User login
I-SPY 2 graduates two neoadjuvant treatments to phase III trials
Taking a step toward the goal of personalized medicine, investigators in the multicenter, adaptive I-SPY 2 trial report that tailoring neoadjuvant therapy combinations to specific cancer subtypes in women with high-risk breast cancer will likely result in higher rates of pathological complete responses for at least two subtypes, including patients with triple-negative disease.
Among women with triple-negative breast cancer (tumors lacking human epidermal growth factor receptor 2 [HER2], estrogen, and progesterone receptors) in the phase II trial, a combination of the poly (ADP-ribose) polymerase (PARP) inhibitor veliparib and carboplatin added to paclitaxel was associated with an estimated 51% pathological complete response (pCR) rate, compared with 26% for patients treated with weekly paclitaxel alone. The predicted probability of success in a phase III trial with the combination was 88%, reported Hope S. Rugo, MD, director of the Breast Oncology Clinical Trials Program at the University of California San Francisco, and her colleagues in I-SPY 2.
“The experience with veliparib-carboplatin in our trial shows the advantage of an adaptively randomized phase II platform trial for matching therapies with biomarker subsets to better inform the design of phase III trials so that they can be more focused, smaller, and faster. Future patients stand to benefit, but trial participants benefit as well in that exposure to ineffective therapy is minimized,” the investigators wrote (N Engl J Med. 2016 July 7. doi: 10.1056/NEJMoa1513749).
Partial results from this trial were reported at the 2013 San Antonio Breast Cancer Symposium.
On the basis of these phase II data, an ongoing phase III neoadjuvant trial is comparing standard chemotherapy alone, with carboplatin, or with veliparib plus carboplatin as treatment for triple-negative breast cancer, Dr. Rugo and her associates said.
In another arm of I-SPY 2 involving a subset of patients with HER2-positive, hormone receptor–negative cancers, the mean estimated pCR rate was 56% for patients treated with the investigational tyrosine-kinase inhibitor (TKI) neratinib, compared with an estimated 33% among patients treated with anti-HER2 agent trastuzumab (Herceptin). All the participants received standard neoadjuvant therapy, which consisted of 12 cycles of paclitaxel, followed by 4 cycles of doxorubicin and cyclophosphamide. The estimated probability of success in phase III with neratinib was 79%, reported John W. Park, MD, of University of California San Francisco, and his coauthors on behalf of I-SPY 2 investigators (N Engl J Med. 2016 July 7. doi: 10.1056/NEJMoa1513750).
On the basis of this phase II study, and to reflect the current standard of dual HER-targeting, the investigators are proceeding with a phase III trial comparing neratinib as neoadjuvant therapy added to pertuzumab (Perjeta), trastuzumab, and a taxane vs. the three latter drugs, and against a combination of neratinib, trastuzumab, and taxane, all followed by doxorubicin and cyclophosphamide.
Partial results of the phase II trial were reported at the 2013 annual meeting of the American Association for Cancer Research.
Nimble trial, tailored therapies
I-SPY 2 (Investigation of Serial Studies to Predict Your Therapeutic Response through Imaging and Molecular Analysis 2) is an ongoing “platform” trial exploring the use of new drugs combined with a standard neoadjuvant therapy backbone for the treatment of high-risk cancers.
Women with stage II or III breast cancers with tumors 2.5 cm or larger are assessed for one of eight biomarker subtypes according to HER2 (human epidermal growth factor receptor 2) status, hormone receptor status, and genetic risk factors based on a 70-gene assay. The patients are then randomized within each biomarker subtype to receive standard therapy with or without an investigational agent.
Each sub-trial has a primary endpoint of an improvement in pathological complete response compared with the standard of care. Changes in tumor volume on MRI are used to predict whether patients will achieve a pCR. Regimens that have a high Bayesian predictive probability of success in a subsequent phase III neoadjuvant trial within the biomarker signature in which they performed well are eligible for moving on to phase III trials.
Triple-negative disease
Among the subgroup of patients with triple-negative disease, 72 were assigned to receive veliparib 50 mg by mouth twice daily for 12 weeks, plus carboplatin at a dose intended to achieve a pharmacologic area under the concentration versus time curve of 6 mg/hour per liter on weeks 1, 4, 7, and 10, plus intravenous paclitaxel at a dose of 80 mg/m2. An additional 44 patients (controls) were randomized to receive paclitaxel alone.
Following paclitaxel alone or with the combination, all patients received doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 every 2-3 weeks for four doses, followed by myeloid growth factor support as appropriate. Following treatment, all patients underwent surgery that included axillary node sampling in accordance with National Comprehensive Cancer Network (NCCN) and local practice guidelines. Adjuvant radiation and endocrine therapy were recommended in accordance with standard guidelines.
The rate of grade 3 or greater hematologic toxic effects in this trial arm was higher in patients treated with veliparib-carboplatin, with neutropenia rates of 71% versus 2% for controls. Adverse events occurring only in patients on veliparib-carboplatin were thrombocytopenia in 21%, anemia in 28%, and febrile neutropenia in 1%. Among patients who had received the combination, toxic effects were higher during doxorubicin-cyclophosphamide therapy.
HR-negative disease
Patients with hormone receptor–negative disease received standard neoadjuvant chemotherapy with 12 weekly cycles of paclitaxel followed by 4 cycles of doxorubicin and cyclophosphamide as described before, with or without oral neratinib 240 mg per day. Patients in the control group who had HER2-positive cancers also received trastuzumab for the first 12 weeks with a loading dose of 4 mg per kilogram of body weight in the first cycle, followed by a maintenance dose of 2 mg per kilogram in cycles 2 through 12.
Surgery, including sentinel-node dissection in patients with node-negative cancer and axillary-node dissection in those with node-positive cancer at diagnosis, was performed according to NCCN and local practice guidelines, and adjuvant radiation and endocrine therapy were recommended according to standard guidelines.
The protocol was modified to included diarrhea prophylaxis with loperamide among patients assigned to receive neratinib.
A total of 127 patients were assigned to neratinib, and 115 of these patients were evaluable for response. Controls included 84 patients, of whom 78 were evaluable. At baseline, more patients in the neratinib group had HER2-positive tumors. Neratinib reached the prespecified efficacy threshold only within the HER2-positive, HR-negative group.
Diarrhea was the most common adverse event, with grade 3 or greater diarrhea occurring among 38% of patients assigned to neratinib. Vomiting and elevated liver enzymes were also more frequent with neratinib.
I-SPY 2 is supported by QuantumLeap Healthcare Collaborative, the Foundation for the National Institutes of Health (from 2010 through 2012) and the National Cancer Institute. Dr. Park reported receiving lecture fees and travel support from Genentech and Pfizer, and receiving royalties from patents. Dr. Rugo reported grants to her institution from BioMarin, and unpaid steering committee participation for BioMarin and AbbVie. Multiple co-authors reported financial relationships of various kinds.
These two multicenter trials may ultimately lead to changes in treatment in the years ahead. The investigators created a collaborative culture around these studies, and the work that appears in the Journal would not have been possible absent that spirit of cooperation and collective creativity. However, these trials were not designed to predict the ultimate success of either neratinib or carboplatin-veliparib in improving disease-free or overall survival. Instead, they predict a positive result with the use of pathological complete response rate as an endpoint in a definitive neoadjuvant study.
Clinicians should remember that pathological complete response rate itself is not a clinically meaningful endpoint; its value is as a surrogate for outcome. Although pathological complete response rate is consistently associated with a decreased risk of relapse and death for individual patients, even substantial improvements in pathological complete response rate in neoadjuvant trials have not consistently translated into improvement in long-term outcomes. The reasons for this are myriad, including the molecular heterogeneity of breast cancer and the possible effect of postsurgical interventions. Most importantly, pathological complete response rate will correlate with survival outcomes only if the neoadjuvant agents leading to the improvement in pathological complete response also eradicate resistant tumor clones.
At this time, improvements in pathological complete response rates as reported in neoadjuvant studies – whether the studies are exploratory, such as I-SPY 2, or more definitive – should not change clinical practice; rather, we should wait for the definitive clinical trials that result from them. Nonetheless, standard neoadjuvant therapy remains a sound clinical approach with the potential to individualize therapy. It also remains a valuable research tool that has the potential to help us develop hypotheses and explore mechanisms of drug resistance.
Lisa A. Carey, MD., is with the UNC Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, and Eric P. Winer, MD, is with the Breast Oncology Program, Dana-Farber Cancer Center, Boston. These comments are excerpted from an editorial (N Engl J Med. 2016 July 7. doi: 10.1056/NEJMe1603691).
These two multicenter trials may ultimately lead to changes in treatment in the years ahead. The investigators created a collaborative culture around these studies, and the work that appears in the Journal would not have been possible absent that spirit of cooperation and collective creativity. However, these trials were not designed to predict the ultimate success of either neratinib or carboplatin-veliparib in improving disease-free or overall survival. Instead, they predict a positive result with the use of pathological complete response rate as an endpoint in a definitive neoadjuvant study.
Clinicians should remember that pathological complete response rate itself is not a clinically meaningful endpoint; its value is as a surrogate for outcome. Although pathological complete response rate is consistently associated with a decreased risk of relapse and death for individual patients, even substantial improvements in pathological complete response rate in neoadjuvant trials have not consistently translated into improvement in long-term outcomes. The reasons for this are myriad, including the molecular heterogeneity of breast cancer and the possible effect of postsurgical interventions. Most importantly, pathological complete response rate will correlate with survival outcomes only if the neoadjuvant agents leading to the improvement in pathological complete response also eradicate resistant tumor clones.
At this time, improvements in pathological complete response rates as reported in neoadjuvant studies – whether the studies are exploratory, such as I-SPY 2, or more definitive – should not change clinical practice; rather, we should wait for the definitive clinical trials that result from them. Nonetheless, standard neoadjuvant therapy remains a sound clinical approach with the potential to individualize therapy. It also remains a valuable research tool that has the potential to help us develop hypotheses and explore mechanisms of drug resistance.
Lisa A. Carey, MD., is with the UNC Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, and Eric P. Winer, MD, is with the Breast Oncology Program, Dana-Farber Cancer Center, Boston. These comments are excerpted from an editorial (N Engl J Med. 2016 July 7. doi: 10.1056/NEJMe1603691).
These two multicenter trials may ultimately lead to changes in treatment in the years ahead. The investigators created a collaborative culture around these studies, and the work that appears in the Journal would not have been possible absent that spirit of cooperation and collective creativity. However, these trials were not designed to predict the ultimate success of either neratinib or carboplatin-veliparib in improving disease-free or overall survival. Instead, they predict a positive result with the use of pathological complete response rate as an endpoint in a definitive neoadjuvant study.
Clinicians should remember that pathological complete response rate itself is not a clinically meaningful endpoint; its value is as a surrogate for outcome. Although pathological complete response rate is consistently associated with a decreased risk of relapse and death for individual patients, even substantial improvements in pathological complete response rate in neoadjuvant trials have not consistently translated into improvement in long-term outcomes. The reasons for this are myriad, including the molecular heterogeneity of breast cancer and the possible effect of postsurgical interventions. Most importantly, pathological complete response rate will correlate with survival outcomes only if the neoadjuvant agents leading to the improvement in pathological complete response also eradicate resistant tumor clones.
At this time, improvements in pathological complete response rates as reported in neoadjuvant studies – whether the studies are exploratory, such as I-SPY 2, or more definitive – should not change clinical practice; rather, we should wait for the definitive clinical trials that result from them. Nonetheless, standard neoadjuvant therapy remains a sound clinical approach with the potential to individualize therapy. It also remains a valuable research tool that has the potential to help us develop hypotheses and explore mechanisms of drug resistance.
Lisa A. Carey, MD., is with the UNC Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, and Eric P. Winer, MD, is with the Breast Oncology Program, Dana-Farber Cancer Center, Boston. These comments are excerpted from an editorial (N Engl J Med. 2016 July 7. doi: 10.1056/NEJMe1603691).
Taking a step toward the goal of personalized medicine, investigators in the multicenter, adaptive I-SPY 2 trial report that tailoring neoadjuvant therapy combinations to specific cancer subtypes in women with high-risk breast cancer will likely result in higher rates of pathological complete responses for at least two subtypes, including patients with triple-negative disease.
Among women with triple-negative breast cancer (tumors lacking human epidermal growth factor receptor 2 [HER2], estrogen, and progesterone receptors) in the phase II trial, a combination of the poly (ADP-ribose) polymerase (PARP) inhibitor veliparib and carboplatin added to paclitaxel was associated with an estimated 51% pathological complete response (pCR) rate, compared with 26% for patients treated with weekly paclitaxel alone. The predicted probability of success in a phase III trial with the combination was 88%, reported Hope S. Rugo, MD, director of the Breast Oncology Clinical Trials Program at the University of California San Francisco, and her colleagues in I-SPY 2.
“The experience with veliparib-carboplatin in our trial shows the advantage of an adaptively randomized phase II platform trial for matching therapies with biomarker subsets to better inform the design of phase III trials so that they can be more focused, smaller, and faster. Future patients stand to benefit, but trial participants benefit as well in that exposure to ineffective therapy is minimized,” the investigators wrote (N Engl J Med. 2016 July 7. doi: 10.1056/NEJMoa1513749).
Partial results from this trial were reported at the 2013 San Antonio Breast Cancer Symposium.
On the basis of these phase II data, an ongoing phase III neoadjuvant trial is comparing standard chemotherapy alone, with carboplatin, or with veliparib plus carboplatin as treatment for triple-negative breast cancer, Dr. Rugo and her associates said.
In another arm of I-SPY 2 involving a subset of patients with HER2-positive, hormone receptor–negative cancers, the mean estimated pCR rate was 56% for patients treated with the investigational tyrosine-kinase inhibitor (TKI) neratinib, compared with an estimated 33% among patients treated with anti-HER2 agent trastuzumab (Herceptin). All the participants received standard neoadjuvant therapy, which consisted of 12 cycles of paclitaxel, followed by 4 cycles of doxorubicin and cyclophosphamide. The estimated probability of success in phase III with neratinib was 79%, reported John W. Park, MD, of University of California San Francisco, and his coauthors on behalf of I-SPY 2 investigators (N Engl J Med. 2016 July 7. doi: 10.1056/NEJMoa1513750).
On the basis of this phase II study, and to reflect the current standard of dual HER-targeting, the investigators are proceeding with a phase III trial comparing neratinib as neoadjuvant therapy added to pertuzumab (Perjeta), trastuzumab, and a taxane vs. the three latter drugs, and against a combination of neratinib, trastuzumab, and taxane, all followed by doxorubicin and cyclophosphamide.
Partial results of the phase II trial were reported at the 2013 annual meeting of the American Association for Cancer Research.
Nimble trial, tailored therapies
I-SPY 2 (Investigation of Serial Studies to Predict Your Therapeutic Response through Imaging and Molecular Analysis 2) is an ongoing “platform” trial exploring the use of new drugs combined with a standard neoadjuvant therapy backbone for the treatment of high-risk cancers.
Women with stage II or III breast cancers with tumors 2.5 cm or larger are assessed for one of eight biomarker subtypes according to HER2 (human epidermal growth factor receptor 2) status, hormone receptor status, and genetic risk factors based on a 70-gene assay. The patients are then randomized within each biomarker subtype to receive standard therapy with or without an investigational agent.
Each sub-trial has a primary endpoint of an improvement in pathological complete response compared with the standard of care. Changes in tumor volume on MRI are used to predict whether patients will achieve a pCR. Regimens that have a high Bayesian predictive probability of success in a subsequent phase III neoadjuvant trial within the biomarker signature in which they performed well are eligible for moving on to phase III trials.
Triple-negative disease
Among the subgroup of patients with triple-negative disease, 72 were assigned to receive veliparib 50 mg by mouth twice daily for 12 weeks, plus carboplatin at a dose intended to achieve a pharmacologic area under the concentration versus time curve of 6 mg/hour per liter on weeks 1, 4, 7, and 10, plus intravenous paclitaxel at a dose of 80 mg/m2. An additional 44 patients (controls) were randomized to receive paclitaxel alone.
Following paclitaxel alone or with the combination, all patients received doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 every 2-3 weeks for four doses, followed by myeloid growth factor support as appropriate. Following treatment, all patients underwent surgery that included axillary node sampling in accordance with National Comprehensive Cancer Network (NCCN) and local practice guidelines. Adjuvant radiation and endocrine therapy were recommended in accordance with standard guidelines.
The rate of grade 3 or greater hematologic toxic effects in this trial arm was higher in patients treated with veliparib-carboplatin, with neutropenia rates of 71% versus 2% for controls. Adverse events occurring only in patients on veliparib-carboplatin were thrombocytopenia in 21%, anemia in 28%, and febrile neutropenia in 1%. Among patients who had received the combination, toxic effects were higher during doxorubicin-cyclophosphamide therapy.
HR-negative disease
Patients with hormone receptor–negative disease received standard neoadjuvant chemotherapy with 12 weekly cycles of paclitaxel followed by 4 cycles of doxorubicin and cyclophosphamide as described before, with or without oral neratinib 240 mg per day. Patients in the control group who had HER2-positive cancers also received trastuzumab for the first 12 weeks with a loading dose of 4 mg per kilogram of body weight in the first cycle, followed by a maintenance dose of 2 mg per kilogram in cycles 2 through 12.
Surgery, including sentinel-node dissection in patients with node-negative cancer and axillary-node dissection in those with node-positive cancer at diagnosis, was performed according to NCCN and local practice guidelines, and adjuvant radiation and endocrine therapy were recommended according to standard guidelines.
The protocol was modified to included diarrhea prophylaxis with loperamide among patients assigned to receive neratinib.
A total of 127 patients were assigned to neratinib, and 115 of these patients were evaluable for response. Controls included 84 patients, of whom 78 were evaluable. At baseline, more patients in the neratinib group had HER2-positive tumors. Neratinib reached the prespecified efficacy threshold only within the HER2-positive, HR-negative group.
Diarrhea was the most common adverse event, with grade 3 or greater diarrhea occurring among 38% of patients assigned to neratinib. Vomiting and elevated liver enzymes were also more frequent with neratinib.
I-SPY 2 is supported by QuantumLeap Healthcare Collaborative, the Foundation for the National Institutes of Health (from 2010 through 2012) and the National Cancer Institute. Dr. Park reported receiving lecture fees and travel support from Genentech and Pfizer, and receiving royalties from patents. Dr. Rugo reported grants to her institution from BioMarin, and unpaid steering committee participation for BioMarin and AbbVie. Multiple co-authors reported financial relationships of various kinds.
Taking a step toward the goal of personalized medicine, investigators in the multicenter, adaptive I-SPY 2 trial report that tailoring neoadjuvant therapy combinations to specific cancer subtypes in women with high-risk breast cancer will likely result in higher rates of pathological complete responses for at least two subtypes, including patients with triple-negative disease.
Among women with triple-negative breast cancer (tumors lacking human epidermal growth factor receptor 2 [HER2], estrogen, and progesterone receptors) in the phase II trial, a combination of the poly (ADP-ribose) polymerase (PARP) inhibitor veliparib and carboplatin added to paclitaxel was associated with an estimated 51% pathological complete response (pCR) rate, compared with 26% for patients treated with weekly paclitaxel alone. The predicted probability of success in a phase III trial with the combination was 88%, reported Hope S. Rugo, MD, director of the Breast Oncology Clinical Trials Program at the University of California San Francisco, and her colleagues in I-SPY 2.
“The experience with veliparib-carboplatin in our trial shows the advantage of an adaptively randomized phase II platform trial for matching therapies with biomarker subsets to better inform the design of phase III trials so that they can be more focused, smaller, and faster. Future patients stand to benefit, but trial participants benefit as well in that exposure to ineffective therapy is minimized,” the investigators wrote (N Engl J Med. 2016 July 7. doi: 10.1056/NEJMoa1513749).
Partial results from this trial were reported at the 2013 San Antonio Breast Cancer Symposium.
On the basis of these phase II data, an ongoing phase III neoadjuvant trial is comparing standard chemotherapy alone, with carboplatin, or with veliparib plus carboplatin as treatment for triple-negative breast cancer, Dr. Rugo and her associates said.
In another arm of I-SPY 2 involving a subset of patients with HER2-positive, hormone receptor–negative cancers, the mean estimated pCR rate was 56% for patients treated with the investigational tyrosine-kinase inhibitor (TKI) neratinib, compared with an estimated 33% among patients treated with anti-HER2 agent trastuzumab (Herceptin). All the participants received standard neoadjuvant therapy, which consisted of 12 cycles of paclitaxel, followed by 4 cycles of doxorubicin and cyclophosphamide. The estimated probability of success in phase III with neratinib was 79%, reported John W. Park, MD, of University of California San Francisco, and his coauthors on behalf of I-SPY 2 investigators (N Engl J Med. 2016 July 7. doi: 10.1056/NEJMoa1513750).
On the basis of this phase II study, and to reflect the current standard of dual HER-targeting, the investigators are proceeding with a phase III trial comparing neratinib as neoadjuvant therapy added to pertuzumab (Perjeta), trastuzumab, and a taxane vs. the three latter drugs, and against a combination of neratinib, trastuzumab, and taxane, all followed by doxorubicin and cyclophosphamide.
Partial results of the phase II trial were reported at the 2013 annual meeting of the American Association for Cancer Research.
Nimble trial, tailored therapies
I-SPY 2 (Investigation of Serial Studies to Predict Your Therapeutic Response through Imaging and Molecular Analysis 2) is an ongoing “platform” trial exploring the use of new drugs combined with a standard neoadjuvant therapy backbone for the treatment of high-risk cancers.
Women with stage II or III breast cancers with tumors 2.5 cm or larger are assessed for one of eight biomarker subtypes according to HER2 (human epidermal growth factor receptor 2) status, hormone receptor status, and genetic risk factors based on a 70-gene assay. The patients are then randomized within each biomarker subtype to receive standard therapy with or without an investigational agent.
Each sub-trial has a primary endpoint of an improvement in pathological complete response compared with the standard of care. Changes in tumor volume on MRI are used to predict whether patients will achieve a pCR. Regimens that have a high Bayesian predictive probability of success in a subsequent phase III neoadjuvant trial within the biomarker signature in which they performed well are eligible for moving on to phase III trials.
Triple-negative disease
Among the subgroup of patients with triple-negative disease, 72 were assigned to receive veliparib 50 mg by mouth twice daily for 12 weeks, plus carboplatin at a dose intended to achieve a pharmacologic area under the concentration versus time curve of 6 mg/hour per liter on weeks 1, 4, 7, and 10, plus intravenous paclitaxel at a dose of 80 mg/m2. An additional 44 patients (controls) were randomized to receive paclitaxel alone.
Following paclitaxel alone or with the combination, all patients received doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 every 2-3 weeks for four doses, followed by myeloid growth factor support as appropriate. Following treatment, all patients underwent surgery that included axillary node sampling in accordance with National Comprehensive Cancer Network (NCCN) and local practice guidelines. Adjuvant radiation and endocrine therapy were recommended in accordance with standard guidelines.
The rate of grade 3 or greater hematologic toxic effects in this trial arm was higher in patients treated with veliparib-carboplatin, with neutropenia rates of 71% versus 2% for controls. Adverse events occurring only in patients on veliparib-carboplatin were thrombocytopenia in 21%, anemia in 28%, and febrile neutropenia in 1%. Among patients who had received the combination, toxic effects were higher during doxorubicin-cyclophosphamide therapy.
HR-negative disease
Patients with hormone receptor–negative disease received standard neoadjuvant chemotherapy with 12 weekly cycles of paclitaxel followed by 4 cycles of doxorubicin and cyclophosphamide as described before, with or without oral neratinib 240 mg per day. Patients in the control group who had HER2-positive cancers also received trastuzumab for the first 12 weeks with a loading dose of 4 mg per kilogram of body weight in the first cycle, followed by a maintenance dose of 2 mg per kilogram in cycles 2 through 12.
Surgery, including sentinel-node dissection in patients with node-negative cancer and axillary-node dissection in those with node-positive cancer at diagnosis, was performed according to NCCN and local practice guidelines, and adjuvant radiation and endocrine therapy were recommended according to standard guidelines.
The protocol was modified to included diarrhea prophylaxis with loperamide among patients assigned to receive neratinib.
A total of 127 patients were assigned to neratinib, and 115 of these patients were evaluable for response. Controls included 84 patients, of whom 78 were evaluable. At baseline, more patients in the neratinib group had HER2-positive tumors. Neratinib reached the prespecified efficacy threshold only within the HER2-positive, HR-negative group.
Diarrhea was the most common adverse event, with grade 3 or greater diarrhea occurring among 38% of patients assigned to neratinib. Vomiting and elevated liver enzymes were also more frequent with neratinib.
I-SPY 2 is supported by QuantumLeap Healthcare Collaborative, the Foundation for the National Institutes of Health (from 2010 through 2012) and the National Cancer Institute. Dr. Park reported receiving lecture fees and travel support from Genentech and Pfizer, and receiving royalties from patents. Dr. Rugo reported grants to her institution from BioMarin, and unpaid steering committee participation for BioMarin and AbbVie. Multiple co-authors reported financial relationships of various kinds.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Two neoadjuvant therapy combinations – one for triple negative breast cancer and one for HER2-positive breast cancer – have a high chance of success in a phase III trial, according to results of an adaptive phase II trial.
Major finding: The predicted probability of success in phase III trials with veliparib, carboplatin, and paclitaxel was 88% in patients with triple-negative breast cancer, and 79% with neratinib and standard chemotherapy for HER2-positive patients.
Data source: I-SPY 2, a multicenter, adaptive randomization study of patients with various subtypes of breast cancer.
Disclosures: I-SPY 2 is supported by QuantumLeap Healthcare Collaborative, the Foundation for the National Institutes of Health (from 2010 through 2012) and the National Cancer Institute. Dr. Park reported receiving lecture fees and travel support from Genentech and Pfizer, and receiving royalties from patents. Dr. Rugo reported grants to her institution from BioMarin, and unpaid steering committee participation for BioMarin and AbbVie. Multiple coauthors reported financial relationships of various kinds.
What Is the Best Management Strategy for Postoperative Atrial Fibrillation?
Clinical question: What is the best management strategy for postoperative atrial fibrillation?
Bottom line: For new-onset atrial fibrillation (AF) following cardiac surgery, both rate control and rhythm control are reasonable strategies. There is no a clear advantage of one over the other. (LOE = 1b)
Reference: Gillinov AM, Bagiella E, Moskowitz AJ, et al. Rate control versus rhythm control for atrial fibrillation after cardiac surgery. N Engl J Med 2016;374(20):1911–1921.
Study design: Randomized controlled trial (nonblinded)
Funding source: Government
Allocation: Concealed
Setting: Inpatient (any location) with outpatient follow-up
Synopsis
Postoperative AF is a common complication of cardiac surgery. In this trial, investigators identified more than 2000 patients who were undergoing coronary-artery bypass grafting and/or cardiac valve surgery. Of these patients, one-third developed new-onset AF and were randomized to receive either rate control or rhythm control.
In the rate-control group, patients received medications to slow heart rate to less than 100 beats per minute. If sinus rhythm was not achieved, these patients could then receive rhythm control per their physician's discretion. In the rhythm-control group, patients received amiodarone with or without rate-lowering medication, followed by cardioversion if AF persisted for 24 to 48 hours. The crossover rate in both groups was approximately 25% due to either drug ineffectiveness in the rate-control group or drug side effects in the rhythm-control group. All patients who remained in AF after 48 hours received anticoagulation.
The 2 groups were similar at baseline: mean age was 69 years, 75% were male, and 94% were white. Intention-to-treat analysis was used to test the primary endpoint of number of days in the emergency department or hospital within 60 days after randomization. There was no significant difference detected in this outcome between the 2 groups, even when the initial length of stay was adjusted for discharge readiness from an AF perspective. A sensitivity analysis accounting for the large number of crossovers also confirmed this finding. More than 90% of patients in both groups had a stable heart rhythm at the 60-day follow-up. Complication rates and 30-day readmission rates were also similar in the 2 groups.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question: What is the best management strategy for postoperative atrial fibrillation?
Bottom line: For new-onset atrial fibrillation (AF) following cardiac surgery, both rate control and rhythm control are reasonable strategies. There is no a clear advantage of one over the other. (LOE = 1b)
Reference: Gillinov AM, Bagiella E, Moskowitz AJ, et al. Rate control versus rhythm control for atrial fibrillation after cardiac surgery. N Engl J Med 2016;374(20):1911–1921.
Study design: Randomized controlled trial (nonblinded)
Funding source: Government
Allocation: Concealed
Setting: Inpatient (any location) with outpatient follow-up
Synopsis
Postoperative AF is a common complication of cardiac surgery. In this trial, investigators identified more than 2000 patients who were undergoing coronary-artery bypass grafting and/or cardiac valve surgery. Of these patients, one-third developed new-onset AF and were randomized to receive either rate control or rhythm control.
In the rate-control group, patients received medications to slow heart rate to less than 100 beats per minute. If sinus rhythm was not achieved, these patients could then receive rhythm control per their physician's discretion. In the rhythm-control group, patients received amiodarone with or without rate-lowering medication, followed by cardioversion if AF persisted for 24 to 48 hours. The crossover rate in both groups was approximately 25% due to either drug ineffectiveness in the rate-control group or drug side effects in the rhythm-control group. All patients who remained in AF after 48 hours received anticoagulation.
The 2 groups were similar at baseline: mean age was 69 years, 75% were male, and 94% were white. Intention-to-treat analysis was used to test the primary endpoint of number of days in the emergency department or hospital within 60 days after randomization. There was no significant difference detected in this outcome between the 2 groups, even when the initial length of stay was adjusted for discharge readiness from an AF perspective. A sensitivity analysis accounting for the large number of crossovers also confirmed this finding. More than 90% of patients in both groups had a stable heart rhythm at the 60-day follow-up. Complication rates and 30-day readmission rates were also similar in the 2 groups.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question: What is the best management strategy for postoperative atrial fibrillation?
Bottom line: For new-onset atrial fibrillation (AF) following cardiac surgery, both rate control and rhythm control are reasonable strategies. There is no a clear advantage of one over the other. (LOE = 1b)
Reference: Gillinov AM, Bagiella E, Moskowitz AJ, et al. Rate control versus rhythm control for atrial fibrillation after cardiac surgery. N Engl J Med 2016;374(20):1911–1921.
Study design: Randomized controlled trial (nonblinded)
Funding source: Government
Allocation: Concealed
Setting: Inpatient (any location) with outpatient follow-up
Synopsis
Postoperative AF is a common complication of cardiac surgery. In this trial, investigators identified more than 2000 patients who were undergoing coronary-artery bypass grafting and/or cardiac valve surgery. Of these patients, one-third developed new-onset AF and were randomized to receive either rate control or rhythm control.
In the rate-control group, patients received medications to slow heart rate to less than 100 beats per minute. If sinus rhythm was not achieved, these patients could then receive rhythm control per their physician's discretion. In the rhythm-control group, patients received amiodarone with or without rate-lowering medication, followed by cardioversion if AF persisted for 24 to 48 hours. The crossover rate in both groups was approximately 25% due to either drug ineffectiveness in the rate-control group or drug side effects in the rhythm-control group. All patients who remained in AF after 48 hours received anticoagulation.
The 2 groups were similar at baseline: mean age was 69 years, 75% were male, and 94% were white. Intention-to-treat analysis was used to test the primary endpoint of number of days in the emergency department or hospital within 60 days after randomization. There was no significant difference detected in this outcome between the 2 groups, even when the initial length of stay was adjusted for discharge readiness from an AF perspective. A sensitivity analysis accounting for the large number of crossovers also confirmed this finding. More than 90% of patients in both groups had a stable heart rhythm at the 60-day follow-up. Complication rates and 30-day readmission rates were also similar in the 2 groups.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Early Initiation of Renal Replacement Therapy Improves Mortality in Critically Ill Patients with Acute Kidney Injury
Clinical question: For critically ill patients with acute kidney injury, does early initiation of renal replacement therapy improve mortality?
Bottom line: In this single-center study, early initiation of renal replacement therapy (RRT) in critically ill patients with acute kidney injury (AKI) decreased the number of deaths at 90 days. Larger studies are required to confirm this finding. Although some patients may prefer to avoid dialysis and its inherent risks, this preference must be balanced with the greater risk of mortality that may occur by not undergoing this treatment early on. (LOE = 1b)
Reference: Zarbock A, Kellum JA, Schmidt C, et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury. JAMA 2016;315(20):2190–2199.
Study design: Randomized controlled trial (nonblinded)
Funding source: Foundation
Allocation: Concealed
Setting: Inpatient (ICU only)
Synopsis
To study the optimal time for initiation of RRT for critically ill patients with AKI, these authors recruited patients with severe sepsis, pressor requirements, refractory fluid overload, or nonrenal organ dysfunction who developed stage 2 AKI (urine output < 0.5 mL/kg/h for more than 12 hours or a 2-fold increase in serum creatinine from baseline). Patients with chronic kidney disease, glomerulonephritis, interstitial nephritis, vasculitis, and postrenal obstruction were excluded, among others.
Overall, 231 patients were randomized to receive either early RRT or delayed RRT. RRT was delivered initially as continuous venovenous hemodiafiltration and could be changed to an intermittent procedure such as intermittent hemodialysis or sustained low-efficiency daily dialysis if renal recovery did not occur after 7 days. Early RRT was initiated within 8 hours of diagnosis of stage 2 AKI while delayed RRT was initiated within 12 hours after patients had developed stage 3 AKI (urine output < 0.3mL/kg/h for more than 24 hours or a 3-fold increase in serum creatinine from baseline) or if patients had an absolute indication for RRT. Patients in the 2 groups had similar baseline Sequential Organ Failure Assessment scores and almost all were surgical patients. Although all patients in the early group received RRT, 9% of patients in the delayed group did not, mostly because they did not progress to stage 3 AKI.
Early RRT resulted in a significantly decreased 90-day mortality rate as compared with delayed RRT (39% vs 55%; P = .03). Patients in the early group also had a decreased duration of RRT (9 days vs 25 days; P = .04), decreased length of hospital stay (51 days vs 82 days; P < .001), and greater recovery of renal function at 90 days (54% vs 39%; P = .02). The authors postulate that initiating early RRT may prevent further injury to the kidneys and other organs by reducing systemic inflammation.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question: For critically ill patients with acute kidney injury, does early initiation of renal replacement therapy improve mortality?
Bottom line: In this single-center study, early initiation of renal replacement therapy (RRT) in critically ill patients with acute kidney injury (AKI) decreased the number of deaths at 90 days. Larger studies are required to confirm this finding. Although some patients may prefer to avoid dialysis and its inherent risks, this preference must be balanced with the greater risk of mortality that may occur by not undergoing this treatment early on. (LOE = 1b)
Reference: Zarbock A, Kellum JA, Schmidt C, et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury. JAMA 2016;315(20):2190–2199.
Study design: Randomized controlled trial (nonblinded)
Funding source: Foundation
Allocation: Concealed
Setting: Inpatient (ICU only)
Synopsis
To study the optimal time for initiation of RRT for critically ill patients with AKI, these authors recruited patients with severe sepsis, pressor requirements, refractory fluid overload, or nonrenal organ dysfunction who developed stage 2 AKI (urine output < 0.5 mL/kg/h for more than 12 hours or a 2-fold increase in serum creatinine from baseline). Patients with chronic kidney disease, glomerulonephritis, interstitial nephritis, vasculitis, and postrenal obstruction were excluded, among others.
Overall, 231 patients were randomized to receive either early RRT or delayed RRT. RRT was delivered initially as continuous venovenous hemodiafiltration and could be changed to an intermittent procedure such as intermittent hemodialysis or sustained low-efficiency daily dialysis if renal recovery did not occur after 7 days. Early RRT was initiated within 8 hours of diagnosis of stage 2 AKI while delayed RRT was initiated within 12 hours after patients had developed stage 3 AKI (urine output < 0.3mL/kg/h for more than 24 hours or a 3-fold increase in serum creatinine from baseline) or if patients had an absolute indication for RRT. Patients in the 2 groups had similar baseline Sequential Organ Failure Assessment scores and almost all were surgical patients. Although all patients in the early group received RRT, 9% of patients in the delayed group did not, mostly because they did not progress to stage 3 AKI.
Early RRT resulted in a significantly decreased 90-day mortality rate as compared with delayed RRT (39% vs 55%; P = .03). Patients in the early group also had a decreased duration of RRT (9 days vs 25 days; P = .04), decreased length of hospital stay (51 days vs 82 days; P < .001), and greater recovery of renal function at 90 days (54% vs 39%; P = .02). The authors postulate that initiating early RRT may prevent further injury to the kidneys and other organs by reducing systemic inflammation.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question: For critically ill patients with acute kidney injury, does early initiation of renal replacement therapy improve mortality?
Bottom line: In this single-center study, early initiation of renal replacement therapy (RRT) in critically ill patients with acute kidney injury (AKI) decreased the number of deaths at 90 days. Larger studies are required to confirm this finding. Although some patients may prefer to avoid dialysis and its inherent risks, this preference must be balanced with the greater risk of mortality that may occur by not undergoing this treatment early on. (LOE = 1b)
Reference: Zarbock A, Kellum JA, Schmidt C, et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury. JAMA 2016;315(20):2190–2199.
Study design: Randomized controlled trial (nonblinded)
Funding source: Foundation
Allocation: Concealed
Setting: Inpatient (ICU only)
Synopsis
To study the optimal time for initiation of RRT for critically ill patients with AKI, these authors recruited patients with severe sepsis, pressor requirements, refractory fluid overload, or nonrenal organ dysfunction who developed stage 2 AKI (urine output < 0.5 mL/kg/h for more than 12 hours or a 2-fold increase in serum creatinine from baseline). Patients with chronic kidney disease, glomerulonephritis, interstitial nephritis, vasculitis, and postrenal obstruction were excluded, among others.
Overall, 231 patients were randomized to receive either early RRT or delayed RRT. RRT was delivered initially as continuous venovenous hemodiafiltration and could be changed to an intermittent procedure such as intermittent hemodialysis or sustained low-efficiency daily dialysis if renal recovery did not occur after 7 days. Early RRT was initiated within 8 hours of diagnosis of stage 2 AKI while delayed RRT was initiated within 12 hours after patients had developed stage 3 AKI (urine output < 0.3mL/kg/h for more than 24 hours or a 3-fold increase in serum creatinine from baseline) or if patients had an absolute indication for RRT. Patients in the 2 groups had similar baseline Sequential Organ Failure Assessment scores and almost all were surgical patients. Although all patients in the early group received RRT, 9% of patients in the delayed group did not, mostly because they did not progress to stage 3 AKI.
Early RRT resulted in a significantly decreased 90-day mortality rate as compared with delayed RRT (39% vs 55%; P = .03). Patients in the early group also had a decreased duration of RRT (9 days vs 25 days; P = .04), decreased length of hospital stay (51 days vs 82 days; P < .001), and greater recovery of renal function at 90 days (54% vs 39%; P = .02). The authors postulate that initiating early RRT may prevent further injury to the kidneys and other organs by reducing systemic inflammation.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
New fragility fracture recommendations emphasize coordination of care
LONDON – The European League Against Rheumatism and the European Federation of National Associations of Orthopaedics and Traumatology have joined forces to develop recommendations for the prevention and management of fragility fractures.
Such fractures are common in men and women over the age of 50 years and can lead to repeat fracture in some patients. The recommendations are unique as they are the first to consider both acute orthopedic and postfracture rheumatologic care, said Willem F. Lems, MD, PhD, of the Amsterdam Rheumatology and Immunology Centre.
At the European Congress of Rheumatology, Dr. Lems provided an overview of the draft recommendations, noting that there would be several overarching principles, one of which recognized the multidisciplinary nature of caring for someone with a fragility fracture. An important point is not who is taking care of the patient, but that the patient is given the best possible care within the multidisciplinary framework.
What constitutes optimal care of course depends on the clinical situation, notably the type of fracture and the age of the patient, and optimal care in all phases of presentation (pre-, peri- and postoperative) can have an important effect on a patient’s outcome. The prevention of subsequent fractures is a key focus, with the recommendation that all patients should be investigated systematically and those deemed at high risk for another fracture should be prescribed both pharmacologic and nonpharmacologic interventions as appropriate. Patient education is also considered important.
As for all EULAR-developed recommendations, standard procedures were followed that involved convening an expert scientific advisory committee and using the Delphi technique to come up with the most important research questions that would be used to formulate the final 10 recommendations. Four of the recommendations cover the acute care setting and six provide advice on postfracture care.
The first of the acute care recommendations looks at pre- and perioperative management of a fragility fracture and highlights that, within 24-48 hours of admission, patients should receive adequate pain and fluid management and treatment, including early surgery if appropriate. This is based on evidence that better outcomes can be achieved in terms of both morbidity and mortality if patients can be seen and managed quickly.
Another of the acute care recommendations focuses on orthogeriatric care, noting that the orthopedic surgeon and a dedicated orthogeriatric team should work together, particularly for elderly patients who have suffered a hip fracture. Key elements here are the management of and prevention of delirium, deep vein thrombosis, pressure sores, and malnutrition.
As for actual fracture treatment, a balanced approach is advised when deciding upon a surgical or nonsurgical approach, especially because this is likely to be an older population with other comorbidities. Only one in three vertebral fractures are symptomatic and only about 10% of patients will be hospitalized for pain. Analgesics, modifying activities, and bracing can be options here. Surgical options for distal radial fracture, hip fracture, and trochanteric and femoral neck fractures are included.
The fourth recommendation looks at the organization of postfracture care and the need for a systematic approach to identify those who may be at risk for subsequent fractures, starting with the suggestion that any patient older than 50 years with a recent fracture should be assessed. The fifth recommendation addresses ways to evaluate this risk, such as looking at the clinical risk factors, performing bone scans and imaging, and screening for underlying osteoporosis or metabolic disorders.
Implementation is the next step, and the sixth recommendation suggests ways these recommendations could be integrated into routine practice. Often one of the biggest barriers to effective postfracture care is the lack of patient, and sometimes clinician, awareness of the risk for a subsequent fracture. This recommendation looks at the role of a possible local fracture liaison service or facilitator to coordinate between the various members of the multidisciplinary team from secondary (orthopedic surgeons, rheumatologists, endocrinologists, and geriatricians) to primary care.
The seventh recommendation addresses rehabilitation and the need to initiate physical training and muscle strengthening as early as possible after the initial fracture, with long-term continuation of balance training and fall prevention.
The final three recommendations focus on how to educate patients about their risk factors, need for follow-up, and the duration of any pharmacologic or nonpharmacologic therapy that they may need. Nonpharmacologic options might include stopping smoking, limiting alcohol intake, as well as taking supplements such as calcium or vitamin D. There will be specific guidance on the use of calcium and vitamin D, which have both pros and cons, but the optimal dosage appears to be 1,000–1,200 mg/day for calcium and 800 IU/day for vitamin D.
Pharmacologic options to prevent subsequent fragility fractures include the bisphosphonates alendronate, risedronate, and zoledronic acid (Reclast), and also the monoclonal antibody denosumab (Prolia). These are the only drugs that have been shown to reduced the risk for vertebral, nonvertebral, and hip fractures in primary analyses. Adherence, tolerance, and regular monitoring are key, and a five-step plan is suggested to aid clinical decision making that covers case finding, risk evaluation, differential diagnosis, treatment, and follow-up.
The recommendations are being finalized and should be available for publication later this year. The recommendations task force also plans to propose a research agenda.
Dr. Lems had no relevant disclosures.
LONDON – The European League Against Rheumatism and the European Federation of National Associations of Orthopaedics and Traumatology have joined forces to develop recommendations for the prevention and management of fragility fractures.
Such fractures are common in men and women over the age of 50 years and can lead to repeat fracture in some patients. The recommendations are unique as they are the first to consider both acute orthopedic and postfracture rheumatologic care, said Willem F. Lems, MD, PhD, of the Amsterdam Rheumatology and Immunology Centre.
At the European Congress of Rheumatology, Dr. Lems provided an overview of the draft recommendations, noting that there would be several overarching principles, one of which recognized the multidisciplinary nature of caring for someone with a fragility fracture. An important point is not who is taking care of the patient, but that the patient is given the best possible care within the multidisciplinary framework.
What constitutes optimal care of course depends on the clinical situation, notably the type of fracture and the age of the patient, and optimal care in all phases of presentation (pre-, peri- and postoperative) can have an important effect on a patient’s outcome. The prevention of subsequent fractures is a key focus, with the recommendation that all patients should be investigated systematically and those deemed at high risk for another fracture should be prescribed both pharmacologic and nonpharmacologic interventions as appropriate. Patient education is also considered important.
As for all EULAR-developed recommendations, standard procedures were followed that involved convening an expert scientific advisory committee and using the Delphi technique to come up with the most important research questions that would be used to formulate the final 10 recommendations. Four of the recommendations cover the acute care setting and six provide advice on postfracture care.
The first of the acute care recommendations looks at pre- and perioperative management of a fragility fracture and highlights that, within 24-48 hours of admission, patients should receive adequate pain and fluid management and treatment, including early surgery if appropriate. This is based on evidence that better outcomes can be achieved in terms of both morbidity and mortality if patients can be seen and managed quickly.
Another of the acute care recommendations focuses on orthogeriatric care, noting that the orthopedic surgeon and a dedicated orthogeriatric team should work together, particularly for elderly patients who have suffered a hip fracture. Key elements here are the management of and prevention of delirium, deep vein thrombosis, pressure sores, and malnutrition.
As for actual fracture treatment, a balanced approach is advised when deciding upon a surgical or nonsurgical approach, especially because this is likely to be an older population with other comorbidities. Only one in three vertebral fractures are symptomatic and only about 10% of patients will be hospitalized for pain. Analgesics, modifying activities, and bracing can be options here. Surgical options for distal radial fracture, hip fracture, and trochanteric and femoral neck fractures are included.
The fourth recommendation looks at the organization of postfracture care and the need for a systematic approach to identify those who may be at risk for subsequent fractures, starting with the suggestion that any patient older than 50 years with a recent fracture should be assessed. The fifth recommendation addresses ways to evaluate this risk, such as looking at the clinical risk factors, performing bone scans and imaging, and screening for underlying osteoporosis or metabolic disorders.
Implementation is the next step, and the sixth recommendation suggests ways these recommendations could be integrated into routine practice. Often one of the biggest barriers to effective postfracture care is the lack of patient, and sometimes clinician, awareness of the risk for a subsequent fracture. This recommendation looks at the role of a possible local fracture liaison service or facilitator to coordinate between the various members of the multidisciplinary team from secondary (orthopedic surgeons, rheumatologists, endocrinologists, and geriatricians) to primary care.
The seventh recommendation addresses rehabilitation and the need to initiate physical training and muscle strengthening as early as possible after the initial fracture, with long-term continuation of balance training and fall prevention.
The final three recommendations focus on how to educate patients about their risk factors, need for follow-up, and the duration of any pharmacologic or nonpharmacologic therapy that they may need. Nonpharmacologic options might include stopping smoking, limiting alcohol intake, as well as taking supplements such as calcium or vitamin D. There will be specific guidance on the use of calcium and vitamin D, which have both pros and cons, but the optimal dosage appears to be 1,000–1,200 mg/day for calcium and 800 IU/day for vitamin D.
Pharmacologic options to prevent subsequent fragility fractures include the bisphosphonates alendronate, risedronate, and zoledronic acid (Reclast), and also the monoclonal antibody denosumab (Prolia). These are the only drugs that have been shown to reduced the risk for vertebral, nonvertebral, and hip fractures in primary analyses. Adherence, tolerance, and regular monitoring are key, and a five-step plan is suggested to aid clinical decision making that covers case finding, risk evaluation, differential diagnosis, treatment, and follow-up.
The recommendations are being finalized and should be available for publication later this year. The recommendations task force also plans to propose a research agenda.
Dr. Lems had no relevant disclosures.
LONDON – The European League Against Rheumatism and the European Federation of National Associations of Orthopaedics and Traumatology have joined forces to develop recommendations for the prevention and management of fragility fractures.
Such fractures are common in men and women over the age of 50 years and can lead to repeat fracture in some patients. The recommendations are unique as they are the first to consider both acute orthopedic and postfracture rheumatologic care, said Willem F. Lems, MD, PhD, of the Amsterdam Rheumatology and Immunology Centre.
At the European Congress of Rheumatology, Dr. Lems provided an overview of the draft recommendations, noting that there would be several overarching principles, one of which recognized the multidisciplinary nature of caring for someone with a fragility fracture. An important point is not who is taking care of the patient, but that the patient is given the best possible care within the multidisciplinary framework.
What constitutes optimal care of course depends on the clinical situation, notably the type of fracture and the age of the patient, and optimal care in all phases of presentation (pre-, peri- and postoperative) can have an important effect on a patient’s outcome. The prevention of subsequent fractures is a key focus, with the recommendation that all patients should be investigated systematically and those deemed at high risk for another fracture should be prescribed both pharmacologic and nonpharmacologic interventions as appropriate. Patient education is also considered important.
As for all EULAR-developed recommendations, standard procedures were followed that involved convening an expert scientific advisory committee and using the Delphi technique to come up with the most important research questions that would be used to formulate the final 10 recommendations. Four of the recommendations cover the acute care setting and six provide advice on postfracture care.
The first of the acute care recommendations looks at pre- and perioperative management of a fragility fracture and highlights that, within 24-48 hours of admission, patients should receive adequate pain and fluid management and treatment, including early surgery if appropriate. This is based on evidence that better outcomes can be achieved in terms of both morbidity and mortality if patients can be seen and managed quickly.
Another of the acute care recommendations focuses on orthogeriatric care, noting that the orthopedic surgeon and a dedicated orthogeriatric team should work together, particularly for elderly patients who have suffered a hip fracture. Key elements here are the management of and prevention of delirium, deep vein thrombosis, pressure sores, and malnutrition.
As for actual fracture treatment, a balanced approach is advised when deciding upon a surgical or nonsurgical approach, especially because this is likely to be an older population with other comorbidities. Only one in three vertebral fractures are symptomatic and only about 10% of patients will be hospitalized for pain. Analgesics, modifying activities, and bracing can be options here. Surgical options for distal radial fracture, hip fracture, and trochanteric and femoral neck fractures are included.
The fourth recommendation looks at the organization of postfracture care and the need for a systematic approach to identify those who may be at risk for subsequent fractures, starting with the suggestion that any patient older than 50 years with a recent fracture should be assessed. The fifth recommendation addresses ways to evaluate this risk, such as looking at the clinical risk factors, performing bone scans and imaging, and screening for underlying osteoporosis or metabolic disorders.
Implementation is the next step, and the sixth recommendation suggests ways these recommendations could be integrated into routine practice. Often one of the biggest barriers to effective postfracture care is the lack of patient, and sometimes clinician, awareness of the risk for a subsequent fracture. This recommendation looks at the role of a possible local fracture liaison service or facilitator to coordinate between the various members of the multidisciplinary team from secondary (orthopedic surgeons, rheumatologists, endocrinologists, and geriatricians) to primary care.
The seventh recommendation addresses rehabilitation and the need to initiate physical training and muscle strengthening as early as possible after the initial fracture, with long-term continuation of balance training and fall prevention.
The final three recommendations focus on how to educate patients about their risk factors, need for follow-up, and the duration of any pharmacologic or nonpharmacologic therapy that they may need. Nonpharmacologic options might include stopping smoking, limiting alcohol intake, as well as taking supplements such as calcium or vitamin D. There will be specific guidance on the use of calcium and vitamin D, which have both pros and cons, but the optimal dosage appears to be 1,000–1,200 mg/day for calcium and 800 IU/day for vitamin D.
Pharmacologic options to prevent subsequent fragility fractures include the bisphosphonates alendronate, risedronate, and zoledronic acid (Reclast), and also the monoclonal antibody denosumab (Prolia). These are the only drugs that have been shown to reduced the risk for vertebral, nonvertebral, and hip fractures in primary analyses. Adherence, tolerance, and regular monitoring are key, and a five-step plan is suggested to aid clinical decision making that covers case finding, risk evaluation, differential diagnosis, treatment, and follow-up.
The recommendations are being finalized and should be available for publication later this year. The recommendations task force also plans to propose a research agenda.
Dr. Lems had no relevant disclosures.
AT THE EULAR 2016 CONGRESS
Cutaneous T-Cell Lymphoma in a Patient With Celiac Disease
To the Editor:
Mycosis fungoides (MF) is the most common form of a heterogeneous group of non-Hodgkin lymphomas known as cutaneous T-cell lymphomas. Celiac disease (CD) is associated with increased risk for development of enteropathy-associated T-cell lymphoma and other intraintestinal and extraintestinal non-Hodgkin lymphomas, but a firm association between CD and MF has not been established.1 The first and second cases of concomitant MF and CD were reported in 1985 and 2009 by Coulson and Sanderson2 and Moreira et al,3 respectively. Two other reports of celiac-associated dermatitis herpetiformis and MF exist.4,5 We report a patient with a unique constellation of MF, CD, and Sjögren syndrome (SS).
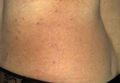
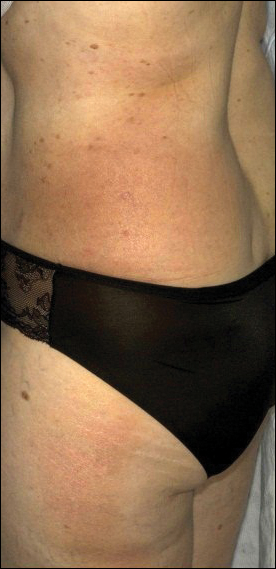
A 54-year-old woman presented with a worsening nonpruritic, slightly tender, eczematous patch on the back of 19 years’ duration. She had a history of SS diagnosed by salivary gland biopsy. She also had a diagnosis of CD confirmed with positive antigliadin IgA antibodies, with a dramatic improvement in symptoms on a gluten-free diet (GFD) after having abdominal pain and diarrhea for many years. She had no evidence of dermatitis herpetiformis. Recently, more red-brown areas of confluent light pink erythema without clear-cut borders had appeared on the axillae, trunk, and thigh (Figure). The patient also noted new lesions and more erythema of the patches when not adhering to a GFD. A biopsy specimen from the left side of the lateral trunk revealed a bandlike lymphocytic infiltrate with irregular nuclear contours displaying epidermotropism with a few Pautrier microabscesses. Immunohistochemistry showed strong CD3 and CD4 positivity with loss of CD7 and scattered CD8 staining. Peripheral blood flow cytometry showed no aberrant cell populations. The patient was diagnosed with MF stage IB and treated with topical corticosteroids and natural light with improvement.
It has been hypothesized that early MF is an autoimmune process caused by dysregulation of a lymphocytic reaction against chronic exogenous or endogenous antigens.4,5 The association of MF with CD supports the possibility of lymphocytic stimulation by a persistent antigen (ie, gluten) in the gastrointestinal tract. Porter et al4 suggested that in susceptible individuals, the resulting clonal T cells may migrate into the epidermis, causing MF. This theory also is supported by the finding that adherence to a GFD leads to decreased risk for malignancy and morbidity.6 In our patient, the chronic autoimmune stimulation in SS could be a factor in the pathogenesis of MF. Additionally, SS, CD, and MF are all strongly associated with increased incidence of specific but different HLA class II antigens. Mycosis fungoides is associated with HLA-DR5 and DQB1*03 alleles, CD with HLA-DQ2 and DQ8, and SS with HLA-DR15 and DR3. We do not know the HLA type of our patient, but she likely possessed multiple alleles, leading to the unique aggregation of diseases.
Furthermore, studies have shown that lymphocytes in CD patients display impaired regulatory T-cell function, causing increased incidence of autoimmune diseases and malignancy.7,8 By this theory, the occurrence of MF in patients is facilitated by the inability of CD lymphocytes to control the abnormal T-cell proliferation in the skin. Interestingly, the finding of SS in our patient supports the possibility of impaired regulatory T-cell function.
Although the occurrence of both MF and CD in our patient could be coincidental, the possibility of correlation must be considered as more cases are documented.
- Catassi C, Fabiani E, Corrao G, et al; Italian Working Group on Coeliac Disease and Non-Hodgkin’s-Lymphoma. Risk of non-Hodgkin Lymphoma in celiac disease. JAMA. 2002;287:1413-1419.
- Coulson IH, Sanderson KV. T-cell lymphoma presenting as tumour d’emblée mycosis fungoides associated with coeliac disease. J R Soc Med. 1985;78(suppl 11):23-24.
- Moreira AI, Menezes N, Varela P, et al. Primary cutaneous peripheral T cell lymphoma and celiac disease [in Portuguese]. Rev Assoc Med Bras. 2009;55:253-256.
- Porter WM, Dawe SA, Bunker CB. Dermatitis herpetiformis and cutaneous T-cell lymphoma. Clin Exp Dermatol. 2001;26:304-305.
- Sun G, Berthelot C, Duvic M. A second case of dermatitis herpetiformis and cutaneous T-cell lymphoma. Clin Exp Dermatol. 2008;33:506-507.
- Holmes GK, Prior P, Lane MR, et al. Malignancy in coeliac disease—effect of a gluten free diet. Gut. 1989;30:333-338.
- Granzotto M, dal Bo S, Quaglia S, et al. Regulatory T-cell function is impaired in celiac disease. Dig Dis Sci. 2009;54:1513-1519.
- Roychoudhuri R, Hirahara K, Mousavi K, et al. BACH2 represses effector programs to stabilize T(reg)-mediated immune homeostasis [published online June 2, 2013]. Nature. 2013;498:506-510.
To the Editor:
Mycosis fungoides (MF) is the most common form of a heterogeneous group of non-Hodgkin lymphomas known as cutaneous T-cell lymphomas. Celiac disease (CD) is associated with increased risk for development of enteropathy-associated T-cell lymphoma and other intraintestinal and extraintestinal non-Hodgkin lymphomas, but a firm association between CD and MF has not been established.1 The first and second cases of concomitant MF and CD were reported in 1985 and 2009 by Coulson and Sanderson2 and Moreira et al,3 respectively. Two other reports of celiac-associated dermatitis herpetiformis and MF exist.4,5 We report a patient with a unique constellation of MF, CD, and Sjögren syndrome (SS).
A 54-year-old woman presented with a worsening nonpruritic, slightly tender, eczematous patch on the back of 19 years’ duration. She had a history of SS diagnosed by salivary gland biopsy. She also had a diagnosis of CD confirmed with positive antigliadin IgA antibodies, with a dramatic improvement in symptoms on a gluten-free diet (GFD) after having abdominal pain and diarrhea for many years. She had no evidence of dermatitis herpetiformis. Recently, more red-brown areas of confluent light pink erythema without clear-cut borders had appeared on the axillae, trunk, and thigh (Figure). The patient also noted new lesions and more erythema of the patches when not adhering to a GFD. A biopsy specimen from the left side of the lateral trunk revealed a bandlike lymphocytic infiltrate with irregular nuclear contours displaying epidermotropism with a few Pautrier microabscesses. Immunohistochemistry showed strong CD3 and CD4 positivity with loss of CD7 and scattered CD8 staining. Peripheral blood flow cytometry showed no aberrant cell populations. The patient was diagnosed with MF stage IB and treated with topical corticosteroids and natural light with improvement.

It has been hypothesized that early MF is an autoimmune process caused by dysregulation of a lymphocytic reaction against chronic exogenous or endogenous antigens.4,5 The association of MF with CD supports the possibility of lymphocytic stimulation by a persistent antigen (ie, gluten) in the gastrointestinal tract. Porter et al4 suggested that in susceptible individuals, the resulting clonal T cells may migrate into the epidermis, causing MF. This theory also is supported by the finding that adherence to a GFD leads to decreased risk for malignancy and morbidity.6 In our patient, the chronic autoimmune stimulation in SS could be a factor in the pathogenesis of MF. Additionally, SS, CD, and MF are all strongly associated with increased incidence of specific but different HLA class II antigens. Mycosis fungoides is associated with HLA-DR5 and DQB1*03 alleles, CD with HLA-DQ2 and DQ8, and SS with HLA-DR15 and DR3. We do not know the HLA type of our patient, but she likely possessed multiple alleles, leading to the unique aggregation of diseases.
Furthermore, studies have shown that lymphocytes in CD patients display impaired regulatory T-cell function, causing increased incidence of autoimmune diseases and malignancy.7,8 By this theory, the occurrence of MF in patients is facilitated by the inability of CD lymphocytes to control the abnormal T-cell proliferation in the skin. Interestingly, the finding of SS in our patient supports the possibility of impaired regulatory T-cell function.
Although the occurrence of both MF and CD in our patient could be coincidental, the possibility of correlation must be considered as more cases are documented.
To the Editor:
Mycosis fungoides (MF) is the most common form of a heterogeneous group of non-Hodgkin lymphomas known as cutaneous T-cell lymphomas. Celiac disease (CD) is associated with increased risk for development of enteropathy-associated T-cell lymphoma and other intraintestinal and extraintestinal non-Hodgkin lymphomas, but a firm association between CD and MF has not been established.1 The first and second cases of concomitant MF and CD were reported in 1985 and 2009 by Coulson and Sanderson2 and Moreira et al,3 respectively. Two other reports of celiac-associated dermatitis herpetiformis and MF exist.4,5 We report a patient with a unique constellation of MF, CD, and Sjögren syndrome (SS).
A 54-year-old woman presented with a worsening nonpruritic, slightly tender, eczematous patch on the back of 19 years’ duration. She had a history of SS diagnosed by salivary gland biopsy. She also had a diagnosis of CD confirmed with positive antigliadin IgA antibodies, with a dramatic improvement in symptoms on a gluten-free diet (GFD) after having abdominal pain and diarrhea for many years. She had no evidence of dermatitis herpetiformis. Recently, more red-brown areas of confluent light pink erythema without clear-cut borders had appeared on the axillae, trunk, and thigh (Figure). The patient also noted new lesions and more erythema of the patches when not adhering to a GFD. A biopsy specimen from the left side of the lateral trunk revealed a bandlike lymphocytic infiltrate with irregular nuclear contours displaying epidermotropism with a few Pautrier microabscesses. Immunohistochemistry showed strong CD3 and CD4 positivity with loss of CD7 and scattered CD8 staining. Peripheral blood flow cytometry showed no aberrant cell populations. The patient was diagnosed with MF stage IB and treated with topical corticosteroids and natural light with improvement.

It has been hypothesized that early MF is an autoimmune process caused by dysregulation of a lymphocytic reaction against chronic exogenous or endogenous antigens.4,5 The association of MF with CD supports the possibility of lymphocytic stimulation by a persistent antigen (ie, gluten) in the gastrointestinal tract. Porter et al4 suggested that in susceptible individuals, the resulting clonal T cells may migrate into the epidermis, causing MF. This theory also is supported by the finding that adherence to a GFD leads to decreased risk for malignancy and morbidity.6 In our patient, the chronic autoimmune stimulation in SS could be a factor in the pathogenesis of MF. Additionally, SS, CD, and MF are all strongly associated with increased incidence of specific but different HLA class II antigens. Mycosis fungoides is associated with HLA-DR5 and DQB1*03 alleles, CD with HLA-DQ2 and DQ8, and SS with HLA-DR15 and DR3. We do not know the HLA type of our patient, but she likely possessed multiple alleles, leading to the unique aggregation of diseases.
Furthermore, studies have shown that lymphocytes in CD patients display impaired regulatory T-cell function, causing increased incidence of autoimmune diseases and malignancy.7,8 By this theory, the occurrence of MF in patients is facilitated by the inability of CD lymphocytes to control the abnormal T-cell proliferation in the skin. Interestingly, the finding of SS in our patient supports the possibility of impaired regulatory T-cell function.
Although the occurrence of both MF and CD in our patient could be coincidental, the possibility of correlation must be considered as more cases are documented.
- Catassi C, Fabiani E, Corrao G, et al; Italian Working Group on Coeliac Disease and Non-Hodgkin’s-Lymphoma. Risk of non-Hodgkin Lymphoma in celiac disease. JAMA. 2002;287:1413-1419.
- Coulson IH, Sanderson KV. T-cell lymphoma presenting as tumour d’emblée mycosis fungoides associated with coeliac disease. J R Soc Med. 1985;78(suppl 11):23-24.
- Moreira AI, Menezes N, Varela P, et al. Primary cutaneous peripheral T cell lymphoma and celiac disease [in Portuguese]. Rev Assoc Med Bras. 2009;55:253-256.
- Porter WM, Dawe SA, Bunker CB. Dermatitis herpetiformis and cutaneous T-cell lymphoma. Clin Exp Dermatol. 2001;26:304-305.
- Sun G, Berthelot C, Duvic M. A second case of dermatitis herpetiformis and cutaneous T-cell lymphoma. Clin Exp Dermatol. 2008;33:506-507.
- Holmes GK, Prior P, Lane MR, et al. Malignancy in coeliac disease—effect of a gluten free diet. Gut. 1989;30:333-338.
- Granzotto M, dal Bo S, Quaglia S, et al. Regulatory T-cell function is impaired in celiac disease. Dig Dis Sci. 2009;54:1513-1519.
- Roychoudhuri R, Hirahara K, Mousavi K, et al. BACH2 represses effector programs to stabilize T(reg)-mediated immune homeostasis [published online June 2, 2013]. Nature. 2013;498:506-510.
- Catassi C, Fabiani E, Corrao G, et al; Italian Working Group on Coeliac Disease and Non-Hodgkin’s-Lymphoma. Risk of non-Hodgkin Lymphoma in celiac disease. JAMA. 2002;287:1413-1419.
- Coulson IH, Sanderson KV. T-cell lymphoma presenting as tumour d’emblée mycosis fungoides associated with coeliac disease. J R Soc Med. 1985;78(suppl 11):23-24.
- Moreira AI, Menezes N, Varela P, et al. Primary cutaneous peripheral T cell lymphoma and celiac disease [in Portuguese]. Rev Assoc Med Bras. 2009;55:253-256.
- Porter WM, Dawe SA, Bunker CB. Dermatitis herpetiformis and cutaneous T-cell lymphoma. Clin Exp Dermatol. 2001;26:304-305.
- Sun G, Berthelot C, Duvic M. A second case of dermatitis herpetiformis and cutaneous T-cell lymphoma. Clin Exp Dermatol. 2008;33:506-507.
- Holmes GK, Prior P, Lane MR, et al. Malignancy in coeliac disease—effect of a gluten free diet. Gut. 1989;30:333-338.
- Granzotto M, dal Bo S, Quaglia S, et al. Regulatory T-cell function is impaired in celiac disease. Dig Dis Sci. 2009;54:1513-1519.
- Roychoudhuri R, Hirahara K, Mousavi K, et al. BACH2 represses effector programs to stabilize T(reg)-mediated immune homeostasis [published online June 2, 2013]. Nature. 2013;498:506-510.
Practice Points
- Mycosis fungoides, the most common type of cutaneous T-cell lymphoma, is an entity for which the pathogenesis is largely unknown.
- Our case and other cases of celiac disease and mycosis fungoides seem to support the immunologic hypothesis of lymphocytic stimulation by a persistent antigen.
Nonpainful Ulcerations on the Nose and Forehead
The Diagnosis: Trigeminal Trophic Syndrome
Trigeminal trophic syndrome (TTS) is a rare condition occurring after injury to the sensory roots of the trigeminal nerve. Trigeminal trophic syndrome was first described in 1933 by Loveman1 as a complication of ablative treatment of trigeminal neuralgia; however, it has been observed with lesions to the central or peripheral nervous system that damage sensory components of the trigeminal nerve. In our patient, the cause was likely an infarction of the posterior inferior cerebellar artery, which supplies both the cerebellum and the trigeminal nuclei in the brain stem.
Other possible causes of TTS include injury from herpes zoster ophthalmicus; vertebrobasilar insufficiency; trauma; Mycobacterium leprae neuritis; spinal cord degeneration; syringobulbia; or tumors such as astrocytomas, meningiomas, and acoustic neuromas.2 The most typical presenting manifestation is a unilateral, crescent-shaped ulceration of the nasal ala, specifically where cartilage is lacking.3 Less frequently, it affects the upper lip, cheeks, forehead, scalp, and ear. It characteristically spares the nasal tip, which derives sensory innervation from the medial branch of the anterior ethmoidal nerve.4 The affected dermatomal distribution can show anesthesia or paresthesia, promoting the urge to touch, pick, or rub the area.
Histology of the TTS lesion is nondiagnostic, usually showing a mixed dermal inflammation without evidence of infection, vasculitis, or malignancy. In our patient, Gram stain, Grocott-Gomori methenamine-silver stain, and immunohistochemical stains to rule out leukemia or lymphoma were all negative. In addition, flow cytometry of the forehead tissue also was normal. Tests for human immunodeficiency virus, herpes simplex virus types 1 and 2, hepatitis, syphilis, histoplasmosis, blastomycosis, coccidioides, and tuberculosis were negative.During the biopsy, the patient was noted to have anesthesia of the skin. He also admitted to facial manipulation and was noted to have blood and tissue under the fingernails on mornings when the lesion was left uncovered overnight.
Treatment of TTS often can be difficult, especially if patients do not admit to wound manipulation or if manipulation occurs during sleep. Prevention of further ulceration can sometimes be achieved by occlusive dressing alone with mupirocin. Physical barriers can be supplemented with medications such as amitriptyline, carbamazepine, diazepam, chlorpromazine, and gabapentin to attempt to reduce paresthesia.2 Psychiatric consultation can sometimes be necessary and helpful.3 Additionally, negative pressure wound therapy has been used with success in the pediatric setting when digital manipulation is difficult to control.5 More aggressive treatments include transcutaneous electrical stimulation, stellate ganglionectomy, radiotherapy, and innervated rotation flaps.6,7
In summary, recognition of this relatively rare cause of unilateral facial erosions is critical, both to prevent counterproductive treatments such as steroids and to initiate measures to prevent further trauma to the lesion.
- Loveman A. An unusual dermatosis following section of the fifth cranial nerve. Arch Dermatol Syph. 1933;28:369-375.
- Monrad SU, Terrell JE, Aronoff DM. The trigeminal trophic syndrome: an unusual cause of nasal ulceration. J Am Acad Dermatol. 2004;50:949-952.
- Finlay AY. Trigeminal trophic syndrome. Arch Dermatol. 1979;115:1118.
- Setyadi HG, Cohen PR, Schulze KE, et al. Trigeminal trophic syndrome. South Med J. 2007;100:43-48.
- Fredeking AE, Silverman RA. Successful treatment of trigeminal trophic syndrome in a 6-year-old boy with negative pressure wound therapy. Arch Dermatol. 2008;144:984-986.
- Sadeghi P, Papay FA, Vidimos AT. Trigeminal trophic syndrome—report of four cases and review of the literature. Dermatol Surg. 2004;30:807-812.
- Willis M, Shockley W, Mobley SR. Treatment options in trigeminal trophic syndrome: a multi-institutional case series. Laryngoscope. 2011;121:712-716.
The Diagnosis: Trigeminal Trophic Syndrome
Trigeminal trophic syndrome (TTS) is a rare condition occurring after injury to the sensory roots of the trigeminal nerve. Trigeminal trophic syndrome was first described in 1933 by Loveman1 as a complication of ablative treatment of trigeminal neuralgia; however, it has been observed with lesions to the central or peripheral nervous system that damage sensory components of the trigeminal nerve. In our patient, the cause was likely an infarction of the posterior inferior cerebellar artery, which supplies both the cerebellum and the trigeminal nuclei in the brain stem.
Other possible causes of TTS include injury from herpes zoster ophthalmicus; vertebrobasilar insufficiency; trauma; Mycobacterium leprae neuritis; spinal cord degeneration; syringobulbia; or tumors such as astrocytomas, meningiomas, and acoustic neuromas.2 The most typical presenting manifestation is a unilateral, crescent-shaped ulceration of the nasal ala, specifically where cartilage is lacking.3 Less frequently, it affects the upper lip, cheeks, forehead, scalp, and ear. It characteristically spares the nasal tip, which derives sensory innervation from the medial branch of the anterior ethmoidal nerve.4 The affected dermatomal distribution can show anesthesia or paresthesia, promoting the urge to touch, pick, or rub the area.
Histology of the TTS lesion is nondiagnostic, usually showing a mixed dermal inflammation without evidence of infection, vasculitis, or malignancy. In our patient, Gram stain, Grocott-Gomori methenamine-silver stain, and immunohistochemical stains to rule out leukemia or lymphoma were all negative. In addition, flow cytometry of the forehead tissue also was normal. Tests for human immunodeficiency virus, herpes simplex virus types 1 and 2, hepatitis, syphilis, histoplasmosis, blastomycosis, coccidioides, and tuberculosis were negative.During the biopsy, the patient was noted to have anesthesia of the skin. He also admitted to facial manipulation and was noted to have blood and tissue under the fingernails on mornings when the lesion was left uncovered overnight.
Treatment of TTS often can be difficult, especially if patients do not admit to wound manipulation or if manipulation occurs during sleep. Prevention of further ulceration can sometimes be achieved by occlusive dressing alone with mupirocin. Physical barriers can be supplemented with medications such as amitriptyline, carbamazepine, diazepam, chlorpromazine, and gabapentin to attempt to reduce paresthesia.2 Psychiatric consultation can sometimes be necessary and helpful.3 Additionally, negative pressure wound therapy has been used with success in the pediatric setting when digital manipulation is difficult to control.5 More aggressive treatments include transcutaneous electrical stimulation, stellate ganglionectomy, radiotherapy, and innervated rotation flaps.6,7
In summary, recognition of this relatively rare cause of unilateral facial erosions is critical, both to prevent counterproductive treatments such as steroids and to initiate measures to prevent further trauma to the lesion.
The Diagnosis: Trigeminal Trophic Syndrome
Trigeminal trophic syndrome (TTS) is a rare condition occurring after injury to the sensory roots of the trigeminal nerve. Trigeminal trophic syndrome was first described in 1933 by Loveman1 as a complication of ablative treatment of trigeminal neuralgia; however, it has been observed with lesions to the central or peripheral nervous system that damage sensory components of the trigeminal nerve. In our patient, the cause was likely an infarction of the posterior inferior cerebellar artery, which supplies both the cerebellum and the trigeminal nuclei in the brain stem.
Other possible causes of TTS include injury from herpes zoster ophthalmicus; vertebrobasilar insufficiency; trauma; Mycobacterium leprae neuritis; spinal cord degeneration; syringobulbia; or tumors such as astrocytomas, meningiomas, and acoustic neuromas.2 The most typical presenting manifestation is a unilateral, crescent-shaped ulceration of the nasal ala, specifically where cartilage is lacking.3 Less frequently, it affects the upper lip, cheeks, forehead, scalp, and ear. It characteristically spares the nasal tip, which derives sensory innervation from the medial branch of the anterior ethmoidal nerve.4 The affected dermatomal distribution can show anesthesia or paresthesia, promoting the urge to touch, pick, or rub the area.
Histology of the TTS lesion is nondiagnostic, usually showing a mixed dermal inflammation without evidence of infection, vasculitis, or malignancy. In our patient, Gram stain, Grocott-Gomori methenamine-silver stain, and immunohistochemical stains to rule out leukemia or lymphoma were all negative. In addition, flow cytometry of the forehead tissue also was normal. Tests for human immunodeficiency virus, herpes simplex virus types 1 and 2, hepatitis, syphilis, histoplasmosis, blastomycosis, coccidioides, and tuberculosis were negative.During the biopsy, the patient was noted to have anesthesia of the skin. He also admitted to facial manipulation and was noted to have blood and tissue under the fingernails on mornings when the lesion was left uncovered overnight.
Treatment of TTS often can be difficult, especially if patients do not admit to wound manipulation or if manipulation occurs during sleep. Prevention of further ulceration can sometimes be achieved by occlusive dressing alone with mupirocin. Physical barriers can be supplemented with medications such as amitriptyline, carbamazepine, diazepam, chlorpromazine, and gabapentin to attempt to reduce paresthesia.2 Psychiatric consultation can sometimes be necessary and helpful.3 Additionally, negative pressure wound therapy has been used with success in the pediatric setting when digital manipulation is difficult to control.5 More aggressive treatments include transcutaneous electrical stimulation, stellate ganglionectomy, radiotherapy, and innervated rotation flaps.6,7
In summary, recognition of this relatively rare cause of unilateral facial erosions is critical, both to prevent counterproductive treatments such as steroids and to initiate measures to prevent further trauma to the lesion.
- Loveman A. An unusual dermatosis following section of the fifth cranial nerve. Arch Dermatol Syph. 1933;28:369-375.
- Monrad SU, Terrell JE, Aronoff DM. The trigeminal trophic syndrome: an unusual cause of nasal ulceration. J Am Acad Dermatol. 2004;50:949-952.
- Finlay AY. Trigeminal trophic syndrome. Arch Dermatol. 1979;115:1118.
- Setyadi HG, Cohen PR, Schulze KE, et al. Trigeminal trophic syndrome. South Med J. 2007;100:43-48.
- Fredeking AE, Silverman RA. Successful treatment of trigeminal trophic syndrome in a 6-year-old boy with negative pressure wound therapy. Arch Dermatol. 2008;144:984-986.
- Sadeghi P, Papay FA, Vidimos AT. Trigeminal trophic syndrome—report of four cases and review of the literature. Dermatol Surg. 2004;30:807-812.
- Willis M, Shockley W, Mobley SR. Treatment options in trigeminal trophic syndrome: a multi-institutional case series. Laryngoscope. 2011;121:712-716.
- Loveman A. An unusual dermatosis following section of the fifth cranial nerve. Arch Dermatol Syph. 1933;28:369-375.
- Monrad SU, Terrell JE, Aronoff DM. The trigeminal trophic syndrome: an unusual cause of nasal ulceration. J Am Acad Dermatol. 2004;50:949-952.
- Finlay AY. Trigeminal trophic syndrome. Arch Dermatol. 1979;115:1118.
- Setyadi HG, Cohen PR, Schulze KE, et al. Trigeminal trophic syndrome. South Med J. 2007;100:43-48.
- Fredeking AE, Silverman RA. Successful treatment of trigeminal trophic syndrome in a 6-year-old boy with negative pressure wound therapy. Arch Dermatol. 2008;144:984-986.
- Sadeghi P, Papay FA, Vidimos AT. Trigeminal trophic syndrome—report of four cases and review of the literature. Dermatol Surg. 2004;30:807-812.
- Willis M, Shockley W, Mobley SR. Treatment options in trigeminal trophic syndrome: a multi-institutional case series. Laryngoscope. 2011;121:712-716.
A 57-year-old man with a history of multiple cerebrovascular accidents was transferred from an outside hospital to our inpatient rheumatology service with nonpainful erosions of the forehead and nasal ala of 6 months’ duration. The patient reported that he initially developed a sore on the nose months prior to presentation with worsening sensations of itching and tingling on the forehead and nose. He also noted a headache and gradual loss of vision in the right eye. The patient was immunocompetent and denied arthralgia or any other skin lesions.
Adalimumab approved to treat noninfectious uveitis
The U.S. Food and Drug Administration has approved adalimumab for treatment of noninfectious intermediate, posterior, and panuveitis in adults, making it the only approved drug for the condition that is not a corticosteroid.
The approval marks the 10th approved indication for adalimumab (Humira) in the United States. It was recently approved for this indication in the European Union as well.
Patients on adalimumab had about half the risk of those on placebo to experience treatment failure (a combination of uveitis flare and decrease in visual acuity) in two pivotal phase III studies, VISUAL-I (hazard ratio, 0.5 for VISUAL-I; P less than .001) and VISUAL-II (HR, 0.57; P = .004). In the trials, patients were treated with an 80-mg loading dose of adalimumab at baseline, followed by a 40-mg subcutaneous injection at week 1 and then 40 mg every other week for up to 80 weeks.
“These approvals provide a valuable option for patients experiencing flare and vision impairment associated with this group of inflammatory diseases of the eye,” Glenn J. Jaffe, MD, of Duke University, Durham, N.C., said in an announcement from the manufacturer of adalimumab, AbbVie. “Data from the robust VISUAL clinical trial program demonstrate the value of Humira as a treatment option for patients with these serious diseases.”
The U.S. Food and Drug Administration has approved adalimumab for treatment of noninfectious intermediate, posterior, and panuveitis in adults, making it the only approved drug for the condition that is not a corticosteroid.
The approval marks the 10th approved indication for adalimumab (Humira) in the United States. It was recently approved for this indication in the European Union as well.
Patients on adalimumab had about half the risk of those on placebo to experience treatment failure (a combination of uveitis flare and decrease in visual acuity) in two pivotal phase III studies, VISUAL-I (hazard ratio, 0.5 for VISUAL-I; P less than .001) and VISUAL-II (HR, 0.57; P = .004). In the trials, patients were treated with an 80-mg loading dose of adalimumab at baseline, followed by a 40-mg subcutaneous injection at week 1 and then 40 mg every other week for up to 80 weeks.
“These approvals provide a valuable option for patients experiencing flare and vision impairment associated with this group of inflammatory diseases of the eye,” Glenn J. Jaffe, MD, of Duke University, Durham, N.C., said in an announcement from the manufacturer of adalimumab, AbbVie. “Data from the robust VISUAL clinical trial program demonstrate the value of Humira as a treatment option for patients with these serious diseases.”
The U.S. Food and Drug Administration has approved adalimumab for treatment of noninfectious intermediate, posterior, and panuveitis in adults, making it the only approved drug for the condition that is not a corticosteroid.
The approval marks the 10th approved indication for adalimumab (Humira) in the United States. It was recently approved for this indication in the European Union as well.
Patients on adalimumab had about half the risk of those on placebo to experience treatment failure (a combination of uveitis flare and decrease in visual acuity) in two pivotal phase III studies, VISUAL-I (hazard ratio, 0.5 for VISUAL-I; P less than .001) and VISUAL-II (HR, 0.57; P = .004). In the trials, patients were treated with an 80-mg loading dose of adalimumab at baseline, followed by a 40-mg subcutaneous injection at week 1 and then 40 mg every other week for up to 80 weeks.
“These approvals provide a valuable option for patients experiencing flare and vision impairment associated with this group of inflammatory diseases of the eye,” Glenn J. Jaffe, MD, of Duke University, Durham, N.C., said in an announcement from the manufacturer of adalimumab, AbbVie. “Data from the robust VISUAL clinical trial program demonstrate the value of Humira as a treatment option for patients with these serious diseases.”
Using Co-Design Methods to Create a Patient-Oriented Discharge Summary
From the OpenLab, University Health Network, Toronto, Canada (Dr. Hahn-Goldberg, Dr. Okrainec, Dr. Abrams, Mr. Huynh); School of Health Policy and Management, York University, Toronto (Dr. Hahn-Goldberg); Toronto Central Local Health Integration Network (Ms. Damba); Centre for Addiction and Mental Health, Toronto (Ms. Solomon); and the Department of Medicine, University Health Network, Toronto (Dr. Okrainec, Dr. Abrams).
Abstract
- Objective: To describe the co-design process we under-took to create a patient-oriented discharge summary (PODS) with patients, caregivers, and providers.
- Method: Descriptive report.
- Results: We designed and produced a prototype PODS, based on best practices in information design, graphic design, and patient education. Through a co-design process, patients, health care providers, designers and system planners worked together to establish what content needed to be included, as well as how it would be organized and presented. From an initial prototype, we then refined the PODS through an iterative participatory design process involving patients, including those from hard-to-reach groups such as patients with language barriers and/or low health literacy and patients with a primary psychiatric diagnosis.
- Conclusion: Co-design events and targeted focus groups are very useful for engaging patients and caregivers in the design and development of solutions aimed at improving their experience of care. It is important to include all users, especially those who are harder to reach, such as patients with language barriers and mental health conditions. Engaging health care providers is essential to ensure feasibility of those solutions.
Traditional discharge summaries are written primarily for the patient’s primary care provider and are not designed as tools to support communication between the clinician and patient regarding instructions for patients to follow at home post discharge. A more patient-centered version of the discharge summary is needed to complement the traditional format.
To enhance our patients’ care experience in the post-discharge period, we set out to co-design a patient-oriented discharge summary (PODS) with patients, caregivers, and providers. The main objective of this project was to develop a prototype PODS that not only addressed critical information that patients felt was the most important to know following discharge, but also provided this information in the most comprehensible format at hospitals within the Toronto area. The project focused on reformatting patient-care instructions for patients discharged from inpatient medical wards as these instructions presented the best opportunity for improvement.
This project drew on work done in countries such as India, South Africa, and Pakistan, where challenges with general and health literacy have led to the introduction of simplified discharge forms and medication instructions that place a greater emphasis on visual communication to improve information comprehension. For example, the use of pictograms in patient materials has been shown to increase patient understanding of and compliance with care instructions in these countries [1–3].
We have provided an overview of the PODS project elsewhere [4]. In this article, we describe the co-design process we undertook with patients, health care providers, and designers in creating a PODS.
Design Methods
We used several methods to design and develop the PODS and to engage multiple stakeholders in the design process. Among these methods were innovative techniques for understanding the patient experience of discharge, such as cultural probes [5], where patients were given journals and cameras to document their time at home after discharge, as described by Hahn-Goldberg et al [4]. Other key methods for determining the design of the PODS included:
Patient education consultation. A patient education representative with training and expertise in designing materials for patients with low health literacy was added to the team advisory committee to act as a consultant at all stages of the study. This helped to ensure that we would be following best practices in design for our target population.
Review of literature. We reviewed the literature pertaining to design for patients with low health literacy and language barriers, including the resources available through the patient education department at the University Health Network.
Review of Toronto Central Local Health Integration Network (TCLHIN) hospitals’ tools: current discharge summaries, components they included and how they were formatted.
Co-design event. We held an interprofessional design event where teams of patients, health care providers, and designers worked together to create draft PODS for 4 hypothetical patient cases.
Focus groups and surveys. We used feedback from focus groups and surveys to revise and improve the first PODS prototype.
Insights from the Literature
Studies have shown that multiple interventions tend to increase adherence, that self-management should be encouraged, and that modes of communication other than verbal must be used [6]. Visual cues, such as pictures or symbols, are useful to help with recall of medications and instructions for people with language barriers or limited health literacy [7]. Simplified written instructions and larger fonts have been found to be effective in patients with language barriers or limited health literacy, as have use of illustrated medication schedules [8]. Other guidelines include using short words and sentences, writing directly to the reader, listing important points in list format, and using left justification so there is even spacing between words.
The literature is consistent with our findings from speaking with patient education representatives and patients. Patients and caregivers noted that the PODS should be written in plain language, use large fonts, include illustrations of care and medication schedules, and include headings that are meaningful to the patient. Patients also expressed the desire for charts and lists that they could use while completing their follow-up care plan.
“My mom made me a chart of when to drink water and how much (patient).”
“We were given a sheet to record all feedings (caregiver).”
“We can provide a list of patient meds in a grid format with days of the week and times of day. We use our judgement to give this to patients. It is not standard practice (pharmacist).”
“A discharge form in ‘plain English’ should be standardized (patient).”
The Co-design Event
Solutions resulting from the design event ranged from more traditional discharge summaries that were enhanced with multiple languages and images to make things clear for patients, to solutions including interactive patient portals. There were solutions that came with stickers to color-code your medications, areas for patients to write notes, and checklists for them to keep track of all their follow-up plans.
Refining the Design Using Focus Groups and Surveys
The ideas and concepts generated during the design event were analyzed by the interdisciplinary advisory team at OpenLab. In addition, several team sessions were held with health care
This is a great piece. You guys are doing an awesome job. This would have saved me so much anxiety and fear of doing something wrong when I was discharged. I didn’t want to bother my doctors and went on a hope and prayer. Even my home care people weren’t always sure of what to do. Again this would be a great step forward in easing patients’ fears, especially senior citizens. GREAT WORK. THANKS FOR CARING.
Discussion
Innovative methods such as co-design events and targeted focus groups are very useful for
Future Plans
The PODS template has now been adapted and implemented in several hospitals in Toronto, Canada, using a supported early adopter process [10]. Future plans are to test the impact of the PODS on patient experience and health outcomes using a randomized controlled trial. Also, for now, we have focused on a paper version of PODS, but with the increasing prevalence of electronic health records and consumer-oriented health care apps, future consideration for a digital and mobile version of PODS is warranted.
Conclusion
Patients need to be prepared for discharge so that they can engage in supported self-management once they return home.
Corresponding author: Shoshana Hahn-Goldberg, PhD, 294 Mullen Dr., Thornhill, ON L4J 2P2.
Funding/support: The PODS project has been funded by the Toronto Central Local Health Integration Network.
Financial disclosures: None.
1. Rajesh R, Vidyasagar S, Varma M, Sharma S. Design And evaluation of pictograms for communicating information about adverse drug reactions to antiretroviral therapy in Indian human immunodeficiency virus positive patients. J Pharm Biomed Sci 2012;16:1–11.
2. Dowse R, Ehlers MS. The evaluation of pharmaceutical pictograms in a low-literate South African population. Patient Educ Couns 2001;45:87–99.
3. Clayton M, Syed F, Rashid A, Fayyaz U. Improving illiterate patients’ understanding and adherence to discharge medications. BMJ Qual Improv Rep 2012;1:u496.w167.
4. Hahn-Goldberg S, Okrainec K, Huynh T, et al. Co-creating patient-oriented instructions with patients, caregivers, and providers. J Hosp Med 2015;10:804–7.
5. Gaver B, Dunne T, Pacenti E. Design: cultural probes. Interactions 1999;6:21–9.
6. Sheridan SL, Halpern DJ, Viera AJ, et al. Interventions for individuals with low health literacy: a systematic review. J Health Commun 2011;16 Suppl 3:30–54.
7. Schillinger D, Machtinger EL, Wang F, et al. Language, literacy, and communication regarding medications in an anticoagulation clinic: A comparison of verbal vs. visual assessment. J Health Commun 2006;11:651–4.
8. Chugh A, Williams MV, Grigsby J, Coleman EA. Better transitions: Improving comprehension of discharge instructions. Front Health Serv Manage 2009;25:11–32.
9. IDEO. Design thinking for educators toolkit. 2nd ed. New York: IDEObooks; 2013.
10. Hahn-Goldberg S, Okrainec K, Damba C, et al. Implementing patient oriented discharge summaries (PODS): A multi-site pilot across early adopter hospitals. Healthc Q 2016;19:42–8.
From the OpenLab, University Health Network, Toronto, Canada (Dr. Hahn-Goldberg, Dr. Okrainec, Dr. Abrams, Mr. Huynh); School of Health Policy and Management, York University, Toronto (Dr. Hahn-Goldberg); Toronto Central Local Health Integration Network (Ms. Damba); Centre for Addiction and Mental Health, Toronto (Ms. Solomon); and the Department of Medicine, University Health Network, Toronto (Dr. Okrainec, Dr. Abrams).
Abstract
- Objective: To describe the co-design process we under-took to create a patient-oriented discharge summary (PODS) with patients, caregivers, and providers.
- Method: Descriptive report.
- Results: We designed and produced a prototype PODS, based on best practices in information design, graphic design, and patient education. Through a co-design process, patients, health care providers, designers and system planners worked together to establish what content needed to be included, as well as how it would be organized and presented. From an initial prototype, we then refined the PODS through an iterative participatory design process involving patients, including those from hard-to-reach groups such as patients with language barriers and/or low health literacy and patients with a primary psychiatric diagnosis.
- Conclusion: Co-design events and targeted focus groups are very useful for engaging patients and caregivers in the design and development of solutions aimed at improving their experience of care. It is important to include all users, especially those who are harder to reach, such as patients with language barriers and mental health conditions. Engaging health care providers is essential to ensure feasibility of those solutions.
Traditional discharge summaries are written primarily for the patient’s primary care provider and are not designed as tools to support communication between the clinician and patient regarding instructions for patients to follow at home post discharge. A more patient-centered version of the discharge summary is needed to complement the traditional format.
To enhance our patients’ care experience in the post-discharge period, we set out to co-design a patient-oriented discharge summary (PODS) with patients, caregivers, and providers. The main objective of this project was to develop a prototype PODS that not only addressed critical information that patients felt was the most important to know following discharge, but also provided this information in the most comprehensible format at hospitals within the Toronto area. The project focused on reformatting patient-care instructions for patients discharged from inpatient medical wards as these instructions presented the best opportunity for improvement.
This project drew on work done in countries such as India, South Africa, and Pakistan, where challenges with general and health literacy have led to the introduction of simplified discharge forms and medication instructions that place a greater emphasis on visual communication to improve information comprehension. For example, the use of pictograms in patient materials has been shown to increase patient understanding of and compliance with care instructions in these countries [1–3].
We have provided an overview of the PODS project elsewhere [4]. In this article, we describe the co-design process we undertook with patients, health care providers, and designers in creating a PODS.
Design Methods
We used several methods to design and develop the PODS and to engage multiple stakeholders in the design process. Among these methods were innovative techniques for understanding the patient experience of discharge, such as cultural probes [5], where patients were given journals and cameras to document their time at home after discharge, as described by Hahn-Goldberg et al [4]. Other key methods for determining the design of the PODS included:
Patient education consultation. A patient education representative with training and expertise in designing materials for patients with low health literacy was added to the team advisory committee to act as a consultant at all stages of the study. This helped to ensure that we would be following best practices in design for our target population.
Review of literature. We reviewed the literature pertaining to design for patients with low health literacy and language barriers, including the resources available through the patient education department at the University Health Network.
Review of Toronto Central Local Health Integration Network (TCLHIN) hospitals’ tools: current discharge summaries, components they included and how they were formatted.
Co-design event. We held an interprofessional design event where teams of patients, health care providers, and designers worked together to create draft PODS for 4 hypothetical patient cases.
Focus groups and surveys. We used feedback from focus groups and surveys to revise and improve the first PODS prototype.
Insights from the Literature
Studies have shown that multiple interventions tend to increase adherence, that self-management should be encouraged, and that modes of communication other than verbal must be used [6]. Visual cues, such as pictures or symbols, are useful to help with recall of medications and instructions for people with language barriers or limited health literacy [7]. Simplified written instructions and larger fonts have been found to be effective in patients with language barriers or limited health literacy, as have use of illustrated medication schedules [8]. Other guidelines include using short words and sentences, writing directly to the reader, listing important points in list format, and using left justification so there is even spacing between words.
The literature is consistent with our findings from speaking with patient education representatives and patients. Patients and caregivers noted that the PODS should be written in plain language, use large fonts, include illustrations of care and medication schedules, and include headings that are meaningful to the patient. Patients also expressed the desire for charts and lists that they could use while completing their follow-up care plan.
“My mom made me a chart of when to drink water and how much (patient).”
“We were given a sheet to record all feedings (caregiver).”
“We can provide a list of patient meds in a grid format with days of the week and times of day. We use our judgement to give this to patients. It is not standard practice (pharmacist).”
“A discharge form in ‘plain English’ should be standardized (patient).”
The Co-design Event
Solutions resulting from the design event ranged from more traditional discharge summaries that were enhanced with multiple languages and images to make things clear for patients, to solutions including interactive patient portals. There were solutions that came with stickers to color-code your medications, areas for patients to write notes, and checklists for them to keep track of all their follow-up plans.
Refining the Design Using Focus Groups and Surveys
The ideas and concepts generated during the design event were analyzed by the interdisciplinary advisory team at OpenLab. In addition, several team sessions were held with health care
This is a great piece. You guys are doing an awesome job. This would have saved me so much anxiety and fear of doing something wrong when I was discharged. I didn’t want to bother my doctors and went on a hope and prayer. Even my home care people weren’t always sure of what to do. Again this would be a great step forward in easing patients’ fears, especially senior citizens. GREAT WORK. THANKS FOR CARING.
Discussion
Innovative methods such as co-design events and targeted focus groups are very useful for
Future Plans
The PODS template has now been adapted and implemented in several hospitals in Toronto, Canada, using a supported early adopter process [10]. Future plans are to test the impact of the PODS on patient experience and health outcomes using a randomized controlled trial. Also, for now, we have focused on a paper version of PODS, but with the increasing prevalence of electronic health records and consumer-oriented health care apps, future consideration for a digital and mobile version of PODS is warranted.
Conclusion
Patients need to be prepared for discharge so that they can engage in supported self-management once they return home.
Corresponding author: Shoshana Hahn-Goldberg, PhD, 294 Mullen Dr., Thornhill, ON L4J 2P2.
Funding/support: The PODS project has been funded by the Toronto Central Local Health Integration Network.
Financial disclosures: None.
From the OpenLab, University Health Network, Toronto, Canada (Dr. Hahn-Goldberg, Dr. Okrainec, Dr. Abrams, Mr. Huynh); School of Health Policy and Management, York University, Toronto (Dr. Hahn-Goldberg); Toronto Central Local Health Integration Network (Ms. Damba); Centre for Addiction and Mental Health, Toronto (Ms. Solomon); and the Department of Medicine, University Health Network, Toronto (Dr. Okrainec, Dr. Abrams).
Abstract
- Objective: To describe the co-design process we under-took to create a patient-oriented discharge summary (PODS) with patients, caregivers, and providers.
- Method: Descriptive report.
- Results: We designed and produced a prototype PODS, based on best practices in information design, graphic design, and patient education. Through a co-design process, patients, health care providers, designers and system planners worked together to establish what content needed to be included, as well as how it would be organized and presented. From an initial prototype, we then refined the PODS through an iterative participatory design process involving patients, including those from hard-to-reach groups such as patients with language barriers and/or low health literacy and patients with a primary psychiatric diagnosis.
- Conclusion: Co-design events and targeted focus groups are very useful for engaging patients and caregivers in the design and development of solutions aimed at improving their experience of care. It is important to include all users, especially those who are harder to reach, such as patients with language barriers and mental health conditions. Engaging health care providers is essential to ensure feasibility of those solutions.
Traditional discharge summaries are written primarily for the patient’s primary care provider and are not designed as tools to support communication between the clinician and patient regarding instructions for patients to follow at home post discharge. A more patient-centered version of the discharge summary is needed to complement the traditional format.
To enhance our patients’ care experience in the post-discharge period, we set out to co-design a patient-oriented discharge summary (PODS) with patients, caregivers, and providers. The main objective of this project was to develop a prototype PODS that not only addressed critical information that patients felt was the most important to know following discharge, but also provided this information in the most comprehensible format at hospitals within the Toronto area. The project focused on reformatting patient-care instructions for patients discharged from inpatient medical wards as these instructions presented the best opportunity for improvement.
This project drew on work done in countries such as India, South Africa, and Pakistan, where challenges with general and health literacy have led to the introduction of simplified discharge forms and medication instructions that place a greater emphasis on visual communication to improve information comprehension. For example, the use of pictograms in patient materials has been shown to increase patient understanding of and compliance with care instructions in these countries [1–3].
We have provided an overview of the PODS project elsewhere [4]. In this article, we describe the co-design process we undertook with patients, health care providers, and designers in creating a PODS.
Design Methods
We used several methods to design and develop the PODS and to engage multiple stakeholders in the design process. Among these methods were innovative techniques for understanding the patient experience of discharge, such as cultural probes [5], where patients were given journals and cameras to document their time at home after discharge, as described by Hahn-Goldberg et al [4]. Other key methods for determining the design of the PODS included:
Patient education consultation. A patient education representative with training and expertise in designing materials for patients with low health literacy was added to the team advisory committee to act as a consultant at all stages of the study. This helped to ensure that we would be following best practices in design for our target population.
Review of literature. We reviewed the literature pertaining to design for patients with low health literacy and language barriers, including the resources available through the patient education department at the University Health Network.
Review of Toronto Central Local Health Integration Network (TCLHIN) hospitals’ tools: current discharge summaries, components they included and how they were formatted.
Co-design event. We held an interprofessional design event where teams of patients, health care providers, and designers worked together to create draft PODS for 4 hypothetical patient cases.
Focus groups and surveys. We used feedback from focus groups and surveys to revise and improve the first PODS prototype.
Insights from the Literature
Studies have shown that multiple interventions tend to increase adherence, that self-management should be encouraged, and that modes of communication other than verbal must be used [6]. Visual cues, such as pictures or symbols, are useful to help with recall of medications and instructions for people with language barriers or limited health literacy [7]. Simplified written instructions and larger fonts have been found to be effective in patients with language barriers or limited health literacy, as have use of illustrated medication schedules [8]. Other guidelines include using short words and sentences, writing directly to the reader, listing important points in list format, and using left justification so there is even spacing between words.
The literature is consistent with our findings from speaking with patient education representatives and patients. Patients and caregivers noted that the PODS should be written in plain language, use large fonts, include illustrations of care and medication schedules, and include headings that are meaningful to the patient. Patients also expressed the desire for charts and lists that they could use while completing their follow-up care plan.
“My mom made me a chart of when to drink water and how much (patient).”
“We were given a sheet to record all feedings (caregiver).”
“We can provide a list of patient meds in a grid format with days of the week and times of day. We use our judgement to give this to patients. It is not standard practice (pharmacist).”
“A discharge form in ‘plain English’ should be standardized (patient).”
The Co-design Event
Solutions resulting from the design event ranged from more traditional discharge summaries that were enhanced with multiple languages and images to make things clear for patients, to solutions including interactive patient portals. There were solutions that came with stickers to color-code your medications, areas for patients to write notes, and checklists for them to keep track of all their follow-up plans.
Refining the Design Using Focus Groups and Surveys
The ideas and concepts generated during the design event were analyzed by the interdisciplinary advisory team at OpenLab. In addition, several team sessions were held with health care
This is a great piece. You guys are doing an awesome job. This would have saved me so much anxiety and fear of doing something wrong when I was discharged. I didn’t want to bother my doctors and went on a hope and prayer. Even my home care people weren’t always sure of what to do. Again this would be a great step forward in easing patients’ fears, especially senior citizens. GREAT WORK. THANKS FOR CARING.
Discussion
Innovative methods such as co-design events and targeted focus groups are very useful for
Future Plans
The PODS template has now been adapted and implemented in several hospitals in Toronto, Canada, using a supported early adopter process [10]. Future plans are to test the impact of the PODS on patient experience and health outcomes using a randomized controlled trial. Also, for now, we have focused on a paper version of PODS, but with the increasing prevalence of electronic health records and consumer-oriented health care apps, future consideration for a digital and mobile version of PODS is warranted.
Conclusion
Patients need to be prepared for discharge so that they can engage in supported self-management once they return home.
Corresponding author: Shoshana Hahn-Goldberg, PhD, 294 Mullen Dr., Thornhill, ON L4J 2P2.
Funding/support: The PODS project has been funded by the Toronto Central Local Health Integration Network.
Financial disclosures: None.
1. Rajesh R, Vidyasagar S, Varma M, Sharma S. Design And evaluation of pictograms for communicating information about adverse drug reactions to antiretroviral therapy in Indian human immunodeficiency virus positive patients. J Pharm Biomed Sci 2012;16:1–11.
2. Dowse R, Ehlers MS. The evaluation of pharmaceutical pictograms in a low-literate South African population. Patient Educ Couns 2001;45:87–99.
3. Clayton M, Syed F, Rashid A, Fayyaz U. Improving illiterate patients’ understanding and adherence to discharge medications. BMJ Qual Improv Rep 2012;1:u496.w167.
4. Hahn-Goldberg S, Okrainec K, Huynh T, et al. Co-creating patient-oriented instructions with patients, caregivers, and providers. J Hosp Med 2015;10:804–7.
5. Gaver B, Dunne T, Pacenti E. Design: cultural probes. Interactions 1999;6:21–9.
6. Sheridan SL, Halpern DJ, Viera AJ, et al. Interventions for individuals with low health literacy: a systematic review. J Health Commun 2011;16 Suppl 3:30–54.
7. Schillinger D, Machtinger EL, Wang F, et al. Language, literacy, and communication regarding medications in an anticoagulation clinic: A comparison of verbal vs. visual assessment. J Health Commun 2006;11:651–4.
8. Chugh A, Williams MV, Grigsby J, Coleman EA. Better transitions: Improving comprehension of discharge instructions. Front Health Serv Manage 2009;25:11–32.
9. IDEO. Design thinking for educators toolkit. 2nd ed. New York: IDEObooks; 2013.
10. Hahn-Goldberg S, Okrainec K, Damba C, et al. Implementing patient oriented discharge summaries (PODS): A multi-site pilot across early adopter hospitals. Healthc Q 2016;19:42–8.
1. Rajesh R, Vidyasagar S, Varma M, Sharma S. Design And evaluation of pictograms for communicating information about adverse drug reactions to antiretroviral therapy in Indian human immunodeficiency virus positive patients. J Pharm Biomed Sci 2012;16:1–11.
2. Dowse R, Ehlers MS. The evaluation of pharmaceutical pictograms in a low-literate South African population. Patient Educ Couns 2001;45:87–99.
3. Clayton M, Syed F, Rashid A, Fayyaz U. Improving illiterate patients’ understanding and adherence to discharge medications. BMJ Qual Improv Rep 2012;1:u496.w167.
4. Hahn-Goldberg S, Okrainec K, Huynh T, et al. Co-creating patient-oriented instructions with patients, caregivers, and providers. J Hosp Med 2015;10:804–7.
5. Gaver B, Dunne T, Pacenti E. Design: cultural probes. Interactions 1999;6:21–9.
6. Sheridan SL, Halpern DJ, Viera AJ, et al. Interventions for individuals with low health literacy: a systematic review. J Health Commun 2011;16 Suppl 3:30–54.
7. Schillinger D, Machtinger EL, Wang F, et al. Language, literacy, and communication regarding medications in an anticoagulation clinic: A comparison of verbal vs. visual assessment. J Health Commun 2006;11:651–4.
8. Chugh A, Williams MV, Grigsby J, Coleman EA. Better transitions: Improving comprehension of discharge instructions. Front Health Serv Manage 2009;25:11–32.
9. IDEO. Design thinking for educators toolkit. 2nd ed. New York: IDEObooks; 2013.
10. Hahn-Goldberg S, Okrainec K, Damba C, et al. Implementing patient oriented discharge summaries (PODS): A multi-site pilot across early adopter hospitals. Healthc Q 2016;19:42–8.
Feds Raise Buprenorphine Patient Loads
Qualified physicians soon will be allowed to provide medication-assisted treatment (MAT) with buprenorphine to nearly triple the number of patients under a new rule by the Substance Abuse and Mental Health Services Administration (SAMHSA).
The rule, announced by the Health & Human Services department on July 6, allows qualified practitioners to prescribe buprenorphine to up to 275 patients, up from the previous limit of 100. Raising the cap will mean treatment of 10,000-70,000 more patients within the first year, according to HHS. The rule takes effect Aug. 5.
In addition, HHS plans to eliminate pain management questions from the payment scoring calculation of the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey. The removal aims to relieve pressure on clinicians to overprescribe opioids since scores on the HCAHPS survey are tied to Medicare payments to hospitals. Hospitals would continue to use the questions to survey patients about their inpatient pain management experience, but the questions would not affect the level of payment that hospitals receive, HHS Secretary Sylvia M. Burwell said during a press conference. The changes are part of a number of steps announced by HHS to build on the agency’s Opioid Initiative.
“Together, these announcements will help us take additional steps forward,” Ms. Burwell said. “They increase access to help more people receive the evidence-based treatment they need. They help providers safely prescribe while helping their patients manage chronic pain, and they fill in the gaps of our understanding of this epidemic and how best to fight it.”
The agency also released a report on ongoing, federally funded opioid misuse and pain treatment research. The report is designed to help stakeholders and external funders of research in avoiding unnecessary duplication of research currently underway, according to HHS. The agency plans to launch more than a dozen new scientific studies on opioid misuse and pain treatment in the near future, Ms. Burwell said.
Another new rule mandates that Indian Health Service (IHS) clinicians and pharmacists check their state Prescription Drug Monitoring Program database prior to prescribing or dispensing any opioid for more than 7 days. The new policy is effective immediately for IHS clinicians authorized to prescribe opioids.
Amid the new steps, Secretary Burwell and others called on Congress to approve the President Obama’s proposed $1.1 billion in new funding to further address prescription opioid abuse and heroin use. Legislators are meeting July 6 to weigh final legislation aimed at the opioid epidemic, but have thus far, not fully supported the president’s proposed funding. In a July 5 letter, Democrats vowed to oppose the bill unless it included more money to treat addicted patients.
“If you want treatment for an opioid use disorder, you should be able to access it when you need it,” Michael Botticelli, director of National Drug Control Policy said during the press conference. “There is still time for Congress to do what’s right.”
Qualified physicians soon will be allowed to provide medication-assisted treatment (MAT) with buprenorphine to nearly triple the number of patients under a new rule by the Substance Abuse and Mental Health Services Administration (SAMHSA).
The rule, announced by the Health & Human Services department on July 6, allows qualified practitioners to prescribe buprenorphine to up to 275 patients, up from the previous limit of 100. Raising the cap will mean treatment of 10,000-70,000 more patients within the first year, according to HHS. The rule takes effect Aug. 5.
In addition, HHS plans to eliminate pain management questions from the payment scoring calculation of the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey. The removal aims to relieve pressure on clinicians to overprescribe opioids since scores on the HCAHPS survey are tied to Medicare payments to hospitals. Hospitals would continue to use the questions to survey patients about their inpatient pain management experience, but the questions would not affect the level of payment that hospitals receive, HHS Secretary Sylvia M. Burwell said during a press conference. The changes are part of a number of steps announced by HHS to build on the agency’s Opioid Initiative.
“Together, these announcements will help us take additional steps forward,” Ms. Burwell said. “They increase access to help more people receive the evidence-based treatment they need. They help providers safely prescribe while helping their patients manage chronic pain, and they fill in the gaps of our understanding of this epidemic and how best to fight it.”
The agency also released a report on ongoing, federally funded opioid misuse and pain treatment research. The report is designed to help stakeholders and external funders of research in avoiding unnecessary duplication of research currently underway, according to HHS. The agency plans to launch more than a dozen new scientific studies on opioid misuse and pain treatment in the near future, Ms. Burwell said.
Another new rule mandates that Indian Health Service (IHS) clinicians and pharmacists check their state Prescription Drug Monitoring Program database prior to prescribing or dispensing any opioid for more than 7 days. The new policy is effective immediately for IHS clinicians authorized to prescribe opioids.
Amid the new steps, Secretary Burwell and others called on Congress to approve the President Obama’s proposed $1.1 billion in new funding to further address prescription opioid abuse and heroin use. Legislators are meeting July 6 to weigh final legislation aimed at the opioid epidemic, but have thus far, not fully supported the president’s proposed funding. In a July 5 letter, Democrats vowed to oppose the bill unless it included more money to treat addicted patients.
“If you want treatment for an opioid use disorder, you should be able to access it when you need it,” Michael Botticelli, director of National Drug Control Policy said during the press conference. “There is still time for Congress to do what’s right.”
Qualified physicians soon will be allowed to provide medication-assisted treatment (MAT) with buprenorphine to nearly triple the number of patients under a new rule by the Substance Abuse and Mental Health Services Administration (SAMHSA).
The rule, announced by the Health & Human Services department on July 6, allows qualified practitioners to prescribe buprenorphine to up to 275 patients, up from the previous limit of 100. Raising the cap will mean treatment of 10,000-70,000 more patients within the first year, according to HHS. The rule takes effect Aug. 5.
In addition, HHS plans to eliminate pain management questions from the payment scoring calculation of the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey. The removal aims to relieve pressure on clinicians to overprescribe opioids since scores on the HCAHPS survey are tied to Medicare payments to hospitals. Hospitals would continue to use the questions to survey patients about their inpatient pain management experience, but the questions would not affect the level of payment that hospitals receive, HHS Secretary Sylvia M. Burwell said during a press conference. The changes are part of a number of steps announced by HHS to build on the agency’s Opioid Initiative.
“Together, these announcements will help us take additional steps forward,” Ms. Burwell said. “They increase access to help more people receive the evidence-based treatment they need. They help providers safely prescribe while helping their patients manage chronic pain, and they fill in the gaps of our understanding of this epidemic and how best to fight it.”
The agency also released a report on ongoing, federally funded opioid misuse and pain treatment research. The report is designed to help stakeholders and external funders of research in avoiding unnecessary duplication of research currently underway, according to HHS. The agency plans to launch more than a dozen new scientific studies on opioid misuse and pain treatment in the near future, Ms. Burwell said.
Another new rule mandates that Indian Health Service (IHS) clinicians and pharmacists check their state Prescription Drug Monitoring Program database prior to prescribing or dispensing any opioid for more than 7 days. The new policy is effective immediately for IHS clinicians authorized to prescribe opioids.
Amid the new steps, Secretary Burwell and others called on Congress to approve the President Obama’s proposed $1.1 billion in new funding to further address prescription opioid abuse and heroin use. Legislators are meeting July 6 to weigh final legislation aimed at the opioid epidemic, but have thus far, not fully supported the president’s proposed funding. In a July 5 letter, Democrats vowed to oppose the bill unless it included more money to treat addicted patients.
“If you want treatment for an opioid use disorder, you should be able to access it when you need it,” Michael Botticelli, director of National Drug Control Policy said during the press conference. “There is still time for Congress to do what’s right.”
Feds raise buprenorphine patient loads
Qualified physicians soon will be allowed to provide medication-assisted treatment (MAT) with buprenorphine to nearly triple the number of patients under a new rule by the Substance Abuse and Mental Health Services Administration (SAMHSA).
The rule, announced by the Health & Human Services department on July 6, allows qualified practitioners to prescribe buprenorphine to up to 275 patients, up from the previous limit of 100. Raising the cap will mean treatment of 10,000-70,000 more patients within the first year, according to HHS. The rule takes effect Aug. 5.
In addition, HHS plans to eliminate pain management questions from the payment scoring calculation of the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey. The removal aims to relieve pressure on clinicians to overprescribe opioids since scores on the HCAHPS survey are tied to Medicare payments to hospitals. Hospitals would continue to use the questions to survey patients about their inpatient pain management experience, but the questions would not affect the level of payment that hospitals receive, HHS Secretary Sylvia M. Burwell said during a press conference. The changes are part of a number of steps announced by HHS to build on the agency’s Opioid Initiative.
“Together, these announcements will help us take additional steps forward,” Ms. Burwell said. “They increase access to help more people receive the evidence-based treatment they need. They help providers safely prescribe while helping their patients manage chronic pain, and they fill in the gaps of our understanding of this epidemic and how best to fight it.”
The agency also released a report on ongoing, federally funded opioid misuse and pain treatment research. The report is designed to help stakeholders and external funders of research in avoiding unnecessary duplication of research currently underway, according to HHS. The agency plans to launch more than a dozen new scientific studies on opioid misuse and pain treatment in the near future, Ms. Burwell said.
Another new rule mandates that Indian Health Service (IHS) clinicians and pharmacists check their state Prescription Drug Monitoring Program database prior to prescribing or dispensing any opioid for more than 7 days. The new policy is effective immediately for IHS clinicians authorized to prescribe opioids.
Amid the new steps, Secretary Burwell and others called on Congress to approve the President Obama’s proposed $1.1 billion in new funding to further address prescription opioid abuse and heroin use. Legislators are meeting July 6 to weigh final legislation aimed at the opioid epidemic, but have thus far, not fully supported the president’s proposed funding. In a July 5 letter, Democrats vowed to oppose the bill unless it included more money to treat addicted patients.
“If you want treatment for an opioid use disorder, you should be able to access it when you need it,” Michael Botticelli, director of National Drug Control Policy said during the press conference. “There is still time for Congress to do what’s right.”
On Twitter @legal_med
Qualified physicians soon will be allowed to provide medication-assisted treatment (MAT) with buprenorphine to nearly triple the number of patients under a new rule by the Substance Abuse and Mental Health Services Administration (SAMHSA).
The rule, announced by the Health & Human Services department on July 6, allows qualified practitioners to prescribe buprenorphine to up to 275 patients, up from the previous limit of 100. Raising the cap will mean treatment of 10,000-70,000 more patients within the first year, according to HHS. The rule takes effect Aug. 5.
In addition, HHS plans to eliminate pain management questions from the payment scoring calculation of the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey. The removal aims to relieve pressure on clinicians to overprescribe opioids since scores on the HCAHPS survey are tied to Medicare payments to hospitals. Hospitals would continue to use the questions to survey patients about their inpatient pain management experience, but the questions would not affect the level of payment that hospitals receive, HHS Secretary Sylvia M. Burwell said during a press conference. The changes are part of a number of steps announced by HHS to build on the agency’s Opioid Initiative.
“Together, these announcements will help us take additional steps forward,” Ms. Burwell said. “They increase access to help more people receive the evidence-based treatment they need. They help providers safely prescribe while helping their patients manage chronic pain, and they fill in the gaps of our understanding of this epidemic and how best to fight it.”
The agency also released a report on ongoing, federally funded opioid misuse and pain treatment research. The report is designed to help stakeholders and external funders of research in avoiding unnecessary duplication of research currently underway, according to HHS. The agency plans to launch more than a dozen new scientific studies on opioid misuse and pain treatment in the near future, Ms. Burwell said.
Another new rule mandates that Indian Health Service (IHS) clinicians and pharmacists check their state Prescription Drug Monitoring Program database prior to prescribing or dispensing any opioid for more than 7 days. The new policy is effective immediately for IHS clinicians authorized to prescribe opioids.
Amid the new steps, Secretary Burwell and others called on Congress to approve the President Obama’s proposed $1.1 billion in new funding to further address prescription opioid abuse and heroin use. Legislators are meeting July 6 to weigh final legislation aimed at the opioid epidemic, but have thus far, not fully supported the president’s proposed funding. In a July 5 letter, Democrats vowed to oppose the bill unless it included more money to treat addicted patients.
“If you want treatment for an opioid use disorder, you should be able to access it when you need it,” Michael Botticelli, director of National Drug Control Policy said during the press conference. “There is still time for Congress to do what’s right.”
On Twitter @legal_med
Qualified physicians soon will be allowed to provide medication-assisted treatment (MAT) with buprenorphine to nearly triple the number of patients under a new rule by the Substance Abuse and Mental Health Services Administration (SAMHSA).
The rule, announced by the Health & Human Services department on July 6, allows qualified practitioners to prescribe buprenorphine to up to 275 patients, up from the previous limit of 100. Raising the cap will mean treatment of 10,000-70,000 more patients within the first year, according to HHS. The rule takes effect Aug. 5.
In addition, HHS plans to eliminate pain management questions from the payment scoring calculation of the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey. The removal aims to relieve pressure on clinicians to overprescribe opioids since scores on the HCAHPS survey are tied to Medicare payments to hospitals. Hospitals would continue to use the questions to survey patients about their inpatient pain management experience, but the questions would not affect the level of payment that hospitals receive, HHS Secretary Sylvia M. Burwell said during a press conference. The changes are part of a number of steps announced by HHS to build on the agency’s Opioid Initiative.
“Together, these announcements will help us take additional steps forward,” Ms. Burwell said. “They increase access to help more people receive the evidence-based treatment they need. They help providers safely prescribe while helping their patients manage chronic pain, and they fill in the gaps of our understanding of this epidemic and how best to fight it.”
The agency also released a report on ongoing, federally funded opioid misuse and pain treatment research. The report is designed to help stakeholders and external funders of research in avoiding unnecessary duplication of research currently underway, according to HHS. The agency plans to launch more than a dozen new scientific studies on opioid misuse and pain treatment in the near future, Ms. Burwell said.
Another new rule mandates that Indian Health Service (IHS) clinicians and pharmacists check their state Prescription Drug Monitoring Program database prior to prescribing or dispensing any opioid for more than 7 days. The new policy is effective immediately for IHS clinicians authorized to prescribe opioids.
Amid the new steps, Secretary Burwell and others called on Congress to approve the President Obama’s proposed $1.1 billion in new funding to further address prescription opioid abuse and heroin use. Legislators are meeting July 6 to weigh final legislation aimed at the opioid epidemic, but have thus far, not fully supported the president’s proposed funding. In a July 5 letter, Democrats vowed to oppose the bill unless it included more money to treat addicted patients.
“If you want treatment for an opioid use disorder, you should be able to access it when you need it,” Michael Botticelli, director of National Drug Control Policy said during the press conference. “There is still time for Congress to do what’s right.”
On Twitter @legal_med