User login
Reticulated erythematous patch on teenager’s foot
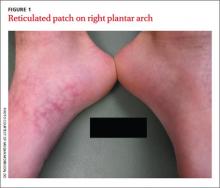
An 18-year-old Caucasian male sought care for an ill-defined reticulated patch on his right plantar arch (FIGURE 1). The patient said that the lesion had gradually appeared 2 years earlier, had grown slowly, and was occasionally itchy. Physical exam revealed a lacy violaceous, hyperpigmented, reticulated patch that was blanchable and nontender to palpation.
Our patient denied having a history of trauma to the area or a coagulation or connective tissue disorder. The lesion didn’t vary with temperature or season, and there were no known triggers. The patient’s left plantar arch was unchanged.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Erythema ab igne
Upon further questioning, the patient acknowledged that he occasionally rested his bare feet around a portable heater under his desk while using his computer for a few hours each day (FIGURE 2). He often kept his right foot on the heater while he let his left foot rest on the ground. A punch biopsy was performed; the findings, when combined with the patient’s report of having exposed his foot to heat, supported the diagnosis of erythema ab igne (EAI).
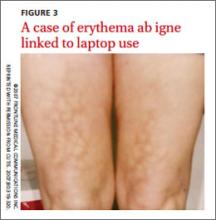
EAI commonly presents as an asymptomatic reticulated erythematous to violaceous patch in an area of the body that has been in contact with heat.1 It originally was described on the bilateral anterior lower extremities after prolonged exposure to burning stoves or open fires.1 With the advent of central heating, these presentations have decreased, but there has been a resurgence of EAI with atypical distributions as a result of evolving technology and new heating sources. Reported causes of EAI include heating pads,1,2 laptop computers3 (FIGURE 3), car seat heaters,4 hot water bottles, popcorn bags, cell phones,5 and space heaters that have resulted in patches on the breast, thighs, arms, and, in our patient, foot.1-5
Blood work, biopsy can help narrow the differential
The differential for EAI includes livedo reticularis, livedo racemosa, cutis marmorata, and cutis marmorata telangiectasia. Livedo reticularis can be associated with autoimmune conditions and coagulopathies. Livedo racemosa is a typical sign of Sneddon’s syndrome and can be seen in up to 70% of patients with antiphospholipid-antibody syndrome and systemic lupus erythematosus. Diagnosis of these conditions is confirmed by elevated coagulation factors, presence of autoimmune antibodies, or history of cerebrovascular accident.6 These tests would be normal in EAI.
Histopathologic changes observed in EAI include an atrophic epidermis with an interface dermatitis, vasodilation, and dermal pigmentation. Necrotic keratinocytes and focal hyperkeratosis can be noted, along with squamous atypia. Although these changes are nonspecific, they can be used to confirm an EAI diagnosis in patients for whom the affected area has been exposed to a heat source.
Histologically, EAI is similar to actinic keratosis, with epidermal changes showing squamous atypia.2 Due to the similarities, these lesions are sometimes referred to as “thermal keratosis.” Some researchers have suggested that the thermal heat may induce epithelial changes in the same way that ultraviolet light produces epithelial changes.7
Rarely, EAI can turn into cancer. There have been a few reported cases of EAI transforming into squamous cell carcinoma or Merkel cell carcinoma; squamous cell carcinoma is more common, and tends to occur after a long latent period (up to 30 years).7-9 EAI lesions often begin as a chronic ulcer and tend not to heal. If the lesion continues to evolve (ie, ulcerate), a biopsy may be warranted to rule out a malignant transformation.
Eliminate heat exposure, consider a topical treatment
Treatment of acute EAI involves eliminating the offending heat source. The hyperpigmentation will slowly resolve over months to years.4 Persistent exposure to heat sources can lead to chronic EAI, which is more difficult to eliminate.
Because hyperpigmentation can be visually unappealing and emotionally distressing, some patients prefer active treatment. EAI has been effectively treated with 4% hydroquinone topical cream twice a day and tretinoin topical cream at night.2,10,11 Lesions that have epithelial atypia have improved with 5-fluorouracil topical cream.7
EAI also has been successfully treated with laser therapy with the 1064-nm Q-switched Nd:YAG laser with low fluence at 2-week intervals.9
Our patient declined topical therapy. He improved after a few months of avoiding the heater under his desk.
CORRESPONDENCE
Megan Morrison, DO, 5333 McAuley Drive Suite R-5003, Ypsilanti, MI 48197; [email protected]
1. Huynh N, Sarma D, Huerter C. Erythema ab igne: a case report and review of the literature. Cutis. 2011;88:290-292.
2. Tan S, Bertucci V. Erythema ab igne: an old condition new again. CMAJ. 2000;162:77-78.
3. Fu LW, Vender R. Erythema ab igne caused by laptop computer gaming—a case report. Int J Dermatol. 2012;51:716-717.
4. Brodell D, Mostow EN. Automobile seat heater-induced erythema ab igne. Arch Dermtol. 2012;148:264-265.
5. Dela Rosa K, Satter EK. Erythematous patches on the chest. Arch Dermatol. 2012;148:113-118.
6. Uthman IW, Khamashta MA. Livedo racemosa: a striking dermatological sign for antiphospholipid syndrome. J Rheumatol. 2006;33:2379-2382.
7. Bilic M, Adams B. Erythema ab igne induced by a laptop computer. J Am Acad Dermatol. 2004;50:973-974.
8. Jones CS, Tyring SK, Lee PC, et al. Development of neuroendocrine (Merkel cell) carcinoma mixed with squamous cell carcinoma in erythema ab igne. Arch Dermatol. 1998;124:110-113.
9. Cho S, Jung JY, Lee JH. Erythema ab igne successfully treated using 1,064-nm Q-switched neodymium-doped yttrium aluminum garnet laser with low fluence. Dermatol Surg. 2011;37:551-553.
10. Cardona LFC, Parsons AC, Sangueza OP. Erythematous lesions on the back of a man: challenge. Erythema ab igne. Am J Dermatopathol. 2011;33:185,199.
11. Sahl WJ, Taira JW. Erythema ab igne: treatment with 5-fluorouracil cream. J Am Acad Dermatol. 1992;27:109-110.
An 18-year-old Caucasian male sought care for an ill-defined reticulated patch on his right plantar arch (FIGURE 1). The patient said that the lesion had gradually appeared 2 years earlier, had grown slowly, and was occasionally itchy. Physical exam revealed a lacy violaceous, hyperpigmented, reticulated patch that was blanchable and nontender to palpation.
Our patient denied having a history of trauma to the area or a coagulation or connective tissue disorder. The lesion didn’t vary with temperature or season, and there were no known triggers. The patient’s left plantar arch was unchanged.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Erythema ab igne
Upon further questioning, the patient acknowledged that he occasionally rested his bare feet around a portable heater under his desk while using his computer for a few hours each day (FIGURE 2). He often kept his right foot on the heater while he let his left foot rest on the ground. A punch biopsy was performed; the findings, when combined with the patient’s report of having exposed his foot to heat, supported the diagnosis of erythema ab igne (EAI).
EAI commonly presents as an asymptomatic reticulated erythematous to violaceous patch in an area of the body that has been in contact with heat.1 It originally was described on the bilateral anterior lower extremities after prolonged exposure to burning stoves or open fires.1 With the advent of central heating, these presentations have decreased, but there has been a resurgence of EAI with atypical distributions as a result of evolving technology and new heating sources. Reported causes of EAI include heating pads,1,2 laptop computers3 (FIGURE 3), car seat heaters,4 hot water bottles, popcorn bags, cell phones,5 and space heaters that have resulted in patches on the breast, thighs, arms, and, in our patient, foot.1-5
Blood work, biopsy can help narrow the differential
The differential for EAI includes livedo reticularis, livedo racemosa, cutis marmorata, and cutis marmorata telangiectasia. Livedo reticularis can be associated with autoimmune conditions and coagulopathies. Livedo racemosa is a typical sign of Sneddon’s syndrome and can be seen in up to 70% of patients with antiphospholipid-antibody syndrome and systemic lupus erythematosus. Diagnosis of these conditions is confirmed by elevated coagulation factors, presence of autoimmune antibodies, or history of cerebrovascular accident.6 These tests would be normal in EAI.
Histopathologic changes observed in EAI include an atrophic epidermis with an interface dermatitis, vasodilation, and dermal pigmentation. Necrotic keratinocytes and focal hyperkeratosis can be noted, along with squamous atypia. Although these changes are nonspecific, they can be used to confirm an EAI diagnosis in patients for whom the affected area has been exposed to a heat source.
Histologically, EAI is similar to actinic keratosis, with epidermal changes showing squamous atypia.2 Due to the similarities, these lesions are sometimes referred to as “thermal keratosis.” Some researchers have suggested that the thermal heat may induce epithelial changes in the same way that ultraviolet light produces epithelial changes.7
Rarely, EAI can turn into cancer. There have been a few reported cases of EAI transforming into squamous cell carcinoma or Merkel cell carcinoma; squamous cell carcinoma is more common, and tends to occur after a long latent period (up to 30 years).7-9 EAI lesions often begin as a chronic ulcer and tend not to heal. If the lesion continues to evolve (ie, ulcerate), a biopsy may be warranted to rule out a malignant transformation.
Eliminate heat exposure, consider a topical treatment
Treatment of acute EAI involves eliminating the offending heat source. The hyperpigmentation will slowly resolve over months to years.4 Persistent exposure to heat sources can lead to chronic EAI, which is more difficult to eliminate.
Because hyperpigmentation can be visually unappealing and emotionally distressing, some patients prefer active treatment. EAI has been effectively treated with 4% hydroquinone topical cream twice a day and tretinoin topical cream at night.2,10,11 Lesions that have epithelial atypia have improved with 5-fluorouracil topical cream.7
EAI also has been successfully treated with laser therapy with the 1064-nm Q-switched Nd:YAG laser with low fluence at 2-week intervals.9
Our patient declined topical therapy. He improved after a few months of avoiding the heater under his desk.
CORRESPONDENCE
Megan Morrison, DO, 5333 McAuley Drive Suite R-5003, Ypsilanti, MI 48197; [email protected]
An 18-year-old Caucasian male sought care for an ill-defined reticulated patch on his right plantar arch (FIGURE 1). The patient said that the lesion had gradually appeared 2 years earlier, had grown slowly, and was occasionally itchy. Physical exam revealed a lacy violaceous, hyperpigmented, reticulated patch that was blanchable and nontender to palpation.
Our patient denied having a history of trauma to the area or a coagulation or connective tissue disorder. The lesion didn’t vary with temperature or season, and there were no known triggers. The patient’s left plantar arch was unchanged.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Erythema ab igne
Upon further questioning, the patient acknowledged that he occasionally rested his bare feet around a portable heater under his desk while using his computer for a few hours each day (FIGURE 2). He often kept his right foot on the heater while he let his left foot rest on the ground. A punch biopsy was performed; the findings, when combined with the patient’s report of having exposed his foot to heat, supported the diagnosis of erythema ab igne (EAI).
EAI commonly presents as an asymptomatic reticulated erythematous to violaceous patch in an area of the body that has been in contact with heat.1 It originally was described on the bilateral anterior lower extremities after prolonged exposure to burning stoves or open fires.1 With the advent of central heating, these presentations have decreased, but there has been a resurgence of EAI with atypical distributions as a result of evolving technology and new heating sources. Reported causes of EAI include heating pads,1,2 laptop computers3 (FIGURE 3), car seat heaters,4 hot water bottles, popcorn bags, cell phones,5 and space heaters that have resulted in patches on the breast, thighs, arms, and, in our patient, foot.1-5
Blood work, biopsy can help narrow the differential
The differential for EAI includes livedo reticularis, livedo racemosa, cutis marmorata, and cutis marmorata telangiectasia. Livedo reticularis can be associated with autoimmune conditions and coagulopathies. Livedo racemosa is a typical sign of Sneddon’s syndrome and can be seen in up to 70% of patients with antiphospholipid-antibody syndrome and systemic lupus erythematosus. Diagnosis of these conditions is confirmed by elevated coagulation factors, presence of autoimmune antibodies, or history of cerebrovascular accident.6 These tests would be normal in EAI.
Histopathologic changes observed in EAI include an atrophic epidermis with an interface dermatitis, vasodilation, and dermal pigmentation. Necrotic keratinocytes and focal hyperkeratosis can be noted, along with squamous atypia. Although these changes are nonspecific, they can be used to confirm an EAI diagnosis in patients for whom the affected area has been exposed to a heat source.
Histologically, EAI is similar to actinic keratosis, with epidermal changes showing squamous atypia.2 Due to the similarities, these lesions are sometimes referred to as “thermal keratosis.” Some researchers have suggested that the thermal heat may induce epithelial changes in the same way that ultraviolet light produces epithelial changes.7
Rarely, EAI can turn into cancer. There have been a few reported cases of EAI transforming into squamous cell carcinoma or Merkel cell carcinoma; squamous cell carcinoma is more common, and tends to occur after a long latent period (up to 30 years).7-9 EAI lesions often begin as a chronic ulcer and tend not to heal. If the lesion continues to evolve (ie, ulcerate), a biopsy may be warranted to rule out a malignant transformation.
Eliminate heat exposure, consider a topical treatment
Treatment of acute EAI involves eliminating the offending heat source. The hyperpigmentation will slowly resolve over months to years.4 Persistent exposure to heat sources can lead to chronic EAI, which is more difficult to eliminate.
Because hyperpigmentation can be visually unappealing and emotionally distressing, some patients prefer active treatment. EAI has been effectively treated with 4% hydroquinone topical cream twice a day and tretinoin topical cream at night.2,10,11 Lesions that have epithelial atypia have improved with 5-fluorouracil topical cream.7
EAI also has been successfully treated with laser therapy with the 1064-nm Q-switched Nd:YAG laser with low fluence at 2-week intervals.9
Our patient declined topical therapy. He improved after a few months of avoiding the heater under his desk.
CORRESPONDENCE
Megan Morrison, DO, 5333 McAuley Drive Suite R-5003, Ypsilanti, MI 48197; [email protected]
1. Huynh N, Sarma D, Huerter C. Erythema ab igne: a case report and review of the literature. Cutis. 2011;88:290-292.
2. Tan S, Bertucci V. Erythema ab igne: an old condition new again. CMAJ. 2000;162:77-78.
3. Fu LW, Vender R. Erythema ab igne caused by laptop computer gaming—a case report. Int J Dermatol. 2012;51:716-717.
4. Brodell D, Mostow EN. Automobile seat heater-induced erythema ab igne. Arch Dermtol. 2012;148:264-265.
5. Dela Rosa K, Satter EK. Erythematous patches on the chest. Arch Dermatol. 2012;148:113-118.
6. Uthman IW, Khamashta MA. Livedo racemosa: a striking dermatological sign for antiphospholipid syndrome. J Rheumatol. 2006;33:2379-2382.
7. Bilic M, Adams B. Erythema ab igne induced by a laptop computer. J Am Acad Dermatol. 2004;50:973-974.
8. Jones CS, Tyring SK, Lee PC, et al. Development of neuroendocrine (Merkel cell) carcinoma mixed with squamous cell carcinoma in erythema ab igne. Arch Dermatol. 1998;124:110-113.
9. Cho S, Jung JY, Lee JH. Erythema ab igne successfully treated using 1,064-nm Q-switched neodymium-doped yttrium aluminum garnet laser with low fluence. Dermatol Surg. 2011;37:551-553.
10. Cardona LFC, Parsons AC, Sangueza OP. Erythematous lesions on the back of a man: challenge. Erythema ab igne. Am J Dermatopathol. 2011;33:185,199.
11. Sahl WJ, Taira JW. Erythema ab igne: treatment with 5-fluorouracil cream. J Am Acad Dermatol. 1992;27:109-110.
1. Huynh N, Sarma D, Huerter C. Erythema ab igne: a case report and review of the literature. Cutis. 2011;88:290-292.
2. Tan S, Bertucci V. Erythema ab igne: an old condition new again. CMAJ. 2000;162:77-78.
3. Fu LW, Vender R. Erythema ab igne caused by laptop computer gaming—a case report. Int J Dermatol. 2012;51:716-717.
4. Brodell D, Mostow EN. Automobile seat heater-induced erythema ab igne. Arch Dermtol. 2012;148:264-265.
5. Dela Rosa K, Satter EK. Erythematous patches on the chest. Arch Dermatol. 2012;148:113-118.
6. Uthman IW, Khamashta MA. Livedo racemosa: a striking dermatological sign for antiphospholipid syndrome. J Rheumatol. 2006;33:2379-2382.
7. Bilic M, Adams B. Erythema ab igne induced by a laptop computer. J Am Acad Dermatol. 2004;50:973-974.
8. Jones CS, Tyring SK, Lee PC, et al. Development of neuroendocrine (Merkel cell) carcinoma mixed with squamous cell carcinoma in erythema ab igne. Arch Dermatol. 1998;124:110-113.
9. Cho S, Jung JY, Lee JH. Erythema ab igne successfully treated using 1,064-nm Q-switched neodymium-doped yttrium aluminum garnet laser with low fluence. Dermatol Surg. 2011;37:551-553.
10. Cardona LFC, Parsons AC, Sangueza OP. Erythematous lesions on the back of a man: challenge. Erythema ab igne. Am J Dermatopathol. 2011;33:185,199.
11. Sahl WJ, Taira JW. Erythema ab igne: treatment with 5-fluorouracil cream. J Am Acad Dermatol. 1992;27:109-110.
Fever, wet cough, rash—Dx?
THE CASE
An 8-month-old Afghan-American girl was brought to the emergency department (ED) for evaluation of a fever and cough. She had been a full-term newborn and was otherwise healthy and up-to-date on routine immunizations. The patient was alert and crying, but consolable. The patient’s pulse was 140 beats/min, axillary temperature was 100.3°F, and respiratory rate was 25 breaths/min. She had rhinorrhea and scattered rhonchi on lung examination; no abnormal skin findings were reported. A chest x-ray showed nonspecific perihilar streaking without consolidation, which the ED physician interpreted as likely reflecting a viral or reactive airway disease. The patient was diagnosed with possible atypical pneumonia and prescribed a course of oral azithromycin (5 mg/kg/d for 7 days).
Two days later, the baby’s parents brought her to our outpatient office because she still had a fever and had developed a rash that had moved from her face to her trunk to her upper arms. The girl also had a wet cough, rhinorrhea, pharyngitis, emesis, nonbloody diarrhea, and poor fluid intake with low urine output. She was fussy and unable to produce tears while crying.
She had an axillary temperature of 100.5°F and a respiratory rate of 60 breaths/min. She also had mild facial edema, copious nasal discharge, erythematous ear canals with opaque, bulging tympanic membranes, right eye discharge, tachycardia, and tachypnea. The patient had pink to violaceous blanching papules and plaques of varied size and shape on her face, chest, abdomen, back, genitals, and upper arms. The plaques were surrounded by halos. She had no lesions on her oral mucosa, palms, or soles.
The parents indicated that the baby’s fever and accompanying symptoms had started 5 days after she and her mother had returned from a 6-week trip to Kabul, Afghanistan to visit family. They stayed in air-conditioned housing, didn’t travel rurally, and had no known exposure to illness. The patient had taken malaria prophylaxis as prescribed.
Due to the appearance of the patient’s rash and the fact that it had appeared soon after she started an antibiotic, we suspected she had a drug allergy that was complicating an upper respiratory viral syndrome with moderate (7%-10% loss of body weight) dehydration. However, given the history of travel along with the presence of cough, rhinorrhea, diarrhea, and a descending rash beginning on the face, we also considered measles.
We instructed the parents to immediately take their daughter to the regional children’s medical center for intravenous fluids and further evaluation. However, possibly due to miscommunication or cultural barriers, they did not go to the children’s hospital ED.
THE DIAGNOSIS
The next day, the Centers for Disease Control and Prevention (CDC) notified us that there had been a case of measles in a child who had been on the same return flight from Afghanistan as our patient. The CDC also confirmed a recent measles outbreak in Kabul.
The local public health department immediately reached out to the patient’s parents, tested the infant, and quarantined the family. Subsequent serologic and polymerase chain reaction (PCR) testing confirmed measles.
DISCUSSION
Measles (English measles/rubeola) is a highly contagious morbillivirus in the paramyxovirus family that spreads quickly through respiratory droplets and remains suspended in nonventilated waiting rooms after an infected patient has left.1
Measles is a leading cause of vaccine-preventable childhood mortality in the world, accounting for an estimated 46% of 1.7 million deaths in 2000.2 Measles disproportionately affects poorer communities, where vaccines may not be available. If just 10% of the population is not immunized, outbreaks can occur.3
Fortunately, thanks to increased immunization, the number of deaths due to measles worldwide has been on the decline, from approximately 733,000 in 2001 to 164,000 in 2008.3,4 Measles is no longer endemic in the United States and is near elimination in the Western Hemisphere if vaccination coverage remains high.
Vaccination. If not traveling internationally, children should receive measles-mumps-rubella (MMR) vaccination between 12 and 15 months and the second dose should be given before they reach age 4.5 However, the CDC reported that in 2014, the number of measles cases in the United States had reached a 20-year high, with 593 cases reported as of August 8.6 Many of these cases involved Americans who were not vaccinated before traveling to countries where the disease was prevalent.4
Before traveling internationally, infants ages 6 to 11 months should receive one MMR vaccination and children >12 months should receive 2 doses before leaving the United States.5
Look for fever, rash, and “the 3 Cs”
During its incubation period, the measles virus replicates in the epithelial cells and spreads first to the local lymphatics and then hematogenously to multiple organs.4 A fever typically develops 10 days after exposure; the rash develops about 4 days later.4
The measles rash is maculopapular and starts on the face, progresses to the trunk and then limbs, and coalesces (FIGURE). The rash typically lasts 3 to 5 days and clears in the same distribution that it appeared.3 The rash is part of a classic clinical presentation that also includes the “3 Cs” (cough, coryza [rhinorrhea], and conjunctivitis). In addition, patients may develop diarrhea and/or Koplik spots, an enanthem of small blue-white haloed lesions on the buccal mucosa (not palate) that are an early manifestation of illness.
Complications occur in around 40% of patients.7 Pneumonia is most common; other complications include croup and otitis media. Stomatitis may hinder children from eating. Rare but serious complications include late central nervous system manifestations such as encephalomyelitis, which affects 1/1000 people with measles.7 Measles inclusion body encephalitis and subacute sclerosing panencephalitis may emerge months to years after the acute infection and can cause progressive cognitive deterioration and death.7
Timing of fever helps narrow the diagnosis
The differential diagnosis for fever and rash in a returning traveler is broad (TABLE 1)8-10 and can be narrowed by a thorough history and exam (TABLE 2).10,11 Reportable public health conditions must be considered in all returning travelers who present with fever, particularly malaria, due to the possibility of acute deterioration.12,13 The timing of fever in relation to travel helps narrow the differential diagnosis. If the incubation period is <21 days, many viral infections (including measles, dengue fever, and chikungunya), malaria (especially falciparum), typhoid fever, leptospirosis, and rickettsial diseases should receive top consideration. If the period is >21 days, other causes are more likely.14
TABLE 2
Taking a returning traveler's history: What to ask10,11
Personal history
Travel history
|
The diagnosis of measles can be confirmed by serologic testing for measles-specific immunoglobulin M (IgM) antibodies (which may not be detected until 4 or more days after the onset of rash) or a 4-fold rise in immunoglobulin G. Detection of measles ribonucleic acid by PCR assay also can provide confirmation.3
Vitamin A can lower risk of mortality, blindness
Treatment of measles consists of supportive care and administration of vitamin A—regardless of the patient’s nutritional status. Vitamin A reduces mortality, decreases the risk of corneal damage, and promotes more rapid recovery and shortened hospital stays.1,15 World Health Organization guidelines recommend administering specific dosages of vitamin A on 2 consecutive days based on the patient’s age (TABLE 3).16 For patients with an underlying vitamin A deficiency, a third dose 2 to 4 weeks later is recommended.17
Our patient
We prescribed vitamin A for our patient but did not administer it. The patient did not follow up and we were not able to confirm the outcome.
THE TAKEAWAY
Before patients travel, counsel them on the need for appropriate immunizations. The MMR vaccine should be given to any child older than age 6 months who will be traveling to a high-risk setting. Health-related information for people who plan to travel is available from the CDC at http://wwwnc.cdc.gov/travel and the US Department of State at http://travel.state.gov/content/passports/english/country.html.
To evaluate fever and rash in an individual returning from travel, take a thorough personal and travel history. Suspect measles in patients who present with cough, rhinorrhea, conjunctivitis, diarrhea, and a descending rash that began on the face. The diagnosis can be confirmed with serologic or PCR testing. Treatment should include supportive measures and vitamin A, regardless of the patient’s nutritional status.
1. Centers for Disease Control and Prevention (CDC). Update: global measles control and mortality reduction—worldwide, 1991-2001. MMWR Morb Mortal Wkly Rep. 2003;52:471-475.
2. Moss WJ, Griffin DE. Measles. Lancet. 2012;379:153-164.
3. Centers for Disease Control and Prevention. Measles. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/pubs/pinkbook/downloads/meas.pdf. Accessed July 24, 2014.
4. Mackell SM. Vaccine recommendations for infants & children. Centers for Disease Control and Prevention Website. Available at: http://wwwnc.cdc.gov/travel/yellowbook/2014/chapter-7-international-travel-infants-children/vaccine-recommendations-for-infants-and-children. Accessed August 8, 2014.
5. Centers for Disease Control and Prevention. Measles cases and outbreaks. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/measles/cases-outbreaks.html. Accessed August 11, 2014.
6. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 5th ed. Philadelphia, PA: Mosby; 2009.
7. Moss WJ. Measles. Magill AJ, Ryan ET, Solomon T, et al. Hunter’s Tropical Medicine and Emerging Infectious Disease. 9th ed. Philadelphia, PA: Saunders Elsevier Inc; 2012.
8. McKinnon HD, Howard T. Evaluating the febrile patient with a rash. [published correction appears in American Academy of Family Physicians Web site. Available at: http://www.aafp.org/afp/2000/0815/p804.html]. Am Fam Physician. 2000;62:804-816.
9. Wilson ME. Fever in returned travelers. Centers for Disease Control and Prevention Web site. Available at: http://wwwnc.cdc.gov/travel/yellowbook/2012/chapter-5-post-travel-evaluation/fever-in-returned-travelers.htm. Updated August 1, 2013. Accessed July 24, 2014.
10. Lopez FA, Sanders CV. Fever and rash in the immunocompetent patient. UpToDate Web site. Available at: http://www.uptodate. com/contents/fever-and-rash-in-the-immunocompetent-patient. Updated June 23, 2014. Accessed July 24, 2014.
11. Feder HM Jr, Mansilla-River K. Fever in returning travelers: a case-based approach. Am Fam Physician. 2013;88:524-530.
12. Centers for Disease Control and Prevention (CDC). Malaria deaths following inappropriate malaria chemoprophylaxis— United States, 2001. MMWR Morb Mortal Wkly Rep. 2001;50: 597-599.
13. Centers for Disease Control and Prevention. MMWR: Summary of notifiable diseases. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/mmwr/mmwr_ nd/index.html. Accessed July 24, 2014.
14. Lo Re V 3rd, Gluckman SJ. Fever in the returned traveler. Am Fam Physician. 2003;68:1343-1350.
15. Huiming Y, Chaomin W, Meng M. Vitamin A for treating measles in children. Cochrane Database Syst Rev. 2005;(4):CD001479.
16. World Health Organization. WHO guidelines for epidemic preparedness and response to measles outbreaks. World Health Organization Web site. Available at: http://www.who.int/csr/ resources/publications/measles/whocdscsrisr991.pdf. Accessed July 24, 2014.
17. Fiebelkorn AP, Goodson JL. Infectious diseases related to travel. Centers for Disease Control and Prevention Web site. Available at: http://wwwnc.cdc.gov/travel/yellowbook/2014/chapter-3-infectious-diseases-related-to-travel/measles-rubeola. Accessed August 19, 2014.
THE CASE
An 8-month-old Afghan-American girl was brought to the emergency department (ED) for evaluation of a fever and cough. She had been a full-term newborn and was otherwise healthy and up-to-date on routine immunizations. The patient was alert and crying, but consolable. The patient’s pulse was 140 beats/min, axillary temperature was 100.3°F, and respiratory rate was 25 breaths/min. She had rhinorrhea and scattered rhonchi on lung examination; no abnormal skin findings were reported. A chest x-ray showed nonspecific perihilar streaking without consolidation, which the ED physician interpreted as likely reflecting a viral or reactive airway disease. The patient was diagnosed with possible atypical pneumonia and prescribed a course of oral azithromycin (5 mg/kg/d for 7 days).
Two days later, the baby’s parents brought her to our outpatient office because she still had a fever and had developed a rash that had moved from her face to her trunk to her upper arms. The girl also had a wet cough, rhinorrhea, pharyngitis, emesis, nonbloody diarrhea, and poor fluid intake with low urine output. She was fussy and unable to produce tears while crying.
She had an axillary temperature of 100.5°F and a respiratory rate of 60 breaths/min. She also had mild facial edema, copious nasal discharge, erythematous ear canals with opaque, bulging tympanic membranes, right eye discharge, tachycardia, and tachypnea. The patient had pink to violaceous blanching papules and plaques of varied size and shape on her face, chest, abdomen, back, genitals, and upper arms. The plaques were surrounded by halos. She had no lesions on her oral mucosa, palms, or soles.
The parents indicated that the baby’s fever and accompanying symptoms had started 5 days after she and her mother had returned from a 6-week trip to Kabul, Afghanistan to visit family. They stayed in air-conditioned housing, didn’t travel rurally, and had no known exposure to illness. The patient had taken malaria prophylaxis as prescribed.
Due to the appearance of the patient’s rash and the fact that it had appeared soon after she started an antibiotic, we suspected she had a drug allergy that was complicating an upper respiratory viral syndrome with moderate (7%-10% loss of body weight) dehydration. However, given the history of travel along with the presence of cough, rhinorrhea, diarrhea, and a descending rash beginning on the face, we also considered measles.
We instructed the parents to immediately take their daughter to the regional children’s medical center for intravenous fluids and further evaluation. However, possibly due to miscommunication or cultural barriers, they did not go to the children’s hospital ED.
THE DIAGNOSIS
The next day, the Centers for Disease Control and Prevention (CDC) notified us that there had been a case of measles in a child who had been on the same return flight from Afghanistan as our patient. The CDC also confirmed a recent measles outbreak in Kabul.
The local public health department immediately reached out to the patient’s parents, tested the infant, and quarantined the family. Subsequent serologic and polymerase chain reaction (PCR) testing confirmed measles.
DISCUSSION
Measles (English measles/rubeola) is a highly contagious morbillivirus in the paramyxovirus family that spreads quickly through respiratory droplets and remains suspended in nonventilated waiting rooms after an infected patient has left.1
Measles is a leading cause of vaccine-preventable childhood mortality in the world, accounting for an estimated 46% of 1.7 million deaths in 2000.2 Measles disproportionately affects poorer communities, where vaccines may not be available. If just 10% of the population is not immunized, outbreaks can occur.3
Fortunately, thanks to increased immunization, the number of deaths due to measles worldwide has been on the decline, from approximately 733,000 in 2001 to 164,000 in 2008.3,4 Measles is no longer endemic in the United States and is near elimination in the Western Hemisphere if vaccination coverage remains high.
Vaccination. If not traveling internationally, children should receive measles-mumps-rubella (MMR) vaccination between 12 and 15 months and the second dose should be given before they reach age 4.5 However, the CDC reported that in 2014, the number of measles cases in the United States had reached a 20-year high, with 593 cases reported as of August 8.6 Many of these cases involved Americans who were not vaccinated before traveling to countries where the disease was prevalent.4
Before traveling internationally, infants ages 6 to 11 months should receive one MMR vaccination and children >12 months should receive 2 doses before leaving the United States.5
Look for fever, rash, and “the 3 Cs”
During its incubation period, the measles virus replicates in the epithelial cells and spreads first to the local lymphatics and then hematogenously to multiple organs.4 A fever typically develops 10 days after exposure; the rash develops about 4 days later.4
The measles rash is maculopapular and starts on the face, progresses to the trunk and then limbs, and coalesces (FIGURE). The rash typically lasts 3 to 5 days and clears in the same distribution that it appeared.3 The rash is part of a classic clinical presentation that also includes the “3 Cs” (cough, coryza [rhinorrhea], and conjunctivitis). In addition, patients may develop diarrhea and/or Koplik spots, an enanthem of small blue-white haloed lesions on the buccal mucosa (not palate) that are an early manifestation of illness.
Complications occur in around 40% of patients.7 Pneumonia is most common; other complications include croup and otitis media. Stomatitis may hinder children from eating. Rare but serious complications include late central nervous system manifestations such as encephalomyelitis, which affects 1/1000 people with measles.7 Measles inclusion body encephalitis and subacute sclerosing panencephalitis may emerge months to years after the acute infection and can cause progressive cognitive deterioration and death.7
Timing of fever helps narrow the diagnosis
The differential diagnosis for fever and rash in a returning traveler is broad (TABLE 1)8-10 and can be narrowed by a thorough history and exam (TABLE 2).10,11 Reportable public health conditions must be considered in all returning travelers who present with fever, particularly malaria, due to the possibility of acute deterioration.12,13 The timing of fever in relation to travel helps narrow the differential diagnosis. If the incubation period is <21 days, many viral infections (including measles, dengue fever, and chikungunya), malaria (especially falciparum), typhoid fever, leptospirosis, and rickettsial diseases should receive top consideration. If the period is >21 days, other causes are more likely.14
TABLE 2
Taking a returning traveler's history: What to ask10,11
Personal history
Travel history
|
The diagnosis of measles can be confirmed by serologic testing for measles-specific immunoglobulin M (IgM) antibodies (which may not be detected until 4 or more days after the onset of rash) or a 4-fold rise in immunoglobulin G. Detection of measles ribonucleic acid by PCR assay also can provide confirmation.3
Vitamin A can lower risk of mortality, blindness
Treatment of measles consists of supportive care and administration of vitamin A—regardless of the patient’s nutritional status. Vitamin A reduces mortality, decreases the risk of corneal damage, and promotes more rapid recovery and shortened hospital stays.1,15 World Health Organization guidelines recommend administering specific dosages of vitamin A on 2 consecutive days based on the patient’s age (TABLE 3).16 For patients with an underlying vitamin A deficiency, a third dose 2 to 4 weeks later is recommended.17
Our patient
We prescribed vitamin A for our patient but did not administer it. The patient did not follow up and we were not able to confirm the outcome.
THE TAKEAWAY
Before patients travel, counsel them on the need for appropriate immunizations. The MMR vaccine should be given to any child older than age 6 months who will be traveling to a high-risk setting. Health-related information for people who plan to travel is available from the CDC at http://wwwnc.cdc.gov/travel and the US Department of State at http://travel.state.gov/content/passports/english/country.html.
To evaluate fever and rash in an individual returning from travel, take a thorough personal and travel history. Suspect measles in patients who present with cough, rhinorrhea, conjunctivitis, diarrhea, and a descending rash that began on the face. The diagnosis can be confirmed with serologic or PCR testing. Treatment should include supportive measures and vitamin A, regardless of the patient’s nutritional status.
THE CASE
An 8-month-old Afghan-American girl was brought to the emergency department (ED) for evaluation of a fever and cough. She had been a full-term newborn and was otherwise healthy and up-to-date on routine immunizations. The patient was alert and crying, but consolable. The patient’s pulse was 140 beats/min, axillary temperature was 100.3°F, and respiratory rate was 25 breaths/min. She had rhinorrhea and scattered rhonchi on lung examination; no abnormal skin findings were reported. A chest x-ray showed nonspecific perihilar streaking without consolidation, which the ED physician interpreted as likely reflecting a viral or reactive airway disease. The patient was diagnosed with possible atypical pneumonia and prescribed a course of oral azithromycin (5 mg/kg/d for 7 days).
Two days later, the baby’s parents brought her to our outpatient office because she still had a fever and had developed a rash that had moved from her face to her trunk to her upper arms. The girl also had a wet cough, rhinorrhea, pharyngitis, emesis, nonbloody diarrhea, and poor fluid intake with low urine output. She was fussy and unable to produce tears while crying.
She had an axillary temperature of 100.5°F and a respiratory rate of 60 breaths/min. She also had mild facial edema, copious nasal discharge, erythematous ear canals with opaque, bulging tympanic membranes, right eye discharge, tachycardia, and tachypnea. The patient had pink to violaceous blanching papules and plaques of varied size and shape on her face, chest, abdomen, back, genitals, and upper arms. The plaques were surrounded by halos. She had no lesions on her oral mucosa, palms, or soles.
The parents indicated that the baby’s fever and accompanying symptoms had started 5 days after she and her mother had returned from a 6-week trip to Kabul, Afghanistan to visit family. They stayed in air-conditioned housing, didn’t travel rurally, and had no known exposure to illness. The patient had taken malaria prophylaxis as prescribed.
Due to the appearance of the patient’s rash and the fact that it had appeared soon after she started an antibiotic, we suspected she had a drug allergy that was complicating an upper respiratory viral syndrome with moderate (7%-10% loss of body weight) dehydration. However, given the history of travel along with the presence of cough, rhinorrhea, diarrhea, and a descending rash beginning on the face, we also considered measles.
We instructed the parents to immediately take their daughter to the regional children’s medical center for intravenous fluids and further evaluation. However, possibly due to miscommunication or cultural barriers, they did not go to the children’s hospital ED.
THE DIAGNOSIS
The next day, the Centers for Disease Control and Prevention (CDC) notified us that there had been a case of measles in a child who had been on the same return flight from Afghanistan as our patient. The CDC also confirmed a recent measles outbreak in Kabul.
The local public health department immediately reached out to the patient’s parents, tested the infant, and quarantined the family. Subsequent serologic and polymerase chain reaction (PCR) testing confirmed measles.
DISCUSSION
Measles (English measles/rubeola) is a highly contagious morbillivirus in the paramyxovirus family that spreads quickly through respiratory droplets and remains suspended in nonventilated waiting rooms after an infected patient has left.1
Measles is a leading cause of vaccine-preventable childhood mortality in the world, accounting for an estimated 46% of 1.7 million deaths in 2000.2 Measles disproportionately affects poorer communities, where vaccines may not be available. If just 10% of the population is not immunized, outbreaks can occur.3
Fortunately, thanks to increased immunization, the number of deaths due to measles worldwide has been on the decline, from approximately 733,000 in 2001 to 164,000 in 2008.3,4 Measles is no longer endemic in the United States and is near elimination in the Western Hemisphere if vaccination coverage remains high.
Vaccination. If not traveling internationally, children should receive measles-mumps-rubella (MMR) vaccination between 12 and 15 months and the second dose should be given before they reach age 4.5 However, the CDC reported that in 2014, the number of measles cases in the United States had reached a 20-year high, with 593 cases reported as of August 8.6 Many of these cases involved Americans who were not vaccinated before traveling to countries where the disease was prevalent.4
Before traveling internationally, infants ages 6 to 11 months should receive one MMR vaccination and children >12 months should receive 2 doses before leaving the United States.5
Look for fever, rash, and “the 3 Cs”
During its incubation period, the measles virus replicates in the epithelial cells and spreads first to the local lymphatics and then hematogenously to multiple organs.4 A fever typically develops 10 days after exposure; the rash develops about 4 days later.4
The measles rash is maculopapular and starts on the face, progresses to the trunk and then limbs, and coalesces (FIGURE). The rash typically lasts 3 to 5 days and clears in the same distribution that it appeared.3 The rash is part of a classic clinical presentation that also includes the “3 Cs” (cough, coryza [rhinorrhea], and conjunctivitis). In addition, patients may develop diarrhea and/or Koplik spots, an enanthem of small blue-white haloed lesions on the buccal mucosa (not palate) that are an early manifestation of illness.
Complications occur in around 40% of patients.7 Pneumonia is most common; other complications include croup and otitis media. Stomatitis may hinder children from eating. Rare but serious complications include late central nervous system manifestations such as encephalomyelitis, which affects 1/1000 people with measles.7 Measles inclusion body encephalitis and subacute sclerosing panencephalitis may emerge months to years after the acute infection and can cause progressive cognitive deterioration and death.7
Timing of fever helps narrow the diagnosis
The differential diagnosis for fever and rash in a returning traveler is broad (TABLE 1)8-10 and can be narrowed by a thorough history and exam (TABLE 2).10,11 Reportable public health conditions must be considered in all returning travelers who present with fever, particularly malaria, due to the possibility of acute deterioration.12,13 The timing of fever in relation to travel helps narrow the differential diagnosis. If the incubation period is <21 days, many viral infections (including measles, dengue fever, and chikungunya), malaria (especially falciparum), typhoid fever, leptospirosis, and rickettsial diseases should receive top consideration. If the period is >21 days, other causes are more likely.14
TABLE 2
Taking a returning traveler's history: What to ask10,11
Personal history
Travel history
|
The diagnosis of measles can be confirmed by serologic testing for measles-specific immunoglobulin M (IgM) antibodies (which may not be detected until 4 or more days after the onset of rash) or a 4-fold rise in immunoglobulin G. Detection of measles ribonucleic acid by PCR assay also can provide confirmation.3
Vitamin A can lower risk of mortality, blindness
Treatment of measles consists of supportive care and administration of vitamin A—regardless of the patient’s nutritional status. Vitamin A reduces mortality, decreases the risk of corneal damage, and promotes more rapid recovery and shortened hospital stays.1,15 World Health Organization guidelines recommend administering specific dosages of vitamin A on 2 consecutive days based on the patient’s age (TABLE 3).16 For patients with an underlying vitamin A deficiency, a third dose 2 to 4 weeks later is recommended.17
Our patient
We prescribed vitamin A for our patient but did not administer it. The patient did not follow up and we were not able to confirm the outcome.
THE TAKEAWAY
Before patients travel, counsel them on the need for appropriate immunizations. The MMR vaccine should be given to any child older than age 6 months who will be traveling to a high-risk setting. Health-related information for people who plan to travel is available from the CDC at http://wwwnc.cdc.gov/travel and the US Department of State at http://travel.state.gov/content/passports/english/country.html.
To evaluate fever and rash in an individual returning from travel, take a thorough personal and travel history. Suspect measles in patients who present with cough, rhinorrhea, conjunctivitis, diarrhea, and a descending rash that began on the face. The diagnosis can be confirmed with serologic or PCR testing. Treatment should include supportive measures and vitamin A, regardless of the patient’s nutritional status.
1. Centers for Disease Control and Prevention (CDC). Update: global measles control and mortality reduction—worldwide, 1991-2001. MMWR Morb Mortal Wkly Rep. 2003;52:471-475.
2. Moss WJ, Griffin DE. Measles. Lancet. 2012;379:153-164.
3. Centers for Disease Control and Prevention. Measles. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/pubs/pinkbook/downloads/meas.pdf. Accessed July 24, 2014.
4. Mackell SM. Vaccine recommendations for infants & children. Centers for Disease Control and Prevention Website. Available at: http://wwwnc.cdc.gov/travel/yellowbook/2014/chapter-7-international-travel-infants-children/vaccine-recommendations-for-infants-and-children. Accessed August 8, 2014.
5. Centers for Disease Control and Prevention. Measles cases and outbreaks. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/measles/cases-outbreaks.html. Accessed August 11, 2014.
6. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 5th ed. Philadelphia, PA: Mosby; 2009.
7. Moss WJ. Measles. Magill AJ, Ryan ET, Solomon T, et al. Hunter’s Tropical Medicine and Emerging Infectious Disease. 9th ed. Philadelphia, PA: Saunders Elsevier Inc; 2012.
8. McKinnon HD, Howard T. Evaluating the febrile patient with a rash. [published correction appears in American Academy of Family Physicians Web site. Available at: http://www.aafp.org/afp/2000/0815/p804.html]. Am Fam Physician. 2000;62:804-816.
9. Wilson ME. Fever in returned travelers. Centers for Disease Control and Prevention Web site. Available at: http://wwwnc.cdc.gov/travel/yellowbook/2012/chapter-5-post-travel-evaluation/fever-in-returned-travelers.htm. Updated August 1, 2013. Accessed July 24, 2014.
10. Lopez FA, Sanders CV. Fever and rash in the immunocompetent patient. UpToDate Web site. Available at: http://www.uptodate. com/contents/fever-and-rash-in-the-immunocompetent-patient. Updated June 23, 2014. Accessed July 24, 2014.
11. Feder HM Jr, Mansilla-River K. Fever in returning travelers: a case-based approach. Am Fam Physician. 2013;88:524-530.
12. Centers for Disease Control and Prevention (CDC). Malaria deaths following inappropriate malaria chemoprophylaxis— United States, 2001. MMWR Morb Mortal Wkly Rep. 2001;50: 597-599.
13. Centers for Disease Control and Prevention. MMWR: Summary of notifiable diseases. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/mmwr/mmwr_ nd/index.html. Accessed July 24, 2014.
14. Lo Re V 3rd, Gluckman SJ. Fever in the returned traveler. Am Fam Physician. 2003;68:1343-1350.
15. Huiming Y, Chaomin W, Meng M. Vitamin A for treating measles in children. Cochrane Database Syst Rev. 2005;(4):CD001479.
16. World Health Organization. WHO guidelines for epidemic preparedness and response to measles outbreaks. World Health Organization Web site. Available at: http://www.who.int/csr/ resources/publications/measles/whocdscsrisr991.pdf. Accessed July 24, 2014.
17. Fiebelkorn AP, Goodson JL. Infectious diseases related to travel. Centers for Disease Control and Prevention Web site. Available at: http://wwwnc.cdc.gov/travel/yellowbook/2014/chapter-3-infectious-diseases-related-to-travel/measles-rubeola. Accessed August 19, 2014.
1. Centers for Disease Control and Prevention (CDC). Update: global measles control and mortality reduction—worldwide, 1991-2001. MMWR Morb Mortal Wkly Rep. 2003;52:471-475.
2. Moss WJ, Griffin DE. Measles. Lancet. 2012;379:153-164.
3. Centers for Disease Control and Prevention. Measles. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/pubs/pinkbook/downloads/meas.pdf. Accessed July 24, 2014.
4. Mackell SM. Vaccine recommendations for infants & children. Centers for Disease Control and Prevention Website. Available at: http://wwwnc.cdc.gov/travel/yellowbook/2014/chapter-7-international-travel-infants-children/vaccine-recommendations-for-infants-and-children. Accessed August 8, 2014.
5. Centers for Disease Control and Prevention. Measles cases and outbreaks. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/measles/cases-outbreaks.html. Accessed August 11, 2014.
6. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 5th ed. Philadelphia, PA: Mosby; 2009.
7. Moss WJ. Measles. Magill AJ, Ryan ET, Solomon T, et al. Hunter’s Tropical Medicine and Emerging Infectious Disease. 9th ed. Philadelphia, PA: Saunders Elsevier Inc; 2012.
8. McKinnon HD, Howard T. Evaluating the febrile patient with a rash. [published correction appears in American Academy of Family Physicians Web site. Available at: http://www.aafp.org/afp/2000/0815/p804.html]. Am Fam Physician. 2000;62:804-816.
9. Wilson ME. Fever in returned travelers. Centers for Disease Control and Prevention Web site. Available at: http://wwwnc.cdc.gov/travel/yellowbook/2012/chapter-5-post-travel-evaluation/fever-in-returned-travelers.htm. Updated August 1, 2013. Accessed July 24, 2014.
10. Lopez FA, Sanders CV. Fever and rash in the immunocompetent patient. UpToDate Web site. Available at: http://www.uptodate. com/contents/fever-and-rash-in-the-immunocompetent-patient. Updated June 23, 2014. Accessed July 24, 2014.
11. Feder HM Jr, Mansilla-River K. Fever in returning travelers: a case-based approach. Am Fam Physician. 2013;88:524-530.
12. Centers for Disease Control and Prevention (CDC). Malaria deaths following inappropriate malaria chemoprophylaxis— United States, 2001. MMWR Morb Mortal Wkly Rep. 2001;50: 597-599.
13. Centers for Disease Control and Prevention. MMWR: Summary of notifiable diseases. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/mmwr/mmwr_ nd/index.html. Accessed July 24, 2014.
14. Lo Re V 3rd, Gluckman SJ. Fever in the returned traveler. Am Fam Physician. 2003;68:1343-1350.
15. Huiming Y, Chaomin W, Meng M. Vitamin A for treating measles in children. Cochrane Database Syst Rev. 2005;(4):CD001479.
16. World Health Organization. WHO guidelines for epidemic preparedness and response to measles outbreaks. World Health Organization Web site. Available at: http://www.who.int/csr/ resources/publications/measles/whocdscsrisr991.pdf. Accessed July 24, 2014.
17. Fiebelkorn AP, Goodson JL. Infectious diseases related to travel. Centers for Disease Control and Prevention Web site. Available at: http://wwwnc.cdc.gov/travel/yellowbook/2014/chapter-3-infectious-diseases-related-to-travel/measles-rubeola. Accessed August 19, 2014.
Unhealthy drug use: How to screen, when to intervene
› Implement screening and brief intervention (SBI) for unhealthy drug use among adults in primary care. C
› Consult the National Institute on Drug Abuse’s Screening for Drug Use in General Medical Settings Resource Guide for step-by-step recommendations for implementing a drug SBI. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Joe M, age 54, comes to your office for his annual physical examination. As part of your routine screening, you ask him, “In the past year, how often have you used alcohol, tobacco, prescription drugs for nonmedical reasons, or illegal drugs?” Mr. M replies that he does not use tobacco and has not used prescription drugs for nonmedical reasons, but drinks alcohol weekly and uses cannabis and cocaine monthly.
If Mr. M were your patient, what would your next steps be?
One promising approach to alleviate substance use problems is screening and brief intervention (SBI), and—when appropriate—referral to an addiction treatment program. With strong evidence of efficacy, alcohol and tobacco SBIs have become recommended “usual” care for adults in primary care settings.1,2 Strategies for applying SBI to unhealthy drug use (“drug” SBI) in primary care have been a natural extension of the evidence that supports alcohol and tobacco SBIs.
Screening for unhealthy drug use consists of a quick risk appraisal, typically via a brief questionnaire.3-5 Patients with a positive screen then receive a more detailed assessment to estimate the extent of their substance use and severity of its consequences. If appropriate, this is followed with a brief intervention (BI), which is a time-limited, patient-centered counseling session designed to reduce substance use and/or related harm.4-6
So how can you make use of a drug SBI in your office setting?
Drug screening: What the evidence says
Currently, evidence on drug SBI is limited. The US Preventive Services Task Force (USPSTF) found insufficient evidence to recommend for or against universal drug SBI.4,7,8 The scarcity of validated screening and assessment tools that are brief enough to be used in primary care, patients’ use of multiple drugs, and confidentiality concerns likely contribute to the relative lack of research in this area.3,6,9
To our knowledge, results of only 5 randomized controlled trials (RCTs) of drug SBI that included universal screening have been published in English. Here is what these researchers found:
Bernstein et al10 investigated the efficacy of SBI for cocaine and heroin use among 23,699 adults in urgent care, women’s health, and homeless clinic settings. They randomized 1175 patients who screened positive on the Drug Abuse Screening Test11 to receive a single BI session or a handout. At 6 months, patients in the BI group were 1.5 times more likely than controls to be abstinent from cocaine (22% vs 17%; P=.045) and heroin (40% vs 31%; P=.050).
Zahradnik et al12 examined the efficacy of SBI in reducing the use of potentially addictive prescription drugs by hospitalized patients. After researchers screened 6000 inpatients, 126 patients who used, abused, or were dependent on prescription medications were randomized to receive 2 BI sessions or an information booklet. At 3 months, 52% of patients in the BI group had a ≥25% reduction in their daily doses of prescription drugs, compared to 30% in the control group (P=.017),12 However, this difference was not maintained at 12 months.13
Humeniuk et al14 evaluated the efficacy of SBI among primary care patients ages 16 to 62 years in Australia, Brazil, India, and the United States who used cannabis, cocaine, amphetamines, or opioids. Patients were screened and assessed using the World Health Organization Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST).15 Patients whose scores indicated they had a moderate risk for problem use (N=731) were randomly assigned to receive a BI or usual care. At 3 months, patients in the BI group reported a reduction in total score for “illicit substance involvement” compared to controls (P<.001). However, country-specific analyses found that BI did not have a statistically significant effect on drug use by those in the United States (N=218), possibly due to protocol differences and a greater exposure to previous substance use treatment among US patients.14
Saitz et al16 investigated the efficacy of drug SBI among primary care patients (N=528) who had been screened using the ASSIST. The most commonly used drugs were marijuana (63% of patients), cocaine (19%), and opioids (17%). Patients were randomly assigned to a 10- to 15-minute BI, a 30- to 45-minute intervention, or no intervention. BI did not show efficacy for decreasing drug use at 6-month follow-up.
Roy-Byrne et al17 screened 10,337 primary care patients of “safety net” clinics serving low-income populations. Of 1621 patients who screened positive for problem drug use, 868 were enrolled and randomly assigned to either a BI group (one-time BI using motivational interviewing, a telephone booster session, and a handout, which included relevant drug-use related information and a list of substance abuse resources) or enhanced care as usual (usual care plus a handout). Over 12 months of follow-up, there were no differences between groups in drug use or related consequences. However, a subgroup analysis suggested that compared to enhanced usual care, BI may help reduce emergency department use and increase admissions to specialized drug treatment programs among those with severe drug problems.
In addition to these 5 RCTs, a large, prospective, uncontrolled trial looked at the efficacy of drug BI among 459,599 patients from various medical settings, including primary care.18 Twenty-three percent of patients screened positive for illicit drug use and were recommended BI (16%), brief treatment (3%) or specialty treatment (4%). At a 6-month follow-up, drug use among these patients decreased by 68% and heavy alcohol use decreased by 39% (P<.001). In addition, general health, mental health, employment, housing status, and criminal behavior improved among patients recommended for brief or specialty treatments (P<.001). Although this trial lent support for the efficacy of drug SBI in primary care, it was limited by the lack of a control group and low follow-up rates at some sites.
A step-by-step approach to drug screening
Although a variety of instruments can be used to screen and assess patients for unhealthy drug use, few have been validated in primary care (TABLE 1).11,15,19-27 Despite limited evidence, multiple professional organizations, including the American Academy of Family Physicians28 and the American Psychiatric Association,26 support routine implementation of drug SBI in primary care.
The National Institute on Drug Abuse (NIDA)’s Screening for Drug Use in General Medical Settings Resource Guide19 provides a step-by-step approach to drug SBI in primary care and other general medical settings. Primarily focused on drug SBI in adults, the NIDA guide details the use of the NIDA Quick Screen and the NIDA-Modified ASSIST (NM ASSIST). These tools are available as a PDF that you can print out and complete manually (http://www.drugabuse.gov/sites/default/ files/pdf/nmassist.pdf) or as a series of forms you can complete online (http://www.drugabuse.gov/nmassist). The NIDA guide also conveniently incorporates links to alcohol and tobacco SBI recommendations.
What to ask first. Following the NIDA algorithm, first screen patients with the Quick Screen, which consists of a single question about substance use: “In the past year, how often have you used alcohol, tobacco products, prescription drugs for nonmedical reasons, or illegal drugs?" (TABLE 2).19,29-32
A negative Quick Screen (a “never” response for all substances) completes the process. Patients with a negative screen should be praised and encouraged to continue their healthy lifestyle, then rescreened annually.
For patients who respond “Yes” to heavy drinking or any tobacco use, the NIDA guide recommends proceeding with an alcohol29 or tobacco30 SBI, respectively, and provides links to appropriate resources (TABLE 2).19,29-32 Those who screen positive for drugs (“Yes” to any drug use in the past year) should receive a detailed assessment using the NM ASSIST32 to determine their risk level for developing a substance use disorder. The NM ASSIST includes 8 questions about the patient’s desire for, use of, and problems related to the use of a wide range of drugs, including cannabis, cocaine, methamphetamine, hallucinogens, and other substances (eg, “In the past 3 months, how often have you used the following substances?” “How often have you had a strong desire or urge to use this substance?” “How often has your use of this substance led to health, social, legal or financial problems?”). The score on the NM ASSIST is used to calculate the patient’s risk level as low, moderate, or high.
For patients who use more than one drug, this risk level is scored separately for each drug and may differ from drug to drug. Multi-drug assessment can become time-consuming and may not be appropriate in some patients, especially if time is an issue (eg, the patient would like to focus on other concerns) or the patient is not interested in addressing certain drugs. In general, the decision about which substances to address should be clinically-driven, tailored to the needs of an individual patient. Focusing on the substance with the highest risk score or associated with the patient’s expressed greatest motivation to change may produce the best results.
CASE › Based on Mr. M’s response to your Quick Screen indicating he drinks alcohol and uses illicit drugs, you administer the NM ASSIST to perform a detailed assessment. His answer to a screening question for problematic alcohol use is negative (In the past year, he has not had >4 drinks in a day). Next, you calculate his NM ASSIST-based risk scores for cannabis and cocaine, and determine he is at moderate risk for developing problems due to cannabis use and at high risk for developing problems based on his use of cocaine. You also note Mr. M’s blood pressure (BP) is elevated (155/90 mm hg).
Conducting a brief intervention
Depending on the patient’s risk level for developing a substance use disorder, he or she should receive either brief advice (for those at low risk) or a BI (for those at moderate or high risk) and, if needed, a referral to treatment. Two popular approaches are FRAMES (Feedback, Responsibility, Advice, Menu of Strategies, Empathy, Self-efficacy) and the NIDA-recommended 5 As intervention. The latter approach entails Asking the patient about his drug use (via the Quick Screen); Advising the patient about his drug use by providing specific medical advice on why he should stop or cut down, and how; Assessing the patient’s readiness to quit or reduce use; Assisting the patient in making a change by creating a plan with specific goals; and Arranging a follow-up visit or specialty assessment and treatment by making referrals as appropriate.19
What about children and adolescents? Implementing a drug SBI in young patients often entails overcoming unique challenges and ethical dilemmas. Although the American Academy of Pediatrics recommends SBI for unhealthy drug and alcohol use among children and adolescents,33,34 the USPSTF did not find sufficient evidence to recommend the practice.1,8,35 Screening for drug use in minors often is complicated by questions about the age at which to start routine screening and issues related to confidentiality and parental involvement. The Center for Adolescent Health and the Law and the National Institute on Alcohol Abuse and Alcoholism provide useful resources related to youth SBI, including guidance on when to consider breeching a child’s confidentiality by engaging parents or guardians (TABLE 3).
TABLE 3
Resources
NIDA Resource Guide NIDA-Modified ASSIST Coding for SBI reimbursement SAMHSA’s Treatment Services Locator NIDA’s List of Community Treatment Programs SAMHSA Opioid Overdose Toolkit Buprenorphine training program Center for Adolescent Health and the Law NIAAA Alcohol Screening and Brief Intervention for Youth |
Help is available for securing treatment, reimbursement
In addition to NIDA, many other organizations offer resources to assist clinicians in using drug SBI and helping patients obtain treatment (TABLE 3). For reimbursement, the Centers for Medicare and Medicaid Services has adopted billing codes for SBI services.36,37 The Substance Abuse and Mental Health Services Administration (SAMHSA)’s Behavioral Health Treatment Services Locator and NIDA’s National Drug Abuse Treatment Clinical Trials Network List of Associated Community Treatment Programs can assist clinicians and patients in finding specialty treatment programs. Self-help groups such as Narcotics Anonymous, Alcoholic Anonymous, or Self-Managment and Recovery Training may help alleviate problems related to insurance coverage, location, and/or timing of services.
SAMHSA’s Opioid Overdose Toolkit provides guidance to clinicians and patients on ways to reduce the risk of overdose. Physicians also can complete a short training program in office-based buprenorphine maintenance therapy to provide evidence-based care to patients with opioid dependence; more details about this program are available from http://www.buppractice.com.
CASE › You decide to use the 5 as intervention with Mr. M. You explain to him that he is at high risk of developing a substance use disorder. You also discuss his elevated BP and the possible negative effects of drug use, especially cocaine, on BP. You advise him that medically it is in his best interest to stop using cocaine and stop or reduce using cannabis. When you ask Mr. M about his readiness to change his drug use, he expresses moderate interest in stopping cocaine but is not willing to reduce his cannabis use. At this time, he is not willing to discuss these issues further (“I’ll think about that”) or create a specific plan. You assure him of your ongoing support, provide him with resources on specialty treatment programs should he wish to consider those, and schedule a follow-up visit in 2 weeks to address BP and, if the patient agrees, drug use.
CORRESPONDENCE
Aleksandra Zgierska, MD, Phd, Department of Family Medicine, University of Wisconsin School of Medicine and Public Health, 1100 Delaplaine Court, Madison, WI 53715-1896; [email protected]
1. US Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/ uspsdrin.htm. Accessed March 4, 2013.
2. US Preventive Services Task Force. Counseling and interventions to prevent tobacco use and tobacco-caused disease in adults and pregnant women. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspstbac2.htm. Accessed March 4, 2014.
3. Saitz R, Alford DP, Bernstein J, et al. Screening and brief intervention for unhealthy drug use in primary care settings: randomized clinical trials are needed. J Addict Med. 2010;4: 123-130.
4. Pilowsky DJ, Wu LT. Screening for alcohol and drug use disorders among adults in primary care: a review. Subst Abuse Rehabil. 2012;3:25-34.
5. Substance Abuse and Mental Health Services Administration. Screening, Brief Intervention, and Referral to Treatment (SBIRT). Substance Abuse and Mental Health Services Administration Web site. Available at: http://www.samhsa.gov/ prevention/sbirt/. Accessed March 4, 2014.
6. Squires LE, Alford DP, Bernstein J, et al. Clinical case discussion: screening and brief intervention for drug use in primary care. J Addict Med. 2010;4:131-136.
7. Krupski A, Joesch JM, Dunn C, et al. Testing the effects of brief intervention in primary care for problem drug use in a randomized controlled trial: rationale, design, and methods. Addict Sci Clin Pract. 2012;7:27.
8. US Preventive Services Task Force. Screening for illicit drug use. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsdrug.htm. Accessed March 4, 2014.
9. Lanier D, Ko S. Screening in Primary Care Settings for Illicit Drug Use: Assessment of Screening Instruments—A Supplemental Evidence Update for the U.S. Preventive Services Task Force. AHRQ Publication No. 08-05108-EF-2. Rockville, MD: Agency for Healthcare Research and Quality; 2008.
10. Bernstein J, Bernstein E, Tassiopoulos K, et al. Brief motivational intervention at a clinic visit reduces cocaine and heroin use. Drug Alcohol Depend. 2005;77:49-59.
11. Skinner HA. The drug abuse screening test. Addict Behav. 1982;7:363-371.
12. Zahradnik A, Otto C, Crackau B, et al. Randomized controlled trial of a brief intervention for problematic prescription drug use in non-treatment-seeking patients. Addiction. 2009;104:109-117.
13. Otto C, Crackau B, Löhrmann I, et al. Brief intervention in general hospital for problematic prescription drug use: 12-month outcome. Drug Alcohol Depend. 2009;105:221-226.
14. Humeniuk R, Ali R, Babor T, et al. A randomized controlled trial of a brief intervention for illicit drugs linked to the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) in clients recruited from primary health-care settings in four countries. Addiction. 2012;107:957-966.
15. WHO ASSIST Working Group. The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): development, reliability and feasibility. Addiction. 2002;97:1183-1194.
16. Saitz R, Palfai TP, Cheng DM, et al. Screening and brief intervention for drug use in primary care: the Assessing Screening Plus brief Intervention’s Resulting Efficacy to stop drug use (ASPIRE) randomized trial. Addict Sci Clin Pract. 2013;8(suppl 1):A61.
17. Roy-Byrne P, Bumgardner K, Krupski A, et al. Brief intervention for problem drug use in safety-net primary care settings: a randomized clinical trial. JAMA. 2014;312(5):492-501.
18. Madras BK, Compton WM, Avula D, et al. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug Alcohol Depend. 2009;99:280-295.
19. National Institute on Drug Abuse. Resource guide: Screening for drug use in general medical settings. National Institute on Drug Abuse Web site. Available at: http://www.drugabuse. gov/publications/resource-guide. Accessed March 8, 2014.
20. Saitz R, Cheng DM, Allensworth-Davies D, et al. The ability of single screening questions for unhealthy alcohol and other drug use to identify substance dependence in primary care. J Stud Alcohol Drugs. 2014;75:153-157.
21. Newcombe DA, Humeniuk RE, Ali R. Validation of the World Health Organization Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): report of results from the Australian site. Drug Alcohol Rev. 2005;24:217-226.
22. Humeniuk R, Ali R, Babor TF, et al. Validation of the Alcohol, Smoking And Substance Involvement Screening Test (ASSIST). Addiction. 2008;103:1039-1047.
23. Mdege ND, Lang J. Screening instruments for detecting illicit drug use/abuse that could be useful in general hospital wards: a systematic review. Addict Behav. 2011;36:1111-1119.
24. Cassidy CM, Schmitz N, Malla A. Validation of the alcohol use disorders identification test and the drug abuse screening test in first episode psychosis. Can J Psychiatry. 2008;53:26-33.
25. Brown RL, Rounds LA. Conjoint screening questionnaires for alcohol and other drug abuse: criterion validity in a primary care practice. Wis Med J. 1995;94:135-140.
26. American Psychiatric Association. Position statement on substance use disorders. American Psychiatric Association Web site. Available at: http://www.psychiatry.org/File%20Library/Advocacy%20and%20Newsroom/Position%20Statements/ps2012_Substance.pdf. Accessed March 4, 2014.
27. Smith PC, Schmidt SM, Allensworth-Davies D, et al. A single-question screening test for drug use in primary care. Arch Intern Med. 2010;170:1155-1160.
28. American Academy of Family Physicians. Substance abuse and addiction. American Academy of Family Physicians Web site. Available at: http://www.aafp.org/about/policies/all/substance-abuse.html. Accessed March 4, 2014.
29. National Institute on Alcohol Abuse and Alcoholism. Helping patients who drink too much: A clinician’s guide. National Institute on Alcohol Abuse and Alcoholism Web site. Available at: http://pubs.niaaa.nih.gov/publications/Practitioner/CliniciansGuide2005/clinicians_guide.htm. Accessed March 4, 2014.
30. US Department of Health and Human Services Public Health Service. Helping smokers quit: A guide for clinicians. US Department of Health and Human Services Public Health Service Web site. Available at: http://www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/tobacco/clinicians//clinhlpsmkqt/. Accessed March 4, 2014.
31. National Institute on Alcohol Abuse and Alcoholism. A Pocket Guide for Alcohol Screening and Brief Intervention. National Institute on Alcohol Abuse and Alcoholism Web site. Available at: http://pubs.niaaa.nih.gov/publications/Practitioner/pocketguide/pocket_guide.htm. Accessed July 30, 2014.
32. National Institute on Drug Abuse. NIDA-Quick Screen V1.0. National Institute on Drug Abuse Web site. Available at: http://www.drugabuse.gov/sites/default/files/pdf/nmassist.pdf. Accessed March 4, 2014.
33. Committee on Substance Abuse, Levy SJ, Kokotailo PK. Substance use screening, brief intervention, and referral to treatment for pediatricians. Pediatrics. 2011;128:e1330-e1340.
34. Kulig JW; American Academy of Pediatrics Committee on Substance Abuse. Tobacco, alcohol, and other drugs: the role of the pediatrician in prevention, identification, and management of substance abuse. Pediatrics. 2005;115:816-821.
35. US Preventive Services Task Force. Primary care behavioral interventions to reduce the nonmedical use of drugs in children and adolescents. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsnonmed.htm. Accessed March 4, 2014.
36. Centers for Medicare & Medicaid Services. Screening, Brief Intervention, and Referral to Treatment (SBIRT) services. Centers for Medicare & Medicaid Services Web site. Available at: http://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/sbirt_factsheet_icn904084.pdf. Accessed March 4, 2014.
37. Substance Abuse and Mental Health Services Administration. Coding for screening and brief intervention reimbursement. Substance Abuse and Mental Health Services Administration Web site. Available at: http://beta.samhsa.gov/sbirt/coding-reimbursement. Accessed August 4, 2014.
› Implement screening and brief intervention (SBI) for unhealthy drug use among adults in primary care. C
› Consult the National Institute on Drug Abuse’s Screening for Drug Use in General Medical Settings Resource Guide for step-by-step recommendations for implementing a drug SBI. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Joe M, age 54, comes to your office for his annual physical examination. As part of your routine screening, you ask him, “In the past year, how often have you used alcohol, tobacco, prescription drugs for nonmedical reasons, or illegal drugs?” Mr. M replies that he does not use tobacco and has not used prescription drugs for nonmedical reasons, but drinks alcohol weekly and uses cannabis and cocaine monthly.
If Mr. M were your patient, what would your next steps be?
One promising approach to alleviate substance use problems is screening and brief intervention (SBI), and—when appropriate—referral to an addiction treatment program. With strong evidence of efficacy, alcohol and tobacco SBIs have become recommended “usual” care for adults in primary care settings.1,2 Strategies for applying SBI to unhealthy drug use (“drug” SBI) in primary care have been a natural extension of the evidence that supports alcohol and tobacco SBIs.
Screening for unhealthy drug use consists of a quick risk appraisal, typically via a brief questionnaire.3-5 Patients with a positive screen then receive a more detailed assessment to estimate the extent of their substance use and severity of its consequences. If appropriate, this is followed with a brief intervention (BI), which is a time-limited, patient-centered counseling session designed to reduce substance use and/or related harm.4-6
So how can you make use of a drug SBI in your office setting?
Drug screening: What the evidence says
Currently, evidence on drug SBI is limited. The US Preventive Services Task Force (USPSTF) found insufficient evidence to recommend for or against universal drug SBI.4,7,8 The scarcity of validated screening and assessment tools that are brief enough to be used in primary care, patients’ use of multiple drugs, and confidentiality concerns likely contribute to the relative lack of research in this area.3,6,9
To our knowledge, results of only 5 randomized controlled trials (RCTs) of drug SBI that included universal screening have been published in English. Here is what these researchers found:
Bernstein et al10 investigated the efficacy of SBI for cocaine and heroin use among 23,699 adults in urgent care, women’s health, and homeless clinic settings. They randomized 1175 patients who screened positive on the Drug Abuse Screening Test11 to receive a single BI session or a handout. At 6 months, patients in the BI group were 1.5 times more likely than controls to be abstinent from cocaine (22% vs 17%; P=.045) and heroin (40% vs 31%; P=.050).
Zahradnik et al12 examined the efficacy of SBI in reducing the use of potentially addictive prescription drugs by hospitalized patients. After researchers screened 6000 inpatients, 126 patients who used, abused, or were dependent on prescription medications were randomized to receive 2 BI sessions or an information booklet. At 3 months, 52% of patients in the BI group had a ≥25% reduction in their daily doses of prescription drugs, compared to 30% in the control group (P=.017),12 However, this difference was not maintained at 12 months.13
Humeniuk et al14 evaluated the efficacy of SBI among primary care patients ages 16 to 62 years in Australia, Brazil, India, and the United States who used cannabis, cocaine, amphetamines, or opioids. Patients were screened and assessed using the World Health Organization Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST).15 Patients whose scores indicated they had a moderate risk for problem use (N=731) were randomly assigned to receive a BI or usual care. At 3 months, patients in the BI group reported a reduction in total score for “illicit substance involvement” compared to controls (P<.001). However, country-specific analyses found that BI did not have a statistically significant effect on drug use by those in the United States (N=218), possibly due to protocol differences and a greater exposure to previous substance use treatment among US patients.14
Saitz et al16 investigated the efficacy of drug SBI among primary care patients (N=528) who had been screened using the ASSIST. The most commonly used drugs were marijuana (63% of patients), cocaine (19%), and opioids (17%). Patients were randomly assigned to a 10- to 15-minute BI, a 30- to 45-minute intervention, or no intervention. BI did not show efficacy for decreasing drug use at 6-month follow-up.
Roy-Byrne et al17 screened 10,337 primary care patients of “safety net” clinics serving low-income populations. Of 1621 patients who screened positive for problem drug use, 868 were enrolled and randomly assigned to either a BI group (one-time BI using motivational interviewing, a telephone booster session, and a handout, which included relevant drug-use related information and a list of substance abuse resources) or enhanced care as usual (usual care plus a handout). Over 12 months of follow-up, there were no differences between groups in drug use or related consequences. However, a subgroup analysis suggested that compared to enhanced usual care, BI may help reduce emergency department use and increase admissions to specialized drug treatment programs among those with severe drug problems.
In addition to these 5 RCTs, a large, prospective, uncontrolled trial looked at the efficacy of drug BI among 459,599 patients from various medical settings, including primary care.18 Twenty-three percent of patients screened positive for illicit drug use and were recommended BI (16%), brief treatment (3%) or specialty treatment (4%). At a 6-month follow-up, drug use among these patients decreased by 68% and heavy alcohol use decreased by 39% (P<.001). In addition, general health, mental health, employment, housing status, and criminal behavior improved among patients recommended for brief or specialty treatments (P<.001). Although this trial lent support for the efficacy of drug SBI in primary care, it was limited by the lack of a control group and low follow-up rates at some sites.
A step-by-step approach to drug screening
Although a variety of instruments can be used to screen and assess patients for unhealthy drug use, few have been validated in primary care (TABLE 1).11,15,19-27 Despite limited evidence, multiple professional organizations, including the American Academy of Family Physicians28 and the American Psychiatric Association,26 support routine implementation of drug SBI in primary care.
The National Institute on Drug Abuse (NIDA)’s Screening for Drug Use in General Medical Settings Resource Guide19 provides a step-by-step approach to drug SBI in primary care and other general medical settings. Primarily focused on drug SBI in adults, the NIDA guide details the use of the NIDA Quick Screen and the NIDA-Modified ASSIST (NM ASSIST). These tools are available as a PDF that you can print out and complete manually (http://www.drugabuse.gov/sites/default/ files/pdf/nmassist.pdf) or as a series of forms you can complete online (http://www.drugabuse.gov/nmassist). The NIDA guide also conveniently incorporates links to alcohol and tobacco SBI recommendations.
What to ask first. Following the NIDA algorithm, first screen patients with the Quick Screen, which consists of a single question about substance use: “In the past year, how often have you used alcohol, tobacco products, prescription drugs for nonmedical reasons, or illegal drugs?" (TABLE 2).19,29-32
A negative Quick Screen (a “never” response for all substances) completes the process. Patients with a negative screen should be praised and encouraged to continue their healthy lifestyle, then rescreened annually.
For patients who respond “Yes” to heavy drinking or any tobacco use, the NIDA guide recommends proceeding with an alcohol29 or tobacco30 SBI, respectively, and provides links to appropriate resources (TABLE 2).19,29-32 Those who screen positive for drugs (“Yes” to any drug use in the past year) should receive a detailed assessment using the NM ASSIST32 to determine their risk level for developing a substance use disorder. The NM ASSIST includes 8 questions about the patient’s desire for, use of, and problems related to the use of a wide range of drugs, including cannabis, cocaine, methamphetamine, hallucinogens, and other substances (eg, “In the past 3 months, how often have you used the following substances?” “How often have you had a strong desire or urge to use this substance?” “How often has your use of this substance led to health, social, legal or financial problems?”). The score on the NM ASSIST is used to calculate the patient’s risk level as low, moderate, or high.
For patients who use more than one drug, this risk level is scored separately for each drug and may differ from drug to drug. Multi-drug assessment can become time-consuming and may not be appropriate in some patients, especially if time is an issue (eg, the patient would like to focus on other concerns) or the patient is not interested in addressing certain drugs. In general, the decision about which substances to address should be clinically-driven, tailored to the needs of an individual patient. Focusing on the substance with the highest risk score or associated with the patient’s expressed greatest motivation to change may produce the best results.
CASE › Based on Mr. M’s response to your Quick Screen indicating he drinks alcohol and uses illicit drugs, you administer the NM ASSIST to perform a detailed assessment. His answer to a screening question for problematic alcohol use is negative (In the past year, he has not had >4 drinks in a day). Next, you calculate his NM ASSIST-based risk scores for cannabis and cocaine, and determine he is at moderate risk for developing problems due to cannabis use and at high risk for developing problems based on his use of cocaine. You also note Mr. M’s blood pressure (BP) is elevated (155/90 mm hg).
Conducting a brief intervention
Depending on the patient’s risk level for developing a substance use disorder, he or she should receive either brief advice (for those at low risk) or a BI (for those at moderate or high risk) and, if needed, a referral to treatment. Two popular approaches are FRAMES (Feedback, Responsibility, Advice, Menu of Strategies, Empathy, Self-efficacy) and the NIDA-recommended 5 As intervention. The latter approach entails Asking the patient about his drug use (via the Quick Screen); Advising the patient about his drug use by providing specific medical advice on why he should stop or cut down, and how; Assessing the patient’s readiness to quit or reduce use; Assisting the patient in making a change by creating a plan with specific goals; and Arranging a follow-up visit or specialty assessment and treatment by making referrals as appropriate.19
What about children and adolescents? Implementing a drug SBI in young patients often entails overcoming unique challenges and ethical dilemmas. Although the American Academy of Pediatrics recommends SBI for unhealthy drug and alcohol use among children and adolescents,33,34 the USPSTF did not find sufficient evidence to recommend the practice.1,8,35 Screening for drug use in minors often is complicated by questions about the age at which to start routine screening and issues related to confidentiality and parental involvement. The Center for Adolescent Health and the Law and the National Institute on Alcohol Abuse and Alcoholism provide useful resources related to youth SBI, including guidance on when to consider breeching a child’s confidentiality by engaging parents or guardians (TABLE 3).
TABLE 3
Resources
NIDA Resource Guide NIDA-Modified ASSIST Coding for SBI reimbursement SAMHSA’s Treatment Services Locator NIDA’s List of Community Treatment Programs SAMHSA Opioid Overdose Toolkit Buprenorphine training program Center for Adolescent Health and the Law NIAAA Alcohol Screening and Brief Intervention for Youth |
Help is available for securing treatment, reimbursement
In addition to NIDA, many other organizations offer resources to assist clinicians in using drug SBI and helping patients obtain treatment (TABLE 3). For reimbursement, the Centers for Medicare and Medicaid Services has adopted billing codes for SBI services.36,37 The Substance Abuse and Mental Health Services Administration (SAMHSA)’s Behavioral Health Treatment Services Locator and NIDA’s National Drug Abuse Treatment Clinical Trials Network List of Associated Community Treatment Programs can assist clinicians and patients in finding specialty treatment programs. Self-help groups such as Narcotics Anonymous, Alcoholic Anonymous, or Self-Managment and Recovery Training may help alleviate problems related to insurance coverage, location, and/or timing of services.
SAMHSA’s Opioid Overdose Toolkit provides guidance to clinicians and patients on ways to reduce the risk of overdose. Physicians also can complete a short training program in office-based buprenorphine maintenance therapy to provide evidence-based care to patients with opioid dependence; more details about this program are available from http://www.buppractice.com.
CASE › You decide to use the 5 as intervention with Mr. M. You explain to him that he is at high risk of developing a substance use disorder. You also discuss his elevated BP and the possible negative effects of drug use, especially cocaine, on BP. You advise him that medically it is in his best interest to stop using cocaine and stop or reduce using cannabis. When you ask Mr. M about his readiness to change his drug use, he expresses moderate interest in stopping cocaine but is not willing to reduce his cannabis use. At this time, he is not willing to discuss these issues further (“I’ll think about that”) or create a specific plan. You assure him of your ongoing support, provide him with resources on specialty treatment programs should he wish to consider those, and schedule a follow-up visit in 2 weeks to address BP and, if the patient agrees, drug use.
CORRESPONDENCE
Aleksandra Zgierska, MD, Phd, Department of Family Medicine, University of Wisconsin School of Medicine and Public Health, 1100 Delaplaine Court, Madison, WI 53715-1896; [email protected]
› Implement screening and brief intervention (SBI) for unhealthy drug use among adults in primary care. C
› Consult the National Institute on Drug Abuse’s Screening for Drug Use in General Medical Settings Resource Guide for step-by-step recommendations for implementing a drug SBI. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Joe M, age 54, comes to your office for his annual physical examination. As part of your routine screening, you ask him, “In the past year, how often have you used alcohol, tobacco, prescription drugs for nonmedical reasons, or illegal drugs?” Mr. M replies that he does not use tobacco and has not used prescription drugs for nonmedical reasons, but drinks alcohol weekly and uses cannabis and cocaine monthly.
If Mr. M were your patient, what would your next steps be?
One promising approach to alleviate substance use problems is screening and brief intervention (SBI), and—when appropriate—referral to an addiction treatment program. With strong evidence of efficacy, alcohol and tobacco SBIs have become recommended “usual” care for adults in primary care settings.1,2 Strategies for applying SBI to unhealthy drug use (“drug” SBI) in primary care have been a natural extension of the evidence that supports alcohol and tobacco SBIs.
Screening for unhealthy drug use consists of a quick risk appraisal, typically via a brief questionnaire.3-5 Patients with a positive screen then receive a more detailed assessment to estimate the extent of their substance use and severity of its consequences. If appropriate, this is followed with a brief intervention (BI), which is a time-limited, patient-centered counseling session designed to reduce substance use and/or related harm.4-6
So how can you make use of a drug SBI in your office setting?
Drug screening: What the evidence says
Currently, evidence on drug SBI is limited. The US Preventive Services Task Force (USPSTF) found insufficient evidence to recommend for or against universal drug SBI.4,7,8 The scarcity of validated screening and assessment tools that are brief enough to be used in primary care, patients’ use of multiple drugs, and confidentiality concerns likely contribute to the relative lack of research in this area.3,6,9
To our knowledge, results of only 5 randomized controlled trials (RCTs) of drug SBI that included universal screening have been published in English. Here is what these researchers found:
Bernstein et al10 investigated the efficacy of SBI for cocaine and heroin use among 23,699 adults in urgent care, women’s health, and homeless clinic settings. They randomized 1175 patients who screened positive on the Drug Abuse Screening Test11 to receive a single BI session or a handout. At 6 months, patients in the BI group were 1.5 times more likely than controls to be abstinent from cocaine (22% vs 17%; P=.045) and heroin (40% vs 31%; P=.050).
Zahradnik et al12 examined the efficacy of SBI in reducing the use of potentially addictive prescription drugs by hospitalized patients. After researchers screened 6000 inpatients, 126 patients who used, abused, or were dependent on prescription medications were randomized to receive 2 BI sessions or an information booklet. At 3 months, 52% of patients in the BI group had a ≥25% reduction in their daily doses of prescription drugs, compared to 30% in the control group (P=.017),12 However, this difference was not maintained at 12 months.13
Humeniuk et al14 evaluated the efficacy of SBI among primary care patients ages 16 to 62 years in Australia, Brazil, India, and the United States who used cannabis, cocaine, amphetamines, or opioids. Patients were screened and assessed using the World Health Organization Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST).15 Patients whose scores indicated they had a moderate risk for problem use (N=731) were randomly assigned to receive a BI or usual care. At 3 months, patients in the BI group reported a reduction in total score for “illicit substance involvement” compared to controls (P<.001). However, country-specific analyses found that BI did not have a statistically significant effect on drug use by those in the United States (N=218), possibly due to protocol differences and a greater exposure to previous substance use treatment among US patients.14
Saitz et al16 investigated the efficacy of drug SBI among primary care patients (N=528) who had been screened using the ASSIST. The most commonly used drugs were marijuana (63% of patients), cocaine (19%), and opioids (17%). Patients were randomly assigned to a 10- to 15-minute BI, a 30- to 45-minute intervention, or no intervention. BI did not show efficacy for decreasing drug use at 6-month follow-up.
Roy-Byrne et al17 screened 10,337 primary care patients of “safety net” clinics serving low-income populations. Of 1621 patients who screened positive for problem drug use, 868 were enrolled and randomly assigned to either a BI group (one-time BI using motivational interviewing, a telephone booster session, and a handout, which included relevant drug-use related information and a list of substance abuse resources) or enhanced care as usual (usual care plus a handout). Over 12 months of follow-up, there were no differences between groups in drug use or related consequences. However, a subgroup analysis suggested that compared to enhanced usual care, BI may help reduce emergency department use and increase admissions to specialized drug treatment programs among those with severe drug problems.
In addition to these 5 RCTs, a large, prospective, uncontrolled trial looked at the efficacy of drug BI among 459,599 patients from various medical settings, including primary care.18 Twenty-three percent of patients screened positive for illicit drug use and were recommended BI (16%), brief treatment (3%) or specialty treatment (4%). At a 6-month follow-up, drug use among these patients decreased by 68% and heavy alcohol use decreased by 39% (P<.001). In addition, general health, mental health, employment, housing status, and criminal behavior improved among patients recommended for brief or specialty treatments (P<.001). Although this trial lent support for the efficacy of drug SBI in primary care, it was limited by the lack of a control group and low follow-up rates at some sites.
A step-by-step approach to drug screening
Although a variety of instruments can be used to screen and assess patients for unhealthy drug use, few have been validated in primary care (TABLE 1).11,15,19-27 Despite limited evidence, multiple professional organizations, including the American Academy of Family Physicians28 and the American Psychiatric Association,26 support routine implementation of drug SBI in primary care.
The National Institute on Drug Abuse (NIDA)’s Screening for Drug Use in General Medical Settings Resource Guide19 provides a step-by-step approach to drug SBI in primary care and other general medical settings. Primarily focused on drug SBI in adults, the NIDA guide details the use of the NIDA Quick Screen and the NIDA-Modified ASSIST (NM ASSIST). These tools are available as a PDF that you can print out and complete manually (http://www.drugabuse.gov/sites/default/ files/pdf/nmassist.pdf) or as a series of forms you can complete online (http://www.drugabuse.gov/nmassist). The NIDA guide also conveniently incorporates links to alcohol and tobacco SBI recommendations.
What to ask first. Following the NIDA algorithm, first screen patients with the Quick Screen, which consists of a single question about substance use: “In the past year, how often have you used alcohol, tobacco products, prescription drugs for nonmedical reasons, or illegal drugs?" (TABLE 2).19,29-32
A negative Quick Screen (a “never” response for all substances) completes the process. Patients with a negative screen should be praised and encouraged to continue their healthy lifestyle, then rescreened annually.
For patients who respond “Yes” to heavy drinking or any tobacco use, the NIDA guide recommends proceeding with an alcohol29 or tobacco30 SBI, respectively, and provides links to appropriate resources (TABLE 2).19,29-32 Those who screen positive for drugs (“Yes” to any drug use in the past year) should receive a detailed assessment using the NM ASSIST32 to determine their risk level for developing a substance use disorder. The NM ASSIST includes 8 questions about the patient’s desire for, use of, and problems related to the use of a wide range of drugs, including cannabis, cocaine, methamphetamine, hallucinogens, and other substances (eg, “In the past 3 months, how often have you used the following substances?” “How often have you had a strong desire or urge to use this substance?” “How often has your use of this substance led to health, social, legal or financial problems?”). The score on the NM ASSIST is used to calculate the patient’s risk level as low, moderate, or high.
For patients who use more than one drug, this risk level is scored separately for each drug and may differ from drug to drug. Multi-drug assessment can become time-consuming and may not be appropriate in some patients, especially if time is an issue (eg, the patient would like to focus on other concerns) or the patient is not interested in addressing certain drugs. In general, the decision about which substances to address should be clinically-driven, tailored to the needs of an individual patient. Focusing on the substance with the highest risk score or associated with the patient’s expressed greatest motivation to change may produce the best results.
CASE › Based on Mr. M’s response to your Quick Screen indicating he drinks alcohol and uses illicit drugs, you administer the NM ASSIST to perform a detailed assessment. His answer to a screening question for problematic alcohol use is negative (In the past year, he has not had >4 drinks in a day). Next, you calculate his NM ASSIST-based risk scores for cannabis and cocaine, and determine he is at moderate risk for developing problems due to cannabis use and at high risk for developing problems based on his use of cocaine. You also note Mr. M’s blood pressure (BP) is elevated (155/90 mm hg).
Conducting a brief intervention
Depending on the patient’s risk level for developing a substance use disorder, he or she should receive either brief advice (for those at low risk) or a BI (for those at moderate or high risk) and, if needed, a referral to treatment. Two popular approaches are FRAMES (Feedback, Responsibility, Advice, Menu of Strategies, Empathy, Self-efficacy) and the NIDA-recommended 5 As intervention. The latter approach entails Asking the patient about his drug use (via the Quick Screen); Advising the patient about his drug use by providing specific medical advice on why he should stop or cut down, and how; Assessing the patient’s readiness to quit or reduce use; Assisting the patient in making a change by creating a plan with specific goals; and Arranging a follow-up visit or specialty assessment and treatment by making referrals as appropriate.19
What about children and adolescents? Implementing a drug SBI in young patients often entails overcoming unique challenges and ethical dilemmas. Although the American Academy of Pediatrics recommends SBI for unhealthy drug and alcohol use among children and adolescents,33,34 the USPSTF did not find sufficient evidence to recommend the practice.1,8,35 Screening for drug use in minors often is complicated by questions about the age at which to start routine screening and issues related to confidentiality and parental involvement. The Center for Adolescent Health and the Law and the National Institute on Alcohol Abuse and Alcoholism provide useful resources related to youth SBI, including guidance on when to consider breeching a child’s confidentiality by engaging parents or guardians (TABLE 3).
TABLE 3
Resources
NIDA Resource Guide NIDA-Modified ASSIST Coding for SBI reimbursement SAMHSA’s Treatment Services Locator NIDA’s List of Community Treatment Programs SAMHSA Opioid Overdose Toolkit Buprenorphine training program Center for Adolescent Health and the Law NIAAA Alcohol Screening and Brief Intervention for Youth |
Help is available for securing treatment, reimbursement
In addition to NIDA, many other organizations offer resources to assist clinicians in using drug SBI and helping patients obtain treatment (TABLE 3). For reimbursement, the Centers for Medicare and Medicaid Services has adopted billing codes for SBI services.36,37 The Substance Abuse and Mental Health Services Administration (SAMHSA)’s Behavioral Health Treatment Services Locator and NIDA’s National Drug Abuse Treatment Clinical Trials Network List of Associated Community Treatment Programs can assist clinicians and patients in finding specialty treatment programs. Self-help groups such as Narcotics Anonymous, Alcoholic Anonymous, or Self-Managment and Recovery Training may help alleviate problems related to insurance coverage, location, and/or timing of services.
SAMHSA’s Opioid Overdose Toolkit provides guidance to clinicians and patients on ways to reduce the risk of overdose. Physicians also can complete a short training program in office-based buprenorphine maintenance therapy to provide evidence-based care to patients with opioid dependence; more details about this program are available from http://www.buppractice.com.
CASE › You decide to use the 5 as intervention with Mr. M. You explain to him that he is at high risk of developing a substance use disorder. You also discuss his elevated BP and the possible negative effects of drug use, especially cocaine, on BP. You advise him that medically it is in his best interest to stop using cocaine and stop or reduce using cannabis. When you ask Mr. M about his readiness to change his drug use, he expresses moderate interest in stopping cocaine but is not willing to reduce his cannabis use. At this time, he is not willing to discuss these issues further (“I’ll think about that”) or create a specific plan. You assure him of your ongoing support, provide him with resources on specialty treatment programs should he wish to consider those, and schedule a follow-up visit in 2 weeks to address BP and, if the patient agrees, drug use.
CORRESPONDENCE
Aleksandra Zgierska, MD, Phd, Department of Family Medicine, University of Wisconsin School of Medicine and Public Health, 1100 Delaplaine Court, Madison, WI 53715-1896; [email protected]
1. US Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/ uspsdrin.htm. Accessed March 4, 2013.
2. US Preventive Services Task Force. Counseling and interventions to prevent tobacco use and tobacco-caused disease in adults and pregnant women. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspstbac2.htm. Accessed March 4, 2014.
3. Saitz R, Alford DP, Bernstein J, et al. Screening and brief intervention for unhealthy drug use in primary care settings: randomized clinical trials are needed. J Addict Med. 2010;4: 123-130.
4. Pilowsky DJ, Wu LT. Screening for alcohol and drug use disorders among adults in primary care: a review. Subst Abuse Rehabil. 2012;3:25-34.
5. Substance Abuse and Mental Health Services Administration. Screening, Brief Intervention, and Referral to Treatment (SBIRT). Substance Abuse and Mental Health Services Administration Web site. Available at: http://www.samhsa.gov/ prevention/sbirt/. Accessed March 4, 2014.
6. Squires LE, Alford DP, Bernstein J, et al. Clinical case discussion: screening and brief intervention for drug use in primary care. J Addict Med. 2010;4:131-136.
7. Krupski A, Joesch JM, Dunn C, et al. Testing the effects of brief intervention in primary care for problem drug use in a randomized controlled trial: rationale, design, and methods. Addict Sci Clin Pract. 2012;7:27.
8. US Preventive Services Task Force. Screening for illicit drug use. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsdrug.htm. Accessed March 4, 2014.
9. Lanier D, Ko S. Screening in Primary Care Settings for Illicit Drug Use: Assessment of Screening Instruments—A Supplemental Evidence Update for the U.S. Preventive Services Task Force. AHRQ Publication No. 08-05108-EF-2. Rockville, MD: Agency for Healthcare Research and Quality; 2008.
10. Bernstein J, Bernstein E, Tassiopoulos K, et al. Brief motivational intervention at a clinic visit reduces cocaine and heroin use. Drug Alcohol Depend. 2005;77:49-59.
11. Skinner HA. The drug abuse screening test. Addict Behav. 1982;7:363-371.
12. Zahradnik A, Otto C, Crackau B, et al. Randomized controlled trial of a brief intervention for problematic prescription drug use in non-treatment-seeking patients. Addiction. 2009;104:109-117.
13. Otto C, Crackau B, Löhrmann I, et al. Brief intervention in general hospital for problematic prescription drug use: 12-month outcome. Drug Alcohol Depend. 2009;105:221-226.
14. Humeniuk R, Ali R, Babor T, et al. A randomized controlled trial of a brief intervention for illicit drugs linked to the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) in clients recruited from primary health-care settings in four countries. Addiction. 2012;107:957-966.
15. WHO ASSIST Working Group. The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): development, reliability and feasibility. Addiction. 2002;97:1183-1194.
16. Saitz R, Palfai TP, Cheng DM, et al. Screening and brief intervention for drug use in primary care: the Assessing Screening Plus brief Intervention’s Resulting Efficacy to stop drug use (ASPIRE) randomized trial. Addict Sci Clin Pract. 2013;8(suppl 1):A61.
17. Roy-Byrne P, Bumgardner K, Krupski A, et al. Brief intervention for problem drug use in safety-net primary care settings: a randomized clinical trial. JAMA. 2014;312(5):492-501.
18. Madras BK, Compton WM, Avula D, et al. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug Alcohol Depend. 2009;99:280-295.
19. National Institute on Drug Abuse. Resource guide: Screening for drug use in general medical settings. National Institute on Drug Abuse Web site. Available at: http://www.drugabuse. gov/publications/resource-guide. Accessed March 8, 2014.
20. Saitz R, Cheng DM, Allensworth-Davies D, et al. The ability of single screening questions for unhealthy alcohol and other drug use to identify substance dependence in primary care. J Stud Alcohol Drugs. 2014;75:153-157.
21. Newcombe DA, Humeniuk RE, Ali R. Validation of the World Health Organization Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): report of results from the Australian site. Drug Alcohol Rev. 2005;24:217-226.
22. Humeniuk R, Ali R, Babor TF, et al. Validation of the Alcohol, Smoking And Substance Involvement Screening Test (ASSIST). Addiction. 2008;103:1039-1047.
23. Mdege ND, Lang J. Screening instruments for detecting illicit drug use/abuse that could be useful in general hospital wards: a systematic review. Addict Behav. 2011;36:1111-1119.
24. Cassidy CM, Schmitz N, Malla A. Validation of the alcohol use disorders identification test and the drug abuse screening test in first episode psychosis. Can J Psychiatry. 2008;53:26-33.
25. Brown RL, Rounds LA. Conjoint screening questionnaires for alcohol and other drug abuse: criterion validity in a primary care practice. Wis Med J. 1995;94:135-140.
26. American Psychiatric Association. Position statement on substance use disorders. American Psychiatric Association Web site. Available at: http://www.psychiatry.org/File%20Library/Advocacy%20and%20Newsroom/Position%20Statements/ps2012_Substance.pdf. Accessed March 4, 2014.
27. Smith PC, Schmidt SM, Allensworth-Davies D, et al. A single-question screening test for drug use in primary care. Arch Intern Med. 2010;170:1155-1160.
28. American Academy of Family Physicians. Substance abuse and addiction. American Academy of Family Physicians Web site. Available at: http://www.aafp.org/about/policies/all/substance-abuse.html. Accessed March 4, 2014.
29. National Institute on Alcohol Abuse and Alcoholism. Helping patients who drink too much: A clinician’s guide. National Institute on Alcohol Abuse and Alcoholism Web site. Available at: http://pubs.niaaa.nih.gov/publications/Practitioner/CliniciansGuide2005/clinicians_guide.htm. Accessed March 4, 2014.
30. US Department of Health and Human Services Public Health Service. Helping smokers quit: A guide for clinicians. US Department of Health and Human Services Public Health Service Web site. Available at: http://www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/tobacco/clinicians//clinhlpsmkqt/. Accessed March 4, 2014.
31. National Institute on Alcohol Abuse and Alcoholism. A Pocket Guide for Alcohol Screening and Brief Intervention. National Institute on Alcohol Abuse and Alcoholism Web site. Available at: http://pubs.niaaa.nih.gov/publications/Practitioner/pocketguide/pocket_guide.htm. Accessed July 30, 2014.
32. National Institute on Drug Abuse. NIDA-Quick Screen V1.0. National Institute on Drug Abuse Web site. Available at: http://www.drugabuse.gov/sites/default/files/pdf/nmassist.pdf. Accessed March 4, 2014.
33. Committee on Substance Abuse, Levy SJ, Kokotailo PK. Substance use screening, brief intervention, and referral to treatment for pediatricians. Pediatrics. 2011;128:e1330-e1340.
34. Kulig JW; American Academy of Pediatrics Committee on Substance Abuse. Tobacco, alcohol, and other drugs: the role of the pediatrician in prevention, identification, and management of substance abuse. Pediatrics. 2005;115:816-821.
35. US Preventive Services Task Force. Primary care behavioral interventions to reduce the nonmedical use of drugs in children and adolescents. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsnonmed.htm. Accessed March 4, 2014.
36. Centers for Medicare & Medicaid Services. Screening, Brief Intervention, and Referral to Treatment (SBIRT) services. Centers for Medicare & Medicaid Services Web site. Available at: http://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/sbirt_factsheet_icn904084.pdf. Accessed March 4, 2014.
37. Substance Abuse and Mental Health Services Administration. Coding for screening and brief intervention reimbursement. Substance Abuse and Mental Health Services Administration Web site. Available at: http://beta.samhsa.gov/sbirt/coding-reimbursement. Accessed August 4, 2014.
1. US Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/ uspsdrin.htm. Accessed March 4, 2013.
2. US Preventive Services Task Force. Counseling and interventions to prevent tobacco use and tobacco-caused disease in adults and pregnant women. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspstbac2.htm. Accessed March 4, 2014.
3. Saitz R, Alford DP, Bernstein J, et al. Screening and brief intervention for unhealthy drug use in primary care settings: randomized clinical trials are needed. J Addict Med. 2010;4: 123-130.
4. Pilowsky DJ, Wu LT. Screening for alcohol and drug use disorders among adults in primary care: a review. Subst Abuse Rehabil. 2012;3:25-34.
5. Substance Abuse and Mental Health Services Administration. Screening, Brief Intervention, and Referral to Treatment (SBIRT). Substance Abuse and Mental Health Services Administration Web site. Available at: http://www.samhsa.gov/ prevention/sbirt/. Accessed March 4, 2014.
6. Squires LE, Alford DP, Bernstein J, et al. Clinical case discussion: screening and brief intervention for drug use in primary care. J Addict Med. 2010;4:131-136.
7. Krupski A, Joesch JM, Dunn C, et al. Testing the effects of brief intervention in primary care for problem drug use in a randomized controlled trial: rationale, design, and methods. Addict Sci Clin Pract. 2012;7:27.
8. US Preventive Services Task Force. Screening for illicit drug use. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsdrug.htm. Accessed March 4, 2014.
9. Lanier D, Ko S. Screening in Primary Care Settings for Illicit Drug Use: Assessment of Screening Instruments—A Supplemental Evidence Update for the U.S. Preventive Services Task Force. AHRQ Publication No. 08-05108-EF-2. Rockville, MD: Agency for Healthcare Research and Quality; 2008.
10. Bernstein J, Bernstein E, Tassiopoulos K, et al. Brief motivational intervention at a clinic visit reduces cocaine and heroin use. Drug Alcohol Depend. 2005;77:49-59.
11. Skinner HA. The drug abuse screening test. Addict Behav. 1982;7:363-371.
12. Zahradnik A, Otto C, Crackau B, et al. Randomized controlled trial of a brief intervention for problematic prescription drug use in non-treatment-seeking patients. Addiction. 2009;104:109-117.
13. Otto C, Crackau B, Löhrmann I, et al. Brief intervention in general hospital for problematic prescription drug use: 12-month outcome. Drug Alcohol Depend. 2009;105:221-226.
14. Humeniuk R, Ali R, Babor T, et al. A randomized controlled trial of a brief intervention for illicit drugs linked to the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) in clients recruited from primary health-care settings in four countries. Addiction. 2012;107:957-966.
15. WHO ASSIST Working Group. The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): development, reliability and feasibility. Addiction. 2002;97:1183-1194.
16. Saitz R, Palfai TP, Cheng DM, et al. Screening and brief intervention for drug use in primary care: the Assessing Screening Plus brief Intervention’s Resulting Efficacy to stop drug use (ASPIRE) randomized trial. Addict Sci Clin Pract. 2013;8(suppl 1):A61.
17. Roy-Byrne P, Bumgardner K, Krupski A, et al. Brief intervention for problem drug use in safety-net primary care settings: a randomized clinical trial. JAMA. 2014;312(5):492-501.
18. Madras BK, Compton WM, Avula D, et al. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug Alcohol Depend. 2009;99:280-295.
19. National Institute on Drug Abuse. Resource guide: Screening for drug use in general medical settings. National Institute on Drug Abuse Web site. Available at: http://www.drugabuse. gov/publications/resource-guide. Accessed March 8, 2014.
20. Saitz R, Cheng DM, Allensworth-Davies D, et al. The ability of single screening questions for unhealthy alcohol and other drug use to identify substance dependence in primary care. J Stud Alcohol Drugs. 2014;75:153-157.
21. Newcombe DA, Humeniuk RE, Ali R. Validation of the World Health Organization Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): report of results from the Australian site. Drug Alcohol Rev. 2005;24:217-226.
22. Humeniuk R, Ali R, Babor TF, et al. Validation of the Alcohol, Smoking And Substance Involvement Screening Test (ASSIST). Addiction. 2008;103:1039-1047.
23. Mdege ND, Lang J. Screening instruments for detecting illicit drug use/abuse that could be useful in general hospital wards: a systematic review. Addict Behav. 2011;36:1111-1119.
24. Cassidy CM, Schmitz N, Malla A. Validation of the alcohol use disorders identification test and the drug abuse screening test in first episode psychosis. Can J Psychiatry. 2008;53:26-33.
25. Brown RL, Rounds LA. Conjoint screening questionnaires for alcohol and other drug abuse: criterion validity in a primary care practice. Wis Med J. 1995;94:135-140.
26. American Psychiatric Association. Position statement on substance use disorders. American Psychiatric Association Web site. Available at: http://www.psychiatry.org/File%20Library/Advocacy%20and%20Newsroom/Position%20Statements/ps2012_Substance.pdf. Accessed March 4, 2014.
27. Smith PC, Schmidt SM, Allensworth-Davies D, et al. A single-question screening test for drug use in primary care. Arch Intern Med. 2010;170:1155-1160.
28. American Academy of Family Physicians. Substance abuse and addiction. American Academy of Family Physicians Web site. Available at: http://www.aafp.org/about/policies/all/substance-abuse.html. Accessed March 4, 2014.
29. National Institute on Alcohol Abuse and Alcoholism. Helping patients who drink too much: A clinician’s guide. National Institute on Alcohol Abuse and Alcoholism Web site. Available at: http://pubs.niaaa.nih.gov/publications/Practitioner/CliniciansGuide2005/clinicians_guide.htm. Accessed March 4, 2014.
30. US Department of Health and Human Services Public Health Service. Helping smokers quit: A guide for clinicians. US Department of Health and Human Services Public Health Service Web site. Available at: http://www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/tobacco/clinicians//clinhlpsmkqt/. Accessed March 4, 2014.
31. National Institute on Alcohol Abuse and Alcoholism. A Pocket Guide for Alcohol Screening and Brief Intervention. National Institute on Alcohol Abuse and Alcoholism Web site. Available at: http://pubs.niaaa.nih.gov/publications/Practitioner/pocketguide/pocket_guide.htm. Accessed July 30, 2014.
32. National Institute on Drug Abuse. NIDA-Quick Screen V1.0. National Institute on Drug Abuse Web site. Available at: http://www.drugabuse.gov/sites/default/files/pdf/nmassist.pdf. Accessed March 4, 2014.
33. Committee on Substance Abuse, Levy SJ, Kokotailo PK. Substance use screening, brief intervention, and referral to treatment for pediatricians. Pediatrics. 2011;128:e1330-e1340.
34. Kulig JW; American Academy of Pediatrics Committee on Substance Abuse. Tobacco, alcohol, and other drugs: the role of the pediatrician in prevention, identification, and management of substance abuse. Pediatrics. 2005;115:816-821.
35. US Preventive Services Task Force. Primary care behavioral interventions to reduce the nonmedical use of drugs in children and adolescents. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsnonmed.htm. Accessed March 4, 2014.
36. Centers for Medicare & Medicaid Services. Screening, Brief Intervention, and Referral to Treatment (SBIRT) services. Centers for Medicare & Medicaid Services Web site. Available at: http://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/sbirt_factsheet_icn904084.pdf. Accessed March 4, 2014.
37. Substance Abuse and Mental Health Services Administration. Coding for screening and brief intervention reimbursement. Substance Abuse and Mental Health Services Administration Web site. Available at: http://beta.samhsa.gov/sbirt/coding-reimbursement. Accessed August 4, 2014.
Why celiac disease is so easy to miss
› Do not rely on symptoms or symptom response to
a gluten-free diet alone
to diagnose celiac disease (CD); this approach does not differentiate CD from non-celiac gluten sensitivity. B
› Use HLA-DQ2 and -DQ8 genotype testing to effectively rule out the disease in selected clinical situations. B
› Test for CD in any
patient who has unexplained elevated serum aminotransferase levels, even in the absence of CD symptoms. A
› Screen all first-degree relatives of patients with
CD by testing for immunoglobulin A (IgA) tissue transglutaminase antibodies and serum IgA levels. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › It was a clinical conundrum. A 2011 case study1 described a 33-year-old woman with a 10-year history of progressive, debilitating pain and weakness. The patient had not received a unifying diagnosis or effective treatment despite multiple diagnostic tests and different recommendations from multiple specialists. The diagnosis remained elusive until a rheumatologist agreed to reexamine the case.
While reviewing the woman’s thick chart, the rheumatologist noted a series of negative results from upper and lower endoscopies and abdominal scans. Further investigation revealed an almost obfuscated clue—blood tests performed 2 years earlier that were positive for celiac disease (CD). However, a small intestine biopsy, which normally is done to confirm the diagnosis, was never performed.
The rheumatologist made a tentative diagnosis of CD and referred her to a nutritionist, who recommended the patient adhere to a strict gluten-free diet. Within 3 months, the patient experienced marked improvement and returned to work.
CD is an often-missed diagnosis. According to a study based on National Health and Nutrition Examination Survey data, only 17% of patients with CD are aware they have the disease.2 As such, it is imperative that primary care physicians familiarize themselves with CD’s myriad clinical presentations, diagnosis, and treatment.3-6
Gluten triggers an immune response in genetically susceptible patients
CD initially was known as “celiac sprue” because it shares characteristics with tropical sprue—diarrhea, malabsorption, and emaciation. It is a unique T-cell autoimmune enteropathy that is precipitated in genetically susceptible individuals by the ingestion of gluten, the major storage protein of wheat, barley, and rye.3,7
Upon ingestion, gluten breaks down to gliadin, which provokes an immune response in the intestinal mucosa of patients with CD. This response results in an inflammatory reaction, primarily in the upper small intestine, that destroys the absorption surface and causes villous atrophy, leading to nutrient malabsorption and chronic diarrhea.8 CD is associated with significant morbidity due to an abnormal excretion of fat (steatorrhea) and varying degrees of malabsorption of vitamins A, D, and K, as well as B complex vitamins including B12 and folate; carbohydrates; protein; water; and minerals such as magnesium, calcium, and iron.9
CD develops only in individuals who possess alleles that encode for HLA-DQ2 or HLA-DQ8 proteins, products of 2 of the HLA genes. And while 30% of Caucasians carry the HLA-DQ2 allele and virtually 100% consume wheat, only 1 in 100 will develop CD.3,10,11 Although the genes are necessary, it is the interplay between genes (both HLA and non-HLA associated) and environment (ie, gluten) that leads to the intestinal mucosa damage typical of the disease. The HLA-DQ region also is associated with increased risk of type 1 diabetes, which might explain the correlation of CD to a host of other autoimmune disorders, including Graves’ disease and rheumatoid arthritis.8,10,11
Increased prevalence reflects better recognition of celiac disease
CD affects .6% to 1% of the population worldwide, with wide regional variation.3 Before the development of serologic assays in the 1970s, CD was a clinical diagnosis based on classic symptoms. With the advent of assays for immunoglobulin A (IgA) antibodies, the prevalence of CD has drastically increased to the current estimates of 1:250 to 1:500.4,5 The prevalence will continue to increase as clinicians become more aware of the different presentations of the disease, which are described below.
CD runs in families. Most patients with CD have a family history of the disease based on inheritance of the HLA alleles. A US study determined that the prevalence of CD was 1:22 in first-degree relatives and 1:39 in second-degree relatives of patients with biopsy-proven CD.12
Less than half of patients have GI symptoms
The classic presentation of CD involves a constellation of signs and symptoms of malabsorption: diarrhea, muscle wasting, and weight loss. Other typical gastrointestinal (GI) symptoms include bloating, flatulence, and abdominal pain.
Recognizing CD can be challenging, however, because <50% of patients diagnosed with CD present with these classic GI symptoms.3 About 50% of CD patients present with extra-intestinal symptoms, such as iron deficiency anemia, aphthous stomatitis, chronic fatigue, osteopenia, and dental enamel hypoplasia.3,8,13 Other possible non-GI symptoms include abnormal liver function test results and skin disorders such as dermatitis herpetiformis, a pruritic rash with cutaneous IgA deposits.3,8 In addition, many patients are asymptomatic.14 This highly variable clinical picture is due to the genetic and immunologic basis of the disease, extent of mucosal injury, and patients’ dietary habits, gender, and age of onset.15 A common clue that suggests a patient may have CD is unexplained iron deficiency anemia that does not improve with oral iron supplementation.4,13
Because symptoms may be intermittent, a patient may delay seeking care until he or she develops secondary manifestations, which often are debilitating and overshadow the GI complaints. Chronic complications of untreated CD include lymphoma and adenocarcinomas of the jejunum, recurrent miscarriages, neurologic disorders, osteoporosis, and hyposplenism.3,4,8
Since CD can manifest with widely varying symptoms, some researchers believe the disease should be classified into 3 categories based on presentation: classic CD, which presents with diarrhea, weight loss, malabsorption, and vitamin deficiency; atypical CD, which presents with minimal GI symptoms but can include anemia, neurologic symptoms, arthritis, or infertility; and asymptomatic CD, which typically displays no symptoms but usually is identified on incidental screening.3,8,16 Non-celiac gluten sensitivity is a distinct condition in which the body reacts adversely to gluten; it is not an autoimmune disease with an inflammatory response.
Order serologic testing for at-risk patients
Because CD remains underdiagnosed,16 taking a thorough family history and dietary history and making sure to at least consider CD as a part of a differential diagnosis is important. Although population-based screening has been proposed, its benefits and cost-effectiveness remain unproven. As a result, serologic testing of at-risk groups—individuals with conditions known to be associated with CD—remains the current standard.3 The TABLE lists groups for whom serologic testing for CD is indicated.16,17
In addition, the American College of Gastroenterology (ACG) and the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) provide guidance on the diagnosis and treatment of adults and children with CD. (An ACG diagnostic algorithm is available at http://www.nature.com/ajg/journal/v108/n5/pdf/ajg201379a.pdf.)
Adults. For patients who are consuming a diet that includes gluten and have symptoms that suggest CD, the ACG guidelines recommend initial testing for IgA tissue transglutaminase (tTG) antibodies.16 The IgA tTG has a sensitivity and specificity >95%.16 An alternative test, the IgA endomysial (IgA EMA) test, has similar sensitivity but is time-consuming and its accuracy depends on the experience and skill of the laboratory technician. A negative result for either test has a high negative predictive value for CD.3,16
IgA deficiency is much more common in patients with CD than in the general population and can result in a false negative test for tTG and EMA. Therefore, consider taking a baseline IgA measurement first. If the patient has an IgA deficiency, the test you’ll use next will change: The preferred test for CD is either immunoglobulin G (IgG) tTG or IgG deamidated gliadin peptides (DGP).3,16
If a patient is already gluten-free... To rule out CD in patients who are already consuming a gluten-free diet, order HLA-DQ2 and HLA-DQ8 testing because these markers have a specificity >99%; if the HLA test is negative, the disease is excluded.8,16
Children. NASPGHAN recommends taking a baseline IgA measurement in children at risk for CD and then testing for IgA tTG antibodies, but not until patients are 3 years old and have been on a diet that includes gluten for at least 1 year.17 Repeat testing at a later date it is recommended for those with negative results because some evidence suggests that in certain patients, later serologic testing will be positive. Alternatively, you may offer HLA testing. If the HLA test is negative, CD can be excluded >99% of the time.
Diagnosis usually is confirmed by intestinal biopsy
Positive results on serologic testing should be confirmed with a biopsy of the small bowel; findings characteristic of CD include an increased number of intraepithelial lymphocytes (>25 per 100 enterocytes), elongation of the crypts, and partial to total villous atrophy.4 Final confirmation of CD is resolution of symptoms by consuming a gluten-free diet.3,8
Alternate approaches to confirming the diagnosis. Although intestinal biopsy has long been considered the gold standard for diagnosis of CD, this may change. In 2012, the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition proposed that the biopsy may not be necessary in children with the following 3 characteristics: classic intestinal symptoms of CD, IgA tTG levels >10 times higher than normal, and a positive HLA-DQ2.18
Catassi and Fasano19 have proposed shifting from relying on algorithms and intestinal biopsy to a quantitative approach. They suggest using the “4 out of 5” rule, meaning the diagnosis of CD can be confirmed if at least 4 of the following 5 criteria are satisfied: typical CD symptoms, a positive IgA tTG, a positive HLA-DQ2 or -DQ8, celiac enteropathy on small bowel biopsy, and response to a gluten-free diet.19
The only proven treatment: A gluten-free diet
Lifelong adherence to a gluten-free diet is the only effective treatment for CD.14,16 Previously, patients with CD were advised to also avoid oats, but most evidence supports the safety of oats (<2 oz/d), provided there is no cross-contamination with gluten.14 Adhering to a strict gluten-free diet can be challenging because cereal flours are ubiquitous in western foods, and some foods may be cross-contaminated. The Celiac Disease Foundation (http://www.celiac.org) offers guidance on maintaining a gluten-free diet.
Because avoiding gluten has become popular even among people who don’t have CD, product labeling that includes information on gluten content has become pervasive. However, determining which items contain gluten depends on accurate labeling, a standard that often is not met in many countries; in the United States, such labeling began to be phased in starting in July 2014.20 As a result, CD patients may unwittingly be exposed to gluten over the long term, which can result in greater morbidity and mortality. Unless a food is labeled “gluten-free," it is best to check with the manufacturer.
Compliance with a gluten-free diet can be monitored by following IgA tTG titers every 1 to 2 years, as these values normalize after a patient has been adhering to the diet for 6 to 24 months.3,16
In addition to lifelong adherence to a gluten-free diet, a National Institutes of Health Consensus Development Conference recommended that management of patients with CD should include21:
- consultation with a skilled dietitian
- education about the disease
- continuous long-term follow-up by a multidisciplinary team
- identification and follow-up of abnormalities found at baseline, such as abnormal liver function test results
- treatment of nutritional deficiencies.
The ACG also recommends that CD patients receive a dual energy x-ray scan for follow-up of osteopenia and a pneumococcal vaccine because functional hyposplenism is associated with CD, and pneumonia is a common complication of hyposplenism.16,18
Compared to infants who are breastfed and don’t receive gluten until ages 4 to 6 months, infants who are fed gluten in their first 3 months have a significantly increased risk of developing antibodies that are associated with celiac disease (CD).22 Recent studies suggest that the effects of breast milk on the microbiota composition of the intestine may help explain this difference. Breast milk selectively stimulates the growth of specific bacteria, including bifidobacteria, which are relatively depleted in children with CD.23,24 Researchers believe breastfeeding and delaying introduction of gluten-containing foods until 4 to 6 months of age might protect against CD.25
Should you recommend a gluten-free diet for other patients? Because avoiding gluten is now popular and many gluten-free products are marketed as “health food,” physicians may be reluctant to recommend a gluten-free diet for patients who have vague abdominal symptoms but negative CD test results. Despite the current popularity of “going gluten-free,” the reality is that in addition to CD, many other diseases may be helped by a gluten-free diet, such as dermatitis herpetiformis, irritable bowel syndrome, and neurologic diseases such as gluten-sensitive ataxia.19 In the end, whether to adopt a gluten-free diet is a decision that you and your patient will need to make together.
Researchers are searching for additional treatments
Because many patients find it difficult to adhere to a gluten-free diet, researchers are investigating several alternative treatments, including a derivative from cholera toxin that inhibits the opening of intestinal epithelial junctions, thereby reducing the resultant inflammatory response, and a desensitizing vaccine.19,22,23 Another intriguing approach involves using the parasite Necator americanus to modulate the immune response to gluten.18 Finally, certain infant feeding practices, including breastfeeding and delaying introduction of gluten to the diet, may minimize the risk of developing CD. (See "A link between infant feeding practices and the risk of CD?" above.22-25)
CORRESPONDENCE
Patrick T. Dowling, MD, MPH, Department of Family Medicine, 50-078 Center for Health Sciences, David Geffen School of Medicine at UCLA, Los Angeles, CA 90095-1683; [email protected]
1. Sanders L. Hurt all over. New York Times Sunday Magazine. November 11, 2011:MM22.
2. Rubio-Tapia A, Ludvigsson JF, Brantner TL, et al. The prevalence of celiac disease in the United States. Am J Gastroenterol. 2012;107:1538-1544.
3. Fasano A, Catassi C. Clinical practice. Celiac disease. N Engl J Med. 2012;367:2419-2426.
4. Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357: 1731-1743.
5. Fasano A, Berti I, Gerarduzzi T, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163:286-292.
6. Mustalahti K, Catassi C, Reunanen A, et al; Coeliac EU Cluster, Project Epidemiology. The prevalence of celiac disease in Europe: results of centralized, international mass screening project. Ann Med. 2010;42:587-595.
7. Farrel R, Kelly C. Celiac disease and refractory celiac disease. In: Feldman M, Friedman LS, Brandt LJ. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. 9th ed. Philadelphia, PA: Saunders; 2010: 1797-1820.
8. Gujral N, Freeman HJ, Thomson Ab. Celiac disease: prevalence, diagnosis, pathogenesis and treatment. World J Gastroenterol. 2012;18:6036-6059.
9. Sleisenger MH. Diseases of malabsorption. In: Beeson PB, McDermott W. Cecil-Loeb Textbook of Medicine. 13th ed. Philadelphia, PA: WB Saunders Company; 1971:1285-1291.
10. Reilly NR, Green PH. Epidemiology and clinical presentations of celiac disease. Sem Immunopathol. 2012;34:473-478.
11. Kagnoff MF. Celiac disease. A gastrointestinal disease with environmental, genetic, and immunologic components. Gastroenterol Clin North Am. 1992;21:405-425.
12. Riddle MS, Murray JA, Porter CK. The incidence and risk of celiac disease in a healthy US adult population. Am J Gastroenterol. 2012;107:1248-1255.
13. Rampertab SD, Pooran N, Brar P, et al. Trends in the presentation of celiac disease. Am J Med. 2006;119:355.e9-355.e14.
14. Pietzak M. Celiac disease, wheat allergy, and gluten sensitivity: when gluten free is not a fad. JPEN J Parenter Enteral Nutr. 2012;36(1 suppl):68S-75S.
15. Sapone A, Bai JC, Ciacci C, et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 2012;10:13.
16. Rubio-Tapia A, Hill ID, Kelly CP, et al; American College of Gastroenterology. ACG clinical guidelines: diagnosis and management celiac disease. Am J Gastroenterol. 2013;108:656-676.
17. Hill ID, Dirks MH, Liptak GS, et al; North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. Guidelines for the diagnosis and treatment of celiac disease in children: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroentertol Nutr. 2005;40:1-19.
18. Husby S, Koletsko S, Korponay-Szabó IR, et al; ESPGHAN Working Group on Coeliac Disease Diagnosis; ESPGHAN Gastoenterology Committee; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. European Society for Pediatric Gastroenterology, Hepatology and Nutrition Guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136-160.
19. Catassi C, Fasano A. Celiac disease diagnosis: simple rules are better than complicated algorithms. Am J Med. 2010;123:691-693.
20. US Food and Drug Administration. Foods labeled gluten-free must now meet FDA's definition. Available at: http://www.fda. gov/Food/NewsEvents/ConstituentUpdates/ucm407867.htm. Accessed August 13, 2014.
21. National Institutes of Health Consensus Development Conference on Celiac Disease. National Institutes of Health Consensus Development Conference Statement. Available at: http://consensus.nih.gov/2004/2004celiacdisease118html.htm. Accessed August 13, 2014.
22. Norris JM, Barriga K, Hoffenberg EJ, et al. Risk of celiac disease autoimmunity and timing of gluten introduction in the diet of infants at increased risk of disease. JAMA. 2005;293:2343-2351.
23. Pozo-Rubio T, Olivares M, Nova E, et al. Immune development and intestinal microbiota in celiac disease. Clin Dev Immunol. 2012;2012:654143
24. Ivarsson A, Myléus A, Norström F, et al. Prevalence of childhood celiac disease and changes in infant feeding. Pediatrics. 2013;131:687-694.
25. Cataldo F, Montalto G. Celiac disease in the developing countries: a new and challenging public health problem. World J Gastroenterol. 2007;13:2153-2159.
› Do not rely on symptoms or symptom response to
a gluten-free diet alone
to diagnose celiac disease (CD); this approach does not differentiate CD from non-celiac gluten sensitivity. B
› Use HLA-DQ2 and -DQ8 genotype testing to effectively rule out the disease in selected clinical situations. B
› Test for CD in any
patient who has unexplained elevated serum aminotransferase levels, even in the absence of CD symptoms. A
› Screen all first-degree relatives of patients with
CD by testing for immunoglobulin A (IgA) tissue transglutaminase antibodies and serum IgA levels. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › It was a clinical conundrum. A 2011 case study1 described a 33-year-old woman with a 10-year history of progressive, debilitating pain and weakness. The patient had not received a unifying diagnosis or effective treatment despite multiple diagnostic tests and different recommendations from multiple specialists. The diagnosis remained elusive until a rheumatologist agreed to reexamine the case.
While reviewing the woman’s thick chart, the rheumatologist noted a series of negative results from upper and lower endoscopies and abdominal scans. Further investigation revealed an almost obfuscated clue—blood tests performed 2 years earlier that were positive for celiac disease (CD). However, a small intestine biopsy, which normally is done to confirm the diagnosis, was never performed.
The rheumatologist made a tentative diagnosis of CD and referred her to a nutritionist, who recommended the patient adhere to a strict gluten-free diet. Within 3 months, the patient experienced marked improvement and returned to work.
CD is an often-missed diagnosis. According to a study based on National Health and Nutrition Examination Survey data, only 17% of patients with CD are aware they have the disease.2 As such, it is imperative that primary care physicians familiarize themselves with CD’s myriad clinical presentations, diagnosis, and treatment.3-6
Gluten triggers an immune response in genetically susceptible patients
CD initially was known as “celiac sprue” because it shares characteristics with tropical sprue—diarrhea, malabsorption, and emaciation. It is a unique T-cell autoimmune enteropathy that is precipitated in genetically susceptible individuals by the ingestion of gluten, the major storage protein of wheat, barley, and rye.3,7
Upon ingestion, gluten breaks down to gliadin, which provokes an immune response in the intestinal mucosa of patients with CD. This response results in an inflammatory reaction, primarily in the upper small intestine, that destroys the absorption surface and causes villous atrophy, leading to nutrient malabsorption and chronic diarrhea.8 CD is associated with significant morbidity due to an abnormal excretion of fat (steatorrhea) and varying degrees of malabsorption of vitamins A, D, and K, as well as B complex vitamins including B12 and folate; carbohydrates; protein; water; and minerals such as magnesium, calcium, and iron.9
CD develops only in individuals who possess alleles that encode for HLA-DQ2 or HLA-DQ8 proteins, products of 2 of the HLA genes. And while 30% of Caucasians carry the HLA-DQ2 allele and virtually 100% consume wheat, only 1 in 100 will develop CD.3,10,11 Although the genes are necessary, it is the interplay between genes (both HLA and non-HLA associated) and environment (ie, gluten) that leads to the intestinal mucosa damage typical of the disease. The HLA-DQ region also is associated with increased risk of type 1 diabetes, which might explain the correlation of CD to a host of other autoimmune disorders, including Graves’ disease and rheumatoid arthritis.8,10,11
Increased prevalence reflects better recognition of celiac disease
CD affects .6% to 1% of the population worldwide, with wide regional variation.3 Before the development of serologic assays in the 1970s, CD was a clinical diagnosis based on classic symptoms. With the advent of assays for immunoglobulin A (IgA) antibodies, the prevalence of CD has drastically increased to the current estimates of 1:250 to 1:500.4,5 The prevalence will continue to increase as clinicians become more aware of the different presentations of the disease, which are described below.
CD runs in families. Most patients with CD have a family history of the disease based on inheritance of the HLA alleles. A US study determined that the prevalence of CD was 1:22 in first-degree relatives and 1:39 in second-degree relatives of patients with biopsy-proven CD.12
Less than half of patients have GI symptoms
The classic presentation of CD involves a constellation of signs and symptoms of malabsorption: diarrhea, muscle wasting, and weight loss. Other typical gastrointestinal (GI) symptoms include bloating, flatulence, and abdominal pain.
Recognizing CD can be challenging, however, because <50% of patients diagnosed with CD present with these classic GI symptoms.3 About 50% of CD patients present with extra-intestinal symptoms, such as iron deficiency anemia, aphthous stomatitis, chronic fatigue, osteopenia, and dental enamel hypoplasia.3,8,13 Other possible non-GI symptoms include abnormal liver function test results and skin disorders such as dermatitis herpetiformis, a pruritic rash with cutaneous IgA deposits.3,8 In addition, many patients are asymptomatic.14 This highly variable clinical picture is due to the genetic and immunologic basis of the disease, extent of mucosal injury, and patients’ dietary habits, gender, and age of onset.15 A common clue that suggests a patient may have CD is unexplained iron deficiency anemia that does not improve with oral iron supplementation.4,13
Because symptoms may be intermittent, a patient may delay seeking care until he or she develops secondary manifestations, which often are debilitating and overshadow the GI complaints. Chronic complications of untreated CD include lymphoma and adenocarcinomas of the jejunum, recurrent miscarriages, neurologic disorders, osteoporosis, and hyposplenism.3,4,8
Since CD can manifest with widely varying symptoms, some researchers believe the disease should be classified into 3 categories based on presentation: classic CD, which presents with diarrhea, weight loss, malabsorption, and vitamin deficiency; atypical CD, which presents with minimal GI symptoms but can include anemia, neurologic symptoms, arthritis, or infertility; and asymptomatic CD, which typically displays no symptoms but usually is identified on incidental screening.3,8,16 Non-celiac gluten sensitivity is a distinct condition in which the body reacts adversely to gluten; it is not an autoimmune disease with an inflammatory response.
Order serologic testing for at-risk patients
Because CD remains underdiagnosed,16 taking a thorough family history and dietary history and making sure to at least consider CD as a part of a differential diagnosis is important. Although population-based screening has been proposed, its benefits and cost-effectiveness remain unproven. As a result, serologic testing of at-risk groups—individuals with conditions known to be associated with CD—remains the current standard.3 The TABLE lists groups for whom serologic testing for CD is indicated.16,17
In addition, the American College of Gastroenterology (ACG) and the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) provide guidance on the diagnosis and treatment of adults and children with CD. (An ACG diagnostic algorithm is available at http://www.nature.com/ajg/journal/v108/n5/pdf/ajg201379a.pdf.)
Adults. For patients who are consuming a diet that includes gluten and have symptoms that suggest CD, the ACG guidelines recommend initial testing for IgA tissue transglutaminase (tTG) antibodies.16 The IgA tTG has a sensitivity and specificity >95%.16 An alternative test, the IgA endomysial (IgA EMA) test, has similar sensitivity but is time-consuming and its accuracy depends on the experience and skill of the laboratory technician. A negative result for either test has a high negative predictive value for CD.3,16
IgA deficiency is much more common in patients with CD than in the general population and can result in a false negative test for tTG and EMA. Therefore, consider taking a baseline IgA measurement first. If the patient has an IgA deficiency, the test you’ll use next will change: The preferred test for CD is either immunoglobulin G (IgG) tTG or IgG deamidated gliadin peptides (DGP).3,16
If a patient is already gluten-free... To rule out CD in patients who are already consuming a gluten-free diet, order HLA-DQ2 and HLA-DQ8 testing because these markers have a specificity >99%; if the HLA test is negative, the disease is excluded.8,16
Children. NASPGHAN recommends taking a baseline IgA measurement in children at risk for CD and then testing for IgA tTG antibodies, but not until patients are 3 years old and have been on a diet that includes gluten for at least 1 year.17 Repeat testing at a later date it is recommended for those with negative results because some evidence suggests that in certain patients, later serologic testing will be positive. Alternatively, you may offer HLA testing. If the HLA test is negative, CD can be excluded >99% of the time.
Diagnosis usually is confirmed by intestinal biopsy
Positive results on serologic testing should be confirmed with a biopsy of the small bowel; findings characteristic of CD include an increased number of intraepithelial lymphocytes (>25 per 100 enterocytes), elongation of the crypts, and partial to total villous atrophy.4 Final confirmation of CD is resolution of symptoms by consuming a gluten-free diet.3,8
Alternate approaches to confirming the diagnosis. Although intestinal biopsy has long been considered the gold standard for diagnosis of CD, this may change. In 2012, the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition proposed that the biopsy may not be necessary in children with the following 3 characteristics: classic intestinal symptoms of CD, IgA tTG levels >10 times higher than normal, and a positive HLA-DQ2.18
Catassi and Fasano19 have proposed shifting from relying on algorithms and intestinal biopsy to a quantitative approach. They suggest using the “4 out of 5” rule, meaning the diagnosis of CD can be confirmed if at least 4 of the following 5 criteria are satisfied: typical CD symptoms, a positive IgA tTG, a positive HLA-DQ2 or -DQ8, celiac enteropathy on small bowel biopsy, and response to a gluten-free diet.19
The only proven treatment: A gluten-free diet
Lifelong adherence to a gluten-free diet is the only effective treatment for CD.14,16 Previously, patients with CD were advised to also avoid oats, but most evidence supports the safety of oats (<2 oz/d), provided there is no cross-contamination with gluten.14 Adhering to a strict gluten-free diet can be challenging because cereal flours are ubiquitous in western foods, and some foods may be cross-contaminated. The Celiac Disease Foundation (http://www.celiac.org) offers guidance on maintaining a gluten-free diet.
Because avoiding gluten has become popular even among people who don’t have CD, product labeling that includes information on gluten content has become pervasive. However, determining which items contain gluten depends on accurate labeling, a standard that often is not met in many countries; in the United States, such labeling began to be phased in starting in July 2014.20 As a result, CD patients may unwittingly be exposed to gluten over the long term, which can result in greater morbidity and mortality. Unless a food is labeled “gluten-free," it is best to check with the manufacturer.
Compliance with a gluten-free diet can be monitored by following IgA tTG titers every 1 to 2 years, as these values normalize after a patient has been adhering to the diet for 6 to 24 months.3,16
In addition to lifelong adherence to a gluten-free diet, a National Institutes of Health Consensus Development Conference recommended that management of patients with CD should include21:
- consultation with a skilled dietitian
- education about the disease
- continuous long-term follow-up by a multidisciplinary team
- identification and follow-up of abnormalities found at baseline, such as abnormal liver function test results
- treatment of nutritional deficiencies.
The ACG also recommends that CD patients receive a dual energy x-ray scan for follow-up of osteopenia and a pneumococcal vaccine because functional hyposplenism is associated with CD, and pneumonia is a common complication of hyposplenism.16,18
Compared to infants who are breastfed and don’t receive gluten until ages 4 to 6 months, infants who are fed gluten in their first 3 months have a significantly increased risk of developing antibodies that are associated with celiac disease (CD).22 Recent studies suggest that the effects of breast milk on the microbiota composition of the intestine may help explain this difference. Breast milk selectively stimulates the growth of specific bacteria, including bifidobacteria, which are relatively depleted in children with CD.23,24 Researchers believe breastfeeding and delaying introduction of gluten-containing foods until 4 to 6 months of age might protect against CD.25
Should you recommend a gluten-free diet for other patients? Because avoiding gluten is now popular and many gluten-free products are marketed as “health food,” physicians may be reluctant to recommend a gluten-free diet for patients who have vague abdominal symptoms but negative CD test results. Despite the current popularity of “going gluten-free,” the reality is that in addition to CD, many other diseases may be helped by a gluten-free diet, such as dermatitis herpetiformis, irritable bowel syndrome, and neurologic diseases such as gluten-sensitive ataxia.19 In the end, whether to adopt a gluten-free diet is a decision that you and your patient will need to make together.
Researchers are searching for additional treatments
Because many patients find it difficult to adhere to a gluten-free diet, researchers are investigating several alternative treatments, including a derivative from cholera toxin that inhibits the opening of intestinal epithelial junctions, thereby reducing the resultant inflammatory response, and a desensitizing vaccine.19,22,23 Another intriguing approach involves using the parasite Necator americanus to modulate the immune response to gluten.18 Finally, certain infant feeding practices, including breastfeeding and delaying introduction of gluten to the diet, may minimize the risk of developing CD. (See "A link between infant feeding practices and the risk of CD?" above.22-25)
CORRESPONDENCE
Patrick T. Dowling, MD, MPH, Department of Family Medicine, 50-078 Center for Health Sciences, David Geffen School of Medicine at UCLA, Los Angeles, CA 90095-1683; [email protected]
› Do not rely on symptoms or symptom response to
a gluten-free diet alone
to diagnose celiac disease (CD); this approach does not differentiate CD from non-celiac gluten sensitivity. B
› Use HLA-DQ2 and -DQ8 genotype testing to effectively rule out the disease in selected clinical situations. B
› Test for CD in any
patient who has unexplained elevated serum aminotransferase levels, even in the absence of CD symptoms. A
› Screen all first-degree relatives of patients with
CD by testing for immunoglobulin A (IgA) tissue transglutaminase antibodies and serum IgA levels. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › It was a clinical conundrum. A 2011 case study1 described a 33-year-old woman with a 10-year history of progressive, debilitating pain and weakness. The patient had not received a unifying diagnosis or effective treatment despite multiple diagnostic tests and different recommendations from multiple specialists. The diagnosis remained elusive until a rheumatologist agreed to reexamine the case.
While reviewing the woman’s thick chart, the rheumatologist noted a series of negative results from upper and lower endoscopies and abdominal scans. Further investigation revealed an almost obfuscated clue—blood tests performed 2 years earlier that were positive for celiac disease (CD). However, a small intestine biopsy, which normally is done to confirm the diagnosis, was never performed.
The rheumatologist made a tentative diagnosis of CD and referred her to a nutritionist, who recommended the patient adhere to a strict gluten-free diet. Within 3 months, the patient experienced marked improvement and returned to work.
CD is an often-missed diagnosis. According to a study based on National Health and Nutrition Examination Survey data, only 17% of patients with CD are aware they have the disease.2 As such, it is imperative that primary care physicians familiarize themselves with CD’s myriad clinical presentations, diagnosis, and treatment.3-6
Gluten triggers an immune response in genetically susceptible patients
CD initially was known as “celiac sprue” because it shares characteristics with tropical sprue—diarrhea, malabsorption, and emaciation. It is a unique T-cell autoimmune enteropathy that is precipitated in genetically susceptible individuals by the ingestion of gluten, the major storage protein of wheat, barley, and rye.3,7
Upon ingestion, gluten breaks down to gliadin, which provokes an immune response in the intestinal mucosa of patients with CD. This response results in an inflammatory reaction, primarily in the upper small intestine, that destroys the absorption surface and causes villous atrophy, leading to nutrient malabsorption and chronic diarrhea.8 CD is associated with significant morbidity due to an abnormal excretion of fat (steatorrhea) and varying degrees of malabsorption of vitamins A, D, and K, as well as B complex vitamins including B12 and folate; carbohydrates; protein; water; and minerals such as magnesium, calcium, and iron.9
CD develops only in individuals who possess alleles that encode for HLA-DQ2 or HLA-DQ8 proteins, products of 2 of the HLA genes. And while 30% of Caucasians carry the HLA-DQ2 allele and virtually 100% consume wheat, only 1 in 100 will develop CD.3,10,11 Although the genes are necessary, it is the interplay between genes (both HLA and non-HLA associated) and environment (ie, gluten) that leads to the intestinal mucosa damage typical of the disease. The HLA-DQ region also is associated with increased risk of type 1 diabetes, which might explain the correlation of CD to a host of other autoimmune disorders, including Graves’ disease and rheumatoid arthritis.8,10,11
Increased prevalence reflects better recognition of celiac disease
CD affects .6% to 1% of the population worldwide, with wide regional variation.3 Before the development of serologic assays in the 1970s, CD was a clinical diagnosis based on classic symptoms. With the advent of assays for immunoglobulin A (IgA) antibodies, the prevalence of CD has drastically increased to the current estimates of 1:250 to 1:500.4,5 The prevalence will continue to increase as clinicians become more aware of the different presentations of the disease, which are described below.
CD runs in families. Most patients with CD have a family history of the disease based on inheritance of the HLA alleles. A US study determined that the prevalence of CD was 1:22 in first-degree relatives and 1:39 in second-degree relatives of patients with biopsy-proven CD.12
Less than half of patients have GI symptoms
The classic presentation of CD involves a constellation of signs and symptoms of malabsorption: diarrhea, muscle wasting, and weight loss. Other typical gastrointestinal (GI) symptoms include bloating, flatulence, and abdominal pain.
Recognizing CD can be challenging, however, because <50% of patients diagnosed with CD present with these classic GI symptoms.3 About 50% of CD patients present with extra-intestinal symptoms, such as iron deficiency anemia, aphthous stomatitis, chronic fatigue, osteopenia, and dental enamel hypoplasia.3,8,13 Other possible non-GI symptoms include abnormal liver function test results and skin disorders such as dermatitis herpetiformis, a pruritic rash with cutaneous IgA deposits.3,8 In addition, many patients are asymptomatic.14 This highly variable clinical picture is due to the genetic and immunologic basis of the disease, extent of mucosal injury, and patients’ dietary habits, gender, and age of onset.15 A common clue that suggests a patient may have CD is unexplained iron deficiency anemia that does not improve with oral iron supplementation.4,13
Because symptoms may be intermittent, a patient may delay seeking care until he or she develops secondary manifestations, which often are debilitating and overshadow the GI complaints. Chronic complications of untreated CD include lymphoma and adenocarcinomas of the jejunum, recurrent miscarriages, neurologic disorders, osteoporosis, and hyposplenism.3,4,8
Since CD can manifest with widely varying symptoms, some researchers believe the disease should be classified into 3 categories based on presentation: classic CD, which presents with diarrhea, weight loss, malabsorption, and vitamin deficiency; atypical CD, which presents with minimal GI symptoms but can include anemia, neurologic symptoms, arthritis, or infertility; and asymptomatic CD, which typically displays no symptoms but usually is identified on incidental screening.3,8,16 Non-celiac gluten sensitivity is a distinct condition in which the body reacts adversely to gluten; it is not an autoimmune disease with an inflammatory response.
Order serologic testing for at-risk patients
Because CD remains underdiagnosed,16 taking a thorough family history and dietary history and making sure to at least consider CD as a part of a differential diagnosis is important. Although population-based screening has been proposed, its benefits and cost-effectiveness remain unproven. As a result, serologic testing of at-risk groups—individuals with conditions known to be associated with CD—remains the current standard.3 The TABLE lists groups for whom serologic testing for CD is indicated.16,17
In addition, the American College of Gastroenterology (ACG) and the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) provide guidance on the diagnosis and treatment of adults and children with CD. (An ACG diagnostic algorithm is available at http://www.nature.com/ajg/journal/v108/n5/pdf/ajg201379a.pdf.)
Adults. For patients who are consuming a diet that includes gluten and have symptoms that suggest CD, the ACG guidelines recommend initial testing for IgA tissue transglutaminase (tTG) antibodies.16 The IgA tTG has a sensitivity and specificity >95%.16 An alternative test, the IgA endomysial (IgA EMA) test, has similar sensitivity but is time-consuming and its accuracy depends on the experience and skill of the laboratory technician. A negative result for either test has a high negative predictive value for CD.3,16
IgA deficiency is much more common in patients with CD than in the general population and can result in a false negative test for tTG and EMA. Therefore, consider taking a baseline IgA measurement first. If the patient has an IgA deficiency, the test you’ll use next will change: The preferred test for CD is either immunoglobulin G (IgG) tTG or IgG deamidated gliadin peptides (DGP).3,16
If a patient is already gluten-free... To rule out CD in patients who are already consuming a gluten-free diet, order HLA-DQ2 and HLA-DQ8 testing because these markers have a specificity >99%; if the HLA test is negative, the disease is excluded.8,16
Children. NASPGHAN recommends taking a baseline IgA measurement in children at risk for CD and then testing for IgA tTG antibodies, but not until patients are 3 years old and have been on a diet that includes gluten for at least 1 year.17 Repeat testing at a later date it is recommended for those with negative results because some evidence suggests that in certain patients, later serologic testing will be positive. Alternatively, you may offer HLA testing. If the HLA test is negative, CD can be excluded >99% of the time.
Diagnosis usually is confirmed by intestinal biopsy
Positive results on serologic testing should be confirmed with a biopsy of the small bowel; findings characteristic of CD include an increased number of intraepithelial lymphocytes (>25 per 100 enterocytes), elongation of the crypts, and partial to total villous atrophy.4 Final confirmation of CD is resolution of symptoms by consuming a gluten-free diet.3,8
Alternate approaches to confirming the diagnosis. Although intestinal biopsy has long been considered the gold standard for diagnosis of CD, this may change. In 2012, the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition proposed that the biopsy may not be necessary in children with the following 3 characteristics: classic intestinal symptoms of CD, IgA tTG levels >10 times higher than normal, and a positive HLA-DQ2.18
Catassi and Fasano19 have proposed shifting from relying on algorithms and intestinal biopsy to a quantitative approach. They suggest using the “4 out of 5” rule, meaning the diagnosis of CD can be confirmed if at least 4 of the following 5 criteria are satisfied: typical CD symptoms, a positive IgA tTG, a positive HLA-DQ2 or -DQ8, celiac enteropathy on small bowel biopsy, and response to a gluten-free diet.19
The only proven treatment: A gluten-free diet
Lifelong adherence to a gluten-free diet is the only effective treatment for CD.14,16 Previously, patients with CD were advised to also avoid oats, but most evidence supports the safety of oats (<2 oz/d), provided there is no cross-contamination with gluten.14 Adhering to a strict gluten-free diet can be challenging because cereal flours are ubiquitous in western foods, and some foods may be cross-contaminated. The Celiac Disease Foundation (http://www.celiac.org) offers guidance on maintaining a gluten-free diet.
Because avoiding gluten has become popular even among people who don’t have CD, product labeling that includes information on gluten content has become pervasive. However, determining which items contain gluten depends on accurate labeling, a standard that often is not met in many countries; in the United States, such labeling began to be phased in starting in July 2014.20 As a result, CD patients may unwittingly be exposed to gluten over the long term, which can result in greater morbidity and mortality. Unless a food is labeled “gluten-free," it is best to check with the manufacturer.
Compliance with a gluten-free diet can be monitored by following IgA tTG titers every 1 to 2 years, as these values normalize after a patient has been adhering to the diet for 6 to 24 months.3,16
In addition to lifelong adherence to a gluten-free diet, a National Institutes of Health Consensus Development Conference recommended that management of patients with CD should include21:
- consultation with a skilled dietitian
- education about the disease
- continuous long-term follow-up by a multidisciplinary team
- identification and follow-up of abnormalities found at baseline, such as abnormal liver function test results
- treatment of nutritional deficiencies.
The ACG also recommends that CD patients receive a dual energy x-ray scan for follow-up of osteopenia and a pneumococcal vaccine because functional hyposplenism is associated with CD, and pneumonia is a common complication of hyposplenism.16,18
Compared to infants who are breastfed and don’t receive gluten until ages 4 to 6 months, infants who are fed gluten in their first 3 months have a significantly increased risk of developing antibodies that are associated with celiac disease (CD).22 Recent studies suggest that the effects of breast milk on the microbiota composition of the intestine may help explain this difference. Breast milk selectively stimulates the growth of specific bacteria, including bifidobacteria, which are relatively depleted in children with CD.23,24 Researchers believe breastfeeding and delaying introduction of gluten-containing foods until 4 to 6 months of age might protect against CD.25
Should you recommend a gluten-free diet for other patients? Because avoiding gluten is now popular and many gluten-free products are marketed as “health food,” physicians may be reluctant to recommend a gluten-free diet for patients who have vague abdominal symptoms but negative CD test results. Despite the current popularity of “going gluten-free,” the reality is that in addition to CD, many other diseases may be helped by a gluten-free diet, such as dermatitis herpetiformis, irritable bowel syndrome, and neurologic diseases such as gluten-sensitive ataxia.19 In the end, whether to adopt a gluten-free diet is a decision that you and your patient will need to make together.
Researchers are searching for additional treatments
Because many patients find it difficult to adhere to a gluten-free diet, researchers are investigating several alternative treatments, including a derivative from cholera toxin that inhibits the opening of intestinal epithelial junctions, thereby reducing the resultant inflammatory response, and a desensitizing vaccine.19,22,23 Another intriguing approach involves using the parasite Necator americanus to modulate the immune response to gluten.18 Finally, certain infant feeding practices, including breastfeeding and delaying introduction of gluten to the diet, may minimize the risk of developing CD. (See "A link between infant feeding practices and the risk of CD?" above.22-25)
CORRESPONDENCE
Patrick T. Dowling, MD, MPH, Department of Family Medicine, 50-078 Center for Health Sciences, David Geffen School of Medicine at UCLA, Los Angeles, CA 90095-1683; [email protected]
1. Sanders L. Hurt all over. New York Times Sunday Magazine. November 11, 2011:MM22.
2. Rubio-Tapia A, Ludvigsson JF, Brantner TL, et al. The prevalence of celiac disease in the United States. Am J Gastroenterol. 2012;107:1538-1544.
3. Fasano A, Catassi C. Clinical practice. Celiac disease. N Engl J Med. 2012;367:2419-2426.
4. Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357: 1731-1743.
5. Fasano A, Berti I, Gerarduzzi T, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163:286-292.
6. Mustalahti K, Catassi C, Reunanen A, et al; Coeliac EU Cluster, Project Epidemiology. The prevalence of celiac disease in Europe: results of centralized, international mass screening project. Ann Med. 2010;42:587-595.
7. Farrel R, Kelly C. Celiac disease and refractory celiac disease. In: Feldman M, Friedman LS, Brandt LJ. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. 9th ed. Philadelphia, PA: Saunders; 2010: 1797-1820.
8. Gujral N, Freeman HJ, Thomson Ab. Celiac disease: prevalence, diagnosis, pathogenesis and treatment. World J Gastroenterol. 2012;18:6036-6059.
9. Sleisenger MH. Diseases of malabsorption. In: Beeson PB, McDermott W. Cecil-Loeb Textbook of Medicine. 13th ed. Philadelphia, PA: WB Saunders Company; 1971:1285-1291.
10. Reilly NR, Green PH. Epidemiology and clinical presentations of celiac disease. Sem Immunopathol. 2012;34:473-478.
11. Kagnoff MF. Celiac disease. A gastrointestinal disease with environmental, genetic, and immunologic components. Gastroenterol Clin North Am. 1992;21:405-425.
12. Riddle MS, Murray JA, Porter CK. The incidence and risk of celiac disease in a healthy US adult population. Am J Gastroenterol. 2012;107:1248-1255.
13. Rampertab SD, Pooran N, Brar P, et al. Trends in the presentation of celiac disease. Am J Med. 2006;119:355.e9-355.e14.
14. Pietzak M. Celiac disease, wheat allergy, and gluten sensitivity: when gluten free is not a fad. JPEN J Parenter Enteral Nutr. 2012;36(1 suppl):68S-75S.
15. Sapone A, Bai JC, Ciacci C, et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 2012;10:13.
16. Rubio-Tapia A, Hill ID, Kelly CP, et al; American College of Gastroenterology. ACG clinical guidelines: diagnosis and management celiac disease. Am J Gastroenterol. 2013;108:656-676.
17. Hill ID, Dirks MH, Liptak GS, et al; North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. Guidelines for the diagnosis and treatment of celiac disease in children: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroentertol Nutr. 2005;40:1-19.
18. Husby S, Koletsko S, Korponay-Szabó IR, et al; ESPGHAN Working Group on Coeliac Disease Diagnosis; ESPGHAN Gastoenterology Committee; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. European Society for Pediatric Gastroenterology, Hepatology and Nutrition Guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136-160.
19. Catassi C, Fasano A. Celiac disease diagnosis: simple rules are better than complicated algorithms. Am J Med. 2010;123:691-693.
20. US Food and Drug Administration. Foods labeled gluten-free must now meet FDA's definition. Available at: http://www.fda. gov/Food/NewsEvents/ConstituentUpdates/ucm407867.htm. Accessed August 13, 2014.
21. National Institutes of Health Consensus Development Conference on Celiac Disease. National Institutes of Health Consensus Development Conference Statement. Available at: http://consensus.nih.gov/2004/2004celiacdisease118html.htm. Accessed August 13, 2014.
22. Norris JM, Barriga K, Hoffenberg EJ, et al. Risk of celiac disease autoimmunity and timing of gluten introduction in the diet of infants at increased risk of disease. JAMA. 2005;293:2343-2351.
23. Pozo-Rubio T, Olivares M, Nova E, et al. Immune development and intestinal microbiota in celiac disease. Clin Dev Immunol. 2012;2012:654143
24. Ivarsson A, Myléus A, Norström F, et al. Prevalence of childhood celiac disease and changes in infant feeding. Pediatrics. 2013;131:687-694.
25. Cataldo F, Montalto G. Celiac disease in the developing countries: a new and challenging public health problem. World J Gastroenterol. 2007;13:2153-2159.
1. Sanders L. Hurt all over. New York Times Sunday Magazine. November 11, 2011:MM22.
2. Rubio-Tapia A, Ludvigsson JF, Brantner TL, et al. The prevalence of celiac disease in the United States. Am J Gastroenterol. 2012;107:1538-1544.
3. Fasano A, Catassi C. Clinical practice. Celiac disease. N Engl J Med. 2012;367:2419-2426.
4. Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357: 1731-1743.
5. Fasano A, Berti I, Gerarduzzi T, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163:286-292.
6. Mustalahti K, Catassi C, Reunanen A, et al; Coeliac EU Cluster, Project Epidemiology. The prevalence of celiac disease in Europe: results of centralized, international mass screening project. Ann Med. 2010;42:587-595.
7. Farrel R, Kelly C. Celiac disease and refractory celiac disease. In: Feldman M, Friedman LS, Brandt LJ. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. 9th ed. Philadelphia, PA: Saunders; 2010: 1797-1820.
8. Gujral N, Freeman HJ, Thomson Ab. Celiac disease: prevalence, diagnosis, pathogenesis and treatment. World J Gastroenterol. 2012;18:6036-6059.
9. Sleisenger MH. Diseases of malabsorption. In: Beeson PB, McDermott W. Cecil-Loeb Textbook of Medicine. 13th ed. Philadelphia, PA: WB Saunders Company; 1971:1285-1291.
10. Reilly NR, Green PH. Epidemiology and clinical presentations of celiac disease. Sem Immunopathol. 2012;34:473-478.
11. Kagnoff MF. Celiac disease. A gastrointestinal disease with environmental, genetic, and immunologic components. Gastroenterol Clin North Am. 1992;21:405-425.
12. Riddle MS, Murray JA, Porter CK. The incidence and risk of celiac disease in a healthy US adult population. Am J Gastroenterol. 2012;107:1248-1255.
13. Rampertab SD, Pooran N, Brar P, et al. Trends in the presentation of celiac disease. Am J Med. 2006;119:355.e9-355.e14.
14. Pietzak M. Celiac disease, wheat allergy, and gluten sensitivity: when gluten free is not a fad. JPEN J Parenter Enteral Nutr. 2012;36(1 suppl):68S-75S.
15. Sapone A, Bai JC, Ciacci C, et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 2012;10:13.
16. Rubio-Tapia A, Hill ID, Kelly CP, et al; American College of Gastroenterology. ACG clinical guidelines: diagnosis and management celiac disease. Am J Gastroenterol. 2013;108:656-676.
17. Hill ID, Dirks MH, Liptak GS, et al; North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. Guidelines for the diagnosis and treatment of celiac disease in children: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroentertol Nutr. 2005;40:1-19.
18. Husby S, Koletsko S, Korponay-Szabó IR, et al; ESPGHAN Working Group on Coeliac Disease Diagnosis; ESPGHAN Gastoenterology Committee; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. European Society for Pediatric Gastroenterology, Hepatology and Nutrition Guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136-160.
19. Catassi C, Fasano A. Celiac disease diagnosis: simple rules are better than complicated algorithms. Am J Med. 2010;123:691-693.
20. US Food and Drug Administration. Foods labeled gluten-free must now meet FDA's definition. Available at: http://www.fda. gov/Food/NewsEvents/ConstituentUpdates/ucm407867.htm. Accessed August 13, 2014.
21. National Institutes of Health Consensus Development Conference on Celiac Disease. National Institutes of Health Consensus Development Conference Statement. Available at: http://consensus.nih.gov/2004/2004celiacdisease118html.htm. Accessed August 13, 2014.
22. Norris JM, Barriga K, Hoffenberg EJ, et al. Risk of celiac disease autoimmunity and timing of gluten introduction in the diet of infants at increased risk of disease. JAMA. 2005;293:2343-2351.
23. Pozo-Rubio T, Olivares M, Nova E, et al. Immune development and intestinal microbiota in celiac disease. Clin Dev Immunol. 2012;2012:654143
24. Ivarsson A, Myléus A, Norström F, et al. Prevalence of childhood celiac disease and changes in infant feeding. Pediatrics. 2013;131:687-694.
25. Cataldo F, Montalto G. Celiac disease in the developing countries: a new and challenging public health problem. World J Gastroenterol. 2007;13:2153-2159.
Stress testing
To the Editor: I was delighted to see an article addressing the overuse of stress tests in asymptomatic individuals.1 I still think, however, that one could really look at the issue a little further. In truly asymptomatic individuals, even those with established coronary heart disease, what is the value of the “annual stress echocardiogram,” often done in cardiologist’s offices? I was perturbed a bit by the statement, “a physician may consider ordering exercise electrocardiography in asymptomatic adults at intermediate risk of coronary heart disease.” Are there data to suggest the number needed to treat or the number needed to harm? I was sobered by the results of the Detection of Ischemia in Asymptomatic Diabetics trial,2 which showed no benefit in screening patients with type 2 diabetes with stress myocardial perfusion imaging (a technique probably more costly but more accurate than stress echocardiography).
I understand that bold statements about the lack of usefulness of the stress test in asymptomatic individuals might be misinterpreted by payers as a justification for denying coverage, but it would provide more help for those of us in primary care who are trying to dissuade patients from inappropriate and potentially harmful testing.
- Smith CD, Alguire PC. Is cardiac stress testing appropriate in asymptomatic adults at low risk? Cleve Clin J Med 2014; 81:405–406.
- Young LH, Wackers FJ, Chyun DA, et al; DIAD Investigators. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA 2009; 301:1547–1555.
To the Editor: I was delighted to see an article addressing the overuse of stress tests in asymptomatic individuals.1 I still think, however, that one could really look at the issue a little further. In truly asymptomatic individuals, even those with established coronary heart disease, what is the value of the “annual stress echocardiogram,” often done in cardiologist’s offices? I was perturbed a bit by the statement, “a physician may consider ordering exercise electrocardiography in asymptomatic adults at intermediate risk of coronary heart disease.” Are there data to suggest the number needed to treat or the number needed to harm? I was sobered by the results of the Detection of Ischemia in Asymptomatic Diabetics trial,2 which showed no benefit in screening patients with type 2 diabetes with stress myocardial perfusion imaging (a technique probably more costly but more accurate than stress echocardiography).
I understand that bold statements about the lack of usefulness of the stress test in asymptomatic individuals might be misinterpreted by payers as a justification for denying coverage, but it would provide more help for those of us in primary care who are trying to dissuade patients from inappropriate and potentially harmful testing.
To the Editor: I was delighted to see an article addressing the overuse of stress tests in asymptomatic individuals.1 I still think, however, that one could really look at the issue a little further. In truly asymptomatic individuals, even those with established coronary heart disease, what is the value of the “annual stress echocardiogram,” often done in cardiologist’s offices? I was perturbed a bit by the statement, “a physician may consider ordering exercise electrocardiography in asymptomatic adults at intermediate risk of coronary heart disease.” Are there data to suggest the number needed to treat or the number needed to harm? I was sobered by the results of the Detection of Ischemia in Asymptomatic Diabetics trial,2 which showed no benefit in screening patients with type 2 diabetes with stress myocardial perfusion imaging (a technique probably more costly but more accurate than stress echocardiography).
I understand that bold statements about the lack of usefulness of the stress test in asymptomatic individuals might be misinterpreted by payers as a justification for denying coverage, but it would provide more help for those of us in primary care who are trying to dissuade patients from inappropriate and potentially harmful testing.
- Smith CD, Alguire PC. Is cardiac stress testing appropriate in asymptomatic adults at low risk? Cleve Clin J Med 2014; 81:405–406.
- Young LH, Wackers FJ, Chyun DA, et al; DIAD Investigators. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA 2009; 301:1547–1555.
- Smith CD, Alguire PC. Is cardiac stress testing appropriate in asymptomatic adults at low risk? Cleve Clin J Med 2014; 81:405–406.
- Young LH, Wackers FJ, Chyun DA, et al; DIAD Investigators. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA 2009; 301:1547–1555.
In reply: Stress testing
In Reply: Thanks so much for sharing your thoughts on our article. We share your frustration with the lack of evidence to support the decision to avoid stress testing in all asymptomatic individuals. In fact, there is no direct evidence that the identification and treatment of screening-detected, asymptomatic coronary artery disease will decrease mortality risk and improve outcomes in patients with no history of coronary artery disease.
The focus of our article was to review the available evidence and guidelines on stress testing low-risk, asymptomatic patients. The statement in the article that you cite, “a physician may consider ordering exercise electrocardiography in asymptomatic adults with intermediate risk of coronary heart disease,” was pulled from the 2010 American College of Cardiology/American Heart Association guideline1 in an attempt to summarize recent guidelines on this issue. Unfortunately, there is currently insufficient evidence to recommend for or against screening in patients at intermediate risk for coronary heart disease. As a result, the decision to perform stress testing in an asymptomatic patient at intermediate risk should include an informed discussion between the physician and patient. In contrast, there is considerable evidence supporting the recommendation not to screen in asymptomatic low-risk individuals, which is the main conclusion of our article.
- Greenland P, Alpert JS, Beller GA, et al; American College of Cardiology Foundation. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2010; 56:e50–e103.
In Reply: Thanks so much for sharing your thoughts on our article. We share your frustration with the lack of evidence to support the decision to avoid stress testing in all asymptomatic individuals. In fact, there is no direct evidence that the identification and treatment of screening-detected, asymptomatic coronary artery disease will decrease mortality risk and improve outcomes in patients with no history of coronary artery disease.
The focus of our article was to review the available evidence and guidelines on stress testing low-risk, asymptomatic patients. The statement in the article that you cite, “a physician may consider ordering exercise electrocardiography in asymptomatic adults with intermediate risk of coronary heart disease,” was pulled from the 2010 American College of Cardiology/American Heart Association guideline1 in an attempt to summarize recent guidelines on this issue. Unfortunately, there is currently insufficient evidence to recommend for or against screening in patients at intermediate risk for coronary heart disease. As a result, the decision to perform stress testing in an asymptomatic patient at intermediate risk should include an informed discussion between the physician and patient. In contrast, there is considerable evidence supporting the recommendation not to screen in asymptomatic low-risk individuals, which is the main conclusion of our article.
In Reply: Thanks so much for sharing your thoughts on our article. We share your frustration with the lack of evidence to support the decision to avoid stress testing in all asymptomatic individuals. In fact, there is no direct evidence that the identification and treatment of screening-detected, asymptomatic coronary artery disease will decrease mortality risk and improve outcomes in patients with no history of coronary artery disease.
The focus of our article was to review the available evidence and guidelines on stress testing low-risk, asymptomatic patients. The statement in the article that you cite, “a physician may consider ordering exercise electrocardiography in asymptomatic adults with intermediate risk of coronary heart disease,” was pulled from the 2010 American College of Cardiology/American Heart Association guideline1 in an attempt to summarize recent guidelines on this issue. Unfortunately, there is currently insufficient evidence to recommend for or against screening in patients at intermediate risk for coronary heart disease. As a result, the decision to perform stress testing in an asymptomatic patient at intermediate risk should include an informed discussion between the physician and patient. In contrast, there is considerable evidence supporting the recommendation not to screen in asymptomatic low-risk individuals, which is the main conclusion of our article.
- Greenland P, Alpert JS, Beller GA, et al; American College of Cardiology Foundation. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2010; 56:e50–e103.
- Greenland P, Alpert JS, Beller GA, et al; American College of Cardiology Foundation. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2010; 56:e50–e103.
Method could improve malaria diagnosis

red blood cell; Credit: St Jude
Children’s Research Hospital
Researchers have found they can diagnose malaria using magnetic fields to detect a byproduct of malarial metabolism.
They used magnetic resonance relaxometry (MRR) to detect a parasitic waste product called hemozoin in malaria-infected red blood cells from mice and humans.
The team said MRR is more sensitive than other methods of detecting malaria, can be carried out using a portable benchtop system, and costs less than 10 cents per test.
Jongyoon Han, PhD, of the Massachusetts Institute of Technology in Cambridge, and his colleagues described the technique in Nature Medicine.
When malaria parasites infect red blood cells, they feed on the nutrient-rich hemoglobin. As hemoglobin breaks down, it releases iron, which can be toxic, so the parasite converts the iron into hemozoin—a weakly paramagnetic crystallite.
Those crystals interfere with the normal magnetic spins of hydrogen atoms. When exposed to a powerful magnetic field, hydrogen atoms align their spins in the same direction.
When a second, smaller field perturbs the atoms, they should all change their spins in synchrony. But if another magnetic particle, such as hemozoin, is present, this synchrony is disrupted through a process called relaxation. The more magnetic particles present, the more quickly the synchrony is disrupted.
“What we are trying to really measure is how the hydrogen’s nuclear magnetic resonance is affected by the proximity of other magnetic particles,” Dr Han said.
This MRR technique enables malaria diagnosis because hemozoin crystals are produced in all 4 stages of malaria infection and are generated by all known species of the Plasmodium parasite. Furthermore, the amount of hemozoin can reveal how severe the infection is, or whether it is responding to treatment.
Dr Han and his colleagues found they could use MRR to detect Plasmodium falciparum infection to as low as 0.0002% parasitemia in 750 nl of cultured blood in less than 5 minutes.
They also detected Plasmodium berghei in mice, allowing for reliable estimation of parasitemia to as low as 0.0001%.
The device the researchers used in this study is small enough to sit on a table or lab bench, but they are working on a portable version the size of a small electronic tablet.
“This system can be built at a very low cost, relative to the million-dollar MRI machines used in a hospital,” said study author Weng Kung Peng, PhD, of the Singapore-MIT Alliance for Research and Technology Centre in Singapore.
“Furthermore, since this technique does not rely on expensive labeling with chemical reagents, we are able to get each diagnostic test done at a cost of less than 10 cents.”
The researchers are launching a company to make this technology available at an affordable price. The team is also running field tests in Southeast Asia and exploring powering the device on solar energy. ![]()

red blood cell; Credit: St Jude
Children’s Research Hospital
Researchers have found they can diagnose malaria using magnetic fields to detect a byproduct of malarial metabolism.
They used magnetic resonance relaxometry (MRR) to detect a parasitic waste product called hemozoin in malaria-infected red blood cells from mice and humans.
The team said MRR is more sensitive than other methods of detecting malaria, can be carried out using a portable benchtop system, and costs less than 10 cents per test.
Jongyoon Han, PhD, of the Massachusetts Institute of Technology in Cambridge, and his colleagues described the technique in Nature Medicine.
When malaria parasites infect red blood cells, they feed on the nutrient-rich hemoglobin. As hemoglobin breaks down, it releases iron, which can be toxic, so the parasite converts the iron into hemozoin—a weakly paramagnetic crystallite.
Those crystals interfere with the normal magnetic spins of hydrogen atoms. When exposed to a powerful magnetic field, hydrogen atoms align their spins in the same direction.
When a second, smaller field perturbs the atoms, they should all change their spins in synchrony. But if another magnetic particle, such as hemozoin, is present, this synchrony is disrupted through a process called relaxation. The more magnetic particles present, the more quickly the synchrony is disrupted.
“What we are trying to really measure is how the hydrogen’s nuclear magnetic resonance is affected by the proximity of other magnetic particles,” Dr Han said.
This MRR technique enables malaria diagnosis because hemozoin crystals are produced in all 4 stages of malaria infection and are generated by all known species of the Plasmodium parasite. Furthermore, the amount of hemozoin can reveal how severe the infection is, or whether it is responding to treatment.
Dr Han and his colleagues found they could use MRR to detect Plasmodium falciparum infection to as low as 0.0002% parasitemia in 750 nl of cultured blood in less than 5 minutes.
They also detected Plasmodium berghei in mice, allowing for reliable estimation of parasitemia to as low as 0.0001%.
The device the researchers used in this study is small enough to sit on a table or lab bench, but they are working on a portable version the size of a small electronic tablet.
“This system can be built at a very low cost, relative to the million-dollar MRI machines used in a hospital,” said study author Weng Kung Peng, PhD, of the Singapore-MIT Alliance for Research and Technology Centre in Singapore.
“Furthermore, since this technique does not rely on expensive labeling with chemical reagents, we are able to get each diagnostic test done at a cost of less than 10 cents.”
The researchers are launching a company to make this technology available at an affordable price. The team is also running field tests in Southeast Asia and exploring powering the device on solar energy. ![]()

red blood cell; Credit: St Jude
Children’s Research Hospital
Researchers have found they can diagnose malaria using magnetic fields to detect a byproduct of malarial metabolism.
They used magnetic resonance relaxometry (MRR) to detect a parasitic waste product called hemozoin in malaria-infected red blood cells from mice and humans.
The team said MRR is more sensitive than other methods of detecting malaria, can be carried out using a portable benchtop system, and costs less than 10 cents per test.
Jongyoon Han, PhD, of the Massachusetts Institute of Technology in Cambridge, and his colleagues described the technique in Nature Medicine.
When malaria parasites infect red blood cells, they feed on the nutrient-rich hemoglobin. As hemoglobin breaks down, it releases iron, which can be toxic, so the parasite converts the iron into hemozoin—a weakly paramagnetic crystallite.
Those crystals interfere with the normal magnetic spins of hydrogen atoms. When exposed to a powerful magnetic field, hydrogen atoms align their spins in the same direction.
When a second, smaller field perturbs the atoms, they should all change their spins in synchrony. But if another magnetic particle, such as hemozoin, is present, this synchrony is disrupted through a process called relaxation. The more magnetic particles present, the more quickly the synchrony is disrupted.
“What we are trying to really measure is how the hydrogen’s nuclear magnetic resonance is affected by the proximity of other magnetic particles,” Dr Han said.
This MRR technique enables malaria diagnosis because hemozoin crystals are produced in all 4 stages of malaria infection and are generated by all known species of the Plasmodium parasite. Furthermore, the amount of hemozoin can reveal how severe the infection is, or whether it is responding to treatment.
Dr Han and his colleagues found they could use MRR to detect Plasmodium falciparum infection to as low as 0.0002% parasitemia in 750 nl of cultured blood in less than 5 minutes.
They also detected Plasmodium berghei in mice, allowing for reliable estimation of parasitemia to as low as 0.0001%.
The device the researchers used in this study is small enough to sit on a table or lab bench, but they are working on a portable version the size of a small electronic tablet.
“This system can be built at a very low cost, relative to the million-dollar MRI machines used in a hospital,” said study author Weng Kung Peng, PhD, of the Singapore-MIT Alliance for Research and Technology Centre in Singapore.
“Furthermore, since this technique does not rely on expensive labeling with chemical reagents, we are able to get each diagnostic test done at a cost of less than 10 cents.”
The researchers are launching a company to make this technology available at an affordable price. The team is also running field tests in Southeast Asia and exploring powering the device on solar energy. ![]()
Management of Gastroenteropancreatic Neuroendocrine Tumors
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Neuroendocrine tumors (NETs) are a rare, heterogeneous group of neoplasms that arise from neuroendocrine cells located throughout the body. These tumors are characterized by variable but most often indolent biologic behavior. They are also classically characterized by their ability to secrete peptides, resulting in distinctive hormonal syndromes. Although NETs have been considered rare, recent studies suggest that they are more common than previously suspected. An analysis of the Surveillance, Epidemiology, and End Results (SEER) database demonstrated a significant increase in the incidence of NETs over time with an age-adjusted annual incidence in the United States of 5.25 cases per 100,000 population. The increase in incidence is likely attributable to increasing awareness, improved diagnostic strategies, and possibly other undetermined environmental and genetic factors.
To read the full article in PDF:
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Neuroendocrine tumors (NETs) are a rare, heterogeneous group of neoplasms that arise from neuroendocrine cells located throughout the body. These tumors are characterized by variable but most often indolent biologic behavior. They are also classically characterized by their ability to secrete peptides, resulting in distinctive hormonal syndromes. Although NETs have been considered rare, recent studies suggest that they are more common than previously suspected. An analysis of the Surveillance, Epidemiology, and End Results (SEER) database demonstrated a significant increase in the incidence of NETs over time with an age-adjusted annual incidence in the United States of 5.25 cases per 100,000 population. The increase in incidence is likely attributable to increasing awareness, improved diagnostic strategies, and possibly other undetermined environmental and genetic factors.
To read the full article in PDF:
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Neuroendocrine tumors (NETs) are a rare, heterogeneous group of neoplasms that arise from neuroendocrine cells located throughout the body. These tumors are characterized by variable but most often indolent biologic behavior. They are also classically characterized by their ability to secrete peptides, resulting in distinctive hormonal syndromes. Although NETs have been considered rare, recent studies suggest that they are more common than previously suspected. An analysis of the Surveillance, Epidemiology, and End Results (SEER) database demonstrated a significant increase in the incidence of NETs over time with an age-adjusted annual incidence in the United States of 5.25 cases per 100,000 population. The increase in incidence is likely attributable to increasing awareness, improved diagnostic strategies, and possibly other undetermined environmental and genetic factors.
To read the full article in PDF:
Radon and lung cancer: Assessing and mitigating the risk
In 1984, a worker at a Pennsylvania nuclear power plant triggered the radiation detector as he was getting ready to go home. This would not be unusual for such a facility, but there was no nuclear fuel on site at the time. The alarm went off every time he left work.
One day, he triggered the alarm as he crossed the detector on arriving at the plant, leading him to suspect that he was bringing radiation from home. He eventually convinced the plant’s health physicists to check his home, although at first they were opposed to the idea. The results revealed high concentrations of radon everywhere, especially in his basement.
Radon was already known to be associated with health risks in underground miners at that time. This event revealed that a naturally occurring radioactive gas could be found in households at potentially hazardous concentrations.
The incident captured the public’s attention, and the Environmental Protection Agency (EPA) and the US Centers for Disease Control and Prevention (CDC) recommended that nearly all homes be tested.1,2 In 1988, the International Agency for Research on Cancer classified radon as a human carcinogen, and Congress passed the Indoor Radon Abatement Act in response to growing concern over health risks.3 This law funded state and federal measures to survey schools and federal buildings for radon levels, to educate citizens, and to develop programs for technical assistance. The long-term goal was to reduce indoor levels nationwide to no more than outdoor levels.
Radon is still considered an important public health hazard. From 15,000 to 21,000 people are estimated to die of lung cancer as a result of radon exposure each year in the United States, making it the second most common cause of lung cancer, behind smoking.4
Considering the relevance of this issue, this article will review the unique characteristics of radon as a risk factor for lung cancer.
WHAT IS RADON?
Radon is a noble gas that occurs naturally as a decay product of uranium 238 and thorium 232. It is colorless, tasteless, and imperceptible to our senses. Its most common isotope is radon 222 (222Rn), which has a half-life of 3.8 days and decays by emitting an alpha particle to become polonium 218. The decay chain continues through several intermediate steps until the stable isotope lead 206 is formed (Figure 1). Two of the isotopes in this chain, polonium 218 and polonium 214, also emit alpha particles.5–7
Radon is primarily formed in soil. Its most important precursor, uranium 238, is ubiquitous, found in most soils and rocks in various concentrations. Radon can also be found in surface water, metal mines (uranium, phosphorus, silver, gold), residue of coal combustion, and natural gas.
Outdoor levels are usually much lower than indoor levels, as radon dissipates very quickly. Indoor radon mostly comes from the soil under the house or building, but it can also originate from coal combustion, gas appliances, and water (especially from private wells). In municipal water systems or surface reservoirs, most of the radon dissipates into the air or decays before the water reaches homes.8,9
Radon’s only commercial application in the United States is in calibrating measuring instruments. In the past, it was used in radiography and to treat cancer but was later replaced by other radiation sources that cost less and pose less hazard of alpha radiation.10
HOW RADON CAN HARM
Alpha particles, emitted by radon 222 and its progenies polonium 218 and polonium 214, are highly effective in damaging tissues. Although they do not travel far or fast, with their two protons and two neutrons, alpha particles are heavy and therefore can cause considerable damage at short range. Although alpha particles can be stopped by a thin barrier such as a piece of paper or the skin, if the source is inhaled or ingested and lodges against mucosal linings, the alpha particles emitted can destroy cells.11
The main route of radon exposure is by inhalation. Since radon is biologically inert, it is readily exhaled after it reaches the lungs. However, radon’s progenies can also be inhaled, either as free particles or attached to airborne particles such as dust, which they tend to attract as a result of their charged state. This attached fraction is believed to be more carcinogenic because it tends to deposit on the respiratory epithelium, notably in the carinae of bronchi. The smaller the dust particle, the deeper it can travel into the lung. The radiation emissions damage the genetic material of cells lining the airways, with the potential to result in lung cancer if the repair process is incomplete.5,8,9
Other routes of exposure include ingestion and dermal exposure. Radon and its progenies can be swallowed in drinking water, passing through the stomach walls and bowels and entering the blood.12 Dermal exposure is not considered a significant route unless the dermis is exposed, since in usual circumstances the skin protects the body from alpha radiation.13
Possible biologic mechanisms by which radon exposure might increase the risk of cancer include gene mutations, chromosome aberrations, generation of reactive oxygen species, up- or down-regulation of cytokines, and production of proteins associated with cell-cycle regulation.14–16
HOW IS RADON MEASURED?
Several devices are commercially available to measure radon levels at home. The most common ones are activated charcoal detectors, electret ion chambers, alpha-track detectors, electronic integrating devices, and continuous monitors. There is no evidence that one device is better than another, but devices that measure radon gas are usually preferred over those that measure decay products because they are simpler to use and more cost-effective. These devices are divided into those used for short-term testing (2–90 days) and long-term testing (Table 1).17
Radon levels can be expressed as follows:
Working levels. One working level (WL) is any combination of radon progeny in 1 L of air that ultimately releases 1.3 × 105 MeV of alpha energy during decay. In studies of miners, the radon progeny concentrations are generally expressed in WL. The cumulative exposure of an individual to this concentration over a “working month” of 170 hours is defined as a working level month (WLM).
Picocuries per liter. In the United States, the rate of decay is commonly reported in picocuries per liter (pCi/L): 1 pCi/L translates to 0.005 WL under usual conditions. The outdoor radon level is normally around 0.4 pCi/L.
Becquerel per cubic meter (Bq/m3) is an International System unit of measure: 1 WL corresponds to 3.7 × 103 Bq/m3, and 1 pCi/L is equivalent to 37 Bq/m3.
Different areas have different radon levels
The Indoor Radon Abatement Act of 1988 helped identify areas in the United States that have the potential for elevated indoor radon levels. An estimated 6 million homes have concentrations greater than 4 pCi/L.
To assist in implementing radon-reducing strategies and allocation of resources, the EPA has created a map (Figure 2) that classifies counties according to the predicted indoor level.18
WHAT IS THE RELATIONSHIP BETWEEN RADON AND LUNG CANCER?
Determining the degree to which radon exposure contributes to lung cancer is a complex task. Radon can be found nearly everywhere, and there are diurnal, seasonal, and random year-to-year variations in the concentration of radon in indoor air.
A minority view
Not everyone agrees that radon is completely bad. For centuries, people have flocked to spas to “take the waters,” and the water at many of these spas has been found to contain radon. In the early 20th century, radiation was touted as having medicinal benefits, and people in many places in the world still go to “radon spas” (some of them in abandoned uranium mines) to help treat conditions such as arthritis and to feel invigorated and energized.
In 2006, a report by Zdrojewicz and Strzelczyk19 urged the medical community to keep an open mind about the possibility that radon exposure may be beneficial in very low doses, perhaps by stimulating repair mechanisms. This concept, called hormesis, differs from the mainstream view that cancer risk rises linearly with radiation dose, with no minimum threshold level (see below).
Risk in miners
As early as in the 16th century, metal miners in central Europe were noted to have a high rate of death from respiratory disease. Radon was discovered in 1900, and in the 20th century lung cancer was linked to high levels of radon detected in uranium mines.
Several small studies of underground miners exposed to high concentrations of radon consistently demonstrated an increased risk of lung cancer.
The Committee on the Biological Effects of Ionizing Radiation (BEIR VI 1999) reviewed 11 major cohort studies of miners. The studies included more than 60,000 miners in Europe, North America, Asia, and Australia, of whom 2,600 died of lung cancer. Lung cancer rates increased linearly with cumulative radon exposure, and the estimated average increase in the lung cancer death rate per WLM in the combined studies was 0.44% (95% confidence interval [CI] 0.20–1.00%). The percentage increase in the lung cancer death rate per WLM varied with time since exposure, with the highest increase in risk during the 5 to 14 years after exposure.4,17 Furthermore, the increase in risk was higher in younger miners, who were exposed to a relatively low radon concentration.
Risk in the general population
The magnitude of the risk in miners led to concern about radon exposure as a cause of lung cancer in the general population. Statistical models were generated that suggested a causal link between radon exposure and lung cancer. Although extrapolation of the results from miners caused controversy, the BEIR VI estimation of risk was validated by studies in the general population.7,20–23
Since the 1980s, several small case-control studies with limited power examined the relationship between indoor radon and lung cancer in the general population. In these studies, individuals who had developed lung cancer were compared with controls who had not developed the disease but who otherwise represented the population from which the cases of lung cancer came.
To improve the statistical power, the investigators of the major studies in Europe, North America, and China pooled the results in separate analyses (Table 2).7,20–23 The average radon concentration to which each individual had been exposed over the previous decades was estimated by measuring the radon concentration at their present and previous homes. On the basis of information from the uranium miners, these studies assumed that the period of exposure was the 30 years ending 5 years before the diagnosis or at death from lung cancer.
The results provided convincing evidence that radon exposure is a cause of lung cancer in the general population and substantiated the extrapolation from the studies of miners. Further, the results of all three pooled analyses were consistent with a linear dose-response relationship with no threshold, suggesting an increased risk of lung cancer even with a radon level below 4 pCi/L (200 Bq/m3), which is the concentration at which mitigation actions are recommended in many countries.17
The North American pooled analysis included 3,662 cases and 4,966 controls from seven studies in the United States and Canada. When data from all studies were combined, the risk of lung cancer was found to increase by 11% per 100-Bq/m3 (about 2.7-pCi/L) increase in measured radon concentration (95% CI 0%–28%). The estimated increase in lung cancer was independent of age, sex, or smoking history.7,20
The Chinese pooled data22 demonstrated a 13% (95% CI 1%–36%) increased risk per 100 Bq/m3.
In the European study, the risk of lung cancer increased by 8% per 100 Bq/m3 (95% CI 3%–16%). The European investigators repeated the analysis, taking into account the random year-to-year variability in measured radon concentration, finding the final estimated risk was an increase of 16% per 100 Bq/m3 using long-term average concentration.21
The combined estimate21,24 from the three pooling studies based on measured radon concentration is an increased risk of lung cancer of 10% per 100 Bq/m3.
Synergistic risk with smoking
Radon exposure was independently associated with lung cancer, and the relationship with cigarette smoking is believed to be synergistic. The radon progeny particles attach themselves to smoke and dust and are then deposited in the bronchial epithelium.25
In the pool of European case-control studies, the cancer risk for current smokers of 15 to 24 cigarettes per day relative to that in never-smokers was 25.8 (95% CI 21–31). Assuming that in the same analysis the lung cancer risk increased by 16% per 100 Bq/m3 of usual radon concentration regardless of smoking status, the cumulative absolute risk by age 75 would be 0.67% in those who never smoked and 16% in smokers at usual radon levels of 400 Bq/m3 (11 pCi/L).21
Rates of all lung cancer subtypes increased
Radon exposure is not associated with a specific histologic subtype of lung cancer. It has been speculated that the incidence of the small-cell subtype might be slightly increased because radon tends to deposit in the more central bronchial carinae.20,21 However, all subtypes have been described in association with radon, the most common being adenocarcinoma and squamous cell carcinoma.26–28
EFFECT OF MITIGATION MEASURES
The US Surgeon General and the EPA recommend that all homes be tested.18 Short-term tests should be used first, keeping in mind that diurnal and seasonal variations may occur.
The World Health Organization has proposed a reference level of 100 Bq/m3 (2.7 pCi/L) to minimize health hazards from indoor radon exposure.17 If this level cannot be reached under the country-specific conditions, the chosen reference level should not exceed 300 Bq/m3 (8 pCi/L).
In the United States, if the result of home testing is higher than 4 pCi/L, a follow-up measurement should be done using a different short-term test or a long-term test. If the follow-up result confirms a level of more than 4 pCi/L, mitigating actions are recommended. The goal is to reduce the indoor radon level as much as possible—down to zero or at least comparable to outdoor levels (national average 0.4 pCi/L).18
A variety of radon mitigation strategies have been used, with different rates of efficacy (Table 3). The optimal strategy depends on the likely source or cause, construction characteristics, soil, and climate.29 Table 4 lists resources for the general public about testing and mitigation measures.
How beneficial is radon mitigation?
Although it is logical to try to reduce the indoor radon concentration, there is no strong evidence yet that this intervention decreases the incidence of lung cancer in the general population.
Using the BEIR VI risk model, Méndez et al30 estimated a 21% reduction in the annual radon-related lung cancer mortality rate by 2100 if all households were compliant with government recommendations (mitigation actions at levels of 4 pCi/L) and assuming that the percentage of cigarette smokers remained constant.
On the other hand, if the number of smokers continues to decline, the benefits from radon mitigation may be less. The expected benefit from mitigation in this scenario is a reduction of 12% in annual radon-related deaths by the year 2100.30 However, it will be challenging to determine whether the expected decline in the incidence of lung cancer and lung cancer deaths is truly attributable to mitigation measures.
MANAGING PATIENTS EXPOSED TO RADON
Screen for lung cancer in smokers only
The National Lung Screening Study (NLST) was a large multicenter trial of annual low-dose computed tomography (CT) to screen for lung cancer in a cohort at high risk: age 55 to 74, at least a 30 pack-year history of smoking in a current smoker, or a former smoker who quit within the past 15 years. The trial demonstrated a 20% reduction in lung cancer deaths in the CT screening group.31
Since the publication of the NLST results, many societies have endorsed screening for lung cancer with low-dose CT using the study criteria. The National Comprehensive Cancer Network (NCCN) expanded these criteria and has recommended screening in patients over age 50 who have a history of smoking and one additional risk factor, such as radon exposure.
However, radon exposure has not been incorporated into a lung cancer risk-prediction model, and there is no empirical evidence suggesting that people who have such a history would benefit from screening.32,33 The joint guidelines of the American College of Chest Physicians and American Society of Clinical Oncology recommend annual low-dose CT screening only for patients who meet the NLST criteria.34
What to do about indeterminate lung nodules
The widely used guidelines from the Fleischner Society35 on how to manage small lung nodules stratify patients into groups at low and high risk of developing lung cancer on the basis of risk factors. The guidelines apply to adults age 35 and older in whom an indeterminate solid nodule was recently detected.
If a patient is at high risk, the recommended approach includes follow-up in shorter intervals depending on the nodule size. History of smoking is recognized as a major risk factor, and the statement also lists family history and exposure to asbestos, uranium, and radon.35
Although the association of radon with lung cancer has been shown in epidemiologic studies, radon exposure has not been included in validated statistical models that assess the probability that an indeterminate lung nodule is malignant. We would expect the risk to be higher in miners, who suffer a more intense exposure to higher levels of radon, than in the general population, which has a low and constantly variable residential exposure. Furthermore, there are no data to support a more aggressive follow-up approach in patients with indeterminate lung nodules and a history of radon exposure.
RADON AND OTHER CANCERS
When a person is exposed to radon, the bronchial epithelium receives the highest dose of ionizing radiation, but other organs such as the kidneys, stomach, and bone marrow may receive doses as well, although lower. Several studies have looked into possible associations, but there is no strong evidence to suggest an increased mortality rate related to radon from cancers other than lung.24,36 However, there seems to be a positive association between radon and the incidence of lymphoproliferative disorders in uranium miners.37,38
Radon can be measured in drinking water, and a few studies have looked at a possible association with gastrointestinal malignancies. The results did not reveal a consistent positive correlation.39,40 The risk of cancer from exposure to radon in the public water supply is likely small and mostly from the transfer of radon particles into the air and not from drinking the water. On the other hand, the risk could be higher with private wells, where radon levels are variable and are possibly higher than from public sources.41
DATA ARE INSUFFICIENT TO GUIDE MANAGEMENT
Radon is a naturally occurring and ubiquitous radioactive gas that can cause tissue damage. Cohort and case-control studies have demonstrated that radon exposure is associated with increased risk of lung cancer. It is recommended that radon levels be measured in every home in the United States and mitigation measures instituted if levels exceed 4 pCi/L.
There are insufficient data to help guide the management of patients with a history of radon exposure, and prospective studies are needed to better understand the individual risk of developing lung cancer and the appropriate management of such patients.
Smoking cessation is an integral part of lung cancer risk reduction from radon exposure.
- Berreby D. The radon raiders: turning perils into profits. The New York Times 1987. www.nytimes.com/1987/07/26/business/the-radon-raiders-turning-perils-into-profits.html?src=pm&pagewanted=1. Accessed August 5, 2014.
- Lewis RK. A history of radon—1470 to 1984. www.ohio-radonpro.com/Radon_History.html. Accessed August 5, 2014.
- World Health Organization (WHO). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Manmade mineral fibres and radon. Summary of data reported and evaluation. http://monographs.iarc.fr/ENG/Monographs/vol43/volume43.pdf. Accessed August 5, 2014.
- Committee on Health Risks of Exposure to Radon (BEIR VI). Health effects of exposure to radon: BEIR VI. Washington, DC: National Academies Press; 1999.
- Samet JM. Radon and lung cancer. J Natl Cancer Inst 1989; 81:745–757.
- Lewis RJ, Lewis Sr RJ. Hawley’s condensed chemical dictionary. 14thed. New York: Wiley-Interscience; 2001.
- Krewski D, Lubin JH, Zielinski JM, et al. Residential radon and risk of lung cancer: a combined analysis of 7 North American case-control studies. Epidemiology 2005; 16:137–145.
- Darby S, Hill D, Doll R. Radon: a likely carcinogen at all exposures. Ann Oncol 2001; 12:1341–1351.
- Sethi TK, El-Ghamry MN, Kloecker GH. Radon and lung cancer. Clin Adv Hematol Oncol 2012; 10:157–164.
- Morrison A. Use of radon for industrial radiography. Can J Res 1945; 23:413–419.
- Narayanan PK, Goodwin EH, Lehnert BE. Alpha particles initiate biological production of superoxide anions and hydrogen peroxide in human cells. Cancer Res 1997; 57:3963–3971.
- Ishikawa T, Narazaki Y, Yasuoka Y, Tokonami S, Yamada Y. Bio-kinetics of radon ingested from drinking water. Radiat Prot Dosimetry 2003; 105:65–70.
- Ishikawa T, Yamada Y, Fukutsu K, Tokonami S. Deposition and clearance for radon progeny in the human respiratory tract. Radiat Prot Dosimetry 2003; 105:143–148.
- Farkas A, Hofmann W, Balásházy I, Szoke I, Madas BG, Moustafa M. Effect of site-specific bronchial radon progeny deposition on the spatial and temporal distributions of cellular responses. Radiat Environ Biophys 2011; 50:281–297.
- Robertson A, Allen J, Laney R, Curnow A. The cellular and molecular carcinogenic effects of radon exposure: a review. Int J Mol Sci 2013; 14:14024–14063.
- Chauhan V, Howland M, Wilkins R. Effects of alpha-particle radiation on microRNA responses in human cell-lines. Open Biochem J 2012; 6:16–22.
- World Health Organization (WHO). WHO handbook on indoor radon: a public health perspective; 2009. www.nrsb.org/pdf/WHO%20Radon%20Handbook.pdf. Accessed August 5, 2014.
- United States Environmental Protection Agency (EPA). www.epa.gov/radon/. Accessed August 5, 2014.
- Zdrojewicz Z, Strzelczyk JJ. Radon treatment controversy. Dose Response 2006; 4:106–118.
- Krewski D, Lubin JH, Zielinski JM, et al. A combined analysis of North American case-control studies of residential radon and lung cancer. J Toxicol Environ Health A 2006; 69:533–597.
- Darby S, Hill D, Auvinen A, et al. Radon in homes and risk of lung cancer: collaborative analysis of individual data from 13 European case-control studies. BMJ 2005; 330:223.
- Lubin JH, Wang ZY, Boice JD, et al. Risk of lung cancer and residential radon in China: pooled results of two studies. Int J Cancer 2004; 109:132–137.
- Darby S, Hill D, Deo H, et al. Residential radon and lung cancer—detailed results of a collaborative analysis of individual data on 7,148 persons with lung cancer and 14,208 persons without lung cancer from 13 epidemiologic studies in Europe. Scand J Work Environ Health 2006; 32(suppl 1):1–83.
- Darby SC, Whitley E, Howe GR, et al. Radon and cancers other than lung cancer in underground miners: a collaborative analysis of 11 studies. J Natl Cancer Inst 1995; 87:378–384.
- Baias PF, Hofmann W, Winkler-Heil R, Cosma C, Duliu OG. Lung dosimetry for inhaled radon progeny in smokers. Radiat Prot Dosimetry 2010; 138:111–118.
- Land CE, Shimosato Y, Saccomanno G, et al. Radiation-associated lung cancer: a comparison of the histology of lung cancers in uranium miners and survivors of the atomic bombings of Hiroshima and Nagasaki. Radiat Res 1993; 134:234–243.
- Kreuzer M, Müller KM, Brachner A, et al. Histopathologic findings of lung carcinoma in German uranium miners. Cancer 2000; 89:2613–2621.
- Saccomanno G, Auerbach O, Kuschner M, et al. A comparison between the localization of lung tumors in uranium miners and in nonminers from 1947 to 1991. Cancer 1996; 77:1278–1283.
- Rahman NM, Tracy BL. Radon control systems in existing and new construction: a review. Radiat Prot Dosimetry 2009; 135:243–255.
- Méndez D, Alshanqeety O, Warner KE, Lantz PM, Courant PN. The impact of declining smoking on radon-related lung cancer in the United States. Am J Public Health 2011; 101:310–314.
- National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365:395–409.
- Wood DE, Eapen GA, Ettinger DS, et al. Lung cancer screening. J Natl Compr Canc Netw 2012; 10:240–265.
- Ettinger DS, Akerley W, Borghaei H, et al; NCCN (National Comprehensive Cancer Network). Non-small cell lung cancer. J Natl Compr Canc Netw 2012; 10:1236–1271.
- Detterbeck FC, Mazzone PJ, Naidich DP, Bach PB. Screening for lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143(suppl 5):e78S–e92S.
- MacMahon H, Austin JH, Gamsu G, et al; Fleischner Society. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005; 237:395–400.
- Darby SC, Radford EP, Whitley E. Radon exposure and cancers other than lung cancer in Swedish iron miners. Environ Health Perspect 1995; 103(suppl 2):45–47.
- Laurier D, Tirmarche M, Mitton N, et al. An update of cancer mortality among the French cohort of uranium miners: extended follow-up and new source of data for causes of death. Eur J Epidemiol 2004; 19:139–146.
- Rericha V, Kulich M, Rericha R, Shore DL, Sandler DP. Incidence of leukemia, lymphoma, and multiple myeloma in Czech uranium miners: a case-cohort study. Environ Health Perspect 2006; 114:818–822.
- Auvinen A, Salonen L, Pekkanen J, Pukkala E, Ilus T, Kurttio P. Radon and other natural radionuclides in drinking water and risk of stomach cancer: a case-cohort study in Finland. Int J Cancer 2005; 114:109–113.
- Kjellberg S, Wiseman JS. The relationship of radon to gastrointestinal malignancies. Am Surg 1995; 61:822–825.
- Cappello MA, Ferraro A, Mendelsohn AB, Prehn AW. Radon-contaminated drinking water from private wells: an environmental health assessment examining a rural Colorado mountain community’s exposure. J Environ Health 2013; 76:18–24.
In 1984, a worker at a Pennsylvania nuclear power plant triggered the radiation detector as he was getting ready to go home. This would not be unusual for such a facility, but there was no nuclear fuel on site at the time. The alarm went off every time he left work.
One day, he triggered the alarm as he crossed the detector on arriving at the plant, leading him to suspect that he was bringing radiation from home. He eventually convinced the plant’s health physicists to check his home, although at first they were opposed to the idea. The results revealed high concentrations of radon everywhere, especially in his basement.
Radon was already known to be associated with health risks in underground miners at that time. This event revealed that a naturally occurring radioactive gas could be found in households at potentially hazardous concentrations.
The incident captured the public’s attention, and the Environmental Protection Agency (EPA) and the US Centers for Disease Control and Prevention (CDC) recommended that nearly all homes be tested.1,2 In 1988, the International Agency for Research on Cancer classified radon as a human carcinogen, and Congress passed the Indoor Radon Abatement Act in response to growing concern over health risks.3 This law funded state and federal measures to survey schools and federal buildings for radon levels, to educate citizens, and to develop programs for technical assistance. The long-term goal was to reduce indoor levels nationwide to no more than outdoor levels.
Radon is still considered an important public health hazard. From 15,000 to 21,000 people are estimated to die of lung cancer as a result of radon exposure each year in the United States, making it the second most common cause of lung cancer, behind smoking.4
Considering the relevance of this issue, this article will review the unique characteristics of radon as a risk factor for lung cancer.
WHAT IS RADON?
Radon is a noble gas that occurs naturally as a decay product of uranium 238 and thorium 232. It is colorless, tasteless, and imperceptible to our senses. Its most common isotope is radon 222 (222Rn), which has a half-life of 3.8 days and decays by emitting an alpha particle to become polonium 218. The decay chain continues through several intermediate steps until the stable isotope lead 206 is formed (Figure 1). Two of the isotopes in this chain, polonium 218 and polonium 214, also emit alpha particles.5–7
Radon is primarily formed in soil. Its most important precursor, uranium 238, is ubiquitous, found in most soils and rocks in various concentrations. Radon can also be found in surface water, metal mines (uranium, phosphorus, silver, gold), residue of coal combustion, and natural gas.
Outdoor levels are usually much lower than indoor levels, as radon dissipates very quickly. Indoor radon mostly comes from the soil under the house or building, but it can also originate from coal combustion, gas appliances, and water (especially from private wells). In municipal water systems or surface reservoirs, most of the radon dissipates into the air or decays before the water reaches homes.8,9
Radon’s only commercial application in the United States is in calibrating measuring instruments. In the past, it was used in radiography and to treat cancer but was later replaced by other radiation sources that cost less and pose less hazard of alpha radiation.10
HOW RADON CAN HARM
Alpha particles, emitted by radon 222 and its progenies polonium 218 and polonium 214, are highly effective in damaging tissues. Although they do not travel far or fast, with their two protons and two neutrons, alpha particles are heavy and therefore can cause considerable damage at short range. Although alpha particles can be stopped by a thin barrier such as a piece of paper or the skin, if the source is inhaled or ingested and lodges against mucosal linings, the alpha particles emitted can destroy cells.11
The main route of radon exposure is by inhalation. Since radon is biologically inert, it is readily exhaled after it reaches the lungs. However, radon’s progenies can also be inhaled, either as free particles or attached to airborne particles such as dust, which they tend to attract as a result of their charged state. This attached fraction is believed to be more carcinogenic because it tends to deposit on the respiratory epithelium, notably in the carinae of bronchi. The smaller the dust particle, the deeper it can travel into the lung. The radiation emissions damage the genetic material of cells lining the airways, with the potential to result in lung cancer if the repair process is incomplete.5,8,9
Other routes of exposure include ingestion and dermal exposure. Radon and its progenies can be swallowed in drinking water, passing through the stomach walls and bowels and entering the blood.12 Dermal exposure is not considered a significant route unless the dermis is exposed, since in usual circumstances the skin protects the body from alpha radiation.13
Possible biologic mechanisms by which radon exposure might increase the risk of cancer include gene mutations, chromosome aberrations, generation of reactive oxygen species, up- or down-regulation of cytokines, and production of proteins associated with cell-cycle regulation.14–16
HOW IS RADON MEASURED?
Several devices are commercially available to measure radon levels at home. The most common ones are activated charcoal detectors, electret ion chambers, alpha-track detectors, electronic integrating devices, and continuous monitors. There is no evidence that one device is better than another, but devices that measure radon gas are usually preferred over those that measure decay products because they are simpler to use and more cost-effective. These devices are divided into those used for short-term testing (2–90 days) and long-term testing (Table 1).17
Radon levels can be expressed as follows:
Working levels. One working level (WL) is any combination of radon progeny in 1 L of air that ultimately releases 1.3 × 105 MeV of alpha energy during decay. In studies of miners, the radon progeny concentrations are generally expressed in WL. The cumulative exposure of an individual to this concentration over a “working month” of 170 hours is defined as a working level month (WLM).
Picocuries per liter. In the United States, the rate of decay is commonly reported in picocuries per liter (pCi/L): 1 pCi/L translates to 0.005 WL under usual conditions. The outdoor radon level is normally around 0.4 pCi/L.
Becquerel per cubic meter (Bq/m3) is an International System unit of measure: 1 WL corresponds to 3.7 × 103 Bq/m3, and 1 pCi/L is equivalent to 37 Bq/m3.
Different areas have different radon levels
The Indoor Radon Abatement Act of 1988 helped identify areas in the United States that have the potential for elevated indoor radon levels. An estimated 6 million homes have concentrations greater than 4 pCi/L.
To assist in implementing radon-reducing strategies and allocation of resources, the EPA has created a map (Figure 2) that classifies counties according to the predicted indoor level.18
WHAT IS THE RELATIONSHIP BETWEEN RADON AND LUNG CANCER?
Determining the degree to which radon exposure contributes to lung cancer is a complex task. Radon can be found nearly everywhere, and there are diurnal, seasonal, and random year-to-year variations in the concentration of radon in indoor air.
A minority view
Not everyone agrees that radon is completely bad. For centuries, people have flocked to spas to “take the waters,” and the water at many of these spas has been found to contain radon. In the early 20th century, radiation was touted as having medicinal benefits, and people in many places in the world still go to “radon spas” (some of them in abandoned uranium mines) to help treat conditions such as arthritis and to feel invigorated and energized.
In 2006, a report by Zdrojewicz and Strzelczyk19 urged the medical community to keep an open mind about the possibility that radon exposure may be beneficial in very low doses, perhaps by stimulating repair mechanisms. This concept, called hormesis, differs from the mainstream view that cancer risk rises linearly with radiation dose, with no minimum threshold level (see below).
Risk in miners
As early as in the 16th century, metal miners in central Europe were noted to have a high rate of death from respiratory disease. Radon was discovered in 1900, and in the 20th century lung cancer was linked to high levels of radon detected in uranium mines.
Several small studies of underground miners exposed to high concentrations of radon consistently demonstrated an increased risk of lung cancer.
The Committee on the Biological Effects of Ionizing Radiation (BEIR VI 1999) reviewed 11 major cohort studies of miners. The studies included more than 60,000 miners in Europe, North America, Asia, and Australia, of whom 2,600 died of lung cancer. Lung cancer rates increased linearly with cumulative radon exposure, and the estimated average increase in the lung cancer death rate per WLM in the combined studies was 0.44% (95% confidence interval [CI] 0.20–1.00%). The percentage increase in the lung cancer death rate per WLM varied with time since exposure, with the highest increase in risk during the 5 to 14 years after exposure.4,17 Furthermore, the increase in risk was higher in younger miners, who were exposed to a relatively low radon concentration.
Risk in the general population
The magnitude of the risk in miners led to concern about radon exposure as a cause of lung cancer in the general population. Statistical models were generated that suggested a causal link between radon exposure and lung cancer. Although extrapolation of the results from miners caused controversy, the BEIR VI estimation of risk was validated by studies in the general population.7,20–23
Since the 1980s, several small case-control studies with limited power examined the relationship between indoor radon and lung cancer in the general population. In these studies, individuals who had developed lung cancer were compared with controls who had not developed the disease but who otherwise represented the population from which the cases of lung cancer came.
To improve the statistical power, the investigators of the major studies in Europe, North America, and China pooled the results in separate analyses (Table 2).7,20–23 The average radon concentration to which each individual had been exposed over the previous decades was estimated by measuring the radon concentration at their present and previous homes. On the basis of information from the uranium miners, these studies assumed that the period of exposure was the 30 years ending 5 years before the diagnosis or at death from lung cancer.
The results provided convincing evidence that radon exposure is a cause of lung cancer in the general population and substantiated the extrapolation from the studies of miners. Further, the results of all three pooled analyses were consistent with a linear dose-response relationship with no threshold, suggesting an increased risk of lung cancer even with a radon level below 4 pCi/L (200 Bq/m3), which is the concentration at which mitigation actions are recommended in many countries.17
The North American pooled analysis included 3,662 cases and 4,966 controls from seven studies in the United States and Canada. When data from all studies were combined, the risk of lung cancer was found to increase by 11% per 100-Bq/m3 (about 2.7-pCi/L) increase in measured radon concentration (95% CI 0%–28%). The estimated increase in lung cancer was independent of age, sex, or smoking history.7,20
The Chinese pooled data22 demonstrated a 13% (95% CI 1%–36%) increased risk per 100 Bq/m3.
In the European study, the risk of lung cancer increased by 8% per 100 Bq/m3 (95% CI 3%–16%). The European investigators repeated the analysis, taking into account the random year-to-year variability in measured radon concentration, finding the final estimated risk was an increase of 16% per 100 Bq/m3 using long-term average concentration.21
The combined estimate21,24 from the three pooling studies based on measured radon concentration is an increased risk of lung cancer of 10% per 100 Bq/m3.
Synergistic risk with smoking
Radon exposure was independently associated with lung cancer, and the relationship with cigarette smoking is believed to be synergistic. The radon progeny particles attach themselves to smoke and dust and are then deposited in the bronchial epithelium.25
In the pool of European case-control studies, the cancer risk for current smokers of 15 to 24 cigarettes per day relative to that in never-smokers was 25.8 (95% CI 21–31). Assuming that in the same analysis the lung cancer risk increased by 16% per 100 Bq/m3 of usual radon concentration regardless of smoking status, the cumulative absolute risk by age 75 would be 0.67% in those who never smoked and 16% in smokers at usual radon levels of 400 Bq/m3 (11 pCi/L).21
Rates of all lung cancer subtypes increased
Radon exposure is not associated with a specific histologic subtype of lung cancer. It has been speculated that the incidence of the small-cell subtype might be slightly increased because radon tends to deposit in the more central bronchial carinae.20,21 However, all subtypes have been described in association with radon, the most common being adenocarcinoma and squamous cell carcinoma.26–28
EFFECT OF MITIGATION MEASURES
The US Surgeon General and the EPA recommend that all homes be tested.18 Short-term tests should be used first, keeping in mind that diurnal and seasonal variations may occur.
The World Health Organization has proposed a reference level of 100 Bq/m3 (2.7 pCi/L) to minimize health hazards from indoor radon exposure.17 If this level cannot be reached under the country-specific conditions, the chosen reference level should not exceed 300 Bq/m3 (8 pCi/L).
In the United States, if the result of home testing is higher than 4 pCi/L, a follow-up measurement should be done using a different short-term test or a long-term test. If the follow-up result confirms a level of more than 4 pCi/L, mitigating actions are recommended. The goal is to reduce the indoor radon level as much as possible—down to zero or at least comparable to outdoor levels (national average 0.4 pCi/L).18
A variety of radon mitigation strategies have been used, with different rates of efficacy (Table 3). The optimal strategy depends on the likely source or cause, construction characteristics, soil, and climate.29 Table 4 lists resources for the general public about testing and mitigation measures.
How beneficial is radon mitigation?
Although it is logical to try to reduce the indoor radon concentration, there is no strong evidence yet that this intervention decreases the incidence of lung cancer in the general population.
Using the BEIR VI risk model, Méndez et al30 estimated a 21% reduction in the annual radon-related lung cancer mortality rate by 2100 if all households were compliant with government recommendations (mitigation actions at levels of 4 pCi/L) and assuming that the percentage of cigarette smokers remained constant.
On the other hand, if the number of smokers continues to decline, the benefits from radon mitigation may be less. The expected benefit from mitigation in this scenario is a reduction of 12% in annual radon-related deaths by the year 2100.30 However, it will be challenging to determine whether the expected decline in the incidence of lung cancer and lung cancer deaths is truly attributable to mitigation measures.
MANAGING PATIENTS EXPOSED TO RADON
Screen for lung cancer in smokers only
The National Lung Screening Study (NLST) was a large multicenter trial of annual low-dose computed tomography (CT) to screen for lung cancer in a cohort at high risk: age 55 to 74, at least a 30 pack-year history of smoking in a current smoker, or a former smoker who quit within the past 15 years. The trial demonstrated a 20% reduction in lung cancer deaths in the CT screening group.31
Since the publication of the NLST results, many societies have endorsed screening for lung cancer with low-dose CT using the study criteria. The National Comprehensive Cancer Network (NCCN) expanded these criteria and has recommended screening in patients over age 50 who have a history of smoking and one additional risk factor, such as radon exposure.
However, radon exposure has not been incorporated into a lung cancer risk-prediction model, and there is no empirical evidence suggesting that people who have such a history would benefit from screening.32,33 The joint guidelines of the American College of Chest Physicians and American Society of Clinical Oncology recommend annual low-dose CT screening only for patients who meet the NLST criteria.34
What to do about indeterminate lung nodules
The widely used guidelines from the Fleischner Society35 on how to manage small lung nodules stratify patients into groups at low and high risk of developing lung cancer on the basis of risk factors. The guidelines apply to adults age 35 and older in whom an indeterminate solid nodule was recently detected.
If a patient is at high risk, the recommended approach includes follow-up in shorter intervals depending on the nodule size. History of smoking is recognized as a major risk factor, and the statement also lists family history and exposure to asbestos, uranium, and radon.35
Although the association of radon with lung cancer has been shown in epidemiologic studies, radon exposure has not been included in validated statistical models that assess the probability that an indeterminate lung nodule is malignant. We would expect the risk to be higher in miners, who suffer a more intense exposure to higher levels of radon, than in the general population, which has a low and constantly variable residential exposure. Furthermore, there are no data to support a more aggressive follow-up approach in patients with indeterminate lung nodules and a history of radon exposure.
RADON AND OTHER CANCERS
When a person is exposed to radon, the bronchial epithelium receives the highest dose of ionizing radiation, but other organs such as the kidneys, stomach, and bone marrow may receive doses as well, although lower. Several studies have looked into possible associations, but there is no strong evidence to suggest an increased mortality rate related to radon from cancers other than lung.24,36 However, there seems to be a positive association between radon and the incidence of lymphoproliferative disorders in uranium miners.37,38
Radon can be measured in drinking water, and a few studies have looked at a possible association with gastrointestinal malignancies. The results did not reveal a consistent positive correlation.39,40 The risk of cancer from exposure to radon in the public water supply is likely small and mostly from the transfer of radon particles into the air and not from drinking the water. On the other hand, the risk could be higher with private wells, where radon levels are variable and are possibly higher than from public sources.41
DATA ARE INSUFFICIENT TO GUIDE MANAGEMENT
Radon is a naturally occurring and ubiquitous radioactive gas that can cause tissue damage. Cohort and case-control studies have demonstrated that radon exposure is associated with increased risk of lung cancer. It is recommended that radon levels be measured in every home in the United States and mitigation measures instituted if levels exceed 4 pCi/L.
There are insufficient data to help guide the management of patients with a history of radon exposure, and prospective studies are needed to better understand the individual risk of developing lung cancer and the appropriate management of such patients.
Smoking cessation is an integral part of lung cancer risk reduction from radon exposure.
In 1984, a worker at a Pennsylvania nuclear power plant triggered the radiation detector as he was getting ready to go home. This would not be unusual for such a facility, but there was no nuclear fuel on site at the time. The alarm went off every time he left work.
One day, he triggered the alarm as he crossed the detector on arriving at the plant, leading him to suspect that he was bringing radiation from home. He eventually convinced the plant’s health physicists to check his home, although at first they were opposed to the idea. The results revealed high concentrations of radon everywhere, especially in his basement.
Radon was already known to be associated with health risks in underground miners at that time. This event revealed that a naturally occurring radioactive gas could be found in households at potentially hazardous concentrations.
The incident captured the public’s attention, and the Environmental Protection Agency (EPA) and the US Centers for Disease Control and Prevention (CDC) recommended that nearly all homes be tested.1,2 In 1988, the International Agency for Research on Cancer classified radon as a human carcinogen, and Congress passed the Indoor Radon Abatement Act in response to growing concern over health risks.3 This law funded state and federal measures to survey schools and federal buildings for radon levels, to educate citizens, and to develop programs for technical assistance. The long-term goal was to reduce indoor levels nationwide to no more than outdoor levels.
Radon is still considered an important public health hazard. From 15,000 to 21,000 people are estimated to die of lung cancer as a result of radon exposure each year in the United States, making it the second most common cause of lung cancer, behind smoking.4
Considering the relevance of this issue, this article will review the unique characteristics of radon as a risk factor for lung cancer.
WHAT IS RADON?
Radon is a noble gas that occurs naturally as a decay product of uranium 238 and thorium 232. It is colorless, tasteless, and imperceptible to our senses. Its most common isotope is radon 222 (222Rn), which has a half-life of 3.8 days and decays by emitting an alpha particle to become polonium 218. The decay chain continues through several intermediate steps until the stable isotope lead 206 is formed (Figure 1). Two of the isotopes in this chain, polonium 218 and polonium 214, also emit alpha particles.5–7
Radon is primarily formed in soil. Its most important precursor, uranium 238, is ubiquitous, found in most soils and rocks in various concentrations. Radon can also be found in surface water, metal mines (uranium, phosphorus, silver, gold), residue of coal combustion, and natural gas.
Outdoor levels are usually much lower than indoor levels, as radon dissipates very quickly. Indoor radon mostly comes from the soil under the house or building, but it can also originate from coal combustion, gas appliances, and water (especially from private wells). In municipal water systems or surface reservoirs, most of the radon dissipates into the air or decays before the water reaches homes.8,9
Radon’s only commercial application in the United States is in calibrating measuring instruments. In the past, it was used in radiography and to treat cancer but was later replaced by other radiation sources that cost less and pose less hazard of alpha radiation.10
HOW RADON CAN HARM
Alpha particles, emitted by radon 222 and its progenies polonium 218 and polonium 214, are highly effective in damaging tissues. Although they do not travel far or fast, with their two protons and two neutrons, alpha particles are heavy and therefore can cause considerable damage at short range. Although alpha particles can be stopped by a thin barrier such as a piece of paper or the skin, if the source is inhaled or ingested and lodges against mucosal linings, the alpha particles emitted can destroy cells.11
The main route of radon exposure is by inhalation. Since radon is biologically inert, it is readily exhaled after it reaches the lungs. However, radon’s progenies can also be inhaled, either as free particles or attached to airborne particles such as dust, which they tend to attract as a result of their charged state. This attached fraction is believed to be more carcinogenic because it tends to deposit on the respiratory epithelium, notably in the carinae of bronchi. The smaller the dust particle, the deeper it can travel into the lung. The radiation emissions damage the genetic material of cells lining the airways, with the potential to result in lung cancer if the repair process is incomplete.5,8,9
Other routes of exposure include ingestion and dermal exposure. Radon and its progenies can be swallowed in drinking water, passing through the stomach walls and bowels and entering the blood.12 Dermal exposure is not considered a significant route unless the dermis is exposed, since in usual circumstances the skin protects the body from alpha radiation.13
Possible biologic mechanisms by which radon exposure might increase the risk of cancer include gene mutations, chromosome aberrations, generation of reactive oxygen species, up- or down-regulation of cytokines, and production of proteins associated with cell-cycle regulation.14–16
HOW IS RADON MEASURED?
Several devices are commercially available to measure radon levels at home. The most common ones are activated charcoal detectors, electret ion chambers, alpha-track detectors, electronic integrating devices, and continuous monitors. There is no evidence that one device is better than another, but devices that measure radon gas are usually preferred over those that measure decay products because they are simpler to use and more cost-effective. These devices are divided into those used for short-term testing (2–90 days) and long-term testing (Table 1).17
Radon levels can be expressed as follows:
Working levels. One working level (WL) is any combination of radon progeny in 1 L of air that ultimately releases 1.3 × 105 MeV of alpha energy during decay. In studies of miners, the radon progeny concentrations are generally expressed in WL. The cumulative exposure of an individual to this concentration over a “working month” of 170 hours is defined as a working level month (WLM).
Picocuries per liter. In the United States, the rate of decay is commonly reported in picocuries per liter (pCi/L): 1 pCi/L translates to 0.005 WL under usual conditions. The outdoor radon level is normally around 0.4 pCi/L.
Becquerel per cubic meter (Bq/m3) is an International System unit of measure: 1 WL corresponds to 3.7 × 103 Bq/m3, and 1 pCi/L is equivalent to 37 Bq/m3.
Different areas have different radon levels
The Indoor Radon Abatement Act of 1988 helped identify areas in the United States that have the potential for elevated indoor radon levels. An estimated 6 million homes have concentrations greater than 4 pCi/L.
To assist in implementing radon-reducing strategies and allocation of resources, the EPA has created a map (Figure 2) that classifies counties according to the predicted indoor level.18
WHAT IS THE RELATIONSHIP BETWEEN RADON AND LUNG CANCER?
Determining the degree to which radon exposure contributes to lung cancer is a complex task. Radon can be found nearly everywhere, and there are diurnal, seasonal, and random year-to-year variations in the concentration of radon in indoor air.
A minority view
Not everyone agrees that radon is completely bad. For centuries, people have flocked to spas to “take the waters,” and the water at many of these spas has been found to contain radon. In the early 20th century, radiation was touted as having medicinal benefits, and people in many places in the world still go to “radon spas” (some of them in abandoned uranium mines) to help treat conditions such as arthritis and to feel invigorated and energized.
In 2006, a report by Zdrojewicz and Strzelczyk19 urged the medical community to keep an open mind about the possibility that radon exposure may be beneficial in very low doses, perhaps by stimulating repair mechanisms. This concept, called hormesis, differs from the mainstream view that cancer risk rises linearly with radiation dose, with no minimum threshold level (see below).
Risk in miners
As early as in the 16th century, metal miners in central Europe were noted to have a high rate of death from respiratory disease. Radon was discovered in 1900, and in the 20th century lung cancer was linked to high levels of radon detected in uranium mines.
Several small studies of underground miners exposed to high concentrations of radon consistently demonstrated an increased risk of lung cancer.
The Committee on the Biological Effects of Ionizing Radiation (BEIR VI 1999) reviewed 11 major cohort studies of miners. The studies included more than 60,000 miners in Europe, North America, Asia, and Australia, of whom 2,600 died of lung cancer. Lung cancer rates increased linearly with cumulative radon exposure, and the estimated average increase in the lung cancer death rate per WLM in the combined studies was 0.44% (95% confidence interval [CI] 0.20–1.00%). The percentage increase in the lung cancer death rate per WLM varied with time since exposure, with the highest increase in risk during the 5 to 14 years after exposure.4,17 Furthermore, the increase in risk was higher in younger miners, who were exposed to a relatively low radon concentration.
Risk in the general population
The magnitude of the risk in miners led to concern about radon exposure as a cause of lung cancer in the general population. Statistical models were generated that suggested a causal link between radon exposure and lung cancer. Although extrapolation of the results from miners caused controversy, the BEIR VI estimation of risk was validated by studies in the general population.7,20–23
Since the 1980s, several small case-control studies with limited power examined the relationship between indoor radon and lung cancer in the general population. In these studies, individuals who had developed lung cancer were compared with controls who had not developed the disease but who otherwise represented the population from which the cases of lung cancer came.
To improve the statistical power, the investigators of the major studies in Europe, North America, and China pooled the results in separate analyses (Table 2).7,20–23 The average radon concentration to which each individual had been exposed over the previous decades was estimated by measuring the radon concentration at their present and previous homes. On the basis of information from the uranium miners, these studies assumed that the period of exposure was the 30 years ending 5 years before the diagnosis or at death from lung cancer.
The results provided convincing evidence that radon exposure is a cause of lung cancer in the general population and substantiated the extrapolation from the studies of miners. Further, the results of all three pooled analyses were consistent with a linear dose-response relationship with no threshold, suggesting an increased risk of lung cancer even with a radon level below 4 pCi/L (200 Bq/m3), which is the concentration at which mitigation actions are recommended in many countries.17
The North American pooled analysis included 3,662 cases and 4,966 controls from seven studies in the United States and Canada. When data from all studies were combined, the risk of lung cancer was found to increase by 11% per 100-Bq/m3 (about 2.7-pCi/L) increase in measured radon concentration (95% CI 0%–28%). The estimated increase in lung cancer was independent of age, sex, or smoking history.7,20
The Chinese pooled data22 demonstrated a 13% (95% CI 1%–36%) increased risk per 100 Bq/m3.
In the European study, the risk of lung cancer increased by 8% per 100 Bq/m3 (95% CI 3%–16%). The European investigators repeated the analysis, taking into account the random year-to-year variability in measured radon concentration, finding the final estimated risk was an increase of 16% per 100 Bq/m3 using long-term average concentration.21
The combined estimate21,24 from the three pooling studies based on measured radon concentration is an increased risk of lung cancer of 10% per 100 Bq/m3.
Synergistic risk with smoking
Radon exposure was independently associated with lung cancer, and the relationship with cigarette smoking is believed to be synergistic. The radon progeny particles attach themselves to smoke and dust and are then deposited in the bronchial epithelium.25
In the pool of European case-control studies, the cancer risk for current smokers of 15 to 24 cigarettes per day relative to that in never-smokers was 25.8 (95% CI 21–31). Assuming that in the same analysis the lung cancer risk increased by 16% per 100 Bq/m3 of usual radon concentration regardless of smoking status, the cumulative absolute risk by age 75 would be 0.67% in those who never smoked and 16% in smokers at usual radon levels of 400 Bq/m3 (11 pCi/L).21
Rates of all lung cancer subtypes increased
Radon exposure is not associated with a specific histologic subtype of lung cancer. It has been speculated that the incidence of the small-cell subtype might be slightly increased because radon tends to deposit in the more central bronchial carinae.20,21 However, all subtypes have been described in association with radon, the most common being adenocarcinoma and squamous cell carcinoma.26–28
EFFECT OF MITIGATION MEASURES
The US Surgeon General and the EPA recommend that all homes be tested.18 Short-term tests should be used first, keeping in mind that diurnal and seasonal variations may occur.
The World Health Organization has proposed a reference level of 100 Bq/m3 (2.7 pCi/L) to minimize health hazards from indoor radon exposure.17 If this level cannot be reached under the country-specific conditions, the chosen reference level should not exceed 300 Bq/m3 (8 pCi/L).
In the United States, if the result of home testing is higher than 4 pCi/L, a follow-up measurement should be done using a different short-term test or a long-term test. If the follow-up result confirms a level of more than 4 pCi/L, mitigating actions are recommended. The goal is to reduce the indoor radon level as much as possible—down to zero or at least comparable to outdoor levels (national average 0.4 pCi/L).18
A variety of radon mitigation strategies have been used, with different rates of efficacy (Table 3). The optimal strategy depends on the likely source or cause, construction characteristics, soil, and climate.29 Table 4 lists resources for the general public about testing and mitigation measures.
How beneficial is radon mitigation?
Although it is logical to try to reduce the indoor radon concentration, there is no strong evidence yet that this intervention decreases the incidence of lung cancer in the general population.
Using the BEIR VI risk model, Méndez et al30 estimated a 21% reduction in the annual radon-related lung cancer mortality rate by 2100 if all households were compliant with government recommendations (mitigation actions at levels of 4 pCi/L) and assuming that the percentage of cigarette smokers remained constant.
On the other hand, if the number of smokers continues to decline, the benefits from radon mitigation may be less. The expected benefit from mitigation in this scenario is a reduction of 12% in annual radon-related deaths by the year 2100.30 However, it will be challenging to determine whether the expected decline in the incidence of lung cancer and lung cancer deaths is truly attributable to mitigation measures.
MANAGING PATIENTS EXPOSED TO RADON
Screen for lung cancer in smokers only
The National Lung Screening Study (NLST) was a large multicenter trial of annual low-dose computed tomography (CT) to screen for lung cancer in a cohort at high risk: age 55 to 74, at least a 30 pack-year history of smoking in a current smoker, or a former smoker who quit within the past 15 years. The trial demonstrated a 20% reduction in lung cancer deaths in the CT screening group.31
Since the publication of the NLST results, many societies have endorsed screening for lung cancer with low-dose CT using the study criteria. The National Comprehensive Cancer Network (NCCN) expanded these criteria and has recommended screening in patients over age 50 who have a history of smoking and one additional risk factor, such as radon exposure.
However, radon exposure has not been incorporated into a lung cancer risk-prediction model, and there is no empirical evidence suggesting that people who have such a history would benefit from screening.32,33 The joint guidelines of the American College of Chest Physicians and American Society of Clinical Oncology recommend annual low-dose CT screening only for patients who meet the NLST criteria.34
What to do about indeterminate lung nodules
The widely used guidelines from the Fleischner Society35 on how to manage small lung nodules stratify patients into groups at low and high risk of developing lung cancer on the basis of risk factors. The guidelines apply to adults age 35 and older in whom an indeterminate solid nodule was recently detected.
If a patient is at high risk, the recommended approach includes follow-up in shorter intervals depending on the nodule size. History of smoking is recognized as a major risk factor, and the statement also lists family history and exposure to asbestos, uranium, and radon.35
Although the association of radon with lung cancer has been shown in epidemiologic studies, radon exposure has not been included in validated statistical models that assess the probability that an indeterminate lung nodule is malignant. We would expect the risk to be higher in miners, who suffer a more intense exposure to higher levels of radon, than in the general population, which has a low and constantly variable residential exposure. Furthermore, there are no data to support a more aggressive follow-up approach in patients with indeterminate lung nodules and a history of radon exposure.
RADON AND OTHER CANCERS
When a person is exposed to radon, the bronchial epithelium receives the highest dose of ionizing radiation, but other organs such as the kidneys, stomach, and bone marrow may receive doses as well, although lower. Several studies have looked into possible associations, but there is no strong evidence to suggest an increased mortality rate related to radon from cancers other than lung.24,36 However, there seems to be a positive association between radon and the incidence of lymphoproliferative disorders in uranium miners.37,38
Radon can be measured in drinking water, and a few studies have looked at a possible association with gastrointestinal malignancies. The results did not reveal a consistent positive correlation.39,40 The risk of cancer from exposure to radon in the public water supply is likely small and mostly from the transfer of radon particles into the air and not from drinking the water. On the other hand, the risk could be higher with private wells, where radon levels are variable and are possibly higher than from public sources.41
DATA ARE INSUFFICIENT TO GUIDE MANAGEMENT
Radon is a naturally occurring and ubiquitous radioactive gas that can cause tissue damage. Cohort and case-control studies have demonstrated that radon exposure is associated with increased risk of lung cancer. It is recommended that radon levels be measured in every home in the United States and mitigation measures instituted if levels exceed 4 pCi/L.
There are insufficient data to help guide the management of patients with a history of radon exposure, and prospective studies are needed to better understand the individual risk of developing lung cancer and the appropriate management of such patients.
Smoking cessation is an integral part of lung cancer risk reduction from radon exposure.
- Berreby D. The radon raiders: turning perils into profits. The New York Times 1987. www.nytimes.com/1987/07/26/business/the-radon-raiders-turning-perils-into-profits.html?src=pm&pagewanted=1. Accessed August 5, 2014.
- Lewis RK. A history of radon—1470 to 1984. www.ohio-radonpro.com/Radon_History.html. Accessed August 5, 2014.
- World Health Organization (WHO). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Manmade mineral fibres and radon. Summary of data reported and evaluation. http://monographs.iarc.fr/ENG/Monographs/vol43/volume43.pdf. Accessed August 5, 2014.
- Committee on Health Risks of Exposure to Radon (BEIR VI). Health effects of exposure to radon: BEIR VI. Washington, DC: National Academies Press; 1999.
- Samet JM. Radon and lung cancer. J Natl Cancer Inst 1989; 81:745–757.
- Lewis RJ, Lewis Sr RJ. Hawley’s condensed chemical dictionary. 14thed. New York: Wiley-Interscience; 2001.
- Krewski D, Lubin JH, Zielinski JM, et al. Residential radon and risk of lung cancer: a combined analysis of 7 North American case-control studies. Epidemiology 2005; 16:137–145.
- Darby S, Hill D, Doll R. Radon: a likely carcinogen at all exposures. Ann Oncol 2001; 12:1341–1351.
- Sethi TK, El-Ghamry MN, Kloecker GH. Radon and lung cancer. Clin Adv Hematol Oncol 2012; 10:157–164.
- Morrison A. Use of radon for industrial radiography. Can J Res 1945; 23:413–419.
- Narayanan PK, Goodwin EH, Lehnert BE. Alpha particles initiate biological production of superoxide anions and hydrogen peroxide in human cells. Cancer Res 1997; 57:3963–3971.
- Ishikawa T, Narazaki Y, Yasuoka Y, Tokonami S, Yamada Y. Bio-kinetics of radon ingested from drinking water. Radiat Prot Dosimetry 2003; 105:65–70.
- Ishikawa T, Yamada Y, Fukutsu K, Tokonami S. Deposition and clearance for radon progeny in the human respiratory tract. Radiat Prot Dosimetry 2003; 105:143–148.
- Farkas A, Hofmann W, Balásházy I, Szoke I, Madas BG, Moustafa M. Effect of site-specific bronchial radon progeny deposition on the spatial and temporal distributions of cellular responses. Radiat Environ Biophys 2011; 50:281–297.
- Robertson A, Allen J, Laney R, Curnow A. The cellular and molecular carcinogenic effects of radon exposure: a review. Int J Mol Sci 2013; 14:14024–14063.
- Chauhan V, Howland M, Wilkins R. Effects of alpha-particle radiation on microRNA responses in human cell-lines. Open Biochem J 2012; 6:16–22.
- World Health Organization (WHO). WHO handbook on indoor radon: a public health perspective; 2009. www.nrsb.org/pdf/WHO%20Radon%20Handbook.pdf. Accessed August 5, 2014.
- United States Environmental Protection Agency (EPA). www.epa.gov/radon/. Accessed August 5, 2014.
- Zdrojewicz Z, Strzelczyk JJ. Radon treatment controversy. Dose Response 2006; 4:106–118.
- Krewski D, Lubin JH, Zielinski JM, et al. A combined analysis of North American case-control studies of residential radon and lung cancer. J Toxicol Environ Health A 2006; 69:533–597.
- Darby S, Hill D, Auvinen A, et al. Radon in homes and risk of lung cancer: collaborative analysis of individual data from 13 European case-control studies. BMJ 2005; 330:223.
- Lubin JH, Wang ZY, Boice JD, et al. Risk of lung cancer and residential radon in China: pooled results of two studies. Int J Cancer 2004; 109:132–137.
- Darby S, Hill D, Deo H, et al. Residential radon and lung cancer—detailed results of a collaborative analysis of individual data on 7,148 persons with lung cancer and 14,208 persons without lung cancer from 13 epidemiologic studies in Europe. Scand J Work Environ Health 2006; 32(suppl 1):1–83.
- Darby SC, Whitley E, Howe GR, et al. Radon and cancers other than lung cancer in underground miners: a collaborative analysis of 11 studies. J Natl Cancer Inst 1995; 87:378–384.
- Baias PF, Hofmann W, Winkler-Heil R, Cosma C, Duliu OG. Lung dosimetry for inhaled radon progeny in smokers. Radiat Prot Dosimetry 2010; 138:111–118.
- Land CE, Shimosato Y, Saccomanno G, et al. Radiation-associated lung cancer: a comparison of the histology of lung cancers in uranium miners and survivors of the atomic bombings of Hiroshima and Nagasaki. Radiat Res 1993; 134:234–243.
- Kreuzer M, Müller KM, Brachner A, et al. Histopathologic findings of lung carcinoma in German uranium miners. Cancer 2000; 89:2613–2621.
- Saccomanno G, Auerbach O, Kuschner M, et al. A comparison between the localization of lung tumors in uranium miners and in nonminers from 1947 to 1991. Cancer 1996; 77:1278–1283.
- Rahman NM, Tracy BL. Radon control systems in existing and new construction: a review. Radiat Prot Dosimetry 2009; 135:243–255.
- Méndez D, Alshanqeety O, Warner KE, Lantz PM, Courant PN. The impact of declining smoking on radon-related lung cancer in the United States. Am J Public Health 2011; 101:310–314.
- National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365:395–409.
- Wood DE, Eapen GA, Ettinger DS, et al. Lung cancer screening. J Natl Compr Canc Netw 2012; 10:240–265.
- Ettinger DS, Akerley W, Borghaei H, et al; NCCN (National Comprehensive Cancer Network). Non-small cell lung cancer. J Natl Compr Canc Netw 2012; 10:1236–1271.
- Detterbeck FC, Mazzone PJ, Naidich DP, Bach PB. Screening for lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143(suppl 5):e78S–e92S.
- MacMahon H, Austin JH, Gamsu G, et al; Fleischner Society. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005; 237:395–400.
- Darby SC, Radford EP, Whitley E. Radon exposure and cancers other than lung cancer in Swedish iron miners. Environ Health Perspect 1995; 103(suppl 2):45–47.
- Laurier D, Tirmarche M, Mitton N, et al. An update of cancer mortality among the French cohort of uranium miners: extended follow-up and new source of data for causes of death. Eur J Epidemiol 2004; 19:139–146.
- Rericha V, Kulich M, Rericha R, Shore DL, Sandler DP. Incidence of leukemia, lymphoma, and multiple myeloma in Czech uranium miners: a case-cohort study. Environ Health Perspect 2006; 114:818–822.
- Auvinen A, Salonen L, Pekkanen J, Pukkala E, Ilus T, Kurttio P. Radon and other natural radionuclides in drinking water and risk of stomach cancer: a case-cohort study in Finland. Int J Cancer 2005; 114:109–113.
- Kjellberg S, Wiseman JS. The relationship of radon to gastrointestinal malignancies. Am Surg 1995; 61:822–825.
- Cappello MA, Ferraro A, Mendelsohn AB, Prehn AW. Radon-contaminated drinking water from private wells: an environmental health assessment examining a rural Colorado mountain community’s exposure. J Environ Health 2013; 76:18–24.
- Berreby D. The radon raiders: turning perils into profits. The New York Times 1987. www.nytimes.com/1987/07/26/business/the-radon-raiders-turning-perils-into-profits.html?src=pm&pagewanted=1. Accessed August 5, 2014.
- Lewis RK. A history of radon—1470 to 1984. www.ohio-radonpro.com/Radon_History.html. Accessed August 5, 2014.
- World Health Organization (WHO). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Manmade mineral fibres and radon. Summary of data reported and evaluation. http://monographs.iarc.fr/ENG/Monographs/vol43/volume43.pdf. Accessed August 5, 2014.
- Committee on Health Risks of Exposure to Radon (BEIR VI). Health effects of exposure to radon: BEIR VI. Washington, DC: National Academies Press; 1999.
- Samet JM. Radon and lung cancer. J Natl Cancer Inst 1989; 81:745–757.
- Lewis RJ, Lewis Sr RJ. Hawley’s condensed chemical dictionary. 14thed. New York: Wiley-Interscience; 2001.
- Krewski D, Lubin JH, Zielinski JM, et al. Residential radon and risk of lung cancer: a combined analysis of 7 North American case-control studies. Epidemiology 2005; 16:137–145.
- Darby S, Hill D, Doll R. Radon: a likely carcinogen at all exposures. Ann Oncol 2001; 12:1341–1351.
- Sethi TK, El-Ghamry MN, Kloecker GH. Radon and lung cancer. Clin Adv Hematol Oncol 2012; 10:157–164.
- Morrison A. Use of radon for industrial radiography. Can J Res 1945; 23:413–419.
- Narayanan PK, Goodwin EH, Lehnert BE. Alpha particles initiate biological production of superoxide anions and hydrogen peroxide in human cells. Cancer Res 1997; 57:3963–3971.
- Ishikawa T, Narazaki Y, Yasuoka Y, Tokonami S, Yamada Y. Bio-kinetics of radon ingested from drinking water. Radiat Prot Dosimetry 2003; 105:65–70.
- Ishikawa T, Yamada Y, Fukutsu K, Tokonami S. Deposition and clearance for radon progeny in the human respiratory tract. Radiat Prot Dosimetry 2003; 105:143–148.
- Farkas A, Hofmann W, Balásházy I, Szoke I, Madas BG, Moustafa M. Effect of site-specific bronchial radon progeny deposition on the spatial and temporal distributions of cellular responses. Radiat Environ Biophys 2011; 50:281–297.
- Robertson A, Allen J, Laney R, Curnow A. The cellular and molecular carcinogenic effects of radon exposure: a review. Int J Mol Sci 2013; 14:14024–14063.
- Chauhan V, Howland M, Wilkins R. Effects of alpha-particle radiation on microRNA responses in human cell-lines. Open Biochem J 2012; 6:16–22.
- World Health Organization (WHO). WHO handbook on indoor radon: a public health perspective; 2009. www.nrsb.org/pdf/WHO%20Radon%20Handbook.pdf. Accessed August 5, 2014.
- United States Environmental Protection Agency (EPA). www.epa.gov/radon/. Accessed August 5, 2014.
- Zdrojewicz Z, Strzelczyk JJ. Radon treatment controversy. Dose Response 2006; 4:106–118.
- Krewski D, Lubin JH, Zielinski JM, et al. A combined analysis of North American case-control studies of residential radon and lung cancer. J Toxicol Environ Health A 2006; 69:533–597.
- Darby S, Hill D, Auvinen A, et al. Radon in homes and risk of lung cancer: collaborative analysis of individual data from 13 European case-control studies. BMJ 2005; 330:223.
- Lubin JH, Wang ZY, Boice JD, et al. Risk of lung cancer and residential radon in China: pooled results of two studies. Int J Cancer 2004; 109:132–137.
- Darby S, Hill D, Deo H, et al. Residential radon and lung cancer—detailed results of a collaborative analysis of individual data on 7,148 persons with lung cancer and 14,208 persons without lung cancer from 13 epidemiologic studies in Europe. Scand J Work Environ Health 2006; 32(suppl 1):1–83.
- Darby SC, Whitley E, Howe GR, et al. Radon and cancers other than lung cancer in underground miners: a collaborative analysis of 11 studies. J Natl Cancer Inst 1995; 87:378–384.
- Baias PF, Hofmann W, Winkler-Heil R, Cosma C, Duliu OG. Lung dosimetry for inhaled radon progeny in smokers. Radiat Prot Dosimetry 2010; 138:111–118.
- Land CE, Shimosato Y, Saccomanno G, et al. Radiation-associated lung cancer: a comparison of the histology of lung cancers in uranium miners and survivors of the atomic bombings of Hiroshima and Nagasaki. Radiat Res 1993; 134:234–243.
- Kreuzer M, Müller KM, Brachner A, et al. Histopathologic findings of lung carcinoma in German uranium miners. Cancer 2000; 89:2613–2621.
- Saccomanno G, Auerbach O, Kuschner M, et al. A comparison between the localization of lung tumors in uranium miners and in nonminers from 1947 to 1991. Cancer 1996; 77:1278–1283.
- Rahman NM, Tracy BL. Radon control systems in existing and new construction: a review. Radiat Prot Dosimetry 2009; 135:243–255.
- Méndez D, Alshanqeety O, Warner KE, Lantz PM, Courant PN. The impact of declining smoking on radon-related lung cancer in the United States. Am J Public Health 2011; 101:310–314.
- National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365:395–409.
- Wood DE, Eapen GA, Ettinger DS, et al. Lung cancer screening. J Natl Compr Canc Netw 2012; 10:240–265.
- Ettinger DS, Akerley W, Borghaei H, et al; NCCN (National Comprehensive Cancer Network). Non-small cell lung cancer. J Natl Compr Canc Netw 2012; 10:1236–1271.
- Detterbeck FC, Mazzone PJ, Naidich DP, Bach PB. Screening for lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143(suppl 5):e78S–e92S.
- MacMahon H, Austin JH, Gamsu G, et al; Fleischner Society. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005; 237:395–400.
- Darby SC, Radford EP, Whitley E. Radon exposure and cancers other than lung cancer in Swedish iron miners. Environ Health Perspect 1995; 103(suppl 2):45–47.
- Laurier D, Tirmarche M, Mitton N, et al. An update of cancer mortality among the French cohort of uranium miners: extended follow-up and new source of data for causes of death. Eur J Epidemiol 2004; 19:139–146.
- Rericha V, Kulich M, Rericha R, Shore DL, Sandler DP. Incidence of leukemia, lymphoma, and multiple myeloma in Czech uranium miners: a case-cohort study. Environ Health Perspect 2006; 114:818–822.
- Auvinen A, Salonen L, Pekkanen J, Pukkala E, Ilus T, Kurttio P. Radon and other natural radionuclides in drinking water and risk of stomach cancer: a case-cohort study in Finland. Int J Cancer 2005; 114:109–113.
- Kjellberg S, Wiseman JS. The relationship of radon to gastrointestinal malignancies. Am Surg 1995; 61:822–825.
- Cappello MA, Ferraro A, Mendelsohn AB, Prehn AW. Radon-contaminated drinking water from private wells: an environmental health assessment examining a rural Colorado mountain community’s exposure. J Environ Health 2013; 76:18–24.
KEY POINTS
- Radon is a noble gas that occurs naturally as a decay product of uranium 238 and thorium 232.
- Radon 222 decays to polonium 218 and then, after several intermediate steps, to polonium 214, both of which emit alpha particles, which are highly effective in damaging tissues.
- Radon exposure is associated with increased lung cancer incidence in underground miners. In the general population, it is estimated to be the second most common cause of lung cancer, after cigarette smoking.
- There is no evidence yet of a benefit of lung cancer screening based on radon exposure.
The protein-sparing modified fast for obese patients with type 2 diabetes: What to expect
Eighty percent of people with type 2 diabetes mellitus are obese or overweight.1 Excess adipose tissue can lead to endocrine dysregulation,2 contributing to the pathogenesis of type 2 diabetes, and obesity is one of the strongest predictors of this disease.3
For obese people with type 2 diabetes, diet and exercise can lead to weight loss and many other benefits, such as better glycemic control, less insulin resistance, lower risk of diabetes-related comorbidities and complications, fewer diabetic medications needed, and lower health care costs.4–7 Intensive lifestyle interventions have also been shown to induce partial remission of diabetes and to prevent the onset of type 2 diabetes in people at high risk of it.5–7
A very-low-calorie diet is one of many dietary options available to patients with type 2 diabetes who are overweight or obese. The protein-sparing modified fast (PSMF) is a type of very-low-calorie diet with a high protein content and simultaneous restriction of carbohydrate and fat.8,9 It was developed in the 1970s, and since then various permutations have been used in weight loss and health care clinics worldwide.
MOSTLY PROTEIN, VERY LITTLE CARBOHYDRATE AND FAT
The PSMF is a medically supervised diet that provides less than 800 kcal/day during an initial intensive phase of about 6 months, followed by the gradual reintroduction of calories during a refeeding phase of about 6 to 8 weeks.10
During the intensive phase, patients obtain most of their calories from protein, approximately 1.2 to 1.5 g/kg of ideal body weight per day. At the same time, carbohydrate intake is restricted to less than 20 to 50 g/day; additional fats outside of protein sources are not allowed.9 Thus, the PSMF shares features of both very-low-calorie diets and very-low-carbohydrate ketogenic diets (eg, the Atkins diet), though some differences exist among the three (Figure 1).
Patients rapidly lose weight during the intensive phase, typically between 1 and 3 kg per week, with even greater losses during the first 2 weeks.8,9 Weight loss typically plateaus within 6 months, at which point patients begin the refeeding period. During refeeding, complex carbohydrates and low-glycemic, high-fiber cereals, fruits, vegetables, and fats are gradually reintroduced. Meanwhile, protein intake is reduced to individually tailored amounts as part of a weight-maintenance diet.
LIPOLYSIS, KETOSIS, DIURESIS
The specific macronutrient composition of the PSMF during the intensive phase is designed so that patients enter ketosis and lose as much fat as they can while preserving lean body mass.9,11 Figure 2 illustrates the mechanisms of ketosis and the metabolic impact of the PSMF.
With dietary carbohydrate restriction, serum glucose and insulin levels decline and glycogen stores are depleted. The drop in serum insulin allows lipolysis to occur, resulting in loss of adipose tissue and production of ketone bodies in the liver. Ketone bodies become the primary source of energy for the brain and other tissues during fasting and have metabolic and neuroprotective benefits.12,13
Some studies suggest that ketosis also suppresses appetite, helping curb total caloric intake throughout the diet.14 Protein itself may increase satiety.15
Glycogen in the liver is bound to water, so the depletion of glycogen also results in loss of attached water. As a result, diuresis contributes significantly to the initial weight loss within the first 2 weeks on the PSMF.9
WHO IS A CANDIDATE FOR THE PSMF?
The PSMF is indicated only for adults with a body mass index (BMI) of at least 30 kg/m2 or a BMI of at least 27 kg/m2 and at least one comorbidity such as type 2 diabetes, hypertension, dyslipidemia, obstructive sleep apnea, osteoarthritis, or fatty liver.12 Patients must also be sufficiently committed and motivated to make the intensive dietary and behavioral changes the program calls for.
The PSMF should be considered when more conventional low-calorie approaches to weight loss fail or when patients become discouraged by the slower results seen with traditional diets.8 Patients undergoing a PSMF are usually encouraged by the initial period of rapid weight loss, and such diets have lower dropout rates.16
This diet may also be recommended for obese patients who have poorly controlled type 2 diabetes and growing resistance to medications, to bring down the blood glucose level. Another use is before bariatric surgery to reduce the risk of obesity-related complications.8 Patients who regain weight after bariatric surgery may also benefit.
MEAL REPLACEMENTS OR A DIET PLAN?
The PSMF program at Cleveland Clinic is based on modified preparation and selection of conventional foods. Details of the program are described in Table 1. Protein sources must be of high biologic value, containing the right mix of essential amino acids (eg, lean meat, fish, poultry, egg whites).9
Some commercially available very-low-calorie diets (eg, OPTIFAST, Medifast) that are advertised as PSMFs consist mainly of meal replacements. In the program at Cleveland Clinic, meal replacements in the form of commercial high-protein shakes or bars can be used occasionally for convenience and to maintain adherence to the diet.
However, preparation of PSMF meals from natural, conventional foods is thought to play an important role in long-term behavior modification and so is strongly encouraged. Patients learn low-fat cooking methods, portion control, and how to make appropriate choices in shopping, eating, and dining out. These lessons are valuable for those who struggle with long-term weight loss. Learning these behaviors through the program may help ease the transition to the weight-maintenance phase and beyond. For some patients, cooking is also a source of enjoyment, as is the sight, smell, and taste of nonliquid foods.10
In addition, patients appreciate being able to eat the same foods as others in their household, except for omitting high-carbohydrate foods. It has also been reported that patients on a food-based PSMF were significantly less hungry and preoccupied with eating than those on a liquid formula diet.17
CONTRAINDICATIONS AND SAFETY CONCERNS
Contraindications to the PSMF include a BMI less than 27 kg/m2, recent myocardial infarction, angina, significant arrhythmia, decompensated congestive heart failure, cerebrovascular insufficiency or recent stroke, end-stage renal disease, liver failure, malignancy, major psychiatric illness, pregnancy or lactation, and wasting disorders. It is also not recommended for patients under age 16 or over age 65.
In view of the risk of diabetic ketoacidosis and the difficulty of titrating required doses ofinsulin, patients with type 1 diabetes mellitus are usually not advised to undergo a low-carbohydrate or very-low-calorie diet.8,12 However, we and others have found that the PSMF can be used in some obese patients with type 1 diabetes if it is combined with appropriate education and careful monitoring.12
Major concerns about the safety of the PSMF stem from experiences with the first very-low-calorie diets in the 1970s, which were associated with fatal cardiac arrhythmias and sudden death.18 These early diets used liquid formulas with hydrolyzed collagen protein of poor biologic value and were deficient in many vitamins and minerals. Today’s very-low-calorie diets use protein sources of high biologic value (chiefly animal, soy, and egg for the PSMF) and are supplemented with necessary vitamins and minerals, reducing the risk of electrolyte and cardiac abnormalities.9,19,20 Furthermore, before starting the PSMF all patients must have an electrocardiogram to be sure they have no arrhythmias (eg, heart block, QT interval prolongation) or ischemia.
Relative contraindications
A known history of cholelithiasis is a relative contraindication to a very-low-calorie diet and may be of concern for some patients and providers. While obesity itself is already a risk factor for gallstones, gallstone formation has also been associated with bile stasis, which occurs from rapid weight loss with liquid formula diets of low fat intake (< 10 g/day).21 However, in the PSMF, fat intake from protein sources, though low (45–70 g/day), is considered high enough to allow adequate gallbladder contraction, thus decreasing the risk of gallstone formation.22
Gout is another relative contraindication, as hyperuricemia with risk of gout is also linked to high-protein diets.9 Palgi et al23 found that uric acid levels rose by a mean of 0.4 mg/dL during the diet. The risk of gout, however, seemed to be small, occurring in fewer than 1% of patients in the study. Furthermore, in a recent study by Li et al,24 uric acid levels were found to significantly decrease in patients on a high-protein, very-low-calorie diet. Nonetheless, uric acid levels should be monitored regularly in patients on the PSMF.
SIDE EFFECTS OF THE DIET
Common side effects of the PSMF include headache, fatigue, orthostatic hypotension, muscle cramps, cold intolerance, constipation, diarrhea, fatigue, halitosis, menstrual changes, and hair thinning. Most of these are transient and may be alleviated by adjusting fluid, salt, and supplement intake. Other side effects may disappear as the patient is weaned off the diet.8,9
REGULAR FOLLOW-UP WITH HEALTH CARE PROVIDERS
Current PSMF programs are considered safe when used in combination with regular follow-up with health care providers.8,12
At Cleveland Clinic, patients meet with a dietitian twice in the first month and monthly thereafter (or more frequently if needed) for weight monitoring and education on nutrition and behavior modification (Table 1). Since the PSMF does not provide complete nutrition, daily supplementation with vitamins and minerals is required.
Daily exercise is encouraged throughout the program to increase fitness and to help keep the weight off during the refeeding phase and after.
Patients also meet every 6 to 8 weeks with the referring nurse practitioner or physician for further monitoring and evaluation of vital signs, laboratory results, and side effects. The PSMF protocol at Cleveland Clinic enables both primary care physicians and specialists (including nurse practitioners) within our network to monitor the patient’s status. Use of a common electronic medical record system is particularly valuable for easy communication between providers. If a primary care physician feels unable to appropriately counsel and supervise a patient in the PSMF program, referral to an endocrinologist or weight loss specialist is recommended.
In addition to baseline electrocardiography and monitoring of uric acid levels, a comprehensive metabolic panel is drawn at baseline, twice in the first month, and monthly thereafter to check for electrolyte imbalances and metabolic and tissue dysfunction such as dehydration, excessive protein loss, and liver or kidney injury.
Patients should not attempt the PSMF without medical supervision. Many patients have friends or family members who want to try the PSMF along with them, but this can be dangerous, especially for those with hypertension or type 2 diabetes. The medications prescribed for these conditions can result in hypotension or hypoglycemia during the PSMF.
Although there are no standard guidelines for adjusting medication use before starting a patient on the PSMF, it is logical to taper off or discontinue antihypertensive agents in patients with tightly controlled hypertension to avoid possible dehydration and hypotension during the first few diuresis-inducing weeks of the diet. In particular, diuretic agents should be discontinued to prevent further electrolyte imbalance and fluid shifts.
Similarly, in patients with tightly controlled type 2 diabetes (hemoglobin A1c < 7.0%), oral hypoglycemic agents and insulin therapy should be reduced before starting the diet to avoid potential hypoglycemia. During the course of the diet, providers should then adjust medication dosages based on follow-up vital signs and laboratory results and daily glucose monitoring.8
EFFECTS OF THE PSMF IN PATIENTS WITH TYPE 2 DIABETES
Though few formal studies have been done, the PSMF may have major effects on hyperglycemia, cardiovascular risk factors, and diabetic nephropathy in obese patients with type 2 diabetes, at least in the short term (Table 2).
Weight loss
In one of the first PSMF studies,23 in 668 patients with or without type 2 diabetes (baseline weight 98 kg), the mean weight loss was 21 kg after the intensive phase and 19 kg by the end of the refeeding phase.
In another observational report,25 25% to 30% of patients lost even more weight, averaging 38.6 kg of weight loss. Typically, the higher the baseline weight, the greater the weight loss during the PSMF.23
Patients with type 2 diabetes lost a similar amount of weight (8.5 kg) compared with those without diabetes (9.4 kg, P = .64) in a study of meal-replacement PSMF (using OPTIFAST shakes and bars).26 In a large meal-replacement study of 2,093 patients, Li et al24 found that weight loss was similar between diabetic, prediabetic, and nondiabetic patients. Weight loss was also closely maintained in those patients who stayed on the diet for 12 months.
In a PSMF study in which all the participants had type 2 diabetes, the mean weight loss was 18.6 kg. Although the patients regained some of this weight, at 1 year they still weighed 8.6 kg less than at baseline. However, a conventional, balanced, low-calorie diet resulted in similar amounts of weight loss after 1 year.27 Furthermore, a second round of the PSMF did not result in significant additional weight loss but rather weight maintenance.28
Fat loss and smaller waist circumference
Most of the weight lost during a PSMF is from fat tissue.11,26 Abdominal (visceral) fat may be lost first, which is desirable for patients with type 2 diabetes, since a higher degree of abdominal fat is linked to insulin resistance.2,29
After a meal-replacement PSMF, waist circumference decreased significantly in patients both with and without type 2 diabetes.24,26 However, in one study, less fat was lost per unit of change of BMI in the group with type 2 diabetes than in the nondiabetic group.26 Since insulin inhibits lipolysis, it is possible that exogenous insulin use in diabetic patients may prevent greater reductions in fat mass, though this is likely not the only mechanism.26
Lower fasting serum glucose
Fasting serum glucose levels decreased significantly from baseline in patients with type 2 diabetes after a PSMF in all studies that measured this variable.23–28,30,31 Changes in fasting glucose are immediate and are associated with caloric restriction rather than weight loss itself.30,32 Furthermore, the observed decrease in serum glucose is even more impressive in view of the withdrawal or reduction of doses of insulin and oral hypoglycemic agents before starting the diet.
In a study that compared glycemic control in a PSMF diet vs a balanced low-calorie diet, the fasting serum glucose in the PSMF group declined 46%, from 255.9 mg/dL at baseline to 138.7 mg/dL at 20 weeks (P = .001). After 1 year, it had risen back to 187.4 mg/dL, which was still 27% lower than at baseline (P = .023). These results compared favorably with those in the low-calorie diet group (P < .05), which saw fasting serum glucose decline 27% after 20 weeks (from 230.6 mg/dL at baseline to 167.6 mg/dL) and then rise to 5% over baseline (243.2 mg/dL) after 1 year.27
In a later study, the decrease in fasting serum glucose was not maintained at 1 year, but a significantly higher percentage (55%) of participants in the PSMF group were still able to remain free of diabetic medications compared with those who followed a balanced low-calorie diet (31%, P = .01).28
Decrease in hemoglobin A1c
Declines in fasting serum glucose corresponded with short-term declines in hemoglobin A1c in several reports.27–31 Hemoglobin A1c declined significantly from an average of 10.4% to 7.3% (P = .001) after PSMF intervention in patients with type 2 diabetes. In contrast, hemoglobin A1c in the low-calorie diet control group declined from 10.4% to 8.6%.27 One year later, hemoglobin A1c remained lower than at baseline in the PSMF group (final 9.2%) and continued to compare favorably against the control group (final 11.8%, between-group P = .001). However, these 1-year post-intervention improvements were not seen in a second, more intensive study.28
Less insulin resistance
In several studies, fasting serum insulin levels declined along with serum glucose levels, implying decreased insulin resistance.25,27,28,30,31 In addition, insulin output was enhanced during glucose challenge after completion of the PSMF, suggesting possible improved (though still impaired) pancreatic beta-cell capacity.25,27,30
Improved lipid profile
The most common effect of the PSMF on the lipid profile is a significant decrease in triglycerides in patients both with and without type 2 diabetes.8,23,24,28 In addition, high-density lipoprotein cholesterol increased in two studies following PSMF intervention or after 1-year of follow-up.24,27,28 Total cholesterol and low-density lipoprotein cholesterol levels also improved after the PSMF, but these changes were not always maintained at follow-up visits.8,24,28
Lower blood pressure
Improvements in both systolic and diastolic blood pressure were noted in two studies, with mean decreases of 6 mm Hg to 13 mm Hg systolic and 8 mm Hg diastolic after PSMF intervention.23,28 In a third study, reductions in blood pressure were less dramatic, and only changes in diastolic but not systolic blood pressure remained significant at 12 months.24 While improvements were not observed in a fourth study, patients in this study also had impaired kidney function caused by diabetic nephropathy, and changes in medication were not taken into account.31
Kidney function tests
In a small study, Friedman et al showed that 12 weeks of the PSMF in six patients with advanced diabetic nephropathy (stage 3B or stage 4 chronic kidney disease) led to a loss of 12% of body weight (P = .03) as well as significant reductions in serum creatinine and cystatin C levels (P < .05).31 In addition, albuminuria decreased by 30% (P = .08). Side effects were minimal, and the diet was well tolerated despite its high protein content, which is a concern in patients with impaired kidney function.
Thus, weight loss via the PSMF may still be beneficial in type 2 diabetic patients with chronic kidney disease and may even improve the course of progression of diabetic nephropathy.
Long-term weight loss is elusive
Long-term weight loss has been an elusive goal for many diet programs. In a study using a very-low-calorie diet in obese patients with type 2 diabetes, substantial weight loss was maintained in half of the patients at 3 years after the intervention, but nearly all of the patients had regained most of their weight after 5 years.33
While commitment to behavior modification, maintenance of physical activity, and continued follow-up are all critical factors in sustaining weight loss, new and innovative approaches to battle weight regain are needed.34
Yet despite considerable weight regain in most patients, the Look AHEAD (Action for Health in Diabetes) study showed that participants in intensive lifestyle intervention programs still achieved greater weight loss after 4 years than those receiving standard care.35 Whether this holds true for those in intensive PSMF programs is unknown. In addition, conclusive PSMF studies regarding glycemic control, lipids, and blood pressure beyond 1 year of follow-up are lacking.
A VIABLE OPTION FOR MANY
Adherence to a very-low-calorie, ketogenic PSMF program results in major short-term health benefits for obese patients with type 2 diabetes. These benefits include significant weight loss, often more than 18 kg, within 6 months.23–28 In addition, significant improvements in fasting glucose23–28,30–32 and hemoglobin A1c levels27–31 are linked to the caloric and carbohydrate restriction of the PSMF. Insulin resistance was also attenuated, with possible partial restoration of pancreatic beta-cell capacity.25,27,28,30,31 In some studies, the PSMF resulted in lower systolic and diastolic blood pressure23,24,28 and triglyceride levels.8,23,24,28 One small study also suggested a possible improvement of diabetic nephropathy.31 Lastly, improvements in glycemia and hypertension were associated with a reduction in the need for antidiabetic and antihypertensive drugs.36
Still, weight loss and many of the associated improvements partially return to baseline levels 1 year after the intervention. Thus, more long-term studies are needed to explore factors for better weight maintenance after the PSMF.
Also, only a few studies have compared the effect of the PSMF between patients with or without type 2 diabetes. One study suggested that fat loss may be reduced in patients with type 2 diabetes.26
In conclusion, despite some risks and safety concerns, PSMF is a viable option for many obese, type 2 diabetic patients as a method of short-term weight loss, with evidence for improvement of glycemic control and cardiovascular risk factors for up to 1 year. To strengthen support for the PSMF, however, further research is warranted on the diet’s long-term effects in patients with type 2 diabetes and also in nondiabetic patients.
Acknowledgments: Many thanks to Cheryl Reitz, RD, LD, CDE, and Dawn Noe, RD, LD, CDE, for providing their expertise on the PSMF protocols carried out at Cleveland Clinic. Additional thanks to Tejas Kashyap for his initial assistance with this review.
- Smyth S, Heron A. Diabetes and obesity: the twin epidemics. Nat Med 2006; 12:75–80.
- Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest 2000; 106:473–481.
- Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med 2001; 345:790–797.
- Andrews RC, Cooper AR, Montgomery AA, et al. Diet or diet plus physical activity versus usual care in patients with newly diagnosed type 2 diabetes: the Early ACTID randomised controlled trial. Lancet 2011; 378:129–139.
- Lindström J, Louheranta A, Mannelin M, et al; Finnish Diabetes Prevention Study Group. The Finnish Diabetes Prevention Study (DPS): lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care 2003; 26:3230–3236.
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346:393–403.
- Gregg EW, Chen H, Wagenknecht LE, et al; Look AHEAD Research Group. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA 2012; 308:2489–2496.
- Henry RR, Gumbiner B. Benefits and limitations of very-low-calorie diet therapy in obese NIDDM. Diabetes Care 1991; 14:802–823.
- Bistrian BR. Clinical use of a protein-sparing modified fast. JAMA 1978; 240:2299–2302.
- Walters JK, Hoogwerf BJ, Reddy SS. The protein-sparing modified fast for obesity-related medical problems. Cleve Clin J Med 1997; 64:242–244.
- Van Gaal LF, Snyders D, De Leeuw IH, Bekaert JL. Anthropometric and calorimetric evidence for the protein sparing effects of a new protein supplemented low calorie preparation. Am J Clin Nutr 1985; 41:540–544.
- Baker S, Jerums G, Proietto J. Effects and clinical potential of very-low-calorie diets (VLCDs) in type 2 diabetes. Diabetes Res Clin Pract 2009; 85:235–242.
- Shimazu T, Hirschey MD, Newman J, et al. Suppression of oxidative stress by ß-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 2013; 339:211–214.
- Johnstone AM, Horgan GW, Murison SD, Bremner DM, Lobley GE. Effects of a high-protein ketogenic diet on hunger, appetite, and weight loss in obese men feeding ad libitum. Am J Clin Nutr 2008; 87:44–55.
- Westerterp-Plantenga MS, Lemmens SG, Westerterp KR. Dietary protein—its role in satiety, energetics, weight loss and health. Br J Nutr 2012; 108(suppl 2):S105–S112.
- Hemmingsson E, Johansson K, Eriksson J, Sundström J, Neovius M, Marcus C. Weight loss and dropout during a commercial weight-loss program including a very-low-calorie diet, a low-calorie diet, or restricted normal food: observational cohort study. Am J Clin Nutr 2012; 96:953–961.
- Wadden TA, Stunkard AJ, Brownell KD, Day SC. A comparison of two very-low-calorie diets: protein-sparing-modified fast versus protein-formula-liquid diet. Am J Clin Nutr 1985; 41:533–539.
- Isner JM, Sours HE, Paris AL, Ferrans VJ, Roberts WC. Sudden, unexpected death in avid dieters using the liquid-protein-modified-fast diet. Observations in 17 patients and the role of the prolonged QT interval. Circulation 1979; 60:1401–1412.
- Henry RR, Wiest-Kent TA, Scheaffer L, Kolterman OG, Olefsky JM. Metabolic consequences of very-low-calorie diet therapy in obese non-insulin-dependent diabetic and nondiabetic subjects. Diabetes 1986; 35:155–164.
- Seim HC, Mitchell JE, Pomeroy C, de Zwaan M. Electrocardiographic findings associated with very low calorie dieting. Int J Obes Relat Metab Disord 1995; 19:817–819.
- Johansson K, Sundström J, Marcus C, Hemmingsson E, Neovius M. Risk of symptomatic gallstones and cholecystectomy after a very-low-calorie diet or low-calorie diet in a commercial weight loss program: 1-year matched cohort study. Int J Obes (Lond) 2014; 38:279–284.
- Festi D, Colecchia A, Orsini M, et al. Gallbladder motility and gallstone formation in obese patients following very low calorie diets. Use it (fat) to lose it (well). Int J Obes Relat Metab Disord 1998; 22:592–600.
- Palgi A, Read JL, Greenberg I, Hoefer MA, Bistrian BR, Blackburn GL. Multidisciplinary treatment of obesity with a protein-sparing modified fast: results in 668 outpatients. Am J Public Health 1985; 75:1190–1194.
- Li Z, Tseng CH, Li Q, Deng ML, Wang M, Heber D. Clinical efficacy of a medically supervised outpatient high-protein, low-calorie diet program is equivalent in prediabetic, diabetic and normoglycemic obese patients. Nutr Diabetes 2014 Feb 10; 4:e105.
- Genuth S. Supplemented fasting in the treatment of obesity and diabetes. Am J Clin Nutr 1979; 32:2579–2586.
- Baker ST, Jerums G, Prendergast LA, Panagiotopoulos S, Strauss BJ, Proietto J. Less fat reduction per unit weight loss in type 2 diabetic compared with nondiabetic obese individuals completing a very-low-calorie diet program. Metabolism 2012; 61:873–882.
- Wing RR, Marcus MD, Salata R, Epstein LH, Miaskiewicz S, Blair EH. Effects of a very-low-calorie diet on long-term glycemic control in obese type 2 diabetic subjects. Arch Intern Med 1991; 151:1334–1340.
- Wing RR, Blair E, Marcus M, Epstein LH, Harvey J. Year-long weight loss treatment for obese patients with type II diabetes: does including an intermittent very-low-calorie diet improve outcome? Am J Med 1994; 97:354–362.
- Kawamura II, Chen CC, Yamazaki K, Miyazawa Y, Isono K. A clinical study of protein sparing modified fast (PSMF) administered preoperatively to morbidly obese patients: comparison of PSMF with natural food products to originally prepared PSMF. Obes Surg 1992; 2:33–40.
- Hughes TA, Gwynne JT, Switzer BR, Herbst C, White G. Effects of caloric restriction and weight loss on glycemic control, insulin release and resistance, and atherosclerotic risk in obese patients with type II diabetes mellitus. Am J Med 1984; 77:7–17.
- Friedman AN, Chambers M, Kamendulis LM, Temmerman J. Short-term changes after a weight reduction intervention in advanced diabetic nephropathy. Clin J Am Soc Nephrol 2013; 8:1892–1898.
- Wing RR, Blair EH, Bononi P, Marcus MD, Watanabe R, Bergman RN. Caloric restriction per se is a significant factor in improvements in glycemic control and insulin sensitivity during weight loss in obese NIDDM patients. Diabetes Care 1994; 17:30–36.
- Paisey RB, Frost J, Harvey P, et al. Five year results of a prospective very low calorie diet or conventional weight loss programme in type 2 diabetes. J Hum Nutr Diet 2002; 15:121–127.
- Blackburn GL. Weight of the nation: moving forward, reversing the trend using medical care. Am J Clin Nutr 2012; 96:949–950.
- Wadden TA, Neiberg RH, Wing RR, et al; Look AHEAD Research Group. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity (Silver Spring) 2011; 19:1987–1998.
- Redmon JB, Bertoni AG, Connelly S, et al; Look AHEAD Research Group. Effect of the Look AHEAD intervention on medication use and related cost to treat cardiovascular disease risk factors in individuals with type 2 diabetes. Diabetes Care 2010; 33:1153–1158.
Eighty percent of people with type 2 diabetes mellitus are obese or overweight.1 Excess adipose tissue can lead to endocrine dysregulation,2 contributing to the pathogenesis of type 2 diabetes, and obesity is one of the strongest predictors of this disease.3
For obese people with type 2 diabetes, diet and exercise can lead to weight loss and many other benefits, such as better glycemic control, less insulin resistance, lower risk of diabetes-related comorbidities and complications, fewer diabetic medications needed, and lower health care costs.4–7 Intensive lifestyle interventions have also been shown to induce partial remission of diabetes and to prevent the onset of type 2 diabetes in people at high risk of it.5–7
A very-low-calorie diet is one of many dietary options available to patients with type 2 diabetes who are overweight or obese. The protein-sparing modified fast (PSMF) is a type of very-low-calorie diet with a high protein content and simultaneous restriction of carbohydrate and fat.8,9 It was developed in the 1970s, and since then various permutations have been used in weight loss and health care clinics worldwide.
MOSTLY PROTEIN, VERY LITTLE CARBOHYDRATE AND FAT
The PSMF is a medically supervised diet that provides less than 800 kcal/day during an initial intensive phase of about 6 months, followed by the gradual reintroduction of calories during a refeeding phase of about 6 to 8 weeks.10
During the intensive phase, patients obtain most of their calories from protein, approximately 1.2 to 1.5 g/kg of ideal body weight per day. At the same time, carbohydrate intake is restricted to less than 20 to 50 g/day; additional fats outside of protein sources are not allowed.9 Thus, the PSMF shares features of both very-low-calorie diets and very-low-carbohydrate ketogenic diets (eg, the Atkins diet), though some differences exist among the three (Figure 1).
Patients rapidly lose weight during the intensive phase, typically between 1 and 3 kg per week, with even greater losses during the first 2 weeks.8,9 Weight loss typically plateaus within 6 months, at which point patients begin the refeeding period. During refeeding, complex carbohydrates and low-glycemic, high-fiber cereals, fruits, vegetables, and fats are gradually reintroduced. Meanwhile, protein intake is reduced to individually tailored amounts as part of a weight-maintenance diet.
LIPOLYSIS, KETOSIS, DIURESIS
The specific macronutrient composition of the PSMF during the intensive phase is designed so that patients enter ketosis and lose as much fat as they can while preserving lean body mass.9,11 Figure 2 illustrates the mechanisms of ketosis and the metabolic impact of the PSMF.
With dietary carbohydrate restriction, serum glucose and insulin levels decline and glycogen stores are depleted. The drop in serum insulin allows lipolysis to occur, resulting in loss of adipose tissue and production of ketone bodies in the liver. Ketone bodies become the primary source of energy for the brain and other tissues during fasting and have metabolic and neuroprotective benefits.12,13
Some studies suggest that ketosis also suppresses appetite, helping curb total caloric intake throughout the diet.14 Protein itself may increase satiety.15
Glycogen in the liver is bound to water, so the depletion of glycogen also results in loss of attached water. As a result, diuresis contributes significantly to the initial weight loss within the first 2 weeks on the PSMF.9
WHO IS A CANDIDATE FOR THE PSMF?
The PSMF is indicated only for adults with a body mass index (BMI) of at least 30 kg/m2 or a BMI of at least 27 kg/m2 and at least one comorbidity such as type 2 diabetes, hypertension, dyslipidemia, obstructive sleep apnea, osteoarthritis, or fatty liver.12 Patients must also be sufficiently committed and motivated to make the intensive dietary and behavioral changes the program calls for.
The PSMF should be considered when more conventional low-calorie approaches to weight loss fail or when patients become discouraged by the slower results seen with traditional diets.8 Patients undergoing a PSMF are usually encouraged by the initial period of rapid weight loss, and such diets have lower dropout rates.16
This diet may also be recommended for obese patients who have poorly controlled type 2 diabetes and growing resistance to medications, to bring down the blood glucose level. Another use is before bariatric surgery to reduce the risk of obesity-related complications.8 Patients who regain weight after bariatric surgery may also benefit.
MEAL REPLACEMENTS OR A DIET PLAN?
The PSMF program at Cleveland Clinic is based on modified preparation and selection of conventional foods. Details of the program are described in Table 1. Protein sources must be of high biologic value, containing the right mix of essential amino acids (eg, lean meat, fish, poultry, egg whites).9
Some commercially available very-low-calorie diets (eg, OPTIFAST, Medifast) that are advertised as PSMFs consist mainly of meal replacements. In the program at Cleveland Clinic, meal replacements in the form of commercial high-protein shakes or bars can be used occasionally for convenience and to maintain adherence to the diet.
However, preparation of PSMF meals from natural, conventional foods is thought to play an important role in long-term behavior modification and so is strongly encouraged. Patients learn low-fat cooking methods, portion control, and how to make appropriate choices in shopping, eating, and dining out. These lessons are valuable for those who struggle with long-term weight loss. Learning these behaviors through the program may help ease the transition to the weight-maintenance phase and beyond. For some patients, cooking is also a source of enjoyment, as is the sight, smell, and taste of nonliquid foods.10
In addition, patients appreciate being able to eat the same foods as others in their household, except for omitting high-carbohydrate foods. It has also been reported that patients on a food-based PSMF were significantly less hungry and preoccupied with eating than those on a liquid formula diet.17
CONTRAINDICATIONS AND SAFETY CONCERNS
Contraindications to the PSMF include a BMI less than 27 kg/m2, recent myocardial infarction, angina, significant arrhythmia, decompensated congestive heart failure, cerebrovascular insufficiency or recent stroke, end-stage renal disease, liver failure, malignancy, major psychiatric illness, pregnancy or lactation, and wasting disorders. It is also not recommended for patients under age 16 or over age 65.
In view of the risk of diabetic ketoacidosis and the difficulty of titrating required doses ofinsulin, patients with type 1 diabetes mellitus are usually not advised to undergo a low-carbohydrate or very-low-calorie diet.8,12 However, we and others have found that the PSMF can be used in some obese patients with type 1 diabetes if it is combined with appropriate education and careful monitoring.12
Major concerns about the safety of the PSMF stem from experiences with the first very-low-calorie diets in the 1970s, which were associated with fatal cardiac arrhythmias and sudden death.18 These early diets used liquid formulas with hydrolyzed collagen protein of poor biologic value and were deficient in many vitamins and minerals. Today’s very-low-calorie diets use protein sources of high biologic value (chiefly animal, soy, and egg for the PSMF) and are supplemented with necessary vitamins and minerals, reducing the risk of electrolyte and cardiac abnormalities.9,19,20 Furthermore, before starting the PSMF all patients must have an electrocardiogram to be sure they have no arrhythmias (eg, heart block, QT interval prolongation) or ischemia.
Relative contraindications
A known history of cholelithiasis is a relative contraindication to a very-low-calorie diet and may be of concern for some patients and providers. While obesity itself is already a risk factor for gallstones, gallstone formation has also been associated with bile stasis, which occurs from rapid weight loss with liquid formula diets of low fat intake (< 10 g/day).21 However, in the PSMF, fat intake from protein sources, though low (45–70 g/day), is considered high enough to allow adequate gallbladder contraction, thus decreasing the risk of gallstone formation.22
Gout is another relative contraindication, as hyperuricemia with risk of gout is also linked to high-protein diets.9 Palgi et al23 found that uric acid levels rose by a mean of 0.4 mg/dL during the diet. The risk of gout, however, seemed to be small, occurring in fewer than 1% of patients in the study. Furthermore, in a recent study by Li et al,24 uric acid levels were found to significantly decrease in patients on a high-protein, very-low-calorie diet. Nonetheless, uric acid levels should be monitored regularly in patients on the PSMF.
SIDE EFFECTS OF THE DIET
Common side effects of the PSMF include headache, fatigue, orthostatic hypotension, muscle cramps, cold intolerance, constipation, diarrhea, fatigue, halitosis, menstrual changes, and hair thinning. Most of these are transient and may be alleviated by adjusting fluid, salt, and supplement intake. Other side effects may disappear as the patient is weaned off the diet.8,9
REGULAR FOLLOW-UP WITH HEALTH CARE PROVIDERS
Current PSMF programs are considered safe when used in combination with regular follow-up with health care providers.8,12
At Cleveland Clinic, patients meet with a dietitian twice in the first month and monthly thereafter (or more frequently if needed) for weight monitoring and education on nutrition and behavior modification (Table 1). Since the PSMF does not provide complete nutrition, daily supplementation with vitamins and minerals is required.
Daily exercise is encouraged throughout the program to increase fitness and to help keep the weight off during the refeeding phase and after.
Patients also meet every 6 to 8 weeks with the referring nurse practitioner or physician for further monitoring and evaluation of vital signs, laboratory results, and side effects. The PSMF protocol at Cleveland Clinic enables both primary care physicians and specialists (including nurse practitioners) within our network to monitor the patient’s status. Use of a common electronic medical record system is particularly valuable for easy communication between providers. If a primary care physician feels unable to appropriately counsel and supervise a patient in the PSMF program, referral to an endocrinologist or weight loss specialist is recommended.
In addition to baseline electrocardiography and monitoring of uric acid levels, a comprehensive metabolic panel is drawn at baseline, twice in the first month, and monthly thereafter to check for electrolyte imbalances and metabolic and tissue dysfunction such as dehydration, excessive protein loss, and liver or kidney injury.
Patients should not attempt the PSMF without medical supervision. Many patients have friends or family members who want to try the PSMF along with them, but this can be dangerous, especially for those with hypertension or type 2 diabetes. The medications prescribed for these conditions can result in hypotension or hypoglycemia during the PSMF.
Although there are no standard guidelines for adjusting medication use before starting a patient on the PSMF, it is logical to taper off or discontinue antihypertensive agents in patients with tightly controlled hypertension to avoid possible dehydration and hypotension during the first few diuresis-inducing weeks of the diet. In particular, diuretic agents should be discontinued to prevent further electrolyte imbalance and fluid shifts.
Similarly, in patients with tightly controlled type 2 diabetes (hemoglobin A1c < 7.0%), oral hypoglycemic agents and insulin therapy should be reduced before starting the diet to avoid potential hypoglycemia. During the course of the diet, providers should then adjust medication dosages based on follow-up vital signs and laboratory results and daily glucose monitoring.8
EFFECTS OF THE PSMF IN PATIENTS WITH TYPE 2 DIABETES
Though few formal studies have been done, the PSMF may have major effects on hyperglycemia, cardiovascular risk factors, and diabetic nephropathy in obese patients with type 2 diabetes, at least in the short term (Table 2).
Weight loss
In one of the first PSMF studies,23 in 668 patients with or without type 2 diabetes (baseline weight 98 kg), the mean weight loss was 21 kg after the intensive phase and 19 kg by the end of the refeeding phase.
In another observational report,25 25% to 30% of patients lost even more weight, averaging 38.6 kg of weight loss. Typically, the higher the baseline weight, the greater the weight loss during the PSMF.23
Patients with type 2 diabetes lost a similar amount of weight (8.5 kg) compared with those without diabetes (9.4 kg, P = .64) in a study of meal-replacement PSMF (using OPTIFAST shakes and bars).26 In a large meal-replacement study of 2,093 patients, Li et al24 found that weight loss was similar between diabetic, prediabetic, and nondiabetic patients. Weight loss was also closely maintained in those patients who stayed on the diet for 12 months.
In a PSMF study in which all the participants had type 2 diabetes, the mean weight loss was 18.6 kg. Although the patients regained some of this weight, at 1 year they still weighed 8.6 kg less than at baseline. However, a conventional, balanced, low-calorie diet resulted in similar amounts of weight loss after 1 year.27 Furthermore, a second round of the PSMF did not result in significant additional weight loss but rather weight maintenance.28
Fat loss and smaller waist circumference
Most of the weight lost during a PSMF is from fat tissue.11,26 Abdominal (visceral) fat may be lost first, which is desirable for patients with type 2 diabetes, since a higher degree of abdominal fat is linked to insulin resistance.2,29
After a meal-replacement PSMF, waist circumference decreased significantly in patients both with and without type 2 diabetes.24,26 However, in one study, less fat was lost per unit of change of BMI in the group with type 2 diabetes than in the nondiabetic group.26 Since insulin inhibits lipolysis, it is possible that exogenous insulin use in diabetic patients may prevent greater reductions in fat mass, though this is likely not the only mechanism.26
Lower fasting serum glucose
Fasting serum glucose levels decreased significantly from baseline in patients with type 2 diabetes after a PSMF in all studies that measured this variable.23–28,30,31 Changes in fasting glucose are immediate and are associated with caloric restriction rather than weight loss itself.30,32 Furthermore, the observed decrease in serum glucose is even more impressive in view of the withdrawal or reduction of doses of insulin and oral hypoglycemic agents before starting the diet.
In a study that compared glycemic control in a PSMF diet vs a balanced low-calorie diet, the fasting serum glucose in the PSMF group declined 46%, from 255.9 mg/dL at baseline to 138.7 mg/dL at 20 weeks (P = .001). After 1 year, it had risen back to 187.4 mg/dL, which was still 27% lower than at baseline (P = .023). These results compared favorably with those in the low-calorie diet group (P < .05), which saw fasting serum glucose decline 27% after 20 weeks (from 230.6 mg/dL at baseline to 167.6 mg/dL) and then rise to 5% over baseline (243.2 mg/dL) after 1 year.27
In a later study, the decrease in fasting serum glucose was not maintained at 1 year, but a significantly higher percentage (55%) of participants in the PSMF group were still able to remain free of diabetic medications compared with those who followed a balanced low-calorie diet (31%, P = .01).28
Decrease in hemoglobin A1c
Declines in fasting serum glucose corresponded with short-term declines in hemoglobin A1c in several reports.27–31 Hemoglobin A1c declined significantly from an average of 10.4% to 7.3% (P = .001) after PSMF intervention in patients with type 2 diabetes. In contrast, hemoglobin A1c in the low-calorie diet control group declined from 10.4% to 8.6%.27 One year later, hemoglobin A1c remained lower than at baseline in the PSMF group (final 9.2%) and continued to compare favorably against the control group (final 11.8%, between-group P = .001). However, these 1-year post-intervention improvements were not seen in a second, more intensive study.28
Less insulin resistance
In several studies, fasting serum insulin levels declined along with serum glucose levels, implying decreased insulin resistance.25,27,28,30,31 In addition, insulin output was enhanced during glucose challenge after completion of the PSMF, suggesting possible improved (though still impaired) pancreatic beta-cell capacity.25,27,30
Improved lipid profile
The most common effect of the PSMF on the lipid profile is a significant decrease in triglycerides in patients both with and without type 2 diabetes.8,23,24,28 In addition, high-density lipoprotein cholesterol increased in two studies following PSMF intervention or after 1-year of follow-up.24,27,28 Total cholesterol and low-density lipoprotein cholesterol levels also improved after the PSMF, but these changes were not always maintained at follow-up visits.8,24,28
Lower blood pressure
Improvements in both systolic and diastolic blood pressure were noted in two studies, with mean decreases of 6 mm Hg to 13 mm Hg systolic and 8 mm Hg diastolic after PSMF intervention.23,28 In a third study, reductions in blood pressure were less dramatic, and only changes in diastolic but not systolic blood pressure remained significant at 12 months.24 While improvements were not observed in a fourth study, patients in this study also had impaired kidney function caused by diabetic nephropathy, and changes in medication were not taken into account.31
Kidney function tests
In a small study, Friedman et al showed that 12 weeks of the PSMF in six patients with advanced diabetic nephropathy (stage 3B or stage 4 chronic kidney disease) led to a loss of 12% of body weight (P = .03) as well as significant reductions in serum creatinine and cystatin C levels (P < .05).31 In addition, albuminuria decreased by 30% (P = .08). Side effects were minimal, and the diet was well tolerated despite its high protein content, which is a concern in patients with impaired kidney function.
Thus, weight loss via the PSMF may still be beneficial in type 2 diabetic patients with chronic kidney disease and may even improve the course of progression of diabetic nephropathy.
Long-term weight loss is elusive
Long-term weight loss has been an elusive goal for many diet programs. In a study using a very-low-calorie diet in obese patients with type 2 diabetes, substantial weight loss was maintained in half of the patients at 3 years after the intervention, but nearly all of the patients had regained most of their weight after 5 years.33
While commitment to behavior modification, maintenance of physical activity, and continued follow-up are all critical factors in sustaining weight loss, new and innovative approaches to battle weight regain are needed.34
Yet despite considerable weight regain in most patients, the Look AHEAD (Action for Health in Diabetes) study showed that participants in intensive lifestyle intervention programs still achieved greater weight loss after 4 years than those receiving standard care.35 Whether this holds true for those in intensive PSMF programs is unknown. In addition, conclusive PSMF studies regarding glycemic control, lipids, and blood pressure beyond 1 year of follow-up are lacking.
A VIABLE OPTION FOR MANY
Adherence to a very-low-calorie, ketogenic PSMF program results in major short-term health benefits for obese patients with type 2 diabetes. These benefits include significant weight loss, often more than 18 kg, within 6 months.23–28 In addition, significant improvements in fasting glucose23–28,30–32 and hemoglobin A1c levels27–31 are linked to the caloric and carbohydrate restriction of the PSMF. Insulin resistance was also attenuated, with possible partial restoration of pancreatic beta-cell capacity.25,27,28,30,31 In some studies, the PSMF resulted in lower systolic and diastolic blood pressure23,24,28 and triglyceride levels.8,23,24,28 One small study also suggested a possible improvement of diabetic nephropathy.31 Lastly, improvements in glycemia and hypertension were associated with a reduction in the need for antidiabetic and antihypertensive drugs.36
Still, weight loss and many of the associated improvements partially return to baseline levels 1 year after the intervention. Thus, more long-term studies are needed to explore factors for better weight maintenance after the PSMF.
Also, only a few studies have compared the effect of the PSMF between patients with or without type 2 diabetes. One study suggested that fat loss may be reduced in patients with type 2 diabetes.26
In conclusion, despite some risks and safety concerns, PSMF is a viable option for many obese, type 2 diabetic patients as a method of short-term weight loss, with evidence for improvement of glycemic control and cardiovascular risk factors for up to 1 year. To strengthen support for the PSMF, however, further research is warranted on the diet’s long-term effects in patients with type 2 diabetes and also in nondiabetic patients.
Acknowledgments: Many thanks to Cheryl Reitz, RD, LD, CDE, and Dawn Noe, RD, LD, CDE, for providing their expertise on the PSMF protocols carried out at Cleveland Clinic. Additional thanks to Tejas Kashyap for his initial assistance with this review.
Eighty percent of people with type 2 diabetes mellitus are obese or overweight.1 Excess adipose tissue can lead to endocrine dysregulation,2 contributing to the pathogenesis of type 2 diabetes, and obesity is one of the strongest predictors of this disease.3
For obese people with type 2 diabetes, diet and exercise can lead to weight loss and many other benefits, such as better glycemic control, less insulin resistance, lower risk of diabetes-related comorbidities and complications, fewer diabetic medications needed, and lower health care costs.4–7 Intensive lifestyle interventions have also been shown to induce partial remission of diabetes and to prevent the onset of type 2 diabetes in people at high risk of it.5–7
A very-low-calorie diet is one of many dietary options available to patients with type 2 diabetes who are overweight or obese. The protein-sparing modified fast (PSMF) is a type of very-low-calorie diet with a high protein content and simultaneous restriction of carbohydrate and fat.8,9 It was developed in the 1970s, and since then various permutations have been used in weight loss and health care clinics worldwide.
MOSTLY PROTEIN, VERY LITTLE CARBOHYDRATE AND FAT
The PSMF is a medically supervised diet that provides less than 800 kcal/day during an initial intensive phase of about 6 months, followed by the gradual reintroduction of calories during a refeeding phase of about 6 to 8 weeks.10
During the intensive phase, patients obtain most of their calories from protein, approximately 1.2 to 1.5 g/kg of ideal body weight per day. At the same time, carbohydrate intake is restricted to less than 20 to 50 g/day; additional fats outside of protein sources are not allowed.9 Thus, the PSMF shares features of both very-low-calorie diets and very-low-carbohydrate ketogenic diets (eg, the Atkins diet), though some differences exist among the three (Figure 1).
Patients rapidly lose weight during the intensive phase, typically between 1 and 3 kg per week, with even greater losses during the first 2 weeks.8,9 Weight loss typically plateaus within 6 months, at which point patients begin the refeeding period. During refeeding, complex carbohydrates and low-glycemic, high-fiber cereals, fruits, vegetables, and fats are gradually reintroduced. Meanwhile, protein intake is reduced to individually tailored amounts as part of a weight-maintenance diet.
LIPOLYSIS, KETOSIS, DIURESIS
The specific macronutrient composition of the PSMF during the intensive phase is designed so that patients enter ketosis and lose as much fat as they can while preserving lean body mass.9,11 Figure 2 illustrates the mechanisms of ketosis and the metabolic impact of the PSMF.
With dietary carbohydrate restriction, serum glucose and insulin levels decline and glycogen stores are depleted. The drop in serum insulin allows lipolysis to occur, resulting in loss of adipose tissue and production of ketone bodies in the liver. Ketone bodies become the primary source of energy for the brain and other tissues during fasting and have metabolic and neuroprotective benefits.12,13
Some studies suggest that ketosis also suppresses appetite, helping curb total caloric intake throughout the diet.14 Protein itself may increase satiety.15
Glycogen in the liver is bound to water, so the depletion of glycogen also results in loss of attached water. As a result, diuresis contributes significantly to the initial weight loss within the first 2 weeks on the PSMF.9
WHO IS A CANDIDATE FOR THE PSMF?
The PSMF is indicated only for adults with a body mass index (BMI) of at least 30 kg/m2 or a BMI of at least 27 kg/m2 and at least one comorbidity such as type 2 diabetes, hypertension, dyslipidemia, obstructive sleep apnea, osteoarthritis, or fatty liver.12 Patients must also be sufficiently committed and motivated to make the intensive dietary and behavioral changes the program calls for.
The PSMF should be considered when more conventional low-calorie approaches to weight loss fail or when patients become discouraged by the slower results seen with traditional diets.8 Patients undergoing a PSMF are usually encouraged by the initial period of rapid weight loss, and such diets have lower dropout rates.16
This diet may also be recommended for obese patients who have poorly controlled type 2 diabetes and growing resistance to medications, to bring down the blood glucose level. Another use is before bariatric surgery to reduce the risk of obesity-related complications.8 Patients who regain weight after bariatric surgery may also benefit.
MEAL REPLACEMENTS OR A DIET PLAN?
The PSMF program at Cleveland Clinic is based on modified preparation and selection of conventional foods. Details of the program are described in Table 1. Protein sources must be of high biologic value, containing the right mix of essential amino acids (eg, lean meat, fish, poultry, egg whites).9
Some commercially available very-low-calorie diets (eg, OPTIFAST, Medifast) that are advertised as PSMFs consist mainly of meal replacements. In the program at Cleveland Clinic, meal replacements in the form of commercial high-protein shakes or bars can be used occasionally for convenience and to maintain adherence to the diet.
However, preparation of PSMF meals from natural, conventional foods is thought to play an important role in long-term behavior modification and so is strongly encouraged. Patients learn low-fat cooking methods, portion control, and how to make appropriate choices in shopping, eating, and dining out. These lessons are valuable for those who struggle with long-term weight loss. Learning these behaviors through the program may help ease the transition to the weight-maintenance phase and beyond. For some patients, cooking is also a source of enjoyment, as is the sight, smell, and taste of nonliquid foods.10
In addition, patients appreciate being able to eat the same foods as others in their household, except for omitting high-carbohydrate foods. It has also been reported that patients on a food-based PSMF were significantly less hungry and preoccupied with eating than those on a liquid formula diet.17
CONTRAINDICATIONS AND SAFETY CONCERNS
Contraindications to the PSMF include a BMI less than 27 kg/m2, recent myocardial infarction, angina, significant arrhythmia, decompensated congestive heart failure, cerebrovascular insufficiency or recent stroke, end-stage renal disease, liver failure, malignancy, major psychiatric illness, pregnancy or lactation, and wasting disorders. It is also not recommended for patients under age 16 or over age 65.
In view of the risk of diabetic ketoacidosis and the difficulty of titrating required doses ofinsulin, patients with type 1 diabetes mellitus are usually not advised to undergo a low-carbohydrate or very-low-calorie diet.8,12 However, we and others have found that the PSMF can be used in some obese patients with type 1 diabetes if it is combined with appropriate education and careful monitoring.12
Major concerns about the safety of the PSMF stem from experiences with the first very-low-calorie diets in the 1970s, which were associated with fatal cardiac arrhythmias and sudden death.18 These early diets used liquid formulas with hydrolyzed collagen protein of poor biologic value and were deficient in many vitamins and minerals. Today’s very-low-calorie diets use protein sources of high biologic value (chiefly animal, soy, and egg for the PSMF) and are supplemented with necessary vitamins and minerals, reducing the risk of electrolyte and cardiac abnormalities.9,19,20 Furthermore, before starting the PSMF all patients must have an electrocardiogram to be sure they have no arrhythmias (eg, heart block, QT interval prolongation) or ischemia.
Relative contraindications
A known history of cholelithiasis is a relative contraindication to a very-low-calorie diet and may be of concern for some patients and providers. While obesity itself is already a risk factor for gallstones, gallstone formation has also been associated with bile stasis, which occurs from rapid weight loss with liquid formula diets of low fat intake (< 10 g/day).21 However, in the PSMF, fat intake from protein sources, though low (45–70 g/day), is considered high enough to allow adequate gallbladder contraction, thus decreasing the risk of gallstone formation.22
Gout is another relative contraindication, as hyperuricemia with risk of gout is also linked to high-protein diets.9 Palgi et al23 found that uric acid levels rose by a mean of 0.4 mg/dL during the diet. The risk of gout, however, seemed to be small, occurring in fewer than 1% of patients in the study. Furthermore, in a recent study by Li et al,24 uric acid levels were found to significantly decrease in patients on a high-protein, very-low-calorie diet. Nonetheless, uric acid levels should be monitored regularly in patients on the PSMF.
SIDE EFFECTS OF THE DIET
Common side effects of the PSMF include headache, fatigue, orthostatic hypotension, muscle cramps, cold intolerance, constipation, diarrhea, fatigue, halitosis, menstrual changes, and hair thinning. Most of these are transient and may be alleviated by adjusting fluid, salt, and supplement intake. Other side effects may disappear as the patient is weaned off the diet.8,9
REGULAR FOLLOW-UP WITH HEALTH CARE PROVIDERS
Current PSMF programs are considered safe when used in combination with regular follow-up with health care providers.8,12
At Cleveland Clinic, patients meet with a dietitian twice in the first month and monthly thereafter (or more frequently if needed) for weight monitoring and education on nutrition and behavior modification (Table 1). Since the PSMF does not provide complete nutrition, daily supplementation with vitamins and minerals is required.
Daily exercise is encouraged throughout the program to increase fitness and to help keep the weight off during the refeeding phase and after.
Patients also meet every 6 to 8 weeks with the referring nurse practitioner or physician for further monitoring and evaluation of vital signs, laboratory results, and side effects. The PSMF protocol at Cleveland Clinic enables both primary care physicians and specialists (including nurse practitioners) within our network to monitor the patient’s status. Use of a common electronic medical record system is particularly valuable for easy communication between providers. If a primary care physician feels unable to appropriately counsel and supervise a patient in the PSMF program, referral to an endocrinologist or weight loss specialist is recommended.
In addition to baseline electrocardiography and monitoring of uric acid levels, a comprehensive metabolic panel is drawn at baseline, twice in the first month, and monthly thereafter to check for electrolyte imbalances and metabolic and tissue dysfunction such as dehydration, excessive protein loss, and liver or kidney injury.
Patients should not attempt the PSMF without medical supervision. Many patients have friends or family members who want to try the PSMF along with them, but this can be dangerous, especially for those with hypertension or type 2 diabetes. The medications prescribed for these conditions can result in hypotension or hypoglycemia during the PSMF.
Although there are no standard guidelines for adjusting medication use before starting a patient on the PSMF, it is logical to taper off or discontinue antihypertensive agents in patients with tightly controlled hypertension to avoid possible dehydration and hypotension during the first few diuresis-inducing weeks of the diet. In particular, diuretic agents should be discontinued to prevent further electrolyte imbalance and fluid shifts.
Similarly, in patients with tightly controlled type 2 diabetes (hemoglobin A1c < 7.0%), oral hypoglycemic agents and insulin therapy should be reduced before starting the diet to avoid potential hypoglycemia. During the course of the diet, providers should then adjust medication dosages based on follow-up vital signs and laboratory results and daily glucose monitoring.8
EFFECTS OF THE PSMF IN PATIENTS WITH TYPE 2 DIABETES
Though few formal studies have been done, the PSMF may have major effects on hyperglycemia, cardiovascular risk factors, and diabetic nephropathy in obese patients with type 2 diabetes, at least in the short term (Table 2).
Weight loss
In one of the first PSMF studies,23 in 668 patients with or without type 2 diabetes (baseline weight 98 kg), the mean weight loss was 21 kg after the intensive phase and 19 kg by the end of the refeeding phase.
In another observational report,25 25% to 30% of patients lost even more weight, averaging 38.6 kg of weight loss. Typically, the higher the baseline weight, the greater the weight loss during the PSMF.23
Patients with type 2 diabetes lost a similar amount of weight (8.5 kg) compared with those without diabetes (9.4 kg, P = .64) in a study of meal-replacement PSMF (using OPTIFAST shakes and bars).26 In a large meal-replacement study of 2,093 patients, Li et al24 found that weight loss was similar between diabetic, prediabetic, and nondiabetic patients. Weight loss was also closely maintained in those patients who stayed on the diet for 12 months.
In a PSMF study in which all the participants had type 2 diabetes, the mean weight loss was 18.6 kg. Although the patients regained some of this weight, at 1 year they still weighed 8.6 kg less than at baseline. However, a conventional, balanced, low-calorie diet resulted in similar amounts of weight loss after 1 year.27 Furthermore, a second round of the PSMF did not result in significant additional weight loss but rather weight maintenance.28
Fat loss and smaller waist circumference
Most of the weight lost during a PSMF is from fat tissue.11,26 Abdominal (visceral) fat may be lost first, which is desirable for patients with type 2 diabetes, since a higher degree of abdominal fat is linked to insulin resistance.2,29
After a meal-replacement PSMF, waist circumference decreased significantly in patients both with and without type 2 diabetes.24,26 However, in one study, less fat was lost per unit of change of BMI in the group with type 2 diabetes than in the nondiabetic group.26 Since insulin inhibits lipolysis, it is possible that exogenous insulin use in diabetic patients may prevent greater reductions in fat mass, though this is likely not the only mechanism.26
Lower fasting serum glucose
Fasting serum glucose levels decreased significantly from baseline in patients with type 2 diabetes after a PSMF in all studies that measured this variable.23–28,30,31 Changes in fasting glucose are immediate and are associated with caloric restriction rather than weight loss itself.30,32 Furthermore, the observed decrease in serum glucose is even more impressive in view of the withdrawal or reduction of doses of insulin and oral hypoglycemic agents before starting the diet.
In a study that compared glycemic control in a PSMF diet vs a balanced low-calorie diet, the fasting serum glucose in the PSMF group declined 46%, from 255.9 mg/dL at baseline to 138.7 mg/dL at 20 weeks (P = .001). After 1 year, it had risen back to 187.4 mg/dL, which was still 27% lower than at baseline (P = .023). These results compared favorably with those in the low-calorie diet group (P < .05), which saw fasting serum glucose decline 27% after 20 weeks (from 230.6 mg/dL at baseline to 167.6 mg/dL) and then rise to 5% over baseline (243.2 mg/dL) after 1 year.27
In a later study, the decrease in fasting serum glucose was not maintained at 1 year, but a significantly higher percentage (55%) of participants in the PSMF group were still able to remain free of diabetic medications compared with those who followed a balanced low-calorie diet (31%, P = .01).28
Decrease in hemoglobin A1c
Declines in fasting serum glucose corresponded with short-term declines in hemoglobin A1c in several reports.27–31 Hemoglobin A1c declined significantly from an average of 10.4% to 7.3% (P = .001) after PSMF intervention in patients with type 2 diabetes. In contrast, hemoglobin A1c in the low-calorie diet control group declined from 10.4% to 8.6%.27 One year later, hemoglobin A1c remained lower than at baseline in the PSMF group (final 9.2%) and continued to compare favorably against the control group (final 11.8%, between-group P = .001). However, these 1-year post-intervention improvements were not seen in a second, more intensive study.28
Less insulin resistance
In several studies, fasting serum insulin levels declined along with serum glucose levels, implying decreased insulin resistance.25,27,28,30,31 In addition, insulin output was enhanced during glucose challenge after completion of the PSMF, suggesting possible improved (though still impaired) pancreatic beta-cell capacity.25,27,30
Improved lipid profile
The most common effect of the PSMF on the lipid profile is a significant decrease in triglycerides in patients both with and without type 2 diabetes.8,23,24,28 In addition, high-density lipoprotein cholesterol increased in two studies following PSMF intervention or after 1-year of follow-up.24,27,28 Total cholesterol and low-density lipoprotein cholesterol levels also improved after the PSMF, but these changes were not always maintained at follow-up visits.8,24,28
Lower blood pressure
Improvements in both systolic and diastolic blood pressure were noted in two studies, with mean decreases of 6 mm Hg to 13 mm Hg systolic and 8 mm Hg diastolic after PSMF intervention.23,28 In a third study, reductions in blood pressure were less dramatic, and only changes in diastolic but not systolic blood pressure remained significant at 12 months.24 While improvements were not observed in a fourth study, patients in this study also had impaired kidney function caused by diabetic nephropathy, and changes in medication were not taken into account.31
Kidney function tests
In a small study, Friedman et al showed that 12 weeks of the PSMF in six patients with advanced diabetic nephropathy (stage 3B or stage 4 chronic kidney disease) led to a loss of 12% of body weight (P = .03) as well as significant reductions in serum creatinine and cystatin C levels (P < .05).31 In addition, albuminuria decreased by 30% (P = .08). Side effects were minimal, and the diet was well tolerated despite its high protein content, which is a concern in patients with impaired kidney function.
Thus, weight loss via the PSMF may still be beneficial in type 2 diabetic patients with chronic kidney disease and may even improve the course of progression of diabetic nephropathy.
Long-term weight loss is elusive
Long-term weight loss has been an elusive goal for many diet programs. In a study using a very-low-calorie diet in obese patients with type 2 diabetes, substantial weight loss was maintained in half of the patients at 3 years after the intervention, but nearly all of the patients had regained most of their weight after 5 years.33
While commitment to behavior modification, maintenance of physical activity, and continued follow-up are all critical factors in sustaining weight loss, new and innovative approaches to battle weight regain are needed.34
Yet despite considerable weight regain in most patients, the Look AHEAD (Action for Health in Diabetes) study showed that participants in intensive lifestyle intervention programs still achieved greater weight loss after 4 years than those receiving standard care.35 Whether this holds true for those in intensive PSMF programs is unknown. In addition, conclusive PSMF studies regarding glycemic control, lipids, and blood pressure beyond 1 year of follow-up are lacking.
A VIABLE OPTION FOR MANY
Adherence to a very-low-calorie, ketogenic PSMF program results in major short-term health benefits for obese patients with type 2 diabetes. These benefits include significant weight loss, often more than 18 kg, within 6 months.23–28 In addition, significant improvements in fasting glucose23–28,30–32 and hemoglobin A1c levels27–31 are linked to the caloric and carbohydrate restriction of the PSMF. Insulin resistance was also attenuated, with possible partial restoration of pancreatic beta-cell capacity.25,27,28,30,31 In some studies, the PSMF resulted in lower systolic and diastolic blood pressure23,24,28 and triglyceride levels.8,23,24,28 One small study also suggested a possible improvement of diabetic nephropathy.31 Lastly, improvements in glycemia and hypertension were associated with a reduction in the need for antidiabetic and antihypertensive drugs.36
Still, weight loss and many of the associated improvements partially return to baseline levels 1 year after the intervention. Thus, more long-term studies are needed to explore factors for better weight maintenance after the PSMF.
Also, only a few studies have compared the effect of the PSMF between patients with or without type 2 diabetes. One study suggested that fat loss may be reduced in patients with type 2 diabetes.26
In conclusion, despite some risks and safety concerns, PSMF is a viable option for many obese, type 2 diabetic patients as a method of short-term weight loss, with evidence for improvement of glycemic control and cardiovascular risk factors for up to 1 year. To strengthen support for the PSMF, however, further research is warranted on the diet’s long-term effects in patients with type 2 diabetes and also in nondiabetic patients.
Acknowledgments: Many thanks to Cheryl Reitz, RD, LD, CDE, and Dawn Noe, RD, LD, CDE, for providing their expertise on the PSMF protocols carried out at Cleveland Clinic. Additional thanks to Tejas Kashyap for his initial assistance with this review.
- Smyth S, Heron A. Diabetes and obesity: the twin epidemics. Nat Med 2006; 12:75–80.
- Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest 2000; 106:473–481.
- Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med 2001; 345:790–797.
- Andrews RC, Cooper AR, Montgomery AA, et al. Diet or diet plus physical activity versus usual care in patients with newly diagnosed type 2 diabetes: the Early ACTID randomised controlled trial. Lancet 2011; 378:129–139.
- Lindström J, Louheranta A, Mannelin M, et al; Finnish Diabetes Prevention Study Group. The Finnish Diabetes Prevention Study (DPS): lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care 2003; 26:3230–3236.
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346:393–403.
- Gregg EW, Chen H, Wagenknecht LE, et al; Look AHEAD Research Group. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA 2012; 308:2489–2496.
- Henry RR, Gumbiner B. Benefits and limitations of very-low-calorie diet therapy in obese NIDDM. Diabetes Care 1991; 14:802–823.
- Bistrian BR. Clinical use of a protein-sparing modified fast. JAMA 1978; 240:2299–2302.
- Walters JK, Hoogwerf BJ, Reddy SS. The protein-sparing modified fast for obesity-related medical problems. Cleve Clin J Med 1997; 64:242–244.
- Van Gaal LF, Snyders D, De Leeuw IH, Bekaert JL. Anthropometric and calorimetric evidence for the protein sparing effects of a new protein supplemented low calorie preparation. Am J Clin Nutr 1985; 41:540–544.
- Baker S, Jerums G, Proietto J. Effects and clinical potential of very-low-calorie diets (VLCDs) in type 2 diabetes. Diabetes Res Clin Pract 2009; 85:235–242.
- Shimazu T, Hirschey MD, Newman J, et al. Suppression of oxidative stress by ß-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 2013; 339:211–214.
- Johnstone AM, Horgan GW, Murison SD, Bremner DM, Lobley GE. Effects of a high-protein ketogenic diet on hunger, appetite, and weight loss in obese men feeding ad libitum. Am J Clin Nutr 2008; 87:44–55.
- Westerterp-Plantenga MS, Lemmens SG, Westerterp KR. Dietary protein—its role in satiety, energetics, weight loss and health. Br J Nutr 2012; 108(suppl 2):S105–S112.
- Hemmingsson E, Johansson K, Eriksson J, Sundström J, Neovius M, Marcus C. Weight loss and dropout during a commercial weight-loss program including a very-low-calorie diet, a low-calorie diet, or restricted normal food: observational cohort study. Am J Clin Nutr 2012; 96:953–961.
- Wadden TA, Stunkard AJ, Brownell KD, Day SC. A comparison of two very-low-calorie diets: protein-sparing-modified fast versus protein-formula-liquid diet. Am J Clin Nutr 1985; 41:533–539.
- Isner JM, Sours HE, Paris AL, Ferrans VJ, Roberts WC. Sudden, unexpected death in avid dieters using the liquid-protein-modified-fast diet. Observations in 17 patients and the role of the prolonged QT interval. Circulation 1979; 60:1401–1412.
- Henry RR, Wiest-Kent TA, Scheaffer L, Kolterman OG, Olefsky JM. Metabolic consequences of very-low-calorie diet therapy in obese non-insulin-dependent diabetic and nondiabetic subjects. Diabetes 1986; 35:155–164.
- Seim HC, Mitchell JE, Pomeroy C, de Zwaan M. Electrocardiographic findings associated with very low calorie dieting. Int J Obes Relat Metab Disord 1995; 19:817–819.
- Johansson K, Sundström J, Marcus C, Hemmingsson E, Neovius M. Risk of symptomatic gallstones and cholecystectomy after a very-low-calorie diet or low-calorie diet in a commercial weight loss program: 1-year matched cohort study. Int J Obes (Lond) 2014; 38:279–284.
- Festi D, Colecchia A, Orsini M, et al. Gallbladder motility and gallstone formation in obese patients following very low calorie diets. Use it (fat) to lose it (well). Int J Obes Relat Metab Disord 1998; 22:592–600.
- Palgi A, Read JL, Greenberg I, Hoefer MA, Bistrian BR, Blackburn GL. Multidisciplinary treatment of obesity with a protein-sparing modified fast: results in 668 outpatients. Am J Public Health 1985; 75:1190–1194.
- Li Z, Tseng CH, Li Q, Deng ML, Wang M, Heber D. Clinical efficacy of a medically supervised outpatient high-protein, low-calorie diet program is equivalent in prediabetic, diabetic and normoglycemic obese patients. Nutr Diabetes 2014 Feb 10; 4:e105.
- Genuth S. Supplemented fasting in the treatment of obesity and diabetes. Am J Clin Nutr 1979; 32:2579–2586.
- Baker ST, Jerums G, Prendergast LA, Panagiotopoulos S, Strauss BJ, Proietto J. Less fat reduction per unit weight loss in type 2 diabetic compared with nondiabetic obese individuals completing a very-low-calorie diet program. Metabolism 2012; 61:873–882.
- Wing RR, Marcus MD, Salata R, Epstein LH, Miaskiewicz S, Blair EH. Effects of a very-low-calorie diet on long-term glycemic control in obese type 2 diabetic subjects. Arch Intern Med 1991; 151:1334–1340.
- Wing RR, Blair E, Marcus M, Epstein LH, Harvey J. Year-long weight loss treatment for obese patients with type II diabetes: does including an intermittent very-low-calorie diet improve outcome? Am J Med 1994; 97:354–362.
- Kawamura II, Chen CC, Yamazaki K, Miyazawa Y, Isono K. A clinical study of protein sparing modified fast (PSMF) administered preoperatively to morbidly obese patients: comparison of PSMF with natural food products to originally prepared PSMF. Obes Surg 1992; 2:33–40.
- Hughes TA, Gwynne JT, Switzer BR, Herbst C, White G. Effects of caloric restriction and weight loss on glycemic control, insulin release and resistance, and atherosclerotic risk in obese patients with type II diabetes mellitus. Am J Med 1984; 77:7–17.
- Friedman AN, Chambers M, Kamendulis LM, Temmerman J. Short-term changes after a weight reduction intervention in advanced diabetic nephropathy. Clin J Am Soc Nephrol 2013; 8:1892–1898.
- Wing RR, Blair EH, Bononi P, Marcus MD, Watanabe R, Bergman RN. Caloric restriction per se is a significant factor in improvements in glycemic control and insulin sensitivity during weight loss in obese NIDDM patients. Diabetes Care 1994; 17:30–36.
- Paisey RB, Frost J, Harvey P, et al. Five year results of a prospective very low calorie diet or conventional weight loss programme in type 2 diabetes. J Hum Nutr Diet 2002; 15:121–127.
- Blackburn GL. Weight of the nation: moving forward, reversing the trend using medical care. Am J Clin Nutr 2012; 96:949–950.
- Wadden TA, Neiberg RH, Wing RR, et al; Look AHEAD Research Group. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity (Silver Spring) 2011; 19:1987–1998.
- Redmon JB, Bertoni AG, Connelly S, et al; Look AHEAD Research Group. Effect of the Look AHEAD intervention on medication use and related cost to treat cardiovascular disease risk factors in individuals with type 2 diabetes. Diabetes Care 2010; 33:1153–1158.
- Smyth S, Heron A. Diabetes and obesity: the twin epidemics. Nat Med 2006; 12:75–80.
- Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest 2000; 106:473–481.
- Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med 2001; 345:790–797.
- Andrews RC, Cooper AR, Montgomery AA, et al. Diet or diet plus physical activity versus usual care in patients with newly diagnosed type 2 diabetes: the Early ACTID randomised controlled trial. Lancet 2011; 378:129–139.
- Lindström J, Louheranta A, Mannelin M, et al; Finnish Diabetes Prevention Study Group. The Finnish Diabetes Prevention Study (DPS): lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care 2003; 26:3230–3236.
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346:393–403.
- Gregg EW, Chen H, Wagenknecht LE, et al; Look AHEAD Research Group. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA 2012; 308:2489–2496.
- Henry RR, Gumbiner B. Benefits and limitations of very-low-calorie diet therapy in obese NIDDM. Diabetes Care 1991; 14:802–823.
- Bistrian BR. Clinical use of a protein-sparing modified fast. JAMA 1978; 240:2299–2302.
- Walters JK, Hoogwerf BJ, Reddy SS. The protein-sparing modified fast for obesity-related medical problems. Cleve Clin J Med 1997; 64:242–244.
- Van Gaal LF, Snyders D, De Leeuw IH, Bekaert JL. Anthropometric and calorimetric evidence for the protein sparing effects of a new protein supplemented low calorie preparation. Am J Clin Nutr 1985; 41:540–544.
- Baker S, Jerums G, Proietto J. Effects and clinical potential of very-low-calorie diets (VLCDs) in type 2 diabetes. Diabetes Res Clin Pract 2009; 85:235–242.
- Shimazu T, Hirschey MD, Newman J, et al. Suppression of oxidative stress by ß-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 2013; 339:211–214.
- Johnstone AM, Horgan GW, Murison SD, Bremner DM, Lobley GE. Effects of a high-protein ketogenic diet on hunger, appetite, and weight loss in obese men feeding ad libitum. Am J Clin Nutr 2008; 87:44–55.
- Westerterp-Plantenga MS, Lemmens SG, Westerterp KR. Dietary protein—its role in satiety, energetics, weight loss and health. Br J Nutr 2012; 108(suppl 2):S105–S112.
- Hemmingsson E, Johansson K, Eriksson J, Sundström J, Neovius M, Marcus C. Weight loss and dropout during a commercial weight-loss program including a very-low-calorie diet, a low-calorie diet, or restricted normal food: observational cohort study. Am J Clin Nutr 2012; 96:953–961.
- Wadden TA, Stunkard AJ, Brownell KD, Day SC. A comparison of two very-low-calorie diets: protein-sparing-modified fast versus protein-formula-liquid diet. Am J Clin Nutr 1985; 41:533–539.
- Isner JM, Sours HE, Paris AL, Ferrans VJ, Roberts WC. Sudden, unexpected death in avid dieters using the liquid-protein-modified-fast diet. Observations in 17 patients and the role of the prolonged QT interval. Circulation 1979; 60:1401–1412.
- Henry RR, Wiest-Kent TA, Scheaffer L, Kolterman OG, Olefsky JM. Metabolic consequences of very-low-calorie diet therapy in obese non-insulin-dependent diabetic and nondiabetic subjects. Diabetes 1986; 35:155–164.
- Seim HC, Mitchell JE, Pomeroy C, de Zwaan M. Electrocardiographic findings associated with very low calorie dieting. Int J Obes Relat Metab Disord 1995; 19:817–819.
- Johansson K, Sundström J, Marcus C, Hemmingsson E, Neovius M. Risk of symptomatic gallstones and cholecystectomy after a very-low-calorie diet or low-calorie diet in a commercial weight loss program: 1-year matched cohort study. Int J Obes (Lond) 2014; 38:279–284.
- Festi D, Colecchia A, Orsini M, et al. Gallbladder motility and gallstone formation in obese patients following very low calorie diets. Use it (fat) to lose it (well). Int J Obes Relat Metab Disord 1998; 22:592–600.
- Palgi A, Read JL, Greenberg I, Hoefer MA, Bistrian BR, Blackburn GL. Multidisciplinary treatment of obesity with a protein-sparing modified fast: results in 668 outpatients. Am J Public Health 1985; 75:1190–1194.
- Li Z, Tseng CH, Li Q, Deng ML, Wang M, Heber D. Clinical efficacy of a medically supervised outpatient high-protein, low-calorie diet program is equivalent in prediabetic, diabetic and normoglycemic obese patients. Nutr Diabetes 2014 Feb 10; 4:e105.
- Genuth S. Supplemented fasting in the treatment of obesity and diabetes. Am J Clin Nutr 1979; 32:2579–2586.
- Baker ST, Jerums G, Prendergast LA, Panagiotopoulos S, Strauss BJ, Proietto J. Less fat reduction per unit weight loss in type 2 diabetic compared with nondiabetic obese individuals completing a very-low-calorie diet program. Metabolism 2012; 61:873–882.
- Wing RR, Marcus MD, Salata R, Epstein LH, Miaskiewicz S, Blair EH. Effects of a very-low-calorie diet on long-term glycemic control in obese type 2 diabetic subjects. Arch Intern Med 1991; 151:1334–1340.
- Wing RR, Blair E, Marcus M, Epstein LH, Harvey J. Year-long weight loss treatment for obese patients with type II diabetes: does including an intermittent very-low-calorie diet improve outcome? Am J Med 1994; 97:354–362.
- Kawamura II, Chen CC, Yamazaki K, Miyazawa Y, Isono K. A clinical study of protein sparing modified fast (PSMF) administered preoperatively to morbidly obese patients: comparison of PSMF with natural food products to originally prepared PSMF. Obes Surg 1992; 2:33–40.
- Hughes TA, Gwynne JT, Switzer BR, Herbst C, White G. Effects of caloric restriction and weight loss on glycemic control, insulin release and resistance, and atherosclerotic risk in obese patients with type II diabetes mellitus. Am J Med 1984; 77:7–17.
- Friedman AN, Chambers M, Kamendulis LM, Temmerman J. Short-term changes after a weight reduction intervention in advanced diabetic nephropathy. Clin J Am Soc Nephrol 2013; 8:1892–1898.
- Wing RR, Blair EH, Bononi P, Marcus MD, Watanabe R, Bergman RN. Caloric restriction per se is a significant factor in improvements in glycemic control and insulin sensitivity during weight loss in obese NIDDM patients. Diabetes Care 1994; 17:30–36.
- Paisey RB, Frost J, Harvey P, et al. Five year results of a prospective very low calorie diet or conventional weight loss programme in type 2 diabetes. J Hum Nutr Diet 2002; 15:121–127.
- Blackburn GL. Weight of the nation: moving forward, reversing the trend using medical care. Am J Clin Nutr 2012; 96:949–950.
- Wadden TA, Neiberg RH, Wing RR, et al; Look AHEAD Research Group. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity (Silver Spring) 2011; 19:1987–1998.
- Redmon JB, Bertoni AG, Connelly S, et al; Look AHEAD Research Group. Effect of the Look AHEAD intervention on medication use and related cost to treat cardiovascular disease risk factors in individuals with type 2 diabetes. Diabetes Care 2010; 33:1153–1158.
KEY POINTS
- The PSMF is indicated in patients who have a body mass index (BMI) of 30 kg/m2 or more, or a BMI of 27 kg/m2 or more with one or more comorbidities such as type 2 diabetes.
- The PSMF provides less than 800 kcal/day during an initial intensive phase of about 6 months, with gradual reintroduction of calories during a refeeding phase lasting 6 to 8 weeks.
- Patients on the PSMF under medical supervision rapidly lose fat while maintaining lean body mass.
- Unfortunately, many patients tend to regain weight after completing a PSMF program. Additional strategies are needed to maintain weight loss.