User login
Discovery reveals potential for viral cancer treatment

Image by Eric Smith
Researchers say they have discovered critical details that explain how a cellular response system tells the difference between damage to the body’s own DNA and the foreign DNA of an invading virus.
The team believes this discovery could aid the development of new cancer-selective viral therapies, and it may help explain why aging, cancers, and other diseases
seem to open the door to viral infections.
“Our study reveals fundamental mechanisms that distinguish DNA breaks at cellular and viral genomes to trigger different responses that protect the host,” said Clodagh O’Shea, of the Salk Institute for Biological Studies in La Jolla, California.
“The findings may also explain why certain conditions like aging, cancer chemotherapy, and inflammation make us more susceptible to viral infection.”
Dr O’Shea and Govind Shah, PhD, also of the Salk Institute, reported these findings in Cell.
The pair described how a cluster of proteins known as the MRN complex detects DNA breaks and amplifies its response through histones.
MRN starts a domino effect, activating histones on surrounding chromosomes, which summons a cascade of additional proteins and results in a cell-wide, all-hands-on-deck alarm to help mend the DNA.
If the cell can’t fix the DNA break, it will induce apoptosis—a self-destruct mechanism that helps to prevent mutated cells from replicating and therefore prevents tumor growth.
“What’s interesting is that even a single break transmits a global signal through the cell, halting cell division and growth,” Dr O’Shea said. “This response prevents replication so the cell doesn’t pass on a break.”
Drs O’Shea and Shah also found that, when it comes to defending against DNA viruses, the cell’s response system begins the same way—with MRN detecting breaks. But it never progresses to the global alarm signal in the case of the virus.
Typically, a common DNA virus enters the cell’s nucleus and turns on genes to replicate its own DNA. The cell detects the unauthorized replication, and the MRN complex grabs and selectively neutralizes viral DNA without triggering a global response that would arrest or kill the cell.
So the MRN response to the virus stays localized and only selectively prevents viral, but not cellular, replication.
When both threats to the genome are present, MRN will activate the massive response at the DNA break, and no MRN is left to respond to the virus. This means the virus is effectively ignored while the cell responds to the more massive alarm.
“The requirement of MRN for sensing both cellular and viral genome breaks has profound consequences,” Dr O’Shea said.
“When MRN is recruited to cellular DNA breaks, it can no longer sense and respond to incoming viral genomes. Thus, the act of responding to cellular genome breaks inactivates the host’s defenses to viral replication.”
Dr O’Shea said this may explain why people who have high levels of cellular DNA damage—such as cancer patients—are more susceptible to viral infections.
“Having damaged DNA compromises our cells’ ability to fight viral infection, while having healthy DNA boosts our cells’ ability to catch viral DNA,” Dr Shah said. “Our work implies that we may be able to engineer viruses that selectively kill cancer cells.”
The researchers aim to use this new knowledge to create viruses that are destroyed in normal cells but replicate specifically in cancer cells.
Unlike normal cells, cancer cells almost always have very high levels of DNA damage. In cancer cells, MRN is already so preoccupied with responding to DNA breaks that an engineered virus could sneak in undetected.
“Cancer cells, by definition, have high mutation rates and genomic instability even at the very earliest stages,” Dr O’Shea said. “So you could imagine building a virus that could destroy even the earliest lesions and be used as a prophylactic.” ![]()

Image by Eric Smith
Researchers say they have discovered critical details that explain how a cellular response system tells the difference between damage to the body’s own DNA and the foreign DNA of an invading virus.
The team believes this discovery could aid the development of new cancer-selective viral therapies, and it may help explain why aging, cancers, and other diseases
seem to open the door to viral infections.
“Our study reveals fundamental mechanisms that distinguish DNA breaks at cellular and viral genomes to trigger different responses that protect the host,” said Clodagh O’Shea, of the Salk Institute for Biological Studies in La Jolla, California.
“The findings may also explain why certain conditions like aging, cancer chemotherapy, and inflammation make us more susceptible to viral infection.”
Dr O’Shea and Govind Shah, PhD, also of the Salk Institute, reported these findings in Cell.
The pair described how a cluster of proteins known as the MRN complex detects DNA breaks and amplifies its response through histones.
MRN starts a domino effect, activating histones on surrounding chromosomes, which summons a cascade of additional proteins and results in a cell-wide, all-hands-on-deck alarm to help mend the DNA.
If the cell can’t fix the DNA break, it will induce apoptosis—a self-destruct mechanism that helps to prevent mutated cells from replicating and therefore prevents tumor growth.
“What’s interesting is that even a single break transmits a global signal through the cell, halting cell division and growth,” Dr O’Shea said. “This response prevents replication so the cell doesn’t pass on a break.”
Drs O’Shea and Shah also found that, when it comes to defending against DNA viruses, the cell’s response system begins the same way—with MRN detecting breaks. But it never progresses to the global alarm signal in the case of the virus.
Typically, a common DNA virus enters the cell’s nucleus and turns on genes to replicate its own DNA. The cell detects the unauthorized replication, and the MRN complex grabs and selectively neutralizes viral DNA without triggering a global response that would arrest or kill the cell.
So the MRN response to the virus stays localized and only selectively prevents viral, but not cellular, replication.
When both threats to the genome are present, MRN will activate the massive response at the DNA break, and no MRN is left to respond to the virus. This means the virus is effectively ignored while the cell responds to the more massive alarm.
“The requirement of MRN for sensing both cellular and viral genome breaks has profound consequences,” Dr O’Shea said.
“When MRN is recruited to cellular DNA breaks, it can no longer sense and respond to incoming viral genomes. Thus, the act of responding to cellular genome breaks inactivates the host’s defenses to viral replication.”
Dr O’Shea said this may explain why people who have high levels of cellular DNA damage—such as cancer patients—are more susceptible to viral infections.
“Having damaged DNA compromises our cells’ ability to fight viral infection, while having healthy DNA boosts our cells’ ability to catch viral DNA,” Dr Shah said. “Our work implies that we may be able to engineer viruses that selectively kill cancer cells.”
The researchers aim to use this new knowledge to create viruses that are destroyed in normal cells but replicate specifically in cancer cells.
Unlike normal cells, cancer cells almost always have very high levels of DNA damage. In cancer cells, MRN is already so preoccupied with responding to DNA breaks that an engineered virus could sneak in undetected.
“Cancer cells, by definition, have high mutation rates and genomic instability even at the very earliest stages,” Dr O’Shea said. “So you could imagine building a virus that could destroy even the earliest lesions and be used as a prophylactic.” ![]()

Image by Eric Smith
Researchers say they have discovered critical details that explain how a cellular response system tells the difference between damage to the body’s own DNA and the foreign DNA of an invading virus.
The team believes this discovery could aid the development of new cancer-selective viral therapies, and it may help explain why aging, cancers, and other diseases
seem to open the door to viral infections.
“Our study reveals fundamental mechanisms that distinguish DNA breaks at cellular and viral genomes to trigger different responses that protect the host,” said Clodagh O’Shea, of the Salk Institute for Biological Studies in La Jolla, California.
“The findings may also explain why certain conditions like aging, cancer chemotherapy, and inflammation make us more susceptible to viral infection.”
Dr O’Shea and Govind Shah, PhD, also of the Salk Institute, reported these findings in Cell.
The pair described how a cluster of proteins known as the MRN complex detects DNA breaks and amplifies its response through histones.
MRN starts a domino effect, activating histones on surrounding chromosomes, which summons a cascade of additional proteins and results in a cell-wide, all-hands-on-deck alarm to help mend the DNA.
If the cell can’t fix the DNA break, it will induce apoptosis—a self-destruct mechanism that helps to prevent mutated cells from replicating and therefore prevents tumor growth.
“What’s interesting is that even a single break transmits a global signal through the cell, halting cell division and growth,” Dr O’Shea said. “This response prevents replication so the cell doesn’t pass on a break.”
Drs O’Shea and Shah also found that, when it comes to defending against DNA viruses, the cell’s response system begins the same way—with MRN detecting breaks. But it never progresses to the global alarm signal in the case of the virus.
Typically, a common DNA virus enters the cell’s nucleus and turns on genes to replicate its own DNA. The cell detects the unauthorized replication, and the MRN complex grabs and selectively neutralizes viral DNA without triggering a global response that would arrest or kill the cell.
So the MRN response to the virus stays localized and only selectively prevents viral, but not cellular, replication.
When both threats to the genome are present, MRN will activate the massive response at the DNA break, and no MRN is left to respond to the virus. This means the virus is effectively ignored while the cell responds to the more massive alarm.
“The requirement of MRN for sensing both cellular and viral genome breaks has profound consequences,” Dr O’Shea said.
“When MRN is recruited to cellular DNA breaks, it can no longer sense and respond to incoming viral genomes. Thus, the act of responding to cellular genome breaks inactivates the host’s defenses to viral replication.”
Dr O’Shea said this may explain why people who have high levels of cellular DNA damage—such as cancer patients—are more susceptible to viral infections.
“Having damaged DNA compromises our cells’ ability to fight viral infection, while having healthy DNA boosts our cells’ ability to catch viral DNA,” Dr Shah said. “Our work implies that we may be able to engineer viruses that selectively kill cancer cells.”
The researchers aim to use this new knowledge to create viruses that are destroyed in normal cells but replicate specifically in cancer cells.
Unlike normal cells, cancer cells almost always have very high levels of DNA damage. In cancer cells, MRN is already so preoccupied with responding to DNA breaks that an engineered virus could sneak in undetected.
“Cancer cells, by definition, have high mutation rates and genomic instability even at the very earliest stages,” Dr O’Shea said. “So you could imagine building a virus that could destroy even the earliest lesions and be used as a prophylactic.” ![]()
Cases of liver failure linked to “fat-burning” supplement
In late 2013, there were 45 cases of acute liver failure (ALF) in Hawaii, and 29 of those people reported taking OxyELITE Pro (an herbal dietary supplement marketed for weight reduction and “fat-burning”) 60 days before illness onset. Of 8 initial cases, 2 patients needed urgent liver transplants, one died, and 5 eventually recovered.1 The manufacturer of OxyELITE Pro voluntarily recalled the product after receiving a warning letter from the US Food and Drug Administration (FDA).
One way to prevent situations like this from occurring might be to ban the sale of weight loss or sports enhancement supplements unless they are rigorously tested and approved by the FDA. Voluntary reporting to the FDA is time-consuming and it takes time for the FDA to follow up on these reports.
As primary care physicians, we need to consistently ask patients about their use of supplements, educate them about the potential dangers, and identify those who are experiencing adverse reactions. While we can’t put a stop to the harm that some herbal dietary supplements might inflict on a public eager to embrace quick fixes for weight loss and improved strength, we can be the best first responders.
Linda L. Wong, MD
Honolulu, Hawaii
Reference
1. Centers for Disease Control and Prevention (CDC). Notes from the field: acute hepatitis and liver failure following the use of a dietary supplement intended for weight loss or muscle building—May-October 2013. MMWR Morb Mortal Wkly Rep. 2013;62:817-819.
In late 2013, there were 45 cases of acute liver failure (ALF) in Hawaii, and 29 of those people reported taking OxyELITE Pro (an herbal dietary supplement marketed for weight reduction and “fat-burning”) 60 days before illness onset. Of 8 initial cases, 2 patients needed urgent liver transplants, one died, and 5 eventually recovered.1 The manufacturer of OxyELITE Pro voluntarily recalled the product after receiving a warning letter from the US Food and Drug Administration (FDA).
One way to prevent situations like this from occurring might be to ban the sale of weight loss or sports enhancement supplements unless they are rigorously tested and approved by the FDA. Voluntary reporting to the FDA is time-consuming and it takes time for the FDA to follow up on these reports.
As primary care physicians, we need to consistently ask patients about their use of supplements, educate them about the potential dangers, and identify those who are experiencing adverse reactions. While we can’t put a stop to the harm that some herbal dietary supplements might inflict on a public eager to embrace quick fixes for weight loss and improved strength, we can be the best first responders.
Linda L. Wong, MD
Honolulu, Hawaii
In late 2013, there were 45 cases of acute liver failure (ALF) in Hawaii, and 29 of those people reported taking OxyELITE Pro (an herbal dietary supplement marketed for weight reduction and “fat-burning”) 60 days before illness onset. Of 8 initial cases, 2 patients needed urgent liver transplants, one died, and 5 eventually recovered.1 The manufacturer of OxyELITE Pro voluntarily recalled the product after receiving a warning letter from the US Food and Drug Administration (FDA).
One way to prevent situations like this from occurring might be to ban the sale of weight loss or sports enhancement supplements unless they are rigorously tested and approved by the FDA. Voluntary reporting to the FDA is time-consuming and it takes time for the FDA to follow up on these reports.
As primary care physicians, we need to consistently ask patients about their use of supplements, educate them about the potential dangers, and identify those who are experiencing adverse reactions. While we can’t put a stop to the harm that some herbal dietary supplements might inflict on a public eager to embrace quick fixes for weight loss and improved strength, we can be the best first responders.
Linda L. Wong, MD
Honolulu, Hawaii
Reference
1. Centers for Disease Control and Prevention (CDC). Notes from the field: acute hepatitis and liver failure following the use of a dietary supplement intended for weight loss or muscle building—May-October 2013. MMWR Morb Mortal Wkly Rep. 2013;62:817-819.
Reference
1. Centers for Disease Control and Prevention (CDC). Notes from the field: acute hepatitis and liver failure following the use of a dietary supplement intended for weight loss or muscle building—May-October 2013. MMWR Morb Mortal Wkly Rep. 2013;62:817-819.
Vaccine-derived polio case presents new challenges to eradication
A male immunodeficient patient excreted strains of a vaccine-derived poliovirus for 28 years, a study has found. This case suggests that there are new barriers to polio eradication, according to Glynis Dunn and coauthors of a research article published August 27 in PLoS Pathogens.
Poliovirus strains in the oral polio vaccine (OPV) can be transmitted from person to person in populations with low immunity and can cause outbreaks. The strains can also replicate for long periods of time in an immunodeficient individual. The patient in question received childhood OPV immunizations at 5, 7, and 12 months of age and a booster when he was about 7 years old. His case is the longest-known example of a vaccinated patient excreting the live poliovirus.
The researchers analyzed 185 stool samples that the patient provided between 1995 and 2015. All stools were positive for strain 2 polio virus; the virus titres shed in the stools were “comparable to virus titres shed by healthy vaccinees and paralytics cases infected with vaccine or wild poliovirus.”
The excreted virus began to diverge from the vaccine strain around the time of the individual’s last known OPV vaccination, acquiring various mutations that affected its antigenic structure.
The patient excreted “a highly virulent and antigenically modified type 2 poliovirus at high titres for a period estimated to be 28 years so far ... Provided antibody titres and immunizations coverage are maintained, it is likely that the population will be protected against paralytic disease, but it is also possible that this virus could circulate in populations only using [inactivated polio vaccine] as described in Israel for wild poliovirus, thus representing a possible source of polio reemergence,” according to the authors.
Read the full article in PLoS Pathogens (doi:10.1371/journal.ppat.1005114).
A male immunodeficient patient excreted strains of a vaccine-derived poliovirus for 28 years, a study has found. This case suggests that there are new barriers to polio eradication, according to Glynis Dunn and coauthors of a research article published August 27 in PLoS Pathogens.
Poliovirus strains in the oral polio vaccine (OPV) can be transmitted from person to person in populations with low immunity and can cause outbreaks. The strains can also replicate for long periods of time in an immunodeficient individual. The patient in question received childhood OPV immunizations at 5, 7, and 12 months of age and a booster when he was about 7 years old. His case is the longest-known example of a vaccinated patient excreting the live poliovirus.
The researchers analyzed 185 stool samples that the patient provided between 1995 and 2015. All stools were positive for strain 2 polio virus; the virus titres shed in the stools were “comparable to virus titres shed by healthy vaccinees and paralytics cases infected with vaccine or wild poliovirus.”
The excreted virus began to diverge from the vaccine strain around the time of the individual’s last known OPV vaccination, acquiring various mutations that affected its antigenic structure.
The patient excreted “a highly virulent and antigenically modified type 2 poliovirus at high titres for a period estimated to be 28 years so far ... Provided antibody titres and immunizations coverage are maintained, it is likely that the population will be protected against paralytic disease, but it is also possible that this virus could circulate in populations only using [inactivated polio vaccine] as described in Israel for wild poliovirus, thus representing a possible source of polio reemergence,” according to the authors.
Read the full article in PLoS Pathogens (doi:10.1371/journal.ppat.1005114).
A male immunodeficient patient excreted strains of a vaccine-derived poliovirus for 28 years, a study has found. This case suggests that there are new barriers to polio eradication, according to Glynis Dunn and coauthors of a research article published August 27 in PLoS Pathogens.
Poliovirus strains in the oral polio vaccine (OPV) can be transmitted from person to person in populations with low immunity and can cause outbreaks. The strains can also replicate for long periods of time in an immunodeficient individual. The patient in question received childhood OPV immunizations at 5, 7, and 12 months of age and a booster when he was about 7 years old. His case is the longest-known example of a vaccinated patient excreting the live poliovirus.
The researchers analyzed 185 stool samples that the patient provided between 1995 and 2015. All stools were positive for strain 2 polio virus; the virus titres shed in the stools were “comparable to virus titres shed by healthy vaccinees and paralytics cases infected with vaccine or wild poliovirus.”
The excreted virus began to diverge from the vaccine strain around the time of the individual’s last known OPV vaccination, acquiring various mutations that affected its antigenic structure.
The patient excreted “a highly virulent and antigenically modified type 2 poliovirus at high titres for a period estimated to be 28 years so far ... Provided antibody titres and immunizations coverage are maintained, it is likely that the population will be protected against paralytic disease, but it is also possible that this virus could circulate in populations only using [inactivated polio vaccine] as described in Israel for wild poliovirus, thus representing a possible source of polio reemergence,” according to the authors.
Read the full article in PLoS Pathogens (doi:10.1371/journal.ppat.1005114).
Public misperception about doctors’ wealth
I’m rich. Aren’t you?
In reality, you’re probably not (depending on what your definition of rich is), and I’m not either.
The problem, unfortunately, is the public perception that all doctors are rich. They see raw numbers, hear about well-publicized cases of criminal medical fraud, read about some world-famous brain surgeon and his 5-million-square-foot house, and immediately figure we’re all rolling in dough.
For most of us, though, that’s far from the norm. We struggle with declining reimbursements and increasing overheads: rent, staff salaries, office supplies, etc. By the time you take the myriad expenses out, there often isn’t much left for us. And, like everyone else, we have mortgages, families to take care of, student loans, and grocery bills. While most of us can still support families and a moderate lifestyle, we sure aren’t rich. When you take time into account (60-70 hours per week), my hourly salary isn’t that high.
Yet, the majority of people don’t see it that way. Granted, we may be in a better financial position than some of our patients, but it still amazes me when they ask me to waive copays or other visit costs. I always say no, and some argue, “But you’re a doctor! You can afford it.” Whether I can or can’t is immaterial. I pay my family’s medical bills in full and on time and would never dream of asking for a discount or freebie for any reason. You let one person skip, then another, then another ... and it starts to add up quickly.
I have several patients who are quite wealthy. I wouldn’t ask them to pay an extra copay “because you can afford it,” but that’s no different from others asking me to waive theirs for the same reason.
I just wish patients would see, or the lay press would show, the reality of finances for a modern-day average doctor. I, personally, am sick of people who still affiliate us with Porsches, two homes, and Wednesday-afternoon golfing. A few of those docs may still be around, but they are a rare exception, not the rule. And I don’t see that changing any time soon.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I’m rich. Aren’t you?
In reality, you’re probably not (depending on what your definition of rich is), and I’m not either.
The problem, unfortunately, is the public perception that all doctors are rich. They see raw numbers, hear about well-publicized cases of criminal medical fraud, read about some world-famous brain surgeon and his 5-million-square-foot house, and immediately figure we’re all rolling in dough.
For most of us, though, that’s far from the norm. We struggle with declining reimbursements and increasing overheads: rent, staff salaries, office supplies, etc. By the time you take the myriad expenses out, there often isn’t much left for us. And, like everyone else, we have mortgages, families to take care of, student loans, and grocery bills. While most of us can still support families and a moderate lifestyle, we sure aren’t rich. When you take time into account (60-70 hours per week), my hourly salary isn’t that high.
Yet, the majority of people don’t see it that way. Granted, we may be in a better financial position than some of our patients, but it still amazes me when they ask me to waive copays or other visit costs. I always say no, and some argue, “But you’re a doctor! You can afford it.” Whether I can or can’t is immaterial. I pay my family’s medical bills in full and on time and would never dream of asking for a discount or freebie for any reason. You let one person skip, then another, then another ... and it starts to add up quickly.
I have several patients who are quite wealthy. I wouldn’t ask them to pay an extra copay “because you can afford it,” but that’s no different from others asking me to waive theirs for the same reason.
I just wish patients would see, or the lay press would show, the reality of finances for a modern-day average doctor. I, personally, am sick of people who still affiliate us with Porsches, two homes, and Wednesday-afternoon golfing. A few of those docs may still be around, but they are a rare exception, not the rule. And I don’t see that changing any time soon.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I’m rich. Aren’t you?
In reality, you’re probably not (depending on what your definition of rich is), and I’m not either.
The problem, unfortunately, is the public perception that all doctors are rich. They see raw numbers, hear about well-publicized cases of criminal medical fraud, read about some world-famous brain surgeon and his 5-million-square-foot house, and immediately figure we’re all rolling in dough.
For most of us, though, that’s far from the norm. We struggle with declining reimbursements and increasing overheads: rent, staff salaries, office supplies, etc. By the time you take the myriad expenses out, there often isn’t much left for us. And, like everyone else, we have mortgages, families to take care of, student loans, and grocery bills. While most of us can still support families and a moderate lifestyle, we sure aren’t rich. When you take time into account (60-70 hours per week), my hourly salary isn’t that high.
Yet, the majority of people don’t see it that way. Granted, we may be in a better financial position than some of our patients, but it still amazes me when they ask me to waive copays or other visit costs. I always say no, and some argue, “But you’re a doctor! You can afford it.” Whether I can or can’t is immaterial. I pay my family’s medical bills in full and on time and would never dream of asking for a discount or freebie for any reason. You let one person skip, then another, then another ... and it starts to add up quickly.
I have several patients who are quite wealthy. I wouldn’t ask them to pay an extra copay “because you can afford it,” but that’s no different from others asking me to waive theirs for the same reason.
I just wish patients would see, or the lay press would show, the reality of finances for a modern-day average doctor. I, personally, am sick of people who still affiliate us with Porsches, two homes, and Wednesday-afternoon golfing. A few of those docs may still be around, but they are a rare exception, not the rule. And I don’t see that changing any time soon.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Can You Nail the Diagnosis? (It’s Not Fungal)
A 50-year-old man is sent to dermatology for evaluation of a “fungal infection” affecting both of his thumbnails. In the past several years, treatment with at least two courses of oral terbinafine and numerous topical antifungal creams has failed to produce any improvement in the problem. The patient denies any nail-related symptoms or trauma to his thumbs.
The patient claims to be otherwise healthy. On further questioning, however, he admits to having intermittent joint pain and swelling, especially in one ankle. He adds that for the past several years, he has experienced severe back pain and stiffness in the morning.
EXAMINATION
The patient looks his stated age, is in no acute distress, and is well developed and well nourished.
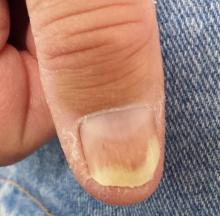
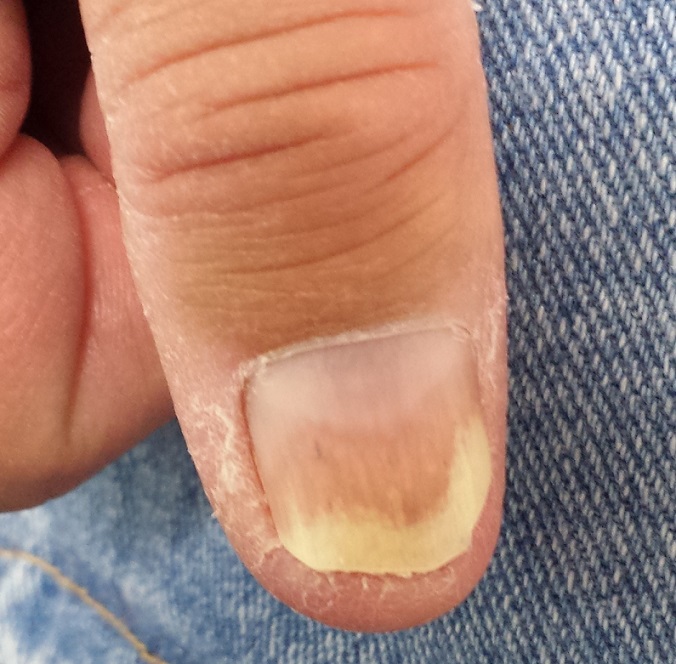
Both thumbnails demonstrate identical changes: separation of the distal one-third of the nail plate from the nail bed and yellowish discoloration of that portion of the nails. In and under the nail plate, fine, short, longitudinal black streaks are seen. Proximally, a well-defined brown band is observed in the subungual areas. The patient’s other nails are unaffected.
Broader examination reveals a salmon-pink rash with white scale covering the periumbilical area. A similar rash is seen in the upper intergluteal area.
What is the diagnosis?
DISCUSSION
All these findings add up to the diagnosis of psoriasis vulgaris, or common psoriasis, a disease that affects almost 3% of the US population and is far less common in persons with darker skin. This inflammatory condition, thought to be of autoimmune origin, manifested in predictable ways in this patient, who most likely inherited the genetic predisposition for the disease.
Although numerous related chromosomal abnormalities have been identified, environmental factors often play a role in psoriasis as well. These include antecedent strep infection, stress, smoking, obesity, alcohol intake, and use of certain medications (eg, beta-blockers, lithium).
Psoriasis is known to affect nails. The changes in this patient are classic: onycholysis (separation of the nail plate from the nail bed), deformed nails (known as dystrophy), yellow-brown discoloration (so-called oil spotting), and often, splinter hemorrhages. Nail pitting, though not seen in this case, is also common.
For some psoriasis patients, nail involvement is the sole manifestation of the disease. But more often, observing these nail changes prompts the provider to look elsewhere for corroborative findings, of which periumbilical and upper intergluteal involvement are typical.
Seen in a diagnostic vacuum, such nail changes are often diagnosed as “fungal infection.” This overlooks the fact that fungal infection is far less common in the fingernails than in toenails. In fact, there are several other items in the differential for nail changes, including lichen planus, eczema, and chronic candidal paronychia.
Had fungal infection (onychomycosis) been a serious possibility, culture or histologic examination of a sample of nail plate could have been confirmatory. First, though, the other items in the differential should have been considered. (I’ve said it before, in a variety of contexts: If your only explanation for discolored and misshapen nails is fungal infection, that’s a problem.)
This patient’s skin disease was initially treated with topical steroids. However, due to his joint symptoms and the possibility of psoriatic arthropathy, he was also referred to rheumatology. It’s entirely possible that he’ll be prescribed a biologic, which will eliminate his otherwise-problematic-to-treat nail psoriasis.
TAKE-HOME LEARNING POINTS
• Since fungal infection of the fingernails is distinctly uncommon, other items in the differential should be considered in such cases.
• Other potential diagnostic explanations for nail changes include lichen planus, eczema, and chronic candidal paronychia.
• Nail changes can be the sole manifestation of psoriasis in a given patient.
• Evaluation should include other areas of involvement that exhibit “classic” signs of psoriasis, including extensor surfaces and periumbilical and upper intergluteal skin.
A 50-year-old man is sent to dermatology for evaluation of a “fungal infection” affecting both of his thumbnails. In the past several years, treatment with at least two courses of oral terbinafine and numerous topical antifungal creams has failed to produce any improvement in the problem. The patient denies any nail-related symptoms or trauma to his thumbs.
The patient claims to be otherwise healthy. On further questioning, however, he admits to having intermittent joint pain and swelling, especially in one ankle. He adds that for the past several years, he has experienced severe back pain and stiffness in the morning.
EXAMINATION
The patient looks his stated age, is in no acute distress, and is well developed and well nourished.
Both thumbnails demonstrate identical changes: separation of the distal one-third of the nail plate from the nail bed and yellowish discoloration of that portion of the nails. In and under the nail plate, fine, short, longitudinal black streaks are seen. Proximally, a well-defined brown band is observed in the subungual areas. The patient’s other nails are unaffected.
Broader examination reveals a salmon-pink rash with white scale covering the periumbilical area. A similar rash is seen in the upper intergluteal area.
What is the diagnosis?
DISCUSSION
All these findings add up to the diagnosis of psoriasis vulgaris, or common psoriasis, a disease that affects almost 3% of the US population and is far less common in persons with darker skin. This inflammatory condition, thought to be of autoimmune origin, manifested in predictable ways in this patient, who most likely inherited the genetic predisposition for the disease.
Although numerous related chromosomal abnormalities have been identified, environmental factors often play a role in psoriasis as well. These include antecedent strep infection, stress, smoking, obesity, alcohol intake, and use of certain medications (eg, beta-blockers, lithium).
Psoriasis is known to affect nails. The changes in this patient are classic: onycholysis (separation of the nail plate from the nail bed), deformed nails (known as dystrophy), yellow-brown discoloration (so-called oil spotting), and often, splinter hemorrhages. Nail pitting, though not seen in this case, is also common.
For some psoriasis patients, nail involvement is the sole manifestation of the disease. But more often, observing these nail changes prompts the provider to look elsewhere for corroborative findings, of which periumbilical and upper intergluteal involvement are typical.
Seen in a diagnostic vacuum, such nail changes are often diagnosed as “fungal infection.” This overlooks the fact that fungal infection is far less common in the fingernails than in toenails. In fact, there are several other items in the differential for nail changes, including lichen planus, eczema, and chronic candidal paronychia.
Had fungal infection (onychomycosis) been a serious possibility, culture or histologic examination of a sample of nail plate could have been confirmatory. First, though, the other items in the differential should have been considered. (I’ve said it before, in a variety of contexts: If your only explanation for discolored and misshapen nails is fungal infection, that’s a problem.)
This patient’s skin disease was initially treated with topical steroids. However, due to his joint symptoms and the possibility of psoriatic arthropathy, he was also referred to rheumatology. It’s entirely possible that he’ll be prescribed a biologic, which will eliminate his otherwise-problematic-to-treat nail psoriasis.
TAKE-HOME LEARNING POINTS
• Since fungal infection of the fingernails is distinctly uncommon, other items in the differential should be considered in such cases.
• Other potential diagnostic explanations for nail changes include lichen planus, eczema, and chronic candidal paronychia.
• Nail changes can be the sole manifestation of psoriasis in a given patient.
• Evaluation should include other areas of involvement that exhibit “classic” signs of psoriasis, including extensor surfaces and periumbilical and upper intergluteal skin.
A 50-year-old man is sent to dermatology for evaluation of a “fungal infection” affecting both of his thumbnails. In the past several years, treatment with at least two courses of oral terbinafine and numerous topical antifungal creams has failed to produce any improvement in the problem. The patient denies any nail-related symptoms or trauma to his thumbs.
The patient claims to be otherwise healthy. On further questioning, however, he admits to having intermittent joint pain and swelling, especially in one ankle. He adds that for the past several years, he has experienced severe back pain and stiffness in the morning.
EXAMINATION
The patient looks his stated age, is in no acute distress, and is well developed and well nourished.
Both thumbnails demonstrate identical changes: separation of the distal one-third of the nail plate from the nail bed and yellowish discoloration of that portion of the nails. In and under the nail plate, fine, short, longitudinal black streaks are seen. Proximally, a well-defined brown band is observed in the subungual areas. The patient’s other nails are unaffected.
Broader examination reveals a salmon-pink rash with white scale covering the periumbilical area. A similar rash is seen in the upper intergluteal area.
What is the diagnosis?
DISCUSSION
All these findings add up to the diagnosis of psoriasis vulgaris, or common psoriasis, a disease that affects almost 3% of the US population and is far less common in persons with darker skin. This inflammatory condition, thought to be of autoimmune origin, manifested in predictable ways in this patient, who most likely inherited the genetic predisposition for the disease.
Although numerous related chromosomal abnormalities have been identified, environmental factors often play a role in psoriasis as well. These include antecedent strep infection, stress, smoking, obesity, alcohol intake, and use of certain medications (eg, beta-blockers, lithium).
Psoriasis is known to affect nails. The changes in this patient are classic: onycholysis (separation of the nail plate from the nail bed), deformed nails (known as dystrophy), yellow-brown discoloration (so-called oil spotting), and often, splinter hemorrhages. Nail pitting, though not seen in this case, is also common.
For some psoriasis patients, nail involvement is the sole manifestation of the disease. But more often, observing these nail changes prompts the provider to look elsewhere for corroborative findings, of which periumbilical and upper intergluteal involvement are typical.
Seen in a diagnostic vacuum, such nail changes are often diagnosed as “fungal infection.” This overlooks the fact that fungal infection is far less common in the fingernails than in toenails. In fact, there are several other items in the differential for nail changes, including lichen planus, eczema, and chronic candidal paronychia.
Had fungal infection (onychomycosis) been a serious possibility, culture or histologic examination of a sample of nail plate could have been confirmatory. First, though, the other items in the differential should have been considered. (I’ve said it before, in a variety of contexts: If your only explanation for discolored and misshapen nails is fungal infection, that’s a problem.)
This patient’s skin disease was initially treated with topical steroids. However, due to his joint symptoms and the possibility of psoriatic arthropathy, he was also referred to rheumatology. It’s entirely possible that he’ll be prescribed a biologic, which will eliminate his otherwise-problematic-to-treat nail psoriasis.
TAKE-HOME LEARNING POINTS
• Since fungal infection of the fingernails is distinctly uncommon, other items in the differential should be considered in such cases.
• Other potential diagnostic explanations for nail changes include lichen planus, eczema, and chronic candidal paronychia.
• Nail changes can be the sole manifestation of psoriasis in a given patient.
• Evaluation should include other areas of involvement that exhibit “classic” signs of psoriasis, including extensor surfaces and periumbilical and upper intergluteal skin.
Hydroxyurea boosts O2 saturations in children with sickle cell disease
Nocturnal and awake oxygen saturations are higher in children whose sickle cell disease (SCD) is treated with hydroxyurea, according to the results of a study by Dr. Indra Narang of the Hospital for Sick Children in Toronto and her colleagues.
“Improving SaO2 [oxygen saturation] may be an important mechanism of action of hydroxyurea therapy. As such, improving SaO2 across the severity spectrum of SCD may be beneficial in decreasing SCD morbidities with an overall improvement in long-term health,” the researchers wrote.
Sickle cell disease is characterized by development of rigid and sickled cells when deoxygenated, which may result in vasoocclusive injury to organs. Hydroxyurea enhances the production of fetal hemoglobin and can lead to less acute chest syndrome and vasoocclusive crises. Furthermore, children with sickle cell disease have a high prevalence of obstructive sleep apnea, which is associated with nocturnal desaturations and may be related to morbidity in SCD.
To evaluate the role of hydroxyurea in nocturnal oxygen saturations in pediatric patients, the researchers conducted a cross-sectional review of pediatric SCD patients referred for polysomnograms at the Hospital for Sick Children in Toronto from May 2007 to May 2014. Polysomnography data were analyzed on 37 children with SCD on hydroxyurea and matched with 104 children with SCD not treated with hydroxyurea. SAO2 was assessed using the Masimo oximeter (Ann Am Thorac Soc. 2015 July;12[7]1044-9).
Obstructive sleep apnea was found in 38% (n = 14) of subjects treated with hydroxyurea versus 52% (n = 54) in the nonhydroxyurea group. In the hydroxyurea group, the median obstructive apnea-hypopnea index was 0.9 events/hr vs. 1.9 events/hr in the nonhydroxyurea group, Dr. Narang and her associates reported.
Compared with the nonhydroxyurea SCD group, the hydroxyurea SCD group had significantly higher median awake (98.6% vs. 96.2%; P less than .0001) and sleep oxygen saturations (98.4% vs. 96.1%; P less than .0001). Likewise, treatment with hydroxyurea was associated with a significantly higher sleep oxygen saturation nadir when compared with the nonhydroxyurea group (91.4% vs. 85%; P = .0002), the investigators said.
Finally, treatment with hydroxyurea was associated with higher hemoglobin levels than no hydroxyurea treatment (P less than .0001) and the hemoglobin levels significantly correlated with sleep, awake, and lowest nocturnal oxygen saturation (P less than .0001).
The authors said that they had no conflicts to disclose.
Nocturnal and awake oxygen saturations are higher in children whose sickle cell disease (SCD) is treated with hydroxyurea, according to the results of a study by Dr. Indra Narang of the Hospital for Sick Children in Toronto and her colleagues.
“Improving SaO2 [oxygen saturation] may be an important mechanism of action of hydroxyurea therapy. As such, improving SaO2 across the severity spectrum of SCD may be beneficial in decreasing SCD morbidities with an overall improvement in long-term health,” the researchers wrote.
Sickle cell disease is characterized by development of rigid and sickled cells when deoxygenated, which may result in vasoocclusive injury to organs. Hydroxyurea enhances the production of fetal hemoglobin and can lead to less acute chest syndrome and vasoocclusive crises. Furthermore, children with sickle cell disease have a high prevalence of obstructive sleep apnea, which is associated with nocturnal desaturations and may be related to morbidity in SCD.
To evaluate the role of hydroxyurea in nocturnal oxygen saturations in pediatric patients, the researchers conducted a cross-sectional review of pediatric SCD patients referred for polysomnograms at the Hospital for Sick Children in Toronto from May 2007 to May 2014. Polysomnography data were analyzed on 37 children with SCD on hydroxyurea and matched with 104 children with SCD not treated with hydroxyurea. SAO2 was assessed using the Masimo oximeter (Ann Am Thorac Soc. 2015 July;12[7]1044-9).
Obstructive sleep apnea was found in 38% (n = 14) of subjects treated with hydroxyurea versus 52% (n = 54) in the nonhydroxyurea group. In the hydroxyurea group, the median obstructive apnea-hypopnea index was 0.9 events/hr vs. 1.9 events/hr in the nonhydroxyurea group, Dr. Narang and her associates reported.
Compared with the nonhydroxyurea SCD group, the hydroxyurea SCD group had significantly higher median awake (98.6% vs. 96.2%; P less than .0001) and sleep oxygen saturations (98.4% vs. 96.1%; P less than .0001). Likewise, treatment with hydroxyurea was associated with a significantly higher sleep oxygen saturation nadir when compared with the nonhydroxyurea group (91.4% vs. 85%; P = .0002), the investigators said.
Finally, treatment with hydroxyurea was associated with higher hemoglobin levels than no hydroxyurea treatment (P less than .0001) and the hemoglobin levels significantly correlated with sleep, awake, and lowest nocturnal oxygen saturation (P less than .0001).
The authors said that they had no conflicts to disclose.
Nocturnal and awake oxygen saturations are higher in children whose sickle cell disease (SCD) is treated with hydroxyurea, according to the results of a study by Dr. Indra Narang of the Hospital for Sick Children in Toronto and her colleagues.
“Improving SaO2 [oxygen saturation] may be an important mechanism of action of hydroxyurea therapy. As such, improving SaO2 across the severity spectrum of SCD may be beneficial in decreasing SCD morbidities with an overall improvement in long-term health,” the researchers wrote.
Sickle cell disease is characterized by development of rigid and sickled cells when deoxygenated, which may result in vasoocclusive injury to organs. Hydroxyurea enhances the production of fetal hemoglobin and can lead to less acute chest syndrome and vasoocclusive crises. Furthermore, children with sickle cell disease have a high prevalence of obstructive sleep apnea, which is associated with nocturnal desaturations and may be related to morbidity in SCD.
To evaluate the role of hydroxyurea in nocturnal oxygen saturations in pediatric patients, the researchers conducted a cross-sectional review of pediatric SCD patients referred for polysomnograms at the Hospital for Sick Children in Toronto from May 2007 to May 2014. Polysomnography data were analyzed on 37 children with SCD on hydroxyurea and matched with 104 children with SCD not treated with hydroxyurea. SAO2 was assessed using the Masimo oximeter (Ann Am Thorac Soc. 2015 July;12[7]1044-9).
Obstructive sleep apnea was found in 38% (n = 14) of subjects treated with hydroxyurea versus 52% (n = 54) in the nonhydroxyurea group. In the hydroxyurea group, the median obstructive apnea-hypopnea index was 0.9 events/hr vs. 1.9 events/hr in the nonhydroxyurea group, Dr. Narang and her associates reported.
Compared with the nonhydroxyurea SCD group, the hydroxyurea SCD group had significantly higher median awake (98.6% vs. 96.2%; P less than .0001) and sleep oxygen saturations (98.4% vs. 96.1%; P less than .0001). Likewise, treatment with hydroxyurea was associated with a significantly higher sleep oxygen saturation nadir when compared with the nonhydroxyurea group (91.4% vs. 85%; P = .0002), the investigators said.
Finally, treatment with hydroxyurea was associated with higher hemoglobin levels than no hydroxyurea treatment (P less than .0001) and the hemoglobin levels significantly correlated with sleep, awake, and lowest nocturnal oxygen saturation (P less than .0001).
The authors said that they had no conflicts to disclose.
FROM ANNALS OF THE AMERICAN THORACIC SOCIETY
Key clinical point: Nocturnal and awake oxygen saturations are higher in patients treated with hydroxyurea.
Major finding: The hydroxyurea SCD group was found to have significantly higher median awake (P less than .0001) and sleep (P less than .0001) oxygen saturation vs. the nonhydroxyurea SCD group and significantly higher sleep oxygen saturation nadir vs. the nonhydroxyurea group (P = .0002).
Data source: A cross-sectional review of 141 pediatric SCD patients referred for polysomnography from May 2007 to May 2014.
Disclosures: The authors said that they had no conflicts to disclose.
Drugs don’t play well together in MF

Photo courtesy of the CDC
Simultaneous administration of lenalidomide and ruxolitinib is not feasible in patients with myelofibrosis (MF), according to research published in haematologica.
Investigators said administering the drugs together proved difficult. Most patients had to stop taking lenalidomide at some point, and many did not restart the drug.
In addition, the study did not meet the predetermined efficacy criteria and was therefore terminated early.
Still, the investigators noted that 17 of 31 patients did respond to treatment, and 10 patients were still taking both drugs at the time of analysis.
The team therefore believes a sequential rather than concomitant treatment approach might work with this combination or for other agents to be combined with ruxolitinib.
Srdan Verstovsek, MD, PhD, of the MD Anderson Cancer Center in Houston, Texas, and his colleagues conducted this research. It was supported by Incyte Corporation (the company developing ruxolitinib) and MD Anderson.
The investigators initiated this study to determine if lenalidomide and ruxolitinib in combination would target distinct clinical and pathological manifestations of MF and prevent treatment-related decreases in blood counts.
They studied the combination in 31 patients with primary MF (n=15), post-polycythemia vera MF (n=12), or post-essential thrombocythemia MF (n=4). The patients’ median age was 66 (range, 37-82), and 21 had received prior treatments (range, 1-3).
The patients received ruxolitinib at 15 mg twice daily in continuous, 28-day cycles, plus 5 mg of lenalidomide once daily on days 1-21. The median follow-up was 28 months (range, 12-35+).
Dosing troubles
In all, 23 patients required dose interruptions of lenalidomide, with or without a dose decrease due to toxicity. Twenty of these interruptions occurred within the first 3 months of therapy, and 14 of the patients never restarted treatment with lenalidomide.
The reasons for dose interruption (or, ultimately, discontinuation) were low platelet count (n=8), low absolute neutrophil count (n=3), anemia (n=3), diarrhea (n=3), financial constraints (n=2), deep vein thrombosis (n=1), skin rash (n=1), transaminitis (n=1), and arthralgia/fever (n=1).
Conversely, 6 patients required an increased dose of ruxolitinib, 3 within the first 3 months. Doses were increased due to leukocytosis (n=2), suboptimal response (n=2), thrombocytosis (n=1), and progressive splenomegaly (n=1).
Discontinuation and early termination
At a median follow-up of 28 months, 25 patients (81%) were still alive, and 16 remained on study. Ten of these patients were taking both drugs, and 6 were taking ruxolitinib only.
For the 15 patients who came off the study, their reasons included concurrent disease (n=3), disease progression (n=2), myelosuppression (n=2), refractory disease (n=3), toxicities (n=2), persistent and severe lower-extremity cellulitis (n=1), non-compliance (n=1), and financial reasons (n=1).
The investigators noted that only 7 patients met the predetermined definition of efficacy—a response to combination treatment within 6 months of initiation without discontinuing either drug.
For the study to continue after the interim analysis, more than 10 patients would have to fulfill those criteria. As they did not, the study was terminated early.
Response
Seventeen patients (55%) achieved an IWG-MRT-defined response of clinical improvement in palpable spleen size. Seven patients had a 100% spleen reduction, and 10 had reduction of 50% or greater.
The median time to clinical improvement in spleen size was 1.8 months (range, 0.4-31), and the median duration of this response was 19 months (range, 3-32+). At last follow-up, 2 patients had lost their response.
One of the 17 spleen responders also achieved an IWG-MRT-defined clinical improvement in hemoglobin (increase of 2 g/dL or greater that was maintained for more than 8 weeks). The time to this response was 28 months, and the response lasted 6 months.
There were differences in response rate, response duration, time to response, and overall survival between patients who required dose interruptions and those who did not. However, none of these differences were statistically significant.
Toxicity
Grade 3/4 myelosuppression occurred in 16 patients, and there was 1 case of lower-extremity thrombosis. The most common non-hematologic adverse events (AEs) were diarrhea (n=8), nausea and vomiting (n=3), abdominal pain (n=3), and constipation (n=3).
Five patients had grade 3/4 non-hematologic AEs—diarrhea, edema, transaminitis, bilirubinemia, and acute kidney injury. Two patients discontinued treatment due to drug-related AEs—grade 2 persistent nausea and grade 3 diarrhea.
Three of the 6 deaths were documented (including 2 that occurred on-study). They were attributed to pneumonia, kidney failure, and possible stroke. ![]()

Photo courtesy of the CDC
Simultaneous administration of lenalidomide and ruxolitinib is not feasible in patients with myelofibrosis (MF), according to research published in haematologica.
Investigators said administering the drugs together proved difficult. Most patients had to stop taking lenalidomide at some point, and many did not restart the drug.
In addition, the study did not meet the predetermined efficacy criteria and was therefore terminated early.
Still, the investigators noted that 17 of 31 patients did respond to treatment, and 10 patients were still taking both drugs at the time of analysis.
The team therefore believes a sequential rather than concomitant treatment approach might work with this combination or for other agents to be combined with ruxolitinib.
Srdan Verstovsek, MD, PhD, of the MD Anderson Cancer Center in Houston, Texas, and his colleagues conducted this research. It was supported by Incyte Corporation (the company developing ruxolitinib) and MD Anderson.
The investigators initiated this study to determine if lenalidomide and ruxolitinib in combination would target distinct clinical and pathological manifestations of MF and prevent treatment-related decreases in blood counts.
They studied the combination in 31 patients with primary MF (n=15), post-polycythemia vera MF (n=12), or post-essential thrombocythemia MF (n=4). The patients’ median age was 66 (range, 37-82), and 21 had received prior treatments (range, 1-3).
The patients received ruxolitinib at 15 mg twice daily in continuous, 28-day cycles, plus 5 mg of lenalidomide once daily on days 1-21. The median follow-up was 28 months (range, 12-35+).
Dosing troubles
In all, 23 patients required dose interruptions of lenalidomide, with or without a dose decrease due to toxicity. Twenty of these interruptions occurred within the first 3 months of therapy, and 14 of the patients never restarted treatment with lenalidomide.
The reasons for dose interruption (or, ultimately, discontinuation) were low platelet count (n=8), low absolute neutrophil count (n=3), anemia (n=3), diarrhea (n=3), financial constraints (n=2), deep vein thrombosis (n=1), skin rash (n=1), transaminitis (n=1), and arthralgia/fever (n=1).
Conversely, 6 patients required an increased dose of ruxolitinib, 3 within the first 3 months. Doses were increased due to leukocytosis (n=2), suboptimal response (n=2), thrombocytosis (n=1), and progressive splenomegaly (n=1).
Discontinuation and early termination
At a median follow-up of 28 months, 25 patients (81%) were still alive, and 16 remained on study. Ten of these patients were taking both drugs, and 6 were taking ruxolitinib only.
For the 15 patients who came off the study, their reasons included concurrent disease (n=3), disease progression (n=2), myelosuppression (n=2), refractory disease (n=3), toxicities (n=2), persistent and severe lower-extremity cellulitis (n=1), non-compliance (n=1), and financial reasons (n=1).
The investigators noted that only 7 patients met the predetermined definition of efficacy—a response to combination treatment within 6 months of initiation without discontinuing either drug.
For the study to continue after the interim analysis, more than 10 patients would have to fulfill those criteria. As they did not, the study was terminated early.
Response
Seventeen patients (55%) achieved an IWG-MRT-defined response of clinical improvement in palpable spleen size. Seven patients had a 100% spleen reduction, and 10 had reduction of 50% or greater.
The median time to clinical improvement in spleen size was 1.8 months (range, 0.4-31), and the median duration of this response was 19 months (range, 3-32+). At last follow-up, 2 patients had lost their response.
One of the 17 spleen responders also achieved an IWG-MRT-defined clinical improvement in hemoglobin (increase of 2 g/dL or greater that was maintained for more than 8 weeks). The time to this response was 28 months, and the response lasted 6 months.
There were differences in response rate, response duration, time to response, and overall survival between patients who required dose interruptions and those who did not. However, none of these differences were statistically significant.
Toxicity
Grade 3/4 myelosuppression occurred in 16 patients, and there was 1 case of lower-extremity thrombosis. The most common non-hematologic adverse events (AEs) were diarrhea (n=8), nausea and vomiting (n=3), abdominal pain (n=3), and constipation (n=3).
Five patients had grade 3/4 non-hematologic AEs—diarrhea, edema, transaminitis, bilirubinemia, and acute kidney injury. Two patients discontinued treatment due to drug-related AEs—grade 2 persistent nausea and grade 3 diarrhea.
Three of the 6 deaths were documented (including 2 that occurred on-study). They were attributed to pneumonia, kidney failure, and possible stroke. ![]()

Photo courtesy of the CDC
Simultaneous administration of lenalidomide and ruxolitinib is not feasible in patients with myelofibrosis (MF), according to research published in haematologica.
Investigators said administering the drugs together proved difficult. Most patients had to stop taking lenalidomide at some point, and many did not restart the drug.
In addition, the study did not meet the predetermined efficacy criteria and was therefore terminated early.
Still, the investigators noted that 17 of 31 patients did respond to treatment, and 10 patients were still taking both drugs at the time of analysis.
The team therefore believes a sequential rather than concomitant treatment approach might work with this combination or for other agents to be combined with ruxolitinib.
Srdan Verstovsek, MD, PhD, of the MD Anderson Cancer Center in Houston, Texas, and his colleagues conducted this research. It was supported by Incyte Corporation (the company developing ruxolitinib) and MD Anderson.
The investigators initiated this study to determine if lenalidomide and ruxolitinib in combination would target distinct clinical and pathological manifestations of MF and prevent treatment-related decreases in blood counts.
They studied the combination in 31 patients with primary MF (n=15), post-polycythemia vera MF (n=12), or post-essential thrombocythemia MF (n=4). The patients’ median age was 66 (range, 37-82), and 21 had received prior treatments (range, 1-3).
The patients received ruxolitinib at 15 mg twice daily in continuous, 28-day cycles, plus 5 mg of lenalidomide once daily on days 1-21. The median follow-up was 28 months (range, 12-35+).
Dosing troubles
In all, 23 patients required dose interruptions of lenalidomide, with or without a dose decrease due to toxicity. Twenty of these interruptions occurred within the first 3 months of therapy, and 14 of the patients never restarted treatment with lenalidomide.
The reasons for dose interruption (or, ultimately, discontinuation) were low platelet count (n=8), low absolute neutrophil count (n=3), anemia (n=3), diarrhea (n=3), financial constraints (n=2), deep vein thrombosis (n=1), skin rash (n=1), transaminitis (n=1), and arthralgia/fever (n=1).
Conversely, 6 patients required an increased dose of ruxolitinib, 3 within the first 3 months. Doses were increased due to leukocytosis (n=2), suboptimal response (n=2), thrombocytosis (n=1), and progressive splenomegaly (n=1).
Discontinuation and early termination
At a median follow-up of 28 months, 25 patients (81%) were still alive, and 16 remained on study. Ten of these patients were taking both drugs, and 6 were taking ruxolitinib only.
For the 15 patients who came off the study, their reasons included concurrent disease (n=3), disease progression (n=2), myelosuppression (n=2), refractory disease (n=3), toxicities (n=2), persistent and severe lower-extremity cellulitis (n=1), non-compliance (n=1), and financial reasons (n=1).
The investigators noted that only 7 patients met the predetermined definition of efficacy—a response to combination treatment within 6 months of initiation without discontinuing either drug.
For the study to continue after the interim analysis, more than 10 patients would have to fulfill those criteria. As they did not, the study was terminated early.
Response
Seventeen patients (55%) achieved an IWG-MRT-defined response of clinical improvement in palpable spleen size. Seven patients had a 100% spleen reduction, and 10 had reduction of 50% or greater.
The median time to clinical improvement in spleen size was 1.8 months (range, 0.4-31), and the median duration of this response was 19 months (range, 3-32+). At last follow-up, 2 patients had lost their response.
One of the 17 spleen responders also achieved an IWG-MRT-defined clinical improvement in hemoglobin (increase of 2 g/dL or greater that was maintained for more than 8 weeks). The time to this response was 28 months, and the response lasted 6 months.
There were differences in response rate, response duration, time to response, and overall survival between patients who required dose interruptions and those who did not. However, none of these differences were statistically significant.
Toxicity
Grade 3/4 myelosuppression occurred in 16 patients, and there was 1 case of lower-extremity thrombosis. The most common non-hematologic adverse events (AEs) were diarrhea (n=8), nausea and vomiting (n=3), abdominal pain (n=3), and constipation (n=3).
Five patients had grade 3/4 non-hematologic AEs—diarrhea, edema, transaminitis, bilirubinemia, and acute kidney injury. Two patients discontinued treatment due to drug-related AEs—grade 2 persistent nausea and grade 3 diarrhea.
Three of the 6 deaths were documented (including 2 that occurred on-study). They were attributed to pneumonia, kidney failure, and possible stroke. ![]()
Database details driver mutations

Photo by Darren Baker
Scientists have created an online database of mutations that have been shown to drive cancers in preclinical or clinical research.
The database, called the Cancer Driver Log (CanDL), currently includes mutations in 62 genes, with hundreds of distinct variants across multiple cancers.
Sameek Roychowdhury, MD, PhD, of The Ohio State University in Columbus, and his colleauges described CanDL in the Journal of Molecular Diagnostics.
“CanDL is a database of gene mutations that have been functionally characterized or have been targeted clinically or preclinically with approved or investigational agents,” Dr Roychowdhury explained.
“Currently, pathology laboratories that sequence tumor tissue must manually research the scientific literature for individual mutations to determine whether they are considered a driver or a passenger to facilitate clinical interpretation.”
“CanDL expedites this time-consuming process by placing key information about known and possible driver mutations that might be effective targets for drug development at their fingertips.”
CanDL entries can be searched by gene or amino acid variants, and they can be downloaded for custom analyses.
The database also includes a mechanism for users to contribute novel driver mutations in open collaboration with the Roychowdhury lab. The team plans to update the database quarterly. ![]()

Photo by Darren Baker
Scientists have created an online database of mutations that have been shown to drive cancers in preclinical or clinical research.
The database, called the Cancer Driver Log (CanDL), currently includes mutations in 62 genes, with hundreds of distinct variants across multiple cancers.
Sameek Roychowdhury, MD, PhD, of The Ohio State University in Columbus, and his colleauges described CanDL in the Journal of Molecular Diagnostics.
“CanDL is a database of gene mutations that have been functionally characterized or have been targeted clinically or preclinically with approved or investigational agents,” Dr Roychowdhury explained.
“Currently, pathology laboratories that sequence tumor tissue must manually research the scientific literature for individual mutations to determine whether they are considered a driver or a passenger to facilitate clinical interpretation.”
“CanDL expedites this time-consuming process by placing key information about known and possible driver mutations that might be effective targets for drug development at their fingertips.”
CanDL entries can be searched by gene or amino acid variants, and they can be downloaded for custom analyses.
The database also includes a mechanism for users to contribute novel driver mutations in open collaboration with the Roychowdhury lab. The team plans to update the database quarterly. ![]()

Photo by Darren Baker
Scientists have created an online database of mutations that have been shown to drive cancers in preclinical or clinical research.
The database, called the Cancer Driver Log (CanDL), currently includes mutations in 62 genes, with hundreds of distinct variants across multiple cancers.
Sameek Roychowdhury, MD, PhD, of The Ohio State University in Columbus, and his colleauges described CanDL in the Journal of Molecular Diagnostics.
“CanDL is a database of gene mutations that have been functionally characterized or have been targeted clinically or preclinically with approved or investigational agents,” Dr Roychowdhury explained.
“Currently, pathology laboratories that sequence tumor tissue must manually research the scientific literature for individual mutations to determine whether they are considered a driver or a passenger to facilitate clinical interpretation.”
“CanDL expedites this time-consuming process by placing key information about known and possible driver mutations that might be effective targets for drug development at their fingertips.”
CanDL entries can be searched by gene or amino acid variants, and they can be downloaded for custom analyses.
The database also includes a mechanism for users to contribute novel driver mutations in open collaboration with the Roychowdhury lab. The team plans to update the database quarterly. ![]()
mAb produces ‘encouraging’ results in rel/ref MM

The anti-CD38 monoclonal antibody daratumumab has demonstrated a “favorable safety profile” and “encouraging efficacy” in patients with relapsed/refractory multiple myeloma (MM), according to researchers.
Results of a phase 1/2 study suggested the drug was most effective at a dose of 16 mg/kg. At this dose, the overall response rate was 36%.
Most adverse events (AEs) were grade 1 or 2, although serious AEs did occur.
“As a single-agent therapy, daratumumab showed significant promise against difficult-to-treat disease in our patients with advanced myeloma who have few other therapeutic options,” said Paul G. Richardson, MD, of the Dana-Farber Cancer Institute in Boston, Massachusetts.
“Because it targets a key receptor and works through different mechanisms than other available agents, it clearly has merited comprehensive testing in larger clinical trials. Preliminary results from these studies have been very encouraging.”
Dr Richardson and his colleagues reported results of the phase 1/2 study in NEJM. The research was previously presented at the 18th Congress of the EHA in 2013. It was sponsored by Janssen Research and Development and Genmab.
Another phase 2 study of single-agent daratumumab in MM was recently presented at the 2015 ASCO Annual Meeting.
Patients and treatment
The current study enrolled patients with relapsed MM or relapsed MM that was refractory to 2 or more prior lines of therapy. The study consisted of 2 parts.
In part 1 (n=30), researchers administered daratumumab in 10 different dosing cohorts at doses ranging from 0.005 to 24 mg/kg of body weight.
In part 2 (n=72), patients received 2 different doses of daratumumab on varying schedules. In schedules A (n=16), B (n=8), and C (n=6), patients received daratumumab at 8 mg/kg in 8 once-weekly infusions and then in twice-monthly infusions for 16 weeks.
In schedules D (n=20) and E (n=22), patients received daratumumab at 16 mg/kg, and, after the first infusion, they had a 3-week washout period to allow for the collection of pharmacokinetic data. They then received weekly treatment for 7 weeks, followed by twice-monthly treatment for 14 weeks.
Safety
There was no maximum tolerated dose identified in part 1 of the study. Infusion-related AEs occurred in 20 patients (63%), serious AEs occurred in 12 patients (37%), and AEs leading to treatment discontinuation occurred in 5 patients (16%).
In part 2, 71% of patients had infusion-related AEs. The most common AEs among patients in both dosing cohorts (8 mg/kg and 16 mg/kg) were fatigue (42%), allergic rhinitis (31%), pyrexia (28%), diarrhea (21%), upper respiratory tract infection (21%), and dyspnea (19%). The most frequent hematologic AE was neutropenia (12%).
Grade 3/4 AEs occurred in 53% of patients in the 8 mg/kg cohort and 26% in the 16 mg/kg cohort. Grade 3/4 AEs that were reported in 2 or more patients included pneumonia (n=5), thrombocytopenia (n=4), neutropenia (n=2), leukopenia (n=2), anemia (n=2), and hyperglycemia (n=2).
Serious AEs occurred in 40% of patients in the 8 mg/kg cohort and 33% in the 16 mg/kg cohort. The most frequent serious AEs were infection-related events.
Efficacy
In part 1, there were no responses among patients who received daratumumab at 2 mg/kg or less (n=18), but responses did occur in patients treated at doses of 4 mg/kg or higher (n=12).
There were 4 partial responses—1 in the 4 mg/kg group, 1 in the 16 mg/kg group, and 2 in the 24 mg/kg group. And there were 3 minimal responses—2 in the 4 mg/kg group and 1 in the 8 mg/kg group.
Three patients had stable disease—1 each in the 8 mg/kg, 16 mg/kg, and 24 mg/kg groups. One patient progressed (16 mg/kg), and 1 was not evaluable (8 mg/kg).
In part 2, the overall response rate was 10% in the 8 mg/kg cohort (3/30) and 36% (15/42) in the 16 mg/kg cohort.
There were 2 complete responses (16 mg/kg), 2 very good partial responses (16 mg/kg), 14 partial responses (3 in the 8 mg/kg cohort and 11 in the 16 mg/kg), and 10 minimal responses (6 in the 8 mg/kg cohort and 4 in the 16 mg/kg cohort).
Thirty-six patients had stable disease (14 in the 8 mg/kg cohort and 22 in the 16 mg/kg cohort). Six patients progressed (all in the 8 mg/kg cohort), and 2 patients were not evaluable (1 in each cohort).
The estimated median progression-free survival was 2.4 months in the 8 mg/kg cohort and 5.6 months in the 16 mg/kg cohort. The overall survival rate at 12 months was 77% in both cohorts. ![]()

The anti-CD38 monoclonal antibody daratumumab has demonstrated a “favorable safety profile” and “encouraging efficacy” in patients with relapsed/refractory multiple myeloma (MM), according to researchers.
Results of a phase 1/2 study suggested the drug was most effective at a dose of 16 mg/kg. At this dose, the overall response rate was 36%.
Most adverse events (AEs) were grade 1 or 2, although serious AEs did occur.
“As a single-agent therapy, daratumumab showed significant promise against difficult-to-treat disease in our patients with advanced myeloma who have few other therapeutic options,” said Paul G. Richardson, MD, of the Dana-Farber Cancer Institute in Boston, Massachusetts.
“Because it targets a key receptor and works through different mechanisms than other available agents, it clearly has merited comprehensive testing in larger clinical trials. Preliminary results from these studies have been very encouraging.”
Dr Richardson and his colleagues reported results of the phase 1/2 study in NEJM. The research was previously presented at the 18th Congress of the EHA in 2013. It was sponsored by Janssen Research and Development and Genmab.
Another phase 2 study of single-agent daratumumab in MM was recently presented at the 2015 ASCO Annual Meeting.
Patients and treatment
The current study enrolled patients with relapsed MM or relapsed MM that was refractory to 2 or more prior lines of therapy. The study consisted of 2 parts.
In part 1 (n=30), researchers administered daratumumab in 10 different dosing cohorts at doses ranging from 0.005 to 24 mg/kg of body weight.
In part 2 (n=72), patients received 2 different doses of daratumumab on varying schedules. In schedules A (n=16), B (n=8), and C (n=6), patients received daratumumab at 8 mg/kg in 8 once-weekly infusions and then in twice-monthly infusions for 16 weeks.
In schedules D (n=20) and E (n=22), patients received daratumumab at 16 mg/kg, and, after the first infusion, they had a 3-week washout period to allow for the collection of pharmacokinetic data. They then received weekly treatment for 7 weeks, followed by twice-monthly treatment for 14 weeks.
Safety
There was no maximum tolerated dose identified in part 1 of the study. Infusion-related AEs occurred in 20 patients (63%), serious AEs occurred in 12 patients (37%), and AEs leading to treatment discontinuation occurred in 5 patients (16%).
In part 2, 71% of patients had infusion-related AEs. The most common AEs among patients in both dosing cohorts (8 mg/kg and 16 mg/kg) were fatigue (42%), allergic rhinitis (31%), pyrexia (28%), diarrhea (21%), upper respiratory tract infection (21%), and dyspnea (19%). The most frequent hematologic AE was neutropenia (12%).
Grade 3/4 AEs occurred in 53% of patients in the 8 mg/kg cohort and 26% in the 16 mg/kg cohort. Grade 3/4 AEs that were reported in 2 or more patients included pneumonia (n=5), thrombocytopenia (n=4), neutropenia (n=2), leukopenia (n=2), anemia (n=2), and hyperglycemia (n=2).
Serious AEs occurred in 40% of patients in the 8 mg/kg cohort and 33% in the 16 mg/kg cohort. The most frequent serious AEs were infection-related events.
Efficacy
In part 1, there were no responses among patients who received daratumumab at 2 mg/kg or less (n=18), but responses did occur in patients treated at doses of 4 mg/kg or higher (n=12).
There were 4 partial responses—1 in the 4 mg/kg group, 1 in the 16 mg/kg group, and 2 in the 24 mg/kg group. And there were 3 minimal responses—2 in the 4 mg/kg group and 1 in the 8 mg/kg group.
Three patients had stable disease—1 each in the 8 mg/kg, 16 mg/kg, and 24 mg/kg groups. One patient progressed (16 mg/kg), and 1 was not evaluable (8 mg/kg).
In part 2, the overall response rate was 10% in the 8 mg/kg cohort (3/30) and 36% (15/42) in the 16 mg/kg cohort.
There were 2 complete responses (16 mg/kg), 2 very good partial responses (16 mg/kg), 14 partial responses (3 in the 8 mg/kg cohort and 11 in the 16 mg/kg), and 10 minimal responses (6 in the 8 mg/kg cohort and 4 in the 16 mg/kg cohort).
Thirty-six patients had stable disease (14 in the 8 mg/kg cohort and 22 in the 16 mg/kg cohort). Six patients progressed (all in the 8 mg/kg cohort), and 2 patients were not evaluable (1 in each cohort).
The estimated median progression-free survival was 2.4 months in the 8 mg/kg cohort and 5.6 months in the 16 mg/kg cohort. The overall survival rate at 12 months was 77% in both cohorts. ![]()

The anti-CD38 monoclonal antibody daratumumab has demonstrated a “favorable safety profile” and “encouraging efficacy” in patients with relapsed/refractory multiple myeloma (MM), according to researchers.
Results of a phase 1/2 study suggested the drug was most effective at a dose of 16 mg/kg. At this dose, the overall response rate was 36%.
Most adverse events (AEs) were grade 1 or 2, although serious AEs did occur.
“As a single-agent therapy, daratumumab showed significant promise against difficult-to-treat disease in our patients with advanced myeloma who have few other therapeutic options,” said Paul G. Richardson, MD, of the Dana-Farber Cancer Institute in Boston, Massachusetts.
“Because it targets a key receptor and works through different mechanisms than other available agents, it clearly has merited comprehensive testing in larger clinical trials. Preliminary results from these studies have been very encouraging.”
Dr Richardson and his colleagues reported results of the phase 1/2 study in NEJM. The research was previously presented at the 18th Congress of the EHA in 2013. It was sponsored by Janssen Research and Development and Genmab.
Another phase 2 study of single-agent daratumumab in MM was recently presented at the 2015 ASCO Annual Meeting.
Patients and treatment
The current study enrolled patients with relapsed MM or relapsed MM that was refractory to 2 or more prior lines of therapy. The study consisted of 2 parts.
In part 1 (n=30), researchers administered daratumumab in 10 different dosing cohorts at doses ranging from 0.005 to 24 mg/kg of body weight.
In part 2 (n=72), patients received 2 different doses of daratumumab on varying schedules. In schedules A (n=16), B (n=8), and C (n=6), patients received daratumumab at 8 mg/kg in 8 once-weekly infusions and then in twice-monthly infusions for 16 weeks.
In schedules D (n=20) and E (n=22), patients received daratumumab at 16 mg/kg, and, after the first infusion, they had a 3-week washout period to allow for the collection of pharmacokinetic data. They then received weekly treatment for 7 weeks, followed by twice-monthly treatment for 14 weeks.
Safety
There was no maximum tolerated dose identified in part 1 of the study. Infusion-related AEs occurred in 20 patients (63%), serious AEs occurred in 12 patients (37%), and AEs leading to treatment discontinuation occurred in 5 patients (16%).
In part 2, 71% of patients had infusion-related AEs. The most common AEs among patients in both dosing cohorts (8 mg/kg and 16 mg/kg) were fatigue (42%), allergic rhinitis (31%), pyrexia (28%), diarrhea (21%), upper respiratory tract infection (21%), and dyspnea (19%). The most frequent hematologic AE was neutropenia (12%).
Grade 3/4 AEs occurred in 53% of patients in the 8 mg/kg cohort and 26% in the 16 mg/kg cohort. Grade 3/4 AEs that were reported in 2 or more patients included pneumonia (n=5), thrombocytopenia (n=4), neutropenia (n=2), leukopenia (n=2), anemia (n=2), and hyperglycemia (n=2).
Serious AEs occurred in 40% of patients in the 8 mg/kg cohort and 33% in the 16 mg/kg cohort. The most frequent serious AEs were infection-related events.
Efficacy
In part 1, there were no responses among patients who received daratumumab at 2 mg/kg or less (n=18), but responses did occur in patients treated at doses of 4 mg/kg or higher (n=12).
There were 4 partial responses—1 in the 4 mg/kg group, 1 in the 16 mg/kg group, and 2 in the 24 mg/kg group. And there were 3 minimal responses—2 in the 4 mg/kg group and 1 in the 8 mg/kg group.
Three patients had stable disease—1 each in the 8 mg/kg, 16 mg/kg, and 24 mg/kg groups. One patient progressed (16 mg/kg), and 1 was not evaluable (8 mg/kg).
In part 2, the overall response rate was 10% in the 8 mg/kg cohort (3/30) and 36% (15/42) in the 16 mg/kg cohort.
There were 2 complete responses (16 mg/kg), 2 very good partial responses (16 mg/kg), 14 partial responses (3 in the 8 mg/kg cohort and 11 in the 16 mg/kg), and 10 minimal responses (6 in the 8 mg/kg cohort and 4 in the 16 mg/kg cohort).
Thirty-six patients had stable disease (14 in the 8 mg/kg cohort and 22 in the 16 mg/kg cohort). Six patients progressed (all in the 8 mg/kg cohort), and 2 patients were not evaluable (1 in each cohort).
The estimated median progression-free survival was 2.4 months in the 8 mg/kg cohort and 5.6 months in the 16 mg/kg cohort. The overall survival rate at 12 months was 77% in both cohorts. ![]()
Team quantifies CAM use among seniors with cancer

Photo by Rhoda Baer
A new study suggests that seniors with cancer may be taking complementary or alternative medicines (CAMs) without their oncologists’ knowledge.
In this single-center study, 27% of senior cancer patients took CAMs at some point during their cancer care.
CAM usage was highest among patients ages 80 to 89, women, Caucasians, and patients with solid tumor malignancies.
Polypharmacy and certain comorbidities were linked to CAM use as well.
Researchers reported these findings in the Journal of Geriatric Oncology.
“Currently, few oncologists are aware of the alternative medicines their patients take,” said study author Ginah Nightingale, PharmD, of Thomas Jefferson University in Philadelphia, Pennsylvania.
“Patients often fail to disclose the CAMs they take because they think they are safe, natural, nontoxic, and not relevant to their cancer care; because they think their doctor will disapprove; or because the doctor doesn’t specifically ask.”
To quantify CAM use in older cancer patients treated at their institution, Dr Nightingale and her colleagues surveyed patients who came to the Senior Adult Oncology Center at Thomas Jefferson University.
In a single visit, patients were seen by a medical oncologist, geriatrician, clinical pharmacist, social worker, and dietician. As part of this assessment, the patients brought in the contents of their medicine cabinets, and the medications they actively used were reviewed and recorded.
A total of 234 patients were included in the final analysis. Their mean age was 79.9 (range, 61–98). Most (87%) had solid tumor malignancies, were Caucasian (74%), and were female (64%).
In all, 26.5% of patients (n=62) had taken at least 1 CAM during their cancer care, with 19.2% taking 1 CAM, 6.4% taking 2, 0.4% taking 3, and 0.4% taking 4 or more CAMs. The highest number of CAMs taken was 10.
CAM usage was highest among patients ages 80 to 89, women, Caucasians, and patients with solid tumor malignancies.
Comorbidities significantly associated with CAM use were vision impairment (P=0.048) and urologic comorbidities (P=0.021). Polypharmacy (concurrent use of 5 or more medications) was significantly associated with CAM use as well (P=0.045).
Some of the commonly used CAMs were mega-dose vitamins or minerals, as well as treatments for macular degeneration, stomach probiotics, and joint health.
The researchers did not examine the potential adverse effects of these medications, but Dr Nightingale said some are known to have a biochemical effect on the body and other drugs.
“It is very important to do a comprehensive screen of all of the medications that older cancer patients take, including CAMs,” she added. “Clear and transparent documentation of CAM use should be recorded in the patient’s medical record. This documentation should indicate that patient-specific communication and/or education was provided so that shared and informed decisions by the patient can be made regarding the continued use of these medications.” ![]()

Photo by Rhoda Baer
A new study suggests that seniors with cancer may be taking complementary or alternative medicines (CAMs) without their oncologists’ knowledge.
In this single-center study, 27% of senior cancer patients took CAMs at some point during their cancer care.
CAM usage was highest among patients ages 80 to 89, women, Caucasians, and patients with solid tumor malignancies.
Polypharmacy and certain comorbidities were linked to CAM use as well.
Researchers reported these findings in the Journal of Geriatric Oncology.
“Currently, few oncologists are aware of the alternative medicines their patients take,” said study author Ginah Nightingale, PharmD, of Thomas Jefferson University in Philadelphia, Pennsylvania.
“Patients often fail to disclose the CAMs they take because they think they are safe, natural, nontoxic, and not relevant to their cancer care; because they think their doctor will disapprove; or because the doctor doesn’t specifically ask.”
To quantify CAM use in older cancer patients treated at their institution, Dr Nightingale and her colleagues surveyed patients who came to the Senior Adult Oncology Center at Thomas Jefferson University.
In a single visit, patients were seen by a medical oncologist, geriatrician, clinical pharmacist, social worker, and dietician. As part of this assessment, the patients brought in the contents of their medicine cabinets, and the medications they actively used were reviewed and recorded.
A total of 234 patients were included in the final analysis. Their mean age was 79.9 (range, 61–98). Most (87%) had solid tumor malignancies, were Caucasian (74%), and were female (64%).
In all, 26.5% of patients (n=62) had taken at least 1 CAM during their cancer care, with 19.2% taking 1 CAM, 6.4% taking 2, 0.4% taking 3, and 0.4% taking 4 or more CAMs. The highest number of CAMs taken was 10.
CAM usage was highest among patients ages 80 to 89, women, Caucasians, and patients with solid tumor malignancies.
Comorbidities significantly associated with CAM use were vision impairment (P=0.048) and urologic comorbidities (P=0.021). Polypharmacy (concurrent use of 5 or more medications) was significantly associated with CAM use as well (P=0.045).
Some of the commonly used CAMs were mega-dose vitamins or minerals, as well as treatments for macular degeneration, stomach probiotics, and joint health.
The researchers did not examine the potential adverse effects of these medications, but Dr Nightingale said some are known to have a biochemical effect on the body and other drugs.
“It is very important to do a comprehensive screen of all of the medications that older cancer patients take, including CAMs,” she added. “Clear and transparent documentation of CAM use should be recorded in the patient’s medical record. This documentation should indicate that patient-specific communication and/or education was provided so that shared and informed decisions by the patient can be made regarding the continued use of these medications.” ![]()

Photo by Rhoda Baer
A new study suggests that seniors with cancer may be taking complementary or alternative medicines (CAMs) without their oncologists’ knowledge.
In this single-center study, 27% of senior cancer patients took CAMs at some point during their cancer care.
CAM usage was highest among patients ages 80 to 89, women, Caucasians, and patients with solid tumor malignancies.
Polypharmacy and certain comorbidities were linked to CAM use as well.
Researchers reported these findings in the Journal of Geriatric Oncology.
“Currently, few oncologists are aware of the alternative medicines their patients take,” said study author Ginah Nightingale, PharmD, of Thomas Jefferson University in Philadelphia, Pennsylvania.
“Patients often fail to disclose the CAMs they take because they think they are safe, natural, nontoxic, and not relevant to their cancer care; because they think their doctor will disapprove; or because the doctor doesn’t specifically ask.”
To quantify CAM use in older cancer patients treated at their institution, Dr Nightingale and her colleagues surveyed patients who came to the Senior Adult Oncology Center at Thomas Jefferson University.
In a single visit, patients were seen by a medical oncologist, geriatrician, clinical pharmacist, social worker, and dietician. As part of this assessment, the patients brought in the contents of their medicine cabinets, and the medications they actively used were reviewed and recorded.
A total of 234 patients were included in the final analysis. Their mean age was 79.9 (range, 61–98). Most (87%) had solid tumor malignancies, were Caucasian (74%), and were female (64%).
In all, 26.5% of patients (n=62) had taken at least 1 CAM during their cancer care, with 19.2% taking 1 CAM, 6.4% taking 2, 0.4% taking 3, and 0.4% taking 4 or more CAMs. The highest number of CAMs taken was 10.
CAM usage was highest among patients ages 80 to 89, women, Caucasians, and patients with solid tumor malignancies.
Comorbidities significantly associated with CAM use were vision impairment (P=0.048) and urologic comorbidities (P=0.021). Polypharmacy (concurrent use of 5 or more medications) was significantly associated with CAM use as well (P=0.045).
Some of the commonly used CAMs were mega-dose vitamins or minerals, as well as treatments for macular degeneration, stomach probiotics, and joint health.
The researchers did not examine the potential adverse effects of these medications, but Dr Nightingale said some are known to have a biochemical effect on the body and other drugs.
“It is very important to do a comprehensive screen of all of the medications that older cancer patients take, including CAMs,” she added. “Clear and transparent documentation of CAM use should be recorded in the patient’s medical record. This documentation should indicate that patient-specific communication and/or education was provided so that shared and informed decisions by the patient can be made regarding the continued use of these medications.” ![]()