User login
A Spontaneous Internal Carotid Artery Dissection Presenting With Headache and Miosis
Internal carotid artery dissection (ICAD) is an uncommon cause of stroke that typically occurs in the setting of (often minor) trauma but can also occur spontaneously. Patients with ICAD typically present with ipsilateral head, face, or neck pain. In approximately half of ICAD cases, an acute partial, painful Horner syndrome is present on examination. Although computed tomography angiography (CTA) is currently the imaging study of choice, magnetic resonance imaging/magnetic resonance angiography (MRI/MRA) and Doppler ultrasound can also be used. Management options include thrombolysis, antiplatelet or anticoagulation therapy, and endovascular or surgical intervention.
Case
A 56-year-old man with a history of migraines presented to the ED with a chief complaint of a 4-day history of right-sided headache. He stated that the pain felt different from his usual migraines and was located behind his right eye. Prior to presentation at the ED, the patient had initially visited an urgent care facility for evaluation. The physician who evaluated the patient at the urgent care facility noted the patient’s left eye appeared dilated and referred him to the ED for evaluation.
The patient further stated that the day prior to presentation, one of his friends had also remarked that the patient’s left eye appeared to be enlarged. The patient denied any visual disturbances, focal weakness, nausea, vomiting, neck pain, or stiffness. His medical history was significant for paroxysmal atrial flutter and hypertension. Regarding medications, the patient was taking dronedarone, clonazepam, omeprazole, and metoprolol.
On physical examination, the patient’s vital signs were: blood pressure, 162/109 mm Hg; heart rate, 85 beats/minute and regular; respiratory rate, 18 breaths/minute; and temperature, 98.6°F. Oxygen saturation was 98% on room air. The patient appeared to be in no acute distress. Ocular examination revealed a dilated left pupil of approximately 5 to 6 mm, and a right pupil measuring approximately 3 mm. Both pupils reacted to light, and the extraocular muscles were intact. The patient’s face appeared symmetrical and had intact sensation. He had normal speech, midline tongue, and good bilateral shoulder shrug. The neck examination revealed normal range of motion with full flexion, without jugular vein distention, lymphadenopathy, or palpable thyroid. The cardiovascular, lung, and abdominal examinations were all normal. The neurological examination showed the patient to be awake, alert, and oriented to person, place, and time. He exhibited 5/5 motor strength in all four extremities, normal gait, and normal finger-to-nose performance; his reflexes were 2+ and symmetrical.
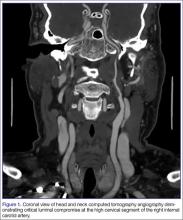
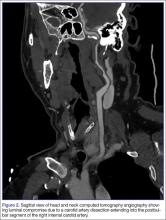
The emergency physician (EP) ordered a stat noncontrast CT scan of the head, complete blood count (CBC), and basic metabolic panel (BMP). The CT scan of the head was interpreted by the radiologist as “no acute intracranial abnormality,” and the CBC and the BMP were normal. Since the EP was concerned about a carotid artery aneurysm or dissection, he ordered a CTA of the head and neck (Figures 1 and 2). The CTA was read as:
Long segment dissection of postbulbar cervical segment of right internal carotid artery, very likely extending into the intracranial segment with critical stenosis at the junction of the high cervical segment and proximal petrous segment. Preserved runoff circulation to the anterior and middle cerebral arteries with robust capacity for collateral support in the context of patent anterior and posterior communicating arteries.
Based on the radiology report, the EP consulted with vascular surgery services, and ordered a Doppler study of the ICAs. The Doppler study demonstrated “arterial thrombus in the right distal extracranial internal carotid artery with hemodynamics suggestive of a distal occlusion/significant obstruction.” The patient was started on an intravenous (IV) heparin drip and admitted to the hospital. Three days later he experienced sudden onset of left arm weakness. An emergent CTA of the head and neck revealed an embolic occlusion of mid-to-distal M1 segment of the right middle cerebral artery. The patient was immediately evaluated by interventional radiology for possible clot removal; however, based on his rapid neurological improvement, he was instead treated medically with aspirin and clopidogrel and continued to show significant neurological improvement. He was discharged home on hospital day 8 on both antiplatelet agents with minimal neurological deficit.
Discussion
Even though ICAD accounts for only 1% to 2% of all strokes, it is responsible for 10% to 25% of strokes in young and middle-aged adults.1-3 The peak incidence for ICAD is in the fifth decade, and it affects men and women equally.4 The extracranial portion of the ICA is the most commonly affected vessel (>90%); dissections of the intracranial portion are associated with greater neurological deficits and have a poorer prognosis.2,5 Cerebral ischemia resulting from a dissection of the extracranial ICA may occur days to weeks after the onset of local symptoms such as head or neck pain, Horner syndrome, or tinnitus.5
In ICAD, a tear in the artery wall causes blood to enter the tunica media of the vessel, forming an intramural hematoma.2,6 This may result in either stenosis of the lumen of the vessel from the enlarging hematoma or an outward aneurysmal dilatation of the vessel that compresses surrounding structures.2,6 Subsequent cerebral ischemia is the result of either arterial embolism or hemodynamic compromise from vessel stenosis.2,3
Causes
Dissections often occur secondary to trauma, though the severity of the trauma may be quite minor.2,6 Seemingly trivial mechanisms that have been associated with dissections include nose blowing, coughing, sudden neck turning, and prolonged telephone conversations. Other known causes are motor vehicle accidents and chiropractic maneuvers. Inherited connective tissue disorders, including Ehlers-Danlos syndrome, Marfan syndrome, fibromuscular dysplasia, and osteogenesis imperfecta, are associated with spontaneous ICAD.2,6
Symptoms
The most common presenting symptom of carotid dissection in approximately two-thirds of patients is ipsilateral head, face, or neck pain,2,6 typically described as sharp, constant, and nonthrobbing. Patients report a subjective bruit in 21% to 39% of cases.7 An acute partial painful Horner syndrome is strongly associated with ICAD, but is present in fewer than half of such patients.6 It is characterized by miosis and ptosis and is the result of compression of the ascending sympathetic fibers that travel alongside the ICA within the carotid sheath. Anhidrosis is not present because the fibers for sweat function in the face travel along the external carotid artery.2
Differential Diagnosis
While there is overlap between strokes caused by ICAD versus plaque, some features can help guide the clinician. Symptomatic carotid disease will frequently present with a history of one or more transient ischemic attacks characterized by focal neurological dysfunction or transient monocular blindness—typically within the previous 6 months.8 This history is not usually present in patients with ICAD. Secondly, pain is a much more prominent symptom in ICAD compared to patients with severe carotid atherosclerosis. The history of trauma, even minor, should make dissection higher on the differential diagnosis. Fortunately, the imaging studies to evaluate for these two diseases are the same.
Assessing Pupillary Asymmetry and Ptosis
Careful attention must be paid to assessing a patient for pupillary asymmetry. In a patient with anisocoria, determining the abnormal pupil may require examination of the patient in both bright and dark lighting conditions. The first step is to examine the patient’s pupils under normal lighting conditions. The next step is to assess each pupil’s response to shining a bright light in each eye. The abnormal pupil is the pupil that does not respond well or at all to bright light shone directly in the eye. If the anisocoria is greatest in bright light, the larger pupil is the abnormal pupil. When the anisocoria is greater in dark conditions, the smaller pupil is the abnormal pupil. In this case, the patient’s abnormal pupil was incorrectly diagnosed as the contralateral larger pupil (ie, left)—highlighting the importance of performing a complete pupillary examination in all patients presenting with neurological symptoms.9
Furthermore, as demonstrated in this case, ptosis in a patient with Horner syndrome caused by an ICAD can be subtle. The ptosis is the result of paralysis of Müeller’s muscle, which is innervated by the sympathetic pathway. The levator palpebrae superioris, which causes the more profound ptosis seen in third nerve palsies, is unaffected.10
Imaging Studies
Once the diagnosis of ICAD is suspected, appropriate vascular imaging must be obtained. Digital subtraction angiography has historically been the gold standard for vascular imaging of the neck vessels, but it has largely been replaced by less invasive and more readily available imaging modalities such as CTA and MRI/MRA.11
Computed Tomography Angiography. This is a widely available, rapid imaging choice and has a sensitivity of 80% to 95% in the detection of ICAD.7 It has a greater ability than MRI to identify dissection features such as intimal flaps, pseudoaneurysms, and high-grade stenosis versus occlusion. One of its disadvantages is the need for iodinated contrast, which can limit the ability to obtain the test in those with renal disease or patients with true allergies to IV contrast material. In addition, a mural hematoma can be mistaken for a noncalcified atherosclerotic plaque in the vessel lumen.6
Magnetic Resonance Imaging and Magnetic Resonance Angiography. Both MRI and MRA are also frequently used to diagnose ICAD. The intramural hematoma displays a hyperdense signal on T1-weighted images and has a characteristic crescent shape adjacent to the lumen.11 Magnetic resonance imaging studies are also sensitive in detecting cerebral ischemia resulting from the dissection. However, the sensitivity of MRI/MRA is highest 2 days after the dissection has occurred.2
Doppler Ultrasound. This is another imaging modality used to detect ICAD—one that is noninvasive, less expensive, requires no contrast material, and is widely available. Limitations of Doppler ultrasound include the inability to scan the distal ICA and a lower sensitivity in detecting dissections that cause low-grade stenosis.2 It is more commonly used for follow-up monitoring of dissections.
Management
There are several options for managing ICAD. In patients with unstable lesions, progressing neurological deficit, or further strokes, endovascular stenting has been shown to have a technical success rate of 99% and a procedural complication rate of 1.3%.12 Similarly, if the patient exhibits symptoms of cerebral ischemia, severe narrowing of the arterial lumen, or an unstable plaque, IV heparin is frequently used, followed by warfarin.13 However, since the majority of carotid and vertebral artery dissections heal spontaneously,14 antithrombotic therapies, including aspirin, clopidogrel or warfarin, are often prescribed to prevent thromboembolic complications.
Conclusion
Diagnosing ICAD requires knowledge of the typical history and presenting features of the disease. Careful attention to the ocular examination must be undertaken in any patient presenting with headache or face or neck pain, because the findings can be subtle. In a patient in whom ICAD is suspected, imaging with CTA or MRI/MRA should be performed. Early consultation with vascular surgery services can help determine the most appropriate treatment strategy.
1. CADISS trial investigators, Markus HS, Hayter E, et al. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol. 2015;14(4):361-367.
2. Thanvi B, Munshi SK, Dawson SL, Robinson TG. Carotid and vertebral artery dissection syndromes. Postgrad Med J. 2005;81(956):383-388.
3. Lucas C, Moulin T, Deplanque D, Tatu L, Chavot D. Stroke patterns of internal carotid artery dissection in 40 patients. Stroke. 1998;29(12):2646-2648.
4. Schievink WI, Mokri B, O’Fallon WM. Recurrent spontaneous cervical-artery dissection. N Engl J Med. 1994;330(6):393-397.
5. Biousse V, D’Anglejan-Chatillon J, Touboul PJ, Amarenco P, Bousser MG. Time course of symptoms in extracranial carotid artery dissections. A series of 80 patients. Stroke. 1995;26(2):235-239.
6. Kasravi N, Leung A, Silver I, Burneo JG. Dissection of the internal carotid artery causing Horner syndrome and palsy of cranial nerve XII. CMAJ. 2010;182(9):E373-E377.
1. Borgman CJ. Horner syndrome secondary to internal carotid artery dissection after a short-distance endurance run: a case study and review. J Optom. 2012;5:209-216.
2. Mohler ER III, Fairman RM. Management of symptomatic carotid atherosclerotic disease. UpToDate Web site. http://www.uptodate.com/contents/management-of-symptomatic-carotid-atherosclerotic-disease. Updated February 24, 2016. Accessed May 6, 2016.
3. Mann J. Anisocoria guidemap. Life in the Fastlane Web site. http://lifeinthefastlane.com/resources/jeff-manns-em-guidemaps/anisocoria-guidemap/. Accessed March 15, 2016.
10. Kedar S, Biousse V, Newman NJ. Horner syndrome. UpToDate Web site. http://www.uptodate.com/contents/horner-syndrome. Updated July 14, 2015. Accessed May 6, 2016.
11. Vertinsky AT, Schwartz NE, Fischbein NJ, Rosenberg J, Albers GW, Zaharchuk G. Comparison of multidetector CT angiography and MR imaging of cervical artery dissection. AJNR Am J Neuroradiol. 2008;29(9):1753-1760.
12. Pham MH, Rahme RJ, Arnaout O, et al. Endovascular stenting of extracranial carotid and vertebral artery dissections: a systematic review of the literature. Neurosurgery. 2011;68(4):856-866.
13. Caplan LR. Dissections of brain-supplying arteries. Nat Clin Pract Neurol. 2008;4(1):34-42.
14. Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898-906.
Internal carotid artery dissection (ICAD) is an uncommon cause of stroke that typically occurs in the setting of (often minor) trauma but can also occur spontaneously. Patients with ICAD typically present with ipsilateral head, face, or neck pain. In approximately half of ICAD cases, an acute partial, painful Horner syndrome is present on examination. Although computed tomography angiography (CTA) is currently the imaging study of choice, magnetic resonance imaging/magnetic resonance angiography (MRI/MRA) and Doppler ultrasound can also be used. Management options include thrombolysis, antiplatelet or anticoagulation therapy, and endovascular or surgical intervention.
Case
A 56-year-old man with a history of migraines presented to the ED with a chief complaint of a 4-day history of right-sided headache. He stated that the pain felt different from his usual migraines and was located behind his right eye. Prior to presentation at the ED, the patient had initially visited an urgent care facility for evaluation. The physician who evaluated the patient at the urgent care facility noted the patient’s left eye appeared dilated and referred him to the ED for evaluation.
The patient further stated that the day prior to presentation, one of his friends had also remarked that the patient’s left eye appeared to be enlarged. The patient denied any visual disturbances, focal weakness, nausea, vomiting, neck pain, or stiffness. His medical history was significant for paroxysmal atrial flutter and hypertension. Regarding medications, the patient was taking dronedarone, clonazepam, omeprazole, and metoprolol.
On physical examination, the patient’s vital signs were: blood pressure, 162/109 mm Hg; heart rate, 85 beats/minute and regular; respiratory rate, 18 breaths/minute; and temperature, 98.6°F. Oxygen saturation was 98% on room air. The patient appeared to be in no acute distress. Ocular examination revealed a dilated left pupil of approximately 5 to 6 mm, and a right pupil measuring approximately 3 mm. Both pupils reacted to light, and the extraocular muscles were intact. The patient’s face appeared symmetrical and had intact sensation. He had normal speech, midline tongue, and good bilateral shoulder shrug. The neck examination revealed normal range of motion with full flexion, without jugular vein distention, lymphadenopathy, or palpable thyroid. The cardiovascular, lung, and abdominal examinations were all normal. The neurological examination showed the patient to be awake, alert, and oriented to person, place, and time. He exhibited 5/5 motor strength in all four extremities, normal gait, and normal finger-to-nose performance; his reflexes were 2+ and symmetrical.
The emergency physician (EP) ordered a stat noncontrast CT scan of the head, complete blood count (CBC), and basic metabolic panel (BMP). The CT scan of the head was interpreted by the radiologist as “no acute intracranial abnormality,” and the CBC and the BMP were normal. Since the EP was concerned about a carotid artery aneurysm or dissection, he ordered a CTA of the head and neck (Figures 1 and 2). The CTA was read as:
Long segment dissection of postbulbar cervical segment of right internal carotid artery, very likely extending into the intracranial segment with critical stenosis at the junction of the high cervical segment and proximal petrous segment. Preserved runoff circulation to the anterior and middle cerebral arteries with robust capacity for collateral support in the context of patent anterior and posterior communicating arteries.
Based on the radiology report, the EP consulted with vascular surgery services, and ordered a Doppler study of the ICAs. The Doppler study demonstrated “arterial thrombus in the right distal extracranial internal carotid artery with hemodynamics suggestive of a distal occlusion/significant obstruction.” The patient was started on an intravenous (IV) heparin drip and admitted to the hospital. Three days later he experienced sudden onset of left arm weakness. An emergent CTA of the head and neck revealed an embolic occlusion of mid-to-distal M1 segment of the right middle cerebral artery. The patient was immediately evaluated by interventional radiology for possible clot removal; however, based on his rapid neurological improvement, he was instead treated medically with aspirin and clopidogrel and continued to show significant neurological improvement. He was discharged home on hospital day 8 on both antiplatelet agents with minimal neurological deficit.
Discussion
Even though ICAD accounts for only 1% to 2% of all strokes, it is responsible for 10% to 25% of strokes in young and middle-aged adults.1-3 The peak incidence for ICAD is in the fifth decade, and it affects men and women equally.4 The extracranial portion of the ICA is the most commonly affected vessel (>90%); dissections of the intracranial portion are associated with greater neurological deficits and have a poorer prognosis.2,5 Cerebral ischemia resulting from a dissection of the extracranial ICA may occur days to weeks after the onset of local symptoms such as head or neck pain, Horner syndrome, or tinnitus.5
In ICAD, a tear in the artery wall causes blood to enter the tunica media of the vessel, forming an intramural hematoma.2,6 This may result in either stenosis of the lumen of the vessel from the enlarging hematoma or an outward aneurysmal dilatation of the vessel that compresses surrounding structures.2,6 Subsequent cerebral ischemia is the result of either arterial embolism or hemodynamic compromise from vessel stenosis.2,3
Causes
Dissections often occur secondary to trauma, though the severity of the trauma may be quite minor.2,6 Seemingly trivial mechanisms that have been associated with dissections include nose blowing, coughing, sudden neck turning, and prolonged telephone conversations. Other known causes are motor vehicle accidents and chiropractic maneuvers. Inherited connective tissue disorders, including Ehlers-Danlos syndrome, Marfan syndrome, fibromuscular dysplasia, and osteogenesis imperfecta, are associated with spontaneous ICAD.2,6
Symptoms
The most common presenting symptom of carotid dissection in approximately two-thirds of patients is ipsilateral head, face, or neck pain,2,6 typically described as sharp, constant, and nonthrobbing. Patients report a subjective bruit in 21% to 39% of cases.7 An acute partial painful Horner syndrome is strongly associated with ICAD, but is present in fewer than half of such patients.6 It is characterized by miosis and ptosis and is the result of compression of the ascending sympathetic fibers that travel alongside the ICA within the carotid sheath. Anhidrosis is not present because the fibers for sweat function in the face travel along the external carotid artery.2
Differential Diagnosis
While there is overlap between strokes caused by ICAD versus plaque, some features can help guide the clinician. Symptomatic carotid disease will frequently present with a history of one or more transient ischemic attacks characterized by focal neurological dysfunction or transient monocular blindness—typically within the previous 6 months.8 This history is not usually present in patients with ICAD. Secondly, pain is a much more prominent symptom in ICAD compared to patients with severe carotid atherosclerosis. The history of trauma, even minor, should make dissection higher on the differential diagnosis. Fortunately, the imaging studies to evaluate for these two diseases are the same.
Assessing Pupillary Asymmetry and Ptosis
Careful attention must be paid to assessing a patient for pupillary asymmetry. In a patient with anisocoria, determining the abnormal pupil may require examination of the patient in both bright and dark lighting conditions. The first step is to examine the patient’s pupils under normal lighting conditions. The next step is to assess each pupil’s response to shining a bright light in each eye. The abnormal pupil is the pupil that does not respond well or at all to bright light shone directly in the eye. If the anisocoria is greatest in bright light, the larger pupil is the abnormal pupil. When the anisocoria is greater in dark conditions, the smaller pupil is the abnormal pupil. In this case, the patient’s abnormal pupil was incorrectly diagnosed as the contralateral larger pupil (ie, left)—highlighting the importance of performing a complete pupillary examination in all patients presenting with neurological symptoms.9
Furthermore, as demonstrated in this case, ptosis in a patient with Horner syndrome caused by an ICAD can be subtle. The ptosis is the result of paralysis of Müeller’s muscle, which is innervated by the sympathetic pathway. The levator palpebrae superioris, which causes the more profound ptosis seen in third nerve palsies, is unaffected.10
Imaging Studies
Once the diagnosis of ICAD is suspected, appropriate vascular imaging must be obtained. Digital subtraction angiography has historically been the gold standard for vascular imaging of the neck vessels, but it has largely been replaced by less invasive and more readily available imaging modalities such as CTA and MRI/MRA.11
Computed Tomography Angiography. This is a widely available, rapid imaging choice and has a sensitivity of 80% to 95% in the detection of ICAD.7 It has a greater ability than MRI to identify dissection features such as intimal flaps, pseudoaneurysms, and high-grade stenosis versus occlusion. One of its disadvantages is the need for iodinated contrast, which can limit the ability to obtain the test in those with renal disease or patients with true allergies to IV contrast material. In addition, a mural hematoma can be mistaken for a noncalcified atherosclerotic plaque in the vessel lumen.6
Magnetic Resonance Imaging and Magnetic Resonance Angiography. Both MRI and MRA are also frequently used to diagnose ICAD. The intramural hematoma displays a hyperdense signal on T1-weighted images and has a characteristic crescent shape adjacent to the lumen.11 Magnetic resonance imaging studies are also sensitive in detecting cerebral ischemia resulting from the dissection. However, the sensitivity of MRI/MRA is highest 2 days after the dissection has occurred.2
Doppler Ultrasound. This is another imaging modality used to detect ICAD—one that is noninvasive, less expensive, requires no contrast material, and is widely available. Limitations of Doppler ultrasound include the inability to scan the distal ICA and a lower sensitivity in detecting dissections that cause low-grade stenosis.2 It is more commonly used for follow-up monitoring of dissections.
Management
There are several options for managing ICAD. In patients with unstable lesions, progressing neurological deficit, or further strokes, endovascular stenting has been shown to have a technical success rate of 99% and a procedural complication rate of 1.3%.12 Similarly, if the patient exhibits symptoms of cerebral ischemia, severe narrowing of the arterial lumen, or an unstable plaque, IV heparin is frequently used, followed by warfarin.13 However, since the majority of carotid and vertebral artery dissections heal spontaneously,14 antithrombotic therapies, including aspirin, clopidogrel or warfarin, are often prescribed to prevent thromboembolic complications.
Conclusion
Diagnosing ICAD requires knowledge of the typical history and presenting features of the disease. Careful attention to the ocular examination must be undertaken in any patient presenting with headache or face or neck pain, because the findings can be subtle. In a patient in whom ICAD is suspected, imaging with CTA or MRI/MRA should be performed. Early consultation with vascular surgery services can help determine the most appropriate treatment strategy.
Internal carotid artery dissection (ICAD) is an uncommon cause of stroke that typically occurs in the setting of (often minor) trauma but can also occur spontaneously. Patients with ICAD typically present with ipsilateral head, face, or neck pain. In approximately half of ICAD cases, an acute partial, painful Horner syndrome is present on examination. Although computed tomography angiography (CTA) is currently the imaging study of choice, magnetic resonance imaging/magnetic resonance angiography (MRI/MRA) and Doppler ultrasound can also be used. Management options include thrombolysis, antiplatelet or anticoagulation therapy, and endovascular or surgical intervention.
Case
A 56-year-old man with a history of migraines presented to the ED with a chief complaint of a 4-day history of right-sided headache. He stated that the pain felt different from his usual migraines and was located behind his right eye. Prior to presentation at the ED, the patient had initially visited an urgent care facility for evaluation. The physician who evaluated the patient at the urgent care facility noted the patient’s left eye appeared dilated and referred him to the ED for evaluation.
The patient further stated that the day prior to presentation, one of his friends had also remarked that the patient’s left eye appeared to be enlarged. The patient denied any visual disturbances, focal weakness, nausea, vomiting, neck pain, or stiffness. His medical history was significant for paroxysmal atrial flutter and hypertension. Regarding medications, the patient was taking dronedarone, clonazepam, omeprazole, and metoprolol.
On physical examination, the patient’s vital signs were: blood pressure, 162/109 mm Hg; heart rate, 85 beats/minute and regular; respiratory rate, 18 breaths/minute; and temperature, 98.6°F. Oxygen saturation was 98% on room air. The patient appeared to be in no acute distress. Ocular examination revealed a dilated left pupil of approximately 5 to 6 mm, and a right pupil measuring approximately 3 mm. Both pupils reacted to light, and the extraocular muscles were intact. The patient’s face appeared symmetrical and had intact sensation. He had normal speech, midline tongue, and good bilateral shoulder shrug. The neck examination revealed normal range of motion with full flexion, without jugular vein distention, lymphadenopathy, or palpable thyroid. The cardiovascular, lung, and abdominal examinations were all normal. The neurological examination showed the patient to be awake, alert, and oriented to person, place, and time. He exhibited 5/5 motor strength in all four extremities, normal gait, and normal finger-to-nose performance; his reflexes were 2+ and symmetrical.
The emergency physician (EP) ordered a stat noncontrast CT scan of the head, complete blood count (CBC), and basic metabolic panel (BMP). The CT scan of the head was interpreted by the radiologist as “no acute intracranial abnormality,” and the CBC and the BMP were normal. Since the EP was concerned about a carotid artery aneurysm or dissection, he ordered a CTA of the head and neck (Figures 1 and 2). The CTA was read as:
Long segment dissection of postbulbar cervical segment of right internal carotid artery, very likely extending into the intracranial segment with critical stenosis at the junction of the high cervical segment and proximal petrous segment. Preserved runoff circulation to the anterior and middle cerebral arteries with robust capacity for collateral support in the context of patent anterior and posterior communicating arteries.
Based on the radiology report, the EP consulted with vascular surgery services, and ordered a Doppler study of the ICAs. The Doppler study demonstrated “arterial thrombus in the right distal extracranial internal carotid artery with hemodynamics suggestive of a distal occlusion/significant obstruction.” The patient was started on an intravenous (IV) heparin drip and admitted to the hospital. Three days later he experienced sudden onset of left arm weakness. An emergent CTA of the head and neck revealed an embolic occlusion of mid-to-distal M1 segment of the right middle cerebral artery. The patient was immediately evaluated by interventional radiology for possible clot removal; however, based on his rapid neurological improvement, he was instead treated medically with aspirin and clopidogrel and continued to show significant neurological improvement. He was discharged home on hospital day 8 on both antiplatelet agents with minimal neurological deficit.
Discussion
Even though ICAD accounts for only 1% to 2% of all strokes, it is responsible for 10% to 25% of strokes in young and middle-aged adults.1-3 The peak incidence for ICAD is in the fifth decade, and it affects men and women equally.4 The extracranial portion of the ICA is the most commonly affected vessel (>90%); dissections of the intracranial portion are associated with greater neurological deficits and have a poorer prognosis.2,5 Cerebral ischemia resulting from a dissection of the extracranial ICA may occur days to weeks after the onset of local symptoms such as head or neck pain, Horner syndrome, or tinnitus.5
In ICAD, a tear in the artery wall causes blood to enter the tunica media of the vessel, forming an intramural hematoma.2,6 This may result in either stenosis of the lumen of the vessel from the enlarging hematoma or an outward aneurysmal dilatation of the vessel that compresses surrounding structures.2,6 Subsequent cerebral ischemia is the result of either arterial embolism or hemodynamic compromise from vessel stenosis.2,3
Causes
Dissections often occur secondary to trauma, though the severity of the trauma may be quite minor.2,6 Seemingly trivial mechanisms that have been associated with dissections include nose blowing, coughing, sudden neck turning, and prolonged telephone conversations. Other known causes are motor vehicle accidents and chiropractic maneuvers. Inherited connective tissue disorders, including Ehlers-Danlos syndrome, Marfan syndrome, fibromuscular dysplasia, and osteogenesis imperfecta, are associated with spontaneous ICAD.2,6
Symptoms
The most common presenting symptom of carotid dissection in approximately two-thirds of patients is ipsilateral head, face, or neck pain,2,6 typically described as sharp, constant, and nonthrobbing. Patients report a subjective bruit in 21% to 39% of cases.7 An acute partial painful Horner syndrome is strongly associated with ICAD, but is present in fewer than half of such patients.6 It is characterized by miosis and ptosis and is the result of compression of the ascending sympathetic fibers that travel alongside the ICA within the carotid sheath. Anhidrosis is not present because the fibers for sweat function in the face travel along the external carotid artery.2
Differential Diagnosis
While there is overlap between strokes caused by ICAD versus plaque, some features can help guide the clinician. Symptomatic carotid disease will frequently present with a history of one or more transient ischemic attacks characterized by focal neurological dysfunction or transient monocular blindness—typically within the previous 6 months.8 This history is not usually present in patients with ICAD. Secondly, pain is a much more prominent symptom in ICAD compared to patients with severe carotid atherosclerosis. The history of trauma, even minor, should make dissection higher on the differential diagnosis. Fortunately, the imaging studies to evaluate for these two diseases are the same.
Assessing Pupillary Asymmetry and Ptosis
Careful attention must be paid to assessing a patient for pupillary asymmetry. In a patient with anisocoria, determining the abnormal pupil may require examination of the patient in both bright and dark lighting conditions. The first step is to examine the patient’s pupils under normal lighting conditions. The next step is to assess each pupil’s response to shining a bright light in each eye. The abnormal pupil is the pupil that does not respond well or at all to bright light shone directly in the eye. If the anisocoria is greatest in bright light, the larger pupil is the abnormal pupil. When the anisocoria is greater in dark conditions, the smaller pupil is the abnormal pupil. In this case, the patient’s abnormal pupil was incorrectly diagnosed as the contralateral larger pupil (ie, left)—highlighting the importance of performing a complete pupillary examination in all patients presenting with neurological symptoms.9
Furthermore, as demonstrated in this case, ptosis in a patient with Horner syndrome caused by an ICAD can be subtle. The ptosis is the result of paralysis of Müeller’s muscle, which is innervated by the sympathetic pathway. The levator palpebrae superioris, which causes the more profound ptosis seen in third nerve palsies, is unaffected.10
Imaging Studies
Once the diagnosis of ICAD is suspected, appropriate vascular imaging must be obtained. Digital subtraction angiography has historically been the gold standard for vascular imaging of the neck vessels, but it has largely been replaced by less invasive and more readily available imaging modalities such as CTA and MRI/MRA.11
Computed Tomography Angiography. This is a widely available, rapid imaging choice and has a sensitivity of 80% to 95% in the detection of ICAD.7 It has a greater ability than MRI to identify dissection features such as intimal flaps, pseudoaneurysms, and high-grade stenosis versus occlusion. One of its disadvantages is the need for iodinated contrast, which can limit the ability to obtain the test in those with renal disease or patients with true allergies to IV contrast material. In addition, a mural hematoma can be mistaken for a noncalcified atherosclerotic plaque in the vessel lumen.6
Magnetic Resonance Imaging and Magnetic Resonance Angiography. Both MRI and MRA are also frequently used to diagnose ICAD. The intramural hematoma displays a hyperdense signal on T1-weighted images and has a characteristic crescent shape adjacent to the lumen.11 Magnetic resonance imaging studies are also sensitive in detecting cerebral ischemia resulting from the dissection. However, the sensitivity of MRI/MRA is highest 2 days after the dissection has occurred.2
Doppler Ultrasound. This is another imaging modality used to detect ICAD—one that is noninvasive, less expensive, requires no contrast material, and is widely available. Limitations of Doppler ultrasound include the inability to scan the distal ICA and a lower sensitivity in detecting dissections that cause low-grade stenosis.2 It is more commonly used for follow-up monitoring of dissections.
Management
There are several options for managing ICAD. In patients with unstable lesions, progressing neurological deficit, or further strokes, endovascular stenting has been shown to have a technical success rate of 99% and a procedural complication rate of 1.3%.12 Similarly, if the patient exhibits symptoms of cerebral ischemia, severe narrowing of the arterial lumen, or an unstable plaque, IV heparin is frequently used, followed by warfarin.13 However, since the majority of carotid and vertebral artery dissections heal spontaneously,14 antithrombotic therapies, including aspirin, clopidogrel or warfarin, are often prescribed to prevent thromboembolic complications.
Conclusion
Diagnosing ICAD requires knowledge of the typical history and presenting features of the disease. Careful attention to the ocular examination must be undertaken in any patient presenting with headache or face or neck pain, because the findings can be subtle. In a patient in whom ICAD is suspected, imaging with CTA or MRI/MRA should be performed. Early consultation with vascular surgery services can help determine the most appropriate treatment strategy.
1. CADISS trial investigators, Markus HS, Hayter E, et al. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol. 2015;14(4):361-367.
2. Thanvi B, Munshi SK, Dawson SL, Robinson TG. Carotid and vertebral artery dissection syndromes. Postgrad Med J. 2005;81(956):383-388.
3. Lucas C, Moulin T, Deplanque D, Tatu L, Chavot D. Stroke patterns of internal carotid artery dissection in 40 patients. Stroke. 1998;29(12):2646-2648.
4. Schievink WI, Mokri B, O’Fallon WM. Recurrent spontaneous cervical-artery dissection. N Engl J Med. 1994;330(6):393-397.
5. Biousse V, D’Anglejan-Chatillon J, Touboul PJ, Amarenco P, Bousser MG. Time course of symptoms in extracranial carotid artery dissections. A series of 80 patients. Stroke. 1995;26(2):235-239.
6. Kasravi N, Leung A, Silver I, Burneo JG. Dissection of the internal carotid artery causing Horner syndrome and palsy of cranial nerve XII. CMAJ. 2010;182(9):E373-E377.
1. Borgman CJ. Horner syndrome secondary to internal carotid artery dissection after a short-distance endurance run: a case study and review. J Optom. 2012;5:209-216.
2. Mohler ER III, Fairman RM. Management of symptomatic carotid atherosclerotic disease. UpToDate Web site. http://www.uptodate.com/contents/management-of-symptomatic-carotid-atherosclerotic-disease. Updated February 24, 2016. Accessed May 6, 2016.
3. Mann J. Anisocoria guidemap. Life in the Fastlane Web site. http://lifeinthefastlane.com/resources/jeff-manns-em-guidemaps/anisocoria-guidemap/. Accessed March 15, 2016.
10. Kedar S, Biousse V, Newman NJ. Horner syndrome. UpToDate Web site. http://www.uptodate.com/contents/horner-syndrome. Updated July 14, 2015. Accessed May 6, 2016.
11. Vertinsky AT, Schwartz NE, Fischbein NJ, Rosenberg J, Albers GW, Zaharchuk G. Comparison of multidetector CT angiography and MR imaging of cervical artery dissection. AJNR Am J Neuroradiol. 2008;29(9):1753-1760.
12. Pham MH, Rahme RJ, Arnaout O, et al. Endovascular stenting of extracranial carotid and vertebral artery dissections: a systematic review of the literature. Neurosurgery. 2011;68(4):856-866.
13. Caplan LR. Dissections of brain-supplying arteries. Nat Clin Pract Neurol. 2008;4(1):34-42.
14. Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898-906.
1. CADISS trial investigators, Markus HS, Hayter E, et al. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol. 2015;14(4):361-367.
2. Thanvi B, Munshi SK, Dawson SL, Robinson TG. Carotid and vertebral artery dissection syndromes. Postgrad Med J. 2005;81(956):383-388.
3. Lucas C, Moulin T, Deplanque D, Tatu L, Chavot D. Stroke patterns of internal carotid artery dissection in 40 patients. Stroke. 1998;29(12):2646-2648.
4. Schievink WI, Mokri B, O’Fallon WM. Recurrent spontaneous cervical-artery dissection. N Engl J Med. 1994;330(6):393-397.
5. Biousse V, D’Anglejan-Chatillon J, Touboul PJ, Amarenco P, Bousser MG. Time course of symptoms in extracranial carotid artery dissections. A series of 80 patients. Stroke. 1995;26(2):235-239.
6. Kasravi N, Leung A, Silver I, Burneo JG. Dissection of the internal carotid artery causing Horner syndrome and palsy of cranial nerve XII. CMAJ. 2010;182(9):E373-E377.
1. Borgman CJ. Horner syndrome secondary to internal carotid artery dissection after a short-distance endurance run: a case study and review. J Optom. 2012;5:209-216.
2. Mohler ER III, Fairman RM. Management of symptomatic carotid atherosclerotic disease. UpToDate Web site. http://www.uptodate.com/contents/management-of-symptomatic-carotid-atherosclerotic-disease. Updated February 24, 2016. Accessed May 6, 2016.
3. Mann J. Anisocoria guidemap. Life in the Fastlane Web site. http://lifeinthefastlane.com/resources/jeff-manns-em-guidemaps/anisocoria-guidemap/. Accessed March 15, 2016.
10. Kedar S, Biousse V, Newman NJ. Horner syndrome. UpToDate Web site. http://www.uptodate.com/contents/horner-syndrome. Updated July 14, 2015. Accessed May 6, 2016.
11. Vertinsky AT, Schwartz NE, Fischbein NJ, Rosenberg J, Albers GW, Zaharchuk G. Comparison of multidetector CT angiography and MR imaging of cervical artery dissection. AJNR Am J Neuroradiol. 2008;29(9):1753-1760.
12. Pham MH, Rahme RJ, Arnaout O, et al. Endovascular stenting of extracranial carotid and vertebral artery dissections: a systematic review of the literature. Neurosurgery. 2011;68(4):856-866.
13. Caplan LR. Dissections of brain-supplying arteries. Nat Clin Pract Neurol. 2008;4(1):34-42.
14. Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898-906.
The Case for Case Reports
In The Case Report Issue, we feature four separate case reports presenting different conditions, much like patients may present in succession to a busy ED. Though considered of lesser importance than other types of peer-reviewed literature in this era of evidence-based medicine, case reports nevertheless fulfill an important role in clinical practice, medical education, and even medical research by identifying and tracking an important cause of a developing disease--especially one with a toxicologic or infectious etiology. In some instances, case reports also identify effective or ineffective treatments (though the latter is more rarely reported) and adverse effects of approved treatments, especially those of a newly introduced “Phase IV” medication.
Often, the ED is the initial setting for many reportable occurrences, and in recent years, patients first presenting to EDs have alerted the entire medical community to serious emerging illnesses such as Legionnaires’ disease, HIV and AIDS, anthrax, and Ebola. Most recently, firsthand reports by a pair of mother/daughter physicians in Brazil linked an alarming appearance of several new cases of microcephaly to a rash that followed a mosquito bite during pregnancy, and ultimately to identification of the mosquito-borne Zika virus.
Similarly, toxicologists at urban poison centers have been able to rapidly link cases of new and puzzling adverse effects and deaths reported by area emergency physicians to a dangerous new street drug or combination of drugs in that area, such as synthetic cannabinoid agonists, or heroin mixed with scopolamine, and then immediately alert other physicians and the public to these dangers.
As recently described by Florek and Dellavalle in Journal of Medical Case Reports (http://bit.ly/28PLi7w), case reports make meaningful contributions to the knowledge and education of medical students, residents, fellows, and (we would add) attendings. Written with the goal of sharing information for medical or scientific purposes, they often serve as a young physician’s first experience with medical writing and provide a solid foundation for manuscript preparation and publication.
Finally, a good ED case report that includes accurate descriptions of all relevant features along with any unique departures from classical presentations, followed by an up-to-date review of current treatments, presents most of us with a vivid means of identifying and remembering the salient features of a clinical problem or disease.
In The Case Report Issue, we feature four separate case reports presenting different conditions, much like patients may present in succession to a busy ED. Though considered of lesser importance than other types of peer-reviewed literature in this era of evidence-based medicine, case reports nevertheless fulfill an important role in clinical practice, medical education, and even medical research by identifying and tracking an important cause of a developing disease--especially one with a toxicologic or infectious etiology. In some instances, case reports also identify effective or ineffective treatments (though the latter is more rarely reported) and adverse effects of approved treatments, especially those of a newly introduced “Phase IV” medication.
Often, the ED is the initial setting for many reportable occurrences, and in recent years, patients first presenting to EDs have alerted the entire medical community to serious emerging illnesses such as Legionnaires’ disease, HIV and AIDS, anthrax, and Ebola. Most recently, firsthand reports by a pair of mother/daughter physicians in Brazil linked an alarming appearance of several new cases of microcephaly to a rash that followed a mosquito bite during pregnancy, and ultimately to identification of the mosquito-borne Zika virus.
Similarly, toxicologists at urban poison centers have been able to rapidly link cases of new and puzzling adverse effects and deaths reported by area emergency physicians to a dangerous new street drug or combination of drugs in that area, such as synthetic cannabinoid agonists, or heroin mixed with scopolamine, and then immediately alert other physicians and the public to these dangers.
As recently described by Florek and Dellavalle in Journal of Medical Case Reports (http://bit.ly/28PLi7w), case reports make meaningful contributions to the knowledge and education of medical students, residents, fellows, and (we would add) attendings. Written with the goal of sharing information for medical or scientific purposes, they often serve as a young physician’s first experience with medical writing and provide a solid foundation for manuscript preparation and publication.
Finally, a good ED case report that includes accurate descriptions of all relevant features along with any unique departures from classical presentations, followed by an up-to-date review of current treatments, presents most of us with a vivid means of identifying and remembering the salient features of a clinical problem or disease.
In The Case Report Issue, we feature four separate case reports presenting different conditions, much like patients may present in succession to a busy ED. Though considered of lesser importance than other types of peer-reviewed literature in this era of evidence-based medicine, case reports nevertheless fulfill an important role in clinical practice, medical education, and even medical research by identifying and tracking an important cause of a developing disease--especially one with a toxicologic or infectious etiology. In some instances, case reports also identify effective or ineffective treatments (though the latter is more rarely reported) and adverse effects of approved treatments, especially those of a newly introduced “Phase IV” medication.
Often, the ED is the initial setting for many reportable occurrences, and in recent years, patients first presenting to EDs have alerted the entire medical community to serious emerging illnesses such as Legionnaires’ disease, HIV and AIDS, anthrax, and Ebola. Most recently, firsthand reports by a pair of mother/daughter physicians in Brazil linked an alarming appearance of several new cases of microcephaly to a rash that followed a mosquito bite during pregnancy, and ultimately to identification of the mosquito-borne Zika virus.
Similarly, toxicologists at urban poison centers have been able to rapidly link cases of new and puzzling adverse effects and deaths reported by area emergency physicians to a dangerous new street drug or combination of drugs in that area, such as synthetic cannabinoid agonists, or heroin mixed with scopolamine, and then immediately alert other physicians and the public to these dangers.
As recently described by Florek and Dellavalle in Journal of Medical Case Reports (http://bit.ly/28PLi7w), case reports make meaningful contributions to the knowledge and education of medical students, residents, fellows, and (we would add) attendings. Written with the goal of sharing information for medical or scientific purposes, they often serve as a young physician’s first experience with medical writing and provide a solid foundation for manuscript preparation and publication.
Finally, a good ED case report that includes accurate descriptions of all relevant features along with any unique departures from classical presentations, followed by an up-to-date review of current treatments, presents most of us with a vivid means of identifying and remembering the salient features of a clinical problem or disease.
First EDition: News for and about the practice of emergency medicine
CDC Issues Advisory About First Mcr-1 Gene in E Coli Found in a Human in the United States
BY JEFF BAUER
The Centers for Disease Control and Prevention (CDC) issued a health advisory to emphasize the importance of taking measures to prevent the transmission of antibiotic-resistant bacteria after a Pennsylvania woman with no recent travel outside of the United States was found to have Escherichia coli (E coli) bacteria carrying the mcr-1 gene. The mcr-1 gene makes bacteria resistant to colistin, which is used as a last-resort antibiotic to treat patients who have multidrug-resistant infections, including carbapenem-resistant Enterobacteriaceae (CRE). The mcr-1 gene exists on a small piece of DNA that is capable of moving from one bacterium to another, which would allow it to spread antibiotic resistance among species. The CDC has been on alert for this gene in the United States ever since its emergence in China in 2015.
The patient in Pennsylvania, who was being treated for a urinary tract infection (UTI), is believed to be the first human case of mcr-1 E coli in the United States. Although the E coli isolate from the patient was also resistant to antibiotics in five additional antibiotic classes (cephalosporins, fluoroquinolones, sulfonamides, aminoglycosides, and tetracyclines), the woman did not have CRE and the bacteria identified were not resistant to all antibiotics. However, the presence of the mcr-1 gene and its ability to share its colistin resistance with other bacteria increases the risk that pan-resistant bacteria could develop. The CDC’s laboratories have developed protocols for testing microorganisms for the mcr-1 gene and the CDC was performing screening tests to see if people in contact with the patient with mcr-1 might be colonized with this organism.
The CDC recommends that clinicians follow Standard and Contact Precautions for any patients colonized or infected with antibiotic-resistant bacteria, including patients found to have mcr-1-mediated resistant organisms. If a patient is found to have Enterobacteriaceae with mcr-1, healthcare facilities and laboratories should immediately notify local or state public health authorities, and inform all clinicians who are caring for the patient. Any room in which a patient with an antibiotic-resistant infection has been placed should receive thorough daily and terminal cleaning.
1. Centers for Disease Control and Prevention. Alert to U.S. healthcare facilities: first mcr-1 gene in E. coli bacteria found in a human in the United States. https://emergency.cdc.gov/han/han00390.asp. Accessed June 23, 2016.
Emergency Medicine Associate Editor Named as Chair of EM at Rutgers
Lewis S. Nelson, MD, has been appointed as the Chair of the Department of Emergency Medicine at Rutgers New Jersey Medical School and Chief of Service of the Emergency Department at University Hospital in Newark. For many years, Dr Nelson has been serving as associate editor, toxicology, for Emergency Medicine, overseeing the journal’s Case Studies in Toxicology department.
HIV Rapid Tests Miss 1 in 7 Infections
BY RICHARD PIZZI
FROM AIDS
apid human immunodeficiency virus (HIV) tests in high-income countries miss about one in seven infections and should be used in combination with fourth-generation enzyme immunoassays (EIA) or nucleic acid amplification tests (NAAT) in clinical settings whenever possible, according to a study in the journal AIDS.
“These infections are likely to be particularly transmissible due to the high HIV viral load in early infection...in high-income countries, rapid tests should be used in combination with fourth-generation EIA or NAAT, except in special circumstances,” the Australian researchers said.
Researchers performed a systematic review and meta-analysis of 18 studies involving 110,122 HIV rapid test results. The primary outcome was the test’s sensitivity for detecting acute or established HIV infections. Sensitivity was calculated by dividing the number of confirmed positive rapid tests by the number of confirmed positive comparator tests. Specificity was calculated by dividing the number of confirmed negative rapid tests by the number of negative comparator tests.
Compared with EIA, the estimated sensitivity of rapid tests was 94.5% (95% confidence interval [CI], 87.4-97.7). Compared with NAAT, the sensitivity of rapid tests was 93.7% (95% CI, 88.7-96.5). The sensitivity of rapid tests in high-income countries was 85.7% (95% CI, 81.9-88.9), and in low-income countries it was 97.7% (95% CI, 95.2-98.9), compared with either EIA or NAAT (P < .01 for difference between settings). Proportions of antibody-negative acute infections were 13.6% (95% CI, 10.1-18.0) and 4.7% (95% CI, 2.8-7.7) in studies from high- and low-income countries, respectively (P < .01).
Rapid tests were less sensitive when used in clinical settings in high-income countries, regardless of whether they were compared with a NAAT or fourth-generation EIA. However, the researchers noted that the discrepancy between high- and low-income countries could be attributed to the higher proportion of acute HIV infections (antibody-negative NAAT positive) in populations tested in high-income countries, which might reflect higher background testing rates or a higher incidence of HIV in men who have sex with men.
1. Tan WS, Chow EP, Fairley CK, Chen MY, Bradshaw CS, Read TR. Sensitivity of HIV rapid tests compared to fourth generation enzyme immunoassays or HIV RNA tests - a systematic review and meta-analysis. AIDS. 2016 Apr 27. [Epub ahead of print]
Two-Step ED Urinary Tract Infection Screening Cuts Catheterization Rate in Half
BY TARA HAELLE
FROM PEDIATRICS
fter implementation of a quality improvement initiative to more effectively screen febrile children for UTIs in the ED, catheterization rates dropped from 63% to 30% over a 6-month period, a study found.
The sustained drop prevented more than 350 young children from catheterization without increasing revisit rates or missing UTIs in the 39% of children who were followed in the care network. This was in a study that compared catheterization rates in 1,520 children aged 6 to 24 months in the year before the intervention and 828 children in the 6 months during the intervention.
“Although urine catheterization remains the gold standard in diagnosing UTIs, it is an invasive procedure that may be avoided in most patients who are being screened,” wrote Dr Jane M. Lavelle of Children’s Hospital of Philadelphia (CHOP) and her associates. Screening for UTIs by this method can be “painful, time consuming, and costly,” they added.
An alternative method to automatic catheterization is a two-step process already included as an option in the American Academy of Pediatrics guidelines: instead of collecting urine through catheterization just once for screening and culture, an ED first noninvasively collects urine with a urine bag for screening in those indicated with evidence-based risk factors, and then catheterizes only those who screen positive.
“Due to the predictive models’ higher sensitivity than specificity for screening, most urine samples will have a negative screen for pyuria or bacteriuria by urine dipstick or microscopy,” the authors wrote.
At baseline, CHOP’s ED was screening 63% of febrile children under age 24 months using catheterization, but screens were most commonly negative and only 4.3% had positive cultures. The authors therefore initiated a switch to the two-step method as a pilot run in one ED area before educating all ED personnel and expanding to the full department in the second month.
Children aged 6 to 24 months comprised approximately 20% of the ED’s more than 90,000 annual patients, and about 22% of these children presented with fever as the primary concern. Children with a history of genitourinary problems or immune deficiency were excluded.
The pilot ran in an “urgent care section of ED where there are typically more children with less complex medical histories and where ‘fever’ is a common complaint,” the investigators said. The staff completed a learning module with assessment and then received in-person and visual reminders of the procedure.
While 69% of 828 febrile young children still underwent screening during the 6-month intervention period, only 16% still underwent urethral catheterization as the initial screening step, typically because of strong clinical indications for a UTI. Another 14% underwent catheterization only after a positive urine screen from an initial noninvasive urine collection or because of an inability to get an adequate urine specimen with the bag. The reduction in catheterization dropped to 55% within 2 weeks of the intervention’s start and spread to other hospital departments. The drop to a 30% catheterization rate remained throughout 18 additional months of monitoring.
The research did not use external funding, and the researchers reported they had no financial disclosures.
1. Lavelle JM, Blackstone MM, Funari MK, et al. Two-step process for ED UTI screening in febrile young children: reducing catheterization rates. Pediatrics. 2016 Jun 2. pii: e20153023. [Epub ahead of print].
CDC Issues Advisory About First Mcr-1 Gene in E Coli Found in a Human in the United States
BY JEFF BAUER
The Centers for Disease Control and Prevention (CDC) issued a health advisory to emphasize the importance of taking measures to prevent the transmission of antibiotic-resistant bacteria after a Pennsylvania woman with no recent travel outside of the United States was found to have Escherichia coli (E coli) bacteria carrying the mcr-1 gene. The mcr-1 gene makes bacteria resistant to colistin, which is used as a last-resort antibiotic to treat patients who have multidrug-resistant infections, including carbapenem-resistant Enterobacteriaceae (CRE). The mcr-1 gene exists on a small piece of DNA that is capable of moving from one bacterium to another, which would allow it to spread antibiotic resistance among species. The CDC has been on alert for this gene in the United States ever since its emergence in China in 2015.
The patient in Pennsylvania, who was being treated for a urinary tract infection (UTI), is believed to be the first human case of mcr-1 E coli in the United States. Although the E coli isolate from the patient was also resistant to antibiotics in five additional antibiotic classes (cephalosporins, fluoroquinolones, sulfonamides, aminoglycosides, and tetracyclines), the woman did not have CRE and the bacteria identified were not resistant to all antibiotics. However, the presence of the mcr-1 gene and its ability to share its colistin resistance with other bacteria increases the risk that pan-resistant bacteria could develop. The CDC’s laboratories have developed protocols for testing microorganisms for the mcr-1 gene and the CDC was performing screening tests to see if people in contact with the patient with mcr-1 might be colonized with this organism.
The CDC recommends that clinicians follow Standard and Contact Precautions for any patients colonized or infected with antibiotic-resistant bacteria, including patients found to have mcr-1-mediated resistant organisms. If a patient is found to have Enterobacteriaceae with mcr-1, healthcare facilities and laboratories should immediately notify local or state public health authorities, and inform all clinicians who are caring for the patient. Any room in which a patient with an antibiotic-resistant infection has been placed should receive thorough daily and terminal cleaning.
1. Centers for Disease Control and Prevention. Alert to U.S. healthcare facilities: first mcr-1 gene in E. coli bacteria found in a human in the United States. https://emergency.cdc.gov/han/han00390.asp. Accessed June 23, 2016.
Emergency Medicine Associate Editor Named as Chair of EM at Rutgers
Lewis S. Nelson, MD, has been appointed as the Chair of the Department of Emergency Medicine at Rutgers New Jersey Medical School and Chief of Service of the Emergency Department at University Hospital in Newark. For many years, Dr Nelson has been serving as associate editor, toxicology, for Emergency Medicine, overseeing the journal’s Case Studies in Toxicology department.
HIV Rapid Tests Miss 1 in 7 Infections
BY RICHARD PIZZI
FROM AIDS
apid human immunodeficiency virus (HIV) tests in high-income countries miss about one in seven infections and should be used in combination with fourth-generation enzyme immunoassays (EIA) or nucleic acid amplification tests (NAAT) in clinical settings whenever possible, according to a study in the journal AIDS.
“These infections are likely to be particularly transmissible due to the high HIV viral load in early infection...in high-income countries, rapid tests should be used in combination with fourth-generation EIA or NAAT, except in special circumstances,” the Australian researchers said.
Researchers performed a systematic review and meta-analysis of 18 studies involving 110,122 HIV rapid test results. The primary outcome was the test’s sensitivity for detecting acute or established HIV infections. Sensitivity was calculated by dividing the number of confirmed positive rapid tests by the number of confirmed positive comparator tests. Specificity was calculated by dividing the number of confirmed negative rapid tests by the number of negative comparator tests.
Compared with EIA, the estimated sensitivity of rapid tests was 94.5% (95% confidence interval [CI], 87.4-97.7). Compared with NAAT, the sensitivity of rapid tests was 93.7% (95% CI, 88.7-96.5). The sensitivity of rapid tests in high-income countries was 85.7% (95% CI, 81.9-88.9), and in low-income countries it was 97.7% (95% CI, 95.2-98.9), compared with either EIA or NAAT (P < .01 for difference between settings). Proportions of antibody-negative acute infections were 13.6% (95% CI, 10.1-18.0) and 4.7% (95% CI, 2.8-7.7) in studies from high- and low-income countries, respectively (P < .01).
Rapid tests were less sensitive when used in clinical settings in high-income countries, regardless of whether they were compared with a NAAT or fourth-generation EIA. However, the researchers noted that the discrepancy between high- and low-income countries could be attributed to the higher proportion of acute HIV infections (antibody-negative NAAT positive) in populations tested in high-income countries, which might reflect higher background testing rates or a higher incidence of HIV in men who have sex with men.
1. Tan WS, Chow EP, Fairley CK, Chen MY, Bradshaw CS, Read TR. Sensitivity of HIV rapid tests compared to fourth generation enzyme immunoassays or HIV RNA tests - a systematic review and meta-analysis. AIDS. 2016 Apr 27. [Epub ahead of print]
Two-Step ED Urinary Tract Infection Screening Cuts Catheterization Rate in Half
BY TARA HAELLE
FROM PEDIATRICS
fter implementation of a quality improvement initiative to more effectively screen febrile children for UTIs in the ED, catheterization rates dropped from 63% to 30% over a 6-month period, a study found.
The sustained drop prevented more than 350 young children from catheterization without increasing revisit rates or missing UTIs in the 39% of children who were followed in the care network. This was in a study that compared catheterization rates in 1,520 children aged 6 to 24 months in the year before the intervention and 828 children in the 6 months during the intervention.
“Although urine catheterization remains the gold standard in diagnosing UTIs, it is an invasive procedure that may be avoided in most patients who are being screened,” wrote Dr Jane M. Lavelle of Children’s Hospital of Philadelphia (CHOP) and her associates. Screening for UTIs by this method can be “painful, time consuming, and costly,” they added.
An alternative method to automatic catheterization is a two-step process already included as an option in the American Academy of Pediatrics guidelines: instead of collecting urine through catheterization just once for screening and culture, an ED first noninvasively collects urine with a urine bag for screening in those indicated with evidence-based risk factors, and then catheterizes only those who screen positive.
“Due to the predictive models’ higher sensitivity than specificity for screening, most urine samples will have a negative screen for pyuria or bacteriuria by urine dipstick or microscopy,” the authors wrote.
At baseline, CHOP’s ED was screening 63% of febrile children under age 24 months using catheterization, but screens were most commonly negative and only 4.3% had positive cultures. The authors therefore initiated a switch to the two-step method as a pilot run in one ED area before educating all ED personnel and expanding to the full department in the second month.
Children aged 6 to 24 months comprised approximately 20% of the ED’s more than 90,000 annual patients, and about 22% of these children presented with fever as the primary concern. Children with a history of genitourinary problems or immune deficiency were excluded.
The pilot ran in an “urgent care section of ED where there are typically more children with less complex medical histories and where ‘fever’ is a common complaint,” the investigators said. The staff completed a learning module with assessment and then received in-person and visual reminders of the procedure.
While 69% of 828 febrile young children still underwent screening during the 6-month intervention period, only 16% still underwent urethral catheterization as the initial screening step, typically because of strong clinical indications for a UTI. Another 14% underwent catheterization only after a positive urine screen from an initial noninvasive urine collection or because of an inability to get an adequate urine specimen with the bag. The reduction in catheterization dropped to 55% within 2 weeks of the intervention’s start and spread to other hospital departments. The drop to a 30% catheterization rate remained throughout 18 additional months of monitoring.
The research did not use external funding, and the researchers reported they had no financial disclosures.
1. Lavelle JM, Blackstone MM, Funari MK, et al. Two-step process for ED UTI screening in febrile young children: reducing catheterization rates. Pediatrics. 2016 Jun 2. pii: e20153023. [Epub ahead of print].
CDC Issues Advisory About First Mcr-1 Gene in E Coli Found in a Human in the United States
BY JEFF BAUER
The Centers for Disease Control and Prevention (CDC) issued a health advisory to emphasize the importance of taking measures to prevent the transmission of antibiotic-resistant bacteria after a Pennsylvania woman with no recent travel outside of the United States was found to have Escherichia coli (E coli) bacteria carrying the mcr-1 gene. The mcr-1 gene makes bacteria resistant to colistin, which is used as a last-resort antibiotic to treat patients who have multidrug-resistant infections, including carbapenem-resistant Enterobacteriaceae (CRE). The mcr-1 gene exists on a small piece of DNA that is capable of moving from one bacterium to another, which would allow it to spread antibiotic resistance among species. The CDC has been on alert for this gene in the United States ever since its emergence in China in 2015.
The patient in Pennsylvania, who was being treated for a urinary tract infection (UTI), is believed to be the first human case of mcr-1 E coli in the United States. Although the E coli isolate from the patient was also resistant to antibiotics in five additional antibiotic classes (cephalosporins, fluoroquinolones, sulfonamides, aminoglycosides, and tetracyclines), the woman did not have CRE and the bacteria identified were not resistant to all antibiotics. However, the presence of the mcr-1 gene and its ability to share its colistin resistance with other bacteria increases the risk that pan-resistant bacteria could develop. The CDC’s laboratories have developed protocols for testing microorganisms for the mcr-1 gene and the CDC was performing screening tests to see if people in contact with the patient with mcr-1 might be colonized with this organism.
The CDC recommends that clinicians follow Standard and Contact Precautions for any patients colonized or infected with antibiotic-resistant bacteria, including patients found to have mcr-1-mediated resistant organisms. If a patient is found to have Enterobacteriaceae with mcr-1, healthcare facilities and laboratories should immediately notify local or state public health authorities, and inform all clinicians who are caring for the patient. Any room in which a patient with an antibiotic-resistant infection has been placed should receive thorough daily and terminal cleaning.
1. Centers for Disease Control and Prevention. Alert to U.S. healthcare facilities: first mcr-1 gene in E. coli bacteria found in a human in the United States. https://emergency.cdc.gov/han/han00390.asp. Accessed June 23, 2016.
Emergency Medicine Associate Editor Named as Chair of EM at Rutgers
Lewis S. Nelson, MD, has been appointed as the Chair of the Department of Emergency Medicine at Rutgers New Jersey Medical School and Chief of Service of the Emergency Department at University Hospital in Newark. For many years, Dr Nelson has been serving as associate editor, toxicology, for Emergency Medicine, overseeing the journal’s Case Studies in Toxicology department.
HIV Rapid Tests Miss 1 in 7 Infections
BY RICHARD PIZZI
FROM AIDS
apid human immunodeficiency virus (HIV) tests in high-income countries miss about one in seven infections and should be used in combination with fourth-generation enzyme immunoassays (EIA) or nucleic acid amplification tests (NAAT) in clinical settings whenever possible, according to a study in the journal AIDS.
“These infections are likely to be particularly transmissible due to the high HIV viral load in early infection...in high-income countries, rapid tests should be used in combination with fourth-generation EIA or NAAT, except in special circumstances,” the Australian researchers said.
Researchers performed a systematic review and meta-analysis of 18 studies involving 110,122 HIV rapid test results. The primary outcome was the test’s sensitivity for detecting acute or established HIV infections. Sensitivity was calculated by dividing the number of confirmed positive rapid tests by the number of confirmed positive comparator tests. Specificity was calculated by dividing the number of confirmed negative rapid tests by the number of negative comparator tests.
Compared with EIA, the estimated sensitivity of rapid tests was 94.5% (95% confidence interval [CI], 87.4-97.7). Compared with NAAT, the sensitivity of rapid tests was 93.7% (95% CI, 88.7-96.5). The sensitivity of rapid tests in high-income countries was 85.7% (95% CI, 81.9-88.9), and in low-income countries it was 97.7% (95% CI, 95.2-98.9), compared with either EIA or NAAT (P < .01 for difference between settings). Proportions of antibody-negative acute infections were 13.6% (95% CI, 10.1-18.0) and 4.7% (95% CI, 2.8-7.7) in studies from high- and low-income countries, respectively (P < .01).
Rapid tests were less sensitive when used in clinical settings in high-income countries, regardless of whether they were compared with a NAAT or fourth-generation EIA. However, the researchers noted that the discrepancy between high- and low-income countries could be attributed to the higher proportion of acute HIV infections (antibody-negative NAAT positive) in populations tested in high-income countries, which might reflect higher background testing rates or a higher incidence of HIV in men who have sex with men.
1. Tan WS, Chow EP, Fairley CK, Chen MY, Bradshaw CS, Read TR. Sensitivity of HIV rapid tests compared to fourth generation enzyme immunoassays or HIV RNA tests - a systematic review and meta-analysis. AIDS. 2016 Apr 27. [Epub ahead of print]
Two-Step ED Urinary Tract Infection Screening Cuts Catheterization Rate in Half
BY TARA HAELLE
FROM PEDIATRICS
fter implementation of a quality improvement initiative to more effectively screen febrile children for UTIs in the ED, catheterization rates dropped from 63% to 30% over a 6-month period, a study found.
The sustained drop prevented more than 350 young children from catheterization without increasing revisit rates or missing UTIs in the 39% of children who were followed in the care network. This was in a study that compared catheterization rates in 1,520 children aged 6 to 24 months in the year before the intervention and 828 children in the 6 months during the intervention.
“Although urine catheterization remains the gold standard in diagnosing UTIs, it is an invasive procedure that may be avoided in most patients who are being screened,” wrote Dr Jane M. Lavelle of Children’s Hospital of Philadelphia (CHOP) and her associates. Screening for UTIs by this method can be “painful, time consuming, and costly,” they added.
An alternative method to automatic catheterization is a two-step process already included as an option in the American Academy of Pediatrics guidelines: instead of collecting urine through catheterization just once for screening and culture, an ED first noninvasively collects urine with a urine bag for screening in those indicated with evidence-based risk factors, and then catheterizes only those who screen positive.
“Due to the predictive models’ higher sensitivity than specificity for screening, most urine samples will have a negative screen for pyuria or bacteriuria by urine dipstick or microscopy,” the authors wrote.
At baseline, CHOP’s ED was screening 63% of febrile children under age 24 months using catheterization, but screens were most commonly negative and only 4.3% had positive cultures. The authors therefore initiated a switch to the two-step method as a pilot run in one ED area before educating all ED personnel and expanding to the full department in the second month.
Children aged 6 to 24 months comprised approximately 20% of the ED’s more than 90,000 annual patients, and about 22% of these children presented with fever as the primary concern. Children with a history of genitourinary problems or immune deficiency were excluded.
The pilot ran in an “urgent care section of ED where there are typically more children with less complex medical histories and where ‘fever’ is a common complaint,” the investigators said. The staff completed a learning module with assessment and then received in-person and visual reminders of the procedure.
While 69% of 828 febrile young children still underwent screening during the 6-month intervention period, only 16% still underwent urethral catheterization as the initial screening step, typically because of strong clinical indications for a UTI. Another 14% underwent catheterization only after a positive urine screen from an initial noninvasive urine collection or because of an inability to get an adequate urine specimen with the bag. The reduction in catheterization dropped to 55% within 2 weeks of the intervention’s start and spread to other hospital departments. The drop to a 30% catheterization rate remained throughout 18 additional months of monitoring.
The research did not use external funding, and the researchers reported they had no financial disclosures.
1. Lavelle JM, Blackstone MM, Funari MK, et al. Two-step process for ED UTI screening in febrile young children: reducing catheterization rates. Pediatrics. 2016 Jun 2. pii: e20153023. [Epub ahead of print].
In Out-of-Hospital Care, IIb or Not IIb…
The 2015 American Heart Association CPR/ACLS update categorizes amiodarone and lidocaine as IIb drugs that “may be considered” for ventricular fibrillation or pulseless ventricular tachycardia unresponsive to CPR, defibrillation, or vasopressors. Out-of-hospital use of these drugs has previously been shown to increase survival rate to hospital admission, but not necessarily to hospital discharge.
The effects of amiodarone and lidocaine on the rate of survival to hospital discharge are addressed in a recent randomized, double-blind, out-of-hospital trial comparing amiodarone, lidocaine, and placebo in the treatment of shock-refractory ventricular fibrillation or pulseless ventricular tachycardia (N Engl J Med. 2016;374[18]:1711-1722). This study was conducted by the Resuscitation Outcomes Consortium (ROC) in 3,026 patients at 10 US and Canadian sites. The ROC authors concluded that “overall, neither amiodarone nor lidocaine resulted in a significantly higher rate of survival or favorable neurologic outcome than the rate with placebo.” But the article raises concerns about its methodology, appropriateness of its primary and secondary outcomes to out-of-hospital (or prehospital) care, and the manner in which its findings were reported.
Because of the condition (unconscious) and circumstances (out of hospital) of the patients at the time medication or placebo must be administered, this NIH-supported trial was conducted under exception from informed consent in emergency research, with FDA and Health Canada oversight, and with approval by trial-site Institutional Review Boards. Notwithstanding the list of regulatory bodies that approved the exception, is the trial appropriate for drugs previously demonstrated to be efficacious in improving survival rates to hospital admission—long considered the goal of prehospital care—when subsequent care from admission to hospital discharge is not standardized or controlled across multiple sites in two countries?
Another concern is the way the results were reported. Will the authors’ conclusion that overall, neither amiodarone nor lidocaine resulted in a significantly higher rate of survival suggest to hurried readers that there is no benefit to any patient to hospital discharge from either antiarrhythmic agent? In the results section, the authors report “active drugs were associated with a survival rate that was significantly higher than the rate with placebo among patients with bystander-witnessed arrest but not among those with unwitnessed arrest.” Also noted in the accompanying editorial entitled “Out-of-Hospital Cardiac Arrest—Are Drugs Ever the Answer?” (N Engl J Med. 2016;374[18]:1781-1782), both drugs were associated with nonsignificant increases in survival rate, fewer subsequent shocks, and less administration of rhythm-control medications or need for CPR during hospitalization, compared with patients’ courses after placebo.
The ROC trial is not the first or only out-of-hospital trial to use survival to hospital discharge as its primary outcome measure. A 1990-1991 study using death or discharge home to determine survival from out-of-hospital cardiac arrests in New York City found that of the 2,329 patients who met entry criteria for that study, overall survival was only 1.4%—which the authors attributed partly to lengthy elapsed time intervals at every step in the chain of survival, lack of adequate bystander CPR, and possibly sociodemographic features common to victims of cardiac arrest in large cities (JAMA. 1994;271[9]:678-683). The poor results led to increases in first responders and AED availability but not the abandonment of properly performed CPR and ACLS. In the ROC trial, length of time from cardiac arrest to administration of medications clearly was shown to be a significant outcome determinant and was emphasized in the accompanying editorial. Here too shouldn’t we concentrate on optimizing the setting and timing of CPR and ACLS measures?
The 2015 American Heart Association CPR/ACLS update categorizes amiodarone and lidocaine as IIb drugs that “may be considered” for ventricular fibrillation or pulseless ventricular tachycardia unresponsive to CPR, defibrillation, or vasopressors. Out-of-hospital use of these drugs has previously been shown to increase survival rate to hospital admission, but not necessarily to hospital discharge.
The effects of amiodarone and lidocaine on the rate of survival to hospital discharge are addressed in a recent randomized, double-blind, out-of-hospital trial comparing amiodarone, lidocaine, and placebo in the treatment of shock-refractory ventricular fibrillation or pulseless ventricular tachycardia (N Engl J Med. 2016;374[18]:1711-1722). This study was conducted by the Resuscitation Outcomes Consortium (ROC) in 3,026 patients at 10 US and Canadian sites. The ROC authors concluded that “overall, neither amiodarone nor lidocaine resulted in a significantly higher rate of survival or favorable neurologic outcome than the rate with placebo.” But the article raises concerns about its methodology, appropriateness of its primary and secondary outcomes to out-of-hospital (or prehospital) care, and the manner in which its findings were reported.
Because of the condition (unconscious) and circumstances (out of hospital) of the patients at the time medication or placebo must be administered, this NIH-supported trial was conducted under exception from informed consent in emergency research, with FDA and Health Canada oversight, and with approval by trial-site Institutional Review Boards. Notwithstanding the list of regulatory bodies that approved the exception, is the trial appropriate for drugs previously demonstrated to be efficacious in improving survival rates to hospital admission—long considered the goal of prehospital care—when subsequent care from admission to hospital discharge is not standardized or controlled across multiple sites in two countries?
Another concern is the way the results were reported. Will the authors’ conclusion that overall, neither amiodarone nor lidocaine resulted in a significantly higher rate of survival suggest to hurried readers that there is no benefit to any patient to hospital discharge from either antiarrhythmic agent? In the results section, the authors report “active drugs were associated with a survival rate that was significantly higher than the rate with placebo among patients with bystander-witnessed arrest but not among those with unwitnessed arrest.” Also noted in the accompanying editorial entitled “Out-of-Hospital Cardiac Arrest—Are Drugs Ever the Answer?” (N Engl J Med. 2016;374[18]:1781-1782), both drugs were associated with nonsignificant increases in survival rate, fewer subsequent shocks, and less administration of rhythm-control medications or need for CPR during hospitalization, compared with patients’ courses after placebo.
The ROC trial is not the first or only out-of-hospital trial to use survival to hospital discharge as its primary outcome measure. A 1990-1991 study using death or discharge home to determine survival from out-of-hospital cardiac arrests in New York City found that of the 2,329 patients who met entry criteria for that study, overall survival was only 1.4%—which the authors attributed partly to lengthy elapsed time intervals at every step in the chain of survival, lack of adequate bystander CPR, and possibly sociodemographic features common to victims of cardiac arrest in large cities (JAMA. 1994;271[9]:678-683). The poor results led to increases in first responders and AED availability but not the abandonment of properly performed CPR and ACLS. In the ROC trial, length of time from cardiac arrest to administration of medications clearly was shown to be a significant outcome determinant and was emphasized in the accompanying editorial. Here too shouldn’t we concentrate on optimizing the setting and timing of CPR and ACLS measures?
The 2015 American Heart Association CPR/ACLS update categorizes amiodarone and lidocaine as IIb drugs that “may be considered” for ventricular fibrillation or pulseless ventricular tachycardia unresponsive to CPR, defibrillation, or vasopressors. Out-of-hospital use of these drugs has previously been shown to increase survival rate to hospital admission, but not necessarily to hospital discharge.
The effects of amiodarone and lidocaine on the rate of survival to hospital discharge are addressed in a recent randomized, double-blind, out-of-hospital trial comparing amiodarone, lidocaine, and placebo in the treatment of shock-refractory ventricular fibrillation or pulseless ventricular tachycardia (N Engl J Med. 2016;374[18]:1711-1722). This study was conducted by the Resuscitation Outcomes Consortium (ROC) in 3,026 patients at 10 US and Canadian sites. The ROC authors concluded that “overall, neither amiodarone nor lidocaine resulted in a significantly higher rate of survival or favorable neurologic outcome than the rate with placebo.” But the article raises concerns about its methodology, appropriateness of its primary and secondary outcomes to out-of-hospital (or prehospital) care, and the manner in which its findings were reported.
Because of the condition (unconscious) and circumstances (out of hospital) of the patients at the time medication or placebo must be administered, this NIH-supported trial was conducted under exception from informed consent in emergency research, with FDA and Health Canada oversight, and with approval by trial-site Institutional Review Boards. Notwithstanding the list of regulatory bodies that approved the exception, is the trial appropriate for drugs previously demonstrated to be efficacious in improving survival rates to hospital admission—long considered the goal of prehospital care—when subsequent care from admission to hospital discharge is not standardized or controlled across multiple sites in two countries?
Another concern is the way the results were reported. Will the authors’ conclusion that overall, neither amiodarone nor lidocaine resulted in a significantly higher rate of survival suggest to hurried readers that there is no benefit to any patient to hospital discharge from either antiarrhythmic agent? In the results section, the authors report “active drugs were associated with a survival rate that was significantly higher than the rate with placebo among patients with bystander-witnessed arrest but not among those with unwitnessed arrest.” Also noted in the accompanying editorial entitled “Out-of-Hospital Cardiac Arrest—Are Drugs Ever the Answer?” (N Engl J Med. 2016;374[18]:1781-1782), both drugs were associated with nonsignificant increases in survival rate, fewer subsequent shocks, and less administration of rhythm-control medications or need for CPR during hospitalization, compared with patients’ courses after placebo.
The ROC trial is not the first or only out-of-hospital trial to use survival to hospital discharge as its primary outcome measure. A 1990-1991 study using death or discharge home to determine survival from out-of-hospital cardiac arrests in New York City found that of the 2,329 patients who met entry criteria for that study, overall survival was only 1.4%—which the authors attributed partly to lengthy elapsed time intervals at every step in the chain of survival, lack of adequate bystander CPR, and possibly sociodemographic features common to victims of cardiac arrest in large cities (JAMA. 1994;271[9]:678-683). The poor results led to increases in first responders and AED availability but not the abandonment of properly performed CPR and ACLS. In the ROC trial, length of time from cardiac arrest to administration of medications clearly was shown to be a significant outcome determinant and was emphasized in the accompanying editorial. Here too shouldn’t we concentrate on optimizing the setting and timing of CPR and ACLS measures?
Pembrolizumab shows signs of efficacy in relapsed/refractory classical Hodgkin lymphoma
PD-1 checkpoint blockade via pembrolizumab is a potential option for classical Hodgkin’s lymphoma that progressed despite brentuximab vedotin therapy, based on a phase Ib, single-arm, open-label, industry-sponsored study of 31 patients.
After a median follow-up of 17 months, five (16%) patients achieved complete remission (90% confidence interval, 7%-31%), and 15 (48%) patients achieved partial remission (90% CI, 33%-64%), for an overall response rate of 65% (48%-79%). Furthermore, 70% of responses lasted at least 24 weeks, reported Philippe Armand, MD, of Dana-Farber Cancer Institute, Boston, and his associates.
“Since the time of study design, it has become apparent that complete responses are not commonly achieved with checkpoint blockade in solid tumors or hematologic malignancies,” they added. “Yet partial responses can be durable, suggesting that the achievement of complete response with checkpoint blockade is not necessary to derive significant clinical benefit.”
Pembrolizumab (Keytruda) is a humanized, highly selective IgG4 anti-PD-1 (programmed death-1) monoclonal antibody approved in the United States for patients with unresectable or metastatic melanoma or metastatic PDL-1 expressing non–small cell lung cancer. Tumor cells in classical Hodgkin lymphoma (HL) often overexpress PD-1 ligands, which “strongly suggests” that HL is PD-1 dependent, the researchers noted (J Clin Oncol. 2016 Jun 27. doi: 10.1200/JCO.2016.67.3467).
The researchers analyzed data for one group of patients within a phase Ib study of pembrolizumab in hematologic malignancies (KEYNOTE-013; NCT01953692). These 31 adults (median age, 32 years; 58% male) all had heavily pretreated classical HL – more than half had received at least five prior lines of therapy, and all had progressed on or after brentuximab vedotin therapy. Most patients (71%) also had received autologous stem cell transplantation. All 31 patients in the study received intravenous pembrolizumab (10 mg/kg) every other week.
Rates of progression-free survival were 69% at 24 weeks and 46% at 52 weeks, the researchers said. “Biomarker analyses demonstrated a high prevalence of PD-L1 and PD-L2 expression, treatment-induced expansion of T cells and natural killer cells, and activation of interferon-gamma, T-cell receptor, and expanded immune-related signaling pathways,” they reported. Those findings indicate that PD-1 blockade activates T-cell and IFN-gamma signaling pathways, which provides “a compelling rationale for the further development of PD-1 blockade in HL,” they concluded.
The most common treatment-related adverse effects were hypothyroidism (16%), diarrhea (16%), nausea (13%), and pneumonitis (10%). Five patients (16%) developed grade 3 treatment-related adverse events, including elevated hepatic transaminases, axillary pain, back pain, joint swelling, colitis, and nephrotic syndrome. Two patients stopped treatment because of adverse effects, but there were no grade 4 events or deaths related to treatment, and no treatment-induced cases of hepatitis, hypophysitis, or uveitis, the researchers noted.
Merck, maker of pembrolizumab, funded the study. Dr. Armand reported financial ties to Merck, Bristol-Myers Squibb, Infinity Pharmaceuticals, Sequenta, Tensha Therapeutics, and Sigma-Tau. Senior author Craig Moskowitz, MD, and five coinvestigators disclosed consulting or advisory relationships or employment with Merck.
PD-1 checkpoint blockade via pembrolizumab is a potential option for classical Hodgkin’s lymphoma that progressed despite brentuximab vedotin therapy, based on a phase Ib, single-arm, open-label, industry-sponsored study of 31 patients.
After a median follow-up of 17 months, five (16%) patients achieved complete remission (90% confidence interval, 7%-31%), and 15 (48%) patients achieved partial remission (90% CI, 33%-64%), for an overall response rate of 65% (48%-79%). Furthermore, 70% of responses lasted at least 24 weeks, reported Philippe Armand, MD, of Dana-Farber Cancer Institute, Boston, and his associates.
“Since the time of study design, it has become apparent that complete responses are not commonly achieved with checkpoint blockade in solid tumors or hematologic malignancies,” they added. “Yet partial responses can be durable, suggesting that the achievement of complete response with checkpoint blockade is not necessary to derive significant clinical benefit.”
Pembrolizumab (Keytruda) is a humanized, highly selective IgG4 anti-PD-1 (programmed death-1) monoclonal antibody approved in the United States for patients with unresectable or metastatic melanoma or metastatic PDL-1 expressing non–small cell lung cancer. Tumor cells in classical Hodgkin lymphoma (HL) often overexpress PD-1 ligands, which “strongly suggests” that HL is PD-1 dependent, the researchers noted (J Clin Oncol. 2016 Jun 27. doi: 10.1200/JCO.2016.67.3467).
The researchers analyzed data for one group of patients within a phase Ib study of pembrolizumab in hematologic malignancies (KEYNOTE-013; NCT01953692). These 31 adults (median age, 32 years; 58% male) all had heavily pretreated classical HL – more than half had received at least five prior lines of therapy, and all had progressed on or after brentuximab vedotin therapy. Most patients (71%) also had received autologous stem cell transplantation. All 31 patients in the study received intravenous pembrolizumab (10 mg/kg) every other week.
Rates of progression-free survival were 69% at 24 weeks and 46% at 52 weeks, the researchers said. “Biomarker analyses demonstrated a high prevalence of PD-L1 and PD-L2 expression, treatment-induced expansion of T cells and natural killer cells, and activation of interferon-gamma, T-cell receptor, and expanded immune-related signaling pathways,” they reported. Those findings indicate that PD-1 blockade activates T-cell and IFN-gamma signaling pathways, which provides “a compelling rationale for the further development of PD-1 blockade in HL,” they concluded.
The most common treatment-related adverse effects were hypothyroidism (16%), diarrhea (16%), nausea (13%), and pneumonitis (10%). Five patients (16%) developed grade 3 treatment-related adverse events, including elevated hepatic transaminases, axillary pain, back pain, joint swelling, colitis, and nephrotic syndrome. Two patients stopped treatment because of adverse effects, but there were no grade 4 events or deaths related to treatment, and no treatment-induced cases of hepatitis, hypophysitis, or uveitis, the researchers noted.
Merck, maker of pembrolizumab, funded the study. Dr. Armand reported financial ties to Merck, Bristol-Myers Squibb, Infinity Pharmaceuticals, Sequenta, Tensha Therapeutics, and Sigma-Tau. Senior author Craig Moskowitz, MD, and five coinvestigators disclosed consulting or advisory relationships or employment with Merck.
PD-1 checkpoint blockade via pembrolizumab is a potential option for classical Hodgkin’s lymphoma that progressed despite brentuximab vedotin therapy, based on a phase Ib, single-arm, open-label, industry-sponsored study of 31 patients.
After a median follow-up of 17 months, five (16%) patients achieved complete remission (90% confidence interval, 7%-31%), and 15 (48%) patients achieved partial remission (90% CI, 33%-64%), for an overall response rate of 65% (48%-79%). Furthermore, 70% of responses lasted at least 24 weeks, reported Philippe Armand, MD, of Dana-Farber Cancer Institute, Boston, and his associates.
“Since the time of study design, it has become apparent that complete responses are not commonly achieved with checkpoint blockade in solid tumors or hematologic malignancies,” they added. “Yet partial responses can be durable, suggesting that the achievement of complete response with checkpoint blockade is not necessary to derive significant clinical benefit.”
Pembrolizumab (Keytruda) is a humanized, highly selective IgG4 anti-PD-1 (programmed death-1) monoclonal antibody approved in the United States for patients with unresectable or metastatic melanoma or metastatic PDL-1 expressing non–small cell lung cancer. Tumor cells in classical Hodgkin lymphoma (HL) often overexpress PD-1 ligands, which “strongly suggests” that HL is PD-1 dependent, the researchers noted (J Clin Oncol. 2016 Jun 27. doi: 10.1200/JCO.2016.67.3467).
The researchers analyzed data for one group of patients within a phase Ib study of pembrolizumab in hematologic malignancies (KEYNOTE-013; NCT01953692). These 31 adults (median age, 32 years; 58% male) all had heavily pretreated classical HL – more than half had received at least five prior lines of therapy, and all had progressed on or after brentuximab vedotin therapy. Most patients (71%) also had received autologous stem cell transplantation. All 31 patients in the study received intravenous pembrolizumab (10 mg/kg) every other week.
Rates of progression-free survival were 69% at 24 weeks and 46% at 52 weeks, the researchers said. “Biomarker analyses demonstrated a high prevalence of PD-L1 and PD-L2 expression, treatment-induced expansion of T cells and natural killer cells, and activation of interferon-gamma, T-cell receptor, and expanded immune-related signaling pathways,” they reported. Those findings indicate that PD-1 blockade activates T-cell and IFN-gamma signaling pathways, which provides “a compelling rationale for the further development of PD-1 blockade in HL,” they concluded.
The most common treatment-related adverse effects were hypothyroidism (16%), diarrhea (16%), nausea (13%), and pneumonitis (10%). Five patients (16%) developed grade 3 treatment-related adverse events, including elevated hepatic transaminases, axillary pain, back pain, joint swelling, colitis, and nephrotic syndrome. Two patients stopped treatment because of adverse effects, but there were no grade 4 events or deaths related to treatment, and no treatment-induced cases of hepatitis, hypophysitis, or uveitis, the researchers noted.
Merck, maker of pembrolizumab, funded the study. Dr. Armand reported financial ties to Merck, Bristol-Myers Squibb, Infinity Pharmaceuticals, Sequenta, Tensha Therapeutics, and Sigma-Tau. Senior author Craig Moskowitz, MD, and five coinvestigators disclosed consulting or advisory relationships or employment with Merck.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Pembrolizumab is a potential treatment option for relapsed/refractory classical Hodgkin lymphoma.
Major finding: The complete response rate was 16%, the overall response rate was 65%, and 70% of responses lasted at least 24 weeks.
Data source: A single-arm, open-label, phase Ib study of pembrolizumab (10 mg/kg every other week) in 31 patients with heavily pretreated classical Hodgkin lymphoma that had progressed on or after brentuximab vedotin.
Disclosures: Merck, maker of pembrolizumab, funded the study. Dr. Armand reported financial ties to Merck, Bristol-Myers Squibb, Infinity Pharmaceuticals, Sequenta, Tensha Therapeutics, and Sigma-Tau. Senior author Craig Moskowitz, MD, and five coinvestigators disclosed consulting or advisory relationships or employment with Merck.
CT Scans Reliable Determinants of Blunt Trauma
NEW YORK - CT scans identify all clinically significant cervical spine injuries in intoxicated patients with blunt trauma, according to a new study.
"I don't think any of the results were particularly surprising to any of us who regularly do trauma care, but what I do think is remarkable about them is that they dispel several long-held myths about the c-spine, intoxicated patients, and the clearance process," Dr. Matthew J. Martin from Legacy Emanuel Medical Center, Portland, Oregon told Reuters Health.
"I think it again confirms that modern CT scan is highly reliable for identifying significant c-spine injuries, but also that the majority of so called 'intoxicated' patients are examinable enough to determine whether the collar can be removed (when combined with the CT scan)," he said.
Up to half of trauma patients are intoxicated, making clearance of the cervical spine a commonly encountered dilemma with both medical and medicolegal implications. Most guidelines indicate that the cervical spine should not be cleared in such patients, resulting in prolonged immobilization or additional imaging even in the face of a normal CT scan.
Dr. Martin's team examined cervical spine clearance practices for intoxicated trauma patients, examined the reliability of cervical spine CT scans for identifying clinically significant injuries (CSIs), and looked for CSIs that might have been missed by CT scans.
Among 1,429 patients who had an alcohol or drug screen performed, 44.2% were intoxicated, the researchers report in JAMA Surgery, online June 15.
Cervical spine injuries were identified in 11.3% of the sober group, 8.1% of the alcohol-intoxicated group, and 12.0% of the drug-intoxicated group.
CT scans yielded negative predictive values of 99.2% for all injuries and 99.8% for unstable injuries. There were five false-negative CT scans, including four central cord syndromes without associated fractures and one potentially unstable injury in a drug-intoxicated patient who presented with clear quadriplegia on examination.
Half of the intoxicated patients were admitted with continued cervical spine immobilization only on the basis of their intoxication. There were no missed CSIs in this group, and all patients were discharged without evidence of an injury or neurologic deficit. They underwent cervical spine immobilization for an average of 15.1 hours, about four times the average time to cervical spine clearance among sober patients (3.7 hours).
"The finding of how long we are keeping these patients in a c-collar based solely on intoxication should raise some eyebrows, and identifies an easy target for process improvement," Dr. Martin said.
"Cervical collars and immobilization are not therapeutic for the vast majority of c-spine injuries; they are really only to prevent inadvertent motion of an unstable c-spine injury," Dr. Martin said. "This is exceedingly rare in a patient who presents with no gross motor deficit, and a high quality CT scan will identify these unstable injuries very reliably. In addition, there are multiple adverse effects of prolonged immobilization, and even of getting an MRI."
"When these are factored in, I think the risk:benefit analysis falls squarely on the side of early clearance based on CT scan," he concluded.
"A key point is that this should be done by experts who are familiar with not only the global concept (the collar can be removed with a negative CT scan), but also the finer points where you could potentially cause harm, or where you should not remove the collar," Dr. Martin added. "This is where a very clear written protocol comes into play and reduces variation or errors that could cause patient harm."
"The results of this study suggest that it is unnecessary to delay cervical spine clearance until intoxicated patients are sober or until magnetic resonance imaging is performed," write Dr. Olubode A. Olufajo and Dr. Ali Salim from Brigham and Women's Hospital, Boston, in a related editorial. "However, caution must be taken in making conclusions based on these data."
"Although the authors conducted the study at an institution with high-quality CT technology and well-trained radiologists, they still recorded a false-negative CT report consistent with a misread," they note. "With the higher potential for this nature of error in lower-resourced settings, it becomes important to compare the costs and benefits of early removal of cervical collars."
They wonder, "With our knowledge that intoxicated patients form up to half of the population of trauma patients, is it really safe to risk irreversible injuries in 1% of the population to save a few hours in cervical clearance times?"
Dr. Stephen Asha from the University of New South Wales in Sydney, Australia, who has reported on various aspects of cervical spine imaging, told Reuters Health by email, "I think this study confirms what clinical experience as well as much of the more recent studies on cervical spine CT scanning tells us, which is that if there is nothing abnormal detected on a new generation, multi-slice CT, then the neck can be cleared."
"Of course there were a few missed injuries, but this needs to be put into context: no one just does a test in isolation, it is always combined with a clinical assessment, and a consideration the mechanism of injury," said Dr. Asha, who was not involved in the new work. "In this case there were five injuries not apparent on the CT scan, but all had obvious spinal cord injury on clinical examination before the CT was done, so these injuries were never going to be missed in a real clinical setting."
"MRI use should be carefully considered because the problem with MRI is that it can be over-sensitive, demonstrating abnormal signal suggesting ligamentous injury in patient who simply have a ligamentous 'strain,'" Dr. Asha explained. "The false-positive results then lead to further periods of inappropriate immobilization and testing, with the accompanying costs, inconvenience, and complications."
"In patients in whom the clinical assessment raises no concerns for injury, then a normal CT should herald the end of investigations," he said. "MRI should be reserved for those where the clinical assessment is abnormal or where the CT is abnormal and further evaluation for ligamentous or spinal injury is required."
Dr. Asha concluded, "If the clinical exam is not concerning and the CT is normal, then clear the neck."
SOURCE: http://bit.ly/28MxHxA and http://bit.ly/28MxHO5
JAMA Surg 2016.
NEW YORK - CT scans identify all clinically significant cervical spine injuries in intoxicated patients with blunt trauma, according to a new study.
"I don't think any of the results were particularly surprising to any of us who regularly do trauma care, but what I do think is remarkable about them is that they dispel several long-held myths about the c-spine, intoxicated patients, and the clearance process," Dr. Matthew J. Martin from Legacy Emanuel Medical Center, Portland, Oregon told Reuters Health.
"I think it again confirms that modern CT scan is highly reliable for identifying significant c-spine injuries, but also that the majority of so called 'intoxicated' patients are examinable enough to determine whether the collar can be removed (when combined with the CT scan)," he said.
Up to half of trauma patients are intoxicated, making clearance of the cervical spine a commonly encountered dilemma with both medical and medicolegal implications. Most guidelines indicate that the cervical spine should not be cleared in such patients, resulting in prolonged immobilization or additional imaging even in the face of a normal CT scan.
Dr. Martin's team examined cervical spine clearance practices for intoxicated trauma patients, examined the reliability of cervical spine CT scans for identifying clinically significant injuries (CSIs), and looked for CSIs that might have been missed by CT scans.
Among 1,429 patients who had an alcohol or drug screen performed, 44.2% were intoxicated, the researchers report in JAMA Surgery, online June 15.
Cervical spine injuries were identified in 11.3% of the sober group, 8.1% of the alcohol-intoxicated group, and 12.0% of the drug-intoxicated group.
CT scans yielded negative predictive values of 99.2% for all injuries and 99.8% for unstable injuries. There were five false-negative CT scans, including four central cord syndromes without associated fractures and one potentially unstable injury in a drug-intoxicated patient who presented with clear quadriplegia on examination.
Half of the intoxicated patients were admitted with continued cervical spine immobilization only on the basis of their intoxication. There were no missed CSIs in this group, and all patients were discharged without evidence of an injury or neurologic deficit. They underwent cervical spine immobilization for an average of 15.1 hours, about four times the average time to cervical spine clearance among sober patients (3.7 hours).
"The finding of how long we are keeping these patients in a c-collar based solely on intoxication should raise some eyebrows, and identifies an easy target for process improvement," Dr. Martin said.
"Cervical collars and immobilization are not therapeutic for the vast majority of c-spine injuries; they are really only to prevent inadvertent motion of an unstable c-spine injury," Dr. Martin said. "This is exceedingly rare in a patient who presents with no gross motor deficit, and a high quality CT scan will identify these unstable injuries very reliably. In addition, there are multiple adverse effects of prolonged immobilization, and even of getting an MRI."
"When these are factored in, I think the risk:benefit analysis falls squarely on the side of early clearance based on CT scan," he concluded.
"A key point is that this should be done by experts who are familiar with not only the global concept (the collar can be removed with a negative CT scan), but also the finer points where you could potentially cause harm, or where you should not remove the collar," Dr. Martin added. "This is where a very clear written protocol comes into play and reduces variation or errors that could cause patient harm."
"The results of this study suggest that it is unnecessary to delay cervical spine clearance until intoxicated patients are sober or until magnetic resonance imaging is performed," write Dr. Olubode A. Olufajo and Dr. Ali Salim from Brigham and Women's Hospital, Boston, in a related editorial. "However, caution must be taken in making conclusions based on these data."
"Although the authors conducted the study at an institution with high-quality CT technology and well-trained radiologists, they still recorded a false-negative CT report consistent with a misread," they note. "With the higher potential for this nature of error in lower-resourced settings, it becomes important to compare the costs and benefits of early removal of cervical collars."
They wonder, "With our knowledge that intoxicated patients form up to half of the population of trauma patients, is it really safe to risk irreversible injuries in 1% of the population to save a few hours in cervical clearance times?"
Dr. Stephen Asha from the University of New South Wales in Sydney, Australia, who has reported on various aspects of cervical spine imaging, told Reuters Health by email, "I think this study confirms what clinical experience as well as much of the more recent studies on cervical spine CT scanning tells us, which is that if there is nothing abnormal detected on a new generation, multi-slice CT, then the neck can be cleared."
"Of course there were a few missed injuries, but this needs to be put into context: no one just does a test in isolation, it is always combined with a clinical assessment, and a consideration the mechanism of injury," said Dr. Asha, who was not involved in the new work. "In this case there were five injuries not apparent on the CT scan, but all had obvious spinal cord injury on clinical examination before the CT was done, so these injuries were never going to be missed in a real clinical setting."
"MRI use should be carefully considered because the problem with MRI is that it can be over-sensitive, demonstrating abnormal signal suggesting ligamentous injury in patient who simply have a ligamentous 'strain,'" Dr. Asha explained. "The false-positive results then lead to further periods of inappropriate immobilization and testing, with the accompanying costs, inconvenience, and complications."
"In patients in whom the clinical assessment raises no concerns for injury, then a normal CT should herald the end of investigations," he said. "MRI should be reserved for those where the clinical assessment is abnormal or where the CT is abnormal and further evaluation for ligamentous or spinal injury is required."
Dr. Asha concluded, "If the clinical exam is not concerning and the CT is normal, then clear the neck."
SOURCE: http://bit.ly/28MxHxA and http://bit.ly/28MxHO5
JAMA Surg 2016.
NEW YORK - CT scans identify all clinically significant cervical spine injuries in intoxicated patients with blunt trauma, according to a new study.
"I don't think any of the results were particularly surprising to any of us who regularly do trauma care, but what I do think is remarkable about them is that they dispel several long-held myths about the c-spine, intoxicated patients, and the clearance process," Dr. Matthew J. Martin from Legacy Emanuel Medical Center, Portland, Oregon told Reuters Health.
"I think it again confirms that modern CT scan is highly reliable for identifying significant c-spine injuries, but also that the majority of so called 'intoxicated' patients are examinable enough to determine whether the collar can be removed (when combined with the CT scan)," he said.
Up to half of trauma patients are intoxicated, making clearance of the cervical spine a commonly encountered dilemma with both medical and medicolegal implications. Most guidelines indicate that the cervical spine should not be cleared in such patients, resulting in prolonged immobilization or additional imaging even in the face of a normal CT scan.
Dr. Martin's team examined cervical spine clearance practices for intoxicated trauma patients, examined the reliability of cervical spine CT scans for identifying clinically significant injuries (CSIs), and looked for CSIs that might have been missed by CT scans.
Among 1,429 patients who had an alcohol or drug screen performed, 44.2% were intoxicated, the researchers report in JAMA Surgery, online June 15.
Cervical spine injuries were identified in 11.3% of the sober group, 8.1% of the alcohol-intoxicated group, and 12.0% of the drug-intoxicated group.
CT scans yielded negative predictive values of 99.2% for all injuries and 99.8% for unstable injuries. There were five false-negative CT scans, including four central cord syndromes without associated fractures and one potentially unstable injury in a drug-intoxicated patient who presented with clear quadriplegia on examination.
Half of the intoxicated patients were admitted with continued cervical spine immobilization only on the basis of their intoxication. There were no missed CSIs in this group, and all patients were discharged without evidence of an injury or neurologic deficit. They underwent cervical spine immobilization for an average of 15.1 hours, about four times the average time to cervical spine clearance among sober patients (3.7 hours).
"The finding of how long we are keeping these patients in a c-collar based solely on intoxication should raise some eyebrows, and identifies an easy target for process improvement," Dr. Martin said.
"Cervical collars and immobilization are not therapeutic for the vast majority of c-spine injuries; they are really only to prevent inadvertent motion of an unstable c-spine injury," Dr. Martin said. "This is exceedingly rare in a patient who presents with no gross motor deficit, and a high quality CT scan will identify these unstable injuries very reliably. In addition, there are multiple adverse effects of prolonged immobilization, and even of getting an MRI."
"When these are factored in, I think the risk:benefit analysis falls squarely on the side of early clearance based on CT scan," he concluded.
"A key point is that this should be done by experts who are familiar with not only the global concept (the collar can be removed with a negative CT scan), but also the finer points where you could potentially cause harm, or where you should not remove the collar," Dr. Martin added. "This is where a very clear written protocol comes into play and reduces variation or errors that could cause patient harm."
"The results of this study suggest that it is unnecessary to delay cervical spine clearance until intoxicated patients are sober or until magnetic resonance imaging is performed," write Dr. Olubode A. Olufajo and Dr. Ali Salim from Brigham and Women's Hospital, Boston, in a related editorial. "However, caution must be taken in making conclusions based on these data."
"Although the authors conducted the study at an institution with high-quality CT technology and well-trained radiologists, they still recorded a false-negative CT report consistent with a misread," they note. "With the higher potential for this nature of error in lower-resourced settings, it becomes important to compare the costs and benefits of early removal of cervical collars."
They wonder, "With our knowledge that intoxicated patients form up to half of the population of trauma patients, is it really safe to risk irreversible injuries in 1% of the population to save a few hours in cervical clearance times?"
Dr. Stephen Asha from the University of New South Wales in Sydney, Australia, who has reported on various aspects of cervical spine imaging, told Reuters Health by email, "I think this study confirms what clinical experience as well as much of the more recent studies on cervical spine CT scanning tells us, which is that if there is nothing abnormal detected on a new generation, multi-slice CT, then the neck can be cleared."
"Of course there were a few missed injuries, but this needs to be put into context: no one just does a test in isolation, it is always combined with a clinical assessment, and a consideration the mechanism of injury," said Dr. Asha, who was not involved in the new work. "In this case there were five injuries not apparent on the CT scan, but all had obvious spinal cord injury on clinical examination before the CT was done, so these injuries were never going to be missed in a real clinical setting."
"MRI use should be carefully considered because the problem with MRI is that it can be over-sensitive, demonstrating abnormal signal suggesting ligamentous injury in patient who simply have a ligamentous 'strain,'" Dr. Asha explained. "The false-positive results then lead to further periods of inappropriate immobilization and testing, with the accompanying costs, inconvenience, and complications."
"In patients in whom the clinical assessment raises no concerns for injury, then a normal CT should herald the end of investigations," he said. "MRI should be reserved for those where the clinical assessment is abnormal or where the CT is abnormal and further evaluation for ligamentous or spinal injury is required."
Dr. Asha concluded, "If the clinical exam is not concerning and the CT is normal, then clear the neck."
SOURCE: http://bit.ly/28MxHxA and http://bit.ly/28MxHO5
JAMA Surg 2016.
When Opioids Mix With Pregnancy, What’s Best?
Hand-wringing stories about the opioid epidemic are flooding the popular press – and clincians are seeing the headlines reflected in their practices.
As clinics fill with more and more pregnant patients who have opioid use disorder, both ob.gyns. and family physicians who incorporate obstetrics are facing a steep learning curve in dealing with the medical and ethical challenges these patients bring to their clinic visits. Though there’s no panacea, collaboration with community and family stakeholders and a comprehensive care model incorporating best practices can optimize outcomes for these fragile patients.
On May 23, 2016, the American College of Obstetricians and Gynecologists (ACOG) issued a statement affirming medically assisted treatment (MAT) as the recommended standard of care for pregnant women with opioid use disorders. In a statement, Hal Lawrence, MD, ACOG’s executive vice president and CEO, said, “Robust evidence has demonstrated that maintenance therapy during pregnancy can improve outcomes.”
Methadone has been the mainstay MAT medication, in part because its long time on the market means that favorable data are more robust than for buprenorphine. For patients in rural areas facing transportation challenges, however, or for those whose jobs or caregiving duties make a daily visit to a methadone clinic difficult, buprenorphine may be the better option. Additionally, especially in smaller communities, an oral self-administered medication avoids the obvious stigma of methadone clinic visits.
In making efforts to reduce maternal opioid dependence a 2016 legislative priority, ACOG voiced opposition to any legislation that might be punitive for women with opioid use disorder, as well as for babies born with neonatal abstinence syndrome; however, ACOG also supports public health efforts to reduce these conditions.
Wanda Filer, MD, president of the American Academy of Family Physicians, concurred in an interview. “We do not support criminalization or incarceration of pregnant women with substance use disorders,” stressed Dr. Filer, who noted that the AAFP does not have a formal policy statement at this point.
Mishka Terplan, MD, an ob.gyn. who also is an addiction medicine specialist and has helped shape ACOG policy in this area, said in an interview that the maternal-fetal-placental unit has a “complicated and unique biology.” About targeted legislation – or reinterpretation of existing legislation – that incarcerates pregnant women with substance use disorder, he said, “These laws in effect cleave that unit. … To me, it’s unnatural, and not in the interests of the mom.”
Most laws that target women who are pregnant and have substance use disorder are on the state rather than the federal level, said Dr. Terplan, medical director of Behavioral Health System Baltimore.
Currently, three states allow involuntary commitment for treatment of pregnant women with substance use disorder, he said. Other states will classify substance use in pregnancy as child abuse, or use “chemical endangerment” statutes as a vehicle for incarceration or prosecution. Additionally, Medicaid provisions or limitations on access to MAT may vary by state, so physicians must be familiar with their local legal landscape in these cases, he said.
Community resources, critical to providing holistic care for this fragile population, are also region specific. In interviews, two physicians caring for pregnant women with opioid use disorder talked about how their practices are tailored to their communities. Understanding which resources are available and what’s possible for their patients informs how they care for these challenging patients.
La Crosse, Wis., is situated along the eastern side of the Mississippi River, close to the Minnesota-Iowa border. Though the college town has about 100,000 people in its urban area, the surrounding area gets very rural, very quickly. Gundersen Health System, based in La Crosse, has over two dozen clinics and a handful of hospitals in three states and is the practice home for Charles Schauberger, MD, an ob.gyn. who specializes in caring for pregnant women with substance use disorder.
Dr. Schauberger sees a broad range of patients with a wide demographic and urban-rural mix. He estimates that about two-thirds of his patients have a history of previous treatment for substance use disorder, while the opioid use is a fairly new development in other one-third. And most of them face many other challenges. “Many of my patients have high concerns about housing insecurity. They do a lot of couchsurfing,” said Dr. Schauberger.
His patient panel’s high no-show rate reflects the chaotic lives and transportation challenges of many of his patients, and it’s not uncommon for Dr. Schauberger’s patients to come from jail to his office for prenatal care. “It’s about not putting up barriers” for these women, he said. “I might see one patient for just one prenatal visit. Another one, we might see 20 times. We take what we can get.” His staff and partners all realize that flexibility is key to maximizing the chance for a good outcome, he said.
Using a collaborative care model, Dr. Schauberger and his nonphysician colleagues will see patients together. “We’ll often have two or three staff members in the room – at least the social worker, the care coordinator, and myself,” he said. Recognizing that “the patient, not the doctor, is at the center of the care model” is critical for making things work, he said. “It’s important for everyone on the team to be aware of that.”
The collaborative model helps to address the many nonmedical challenges that can contribute to ongoing issues with substance use. Family dynamics and the presence – or absence – of a support system can make a big difference in adherence to a treatment plan during pregnancy and in the postpartum period. “The social, political, and legal issues are really what are important. … We try to get them into the system as soon as possible,” he said. His practice has a close connection with the addiction team at Gundersen Health, which dispenses methadone, as does a private addiction clinic in town, said Dr. Schauberger, who prescribes buprenorphine.
Wisconsin is one of the three states where pregnant women can be committed for coerced treatment, said Dr. Terplan. Dr. Schauberger has a good connection with his legal system locally, and he views any possibility of incarceration or commitment for substance use in pregnancy as “a serious problem that we need to avoid.”
Not all physicians practice in an environment where they have the luxury of specialization, and Dr. Schauberger said that the demand is too high in many places for primary care providers not to manage the care of pregnant women with substance use disorders. Though these women are high risk, “it is a type of high risk that a family medicine doctor should be able to take care of without problem. Primarily the risks are premature labor and intrauterine growth retardation, which are both problems that most family medicine doctors that do obstetrics should be able to identify and follow,” he said.
This fits with the mission of family practice, said Dr. Filer. As a specialty, “family practice is tied in to community resources,” she said. “Knowing your referral network is vital.” She also sees a growing trend of AAFP members who have completed addiction medicine fellowships. “This reflects a practice need – and a community need,” she said.
One family practice physician’s rural southeast Nebraska practice necessitates a creative and flexible approach to caring for pregnant women with opioid use disorder. Robert Wergin, MD, the only physician in the small town of Milford, Neb., has a cradle-to-grave practice that includes obstetrics. When on call, he’s also the emergency physician at the 25-bed critical-access hospital that serves his area. The opioid epidemic touches his pregnant patients frequently.
Dr. Wergin is the current board chair of AAFP, as well as a past president of the organization. A Nebraska native, he draws his resources from the community, often tapping local pastors for help. He recently referred a pregnant woman to a church food pantry, since her food aid for the month had run out and she had another 10 days to face with an empty larder. “You’ve got to look at the comprehensive picture, and really know the patient to understand barriers to care, such as transportation problems and poverty.”
The area has no methadone clinic, and the few buprenorphine prescribers in the area usually have full practices and waiting lists. This means that optimal MAT may not be achievable. When he can, he sends patients to Lincoln, Neb., about 30 miles away, for treatment. If that’s not feasible, and the problem is identified early in the pregnancy, Dr. Wergin may help patients taper to eliminate or reduce opioid use.
An opinion reaffirmed in 2014 by ACOG recognized that MAT may not be accessible to all. ACOG’s Committee on Health Care for Underserved Women, together with the American Society of Addiction Medicine, wrote, “If the alternative to medically supervised withdrawal is continued illicit drug use, then a medically supervised withdrawal in the first trimester is preferable to waiting until the second trimester.”
Dr. Wergin also works hard to incorporate family supports into his care of substance-using pregnant women. “Comprehensive care is really important,” he said. “Somehow, you have to get a buy-in from the patient,” and sometimes a supportive mother or partner can make a big difference in maternal and neonatal outcomes, he explained. Rapport and trust are critical, and his career-long presence in the community has helped build that trust with the families for whom he cares, he said.
Planning for delivery of an infant who’s likely to exhibit neonatal abstinence syndrome is a particular challenge for Dr. Wergin. His local hospital has a staff of generalist nurses, no NICU, and no ventilators appropriate for critically ill infants. Optimally, there’s time for transport to a tertiary care center in Lincoln before delivery. Inevitably, though, some deliveries can’t wait. “If you’ve got a withdrawing baby, the earlier they get to a higher level of care, the better,” said Dr. Wergin. “You have to prepare as best you can, and coordinate a transfer soon.”
Dr. Filer said that the real value proposition offered by family physicians in addressing the social and medical complexity of substance use in pregnancy is the wrap-around care the specialty offers. “We can leverage the family and support systems … to help these women find a way to become whole again – whole in their work, in their family, in parenting,” she said.
Hand-wringing stories about the opioid epidemic are flooding the popular press – and clincians are seeing the headlines reflected in their practices.
As clinics fill with more and more pregnant patients who have opioid use disorder, both ob.gyns. and family physicians who incorporate obstetrics are facing a steep learning curve in dealing with the medical and ethical challenges these patients bring to their clinic visits. Though there’s no panacea, collaboration with community and family stakeholders and a comprehensive care model incorporating best practices can optimize outcomes for these fragile patients.
On May 23, 2016, the American College of Obstetricians and Gynecologists (ACOG) issued a statement affirming medically assisted treatment (MAT) as the recommended standard of care for pregnant women with opioid use disorders. In a statement, Hal Lawrence, MD, ACOG’s executive vice president and CEO, said, “Robust evidence has demonstrated that maintenance therapy during pregnancy can improve outcomes.”
Methadone has been the mainstay MAT medication, in part because its long time on the market means that favorable data are more robust than for buprenorphine. For patients in rural areas facing transportation challenges, however, or for those whose jobs or caregiving duties make a daily visit to a methadone clinic difficult, buprenorphine may be the better option. Additionally, especially in smaller communities, an oral self-administered medication avoids the obvious stigma of methadone clinic visits.
In making efforts to reduce maternal opioid dependence a 2016 legislative priority, ACOG voiced opposition to any legislation that might be punitive for women with opioid use disorder, as well as for babies born with neonatal abstinence syndrome; however, ACOG also supports public health efforts to reduce these conditions.
Wanda Filer, MD, president of the American Academy of Family Physicians, concurred in an interview. “We do not support criminalization or incarceration of pregnant women with substance use disorders,” stressed Dr. Filer, who noted that the AAFP does not have a formal policy statement at this point.
Mishka Terplan, MD, an ob.gyn. who also is an addiction medicine specialist and has helped shape ACOG policy in this area, said in an interview that the maternal-fetal-placental unit has a “complicated and unique biology.” About targeted legislation – or reinterpretation of existing legislation – that incarcerates pregnant women with substance use disorder, he said, “These laws in effect cleave that unit. … To me, it’s unnatural, and not in the interests of the mom.”
Most laws that target women who are pregnant and have substance use disorder are on the state rather than the federal level, said Dr. Terplan, medical director of Behavioral Health System Baltimore.
Currently, three states allow involuntary commitment for treatment of pregnant women with substance use disorder, he said. Other states will classify substance use in pregnancy as child abuse, or use “chemical endangerment” statutes as a vehicle for incarceration or prosecution. Additionally, Medicaid provisions or limitations on access to MAT may vary by state, so physicians must be familiar with their local legal landscape in these cases, he said.
Community resources, critical to providing holistic care for this fragile population, are also region specific. In interviews, two physicians caring for pregnant women with opioid use disorder talked about how their practices are tailored to their communities. Understanding which resources are available and what’s possible for their patients informs how they care for these challenging patients.
La Crosse, Wis., is situated along the eastern side of the Mississippi River, close to the Minnesota-Iowa border. Though the college town has about 100,000 people in its urban area, the surrounding area gets very rural, very quickly. Gundersen Health System, based in La Crosse, has over two dozen clinics and a handful of hospitals in three states and is the practice home for Charles Schauberger, MD, an ob.gyn. who specializes in caring for pregnant women with substance use disorder.
Dr. Schauberger sees a broad range of patients with a wide demographic and urban-rural mix. He estimates that about two-thirds of his patients have a history of previous treatment for substance use disorder, while the opioid use is a fairly new development in other one-third. And most of them face many other challenges. “Many of my patients have high concerns about housing insecurity. They do a lot of couchsurfing,” said Dr. Schauberger.
His patient panel’s high no-show rate reflects the chaotic lives and transportation challenges of many of his patients, and it’s not uncommon for Dr. Schauberger’s patients to come from jail to his office for prenatal care. “It’s about not putting up barriers” for these women, he said. “I might see one patient for just one prenatal visit. Another one, we might see 20 times. We take what we can get.” His staff and partners all realize that flexibility is key to maximizing the chance for a good outcome, he said.
Using a collaborative care model, Dr. Schauberger and his nonphysician colleagues will see patients together. “We’ll often have two or three staff members in the room – at least the social worker, the care coordinator, and myself,” he said. Recognizing that “the patient, not the doctor, is at the center of the care model” is critical for making things work, he said. “It’s important for everyone on the team to be aware of that.”
The collaborative model helps to address the many nonmedical challenges that can contribute to ongoing issues with substance use. Family dynamics and the presence – or absence – of a support system can make a big difference in adherence to a treatment plan during pregnancy and in the postpartum period. “The social, political, and legal issues are really what are important. … We try to get them into the system as soon as possible,” he said. His practice has a close connection with the addiction team at Gundersen Health, which dispenses methadone, as does a private addiction clinic in town, said Dr. Schauberger, who prescribes buprenorphine.
Wisconsin is one of the three states where pregnant women can be committed for coerced treatment, said Dr. Terplan. Dr. Schauberger has a good connection with his legal system locally, and he views any possibility of incarceration or commitment for substance use in pregnancy as “a serious problem that we need to avoid.”
Not all physicians practice in an environment where they have the luxury of specialization, and Dr. Schauberger said that the demand is too high in many places for primary care providers not to manage the care of pregnant women with substance use disorders. Though these women are high risk, “it is a type of high risk that a family medicine doctor should be able to take care of without problem. Primarily the risks are premature labor and intrauterine growth retardation, which are both problems that most family medicine doctors that do obstetrics should be able to identify and follow,” he said.
This fits with the mission of family practice, said Dr. Filer. As a specialty, “family practice is tied in to community resources,” she said. “Knowing your referral network is vital.” She also sees a growing trend of AAFP members who have completed addiction medicine fellowships. “This reflects a practice need – and a community need,” she said.
One family practice physician’s rural southeast Nebraska practice necessitates a creative and flexible approach to caring for pregnant women with opioid use disorder. Robert Wergin, MD, the only physician in the small town of Milford, Neb., has a cradle-to-grave practice that includes obstetrics. When on call, he’s also the emergency physician at the 25-bed critical-access hospital that serves his area. The opioid epidemic touches his pregnant patients frequently.
Dr. Wergin is the current board chair of AAFP, as well as a past president of the organization. A Nebraska native, he draws his resources from the community, often tapping local pastors for help. He recently referred a pregnant woman to a church food pantry, since her food aid for the month had run out and she had another 10 days to face with an empty larder. “You’ve got to look at the comprehensive picture, and really know the patient to understand barriers to care, such as transportation problems and poverty.”
The area has no methadone clinic, and the few buprenorphine prescribers in the area usually have full practices and waiting lists. This means that optimal MAT may not be achievable. When he can, he sends patients to Lincoln, Neb., about 30 miles away, for treatment. If that’s not feasible, and the problem is identified early in the pregnancy, Dr. Wergin may help patients taper to eliminate or reduce opioid use.
An opinion reaffirmed in 2014 by ACOG recognized that MAT may not be accessible to all. ACOG’s Committee on Health Care for Underserved Women, together with the American Society of Addiction Medicine, wrote, “If the alternative to medically supervised withdrawal is continued illicit drug use, then a medically supervised withdrawal in the first trimester is preferable to waiting until the second trimester.”
Dr. Wergin also works hard to incorporate family supports into his care of substance-using pregnant women. “Comprehensive care is really important,” he said. “Somehow, you have to get a buy-in from the patient,” and sometimes a supportive mother or partner can make a big difference in maternal and neonatal outcomes, he explained. Rapport and trust are critical, and his career-long presence in the community has helped build that trust with the families for whom he cares, he said.
Planning for delivery of an infant who’s likely to exhibit neonatal abstinence syndrome is a particular challenge for Dr. Wergin. His local hospital has a staff of generalist nurses, no NICU, and no ventilators appropriate for critically ill infants. Optimally, there’s time for transport to a tertiary care center in Lincoln before delivery. Inevitably, though, some deliveries can’t wait. “If you’ve got a withdrawing baby, the earlier they get to a higher level of care, the better,” said Dr. Wergin. “You have to prepare as best you can, and coordinate a transfer soon.”
Dr. Filer said that the real value proposition offered by family physicians in addressing the social and medical complexity of substance use in pregnancy is the wrap-around care the specialty offers. “We can leverage the family and support systems … to help these women find a way to become whole again – whole in their work, in their family, in parenting,” she said.
Hand-wringing stories about the opioid epidemic are flooding the popular press – and clincians are seeing the headlines reflected in their practices.
As clinics fill with more and more pregnant patients who have opioid use disorder, both ob.gyns. and family physicians who incorporate obstetrics are facing a steep learning curve in dealing with the medical and ethical challenges these patients bring to their clinic visits. Though there’s no panacea, collaboration with community and family stakeholders and a comprehensive care model incorporating best practices can optimize outcomes for these fragile patients.
On May 23, 2016, the American College of Obstetricians and Gynecologists (ACOG) issued a statement affirming medically assisted treatment (MAT) as the recommended standard of care for pregnant women with opioid use disorders. In a statement, Hal Lawrence, MD, ACOG’s executive vice president and CEO, said, “Robust evidence has demonstrated that maintenance therapy during pregnancy can improve outcomes.”
Methadone has been the mainstay MAT medication, in part because its long time on the market means that favorable data are more robust than for buprenorphine. For patients in rural areas facing transportation challenges, however, or for those whose jobs or caregiving duties make a daily visit to a methadone clinic difficult, buprenorphine may be the better option. Additionally, especially in smaller communities, an oral self-administered medication avoids the obvious stigma of methadone clinic visits.
In making efforts to reduce maternal opioid dependence a 2016 legislative priority, ACOG voiced opposition to any legislation that might be punitive for women with opioid use disorder, as well as for babies born with neonatal abstinence syndrome; however, ACOG also supports public health efforts to reduce these conditions.
Wanda Filer, MD, president of the American Academy of Family Physicians, concurred in an interview. “We do not support criminalization or incarceration of pregnant women with substance use disorders,” stressed Dr. Filer, who noted that the AAFP does not have a formal policy statement at this point.
Mishka Terplan, MD, an ob.gyn. who also is an addiction medicine specialist and has helped shape ACOG policy in this area, said in an interview that the maternal-fetal-placental unit has a “complicated and unique biology.” About targeted legislation – or reinterpretation of existing legislation – that incarcerates pregnant women with substance use disorder, he said, “These laws in effect cleave that unit. … To me, it’s unnatural, and not in the interests of the mom.”
Most laws that target women who are pregnant and have substance use disorder are on the state rather than the federal level, said Dr. Terplan, medical director of Behavioral Health System Baltimore.
Currently, three states allow involuntary commitment for treatment of pregnant women with substance use disorder, he said. Other states will classify substance use in pregnancy as child abuse, or use “chemical endangerment” statutes as a vehicle for incarceration or prosecution. Additionally, Medicaid provisions or limitations on access to MAT may vary by state, so physicians must be familiar with their local legal landscape in these cases, he said.
Community resources, critical to providing holistic care for this fragile population, are also region specific. In interviews, two physicians caring for pregnant women with opioid use disorder talked about how their practices are tailored to their communities. Understanding which resources are available and what’s possible for their patients informs how they care for these challenging patients.
La Crosse, Wis., is situated along the eastern side of the Mississippi River, close to the Minnesota-Iowa border. Though the college town has about 100,000 people in its urban area, the surrounding area gets very rural, very quickly. Gundersen Health System, based in La Crosse, has over two dozen clinics and a handful of hospitals in three states and is the practice home for Charles Schauberger, MD, an ob.gyn. who specializes in caring for pregnant women with substance use disorder.
Dr. Schauberger sees a broad range of patients with a wide demographic and urban-rural mix. He estimates that about two-thirds of his patients have a history of previous treatment for substance use disorder, while the opioid use is a fairly new development in other one-third. And most of them face many other challenges. “Many of my patients have high concerns about housing insecurity. They do a lot of couchsurfing,” said Dr. Schauberger.
His patient panel’s high no-show rate reflects the chaotic lives and transportation challenges of many of his patients, and it’s not uncommon for Dr. Schauberger’s patients to come from jail to his office for prenatal care. “It’s about not putting up barriers” for these women, he said. “I might see one patient for just one prenatal visit. Another one, we might see 20 times. We take what we can get.” His staff and partners all realize that flexibility is key to maximizing the chance for a good outcome, he said.
Using a collaborative care model, Dr. Schauberger and his nonphysician colleagues will see patients together. “We’ll often have two or three staff members in the room – at least the social worker, the care coordinator, and myself,” he said. Recognizing that “the patient, not the doctor, is at the center of the care model” is critical for making things work, he said. “It’s important for everyone on the team to be aware of that.”
The collaborative model helps to address the many nonmedical challenges that can contribute to ongoing issues with substance use. Family dynamics and the presence – or absence – of a support system can make a big difference in adherence to a treatment plan during pregnancy and in the postpartum period. “The social, political, and legal issues are really what are important. … We try to get them into the system as soon as possible,” he said. His practice has a close connection with the addiction team at Gundersen Health, which dispenses methadone, as does a private addiction clinic in town, said Dr. Schauberger, who prescribes buprenorphine.
Wisconsin is one of the three states where pregnant women can be committed for coerced treatment, said Dr. Terplan. Dr. Schauberger has a good connection with his legal system locally, and he views any possibility of incarceration or commitment for substance use in pregnancy as “a serious problem that we need to avoid.”
Not all physicians practice in an environment where they have the luxury of specialization, and Dr. Schauberger said that the demand is too high in many places for primary care providers not to manage the care of pregnant women with substance use disorders. Though these women are high risk, “it is a type of high risk that a family medicine doctor should be able to take care of without problem. Primarily the risks are premature labor and intrauterine growth retardation, which are both problems that most family medicine doctors that do obstetrics should be able to identify and follow,” he said.
This fits with the mission of family practice, said Dr. Filer. As a specialty, “family practice is tied in to community resources,” she said. “Knowing your referral network is vital.” She also sees a growing trend of AAFP members who have completed addiction medicine fellowships. “This reflects a practice need – and a community need,” she said.
One family practice physician’s rural southeast Nebraska practice necessitates a creative and flexible approach to caring for pregnant women with opioid use disorder. Robert Wergin, MD, the only physician in the small town of Milford, Neb., has a cradle-to-grave practice that includes obstetrics. When on call, he’s also the emergency physician at the 25-bed critical-access hospital that serves his area. The opioid epidemic touches his pregnant patients frequently.
Dr. Wergin is the current board chair of AAFP, as well as a past president of the organization. A Nebraska native, he draws his resources from the community, often tapping local pastors for help. He recently referred a pregnant woman to a church food pantry, since her food aid for the month had run out and she had another 10 days to face with an empty larder. “You’ve got to look at the comprehensive picture, and really know the patient to understand barriers to care, such as transportation problems and poverty.”
The area has no methadone clinic, and the few buprenorphine prescribers in the area usually have full practices and waiting lists. This means that optimal MAT may not be achievable. When he can, he sends patients to Lincoln, Neb., about 30 miles away, for treatment. If that’s not feasible, and the problem is identified early in the pregnancy, Dr. Wergin may help patients taper to eliminate or reduce opioid use.
An opinion reaffirmed in 2014 by ACOG recognized that MAT may not be accessible to all. ACOG’s Committee on Health Care for Underserved Women, together with the American Society of Addiction Medicine, wrote, “If the alternative to medically supervised withdrawal is continued illicit drug use, then a medically supervised withdrawal in the first trimester is preferable to waiting until the second trimester.”
Dr. Wergin also works hard to incorporate family supports into his care of substance-using pregnant women. “Comprehensive care is really important,” he said. “Somehow, you have to get a buy-in from the patient,” and sometimes a supportive mother or partner can make a big difference in maternal and neonatal outcomes, he explained. Rapport and trust are critical, and his career-long presence in the community has helped build that trust with the families for whom he cares, he said.
Planning for delivery of an infant who’s likely to exhibit neonatal abstinence syndrome is a particular challenge for Dr. Wergin. His local hospital has a staff of generalist nurses, no NICU, and no ventilators appropriate for critically ill infants. Optimally, there’s time for transport to a tertiary care center in Lincoln before delivery. Inevitably, though, some deliveries can’t wait. “If you’ve got a withdrawing baby, the earlier they get to a higher level of care, the better,” said Dr. Wergin. “You have to prepare as best you can, and coordinate a transfer soon.”
Dr. Filer said that the real value proposition offered by family physicians in addressing the social and medical complexity of substance use in pregnancy is the wrap-around care the specialty offers. “We can leverage the family and support systems … to help these women find a way to become whole again – whole in their work, in their family, in parenting,” she said.
Comparison of Pneumatic Broadband Light Plus Adapalene Gel 0.3% Versus Adapalene Gel 0.3% Monotherapy in the Treatment of Mild to Moderate Acne
Acne is a common and distressing condition that typically presents in adolescents and young adults and has been associated with not only medical but also emotional and aesthetic consequences. Acne treatments that offer faster improvement are the coveted goal. Although clinical studies support the use of combination therapy with topical retinoids and antibiotics, the overuse of antibiotics raises caution for bacterial resistance.1 Therefore, adjunctive treatments such as chemical peels, light therapy, and laser treatments can hasten the response to traditional acne treatments and in some cases may potentially decrease use of both oral and topical antibiotics.
Light therapy, particularly with visible light, may improve acne outcomes. Pneumatic broadband light (PBBL) is a light treatment in the broadband range (400–1200 nm) combined with a vacuum. The suction created by the vacuum has several effects on acne lesions, such as creating a mechanical lysis of thin-walled pustules and dislodging pore impaction. The blue light with a wavelength of 410 nm targets endogenous porphyrins in Propionibacterium acnes and elicits singlet oxygen production, resulting in bacterial destruction.2,3 Studies showed that PBBL alone was effective in most patients with mild to moderate acne and caused minimal side effects.2-4
We sought to determine if PBBL combined with a topical retinoid can accelerate and prolong acne improvement. We evaluated the efficacy, safety, and tolerability of PBBL plus adapalene gel 0.3% versus adapalene gel 0.3% monotherapy in patients with mild to moderate acne.
METHODSPatient Population
Patients with mild to moderate acne were eligible for the study if they were 18 years or older at screening, in good health, had stopped oral isotretinoin for at least 1 year prior to treatment initiation, and were not taking oral or topical antibiotics or using any topical retinoid derivatives for at least 1 month prior to treatment initiation. Inclusion criteria included at least 10 acne lesions on the face. Patients were excluded if they had a history of receiving PBBL treatment; had a history of scarring, hypopigmentation, or hyperpigmentation from laser or light treatments; and/or were pregnant or refused use of contraception during the study period.
Study Design
This single-blind, randomized, split-face study was approved by the institutional review board of the University of Pennsylvania (Philadelphia, Pennsylvania). All participants provided informed consent before entering the study. Each participant was randomly assigned to receive PBBL on one side of the face for 6 consecutive weeks and apply adapalene gel 0.3% to both sides of the face nightly for 10 weeks. Pneumatic broadband light treatment was performed using the following settings: starting power 2 (approximately 4–6 J/cm2) and vacuum setting 3 (negative pressure, approximately 3 lb/in2). The power setting was increased to a maximum of 6 (12–14 J/cm2) at subsequent visits depending on tolerability of the participants.
All participants visited the clinic weekly for 6 weeks and also returned for follow-up at week 10 (4 weeks following last PBBL treatment). At each visit, the participants completed satisfaction questionnaires and were assessed by a dermatologist evaluator using several parameters including the modified Global Acne Grading Score (mGAGS), clinical photography, participant self-assessment, physician assessment, and Wong Baker FACES Pain Rating Scale (WBPRS). The physician evaluator was blinded to the side of the face receiving PBBL treatment. Clinical photographs were taken to compare the clinical outcome at each visit versus baseline.
Efficacy Evaluation
Acne Counts
The blinded evaluator counted acne lesions and assessed the mGAGS at each visit prior to administration of the PBBL treatment. Acne lesions were counted separately as noninflammatory (comedones) and inflammatory (papules, pustules, nodules) on the forehead, cheeks, nose, and chin.
Modified Global Acne Grading Score
The modified Global Acne Grading Score was modified from the Global Acne Grading Scale (GAGS) that has previously been used to evaluate acne severity.5 The original GAGS used the type and location of the acne lesions. The GAGS considers 6 locations on the face, chest, and upper back, with a grading factor for each location (forehead=2; cheeks=2; nose=1; chin=1). Another grading factor represented the lesion type (0=no lesion; 1=comedone; 2=papule; 3=pustule; 4=nodule). The local score was calculated by multiplying the location grading factor by the lesion type grading factor. The total score was the sum of the individual local scores for the 4 locations.
Given that the number of acne lesions is important, we modified the GAGS by adding a grading factor that represented the number of lesions to improve the accuracy of the test (1=0–10 lesions; 2=11–20 lesions; 3=21–30 lesions; 4=≥31 lesions). The local score of mGAGS was calculated by multiplying the grading factors for location, lesion type, and number of lesions. Each local score was then added to yield a total score. The mGAGS may be useful and more accurate to determine the severity of acne (0=none; 1–44=mild; 45–80=moderate; 81–132=severe; 133–176=very severe).
Participant Self-assessment
Participants assessed their acne lesions using an 11-point rating scale (–5=100% worsening; –4=76%–99% worsening; –3=51%–75% worsening; –2=26%–50% worsening; –1=1%–25% worsening; 0=no improvement; 1=1%–25% improvement; 2=26%–50% improvement; 3=51%–75% improvement; 4=76%–99% improvement; 5=100% acne clear) to compare their acne at each treatment visit and week 10 follow-up with a baseline photograph.
Physician Assessment
The blinded evaluator assessed acne lesions on the face using the same 11-point rating scale that was used for participant self-assessment. For each participant, assessments were made at each treatment visit and week 10 follow-up by comparing baseline photographs.
Safety Evaluation
The WBPRS score, a standardized 6-point scale (0=no pain; 1=hurts a little bit; 2=hurts a little bit more; 3=hurts even more; 4=hurts whole lot; 5=hurt worst),6 was used to evaluate pain toleration during PBBL treatments and was recorded along with adverse events throughout the study.
Statistical Analysis
Based on data from 2 prior studies,3,7 we expected that the favorable clinical outcome of adapalene gel 0.3% and PBBL therapy would be 23% and 78%, respectively. If the adjunctive therapy with PBBL was beneficial, the favorable outcome would be higher than 78%. To be able to detect this difference, the sample size of 11 patients was needed when 5% type I error and 20% type II error were accepted.
Categorical variables were expressed as percentages, while continuous variables were expressed in terms of median (range). The clinical outcomes between both treatment groups were compared using the Wilcoxon signed rank test. A 2-tailed P value of ≤.05 was considered statistically significant. All statistical calculations were performed using STATA software version 10.0.
RESULTS
Baseline Characteristics
Four male and 7 female patients aged 18 to 35 years (median, 23 years) with mild to moderate acne were enrolled in the study. Of the 11 participants, 7 were white, 2 were black, 1 was Asian, and 1 was Latin American. Baseline characteristics of both sides of the face were comparable in all participants (Table 1). Eight participants (73%) completed the study. Two black participants withdrew from the study due to hyperpigmentation following PBBL treatment; 1 participant did not return for follow-up at week 10, as she was out of the country.
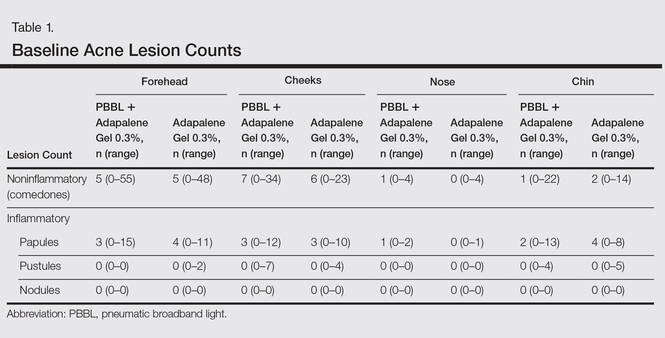
Lesion Counts
At week 3, reduction in noninflammatory lesions was significantly greater on the side receiving the combination therapy compared to the monotherapy side (P=.04)(Table 2). However, there was no significant difference between the combination therapy and the adapalene monotherapy sides in the reduction of noninflammatory and inflammatory lesions at week 4 (Figure 1). There was a remarkable improvement of the combination therapy and adapalene monotherapy sides in acne lesions, but there was no significant difference between the combination therapy and the adapalene monotherapy sides (Figure 2).
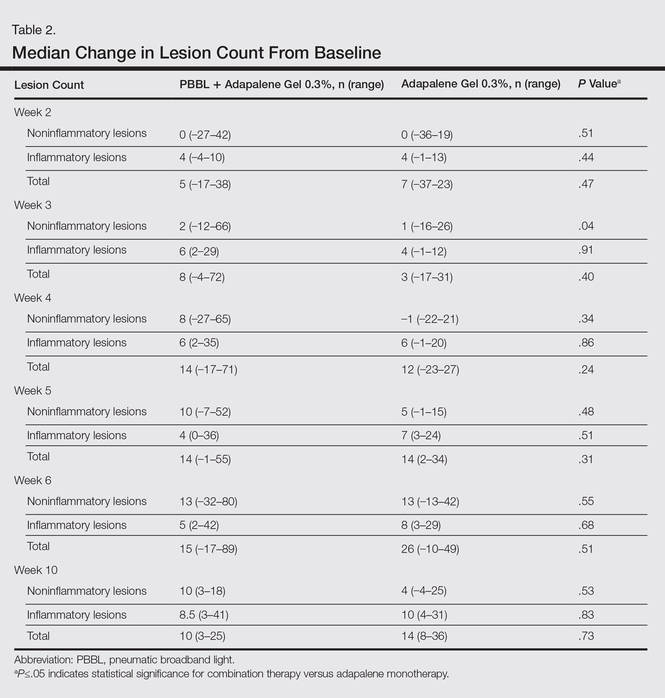
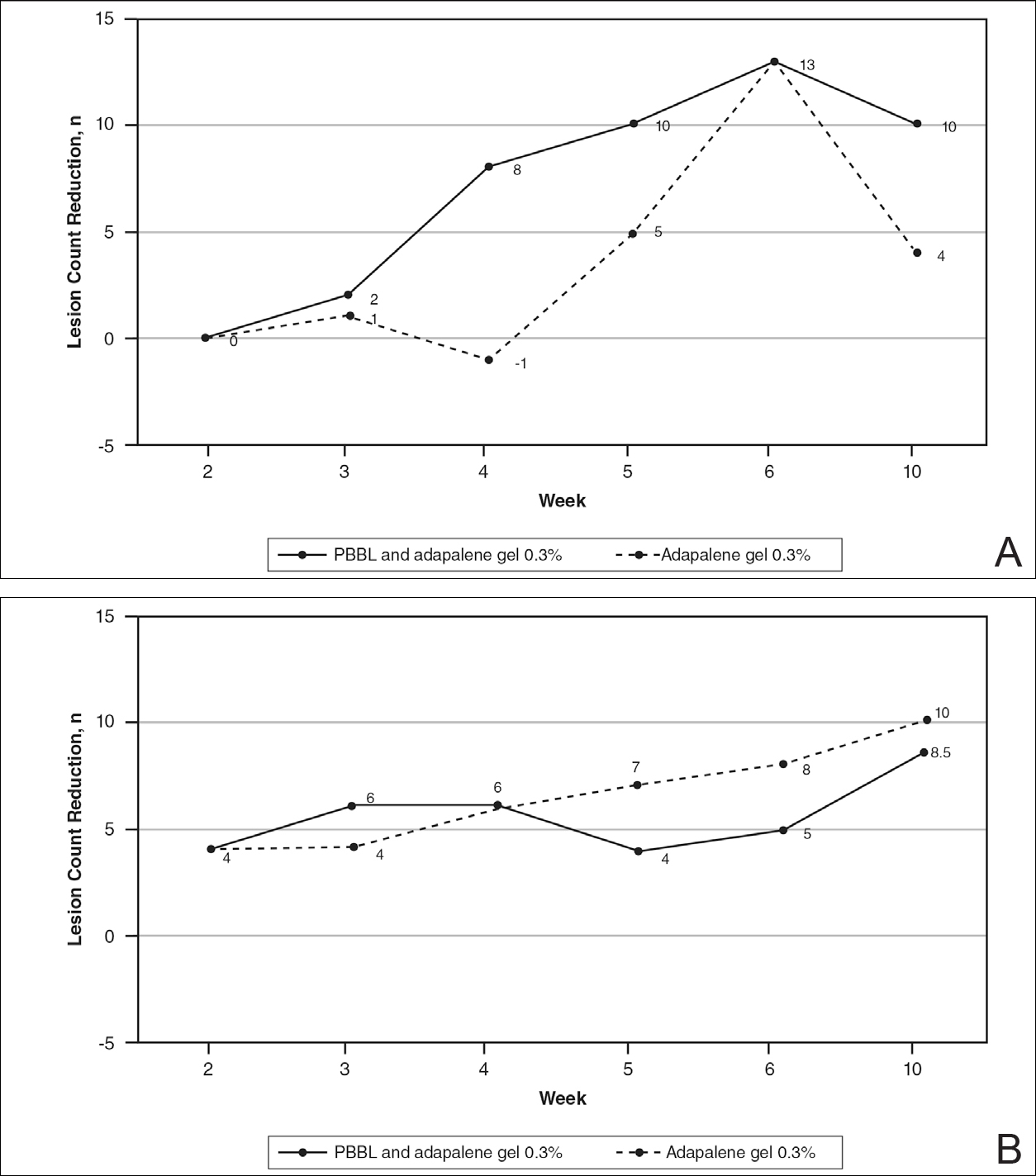
Modified Global Acne Grading Score
At weeks 3 and 4, the improvement of mGAGS was significantly greater on the side treated with the combination therapy (P=.05). However, this significant difference was not sustained (Table 3).
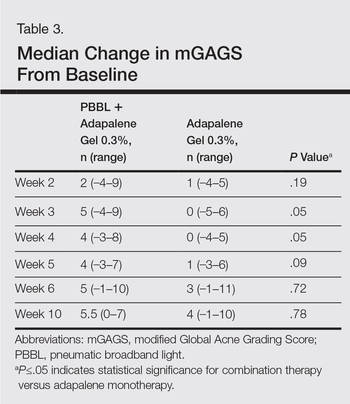
Participant Self-assessment and Physician Assessment
The rate of acne improvement according to participant self-assessment was slightly higher on the side receiving the combination therapy compared to the monotherapy side at week 2 (26%–50% vs 1%–25%) and week 6 (76%–99% vs 51%–75%). However, there was no statistically significant difference. For the physician assessment, there was no significant difference between the monotherapy and combination therapy sides.
Safety
The median WBPRS score was 1 (hurts a little bit) throughout all PBBL treatment visits. The maximum score was highest at week 1 (4=hurts whole lot) and subsequently decreased to 2 (hurts a little bit more) at week 6.
After the PBBL treatment, all participants experienced transient erythema in the treatment area. All participants noted their skin had become drier than usual from adapalene, except 1 participant (11%) who reported very dry skin on areas where adapalene gel 0.3% had been applied. However, the dryness was tolerable and relief was reported following application of a moisturizer. No participants withdrew from the study due to skin dryness.
Both black participants experienced hyperpigmentation caused by PBBL (1 on the treatment sites, the other on the test spot) and withdrew from the study. The hyperpigmentation resolved over time following application of a topical bleaching cream. One patient experienced purpura following PBBL treatment at week 4, which was associated with an increase in PBBL power. No other side effects (eg, scaling, stinging, burning, vesicle formation, blistering, crusting, scarring) were observed.
COMMENT
This 10-week study demonstrated that PBBL initially improved the appearance of acne in the first month of treatment, as determined by the significantly greater reduction in mGAGS for the combination side versus the adapalene monotherapy side. Differences in the reduction of acne lesions were not significant between the 2 treatments, except for noninflammatory lesion reduction at week 3. Analysis of physician assessment with photographs revealed acne improvement from baseline in the first month but no additional effects with the PBBL treatment at the end of study. Similarly, participant assessment indicated an improvement by week 2 with the combination therapy compared to adapalene monotherapy in their assessment of acne lesion reductions from baseline. By the end of the study, there was no significant difference between monotherapy and combination therapy.
These findings illustrate that combination therapy with PBBL plus adapalene improved the appearance of acne lesions within the first month of treatment, but there were no further signs of improvement at weeks 5 and 6. These results are consistent with at least 2 other studies that demonstrated acne reduction within the first 3 weeks of PBBL treatment.2,4 The current study was completed as planned with 6 weeks of combination therapy and patients continued adapalene application until the last follow-up visit in week 10. The length of the combination treatment was enough to determine that extension of treatment would not be necessary to gain any further benefits in this study. Because of the small sample size, we would not be able to detect any significant differences, as the difference between the combination therapy and the adapalene monotherapy was less than 55%. Therefore, a future study with a larger sample size is needed to draw a better conclusion.
Pneumatic broadband light has shown impressive results in acne treatment. However, some side effects need to be considered. Minimal adverse events have been reported such as erythema, dryness, peeling, burning, and itching.2-4 In this study, we found that all patients experienced transient erythema during and after PBBL treatment, but this effect disappeared in minutes. Purpura can occur if a higher power of PBBL is performed (6 or greater). Black patients experienced hyperpigmentation that can occur in darker skin types, as reported when light therapy is performed despite using the correct skin type tips.8 Therefore, care must be used in darker skin types, and we advocate a skin test in this population prior to general use.
Our study showed that PBBL can be safely combined with adapalene gel 0.3% and is well tolerated in the treatment of mild to moderate facial acne vulgaris for patients with Fitzpatrick skin types I to III. The combination of PBBL and adapalene reduces acne severity, as shown by the reduction in mGAGS during the first month of treatment. Patients noted faster improvement in their acne lesions with this combination. Although this study was limited by a relatively small sample size, this information may be useful in getting patients to be compliant overall, as they appeared to see results sooner, giving other therapies time to initiate their effect. It appears that 4 consecutive weekly treatments are enough to see that effect. Additionally, this combination therapy provides results without having to resort to oral antibiotics, as many patients today are concerned about creating future antibiotic resistance.
Conclusion
Adapalene gel 0.3% can be safely combined with PBBL for treatment of mild to moderate acne. Although the benefits of this combination therapy can be seen after 4 consecutive weekly treatments, the beneficial effect is not sustained.
Acknowledgment
The authors would like to thank Joyce Okawa, RN (Philadelphia, Pennsylvania), for her assistance in the submission to the University of Pennsylvania institutional review board.
- Thiboutot D, Gollnick H, Bettoli V, et al. New insights into the management of acne: an update from the Global Alliance to Improve Outcomes in Acne group. J Am Acad Dermatol. 2009;60(5 suppl):S1-S50.
- Gold MH, Biron J. Efficacy of a novel combination of pneumatic energy and broadband light for the treatment of acne. J Drugs Dermatol. 2008;7:639-642.
- Shamban AT, Enokibori M, Narurkar V, et al. Photopneumatic technology for the treatment of acne vulgaris. J Drugs Dermatol. 2008;7:139-145.
- Wanitphakdeedecha R, Tanzi EL, Alster TS. Photopneumatic therapy for the treatment of acne. J Drugs Dermatol. 2009;8:239-241.
- Doshi A, Zaheer A, Stiller MJ. A comparison of current acne grading systems and proposal of a novel system. Int J Dermatol. 1997;36:416-418.
- Wong DL, Baker CM. Pain in children: comparison of assessment scales. Pediatr Nurs. 1988;14:9-17.
- Thiboutot D, Pariser DM, Egan N, et al. Adapalene gel 0.3% for the treatment of acne vulgaris: a multicenter, randomized, double-blind, controlled, phase III trial. J Am Acad Dermatol. 2006;54:242-250.
- Yeung CK, Shek SY, Bjerring P, et al. A comparative study of intense pulsed light alone and its combination with photodynamic therapy for the treatment of facial acne in Asian skin. Lasers Surg Med. 2007;39:1-6.
Acne is a common and distressing condition that typically presents in adolescents and young adults and has been associated with not only medical but also emotional and aesthetic consequences. Acne treatments that offer faster improvement are the coveted goal. Although clinical studies support the use of combination therapy with topical retinoids and antibiotics, the overuse of antibiotics raises caution for bacterial resistance.1 Therefore, adjunctive treatments such as chemical peels, light therapy, and laser treatments can hasten the response to traditional acne treatments and in some cases may potentially decrease use of both oral and topical antibiotics.
Light therapy, particularly with visible light, may improve acne outcomes. Pneumatic broadband light (PBBL) is a light treatment in the broadband range (400–1200 nm) combined with a vacuum. The suction created by the vacuum has several effects on acne lesions, such as creating a mechanical lysis of thin-walled pustules and dislodging pore impaction. The blue light with a wavelength of 410 nm targets endogenous porphyrins in Propionibacterium acnes and elicits singlet oxygen production, resulting in bacterial destruction.2,3 Studies showed that PBBL alone was effective in most patients with mild to moderate acne and caused minimal side effects.2-4
We sought to determine if PBBL combined with a topical retinoid can accelerate and prolong acne improvement. We evaluated the efficacy, safety, and tolerability of PBBL plus adapalene gel 0.3% versus adapalene gel 0.3% monotherapy in patients with mild to moderate acne.
METHODSPatient Population
Patients with mild to moderate acne were eligible for the study if they were 18 years or older at screening, in good health, had stopped oral isotretinoin for at least 1 year prior to treatment initiation, and were not taking oral or topical antibiotics or using any topical retinoid derivatives for at least 1 month prior to treatment initiation. Inclusion criteria included at least 10 acne lesions on the face. Patients were excluded if they had a history of receiving PBBL treatment; had a history of scarring, hypopigmentation, or hyperpigmentation from laser or light treatments; and/or were pregnant or refused use of contraception during the study period.
Study Design
This single-blind, randomized, split-face study was approved by the institutional review board of the University of Pennsylvania (Philadelphia, Pennsylvania). All participants provided informed consent before entering the study. Each participant was randomly assigned to receive PBBL on one side of the face for 6 consecutive weeks and apply adapalene gel 0.3% to both sides of the face nightly for 10 weeks. Pneumatic broadband light treatment was performed using the following settings: starting power 2 (approximately 4–6 J/cm2) and vacuum setting 3 (negative pressure, approximately 3 lb/in2). The power setting was increased to a maximum of 6 (12–14 J/cm2) at subsequent visits depending on tolerability of the participants.
All participants visited the clinic weekly for 6 weeks and also returned for follow-up at week 10 (4 weeks following last PBBL treatment). At each visit, the participants completed satisfaction questionnaires and were assessed by a dermatologist evaluator using several parameters including the modified Global Acne Grading Score (mGAGS), clinical photography, participant self-assessment, physician assessment, and Wong Baker FACES Pain Rating Scale (WBPRS). The physician evaluator was blinded to the side of the face receiving PBBL treatment. Clinical photographs were taken to compare the clinical outcome at each visit versus baseline.
Efficacy Evaluation
Acne Counts
The blinded evaluator counted acne lesions and assessed the mGAGS at each visit prior to administration of the PBBL treatment. Acne lesions were counted separately as noninflammatory (comedones) and inflammatory (papules, pustules, nodules) on the forehead, cheeks, nose, and chin.
Modified Global Acne Grading Score
The modified Global Acne Grading Score was modified from the Global Acne Grading Scale (GAGS) that has previously been used to evaluate acne severity.5 The original GAGS used the type and location of the acne lesions. The GAGS considers 6 locations on the face, chest, and upper back, with a grading factor for each location (forehead=2; cheeks=2; nose=1; chin=1). Another grading factor represented the lesion type (0=no lesion; 1=comedone; 2=papule; 3=pustule; 4=nodule). The local score was calculated by multiplying the location grading factor by the lesion type grading factor. The total score was the sum of the individual local scores for the 4 locations.
Given that the number of acne lesions is important, we modified the GAGS by adding a grading factor that represented the number of lesions to improve the accuracy of the test (1=0–10 lesions; 2=11–20 lesions; 3=21–30 lesions; 4=≥31 lesions). The local score of mGAGS was calculated by multiplying the grading factors for location, lesion type, and number of lesions. Each local score was then added to yield a total score. The mGAGS may be useful and more accurate to determine the severity of acne (0=none; 1–44=mild; 45–80=moderate; 81–132=severe; 133–176=very severe).
Participant Self-assessment
Participants assessed their acne lesions using an 11-point rating scale (–5=100% worsening; –4=76%–99% worsening; –3=51%–75% worsening; –2=26%–50% worsening; –1=1%–25% worsening; 0=no improvement; 1=1%–25% improvement; 2=26%–50% improvement; 3=51%–75% improvement; 4=76%–99% improvement; 5=100% acne clear) to compare their acne at each treatment visit and week 10 follow-up with a baseline photograph.
Physician Assessment
The blinded evaluator assessed acne lesions on the face using the same 11-point rating scale that was used for participant self-assessment. For each participant, assessments were made at each treatment visit and week 10 follow-up by comparing baseline photographs.
Safety Evaluation
The WBPRS score, a standardized 6-point scale (0=no pain; 1=hurts a little bit; 2=hurts a little bit more; 3=hurts even more; 4=hurts whole lot; 5=hurt worst),6 was used to evaluate pain toleration during PBBL treatments and was recorded along with adverse events throughout the study.
Statistical Analysis
Based on data from 2 prior studies,3,7 we expected that the favorable clinical outcome of adapalene gel 0.3% and PBBL therapy would be 23% and 78%, respectively. If the adjunctive therapy with PBBL was beneficial, the favorable outcome would be higher than 78%. To be able to detect this difference, the sample size of 11 patients was needed when 5% type I error and 20% type II error were accepted.
Categorical variables were expressed as percentages, while continuous variables were expressed in terms of median (range). The clinical outcomes between both treatment groups were compared using the Wilcoxon signed rank test. A 2-tailed P value of ≤.05 was considered statistically significant. All statistical calculations were performed using STATA software version 10.0.
RESULTS
Baseline Characteristics
Four male and 7 female patients aged 18 to 35 years (median, 23 years) with mild to moderate acne were enrolled in the study. Of the 11 participants, 7 were white, 2 were black, 1 was Asian, and 1 was Latin American. Baseline characteristics of both sides of the face were comparable in all participants (Table 1). Eight participants (73%) completed the study. Two black participants withdrew from the study due to hyperpigmentation following PBBL treatment; 1 participant did not return for follow-up at week 10, as she was out of the country.

Lesion Counts
At week 3, reduction in noninflammatory lesions was significantly greater on the side receiving the combination therapy compared to the monotherapy side (P=.04)(Table 2). However, there was no significant difference between the combination therapy and the adapalene monotherapy sides in the reduction of noninflammatory and inflammatory lesions at week 4 (Figure 1). There was a remarkable improvement of the combination therapy and adapalene monotherapy sides in acne lesions, but there was no significant difference between the combination therapy and the adapalene monotherapy sides (Figure 2).



Modified Global Acne Grading Score
At weeks 3 and 4, the improvement of mGAGS was significantly greater on the side treated with the combination therapy (P=.05). However, this significant difference was not sustained (Table 3).

Participant Self-assessment and Physician Assessment
The rate of acne improvement according to participant self-assessment was slightly higher on the side receiving the combination therapy compared to the monotherapy side at week 2 (26%–50% vs 1%–25%) and week 6 (76%–99% vs 51%–75%). However, there was no statistically significant difference. For the physician assessment, there was no significant difference between the monotherapy and combination therapy sides.
Safety
The median WBPRS score was 1 (hurts a little bit) throughout all PBBL treatment visits. The maximum score was highest at week 1 (4=hurts whole lot) and subsequently decreased to 2 (hurts a little bit more) at week 6.
After the PBBL treatment, all participants experienced transient erythema in the treatment area. All participants noted their skin had become drier than usual from adapalene, except 1 participant (11%) who reported very dry skin on areas where adapalene gel 0.3% had been applied. However, the dryness was tolerable and relief was reported following application of a moisturizer. No participants withdrew from the study due to skin dryness.
Both black participants experienced hyperpigmentation caused by PBBL (1 on the treatment sites, the other on the test spot) and withdrew from the study. The hyperpigmentation resolved over time following application of a topical bleaching cream. One patient experienced purpura following PBBL treatment at week 4, which was associated with an increase in PBBL power. No other side effects (eg, scaling, stinging, burning, vesicle formation, blistering, crusting, scarring) were observed.
COMMENT
This 10-week study demonstrated that PBBL initially improved the appearance of acne in the first month of treatment, as determined by the significantly greater reduction in mGAGS for the combination side versus the adapalene monotherapy side. Differences in the reduction of acne lesions were not significant between the 2 treatments, except for noninflammatory lesion reduction at week 3. Analysis of physician assessment with photographs revealed acne improvement from baseline in the first month but no additional effects with the PBBL treatment at the end of study. Similarly, participant assessment indicated an improvement by week 2 with the combination therapy compared to adapalene monotherapy in their assessment of acne lesion reductions from baseline. By the end of the study, there was no significant difference between monotherapy and combination therapy.
These findings illustrate that combination therapy with PBBL plus adapalene improved the appearance of acne lesions within the first month of treatment, but there were no further signs of improvement at weeks 5 and 6. These results are consistent with at least 2 other studies that demonstrated acne reduction within the first 3 weeks of PBBL treatment.2,4 The current study was completed as planned with 6 weeks of combination therapy and patients continued adapalene application until the last follow-up visit in week 10. The length of the combination treatment was enough to determine that extension of treatment would not be necessary to gain any further benefits in this study. Because of the small sample size, we would not be able to detect any significant differences, as the difference between the combination therapy and the adapalene monotherapy was less than 55%. Therefore, a future study with a larger sample size is needed to draw a better conclusion.
Pneumatic broadband light has shown impressive results in acne treatment. However, some side effects need to be considered. Minimal adverse events have been reported such as erythema, dryness, peeling, burning, and itching.2-4 In this study, we found that all patients experienced transient erythema during and after PBBL treatment, but this effect disappeared in minutes. Purpura can occur if a higher power of PBBL is performed (6 or greater). Black patients experienced hyperpigmentation that can occur in darker skin types, as reported when light therapy is performed despite using the correct skin type tips.8 Therefore, care must be used in darker skin types, and we advocate a skin test in this population prior to general use.
Our study showed that PBBL can be safely combined with adapalene gel 0.3% and is well tolerated in the treatment of mild to moderate facial acne vulgaris for patients with Fitzpatrick skin types I to III. The combination of PBBL and adapalene reduces acne severity, as shown by the reduction in mGAGS during the first month of treatment. Patients noted faster improvement in their acne lesions with this combination. Although this study was limited by a relatively small sample size, this information may be useful in getting patients to be compliant overall, as they appeared to see results sooner, giving other therapies time to initiate their effect. It appears that 4 consecutive weekly treatments are enough to see that effect. Additionally, this combination therapy provides results without having to resort to oral antibiotics, as many patients today are concerned about creating future antibiotic resistance.
Conclusion
Adapalene gel 0.3% can be safely combined with PBBL for treatment of mild to moderate acne. Although the benefits of this combination therapy can be seen after 4 consecutive weekly treatments, the beneficial effect is not sustained.
Acknowledgment
The authors would like to thank Joyce Okawa, RN (Philadelphia, Pennsylvania), for her assistance in the submission to the University of Pennsylvania institutional review board.
Acne is a common and distressing condition that typically presents in adolescents and young adults and has been associated with not only medical but also emotional and aesthetic consequences. Acne treatments that offer faster improvement are the coveted goal. Although clinical studies support the use of combination therapy with topical retinoids and antibiotics, the overuse of antibiotics raises caution for bacterial resistance.1 Therefore, adjunctive treatments such as chemical peels, light therapy, and laser treatments can hasten the response to traditional acne treatments and in some cases may potentially decrease use of both oral and topical antibiotics.
Light therapy, particularly with visible light, may improve acne outcomes. Pneumatic broadband light (PBBL) is a light treatment in the broadband range (400–1200 nm) combined with a vacuum. The suction created by the vacuum has several effects on acne lesions, such as creating a mechanical lysis of thin-walled pustules and dislodging pore impaction. The blue light with a wavelength of 410 nm targets endogenous porphyrins in Propionibacterium acnes and elicits singlet oxygen production, resulting in bacterial destruction.2,3 Studies showed that PBBL alone was effective in most patients with mild to moderate acne and caused minimal side effects.2-4
We sought to determine if PBBL combined with a topical retinoid can accelerate and prolong acne improvement. We evaluated the efficacy, safety, and tolerability of PBBL plus adapalene gel 0.3% versus adapalene gel 0.3% monotherapy in patients with mild to moderate acne.
METHODSPatient Population
Patients with mild to moderate acne were eligible for the study if they were 18 years or older at screening, in good health, had stopped oral isotretinoin for at least 1 year prior to treatment initiation, and were not taking oral or topical antibiotics or using any topical retinoid derivatives for at least 1 month prior to treatment initiation. Inclusion criteria included at least 10 acne lesions on the face. Patients were excluded if they had a history of receiving PBBL treatment; had a history of scarring, hypopigmentation, or hyperpigmentation from laser or light treatments; and/or were pregnant or refused use of contraception during the study period.
Study Design
This single-blind, randomized, split-face study was approved by the institutional review board of the University of Pennsylvania (Philadelphia, Pennsylvania). All participants provided informed consent before entering the study. Each participant was randomly assigned to receive PBBL on one side of the face for 6 consecutive weeks and apply adapalene gel 0.3% to both sides of the face nightly for 10 weeks. Pneumatic broadband light treatment was performed using the following settings: starting power 2 (approximately 4–6 J/cm2) and vacuum setting 3 (negative pressure, approximately 3 lb/in2). The power setting was increased to a maximum of 6 (12–14 J/cm2) at subsequent visits depending on tolerability of the participants.
All participants visited the clinic weekly for 6 weeks and also returned for follow-up at week 10 (4 weeks following last PBBL treatment). At each visit, the participants completed satisfaction questionnaires and were assessed by a dermatologist evaluator using several parameters including the modified Global Acne Grading Score (mGAGS), clinical photography, participant self-assessment, physician assessment, and Wong Baker FACES Pain Rating Scale (WBPRS). The physician evaluator was blinded to the side of the face receiving PBBL treatment. Clinical photographs were taken to compare the clinical outcome at each visit versus baseline.
Efficacy Evaluation
Acne Counts
The blinded evaluator counted acne lesions and assessed the mGAGS at each visit prior to administration of the PBBL treatment. Acne lesions were counted separately as noninflammatory (comedones) and inflammatory (papules, pustules, nodules) on the forehead, cheeks, nose, and chin.
Modified Global Acne Grading Score
The modified Global Acne Grading Score was modified from the Global Acne Grading Scale (GAGS) that has previously been used to evaluate acne severity.5 The original GAGS used the type and location of the acne lesions. The GAGS considers 6 locations on the face, chest, and upper back, with a grading factor for each location (forehead=2; cheeks=2; nose=1; chin=1). Another grading factor represented the lesion type (0=no lesion; 1=comedone; 2=papule; 3=pustule; 4=nodule). The local score was calculated by multiplying the location grading factor by the lesion type grading factor. The total score was the sum of the individual local scores for the 4 locations.
Given that the number of acne lesions is important, we modified the GAGS by adding a grading factor that represented the number of lesions to improve the accuracy of the test (1=0–10 lesions; 2=11–20 lesions; 3=21–30 lesions; 4=≥31 lesions). The local score of mGAGS was calculated by multiplying the grading factors for location, lesion type, and number of lesions. Each local score was then added to yield a total score. The mGAGS may be useful and more accurate to determine the severity of acne (0=none; 1–44=mild; 45–80=moderate; 81–132=severe; 133–176=very severe).
Participant Self-assessment
Participants assessed their acne lesions using an 11-point rating scale (–5=100% worsening; –4=76%–99% worsening; –3=51%–75% worsening; –2=26%–50% worsening; –1=1%–25% worsening; 0=no improvement; 1=1%–25% improvement; 2=26%–50% improvement; 3=51%–75% improvement; 4=76%–99% improvement; 5=100% acne clear) to compare their acne at each treatment visit and week 10 follow-up with a baseline photograph.
Physician Assessment
The blinded evaluator assessed acne lesions on the face using the same 11-point rating scale that was used for participant self-assessment. For each participant, assessments were made at each treatment visit and week 10 follow-up by comparing baseline photographs.
Safety Evaluation
The WBPRS score, a standardized 6-point scale (0=no pain; 1=hurts a little bit; 2=hurts a little bit more; 3=hurts even more; 4=hurts whole lot; 5=hurt worst),6 was used to evaluate pain toleration during PBBL treatments and was recorded along with adverse events throughout the study.
Statistical Analysis
Based on data from 2 prior studies,3,7 we expected that the favorable clinical outcome of adapalene gel 0.3% and PBBL therapy would be 23% and 78%, respectively. If the adjunctive therapy with PBBL was beneficial, the favorable outcome would be higher than 78%. To be able to detect this difference, the sample size of 11 patients was needed when 5% type I error and 20% type II error were accepted.
Categorical variables were expressed as percentages, while continuous variables were expressed in terms of median (range). The clinical outcomes between both treatment groups were compared using the Wilcoxon signed rank test. A 2-tailed P value of ≤.05 was considered statistically significant. All statistical calculations were performed using STATA software version 10.0.
RESULTS
Baseline Characteristics
Four male and 7 female patients aged 18 to 35 years (median, 23 years) with mild to moderate acne were enrolled in the study. Of the 11 participants, 7 were white, 2 were black, 1 was Asian, and 1 was Latin American. Baseline characteristics of both sides of the face were comparable in all participants (Table 1). Eight participants (73%) completed the study. Two black participants withdrew from the study due to hyperpigmentation following PBBL treatment; 1 participant did not return for follow-up at week 10, as she was out of the country.

Lesion Counts
At week 3, reduction in noninflammatory lesions was significantly greater on the side receiving the combination therapy compared to the monotherapy side (P=.04)(Table 2). However, there was no significant difference between the combination therapy and the adapalene monotherapy sides in the reduction of noninflammatory and inflammatory lesions at week 4 (Figure 1). There was a remarkable improvement of the combination therapy and adapalene monotherapy sides in acne lesions, but there was no significant difference between the combination therapy and the adapalene monotherapy sides (Figure 2).



Modified Global Acne Grading Score
At weeks 3 and 4, the improvement of mGAGS was significantly greater on the side treated with the combination therapy (P=.05). However, this significant difference was not sustained (Table 3).

Participant Self-assessment and Physician Assessment
The rate of acne improvement according to participant self-assessment was slightly higher on the side receiving the combination therapy compared to the monotherapy side at week 2 (26%–50% vs 1%–25%) and week 6 (76%–99% vs 51%–75%). However, there was no statistically significant difference. For the physician assessment, there was no significant difference between the monotherapy and combination therapy sides.
Safety
The median WBPRS score was 1 (hurts a little bit) throughout all PBBL treatment visits. The maximum score was highest at week 1 (4=hurts whole lot) and subsequently decreased to 2 (hurts a little bit more) at week 6.
After the PBBL treatment, all participants experienced transient erythema in the treatment area. All participants noted their skin had become drier than usual from adapalene, except 1 participant (11%) who reported very dry skin on areas where adapalene gel 0.3% had been applied. However, the dryness was tolerable and relief was reported following application of a moisturizer. No participants withdrew from the study due to skin dryness.
Both black participants experienced hyperpigmentation caused by PBBL (1 on the treatment sites, the other on the test spot) and withdrew from the study. The hyperpigmentation resolved over time following application of a topical bleaching cream. One patient experienced purpura following PBBL treatment at week 4, which was associated with an increase in PBBL power. No other side effects (eg, scaling, stinging, burning, vesicle formation, blistering, crusting, scarring) were observed.
COMMENT
This 10-week study demonstrated that PBBL initially improved the appearance of acne in the first month of treatment, as determined by the significantly greater reduction in mGAGS for the combination side versus the adapalene monotherapy side. Differences in the reduction of acne lesions were not significant between the 2 treatments, except for noninflammatory lesion reduction at week 3. Analysis of physician assessment with photographs revealed acne improvement from baseline in the first month but no additional effects with the PBBL treatment at the end of study. Similarly, participant assessment indicated an improvement by week 2 with the combination therapy compared to adapalene monotherapy in their assessment of acne lesion reductions from baseline. By the end of the study, there was no significant difference between monotherapy and combination therapy.
These findings illustrate that combination therapy with PBBL plus adapalene improved the appearance of acne lesions within the first month of treatment, but there were no further signs of improvement at weeks 5 and 6. These results are consistent with at least 2 other studies that demonstrated acne reduction within the first 3 weeks of PBBL treatment.2,4 The current study was completed as planned with 6 weeks of combination therapy and patients continued adapalene application until the last follow-up visit in week 10. The length of the combination treatment was enough to determine that extension of treatment would not be necessary to gain any further benefits in this study. Because of the small sample size, we would not be able to detect any significant differences, as the difference between the combination therapy and the adapalene monotherapy was less than 55%. Therefore, a future study with a larger sample size is needed to draw a better conclusion.
Pneumatic broadband light has shown impressive results in acne treatment. However, some side effects need to be considered. Minimal adverse events have been reported such as erythema, dryness, peeling, burning, and itching.2-4 In this study, we found that all patients experienced transient erythema during and after PBBL treatment, but this effect disappeared in minutes. Purpura can occur if a higher power of PBBL is performed (6 or greater). Black patients experienced hyperpigmentation that can occur in darker skin types, as reported when light therapy is performed despite using the correct skin type tips.8 Therefore, care must be used in darker skin types, and we advocate a skin test in this population prior to general use.
Our study showed that PBBL can be safely combined with adapalene gel 0.3% and is well tolerated in the treatment of mild to moderate facial acne vulgaris for patients with Fitzpatrick skin types I to III. The combination of PBBL and adapalene reduces acne severity, as shown by the reduction in mGAGS during the first month of treatment. Patients noted faster improvement in their acne lesions with this combination. Although this study was limited by a relatively small sample size, this information may be useful in getting patients to be compliant overall, as they appeared to see results sooner, giving other therapies time to initiate their effect. It appears that 4 consecutive weekly treatments are enough to see that effect. Additionally, this combination therapy provides results without having to resort to oral antibiotics, as many patients today are concerned about creating future antibiotic resistance.
Conclusion
Adapalene gel 0.3% can be safely combined with PBBL for treatment of mild to moderate acne. Although the benefits of this combination therapy can be seen after 4 consecutive weekly treatments, the beneficial effect is not sustained.
Acknowledgment
The authors would like to thank Joyce Okawa, RN (Philadelphia, Pennsylvania), for her assistance in the submission to the University of Pennsylvania institutional review board.
- Thiboutot D, Gollnick H, Bettoli V, et al. New insights into the management of acne: an update from the Global Alliance to Improve Outcomes in Acne group. J Am Acad Dermatol. 2009;60(5 suppl):S1-S50.
- Gold MH, Biron J. Efficacy of a novel combination of pneumatic energy and broadband light for the treatment of acne. J Drugs Dermatol. 2008;7:639-642.
- Shamban AT, Enokibori M, Narurkar V, et al. Photopneumatic technology for the treatment of acne vulgaris. J Drugs Dermatol. 2008;7:139-145.
- Wanitphakdeedecha R, Tanzi EL, Alster TS. Photopneumatic therapy for the treatment of acne. J Drugs Dermatol. 2009;8:239-241.
- Doshi A, Zaheer A, Stiller MJ. A comparison of current acne grading systems and proposal of a novel system. Int J Dermatol. 1997;36:416-418.
- Wong DL, Baker CM. Pain in children: comparison of assessment scales. Pediatr Nurs. 1988;14:9-17.
- Thiboutot D, Pariser DM, Egan N, et al. Adapalene gel 0.3% for the treatment of acne vulgaris: a multicenter, randomized, double-blind, controlled, phase III trial. J Am Acad Dermatol. 2006;54:242-250.
- Yeung CK, Shek SY, Bjerring P, et al. A comparative study of intense pulsed light alone and its combination with photodynamic therapy for the treatment of facial acne in Asian skin. Lasers Surg Med. 2007;39:1-6.
- Thiboutot D, Gollnick H, Bettoli V, et al. New insights into the management of acne: an update from the Global Alliance to Improve Outcomes in Acne group. J Am Acad Dermatol. 2009;60(5 suppl):S1-S50.
- Gold MH, Biron J. Efficacy of a novel combination of pneumatic energy and broadband light for the treatment of acne. J Drugs Dermatol. 2008;7:639-642.
- Shamban AT, Enokibori M, Narurkar V, et al. Photopneumatic technology for the treatment of acne vulgaris. J Drugs Dermatol. 2008;7:139-145.
- Wanitphakdeedecha R, Tanzi EL, Alster TS. Photopneumatic therapy for the treatment of acne. J Drugs Dermatol. 2009;8:239-241.
- Doshi A, Zaheer A, Stiller MJ. A comparison of current acne grading systems and proposal of a novel system. Int J Dermatol. 1997;36:416-418.
- Wong DL, Baker CM. Pain in children: comparison of assessment scales. Pediatr Nurs. 1988;14:9-17.
- Thiboutot D, Pariser DM, Egan N, et al. Adapalene gel 0.3% for the treatment of acne vulgaris: a multicenter, randomized, double-blind, controlled, phase III trial. J Am Acad Dermatol. 2006;54:242-250.
- Yeung CK, Shek SY, Bjerring P, et al. A comparative study of intense pulsed light alone and its combination with photodynamic therapy for the treatment of facial acne in Asian skin. Lasers Surg Med. 2007;39:1-6.
Practice Points
- Compliance is achieved when patients can see improvements with their acne treatments quickly.
- Combination therapy achieves the goal of a quicker visual improvement of acneform pustules and papules with pneumatic broadband light while topical acne treatments have a chance to work, thus increasing compliance.
Diet and Acne: Where Are We?
Over the past few years there have been studies published that support a relationship between acne and nutritional factors. Most suggest that high-glycemic-load diets and milk/dairy consumption might promote the development or exacerbation of acne vulgaris. So, what’s the mechanism? Some investigators believe that a high-glycemic-index diet induces hyperinsulinemia, which in turn elicits endocrine responses such as increasing androgen synthesis, ultimately inducing acne through mediators such as androgens, insulinlike growth factor (IGF) 1, and IGF binding protein 3. Insulinlike growth factor 1 itself can induce keratinocyte proliferation, sebocyte proliferation, and sebum production. We know that acne can be related to some endocrine diseases, such as polycystic ovary syndrome, which is characterized by peripheral insulin resistance and hyperinsulinemia, as well as acne, hirsutism, and androgenic alopecia.
In a study published by Çerman et al (J Am Acad Dermatol. 2016;75:155-162), investigators aimed to support the relationship between acne and diet and proposed that adiponectin levels, an adipocyte-derived hormone with established anti-inflammatory, antioxidant, and antidiabetic effects, are inversely associated with glycemic intake. Adiponectin inhibits proinflammatory cytokines, downregulates adhesion molecule expression, suppresses toll-like receptors and their ligands, and increases insulin sensitivity. In this small study of 50 patients with acne matched to 36 healthy controls, mean (SD) serum adiponectin concentrations were significantly lower in the patients with acne vulgaris than in the healthy controls (9.93 [2.29] ng/mL_1 vs 11.28 [2.74] ng/mL_1; P=.015), though milk and dairy product consumption, serum glucose, insulin, IGF-1, IGF binding protein 3, and homeostasis model assessment of insulin resistance values of the acne vulgaris and control groups did not differ significantly. The authors argued that this finding supports low-glycemic-load diets given the inverse correlation with adiponectin concentrations.
For every promising study comes one that may refute it. A study published online in February 2016 in Human & Experimental Toxicology aimed to evaluate several adipokines (adipocyte-derived cytokines) such as leptin, adiponectin, ghrelin and adiponectin levels, and adiponectin and leptin rates that indicate insulin resistance in nonobese patients with severe acne vulgaris. Although this study was smaller (30 acne patients and 15 controls), investigators found no difference between the 2 groups for any of these adipokines. It is important to note that patients studied were nonobese, nondiabetic, and glycemic load was not taken into account, so it is possible that this correlation is more significant for patients with factors such as insulin resistance and obesity.
What’s the issue?
Regardless of these findings, we have enough evidence to support that eating poorly can worsen acne and have other effects on the body. Are we all in agreement with this conclusion? Eating poorly is bad for more than just acne. High glycemic load leads to a proinflammatory state. Think psoriasis here. Chronic inflammation is detrimental for every organ system. Therefore, in addition to focusing on the pathways and elucidating the biology, let’s also design curricula to train current and future dermatologists how to counsel patients on diet or at the very least create resources to enable us to guide our patients. I published a survey study (J Am Acad Dermatol. 2014;71:1028-1029) showing that dermatologists are not comfortable counseling patients, specifically psoriasis patients, on diet, smoking, and drinking alcohol. It is time to create these tools. Do you want these types of resources?
Over the past few years there have been studies published that support a relationship between acne and nutritional factors. Most suggest that high-glycemic-load diets and milk/dairy consumption might promote the development or exacerbation of acne vulgaris. So, what’s the mechanism? Some investigators believe that a high-glycemic-index diet induces hyperinsulinemia, which in turn elicits endocrine responses such as increasing androgen synthesis, ultimately inducing acne through mediators such as androgens, insulinlike growth factor (IGF) 1, and IGF binding protein 3. Insulinlike growth factor 1 itself can induce keratinocyte proliferation, sebocyte proliferation, and sebum production. We know that acne can be related to some endocrine diseases, such as polycystic ovary syndrome, which is characterized by peripheral insulin resistance and hyperinsulinemia, as well as acne, hirsutism, and androgenic alopecia.
In a study published by Çerman et al (J Am Acad Dermatol. 2016;75:155-162), investigators aimed to support the relationship between acne and diet and proposed that adiponectin levels, an adipocyte-derived hormone with established anti-inflammatory, antioxidant, and antidiabetic effects, are inversely associated with glycemic intake. Adiponectin inhibits proinflammatory cytokines, downregulates adhesion molecule expression, suppresses toll-like receptors and their ligands, and increases insulin sensitivity. In this small study of 50 patients with acne matched to 36 healthy controls, mean (SD) serum adiponectin concentrations were significantly lower in the patients with acne vulgaris than in the healthy controls (9.93 [2.29] ng/mL_1 vs 11.28 [2.74] ng/mL_1; P=.015), though milk and dairy product consumption, serum glucose, insulin, IGF-1, IGF binding protein 3, and homeostasis model assessment of insulin resistance values of the acne vulgaris and control groups did not differ significantly. The authors argued that this finding supports low-glycemic-load diets given the inverse correlation with adiponectin concentrations.
For every promising study comes one that may refute it. A study published online in February 2016 in Human & Experimental Toxicology aimed to evaluate several adipokines (adipocyte-derived cytokines) such as leptin, adiponectin, ghrelin and adiponectin levels, and adiponectin and leptin rates that indicate insulin resistance in nonobese patients with severe acne vulgaris. Although this study was smaller (30 acne patients and 15 controls), investigators found no difference between the 2 groups for any of these adipokines. It is important to note that patients studied were nonobese, nondiabetic, and glycemic load was not taken into account, so it is possible that this correlation is more significant for patients with factors such as insulin resistance and obesity.
What’s the issue?
Regardless of these findings, we have enough evidence to support that eating poorly can worsen acne and have other effects on the body. Are we all in agreement with this conclusion? Eating poorly is bad for more than just acne. High glycemic load leads to a proinflammatory state. Think psoriasis here. Chronic inflammation is detrimental for every organ system. Therefore, in addition to focusing on the pathways and elucidating the biology, let’s also design curricula to train current and future dermatologists how to counsel patients on diet or at the very least create resources to enable us to guide our patients. I published a survey study (J Am Acad Dermatol. 2014;71:1028-1029) showing that dermatologists are not comfortable counseling patients, specifically psoriasis patients, on diet, smoking, and drinking alcohol. It is time to create these tools. Do you want these types of resources?
Over the past few years there have been studies published that support a relationship between acne and nutritional factors. Most suggest that high-glycemic-load diets and milk/dairy consumption might promote the development or exacerbation of acne vulgaris. So, what’s the mechanism? Some investigators believe that a high-glycemic-index diet induces hyperinsulinemia, which in turn elicits endocrine responses such as increasing androgen synthesis, ultimately inducing acne through mediators such as androgens, insulinlike growth factor (IGF) 1, and IGF binding protein 3. Insulinlike growth factor 1 itself can induce keratinocyte proliferation, sebocyte proliferation, and sebum production. We know that acne can be related to some endocrine diseases, such as polycystic ovary syndrome, which is characterized by peripheral insulin resistance and hyperinsulinemia, as well as acne, hirsutism, and androgenic alopecia.
In a study published by Çerman et al (J Am Acad Dermatol. 2016;75:155-162), investigators aimed to support the relationship between acne and diet and proposed that adiponectin levels, an adipocyte-derived hormone with established anti-inflammatory, antioxidant, and antidiabetic effects, are inversely associated with glycemic intake. Adiponectin inhibits proinflammatory cytokines, downregulates adhesion molecule expression, suppresses toll-like receptors and their ligands, and increases insulin sensitivity. In this small study of 50 patients with acne matched to 36 healthy controls, mean (SD) serum adiponectin concentrations were significantly lower in the patients with acne vulgaris than in the healthy controls (9.93 [2.29] ng/mL_1 vs 11.28 [2.74] ng/mL_1; P=.015), though milk and dairy product consumption, serum glucose, insulin, IGF-1, IGF binding protein 3, and homeostasis model assessment of insulin resistance values of the acne vulgaris and control groups did not differ significantly. The authors argued that this finding supports low-glycemic-load diets given the inverse correlation with adiponectin concentrations.
For every promising study comes one that may refute it. A study published online in February 2016 in Human & Experimental Toxicology aimed to evaluate several adipokines (adipocyte-derived cytokines) such as leptin, adiponectin, ghrelin and adiponectin levels, and adiponectin and leptin rates that indicate insulin resistance in nonobese patients with severe acne vulgaris. Although this study was smaller (30 acne patients and 15 controls), investigators found no difference between the 2 groups for any of these adipokines. It is important to note that patients studied were nonobese, nondiabetic, and glycemic load was not taken into account, so it is possible that this correlation is more significant for patients with factors such as insulin resistance and obesity.
What’s the issue?
Regardless of these findings, we have enough evidence to support that eating poorly can worsen acne and have other effects on the body. Are we all in agreement with this conclusion? Eating poorly is bad for more than just acne. High glycemic load leads to a proinflammatory state. Think psoriasis here. Chronic inflammation is detrimental for every organ system. Therefore, in addition to focusing on the pathways and elucidating the biology, let’s also design curricula to train current and future dermatologists how to counsel patients on diet or at the very least create resources to enable us to guide our patients. I published a survey study (J Am Acad Dermatol. 2014;71:1028-1029) showing that dermatologists are not comfortable counseling patients, specifically psoriasis patients, on diet, smoking, and drinking alcohol. It is time to create these tools. Do you want these types of resources?
Little evidence of ‘slippery slope’ with euthanasia or physician-assisted suicide
While euthanasia and physician-assisted suicide increasingly are being legalized around the world, there appears to be little evidence that vulnerable patients are being targeted by abuse of the practice, researchers say.
A review published in the July 5 edition of JAMA examined surveys and death certification studies from countries and jurisdictions where physician-assisted suicide, euthanasia or both have been legalized, to explore attitudes and practices.
Currently, physician-assisted suicide is legal in five U.S. states – California, Montana, Oregon, Vermont, and Washington – while euthanasia or physician-assisted suicide is legal in Belgium, Canada, Colombia, Luxembourg, the Netherlands, and Switzerland, (JAMA 2016;316:79-90. doi: 10.1001/jama.2016.8499).
The review found evidence suggesting that in the United States, physician-assisted suicide accounts for less than 0.4% of all deaths. In Belgium and the Netherlands, that prevalence is much higher; the most recent studies suggested that 4.6% of all deaths in Belgium and 2.9% of all deaths in the Netherlands can be attributed to euthanasia or physician-assisted suicide, and there is the suggestion that this proportion is increasing over time.
The authors also addressed the so-called “slippery slope” argument, which posits that legalization of euthanasia or physician-assisted suicide would see its expansion to include patients who had not explicitly requested it. They noted that the incidence of deaths resulting from administration of lethal drugs without explicit patient consent appeared to have declined from 0.8% of deaths in the Netherlands in 1990 to 0.2% of deaths in 2010.
Similarly in Belgium, these deaths without explicit patient consent were estimated to be around 3.2% of deaths before legalization and 1.7% of deaths in 2013, after legalization.
“There is much debate concerning performing euthanasia, physician-assisted suicide, or other life-ending procedures on patients with dementia or chronic mental illness, who are minors, who are just “tired of life,” or who are socioeconomically vulnerable,” wrote Dr. Ezekiel J. Emanuel, vice provost for global initiatives and professor and chair of the department of medical ethics and health policy medical ethics and health policy at the University of Pennsylvania in Philadelphia, and coauthors.
However they cited a survey of Dutch physicians which found that only 2% of requests for euthanasia or physician-assisted suicide were from patients with a psychiatric disease, 4% were from patients with dementia, and 3% were from patients with neither a serious physical or psychiatric disease.
“In the United States, the concern that minorities, the disabled, the poor, or other socioeconomically marginalized groups might be pressured to accept [physician-assisted suicide] does not seem to be borne out,” the authors wrote. “The demographic profile of patients in the United States who have received these interventions is white, well-educated, and well-insured.”
The majority of cases of physician-assisted suicide or euthanasia in the Netherlands and Belgium – 70% – occur in patients with cancer, and 6% are among individuals with a neurodegenerative disease.
The authors also examined changing attitudes among the general public and among physicians towards physician-assisted suicide and euthanasia. They found that public support has declined slightly in the United States, from 75% in 2005 to 64% in 2012, but pointed out that assessing attitudes was challenging because of “framing effects” in surveys.
“Support varies substantially depending on the wording of survey questions; the provision of details about the patients, their prognosis, their medical diagnosis, and symptoms; how the interventions are characterized; and whether the questions are focused on ethical acceptability, legalization, or some other endorsement.”
Support for euthanasia and physician-assisted suicide has increased in most Western European nations but has either plateaued or decreased in much of Central and Eastern Europe.
“These changes seem correlated with a strong decline in religiosity in Western Europe and an increase in religiosity in post-communist Eastern Europe,” the authors suggested.
Physician surveys experience the same framing issues, but the authors cited a 2014 Medscape survey of physicians in seven countries which found that 54% of U.S. respondents agreed that physician-assisted suicide should be allowed, compared with 47% of respondents in Germany and the U.K., 42% in Italy, 30% in France, and 36% in Spain.
No conflicts of interest were reported.
While euthanasia and physician-assisted suicide increasingly are being legalized around the world, there appears to be little evidence that vulnerable patients are being targeted by abuse of the practice, researchers say.
A review published in the July 5 edition of JAMA examined surveys and death certification studies from countries and jurisdictions where physician-assisted suicide, euthanasia or both have been legalized, to explore attitudes and practices.
Currently, physician-assisted suicide is legal in five U.S. states – California, Montana, Oregon, Vermont, and Washington – while euthanasia or physician-assisted suicide is legal in Belgium, Canada, Colombia, Luxembourg, the Netherlands, and Switzerland, (JAMA 2016;316:79-90. doi: 10.1001/jama.2016.8499).
The review found evidence suggesting that in the United States, physician-assisted suicide accounts for less than 0.4% of all deaths. In Belgium and the Netherlands, that prevalence is much higher; the most recent studies suggested that 4.6% of all deaths in Belgium and 2.9% of all deaths in the Netherlands can be attributed to euthanasia or physician-assisted suicide, and there is the suggestion that this proportion is increasing over time.
The authors also addressed the so-called “slippery slope” argument, which posits that legalization of euthanasia or physician-assisted suicide would see its expansion to include patients who had not explicitly requested it. They noted that the incidence of deaths resulting from administration of lethal drugs without explicit patient consent appeared to have declined from 0.8% of deaths in the Netherlands in 1990 to 0.2% of deaths in 2010.
Similarly in Belgium, these deaths without explicit patient consent were estimated to be around 3.2% of deaths before legalization and 1.7% of deaths in 2013, after legalization.
“There is much debate concerning performing euthanasia, physician-assisted suicide, or other life-ending procedures on patients with dementia or chronic mental illness, who are minors, who are just “tired of life,” or who are socioeconomically vulnerable,” wrote Dr. Ezekiel J. Emanuel, vice provost for global initiatives and professor and chair of the department of medical ethics and health policy medical ethics and health policy at the University of Pennsylvania in Philadelphia, and coauthors.
However they cited a survey of Dutch physicians which found that only 2% of requests for euthanasia or physician-assisted suicide were from patients with a psychiatric disease, 4% were from patients with dementia, and 3% were from patients with neither a serious physical or psychiatric disease.
“In the United States, the concern that minorities, the disabled, the poor, or other socioeconomically marginalized groups might be pressured to accept [physician-assisted suicide] does not seem to be borne out,” the authors wrote. “The demographic profile of patients in the United States who have received these interventions is white, well-educated, and well-insured.”
The majority of cases of physician-assisted suicide or euthanasia in the Netherlands and Belgium – 70% – occur in patients with cancer, and 6% are among individuals with a neurodegenerative disease.
The authors also examined changing attitudes among the general public and among physicians towards physician-assisted suicide and euthanasia. They found that public support has declined slightly in the United States, from 75% in 2005 to 64% in 2012, but pointed out that assessing attitudes was challenging because of “framing effects” in surveys.
“Support varies substantially depending on the wording of survey questions; the provision of details about the patients, their prognosis, their medical diagnosis, and symptoms; how the interventions are characterized; and whether the questions are focused on ethical acceptability, legalization, or some other endorsement.”
Support for euthanasia and physician-assisted suicide has increased in most Western European nations but has either plateaued or decreased in much of Central and Eastern Europe.
“These changes seem correlated with a strong decline in religiosity in Western Europe and an increase in religiosity in post-communist Eastern Europe,” the authors suggested.
Physician surveys experience the same framing issues, but the authors cited a 2014 Medscape survey of physicians in seven countries which found that 54% of U.S. respondents agreed that physician-assisted suicide should be allowed, compared with 47% of respondents in Germany and the U.K., 42% in Italy, 30% in France, and 36% in Spain.
No conflicts of interest were reported.
While euthanasia and physician-assisted suicide increasingly are being legalized around the world, there appears to be little evidence that vulnerable patients are being targeted by abuse of the practice, researchers say.
A review published in the July 5 edition of JAMA examined surveys and death certification studies from countries and jurisdictions where physician-assisted suicide, euthanasia or both have been legalized, to explore attitudes and practices.
Currently, physician-assisted suicide is legal in five U.S. states – California, Montana, Oregon, Vermont, and Washington – while euthanasia or physician-assisted suicide is legal in Belgium, Canada, Colombia, Luxembourg, the Netherlands, and Switzerland, (JAMA 2016;316:79-90. doi: 10.1001/jama.2016.8499).
The review found evidence suggesting that in the United States, physician-assisted suicide accounts for less than 0.4% of all deaths. In Belgium and the Netherlands, that prevalence is much higher; the most recent studies suggested that 4.6% of all deaths in Belgium and 2.9% of all deaths in the Netherlands can be attributed to euthanasia or physician-assisted suicide, and there is the suggestion that this proportion is increasing over time.
The authors also addressed the so-called “slippery slope” argument, which posits that legalization of euthanasia or physician-assisted suicide would see its expansion to include patients who had not explicitly requested it. They noted that the incidence of deaths resulting from administration of lethal drugs without explicit patient consent appeared to have declined from 0.8% of deaths in the Netherlands in 1990 to 0.2% of deaths in 2010.
Similarly in Belgium, these deaths without explicit patient consent were estimated to be around 3.2% of deaths before legalization and 1.7% of deaths in 2013, after legalization.
“There is much debate concerning performing euthanasia, physician-assisted suicide, or other life-ending procedures on patients with dementia or chronic mental illness, who are minors, who are just “tired of life,” or who are socioeconomically vulnerable,” wrote Dr. Ezekiel J. Emanuel, vice provost for global initiatives and professor and chair of the department of medical ethics and health policy medical ethics and health policy at the University of Pennsylvania in Philadelphia, and coauthors.
However they cited a survey of Dutch physicians which found that only 2% of requests for euthanasia or physician-assisted suicide were from patients with a psychiatric disease, 4% were from patients with dementia, and 3% were from patients with neither a serious physical or psychiatric disease.
“In the United States, the concern that minorities, the disabled, the poor, or other socioeconomically marginalized groups might be pressured to accept [physician-assisted suicide] does not seem to be borne out,” the authors wrote. “The demographic profile of patients in the United States who have received these interventions is white, well-educated, and well-insured.”
The majority of cases of physician-assisted suicide or euthanasia in the Netherlands and Belgium – 70% – occur in patients with cancer, and 6% are among individuals with a neurodegenerative disease.
The authors also examined changing attitudes among the general public and among physicians towards physician-assisted suicide and euthanasia. They found that public support has declined slightly in the United States, from 75% in 2005 to 64% in 2012, but pointed out that assessing attitudes was challenging because of “framing effects” in surveys.
“Support varies substantially depending on the wording of survey questions; the provision of details about the patients, their prognosis, their medical diagnosis, and symptoms; how the interventions are characterized; and whether the questions are focused on ethical acceptability, legalization, or some other endorsement.”
Support for euthanasia and physician-assisted suicide has increased in most Western European nations but has either plateaued or decreased in much of Central and Eastern Europe.
“These changes seem correlated with a strong decline in religiosity in Western Europe and an increase in religiosity in post-communist Eastern Europe,” the authors suggested.
Physician surveys experience the same framing issues, but the authors cited a 2014 Medscape survey of physicians in seven countries which found that 54% of U.S. respondents agreed that physician-assisted suicide should be allowed, compared with 47% of respondents in Germany and the U.K., 42% in Italy, 30% in France, and 36% in Spain.
No conflicts of interest were reported.
FROM JAMA
Key clinical point: While euthanasia and physician-assisted suicide are being legalized worldwide, there is little evidence that patients are being targeted by abuse of the practice.
Major finding: Euthanasia and physician-assisted suicide account for less than 0.4% of deaths in the United States; most such deaths are among patients who are white, well-educated, and well-insured.
Data source: Review of surveys and death certificate studies in countries where physician-assisted suicide or euthanasia is legal.
Disclosures: No conflicts of interest were disclosed.