User login
Inspiring Americans
With the understanding that healing after war is a long-term process, Welcome Back Veterans (http://www.welcomebackveterans.org), founded by Major League Baseball Charities, the nonprofit McCormick Foundation, and the charitable Entertainment Industry Foundation, was created to “inspire Americans to reach out and help our returning veterans and their families.”
Welcome Back Veterans offers resources on PTSD, including an overview and related statistics, a detailed symptoms list, treatment information, support group names and phone numbers, and a reading list of books on PTSD, all vetted by the organization.
Highlighted veteran stories are posted in the Latest News, which are also accessible via e-mail sign-ups and RSS feeds. A robust list of family resources is available, as well as many inspiring active-duty military personnel and veteran videos.
In addition to the online resources provided for free on the website, Welcome Back Veterans is funding programs at various institutions throughout the U.S. The McCormick Foundation donation page may be accessed directly through
the homepage of Welcome Back Veterans.
With the understanding that healing after war is a long-term process, Welcome Back Veterans (http://www.welcomebackveterans.org), founded by Major League Baseball Charities, the nonprofit McCormick Foundation, and the charitable Entertainment Industry Foundation, was created to “inspire Americans to reach out and help our returning veterans and their families.”
Welcome Back Veterans offers resources on PTSD, including an overview and related statistics, a detailed symptoms list, treatment information, support group names and phone numbers, and a reading list of books on PTSD, all vetted by the organization.
Highlighted veteran stories are posted in the Latest News, which are also accessible via e-mail sign-ups and RSS feeds. A robust list of family resources is available, as well as many inspiring active-duty military personnel and veteran videos.
In addition to the online resources provided for free on the website, Welcome Back Veterans is funding programs at various institutions throughout the U.S. The McCormick Foundation donation page may be accessed directly through
the homepage of Welcome Back Veterans.
With the understanding that healing after war is a long-term process, Welcome Back Veterans (http://www.welcomebackveterans.org), founded by Major League Baseball Charities, the nonprofit McCormick Foundation, and the charitable Entertainment Industry Foundation, was created to “inspire Americans to reach out and help our returning veterans and their families.”
Welcome Back Veterans offers resources on PTSD, including an overview and related statistics, a detailed symptoms list, treatment information, support group names and phone numbers, and a reading list of books on PTSD, all vetted by the organization.
Highlighted veteran stories are posted in the Latest News, which are also accessible via e-mail sign-ups and RSS feeds. A robust list of family resources is available, as well as many inspiring active-duty military personnel and veteran videos.
In addition to the online resources provided for free on the website, Welcome Back Veterans is funding programs at various institutions throughout the U.S. The McCormick Foundation donation page may be accessed directly through
the homepage of Welcome Back Veterans.
Molecule enables ‘robust’ expansion of cord blood cells

Credit: NHS
Investigators say they have identified a molecule that allows for robust ex vivo expansion of human cord blood (CB) cells.
CB cells expanded with this molecule, known as UM171, were capable of human hematopoietic reconstitution in NSG mice, an effect that lasted more than 6 months.
The researchers believe UM171 acts by enhancing the long-term-hematopoietic stem cell (LT-HSC) self-renewal machinery independently of AhR suppression.
Guy Sauvageau, MD, PhD, of the University of Montreal in Quebec, Canada, and his colleagues identified UM171 and described the discovery in Science.
The team first screened a library of 5280 low-molecular-weight compounds looking for those with the ability to expand human CD34+CD45RA- mobilized peripheral blood cells, which are enriched in LT-HSCs.
They got 7 hits, and only 2 of these—UM729 and UM118428—did not suppress the AhR pathway. The researchers selected UM729 for further characterization and optimization because it demonstrated superior activity in expanding CD34+CD45RA- cells.
The investigators analyzed more than 300 newly synthesized analogs of UM729 and identified one that was 10 to 20 times more potent than UM729. That compound was UM171.
UM171 could expand CD34+CD45RA- cells at concentrations of 17 nM to 19 nM. The highest expansion of multipotent progenitors and long-term culture-initiating cells occurred on day 12.
The effect of UM171 required its constant presence in the media, and the compound lacked direct mitogenic activity. UM171 did not affect the division rate of phenotypically primitive cell populations.
The researchers compared UM171 to SR1 (a compound known to promote self-renewal of HSCs) in fed-batch culture. They found that frequencies of CD34+ CB cells were similar in cultures containing SR1 and those containing UM171. But CD34+CD45RA- cells were more abundant with UM171 (P<0.005).
The team then evaluated LT-HSC populations. Twenty weeks after CD34+ CB cells were transplanted into mice, LT-HSC frequencies were similar in mice that received control and SR1-expanded cells. But LT-HSC frequencies were 13-fold higher in the mice that received UM171-expanded cells.
Next, the investigators assessed human hematopoietic reconstitution in NSG mice transplanted with fresh or expanded cells. They observed 2 patterns of reconstitution. One was from predominantly lymphomyeloid LT-HSCs that occurred with high cell doses in most conditions.
And the other was from LT-HSCs that display a lymphoid-deficient differentiation phenotype mostly observed with UM171 treatment, with or without SR1. However, UM171 did not negatively affect B lymphopoiesis or the frequency or number of lymphomyeloid LT-HSCs.
The impact of UM171 on LT-HSCs was preserved at 30 weeks post-transplant. And myeloid cell output was slightly augmented, a phenomenon that has been observed with normal, unexpanded cells.
The researchers also transplanted UM171-treated LT-HSC populations into secondary recipients. And they found the cells were still competent, but they had no advantage over unmanipulated CD34+ cells.
A clinical study of UM171 and a new type of bioreactor developed for stem cell culture is set to begin this December. The cells will be expanded at Maisonneuve-Rosemont Hospital, and grafts will be distributed to patients in Montreal, Quebec City, and Vancouver. The investigators expect tangible results will be available in December 2015. ![]()

Credit: NHS
Investigators say they have identified a molecule that allows for robust ex vivo expansion of human cord blood (CB) cells.
CB cells expanded with this molecule, known as UM171, were capable of human hematopoietic reconstitution in NSG mice, an effect that lasted more than 6 months.
The researchers believe UM171 acts by enhancing the long-term-hematopoietic stem cell (LT-HSC) self-renewal machinery independently of AhR suppression.
Guy Sauvageau, MD, PhD, of the University of Montreal in Quebec, Canada, and his colleagues identified UM171 and described the discovery in Science.
The team first screened a library of 5280 low-molecular-weight compounds looking for those with the ability to expand human CD34+CD45RA- mobilized peripheral blood cells, which are enriched in LT-HSCs.
They got 7 hits, and only 2 of these—UM729 and UM118428—did not suppress the AhR pathway. The researchers selected UM729 for further characterization and optimization because it demonstrated superior activity in expanding CD34+CD45RA- cells.
The investigators analyzed more than 300 newly synthesized analogs of UM729 and identified one that was 10 to 20 times more potent than UM729. That compound was UM171.
UM171 could expand CD34+CD45RA- cells at concentrations of 17 nM to 19 nM. The highest expansion of multipotent progenitors and long-term culture-initiating cells occurred on day 12.
The effect of UM171 required its constant presence in the media, and the compound lacked direct mitogenic activity. UM171 did not affect the division rate of phenotypically primitive cell populations.
The researchers compared UM171 to SR1 (a compound known to promote self-renewal of HSCs) in fed-batch culture. They found that frequencies of CD34+ CB cells were similar in cultures containing SR1 and those containing UM171. But CD34+CD45RA- cells were more abundant with UM171 (P<0.005).
The team then evaluated LT-HSC populations. Twenty weeks after CD34+ CB cells were transplanted into mice, LT-HSC frequencies were similar in mice that received control and SR1-expanded cells. But LT-HSC frequencies were 13-fold higher in the mice that received UM171-expanded cells.
Next, the investigators assessed human hematopoietic reconstitution in NSG mice transplanted with fresh or expanded cells. They observed 2 patterns of reconstitution. One was from predominantly lymphomyeloid LT-HSCs that occurred with high cell doses in most conditions.
And the other was from LT-HSCs that display a lymphoid-deficient differentiation phenotype mostly observed with UM171 treatment, with or without SR1. However, UM171 did not negatively affect B lymphopoiesis or the frequency or number of lymphomyeloid LT-HSCs.
The impact of UM171 on LT-HSCs was preserved at 30 weeks post-transplant. And myeloid cell output was slightly augmented, a phenomenon that has been observed with normal, unexpanded cells.
The researchers also transplanted UM171-treated LT-HSC populations into secondary recipients. And they found the cells were still competent, but they had no advantage over unmanipulated CD34+ cells.
A clinical study of UM171 and a new type of bioreactor developed for stem cell culture is set to begin this December. The cells will be expanded at Maisonneuve-Rosemont Hospital, and grafts will be distributed to patients in Montreal, Quebec City, and Vancouver. The investigators expect tangible results will be available in December 2015. ![]()

Credit: NHS
Investigators say they have identified a molecule that allows for robust ex vivo expansion of human cord blood (CB) cells.
CB cells expanded with this molecule, known as UM171, were capable of human hematopoietic reconstitution in NSG mice, an effect that lasted more than 6 months.
The researchers believe UM171 acts by enhancing the long-term-hematopoietic stem cell (LT-HSC) self-renewal machinery independently of AhR suppression.
Guy Sauvageau, MD, PhD, of the University of Montreal in Quebec, Canada, and his colleagues identified UM171 and described the discovery in Science.
The team first screened a library of 5280 low-molecular-weight compounds looking for those with the ability to expand human CD34+CD45RA- mobilized peripheral blood cells, which are enriched in LT-HSCs.
They got 7 hits, and only 2 of these—UM729 and UM118428—did not suppress the AhR pathway. The researchers selected UM729 for further characterization and optimization because it demonstrated superior activity in expanding CD34+CD45RA- cells.
The investigators analyzed more than 300 newly synthesized analogs of UM729 and identified one that was 10 to 20 times more potent than UM729. That compound was UM171.
UM171 could expand CD34+CD45RA- cells at concentrations of 17 nM to 19 nM. The highest expansion of multipotent progenitors and long-term culture-initiating cells occurred on day 12.
The effect of UM171 required its constant presence in the media, and the compound lacked direct mitogenic activity. UM171 did not affect the division rate of phenotypically primitive cell populations.
The researchers compared UM171 to SR1 (a compound known to promote self-renewal of HSCs) in fed-batch culture. They found that frequencies of CD34+ CB cells were similar in cultures containing SR1 and those containing UM171. But CD34+CD45RA- cells were more abundant with UM171 (P<0.005).
The team then evaluated LT-HSC populations. Twenty weeks after CD34+ CB cells were transplanted into mice, LT-HSC frequencies were similar in mice that received control and SR1-expanded cells. But LT-HSC frequencies were 13-fold higher in the mice that received UM171-expanded cells.
Next, the investigators assessed human hematopoietic reconstitution in NSG mice transplanted with fresh or expanded cells. They observed 2 patterns of reconstitution. One was from predominantly lymphomyeloid LT-HSCs that occurred with high cell doses in most conditions.
And the other was from LT-HSCs that display a lymphoid-deficient differentiation phenotype mostly observed with UM171 treatment, with or without SR1. However, UM171 did not negatively affect B lymphopoiesis or the frequency or number of lymphomyeloid LT-HSCs.
The impact of UM171 on LT-HSCs was preserved at 30 weeks post-transplant. And myeloid cell output was slightly augmented, a phenomenon that has been observed with normal, unexpanded cells.
The researchers also transplanted UM171-treated LT-HSC populations into secondary recipients. And they found the cells were still competent, but they had no advantage over unmanipulated CD34+ cells.
A clinical study of UM171 and a new type of bioreactor developed for stem cell culture is set to begin this December. The cells will be expanded at Maisonneuve-Rosemont Hospital, and grafts will be distributed to patients in Montreal, Quebec City, and Vancouver. The investigators expect tangible results will be available in December 2015. ![]()
Predicting chemo drugs’ effects on male fertility

Credit: Rhoda Baer
A mathematical formula may help physicians predict which cancer survivors are likely to have normal sperm production following chemotherapy with alkylating agents.
Researchers developed the formula to calculate survivors’ cumulative treatment exposure to alkylating agents as a cyclophosphamide-equivalent dose (CED).
The team used this formula to evaluate a cohort of cancer survivors and found that CEDs of 4 g/m2 or less were associated with normal sperm concentrations.
An account of this research was published in The Lancet Oncology.
“Based on these results, we would recommend pretreatment fertility preservation be offered, whenever clinically possible, to any male whose projected treatment is expected to include a cyclophosphamide-equivalent dose greater than 4 g/m2,” said study author Daniel Green, MD, of St Jude Children’s Research Hospital in Memphis, Tennessee.
Dr Green and his colleagues had studied 214 male survivors of childhood cancer who had a median age of 29. Subjects had received cyclophosphamide and other alkylating agents, but their treatment did not include radiation.
The researchers performed semen analysis in the survivors a median of 21 years from their diagnosis. The analysis showed that 47.6% of survivors had normal sperm concentrations (at least 15 million per milliliter), 27.6% had low sperm counts, and 24.8% produced no sperm.
To determine the impact of alkylating agents on the survivors’ sperm production, the researchers calculated each survivor’s cumulative treatment exposure as a CED.
They did this using the following formula: CED (mg/m2)= 1.0 (cumulative cyclophosphamide dose [mg/m2]) + 0.244 (cumulative ifosfamide dose [mg/m2]) + 0.857 (cumulative procarbazine dose [mg/m2]) + 14.286 (cumulative chlorambucil dose [mg/m2]) + 15.0 (cumulative carmustine dose [mg/m2]) + 16.0 (cumulative lomustine dose [mg/m2]) + 40 (cumulative melphalan dose [mg/m2]) + 50 (cumulative thiotepa dose [mg/m2]) + 100 cumulative chlormethine dose [mg/m2]) + 8.823 (cumulative busulfan dose [mg/m2]).
The researchers could not identify a uniformly safe or toxic dose of these drugs, but they found that survivor sperm concentrations generally decreased as cumulative exposure to alkylating agents increased.
And CEDs of 4 g/m2 or less were associated with normal sperm concentrations. Almost 89% of survivors whose CED was 4 g/m2 or less had a normal sperm count. Their sperm were also more likely than sperm from other survivors to look and behave normally.
Dr Green did note that, although a lower cumulative dose of alkylating agents was associated with normal sperm production, outcomes for individual patients were unpredictable and varied. ![]()

Credit: Rhoda Baer
A mathematical formula may help physicians predict which cancer survivors are likely to have normal sperm production following chemotherapy with alkylating agents.
Researchers developed the formula to calculate survivors’ cumulative treatment exposure to alkylating agents as a cyclophosphamide-equivalent dose (CED).
The team used this formula to evaluate a cohort of cancer survivors and found that CEDs of 4 g/m2 or less were associated with normal sperm concentrations.
An account of this research was published in The Lancet Oncology.
“Based on these results, we would recommend pretreatment fertility preservation be offered, whenever clinically possible, to any male whose projected treatment is expected to include a cyclophosphamide-equivalent dose greater than 4 g/m2,” said study author Daniel Green, MD, of St Jude Children’s Research Hospital in Memphis, Tennessee.
Dr Green and his colleagues had studied 214 male survivors of childhood cancer who had a median age of 29. Subjects had received cyclophosphamide and other alkylating agents, but their treatment did not include radiation.
The researchers performed semen analysis in the survivors a median of 21 years from their diagnosis. The analysis showed that 47.6% of survivors had normal sperm concentrations (at least 15 million per milliliter), 27.6% had low sperm counts, and 24.8% produced no sperm.
To determine the impact of alkylating agents on the survivors’ sperm production, the researchers calculated each survivor’s cumulative treatment exposure as a CED.
They did this using the following formula: CED (mg/m2)= 1.0 (cumulative cyclophosphamide dose [mg/m2]) + 0.244 (cumulative ifosfamide dose [mg/m2]) + 0.857 (cumulative procarbazine dose [mg/m2]) + 14.286 (cumulative chlorambucil dose [mg/m2]) + 15.0 (cumulative carmustine dose [mg/m2]) + 16.0 (cumulative lomustine dose [mg/m2]) + 40 (cumulative melphalan dose [mg/m2]) + 50 (cumulative thiotepa dose [mg/m2]) + 100 cumulative chlormethine dose [mg/m2]) + 8.823 (cumulative busulfan dose [mg/m2]).
The researchers could not identify a uniformly safe or toxic dose of these drugs, but they found that survivor sperm concentrations generally decreased as cumulative exposure to alkylating agents increased.
And CEDs of 4 g/m2 or less were associated with normal sperm concentrations. Almost 89% of survivors whose CED was 4 g/m2 or less had a normal sperm count. Their sperm were also more likely than sperm from other survivors to look and behave normally.
Dr Green did note that, although a lower cumulative dose of alkylating agents was associated with normal sperm production, outcomes for individual patients were unpredictable and varied. ![]()

Credit: Rhoda Baer
A mathematical formula may help physicians predict which cancer survivors are likely to have normal sperm production following chemotherapy with alkylating agents.
Researchers developed the formula to calculate survivors’ cumulative treatment exposure to alkylating agents as a cyclophosphamide-equivalent dose (CED).
The team used this formula to evaluate a cohort of cancer survivors and found that CEDs of 4 g/m2 or less were associated with normal sperm concentrations.
An account of this research was published in The Lancet Oncology.
“Based on these results, we would recommend pretreatment fertility preservation be offered, whenever clinically possible, to any male whose projected treatment is expected to include a cyclophosphamide-equivalent dose greater than 4 g/m2,” said study author Daniel Green, MD, of St Jude Children’s Research Hospital in Memphis, Tennessee.
Dr Green and his colleagues had studied 214 male survivors of childhood cancer who had a median age of 29. Subjects had received cyclophosphamide and other alkylating agents, but their treatment did not include radiation.
The researchers performed semen analysis in the survivors a median of 21 years from their diagnosis. The analysis showed that 47.6% of survivors had normal sperm concentrations (at least 15 million per milliliter), 27.6% had low sperm counts, and 24.8% produced no sperm.
To determine the impact of alkylating agents on the survivors’ sperm production, the researchers calculated each survivor’s cumulative treatment exposure as a CED.
They did this using the following formula: CED (mg/m2)= 1.0 (cumulative cyclophosphamide dose [mg/m2]) + 0.244 (cumulative ifosfamide dose [mg/m2]) + 0.857 (cumulative procarbazine dose [mg/m2]) + 14.286 (cumulative chlorambucil dose [mg/m2]) + 15.0 (cumulative carmustine dose [mg/m2]) + 16.0 (cumulative lomustine dose [mg/m2]) + 40 (cumulative melphalan dose [mg/m2]) + 50 (cumulative thiotepa dose [mg/m2]) + 100 cumulative chlormethine dose [mg/m2]) + 8.823 (cumulative busulfan dose [mg/m2]).
The researchers could not identify a uniformly safe or toxic dose of these drugs, but they found that survivor sperm concentrations generally decreased as cumulative exposure to alkylating agents increased.
And CEDs of 4 g/m2 or less were associated with normal sperm concentrations. Almost 89% of survivors whose CED was 4 g/m2 or less had a normal sperm count. Their sperm were also more likely than sperm from other survivors to look and behave normally.
Dr Green did note that, although a lower cumulative dose of alkylating agents was associated with normal sperm production, outcomes for individual patients were unpredictable and varied. ![]()
Disrupting equilibrium to fight AML

Credit: Lance Liotta
Understanding the relationship between mutated and wild-type genes could lead to new therapies for acute myeloid leukemia (AML), according to researchers.
The group discovered that RUNX1/ETO, generated by the chromosomal translocation t(8;21), regulates a leukemia-propagating transcriptional network via a dynamic equilibrium with RUNX1.
But depleting RUNX1/ETO prompts the formation of a transcriptional network that promotes myeloid differentiation.
Constanze Bonifer, PhD, of the University of Birmingham in the UK, and her colleagues detailed this work in Cell Reports.
The researchers likened normal hematopoiesis to a production line, with genes acting as regulators to control each step of blood formation. When a mutation occurs in the relevant regulator genes, the finely balanced order of the production line is disrupted, with drastic consequences.
Using digital footprinting and chromatin immunoprecipitation sequencing, the team showed that RUNX1/ETO switches off hundreds of other genes, many of them regulators themselves. As a consequence of the drastically altered production line, normal blood formation cannot happen, and leukemic cells form.
“Understanding how these rogue regulators operate is essential,” Dr Bonifer said. “[T]hese leukemic cells have one mutated gene and one unchanged one that would make the normal regulator. What happens in the leukemic cell is fundamentally a battle for supremacy between the two regulators, and the mutated one wins much of the time.”
“This is compounded by the normal regulator, which tries to compensate for defeat, and, in doing so, changes the output of genes that would be otherwise unaffected by the abnormal regulator. Quite simply, the result is a real mess. The cells are confused and can’t develop into mature blood cells.”
More specifically, the researchers found that the transcriptional program underlying leukemic propagation is regulated by a dynamic equilibrium between RUNX1/ETO and RUNX1 complexes.
Disrupting this equilibrium in t(8;21) cells by depleting RUNX1/ETO led to a global redistribution of transcription factor complexes within pre-existing open chromatin. And this prompted the formation of a transcriptional network that drives myeloid differentiation.
“If targeting [RUNX1/ETO] can reverse the changes it is making to the cellular production line, then it would ultimately point towards new avenues for a more precise treatment of leukemia,” said study author Olaf Heidenreich, PhD, of Newcastle University in the UK.
“Knowing that the production line can be restored to normal function gives us real hope. Of course, that is much easier to do in the lab than it is in the human body. But now we know how this works, we can look to deliver inhibitors to those mutated regulators. Creating one that works is the next step we have to overcome.” ![]()

Credit: Lance Liotta
Understanding the relationship between mutated and wild-type genes could lead to new therapies for acute myeloid leukemia (AML), according to researchers.
The group discovered that RUNX1/ETO, generated by the chromosomal translocation t(8;21), regulates a leukemia-propagating transcriptional network via a dynamic equilibrium with RUNX1.
But depleting RUNX1/ETO prompts the formation of a transcriptional network that promotes myeloid differentiation.
Constanze Bonifer, PhD, of the University of Birmingham in the UK, and her colleagues detailed this work in Cell Reports.
The researchers likened normal hematopoiesis to a production line, with genes acting as regulators to control each step of blood formation. When a mutation occurs in the relevant regulator genes, the finely balanced order of the production line is disrupted, with drastic consequences.
Using digital footprinting and chromatin immunoprecipitation sequencing, the team showed that RUNX1/ETO switches off hundreds of other genes, many of them regulators themselves. As a consequence of the drastically altered production line, normal blood formation cannot happen, and leukemic cells form.
“Understanding how these rogue regulators operate is essential,” Dr Bonifer said. “[T]hese leukemic cells have one mutated gene and one unchanged one that would make the normal regulator. What happens in the leukemic cell is fundamentally a battle for supremacy between the two regulators, and the mutated one wins much of the time.”
“This is compounded by the normal regulator, which tries to compensate for defeat, and, in doing so, changes the output of genes that would be otherwise unaffected by the abnormal regulator. Quite simply, the result is a real mess. The cells are confused and can’t develop into mature blood cells.”
More specifically, the researchers found that the transcriptional program underlying leukemic propagation is regulated by a dynamic equilibrium between RUNX1/ETO and RUNX1 complexes.
Disrupting this equilibrium in t(8;21) cells by depleting RUNX1/ETO led to a global redistribution of transcription factor complexes within pre-existing open chromatin. And this prompted the formation of a transcriptional network that drives myeloid differentiation.
“If targeting [RUNX1/ETO] can reverse the changes it is making to the cellular production line, then it would ultimately point towards new avenues for a more precise treatment of leukemia,” said study author Olaf Heidenreich, PhD, of Newcastle University in the UK.
“Knowing that the production line can be restored to normal function gives us real hope. Of course, that is much easier to do in the lab than it is in the human body. But now we know how this works, we can look to deliver inhibitors to those mutated regulators. Creating one that works is the next step we have to overcome.” ![]()

Credit: Lance Liotta
Understanding the relationship between mutated and wild-type genes could lead to new therapies for acute myeloid leukemia (AML), according to researchers.
The group discovered that RUNX1/ETO, generated by the chromosomal translocation t(8;21), regulates a leukemia-propagating transcriptional network via a dynamic equilibrium with RUNX1.
But depleting RUNX1/ETO prompts the formation of a transcriptional network that promotes myeloid differentiation.
Constanze Bonifer, PhD, of the University of Birmingham in the UK, and her colleagues detailed this work in Cell Reports.
The researchers likened normal hematopoiesis to a production line, with genes acting as regulators to control each step of blood formation. When a mutation occurs in the relevant regulator genes, the finely balanced order of the production line is disrupted, with drastic consequences.
Using digital footprinting and chromatin immunoprecipitation sequencing, the team showed that RUNX1/ETO switches off hundreds of other genes, many of them regulators themselves. As a consequence of the drastically altered production line, normal blood formation cannot happen, and leukemic cells form.
“Understanding how these rogue regulators operate is essential,” Dr Bonifer said. “[T]hese leukemic cells have one mutated gene and one unchanged one that would make the normal regulator. What happens in the leukemic cell is fundamentally a battle for supremacy between the two regulators, and the mutated one wins much of the time.”
“This is compounded by the normal regulator, which tries to compensate for defeat, and, in doing so, changes the output of genes that would be otherwise unaffected by the abnormal regulator. Quite simply, the result is a real mess. The cells are confused and can’t develop into mature blood cells.”
More specifically, the researchers found that the transcriptional program underlying leukemic propagation is regulated by a dynamic equilibrium between RUNX1/ETO and RUNX1 complexes.
Disrupting this equilibrium in t(8;21) cells by depleting RUNX1/ETO led to a global redistribution of transcription factor complexes within pre-existing open chromatin. And this prompted the formation of a transcriptional network that drives myeloid differentiation.
“If targeting [RUNX1/ETO] can reverse the changes it is making to the cellular production line, then it would ultimately point towards new avenues for a more precise treatment of leukemia,” said study author Olaf Heidenreich, PhD, of Newcastle University in the UK.
“Knowing that the production line can be restored to normal function gives us real hope. Of course, that is much easier to do in the lab than it is in the human body. But now we know how this works, we can look to deliver inhibitors to those mutated regulators. Creating one that works is the next step we have to overcome.” ![]()
Longer duration of triple therapy confers no benefit, trial suggests

Credit: Sage Ross
WASHINGTON, DC—Six weeks of triple anticoagulant therapy may be sufficient in patients who have received a drug-eluting stent.
Results of the ISAR-TRIPLE trial revealed no significant differences in net clinical outcomes for patients who received 6 weeks of triple anticoagulant therapy and those who received 6 months of the therapy.
Nikolaus Sarafoff, MD, of Deutsches Herzzentrum Munich and Klinikum der Universität Munich in Germany, presented these results at TCT 2014.
“The shortening of triple therapy neither reduced the incidence of TIMI major bleeding nor increased the incidence of the composite of ischemic events,” Dr Sarafoff said. “These results suggest that physicians should weigh the trade-off between ischemic and bleeding risk when choosing the shorter or longer duration of triple therapy.”
For the ISAR-TRIPLE trial, Dr Sarafoff and his colleagues randomized 614 patients in a 1:1 fashion to either 6 weeks or 6 months of clopidogrel therapy, in addition to aspirin plus an oral anticoagulant (phenprocoumon or warfarin).
The primary endpoint was a composite of death, myocardial infarction, definite stent thrombosis, stroke, or TIMI major bleeding at 9 months.
As secondary endpoints, the researchers assessed TIMI major bleeding separately from a composite endpoint of cardiac death, myocardial infarction, stent thrombosis, or ischemic stroke.
The primary endpoint occurred in 9.8% of patients in the 6-week treatment group and 8.8% of patients in the 6-month group (P=0.63.)
The composite of cardiac death, myocardial infarction, stent thrombosis, or ischemic stroke occurred at a similar rate in both the 6-week and 6-month groups—4.0% and 4.3%, respectively (P=0.87).
And the same was true for TIMI major bleeding, which occurred in 5.3% of patients in the 6-week group and 4.0% in the 6-month group (P=0.44).
This trial was funded by Deutsches Herzzentrum München. Dr Sarafoff reported fees for lectures or traveling from Lilly/Daiichi Sankyo, Boehringer Ingelheim, Bayer Healthcare, Boston Scientific, Biotronik, and Medtronic. ![]()

Credit: Sage Ross
WASHINGTON, DC—Six weeks of triple anticoagulant therapy may be sufficient in patients who have received a drug-eluting stent.
Results of the ISAR-TRIPLE trial revealed no significant differences in net clinical outcomes for patients who received 6 weeks of triple anticoagulant therapy and those who received 6 months of the therapy.
Nikolaus Sarafoff, MD, of Deutsches Herzzentrum Munich and Klinikum der Universität Munich in Germany, presented these results at TCT 2014.
“The shortening of triple therapy neither reduced the incidence of TIMI major bleeding nor increased the incidence of the composite of ischemic events,” Dr Sarafoff said. “These results suggest that physicians should weigh the trade-off between ischemic and bleeding risk when choosing the shorter or longer duration of triple therapy.”
For the ISAR-TRIPLE trial, Dr Sarafoff and his colleagues randomized 614 patients in a 1:1 fashion to either 6 weeks or 6 months of clopidogrel therapy, in addition to aspirin plus an oral anticoagulant (phenprocoumon or warfarin).
The primary endpoint was a composite of death, myocardial infarction, definite stent thrombosis, stroke, or TIMI major bleeding at 9 months.
As secondary endpoints, the researchers assessed TIMI major bleeding separately from a composite endpoint of cardiac death, myocardial infarction, stent thrombosis, or ischemic stroke.
The primary endpoint occurred in 9.8% of patients in the 6-week treatment group and 8.8% of patients in the 6-month group (P=0.63.)
The composite of cardiac death, myocardial infarction, stent thrombosis, or ischemic stroke occurred at a similar rate in both the 6-week and 6-month groups—4.0% and 4.3%, respectively (P=0.87).
And the same was true for TIMI major bleeding, which occurred in 5.3% of patients in the 6-week group and 4.0% in the 6-month group (P=0.44).
This trial was funded by Deutsches Herzzentrum München. Dr Sarafoff reported fees for lectures or traveling from Lilly/Daiichi Sankyo, Boehringer Ingelheim, Bayer Healthcare, Boston Scientific, Biotronik, and Medtronic. ![]()

Credit: Sage Ross
WASHINGTON, DC—Six weeks of triple anticoagulant therapy may be sufficient in patients who have received a drug-eluting stent.
Results of the ISAR-TRIPLE trial revealed no significant differences in net clinical outcomes for patients who received 6 weeks of triple anticoagulant therapy and those who received 6 months of the therapy.
Nikolaus Sarafoff, MD, of Deutsches Herzzentrum Munich and Klinikum der Universität Munich in Germany, presented these results at TCT 2014.
“The shortening of triple therapy neither reduced the incidence of TIMI major bleeding nor increased the incidence of the composite of ischemic events,” Dr Sarafoff said. “These results suggest that physicians should weigh the trade-off between ischemic and bleeding risk when choosing the shorter or longer duration of triple therapy.”
For the ISAR-TRIPLE trial, Dr Sarafoff and his colleagues randomized 614 patients in a 1:1 fashion to either 6 weeks or 6 months of clopidogrel therapy, in addition to aspirin plus an oral anticoagulant (phenprocoumon or warfarin).
The primary endpoint was a composite of death, myocardial infarction, definite stent thrombosis, stroke, or TIMI major bleeding at 9 months.
As secondary endpoints, the researchers assessed TIMI major bleeding separately from a composite endpoint of cardiac death, myocardial infarction, stent thrombosis, or ischemic stroke.
The primary endpoint occurred in 9.8% of patients in the 6-week treatment group and 8.8% of patients in the 6-month group (P=0.63.)
The composite of cardiac death, myocardial infarction, stent thrombosis, or ischemic stroke occurred at a similar rate in both the 6-week and 6-month groups—4.0% and 4.3%, respectively (P=0.87).
And the same was true for TIMI major bleeding, which occurred in 5.3% of patients in the 6-week group and 4.0% in the 6-month group (P=0.44).
This trial was funded by Deutsches Herzzentrum München. Dr Sarafoff reported fees for lectures or traveling from Lilly/Daiichi Sankyo, Boehringer Ingelheim, Bayer Healthcare, Boston Scientific, Biotronik, and Medtronic. ![]()
Shorter Stay, Fewer Interventions With Initial Cholecystectomy for Choledocholithiasis
Clinical question
Which strategy — initial cholecystectomy or common duct endoscopic investigation followed by cholecystectomy — leads to better outcomes in the treatment of suspected common duct stones?
Bottom line
As compared with a strategy of sequential common duct endoscopy followed by surgery, the use of initial cholecystectomy with intraoperative cholangiogram for patients at intermediate risk of choledocholithiasis leads to shorter hospital length of stay and fewer interventions with otherwise comparable outcomes. (LOE = 1b)
Reference
Iranmanesh P, Frossard J, Mugnier-Konrad B, et al. Initial cholecystectomy vs. sequential common duct endoscopic assessment and subsequent cholecystectomy for suspected gallstone migration. JAMA 2014;312(2):137-144.
Study design
Randomized controlled trial (nonblinded)
Funding source
Unknown/not stated
Allocation
Concealed
Setting
Inpatient (any location) with outpatient follow-up
Synopsis
The role of common duct endoscopic investigation prior to cholecystectomy for patients at intermediate risk of choledocholithiasis is questionable as stones often migrate into the duodenum without such intervention. Using concealed allocation, investigators randomized 100 patients who presented to an emergency department with clinical suspicion of choledocholithiasis (right upper quadrant or epigastric abdominal pain with elevated liver function test results and ultrasound-confirmed gallstone) to 1 of 2 groups. The study group underwent primary cholecystectomy with intraoperative cholangiogram, while the classical treatment group first had common duct exploration with magnetic resonance cholangiopancreatography (MRCP), endoscopic ultrasound (EUS), and endoscopic retrograde cholangiopancreatography (ERCP), if needed, followed by cholecystectomy. The authors excluded patients with severe sepsis, pancreatitis, bilirubinemia greater than 4 mg/dL, or proven common duct stones on admission. The 2 groups had similar baseline characteristics, including similar preoperative health status scores. Overall, the number of confirmed common duct stones in each group was approximately 20%. Patients in the cholecystectomy-first group had a significantly shorter hospital length of stay than the classical treatment group (5 days vs 8 days; P < .001) and also had fewer common duct investigations (MRCP, EUS, or ERCP; 25 vs 71; P < .001). There were no significant differences detected between the 2 groups in quality-of-life assessments or in surgical or medical complications within 6 months.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question
Which strategy — initial cholecystectomy or common duct endoscopic investigation followed by cholecystectomy — leads to better outcomes in the treatment of suspected common duct stones?
Bottom line
As compared with a strategy of sequential common duct endoscopy followed by surgery, the use of initial cholecystectomy with intraoperative cholangiogram for patients at intermediate risk of choledocholithiasis leads to shorter hospital length of stay and fewer interventions with otherwise comparable outcomes. (LOE = 1b)
Reference
Iranmanesh P, Frossard J, Mugnier-Konrad B, et al. Initial cholecystectomy vs. sequential common duct endoscopic assessment and subsequent cholecystectomy for suspected gallstone migration. JAMA 2014;312(2):137-144.
Study design
Randomized controlled trial (nonblinded)
Funding source
Unknown/not stated
Allocation
Concealed
Setting
Inpatient (any location) with outpatient follow-up
Synopsis
The role of common duct endoscopic investigation prior to cholecystectomy for patients at intermediate risk of choledocholithiasis is questionable as stones often migrate into the duodenum without such intervention. Using concealed allocation, investigators randomized 100 patients who presented to an emergency department with clinical suspicion of choledocholithiasis (right upper quadrant or epigastric abdominal pain with elevated liver function test results and ultrasound-confirmed gallstone) to 1 of 2 groups. The study group underwent primary cholecystectomy with intraoperative cholangiogram, while the classical treatment group first had common duct exploration with magnetic resonance cholangiopancreatography (MRCP), endoscopic ultrasound (EUS), and endoscopic retrograde cholangiopancreatography (ERCP), if needed, followed by cholecystectomy. The authors excluded patients with severe sepsis, pancreatitis, bilirubinemia greater than 4 mg/dL, or proven common duct stones on admission. The 2 groups had similar baseline characteristics, including similar preoperative health status scores. Overall, the number of confirmed common duct stones in each group was approximately 20%. Patients in the cholecystectomy-first group had a significantly shorter hospital length of stay than the classical treatment group (5 days vs 8 days; P < .001) and also had fewer common duct investigations (MRCP, EUS, or ERCP; 25 vs 71; P < .001). There were no significant differences detected between the 2 groups in quality-of-life assessments or in surgical or medical complications within 6 months.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question
Which strategy — initial cholecystectomy or common duct endoscopic investigation followed by cholecystectomy — leads to better outcomes in the treatment of suspected common duct stones?
Bottom line
As compared with a strategy of sequential common duct endoscopy followed by surgery, the use of initial cholecystectomy with intraoperative cholangiogram for patients at intermediate risk of choledocholithiasis leads to shorter hospital length of stay and fewer interventions with otherwise comparable outcomes. (LOE = 1b)
Reference
Iranmanesh P, Frossard J, Mugnier-Konrad B, et al. Initial cholecystectomy vs. sequential common duct endoscopic assessment and subsequent cholecystectomy for suspected gallstone migration. JAMA 2014;312(2):137-144.
Study design
Randomized controlled trial (nonblinded)
Funding source
Unknown/not stated
Allocation
Concealed
Setting
Inpatient (any location) with outpatient follow-up
Synopsis
The role of common duct endoscopic investigation prior to cholecystectomy for patients at intermediate risk of choledocholithiasis is questionable as stones often migrate into the duodenum without such intervention. Using concealed allocation, investigators randomized 100 patients who presented to an emergency department with clinical suspicion of choledocholithiasis (right upper quadrant or epigastric abdominal pain with elevated liver function test results and ultrasound-confirmed gallstone) to 1 of 2 groups. The study group underwent primary cholecystectomy with intraoperative cholangiogram, while the classical treatment group first had common duct exploration with magnetic resonance cholangiopancreatography (MRCP), endoscopic ultrasound (EUS), and endoscopic retrograde cholangiopancreatography (ERCP), if needed, followed by cholecystectomy. The authors excluded patients with severe sepsis, pancreatitis, bilirubinemia greater than 4 mg/dL, or proven common duct stones on admission. The 2 groups had similar baseline characteristics, including similar preoperative health status scores. Overall, the number of confirmed common duct stones in each group was approximately 20%. Patients in the cholecystectomy-first group had a significantly shorter hospital length of stay than the classical treatment group (5 days vs 8 days; P < .001) and also had fewer common duct investigations (MRCP, EUS, or ERCP; 25 vs 71; P < .001). There were no significant differences detected between the 2 groups in quality-of-life assessments or in surgical or medical complications within 6 months.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
No Harm in Stopping Antibiotics After Cholecystectomy for Acute Cholecystitis
Clinical question
Does stopping antibiotic treatment after cholecystectomy for mild to moderate acute calculous cholecystitis affect outcomes?
Bottom line
Stopping antibiotic treatment after cholecystectomy for mild to moderate acute cholecystitis does not increase postoperative infection rates compared with a strategy of 5 days of postoperative antibiotics. (LOE = 1b)
Reference
Regimbeau JM, Fuks D, Pautrat K, et al, for the FRENCH Study Group. Effect of postoperative antibiotic administration on postoperative infection following cholecystectomy for acute calculous cholecystitis. JAMA 2014;312(2):145-154.
Study design
Randomized controlled trial (nonblinded)
Funding source
Government
Allocation
Concealed
Setting
Inpatient (any location) with outpatient follow-up
Synopsis
Using concealed allocation, these investigators randomized 414 adult patients who presented to an emergency department with mild or moderate acute calculous cholecystitis requiring cholecystectomy into 2 groups: (1) continue taking antibiotics or (2) stop taking antibiotics during the postoperative period. Those with severe cholecystitis, defined as concomitant dysfunction of other organ systems, were excluded, as were those with acute pancreatitis, cholangitis, biliary peritonitis, or cirrhosis. All study patients received amoxicillin plus clavulanic acid 3 times a day from admission to day of surgery. The treatment group continued the same antibiotic regimen for 5 days after surgery, while the nontreatment group received no further antibiotics. The 2 groups were well balanced, with a mean age of 56 years and mean duration of preoperative antibiotics of 2 days. Approximately half the patients in each group had mild cholecystitis. For the primary outcome of postoperative surgical site or distant site infections at 4 weeks, there was no significant difference detected between the 2 groups in either the intention-to-treat or per-protocol analyses (intention-to-treat: 17% for nontreatment vs 15% for antibiotic group; per-protocol: 13% for both groups). This held true when the outcomes were analyzed according to severity of cholecystitis or duration of preoperative antibiotic use.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question
Does stopping antibiotic treatment after cholecystectomy for mild to moderate acute calculous cholecystitis affect outcomes?
Bottom line
Stopping antibiotic treatment after cholecystectomy for mild to moderate acute cholecystitis does not increase postoperative infection rates compared with a strategy of 5 days of postoperative antibiotics. (LOE = 1b)
Reference
Regimbeau JM, Fuks D, Pautrat K, et al, for the FRENCH Study Group. Effect of postoperative antibiotic administration on postoperative infection following cholecystectomy for acute calculous cholecystitis. JAMA 2014;312(2):145-154.
Study design
Randomized controlled trial (nonblinded)
Funding source
Government
Allocation
Concealed
Setting
Inpatient (any location) with outpatient follow-up
Synopsis
Using concealed allocation, these investigators randomized 414 adult patients who presented to an emergency department with mild or moderate acute calculous cholecystitis requiring cholecystectomy into 2 groups: (1) continue taking antibiotics or (2) stop taking antibiotics during the postoperative period. Those with severe cholecystitis, defined as concomitant dysfunction of other organ systems, were excluded, as were those with acute pancreatitis, cholangitis, biliary peritonitis, or cirrhosis. All study patients received amoxicillin plus clavulanic acid 3 times a day from admission to day of surgery. The treatment group continued the same antibiotic regimen for 5 days after surgery, while the nontreatment group received no further antibiotics. The 2 groups were well balanced, with a mean age of 56 years and mean duration of preoperative antibiotics of 2 days. Approximately half the patients in each group had mild cholecystitis. For the primary outcome of postoperative surgical site or distant site infections at 4 weeks, there was no significant difference detected between the 2 groups in either the intention-to-treat or per-protocol analyses (intention-to-treat: 17% for nontreatment vs 15% for antibiotic group; per-protocol: 13% for both groups). This held true when the outcomes were analyzed according to severity of cholecystitis or duration of preoperative antibiotic use.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question
Does stopping antibiotic treatment after cholecystectomy for mild to moderate acute calculous cholecystitis affect outcomes?
Bottom line
Stopping antibiotic treatment after cholecystectomy for mild to moderate acute cholecystitis does not increase postoperative infection rates compared with a strategy of 5 days of postoperative antibiotics. (LOE = 1b)
Reference
Regimbeau JM, Fuks D, Pautrat K, et al, for the FRENCH Study Group. Effect of postoperative antibiotic administration on postoperative infection following cholecystectomy for acute calculous cholecystitis. JAMA 2014;312(2):145-154.
Study design
Randomized controlled trial (nonblinded)
Funding source
Government
Allocation
Concealed
Setting
Inpatient (any location) with outpatient follow-up
Synopsis
Using concealed allocation, these investigators randomized 414 adult patients who presented to an emergency department with mild or moderate acute calculous cholecystitis requiring cholecystectomy into 2 groups: (1) continue taking antibiotics or (2) stop taking antibiotics during the postoperative period. Those with severe cholecystitis, defined as concomitant dysfunction of other organ systems, were excluded, as were those with acute pancreatitis, cholangitis, biliary peritonitis, or cirrhosis. All study patients received amoxicillin plus clavulanic acid 3 times a day from admission to day of surgery. The treatment group continued the same antibiotic regimen for 5 days after surgery, while the nontreatment group received no further antibiotics. The 2 groups were well balanced, with a mean age of 56 years and mean duration of preoperative antibiotics of 2 days. Approximately half the patients in each group had mild cholecystitis. For the primary outcome of postoperative surgical site or distant site infections at 4 weeks, there was no significant difference detected between the 2 groups in either the intention-to-treat or per-protocol analyses (intention-to-treat: 17% for nontreatment vs 15% for antibiotic group; per-protocol: 13% for both groups). This held true when the outcomes were analyzed according to severity of cholecystitis or duration of preoperative antibiotic use.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Knee Surgery Not Necessary for Middle-Aged Patients with Mild Osteoarthritis
New meta-analysis evidence suggests that there is no benefit to having arthroscopic meniscal debridement for degenerative meniscal tears in comparison with nonoperative treatments or sham treatments in middle-aged patients with mild or no concomitant osteoarthritis. The findings were published August 25 online ahead of print in the Canadian Medical Association Journal.
“Doctors need to carefully weigh the costs and benefits when deciding who should undergo such surgery,” said Moin Khan, MD, lead author of the study and research fellow in orthopedic surgery in the Michael G. DeGroote School of Medicine at McMaster University in Hamilton, Ontario.
Dr. Khan and colleagues conducted a meta-analysis of 7 randomized controlled trials published between 1946 and January 20, 2014, on arthroscopic partial meniscectomy in patients with mild to no osteoarthritis compared with nonoperative treatments. Two reviewers independently screened all abstracts and titles for eligibility. In total, there were 811 knees in 805 patients with a mean age of 56 years. The pooled treatment effect of arthroscopic surgery did not show a significant or minimally important difference between treatment arms for long-term functional outcomes. Short-term functional outcomes between groups were significant but did not exceed the threshold for minimally important difference. Arthroscopic surgery did not result in a significant improvement in either short- or long-term pain scores.
“This study shows that surgery should not be the initial option for middle-age or older patients, as there is limited evidence supporting partial meniscectomy surgery for meniscus tears,” Dr. Khan said. “Other treatments should be used first.”
“Arthroscopic debridement or washout of knee osteoarthritis has come under lots of scrutiny based upon trials that suggest patients get no benefit from the procedure. We’re concerned that many surgeons worldwide may still be doing this procedure,” stated the researchers.
Suggested Reading
Khan M, Evaniew N, Bedi A, et al. Arthroscopic surgery for degenerative tears of the meniscus: a systematic review and meta-analysis. CMAJ. 2014 Aug 25. pii: cmaj.140433. [Epub ahead of print]
New meta-analysis evidence suggests that there is no benefit to having arthroscopic meniscal debridement for degenerative meniscal tears in comparison with nonoperative treatments or sham treatments in middle-aged patients with mild or no concomitant osteoarthritis. The findings were published August 25 online ahead of print in the Canadian Medical Association Journal.
“Doctors need to carefully weigh the costs and benefits when deciding who should undergo such surgery,” said Moin Khan, MD, lead author of the study and research fellow in orthopedic surgery in the Michael G. DeGroote School of Medicine at McMaster University in Hamilton, Ontario.
Dr. Khan and colleagues conducted a meta-analysis of 7 randomized controlled trials published between 1946 and January 20, 2014, on arthroscopic partial meniscectomy in patients with mild to no osteoarthritis compared with nonoperative treatments. Two reviewers independently screened all abstracts and titles for eligibility. In total, there were 811 knees in 805 patients with a mean age of 56 years. The pooled treatment effect of arthroscopic surgery did not show a significant or minimally important difference between treatment arms for long-term functional outcomes. Short-term functional outcomes between groups were significant but did not exceed the threshold for minimally important difference. Arthroscopic surgery did not result in a significant improvement in either short- or long-term pain scores.
“This study shows that surgery should not be the initial option for middle-age or older patients, as there is limited evidence supporting partial meniscectomy surgery for meniscus tears,” Dr. Khan said. “Other treatments should be used first.”
“Arthroscopic debridement or washout of knee osteoarthritis has come under lots of scrutiny based upon trials that suggest patients get no benefit from the procedure. We’re concerned that many surgeons worldwide may still be doing this procedure,” stated the researchers.
New meta-analysis evidence suggests that there is no benefit to having arthroscopic meniscal debridement for degenerative meniscal tears in comparison with nonoperative treatments or sham treatments in middle-aged patients with mild or no concomitant osteoarthritis. The findings were published August 25 online ahead of print in the Canadian Medical Association Journal.
“Doctors need to carefully weigh the costs and benefits when deciding who should undergo such surgery,” said Moin Khan, MD, lead author of the study and research fellow in orthopedic surgery in the Michael G. DeGroote School of Medicine at McMaster University in Hamilton, Ontario.
Dr. Khan and colleagues conducted a meta-analysis of 7 randomized controlled trials published between 1946 and January 20, 2014, on arthroscopic partial meniscectomy in patients with mild to no osteoarthritis compared with nonoperative treatments. Two reviewers independently screened all abstracts and titles for eligibility. In total, there were 811 knees in 805 patients with a mean age of 56 years. The pooled treatment effect of arthroscopic surgery did not show a significant or minimally important difference between treatment arms for long-term functional outcomes. Short-term functional outcomes between groups were significant but did not exceed the threshold for minimally important difference. Arthroscopic surgery did not result in a significant improvement in either short- or long-term pain scores.
“This study shows that surgery should not be the initial option for middle-age or older patients, as there is limited evidence supporting partial meniscectomy surgery for meniscus tears,” Dr. Khan said. “Other treatments should be used first.”
“Arthroscopic debridement or washout of knee osteoarthritis has come under lots of scrutiny based upon trials that suggest patients get no benefit from the procedure. We’re concerned that many surgeons worldwide may still be doing this procedure,” stated the researchers.
Suggested Reading
Khan M, Evaniew N, Bedi A, et al. Arthroscopic surgery for degenerative tears of the meniscus: a systematic review and meta-analysis. CMAJ. 2014 Aug 25. pii: cmaj.140433. [Epub ahead of print]
Suggested Reading
Khan M, Evaniew N, Bedi A, et al. Arthroscopic surgery for degenerative tears of the meniscus: a systematic review and meta-analysis. CMAJ. 2014 Aug 25. pii: cmaj.140433. [Epub ahead of print]
Baby Has Rash; Parents Feel Itchy
A 5-month-old baby is brought in by his parents for evaluation of a rash that manifested on his hands several weeks ago. It then spread to his arms and trunk and is now essentially everywhere except his face. Despite a number of treatment attempts, including use of oral antibiotics (cephalexin suspension 125/5 cc) and OTC topical steroid creams, the problem has persisted.
Prior to dermatology, they had consulted a pediatrician. He suggested the child might have scabies, for which he prescribed permethrin cream. The parents tried it, but it made little if any difference.
Neither the child nor his parents are atopic. However, both parents have recently started to feel itchy.
EXAMINATION
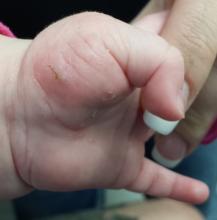
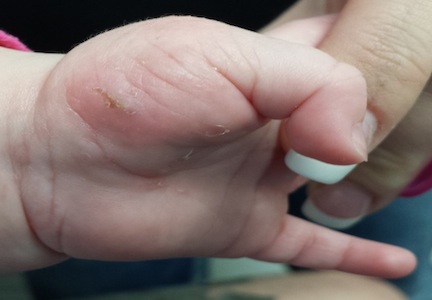
The child is afebrile and in no acute distress. Hundreds of tiny papules are scattered on his trunk, arms, and legs, with a particular concentration on his palms. Several of the papules, on closer examination, prove to be vesicles (ie, filled with clear fluid).
One of these lesions, on the child’s volar wrist, is scraped and the sample examined under the microscope. Magnification at 10x power reveals an adult scabies organism and a number of rugby ball–shaped eggs.
Both parents are also examined and found to have probable scabies as well. The mother’s lesions are concentrated around the anterior axillary areas and waistline. The father’s are on his volar wrists and penis.
What is the diagnosis?
DISCUSSION
This case nicely illustrates several issues revolving around the diagnosis of scabies. One might think this would be a simple matter: Diagnose, then treat. Alas, it is seldom so.
For one thing, the diagnosis of scabies needs to be confirmed, whenever possible, with microscopic findings of scabetic elements. Without this, patient and provider confidence are lacking—a situation that often leads to shotgun treatment.
In addition, had the diagnosis been confirmed prior to presentation to dermatology, the previously consulted providers might have considered treating the whole family and trying to identify the source of the infestation. Both of these are absolutely crucial to successful treatment.
Several factors make the diagnosis of scabies difficult in infants. Any part of an infant’s thin, soft, relatively hairless skin is fair game (whereas, in adults, scabies rarely affects skin above the neck). Furthermore, although infants with scabies undoubtedly itch—probably just as much as adults—they are totally inept excoriators and even worse historians. In contrast, adults with scabies will scratch continuously while in the exam room and complain bitterly 24/7.
Once the diagnosis is established, a crucial element of dealing effectively with scabies is education—in this case, of the parents. They must understand the nature of the problem in specific terms. For example, scabies cannot be caught from or given to nonhuman hosts (eg, animals). And while I advise affected families to clean areas such as beds, sofas, and bathrooms, I also emphasize that the organism does not reside in or multiply on inanimate objects. Despite my best efforts, though, some families become almost hysterical: steam-cleaning every surface, calling pest control, washing bedding and towels multiple times, and calling me three times a day.
Families must also understand that treatment of all household members must be coordinated and done twice, seven to 10 days apart, in order to kill freshly hatched organisms. This child was treated with permethrin 5% cream, applied to the entire body and left on overnight, then washed off the next morning (twice per the schedule outlined above). In addition to permethrin, the adults were treated with ivermectin (200 mcg/kg) on the same schedule. Even with these extensive measures, recurrence would not be surprising.
Most often, when treatment “fails,” it is because the diagnosis was not scabies in the first place. In confirmed cases, treatment will be unsuccessful if all family members are not adequately (and concurrently) treated. Another problem occurs when the actual source is outside the home (daycare, sleepovers, sexual partner) and remains unidentified—dooming the family to recurrence. (Institutional scabies—from nursing homes, group living, etc—can be far more difficult to deal with and is beyond the scope of this article.)
The differential for scabies includes—most significantly—atopic dermatitis, which it can closely resemble.
TAKE-HOME LEARNING POINTS
• Scabies can show up almost anywhere on an infant’s body, because the skin is so thin, hairless, and soft.
• If the baby has scabies, chances are the parents and siblings have it too.
• Someone brings scabies into the family, and unless the source is identified and treated, the problem will recur.
• Microscopic examination (KOH) for scabetic elements is a crucial component of diagnosis and treatment.
• Scabies sarcoptes var humani is species-specific and cannot be given to or caught from an animal.
• Permethrin cream 5% is considered safe for infants ages 2 months and older.
A 5-month-old baby is brought in by his parents for evaluation of a rash that manifested on his hands several weeks ago. It then spread to his arms and trunk and is now essentially everywhere except his face. Despite a number of treatment attempts, including use of oral antibiotics (cephalexin suspension 125/5 cc) and OTC topical steroid creams, the problem has persisted.
Prior to dermatology, they had consulted a pediatrician. He suggested the child might have scabies, for which he prescribed permethrin cream. The parents tried it, but it made little if any difference.
Neither the child nor his parents are atopic. However, both parents have recently started to feel itchy.
EXAMINATION
The child is afebrile and in no acute distress. Hundreds of tiny papules are scattered on his trunk, arms, and legs, with a particular concentration on his palms. Several of the papules, on closer examination, prove to be vesicles (ie, filled with clear fluid).
One of these lesions, on the child’s volar wrist, is scraped and the sample examined under the microscope. Magnification at 10x power reveals an adult scabies organism and a number of rugby ball–shaped eggs.
Both parents are also examined and found to have probable scabies as well. The mother’s lesions are concentrated around the anterior axillary areas and waistline. The father’s are on his volar wrists and penis.
What is the diagnosis?
DISCUSSION
This case nicely illustrates several issues revolving around the diagnosis of scabies. One might think this would be a simple matter: Diagnose, then treat. Alas, it is seldom so.
For one thing, the diagnosis of scabies needs to be confirmed, whenever possible, with microscopic findings of scabetic elements. Without this, patient and provider confidence are lacking—a situation that often leads to shotgun treatment.
In addition, had the diagnosis been confirmed prior to presentation to dermatology, the previously consulted providers might have considered treating the whole family and trying to identify the source of the infestation. Both of these are absolutely crucial to successful treatment.
Several factors make the diagnosis of scabies difficult in infants. Any part of an infant’s thin, soft, relatively hairless skin is fair game (whereas, in adults, scabies rarely affects skin above the neck). Furthermore, although infants with scabies undoubtedly itch—probably just as much as adults—they are totally inept excoriators and even worse historians. In contrast, adults with scabies will scratch continuously while in the exam room and complain bitterly 24/7.
Once the diagnosis is established, a crucial element of dealing effectively with scabies is education—in this case, of the parents. They must understand the nature of the problem in specific terms. For example, scabies cannot be caught from or given to nonhuman hosts (eg, animals). And while I advise affected families to clean areas such as beds, sofas, and bathrooms, I also emphasize that the organism does not reside in or multiply on inanimate objects. Despite my best efforts, though, some families become almost hysterical: steam-cleaning every surface, calling pest control, washing bedding and towels multiple times, and calling me three times a day.
Families must also understand that treatment of all household members must be coordinated and done twice, seven to 10 days apart, in order to kill freshly hatched organisms. This child was treated with permethrin 5% cream, applied to the entire body and left on overnight, then washed off the next morning (twice per the schedule outlined above). In addition to permethrin, the adults were treated with ivermectin (200 mcg/kg) on the same schedule. Even with these extensive measures, recurrence would not be surprising.
Most often, when treatment “fails,” it is because the diagnosis was not scabies in the first place. In confirmed cases, treatment will be unsuccessful if all family members are not adequately (and concurrently) treated. Another problem occurs when the actual source is outside the home (daycare, sleepovers, sexual partner) and remains unidentified—dooming the family to recurrence. (Institutional scabies—from nursing homes, group living, etc—can be far more difficult to deal with and is beyond the scope of this article.)
The differential for scabies includes—most significantly—atopic dermatitis, which it can closely resemble.
TAKE-HOME LEARNING POINTS
• Scabies can show up almost anywhere on an infant’s body, because the skin is so thin, hairless, and soft.
• If the baby has scabies, chances are the parents and siblings have it too.
• Someone brings scabies into the family, and unless the source is identified and treated, the problem will recur.
• Microscopic examination (KOH) for scabetic elements is a crucial component of diagnosis and treatment.
• Scabies sarcoptes var humani is species-specific and cannot be given to or caught from an animal.
• Permethrin cream 5% is considered safe for infants ages 2 months and older.
A 5-month-old baby is brought in by his parents for evaluation of a rash that manifested on his hands several weeks ago. It then spread to his arms and trunk and is now essentially everywhere except his face. Despite a number of treatment attempts, including use of oral antibiotics (cephalexin suspension 125/5 cc) and OTC topical steroid creams, the problem has persisted.
Prior to dermatology, they had consulted a pediatrician. He suggested the child might have scabies, for which he prescribed permethrin cream. The parents tried it, but it made little if any difference.
Neither the child nor his parents are atopic. However, both parents have recently started to feel itchy.
EXAMINATION
The child is afebrile and in no acute distress. Hundreds of tiny papules are scattered on his trunk, arms, and legs, with a particular concentration on his palms. Several of the papules, on closer examination, prove to be vesicles (ie, filled with clear fluid).
One of these lesions, on the child’s volar wrist, is scraped and the sample examined under the microscope. Magnification at 10x power reveals an adult scabies organism and a number of rugby ball–shaped eggs.
Both parents are also examined and found to have probable scabies as well. The mother’s lesions are concentrated around the anterior axillary areas and waistline. The father’s are on his volar wrists and penis.
What is the diagnosis?
DISCUSSION
This case nicely illustrates several issues revolving around the diagnosis of scabies. One might think this would be a simple matter: Diagnose, then treat. Alas, it is seldom so.
For one thing, the diagnosis of scabies needs to be confirmed, whenever possible, with microscopic findings of scabetic elements. Without this, patient and provider confidence are lacking—a situation that often leads to shotgun treatment.
In addition, had the diagnosis been confirmed prior to presentation to dermatology, the previously consulted providers might have considered treating the whole family and trying to identify the source of the infestation. Both of these are absolutely crucial to successful treatment.
Several factors make the diagnosis of scabies difficult in infants. Any part of an infant’s thin, soft, relatively hairless skin is fair game (whereas, in adults, scabies rarely affects skin above the neck). Furthermore, although infants with scabies undoubtedly itch—probably just as much as adults—they are totally inept excoriators and even worse historians. In contrast, adults with scabies will scratch continuously while in the exam room and complain bitterly 24/7.
Once the diagnosis is established, a crucial element of dealing effectively with scabies is education—in this case, of the parents. They must understand the nature of the problem in specific terms. For example, scabies cannot be caught from or given to nonhuman hosts (eg, animals). And while I advise affected families to clean areas such as beds, sofas, and bathrooms, I also emphasize that the organism does not reside in or multiply on inanimate objects. Despite my best efforts, though, some families become almost hysterical: steam-cleaning every surface, calling pest control, washing bedding and towels multiple times, and calling me three times a day.
Families must also understand that treatment of all household members must be coordinated and done twice, seven to 10 days apart, in order to kill freshly hatched organisms. This child was treated with permethrin 5% cream, applied to the entire body and left on overnight, then washed off the next morning (twice per the schedule outlined above). In addition to permethrin, the adults were treated with ivermectin (200 mcg/kg) on the same schedule. Even with these extensive measures, recurrence would not be surprising.
Most often, when treatment “fails,” it is because the diagnosis was not scabies in the first place. In confirmed cases, treatment will be unsuccessful if all family members are not adequately (and concurrently) treated. Another problem occurs when the actual source is outside the home (daycare, sleepovers, sexual partner) and remains unidentified—dooming the family to recurrence. (Institutional scabies—from nursing homes, group living, etc—can be far more difficult to deal with and is beyond the scope of this article.)
The differential for scabies includes—most significantly—atopic dermatitis, which it can closely resemble.
TAKE-HOME LEARNING POINTS
• Scabies can show up almost anywhere on an infant’s body, because the skin is so thin, hairless, and soft.
• If the baby has scabies, chances are the parents and siblings have it too.
• Someone brings scabies into the family, and unless the source is identified and treated, the problem will recur.
• Microscopic examination (KOH) for scabetic elements is a crucial component of diagnosis and treatment.
• Scabies sarcoptes var humani is species-specific and cannot be given to or caught from an animal.
• Permethrin cream 5% is considered safe for infants ages 2 months and older.
Care your way to LOS solutions
High-quality care, optimal length of stay (LOS), patient satisfaction, cost-effectiveness – all part of the hospitalists’ creed, our raison d’être. But with these exist national, as well as local imperatives, some of which carry penalties and/or rewards. Public and private organizations devote a huge amount of resources into setting higher and higher bars of excellence for physicians. Individual hospitals adapt and tweak the methods of other centers that have outstanding track records in hopes they, too, may enjoy similar success. Yet, at the end of the day, we are the foot soldiers.
Insurers should not mandate the care we provide. Government should not have to tell us what is acceptable practice and what is not. And hospital administrators – God bless them – should not have to stab blindly in the dark for solutions to the problems that plague their individual institutions. After all, we physicians are at the patients’ bedsides. We talk to them and their families, consult effective and efficient specialists, write orders to take care of them, and ultimately discharge them to their next phase in care.
There is a tremendous amount of low-hanging fruit we easily could seize upon to make our hospitals run more smoothly and make our patients much happier (though the processes and procedures that make one institution ineffective may not plague the next).
For instance, many hospitals have a peak time for admissions, as well as for discharges, and these two times frequently do not coincide. As a result, there may be a backlog of patients in the emergency department (ED) awaiting a clean bed. Invariably, meanwhile, there are patients pacing the halls anxiously waiting for the doctor to arrive to discharge them. But if that doctor is busy seeing a new or very sick patient, that discharge may just have to wait, sometimes for several hours. Here, I have learned to try to look for opportunities instead of focusing on obstacles.
If I anticipate that a patient will be discharged the following day, I try prepare the discharge summary and patient instruction sheet, and to write the prescriptions a day in advance (when time permits). That way, on the following day, instead of devoting 45 minutes to reviewing the records of a lengthy hospital stay, I can simply check on the patient to confirm that she has no new problems and that her examination is stable. Within seconds, I can type in a discharge order and move along to the next patient. Even in the midst of a very busy day, I can typically work in this type of visit fairly early.
On the other hand, if the same patient is likely to be discharged the day after I leave the service, the same preparation by me can save my partner a great deal of time the next day. If everything is already done except the official discharge order, she, too, can likely discharge the patient early in the day, instead of late in the evening after she learns the entire service. (Who likes going home in the dark anyway?)
The patient is happier. The administration is happier to have more beds freed up earlier. The little old lady in the ED with a comminuted hip fracture will get a nice warm bed quicker, and the rounder is less stressed. Everyone wins!
Listening to our patients’ desires, not just their needs can also go a long way in patient satisfaction.
I recently had a patient who was visiting from the other side of the country who, unfortunately, wound up in our ED for cellulitis. She was part of a historical group from California who had traveled to the Washington, D.C., area to attend a national function. The event was to culminate in a banquet that evening – a banquet that she was going to miss. When I saw her, she acknowledged she was getting better on the intravenous vancomycin that was started in the ED the night before, and though the line of demarcation drawn by my partner clearly showed her infection was improving, she still had mild-moderate cellulitis. Her history of methicillin-resistant Staphylococcus aureus (MRSA) made me uncomfortable discharging her on a regimen that would “probably” cover MRSA, and we all know that linezolid (Zyvox) can be incredibly expensive if not on a patient’s formulary. There we were at 5 p.m. on a Saturday. Who would be reachable for a prior authorization?
As I looked down at her sad face and saw the disappointment in her eyes, I had to do something! She was in the area for a great cause; the hospitalization was an unexpected nuisance that threatened to destroy her entire trip. The solution was simple. I called her pharmacist in California and found out that her copay for Zyvox was an affordable $30, so I could safely discharge her in time for her banquet. While that falls far short of an near-miracle that changed a life, my simple effort made a big difference for her.
The point is that when we focus on the patient’s entire needs – not just the disease that brought them to the hospital in the first place – we can create solutions to many of their problems. Sometimes it’s the finishing touches, not just the medical care, that patients remember most.
Dr. Hester is a hospitalist at Baltimore-Washington Medical Center in Glen Burnie, Md. She is the creator of the Patient Whiz, a patient-engagement app for iOS. Reach her at [email protected].
High-quality care, optimal length of stay (LOS), patient satisfaction, cost-effectiveness – all part of the hospitalists’ creed, our raison d’être. But with these exist national, as well as local imperatives, some of which carry penalties and/or rewards. Public and private organizations devote a huge amount of resources into setting higher and higher bars of excellence for physicians. Individual hospitals adapt and tweak the methods of other centers that have outstanding track records in hopes they, too, may enjoy similar success. Yet, at the end of the day, we are the foot soldiers.
Insurers should not mandate the care we provide. Government should not have to tell us what is acceptable practice and what is not. And hospital administrators – God bless them – should not have to stab blindly in the dark for solutions to the problems that plague their individual institutions. After all, we physicians are at the patients’ bedsides. We talk to them and their families, consult effective and efficient specialists, write orders to take care of them, and ultimately discharge them to their next phase in care.
There is a tremendous amount of low-hanging fruit we easily could seize upon to make our hospitals run more smoothly and make our patients much happier (though the processes and procedures that make one institution ineffective may not plague the next).
For instance, many hospitals have a peak time for admissions, as well as for discharges, and these two times frequently do not coincide. As a result, there may be a backlog of patients in the emergency department (ED) awaiting a clean bed. Invariably, meanwhile, there are patients pacing the halls anxiously waiting for the doctor to arrive to discharge them. But if that doctor is busy seeing a new or very sick patient, that discharge may just have to wait, sometimes for several hours. Here, I have learned to try to look for opportunities instead of focusing on obstacles.
If I anticipate that a patient will be discharged the following day, I try prepare the discharge summary and patient instruction sheet, and to write the prescriptions a day in advance (when time permits). That way, on the following day, instead of devoting 45 minutes to reviewing the records of a lengthy hospital stay, I can simply check on the patient to confirm that she has no new problems and that her examination is stable. Within seconds, I can type in a discharge order and move along to the next patient. Even in the midst of a very busy day, I can typically work in this type of visit fairly early.
On the other hand, if the same patient is likely to be discharged the day after I leave the service, the same preparation by me can save my partner a great deal of time the next day. If everything is already done except the official discharge order, she, too, can likely discharge the patient early in the day, instead of late in the evening after she learns the entire service. (Who likes going home in the dark anyway?)
The patient is happier. The administration is happier to have more beds freed up earlier. The little old lady in the ED with a comminuted hip fracture will get a nice warm bed quicker, and the rounder is less stressed. Everyone wins!
Listening to our patients’ desires, not just their needs can also go a long way in patient satisfaction.
I recently had a patient who was visiting from the other side of the country who, unfortunately, wound up in our ED for cellulitis. She was part of a historical group from California who had traveled to the Washington, D.C., area to attend a national function. The event was to culminate in a banquet that evening – a banquet that she was going to miss. When I saw her, she acknowledged she was getting better on the intravenous vancomycin that was started in the ED the night before, and though the line of demarcation drawn by my partner clearly showed her infection was improving, she still had mild-moderate cellulitis. Her history of methicillin-resistant Staphylococcus aureus (MRSA) made me uncomfortable discharging her on a regimen that would “probably” cover MRSA, and we all know that linezolid (Zyvox) can be incredibly expensive if not on a patient’s formulary. There we were at 5 p.m. on a Saturday. Who would be reachable for a prior authorization?
As I looked down at her sad face and saw the disappointment in her eyes, I had to do something! She was in the area for a great cause; the hospitalization was an unexpected nuisance that threatened to destroy her entire trip. The solution was simple. I called her pharmacist in California and found out that her copay for Zyvox was an affordable $30, so I could safely discharge her in time for her banquet. While that falls far short of an near-miracle that changed a life, my simple effort made a big difference for her.
The point is that when we focus on the patient’s entire needs – not just the disease that brought them to the hospital in the first place – we can create solutions to many of their problems. Sometimes it’s the finishing touches, not just the medical care, that patients remember most.
Dr. Hester is a hospitalist at Baltimore-Washington Medical Center in Glen Burnie, Md. She is the creator of the Patient Whiz, a patient-engagement app for iOS. Reach her at [email protected].
High-quality care, optimal length of stay (LOS), patient satisfaction, cost-effectiveness – all part of the hospitalists’ creed, our raison d’être. But with these exist national, as well as local imperatives, some of which carry penalties and/or rewards. Public and private organizations devote a huge amount of resources into setting higher and higher bars of excellence for physicians. Individual hospitals adapt and tweak the methods of other centers that have outstanding track records in hopes they, too, may enjoy similar success. Yet, at the end of the day, we are the foot soldiers.
Insurers should not mandate the care we provide. Government should not have to tell us what is acceptable practice and what is not. And hospital administrators – God bless them – should not have to stab blindly in the dark for solutions to the problems that plague their individual institutions. After all, we physicians are at the patients’ bedsides. We talk to them and their families, consult effective and efficient specialists, write orders to take care of them, and ultimately discharge them to their next phase in care.
There is a tremendous amount of low-hanging fruit we easily could seize upon to make our hospitals run more smoothly and make our patients much happier (though the processes and procedures that make one institution ineffective may not plague the next).
For instance, many hospitals have a peak time for admissions, as well as for discharges, and these two times frequently do not coincide. As a result, there may be a backlog of patients in the emergency department (ED) awaiting a clean bed. Invariably, meanwhile, there are patients pacing the halls anxiously waiting for the doctor to arrive to discharge them. But if that doctor is busy seeing a new or very sick patient, that discharge may just have to wait, sometimes for several hours. Here, I have learned to try to look for opportunities instead of focusing on obstacles.
If I anticipate that a patient will be discharged the following day, I try prepare the discharge summary and patient instruction sheet, and to write the prescriptions a day in advance (when time permits). That way, on the following day, instead of devoting 45 minutes to reviewing the records of a lengthy hospital stay, I can simply check on the patient to confirm that she has no new problems and that her examination is stable. Within seconds, I can type in a discharge order and move along to the next patient. Even in the midst of a very busy day, I can typically work in this type of visit fairly early.
On the other hand, if the same patient is likely to be discharged the day after I leave the service, the same preparation by me can save my partner a great deal of time the next day. If everything is already done except the official discharge order, she, too, can likely discharge the patient early in the day, instead of late in the evening after she learns the entire service. (Who likes going home in the dark anyway?)
The patient is happier. The administration is happier to have more beds freed up earlier. The little old lady in the ED with a comminuted hip fracture will get a nice warm bed quicker, and the rounder is less stressed. Everyone wins!
Listening to our patients’ desires, not just their needs can also go a long way in patient satisfaction.
I recently had a patient who was visiting from the other side of the country who, unfortunately, wound up in our ED for cellulitis. She was part of a historical group from California who had traveled to the Washington, D.C., area to attend a national function. The event was to culminate in a banquet that evening – a banquet that she was going to miss. When I saw her, she acknowledged she was getting better on the intravenous vancomycin that was started in the ED the night before, and though the line of demarcation drawn by my partner clearly showed her infection was improving, she still had mild-moderate cellulitis. Her history of methicillin-resistant Staphylococcus aureus (MRSA) made me uncomfortable discharging her on a regimen that would “probably” cover MRSA, and we all know that linezolid (Zyvox) can be incredibly expensive if not on a patient’s formulary. There we were at 5 p.m. on a Saturday. Who would be reachable for a prior authorization?
As I looked down at her sad face and saw the disappointment in her eyes, I had to do something! She was in the area for a great cause; the hospitalization was an unexpected nuisance that threatened to destroy her entire trip. The solution was simple. I called her pharmacist in California and found out that her copay for Zyvox was an affordable $30, so I could safely discharge her in time for her banquet. While that falls far short of an near-miracle that changed a life, my simple effort made a big difference for her.
The point is that when we focus on the patient’s entire needs – not just the disease that brought them to the hospital in the first place – we can create solutions to many of their problems. Sometimes it’s the finishing touches, not just the medical care, that patients remember most.
Dr. Hester is a hospitalist at Baltimore-Washington Medical Center in Glen Burnie, Md. She is the creator of the Patient Whiz, a patient-engagement app for iOS. Reach her at [email protected].