User login
Drug combinations found to increase upper gastrointestinal bleeding risk
Combining nonsteroidal anti-inflammatory drugs with selective serotonin reuptake inhibitors increased the risk of upper gastrointestinal bleeding by up to 190% beyond the baseline risk found for NSAID monotherapy, researchers reported in the October issue of Gastroenterology.
Patients also faced excess risks of upper GI bleeding when they took corticosteroids, aldosterone antagonists, or anticoagulants together with low-dose aspirin or nonselective NSAIDs, although the effect was not seen for COX-2 inhibitors, said Dr. Gwen Masclee at Erasmus Medical Center in Rotterdam, the Netherlands and her associates.
Source: American Gastroenterological Association
The findings should help clinicians tailor treatments to minimize chances of upper gastrointestinal bleeding, particularly for elderly patients who often take multiple drugs, the investigators said (Gastroenterology 2014 [doi:10.1053/j.gastro.2014.06.007]).
The researchers analyzed 114,835 cases of upper gastrointestinal bleeding, including all gastroduodenal ulcers and hemorrhages extracted from seven electronic health record databases from the Netherlands, Italy, and Denmark. Three databases included primary care data, and four were administrative claims data, the investigators said. Cases served as their own controls, they noted.
Monotherapy with prescription nonselective NSAIDs increased the chances of an upper gastrointestinal bleed by 4.3 times, compared with not using any of the drugs studied (95% confidence interval, 4.1-4.4), the researchers said. Notably, bleeding risk from taking either nonselective NSAIDs or corticosteroids was the same, they said, adding that previous studies have yielded inconsistent findings on the topic. The incidence ratios for monotherapy with low-dose aspirin and COX-2 inhibitors were slightly lower at 3.1 (95% CI, 2.9-3.2) and 2.9 (95% CI, 2.7-3.2), respectively, they added.
Combining nonselective NSAIDs, COX-2 inhibitors, or low-dose aspirin with SSRIs led to excess risks of upper gastrointestinal bleeding of 1.6 (95% CI, 0.5-2.6), 1.9 (95% CI, 0.2-3.4), and 0.49 (–0.05-1.03), respectively, the researchers reported. "From a biological point of view, this interaction seems plausible because SSRIs decrease the serotonin level, resulting in impaired thrombocyte aggregation and an increased risk of bleeding in general," they said.
Corticosteroids combined with nonselective NSAIDs led to the greatest increases in bleeding risk, with an incidence ratio of 12.8 (95% CI, 11.1-14.7), compared with nonuse of any drug studied, and an excess risk of 5.5 (3.7-7.3), compared with NSAID use alone, said the researchers. Adding aldosterone antagonists to nonselective NSAIDs led to an excess risk of 4.46, compared with using nonselective NSAIDs alone, they reported (95% CI, 1.79-7.13).
Because the study did not capture over-the-counter NSAID prescriptions, it could have underestimated use of these drugs, the investigators said. Also, changes in health or NSAID use during the study could have created residual confounding, although sensitivity analyses did not reveal problems, they reported. They added that misclassification of some data could have led them to underestimate risks. "Finally, we did not take any carryover effect or dose of drug exposure into account, which potentially limits the generalizability concerning causality of the associations," they concluded.
Five authors reported employment or other financial support from Erasmus University Medical Center, AstraZeneca, Janssen, PHARMO Institute, and the European Medicines Agency. The other authors reported no relevant conflicts of interests.
Gastrointestinal toxicity is the major issue limiting nonsteroidal anti-inflammatory use. The excess annual risk of upper gastrointestinal bleeding per 1,000 patients is about 1 with low-dose aspirin, about 2 with coxibs, and about 4-6 with traditional NSAIDs (ibuprofen, naproxen). However, the risk of upper gastrointestinal bleeding increases markedly with several factors, including the use of concomitant medications.
Ideally, large randomized trials comparing NSAIDs with and without a concomitant medication would inform our assessment of risk. However, few such trials are available, so we commonly rely on observational database studies, such as that of Masclee et al. These studies have the important benefit of large sample size and "real world" results, but also have potential limitations, including reliability of data (for example, accuracy of diagnostic coding) and potential bias because of unequal distribution of confounding factors between cases and controls.
Masclee et al. report significant synergy (more than additive risk) of traditional NSAIDs with corticosteroids, SSRIs, aldosterone antagonists, and antithrombotic agents other than low-dose aspirin (although risk was increased with traditional NSAIDs plus low-dose aspirin). Low-dose aspirin was synergistic with antithrombotic agents and corticosteroids, while coxibs were synergistic with low-dose aspirin and SSRIs.
The results of Masclee et al. support current North American guidelines, which suggest use of proton pump inhibitors or misoprostol for traditional NSAID users taking concomitant medications such as antithrombotics, corticosteroids, or SSRIs, and use of PPIs for low-dose-aspirin users taking antithrombotics or taking corticosteroids if greater than or equal to 60 years old. Their results also suggest further evaluation of aldosterone antagonists is warranted as another possible risk factor.
Dr. Loren Laine is professor of medicine, department of internal medicine, Yale University, New Haven, Conn. He is on the Data Safety Monitoring Boards of Eisai, BMS, and Bayer; and is a consultant for AstraZeneca.
Gastrointestinal toxicity is the major issue limiting nonsteroidal anti-inflammatory use. The excess annual risk of upper gastrointestinal bleeding per 1,000 patients is about 1 with low-dose aspirin, about 2 with coxibs, and about 4-6 with traditional NSAIDs (ibuprofen, naproxen). However, the risk of upper gastrointestinal bleeding increases markedly with several factors, including the use of concomitant medications.
Ideally, large randomized trials comparing NSAIDs with and without a concomitant medication would inform our assessment of risk. However, few such trials are available, so we commonly rely on observational database studies, such as that of Masclee et al. These studies have the important benefit of large sample size and "real world" results, but also have potential limitations, including reliability of data (for example, accuracy of diagnostic coding) and potential bias because of unequal distribution of confounding factors between cases and controls.
Masclee et al. report significant synergy (more than additive risk) of traditional NSAIDs with corticosteroids, SSRIs, aldosterone antagonists, and antithrombotic agents other than low-dose aspirin (although risk was increased with traditional NSAIDs plus low-dose aspirin). Low-dose aspirin was synergistic with antithrombotic agents and corticosteroids, while coxibs were synergistic with low-dose aspirin and SSRIs.
The results of Masclee et al. support current North American guidelines, which suggest use of proton pump inhibitors or misoprostol for traditional NSAID users taking concomitant medications such as antithrombotics, corticosteroids, or SSRIs, and use of PPIs for low-dose-aspirin users taking antithrombotics or taking corticosteroids if greater than or equal to 60 years old. Their results also suggest further evaluation of aldosterone antagonists is warranted as another possible risk factor.
Dr. Loren Laine is professor of medicine, department of internal medicine, Yale University, New Haven, Conn. He is on the Data Safety Monitoring Boards of Eisai, BMS, and Bayer; and is a consultant for AstraZeneca.
Gastrointestinal toxicity is the major issue limiting nonsteroidal anti-inflammatory use. The excess annual risk of upper gastrointestinal bleeding per 1,000 patients is about 1 with low-dose aspirin, about 2 with coxibs, and about 4-6 with traditional NSAIDs (ibuprofen, naproxen). However, the risk of upper gastrointestinal bleeding increases markedly with several factors, including the use of concomitant medications.
Ideally, large randomized trials comparing NSAIDs with and without a concomitant medication would inform our assessment of risk. However, few such trials are available, so we commonly rely on observational database studies, such as that of Masclee et al. These studies have the important benefit of large sample size and "real world" results, but also have potential limitations, including reliability of data (for example, accuracy of diagnostic coding) and potential bias because of unequal distribution of confounding factors between cases and controls.
Masclee et al. report significant synergy (more than additive risk) of traditional NSAIDs with corticosteroids, SSRIs, aldosterone antagonists, and antithrombotic agents other than low-dose aspirin (although risk was increased with traditional NSAIDs plus low-dose aspirin). Low-dose aspirin was synergistic with antithrombotic agents and corticosteroids, while coxibs were synergistic with low-dose aspirin and SSRIs.
The results of Masclee et al. support current North American guidelines, which suggest use of proton pump inhibitors or misoprostol for traditional NSAID users taking concomitant medications such as antithrombotics, corticosteroids, or SSRIs, and use of PPIs for low-dose-aspirin users taking antithrombotics or taking corticosteroids if greater than or equal to 60 years old. Their results also suggest further evaluation of aldosterone antagonists is warranted as another possible risk factor.
Dr. Loren Laine is professor of medicine, department of internal medicine, Yale University, New Haven, Conn. He is on the Data Safety Monitoring Boards of Eisai, BMS, and Bayer; and is a consultant for AstraZeneca.
Combining nonsteroidal anti-inflammatory drugs with selective serotonin reuptake inhibitors increased the risk of upper gastrointestinal bleeding by up to 190% beyond the baseline risk found for NSAID monotherapy, researchers reported in the October issue of Gastroenterology.
Patients also faced excess risks of upper GI bleeding when they took corticosteroids, aldosterone antagonists, or anticoagulants together with low-dose aspirin or nonselective NSAIDs, although the effect was not seen for COX-2 inhibitors, said Dr. Gwen Masclee at Erasmus Medical Center in Rotterdam, the Netherlands and her associates.
Source: American Gastroenterological Association
The findings should help clinicians tailor treatments to minimize chances of upper gastrointestinal bleeding, particularly for elderly patients who often take multiple drugs, the investigators said (Gastroenterology 2014 [doi:10.1053/j.gastro.2014.06.007]).
The researchers analyzed 114,835 cases of upper gastrointestinal bleeding, including all gastroduodenal ulcers and hemorrhages extracted from seven electronic health record databases from the Netherlands, Italy, and Denmark. Three databases included primary care data, and four were administrative claims data, the investigators said. Cases served as their own controls, they noted.
Monotherapy with prescription nonselective NSAIDs increased the chances of an upper gastrointestinal bleed by 4.3 times, compared with not using any of the drugs studied (95% confidence interval, 4.1-4.4), the researchers said. Notably, bleeding risk from taking either nonselective NSAIDs or corticosteroids was the same, they said, adding that previous studies have yielded inconsistent findings on the topic. The incidence ratios for monotherapy with low-dose aspirin and COX-2 inhibitors were slightly lower at 3.1 (95% CI, 2.9-3.2) and 2.9 (95% CI, 2.7-3.2), respectively, they added.
Combining nonselective NSAIDs, COX-2 inhibitors, or low-dose aspirin with SSRIs led to excess risks of upper gastrointestinal bleeding of 1.6 (95% CI, 0.5-2.6), 1.9 (95% CI, 0.2-3.4), and 0.49 (–0.05-1.03), respectively, the researchers reported. "From a biological point of view, this interaction seems plausible because SSRIs decrease the serotonin level, resulting in impaired thrombocyte aggregation and an increased risk of bleeding in general," they said.
Corticosteroids combined with nonselective NSAIDs led to the greatest increases in bleeding risk, with an incidence ratio of 12.8 (95% CI, 11.1-14.7), compared with nonuse of any drug studied, and an excess risk of 5.5 (3.7-7.3), compared with NSAID use alone, said the researchers. Adding aldosterone antagonists to nonselective NSAIDs led to an excess risk of 4.46, compared with using nonselective NSAIDs alone, they reported (95% CI, 1.79-7.13).
Because the study did not capture over-the-counter NSAID prescriptions, it could have underestimated use of these drugs, the investigators said. Also, changes in health or NSAID use during the study could have created residual confounding, although sensitivity analyses did not reveal problems, they reported. They added that misclassification of some data could have led them to underestimate risks. "Finally, we did not take any carryover effect or dose of drug exposure into account, which potentially limits the generalizability concerning causality of the associations," they concluded.
Five authors reported employment or other financial support from Erasmus University Medical Center, AstraZeneca, Janssen, PHARMO Institute, and the European Medicines Agency. The other authors reported no relevant conflicts of interests.
Combining nonsteroidal anti-inflammatory drugs with selective serotonin reuptake inhibitors increased the risk of upper gastrointestinal bleeding by up to 190% beyond the baseline risk found for NSAID monotherapy, researchers reported in the October issue of Gastroenterology.
Patients also faced excess risks of upper GI bleeding when they took corticosteroids, aldosterone antagonists, or anticoagulants together with low-dose aspirin or nonselective NSAIDs, although the effect was not seen for COX-2 inhibitors, said Dr. Gwen Masclee at Erasmus Medical Center in Rotterdam, the Netherlands and her associates.
Source: American Gastroenterological Association
The findings should help clinicians tailor treatments to minimize chances of upper gastrointestinal bleeding, particularly for elderly patients who often take multiple drugs, the investigators said (Gastroenterology 2014 [doi:10.1053/j.gastro.2014.06.007]).
The researchers analyzed 114,835 cases of upper gastrointestinal bleeding, including all gastroduodenal ulcers and hemorrhages extracted from seven electronic health record databases from the Netherlands, Italy, and Denmark. Three databases included primary care data, and four were administrative claims data, the investigators said. Cases served as their own controls, they noted.
Monotherapy with prescription nonselective NSAIDs increased the chances of an upper gastrointestinal bleed by 4.3 times, compared with not using any of the drugs studied (95% confidence interval, 4.1-4.4), the researchers said. Notably, bleeding risk from taking either nonselective NSAIDs or corticosteroids was the same, they said, adding that previous studies have yielded inconsistent findings on the topic. The incidence ratios for monotherapy with low-dose aspirin and COX-2 inhibitors were slightly lower at 3.1 (95% CI, 2.9-3.2) and 2.9 (95% CI, 2.7-3.2), respectively, they added.
Combining nonselective NSAIDs, COX-2 inhibitors, or low-dose aspirin with SSRIs led to excess risks of upper gastrointestinal bleeding of 1.6 (95% CI, 0.5-2.6), 1.9 (95% CI, 0.2-3.4), and 0.49 (–0.05-1.03), respectively, the researchers reported. "From a biological point of view, this interaction seems plausible because SSRIs decrease the serotonin level, resulting in impaired thrombocyte aggregation and an increased risk of bleeding in general," they said.
Corticosteroids combined with nonselective NSAIDs led to the greatest increases in bleeding risk, with an incidence ratio of 12.8 (95% CI, 11.1-14.7), compared with nonuse of any drug studied, and an excess risk of 5.5 (3.7-7.3), compared with NSAID use alone, said the researchers. Adding aldosterone antagonists to nonselective NSAIDs led to an excess risk of 4.46, compared with using nonselective NSAIDs alone, they reported (95% CI, 1.79-7.13).
Because the study did not capture over-the-counter NSAID prescriptions, it could have underestimated use of these drugs, the investigators said. Also, changes in health or NSAID use during the study could have created residual confounding, although sensitivity analyses did not reveal problems, they reported. They added that misclassification of some data could have led them to underestimate risks. "Finally, we did not take any carryover effect or dose of drug exposure into account, which potentially limits the generalizability concerning causality of the associations," they concluded.
Five authors reported employment or other financial support from Erasmus University Medical Center, AstraZeneca, Janssen, PHARMO Institute, and the European Medicines Agency. The other authors reported no relevant conflicts of interests.
FROM GASTROENTEROLOGY
Key clinical point: Excess risk of upper gastrointestinal bleeding occurred with combinations of NSAIDs and selective serotonin reuptake inhibitors, and with combinations of nonselective NSAIDs or low-dose aspirin with corticosteroids, aldosterone antagonists, or anticoagulants.
Major finding: Excess risks from combining SSRIs with nonselective NSAIDs, COX-2 inhibitors, or low-dose aspirin were 1.62 (95% CI, 0.58-2.66), 1.86 (95% CI, 0.28- 3.44), and 0.49 (95% CI, –0.05-1.03), respectively.
Data source: Case series analysis of 114,835 patients with upper gastrointestinal bleeding. Patients were identified from seven electronic health record databases from the Netherlands, Italy, and Denmark, and cases served as their own controls.
Disclosures: Five authors reported employment or other financial support from Erasmus University Medical Center, AstraZeneca, Janssen, PHARMO Institute, and the European Medicines Agency. The other authors reported no relevant conflicts of interests.
Pediatric IBD rose by more than 40% in 15 years
Pediatric inflammatory bowel disease grew by more than 40% in a 15-year period in Ontario, Canada, according to a retrospective cohort study published in the October issue of Gastroenterology.
Although rates of inflammatory bowel disease (IBD) rose in children and adolescents of all ages, the steepest increase occurred in children with very-early-onset IBD (VEO-IBD), defined as disease diagnosed before they were 10 years old, said Dr. Eric Benchimol at the University of Ottawa and his associates. But these patients also tended to use fewer health services and have fewer surgeries for IBD, compared with older children with the disease, the investigators said (Gastroenterology 2014 October [doi.org/10.1053/j.gastro.2014.06.023]).
Source: American Gastroenterological Association
The findings add to research indicating that VEO-IBD is a distinct form of IBD and indicate the need to assess subgroups of these patients to look at phenotype, genotype, intestinal microbiome, and treatment response, the investigators said.
For the study, researchers created a cohort based on an algorithm of health care visits that identified all children and adolescents in Ontario diagnosed with IBD before age 18 years. The analysis included 7,143 patients with IBD, among whom about 14% had VEO-IBD, the investigators reported.
The overall rate of IBD in children up to 18 years old increased from 9.4 to 13.2 cases per 100,000 population from 1994 through 2009 (P less than .0001), the researchers said. And the yearly increase in VEO-IBD averaged 7.4% – more than three times greater than the 2.2% average annual rise among children diagnosed at 10 years and older, the investigators reported.
But health care utilization trends did not mirror changes in incidence, Dr. Benchimol and associates reported. For example, children diagnosed before they were 6 years old had significantly fewer outpatient visits for IBD, compared with children diagnosed at 10 years and older (odds ratio for girls, 0.67; 95% confidence interval, 0.58-0.78; OR for boys, 0.86; 95% CI, 0.75-0.98). Furthermore, patients diagnosed before age 6 years were less likely to be hospitalized for IBD than were older children with the disease (hazard ratio for girls, 0.70; 95% CI, 0.56-0.87; HR for boys, 1.12; 95% CI, 0.94-1.33), the investigators said.
The likelihood of undergoing intestinal resection also was lower for children diagnosed before age 6 years with Crohn’s disease, compared with older girls (HR, 0.35; 95% CI, 0.16-0.78) and boys (HR, 0.59; 95% CI, 0.34-0.99), said the researchers. And patients diagnosed before age 6 years with ulcerative colitis were less likely to undergo colectomy than were older girls (HR, 0.88; 95% CI, 0.47-1.63) and boys (HR, 0.42; 95% CI, 0.21-0.85). In contrast, rates of IBD-related surgery and hospitalization were similar between children diagnosed at 6-9.9 years of age and those diagnosed at age 10 up to 18 years, the investigators said.
A cohort study from the United States also found a lower likelihood of surgery in children with VEO-IBD, the researchers noted. Large-bowel involvement without ileal disease is prominent in young children with IBD, and these patients might be unlikely to undergo resection because colectomy requires a permanent ostotomy, they added.
The work was supported by the American College of Gastroenterology, the Ontario Ministry of Health and Long-Term Care, the Canadian Institutes of Health Research, the Crohn’s and Colitis Foundation of Canada, the National Institutes of Health, the Wolpow Family Chair in IBD Treatment and Research, the Ontario Ministry of Research and Innovation, and the Leona M. and Harry B. Helmsley Charitable Trust. The authors reported no conflicts of interest.
Pediatric inflammatory bowel disease grew by more than 40% in a 15-year period in Ontario, Canada, according to a retrospective cohort study published in the October issue of Gastroenterology.
Although rates of inflammatory bowel disease (IBD) rose in children and adolescents of all ages, the steepest increase occurred in children with very-early-onset IBD (VEO-IBD), defined as disease diagnosed before they were 10 years old, said Dr. Eric Benchimol at the University of Ottawa and his associates. But these patients also tended to use fewer health services and have fewer surgeries for IBD, compared with older children with the disease, the investigators said (Gastroenterology 2014 October [doi.org/10.1053/j.gastro.2014.06.023]).
Source: American Gastroenterological Association
The findings add to research indicating that VEO-IBD is a distinct form of IBD and indicate the need to assess subgroups of these patients to look at phenotype, genotype, intestinal microbiome, and treatment response, the investigators said.
For the study, researchers created a cohort based on an algorithm of health care visits that identified all children and adolescents in Ontario diagnosed with IBD before age 18 years. The analysis included 7,143 patients with IBD, among whom about 14% had VEO-IBD, the investigators reported.
The overall rate of IBD in children up to 18 years old increased from 9.4 to 13.2 cases per 100,000 population from 1994 through 2009 (P less than .0001), the researchers said. And the yearly increase in VEO-IBD averaged 7.4% – more than three times greater than the 2.2% average annual rise among children diagnosed at 10 years and older, the investigators reported.
But health care utilization trends did not mirror changes in incidence, Dr. Benchimol and associates reported. For example, children diagnosed before they were 6 years old had significantly fewer outpatient visits for IBD, compared with children diagnosed at 10 years and older (odds ratio for girls, 0.67; 95% confidence interval, 0.58-0.78; OR for boys, 0.86; 95% CI, 0.75-0.98). Furthermore, patients diagnosed before age 6 years were less likely to be hospitalized for IBD than were older children with the disease (hazard ratio for girls, 0.70; 95% CI, 0.56-0.87; HR for boys, 1.12; 95% CI, 0.94-1.33), the investigators said.
The likelihood of undergoing intestinal resection also was lower for children diagnosed before age 6 years with Crohn’s disease, compared with older girls (HR, 0.35; 95% CI, 0.16-0.78) and boys (HR, 0.59; 95% CI, 0.34-0.99), said the researchers. And patients diagnosed before age 6 years with ulcerative colitis were less likely to undergo colectomy than were older girls (HR, 0.88; 95% CI, 0.47-1.63) and boys (HR, 0.42; 95% CI, 0.21-0.85). In contrast, rates of IBD-related surgery and hospitalization were similar between children diagnosed at 6-9.9 years of age and those diagnosed at age 10 up to 18 years, the investigators said.
A cohort study from the United States also found a lower likelihood of surgery in children with VEO-IBD, the researchers noted. Large-bowel involvement without ileal disease is prominent in young children with IBD, and these patients might be unlikely to undergo resection because colectomy requires a permanent ostotomy, they added.
The work was supported by the American College of Gastroenterology, the Ontario Ministry of Health and Long-Term Care, the Canadian Institutes of Health Research, the Crohn’s and Colitis Foundation of Canada, the National Institutes of Health, the Wolpow Family Chair in IBD Treatment and Research, the Ontario Ministry of Research and Innovation, and the Leona M. and Harry B. Helmsley Charitable Trust. The authors reported no conflicts of interest.
Pediatric inflammatory bowel disease grew by more than 40% in a 15-year period in Ontario, Canada, according to a retrospective cohort study published in the October issue of Gastroenterology.
Although rates of inflammatory bowel disease (IBD) rose in children and adolescents of all ages, the steepest increase occurred in children with very-early-onset IBD (VEO-IBD), defined as disease diagnosed before they were 10 years old, said Dr. Eric Benchimol at the University of Ottawa and his associates. But these patients also tended to use fewer health services and have fewer surgeries for IBD, compared with older children with the disease, the investigators said (Gastroenterology 2014 October [doi.org/10.1053/j.gastro.2014.06.023]).
Source: American Gastroenterological Association
The findings add to research indicating that VEO-IBD is a distinct form of IBD and indicate the need to assess subgroups of these patients to look at phenotype, genotype, intestinal microbiome, and treatment response, the investigators said.
For the study, researchers created a cohort based on an algorithm of health care visits that identified all children and adolescents in Ontario diagnosed with IBD before age 18 years. The analysis included 7,143 patients with IBD, among whom about 14% had VEO-IBD, the investigators reported.
The overall rate of IBD in children up to 18 years old increased from 9.4 to 13.2 cases per 100,000 population from 1994 through 2009 (P less than .0001), the researchers said. And the yearly increase in VEO-IBD averaged 7.4% – more than three times greater than the 2.2% average annual rise among children diagnosed at 10 years and older, the investigators reported.
But health care utilization trends did not mirror changes in incidence, Dr. Benchimol and associates reported. For example, children diagnosed before they were 6 years old had significantly fewer outpatient visits for IBD, compared with children diagnosed at 10 years and older (odds ratio for girls, 0.67; 95% confidence interval, 0.58-0.78; OR for boys, 0.86; 95% CI, 0.75-0.98). Furthermore, patients diagnosed before age 6 years were less likely to be hospitalized for IBD than were older children with the disease (hazard ratio for girls, 0.70; 95% CI, 0.56-0.87; HR for boys, 1.12; 95% CI, 0.94-1.33), the investigators said.
The likelihood of undergoing intestinal resection also was lower for children diagnosed before age 6 years with Crohn’s disease, compared with older girls (HR, 0.35; 95% CI, 0.16-0.78) and boys (HR, 0.59; 95% CI, 0.34-0.99), said the researchers. And patients diagnosed before age 6 years with ulcerative colitis were less likely to undergo colectomy than were older girls (HR, 0.88; 95% CI, 0.47-1.63) and boys (HR, 0.42; 95% CI, 0.21-0.85). In contrast, rates of IBD-related surgery and hospitalization were similar between children diagnosed at 6-9.9 years of age and those diagnosed at age 10 up to 18 years, the investigators said.
A cohort study from the United States also found a lower likelihood of surgery in children with VEO-IBD, the researchers noted. Large-bowel involvement without ileal disease is prominent in young children with IBD, and these patients might be unlikely to undergo resection because colectomy requires a permanent ostotomy, they added.
The work was supported by the American College of Gastroenterology, the Ontario Ministry of Health and Long-Term Care, the Canadian Institutes of Health Research, the Crohn’s and Colitis Foundation of Canada, the National Institutes of Health, the Wolpow Family Chair in IBD Treatment and Research, the Ontario Ministry of Research and Innovation, and the Leona M. and Harry B. Helmsley Charitable Trust. The authors reported no conflicts of interest.
FROM GASTROENTEROLOGY
Key clinical point: Although the steepest rise in inflammatory bowel disease occurred in children diagnosed before age 10 years, children diagnosed before age 6 years had the lowest rates of IBD-related outpatient visits, hospitalizations, and surgeries.
Major finding: Rates of pediatric IBD increased by more than 40% between 1994 and 2009 in Ontario, Canada. Rates rose by an average of 7.4% annually in children diagnosed before age 10 years, compared with 2.2% for children diagnosed from 10 years to before 18 years of age. Rates of outpatient visits, hospitalizations, and IBD-related surgeries were significantly lower in children diagnosed before age 6 years, compared with children diagnosed at 10 years or older.
Data Source: Retrospective study of the Ontario Crohn’s and Colitis Cohort, which included 7,143 children and adolescents with IBD diagnosed between 1994 and 2009 in Ontario, Canada.
Disclosures: The work was supported by grants and researcher awards from the American College of Gastroenterology, the Ontario Ministry of Health and Long-Term Care, the Canadian Institutes of Health Research, the Crohn’s and Colitis Foundation of Canada, the National Institutes of Health, the Wolpow Family Chair in IBD Treatment and Research, the Ontario Ministry of Research and Innovation, and the Leona M. and Harry B. Helmsley Charitable Trust. The authors reported no conflicts of interest.
Metastatic Brain Tumors
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Systemic cancer can affect the central nervous system in several different ways, including direct tumor metastasis and indirect remote effects. Intracranial metastasis can involve the skull, dura, and leptomeninges (arachnoid and pia mater), as well as the brain parenchyma. Of these, parenchymal brain metastases are the most common and have been found in as many as 24% of cancer patients in autopsy studies. It has been reported that metastatic brain tumors outnumber primary brain tumors 10 to 1.
To read the full article in PDF:
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Systemic cancer can affect the central nervous system in several different ways, including direct tumor metastasis and indirect remote effects. Intracranial metastasis can involve the skull, dura, and leptomeninges (arachnoid and pia mater), as well as the brain parenchyma. Of these, parenchymal brain metastases are the most common and have been found in as many as 24% of cancer patients in autopsy studies. It has been reported that metastatic brain tumors outnumber primary brain tumors 10 to 1.
To read the full article in PDF:
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Systemic cancer can affect the central nervous system in several different ways, including direct tumor metastasis and indirect remote effects. Intracranial metastasis can involve the skull, dura, and leptomeninges (arachnoid and pia mater), as well as the brain parenchyma. Of these, parenchymal brain metastases are the most common and have been found in as many as 24% of cancer patients in autopsy studies. It has been reported that metastatic brain tumors outnumber primary brain tumors 10 to 1.
To read the full article in PDF:
Quality and Safety During Off Hours
Patients experience acute illness at all hours of the day. In acute care hospitals, over 60% of patient admissions occur outside of normal business hours, or the off hours.[1, 2] Similarly, the acute decompensation of patients already admitted to hospital‐based units is frequent, with 90% of rapid responses occurring between 9 pm and 6 am.[3] Research suggests worse hospital performance during off hours, including increased patient falls, in‐hospital cardiac arrest mortality, and severity of hospital employee injuries.[2, 4, 5, 6, 7]
Although hospital‐based services should match care demand, the disparity between patient acuity and hospital capability at night is significant. Off hours typically have lower staffing of nurses, and attending and housestaff physicians, and ancillary staff as well as limited availability of consultative and supportive services.[8] Additionally, off‐hours providers are subject to the physiological effects of imbalanced circadian rhythms, including fatigue, attenuating their abilities to provide high‐quality care. The significant patient care needs mandate continuous patient care delivery without compromising quality or safety. To achieve this, further defining the barriers to delivering quality care during off hours is essential to improvement efforts in medicine‐based units.
Previous investigations have found increased occurrence and severity of worker accidents, increased potential for higher occurrence of preventable adverse patient events, and decreased performance during off hours.[4, 9, 10] Additionally, detrimental effects of off‐hours care may be further magnified by rotating employees through both day and night shifts, a common practice in academic hospitals.[11, 12] Potentially modifiable outcomes, such as patient fall rate and in‐hospital cardiac arrest survival differ markedly between day and night shifts.[6, 13] These studies primarily report on specific diseases, such as myocardial infarction and stroke, and are investigated from the perspective of hospital‐level outcomes.
To our knowledge, no study has reported provider‐perceived quality and safety issues occurring during off hours in an academic setting. Likewise, although off‐hours collaborative care requires shared, interprofessional conceptualization regarding care delivery, this perspective has not been reported. Understanding the similarities and differences between provider perceptions will allow the construction of an interprofessional team mental model, facilitating the design of future quality improvement initiatives.[14, 15] Our objectives were to: (1) identify off‐hours quality and safety issues, (2) assess which issues are perceived as most significant, and (3) evaluate differences in perceptions of these issues between nurses, and attending and housestaff physicians.
METHODS
Study Design
To investigate quality and safety issues occurring during off hours, we employed a prospective, mixed‐methods sequential exploratory study design, involving an initial qualitative analysis of adverse events followed by quantitative survey assessment.[16] We chose a mixed‐methods approach because provider‐perceived off‐hours issues had not been explicitly identified in the literature, requiring preliminary qualitative assessment. For the purpose of this study, we defined off hours as the 7 pm to 7am time period, which overlapped night shifts for both nurses and physicians. The study was approved by the institutional review board as a quality improvement project.
Study Setting
The study was conducted at a 378‐bed, university‐based acute care hospital in central Pennsylvania. There are a total of 64 internal medicine beds located in 2 units: a general medicine unit (44 beds, staffed by 60 nurses, nurse‐to‐patient ratio 1:4) and an intermediate care unit (20 beds, staffed by 41 nurses, nurse‐to‐patient ratio 1:3). The medicine residency program consists of 69 residents and 14 combined internal medicinepediatrics residents. During the day, 3 teaching teams and 1 nonteaching team care for all medicine patients. Overnight, 3 junior/senior level residents admit patients to the medicine service, whereas 2 interns provide cross‐coverage for all medicine and specialty service patients. Starting in September 2012 (before data collection), an overnight faculty‐level academic hospitalist, or nocturnist, provided on‐site housestaff supervision.
Qualitative Data Collection
For the qualitative analysis, we used 2 methods to develop our database. First, we created an electronic survey (see Supporting Information, Appendix 1, in the online version of this article) to identify near misses/adverse events occurring overnight, distributed to the nocturnist, 3 daytime hospitalists, and unit charge nurses following each shift (October 2012March 2013). The survey items were developed for the purpose of this study, with several items modified from a previously published survey.[17] Second, residency program directors recorded field notes during end‐of‐rotation debriefings (1 hour) with departing overnight housestaff, which were then dictated and transcribed. The subsequent analysis from these sources informed the quantitative survey (see Supporting Information, Appendix 2, in the online version of this article).
Survey Instrument
Three months after the initiation of qualitative data collection, 1 investigator (J.D.G.) developed a preliminary codebook to identify categories and themes. From this codebook, the research team drafted a survey instrument (the complete qualitative analysis occurred after survey development). To maintain focus on systematic quality improvement, items related to perceived mismanagement, relationship tensions, and professionalism were excluded. The survey was pilot‐tested with 5 faculty physicians and 2 nursing staff, prompting several modifications to improve clarity. Primary demographic items included provider role (nurse, attending physician, or housestaff physician) and years in current role. The 24 survey items were grouped into 5 different categories: (1) Quality of Care Delivery, (2) Communication and Coordination, (3) Staffing and Supervision, (4) Patient Transfers, and (5) Consulting Service Issues. Each item was investigated on a 7‐point scale (1=lowest rating, 7=highest rating). Descriptive text was provided at the extremes (choices 1 and 7), whereas intermediary values (26) did not have descriptive cues. The descriptive anchors for Quality of Care Delivery and Patient Transfers were 1=never and 7=always, whereas the descriptive anchors Communication and Coordination and Staffing and Supervision were 1=poor and 7=superior; Consulting Service Issues used a mix of both. Providers with off‐hours experience were asked to rank 4 time periods (710 pm, 10 pm1 am, 14 am, 47 am) regarding quality of care delivery in the medicine units (1=best, 4=worst). We asked both daytime and nighttime providers about perceptions of off‐hours care because, given the boundary spanning the nature of medical care across work shifts, daytime providers frequently identify issues not apparent until hours (or even days) after completion of a night shift. A similar design was used in prior work investigating safety at night.[17]
Quantitative Data Collection
In June of 2013, we emailed a survey link (
Data Analysis
Using the preliminary codebook, 2 investigators (J.D.G., E.M.) jointly analyzed a segment of the dataset using Atlas.ti 6.0 (Scientific Software, Berlin, Germany). Two investigators independently coded the data, compared codes for agreement, and updated the codebook. The remaining data were coded independently, with regular adjudication sessions to modify the codebook. All investigators reviewed and agreed upon themes and representative quotations.
Descriptive statistics, Pearson correlation statistics, Kruskal‐Wallis tests, and signed rank tests (with Bonferroni correction) were used to report group characteristics, correlate rank order, make comparisons between groups (nursing staff, and attending and housestaff physicians; day/night providers), and compare quality rankings by time period, respectively. The data were analyzed using SAS 9.3 (SAS Institute Inc., Cary, NC) and Stata/IC‐8 (StataCorp, College Park, TX).
RESULTS
Qualitative Analysis of Off‐Hours' Adverse Events and Near Misses
A total of 190 events were reported by daytime attending physicians (n=100), nocturnists (n=60), and nighttime charge nurses (n=30). Although questions asked participants to describe near misses/adverse events, respondents also reported a number of global quality issues not related to specific events. Similarly, debriefing sessions with housestaff (n=5) addressed both specific overnight events and residency‐related issues. Seven themes were identified: (1) perceived mismanagement, (2) quality of delivery processes, (3) communication and coordination, (4) staffing and supervision, (5) patient transfers, (6) consulting service issues, and, (7) professionalism/relational tensions. Table 1 lists the code frequencies and exemplary quotations.
| Category and Themes | Code Frequency No. (% of 322) | Representative Quotation |
|---|---|---|
| ||
| Perceived mismanagement | 97 (30) | We had a new admission to the general medicine unit with atrial flutter and rapid ventricular response who did not receive rate controlling agents but rather received diuretics. [The patient's] heart rate remained between 110 and 130 overnight, with a troponin rise in the am likely from demand. The attending note states rate controllers and discussed with housestaff, but this was not performed. |
| Quality of delivery processes | 63 (20) | One patient had a delay in MRI scanning in the off hours due to the scanner being down and scheduling. When the patient went down, there seemed to be little attempt to make sure patient went through scanner; unclear if housestaff called or not to come to assist. Now, the delay in care is even further along. |
| Communication and coordination | 50 (16) | A patient was transferred to the intermediate care unit with hypercarbic respiratory failure. The patient had delay of >1 hour to receive IV Bumex because pharmacy would not release the dose from Pyxis, and the nurse did not let us know there was a delay. When I asked the nurse why, she responded because she's not the only patient I have. I pointed out that the patient was in failure and needed Bumex, stat. If we had not clearly communicated either verbally or via computer, she should let us know how to do that better. |
| Staffing and supervision | 39 (12) | A patient was admitted DNR/DNI with advanced dementia, new on BiPaP at 100%, and hypotensive. The team's intern [identified] the need for interventions, including a central line. This was discussed with overloaded intensive care unit resident. The intern struggled until another resident assisted along with the night attending. Issues included: initial triage, no resident backup for team, and attending backup. I should have been more hands on in the moment to assist the intern navigating the system of care. Many issues here, but no senior resident was involved in care until [late]. |
| Patient transfers | 38 (12) | One patient went from the emergency department [to us] on the 5th floor at 7:45 pm. The ED placed an order for packed red blood cells and it was written at 4:45 pm. When patient arrived on our floor at 7:45 pm, the transfusion had not been started. The floor nurse started it at 8:10 pm . |
| Consulting services | 18 (6) | Regarding a new outside hospital transfer, the medicine team was informed that [the consulting service] would place official consult on the chart when imaging studies from the outside institution were available. Despite this, the consult was still not done after 36 hours, and [we are] still waiting. We contacted service several times. |
| Professionalism and relational tensions | 17 (5) | [One admission from the emergency department] involved a patient who received subcutaneous insulin for hyperkalemia as opposed to intravenous insulin. When brought to [their] attention, they became very confrontational and abrupt and denied having ordered or administered it that way, although it was documented in the EMR. |
Perceived Mismanagement
Participants commonly questioned the decision making, diagnosis, or management of off‐hours providers. Concerns included the response to acute illness (eg, delay in calling a code), treatment decisions (eg, diuresis in a patient with urinary retention), or omission of necessary actions (eg, no cultures ordered for septicemia).
Quality of Delivery Processes
Participants frequently described quality of care delivery issues primarily related to timeliness or delays in delivery processes (34/63 coding references), or patient safety issues (29/63 coding references). Described events revealed concerns about the timeliness of lab reporting, imaging, blood draws, and medication ordering/processing.
Communication and Coordination
Breakdowns in communication and coordination often threatened patient safety. Identified issues included poor communication between primary physicians, nurses, consulting services, and emergency department (ED) providers, as well as documentation within the electronic medical record.
Staffing and Supervision
Several events highlighted staffing or supervision limitations, such as perceived low nursing or physician staffing levels. The degree of nocturnist supervision was polarizing, with both increased and decreased levels of supervision reported as limiting care delivery (or housestaff education).
Patient Transfers
Patient transfers to medicine units from the ED, other inpatient units, or outside hospitals, were identified several times as an influential factor. The care transition and need for information exchange led to a perceived compromise in quality or safety.
Consulting Service Issues
Several examples highlighted perceived issues related to the communication, coordination, or timeliness of consultant services in providing care.
Professionalism/Relational Tensions
Last, providers described situations in which they perceived lack of professionalism or relational tensions between providers, either in regard to interactions or clinical decisions in patient care.
Quantitative Results
Of 214 surveys sent, data were collected from 160 respondents (75% response), including 64/101 nursing staff (63% response), 25/28 attending physicians (80% response), and 71/85 housestaff physicians (84% response). Table 2 describes the participant demographics.
| Variable | No. (%) |
|---|---|
| |
| Nursing staff | 64 (40) |
| Intermediate care unit | 20 |
| General medicine ward | 44 |
| All night shifts | 16 |
| Mix of day and night shifts | 26 |
| Years of experience, mean (SD) | 7.7 (9.7) |
| Attending physicians | 25 (16) |
| No. providing care only at night | 4 |
| No. of weeks as overnight hospitalist in past year, mean (SD) | 11.5 (4.1) |
| No. providing care only during the day | 21 |
| Years since residency graduation, mean (SD) | 9.0 (8.5) |
| Medicine residents | 71 (44) |
| Intern | 27 |
| Junior resident | 23 |
| Senior resident* | 21 |
Off‐Hours Quality and Safety Issues
Ratings and comparisons of the 24 items are shown in Table 3. For all items, the mean rating was below 5 (7‐point scale). Lowest‐rated (least optimal) items were: timeliness, safety, and communication involved with patients admitted from the ED, number of attending physicians, and timeliness of consults and blood draws. Highest‐rated (more optimal) items were: timely reporting of labs, timely identification of deteriorating status, medication ordering and processing, communication between physicians, and safety and communication involved with intraservice transfers.
| Category and Survey Item, Mean (SD)* | Total (160) | Providers With Night Experience | Nighttime Providers (116) | Daytime Providers (44) | P Value | |||
|---|---|---|---|---|---|---|---|---|
| Nurses (41) | Attending Physicians (4) | Housestaff (71) | P Value | |||||
| ||||||||
| Quality of care delivery | ||||||||
| Timely reporting of labs at night | 4.70 (1.39) | 5.12 (1.50) | 4.50 (1.00) | 4.61 (1.47) | 0.11 | 4.78 (1.48) | 4.48 (1.11) | 0.09 |
| Timely identification of deteriorating status | 4.67 (1.34) | 4.88 (1.36) | 5.00 (0.82) | 4.85 (1.20) | 0.93 | 4.86 (1.24) | 4.16 (1.45) | 0.006 |
| Medication ordering and processing | 4.63 (1.13) | 4.88 (1.25) | 5.25 (0.50) | 4.66 (1.08) | 0.19 | 4.76 (1.13) | 4.27 (1.06) | 0.01 |
| Timely completion of imaging at night | 4.29 (1.32) | 4.32 (1.46) | 4.75 (0.96) | 4.39 (1.29) | 0.88 | 4.38 (1.34) | 4.05 (1.26) | 0.12 |
| Timely reporting of results at night | 4.19 (1.43) | 4.27 (1.53) | 4.00 (1.83) | 4.11 (1.44) | 0.84 | 4.16 (1.47) | 4.27 (1.30) | 0.76 |
| Timely med release from pharmacy at night | 4.16 (1.29) | 4.00 (1.32) | 4.50 (0.58) | 4.28 (1.29) | 0.44 | 4.19 (1.28) | 4.09 (1.31) | 0.90 |
| Timely blood draws at night | 3.96 (1.52) | 4.63 (1.44) | 4.50 (0.58) | 3.53 (1.49) | <0.001 | 3.96 (1.54) | 3.98 (1.47) | 0.98 |
| Communication and coordination | ||||||||
| Communication between physicians | 4.63 (1.26) | 4.29 (1.23) | 6.00 (1.15) | 5.14 (1.12) | <0.001 | 4.87 (1.24) | 3.98 (1.09) | <0.001 |
| Communication between nursing and pharmacy | 4.39 (1.27) | 4.83 (1.41) | 5.00 (0.82) | 4.27 (1.29) | 0.04 | 4.49 (1.34) | 4.11 (4.11) | 0.08 |
| Communication between nursing and physicians | 4.39 (1.28) | 4.44 (1.36) | 5.00 (0.82) | 4.58 (1.31) | 0.64 | 4.54 (1.31) | 3.98 (1.13) | 0.01 |
| Documentation in medical record | 4.33 (1.36) | 5.00 (1.36) | 6.00 (0.82) | 4.23 1.19) | <0.001 | 4.56 (1.31) | 3.70 (1.30) | <0.001 |
| Ease of contacting primary providers at night | 4.31 (1.29) | 4.46 (1.27) | 6.00 (0.00) | 4.54 (1.18) | 0.02 | 4.56 (1.22) | 3.66 (1.27) | <0.001 |
| Staffing and supervision | ||||||||
| No. of nursing staff | 4.51 (1.27) | 4.54 (1.50) | 5.50 (0.58) | 4.59 (1.21) | 0.25 | 4.60 (1.31) | 4.25 (1.14) | 0.025 |
| Supervision of housestaff | 4.43 (1.34) | 4.56 (1.40) | 6.25 (0.50) | 4.55 (1.34) | 0.03 | 4.61 (1.37) | 3.95 (1.14) | 0.002 |
| No. of housestaff | 4.09 (1.39) | 4.27 (1.40) | 4.50 (1.29) | 4.11 (1.44) | 0.70 | 4.18 (1.41) | 3.86 (1.32) | 0.12 |
| No. of ancillary staff | 4.00 (1.40) | 4.27 (1.53) | 5.75 (0.96) | 3.85 (1.40) | 0.02 | 4.06 (1.48) | 3.84 (1.18) | 0.27 |
| No. of attending physicians | 3.79 (1.50) | 3.49 (1.76) | 5.25 (0.96) | 3.89 (1.43) | 0.07 | 3.79 (1.57) | 3.80 (1.32) | 0.98 |
| Patient transfers | ||||||||
| For patients accepted to medicine from another medicine unit | ||||||||
| Timely and safe patient transfers | 4.56 (1.28) | 5.15 (1.11) | 4.75 (0.50) | 4.55 (1.23) | 0.025 | 4.77 (1.20) | 4.00 (1.33) | 0.001 |
| High quality communication between providers | 4.55 (1.35) | 5.34 (1.13) | 5.00 (0.82) | 4.49 (1.22) | 0.001 | 4.81 (1.24) | 3.86 (1.41) | <0.001 |
| For patients admitted from emergency department to medicine unit | ||||||||
| Appropriate testing and treatment | 4.16 (1.34) | 4.15 (1.30) | 4.00 (1.83) | 4.21 (1.43) | 0.96 | 4.18 (1.39) | 4.11 (1.20) | 0.66 |
| Timely and safe transfers | 3.89 (1.38) | 3.63 (1.50) | 5.50 (0.58) | 4.08 (1.32) | 0.02 | 3.97 (1.40) | 3.68 1.29) | 0.23 |
| High‐quality communication between providers | 2.93 (1.38) | 2.56 (1.23) | 3.75 (1.26) | 3.00 (1.39) | 0.08 | 2.87 (1.35) | 3.07 (1.47) | 0.41 |
| Consulting service issues | ||||||||
| Timely consults at night | 4.04 (1.35) | 4.27 (1.28) | 4.00 (0.82) | 4.10 (1.47) | 0.69 | 4.16 (1.38) | 3.73 (1.25) | 0.053 |
| Communication between consults and physicians | 3.93 (1.40) | 3.46 (1.45) | 5.75 (1.26) | 4.35 (1.27) | <0.001 | 4.09 (1.42) | 3.50 (1.27) | 0.016 |
Comparisons Between Professional Groups With Night Experience
Of the 24 items, 11 showed statistically significant differences between groups (P<0.05). Items with the largest difference between groups included: timely blood draws at night (housestaff physicians lowest), communication between physicians (nursing lowest), documentation in medical record (housestaff physicians lowest), and communication between consults and physicians (nursing lowest). The rank order between housestaff physicians and nurses, and housestaff and attending physicians showed moderately positive correlations (r=0.61, P=0.002 and r=0.47, P=0.022, respectively). The correlation between nurses and attending physicians showed a weak correlation (r=0.19, P=0.375).
Comparisons Between Front‐Line Providers With and Without Night Experience
Of the 24 items, 12 showed statistically significant differences between groups (P<0.05), with day providers reporting lower ratings in all 12. Items with the largest difference between groups included: communication between consults and physicians, ease of contacting providers, communication between providers, documentation, and safety and communication related to transfers from other units. The rank order between night and day groups showed a statistically significant positive correlation (r=0.65, P=0.001).
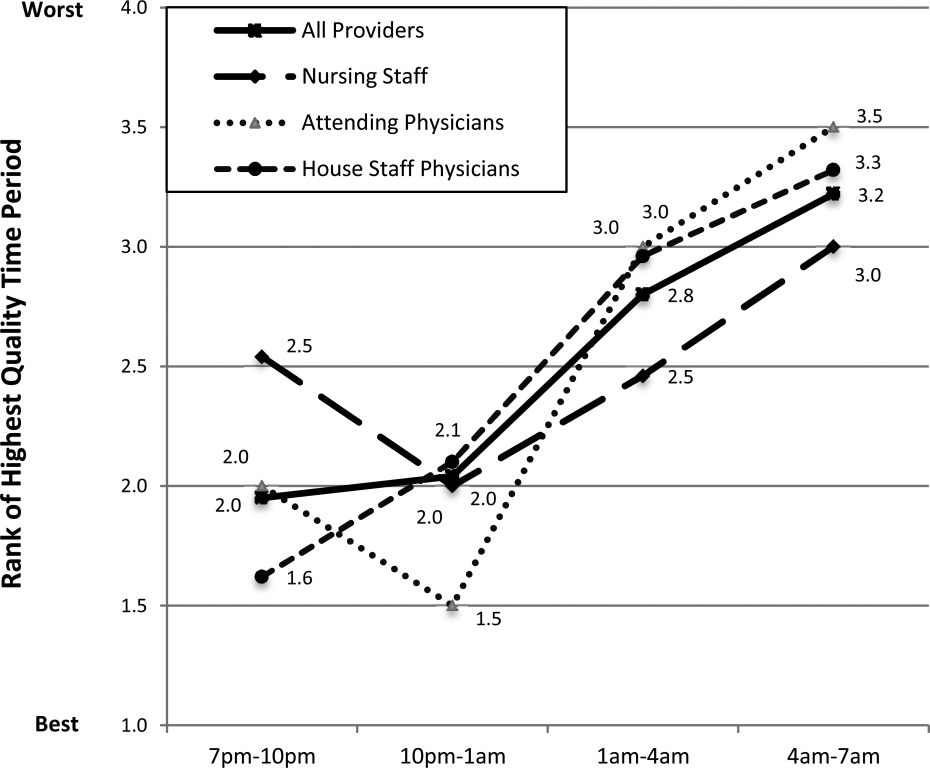
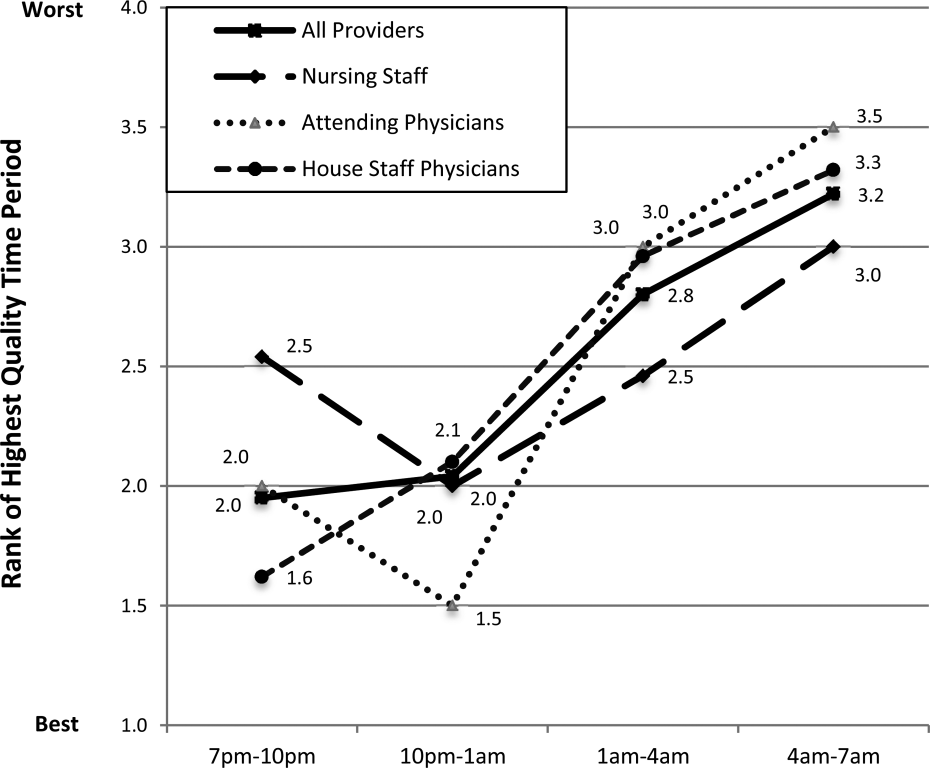
Perceived Highest Quality of Care Time Period During Off Hours
Compared with other time periods, all providers ranked 4 to 7 am as the period with the lowest quality of care delivery (mean rank 3.2, P0.001) (Figure 1). Nursing staff and attending physicians both ranked the 10 pm to 1 am time period as the best period (mean of 2.0 and 1.5, respectively), whereas housestaff physicians ranked the 7 to 10 pm as the best time period (mean 1.62). The only statistical difference between provider groups for any given time period was the 7 to 10 pm time period (P=0.002).
DISCUSSION
In this prospective, mixed‐methods study evaluating perceived off‐hours quality and safety issues, several themes were identified, including perceived mismanagement, insufficient quality of delivery processes, communication/coordination breakdowns, and staffing and supervision issues. In the quantitative analysis, lowest‐rated items (lowest quality) related to timeliness/safety/communication involved with ED transfers, number of attending physicians, and timeliness of consults and blood draws. Highest‐rated items (highest quality) related to timeliness of lab reporting and identification of deteriorating patients, medication ordering/processing, communication between physicians, and safety/communication during intraservice transfers. In general, day providers reported lower ratings than night providers on nearly all quality‐related items. Nursing staff reported the lowest ratings regarding communication between physicians and consults, whereas housestaff physicians reported the lowest ratings regarding documentation in the medical record and timely blood draws. These between‐group differences reveal the lack of shared conceptual understanding regarding off‐hours care delivery.
Our qualitative results reveal several significant issues related to care delivery during off hours, many of which are not obtainable by hospital‐level data or chart review.[18] For hospital‐based medicine units, an understanding of the structure‐ and process‐related factors associated with events is required for quality improvement efforts. Although the primary focus for this work was the off hours, it is plausible that providers may have identified similar issues as important issues during daytime hours. Our study was not designed to investigate if these perceived issues are specific to off hours, or if these issues are an accurate reflection of objective events occurring during this time period. We believe this topic deserves further investigation, as understanding if these off‐hours perceptions are unique to this time period would change the scope of future quality improvement initiatives.
The most significant finding in the quantitative results was the vulnerability in quality and safety during patient admissions from the ED, specifically in relation to communication and timeliness of transfer. Between‐unit handoffs for patients admitted from the ED to medicine units have been identified as particularly vulnerable to breakdowns in the communication process.[19, 20, 21, 22] There are multiple etiologies, including clinical uncertainty, higher acuity in patient illness early in hospitalization, and cultural differences between services.[23] Additionally, patterns of communication and standardized handoff processes are often insufficient. In our hospital system, the transfer process relies primarily upon synchronous communication methods without standardized, asynchronous information exchange. We hypothesize front‐line providers perceive this lack of standardization as a primary threat to quality. Because approximately 60% of new patient admissions from the ED to medicine service (both in our hospital and in prior studies) occur during off hours, these findings highlight a need for subsequent study and quality improvement efforts.[24]
During the time of this study, our medicine units were staffed at night by 5 medicine housestaff physicians and 1 academic hospitalist, or nocturnist. In efforts to improve quality and safety during off hours, our hospital, as well as other health systems, implemented the nocturnist position, a faculty‐level attending physician to provide off‐hours clinical care and housestaff supervision.[25] Although participants reported a moderate rating of housestaff supervision, participants provided lower scores for staffing numbers of nurses, and housestaff and attending physicians, despite nocturnist presence. With both increased off‐hours supervision in our hospital and increasing use of faculty‐level physicians in other academic programs, these results provide context for the anticipated level of overnight housestaff supervision.[26, 27] To our knowledge, this is the first study to investigate perceived overnight quality issues on medicine units following such staffing models. Although this model of direct, on‐site supervision in academic medicine programs may help offset staffing and supervisory issues during off hours, the nocturnist role is insufficient to offset threats to quality/safety already inherent within the system. Furthermore, prospective trials following implementation of nocturnist systems have shown mixed results in improving patient outcomes.[28] These findings have led some to question whether resources dedicated to nocturnist staffing may be better allocated to other overnight initiatives, highlighting the need for a more subtle understanding of quality issues to design targeted interventions.[29]
A notable finding from this work is that providers without night experience reported lower scores for 20 of 24 items, highlighting their perceptions of the quality of care delivery during off hours are lower than those who experience this environment. Although day providers are not directly experiencing off‐hours delivery processes, these providers receive and detect the results from care delivery at night.[17] Most nurse, physician, and hospital leaders are present in the hospital only during day hours, requiring these individuals to account for differences in perceived and actual care delivered overnight.[1] These individuals make critical decisions pertaining to process changes and quality improvement efforts in these units. We believe these results raise awareness for leadership decisions and quality improvement efforts in medicine service units, specifically to focus on overnight issues beyond staffing issues alone.
All respondent groups ranked the latter half of the shift (17 am) as lower in quality compared to the first 6 hours (7 pm1 am). This finding is contrary to our hypothesis that earlier time periods, during the majority of patient admissions (and presumed higher workload for all providers), would be perceived as lower quality. Reasons for this finding are unknown, but may relate to end‐of‐shift tasks, sign‐out preparation, provider fatigue, or disease‐related concerns (eg, increased incidence of stroke and myocardial infarction) during the latter portions of night shifts. One study identified a decrease in nursing clinical judgments from the beginning to end of 12‐hour shifts, with a potential suggested mechanism of decrease in ability to maintain attention during judgments.[30] Additionally, in a study by Folkard et al., risk was highest within the first several hours and fell substantially thereafter during a shift.[9] To our knowledge, no work has investigated perceived or objective quality outcomes by time period during the off‐hours shift in medicine units. Further work could help delineate why provider‐perceived compromises in quality occur late in off‐hours shifts and whether this correlates to safety events.
There are several limitations to our study. First, although all surveys were pilot tested for content validity, the construct validity was not rigorously assessed. Second, although data were collected from all participant groups, the collection methods were unbalanced, favoring attending‐level physician perspectives. Although the relative incidence of vulnerabilities in quality and safety should be interpreted with caution, our methods and general taxonomy provide a framework for developing and monitoring the perceptions of future interventions. Due to limitations in infrastructure, our findings could not be independently validated through review of reported adverse events, but previous investigations have found the vast majority of adverse events are not detected by standard anonymous reporting.[31, 32, 33] Our methodology (used in our prior work) may provide an independent means of detecting causes of poor quality not easily observed through routine surveillance.[22] Although many survey items showed statistical differences between provider groups, the clinical significance is subject to interpretation. Last, the perceptions and events related to our institution may not be fully generalizable to other academic programs or service lines, particularly in community‐based, nonteaching hospitals.
In conclusion, our results suggest a significant discrepancy between the concerns of day and night providers regarding the quality of care delivered to inpatients during the off hours, specifically with issues related to communication, quality‐of‐care delivery processes, and patient transfers from the ED. Although specific concerns may be institution‐ (and service line‐) dependent, appropriately designing initiatives to improve the quality of care delivered overnight will need to take the perspectives of both provider groups into account. Additionally, educational initiatives should focus on achieving a shared mental model among all providers to improve collaboration and performance.
Acknowledgements
The authors thank the nurses, internal medicine housestaff physicians, and general internal medicine attending physicians at the Penn State Hershey Medical Center for their participation in this study.
Disclosure: Nothing to report.
- . Like night and day—shedding light on off‐hours care. N Engl J Med. 2008;358(20):2091–2093.
- , , , . Call nights and patient care. J Gen Intern Med. 1992;7(4):405–410.
- , , , et al. Uncovering system errors using a rapid response team: cross‐coverage caught in the crossfire. Discussion. J Trauma. 2009;67(1):173–179.
- , . The impact of shift work on the risk and severity of injuries for hospital employees: an analysis using Oregon workers' compensation data. Occup Med (Lond). 2004;54(8):556–563.
- , . Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med. 2001;345(9):663–668.
- , , , et al. The association of shift‐level nurse staffing with adverse patient events. J Nurs Adm. 2011;41(2):64–70.
- , , , et al. Heart disease and stroke statistics—2010 update A report from the American Heart Association. Circulation. 2010;121(7):e46–e215.
- , , , , O'Neil E. Minimum Nurse Staffing Ratios In California Acute Care Hospitals. Oakland, CA: California Workforce Initiative; 2000.
- , . Shift work, safety and productivity. Occup Med (Lond). 2003;53(2):95–101.
- , , . Increased injuries on night shift. Lancet. 1994;344(8930):1137–1139.
- , . Shift and night work and long working hours‐a systematic review of safety implications. Scand J Work Environ Health. 2011:37(3):173–185.
- , , , et al. Rotating shift work, sleep, and accidents related to sleepiness in hospital nurses. Am J Public Health. 1992;82(7):1011–1014.
- , , , et al. Survival from in‐hospital cardiac arrest during nights and weekends. JAMA. 2008;299(7):785–792.
- , , , , . The influence of shared mental models on team process and performance. J Appl Psychol. 2000;85(2):273.
- , . Team mental models and their potential to improve teamwork and safety: a review and implications for future research in healthcare. Saf Sci. 2012;50(5):1344–1354.
- . Editorial: mapping the field of mixed methods research. J Mix Methods Res. 2009;3(2):95–108.
- , . Decreasing adverse events through night talks: an interdisciplinary, hospital‐based quality improvement project. Perm J. Fall 2009;13(4):16–22.
- , , , et al. “Global trigger tool” shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff (Millwood). 2011;30(4):581–589.
- , , , , , . Dropping the baton: a qualitative analysis of failures during the transition from emergency department to inpatient care. Ann Emerg Med. 2009;53(6):701–710.e704.
- . Smoothing transitions. Joint Commission targets patient handoffs. Mod Healthc. 2010;40(43):8–9.
- , , , , , . The patient handoff: a comprehensive curricular blueprint for resident education to improve continuity of care. Acad Med. 2012;87(4):411–418.
- , , , , , . Patient care transitions from the emergency department to the medicine ward: evaluation of a standardized electronic signout tool. Int J Qual Health Care. 2014;26(4):337–347.
- , . The unappreciated challenges of between‐unit handoffs: negotiating and coordinating across boundaries. Ann Emerg Med. 2013;61(2):155–160.
- , , , , , . The association between night or weekend admission and hospitalization‐relevant patient outcomes. J Hosp Med. 2011;6(1):10–14.
- . Middle‐of‐the‐night medicine is rarely patient‐centred. CMAJ. 2011;183(13):1467–1468.
- , , , et al. Survey of overnight academic hospitalist supervision of trainees. J Hosp Med. 2012;7(7):521–523.
- , , , , , . Effects of increased overnight supervision on resident education, decision‐making, and autonomy. J Hosp Med. 2012;7(8):606–610.
- , , , et al. A randomized trial of nighttime physician staffing in an intensive care unit. N Engl J Med. 2013;368(23):2201–2209.
- . Intensivists at night: putting resources in the right place. Crit Care. 2013;17(5):1008.
- , , . Changes in nurses' decision making during a 12‐h day shift. Occup Med (Lond). 2013;63(1):60–65.
- , , , , , . The incident reporting system does not detect adverse drug events: a problem for quality improvement. Jt Comm J Qual Improv. 1995;21(10):541–548.
- , , , , . An evaluation of adverse incident reporting. J Eval Clin Pract. 1999;5(1):5–12.
- , . Reporting and preventing medical mishaps: lessons from non‐medical near miss reporting systems. BMJ. 2000;320(7237):759–763.
Patients experience acute illness at all hours of the day. In acute care hospitals, over 60% of patient admissions occur outside of normal business hours, or the off hours.[1, 2] Similarly, the acute decompensation of patients already admitted to hospital‐based units is frequent, with 90% of rapid responses occurring between 9 pm and 6 am.[3] Research suggests worse hospital performance during off hours, including increased patient falls, in‐hospital cardiac arrest mortality, and severity of hospital employee injuries.[2, 4, 5, 6, 7]
Although hospital‐based services should match care demand, the disparity between patient acuity and hospital capability at night is significant. Off hours typically have lower staffing of nurses, and attending and housestaff physicians, and ancillary staff as well as limited availability of consultative and supportive services.[8] Additionally, off‐hours providers are subject to the physiological effects of imbalanced circadian rhythms, including fatigue, attenuating their abilities to provide high‐quality care. The significant patient care needs mandate continuous patient care delivery without compromising quality or safety. To achieve this, further defining the barriers to delivering quality care during off hours is essential to improvement efforts in medicine‐based units.
Previous investigations have found increased occurrence and severity of worker accidents, increased potential for higher occurrence of preventable adverse patient events, and decreased performance during off hours.[4, 9, 10] Additionally, detrimental effects of off‐hours care may be further magnified by rotating employees through both day and night shifts, a common practice in academic hospitals.[11, 12] Potentially modifiable outcomes, such as patient fall rate and in‐hospital cardiac arrest survival differ markedly between day and night shifts.[6, 13] These studies primarily report on specific diseases, such as myocardial infarction and stroke, and are investigated from the perspective of hospital‐level outcomes.
To our knowledge, no study has reported provider‐perceived quality and safety issues occurring during off hours in an academic setting. Likewise, although off‐hours collaborative care requires shared, interprofessional conceptualization regarding care delivery, this perspective has not been reported. Understanding the similarities and differences between provider perceptions will allow the construction of an interprofessional team mental model, facilitating the design of future quality improvement initiatives.[14, 15] Our objectives were to: (1) identify off‐hours quality and safety issues, (2) assess which issues are perceived as most significant, and (3) evaluate differences in perceptions of these issues between nurses, and attending and housestaff physicians.
METHODS
Study Design
To investigate quality and safety issues occurring during off hours, we employed a prospective, mixed‐methods sequential exploratory study design, involving an initial qualitative analysis of adverse events followed by quantitative survey assessment.[16] We chose a mixed‐methods approach because provider‐perceived off‐hours issues had not been explicitly identified in the literature, requiring preliminary qualitative assessment. For the purpose of this study, we defined off hours as the 7 pm to 7am time period, which overlapped night shifts for both nurses and physicians. The study was approved by the institutional review board as a quality improvement project.
Study Setting
The study was conducted at a 378‐bed, university‐based acute care hospital in central Pennsylvania. There are a total of 64 internal medicine beds located in 2 units: a general medicine unit (44 beds, staffed by 60 nurses, nurse‐to‐patient ratio 1:4) and an intermediate care unit (20 beds, staffed by 41 nurses, nurse‐to‐patient ratio 1:3). The medicine residency program consists of 69 residents and 14 combined internal medicinepediatrics residents. During the day, 3 teaching teams and 1 nonteaching team care for all medicine patients. Overnight, 3 junior/senior level residents admit patients to the medicine service, whereas 2 interns provide cross‐coverage for all medicine and specialty service patients. Starting in September 2012 (before data collection), an overnight faculty‐level academic hospitalist, or nocturnist, provided on‐site housestaff supervision.
Qualitative Data Collection
For the qualitative analysis, we used 2 methods to develop our database. First, we created an electronic survey (see Supporting Information, Appendix 1, in the online version of this article) to identify near misses/adverse events occurring overnight, distributed to the nocturnist, 3 daytime hospitalists, and unit charge nurses following each shift (October 2012March 2013). The survey items were developed for the purpose of this study, with several items modified from a previously published survey.[17] Second, residency program directors recorded field notes during end‐of‐rotation debriefings (1 hour) with departing overnight housestaff, which were then dictated and transcribed. The subsequent analysis from these sources informed the quantitative survey (see Supporting Information, Appendix 2, in the online version of this article).
Survey Instrument
Three months after the initiation of qualitative data collection, 1 investigator (J.D.G.) developed a preliminary codebook to identify categories and themes. From this codebook, the research team drafted a survey instrument (the complete qualitative analysis occurred after survey development). To maintain focus on systematic quality improvement, items related to perceived mismanagement, relationship tensions, and professionalism were excluded. The survey was pilot‐tested with 5 faculty physicians and 2 nursing staff, prompting several modifications to improve clarity. Primary demographic items included provider role (nurse, attending physician, or housestaff physician) and years in current role. The 24 survey items were grouped into 5 different categories: (1) Quality of Care Delivery, (2) Communication and Coordination, (3) Staffing and Supervision, (4) Patient Transfers, and (5) Consulting Service Issues. Each item was investigated on a 7‐point scale (1=lowest rating, 7=highest rating). Descriptive text was provided at the extremes (choices 1 and 7), whereas intermediary values (26) did not have descriptive cues. The descriptive anchors for Quality of Care Delivery and Patient Transfers were 1=never and 7=always, whereas the descriptive anchors Communication and Coordination and Staffing and Supervision were 1=poor and 7=superior; Consulting Service Issues used a mix of both. Providers with off‐hours experience were asked to rank 4 time periods (710 pm, 10 pm1 am, 14 am, 47 am) regarding quality of care delivery in the medicine units (1=best, 4=worst). We asked both daytime and nighttime providers about perceptions of off‐hours care because, given the boundary spanning the nature of medical care across work shifts, daytime providers frequently identify issues not apparent until hours (or even days) after completion of a night shift. A similar design was used in prior work investigating safety at night.[17]
Quantitative Data Collection
In June of 2013, we emailed a survey link (
Data Analysis
Using the preliminary codebook, 2 investigators (J.D.G., E.M.) jointly analyzed a segment of the dataset using Atlas.ti 6.0 (Scientific Software, Berlin, Germany). Two investigators independently coded the data, compared codes for agreement, and updated the codebook. The remaining data were coded independently, with regular adjudication sessions to modify the codebook. All investigators reviewed and agreed upon themes and representative quotations.
Descriptive statistics, Pearson correlation statistics, Kruskal‐Wallis tests, and signed rank tests (with Bonferroni correction) were used to report group characteristics, correlate rank order, make comparisons between groups (nursing staff, and attending and housestaff physicians; day/night providers), and compare quality rankings by time period, respectively. The data were analyzed using SAS 9.3 (SAS Institute Inc., Cary, NC) and Stata/IC‐8 (StataCorp, College Park, TX).
RESULTS
Qualitative Analysis of Off‐Hours' Adverse Events and Near Misses
A total of 190 events were reported by daytime attending physicians (n=100), nocturnists (n=60), and nighttime charge nurses (n=30). Although questions asked participants to describe near misses/adverse events, respondents also reported a number of global quality issues not related to specific events. Similarly, debriefing sessions with housestaff (n=5) addressed both specific overnight events and residency‐related issues. Seven themes were identified: (1) perceived mismanagement, (2) quality of delivery processes, (3) communication and coordination, (4) staffing and supervision, (5) patient transfers, (6) consulting service issues, and, (7) professionalism/relational tensions. Table 1 lists the code frequencies and exemplary quotations.
| Category and Themes | Code Frequency No. (% of 322) | Representative Quotation |
|---|---|---|
| ||
| Perceived mismanagement | 97 (30) | We had a new admission to the general medicine unit with atrial flutter and rapid ventricular response who did not receive rate controlling agents but rather received diuretics. [The patient's] heart rate remained between 110 and 130 overnight, with a troponin rise in the am likely from demand. The attending note states rate controllers and discussed with housestaff, but this was not performed. |
| Quality of delivery processes | 63 (20) | One patient had a delay in MRI scanning in the off hours due to the scanner being down and scheduling. When the patient went down, there seemed to be little attempt to make sure patient went through scanner; unclear if housestaff called or not to come to assist. Now, the delay in care is even further along. |
| Communication and coordination | 50 (16) | A patient was transferred to the intermediate care unit with hypercarbic respiratory failure. The patient had delay of >1 hour to receive IV Bumex because pharmacy would not release the dose from Pyxis, and the nurse did not let us know there was a delay. When I asked the nurse why, she responded because she's not the only patient I have. I pointed out that the patient was in failure and needed Bumex, stat. If we had not clearly communicated either verbally or via computer, she should let us know how to do that better. |
| Staffing and supervision | 39 (12) | A patient was admitted DNR/DNI with advanced dementia, new on BiPaP at 100%, and hypotensive. The team's intern [identified] the need for interventions, including a central line. This was discussed with overloaded intensive care unit resident. The intern struggled until another resident assisted along with the night attending. Issues included: initial triage, no resident backup for team, and attending backup. I should have been more hands on in the moment to assist the intern navigating the system of care. Many issues here, but no senior resident was involved in care until [late]. |
| Patient transfers | 38 (12) | One patient went from the emergency department [to us] on the 5th floor at 7:45 pm. The ED placed an order for packed red blood cells and it was written at 4:45 pm. When patient arrived on our floor at 7:45 pm, the transfusion had not been started. The floor nurse started it at 8:10 pm . |
| Consulting services | 18 (6) | Regarding a new outside hospital transfer, the medicine team was informed that [the consulting service] would place official consult on the chart when imaging studies from the outside institution were available. Despite this, the consult was still not done after 36 hours, and [we are] still waiting. We contacted service several times. |
| Professionalism and relational tensions | 17 (5) | [One admission from the emergency department] involved a patient who received subcutaneous insulin for hyperkalemia as opposed to intravenous insulin. When brought to [their] attention, they became very confrontational and abrupt and denied having ordered or administered it that way, although it was documented in the EMR. |
Perceived Mismanagement
Participants commonly questioned the decision making, diagnosis, or management of off‐hours providers. Concerns included the response to acute illness (eg, delay in calling a code), treatment decisions (eg, diuresis in a patient with urinary retention), or omission of necessary actions (eg, no cultures ordered for septicemia).
Quality of Delivery Processes
Participants frequently described quality of care delivery issues primarily related to timeliness or delays in delivery processes (34/63 coding references), or patient safety issues (29/63 coding references). Described events revealed concerns about the timeliness of lab reporting, imaging, blood draws, and medication ordering/processing.
Communication and Coordination
Breakdowns in communication and coordination often threatened patient safety. Identified issues included poor communication between primary physicians, nurses, consulting services, and emergency department (ED) providers, as well as documentation within the electronic medical record.
Staffing and Supervision
Several events highlighted staffing or supervision limitations, such as perceived low nursing or physician staffing levels. The degree of nocturnist supervision was polarizing, with both increased and decreased levels of supervision reported as limiting care delivery (or housestaff education).
Patient Transfers
Patient transfers to medicine units from the ED, other inpatient units, or outside hospitals, were identified several times as an influential factor. The care transition and need for information exchange led to a perceived compromise in quality or safety.
Consulting Service Issues
Several examples highlighted perceived issues related to the communication, coordination, or timeliness of consultant services in providing care.
Professionalism/Relational Tensions
Last, providers described situations in which they perceived lack of professionalism or relational tensions between providers, either in regard to interactions or clinical decisions in patient care.
Quantitative Results
Of 214 surveys sent, data were collected from 160 respondents (75% response), including 64/101 nursing staff (63% response), 25/28 attending physicians (80% response), and 71/85 housestaff physicians (84% response). Table 2 describes the participant demographics.
| Variable | No. (%) |
|---|---|
| |
| Nursing staff | 64 (40) |
| Intermediate care unit | 20 |
| General medicine ward | 44 |
| All night shifts | 16 |
| Mix of day and night shifts | 26 |
| Years of experience, mean (SD) | 7.7 (9.7) |
| Attending physicians | 25 (16) |
| No. providing care only at night | 4 |
| No. of weeks as overnight hospitalist in past year, mean (SD) | 11.5 (4.1) |
| No. providing care only during the day | 21 |
| Years since residency graduation, mean (SD) | 9.0 (8.5) |
| Medicine residents | 71 (44) |
| Intern | 27 |
| Junior resident | 23 |
| Senior resident* | 21 |
Off‐Hours Quality and Safety Issues
Ratings and comparisons of the 24 items are shown in Table 3. For all items, the mean rating was below 5 (7‐point scale). Lowest‐rated (least optimal) items were: timeliness, safety, and communication involved with patients admitted from the ED, number of attending physicians, and timeliness of consults and blood draws. Highest‐rated (more optimal) items were: timely reporting of labs, timely identification of deteriorating status, medication ordering and processing, communication between physicians, and safety and communication involved with intraservice transfers.
| Category and Survey Item, Mean (SD)* | Total (160) | Providers With Night Experience | Nighttime Providers (116) | Daytime Providers (44) | P Value | |||
|---|---|---|---|---|---|---|---|---|
| Nurses (41) | Attending Physicians (4) | Housestaff (71) | P Value | |||||
| ||||||||
| Quality of care delivery | ||||||||
| Timely reporting of labs at night | 4.70 (1.39) | 5.12 (1.50) | 4.50 (1.00) | 4.61 (1.47) | 0.11 | 4.78 (1.48) | 4.48 (1.11) | 0.09 |
| Timely identification of deteriorating status | 4.67 (1.34) | 4.88 (1.36) | 5.00 (0.82) | 4.85 (1.20) | 0.93 | 4.86 (1.24) | 4.16 (1.45) | 0.006 |
| Medication ordering and processing | 4.63 (1.13) | 4.88 (1.25) | 5.25 (0.50) | 4.66 (1.08) | 0.19 | 4.76 (1.13) | 4.27 (1.06) | 0.01 |
| Timely completion of imaging at night | 4.29 (1.32) | 4.32 (1.46) | 4.75 (0.96) | 4.39 (1.29) | 0.88 | 4.38 (1.34) | 4.05 (1.26) | 0.12 |
| Timely reporting of results at night | 4.19 (1.43) | 4.27 (1.53) | 4.00 (1.83) | 4.11 (1.44) | 0.84 | 4.16 (1.47) | 4.27 (1.30) | 0.76 |
| Timely med release from pharmacy at night | 4.16 (1.29) | 4.00 (1.32) | 4.50 (0.58) | 4.28 (1.29) | 0.44 | 4.19 (1.28) | 4.09 (1.31) | 0.90 |
| Timely blood draws at night | 3.96 (1.52) | 4.63 (1.44) | 4.50 (0.58) | 3.53 (1.49) | <0.001 | 3.96 (1.54) | 3.98 (1.47) | 0.98 |
| Communication and coordination | ||||||||
| Communication between physicians | 4.63 (1.26) | 4.29 (1.23) | 6.00 (1.15) | 5.14 (1.12) | <0.001 | 4.87 (1.24) | 3.98 (1.09) | <0.001 |
| Communication between nursing and pharmacy | 4.39 (1.27) | 4.83 (1.41) | 5.00 (0.82) | 4.27 (1.29) | 0.04 | 4.49 (1.34) | 4.11 (4.11) | 0.08 |
| Communication between nursing and physicians | 4.39 (1.28) | 4.44 (1.36) | 5.00 (0.82) | 4.58 (1.31) | 0.64 | 4.54 (1.31) | 3.98 (1.13) | 0.01 |
| Documentation in medical record | 4.33 (1.36) | 5.00 (1.36) | 6.00 (0.82) | 4.23 1.19) | <0.001 | 4.56 (1.31) | 3.70 (1.30) | <0.001 |
| Ease of contacting primary providers at night | 4.31 (1.29) | 4.46 (1.27) | 6.00 (0.00) | 4.54 (1.18) | 0.02 | 4.56 (1.22) | 3.66 (1.27) | <0.001 |
| Staffing and supervision | ||||||||
| No. of nursing staff | 4.51 (1.27) | 4.54 (1.50) | 5.50 (0.58) | 4.59 (1.21) | 0.25 | 4.60 (1.31) | 4.25 (1.14) | 0.025 |
| Supervision of housestaff | 4.43 (1.34) | 4.56 (1.40) | 6.25 (0.50) | 4.55 (1.34) | 0.03 | 4.61 (1.37) | 3.95 (1.14) | 0.002 |
| No. of housestaff | 4.09 (1.39) | 4.27 (1.40) | 4.50 (1.29) | 4.11 (1.44) | 0.70 | 4.18 (1.41) | 3.86 (1.32) | 0.12 |
| No. of ancillary staff | 4.00 (1.40) | 4.27 (1.53) | 5.75 (0.96) | 3.85 (1.40) | 0.02 | 4.06 (1.48) | 3.84 (1.18) | 0.27 |
| No. of attending physicians | 3.79 (1.50) | 3.49 (1.76) | 5.25 (0.96) | 3.89 (1.43) | 0.07 | 3.79 (1.57) | 3.80 (1.32) | 0.98 |
| Patient transfers | ||||||||
| For patients accepted to medicine from another medicine unit | ||||||||
| Timely and safe patient transfers | 4.56 (1.28) | 5.15 (1.11) | 4.75 (0.50) | 4.55 (1.23) | 0.025 | 4.77 (1.20) | 4.00 (1.33) | 0.001 |
| High quality communication between providers | 4.55 (1.35) | 5.34 (1.13) | 5.00 (0.82) | 4.49 (1.22) | 0.001 | 4.81 (1.24) | 3.86 (1.41) | <0.001 |
| For patients admitted from emergency department to medicine unit | ||||||||
| Appropriate testing and treatment | 4.16 (1.34) | 4.15 (1.30) | 4.00 (1.83) | 4.21 (1.43) | 0.96 | 4.18 (1.39) | 4.11 (1.20) | 0.66 |
| Timely and safe transfers | 3.89 (1.38) | 3.63 (1.50) | 5.50 (0.58) | 4.08 (1.32) | 0.02 | 3.97 (1.40) | 3.68 1.29) | 0.23 |
| High‐quality communication between providers | 2.93 (1.38) | 2.56 (1.23) | 3.75 (1.26) | 3.00 (1.39) | 0.08 | 2.87 (1.35) | 3.07 (1.47) | 0.41 |
| Consulting service issues | ||||||||
| Timely consults at night | 4.04 (1.35) | 4.27 (1.28) | 4.00 (0.82) | 4.10 (1.47) | 0.69 | 4.16 (1.38) | 3.73 (1.25) | 0.053 |
| Communication between consults and physicians | 3.93 (1.40) | 3.46 (1.45) | 5.75 (1.26) | 4.35 (1.27) | <0.001 | 4.09 (1.42) | 3.50 (1.27) | 0.016 |
Comparisons Between Professional Groups With Night Experience
Of the 24 items, 11 showed statistically significant differences between groups (P<0.05). Items with the largest difference between groups included: timely blood draws at night (housestaff physicians lowest), communication between physicians (nursing lowest), documentation in medical record (housestaff physicians lowest), and communication between consults and physicians (nursing lowest). The rank order between housestaff physicians and nurses, and housestaff and attending physicians showed moderately positive correlations (r=0.61, P=0.002 and r=0.47, P=0.022, respectively). The correlation between nurses and attending physicians showed a weak correlation (r=0.19, P=0.375).
Comparisons Between Front‐Line Providers With and Without Night Experience
Of the 24 items, 12 showed statistically significant differences between groups (P<0.05), with day providers reporting lower ratings in all 12. Items with the largest difference between groups included: communication between consults and physicians, ease of contacting providers, communication between providers, documentation, and safety and communication related to transfers from other units. The rank order between night and day groups showed a statistically significant positive correlation (r=0.65, P=0.001).
Perceived Highest Quality of Care Time Period During Off Hours
Compared with other time periods, all providers ranked 4 to 7 am as the period with the lowest quality of care delivery (mean rank 3.2, P0.001) (Figure 1). Nursing staff and attending physicians both ranked the 10 pm to 1 am time period as the best period (mean of 2.0 and 1.5, respectively), whereas housestaff physicians ranked the 7 to 10 pm as the best time period (mean 1.62). The only statistical difference between provider groups for any given time period was the 7 to 10 pm time period (P=0.002).

DISCUSSION
In this prospective, mixed‐methods study evaluating perceived off‐hours quality and safety issues, several themes were identified, including perceived mismanagement, insufficient quality of delivery processes, communication/coordination breakdowns, and staffing and supervision issues. In the quantitative analysis, lowest‐rated items (lowest quality) related to timeliness/safety/communication involved with ED transfers, number of attending physicians, and timeliness of consults and blood draws. Highest‐rated items (highest quality) related to timeliness of lab reporting and identification of deteriorating patients, medication ordering/processing, communication between physicians, and safety/communication during intraservice transfers. In general, day providers reported lower ratings than night providers on nearly all quality‐related items. Nursing staff reported the lowest ratings regarding communication between physicians and consults, whereas housestaff physicians reported the lowest ratings regarding documentation in the medical record and timely blood draws. These between‐group differences reveal the lack of shared conceptual understanding regarding off‐hours care delivery.
Our qualitative results reveal several significant issues related to care delivery during off hours, many of which are not obtainable by hospital‐level data or chart review.[18] For hospital‐based medicine units, an understanding of the structure‐ and process‐related factors associated with events is required for quality improvement efforts. Although the primary focus for this work was the off hours, it is plausible that providers may have identified similar issues as important issues during daytime hours. Our study was not designed to investigate if these perceived issues are specific to off hours, or if these issues are an accurate reflection of objective events occurring during this time period. We believe this topic deserves further investigation, as understanding if these off‐hours perceptions are unique to this time period would change the scope of future quality improvement initiatives.
The most significant finding in the quantitative results was the vulnerability in quality and safety during patient admissions from the ED, specifically in relation to communication and timeliness of transfer. Between‐unit handoffs for patients admitted from the ED to medicine units have been identified as particularly vulnerable to breakdowns in the communication process.[19, 20, 21, 22] There are multiple etiologies, including clinical uncertainty, higher acuity in patient illness early in hospitalization, and cultural differences between services.[23] Additionally, patterns of communication and standardized handoff processes are often insufficient. In our hospital system, the transfer process relies primarily upon synchronous communication methods without standardized, asynchronous information exchange. We hypothesize front‐line providers perceive this lack of standardization as a primary threat to quality. Because approximately 60% of new patient admissions from the ED to medicine service (both in our hospital and in prior studies) occur during off hours, these findings highlight a need for subsequent study and quality improvement efforts.[24]
During the time of this study, our medicine units were staffed at night by 5 medicine housestaff physicians and 1 academic hospitalist, or nocturnist. In efforts to improve quality and safety during off hours, our hospital, as well as other health systems, implemented the nocturnist position, a faculty‐level attending physician to provide off‐hours clinical care and housestaff supervision.[25] Although participants reported a moderate rating of housestaff supervision, participants provided lower scores for staffing numbers of nurses, and housestaff and attending physicians, despite nocturnist presence. With both increased off‐hours supervision in our hospital and increasing use of faculty‐level physicians in other academic programs, these results provide context for the anticipated level of overnight housestaff supervision.[26, 27] To our knowledge, this is the first study to investigate perceived overnight quality issues on medicine units following such staffing models. Although this model of direct, on‐site supervision in academic medicine programs may help offset staffing and supervisory issues during off hours, the nocturnist role is insufficient to offset threats to quality/safety already inherent within the system. Furthermore, prospective trials following implementation of nocturnist systems have shown mixed results in improving patient outcomes.[28] These findings have led some to question whether resources dedicated to nocturnist staffing may be better allocated to other overnight initiatives, highlighting the need for a more subtle understanding of quality issues to design targeted interventions.[29]
A notable finding from this work is that providers without night experience reported lower scores for 20 of 24 items, highlighting their perceptions of the quality of care delivery during off hours are lower than those who experience this environment. Although day providers are not directly experiencing off‐hours delivery processes, these providers receive and detect the results from care delivery at night.[17] Most nurse, physician, and hospital leaders are present in the hospital only during day hours, requiring these individuals to account for differences in perceived and actual care delivered overnight.[1] These individuals make critical decisions pertaining to process changes and quality improvement efforts in these units. We believe these results raise awareness for leadership decisions and quality improvement efforts in medicine service units, specifically to focus on overnight issues beyond staffing issues alone.
All respondent groups ranked the latter half of the shift (17 am) as lower in quality compared to the first 6 hours (7 pm1 am). This finding is contrary to our hypothesis that earlier time periods, during the majority of patient admissions (and presumed higher workload for all providers), would be perceived as lower quality. Reasons for this finding are unknown, but may relate to end‐of‐shift tasks, sign‐out preparation, provider fatigue, or disease‐related concerns (eg, increased incidence of stroke and myocardial infarction) during the latter portions of night shifts. One study identified a decrease in nursing clinical judgments from the beginning to end of 12‐hour shifts, with a potential suggested mechanism of decrease in ability to maintain attention during judgments.[30] Additionally, in a study by Folkard et al., risk was highest within the first several hours and fell substantially thereafter during a shift.[9] To our knowledge, no work has investigated perceived or objective quality outcomes by time period during the off‐hours shift in medicine units. Further work could help delineate why provider‐perceived compromises in quality occur late in off‐hours shifts and whether this correlates to safety events.
There are several limitations to our study. First, although all surveys were pilot tested for content validity, the construct validity was not rigorously assessed. Second, although data were collected from all participant groups, the collection methods were unbalanced, favoring attending‐level physician perspectives. Although the relative incidence of vulnerabilities in quality and safety should be interpreted with caution, our methods and general taxonomy provide a framework for developing and monitoring the perceptions of future interventions. Due to limitations in infrastructure, our findings could not be independently validated through review of reported adverse events, but previous investigations have found the vast majority of adverse events are not detected by standard anonymous reporting.[31, 32, 33] Our methodology (used in our prior work) may provide an independent means of detecting causes of poor quality not easily observed through routine surveillance.[22] Although many survey items showed statistical differences between provider groups, the clinical significance is subject to interpretation. Last, the perceptions and events related to our institution may not be fully generalizable to other academic programs or service lines, particularly in community‐based, nonteaching hospitals.
In conclusion, our results suggest a significant discrepancy between the concerns of day and night providers regarding the quality of care delivered to inpatients during the off hours, specifically with issues related to communication, quality‐of‐care delivery processes, and patient transfers from the ED. Although specific concerns may be institution‐ (and service line‐) dependent, appropriately designing initiatives to improve the quality of care delivered overnight will need to take the perspectives of both provider groups into account. Additionally, educational initiatives should focus on achieving a shared mental model among all providers to improve collaboration and performance.
Acknowledgements
The authors thank the nurses, internal medicine housestaff physicians, and general internal medicine attending physicians at the Penn State Hershey Medical Center for their participation in this study.
Disclosure: Nothing to report.
Patients experience acute illness at all hours of the day. In acute care hospitals, over 60% of patient admissions occur outside of normal business hours, or the off hours.[1, 2] Similarly, the acute decompensation of patients already admitted to hospital‐based units is frequent, with 90% of rapid responses occurring between 9 pm and 6 am.[3] Research suggests worse hospital performance during off hours, including increased patient falls, in‐hospital cardiac arrest mortality, and severity of hospital employee injuries.[2, 4, 5, 6, 7]
Although hospital‐based services should match care demand, the disparity between patient acuity and hospital capability at night is significant. Off hours typically have lower staffing of nurses, and attending and housestaff physicians, and ancillary staff as well as limited availability of consultative and supportive services.[8] Additionally, off‐hours providers are subject to the physiological effects of imbalanced circadian rhythms, including fatigue, attenuating their abilities to provide high‐quality care. The significant patient care needs mandate continuous patient care delivery without compromising quality or safety. To achieve this, further defining the barriers to delivering quality care during off hours is essential to improvement efforts in medicine‐based units.
Previous investigations have found increased occurrence and severity of worker accidents, increased potential for higher occurrence of preventable adverse patient events, and decreased performance during off hours.[4, 9, 10] Additionally, detrimental effects of off‐hours care may be further magnified by rotating employees through both day and night shifts, a common practice in academic hospitals.[11, 12] Potentially modifiable outcomes, such as patient fall rate and in‐hospital cardiac arrest survival differ markedly between day and night shifts.[6, 13] These studies primarily report on specific diseases, such as myocardial infarction and stroke, and are investigated from the perspective of hospital‐level outcomes.
To our knowledge, no study has reported provider‐perceived quality and safety issues occurring during off hours in an academic setting. Likewise, although off‐hours collaborative care requires shared, interprofessional conceptualization regarding care delivery, this perspective has not been reported. Understanding the similarities and differences between provider perceptions will allow the construction of an interprofessional team mental model, facilitating the design of future quality improvement initiatives.[14, 15] Our objectives were to: (1) identify off‐hours quality and safety issues, (2) assess which issues are perceived as most significant, and (3) evaluate differences in perceptions of these issues between nurses, and attending and housestaff physicians.
METHODS
Study Design
To investigate quality and safety issues occurring during off hours, we employed a prospective, mixed‐methods sequential exploratory study design, involving an initial qualitative analysis of adverse events followed by quantitative survey assessment.[16] We chose a mixed‐methods approach because provider‐perceived off‐hours issues had not been explicitly identified in the literature, requiring preliminary qualitative assessment. For the purpose of this study, we defined off hours as the 7 pm to 7am time period, which overlapped night shifts for both nurses and physicians. The study was approved by the institutional review board as a quality improvement project.
Study Setting
The study was conducted at a 378‐bed, university‐based acute care hospital in central Pennsylvania. There are a total of 64 internal medicine beds located in 2 units: a general medicine unit (44 beds, staffed by 60 nurses, nurse‐to‐patient ratio 1:4) and an intermediate care unit (20 beds, staffed by 41 nurses, nurse‐to‐patient ratio 1:3). The medicine residency program consists of 69 residents and 14 combined internal medicinepediatrics residents. During the day, 3 teaching teams and 1 nonteaching team care for all medicine patients. Overnight, 3 junior/senior level residents admit patients to the medicine service, whereas 2 interns provide cross‐coverage for all medicine and specialty service patients. Starting in September 2012 (before data collection), an overnight faculty‐level academic hospitalist, or nocturnist, provided on‐site housestaff supervision.
Qualitative Data Collection
For the qualitative analysis, we used 2 methods to develop our database. First, we created an electronic survey (see Supporting Information, Appendix 1, in the online version of this article) to identify near misses/adverse events occurring overnight, distributed to the nocturnist, 3 daytime hospitalists, and unit charge nurses following each shift (October 2012March 2013). The survey items were developed for the purpose of this study, with several items modified from a previously published survey.[17] Second, residency program directors recorded field notes during end‐of‐rotation debriefings (1 hour) with departing overnight housestaff, which were then dictated and transcribed. The subsequent analysis from these sources informed the quantitative survey (see Supporting Information, Appendix 2, in the online version of this article).
Survey Instrument
Three months after the initiation of qualitative data collection, 1 investigator (J.D.G.) developed a preliminary codebook to identify categories and themes. From this codebook, the research team drafted a survey instrument (the complete qualitative analysis occurred after survey development). To maintain focus on systematic quality improvement, items related to perceived mismanagement, relationship tensions, and professionalism were excluded. The survey was pilot‐tested with 5 faculty physicians and 2 nursing staff, prompting several modifications to improve clarity. Primary demographic items included provider role (nurse, attending physician, or housestaff physician) and years in current role. The 24 survey items were grouped into 5 different categories: (1) Quality of Care Delivery, (2) Communication and Coordination, (3) Staffing and Supervision, (4) Patient Transfers, and (5) Consulting Service Issues. Each item was investigated on a 7‐point scale (1=lowest rating, 7=highest rating). Descriptive text was provided at the extremes (choices 1 and 7), whereas intermediary values (26) did not have descriptive cues. The descriptive anchors for Quality of Care Delivery and Patient Transfers were 1=never and 7=always, whereas the descriptive anchors Communication and Coordination and Staffing and Supervision were 1=poor and 7=superior; Consulting Service Issues used a mix of both. Providers with off‐hours experience were asked to rank 4 time periods (710 pm, 10 pm1 am, 14 am, 47 am) regarding quality of care delivery in the medicine units (1=best, 4=worst). We asked both daytime and nighttime providers about perceptions of off‐hours care because, given the boundary spanning the nature of medical care across work shifts, daytime providers frequently identify issues not apparent until hours (or even days) after completion of a night shift. A similar design was used in prior work investigating safety at night.[17]
Quantitative Data Collection
In June of 2013, we emailed a survey link (
Data Analysis
Using the preliminary codebook, 2 investigators (J.D.G., E.M.) jointly analyzed a segment of the dataset using Atlas.ti 6.0 (Scientific Software, Berlin, Germany). Two investigators independently coded the data, compared codes for agreement, and updated the codebook. The remaining data were coded independently, with regular adjudication sessions to modify the codebook. All investigators reviewed and agreed upon themes and representative quotations.
Descriptive statistics, Pearson correlation statistics, Kruskal‐Wallis tests, and signed rank tests (with Bonferroni correction) were used to report group characteristics, correlate rank order, make comparisons between groups (nursing staff, and attending and housestaff physicians; day/night providers), and compare quality rankings by time period, respectively. The data were analyzed using SAS 9.3 (SAS Institute Inc., Cary, NC) and Stata/IC‐8 (StataCorp, College Park, TX).
RESULTS
Qualitative Analysis of Off‐Hours' Adverse Events and Near Misses
A total of 190 events were reported by daytime attending physicians (n=100), nocturnists (n=60), and nighttime charge nurses (n=30). Although questions asked participants to describe near misses/adverse events, respondents also reported a number of global quality issues not related to specific events. Similarly, debriefing sessions with housestaff (n=5) addressed both specific overnight events and residency‐related issues. Seven themes were identified: (1) perceived mismanagement, (2) quality of delivery processes, (3) communication and coordination, (4) staffing and supervision, (5) patient transfers, (6) consulting service issues, and, (7) professionalism/relational tensions. Table 1 lists the code frequencies and exemplary quotations.
| Category and Themes | Code Frequency No. (% of 322) | Representative Quotation |
|---|---|---|
| ||
| Perceived mismanagement | 97 (30) | We had a new admission to the general medicine unit with atrial flutter and rapid ventricular response who did not receive rate controlling agents but rather received diuretics. [The patient's] heart rate remained between 110 and 130 overnight, with a troponin rise in the am likely from demand. The attending note states rate controllers and discussed with housestaff, but this was not performed. |
| Quality of delivery processes | 63 (20) | One patient had a delay in MRI scanning in the off hours due to the scanner being down and scheduling. When the patient went down, there seemed to be little attempt to make sure patient went through scanner; unclear if housestaff called or not to come to assist. Now, the delay in care is even further along. |
| Communication and coordination | 50 (16) | A patient was transferred to the intermediate care unit with hypercarbic respiratory failure. The patient had delay of >1 hour to receive IV Bumex because pharmacy would not release the dose from Pyxis, and the nurse did not let us know there was a delay. When I asked the nurse why, she responded because she's not the only patient I have. I pointed out that the patient was in failure and needed Bumex, stat. If we had not clearly communicated either verbally or via computer, she should let us know how to do that better. |
| Staffing and supervision | 39 (12) | A patient was admitted DNR/DNI with advanced dementia, new on BiPaP at 100%, and hypotensive. The team's intern [identified] the need for interventions, including a central line. This was discussed with overloaded intensive care unit resident. The intern struggled until another resident assisted along with the night attending. Issues included: initial triage, no resident backup for team, and attending backup. I should have been more hands on in the moment to assist the intern navigating the system of care. Many issues here, but no senior resident was involved in care until [late]. |
| Patient transfers | 38 (12) | One patient went from the emergency department [to us] on the 5th floor at 7:45 pm. The ED placed an order for packed red blood cells and it was written at 4:45 pm. When patient arrived on our floor at 7:45 pm, the transfusion had not been started. The floor nurse started it at 8:10 pm . |
| Consulting services | 18 (6) | Regarding a new outside hospital transfer, the medicine team was informed that [the consulting service] would place official consult on the chart when imaging studies from the outside institution were available. Despite this, the consult was still not done after 36 hours, and [we are] still waiting. We contacted service several times. |
| Professionalism and relational tensions | 17 (5) | [One admission from the emergency department] involved a patient who received subcutaneous insulin for hyperkalemia as opposed to intravenous insulin. When brought to [their] attention, they became very confrontational and abrupt and denied having ordered or administered it that way, although it was documented in the EMR. |
Perceived Mismanagement
Participants commonly questioned the decision making, diagnosis, or management of off‐hours providers. Concerns included the response to acute illness (eg, delay in calling a code), treatment decisions (eg, diuresis in a patient with urinary retention), or omission of necessary actions (eg, no cultures ordered for septicemia).
Quality of Delivery Processes
Participants frequently described quality of care delivery issues primarily related to timeliness or delays in delivery processes (34/63 coding references), or patient safety issues (29/63 coding references). Described events revealed concerns about the timeliness of lab reporting, imaging, blood draws, and medication ordering/processing.
Communication and Coordination
Breakdowns in communication and coordination often threatened patient safety. Identified issues included poor communication between primary physicians, nurses, consulting services, and emergency department (ED) providers, as well as documentation within the electronic medical record.
Staffing and Supervision
Several events highlighted staffing or supervision limitations, such as perceived low nursing or physician staffing levels. The degree of nocturnist supervision was polarizing, with both increased and decreased levels of supervision reported as limiting care delivery (or housestaff education).
Patient Transfers
Patient transfers to medicine units from the ED, other inpatient units, or outside hospitals, were identified several times as an influential factor. The care transition and need for information exchange led to a perceived compromise in quality or safety.
Consulting Service Issues
Several examples highlighted perceived issues related to the communication, coordination, or timeliness of consultant services in providing care.
Professionalism/Relational Tensions
Last, providers described situations in which they perceived lack of professionalism or relational tensions between providers, either in regard to interactions or clinical decisions in patient care.
Quantitative Results
Of 214 surveys sent, data were collected from 160 respondents (75% response), including 64/101 nursing staff (63% response), 25/28 attending physicians (80% response), and 71/85 housestaff physicians (84% response). Table 2 describes the participant demographics.
| Variable | No. (%) |
|---|---|
| |
| Nursing staff | 64 (40) |
| Intermediate care unit | 20 |
| General medicine ward | 44 |
| All night shifts | 16 |
| Mix of day and night shifts | 26 |
| Years of experience, mean (SD) | 7.7 (9.7) |
| Attending physicians | 25 (16) |
| No. providing care only at night | 4 |
| No. of weeks as overnight hospitalist in past year, mean (SD) | 11.5 (4.1) |
| No. providing care only during the day | 21 |
| Years since residency graduation, mean (SD) | 9.0 (8.5) |
| Medicine residents | 71 (44) |
| Intern | 27 |
| Junior resident | 23 |
| Senior resident* | 21 |
Off‐Hours Quality and Safety Issues
Ratings and comparisons of the 24 items are shown in Table 3. For all items, the mean rating was below 5 (7‐point scale). Lowest‐rated (least optimal) items were: timeliness, safety, and communication involved with patients admitted from the ED, number of attending physicians, and timeliness of consults and blood draws. Highest‐rated (more optimal) items were: timely reporting of labs, timely identification of deteriorating status, medication ordering and processing, communication between physicians, and safety and communication involved with intraservice transfers.
| Category and Survey Item, Mean (SD)* | Total (160) | Providers With Night Experience | Nighttime Providers (116) | Daytime Providers (44) | P Value | |||
|---|---|---|---|---|---|---|---|---|
| Nurses (41) | Attending Physicians (4) | Housestaff (71) | P Value | |||||
| ||||||||
| Quality of care delivery | ||||||||
| Timely reporting of labs at night | 4.70 (1.39) | 5.12 (1.50) | 4.50 (1.00) | 4.61 (1.47) | 0.11 | 4.78 (1.48) | 4.48 (1.11) | 0.09 |
| Timely identification of deteriorating status | 4.67 (1.34) | 4.88 (1.36) | 5.00 (0.82) | 4.85 (1.20) | 0.93 | 4.86 (1.24) | 4.16 (1.45) | 0.006 |
| Medication ordering and processing | 4.63 (1.13) | 4.88 (1.25) | 5.25 (0.50) | 4.66 (1.08) | 0.19 | 4.76 (1.13) | 4.27 (1.06) | 0.01 |
| Timely completion of imaging at night | 4.29 (1.32) | 4.32 (1.46) | 4.75 (0.96) | 4.39 (1.29) | 0.88 | 4.38 (1.34) | 4.05 (1.26) | 0.12 |
| Timely reporting of results at night | 4.19 (1.43) | 4.27 (1.53) | 4.00 (1.83) | 4.11 (1.44) | 0.84 | 4.16 (1.47) | 4.27 (1.30) | 0.76 |
| Timely med release from pharmacy at night | 4.16 (1.29) | 4.00 (1.32) | 4.50 (0.58) | 4.28 (1.29) | 0.44 | 4.19 (1.28) | 4.09 (1.31) | 0.90 |
| Timely blood draws at night | 3.96 (1.52) | 4.63 (1.44) | 4.50 (0.58) | 3.53 (1.49) | <0.001 | 3.96 (1.54) | 3.98 (1.47) | 0.98 |
| Communication and coordination | ||||||||
| Communication between physicians | 4.63 (1.26) | 4.29 (1.23) | 6.00 (1.15) | 5.14 (1.12) | <0.001 | 4.87 (1.24) | 3.98 (1.09) | <0.001 |
| Communication between nursing and pharmacy | 4.39 (1.27) | 4.83 (1.41) | 5.00 (0.82) | 4.27 (1.29) | 0.04 | 4.49 (1.34) | 4.11 (4.11) | 0.08 |
| Communication between nursing and physicians | 4.39 (1.28) | 4.44 (1.36) | 5.00 (0.82) | 4.58 (1.31) | 0.64 | 4.54 (1.31) | 3.98 (1.13) | 0.01 |
| Documentation in medical record | 4.33 (1.36) | 5.00 (1.36) | 6.00 (0.82) | 4.23 1.19) | <0.001 | 4.56 (1.31) | 3.70 (1.30) | <0.001 |
| Ease of contacting primary providers at night | 4.31 (1.29) | 4.46 (1.27) | 6.00 (0.00) | 4.54 (1.18) | 0.02 | 4.56 (1.22) | 3.66 (1.27) | <0.001 |
| Staffing and supervision | ||||||||
| No. of nursing staff | 4.51 (1.27) | 4.54 (1.50) | 5.50 (0.58) | 4.59 (1.21) | 0.25 | 4.60 (1.31) | 4.25 (1.14) | 0.025 |
| Supervision of housestaff | 4.43 (1.34) | 4.56 (1.40) | 6.25 (0.50) | 4.55 (1.34) | 0.03 | 4.61 (1.37) | 3.95 (1.14) | 0.002 |
| No. of housestaff | 4.09 (1.39) | 4.27 (1.40) | 4.50 (1.29) | 4.11 (1.44) | 0.70 | 4.18 (1.41) | 3.86 (1.32) | 0.12 |
| No. of ancillary staff | 4.00 (1.40) | 4.27 (1.53) | 5.75 (0.96) | 3.85 (1.40) | 0.02 | 4.06 (1.48) | 3.84 (1.18) | 0.27 |
| No. of attending physicians | 3.79 (1.50) | 3.49 (1.76) | 5.25 (0.96) | 3.89 (1.43) | 0.07 | 3.79 (1.57) | 3.80 (1.32) | 0.98 |
| Patient transfers | ||||||||
| For patients accepted to medicine from another medicine unit | ||||||||
| Timely and safe patient transfers | 4.56 (1.28) | 5.15 (1.11) | 4.75 (0.50) | 4.55 (1.23) | 0.025 | 4.77 (1.20) | 4.00 (1.33) | 0.001 |
| High quality communication between providers | 4.55 (1.35) | 5.34 (1.13) | 5.00 (0.82) | 4.49 (1.22) | 0.001 | 4.81 (1.24) | 3.86 (1.41) | <0.001 |
| For patients admitted from emergency department to medicine unit | ||||||||
| Appropriate testing and treatment | 4.16 (1.34) | 4.15 (1.30) | 4.00 (1.83) | 4.21 (1.43) | 0.96 | 4.18 (1.39) | 4.11 (1.20) | 0.66 |
| Timely and safe transfers | 3.89 (1.38) | 3.63 (1.50) | 5.50 (0.58) | 4.08 (1.32) | 0.02 | 3.97 (1.40) | 3.68 1.29) | 0.23 |
| High‐quality communication between providers | 2.93 (1.38) | 2.56 (1.23) | 3.75 (1.26) | 3.00 (1.39) | 0.08 | 2.87 (1.35) | 3.07 (1.47) | 0.41 |
| Consulting service issues | ||||||||
| Timely consults at night | 4.04 (1.35) | 4.27 (1.28) | 4.00 (0.82) | 4.10 (1.47) | 0.69 | 4.16 (1.38) | 3.73 (1.25) | 0.053 |
| Communication between consults and physicians | 3.93 (1.40) | 3.46 (1.45) | 5.75 (1.26) | 4.35 (1.27) | <0.001 | 4.09 (1.42) | 3.50 (1.27) | 0.016 |
Comparisons Between Professional Groups With Night Experience
Of the 24 items, 11 showed statistically significant differences between groups (P<0.05). Items with the largest difference between groups included: timely blood draws at night (housestaff physicians lowest), communication between physicians (nursing lowest), documentation in medical record (housestaff physicians lowest), and communication between consults and physicians (nursing lowest). The rank order between housestaff physicians and nurses, and housestaff and attending physicians showed moderately positive correlations (r=0.61, P=0.002 and r=0.47, P=0.022, respectively). The correlation between nurses and attending physicians showed a weak correlation (r=0.19, P=0.375).
Comparisons Between Front‐Line Providers With and Without Night Experience
Of the 24 items, 12 showed statistically significant differences between groups (P<0.05), with day providers reporting lower ratings in all 12. Items with the largest difference between groups included: communication between consults and physicians, ease of contacting providers, communication between providers, documentation, and safety and communication related to transfers from other units. The rank order between night and day groups showed a statistically significant positive correlation (r=0.65, P=0.001).
Perceived Highest Quality of Care Time Period During Off Hours
Compared with other time periods, all providers ranked 4 to 7 am as the period with the lowest quality of care delivery (mean rank 3.2, P0.001) (Figure 1). Nursing staff and attending physicians both ranked the 10 pm to 1 am time period as the best period (mean of 2.0 and 1.5, respectively), whereas housestaff physicians ranked the 7 to 10 pm as the best time period (mean 1.62). The only statistical difference between provider groups for any given time period was the 7 to 10 pm time period (P=0.002).

DISCUSSION
In this prospective, mixed‐methods study evaluating perceived off‐hours quality and safety issues, several themes were identified, including perceived mismanagement, insufficient quality of delivery processes, communication/coordination breakdowns, and staffing and supervision issues. In the quantitative analysis, lowest‐rated items (lowest quality) related to timeliness/safety/communication involved with ED transfers, number of attending physicians, and timeliness of consults and blood draws. Highest‐rated items (highest quality) related to timeliness of lab reporting and identification of deteriorating patients, medication ordering/processing, communication between physicians, and safety/communication during intraservice transfers. In general, day providers reported lower ratings than night providers on nearly all quality‐related items. Nursing staff reported the lowest ratings regarding communication between physicians and consults, whereas housestaff physicians reported the lowest ratings regarding documentation in the medical record and timely blood draws. These between‐group differences reveal the lack of shared conceptual understanding regarding off‐hours care delivery.
Our qualitative results reveal several significant issues related to care delivery during off hours, many of which are not obtainable by hospital‐level data or chart review.[18] For hospital‐based medicine units, an understanding of the structure‐ and process‐related factors associated with events is required for quality improvement efforts. Although the primary focus for this work was the off hours, it is plausible that providers may have identified similar issues as important issues during daytime hours. Our study was not designed to investigate if these perceived issues are specific to off hours, or if these issues are an accurate reflection of objective events occurring during this time period. We believe this topic deserves further investigation, as understanding if these off‐hours perceptions are unique to this time period would change the scope of future quality improvement initiatives.
The most significant finding in the quantitative results was the vulnerability in quality and safety during patient admissions from the ED, specifically in relation to communication and timeliness of transfer. Between‐unit handoffs for patients admitted from the ED to medicine units have been identified as particularly vulnerable to breakdowns in the communication process.[19, 20, 21, 22] There are multiple etiologies, including clinical uncertainty, higher acuity in patient illness early in hospitalization, and cultural differences between services.[23] Additionally, patterns of communication and standardized handoff processes are often insufficient. In our hospital system, the transfer process relies primarily upon synchronous communication methods without standardized, asynchronous information exchange. We hypothesize front‐line providers perceive this lack of standardization as a primary threat to quality. Because approximately 60% of new patient admissions from the ED to medicine service (both in our hospital and in prior studies) occur during off hours, these findings highlight a need for subsequent study and quality improvement efforts.[24]
During the time of this study, our medicine units were staffed at night by 5 medicine housestaff physicians and 1 academic hospitalist, or nocturnist. In efforts to improve quality and safety during off hours, our hospital, as well as other health systems, implemented the nocturnist position, a faculty‐level attending physician to provide off‐hours clinical care and housestaff supervision.[25] Although participants reported a moderate rating of housestaff supervision, participants provided lower scores for staffing numbers of nurses, and housestaff and attending physicians, despite nocturnist presence. With both increased off‐hours supervision in our hospital and increasing use of faculty‐level physicians in other academic programs, these results provide context for the anticipated level of overnight housestaff supervision.[26, 27] To our knowledge, this is the first study to investigate perceived overnight quality issues on medicine units following such staffing models. Although this model of direct, on‐site supervision in academic medicine programs may help offset staffing and supervisory issues during off hours, the nocturnist role is insufficient to offset threats to quality/safety already inherent within the system. Furthermore, prospective trials following implementation of nocturnist systems have shown mixed results in improving patient outcomes.[28] These findings have led some to question whether resources dedicated to nocturnist staffing may be better allocated to other overnight initiatives, highlighting the need for a more subtle understanding of quality issues to design targeted interventions.[29]
A notable finding from this work is that providers without night experience reported lower scores for 20 of 24 items, highlighting their perceptions of the quality of care delivery during off hours are lower than those who experience this environment. Although day providers are not directly experiencing off‐hours delivery processes, these providers receive and detect the results from care delivery at night.[17] Most nurse, physician, and hospital leaders are present in the hospital only during day hours, requiring these individuals to account for differences in perceived and actual care delivered overnight.[1] These individuals make critical decisions pertaining to process changes and quality improvement efforts in these units. We believe these results raise awareness for leadership decisions and quality improvement efforts in medicine service units, specifically to focus on overnight issues beyond staffing issues alone.
All respondent groups ranked the latter half of the shift (17 am) as lower in quality compared to the first 6 hours (7 pm1 am). This finding is contrary to our hypothesis that earlier time periods, during the majority of patient admissions (and presumed higher workload for all providers), would be perceived as lower quality. Reasons for this finding are unknown, but may relate to end‐of‐shift tasks, sign‐out preparation, provider fatigue, or disease‐related concerns (eg, increased incidence of stroke and myocardial infarction) during the latter portions of night shifts. One study identified a decrease in nursing clinical judgments from the beginning to end of 12‐hour shifts, with a potential suggested mechanism of decrease in ability to maintain attention during judgments.[30] Additionally, in a study by Folkard et al., risk was highest within the first several hours and fell substantially thereafter during a shift.[9] To our knowledge, no work has investigated perceived or objective quality outcomes by time period during the off‐hours shift in medicine units. Further work could help delineate why provider‐perceived compromises in quality occur late in off‐hours shifts and whether this correlates to safety events.
There are several limitations to our study. First, although all surveys were pilot tested for content validity, the construct validity was not rigorously assessed. Second, although data were collected from all participant groups, the collection methods were unbalanced, favoring attending‐level physician perspectives. Although the relative incidence of vulnerabilities in quality and safety should be interpreted with caution, our methods and general taxonomy provide a framework for developing and monitoring the perceptions of future interventions. Due to limitations in infrastructure, our findings could not be independently validated through review of reported adverse events, but previous investigations have found the vast majority of adverse events are not detected by standard anonymous reporting.[31, 32, 33] Our methodology (used in our prior work) may provide an independent means of detecting causes of poor quality not easily observed through routine surveillance.[22] Although many survey items showed statistical differences between provider groups, the clinical significance is subject to interpretation. Last, the perceptions and events related to our institution may not be fully generalizable to other academic programs or service lines, particularly in community‐based, nonteaching hospitals.
In conclusion, our results suggest a significant discrepancy between the concerns of day and night providers regarding the quality of care delivered to inpatients during the off hours, specifically with issues related to communication, quality‐of‐care delivery processes, and patient transfers from the ED. Although specific concerns may be institution‐ (and service line‐) dependent, appropriately designing initiatives to improve the quality of care delivered overnight will need to take the perspectives of both provider groups into account. Additionally, educational initiatives should focus on achieving a shared mental model among all providers to improve collaboration and performance.
Acknowledgements
The authors thank the nurses, internal medicine housestaff physicians, and general internal medicine attending physicians at the Penn State Hershey Medical Center for their participation in this study.
Disclosure: Nothing to report.
- . Like night and day—shedding light on off‐hours care. N Engl J Med. 2008;358(20):2091–2093.
- , , , . Call nights and patient care. J Gen Intern Med. 1992;7(4):405–410.
- , , , et al. Uncovering system errors using a rapid response team: cross‐coverage caught in the crossfire. Discussion. J Trauma. 2009;67(1):173–179.
- , . The impact of shift work on the risk and severity of injuries for hospital employees: an analysis using Oregon workers' compensation data. Occup Med (Lond). 2004;54(8):556–563.
- , . Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med. 2001;345(9):663–668.
- , , , et al. The association of shift‐level nurse staffing with adverse patient events. J Nurs Adm. 2011;41(2):64–70.
- , , , et al. Heart disease and stroke statistics—2010 update A report from the American Heart Association. Circulation. 2010;121(7):e46–e215.
- , , , , O'Neil E. Minimum Nurse Staffing Ratios In California Acute Care Hospitals. Oakland, CA: California Workforce Initiative; 2000.
- , . Shift work, safety and productivity. Occup Med (Lond). 2003;53(2):95–101.
- , , . Increased injuries on night shift. Lancet. 1994;344(8930):1137–1139.
- , . Shift and night work and long working hours‐a systematic review of safety implications. Scand J Work Environ Health. 2011:37(3):173–185.
- , , , et al. Rotating shift work, sleep, and accidents related to sleepiness in hospital nurses. Am J Public Health. 1992;82(7):1011–1014.
- , , , et al. Survival from in‐hospital cardiac arrest during nights and weekends. JAMA. 2008;299(7):785–792.
- , , , , . The influence of shared mental models on team process and performance. J Appl Psychol. 2000;85(2):273.
- , . Team mental models and their potential to improve teamwork and safety: a review and implications for future research in healthcare. Saf Sci. 2012;50(5):1344–1354.
- . Editorial: mapping the field of mixed methods research. J Mix Methods Res. 2009;3(2):95–108.
- , . Decreasing adverse events through night talks: an interdisciplinary, hospital‐based quality improvement project. Perm J. Fall 2009;13(4):16–22.
- , , , et al. “Global trigger tool” shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff (Millwood). 2011;30(4):581–589.
- , , , , , . Dropping the baton: a qualitative analysis of failures during the transition from emergency department to inpatient care. Ann Emerg Med. 2009;53(6):701–710.e704.
- . Smoothing transitions. Joint Commission targets patient handoffs. Mod Healthc. 2010;40(43):8–9.
- , , , , , . The patient handoff: a comprehensive curricular blueprint for resident education to improve continuity of care. Acad Med. 2012;87(4):411–418.
- , , , , , . Patient care transitions from the emergency department to the medicine ward: evaluation of a standardized electronic signout tool. Int J Qual Health Care. 2014;26(4):337–347.
- , . The unappreciated challenges of between‐unit handoffs: negotiating and coordinating across boundaries. Ann Emerg Med. 2013;61(2):155–160.
- , , , , , . The association between night or weekend admission and hospitalization‐relevant patient outcomes. J Hosp Med. 2011;6(1):10–14.
- . Middle‐of‐the‐night medicine is rarely patient‐centred. CMAJ. 2011;183(13):1467–1468.
- , , , et al. Survey of overnight academic hospitalist supervision of trainees. J Hosp Med. 2012;7(7):521–523.
- , , , , , . Effects of increased overnight supervision on resident education, decision‐making, and autonomy. J Hosp Med. 2012;7(8):606–610.
- , , , et al. A randomized trial of nighttime physician staffing in an intensive care unit. N Engl J Med. 2013;368(23):2201–2209.
- . Intensivists at night: putting resources in the right place. Crit Care. 2013;17(5):1008.
- , , . Changes in nurses' decision making during a 12‐h day shift. Occup Med (Lond). 2013;63(1):60–65.
- , , , , , . The incident reporting system does not detect adverse drug events: a problem for quality improvement. Jt Comm J Qual Improv. 1995;21(10):541–548.
- , , , , . An evaluation of adverse incident reporting. J Eval Clin Pract. 1999;5(1):5–12.
- , . Reporting and preventing medical mishaps: lessons from non‐medical near miss reporting systems. BMJ. 2000;320(7237):759–763.
- . Like night and day—shedding light on off‐hours care. N Engl J Med. 2008;358(20):2091–2093.
- , , , . Call nights and patient care. J Gen Intern Med. 1992;7(4):405–410.
- , , , et al. Uncovering system errors using a rapid response team: cross‐coverage caught in the crossfire. Discussion. J Trauma. 2009;67(1):173–179.
- , . The impact of shift work on the risk and severity of injuries for hospital employees: an analysis using Oregon workers' compensation data. Occup Med (Lond). 2004;54(8):556–563.
- , . Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med. 2001;345(9):663–668.
- , , , et al. The association of shift‐level nurse staffing with adverse patient events. J Nurs Adm. 2011;41(2):64–70.
- , , , et al. Heart disease and stroke statistics—2010 update A report from the American Heart Association. Circulation. 2010;121(7):e46–e215.
- , , , , O'Neil E. Minimum Nurse Staffing Ratios In California Acute Care Hospitals. Oakland, CA: California Workforce Initiative; 2000.
- , . Shift work, safety and productivity. Occup Med (Lond). 2003;53(2):95–101.
- , , . Increased injuries on night shift. Lancet. 1994;344(8930):1137–1139.
- , . Shift and night work and long working hours‐a systematic review of safety implications. Scand J Work Environ Health. 2011:37(3):173–185.
- , , , et al. Rotating shift work, sleep, and accidents related to sleepiness in hospital nurses. Am J Public Health. 1992;82(7):1011–1014.
- , , , et al. Survival from in‐hospital cardiac arrest during nights and weekends. JAMA. 2008;299(7):785–792.
- , , , , . The influence of shared mental models on team process and performance. J Appl Psychol. 2000;85(2):273.
- , . Team mental models and their potential to improve teamwork and safety: a review and implications for future research in healthcare. Saf Sci. 2012;50(5):1344–1354.
- . Editorial: mapping the field of mixed methods research. J Mix Methods Res. 2009;3(2):95–108.
- , . Decreasing adverse events through night talks: an interdisciplinary, hospital‐based quality improvement project. Perm J. Fall 2009;13(4):16–22.
- , , , et al. “Global trigger tool” shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff (Millwood). 2011;30(4):581–589.
- , , , , , . Dropping the baton: a qualitative analysis of failures during the transition from emergency department to inpatient care. Ann Emerg Med. 2009;53(6):701–710.e704.
- . Smoothing transitions. Joint Commission targets patient handoffs. Mod Healthc. 2010;40(43):8–9.
- , , , , , . The patient handoff: a comprehensive curricular blueprint for resident education to improve continuity of care. Acad Med. 2012;87(4):411–418.
- , , , , , . Patient care transitions from the emergency department to the medicine ward: evaluation of a standardized electronic signout tool. Int J Qual Health Care. 2014;26(4):337–347.
- , . The unappreciated challenges of between‐unit handoffs: negotiating and coordinating across boundaries. Ann Emerg Med. 2013;61(2):155–160.
- , , , , , . The association between night or weekend admission and hospitalization‐relevant patient outcomes. J Hosp Med. 2011;6(1):10–14.
- . Middle‐of‐the‐night medicine is rarely patient‐centred. CMAJ. 2011;183(13):1467–1468.
- , , , et al. Survey of overnight academic hospitalist supervision of trainees. J Hosp Med. 2012;7(7):521–523.
- , , , , , . Effects of increased overnight supervision on resident education, decision‐making, and autonomy. J Hosp Med. 2012;7(8):606–610.
- , , , et al. A randomized trial of nighttime physician staffing in an intensive care unit. N Engl J Med. 2013;368(23):2201–2209.
- . Intensivists at night: putting resources in the right place. Crit Care. 2013;17(5):1008.
- , , . Changes in nurses' decision making during a 12‐h day shift. Occup Med (Lond). 2013;63(1):60–65.
- , , , , , . The incident reporting system does not detect adverse drug events: a problem for quality improvement. Jt Comm J Qual Improv. 1995;21(10):541–548.
- , , , , . An evaluation of adverse incident reporting. J Eval Clin Pract. 1999;5(1):5–12.
- , . Reporting and preventing medical mishaps: lessons from non‐medical near miss reporting systems. BMJ. 2000;320(7237):759–763.
© 2014 Society of Hospital Medicine
Munchausen Syndrome by Adult Proxy
Asher first described Munchausen syndrome by proxy over 60 years ago. Like the famous Baron von Munchausen, the persons affected have always traveled widely; and their stories like those attributed to him, are both dramatic and untruthful.[1] Munchausen syndrome is a psychiatric disorder in which a patient intentionally induces or feigns symptoms of physical or psychiatric illness to assume the sick role. In 1977, Meadow described the first case in which a caregiverperpetrator deliberately produced physical symptoms in a child for proxy gratification.[2] Unlike malingering, in which external incentives drive conscious symptom falsification, Munchausen syndrome by proxy (MSBP) is associated with fulfillment of the abuser's own psychological need for garnering praise from medical staff for devoted care given a sick child.[3, 4]
MSBP was once considered vanishingly rare. Many experts now believe it is more common, with a reported annual incidence of 0.4/100,000 in children younger than 16 years, and 2/100,000 in children younger than 1 year.[5] It is a disorder in which a parent, often the mother (94%99%)[6] and often with training or interest in the medical field,[5] is the perpetrator. The medical team caring for her child often views her as unusually helpful, and she is frequently psychiatrically ill with disorders such as depression, personality disorder, or prior personal history of somatoform or factitious disorder.[7, 8] The perpetrator typically inflicts physical harm, although occasionally she may simply lie about symptoms or tamper with laboratory samples.[5] The most common methods of inflicting harm are poisoning and suffocation. Overall mortality is 6% to 9%.[6, 9]
Although a large body of literature addresses pediatric cases, there is little to guide clinicians when victims are adults. An obvious reason may be that MSBP with adult proxies (MSB‐AP) has been reported so rarely, although we believe it is under‐recognized and more common than thought. The primary objective of this review was to identify all published cases of MSB‐AP, and synthesize them to characterize victims and perpetrators, modes of deceit, and relationships between victims and perpetrators so that clinicians will be better equipped to recognize such cases or at least include MSB‐AP in the differential of possibilities when symptoms and history are inconsistent.
METHODS
The Mayo Clinic Rochester Institutional Review Board approved this study. The databases of Ovid MEDLINE, Ovid EMBASE, PubMed, Web of Knowledge, and PsychINFO were searched from inception through April 2014 to identify all published cases of Munchausen by proxy in patients 18 years or older. The following search terms were used: Munchausen syndrome by proxy, factitious disorder by proxy, Munchausen syndrome, and factitious disorder. Reports were included when they described single or multiple cases of MSBP with victims aged at least 18 years. The search was not limited to articles published in English. Bibliographies of selected articles were reviewed for reports identifying additional cases.
RESULTS
We found 10 reports describing 11 cases of MSB‐AP and 1 report describing 2 unique cases of MSB‐AP (Tables 1 and 2). Two case reports were published in French[10, 11] and 1 in Polish.[12] Sigal et al.[13] describes 2 different victims with a common perpetrator, and another report[14] describes the same perpetrator with a third victim. One case, though cited as MSB‐AP in the literature was excluded because it did not meet the criteria for the disorder. In this case, the wife of a 28‐year‐old alcoholic male poured acid on him while he was inebriated, ostensibly to vent frustration and coerce him into sobriety.[15, 16]
| Author | Gender | Age, y | Presenting Features | Occupation/Education | Outcome |
|---|---|---|---|---|---|
| |||||
| Sigal M et al. (1986)[13] | F | 20s | Abscesses (skin) | NP | Death |
| F | 21 | Abscesses (skin) | Child care | Paraplegia | |
| Sigal MD et al. (1991)[14] | M | NP | Rash | NP | Abuse stopped |
| Smith NJ et al. (1989)[19] | M | 69 | None | Retired businessman | Continued fabrication |
| Krebs MO et al. (1996)[10] | M | 40s | Coma | Businessman | Abuse stopped |
| Ben‐Chetrit E et al. (1998)[20] | F | 73 | Coma | NP | Abuse stopped |
| Feldman KW et al. (1998)[8] | F | 21 | NP | Developmental delay | NP |
| Chodorowsk Z et al. (2003)[12] | F | 80 | Syncope | NP | Abuse stopped |
| Strubel D et al. (2003)[11] | F | 82 | None | NP | NP |
| Granot R et al. (2004)[21] | M | 71 | Coma | NP | Abuse stopped |
| Deimel GW et al. (2012)[17] | F | 23 | Rash | High school graduate | Continued abuse |
| F | 21 | Recurrent bacteremia | College student | Death | |
| Singh A et al. (2013)[22] | F | 79 | Fluid overload/false symptom history | Retired | Continued |
| Author | Gender | Age, y | Relationship | Occupation | Mode of Abuse | Outcome When Confronted |
|---|---|---|---|---|---|---|
| ||||||
| Sigal M et al. (1986)[13] | M | 26 | Husbanda | Businessman | Poisoningb followed by subcutaneous gasoline injection | Confession and incarceration |
| M | 29 | Boyfrienda | Businessman | Poisoningb followed by subcutaneous gasoline injection | Confession and incarceration | |
| Sigal MD et al. (1991)[14] | M | 34 | Cellmatea | Worked in medical clinic where incarcerated | Poisoningc followed by subcutaneous turpentine injection | Confession and attempted murder conviction |
| Smith NJ et al. (1989)[19] | F | 55 | Companion | Nurse | False history of hematuria, weakness, headaches | Denial |
| Krebs MO et al. (1996)[10] | F | 47 | Wife | Nurse | Tranquilizer injections | Confession and placed on probation |
| Ben‐Chetrit E et al. (1998)[20] | F | NP | Daughter | Nurse | Insulin injections | Denial |
| Feldman KW et al. (1998)[8] | F | NP | Mother | Business woman | False history of Batten's disease | NP |
| Chodorowsk Z et al. (2003)[12] | F | NP | Granddaughter | NP | Poisoningb | Denial |
| Strubel D et al. (2003)[11] | M | NP | Son | NP | False history of memory loss | NP |
| Granot R et al. (2004)[21] | F | NP | Wife | Hospital employee | Poisoningb | Confession |
| Deimel GW et al. (2012)[17] | F | NP | Mother | Unemployed chronic medical problems | Toxin application to skin | Denial |
| F | NP | Mother | Medical office receptionist | Intravenous injection unknown substance | Denial | |
| Singh A et al. (2013)[22] | M | NP | Son | NP | Fluid administration in context of fluid restriction/erratic medication administration/falsifying severity of symptoms | Denial |
Of the 13 victims, 9 (69%) were women and 4 (31%) were men. Of the ages reported, the median age was 69 years and the mean age was 51 (range, 2182 years). Exact age was not reported in 3 cases. Lying about signs and symptoms, but not actually inducing injury, occurred in 3 cases (23%), whereas in 10 cases (77%), the victims presented with physical findings, including coma (3), rash (2), skin abscesses (2), syncope (1), recurrent bacteremia (1), and fluid overload (1). Seven (54%) of the victims were poisoned, 2 via drug injection and 5 by beverage/food contamination. A perpetrator sedated 3 victims and subsequently injected them, 2 with gasoline and another with turpentine. Two of the victims were involved in business, 1 worked in childcare, 1 attended beauty school after graduating from high school, 1 attended college, and 1 was developmentally delayed. Victim education or occupation was not reported in 7 cases.
Of the 11 perpetrators, 8 (73%) were women, and 3 (27%) were men (note that the same male perpetrator had 3 victims). Median age was 34 years (range, 2655 years), although exact age was not reported in 4 cases. The perpetrator was the victim's mother in 3 cases, wife in 2 cases, son in 2 cases, and daughter, granddaughter, husband, companion, boyfriend, or prison cellmate in 1 case each. Five (38%) worked in healthcare.
All of the perpetrators were highly involved, even overly involved, in the care of their victims, frequently present, sometimes hovering, in hospital settings, and were viewed as generally helpful, if not overintrusive, by hospital staff. When confronted, 3 perpetrators confessed, 3 denied abuse that then ceased, and 4 more denied abuse that continued, culminating in death in 1 case. In 1 case, the outcome was not reported.[8] At least 3 victims remained with their perpetrators. Two perpetrators were criminally charged, 1 receiving probation and the other incarceration. The latter began abusing his cellmate, behavior that did not stop until he was confronted in prison.
CONCLUSION/DISCUSSION
Our primary objective was to locate and review all published cases of MSB‐AP. Our secondary aim was to describe salient characteristics of perpetrators, victims, and fabricated diseases in hopes of helping clinicians better recognize this disorder.
Our review shows that perpetrators were exclusively the victims' caregivers, including mothers, wives, husbands, daughters, granddaughters, or companions. These perpetrators, many with healthcare backgrounds, were attentive, helpful, and excessively present. In the majority of cases, hidden physical abuse yielded visible disease. Less commonly, perpetrators lied about symptoms rather than actually creating signs of disease. The most common mode of disease instigation involved poisoning through beverage/food contamination or subcutaneous injection. Geriatric and developmentally delayed persons appeared particularly vulnerable to victimization. Of the 13 victims, 5 were geriatric and 1 was developmentally delayed.
The adult cases we report are similar to child cases in that the perpetrators are caregivers; however, the caregivers of the adults are a more diverse group. Other similarities between adult and child cases are that physical signs occur more often than simply falsifying information, and poisoning is the most common method of disease fabrication. Suffocation, although common in child cases, has not been reported in adults. Though present in only a minority of cases, another feature distinguishing these cases from those reported in the pediatric literature is the presence of collusion between the perpetrator and victim. When MSBP was first described, Meadow believed that victims would reach an age at which the disorder would cease because they would fight back or report the abuse.[2] In 7 of the adult cases, the victims were unknowingly poisoned; however, in 2 cases,[17] the victims knew what their mothers were doing to them and yet denied that they were harming them. To explain this collusion, Deimel et al. proposed Stockholm syndrome, a condition in which a victim holds a perpetrator in high regard, despite experiencing at their hands what others might consider brainwashing and torture.
The data from the individual cases are sometimes frustratingly incomplete, with inconsistent reporting of dyad demographics and outcomes across the 13 cases, which compromises efforts to compare and contrast them. However, because no published studies have thoroughly reviewed all existing cases of MSB‐AP, we believe our review provides important insights into this condition by consolidating available information. It is our hope that by characterizing perpetrators, victims, and common presentations, we will raise awareness about this condition among healthcare providers so that it may be included in the differential diagnosis when they encounter this dyad: a patient's medical problems do not respond as expected to therapy and a caregivers appears overly involved or attention seeking.
The diagnosis of a factitious disorder often presents an immense clinical challenge and generally involves a multidisciplinary approach.[18] In addition to the incomplete data for existing cases in the literature, we recognize the ongoing difficulties in precise diagnosis of this disorder. Because a hallmark of pathology is secrecy at the outset and often denial, and even abrupt transition of care, upon confrontation, it is often very difficult, especially early on, to uncover patterns of perpetration, let alone posit a motive. We recognize that there may be some perpetrators who are motivated by something other than purely psychological end points, such as financial reward or even sexual victimization. And when alternate care venues are sought, clinicians are often left wondering. Further, the damage that may come to a therapeutic relationship by prematurely diagnosing MSB‐AP is important to keep in mind. Hospitalists who suspect MSB‐AP should consult psychiatry. Although MSB‐AP is a diagnosis of exclusion and often based on circumstantial evidence, psychiatry can assist in diagnosing this disorder and, in the event of a confession, provide immediate therapeutic intervention. Social services can aid in a vulnerable adult investigation for patients who do not have capacity.
When Meadow first described MSBP, he ended his article by asking Is this degree of falsification rare or is it under‐recognized? Time has answered Meadow's question. Now we ask the same question with regard to MSB‐AP, is it rare or under‐recognized? We must remain vigilant for this disorder. Early recognition can prevent healthcare providers from unknowingly perpetuating victimization by treating caregiver‐induced pathology as if legitimate, thereby satisfying the perpetrator's psychological needs. Despite Meadow's assertion that proxies outgrow their victimization, our review warns that advanced age does not preclude vulnerability and in some cases, may actually increase it. In the future, the incidence and prevalence of MSB‐AP is likely to increase as medical technology allows greater survival of cognitively impaired populations who are dependent on others for care. The elderly and developmentally delayed may be especially at risk.
ACKNOWLEDGMENTS
Disclosures: M.C.B., M.B.W., and M.I.L. report no conflicts of interest. J.M.B. receives payment for lectures, including service on speakers bureaus, from nonprofit continuing medical education organizations and universities for occasional lectures; however, this funding is not relevant to this review.
- . Munchausen syndrome. Lancet. 1951(1):339–341.
- . Munchausen syndrome by proxy. The hinterland of child abuse. Lancet. 1977;2(8033):343–345.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision. 4th ed. Washington, DC: American Psychiatric Press; 2000.
- , , , et al. Position paper: definitional issues in Munchausen by proxy. Child Maltreat. 2002;7(2):105–111.
- , , , . Epidemiology of Munchausen syndrome by proxy, non‐accidental poisoning, and non‐accidental suffocation. Arch Dis Child. 1996;75(1):57–61.
- . Web of deceit: a literature review of Munchausen syndrome by proxy. Child Abuse Negl. 1987;11(4):547–563.
- , . Psychopathology of perpetrators of fabricated or induced illness in children: case series. Br J Psychiatry. 2011;199(2):113–118.
- , . The central venous catheter as a source of medical chaos in Munchausen syndrome by proxy. J Pediatr Surg. 1998;33(4):623–627.
- , . Munchausen syndrome by proxy: diagnosis and prevalence. Am J Orthopsychiatry. 1993;63(2):318–321.
- , , , . Munchhausen syndrome by proxy between two adults [in French]. Presse Med. 1996;25(12):583–586.
- , , . Munchhausen syndrome by proxy in an old woman [in French]. Revue Geriatr. 2003;28:425–428.
- , , , . Consciousness disturbances: a case report of Munchausen by proxy syndrome in an elderly patient [in Polish]. Przegl Lek. 2003;60(4):307–308.
- , , . Munchausen syndrome by adult proxy: a perpetrator abusing two adults. J Nerv Ment Dis. 1986;174(11):696–698.
- , , . Munchausen syndrome by adult proxy revisited. Isr J Psychiatry Relat Sci. 1991;28(1):33–36.
- , , , , , . Otolaryngology fantastica: the ear, nose, and throat manifestations of Munchausen's syndrome. Laryngoscope. 2012;122(1):51–57.
- . Witchcraft's syndrome: Munchausen's syndrome by proxy. Int J Dermatol. 1998;37(3):229–230.
- , , , , , . Munchausen syndrome by proxy: an adult dyad. Psychosomatics. 2012;53(3):294–299.
- , . Factitious disorders and malingering: challenges for clinical assessment and management. Lancet. 2014;383(9926):1422–1432.
- , . More in sickness than in health: a case study of Munchausen by proxy in the elderly. J Fam Ther. 1989;11(4):321–334.
- , . Recurrent hypoglycaemia in multiple myeloma: a case of Munchausen syndrome by proxy in an elderly patient. J Intern Med. 1998;244(2):175–178.
- , , , , . Idiopathic recurrent stupor: a warning. J Neurol Neurosurg Psychiatry. 2004;75(3):368–369.
- , , . Munchausen by proxy in older adults: A case report. Maced J Med Sci. 2013;6(2):178–181.
Asher first described Munchausen syndrome by proxy over 60 years ago. Like the famous Baron von Munchausen, the persons affected have always traveled widely; and their stories like those attributed to him, are both dramatic and untruthful.[1] Munchausen syndrome is a psychiatric disorder in which a patient intentionally induces or feigns symptoms of physical or psychiatric illness to assume the sick role. In 1977, Meadow described the first case in which a caregiverperpetrator deliberately produced physical symptoms in a child for proxy gratification.[2] Unlike malingering, in which external incentives drive conscious symptom falsification, Munchausen syndrome by proxy (MSBP) is associated with fulfillment of the abuser's own psychological need for garnering praise from medical staff for devoted care given a sick child.[3, 4]
MSBP was once considered vanishingly rare. Many experts now believe it is more common, with a reported annual incidence of 0.4/100,000 in children younger than 16 years, and 2/100,000 in children younger than 1 year.[5] It is a disorder in which a parent, often the mother (94%99%)[6] and often with training or interest in the medical field,[5] is the perpetrator. The medical team caring for her child often views her as unusually helpful, and she is frequently psychiatrically ill with disorders such as depression, personality disorder, or prior personal history of somatoform or factitious disorder.[7, 8] The perpetrator typically inflicts physical harm, although occasionally she may simply lie about symptoms or tamper with laboratory samples.[5] The most common methods of inflicting harm are poisoning and suffocation. Overall mortality is 6% to 9%.[6, 9]
Although a large body of literature addresses pediatric cases, there is little to guide clinicians when victims are adults. An obvious reason may be that MSBP with adult proxies (MSB‐AP) has been reported so rarely, although we believe it is under‐recognized and more common than thought. The primary objective of this review was to identify all published cases of MSB‐AP, and synthesize them to characterize victims and perpetrators, modes of deceit, and relationships between victims and perpetrators so that clinicians will be better equipped to recognize such cases or at least include MSB‐AP in the differential of possibilities when symptoms and history are inconsistent.
METHODS
The Mayo Clinic Rochester Institutional Review Board approved this study. The databases of Ovid MEDLINE, Ovid EMBASE, PubMed, Web of Knowledge, and PsychINFO were searched from inception through April 2014 to identify all published cases of Munchausen by proxy in patients 18 years or older. The following search terms were used: Munchausen syndrome by proxy, factitious disorder by proxy, Munchausen syndrome, and factitious disorder. Reports were included when they described single or multiple cases of MSBP with victims aged at least 18 years. The search was not limited to articles published in English. Bibliographies of selected articles were reviewed for reports identifying additional cases.
RESULTS
We found 10 reports describing 11 cases of MSB‐AP and 1 report describing 2 unique cases of MSB‐AP (Tables 1 and 2). Two case reports were published in French[10, 11] and 1 in Polish.[12] Sigal et al.[13] describes 2 different victims with a common perpetrator, and another report[14] describes the same perpetrator with a third victim. One case, though cited as MSB‐AP in the literature was excluded because it did not meet the criteria for the disorder. In this case, the wife of a 28‐year‐old alcoholic male poured acid on him while he was inebriated, ostensibly to vent frustration and coerce him into sobriety.[15, 16]
| Author | Gender | Age, y | Presenting Features | Occupation/Education | Outcome |
|---|---|---|---|---|---|
| |||||
| Sigal M et al. (1986)[13] | F | 20s | Abscesses (skin) | NP | Death |
| F | 21 | Abscesses (skin) | Child care | Paraplegia | |
| Sigal MD et al. (1991)[14] | M | NP | Rash | NP | Abuse stopped |
| Smith NJ et al. (1989)[19] | M | 69 | None | Retired businessman | Continued fabrication |
| Krebs MO et al. (1996)[10] | M | 40s | Coma | Businessman | Abuse stopped |
| Ben‐Chetrit E et al. (1998)[20] | F | 73 | Coma | NP | Abuse stopped |
| Feldman KW et al. (1998)[8] | F | 21 | NP | Developmental delay | NP |
| Chodorowsk Z et al. (2003)[12] | F | 80 | Syncope | NP | Abuse stopped |
| Strubel D et al. (2003)[11] | F | 82 | None | NP | NP |
| Granot R et al. (2004)[21] | M | 71 | Coma | NP | Abuse stopped |
| Deimel GW et al. (2012)[17] | F | 23 | Rash | High school graduate | Continued abuse |
| F | 21 | Recurrent bacteremia | College student | Death | |
| Singh A et al. (2013)[22] | F | 79 | Fluid overload/false symptom history | Retired | Continued |
| Author | Gender | Age, y | Relationship | Occupation | Mode of Abuse | Outcome When Confronted |
|---|---|---|---|---|---|---|
| ||||||
| Sigal M et al. (1986)[13] | M | 26 | Husbanda | Businessman | Poisoningb followed by subcutaneous gasoline injection | Confession and incarceration |
| M | 29 | Boyfrienda | Businessman | Poisoningb followed by subcutaneous gasoline injection | Confession and incarceration | |
| Sigal MD et al. (1991)[14] | M | 34 | Cellmatea | Worked in medical clinic where incarcerated | Poisoningc followed by subcutaneous turpentine injection | Confession and attempted murder conviction |
| Smith NJ et al. (1989)[19] | F | 55 | Companion | Nurse | False history of hematuria, weakness, headaches | Denial |
| Krebs MO et al. (1996)[10] | F | 47 | Wife | Nurse | Tranquilizer injections | Confession and placed on probation |
| Ben‐Chetrit E et al. (1998)[20] | F | NP | Daughter | Nurse | Insulin injections | Denial |
| Feldman KW et al. (1998)[8] | F | NP | Mother | Business woman | False history of Batten's disease | NP |
| Chodorowsk Z et al. (2003)[12] | F | NP | Granddaughter | NP | Poisoningb | Denial |
| Strubel D et al. (2003)[11] | M | NP | Son | NP | False history of memory loss | NP |
| Granot R et al. (2004)[21] | F | NP | Wife | Hospital employee | Poisoningb | Confession |
| Deimel GW et al. (2012)[17] | F | NP | Mother | Unemployed chronic medical problems | Toxin application to skin | Denial |
| F | NP | Mother | Medical office receptionist | Intravenous injection unknown substance | Denial | |
| Singh A et al. (2013)[22] | M | NP | Son | NP | Fluid administration in context of fluid restriction/erratic medication administration/falsifying severity of symptoms | Denial |
Of the 13 victims, 9 (69%) were women and 4 (31%) were men. Of the ages reported, the median age was 69 years and the mean age was 51 (range, 2182 years). Exact age was not reported in 3 cases. Lying about signs and symptoms, but not actually inducing injury, occurred in 3 cases (23%), whereas in 10 cases (77%), the victims presented with physical findings, including coma (3), rash (2), skin abscesses (2), syncope (1), recurrent bacteremia (1), and fluid overload (1). Seven (54%) of the victims were poisoned, 2 via drug injection and 5 by beverage/food contamination. A perpetrator sedated 3 victims and subsequently injected them, 2 with gasoline and another with turpentine. Two of the victims were involved in business, 1 worked in childcare, 1 attended beauty school after graduating from high school, 1 attended college, and 1 was developmentally delayed. Victim education or occupation was not reported in 7 cases.
Of the 11 perpetrators, 8 (73%) were women, and 3 (27%) were men (note that the same male perpetrator had 3 victims). Median age was 34 years (range, 2655 years), although exact age was not reported in 4 cases. The perpetrator was the victim's mother in 3 cases, wife in 2 cases, son in 2 cases, and daughter, granddaughter, husband, companion, boyfriend, or prison cellmate in 1 case each. Five (38%) worked in healthcare.
All of the perpetrators were highly involved, even overly involved, in the care of their victims, frequently present, sometimes hovering, in hospital settings, and were viewed as generally helpful, if not overintrusive, by hospital staff. When confronted, 3 perpetrators confessed, 3 denied abuse that then ceased, and 4 more denied abuse that continued, culminating in death in 1 case. In 1 case, the outcome was not reported.[8] At least 3 victims remained with their perpetrators. Two perpetrators were criminally charged, 1 receiving probation and the other incarceration. The latter began abusing his cellmate, behavior that did not stop until he was confronted in prison.
CONCLUSION/DISCUSSION
Our primary objective was to locate and review all published cases of MSB‐AP. Our secondary aim was to describe salient characteristics of perpetrators, victims, and fabricated diseases in hopes of helping clinicians better recognize this disorder.
Our review shows that perpetrators were exclusively the victims' caregivers, including mothers, wives, husbands, daughters, granddaughters, or companions. These perpetrators, many with healthcare backgrounds, were attentive, helpful, and excessively present. In the majority of cases, hidden physical abuse yielded visible disease. Less commonly, perpetrators lied about symptoms rather than actually creating signs of disease. The most common mode of disease instigation involved poisoning through beverage/food contamination or subcutaneous injection. Geriatric and developmentally delayed persons appeared particularly vulnerable to victimization. Of the 13 victims, 5 were geriatric and 1 was developmentally delayed.
The adult cases we report are similar to child cases in that the perpetrators are caregivers; however, the caregivers of the adults are a more diverse group. Other similarities between adult and child cases are that physical signs occur more often than simply falsifying information, and poisoning is the most common method of disease fabrication. Suffocation, although common in child cases, has not been reported in adults. Though present in only a minority of cases, another feature distinguishing these cases from those reported in the pediatric literature is the presence of collusion between the perpetrator and victim. When MSBP was first described, Meadow believed that victims would reach an age at which the disorder would cease because they would fight back or report the abuse.[2] In 7 of the adult cases, the victims were unknowingly poisoned; however, in 2 cases,[17] the victims knew what their mothers were doing to them and yet denied that they were harming them. To explain this collusion, Deimel et al. proposed Stockholm syndrome, a condition in which a victim holds a perpetrator in high regard, despite experiencing at their hands what others might consider brainwashing and torture.
The data from the individual cases are sometimes frustratingly incomplete, with inconsistent reporting of dyad demographics and outcomes across the 13 cases, which compromises efforts to compare and contrast them. However, because no published studies have thoroughly reviewed all existing cases of MSB‐AP, we believe our review provides important insights into this condition by consolidating available information. It is our hope that by characterizing perpetrators, victims, and common presentations, we will raise awareness about this condition among healthcare providers so that it may be included in the differential diagnosis when they encounter this dyad: a patient's medical problems do not respond as expected to therapy and a caregivers appears overly involved or attention seeking.
The diagnosis of a factitious disorder often presents an immense clinical challenge and generally involves a multidisciplinary approach.[18] In addition to the incomplete data for existing cases in the literature, we recognize the ongoing difficulties in precise diagnosis of this disorder. Because a hallmark of pathology is secrecy at the outset and often denial, and even abrupt transition of care, upon confrontation, it is often very difficult, especially early on, to uncover patterns of perpetration, let alone posit a motive. We recognize that there may be some perpetrators who are motivated by something other than purely psychological end points, such as financial reward or even sexual victimization. And when alternate care venues are sought, clinicians are often left wondering. Further, the damage that may come to a therapeutic relationship by prematurely diagnosing MSB‐AP is important to keep in mind. Hospitalists who suspect MSB‐AP should consult psychiatry. Although MSB‐AP is a diagnosis of exclusion and often based on circumstantial evidence, psychiatry can assist in diagnosing this disorder and, in the event of a confession, provide immediate therapeutic intervention. Social services can aid in a vulnerable adult investigation for patients who do not have capacity.
When Meadow first described MSBP, he ended his article by asking Is this degree of falsification rare or is it under‐recognized? Time has answered Meadow's question. Now we ask the same question with regard to MSB‐AP, is it rare or under‐recognized? We must remain vigilant for this disorder. Early recognition can prevent healthcare providers from unknowingly perpetuating victimization by treating caregiver‐induced pathology as if legitimate, thereby satisfying the perpetrator's psychological needs. Despite Meadow's assertion that proxies outgrow their victimization, our review warns that advanced age does not preclude vulnerability and in some cases, may actually increase it. In the future, the incidence and prevalence of MSB‐AP is likely to increase as medical technology allows greater survival of cognitively impaired populations who are dependent on others for care. The elderly and developmentally delayed may be especially at risk.
ACKNOWLEDGMENTS
Disclosures: M.C.B., M.B.W., and M.I.L. report no conflicts of interest. J.M.B. receives payment for lectures, including service on speakers bureaus, from nonprofit continuing medical education organizations and universities for occasional lectures; however, this funding is not relevant to this review.
Asher first described Munchausen syndrome by proxy over 60 years ago. Like the famous Baron von Munchausen, the persons affected have always traveled widely; and their stories like those attributed to him, are both dramatic and untruthful.[1] Munchausen syndrome is a psychiatric disorder in which a patient intentionally induces or feigns symptoms of physical or psychiatric illness to assume the sick role. In 1977, Meadow described the first case in which a caregiverperpetrator deliberately produced physical symptoms in a child for proxy gratification.[2] Unlike malingering, in which external incentives drive conscious symptom falsification, Munchausen syndrome by proxy (MSBP) is associated with fulfillment of the abuser's own psychological need for garnering praise from medical staff for devoted care given a sick child.[3, 4]
MSBP was once considered vanishingly rare. Many experts now believe it is more common, with a reported annual incidence of 0.4/100,000 in children younger than 16 years, and 2/100,000 in children younger than 1 year.[5] It is a disorder in which a parent, often the mother (94%99%)[6] and often with training or interest in the medical field,[5] is the perpetrator. The medical team caring for her child often views her as unusually helpful, and she is frequently psychiatrically ill with disorders such as depression, personality disorder, or prior personal history of somatoform or factitious disorder.[7, 8] The perpetrator typically inflicts physical harm, although occasionally she may simply lie about symptoms or tamper with laboratory samples.[5] The most common methods of inflicting harm are poisoning and suffocation. Overall mortality is 6% to 9%.[6, 9]
Although a large body of literature addresses pediatric cases, there is little to guide clinicians when victims are adults. An obvious reason may be that MSBP with adult proxies (MSB‐AP) has been reported so rarely, although we believe it is under‐recognized and more common than thought. The primary objective of this review was to identify all published cases of MSB‐AP, and synthesize them to characterize victims and perpetrators, modes of deceit, and relationships between victims and perpetrators so that clinicians will be better equipped to recognize such cases or at least include MSB‐AP in the differential of possibilities when symptoms and history are inconsistent.
METHODS
The Mayo Clinic Rochester Institutional Review Board approved this study. The databases of Ovid MEDLINE, Ovid EMBASE, PubMed, Web of Knowledge, and PsychINFO were searched from inception through April 2014 to identify all published cases of Munchausen by proxy in patients 18 years or older. The following search terms were used: Munchausen syndrome by proxy, factitious disorder by proxy, Munchausen syndrome, and factitious disorder. Reports were included when they described single or multiple cases of MSBP with victims aged at least 18 years. The search was not limited to articles published in English. Bibliographies of selected articles were reviewed for reports identifying additional cases.
RESULTS
We found 10 reports describing 11 cases of MSB‐AP and 1 report describing 2 unique cases of MSB‐AP (Tables 1 and 2). Two case reports were published in French[10, 11] and 1 in Polish.[12] Sigal et al.[13] describes 2 different victims with a common perpetrator, and another report[14] describes the same perpetrator with a third victim. One case, though cited as MSB‐AP in the literature was excluded because it did not meet the criteria for the disorder. In this case, the wife of a 28‐year‐old alcoholic male poured acid on him while he was inebriated, ostensibly to vent frustration and coerce him into sobriety.[15, 16]
| Author | Gender | Age, y | Presenting Features | Occupation/Education | Outcome |
|---|---|---|---|---|---|
| |||||
| Sigal M et al. (1986)[13] | F | 20s | Abscesses (skin) | NP | Death |
| F | 21 | Abscesses (skin) | Child care | Paraplegia | |
| Sigal MD et al. (1991)[14] | M | NP | Rash | NP | Abuse stopped |
| Smith NJ et al. (1989)[19] | M | 69 | None | Retired businessman | Continued fabrication |
| Krebs MO et al. (1996)[10] | M | 40s | Coma | Businessman | Abuse stopped |
| Ben‐Chetrit E et al. (1998)[20] | F | 73 | Coma | NP | Abuse stopped |
| Feldman KW et al. (1998)[8] | F | 21 | NP | Developmental delay | NP |
| Chodorowsk Z et al. (2003)[12] | F | 80 | Syncope | NP | Abuse stopped |
| Strubel D et al. (2003)[11] | F | 82 | None | NP | NP |
| Granot R et al. (2004)[21] | M | 71 | Coma | NP | Abuse stopped |
| Deimel GW et al. (2012)[17] | F | 23 | Rash | High school graduate | Continued abuse |
| F | 21 | Recurrent bacteremia | College student | Death | |
| Singh A et al. (2013)[22] | F | 79 | Fluid overload/false symptom history | Retired | Continued |
| Author | Gender | Age, y | Relationship | Occupation | Mode of Abuse | Outcome When Confronted |
|---|---|---|---|---|---|---|
| ||||||
| Sigal M et al. (1986)[13] | M | 26 | Husbanda | Businessman | Poisoningb followed by subcutaneous gasoline injection | Confession and incarceration |
| M | 29 | Boyfrienda | Businessman | Poisoningb followed by subcutaneous gasoline injection | Confession and incarceration | |
| Sigal MD et al. (1991)[14] | M | 34 | Cellmatea | Worked in medical clinic where incarcerated | Poisoningc followed by subcutaneous turpentine injection | Confession and attempted murder conviction |
| Smith NJ et al. (1989)[19] | F | 55 | Companion | Nurse | False history of hematuria, weakness, headaches | Denial |
| Krebs MO et al. (1996)[10] | F | 47 | Wife | Nurse | Tranquilizer injections | Confession and placed on probation |
| Ben‐Chetrit E et al. (1998)[20] | F | NP | Daughter | Nurse | Insulin injections | Denial |
| Feldman KW et al. (1998)[8] | F | NP | Mother | Business woman | False history of Batten's disease | NP |
| Chodorowsk Z et al. (2003)[12] | F | NP | Granddaughter | NP | Poisoningb | Denial |
| Strubel D et al. (2003)[11] | M | NP | Son | NP | False history of memory loss | NP |
| Granot R et al. (2004)[21] | F | NP | Wife | Hospital employee | Poisoningb | Confession |
| Deimel GW et al. (2012)[17] | F | NP | Mother | Unemployed chronic medical problems | Toxin application to skin | Denial |
| F | NP | Mother | Medical office receptionist | Intravenous injection unknown substance | Denial | |
| Singh A et al. (2013)[22] | M | NP | Son | NP | Fluid administration in context of fluid restriction/erratic medication administration/falsifying severity of symptoms | Denial |
Of the 13 victims, 9 (69%) were women and 4 (31%) were men. Of the ages reported, the median age was 69 years and the mean age was 51 (range, 2182 years). Exact age was not reported in 3 cases. Lying about signs and symptoms, but not actually inducing injury, occurred in 3 cases (23%), whereas in 10 cases (77%), the victims presented with physical findings, including coma (3), rash (2), skin abscesses (2), syncope (1), recurrent bacteremia (1), and fluid overload (1). Seven (54%) of the victims were poisoned, 2 via drug injection and 5 by beverage/food contamination. A perpetrator sedated 3 victims and subsequently injected them, 2 with gasoline and another with turpentine. Two of the victims were involved in business, 1 worked in childcare, 1 attended beauty school after graduating from high school, 1 attended college, and 1 was developmentally delayed. Victim education or occupation was not reported in 7 cases.
Of the 11 perpetrators, 8 (73%) were women, and 3 (27%) were men (note that the same male perpetrator had 3 victims). Median age was 34 years (range, 2655 years), although exact age was not reported in 4 cases. The perpetrator was the victim's mother in 3 cases, wife in 2 cases, son in 2 cases, and daughter, granddaughter, husband, companion, boyfriend, or prison cellmate in 1 case each. Five (38%) worked in healthcare.
All of the perpetrators were highly involved, even overly involved, in the care of their victims, frequently present, sometimes hovering, in hospital settings, and were viewed as generally helpful, if not overintrusive, by hospital staff. When confronted, 3 perpetrators confessed, 3 denied abuse that then ceased, and 4 more denied abuse that continued, culminating in death in 1 case. In 1 case, the outcome was not reported.[8] At least 3 victims remained with their perpetrators. Two perpetrators were criminally charged, 1 receiving probation and the other incarceration. The latter began abusing his cellmate, behavior that did not stop until he was confronted in prison.
CONCLUSION/DISCUSSION
Our primary objective was to locate and review all published cases of MSB‐AP. Our secondary aim was to describe salient characteristics of perpetrators, victims, and fabricated diseases in hopes of helping clinicians better recognize this disorder.
Our review shows that perpetrators were exclusively the victims' caregivers, including mothers, wives, husbands, daughters, granddaughters, or companions. These perpetrators, many with healthcare backgrounds, were attentive, helpful, and excessively present. In the majority of cases, hidden physical abuse yielded visible disease. Less commonly, perpetrators lied about symptoms rather than actually creating signs of disease. The most common mode of disease instigation involved poisoning through beverage/food contamination or subcutaneous injection. Geriatric and developmentally delayed persons appeared particularly vulnerable to victimization. Of the 13 victims, 5 were geriatric and 1 was developmentally delayed.
The adult cases we report are similar to child cases in that the perpetrators are caregivers; however, the caregivers of the adults are a more diverse group. Other similarities between adult and child cases are that physical signs occur more often than simply falsifying information, and poisoning is the most common method of disease fabrication. Suffocation, although common in child cases, has not been reported in adults. Though present in only a minority of cases, another feature distinguishing these cases from those reported in the pediatric literature is the presence of collusion between the perpetrator and victim. When MSBP was first described, Meadow believed that victims would reach an age at which the disorder would cease because they would fight back or report the abuse.[2] In 7 of the adult cases, the victims were unknowingly poisoned; however, in 2 cases,[17] the victims knew what their mothers were doing to them and yet denied that they were harming them. To explain this collusion, Deimel et al. proposed Stockholm syndrome, a condition in which a victim holds a perpetrator in high regard, despite experiencing at their hands what others might consider brainwashing and torture.
The data from the individual cases are sometimes frustratingly incomplete, with inconsistent reporting of dyad demographics and outcomes across the 13 cases, which compromises efforts to compare and contrast them. However, because no published studies have thoroughly reviewed all existing cases of MSB‐AP, we believe our review provides important insights into this condition by consolidating available information. It is our hope that by characterizing perpetrators, victims, and common presentations, we will raise awareness about this condition among healthcare providers so that it may be included in the differential diagnosis when they encounter this dyad: a patient's medical problems do not respond as expected to therapy and a caregivers appears overly involved or attention seeking.
The diagnosis of a factitious disorder often presents an immense clinical challenge and generally involves a multidisciplinary approach.[18] In addition to the incomplete data for existing cases in the literature, we recognize the ongoing difficulties in precise diagnosis of this disorder. Because a hallmark of pathology is secrecy at the outset and often denial, and even abrupt transition of care, upon confrontation, it is often very difficult, especially early on, to uncover patterns of perpetration, let alone posit a motive. We recognize that there may be some perpetrators who are motivated by something other than purely psychological end points, such as financial reward or even sexual victimization. And when alternate care venues are sought, clinicians are often left wondering. Further, the damage that may come to a therapeutic relationship by prematurely diagnosing MSB‐AP is important to keep in mind. Hospitalists who suspect MSB‐AP should consult psychiatry. Although MSB‐AP is a diagnosis of exclusion and often based on circumstantial evidence, psychiatry can assist in diagnosing this disorder and, in the event of a confession, provide immediate therapeutic intervention. Social services can aid in a vulnerable adult investigation for patients who do not have capacity.
When Meadow first described MSBP, he ended his article by asking Is this degree of falsification rare or is it under‐recognized? Time has answered Meadow's question. Now we ask the same question with regard to MSB‐AP, is it rare or under‐recognized? We must remain vigilant for this disorder. Early recognition can prevent healthcare providers from unknowingly perpetuating victimization by treating caregiver‐induced pathology as if legitimate, thereby satisfying the perpetrator's psychological needs. Despite Meadow's assertion that proxies outgrow their victimization, our review warns that advanced age does not preclude vulnerability and in some cases, may actually increase it. In the future, the incidence and prevalence of MSB‐AP is likely to increase as medical technology allows greater survival of cognitively impaired populations who are dependent on others for care. The elderly and developmentally delayed may be especially at risk.
ACKNOWLEDGMENTS
Disclosures: M.C.B., M.B.W., and M.I.L. report no conflicts of interest. J.M.B. receives payment for lectures, including service on speakers bureaus, from nonprofit continuing medical education organizations and universities for occasional lectures; however, this funding is not relevant to this review.
- . Munchausen syndrome. Lancet. 1951(1):339–341.
- . Munchausen syndrome by proxy. The hinterland of child abuse. Lancet. 1977;2(8033):343–345.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision. 4th ed. Washington, DC: American Psychiatric Press; 2000.
- , , , et al. Position paper: definitional issues in Munchausen by proxy. Child Maltreat. 2002;7(2):105–111.
- , , , . Epidemiology of Munchausen syndrome by proxy, non‐accidental poisoning, and non‐accidental suffocation. Arch Dis Child. 1996;75(1):57–61.
- . Web of deceit: a literature review of Munchausen syndrome by proxy. Child Abuse Negl. 1987;11(4):547–563.
- , . Psychopathology of perpetrators of fabricated or induced illness in children: case series. Br J Psychiatry. 2011;199(2):113–118.
- , . The central venous catheter as a source of medical chaos in Munchausen syndrome by proxy. J Pediatr Surg. 1998;33(4):623–627.
- , . Munchausen syndrome by proxy: diagnosis and prevalence. Am J Orthopsychiatry. 1993;63(2):318–321.
- , , , . Munchhausen syndrome by proxy between two adults [in French]. Presse Med. 1996;25(12):583–586.
- , , . Munchhausen syndrome by proxy in an old woman [in French]. Revue Geriatr. 2003;28:425–428.
- , , , . Consciousness disturbances: a case report of Munchausen by proxy syndrome in an elderly patient [in Polish]. Przegl Lek. 2003;60(4):307–308.
- , , . Munchausen syndrome by adult proxy: a perpetrator abusing two adults. J Nerv Ment Dis. 1986;174(11):696–698.
- , , . Munchausen syndrome by adult proxy revisited. Isr J Psychiatry Relat Sci. 1991;28(1):33–36.
- , , , , , . Otolaryngology fantastica: the ear, nose, and throat manifestations of Munchausen's syndrome. Laryngoscope. 2012;122(1):51–57.
- . Witchcraft's syndrome: Munchausen's syndrome by proxy. Int J Dermatol. 1998;37(3):229–230.
- , , , , , . Munchausen syndrome by proxy: an adult dyad. Psychosomatics. 2012;53(3):294–299.
- , . Factitious disorders and malingering: challenges for clinical assessment and management. Lancet. 2014;383(9926):1422–1432.
- , . More in sickness than in health: a case study of Munchausen by proxy in the elderly. J Fam Ther. 1989;11(4):321–334.
- , . Recurrent hypoglycaemia in multiple myeloma: a case of Munchausen syndrome by proxy in an elderly patient. J Intern Med. 1998;244(2):175–178.
- , , , , . Idiopathic recurrent stupor: a warning. J Neurol Neurosurg Psychiatry. 2004;75(3):368–369.
- , , . Munchausen by proxy in older adults: A case report. Maced J Med Sci. 2013;6(2):178–181.
- . Munchausen syndrome. Lancet. 1951(1):339–341.
- . Munchausen syndrome by proxy. The hinterland of child abuse. Lancet. 1977;2(8033):343–345.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision. 4th ed. Washington, DC: American Psychiatric Press; 2000.
- , , , et al. Position paper: definitional issues in Munchausen by proxy. Child Maltreat. 2002;7(2):105–111.
- , , , . Epidemiology of Munchausen syndrome by proxy, non‐accidental poisoning, and non‐accidental suffocation. Arch Dis Child. 1996;75(1):57–61.
- . Web of deceit: a literature review of Munchausen syndrome by proxy. Child Abuse Negl. 1987;11(4):547–563.
- , . Psychopathology of perpetrators of fabricated or induced illness in children: case series. Br J Psychiatry. 2011;199(2):113–118.
- , . The central venous catheter as a source of medical chaos in Munchausen syndrome by proxy. J Pediatr Surg. 1998;33(4):623–627.
- , . Munchausen syndrome by proxy: diagnosis and prevalence. Am J Orthopsychiatry. 1993;63(2):318–321.
- , , , . Munchhausen syndrome by proxy between two adults [in French]. Presse Med. 1996;25(12):583–586.
- , , . Munchhausen syndrome by proxy in an old woman [in French]. Revue Geriatr. 2003;28:425–428.
- , , , . Consciousness disturbances: a case report of Munchausen by proxy syndrome in an elderly patient [in Polish]. Przegl Lek. 2003;60(4):307–308.
- , , . Munchausen syndrome by adult proxy: a perpetrator abusing two adults. J Nerv Ment Dis. 1986;174(11):696–698.
- , , . Munchausen syndrome by adult proxy revisited. Isr J Psychiatry Relat Sci. 1991;28(1):33–36.
- , , , , , . Otolaryngology fantastica: the ear, nose, and throat manifestations of Munchausen's syndrome. Laryngoscope. 2012;122(1):51–57.
- . Witchcraft's syndrome: Munchausen's syndrome by proxy. Int J Dermatol. 1998;37(3):229–230.
- , , , , , . Munchausen syndrome by proxy: an adult dyad. Psychosomatics. 2012;53(3):294–299.
- , . Factitious disorders and malingering: challenges for clinical assessment and management. Lancet. 2014;383(9926):1422–1432.
- , . More in sickness than in health: a case study of Munchausen by proxy in the elderly. J Fam Ther. 1989;11(4):321–334.
- , . Recurrent hypoglycaemia in multiple myeloma: a case of Munchausen syndrome by proxy in an elderly patient. J Intern Med. 1998;244(2):175–178.
- , , , , . Idiopathic recurrent stupor: a warning. J Neurol Neurosurg Psychiatry. 2004;75(3):368–369.
- , , . Munchausen by proxy in older adults: A case report. Maced J Med Sci. 2013;6(2):178–181.
© 2014 Society of Hospital Medicine
Managing snoring: When to consider surgery
Snoring can range in significance from disturbing a bed partner to being a symptom of obstructive sleep apnea, a risk factor for cardiac disease and stroke. Snoring that is unrelated to obstructive sleep apnea may respond to a combination of nonsurgical treatments. However, if the problem persists despite conservative therapy, then surgical options may be considered.
This article explores why people snore, provides guidance for evaluating it and ruling out obstructive sleep apnea, and describes the available surgical treatments. Snoring associated with obstructive sleep apnea requires a different surgical treatment strategy that is beyond the scope of this article.
WHY PEOPLE SNORE
Humans go through four stages of sleep in each sleep cycle (and four or five cycles per night), and each stage has unique physiologic characteristics. As we progress deeper into sleep with each successive stage, the skeletal muscles of the body relax and eventually become atonic, except for the respiratory and ocular muscles. Soft tissues of the upper aerodigestive tract also lose their muscular tone.
Snoring is an undesirable vibratory sound that originates from the soft tissues of the upper respiratory tract during sleep, as airflow causes the relaxed tissues to vibrate.
The upper airway can be obstructed by the nasal septum, inferior nasal turbinates, adenoids, tonsils, uvula, soft palate, and base of the tongue—and often by more than one (Figure 1).3 In rare cases, obstruction can occur at the level of the larynx, such as from a tumor, laryngomalacia, or a laryngeal defect.
A SPECTRUM OF SLEEP-DISORDERED BREATHING
The American Academy of Sleep Medicine’s International Classification of Sleep Disorders1 defines a number of sleep disorders. In clinical practice, the first-line diagnostic test for sleep disorders is polysomnography.
Snoring is only one sign of sleep-disordered breathing; others are excessive daytime somnolence, restless sleep, and witnessed apnea.
Considerable evidence links obstructive sleep apnea with serious medical problems including hypertension, coronary artery disease, heart failure, cardiac arrhythmia, and stroke.2 Others include mood disorders, decreased libido, and cognitive impairment, with changes in attention, concentration, executive function, and fine-motor coordination.4 Therefore, ruling out obstructive sleep apnea is essential before pursuing interventions for primary snoring, although both disorders may warrant surgery.
PATIENT HISTORY
Most patients who present to the office because of snoring have snored for many years. Many seek medical attention at the request of a long-suffering bed partner.
Associated symptoms in primary snoring may include mouth breathing, chronic nasal congestion, and morning dry throat. Witnessed apnea, frequent awakenings during sleep, restless sleep, daytime somnolence, frequents naps, and memory impairment may be signs of more significant sleep-disordered breathing, such as obstructive sleep apnea.
The Epworth sleepiness scale may help quantify the severity of daytime somnolence.5 It is measured in a short questionnaire in which the patient indicates, on a scale of 0 to 3, his or her likelihood of dozing in a variety of situations.
The STOP-BANG questionnaire consists of eight yes-no questions:
- Snore: Have you been told that you snore?
- Tired: Are you often tired during the day?
- Obstruction: Do you know if you stop breathing, or has anyone witnessed you stop breathing while you are asleep?
- Pressure: Do you have high blood pressure or are you on medication to control high blood pressure?
- Body mass index: Is your body mass index higher than 35 kg/m2?
- Age: Are you age 50 or older?
- Neck: Do you have a neck circumference greater than 17 inches (men) or greater than 16 inches (women)?
- Gender: Are you male?
A score of three or higher has shown a sensitivity of 93% for detecting moderate obstructive sleep apnea and 100% for severe obstructive sleep apnea.6
SEARCHING FOR ANATOMIC CAUSES OF SNORING
A thorough physical examination should be done, focusing on potential anatomic causes of snoring. Nasal septal deviation or inferior turbinate hypertrophy with mucosal congestion may contribute to chronic mouth breathing secondary to nasal obstruction. Patients with a body mass index over 35 kg/m2 and neck circumference over 17 inches (16 inches in women) are at higher risk of obstructive sleep apnea.
Indirect mirror examination or flexible transnasal endoscopy may reveal obstructing or persistent adenoid lymphoid tissue, particularly in young adults. Transnasal endoscopy may also reveal dynamic collapse of the palate and lateral oropharyngeal wall or fullness of the tongue base with subsequent narrowing of the oropharynx.
Examination of the oral cavity may reveal a disproportionately large tongue, a narrow opening into the oropharynx, or tonsillar hypertrophy. The Friedman classification (Figure 2), also called the modified Mallampati scale, can be used to describe the findings on physical examination of the palate and tongue in a systematic way. There are four grades of increasing severity, and the higher the grade, the less likely that surgery will succeed in patients with obstructive sleep apnea.7 The mouth is examined with the tongue in a relaxed position; in contrast, the original Mallampati classification, which is often used by anesthesiologists in assessing the oral airway, is assessed with the tongue protruding.
During flexible endoscopy, asking the patient to attempt to recreate the snoring can sometimes reveal the causative anatomic structure, which is usually the soft palate.
SLEEP STUDIES
A full diagnostic workup should include a sleep study if obstructive sleep apnea cannot be ruled out by the history and examination. Sleep studies include either polysomnography in a sleep laboratory or a home sleep test. They allow the clinician to further evaluate the severity of sleep-disordered breathing and to distinguish primary snoring from obstructive sleep apnea. This is particularly important if elective surgical intervention is planned. Sleep studies can also be used to evaluate for other sleep disorders.
Apnea is considered obstructive when polysomnography reveals episodes of no oral or nasal airflow with continued inspiratory effort, evidenced by abdominal or thoracic muscle activity. Hypopnea is defined as a 30% or greater reduction in airflow lasting at least 10 seconds, with an associated 4% or greater oxygen desaturation.1 The combined number of apnea and hypopnea events per hour, or apnea-hypopnea index, is used clinically to quantify the severity of sleep-disordered breathing.
Primary snoring is diagnosed if the apnea-hypopnea index is 5 or less. Obstructive sleep apnea is considered mild when the apnea-hypopnea index is greater than 5 but less than 15, moderate from 15 to 30, and severe if over 30.
LIMITED ROLE FOR IMAGING
Cephalometric radiography (plain radiography of the airways) has limited value in the workup of primary snoring and is discouraged. Imaging is most useful in assessing craniofacial skeletal abnormalities. Lateral airway images can help in diagnosing adenoid hypertrophy in children. However, flexible nasopharyngoscopy can obtain this information by direct visualization with no radiation exposure.
Computed tomography and magnetic resonance imaging are seldom used in the workup of snoring because they do little to guide therapeutic intervention, are expensive, and, in the case of computed tomography, expose the patient to unnecessary radiation. Imaging does a have a role when planning surgical intervention of obstruction that involves the maxillofacial skeleton.
NONSURGICAL MANAGEMENT
The primary goal of therapy for snoring is to eliminate or reduce noise levels.
Although no study to date has analyzed the efficacy of nonsurgical management, several treatments are aimed at the root causes of snoring in an attempt to decrease it.
Intranasal topical steroids reduce inflammation of the nasal mucosa that occurs with allergic and nonallergic rhinitis, thereby opening up the nasal airway. They may reduce snoring in a small number of cases. These drugs must often be used in the long term to maintain their efficacy.
Devices. Other than continuous positive airway pressure (CPAP), the only currently available nonsurgical device approved by the US Food and Drug Administration for the treatment of snoring and obstructive sleep apnea is an oral dental appliance, which is customized to the patient’s dentition to relieve upper-airway obstruction by soft tissues of the oral cavity. The lower jaw is forced anteriorly, pulling the tongue and attached soft tissues forward. Custom-fitted oral appliances are an effective option for mild to moderate sleep apnea and associated snoring, and are more effective than thermoplastic “boil-and-bite” devices.8 These can easily be used in patients who have primary snoring.
Over-the-counter remedies such as nasal strips and head-positioning pillows have not been shown to be efficacious for snoring.9
Weight loss. Patients should be encouraged to join a weight-management program if overweight.
Sleep on the side, not on the back. Changing the sleep position may be useful in patients who have positional symptoms. Snoring is often worse in the supine position because gravity acting on the palate and tongue causes narrowing of the airway. “Positional therapy” employs devices to force patients to sleep in a lateral decubitus position to counter the effects of gravity.
Alcohol cessation. Alcohol has a relaxing effect on the muscles of the upper-respiratory tract, and abstaining from alcohol may therefore reduce snoring.
INDICATIONS FOR SURGERY
Surgery can decrease the noise level of snoring and thus bring relief for the patient’s bed partner.
Assessment of the upper airway may suggest the appropriate treatment, depending on whether the patient has nasal obstruction, adenoid hypertrophy, or palatal movement. A sleep study, if not previously done, should be done before surgery to rule out obstructive sleep apnea.
Many patients opt for surgery after noninvasive forms of treatment have proven ineffective or difficult to tolerate. When medical therapy for snoring has been unsuccessful, a discussion of the benefits, risks, and alternatives to surgery must take place between the patient and the surgeon.
SURGICAL PROCEDURES
Septoplasty
Septoplasty—straightening the nasal septum to improve the nasal airway—is an outpatient procedure. Although a deviated septum alone is not often the sole cause of snoring, most otolaryngologists agree that the septum should be addressed before or concomitantly with any palatal surgery for sleep-disordered breathing.
Nasal congestion often comes from a deviated bony or cartilaginous septum, enlarged turbinates, or bone spurs. Septal deviation may be developmental or the result of trauma to the nose.
Complications of septoplasty are rare but include septal perforation, scar-band formation, septal hematoma, epistaxis, and infection.
Radiofrequency ablation of the inferior turbinates
Hypertrophy of the inferior turbinate is the most common cause of nasal obstruction, followed by structural deformity of the nasal airway by septal deviation.3 Many patients report fixed or fluctuating nasal congestion and chronic mouth-breathing. The causes of turbinate congestion or enlargement include allergic rhinitis, upper-respiratory infection, and chronic rhinitis. In most cases, turbinate hypertrophy occurs at the level of the submucosa.
Radiofrequency ablation uses radiofrequency energy to generate heat at approximately 85°C (185°F) to create finely controlled coagulative lesions. The lesions are naturally resorbed in 3 to 8 weeks, inducing fibrosis, reducing excess tissue volume, and thus opening the airway. The procedure can be repeated several times to achieve optimal results. Radiofrequency ablation can also be used to reduce anatomic obstruction in other parts of the airway, such as the soft palate and the base of tongue.
Submucosal radiofrequency ablation of the inferior turbinate is a simple office-based procedure. It is often combined with septoplasty to optimize the nasal airway.
Mild to moderate edema with subsequent nasal obstruction and thick mucus formation can be expected the first week after the procedure. The risk of postoperative bleeding and infection is low. When performed with septoplasty, there is a low risk that scar tissue, or synechiae, may form between the turbinate and the septum.
Radiofrequency ablation of the palate
The soft palate is the most common anatomic source of snoring, and radiofrequency ablation can be applied to it as well. As with radiofrequency ablation in other areas, coagulative necrosis leads to fibrosis, and the soft tissue eventually contracts in volume with increased stiffness, thereby resulting in less tissue elasticity and vibration.
Carroll et al10 reported that nasal surgery combined with radiofrequency ablation of either the palate or the base of the tongue completely resolved snoring (according to the patient’s bed partner) in 42% of cases and improved it in 52%, with few complications. Also, patients who received more than one radiofrequency ablation application were more than twice as likely to have resolution of their snoring.
A systematic review of palatal radiofrequency ablation for snoring found that it is safe with minimal complication rates and reduces snoring in short-term follow-up.11 The authors reviewed 30 studies: two randomized controlled trials, four clinical controlled trials, and 24 prospective uncontrolled studies. The only placebo-controlled randomized controlled trial found soft-palate radiofrequency ablation to be superior to placebo. In these studies, follow-up varied from 6 weeks to 26 months. However, the relapse rate was as high as 50% at a mean follow-up time of 13.2 months.
Thus, most of the information in this review has come from observational studies with short follow-up time. In another study, however, the authors presented a 5-year follow-up of palatal radiofrequency ablation that showed persistent and satisfying reduction of snoring.12
Injection snoreplasty
Alternative procedures have been used to reduce palatal flutter that leads to snoring.
Injection snoreplasty was first described by Brietzke and Mair al in 2001.13 Sodium tetradecyl sulfate, a sclerotherapy agent, is injected directly into the submucosal layer of the soft palate to induce scarring and reduce or eliminate snoring caused by the soft palate.
In a cohort study of 25 patients, the subjective success rate was 75% (13 patients) as far out as 19 months.14 In a separate cohort of 17 patients, home polysomnography with audio recordings was done before and after treatment in patients who underwent injection snoreplasty. Twelve (17%) of these patients had a significant reduction in the proportion of palatal snoring, loudness, and flutter frequency. Long-term success and snoring relapse rates of injection snoreplasty were reported to be similar to those of other current treatments.14
Pillar implants
The Pillar implant (Medtronic) was approved by the US Food and Drug Administration in 2002 for snoring and in 2004 for mild to moderate obstructive sleep apnea.
The implant, made of a woven polyester material, is designed to reduce vibration of the soft palate by increasing its stiffness. The implant induces a chronic inflammatory response that is thought to result in the formation of a fibrous capsule, which may also play a role in palatal stiffening. Three thin implants are inserted into the paramedian soft palate in a parallel orientation. This is an outpatient procedure done in the office.
The short-term benefits of the Pillar implant procedure have been well documented.15,16 A meta-analysis of seven case-controlled studies that included 174 patients found the Pillar implant significantly decreased the loudness of snoring by 59%.15 The major disadvantage of Pillar implants was their high extrusion rate, which was reported to be 9.3%.15 While statistically significant improvement has been shown at up to 1 year, a recent longitudinal study suggests a clinical deterioration in snoring scale scores by 4 years after the procedure.16
Laser-assisted uvulopalatoplasty
Laser-assisted uvulopalatoplasty is a staged office-based procedure that involves removal of excess uvular mucosa and the creation of transpalatal vertical troughs to widen the retropalatal airway for the treatment of snoring and mild obstructive sleep apnea. The treatment typically requires about three sessions. It aims to mimic the palatal appearance of uvulopalatopharyngoplasty used to treat obstructive sleep apnea and has been proposed to have similar surgical outcomes in properly selected patients.
Krespi and Kaeker,17 in 1994, were among the first to describe the technique in the United States.
Kyrmizakis et al,18 in a retrospective study of 59 patients with habitual snoring who underwent laser-assisted uvulopalatoplasty, showed that a significant number of patients benefited from the procedure. During a follow-up ranging from 6 months to 5 years (mean 40 months), 91.5% of the patients with habitual snoring reported significant short-term improvement based on a posttreatment questionnaire, and 79.7% reported long-term subjective improvement.
Unfortunately, most of the studies have been small, and thus there is some controversy about the efficacy of laser-assisted uvulopalatoplasty, particularly in patients with obstructive sleep apnea. The most significant complication during healing is pain, which may deter patients from completing the full course of treatment.
- American Academy of Sleep Medicine. International Classification of Sleep Disorders – Second Edition (ICSD-2). American Academy of Sleep Medicine 2005, 0965722023 978-0965722025.
- Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA 2003; 290:1906–1914.
- Clemente CD. Anatomy: A Regional Atlas of the Human Body. Philadelphia; Lippincott Williams & Wilkins; 2010:752.
- Jackson ML, Howard ME, Barnes M. Cognition and daytime functioning in sleep-related breathing disorders. Prog Brain Res 2011; 190:53–68.
- Damiani MF, Quaranta VN, Falcone VA, et al. The Epworth Sleepiness Scale: conventional self vs physician administration. Chest 2013; 143:1569–1575.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Friedman M, Ibrahim H, Bass L. Clinical staging for sleep-disordered breathing. Otolaryngol Head Neck Surg 2002; 127:13–21.
- Vanderveken OM, Devolder A, Marklund M, et al. Comparison of a custom-made and a thermoplastic oral appliance for the treatment of mild sleep apnea. Am J Respir Crit Care Med 2008; 178:197–202.
- Michaelson P, Mair EA. Popular snore aids: do they work? Otolaryngol Head Neck Surg 2004; 130:649–658.
- Carroll W, Wilhoit CS, Intaphan J, Nguyen SA, Gillespie MB. Snoring management with nasal surgery and upper airway radiofrequency ablation. Otolaryngol Head Neck Surg 2012; 146:1023–1027.
- Bäck LJ, Hytönen ML, Roine RP, Malmivaara AOV. Radiofrequency ablation treatment of soft palate for patients with snoring: a systematic review of effectiveness and adverse effects. Laryngoscope 2009, 119:1241–1250.
- DeVito A, Frassinet S, Panatta ML, Montevecchi F, Canzi P, Vicini C. Multilevel radiofrequency ablation for snoring and OSAHS patients therapy: long-term outcomes. Eur Arch Otolaryngol 2012; 269:321–330.
- Brietzke SE, Mair EA. Injection snoreplasty: how to treat snoring without all the pain and expense. Otolaryngol Head Neck Surg 2001; 124:503–510.
- Brietzke SE, Mair EA. Injection snoreplasty: extended follow-up and new objective data. Otolaryngol Head Neck Surg 2003; 128:605–615.
- Choi JH, Kim SN, Cho JH. Efficacy of the Pillar implant in the treatment of snoring and mild-to-moderate obstructive sleep apnea: a meta-analysis. Laryngoscope 2013; 123:269–276.
- Rotenberg BW, Luu K. Four-year outcomes of palatal implants for primary snoring treatment: a prospective longitudinal study. Laryngoscope 2012; 122:696–699.
- Krespi YP, Kacker A. Laser-assisted uvulopalatoplasty revisited. Otolaryngol Clin North Am 2003; 36:495–500.
- Kyrmizakis DE, Chimona TS, Papadakis CE, et al. Laser-assisted uvulopalatoplasty for the treatment of snoring and mild obstructive sleep apnea syndrome. J Otolaryngol 2003; 32:174–179.
Snoring can range in significance from disturbing a bed partner to being a symptom of obstructive sleep apnea, a risk factor for cardiac disease and stroke. Snoring that is unrelated to obstructive sleep apnea may respond to a combination of nonsurgical treatments. However, if the problem persists despite conservative therapy, then surgical options may be considered.
This article explores why people snore, provides guidance for evaluating it and ruling out obstructive sleep apnea, and describes the available surgical treatments. Snoring associated with obstructive sleep apnea requires a different surgical treatment strategy that is beyond the scope of this article.
WHY PEOPLE SNORE
Humans go through four stages of sleep in each sleep cycle (and four or five cycles per night), and each stage has unique physiologic characteristics. As we progress deeper into sleep with each successive stage, the skeletal muscles of the body relax and eventually become atonic, except for the respiratory and ocular muscles. Soft tissues of the upper aerodigestive tract also lose their muscular tone.
Snoring is an undesirable vibratory sound that originates from the soft tissues of the upper respiratory tract during sleep, as airflow causes the relaxed tissues to vibrate.
The upper airway can be obstructed by the nasal septum, inferior nasal turbinates, adenoids, tonsils, uvula, soft palate, and base of the tongue—and often by more than one (Figure 1).3 In rare cases, obstruction can occur at the level of the larynx, such as from a tumor, laryngomalacia, or a laryngeal defect.
A SPECTRUM OF SLEEP-DISORDERED BREATHING
The American Academy of Sleep Medicine’s International Classification of Sleep Disorders1 defines a number of sleep disorders. In clinical practice, the first-line diagnostic test for sleep disorders is polysomnography.
Snoring is only one sign of sleep-disordered breathing; others are excessive daytime somnolence, restless sleep, and witnessed apnea.
Considerable evidence links obstructive sleep apnea with serious medical problems including hypertension, coronary artery disease, heart failure, cardiac arrhythmia, and stroke.2 Others include mood disorders, decreased libido, and cognitive impairment, with changes in attention, concentration, executive function, and fine-motor coordination.4 Therefore, ruling out obstructive sleep apnea is essential before pursuing interventions for primary snoring, although both disorders may warrant surgery.
PATIENT HISTORY
Most patients who present to the office because of snoring have snored for many years. Many seek medical attention at the request of a long-suffering bed partner.
Associated symptoms in primary snoring may include mouth breathing, chronic nasal congestion, and morning dry throat. Witnessed apnea, frequent awakenings during sleep, restless sleep, daytime somnolence, frequents naps, and memory impairment may be signs of more significant sleep-disordered breathing, such as obstructive sleep apnea.
The Epworth sleepiness scale may help quantify the severity of daytime somnolence.5 It is measured in a short questionnaire in which the patient indicates, on a scale of 0 to 3, his or her likelihood of dozing in a variety of situations.
The STOP-BANG questionnaire consists of eight yes-no questions:
- Snore: Have you been told that you snore?
- Tired: Are you often tired during the day?
- Obstruction: Do you know if you stop breathing, or has anyone witnessed you stop breathing while you are asleep?
- Pressure: Do you have high blood pressure or are you on medication to control high blood pressure?
- Body mass index: Is your body mass index higher than 35 kg/m2?
- Age: Are you age 50 or older?
- Neck: Do you have a neck circumference greater than 17 inches (men) or greater than 16 inches (women)?
- Gender: Are you male?
A score of three or higher has shown a sensitivity of 93% for detecting moderate obstructive sleep apnea and 100% for severe obstructive sleep apnea.6
SEARCHING FOR ANATOMIC CAUSES OF SNORING
A thorough physical examination should be done, focusing on potential anatomic causes of snoring. Nasal septal deviation or inferior turbinate hypertrophy with mucosal congestion may contribute to chronic mouth breathing secondary to nasal obstruction. Patients with a body mass index over 35 kg/m2 and neck circumference over 17 inches (16 inches in women) are at higher risk of obstructive sleep apnea.
Indirect mirror examination or flexible transnasal endoscopy may reveal obstructing or persistent adenoid lymphoid tissue, particularly in young adults. Transnasal endoscopy may also reveal dynamic collapse of the palate and lateral oropharyngeal wall or fullness of the tongue base with subsequent narrowing of the oropharynx.
Examination of the oral cavity may reveal a disproportionately large tongue, a narrow opening into the oropharynx, or tonsillar hypertrophy. The Friedman classification (Figure 2), also called the modified Mallampati scale, can be used to describe the findings on physical examination of the palate and tongue in a systematic way. There are four grades of increasing severity, and the higher the grade, the less likely that surgery will succeed in patients with obstructive sleep apnea.7 The mouth is examined with the tongue in a relaxed position; in contrast, the original Mallampati classification, which is often used by anesthesiologists in assessing the oral airway, is assessed with the tongue protruding.
During flexible endoscopy, asking the patient to attempt to recreate the snoring can sometimes reveal the causative anatomic structure, which is usually the soft palate.
SLEEP STUDIES
A full diagnostic workup should include a sleep study if obstructive sleep apnea cannot be ruled out by the history and examination. Sleep studies include either polysomnography in a sleep laboratory or a home sleep test. They allow the clinician to further evaluate the severity of sleep-disordered breathing and to distinguish primary snoring from obstructive sleep apnea. This is particularly important if elective surgical intervention is planned. Sleep studies can also be used to evaluate for other sleep disorders.
Apnea is considered obstructive when polysomnography reveals episodes of no oral or nasal airflow with continued inspiratory effort, evidenced by abdominal or thoracic muscle activity. Hypopnea is defined as a 30% or greater reduction in airflow lasting at least 10 seconds, with an associated 4% or greater oxygen desaturation.1 The combined number of apnea and hypopnea events per hour, or apnea-hypopnea index, is used clinically to quantify the severity of sleep-disordered breathing.
Primary snoring is diagnosed if the apnea-hypopnea index is 5 or less. Obstructive sleep apnea is considered mild when the apnea-hypopnea index is greater than 5 but less than 15, moderate from 15 to 30, and severe if over 30.
LIMITED ROLE FOR IMAGING
Cephalometric radiography (plain radiography of the airways) has limited value in the workup of primary snoring and is discouraged. Imaging is most useful in assessing craniofacial skeletal abnormalities. Lateral airway images can help in diagnosing adenoid hypertrophy in children. However, flexible nasopharyngoscopy can obtain this information by direct visualization with no radiation exposure.
Computed tomography and magnetic resonance imaging are seldom used in the workup of snoring because they do little to guide therapeutic intervention, are expensive, and, in the case of computed tomography, expose the patient to unnecessary radiation. Imaging does a have a role when planning surgical intervention of obstruction that involves the maxillofacial skeleton.
NONSURGICAL MANAGEMENT
The primary goal of therapy for snoring is to eliminate or reduce noise levels.
Although no study to date has analyzed the efficacy of nonsurgical management, several treatments are aimed at the root causes of snoring in an attempt to decrease it.
Intranasal topical steroids reduce inflammation of the nasal mucosa that occurs with allergic and nonallergic rhinitis, thereby opening up the nasal airway. They may reduce snoring in a small number of cases. These drugs must often be used in the long term to maintain their efficacy.
Devices. Other than continuous positive airway pressure (CPAP), the only currently available nonsurgical device approved by the US Food and Drug Administration for the treatment of snoring and obstructive sleep apnea is an oral dental appliance, which is customized to the patient’s dentition to relieve upper-airway obstruction by soft tissues of the oral cavity. The lower jaw is forced anteriorly, pulling the tongue and attached soft tissues forward. Custom-fitted oral appliances are an effective option for mild to moderate sleep apnea and associated snoring, and are more effective than thermoplastic “boil-and-bite” devices.8 These can easily be used in patients who have primary snoring.
Over-the-counter remedies such as nasal strips and head-positioning pillows have not been shown to be efficacious for snoring.9
Weight loss. Patients should be encouraged to join a weight-management program if overweight.
Sleep on the side, not on the back. Changing the sleep position may be useful in patients who have positional symptoms. Snoring is often worse in the supine position because gravity acting on the palate and tongue causes narrowing of the airway. “Positional therapy” employs devices to force patients to sleep in a lateral decubitus position to counter the effects of gravity.
Alcohol cessation. Alcohol has a relaxing effect on the muscles of the upper-respiratory tract, and abstaining from alcohol may therefore reduce snoring.
INDICATIONS FOR SURGERY
Surgery can decrease the noise level of snoring and thus bring relief for the patient’s bed partner.
Assessment of the upper airway may suggest the appropriate treatment, depending on whether the patient has nasal obstruction, adenoid hypertrophy, or palatal movement. A sleep study, if not previously done, should be done before surgery to rule out obstructive sleep apnea.
Many patients opt for surgery after noninvasive forms of treatment have proven ineffective or difficult to tolerate. When medical therapy for snoring has been unsuccessful, a discussion of the benefits, risks, and alternatives to surgery must take place between the patient and the surgeon.
SURGICAL PROCEDURES
Septoplasty
Septoplasty—straightening the nasal septum to improve the nasal airway—is an outpatient procedure. Although a deviated septum alone is not often the sole cause of snoring, most otolaryngologists agree that the septum should be addressed before or concomitantly with any palatal surgery for sleep-disordered breathing.
Nasal congestion often comes from a deviated bony or cartilaginous septum, enlarged turbinates, or bone spurs. Septal deviation may be developmental or the result of trauma to the nose.
Complications of septoplasty are rare but include septal perforation, scar-band formation, septal hematoma, epistaxis, and infection.
Radiofrequency ablation of the inferior turbinates
Hypertrophy of the inferior turbinate is the most common cause of nasal obstruction, followed by structural deformity of the nasal airway by septal deviation.3 Many patients report fixed or fluctuating nasal congestion and chronic mouth-breathing. The causes of turbinate congestion or enlargement include allergic rhinitis, upper-respiratory infection, and chronic rhinitis. In most cases, turbinate hypertrophy occurs at the level of the submucosa.
Radiofrequency ablation uses radiofrequency energy to generate heat at approximately 85°C (185°F) to create finely controlled coagulative lesions. The lesions are naturally resorbed in 3 to 8 weeks, inducing fibrosis, reducing excess tissue volume, and thus opening the airway. The procedure can be repeated several times to achieve optimal results. Radiofrequency ablation can also be used to reduce anatomic obstruction in other parts of the airway, such as the soft palate and the base of tongue.
Submucosal radiofrequency ablation of the inferior turbinate is a simple office-based procedure. It is often combined with septoplasty to optimize the nasal airway.
Mild to moderate edema with subsequent nasal obstruction and thick mucus formation can be expected the first week after the procedure. The risk of postoperative bleeding and infection is low. When performed with septoplasty, there is a low risk that scar tissue, or synechiae, may form between the turbinate and the septum.
Radiofrequency ablation of the palate
The soft palate is the most common anatomic source of snoring, and radiofrequency ablation can be applied to it as well. As with radiofrequency ablation in other areas, coagulative necrosis leads to fibrosis, and the soft tissue eventually contracts in volume with increased stiffness, thereby resulting in less tissue elasticity and vibration.
Carroll et al10 reported that nasal surgery combined with radiofrequency ablation of either the palate or the base of the tongue completely resolved snoring (according to the patient’s bed partner) in 42% of cases and improved it in 52%, with few complications. Also, patients who received more than one radiofrequency ablation application were more than twice as likely to have resolution of their snoring.
A systematic review of palatal radiofrequency ablation for snoring found that it is safe with minimal complication rates and reduces snoring in short-term follow-up.11 The authors reviewed 30 studies: two randomized controlled trials, four clinical controlled trials, and 24 prospective uncontrolled studies. The only placebo-controlled randomized controlled trial found soft-palate radiofrequency ablation to be superior to placebo. In these studies, follow-up varied from 6 weeks to 26 months. However, the relapse rate was as high as 50% at a mean follow-up time of 13.2 months.
Thus, most of the information in this review has come from observational studies with short follow-up time. In another study, however, the authors presented a 5-year follow-up of palatal radiofrequency ablation that showed persistent and satisfying reduction of snoring.12
Injection snoreplasty
Alternative procedures have been used to reduce palatal flutter that leads to snoring.
Injection snoreplasty was first described by Brietzke and Mair al in 2001.13 Sodium tetradecyl sulfate, a sclerotherapy agent, is injected directly into the submucosal layer of the soft palate to induce scarring and reduce or eliminate snoring caused by the soft palate.
In a cohort study of 25 patients, the subjective success rate was 75% (13 patients) as far out as 19 months.14 In a separate cohort of 17 patients, home polysomnography with audio recordings was done before and after treatment in patients who underwent injection snoreplasty. Twelve (17%) of these patients had a significant reduction in the proportion of palatal snoring, loudness, and flutter frequency. Long-term success and snoring relapse rates of injection snoreplasty were reported to be similar to those of other current treatments.14
Pillar implants
The Pillar implant (Medtronic) was approved by the US Food and Drug Administration in 2002 for snoring and in 2004 for mild to moderate obstructive sleep apnea.
The implant, made of a woven polyester material, is designed to reduce vibration of the soft palate by increasing its stiffness. The implant induces a chronic inflammatory response that is thought to result in the formation of a fibrous capsule, which may also play a role in palatal stiffening. Three thin implants are inserted into the paramedian soft palate in a parallel orientation. This is an outpatient procedure done in the office.
The short-term benefits of the Pillar implant procedure have been well documented.15,16 A meta-analysis of seven case-controlled studies that included 174 patients found the Pillar implant significantly decreased the loudness of snoring by 59%.15 The major disadvantage of Pillar implants was their high extrusion rate, which was reported to be 9.3%.15 While statistically significant improvement has been shown at up to 1 year, a recent longitudinal study suggests a clinical deterioration in snoring scale scores by 4 years after the procedure.16
Laser-assisted uvulopalatoplasty
Laser-assisted uvulopalatoplasty is a staged office-based procedure that involves removal of excess uvular mucosa and the creation of transpalatal vertical troughs to widen the retropalatal airway for the treatment of snoring and mild obstructive sleep apnea. The treatment typically requires about three sessions. It aims to mimic the palatal appearance of uvulopalatopharyngoplasty used to treat obstructive sleep apnea and has been proposed to have similar surgical outcomes in properly selected patients.
Krespi and Kaeker,17 in 1994, were among the first to describe the technique in the United States.
Kyrmizakis et al,18 in a retrospective study of 59 patients with habitual snoring who underwent laser-assisted uvulopalatoplasty, showed that a significant number of patients benefited from the procedure. During a follow-up ranging from 6 months to 5 years (mean 40 months), 91.5% of the patients with habitual snoring reported significant short-term improvement based on a posttreatment questionnaire, and 79.7% reported long-term subjective improvement.
Unfortunately, most of the studies have been small, and thus there is some controversy about the efficacy of laser-assisted uvulopalatoplasty, particularly in patients with obstructive sleep apnea. The most significant complication during healing is pain, which may deter patients from completing the full course of treatment.
Snoring can range in significance from disturbing a bed partner to being a symptom of obstructive sleep apnea, a risk factor for cardiac disease and stroke. Snoring that is unrelated to obstructive sleep apnea may respond to a combination of nonsurgical treatments. However, if the problem persists despite conservative therapy, then surgical options may be considered.
This article explores why people snore, provides guidance for evaluating it and ruling out obstructive sleep apnea, and describes the available surgical treatments. Snoring associated with obstructive sleep apnea requires a different surgical treatment strategy that is beyond the scope of this article.
WHY PEOPLE SNORE
Humans go through four stages of sleep in each sleep cycle (and four or five cycles per night), and each stage has unique physiologic characteristics. As we progress deeper into sleep with each successive stage, the skeletal muscles of the body relax and eventually become atonic, except for the respiratory and ocular muscles. Soft tissues of the upper aerodigestive tract also lose their muscular tone.
Snoring is an undesirable vibratory sound that originates from the soft tissues of the upper respiratory tract during sleep, as airflow causes the relaxed tissues to vibrate.
The upper airway can be obstructed by the nasal septum, inferior nasal turbinates, adenoids, tonsils, uvula, soft palate, and base of the tongue—and often by more than one (Figure 1).3 In rare cases, obstruction can occur at the level of the larynx, such as from a tumor, laryngomalacia, or a laryngeal defect.
A SPECTRUM OF SLEEP-DISORDERED BREATHING
The American Academy of Sleep Medicine’s International Classification of Sleep Disorders1 defines a number of sleep disorders. In clinical practice, the first-line diagnostic test for sleep disorders is polysomnography.
Snoring is only one sign of sleep-disordered breathing; others are excessive daytime somnolence, restless sleep, and witnessed apnea.
Considerable evidence links obstructive sleep apnea with serious medical problems including hypertension, coronary artery disease, heart failure, cardiac arrhythmia, and stroke.2 Others include mood disorders, decreased libido, and cognitive impairment, with changes in attention, concentration, executive function, and fine-motor coordination.4 Therefore, ruling out obstructive sleep apnea is essential before pursuing interventions for primary snoring, although both disorders may warrant surgery.
PATIENT HISTORY
Most patients who present to the office because of snoring have snored for many years. Many seek medical attention at the request of a long-suffering bed partner.
Associated symptoms in primary snoring may include mouth breathing, chronic nasal congestion, and morning dry throat. Witnessed apnea, frequent awakenings during sleep, restless sleep, daytime somnolence, frequents naps, and memory impairment may be signs of more significant sleep-disordered breathing, such as obstructive sleep apnea.
The Epworth sleepiness scale may help quantify the severity of daytime somnolence.5 It is measured in a short questionnaire in which the patient indicates, on a scale of 0 to 3, his or her likelihood of dozing in a variety of situations.
The STOP-BANG questionnaire consists of eight yes-no questions:
- Snore: Have you been told that you snore?
- Tired: Are you often tired during the day?
- Obstruction: Do you know if you stop breathing, or has anyone witnessed you stop breathing while you are asleep?
- Pressure: Do you have high blood pressure or are you on medication to control high blood pressure?
- Body mass index: Is your body mass index higher than 35 kg/m2?
- Age: Are you age 50 or older?
- Neck: Do you have a neck circumference greater than 17 inches (men) or greater than 16 inches (women)?
- Gender: Are you male?
A score of three or higher has shown a sensitivity of 93% for detecting moderate obstructive sleep apnea and 100% for severe obstructive sleep apnea.6
SEARCHING FOR ANATOMIC CAUSES OF SNORING
A thorough physical examination should be done, focusing on potential anatomic causes of snoring. Nasal septal deviation or inferior turbinate hypertrophy with mucosal congestion may contribute to chronic mouth breathing secondary to nasal obstruction. Patients with a body mass index over 35 kg/m2 and neck circumference over 17 inches (16 inches in women) are at higher risk of obstructive sleep apnea.
Indirect mirror examination or flexible transnasal endoscopy may reveal obstructing or persistent adenoid lymphoid tissue, particularly in young adults. Transnasal endoscopy may also reveal dynamic collapse of the palate and lateral oropharyngeal wall or fullness of the tongue base with subsequent narrowing of the oropharynx.
Examination of the oral cavity may reveal a disproportionately large tongue, a narrow opening into the oropharynx, or tonsillar hypertrophy. The Friedman classification (Figure 2), also called the modified Mallampati scale, can be used to describe the findings on physical examination of the palate and tongue in a systematic way. There are four grades of increasing severity, and the higher the grade, the less likely that surgery will succeed in patients with obstructive sleep apnea.7 The mouth is examined with the tongue in a relaxed position; in contrast, the original Mallampati classification, which is often used by anesthesiologists in assessing the oral airway, is assessed with the tongue protruding.
During flexible endoscopy, asking the patient to attempt to recreate the snoring can sometimes reveal the causative anatomic structure, which is usually the soft palate.
SLEEP STUDIES
A full diagnostic workup should include a sleep study if obstructive sleep apnea cannot be ruled out by the history and examination. Sleep studies include either polysomnography in a sleep laboratory or a home sleep test. They allow the clinician to further evaluate the severity of sleep-disordered breathing and to distinguish primary snoring from obstructive sleep apnea. This is particularly important if elective surgical intervention is planned. Sleep studies can also be used to evaluate for other sleep disorders.
Apnea is considered obstructive when polysomnography reveals episodes of no oral or nasal airflow with continued inspiratory effort, evidenced by abdominal or thoracic muscle activity. Hypopnea is defined as a 30% or greater reduction in airflow lasting at least 10 seconds, with an associated 4% or greater oxygen desaturation.1 The combined number of apnea and hypopnea events per hour, or apnea-hypopnea index, is used clinically to quantify the severity of sleep-disordered breathing.
Primary snoring is diagnosed if the apnea-hypopnea index is 5 or less. Obstructive sleep apnea is considered mild when the apnea-hypopnea index is greater than 5 but less than 15, moderate from 15 to 30, and severe if over 30.
LIMITED ROLE FOR IMAGING
Cephalometric radiography (plain radiography of the airways) has limited value in the workup of primary snoring and is discouraged. Imaging is most useful in assessing craniofacial skeletal abnormalities. Lateral airway images can help in diagnosing adenoid hypertrophy in children. However, flexible nasopharyngoscopy can obtain this information by direct visualization with no radiation exposure.
Computed tomography and magnetic resonance imaging are seldom used in the workup of snoring because they do little to guide therapeutic intervention, are expensive, and, in the case of computed tomography, expose the patient to unnecessary radiation. Imaging does a have a role when planning surgical intervention of obstruction that involves the maxillofacial skeleton.
NONSURGICAL MANAGEMENT
The primary goal of therapy for snoring is to eliminate or reduce noise levels.
Although no study to date has analyzed the efficacy of nonsurgical management, several treatments are aimed at the root causes of snoring in an attempt to decrease it.
Intranasal topical steroids reduce inflammation of the nasal mucosa that occurs with allergic and nonallergic rhinitis, thereby opening up the nasal airway. They may reduce snoring in a small number of cases. These drugs must often be used in the long term to maintain their efficacy.
Devices. Other than continuous positive airway pressure (CPAP), the only currently available nonsurgical device approved by the US Food and Drug Administration for the treatment of snoring and obstructive sleep apnea is an oral dental appliance, which is customized to the patient’s dentition to relieve upper-airway obstruction by soft tissues of the oral cavity. The lower jaw is forced anteriorly, pulling the tongue and attached soft tissues forward. Custom-fitted oral appliances are an effective option for mild to moderate sleep apnea and associated snoring, and are more effective than thermoplastic “boil-and-bite” devices.8 These can easily be used in patients who have primary snoring.
Over-the-counter remedies such as nasal strips and head-positioning pillows have not been shown to be efficacious for snoring.9
Weight loss. Patients should be encouraged to join a weight-management program if overweight.
Sleep on the side, not on the back. Changing the sleep position may be useful in patients who have positional symptoms. Snoring is often worse in the supine position because gravity acting on the palate and tongue causes narrowing of the airway. “Positional therapy” employs devices to force patients to sleep in a lateral decubitus position to counter the effects of gravity.
Alcohol cessation. Alcohol has a relaxing effect on the muscles of the upper-respiratory tract, and abstaining from alcohol may therefore reduce snoring.
INDICATIONS FOR SURGERY
Surgery can decrease the noise level of snoring and thus bring relief for the patient’s bed partner.
Assessment of the upper airway may suggest the appropriate treatment, depending on whether the patient has nasal obstruction, adenoid hypertrophy, or palatal movement. A sleep study, if not previously done, should be done before surgery to rule out obstructive sleep apnea.
Many patients opt for surgery after noninvasive forms of treatment have proven ineffective or difficult to tolerate. When medical therapy for snoring has been unsuccessful, a discussion of the benefits, risks, and alternatives to surgery must take place between the patient and the surgeon.
SURGICAL PROCEDURES
Septoplasty
Septoplasty—straightening the nasal septum to improve the nasal airway—is an outpatient procedure. Although a deviated septum alone is not often the sole cause of snoring, most otolaryngologists agree that the septum should be addressed before or concomitantly with any palatal surgery for sleep-disordered breathing.
Nasal congestion often comes from a deviated bony or cartilaginous septum, enlarged turbinates, or bone spurs. Septal deviation may be developmental or the result of trauma to the nose.
Complications of septoplasty are rare but include septal perforation, scar-band formation, septal hematoma, epistaxis, and infection.
Radiofrequency ablation of the inferior turbinates
Hypertrophy of the inferior turbinate is the most common cause of nasal obstruction, followed by structural deformity of the nasal airway by septal deviation.3 Many patients report fixed or fluctuating nasal congestion and chronic mouth-breathing. The causes of turbinate congestion or enlargement include allergic rhinitis, upper-respiratory infection, and chronic rhinitis. In most cases, turbinate hypertrophy occurs at the level of the submucosa.
Radiofrequency ablation uses radiofrequency energy to generate heat at approximately 85°C (185°F) to create finely controlled coagulative lesions. The lesions are naturally resorbed in 3 to 8 weeks, inducing fibrosis, reducing excess tissue volume, and thus opening the airway. The procedure can be repeated several times to achieve optimal results. Radiofrequency ablation can also be used to reduce anatomic obstruction in other parts of the airway, such as the soft palate and the base of tongue.
Submucosal radiofrequency ablation of the inferior turbinate is a simple office-based procedure. It is often combined with septoplasty to optimize the nasal airway.
Mild to moderate edema with subsequent nasal obstruction and thick mucus formation can be expected the first week after the procedure. The risk of postoperative bleeding and infection is low. When performed with septoplasty, there is a low risk that scar tissue, or synechiae, may form between the turbinate and the septum.
Radiofrequency ablation of the palate
The soft palate is the most common anatomic source of snoring, and radiofrequency ablation can be applied to it as well. As with radiofrequency ablation in other areas, coagulative necrosis leads to fibrosis, and the soft tissue eventually contracts in volume with increased stiffness, thereby resulting in less tissue elasticity and vibration.
Carroll et al10 reported that nasal surgery combined with radiofrequency ablation of either the palate or the base of the tongue completely resolved snoring (according to the patient’s bed partner) in 42% of cases and improved it in 52%, with few complications. Also, patients who received more than one radiofrequency ablation application were more than twice as likely to have resolution of their snoring.
A systematic review of palatal radiofrequency ablation for snoring found that it is safe with minimal complication rates and reduces snoring in short-term follow-up.11 The authors reviewed 30 studies: two randomized controlled trials, four clinical controlled trials, and 24 prospective uncontrolled studies. The only placebo-controlled randomized controlled trial found soft-palate radiofrequency ablation to be superior to placebo. In these studies, follow-up varied from 6 weeks to 26 months. However, the relapse rate was as high as 50% at a mean follow-up time of 13.2 months.
Thus, most of the information in this review has come from observational studies with short follow-up time. In another study, however, the authors presented a 5-year follow-up of palatal radiofrequency ablation that showed persistent and satisfying reduction of snoring.12
Injection snoreplasty
Alternative procedures have been used to reduce palatal flutter that leads to snoring.
Injection snoreplasty was first described by Brietzke and Mair al in 2001.13 Sodium tetradecyl sulfate, a sclerotherapy agent, is injected directly into the submucosal layer of the soft palate to induce scarring and reduce or eliminate snoring caused by the soft palate.
In a cohort study of 25 patients, the subjective success rate was 75% (13 patients) as far out as 19 months.14 In a separate cohort of 17 patients, home polysomnography with audio recordings was done before and after treatment in patients who underwent injection snoreplasty. Twelve (17%) of these patients had a significant reduction in the proportion of palatal snoring, loudness, and flutter frequency. Long-term success and snoring relapse rates of injection snoreplasty were reported to be similar to those of other current treatments.14
Pillar implants
The Pillar implant (Medtronic) was approved by the US Food and Drug Administration in 2002 for snoring and in 2004 for mild to moderate obstructive sleep apnea.
The implant, made of a woven polyester material, is designed to reduce vibration of the soft palate by increasing its stiffness. The implant induces a chronic inflammatory response that is thought to result in the formation of a fibrous capsule, which may also play a role in palatal stiffening. Three thin implants are inserted into the paramedian soft palate in a parallel orientation. This is an outpatient procedure done in the office.
The short-term benefits of the Pillar implant procedure have been well documented.15,16 A meta-analysis of seven case-controlled studies that included 174 patients found the Pillar implant significantly decreased the loudness of snoring by 59%.15 The major disadvantage of Pillar implants was their high extrusion rate, which was reported to be 9.3%.15 While statistically significant improvement has been shown at up to 1 year, a recent longitudinal study suggests a clinical deterioration in snoring scale scores by 4 years after the procedure.16
Laser-assisted uvulopalatoplasty
Laser-assisted uvulopalatoplasty is a staged office-based procedure that involves removal of excess uvular mucosa and the creation of transpalatal vertical troughs to widen the retropalatal airway for the treatment of snoring and mild obstructive sleep apnea. The treatment typically requires about three sessions. It aims to mimic the palatal appearance of uvulopalatopharyngoplasty used to treat obstructive sleep apnea and has been proposed to have similar surgical outcomes in properly selected patients.
Krespi and Kaeker,17 in 1994, were among the first to describe the technique in the United States.
Kyrmizakis et al,18 in a retrospective study of 59 patients with habitual snoring who underwent laser-assisted uvulopalatoplasty, showed that a significant number of patients benefited from the procedure. During a follow-up ranging from 6 months to 5 years (mean 40 months), 91.5% of the patients with habitual snoring reported significant short-term improvement based on a posttreatment questionnaire, and 79.7% reported long-term subjective improvement.
Unfortunately, most of the studies have been small, and thus there is some controversy about the efficacy of laser-assisted uvulopalatoplasty, particularly in patients with obstructive sleep apnea. The most significant complication during healing is pain, which may deter patients from completing the full course of treatment.
- American Academy of Sleep Medicine. International Classification of Sleep Disorders – Second Edition (ICSD-2). American Academy of Sleep Medicine 2005, 0965722023 978-0965722025.
- Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA 2003; 290:1906–1914.
- Clemente CD. Anatomy: A Regional Atlas of the Human Body. Philadelphia; Lippincott Williams & Wilkins; 2010:752.
- Jackson ML, Howard ME, Barnes M. Cognition and daytime functioning in sleep-related breathing disorders. Prog Brain Res 2011; 190:53–68.
- Damiani MF, Quaranta VN, Falcone VA, et al. The Epworth Sleepiness Scale: conventional self vs physician administration. Chest 2013; 143:1569–1575.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Friedman M, Ibrahim H, Bass L. Clinical staging for sleep-disordered breathing. Otolaryngol Head Neck Surg 2002; 127:13–21.
- Vanderveken OM, Devolder A, Marklund M, et al. Comparison of a custom-made and a thermoplastic oral appliance for the treatment of mild sleep apnea. Am J Respir Crit Care Med 2008; 178:197–202.
- Michaelson P, Mair EA. Popular snore aids: do they work? Otolaryngol Head Neck Surg 2004; 130:649–658.
- Carroll W, Wilhoit CS, Intaphan J, Nguyen SA, Gillespie MB. Snoring management with nasal surgery and upper airway radiofrequency ablation. Otolaryngol Head Neck Surg 2012; 146:1023–1027.
- Bäck LJ, Hytönen ML, Roine RP, Malmivaara AOV. Radiofrequency ablation treatment of soft palate for patients with snoring: a systematic review of effectiveness and adverse effects. Laryngoscope 2009, 119:1241–1250.
- DeVito A, Frassinet S, Panatta ML, Montevecchi F, Canzi P, Vicini C. Multilevel radiofrequency ablation for snoring and OSAHS patients therapy: long-term outcomes. Eur Arch Otolaryngol 2012; 269:321–330.
- Brietzke SE, Mair EA. Injection snoreplasty: how to treat snoring without all the pain and expense. Otolaryngol Head Neck Surg 2001; 124:503–510.
- Brietzke SE, Mair EA. Injection snoreplasty: extended follow-up and new objective data. Otolaryngol Head Neck Surg 2003; 128:605–615.
- Choi JH, Kim SN, Cho JH. Efficacy of the Pillar implant in the treatment of snoring and mild-to-moderate obstructive sleep apnea: a meta-analysis. Laryngoscope 2013; 123:269–276.
- Rotenberg BW, Luu K. Four-year outcomes of palatal implants for primary snoring treatment: a prospective longitudinal study. Laryngoscope 2012; 122:696–699.
- Krespi YP, Kacker A. Laser-assisted uvulopalatoplasty revisited. Otolaryngol Clin North Am 2003; 36:495–500.
- Kyrmizakis DE, Chimona TS, Papadakis CE, et al. Laser-assisted uvulopalatoplasty for the treatment of snoring and mild obstructive sleep apnea syndrome. J Otolaryngol 2003; 32:174–179.
- American Academy of Sleep Medicine. International Classification of Sleep Disorders – Second Edition (ICSD-2). American Academy of Sleep Medicine 2005, 0965722023 978-0965722025.
- Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA 2003; 290:1906–1914.
- Clemente CD. Anatomy: A Regional Atlas of the Human Body. Philadelphia; Lippincott Williams & Wilkins; 2010:752.
- Jackson ML, Howard ME, Barnes M. Cognition and daytime functioning in sleep-related breathing disorders. Prog Brain Res 2011; 190:53–68.
- Damiani MF, Quaranta VN, Falcone VA, et al. The Epworth Sleepiness Scale: conventional self vs physician administration. Chest 2013; 143:1569–1575.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Friedman M, Ibrahim H, Bass L. Clinical staging for sleep-disordered breathing. Otolaryngol Head Neck Surg 2002; 127:13–21.
- Vanderveken OM, Devolder A, Marklund M, et al. Comparison of a custom-made and a thermoplastic oral appliance for the treatment of mild sleep apnea. Am J Respir Crit Care Med 2008; 178:197–202.
- Michaelson P, Mair EA. Popular snore aids: do they work? Otolaryngol Head Neck Surg 2004; 130:649–658.
- Carroll W, Wilhoit CS, Intaphan J, Nguyen SA, Gillespie MB. Snoring management with nasal surgery and upper airway radiofrequency ablation. Otolaryngol Head Neck Surg 2012; 146:1023–1027.
- Bäck LJ, Hytönen ML, Roine RP, Malmivaara AOV. Radiofrequency ablation treatment of soft palate for patients with snoring: a systematic review of effectiveness and adverse effects. Laryngoscope 2009, 119:1241–1250.
- DeVito A, Frassinet S, Panatta ML, Montevecchi F, Canzi P, Vicini C. Multilevel radiofrequency ablation for snoring and OSAHS patients therapy: long-term outcomes. Eur Arch Otolaryngol 2012; 269:321–330.
- Brietzke SE, Mair EA. Injection snoreplasty: how to treat snoring without all the pain and expense. Otolaryngol Head Neck Surg 2001; 124:503–510.
- Brietzke SE, Mair EA. Injection snoreplasty: extended follow-up and new objective data. Otolaryngol Head Neck Surg 2003; 128:605–615.
- Choi JH, Kim SN, Cho JH. Efficacy of the Pillar implant in the treatment of snoring and mild-to-moderate obstructive sleep apnea: a meta-analysis. Laryngoscope 2013; 123:269–276.
- Rotenberg BW, Luu K. Four-year outcomes of palatal implants for primary snoring treatment: a prospective longitudinal study. Laryngoscope 2012; 122:696–699.
- Krespi YP, Kacker A. Laser-assisted uvulopalatoplasty revisited. Otolaryngol Clin North Am 2003; 36:495–500.
- Kyrmizakis DE, Chimona TS, Papadakis CE, et al. Laser-assisted uvulopalatoplasty for the treatment of snoring and mild obstructive sleep apnea syndrome. J Otolaryngol 2003; 32:174–179.
KEY POINTS
- The treatment of snoring begins with a thorough history and physical examination.
- Polysomnography is almost always necessary to rule out other sleep disorders, such as obstructive sleep apnea. This is particularly important if an elective surgical intervention is planned.
- Surgical procedures for snoring include septoplasty with or without radiofrequency ablation of the upper airway, injection snoreplasty, Pillar implants, and laser-assisted uvulopalatoplasty.
- Although studies indicate that these procedures are effective, no well-controlled study has compared one procedure against another. The choice of procedure is often determined by the expertise of the surgeon, and the outcome is highly dependent on the skill of the surgeon.
Keeping up with immunizations for adults
A 58-year-old man with a history of irritable bowel syndrome and diabetes presents for an evaluation in early November. He is taking metformin and insulin glargine 10 units. He smokes 1 pack per day. He believes that his childhood immunizations were completed, but he has no records. He thinks his last “shot” was 15 years ago when he cut his hand on some wood.
Which immunizations, if any, would be most appropriate for this patient?
The explosion of new vaccines, new formulations, and new combinations made available in recent years makes managing immunizations a challenge. This article reviews common immunizations and current recommendations for their appropriate use.
Immunization recommendations (Table 1) are made predominantly by the Advisory Committee on Immunization Practices (ACIP) of the US Centers for Disease Control and Prevention (CDC). The last 15 years have seen the arrival of new vaccines (eg, varicella, hepatitis A, pneumococcal, and human papillomavirus), new formulations (eg, intranasal influenza), and new combinations.
To keep clinicians abreast of new indications, the ACIP issues immunization schedules annually for children and adults, available online and downloadable for easy reference.1 For adults, the ACIP provides schedules based on age and medical condition. The schedule for medical conditions offers specific information regarding immunization and pregnancy, human immunodeficiency virus (HIV) infection, kidney failure, heart disease, asplenia, and other conditions. The ACIP also provides guidance on contraindications; for example, pregnant and immunocompromised patients should not receive the live-attenuated vaccines, ie, zoster, varicella, and combined measles, mumps, and rubella [MMR]).
Adult awareness of vaccines is low, as are vaccination rates: in people older than 60, the vaccination rate is about 70% for influenza, 60% for pneumococcus, 50% for tetanus, and 15% for zoster. The lack of vaccine awareness and the availability of new vaccines and indications have made it difficult to manage immunizations in the primary care setting. The electronic medical record is useful for tracking patient vaccine needs. Ideally, keeping up with immunizations should be a routine part of visits provided by a physician’s care team and does not always require direct physician coordination.
TETANUS, DIPHTHERIA, PERTUSSIS EVERY 10 YEARS
Tetanus (also called “lockjaw”) is a nervous system disorder characterized by muscle spasms. Caused by infection with Clostridium tetani, it is a rare disease in the United States thanks to widespread immunization, and it causes fewer than 50 cases annually. Worldwide, the incidence is about 1 million cases a year with 200,000 to 300,000 deaths.
Diphtheria (formerly sometimes called “throat distemper”) is caused by the gram-positive bacillus Corynebacterium diphtheriae and can occur as a respiratory illness or as a milder cutaneous form. The last outbreak in the United States was in Seattle in the 1970s, with the last reported case in 2003. The ACIP recommends booster shots for tetanus and diphtheria every 10 years following completion of the primary series.
Pertussis or whooping cough, caused by Bordetella pertussis infection, is a highly contagious disease increasingly seen in adults in the United States. It causes few deaths but high morbidity, with coughing that can persist up to 3 months. Coughing can be severe enough to cause vomiting, a characteristic sign.
In July 2012, the CDC reported that the United States was at a 50-year high for pertussis, with 18,000 cases reported and 8 deaths.2 In Washington State alone, more than 2,520 cases had been seen through June 16 of that year, a 1,300% increase over the previous year. Rates were high in older children and adolescents despite previous vaccination, suggesting an early waning of immunity.
The ACIP recommends a single dose of the combination of high-dose tetanus and low-dose diphtheria and pertussis vaccines (Tdap) for all adults regardless of age and for all pregnant women with each pregnancy between 27 and 36 weeks of gestation. A dose of Tdap counts as the tetanus-diphtheria booster shot that is recommended every 10 years.
The patient described above is due for his tetanus-diphtheria booster and so should be given Tdap.
MEASLES, MUMPS, RUBELLA FOR THOSE BORN AFTER 1957
Measles remains a problem in the developing world, with an estimated average of 330 deaths daily. The number of cases fell 99% in the United States following the vaccination program that started in the early 1960s. Before the measles vaccine was available, an estimated 90% of children acquired measles by age 15.
The clinical syndrome consists of fever, conjunctivitis, cough, rash, and the characteristic Koplik spots—small white spots occurring on the inside of cheeks early in the disease course.
During the first 5 months of 2014, the CDC reported 334 cases of measles in the United States in 18 states, with most people affected being unvaccinated.3 In comparison, from 2001 to 2008, the number of cases averaged 56 annually.
Many of the recent cases were associated with infections brought from the Philippines. The increased number of measles cases underscores the need for vaccination to prevent measles and its complications.
Mumps is an acute, self-limited viral syndrome, and parotitis is the hallmark. Vaccination led to a 99% decline in cases in the United States. Although complications are rare, they can be serious and include orchitis (with risk of sterility), meningoencephalitis, and deafness.
Mumps outbreaks still occur, especially in close-contact settings such as schools, colleges, and camps. During the first half of 2014, central Ohio had more than 400 cases linked to The Ohio State University.
Rubella, also known as German measles, is a generally mild infection but is associated with congenital rubella syndrome. If a woman is infected with rubella in the first trimester of pregnancy, the risk of miscarriage is greater than 80%, as is the risk of birth defects, including hearing loss, developmental delay, growth retardation, and cardiac and eye defects.
Recommendations for MMR vaccination. People born before 1957 are considered immune to measles and usually to mumps. Health care workers should document immunity before assuming no vaccination is needed.
People born in 1957 or after should have one dose of MMR vaccine unless immunity is documented or unless there is a contraindication such as immunosuppression. A second dose is recommended for those born in or after 1957 who are considered to be at high risk: eg, health care workers, students entering college, and international travelers. The second dose should be given 4 weeks after the first.
Women of childbearing age should be screened for immunity to rubella. Susceptible women should receive MMR, although not during pregnancy and not if they may get pregnant within 4 weeks.
The patient described above was born before 1957, and so he is probably immune to measles and mumps.
HEPATITIS B FOR THOSE AT RISK
Hepatitis B vaccination is recommended for all adolescents and adults at increased risk: eg, men who have sex with men, intravenous drug users, people with multiple sexual partners, health care workers, patients with end-stage renal disease on hemodialysis, patients with chronic liver disease, and those with diabetes (age < 60).
Immunization consists of a series of three shots (at 0, 1–2, and 4–6 months). Booster doses are not recommended. Postvaccination testing for immunity is available and is recommended for health care workers, patients on hemodialysis, patients with HIV infection or who are otherwise immunocompromised, and sexual partners of people who are positive for hepatitis B surface antigen. Nonresponders should be revaccinated with the entire three-shot schedule. Hepatitis B vaccination is safe in pregnancy.
The patient described above has diabetes and so is a candidate for vaccination.
HEPATITIS A: A SLIGHTLY DIFFERENT RISK GROUP
Hepatitis A vaccination is recommended only for at-risk populations: international travelers; intravenous drug users; men who have sex with men; patients with clotting disorders, chronic liver disease, or hepatitis C infection; international adoptees; and laboratory personnel working with hepatitis A virus. The vaccination is given in two doses with a minimum interval of 6 months between doses.
A hepatitis A and hepatitis B combination vaccine (Twinrix) is also available. It is given in three doses, at 0, 1, and 6 months.
ANNUAL INFLUENZA VACCINE FOR ALL
In 2010, the ACIP recommended a policy of universal annual vaccination for everyone age 6 months and older. Some patients are at especially high risk themselves or are at high risk of exposing others and so are given higher priority during vaccine shortages—ie, patients who are immunosuppressed or have other medical risk factors, health care workers, household members of at-risk patients, and pregnant women after 13 weeks of gestation.
There are few contraindications, so almost everyone should be encouraged to receive the influenza vaccine. The flu shot does not cause the flu, but it may cause soreness at the injection site. Those with severe egg allergy should not receive the standard flu shot; a recombinant vaccine that does not use egg culture is available.
The standard flu shot is an inactivated influenza vaccine. In the past, most formulations were trivalent, but quadrivalent formulations are becoming more common. Special high-dose formulations are believed to elicit a better immune response and can be recommended for people over age 65. Intradermal and intramuscular formulations are available.
An intranasal live-attenuated influenza vaccine is also available and may be used for people ages 2 through 49. It should not be given to immunosuppressed people or to pregnant women.
Our patient should get a flu shot.
PNEUMOCOCCAL VACCINE FOR THOSE AGE 65 AND OLDER OR AT RISK
Two formulations are now available for pneumococcal immunization. The standard is a 23-valent polysaccharide vaccine (PPSV23; Pneumovax) indicated for people age 65 and older.
Patients under age 65 can receive PPSV23 if they have chronic lung disease, chronic cardiovascular disease, diabetes, chronic liver disease, or alcoholism or are a resident of a nursing home or an active smoker.
Our patient is a candidate for PPSV23 since he smokes and has diabetes.
The other formulation is a conjugate 13-valent vaccine (PCV13; Prevnar 13). Patients over age 19 at high risk should be given PCV13 plus the PPSV23 8 weeks later. Those who already received PPSV23 should be given PCV13 vaccine more than 1 year later. Candidates for PCV13 are those with immunocompromising conditions (including chronic renal failure and nephrotic syndrome), functional or anatomic asplenia, cerebrospinal fluid leaks, or cochlear implants.
The current revaccination schedule for PPSV23 is as follows:
- One-time revaccination 5 years after the first dose in patients with chronic renal failure, nephrotic syndrome, asplenia, or an immunosuppressive condition
- One-time revaccination for patients age 65 or older if they were younger than 65 when first immunized (with one or two doses of PPSV23) and 5 years have passed
- No revaccination is needed for people vaccinated with PPSV23 after age 65.
HUMAN PAPILLOMAVIRUS VACCINE
Human papillomavirus is the most common sexually transmitted infection in the United States and is strongly associated with cervical cancer. Immunization is now indicated for both sexes, generally between the ages of 9 and 26. Two vaccines are available: the quadrivalent formulation (Gardasil) for males or females and the bivalent formulation (Cervarix) for females only.
Immunization should be given in three doses: at 0, 1 to 2 months, and 6 months. It can be given to patients who are immunocompromised as a result of infection (including HIV infection), disease, or medications, or who have a history of genital warts, an abnormal Papanicolaou test, or a positive human papillomavirus DNA test.
It is hoped that immunization will lead to a significant decrease in cervical cancer rates. Eradication is unlikely because other papillomavirus strains also can lead to cancer, so cancer screening is still warranted. For men who have sex with men, it is hoped that immunization will prevent condyloma and anal cancer.
CHICKENPOX AND SHINGLES VACCINES
Varicella vaccine (Varivax) contains a live-attenuated virus to protect against chickenpox. It is recommended for all adults who have no evidence of immunity. Immunity is assumed with a history of chickenpox, being born before 1980, or having positive titers. Vaccination should be emphasized for those who come in contact with patients at high risk of severe disease (eg, health care workers, family contacts of immunocompromised patients) and in individuals with a high risk of personal exposure (eg, teachers, day care workers).
The vaccine is given in two doses, 4 to 8 weeks apart. Women who are pregnant or who may get pregnant within 4 weeks should not be vaccinated.
The shingles vaccine (Zostavax) is a larger dose of the varicella vaccine and reduces the incidence of shingles by 50% and postherpetic neuralgia by 66%.4 It was approved by the US Food and Drug Administration in May 2006 for people starting at age 50, but was recommended by ACIP in October 2006 for people age 60 and older; as a result, some insurance companies deny coverage for patients ages 50 through 59.
The shingles vaccine can be given to patients who have already had shingles. Pregnancy and severe immunodeficiency are contraindications.
Our patient, 58 years old, could be considered for shingles vaccine if covered by his insurance company or if he wishes to pay for it.
MENINGOCOCCUS VACCINE
Meningococcal immunization is recommended for people at high risk: college students who plan to live in dormitories, adults without a spleen or with complement deficiencies or HIV infection, or travelers to the “meningitis belt” of sub-Saharan Africa.
Two types of meningococcal vaccine are available: the conjugate quadrivalent vaccine (MCV4) for people age 55 and younger, and the polysaccharide quadrivalent vaccine (MPSV4) for people over age 56.
- US Centers for Disease Control and Prevention (CDC). Adult immunization schedules. www.cdc.gov/vaccines/schedules/hcp/adult.html. Accessed August 21, 2014.
- US Centers for Disease Control and Prevention (CDC). Pertussis epidemic—Washington, 2012. MMWR Morb Mortal Wkly Rep 2012; 61:517–522.
- US Centers for Disease Control and Prevention (CDC). Measles cases and outbreaks, January 1 to August 15, 2014. www.cdc.gov/measles/cases-outbreaks.html. Accessed August 21, 2014.
- Oxman MN, Levin MJ, Johnson MS, et al; for the Shingles Prevention Study Group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352:2271–2284.
A 58-year-old man with a history of irritable bowel syndrome and diabetes presents for an evaluation in early November. He is taking metformin and insulin glargine 10 units. He smokes 1 pack per day. He believes that his childhood immunizations were completed, but he has no records. He thinks his last “shot” was 15 years ago when he cut his hand on some wood.
Which immunizations, if any, would be most appropriate for this patient?
The explosion of new vaccines, new formulations, and new combinations made available in recent years makes managing immunizations a challenge. This article reviews common immunizations and current recommendations for their appropriate use.
Immunization recommendations (Table 1) are made predominantly by the Advisory Committee on Immunization Practices (ACIP) of the US Centers for Disease Control and Prevention (CDC). The last 15 years have seen the arrival of new vaccines (eg, varicella, hepatitis A, pneumococcal, and human papillomavirus), new formulations (eg, intranasal influenza), and new combinations.
To keep clinicians abreast of new indications, the ACIP issues immunization schedules annually for children and adults, available online and downloadable for easy reference.1 For adults, the ACIP provides schedules based on age and medical condition. The schedule for medical conditions offers specific information regarding immunization and pregnancy, human immunodeficiency virus (HIV) infection, kidney failure, heart disease, asplenia, and other conditions. The ACIP also provides guidance on contraindications; for example, pregnant and immunocompromised patients should not receive the live-attenuated vaccines, ie, zoster, varicella, and combined measles, mumps, and rubella [MMR]).
Adult awareness of vaccines is low, as are vaccination rates: in people older than 60, the vaccination rate is about 70% for influenza, 60% for pneumococcus, 50% for tetanus, and 15% for zoster. The lack of vaccine awareness and the availability of new vaccines and indications have made it difficult to manage immunizations in the primary care setting. The electronic medical record is useful for tracking patient vaccine needs. Ideally, keeping up with immunizations should be a routine part of visits provided by a physician’s care team and does not always require direct physician coordination.
TETANUS, DIPHTHERIA, PERTUSSIS EVERY 10 YEARS
Tetanus (also called “lockjaw”) is a nervous system disorder characterized by muscle spasms. Caused by infection with Clostridium tetani, it is a rare disease in the United States thanks to widespread immunization, and it causes fewer than 50 cases annually. Worldwide, the incidence is about 1 million cases a year with 200,000 to 300,000 deaths.
Diphtheria (formerly sometimes called “throat distemper”) is caused by the gram-positive bacillus Corynebacterium diphtheriae and can occur as a respiratory illness or as a milder cutaneous form. The last outbreak in the United States was in Seattle in the 1970s, with the last reported case in 2003. The ACIP recommends booster shots for tetanus and diphtheria every 10 years following completion of the primary series.
Pertussis or whooping cough, caused by Bordetella pertussis infection, is a highly contagious disease increasingly seen in adults in the United States. It causes few deaths but high morbidity, with coughing that can persist up to 3 months. Coughing can be severe enough to cause vomiting, a characteristic sign.
In July 2012, the CDC reported that the United States was at a 50-year high for pertussis, with 18,000 cases reported and 8 deaths.2 In Washington State alone, more than 2,520 cases had been seen through June 16 of that year, a 1,300% increase over the previous year. Rates were high in older children and adolescents despite previous vaccination, suggesting an early waning of immunity.
The ACIP recommends a single dose of the combination of high-dose tetanus and low-dose diphtheria and pertussis vaccines (Tdap) for all adults regardless of age and for all pregnant women with each pregnancy between 27 and 36 weeks of gestation. A dose of Tdap counts as the tetanus-diphtheria booster shot that is recommended every 10 years.
The patient described above is due for his tetanus-diphtheria booster and so should be given Tdap.
MEASLES, MUMPS, RUBELLA FOR THOSE BORN AFTER 1957
Measles remains a problem in the developing world, with an estimated average of 330 deaths daily. The number of cases fell 99% in the United States following the vaccination program that started in the early 1960s. Before the measles vaccine was available, an estimated 90% of children acquired measles by age 15.
The clinical syndrome consists of fever, conjunctivitis, cough, rash, and the characteristic Koplik spots—small white spots occurring on the inside of cheeks early in the disease course.
During the first 5 months of 2014, the CDC reported 334 cases of measles in the United States in 18 states, with most people affected being unvaccinated.3 In comparison, from 2001 to 2008, the number of cases averaged 56 annually.
Many of the recent cases were associated with infections brought from the Philippines. The increased number of measles cases underscores the need for vaccination to prevent measles and its complications.
Mumps is an acute, self-limited viral syndrome, and parotitis is the hallmark. Vaccination led to a 99% decline in cases in the United States. Although complications are rare, they can be serious and include orchitis (with risk of sterility), meningoencephalitis, and deafness.
Mumps outbreaks still occur, especially in close-contact settings such as schools, colleges, and camps. During the first half of 2014, central Ohio had more than 400 cases linked to The Ohio State University.
Rubella, also known as German measles, is a generally mild infection but is associated with congenital rubella syndrome. If a woman is infected with rubella in the first trimester of pregnancy, the risk of miscarriage is greater than 80%, as is the risk of birth defects, including hearing loss, developmental delay, growth retardation, and cardiac and eye defects.
Recommendations for MMR vaccination. People born before 1957 are considered immune to measles and usually to mumps. Health care workers should document immunity before assuming no vaccination is needed.
People born in 1957 or after should have one dose of MMR vaccine unless immunity is documented or unless there is a contraindication such as immunosuppression. A second dose is recommended for those born in or after 1957 who are considered to be at high risk: eg, health care workers, students entering college, and international travelers. The second dose should be given 4 weeks after the first.
Women of childbearing age should be screened for immunity to rubella. Susceptible women should receive MMR, although not during pregnancy and not if they may get pregnant within 4 weeks.
The patient described above was born before 1957, and so he is probably immune to measles and mumps.
HEPATITIS B FOR THOSE AT RISK
Hepatitis B vaccination is recommended for all adolescents and adults at increased risk: eg, men who have sex with men, intravenous drug users, people with multiple sexual partners, health care workers, patients with end-stage renal disease on hemodialysis, patients with chronic liver disease, and those with diabetes (age < 60).
Immunization consists of a series of three shots (at 0, 1–2, and 4–6 months). Booster doses are not recommended. Postvaccination testing for immunity is available and is recommended for health care workers, patients on hemodialysis, patients with HIV infection or who are otherwise immunocompromised, and sexual partners of people who are positive for hepatitis B surface antigen. Nonresponders should be revaccinated with the entire three-shot schedule. Hepatitis B vaccination is safe in pregnancy.
The patient described above has diabetes and so is a candidate for vaccination.
HEPATITIS A: A SLIGHTLY DIFFERENT RISK GROUP
Hepatitis A vaccination is recommended only for at-risk populations: international travelers; intravenous drug users; men who have sex with men; patients with clotting disorders, chronic liver disease, or hepatitis C infection; international adoptees; and laboratory personnel working with hepatitis A virus. The vaccination is given in two doses with a minimum interval of 6 months between doses.
A hepatitis A and hepatitis B combination vaccine (Twinrix) is also available. It is given in three doses, at 0, 1, and 6 months.
ANNUAL INFLUENZA VACCINE FOR ALL
In 2010, the ACIP recommended a policy of universal annual vaccination for everyone age 6 months and older. Some patients are at especially high risk themselves or are at high risk of exposing others and so are given higher priority during vaccine shortages—ie, patients who are immunosuppressed or have other medical risk factors, health care workers, household members of at-risk patients, and pregnant women after 13 weeks of gestation.
There are few contraindications, so almost everyone should be encouraged to receive the influenza vaccine. The flu shot does not cause the flu, but it may cause soreness at the injection site. Those with severe egg allergy should not receive the standard flu shot; a recombinant vaccine that does not use egg culture is available.
The standard flu shot is an inactivated influenza vaccine. In the past, most formulations were trivalent, but quadrivalent formulations are becoming more common. Special high-dose formulations are believed to elicit a better immune response and can be recommended for people over age 65. Intradermal and intramuscular formulations are available.
An intranasal live-attenuated influenza vaccine is also available and may be used for people ages 2 through 49. It should not be given to immunosuppressed people or to pregnant women.
Our patient should get a flu shot.
PNEUMOCOCCAL VACCINE FOR THOSE AGE 65 AND OLDER OR AT RISK
Two formulations are now available for pneumococcal immunization. The standard is a 23-valent polysaccharide vaccine (PPSV23; Pneumovax) indicated for people age 65 and older.
Patients under age 65 can receive PPSV23 if they have chronic lung disease, chronic cardiovascular disease, diabetes, chronic liver disease, or alcoholism or are a resident of a nursing home or an active smoker.
Our patient is a candidate for PPSV23 since he smokes and has diabetes.
The other formulation is a conjugate 13-valent vaccine (PCV13; Prevnar 13). Patients over age 19 at high risk should be given PCV13 plus the PPSV23 8 weeks later. Those who already received PPSV23 should be given PCV13 vaccine more than 1 year later. Candidates for PCV13 are those with immunocompromising conditions (including chronic renal failure and nephrotic syndrome), functional or anatomic asplenia, cerebrospinal fluid leaks, or cochlear implants.
The current revaccination schedule for PPSV23 is as follows:
- One-time revaccination 5 years after the first dose in patients with chronic renal failure, nephrotic syndrome, asplenia, or an immunosuppressive condition
- One-time revaccination for patients age 65 or older if they were younger than 65 when first immunized (with one or two doses of PPSV23) and 5 years have passed
- No revaccination is needed for people vaccinated with PPSV23 after age 65.
HUMAN PAPILLOMAVIRUS VACCINE
Human papillomavirus is the most common sexually transmitted infection in the United States and is strongly associated with cervical cancer. Immunization is now indicated for both sexes, generally between the ages of 9 and 26. Two vaccines are available: the quadrivalent formulation (Gardasil) for males or females and the bivalent formulation (Cervarix) for females only.
Immunization should be given in three doses: at 0, 1 to 2 months, and 6 months. It can be given to patients who are immunocompromised as a result of infection (including HIV infection), disease, or medications, or who have a history of genital warts, an abnormal Papanicolaou test, or a positive human papillomavirus DNA test.
It is hoped that immunization will lead to a significant decrease in cervical cancer rates. Eradication is unlikely because other papillomavirus strains also can lead to cancer, so cancer screening is still warranted. For men who have sex with men, it is hoped that immunization will prevent condyloma and anal cancer.
CHICKENPOX AND SHINGLES VACCINES
Varicella vaccine (Varivax) contains a live-attenuated virus to protect against chickenpox. It is recommended for all adults who have no evidence of immunity. Immunity is assumed with a history of chickenpox, being born before 1980, or having positive titers. Vaccination should be emphasized for those who come in contact with patients at high risk of severe disease (eg, health care workers, family contacts of immunocompromised patients) and in individuals with a high risk of personal exposure (eg, teachers, day care workers).
The vaccine is given in two doses, 4 to 8 weeks apart. Women who are pregnant or who may get pregnant within 4 weeks should not be vaccinated.
The shingles vaccine (Zostavax) is a larger dose of the varicella vaccine and reduces the incidence of shingles by 50% and postherpetic neuralgia by 66%.4 It was approved by the US Food and Drug Administration in May 2006 for people starting at age 50, but was recommended by ACIP in October 2006 for people age 60 and older; as a result, some insurance companies deny coverage for patients ages 50 through 59.
The shingles vaccine can be given to patients who have already had shingles. Pregnancy and severe immunodeficiency are contraindications.
Our patient, 58 years old, could be considered for shingles vaccine if covered by his insurance company or if he wishes to pay for it.
MENINGOCOCCUS VACCINE
Meningococcal immunization is recommended for people at high risk: college students who plan to live in dormitories, adults without a spleen or with complement deficiencies or HIV infection, or travelers to the “meningitis belt” of sub-Saharan Africa.
Two types of meningococcal vaccine are available: the conjugate quadrivalent vaccine (MCV4) for people age 55 and younger, and the polysaccharide quadrivalent vaccine (MPSV4) for people over age 56.
A 58-year-old man with a history of irritable bowel syndrome and diabetes presents for an evaluation in early November. He is taking metformin and insulin glargine 10 units. He smokes 1 pack per day. He believes that his childhood immunizations were completed, but he has no records. He thinks his last “shot” was 15 years ago when he cut his hand on some wood.
Which immunizations, if any, would be most appropriate for this patient?
The explosion of new vaccines, new formulations, and new combinations made available in recent years makes managing immunizations a challenge. This article reviews common immunizations and current recommendations for their appropriate use.
Immunization recommendations (Table 1) are made predominantly by the Advisory Committee on Immunization Practices (ACIP) of the US Centers for Disease Control and Prevention (CDC). The last 15 years have seen the arrival of new vaccines (eg, varicella, hepatitis A, pneumococcal, and human papillomavirus), new formulations (eg, intranasal influenza), and new combinations.
To keep clinicians abreast of new indications, the ACIP issues immunization schedules annually for children and adults, available online and downloadable for easy reference.1 For adults, the ACIP provides schedules based on age and medical condition. The schedule for medical conditions offers specific information regarding immunization and pregnancy, human immunodeficiency virus (HIV) infection, kidney failure, heart disease, asplenia, and other conditions. The ACIP also provides guidance on contraindications; for example, pregnant and immunocompromised patients should not receive the live-attenuated vaccines, ie, zoster, varicella, and combined measles, mumps, and rubella [MMR]).
Adult awareness of vaccines is low, as are vaccination rates: in people older than 60, the vaccination rate is about 70% for influenza, 60% for pneumococcus, 50% for tetanus, and 15% for zoster. The lack of vaccine awareness and the availability of new vaccines and indications have made it difficult to manage immunizations in the primary care setting. The electronic medical record is useful for tracking patient vaccine needs. Ideally, keeping up with immunizations should be a routine part of visits provided by a physician’s care team and does not always require direct physician coordination.
TETANUS, DIPHTHERIA, PERTUSSIS EVERY 10 YEARS
Tetanus (also called “lockjaw”) is a nervous system disorder characterized by muscle spasms. Caused by infection with Clostridium tetani, it is a rare disease in the United States thanks to widespread immunization, and it causes fewer than 50 cases annually. Worldwide, the incidence is about 1 million cases a year with 200,000 to 300,000 deaths.
Diphtheria (formerly sometimes called “throat distemper”) is caused by the gram-positive bacillus Corynebacterium diphtheriae and can occur as a respiratory illness or as a milder cutaneous form. The last outbreak in the United States was in Seattle in the 1970s, with the last reported case in 2003. The ACIP recommends booster shots for tetanus and diphtheria every 10 years following completion of the primary series.
Pertussis or whooping cough, caused by Bordetella pertussis infection, is a highly contagious disease increasingly seen in adults in the United States. It causes few deaths but high morbidity, with coughing that can persist up to 3 months. Coughing can be severe enough to cause vomiting, a characteristic sign.
In July 2012, the CDC reported that the United States was at a 50-year high for pertussis, with 18,000 cases reported and 8 deaths.2 In Washington State alone, more than 2,520 cases had been seen through June 16 of that year, a 1,300% increase over the previous year. Rates were high in older children and adolescents despite previous vaccination, suggesting an early waning of immunity.
The ACIP recommends a single dose of the combination of high-dose tetanus and low-dose diphtheria and pertussis vaccines (Tdap) for all adults regardless of age and for all pregnant women with each pregnancy between 27 and 36 weeks of gestation. A dose of Tdap counts as the tetanus-diphtheria booster shot that is recommended every 10 years.
The patient described above is due for his tetanus-diphtheria booster and so should be given Tdap.
MEASLES, MUMPS, RUBELLA FOR THOSE BORN AFTER 1957
Measles remains a problem in the developing world, with an estimated average of 330 deaths daily. The number of cases fell 99% in the United States following the vaccination program that started in the early 1960s. Before the measles vaccine was available, an estimated 90% of children acquired measles by age 15.
The clinical syndrome consists of fever, conjunctivitis, cough, rash, and the characteristic Koplik spots—small white spots occurring on the inside of cheeks early in the disease course.
During the first 5 months of 2014, the CDC reported 334 cases of measles in the United States in 18 states, with most people affected being unvaccinated.3 In comparison, from 2001 to 2008, the number of cases averaged 56 annually.
Many of the recent cases were associated with infections brought from the Philippines. The increased number of measles cases underscores the need for vaccination to prevent measles and its complications.
Mumps is an acute, self-limited viral syndrome, and parotitis is the hallmark. Vaccination led to a 99% decline in cases in the United States. Although complications are rare, they can be serious and include orchitis (with risk of sterility), meningoencephalitis, and deafness.
Mumps outbreaks still occur, especially in close-contact settings such as schools, colleges, and camps. During the first half of 2014, central Ohio had more than 400 cases linked to The Ohio State University.
Rubella, also known as German measles, is a generally mild infection but is associated with congenital rubella syndrome. If a woman is infected with rubella in the first trimester of pregnancy, the risk of miscarriage is greater than 80%, as is the risk of birth defects, including hearing loss, developmental delay, growth retardation, and cardiac and eye defects.
Recommendations for MMR vaccination. People born before 1957 are considered immune to measles and usually to mumps. Health care workers should document immunity before assuming no vaccination is needed.
People born in 1957 or after should have one dose of MMR vaccine unless immunity is documented or unless there is a contraindication such as immunosuppression. A second dose is recommended for those born in or after 1957 who are considered to be at high risk: eg, health care workers, students entering college, and international travelers. The second dose should be given 4 weeks after the first.
Women of childbearing age should be screened for immunity to rubella. Susceptible women should receive MMR, although not during pregnancy and not if they may get pregnant within 4 weeks.
The patient described above was born before 1957, and so he is probably immune to measles and mumps.
HEPATITIS B FOR THOSE AT RISK
Hepatitis B vaccination is recommended for all adolescents and adults at increased risk: eg, men who have sex with men, intravenous drug users, people with multiple sexual partners, health care workers, patients with end-stage renal disease on hemodialysis, patients with chronic liver disease, and those with diabetes (age < 60).
Immunization consists of a series of three shots (at 0, 1–2, and 4–6 months). Booster doses are not recommended. Postvaccination testing for immunity is available and is recommended for health care workers, patients on hemodialysis, patients with HIV infection or who are otherwise immunocompromised, and sexual partners of people who are positive for hepatitis B surface antigen. Nonresponders should be revaccinated with the entire three-shot schedule. Hepatitis B vaccination is safe in pregnancy.
The patient described above has diabetes and so is a candidate for vaccination.
HEPATITIS A: A SLIGHTLY DIFFERENT RISK GROUP
Hepatitis A vaccination is recommended only for at-risk populations: international travelers; intravenous drug users; men who have sex with men; patients with clotting disorders, chronic liver disease, or hepatitis C infection; international adoptees; and laboratory personnel working with hepatitis A virus. The vaccination is given in two doses with a minimum interval of 6 months between doses.
A hepatitis A and hepatitis B combination vaccine (Twinrix) is also available. It is given in three doses, at 0, 1, and 6 months.
ANNUAL INFLUENZA VACCINE FOR ALL
In 2010, the ACIP recommended a policy of universal annual vaccination for everyone age 6 months and older. Some patients are at especially high risk themselves or are at high risk of exposing others and so are given higher priority during vaccine shortages—ie, patients who are immunosuppressed or have other medical risk factors, health care workers, household members of at-risk patients, and pregnant women after 13 weeks of gestation.
There are few contraindications, so almost everyone should be encouraged to receive the influenza vaccine. The flu shot does not cause the flu, but it may cause soreness at the injection site. Those with severe egg allergy should not receive the standard flu shot; a recombinant vaccine that does not use egg culture is available.
The standard flu shot is an inactivated influenza vaccine. In the past, most formulations were trivalent, but quadrivalent formulations are becoming more common. Special high-dose formulations are believed to elicit a better immune response and can be recommended for people over age 65. Intradermal and intramuscular formulations are available.
An intranasal live-attenuated influenza vaccine is also available and may be used for people ages 2 through 49. It should not be given to immunosuppressed people or to pregnant women.
Our patient should get a flu shot.
PNEUMOCOCCAL VACCINE FOR THOSE AGE 65 AND OLDER OR AT RISK
Two formulations are now available for pneumococcal immunization. The standard is a 23-valent polysaccharide vaccine (PPSV23; Pneumovax) indicated for people age 65 and older.
Patients under age 65 can receive PPSV23 if they have chronic lung disease, chronic cardiovascular disease, diabetes, chronic liver disease, or alcoholism or are a resident of a nursing home or an active smoker.
Our patient is a candidate for PPSV23 since he smokes and has diabetes.
The other formulation is a conjugate 13-valent vaccine (PCV13; Prevnar 13). Patients over age 19 at high risk should be given PCV13 plus the PPSV23 8 weeks later. Those who already received PPSV23 should be given PCV13 vaccine more than 1 year later. Candidates for PCV13 are those with immunocompromising conditions (including chronic renal failure and nephrotic syndrome), functional or anatomic asplenia, cerebrospinal fluid leaks, or cochlear implants.
The current revaccination schedule for PPSV23 is as follows:
- One-time revaccination 5 years after the first dose in patients with chronic renal failure, nephrotic syndrome, asplenia, or an immunosuppressive condition
- One-time revaccination for patients age 65 or older if they were younger than 65 when first immunized (with one or two doses of PPSV23) and 5 years have passed
- No revaccination is needed for people vaccinated with PPSV23 after age 65.
HUMAN PAPILLOMAVIRUS VACCINE
Human papillomavirus is the most common sexually transmitted infection in the United States and is strongly associated with cervical cancer. Immunization is now indicated for both sexes, generally between the ages of 9 and 26. Two vaccines are available: the quadrivalent formulation (Gardasil) for males or females and the bivalent formulation (Cervarix) for females only.
Immunization should be given in three doses: at 0, 1 to 2 months, and 6 months. It can be given to patients who are immunocompromised as a result of infection (including HIV infection), disease, or medications, or who have a history of genital warts, an abnormal Papanicolaou test, or a positive human papillomavirus DNA test.
It is hoped that immunization will lead to a significant decrease in cervical cancer rates. Eradication is unlikely because other papillomavirus strains also can lead to cancer, so cancer screening is still warranted. For men who have sex with men, it is hoped that immunization will prevent condyloma and anal cancer.
CHICKENPOX AND SHINGLES VACCINES
Varicella vaccine (Varivax) contains a live-attenuated virus to protect against chickenpox. It is recommended for all adults who have no evidence of immunity. Immunity is assumed with a history of chickenpox, being born before 1980, or having positive titers. Vaccination should be emphasized for those who come in contact with patients at high risk of severe disease (eg, health care workers, family contacts of immunocompromised patients) and in individuals with a high risk of personal exposure (eg, teachers, day care workers).
The vaccine is given in two doses, 4 to 8 weeks apart. Women who are pregnant or who may get pregnant within 4 weeks should not be vaccinated.
The shingles vaccine (Zostavax) is a larger dose of the varicella vaccine and reduces the incidence of shingles by 50% and postherpetic neuralgia by 66%.4 It was approved by the US Food and Drug Administration in May 2006 for people starting at age 50, but was recommended by ACIP in October 2006 for people age 60 and older; as a result, some insurance companies deny coverage for patients ages 50 through 59.
The shingles vaccine can be given to patients who have already had shingles. Pregnancy and severe immunodeficiency are contraindications.
Our patient, 58 years old, could be considered for shingles vaccine if covered by his insurance company or if he wishes to pay for it.
MENINGOCOCCUS VACCINE
Meningococcal immunization is recommended for people at high risk: college students who plan to live in dormitories, adults without a spleen or with complement deficiencies or HIV infection, or travelers to the “meningitis belt” of sub-Saharan Africa.
Two types of meningococcal vaccine are available: the conjugate quadrivalent vaccine (MCV4) for people age 55 and younger, and the polysaccharide quadrivalent vaccine (MPSV4) for people over age 56.
- US Centers for Disease Control and Prevention (CDC). Adult immunization schedules. www.cdc.gov/vaccines/schedules/hcp/adult.html. Accessed August 21, 2014.
- US Centers for Disease Control and Prevention (CDC). Pertussis epidemic—Washington, 2012. MMWR Morb Mortal Wkly Rep 2012; 61:517–522.
- US Centers for Disease Control and Prevention (CDC). Measles cases and outbreaks, January 1 to August 15, 2014. www.cdc.gov/measles/cases-outbreaks.html. Accessed August 21, 2014.
- Oxman MN, Levin MJ, Johnson MS, et al; for the Shingles Prevention Study Group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352:2271–2284.
- US Centers for Disease Control and Prevention (CDC). Adult immunization schedules. www.cdc.gov/vaccines/schedules/hcp/adult.html. Accessed August 21, 2014.
- US Centers for Disease Control and Prevention (CDC). Pertussis epidemic—Washington, 2012. MMWR Morb Mortal Wkly Rep 2012; 61:517–522.
- US Centers for Disease Control and Prevention (CDC). Measles cases and outbreaks, January 1 to August 15, 2014. www.cdc.gov/measles/cases-outbreaks.html. Accessed August 21, 2014.
- Oxman MN, Levin MJ, Johnson MS, et al; for the Shingles Prevention Study Group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352:2271–2284.
KEY POINTS
- Information on immunization schedules, including an app for mobile devices, is available at www.cdc.gov/vaccines/schedules/hcp/adult.html.
- Vaccination rates in adults are low, and appropriate vaccinations should be encouraged. The electronic medical record can help remind us when vaccinations are due.
- The live-attenuated vaccines, ie, zoster, varicella, and combined measles, mumps, and rubella, are contraindicated during pregnancy and in immunocompromised patients.
Terry nails in a patient with chronic alcoholic liver disease
A 45-year-old man with chronic alcoholism for the past 20 years and chronic liver disease for the past 2 years was admitted to the hospital with abdominal distention, yellowish discoloration of the eyes, itching all over the body, decreased appetite, and fresh rectal bleeding.
He had the classic signs of chronic liver disease, including icterus, pallor, parotid swelling, gynecomastia, spider angiomata, sparse axillary and pubic hair, transverse stretched umbilicus, divarication of the rectus abdominis muscles, caput medusae, small testes, and bilateral pedal edema.
Examination of the fingernails revealed a distal thin pink-to-brown transverse band 0.5 to 2.0 mm in width, a white nail bed, and no lunula (Figure 1)—the characteristic findings of Terry nails.
Systemic examination revealed moderate ascites (shifting dullness present), splenomegaly, and external hemorrhoids suggestive of portal hypertension.
TERRY NAILS
In 1954, Dr. Richard Terry first reported the finding of a white nail bed with ground-glass opacity in patients who had hepatic cirrhosis.1 The condition is bilaterally symmetrical, with a tendency to be more marked in the thumb and forefinger.1
In 1984, Holzberg and Walker2 consecutively studied 512 hospitalized patients and observed Terry nails in 25.2% of them. Based on their findings, they redefined the criteria for Terry nail as follows:
- Distal thin pink-to-brown transverse band, 0.5 to 3.0 mm in width
- Decreased venous return not obscuring the distal band
- White or light pink proximal nail
- Lunula possibly absent
- At least 4 of 10 nails with the above criteria.
Patients who do not have the findings on all fingernails commonly have involvement of the thumb and forefinger. Holzberg and Walker confirmed a statistically significant association of Terry nails with cirrhosis, chronic heart failure, and adult-onset diabetes, especially in younger patients.2 Terry nails have also been observed in thyrotoxicosis, pulmonary eosinophilia, malnutrition, actinic keratosis, and advanced age.1–4
Using the updated diagnostic criteria, Park et al5 studied fingernails in 444 medical inpatients with chronic systemic disease, and only 30.6% had Terry nails. There were statistically significant associations with cirrhosis (57%), congestive heart failure (51.5%), and diabetes mellitus (49%); the associations with chronic renal failure (19%) and cancer (18%) were not statistically significant.5 They were more common in older patients. The average number of nails affected per patient tended to be higher in frequency close to the thumb; 28.7% patients had all nails affected.5
Terry nails should alert the clinician to the possibility of an underlying systemic disease, especially advanced liver disease. Possible explanations for the clinical changes observed in Terry nails include abnormal steroid metabolism, abnormal estrogen-androgen ratio, alteration of nail bed-to-nail plate attachment, hypoalbuminemia, increased digital blood flow, and overgrowth of the connective tissue between nail bed and the growth plate. The pathologic study of longitudinal nail biopsy specimens shows telangiectasia in the upper dermis of the distal nail band.3
Important differential diagnoses are Lindsay (half-and-half) nails, associated with chronic renal failure, and Neapolitan nails, associated with aging.3
- Terry R. White nails in hepatic cirrhosis. Lancet 1954; 266:757–759.
- Holzberg M, Walker HK. Terry’s nails: revised definition and new correlations. Lancet 1984; 1:896–899.
- Holzberg M. The nail in systemic disease. In:Baran R, de Berker DAR, Holzberg M, Thomas L, editors. Baran & Dawber’s Diseases of the Nails and Their Management, 4th ed. Oxford, UK: Blackwell Publishing Ltd.; 2012.
- Nia AM, Ederer S, Dahlem KM, Gassanov N, Er F. Terry’s nails: a window to systemic diseases. Am J Med 2011; 124:602–604.
- Park KY, Kim SW, Cho JS. Research on the frequency of Terry’s nail in the medical inpatients with chronic illnesses. Korean J Dermatol 1992; 30:864–870.
A 45-year-old man with chronic alcoholism for the past 20 years and chronic liver disease for the past 2 years was admitted to the hospital with abdominal distention, yellowish discoloration of the eyes, itching all over the body, decreased appetite, and fresh rectal bleeding.
He had the classic signs of chronic liver disease, including icterus, pallor, parotid swelling, gynecomastia, spider angiomata, sparse axillary and pubic hair, transverse stretched umbilicus, divarication of the rectus abdominis muscles, caput medusae, small testes, and bilateral pedal edema.
Examination of the fingernails revealed a distal thin pink-to-brown transverse band 0.5 to 2.0 mm in width, a white nail bed, and no lunula (Figure 1)—the characteristic findings of Terry nails.
Systemic examination revealed moderate ascites (shifting dullness present), splenomegaly, and external hemorrhoids suggestive of portal hypertension.
TERRY NAILS
In 1954, Dr. Richard Terry first reported the finding of a white nail bed with ground-glass opacity in patients who had hepatic cirrhosis.1 The condition is bilaterally symmetrical, with a tendency to be more marked in the thumb and forefinger.1
In 1984, Holzberg and Walker2 consecutively studied 512 hospitalized patients and observed Terry nails in 25.2% of them. Based on their findings, they redefined the criteria for Terry nail as follows:
- Distal thin pink-to-brown transverse band, 0.5 to 3.0 mm in width
- Decreased venous return not obscuring the distal band
- White or light pink proximal nail
- Lunula possibly absent
- At least 4 of 10 nails with the above criteria.
Patients who do not have the findings on all fingernails commonly have involvement of the thumb and forefinger. Holzberg and Walker confirmed a statistically significant association of Terry nails with cirrhosis, chronic heart failure, and adult-onset diabetes, especially in younger patients.2 Terry nails have also been observed in thyrotoxicosis, pulmonary eosinophilia, malnutrition, actinic keratosis, and advanced age.1–4
Using the updated diagnostic criteria, Park et al5 studied fingernails in 444 medical inpatients with chronic systemic disease, and only 30.6% had Terry nails. There were statistically significant associations with cirrhosis (57%), congestive heart failure (51.5%), and diabetes mellitus (49%); the associations with chronic renal failure (19%) and cancer (18%) were not statistically significant.5 They were more common in older patients. The average number of nails affected per patient tended to be higher in frequency close to the thumb; 28.7% patients had all nails affected.5
Terry nails should alert the clinician to the possibility of an underlying systemic disease, especially advanced liver disease. Possible explanations for the clinical changes observed in Terry nails include abnormal steroid metabolism, abnormal estrogen-androgen ratio, alteration of nail bed-to-nail plate attachment, hypoalbuminemia, increased digital blood flow, and overgrowth of the connective tissue between nail bed and the growth plate. The pathologic study of longitudinal nail biopsy specimens shows telangiectasia in the upper dermis of the distal nail band.3
Important differential diagnoses are Lindsay (half-and-half) nails, associated with chronic renal failure, and Neapolitan nails, associated with aging.3
A 45-year-old man with chronic alcoholism for the past 20 years and chronic liver disease for the past 2 years was admitted to the hospital with abdominal distention, yellowish discoloration of the eyes, itching all over the body, decreased appetite, and fresh rectal bleeding.
He had the classic signs of chronic liver disease, including icterus, pallor, parotid swelling, gynecomastia, spider angiomata, sparse axillary and pubic hair, transverse stretched umbilicus, divarication of the rectus abdominis muscles, caput medusae, small testes, and bilateral pedal edema.
Examination of the fingernails revealed a distal thin pink-to-brown transverse band 0.5 to 2.0 mm in width, a white nail bed, and no lunula (Figure 1)—the characteristic findings of Terry nails.
Systemic examination revealed moderate ascites (shifting dullness present), splenomegaly, and external hemorrhoids suggestive of portal hypertension.
TERRY NAILS
In 1954, Dr. Richard Terry first reported the finding of a white nail bed with ground-glass opacity in patients who had hepatic cirrhosis.1 The condition is bilaterally symmetrical, with a tendency to be more marked in the thumb and forefinger.1
In 1984, Holzberg and Walker2 consecutively studied 512 hospitalized patients and observed Terry nails in 25.2% of them. Based on their findings, they redefined the criteria for Terry nail as follows:
- Distal thin pink-to-brown transverse band, 0.5 to 3.0 mm in width
- Decreased venous return not obscuring the distal band
- White or light pink proximal nail
- Lunula possibly absent
- At least 4 of 10 nails with the above criteria.
Patients who do not have the findings on all fingernails commonly have involvement of the thumb and forefinger. Holzberg and Walker confirmed a statistically significant association of Terry nails with cirrhosis, chronic heart failure, and adult-onset diabetes, especially in younger patients.2 Terry nails have also been observed in thyrotoxicosis, pulmonary eosinophilia, malnutrition, actinic keratosis, and advanced age.1–4
Using the updated diagnostic criteria, Park et al5 studied fingernails in 444 medical inpatients with chronic systemic disease, and only 30.6% had Terry nails. There were statistically significant associations with cirrhosis (57%), congestive heart failure (51.5%), and diabetes mellitus (49%); the associations with chronic renal failure (19%) and cancer (18%) were not statistically significant.5 They were more common in older patients. The average number of nails affected per patient tended to be higher in frequency close to the thumb; 28.7% patients had all nails affected.5
Terry nails should alert the clinician to the possibility of an underlying systemic disease, especially advanced liver disease. Possible explanations for the clinical changes observed in Terry nails include abnormal steroid metabolism, abnormal estrogen-androgen ratio, alteration of nail bed-to-nail plate attachment, hypoalbuminemia, increased digital blood flow, and overgrowth of the connective tissue between nail bed and the growth plate. The pathologic study of longitudinal nail biopsy specimens shows telangiectasia in the upper dermis of the distal nail band.3
Important differential diagnoses are Lindsay (half-and-half) nails, associated with chronic renal failure, and Neapolitan nails, associated with aging.3
- Terry R. White nails in hepatic cirrhosis. Lancet 1954; 266:757–759.
- Holzberg M, Walker HK. Terry’s nails: revised definition and new correlations. Lancet 1984; 1:896–899.
- Holzberg M. The nail in systemic disease. In:Baran R, de Berker DAR, Holzberg M, Thomas L, editors. Baran & Dawber’s Diseases of the Nails and Their Management, 4th ed. Oxford, UK: Blackwell Publishing Ltd.; 2012.
- Nia AM, Ederer S, Dahlem KM, Gassanov N, Er F. Terry’s nails: a window to systemic diseases. Am J Med 2011; 124:602–604.
- Park KY, Kim SW, Cho JS. Research on the frequency of Terry’s nail in the medical inpatients with chronic illnesses. Korean J Dermatol 1992; 30:864–870.
- Terry R. White nails in hepatic cirrhosis. Lancet 1954; 266:757–759.
- Holzberg M, Walker HK. Terry’s nails: revised definition and new correlations. Lancet 1984; 1:896–899.
- Holzberg M. The nail in systemic disease. In:Baran R, de Berker DAR, Holzberg M, Thomas L, editors. Baran & Dawber’s Diseases of the Nails and Their Management, 4th ed. Oxford, UK: Blackwell Publishing Ltd.; 2012.
- Nia AM, Ederer S, Dahlem KM, Gassanov N, Er F. Terry’s nails: a window to systemic diseases. Am J Med 2011; 124:602–604.
- Park KY, Kim SW, Cho JS. Research on the frequency of Terry’s nail in the medical inpatients with chronic illnesses. Korean J Dermatol 1992; 30:864–870.
Should all patients have a resting 12-lead ECG before elective noncardiac surgery?
A 55-year-old lawyer with hypertension well controlled on lisinopril and amlodipine is scheduled for elective hernia repair under general anesthesia. His surgeon has referred him for a preoperative evaluation. He has never smoked, runs 4 miles on days off from work, and enjoys long hiking trips. On examination, his body mass index is 26 kg/m2 and his blood pressure is 130/78 mm Hg; his cardiac examination and the rest of the clinical examination are unremarkable. He asks if he should have an electrocardiogram (ECG) as a part of his workup.
A preoperative ECG is not routinely recommended in all asymptomatic patients undergoing noncardiac surgery.
Consider obtaining an ECG in patients planning to undergo a high-risk surgical procedure, especially if they have one or more clinical risk factors for coronary artery disease, and in patients undergoing elevated-cardiac-risk surgery who are known to have coronary artery disease, chronic heart failure, peripheral arterial disease, or cerebrovascular disease. However, a preoperative ECG is not routinely recommended for patients perceived to be at low cardiac risk who are planning to undergo low-risk surgery. In those patients it could delay surgery unnecessarily, cause further unnecessary testing, drive up costs, and increase patient anxiety.
Here we discuss the perioperative cardiac risk based on type of surgery and patient characteristics and summarize the current guidelines and recommendations on obtaining a preoperative 12-lead ECG in patients undergoing noncardiac surgery.
RISK DEPENDS ON TYPE OF SURGERY AND PATIENT FACTORS
Physicians, including primary care physicians, hospitalists, cardiologists, and anesthesiologists, are routinely asked to evaluate patients before surgical procedures. The purpose of the preoperative evaluation is to optimize existing medical conditions, to identify undiagnosed conditions that can increase risk of perioperative morbidity and death, and to suggest strategies to mitigate risk.1,2
Cardiac risk is multifactorial, and risk factors for postoperative adverse cardiac events include the type of surgery and patient factors.1,3
Cardiac risk based on type of surgery
Low-risk procedures are those in which the risk of a perioperative major adverse cardiac event is less than 1%.1,4 Examples:
- Ambulatory surgery
- Breast or plastic surgery
- Cataract surgery
- Endoscopic procedures.
Elevated-risk procedures are those in which the risk is 1% or higher. Examples:
- Intraperitoneal surgery
- Intrathoracic surgery
- Carotid endarterectomy
- Head and neck surgery
- Orthopedic surgery
- Prostate surgery
- Aortic surgery
- Major vascular surgery
- Peripheral arterial surgery.
Cardiac risk based on patient factors
The 2014 American College of Cardiology and American Heart Association (ACC/AHA) perioperative guidelines list a number of clinical risk factors for perioperative cardiac morbidity and death.1 These include coronary artery disease, chronic heart failure, clinically suspected moderate or greater degrees of valvular heart disease, arrhythmias, conduction disorders, pulmonary vascular disease, and adult congenital heart disease.
Patients with these conditions and patients with unstable coronary syndromes warrant preoperative ECGs and sometimes even urgent interventions before any nonemergency surgery, provided such interventions would affect decision-making and perioperative care.1
The risk of perioperative cardiac morbidity and death can be calculated using either the Revised Cardiac Risk Index scoring system or the American College of Surgeons National Surgical Quality Improvement Program calculator.157 The former is fairly simple, validated, and accepted, while the latter requires use of online calculators (eg, www.surgicalriskcalculator.com/miorcardiacarrest, www.riskcalculator.facs.org).
The Revised Cardiac Risk Index has six clinical predictors of major perioperative cardiac events:
- History of cerebrovascular disease
- Prior or current compensated congestive heart failure
- History of coronary artery disease
- Insulin-dependent diabetes mellitus
- Renal insufficiency, defined as a serum creatinine level of 2 mg/dL or higher
- Patient undergoing suprainguinal vascular, intraperitoneal, or intrathoracic surgery.
A patient who has 0 or 1 of these predictors would have a low risk of a major adverse cardiac event, whereas a patient with 2 or more would have elevated risk. These risk factors must be taken into consideration to determine the need, if any, for a preoperative ECG.
What an ECG can tell us
Abnormalities such as left ventricular hypertrophy, ST-segment depression, and pathologic Q waves on a preoperative ECG in a patient undergoing an elevated-risk surgical procedure may predict adverse perioperative cardiac events.3,6
In a retrospective study of 23,036 patients, Noordzij et al7 found that in patients undergoing elevated-risk surgery, those with an abnormal preoperative ECG had a higher incidence of cardiovascular death than those with a normal ECG. However, a preoperative ECG was obtained only in patients with established coronary artery disease or risk factors for cardiovascular disease. Hence, although an abnormal ECG in such patients undergoing elevated-risk surgery was predictive of adverse postoperative cardiac outcomes, we cannot say that the same would apply to patients without these characteristics undergoing elevated-risk surgery.
In a prospective observational study of patients with known coronary artery disease undergoing major noncardiac surgery, a preoperative ECG was found to contain prognostic information and was predictive of long-term outcome independent of clinical findings and perioperative ischemia.8
CURRENT GUIDELINES AND RECOMMENDATIONS
Several guidelines address whether to order a preoperative ECG but are mostly based on low-level evidence and expert opinion.1,2,6,9
Current guidelines recommend obtaining a preoperative ECG in patients with known coronary, peripheral arterial, or cerebrovascular disease.1,6,9
Obesity and associated comorbidities such as coronary artery disease, heart failure, systemic hypertension, and sleep apnea can predispose to increased perioperative complications. A preoperative 12-lead ECG is reasonable in morbidly obese patients (body mass index ≥ 40 kg/m2) and in obese patients (body mass index ≥ 30 kg/m2) with at least one risk factor for coronary artery disease or poor exercise tolerance, or both.10
Liu et al11 looked at the predictive value of a preoperative 12-lead ECG in 513 elderly patients (age ≥ 70) undergoing noncardiac surgery and found that electrocardiographic abnormalities were not predictive of adverse cardiac outcomes. In this study, although electrocardiographic abnormalities were common (noted in 75% of the patients), they were nonspecific and less useful in predicting postoperative cardiac complications than was the presence of comorbidities.11 Age alone as a cutoff for obtaining a preoperative ECG is not predictive of postoperative outcomes and a preoperative ECG is not warranted in all elderly patients. This is also reflected in current ACC/AHA guidelines on perioperative cardiovascular evaluation1 and is a change from prior ACC/AHA guidelines when age was used as a criterion for preoperative ECGs.12
Current guidelines do not recommend getting a preoperative ECG in asymptomatic patients undergoing low-cardiac-risk surgery.1,4,9
Although the ideal time for ordering an ECG before a planned surgery is unknown, obtaining one within 90 days before the surgery is considered adequate in stable patients in whom an ECG is indicated.1
BACK TO OUR PATIENT
On the basis of current evidence, our patient does not need a preoperative ECG, as it is unlikely to alter his perioperative management and instead may delay his surgery unnecessarily if any nonspecific changes prompt further cardiac workup.
CLINICAL BOTTOM LINE
Although frequently ordered in clinical practice, preoperative electrocardiography has a limited role in predicting postoperative outcome and should be ordered only in the appropriate clinical setting.1 Moreover, there is little evidence that outcomes are better if we obtain an ECG before surgery. The clinician should consider patient factors and the type of surgery before ordering diagnostic tests, including electrocardiography.
In asymptomatic patients undergoing nonemergent surgery:
- It is reasonable to obtain a preoperative ECG in patients with known coronary artery disease, significant arrhythmia, peripheral arterial disease, cerebrovascular disease, chronic heart failure, or other significant structural heart disease undergoing elevated-cardiac-risk surgery.
- Do not order a preoperative ECG in asymptomatic patients undergoing low-risk surgery.
- Obtaining a preoperative ECG is reasonable in morbidly obese patients and in obese patients with one or more risk factors for coronary artery disease, or poor exercise tolerance, undergoing high-risk surgery.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. J Am Coll Cardiol 2014; Jul 29. 10.1016/j.jacc.2014.07.944. [Epub ahead of print]
- Feely MA, Collins CS, Daniels PR, Kebede EB, Jatoi A, Mauck KF. Preoperative testing before noncardiac surgery: guidelines and recommendations. Am Fam Physician 2013; 87:414–418.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Task Force for Preoperative Cardiac Risk Assessment and Perioperative Cardiac Management in Non-cardiac Surgery; European Society of Cardiology (ESC); Poldermans D, Bax JJ, Boersma E, et al. Guidelines for pre-operative cardiac risk assessment and perioperative cardiac management in non-cardiac surgery. Eur Heart J 2009; 30:2769–2812.
- Bilimoria KY, Liu Y, Paruch JL, Zhou L, Kmiecik TE, Ko CY, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 2013; 217:833–842.
- Landesberg G, Einav S, Christopherson R, et al. Perioperative ischemia and cardiac complications in major vascular surgery: importance of the preoperative twelve-lead electrocardiogram. J Vasc Surg 1997; 26:570–578.
- Noordzij PG, Boersma E, Bax JJ, et al. Prognostic value of routine preoperative electrocardiography in patients undergoing noncardiac surgery. Am J Cardiol 2006; 97:1103–1106.
- Jeger RV, Probst C, Arsenic R, et al. Long-term prognostic value of the preoperative 12-lead electrocardiogram before major noncardiac surgery in coronary artery disease. Am Heart J 2006; 151:508–513.
- Committee on Standards and Practice Parameters; Apfelbaum JL, Connis RT, Nickinovich DG, Pasternak LR, Arens JF, Caplan RA, et al. Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology 2012; 116:522–538.
- Poirier P, Alpert MA, Fleisher LA, et al. Cardiovascular evaluation and management of severely obese patients undergoing surgery: a science advisory from the American Heart Association. Circulation 2009; 120:86–95.
- Liu LL, Dzankic S, Leung JM. Preoperative electrocardiogram abnormalities do not predict postoperative cardiac complications in geriatric surgical patients. J Am Geriatr Soc 2002; 50:1186–1191.
- Eagle KA, Berger PB, Calkins H, et al; American College of Cardiology; American Heart Association. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary. J Am Coll Cardiol 2002; 39:542–553.
A 55-year-old lawyer with hypertension well controlled on lisinopril and amlodipine is scheduled for elective hernia repair under general anesthesia. His surgeon has referred him for a preoperative evaluation. He has never smoked, runs 4 miles on days off from work, and enjoys long hiking trips. On examination, his body mass index is 26 kg/m2 and his blood pressure is 130/78 mm Hg; his cardiac examination and the rest of the clinical examination are unremarkable. He asks if he should have an electrocardiogram (ECG) as a part of his workup.
A preoperative ECG is not routinely recommended in all asymptomatic patients undergoing noncardiac surgery.
Consider obtaining an ECG in patients planning to undergo a high-risk surgical procedure, especially if they have one or more clinical risk factors for coronary artery disease, and in patients undergoing elevated-cardiac-risk surgery who are known to have coronary artery disease, chronic heart failure, peripheral arterial disease, or cerebrovascular disease. However, a preoperative ECG is not routinely recommended for patients perceived to be at low cardiac risk who are planning to undergo low-risk surgery. In those patients it could delay surgery unnecessarily, cause further unnecessary testing, drive up costs, and increase patient anxiety.
Here we discuss the perioperative cardiac risk based on type of surgery and patient characteristics and summarize the current guidelines and recommendations on obtaining a preoperative 12-lead ECG in patients undergoing noncardiac surgery.
RISK DEPENDS ON TYPE OF SURGERY AND PATIENT FACTORS
Physicians, including primary care physicians, hospitalists, cardiologists, and anesthesiologists, are routinely asked to evaluate patients before surgical procedures. The purpose of the preoperative evaluation is to optimize existing medical conditions, to identify undiagnosed conditions that can increase risk of perioperative morbidity and death, and to suggest strategies to mitigate risk.1,2
Cardiac risk is multifactorial, and risk factors for postoperative adverse cardiac events include the type of surgery and patient factors.1,3
Cardiac risk based on type of surgery
Low-risk procedures are those in which the risk of a perioperative major adverse cardiac event is less than 1%.1,4 Examples:
- Ambulatory surgery
- Breast or plastic surgery
- Cataract surgery
- Endoscopic procedures.
Elevated-risk procedures are those in which the risk is 1% or higher. Examples:
- Intraperitoneal surgery
- Intrathoracic surgery
- Carotid endarterectomy
- Head and neck surgery
- Orthopedic surgery
- Prostate surgery
- Aortic surgery
- Major vascular surgery
- Peripheral arterial surgery.
Cardiac risk based on patient factors
The 2014 American College of Cardiology and American Heart Association (ACC/AHA) perioperative guidelines list a number of clinical risk factors for perioperative cardiac morbidity and death.1 These include coronary artery disease, chronic heart failure, clinically suspected moderate or greater degrees of valvular heart disease, arrhythmias, conduction disorders, pulmonary vascular disease, and adult congenital heart disease.
Patients with these conditions and patients with unstable coronary syndromes warrant preoperative ECGs and sometimes even urgent interventions before any nonemergency surgery, provided such interventions would affect decision-making and perioperative care.1
The risk of perioperative cardiac morbidity and death can be calculated using either the Revised Cardiac Risk Index scoring system or the American College of Surgeons National Surgical Quality Improvement Program calculator.157 The former is fairly simple, validated, and accepted, while the latter requires use of online calculators (eg, www.surgicalriskcalculator.com/miorcardiacarrest, www.riskcalculator.facs.org).
The Revised Cardiac Risk Index has six clinical predictors of major perioperative cardiac events:
- History of cerebrovascular disease
- Prior or current compensated congestive heart failure
- History of coronary artery disease
- Insulin-dependent diabetes mellitus
- Renal insufficiency, defined as a serum creatinine level of 2 mg/dL or higher
- Patient undergoing suprainguinal vascular, intraperitoneal, or intrathoracic surgery.
A patient who has 0 or 1 of these predictors would have a low risk of a major adverse cardiac event, whereas a patient with 2 or more would have elevated risk. These risk factors must be taken into consideration to determine the need, if any, for a preoperative ECG.
What an ECG can tell us
Abnormalities such as left ventricular hypertrophy, ST-segment depression, and pathologic Q waves on a preoperative ECG in a patient undergoing an elevated-risk surgical procedure may predict adverse perioperative cardiac events.3,6
In a retrospective study of 23,036 patients, Noordzij et al7 found that in patients undergoing elevated-risk surgery, those with an abnormal preoperative ECG had a higher incidence of cardiovascular death than those with a normal ECG. However, a preoperative ECG was obtained only in patients with established coronary artery disease or risk factors for cardiovascular disease. Hence, although an abnormal ECG in such patients undergoing elevated-risk surgery was predictive of adverse postoperative cardiac outcomes, we cannot say that the same would apply to patients without these characteristics undergoing elevated-risk surgery.
In a prospective observational study of patients with known coronary artery disease undergoing major noncardiac surgery, a preoperative ECG was found to contain prognostic information and was predictive of long-term outcome independent of clinical findings and perioperative ischemia.8
CURRENT GUIDELINES AND RECOMMENDATIONS
Several guidelines address whether to order a preoperative ECG but are mostly based on low-level evidence and expert opinion.1,2,6,9
Current guidelines recommend obtaining a preoperative ECG in patients with known coronary, peripheral arterial, or cerebrovascular disease.1,6,9
Obesity and associated comorbidities such as coronary artery disease, heart failure, systemic hypertension, and sleep apnea can predispose to increased perioperative complications. A preoperative 12-lead ECG is reasonable in morbidly obese patients (body mass index ≥ 40 kg/m2) and in obese patients (body mass index ≥ 30 kg/m2) with at least one risk factor for coronary artery disease or poor exercise tolerance, or both.10
Liu et al11 looked at the predictive value of a preoperative 12-lead ECG in 513 elderly patients (age ≥ 70) undergoing noncardiac surgery and found that electrocardiographic abnormalities were not predictive of adverse cardiac outcomes. In this study, although electrocardiographic abnormalities were common (noted in 75% of the patients), they were nonspecific and less useful in predicting postoperative cardiac complications than was the presence of comorbidities.11 Age alone as a cutoff for obtaining a preoperative ECG is not predictive of postoperative outcomes and a preoperative ECG is not warranted in all elderly patients. This is also reflected in current ACC/AHA guidelines on perioperative cardiovascular evaluation1 and is a change from prior ACC/AHA guidelines when age was used as a criterion for preoperative ECGs.12
Current guidelines do not recommend getting a preoperative ECG in asymptomatic patients undergoing low-cardiac-risk surgery.1,4,9
Although the ideal time for ordering an ECG before a planned surgery is unknown, obtaining one within 90 days before the surgery is considered adequate in stable patients in whom an ECG is indicated.1
BACK TO OUR PATIENT
On the basis of current evidence, our patient does not need a preoperative ECG, as it is unlikely to alter his perioperative management and instead may delay his surgery unnecessarily if any nonspecific changes prompt further cardiac workup.
CLINICAL BOTTOM LINE
Although frequently ordered in clinical practice, preoperative electrocardiography has a limited role in predicting postoperative outcome and should be ordered only in the appropriate clinical setting.1 Moreover, there is little evidence that outcomes are better if we obtain an ECG before surgery. The clinician should consider patient factors and the type of surgery before ordering diagnostic tests, including electrocardiography.
In asymptomatic patients undergoing nonemergent surgery:
- It is reasonable to obtain a preoperative ECG in patients with known coronary artery disease, significant arrhythmia, peripheral arterial disease, cerebrovascular disease, chronic heart failure, or other significant structural heart disease undergoing elevated-cardiac-risk surgery.
- Do not order a preoperative ECG in asymptomatic patients undergoing low-risk surgery.
- Obtaining a preoperative ECG is reasonable in morbidly obese patients and in obese patients with one or more risk factors for coronary artery disease, or poor exercise tolerance, undergoing high-risk surgery.
A 55-year-old lawyer with hypertension well controlled on lisinopril and amlodipine is scheduled for elective hernia repair under general anesthesia. His surgeon has referred him for a preoperative evaluation. He has never smoked, runs 4 miles on days off from work, and enjoys long hiking trips. On examination, his body mass index is 26 kg/m2 and his blood pressure is 130/78 mm Hg; his cardiac examination and the rest of the clinical examination are unremarkable. He asks if he should have an electrocardiogram (ECG) as a part of his workup.
A preoperative ECG is not routinely recommended in all asymptomatic patients undergoing noncardiac surgery.
Consider obtaining an ECG in patients planning to undergo a high-risk surgical procedure, especially if they have one or more clinical risk factors for coronary artery disease, and in patients undergoing elevated-cardiac-risk surgery who are known to have coronary artery disease, chronic heart failure, peripheral arterial disease, or cerebrovascular disease. However, a preoperative ECG is not routinely recommended for patients perceived to be at low cardiac risk who are planning to undergo low-risk surgery. In those patients it could delay surgery unnecessarily, cause further unnecessary testing, drive up costs, and increase patient anxiety.
Here we discuss the perioperative cardiac risk based on type of surgery and patient characteristics and summarize the current guidelines and recommendations on obtaining a preoperative 12-lead ECG in patients undergoing noncardiac surgery.
RISK DEPENDS ON TYPE OF SURGERY AND PATIENT FACTORS
Physicians, including primary care physicians, hospitalists, cardiologists, and anesthesiologists, are routinely asked to evaluate patients before surgical procedures. The purpose of the preoperative evaluation is to optimize existing medical conditions, to identify undiagnosed conditions that can increase risk of perioperative morbidity and death, and to suggest strategies to mitigate risk.1,2
Cardiac risk is multifactorial, and risk factors for postoperative adverse cardiac events include the type of surgery and patient factors.1,3
Cardiac risk based on type of surgery
Low-risk procedures are those in which the risk of a perioperative major adverse cardiac event is less than 1%.1,4 Examples:
- Ambulatory surgery
- Breast or plastic surgery
- Cataract surgery
- Endoscopic procedures.
Elevated-risk procedures are those in which the risk is 1% or higher. Examples:
- Intraperitoneal surgery
- Intrathoracic surgery
- Carotid endarterectomy
- Head and neck surgery
- Orthopedic surgery
- Prostate surgery
- Aortic surgery
- Major vascular surgery
- Peripheral arterial surgery.
Cardiac risk based on patient factors
The 2014 American College of Cardiology and American Heart Association (ACC/AHA) perioperative guidelines list a number of clinical risk factors for perioperative cardiac morbidity and death.1 These include coronary artery disease, chronic heart failure, clinically suspected moderate or greater degrees of valvular heart disease, arrhythmias, conduction disorders, pulmonary vascular disease, and adult congenital heart disease.
Patients with these conditions and patients with unstable coronary syndromes warrant preoperative ECGs and sometimes even urgent interventions before any nonemergency surgery, provided such interventions would affect decision-making and perioperative care.1
The risk of perioperative cardiac morbidity and death can be calculated using either the Revised Cardiac Risk Index scoring system or the American College of Surgeons National Surgical Quality Improvement Program calculator.157 The former is fairly simple, validated, and accepted, while the latter requires use of online calculators (eg, www.surgicalriskcalculator.com/miorcardiacarrest, www.riskcalculator.facs.org).
The Revised Cardiac Risk Index has six clinical predictors of major perioperative cardiac events:
- History of cerebrovascular disease
- Prior or current compensated congestive heart failure
- History of coronary artery disease
- Insulin-dependent diabetes mellitus
- Renal insufficiency, defined as a serum creatinine level of 2 mg/dL or higher
- Patient undergoing suprainguinal vascular, intraperitoneal, or intrathoracic surgery.
A patient who has 0 or 1 of these predictors would have a low risk of a major adverse cardiac event, whereas a patient with 2 or more would have elevated risk. These risk factors must be taken into consideration to determine the need, if any, for a preoperative ECG.
What an ECG can tell us
Abnormalities such as left ventricular hypertrophy, ST-segment depression, and pathologic Q waves on a preoperative ECG in a patient undergoing an elevated-risk surgical procedure may predict adverse perioperative cardiac events.3,6
In a retrospective study of 23,036 patients, Noordzij et al7 found that in patients undergoing elevated-risk surgery, those with an abnormal preoperative ECG had a higher incidence of cardiovascular death than those with a normal ECG. However, a preoperative ECG was obtained only in patients with established coronary artery disease or risk factors for cardiovascular disease. Hence, although an abnormal ECG in such patients undergoing elevated-risk surgery was predictive of adverse postoperative cardiac outcomes, we cannot say that the same would apply to patients without these characteristics undergoing elevated-risk surgery.
In a prospective observational study of patients with known coronary artery disease undergoing major noncardiac surgery, a preoperative ECG was found to contain prognostic information and was predictive of long-term outcome independent of clinical findings and perioperative ischemia.8
CURRENT GUIDELINES AND RECOMMENDATIONS
Several guidelines address whether to order a preoperative ECG but are mostly based on low-level evidence and expert opinion.1,2,6,9
Current guidelines recommend obtaining a preoperative ECG in patients with known coronary, peripheral arterial, or cerebrovascular disease.1,6,9
Obesity and associated comorbidities such as coronary artery disease, heart failure, systemic hypertension, and sleep apnea can predispose to increased perioperative complications. A preoperative 12-lead ECG is reasonable in morbidly obese patients (body mass index ≥ 40 kg/m2) and in obese patients (body mass index ≥ 30 kg/m2) with at least one risk factor for coronary artery disease or poor exercise tolerance, or both.10
Liu et al11 looked at the predictive value of a preoperative 12-lead ECG in 513 elderly patients (age ≥ 70) undergoing noncardiac surgery and found that electrocardiographic abnormalities were not predictive of adverse cardiac outcomes. In this study, although electrocardiographic abnormalities were common (noted in 75% of the patients), they were nonspecific and less useful in predicting postoperative cardiac complications than was the presence of comorbidities.11 Age alone as a cutoff for obtaining a preoperative ECG is not predictive of postoperative outcomes and a preoperative ECG is not warranted in all elderly patients. This is also reflected in current ACC/AHA guidelines on perioperative cardiovascular evaluation1 and is a change from prior ACC/AHA guidelines when age was used as a criterion for preoperative ECGs.12
Current guidelines do not recommend getting a preoperative ECG in asymptomatic patients undergoing low-cardiac-risk surgery.1,4,9
Although the ideal time for ordering an ECG before a planned surgery is unknown, obtaining one within 90 days before the surgery is considered adequate in stable patients in whom an ECG is indicated.1
BACK TO OUR PATIENT
On the basis of current evidence, our patient does not need a preoperative ECG, as it is unlikely to alter his perioperative management and instead may delay his surgery unnecessarily if any nonspecific changes prompt further cardiac workup.
CLINICAL BOTTOM LINE
Although frequently ordered in clinical practice, preoperative electrocardiography has a limited role in predicting postoperative outcome and should be ordered only in the appropriate clinical setting.1 Moreover, there is little evidence that outcomes are better if we obtain an ECG before surgery. The clinician should consider patient factors and the type of surgery before ordering diagnostic tests, including electrocardiography.
In asymptomatic patients undergoing nonemergent surgery:
- It is reasonable to obtain a preoperative ECG in patients with known coronary artery disease, significant arrhythmia, peripheral arterial disease, cerebrovascular disease, chronic heart failure, or other significant structural heart disease undergoing elevated-cardiac-risk surgery.
- Do not order a preoperative ECG in asymptomatic patients undergoing low-risk surgery.
- Obtaining a preoperative ECG is reasonable in morbidly obese patients and in obese patients with one or more risk factors for coronary artery disease, or poor exercise tolerance, undergoing high-risk surgery.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. J Am Coll Cardiol 2014; Jul 29. 10.1016/j.jacc.2014.07.944. [Epub ahead of print]
- Feely MA, Collins CS, Daniels PR, Kebede EB, Jatoi A, Mauck KF. Preoperative testing before noncardiac surgery: guidelines and recommendations. Am Fam Physician 2013; 87:414–418.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Task Force for Preoperative Cardiac Risk Assessment and Perioperative Cardiac Management in Non-cardiac Surgery; European Society of Cardiology (ESC); Poldermans D, Bax JJ, Boersma E, et al. Guidelines for pre-operative cardiac risk assessment and perioperative cardiac management in non-cardiac surgery. Eur Heart J 2009; 30:2769–2812.
- Bilimoria KY, Liu Y, Paruch JL, Zhou L, Kmiecik TE, Ko CY, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 2013; 217:833–842.
- Landesberg G, Einav S, Christopherson R, et al. Perioperative ischemia and cardiac complications in major vascular surgery: importance of the preoperative twelve-lead electrocardiogram. J Vasc Surg 1997; 26:570–578.
- Noordzij PG, Boersma E, Bax JJ, et al. Prognostic value of routine preoperative electrocardiography in patients undergoing noncardiac surgery. Am J Cardiol 2006; 97:1103–1106.
- Jeger RV, Probst C, Arsenic R, et al. Long-term prognostic value of the preoperative 12-lead electrocardiogram before major noncardiac surgery in coronary artery disease. Am Heart J 2006; 151:508–513.
- Committee on Standards and Practice Parameters; Apfelbaum JL, Connis RT, Nickinovich DG, Pasternak LR, Arens JF, Caplan RA, et al. Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology 2012; 116:522–538.
- Poirier P, Alpert MA, Fleisher LA, et al. Cardiovascular evaluation and management of severely obese patients undergoing surgery: a science advisory from the American Heart Association. Circulation 2009; 120:86–95.
- Liu LL, Dzankic S, Leung JM. Preoperative electrocardiogram abnormalities do not predict postoperative cardiac complications in geriatric surgical patients. J Am Geriatr Soc 2002; 50:1186–1191.
- Eagle KA, Berger PB, Calkins H, et al; American College of Cardiology; American Heart Association. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary. J Am Coll Cardiol 2002; 39:542–553.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. J Am Coll Cardiol 2014; Jul 29. 10.1016/j.jacc.2014.07.944. [Epub ahead of print]
- Feely MA, Collins CS, Daniels PR, Kebede EB, Jatoi A, Mauck KF. Preoperative testing before noncardiac surgery: guidelines and recommendations. Am Fam Physician 2013; 87:414–418.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Task Force for Preoperative Cardiac Risk Assessment and Perioperative Cardiac Management in Non-cardiac Surgery; European Society of Cardiology (ESC); Poldermans D, Bax JJ, Boersma E, et al. Guidelines for pre-operative cardiac risk assessment and perioperative cardiac management in non-cardiac surgery. Eur Heart J 2009; 30:2769–2812.
- Bilimoria KY, Liu Y, Paruch JL, Zhou L, Kmiecik TE, Ko CY, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 2013; 217:833–842.
- Landesberg G, Einav S, Christopherson R, et al. Perioperative ischemia and cardiac complications in major vascular surgery: importance of the preoperative twelve-lead electrocardiogram. J Vasc Surg 1997; 26:570–578.
- Noordzij PG, Boersma E, Bax JJ, et al. Prognostic value of routine preoperative electrocardiography in patients undergoing noncardiac surgery. Am J Cardiol 2006; 97:1103–1106.
- Jeger RV, Probst C, Arsenic R, et al. Long-term prognostic value of the preoperative 12-lead electrocardiogram before major noncardiac surgery in coronary artery disease. Am Heart J 2006; 151:508–513.
- Committee on Standards and Practice Parameters; Apfelbaum JL, Connis RT, Nickinovich DG, Pasternak LR, Arens JF, Caplan RA, et al. Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology 2012; 116:522–538.
- Poirier P, Alpert MA, Fleisher LA, et al. Cardiovascular evaluation and management of severely obese patients undergoing surgery: a science advisory from the American Heart Association. Circulation 2009; 120:86–95.
- Liu LL, Dzankic S, Leung JM. Preoperative electrocardiogram abnormalities do not predict postoperative cardiac complications in geriatric surgical patients. J Am Geriatr Soc 2002; 50:1186–1191.
- Eagle KA, Berger PB, Calkins H, et al; American College of Cardiology; American Heart Association. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary. J Am Coll Cardiol 2002; 39:542–553.
To dream the maybe possible dream: A breast cancer vaccine
The Journal generally does not publish articles on topics not yet clinically relevant. But since the topic of immunization is so often in the news, and since immunotherapeutic strategies against cancer continue to be tested in clinical trials, we decided to include in this issue an edited transcript of a Medicine Grand Rounds presentation at Cleveland Clinic by Dr. Vincent Tuohy on a novel strategy to develop a vaccine against a particularly virulent form of breast cancer.
The immune system’s response to cancer is complex. Melanoma and renal cell carcinoma seem particularly susceptible to suppression by the native or augmented immune response. But most cancers seem to grow—and many metastasize—seemingly unaffected by our immune system, and sometimes even in the presence of detectable antitumor cell-directed lymphocytes and antibodies. Many attempts at devising human antitumor vaccines and immunotherapies have failed. On the other hand, we have seen the successful development of effective monoclonal antibody therapies (eg, rituximab for B cell lymphoma), immunomodulatory treatments for patients with advanced disease, and vaccines against viruses that cause cancer, ie, human papillomavirus and hepatitis B.
To fully appreciate the nuances of Dr. Tuohy’s proposed strategy, which has not yet been tested in clinical trials, and the complexities of tumor immunology, a very brief primer on the challenges is in order.
CHALLENGES TO DEVELOPING CANCER IMMUNOTHERAPY
Solid tumors can be triggered by multiple mechanisms, alone or in combination, including viruses, spontaneous mutations, overexpression of tumor promoters, and underexpression of tumor suppressors. Once growing, the solid tumor establishes its own rogue growth community, complete with a new infrastructure to supply nutrition and oxygen, potential means for expansion locally and distally, and a system to defend itself from the body’s immune system. The last of these poses specific challenges to successful spontaneous immune surveillance and to immunotherapy designed to kill cancer cells.
Microbial pathogens trigger both a nonspecific (innate) and a specific immune response in the human body. Initially, the immune system is nonspecifically “revved up,” triggered by shared “danger signals” associated with the perceived pathogen and its specific antigens. Then, specialized cells including dendritic cells locally and in proximate lymph nodes are primed to present the de novo antigens in a way that generates a specific and maturing immune response capable of getting rid of the pathogen. Tumors are also pathogenic and in some ways “foreign.” However, they are also similar to normal tissue and interact quite differently with the immune response in ways that enhance their likelihood of growth and survival. Tumor cells often do not send a danger signal to the immune system akin to what is generated by a staphylococcal or mycobacterial invader.
Tumor cells express specific antigens on their surface, such as viral proteins, cancer-associated mutated proteins, and overexpressed differentiated or undifferentiated antigens, including in some cases what Dr. Tuohy discusses as “retired proteins.” But some of these antigens may also be expressed in normal tissues, especially in an environment of resolving inflammation. Some signal the immune system to down-regulate what could otherwise be a vigorous self-destructive response every time there was inflammation.
The dendritic cell activation of the potential antitumor T-cell response and the antitumor T-cell response itself seem to be systematically blunted by many tumors. Reversal of this blunting represents one strategy currently used with very modest clinical success in treating advanced melanoma. Some newly generated tumor-antigen–recognizing T cells may in fact exert suppressor (or regulator) and not cytotoxic activity. Some tumors exhibit systemic immunosuppressive activity; this can be manifested not only by unchecked tumor growth, but also by an increased susceptibility to certain infections.
PITFALLS OF MESSING WITH THE IMMUNE SYSTEM
Messing with the immune system is not without pitfalls. Not all toxicities will be predicted by preclinical animal studies, and human immunity is not a mirror image of rodents’ or even other primates’ immune systems. Augmentation of an antitumor response, in part from the interplay of the complexities noted above, may lead to destruction of normal tissue elsewhere, or even to disruption of tolerance with the expression of autoimmunity. The toxicities will be different than the somewhat predictable toxicities from traditional antiproliferative chemotherapies—witness the striking systemic toxicity from interleukin 2-based therapies.
Whether Dr. Tuohy’s approach to developing a tumor vaccine will ultimately reach our formularies remains to be seen. The work is in an extremely preliminary phase. But the concept of immunotherapy for cancer remains an active area of research that is worth keeping an eye on.
The Journal generally does not publish articles on topics not yet clinically relevant. But since the topic of immunization is so often in the news, and since immunotherapeutic strategies against cancer continue to be tested in clinical trials, we decided to include in this issue an edited transcript of a Medicine Grand Rounds presentation at Cleveland Clinic by Dr. Vincent Tuohy on a novel strategy to develop a vaccine against a particularly virulent form of breast cancer.
The immune system’s response to cancer is complex. Melanoma and renal cell carcinoma seem particularly susceptible to suppression by the native or augmented immune response. But most cancers seem to grow—and many metastasize—seemingly unaffected by our immune system, and sometimes even in the presence of detectable antitumor cell-directed lymphocytes and antibodies. Many attempts at devising human antitumor vaccines and immunotherapies have failed. On the other hand, we have seen the successful development of effective monoclonal antibody therapies (eg, rituximab for B cell lymphoma), immunomodulatory treatments for patients with advanced disease, and vaccines against viruses that cause cancer, ie, human papillomavirus and hepatitis B.
To fully appreciate the nuances of Dr. Tuohy’s proposed strategy, which has not yet been tested in clinical trials, and the complexities of tumor immunology, a very brief primer on the challenges is in order.
CHALLENGES TO DEVELOPING CANCER IMMUNOTHERAPY
Solid tumors can be triggered by multiple mechanisms, alone or in combination, including viruses, spontaneous mutations, overexpression of tumor promoters, and underexpression of tumor suppressors. Once growing, the solid tumor establishes its own rogue growth community, complete with a new infrastructure to supply nutrition and oxygen, potential means for expansion locally and distally, and a system to defend itself from the body’s immune system. The last of these poses specific challenges to successful spontaneous immune surveillance and to immunotherapy designed to kill cancer cells.
Microbial pathogens trigger both a nonspecific (innate) and a specific immune response in the human body. Initially, the immune system is nonspecifically “revved up,” triggered by shared “danger signals” associated with the perceived pathogen and its specific antigens. Then, specialized cells including dendritic cells locally and in proximate lymph nodes are primed to present the de novo antigens in a way that generates a specific and maturing immune response capable of getting rid of the pathogen. Tumors are also pathogenic and in some ways “foreign.” However, they are also similar to normal tissue and interact quite differently with the immune response in ways that enhance their likelihood of growth and survival. Tumor cells often do not send a danger signal to the immune system akin to what is generated by a staphylococcal or mycobacterial invader.
Tumor cells express specific antigens on their surface, such as viral proteins, cancer-associated mutated proteins, and overexpressed differentiated or undifferentiated antigens, including in some cases what Dr. Tuohy discusses as “retired proteins.” But some of these antigens may also be expressed in normal tissues, especially in an environment of resolving inflammation. Some signal the immune system to down-regulate what could otherwise be a vigorous self-destructive response every time there was inflammation.
The dendritic cell activation of the potential antitumor T-cell response and the antitumor T-cell response itself seem to be systematically blunted by many tumors. Reversal of this blunting represents one strategy currently used with very modest clinical success in treating advanced melanoma. Some newly generated tumor-antigen–recognizing T cells may in fact exert suppressor (or regulator) and not cytotoxic activity. Some tumors exhibit systemic immunosuppressive activity; this can be manifested not only by unchecked tumor growth, but also by an increased susceptibility to certain infections.
PITFALLS OF MESSING WITH THE IMMUNE SYSTEM
Messing with the immune system is not without pitfalls. Not all toxicities will be predicted by preclinical animal studies, and human immunity is not a mirror image of rodents’ or even other primates’ immune systems. Augmentation of an antitumor response, in part from the interplay of the complexities noted above, may lead to destruction of normal tissue elsewhere, or even to disruption of tolerance with the expression of autoimmunity. The toxicities will be different than the somewhat predictable toxicities from traditional antiproliferative chemotherapies—witness the striking systemic toxicity from interleukin 2-based therapies.
Whether Dr. Tuohy’s approach to developing a tumor vaccine will ultimately reach our formularies remains to be seen. The work is in an extremely preliminary phase. But the concept of immunotherapy for cancer remains an active area of research that is worth keeping an eye on.
The Journal generally does not publish articles on topics not yet clinically relevant. But since the topic of immunization is so often in the news, and since immunotherapeutic strategies against cancer continue to be tested in clinical trials, we decided to include in this issue an edited transcript of a Medicine Grand Rounds presentation at Cleveland Clinic by Dr. Vincent Tuohy on a novel strategy to develop a vaccine against a particularly virulent form of breast cancer.
The immune system’s response to cancer is complex. Melanoma and renal cell carcinoma seem particularly susceptible to suppression by the native or augmented immune response. But most cancers seem to grow—and many metastasize—seemingly unaffected by our immune system, and sometimes even in the presence of detectable antitumor cell-directed lymphocytes and antibodies. Many attempts at devising human antitumor vaccines and immunotherapies have failed. On the other hand, we have seen the successful development of effective monoclonal antibody therapies (eg, rituximab for B cell lymphoma), immunomodulatory treatments for patients with advanced disease, and vaccines against viruses that cause cancer, ie, human papillomavirus and hepatitis B.
To fully appreciate the nuances of Dr. Tuohy’s proposed strategy, which has not yet been tested in clinical trials, and the complexities of tumor immunology, a very brief primer on the challenges is in order.
CHALLENGES TO DEVELOPING CANCER IMMUNOTHERAPY
Solid tumors can be triggered by multiple mechanisms, alone or in combination, including viruses, spontaneous mutations, overexpression of tumor promoters, and underexpression of tumor suppressors. Once growing, the solid tumor establishes its own rogue growth community, complete with a new infrastructure to supply nutrition and oxygen, potential means for expansion locally and distally, and a system to defend itself from the body’s immune system. The last of these poses specific challenges to successful spontaneous immune surveillance and to immunotherapy designed to kill cancer cells.
Microbial pathogens trigger both a nonspecific (innate) and a specific immune response in the human body. Initially, the immune system is nonspecifically “revved up,” triggered by shared “danger signals” associated with the perceived pathogen and its specific antigens. Then, specialized cells including dendritic cells locally and in proximate lymph nodes are primed to present the de novo antigens in a way that generates a specific and maturing immune response capable of getting rid of the pathogen. Tumors are also pathogenic and in some ways “foreign.” However, they are also similar to normal tissue and interact quite differently with the immune response in ways that enhance their likelihood of growth and survival. Tumor cells often do not send a danger signal to the immune system akin to what is generated by a staphylococcal or mycobacterial invader.
Tumor cells express specific antigens on their surface, such as viral proteins, cancer-associated mutated proteins, and overexpressed differentiated or undifferentiated antigens, including in some cases what Dr. Tuohy discusses as “retired proteins.” But some of these antigens may also be expressed in normal tissues, especially in an environment of resolving inflammation. Some signal the immune system to down-regulate what could otherwise be a vigorous self-destructive response every time there was inflammation.
The dendritic cell activation of the potential antitumor T-cell response and the antitumor T-cell response itself seem to be systematically blunted by many tumors. Reversal of this blunting represents one strategy currently used with very modest clinical success in treating advanced melanoma. Some newly generated tumor-antigen–recognizing T cells may in fact exert suppressor (or regulator) and not cytotoxic activity. Some tumors exhibit systemic immunosuppressive activity; this can be manifested not only by unchecked tumor growth, but also by an increased susceptibility to certain infections.
PITFALLS OF MESSING WITH THE IMMUNE SYSTEM
Messing with the immune system is not without pitfalls. Not all toxicities will be predicted by preclinical animal studies, and human immunity is not a mirror image of rodents’ or even other primates’ immune systems. Augmentation of an antitumor response, in part from the interplay of the complexities noted above, may lead to destruction of normal tissue elsewhere, or even to disruption of tolerance with the expression of autoimmunity. The toxicities will be different than the somewhat predictable toxicities from traditional antiproliferative chemotherapies—witness the striking systemic toxicity from interleukin 2-based therapies.
Whether Dr. Tuohy’s approach to developing a tumor vaccine will ultimately reach our formularies remains to be seen. The work is in an extremely preliminary phase. But the concept of immunotherapy for cancer remains an active area of research that is worth keeping an eye on.