User login
Preventing Preterm Birth: The Most Important Challenge in Today’s Obstetrics
Vincenzo Berghella, MD
Professor
Division of Maternal-Fetal Medicine
Department of Obstetrics and Gynecology
Sidney Kimmel Medical College of Thomas
Jefferson University
Philadelphia, PA
Cynthia Gyamfi-Bannerman, MD, MSc
Ellen Jacobson Levine and Eugene Jacobson
Associate Professor of Women’s Health
in OBGYN
Department of Obstetrics and Gynecology
Division of Maternal-Fetal Medicine
Columbia University Medical Center
New York, NY
David L. Gandell, MD
Clinical Professor of Obstetrics
and Gynecology
Department of Obstetrics and Gynecology
Strong Memorial Hospital
University of Rochester School
of Medicine and Dentistry
Rochester, NY
Tracy A. Manuck, MD, MSCI
Assistant Professor
University of North Carolina
Department of Obstetrics and Gynecology
Division of Maternal Fetal Medicine
Medical Director, UNC Prematurity
Prevention Program
University of North Carolina-Chapel Hill
Chapel Hill, NC
Daniel O’Keeffe, MD
Executive Vice President
Society for Maternal-Fetal Medicine
Washington, DC
Ashley S. Roman, MD, MPH
Clinical Assistant Professor
Division of Maternal Fetal Medicine
Department of Obstetrics and Gynecology
Director, Division of Maternal Fetal Medicine
NYU School of Medicine
NYU Langone Medical Center,
New York, NY
Click here to read the supplement
To receive CME credit, please read the article and go to www.omniaeducation.com/obg2016 to complete the online evaluation.
Vincenzo Berghella, MD
Professor
Division of Maternal-Fetal Medicine
Department of Obstetrics and Gynecology
Sidney Kimmel Medical College of Thomas
Jefferson University
Philadelphia, PA
Cynthia Gyamfi-Bannerman, MD, MSc
Ellen Jacobson Levine and Eugene Jacobson
Associate Professor of Women’s Health
in OBGYN
Department of Obstetrics and Gynecology
Division of Maternal-Fetal Medicine
Columbia University Medical Center
New York, NY
David L. Gandell, MD
Clinical Professor of Obstetrics
and Gynecology
Department of Obstetrics and Gynecology
Strong Memorial Hospital
University of Rochester School
of Medicine and Dentistry
Rochester, NY
Tracy A. Manuck, MD, MSCI
Assistant Professor
University of North Carolina
Department of Obstetrics and Gynecology
Division of Maternal Fetal Medicine
Medical Director, UNC Prematurity
Prevention Program
University of North Carolina-Chapel Hill
Chapel Hill, NC
Daniel O’Keeffe, MD
Executive Vice President
Society for Maternal-Fetal Medicine
Washington, DC
Ashley S. Roman, MD, MPH
Clinical Assistant Professor
Division of Maternal Fetal Medicine
Department of Obstetrics and Gynecology
Director, Division of Maternal Fetal Medicine
NYU School of Medicine
NYU Langone Medical Center,
New York, NY
Click here to read the supplement
To receive CME credit, please read the article and go to www.omniaeducation.com/obg2016 to complete the online evaluation.
Vincenzo Berghella, MD
Professor
Division of Maternal-Fetal Medicine
Department of Obstetrics and Gynecology
Sidney Kimmel Medical College of Thomas
Jefferson University
Philadelphia, PA
Cynthia Gyamfi-Bannerman, MD, MSc
Ellen Jacobson Levine and Eugene Jacobson
Associate Professor of Women’s Health
in OBGYN
Department of Obstetrics and Gynecology
Division of Maternal-Fetal Medicine
Columbia University Medical Center
New York, NY
David L. Gandell, MD
Clinical Professor of Obstetrics
and Gynecology
Department of Obstetrics and Gynecology
Strong Memorial Hospital
University of Rochester School
of Medicine and Dentistry
Rochester, NY
Tracy A. Manuck, MD, MSCI
Assistant Professor
University of North Carolina
Department of Obstetrics and Gynecology
Division of Maternal Fetal Medicine
Medical Director, UNC Prematurity
Prevention Program
University of North Carolina-Chapel Hill
Chapel Hill, NC
Daniel O’Keeffe, MD
Executive Vice President
Society for Maternal-Fetal Medicine
Washington, DC
Ashley S. Roman, MD, MPH
Clinical Assistant Professor
Division of Maternal Fetal Medicine
Department of Obstetrics and Gynecology
Director, Division of Maternal Fetal Medicine
NYU School of Medicine
NYU Langone Medical Center,
New York, NY
Click here to read the supplement
To receive CME credit, please read the article and go to www.omniaeducation.com/obg2016 to complete the online evaluation.
Chronic Daily Headache Is Common Among Younger Children
SAN DIEGO—Chronic daily headache is common in younger children and manifests with similar characteristics as it does among older children, according to research presented at the 58th Annual Scientific Meeting of the American Headache Society. Because younger children often present for evaluation later in the disease process, compared with older children, earlier diagnosis would decrease morbidity significantly. Greater effort is needed to decrease medication overuse and school absenteeism, which contribute to headache chronification, said Emine Tunc, MD, research scholar at the Cleveland Clinic Foundation.
To increase the awareness of chronic headache among children between ages 3 and 11, Dr. Tunc and colleagues performed a literature review. The group identified 29 articles that examined the prevalence and characteristics of chronic daily headache in children. Six of them included information specific to younger children.
Dr. Tunc and her colleague A. David Rothner, MD, a child neurologist at the Cleveland Clinic, also reviewed the charts of patients who presented to the Cleveland Clinic's headache clinic for evaluation between May 2014 and May 2015. They defined chronic daily headache as more than 15 days of headache per month for at least two months and excluded patients with secondary headache disorders.
Pediatric Incidence of Chronic Daily Headache
The mean frequency of chronic daily headache in children and adolescents reported in the literature ranged from 8% to 30%, with a weighted mean of 23%. Between 28% and 56% of patients in the literature were younger than 12. Dr. Tunc and colleagues identified 327 patients with primary headache in their chart review. Of those patients, 57% had chronic daily headache. Approximately 28% of patients with chronic daily headache were younger than 12. The most common type of chronic daily headache was chronic migraine (a combination of migraine and tension-type headaches).
In the literature review and in the chart review, the male-to-female ratio was 1:2 among older patients (ie, between ages 11 and 18), which is similar to the ratio among adults, and 1:1 among younger patients (ie, ages 3 to 11). "This follows the concept that males develop headaches earlier than females, and later on, females [predominate]," said Dr. Tunc.
Younger Children Present Later in the Disease Process
The most interesting finding, she added, was that younger patients had greater duration of illness before initial evaluation, compared with older patients. One possible explanation for this finding is that children younger than 11 may not be articulate enough to state that they are having a headache, or parents may misinterpret signs and symptoms of headaches (eg, cessation of play and retreat to a dark, quiet room). In addition, parents may doubt that their young children are having headaches and may ignore them.
The chart review also showed that 61% of younger children and 52% of older children had medication overuse. "This difference between younger and older children is not statistically significant, but we were not expecting to see this at all," said Dr. Tunc. "One possible explanation, which we come across most commonly, is that parents are eager to stop the pain of little ones, but they are less concerned in older children. But the information that they are lacking is that pain killers themselves can initiate headaches."
About 38% of younger patients and 59% of older patients underwent brain MRI. All MRI results were within normal limits. "This proves to us that if there is no neurologic symptom and the physical exam is not concerning for underlying neurologic diseases such as tumor or increased CSF, MRI is not going to help us to diagnose these patients," said Dr. Tunc. "A comprehensive history and a really good physical exam will help us to diagnose these patients without an MRI."
Finally, the investigators observed that 30% of younger patients and 41% of older patients had excessive school absences, which they defined as missing more than 10 full days of school in the previous three months. "This school absenteeism is not only a result of the headaches, but also contributes to the chronicity of the headaches and makes those headaches harder to treat," said Dr. Tunc.
"As a next step, we should find ways to share this information with the pediatricians," she continued. Pediatricians are the first physicians to see children with headache and should be able to provide early diagnosis, treatment, or at least refer patients to a headache specialist or child neurologist, Dr. Tunc concluded.
—Erik Greb
Suggested Reading
Bigal ME, Rapoport AM, Tepper SJ, et al. The classification of chronic daily headache in adolescents--a comparison between the second edition of the International Classification of Headache Disorders and alternative diagnostic criteria. Headache. 2005;45(5):582-589.
Gladstein J, Rothner AD. Chronic daily headache in children and adolescents. Semin Pediatr Neurol. 2010;17(2):88-92.
Seshia SS. Chronic daily headache in children and adolescents. Can J Neurol Sci. 2004;31(3):319-323.
SAN DIEGO—Chronic daily headache is common in younger children and manifests with similar characteristics as it does among older children, according to research presented at the 58th Annual Scientific Meeting of the American Headache Society. Because younger children often present for evaluation later in the disease process, compared with older children, earlier diagnosis would decrease morbidity significantly. Greater effort is needed to decrease medication overuse and school absenteeism, which contribute to headache chronification, said Emine Tunc, MD, research scholar at the Cleveland Clinic Foundation.
To increase the awareness of chronic headache among children between ages 3 and 11, Dr. Tunc and colleagues performed a literature review. The group identified 29 articles that examined the prevalence and characteristics of chronic daily headache in children. Six of them included information specific to younger children.
Dr. Tunc and her colleague A. David Rothner, MD, a child neurologist at the Cleveland Clinic, also reviewed the charts of patients who presented to the Cleveland Clinic's headache clinic for evaluation between May 2014 and May 2015. They defined chronic daily headache as more than 15 days of headache per month for at least two months and excluded patients with secondary headache disorders.
Pediatric Incidence of Chronic Daily Headache
The mean frequency of chronic daily headache in children and adolescents reported in the literature ranged from 8% to 30%, with a weighted mean of 23%. Between 28% and 56% of patients in the literature were younger than 12. Dr. Tunc and colleagues identified 327 patients with primary headache in their chart review. Of those patients, 57% had chronic daily headache. Approximately 28% of patients with chronic daily headache were younger than 12. The most common type of chronic daily headache was chronic migraine (a combination of migraine and tension-type headaches).
In the literature review and in the chart review, the male-to-female ratio was 1:2 among older patients (ie, between ages 11 and 18), which is similar to the ratio among adults, and 1:1 among younger patients (ie, ages 3 to 11). "This follows the concept that males develop headaches earlier than females, and later on, females [predominate]," said Dr. Tunc.
Younger Children Present Later in the Disease Process
The most interesting finding, she added, was that younger patients had greater duration of illness before initial evaluation, compared with older patients. One possible explanation for this finding is that children younger than 11 may not be articulate enough to state that they are having a headache, or parents may misinterpret signs and symptoms of headaches (eg, cessation of play and retreat to a dark, quiet room). In addition, parents may doubt that their young children are having headaches and may ignore them.
The chart review also showed that 61% of younger children and 52% of older children had medication overuse. "This difference between younger and older children is not statistically significant, but we were not expecting to see this at all," said Dr. Tunc. "One possible explanation, which we come across most commonly, is that parents are eager to stop the pain of little ones, but they are less concerned in older children. But the information that they are lacking is that pain killers themselves can initiate headaches."
About 38% of younger patients and 59% of older patients underwent brain MRI. All MRI results were within normal limits. "This proves to us that if there is no neurologic symptom and the physical exam is not concerning for underlying neurologic diseases such as tumor or increased CSF, MRI is not going to help us to diagnose these patients," said Dr. Tunc. "A comprehensive history and a really good physical exam will help us to diagnose these patients without an MRI."
Finally, the investigators observed that 30% of younger patients and 41% of older patients had excessive school absences, which they defined as missing more than 10 full days of school in the previous three months. "This school absenteeism is not only a result of the headaches, but also contributes to the chronicity of the headaches and makes those headaches harder to treat," said Dr. Tunc.
"As a next step, we should find ways to share this information with the pediatricians," she continued. Pediatricians are the first physicians to see children with headache and should be able to provide early diagnosis, treatment, or at least refer patients to a headache specialist or child neurologist, Dr. Tunc concluded.
—Erik Greb
SAN DIEGO—Chronic daily headache is common in younger children and manifests with similar characteristics as it does among older children, according to research presented at the 58th Annual Scientific Meeting of the American Headache Society. Because younger children often present for evaluation later in the disease process, compared with older children, earlier diagnosis would decrease morbidity significantly. Greater effort is needed to decrease medication overuse and school absenteeism, which contribute to headache chronification, said Emine Tunc, MD, research scholar at the Cleveland Clinic Foundation.
To increase the awareness of chronic headache among children between ages 3 and 11, Dr. Tunc and colleagues performed a literature review. The group identified 29 articles that examined the prevalence and characteristics of chronic daily headache in children. Six of them included information specific to younger children.
Dr. Tunc and her colleague A. David Rothner, MD, a child neurologist at the Cleveland Clinic, also reviewed the charts of patients who presented to the Cleveland Clinic's headache clinic for evaluation between May 2014 and May 2015. They defined chronic daily headache as more than 15 days of headache per month for at least two months and excluded patients with secondary headache disorders.
Pediatric Incidence of Chronic Daily Headache
The mean frequency of chronic daily headache in children and adolescents reported in the literature ranged from 8% to 30%, with a weighted mean of 23%. Between 28% and 56% of patients in the literature were younger than 12. Dr. Tunc and colleagues identified 327 patients with primary headache in their chart review. Of those patients, 57% had chronic daily headache. Approximately 28% of patients with chronic daily headache were younger than 12. The most common type of chronic daily headache was chronic migraine (a combination of migraine and tension-type headaches).
In the literature review and in the chart review, the male-to-female ratio was 1:2 among older patients (ie, between ages 11 and 18), which is similar to the ratio among adults, and 1:1 among younger patients (ie, ages 3 to 11). "This follows the concept that males develop headaches earlier than females, and later on, females [predominate]," said Dr. Tunc.
Younger Children Present Later in the Disease Process
The most interesting finding, she added, was that younger patients had greater duration of illness before initial evaluation, compared with older patients. One possible explanation for this finding is that children younger than 11 may not be articulate enough to state that they are having a headache, or parents may misinterpret signs and symptoms of headaches (eg, cessation of play and retreat to a dark, quiet room). In addition, parents may doubt that their young children are having headaches and may ignore them.
The chart review also showed that 61% of younger children and 52% of older children had medication overuse. "This difference between younger and older children is not statistically significant, but we were not expecting to see this at all," said Dr. Tunc. "One possible explanation, which we come across most commonly, is that parents are eager to stop the pain of little ones, but they are less concerned in older children. But the information that they are lacking is that pain killers themselves can initiate headaches."
About 38% of younger patients and 59% of older patients underwent brain MRI. All MRI results were within normal limits. "This proves to us that if there is no neurologic symptom and the physical exam is not concerning for underlying neurologic diseases such as tumor or increased CSF, MRI is not going to help us to diagnose these patients," said Dr. Tunc. "A comprehensive history and a really good physical exam will help us to diagnose these patients without an MRI."
Finally, the investigators observed that 30% of younger patients and 41% of older patients had excessive school absences, which they defined as missing more than 10 full days of school in the previous three months. "This school absenteeism is not only a result of the headaches, but also contributes to the chronicity of the headaches and makes those headaches harder to treat," said Dr. Tunc.
"As a next step, we should find ways to share this information with the pediatricians," she continued. Pediatricians are the first physicians to see children with headache and should be able to provide early diagnosis, treatment, or at least refer patients to a headache specialist or child neurologist, Dr. Tunc concluded.
—Erik Greb
Suggested Reading
Bigal ME, Rapoport AM, Tepper SJ, et al. The classification of chronic daily headache in adolescents--a comparison between the second edition of the International Classification of Headache Disorders and alternative diagnostic criteria. Headache. 2005;45(5):582-589.
Gladstein J, Rothner AD. Chronic daily headache in children and adolescents. Semin Pediatr Neurol. 2010;17(2):88-92.
Seshia SS. Chronic daily headache in children and adolescents. Can J Neurol Sci. 2004;31(3):319-323.
Suggested Reading
Bigal ME, Rapoport AM, Tepper SJ, et al. The classification of chronic daily headache in adolescents--a comparison between the second edition of the International Classification of Headache Disorders and alternative diagnostic criteria. Headache. 2005;45(5):582-589.
Gladstein J, Rothner AD. Chronic daily headache in children and adolescents. Semin Pediatr Neurol. 2010;17(2):88-92.
Seshia SS. Chronic daily headache in children and adolescents. Can J Neurol Sci. 2004;31(3):319-323.
Primary care physicians diagnose most pediatric thyroid conditions
Primary care physicians can play an important role in managing thyroid disease in children and teens by proactive screening and evaluation, based on data from a literature review of 83 articles published between Jan. 1, 2010, and Dec. 31, 2015. The review was published online Aug. 29 in JAMA Pediatrics.
“Early diagnosis and treatment of thyroid hormone deficiency is crucial to ensure normal development and cognition,” wrote Dr. Patrick Hanley of the Children’s Hospital of Philadelphia and his colleagues.
Thyroid dysgenesis accounts for 80%-85% of cases of primary congenital hypothyroidism, and many newborns with the condition are asymptomatic at birth because of protection by maternal thyroid hormones. Early signs of thyroid problems include a hoarse cry, prolonged jaundice, lethargy, poor feeding, and constipation, the researchers said (JAMA Pediatr. 2016. doi:10.1001/jamapediatrics.2016.0486).
“Once the diagnosis has been made, additional testing can be considered to determine the etiology of the hypothyroidism so that the family can receive anticipatory guidance in regard to the potential need for lifelong thyroid hormone replacement therapy,” the researchers wrote.
The treatment of choice for congenital hypothyroidism is levothyroxine at a starting dose of 10-15 mcg/kg once daily, they noted.
Read the full study here: http://archpedi.jamanetwork.com/article.aspx?doi=10.1001/jamapediatrics.2016.0486.
Primary care physicians can play an important role in managing thyroid disease in children and teens by proactive screening and evaluation, based on data from a literature review of 83 articles published between Jan. 1, 2010, and Dec. 31, 2015. The review was published online Aug. 29 in JAMA Pediatrics.
“Early diagnosis and treatment of thyroid hormone deficiency is crucial to ensure normal development and cognition,” wrote Dr. Patrick Hanley of the Children’s Hospital of Philadelphia and his colleagues.
Thyroid dysgenesis accounts for 80%-85% of cases of primary congenital hypothyroidism, and many newborns with the condition are asymptomatic at birth because of protection by maternal thyroid hormones. Early signs of thyroid problems include a hoarse cry, prolonged jaundice, lethargy, poor feeding, and constipation, the researchers said (JAMA Pediatr. 2016. doi:10.1001/jamapediatrics.2016.0486).
“Once the diagnosis has been made, additional testing can be considered to determine the etiology of the hypothyroidism so that the family can receive anticipatory guidance in regard to the potential need for lifelong thyroid hormone replacement therapy,” the researchers wrote.
The treatment of choice for congenital hypothyroidism is levothyroxine at a starting dose of 10-15 mcg/kg once daily, they noted.
Read the full study here: http://archpedi.jamanetwork.com/article.aspx?doi=10.1001/jamapediatrics.2016.0486.
Primary care physicians can play an important role in managing thyroid disease in children and teens by proactive screening and evaluation, based on data from a literature review of 83 articles published between Jan. 1, 2010, and Dec. 31, 2015. The review was published online Aug. 29 in JAMA Pediatrics.
“Early diagnosis and treatment of thyroid hormone deficiency is crucial to ensure normal development and cognition,” wrote Dr. Patrick Hanley of the Children’s Hospital of Philadelphia and his colleagues.
Thyroid dysgenesis accounts for 80%-85% of cases of primary congenital hypothyroidism, and many newborns with the condition are asymptomatic at birth because of protection by maternal thyroid hormones. Early signs of thyroid problems include a hoarse cry, prolonged jaundice, lethargy, poor feeding, and constipation, the researchers said (JAMA Pediatr. 2016. doi:10.1001/jamapediatrics.2016.0486).
“Once the diagnosis has been made, additional testing can be considered to determine the etiology of the hypothyroidism so that the family can receive anticipatory guidance in regard to the potential need for lifelong thyroid hormone replacement therapy,” the researchers wrote.
The treatment of choice for congenital hypothyroidism is levothyroxine at a starting dose of 10-15 mcg/kg once daily, they noted.
Read the full study here: http://archpedi.jamanetwork.com/article.aspx?doi=10.1001/jamapediatrics.2016.0486.
FROM JAMA PEDIATRICS
New Recommendations for Transitioning Youths With Brain Disorders to Adult Care
A new consensus statement provides recommendations for transitioning adolescents and young adults with neurologic disorders to adult care. Created by the Child Neurology Foundation, the recommendations were published online ahead of print July 27 in Neurology.
"The goal of this research is to help make the transition easier for millions of parents and caregivers of children who are reaching the age where they will be dealing with complex teenage and adult life situations," said study author Lawrence W. Brown, MD, a pediatric neurologist at the Children's Hospital of Philadelphia.
The consensus statement is the work of the Neurology Transition Consensus Panel, an interdisciplinary team including child neurologists, child neurology nurses, adult neurologists, rehabilitation specialists, patients, and family members of neurology patients.
The research team reviewed studies published within the last 10 years focused mainly on transition of care for those with neurologic disorders. Older studies and book chapters that were relevant to transition in general were also included. The consensus statement addresses the great majority of adolescents and young adults whose care shifts from pediatric to adult providers. The paper does not directly address children with brain diseases who are not expected to live to adulthood or those children who are managed by adult neurologists. However, it strongly recommends that adult models of care be introduced even if providers are unchanged.
The consensus statement identifies the following eight best practices for making the transition from pediatric to adult care as smooth as possible:
· Expectation of future transition to the adult care system with the youth and family is discussed before age 13.
· Self-management skills should be assessed at age 12 and reevaluated on a yearly basis.
· Yearly transition planning sessions should also address the youth's medical condition, including current medications and potential side effects; signs and symptoms of concern; genetic counseling and reproductive implications of the condition; issues of puberty and sexuality; driving, alcohol, substance use, and other risks; and emotional or psychological concerns and wellness.
· If appropriate, a discussion with the caregivers regarding the youth's expected legal competency (ie, whether there is a need for legal guardianship and powers of attorney) should begin by age 14. If the youth's expected legal competency is unclear, assessment of that capacity should be made yearly.
· A comprehensive transition plan should be developed by age 14 in collaboration with the youth, caregivers, other health care providers, school personnel, vocational professionals, community services providers, and legal services regarding all aspects of health, financial, and legal care. This is usually the responsibility of the primary care provider.
· The child neurology team is responsible for the neurologic component of the comprehensive transition plan and should update it annually.
· Adult providers are identified in collaboration with the youth and caregivers prior to the anticipated time of transfer. A medical transfer packet is prepared for the adult provider and provided to the youth; it includes the transition plan and medical summary with pertinent history, diagnostic evaluations, previous drug trials, current medications, and protocol for emergency care.
· The child neurology team communicates directly with the new adult provider to ensure smooth completion of the transition process, which is finalized after the first appointment. It is recommended that the child neurologist be available to the youth and the adult provider for continuity and support.
"We believe that following these steps will make a real difference in the lives of our patients as they become independent and self-sufficient adults to the greatest extent possible," said Dr. Brown.
The consensus statement was endorsed by the American Academy of Neurology, the Child Neurology Society, and the American Academy of Pediatrics and was cosponsored by Eisai.
Suggested Reading
American Academy of Pediatrics, American Academy of Family Physicians, American College of Physicians, et al. Supporting the health care transition from adolescence to adulthood in the medical home. Pediatrics. 2011;128(1):182-200.
Brown LW, Camfield P, Capers M, et al. The neurologist's role in supporting transition to adult health care. Neurology. 2016 July 27 [Epub ahead of print].
A new consensus statement provides recommendations for transitioning adolescents and young adults with neurologic disorders to adult care. Created by the Child Neurology Foundation, the recommendations were published online ahead of print July 27 in Neurology.
"The goal of this research is to help make the transition easier for millions of parents and caregivers of children who are reaching the age where they will be dealing with complex teenage and adult life situations," said study author Lawrence W. Brown, MD, a pediatric neurologist at the Children's Hospital of Philadelphia.
The consensus statement is the work of the Neurology Transition Consensus Panel, an interdisciplinary team including child neurologists, child neurology nurses, adult neurologists, rehabilitation specialists, patients, and family members of neurology patients.
The research team reviewed studies published within the last 10 years focused mainly on transition of care for those with neurologic disorders. Older studies and book chapters that were relevant to transition in general were also included. The consensus statement addresses the great majority of adolescents and young adults whose care shifts from pediatric to adult providers. The paper does not directly address children with brain diseases who are not expected to live to adulthood or those children who are managed by adult neurologists. However, it strongly recommends that adult models of care be introduced even if providers are unchanged.
The consensus statement identifies the following eight best practices for making the transition from pediatric to adult care as smooth as possible:
· Expectation of future transition to the adult care system with the youth and family is discussed before age 13.
· Self-management skills should be assessed at age 12 and reevaluated on a yearly basis.
· Yearly transition planning sessions should also address the youth's medical condition, including current medications and potential side effects; signs and symptoms of concern; genetic counseling and reproductive implications of the condition; issues of puberty and sexuality; driving, alcohol, substance use, and other risks; and emotional or psychological concerns and wellness.
· If appropriate, a discussion with the caregivers regarding the youth's expected legal competency (ie, whether there is a need for legal guardianship and powers of attorney) should begin by age 14. If the youth's expected legal competency is unclear, assessment of that capacity should be made yearly.
· A comprehensive transition plan should be developed by age 14 in collaboration with the youth, caregivers, other health care providers, school personnel, vocational professionals, community services providers, and legal services regarding all aspects of health, financial, and legal care. This is usually the responsibility of the primary care provider.
· The child neurology team is responsible for the neurologic component of the comprehensive transition plan and should update it annually.
· Adult providers are identified in collaboration with the youth and caregivers prior to the anticipated time of transfer. A medical transfer packet is prepared for the adult provider and provided to the youth; it includes the transition plan and medical summary with pertinent history, diagnostic evaluations, previous drug trials, current medications, and protocol for emergency care.
· The child neurology team communicates directly with the new adult provider to ensure smooth completion of the transition process, which is finalized after the first appointment. It is recommended that the child neurologist be available to the youth and the adult provider for continuity and support.
"We believe that following these steps will make a real difference in the lives of our patients as they become independent and self-sufficient adults to the greatest extent possible," said Dr. Brown.
The consensus statement was endorsed by the American Academy of Neurology, the Child Neurology Society, and the American Academy of Pediatrics and was cosponsored by Eisai.
A new consensus statement provides recommendations for transitioning adolescents and young adults with neurologic disorders to adult care. Created by the Child Neurology Foundation, the recommendations were published online ahead of print July 27 in Neurology.
"The goal of this research is to help make the transition easier for millions of parents and caregivers of children who are reaching the age where they will be dealing with complex teenage and adult life situations," said study author Lawrence W. Brown, MD, a pediatric neurologist at the Children's Hospital of Philadelphia.
The consensus statement is the work of the Neurology Transition Consensus Panel, an interdisciplinary team including child neurologists, child neurology nurses, adult neurologists, rehabilitation specialists, patients, and family members of neurology patients.
The research team reviewed studies published within the last 10 years focused mainly on transition of care for those with neurologic disorders. Older studies and book chapters that were relevant to transition in general were also included. The consensus statement addresses the great majority of adolescents and young adults whose care shifts from pediatric to adult providers. The paper does not directly address children with brain diseases who are not expected to live to adulthood or those children who are managed by adult neurologists. However, it strongly recommends that adult models of care be introduced even if providers are unchanged.
The consensus statement identifies the following eight best practices for making the transition from pediatric to adult care as smooth as possible:
· Expectation of future transition to the adult care system with the youth and family is discussed before age 13.
· Self-management skills should be assessed at age 12 and reevaluated on a yearly basis.
· Yearly transition planning sessions should also address the youth's medical condition, including current medications and potential side effects; signs and symptoms of concern; genetic counseling and reproductive implications of the condition; issues of puberty and sexuality; driving, alcohol, substance use, and other risks; and emotional or psychological concerns and wellness.
· If appropriate, a discussion with the caregivers regarding the youth's expected legal competency (ie, whether there is a need for legal guardianship and powers of attorney) should begin by age 14. If the youth's expected legal competency is unclear, assessment of that capacity should be made yearly.
· A comprehensive transition plan should be developed by age 14 in collaboration with the youth, caregivers, other health care providers, school personnel, vocational professionals, community services providers, and legal services regarding all aspects of health, financial, and legal care. This is usually the responsibility of the primary care provider.
· The child neurology team is responsible for the neurologic component of the comprehensive transition plan and should update it annually.
· Adult providers are identified in collaboration with the youth and caregivers prior to the anticipated time of transfer. A medical transfer packet is prepared for the adult provider and provided to the youth; it includes the transition plan and medical summary with pertinent history, diagnostic evaluations, previous drug trials, current medications, and protocol for emergency care.
· The child neurology team communicates directly with the new adult provider to ensure smooth completion of the transition process, which is finalized after the first appointment. It is recommended that the child neurologist be available to the youth and the adult provider for continuity and support.
"We believe that following these steps will make a real difference in the lives of our patients as they become independent and self-sufficient adults to the greatest extent possible," said Dr. Brown.
The consensus statement was endorsed by the American Academy of Neurology, the Child Neurology Society, and the American Academy of Pediatrics and was cosponsored by Eisai.
Suggested Reading
American Academy of Pediatrics, American Academy of Family Physicians, American College of Physicians, et al. Supporting the health care transition from adolescence to adulthood in the medical home. Pediatrics. 2011;128(1):182-200.
Brown LW, Camfield P, Capers M, et al. The neurologist's role in supporting transition to adult health care. Neurology. 2016 July 27 [Epub ahead of print].
Suggested Reading
American Academy of Pediatrics, American Academy of Family Physicians, American College of Physicians, et al. Supporting the health care transition from adolescence to adulthood in the medical home. Pediatrics. 2011;128(1):182-200.
Brown LW, Camfield P, Capers M, et al. The neurologist's role in supporting transition to adult health care. Neurology. 2016 July 27 [Epub ahead of print].
Clinical Update: Improving Patient Care by Reducing Retained Sponges During Vaginal Deliveries
This supplement is sponsored by Medtronic.
The retained surgical sponge or gauze after surgical intervention or a birth can have serious consequences for patients and poses a challenging problem for clinicians. This supplement explores the causes and consequences of sponge retention and how the Situate™ Delivery System can help reduce the incidence of gossypiboma, which is responsible for approximately 2000 cases of retained surgical items each year in the US.
Hector Chapa, MD
Clinical Assistant Professor
Texas A&M College of Medicine – College Station
Department of ObGyn, Brazos Valley ObGyn
ObGyn Hospitalist Group
CHI St. Joseph Regional Hospital
Bryan, Texas
Click here to read the Clinical Update
This supplement is sponsored by Medtronic.
The retained surgical sponge or gauze after surgical intervention or a birth can have serious consequences for patients and poses a challenging problem for clinicians. This supplement explores the causes and consequences of sponge retention and how the Situate™ Delivery System can help reduce the incidence of gossypiboma, which is responsible for approximately 2000 cases of retained surgical items each year in the US.
Hector Chapa, MD
Clinical Assistant Professor
Texas A&M College of Medicine – College Station
Department of ObGyn, Brazos Valley ObGyn
ObGyn Hospitalist Group
CHI St. Joseph Regional Hospital
Bryan, Texas
Click here to read the Clinical Update
This supplement is sponsored by Medtronic.
The retained surgical sponge or gauze after surgical intervention or a birth can have serious consequences for patients and poses a challenging problem for clinicians. This supplement explores the causes and consequences of sponge retention and how the Situate™ Delivery System can help reduce the incidence of gossypiboma, which is responsible for approximately 2000 cases of retained surgical items each year in the US.
Hector Chapa, MD
Clinical Assistant Professor
Texas A&M College of Medicine – College Station
Department of ObGyn, Brazos Valley ObGyn
ObGyn Hospitalist Group
CHI St. Joseph Regional Hospital
Bryan, Texas
Click here to read the Clinical Update
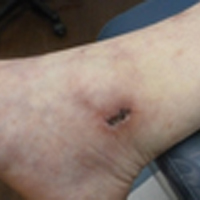
Painful Ulcerations Above the Malleoli
The Diagnosis: Livedoid Vasculopathy
Livedoid vasculopathy (LV) is a rare cutaneous disorder that most commonly affects the lower legs. It has an estimated incidence of 1 case per 100,000 per year and predominantly affects women.1 The disease pathogenesis is not fully understood but is thought to involve thrombosis and occlusion of dermal vessels resulting in tissue hypoxia.2 Both inherited and acquired thrombophilic conditions frequently are seen in patients with LV.3,4 Livedoid vasculopathy also has been described as idiopathic5 and is associated with immune complex deposition.6 However, the number of cases of idiopathic LV may be overestimated; as technological advancements to detect coagulation abnormalities improve, it is hypothesized that this entity will be identified less often.2,4
Livedoid vasculopathy has been described in the literature using the term PPURPLE (painful purpuric ulcers with reticular pattern of lower extremities).7 The triad of livedo racemosa, recurrent painful ulcerations, and residual healing with atrophie blanche characterizes the clinical manifestations of LV; however, all 3 characteristics do not need to appear simultaneously for a diagnosis to be made. The condition has a chronic course with spontaneous remissions and exacerbations. Episodic ulcerations occur, especially in the summertime, and heal slowly, leaving behind atrophic, porcelain white, stellate-shaped scars called atrophie blanche. Livedo racemosa also may be seen in Sneddon syndrome; however, these patients experience neurologic symptoms secondary to cerebrovascular occlusion. In contrast to livedo racemosa, acquired livedo reticularis represents a physiologic hypoperfusion pattern that occurs in response to cold exposure.8 A localized sharp pain, known as angina cutis, typically precedes the clinical symptom of painful ulcerations.9 Atrophie blanche once was thought to be specific to LV but has been seen in other diseases such as systemic lupus erythematosus and chronic venous insufficiency.2
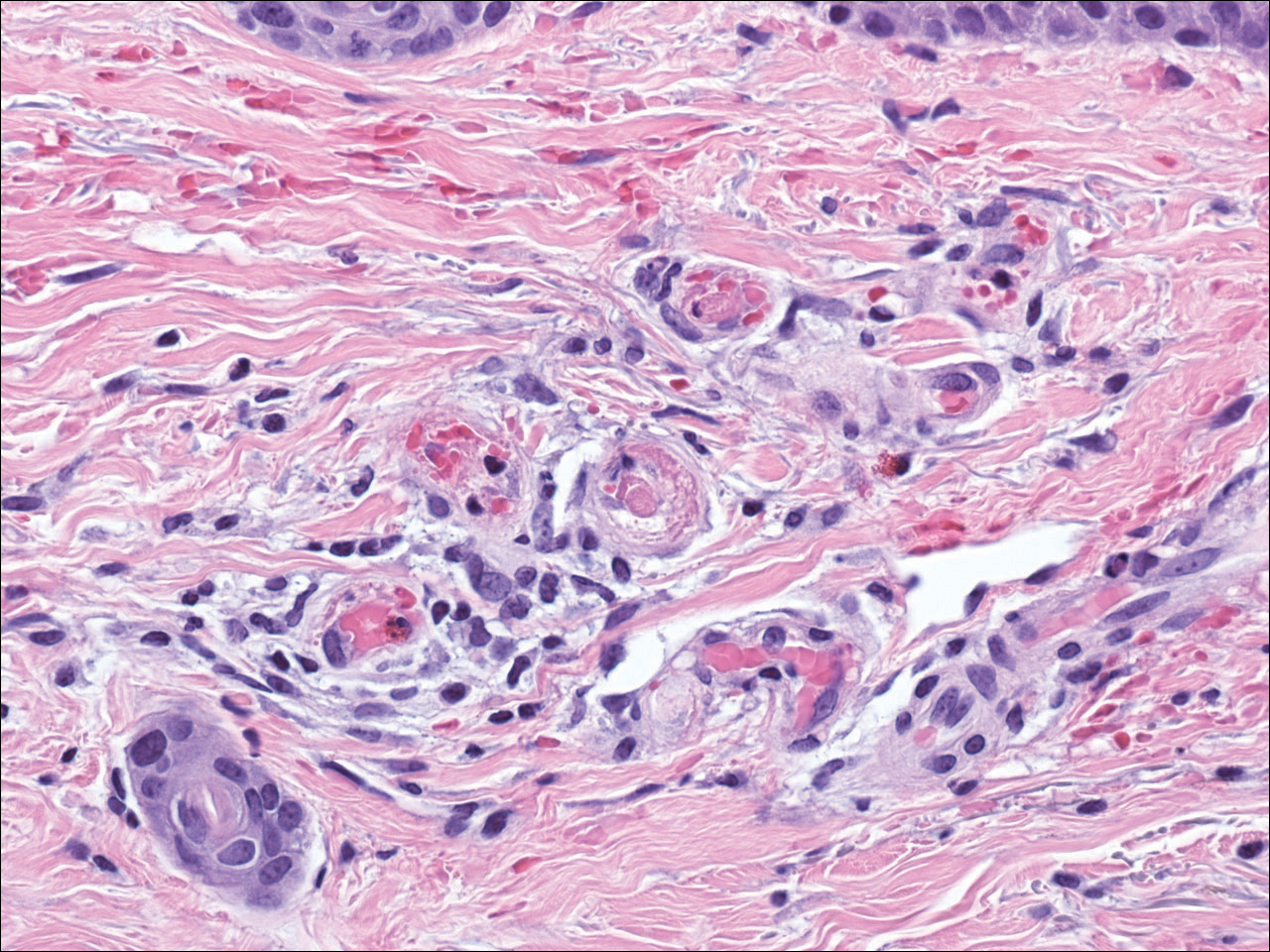
The diagnosis of LV is based on identification of characteristic clinical features and skin biopsy. In almost all biopsy specimens, histopathology reveals fibrinoid occlusion of vessels in the superficial and mid dermis.4 Other findings may include epidermal necrosis and vessel wall hyalinization and infarction2 (Figure). Because LV is commonly misdiagnosed as vasculitis, the absence of hallmark features of vasculitis such as neutrophilic infiltrate of blood vessel walls and fibrinoid necrosis suggest the diagnosis. Extensive laboratory evaluation for inherited and acquired coagulation abnormalities should be performed.
Treatment of LV is difficult, as there is currently no consensus on optimal therapy. The mainstay of therapy is to reduce pain, prevent infection, and reduce ulceration and development of atrophie blanche. Underlying causes should be identified and appropriately treated. Because the primary pathogenesis of LV is considered to be a hypercoagulable state, first-line treatment often includes therapies to enhance blood flow and prevent thrombosis such as smoking cessation, antiplatelet therapy, and pentoxifylline. Vasodilating agents, anti-inflammatory agents, anticoagulation, and fibrinolytic therapy also have been used with varying degrees of success.7
- Fritsch P, Zelger B. Livedo vasculitis [in German]. Hautarzt. 1995;46:215-224; quiz 222-223.
- Kerk N, Goerge T. Livedoid vasculopathy—a thrombotic disease. Vasa. 2013;42:317-322.
- Stevanovic DV. Atrophie blanche. a sign of dermal blood occlusion. Arch Dermatol. 1974;109:858-862.
- Hairston BR, Davis MD, Pittelkow MR, et al. Livedoid vasculopathy: further evidence for procoagulant pathogenesis. Arch Dermatol. 2006;142:1413-1418.
- Shornick JK, Nicholes BK, Bergstresser PR, et al. Idiopathic atrophie blanche. J Am Acad Dermatol. 1983;8:792-798.
- Feldaker M, Hines EA Jr, Kierland RR. Livedo reticularis with ulcerations. Circulation. 1956;13:196-216.
- Callen JP. Livedoid vasculopathy: what it is and how the patient should be evaluated and treated. Arch Dermatol. 2006;142:1481-1482.
- Copeman PW. Livedo reticularis. signs in the skin of disturbance of blood viscosity and of blood flow. Br J Dermatol. 1975;93:519-529.
- Goerge T. Livedoid vasculopathy. pathogenesis, diagnosis and treatment of cutaneous infarction [in German]. Hautarzt. 2011;62:627-634; quiz 635.
The Diagnosis: Livedoid Vasculopathy
Livedoid vasculopathy (LV) is a rare cutaneous disorder that most commonly affects the lower legs. It has an estimated incidence of 1 case per 100,000 per year and predominantly affects women.1 The disease pathogenesis is not fully understood but is thought to involve thrombosis and occlusion of dermal vessels resulting in tissue hypoxia.2 Both inherited and acquired thrombophilic conditions frequently are seen in patients with LV.3,4 Livedoid vasculopathy also has been described as idiopathic5 and is associated with immune complex deposition.6 However, the number of cases of idiopathic LV may be overestimated; as technological advancements to detect coagulation abnormalities improve, it is hypothesized that this entity will be identified less often.2,4
Livedoid vasculopathy has been described in the literature using the term PPURPLE (painful purpuric ulcers with reticular pattern of lower extremities).7 The triad of livedo racemosa, recurrent painful ulcerations, and residual healing with atrophie blanche characterizes the clinical manifestations of LV; however, all 3 characteristics do not need to appear simultaneously for a diagnosis to be made. The condition has a chronic course with spontaneous remissions and exacerbations. Episodic ulcerations occur, especially in the summertime, and heal slowly, leaving behind atrophic, porcelain white, stellate-shaped scars called atrophie blanche. Livedo racemosa also may be seen in Sneddon syndrome; however, these patients experience neurologic symptoms secondary to cerebrovascular occlusion. In contrast to livedo racemosa, acquired livedo reticularis represents a physiologic hypoperfusion pattern that occurs in response to cold exposure.8 A localized sharp pain, known as angina cutis, typically precedes the clinical symptom of painful ulcerations.9 Atrophie blanche once was thought to be specific to LV but has been seen in other diseases such as systemic lupus erythematosus and chronic venous insufficiency.2
The diagnosis of LV is based on identification of characteristic clinical features and skin biopsy. In almost all biopsy specimens, histopathology reveals fibrinoid occlusion of vessels in the superficial and mid dermis.4 Other findings may include epidermal necrosis and vessel wall hyalinization and infarction2 (Figure). Because LV is commonly misdiagnosed as vasculitis, the absence of hallmark features of vasculitis such as neutrophilic infiltrate of blood vessel walls and fibrinoid necrosis suggest the diagnosis. Extensive laboratory evaluation for inherited and acquired coagulation abnormalities should be performed.

Treatment of LV is difficult, as there is currently no consensus on optimal therapy. The mainstay of therapy is to reduce pain, prevent infection, and reduce ulceration and development of atrophie blanche. Underlying causes should be identified and appropriately treated. Because the primary pathogenesis of LV is considered to be a hypercoagulable state, first-line treatment often includes therapies to enhance blood flow and prevent thrombosis such as smoking cessation, antiplatelet therapy, and pentoxifylline. Vasodilating agents, anti-inflammatory agents, anticoagulation, and fibrinolytic therapy also have been used with varying degrees of success.7
The Diagnosis: Livedoid Vasculopathy
Livedoid vasculopathy (LV) is a rare cutaneous disorder that most commonly affects the lower legs. It has an estimated incidence of 1 case per 100,000 per year and predominantly affects women.1 The disease pathogenesis is not fully understood but is thought to involve thrombosis and occlusion of dermal vessels resulting in tissue hypoxia.2 Both inherited and acquired thrombophilic conditions frequently are seen in patients with LV.3,4 Livedoid vasculopathy also has been described as idiopathic5 and is associated with immune complex deposition.6 However, the number of cases of idiopathic LV may be overestimated; as technological advancements to detect coagulation abnormalities improve, it is hypothesized that this entity will be identified less often.2,4
Livedoid vasculopathy has been described in the literature using the term PPURPLE (painful purpuric ulcers with reticular pattern of lower extremities).7 The triad of livedo racemosa, recurrent painful ulcerations, and residual healing with atrophie blanche characterizes the clinical manifestations of LV; however, all 3 characteristics do not need to appear simultaneously for a diagnosis to be made. The condition has a chronic course with spontaneous remissions and exacerbations. Episodic ulcerations occur, especially in the summertime, and heal slowly, leaving behind atrophic, porcelain white, stellate-shaped scars called atrophie blanche. Livedo racemosa also may be seen in Sneddon syndrome; however, these patients experience neurologic symptoms secondary to cerebrovascular occlusion. In contrast to livedo racemosa, acquired livedo reticularis represents a physiologic hypoperfusion pattern that occurs in response to cold exposure.8 A localized sharp pain, known as angina cutis, typically precedes the clinical symptom of painful ulcerations.9 Atrophie blanche once was thought to be specific to LV but has been seen in other diseases such as systemic lupus erythematosus and chronic venous insufficiency.2
The diagnosis of LV is based on identification of characteristic clinical features and skin biopsy. In almost all biopsy specimens, histopathology reveals fibrinoid occlusion of vessels in the superficial and mid dermis.4 Other findings may include epidermal necrosis and vessel wall hyalinization and infarction2 (Figure). Because LV is commonly misdiagnosed as vasculitis, the absence of hallmark features of vasculitis such as neutrophilic infiltrate of blood vessel walls and fibrinoid necrosis suggest the diagnosis. Extensive laboratory evaluation for inherited and acquired coagulation abnormalities should be performed.

Treatment of LV is difficult, as there is currently no consensus on optimal therapy. The mainstay of therapy is to reduce pain, prevent infection, and reduce ulceration and development of atrophie blanche. Underlying causes should be identified and appropriately treated. Because the primary pathogenesis of LV is considered to be a hypercoagulable state, first-line treatment often includes therapies to enhance blood flow and prevent thrombosis such as smoking cessation, antiplatelet therapy, and pentoxifylline. Vasodilating agents, anti-inflammatory agents, anticoagulation, and fibrinolytic therapy also have been used with varying degrees of success.7
- Fritsch P, Zelger B. Livedo vasculitis [in German]. Hautarzt. 1995;46:215-224; quiz 222-223.
- Kerk N, Goerge T. Livedoid vasculopathy—a thrombotic disease. Vasa. 2013;42:317-322.
- Stevanovic DV. Atrophie blanche. a sign of dermal blood occlusion. Arch Dermatol. 1974;109:858-862.
- Hairston BR, Davis MD, Pittelkow MR, et al. Livedoid vasculopathy: further evidence for procoagulant pathogenesis. Arch Dermatol. 2006;142:1413-1418.
- Shornick JK, Nicholes BK, Bergstresser PR, et al. Idiopathic atrophie blanche. J Am Acad Dermatol. 1983;8:792-798.
- Feldaker M, Hines EA Jr, Kierland RR. Livedo reticularis with ulcerations. Circulation. 1956;13:196-216.
- Callen JP. Livedoid vasculopathy: what it is and how the patient should be evaluated and treated. Arch Dermatol. 2006;142:1481-1482.
- Copeman PW. Livedo reticularis. signs in the skin of disturbance of blood viscosity and of blood flow. Br J Dermatol. 1975;93:519-529.
- Goerge T. Livedoid vasculopathy. pathogenesis, diagnosis and treatment of cutaneous infarction [in German]. Hautarzt. 2011;62:627-634; quiz 635.
- Fritsch P, Zelger B. Livedo vasculitis [in German]. Hautarzt. 1995;46:215-224; quiz 222-223.
- Kerk N, Goerge T. Livedoid vasculopathy—a thrombotic disease. Vasa. 2013;42:317-322.
- Stevanovic DV. Atrophie blanche. a sign of dermal blood occlusion. Arch Dermatol. 1974;109:858-862.
- Hairston BR, Davis MD, Pittelkow MR, et al. Livedoid vasculopathy: further evidence for procoagulant pathogenesis. Arch Dermatol. 2006;142:1413-1418.
- Shornick JK, Nicholes BK, Bergstresser PR, et al. Idiopathic atrophie blanche. J Am Acad Dermatol. 1983;8:792-798.
- Feldaker M, Hines EA Jr, Kierland RR. Livedo reticularis with ulcerations. Circulation. 1956;13:196-216.
- Callen JP. Livedoid vasculopathy: what it is and how the patient should be evaluated and treated. Arch Dermatol. 2006;142:1481-1482.
- Copeman PW. Livedo reticularis. signs in the skin of disturbance of blood viscosity and of blood flow. Br J Dermatol. 1975;93:519-529.
- Goerge T. Livedoid vasculopathy. pathogenesis, diagnosis and treatment of cutaneous infarction [in German]. Hautarzt. 2011;62:627-634; quiz 635.
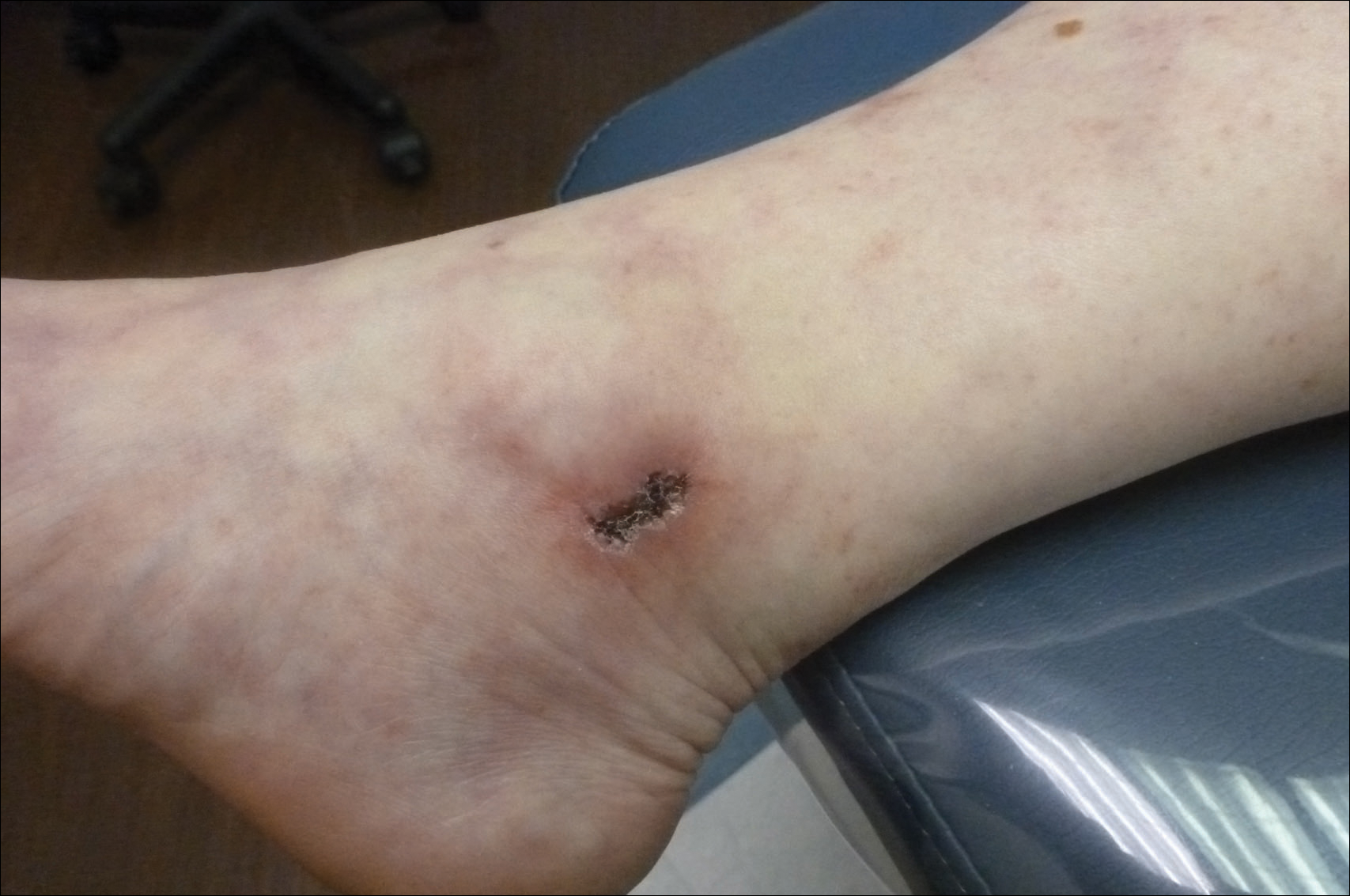
A 58-year-old woman presented in the summertime with skin discoloration of the bilateral lower legs and painful ulcerations above the medial and lateral malleoli of 15 years’ duration. She denied any recent trauma to the area or change in skin lesion appearance with cold exposure. Extensive laboratory evaluation for inherited and acquired coagulation abnormalities was negative. A punch biopsy specimen obtained from the left anterior lower leg revealed vascular thrombi with extravasated erythrocytes and a sparse perivascular inflammatory cell infiltrate.
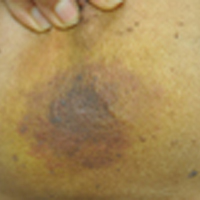
Enlarging Breast Lesion
The Diagnosis: Radiation-Associated Angiosarcoma
At the time of presentation, a 4-mm lesional punch biopsy was obtained (Figure), which revealed an epithelioid neoplasm within the dermis expressing CD31 and CD34, and staining negatively for S-100, CD45, and estrogen and progesterone receptors. The histologic and immunophenotypic findings were compatible with the diagnosis of angiosarcoma. Given the patient’s history of radiation for breast carcinoma several years ago, this tumor was consistent with radiation-associated angiosarcoma (RAAS).
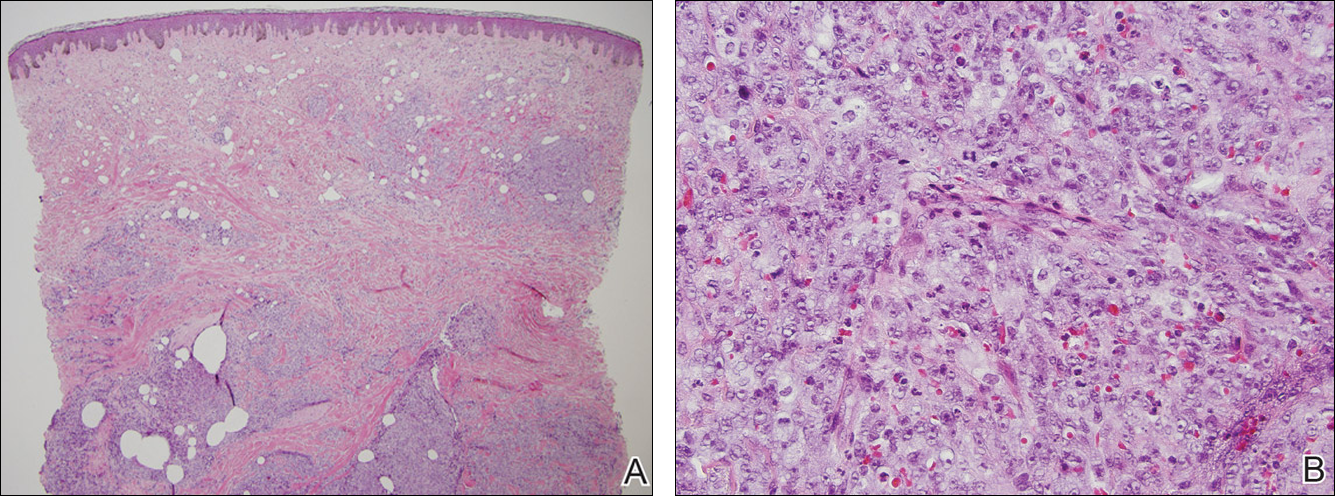
Development of secondary angiosarcoma has been linked to both prior radiation (RAAS) and chronic lymphedema (Stewart-Treves syndrome).1 Radiation-associated angiosarcoma is defined as a “pathologically confirmed breast or chest wall angiosarcoma arising within a previously irradiated field.”2 The incidence of RAAS is estimated to be 0.9 per 1000 individuals following radiation treatment of breast cancer over the subsequent 15 years and a mean time from radiation to development of 7 years.1 Incidence is expected to increase in the future due to improved likelihood of surviving early-stage breast carcinoma and the increased use of external beam radiation therapy for management of breast cancer.
Differentiating between primary and secondary angiosarcoma of the breast is important. Although primary breast angiosarcoma usually arises in women aged 30 to 40 years, RAAS tends to arise in older women (mean age, 68 years) and is seen only in those women with prior radiation.2 Additionally, high-level amplification of MYC, a known photo-oncogene, on chromosome 8 is a key genetic alteration of RAAS that helps to distinguish it from primary angiosarcoma, though this variance may be present in only half of RAAS cases.3 Immunohistochemical analysis of tumor cells for MYC expression correlates well with this amplification and also is helpful in distinguishing atypical vascular lesions from RAAS.4 Atypical vascular lesions, similar to RAAS, occur years after radiation exposure and may have a similar clinical presentation. Atypical vascular lesions do not progress to angiosarcoma in reported cases, but clinical and histologic overlap with RAAS make the diagnosis difficult.5 In these cases, analysis with fluorescence in situ hybridization or immunohistochemistry for the MYC amplification is important to differentiate these tumors.6
At the time of presentation, the majority of patients with RAAS of the breast have localized disease, often with a variable presentation. In all known cases, there have been skin changes present, emphasizing the importance of both patient and clinician vigilance on a regular basis in at-risk individuals. In one study, the most common presentation was breast ecchymosis, which was observed in 55% of patients.7 These lesions involve the dermis and are commonly mistaken for benign conditions such as infection or hemorrhage.2 In 2 other studies, RAAS most often manifested as a skin nodule or apparent tumor, closely followed by either a rash or bruiselike presentation.1,2
The overall recommendation for management of patients with ecchymotic skin lesions in previously irradiated regions is to obtain a biopsy specimen for tissue diagnosis. Although there is no standard of care for the management of RAAS, a multidisciplinary approach involving specialists from oncology, surgical oncology, and radiation oncology is recommended. Most often, radical surgery encompassing both the breast parenchyma and the at-risk radiated skin is performed. Extensive surgery has demonstrated the best survival benefits compared to mastectomy alone.7 Chemotherapeutics also may be used as adjuncts to surgery, which have been determined to decrease local recurrence rates but have no proven survival benefits.2 Adverse prognostic factors for survival are tumor size greater than 10 cm and development of local and/or distant metastases.2 Following the diagnosis of RAAS, our patient underwent radical mastectomy with adjuvant chemotherapy and remained disease free 6 months after surgery.
In summary, RAAS is a well-known, albeit relatively uncommon, consequence of radiation therapy. Dermatologists, oncologists, and primary care providers play an important role in recognizing this entity when evaluating patients with ecchymotic lesions as well as nodules or tumors within an irradiated field. Biopsy should be obtained promptly to prevent delay in diagnosis and to expedite referral to appropriate specialists for further evaluation and treatment.
- Seinen JM, Emelie S, Verstappen V, et al. Radiation-associated angiosarcoma after breast cancer: high recurrence rate and poor survival despite surgical treatment with R0 resection. Ann Surg Oncol. 2012;19:2700-2706.
- Torres KE, Ravi V, Kin K, et al. Long-term outcomes in patients with radiation-associated angiosarcomas of the breast following surgery and radiotherapy for breast cancer. Ann Surg Oncol. 2013;20:1267-1274.
- Manner J, Radlwimmer B, Hohenberger P, et al. MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol. 2010;176:34-39.
- Ginter PS, Mosquera JM, MacDonald TY, et al. Diagnostic utility of MYC amplification and anti-MYC immunohistochemistry in atypical vascular lesions, primary or radiation-induced mammary angiosarcomas, and primary angiosarcomas of other sites. Hum Pathol. 2014;45:709-716.
- Mentzel T, Schildhaus HU, Palmedo G, et al. Postradiation cutaneous angiosarcoma after treatment of breast carcinoma is characterized by MYC amplification in contrast to atypical vascular lesions after radiotherapy and control cases: clinicopathological immunohistochemical and molecular analysis of 66 cases. Mod Pathol. 2012;25:75-85.
- Fernandez AP, Sun Y, Tubbs RR, et al. FISH for MYC amplification and anti-MYC immunohistochemistry: useful diagnostic tools in the assessment of secondary angiosarcoma and atypical vascular proliferations. J Cutan Pathol. 2012;39:234-242.
- Morgan EA, Kozono DE, Wang Q, et al. Cutaneous radiation-associated angiosarcoma of the breast: poor prognosis in a rare secondary malignancy. Ann Surg Oncol. 2012;19:3801-3808.
The Diagnosis: Radiation-Associated Angiosarcoma
At the time of presentation, a 4-mm lesional punch biopsy was obtained (Figure), which revealed an epithelioid neoplasm within the dermis expressing CD31 and CD34, and staining negatively for S-100, CD45, and estrogen and progesterone receptors. The histologic and immunophenotypic findings were compatible with the diagnosis of angiosarcoma. Given the patient’s history of radiation for breast carcinoma several years ago, this tumor was consistent with radiation-associated angiosarcoma (RAAS).

Development of secondary angiosarcoma has been linked to both prior radiation (RAAS) and chronic lymphedema (Stewart-Treves syndrome).1 Radiation-associated angiosarcoma is defined as a “pathologically confirmed breast or chest wall angiosarcoma arising within a previously irradiated field.”2 The incidence of RAAS is estimated to be 0.9 per 1000 individuals following radiation treatment of breast cancer over the subsequent 15 years and a mean time from radiation to development of 7 years.1 Incidence is expected to increase in the future due to improved likelihood of surviving early-stage breast carcinoma and the increased use of external beam radiation therapy for management of breast cancer.
Differentiating between primary and secondary angiosarcoma of the breast is important. Although primary breast angiosarcoma usually arises in women aged 30 to 40 years, RAAS tends to arise in older women (mean age, 68 years) and is seen only in those women with prior radiation.2 Additionally, high-level amplification of MYC, a known photo-oncogene, on chromosome 8 is a key genetic alteration of RAAS that helps to distinguish it from primary angiosarcoma, though this variance may be present in only half of RAAS cases.3 Immunohistochemical analysis of tumor cells for MYC expression correlates well with this amplification and also is helpful in distinguishing atypical vascular lesions from RAAS.4 Atypical vascular lesions, similar to RAAS, occur years after radiation exposure and may have a similar clinical presentation. Atypical vascular lesions do not progress to angiosarcoma in reported cases, but clinical and histologic overlap with RAAS make the diagnosis difficult.5 In these cases, analysis with fluorescence in situ hybridization or immunohistochemistry for the MYC amplification is important to differentiate these tumors.6
At the time of presentation, the majority of patients with RAAS of the breast have localized disease, often with a variable presentation. In all known cases, there have been skin changes present, emphasizing the importance of both patient and clinician vigilance on a regular basis in at-risk individuals. In one study, the most common presentation was breast ecchymosis, which was observed in 55% of patients.7 These lesions involve the dermis and are commonly mistaken for benign conditions such as infection or hemorrhage.2 In 2 other studies, RAAS most often manifested as a skin nodule or apparent tumor, closely followed by either a rash or bruiselike presentation.1,2
The overall recommendation for management of patients with ecchymotic skin lesions in previously irradiated regions is to obtain a biopsy specimen for tissue diagnosis. Although there is no standard of care for the management of RAAS, a multidisciplinary approach involving specialists from oncology, surgical oncology, and radiation oncology is recommended. Most often, radical surgery encompassing both the breast parenchyma and the at-risk radiated skin is performed. Extensive surgery has demonstrated the best survival benefits compared to mastectomy alone.7 Chemotherapeutics also may be used as adjuncts to surgery, which have been determined to decrease local recurrence rates but have no proven survival benefits.2 Adverse prognostic factors for survival are tumor size greater than 10 cm and development of local and/or distant metastases.2 Following the diagnosis of RAAS, our patient underwent radical mastectomy with adjuvant chemotherapy and remained disease free 6 months after surgery.
In summary, RAAS is a well-known, albeit relatively uncommon, consequence of radiation therapy. Dermatologists, oncologists, and primary care providers play an important role in recognizing this entity when evaluating patients with ecchymotic lesions as well as nodules or tumors within an irradiated field. Biopsy should be obtained promptly to prevent delay in diagnosis and to expedite referral to appropriate specialists for further evaluation and treatment.
The Diagnosis: Radiation-Associated Angiosarcoma
At the time of presentation, a 4-mm lesional punch biopsy was obtained (Figure), which revealed an epithelioid neoplasm within the dermis expressing CD31 and CD34, and staining negatively for S-100, CD45, and estrogen and progesterone receptors. The histologic and immunophenotypic findings were compatible with the diagnosis of angiosarcoma. Given the patient’s history of radiation for breast carcinoma several years ago, this tumor was consistent with radiation-associated angiosarcoma (RAAS).

Development of secondary angiosarcoma has been linked to both prior radiation (RAAS) and chronic lymphedema (Stewart-Treves syndrome).1 Radiation-associated angiosarcoma is defined as a “pathologically confirmed breast or chest wall angiosarcoma arising within a previously irradiated field.”2 The incidence of RAAS is estimated to be 0.9 per 1000 individuals following radiation treatment of breast cancer over the subsequent 15 years and a mean time from radiation to development of 7 years.1 Incidence is expected to increase in the future due to improved likelihood of surviving early-stage breast carcinoma and the increased use of external beam radiation therapy for management of breast cancer.
Differentiating between primary and secondary angiosarcoma of the breast is important. Although primary breast angiosarcoma usually arises in women aged 30 to 40 years, RAAS tends to arise in older women (mean age, 68 years) and is seen only in those women with prior radiation.2 Additionally, high-level amplification of MYC, a known photo-oncogene, on chromosome 8 is a key genetic alteration of RAAS that helps to distinguish it from primary angiosarcoma, though this variance may be present in only half of RAAS cases.3 Immunohistochemical analysis of tumor cells for MYC expression correlates well with this amplification and also is helpful in distinguishing atypical vascular lesions from RAAS.4 Atypical vascular lesions, similar to RAAS, occur years after radiation exposure and may have a similar clinical presentation. Atypical vascular lesions do not progress to angiosarcoma in reported cases, but clinical and histologic overlap with RAAS make the diagnosis difficult.5 In these cases, analysis with fluorescence in situ hybridization or immunohistochemistry for the MYC amplification is important to differentiate these tumors.6
At the time of presentation, the majority of patients with RAAS of the breast have localized disease, often with a variable presentation. In all known cases, there have been skin changes present, emphasizing the importance of both patient and clinician vigilance on a regular basis in at-risk individuals. In one study, the most common presentation was breast ecchymosis, which was observed in 55% of patients.7 These lesions involve the dermis and are commonly mistaken for benign conditions such as infection or hemorrhage.2 In 2 other studies, RAAS most often manifested as a skin nodule or apparent tumor, closely followed by either a rash or bruiselike presentation.1,2
The overall recommendation for management of patients with ecchymotic skin lesions in previously irradiated regions is to obtain a biopsy specimen for tissue diagnosis. Although there is no standard of care for the management of RAAS, a multidisciplinary approach involving specialists from oncology, surgical oncology, and radiation oncology is recommended. Most often, radical surgery encompassing both the breast parenchyma and the at-risk radiated skin is performed. Extensive surgery has demonstrated the best survival benefits compared to mastectomy alone.7 Chemotherapeutics also may be used as adjuncts to surgery, which have been determined to decrease local recurrence rates but have no proven survival benefits.2 Adverse prognostic factors for survival are tumor size greater than 10 cm and development of local and/or distant metastases.2 Following the diagnosis of RAAS, our patient underwent radical mastectomy with adjuvant chemotherapy and remained disease free 6 months after surgery.
In summary, RAAS is a well-known, albeit relatively uncommon, consequence of radiation therapy. Dermatologists, oncologists, and primary care providers play an important role in recognizing this entity when evaluating patients with ecchymotic lesions as well as nodules or tumors within an irradiated field. Biopsy should be obtained promptly to prevent delay in diagnosis and to expedite referral to appropriate specialists for further evaluation and treatment.
- Seinen JM, Emelie S, Verstappen V, et al. Radiation-associated angiosarcoma after breast cancer: high recurrence rate and poor survival despite surgical treatment with R0 resection. Ann Surg Oncol. 2012;19:2700-2706.
- Torres KE, Ravi V, Kin K, et al. Long-term outcomes in patients with radiation-associated angiosarcomas of the breast following surgery and radiotherapy for breast cancer. Ann Surg Oncol. 2013;20:1267-1274.
- Manner J, Radlwimmer B, Hohenberger P, et al. MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol. 2010;176:34-39.
- Ginter PS, Mosquera JM, MacDonald TY, et al. Diagnostic utility of MYC amplification and anti-MYC immunohistochemistry in atypical vascular lesions, primary or radiation-induced mammary angiosarcomas, and primary angiosarcomas of other sites. Hum Pathol. 2014;45:709-716.
- Mentzel T, Schildhaus HU, Palmedo G, et al. Postradiation cutaneous angiosarcoma after treatment of breast carcinoma is characterized by MYC amplification in contrast to atypical vascular lesions after radiotherapy and control cases: clinicopathological immunohistochemical and molecular analysis of 66 cases. Mod Pathol. 2012;25:75-85.
- Fernandez AP, Sun Y, Tubbs RR, et al. FISH for MYC amplification and anti-MYC immunohistochemistry: useful diagnostic tools in the assessment of secondary angiosarcoma and atypical vascular proliferations. J Cutan Pathol. 2012;39:234-242.
- Morgan EA, Kozono DE, Wang Q, et al. Cutaneous radiation-associated angiosarcoma of the breast: poor prognosis in a rare secondary malignancy. Ann Surg Oncol. 2012;19:3801-3808.
- Seinen JM, Emelie S, Verstappen V, et al. Radiation-associated angiosarcoma after breast cancer: high recurrence rate and poor survival despite surgical treatment with R0 resection. Ann Surg Oncol. 2012;19:2700-2706.
- Torres KE, Ravi V, Kin K, et al. Long-term outcomes in patients with radiation-associated angiosarcomas of the breast following surgery and radiotherapy for breast cancer. Ann Surg Oncol. 2013;20:1267-1274.
- Manner J, Radlwimmer B, Hohenberger P, et al. MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol. 2010;176:34-39.
- Ginter PS, Mosquera JM, MacDonald TY, et al. Diagnostic utility of MYC amplification and anti-MYC immunohistochemistry in atypical vascular lesions, primary or radiation-induced mammary angiosarcomas, and primary angiosarcomas of other sites. Hum Pathol. 2014;45:709-716.
- Mentzel T, Schildhaus HU, Palmedo G, et al. Postradiation cutaneous angiosarcoma after treatment of breast carcinoma is characterized by MYC amplification in contrast to atypical vascular lesions after radiotherapy and control cases: clinicopathological immunohistochemical and molecular analysis of 66 cases. Mod Pathol. 2012;25:75-85.
- Fernandez AP, Sun Y, Tubbs RR, et al. FISH for MYC amplification and anti-MYC immunohistochemistry: useful diagnostic tools in the assessment of secondary angiosarcoma and atypical vascular proliferations. J Cutan Pathol. 2012;39:234-242.
- Morgan EA, Kozono DE, Wang Q, et al. Cutaneous radiation-associated angiosarcoma of the breast: poor prognosis in a rare secondary malignancy. Ann Surg Oncol. 2012;19:3801-3808.

A 75-year-old woman with a history of stage II invasive ductal carcinoma of the right breast presented to the dermatology clinic with an enlarging, indurated, ecchymotic plaque on the inferior aspect of the right breast of 2 months’ duration. The patient underwent a lumpectomy, radiation, and adjuvant chemotherapy 13 years prior to presentation. Review of systems was otherwise noncontributory.
Patients Concerned about Hospitalist Service Handovers
Background: Service handovers contribute to discontinuity of care in hospitalized patients. Research on hospitalist service handovers is limited, and no previous study has examined the service handover from the patient’s perspective.
Study design: Interview-based, qualitative analysis.
Setting: Urban academic medical center.
Synopsis: Researchers interviewed 40 hospitalized patients using a semi-structured nine-question interview regarding their attending hospitalist service change. The constant comparative method was used to identify recurrent themes in patient responses. The research team identified six themes representative of patient concerns during service change: physician-patient communication, transparency in communication, hospitalist-specialist communication, new opportunities due to transition, bedside manner, and indifference toward the transition.
Authors used the six themes to develop a model for the ideal service handover, utilizing open lines of communication, facilitated by multiple modalities and disciplines, and recognizing the patient’s role as the primary stakeholder in the transition of care.
Bottom line: Incorporating patients’ perspective presents an opportunity to improve communication and efficiency during hospitalist service transitions.
Citation: Wray CM, Farnan JM, Arora VM, Meltzer DO. A qualitative analysis of patients’ experience with hospitalist service handovers [published online ahead of print May 11, 2016]. J Hosp Med. doi:10.1002/jhm.2608.
Background: Service handovers contribute to discontinuity of care in hospitalized patients. Research on hospitalist service handovers is limited, and no previous study has examined the service handover from the patient’s perspective.
Study design: Interview-based, qualitative analysis.
Setting: Urban academic medical center.
Synopsis: Researchers interviewed 40 hospitalized patients using a semi-structured nine-question interview regarding their attending hospitalist service change. The constant comparative method was used to identify recurrent themes in patient responses. The research team identified six themes representative of patient concerns during service change: physician-patient communication, transparency in communication, hospitalist-specialist communication, new opportunities due to transition, bedside manner, and indifference toward the transition.
Authors used the six themes to develop a model for the ideal service handover, utilizing open lines of communication, facilitated by multiple modalities and disciplines, and recognizing the patient’s role as the primary stakeholder in the transition of care.
Bottom line: Incorporating patients’ perspective presents an opportunity to improve communication and efficiency during hospitalist service transitions.
Citation: Wray CM, Farnan JM, Arora VM, Meltzer DO. A qualitative analysis of patients’ experience with hospitalist service handovers [published online ahead of print May 11, 2016]. J Hosp Med. doi:10.1002/jhm.2608.
Background: Service handovers contribute to discontinuity of care in hospitalized patients. Research on hospitalist service handovers is limited, and no previous study has examined the service handover from the patient’s perspective.
Study design: Interview-based, qualitative analysis.
Setting: Urban academic medical center.
Synopsis: Researchers interviewed 40 hospitalized patients using a semi-structured nine-question interview regarding their attending hospitalist service change. The constant comparative method was used to identify recurrent themes in patient responses. The research team identified six themes representative of patient concerns during service change: physician-patient communication, transparency in communication, hospitalist-specialist communication, new opportunities due to transition, bedside manner, and indifference toward the transition.
Authors used the six themes to develop a model for the ideal service handover, utilizing open lines of communication, facilitated by multiple modalities and disciplines, and recognizing the patient’s role as the primary stakeholder in the transition of care.
Bottom line: Incorporating patients’ perspective presents an opportunity to improve communication and efficiency during hospitalist service transitions.
Citation: Wray CM, Farnan JM, Arora VM, Meltzer DO. A qualitative analysis of patients’ experience with hospitalist service handovers [published online ahead of print May 11, 2016]. J Hosp Med. doi:10.1002/jhm.2608.
Warfarin Reduces Risk of Ischemic Stroke in High-Risk Patients
Background: For patients with atrial fibrillation and history of intracranial hemorrhage (ICH), the risk of further ICH and the benefit of antithrombotic agents for stroke risk reduction remain unclear.
Study design: Retrospective cohort study.
Setting: National Health Research Institutes, Taiwan.
Synopsis: Using the National Health Insurance Research Database in Taiwan, researchers identified 307,640 patients with atrial fibrillation and a CHA2DS2-VASc score >/= 2. Of this group, 12,917 patients with a history of ICH were identified and separated into three groups: no treatment, antiplatelet treatment, or warfarin. Among the no treatment group, the rate of ICH and ischemic cerebrovascular accident were 4.2 and 5.8 per 100 person-years, respectively. Among patients on antiplatelet therapy, the rates were 5.3% and 5.2%, respectively. Among patients on warfarin, the number needed to treat (NNT) for preventing one ischemic stroke was lower than the number needed to harm (NNH) for producing one ICH among patients with a CHA2DS2-VASc score >/= 6. In patients with lower CHA2DS2-VASc scores, the NNT was higher than NNH.
Bottom line: Treatment with warfarin may benefit patients with atrial fibrillation and prior ICH with CHA2DS2-VASc scores >/= 6, but risk likely outweighs benefit in patients with lower scores.
Citation: Chao TF, Liu CJ, Liao JN, et al. Use of oral anticoagulants for stroke prevention in patients with atrial fibrillation who have a history of intracranial hemorrhage. Circulation. 2016;133(16):1540-1547.
Background: For patients with atrial fibrillation and history of intracranial hemorrhage (ICH), the risk of further ICH and the benefit of antithrombotic agents for stroke risk reduction remain unclear.
Study design: Retrospective cohort study.
Setting: National Health Research Institutes, Taiwan.
Synopsis: Using the National Health Insurance Research Database in Taiwan, researchers identified 307,640 patients with atrial fibrillation and a CHA2DS2-VASc score >/= 2. Of this group, 12,917 patients with a history of ICH were identified and separated into three groups: no treatment, antiplatelet treatment, or warfarin. Among the no treatment group, the rate of ICH and ischemic cerebrovascular accident were 4.2 and 5.8 per 100 person-years, respectively. Among patients on antiplatelet therapy, the rates were 5.3% and 5.2%, respectively. Among patients on warfarin, the number needed to treat (NNT) for preventing one ischemic stroke was lower than the number needed to harm (NNH) for producing one ICH among patients with a CHA2DS2-VASc score >/= 6. In patients with lower CHA2DS2-VASc scores, the NNT was higher than NNH.
Bottom line: Treatment with warfarin may benefit patients with atrial fibrillation and prior ICH with CHA2DS2-VASc scores >/= 6, but risk likely outweighs benefit in patients with lower scores.
Citation: Chao TF, Liu CJ, Liao JN, et al. Use of oral anticoagulants for stroke prevention in patients with atrial fibrillation who have a history of intracranial hemorrhage. Circulation. 2016;133(16):1540-1547.
Background: For patients with atrial fibrillation and history of intracranial hemorrhage (ICH), the risk of further ICH and the benefit of antithrombotic agents for stroke risk reduction remain unclear.
Study design: Retrospective cohort study.
Setting: National Health Research Institutes, Taiwan.
Synopsis: Using the National Health Insurance Research Database in Taiwan, researchers identified 307,640 patients with atrial fibrillation and a CHA2DS2-VASc score >/= 2. Of this group, 12,917 patients with a history of ICH were identified and separated into three groups: no treatment, antiplatelet treatment, or warfarin. Among the no treatment group, the rate of ICH and ischemic cerebrovascular accident were 4.2 and 5.8 per 100 person-years, respectively. Among patients on antiplatelet therapy, the rates were 5.3% and 5.2%, respectively. Among patients on warfarin, the number needed to treat (NNT) for preventing one ischemic stroke was lower than the number needed to harm (NNH) for producing one ICH among patients with a CHA2DS2-VASc score >/= 6. In patients with lower CHA2DS2-VASc scores, the NNT was higher than NNH.
Bottom line: Treatment with warfarin may benefit patients with atrial fibrillation and prior ICH with CHA2DS2-VASc scores >/= 6, but risk likely outweighs benefit in patients with lower scores.
Citation: Chao TF, Liu CJ, Liao JN, et al. Use of oral anticoagulants for stroke prevention in patients with atrial fibrillation who have a history of intracranial hemorrhage. Circulation. 2016;133(16):1540-1547.
Identifying and targeting malignant aging in sAML

Photo courtesy of the University
of California San Diego
Researchers say they have identified RNA-based biomarkers that distinguish normal, aged hematopoietic stem and progenitor cells (HSPCs) from leukemia stem cells (LSCs) associated with secondary acute myeloid leukemia (sAML).
The team believes their discovery may provide a new way to predict leukemic relapse and identify targets for drug development.
Catriona Jamieson, MD, PhD, of the University of California San Diego in La Jolla, and her colleagues described the discovery in Cell Stem Cell.
The researchers noted that age-related hematopoietic stem cell exhaustion and myeloid-lineage skewing promote the transformation of hematopoietic progenitors into therapy-resistant LSCs in sAML. However, the contribution of RNA processing alterations to HSPC aging and LSC generation remains unclear.
With this study, Dr Jamieson and her colleagues discovered RNA splice isoform expression patterns that distinguished normal, aged HSPCs from sAML LSCs.
The team said the aged HSPCs displayed pro-apoptotic BCL2 splice isoform switching, while sAML LSCs favored pro-survival expression of BCL2L1 (BCL-XL).
“These splicing signatures could potentially be used as clinical biomarkers to detect blood stem cells that show signs of early aging or leukemia and to monitor patient responses to treatment,” said study author Leslie Crews, PhD, of the University of California San Diego.
“By being able to distinguish benign from malignant aging based on distinctive RNA splicing patterns, we can develop therapeutic strategies that selectively target leukemia stem cells while sparing normal hematopoietic stem cells,” Dr Jamieson added.
In fact, she and her colleagues were able to show that treatment with a pharmacologic splicing modulator known as 17S-FD-895 could eradicate sAML LSCs while sparing normal HSPCs. The compound reversed pro-survival splice isoform switching and impaired LSC maintenance.
“Our findings show that RNA splicing is a unique therapeutic vulnerability for secondary AML,” Dr Jamieson said. “RNA-splicing-targeted therapies may be a potent and selective way to clear leukemia stem cells and prevent relapse.” ![]()

Photo courtesy of the University
of California San Diego
Researchers say they have identified RNA-based biomarkers that distinguish normal, aged hematopoietic stem and progenitor cells (HSPCs) from leukemia stem cells (LSCs) associated with secondary acute myeloid leukemia (sAML).
The team believes their discovery may provide a new way to predict leukemic relapse and identify targets for drug development.
Catriona Jamieson, MD, PhD, of the University of California San Diego in La Jolla, and her colleagues described the discovery in Cell Stem Cell.
The researchers noted that age-related hematopoietic stem cell exhaustion and myeloid-lineage skewing promote the transformation of hematopoietic progenitors into therapy-resistant LSCs in sAML. However, the contribution of RNA processing alterations to HSPC aging and LSC generation remains unclear.
With this study, Dr Jamieson and her colleagues discovered RNA splice isoform expression patterns that distinguished normal, aged HSPCs from sAML LSCs.
The team said the aged HSPCs displayed pro-apoptotic BCL2 splice isoform switching, while sAML LSCs favored pro-survival expression of BCL2L1 (BCL-XL).
“These splicing signatures could potentially be used as clinical biomarkers to detect blood stem cells that show signs of early aging or leukemia and to monitor patient responses to treatment,” said study author Leslie Crews, PhD, of the University of California San Diego.
“By being able to distinguish benign from malignant aging based on distinctive RNA splicing patterns, we can develop therapeutic strategies that selectively target leukemia stem cells while sparing normal hematopoietic stem cells,” Dr Jamieson added.
In fact, she and her colleagues were able to show that treatment with a pharmacologic splicing modulator known as 17S-FD-895 could eradicate sAML LSCs while sparing normal HSPCs. The compound reversed pro-survival splice isoform switching and impaired LSC maintenance.
“Our findings show that RNA splicing is a unique therapeutic vulnerability for secondary AML,” Dr Jamieson said. “RNA-splicing-targeted therapies may be a potent and selective way to clear leukemia stem cells and prevent relapse.” ![]()

Photo courtesy of the University
of California San Diego
Researchers say they have identified RNA-based biomarkers that distinguish normal, aged hematopoietic stem and progenitor cells (HSPCs) from leukemia stem cells (LSCs) associated with secondary acute myeloid leukemia (sAML).
The team believes their discovery may provide a new way to predict leukemic relapse and identify targets for drug development.
Catriona Jamieson, MD, PhD, of the University of California San Diego in La Jolla, and her colleagues described the discovery in Cell Stem Cell.
The researchers noted that age-related hematopoietic stem cell exhaustion and myeloid-lineage skewing promote the transformation of hematopoietic progenitors into therapy-resistant LSCs in sAML. However, the contribution of RNA processing alterations to HSPC aging and LSC generation remains unclear.
With this study, Dr Jamieson and her colleagues discovered RNA splice isoform expression patterns that distinguished normal, aged HSPCs from sAML LSCs.
The team said the aged HSPCs displayed pro-apoptotic BCL2 splice isoform switching, while sAML LSCs favored pro-survival expression of BCL2L1 (BCL-XL).
“These splicing signatures could potentially be used as clinical biomarkers to detect blood stem cells that show signs of early aging or leukemia and to monitor patient responses to treatment,” said study author Leslie Crews, PhD, of the University of California San Diego.
“By being able to distinguish benign from malignant aging based on distinctive RNA splicing patterns, we can develop therapeutic strategies that selectively target leukemia stem cells while sparing normal hematopoietic stem cells,” Dr Jamieson added.
In fact, she and her colleagues were able to show that treatment with a pharmacologic splicing modulator known as 17S-FD-895 could eradicate sAML LSCs while sparing normal HSPCs. The compound reversed pro-survival splice isoform switching and impaired LSC maintenance.
“Our findings show that RNA splicing is a unique therapeutic vulnerability for secondary AML,” Dr Jamieson said. “RNA-splicing-targeted therapies may be a potent and selective way to clear leukemia stem cells and prevent relapse.” ![]()