User login
Study supports new gold standard for FL

PET-CT should be the new standard for response assessment in patients with follicular lymphoma (FL), according to researchers.
The group found evidence suggesting that PET-CT is more accurate than conventional CT in measuring treatment response and predicting survival in patients with FL.
“Our findings have important implications for patients with follicular lymphoma,” said study author Judith Trotman, MBChB, of the University of Sydney in Australia.
“Compared to conventional CT scanning, PET-CT is more accurate in mapping out the lymphoma and better identifies the majority of patients who have a prolonged remission after treatment.”
Dr Trotman and her colleagues reported these findings in The Lancet Haematology. The results will also be discussed at the International Workshop on PET in Lymphoma in Menton, France, which is taking place September 19-20.
By assessing imaging performed in 3 clinical trials, the researchers examined the link between PET-CT status and survival following first-line immunochemotherapy for advanced FL.
Independent, masked reviewers evaluated scans of 246 patients who underwent both PET-CT and traditional CT imaging within 3 months of their last dose of therapy. Patients were followed for a median of 54.8 months.
Seventeen percent of patients had a positive post-induction PET scan, according to a cutoff of 4 or higher on the 5PS.
When the researchers compared patients with a positive PET scan to those with a negative scan, the hazard ratio (HR) for progression-free survival (PFS) was 3.9 (P<0.0001). For overall survival, the HR was 6.7 (P=0.0002).
The 4-year PFS was 23.2% in patients with a positive PET scan and 63.4% in those who had a negative PET scan (P<0.0001). The 4-year overall survival was 87.2% and 97.1%, respectively (P<0.0001).
The researchers also discovered that conventional CT-based response—complete response or unconfirmed complete response compared to partial response—was weakly predictive of PFS. The HR was 1.7 (P=0.017).
“Our study shows that PET-CT is much better in evaluating treatment response and is an early predictor of survival,” Dr Trotman said. “This greater accuracy will assist physicians to more effectively monitor their patients.”
“We expect this research will result in PET-CT imaging replacing CT, becoming the new gold standard to evaluate patients with follicular lymphoma after treatment. Importantly, it will be a platform for future studies of response-adapted therapies aimed to improve the poor outcomes for those patients who remain PET-positive.”
This study may also pave the way for several new research opportunities, according to Bruce Cheson, MD, of Georgetown University in Washington DC, who wrote a comment article related to this study.
“One such possibility would be to assess if an early reaction to the PET scan result improves patient outcome,” he wrote. “Thus, patients with a positive PET scan after induction therapy could be randomly assigned to either deferred treatment until disease progression or immediate intervention.”
“A preferable alternative would be to introduce a unique agent at that time, such as the newly developed small molecules (eg, idelalisib, ibrutinib, or ABT-199) in a novel combination.” ![]()

PET-CT should be the new standard for response assessment in patients with follicular lymphoma (FL), according to researchers.
The group found evidence suggesting that PET-CT is more accurate than conventional CT in measuring treatment response and predicting survival in patients with FL.
“Our findings have important implications for patients with follicular lymphoma,” said study author Judith Trotman, MBChB, of the University of Sydney in Australia.
“Compared to conventional CT scanning, PET-CT is more accurate in mapping out the lymphoma and better identifies the majority of patients who have a prolonged remission after treatment.”
Dr Trotman and her colleagues reported these findings in The Lancet Haematology. The results will also be discussed at the International Workshop on PET in Lymphoma in Menton, France, which is taking place September 19-20.
By assessing imaging performed in 3 clinical trials, the researchers examined the link between PET-CT status and survival following first-line immunochemotherapy for advanced FL.
Independent, masked reviewers evaluated scans of 246 patients who underwent both PET-CT and traditional CT imaging within 3 months of their last dose of therapy. Patients were followed for a median of 54.8 months.
Seventeen percent of patients had a positive post-induction PET scan, according to a cutoff of 4 or higher on the 5PS.
When the researchers compared patients with a positive PET scan to those with a negative scan, the hazard ratio (HR) for progression-free survival (PFS) was 3.9 (P<0.0001). For overall survival, the HR was 6.7 (P=0.0002).
The 4-year PFS was 23.2% in patients with a positive PET scan and 63.4% in those who had a negative PET scan (P<0.0001). The 4-year overall survival was 87.2% and 97.1%, respectively (P<0.0001).
The researchers also discovered that conventional CT-based response—complete response or unconfirmed complete response compared to partial response—was weakly predictive of PFS. The HR was 1.7 (P=0.017).
“Our study shows that PET-CT is much better in evaluating treatment response and is an early predictor of survival,” Dr Trotman said. “This greater accuracy will assist physicians to more effectively monitor their patients.”
“We expect this research will result in PET-CT imaging replacing CT, becoming the new gold standard to evaluate patients with follicular lymphoma after treatment. Importantly, it will be a platform for future studies of response-adapted therapies aimed to improve the poor outcomes for those patients who remain PET-positive.”
This study may also pave the way for several new research opportunities, according to Bruce Cheson, MD, of Georgetown University in Washington DC, who wrote a comment article related to this study.
“One such possibility would be to assess if an early reaction to the PET scan result improves patient outcome,” he wrote. “Thus, patients with a positive PET scan after induction therapy could be randomly assigned to either deferred treatment until disease progression or immediate intervention.”
“A preferable alternative would be to introduce a unique agent at that time, such as the newly developed small molecules (eg, idelalisib, ibrutinib, or ABT-199) in a novel combination.” ![]()

PET-CT should be the new standard for response assessment in patients with follicular lymphoma (FL), according to researchers.
The group found evidence suggesting that PET-CT is more accurate than conventional CT in measuring treatment response and predicting survival in patients with FL.
“Our findings have important implications for patients with follicular lymphoma,” said study author Judith Trotman, MBChB, of the University of Sydney in Australia.
“Compared to conventional CT scanning, PET-CT is more accurate in mapping out the lymphoma and better identifies the majority of patients who have a prolonged remission after treatment.”
Dr Trotman and her colleagues reported these findings in The Lancet Haematology. The results will also be discussed at the International Workshop on PET in Lymphoma in Menton, France, which is taking place September 19-20.
By assessing imaging performed in 3 clinical trials, the researchers examined the link between PET-CT status and survival following first-line immunochemotherapy for advanced FL.
Independent, masked reviewers evaluated scans of 246 patients who underwent both PET-CT and traditional CT imaging within 3 months of their last dose of therapy. Patients were followed for a median of 54.8 months.
Seventeen percent of patients had a positive post-induction PET scan, according to a cutoff of 4 or higher on the 5PS.
When the researchers compared patients with a positive PET scan to those with a negative scan, the hazard ratio (HR) for progression-free survival (PFS) was 3.9 (P<0.0001). For overall survival, the HR was 6.7 (P=0.0002).
The 4-year PFS was 23.2% in patients with a positive PET scan and 63.4% in those who had a negative PET scan (P<0.0001). The 4-year overall survival was 87.2% and 97.1%, respectively (P<0.0001).
The researchers also discovered that conventional CT-based response—complete response or unconfirmed complete response compared to partial response—was weakly predictive of PFS. The HR was 1.7 (P=0.017).
“Our study shows that PET-CT is much better in evaluating treatment response and is an early predictor of survival,” Dr Trotman said. “This greater accuracy will assist physicians to more effectively monitor their patients.”
“We expect this research will result in PET-CT imaging replacing CT, becoming the new gold standard to evaluate patients with follicular lymphoma after treatment. Importantly, it will be a platform for future studies of response-adapted therapies aimed to improve the poor outcomes for those patients who remain PET-positive.”
This study may also pave the way for several new research opportunities, according to Bruce Cheson, MD, of Georgetown University in Washington DC, who wrote a comment article related to this study.
“One such possibility would be to assess if an early reaction to the PET scan result improves patient outcome,” he wrote. “Thus, patients with a positive PET scan after induction therapy could be randomly assigned to either deferred treatment until disease progression or immediate intervention.”
“A preferable alternative would be to introduce a unique agent at that time, such as the newly developed small molecules (eg, idelalisib, ibrutinib, or ABT-199) in a novel combination.” ![]()
Group investigates link between implants and ALCL
![]()
Credit: FDA
A newly published review provides insight into anaplastic large-cell lymphoma (ALCL) associated with breast implants.
Previous reports have demonstrated a link between breast implants and ALCL, but the reasons why implants may contribute to this type of lymphoma have remained unclear.
So Suzanne Turner, PhD, of the University of Cambridge in the UK, and her colleagues evaluated the 71 recorded cases of implant-associated ALCL (iALCL).
The researchers recounted their findings in Mutation Research/Reviews in Mutations Research.
The team calculated that the absolute risk of developing iALCL is low, ranging from 1:500,000 to 1:3,000,000 patients with breast implants per year.
Among the 71 cases of iALCL, the average patient was 50 years of age. The average time from breast augmentation/reconstruction to iALCL diagnosis was 10 years.
Most of the cases presented in the capsule surrounding the implant, as part of the periprosthetic fluid or the capsule itself.
The majority of cases were ALK-negative but had a good prognosis. Of the 49 cases where information on the patients’ progress was available, there were 5 deaths.
Some patients received chemotherapy and radiotherapy, but many achieved remission once the implant and surrounding tissue were removed. This suggests it is the body’s abnormal immune response to the implant that is causing iALCL, the researchers said.
There is some evidence to suggest that having any type of prosthetic implant can increase the risk of lymphoma. And some researchers have suggested that lifestyle differences between patients with and without breast implants may be at the root of iALCL.
But Dr Turner and her colleagues said the biological features of lymphomas in the presence of implants, such as the lack of ALK and the proximity to the implant, suggest a real link.
“It’s becoming clear that implant-related ALCL is a distinct clinical entity in itself,” Dr Turner said. “There are still unanswered questions, and only by getting to the bottom of this very rare disease will we be able to find alternative ways to treat it.” ![]()
![]()
Credit: FDA
A newly published review provides insight into anaplastic large-cell lymphoma (ALCL) associated with breast implants.
Previous reports have demonstrated a link between breast implants and ALCL, but the reasons why implants may contribute to this type of lymphoma have remained unclear.
So Suzanne Turner, PhD, of the University of Cambridge in the UK, and her colleagues evaluated the 71 recorded cases of implant-associated ALCL (iALCL).
The researchers recounted their findings in Mutation Research/Reviews in Mutations Research.
The team calculated that the absolute risk of developing iALCL is low, ranging from 1:500,000 to 1:3,000,000 patients with breast implants per year.
Among the 71 cases of iALCL, the average patient was 50 years of age. The average time from breast augmentation/reconstruction to iALCL diagnosis was 10 years.
Most of the cases presented in the capsule surrounding the implant, as part of the periprosthetic fluid or the capsule itself.
The majority of cases were ALK-negative but had a good prognosis. Of the 49 cases where information on the patients’ progress was available, there were 5 deaths.
Some patients received chemotherapy and radiotherapy, but many achieved remission once the implant and surrounding tissue were removed. This suggests it is the body’s abnormal immune response to the implant that is causing iALCL, the researchers said.
There is some evidence to suggest that having any type of prosthetic implant can increase the risk of lymphoma. And some researchers have suggested that lifestyle differences between patients with and without breast implants may be at the root of iALCL.
But Dr Turner and her colleagues said the biological features of lymphomas in the presence of implants, such as the lack of ALK and the proximity to the implant, suggest a real link.
“It’s becoming clear that implant-related ALCL is a distinct clinical entity in itself,” Dr Turner said. “There are still unanswered questions, and only by getting to the bottom of this very rare disease will we be able to find alternative ways to treat it.” ![]()
![]()
Credit: FDA
A newly published review provides insight into anaplastic large-cell lymphoma (ALCL) associated with breast implants.
Previous reports have demonstrated a link between breast implants and ALCL, but the reasons why implants may contribute to this type of lymphoma have remained unclear.
So Suzanne Turner, PhD, of the University of Cambridge in the UK, and her colleagues evaluated the 71 recorded cases of implant-associated ALCL (iALCL).
The researchers recounted their findings in Mutation Research/Reviews in Mutations Research.
The team calculated that the absolute risk of developing iALCL is low, ranging from 1:500,000 to 1:3,000,000 patients with breast implants per year.
Among the 71 cases of iALCL, the average patient was 50 years of age. The average time from breast augmentation/reconstruction to iALCL diagnosis was 10 years.
Most of the cases presented in the capsule surrounding the implant, as part of the periprosthetic fluid or the capsule itself.
The majority of cases were ALK-negative but had a good prognosis. Of the 49 cases where information on the patients’ progress was available, there were 5 deaths.
Some patients received chemotherapy and radiotherapy, but many achieved remission once the implant and surrounding tissue were removed. This suggests it is the body’s abnormal immune response to the implant that is causing iALCL, the researchers said.
There is some evidence to suggest that having any type of prosthetic implant can increase the risk of lymphoma. And some researchers have suggested that lifestyle differences between patients with and without breast implants may be at the root of iALCL.
But Dr Turner and her colleagues said the biological features of lymphomas in the presence of implants, such as the lack of ALK and the proximity to the implant, suggest a real link.
“It’s becoming clear that implant-related ALCL is a distinct clinical entity in itself,” Dr Turner said. “There are still unanswered questions, and only by getting to the bottom of this very rare disease will we be able to find alternative ways to treat it.” ![]()
Shorter duration of DAPT appears safe

Credit: Andre E.X. Brown
WASHINGTON, DC—New research indicates that patients may only need 6 months of dual antiplatelet therapy (DAPT) after receiving a second-generation drug-eluting stent.
Results of the SECURITY trial showed that patients had similar outcomes whether they received DAPT for 6 months or a full year.
The proportion of patients who met a composite endpoint of cardiac, thrombotic, and bleeding events was low among both treatment groups at the 12- and 24-month time points.
Antonio Colombo, MD, of San Raffaele Scientific Institute in Milan, Italy, and his colleagues reported these findings at TCT 2014 and in the Journal of the American College of Cardiology.
SECURITY was a randomized, multicenter study that aimed to address the need for prolonged use of DAPT following the implantation of a second-generation drug-eluting stent for patients without high-risk acute coronary syndromes.
Patients with a diagnosis of stable or unstable angina or documented silent ischemia undergoing revascularization with at least 1 second-generation drug-eluting stent were eligible for the study. The stents used were the Endeavor Resolute (Medtronic), Xience (Abbott), Promus (Boston Scientific), Nobori (Terumo Corporation), and the Biomatrix (Biosensors Europe SA).
Of 1399 patients, 682 were randomized to 6 months of DAPT, and 717 were randomized to 12 months of treatment. They received clopidogrel at 75 mg per day for at least 3 days before the procedure or a pre-procedural loading dose of a minimum of 300 mg of clopidogrel, if they were not on chronic clopidogrel therapy.
In the post-procedure period, patients received 75 mg of clopidogrel for 6 or 12 months, according to randomization. They also received aspirin indefinitely. And once the new antiplatelet compounds prasugrel and ticagrelor hit the market, they were allowed as treatment options in a protocol amendment.
At 12 months, the incidence of the primary endpoint—the composite of cardiac death, myocardial infarction (MI), stroke, definite or probable stent thrombosis, or BARC type 3 or 5 bleeding—was 4.5% in the 6-month DAPT group and 3.7% in the 12-month DAPT group (P=0.469).
After 24 months, the 6-month and 12-month groups still showed similar incidences of cardiac death (0.9% vs 0.8%, P=0.925), MI (3.1% vs 2.6%, P=0.636), stroke (0.9% vs 0.4%, P=0.636), stent thrombosis (0.4% vs 0.4%, P=0.951), and BARC 3 or 5 bleeding (0.7% vs 1.1%, P=0.496).
Rates of the secondary endpoint—a composite of cardiac death, spontaneous MI, stroke, definite or probable stent thrombosis, or BARC type 2, 3, or 5 bleeding at 12 and 24 months—were also similar among the 6-month and 12-month treatment groups.
At 12 months, the incidence of the secondary composite endpoint was 5.3% in the 6-month group and 4.0% in the 12-month group (P=0.273). In the year that followed, both groups had lower incidences of secondary composite endpoints—1.5% and 2.2%, respectively (P=0.289).
A multivariable analysis showed that an age of 75 years or older, the type of stent used, the mean number of stents implanted, the mean stent length, and the mean stent size were all significant independent predictors of the primary endpoint.
The SECURITY trial was funded by grants from Medtronic and Terumo, makers of 2 brands of drug-eluting stents used in the study. ![]()

Credit: Andre E.X. Brown
WASHINGTON, DC—New research indicates that patients may only need 6 months of dual antiplatelet therapy (DAPT) after receiving a second-generation drug-eluting stent.
Results of the SECURITY trial showed that patients had similar outcomes whether they received DAPT for 6 months or a full year.
The proportion of patients who met a composite endpoint of cardiac, thrombotic, and bleeding events was low among both treatment groups at the 12- and 24-month time points.
Antonio Colombo, MD, of San Raffaele Scientific Institute in Milan, Italy, and his colleagues reported these findings at TCT 2014 and in the Journal of the American College of Cardiology.
SECURITY was a randomized, multicenter study that aimed to address the need for prolonged use of DAPT following the implantation of a second-generation drug-eluting stent for patients without high-risk acute coronary syndromes.
Patients with a diagnosis of stable or unstable angina or documented silent ischemia undergoing revascularization with at least 1 second-generation drug-eluting stent were eligible for the study. The stents used were the Endeavor Resolute (Medtronic), Xience (Abbott), Promus (Boston Scientific), Nobori (Terumo Corporation), and the Biomatrix (Biosensors Europe SA).
Of 1399 patients, 682 were randomized to 6 months of DAPT, and 717 were randomized to 12 months of treatment. They received clopidogrel at 75 mg per day for at least 3 days before the procedure or a pre-procedural loading dose of a minimum of 300 mg of clopidogrel, if they were not on chronic clopidogrel therapy.
In the post-procedure period, patients received 75 mg of clopidogrel for 6 or 12 months, according to randomization. They also received aspirin indefinitely. And once the new antiplatelet compounds prasugrel and ticagrelor hit the market, they were allowed as treatment options in a protocol amendment.
At 12 months, the incidence of the primary endpoint—the composite of cardiac death, myocardial infarction (MI), stroke, definite or probable stent thrombosis, or BARC type 3 or 5 bleeding—was 4.5% in the 6-month DAPT group and 3.7% in the 12-month DAPT group (P=0.469).
After 24 months, the 6-month and 12-month groups still showed similar incidences of cardiac death (0.9% vs 0.8%, P=0.925), MI (3.1% vs 2.6%, P=0.636), stroke (0.9% vs 0.4%, P=0.636), stent thrombosis (0.4% vs 0.4%, P=0.951), and BARC 3 or 5 bleeding (0.7% vs 1.1%, P=0.496).
Rates of the secondary endpoint—a composite of cardiac death, spontaneous MI, stroke, definite or probable stent thrombosis, or BARC type 2, 3, or 5 bleeding at 12 and 24 months—were also similar among the 6-month and 12-month treatment groups.
At 12 months, the incidence of the secondary composite endpoint was 5.3% in the 6-month group and 4.0% in the 12-month group (P=0.273). In the year that followed, both groups had lower incidences of secondary composite endpoints—1.5% and 2.2%, respectively (P=0.289).
A multivariable analysis showed that an age of 75 years or older, the type of stent used, the mean number of stents implanted, the mean stent length, and the mean stent size were all significant independent predictors of the primary endpoint.
The SECURITY trial was funded by grants from Medtronic and Terumo, makers of 2 brands of drug-eluting stents used in the study. ![]()

Credit: Andre E.X. Brown
WASHINGTON, DC—New research indicates that patients may only need 6 months of dual antiplatelet therapy (DAPT) after receiving a second-generation drug-eluting stent.
Results of the SECURITY trial showed that patients had similar outcomes whether they received DAPT for 6 months or a full year.
The proportion of patients who met a composite endpoint of cardiac, thrombotic, and bleeding events was low among both treatment groups at the 12- and 24-month time points.
Antonio Colombo, MD, of San Raffaele Scientific Institute in Milan, Italy, and his colleagues reported these findings at TCT 2014 and in the Journal of the American College of Cardiology.
SECURITY was a randomized, multicenter study that aimed to address the need for prolonged use of DAPT following the implantation of a second-generation drug-eluting stent for patients without high-risk acute coronary syndromes.
Patients with a diagnosis of stable or unstable angina or documented silent ischemia undergoing revascularization with at least 1 second-generation drug-eluting stent were eligible for the study. The stents used were the Endeavor Resolute (Medtronic), Xience (Abbott), Promus (Boston Scientific), Nobori (Terumo Corporation), and the Biomatrix (Biosensors Europe SA).
Of 1399 patients, 682 were randomized to 6 months of DAPT, and 717 were randomized to 12 months of treatment. They received clopidogrel at 75 mg per day for at least 3 days before the procedure or a pre-procedural loading dose of a minimum of 300 mg of clopidogrel, if they were not on chronic clopidogrel therapy.
In the post-procedure period, patients received 75 mg of clopidogrel for 6 or 12 months, according to randomization. They also received aspirin indefinitely. And once the new antiplatelet compounds prasugrel and ticagrelor hit the market, they were allowed as treatment options in a protocol amendment.
At 12 months, the incidence of the primary endpoint—the composite of cardiac death, myocardial infarction (MI), stroke, definite or probable stent thrombosis, or BARC type 3 or 5 bleeding—was 4.5% in the 6-month DAPT group and 3.7% in the 12-month DAPT group (P=0.469).
After 24 months, the 6-month and 12-month groups still showed similar incidences of cardiac death (0.9% vs 0.8%, P=0.925), MI (3.1% vs 2.6%, P=0.636), stroke (0.9% vs 0.4%, P=0.636), stent thrombosis (0.4% vs 0.4%, P=0.951), and BARC 3 or 5 bleeding (0.7% vs 1.1%, P=0.496).
Rates of the secondary endpoint—a composite of cardiac death, spontaneous MI, stroke, definite or probable stent thrombosis, or BARC type 2, 3, or 5 bleeding at 12 and 24 months—were also similar among the 6-month and 12-month treatment groups.
At 12 months, the incidence of the secondary composite endpoint was 5.3% in the 6-month group and 4.0% in the 12-month group (P=0.273). In the year that followed, both groups had lower incidences of secondary composite endpoints—1.5% and 2.2%, respectively (P=0.289).
A multivariable analysis showed that an age of 75 years or older, the type of stent used, the mean number of stents implanted, the mean stent length, and the mean stent size were all significant independent predictors of the primary endpoint.
The SECURITY trial was funded by grants from Medtronic and Terumo, makers of 2 brands of drug-eluting stents used in the study. ![]()
Bivalirudin bests heparin in BRIGHT trial

Credit: Bill Branson
WASHINGTON, DC—The latest results from the BRIGHT trial suggest bivalirudin confers benefits over heparin monotherapy and heparin plus tirofiban for patients with acute myocardial infarction undergoing percutaneous coronary intervention.
Patients who received bivalirudin had a significantly lower incidence of net adverse clinical events (NACE), a composite of death from any cause, reinfarction, ischemia-driven target vessel revascularization, stroke, or any bleeding, both at 30 days and at 1 year.
Bivalirudin conferred a lower risk of bleeding at both time points as well.
However, there were no significant differences between the treatment arms with regard to stent thrombosis or major adverse cardiac and cerebral events (MACCE), a composite of death from any cause, reinfarction, ischemia-driven target vessel revascularization, or stroke.
Yaling Han, MD, of the General Hospital of Shenyang Military Region in China, presented these data at TCT 2014.
The BRIGHT trial included 2194 patients who had acute myocardial infarction and were eligible for emergency percutaneous coronary intervention. They were randomized to receive bivalirudin alone (n=735), heparin alone (n=729), or heparin plus tirofiban (n=730).
The primary endpoint was NACE at 30 days. Secondary endpoints were NACE at 1 year, any bleeding at 30 days and 1 year, and MACCE at 30 days and 1 year. Safety endpoints were thrombocytopenia at 30 days and stent thrombosis at 30 days and 1 year.
Bivalirudin was superior to both heparin monotherapy and heparin plus tirofiban in reducing the primary composite endpoint. At 30 days, NACE had occurred in 8.8%, 13.2%, and 17.0% of patients, respectively (P<0.001).
Bivalirudin was also superior with regard to bleeding at 30 days. Bleeding occurred in 4.1% of patients in the bivalirudin arm, 7.5% in the heparin arm, and 12.3% in the heparin-tirofiban arm (P<0.001).
However, there was no significant difference between the arms for the incidence of MACCE at 30 days—5.0%, 5.8%, and 4.9%, respectively (P=0.74). Likewise, there was no significant difference in the rate of stent thrombosis at 30 days—0.6%, 0.9%, and 0.7%, respectively (P=0.77).
There were differences between the arms in the 30-day thrombocytopenia rate, which was 0.1% in the bivalirudin arm, 0.7% in the heparin arm, and 1.1% in the heparin-tirofiban arm (P=0.04 for bivalirudin vs pooled heparin, P=0.12 for bivalirudin vs heparin, P=0.02 for bivalirudin vs heparin-tirofiban).
The differences between the treatment arms at 1 year were similar to those observed at the 30-day mark. Bivalirudin remained significantly superior when it came to NACE and bleeding, but there were no significant differences with regard to MACCE and stent thrombosis.
At 1-year, NACE had occurred in 12.8% of patients in the bivalirudin arm, 16.5% in the heparin arm, and 20.5% in the heparin-tirofiban arm (P<0.001). Bleeding had occurred in 6.3%, 9.9%, and 14.2% of patients, respectively (P<0.001).
The incidence of MACCE was 6.7% in the bivalirudin arm, 7.3% in the heparin arm, and 6.8%, in the heparin-tirofiban arm. And stent thrombosis had occurred in 1.2%, 1.9%, and 1.2% of patients, respectively.
This research was funded by Salubris Pharmaceutical Co. Ltd, makers of bivalirudin, and the National Key Science and Technology R&D project of the 12th Five-Year Plan. ![]()

Credit: Bill Branson
WASHINGTON, DC—The latest results from the BRIGHT trial suggest bivalirudin confers benefits over heparin monotherapy and heparin plus tirofiban for patients with acute myocardial infarction undergoing percutaneous coronary intervention.
Patients who received bivalirudin had a significantly lower incidence of net adverse clinical events (NACE), a composite of death from any cause, reinfarction, ischemia-driven target vessel revascularization, stroke, or any bleeding, both at 30 days and at 1 year.
Bivalirudin conferred a lower risk of bleeding at both time points as well.
However, there were no significant differences between the treatment arms with regard to stent thrombosis or major adverse cardiac and cerebral events (MACCE), a composite of death from any cause, reinfarction, ischemia-driven target vessel revascularization, or stroke.
Yaling Han, MD, of the General Hospital of Shenyang Military Region in China, presented these data at TCT 2014.
The BRIGHT trial included 2194 patients who had acute myocardial infarction and were eligible for emergency percutaneous coronary intervention. They were randomized to receive bivalirudin alone (n=735), heparin alone (n=729), or heparin plus tirofiban (n=730).
The primary endpoint was NACE at 30 days. Secondary endpoints were NACE at 1 year, any bleeding at 30 days and 1 year, and MACCE at 30 days and 1 year. Safety endpoints were thrombocytopenia at 30 days and stent thrombosis at 30 days and 1 year.
Bivalirudin was superior to both heparin monotherapy and heparin plus tirofiban in reducing the primary composite endpoint. At 30 days, NACE had occurred in 8.8%, 13.2%, and 17.0% of patients, respectively (P<0.001).
Bivalirudin was also superior with regard to bleeding at 30 days. Bleeding occurred in 4.1% of patients in the bivalirudin arm, 7.5% in the heparin arm, and 12.3% in the heparin-tirofiban arm (P<0.001).
However, there was no significant difference between the arms for the incidence of MACCE at 30 days—5.0%, 5.8%, and 4.9%, respectively (P=0.74). Likewise, there was no significant difference in the rate of stent thrombosis at 30 days—0.6%, 0.9%, and 0.7%, respectively (P=0.77).
There were differences between the arms in the 30-day thrombocytopenia rate, which was 0.1% in the bivalirudin arm, 0.7% in the heparin arm, and 1.1% in the heparin-tirofiban arm (P=0.04 for bivalirudin vs pooled heparin, P=0.12 for bivalirudin vs heparin, P=0.02 for bivalirudin vs heparin-tirofiban).
The differences between the treatment arms at 1 year were similar to those observed at the 30-day mark. Bivalirudin remained significantly superior when it came to NACE and bleeding, but there were no significant differences with regard to MACCE and stent thrombosis.
At 1-year, NACE had occurred in 12.8% of patients in the bivalirudin arm, 16.5% in the heparin arm, and 20.5% in the heparin-tirofiban arm (P<0.001). Bleeding had occurred in 6.3%, 9.9%, and 14.2% of patients, respectively (P<0.001).
The incidence of MACCE was 6.7% in the bivalirudin arm, 7.3% in the heparin arm, and 6.8%, in the heparin-tirofiban arm. And stent thrombosis had occurred in 1.2%, 1.9%, and 1.2% of patients, respectively.
This research was funded by Salubris Pharmaceutical Co. Ltd, makers of bivalirudin, and the National Key Science and Technology R&D project of the 12th Five-Year Plan. ![]()

Credit: Bill Branson
WASHINGTON, DC—The latest results from the BRIGHT trial suggest bivalirudin confers benefits over heparin monotherapy and heparin plus tirofiban for patients with acute myocardial infarction undergoing percutaneous coronary intervention.
Patients who received bivalirudin had a significantly lower incidence of net adverse clinical events (NACE), a composite of death from any cause, reinfarction, ischemia-driven target vessel revascularization, stroke, or any bleeding, both at 30 days and at 1 year.
Bivalirudin conferred a lower risk of bleeding at both time points as well.
However, there were no significant differences between the treatment arms with regard to stent thrombosis or major adverse cardiac and cerebral events (MACCE), a composite of death from any cause, reinfarction, ischemia-driven target vessel revascularization, or stroke.
Yaling Han, MD, of the General Hospital of Shenyang Military Region in China, presented these data at TCT 2014.
The BRIGHT trial included 2194 patients who had acute myocardial infarction and were eligible for emergency percutaneous coronary intervention. They were randomized to receive bivalirudin alone (n=735), heparin alone (n=729), or heparin plus tirofiban (n=730).
The primary endpoint was NACE at 30 days. Secondary endpoints were NACE at 1 year, any bleeding at 30 days and 1 year, and MACCE at 30 days and 1 year. Safety endpoints were thrombocytopenia at 30 days and stent thrombosis at 30 days and 1 year.
Bivalirudin was superior to both heparin monotherapy and heparin plus tirofiban in reducing the primary composite endpoint. At 30 days, NACE had occurred in 8.8%, 13.2%, and 17.0% of patients, respectively (P<0.001).
Bivalirudin was also superior with regard to bleeding at 30 days. Bleeding occurred in 4.1% of patients in the bivalirudin arm, 7.5% in the heparin arm, and 12.3% in the heparin-tirofiban arm (P<0.001).
However, there was no significant difference between the arms for the incidence of MACCE at 30 days—5.0%, 5.8%, and 4.9%, respectively (P=0.74). Likewise, there was no significant difference in the rate of stent thrombosis at 30 days—0.6%, 0.9%, and 0.7%, respectively (P=0.77).
There were differences between the arms in the 30-day thrombocytopenia rate, which was 0.1% in the bivalirudin arm, 0.7% in the heparin arm, and 1.1% in the heparin-tirofiban arm (P=0.04 for bivalirudin vs pooled heparin, P=0.12 for bivalirudin vs heparin, P=0.02 for bivalirudin vs heparin-tirofiban).
The differences between the treatment arms at 1 year were similar to those observed at the 30-day mark. Bivalirudin remained significantly superior when it came to NACE and bleeding, but there were no significant differences with regard to MACCE and stent thrombosis.
At 1-year, NACE had occurred in 12.8% of patients in the bivalirudin arm, 16.5% in the heparin arm, and 20.5% in the heparin-tirofiban arm (P<0.001). Bleeding had occurred in 6.3%, 9.9%, and 14.2% of patients, respectively (P<0.001).
The incidence of MACCE was 6.7% in the bivalirudin arm, 7.3% in the heparin arm, and 6.8%, in the heparin-tirofiban arm. And stent thrombosis had occurred in 1.2%, 1.9%, and 1.2% of patients, respectively.
This research was funded by Salubris Pharmaceutical Co. Ltd, makers of bivalirudin, and the National Key Science and Technology R&D project of the 12th Five-Year Plan. ![]()
Honey
Honeybees (Apis mellifera, A. cerana, A. dorsata, A. floria, A. andreniformis, A. koschevnikov, and A. laborisa) play a key role in propagating numerous plants, flower nectar, and flower pollen as well as in pollinating approximately one-third of common agricultural crops, including fruits, vegetables, nuts, and seeds (Time magazine; Proc. Biol. Sci. 2007;274[1608]:303-13). Indeed, the honeybee is the lone insect that produces food regularly consumed by human beings (Am. J. Ther. 2014;21:304-23). Honey, which contains more than 180 compounds, is produced by honeybees from flower nectar. This sweet food product is supersaturated in sugar, and also contains phenolic acids, flavonoids, ascorbic acid, alpha-tocopherol, carotenoids, the enzymes glucose oxidase and catalase, organic and amino acids, and proteins (J. Food Sci. 2008;73:R117-24). Honey has been used since ancient times in Ayurvedic medicine to treat diabetes and has long been used to treat infected wounds (Ayu 2012;33:178-82; Clin. Infect. Dis. 2009;49:1541-9). Currently, honey is used in Ayurvedic medicine to treat acne, and it is incorporated in various cosmetic formulations such as facial washes, skin moisturizers, and hair conditioners (Ayu 2012;33:178-82).
History
For at least 2,700 years, traditional medical practice has included the use of topically applied honey for various conditions, with many modern researchers retrospectively attributing this usage to the antibacterial activity of honey (Am. J. Ther. 2014;21:304-23; Clin. Infect. Dis. 2008;46:1677-82). Honey served as a potent anti-inflammatory and antibacterial agent in folk remedies in ancient Egypt, Greece, and Rome, with written references to the medical application of bee products dating back to ancientEgypt, India, and China (Am. J. Ther. 2014;21:304-23; Cancer Res. 1993;53:1255-61; Evid. Based Complement. Alternat. Med. 2013;2013:697390)). For more than 4,000 years, honey has been used in Ayurvedic medicine, and its use has been traced to the Xin dynasty in China (Am. J. Ther. 2014;21:304-23). The antibacterial characteristics of honey were first reported in 1892 (IUBMB Life 2012;64:48-55). Russia and Germany used honey for wound treatment through World War I. The traditional medical application of honey began to subside with the advent of antibiotics in the 1940s(Burns 2013; 39:1514-25; Int. J. Clin. Pract. 2007;61:1705-7).
Chemistry
Myriad biological functions are associated with honey (antibacterial, antioxidant, antitumor, anti-inflammatory, antibrowning, and antiviral) and ascribed mainly to its constituent phenolic compounds, such as flavonoids, including chrysin (J. Food Sci. 2008;73:R117-24). Indeed, medical grade honeys such as manuka honey (a monofloral honey derived from Leptospermum scoparium, a member of the Myrtaceae family, native to New Zealand) and Medihoney® (a standardized mix of Australian and New Zealand honeys) are rich in flavonoids (Int. J. Clin. Pract. 2007;61:1705-7;J. Int. Acad. Periodontol. 2004;6:63-7; Evid. Based Complement. Alternat. Med. 2009;6:165-73;J. Agric. Food Chem. 2012;60:7229-37). Honey has a pH ranging from 3.2 to 4.5 and an acidity level that stymies the growth of many microorganisms (Burns 2013;39:1514-25; J. Clin. Nurs. 2008;17:2604-23; Nurs. Times. 2006;102:40-2; Br. J. Community Nurs. 2004;Suppl:S21-7 ).
Antibacterial activity
In 2008, Kwakman et al. found that within 24 hours, 10%-40% (vol/vol) medical grade honey (Revamil) destroyed antibiotic-susceptible and antibiotic-resistant isolates of Staphylococcus aureus,S. epidermidis, Enterococcus faecium, Escherichia coli, Pseudomonas aeruginosa, Enterobacter cloacae, and Klebsiella oxytoca. After 2 days of honey application, they also observed a 100-fold decrease in forearm skin colonization in healthy volunteers, with the number of positive skin cultures declining by 76%. The researchers concluded that Revamil exhibits significant potential to prevent or treat infections, including those spawned by multidrug-resistant bacteria (Clin. Infect. Dis. 2008;46:1677-82). Honey has been demonstrated to be clinically effective in treating several kinds of wound infections, reducing skin colonization of multiple bacteria, including methicillin-resistant S. aureus (Clin. Infect. Dis. 2008;46:1677-82) and enhancing wound healing, without provoking adverse effects ( Clin. Infect. Dis. 2009;49:1541-9). Manuka honey and Medihoney are the main forms of medical grade honey used in clinical practice. Nonmedical grade honey may contain viable bacterial spores (including clostridia), and manifest less predictable antibacterial properties (Clin. Infect. Dis. 2009;49:1541-9).
Honey is used in over-the-counter products as a moisturizing agent and in hair-conditioning products based on its strong humectant properties. It is also used in home remedies to treat burns, wounds, eczema, and dermatitis, especially in Asia (Ayu 2012;33:178-8).
Seborrheic dermatitis/dandruff
In 2001, Al-Waili assessed the potential of topically applied crude honey (90% honey diluted in warm water) to treat chronic seborrheic dermatitis of the scalp, face, and chest in 30 patients (20 males and 10 females, aged 15-60 years). Over the initial 4 weeks of treatment, honey was gently rubbed onto lesions every other day for 2-3 minutes at a time, with the ointment left on for 3 hours before gentle warm-water rinsing. Then, in a 6-month prophylactic phase, the participants were divided into a once-weekly treatment group and a control group. Skin lesions healed completely within 2 weeks in the treatment group, after significant reductions in itching and scaling in just the first week. Subjective improvements in hair loss were also reported. Relapse was observed in 12 of the 15 subjects in the control group within 2-4 months of therapy cessation and none in the treatment group. The author concluded that weekly use of crude honey significantly improves seborrheic dermatitis symptoms and related hair loss (Eur. J. Med. Res. 2001;6:306-8).
Wound healing
In February 2013, Jull published a review of 25 randomized and quasirandomized trials evaluating honey in the treatment of acute or chronic wounds, finding that honey might delay healing in partial- and full-thickness burns, compared with early excision and grafting, but it does not significantly enhance healing of chronic venous leg ulcers. They suggested that while honey may prove to be more effective than some conventional dressings for such ulcers, evidence is currently insufficient to support this claim ( Cochrane Database Syst. Rev. 2013;2:CD005083). Later that year, Vandamme et al. identified 55 studies in a literature review suggesting that honey stimulates healing of burns, ulcers, and other wounds. They also found, despite some methodologic concerns, that honey exerts antibacterial activity in burn treatment and deodorizing, debridement, anti-inflammatory, and analgesic activity ( Burns 2013;39:1514-25).
Conclusion
Honey has a long history of traditional medicinal use and has been found to display significant biologic activity, including antibacterial, antioxidant, antitumor, anti-inflammatory, antibrowning, and antiviral. The antibacterial properties of honey are particularly compelling. While more research, in the form of randomized, controlled trials, is needed prior to incorporating bee products into the dermatologic armamentarium as first-line therapies, the potential of honey usage for skin care is promising.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (McGraw-Hill, April 2002), and a book for consumers, “The Skin Type Solution” (Bantam, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever.
Honeybees (Apis mellifera, A. cerana, A. dorsata, A. floria, A. andreniformis, A. koschevnikov, and A. laborisa) play a key role in propagating numerous plants, flower nectar, and flower pollen as well as in pollinating approximately one-third of common agricultural crops, including fruits, vegetables, nuts, and seeds (Time magazine; Proc. Biol. Sci. 2007;274[1608]:303-13). Indeed, the honeybee is the lone insect that produces food regularly consumed by human beings (Am. J. Ther. 2014;21:304-23). Honey, which contains more than 180 compounds, is produced by honeybees from flower nectar. This sweet food product is supersaturated in sugar, and also contains phenolic acids, flavonoids, ascorbic acid, alpha-tocopherol, carotenoids, the enzymes glucose oxidase and catalase, organic and amino acids, and proteins (J. Food Sci. 2008;73:R117-24). Honey has been used since ancient times in Ayurvedic medicine to treat diabetes and has long been used to treat infected wounds (Ayu 2012;33:178-82; Clin. Infect. Dis. 2009;49:1541-9). Currently, honey is used in Ayurvedic medicine to treat acne, and it is incorporated in various cosmetic formulations such as facial washes, skin moisturizers, and hair conditioners (Ayu 2012;33:178-82).
History
For at least 2,700 years, traditional medical practice has included the use of topically applied honey for various conditions, with many modern researchers retrospectively attributing this usage to the antibacterial activity of honey (Am. J. Ther. 2014;21:304-23; Clin. Infect. Dis. 2008;46:1677-82). Honey served as a potent anti-inflammatory and antibacterial agent in folk remedies in ancient Egypt, Greece, and Rome, with written references to the medical application of bee products dating back to ancientEgypt, India, and China (Am. J. Ther. 2014;21:304-23; Cancer Res. 1993;53:1255-61; Evid. Based Complement. Alternat. Med. 2013;2013:697390)). For more than 4,000 years, honey has been used in Ayurvedic medicine, and its use has been traced to the Xin dynasty in China (Am. J. Ther. 2014;21:304-23). The antibacterial characteristics of honey were first reported in 1892 (IUBMB Life 2012;64:48-55). Russia and Germany used honey for wound treatment through World War I. The traditional medical application of honey began to subside with the advent of antibiotics in the 1940s(Burns 2013; 39:1514-25; Int. J. Clin. Pract. 2007;61:1705-7).
Chemistry
Myriad biological functions are associated with honey (antibacterial, antioxidant, antitumor, anti-inflammatory, antibrowning, and antiviral) and ascribed mainly to its constituent phenolic compounds, such as flavonoids, including chrysin (J. Food Sci. 2008;73:R117-24). Indeed, medical grade honeys such as manuka honey (a monofloral honey derived from Leptospermum scoparium, a member of the Myrtaceae family, native to New Zealand) and Medihoney® (a standardized mix of Australian and New Zealand honeys) are rich in flavonoids (Int. J. Clin. Pract. 2007;61:1705-7;J. Int. Acad. Periodontol. 2004;6:63-7; Evid. Based Complement. Alternat. Med. 2009;6:165-73;J. Agric. Food Chem. 2012;60:7229-37). Honey has a pH ranging from 3.2 to 4.5 and an acidity level that stymies the growth of many microorganisms (Burns 2013;39:1514-25; J. Clin. Nurs. 2008;17:2604-23; Nurs. Times. 2006;102:40-2; Br. J. Community Nurs. 2004;Suppl:S21-7 ).
Antibacterial activity
In 2008, Kwakman et al. found that within 24 hours, 10%-40% (vol/vol) medical grade honey (Revamil) destroyed antibiotic-susceptible and antibiotic-resistant isolates of Staphylococcus aureus,S. epidermidis, Enterococcus faecium, Escherichia coli, Pseudomonas aeruginosa, Enterobacter cloacae, and Klebsiella oxytoca. After 2 days of honey application, they also observed a 100-fold decrease in forearm skin colonization in healthy volunteers, with the number of positive skin cultures declining by 76%. The researchers concluded that Revamil exhibits significant potential to prevent or treat infections, including those spawned by multidrug-resistant bacteria (Clin. Infect. Dis. 2008;46:1677-82). Honey has been demonstrated to be clinically effective in treating several kinds of wound infections, reducing skin colonization of multiple bacteria, including methicillin-resistant S. aureus (Clin. Infect. Dis. 2008;46:1677-82) and enhancing wound healing, without provoking adverse effects ( Clin. Infect. Dis. 2009;49:1541-9). Manuka honey and Medihoney are the main forms of medical grade honey used in clinical practice. Nonmedical grade honey may contain viable bacterial spores (including clostridia), and manifest less predictable antibacterial properties (Clin. Infect. Dis. 2009;49:1541-9).
Honey is used in over-the-counter products as a moisturizing agent and in hair-conditioning products based on its strong humectant properties. It is also used in home remedies to treat burns, wounds, eczema, and dermatitis, especially in Asia (Ayu 2012;33:178-8).
Seborrheic dermatitis/dandruff
In 2001, Al-Waili assessed the potential of topically applied crude honey (90% honey diluted in warm water) to treat chronic seborrheic dermatitis of the scalp, face, and chest in 30 patients (20 males and 10 females, aged 15-60 years). Over the initial 4 weeks of treatment, honey was gently rubbed onto lesions every other day for 2-3 minutes at a time, with the ointment left on for 3 hours before gentle warm-water rinsing. Then, in a 6-month prophylactic phase, the participants were divided into a once-weekly treatment group and a control group. Skin lesions healed completely within 2 weeks in the treatment group, after significant reductions in itching and scaling in just the first week. Subjective improvements in hair loss were also reported. Relapse was observed in 12 of the 15 subjects in the control group within 2-4 months of therapy cessation and none in the treatment group. The author concluded that weekly use of crude honey significantly improves seborrheic dermatitis symptoms and related hair loss (Eur. J. Med. Res. 2001;6:306-8).
Wound healing
In February 2013, Jull published a review of 25 randomized and quasirandomized trials evaluating honey in the treatment of acute or chronic wounds, finding that honey might delay healing in partial- and full-thickness burns, compared with early excision and grafting, but it does not significantly enhance healing of chronic venous leg ulcers. They suggested that while honey may prove to be more effective than some conventional dressings for such ulcers, evidence is currently insufficient to support this claim ( Cochrane Database Syst. Rev. 2013;2:CD005083). Later that year, Vandamme et al. identified 55 studies in a literature review suggesting that honey stimulates healing of burns, ulcers, and other wounds. They also found, despite some methodologic concerns, that honey exerts antibacterial activity in burn treatment and deodorizing, debridement, anti-inflammatory, and analgesic activity ( Burns 2013;39:1514-25).
Conclusion
Honey has a long history of traditional medicinal use and has been found to display significant biologic activity, including antibacterial, antioxidant, antitumor, anti-inflammatory, antibrowning, and antiviral. The antibacterial properties of honey are particularly compelling. While more research, in the form of randomized, controlled trials, is needed prior to incorporating bee products into the dermatologic armamentarium as first-line therapies, the potential of honey usage for skin care is promising.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (McGraw-Hill, April 2002), and a book for consumers, “The Skin Type Solution” (Bantam, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever.
Honeybees (Apis mellifera, A. cerana, A. dorsata, A. floria, A. andreniformis, A. koschevnikov, and A. laborisa) play a key role in propagating numerous plants, flower nectar, and flower pollen as well as in pollinating approximately one-third of common agricultural crops, including fruits, vegetables, nuts, and seeds (Time magazine; Proc. Biol. Sci. 2007;274[1608]:303-13). Indeed, the honeybee is the lone insect that produces food regularly consumed by human beings (Am. J. Ther. 2014;21:304-23). Honey, which contains more than 180 compounds, is produced by honeybees from flower nectar. This sweet food product is supersaturated in sugar, and also contains phenolic acids, flavonoids, ascorbic acid, alpha-tocopherol, carotenoids, the enzymes glucose oxidase and catalase, organic and amino acids, and proteins (J. Food Sci. 2008;73:R117-24). Honey has been used since ancient times in Ayurvedic medicine to treat diabetes and has long been used to treat infected wounds (Ayu 2012;33:178-82; Clin. Infect. Dis. 2009;49:1541-9). Currently, honey is used in Ayurvedic medicine to treat acne, and it is incorporated in various cosmetic formulations such as facial washes, skin moisturizers, and hair conditioners (Ayu 2012;33:178-82).
History
For at least 2,700 years, traditional medical practice has included the use of topically applied honey for various conditions, with many modern researchers retrospectively attributing this usage to the antibacterial activity of honey (Am. J. Ther. 2014;21:304-23; Clin. Infect. Dis. 2008;46:1677-82). Honey served as a potent anti-inflammatory and antibacterial agent in folk remedies in ancient Egypt, Greece, and Rome, with written references to the medical application of bee products dating back to ancientEgypt, India, and China (Am. J. Ther. 2014;21:304-23; Cancer Res. 1993;53:1255-61; Evid. Based Complement. Alternat. Med. 2013;2013:697390)). For more than 4,000 years, honey has been used in Ayurvedic medicine, and its use has been traced to the Xin dynasty in China (Am. J. Ther. 2014;21:304-23). The antibacterial characteristics of honey were first reported in 1892 (IUBMB Life 2012;64:48-55). Russia and Germany used honey for wound treatment through World War I. The traditional medical application of honey began to subside with the advent of antibiotics in the 1940s(Burns 2013; 39:1514-25; Int. J. Clin. Pract. 2007;61:1705-7).
Chemistry
Myriad biological functions are associated with honey (antibacterial, antioxidant, antitumor, anti-inflammatory, antibrowning, and antiviral) and ascribed mainly to its constituent phenolic compounds, such as flavonoids, including chrysin (J. Food Sci. 2008;73:R117-24). Indeed, medical grade honeys such as manuka honey (a monofloral honey derived from Leptospermum scoparium, a member of the Myrtaceae family, native to New Zealand) and Medihoney® (a standardized mix of Australian and New Zealand honeys) are rich in flavonoids (Int. J. Clin. Pract. 2007;61:1705-7;J. Int. Acad. Periodontol. 2004;6:63-7; Evid. Based Complement. Alternat. Med. 2009;6:165-73;J. Agric. Food Chem. 2012;60:7229-37). Honey has a pH ranging from 3.2 to 4.5 and an acidity level that stymies the growth of many microorganisms (Burns 2013;39:1514-25; J. Clin. Nurs. 2008;17:2604-23; Nurs. Times. 2006;102:40-2; Br. J. Community Nurs. 2004;Suppl:S21-7 ).
Antibacterial activity
In 2008, Kwakman et al. found that within 24 hours, 10%-40% (vol/vol) medical grade honey (Revamil) destroyed antibiotic-susceptible and antibiotic-resistant isolates of Staphylococcus aureus,S. epidermidis, Enterococcus faecium, Escherichia coli, Pseudomonas aeruginosa, Enterobacter cloacae, and Klebsiella oxytoca. After 2 days of honey application, they also observed a 100-fold decrease in forearm skin colonization in healthy volunteers, with the number of positive skin cultures declining by 76%. The researchers concluded that Revamil exhibits significant potential to prevent or treat infections, including those spawned by multidrug-resistant bacteria (Clin. Infect. Dis. 2008;46:1677-82). Honey has been demonstrated to be clinically effective in treating several kinds of wound infections, reducing skin colonization of multiple bacteria, including methicillin-resistant S. aureus (Clin. Infect. Dis. 2008;46:1677-82) and enhancing wound healing, without provoking adverse effects ( Clin. Infect. Dis. 2009;49:1541-9). Manuka honey and Medihoney are the main forms of medical grade honey used in clinical practice. Nonmedical grade honey may contain viable bacterial spores (including clostridia), and manifest less predictable antibacterial properties (Clin. Infect. Dis. 2009;49:1541-9).
Honey is used in over-the-counter products as a moisturizing agent and in hair-conditioning products based on its strong humectant properties. It is also used in home remedies to treat burns, wounds, eczema, and dermatitis, especially in Asia (Ayu 2012;33:178-8).
Seborrheic dermatitis/dandruff
In 2001, Al-Waili assessed the potential of topically applied crude honey (90% honey diluted in warm water) to treat chronic seborrheic dermatitis of the scalp, face, and chest in 30 patients (20 males and 10 females, aged 15-60 years). Over the initial 4 weeks of treatment, honey was gently rubbed onto lesions every other day for 2-3 minutes at a time, with the ointment left on for 3 hours before gentle warm-water rinsing. Then, in a 6-month prophylactic phase, the participants were divided into a once-weekly treatment group and a control group. Skin lesions healed completely within 2 weeks in the treatment group, after significant reductions in itching and scaling in just the first week. Subjective improvements in hair loss were also reported. Relapse was observed in 12 of the 15 subjects in the control group within 2-4 months of therapy cessation and none in the treatment group. The author concluded that weekly use of crude honey significantly improves seborrheic dermatitis symptoms and related hair loss (Eur. J. Med. Res. 2001;6:306-8).
Wound healing
In February 2013, Jull published a review of 25 randomized and quasirandomized trials evaluating honey in the treatment of acute or chronic wounds, finding that honey might delay healing in partial- and full-thickness burns, compared with early excision and grafting, but it does not significantly enhance healing of chronic venous leg ulcers. They suggested that while honey may prove to be more effective than some conventional dressings for such ulcers, evidence is currently insufficient to support this claim ( Cochrane Database Syst. Rev. 2013;2:CD005083). Later that year, Vandamme et al. identified 55 studies in a literature review suggesting that honey stimulates healing of burns, ulcers, and other wounds. They also found, despite some methodologic concerns, that honey exerts antibacterial activity in burn treatment and deodorizing, debridement, anti-inflammatory, and analgesic activity ( Burns 2013;39:1514-25).
Conclusion
Honey has a long history of traditional medicinal use and has been found to display significant biologic activity, including antibacterial, antioxidant, antitumor, anti-inflammatory, antibrowning, and antiviral. The antibacterial properties of honey are particularly compelling. While more research, in the form of randomized, controlled trials, is needed prior to incorporating bee products into the dermatologic armamentarium as first-line therapies, the potential of honey usage for skin care is promising.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (McGraw-Hill, April 2002), and a book for consumers, “The Skin Type Solution” (Bantam, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever.
Depressed, suicidal, and brittle in her bones
CASE Broken down
Ms. E, age 20, is a college student who has had major depressive disorder for several years and a genetic bone disease (osteogenesis imperfecta, mixed type III and IV). She presents with depression, anxiety, and suicidal ideation. She reports recent worsening of her depressive symptoms, including anhedonia, excessive sleep, difficulty concentrating, and feeling overwhelmed, hopeless, and worthless. She also describes frequent thoughts of suicide with the plan of putting herself in oncoming traffic, although she has no history of suicide attempts.
Previously, her primary care physician prescribed lorazepam, 0.5 mg, as needed for anxiety, and sertraline, 100 mg/d, for depression and anxiety. She experienced only partial improvement in symptoms, however.
In addition to depressive symptoms, Ms. E describes manic symptoms lasting for as long as 3 to 5 days, including decreased need for sleep, increased energy, pressured speech, racing thoughts, distractibility, spending excessive money on cosmetics, and risking her safety—given her skeletal disorder— by participating in high-impact stage-combat classes. She denies auditory and visual hallucinations, homicidal ideation, and delusions.
The medical history is significant for osteogenesis imperfecta, which has caused 62 fractures and required 16 surgeries. Ms. E is a theater major who, despite her short stature and wheelchair use, reports enjoying her acting career and says she does not feel demoralized by her medical condition. She describes overcoming her physical disabilities with pride and confidence. However, her recent worsening mood symptoms have left her unable to concentrate and feeling overwhelmed with school.
Ms. E is voluntarily admitted to an inpatient psychiatric unit with a diagnosis of bipolar I disorder with rapid cycling, most recent episode mixed. Because of her bone fragility, the treatment team considers what would be an appropriate course of drug treatment to control bipolar symptoms while minimizing risk of bone loss.
Which medications are associated with decreased bone mineral density?
a) citalopram
b) haloperidol
c) carbamazepine
d) paliperidone
e) all of the above
The authors’ observations
Osteogenesis imperfecta is a genetic condition caused by mutations in genes implicated in collagen production. As a result, bones are brittle and prone to fracture. Different classes of psychotropics have been shown to increase risk of bone fractures through a variety of mechanisms. Clinicians often must choose appropriate pharmacotherapy for patients at high risk of fracture, including postmenopausal women, older patients, malnourished persons, and those with hormonal deficiencies leading to osteoporosis.
To assist our clinical decision-making, we reviewed the literature to establish appropriate management of a patient with increased bone fragility and new-onset bipolar disorder. We considered all classes of medications used to treat bipolar disorder, including antipsychotics, antidepressants, lithium, and anticonvulsants.
Antipsychotics
In population-based studies, prolactin-elevating antipsychotics have been associated with decreased bone mineral density and increased risk of fracture.1 Additional studies on geriatric and non-geriatric populations have supported these findings.2,3
The mechanism through which fracture risk is increased likely is related to antipsychotics’ effect on serum prolactin and cortisol levels. Antipsychotics act as antagonists on D2 receptors in the hypothalamic tubero-infundibular pathway, therefore preventing inhibition of prolactin. Long-term elevation in serum prolactin can cause loss of bone mineral density through secondary hypogonadism and direct effects on target tissues. Additional modifying factors include smoking and estrogen use.
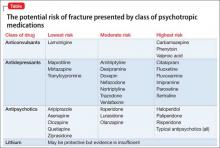
The degree to which antipsychotics increase fracture risk might be related to the degree of serum prolactin elevation.4 Antipsychotics previously have been grouped by the degree of prolactin elevation, categorizing them as high, medium, and low or no potential to elevate serum prolactin.4 Based on this classification, typical antipsychotics, risperidone, and paliperidone have the highest potential to elevate prolactin. Accordingly, antipsychotics with the lowest fracture risk are those that have the lowest risk of serum prolactin elevation: ziprasidone, asenapine, quetiapine, and clozapine. Aripiprazole may lower prolactin in some patients. This is supported by studies noting reduced bone mineral density5,6 and increased risk of fracture1 with high-potential vs low- or no-potential antipsychotics. Because of these findings, it is crucial to consider the potential risk of prolactin elevation when treating patients at increased risk of fracture. Providers should consider low/no potential antipsychotic medications before considering those with medium or high potential (Table).
Antidepressants
In a meta-analysis, antidepressants were shown to increase fracture risk by 70% to 90%.2 However, the relative risk varies by antidepressant class. Several studies have shown that selective serotonin reuptake inhibitors (SSRIs) are associated with a higher risk of fracture compared with tricyclic antidepressants (TCAs).7 In addition, antidepressants with a high affinity for the serotonin transporter, including citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and imipramine, have been associated with greater risk of osteoporotic fracture compared with those with low affinity.8
The mechanisms by which antidepressants increase fracture risk are complex, although the strongest evidence implicates a direct effect on bone metabolism via the 5-HTT receptor. This receptor, found on osteoblasts and osteoclasts, plays an important role in bone metabolism; it is through this receptor that SSRIs might inhibit osteoblasts and promote osteoclast activity, thereby disrupting bone microarchitecture. Additional studies are needed to further describe the mechanism of the association among antidepressants, bone mineral density, and fracture risk.
Fracture risk is associated with duration of use rather than dosage. Population-based studies show a higher fracture risk for new users of TCAs compared with continuous users, and the risk of fracture with SSRIs seems to increase slightly over time.9 No association has been identified between fracture risk and antidepressant dosage. According to the literature, drugs with low affinity for the serotonin transporter, such as maprotiline and mirtazapine, likely are the safest antidepressants for patients at increased risk of fracture. Options also include other TCAs and any antidepressant with low affinity for the serotonin receptor.7,8
Lithium
Studies on lithium and bone mineral density have shown mixed results. Older studies found that lithium had a negative or no effect on bone mineral density or the parathyroid hormone level.10 More recent investigations, however, suggest that the drug has a protective effect on bone mineral density, although this has not been replicated in all studies.
In a mouse model, lithium has been shown to enhance bone formation and improve bone mass, at least in part by activation of the Wnt signaling pathway through an inhibitory effect on glycogen synthase kinase-3β.11 In humans, lithium-treated adults had lower serum alkaline phosphate, osteocalcin, and C-telopeptide levels compared with controls, suggesting a state of decreased bone remodeling and increased turnover.12 There is a paucity of clinical data on the effect of lithium on fracture risk. Additional studies are necessary to elucidate lithium’s mechanism on bone mineral density and determine the magnitude of the clinical effect.
Anticonvulsants
The association among anticonvulsants, decreased bone mineral density, and increased risk of fracture is well-established in the literature.13 However, causality is difficult to determine, because many studies were of patients with a seizure disorder, who often have additional risk factors for fracture, including seizure-related trauma, drowsiness, and slowed reflexes.
Mechanisms through which anticonvulsants increase fracture risk include increased bone resorption, secondary hypoparathyroidism, and pseudohypoparathyroidism. Markers of bone resorption were elevated in patients receiving an antiepileptic.14 This effect might be enhanced by co-administration of cytochrome P450 (CYP450) enzyme-inducing anticonvulsants and CYP450 enzyme-inhibiting medications, such as valproate. Long-term treatment with valproate may produce reduction of bone mass and increased risk of fractures; however, other studies disagree with this finding.15
In addition to CYP450-inducing effects, phenytoin, carbamezapine, and phenobarbital can increase catabolism of vitamin D, which is associated with osteomalacia.14 This results in decreased intestinal absorption of calcium, hypocalcemia, and secondary hyperparathyroidism, which also increases fracture risk. Anticonvulsants also might increase resistance to pseudohypoparathyroidism and inhibit calcitonin secretion.
Lamotrigine has not been shown to interfere with bone accrual16 and may be a safer mood stabilizer for patients at high risk of fracture. For patients at increased risk of fracture, it is important to select an anticonvulsant wisely to minimize fracture risk.
How would you treat Ms. E during her hospitalization for bipolar disorder?
a) carbamazepine
b) lithium
c) risperidone
d) mirtazapine
TREATMENT Minimizing polypharmacy
Because many pharmacotherapeutic options for managing bipolar disorder can increase the risk of fracture, clinicians must be aware of the relative risk of each class of medication and each individual drug. We initiated lithium, 300 mg, 3 times a day, to stabilize Ms. E’s mood. Although clinical data are inconclusive regarding lithium’s effect on fracture risk, we felt that the benefit of acute mood stabilization outweighed the risk of decreased bone mineral index.
We selected aripiprazole, 10 mg/d, as an adjunctive treatment because of its minimal effect on serum prolactin levels.4 We considered prescribing an antidepressant but decided against it because we were concerned about manic switching.
Polypharmacy is another important consideration for Ms. E. Several studies have identified polypharmacy, particularly with antipsychotics, as an independent risk factor for fracture.3 Therefore, we sought to minimize the number of medications Ms. E receives. Although lithium monotherapy is an option, we thought that her mood symptoms were severe enough that the risk of inadequately treating her bipolar symptoms outweighed the additional risk of fracture from dual therapy with lithium and aripiprazole. Untreated or inadequately treated depression is associated with a higher fracture risk. Therefore, we avoided prescribing >2 medications to mitigate any excessive risk of fracture from polypharmacy.
Bottom Line
Different classes of medications—antipsychotics, anticonvulsants, antidepressants, and lithium—used for treating bipolar disorder have been shown to increase risk of bone fracture through a variety of mechanisms. Anticonvulsants and prolactin-elevating antipsychotics are associated with increased fracture risk; evidence on lithium is mixed. Fracture risk with antidepressants is associated with duration of use, rather than dosage.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Howard L, Kirkwood G, Leese M. Risk of hip fracture in patients with a history of schizophrenia. Br J Psychiatry. 2007;190:129-134.
2. Takkouche B, Montes-Martínez A, Gill SS, et al. Psychotropic medications and the risk of fracture: a meta-analysis. Drug Saf. 2007;30(2):171-184.
3. Sørensen HJ, Jensen SO, Nielsen J. Schizophrenia, antipsychotics and risk of hip fracture: a population-based analysis. Eur Neuropsychopharmacol. 2013;23(8):872-878.
4. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
5. Bilici M, Cakirbay H, Guler M, et al. Classical and atypical neuroleptics, and bone mineral density, in patients with schizophrenia. Int J Neurosci. 2002;112(7):817-828.
6. Becker D, Liver O, Mester R, et al. Risperidone, but not olanzapine, decreases bone mineral density in female premenopausal schizophrenia patients. J Clin Psychiatry. 2003;64(7):761-766.
7. Bolton JM, Metge C, Lix L, et al. Fracture risk from psychotropic medications: a population-based analysis. J Clin Psychopharmacol. 2008;28(4):384-391.
8. Verdel BM, Souverein PC, Egberts TC, et al. Use of antidepressant drugs and risk of osteoporotic and non-osteoporotic fractures. Bone. 2010;47(3):604-609.
9. Diem SJ, Ruppert K, Cauley JA. Rates of bone loss among women initiating antidepressant medication use in midlife. J Clin Endocrinol Metab. 2013;(11):4355-4363.
10. Plenge P, Rafaelsen OJ. Lithium effects on calcium, magnesium and phosphate in man: effects on balance, bone mineral content, faecal and urinary excretion. Acta Psychiatr Scand. 1982;66(5):361-373.
11. Clément-Lacroix P, Ai M, Morvan F, et al. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci U S A. 2005;102(48):17406-17411.
12. Zamani A, Omrani GR, Nasab MM. Lithium’s effect on bone mineral density. Bone. 2009;44(2):331-334.
13. Swanton J, Simister R, Altmann D, et al. Bone mineral density in institutionalised patients with refractory epilepsy. Seizure. 2007;16(6):538-541.
14. Pack AM, Morrell MJ. Epilepsy and bone health in adults. Epilepsy Behav. 2004;5(suppl 2):S24-S29.
15. Pack AM. Bone disease in epilepsy. Curr Neurol Neurosci Rep. 2004;4(4):329-334.
16. Sheth RD, Hermann BP. Bone mineral density with lamotrigine monotherapy for epilepsy. Pediatr Neurol. 2007;37(4):250-254.
CASE Broken down
Ms. E, age 20, is a college student who has had major depressive disorder for several years and a genetic bone disease (osteogenesis imperfecta, mixed type III and IV). She presents with depression, anxiety, and suicidal ideation. She reports recent worsening of her depressive symptoms, including anhedonia, excessive sleep, difficulty concentrating, and feeling overwhelmed, hopeless, and worthless. She also describes frequent thoughts of suicide with the plan of putting herself in oncoming traffic, although she has no history of suicide attempts.
Previously, her primary care physician prescribed lorazepam, 0.5 mg, as needed for anxiety, and sertraline, 100 mg/d, for depression and anxiety. She experienced only partial improvement in symptoms, however.
In addition to depressive symptoms, Ms. E describes manic symptoms lasting for as long as 3 to 5 days, including decreased need for sleep, increased energy, pressured speech, racing thoughts, distractibility, spending excessive money on cosmetics, and risking her safety—given her skeletal disorder— by participating in high-impact stage-combat classes. She denies auditory and visual hallucinations, homicidal ideation, and delusions.
The medical history is significant for osteogenesis imperfecta, which has caused 62 fractures and required 16 surgeries. Ms. E is a theater major who, despite her short stature and wheelchair use, reports enjoying her acting career and says she does not feel demoralized by her medical condition. She describes overcoming her physical disabilities with pride and confidence. However, her recent worsening mood symptoms have left her unable to concentrate and feeling overwhelmed with school.
Ms. E is voluntarily admitted to an inpatient psychiatric unit with a diagnosis of bipolar I disorder with rapid cycling, most recent episode mixed. Because of her bone fragility, the treatment team considers what would be an appropriate course of drug treatment to control bipolar symptoms while minimizing risk of bone loss.
Which medications are associated with decreased bone mineral density?
a) citalopram
b) haloperidol
c) carbamazepine
d) paliperidone
e) all of the above
The authors’ observations
Osteogenesis imperfecta is a genetic condition caused by mutations in genes implicated in collagen production. As a result, bones are brittle and prone to fracture. Different classes of psychotropics have been shown to increase risk of bone fractures through a variety of mechanisms. Clinicians often must choose appropriate pharmacotherapy for patients at high risk of fracture, including postmenopausal women, older patients, malnourished persons, and those with hormonal deficiencies leading to osteoporosis.
To assist our clinical decision-making, we reviewed the literature to establish appropriate management of a patient with increased bone fragility and new-onset bipolar disorder. We considered all classes of medications used to treat bipolar disorder, including antipsychotics, antidepressants, lithium, and anticonvulsants.
Antipsychotics
In population-based studies, prolactin-elevating antipsychotics have been associated with decreased bone mineral density and increased risk of fracture.1 Additional studies on geriatric and non-geriatric populations have supported these findings.2,3
The mechanism through which fracture risk is increased likely is related to antipsychotics’ effect on serum prolactin and cortisol levels. Antipsychotics act as antagonists on D2 receptors in the hypothalamic tubero-infundibular pathway, therefore preventing inhibition of prolactin. Long-term elevation in serum prolactin can cause loss of bone mineral density through secondary hypogonadism and direct effects on target tissues. Additional modifying factors include smoking and estrogen use.
The degree to which antipsychotics increase fracture risk might be related to the degree of serum prolactin elevation.4 Antipsychotics previously have been grouped by the degree of prolactin elevation, categorizing them as high, medium, and low or no potential to elevate serum prolactin.4 Based on this classification, typical antipsychotics, risperidone, and paliperidone have the highest potential to elevate prolactin. Accordingly, antipsychotics with the lowest fracture risk are those that have the lowest risk of serum prolactin elevation: ziprasidone, asenapine, quetiapine, and clozapine. Aripiprazole may lower prolactin in some patients. This is supported by studies noting reduced bone mineral density5,6 and increased risk of fracture1 with high-potential vs low- or no-potential antipsychotics. Because of these findings, it is crucial to consider the potential risk of prolactin elevation when treating patients at increased risk of fracture. Providers should consider low/no potential antipsychotic medications before considering those with medium or high potential (Table).
Antidepressants
In a meta-analysis, antidepressants were shown to increase fracture risk by 70% to 90%.2 However, the relative risk varies by antidepressant class. Several studies have shown that selective serotonin reuptake inhibitors (SSRIs) are associated with a higher risk of fracture compared with tricyclic antidepressants (TCAs).7 In addition, antidepressants with a high affinity for the serotonin transporter, including citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and imipramine, have been associated with greater risk of osteoporotic fracture compared with those with low affinity.8
The mechanisms by which antidepressants increase fracture risk are complex, although the strongest evidence implicates a direct effect on bone metabolism via the 5-HTT receptor. This receptor, found on osteoblasts and osteoclasts, plays an important role in bone metabolism; it is through this receptor that SSRIs might inhibit osteoblasts and promote osteoclast activity, thereby disrupting bone microarchitecture. Additional studies are needed to further describe the mechanism of the association among antidepressants, bone mineral density, and fracture risk.
Fracture risk is associated with duration of use rather than dosage. Population-based studies show a higher fracture risk for new users of TCAs compared with continuous users, and the risk of fracture with SSRIs seems to increase slightly over time.9 No association has been identified between fracture risk and antidepressant dosage. According to the literature, drugs with low affinity for the serotonin transporter, such as maprotiline and mirtazapine, likely are the safest antidepressants for patients at increased risk of fracture. Options also include other TCAs and any antidepressant with low affinity for the serotonin receptor.7,8
Lithium
Studies on lithium and bone mineral density have shown mixed results. Older studies found that lithium had a negative or no effect on bone mineral density or the parathyroid hormone level.10 More recent investigations, however, suggest that the drug has a protective effect on bone mineral density, although this has not been replicated in all studies.
In a mouse model, lithium has been shown to enhance bone formation and improve bone mass, at least in part by activation of the Wnt signaling pathway through an inhibitory effect on glycogen synthase kinase-3β.11 In humans, lithium-treated adults had lower serum alkaline phosphate, osteocalcin, and C-telopeptide levels compared with controls, suggesting a state of decreased bone remodeling and increased turnover.12 There is a paucity of clinical data on the effect of lithium on fracture risk. Additional studies are necessary to elucidate lithium’s mechanism on bone mineral density and determine the magnitude of the clinical effect.
Anticonvulsants
The association among anticonvulsants, decreased bone mineral density, and increased risk of fracture is well-established in the literature.13 However, causality is difficult to determine, because many studies were of patients with a seizure disorder, who often have additional risk factors for fracture, including seizure-related trauma, drowsiness, and slowed reflexes.
Mechanisms through which anticonvulsants increase fracture risk include increased bone resorption, secondary hypoparathyroidism, and pseudohypoparathyroidism. Markers of bone resorption were elevated in patients receiving an antiepileptic.14 This effect might be enhanced by co-administration of cytochrome P450 (CYP450) enzyme-inducing anticonvulsants and CYP450 enzyme-inhibiting medications, such as valproate. Long-term treatment with valproate may produce reduction of bone mass and increased risk of fractures; however, other studies disagree with this finding.15
In addition to CYP450-inducing effects, phenytoin, carbamezapine, and phenobarbital can increase catabolism of vitamin D, which is associated with osteomalacia.14 This results in decreased intestinal absorption of calcium, hypocalcemia, and secondary hyperparathyroidism, which also increases fracture risk. Anticonvulsants also might increase resistance to pseudohypoparathyroidism and inhibit calcitonin secretion.
Lamotrigine has not been shown to interfere with bone accrual16 and may be a safer mood stabilizer for patients at high risk of fracture. For patients at increased risk of fracture, it is important to select an anticonvulsant wisely to minimize fracture risk.
How would you treat Ms. E during her hospitalization for bipolar disorder?
a) carbamazepine
b) lithium
c) risperidone
d) mirtazapine
TREATMENT Minimizing polypharmacy
Because many pharmacotherapeutic options for managing bipolar disorder can increase the risk of fracture, clinicians must be aware of the relative risk of each class of medication and each individual drug. We initiated lithium, 300 mg, 3 times a day, to stabilize Ms. E’s mood. Although clinical data are inconclusive regarding lithium’s effect on fracture risk, we felt that the benefit of acute mood stabilization outweighed the risk of decreased bone mineral index.
We selected aripiprazole, 10 mg/d, as an adjunctive treatment because of its minimal effect on serum prolactin levels.4 We considered prescribing an antidepressant but decided against it because we were concerned about manic switching.
Polypharmacy is another important consideration for Ms. E. Several studies have identified polypharmacy, particularly with antipsychotics, as an independent risk factor for fracture.3 Therefore, we sought to minimize the number of medications Ms. E receives. Although lithium monotherapy is an option, we thought that her mood symptoms were severe enough that the risk of inadequately treating her bipolar symptoms outweighed the additional risk of fracture from dual therapy with lithium and aripiprazole. Untreated or inadequately treated depression is associated with a higher fracture risk. Therefore, we avoided prescribing >2 medications to mitigate any excessive risk of fracture from polypharmacy.
Bottom Line
Different classes of medications—antipsychotics, anticonvulsants, antidepressants, and lithium—used for treating bipolar disorder have been shown to increase risk of bone fracture through a variety of mechanisms. Anticonvulsants and prolactin-elevating antipsychotics are associated with increased fracture risk; evidence on lithium is mixed. Fracture risk with antidepressants is associated with duration of use, rather than dosage.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Broken down
Ms. E, age 20, is a college student who has had major depressive disorder for several years and a genetic bone disease (osteogenesis imperfecta, mixed type III and IV). She presents with depression, anxiety, and suicidal ideation. She reports recent worsening of her depressive symptoms, including anhedonia, excessive sleep, difficulty concentrating, and feeling overwhelmed, hopeless, and worthless. She also describes frequent thoughts of suicide with the plan of putting herself in oncoming traffic, although she has no history of suicide attempts.
Previously, her primary care physician prescribed lorazepam, 0.5 mg, as needed for anxiety, and sertraline, 100 mg/d, for depression and anxiety. She experienced only partial improvement in symptoms, however.
In addition to depressive symptoms, Ms. E describes manic symptoms lasting for as long as 3 to 5 days, including decreased need for sleep, increased energy, pressured speech, racing thoughts, distractibility, spending excessive money on cosmetics, and risking her safety—given her skeletal disorder— by participating in high-impact stage-combat classes. She denies auditory and visual hallucinations, homicidal ideation, and delusions.
The medical history is significant for osteogenesis imperfecta, which has caused 62 fractures and required 16 surgeries. Ms. E is a theater major who, despite her short stature and wheelchair use, reports enjoying her acting career and says she does not feel demoralized by her medical condition. She describes overcoming her physical disabilities with pride and confidence. However, her recent worsening mood symptoms have left her unable to concentrate and feeling overwhelmed with school.
Ms. E is voluntarily admitted to an inpatient psychiatric unit with a diagnosis of bipolar I disorder with rapid cycling, most recent episode mixed. Because of her bone fragility, the treatment team considers what would be an appropriate course of drug treatment to control bipolar symptoms while minimizing risk of bone loss.
Which medications are associated with decreased bone mineral density?
a) citalopram
b) haloperidol
c) carbamazepine
d) paliperidone
e) all of the above
The authors’ observations
Osteogenesis imperfecta is a genetic condition caused by mutations in genes implicated in collagen production. As a result, bones are brittle and prone to fracture. Different classes of psychotropics have been shown to increase risk of bone fractures through a variety of mechanisms. Clinicians often must choose appropriate pharmacotherapy for patients at high risk of fracture, including postmenopausal women, older patients, malnourished persons, and those with hormonal deficiencies leading to osteoporosis.
To assist our clinical decision-making, we reviewed the literature to establish appropriate management of a patient with increased bone fragility and new-onset bipolar disorder. We considered all classes of medications used to treat bipolar disorder, including antipsychotics, antidepressants, lithium, and anticonvulsants.
Antipsychotics
In population-based studies, prolactin-elevating antipsychotics have been associated with decreased bone mineral density and increased risk of fracture.1 Additional studies on geriatric and non-geriatric populations have supported these findings.2,3
The mechanism through which fracture risk is increased likely is related to antipsychotics’ effect on serum prolactin and cortisol levels. Antipsychotics act as antagonists on D2 receptors in the hypothalamic tubero-infundibular pathway, therefore preventing inhibition of prolactin. Long-term elevation in serum prolactin can cause loss of bone mineral density through secondary hypogonadism and direct effects on target tissues. Additional modifying factors include smoking and estrogen use.
The degree to which antipsychotics increase fracture risk might be related to the degree of serum prolactin elevation.4 Antipsychotics previously have been grouped by the degree of prolactin elevation, categorizing them as high, medium, and low or no potential to elevate serum prolactin.4 Based on this classification, typical antipsychotics, risperidone, and paliperidone have the highest potential to elevate prolactin. Accordingly, antipsychotics with the lowest fracture risk are those that have the lowest risk of serum prolactin elevation: ziprasidone, asenapine, quetiapine, and clozapine. Aripiprazole may lower prolactin in some patients. This is supported by studies noting reduced bone mineral density5,6 and increased risk of fracture1 with high-potential vs low- or no-potential antipsychotics. Because of these findings, it is crucial to consider the potential risk of prolactin elevation when treating patients at increased risk of fracture. Providers should consider low/no potential antipsychotic medications before considering those with medium or high potential (Table).
Antidepressants
In a meta-analysis, antidepressants were shown to increase fracture risk by 70% to 90%.2 However, the relative risk varies by antidepressant class. Several studies have shown that selective serotonin reuptake inhibitors (SSRIs) are associated with a higher risk of fracture compared with tricyclic antidepressants (TCAs).7 In addition, antidepressants with a high affinity for the serotonin transporter, including citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and imipramine, have been associated with greater risk of osteoporotic fracture compared with those with low affinity.8
The mechanisms by which antidepressants increase fracture risk are complex, although the strongest evidence implicates a direct effect on bone metabolism via the 5-HTT receptor. This receptor, found on osteoblasts and osteoclasts, plays an important role in bone metabolism; it is through this receptor that SSRIs might inhibit osteoblasts and promote osteoclast activity, thereby disrupting bone microarchitecture. Additional studies are needed to further describe the mechanism of the association among antidepressants, bone mineral density, and fracture risk.
Fracture risk is associated with duration of use rather than dosage. Population-based studies show a higher fracture risk for new users of TCAs compared with continuous users, and the risk of fracture with SSRIs seems to increase slightly over time.9 No association has been identified between fracture risk and antidepressant dosage. According to the literature, drugs with low affinity for the serotonin transporter, such as maprotiline and mirtazapine, likely are the safest antidepressants for patients at increased risk of fracture. Options also include other TCAs and any antidepressant with low affinity for the serotonin receptor.7,8
Lithium
Studies on lithium and bone mineral density have shown mixed results. Older studies found that lithium had a negative or no effect on bone mineral density or the parathyroid hormone level.10 More recent investigations, however, suggest that the drug has a protective effect on bone mineral density, although this has not been replicated in all studies.
In a mouse model, lithium has been shown to enhance bone formation and improve bone mass, at least in part by activation of the Wnt signaling pathway through an inhibitory effect on glycogen synthase kinase-3β.11 In humans, lithium-treated adults had lower serum alkaline phosphate, osteocalcin, and C-telopeptide levels compared with controls, suggesting a state of decreased bone remodeling and increased turnover.12 There is a paucity of clinical data on the effect of lithium on fracture risk. Additional studies are necessary to elucidate lithium’s mechanism on bone mineral density and determine the magnitude of the clinical effect.
Anticonvulsants
The association among anticonvulsants, decreased bone mineral density, and increased risk of fracture is well-established in the literature.13 However, causality is difficult to determine, because many studies were of patients with a seizure disorder, who often have additional risk factors for fracture, including seizure-related trauma, drowsiness, and slowed reflexes.
Mechanisms through which anticonvulsants increase fracture risk include increased bone resorption, secondary hypoparathyroidism, and pseudohypoparathyroidism. Markers of bone resorption were elevated in patients receiving an antiepileptic.14 This effect might be enhanced by co-administration of cytochrome P450 (CYP450) enzyme-inducing anticonvulsants and CYP450 enzyme-inhibiting medications, such as valproate. Long-term treatment with valproate may produce reduction of bone mass and increased risk of fractures; however, other studies disagree with this finding.15
In addition to CYP450-inducing effects, phenytoin, carbamezapine, and phenobarbital can increase catabolism of vitamin D, which is associated with osteomalacia.14 This results in decreased intestinal absorption of calcium, hypocalcemia, and secondary hyperparathyroidism, which also increases fracture risk. Anticonvulsants also might increase resistance to pseudohypoparathyroidism and inhibit calcitonin secretion.
Lamotrigine has not been shown to interfere with bone accrual16 and may be a safer mood stabilizer for patients at high risk of fracture. For patients at increased risk of fracture, it is important to select an anticonvulsant wisely to minimize fracture risk.
How would you treat Ms. E during her hospitalization for bipolar disorder?
a) carbamazepine
b) lithium
c) risperidone
d) mirtazapine
TREATMENT Minimizing polypharmacy
Because many pharmacotherapeutic options for managing bipolar disorder can increase the risk of fracture, clinicians must be aware of the relative risk of each class of medication and each individual drug. We initiated lithium, 300 mg, 3 times a day, to stabilize Ms. E’s mood. Although clinical data are inconclusive regarding lithium’s effect on fracture risk, we felt that the benefit of acute mood stabilization outweighed the risk of decreased bone mineral index.
We selected aripiprazole, 10 mg/d, as an adjunctive treatment because of its minimal effect on serum prolactin levels.4 We considered prescribing an antidepressant but decided against it because we were concerned about manic switching.
Polypharmacy is another important consideration for Ms. E. Several studies have identified polypharmacy, particularly with antipsychotics, as an independent risk factor for fracture.3 Therefore, we sought to minimize the number of medications Ms. E receives. Although lithium monotherapy is an option, we thought that her mood symptoms were severe enough that the risk of inadequately treating her bipolar symptoms outweighed the additional risk of fracture from dual therapy with lithium and aripiprazole. Untreated or inadequately treated depression is associated with a higher fracture risk. Therefore, we avoided prescribing >2 medications to mitigate any excessive risk of fracture from polypharmacy.
Bottom Line
Different classes of medications—antipsychotics, anticonvulsants, antidepressants, and lithium—used for treating bipolar disorder have been shown to increase risk of bone fracture through a variety of mechanisms. Anticonvulsants and prolactin-elevating antipsychotics are associated with increased fracture risk; evidence on lithium is mixed. Fracture risk with antidepressants is associated with duration of use, rather than dosage.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Howard L, Kirkwood G, Leese M. Risk of hip fracture in patients with a history of schizophrenia. Br J Psychiatry. 2007;190:129-134.
2. Takkouche B, Montes-Martínez A, Gill SS, et al. Psychotropic medications and the risk of fracture: a meta-analysis. Drug Saf. 2007;30(2):171-184.
3. Sørensen HJ, Jensen SO, Nielsen J. Schizophrenia, antipsychotics and risk of hip fracture: a population-based analysis. Eur Neuropsychopharmacol. 2013;23(8):872-878.
4. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
5. Bilici M, Cakirbay H, Guler M, et al. Classical and atypical neuroleptics, and bone mineral density, in patients with schizophrenia. Int J Neurosci. 2002;112(7):817-828.
6. Becker D, Liver O, Mester R, et al. Risperidone, but not olanzapine, decreases bone mineral density in female premenopausal schizophrenia patients. J Clin Psychiatry. 2003;64(7):761-766.
7. Bolton JM, Metge C, Lix L, et al. Fracture risk from psychotropic medications: a population-based analysis. J Clin Psychopharmacol. 2008;28(4):384-391.
8. Verdel BM, Souverein PC, Egberts TC, et al. Use of antidepressant drugs and risk of osteoporotic and non-osteoporotic fractures. Bone. 2010;47(3):604-609.
9. Diem SJ, Ruppert K, Cauley JA. Rates of bone loss among women initiating antidepressant medication use in midlife. J Clin Endocrinol Metab. 2013;(11):4355-4363.
10. Plenge P, Rafaelsen OJ. Lithium effects on calcium, magnesium and phosphate in man: effects on balance, bone mineral content, faecal and urinary excretion. Acta Psychiatr Scand. 1982;66(5):361-373.
11. Clément-Lacroix P, Ai M, Morvan F, et al. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci U S A. 2005;102(48):17406-17411.
12. Zamani A, Omrani GR, Nasab MM. Lithium’s effect on bone mineral density. Bone. 2009;44(2):331-334.
13. Swanton J, Simister R, Altmann D, et al. Bone mineral density in institutionalised patients with refractory epilepsy. Seizure. 2007;16(6):538-541.
14. Pack AM, Morrell MJ. Epilepsy and bone health in adults. Epilepsy Behav. 2004;5(suppl 2):S24-S29.
15. Pack AM. Bone disease in epilepsy. Curr Neurol Neurosci Rep. 2004;4(4):329-334.
16. Sheth RD, Hermann BP. Bone mineral density with lamotrigine monotherapy for epilepsy. Pediatr Neurol. 2007;37(4):250-254.
1. Howard L, Kirkwood G, Leese M. Risk of hip fracture in patients with a history of schizophrenia. Br J Psychiatry. 2007;190:129-134.
2. Takkouche B, Montes-Martínez A, Gill SS, et al. Psychotropic medications and the risk of fracture: a meta-analysis. Drug Saf. 2007;30(2):171-184.
3. Sørensen HJ, Jensen SO, Nielsen J. Schizophrenia, antipsychotics and risk of hip fracture: a population-based analysis. Eur Neuropsychopharmacol. 2013;23(8):872-878.
4. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
5. Bilici M, Cakirbay H, Guler M, et al. Classical and atypical neuroleptics, and bone mineral density, in patients with schizophrenia. Int J Neurosci. 2002;112(7):817-828.
6. Becker D, Liver O, Mester R, et al. Risperidone, but not olanzapine, decreases bone mineral density in female premenopausal schizophrenia patients. J Clin Psychiatry. 2003;64(7):761-766.
7. Bolton JM, Metge C, Lix L, et al. Fracture risk from psychotropic medications: a population-based analysis. J Clin Psychopharmacol. 2008;28(4):384-391.
8. Verdel BM, Souverein PC, Egberts TC, et al. Use of antidepressant drugs and risk of osteoporotic and non-osteoporotic fractures. Bone. 2010;47(3):604-609.
9. Diem SJ, Ruppert K, Cauley JA. Rates of bone loss among women initiating antidepressant medication use in midlife. J Clin Endocrinol Metab. 2013;(11):4355-4363.
10. Plenge P, Rafaelsen OJ. Lithium effects on calcium, magnesium and phosphate in man: effects on balance, bone mineral content, faecal and urinary excretion. Acta Psychiatr Scand. 1982;66(5):361-373.
11. Clément-Lacroix P, Ai M, Morvan F, et al. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci U S A. 2005;102(48):17406-17411.
12. Zamani A, Omrani GR, Nasab MM. Lithium’s effect on bone mineral density. Bone. 2009;44(2):331-334.
13. Swanton J, Simister R, Altmann D, et al. Bone mineral density in institutionalised patients with refractory epilepsy. Seizure. 2007;16(6):538-541.
14. Pack AM, Morrell MJ. Epilepsy and bone health in adults. Epilepsy Behav. 2004;5(suppl 2):S24-S29.
15. Pack AM. Bone disease in epilepsy. Curr Neurol Neurosci Rep. 2004;4(4):329-334.
16. Sheth RD, Hermann BP. Bone mineral density with lamotrigine monotherapy for epilepsy. Pediatr Neurol. 2007;37(4):250-254.
Man Awakens With "Fluttering" in His Chest
ANSWER
This ECG is consistent with coarse atrial fibrillation with a rapid ventricular response and a nonspecific T-wave abnormality. The patient’s presentation is strongly suggestive of lone atrial fibrillation: This was the first incidence, it occurred in the absence of an existing heart condition, and it presented with an abrupt onset of increased heart rate and dyspnea.
Lone atrial fibrillation most commonly occurs in men in their 40s and 50s. It is vagally mediated, occurring during sleep or relaxation and after food and/or alcohol consumption.
The patient was cardioverted to normal sinus rhythm in the ED without difficulty, and follow-up was arranged.
ANSWER
This ECG is consistent with coarse atrial fibrillation with a rapid ventricular response and a nonspecific T-wave abnormality. The patient’s presentation is strongly suggestive of lone atrial fibrillation: This was the first incidence, it occurred in the absence of an existing heart condition, and it presented with an abrupt onset of increased heart rate and dyspnea.
Lone atrial fibrillation most commonly occurs in men in their 40s and 50s. It is vagally mediated, occurring during sleep or relaxation and after food and/or alcohol consumption.
The patient was cardioverted to normal sinus rhythm in the ED without difficulty, and follow-up was arranged.
ANSWER
This ECG is consistent with coarse atrial fibrillation with a rapid ventricular response and a nonspecific T-wave abnormality. The patient’s presentation is strongly suggestive of lone atrial fibrillation: This was the first incidence, it occurred in the absence of an existing heart condition, and it presented with an abrupt onset of increased heart rate and dyspnea.
Lone atrial fibrillation most commonly occurs in men in their 40s and 50s. It is vagally mediated, occurring during sleep or relaxation and after food and/or alcohol consumption.
The patient was cardioverted to normal sinus rhythm in the ED without difficulty, and follow-up was arranged.
A 56-year-old man presents to the emergency department (ED) complaining of shortness of breath and a rapid heart rate. He went to bed at his regular time (10:30 pm) last night and woke up at 3:30 am with a fluttering sensation in his chest. He checked his pulse; it was 120 beats/min. Alarmed, he got out of bed and noted he was short of breath as he walked to the bathroom. He went back to bed, but after approximately 20 minutes without relief, he decided to call his son to take him to the ED. The time from onset of symptoms until arrival at the ED was two hours. During that time, his symptoms did not change. When you examine the patient, he states that he is typically in excellent health and has never experienced either shortness of breath or a rapid heart rate before. He denies a history of cardiac or pulmonary disease and has never had chest pain, syncope, or near-syncope. Medical history is unremarkable. Surgical history is remarkable for a tonsillectomy in childhood and an appendectomy for acute appendicitis at age 18. The patient has no known drug allergies and is taking ibuprofen for a recent ankle sprain but is on no other medications. He works as a certified public accountant and has a sedentary lifestyle. He drinks two to three glasses of wine each evening, does not smoke, and denies recreational or naturopathic medication use. He is a widower (his wife died of breast cancer at age 44) and has one son who lives in the same housing complex. The review of systems is remarkable for a recent left ankle sprain, which occurred when the patient slipped on the carpet at home. Vital signs include a blood pressure of 144/84 mm Hg; pulse, 130 beats/min; respiratory rate, 18 breaths/min-1; O2 saturation, 98%; and temperature, 98.9°F. His height is 5 ft 9 in and his weight, 223 lb. The physical exam reveals an obese white male in mild distress. The HEENT exam reveals corrective lenses and the absence of tonsils. The neck shows no evidence of thyromegaly or jugular venous distention. The lungs are clear in all fields. The cardiac rhythm is irregular with a rate of 130 beats/min. There are no murmurs or extra heart sounds audible. The abdomen is obese and nontender, with no palpable masses. An old surgical scar is evident in the right lower quadrant, consistent with his history of an appendectomy. The lower extremities show no evidence of peripheral edema. Mild discomfort is present with examination of the left ankle. Peripheral pulses are strong and equal, and the neurologic exam is intact. An ECG is obtained that reveals a ventricular rate of 131 beats/min; PR interval, not measured; QRS duration, 82 ms; QT/QTc interval, 374/552 ms; no P axis; R axis, 68°; and T axis, 36°. What is your interpretation of this ECG?
E-mailing patients
I’ve never lived in a world without e-mail. No, I’m not one of those millennial kids; e-mail has been around for a long time. Sending messages between computers dates to the 1960s, but most people consider 1971 to be the birth of e-mail. That’s when Ray Tomlinson added the @ symbol to separate users’ names from their e-mail addresses.
Today, e-mail is ubiquitous. You can e-mail your mother, your colleagues, or your cable company. You can even e-mail the president of the United States. Other than the pope and most physicians, there aren’t many people you cannot e-mail. (Although, interestingly, you can reach His Holiness on Twitter @Pontifex.)
We physicians have historically had a few good reasons to avoid e-mailing patients, but many of those objections are unwarranted. As part the meaningful use EHR incentive program from the Centers for Medicare & Medicaid Services, secure messaging will now be required to be eligible for rewards. Although many physicians cite security as a concern, most electronic medical record systems now have patient portals that allow for secure, safe messaging. Encroachment into private time, however, is still a concern for many physicians.
At Kaiser Permanente (KP), we’ve been using secure e-mails with our patients for more than 5 years. When we started, I had some of the same concerns as most doctors: When am I going to have time to do this? What types of questions will patients send? As it turns out, the system has been wildly popular for patients. In 2013 alone, we replied to more than 14 million patient messages. We encourage our patients to use e-mail to stay connected with us, because it leads to improved patient experiences and improved outcomes.
Managing e-mail in-boxes is difficult work, and we KP physicians constantly try to find ways to be more efficient. E-mail does sometimes encroach on my personal time, but I’ve discovered that’s okay. As it turns out, e-mail encroaches on my entrepreneurial brother’s personal time, my financial planner’s personal time, and my plumber’s personal time. Being always connected is a modern luxury and a curse. It’s also part of being a professional.
Here are some steps I’ve taken to manage my patient e-mails. First, I always remember that this electronic message is connected to a real person with real worry. Second, I remember how appreciative patients are to get a message from their doctor. E-mail a patient after 8 p.m., and they will never forget you. Third, clearly delineate time to take care of business. It never feels burdensome in part because I am in control. I choose to e-mail patients not because I have to but because I’m that doctor and it makes me feel good.
This weekend, for example, I did patient messages in a Jackson Hole, Wyo., coffee shop while on vacation. Just as I opened my computer, I noticed a young guy in a fleece jacket next to me checking his e-mail while his wife and two kids enjoyed muffins and hot cocoa. While I was waiting for my wife, Susan, to order our lattes, I overheard him make a call to his office: “Yes, I’m out, but why don’t you e-mail me that and I’ll get right back to you.”
I’m right with you, buddy, I think. I use my token and the wifi there in Wyoming to access my patient e-mails. There are only five. The messages are like most I receive: “I have a new spot,” or “The cream you gave me isn’t working,” or “My acne is better, so should I reduce the spironolactone?” I hammer replies out in 10 minutes.
My wife returns with lattes and opens the local paper while I review 14 biopsy results from 2 days ago. For most of them, I use a template and the secure e-mail to send patients their results. I then send a few notes to some patients, advising them to follow up with me for excisional surgeries.
The work I was doing was not additive; the questions my patients sent would have had to be addressed at some time. In fact, if they had called, then they would have left a message with a nurse who would have sent a message to me, which I would have had to reply to, and then send the message back to the nurse who would have to reply to the patient.
Despite our love/hate relationship with it, e-mail has been one of the great innovations of the 20th century, and it is the primary form of communication in the business world. According to one study, more than 100 billion business e-mails were sent and received every day in 2013. Yet, fewer than one-third of physicians use e-mail to communicate with their patients.Personally, I have found patients to be generally understanding, courteous, and appreciative of e-mail. Of course, there are a few who don’t follow good etiquette. (One of my primary care colleagues relates a story of a patient who e-mailed her every time she had a bowel movement. Gastroenteritis can significantly add to e-mail burden, apparently.)
There’s no doubt that e-mail will soon become the primary way to communicate with patients. Based on our experience at KP, this will ultimately be to the benefit of both doctors and patients. A June 2014 survey by Catalyst Healthcare Research showed that 93% of patients preferred to see a physician who offers e-mail communication with his or her patients. More than one-quarter of those respondents said they’d be willing to pay a $25 charge for such communication. It’s not surprising; as with all businesses, not just medicine, that patients want more channels of communication, not fewer. Fortunately for them, many of today’s medical residents are being trained to use electronic communication with patients. For instance, a 2013 study published in the Postgraduate Medical Journal found that 57% of residents used e-mail to communicate with patients.
My wife finished reading the Jackson Hole Daily newspaper and outlined our hike to Taggart Lake. And I finished answering my messages. The guy sitting next to me is still tapping away at his keyboard. I make eye contact and say, “Almost done?” “Yup,” he replies, “Better for me to just knock it out now, because I’ll just have to deal with it on Monday.” I agree.
Susan and I pack up and head for the trail, which is thankfully connection free. Let’s just hope we don’t run into any bears.
Dr. Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego, and volunteer clinical assistant professor at the University of California, San Diego. Dr. Benabio is @dermdoc on Twitter.
I’ve never lived in a world without e-mail. No, I’m not one of those millennial kids; e-mail has been around for a long time. Sending messages between computers dates to the 1960s, but most people consider 1971 to be the birth of e-mail. That’s when Ray Tomlinson added the @ symbol to separate users’ names from their e-mail addresses.
Today, e-mail is ubiquitous. You can e-mail your mother, your colleagues, or your cable company. You can even e-mail the president of the United States. Other than the pope and most physicians, there aren’t many people you cannot e-mail. (Although, interestingly, you can reach His Holiness on Twitter @Pontifex.)
We physicians have historically had a few good reasons to avoid e-mailing patients, but many of those objections are unwarranted. As part the meaningful use EHR incentive program from the Centers for Medicare & Medicaid Services, secure messaging will now be required to be eligible for rewards. Although many physicians cite security as a concern, most electronic medical record systems now have patient portals that allow for secure, safe messaging. Encroachment into private time, however, is still a concern for many physicians.
At Kaiser Permanente (KP), we’ve been using secure e-mails with our patients for more than 5 years. When we started, I had some of the same concerns as most doctors: When am I going to have time to do this? What types of questions will patients send? As it turns out, the system has been wildly popular for patients. In 2013 alone, we replied to more than 14 million patient messages. We encourage our patients to use e-mail to stay connected with us, because it leads to improved patient experiences and improved outcomes.
Managing e-mail in-boxes is difficult work, and we KP physicians constantly try to find ways to be more efficient. E-mail does sometimes encroach on my personal time, but I’ve discovered that’s okay. As it turns out, e-mail encroaches on my entrepreneurial brother’s personal time, my financial planner’s personal time, and my plumber’s personal time. Being always connected is a modern luxury and a curse. It’s also part of being a professional.
Here are some steps I’ve taken to manage my patient e-mails. First, I always remember that this electronic message is connected to a real person with real worry. Second, I remember how appreciative patients are to get a message from their doctor. E-mail a patient after 8 p.m., and they will never forget you. Third, clearly delineate time to take care of business. It never feels burdensome in part because I am in control. I choose to e-mail patients not because I have to but because I’m that doctor and it makes me feel good.
This weekend, for example, I did patient messages in a Jackson Hole, Wyo., coffee shop while on vacation. Just as I opened my computer, I noticed a young guy in a fleece jacket next to me checking his e-mail while his wife and two kids enjoyed muffins and hot cocoa. While I was waiting for my wife, Susan, to order our lattes, I overheard him make a call to his office: “Yes, I’m out, but why don’t you e-mail me that and I’ll get right back to you.”
I’m right with you, buddy, I think. I use my token and the wifi there in Wyoming to access my patient e-mails. There are only five. The messages are like most I receive: “I have a new spot,” or “The cream you gave me isn’t working,” or “My acne is better, so should I reduce the spironolactone?” I hammer replies out in 10 minutes.
My wife returns with lattes and opens the local paper while I review 14 biopsy results from 2 days ago. For most of them, I use a template and the secure e-mail to send patients their results. I then send a few notes to some patients, advising them to follow up with me for excisional surgeries.
The work I was doing was not additive; the questions my patients sent would have had to be addressed at some time. In fact, if they had called, then they would have left a message with a nurse who would have sent a message to me, which I would have had to reply to, and then send the message back to the nurse who would have to reply to the patient.
Despite our love/hate relationship with it, e-mail has been one of the great innovations of the 20th century, and it is the primary form of communication in the business world. According to one study, more than 100 billion business e-mails were sent and received every day in 2013. Yet, fewer than one-third of physicians use e-mail to communicate with their patients.Personally, I have found patients to be generally understanding, courteous, and appreciative of e-mail. Of course, there are a few who don’t follow good etiquette. (One of my primary care colleagues relates a story of a patient who e-mailed her every time she had a bowel movement. Gastroenteritis can significantly add to e-mail burden, apparently.)
There’s no doubt that e-mail will soon become the primary way to communicate with patients. Based on our experience at KP, this will ultimately be to the benefit of both doctors and patients. A June 2014 survey by Catalyst Healthcare Research showed that 93% of patients preferred to see a physician who offers e-mail communication with his or her patients. More than one-quarter of those respondents said they’d be willing to pay a $25 charge for such communication. It’s not surprising; as with all businesses, not just medicine, that patients want more channels of communication, not fewer. Fortunately for them, many of today’s medical residents are being trained to use electronic communication with patients. For instance, a 2013 study published in the Postgraduate Medical Journal found that 57% of residents used e-mail to communicate with patients.
My wife finished reading the Jackson Hole Daily newspaper and outlined our hike to Taggart Lake. And I finished answering my messages. The guy sitting next to me is still tapping away at his keyboard. I make eye contact and say, “Almost done?” “Yup,” he replies, “Better for me to just knock it out now, because I’ll just have to deal with it on Monday.” I agree.
Susan and I pack up and head for the trail, which is thankfully connection free. Let’s just hope we don’t run into any bears.
Dr. Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego, and volunteer clinical assistant professor at the University of California, San Diego. Dr. Benabio is @dermdoc on Twitter.
I’ve never lived in a world without e-mail. No, I’m not one of those millennial kids; e-mail has been around for a long time. Sending messages between computers dates to the 1960s, but most people consider 1971 to be the birth of e-mail. That’s when Ray Tomlinson added the @ symbol to separate users’ names from their e-mail addresses.
Today, e-mail is ubiquitous. You can e-mail your mother, your colleagues, or your cable company. You can even e-mail the president of the United States. Other than the pope and most physicians, there aren’t many people you cannot e-mail. (Although, interestingly, you can reach His Holiness on Twitter @Pontifex.)
We physicians have historically had a few good reasons to avoid e-mailing patients, but many of those objections are unwarranted. As part the meaningful use EHR incentive program from the Centers for Medicare & Medicaid Services, secure messaging will now be required to be eligible for rewards. Although many physicians cite security as a concern, most electronic medical record systems now have patient portals that allow for secure, safe messaging. Encroachment into private time, however, is still a concern for many physicians.
At Kaiser Permanente (KP), we’ve been using secure e-mails with our patients for more than 5 years. When we started, I had some of the same concerns as most doctors: When am I going to have time to do this? What types of questions will patients send? As it turns out, the system has been wildly popular for patients. In 2013 alone, we replied to more than 14 million patient messages. We encourage our patients to use e-mail to stay connected with us, because it leads to improved patient experiences and improved outcomes.
Managing e-mail in-boxes is difficult work, and we KP physicians constantly try to find ways to be more efficient. E-mail does sometimes encroach on my personal time, but I’ve discovered that’s okay. As it turns out, e-mail encroaches on my entrepreneurial brother’s personal time, my financial planner’s personal time, and my plumber’s personal time. Being always connected is a modern luxury and a curse. It’s also part of being a professional.
Here are some steps I’ve taken to manage my patient e-mails. First, I always remember that this electronic message is connected to a real person with real worry. Second, I remember how appreciative patients are to get a message from their doctor. E-mail a patient after 8 p.m., and they will never forget you. Third, clearly delineate time to take care of business. It never feels burdensome in part because I am in control. I choose to e-mail patients not because I have to but because I’m that doctor and it makes me feel good.
This weekend, for example, I did patient messages in a Jackson Hole, Wyo., coffee shop while on vacation. Just as I opened my computer, I noticed a young guy in a fleece jacket next to me checking his e-mail while his wife and two kids enjoyed muffins and hot cocoa. While I was waiting for my wife, Susan, to order our lattes, I overheard him make a call to his office: “Yes, I’m out, but why don’t you e-mail me that and I’ll get right back to you.”
I’m right with you, buddy, I think. I use my token and the wifi there in Wyoming to access my patient e-mails. There are only five. The messages are like most I receive: “I have a new spot,” or “The cream you gave me isn’t working,” or “My acne is better, so should I reduce the spironolactone?” I hammer replies out in 10 minutes.
My wife returns with lattes and opens the local paper while I review 14 biopsy results from 2 days ago. For most of them, I use a template and the secure e-mail to send patients their results. I then send a few notes to some patients, advising them to follow up with me for excisional surgeries.
The work I was doing was not additive; the questions my patients sent would have had to be addressed at some time. In fact, if they had called, then they would have left a message with a nurse who would have sent a message to me, which I would have had to reply to, and then send the message back to the nurse who would have to reply to the patient.
Despite our love/hate relationship with it, e-mail has been one of the great innovations of the 20th century, and it is the primary form of communication in the business world. According to one study, more than 100 billion business e-mails were sent and received every day in 2013. Yet, fewer than one-third of physicians use e-mail to communicate with their patients.Personally, I have found patients to be generally understanding, courteous, and appreciative of e-mail. Of course, there are a few who don’t follow good etiquette. (One of my primary care colleagues relates a story of a patient who e-mailed her every time she had a bowel movement. Gastroenteritis can significantly add to e-mail burden, apparently.)
There’s no doubt that e-mail will soon become the primary way to communicate with patients. Based on our experience at KP, this will ultimately be to the benefit of both doctors and patients. A June 2014 survey by Catalyst Healthcare Research showed that 93% of patients preferred to see a physician who offers e-mail communication with his or her patients. More than one-quarter of those respondents said they’d be willing to pay a $25 charge for such communication. It’s not surprising; as with all businesses, not just medicine, that patients want more channels of communication, not fewer. Fortunately for them, many of today’s medical residents are being trained to use electronic communication with patients. For instance, a 2013 study published in the Postgraduate Medical Journal found that 57% of residents used e-mail to communicate with patients.
My wife finished reading the Jackson Hole Daily newspaper and outlined our hike to Taggart Lake. And I finished answering my messages. The guy sitting next to me is still tapping away at his keyboard. I make eye contact and say, “Almost done?” “Yup,” he replies, “Better for me to just knock it out now, because I’ll just have to deal with it on Monday.” I agree.
Susan and I pack up and head for the trail, which is thankfully connection free. Let’s just hope we don’t run into any bears.
Dr. Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego, and volunteer clinical assistant professor at the University of California, San Diego. Dr. Benabio is @dermdoc on Twitter.
Sedative-hypnotics for sleepless geriatric patients
Mr. R, 75, is having difficulty sleeping. When he goes to bed, he lies there for what seems like forever, unable to fall asleep. He feels “so tired” and ends up taking naps during the day, but he cannot break this cycle. He has tried using over-the-counter products with little relief.
Mr. R’s primary care physician prescribes zaleplon, 10 mg/d, and asks him to call the clinic in 2 weeks to discuss his progress. He takes zaleplon as directed for several nights and begins to feel “sluggish” during the day, both mentally and physically, despite reporting an increase in the overall amount of sleep at night.
Sedative-hypnotic drugs are among the most commonly used medications in the United States. Use of these drugs, as well as anxiolytics, has increased from 2.8% between 1988 and 1994 to 4.7% between 2007 and 2010, according to the Department of Health and Human Services.1 In 2011, drugs categorized as sedative-hypnotics or antipsychotics were involved in 6.1% of all human exposures identified in the American Association of Poison Control Centers’ National Poison Data System.2 Therefore, an understanding of clinical and pharmacological variables related to safe and effective use is important for clinicians prescribing and monitoring therapy with these agents.
Neuropsychiatric disorders are prevalent among geriatric patients and are associated with age-related physiologic changes in the CNS.3 Such changes involve:
• neuroanatomy (brain atrophy, decreased neuronal density, increased plaque formation)
• neurotransmitters (reduced cholinergic transmission, decreased synthesis of dopamine and catecholamines), and
• neurophysiology (reduced cerebral blood flow).
These physiologic processes manifest as alterations in mental status, reflexes, sensation, gait, balance, and sleep. Examples of sleep changes among geriatric patients include decreased sleep efficiency, more frequent awakenings, and more variable sleep duration.3,4 Sleep disorders also may be related to mental disorders and other medical conditions.5 For example, the prevalence of sleep-related respiratory disorders, such as obstructive sleep apnea and central sleep apnea, increases with age.6
Sleep disorders are common among geriatric patients. In a large epidemiologic study of sleep complaints in patients age ≥65, more than one-half of patients had at least 1 sleep complaint (ie, difficulty falling asleep, trouble waking up, early awakening, need for naps, and feeling ill-rested).7 As many as 34% of patients reported symptoms of insomnia. In an analysis of National Ambulatory Medical Survey Data over 6 years, 24.8% to 27.9% of sleep-related medical office visits were attributed to patients age ≥65.8
Pharmacology in aging
Prescribing sedative-hypnotic drugs is not routinely recommended for older patients with a sleep disorder. Geriatric patients, compared with younger patients, are at higher risk of iatrogenic complications because of polypharmacy, comorbidities, relative renal and hepatic insufficiency, and other physiologic changes leading to alterations in drug exposure and metabolism (Table 1).9-12
Aging is associated with changes in body composition, including an increase in total body fat and decrease in lean body mass and total body water. These changes, as well as a prolonged GI transit time, decrease in active gut transporters, decreased blood perfusion, and decrease in plasma proteins such as albumin (because of reduced liver function or malnutrition), may lead to alteration in drug absorption patterns and may increase the volume of distribution for lipophilic drugs. Additionally, the elimination half-life of some drugs may increase with age because of larger volumes of distribution and reduction in hepatic or renal clearance.
The clinical significance of these changes is not well established. Although the process of drug absorption can change with age, the amount of drug absorbed might not be significantly affected. An increase in the volume of distribution and reduction in drug metabolism and clearance might lead to increasing amounts of circulating drug and duration of drug exposure, putting geriatric patients at an increased risk for adverse effects and drug toxicity.9
Among these mechanisms, Dolder et al11 hypothesized that drug metabolism catalyzed by cytochrome P450 (CYP) enzymes and renal excretion may be of greatest concern. Although in vitro studies suggest that concentration of CYP enzymes does not decline with age, in vivo studies have demonstrated reduced CYP activity in geriatric patients.11,12 Theoretically, a reduction in CYP activity would increase the bioavailability of drugs, especially those that are subject to extensive first-pass (ie, hepatic) metabolism, and may lead to a reduction in systemic clearance.
Independent of metabolic changes, geriatric patients are at risk of reduced renal clearance because of age-related changes in glomerular filtration rate. Pharmacodynamic changes might be observed in older patients and could be a concern even in the setting of unaltered pharmacokinetic factors.9 These changes usually require administering smaller drug dosages.
Sedative-hypnotic medications
Sedative-hypnotic agents include several barbiturates, benzodiazepines (BZDs), non-BZD benzodiazepine-receptor agonists (BzRAs), a melatonin-receptor agonist (ie, ramelteon), and an orexin-receptor antagonist (ie, suvorexant).13,14Table 214-29 summarizes selected sedative-hypnotic drugs. Additional drug classes used to treat insomnia include:
• sedating antidepressants (trazodone, amitriptyline, doxepin, mirtazapine)
• antiepileptic drugs (gabapentin, tiagabine)
• atypical antipsychotics (quetiapine, olanzapine).
FDA-approved agents for treating insomnia include amobarbital, butabarbital, pentobarbital, phenobarbital, secobarbital, chloral hydrate, diphenhydramine, doxylamine, doxepin, estazolam, flurazepam, lorazepam, quazepam, temazepam, triazolam, eszopiclone, zaleplon, zolpidem, ramelteon, and suvorexant. Not all of these drugs are recommended for use in geriatric patients. Barbiturates, for example, should be avoided.30
Pharmacokinetic characteristics vary among drugs and drug classes. Choice of pharmacotherapy should account for patient and drug characteristics and the specific sleep complaint. Sleep disorders may be variously characterized as difficulty with sleep initiation, duration, consolidation, or quality.13 Therefore, onset and duration of effect are important drug-related considerations. Sedative-hypnotic drugs with a short time-to-onset may be ideal for patients with sleep-onset insomnia.
The drugs’ duration of effect (eg, presence of active metabolites, long elimination half-life) also must be reviewed. A long elimination half-life may lead to increased drug exposure and unwanted side effects such as residual daytime drowsiness. Despite this, sedative-hypnotic drugs with a longer duration of effect (eg, intermediate- or long-acting drugs) may be best for patients with insomnia defined by difficulty maintaining sleep.
Benzodiazepines vary in their time to onset of effect, rate of elimination, and metabolism.15-21 BZDs that are FDA- approved for use as sedative-hypnotics are listed in Table 2.14-29 These BZDs have different onsets of effect as evidenced by time to achieve maximum plasma concentration (Tmax), ranging from 0.5 hours (flurazepam) to 2 hours (estazolam, quazepam, triazolam). The elimination half-life varies widely among these medications, from 1.5 hours (triazolam) to >100 hours (flurazepam). Flurazepam’s long half-life is attributable to its active major metabolite. Although most BZDs are metabolized hepatically, temazepam is subject to minimal hepatic metabolism.
Benzodiazepine-receptor agonists. There is substantial variation in the pharmacokinetic characteristics of BzRAs.15,16,22-28 There also are differences among the zolpidem dosage forms; sublingual formulations have the shortest onset of effect. Eszopiclone and zaleplon have low protein binding compared with zolpidem. Elimination half-lives vary among drugs with the shortest attributed to zaleplon (1 hour) and longest to eszopiclone (6 hours). All BzRAs are subject to extensive hepatic metabolism.
Ramelteon. Singular in its class, ramelteon is a treatment option for insomnia.29 This drug has a short onset of effect, moderate protein binding, and extensive hepatic metabolism. Ramelteon is primarily excreted in the urine as its metabolites, and the drug half-life is relatively short.
Suvorexant is the latest addition to the sedative-hypnotic armamentarium, approved by the FDA in August 2014 for difficulty with sleep onset and/or sleep maintenance.14 As an orexin-receptor antagonist, suvorexant represents a novel pharmacologic class. Suvorexant exhibits moderately rapid absorption with time to peak concentration ranging from 30 minutes to 6 hours in fasting conditions; absorption is delayed when taken with or soon after a meal. The drug is highly protein bound and extensively metabolized, primarily through CYP3A. The manufacturer recommends dose reduction (5 mg at bedtime) in patients taking moderate CYP3A inhibitors and avoiding suvorexant in patients taking strong CYP3A inhibitors. Suvorexant is primarily excreted through feces and the mean half-life is relatively long.
Considering these characteristics and age-related physiologic changes, the practitioner should be concerned about drugs that undergo extensive hepatic metabolism. Age-related reductions in CYP activity may lead to an increase in drug bioavailability and a decrease in the systemic clearance,11 which might be associated with an increase in elimination half-life and duration of action. Dosage adjustments are recommended for several BZDs (lower initial and maximum dosages for most agents) and BzRAs.17-28 No dosage adjustments for ramelteon or suvorexant in geriatric patients have been specified14,29; the manufacturers for both products assert that no differences in safety and efficacy have been observed between older and younger adult patients.
Alternative and complementary medications
Several non-prescription products, including over-the-counter drugs (eg, diphenhy-dramine, doxylamine) and herbal therapies (eg, melatonin, valerian), are used for their sedative-hypnotic properties. There is a lack of evidence supporting using diphenhydra-mine in patients with chronic insomnia, and tolerance to its hypnotic effect has been reported with repeated use.31 Concerns about anticholinergic toxicity and CNS depression limit its use in geriatric patients. Among herbal therapies, melatonin may have the strongest evidence for its ability to alleviate sleep disorders in geriatric patients32; however, meta-analyses have demonstrated small effects of melatonin on sleep latency and minimal differences in wake time after sleep onset and total sleep time.13
Clinical practice guidelines
Non-pharmacotherapeutic interventions, such as behavioral (eg, sleep hygiene measures) and psychological therapy, are recommended for initial management of sleep disorders in geriatric patients.13,33 In conjunction, the American Medical Directors Association (AMDA) recommends address ing underlying causes and exacerbating factors (eg, medical condition or medication).33 The AMDA recommends avoiding long-term pharmacotherapy and advises caution with BZD-hypnotic drugs, tricyclic antidepressants, and antihistamines. The American Academy of Sleep Medicine (AASM) recommends an initial treatment period of 2 to 4 weeks, followed by re-evaluation of continued need for treatment.13 The AASM recommends short- or intermediate-acting BzRAs or ramelteon for initial pharmacologic management of primary insomnias and insomnias comorbid with other conditions. The AASM also recommends specific dosages of BzRAs and BZDs for geriatric patients, which coincide with manufacturer-recommended dosages (Table 2).14-29
Barbiturates, chloral hydrate, and non-barbiturate, non-BZD drugs such as meprobamate are not recommended because of potential significant adverse effects and tolerance/dependence, and low therapeutic index. The AASM advises caution when using prescription drugs off-label for insomnia (eg, antidepressants, antiepileptics, antipsychotics) and recommends avoiding them, if possible, because of limited evidence supporting their use.13
Safety concerns
Two commonly used references contain recommendations for sedative-hypnotic medication use in geriatric patients.30,34 According to Gallagher et al’s34 Screening Tool of Older Person’s Prescriptions (STOPP), long-term (>1 month) use of long-acting BZDs (eg, flurazepam, diazepam) and prolonged use (>1 week) of first-generation antihistamines (eg, diphenhydramine, doxylamine) should be avoided in patients age ≥65 because of the risk of sedation, confusion, and anticholinergic side effects. STOPP recognizes that any use of BZDs, neuroleptics, or first-generation antihistamines may contribute to postural imbalance; therefore these agents are not recommended in older patients at risk for falls.
In the 2012 American Geriatrics Society (AGS) Beers Criteria, the AGS recommends avoiding barbiturates in older adults because of the high rate of physical dependence, tolerance to sleep effects, and overdose risk at low dosages.30 The AGS also recommends avoiding BZDs, stating that older adults have increased sensitivity to these agents and are at an increased risk of cognitive impairment, delirium, falls, fractures, and motor vehicle accidents when taking these drugs. Non-BZD BzRAs also should not be prescribed to patients with a history of falls or fractures, unless safer alternatives are not available.
The FDA has issued several advisory reports regarding sedative-hypnotic drugs. In 2007, all manufacturers of sedative-hypnotic drugs were required to modify their product labeling to include stronger language about potential risks.35 Among these changes, warnings for anaphylaxis and complex sleep-related behaviors were added. Also, the FDA requested that manufacturers of sedative-hypnotic drugs develop and provide patient medication guides, advising consumers on the potential risks and precautions associated with these drugs. More recently, the FDA announced changes to dosing recommendations for zolpidem-containing products because of the risk of impaired mental alertness36; manufacturers were required to lower the recommended dosages for each product.
Manufacturers of FDA-approved sedative-hypnotic drugs urge caution when prescribing these medications for geriatric patients, citing the potential for increased sensitivity, manifesting as marked excitement, depression, or confusion (eg, barbiturates), and greater risk for dosage-related adverse effects (eg, oversedation, dizziness, confusion, impaired psychomotor performance, ataxia).17-29
Use in clinical practice
Several variables should be considered when evaluating appropriateness of pharmacotherapy, including characteristics of the drug and the patient. Geriatric patients may be prone to comorbidities resulting from age-related physiologic changes. These diseases may be confounding (ie, contributing to sleep disorders); examples include medical illnesses, such as hyperthyroidism and arthritis, and psychiatric illnesses, such as depression and anxiety.37 Other conditions, such as renal and hepatic dysfunction, may lead to alteration in drug exposure. These conditions should be assessed through routine renal function tests (eg, serum creatinine and glomerular filtration rate) and liver function tests (eg, serum albumin and liver transaminases).
Multiple comorbidities suggest a higher likelihood of polypharmacy, leading to other drug-related issues (eg, drug-drug interactions). Although these issues may guide therapy by restricting medication options, their potential contribution to the underlying sleep complaints should be considered.37 Several drugs commonly used by geriatric patients may affect wakefulness (eg, analgesics, antidepressants, and antihypertensives [sedating], and thyroid hormones, corticosteroids, and CNS stimulants [alerting]).
CASE CONTINUED
In Mr. R’s case, zaleplon was initiated at 10 mg/d. Because of his age and the nature of his sleep disorder, the choice of sedative-hypnotic was suitable; however, the prescribed dosage was inappropriate. The sluggishness Mr. R experienced likely was a manifestation of increased exposure to the drug. According to manufacturer and AASM recommendations, a more appropriate dosage is 5 mg/d.13,23 Mr. R’s medical history and current medications, and his hepatic and renal function, should be assessed. If Mr. R continues to have issues with sleep initiation, zaleplon, 5 mg at bedtime, should be considered.
Related Resources
• Institute for Safe Medication Practices. www.ismp.org.
• MedWatch: The FDA Safety Information and Adverse Event Reporting Program. www.fda.gov/Safety/MedWatch/default.htm.
Drug Brand Names
Amitriptyline • Elavil Mirtazapine • Remeron
Amobarbital • Amytal Olanzapine • Zyprexa
Butabarbital • Butisol Pentobarbital • Nembutal
Chloral hydrate • Somnote Phenobarbital • Luminal
Diazepam • Valium Quazepam • Doral
Diphenhydramine • Benadryl, others Quetiapine • Seroquel
Doxepin • Silenor Ramelteon • Rozerem
Doxylamine • Unisom, others Secobarbital • Seconal
Estazolam • ProSom Suvorexant • Belsomra
Eszopiclone • Lunesta Temazepam • Restoril
Flurazepam • Dalmane Tiagabine • Gabitril
Gabapentin • Neurontin, Trazodone • Desyrel
Gralise, Horizant Zaleplon • Sonata
Lorazepam • Ativan Zolpidem • Ambien, Edluar,
Meprobamate • Equanil Intermezzo, Zolpimist
Acknowledgement
Vicki L. Ellingrod, PharmD, FCCP, is the series editor of Savvy Psychopharmacology.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. National Center for Health Statistics. Health, United States, 2012, with special feature on emergency care. http://www. cdc.gov/nchs/data/hus/hus12.pdf. Published May 2013. Accessed August 22, 2014.
2. Bronstein AC, Spyker DA, Cantilena LR Jr, et al. 2011 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 29th annual report. Clin Toxicol (Phila). 2012;50(10): 911-1164.
3. Inouye SK. Neuropsychiatric aspects of aging. In: Goldman L, Schafer AI, eds. Goldman’s cecil medicine. 24th ed. Philadelphia, PA: Elsevier Saunders; 2011:114-116.
4. Diagnostic and statistical manual of mental disorders, 4th ed. Washington, DC: American Psychiatric Association; 1994.
5. American Psychiatric Association. Highlights of changes from DSM-IV-TR to DSM-5. http://www.psychiatry. org/File%20Library/Practice/DSM/DSM-5/Changes-from-DSM-IV-TR--to-DSM-5.pdf. 2013. Accessed August 22, 2014.
6. Edwards BA, O’Driscoll DM, Ali A, et al. Aging and sleep: physiology and pathophysiology. Semin Respir Crit Care Med. 2010;31(5):618-633.
7. Foley DJ, Monjan AA, Brown SL, et al. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18(6):425-432.
8. Morlock RJ, Tan M, Mitchell DY. Patient characteristics and patterns of drug use for sleep complaints in the United States: analysis of National Ambulatory Medical Survey Data, 1997-2002. Clin Ther. 2006;28(7):1044-1053.
9. Diasio RB. Principles of drug therapy. In: Goldman L, Schafer AI, eds. Goldman’s Cecil medicine. 24th ed. Philadelphia, PA: Elsevier Saunders; 2011:124-132.
10. Hilmer SN, Ford GA. General principles of pharmacology. In: Halter JB, Ouslander JG, Tinetti ME, et al, eds. Hazzard’s geriatric medicine and gerontology. 6th ed. New York, NY: McGraw-Hill; 2009:103-122.
11. Dolder C, Nelson M, McKinsey J. Use of non-benzodiazepine hypnotics in the elderly: are all agents the same? CNS Drugs. 2007;21(5):389-405.
12. Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev. 2009;41(2):67-76.
13. Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487-504.
14. Belsomra [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.; 2014.
15. Micromedex Healthcare Series. Greenwood Village, CO: Thomson Healthcare. http://micromedex.com. Accessed August 22, 2014.
16. Lexicomp. St. Louis, MO: Wolters Kluwer Health. http:// www.lexi.com. Accessed August 22, 2014.
17. Estazolam [package insert]. Corona, CA: Watson Pharma, Inc; 2008.
18. Flurazepam [package insert]. Eatontown, NJ: West-Ward Pharmaceutical Corp; 2010.
19. Doral [package insert]. Las Vegas, NV: Nuro Pharma, Inc; 2013.
20. Restoril [package insert]. Hazelwood, MO: Mallinckrodt Inc; 2010.
21. Halcion [package insert]. New York, NY: Pharmacia & Upjohn Co; 2013.
22. Lunesta [package insert]. Marlborough, MA: Sunovion Pharmaceuticals Inc; 2012.
23. Sonata [package insert]. New York, NY: Pfizer Inc; 2013.
24. Ambien [package insert]. Bridgewater, NJ: Sanofi-Aventis; 2013.
25. Ambien CR [package insert]. Bridgewater, NJ: Sanofi- Aventis; 2013.
26. Edluar [package insert]. Somerset, NJ: Meda Pharmaceuticals Inc; 2009.
27. Intermezzo [package insert]. Point Richmond, CA: Transcept Pharmaceuticals, Inc; 2011.
28. Zolpimist [package insert]. Richmond, VA: ECR Pharmaceuticals; 2013.
29. Rozerem [package insert]. Deerfield, IL: Takeda Pharmaceuticals America, Inc; 2010.
30. The American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
31. Kirkwood CK, Melton ST. Insomnia, drowsiness, and fatigue. In: Krinsky DL, Berardi RR, Ferreri SP, et al, eds. Handbook of nonprescription drugs: an interactive approach to self-care. 17th ed. Washington, DC: American Pharmacists Association; 2012:867-884.
32. Insomnia. In: Natural Standard. Somerville, MA: Natural Standard. https://naturalmedicines.therapeuticresearch. com/databases/medical-conditions/i/insomnia.aspx. Accessed August 22, 2014.
33. American Medical Directors Association. Clinical practice guideline: sleep disorders. Columbia, MD: American Medical Directors Association; 2006.
34. Gallagher P, Ryan C, Byrne S, et al. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72-83.
35. Food and Drug Administration. FDA requests label change for all sleep disorder drug products. http://www.fda. gov/newsevents/newsroom/pressannouncements/2007/ ucm108868.htm. Published March 14, 2007. Accessed August 22, 2014.
36. Food and Drug Administration. FDA drug safety communication: risk of next-morning impairment after use of insomnia drugs; FDA requires lower recommended doses for certain drugs containing zolpidem (Ambien, Ambien CR, Edluar, and Zolpimist). http://www.fda.gov/drugs/ drugsafety/ucm334033.htm. Published January 10, 2013. Accessed August 22, 2014.
37. Cohen-Zion M, Ancoli-Israel S. Sleep disorders. In: Hazzard’s geriatric medicine and gerontology. 6th ed. New York, NY: McGraw-Hill; 2009:671-682.
Mr. R, 75, is having difficulty sleeping. When he goes to bed, he lies there for what seems like forever, unable to fall asleep. He feels “so tired” and ends up taking naps during the day, but he cannot break this cycle. He has tried using over-the-counter products with little relief.
Mr. R’s primary care physician prescribes zaleplon, 10 mg/d, and asks him to call the clinic in 2 weeks to discuss his progress. He takes zaleplon as directed for several nights and begins to feel “sluggish” during the day, both mentally and physically, despite reporting an increase in the overall amount of sleep at night.
Sedative-hypnotic drugs are among the most commonly used medications in the United States. Use of these drugs, as well as anxiolytics, has increased from 2.8% between 1988 and 1994 to 4.7% between 2007 and 2010, according to the Department of Health and Human Services.1 In 2011, drugs categorized as sedative-hypnotics or antipsychotics were involved in 6.1% of all human exposures identified in the American Association of Poison Control Centers’ National Poison Data System.2 Therefore, an understanding of clinical and pharmacological variables related to safe and effective use is important for clinicians prescribing and monitoring therapy with these agents.
Neuropsychiatric disorders are prevalent among geriatric patients and are associated with age-related physiologic changes in the CNS.3 Such changes involve:
• neuroanatomy (brain atrophy, decreased neuronal density, increased plaque formation)
• neurotransmitters (reduced cholinergic transmission, decreased synthesis of dopamine and catecholamines), and
• neurophysiology (reduced cerebral blood flow).
These physiologic processes manifest as alterations in mental status, reflexes, sensation, gait, balance, and sleep. Examples of sleep changes among geriatric patients include decreased sleep efficiency, more frequent awakenings, and more variable sleep duration.3,4 Sleep disorders also may be related to mental disorders and other medical conditions.5 For example, the prevalence of sleep-related respiratory disorders, such as obstructive sleep apnea and central sleep apnea, increases with age.6
Sleep disorders are common among geriatric patients. In a large epidemiologic study of sleep complaints in patients age ≥65, more than one-half of patients had at least 1 sleep complaint (ie, difficulty falling asleep, trouble waking up, early awakening, need for naps, and feeling ill-rested).7 As many as 34% of patients reported symptoms of insomnia. In an analysis of National Ambulatory Medical Survey Data over 6 years, 24.8% to 27.9% of sleep-related medical office visits were attributed to patients age ≥65.8
Pharmacology in aging
Prescribing sedative-hypnotic drugs is not routinely recommended for older patients with a sleep disorder. Geriatric patients, compared with younger patients, are at higher risk of iatrogenic complications because of polypharmacy, comorbidities, relative renal and hepatic insufficiency, and other physiologic changes leading to alterations in drug exposure and metabolism (Table 1).9-12
Aging is associated with changes in body composition, including an increase in total body fat and decrease in lean body mass and total body water. These changes, as well as a prolonged GI transit time, decrease in active gut transporters, decreased blood perfusion, and decrease in plasma proteins such as albumin (because of reduced liver function or malnutrition), may lead to alteration in drug absorption patterns and may increase the volume of distribution for lipophilic drugs. Additionally, the elimination half-life of some drugs may increase with age because of larger volumes of distribution and reduction in hepatic or renal clearance.
The clinical significance of these changes is not well established. Although the process of drug absorption can change with age, the amount of drug absorbed might not be significantly affected. An increase in the volume of distribution and reduction in drug metabolism and clearance might lead to increasing amounts of circulating drug and duration of drug exposure, putting geriatric patients at an increased risk for adverse effects and drug toxicity.9
Among these mechanisms, Dolder et al11 hypothesized that drug metabolism catalyzed by cytochrome P450 (CYP) enzymes and renal excretion may be of greatest concern. Although in vitro studies suggest that concentration of CYP enzymes does not decline with age, in vivo studies have demonstrated reduced CYP activity in geriatric patients.11,12 Theoretically, a reduction in CYP activity would increase the bioavailability of drugs, especially those that are subject to extensive first-pass (ie, hepatic) metabolism, and may lead to a reduction in systemic clearance.
Independent of metabolic changes, geriatric patients are at risk of reduced renal clearance because of age-related changes in glomerular filtration rate. Pharmacodynamic changes might be observed in older patients and could be a concern even in the setting of unaltered pharmacokinetic factors.9 These changes usually require administering smaller drug dosages.
Sedative-hypnotic medications
Sedative-hypnotic agents include several barbiturates, benzodiazepines (BZDs), non-BZD benzodiazepine-receptor agonists (BzRAs), a melatonin-receptor agonist (ie, ramelteon), and an orexin-receptor antagonist (ie, suvorexant).13,14Table 214-29 summarizes selected sedative-hypnotic drugs. Additional drug classes used to treat insomnia include:
• sedating antidepressants (trazodone, amitriptyline, doxepin, mirtazapine)
• antiepileptic drugs (gabapentin, tiagabine)
• atypical antipsychotics (quetiapine, olanzapine).
FDA-approved agents for treating insomnia include amobarbital, butabarbital, pentobarbital, phenobarbital, secobarbital, chloral hydrate, diphenhydramine, doxylamine, doxepin, estazolam, flurazepam, lorazepam, quazepam, temazepam, triazolam, eszopiclone, zaleplon, zolpidem, ramelteon, and suvorexant. Not all of these drugs are recommended for use in geriatric patients. Barbiturates, for example, should be avoided.30
Pharmacokinetic characteristics vary among drugs and drug classes. Choice of pharmacotherapy should account for patient and drug characteristics and the specific sleep complaint. Sleep disorders may be variously characterized as difficulty with sleep initiation, duration, consolidation, or quality.13 Therefore, onset and duration of effect are important drug-related considerations. Sedative-hypnotic drugs with a short time-to-onset may be ideal for patients with sleep-onset insomnia.
The drugs’ duration of effect (eg, presence of active metabolites, long elimination half-life) also must be reviewed. A long elimination half-life may lead to increased drug exposure and unwanted side effects such as residual daytime drowsiness. Despite this, sedative-hypnotic drugs with a longer duration of effect (eg, intermediate- or long-acting drugs) may be best for patients with insomnia defined by difficulty maintaining sleep.
Benzodiazepines vary in their time to onset of effect, rate of elimination, and metabolism.15-21 BZDs that are FDA- approved for use as sedative-hypnotics are listed in Table 2.14-29 These BZDs have different onsets of effect as evidenced by time to achieve maximum plasma concentration (Tmax), ranging from 0.5 hours (flurazepam) to 2 hours (estazolam, quazepam, triazolam). The elimination half-life varies widely among these medications, from 1.5 hours (triazolam) to >100 hours (flurazepam). Flurazepam’s long half-life is attributable to its active major metabolite. Although most BZDs are metabolized hepatically, temazepam is subject to minimal hepatic metabolism.
Benzodiazepine-receptor agonists. There is substantial variation in the pharmacokinetic characteristics of BzRAs.15,16,22-28 There also are differences among the zolpidem dosage forms; sublingual formulations have the shortest onset of effect. Eszopiclone and zaleplon have low protein binding compared with zolpidem. Elimination half-lives vary among drugs with the shortest attributed to zaleplon (1 hour) and longest to eszopiclone (6 hours). All BzRAs are subject to extensive hepatic metabolism.
Ramelteon. Singular in its class, ramelteon is a treatment option for insomnia.29 This drug has a short onset of effect, moderate protein binding, and extensive hepatic metabolism. Ramelteon is primarily excreted in the urine as its metabolites, and the drug half-life is relatively short.
Suvorexant is the latest addition to the sedative-hypnotic armamentarium, approved by the FDA in August 2014 for difficulty with sleep onset and/or sleep maintenance.14 As an orexin-receptor antagonist, suvorexant represents a novel pharmacologic class. Suvorexant exhibits moderately rapid absorption with time to peak concentration ranging from 30 minutes to 6 hours in fasting conditions; absorption is delayed when taken with or soon after a meal. The drug is highly protein bound and extensively metabolized, primarily through CYP3A. The manufacturer recommends dose reduction (5 mg at bedtime) in patients taking moderate CYP3A inhibitors and avoiding suvorexant in patients taking strong CYP3A inhibitors. Suvorexant is primarily excreted through feces and the mean half-life is relatively long.
Considering these characteristics and age-related physiologic changes, the practitioner should be concerned about drugs that undergo extensive hepatic metabolism. Age-related reductions in CYP activity may lead to an increase in drug bioavailability and a decrease in the systemic clearance,11 which might be associated with an increase in elimination half-life and duration of action. Dosage adjustments are recommended for several BZDs (lower initial and maximum dosages for most agents) and BzRAs.17-28 No dosage adjustments for ramelteon or suvorexant in geriatric patients have been specified14,29; the manufacturers for both products assert that no differences in safety and efficacy have been observed between older and younger adult patients.
Alternative and complementary medications
Several non-prescription products, including over-the-counter drugs (eg, diphenhy-dramine, doxylamine) and herbal therapies (eg, melatonin, valerian), are used for their sedative-hypnotic properties. There is a lack of evidence supporting using diphenhydra-mine in patients with chronic insomnia, and tolerance to its hypnotic effect has been reported with repeated use.31 Concerns about anticholinergic toxicity and CNS depression limit its use in geriatric patients. Among herbal therapies, melatonin may have the strongest evidence for its ability to alleviate sleep disorders in geriatric patients32; however, meta-analyses have demonstrated small effects of melatonin on sleep latency and minimal differences in wake time after sleep onset and total sleep time.13
Clinical practice guidelines
Non-pharmacotherapeutic interventions, such as behavioral (eg, sleep hygiene measures) and psychological therapy, are recommended for initial management of sleep disorders in geriatric patients.13,33 In conjunction, the American Medical Directors Association (AMDA) recommends address ing underlying causes and exacerbating factors (eg, medical condition or medication).33 The AMDA recommends avoiding long-term pharmacotherapy and advises caution with BZD-hypnotic drugs, tricyclic antidepressants, and antihistamines. The American Academy of Sleep Medicine (AASM) recommends an initial treatment period of 2 to 4 weeks, followed by re-evaluation of continued need for treatment.13 The AASM recommends short- or intermediate-acting BzRAs or ramelteon for initial pharmacologic management of primary insomnias and insomnias comorbid with other conditions. The AASM also recommends specific dosages of BzRAs and BZDs for geriatric patients, which coincide with manufacturer-recommended dosages (Table 2).14-29
Barbiturates, chloral hydrate, and non-barbiturate, non-BZD drugs such as meprobamate are not recommended because of potential significant adverse effects and tolerance/dependence, and low therapeutic index. The AASM advises caution when using prescription drugs off-label for insomnia (eg, antidepressants, antiepileptics, antipsychotics) and recommends avoiding them, if possible, because of limited evidence supporting their use.13
Safety concerns
Two commonly used references contain recommendations for sedative-hypnotic medication use in geriatric patients.30,34 According to Gallagher et al’s34 Screening Tool of Older Person’s Prescriptions (STOPP), long-term (>1 month) use of long-acting BZDs (eg, flurazepam, diazepam) and prolonged use (>1 week) of first-generation antihistamines (eg, diphenhydramine, doxylamine) should be avoided in patients age ≥65 because of the risk of sedation, confusion, and anticholinergic side effects. STOPP recognizes that any use of BZDs, neuroleptics, or first-generation antihistamines may contribute to postural imbalance; therefore these agents are not recommended in older patients at risk for falls.
In the 2012 American Geriatrics Society (AGS) Beers Criteria, the AGS recommends avoiding barbiturates in older adults because of the high rate of physical dependence, tolerance to sleep effects, and overdose risk at low dosages.30 The AGS also recommends avoiding BZDs, stating that older adults have increased sensitivity to these agents and are at an increased risk of cognitive impairment, delirium, falls, fractures, and motor vehicle accidents when taking these drugs. Non-BZD BzRAs also should not be prescribed to patients with a history of falls or fractures, unless safer alternatives are not available.
The FDA has issued several advisory reports regarding sedative-hypnotic drugs. In 2007, all manufacturers of sedative-hypnotic drugs were required to modify their product labeling to include stronger language about potential risks.35 Among these changes, warnings for anaphylaxis and complex sleep-related behaviors were added. Also, the FDA requested that manufacturers of sedative-hypnotic drugs develop and provide patient medication guides, advising consumers on the potential risks and precautions associated with these drugs. More recently, the FDA announced changes to dosing recommendations for zolpidem-containing products because of the risk of impaired mental alertness36; manufacturers were required to lower the recommended dosages for each product.
Manufacturers of FDA-approved sedative-hypnotic drugs urge caution when prescribing these medications for geriatric patients, citing the potential for increased sensitivity, manifesting as marked excitement, depression, or confusion (eg, barbiturates), and greater risk for dosage-related adverse effects (eg, oversedation, dizziness, confusion, impaired psychomotor performance, ataxia).17-29
Use in clinical practice
Several variables should be considered when evaluating appropriateness of pharmacotherapy, including characteristics of the drug and the patient. Geriatric patients may be prone to comorbidities resulting from age-related physiologic changes. These diseases may be confounding (ie, contributing to sleep disorders); examples include medical illnesses, such as hyperthyroidism and arthritis, and psychiatric illnesses, such as depression and anxiety.37 Other conditions, such as renal and hepatic dysfunction, may lead to alteration in drug exposure. These conditions should be assessed through routine renal function tests (eg, serum creatinine and glomerular filtration rate) and liver function tests (eg, serum albumin and liver transaminases).
Multiple comorbidities suggest a higher likelihood of polypharmacy, leading to other drug-related issues (eg, drug-drug interactions). Although these issues may guide therapy by restricting medication options, their potential contribution to the underlying sleep complaints should be considered.37 Several drugs commonly used by geriatric patients may affect wakefulness (eg, analgesics, antidepressants, and antihypertensives [sedating], and thyroid hormones, corticosteroids, and CNS stimulants [alerting]).
CASE CONTINUED
In Mr. R’s case, zaleplon was initiated at 10 mg/d. Because of his age and the nature of his sleep disorder, the choice of sedative-hypnotic was suitable; however, the prescribed dosage was inappropriate. The sluggishness Mr. R experienced likely was a manifestation of increased exposure to the drug. According to manufacturer and AASM recommendations, a more appropriate dosage is 5 mg/d.13,23 Mr. R’s medical history and current medications, and his hepatic and renal function, should be assessed. If Mr. R continues to have issues with sleep initiation, zaleplon, 5 mg at bedtime, should be considered.
Related Resources
• Institute for Safe Medication Practices. www.ismp.org.
• MedWatch: The FDA Safety Information and Adverse Event Reporting Program. www.fda.gov/Safety/MedWatch/default.htm.
Drug Brand Names
Amitriptyline • Elavil Mirtazapine • Remeron
Amobarbital • Amytal Olanzapine • Zyprexa
Butabarbital • Butisol Pentobarbital • Nembutal
Chloral hydrate • Somnote Phenobarbital • Luminal
Diazepam • Valium Quazepam • Doral
Diphenhydramine • Benadryl, others Quetiapine • Seroquel
Doxepin • Silenor Ramelteon • Rozerem
Doxylamine • Unisom, others Secobarbital • Seconal
Estazolam • ProSom Suvorexant • Belsomra
Eszopiclone • Lunesta Temazepam • Restoril
Flurazepam • Dalmane Tiagabine • Gabitril
Gabapentin • Neurontin, Trazodone • Desyrel
Gralise, Horizant Zaleplon • Sonata
Lorazepam • Ativan Zolpidem • Ambien, Edluar,
Meprobamate • Equanil Intermezzo, Zolpimist
Acknowledgement
Vicki L. Ellingrod, PharmD, FCCP, is the series editor of Savvy Psychopharmacology.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Mr. R, 75, is having difficulty sleeping. When he goes to bed, he lies there for what seems like forever, unable to fall asleep. He feels “so tired” and ends up taking naps during the day, but he cannot break this cycle. He has tried using over-the-counter products with little relief.
Mr. R’s primary care physician prescribes zaleplon, 10 mg/d, and asks him to call the clinic in 2 weeks to discuss his progress. He takes zaleplon as directed for several nights and begins to feel “sluggish” during the day, both mentally and physically, despite reporting an increase in the overall amount of sleep at night.
Sedative-hypnotic drugs are among the most commonly used medications in the United States. Use of these drugs, as well as anxiolytics, has increased from 2.8% between 1988 and 1994 to 4.7% between 2007 and 2010, according to the Department of Health and Human Services.1 In 2011, drugs categorized as sedative-hypnotics or antipsychotics were involved in 6.1% of all human exposures identified in the American Association of Poison Control Centers’ National Poison Data System.2 Therefore, an understanding of clinical and pharmacological variables related to safe and effective use is important for clinicians prescribing and monitoring therapy with these agents.
Neuropsychiatric disorders are prevalent among geriatric patients and are associated with age-related physiologic changes in the CNS.3 Such changes involve:
• neuroanatomy (brain atrophy, decreased neuronal density, increased plaque formation)
• neurotransmitters (reduced cholinergic transmission, decreased synthesis of dopamine and catecholamines), and
• neurophysiology (reduced cerebral blood flow).
These physiologic processes manifest as alterations in mental status, reflexes, sensation, gait, balance, and sleep. Examples of sleep changes among geriatric patients include decreased sleep efficiency, more frequent awakenings, and more variable sleep duration.3,4 Sleep disorders also may be related to mental disorders and other medical conditions.5 For example, the prevalence of sleep-related respiratory disorders, such as obstructive sleep apnea and central sleep apnea, increases with age.6
Sleep disorders are common among geriatric patients. In a large epidemiologic study of sleep complaints in patients age ≥65, more than one-half of patients had at least 1 sleep complaint (ie, difficulty falling asleep, trouble waking up, early awakening, need for naps, and feeling ill-rested).7 As many as 34% of patients reported symptoms of insomnia. In an analysis of National Ambulatory Medical Survey Data over 6 years, 24.8% to 27.9% of sleep-related medical office visits were attributed to patients age ≥65.8
Pharmacology in aging
Prescribing sedative-hypnotic drugs is not routinely recommended for older patients with a sleep disorder. Geriatric patients, compared with younger patients, are at higher risk of iatrogenic complications because of polypharmacy, comorbidities, relative renal and hepatic insufficiency, and other physiologic changes leading to alterations in drug exposure and metabolism (Table 1).9-12
Aging is associated with changes in body composition, including an increase in total body fat and decrease in lean body mass and total body water. These changes, as well as a prolonged GI transit time, decrease in active gut transporters, decreased blood perfusion, and decrease in plasma proteins such as albumin (because of reduced liver function or malnutrition), may lead to alteration in drug absorption patterns and may increase the volume of distribution for lipophilic drugs. Additionally, the elimination half-life of some drugs may increase with age because of larger volumes of distribution and reduction in hepatic or renal clearance.
The clinical significance of these changes is not well established. Although the process of drug absorption can change with age, the amount of drug absorbed might not be significantly affected. An increase in the volume of distribution and reduction in drug metabolism and clearance might lead to increasing amounts of circulating drug and duration of drug exposure, putting geriatric patients at an increased risk for adverse effects and drug toxicity.9
Among these mechanisms, Dolder et al11 hypothesized that drug metabolism catalyzed by cytochrome P450 (CYP) enzymes and renal excretion may be of greatest concern. Although in vitro studies suggest that concentration of CYP enzymes does not decline with age, in vivo studies have demonstrated reduced CYP activity in geriatric patients.11,12 Theoretically, a reduction in CYP activity would increase the bioavailability of drugs, especially those that are subject to extensive first-pass (ie, hepatic) metabolism, and may lead to a reduction in systemic clearance.
Independent of metabolic changes, geriatric patients are at risk of reduced renal clearance because of age-related changes in glomerular filtration rate. Pharmacodynamic changes might be observed in older patients and could be a concern even in the setting of unaltered pharmacokinetic factors.9 These changes usually require administering smaller drug dosages.
Sedative-hypnotic medications
Sedative-hypnotic agents include several barbiturates, benzodiazepines (BZDs), non-BZD benzodiazepine-receptor agonists (BzRAs), a melatonin-receptor agonist (ie, ramelteon), and an orexin-receptor antagonist (ie, suvorexant).13,14Table 214-29 summarizes selected sedative-hypnotic drugs. Additional drug classes used to treat insomnia include:
• sedating antidepressants (trazodone, amitriptyline, doxepin, mirtazapine)
• antiepileptic drugs (gabapentin, tiagabine)
• atypical antipsychotics (quetiapine, olanzapine).
FDA-approved agents for treating insomnia include amobarbital, butabarbital, pentobarbital, phenobarbital, secobarbital, chloral hydrate, diphenhydramine, doxylamine, doxepin, estazolam, flurazepam, lorazepam, quazepam, temazepam, triazolam, eszopiclone, zaleplon, zolpidem, ramelteon, and suvorexant. Not all of these drugs are recommended for use in geriatric patients. Barbiturates, for example, should be avoided.30
Pharmacokinetic characteristics vary among drugs and drug classes. Choice of pharmacotherapy should account for patient and drug characteristics and the specific sleep complaint. Sleep disorders may be variously characterized as difficulty with sleep initiation, duration, consolidation, or quality.13 Therefore, onset and duration of effect are important drug-related considerations. Sedative-hypnotic drugs with a short time-to-onset may be ideal for patients with sleep-onset insomnia.
The drugs’ duration of effect (eg, presence of active metabolites, long elimination half-life) also must be reviewed. A long elimination half-life may lead to increased drug exposure and unwanted side effects such as residual daytime drowsiness. Despite this, sedative-hypnotic drugs with a longer duration of effect (eg, intermediate- or long-acting drugs) may be best for patients with insomnia defined by difficulty maintaining sleep.
Benzodiazepines vary in their time to onset of effect, rate of elimination, and metabolism.15-21 BZDs that are FDA- approved for use as sedative-hypnotics are listed in Table 2.14-29 These BZDs have different onsets of effect as evidenced by time to achieve maximum plasma concentration (Tmax), ranging from 0.5 hours (flurazepam) to 2 hours (estazolam, quazepam, triazolam). The elimination half-life varies widely among these medications, from 1.5 hours (triazolam) to >100 hours (flurazepam). Flurazepam’s long half-life is attributable to its active major metabolite. Although most BZDs are metabolized hepatically, temazepam is subject to minimal hepatic metabolism.
Benzodiazepine-receptor agonists. There is substantial variation in the pharmacokinetic characteristics of BzRAs.15,16,22-28 There also are differences among the zolpidem dosage forms; sublingual formulations have the shortest onset of effect. Eszopiclone and zaleplon have low protein binding compared with zolpidem. Elimination half-lives vary among drugs with the shortest attributed to zaleplon (1 hour) and longest to eszopiclone (6 hours). All BzRAs are subject to extensive hepatic metabolism.
Ramelteon. Singular in its class, ramelteon is a treatment option for insomnia.29 This drug has a short onset of effect, moderate protein binding, and extensive hepatic metabolism. Ramelteon is primarily excreted in the urine as its metabolites, and the drug half-life is relatively short.
Suvorexant is the latest addition to the sedative-hypnotic armamentarium, approved by the FDA in August 2014 for difficulty with sleep onset and/or sleep maintenance.14 As an orexin-receptor antagonist, suvorexant represents a novel pharmacologic class. Suvorexant exhibits moderately rapid absorption with time to peak concentration ranging from 30 minutes to 6 hours in fasting conditions; absorption is delayed when taken with or soon after a meal. The drug is highly protein bound and extensively metabolized, primarily through CYP3A. The manufacturer recommends dose reduction (5 mg at bedtime) in patients taking moderate CYP3A inhibitors and avoiding suvorexant in patients taking strong CYP3A inhibitors. Suvorexant is primarily excreted through feces and the mean half-life is relatively long.
Considering these characteristics and age-related physiologic changes, the practitioner should be concerned about drugs that undergo extensive hepatic metabolism. Age-related reductions in CYP activity may lead to an increase in drug bioavailability and a decrease in the systemic clearance,11 which might be associated with an increase in elimination half-life and duration of action. Dosage adjustments are recommended for several BZDs (lower initial and maximum dosages for most agents) and BzRAs.17-28 No dosage adjustments for ramelteon or suvorexant in geriatric patients have been specified14,29; the manufacturers for both products assert that no differences in safety and efficacy have been observed between older and younger adult patients.
Alternative and complementary medications
Several non-prescription products, including over-the-counter drugs (eg, diphenhy-dramine, doxylamine) and herbal therapies (eg, melatonin, valerian), are used for their sedative-hypnotic properties. There is a lack of evidence supporting using diphenhydra-mine in patients with chronic insomnia, and tolerance to its hypnotic effect has been reported with repeated use.31 Concerns about anticholinergic toxicity and CNS depression limit its use in geriatric patients. Among herbal therapies, melatonin may have the strongest evidence for its ability to alleviate sleep disorders in geriatric patients32; however, meta-analyses have demonstrated small effects of melatonin on sleep latency and minimal differences in wake time after sleep onset and total sleep time.13
Clinical practice guidelines
Non-pharmacotherapeutic interventions, such as behavioral (eg, sleep hygiene measures) and psychological therapy, are recommended for initial management of sleep disorders in geriatric patients.13,33 In conjunction, the American Medical Directors Association (AMDA) recommends address ing underlying causes and exacerbating factors (eg, medical condition or medication).33 The AMDA recommends avoiding long-term pharmacotherapy and advises caution with BZD-hypnotic drugs, tricyclic antidepressants, and antihistamines. The American Academy of Sleep Medicine (AASM) recommends an initial treatment period of 2 to 4 weeks, followed by re-evaluation of continued need for treatment.13 The AASM recommends short- or intermediate-acting BzRAs or ramelteon for initial pharmacologic management of primary insomnias and insomnias comorbid with other conditions. The AASM also recommends specific dosages of BzRAs and BZDs for geriatric patients, which coincide with manufacturer-recommended dosages (Table 2).14-29
Barbiturates, chloral hydrate, and non-barbiturate, non-BZD drugs such as meprobamate are not recommended because of potential significant adverse effects and tolerance/dependence, and low therapeutic index. The AASM advises caution when using prescription drugs off-label for insomnia (eg, antidepressants, antiepileptics, antipsychotics) and recommends avoiding them, if possible, because of limited evidence supporting their use.13
Safety concerns
Two commonly used references contain recommendations for sedative-hypnotic medication use in geriatric patients.30,34 According to Gallagher et al’s34 Screening Tool of Older Person’s Prescriptions (STOPP), long-term (>1 month) use of long-acting BZDs (eg, flurazepam, diazepam) and prolonged use (>1 week) of first-generation antihistamines (eg, diphenhydramine, doxylamine) should be avoided in patients age ≥65 because of the risk of sedation, confusion, and anticholinergic side effects. STOPP recognizes that any use of BZDs, neuroleptics, or first-generation antihistamines may contribute to postural imbalance; therefore these agents are not recommended in older patients at risk for falls.
In the 2012 American Geriatrics Society (AGS) Beers Criteria, the AGS recommends avoiding barbiturates in older adults because of the high rate of physical dependence, tolerance to sleep effects, and overdose risk at low dosages.30 The AGS also recommends avoiding BZDs, stating that older adults have increased sensitivity to these agents and are at an increased risk of cognitive impairment, delirium, falls, fractures, and motor vehicle accidents when taking these drugs. Non-BZD BzRAs also should not be prescribed to patients with a history of falls or fractures, unless safer alternatives are not available.
The FDA has issued several advisory reports regarding sedative-hypnotic drugs. In 2007, all manufacturers of sedative-hypnotic drugs were required to modify their product labeling to include stronger language about potential risks.35 Among these changes, warnings for anaphylaxis and complex sleep-related behaviors were added. Also, the FDA requested that manufacturers of sedative-hypnotic drugs develop and provide patient medication guides, advising consumers on the potential risks and precautions associated with these drugs. More recently, the FDA announced changes to dosing recommendations for zolpidem-containing products because of the risk of impaired mental alertness36; manufacturers were required to lower the recommended dosages for each product.
Manufacturers of FDA-approved sedative-hypnotic drugs urge caution when prescribing these medications for geriatric patients, citing the potential for increased sensitivity, manifesting as marked excitement, depression, or confusion (eg, barbiturates), and greater risk for dosage-related adverse effects (eg, oversedation, dizziness, confusion, impaired psychomotor performance, ataxia).17-29
Use in clinical practice
Several variables should be considered when evaluating appropriateness of pharmacotherapy, including characteristics of the drug and the patient. Geriatric patients may be prone to comorbidities resulting from age-related physiologic changes. These diseases may be confounding (ie, contributing to sleep disorders); examples include medical illnesses, such as hyperthyroidism and arthritis, and psychiatric illnesses, such as depression and anxiety.37 Other conditions, such as renal and hepatic dysfunction, may lead to alteration in drug exposure. These conditions should be assessed through routine renal function tests (eg, serum creatinine and glomerular filtration rate) and liver function tests (eg, serum albumin and liver transaminases).
Multiple comorbidities suggest a higher likelihood of polypharmacy, leading to other drug-related issues (eg, drug-drug interactions). Although these issues may guide therapy by restricting medication options, their potential contribution to the underlying sleep complaints should be considered.37 Several drugs commonly used by geriatric patients may affect wakefulness (eg, analgesics, antidepressants, and antihypertensives [sedating], and thyroid hormones, corticosteroids, and CNS stimulants [alerting]).
CASE CONTINUED
In Mr. R’s case, zaleplon was initiated at 10 mg/d. Because of his age and the nature of his sleep disorder, the choice of sedative-hypnotic was suitable; however, the prescribed dosage was inappropriate. The sluggishness Mr. R experienced likely was a manifestation of increased exposure to the drug. According to manufacturer and AASM recommendations, a more appropriate dosage is 5 mg/d.13,23 Mr. R’s medical history and current medications, and his hepatic and renal function, should be assessed. If Mr. R continues to have issues with sleep initiation, zaleplon, 5 mg at bedtime, should be considered.
Related Resources
• Institute for Safe Medication Practices. www.ismp.org.
• MedWatch: The FDA Safety Information and Adverse Event Reporting Program. www.fda.gov/Safety/MedWatch/default.htm.
Drug Brand Names
Amitriptyline • Elavil Mirtazapine • Remeron
Amobarbital • Amytal Olanzapine • Zyprexa
Butabarbital • Butisol Pentobarbital • Nembutal
Chloral hydrate • Somnote Phenobarbital • Luminal
Diazepam • Valium Quazepam • Doral
Diphenhydramine • Benadryl, others Quetiapine • Seroquel
Doxepin • Silenor Ramelteon • Rozerem
Doxylamine • Unisom, others Secobarbital • Seconal
Estazolam • ProSom Suvorexant • Belsomra
Eszopiclone • Lunesta Temazepam • Restoril
Flurazepam • Dalmane Tiagabine • Gabitril
Gabapentin • Neurontin, Trazodone • Desyrel
Gralise, Horizant Zaleplon • Sonata
Lorazepam • Ativan Zolpidem • Ambien, Edluar,
Meprobamate • Equanil Intermezzo, Zolpimist
Acknowledgement
Vicki L. Ellingrod, PharmD, FCCP, is the series editor of Savvy Psychopharmacology.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. National Center for Health Statistics. Health, United States, 2012, with special feature on emergency care. http://www. cdc.gov/nchs/data/hus/hus12.pdf. Published May 2013. Accessed August 22, 2014.
2. Bronstein AC, Spyker DA, Cantilena LR Jr, et al. 2011 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 29th annual report. Clin Toxicol (Phila). 2012;50(10): 911-1164.
3. Inouye SK. Neuropsychiatric aspects of aging. In: Goldman L, Schafer AI, eds. Goldman’s cecil medicine. 24th ed. Philadelphia, PA: Elsevier Saunders; 2011:114-116.
4. Diagnostic and statistical manual of mental disorders, 4th ed. Washington, DC: American Psychiatric Association; 1994.
5. American Psychiatric Association. Highlights of changes from DSM-IV-TR to DSM-5. http://www.psychiatry. org/File%20Library/Practice/DSM/DSM-5/Changes-from-DSM-IV-TR--to-DSM-5.pdf. 2013. Accessed August 22, 2014.
6. Edwards BA, O’Driscoll DM, Ali A, et al. Aging and sleep: physiology and pathophysiology. Semin Respir Crit Care Med. 2010;31(5):618-633.
7. Foley DJ, Monjan AA, Brown SL, et al. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18(6):425-432.
8. Morlock RJ, Tan M, Mitchell DY. Patient characteristics and patterns of drug use for sleep complaints in the United States: analysis of National Ambulatory Medical Survey Data, 1997-2002. Clin Ther. 2006;28(7):1044-1053.
9. Diasio RB. Principles of drug therapy. In: Goldman L, Schafer AI, eds. Goldman’s Cecil medicine. 24th ed. Philadelphia, PA: Elsevier Saunders; 2011:124-132.
10. Hilmer SN, Ford GA. General principles of pharmacology. In: Halter JB, Ouslander JG, Tinetti ME, et al, eds. Hazzard’s geriatric medicine and gerontology. 6th ed. New York, NY: McGraw-Hill; 2009:103-122.
11. Dolder C, Nelson M, McKinsey J. Use of non-benzodiazepine hypnotics in the elderly: are all agents the same? CNS Drugs. 2007;21(5):389-405.
12. Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev. 2009;41(2):67-76.
13. Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487-504.
14. Belsomra [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.; 2014.
15. Micromedex Healthcare Series. Greenwood Village, CO: Thomson Healthcare. http://micromedex.com. Accessed August 22, 2014.
16. Lexicomp. St. Louis, MO: Wolters Kluwer Health. http:// www.lexi.com. Accessed August 22, 2014.
17. Estazolam [package insert]. Corona, CA: Watson Pharma, Inc; 2008.
18. Flurazepam [package insert]. Eatontown, NJ: West-Ward Pharmaceutical Corp; 2010.
19. Doral [package insert]. Las Vegas, NV: Nuro Pharma, Inc; 2013.
20. Restoril [package insert]. Hazelwood, MO: Mallinckrodt Inc; 2010.
21. Halcion [package insert]. New York, NY: Pharmacia & Upjohn Co; 2013.
22. Lunesta [package insert]. Marlborough, MA: Sunovion Pharmaceuticals Inc; 2012.
23. Sonata [package insert]. New York, NY: Pfizer Inc; 2013.
24. Ambien [package insert]. Bridgewater, NJ: Sanofi-Aventis; 2013.
25. Ambien CR [package insert]. Bridgewater, NJ: Sanofi- Aventis; 2013.
26. Edluar [package insert]. Somerset, NJ: Meda Pharmaceuticals Inc; 2009.
27. Intermezzo [package insert]. Point Richmond, CA: Transcept Pharmaceuticals, Inc; 2011.
28. Zolpimist [package insert]. Richmond, VA: ECR Pharmaceuticals; 2013.
29. Rozerem [package insert]. Deerfield, IL: Takeda Pharmaceuticals America, Inc; 2010.
30. The American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
31. Kirkwood CK, Melton ST. Insomnia, drowsiness, and fatigue. In: Krinsky DL, Berardi RR, Ferreri SP, et al, eds. Handbook of nonprescription drugs: an interactive approach to self-care. 17th ed. Washington, DC: American Pharmacists Association; 2012:867-884.
32. Insomnia. In: Natural Standard. Somerville, MA: Natural Standard. https://naturalmedicines.therapeuticresearch. com/databases/medical-conditions/i/insomnia.aspx. Accessed August 22, 2014.
33. American Medical Directors Association. Clinical practice guideline: sleep disorders. Columbia, MD: American Medical Directors Association; 2006.
34. Gallagher P, Ryan C, Byrne S, et al. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72-83.
35. Food and Drug Administration. FDA requests label change for all sleep disorder drug products. http://www.fda. gov/newsevents/newsroom/pressannouncements/2007/ ucm108868.htm. Published March 14, 2007. Accessed August 22, 2014.
36. Food and Drug Administration. FDA drug safety communication: risk of next-morning impairment after use of insomnia drugs; FDA requires lower recommended doses for certain drugs containing zolpidem (Ambien, Ambien CR, Edluar, and Zolpimist). http://www.fda.gov/drugs/ drugsafety/ucm334033.htm. Published January 10, 2013. Accessed August 22, 2014.
37. Cohen-Zion M, Ancoli-Israel S. Sleep disorders. In: Hazzard’s geriatric medicine and gerontology. 6th ed. New York, NY: McGraw-Hill; 2009:671-682.
1. National Center for Health Statistics. Health, United States, 2012, with special feature on emergency care. http://www. cdc.gov/nchs/data/hus/hus12.pdf. Published May 2013. Accessed August 22, 2014.
2. Bronstein AC, Spyker DA, Cantilena LR Jr, et al. 2011 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 29th annual report. Clin Toxicol (Phila). 2012;50(10): 911-1164.
3. Inouye SK. Neuropsychiatric aspects of aging. In: Goldman L, Schafer AI, eds. Goldman’s cecil medicine. 24th ed. Philadelphia, PA: Elsevier Saunders; 2011:114-116.
4. Diagnostic and statistical manual of mental disorders, 4th ed. Washington, DC: American Psychiatric Association; 1994.
5. American Psychiatric Association. Highlights of changes from DSM-IV-TR to DSM-5. http://www.psychiatry. org/File%20Library/Practice/DSM/DSM-5/Changes-from-DSM-IV-TR--to-DSM-5.pdf. 2013. Accessed August 22, 2014.
6. Edwards BA, O’Driscoll DM, Ali A, et al. Aging and sleep: physiology and pathophysiology. Semin Respir Crit Care Med. 2010;31(5):618-633.
7. Foley DJ, Monjan AA, Brown SL, et al. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18(6):425-432.
8. Morlock RJ, Tan M, Mitchell DY. Patient characteristics and patterns of drug use for sleep complaints in the United States: analysis of National Ambulatory Medical Survey Data, 1997-2002. Clin Ther. 2006;28(7):1044-1053.
9. Diasio RB. Principles of drug therapy. In: Goldman L, Schafer AI, eds. Goldman’s Cecil medicine. 24th ed. Philadelphia, PA: Elsevier Saunders; 2011:124-132.
10. Hilmer SN, Ford GA. General principles of pharmacology. In: Halter JB, Ouslander JG, Tinetti ME, et al, eds. Hazzard’s geriatric medicine and gerontology. 6th ed. New York, NY: McGraw-Hill; 2009:103-122.
11. Dolder C, Nelson M, McKinsey J. Use of non-benzodiazepine hypnotics in the elderly: are all agents the same? CNS Drugs. 2007;21(5):389-405.
12. Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev. 2009;41(2):67-76.
13. Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487-504.
14. Belsomra [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.; 2014.
15. Micromedex Healthcare Series. Greenwood Village, CO: Thomson Healthcare. http://micromedex.com. Accessed August 22, 2014.
16. Lexicomp. St. Louis, MO: Wolters Kluwer Health. http:// www.lexi.com. Accessed August 22, 2014.
17. Estazolam [package insert]. Corona, CA: Watson Pharma, Inc; 2008.
18. Flurazepam [package insert]. Eatontown, NJ: West-Ward Pharmaceutical Corp; 2010.
19. Doral [package insert]. Las Vegas, NV: Nuro Pharma, Inc; 2013.
20. Restoril [package insert]. Hazelwood, MO: Mallinckrodt Inc; 2010.
21. Halcion [package insert]. New York, NY: Pharmacia & Upjohn Co; 2013.
22. Lunesta [package insert]. Marlborough, MA: Sunovion Pharmaceuticals Inc; 2012.
23. Sonata [package insert]. New York, NY: Pfizer Inc; 2013.
24. Ambien [package insert]. Bridgewater, NJ: Sanofi-Aventis; 2013.
25. Ambien CR [package insert]. Bridgewater, NJ: Sanofi- Aventis; 2013.
26. Edluar [package insert]. Somerset, NJ: Meda Pharmaceuticals Inc; 2009.
27. Intermezzo [package insert]. Point Richmond, CA: Transcept Pharmaceuticals, Inc; 2011.
28. Zolpimist [package insert]. Richmond, VA: ECR Pharmaceuticals; 2013.
29. Rozerem [package insert]. Deerfield, IL: Takeda Pharmaceuticals America, Inc; 2010.
30. The American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
31. Kirkwood CK, Melton ST. Insomnia, drowsiness, and fatigue. In: Krinsky DL, Berardi RR, Ferreri SP, et al, eds. Handbook of nonprescription drugs: an interactive approach to self-care. 17th ed. Washington, DC: American Pharmacists Association; 2012:867-884.
32. Insomnia. In: Natural Standard. Somerville, MA: Natural Standard. https://naturalmedicines.therapeuticresearch. com/databases/medical-conditions/i/insomnia.aspx. Accessed August 22, 2014.
33. American Medical Directors Association. Clinical practice guideline: sleep disorders. Columbia, MD: American Medical Directors Association; 2006.
34. Gallagher P, Ryan C, Byrne S, et al. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72-83.
35. Food and Drug Administration. FDA requests label change for all sleep disorder drug products. http://www.fda. gov/newsevents/newsroom/pressannouncements/2007/ ucm108868.htm. Published March 14, 2007. Accessed August 22, 2014.
36. Food and Drug Administration. FDA drug safety communication: risk of next-morning impairment after use of insomnia drugs; FDA requires lower recommended doses for certain drugs containing zolpidem (Ambien, Ambien CR, Edluar, and Zolpimist). http://www.fda.gov/drugs/ drugsafety/ucm334033.htm. Published January 10, 2013. Accessed August 22, 2014.
37. Cohen-Zion M, Ancoli-Israel S. Sleep disorders. In: Hazzard’s geriatric medicine and gerontology. 6th ed. New York, NY: McGraw-Hill; 2009:671-682.
Managing Your Practice: What is your practice worth?
At least once during your career, you probably will have to put a value on your practice. The need arises more often than you might think – if you sell it, of course (more on that next month); but also for estate planning, preparation of financial statements, or divorce negotiations; or when an associate joins or leaves your office; or if you have occasion to combine or partner your practice with one or more others, as I will discuss in detail in a future issue.
As you might guess, a medical practice is trickier to value than an ordinary business, and usually requires the services of an experienced professional appraiser. Entire books have been written about the process, so I can’t hope to cover it completely in a few hundred words, but three basic yardsticks are essential for a practice appraisal:
Tangible assets: equipment, cash, accounts receivable, and other property owned by the practice.
Liabilities: accounts payable, outstanding loans, and anything else owed to others.
Intangible assets: sometimes called “good will” – the reputation of the physicians, the location and name recognition of the practice, the loyalty and volume of patients, and other, well, intangibles.
Armed with those numbers, an appraiser can then determine the “equity,” or book value, of the practice.
Valuing tangible assets is comparatively straightforward, but there are several ways to do it, and when reviewing a practice appraisal you should ask which of them was used. Depreciated value is the book value of equipment and supplies as determined by their purchase price, less the amount their value has decreased since purchase. Remaining useful life value estimates how long the equipment can be expected to last. Market (or replacement) value is the amount it would cost on the open market to replace all equipment and supplies.
Intangible assets are more difficult to value. Many components are analyzed, including location, interior and exterior decor, accessibility to patients, age and functional status of equipment, systems in place to promote efficiency, reasons patients come back (if they do), and the overall reputation of the practice in the community. Other important factors include the “payer mix” (what percentage pays cash, how many third-party contracts are in place and how well they pay, etc.), the extent and strength of the referral base, and the presence of clinical studies or other supplemental income streams.
It is also important to determine to what extent intangible assets are transferrable. For example, unique skills with a laser, neurotoxins, or filler substances (or extraordinary personal charisma) may increase your practice’s value to you, but they are worthless to the next owner, and he or she will be unwilling to pay for them unless your services become part of the deal.
Once again, there are many ways to estimate intangible asset value, and once again you should ask which were used. Cash flow analysis works on the assumption that cash flow is a measure of intangible value. Capitalization of earnings puts a value, or capitalization, on the practice’s income streams using a variety of assumptions. Guideline comparison uses various databases to compare your practice with other, similar ones that have changed hands in the past.
Two newer techniques, which some consider to provide a better estimate of intangible assets, are the replacement method, which estimates the costs of starting the practice over again in the current market; and the excess earnings method, which measures how far above average your practice’s earnings are (and thus its overall value).
Asset-based valuation is the most popular, but by no means the only, method available. Income-based valuation looks at the source and strength of a practice’s income stream as a creator of value, as well as whether or not the income stream under a different owner would mirror its present one. This in turn becomes the basis for an understanding of the fair market value of both tangible and intangible assets. Market valuation combines the asset-based and income-based approaches, along with an analysis of sales and mergers of comparable practices in the community, to determine the value of a practice in its local market.
Whatever methods are used, it is important that the appraisal be done by an experienced financial consultant, that all techniques used in the valuation be divulged and explained, and that documentation is supplied to support the conclusions reached. This is especially important if the appraisal will be relied upon in the sale or merger of the practice. I’ll talk about sales and mergers over the next several columns.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Skin & Allergy News.
At least once during your career, you probably will have to put a value on your practice. The need arises more often than you might think – if you sell it, of course (more on that next month); but also for estate planning, preparation of financial statements, or divorce negotiations; or when an associate joins or leaves your office; or if you have occasion to combine or partner your practice with one or more others, as I will discuss in detail in a future issue.
As you might guess, a medical practice is trickier to value than an ordinary business, and usually requires the services of an experienced professional appraiser. Entire books have been written about the process, so I can’t hope to cover it completely in a few hundred words, but three basic yardsticks are essential for a practice appraisal:
Tangible assets: equipment, cash, accounts receivable, and other property owned by the practice.
Liabilities: accounts payable, outstanding loans, and anything else owed to others.
Intangible assets: sometimes called “good will” – the reputation of the physicians, the location and name recognition of the practice, the loyalty and volume of patients, and other, well, intangibles.
Armed with those numbers, an appraiser can then determine the “equity,” or book value, of the practice.
Valuing tangible assets is comparatively straightforward, but there are several ways to do it, and when reviewing a practice appraisal you should ask which of them was used. Depreciated value is the book value of equipment and supplies as determined by their purchase price, less the amount their value has decreased since purchase. Remaining useful life value estimates how long the equipment can be expected to last. Market (or replacement) value is the amount it would cost on the open market to replace all equipment and supplies.
Intangible assets are more difficult to value. Many components are analyzed, including location, interior and exterior decor, accessibility to patients, age and functional status of equipment, systems in place to promote efficiency, reasons patients come back (if they do), and the overall reputation of the practice in the community. Other important factors include the “payer mix” (what percentage pays cash, how many third-party contracts are in place and how well they pay, etc.), the extent and strength of the referral base, and the presence of clinical studies or other supplemental income streams.
It is also important to determine to what extent intangible assets are transferrable. For example, unique skills with a laser, neurotoxins, or filler substances (or extraordinary personal charisma) may increase your practice’s value to you, but they are worthless to the next owner, and he or she will be unwilling to pay for them unless your services become part of the deal.
Once again, there are many ways to estimate intangible asset value, and once again you should ask which were used. Cash flow analysis works on the assumption that cash flow is a measure of intangible value. Capitalization of earnings puts a value, or capitalization, on the practice’s income streams using a variety of assumptions. Guideline comparison uses various databases to compare your practice with other, similar ones that have changed hands in the past.
Two newer techniques, which some consider to provide a better estimate of intangible assets, are the replacement method, which estimates the costs of starting the practice over again in the current market; and the excess earnings method, which measures how far above average your practice’s earnings are (and thus its overall value).
Asset-based valuation is the most popular, but by no means the only, method available. Income-based valuation looks at the source and strength of a practice’s income stream as a creator of value, as well as whether or not the income stream under a different owner would mirror its present one. This in turn becomes the basis for an understanding of the fair market value of both tangible and intangible assets. Market valuation combines the asset-based and income-based approaches, along with an analysis of sales and mergers of comparable practices in the community, to determine the value of a practice in its local market.
Whatever methods are used, it is important that the appraisal be done by an experienced financial consultant, that all techniques used in the valuation be divulged and explained, and that documentation is supplied to support the conclusions reached. This is especially important if the appraisal will be relied upon in the sale or merger of the practice. I’ll talk about sales and mergers over the next several columns.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Skin & Allergy News.
At least once during your career, you probably will have to put a value on your practice. The need arises more often than you might think – if you sell it, of course (more on that next month); but also for estate planning, preparation of financial statements, or divorce negotiations; or when an associate joins or leaves your office; or if you have occasion to combine or partner your practice with one or more others, as I will discuss in detail in a future issue.
As you might guess, a medical practice is trickier to value than an ordinary business, and usually requires the services of an experienced professional appraiser. Entire books have been written about the process, so I can’t hope to cover it completely in a few hundred words, but three basic yardsticks are essential for a practice appraisal:
Tangible assets: equipment, cash, accounts receivable, and other property owned by the practice.
Liabilities: accounts payable, outstanding loans, and anything else owed to others.
Intangible assets: sometimes called “good will” – the reputation of the physicians, the location and name recognition of the practice, the loyalty and volume of patients, and other, well, intangibles.
Armed with those numbers, an appraiser can then determine the “equity,” or book value, of the practice.
Valuing tangible assets is comparatively straightforward, but there are several ways to do it, and when reviewing a practice appraisal you should ask which of them was used. Depreciated value is the book value of equipment and supplies as determined by their purchase price, less the amount their value has decreased since purchase. Remaining useful life value estimates how long the equipment can be expected to last. Market (or replacement) value is the amount it would cost on the open market to replace all equipment and supplies.
Intangible assets are more difficult to value. Many components are analyzed, including location, interior and exterior decor, accessibility to patients, age and functional status of equipment, systems in place to promote efficiency, reasons patients come back (if they do), and the overall reputation of the practice in the community. Other important factors include the “payer mix” (what percentage pays cash, how many third-party contracts are in place and how well they pay, etc.), the extent and strength of the referral base, and the presence of clinical studies or other supplemental income streams.
It is also important to determine to what extent intangible assets are transferrable. For example, unique skills with a laser, neurotoxins, or filler substances (or extraordinary personal charisma) may increase your practice’s value to you, but they are worthless to the next owner, and he or she will be unwilling to pay for them unless your services become part of the deal.
Once again, there are many ways to estimate intangible asset value, and once again you should ask which were used. Cash flow analysis works on the assumption that cash flow is a measure of intangible value. Capitalization of earnings puts a value, or capitalization, on the practice’s income streams using a variety of assumptions. Guideline comparison uses various databases to compare your practice with other, similar ones that have changed hands in the past.
Two newer techniques, which some consider to provide a better estimate of intangible assets, are the replacement method, which estimates the costs of starting the practice over again in the current market; and the excess earnings method, which measures how far above average your practice’s earnings are (and thus its overall value).
Asset-based valuation is the most popular, but by no means the only, method available. Income-based valuation looks at the source and strength of a practice’s income stream as a creator of value, as well as whether or not the income stream under a different owner would mirror its present one. This in turn becomes the basis for an understanding of the fair market value of both tangible and intangible assets. Market valuation combines the asset-based and income-based approaches, along with an analysis of sales and mergers of comparable practices in the community, to determine the value of a practice in its local market.
Whatever methods are used, it is important that the appraisal be done by an experienced financial consultant, that all techniques used in the valuation be divulged and explained, and that documentation is supplied to support the conclusions reached. This is especially important if the appraisal will be relied upon in the sale or merger of the practice. I’ll talk about sales and mergers over the next several columns.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Skin & Allergy News.