User login
HCQ eye toxicity needs experience to assess
LONDON – Retinopathy in patients taking long-term hydroxychloroquine for rheumatic conditions requires assessment by those experienced with specialized ophthalmic imaging, according to study findings presented at the European Congress of Rheumatology.
Nonspecific abnormalities, which often are unrelated to hydroxychloroquine (HCQ), can be seen with many of the tests recommended by current ophthalmology guidelines. These changes need “careful interpretation by retina specialists,” the study’s investigators wrote in a poster presentation.
HCQ is used widely for the treatment of systemic lupus erythematosus (SLE), rheumatoid arthritis, and many other inflammatory or autoimmune conditions, but it can cause irreversible eye damage and is often associated with prolonged (greater than 5 years) use. Specifically, it can cause a type of end-stage retinopathy called bull’s-eye maculopathy, which is where the fovea becomes hyperpigmented, much like the bull’s-eye on a dartboard. This can lead to substantial vision loss (blind spots) if not caught early.
Although it is reasonably rare to develop end-stage retinopathy, there is currently no treatment for HCQ-induced retinopathy. Stopping the drug may not necessarily stop the retinal damage, and drug withdrawal may not be an option in many patients given the lack of alternative options to treat the symptoms of SLE, study author and ophthalmologist Syed Mahmood Ali Shah, MBBS, MD, said in an interview.
Dr. Shah and his associates at Johns Hopkins University in Baltimore reported on applying the 2011 American Academy of Ophthalmology (AAO) guidelines on screening for HCQ retinopathy (Ophthalmology. 2011;118:415-22) to an academic practice. They also estimated the prevalence of HCQ retinopathy among 135 consecutively treated patients with SLE using recommended tests. The mean duration of HCQ use was 12.5 years.
The 2011 AAO guidelines – which in March 2016 were updated (Ophthalmology 2016 Jun;123:1386-94) – recommended the use of three “ancillary” tests in addition to the usual clinical ophthalmic examination and assessment of visual fields: optical coherence tomography (OCT), fundus autofluorescence (FAF), and multifocal electroretinography (mfERG). Dr. Shah and his colleagues used these three tests together with eye-tracking microperimetry (MP) as a substitute for Humphrey Visual Fields (HVF), which is a common visual field test used in the United States.
One difference between the 2011 guidelines and 2016 revision is that “the baseline exam can now be performed relying [only] on the fundus exam, with additional imaging required only for abnormal patients,” Dr. Shah said. “Overall, the guidelines have not changed on how often and how much you follow up,” he added. “The change is that there is no need to do these tests at baseline unless changes of the fundus are present.” However, OCT has become more widely used in many offices and has been recognized as the most useful objective test and shall be performed if there are any abnormal findings of the fundus.
A total of 266 eyes were examined using these imaging methods and interpreted by experienced retina specialists. Overall, HCQ-related abnormalities were noted in 14 (5%) eyes using OCT, 18 (7%) using FAF, 27 (10%) eyes using mfERG, and 20 (7%) using MP.
MP had the lowest discrepancy between the overall number of eyes with abnormalities (72 [27%] of 266) detected and the number of eyes with abnormalities related to HCQ (20 [28%] of 72), followed by OCT (21% and 25%, respectively), FAF (19% and 35%) and mfERG (37% and 28%). Only four patients (3%) showed changes in all four tests suggestive of HCQ retinopathy.
In the absence of baseline data from the AAO recommended ancillary tests before the use of HCQ, “it may be difficult to interpret changes seen on these tests since most of the screenings are done by regular ophthalmologists who lack the equipment and experience with specialized testing such as mfERG, FAF, and OCT,” Dr. Shah and his coauthors noted. “We found a substantial number of cases with abnormalities unrelated to HCQ.”
Giving some practical advice, Dr. Shah noted that “before a patient starts treatment with HCQ, they should undergo a baseline ophthalmic assessment. Then if the patient complains of any vision changes, even if they have been taking the drug for less than 5 years, they should be reassessed.”
While repeat follow-up is, of course, necessary, he intimated that it is necessary to find a balance of risk and cost in regard to the frequency of screening for drug-related damage. “The American Academy of Ophthalmology currently recommends that a baseline fundus exam be performed shortly after starting HCQ. Ancillary OCT and visual fields shall only be performed if the fundus is abnormal at this baseline exam. However, since most retina specialists get OCT and visual field testing anyway it is wise to look at these as well,” he suggested. After 5 years of using the drug, they must be seen more regularly, and this is the point when ophthalmologists can decide if this should be every 6 months or annually, with the latter recommended by the AAO guidelines for patients with no additional risk factors.
The study was supported by noncommercial grants. Dr. Shah had no conflicts of interest to disclose.
LONDON – Retinopathy in patients taking long-term hydroxychloroquine for rheumatic conditions requires assessment by those experienced with specialized ophthalmic imaging, according to study findings presented at the European Congress of Rheumatology.
Nonspecific abnormalities, which often are unrelated to hydroxychloroquine (HCQ), can be seen with many of the tests recommended by current ophthalmology guidelines. These changes need “careful interpretation by retina specialists,” the study’s investigators wrote in a poster presentation.
HCQ is used widely for the treatment of systemic lupus erythematosus (SLE), rheumatoid arthritis, and many other inflammatory or autoimmune conditions, but it can cause irreversible eye damage and is often associated with prolonged (greater than 5 years) use. Specifically, it can cause a type of end-stage retinopathy called bull’s-eye maculopathy, which is where the fovea becomes hyperpigmented, much like the bull’s-eye on a dartboard. This can lead to substantial vision loss (blind spots) if not caught early.
Although it is reasonably rare to develop end-stage retinopathy, there is currently no treatment for HCQ-induced retinopathy. Stopping the drug may not necessarily stop the retinal damage, and drug withdrawal may not be an option in many patients given the lack of alternative options to treat the symptoms of SLE, study author and ophthalmologist Syed Mahmood Ali Shah, MBBS, MD, said in an interview.
Dr. Shah and his associates at Johns Hopkins University in Baltimore reported on applying the 2011 American Academy of Ophthalmology (AAO) guidelines on screening for HCQ retinopathy (Ophthalmology. 2011;118:415-22) to an academic practice. They also estimated the prevalence of HCQ retinopathy among 135 consecutively treated patients with SLE using recommended tests. The mean duration of HCQ use was 12.5 years.
The 2011 AAO guidelines – which in March 2016 were updated (Ophthalmology 2016 Jun;123:1386-94) – recommended the use of three “ancillary” tests in addition to the usual clinical ophthalmic examination and assessment of visual fields: optical coherence tomography (OCT), fundus autofluorescence (FAF), and multifocal electroretinography (mfERG). Dr. Shah and his colleagues used these three tests together with eye-tracking microperimetry (MP) as a substitute for Humphrey Visual Fields (HVF), which is a common visual field test used in the United States.
One difference between the 2011 guidelines and 2016 revision is that “the baseline exam can now be performed relying [only] on the fundus exam, with additional imaging required only for abnormal patients,” Dr. Shah said. “Overall, the guidelines have not changed on how often and how much you follow up,” he added. “The change is that there is no need to do these tests at baseline unless changes of the fundus are present.” However, OCT has become more widely used in many offices and has been recognized as the most useful objective test and shall be performed if there are any abnormal findings of the fundus.
A total of 266 eyes were examined using these imaging methods and interpreted by experienced retina specialists. Overall, HCQ-related abnormalities were noted in 14 (5%) eyes using OCT, 18 (7%) using FAF, 27 (10%) eyes using mfERG, and 20 (7%) using MP.
MP had the lowest discrepancy between the overall number of eyes with abnormalities (72 [27%] of 266) detected and the number of eyes with abnormalities related to HCQ (20 [28%] of 72), followed by OCT (21% and 25%, respectively), FAF (19% and 35%) and mfERG (37% and 28%). Only four patients (3%) showed changes in all four tests suggestive of HCQ retinopathy.
In the absence of baseline data from the AAO recommended ancillary tests before the use of HCQ, “it may be difficult to interpret changes seen on these tests since most of the screenings are done by regular ophthalmologists who lack the equipment and experience with specialized testing such as mfERG, FAF, and OCT,” Dr. Shah and his coauthors noted. “We found a substantial number of cases with abnormalities unrelated to HCQ.”
Giving some practical advice, Dr. Shah noted that “before a patient starts treatment with HCQ, they should undergo a baseline ophthalmic assessment. Then if the patient complains of any vision changes, even if they have been taking the drug for less than 5 years, they should be reassessed.”
While repeat follow-up is, of course, necessary, he intimated that it is necessary to find a balance of risk and cost in regard to the frequency of screening for drug-related damage. “The American Academy of Ophthalmology currently recommends that a baseline fundus exam be performed shortly after starting HCQ. Ancillary OCT and visual fields shall only be performed if the fundus is abnormal at this baseline exam. However, since most retina specialists get OCT and visual field testing anyway it is wise to look at these as well,” he suggested. After 5 years of using the drug, they must be seen more regularly, and this is the point when ophthalmologists can decide if this should be every 6 months or annually, with the latter recommended by the AAO guidelines for patients with no additional risk factors.
The study was supported by noncommercial grants. Dr. Shah had no conflicts of interest to disclose.
LONDON – Retinopathy in patients taking long-term hydroxychloroquine for rheumatic conditions requires assessment by those experienced with specialized ophthalmic imaging, according to study findings presented at the European Congress of Rheumatology.
Nonspecific abnormalities, which often are unrelated to hydroxychloroquine (HCQ), can be seen with many of the tests recommended by current ophthalmology guidelines. These changes need “careful interpretation by retina specialists,” the study’s investigators wrote in a poster presentation.
HCQ is used widely for the treatment of systemic lupus erythematosus (SLE), rheumatoid arthritis, and many other inflammatory or autoimmune conditions, but it can cause irreversible eye damage and is often associated with prolonged (greater than 5 years) use. Specifically, it can cause a type of end-stage retinopathy called bull’s-eye maculopathy, which is where the fovea becomes hyperpigmented, much like the bull’s-eye on a dartboard. This can lead to substantial vision loss (blind spots) if not caught early.
Although it is reasonably rare to develop end-stage retinopathy, there is currently no treatment for HCQ-induced retinopathy. Stopping the drug may not necessarily stop the retinal damage, and drug withdrawal may not be an option in many patients given the lack of alternative options to treat the symptoms of SLE, study author and ophthalmologist Syed Mahmood Ali Shah, MBBS, MD, said in an interview.
Dr. Shah and his associates at Johns Hopkins University in Baltimore reported on applying the 2011 American Academy of Ophthalmology (AAO) guidelines on screening for HCQ retinopathy (Ophthalmology. 2011;118:415-22) to an academic practice. They also estimated the prevalence of HCQ retinopathy among 135 consecutively treated patients with SLE using recommended tests. The mean duration of HCQ use was 12.5 years.
The 2011 AAO guidelines – which in March 2016 were updated (Ophthalmology 2016 Jun;123:1386-94) – recommended the use of three “ancillary” tests in addition to the usual clinical ophthalmic examination and assessment of visual fields: optical coherence tomography (OCT), fundus autofluorescence (FAF), and multifocal electroretinography (mfERG). Dr. Shah and his colleagues used these three tests together with eye-tracking microperimetry (MP) as a substitute for Humphrey Visual Fields (HVF), which is a common visual field test used in the United States.
One difference between the 2011 guidelines and 2016 revision is that “the baseline exam can now be performed relying [only] on the fundus exam, with additional imaging required only for abnormal patients,” Dr. Shah said. “Overall, the guidelines have not changed on how often and how much you follow up,” he added. “The change is that there is no need to do these tests at baseline unless changes of the fundus are present.” However, OCT has become more widely used in many offices and has been recognized as the most useful objective test and shall be performed if there are any abnormal findings of the fundus.
A total of 266 eyes were examined using these imaging methods and interpreted by experienced retina specialists. Overall, HCQ-related abnormalities were noted in 14 (5%) eyes using OCT, 18 (7%) using FAF, 27 (10%) eyes using mfERG, and 20 (7%) using MP.
MP had the lowest discrepancy between the overall number of eyes with abnormalities (72 [27%] of 266) detected and the number of eyes with abnormalities related to HCQ (20 [28%] of 72), followed by OCT (21% and 25%, respectively), FAF (19% and 35%) and mfERG (37% and 28%). Only four patients (3%) showed changes in all four tests suggestive of HCQ retinopathy.
In the absence of baseline data from the AAO recommended ancillary tests before the use of HCQ, “it may be difficult to interpret changes seen on these tests since most of the screenings are done by regular ophthalmologists who lack the equipment and experience with specialized testing such as mfERG, FAF, and OCT,” Dr. Shah and his coauthors noted. “We found a substantial number of cases with abnormalities unrelated to HCQ.”
Giving some practical advice, Dr. Shah noted that “before a patient starts treatment with HCQ, they should undergo a baseline ophthalmic assessment. Then if the patient complains of any vision changes, even if they have been taking the drug for less than 5 years, they should be reassessed.”
While repeat follow-up is, of course, necessary, he intimated that it is necessary to find a balance of risk and cost in regard to the frequency of screening for drug-related damage. “The American Academy of Ophthalmology currently recommends that a baseline fundus exam be performed shortly after starting HCQ. Ancillary OCT and visual fields shall only be performed if the fundus is abnormal at this baseline exam. However, since most retina specialists get OCT and visual field testing anyway it is wise to look at these as well,” he suggested. After 5 years of using the drug, they must be seen more regularly, and this is the point when ophthalmologists can decide if this should be every 6 months or annually, with the latter recommended by the AAO guidelines for patients with no additional risk factors.
The study was supported by noncommercial grants. Dr. Shah had no conflicts of interest to disclose.
AT THE EULAR 2016 CONGRESS
Key clinical point: Several eye abnormalities can be mistaken for hydroxychloroquine-related eye toxicity, making specialist ophthalmic assessment paramount.
Major finding: Only four patients (3%) showed changes in all four tests suggestive of HCQ retinopathy.
Data source: Observational study of 135 patients with SLE being seen for suspected hydroxychloroquine-related retinopathy at an academic practice
Disclosures: The study was supported by noncommercial grants. Dr. Shah had no conflicts of interest to disclose.
mtDNA level predicts IVF embryo viability
HELSINKI – Mitochondrial DNA level appears to be a useful biomarker for in vitro fertilization embryo viability, according to findings from a blinded prospective non-selection study.
An analysis of 280 chromosomally normal blastocysts showed that 15 (5.4%) contained unusually high levels of mitochondrial DNA (mtDNA) and the remaining blastocysts had normal or low mtDNA levels. Of 111 of the blastocyst transfers for which outcome data were available, 78 (70%) led to ongoing pregnancies, and all of those involved blastocysts with normal or low mtDNA levels, while 8 of the 33 blastocysts that failed to implant (24%) had unusually high mtDNA levels, Elpida Fragouli, PhD, reported at the annual meeting of the European Society of Human Reproduction and Embryology.
Thus, the ongoing pregnancy rate for morphologically good, euploid blastocysts was 76% for those with normal/low mtDNA levels, compared with 0% for those with elevated mtDNA levels – a highly statistically significant difference. The overall pregnancy rate was 70%, said Dr. Fragouli of Reprogenetics UK and the University of Oxford.
The blastocysts in the study were generated by 143 couples who underwent IVF in a single clinic. All blastocysts were biopsied and shown to be chromosomally normal using preimplantation genetic screening.
“The study demonstrates that mitochondrial DNA levels are highly predictive of an embryo’s implantation potential,” Dr. Fragouli said, noting that the “very robust” findings could potentially enhance embryo selection and improve IVF outcomes.
The methodology used in the study has been extensively validated, she said. However, a randomized clinical trial will be necessary to determine the true extent of any clinical benefit, she added, noting that research is also needed to improve understanding of the biology of mtDNA expansion.
The findings are of particular interest, because while it is well known that chromosomal abnormality in embryos is common and increases with age, and is the main cause of implantation failure, it has been less clear why about a third of euploid embryos fail to produce a pregnancy.
“The combination of chromosome analysis and mitochondrial assessment may now represent the most accurate and predictive measure of embryo viability with great potential for improving IVF outcome,” according to an ESHRE press release on the findings.
Levels of mtDNA can be quickly measured using polymerase chain reaction. Next generation sequencing can also be used, Dr. Fragouli noted. However, since aneuploidy remains the most common cause of embryo implantation failure, mtDNA and chromosome testing would be necessary.
“Mitochondrial analysis does not replace [aneuploidy screening]. It is the combination of the two methods ... that is so powerful,” she said, noting that efforts are underway to develop an approach to assessing chromosome content and mtDNA simultaneously to reduce the extra cost.
The group has started offering mtDNA quantification clinically in the United States and has applied to the Human Fertilisation and Embryology Authority for a license to use the testing in the United Kingdom.
Reprogenetics provided funding for this study.
HELSINKI – Mitochondrial DNA level appears to be a useful biomarker for in vitro fertilization embryo viability, according to findings from a blinded prospective non-selection study.
An analysis of 280 chromosomally normal blastocysts showed that 15 (5.4%) contained unusually high levels of mitochondrial DNA (mtDNA) and the remaining blastocysts had normal or low mtDNA levels. Of 111 of the blastocyst transfers for which outcome data were available, 78 (70%) led to ongoing pregnancies, and all of those involved blastocysts with normal or low mtDNA levels, while 8 of the 33 blastocysts that failed to implant (24%) had unusually high mtDNA levels, Elpida Fragouli, PhD, reported at the annual meeting of the European Society of Human Reproduction and Embryology.
Thus, the ongoing pregnancy rate for morphologically good, euploid blastocysts was 76% for those with normal/low mtDNA levels, compared with 0% for those with elevated mtDNA levels – a highly statistically significant difference. The overall pregnancy rate was 70%, said Dr. Fragouli of Reprogenetics UK and the University of Oxford.
The blastocysts in the study were generated by 143 couples who underwent IVF in a single clinic. All blastocysts were biopsied and shown to be chromosomally normal using preimplantation genetic screening.
“The study demonstrates that mitochondrial DNA levels are highly predictive of an embryo’s implantation potential,” Dr. Fragouli said, noting that the “very robust” findings could potentially enhance embryo selection and improve IVF outcomes.
The methodology used in the study has been extensively validated, she said. However, a randomized clinical trial will be necessary to determine the true extent of any clinical benefit, she added, noting that research is also needed to improve understanding of the biology of mtDNA expansion.
The findings are of particular interest, because while it is well known that chromosomal abnormality in embryos is common and increases with age, and is the main cause of implantation failure, it has been less clear why about a third of euploid embryos fail to produce a pregnancy.
“The combination of chromosome analysis and mitochondrial assessment may now represent the most accurate and predictive measure of embryo viability with great potential for improving IVF outcome,” according to an ESHRE press release on the findings.
Levels of mtDNA can be quickly measured using polymerase chain reaction. Next generation sequencing can also be used, Dr. Fragouli noted. However, since aneuploidy remains the most common cause of embryo implantation failure, mtDNA and chromosome testing would be necessary.
“Mitochondrial analysis does not replace [aneuploidy screening]. It is the combination of the two methods ... that is so powerful,” she said, noting that efforts are underway to develop an approach to assessing chromosome content and mtDNA simultaneously to reduce the extra cost.
The group has started offering mtDNA quantification clinically in the United States and has applied to the Human Fertilisation and Embryology Authority for a license to use the testing in the United Kingdom.
Reprogenetics provided funding for this study.
HELSINKI – Mitochondrial DNA level appears to be a useful biomarker for in vitro fertilization embryo viability, according to findings from a blinded prospective non-selection study.
An analysis of 280 chromosomally normal blastocysts showed that 15 (5.4%) contained unusually high levels of mitochondrial DNA (mtDNA) and the remaining blastocysts had normal or low mtDNA levels. Of 111 of the blastocyst transfers for which outcome data were available, 78 (70%) led to ongoing pregnancies, and all of those involved blastocysts with normal or low mtDNA levels, while 8 of the 33 blastocysts that failed to implant (24%) had unusually high mtDNA levels, Elpida Fragouli, PhD, reported at the annual meeting of the European Society of Human Reproduction and Embryology.
Thus, the ongoing pregnancy rate for morphologically good, euploid blastocysts was 76% for those with normal/low mtDNA levels, compared with 0% for those with elevated mtDNA levels – a highly statistically significant difference. The overall pregnancy rate was 70%, said Dr. Fragouli of Reprogenetics UK and the University of Oxford.
The blastocysts in the study were generated by 143 couples who underwent IVF in a single clinic. All blastocysts were biopsied and shown to be chromosomally normal using preimplantation genetic screening.
“The study demonstrates that mitochondrial DNA levels are highly predictive of an embryo’s implantation potential,” Dr. Fragouli said, noting that the “very robust” findings could potentially enhance embryo selection and improve IVF outcomes.
The methodology used in the study has been extensively validated, she said. However, a randomized clinical trial will be necessary to determine the true extent of any clinical benefit, she added, noting that research is also needed to improve understanding of the biology of mtDNA expansion.
The findings are of particular interest, because while it is well known that chromosomal abnormality in embryos is common and increases with age, and is the main cause of implantation failure, it has been less clear why about a third of euploid embryos fail to produce a pregnancy.
“The combination of chromosome analysis and mitochondrial assessment may now represent the most accurate and predictive measure of embryo viability with great potential for improving IVF outcome,” according to an ESHRE press release on the findings.
Levels of mtDNA can be quickly measured using polymerase chain reaction. Next generation sequencing can also be used, Dr. Fragouli noted. However, since aneuploidy remains the most common cause of embryo implantation failure, mtDNA and chromosome testing would be necessary.
“Mitochondrial analysis does not replace [aneuploidy screening]. It is the combination of the two methods ... that is so powerful,” she said, noting that efforts are underway to develop an approach to assessing chromosome content and mtDNA simultaneously to reduce the extra cost.
The group has started offering mtDNA quantification clinically in the United States and has applied to the Human Fertilisation and Embryology Authority for a license to use the testing in the United Kingdom.
Reprogenetics provided funding for this study.
AT ESHRE 2016
Key clinical point: Mitochondrial DNA level may offer a way to assess embryo viability when doing in vitro fertilization.
Major finding: The ongoing pregnancy rate for euploid blastocysts was 76% for those with normal/low mtDNA levels, compared with 0% for those with elevated mtDNA levels.
Data source: A blinded prospective non-selection study of 280 blastocysts.
Disclosures: Reprogenetics provided funding for this study.
Pediatric Cancer Survivors at Increased Risk for Endocrine Abnormalities
Patients who survived pediatric-onset cancer are at increased risk for developing or experiencing endocrine abnormalities.
Risk was significantly higher in survivors who underwent high-risk therapeutic exposures compared with survivors not so exposed. Moreover, the incidence and prevalence of endocrine abnormalities increased across the lifespan of survivors, reported Sogol Mostoufi-Moab, MD, of University of Pennsylvania, Philadelphia, and his associates (J Clin Oncol. 2016 Jul. doi: 10.1200/JCO.2016.66.6545).
A total of 14,290 patients met the study’s eligibility requirements, which included a diagnosis of cancer before age 21 years and 5-year survival following diagnosis. Cancer diagnoses included leukemia, Hodgkin and non-Hodgkin lymphoma, Wilms tumor, neuroblastoma, sarcoma, bone malignancy, and central nervous system malignancy. Baseline and follow-up questionnaires collected endocrine-related outcomes of interest, demographic information, and medical histories for both cancer survivors and their siblings (n = 4,031). For survivors, median age at diagnosis was 6 years and median age at last follow-up was 32 years. For siblings, median age at last follow-up was 34 years.
Overall 44% of cancer survivors had at least one endocrinopathy, 16.7% had at least two, and 6.6% had three or more. Survivors of Hodgkin lymphoma had the highest frequency of endocrine abnormality (60.1%) followed by survivors of CNS malignancy (54%), leukemia (45.6%), sarcoma (41.3%), non-Hodgkin lymphoma (39.7%), and neuroblastoma (31.9%).
Specifically, thyroid disorders were more frequent among cancer survivors than among their siblings: underactive thyroid (hazard ratio, 2.2; 95% confidence interval, 1.8-2.7), overactive thyroid (HR, 2.4; 95% CI, 1.7-3.3), thyroid nodules (HR, 3.9; 95% CI, 2.9-5.4), and thyroid cancer (HR 2.5; 95% CI, 1.2-5.3).
Compared to their siblings, cancer survivors showed increased risk of developing diabetes (RR, 1.8; 95% CI, 1.4-2.3).
Among survivors, those exposed to high-risk therapies (defined by the Children’s Oncology Group’s Long-Term Follow-Up Guidelinesfor Survivors of Childhood, Adolescent, and Young Adult Cancers) were at a greater risk of developing primary hypothyroidism (HR, 6.6; 95% CI, 5.6-7.8) central hypothyroidism (HR, 3.9; 95% CI, 2.9-5.2), an overactive thyroid (HR, 1.8; 95% CI, 1.2-2.8), thyroid nodules (HR, 6.3; 95% CI, 5.2-7.5), and thyroid cancer (HR, 9.2; 95% CI, 6.2-13.7) compared with survivors not so exposed.
The National Cancer Institute, the Cancer Center Support Grant, and the American Lebanese Syrian Associated Charities of St. Jude Children’s Research Hospital funded the study. Dr. Mostoufi-Moab and nine other investigators had no disclosures to report. Two investigators reported receiving financial compensation or honoraria from Merck or Sandoz.
Patients who survived pediatric-onset cancer are at increased risk for developing or experiencing endocrine abnormalities.
Risk was significantly higher in survivors who underwent high-risk therapeutic exposures compared with survivors not so exposed. Moreover, the incidence and prevalence of endocrine abnormalities increased across the lifespan of survivors, reported Sogol Mostoufi-Moab, MD, of University of Pennsylvania, Philadelphia, and his associates (J Clin Oncol. 2016 Jul. doi: 10.1200/JCO.2016.66.6545).
A total of 14,290 patients met the study’s eligibility requirements, which included a diagnosis of cancer before age 21 years and 5-year survival following diagnosis. Cancer diagnoses included leukemia, Hodgkin and non-Hodgkin lymphoma, Wilms tumor, neuroblastoma, sarcoma, bone malignancy, and central nervous system malignancy. Baseline and follow-up questionnaires collected endocrine-related outcomes of interest, demographic information, and medical histories for both cancer survivors and their siblings (n = 4,031). For survivors, median age at diagnosis was 6 years and median age at last follow-up was 32 years. For siblings, median age at last follow-up was 34 years.
Overall 44% of cancer survivors had at least one endocrinopathy, 16.7% had at least two, and 6.6% had three or more. Survivors of Hodgkin lymphoma had the highest frequency of endocrine abnormality (60.1%) followed by survivors of CNS malignancy (54%), leukemia (45.6%), sarcoma (41.3%), non-Hodgkin lymphoma (39.7%), and neuroblastoma (31.9%).
Specifically, thyroid disorders were more frequent among cancer survivors than among their siblings: underactive thyroid (hazard ratio, 2.2; 95% confidence interval, 1.8-2.7), overactive thyroid (HR, 2.4; 95% CI, 1.7-3.3), thyroid nodules (HR, 3.9; 95% CI, 2.9-5.4), and thyroid cancer (HR 2.5; 95% CI, 1.2-5.3).
Compared to their siblings, cancer survivors showed increased risk of developing diabetes (RR, 1.8; 95% CI, 1.4-2.3).
Among survivors, those exposed to high-risk therapies (defined by the Children’s Oncology Group’s Long-Term Follow-Up Guidelinesfor Survivors of Childhood, Adolescent, and Young Adult Cancers) were at a greater risk of developing primary hypothyroidism (HR, 6.6; 95% CI, 5.6-7.8) central hypothyroidism (HR, 3.9; 95% CI, 2.9-5.2), an overactive thyroid (HR, 1.8; 95% CI, 1.2-2.8), thyroid nodules (HR, 6.3; 95% CI, 5.2-7.5), and thyroid cancer (HR, 9.2; 95% CI, 6.2-13.7) compared with survivors not so exposed.
The National Cancer Institute, the Cancer Center Support Grant, and the American Lebanese Syrian Associated Charities of St. Jude Children’s Research Hospital funded the study. Dr. Mostoufi-Moab and nine other investigators had no disclosures to report. Two investigators reported receiving financial compensation or honoraria from Merck or Sandoz.
Patients who survived pediatric-onset cancer are at increased risk for developing or experiencing endocrine abnormalities.
Risk was significantly higher in survivors who underwent high-risk therapeutic exposures compared with survivors not so exposed. Moreover, the incidence and prevalence of endocrine abnormalities increased across the lifespan of survivors, reported Sogol Mostoufi-Moab, MD, of University of Pennsylvania, Philadelphia, and his associates (J Clin Oncol. 2016 Jul. doi: 10.1200/JCO.2016.66.6545).
A total of 14,290 patients met the study’s eligibility requirements, which included a diagnosis of cancer before age 21 years and 5-year survival following diagnosis. Cancer diagnoses included leukemia, Hodgkin and non-Hodgkin lymphoma, Wilms tumor, neuroblastoma, sarcoma, bone malignancy, and central nervous system malignancy. Baseline and follow-up questionnaires collected endocrine-related outcomes of interest, demographic information, and medical histories for both cancer survivors and their siblings (n = 4,031). For survivors, median age at diagnosis was 6 years and median age at last follow-up was 32 years. For siblings, median age at last follow-up was 34 years.
Overall 44% of cancer survivors had at least one endocrinopathy, 16.7% had at least two, and 6.6% had three or more. Survivors of Hodgkin lymphoma had the highest frequency of endocrine abnormality (60.1%) followed by survivors of CNS malignancy (54%), leukemia (45.6%), sarcoma (41.3%), non-Hodgkin lymphoma (39.7%), and neuroblastoma (31.9%).
Specifically, thyroid disorders were more frequent among cancer survivors than among their siblings: underactive thyroid (hazard ratio, 2.2; 95% confidence interval, 1.8-2.7), overactive thyroid (HR, 2.4; 95% CI, 1.7-3.3), thyroid nodules (HR, 3.9; 95% CI, 2.9-5.4), and thyroid cancer (HR 2.5; 95% CI, 1.2-5.3).
Compared to their siblings, cancer survivors showed increased risk of developing diabetes (RR, 1.8; 95% CI, 1.4-2.3).
Among survivors, those exposed to high-risk therapies (defined by the Children’s Oncology Group’s Long-Term Follow-Up Guidelinesfor Survivors of Childhood, Adolescent, and Young Adult Cancers) were at a greater risk of developing primary hypothyroidism (HR, 6.6; 95% CI, 5.6-7.8) central hypothyroidism (HR, 3.9; 95% CI, 2.9-5.2), an overactive thyroid (HR, 1.8; 95% CI, 1.2-2.8), thyroid nodules (HR, 6.3; 95% CI, 5.2-7.5), and thyroid cancer (HR, 9.2; 95% CI, 6.2-13.7) compared with survivors not so exposed.
The National Cancer Institute, the Cancer Center Support Grant, and the American Lebanese Syrian Associated Charities of St. Jude Children’s Research Hospital funded the study. Dr. Mostoufi-Moab and nine other investigators had no disclosures to report. Two investigators reported receiving financial compensation or honoraria from Merck or Sandoz.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Pediatric cancer survivors at increased risk for endocrine abnormalities
Patients who survived pediatric-onset cancer are at increased risk for developing or experiencing endocrine abnormalities.
Risk was significantly higher in survivors who underwent high-risk therapeutic exposures compared with survivors not so exposed. Moreover, the incidence and prevalence of endocrine abnormalities increased across the lifespan of survivors, reported Sogol Mostoufi-Moab, MD, of University of Pennsylvania, Philadelphia, and his associates (J Clin Oncol. 2016 Jul. doi: 10.1200/JCO.2016.66.6545).
A total of 14,290 patients met the study’s eligibility requirements, which included a diagnosis of cancer before age 21 years and 5-year survival following diagnosis. Cancer diagnoses included leukemia, Hodgkin and non-Hodgkin lymphoma, Wilms tumor, neuroblastoma, sarcoma, bone malignancy, and central nervous system malignancy. Baseline and follow-up questionnaires collected endocrine-related outcomes of interest, demographic information, and medical histories for both cancer survivors and their siblings (n = 4,031). For survivors, median age at diagnosis was 6 years and median age at last follow-up was 32 years. For siblings, median age at last follow-up was 34 years.
Overall 44% of cancer survivors had at least one endocrinopathy, 16.7% had at least two, and 6.6% had three or more. Survivors of Hodgkin lymphoma had the highest frequency of endocrine abnormality (60.1%) followed by survivors of CNS malignancy (54%), leukemia (45.6%), sarcoma (41.3%), non-Hodgkin lymphoma (39.7%), and neuroblastoma (31.9%).
Specifically, thyroid disorders were more frequent among cancer survivors than among their siblings: underactive thyroid (hazard ratio, 2.2; 95% confidence interval, 1.8-2.7), overactive thyroid (HR, 2.4; 95% CI, 1.7-3.3), thyroid nodules (HR, 3.9; 95% CI, 2.9-5.4), and thyroid cancer (HR 2.5; 95% CI, 1.2-5.3).
Compared to their siblings, cancer survivors showed increased risk of developing diabetes (RR, 1.8; 95% CI, 1.4-2.3).
Among survivors, those exposed to high-risk therapies (defined by the Children’s Oncology Group’s Long-Term Follow-Up Guidelinesfor Survivors of Childhood, Adolescent, and Young Adult Cancers) were at a greater risk of developing primary hypothyroidism (HR, 6.6; 95% CI, 5.6-7.8) central hypothyroidism (HR, 3.9; 95% CI, 2.9-5.2), an overactive thyroid (HR, 1.8; 95% CI, 1.2-2.8), thyroid nodules (HR, 6.3; 95% CI, 5.2-7.5), and thyroid cancer (HR, 9.2; 95% CI, 6.2-13.7) compared with survivors not so exposed.
The National Cancer Institute, the Cancer Center Support Grant, and the American Lebanese Syrian Associated Charities of St. Jude Children’s Research Hospital funded the study. Dr. Mostoufi-Moab and nine other investigators had no disclosures to report. Two investigators reported receiving financial compensation or honoraria from Merck or Sandoz.
On Twitter @jessnicolecraig
Patients who survived pediatric-onset cancer are at increased risk for developing or experiencing endocrine abnormalities.
Risk was significantly higher in survivors who underwent high-risk therapeutic exposures compared with survivors not so exposed. Moreover, the incidence and prevalence of endocrine abnormalities increased across the lifespan of survivors, reported Sogol Mostoufi-Moab, MD, of University of Pennsylvania, Philadelphia, and his associates (J Clin Oncol. 2016 Jul. doi: 10.1200/JCO.2016.66.6545).
A total of 14,290 patients met the study’s eligibility requirements, which included a diagnosis of cancer before age 21 years and 5-year survival following diagnosis. Cancer diagnoses included leukemia, Hodgkin and non-Hodgkin lymphoma, Wilms tumor, neuroblastoma, sarcoma, bone malignancy, and central nervous system malignancy. Baseline and follow-up questionnaires collected endocrine-related outcomes of interest, demographic information, and medical histories for both cancer survivors and their siblings (n = 4,031). For survivors, median age at diagnosis was 6 years and median age at last follow-up was 32 years. For siblings, median age at last follow-up was 34 years.
Overall 44% of cancer survivors had at least one endocrinopathy, 16.7% had at least two, and 6.6% had three or more. Survivors of Hodgkin lymphoma had the highest frequency of endocrine abnormality (60.1%) followed by survivors of CNS malignancy (54%), leukemia (45.6%), sarcoma (41.3%), non-Hodgkin lymphoma (39.7%), and neuroblastoma (31.9%).
Specifically, thyroid disorders were more frequent among cancer survivors than among their siblings: underactive thyroid (hazard ratio, 2.2; 95% confidence interval, 1.8-2.7), overactive thyroid (HR, 2.4; 95% CI, 1.7-3.3), thyroid nodules (HR, 3.9; 95% CI, 2.9-5.4), and thyroid cancer (HR 2.5; 95% CI, 1.2-5.3).
Compared to their siblings, cancer survivors showed increased risk of developing diabetes (RR, 1.8; 95% CI, 1.4-2.3).
Among survivors, those exposed to high-risk therapies (defined by the Children’s Oncology Group’s Long-Term Follow-Up Guidelinesfor Survivors of Childhood, Adolescent, and Young Adult Cancers) were at a greater risk of developing primary hypothyroidism (HR, 6.6; 95% CI, 5.6-7.8) central hypothyroidism (HR, 3.9; 95% CI, 2.9-5.2), an overactive thyroid (HR, 1.8; 95% CI, 1.2-2.8), thyroid nodules (HR, 6.3; 95% CI, 5.2-7.5), and thyroid cancer (HR, 9.2; 95% CI, 6.2-13.7) compared with survivors not so exposed.
The National Cancer Institute, the Cancer Center Support Grant, and the American Lebanese Syrian Associated Charities of St. Jude Children’s Research Hospital funded the study. Dr. Mostoufi-Moab and nine other investigators had no disclosures to report. Two investigators reported receiving financial compensation or honoraria from Merck or Sandoz.
On Twitter @jessnicolecraig
Patients who survived pediatric-onset cancer are at increased risk for developing or experiencing endocrine abnormalities.
Risk was significantly higher in survivors who underwent high-risk therapeutic exposures compared with survivors not so exposed. Moreover, the incidence and prevalence of endocrine abnormalities increased across the lifespan of survivors, reported Sogol Mostoufi-Moab, MD, of University of Pennsylvania, Philadelphia, and his associates (J Clin Oncol. 2016 Jul. doi: 10.1200/JCO.2016.66.6545).
A total of 14,290 patients met the study’s eligibility requirements, which included a diagnosis of cancer before age 21 years and 5-year survival following diagnosis. Cancer diagnoses included leukemia, Hodgkin and non-Hodgkin lymphoma, Wilms tumor, neuroblastoma, sarcoma, bone malignancy, and central nervous system malignancy. Baseline and follow-up questionnaires collected endocrine-related outcomes of interest, demographic information, and medical histories for both cancer survivors and their siblings (n = 4,031). For survivors, median age at diagnosis was 6 years and median age at last follow-up was 32 years. For siblings, median age at last follow-up was 34 years.
Overall 44% of cancer survivors had at least one endocrinopathy, 16.7% had at least two, and 6.6% had three or more. Survivors of Hodgkin lymphoma had the highest frequency of endocrine abnormality (60.1%) followed by survivors of CNS malignancy (54%), leukemia (45.6%), sarcoma (41.3%), non-Hodgkin lymphoma (39.7%), and neuroblastoma (31.9%).
Specifically, thyroid disorders were more frequent among cancer survivors than among their siblings: underactive thyroid (hazard ratio, 2.2; 95% confidence interval, 1.8-2.7), overactive thyroid (HR, 2.4; 95% CI, 1.7-3.3), thyroid nodules (HR, 3.9; 95% CI, 2.9-5.4), and thyroid cancer (HR 2.5; 95% CI, 1.2-5.3).
Compared to their siblings, cancer survivors showed increased risk of developing diabetes (RR, 1.8; 95% CI, 1.4-2.3).
Among survivors, those exposed to high-risk therapies (defined by the Children’s Oncology Group’s Long-Term Follow-Up Guidelinesfor Survivors of Childhood, Adolescent, and Young Adult Cancers) were at a greater risk of developing primary hypothyroidism (HR, 6.6; 95% CI, 5.6-7.8) central hypothyroidism (HR, 3.9; 95% CI, 2.9-5.2), an overactive thyroid (HR, 1.8; 95% CI, 1.2-2.8), thyroid nodules (HR, 6.3; 95% CI, 5.2-7.5), and thyroid cancer (HR, 9.2; 95% CI, 6.2-13.7) compared with survivors not so exposed.
The National Cancer Institute, the Cancer Center Support Grant, and the American Lebanese Syrian Associated Charities of St. Jude Children’s Research Hospital funded the study. Dr. Mostoufi-Moab and nine other investigators had no disclosures to report. Two investigators reported receiving financial compensation or honoraria from Merck or Sandoz.
On Twitter @jessnicolecraig
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Survivors of pediatric-onset cancer are at increased risk for developing endocrine abnormalities.
Major finding: Overall, 44% of childhood cancer survivors had at least one endocrinopathy. Survivors of Hodgkin lymphoma had the highest frequency of endocrine abnormality (60.1%) followed by survivors of CNS malignancy (54%), leukemia (45.6%), sarcoma (41.3%), non-Hodgkin lymphoma (39.7%), and neuroblastoma (31.9%).
Data source: A multi-institutional retrospective study of 14,290 men and women who survived pediatric cancer.
Disclosures: The National Cancer Institute, the Cancer Center Support Grant, and the American Lebanese Syrian Associated Charities of St. Jude Children’s Research Hospital funded the study. Dr. Mostoufi-Moab and nine other investigators had no disclosures to report. Two investigators reported receiving financial compensation or honoraria from Merck or Sandoz.
Emergency Ultrasound: Ultrasound-Guided Ulnar, Median, and Radial Nerve Blocks
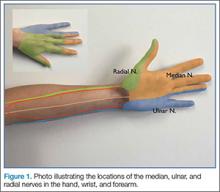
Emergency physicians (EPs) have traditionally used the landmark technique to block the radial, ulnar, and median nerves at the wrist (Figure 1). Many times, however, there is a need to perform the block more proximally. Performing these blocks with real-time ultrasound guidance allows the clinician to visually target the nerve, requires less anesthetic agent, and helps to avoid vascular structures. As with any procedure, employing the appropriate technique, along with practice, increases the success of the block.
Patient Selection
Before performing a nerve block, the EP must first determine if the patient is an appropriate candidate. The EP should be cautious in performing a nerve block on any patient who has paresthesias, tingling, or weakness, as the block will complicate further examinations. Likewise, a nerve block may be contraindicated in a patient in whom compartment syndrome is a concern, since the analgesic effect will inhibit the patient’s ability to sense increasing pain or worsening paresthesias.
Equipment and Preprocedure Care
An ultrasound-guided nerve block is performed using the linear high-frequency probe. Prior to the procedure, standard infection-control measures should be taken—ie, thoroughly cleaning the preinjection site and using a transducer-probe cover. Regarding the choice of anesthetic, either bupivacaine or lidocaine is appropriate; however, bupivacaine will provide a longer duration of analgesia. To administer the anesthetic, we typically use a regular cutting needle or a spinal needle. A review of the literature typically suggests either noncutting needle tips or tips with short bevels. There is a paucity of data on needle tip selection. The use of noncutting needle tips or tips with short bevels may be a better choice than a regular cutting needle or a spinal needle because they may decrease the chance of intraneural injection and consequent nerve injury.
Single- Versus Two-Person Technique
Peripheral nerve blocks can be performed using either a single- or two-person technique. In the one-person technique, the operator manipulates both the probe and the syringe. The two-person technique, however, requires the addition of tubing between the needle and the syringe. This can be done with the addition of a small section of intravenous (IV) tubing or by connecting two pieces of tubing together (the type traditionally placed on IV catheters). The operator holds the needle and the probe while the syringe and injection are controlled by the second person. Then, with the ultrasound machine set at the nerve or soft-tissue presetting, the scan begins by placing the probe in a transverse orientation.
Nerve Location and Identification
As previously noted, the ulnar, median, and radial nerves have traditionally been identified through use of the landmark technique just proximal to the wrist. The nerves can be located initially at these sites and then traced proximally.
Ulnar Nerve
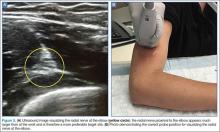
The ulnar nerve is located on the ulnar side of the forearm, just proximal to the wrist. (Figure 2a and 2b). The clinician should begin by fanning the probe at the wrist to find the ulnar artery and locate the nerve bundle. The ulnar nerve is also located on the ulnar side of the ulnar artery. The nerve will diverge from the path of the artery as it is traced proximally. To decrease the chance of an arterial injection/injury, the clinician should administer a nerve block after separating these two structures.
Median Nerve
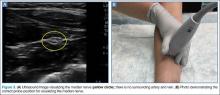
The clinician can employ the landmark approach to help find the nerve; then the scan should begin at the carpal tunnel. On ultrasound, the tendons in the carpal tunnel will appear similar to nerves (ie, round and hyperechoic) compared to surrounding muscle. As one continues to slide the probe up the forearm, the tendons will become muscles and a single hyperechoic structure will remain—the median nerve running in between the flexor digitorum superificialis and the flexor digitorum profundus (Figure 3a and 3b). Since there is no artery alongside the median nerve, it can be traced proximally; therefore, the procedure can be performed in any convenient location.
Radial Nerve
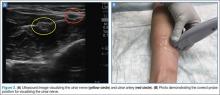
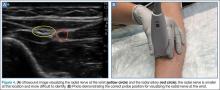
Of the three nerves, the radial nerve is the most challenging to visualize on ultrasound. There are two approaches to performing a radial nerve block. In the first approach, the radial nerve can be found just proximal to the wrist crease on the radial side of the radial artery (Figure 4a and 4b). This nerve is typically much smaller and harder to visualize at this level; it can be traced proximally and the block performed at this location. In the second approach, the radial nerve can be located 3 to 4 cm proximal to the elbow with the probe located anterolaterally (Figure 5a and 5b). In this location, the radial nerve lies between the brachialis and the brachioradialis muscles. In this approach, the nerve is much larger and easier to visualize.
Performing the Block
Prior to performing an anesthetic block at the ulnar, median, or radial nerve at the wrist, the clinician should first place the patient in a sitting or supine position with the appropriate elbow extended. When performing the block at the radial nerve above the elbow, the hand is typically placed in a resting position on the patient’s abdomen. When localizing the nerve, the angle of the transducer can vary the appearance of the nerve dramatically. To ensure the best possible view, the clinician should slowly “rock” the probe back and forth 10° to 20° in plane with the long axis of the arm, making sure the probe is placed as perpendicular as possible to the nerve. Once the nerve is identified, the clinician can follow it up and down the forearm with the probe to identify the best site to perform the block. In the optimal location, there should be a clear path that is as superficial as possible and avoids any vascular structures. We prefer using an in-plane technique to perform the nerve block to visualize the entire needle as it approaches the nerve. Once the site has been determined, the clinician should slowly inject 4 to 5 cc of anesthetic around the nerve, with the objective to partially surround the nerve. There is no need to completely surround the nerve, as doing so is not necessary to achieve a successful block. The clinician should stop immediately if the patient reports pain or if there is increased resistance, because this could indicate an intraneural injection.
Summary
Ultrasound-guided peripheral nerve blocks are an excellent option for providing regional anesthesia to lacerations and wounds that are too large for a local anesthetic. This technique can provide better analgesic relief, enhancing patient care.
Emergency physicians (EPs) have traditionally used the landmark technique to block the radial, ulnar, and median nerves at the wrist (Figure 1). Many times, however, there is a need to perform the block more proximally. Performing these blocks with real-time ultrasound guidance allows the clinician to visually target the nerve, requires less anesthetic agent, and helps to avoid vascular structures. As with any procedure, employing the appropriate technique, along with practice, increases the success of the block.
Patient Selection
Before performing a nerve block, the EP must first determine if the patient is an appropriate candidate. The EP should be cautious in performing a nerve block on any patient who has paresthesias, tingling, or weakness, as the block will complicate further examinations. Likewise, a nerve block may be contraindicated in a patient in whom compartment syndrome is a concern, since the analgesic effect will inhibit the patient’s ability to sense increasing pain or worsening paresthesias.
Equipment and Preprocedure Care
An ultrasound-guided nerve block is performed using the linear high-frequency probe. Prior to the procedure, standard infection-control measures should be taken—ie, thoroughly cleaning the preinjection site and using a transducer-probe cover. Regarding the choice of anesthetic, either bupivacaine or lidocaine is appropriate; however, bupivacaine will provide a longer duration of analgesia. To administer the anesthetic, we typically use a regular cutting needle or a spinal needle. A review of the literature typically suggests either noncutting needle tips or tips with short bevels. There is a paucity of data on needle tip selection. The use of noncutting needle tips or tips with short bevels may be a better choice than a regular cutting needle or a spinal needle because they may decrease the chance of intraneural injection and consequent nerve injury.
Single- Versus Two-Person Technique
Peripheral nerve blocks can be performed using either a single- or two-person technique. In the one-person technique, the operator manipulates both the probe and the syringe. The two-person technique, however, requires the addition of tubing between the needle and the syringe. This can be done with the addition of a small section of intravenous (IV) tubing or by connecting two pieces of tubing together (the type traditionally placed on IV catheters). The operator holds the needle and the probe while the syringe and injection are controlled by the second person. Then, with the ultrasound machine set at the nerve or soft-tissue presetting, the scan begins by placing the probe in a transverse orientation.
Nerve Location and Identification
As previously noted, the ulnar, median, and radial nerves have traditionally been identified through use of the landmark technique just proximal to the wrist. The nerves can be located initially at these sites and then traced proximally.
Ulnar Nerve
The ulnar nerve is located on the ulnar side of the forearm, just proximal to the wrist. (Figure 2a and 2b). The clinician should begin by fanning the probe at the wrist to find the ulnar artery and locate the nerve bundle. The ulnar nerve is also located on the ulnar side of the ulnar artery. The nerve will diverge from the path of the artery as it is traced proximally. To decrease the chance of an arterial injection/injury, the clinician should administer a nerve block after separating these two structures.
Median Nerve
The clinician can employ the landmark approach to help find the nerve; then the scan should begin at the carpal tunnel. On ultrasound, the tendons in the carpal tunnel will appear similar to nerves (ie, round and hyperechoic) compared to surrounding muscle. As one continues to slide the probe up the forearm, the tendons will become muscles and a single hyperechoic structure will remain—the median nerve running in between the flexor digitorum superificialis and the flexor digitorum profundus (Figure 3a and 3b). Since there is no artery alongside the median nerve, it can be traced proximally; therefore, the procedure can be performed in any convenient location.
Radial Nerve
Of the three nerves, the radial nerve is the most challenging to visualize on ultrasound. There are two approaches to performing a radial nerve block. In the first approach, the radial nerve can be found just proximal to the wrist crease on the radial side of the radial artery (Figure 4a and 4b). This nerve is typically much smaller and harder to visualize at this level; it can be traced proximally and the block performed at this location. In the second approach, the radial nerve can be located 3 to 4 cm proximal to the elbow with the probe located anterolaterally (Figure 5a and 5b). In this location, the radial nerve lies between the brachialis and the brachioradialis muscles. In this approach, the nerve is much larger and easier to visualize.
Performing the Block
Prior to performing an anesthetic block at the ulnar, median, or radial nerve at the wrist, the clinician should first place the patient in a sitting or supine position with the appropriate elbow extended. When performing the block at the radial nerve above the elbow, the hand is typically placed in a resting position on the patient’s abdomen. When localizing the nerve, the angle of the transducer can vary the appearance of the nerve dramatically. To ensure the best possible view, the clinician should slowly “rock” the probe back and forth 10° to 20° in plane with the long axis of the arm, making sure the probe is placed as perpendicular as possible to the nerve. Once the nerve is identified, the clinician can follow it up and down the forearm with the probe to identify the best site to perform the block. In the optimal location, there should be a clear path that is as superficial as possible and avoids any vascular structures. We prefer using an in-plane technique to perform the nerve block to visualize the entire needle as it approaches the nerve. Once the site has been determined, the clinician should slowly inject 4 to 5 cc of anesthetic around the nerve, with the objective to partially surround the nerve. There is no need to completely surround the nerve, as doing so is not necessary to achieve a successful block. The clinician should stop immediately if the patient reports pain or if there is increased resistance, because this could indicate an intraneural injection.
Summary
Ultrasound-guided peripheral nerve blocks are an excellent option for providing regional anesthesia to lacerations and wounds that are too large for a local anesthetic. This technique can provide better analgesic relief, enhancing patient care.
Emergency physicians (EPs) have traditionally used the landmark technique to block the radial, ulnar, and median nerves at the wrist (Figure 1). Many times, however, there is a need to perform the block more proximally. Performing these blocks with real-time ultrasound guidance allows the clinician to visually target the nerve, requires less anesthetic agent, and helps to avoid vascular structures. As with any procedure, employing the appropriate technique, along with practice, increases the success of the block.
Patient Selection
Before performing a nerve block, the EP must first determine if the patient is an appropriate candidate. The EP should be cautious in performing a nerve block on any patient who has paresthesias, tingling, or weakness, as the block will complicate further examinations. Likewise, a nerve block may be contraindicated in a patient in whom compartment syndrome is a concern, since the analgesic effect will inhibit the patient’s ability to sense increasing pain or worsening paresthesias.
Equipment and Preprocedure Care
An ultrasound-guided nerve block is performed using the linear high-frequency probe. Prior to the procedure, standard infection-control measures should be taken—ie, thoroughly cleaning the preinjection site and using a transducer-probe cover. Regarding the choice of anesthetic, either bupivacaine or lidocaine is appropriate; however, bupivacaine will provide a longer duration of analgesia. To administer the anesthetic, we typically use a regular cutting needle or a spinal needle. A review of the literature typically suggests either noncutting needle tips or tips with short bevels. There is a paucity of data on needle tip selection. The use of noncutting needle tips or tips with short bevels may be a better choice than a regular cutting needle or a spinal needle because they may decrease the chance of intraneural injection and consequent nerve injury.
Single- Versus Two-Person Technique
Peripheral nerve blocks can be performed using either a single- or two-person technique. In the one-person technique, the operator manipulates both the probe and the syringe. The two-person technique, however, requires the addition of tubing between the needle and the syringe. This can be done with the addition of a small section of intravenous (IV) tubing or by connecting two pieces of tubing together (the type traditionally placed on IV catheters). The operator holds the needle and the probe while the syringe and injection are controlled by the second person. Then, with the ultrasound machine set at the nerve or soft-tissue presetting, the scan begins by placing the probe in a transverse orientation.
Nerve Location and Identification
As previously noted, the ulnar, median, and radial nerves have traditionally been identified through use of the landmark technique just proximal to the wrist. The nerves can be located initially at these sites and then traced proximally.
Ulnar Nerve
The ulnar nerve is located on the ulnar side of the forearm, just proximal to the wrist. (Figure 2a and 2b). The clinician should begin by fanning the probe at the wrist to find the ulnar artery and locate the nerve bundle. The ulnar nerve is also located on the ulnar side of the ulnar artery. The nerve will diverge from the path of the artery as it is traced proximally. To decrease the chance of an arterial injection/injury, the clinician should administer a nerve block after separating these two structures.
Median Nerve
The clinician can employ the landmark approach to help find the nerve; then the scan should begin at the carpal tunnel. On ultrasound, the tendons in the carpal tunnel will appear similar to nerves (ie, round and hyperechoic) compared to surrounding muscle. As one continues to slide the probe up the forearm, the tendons will become muscles and a single hyperechoic structure will remain—the median nerve running in between the flexor digitorum superificialis and the flexor digitorum profundus (Figure 3a and 3b). Since there is no artery alongside the median nerve, it can be traced proximally; therefore, the procedure can be performed in any convenient location.
Radial Nerve
Of the three nerves, the radial nerve is the most challenging to visualize on ultrasound. There are two approaches to performing a radial nerve block. In the first approach, the radial nerve can be found just proximal to the wrist crease on the radial side of the radial artery (Figure 4a and 4b). This nerve is typically much smaller and harder to visualize at this level; it can be traced proximally and the block performed at this location. In the second approach, the radial nerve can be located 3 to 4 cm proximal to the elbow with the probe located anterolaterally (Figure 5a and 5b). In this location, the radial nerve lies between the brachialis and the brachioradialis muscles. In this approach, the nerve is much larger and easier to visualize.
Performing the Block
Prior to performing an anesthetic block at the ulnar, median, or radial nerve at the wrist, the clinician should first place the patient in a sitting or supine position with the appropriate elbow extended. When performing the block at the radial nerve above the elbow, the hand is typically placed in a resting position on the patient’s abdomen. When localizing the nerve, the angle of the transducer can vary the appearance of the nerve dramatically. To ensure the best possible view, the clinician should slowly “rock” the probe back and forth 10° to 20° in plane with the long axis of the arm, making sure the probe is placed as perpendicular as possible to the nerve. Once the nerve is identified, the clinician can follow it up and down the forearm with the probe to identify the best site to perform the block. In the optimal location, there should be a clear path that is as superficial as possible and avoids any vascular structures. We prefer using an in-plane technique to perform the nerve block to visualize the entire needle as it approaches the nerve. Once the site has been determined, the clinician should slowly inject 4 to 5 cc of anesthetic around the nerve, with the objective to partially surround the nerve. There is no need to completely surround the nerve, as doing so is not necessary to achieve a successful block. The clinician should stop immediately if the patient reports pain or if there is increased resistance, because this could indicate an intraneural injection.
Summary
Ultrasound-guided peripheral nerve blocks are an excellent option for providing regional anesthesia to lacerations and wounds that are too large for a local anesthetic. This technique can provide better analgesic relief, enhancing patient care.
Does Optic Nerve Sheath Diameter Ultrasonography Permit Accurate Detection of Real-Time Changes in ICP?
Case Scenarios
Case 1
While working abroad in a resource-limited environment, a patient was brought in after falling and hitting his head. Initially, the patient was awake and alert, but he gradually became minimally responsive, with a Glasgow Coma Scale score of 9. Your facility did not have computed tomography (CT) or magnetic resonance imaging (MRI), but did have a point-of-care ultrasound (US) machine. You measured the patient’s optic nerve sheath diameter (ONSD) with the US and found a diameter of 4.5 mm in each eye. With this clinical change, you wondered if repeat US scans to detect increasing intracranial pressure (ICP) would represent changes in the patient’s condition.
Case 2
A patient who presented with an intracranial hemorrhage was treated with hypertonic saline and was awaiting neurosurgical placement of an extraventicular drain. During this time, a resident who was on a US rotation asked you if she would be able to detect changes in the patient’s ICP using US rather than placing an invasive device. How do you respond?
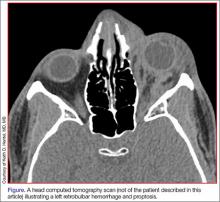
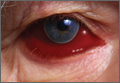
In adults, ICP is normally 10 to 15 mm Hg. It may be pathologically increased in several life-threatening conditions, including traumatic brain injury (TBI), subarachnoid hemorrhage, central venous thrombosis, brain tumor, and abscess. It is also increased by nonacute pathology, such as idiopathic intracranial hypertension (IIH), which also is known as pseudotumor cerebri. In patients with acute pathology, ICP above 20 mm Hg is generally considered an indication for treatment.1 Indications for ICP monitoring in TBI include positive CT findings, patient age greater than 40 years, systemic hypotension, or abnormal flexion/extension in response to pain.2 Other reasons to monitor ICP include the management of pseudotumor cerebri or after ventriculoperitoneal shunt surgery.3
Unfortunately, current methods of ICP monitoring have significant drawbacks and limitations. The gold standard of ICP monitoring—measurement using an intraventricular catheter—increases the risks of infection and hemorrhage, requires the skill of a neurosurgeon, and may be contraindicated due to coagulopathy or thrombocytopenia. It also cannot be done in a prehospital setting and only to a limited extent in the ED.4
Computed tomography scans and MRI can assess elevated ICP, but these tests are expensive, may increase patient radiation exposure, require patient transport, and may not always detect raised ICP. In the appropriate clinical context, signs present on physical examination, such as decorticate/decerebrate posturing, papilledema, or fixed/dilated pupils, may be highly suggestive of an increased ICP, but sensitivity and specificity are inadequate. Delay in diagnosis is also a drawback of imaging and physical examination, as findings may not present until ICP has been persistently elevated.
Given the disadvantages of current means of assessing elevated ICP, several noninvasive methods of measuring ICP are being investigated. These include such techniques as transcranial Doppler, electroencephalogram, pupillometry, and ONSD measurements.5 This article reviews current applications of ultrasonography measurements of the ONSD in assessing elevations in ICP.
ONSD US
Assessment of ICP via measurement of the ONSD has attracted increasing attention, particularly in emergency medicine. Measurements of the ONSD are possible with CT, MRI, and US. Of these modalities, ONSD US has attracted the most interest, due to its low cost, wide availability, and rapidity. It does not require patient transport, and does not expose a patient to additional radiation. In addition, ONSD US has been utilized in low-resource settings, and may be particularly useful in prehospital and mass-casualty situations.6
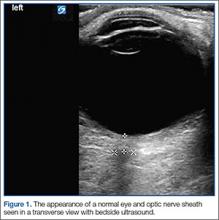
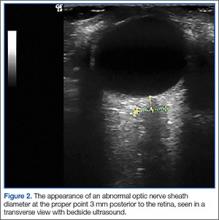
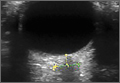
The underlying relationship between ONSD and ICP is a result of the enclosure of the subarachnoid space by the ONS. Increased ICP leads to expansion of the ONS, particularly at 3 mm behind the globe, in the retrobulbar compartment (Figures 1 and 2).7
Unfortunately, it is not possible to precisely determine ICP from an ONSD measurement, because baseline ONSD values and elasticity vary significantly within the population.4,8 As a result, ONSD US has been investigated mostly for its ability to detect qualitative changes—particularly as a screen for elevated ICP. Optic nerve sheath diameter has high discriminative value in its ability to distinguish normal from elevated ICP. In a meta-analysis, Dubourg et al9 showed that the technique had an area under the summary receiver-operating curve of 0.94, signifying excellent test accuracy to diagnose elevated ICPs.
Researchers have attempted to determine a threshold value of ONSD that would serve as a clinically useful predictor of elevated ICP. Currently, this value ranges from 4.8 to 5.9 mm, depending on the study9; 5 mm is commonly used clinically as a threshold.10
Using ONSD US to Monitor Rapid Changes in ICP
While the use of the ONSD technique to screen for elevated ICP is relatively well established, the use of ONSD US to track acute changes in ICP is not as well studied. Serial tracking of acute changes could be useful in a patient at risk for intracranial hypertension secondary to trauma, to monitor the results of treating a patient with IIH, or after ventriculoperitoneal shunt placement.3
In Vivo Data
In 1993, Tamburrelli et al11 performed the first ONSD intrathecal infusion study, using A-scan sonography, and concluded that there was a “direct, biphasic, positive relation between diastolic intracranial pressure and optic nerve diameters” and that the data showed “rapid changes of optic nerve diameters in response to variation of intracranial pressure.”
In 1997, Hansen and Helmke12 recorded ONSD versus ICP data in the first intrathecal infusion test to use B-scan mode sonography. Ultrasonography was performed at 2- to 4-minute intervals. Their data demonstrated a linear relationship between ICP and ONSD over a particular cerebrospinal fluid pressure interval. They noted that “this interval differed between patients: ONS dilation commenced at pressure thresholds between 15 mm Hg and 30 mm Hg and in some patients saturation of the response (constant ONSD) occurred between 30 mm Hg and 40 mm Hg.”
The slope of ONSD versus ICP curve varied considerably by patient, making it impossible to infer an absolute ICP value from an ONSD without prior knowledge of the patient’s ratio. Similar to the data from Tamburrelli et al,11 Hansen and Helmke12 also found that there was no lag in ONSD response to ICP: “Within this interval, no temporal delay of the ONS response was noted.”
The only study comparing real-time ONSD data to gold-standard measurements of rapidly changing ICP in humans was performed by Maissan et al13 in 2015. This study involved a cohort of 18 patients who had suffered TBI and had intraparenchymal probes inserted. Because ICP rises transiently during endotracheal tube suctioning due to irritation of the trachea, the increase and subsequent decrease after suctioning was an ideal time to perform ONSD measurements and compare them to simultaneous gold-standard ICP measurements. The ONSD US measurements were performed 30 to 60 seconds prior to suctioning, during suctioning, and 30 to 60 seconds after suctioning.
Even during this very rapid time course, a strong correlation between ICP and ONSD measurements was demonstrated. The R2 value was 0.80. There was no perceptible “lag” in ONSD change; changes in ICP were immediately reflected in ONSD. Notably, an absolute change of less than 8 to 10 mm Hg in ICP did not affect ONSD, which is consistent with data collected by Hansen and Helmke.12
Therapeutic Lumbar Puncture for IIH
There are two case reports of ONSD US measurements being taken pre- and postlumbar puncture (LP) in patients with IIH. In the first, in 1989 Galetta et al14 used A-scan US to measure pre- and post-LP ONSD in a woman with papilledema secondary to IIH. They found a significant reduction in ONSD bilaterally “within minutes” of performing the LP.14
The second case report was published in 2015 by Singleton et al.15 They recorded ONSD measurements 30 minutes pre- and post-LP in a woman who presented to the ED with symptoms from elevated ICP. After reduction of pressure via LP, they recorded a significant reduction in ONSD bilaterally.15
Cadaver Data
Hansen et al16 evaluated the distensibility and elasticity of the ONS using postmortem optic nerve preparations. The ONSD was recorded 200 seconds after each pressure increase, which was long enough to achieve stable diameters. They found a linear correlation between pressure increases of 5 to 45 mm Hg and ONSD. This would suggest a potential positively correlated change in ONSD with in vivo changes in ICP. However, this still needs further clinical study to better assess measurable changes in living patients.
Conclusion
Published data have consistently demonstrated that changes in ICP are rapidly transmitted to the optic nerve sheath and that there does not appear to be a temporal lag in the ONSD. Based on in vivo data, the relationship between ICP and ONSD appears to be linear only over a range of moderately elevated ICP. According to Hansen and Helmke,12 this range starts at approximately 18 to 30 mm Hg, and ends at approximately 40 to 45 mm Hg. Maissan et al13 observed similar findings: “At low levels, ICP changes (8-10 mm Hg) do not affect the ONSD.”
There is still need for additional research to validate and refine these findings. Only one study has compared gold-standard ICP measurements with ONSD US measurements in real time,13 and the literature on ONSD US in tracking ICP after therapeutic LP in IIH consists of only two case reports.
Thus, with some caveats, ONSD US appears to permit qualitative tracking of ICP in real time. This supports its use in situations where a patient may have rapidly changing ICP, such as close monitoring of patients at risk for elevated ICP in a critical care setting, and response to treatment in patients with IIH.
1. Stocchetti N, Maas AI. Traumatic intracranial hypertension. N Engl J Med. 2014;370(22):2121-2130.
2. Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons; et al. Guidelines for the management of severe traumatic brain injury. VI. Indications for intracranial pressure monitoring. J Neurotrauma. 2007;24(Suppl 1):S37-S44.
3. Choi SH, Min KT, Park EK, Kim MS, Jung JH, Kim H. Ultrasonography of the optic nerve sheath to assess intracranial pressure changes after ventriculo-peritoneal shunt surgery in children with hydrocephalus: a prospective observational study. Anaesthesia. 2015;70(11):1268-1273.
4. Kristiansson H, Nissborg E, Bartek J Jr, Andresen M, Reinstrup P, Romner B. Measuring elevated intracranial pressure through noninvasive methods: a review of the literature. J Neurosurg Anesthesiol. 2013;25(4):372-385.
5. Rajajee V, Thyagarajan P, Rajagopalan RE. Optic nerve ultrasonography for detection of raised intracranial pressure when invasive monitoring is unavailable. Neurol India. 2010;58(5):812-813.
6. Robba C, Baciqaluppi S, Cardim D, Donnelly J, Bertuccio A, Czosnyka M. Non-invasive assessment of intracranial pressure. Acta Neurol Scand. 2016;134(1):4-21.
7. Hansen HC, Helmke K. The subarachnoid space surrounding the optic nerves. An ultrasound study of the optic nerve sheath. Surg Radiol Anat. 1996;18(4):323-328.
8. Hansen HC, Lagrèze W, Krueger O, Helmke K. Dependence of the optic nerve sheath diameter on acutely applied subarachnoidal pressure - an experimental ultrasound study. Acta Ophthalmol. 2011;89(6):e528-e532.
9. Dubourg J, Javouhey E, Geeraerts T, Messerer M, Kassai B. Ultrasonography of optic nerve sheath diameter for detection of raised intracranial pressure: a systematic review and meta-analysis. Intensive Care Med. 2011;37(7):1059-1068.
10. Kimberly HH, Shah S, Marill K, Noble V. Correlation of optic nerve sheath diameter with direct measurement of intracranial pressure. Acad Emerg Med. 2008;15(2):201-204.
11. Tamburrelli C, Anile C, Mangiola A, Falsini B, Palma P. CSF dynamic parameters and changes of optic nerve diameters measured by standardized echography. In: Till P, ed. Ophthalmic Echography 13: Proceedings of the 13th SIDUO Congress, Vienna, Austria, 1990; vol 55. Dordrecht, Netherlands: Springer Netherlands; 1993:101-109.
12. Hansen HC, Helmke K. Validation of the optic nerve sheath response to changing cerebrospinal fluid pressure: ultrasound findings during intrathecal infusion tests. J Neurosurg. 1997;87(1):34-40.
13. Maissan IM, Dirven PJ, Haitsma IK, Hoeks SE, Gommers D, Stolker RJ. Ultrasonographic measured optic nerve sheath diameter as an accurate and quick monitor for changes in intracranial pressure. J Neurosurg. 2015;123(3)743-747.
14. Galetta S, Byrne SF, Smith JL. Echographic correlation of optic nerve sheath size and cerebrospinal fluid pressure. J Clin Neuroophthalmol. 1989;9(2):79-82.
15. Singleton J, Dagan A, Edlow JA, Hoffmann B. Real-time optic nerve sheath diameter reduction measured with bedside ultrasound after therapeutic lumbar puncture in a patient with idiopathic intracranial hypertension. Am J Emerg Med. 2015;33(6):860.e5-e7.
16. Hansen HC, Lagrèze W, Krueger O, Helmke K. Dependence of the optic nerve sheath diameter on acutely applied subarachnoidal pressure—an experimental ultrasound study. Acta Ophthalmol. 2011;89(6):e528-e532.
Case Scenarios
Case 1
While working abroad in a resource-limited environment, a patient was brought in after falling and hitting his head. Initially, the patient was awake and alert, but he gradually became minimally responsive, with a Glasgow Coma Scale score of 9. Your facility did not have computed tomography (CT) or magnetic resonance imaging (MRI), but did have a point-of-care ultrasound (US) machine. You measured the patient’s optic nerve sheath diameter (ONSD) with the US and found a diameter of 4.5 mm in each eye. With this clinical change, you wondered if repeat US scans to detect increasing intracranial pressure (ICP) would represent changes in the patient’s condition.
Case 2
A patient who presented with an intracranial hemorrhage was treated with hypertonic saline and was awaiting neurosurgical placement of an extraventicular drain. During this time, a resident who was on a US rotation asked you if she would be able to detect changes in the patient’s ICP using US rather than placing an invasive device. How do you respond?
In adults, ICP is normally 10 to 15 mm Hg. It may be pathologically increased in several life-threatening conditions, including traumatic brain injury (TBI), subarachnoid hemorrhage, central venous thrombosis, brain tumor, and abscess. It is also increased by nonacute pathology, such as idiopathic intracranial hypertension (IIH), which also is known as pseudotumor cerebri. In patients with acute pathology, ICP above 20 mm Hg is generally considered an indication for treatment.1 Indications for ICP monitoring in TBI include positive CT findings, patient age greater than 40 years, systemic hypotension, or abnormal flexion/extension in response to pain.2 Other reasons to monitor ICP include the management of pseudotumor cerebri or after ventriculoperitoneal shunt surgery.3
Unfortunately, current methods of ICP monitoring have significant drawbacks and limitations. The gold standard of ICP monitoring—measurement using an intraventricular catheter—increases the risks of infection and hemorrhage, requires the skill of a neurosurgeon, and may be contraindicated due to coagulopathy or thrombocytopenia. It also cannot be done in a prehospital setting and only to a limited extent in the ED.4
Computed tomography scans and MRI can assess elevated ICP, but these tests are expensive, may increase patient radiation exposure, require patient transport, and may not always detect raised ICP. In the appropriate clinical context, signs present on physical examination, such as decorticate/decerebrate posturing, papilledema, or fixed/dilated pupils, may be highly suggestive of an increased ICP, but sensitivity and specificity are inadequate. Delay in diagnosis is also a drawback of imaging and physical examination, as findings may not present until ICP has been persistently elevated.
Given the disadvantages of current means of assessing elevated ICP, several noninvasive methods of measuring ICP are being investigated. These include such techniques as transcranial Doppler, electroencephalogram, pupillometry, and ONSD measurements.5 This article reviews current applications of ultrasonography measurements of the ONSD in assessing elevations in ICP.
ONSD US
Assessment of ICP via measurement of the ONSD has attracted increasing attention, particularly in emergency medicine. Measurements of the ONSD are possible with CT, MRI, and US. Of these modalities, ONSD US has attracted the most interest, due to its low cost, wide availability, and rapidity. It does not require patient transport, and does not expose a patient to additional radiation. In addition, ONSD US has been utilized in low-resource settings, and may be particularly useful in prehospital and mass-casualty situations.6
The underlying relationship between ONSD and ICP is a result of the enclosure of the subarachnoid space by the ONS. Increased ICP leads to expansion of the ONS, particularly at 3 mm behind the globe, in the retrobulbar compartment (Figures 1 and 2).7
Unfortunately, it is not possible to precisely determine ICP from an ONSD measurement, because baseline ONSD values and elasticity vary significantly within the population.4,8 As a result, ONSD US has been investigated mostly for its ability to detect qualitative changes—particularly as a screen for elevated ICP. Optic nerve sheath diameter has high discriminative value in its ability to distinguish normal from elevated ICP. In a meta-analysis, Dubourg et al9 showed that the technique had an area under the summary receiver-operating curve of 0.94, signifying excellent test accuracy to diagnose elevated ICPs.
Researchers have attempted to determine a threshold value of ONSD that would serve as a clinically useful predictor of elevated ICP. Currently, this value ranges from 4.8 to 5.9 mm, depending on the study9; 5 mm is commonly used clinically as a threshold.10
Using ONSD US to Monitor Rapid Changes in ICP
While the use of the ONSD technique to screen for elevated ICP is relatively well established, the use of ONSD US to track acute changes in ICP is not as well studied. Serial tracking of acute changes could be useful in a patient at risk for intracranial hypertension secondary to trauma, to monitor the results of treating a patient with IIH, or after ventriculoperitoneal shunt placement.3
In Vivo Data
In 1993, Tamburrelli et al11 performed the first ONSD intrathecal infusion study, using A-scan sonography, and concluded that there was a “direct, biphasic, positive relation between diastolic intracranial pressure and optic nerve diameters” and that the data showed “rapid changes of optic nerve diameters in response to variation of intracranial pressure.”
In 1997, Hansen and Helmke12 recorded ONSD versus ICP data in the first intrathecal infusion test to use B-scan mode sonography. Ultrasonography was performed at 2- to 4-minute intervals. Their data demonstrated a linear relationship between ICP and ONSD over a particular cerebrospinal fluid pressure interval. They noted that “this interval differed between patients: ONS dilation commenced at pressure thresholds between 15 mm Hg and 30 mm Hg and in some patients saturation of the response (constant ONSD) occurred between 30 mm Hg and 40 mm Hg.”
The slope of ONSD versus ICP curve varied considerably by patient, making it impossible to infer an absolute ICP value from an ONSD without prior knowledge of the patient’s ratio. Similar to the data from Tamburrelli et al,11 Hansen and Helmke12 also found that there was no lag in ONSD response to ICP: “Within this interval, no temporal delay of the ONS response was noted.”
The only study comparing real-time ONSD data to gold-standard measurements of rapidly changing ICP in humans was performed by Maissan et al13 in 2015. This study involved a cohort of 18 patients who had suffered TBI and had intraparenchymal probes inserted. Because ICP rises transiently during endotracheal tube suctioning due to irritation of the trachea, the increase and subsequent decrease after suctioning was an ideal time to perform ONSD measurements and compare them to simultaneous gold-standard ICP measurements. The ONSD US measurements were performed 30 to 60 seconds prior to suctioning, during suctioning, and 30 to 60 seconds after suctioning.
Even during this very rapid time course, a strong correlation between ICP and ONSD measurements was demonstrated. The R2 value was 0.80. There was no perceptible “lag” in ONSD change; changes in ICP were immediately reflected in ONSD. Notably, an absolute change of less than 8 to 10 mm Hg in ICP did not affect ONSD, which is consistent with data collected by Hansen and Helmke.12
Therapeutic Lumbar Puncture for IIH
There are two case reports of ONSD US measurements being taken pre- and postlumbar puncture (LP) in patients with IIH. In the first, in 1989 Galetta et al14 used A-scan US to measure pre- and post-LP ONSD in a woman with papilledema secondary to IIH. They found a significant reduction in ONSD bilaterally “within minutes” of performing the LP.14
The second case report was published in 2015 by Singleton et al.15 They recorded ONSD measurements 30 minutes pre- and post-LP in a woman who presented to the ED with symptoms from elevated ICP. After reduction of pressure via LP, they recorded a significant reduction in ONSD bilaterally.15
Cadaver Data
Hansen et al16 evaluated the distensibility and elasticity of the ONS using postmortem optic nerve preparations. The ONSD was recorded 200 seconds after each pressure increase, which was long enough to achieve stable diameters. They found a linear correlation between pressure increases of 5 to 45 mm Hg and ONSD. This would suggest a potential positively correlated change in ONSD with in vivo changes in ICP. However, this still needs further clinical study to better assess measurable changes in living patients.
Conclusion
Published data have consistently demonstrated that changes in ICP are rapidly transmitted to the optic nerve sheath and that there does not appear to be a temporal lag in the ONSD. Based on in vivo data, the relationship between ICP and ONSD appears to be linear only over a range of moderately elevated ICP. According to Hansen and Helmke,12 this range starts at approximately 18 to 30 mm Hg, and ends at approximately 40 to 45 mm Hg. Maissan et al13 observed similar findings: “At low levels, ICP changes (8-10 mm Hg) do not affect the ONSD.”
There is still need for additional research to validate and refine these findings. Only one study has compared gold-standard ICP measurements with ONSD US measurements in real time,13 and the literature on ONSD US in tracking ICP after therapeutic LP in IIH consists of only two case reports.
Thus, with some caveats, ONSD US appears to permit qualitative tracking of ICP in real time. This supports its use in situations where a patient may have rapidly changing ICP, such as close monitoring of patients at risk for elevated ICP in a critical care setting, and response to treatment in patients with IIH.
Case Scenarios
Case 1
While working abroad in a resource-limited environment, a patient was brought in after falling and hitting his head. Initially, the patient was awake and alert, but he gradually became minimally responsive, with a Glasgow Coma Scale score of 9. Your facility did not have computed tomography (CT) or magnetic resonance imaging (MRI), but did have a point-of-care ultrasound (US) machine. You measured the patient’s optic nerve sheath diameter (ONSD) with the US and found a diameter of 4.5 mm in each eye. With this clinical change, you wondered if repeat US scans to detect increasing intracranial pressure (ICP) would represent changes in the patient’s condition.
Case 2
A patient who presented with an intracranial hemorrhage was treated with hypertonic saline and was awaiting neurosurgical placement of an extraventicular drain. During this time, a resident who was on a US rotation asked you if she would be able to detect changes in the patient’s ICP using US rather than placing an invasive device. How do you respond?
In adults, ICP is normally 10 to 15 mm Hg. It may be pathologically increased in several life-threatening conditions, including traumatic brain injury (TBI), subarachnoid hemorrhage, central venous thrombosis, brain tumor, and abscess. It is also increased by nonacute pathology, such as idiopathic intracranial hypertension (IIH), which also is known as pseudotumor cerebri. In patients with acute pathology, ICP above 20 mm Hg is generally considered an indication for treatment.1 Indications for ICP monitoring in TBI include positive CT findings, patient age greater than 40 years, systemic hypotension, or abnormal flexion/extension in response to pain.2 Other reasons to monitor ICP include the management of pseudotumor cerebri or after ventriculoperitoneal shunt surgery.3
Unfortunately, current methods of ICP monitoring have significant drawbacks and limitations. The gold standard of ICP monitoring—measurement using an intraventricular catheter—increases the risks of infection and hemorrhage, requires the skill of a neurosurgeon, and may be contraindicated due to coagulopathy or thrombocytopenia. It also cannot be done in a prehospital setting and only to a limited extent in the ED.4
Computed tomography scans and MRI can assess elevated ICP, but these tests are expensive, may increase patient radiation exposure, require patient transport, and may not always detect raised ICP. In the appropriate clinical context, signs present on physical examination, such as decorticate/decerebrate posturing, papilledema, or fixed/dilated pupils, may be highly suggestive of an increased ICP, but sensitivity and specificity are inadequate. Delay in diagnosis is also a drawback of imaging and physical examination, as findings may not present until ICP has been persistently elevated.
Given the disadvantages of current means of assessing elevated ICP, several noninvasive methods of measuring ICP are being investigated. These include such techniques as transcranial Doppler, electroencephalogram, pupillometry, and ONSD measurements.5 This article reviews current applications of ultrasonography measurements of the ONSD in assessing elevations in ICP.
ONSD US
Assessment of ICP via measurement of the ONSD has attracted increasing attention, particularly in emergency medicine. Measurements of the ONSD are possible with CT, MRI, and US. Of these modalities, ONSD US has attracted the most interest, due to its low cost, wide availability, and rapidity. It does not require patient transport, and does not expose a patient to additional radiation. In addition, ONSD US has been utilized in low-resource settings, and may be particularly useful in prehospital and mass-casualty situations.6
The underlying relationship between ONSD and ICP is a result of the enclosure of the subarachnoid space by the ONS. Increased ICP leads to expansion of the ONS, particularly at 3 mm behind the globe, in the retrobulbar compartment (Figures 1 and 2).7
Unfortunately, it is not possible to precisely determine ICP from an ONSD measurement, because baseline ONSD values and elasticity vary significantly within the population.4,8 As a result, ONSD US has been investigated mostly for its ability to detect qualitative changes—particularly as a screen for elevated ICP. Optic nerve sheath diameter has high discriminative value in its ability to distinguish normal from elevated ICP. In a meta-analysis, Dubourg et al9 showed that the technique had an area under the summary receiver-operating curve of 0.94, signifying excellent test accuracy to diagnose elevated ICPs.
Researchers have attempted to determine a threshold value of ONSD that would serve as a clinically useful predictor of elevated ICP. Currently, this value ranges from 4.8 to 5.9 mm, depending on the study9; 5 mm is commonly used clinically as a threshold.10
Using ONSD US to Monitor Rapid Changes in ICP
While the use of the ONSD technique to screen for elevated ICP is relatively well established, the use of ONSD US to track acute changes in ICP is not as well studied. Serial tracking of acute changes could be useful in a patient at risk for intracranial hypertension secondary to trauma, to monitor the results of treating a patient with IIH, or after ventriculoperitoneal shunt placement.3
In Vivo Data
In 1993, Tamburrelli et al11 performed the first ONSD intrathecal infusion study, using A-scan sonography, and concluded that there was a “direct, biphasic, positive relation between diastolic intracranial pressure and optic nerve diameters” and that the data showed “rapid changes of optic nerve diameters in response to variation of intracranial pressure.”
In 1997, Hansen and Helmke12 recorded ONSD versus ICP data in the first intrathecal infusion test to use B-scan mode sonography. Ultrasonography was performed at 2- to 4-minute intervals. Their data demonstrated a linear relationship between ICP and ONSD over a particular cerebrospinal fluid pressure interval. They noted that “this interval differed between patients: ONS dilation commenced at pressure thresholds between 15 mm Hg and 30 mm Hg and in some patients saturation of the response (constant ONSD) occurred between 30 mm Hg and 40 mm Hg.”
The slope of ONSD versus ICP curve varied considerably by patient, making it impossible to infer an absolute ICP value from an ONSD without prior knowledge of the patient’s ratio. Similar to the data from Tamburrelli et al,11 Hansen and Helmke12 also found that there was no lag in ONSD response to ICP: “Within this interval, no temporal delay of the ONS response was noted.”
The only study comparing real-time ONSD data to gold-standard measurements of rapidly changing ICP in humans was performed by Maissan et al13 in 2015. This study involved a cohort of 18 patients who had suffered TBI and had intraparenchymal probes inserted. Because ICP rises transiently during endotracheal tube suctioning due to irritation of the trachea, the increase and subsequent decrease after suctioning was an ideal time to perform ONSD measurements and compare them to simultaneous gold-standard ICP measurements. The ONSD US measurements were performed 30 to 60 seconds prior to suctioning, during suctioning, and 30 to 60 seconds after suctioning.
Even during this very rapid time course, a strong correlation between ICP and ONSD measurements was demonstrated. The R2 value was 0.80. There was no perceptible “lag” in ONSD change; changes in ICP were immediately reflected in ONSD. Notably, an absolute change of less than 8 to 10 mm Hg in ICP did not affect ONSD, which is consistent with data collected by Hansen and Helmke.12
Therapeutic Lumbar Puncture for IIH
There are two case reports of ONSD US measurements being taken pre- and postlumbar puncture (LP) in patients with IIH. In the first, in 1989 Galetta et al14 used A-scan US to measure pre- and post-LP ONSD in a woman with papilledema secondary to IIH. They found a significant reduction in ONSD bilaterally “within minutes” of performing the LP.14
The second case report was published in 2015 by Singleton et al.15 They recorded ONSD measurements 30 minutes pre- and post-LP in a woman who presented to the ED with symptoms from elevated ICP. After reduction of pressure via LP, they recorded a significant reduction in ONSD bilaterally.15
Cadaver Data
Hansen et al16 evaluated the distensibility and elasticity of the ONS using postmortem optic nerve preparations. The ONSD was recorded 200 seconds after each pressure increase, which was long enough to achieve stable diameters. They found a linear correlation between pressure increases of 5 to 45 mm Hg and ONSD. This would suggest a potential positively correlated change in ONSD with in vivo changes in ICP. However, this still needs further clinical study to better assess measurable changes in living patients.
Conclusion
Published data have consistently demonstrated that changes in ICP are rapidly transmitted to the optic nerve sheath and that there does not appear to be a temporal lag in the ONSD. Based on in vivo data, the relationship between ICP and ONSD appears to be linear only over a range of moderately elevated ICP. According to Hansen and Helmke,12 this range starts at approximately 18 to 30 mm Hg, and ends at approximately 40 to 45 mm Hg. Maissan et al13 observed similar findings: “At low levels, ICP changes (8-10 mm Hg) do not affect the ONSD.”
There is still need for additional research to validate and refine these findings. Only one study has compared gold-standard ICP measurements with ONSD US measurements in real time,13 and the literature on ONSD US in tracking ICP after therapeutic LP in IIH consists of only two case reports.
Thus, with some caveats, ONSD US appears to permit qualitative tracking of ICP in real time. This supports its use in situations where a patient may have rapidly changing ICP, such as close monitoring of patients at risk for elevated ICP in a critical care setting, and response to treatment in patients with IIH.
1. Stocchetti N, Maas AI. Traumatic intracranial hypertension. N Engl J Med. 2014;370(22):2121-2130.
2. Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons; et al. Guidelines for the management of severe traumatic brain injury. VI. Indications for intracranial pressure monitoring. J Neurotrauma. 2007;24(Suppl 1):S37-S44.
3. Choi SH, Min KT, Park EK, Kim MS, Jung JH, Kim H. Ultrasonography of the optic nerve sheath to assess intracranial pressure changes after ventriculo-peritoneal shunt surgery in children with hydrocephalus: a prospective observational study. Anaesthesia. 2015;70(11):1268-1273.
4. Kristiansson H, Nissborg E, Bartek J Jr, Andresen M, Reinstrup P, Romner B. Measuring elevated intracranial pressure through noninvasive methods: a review of the literature. J Neurosurg Anesthesiol. 2013;25(4):372-385.
5. Rajajee V, Thyagarajan P, Rajagopalan RE. Optic nerve ultrasonography for detection of raised intracranial pressure when invasive monitoring is unavailable. Neurol India. 2010;58(5):812-813.
6. Robba C, Baciqaluppi S, Cardim D, Donnelly J, Bertuccio A, Czosnyka M. Non-invasive assessment of intracranial pressure. Acta Neurol Scand. 2016;134(1):4-21.
7. Hansen HC, Helmke K. The subarachnoid space surrounding the optic nerves. An ultrasound study of the optic nerve sheath. Surg Radiol Anat. 1996;18(4):323-328.
8. Hansen HC, Lagrèze W, Krueger O, Helmke K. Dependence of the optic nerve sheath diameter on acutely applied subarachnoidal pressure - an experimental ultrasound study. Acta Ophthalmol. 2011;89(6):e528-e532.
9. Dubourg J, Javouhey E, Geeraerts T, Messerer M, Kassai B. Ultrasonography of optic nerve sheath diameter for detection of raised intracranial pressure: a systematic review and meta-analysis. Intensive Care Med. 2011;37(7):1059-1068.
10. Kimberly HH, Shah S, Marill K, Noble V. Correlation of optic nerve sheath diameter with direct measurement of intracranial pressure. Acad Emerg Med. 2008;15(2):201-204.
11. Tamburrelli C, Anile C, Mangiola A, Falsini B, Palma P. CSF dynamic parameters and changes of optic nerve diameters measured by standardized echography. In: Till P, ed. Ophthalmic Echography 13: Proceedings of the 13th SIDUO Congress, Vienna, Austria, 1990; vol 55. Dordrecht, Netherlands: Springer Netherlands; 1993:101-109.
12. Hansen HC, Helmke K. Validation of the optic nerve sheath response to changing cerebrospinal fluid pressure: ultrasound findings during intrathecal infusion tests. J Neurosurg. 1997;87(1):34-40.
13. Maissan IM, Dirven PJ, Haitsma IK, Hoeks SE, Gommers D, Stolker RJ. Ultrasonographic measured optic nerve sheath diameter as an accurate and quick monitor for changes in intracranial pressure. J Neurosurg. 2015;123(3)743-747.
14. Galetta S, Byrne SF, Smith JL. Echographic correlation of optic nerve sheath size and cerebrospinal fluid pressure. J Clin Neuroophthalmol. 1989;9(2):79-82.
15. Singleton J, Dagan A, Edlow JA, Hoffmann B. Real-time optic nerve sheath diameter reduction measured with bedside ultrasound after therapeutic lumbar puncture in a patient with idiopathic intracranial hypertension. Am J Emerg Med. 2015;33(6):860.e5-e7.
16. Hansen HC, Lagrèze W, Krueger O, Helmke K. Dependence of the optic nerve sheath diameter on acutely applied subarachnoidal pressure—an experimental ultrasound study. Acta Ophthalmol. 2011;89(6):e528-e532.
1. Stocchetti N, Maas AI. Traumatic intracranial hypertension. N Engl J Med. 2014;370(22):2121-2130.
2. Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons; et al. Guidelines for the management of severe traumatic brain injury. VI. Indications for intracranial pressure monitoring. J Neurotrauma. 2007;24(Suppl 1):S37-S44.
3. Choi SH, Min KT, Park EK, Kim MS, Jung JH, Kim H. Ultrasonography of the optic nerve sheath to assess intracranial pressure changes after ventriculo-peritoneal shunt surgery in children with hydrocephalus: a prospective observational study. Anaesthesia. 2015;70(11):1268-1273.
4. Kristiansson H, Nissborg E, Bartek J Jr, Andresen M, Reinstrup P, Romner B. Measuring elevated intracranial pressure through noninvasive methods: a review of the literature. J Neurosurg Anesthesiol. 2013;25(4):372-385.
5. Rajajee V, Thyagarajan P, Rajagopalan RE. Optic nerve ultrasonography for detection of raised intracranial pressure when invasive monitoring is unavailable. Neurol India. 2010;58(5):812-813.
6. Robba C, Baciqaluppi S, Cardim D, Donnelly J, Bertuccio A, Czosnyka M. Non-invasive assessment of intracranial pressure. Acta Neurol Scand. 2016;134(1):4-21.
7. Hansen HC, Helmke K. The subarachnoid space surrounding the optic nerves. An ultrasound study of the optic nerve sheath. Surg Radiol Anat. 1996;18(4):323-328.
8. Hansen HC, Lagrèze W, Krueger O, Helmke K. Dependence of the optic nerve sheath diameter on acutely applied subarachnoidal pressure - an experimental ultrasound study. Acta Ophthalmol. 2011;89(6):e528-e532.
9. Dubourg J, Javouhey E, Geeraerts T, Messerer M, Kassai B. Ultrasonography of optic nerve sheath diameter for detection of raised intracranial pressure: a systematic review and meta-analysis. Intensive Care Med. 2011;37(7):1059-1068.
10. Kimberly HH, Shah S, Marill K, Noble V. Correlation of optic nerve sheath diameter with direct measurement of intracranial pressure. Acad Emerg Med. 2008;15(2):201-204.
11. Tamburrelli C, Anile C, Mangiola A, Falsini B, Palma P. CSF dynamic parameters and changes of optic nerve diameters measured by standardized echography. In: Till P, ed. Ophthalmic Echography 13: Proceedings of the 13th SIDUO Congress, Vienna, Austria, 1990; vol 55. Dordrecht, Netherlands: Springer Netherlands; 1993:101-109.
12. Hansen HC, Helmke K. Validation of the optic nerve sheath response to changing cerebrospinal fluid pressure: ultrasound findings during intrathecal infusion tests. J Neurosurg. 1997;87(1):34-40.
13. Maissan IM, Dirven PJ, Haitsma IK, Hoeks SE, Gommers D, Stolker RJ. Ultrasonographic measured optic nerve sheath diameter as an accurate and quick monitor for changes in intracranial pressure. J Neurosurg. 2015;123(3)743-747.
14. Galetta S, Byrne SF, Smith JL. Echographic correlation of optic nerve sheath size and cerebrospinal fluid pressure. J Clin Neuroophthalmol. 1989;9(2):79-82.
15. Singleton J, Dagan A, Edlow JA, Hoffmann B. Real-time optic nerve sheath diameter reduction measured with bedside ultrasound after therapeutic lumbar puncture in a patient with idiopathic intracranial hypertension. Am J Emerg Med. 2015;33(6):860.e5-e7.
16. Hansen HC, Lagrèze W, Krueger O, Helmke K. Dependence of the optic nerve sheath diameter on acutely applied subarachnoidal pressure—an experimental ultrasound study. Acta Ophthalmol. 2011;89(6):e528-e532.
Invasive Salmonellosis in a 45-Day-Old Infant
The management of a febrile infant is complex and requires obtaining a detailed history of all possible exposures. Published guidelines alone are not always completely accurate for diagnosing or excluding serious illness, and are not a substitute for a thorough examination and history.
Case
The parents of a 45-day-old girl were referred to our regional pediatric hospital by a local community hospital for emergent evaluation of their infant. The day prior, they had taken the infant to the referring ED because of persistent fussiness and subjective fever. They were neither sure of the tests that were obtained during that visit nor why they were instructed to take their daughter to our pediatric facility. They did, however, recall that during the visit to the community ED, the patient had a rectal temperature of 102.7°F, was given an antibiotic injection, and was well appearing and acting normally. Also, at discharge, the infant’s parents were instructed to follow up with the patient’s pediatrician within 24 hours.
Since their daughter’s discharge from the community ED, both parents noted that she seemed more irritable, felt warm, and had not been feeding well. They confirmed that she was an otherwise healthy infant who had been born full term via normal vaginal delivery and without complications.
On initial assessment at our ED, the patient was fussy and had mottled extremities and dusky nail beds. Her vital signs at presentation were: heart rate, 223 beats/minute; respiratory rate, 36 breaths/minute; and rectal temperature, 103.6°F. Oxygen saturation was 96% on room air. The infant was resuscitated with 20 mL/kg of intravenous (IV) normal saline and given oral acetaminophen. Laboratory studies were obtained, and a lumbar puncture (LP) was performed.
She was treated with IV acyclovir, ceftriaxone, and vancomycin. Her complete blood count (CBC) was notable for a white blood cell count (WBC) of 22.60 x 109/L; cerebrospinal fluid (CSF) analysis revealed a WBC of 4.52 x 109/L with 75% neutrophils, and serum glucose of 63 mg/dL.
Since the patient’s parents did not have any paperwork or information from the prior ED visit, our ED contacted the community ED by phone, and a representative provided the following information: the patient had appeared well but was febrile at presentation; laboratory evaluation was obtained, but no LP was performed; she was treated with intramuscular (IM) ceftriaxone and acetaminophen; and she was discharged home in the care of her parents. Regarding laboratory studies performed at the community ED, the only test result made available by phone was the preliminary blood culture report that revealed growth of gram-negative rods with speciation pending, which prompted the referral to our facility.
Based on the information provided by the community ED and our evaluation and work-up, the patient was admitted to the pediatric intensive care unit. Magnetic resonance imaging (MRI) of the brain was performed, which showed debris in the lateral ventricles consistent with ventriculitis—likely secondary to meningitis. The blood, urine, and cerebrospinal fluid (CSF) cultures collected during our evaluation produced no growth; however, blood cultures from the rural ED eventually grew Salmonella.
A further detailed history revealed that the infant and her parents had been living with a family friend who owned an iguana. According to reports, the iguana had free run of the home and often crawled around and across the infant while she was lying on a blanket on the floor. The patient’s parents were not aware of the diseases associated with reptile contact. Due to concerns over the social situation, the patient was kept in the hospital for the entire recommended 21-day course of antibiotic therapy, during which time the parents received assistance finding alternate living arrangements.
Discussion
Current Practice Guidelines for Managing Febrile Infants
Current guidelines from the American Academy of Pediatrics (AAP) and the American College of Emergency Physicians (ACEP) recommend a full sepsis work-up for all neonates (ie, ages 0 to 28 days) who present with a fever (defined as a rectal temperature ≥100.4°F).1,2 The probability of a serious bacterial infection (SBI) in patients in this age group who present with fever is approximately 12%.3 A full sepsis work-up generally includes a CBC, blood cultures, urinalysis with culture, CSF analysis with culture, and stool cultures if diarrhea is present.
Current guidelines for infants 29 to 90 days of age who present with fever differ between professional associations. The AAP and the American Academy of Family Physicians recommend the following for children in this age range: laboratory evaluation with CBC, blood cultures, CSF analysis, urinalysis, and culture. If laboratory evaluation reveals a WBC of less than 15 x 109/L with an absolute neutrophil count of less than 10 x 109/L, along with a normal CSF and urinalysis, the patient can be given IM ceftriaxone and follow-up arranged in 24 hours. This approach is recommended for patients who are otherwise healthy, nontoxic at presentation, and under the care of a responsible adult.4,5 By comparison, the Philadelphia protocol, though suggesting an identical work-up, recommends against the use of antibiotics in infants deemed at low risk for SBI.6
The ACEP does not specifically endorse a management strategy for febrile infants in the 29- to 90-day age group, but instead acknowledges that no age cut-off within this group can be considered absolute when determining management strategy, and suggests that children up to 60 days old should be managed in a manner similar to neonates.2 The published guidelines do not include consideration of specific history exposure in the management recommendations.
Typhoidal Serotypes
Salmonella can be divided into typhoidal (including S typhi and S paratyphi) and nontyphoidal serotypes (NTS), with the two groups manifesting as very different diseases.7 Typhoidal serotypes lead to the disease process known as typhoid, which typically presents with fever, chills, abdominal pain, nonbloody diarrhea or constipation, nausea, anorexia, headache, hepatosplenomegaly, and rose spots.8 These symptoms typically present after a 14-day incubation period and persist for 21 days.9 Humans are the only known infected source of these species, which are spread via the fecal-oral route.10
In contrast, disease from NTS manifests within 12 hours of exposure with watery diarrhea, nausea, vomiting, and fever, with symptoms lasting up to 10 days.11 Both groups cause disease by invading the intestinal epithelium12; however, typhoidal species induce less intestinal inflammation, facilitating bacterial invasion and making systemic disease more likely.13
Transmission
Many animals are known to carry NTS, including reptiles, where Salmonella occurs naturally in their gastrointestinal tract.14 Twenty-five percent of Salmonella infections in children younger than age 5 years have been attributed to contact with a pet,15 with small turtles (shell diameter <4 inches) accounting for 42% of all pet-related Salmonella infections.
Though gastroenteritis is the most common clinical manifestation of infection with NTS, approximately 5% of patients will develop invasive disease, including bacteremia, meningitis, septic arthritis, or osteomyelitis.16 Children with invasive disease are more likely to have been exposed to an iguana, snake, or bearded dragon than to a turtle. If the pet is kept indoors, the risk of invasive disease is more likely. The average age of patients with invasive disease is 62 days, versus 2 years for noninvasive disease.17
Diagnosis
Growth of Salmonella on cultures of stool, blood, urine, or CSF dishes is the mainstay of diagnosis of typhoid and nontyphoidal disease, but bacterial concentrations are higher in bone marrow aspirate, making it superior to blood cultures.18 Biopsy of the rose spots of typhoid may also provide the diagnosis.
Management
Since Salmonella gastroenteritis is usually a self-limited disease, current recommendations reserve treatment with antibiotics for patients with severe disease or who are immunocompromised. When necessary, treatment consists of 7 to 10 days of a fluoroquinolone or third-generation cephalosporin, which is the same regimen suggested for typhoid. Treatment of central nervous system (CNS) salmonellosis consists of at least 3 weeks of a third-generation cephalosporin; the AAP recommends at least 4 weeks of treatment.19
Case Conclusion
Prior to the patient’s transfer to our facility, she was treated empirically with ceftriaxone without prior CSF analysis—an approach that does not follow any current guidelines for the treatment of a febrile infant. Though an LP was not performed until approximately 24 hours after the initial antibiotic was given, the patient demonstrated CSF pleocytosis with no organisms on gram stain and no growth on culture. Given this pleocytosis and Salmonella bacteremia in the context of prior antibiotic treatment, and MRI consistent with CNS involvement, the patient was treated for 21 days for presumed Salmonella meningitis. A CSF analysis performed on her initial visit could have more accurately directed the type and duration of treatment if the findings on subsequent imaging studies and CSF analysis were ambiguous.
Summary
Emergency physicians may underestimate the likelihood of SBI in otherwise well-appearing febrile infants. While certain aspects of the history and physical examination in a febrile, well-appearing infant have been shown to correlate with an increased risk of SBI, no single finding can definitively rule in or rule out the disease.20 Opinions differ as to optimal management strategies for febrile, well-appearing infants outside the neonatal period. However, an appropriate level of clinical suspicion, within the context of a thorough investigation into the infant’s health history and social situation, can aid the clinician and guide treatment and disposition.
1. Baraff LJ, Bass JW, Fleisher GR, et al. Practice guideline for the management of infants and children 0 to 36 months of age with fever without source. Agency for Health Care Policy and Research. Ann Emerg Med. 1993;22(7):1198-1210. Erratum in Ann Emerg Med. 1993;22(9):1490.
2. American College of Emergency Physicians Clinical Policies Committee; American College of Emergency Physicians Clinical Policies Subcommittee on Pediatric Fever. Clinical policy for children younger than three years presenting to the emergency department with fever. Ann Emerg Med. 2003;42(4):530-545.
3. Kadish HA, Loveridge B, Tobey J, Bolte RG, Corneli HM. Applying outpatient protocols in febrile infants 1-28 days of age: can the threshold be lowered? Clin Pediatr (Phila). 2000;39(2):81-88.
4. Sur DK, Bukont EL. Evaluating fever of unidentifiable source in young children. Am Fam Physician. 2007;75(12):1805-1811.
5. Baraff LJ, Lee SI, Schriger DL. Outcomes of bacterial meningitis in children: a meta-analysis. Pediatr Infect Dis J. 1993;12(5):389-394.
6. Baker MD, Bell LM, Avner JR. Outpatient management without antibiotics of fever in selected infants. N Eng J Med. 1993;329(20):1437-1441.
7. Gal-Mor O, Boyle EC, Grassl GA. Same species, different diseases: how and why typhoidal and non-typhoidal Salmonella enterica serovars differ. Front in Microbiol. 2014;5:391.
8. Stuart BM, Pullen RL. Typhoid: clinical analysis of 360 cases. Arch Intern Med (Chic). 1946;78(6):
629-661.
9. Olsen SJ, Bleasdale SC, Magnano AR, et al. Outbreaks of typhoid fever in the United States, 1960-99. Epidemiol Infect. 2003;130(1):13-21.
10. Newton AE, Routh JA, Mahon BE. Typhoid and Paratyphoid Fever. In: Brunette GW, Kozarsky PE, Cohen NJ, et al, eds. CDC Health Information for International Travel. New York, NY: Oxford University Press; 2016. http://wwwnc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/typhoid-paratyphoid-fever. Updated July 10, 2015. Accessed June 13, 2016.
11. McGovern VJ, Slavutin LJ. Pathology of Salmonella colitis. Am J Surg Pathol. 1979;3(6):483-490.
12. Liu SL, Ezaki T, Miura H, Matsui K, Yabuuchi E. Intact motility as a Salmonella typhi invasion-related factor. Infect Immun. 1988;56(8):1967-1973.
13. House D, Wain J, Ho VA, et al. Serology of typhoid fever in an area of endemicity and its relevance to diagnosis. J Clin Microbiol. 2001;39(3):1002-1007.
14. Hoelzer K, Moreno Switt AI, Wiedmann M. Animal contact as a source of human non-typhoidal salmonellosis. Vet Res. 2011;42(1):34.
15. Murphy D, Oshin F. Reptile-associated salmonellosis in children aged under 5 years in South West England. Arch Dis Child. 2015;100(4):364-365.
16. Iwamoto M. Infectious diseases related to travel. In: Brunette GW, Kozarsky PE, Cohen NJ, et al, eds. CDC Health Information for International Travel. New York, NY: Oxford University Press; 2016. http://wwwnc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/salmonellosis-nontyphoidal. Updated July 10, 2015. Accessed June 13, 2016.
17. Meyer Sauteur PM, Relly C, Hug M, Wittenbrink MM, Berger C. Risk factors for invasive reptile-associated salmonellosis in children. Vector-Borne and Zoonotic Dis. 2013;13(6):419-421.
18. Gasem MH, Keuter M, Dolmans WM, Van Der Ven-Jongekrijg J, Djokomoeljanto R, Van Der Meer JW. Persistence of Salmonellae in blood and bone marrow: randomized controlled trial comparing ciprofloxacin and chloramphenicol treatments against enteric fever. Antimicrob Agents Chemother. 2003;47(5):1727-1731.
19. Price EH, de Louvois J, Workman MR. Antibiotics for Salmonella meningitis in children. J Antimicrob Chemother. 2000;46(5) 653-655.
20. Craig JC, Williams GJ, Jones M, et al. The accuracy of clinical symptoms and signs for the diagnosis of serious bacterial infection in young febrile children: prospective cohort study of 15 781 febrile illnesses. BMJ. 2010;340:c1594.
The management of a febrile infant is complex and requires obtaining a detailed history of all possible exposures. Published guidelines alone are not always completely accurate for diagnosing or excluding serious illness, and are not a substitute for a thorough examination and history.
Case
The parents of a 45-day-old girl were referred to our regional pediatric hospital by a local community hospital for emergent evaluation of their infant. The day prior, they had taken the infant to the referring ED because of persistent fussiness and subjective fever. They were neither sure of the tests that were obtained during that visit nor why they were instructed to take their daughter to our pediatric facility. They did, however, recall that during the visit to the community ED, the patient had a rectal temperature of 102.7°F, was given an antibiotic injection, and was well appearing and acting normally. Also, at discharge, the infant’s parents were instructed to follow up with the patient’s pediatrician within 24 hours.
Since their daughter’s discharge from the community ED, both parents noted that she seemed more irritable, felt warm, and had not been feeding well. They confirmed that she was an otherwise healthy infant who had been born full term via normal vaginal delivery and without complications.
On initial assessment at our ED, the patient was fussy and had mottled extremities and dusky nail beds. Her vital signs at presentation were: heart rate, 223 beats/minute; respiratory rate, 36 breaths/minute; and rectal temperature, 103.6°F. Oxygen saturation was 96% on room air. The infant was resuscitated with 20 mL/kg of intravenous (IV) normal saline and given oral acetaminophen. Laboratory studies were obtained, and a lumbar puncture (LP) was performed.
She was treated with IV acyclovir, ceftriaxone, and vancomycin. Her complete blood count (CBC) was notable for a white blood cell count (WBC) of 22.60 x 109/L; cerebrospinal fluid (CSF) analysis revealed a WBC of 4.52 x 109/L with 75% neutrophils, and serum glucose of 63 mg/dL.
Since the patient’s parents did not have any paperwork or information from the prior ED visit, our ED contacted the community ED by phone, and a representative provided the following information: the patient had appeared well but was febrile at presentation; laboratory evaluation was obtained, but no LP was performed; she was treated with intramuscular (IM) ceftriaxone and acetaminophen; and she was discharged home in the care of her parents. Regarding laboratory studies performed at the community ED, the only test result made available by phone was the preliminary blood culture report that revealed growth of gram-negative rods with speciation pending, which prompted the referral to our facility.
Based on the information provided by the community ED and our evaluation and work-up, the patient was admitted to the pediatric intensive care unit. Magnetic resonance imaging (MRI) of the brain was performed, which showed debris in the lateral ventricles consistent with ventriculitis—likely secondary to meningitis. The blood, urine, and cerebrospinal fluid (CSF) cultures collected during our evaluation produced no growth; however, blood cultures from the rural ED eventually grew Salmonella.
A further detailed history revealed that the infant and her parents had been living with a family friend who owned an iguana. According to reports, the iguana had free run of the home and often crawled around and across the infant while she was lying on a blanket on the floor. The patient’s parents were not aware of the diseases associated with reptile contact. Due to concerns over the social situation, the patient was kept in the hospital for the entire recommended 21-day course of antibiotic therapy, during which time the parents received assistance finding alternate living arrangements.
Discussion
Current Practice Guidelines for Managing Febrile Infants
Current guidelines from the American Academy of Pediatrics (AAP) and the American College of Emergency Physicians (ACEP) recommend a full sepsis work-up for all neonates (ie, ages 0 to 28 days) who present with a fever (defined as a rectal temperature ≥100.4°F).1,2 The probability of a serious bacterial infection (SBI) in patients in this age group who present with fever is approximately 12%.3 A full sepsis work-up generally includes a CBC, blood cultures, urinalysis with culture, CSF analysis with culture, and stool cultures if diarrhea is present.
Current guidelines for infants 29 to 90 days of age who present with fever differ between professional associations. The AAP and the American Academy of Family Physicians recommend the following for children in this age range: laboratory evaluation with CBC, blood cultures, CSF analysis, urinalysis, and culture. If laboratory evaluation reveals a WBC of less than 15 x 109/L with an absolute neutrophil count of less than 10 x 109/L, along with a normal CSF and urinalysis, the patient can be given IM ceftriaxone and follow-up arranged in 24 hours. This approach is recommended for patients who are otherwise healthy, nontoxic at presentation, and under the care of a responsible adult.4,5 By comparison, the Philadelphia protocol, though suggesting an identical work-up, recommends against the use of antibiotics in infants deemed at low risk for SBI.6
The ACEP does not specifically endorse a management strategy for febrile infants in the 29- to 90-day age group, but instead acknowledges that no age cut-off within this group can be considered absolute when determining management strategy, and suggests that children up to 60 days old should be managed in a manner similar to neonates.2 The published guidelines do not include consideration of specific history exposure in the management recommendations.
Typhoidal Serotypes
Salmonella can be divided into typhoidal (including S typhi and S paratyphi) and nontyphoidal serotypes (NTS), with the two groups manifesting as very different diseases.7 Typhoidal serotypes lead to the disease process known as typhoid, which typically presents with fever, chills, abdominal pain, nonbloody diarrhea or constipation, nausea, anorexia, headache, hepatosplenomegaly, and rose spots.8 These symptoms typically present after a 14-day incubation period and persist for 21 days.9 Humans are the only known infected source of these species, which are spread via the fecal-oral route.10
In contrast, disease from NTS manifests within 12 hours of exposure with watery diarrhea, nausea, vomiting, and fever, with symptoms lasting up to 10 days.11 Both groups cause disease by invading the intestinal epithelium12; however, typhoidal species induce less intestinal inflammation, facilitating bacterial invasion and making systemic disease more likely.13
Transmission
Many animals are known to carry NTS, including reptiles, where Salmonella occurs naturally in their gastrointestinal tract.14 Twenty-five percent of Salmonella infections in children younger than age 5 years have been attributed to contact with a pet,15 with small turtles (shell diameter <4 inches) accounting for 42% of all pet-related Salmonella infections.
Though gastroenteritis is the most common clinical manifestation of infection with NTS, approximately 5% of patients will develop invasive disease, including bacteremia, meningitis, septic arthritis, or osteomyelitis.16 Children with invasive disease are more likely to have been exposed to an iguana, snake, or bearded dragon than to a turtle. If the pet is kept indoors, the risk of invasive disease is more likely. The average age of patients with invasive disease is 62 days, versus 2 years for noninvasive disease.17
Diagnosis
Growth of Salmonella on cultures of stool, blood, urine, or CSF dishes is the mainstay of diagnosis of typhoid and nontyphoidal disease, but bacterial concentrations are higher in bone marrow aspirate, making it superior to blood cultures.18 Biopsy of the rose spots of typhoid may also provide the diagnosis.
Management
Since Salmonella gastroenteritis is usually a self-limited disease, current recommendations reserve treatment with antibiotics for patients with severe disease or who are immunocompromised. When necessary, treatment consists of 7 to 10 days of a fluoroquinolone or third-generation cephalosporin, which is the same regimen suggested for typhoid. Treatment of central nervous system (CNS) salmonellosis consists of at least 3 weeks of a third-generation cephalosporin; the AAP recommends at least 4 weeks of treatment.19
Case Conclusion
Prior to the patient’s transfer to our facility, she was treated empirically with ceftriaxone without prior CSF analysis—an approach that does not follow any current guidelines for the treatment of a febrile infant. Though an LP was not performed until approximately 24 hours after the initial antibiotic was given, the patient demonstrated CSF pleocytosis with no organisms on gram stain and no growth on culture. Given this pleocytosis and Salmonella bacteremia in the context of prior antibiotic treatment, and MRI consistent with CNS involvement, the patient was treated for 21 days for presumed Salmonella meningitis. A CSF analysis performed on her initial visit could have more accurately directed the type and duration of treatment if the findings on subsequent imaging studies and CSF analysis were ambiguous.
Summary
Emergency physicians may underestimate the likelihood of SBI in otherwise well-appearing febrile infants. While certain aspects of the history and physical examination in a febrile, well-appearing infant have been shown to correlate with an increased risk of SBI, no single finding can definitively rule in or rule out the disease.20 Opinions differ as to optimal management strategies for febrile, well-appearing infants outside the neonatal period. However, an appropriate level of clinical suspicion, within the context of a thorough investigation into the infant’s health history and social situation, can aid the clinician and guide treatment and disposition.
The management of a febrile infant is complex and requires obtaining a detailed history of all possible exposures. Published guidelines alone are not always completely accurate for diagnosing or excluding serious illness, and are not a substitute for a thorough examination and history.
Case
The parents of a 45-day-old girl were referred to our regional pediatric hospital by a local community hospital for emergent evaluation of their infant. The day prior, they had taken the infant to the referring ED because of persistent fussiness and subjective fever. They were neither sure of the tests that were obtained during that visit nor why they were instructed to take their daughter to our pediatric facility. They did, however, recall that during the visit to the community ED, the patient had a rectal temperature of 102.7°F, was given an antibiotic injection, and was well appearing and acting normally. Also, at discharge, the infant’s parents were instructed to follow up with the patient’s pediatrician within 24 hours.
Since their daughter’s discharge from the community ED, both parents noted that she seemed more irritable, felt warm, and had not been feeding well. They confirmed that she was an otherwise healthy infant who had been born full term via normal vaginal delivery and without complications.
On initial assessment at our ED, the patient was fussy and had mottled extremities and dusky nail beds. Her vital signs at presentation were: heart rate, 223 beats/minute; respiratory rate, 36 breaths/minute; and rectal temperature, 103.6°F. Oxygen saturation was 96% on room air. The infant was resuscitated with 20 mL/kg of intravenous (IV) normal saline and given oral acetaminophen. Laboratory studies were obtained, and a lumbar puncture (LP) was performed.
She was treated with IV acyclovir, ceftriaxone, and vancomycin. Her complete blood count (CBC) was notable for a white blood cell count (WBC) of 22.60 x 109/L; cerebrospinal fluid (CSF) analysis revealed a WBC of 4.52 x 109/L with 75% neutrophils, and serum glucose of 63 mg/dL.
Since the patient’s parents did not have any paperwork or information from the prior ED visit, our ED contacted the community ED by phone, and a representative provided the following information: the patient had appeared well but was febrile at presentation; laboratory evaluation was obtained, but no LP was performed; she was treated with intramuscular (IM) ceftriaxone and acetaminophen; and she was discharged home in the care of her parents. Regarding laboratory studies performed at the community ED, the only test result made available by phone was the preliminary blood culture report that revealed growth of gram-negative rods with speciation pending, which prompted the referral to our facility.
Based on the information provided by the community ED and our evaluation and work-up, the patient was admitted to the pediatric intensive care unit. Magnetic resonance imaging (MRI) of the brain was performed, which showed debris in the lateral ventricles consistent with ventriculitis—likely secondary to meningitis. The blood, urine, and cerebrospinal fluid (CSF) cultures collected during our evaluation produced no growth; however, blood cultures from the rural ED eventually grew Salmonella.
A further detailed history revealed that the infant and her parents had been living with a family friend who owned an iguana. According to reports, the iguana had free run of the home and often crawled around and across the infant while she was lying on a blanket on the floor. The patient’s parents were not aware of the diseases associated with reptile contact. Due to concerns over the social situation, the patient was kept in the hospital for the entire recommended 21-day course of antibiotic therapy, during which time the parents received assistance finding alternate living arrangements.
Discussion
Current Practice Guidelines for Managing Febrile Infants
Current guidelines from the American Academy of Pediatrics (AAP) and the American College of Emergency Physicians (ACEP) recommend a full sepsis work-up for all neonates (ie, ages 0 to 28 days) who present with a fever (defined as a rectal temperature ≥100.4°F).1,2 The probability of a serious bacterial infection (SBI) in patients in this age group who present with fever is approximately 12%.3 A full sepsis work-up generally includes a CBC, blood cultures, urinalysis with culture, CSF analysis with culture, and stool cultures if diarrhea is present.
Current guidelines for infants 29 to 90 days of age who present with fever differ between professional associations. The AAP and the American Academy of Family Physicians recommend the following for children in this age range: laboratory evaluation with CBC, blood cultures, CSF analysis, urinalysis, and culture. If laboratory evaluation reveals a WBC of less than 15 x 109/L with an absolute neutrophil count of less than 10 x 109/L, along with a normal CSF and urinalysis, the patient can be given IM ceftriaxone and follow-up arranged in 24 hours. This approach is recommended for patients who are otherwise healthy, nontoxic at presentation, and under the care of a responsible adult.4,5 By comparison, the Philadelphia protocol, though suggesting an identical work-up, recommends against the use of antibiotics in infants deemed at low risk for SBI.6
The ACEP does not specifically endorse a management strategy for febrile infants in the 29- to 90-day age group, but instead acknowledges that no age cut-off within this group can be considered absolute when determining management strategy, and suggests that children up to 60 days old should be managed in a manner similar to neonates.2 The published guidelines do not include consideration of specific history exposure in the management recommendations.
Typhoidal Serotypes
Salmonella can be divided into typhoidal (including S typhi and S paratyphi) and nontyphoidal serotypes (NTS), with the two groups manifesting as very different diseases.7 Typhoidal serotypes lead to the disease process known as typhoid, which typically presents with fever, chills, abdominal pain, nonbloody diarrhea or constipation, nausea, anorexia, headache, hepatosplenomegaly, and rose spots.8 These symptoms typically present after a 14-day incubation period and persist for 21 days.9 Humans are the only known infected source of these species, which are spread via the fecal-oral route.10
In contrast, disease from NTS manifests within 12 hours of exposure with watery diarrhea, nausea, vomiting, and fever, with symptoms lasting up to 10 days.11 Both groups cause disease by invading the intestinal epithelium12; however, typhoidal species induce less intestinal inflammation, facilitating bacterial invasion and making systemic disease more likely.13
Transmission
Many animals are known to carry NTS, including reptiles, where Salmonella occurs naturally in their gastrointestinal tract.14 Twenty-five percent of Salmonella infections in children younger than age 5 years have been attributed to contact with a pet,15 with small turtles (shell diameter <4 inches) accounting for 42% of all pet-related Salmonella infections.
Though gastroenteritis is the most common clinical manifestation of infection with NTS, approximately 5% of patients will develop invasive disease, including bacteremia, meningitis, septic arthritis, or osteomyelitis.16 Children with invasive disease are more likely to have been exposed to an iguana, snake, or bearded dragon than to a turtle. If the pet is kept indoors, the risk of invasive disease is more likely. The average age of patients with invasive disease is 62 days, versus 2 years for noninvasive disease.17
Diagnosis
Growth of Salmonella on cultures of stool, blood, urine, or CSF dishes is the mainstay of diagnosis of typhoid and nontyphoidal disease, but bacterial concentrations are higher in bone marrow aspirate, making it superior to blood cultures.18 Biopsy of the rose spots of typhoid may also provide the diagnosis.
Management
Since Salmonella gastroenteritis is usually a self-limited disease, current recommendations reserve treatment with antibiotics for patients with severe disease or who are immunocompromised. When necessary, treatment consists of 7 to 10 days of a fluoroquinolone or third-generation cephalosporin, which is the same regimen suggested for typhoid. Treatment of central nervous system (CNS) salmonellosis consists of at least 3 weeks of a third-generation cephalosporin; the AAP recommends at least 4 weeks of treatment.19
Case Conclusion
Prior to the patient’s transfer to our facility, she was treated empirically with ceftriaxone without prior CSF analysis—an approach that does not follow any current guidelines for the treatment of a febrile infant. Though an LP was not performed until approximately 24 hours after the initial antibiotic was given, the patient demonstrated CSF pleocytosis with no organisms on gram stain and no growth on culture. Given this pleocytosis and Salmonella bacteremia in the context of prior antibiotic treatment, and MRI consistent with CNS involvement, the patient was treated for 21 days for presumed Salmonella meningitis. A CSF analysis performed on her initial visit could have more accurately directed the type and duration of treatment if the findings on subsequent imaging studies and CSF analysis were ambiguous.
Summary
Emergency physicians may underestimate the likelihood of SBI in otherwise well-appearing febrile infants. While certain aspects of the history and physical examination in a febrile, well-appearing infant have been shown to correlate with an increased risk of SBI, no single finding can definitively rule in or rule out the disease.20 Opinions differ as to optimal management strategies for febrile, well-appearing infants outside the neonatal period. However, an appropriate level of clinical suspicion, within the context of a thorough investigation into the infant’s health history and social situation, can aid the clinician and guide treatment and disposition.
1. Baraff LJ, Bass JW, Fleisher GR, et al. Practice guideline for the management of infants and children 0 to 36 months of age with fever without source. Agency for Health Care Policy and Research. Ann Emerg Med. 1993;22(7):1198-1210. Erratum in Ann Emerg Med. 1993;22(9):1490.
2. American College of Emergency Physicians Clinical Policies Committee; American College of Emergency Physicians Clinical Policies Subcommittee on Pediatric Fever. Clinical policy for children younger than three years presenting to the emergency department with fever. Ann Emerg Med. 2003;42(4):530-545.
3. Kadish HA, Loveridge B, Tobey J, Bolte RG, Corneli HM. Applying outpatient protocols in febrile infants 1-28 days of age: can the threshold be lowered? Clin Pediatr (Phila). 2000;39(2):81-88.
4. Sur DK, Bukont EL. Evaluating fever of unidentifiable source in young children. Am Fam Physician. 2007;75(12):1805-1811.
5. Baraff LJ, Lee SI, Schriger DL. Outcomes of bacterial meningitis in children: a meta-analysis. Pediatr Infect Dis J. 1993;12(5):389-394.
6. Baker MD, Bell LM, Avner JR. Outpatient management without antibiotics of fever in selected infants. N Eng J Med. 1993;329(20):1437-1441.
7. Gal-Mor O, Boyle EC, Grassl GA. Same species, different diseases: how and why typhoidal and non-typhoidal Salmonella enterica serovars differ. Front in Microbiol. 2014;5:391.
8. Stuart BM, Pullen RL. Typhoid: clinical analysis of 360 cases. Arch Intern Med (Chic). 1946;78(6):
629-661.
9. Olsen SJ, Bleasdale SC, Magnano AR, et al. Outbreaks of typhoid fever in the United States, 1960-99. Epidemiol Infect. 2003;130(1):13-21.
10. Newton AE, Routh JA, Mahon BE. Typhoid and Paratyphoid Fever. In: Brunette GW, Kozarsky PE, Cohen NJ, et al, eds. CDC Health Information for International Travel. New York, NY: Oxford University Press; 2016. http://wwwnc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/typhoid-paratyphoid-fever. Updated July 10, 2015. Accessed June 13, 2016.
11. McGovern VJ, Slavutin LJ. Pathology of Salmonella colitis. Am J Surg Pathol. 1979;3(6):483-490.
12. Liu SL, Ezaki T, Miura H, Matsui K, Yabuuchi E. Intact motility as a Salmonella typhi invasion-related factor. Infect Immun. 1988;56(8):1967-1973.
13. House D, Wain J, Ho VA, et al. Serology of typhoid fever in an area of endemicity and its relevance to diagnosis. J Clin Microbiol. 2001;39(3):1002-1007.
14. Hoelzer K, Moreno Switt AI, Wiedmann M. Animal contact as a source of human non-typhoidal salmonellosis. Vet Res. 2011;42(1):34.
15. Murphy D, Oshin F. Reptile-associated salmonellosis in children aged under 5 years in South West England. Arch Dis Child. 2015;100(4):364-365.
16. Iwamoto M. Infectious diseases related to travel. In: Brunette GW, Kozarsky PE, Cohen NJ, et al, eds. CDC Health Information for International Travel. New York, NY: Oxford University Press; 2016. http://wwwnc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/salmonellosis-nontyphoidal. Updated July 10, 2015. Accessed June 13, 2016.
17. Meyer Sauteur PM, Relly C, Hug M, Wittenbrink MM, Berger C. Risk factors for invasive reptile-associated salmonellosis in children. Vector-Borne and Zoonotic Dis. 2013;13(6):419-421.
18. Gasem MH, Keuter M, Dolmans WM, Van Der Ven-Jongekrijg J, Djokomoeljanto R, Van Der Meer JW. Persistence of Salmonellae in blood and bone marrow: randomized controlled trial comparing ciprofloxacin and chloramphenicol treatments against enteric fever. Antimicrob Agents Chemother. 2003;47(5):1727-1731.
19. Price EH, de Louvois J, Workman MR. Antibiotics for Salmonella meningitis in children. J Antimicrob Chemother. 2000;46(5) 653-655.
20. Craig JC, Williams GJ, Jones M, et al. The accuracy of clinical symptoms and signs for the diagnosis of serious bacterial infection in young febrile children: prospective cohort study of 15 781 febrile illnesses. BMJ. 2010;340:c1594.
1. Baraff LJ, Bass JW, Fleisher GR, et al. Practice guideline for the management of infants and children 0 to 36 months of age with fever without source. Agency for Health Care Policy and Research. Ann Emerg Med. 1993;22(7):1198-1210. Erratum in Ann Emerg Med. 1993;22(9):1490.
2. American College of Emergency Physicians Clinical Policies Committee; American College of Emergency Physicians Clinical Policies Subcommittee on Pediatric Fever. Clinical policy for children younger than three years presenting to the emergency department with fever. Ann Emerg Med. 2003;42(4):530-545.
3. Kadish HA, Loveridge B, Tobey J, Bolte RG, Corneli HM. Applying outpatient protocols in febrile infants 1-28 days of age: can the threshold be lowered? Clin Pediatr (Phila). 2000;39(2):81-88.
4. Sur DK, Bukont EL. Evaluating fever of unidentifiable source in young children. Am Fam Physician. 2007;75(12):1805-1811.
5. Baraff LJ, Lee SI, Schriger DL. Outcomes of bacterial meningitis in children: a meta-analysis. Pediatr Infect Dis J. 1993;12(5):389-394.
6. Baker MD, Bell LM, Avner JR. Outpatient management without antibiotics of fever in selected infants. N Eng J Med. 1993;329(20):1437-1441.
7. Gal-Mor O, Boyle EC, Grassl GA. Same species, different diseases: how and why typhoidal and non-typhoidal Salmonella enterica serovars differ. Front in Microbiol. 2014;5:391.
8. Stuart BM, Pullen RL. Typhoid: clinical analysis of 360 cases. Arch Intern Med (Chic). 1946;78(6):
629-661.
9. Olsen SJ, Bleasdale SC, Magnano AR, et al. Outbreaks of typhoid fever in the United States, 1960-99. Epidemiol Infect. 2003;130(1):13-21.
10. Newton AE, Routh JA, Mahon BE. Typhoid and Paratyphoid Fever. In: Brunette GW, Kozarsky PE, Cohen NJ, et al, eds. CDC Health Information for International Travel. New York, NY: Oxford University Press; 2016. http://wwwnc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/typhoid-paratyphoid-fever. Updated July 10, 2015. Accessed June 13, 2016.
11. McGovern VJ, Slavutin LJ. Pathology of Salmonella colitis. Am J Surg Pathol. 1979;3(6):483-490.
12. Liu SL, Ezaki T, Miura H, Matsui K, Yabuuchi E. Intact motility as a Salmonella typhi invasion-related factor. Infect Immun. 1988;56(8):1967-1973.
13. House D, Wain J, Ho VA, et al. Serology of typhoid fever in an area of endemicity and its relevance to diagnosis. J Clin Microbiol. 2001;39(3):1002-1007.
14. Hoelzer K, Moreno Switt AI, Wiedmann M. Animal contact as a source of human non-typhoidal salmonellosis. Vet Res. 2011;42(1):34.
15. Murphy D, Oshin F. Reptile-associated salmonellosis in children aged under 5 years in South West England. Arch Dis Child. 2015;100(4):364-365.
16. Iwamoto M. Infectious diseases related to travel. In: Brunette GW, Kozarsky PE, Cohen NJ, et al, eds. CDC Health Information for International Travel. New York, NY: Oxford University Press; 2016. http://wwwnc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/salmonellosis-nontyphoidal. Updated July 10, 2015. Accessed June 13, 2016.
17. Meyer Sauteur PM, Relly C, Hug M, Wittenbrink MM, Berger C. Risk factors for invasive reptile-associated salmonellosis in children. Vector-Borne and Zoonotic Dis. 2013;13(6):419-421.
18. Gasem MH, Keuter M, Dolmans WM, Van Der Ven-Jongekrijg J, Djokomoeljanto R, Van Der Meer JW. Persistence of Salmonellae in blood and bone marrow: randomized controlled trial comparing ciprofloxacin and chloramphenicol treatments against enteric fever. Antimicrob Agents Chemother. 2003;47(5):1727-1731.
19. Price EH, de Louvois J, Workman MR. Antibiotics for Salmonella meningitis in children. J Antimicrob Chemother. 2000;46(5) 653-655.
20. Craig JC, Williams GJ, Jones M, et al. The accuracy of clinical symptoms and signs for the diagnosis of serious bacterial infection in young febrile children: prospective cohort study of 15 781 febrile illnesses. BMJ. 2010;340:c1594.
Spontaneous Retrobulbar Hemorrhage
Most emergency physicians (EPs) encounter several patients a year with hemorrhages due to factor Xa (FXa) inhibitors. Such bleeding may occur in patterns not previously recognized with traditional anticoagulant therapy. Retrobulbar hemorrhage is typically associated with significant facial or orbital trauma, and spontaneous hemorrhage is a very rare cause of orbital compartment syndrome.1 Retrobulbar hemorrhage can lead to orbital compartment syndrome due to increased orbital pressure within a closed space. Because orbital compartment syndrome can compromise blood flow to the optic nerve or central retinal artery, it is extremely important to decrease orbital pressure as quickly as possible in affected patients. Therefore, canthotomy/cantholysis should be performed sooner rather than later, as 90 minutes of elevated intraocular pressure (IOP) can lead to permanent vision loss.2
Rivaroxaban, one of the relatively new oral anticoagulant agents that inhibit FXa, is used as an alternative therapy to vitamin K antagonists. The FXa agents have been approved to reduce the risk of stroke in patients with nonvalvular atrial fibrillation (AF).3 According to a meta-analysis of rivaroxaban and bleeding risk, rivaroxaban was shown to have no increased risk of major or clinically relevant nonmajor bleeding compared to vitamin K antagonists. Rivaroxaban was also associated with a significant decrease in fatal bleeding (relative risk, 0.48, 95%; confidence interval, 0.31 to 0.74).4
Case Report
A 79-year-old man with a medical history of hypertension, transient ischemic attacks (TIAs), and AF, for which he was taking rivaroxaban, was referred to our ED by a local rural ED for further evaluation and treatment of a retrobulbar hemorrhage. (The patient’s family refused emergency medical services transport from the rural ED.) The patient stated that upon awakening earlier that morning, he felt “pressure” in his right eye and experienced periorbital swelling that continued to worsen throughout the day. He denied any trauma, falls, or strikes to the face or head. The patient’s account and history were confirmed by the family members with whom he resided.
During the patient’s evaluation at the rural ED, a computed tomography (CT) scan of the head was performed, which demonstrated a retrobulbar hematoma on the right side (See the Figure for an example of a CT scan illustrating a retrobulbar hematoma with proptosis). Since the patient’s initial right IOP was 32 mm Hg (normal range, 12-22 mm Hg), ophthalmology services at this institution performed a lateral canthotomy. The patient’s right IOP postsurgery decreased but remained elevated at 27 mm Hg. In addition to surgical intervention, he was given oral acetazolamide and timolol. Then, because the patient was hemodynamically stable, he was referred to our institution for further evaluation.
Upon arrival at our ED, the patient reported slow bleeding from the canthotomy site. He denied any chest pain, shortness of breath, light-headedness, dizziness, or visual changes. Additional history revealed that in addition to taking rivaroxaban, the patient was also on a daily 81-mg aspirin regimen. His vital signs at presentation were: blood pressure (BP), 130/68 mm Hg; heart rate, 75 beats/minute; and respiratory rate, 16 breaths/minute; and temperature, afebrile. Oxygen saturation was 99% on room air.
Physical examination revealed blood oozing from the right eye at the canthotomy site. There was no other evidence of trauma to the eye or head, and IOP of the right eye was normal at 14 mm Hg. Laboratory studies revealed a hemoglobin value of 16.8 g/dL, a hematocrit of 48%, and a white blood cell (WBC) count of 8.8 x 109/L. The basic metabolic profile, including creatinine, was unremarkable. A type and screen blood pretransfusion compatibility test was also ordered.
Since the patient’s ocular hemorrhaging persisted, ophthalmology services were consulted. The ophthalmology examination measured a right IOP of 14 mm Hg and a visual acuity of 20/200. The patient’s pupils were equal, round, and reactive to light, and a subconjunctival hematoma was noted. The ophthalmologist recommended no further surgical interventions at that time.
Due to the continued ocular bleeding, hematology services were also consulted. The hematologist recommended 50 U/kg of intravenous (IV) prothrombin complex concentrate (PCC) to reverse the anticoagulatory effects of rivaroxaban. The patient was given one dose of PCC in the ED. Throughout his ED course, the patient did not experience any deterioration of visual acuity. However, during repeated IOP checks, he experienced one episode of vasovagal syncope with a systolic BP in the 70s. The syncope resolved promptly after the patient was placed in a supine position and was given an IV bolus of normal saline fluid. The patient still had oozing at the incision site, and was admitted to the general medicine floor.
During his inpatient stay, the patient remained hemodynamically stable and did not require transfusion of blood or platelet products. All home anticoagulant medications were discontinued. The patient continued to have some oozing the following morning, and was given an additional dose of IV PCC (50 U/kg), which resolved the bleeding. He remained hemostatic and, based on his history of AF, he was discharged home on warfarin without bridge therapy. Both rivaroxaban and daily aspirin therapy were discontinued. The lateral canthotomy and cantholysis healed without need for surgical intervention. An ophthalmology follow-up clinic visit 1 week after discharge from the hospital revealed an already self-healed incision without ectropion or retraction and with only mild laxity. Given the patient’s history of AF with TIAs while off anticoagulants, the ophthalmologist did not recommend any other surgical intervention that would have required discontinuing the warfarin.
Discussion
With any retrobulbar hematoma, one must be concerned for orbital compartment syndrome. Orbital hemorrhage is the most common cause of orbital compartment syndrome, usually occurring secondary to trauma, surgery, or retrobulbar injection. In this case, spontaneous hemorrhage due to anticoagulation was believed to be the cause—albeit a rare one—of orbital compartment syndrome. Because the orbital space is enclosed and cannot expand, it is vulnerable to compartment syndrome, and subsequent ischemia can lead to permanent vision impairment or complete loss of vision.5 Early recognition and treatment is imperative to preserve vision as an elevated intraorbital pressure for 60 to 100 minutes can lead to permanent visual sequelae.
Management
Treatment of retrobulbar hemorrhage includes lateral canthotomy and cantholysis, which have been shown to reduce IOP an average of 14.2 mm Hg.6 In our patient, IOP in the affected eye was reduced by 18 mm Hg. In addition to the patient’s high IOP at presentation, another concern was the continued hemorrhaging from both the incision site and its potential to exacerbate the underlying retrobulbar hematoma. Management of this condition posed a challenge because this patient was taking a newer anticoagulant, for which there is currently no specific reversal agent. After consultation with hematology services, the patient was given PCC because small studies have suggested that PCC may reverse rivaroxaban-induced anticoagulation.7 While more expensive than fresh frozen plasma, PCC has a high safety profile and should be considered in cases of life-threatening bleeding—especially in patients who have renal failure, as rivaroxaban is renally excreted. The half-life of rivaroxaban is 5 to 9 hours and its effects may last up to 12 hours. An IV dose of 50 U/kg PCC can be effective in reversing rivaroxaban; this dose can be repeated every 12 hours until hemorrhaging abates or until rivaroxaban is cleared.
Potential Factor Xa Reversal Agent
Phase IV trials are underway in the ANNEX-A (Andexanet Alfa a Novel Antidote to the Anticoagulant Effects of FXa inhibitors – Apixaban) and ANNEX-R (Andexanet Alfa a Novel Antidote to the Anticoagulant Effects of FXa inhibitors – Rivaroxaban) studies assessing andexanet alpha, an FXa inhibitor reversal agent and potential FXa inhibitor antidote. Andexanet alpha is a decoy protein that binds to FXa inhibitors in the active site, restoring endogenous FXa and reducing anticoagulant activity.8 This serves as another promising reversal agent for apixaban, edoxaban, and rivaroxaban. With the development of these new FXa reversal agents, EPs will have more options for reversal of anticoagulation in patients with unique hemorrhagic presentations.
Conclusion
Rivaroxaban has the potential to replace warfarin as a “novel” oral anticoagulant of choice for multiple indications, especially as more insurance companies cover the use of the FXa inhibitors. As a result of their increased use, the EP is likely to see an increasing number of patients who present with hemorrhagic consequences of the FXa inhibitors, and in turn must be familiar with the properties of this class of anticoagulants—including potential reversal strategies.
Our case of spontaneous retrobulbar hemorrhage may be one of these new patterns of bleeding to be expected from a novel FXa inhibitor. Therefore, it is imperative that EPs consider retrobulbar hemorrhage and other possible bleeding locations in patients on an FXa inhibitor.
1. McAllister AR, Sobel RK, Allen RC. Spontaneous retrobulbar hemorrhage with subsequent orbital compartment syndrome. University of Iowa Health Care Ophthalmology and Visual Sciences Web site. http://www.eyerounds.org/cases/168-orbital-compartment-syndrome.htm. Accessed June 14, 2016.
2. Winterton JV, Patel K, Mizen KD. Review of management options for a retrobulbar hemorrhage. J Oral Maxillofac Surg. 2007;65(2):296-299.
3. Wasserlauf G, Grandi SM, Filion KB, Eisenberg MJ. Meta-analysis of rivaroxaban and bleeding risk. Am J Cardiol. 2013;112(3):454-460.
4. Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883-891.
5. Kloss BT, Patel R. Orbital compartment syndrome from retrobulbar hemorrhage. Int J Emerg Med. 2010;3(4):521-522.
6. Peak DA. Acute orbital compartment syndrome. Medscape. http://emedicine.medscape.com/article/799528-overview. Updated November 4, 2015. Accessed June 14, 2016.
7. Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124(14):1573-1579.
8. Siegal DM, Curnutte JT, Connolly SJ, et al. Andexanet alfa for the reversal of factor Xa inhibitor activity. N Engl J Med. 2015;373(25):2413-2424.
Most emergency physicians (EPs) encounter several patients a year with hemorrhages due to factor Xa (FXa) inhibitors. Such bleeding may occur in patterns not previously recognized with traditional anticoagulant therapy. Retrobulbar hemorrhage is typically associated with significant facial or orbital trauma, and spontaneous hemorrhage is a very rare cause of orbital compartment syndrome.1 Retrobulbar hemorrhage can lead to orbital compartment syndrome due to increased orbital pressure within a closed space. Because orbital compartment syndrome can compromise blood flow to the optic nerve or central retinal artery, it is extremely important to decrease orbital pressure as quickly as possible in affected patients. Therefore, canthotomy/cantholysis should be performed sooner rather than later, as 90 minutes of elevated intraocular pressure (IOP) can lead to permanent vision loss.2
Rivaroxaban, one of the relatively new oral anticoagulant agents that inhibit FXa, is used as an alternative therapy to vitamin K antagonists. The FXa agents have been approved to reduce the risk of stroke in patients with nonvalvular atrial fibrillation (AF).3 According to a meta-analysis of rivaroxaban and bleeding risk, rivaroxaban was shown to have no increased risk of major or clinically relevant nonmajor bleeding compared to vitamin K antagonists. Rivaroxaban was also associated with a significant decrease in fatal bleeding (relative risk, 0.48, 95%; confidence interval, 0.31 to 0.74).4
Case Report
A 79-year-old man with a medical history of hypertension, transient ischemic attacks (TIAs), and AF, for which he was taking rivaroxaban, was referred to our ED by a local rural ED for further evaluation and treatment of a retrobulbar hemorrhage. (The patient’s family refused emergency medical services transport from the rural ED.) The patient stated that upon awakening earlier that morning, he felt “pressure” in his right eye and experienced periorbital swelling that continued to worsen throughout the day. He denied any trauma, falls, or strikes to the face or head. The patient’s account and history were confirmed by the family members with whom he resided.
During the patient’s evaluation at the rural ED, a computed tomography (CT) scan of the head was performed, which demonstrated a retrobulbar hematoma on the right side (See the Figure for an example of a CT scan illustrating a retrobulbar hematoma with proptosis). Since the patient’s initial right IOP was 32 mm Hg (normal range, 12-22 mm Hg), ophthalmology services at this institution performed a lateral canthotomy. The patient’s right IOP postsurgery decreased but remained elevated at 27 mm Hg. In addition to surgical intervention, he was given oral acetazolamide and timolol. Then, because the patient was hemodynamically stable, he was referred to our institution for further evaluation.
Upon arrival at our ED, the patient reported slow bleeding from the canthotomy site. He denied any chest pain, shortness of breath, light-headedness, dizziness, or visual changes. Additional history revealed that in addition to taking rivaroxaban, the patient was also on a daily 81-mg aspirin regimen. His vital signs at presentation were: blood pressure (BP), 130/68 mm Hg; heart rate, 75 beats/minute; and respiratory rate, 16 breaths/minute; and temperature, afebrile. Oxygen saturation was 99% on room air.
Physical examination revealed blood oozing from the right eye at the canthotomy site. There was no other evidence of trauma to the eye or head, and IOP of the right eye was normal at 14 mm Hg. Laboratory studies revealed a hemoglobin value of 16.8 g/dL, a hematocrit of 48%, and a white blood cell (WBC) count of 8.8 x 109/L. The basic metabolic profile, including creatinine, was unremarkable. A type and screen blood pretransfusion compatibility test was also ordered.
Since the patient’s ocular hemorrhaging persisted, ophthalmology services were consulted. The ophthalmology examination measured a right IOP of 14 mm Hg and a visual acuity of 20/200. The patient’s pupils were equal, round, and reactive to light, and a subconjunctival hematoma was noted. The ophthalmologist recommended no further surgical interventions at that time.
Due to the continued ocular bleeding, hematology services were also consulted. The hematologist recommended 50 U/kg of intravenous (IV) prothrombin complex concentrate (PCC) to reverse the anticoagulatory effects of rivaroxaban. The patient was given one dose of PCC in the ED. Throughout his ED course, the patient did not experience any deterioration of visual acuity. However, during repeated IOP checks, he experienced one episode of vasovagal syncope with a systolic BP in the 70s. The syncope resolved promptly after the patient was placed in a supine position and was given an IV bolus of normal saline fluid. The patient still had oozing at the incision site, and was admitted to the general medicine floor.
During his inpatient stay, the patient remained hemodynamically stable and did not require transfusion of blood or platelet products. All home anticoagulant medications were discontinued. The patient continued to have some oozing the following morning, and was given an additional dose of IV PCC (50 U/kg), which resolved the bleeding. He remained hemostatic and, based on his history of AF, he was discharged home on warfarin without bridge therapy. Both rivaroxaban and daily aspirin therapy were discontinued. The lateral canthotomy and cantholysis healed without need for surgical intervention. An ophthalmology follow-up clinic visit 1 week after discharge from the hospital revealed an already self-healed incision without ectropion or retraction and with only mild laxity. Given the patient’s history of AF with TIAs while off anticoagulants, the ophthalmologist did not recommend any other surgical intervention that would have required discontinuing the warfarin.
Discussion
With any retrobulbar hematoma, one must be concerned for orbital compartment syndrome. Orbital hemorrhage is the most common cause of orbital compartment syndrome, usually occurring secondary to trauma, surgery, or retrobulbar injection. In this case, spontaneous hemorrhage due to anticoagulation was believed to be the cause—albeit a rare one—of orbital compartment syndrome. Because the orbital space is enclosed and cannot expand, it is vulnerable to compartment syndrome, and subsequent ischemia can lead to permanent vision impairment or complete loss of vision.5 Early recognition and treatment is imperative to preserve vision as an elevated intraorbital pressure for 60 to 100 minutes can lead to permanent visual sequelae.
Management
Treatment of retrobulbar hemorrhage includes lateral canthotomy and cantholysis, which have been shown to reduce IOP an average of 14.2 mm Hg.6 In our patient, IOP in the affected eye was reduced by 18 mm Hg. In addition to the patient’s high IOP at presentation, another concern was the continued hemorrhaging from both the incision site and its potential to exacerbate the underlying retrobulbar hematoma. Management of this condition posed a challenge because this patient was taking a newer anticoagulant, for which there is currently no specific reversal agent. After consultation with hematology services, the patient was given PCC because small studies have suggested that PCC may reverse rivaroxaban-induced anticoagulation.7 While more expensive than fresh frozen plasma, PCC has a high safety profile and should be considered in cases of life-threatening bleeding—especially in patients who have renal failure, as rivaroxaban is renally excreted. The half-life of rivaroxaban is 5 to 9 hours and its effects may last up to 12 hours. An IV dose of 50 U/kg PCC can be effective in reversing rivaroxaban; this dose can be repeated every 12 hours until hemorrhaging abates or until rivaroxaban is cleared.
Potential Factor Xa Reversal Agent
Phase IV trials are underway in the ANNEX-A (Andexanet Alfa a Novel Antidote to the Anticoagulant Effects of FXa inhibitors – Apixaban) and ANNEX-R (Andexanet Alfa a Novel Antidote to the Anticoagulant Effects of FXa inhibitors – Rivaroxaban) studies assessing andexanet alpha, an FXa inhibitor reversal agent and potential FXa inhibitor antidote. Andexanet alpha is a decoy protein that binds to FXa inhibitors in the active site, restoring endogenous FXa and reducing anticoagulant activity.8 This serves as another promising reversal agent for apixaban, edoxaban, and rivaroxaban. With the development of these new FXa reversal agents, EPs will have more options for reversal of anticoagulation in patients with unique hemorrhagic presentations.
Conclusion
Rivaroxaban has the potential to replace warfarin as a “novel” oral anticoagulant of choice for multiple indications, especially as more insurance companies cover the use of the FXa inhibitors. As a result of their increased use, the EP is likely to see an increasing number of patients who present with hemorrhagic consequences of the FXa inhibitors, and in turn must be familiar with the properties of this class of anticoagulants—including potential reversal strategies.
Our case of spontaneous retrobulbar hemorrhage may be one of these new patterns of bleeding to be expected from a novel FXa inhibitor. Therefore, it is imperative that EPs consider retrobulbar hemorrhage and other possible bleeding locations in patients on an FXa inhibitor.
Most emergency physicians (EPs) encounter several patients a year with hemorrhages due to factor Xa (FXa) inhibitors. Such bleeding may occur in patterns not previously recognized with traditional anticoagulant therapy. Retrobulbar hemorrhage is typically associated with significant facial or orbital trauma, and spontaneous hemorrhage is a very rare cause of orbital compartment syndrome.1 Retrobulbar hemorrhage can lead to orbital compartment syndrome due to increased orbital pressure within a closed space. Because orbital compartment syndrome can compromise blood flow to the optic nerve or central retinal artery, it is extremely important to decrease orbital pressure as quickly as possible in affected patients. Therefore, canthotomy/cantholysis should be performed sooner rather than later, as 90 minutes of elevated intraocular pressure (IOP) can lead to permanent vision loss.2
Rivaroxaban, one of the relatively new oral anticoagulant agents that inhibit FXa, is used as an alternative therapy to vitamin K antagonists. The FXa agents have been approved to reduce the risk of stroke in patients with nonvalvular atrial fibrillation (AF).3 According to a meta-analysis of rivaroxaban and bleeding risk, rivaroxaban was shown to have no increased risk of major or clinically relevant nonmajor bleeding compared to vitamin K antagonists. Rivaroxaban was also associated with a significant decrease in fatal bleeding (relative risk, 0.48, 95%; confidence interval, 0.31 to 0.74).4
Case Report
A 79-year-old man with a medical history of hypertension, transient ischemic attacks (TIAs), and AF, for which he was taking rivaroxaban, was referred to our ED by a local rural ED for further evaluation and treatment of a retrobulbar hemorrhage. (The patient’s family refused emergency medical services transport from the rural ED.) The patient stated that upon awakening earlier that morning, he felt “pressure” in his right eye and experienced periorbital swelling that continued to worsen throughout the day. He denied any trauma, falls, or strikes to the face or head. The patient’s account and history were confirmed by the family members with whom he resided.
During the patient’s evaluation at the rural ED, a computed tomography (CT) scan of the head was performed, which demonstrated a retrobulbar hematoma on the right side (See the Figure for an example of a CT scan illustrating a retrobulbar hematoma with proptosis). Since the patient’s initial right IOP was 32 mm Hg (normal range, 12-22 mm Hg), ophthalmology services at this institution performed a lateral canthotomy. The patient’s right IOP postsurgery decreased but remained elevated at 27 mm Hg. In addition to surgical intervention, he was given oral acetazolamide and timolol. Then, because the patient was hemodynamically stable, he was referred to our institution for further evaluation.
Upon arrival at our ED, the patient reported slow bleeding from the canthotomy site. He denied any chest pain, shortness of breath, light-headedness, dizziness, or visual changes. Additional history revealed that in addition to taking rivaroxaban, the patient was also on a daily 81-mg aspirin regimen. His vital signs at presentation were: blood pressure (BP), 130/68 mm Hg; heart rate, 75 beats/minute; and respiratory rate, 16 breaths/minute; and temperature, afebrile. Oxygen saturation was 99% on room air.
Physical examination revealed blood oozing from the right eye at the canthotomy site. There was no other evidence of trauma to the eye or head, and IOP of the right eye was normal at 14 mm Hg. Laboratory studies revealed a hemoglobin value of 16.8 g/dL, a hematocrit of 48%, and a white blood cell (WBC) count of 8.8 x 109/L. The basic metabolic profile, including creatinine, was unremarkable. A type and screen blood pretransfusion compatibility test was also ordered.
Since the patient’s ocular hemorrhaging persisted, ophthalmology services were consulted. The ophthalmology examination measured a right IOP of 14 mm Hg and a visual acuity of 20/200. The patient’s pupils were equal, round, and reactive to light, and a subconjunctival hematoma was noted. The ophthalmologist recommended no further surgical interventions at that time.
Due to the continued ocular bleeding, hematology services were also consulted. The hematologist recommended 50 U/kg of intravenous (IV) prothrombin complex concentrate (PCC) to reverse the anticoagulatory effects of rivaroxaban. The patient was given one dose of PCC in the ED. Throughout his ED course, the patient did not experience any deterioration of visual acuity. However, during repeated IOP checks, he experienced one episode of vasovagal syncope with a systolic BP in the 70s. The syncope resolved promptly after the patient was placed in a supine position and was given an IV bolus of normal saline fluid. The patient still had oozing at the incision site, and was admitted to the general medicine floor.
During his inpatient stay, the patient remained hemodynamically stable and did not require transfusion of blood or platelet products. All home anticoagulant medications were discontinued. The patient continued to have some oozing the following morning, and was given an additional dose of IV PCC (50 U/kg), which resolved the bleeding. He remained hemostatic and, based on his history of AF, he was discharged home on warfarin without bridge therapy. Both rivaroxaban and daily aspirin therapy were discontinued. The lateral canthotomy and cantholysis healed without need for surgical intervention. An ophthalmology follow-up clinic visit 1 week after discharge from the hospital revealed an already self-healed incision without ectropion or retraction and with only mild laxity. Given the patient’s history of AF with TIAs while off anticoagulants, the ophthalmologist did not recommend any other surgical intervention that would have required discontinuing the warfarin.
Discussion
With any retrobulbar hematoma, one must be concerned for orbital compartment syndrome. Orbital hemorrhage is the most common cause of orbital compartment syndrome, usually occurring secondary to trauma, surgery, or retrobulbar injection. In this case, spontaneous hemorrhage due to anticoagulation was believed to be the cause—albeit a rare one—of orbital compartment syndrome. Because the orbital space is enclosed and cannot expand, it is vulnerable to compartment syndrome, and subsequent ischemia can lead to permanent vision impairment or complete loss of vision.5 Early recognition and treatment is imperative to preserve vision as an elevated intraorbital pressure for 60 to 100 minutes can lead to permanent visual sequelae.
Management
Treatment of retrobulbar hemorrhage includes lateral canthotomy and cantholysis, which have been shown to reduce IOP an average of 14.2 mm Hg.6 In our patient, IOP in the affected eye was reduced by 18 mm Hg. In addition to the patient’s high IOP at presentation, another concern was the continued hemorrhaging from both the incision site and its potential to exacerbate the underlying retrobulbar hematoma. Management of this condition posed a challenge because this patient was taking a newer anticoagulant, for which there is currently no specific reversal agent. After consultation with hematology services, the patient was given PCC because small studies have suggested that PCC may reverse rivaroxaban-induced anticoagulation.7 While more expensive than fresh frozen plasma, PCC has a high safety profile and should be considered in cases of life-threatening bleeding—especially in patients who have renal failure, as rivaroxaban is renally excreted. The half-life of rivaroxaban is 5 to 9 hours and its effects may last up to 12 hours. An IV dose of 50 U/kg PCC can be effective in reversing rivaroxaban; this dose can be repeated every 12 hours until hemorrhaging abates or until rivaroxaban is cleared.
Potential Factor Xa Reversal Agent
Phase IV trials are underway in the ANNEX-A (Andexanet Alfa a Novel Antidote to the Anticoagulant Effects of FXa inhibitors – Apixaban) and ANNEX-R (Andexanet Alfa a Novel Antidote to the Anticoagulant Effects of FXa inhibitors – Rivaroxaban) studies assessing andexanet alpha, an FXa inhibitor reversal agent and potential FXa inhibitor antidote. Andexanet alpha is a decoy protein that binds to FXa inhibitors in the active site, restoring endogenous FXa and reducing anticoagulant activity.8 This serves as another promising reversal agent for apixaban, edoxaban, and rivaroxaban. With the development of these new FXa reversal agents, EPs will have more options for reversal of anticoagulation in patients with unique hemorrhagic presentations.
Conclusion
Rivaroxaban has the potential to replace warfarin as a “novel” oral anticoagulant of choice for multiple indications, especially as more insurance companies cover the use of the FXa inhibitors. As a result of their increased use, the EP is likely to see an increasing number of patients who present with hemorrhagic consequences of the FXa inhibitors, and in turn must be familiar with the properties of this class of anticoagulants—including potential reversal strategies.
Our case of spontaneous retrobulbar hemorrhage may be one of these new patterns of bleeding to be expected from a novel FXa inhibitor. Therefore, it is imperative that EPs consider retrobulbar hemorrhage and other possible bleeding locations in patients on an FXa inhibitor.
1. McAllister AR, Sobel RK, Allen RC. Spontaneous retrobulbar hemorrhage with subsequent orbital compartment syndrome. University of Iowa Health Care Ophthalmology and Visual Sciences Web site. http://www.eyerounds.org/cases/168-orbital-compartment-syndrome.htm. Accessed June 14, 2016.
2. Winterton JV, Patel K, Mizen KD. Review of management options for a retrobulbar hemorrhage. J Oral Maxillofac Surg. 2007;65(2):296-299.
3. Wasserlauf G, Grandi SM, Filion KB, Eisenberg MJ. Meta-analysis of rivaroxaban and bleeding risk. Am J Cardiol. 2013;112(3):454-460.
4. Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883-891.
5. Kloss BT, Patel R. Orbital compartment syndrome from retrobulbar hemorrhage. Int J Emerg Med. 2010;3(4):521-522.
6. Peak DA. Acute orbital compartment syndrome. Medscape. http://emedicine.medscape.com/article/799528-overview. Updated November 4, 2015. Accessed June 14, 2016.
7. Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124(14):1573-1579.
8. Siegal DM, Curnutte JT, Connolly SJ, et al. Andexanet alfa for the reversal of factor Xa inhibitor activity. N Engl J Med. 2015;373(25):2413-2424.
1. McAllister AR, Sobel RK, Allen RC. Spontaneous retrobulbar hemorrhage with subsequent orbital compartment syndrome. University of Iowa Health Care Ophthalmology and Visual Sciences Web site. http://www.eyerounds.org/cases/168-orbital-compartment-syndrome.htm. Accessed June 14, 2016.
2. Winterton JV, Patel K, Mizen KD. Review of management options for a retrobulbar hemorrhage. J Oral Maxillofac Surg. 2007;65(2):296-299.
3. Wasserlauf G, Grandi SM, Filion KB, Eisenberg MJ. Meta-analysis of rivaroxaban and bleeding risk. Am J Cardiol. 2013;112(3):454-460.
4. Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883-891.
5. Kloss BT, Patel R. Orbital compartment syndrome from retrobulbar hemorrhage. Int J Emerg Med. 2010;3(4):521-522.
6. Peak DA. Acute orbital compartment syndrome. Medscape. http://emedicine.medscape.com/article/799528-overview. Updated November 4, 2015. Accessed June 14, 2016.
7. Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124(14):1573-1579.
8. Siegal DM, Curnutte JT, Connolly SJ, et al. Andexanet alfa for the reversal of factor Xa inhibitor activity. N Engl J Med. 2015;373(25):2413-2424.
This'll Really Get Under Your Skin
A 23-year-old woman presents to dermatology with an itchy rash she has had for several months. Although it manifested on her wrists and finger, the rash moves around and causes itching on her legs, trunk, and arms at various times. It has not affected her breasts or axillae.
The patient has been seen in primary care several times and received the usual topical steroids, antihistamines, and at least three courses of prednisone—none affording much relief.
She denies that anyone else in her household is itching. During her last visit to primary care, they treated her with topical permethrin cream, which was to be left on overnight then washed off. No relief was forthcoming.
EXAMINATION
Scattered areas of faint eczematoid rashes can be seen across her thighs and arms. There are two or three tiny excoriated papules on both volar wrists, but no intact vesicles are observed.
A closer inspection of her palms reveals one tiny linear vesicle on the mid right palm. Vigorous scraping with a #10 blade produces material, which is placed on a slide, covered, filled with potassium hydroxide 10%, and examined under a microscope.
What is the diagnosis?
DISCUSSION
Microscopic evaluation revealed a scabies adult, still moving among the dead cells. The paucity of organisms was probably a result of the prior treatment with permethrin, which confused the issue. When scabies is suspected, there’s only one way to confirm it: Perform a KOH prep. Otherwise, it’s just guesswork.
This particular patient was under the (faulty) impression that because her permethrin treatment failed, she didn’t have scabies. But a single treatment with topical antiscabetic cream is rarely curative. It must be done twice, seven to 10 days apart, to kill organisms newly hatched from eggs lain in the skin. In many cases, oral ivermectin is needed as well.
The itching experienced with scabies is due to an allergic response to scabetic material (droppings, tissue juice). The resultant eczematous rash can be very challenging to deal with.
Three other people live in the patient’s house, and it’s likely that these family members have been infested over several months’ time. They need treatment as well and will require two applications of cream.
Thought should also be given to how the patient acquired the infestation, lest she get it again. This may result in the need to avoid contact with certain people or confront them about their possible role as the source.
TAKE-HOME LEARNING POINTS
• The itching and rash of scabies result from an allergic reaction to the scabetic elements deposited in the skin. This reaction can closely resemble eczema.
• A single treatment of permethrin cream is unlikely to clear scabies. In order to have an effect on the eggs lain in the skin (which hatch in seven to 10 days), two treatments are necessary.
• Many cases of scabies will survive permethrin treatment, so consider adding ivermectin (oral antiscabetic medication) to the regimen.
• All family members living in the same house must be treated at the same time, lest they re-infest one another.
• KOH prep is the gold standard for diagnosing scabies.
A 23-year-old woman presents to dermatology with an itchy rash she has had for several months. Although it manifested on her wrists and finger, the rash moves around and causes itching on her legs, trunk, and arms at various times. It has not affected her breasts or axillae.
The patient has been seen in primary care several times and received the usual topical steroids, antihistamines, and at least three courses of prednisone—none affording much relief.
She denies that anyone else in her household is itching. During her last visit to primary care, they treated her with topical permethrin cream, which was to be left on overnight then washed off. No relief was forthcoming.
EXAMINATION
Scattered areas of faint eczematoid rashes can be seen across her thighs and arms. There are two or three tiny excoriated papules on both volar wrists, but no intact vesicles are observed.
A closer inspection of her palms reveals one tiny linear vesicle on the mid right palm. Vigorous scraping with a #10 blade produces material, which is placed on a slide, covered, filled with potassium hydroxide 10%, and examined under a microscope.
What is the diagnosis?
DISCUSSION
Microscopic evaluation revealed a scabies adult, still moving among the dead cells. The paucity of organisms was probably a result of the prior treatment with permethrin, which confused the issue. When scabies is suspected, there’s only one way to confirm it: Perform a KOH prep. Otherwise, it’s just guesswork.
This particular patient was under the (faulty) impression that because her permethrin treatment failed, she didn’t have scabies. But a single treatment with topical antiscabetic cream is rarely curative. It must be done twice, seven to 10 days apart, to kill organisms newly hatched from eggs lain in the skin. In many cases, oral ivermectin is needed as well.
The itching experienced with scabies is due to an allergic response to scabetic material (droppings, tissue juice). The resultant eczematous rash can be very challenging to deal with.
Three other people live in the patient’s house, and it’s likely that these family members have been infested over several months’ time. They need treatment as well and will require two applications of cream.
Thought should also be given to how the patient acquired the infestation, lest she get it again. This may result in the need to avoid contact with certain people or confront them about their possible role as the source.
TAKE-HOME LEARNING POINTS
• The itching and rash of scabies result from an allergic reaction to the scabetic elements deposited in the skin. This reaction can closely resemble eczema.
• A single treatment of permethrin cream is unlikely to clear scabies. In order to have an effect on the eggs lain in the skin (which hatch in seven to 10 days), two treatments are necessary.
• Many cases of scabies will survive permethrin treatment, so consider adding ivermectin (oral antiscabetic medication) to the regimen.
• All family members living in the same house must be treated at the same time, lest they re-infest one another.
• KOH prep is the gold standard for diagnosing scabies.
A 23-year-old woman presents to dermatology with an itchy rash she has had for several months. Although it manifested on her wrists and finger, the rash moves around and causes itching on her legs, trunk, and arms at various times. It has not affected her breasts or axillae.
The patient has been seen in primary care several times and received the usual topical steroids, antihistamines, and at least three courses of prednisone—none affording much relief.
She denies that anyone else in her household is itching. During her last visit to primary care, they treated her with topical permethrin cream, which was to be left on overnight then washed off. No relief was forthcoming.
EXAMINATION
Scattered areas of faint eczematoid rashes can be seen across her thighs and arms. There are two or three tiny excoriated papules on both volar wrists, but no intact vesicles are observed.
A closer inspection of her palms reveals one tiny linear vesicle on the mid right palm. Vigorous scraping with a #10 blade produces material, which is placed on a slide, covered, filled with potassium hydroxide 10%, and examined under a microscope.
What is the diagnosis?
DISCUSSION
Microscopic evaluation revealed a scabies adult, still moving among the dead cells. The paucity of organisms was probably a result of the prior treatment with permethrin, which confused the issue. When scabies is suspected, there’s only one way to confirm it: Perform a KOH prep. Otherwise, it’s just guesswork.
This particular patient was under the (faulty) impression that because her permethrin treatment failed, she didn’t have scabies. But a single treatment with topical antiscabetic cream is rarely curative. It must be done twice, seven to 10 days apart, to kill organisms newly hatched from eggs lain in the skin. In many cases, oral ivermectin is needed as well.
The itching experienced with scabies is due to an allergic response to scabetic material (droppings, tissue juice). The resultant eczematous rash can be very challenging to deal with.
Three other people live in the patient’s house, and it’s likely that these family members have been infested over several months’ time. They need treatment as well and will require two applications of cream.
Thought should also be given to how the patient acquired the infestation, lest she get it again. This may result in the need to avoid contact with certain people or confront them about their possible role as the source.
TAKE-HOME LEARNING POINTS
• The itching and rash of scabies result from an allergic reaction to the scabetic elements deposited in the skin. This reaction can closely resemble eczema.
• A single treatment of permethrin cream is unlikely to clear scabies. In order to have an effect on the eggs lain in the skin (which hatch in seven to 10 days), two treatments are necessary.
• Many cases of scabies will survive permethrin treatment, so consider adding ivermectin (oral antiscabetic medication) to the regimen.
• All family members living in the same house must be treated at the same time, lest they re-infest one another.
• KOH prep is the gold standard for diagnosing scabies.
Atypical Acute Myocardial Infarction and Concomitant Acute Cerebral Infarct
A 61-year-old woman without any known medical history presented with a chief complaint of right arm numbness and right-sided scalp numbness that had started 2 days earlier. She described a “pins and needles” sensation in her right upper extremity and right scalp, and said the numbness in her scalp was especially noticeable when she combed her hair. The patient denied any chest pain, shortness of breath, weakness in her arms or legs, headache, or blurred vision.
She said that 1 day prior to the onset of the paresthesias, she woke up fatigued and vomited once. Throughout that day, she had symptoms of nausea and fatigue, and sought treatment at an urgent care center that afternoon. At the urgent care center, she was diagnosed with a “stomach virus” and was given an antiemetic. The next day, her nausea improved, but the paresthesias began in her right hand and scalp. On the third day, the patient went to work, but the persistent paresthesias caused her to visit her primary care physician, who sent her to our ED for further work-up.
The patient said she had been in good health until 3 days ago. She reported no medical problems and was taking no medications. The patient denied smoking or using alcohol; her family history was significant only in that her father had a myocardial infarction (MI) while in his 50s.
On physical examination, the patient was alert, oriented, and in no apparent distress. Her body mass index was 28.3 kg/m2. Vital signs were: temperature, 99.2°F; blood pressure, 113/73 mm Hg; heart rate, 93 beats/minute; and respiratory rate, 18 breaths/minute. Oxygen saturation was 95% on room air.
Her head was normocephalic and atraumatic, and her eyes, ears, nose, and throat were normal. Her neck was supple and without jugular vein distension. The cardiac examination revealed normal heart sounds without murmurs, rubs, or gallops. Her lungs were clear without rales, wheezes, or rhonchi. Her abdomen was soft, without tenderness, guarding, or rebound, and she had normal bowel sounds.
Her musculoskeletal examination was normal, with +5/5 strength bilaterally in her upper and lower extremities. The patient’s skin examination also was normal. On neurological examination, her right upper extremity and right side of her face were noted to have decreased sensation via pinprick compared to the left side, but the examination was otherwise normal. The National Institutes of Health Stroke Scale score was 1.
The patient’s electrocardiogram (ECG) showed a normal sinus rhythm (rate, 90 beats/min), a lateral infarct of undetermined age, and a left atrial abnormality. Laboratory evaluation was significant only for a brain natriuretic peptide level of 334 pg/mL, a creatine phosphokinase (CPK) level of 782 IU/L, and a troponin I level of >50 ng/mL (Table). Serial cardiac enzyme levels were obtained and showed a decline of CPK from 782 IU/L to 331 IU/L over the following 36 hours. However, the troponin I levels remained >50 ng/mL for 5 days and then declined to 31.6 ng/mL.
A computed tomography (CT) scan of the brain without contrast revealed an acute to subacute infarct in the left occipital and left thalamic regions (Figure). A stat transthoracic echocardiogram (ECHO) performed in the ED revealed a dilated left ventricle with an ejection fraction of 20% to 25%, along with a hypokinetic anterolateral wall and an akinetic inferolateral wall. No atrial thrombus was visible on the ECHO. Doppler studies of the patient’s lower extremities were negative for deep vein thrombosis. Magnetic resonance imaging of her brain showed an infarct in the posterior circulation distribution involving the left occipital lobes and small areas in the left thalamic and right parietal-occipital regions. Hemorrhagic conversion of the left occipital infarct without mass effect was also noted. The patient was admitted to the neurological intensive care unit for frequent neurological examinations and close monitoring for worsening cerebral hemorrhage.
When the patient had still been in the ED, cardiology services were consulted; the cardiologist initiated a heparin drip with close monitoring of the coagulation studies. Cardiac catheterization was not done immediately because the ECG did not show acute ST elevations. The day after her presentation to the ED, the patient underwent a primary percutaneous coronary catheterization and was found to have a small rudimentary left anterior descending artery, with only small branches supplying the septal region. The right circumflex artery was very large and was supplying the lateral wall. No stents were placed during this procedure. A transesophageal ECHO (TEE) showed no evidence of a left atrial appendage thrombus.
The patient experienced an episode of coffee ground emesis while undergoing the TEE. Her hemoglobin declined from 11.9 g/dL to 7.9 g/dL, which led to a transfusion of 2 U of packed red blood cells and platelets. Heparin was discontinued and a proton pump inhibitor was started; however, no endoscopy was done at that time.
Throughout her stay, the patient was continuously monitored, but no evidence of arrhythmia or atrial fibrillation was found. Upon discharge, the neurologist recommended the patient receive clopidogrel and aspirin therapy for 3 months with subsequent aspirin monotherapy afterward. The patient was discharged after 10 days in the hospital.
Discussion
Although she had an MI, the patient presented here did not experience any chest pain. Her chief complaint in the ED was paresthesias related to her concomitant stroke, and only on further probing did she describe the additional symptoms of fatigability and vomiting.
Since heart disease and stroke share common risk factors and pathophysiology, acute cerebral ischemic events may happen concurrently with MIs. In a review of studies that included approximately 2,900 patients who had an acute stroke, Kerr et al1 found that 20% had elevated troponin levels within 7 days of the stroke. In 2013, the American Heart Association and American Stroke Association published guidelines advising that all patients who present with acute cerebral ischemia have an emergent ECG and baseline troponin level.2 This was in response to evidence that even low positive troponin levels have been associated with an increased risk of mortality.3 Positive troponin levels are especially important because fatal and nonfatal stroke post-MI events have been found to be increasing in frequency for women, even though there has been a significant overall reduction in post-MI mortality.4 Patients who have an ischemic stroke concurrently with an acute MI or soon after have an overall poorer clinical prognosis.5
For emergency physicians (EPs), this is a “chicken or the egg” scenario. It is difficult to determine which came first: the MI or the cerebral ischemia. Similar risk factors can result in an acute embolic event from revascularization, atrial fibrillation without proper anticoagulation, or a poorly functioning left ventricle.6 It is important to remember that regardless of the order of occurrence, the incidence of ischemic stroke is markedly increased in conjunction with an acute MI.7 Several theories have been advanced regarding the relationship between ischemic stroke and acute MI. One theory proposes that elevated troponin levels could be related to a large catecholamine release after a cerebral ischemic stroke, resulting in subsequent myocardial injury or cardiomyopathy.7 However, this theory remains controversial.
Management
The major consideration for the EP is whether or not to give thrombolytics to a patient who presents with concomitant acute MI and ischemic stroke. An acute MI within the 3 months preceding an acute stroke is considered a relative contraindication for intravenous tissue plasminogen activator (tPA).8 It has also been found, albeit rarely, that there is an increased risk of cardiac rupture or tamponade due to the breakdown of the fibrin clot within the necrotic cardiac tissue.8
How should patients with stroke complicated by acute cardiac compromise be managed in the ED? One acute vascular event cannot be ignored while addressing the other. There are no evidence-based guidelines for the management of patients who present with this picture.8 In addition, no published clinical studies have focused on the decision-making process for these patients.8
Immediate percutaneous coronary intervention for the MI performed on such patients would prevent the use of tPA for the acute stroke. Though any anticoagulation increases the risk for postischemic cerebral hemorrhage, heparin is necessary to prevent the formation of a left ventricle thrombus.7 Alternately, mechanical thrombectomy and cardiac catheterization may be combined as emergent treatments for these patients, but performing these two procedures simultaneously is not widely available.
For a patient who presents to the ED within both the cardiac and stroke treatment windows, tPA might be a viable option, and the only one readily valuable.8 However, the EP must be mindful of the varying dosages of tPA and means of administration for different thrombosis sites. Also, care must be taken when treating a patient with dual or triple antiplatelet therapy because of the increased risk of hemorrhage.9 Currently, no safe standardized regimens have been established, and further trials need to be performed.10
If the patient in this case report had presented at our ED with only signs and symptoms of an MI, typically she would have been treated with heparin, aspirin, and an urgent cardiac catheterization. If she had presented with only signs and symptoms of a stroke, she would have been treated with full-dose aspirin and worked up from a neurological perspective. Because she had signs and symptoms of both, she presented a dilemma. She was initially treated with heparin to prevent a thrombus formation, but then later changed to only clopidogrel and aspirin to prevent further episodes of coffee ground emesis or worsening hemorrhagic conversion.
Conclusion
Common risk factors for cardiac and cerebral ischemic events may result in a patient presenting with both acute MI and an acute cerebral ischemic event. There have not been sufficient clinical studies to determine the best decision-making process for these patients. Therefore, patients with this complicated presentation must be assessed on an individual basis. Current treatment options are varied and are based according to history of the present illness, time of presentation to the ED, and the available resources within the hospital.
1. Kerr G, Ray G, Wu O, Stott DJ, Langhorne P. Elevated troponin after stroke: a systematic review. Cerebrovasc Dis. 2009;28(3):220-226.
2. Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870-947.
3. Di Angelantonio E, Fiorelli M, Toni D, et al. Prognostic significance of admission levels of troponin I in patients with acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2005;76(1):76-81.
4. Shiue I, Hristova K, Sharma J. Correspondence: gender and outcome from acute myocardial infarction and secondary stoke. Br J Cardiology. 2014;21:90.
5. Park S, Jung J. Risk factors for acute cardioembolic brain stroke in acute myocardial infarction. Korean Circulation J. 2005;35:353-356.
6. Witt BJ, Ballman KV, Brown RD Jr, Meverden RA, Jacobsen SJ, Roger VL. The incidence of stroke after myocardial infarction: a meta-analysis. Am J Med. 2006;119(4):354.e1-e9.
7. Feher G, Tibold A, Kotlani K, Szapary L. The clinical importance of troponin elevation in ischaemic cerebrovascular events: a clinical review. Journal of Cardiology and Therapy. 2014;1(7):141-149.
8. Maciel R, Palma R, Sousa P, Ferreira F, Nzwalo H. Acute stroke with concomitant acute myocardial infarction: will you thrombolyse? J Stroke. 2015;17(1):84-86.
9. Toyoda K, Yasaka M, Iwade K, et al; Bleeding with Antithrombotic Therapy (BAT) Study Group. Dual antithrombotic therapy increases severe bleeding events in patients with stroke and cardiovascular disease: a prospective, multicenter, observational study. Stroke. 2008;39(6):1740-1745.
10. Omar HR, Mangar D, Camporesi EM. Simultaneous thrombosis of 2 vascular territories: is thrombolytic therapy a better option? Am J Emerg Med. 2013;31(9):1412-1413.
A 61-year-old woman without any known medical history presented with a chief complaint of right arm numbness and right-sided scalp numbness that had started 2 days earlier. She described a “pins and needles” sensation in her right upper extremity and right scalp, and said the numbness in her scalp was especially noticeable when she combed her hair. The patient denied any chest pain, shortness of breath, weakness in her arms or legs, headache, or blurred vision.
She said that 1 day prior to the onset of the paresthesias, she woke up fatigued and vomited once. Throughout that day, she had symptoms of nausea and fatigue, and sought treatment at an urgent care center that afternoon. At the urgent care center, she was diagnosed with a “stomach virus” and was given an antiemetic. The next day, her nausea improved, but the paresthesias began in her right hand and scalp. On the third day, the patient went to work, but the persistent paresthesias caused her to visit her primary care physician, who sent her to our ED for further work-up.
The patient said she had been in good health until 3 days ago. She reported no medical problems and was taking no medications. The patient denied smoking or using alcohol; her family history was significant only in that her father had a myocardial infarction (MI) while in his 50s.
On physical examination, the patient was alert, oriented, and in no apparent distress. Her body mass index was 28.3 kg/m2. Vital signs were: temperature, 99.2°F; blood pressure, 113/73 mm Hg; heart rate, 93 beats/minute; and respiratory rate, 18 breaths/minute. Oxygen saturation was 95% on room air.
Her head was normocephalic and atraumatic, and her eyes, ears, nose, and throat were normal. Her neck was supple and without jugular vein distension. The cardiac examination revealed normal heart sounds without murmurs, rubs, or gallops. Her lungs were clear without rales, wheezes, or rhonchi. Her abdomen was soft, without tenderness, guarding, or rebound, and she had normal bowel sounds.
Her musculoskeletal examination was normal, with +5/5 strength bilaterally in her upper and lower extremities. The patient’s skin examination also was normal. On neurological examination, her right upper extremity and right side of her face were noted to have decreased sensation via pinprick compared to the left side, but the examination was otherwise normal. The National Institutes of Health Stroke Scale score was 1.
The patient’s electrocardiogram (ECG) showed a normal sinus rhythm (rate, 90 beats/min), a lateral infarct of undetermined age, and a left atrial abnormality. Laboratory evaluation was significant only for a brain natriuretic peptide level of 334 pg/mL, a creatine phosphokinase (CPK) level of 782 IU/L, and a troponin I level of >50 ng/mL (Table). Serial cardiac enzyme levels were obtained and showed a decline of CPK from 782 IU/L to 331 IU/L over the following 36 hours. However, the troponin I levels remained >50 ng/mL for 5 days and then declined to 31.6 ng/mL.
A computed tomography (CT) scan of the brain without contrast revealed an acute to subacute infarct in the left occipital and left thalamic regions (Figure). A stat transthoracic echocardiogram (ECHO) performed in the ED revealed a dilated left ventricle with an ejection fraction of 20% to 25%, along with a hypokinetic anterolateral wall and an akinetic inferolateral wall. No atrial thrombus was visible on the ECHO. Doppler studies of the patient’s lower extremities were negative for deep vein thrombosis. Magnetic resonance imaging of her brain showed an infarct in the posterior circulation distribution involving the left occipital lobes and small areas in the left thalamic and right parietal-occipital regions. Hemorrhagic conversion of the left occipital infarct without mass effect was also noted. The patient was admitted to the neurological intensive care unit for frequent neurological examinations and close monitoring for worsening cerebral hemorrhage.
When the patient had still been in the ED, cardiology services were consulted; the cardiologist initiated a heparin drip with close monitoring of the coagulation studies. Cardiac catheterization was not done immediately because the ECG did not show acute ST elevations. The day after her presentation to the ED, the patient underwent a primary percutaneous coronary catheterization and was found to have a small rudimentary left anterior descending artery, with only small branches supplying the septal region. The right circumflex artery was very large and was supplying the lateral wall. No stents were placed during this procedure. A transesophageal ECHO (TEE) showed no evidence of a left atrial appendage thrombus.
The patient experienced an episode of coffee ground emesis while undergoing the TEE. Her hemoglobin declined from 11.9 g/dL to 7.9 g/dL, which led to a transfusion of 2 U of packed red blood cells and platelets. Heparin was discontinued and a proton pump inhibitor was started; however, no endoscopy was done at that time.
Throughout her stay, the patient was continuously monitored, but no evidence of arrhythmia or atrial fibrillation was found. Upon discharge, the neurologist recommended the patient receive clopidogrel and aspirin therapy for 3 months with subsequent aspirin monotherapy afterward. The patient was discharged after 10 days in the hospital.
Discussion
Although she had an MI, the patient presented here did not experience any chest pain. Her chief complaint in the ED was paresthesias related to her concomitant stroke, and only on further probing did she describe the additional symptoms of fatigability and vomiting.
Since heart disease and stroke share common risk factors and pathophysiology, acute cerebral ischemic events may happen concurrently with MIs. In a review of studies that included approximately 2,900 patients who had an acute stroke, Kerr et al1 found that 20% had elevated troponin levels within 7 days of the stroke. In 2013, the American Heart Association and American Stroke Association published guidelines advising that all patients who present with acute cerebral ischemia have an emergent ECG and baseline troponin level.2 This was in response to evidence that even low positive troponin levels have been associated with an increased risk of mortality.3 Positive troponin levels are especially important because fatal and nonfatal stroke post-MI events have been found to be increasing in frequency for women, even though there has been a significant overall reduction in post-MI mortality.4 Patients who have an ischemic stroke concurrently with an acute MI or soon after have an overall poorer clinical prognosis.5
For emergency physicians (EPs), this is a “chicken or the egg” scenario. It is difficult to determine which came first: the MI or the cerebral ischemia. Similar risk factors can result in an acute embolic event from revascularization, atrial fibrillation without proper anticoagulation, or a poorly functioning left ventricle.6 It is important to remember that regardless of the order of occurrence, the incidence of ischemic stroke is markedly increased in conjunction with an acute MI.7 Several theories have been advanced regarding the relationship between ischemic stroke and acute MI. One theory proposes that elevated troponin levels could be related to a large catecholamine release after a cerebral ischemic stroke, resulting in subsequent myocardial injury or cardiomyopathy.7 However, this theory remains controversial.
Management
The major consideration for the EP is whether or not to give thrombolytics to a patient who presents with concomitant acute MI and ischemic stroke. An acute MI within the 3 months preceding an acute stroke is considered a relative contraindication for intravenous tissue plasminogen activator (tPA).8 It has also been found, albeit rarely, that there is an increased risk of cardiac rupture or tamponade due to the breakdown of the fibrin clot within the necrotic cardiac tissue.8
How should patients with stroke complicated by acute cardiac compromise be managed in the ED? One acute vascular event cannot be ignored while addressing the other. There are no evidence-based guidelines for the management of patients who present with this picture.8 In addition, no published clinical studies have focused on the decision-making process for these patients.8
Immediate percutaneous coronary intervention for the MI performed on such patients would prevent the use of tPA for the acute stroke. Though any anticoagulation increases the risk for postischemic cerebral hemorrhage, heparin is necessary to prevent the formation of a left ventricle thrombus.7 Alternately, mechanical thrombectomy and cardiac catheterization may be combined as emergent treatments for these patients, but performing these two procedures simultaneously is not widely available.
For a patient who presents to the ED within both the cardiac and stroke treatment windows, tPA might be a viable option, and the only one readily valuable.8 However, the EP must be mindful of the varying dosages of tPA and means of administration for different thrombosis sites. Also, care must be taken when treating a patient with dual or triple antiplatelet therapy because of the increased risk of hemorrhage.9 Currently, no safe standardized regimens have been established, and further trials need to be performed.10
If the patient in this case report had presented at our ED with only signs and symptoms of an MI, typically she would have been treated with heparin, aspirin, and an urgent cardiac catheterization. If she had presented with only signs and symptoms of a stroke, she would have been treated with full-dose aspirin and worked up from a neurological perspective. Because she had signs and symptoms of both, she presented a dilemma. She was initially treated with heparin to prevent a thrombus formation, but then later changed to only clopidogrel and aspirin to prevent further episodes of coffee ground emesis or worsening hemorrhagic conversion.
Conclusion
Common risk factors for cardiac and cerebral ischemic events may result in a patient presenting with both acute MI and an acute cerebral ischemic event. There have not been sufficient clinical studies to determine the best decision-making process for these patients. Therefore, patients with this complicated presentation must be assessed on an individual basis. Current treatment options are varied and are based according to history of the present illness, time of presentation to the ED, and the available resources within the hospital.
A 61-year-old woman without any known medical history presented with a chief complaint of right arm numbness and right-sided scalp numbness that had started 2 days earlier. She described a “pins and needles” sensation in her right upper extremity and right scalp, and said the numbness in her scalp was especially noticeable when she combed her hair. The patient denied any chest pain, shortness of breath, weakness in her arms or legs, headache, or blurred vision.
She said that 1 day prior to the onset of the paresthesias, she woke up fatigued and vomited once. Throughout that day, she had symptoms of nausea and fatigue, and sought treatment at an urgent care center that afternoon. At the urgent care center, she was diagnosed with a “stomach virus” and was given an antiemetic. The next day, her nausea improved, but the paresthesias began in her right hand and scalp. On the third day, the patient went to work, but the persistent paresthesias caused her to visit her primary care physician, who sent her to our ED for further work-up.
The patient said she had been in good health until 3 days ago. She reported no medical problems and was taking no medications. The patient denied smoking or using alcohol; her family history was significant only in that her father had a myocardial infarction (MI) while in his 50s.
On physical examination, the patient was alert, oriented, and in no apparent distress. Her body mass index was 28.3 kg/m2. Vital signs were: temperature, 99.2°F; blood pressure, 113/73 mm Hg; heart rate, 93 beats/minute; and respiratory rate, 18 breaths/minute. Oxygen saturation was 95% on room air.
Her head was normocephalic and atraumatic, and her eyes, ears, nose, and throat were normal. Her neck was supple and without jugular vein distension. The cardiac examination revealed normal heart sounds without murmurs, rubs, or gallops. Her lungs were clear without rales, wheezes, or rhonchi. Her abdomen was soft, without tenderness, guarding, or rebound, and she had normal bowel sounds.
Her musculoskeletal examination was normal, with +5/5 strength bilaterally in her upper and lower extremities. The patient’s skin examination also was normal. On neurological examination, her right upper extremity and right side of her face were noted to have decreased sensation via pinprick compared to the left side, but the examination was otherwise normal. The National Institutes of Health Stroke Scale score was 1.
The patient’s electrocardiogram (ECG) showed a normal sinus rhythm (rate, 90 beats/min), a lateral infarct of undetermined age, and a left atrial abnormality. Laboratory evaluation was significant only for a brain natriuretic peptide level of 334 pg/mL, a creatine phosphokinase (CPK) level of 782 IU/L, and a troponin I level of >50 ng/mL (Table). Serial cardiac enzyme levels were obtained and showed a decline of CPK from 782 IU/L to 331 IU/L over the following 36 hours. However, the troponin I levels remained >50 ng/mL for 5 days and then declined to 31.6 ng/mL.
A computed tomography (CT) scan of the brain without contrast revealed an acute to subacute infarct in the left occipital and left thalamic regions (Figure). A stat transthoracic echocardiogram (ECHO) performed in the ED revealed a dilated left ventricle with an ejection fraction of 20% to 25%, along with a hypokinetic anterolateral wall and an akinetic inferolateral wall. No atrial thrombus was visible on the ECHO. Doppler studies of the patient’s lower extremities were negative for deep vein thrombosis. Magnetic resonance imaging of her brain showed an infarct in the posterior circulation distribution involving the left occipital lobes and small areas in the left thalamic and right parietal-occipital regions. Hemorrhagic conversion of the left occipital infarct without mass effect was also noted. The patient was admitted to the neurological intensive care unit for frequent neurological examinations and close monitoring for worsening cerebral hemorrhage.
When the patient had still been in the ED, cardiology services were consulted; the cardiologist initiated a heparin drip with close monitoring of the coagulation studies. Cardiac catheterization was not done immediately because the ECG did not show acute ST elevations. The day after her presentation to the ED, the patient underwent a primary percutaneous coronary catheterization and was found to have a small rudimentary left anterior descending artery, with only small branches supplying the septal region. The right circumflex artery was very large and was supplying the lateral wall. No stents were placed during this procedure. A transesophageal ECHO (TEE) showed no evidence of a left atrial appendage thrombus.
The patient experienced an episode of coffee ground emesis while undergoing the TEE. Her hemoglobin declined from 11.9 g/dL to 7.9 g/dL, which led to a transfusion of 2 U of packed red blood cells and platelets. Heparin was discontinued and a proton pump inhibitor was started; however, no endoscopy was done at that time.
Throughout her stay, the patient was continuously monitored, but no evidence of arrhythmia or atrial fibrillation was found. Upon discharge, the neurologist recommended the patient receive clopidogrel and aspirin therapy for 3 months with subsequent aspirin monotherapy afterward. The patient was discharged after 10 days in the hospital.
Discussion
Although she had an MI, the patient presented here did not experience any chest pain. Her chief complaint in the ED was paresthesias related to her concomitant stroke, and only on further probing did she describe the additional symptoms of fatigability and vomiting.
Since heart disease and stroke share common risk factors and pathophysiology, acute cerebral ischemic events may happen concurrently with MIs. In a review of studies that included approximately 2,900 patients who had an acute stroke, Kerr et al1 found that 20% had elevated troponin levels within 7 days of the stroke. In 2013, the American Heart Association and American Stroke Association published guidelines advising that all patients who present with acute cerebral ischemia have an emergent ECG and baseline troponin level.2 This was in response to evidence that even low positive troponin levels have been associated with an increased risk of mortality.3 Positive troponin levels are especially important because fatal and nonfatal stroke post-MI events have been found to be increasing in frequency for women, even though there has been a significant overall reduction in post-MI mortality.4 Patients who have an ischemic stroke concurrently with an acute MI or soon after have an overall poorer clinical prognosis.5
For emergency physicians (EPs), this is a “chicken or the egg” scenario. It is difficult to determine which came first: the MI or the cerebral ischemia. Similar risk factors can result in an acute embolic event from revascularization, atrial fibrillation without proper anticoagulation, or a poorly functioning left ventricle.6 It is important to remember that regardless of the order of occurrence, the incidence of ischemic stroke is markedly increased in conjunction with an acute MI.7 Several theories have been advanced regarding the relationship between ischemic stroke and acute MI. One theory proposes that elevated troponin levels could be related to a large catecholamine release after a cerebral ischemic stroke, resulting in subsequent myocardial injury or cardiomyopathy.7 However, this theory remains controversial.
Management
The major consideration for the EP is whether or not to give thrombolytics to a patient who presents with concomitant acute MI and ischemic stroke. An acute MI within the 3 months preceding an acute stroke is considered a relative contraindication for intravenous tissue plasminogen activator (tPA).8 It has also been found, albeit rarely, that there is an increased risk of cardiac rupture or tamponade due to the breakdown of the fibrin clot within the necrotic cardiac tissue.8
How should patients with stroke complicated by acute cardiac compromise be managed in the ED? One acute vascular event cannot be ignored while addressing the other. There are no evidence-based guidelines for the management of patients who present with this picture.8 In addition, no published clinical studies have focused on the decision-making process for these patients.8
Immediate percutaneous coronary intervention for the MI performed on such patients would prevent the use of tPA for the acute stroke. Though any anticoagulation increases the risk for postischemic cerebral hemorrhage, heparin is necessary to prevent the formation of a left ventricle thrombus.7 Alternately, mechanical thrombectomy and cardiac catheterization may be combined as emergent treatments for these patients, but performing these two procedures simultaneously is not widely available.
For a patient who presents to the ED within both the cardiac and stroke treatment windows, tPA might be a viable option, and the only one readily valuable.8 However, the EP must be mindful of the varying dosages of tPA and means of administration for different thrombosis sites. Also, care must be taken when treating a patient with dual or triple antiplatelet therapy because of the increased risk of hemorrhage.9 Currently, no safe standardized regimens have been established, and further trials need to be performed.10
If the patient in this case report had presented at our ED with only signs and symptoms of an MI, typically she would have been treated with heparin, aspirin, and an urgent cardiac catheterization. If she had presented with only signs and symptoms of a stroke, she would have been treated with full-dose aspirin and worked up from a neurological perspective. Because she had signs and symptoms of both, she presented a dilemma. She was initially treated with heparin to prevent a thrombus formation, but then later changed to only clopidogrel and aspirin to prevent further episodes of coffee ground emesis or worsening hemorrhagic conversion.
Conclusion
Common risk factors for cardiac and cerebral ischemic events may result in a patient presenting with both acute MI and an acute cerebral ischemic event. There have not been sufficient clinical studies to determine the best decision-making process for these patients. Therefore, patients with this complicated presentation must be assessed on an individual basis. Current treatment options are varied and are based according to history of the present illness, time of presentation to the ED, and the available resources within the hospital.
1. Kerr G, Ray G, Wu O, Stott DJ, Langhorne P. Elevated troponin after stroke: a systematic review. Cerebrovasc Dis. 2009;28(3):220-226.
2. Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870-947.
3. Di Angelantonio E, Fiorelli M, Toni D, et al. Prognostic significance of admission levels of troponin I in patients with acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2005;76(1):76-81.
4. Shiue I, Hristova K, Sharma J. Correspondence: gender and outcome from acute myocardial infarction and secondary stoke. Br J Cardiology. 2014;21:90.
5. Park S, Jung J. Risk factors for acute cardioembolic brain stroke in acute myocardial infarction. Korean Circulation J. 2005;35:353-356.
6. Witt BJ, Ballman KV, Brown RD Jr, Meverden RA, Jacobsen SJ, Roger VL. The incidence of stroke after myocardial infarction: a meta-analysis. Am J Med. 2006;119(4):354.e1-e9.
7. Feher G, Tibold A, Kotlani K, Szapary L. The clinical importance of troponin elevation in ischaemic cerebrovascular events: a clinical review. Journal of Cardiology and Therapy. 2014;1(7):141-149.
8. Maciel R, Palma R, Sousa P, Ferreira F, Nzwalo H. Acute stroke with concomitant acute myocardial infarction: will you thrombolyse? J Stroke. 2015;17(1):84-86.
9. Toyoda K, Yasaka M, Iwade K, et al; Bleeding with Antithrombotic Therapy (BAT) Study Group. Dual antithrombotic therapy increases severe bleeding events in patients with stroke and cardiovascular disease: a prospective, multicenter, observational study. Stroke. 2008;39(6):1740-1745.
10. Omar HR, Mangar D, Camporesi EM. Simultaneous thrombosis of 2 vascular territories: is thrombolytic therapy a better option? Am J Emerg Med. 2013;31(9):1412-1413.
1. Kerr G, Ray G, Wu O, Stott DJ, Langhorne P. Elevated troponin after stroke: a systematic review. Cerebrovasc Dis. 2009;28(3):220-226.
2. Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870-947.
3. Di Angelantonio E, Fiorelli M, Toni D, et al. Prognostic significance of admission levels of troponin I in patients with acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2005;76(1):76-81.
4. Shiue I, Hristova K, Sharma J. Correspondence: gender and outcome from acute myocardial infarction and secondary stoke. Br J Cardiology. 2014;21:90.
5. Park S, Jung J. Risk factors for acute cardioembolic brain stroke in acute myocardial infarction. Korean Circulation J. 2005;35:353-356.
6. Witt BJ, Ballman KV, Brown RD Jr, Meverden RA, Jacobsen SJ, Roger VL. The incidence of stroke after myocardial infarction: a meta-analysis. Am J Med. 2006;119(4):354.e1-e9.
7. Feher G, Tibold A, Kotlani K, Szapary L. The clinical importance of troponin elevation in ischaemic cerebrovascular events: a clinical review. Journal of Cardiology and Therapy. 2014;1(7):141-149.
8. Maciel R, Palma R, Sousa P, Ferreira F, Nzwalo H. Acute stroke with concomitant acute myocardial infarction: will you thrombolyse? J Stroke. 2015;17(1):84-86.
9. Toyoda K, Yasaka M, Iwade K, et al; Bleeding with Antithrombotic Therapy (BAT) Study Group. Dual antithrombotic therapy increases severe bleeding events in patients with stroke and cardiovascular disease: a prospective, multicenter, observational study. Stroke. 2008;39(6):1740-1745.
10. Omar HR, Mangar D, Camporesi EM. Simultaneous thrombosis of 2 vascular territories: is thrombolytic therapy a better option? Am J Emerg Med. 2013;31(9):1412-1413.