User login
Penile Rash Worries Man (And Wife)
A 61-year-old man presents with an asymptomatic but worrisome (to him) penile rash that manifested over a two-day period several weeks ago. OTC miconazole cream, triple-antibiotic creams, and most recently, a two-day course of fluconazole (200 mg bid) have not helped.
The patient denies any sexual exposure outside his marriage, as does his wife. Both are in good health in other respects, although the wife has been treated for lymphoma (long since declared cured).
At first, the patient denies any other skin problems. However, with specific questioning, he admits to having a chronic scaly and slightly itchy rash that periodically appears in the same places: bilateral brows, nasolabial folds, various spots in his beard, and both external ear canals.
Additional history taking reveals that the patient has been under a great deal of stress lately. His father recently died, leaving him to deal with a number of issues, and his work shift changed, requiring him to sleep during the day and work at night.
EXAMINATION
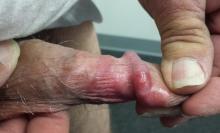
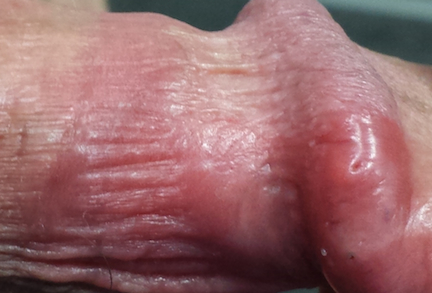
The penile rash is macular, smooth, strikingly red, and shiny. It covers the dorsal, distal penile shaft, spilling onto the glans. It looks wet but is quite dry. His groin, upper intergluteal area, and axillae are free of any changes.
A fine pink rash is seen in the glabella, extending into both brows. Similar areas of focal scaling on pink bases are also noted in the beard area and in both external auditory meati.
What is the diagnosis?
DISCUSSION
This is the way seborrheic dermatitis (SD) acts: It sits around for years, causing minor problems in common areas (eg, face, scalp, ears, and beard). Then the stress of life intrudes, the candle is being burnt from both ends, and SD blossoms, appearing in places such as the chest, groin, suprapubic area, axillae, and genitals.
On the genitals, SD is often bright red, with little if any scale. Why? The moisture and friction particular to the genital and intertriginous (skin on skin) areas prevent scale from accumulating.
Penile rashes generally get everyone’s attention, including that of the patient’s sexual partner(s). For the average nonclinician, the list of possible explanations is infinite—and worrisome. The situation is, in some ways, worse for the average primary care provider, who will almost always decree such a rash a “yeast infection” (though it seldom responds to anti-yeast medications—for good reason: Otherwise healthy circumcised men almost never develop yeast infections anywhere, let alone on the penis).
If you remove “yeast” from the differential, the average primary care provider will be lost. An abbreviated differential list for penile rashes and conditions includes:
• Psoriasis
• Seborrhea
• Contact vs irritant dermatitis
• Eczema/lichen simplex chronicus
• Lichen sclerosis et atrophicus (traditionally termed balanitis xerotica obliterans or BXO when it occurs on the penis)
• Lichen planus
• Reiter syndrome
• Scabies
• Erythroplasia of Queyrat (superficial squamous cell carcinoma)
• Melanoma
• Herpes simplex
• Molluscum
• Pearly penile papules
• Tyson’s glands (prominent pilosebaceous glands on the penile shaft)
• Angiokeratoma of Fordyce
• Condyloma accuminata
• Primary syphilis chancre
• Fixed-drug eruption
(Note that while yeast is not on this list, it certainly could be considered in an uncircumcised hyperglycemic patient.)
The top five items on the list would cover 90% of patients with penile rashes. Seborrheic dermatitis is utterly common but only rarely recognized when it occurs in unusual areas (as in this case).
For this patient, I prescribed topical hydrocortisone 2.5% cream (to be applied bid to all affected areas) and advised him to shampoo the areas daily with ketoconazole-containing shampoo. But the main thing I did for him, and his wife, was provide peace of mind about all the terrible conditions he didn’t have. With treatment, his penile lesions resolved within two weeks.
TAKE-HOME LEARNING POINTS
• Seborrheic dermatitis (SD) is common in the scalp, on the face, and in the ears, but it can also appear on the chest, axillae, or genitals.
• Yeast infections are quite uncommon in otherwise healthy men.
• Stress is often a triggering factor with worsening SD.
• Asking about and finding corroborative areas of involvement (face, ears, brows, scalp) can help in diagnosing atypical cases of SD.
A 61-year-old man presents with an asymptomatic but worrisome (to him) penile rash that manifested over a two-day period several weeks ago. OTC miconazole cream, triple-antibiotic creams, and most recently, a two-day course of fluconazole (200 mg bid) have not helped.
The patient denies any sexual exposure outside his marriage, as does his wife. Both are in good health in other respects, although the wife has been treated for lymphoma (long since declared cured).
At first, the patient denies any other skin problems. However, with specific questioning, he admits to having a chronic scaly and slightly itchy rash that periodically appears in the same places: bilateral brows, nasolabial folds, various spots in his beard, and both external ear canals.
Additional history taking reveals that the patient has been under a great deal of stress lately. His father recently died, leaving him to deal with a number of issues, and his work shift changed, requiring him to sleep during the day and work at night.
EXAMINATION
The penile rash is macular, smooth, strikingly red, and shiny. It covers the dorsal, distal penile shaft, spilling onto the glans. It looks wet but is quite dry. His groin, upper intergluteal area, and axillae are free of any changes.
A fine pink rash is seen in the glabella, extending into both brows. Similar areas of focal scaling on pink bases are also noted in the beard area and in both external auditory meati.
What is the diagnosis?
DISCUSSION
This is the way seborrheic dermatitis (SD) acts: It sits around for years, causing minor problems in common areas (eg, face, scalp, ears, and beard). Then the stress of life intrudes, the candle is being burnt from both ends, and SD blossoms, appearing in places such as the chest, groin, suprapubic area, axillae, and genitals.
On the genitals, SD is often bright red, with little if any scale. Why? The moisture and friction particular to the genital and intertriginous (skin on skin) areas prevent scale from accumulating.
Penile rashes generally get everyone’s attention, including that of the patient’s sexual partner(s). For the average nonclinician, the list of possible explanations is infinite—and worrisome. The situation is, in some ways, worse for the average primary care provider, who will almost always decree such a rash a “yeast infection” (though it seldom responds to anti-yeast medications—for good reason: Otherwise healthy circumcised men almost never develop yeast infections anywhere, let alone on the penis).
If you remove “yeast” from the differential, the average primary care provider will be lost. An abbreviated differential list for penile rashes and conditions includes:
• Psoriasis
• Seborrhea
• Contact vs irritant dermatitis
• Eczema/lichen simplex chronicus
• Lichen sclerosis et atrophicus (traditionally termed balanitis xerotica obliterans or BXO when it occurs on the penis)
• Lichen planus
• Reiter syndrome
• Scabies
• Erythroplasia of Queyrat (superficial squamous cell carcinoma)
• Melanoma
• Herpes simplex
• Molluscum
• Pearly penile papules
• Tyson’s glands (prominent pilosebaceous glands on the penile shaft)
• Angiokeratoma of Fordyce
• Condyloma accuminata
• Primary syphilis chancre
• Fixed-drug eruption
(Note that while yeast is not on this list, it certainly could be considered in an uncircumcised hyperglycemic patient.)
The top five items on the list would cover 90% of patients with penile rashes. Seborrheic dermatitis is utterly common but only rarely recognized when it occurs in unusual areas (as in this case).
For this patient, I prescribed topical hydrocortisone 2.5% cream (to be applied bid to all affected areas) and advised him to shampoo the areas daily with ketoconazole-containing shampoo. But the main thing I did for him, and his wife, was provide peace of mind about all the terrible conditions he didn’t have. With treatment, his penile lesions resolved within two weeks.
TAKE-HOME LEARNING POINTS
• Seborrheic dermatitis (SD) is common in the scalp, on the face, and in the ears, but it can also appear on the chest, axillae, or genitals.
• Yeast infections are quite uncommon in otherwise healthy men.
• Stress is often a triggering factor with worsening SD.
• Asking about and finding corroborative areas of involvement (face, ears, brows, scalp) can help in diagnosing atypical cases of SD.
A 61-year-old man presents with an asymptomatic but worrisome (to him) penile rash that manifested over a two-day period several weeks ago. OTC miconazole cream, triple-antibiotic creams, and most recently, a two-day course of fluconazole (200 mg bid) have not helped.
The patient denies any sexual exposure outside his marriage, as does his wife. Both are in good health in other respects, although the wife has been treated for lymphoma (long since declared cured).
At first, the patient denies any other skin problems. However, with specific questioning, he admits to having a chronic scaly and slightly itchy rash that periodically appears in the same places: bilateral brows, nasolabial folds, various spots in his beard, and both external ear canals.
Additional history taking reveals that the patient has been under a great deal of stress lately. His father recently died, leaving him to deal with a number of issues, and his work shift changed, requiring him to sleep during the day and work at night.
EXAMINATION
The penile rash is macular, smooth, strikingly red, and shiny. It covers the dorsal, distal penile shaft, spilling onto the glans. It looks wet but is quite dry. His groin, upper intergluteal area, and axillae are free of any changes.
A fine pink rash is seen in the glabella, extending into both brows. Similar areas of focal scaling on pink bases are also noted in the beard area and in both external auditory meati.
What is the diagnosis?
DISCUSSION
This is the way seborrheic dermatitis (SD) acts: It sits around for years, causing minor problems in common areas (eg, face, scalp, ears, and beard). Then the stress of life intrudes, the candle is being burnt from both ends, and SD blossoms, appearing in places such as the chest, groin, suprapubic area, axillae, and genitals.
On the genitals, SD is often bright red, with little if any scale. Why? The moisture and friction particular to the genital and intertriginous (skin on skin) areas prevent scale from accumulating.
Penile rashes generally get everyone’s attention, including that of the patient’s sexual partner(s). For the average nonclinician, the list of possible explanations is infinite—and worrisome. The situation is, in some ways, worse for the average primary care provider, who will almost always decree such a rash a “yeast infection” (though it seldom responds to anti-yeast medications—for good reason: Otherwise healthy circumcised men almost never develop yeast infections anywhere, let alone on the penis).
If you remove “yeast” from the differential, the average primary care provider will be lost. An abbreviated differential list for penile rashes and conditions includes:
• Psoriasis
• Seborrhea
• Contact vs irritant dermatitis
• Eczema/lichen simplex chronicus
• Lichen sclerosis et atrophicus (traditionally termed balanitis xerotica obliterans or BXO when it occurs on the penis)
• Lichen planus
• Reiter syndrome
• Scabies
• Erythroplasia of Queyrat (superficial squamous cell carcinoma)
• Melanoma
• Herpes simplex
• Molluscum
• Pearly penile papules
• Tyson’s glands (prominent pilosebaceous glands on the penile shaft)
• Angiokeratoma of Fordyce
• Condyloma accuminata
• Primary syphilis chancre
• Fixed-drug eruption
(Note that while yeast is not on this list, it certainly could be considered in an uncircumcised hyperglycemic patient.)
The top five items on the list would cover 90% of patients with penile rashes. Seborrheic dermatitis is utterly common but only rarely recognized when it occurs in unusual areas (as in this case).
For this patient, I prescribed topical hydrocortisone 2.5% cream (to be applied bid to all affected areas) and advised him to shampoo the areas daily with ketoconazole-containing shampoo. But the main thing I did for him, and his wife, was provide peace of mind about all the terrible conditions he didn’t have. With treatment, his penile lesions resolved within two weeks.
TAKE-HOME LEARNING POINTS
• Seborrheic dermatitis (SD) is common in the scalp, on the face, and in the ears, but it can also appear on the chest, axillae, or genitals.
• Yeast infections are quite uncommon in otherwise healthy men.
• Stress is often a triggering factor with worsening SD.
• Asking about and finding corroborative areas of involvement (face, ears, brows, scalp) can help in diagnosing atypical cases of SD.
New business model puts dermatology ‘back in the hands of the dermatologist’
Dermatology has yet to conquer the cosmetic corner of the specialty. That’s according to Dr. Leslie S. Baumann, of the Miami-based, Skin Type Solutions, who explains a new franchise model she says will help “put dermatology back in the hands of dermatologists.”
In this interview, Dr. Baumann, who writes the Cosmeceutical Critique column for Skin & Allergy News, explains her new franchise method for selling skin care products in the dermatologist’s office, and why she thinks it will “disrupt” business as usual in the retail skin care marketplace, including for online retailers.
The following is an edited transcript of the interview, which you can hear in its entirety here.
SAN: Welcome, Dr. Baumann. You recently wrote that you were helping to “put dermatology back in the hands of dermatologists.” Can you expand on that?
Dr. Baumann: I find that most patients really don’t buy their products from dermatologists. They buy them from Sephora, CVS, or the department store, but it’s dermatologists who have years of training about skin, and consumers don’t realize that the dermatologist’s office is the natural choice for where to buy their skin care products. I know that in some parts of the country, access to dermatologists is limited. I think that if a person can see a dermatologist, then that should be where they go for products as well, but that’s not happening. I think dermatologists have done a poor job in getting the word out that we’re the complete skin care experts.
SAN: So, you’ve created a business model to help with this. Please explain how it works.
Dr. Baumann: I love the science of skin care ingredients, and I want to prescribe the right skin care products to my patients. I was at the University of Miami for about 15 years. We didn’t have a huge staff, and I went through the whole skin care evaluation process and created the correct regimen myself. The original evaluation regimen took about 45 minutes, and I realized that patients would probably purchase products from me at first, but the next time, they would buy them somewhere else.
So, I streamlined my approach to make it faster and not require a lot of staff. I determined that I needed to divide patients into skin types. I came up with 16 main skin types, based on four issues: oily vs. dry; sensitive vs. resistant; pigmented vs. unpigmented; and whether the skin is wrinkle-prone. This was the basis of my book, “The Skin Type Solution,” which was a New York Times Bestseller. This showed me that consumers really care about skin products.
The biggest challenge was to get my staff to be able to correctly diagnose the skin type. It took me years to develop my questionnaire, and we have done all kinds of clinical trials to validate it. Now my staff can administer that questionnaire on an iPad, and it automatically calculates the skin type.
The next step was figuring out which products work for each skin type, and then presetting regimens. I found the best products from the companies that had the best technology, and then I tested those on different skin types. I might have five or six products from four or five different brands for one skin type.
SAN: And you are confident that mixing products from the different brands and all the different ingredients won’t somehow irritate the patient’s skin?
Dr. Baumann: I have done a lot of research on cosmetic ingredients; that’s really my core competency. One company might have the best sunscreen technology, but that doesn’t mean they have the best retinol technology, yet every brand feels that pressure to have lots of different products. However, because I do the research trials for the companies, I know each one’s best technology. So, I find the best technology and apply it like a Rubik’s Cube to each skin type’s need, so they always get the best product, and then I test the product. I have been testing this method since 2005.
SAN: What about eponymous skin care lines?
Dr. Baumann: When you go private label, you hire a formulator the way you’d hire a personal chef. That person may not be the scientist who invented that technology. When you have a private label, there is no way you can achieve the same results as you can when you are sourcing products from the best scientists in the world. I know who these people are because I do the research trials. I also know the ingredient supply companies who have the basic scientists in the lab, tinkering with the cell cultures and looking at the mitochondria, so I know where those basic ingredients go. Because I have a large following after the sale of my book and my online blog, I can get volume discounts from the companies. I created a store in the office, and each shelf is color-coded by skin type so it’s easy to know what products to buy,
My friend, who franchised Blimpie’s sandwich shops, was in my office one day watching customers take everything off the shelf and purchase all of it, and he convinced me to franchise it.
It’s important to understand why we chose this model. If you are a cosmetics company, you are not allowed to tell doctors how much to charge for the products because it violates antitrust laws. This allows some people to buy products and then dump them cheaply on the Internet. We control that by having the doctors sign an agreement that they will not sell the products online, and if they do, we cut them off.
SAN: How do you ensure that the products in your franchises aren’t elsewhere on the Internet?
Dr. Baumann: The plan is that once we have enough doctors in the program, we will negotiate with the manufacturers to create products that are exclusive to us.
SAN: Is this a revolution?
Dr. Baumann: That’s the point. It’s putting the power back in the hands of the dermatologist because we are the authorities on skin care. We need to be the ones that people get all the best news and products from first. Just imagine a world where a great new skin care technology comes out, and the only place you can get it is from the dermatologists. That’s going to drive so much business into the dermatologist’s office, and will help dermatologists build their general and cosmetic practice. The beauty of my system is that it trains your staff to identify the most appropriate products for each patient, but I have selected those products. I tested this method in six dermatologists’ offices, and we found that the product exchange rate went from about 35% to 3%. So, when your patient has a better outcome, they are more likely to trust you, and more likely to refer friends to you.
SAN: But what if a patient doesn’t have access to a dermatologist and the model for purchasing skin care products does change? It sounds like it will be harder for them to get the skin care they need.
Dr. Baumann: That is a great question. It’s a problem I have to figure out how to solve. Right now we’re considering Skype consults. This is not considered practicing medicine, so you can do it across state lines.
SAN: Do you plan to franchise dermatologists only or would you also sell the franchise rights to medical spas and physicians other than dermatologists?
Dr. Baumann: That’s really against my philosophy. I want to bring skin care back to the dermatologist. Did you know that only 15 percent of dermatologists sell skin care products in their practice? It’s difficult for them to set the system up and buy products from the different companies. My company streamlines that process. Another reason many dermatologists don’t sell products is because of ethical concerns. I believe if you are offering patients the best products for their skin types, products they can’t get somewhere else, and at the best price, then that is ethical. When you’re just selling things to make money by taking advantage of the patient-doctor relationship, then I am absolutely against it.
SAN: Why might a dermatologist turn away from this franchise model?
Dr. Baumann: There is no reason not to do it, because the startup costs are minimal. The only reason they might not want to do it is if they have their own skin care brand. A doctor could still sell his or her own brand, but they wouldn’t be able to have it on the Skin Type Solutions shelves, and that gets complicated. Based on patient surveys I’ve done, people don’t really want private skin care labels. I think they feel very suspicious of them. My system solves that problem by helping consumers realize the doctor isn’t pretending they invented these products. This is a more honest approach, in my opinion. That might be controversial, but that’s how I feel.
SAN: It sounds like the cosmetic manufacturers would favor this if you like their line.
Dr. Baumann: My system favors good technology. So the charlatans who are trying to sell stem cell therapies or peptides that don’t work aren’t going to like my system. My system favors the geniuses in the lab who don’t know how to get their technology out there, and I know a lot of them. We’ll be able to find that technology and then launch it through dermatology practices. This helps the genius underdogs who don’t know what to do with what they’ve discovered.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (McGraw-Hill, New York 2002) , and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” will be published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy,Topix Pharmaceuticals, and Unilever.
On Twitter @whitneymcknight
Dermatology has yet to conquer the cosmetic corner of the specialty. That’s according to Dr. Leslie S. Baumann, of the Miami-based, Skin Type Solutions, who explains a new franchise model she says will help “put dermatology back in the hands of dermatologists.”
In this interview, Dr. Baumann, who writes the Cosmeceutical Critique column for Skin & Allergy News, explains her new franchise method for selling skin care products in the dermatologist’s office, and why she thinks it will “disrupt” business as usual in the retail skin care marketplace, including for online retailers.
The following is an edited transcript of the interview, which you can hear in its entirety here.
SAN: Welcome, Dr. Baumann. You recently wrote that you were helping to “put dermatology back in the hands of dermatologists.” Can you expand on that?
Dr. Baumann: I find that most patients really don’t buy their products from dermatologists. They buy them from Sephora, CVS, or the department store, but it’s dermatologists who have years of training about skin, and consumers don’t realize that the dermatologist’s office is the natural choice for where to buy their skin care products. I know that in some parts of the country, access to dermatologists is limited. I think that if a person can see a dermatologist, then that should be where they go for products as well, but that’s not happening. I think dermatologists have done a poor job in getting the word out that we’re the complete skin care experts.
SAN: So, you’ve created a business model to help with this. Please explain how it works.
Dr. Baumann: I love the science of skin care ingredients, and I want to prescribe the right skin care products to my patients. I was at the University of Miami for about 15 years. We didn’t have a huge staff, and I went through the whole skin care evaluation process and created the correct regimen myself. The original evaluation regimen took about 45 minutes, and I realized that patients would probably purchase products from me at first, but the next time, they would buy them somewhere else.
So, I streamlined my approach to make it faster and not require a lot of staff. I determined that I needed to divide patients into skin types. I came up with 16 main skin types, based on four issues: oily vs. dry; sensitive vs. resistant; pigmented vs. unpigmented; and whether the skin is wrinkle-prone. This was the basis of my book, “The Skin Type Solution,” which was a New York Times Bestseller. This showed me that consumers really care about skin products.
The biggest challenge was to get my staff to be able to correctly diagnose the skin type. It took me years to develop my questionnaire, and we have done all kinds of clinical trials to validate it. Now my staff can administer that questionnaire on an iPad, and it automatically calculates the skin type.
The next step was figuring out which products work for each skin type, and then presetting regimens. I found the best products from the companies that had the best technology, and then I tested those on different skin types. I might have five or six products from four or five different brands for one skin type.
SAN: And you are confident that mixing products from the different brands and all the different ingredients won’t somehow irritate the patient’s skin?
Dr. Baumann: I have done a lot of research on cosmetic ingredients; that’s really my core competency. One company might have the best sunscreen technology, but that doesn’t mean they have the best retinol technology, yet every brand feels that pressure to have lots of different products. However, because I do the research trials for the companies, I know each one’s best technology. So, I find the best technology and apply it like a Rubik’s Cube to each skin type’s need, so they always get the best product, and then I test the product. I have been testing this method since 2005.
SAN: What about eponymous skin care lines?
Dr. Baumann: When you go private label, you hire a formulator the way you’d hire a personal chef. That person may not be the scientist who invented that technology. When you have a private label, there is no way you can achieve the same results as you can when you are sourcing products from the best scientists in the world. I know who these people are because I do the research trials. I also know the ingredient supply companies who have the basic scientists in the lab, tinkering with the cell cultures and looking at the mitochondria, so I know where those basic ingredients go. Because I have a large following after the sale of my book and my online blog, I can get volume discounts from the companies. I created a store in the office, and each shelf is color-coded by skin type so it’s easy to know what products to buy,
My friend, who franchised Blimpie’s sandwich shops, was in my office one day watching customers take everything off the shelf and purchase all of it, and he convinced me to franchise it.
It’s important to understand why we chose this model. If you are a cosmetics company, you are not allowed to tell doctors how much to charge for the products because it violates antitrust laws. This allows some people to buy products and then dump them cheaply on the Internet. We control that by having the doctors sign an agreement that they will not sell the products online, and if they do, we cut them off.
SAN: How do you ensure that the products in your franchises aren’t elsewhere on the Internet?
Dr. Baumann: The plan is that once we have enough doctors in the program, we will negotiate with the manufacturers to create products that are exclusive to us.
SAN: Is this a revolution?
Dr. Baumann: That’s the point. It’s putting the power back in the hands of the dermatologist because we are the authorities on skin care. We need to be the ones that people get all the best news and products from first. Just imagine a world where a great new skin care technology comes out, and the only place you can get it is from the dermatologists. That’s going to drive so much business into the dermatologist’s office, and will help dermatologists build their general and cosmetic practice. The beauty of my system is that it trains your staff to identify the most appropriate products for each patient, but I have selected those products. I tested this method in six dermatologists’ offices, and we found that the product exchange rate went from about 35% to 3%. So, when your patient has a better outcome, they are more likely to trust you, and more likely to refer friends to you.
SAN: But what if a patient doesn’t have access to a dermatologist and the model for purchasing skin care products does change? It sounds like it will be harder for them to get the skin care they need.
Dr. Baumann: That is a great question. It’s a problem I have to figure out how to solve. Right now we’re considering Skype consults. This is not considered practicing medicine, so you can do it across state lines.
SAN: Do you plan to franchise dermatologists only or would you also sell the franchise rights to medical spas and physicians other than dermatologists?
Dr. Baumann: That’s really against my philosophy. I want to bring skin care back to the dermatologist. Did you know that only 15 percent of dermatologists sell skin care products in their practice? It’s difficult for them to set the system up and buy products from the different companies. My company streamlines that process. Another reason many dermatologists don’t sell products is because of ethical concerns. I believe if you are offering patients the best products for their skin types, products they can’t get somewhere else, and at the best price, then that is ethical. When you’re just selling things to make money by taking advantage of the patient-doctor relationship, then I am absolutely against it.
SAN: Why might a dermatologist turn away from this franchise model?
Dr. Baumann: There is no reason not to do it, because the startup costs are minimal. The only reason they might not want to do it is if they have their own skin care brand. A doctor could still sell his or her own brand, but they wouldn’t be able to have it on the Skin Type Solutions shelves, and that gets complicated. Based on patient surveys I’ve done, people don’t really want private skin care labels. I think they feel very suspicious of them. My system solves that problem by helping consumers realize the doctor isn’t pretending they invented these products. This is a more honest approach, in my opinion. That might be controversial, but that’s how I feel.
SAN: It sounds like the cosmetic manufacturers would favor this if you like their line.
Dr. Baumann: My system favors good technology. So the charlatans who are trying to sell stem cell therapies or peptides that don’t work aren’t going to like my system. My system favors the geniuses in the lab who don’t know how to get their technology out there, and I know a lot of them. We’ll be able to find that technology and then launch it through dermatology practices. This helps the genius underdogs who don’t know what to do with what they’ve discovered.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (McGraw-Hill, New York 2002) , and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” will be published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy,Topix Pharmaceuticals, and Unilever.
On Twitter @whitneymcknight
Dermatology has yet to conquer the cosmetic corner of the specialty. That’s according to Dr. Leslie S. Baumann, of the Miami-based, Skin Type Solutions, who explains a new franchise model she says will help “put dermatology back in the hands of dermatologists.”
In this interview, Dr. Baumann, who writes the Cosmeceutical Critique column for Skin & Allergy News, explains her new franchise method for selling skin care products in the dermatologist’s office, and why she thinks it will “disrupt” business as usual in the retail skin care marketplace, including for online retailers.
The following is an edited transcript of the interview, which you can hear in its entirety here.
SAN: Welcome, Dr. Baumann. You recently wrote that you were helping to “put dermatology back in the hands of dermatologists.” Can you expand on that?
Dr. Baumann: I find that most patients really don’t buy their products from dermatologists. They buy them from Sephora, CVS, or the department store, but it’s dermatologists who have years of training about skin, and consumers don’t realize that the dermatologist’s office is the natural choice for where to buy their skin care products. I know that in some parts of the country, access to dermatologists is limited. I think that if a person can see a dermatologist, then that should be where they go for products as well, but that’s not happening. I think dermatologists have done a poor job in getting the word out that we’re the complete skin care experts.
SAN: So, you’ve created a business model to help with this. Please explain how it works.
Dr. Baumann: I love the science of skin care ingredients, and I want to prescribe the right skin care products to my patients. I was at the University of Miami for about 15 years. We didn’t have a huge staff, and I went through the whole skin care evaluation process and created the correct regimen myself. The original evaluation regimen took about 45 minutes, and I realized that patients would probably purchase products from me at first, but the next time, they would buy them somewhere else.
So, I streamlined my approach to make it faster and not require a lot of staff. I determined that I needed to divide patients into skin types. I came up with 16 main skin types, based on four issues: oily vs. dry; sensitive vs. resistant; pigmented vs. unpigmented; and whether the skin is wrinkle-prone. This was the basis of my book, “The Skin Type Solution,” which was a New York Times Bestseller. This showed me that consumers really care about skin products.
The biggest challenge was to get my staff to be able to correctly diagnose the skin type. It took me years to develop my questionnaire, and we have done all kinds of clinical trials to validate it. Now my staff can administer that questionnaire on an iPad, and it automatically calculates the skin type.
The next step was figuring out which products work for each skin type, and then presetting regimens. I found the best products from the companies that had the best technology, and then I tested those on different skin types. I might have five or six products from four or five different brands for one skin type.
SAN: And you are confident that mixing products from the different brands and all the different ingredients won’t somehow irritate the patient’s skin?
Dr. Baumann: I have done a lot of research on cosmetic ingredients; that’s really my core competency. One company might have the best sunscreen technology, but that doesn’t mean they have the best retinol technology, yet every brand feels that pressure to have lots of different products. However, because I do the research trials for the companies, I know each one’s best technology. So, I find the best technology and apply it like a Rubik’s Cube to each skin type’s need, so they always get the best product, and then I test the product. I have been testing this method since 2005.
SAN: What about eponymous skin care lines?
Dr. Baumann: When you go private label, you hire a formulator the way you’d hire a personal chef. That person may not be the scientist who invented that technology. When you have a private label, there is no way you can achieve the same results as you can when you are sourcing products from the best scientists in the world. I know who these people are because I do the research trials. I also know the ingredient supply companies who have the basic scientists in the lab, tinkering with the cell cultures and looking at the mitochondria, so I know where those basic ingredients go. Because I have a large following after the sale of my book and my online blog, I can get volume discounts from the companies. I created a store in the office, and each shelf is color-coded by skin type so it’s easy to know what products to buy,
My friend, who franchised Blimpie’s sandwich shops, was in my office one day watching customers take everything off the shelf and purchase all of it, and he convinced me to franchise it.
It’s important to understand why we chose this model. If you are a cosmetics company, you are not allowed to tell doctors how much to charge for the products because it violates antitrust laws. This allows some people to buy products and then dump them cheaply on the Internet. We control that by having the doctors sign an agreement that they will not sell the products online, and if they do, we cut them off.
SAN: How do you ensure that the products in your franchises aren’t elsewhere on the Internet?
Dr. Baumann: The plan is that once we have enough doctors in the program, we will negotiate with the manufacturers to create products that are exclusive to us.
SAN: Is this a revolution?
Dr. Baumann: That’s the point. It’s putting the power back in the hands of the dermatologist because we are the authorities on skin care. We need to be the ones that people get all the best news and products from first. Just imagine a world where a great new skin care technology comes out, and the only place you can get it is from the dermatologists. That’s going to drive so much business into the dermatologist’s office, and will help dermatologists build their general and cosmetic practice. The beauty of my system is that it trains your staff to identify the most appropriate products for each patient, but I have selected those products. I tested this method in six dermatologists’ offices, and we found that the product exchange rate went from about 35% to 3%. So, when your patient has a better outcome, they are more likely to trust you, and more likely to refer friends to you.
SAN: But what if a patient doesn’t have access to a dermatologist and the model for purchasing skin care products does change? It sounds like it will be harder for them to get the skin care they need.
Dr. Baumann: That is a great question. It’s a problem I have to figure out how to solve. Right now we’re considering Skype consults. This is not considered practicing medicine, so you can do it across state lines.
SAN: Do you plan to franchise dermatologists only or would you also sell the franchise rights to medical spas and physicians other than dermatologists?
Dr. Baumann: That’s really against my philosophy. I want to bring skin care back to the dermatologist. Did you know that only 15 percent of dermatologists sell skin care products in their practice? It’s difficult for them to set the system up and buy products from the different companies. My company streamlines that process. Another reason many dermatologists don’t sell products is because of ethical concerns. I believe if you are offering patients the best products for their skin types, products they can’t get somewhere else, and at the best price, then that is ethical. When you’re just selling things to make money by taking advantage of the patient-doctor relationship, then I am absolutely against it.
SAN: Why might a dermatologist turn away from this franchise model?
Dr. Baumann: There is no reason not to do it, because the startup costs are minimal. The only reason they might not want to do it is if they have their own skin care brand. A doctor could still sell his or her own brand, but they wouldn’t be able to have it on the Skin Type Solutions shelves, and that gets complicated. Based on patient surveys I’ve done, people don’t really want private skin care labels. I think they feel very suspicious of them. My system solves that problem by helping consumers realize the doctor isn’t pretending they invented these products. This is a more honest approach, in my opinion. That might be controversial, but that’s how I feel.
SAN: It sounds like the cosmetic manufacturers would favor this if you like their line.
Dr. Baumann: My system favors good technology. So the charlatans who are trying to sell stem cell therapies or peptides that don’t work aren’t going to like my system. My system favors the geniuses in the lab who don’t know how to get their technology out there, and I know a lot of them. We’ll be able to find that technology and then launch it through dermatology practices. This helps the genius underdogs who don’t know what to do with what they’ve discovered.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (McGraw-Hill, New York 2002) , and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” will be published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy,Topix Pharmaceuticals, and Unilever.
On Twitter @whitneymcknight
Lungs donated after asphyxiation, drowning found suitable for transplant
Patients who received lung transplants from donors who died of asphyxiation or drowning had similar survival rates and clinical outcomes as those whose donors died of other causes, according to a large registry analysis in the October issue of The Annals of Thoracic Surgery.
“Asphyxiation or drowning as a donor cause of death should not automatically exclude the organ from transplant consideration,” said Dr. Bryan A. Whitson of Ohio State University, Columbus, and his associates. Donor death from asphyxiation or drowning did not significantly affect rates of airway dehiscence, transplant rejection, posttransplant stroke or dialysis, or long-term survival.
Lungs donated after asphyxiation or drowning should be carefully evaluated for parenchymal injury, microbial contamination, and the possibility of primary graft dysfunction, the researchers cautioned. For example, asphyxiation and drowning can alter lung surfactant levels (Ann. Thorac. Surg. 2014;98:1145-51).
The analysis included 18,205 U.S. adults who underwent lung transplantation between 1987 and 2010, including 309 patients whose donors had reportedly died from drowning or asphyxiation. Patients were identified from the UNOS/OPTN STAR (United Network for Organ Sharing/Organ Procurement and Transplantation Network Standard Transplant Analysis and Research) database, which is overseen by the U.S. Department of Health & Human Services.
Ten-year survival curves did not vary based on donor cause of death, either when analyzed individually or when asphyxiation or drowning was compared with all other causes (P = .52), the researchers said. In fact, pulmonary deaths were significantly less common (5.8%) among recipients whose donors had died of asphyxiation or drowning compared with other causes (9.5%; P = .02).
Donor death from drowning and asphyxiation also did not significantly affect rates of treatment for transplant rejection within the first year after surgery (50.8% vs. 47.4% for all other causes of donor death), or posttransplant rates of stroke (0.7% vs. 2.1%) or dialysis (5.4% vs. 5.2%), the investigators said. However, hospital length of stay averaged 0.8 days longer when donors had died of asphyxiation or drowning compared with other causes (27.3 vs. 26.5 days; P < 0.001).
Dr. Jacques-Pierre Fontaine comments: The shortage of suitable donor lungs remains an important problem. Less than 20% of lungs being offered for donation are being used. The notion that asphyxiation or drowning excludes a patient from being a potential donor is widespread among some clinicians.
This extensive retrospective review of the robust UNOS Database demonstrates that recipients of lungs from donors who died from asphyxiation or drowning have similar 10-year survival and post-transplant complication rates. In carefully selected donors, these lungs may be successfully used. Furthermore, "optimization" of marginal donor lungs may become more prevalent as ex-vivo lung perfusion technology evolves.
Dr. Fontaine specializes in thoracic surgery at the Moffitt Cancer Center in Tampa, Florida.
Dr. Jacques-Pierre Fontaine comments: The shortage of suitable donor lungs remains an important problem. Less than 20% of lungs being offered for donation are being used. The notion that asphyxiation or drowning excludes a patient from being a potential donor is widespread among some clinicians.
This extensive retrospective review of the robust UNOS Database demonstrates that recipients of lungs from donors who died from asphyxiation or drowning have similar 10-year survival and post-transplant complication rates. In carefully selected donors, these lungs may be successfully used. Furthermore, "optimization" of marginal donor lungs may become more prevalent as ex-vivo lung perfusion technology evolves.
Dr. Fontaine specializes in thoracic surgery at the Moffitt Cancer Center in Tampa, Florida.
Dr. Jacques-Pierre Fontaine comments: The shortage of suitable donor lungs remains an important problem. Less than 20% of lungs being offered for donation are being used. The notion that asphyxiation or drowning excludes a patient from being a potential donor is widespread among some clinicians.
This extensive retrospective review of the robust UNOS Database demonstrates that recipients of lungs from donors who died from asphyxiation or drowning have similar 10-year survival and post-transplant complication rates. In carefully selected donors, these lungs may be successfully used. Furthermore, "optimization" of marginal donor lungs may become more prevalent as ex-vivo lung perfusion technology evolves.
Dr. Fontaine specializes in thoracic surgery at the Moffitt Cancer Center in Tampa, Florida.
Patients who received lung transplants from donors who died of asphyxiation or drowning had similar survival rates and clinical outcomes as those whose donors died of other causes, according to a large registry analysis in the October issue of The Annals of Thoracic Surgery.
“Asphyxiation or drowning as a donor cause of death should not automatically exclude the organ from transplant consideration,” said Dr. Bryan A. Whitson of Ohio State University, Columbus, and his associates. Donor death from asphyxiation or drowning did not significantly affect rates of airway dehiscence, transplant rejection, posttransplant stroke or dialysis, or long-term survival.
Lungs donated after asphyxiation or drowning should be carefully evaluated for parenchymal injury, microbial contamination, and the possibility of primary graft dysfunction, the researchers cautioned. For example, asphyxiation and drowning can alter lung surfactant levels (Ann. Thorac. Surg. 2014;98:1145-51).
The analysis included 18,205 U.S. adults who underwent lung transplantation between 1987 and 2010, including 309 patients whose donors had reportedly died from drowning or asphyxiation. Patients were identified from the UNOS/OPTN STAR (United Network for Organ Sharing/Organ Procurement and Transplantation Network Standard Transplant Analysis and Research) database, which is overseen by the U.S. Department of Health & Human Services.
Ten-year survival curves did not vary based on donor cause of death, either when analyzed individually or when asphyxiation or drowning was compared with all other causes (P = .52), the researchers said. In fact, pulmonary deaths were significantly less common (5.8%) among recipients whose donors had died of asphyxiation or drowning compared with other causes (9.5%; P = .02).
Donor death from drowning and asphyxiation also did not significantly affect rates of treatment for transplant rejection within the first year after surgery (50.8% vs. 47.4% for all other causes of donor death), or posttransplant rates of stroke (0.7% vs. 2.1%) or dialysis (5.4% vs. 5.2%), the investigators said. However, hospital length of stay averaged 0.8 days longer when donors had died of asphyxiation or drowning compared with other causes (27.3 vs. 26.5 days; P < 0.001).
Patients who received lung transplants from donors who died of asphyxiation or drowning had similar survival rates and clinical outcomes as those whose donors died of other causes, according to a large registry analysis in the October issue of The Annals of Thoracic Surgery.
“Asphyxiation or drowning as a donor cause of death should not automatically exclude the organ from transplant consideration,” said Dr. Bryan A. Whitson of Ohio State University, Columbus, and his associates. Donor death from asphyxiation or drowning did not significantly affect rates of airway dehiscence, transplant rejection, posttransplant stroke or dialysis, or long-term survival.
Lungs donated after asphyxiation or drowning should be carefully evaluated for parenchymal injury, microbial contamination, and the possibility of primary graft dysfunction, the researchers cautioned. For example, asphyxiation and drowning can alter lung surfactant levels (Ann. Thorac. Surg. 2014;98:1145-51).
The analysis included 18,205 U.S. adults who underwent lung transplantation between 1987 and 2010, including 309 patients whose donors had reportedly died from drowning or asphyxiation. Patients were identified from the UNOS/OPTN STAR (United Network for Organ Sharing/Organ Procurement and Transplantation Network Standard Transplant Analysis and Research) database, which is overseen by the U.S. Department of Health & Human Services.
Ten-year survival curves did not vary based on donor cause of death, either when analyzed individually or when asphyxiation or drowning was compared with all other causes (P = .52), the researchers said. In fact, pulmonary deaths were significantly less common (5.8%) among recipients whose donors had died of asphyxiation or drowning compared with other causes (9.5%; P = .02).
Donor death from drowning and asphyxiation also did not significantly affect rates of treatment for transplant rejection within the first year after surgery (50.8% vs. 47.4% for all other causes of donor death), or posttransplant rates of stroke (0.7% vs. 2.1%) or dialysis (5.4% vs. 5.2%), the investigators said. However, hospital length of stay averaged 0.8 days longer when donors had died of asphyxiation or drowning compared with other causes (27.3 vs. 26.5 days; P < 0.001).
Key clinical point: Lung transplant recipients had good outcomes and long-term survival in cases involving donors who died of asphyxiation or drowning.
Major finding: Pulmonary deaths were significantly less common (5.8%) among recipients whose donors had died of asphyxiation or drowning compared with other causes (9.5%; P = .02).
Data source: Retrospective registry analysis of 18,250 lung transplant recipients.
Disclosures: The authors did not report funding sources or conflicts of interest.
Is Gustav next?
Thursday was a rough day. Not for me, but for my front-desk personnel. I wouldn’t even have known about it, if Nilda hadn’t clued me in.
“I’m a preschool teacher,” she said, after asking about Botox for underarm sweating. “So I have a lot of patience. But your front-desk people are amazing.”
“What do you mean?” I asked her.
“This lady walks in without an appointment,” she said. “Several people are trying to check in, and she just waltzes over and says, ‘The doctor said I could come in whenever I wanted.’”
I smiled. “That’s Harriet. She’s worried that she has an infection. We make allowances for people over 90.”
“And then there was a woman who didn’t even want to be seen,” Nilda went on. “She’d gotten a bill she didn’t approve of, and she kept going on and on.
“Your secretary said she would call the insurance company to look into it, but the woman kept saying, ‘I’ve been a patient here for 20 years, and there’s never been a problem with the insurance.’
“It would have been fine for your secretary to politely tell the woman she’d take care of it, but now she had to get back to patients trying to register. But she didn’t lose her cool, just kept repeating that she would call the patient’s insurer and let her know.”
I thanked Nilda very much for the feedback. “Most people don’t bother to comment unless they have a complaint,” I said, “so I appreciate your taking the time to say something positive. I’ll be sure to pass it on.”
“And I thought preschool children were tough,” said Nilda.
At lunch, I asked the staff what had been going on.
“Must be a full moon,” said Irma, her eyes twinkling. “The registration desk was like a zoo, what with all the new patients and the old ones who hadn’t been here in years re-registering. And in the middle of it all, a lady whose husband had already checked in and sat down kept calling out, ‘Is Gustav next’?”
“The man sitting next to her – must have been Gustav himself – grumbled at her to please keep quiet, but she kept calling out, ‘Is Gustav next?’
“Then Dorit comes in, complaining about her bill. It turns out that her insurance changed in May, but she had forgotten about it, and she didn’t understand what the change would mean for payment. I told her I would call her insurer and find out.
“ ‘I’ve been a patient here for 20 years,’ she kept saying. ‘So don’t overcharge me!’
“I told her I would let her know what her insurer said and promised that we wouldn’t overcharge her on the copay.
“In the meantime, Harriet, the walk-in, kept standing in front of the window saying, ‘Doctor Rockoff said I could come in whenever I want, and my son-in-law took off work to bring me in and he’s waiting outside.’
“And while Harriet was saying that, the lady in the chair kept calling out, ‘Is Gustav next? Is Gustav next?’ ”
I smiled to myself, trying to think of which absurdist playwright could do justice to what went on that morning in my waiting room, and maybe on lots of mornings and afternoons in waiting rooms everywhere.
“You should know,” I told Irma and the rest of the staff, “that one of the patients commented on how well you all did. You handled all that insanity while staying cool and polite. Great job!”
Of course, we have to stay vigilant for rude or discourteous behavior on the part of our staff. But that same staff often protects us from some pretty unreasonable behavior that patients sometimes can throw at them. It makes sense to make a point of telling our front-desk representatives from time to time how much we appreciate the graceful way they handle the guff and allow us to focus on each patient in the exam room.
Meantime, I am working on my new drama, a sequel to Waiting for Godot. I will call it, Is Gustav Next?
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Skin & Allergy News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years.
Thursday was a rough day. Not for me, but for my front-desk personnel. I wouldn’t even have known about it, if Nilda hadn’t clued me in.
“I’m a preschool teacher,” she said, after asking about Botox for underarm sweating. “So I have a lot of patience. But your front-desk people are amazing.”
“What do you mean?” I asked her.
“This lady walks in without an appointment,” she said. “Several people are trying to check in, and she just waltzes over and says, ‘The doctor said I could come in whenever I wanted.’”
I smiled. “That’s Harriet. She’s worried that she has an infection. We make allowances for people over 90.”
“And then there was a woman who didn’t even want to be seen,” Nilda went on. “She’d gotten a bill she didn’t approve of, and she kept going on and on.
“Your secretary said she would call the insurance company to look into it, but the woman kept saying, ‘I’ve been a patient here for 20 years, and there’s never been a problem with the insurance.’
“It would have been fine for your secretary to politely tell the woman she’d take care of it, but now she had to get back to patients trying to register. But she didn’t lose her cool, just kept repeating that she would call the patient’s insurer and let her know.”
I thanked Nilda very much for the feedback. “Most people don’t bother to comment unless they have a complaint,” I said, “so I appreciate your taking the time to say something positive. I’ll be sure to pass it on.”
“And I thought preschool children were tough,” said Nilda.
At lunch, I asked the staff what had been going on.
“Must be a full moon,” said Irma, her eyes twinkling. “The registration desk was like a zoo, what with all the new patients and the old ones who hadn’t been here in years re-registering. And in the middle of it all, a lady whose husband had already checked in and sat down kept calling out, ‘Is Gustav next’?”
“The man sitting next to her – must have been Gustav himself – grumbled at her to please keep quiet, but she kept calling out, ‘Is Gustav next?’
“Then Dorit comes in, complaining about her bill. It turns out that her insurance changed in May, but she had forgotten about it, and she didn’t understand what the change would mean for payment. I told her I would call her insurer and find out.
“ ‘I’ve been a patient here for 20 years,’ she kept saying. ‘So don’t overcharge me!’
“I told her I would let her know what her insurer said and promised that we wouldn’t overcharge her on the copay.
“In the meantime, Harriet, the walk-in, kept standing in front of the window saying, ‘Doctor Rockoff said I could come in whenever I want, and my son-in-law took off work to bring me in and he’s waiting outside.’
“And while Harriet was saying that, the lady in the chair kept calling out, ‘Is Gustav next? Is Gustav next?’ ”
I smiled to myself, trying to think of which absurdist playwright could do justice to what went on that morning in my waiting room, and maybe on lots of mornings and afternoons in waiting rooms everywhere.
“You should know,” I told Irma and the rest of the staff, “that one of the patients commented on how well you all did. You handled all that insanity while staying cool and polite. Great job!”
Of course, we have to stay vigilant for rude or discourteous behavior on the part of our staff. But that same staff often protects us from some pretty unreasonable behavior that patients sometimes can throw at them. It makes sense to make a point of telling our front-desk representatives from time to time how much we appreciate the graceful way they handle the guff and allow us to focus on each patient in the exam room.
Meantime, I am working on my new drama, a sequel to Waiting for Godot. I will call it, Is Gustav Next?
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Skin & Allergy News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years.
Thursday was a rough day. Not for me, but for my front-desk personnel. I wouldn’t even have known about it, if Nilda hadn’t clued me in.
“I’m a preschool teacher,” she said, after asking about Botox for underarm sweating. “So I have a lot of patience. But your front-desk people are amazing.”
“What do you mean?” I asked her.
“This lady walks in without an appointment,” she said. “Several people are trying to check in, and she just waltzes over and says, ‘The doctor said I could come in whenever I wanted.’”
I smiled. “That’s Harriet. She’s worried that she has an infection. We make allowances for people over 90.”
“And then there was a woman who didn’t even want to be seen,” Nilda went on. “She’d gotten a bill she didn’t approve of, and she kept going on and on.
“Your secretary said she would call the insurance company to look into it, but the woman kept saying, ‘I’ve been a patient here for 20 years, and there’s never been a problem with the insurance.’
“It would have been fine for your secretary to politely tell the woman she’d take care of it, but now she had to get back to patients trying to register. But she didn’t lose her cool, just kept repeating that she would call the patient’s insurer and let her know.”
I thanked Nilda very much for the feedback. “Most people don’t bother to comment unless they have a complaint,” I said, “so I appreciate your taking the time to say something positive. I’ll be sure to pass it on.”
“And I thought preschool children were tough,” said Nilda.
At lunch, I asked the staff what had been going on.
“Must be a full moon,” said Irma, her eyes twinkling. “The registration desk was like a zoo, what with all the new patients and the old ones who hadn’t been here in years re-registering. And in the middle of it all, a lady whose husband had already checked in and sat down kept calling out, ‘Is Gustav next’?”
“The man sitting next to her – must have been Gustav himself – grumbled at her to please keep quiet, but she kept calling out, ‘Is Gustav next?’
“Then Dorit comes in, complaining about her bill. It turns out that her insurance changed in May, but she had forgotten about it, and she didn’t understand what the change would mean for payment. I told her I would call her insurer and find out.
“ ‘I’ve been a patient here for 20 years,’ she kept saying. ‘So don’t overcharge me!’
“I told her I would let her know what her insurer said and promised that we wouldn’t overcharge her on the copay.
“In the meantime, Harriet, the walk-in, kept standing in front of the window saying, ‘Doctor Rockoff said I could come in whenever I want, and my son-in-law took off work to bring me in and he’s waiting outside.’
“And while Harriet was saying that, the lady in the chair kept calling out, ‘Is Gustav next? Is Gustav next?’ ”
I smiled to myself, trying to think of which absurdist playwright could do justice to what went on that morning in my waiting room, and maybe on lots of mornings and afternoons in waiting rooms everywhere.
“You should know,” I told Irma and the rest of the staff, “that one of the patients commented on how well you all did. You handled all that insanity while staying cool and polite. Great job!”
Of course, we have to stay vigilant for rude or discourteous behavior on the part of our staff. But that same staff often protects us from some pretty unreasonable behavior that patients sometimes can throw at them. It makes sense to make a point of telling our front-desk representatives from time to time how much we appreciate the graceful way they handle the guff and allow us to focus on each patient in the exam room.
Meantime, I am working on my new drama, a sequel to Waiting for Godot. I will call it, Is Gustav Next?
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Skin & Allergy News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years.
Ibrutinib fights chronic GVHD in mice

Preclinical research suggests the anticancer agent ibrutinib can ameliorate chronic graft-vs-host disease (GVHD).
Ibrutinib reduced the symptoms and progression of chronic GVHD in mouse models, and it decreased the activation of T and B cells isolated from patients with chronic GVHD.
These results indicate that T and B cells drive chronic GVHD and ibrutinib should be explored as a treatment option for human GVHD, according to investigators.
Bruce Blazar, MD, of the University of Minnesota in Minneapolis, and his colleagues described this work in The Journal of Clinical Investigation.
The team noted that CD4+ T cells and B cells mediate chronic GVHD, so it follows that targeting these populations might inhibit chronic GVHD pathogenesis.
As ibrutinib targets Th2 cells and B cells, the investigators decided to test whether the drug could reverse established chronic GVHD in 2 mouse models—a model of T cell–driven sclerodermatous chronic GVHD and an alloantibody-driven, multiorgan-system chronic GVHD model that induces bronchiolar obliterans.
Sclerodermatous chronic GVHD
The researchers first found that ibrutinib can reduce the clinical signs of sclerodermatous chronic GVHD. Fourteen days after treatment began, vehicle-treated mice and those that received cyclosporine exhibited sclerodermatous lesions, hair loss, hunched posture, and scabbing. But ibrutinib-treated mice did not.
Animals treated with ibrutinib had a significantly lower overall intensity of chronic GVHD—as measured by body weight, posture, mobility, hair loss, skin lesions, and vitality—than vehicle-treated mice (P=0.0184).
Ibrutinib also extended the median time to chronic GVHD progression by 14 days when compared to control. Thirty-three percent of ibrutinib-treated mice remained progression-free, compared to 12% of vehicle-treated mice (P<0.02).
Overall survival was 100% among ibrutinib-treated mice, 82% for cyclosporine-treated mice, and 88% for vehicle-treated mice.
The investigators also discovered that prolonged administration of ibrutinib is needed to reap the maximum therapeutic benefit in sclerodermatous chronic GVHD. Withdrawing treatment at day 60 enabled clinical breakthrough of chronic GVHD in a single mouse.
Alloantibody-driven chronic GVHD
In this model, ibrutinib inhibited the development of bronchiolar obliterans, as measured by pulmonary resistance (P=0.0090), elastance (P=0.0019), and compliance (P=0.0071).
In addition, there was less peribroncheolar collagen fibrosis among ibrutinib-treated animals and a significant reduction in pulmonary fibrosis (P<0.0001) compared to vehicle-treated controls.
However, continued therapy was necessary to see a long-term benefit with ibrutinib. Prophylactic ibrutinib given from day -2 to day 28 was not effective against chronic GVHD or bronchiolar obliterans.
Lastly, the investigators found that ibrutinib reduced the overall size, cellularity, and number of germinal center reactions (P<0.001), and the drug eliminated pulmonary immunoglobulin deposition (P<0.001).
Verifying the results
Additional experiments showed that mice lacking Bruton tyrosine kinase and IL-2 inducible T-cell kinase (both of which are inhibited by ibrutinib) did not develop chronic GVHD, which suggests these molecules are necessary for the condition to occur.
The investigators also discovered that ibrutinib reduced the activation of T and B cells from patients with active chronic GVHD.
CD4+ T cells pretreated with ibrutinib had lower surface expression of CD69 after ex vivo T-cell receptor stimulation using anti-CD3 (P=0.033). And purified B cells pretreated with ibrutinib showed lower levels of pBTK-Y223, pPLCγ2-Y1217, and pERK1/2.
Dr Blazar and his colleagues said these results indicate that B cells and T cells drive chronic GVHD and suggest that ibrutinib should be considered for testing in clinical trials of chronic GVHD. ![]()

Preclinical research suggests the anticancer agent ibrutinib can ameliorate chronic graft-vs-host disease (GVHD).
Ibrutinib reduced the symptoms and progression of chronic GVHD in mouse models, and it decreased the activation of T and B cells isolated from patients with chronic GVHD.
These results indicate that T and B cells drive chronic GVHD and ibrutinib should be explored as a treatment option for human GVHD, according to investigators.
Bruce Blazar, MD, of the University of Minnesota in Minneapolis, and his colleagues described this work in The Journal of Clinical Investigation.
The team noted that CD4+ T cells and B cells mediate chronic GVHD, so it follows that targeting these populations might inhibit chronic GVHD pathogenesis.
As ibrutinib targets Th2 cells and B cells, the investigators decided to test whether the drug could reverse established chronic GVHD in 2 mouse models—a model of T cell–driven sclerodermatous chronic GVHD and an alloantibody-driven, multiorgan-system chronic GVHD model that induces bronchiolar obliterans.
Sclerodermatous chronic GVHD
The researchers first found that ibrutinib can reduce the clinical signs of sclerodermatous chronic GVHD. Fourteen days after treatment began, vehicle-treated mice and those that received cyclosporine exhibited sclerodermatous lesions, hair loss, hunched posture, and scabbing. But ibrutinib-treated mice did not.
Animals treated with ibrutinib had a significantly lower overall intensity of chronic GVHD—as measured by body weight, posture, mobility, hair loss, skin lesions, and vitality—than vehicle-treated mice (P=0.0184).
Ibrutinib also extended the median time to chronic GVHD progression by 14 days when compared to control. Thirty-three percent of ibrutinib-treated mice remained progression-free, compared to 12% of vehicle-treated mice (P<0.02).
Overall survival was 100% among ibrutinib-treated mice, 82% for cyclosporine-treated mice, and 88% for vehicle-treated mice.
The investigators also discovered that prolonged administration of ibrutinib is needed to reap the maximum therapeutic benefit in sclerodermatous chronic GVHD. Withdrawing treatment at day 60 enabled clinical breakthrough of chronic GVHD in a single mouse.
Alloantibody-driven chronic GVHD
In this model, ibrutinib inhibited the development of bronchiolar obliterans, as measured by pulmonary resistance (P=0.0090), elastance (P=0.0019), and compliance (P=0.0071).
In addition, there was less peribroncheolar collagen fibrosis among ibrutinib-treated animals and a significant reduction in pulmonary fibrosis (P<0.0001) compared to vehicle-treated controls.
However, continued therapy was necessary to see a long-term benefit with ibrutinib. Prophylactic ibrutinib given from day -2 to day 28 was not effective against chronic GVHD or bronchiolar obliterans.
Lastly, the investigators found that ibrutinib reduced the overall size, cellularity, and number of germinal center reactions (P<0.001), and the drug eliminated pulmonary immunoglobulin deposition (P<0.001).
Verifying the results
Additional experiments showed that mice lacking Bruton tyrosine kinase and IL-2 inducible T-cell kinase (both of which are inhibited by ibrutinib) did not develop chronic GVHD, which suggests these molecules are necessary for the condition to occur.
The investigators also discovered that ibrutinib reduced the activation of T and B cells from patients with active chronic GVHD.
CD4+ T cells pretreated with ibrutinib had lower surface expression of CD69 after ex vivo T-cell receptor stimulation using anti-CD3 (P=0.033). And purified B cells pretreated with ibrutinib showed lower levels of pBTK-Y223, pPLCγ2-Y1217, and pERK1/2.
Dr Blazar and his colleagues said these results indicate that B cells and T cells drive chronic GVHD and suggest that ibrutinib should be considered for testing in clinical trials of chronic GVHD. ![]()

Preclinical research suggests the anticancer agent ibrutinib can ameliorate chronic graft-vs-host disease (GVHD).
Ibrutinib reduced the symptoms and progression of chronic GVHD in mouse models, and it decreased the activation of T and B cells isolated from patients with chronic GVHD.
These results indicate that T and B cells drive chronic GVHD and ibrutinib should be explored as a treatment option for human GVHD, according to investigators.
Bruce Blazar, MD, of the University of Minnesota in Minneapolis, and his colleagues described this work in The Journal of Clinical Investigation.
The team noted that CD4+ T cells and B cells mediate chronic GVHD, so it follows that targeting these populations might inhibit chronic GVHD pathogenesis.
As ibrutinib targets Th2 cells and B cells, the investigators decided to test whether the drug could reverse established chronic GVHD in 2 mouse models—a model of T cell–driven sclerodermatous chronic GVHD and an alloantibody-driven, multiorgan-system chronic GVHD model that induces bronchiolar obliterans.
Sclerodermatous chronic GVHD
The researchers first found that ibrutinib can reduce the clinical signs of sclerodermatous chronic GVHD. Fourteen days after treatment began, vehicle-treated mice and those that received cyclosporine exhibited sclerodermatous lesions, hair loss, hunched posture, and scabbing. But ibrutinib-treated mice did not.
Animals treated with ibrutinib had a significantly lower overall intensity of chronic GVHD—as measured by body weight, posture, mobility, hair loss, skin lesions, and vitality—than vehicle-treated mice (P=0.0184).
Ibrutinib also extended the median time to chronic GVHD progression by 14 days when compared to control. Thirty-three percent of ibrutinib-treated mice remained progression-free, compared to 12% of vehicle-treated mice (P<0.02).
Overall survival was 100% among ibrutinib-treated mice, 82% for cyclosporine-treated mice, and 88% for vehicle-treated mice.
The investigators also discovered that prolonged administration of ibrutinib is needed to reap the maximum therapeutic benefit in sclerodermatous chronic GVHD. Withdrawing treatment at day 60 enabled clinical breakthrough of chronic GVHD in a single mouse.
Alloantibody-driven chronic GVHD
In this model, ibrutinib inhibited the development of bronchiolar obliterans, as measured by pulmonary resistance (P=0.0090), elastance (P=0.0019), and compliance (P=0.0071).
In addition, there was less peribroncheolar collagen fibrosis among ibrutinib-treated animals and a significant reduction in pulmonary fibrosis (P<0.0001) compared to vehicle-treated controls.
However, continued therapy was necessary to see a long-term benefit with ibrutinib. Prophylactic ibrutinib given from day -2 to day 28 was not effective against chronic GVHD or bronchiolar obliterans.
Lastly, the investigators found that ibrutinib reduced the overall size, cellularity, and number of germinal center reactions (P<0.001), and the drug eliminated pulmonary immunoglobulin deposition (P<0.001).
Verifying the results
Additional experiments showed that mice lacking Bruton tyrosine kinase and IL-2 inducible T-cell kinase (both of which are inhibited by ibrutinib) did not develop chronic GVHD, which suggests these molecules are necessary for the condition to occur.
The investigators also discovered that ibrutinib reduced the activation of T and B cells from patients with active chronic GVHD.
CD4+ T cells pretreated with ibrutinib had lower surface expression of CD69 after ex vivo T-cell receptor stimulation using anti-CD3 (P=0.033). And purified B cells pretreated with ibrutinib showed lower levels of pBTK-Y223, pPLCγ2-Y1217, and pERK1/2.
Dr Blazar and his colleagues said these results indicate that B cells and T cells drive chronic GVHD and suggest that ibrutinib should be considered for testing in clinical trials of chronic GVHD. ![]()
Group detects new steps in hematopoiesis

in the bone marrow
Researchers say they have discovered previously undetected steps in hematopoiesis, establishing that a highly complex series of events determine the fate of closely related populations of blood progenitor cells.
Their study revealed thousands of differences in gene expression between blood cell types.
These differences result from many specific events that are crucial for normal blood development, and errors in this process can lead to blood disorders.
The researchers described their work in Science.
The team sequenced RNA from 8 primary human hematopoietic progenitor populations representing the classical myeloid commitment stages of hematopoiesis and the main lymphoid stage.
This revealed 6711 genes and 10,724 transcripts enriched in non-protein-coding elements at early stages of differentiation.
The researchers also discovered the extent to which RNA is cut and pasted together in different ways during hematopoiesis, leading to specific forms of proteins for each of these stages.
“We have identified thousands of novel places where the RNA is processed in an alternative way,” said study author Willem Ouwehand, MD, PhD, of the University of Cambridge in the UK.
Specifically, the team identified 7881 novel splice junctions and 2301 differentially used alternative splicing events enriched in genes involved in regulatory processes.
“Such events changed the amount, structure, and behavior of proteins derived from a single gene,” said study author Wendy Erber, MD, DPhil, of the University of Western Australia in Crawley.
“Alternative proteins could drive stem cells towards becoming different mature blood cells.”
Until this study, hematopoiesis was relatively well understood at the level of DNA. What was not known was how the genetic information in DNA was then transcribed to generate RNA, leading to protein formation.
The researchers illustrated the importance of alternative RNA splicing in blood cell development by studying the role that 2 different forms of the same transcription factor—NFIB—play in megakaryocyte formation.
The team said their findings could have significant applications for patients with blood disorders. The results could aid the design of diagnostics and new therapies, as well as prove valuable for studies in stem cell transplant and for discovering the genetic basis of rare, inherited hematologic disorders. ![]()

in the bone marrow
Researchers say they have discovered previously undetected steps in hematopoiesis, establishing that a highly complex series of events determine the fate of closely related populations of blood progenitor cells.
Their study revealed thousands of differences in gene expression between blood cell types.
These differences result from many specific events that are crucial for normal blood development, and errors in this process can lead to blood disorders.
The researchers described their work in Science.
The team sequenced RNA from 8 primary human hematopoietic progenitor populations representing the classical myeloid commitment stages of hematopoiesis and the main lymphoid stage.
This revealed 6711 genes and 10,724 transcripts enriched in non-protein-coding elements at early stages of differentiation.
The researchers also discovered the extent to which RNA is cut and pasted together in different ways during hematopoiesis, leading to specific forms of proteins for each of these stages.
“We have identified thousands of novel places where the RNA is processed in an alternative way,” said study author Willem Ouwehand, MD, PhD, of the University of Cambridge in the UK.
Specifically, the team identified 7881 novel splice junctions and 2301 differentially used alternative splicing events enriched in genes involved in regulatory processes.
“Such events changed the amount, structure, and behavior of proteins derived from a single gene,” said study author Wendy Erber, MD, DPhil, of the University of Western Australia in Crawley.
“Alternative proteins could drive stem cells towards becoming different mature blood cells.”
Until this study, hematopoiesis was relatively well understood at the level of DNA. What was not known was how the genetic information in DNA was then transcribed to generate RNA, leading to protein formation.
The researchers illustrated the importance of alternative RNA splicing in blood cell development by studying the role that 2 different forms of the same transcription factor—NFIB—play in megakaryocyte formation.
The team said their findings could have significant applications for patients with blood disorders. The results could aid the design of diagnostics and new therapies, as well as prove valuable for studies in stem cell transplant and for discovering the genetic basis of rare, inherited hematologic disorders. ![]()

in the bone marrow
Researchers say they have discovered previously undetected steps in hematopoiesis, establishing that a highly complex series of events determine the fate of closely related populations of blood progenitor cells.
Their study revealed thousands of differences in gene expression between blood cell types.
These differences result from many specific events that are crucial for normal blood development, and errors in this process can lead to blood disorders.
The researchers described their work in Science.
The team sequenced RNA from 8 primary human hematopoietic progenitor populations representing the classical myeloid commitment stages of hematopoiesis and the main lymphoid stage.
This revealed 6711 genes and 10,724 transcripts enriched in non-protein-coding elements at early stages of differentiation.
The researchers also discovered the extent to which RNA is cut and pasted together in different ways during hematopoiesis, leading to specific forms of proteins for each of these stages.
“We have identified thousands of novel places where the RNA is processed in an alternative way,” said study author Willem Ouwehand, MD, PhD, of the University of Cambridge in the UK.
Specifically, the team identified 7881 novel splice junctions and 2301 differentially used alternative splicing events enriched in genes involved in regulatory processes.
“Such events changed the amount, structure, and behavior of proteins derived from a single gene,” said study author Wendy Erber, MD, DPhil, of the University of Western Australia in Crawley.
“Alternative proteins could drive stem cells towards becoming different mature blood cells.”
Until this study, hematopoiesis was relatively well understood at the level of DNA. What was not known was how the genetic information in DNA was then transcribed to generate RNA, leading to protein formation.
The researchers illustrated the importance of alternative RNA splicing in blood cell development by studying the role that 2 different forms of the same transcription factor—NFIB—play in megakaryocyte formation.
The team said their findings could have significant applications for patients with blood disorders. The results could aid the design of diagnostics and new therapies, as well as prove valuable for studies in stem cell transplant and for discovering the genetic basis of rare, inherited hematologic disorders. ![]()
HHS identifies known and likely carcinogens

Credit: Trevor MacInnis
The US Department of Health and Human Services (HHS) has identified 1 chemical substance as a known human carcinogen and 3 additional substances as likely carcinogens.
Ortho-toluidine, which is used to make rubber chemicals, pesticides, and dyes, has been shown to cause urinary bladder cancer and is now listed as a known human carcinogen.
The 3 substances that are likely to be human carcinogens are 1-bromopropane, cumene, and pentachlorophenol.
1-bromopropane is used as a cleaning solvent and spray adhesive. Cumene is used to make phenol and acetone, and it is found in fuel products and tobacco smoke. Pentachlorophenol is a mixture used to preserve wood.
Exposure to pentachlorophenol is associated with an increased risk of non-Hodgkin lymphoma in humans and solid tumor malignancies in mice. Cumene and 1-bromopropane have been linked to solid tumor malignancies in mice as well.
All 4 substances are listed in the HHS’s 13th Report on Carcinogens, a science-based document prepared by the National Toxicology Program (NTP) that identifies chemical, biological, and physical agents considered to be cancer hazards for people living in the US.
The new report has a total of 243 listings, which includes known carcinogens and substances “reasonably anticipated” to be carcinogens.
“Identifying substances in our environment that can make people vulnerable to cancer will help in prevention efforts,” said Linda Birnbaum, PhD, director of the National Institute of Environmental Health Sciences and the NTP.
“This report provides a valuable resource for health regulatory and research agencies, and it empowers the public with information people can use to reduce exposure to cancer-causing substances.”
New known carcinogen
Since 1983, ortho-toluidine has been listed in the HHS’s Report on Carcinogens as reasonably anticipated to be a human carcinogen. However, new cancer studies led the NTP to reevaluate and reclassify ortho-toluidine. It is now classified as a known human carcinogen, based on clinical studies showing it causes urinary bladder cancer.
Ortho-toluidine is a synthetic chemical produced in other countries and imported into the US by several companies in high volumes. It is primarily used to make rubber chemicals, pesticides, and dyes. It is also used in some consumer and medical products.
People are mainly exposed through the workplace, by skin contact and/or inhalation when using ortho-toluidine. They can also be exposed outside the workplace through sources such as tobacco smoke.
Three new substances likely to be carcinogenic
Pentachlorophenol
Pentachlorophenol and byproducts of its synthesis are complex mixtures of chemicals used as wood preservatives. Because virtually everyone exposed to pentachlorophenol is also exposed to its synthesis byproducts, they were evaluated together.
In the US, pentachlorophenol has been regulated since the 1980s as a restricted-use pesticide. It is used industrially for treating utility poles, wood pilings, fence posts, and lumber or timber for construction.
Most exposure has occurred in settings where workers treat lumber or come in contact with treated lumber. People may also be exposed to this mixture from breathing contaminated air or dust, or from contact with contaminated soil.
Exposure to this mixture was associated with an increased risk of non-Hodgkin lymphoma in clinical studies. In mice, it has been shown to cause tumors in the liver and other organs.
1-bromopropane
1-bromopropane is a liquid used as a solvent in many commercial industries. It is used as a cleaner for optics, electronics, and metals, as well as a solvent for aerosol-applied adhesives such as those used in foam cushion manufacturing.
It is also used in dry cleaning and in solvent sprays for aircraft maintenance. Workers in certain occupations may be more exposed to 1-bromopropane than the general population.
The NTP did not identify any clinical studies that evaluated the relationship between human cancer and exposure to 1-bromopropane. However, inhalation exposure to 1-bromopropane in rodents caused tumors in several organs, including the skin, lungs, and large intestine.
Cumene
Cumene is a flammable and volatile liquid with a gasoline-like odor. It is a natural component of coal tar and petroleum, and is found in tobacco smoke. It is used primarily to make acetone and phenol.
People are mainly exposed to cumene through the environment and in workplaces that use or produce cumene. It can be found in emissions from petroleum products.
Inhalation exposure to cumene caused lung tumors in male and female mice, and liver tumors in female mice. The NTP did not identify any clinical studies evaluating the relationship between cancer and exposure to cumene. ![]()

Credit: Trevor MacInnis
The US Department of Health and Human Services (HHS) has identified 1 chemical substance as a known human carcinogen and 3 additional substances as likely carcinogens.
Ortho-toluidine, which is used to make rubber chemicals, pesticides, and dyes, has been shown to cause urinary bladder cancer and is now listed as a known human carcinogen.
The 3 substances that are likely to be human carcinogens are 1-bromopropane, cumene, and pentachlorophenol.
1-bromopropane is used as a cleaning solvent and spray adhesive. Cumene is used to make phenol and acetone, and it is found in fuel products and tobacco smoke. Pentachlorophenol is a mixture used to preserve wood.
Exposure to pentachlorophenol is associated with an increased risk of non-Hodgkin lymphoma in humans and solid tumor malignancies in mice. Cumene and 1-bromopropane have been linked to solid tumor malignancies in mice as well.
All 4 substances are listed in the HHS’s 13th Report on Carcinogens, a science-based document prepared by the National Toxicology Program (NTP) that identifies chemical, biological, and physical agents considered to be cancer hazards for people living in the US.
The new report has a total of 243 listings, which includes known carcinogens and substances “reasonably anticipated” to be carcinogens.
“Identifying substances in our environment that can make people vulnerable to cancer will help in prevention efforts,” said Linda Birnbaum, PhD, director of the National Institute of Environmental Health Sciences and the NTP.
“This report provides a valuable resource for health regulatory and research agencies, and it empowers the public with information people can use to reduce exposure to cancer-causing substances.”
New known carcinogen
Since 1983, ortho-toluidine has been listed in the HHS’s Report on Carcinogens as reasonably anticipated to be a human carcinogen. However, new cancer studies led the NTP to reevaluate and reclassify ortho-toluidine. It is now classified as a known human carcinogen, based on clinical studies showing it causes urinary bladder cancer.
Ortho-toluidine is a synthetic chemical produced in other countries and imported into the US by several companies in high volumes. It is primarily used to make rubber chemicals, pesticides, and dyes. It is also used in some consumer and medical products.
People are mainly exposed through the workplace, by skin contact and/or inhalation when using ortho-toluidine. They can also be exposed outside the workplace through sources such as tobacco smoke.
Three new substances likely to be carcinogenic
Pentachlorophenol
Pentachlorophenol and byproducts of its synthesis are complex mixtures of chemicals used as wood preservatives. Because virtually everyone exposed to pentachlorophenol is also exposed to its synthesis byproducts, they were evaluated together.
In the US, pentachlorophenol has been regulated since the 1980s as a restricted-use pesticide. It is used industrially for treating utility poles, wood pilings, fence posts, and lumber or timber for construction.
Most exposure has occurred in settings where workers treat lumber or come in contact with treated lumber. People may also be exposed to this mixture from breathing contaminated air or dust, or from contact with contaminated soil.
Exposure to this mixture was associated with an increased risk of non-Hodgkin lymphoma in clinical studies. In mice, it has been shown to cause tumors in the liver and other organs.
1-bromopropane
1-bromopropane is a liquid used as a solvent in many commercial industries. It is used as a cleaner for optics, electronics, and metals, as well as a solvent for aerosol-applied adhesives such as those used in foam cushion manufacturing.
It is also used in dry cleaning and in solvent sprays for aircraft maintenance. Workers in certain occupations may be more exposed to 1-bromopropane than the general population.
The NTP did not identify any clinical studies that evaluated the relationship between human cancer and exposure to 1-bromopropane. However, inhalation exposure to 1-bromopropane in rodents caused tumors in several organs, including the skin, lungs, and large intestine.
Cumene
Cumene is a flammable and volatile liquid with a gasoline-like odor. It is a natural component of coal tar and petroleum, and is found in tobacco smoke. It is used primarily to make acetone and phenol.
People are mainly exposed to cumene through the environment and in workplaces that use or produce cumene. It can be found in emissions from petroleum products.
Inhalation exposure to cumene caused lung tumors in male and female mice, and liver tumors in female mice. The NTP did not identify any clinical studies evaluating the relationship between cancer and exposure to cumene. ![]()

Credit: Trevor MacInnis
The US Department of Health and Human Services (HHS) has identified 1 chemical substance as a known human carcinogen and 3 additional substances as likely carcinogens.
Ortho-toluidine, which is used to make rubber chemicals, pesticides, and dyes, has been shown to cause urinary bladder cancer and is now listed as a known human carcinogen.
The 3 substances that are likely to be human carcinogens are 1-bromopropane, cumene, and pentachlorophenol.
1-bromopropane is used as a cleaning solvent and spray adhesive. Cumene is used to make phenol and acetone, and it is found in fuel products and tobacco smoke. Pentachlorophenol is a mixture used to preserve wood.
Exposure to pentachlorophenol is associated with an increased risk of non-Hodgkin lymphoma in humans and solid tumor malignancies in mice. Cumene and 1-bromopropane have been linked to solid tumor malignancies in mice as well.
All 4 substances are listed in the HHS’s 13th Report on Carcinogens, a science-based document prepared by the National Toxicology Program (NTP) that identifies chemical, biological, and physical agents considered to be cancer hazards for people living in the US.
The new report has a total of 243 listings, which includes known carcinogens and substances “reasonably anticipated” to be carcinogens.
“Identifying substances in our environment that can make people vulnerable to cancer will help in prevention efforts,” said Linda Birnbaum, PhD, director of the National Institute of Environmental Health Sciences and the NTP.
“This report provides a valuable resource for health regulatory and research agencies, and it empowers the public with information people can use to reduce exposure to cancer-causing substances.”
New known carcinogen
Since 1983, ortho-toluidine has been listed in the HHS’s Report on Carcinogens as reasonably anticipated to be a human carcinogen. However, new cancer studies led the NTP to reevaluate and reclassify ortho-toluidine. It is now classified as a known human carcinogen, based on clinical studies showing it causes urinary bladder cancer.
Ortho-toluidine is a synthetic chemical produced in other countries and imported into the US by several companies in high volumes. It is primarily used to make rubber chemicals, pesticides, and dyes. It is also used in some consumer and medical products.
People are mainly exposed through the workplace, by skin contact and/or inhalation when using ortho-toluidine. They can also be exposed outside the workplace through sources such as tobacco smoke.
Three new substances likely to be carcinogenic
Pentachlorophenol
Pentachlorophenol and byproducts of its synthesis are complex mixtures of chemicals used as wood preservatives. Because virtually everyone exposed to pentachlorophenol is also exposed to its synthesis byproducts, they were evaluated together.
In the US, pentachlorophenol has been regulated since the 1980s as a restricted-use pesticide. It is used industrially for treating utility poles, wood pilings, fence posts, and lumber or timber for construction.
Most exposure has occurred in settings where workers treat lumber or come in contact with treated lumber. People may also be exposed to this mixture from breathing contaminated air or dust, or from contact with contaminated soil.
Exposure to this mixture was associated with an increased risk of non-Hodgkin lymphoma in clinical studies. In mice, it has been shown to cause tumors in the liver and other organs.
1-bromopropane
1-bromopropane is a liquid used as a solvent in many commercial industries. It is used as a cleaner for optics, electronics, and metals, as well as a solvent for aerosol-applied adhesives such as those used in foam cushion manufacturing.
It is also used in dry cleaning and in solvent sprays for aircraft maintenance. Workers in certain occupations may be more exposed to 1-bromopropane than the general population.
The NTP did not identify any clinical studies that evaluated the relationship between human cancer and exposure to 1-bromopropane. However, inhalation exposure to 1-bromopropane in rodents caused tumors in several organs, including the skin, lungs, and large intestine.
Cumene
Cumene is a flammable and volatile liquid with a gasoline-like odor. It is a natural component of coal tar and petroleum, and is found in tobacco smoke. It is used primarily to make acetone and phenol.
People are mainly exposed to cumene through the environment and in workplaces that use or produce cumene. It can be found in emissions from petroleum products.
Inhalation exposure to cumene caused lung tumors in male and female mice, and liver tumors in female mice. The NTP did not identify any clinical studies evaluating the relationship between cancer and exposure to cumene. ![]()
Results of VTE prophylaxis vary in otolaryngology patients

The effectiveness of thromboprophylaxis in otolaryngology patients undergoing surgery differs based on patient risk and the procedure, a new study suggests.
Overall, the incidence of venous thromboembolism (VTE) was similar between patients who received prophylaxis and those who did not.
However, prophylaxis reduced the incidence of VTE in patients with a high Caprini risk score and those who underwent microvascular free tissue reconstruction.
On the other hand, there was an increased risk of bleeding complications associated with prophylaxis among patients who underwent microvascular free tissue reconstruction and in the cohort overall.
Vinita Bahl, DMD, of the University of Michigan Health System in Ann Arbor, and his colleagues conducted this research and recounted the results in JAMA Otolaryngology–Head & Neck Surgery.
The researchers analyzed 3498 patients treated by surgeons at an academic medical center between September 2003 and June 2010. The team assessed the incidence of VTE and bleeding complications in the 30 days after surgery.
In all, 1482 patients received VTE prophylaxis. Most (96.8%) received subcutaneous unfractionated heparin (5000 IU 2-3 times daily), 3.1% received enoxaparin (30-40 mg daily), and 0.1% received fondaparinux (2.5 mg daily).
The incidence of VTE among all patients was 1.3%—0.74% were deep vein thromboses and 0.66% were pulmonary emboli. The overall incidence of bleeding complications was 2.2%.
VTE occurred in 1.2% of patients who received prophylaxis and 1.3% of patients who did not (P=0.75).
Bleeding complications occurred in 3.5% and 1.2% of patients, respectively (P<0.001).
Patients with Caprini VTE risk scores greater than 7 were less likely to develop a VTE if they received perioperative prophylaxis—5.3% vs 10.4% (P=0.06).
Prophylaxis also decreased the incidence of VTE among patients who underwent free tissue transfer—2.1% vs 7.7% (P=0.002). But it increased bleeding complications—11.9% vs 4.5% (P=0.01).
Bleeding complications were associated with concomitant use of antiplatelet medications and VTE prophylaxis.
In all other patients, prophylaxis did not affect the incidence of VTE or bleeding. VTE occurred in 1% of treated and 0.6% of untreated patients (P=0.12). And bleeding occurred in 1.5% and 0.9% of patients, respectively (P=0.15).
These results suggest the Caprini risk assessment model is an effective tool to stratify otolaryngology patients according to VTE risk, the researchers said.
However, they believe additional research is needed before recommendations can be made for patients undergoing free tissue reconstruction, as prophylaxis reduced their risk of VTE but increased their risk of bleeding. ![]()

The effectiveness of thromboprophylaxis in otolaryngology patients undergoing surgery differs based on patient risk and the procedure, a new study suggests.
Overall, the incidence of venous thromboembolism (VTE) was similar between patients who received prophylaxis and those who did not.
However, prophylaxis reduced the incidence of VTE in patients with a high Caprini risk score and those who underwent microvascular free tissue reconstruction.
On the other hand, there was an increased risk of bleeding complications associated with prophylaxis among patients who underwent microvascular free tissue reconstruction and in the cohort overall.
Vinita Bahl, DMD, of the University of Michigan Health System in Ann Arbor, and his colleagues conducted this research and recounted the results in JAMA Otolaryngology–Head & Neck Surgery.
The researchers analyzed 3498 patients treated by surgeons at an academic medical center between September 2003 and June 2010. The team assessed the incidence of VTE and bleeding complications in the 30 days after surgery.
In all, 1482 patients received VTE prophylaxis. Most (96.8%) received subcutaneous unfractionated heparin (5000 IU 2-3 times daily), 3.1% received enoxaparin (30-40 mg daily), and 0.1% received fondaparinux (2.5 mg daily).
The incidence of VTE among all patients was 1.3%—0.74% were deep vein thromboses and 0.66% were pulmonary emboli. The overall incidence of bleeding complications was 2.2%.
VTE occurred in 1.2% of patients who received prophylaxis and 1.3% of patients who did not (P=0.75).
Bleeding complications occurred in 3.5% and 1.2% of patients, respectively (P<0.001).
Patients with Caprini VTE risk scores greater than 7 were less likely to develop a VTE if they received perioperative prophylaxis—5.3% vs 10.4% (P=0.06).
Prophylaxis also decreased the incidence of VTE among patients who underwent free tissue transfer—2.1% vs 7.7% (P=0.002). But it increased bleeding complications—11.9% vs 4.5% (P=0.01).
Bleeding complications were associated with concomitant use of antiplatelet medications and VTE prophylaxis.
In all other patients, prophylaxis did not affect the incidence of VTE or bleeding. VTE occurred in 1% of treated and 0.6% of untreated patients (P=0.12). And bleeding occurred in 1.5% and 0.9% of patients, respectively (P=0.15).
These results suggest the Caprini risk assessment model is an effective tool to stratify otolaryngology patients according to VTE risk, the researchers said.
However, they believe additional research is needed before recommendations can be made for patients undergoing free tissue reconstruction, as prophylaxis reduced their risk of VTE but increased their risk of bleeding. ![]()

The effectiveness of thromboprophylaxis in otolaryngology patients undergoing surgery differs based on patient risk and the procedure, a new study suggests.
Overall, the incidence of venous thromboembolism (VTE) was similar between patients who received prophylaxis and those who did not.
However, prophylaxis reduced the incidence of VTE in patients with a high Caprini risk score and those who underwent microvascular free tissue reconstruction.
On the other hand, there was an increased risk of bleeding complications associated with prophylaxis among patients who underwent microvascular free tissue reconstruction and in the cohort overall.
Vinita Bahl, DMD, of the University of Michigan Health System in Ann Arbor, and his colleagues conducted this research and recounted the results in JAMA Otolaryngology–Head & Neck Surgery.
The researchers analyzed 3498 patients treated by surgeons at an academic medical center between September 2003 and June 2010. The team assessed the incidence of VTE and bleeding complications in the 30 days after surgery.
In all, 1482 patients received VTE prophylaxis. Most (96.8%) received subcutaneous unfractionated heparin (5000 IU 2-3 times daily), 3.1% received enoxaparin (30-40 mg daily), and 0.1% received fondaparinux (2.5 mg daily).
The incidence of VTE among all patients was 1.3%—0.74% were deep vein thromboses and 0.66% were pulmonary emboli. The overall incidence of bleeding complications was 2.2%.
VTE occurred in 1.2% of patients who received prophylaxis and 1.3% of patients who did not (P=0.75).
Bleeding complications occurred in 3.5% and 1.2% of patients, respectively (P<0.001).
Patients with Caprini VTE risk scores greater than 7 were less likely to develop a VTE if they received perioperative prophylaxis—5.3% vs 10.4% (P=0.06).
Prophylaxis also decreased the incidence of VTE among patients who underwent free tissue transfer—2.1% vs 7.7% (P=0.002). But it increased bleeding complications—11.9% vs 4.5% (P=0.01).
Bleeding complications were associated with concomitant use of antiplatelet medications and VTE prophylaxis.
In all other patients, prophylaxis did not affect the incidence of VTE or bleeding. VTE occurred in 1% of treated and 0.6% of untreated patients (P=0.12). And bleeding occurred in 1.5% and 0.9% of patients, respectively (P=0.15).
These results suggest the Caprini risk assessment model is an effective tool to stratify otolaryngology patients according to VTE risk, the researchers said.
However, they believe additional research is needed before recommendations can be made for patients undergoing free tissue reconstruction, as prophylaxis reduced their risk of VTE but increased their risk of bleeding. ![]()
Gout may predispose people, particularly women, to diabetes
Screen for diabetes and aggressively manage risk factors in patients with gout, especially women, investigators concluded from a retrospective, matched cohort study published Oct. 2 in Annals of the Rheumatic Diseases.
They found that women have a 71% greater risk of developing diabetes if they have gout (hazard ratio, 1.71; 95% confidence interval, 1.51-1.93; P less than .001), and men with gout have a 22% greater risk (HR, 1.22; 95% CI, 1.13-1.31; P less than .001), compared with the general population.
The study “suggests that gout may be independently associated with an increased risk of diabetes. These findings were independent of BMI [body mass index], lifestyle factors, and other known risk factors. The magnitude of excess diabetes risk in gout was significantly larger among women than men, both in risk difference and relative risk, and these findings persisted across all age categories. These findings support aggressive management of risk factors of diabetes in patients with gout,” concluded senior investigator Dr. Hyon K. Choi and his colleagues at Boston University (Ann. Rheum. Dis. 2014 Oct. 2 [doi: 10.1136/annrheumdis-2014-205827]).
Using 15 years’ worth of data from the U.K. Health Improvement Network, which contains the records of about 7.3 million patients, the investigators matched 35,339 patients with newly diagnosed gout with up to 5 control subjects for age, sex, and BMI; they then looked to see who subsequently developed diabetes.
Among patients with gout, there were 10.1 cases of new-onset diabetes in women and 9.5 cases in men per 1,000 person-years. Among the 137,056 controls without gout, there were 5.6 cases of new-onset diabetes in women and 7.2 cases in men per 1,000 person-years.
After adjustment for smoking, alcohol consumption, physician visits, comorbidities, medication use, and BMI as a continuous variable, gout increased the risk of diabetes by 48% in women (HR, 1.48; 95% CI, 1.29-1.68; P less than .001) and by 15% in men (HR, 1.15; 95% CI, 1.06-1.24; P less than .001). The sex difference persisted across age groups.
Gout patients consumed more alcohol, visited their doctor more often, had more health problems, and took steroids and diuretics more frequently than did those who did not have gout. Overall, 72.4% of the gout cases were in men with a mean age of 62.7 years; the rest were in women, but women with gout tended to be a bit older, with a mean age of 67.9 years.
Perhaps, “low-grade inflammation among patients with gout promote[s] the diabetogenic process,” the investigators wrote. “Alternatively, the link may stem from the shared metabolic factors of the two conditions, such as the correlates of the metabolic syndrome or shared genes. Furthermore, the link between hyperuricemia and the risk of type 2 diabetes may originate at the renal level, as insulin resistance and higher insulin levels are known to reduce renal excretion of urate,” they noted.
It’s unclear why women seem to be more affected. “SUA [serum uric acid] levels in men are about 1 mg/dL higher than in women during adulthood, although levels in women increase around natural menopause. Thus, the physiological impact of uric acid levels, which are high enough to cause gout, could be stronger among women than men. Furthermore, female gout patients may have higher SUA levels on average than male gout patients, which could also contribute to a larger association with the risk of diabetes among women,” they suggested.
Dr. Choi previously linked gout to the development of diabetes, but only in men with high cardiovascular risk profiles (Rheumatology 2008;47:1567-70).
The investigators had no disclosures. The work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Screen for diabetes and aggressively manage risk factors in patients with gout, especially women, investigators concluded from a retrospective, matched cohort study published Oct. 2 in Annals of the Rheumatic Diseases.
They found that women have a 71% greater risk of developing diabetes if they have gout (hazard ratio, 1.71; 95% confidence interval, 1.51-1.93; P less than .001), and men with gout have a 22% greater risk (HR, 1.22; 95% CI, 1.13-1.31; P less than .001), compared with the general population.
The study “suggests that gout may be independently associated with an increased risk of diabetes. These findings were independent of BMI [body mass index], lifestyle factors, and other known risk factors. The magnitude of excess diabetes risk in gout was significantly larger among women than men, both in risk difference and relative risk, and these findings persisted across all age categories. These findings support aggressive management of risk factors of diabetes in patients with gout,” concluded senior investigator Dr. Hyon K. Choi and his colleagues at Boston University (Ann. Rheum. Dis. 2014 Oct. 2 [doi: 10.1136/annrheumdis-2014-205827]).
Using 15 years’ worth of data from the U.K. Health Improvement Network, which contains the records of about 7.3 million patients, the investigators matched 35,339 patients with newly diagnosed gout with up to 5 control subjects for age, sex, and BMI; they then looked to see who subsequently developed diabetes.
Among patients with gout, there were 10.1 cases of new-onset diabetes in women and 9.5 cases in men per 1,000 person-years. Among the 137,056 controls without gout, there were 5.6 cases of new-onset diabetes in women and 7.2 cases in men per 1,000 person-years.
After adjustment for smoking, alcohol consumption, physician visits, comorbidities, medication use, and BMI as a continuous variable, gout increased the risk of diabetes by 48% in women (HR, 1.48; 95% CI, 1.29-1.68; P less than .001) and by 15% in men (HR, 1.15; 95% CI, 1.06-1.24; P less than .001). The sex difference persisted across age groups.
Gout patients consumed more alcohol, visited their doctor more often, had more health problems, and took steroids and diuretics more frequently than did those who did not have gout. Overall, 72.4% of the gout cases were in men with a mean age of 62.7 years; the rest were in women, but women with gout tended to be a bit older, with a mean age of 67.9 years.
Perhaps, “low-grade inflammation among patients with gout promote[s] the diabetogenic process,” the investigators wrote. “Alternatively, the link may stem from the shared metabolic factors of the two conditions, such as the correlates of the metabolic syndrome or shared genes. Furthermore, the link between hyperuricemia and the risk of type 2 diabetes may originate at the renal level, as insulin resistance and higher insulin levels are known to reduce renal excretion of urate,” they noted.
It’s unclear why women seem to be more affected. “SUA [serum uric acid] levels in men are about 1 mg/dL higher than in women during adulthood, although levels in women increase around natural menopause. Thus, the physiological impact of uric acid levels, which are high enough to cause gout, could be stronger among women than men. Furthermore, female gout patients may have higher SUA levels on average than male gout patients, which could also contribute to a larger association with the risk of diabetes among women,” they suggested.
Dr. Choi previously linked gout to the development of diabetes, but only in men with high cardiovascular risk profiles (Rheumatology 2008;47:1567-70).
The investigators had no disclosures. The work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Screen for diabetes and aggressively manage risk factors in patients with gout, especially women, investigators concluded from a retrospective, matched cohort study published Oct. 2 in Annals of the Rheumatic Diseases.
They found that women have a 71% greater risk of developing diabetes if they have gout (hazard ratio, 1.71; 95% confidence interval, 1.51-1.93; P less than .001), and men with gout have a 22% greater risk (HR, 1.22; 95% CI, 1.13-1.31; P less than .001), compared with the general population.
The study “suggests that gout may be independently associated with an increased risk of diabetes. These findings were independent of BMI [body mass index], lifestyle factors, and other known risk factors. The magnitude of excess diabetes risk in gout was significantly larger among women than men, both in risk difference and relative risk, and these findings persisted across all age categories. These findings support aggressive management of risk factors of diabetes in patients with gout,” concluded senior investigator Dr. Hyon K. Choi and his colleagues at Boston University (Ann. Rheum. Dis. 2014 Oct. 2 [doi: 10.1136/annrheumdis-2014-205827]).
Using 15 years’ worth of data from the U.K. Health Improvement Network, which contains the records of about 7.3 million patients, the investigators matched 35,339 patients with newly diagnosed gout with up to 5 control subjects for age, sex, and BMI; they then looked to see who subsequently developed diabetes.
Among patients with gout, there were 10.1 cases of new-onset diabetes in women and 9.5 cases in men per 1,000 person-years. Among the 137,056 controls without gout, there were 5.6 cases of new-onset diabetes in women and 7.2 cases in men per 1,000 person-years.
After adjustment for smoking, alcohol consumption, physician visits, comorbidities, medication use, and BMI as a continuous variable, gout increased the risk of diabetes by 48% in women (HR, 1.48; 95% CI, 1.29-1.68; P less than .001) and by 15% in men (HR, 1.15; 95% CI, 1.06-1.24; P less than .001). The sex difference persisted across age groups.
Gout patients consumed more alcohol, visited their doctor more often, had more health problems, and took steroids and diuretics more frequently than did those who did not have gout. Overall, 72.4% of the gout cases were in men with a mean age of 62.7 years; the rest were in women, but women with gout tended to be a bit older, with a mean age of 67.9 years.
Perhaps, “low-grade inflammation among patients with gout promote[s] the diabetogenic process,” the investigators wrote. “Alternatively, the link may stem from the shared metabolic factors of the two conditions, such as the correlates of the metabolic syndrome or shared genes. Furthermore, the link between hyperuricemia and the risk of type 2 diabetes may originate at the renal level, as insulin resistance and higher insulin levels are known to reduce renal excretion of urate,” they noted.
It’s unclear why women seem to be more affected. “SUA [serum uric acid] levels in men are about 1 mg/dL higher than in women during adulthood, although levels in women increase around natural menopause. Thus, the physiological impact of uric acid levels, which are high enough to cause gout, could be stronger among women than men. Furthermore, female gout patients may have higher SUA levels on average than male gout patients, which could also contribute to a larger association with the risk of diabetes among women,” they suggested.
Dr. Choi previously linked gout to the development of diabetes, but only in men with high cardiovascular risk profiles (Rheumatology 2008;47:1567-70).
The investigators had no disclosures. The work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point: Be on the lookout for diabetes in your gout patients.
Major finding: Women have a 71% greater risk of developing diabetes if they have gout (HR, 1.71; 95% CI, 1.51-1.93; P less than .001), and men with gout have a 22% greater risk (HR, 1.22; 95% CI, 1.13-1.31; P less than .001), compared with the general population.
Data source: Retrospective database study of more than 170,000 patients.
Disclosures: The investigators had no disclosures. The work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Urinary incontinence – An individual and societal ill
Urinary incontinence is a major health care concern, both in terms of the numbers of women who are suffering and with respect to societal costs and the impact on health care spending. Approximately 15 years ago, an international group reported in the Journal of the American Medical Association that 200 million people worldwide – 75%-80% of them women – were suffering from urinary incontinence (JAMA 1998;280:951-3).
Since then, a high prevalence of urinary incontinence has been documented in various studies and reports. Experts have estimated, for instance, that between 13 million and 25 million adult Americans experience transient or chronic symptoms, and that approximately half of these patients suffer from severe or bothersome symptoms. Again, the majority of these individuals are women.
Consumer-based research suggests that 25% of women over the age of 18 years experience episodes of urinary incontinence, according to prevalence data collected by the National Association for Continence. In 2001, 10% of women under the age of 65 years and 35% of women over 65 had symptoms of involuntary leakage, according to the National Institute of Diabetes and Digestive and Kidney Diseases. Despite this, nearly two-thirds of patients never discussed bladder health with their health care provider and on average, women wait over 6 years from symptom onset before a diagnosis is established. Moreover, the costs are significant; in 2001, the cost for urinary incontinence in the United States was $16.3 billion (Obstet. Gynecol. 2001;98:398-406).
There are four types of urinary incontinence – urge, stress, mixed, and overflow. Urge incontinence typically is accompanied by urgency. Stress incontinence occurs with the increased abdominal pressure that accompanies effort, exertion, laughing, coughing, and sneezing. Overflow incontinence generally involves continuous urinary loss and incomplete bladder emptying.
Over the next four installments of Master Class in Gynecologic Surgery, I have chosen to feature the workup and treatment of urinary incontinence. For our first installment, I have asked my former resident Dr. Sandra Culbertson, who is now a professor in the department of obstetrics and gynecology at the University of Chicago, to share her knowledge of the optimal approach for evaluating urinary incontinence in the office. As she explains, it is critical to discern the uncomplicated cases of stress urinary incontinence from possibly complicated cases that require more assessment.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller had no relevant financial disclosures.
Urinary incontinence is a major health care concern, both in terms of the numbers of women who are suffering and with respect to societal costs and the impact on health care spending. Approximately 15 years ago, an international group reported in the Journal of the American Medical Association that 200 million people worldwide – 75%-80% of them women – were suffering from urinary incontinence (JAMA 1998;280:951-3).
Since then, a high prevalence of urinary incontinence has been documented in various studies and reports. Experts have estimated, for instance, that between 13 million and 25 million adult Americans experience transient or chronic symptoms, and that approximately half of these patients suffer from severe or bothersome symptoms. Again, the majority of these individuals are women.
Consumer-based research suggests that 25% of women over the age of 18 years experience episodes of urinary incontinence, according to prevalence data collected by the National Association for Continence. In 2001, 10% of women under the age of 65 years and 35% of women over 65 had symptoms of involuntary leakage, according to the National Institute of Diabetes and Digestive and Kidney Diseases. Despite this, nearly two-thirds of patients never discussed bladder health with their health care provider and on average, women wait over 6 years from symptom onset before a diagnosis is established. Moreover, the costs are significant; in 2001, the cost for urinary incontinence in the United States was $16.3 billion (Obstet. Gynecol. 2001;98:398-406).
There are four types of urinary incontinence – urge, stress, mixed, and overflow. Urge incontinence typically is accompanied by urgency. Stress incontinence occurs with the increased abdominal pressure that accompanies effort, exertion, laughing, coughing, and sneezing. Overflow incontinence generally involves continuous urinary loss and incomplete bladder emptying.
Over the next four installments of Master Class in Gynecologic Surgery, I have chosen to feature the workup and treatment of urinary incontinence. For our first installment, I have asked my former resident Dr. Sandra Culbertson, who is now a professor in the department of obstetrics and gynecology at the University of Chicago, to share her knowledge of the optimal approach for evaluating urinary incontinence in the office. As she explains, it is critical to discern the uncomplicated cases of stress urinary incontinence from possibly complicated cases that require more assessment.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller had no relevant financial disclosures.
Urinary incontinence is a major health care concern, both in terms of the numbers of women who are suffering and with respect to societal costs and the impact on health care spending. Approximately 15 years ago, an international group reported in the Journal of the American Medical Association that 200 million people worldwide – 75%-80% of them women – were suffering from urinary incontinence (JAMA 1998;280:951-3).
Since then, a high prevalence of urinary incontinence has been documented in various studies and reports. Experts have estimated, for instance, that between 13 million and 25 million adult Americans experience transient or chronic symptoms, and that approximately half of these patients suffer from severe or bothersome symptoms. Again, the majority of these individuals are women.
Consumer-based research suggests that 25% of women over the age of 18 years experience episodes of urinary incontinence, according to prevalence data collected by the National Association for Continence. In 2001, 10% of women under the age of 65 years and 35% of women over 65 had symptoms of involuntary leakage, according to the National Institute of Diabetes and Digestive and Kidney Diseases. Despite this, nearly two-thirds of patients never discussed bladder health with their health care provider and on average, women wait over 6 years from symptom onset before a diagnosis is established. Moreover, the costs are significant; in 2001, the cost for urinary incontinence in the United States was $16.3 billion (Obstet. Gynecol. 2001;98:398-406).
There are four types of urinary incontinence – urge, stress, mixed, and overflow. Urge incontinence typically is accompanied by urgency. Stress incontinence occurs with the increased abdominal pressure that accompanies effort, exertion, laughing, coughing, and sneezing. Overflow incontinence generally involves continuous urinary loss and incomplete bladder emptying.
Over the next four installments of Master Class in Gynecologic Surgery, I have chosen to feature the workup and treatment of urinary incontinence. For our first installment, I have asked my former resident Dr. Sandra Culbertson, who is now a professor in the department of obstetrics and gynecology at the University of Chicago, to share her knowledge of the optimal approach for evaluating urinary incontinence in the office. As she explains, it is critical to discern the uncomplicated cases of stress urinary incontinence from possibly complicated cases that require more assessment.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller had no relevant financial disclosures.