User login
VIDEO: Moderate LDL, SBP reductions slash cardiovascular events 90% over time
ROME – Combined exposure to low LDL cholesterol and systolic blood pressure is associated with multiplicative and cumulative effects over time, Brian A. Ference, MD, said in a video interview at the annual congress of the European Society of Cardiology.
Indeed, long-term exposure to a combined 1-mmol/L lower LDL cholesterol and 10-mm Hg lower systolic BP was associated with up to a 90% lower risk of major cardiovascular events in the “naturally randomized” study he presented. The investigators used the 102,000 participants’ genetic LDL and BP scores in a Mendelian design.
If these lower LDL and blood pressure levels are sustained over decades, “those cumulative effects multiply, resulting in potentially dramatic reductions in the lifetime risk of cardiovascular events from even modestly lower levels of LDL and systolic blood pressure,” Dr. Ference of Wayne State University, Detroit, told reporter Bruce Jancin.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ROME – Combined exposure to low LDL cholesterol and systolic blood pressure is associated with multiplicative and cumulative effects over time, Brian A. Ference, MD, said in a video interview at the annual congress of the European Society of Cardiology.
Indeed, long-term exposure to a combined 1-mmol/L lower LDL cholesterol and 10-mm Hg lower systolic BP was associated with up to a 90% lower risk of major cardiovascular events in the “naturally randomized” study he presented. The investigators used the 102,000 participants’ genetic LDL and BP scores in a Mendelian design.
If these lower LDL and blood pressure levels are sustained over decades, “those cumulative effects multiply, resulting in potentially dramatic reductions in the lifetime risk of cardiovascular events from even modestly lower levels of LDL and systolic blood pressure,” Dr. Ference of Wayne State University, Detroit, told reporter Bruce Jancin.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ROME – Combined exposure to low LDL cholesterol and systolic blood pressure is associated with multiplicative and cumulative effects over time, Brian A. Ference, MD, said in a video interview at the annual congress of the European Society of Cardiology.
Indeed, long-term exposure to a combined 1-mmol/L lower LDL cholesterol and 10-mm Hg lower systolic BP was associated with up to a 90% lower risk of major cardiovascular events in the “naturally randomized” study he presented. The investigators used the 102,000 participants’ genetic LDL and BP scores in a Mendelian design.
If these lower LDL and blood pressure levels are sustained over decades, “those cumulative effects multiply, resulting in potentially dramatic reductions in the lifetime risk of cardiovascular events from even modestly lower levels of LDL and systolic blood pressure,” Dr. Ference of Wayne State University, Detroit, told reporter Bruce Jancin.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE ESC CONGRESS 2016
Zika outbreak forces better history taking, tracking
ANNAPOLIS, MD. – The definition of taking a thorough travel history has expanded with the spread of Zika.
Physicians “need to focus not only on patients’ travel histories, but also the travel histories and future travel plans of our patients’ sexual partners,” Ilona T. Goldfarb, a perinatologist at Massachusetts General Hospital, Boston, said in an interview. “We cannot rely on our patients to just [offer] that they’ve been in the Caribbean. We have to ask them diligently, and at every visit.”
Dr. Goldfarb added that immigration is a risk for Zika exposure, and may be a barrier to accurate history taking. In these cases, travel history will need to be performed in the patient’s spoken language to ensure accuracy, Dr. Goldfarb said. To track and communicate Zika information, Dr. Goldfarb and her colleagues have developed a three-part response based on their experience with previous infectious disease emergencies:
1. Communication. The practice is in regular communication with the Centers for Disease Control and Prevention and the state public health department to ensure they are up to date on all guidelines.
2. Education. Dr. Goldfarb and her practice colleagues routinely brief each other and patients on any new information, including recommended testing and guidelines, as well as travel warnings.
3. Tracking. Clinicians use a tracking worksheet for every patient screened for potential Zika exposure, allowing them to prospectively and retrospectively review patients. This has already proven useful, Dr. Goldfarb said, when the CDC changed its guidance around testing of asymptomatic patients.
In a presentation at the annual scientific meeting of the Infectious Diseases Society for Obstetrics and Gynecology, Dr. Goldfarb presented the data that she and her colleagues have collected so far on patients with Zika exposure.
As of Aug. 10, 2016, the practice had screened 142 women for Zika virus exposure since January 2016. More than 80% of the exposure came from travel to Zika-endemic areas. There have been few cases of exposure reported through sex, but Dr. Goldfarb said she thinks this type of exposure has been underreported because the link between infection and sex was not known until more recently.
Of the patients screened, 87% were appropriate candidates for Zika virus serum testing under CDC guidelines. There have been four positive serum tests for Zika exposure in Dr. Goldfarb’s patients so far.
In the one live birth, the newborn showed no visible signs of abnormalities. Testing revealed Zika virus RNA in the placenta, but not in the cord blood. The other three pregnancies were either terminated or associated with miscarriages. Again, Zika was detected in the placentas, but not in the fetuses.
Testing protocols have been a moving target since the outbreak began, according to Dr. Goldfarb. Some patients who call or are screened for possible exposure “are not actually eligible for testing because of the time frame or location of travel.”
To make sure that appropriate testing is being performed, Dr. Goldfarb advised designating an in-practice “Zika expert.” In her own practice, Dr. Goldfarb and one other colleague handle all Zika screening and inquiries from patients and colleagues. “This has greatly improved our efficiency and the experience for the patients,” she said in an interview.
The Massachusetts Department of Public Health is piloting a program with Dr. Goldfarb’s practice to determine if using the “designated expert” approach will improve laboratory wait times, compared with the standard protocol of having clinicians obtain approval from a state epidemiologist before sending patient serum samples for testing.
It has taken from 2 to 68 days to receive test results from the state lab, although a period of 17 days is typical, Dr. Goldfarb reported. The process has improved since last March when state health officials ramped up their capacity, she said.
As of Aug. 10, Dr. Goldfarb’s practice has performed 107 ultrasounds for patients with suspected Zika virus, averaging 3 per patient. Across all ultrasounds, there were three abnormalities, including one case of bilateral ventriculomegaly. Earlier in that pregnancy, the patient had tested negative for Zika on real-time reverse transcription polymerase chain reaction testing, which simultaneously screens for dengue, chikungunya, and Zika. Serum testing was also negative for Zika in the two other pregnancies with fetal abnormalities.
Dr. Goldfarb said she couldn’t quantify how much time is being spent on Zika screening and counseling since that is not being tracked, but she estimated that her practice takes between three and five calls or questions per day regarding the virus.
On Twitter @whitneymcknight
ANNAPOLIS, MD. – The definition of taking a thorough travel history has expanded with the spread of Zika.
Physicians “need to focus not only on patients’ travel histories, but also the travel histories and future travel plans of our patients’ sexual partners,” Ilona T. Goldfarb, a perinatologist at Massachusetts General Hospital, Boston, said in an interview. “We cannot rely on our patients to just [offer] that they’ve been in the Caribbean. We have to ask them diligently, and at every visit.”
Dr. Goldfarb added that immigration is a risk for Zika exposure, and may be a barrier to accurate history taking. In these cases, travel history will need to be performed in the patient’s spoken language to ensure accuracy, Dr. Goldfarb said. To track and communicate Zika information, Dr. Goldfarb and her colleagues have developed a three-part response based on their experience with previous infectious disease emergencies:
1. Communication. The practice is in regular communication with the Centers for Disease Control and Prevention and the state public health department to ensure they are up to date on all guidelines.
2. Education. Dr. Goldfarb and her practice colleagues routinely brief each other and patients on any new information, including recommended testing and guidelines, as well as travel warnings.
3. Tracking. Clinicians use a tracking worksheet for every patient screened for potential Zika exposure, allowing them to prospectively and retrospectively review patients. This has already proven useful, Dr. Goldfarb said, when the CDC changed its guidance around testing of asymptomatic patients.
In a presentation at the annual scientific meeting of the Infectious Diseases Society for Obstetrics and Gynecology, Dr. Goldfarb presented the data that she and her colleagues have collected so far on patients with Zika exposure.
As of Aug. 10, 2016, the practice had screened 142 women for Zika virus exposure since January 2016. More than 80% of the exposure came from travel to Zika-endemic areas. There have been few cases of exposure reported through sex, but Dr. Goldfarb said she thinks this type of exposure has been underreported because the link between infection and sex was not known until more recently.
Of the patients screened, 87% were appropriate candidates for Zika virus serum testing under CDC guidelines. There have been four positive serum tests for Zika exposure in Dr. Goldfarb’s patients so far.
In the one live birth, the newborn showed no visible signs of abnormalities. Testing revealed Zika virus RNA in the placenta, but not in the cord blood. The other three pregnancies were either terminated or associated with miscarriages. Again, Zika was detected in the placentas, but not in the fetuses.
Testing protocols have been a moving target since the outbreak began, according to Dr. Goldfarb. Some patients who call or are screened for possible exposure “are not actually eligible for testing because of the time frame or location of travel.”
To make sure that appropriate testing is being performed, Dr. Goldfarb advised designating an in-practice “Zika expert.” In her own practice, Dr. Goldfarb and one other colleague handle all Zika screening and inquiries from patients and colleagues. “This has greatly improved our efficiency and the experience for the patients,” she said in an interview.
The Massachusetts Department of Public Health is piloting a program with Dr. Goldfarb’s practice to determine if using the “designated expert” approach will improve laboratory wait times, compared with the standard protocol of having clinicians obtain approval from a state epidemiologist before sending patient serum samples for testing.
It has taken from 2 to 68 days to receive test results from the state lab, although a period of 17 days is typical, Dr. Goldfarb reported. The process has improved since last March when state health officials ramped up their capacity, she said.
As of Aug. 10, Dr. Goldfarb’s practice has performed 107 ultrasounds for patients with suspected Zika virus, averaging 3 per patient. Across all ultrasounds, there were three abnormalities, including one case of bilateral ventriculomegaly. Earlier in that pregnancy, the patient had tested negative for Zika on real-time reverse transcription polymerase chain reaction testing, which simultaneously screens for dengue, chikungunya, and Zika. Serum testing was also negative for Zika in the two other pregnancies with fetal abnormalities.
Dr. Goldfarb said she couldn’t quantify how much time is being spent on Zika screening and counseling since that is not being tracked, but she estimated that her practice takes between three and five calls or questions per day regarding the virus.
On Twitter @whitneymcknight
ANNAPOLIS, MD. – The definition of taking a thorough travel history has expanded with the spread of Zika.
Physicians “need to focus not only on patients’ travel histories, but also the travel histories and future travel plans of our patients’ sexual partners,” Ilona T. Goldfarb, a perinatologist at Massachusetts General Hospital, Boston, said in an interview. “We cannot rely on our patients to just [offer] that they’ve been in the Caribbean. We have to ask them diligently, and at every visit.”
Dr. Goldfarb added that immigration is a risk for Zika exposure, and may be a barrier to accurate history taking. In these cases, travel history will need to be performed in the patient’s spoken language to ensure accuracy, Dr. Goldfarb said. To track and communicate Zika information, Dr. Goldfarb and her colleagues have developed a three-part response based on their experience with previous infectious disease emergencies:
1. Communication. The practice is in regular communication with the Centers for Disease Control and Prevention and the state public health department to ensure they are up to date on all guidelines.
2. Education. Dr. Goldfarb and her practice colleagues routinely brief each other and patients on any new information, including recommended testing and guidelines, as well as travel warnings.
3. Tracking. Clinicians use a tracking worksheet for every patient screened for potential Zika exposure, allowing them to prospectively and retrospectively review patients. This has already proven useful, Dr. Goldfarb said, when the CDC changed its guidance around testing of asymptomatic patients.
In a presentation at the annual scientific meeting of the Infectious Diseases Society for Obstetrics and Gynecology, Dr. Goldfarb presented the data that she and her colleagues have collected so far on patients with Zika exposure.
As of Aug. 10, 2016, the practice had screened 142 women for Zika virus exposure since January 2016. More than 80% of the exposure came from travel to Zika-endemic areas. There have been few cases of exposure reported through sex, but Dr. Goldfarb said she thinks this type of exposure has been underreported because the link between infection and sex was not known until more recently.
Of the patients screened, 87% were appropriate candidates for Zika virus serum testing under CDC guidelines. There have been four positive serum tests for Zika exposure in Dr. Goldfarb’s patients so far.
In the one live birth, the newborn showed no visible signs of abnormalities. Testing revealed Zika virus RNA in the placenta, but not in the cord blood. The other three pregnancies were either terminated or associated with miscarriages. Again, Zika was detected in the placentas, but not in the fetuses.
Testing protocols have been a moving target since the outbreak began, according to Dr. Goldfarb. Some patients who call or are screened for possible exposure “are not actually eligible for testing because of the time frame or location of travel.”
To make sure that appropriate testing is being performed, Dr. Goldfarb advised designating an in-practice “Zika expert.” In her own practice, Dr. Goldfarb and one other colleague handle all Zika screening and inquiries from patients and colleagues. “This has greatly improved our efficiency and the experience for the patients,” she said in an interview.
The Massachusetts Department of Public Health is piloting a program with Dr. Goldfarb’s practice to determine if using the “designated expert” approach will improve laboratory wait times, compared with the standard protocol of having clinicians obtain approval from a state epidemiologist before sending patient serum samples for testing.
It has taken from 2 to 68 days to receive test results from the state lab, although a period of 17 days is typical, Dr. Goldfarb reported. The process has improved since last March when state health officials ramped up their capacity, she said.
As of Aug. 10, Dr. Goldfarb’s practice has performed 107 ultrasounds for patients with suspected Zika virus, averaging 3 per patient. Across all ultrasounds, there were three abnormalities, including one case of bilateral ventriculomegaly. Earlier in that pregnancy, the patient had tested negative for Zika on real-time reverse transcription polymerase chain reaction testing, which simultaneously screens for dengue, chikungunya, and Zika. Serum testing was also negative for Zika in the two other pregnancies with fetal abnormalities.
Dr. Goldfarb said she couldn’t quantify how much time is being spent on Zika screening and counseling since that is not being tracked, but she estimated that her practice takes between three and five calls or questions per day regarding the virus.
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM IDSOG
Request for Applications for JVS Editorial Positions
The editors of the Journal of Vascular Surgery Publications are seeking nominations, including self-nominations, for multiple editorial positions. All applicants must be active or senior members of the Society for Vascular Surgery. Prior service on the JVS editorial board is preferred. An annual financial stipend is provided for these editorial roles.
More information about each of the positions listed below can be found in this online document.
Assistant editor of abstracts and book reviews. This editor selects non-JVS abstracts and writes short commentaries for those abstracts for publication in the JVS and JVS-VL. This editor will also write and solicit book reviews.
Assistant editor of social media and press releases. This position requires writing short press release articles for the JVS Journals and drafting Facebook, Twitter and LinkedIn posts to promote JVS content.
Assistant editor of reviews for the Journal of Vascular Surgery. The assistant editor solicits review articles and manages the peer-review process for systematic reviews, meta-analyses, and evidence summaries.
Associate basic science editor for the JVS and the JVSVL. This associate editor manages the peer-review process and makes the final decision on manuscript publication for about 200 basic science submissions a year.
Associate editor of the Journal of Vascular Surgery. The JVS associate editor manages the peer-review process of about 600 submissions a year, recommending a final decision to the editors regarding the publication of a submission.
If you would like to apply for one of these editorial positions, please send your name and a brief summary of your qualifications Jessica McEwan, JVS managing editor at: [email protected]. The deadline for applications is Oct. 1, 2016.
The editors of the Journal of Vascular Surgery Publications are seeking nominations, including self-nominations, for multiple editorial positions. All applicants must be active or senior members of the Society for Vascular Surgery. Prior service on the JVS editorial board is preferred. An annual financial stipend is provided for these editorial roles.
More information about each of the positions listed below can be found in this online document.
Assistant editor of abstracts and book reviews. This editor selects non-JVS abstracts and writes short commentaries for those abstracts for publication in the JVS and JVS-VL. This editor will also write and solicit book reviews.
Assistant editor of social media and press releases. This position requires writing short press release articles for the JVS Journals and drafting Facebook, Twitter and LinkedIn posts to promote JVS content.
Assistant editor of reviews for the Journal of Vascular Surgery. The assistant editor solicits review articles and manages the peer-review process for systematic reviews, meta-analyses, and evidence summaries.
Associate basic science editor for the JVS and the JVSVL. This associate editor manages the peer-review process and makes the final decision on manuscript publication for about 200 basic science submissions a year.
Associate editor of the Journal of Vascular Surgery. The JVS associate editor manages the peer-review process of about 600 submissions a year, recommending a final decision to the editors regarding the publication of a submission.
If you would like to apply for one of these editorial positions, please send your name and a brief summary of your qualifications Jessica McEwan, JVS managing editor at: [email protected]. The deadline for applications is Oct. 1, 2016.
The editors of the Journal of Vascular Surgery Publications are seeking nominations, including self-nominations, for multiple editorial positions. All applicants must be active or senior members of the Society for Vascular Surgery. Prior service on the JVS editorial board is preferred. An annual financial stipend is provided for these editorial roles.
More information about each of the positions listed below can be found in this online document.
Assistant editor of abstracts and book reviews. This editor selects non-JVS abstracts and writes short commentaries for those abstracts for publication in the JVS and JVS-VL. This editor will also write and solicit book reviews.
Assistant editor of social media and press releases. This position requires writing short press release articles for the JVS Journals and drafting Facebook, Twitter and LinkedIn posts to promote JVS content.
Assistant editor of reviews for the Journal of Vascular Surgery. The assistant editor solicits review articles and manages the peer-review process for systematic reviews, meta-analyses, and evidence summaries.
Associate basic science editor for the JVS and the JVSVL. This associate editor manages the peer-review process and makes the final decision on manuscript publication for about 200 basic science submissions a year.
Associate editor of the Journal of Vascular Surgery. The JVS associate editor manages the peer-review process of about 600 submissions a year, recommending a final decision to the editors regarding the publication of a submission.
If you would like to apply for one of these editorial positions, please send your name and a brief summary of your qualifications Jessica McEwan, JVS managing editor at: [email protected]. The deadline for applications is Oct. 1, 2016.
Submit Proposals for VAM 2017
All SVS members may submit proposals for invited sessions for the 2017 Vascular Annual Meeting, set for May 31 to June 3 (plenaries and exhibits are June 1 to 3) in San Diego, Calif.
Previously, the process was open only to SVS committees; planners opened the process to all members this year, with any and all ideas for sessions to be reviewed for further development.
Invited sessions include postgraduate courses, breakfast and concurrent sessions and workshops. Initial submissions are due Sept. 16. Learn more here.
All SVS members may submit proposals for invited sessions for the 2017 Vascular Annual Meeting, set for May 31 to June 3 (plenaries and exhibits are June 1 to 3) in San Diego, Calif.
Previously, the process was open only to SVS committees; planners opened the process to all members this year, with any and all ideas for sessions to be reviewed for further development.
Invited sessions include postgraduate courses, breakfast and concurrent sessions and workshops. Initial submissions are due Sept. 16. Learn more here.
All SVS members may submit proposals for invited sessions for the 2017 Vascular Annual Meeting, set for May 31 to June 3 (plenaries and exhibits are June 1 to 3) in San Diego, Calif.
Previously, the process was open only to SVS committees; planners opened the process to all members this year, with any and all ideas for sessions to be reviewed for further development.
Invited sessions include postgraduate courses, breakfast and concurrent sessions and workshops. Initial submissions are due Sept. 16. Learn more here.
VIDEO: PCSK9 inhibitor can nearly replace need for apheresis in HeFH
ROME – Treating patients with heterozygous familial hypercholesterolemia (HeFH) with PCSK9 inhibitors can reduce their need for lipoprotein apheresis and its associated costs, Patrick M. Moriarty, MD, said in a video interview at the annual congress of the European Society of Cardiology.
In the randomized, phase III ODYSSEY ESCAPE trial, HeFH patients who underwent weekly apheresis were treated with either alirocumab, a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor, or placebo. At the end of the study, the alirocumab-treated patients had a 75% greater reduction in need for apheresis, compared with those on placebo – a statistically significant difference.
With a price tag of $50,000-$75,000 a year for lipoprotein apheresis, compared with the roughly $12,000 cost for a PCSK9 inhibitor, this represents a significant savings for patients with HeFH, which occurs in roughly 1 in 200 people worldwide, Dr. Moriarty of the University of Kansas, Kansas City, told reporter Bruce Jancin.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ROME – Treating patients with heterozygous familial hypercholesterolemia (HeFH) with PCSK9 inhibitors can reduce their need for lipoprotein apheresis and its associated costs, Patrick M. Moriarty, MD, said in a video interview at the annual congress of the European Society of Cardiology.
In the randomized, phase III ODYSSEY ESCAPE trial, HeFH patients who underwent weekly apheresis were treated with either alirocumab, a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor, or placebo. At the end of the study, the alirocumab-treated patients had a 75% greater reduction in need for apheresis, compared with those on placebo – a statistically significant difference.
With a price tag of $50,000-$75,000 a year for lipoprotein apheresis, compared with the roughly $12,000 cost for a PCSK9 inhibitor, this represents a significant savings for patients with HeFH, which occurs in roughly 1 in 200 people worldwide, Dr. Moriarty of the University of Kansas, Kansas City, told reporter Bruce Jancin.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ROME – Treating patients with heterozygous familial hypercholesterolemia (HeFH) with PCSK9 inhibitors can reduce their need for lipoprotein apheresis and its associated costs, Patrick M. Moriarty, MD, said in a video interview at the annual congress of the European Society of Cardiology.
In the randomized, phase III ODYSSEY ESCAPE trial, HeFH patients who underwent weekly apheresis were treated with either alirocumab, a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor, or placebo. At the end of the study, the alirocumab-treated patients had a 75% greater reduction in need for apheresis, compared with those on placebo – a statistically significant difference.
With a price tag of $50,000-$75,000 a year for lipoprotein apheresis, compared with the roughly $12,000 cost for a PCSK9 inhibitor, this represents a significant savings for patients with HeFH, which occurs in roughly 1 in 200 people worldwide, Dr. Moriarty of the University of Kansas, Kansas City, told reporter Bruce Jancin.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE ESC CONGRESS 2016
First TOS Reporting Standards Published
SVS members now have access to the first-ever reporting standards for Thoracic Outlet Syndrome, published in the September issue (online) at the Journal of Vascular Surgery. Drs. Karl Illig and Audra Duncan led the TOS Reporting Standards Writing Group, which worked for three years to create the reporting standards. The group hopes that with unified data collection, practice guidelines can follow in five to 10 years.
Access the reporting standards here.
SVS members now have access to the first-ever reporting standards for Thoracic Outlet Syndrome, published in the September issue (online) at the Journal of Vascular Surgery. Drs. Karl Illig and Audra Duncan led the TOS Reporting Standards Writing Group, which worked for three years to create the reporting standards. The group hopes that with unified data collection, practice guidelines can follow in five to 10 years.
Access the reporting standards here.
SVS members now have access to the first-ever reporting standards for Thoracic Outlet Syndrome, published in the September issue (online) at the Journal of Vascular Surgery. Drs. Karl Illig and Audra Duncan led the TOS Reporting Standards Writing Group, which worked for three years to create the reporting standards. The group hopes that with unified data collection, practice guidelines can follow in five to 10 years.
Access the reporting standards here.
Review Course Recordings Available
Just in time for the September recertification exam! Recordings of the 2015 Comprehensive Vascular Review Course are available for purchase.
The 2015 intensive two-day course was led by vascular surgeons with extensive expertise in their respective fields. The recordings include didactic lectures and review of essential VESAP questions.
The recordings also provide a comprehensive update in all areas of vascular surgery. No CME credit is available.
Purchase the recordings here.
Just in time for the September recertification exam! Recordings of the 2015 Comprehensive Vascular Review Course are available for purchase.
The 2015 intensive two-day course was led by vascular surgeons with extensive expertise in their respective fields. The recordings include didactic lectures and review of essential VESAP questions.
The recordings also provide a comprehensive update in all areas of vascular surgery. No CME credit is available.
Purchase the recordings here.
Just in time for the September recertification exam! Recordings of the 2015 Comprehensive Vascular Review Course are available for purchase.
The 2015 intensive two-day course was led by vascular surgeons with extensive expertise in their respective fields. The recordings include didactic lectures and review of essential VESAP questions.
The recordings also provide a comprehensive update in all areas of vascular surgery. No CME credit is available.
Purchase the recordings here.
Psoriasis cardiovascular risk parallels type 2 diabetes
Psoriasis increased the measures of coronary artery calcium at a level similar to that seen in type 2 diabetes mellitus, independent of cardiovascular disease risk factors, based on data from a trio of cross-sectional studies including 387 adults. .
“Psoriasis and type 2 diabetes share similar cardiovascular risk profiles, which may predispose patients to developing coronary atherosclerosis at a relatively young age,” wrote Bobbak Mansouri, MD, of Baylor University Medical Center in Dallas and his associates (JAMA Dermatol. 2016. [doi: 10.1001/jamadermatol.2016.2907]).
The researchers compared coronary artery calcium (CAC) levels in patients with psoriasis, patients with type 2 diabetes, and healthy controls. CAC has become an accepted measure of atherosclerosis and “the cornerstone for screening the risk of future cardiac events and improving cardiovascular risk stratification beyond traditional risk factors, especially in higher-risk groups,” according to the investigators. The average age of the patients was 52 years, 50% were female, and at least 92% were white.
The researchers used a hierarchical Tobit regression analysis to determine the association between disease and CAC level, as measured by the Agatston score. After controlling for confounding variables, including cardiovascular risk factors (low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, fasting blood glucose, systolic blood pressure, and tobacco use), the association with CAC was similar in psoriasis patients and type 2 diabetes patients (Tobit regression ratio [TRR], 0.89 and 0.79, respectively).
In a logistic multivariate regression analysis, patients with either psoriasis or type 2 diabetes were approximately twice as likely to have evidence of CAC as were healthy controls (odds ratio, 2.35 and 2.18, respectively), and psoriasis remained independently associated with the presence of CAC.
“When we added use of systemic or biological therapy to the models, the TRR and OR increased; however, these analyses were exploratory,” wrote Dr. Mansouri and his associates.
The study was limited by factors including the cross-sectional design and lack of diversity in the patient population, the investigators noted. However, the results suggest that “CAC assessment may be considered in patients with psoriasis who have two or more traditional cardiovascular risk factors given the high prevalence of CAC observed in this study,” they said.
Dr. Mansouri disclosed serving on an advisory board and receiving an honorarium from Celgene, maker of the psoriasis drug apremilast (Otezl). Study coauthors disclosed financial relationships with multiple pharmaceutical companies.
Psoriasis increased the measures of coronary artery calcium at a level similar to that seen in type 2 diabetes mellitus, independent of cardiovascular disease risk factors, based on data from a trio of cross-sectional studies including 387 adults. .
“Psoriasis and type 2 diabetes share similar cardiovascular risk profiles, which may predispose patients to developing coronary atherosclerosis at a relatively young age,” wrote Bobbak Mansouri, MD, of Baylor University Medical Center in Dallas and his associates (JAMA Dermatol. 2016. [doi: 10.1001/jamadermatol.2016.2907]).
The researchers compared coronary artery calcium (CAC) levels in patients with psoriasis, patients with type 2 diabetes, and healthy controls. CAC has become an accepted measure of atherosclerosis and “the cornerstone for screening the risk of future cardiac events and improving cardiovascular risk stratification beyond traditional risk factors, especially in higher-risk groups,” according to the investigators. The average age of the patients was 52 years, 50% were female, and at least 92% were white.
The researchers used a hierarchical Tobit regression analysis to determine the association between disease and CAC level, as measured by the Agatston score. After controlling for confounding variables, including cardiovascular risk factors (low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, fasting blood glucose, systolic blood pressure, and tobacco use), the association with CAC was similar in psoriasis patients and type 2 diabetes patients (Tobit regression ratio [TRR], 0.89 and 0.79, respectively).
In a logistic multivariate regression analysis, patients with either psoriasis or type 2 diabetes were approximately twice as likely to have evidence of CAC as were healthy controls (odds ratio, 2.35 and 2.18, respectively), and psoriasis remained independently associated with the presence of CAC.
“When we added use of systemic or biological therapy to the models, the TRR and OR increased; however, these analyses were exploratory,” wrote Dr. Mansouri and his associates.
The study was limited by factors including the cross-sectional design and lack of diversity in the patient population, the investigators noted. However, the results suggest that “CAC assessment may be considered in patients with psoriasis who have two or more traditional cardiovascular risk factors given the high prevalence of CAC observed in this study,” they said.
Dr. Mansouri disclosed serving on an advisory board and receiving an honorarium from Celgene, maker of the psoriasis drug apremilast (Otezl). Study coauthors disclosed financial relationships with multiple pharmaceutical companies.
Psoriasis increased the measures of coronary artery calcium at a level similar to that seen in type 2 diabetes mellitus, independent of cardiovascular disease risk factors, based on data from a trio of cross-sectional studies including 387 adults. .
“Psoriasis and type 2 diabetes share similar cardiovascular risk profiles, which may predispose patients to developing coronary atherosclerosis at a relatively young age,” wrote Bobbak Mansouri, MD, of Baylor University Medical Center in Dallas and his associates (JAMA Dermatol. 2016. [doi: 10.1001/jamadermatol.2016.2907]).
The researchers compared coronary artery calcium (CAC) levels in patients with psoriasis, patients with type 2 diabetes, and healthy controls. CAC has become an accepted measure of atherosclerosis and “the cornerstone for screening the risk of future cardiac events and improving cardiovascular risk stratification beyond traditional risk factors, especially in higher-risk groups,” according to the investigators. The average age of the patients was 52 years, 50% were female, and at least 92% were white.
The researchers used a hierarchical Tobit regression analysis to determine the association between disease and CAC level, as measured by the Agatston score. After controlling for confounding variables, including cardiovascular risk factors (low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, fasting blood glucose, systolic blood pressure, and tobacco use), the association with CAC was similar in psoriasis patients and type 2 diabetes patients (Tobit regression ratio [TRR], 0.89 and 0.79, respectively).
In a logistic multivariate regression analysis, patients with either psoriasis or type 2 diabetes were approximately twice as likely to have evidence of CAC as were healthy controls (odds ratio, 2.35 and 2.18, respectively), and psoriasis remained independently associated with the presence of CAC.
“When we added use of systemic or biological therapy to the models, the TRR and OR increased; however, these analyses were exploratory,” wrote Dr. Mansouri and his associates.
The study was limited by factors including the cross-sectional design and lack of diversity in the patient population, the investigators noted. However, the results suggest that “CAC assessment may be considered in patients with psoriasis who have two or more traditional cardiovascular risk factors given the high prevalence of CAC observed in this study,” they said.
Dr. Mansouri disclosed serving on an advisory board and receiving an honorarium from Celgene, maker of the psoriasis drug apremilast (Otezl). Study coauthors disclosed financial relationships with multiple pharmaceutical companies.
FROM JAMA DERMATOLOGY
Key clinical point: Psoriasis patients have increased coronary artery calcium levels similar to those seen in type 2 diabetes patients, suggesting subclinical atherosclerosis.
Major finding: Psoriasis patients were more than twice as likely (odds ratio, 2.35) to have evidence of coronary artery calcium, compared with healthy controls.
Data source: A set of three single-center, cross-sectional studies totaling 387 adults and including individuals with psoriasis or type 2 diabetes, and healthy controls.
Disclosures: Dr. Mansouri disclosed serving on an advisory board and receiving an honorarium from Celgene, maker of the psoriasis drug apremilast (Otezl). Study coauthors disclosed financial relationships with multiple pharmaceutical companies.
Cycling accounts for the most sports-related ED visits
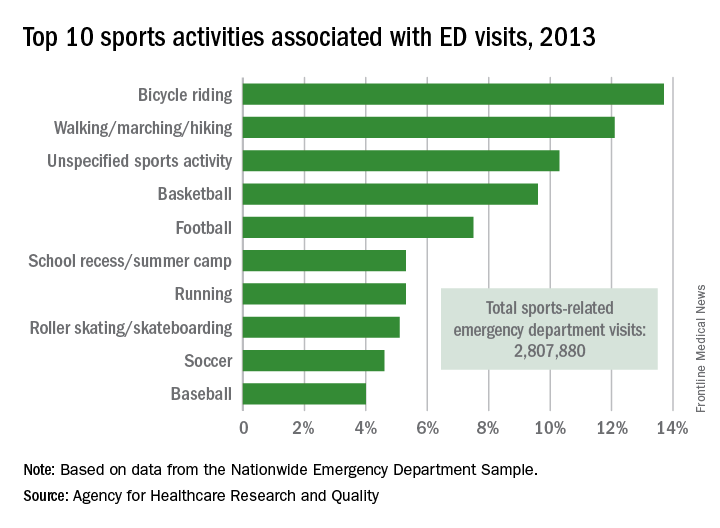
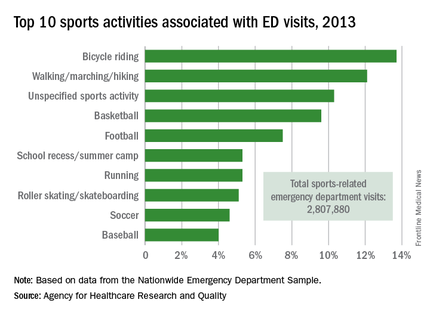
Bicycle riding results in more visits to the emergency department than any other sports activity, according to the Agency for Healthcare Research and Quality.
In 2013, cycling accounted for 13.7% of the 2.8 million ED visits in which the patient was discharged. Walking/marching/hiking was next with 12.1% of ED visits, while unspecified sports activities were third with 10.3% of visits. Team sports took the next two spots: basketball accounted for 9.6% of visits and football accounted for 7.5%, the AHRQ reported.
Among those under age 18 years, who accounted for over 1.5 million ED visits (54.7%) in 2013, football was associated with the most visits for boys (16.4%) and school recess/summer camp led to the most visits (12.8%) for girls. Bicycle riding was the leading reason for males aged 18-44 (16%) and 45-64 (34.9%), while walking/marching/hiking was the leading reason for females aged 18-44 (25.6%) and 45-64 years (49%). For those older than 65 years, walking/marching/hiking was the most common reason for ED visits among both men (54.3%) and women (79.7%), according to data from the Nationwide Emergency Department Sample.
The most common injuries in 2013 for sports-related ED visits (discharged) were sprains (24%), fractures (21%), and superficial injuries (18%). Among the top 10 injury-producing sports activities, cycling had the highest rate of superficial injuries at 26%, school recess/summer camp had the highest fracture rate (42%), and basketball led with a sprain rate of 40%, the AHRQ said.
Bicycle riding results in more visits to the emergency department than any other sports activity, according to the Agency for Healthcare Research and Quality.
In 2013, cycling accounted for 13.7% of the 2.8 million ED visits in which the patient was discharged. Walking/marching/hiking was next with 12.1% of ED visits, while unspecified sports activities were third with 10.3% of visits. Team sports took the next two spots: basketball accounted for 9.6% of visits and football accounted for 7.5%, the AHRQ reported.

Among those under age 18 years, who accounted for over 1.5 million ED visits (54.7%) in 2013, football was associated with the most visits for boys (16.4%) and school recess/summer camp led to the most visits (12.8%) for girls. Bicycle riding was the leading reason for males aged 18-44 (16%) and 45-64 (34.9%), while walking/marching/hiking was the leading reason for females aged 18-44 (25.6%) and 45-64 years (49%). For those older than 65 years, walking/marching/hiking was the most common reason for ED visits among both men (54.3%) and women (79.7%), according to data from the Nationwide Emergency Department Sample.
The most common injuries in 2013 for sports-related ED visits (discharged) were sprains (24%), fractures (21%), and superficial injuries (18%). Among the top 10 injury-producing sports activities, cycling had the highest rate of superficial injuries at 26%, school recess/summer camp had the highest fracture rate (42%), and basketball led with a sprain rate of 40%, the AHRQ said.
Bicycle riding results in more visits to the emergency department than any other sports activity, according to the Agency for Healthcare Research and Quality.
In 2013, cycling accounted for 13.7% of the 2.8 million ED visits in which the patient was discharged. Walking/marching/hiking was next with 12.1% of ED visits, while unspecified sports activities were third with 10.3% of visits. Team sports took the next two spots: basketball accounted for 9.6% of visits and football accounted for 7.5%, the AHRQ reported.

Among those under age 18 years, who accounted for over 1.5 million ED visits (54.7%) in 2013, football was associated with the most visits for boys (16.4%) and school recess/summer camp led to the most visits (12.8%) for girls. Bicycle riding was the leading reason for males aged 18-44 (16%) and 45-64 (34.9%), while walking/marching/hiking was the leading reason for females aged 18-44 (25.6%) and 45-64 years (49%). For those older than 65 years, walking/marching/hiking was the most common reason for ED visits among both men (54.3%) and women (79.7%), according to data from the Nationwide Emergency Department Sample.
The most common injuries in 2013 for sports-related ED visits (discharged) were sprains (24%), fractures (21%), and superficial injuries (18%). Among the top 10 injury-producing sports activities, cycling had the highest rate of superficial injuries at 26%, school recess/summer camp had the highest fracture rate (42%), and basketball led with a sprain rate of 40%, the AHRQ said.
Vaccine refusals and pediatrician dismissals increasing
More parents have been refusing vaccines in recent years than a decade ago, according to surveys of pediatricians by the American Academy of Pediatrics published in Pediatrics Aug. 29.
“In a busy practice, vaccine refusals and delays occur daily (if not multiple times per day),” wrote Catherine Hough-Telford, MD, of the University of Alabama at Birmingham and her colleagues (Pediatrics. 2016 Aug. 29. doi: 10.1542/peds.2016-2127).

“From the perspective of the pediatricians, parents who delay vaccines may do so because of concern for their child’s discomfort and concern about immune system burden, whereas vaccine refusers are more likely to believe that vaccines are unnecessary,” the authors wrote. “Pediatricians report that they continue to provide education to vaccine-refusing and delaying parents at high rates.”
Dr. Hough-Telford’s team compared the national AAP Periodic Surveys of 2006 and 2013 that dealt exclusively with immunizations to learn how currently practicing pediatricians perceived three issues related to vaccination: the prevalence of vaccine refusals and delays, why parents refuse or delay vaccines, and the decision of doctors to dismiss families who refuse to vaccinate.
The researchers excluded pediatricians who did not routinely administer vaccines or otherwise adhere to the Centers for Disease Control and Prevention–recommended immunization schedule for their patients. The 2006 survey had a response rate of 52.6% and included 629 final respondents; the 2013 survey had a response rate of 52.7% and included 627 respondents.
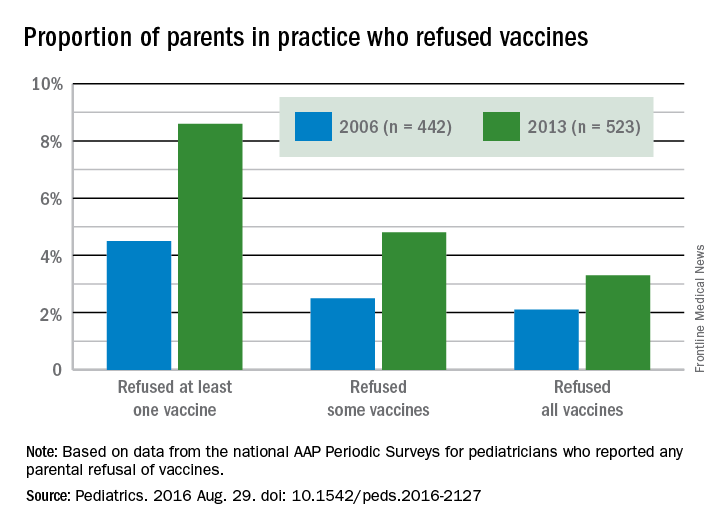
The proportion of pediatricians reporting parental refusals in their practice increased from 74.5% in 2006 to 87% in 2013 (odds ratio, 2.29; P less than .001). These pediatricians estimated in 2013 that 8.6% of their patients refused at least one vaccine, compared with 4.5% in 2006. Similarly, 2.5% of parents refused some vaccines in 2006, the physicians reported, compared with 4.8% in 2013. Those refusing all vaccines increased from 2.1% in 2006 to 3.3% in 2013.
A perceived 73.1% of parents refused vaccines in 2013 because they regarded them as unnecessary, the pediatricians reported, compared with 63.4% in 2006. But parental concern over autism and/or thimerosal dropped from 74.2% of vaccine-refusing parents in 2006 to 64.3% in 2013. Further, the parents refusing vaccines because of safety/side effects concerns dropped from 73.7% in 2006 to 66.6% in 2013, and those worried about their children receiving too many shots more than halved from 42.1% in 2006 to 17% in 2013. Concern among parents about their baby being too small to receive vaccines also dropped.

Physicians estimated that 7.3% of their parents wanted to delay one vaccine, 7.1% wanted to delay multiple vaccines, and 4.3% wanted to delay all vaccines. Urban, inner-city pediatricians were less likely to have parents requesting delays than were parents in other areas, but requests for vaccine delays were geographically similar across different U.S. regions. Only the 2013 survey included questions on delaying vaccines.
Pediatricians reported that 75% of their patients wanted to delay vaccines to reduce discomfort to their child, and 72.5% wanted to delay because they perceived too many vaccines would overburden their child’s immune system.
The percentage of pediatricians who always dismiss patients who continue to refuse vaccines increased from 6.1% in 2006 to 11.7% in 2013; they cited a lack of trust between physician and patient as a major reason (87.4% in 2006; 79.9% in 2013). Further, 80.5% of pediatricians reported in 2013 (the only year asked) that they dismissed vaccine-refusing patients out of concern for their other patients.
Despite no notable geographic differences in dismissals in 2006, the 2013 survey revealed that pediatricians in the West had three to four times greater odds of dismissing patients than those in the Midwest and South. Suburban pediatricians had three times greater odds of dismissing patients than did urban physicians.
The research was funded by the AAP and the CDC Childhood Immunization Support Program. Dr. Kimberlin was a site principal investigator for two multisite studies conducted by GlaxoSmithKline and Gilead.
More parents have been refusing vaccines in recent years than a decade ago, according to surveys of pediatricians by the American Academy of Pediatrics published in Pediatrics Aug. 29.
“In a busy practice, vaccine refusals and delays occur daily (if not multiple times per day),” wrote Catherine Hough-Telford, MD, of the University of Alabama at Birmingham and her colleagues (Pediatrics. 2016 Aug. 29. doi: 10.1542/peds.2016-2127).

“From the perspective of the pediatricians, parents who delay vaccines may do so because of concern for their child’s discomfort and concern about immune system burden, whereas vaccine refusers are more likely to believe that vaccines are unnecessary,” the authors wrote. “Pediatricians report that they continue to provide education to vaccine-refusing and delaying parents at high rates.”
Dr. Hough-Telford’s team compared the national AAP Periodic Surveys of 2006 and 2013 that dealt exclusively with immunizations to learn how currently practicing pediatricians perceived three issues related to vaccination: the prevalence of vaccine refusals and delays, why parents refuse or delay vaccines, and the decision of doctors to dismiss families who refuse to vaccinate.
The researchers excluded pediatricians who did not routinely administer vaccines or otherwise adhere to the Centers for Disease Control and Prevention–recommended immunization schedule for their patients. The 2006 survey had a response rate of 52.6% and included 629 final respondents; the 2013 survey had a response rate of 52.7% and included 627 respondents.
The proportion of pediatricians reporting parental refusals in their practice increased from 74.5% in 2006 to 87% in 2013 (odds ratio, 2.29; P less than .001). These pediatricians estimated in 2013 that 8.6% of their patients refused at least one vaccine, compared with 4.5% in 2006. Similarly, 2.5% of parents refused some vaccines in 2006, the physicians reported, compared with 4.8% in 2013. Those refusing all vaccines increased from 2.1% in 2006 to 3.3% in 2013.
A perceived 73.1% of parents refused vaccines in 2013 because they regarded them as unnecessary, the pediatricians reported, compared with 63.4% in 2006. But parental concern over autism and/or thimerosal dropped from 74.2% of vaccine-refusing parents in 2006 to 64.3% in 2013. Further, the parents refusing vaccines because of safety/side effects concerns dropped from 73.7% in 2006 to 66.6% in 2013, and those worried about their children receiving too many shots more than halved from 42.1% in 2006 to 17% in 2013. Concern among parents about their baby being too small to receive vaccines also dropped.

Physicians estimated that 7.3% of their parents wanted to delay one vaccine, 7.1% wanted to delay multiple vaccines, and 4.3% wanted to delay all vaccines. Urban, inner-city pediatricians were less likely to have parents requesting delays than were parents in other areas, but requests for vaccine delays were geographically similar across different U.S. regions. Only the 2013 survey included questions on delaying vaccines.
Pediatricians reported that 75% of their patients wanted to delay vaccines to reduce discomfort to their child, and 72.5% wanted to delay because they perceived too many vaccines would overburden their child’s immune system.
The percentage of pediatricians who always dismiss patients who continue to refuse vaccines increased from 6.1% in 2006 to 11.7% in 2013; they cited a lack of trust between physician and patient as a major reason (87.4% in 2006; 79.9% in 2013). Further, 80.5% of pediatricians reported in 2013 (the only year asked) that they dismissed vaccine-refusing patients out of concern for their other patients.
Despite no notable geographic differences in dismissals in 2006, the 2013 survey revealed that pediatricians in the West had three to four times greater odds of dismissing patients than those in the Midwest and South. Suburban pediatricians had three times greater odds of dismissing patients than did urban physicians.
The research was funded by the AAP and the CDC Childhood Immunization Support Program. Dr. Kimberlin was a site principal investigator for two multisite studies conducted by GlaxoSmithKline and Gilead.
More parents have been refusing vaccines in recent years than a decade ago, according to surveys of pediatricians by the American Academy of Pediatrics published in Pediatrics Aug. 29.
“In a busy practice, vaccine refusals and delays occur daily (if not multiple times per day),” wrote Catherine Hough-Telford, MD, of the University of Alabama at Birmingham and her colleagues (Pediatrics. 2016 Aug. 29. doi: 10.1542/peds.2016-2127).

“From the perspective of the pediatricians, parents who delay vaccines may do so because of concern for their child’s discomfort and concern about immune system burden, whereas vaccine refusers are more likely to believe that vaccines are unnecessary,” the authors wrote. “Pediatricians report that they continue to provide education to vaccine-refusing and delaying parents at high rates.”
Dr. Hough-Telford’s team compared the national AAP Periodic Surveys of 2006 and 2013 that dealt exclusively with immunizations to learn how currently practicing pediatricians perceived three issues related to vaccination: the prevalence of vaccine refusals and delays, why parents refuse or delay vaccines, and the decision of doctors to dismiss families who refuse to vaccinate.
The researchers excluded pediatricians who did not routinely administer vaccines or otherwise adhere to the Centers for Disease Control and Prevention–recommended immunization schedule for their patients. The 2006 survey had a response rate of 52.6% and included 629 final respondents; the 2013 survey had a response rate of 52.7% and included 627 respondents.
The proportion of pediatricians reporting parental refusals in their practice increased from 74.5% in 2006 to 87% in 2013 (odds ratio, 2.29; P less than .001). These pediatricians estimated in 2013 that 8.6% of their patients refused at least one vaccine, compared with 4.5% in 2006. Similarly, 2.5% of parents refused some vaccines in 2006, the physicians reported, compared with 4.8% in 2013. Those refusing all vaccines increased from 2.1% in 2006 to 3.3% in 2013.
A perceived 73.1% of parents refused vaccines in 2013 because they regarded them as unnecessary, the pediatricians reported, compared with 63.4% in 2006. But parental concern over autism and/or thimerosal dropped from 74.2% of vaccine-refusing parents in 2006 to 64.3% in 2013. Further, the parents refusing vaccines because of safety/side effects concerns dropped from 73.7% in 2006 to 66.6% in 2013, and those worried about their children receiving too many shots more than halved from 42.1% in 2006 to 17% in 2013. Concern among parents about their baby being too small to receive vaccines also dropped.

Physicians estimated that 7.3% of their parents wanted to delay one vaccine, 7.1% wanted to delay multiple vaccines, and 4.3% wanted to delay all vaccines. Urban, inner-city pediatricians were less likely to have parents requesting delays than were parents in other areas, but requests for vaccine delays were geographically similar across different U.S. regions. Only the 2013 survey included questions on delaying vaccines.
Pediatricians reported that 75% of their patients wanted to delay vaccines to reduce discomfort to their child, and 72.5% wanted to delay because they perceived too many vaccines would overburden their child’s immune system.
The percentage of pediatricians who always dismiss patients who continue to refuse vaccines increased from 6.1% in 2006 to 11.7% in 2013; they cited a lack of trust between physician and patient as a major reason (87.4% in 2006; 79.9% in 2013). Further, 80.5% of pediatricians reported in 2013 (the only year asked) that they dismissed vaccine-refusing patients out of concern for their other patients.
Despite no notable geographic differences in dismissals in 2006, the 2013 survey revealed that pediatricians in the West had three to four times greater odds of dismissing patients than those in the Midwest and South. Suburban pediatricians had three times greater odds of dismissing patients than did urban physicians.
The research was funded by the AAP and the CDC Childhood Immunization Support Program. Dr. Kimberlin was a site principal investigator for two multisite studies conducted by GlaxoSmithKline and Gilead.
FROM PEDIATRICS
Key clinical point: More parents have been refusing vaccines, and more pediatricians have been dismissing vaccine-refusing patients, since 2006.
Major finding: 11.7% of pediatricians reported dismissing patients for refusing vaccines in 2013, compared with 6.1% in 2006, and 8.6% of parents refused at least one vaccine in 2013, compared with 4.5% in 2006.
Data source: The findings are based on surveys of practicing members of the American Academy of Pediatrics, with 629 pediatricians in 2006 and 627 pediatricians in 2013.
Disclosures: The research was funded by the AAP and the Centers for Disease Control and Prevention Childhood Immunization Support Program. Dr. Kimberlin was a site principal investigator for two multisite studies conducted by GlaxoSmithKline and Gilead.