section, sunny section over course of game) between the current study compared to the results from 10 years prior4 (Tables 1 and 2). For spectators in the bleacher section, even presumably knowing in advance that seating would be in the sun did not significantly increase hat-wearing behavior. Although skin cancer rates continue to rise, hat-wearing trends remain stable, revealing a concerning trend.
Increased availability of sunscreen has led to improved sun-protective behaviors in many populations.7 In our study, the free hat giveaway had the greatest impact on hat wearing, which suggests that improved availability and access to hats can lead to an important opportunity for sun-protection programs to partner with hat manufacturers to augment their use and protective impact.
Sun avoidance during childhood and adolescence has been shown to decrease the risk for melanoma.1 Young children had the highest rate of hat usage in the current study, possibly due to parental example or dictates. Research has shown the importance of role models in promoting sun safety to young children,8,9 so perhaps use of hats by parents or MLB players contributed to the hat-wearing behavior observed in this subpopulation.
Given the limited change observed in hat-wearing behaviors over the last decade, a knowledge and behavioral gap appears to exist that may be able to be exploited to enhance future sun protection. Also, based on our findings, the MLB and other sun-protection education campaigns may wish to augment their UVR protective messages by offering hat giveaways, which appear to have a notable impact.
Acknowledgment
The authors thank Jessie Skapik, BS (New York, New York), for her independent review of the spectator photographs.
Spectators at baseball games may be exposed to excess solar UV radiation (UVR), which has been linked to the development of both melanoma and nonmelanoma skin cancers.1,2 Although baseball hats traditionally are worn to demonstrate team support, they also may provide some sun protection for the head and face where skin cancers are commonly found.
The importance of protecting the skin from solar UVR has led to sun-protection programs and community education as well as efforts to evaluate the impact of these programs. Major League Baseball (MLB) has partnered with the American Academy of Dermatology since 1999 to promote the importance of sun protection and raise skin cancer awareness through its Play Sun Smart program.3 A study conducted 10 years ago (N=2030) evaluated hat use in spectators at MLB games and noted that less than half of all spectators in seating sections exposed to direct sunlight wore hats.4 The purpose of the current study was to evaluate how public education about sun protection has impacted the use of hats by spectators at MLB games in 2015 compared to the prior study in 2006.
Methods
Data were collected during a 3-game series (2 day games, 1 night game) in August 2015 in New York, New York. During one of the day games, 18,000 fans received a free wide-brimmed hat. High-resolution digital photographs of seating sections were obtained using a camera with a 300-mm lens. Using the same methodology as the prior study,4 sunny and shaded seating sections were photographed during all 3 games (Figure). Photographs of each section were analyzed by an independent reviewer using a high-resolution computer screen. Spectators wearing head coverings—baseball hats, visors, or hats with circumferential brims—were defined as using hats. The number of spectators wearing hats versus not wearing hats was recorded for all identical sections of interest. Bleacher seating was analyzed separately, as spectators presumably knew in advance of the continuous direct sun exposure during day games, and a subset of young children in the bleachers (<10 years of age) also was assessed. A continuously sunny section also was evaluated at the second and sixth innings to see if hats were presumably purchased during exposure. Statistical significance was determined using χ2 tests with P<.05 indicating statistical significance.

Results
This analysis consisted of 3539 spectators. In both the sunny and shaded sections of a day game, there were more spectators wearing hats (49% and 37%, respectively)(P<.001) than in the same sections at night games (35% and 29%, respectively)(Table 1). During the day game, more spectators wore hats in the sunny section than in the adjacent shaded section (49% vs 37%; P<.001). Analysis of the same 2 sections during the night game revealed no significant differences.

Spectators sitting in the bleachers during a day game who presumably knew to anticipate direct sun exposure showed no significant differences in hat-wearing patterns versus the sunny section (44% vs 49%) but were more likely to wear hats compared to those sitting in the bleachers at the night game (44% vs 33%)(P<.001)(Table 1). There was no significant difference in the number of hats worn by spectators in the sunny section in the second inning (43%) versus the same section after continuous sun exposure at the sixth inning (44%)(Table 2). Significantly more children seated in the bleachers during the day game wore hats compared to adults in the same section (64% vs 42%; P<.001)(Table 3). During the hat giveaway day, significantly more spectators wore hats (the majority of which were the free giveaway hats) across all sections studied (P<.001)(Table 4).



Comment
More than 23 million spectators attended daytime MLB games in 2015, with millions more attending minor league and amateur events.5Although sun-protection messages tend to be well understood and received by society, many choose to ignore them.6
In partnership with the American Academy of Dermatology, the MLB’s Play Sun Smart program has promoted UVR risk awareness at sporting events since 1999.3 Those affiliated with MLB teams also receive annual skin cancer screenings in conjunction with a public education effort in May of each season. However, despite the years of sun-protection education, our study found that less than half of attendees wore hats for UVR protection. In fact, there were no significant differences noted across all of the hat-wearing parameters studied (day vs night game, sunny vs shaded section, sunny section over course of game) between the current study compared to the results from 10 years prior4 (Tables 1 and 2). For spectators in the bleacher section, even presumably knowing in advance that seating would be in the sun did not significantly increase hat-wearing behavior. Although skin cancer rates continue to rise, hat-wearing trends remain stable, revealing a concerning trend.
Increased availability of sunscreen has led to improved sun-protective behaviors in many populations.7 In our study, the free hat giveaway had the greatest impact on hat wearing, which suggests that improved availability and access to hats can lead to an important opportunity for sun-protection programs to partner with hat manufacturers to augment their use and protective impact.
Sun avoidance during childhood and adolescence has been shown to decrease the risk for melanoma.1 Young children had the highest rate of hat usage in the current study, possibly due to parental example or dictates. Research has shown the importance of role models in promoting sun safety to young children,8,9 so perhaps use of hats by parents or MLB players contributed to the hat-wearing behavior observed in this subpopulation.
Given the limited change observed in hat-wearing behaviors over the last decade, a knowledge and behavioral gap appears to exist that may be able to be exploited to enhance future sun protection. Also, based on our findings, the MLB and other sun-protection education campaigns may wish to augment their UVR protective messages by offering hat giveaways, which appear to have a notable impact.
Acknowledgment
The authors thank Jessie Skapik, BS (New York, New York), for her independent review of the spectator photographs.
References
1. Rigel DS. Cutaneous ultraviolet exposure and its relationship to the development of skin cancer. J Am Acad Dermatol. 2008;58(5, suppl 2):S129-S132.
2. Lim HW, James WD, Rigel DS, et al. Adverse effects of ultraviolet radiation from the use of indoor tanning equipment: time to ban the tan. J Am Acad Dermatol. 2011;64:893-902.
3. Play Sun Smart. American Academy of Dermatology website. https://www.aad.org/public/spot-skin-cancer/programs/play-sun-smart. Accessed August 25, 2016.
4. Rigel AS, Lebwohl MG. Hat-wearing patterns in persons attending baseball games. J Am Acad Dermatol. 2006;54:918-919.
5. MLB attendance report - 2016. ESPN website. www.espn.go.com/mlb/attendance. Accessed May 20, 2016.
6. Turner D, Harrison SL, Buettner P, et al. Does being a “SunSmart School” influence hat-wearing compliance? an ecological study of hat-wearing rates at Australian primary schools in a region of high sun exposure [published online December 29, 2013]. Prev Med. 2014;60:107-114.
7. Dubas LE, Adams BB. Sunscreen use and availability among female collegiate athletes [published online February 3, 2012]. J Am Acad Dermatol. 2012;67:876.e1-876.e6.
8. O’Riodran DL, Geller AC, Brooks DR, et al. Sunburn reduction through parental role modeling and sunscreen vigilance. J Pediatr. 2003;142:67-72.
9. Turrisi R, Hillhouse J, Heavin S, et al. Examination of the short-term efficacy of a parent-based intervention to prevent skin cancer. J Behav Med. 2004;27:393-412.
References
1. Rigel DS. Cutaneous ultraviolet exposure and its relationship to the development of skin cancer. J Am Acad Dermatol. 2008;58(5, suppl 2):S129-S132.
2. Lim HW, James WD, Rigel DS, et al. Adverse effects of ultraviolet radiation from the use of indoor tanning equipment: time to ban the tan. J Am Acad Dermatol. 2011;64:893-902.
3. Play Sun Smart. American Academy of Dermatology website. https://www.aad.org/public/spot-skin-cancer/programs/play-sun-smart. Accessed August 25, 2016.
4. Rigel AS, Lebwohl MG. Hat-wearing patterns in persons attending baseball games. J Am Acad Dermatol. 2006;54:918-919.
5. MLB attendance report - 2016. ESPN website. www.espn.go.com/mlb/attendance. Accessed May 20, 2016.
6. Turner D, Harrison SL, Buettner P, et al. Does being a “SunSmart School” influence hat-wearing compliance? an ecological study of hat-wearing rates at Australian primary schools in a region of high sun exposure [published online December 29, 2013]. Prev Med. 2014;60:107-114.
7. Dubas LE, Adams BB. Sunscreen use and availability among female collegiate athletes [published online February 3, 2012]. J Am Acad Dermatol. 2012;67:876.e1-876.e6.
8. O’Riodran DL, Geller AC, Brooks DR, et al. Sunburn reduction through parental role modeling and sunscreen vigilance. J Pediatr. 2003;142:67-72.
9. Turrisi R, Hillhouse J, Heavin S, et al. Examination of the short-term efficacy of a parent-based intervention to prevent skin cancer. J Behav Med. 2004;27:393-412.
Practice Points
- With less than half of attendees wearing hats to Major League Baseball games, there has been limited change in hat-wearing behavior over the last decade, possibly due to a knowledge or behavioral gap.
- Improved availability and access to hats can lead to improved sun-protective behaviors.
Mass administration of malaria drugs may cut morbidity during Ebola outbreaks
Mass administration of malaria chemoprevention during Ebola virus disease outbreaks may reduce cases of fever, according to a study published in PLOS ONE.
During the October-December 2014 Ebola virus disease (EVD) outbreak in Liberia, health care services were limited, negatively impacting malaria treatment. Hoping to reduce malaria-associated morbidity, investigators targeted four neighborhoods in Monrovia, Liberia – with a total population of 551,971 – for a mass drug administration (MDA) of malaria chemoprevention. MDA participants were divided into two treatment rounds, with 102,372 households verified as receiving treatment with the drug combination artesunate/amodiaquine by community leaders and a malaria committee in round 1, and 103,497 households verified in round 2.
Incidences of self-reported fever episodes declined significantly after round 1 (1.5%), compared with the month prior to round 1 (4.2%) (P < .0001). Self-reported fever incidences in children younger than 5 years of age (6.9%) and in older household members (3.8%) both decreased, to 1.1% and 1.6%, respectively, after round 1 of the MDA.
The researchers also found that self-reported fever was 4.9% lower after round 1 in household members who took a full course of artesunate/amodiaquine malaria chemoprevention (ASAQ-CP) but only 0.6% lower among household members who did not start or not complete a full course of ASAQ-CP. Still, reported incidence of fever declined in both groups, although the risk difference (RD) was significantly larger among the group that took part in the ASAQ-CP course (P < .001).
“Despite high acceptance and coverage of the MDA and the small impact of side effects, initiation of malaria chemoprevention was low, possibly due to health messaging and behavior in the pre-Ebola outbreak period and the ongoing lack of health care services,” researchers concluded. “Combining MDAs during Ebola outbreaks with longer-term interventions to prevent malaria and to improve access to health care might reduce the proportion of respondents saving their treatment for future malaria episodes.”
Read the full study in PLOS ONE (doi: 10.1371/journal.pone.0161311).
Mass administration of malaria chemoprevention during Ebola virus disease outbreaks may reduce cases of fever, according to a study published in PLOS ONE.
During the October-December 2014 Ebola virus disease (EVD) outbreak in Liberia, health care services were limited, negatively impacting malaria treatment. Hoping to reduce malaria-associated morbidity, investigators targeted four neighborhoods in Monrovia, Liberia – with a total population of 551,971 – for a mass drug administration (MDA) of malaria chemoprevention. MDA participants were divided into two treatment rounds, with 102,372 households verified as receiving treatment with the drug combination artesunate/amodiaquine by community leaders and a malaria committee in round 1, and 103,497 households verified in round 2.
Incidences of self-reported fever episodes declined significantly after round 1 (1.5%), compared with the month prior to round 1 (4.2%) (P < .0001). Self-reported fever incidences in children younger than 5 years of age (6.9%) and in older household members (3.8%) both decreased, to 1.1% and 1.6%, respectively, after round 1 of the MDA.
The researchers also found that self-reported fever was 4.9% lower after round 1 in household members who took a full course of artesunate/amodiaquine malaria chemoprevention (ASAQ-CP) but only 0.6% lower among household members who did not start or not complete a full course of ASAQ-CP. Still, reported incidence of fever declined in both groups, although the risk difference (RD) was significantly larger among the group that took part in the ASAQ-CP course (P < .001).
“Despite high acceptance and coverage of the MDA and the small impact of side effects, initiation of malaria chemoprevention was low, possibly due to health messaging and behavior in the pre-Ebola outbreak period and the ongoing lack of health care services,” researchers concluded. “Combining MDAs during Ebola outbreaks with longer-term interventions to prevent malaria and to improve access to health care might reduce the proportion of respondents saving their treatment for future malaria episodes.”
Read the full study in PLOS ONE (doi: 10.1371/journal.pone.0161311).
Mass administration of malaria chemoprevention during Ebola virus disease outbreaks may reduce cases of fever, according to a study published in PLOS ONE.
During the October-December 2014 Ebola virus disease (EVD) outbreak in Liberia, health care services were limited, negatively impacting malaria treatment. Hoping to reduce malaria-associated morbidity, investigators targeted four neighborhoods in Monrovia, Liberia – with a total population of 551,971 – for a mass drug administration (MDA) of malaria chemoprevention. MDA participants were divided into two treatment rounds, with 102,372 households verified as receiving treatment with the drug combination artesunate/amodiaquine by community leaders and a malaria committee in round 1, and 103,497 households verified in round 2.
Incidences of self-reported fever episodes declined significantly after round 1 (1.5%), compared with the month prior to round 1 (4.2%) (P < .0001). Self-reported fever incidences in children younger than 5 years of age (6.9%) and in older household members (3.8%) both decreased, to 1.1% and 1.6%, respectively, after round 1 of the MDA.
The researchers also found that self-reported fever was 4.9% lower after round 1 in household members who took a full course of artesunate/amodiaquine malaria chemoprevention (ASAQ-CP) but only 0.6% lower among household members who did not start or not complete a full course of ASAQ-CP. Still, reported incidence of fever declined in both groups, although the risk difference (RD) was significantly larger among the group that took part in the ASAQ-CP course (P < .001).
“Despite high acceptance and coverage of the MDA and the small impact of side effects, initiation of malaria chemoprevention was low, possibly due to health messaging and behavior in the pre-Ebola outbreak period and the ongoing lack of health care services,” researchers concluded. “Combining MDAs during Ebola outbreaks with longer-term interventions to prevent malaria and to improve access to health care might reduce the proportion of respondents saving their treatment for future malaria episodes.”
Read the full study in PLOS ONE (doi: 10.1371/journal.pone.0161311).
FROM PLOS ONE
Dasatinib plus venetoclax shows promise in mouse model of Ph+ALL
The combination of dasatinib and venetoclax had a synergistic effect that was associated with lower toxicity than single-agent therapy, based on responses of primary Philadelphia chromosome–positive acute lymphoblastic leukemia (PH+ALL) samples in xenografted immunodeficient mice, according to Jessica T. Leonard, MD.
Dr. Leonard and her colleagues at Oregon Health and Science University in Portland demonstrated that the combination of venetoclax – a selective inhibitor of B cell lymphoma 2 – was highly synergistic with tyrosine kinase inhibitors in vitro. In the preclinical model of PH+ALL, a stepwise reduction in median inhibitory concentration of dasatinib was observed with increasing doses of venetoclax, as was decreased cell viability and induced apoptosis. Dasatinib – a breakpoint cluster region–Abelson kinase inhibitor – has an additional advantage of potentially overcoming venetoclax resistance by blocking a common mechanism of resistance to the agent, the investigators reported Aug. 31 in Science Translational Medicine (2016; 8[354]:354ra114).
The combination boosted antitumor activity against Ph+ALL cells grown in culture. In the mouse model of Ph+ALL, all of the mice in the combination dosing group remained alive during the 4-week treatment period. The combination therapy was well tolerated, and superior to either agent alone with respect to antileukemic efficacy.
The investigators focused on combining venetoclax with dasatinib because it is “the current backbone for the treatment of adult Ph+ALL ... These results lay the foundation for the testing of this combination in patients with Ph+ALL with the goal of improving treatment,” they concluded.
This study was supported in part by the Leukemia & Lymphoma Society and the Newman’s Own Foundation. Dr. Leonard reported having no other disclosures, but various coauthors reported receiving research support from, and/or serving as a consultant or scientific advisory board member to Genentech, the maker of venetoclax, and Bristol-Myers Squibb, the maker of dasatinib, as well as numerous other drug companies.
The combination of dasatinib and venetoclax had a synergistic effect that was associated with lower toxicity than single-agent therapy, based on responses of primary Philadelphia chromosome–positive acute lymphoblastic leukemia (PH+ALL) samples in xenografted immunodeficient mice, according to Jessica T. Leonard, MD.
Dr. Leonard and her colleagues at Oregon Health and Science University in Portland demonstrated that the combination of venetoclax – a selective inhibitor of B cell lymphoma 2 – was highly synergistic with tyrosine kinase inhibitors in vitro. In the preclinical model of PH+ALL, a stepwise reduction in median inhibitory concentration of dasatinib was observed with increasing doses of venetoclax, as was decreased cell viability and induced apoptosis. Dasatinib – a breakpoint cluster region–Abelson kinase inhibitor – has an additional advantage of potentially overcoming venetoclax resistance by blocking a common mechanism of resistance to the agent, the investigators reported Aug. 31 in Science Translational Medicine (2016; 8[354]:354ra114).
The combination boosted antitumor activity against Ph+ALL cells grown in culture. In the mouse model of Ph+ALL, all of the mice in the combination dosing group remained alive during the 4-week treatment period. The combination therapy was well tolerated, and superior to either agent alone with respect to antileukemic efficacy.
The investigators focused on combining venetoclax with dasatinib because it is “the current backbone for the treatment of adult Ph+ALL ... These results lay the foundation for the testing of this combination in patients with Ph+ALL with the goal of improving treatment,” they concluded.
This study was supported in part by the Leukemia & Lymphoma Society and the Newman’s Own Foundation. Dr. Leonard reported having no other disclosures, but various coauthors reported receiving research support from, and/or serving as a consultant or scientific advisory board member to Genentech, the maker of venetoclax, and Bristol-Myers Squibb, the maker of dasatinib, as well as numerous other drug companies.
The combination of dasatinib and venetoclax had a synergistic effect that was associated with lower toxicity than single-agent therapy, based on responses of primary Philadelphia chromosome–positive acute lymphoblastic leukemia (PH+ALL) samples in xenografted immunodeficient mice, according to Jessica T. Leonard, MD.
Dr. Leonard and her colleagues at Oregon Health and Science University in Portland demonstrated that the combination of venetoclax – a selective inhibitor of B cell lymphoma 2 – was highly synergistic with tyrosine kinase inhibitors in vitro. In the preclinical model of PH+ALL, a stepwise reduction in median inhibitory concentration of dasatinib was observed with increasing doses of venetoclax, as was decreased cell viability and induced apoptosis. Dasatinib – a breakpoint cluster region–Abelson kinase inhibitor – has an additional advantage of potentially overcoming venetoclax resistance by blocking a common mechanism of resistance to the agent, the investigators reported Aug. 31 in Science Translational Medicine (2016; 8[354]:354ra114).
The combination boosted antitumor activity against Ph+ALL cells grown in culture. In the mouse model of Ph+ALL, all of the mice in the combination dosing group remained alive during the 4-week treatment period. The combination therapy was well tolerated, and superior to either agent alone with respect to antileukemic efficacy.
The investigators focused on combining venetoclax with dasatinib because it is “the current backbone for the treatment of adult Ph+ALL ... These results lay the foundation for the testing of this combination in patients with Ph+ALL with the goal of improving treatment,” they concluded.
This study was supported in part by the Leukemia & Lymphoma Society and the Newman’s Own Foundation. Dr. Leonard reported having no other disclosures, but various coauthors reported receiving research support from, and/or serving as a consultant or scientific advisory board member to Genentech, the maker of venetoclax, and Bristol-Myers Squibb, the maker of dasatinib, as well as numerous other drug companies.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: The combination of dasatinib and venetoclax shows promise for the treatment of primary Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ALL) samples in xenografted immunodeficient mice and should be further evaluated for patient care.
Major finding: A stepwise reduction in median inhibitory concentration of dasatinib was seen with increasing doses of venetoclax.
Data source: In vitro and in vivo evaluation of BCL-2 inhibition in combination with kinase inhibition in a murine model of Ph+ALL.
Disclosures: This study was supported in part by the Leukemia & Lymphoma Society and the Newman’s Own Foundation. Dr. Leonard reported having no other disclosures, but various coauthors reported receiving research support from, and/or serving as a consultant or scientific advisory board member to Genentech, the maker of venetoclax, and Bristol-Myers Squibb, the maker of dasatinib, as well as numerous other drug companies.
ENSURE-AF trial supports edoxaban for electrical cardioversion
ROME – Results of the largest-ever randomized clinical trial of anticoagulation for electrical cardioversion of patients with nonvalvular atrial fibrillation demonstrate that edoxaban is a safe, effective, and convenient alternative to the standard strategy of enoxaparin as a bridge to warfarin.
The ENSURE-AF trial was a phase IIIb study involving 2,199 patients with atrial fibrillation who underwent electrical cardioversion at 239 sites in the United States and 19 European countries. The key finding: The edoxaban-treated group had rates of thromboembolism and major bleeding at 28-30 days follow-up similar to those of the enoxaparin/warfarin-treated controls.
And edoxaban offered a major practical advantage: Because “edoxaban kicks in within 2 hours, you can do the procedure just 2 hours after initiation of therapy in a patient with a reassuring transesophageal echocardiographic exam, which is definitely not possible with warfarin,” Andreas Goette, MD, observed at the annual congress of the European Society of Cardiology.
Roughly half of participants were treated at centers that don’t routinely use a transesophageal echo-guided management strategy and therefore delayed cardioversion until patients were anticoagulated for at least 3 weeks. The safety and efficacy outcomes were similar regardless of whether or not transesophageal echocardiography (TEE) guidance was used, according to Dr. Goette of St. Vincenz Hospital in Paderborn, Germany.
Edoxaban (Savaysa) was prescribed at 60 mg once daily except in patients weighing 60 kg or less or having a creatinine clearance rate of 15-50 mL/min, in which case they received 30 mg once daily. In the control arm, enoxaparin (Lovenox) was used until warfarin achieved an International Normalized Ratio of 2.0-3.0. Patients in the enoxaparin/warfarin arm spent a mean of 71% of their treatment time within the target INR range.
The primary efficacy outcome was the 28-day composite of stroke or other systemic embolic events, MI, or cardiovascular mortality. The rate was 0.5% in the edoxaban arm and 1.0% in the enoxaparin/warfarin group. In patients whose management strategy was TEE-guided, the rate was 0.3% in the edoxaban group and 0.8% with enoxaparin/warfarin. In non-TEE-guided patients, the rates were 0.6% and 1.2% with edoxaban and warfarin, respectively.
Although rates were consistently numerically lower in the edoxaban group, the differences did not reach statistical significance, Dr. Goette explained.
The combined rate of major or clinically relevant nonmajor bleeding through 30 days was 1.5% with edoxaban and similar at 1.0% with enoxaparin plus warfarin. Three patients in the edoxaban group experienced a major bleeding event, as did five in the comparator arm.
Because anticoagulation with edoxaban is so convenient and allows cardioversion to safely be performed in short order, the ENSURE-AF investigators are in the process of calculating the potential savings in health care costs obtainable through this strategy, the cardiologist said.
ENSURE-AF provides the first prospective randomized data on the use of edoxaban as an alternative to warfarin for pericardioversion anticoagulation. There has been one other randomized trial of a novel oral anticoagulant (NOAC) in this setting, the 1,504-patient X-VeRT trial (Eur Heart J. 2014 Dec 14;35[47]:3346-55), involving rivaroxaban (Xarelto).
Riccardo Cappato, MD, first author of the X-VeRT publication, served as the designated discussant for ENSURE-AF. He noted that the results of the two trials are “completely superimposable.” Rates of the composite efficacy endpoint were identical at 0.5% for both NOACs versus 1.0% for the vitamin K antagonist arms of X-VeRT and ENSURE-AF. The major bleeding rates also were identical for edoxaban and rivaroxaban in the two studies. Moreover, the major bleeding rates associated with warfarin or other vitamin K antagonists were spot-on the same in the two trials.
“It’s a rather unusual situation for such large numbers of patients,” observed Dr. Cappato of Humanitas Research Institute in Milan.
“These data go very clearly in the same direction. I think a good take-home message here for us today is that both of these novel oral anticoagulants can be safely and efficaciously applied to patients undergoing elective cardioversion of nonvalvular atrial fibrillation,” he added.
In an interview, Mark A. Creager, MD, immediate past president of the American Heart Association, said that many U.S. physicians are switching to NOACs for this purpose.
“We are already using the novel oral anticoagulants to facilitate anticoagulation for patients undergoing cardioversion, so ENSURE-AF provides objective evidence that edoxaban is a reasonable drug,” said Dr. Creager, director of the Dartmouth-Hitchcock Heart and Vascular Center in New Hampshire.
The ENSURE-AF trial was funded by Daiichi Sankyo. Dr. Goette and Dr. Cappato reported receiving research grants from and serving as consultants to that company and other pharmaceutical and medical device manufacturers.
Simultaneously with Dr. Goette’s presentation in Rome, the ENSURE-AF results were published online Aug. 30 in The Lancet.
ROME – Results of the largest-ever randomized clinical trial of anticoagulation for electrical cardioversion of patients with nonvalvular atrial fibrillation demonstrate that edoxaban is a safe, effective, and convenient alternative to the standard strategy of enoxaparin as a bridge to warfarin.
The ENSURE-AF trial was a phase IIIb study involving 2,199 patients with atrial fibrillation who underwent electrical cardioversion at 239 sites in the United States and 19 European countries. The key finding: The edoxaban-treated group had rates of thromboembolism and major bleeding at 28-30 days follow-up similar to those of the enoxaparin/warfarin-treated controls.
And edoxaban offered a major practical advantage: Because “edoxaban kicks in within 2 hours, you can do the procedure just 2 hours after initiation of therapy in a patient with a reassuring transesophageal echocardiographic exam, which is definitely not possible with warfarin,” Andreas Goette, MD, observed at the annual congress of the European Society of Cardiology.
Roughly half of participants were treated at centers that don’t routinely use a transesophageal echo-guided management strategy and therefore delayed cardioversion until patients were anticoagulated for at least 3 weeks. The safety and efficacy outcomes were similar regardless of whether or not transesophageal echocardiography (TEE) guidance was used, according to Dr. Goette of St. Vincenz Hospital in Paderborn, Germany.
Edoxaban (Savaysa) was prescribed at 60 mg once daily except in patients weighing 60 kg or less or having a creatinine clearance rate of 15-50 mL/min, in which case they received 30 mg once daily. In the control arm, enoxaparin (Lovenox) was used until warfarin achieved an International Normalized Ratio of 2.0-3.0. Patients in the enoxaparin/warfarin arm spent a mean of 71% of their treatment time within the target INR range.
The primary efficacy outcome was the 28-day composite of stroke or other systemic embolic events, MI, or cardiovascular mortality. The rate was 0.5% in the edoxaban arm and 1.0% in the enoxaparin/warfarin group. In patients whose management strategy was TEE-guided, the rate was 0.3% in the edoxaban group and 0.8% with enoxaparin/warfarin. In non-TEE-guided patients, the rates were 0.6% and 1.2% with edoxaban and warfarin, respectively.
Although rates were consistently numerically lower in the edoxaban group, the differences did not reach statistical significance, Dr. Goette explained.
The combined rate of major or clinically relevant nonmajor bleeding through 30 days was 1.5% with edoxaban and similar at 1.0% with enoxaparin plus warfarin. Three patients in the edoxaban group experienced a major bleeding event, as did five in the comparator arm.
Because anticoagulation with edoxaban is so convenient and allows cardioversion to safely be performed in short order, the ENSURE-AF investigators are in the process of calculating the potential savings in health care costs obtainable through this strategy, the cardiologist said.
ENSURE-AF provides the first prospective randomized data on the use of edoxaban as an alternative to warfarin for pericardioversion anticoagulation. There has been one other randomized trial of a novel oral anticoagulant (NOAC) in this setting, the 1,504-patient X-VeRT trial (Eur Heart J. 2014 Dec 14;35[47]:3346-55), involving rivaroxaban (Xarelto).
Riccardo Cappato, MD, first author of the X-VeRT publication, served as the designated discussant for ENSURE-AF. He noted that the results of the two trials are “completely superimposable.” Rates of the composite efficacy endpoint were identical at 0.5% for both NOACs versus 1.0% for the vitamin K antagonist arms of X-VeRT and ENSURE-AF. The major bleeding rates also were identical for edoxaban and rivaroxaban in the two studies. Moreover, the major bleeding rates associated with warfarin or other vitamin K antagonists were spot-on the same in the two trials.
“It’s a rather unusual situation for such large numbers of patients,” observed Dr. Cappato of Humanitas Research Institute in Milan.
“These data go very clearly in the same direction. I think a good take-home message here for us today is that both of these novel oral anticoagulants can be safely and efficaciously applied to patients undergoing elective cardioversion of nonvalvular atrial fibrillation,” he added.
In an interview, Mark A. Creager, MD, immediate past president of the American Heart Association, said that many U.S. physicians are switching to NOACs for this purpose.
“We are already using the novel oral anticoagulants to facilitate anticoagulation for patients undergoing cardioversion, so ENSURE-AF provides objective evidence that edoxaban is a reasonable drug,” said Dr. Creager, director of the Dartmouth-Hitchcock Heart and Vascular Center in New Hampshire.
The ENSURE-AF trial was funded by Daiichi Sankyo. Dr. Goette and Dr. Cappato reported receiving research grants from and serving as consultants to that company and other pharmaceutical and medical device manufacturers.
Simultaneously with Dr. Goette’s presentation in Rome, the ENSURE-AF results were published online Aug. 30 in The Lancet.
ROME – Results of the largest-ever randomized clinical trial of anticoagulation for electrical cardioversion of patients with nonvalvular atrial fibrillation demonstrate that edoxaban is a safe, effective, and convenient alternative to the standard strategy of enoxaparin as a bridge to warfarin.
The ENSURE-AF trial was a phase IIIb study involving 2,199 patients with atrial fibrillation who underwent electrical cardioversion at 239 sites in the United States and 19 European countries. The key finding: The edoxaban-treated group had rates of thromboembolism and major bleeding at 28-30 days follow-up similar to those of the enoxaparin/warfarin-treated controls.
And edoxaban offered a major practical advantage: Because “edoxaban kicks in within 2 hours, you can do the procedure just 2 hours after initiation of therapy in a patient with a reassuring transesophageal echocardiographic exam, which is definitely not possible with warfarin,” Andreas Goette, MD, observed at the annual congress of the European Society of Cardiology.
Roughly half of participants were treated at centers that don’t routinely use a transesophageal echo-guided management strategy and therefore delayed cardioversion until patients were anticoagulated for at least 3 weeks. The safety and efficacy outcomes were similar regardless of whether or not transesophageal echocardiography (TEE) guidance was used, according to Dr. Goette of St. Vincenz Hospital in Paderborn, Germany.
Edoxaban (Savaysa) was prescribed at 60 mg once daily except in patients weighing 60 kg or less or having a creatinine clearance rate of 15-50 mL/min, in which case they received 30 mg once daily. In the control arm, enoxaparin (Lovenox) was used until warfarin achieved an International Normalized Ratio of 2.0-3.0. Patients in the enoxaparin/warfarin arm spent a mean of 71% of their treatment time within the target INR range.
The primary efficacy outcome was the 28-day composite of stroke or other systemic embolic events, MI, or cardiovascular mortality. The rate was 0.5% in the edoxaban arm and 1.0% in the enoxaparin/warfarin group. In patients whose management strategy was TEE-guided, the rate was 0.3% in the edoxaban group and 0.8% with enoxaparin/warfarin. In non-TEE-guided patients, the rates were 0.6% and 1.2% with edoxaban and warfarin, respectively.
Although rates were consistently numerically lower in the edoxaban group, the differences did not reach statistical significance, Dr. Goette explained.
The combined rate of major or clinically relevant nonmajor bleeding through 30 days was 1.5% with edoxaban and similar at 1.0% with enoxaparin plus warfarin. Three patients in the edoxaban group experienced a major bleeding event, as did five in the comparator arm.
Because anticoagulation with edoxaban is so convenient and allows cardioversion to safely be performed in short order, the ENSURE-AF investigators are in the process of calculating the potential savings in health care costs obtainable through this strategy, the cardiologist said.
ENSURE-AF provides the first prospective randomized data on the use of edoxaban as an alternative to warfarin for pericardioversion anticoagulation. There has been one other randomized trial of a novel oral anticoagulant (NOAC) in this setting, the 1,504-patient X-VeRT trial (Eur Heart J. 2014 Dec 14;35[47]:3346-55), involving rivaroxaban (Xarelto).
Riccardo Cappato, MD, first author of the X-VeRT publication, served as the designated discussant for ENSURE-AF. He noted that the results of the two trials are “completely superimposable.” Rates of the composite efficacy endpoint were identical at 0.5% for both NOACs versus 1.0% for the vitamin K antagonist arms of X-VeRT and ENSURE-AF. The major bleeding rates also were identical for edoxaban and rivaroxaban in the two studies. Moreover, the major bleeding rates associated with warfarin or other vitamin K antagonists were spot-on the same in the two trials.
“It’s a rather unusual situation for such large numbers of patients,” observed Dr. Cappato of Humanitas Research Institute in Milan.
“These data go very clearly in the same direction. I think a good take-home message here for us today is that both of these novel oral anticoagulants can be safely and efficaciously applied to patients undergoing elective cardioversion of nonvalvular atrial fibrillation,” he added.
In an interview, Mark A. Creager, MD, immediate past president of the American Heart Association, said that many U.S. physicians are switching to NOACs for this purpose.
“We are already using the novel oral anticoagulants to facilitate anticoagulation for patients undergoing cardioversion, so ENSURE-AF provides objective evidence that edoxaban is a reasonable drug,” said Dr. Creager, director of the Dartmouth-Hitchcock Heart and Vascular Center in New Hampshire.
The ENSURE-AF trial was funded by Daiichi Sankyo. Dr. Goette and Dr. Cappato reported receiving research grants from and serving as consultants to that company and other pharmaceutical and medical device manufacturers.
Simultaneously with Dr. Goette’s presentation in Rome, the ENSURE-AF results were published online Aug. 30 in The Lancet.
AT THE ESC CONGRESS 2016
Key clinical point: Edoxaban is a safe, effective, and convenient alternative to warfarin for anticoagulation in patients undergoing electrical cardioversion of atrial fibrillation.
Major finding: The composite endpoint of stroke, other systemic embolic events, MI, or cardiovascular death occurred in 0.5% of patients with atrial fibrillation assigned to edoxaban for pericardioversion anticoagulation and in 1.0% on enoxaparin bridging to warfarin.
Data source: A randomized prospective multinational trial of 2,199 patients scheduled for electrical cardioversion of their nonvalvular atrial fibrillation.
Disclosures: The ENSURE-AF trial was funded by Daiichi Sankyo. The presenter reported receiving research grants from and serving as a consultant to that company as well as other pharmaceutical and medical device manufacturers.
Removal from play reduces concussion recovery time in athletes
Sport-related concussion (SRC) recovery time can be reduced if athletes are removed from game participation, according to R.J. Elbin, PhD, of the University of Arkansas, Fayetteville, and his associates.
In the prospective study, 95 athletes sought care for an SRC at a concussion specialty clinic between Sept. 1 and Dec. 1, 2014. The athletes were divided into two groups: those who continued to play after experiencing signs and symptoms of an SRC and those who were immediately removed from play. The played group took longer to recover (44 days) than did the removed group (22 days) (P = .003).
Post hoc analyses revealed that the played group demonstrated significantly worse verbal and visual memory, processing speed, and reaction time, and higher symptoms (all P less than or equal to .001), compared with the removed group at 1-7 days. From 8 to 30 days post injury, the played group demonstrated worse verbal memory (P = .009), visual memory (P less than or equal to .001), processing speed (P = .001), and greater symptoms (P = .001), compared with the removed group.
The study also showed that athletes in the played group were 8.80 times more likely to experience a protracted recovery, compared with athletes in the removed group (21 days or longer) (P less than .001). Athletes participated in a variety of sports including football, soccer, ice hockey, volleyball, field hockey, rugby, basketball, and wrestling.
“This study is the first to show that athletes who continue to play with an SRC experience a longer recovery and more time away from the sport,” researchers concluded. “These findings should be incorporated into SRC education and awareness programs for athletes, coaches, parents, and medical professionals.”
Find the full study in Pediatrics (doi: 10.1542/peds.2016-0910).
Sport-related concussion (SRC) recovery time can be reduced if athletes are removed from game participation, according to R.J. Elbin, PhD, of the University of Arkansas, Fayetteville, and his associates.
In the prospective study, 95 athletes sought care for an SRC at a concussion specialty clinic between Sept. 1 and Dec. 1, 2014. The athletes were divided into two groups: those who continued to play after experiencing signs and symptoms of an SRC and those who were immediately removed from play. The played group took longer to recover (44 days) than did the removed group (22 days) (P = .003).
Post hoc analyses revealed that the played group demonstrated significantly worse verbal and visual memory, processing speed, and reaction time, and higher symptoms (all P less than or equal to .001), compared with the removed group at 1-7 days. From 8 to 30 days post injury, the played group demonstrated worse verbal memory (P = .009), visual memory (P less than or equal to .001), processing speed (P = .001), and greater symptoms (P = .001), compared with the removed group.
The study also showed that athletes in the played group were 8.80 times more likely to experience a protracted recovery, compared with athletes in the removed group (21 days or longer) (P less than .001). Athletes participated in a variety of sports including football, soccer, ice hockey, volleyball, field hockey, rugby, basketball, and wrestling.
“This study is the first to show that athletes who continue to play with an SRC experience a longer recovery and more time away from the sport,” researchers concluded. “These findings should be incorporated into SRC education and awareness programs for athletes, coaches, parents, and medical professionals.”
Find the full study in Pediatrics (doi: 10.1542/peds.2016-0910).
Sport-related concussion (SRC) recovery time can be reduced if athletes are removed from game participation, according to R.J. Elbin, PhD, of the University of Arkansas, Fayetteville, and his associates.
In the prospective study, 95 athletes sought care for an SRC at a concussion specialty clinic between Sept. 1 and Dec. 1, 2014. The athletes were divided into two groups: those who continued to play after experiencing signs and symptoms of an SRC and those who were immediately removed from play. The played group took longer to recover (44 days) than did the removed group (22 days) (P = .003).
Post hoc analyses revealed that the played group demonstrated significantly worse verbal and visual memory, processing speed, and reaction time, and higher symptoms (all P less than or equal to .001), compared with the removed group at 1-7 days. From 8 to 30 days post injury, the played group demonstrated worse verbal memory (P = .009), visual memory (P less than or equal to .001), processing speed (P = .001), and greater symptoms (P = .001), compared with the removed group.
The study also showed that athletes in the played group were 8.80 times more likely to experience a protracted recovery, compared with athletes in the removed group (21 days or longer) (P less than .001). Athletes participated in a variety of sports including football, soccer, ice hockey, volleyball, field hockey, rugby, basketball, and wrestling.
“This study is the first to show that athletes who continue to play with an SRC experience a longer recovery and more time away from the sport,” researchers concluded. “These findings should be incorporated into SRC education and awareness programs for athletes, coaches, parents, and medical professionals.”
Find the full study in Pediatrics (doi: 10.1542/peds.2016-0910).
FROM PEDIATRICS
PAI-1 modifications, early-life LRIs increase asthma risk
A genetic modification of the plasminogen activator inhibitor-1 gene in conjunction with lower respiratory infections during early life was associated with increased risk of asthma, morbidities, and reduced lung function, according to Seong H. Cho, MD, and his associates.
A history of respiratory syncytial virus (RSV) and a history of other lower respiratory infections (LRIs) before the age of 2 were independently associated with asthma in Latino people aged 8-21, with odd ratios of 9.9 and 9.1, respectively, while PAI-1 was not independently associated. In combination, the OR for PAI-1/RSV increased to 17.7, and the OR for PAI-1/other LRIs increased to 11.7.
Lung function was also adversely affected by the joint effect of PAI-1 and early life infection. In patients with PAI-1/LRI, forced expiratory volume in 1 second (FEV1) percent predicted and FEV1/forced vital capacity (FVC) percent predicted were significantly less than in the control group. Similar but less significant results were seen in the PAI-1/RSV group. Recurring hospitalizations were also significantly more likely in the PAI-1/RSV group, with an OR of 3.1.
“Further prospective studies are needed to replicate our RSV-genotype findings in other non-Latino populations, and determine if PAI-1 variants may serve as a biomarker of risk, which may provide impetus for clinical trials of primary prevention of asthma. In the interim, PAI-1 genotype in combination with significant LRI identifies individuals at increased risk of developing asthma,” the investigators wrote.
Find the full study in PLoS One (doi: 10.1371/journal.pone.0157848).
A genetic modification of the plasminogen activator inhibitor-1 gene in conjunction with lower respiratory infections during early life was associated with increased risk of asthma, morbidities, and reduced lung function, according to Seong H. Cho, MD, and his associates.
A history of respiratory syncytial virus (RSV) and a history of other lower respiratory infections (LRIs) before the age of 2 were independently associated with asthma in Latino people aged 8-21, with odd ratios of 9.9 and 9.1, respectively, while PAI-1 was not independently associated. In combination, the OR for PAI-1/RSV increased to 17.7, and the OR for PAI-1/other LRIs increased to 11.7.
Lung function was also adversely affected by the joint effect of PAI-1 and early life infection. In patients with PAI-1/LRI, forced expiratory volume in 1 second (FEV1) percent predicted and FEV1/forced vital capacity (FVC) percent predicted were significantly less than in the control group. Similar but less significant results were seen in the PAI-1/RSV group. Recurring hospitalizations were also significantly more likely in the PAI-1/RSV group, with an OR of 3.1.
“Further prospective studies are needed to replicate our RSV-genotype findings in other non-Latino populations, and determine if PAI-1 variants may serve as a biomarker of risk, which may provide impetus for clinical trials of primary prevention of asthma. In the interim, PAI-1 genotype in combination with significant LRI identifies individuals at increased risk of developing asthma,” the investigators wrote.
Find the full study in PLoS One (doi: 10.1371/journal.pone.0157848).
A genetic modification of the plasminogen activator inhibitor-1 gene in conjunction with lower respiratory infections during early life was associated with increased risk of asthma, morbidities, and reduced lung function, according to Seong H. Cho, MD, and his associates.
A history of respiratory syncytial virus (RSV) and a history of other lower respiratory infections (LRIs) before the age of 2 were independently associated with asthma in Latino people aged 8-21, with odd ratios of 9.9 and 9.1, respectively, while PAI-1 was not independently associated. In combination, the OR for PAI-1/RSV increased to 17.7, and the OR for PAI-1/other LRIs increased to 11.7.
Lung function was also adversely affected by the joint effect of PAI-1 and early life infection. In patients with PAI-1/LRI, forced expiratory volume in 1 second (FEV1) percent predicted and FEV1/forced vital capacity (FVC) percent predicted were significantly less than in the control group. Similar but less significant results were seen in the PAI-1/RSV group. Recurring hospitalizations were also significantly more likely in the PAI-1/RSV group, with an OR of 3.1.
“Further prospective studies are needed to replicate our RSV-genotype findings in other non-Latino populations, and determine if PAI-1 variants may serve as a biomarker of risk, which may provide impetus for clinical trials of primary prevention of asthma. In the interim, PAI-1 genotype in combination with significant LRI identifies individuals at increased risk of developing asthma,” the investigators wrote.
Find the full study in PLoS One (doi: 10.1371/journal.pone.0157848).
FROM PLOS ONE