User login
Acute-Care Surgery Hospitalists: Coming to a Medical Center Near You?
A surgical hospitalist program that's been shown to improve clinical outcomes and reduce inpatient length of stay (LOS) in a non-trauma setting is replicable across the country, says the author of a recent study.
Published in the Journal of the American College of Surgeons, the study reports that a team of surgeons dedicated solely to acute-care surgeries at Sutter Medical Center in Sacramento, Calif., decreased patients' LOS (6.5 days to 5.7 days, P<0.0016), hospital costs ($12,009 to $8306, P<0.0001), and overall complications (21% to 12%, P<0.0001).
Readmissions also "showed a downward trend" but not enough to be statistically significant. The retrospective review looked at the five-year period from 2007 to 2011, which represented the year before the service was initiated and the subsequent four years in which it was practiced.
"By decreasing variation, we improved outcomes and we improved efficiencies," says study author Leon Owens, MD, FACS, president and chief executive officer of the Sacramento-based Surgical Affiliates Management Group, which provided the surgical coverage for Sutter Medical Center. "So we wound up saving money and taking better care of patients. We succeeded because of the uniformity of how we approached the problems and because our team of surgeons is dedicated to doing these surgeries. They do not additionally need to do an elective surgery or deal with other distractions at their office."
Dr. Owens says he believes a well-managed team of acute-care surgeons can improve patient care at any institution that chooses to institute a similar program. "It's not only the brilliance of our doctors but the methodology," he adds. "Our doctors are bright and good, but if you get group-oriented, team-willing, competent surgeons, I believe this is reproducible. We believe this is going to be a common practice across the country. It's just a matter of time to get there." TH
Visit our website for more information on surgical hospitalists.
A surgical hospitalist program that's been shown to improve clinical outcomes and reduce inpatient length of stay (LOS) in a non-trauma setting is replicable across the country, says the author of a recent study.
Published in the Journal of the American College of Surgeons, the study reports that a team of surgeons dedicated solely to acute-care surgeries at Sutter Medical Center in Sacramento, Calif., decreased patients' LOS (6.5 days to 5.7 days, P<0.0016), hospital costs ($12,009 to $8306, P<0.0001), and overall complications (21% to 12%, P<0.0001).
Readmissions also "showed a downward trend" but not enough to be statistically significant. The retrospective review looked at the five-year period from 2007 to 2011, which represented the year before the service was initiated and the subsequent four years in which it was practiced.
"By decreasing variation, we improved outcomes and we improved efficiencies," says study author Leon Owens, MD, FACS, president and chief executive officer of the Sacramento-based Surgical Affiliates Management Group, which provided the surgical coverage for Sutter Medical Center. "So we wound up saving money and taking better care of patients. We succeeded because of the uniformity of how we approached the problems and because our team of surgeons is dedicated to doing these surgeries. They do not additionally need to do an elective surgery or deal with other distractions at their office."
Dr. Owens says he believes a well-managed team of acute-care surgeons can improve patient care at any institution that chooses to institute a similar program. "It's not only the brilliance of our doctors but the methodology," he adds. "Our doctors are bright and good, but if you get group-oriented, team-willing, competent surgeons, I believe this is reproducible. We believe this is going to be a common practice across the country. It's just a matter of time to get there." TH
Visit our website for more information on surgical hospitalists.
A surgical hospitalist program that's been shown to improve clinical outcomes and reduce inpatient length of stay (LOS) in a non-trauma setting is replicable across the country, says the author of a recent study.
Published in the Journal of the American College of Surgeons, the study reports that a team of surgeons dedicated solely to acute-care surgeries at Sutter Medical Center in Sacramento, Calif., decreased patients' LOS (6.5 days to 5.7 days, P<0.0016), hospital costs ($12,009 to $8306, P<0.0001), and overall complications (21% to 12%, P<0.0001).
Readmissions also "showed a downward trend" but not enough to be statistically significant. The retrospective review looked at the five-year period from 2007 to 2011, which represented the year before the service was initiated and the subsequent four years in which it was practiced.
"By decreasing variation, we improved outcomes and we improved efficiencies," says study author Leon Owens, MD, FACS, president and chief executive officer of the Sacramento-based Surgical Affiliates Management Group, which provided the surgical coverage for Sutter Medical Center. "So we wound up saving money and taking better care of patients. We succeeded because of the uniformity of how we approached the problems and because our team of surgeons is dedicated to doing these surgeries. They do not additionally need to do an elective surgery or deal with other distractions at their office."
Dr. Owens says he believes a well-managed team of acute-care surgeons can improve patient care at any institution that chooses to institute a similar program. "It's not only the brilliance of our doctors but the methodology," he adds. "Our doctors are bright and good, but if you get group-oriented, team-willing, competent surgeons, I believe this is reproducible. We believe this is going to be a common practice across the country. It's just a matter of time to get there." TH
Visit our website for more information on surgical hospitalists.
Epidemiology, Consequences of Non-Leg VTE in Critically Ill Patients
Clinical question: Which risk factors are key in the development of non-leg deep vein thromboses (NLDVTs), and what are the expected clinical sequelae from these events?
Background: Critically ill patients are at increased risk of venous thrombosis. Despite adherence to recommended daily thromboprophylaxis, many patients will develop a venous thrombosis in a vein other than the lower extremity. The association between NLDVT and pulmonary embolism (PE) or death is less clearly identified.
Study design: The PROphylaxis for ThromboEmbolism in Critical Care Trial (PROTECT), a multicenter, randomized, blinded, and concealed prospective cohort study occurring between May 2006 and June 2010.
Setting: Sixty-seven international secondary and tertiary care ICUs in both academic and community settings.
Synopsis: Researchers enrolled 3,746 ICU patients in a randomized controlled trial of dalteparin versus standard heparin for thromboprophylaxis. Of these patients, 84 (2.2%) developed a NLDVT. These thromboses were more likely to be deep and located proximally.
Risk factors were assessed using five selected variables: APACHE (acute physiology and chronic health evaluation), BMI, malignancy, and treatment with vasopressors or statins. Outside of indwelling upper extremity central venous catheters, cancer was the only independent predictor of NLDVT.
Compared to patients without any VTE, those with NLDVT were more likely to develop PE (14.9% versus 1.9%) and have longer ICU stays (19 versus nine days). On average, one in seven patients with NLDVT developed PE during the hospital stay. Despite the association with PE, NLDVT was not associated with an increased ICU mortality in an adjusted model. However, the PROTECT trial may have been underpowered to detect a difference. Additional limitations of the study included a relatively small total number of NLDVTs and a lack of standardized screening protocols for both NLDVT and PE.
Bottom line: Despite universal heparin thromboprophylaxis, many medical-surgical critically ill patients may develop NLDVT, placing them at higher risk for longer ICU stays and PE. TH
Citation: Lamontagne F, McIntyre L, Dodek P, et al. Nonleg venous thrombosis in critically ill adults: a nested prospective cohort study. JAMA Intern Med. 2014;174(5):689-696.
Clinical question: Which risk factors are key in the development of non-leg deep vein thromboses (NLDVTs), and what are the expected clinical sequelae from these events?
Background: Critically ill patients are at increased risk of venous thrombosis. Despite adherence to recommended daily thromboprophylaxis, many patients will develop a venous thrombosis in a vein other than the lower extremity. The association between NLDVT and pulmonary embolism (PE) or death is less clearly identified.
Study design: The PROphylaxis for ThromboEmbolism in Critical Care Trial (PROTECT), a multicenter, randomized, blinded, and concealed prospective cohort study occurring between May 2006 and June 2010.
Setting: Sixty-seven international secondary and tertiary care ICUs in both academic and community settings.
Synopsis: Researchers enrolled 3,746 ICU patients in a randomized controlled trial of dalteparin versus standard heparin for thromboprophylaxis. Of these patients, 84 (2.2%) developed a NLDVT. These thromboses were more likely to be deep and located proximally.
Risk factors were assessed using five selected variables: APACHE (acute physiology and chronic health evaluation), BMI, malignancy, and treatment with vasopressors or statins. Outside of indwelling upper extremity central venous catheters, cancer was the only independent predictor of NLDVT.
Compared to patients without any VTE, those with NLDVT were more likely to develop PE (14.9% versus 1.9%) and have longer ICU stays (19 versus nine days). On average, one in seven patients with NLDVT developed PE during the hospital stay. Despite the association with PE, NLDVT was not associated with an increased ICU mortality in an adjusted model. However, the PROTECT trial may have been underpowered to detect a difference. Additional limitations of the study included a relatively small total number of NLDVTs and a lack of standardized screening protocols for both NLDVT and PE.
Bottom line: Despite universal heparin thromboprophylaxis, many medical-surgical critically ill patients may develop NLDVT, placing them at higher risk for longer ICU stays and PE. TH
Citation: Lamontagne F, McIntyre L, Dodek P, et al. Nonleg venous thrombosis in critically ill adults: a nested prospective cohort study. JAMA Intern Med. 2014;174(5):689-696.
Clinical question: Which risk factors are key in the development of non-leg deep vein thromboses (NLDVTs), and what are the expected clinical sequelae from these events?
Background: Critically ill patients are at increased risk of venous thrombosis. Despite adherence to recommended daily thromboprophylaxis, many patients will develop a venous thrombosis in a vein other than the lower extremity. The association between NLDVT and pulmonary embolism (PE) or death is less clearly identified.
Study design: The PROphylaxis for ThromboEmbolism in Critical Care Trial (PROTECT), a multicenter, randomized, blinded, and concealed prospective cohort study occurring between May 2006 and June 2010.
Setting: Sixty-seven international secondary and tertiary care ICUs in both academic and community settings.
Synopsis: Researchers enrolled 3,746 ICU patients in a randomized controlled trial of dalteparin versus standard heparin for thromboprophylaxis. Of these patients, 84 (2.2%) developed a NLDVT. These thromboses were more likely to be deep and located proximally.
Risk factors were assessed using five selected variables: APACHE (acute physiology and chronic health evaluation), BMI, malignancy, and treatment with vasopressors or statins. Outside of indwelling upper extremity central venous catheters, cancer was the only independent predictor of NLDVT.
Compared to patients without any VTE, those with NLDVT were more likely to develop PE (14.9% versus 1.9%) and have longer ICU stays (19 versus nine days). On average, one in seven patients with NLDVT developed PE during the hospital stay. Despite the association with PE, NLDVT was not associated with an increased ICU mortality in an adjusted model. However, the PROTECT trial may have been underpowered to detect a difference. Additional limitations of the study included a relatively small total number of NLDVTs and a lack of standardized screening protocols for both NLDVT and PE.
Bottom line: Despite universal heparin thromboprophylaxis, many medical-surgical critically ill patients may develop NLDVT, placing them at higher risk for longer ICU stays and PE. TH
Citation: Lamontagne F, McIntyre L, Dodek P, et al. Nonleg venous thrombosis in critically ill adults: a nested prospective cohort study. JAMA Intern Med. 2014;174(5):689-696.
Early data indicate D-cycloserine augments effects of virtual reality treatment for PTSD
WASHINGTON – The use of D-cycloserine has promise as a way to augment the beneficial effects of virtual reality therapy of posttraumatic stress disorder, results of two recently published studies show. The results were discussed during a symposium at the annual convention of the American Psychological Association.
In one study of Iraq and Afghanistan veterans, the use of D-cycloserine (DCS) did not provide an advantage overall, compared with alprazolam or placebo. However, DCS was associated with favorable effects on cortisol and startle reactivity, compared with the other two groups, said one of the authors, Tanja Jovanovic, Ph.D. In another trial, a small proof-of concept study, the PTSD remission rate 6 months after treatment was almost 70% among those treated with a combination of virtual reality (VR) therapy and DCS, compared with 17% among those treated with VR therapy and placebo.
DCS, an NMDA (N-methyl-D-aspartate)-receptor partial agonist approved as an antibacterial by the Food and Drug Administration, has been found to enhance exposure therapy for conditions that include social anxiety and acrophobia in previous studies. NMDA also has been found to facilitate extinction learning in animal studies, said Dr. Jovanovic director of the neurophysiology laboratory at the Grady Trauma Project at Emory University, Atlanta.
In the study, 156 veterans of the Iraq and Afghanistan wars were randomized to treatment with DCS (50 mg), alprazolam (0.25 mg), or placebo plus five sessions of virtual reality exposure therapy (after an introductory VR session). Assessments of patients – which included evaluation of PTSD symptoms, psychophysiologic responses, and cortisol reactivity – were performed before treatment and 3, 6, and 12 months after treatment. Monitoring included placing electrodes under the eye to measure the contraction of the eye blink muscle and skin conductance testing during exposure to the VR scenes (two convoy explosion scenes and a city scene), said Dr. Jovanovic, one of the authors of the study, which was published in June (Am. J. Psychiatry 2014;171:640-8).
After five series of VR treatment, PTSD symptoms significantly decreased in all three groups after treatment, based on changes on the Clinician-Administered PTSD Scale (CAPS) score, but the greatest degree of reduction in symptoms at 12 months was observed in the DCS group, she said. Those treated with DCS "showed the biggest decline and actually maintained those gains at 6 months, which we did not see with the other groups."
In addition, those treated with DCS had a reduction in cortisol and startle reactivity that was greater than the changes observed in the two other groups. The magnitude of startle reactivity at baseline was related to the change in the CAPS score 6 months later, "so those gains they are maintaining at 6 months are predicted by their initial response to the virtual reality session pretreatment ... the more reactive, the better they got," Dr. Jovanovic reported.
The same also was true for skin conductance findings: The more reactive they were at baseline in this measure, the more improved the patients were at follow-up, but this association was only evident in the DCS-treated patients, Dr. Jovanovic said.
Measurements of cortisol levels before exposure to the VR scenes, immediately afterward, and 15 minutes afterward determined that cortisol reactivity was attenuated with treatment. Cortisol reactivity significantly dropped in all three groups, from before treatment to the 6-month follow-up but was the lowest in the DCS-treated patients. In the alprazolam-treated group, the higher the cortisol reactivity was before treatment, the worse the outcomes were with treatment, the reverse of what was seen with other psychophysiological measures, she added.
During the same symposium on the use of VR in the treatment of PTSD, JoAnn Difede, Ph.D., professor of psychiatry, Cornell University, New York, described the use of DCS "as a cognitive enhancer" for treatment of people with PTSD related to the World Trade Center attacks in 2001. "We see this as very promising," she said.
In one double-blind, proof-of-concept study, 25 people with PTSD were randomized to DCS (100 mg) or placebo administered 90 minutes before weekly sessions of VR therapy, timed so that plasma concentrations would peak during the session. In the placebo group, 3 dropped out, but none of the 13 patients in the DCS group dropped out (Neuropsychopharmacology 2014; 39:1052-8).
Six months after treatment, 9 of the 13 patients (69%) in the DCS group were in remission, compared with 2 of the 12 (17%) on placebo plus VR exposure. Remission was defined as a CAPS total score of 20 or less, and minimal or no impairment in social, occupational, and other important areas of function, as judged by an independent blinded assessor.
Dr. Difede, also director of the program for anxiety and traumatic stress studies at New York-Presbyterian Hospital, said the two groups began to diverge at the third session, and those who received DCS continued to improve 6 months later. A post-hoc analysis identified a "drastic improvement" in anger and sleep among those treated with DCS, a finding that she and her associates plan to look at more closely. The study presented by Dr. Jovanovic was funded by the National Institute of Mental Health. Dr. Difede received partial funding support from DeWitt-Wallace Fund of the New York Community Trust. The trust was not involved in the design, data collection, or in any other aspects of the study.
WASHINGTON – The use of D-cycloserine has promise as a way to augment the beneficial effects of virtual reality therapy of posttraumatic stress disorder, results of two recently published studies show. The results were discussed during a symposium at the annual convention of the American Psychological Association.
In one study of Iraq and Afghanistan veterans, the use of D-cycloserine (DCS) did not provide an advantage overall, compared with alprazolam or placebo. However, DCS was associated with favorable effects on cortisol and startle reactivity, compared with the other two groups, said one of the authors, Tanja Jovanovic, Ph.D. In another trial, a small proof-of concept study, the PTSD remission rate 6 months after treatment was almost 70% among those treated with a combination of virtual reality (VR) therapy and DCS, compared with 17% among those treated with VR therapy and placebo.
DCS, an NMDA (N-methyl-D-aspartate)-receptor partial agonist approved as an antibacterial by the Food and Drug Administration, has been found to enhance exposure therapy for conditions that include social anxiety and acrophobia in previous studies. NMDA also has been found to facilitate extinction learning in animal studies, said Dr. Jovanovic director of the neurophysiology laboratory at the Grady Trauma Project at Emory University, Atlanta.
In the study, 156 veterans of the Iraq and Afghanistan wars were randomized to treatment with DCS (50 mg), alprazolam (0.25 mg), or placebo plus five sessions of virtual reality exposure therapy (after an introductory VR session). Assessments of patients – which included evaluation of PTSD symptoms, psychophysiologic responses, and cortisol reactivity – were performed before treatment and 3, 6, and 12 months after treatment. Monitoring included placing electrodes under the eye to measure the contraction of the eye blink muscle and skin conductance testing during exposure to the VR scenes (two convoy explosion scenes and a city scene), said Dr. Jovanovic, one of the authors of the study, which was published in June (Am. J. Psychiatry 2014;171:640-8).
After five series of VR treatment, PTSD symptoms significantly decreased in all three groups after treatment, based on changes on the Clinician-Administered PTSD Scale (CAPS) score, but the greatest degree of reduction in symptoms at 12 months was observed in the DCS group, she said. Those treated with DCS "showed the biggest decline and actually maintained those gains at 6 months, which we did not see with the other groups."
In addition, those treated with DCS had a reduction in cortisol and startle reactivity that was greater than the changes observed in the two other groups. The magnitude of startle reactivity at baseline was related to the change in the CAPS score 6 months later, "so those gains they are maintaining at 6 months are predicted by their initial response to the virtual reality session pretreatment ... the more reactive, the better they got," Dr. Jovanovic reported.
The same also was true for skin conductance findings: The more reactive they were at baseline in this measure, the more improved the patients were at follow-up, but this association was only evident in the DCS-treated patients, Dr. Jovanovic said.
Measurements of cortisol levels before exposure to the VR scenes, immediately afterward, and 15 minutes afterward determined that cortisol reactivity was attenuated with treatment. Cortisol reactivity significantly dropped in all three groups, from before treatment to the 6-month follow-up but was the lowest in the DCS-treated patients. In the alprazolam-treated group, the higher the cortisol reactivity was before treatment, the worse the outcomes were with treatment, the reverse of what was seen with other psychophysiological measures, she added.
During the same symposium on the use of VR in the treatment of PTSD, JoAnn Difede, Ph.D., professor of psychiatry, Cornell University, New York, described the use of DCS "as a cognitive enhancer" for treatment of people with PTSD related to the World Trade Center attacks in 2001. "We see this as very promising," she said.
In one double-blind, proof-of-concept study, 25 people with PTSD were randomized to DCS (100 mg) or placebo administered 90 minutes before weekly sessions of VR therapy, timed so that plasma concentrations would peak during the session. In the placebo group, 3 dropped out, but none of the 13 patients in the DCS group dropped out (Neuropsychopharmacology 2014; 39:1052-8).
Six months after treatment, 9 of the 13 patients (69%) in the DCS group were in remission, compared with 2 of the 12 (17%) on placebo plus VR exposure. Remission was defined as a CAPS total score of 20 or less, and minimal or no impairment in social, occupational, and other important areas of function, as judged by an independent blinded assessor.
Dr. Difede, also director of the program for anxiety and traumatic stress studies at New York-Presbyterian Hospital, said the two groups began to diverge at the third session, and those who received DCS continued to improve 6 months later. A post-hoc analysis identified a "drastic improvement" in anger and sleep among those treated with DCS, a finding that she and her associates plan to look at more closely. The study presented by Dr. Jovanovic was funded by the National Institute of Mental Health. Dr. Difede received partial funding support from DeWitt-Wallace Fund of the New York Community Trust. The trust was not involved in the design, data collection, or in any other aspects of the study.
WASHINGTON – The use of D-cycloserine has promise as a way to augment the beneficial effects of virtual reality therapy of posttraumatic stress disorder, results of two recently published studies show. The results were discussed during a symposium at the annual convention of the American Psychological Association.
In one study of Iraq and Afghanistan veterans, the use of D-cycloserine (DCS) did not provide an advantage overall, compared with alprazolam or placebo. However, DCS was associated with favorable effects on cortisol and startle reactivity, compared with the other two groups, said one of the authors, Tanja Jovanovic, Ph.D. In another trial, a small proof-of concept study, the PTSD remission rate 6 months after treatment was almost 70% among those treated with a combination of virtual reality (VR) therapy and DCS, compared with 17% among those treated with VR therapy and placebo.
DCS, an NMDA (N-methyl-D-aspartate)-receptor partial agonist approved as an antibacterial by the Food and Drug Administration, has been found to enhance exposure therapy for conditions that include social anxiety and acrophobia in previous studies. NMDA also has been found to facilitate extinction learning in animal studies, said Dr. Jovanovic director of the neurophysiology laboratory at the Grady Trauma Project at Emory University, Atlanta.
In the study, 156 veterans of the Iraq and Afghanistan wars were randomized to treatment with DCS (50 mg), alprazolam (0.25 mg), or placebo plus five sessions of virtual reality exposure therapy (after an introductory VR session). Assessments of patients – which included evaluation of PTSD symptoms, psychophysiologic responses, and cortisol reactivity – were performed before treatment and 3, 6, and 12 months after treatment. Monitoring included placing electrodes under the eye to measure the contraction of the eye blink muscle and skin conductance testing during exposure to the VR scenes (two convoy explosion scenes and a city scene), said Dr. Jovanovic, one of the authors of the study, which was published in June (Am. J. Psychiatry 2014;171:640-8).
After five series of VR treatment, PTSD symptoms significantly decreased in all three groups after treatment, based on changes on the Clinician-Administered PTSD Scale (CAPS) score, but the greatest degree of reduction in symptoms at 12 months was observed in the DCS group, she said. Those treated with DCS "showed the biggest decline and actually maintained those gains at 6 months, which we did not see with the other groups."
In addition, those treated with DCS had a reduction in cortisol and startle reactivity that was greater than the changes observed in the two other groups. The magnitude of startle reactivity at baseline was related to the change in the CAPS score 6 months later, "so those gains they are maintaining at 6 months are predicted by their initial response to the virtual reality session pretreatment ... the more reactive, the better they got," Dr. Jovanovic reported.
The same also was true for skin conductance findings: The more reactive they were at baseline in this measure, the more improved the patients were at follow-up, but this association was only evident in the DCS-treated patients, Dr. Jovanovic said.
Measurements of cortisol levels before exposure to the VR scenes, immediately afterward, and 15 minutes afterward determined that cortisol reactivity was attenuated with treatment. Cortisol reactivity significantly dropped in all three groups, from before treatment to the 6-month follow-up but was the lowest in the DCS-treated patients. In the alprazolam-treated group, the higher the cortisol reactivity was before treatment, the worse the outcomes were with treatment, the reverse of what was seen with other psychophysiological measures, she added.
During the same symposium on the use of VR in the treatment of PTSD, JoAnn Difede, Ph.D., professor of psychiatry, Cornell University, New York, described the use of DCS "as a cognitive enhancer" for treatment of people with PTSD related to the World Trade Center attacks in 2001. "We see this as very promising," she said.
In one double-blind, proof-of-concept study, 25 people with PTSD were randomized to DCS (100 mg) or placebo administered 90 minutes before weekly sessions of VR therapy, timed so that plasma concentrations would peak during the session. In the placebo group, 3 dropped out, but none of the 13 patients in the DCS group dropped out (Neuropsychopharmacology 2014; 39:1052-8).
Six months after treatment, 9 of the 13 patients (69%) in the DCS group were in remission, compared with 2 of the 12 (17%) on placebo plus VR exposure. Remission was defined as a CAPS total score of 20 or less, and minimal or no impairment in social, occupational, and other important areas of function, as judged by an independent blinded assessor.
Dr. Difede, also director of the program for anxiety and traumatic stress studies at New York-Presbyterian Hospital, said the two groups began to diverge at the third session, and those who received DCS continued to improve 6 months later. A post-hoc analysis identified a "drastic improvement" in anger and sleep among those treated with DCS, a finding that she and her associates plan to look at more closely. The study presented by Dr. Jovanovic was funded by the National Institute of Mental Health. Dr. Difede received partial funding support from DeWitt-Wallace Fund of the New York Community Trust. The trust was not involved in the design, data collection, or in any other aspects of the study.
EXPERT ANALYSIS AT THE 2014 APA CONVENTION
Preventing postoperative neuropathies: Patient positioning for minimally invasive procedures
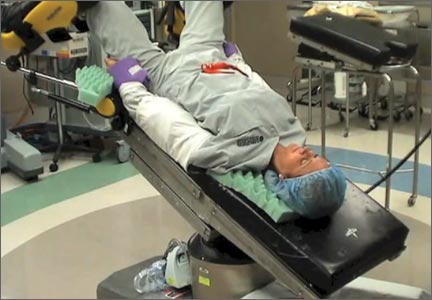
In this comprehensive educational video we review appropriate patient positioning for laparoscopic and robotic surgery to prevent postoperative neuropathies that can be experienced with gynecologic surgery. We also include a case-based review of injuries specific to the brachial plexus, ulnar nerve, and femoral nerve.
Our technique involves the use of a bed sheet, an egg crate foam mattress pad, and boot-type stirrups. We recommend setting up the operating room table to facilitate tucking of the patient’s arms and to prevent slippage of the patient when she is placed in steep Trendelenburg. For all steps involved, see the video.
Tips for setting up the operating room bed include:
- Use of a single bed sheet placed across the head of a bare table with an egg crate foam mattress pad over the sheet to prevent the need for strapping the patient to the bed or the use of shoulder braces to prevent slippage.
- For low dorsal lithotomy positioning, flex the patient’s hips with a trunk-to-thigh angle of approximately 170°, and never more than 180°.
- For arm tucking, remove the arm boards and excess egg crate foam from the patient’s side and placecushioning over the elbow and the wrist. Keep the patient’s hand pronated when tucking and do not allow the arm to hang over the side of the bed.
- If the patient is obese, support the tucked arm by placing the arm boards beneath the arm parallel to the bed.
Next month we continue our series on surgical techniques with a video on why choosing the proper colpotomy cup is critical for successful minimally invasive hysterectomy.

Will you be joining me at the AAGL Global Congress on Minimally Invasive Gynecology in Vancouver this November? Safe patient positioning for minimally invasive surgery and other exciting topics will be discussed. Visit www.aagl.org/globalcongress for more information.
—Dr. Arnold Advincula, AAGL 2014 Scientific Program Chair
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In this comprehensive educational video we review appropriate patient positioning for laparoscopic and robotic surgery to prevent postoperative neuropathies that can be experienced with gynecologic surgery. We also include a case-based review of injuries specific to the brachial plexus, ulnar nerve, and femoral nerve.
Our technique involves the use of a bed sheet, an egg crate foam mattress pad, and boot-type stirrups. We recommend setting up the operating room table to facilitate tucking of the patient’s arms and to prevent slippage of the patient when she is placed in steep Trendelenburg. For all steps involved, see the video.
Tips for setting up the operating room bed include:
- Use of a single bed sheet placed across the head of a bare table with an egg crate foam mattress pad over the sheet to prevent the need for strapping the patient to the bed or the use of shoulder braces to prevent slippage.
- For low dorsal lithotomy positioning, flex the patient’s hips with a trunk-to-thigh angle of approximately 170°, and never more than 180°.
- For arm tucking, remove the arm boards and excess egg crate foam from the patient’s side and placecushioning over the elbow and the wrist. Keep the patient’s hand pronated when tucking and do not allow the arm to hang over the side of the bed.
- If the patient is obese, support the tucked arm by placing the arm boards beneath the arm parallel to the bed.
Next month we continue our series on surgical techniques with a video on why choosing the proper colpotomy cup is critical for successful minimally invasive hysterectomy.

Will you be joining me at the AAGL Global Congress on Minimally Invasive Gynecology in Vancouver this November? Safe patient positioning for minimally invasive surgery and other exciting topics will be discussed. Visit www.aagl.org/globalcongress for more information.
—Dr. Arnold Advincula, AAGL 2014 Scientific Program Chair
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In this comprehensive educational video we review appropriate patient positioning for laparoscopic and robotic surgery to prevent postoperative neuropathies that can be experienced with gynecologic surgery. We also include a case-based review of injuries specific to the brachial plexus, ulnar nerve, and femoral nerve.
Our technique involves the use of a bed sheet, an egg crate foam mattress pad, and boot-type stirrups. We recommend setting up the operating room table to facilitate tucking of the patient’s arms and to prevent slippage of the patient when she is placed in steep Trendelenburg. For all steps involved, see the video.
Tips for setting up the operating room bed include:
- Use of a single bed sheet placed across the head of a bare table with an egg crate foam mattress pad over the sheet to prevent the need for strapping the patient to the bed or the use of shoulder braces to prevent slippage.
- For low dorsal lithotomy positioning, flex the patient’s hips with a trunk-to-thigh angle of approximately 170°, and never more than 180°.
- For arm tucking, remove the arm boards and excess egg crate foam from the patient’s side and placecushioning over the elbow and the wrist. Keep the patient’s hand pronated when tucking and do not allow the arm to hang over the side of the bed.
- If the patient is obese, support the tucked arm by placing the arm boards beneath the arm parallel to the bed.
Next month we continue our series on surgical techniques with a video on why choosing the proper colpotomy cup is critical for successful minimally invasive hysterectomy.

Will you be joining me at the AAGL Global Congress on Minimally Invasive Gynecology in Vancouver this November? Safe patient positioning for minimally invasive surgery and other exciting topics will be discussed. Visit www.aagl.org/globalcongress for more information.
—Dr. Arnold Advincula, AAGL 2014 Scientific Program Chair
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Isolated systolic hypertension linked to angina; isolated diastolic hypertension linked to AAA
Isolated high systolic blood pressure is strongly associated with risk for intracerebral hemorrhage, subarachnoid hemorrhage, and stable angina, whereas isolated high diastolic blood pressure is associated with risk for abdominal aortic aneurysm, according to findings published in the Lancet.
The observations debunk widespread assumptions that isolated systolic and diastolic blood pressures are similarly and consistently associated with cardiovascular diseases, wrote Dr. Eleni Rapsomaniki and associates of the Farr Institute of Health Informatics Research in London (Lancet 2014;383:1899-1911).
"Our data support the possibility that blood pressure functions through different underlying biological mechanisms for different diseases," the researchers wrote. These data can be considered when counseling patients.
The results "also emphasize the limitations of existing blood pressure–lowering strategies," they added. Better implementation of existing blood pressure–lowering treatments and better management of other cardiovascular risk factors as part of global risk estimation could help to mitigate excess risk.
The researchers studied 1.25 million patients, aged 30 years or older, who had no previous history of cardiovascular disease. Baseline blood pressures were recorded during primary care consultations. Patients were classified as having hypertension with a baseline blood pressure reading of 140/90 mm Hg or greater, a previous diagnosis of hypertension, or repeat prescriptions for blood pressure–lowering medications.
Isolated systolic hypertension was defined as a value of 140 mm Hg or higher with a diastolic level of lower than 90 mm Hg. Isolated diastolic hypertension was defined as systolic blood pressure of less than 140 mm Hg and a diastolic level of 90 mm Hg or higher.
Endpoints were defined as the initial presentation of any of 12 cardiovascular diseases.
The primary analysis reported associations of outcomes with a 20/10 mm Hg increase in systolic/diastolic blood pressure by age group (30-59 years, 60-79 years, and 80 years or older), and estimated risks and years of life lost associated with hypertension for the index ages of 30, 60, and 80 years. The secondary analysis reported associations of blood pressure with cardiovascular outcomes, after adjusting for smoking status, diabetes, cholesterol, body mass index, and baseline treatment with hypertension drugs.
Isolated systolic hypertension was strongly associated with intracerebral hemorrhage (hazard ratio, 1.44; 95% confidence interval, 1.32-1.58), subarachnoid hemorrhage (HR, 1.43; CI, 1.25-1.63), and stable angina (HR, 1.41; CI, 1.36-1.46).
Isolated diastolic hypertension was associated with abdominal aortic aneurysm (HR, 1.45; 95% CI, 1.34-1.56).
Peripheral arterial disease had the strongest association with pulse pressure (HR, 1.23; CI, 1.20-1.27).
The lifetime risk of total cardiovascular disease at 30 years of age was 63.3% in people with hypertension and 46·1% in those with healthy blood pressure, with stable and unstable angina as the most frequent outcomes. The mean number of cardiovascular disease–free life years lost in those with hypertension was 5 years at 30 years of age, 3.4 years at 60 years, and 1.6 years at 80 years.
The study was funded by the National Institute for Health Research, the Wellcome Trust, the Medical Research Council Prognosis Research Strategy Partnership, and numerous other sources. The researchers had no competing interests.
This study provides important new information to improve risk assessment, patient counseling, and decision making for patients with hypertension.
Factors that can improve drug compliance and treatment persistence to prescribed therapy ought to be better understood than they are at present. Within 2 years, 35% of patients who start antihypertensive drug therapy discontinue treatment. Furthermore, many patients referred for so-called treatment-resistant hypertension do not take their prescribed medication. Additionally, home blood-pressure monitoring and 24-hour ambulatory blood-pressure monitoring would identify patients susceptible to the white coat effect, improve risk stratification, and increase patient engagement.
People with secondary forms of hypertension can often be offered specific treatment and are thus important to identify, in particular those with apparently treatment-resistant disease. Finally, most patients with remaining uncontrolled hypertension can be well controlled when referred to a specialist
The clinical benefit of improved risk assessment and appropriate treatment might be substantial.
Dr. Thomas Kahan is with the Karolinska Institute in Stockholm, Sweden. He made his remarks in an editorial that accompanied the study. Dr. Kahan reported receiving research grants from Celladon, Medtronic, Pfizer, and Servier.
This study provides important new information to improve risk assessment, patient counseling, and decision making for patients with hypertension.
Factors that can improve drug compliance and treatment persistence to prescribed therapy ought to be better understood than they are at present. Within 2 years, 35% of patients who start antihypertensive drug therapy discontinue treatment. Furthermore, many patients referred for so-called treatment-resistant hypertension do not take their prescribed medication. Additionally, home blood-pressure monitoring and 24-hour ambulatory blood-pressure monitoring would identify patients susceptible to the white coat effect, improve risk stratification, and increase patient engagement.
People with secondary forms of hypertension can often be offered specific treatment and are thus important to identify, in particular those with apparently treatment-resistant disease. Finally, most patients with remaining uncontrolled hypertension can be well controlled when referred to a specialist
The clinical benefit of improved risk assessment and appropriate treatment might be substantial.
Dr. Thomas Kahan is with the Karolinska Institute in Stockholm, Sweden. He made his remarks in an editorial that accompanied the study. Dr. Kahan reported receiving research grants from Celladon, Medtronic, Pfizer, and Servier.
This study provides important new information to improve risk assessment, patient counseling, and decision making for patients with hypertension.
Factors that can improve drug compliance and treatment persistence to prescribed therapy ought to be better understood than they are at present. Within 2 years, 35% of patients who start antihypertensive drug therapy discontinue treatment. Furthermore, many patients referred for so-called treatment-resistant hypertension do not take their prescribed medication. Additionally, home blood-pressure monitoring and 24-hour ambulatory blood-pressure monitoring would identify patients susceptible to the white coat effect, improve risk stratification, and increase patient engagement.
People with secondary forms of hypertension can often be offered specific treatment and are thus important to identify, in particular those with apparently treatment-resistant disease. Finally, most patients with remaining uncontrolled hypertension can be well controlled when referred to a specialist
The clinical benefit of improved risk assessment and appropriate treatment might be substantial.
Dr. Thomas Kahan is with the Karolinska Institute in Stockholm, Sweden. He made his remarks in an editorial that accompanied the study. Dr. Kahan reported receiving research grants from Celladon, Medtronic, Pfizer, and Servier.
Isolated high systolic blood pressure is strongly associated with risk for intracerebral hemorrhage, subarachnoid hemorrhage, and stable angina, whereas isolated high diastolic blood pressure is associated with risk for abdominal aortic aneurysm, according to findings published in the Lancet.
The observations debunk widespread assumptions that isolated systolic and diastolic blood pressures are similarly and consistently associated with cardiovascular diseases, wrote Dr. Eleni Rapsomaniki and associates of the Farr Institute of Health Informatics Research in London (Lancet 2014;383:1899-1911).
"Our data support the possibility that blood pressure functions through different underlying biological mechanisms for different diseases," the researchers wrote. These data can be considered when counseling patients.
The results "also emphasize the limitations of existing blood pressure–lowering strategies," they added. Better implementation of existing blood pressure–lowering treatments and better management of other cardiovascular risk factors as part of global risk estimation could help to mitigate excess risk.
The researchers studied 1.25 million patients, aged 30 years or older, who had no previous history of cardiovascular disease. Baseline blood pressures were recorded during primary care consultations. Patients were classified as having hypertension with a baseline blood pressure reading of 140/90 mm Hg or greater, a previous diagnosis of hypertension, or repeat prescriptions for blood pressure–lowering medications.
Isolated systolic hypertension was defined as a value of 140 mm Hg or higher with a diastolic level of lower than 90 mm Hg. Isolated diastolic hypertension was defined as systolic blood pressure of less than 140 mm Hg and a diastolic level of 90 mm Hg or higher.
Endpoints were defined as the initial presentation of any of 12 cardiovascular diseases.
The primary analysis reported associations of outcomes with a 20/10 mm Hg increase in systolic/diastolic blood pressure by age group (30-59 years, 60-79 years, and 80 years or older), and estimated risks and years of life lost associated with hypertension for the index ages of 30, 60, and 80 years. The secondary analysis reported associations of blood pressure with cardiovascular outcomes, after adjusting for smoking status, diabetes, cholesterol, body mass index, and baseline treatment with hypertension drugs.
Isolated systolic hypertension was strongly associated with intracerebral hemorrhage (hazard ratio, 1.44; 95% confidence interval, 1.32-1.58), subarachnoid hemorrhage (HR, 1.43; CI, 1.25-1.63), and stable angina (HR, 1.41; CI, 1.36-1.46).
Isolated diastolic hypertension was associated with abdominal aortic aneurysm (HR, 1.45; 95% CI, 1.34-1.56).
Peripheral arterial disease had the strongest association with pulse pressure (HR, 1.23; CI, 1.20-1.27).
The lifetime risk of total cardiovascular disease at 30 years of age was 63.3% in people with hypertension and 46·1% in those with healthy blood pressure, with stable and unstable angina as the most frequent outcomes. The mean number of cardiovascular disease–free life years lost in those with hypertension was 5 years at 30 years of age, 3.4 years at 60 years, and 1.6 years at 80 years.
The study was funded by the National Institute for Health Research, the Wellcome Trust, the Medical Research Council Prognosis Research Strategy Partnership, and numerous other sources. The researchers had no competing interests.
Isolated high systolic blood pressure is strongly associated with risk for intracerebral hemorrhage, subarachnoid hemorrhage, and stable angina, whereas isolated high diastolic blood pressure is associated with risk for abdominal aortic aneurysm, according to findings published in the Lancet.
The observations debunk widespread assumptions that isolated systolic and diastolic blood pressures are similarly and consistently associated with cardiovascular diseases, wrote Dr. Eleni Rapsomaniki and associates of the Farr Institute of Health Informatics Research in London (Lancet 2014;383:1899-1911).
"Our data support the possibility that blood pressure functions through different underlying biological mechanisms for different diseases," the researchers wrote. These data can be considered when counseling patients.
The results "also emphasize the limitations of existing blood pressure–lowering strategies," they added. Better implementation of existing blood pressure–lowering treatments and better management of other cardiovascular risk factors as part of global risk estimation could help to mitigate excess risk.
The researchers studied 1.25 million patients, aged 30 years or older, who had no previous history of cardiovascular disease. Baseline blood pressures were recorded during primary care consultations. Patients were classified as having hypertension with a baseline blood pressure reading of 140/90 mm Hg or greater, a previous diagnosis of hypertension, or repeat prescriptions for blood pressure–lowering medications.
Isolated systolic hypertension was defined as a value of 140 mm Hg or higher with a diastolic level of lower than 90 mm Hg. Isolated diastolic hypertension was defined as systolic blood pressure of less than 140 mm Hg and a diastolic level of 90 mm Hg or higher.
Endpoints were defined as the initial presentation of any of 12 cardiovascular diseases.
The primary analysis reported associations of outcomes with a 20/10 mm Hg increase in systolic/diastolic blood pressure by age group (30-59 years, 60-79 years, and 80 years or older), and estimated risks and years of life lost associated with hypertension for the index ages of 30, 60, and 80 years. The secondary analysis reported associations of blood pressure with cardiovascular outcomes, after adjusting for smoking status, diabetes, cholesterol, body mass index, and baseline treatment with hypertension drugs.
Isolated systolic hypertension was strongly associated with intracerebral hemorrhage (hazard ratio, 1.44; 95% confidence interval, 1.32-1.58), subarachnoid hemorrhage (HR, 1.43; CI, 1.25-1.63), and stable angina (HR, 1.41; CI, 1.36-1.46).
Isolated diastolic hypertension was associated with abdominal aortic aneurysm (HR, 1.45; 95% CI, 1.34-1.56).
Peripheral arterial disease had the strongest association with pulse pressure (HR, 1.23; CI, 1.20-1.27).
The lifetime risk of total cardiovascular disease at 30 years of age was 63.3% in people with hypertension and 46·1% in those with healthy blood pressure, with stable and unstable angina as the most frequent outcomes. The mean number of cardiovascular disease–free life years lost in those with hypertension was 5 years at 30 years of age, 3.4 years at 60 years, and 1.6 years at 80 years.
The study was funded by the National Institute for Health Research, the Wellcome Trust, the Medical Research Council Prognosis Research Strategy Partnership, and numerous other sources. The researchers had no competing interests.
FROM THE LANCET
Key clinical point: Isolated systolic and diastolic blood pressures are associated with increased risks for different types of cardiovascular disease.
Major finding: High systolic BP was strongly associated with intracerebral hemorrhage (HR, 1.44) and stable angina (HR, 1.41). High diastolic BP was associated with abdominal aortic aneurysm (HR, 1.45).
Data source: A study of the association of blood pressure with 12 cardiovascular diseases in 1.25 million patients in the CALIBER program, 30 years of age and older with no previous diagnosis of CVD.
Disclosures: The study was largely funded by the National Institute for Health Research, the Wellcome Trust, and the Medical Research Council Prognosis Research Strategy Partnership. The researchers declared no competing interests.
Evidence questioned in debate over monitoring dabigatran levels to avert bleeds
The manufacturer of the oral anticoagulant dabigatran "withheld analyses that calculate how many major bleeds dose adjustment could prevent," the BMJ charges in an article based on internal documents released during litigation in the United States and freedom of information act requests obtained by the journal.
Boehringer Ingelheim "found that if the plasma levels of the drug were measured and the dose was adjusted accordingly major bleeds could be reduced by 30%-40%, compared with well-controlled warfarin," according to the article (BMJ 2014;349:g4670), which was published along with an editorial and an analysis. The article further stated that the manufacturer "has failed to share with regulators information about the potential benefits of monitoring anticoagulant activity and adjusting the dose to make sure the drug is working as safely and effectively as possible."
In the analysis, Thomas Moore, senior scientist at the Institute for Safe Medication Practices, Horsham, Pa., and his coauthors said that "the bleeding risk of dabigatran can be reduced and efficacy improved by individualizing the dose in patients based on plasma level, age, and kidney function" (BMJ 2014;349:g4517[doi:10.1136/bmj.g4517]).
Dr. Rita Redberg and Dr. Blake Charlton of the University of California, San Francisco, wrote in an accompanying editorial that the analysis "illuminates a lack of transparency about the safety of unmonitored dabigatran, compounded by the drug’s fickle pharmacokinetics, which can cause a fivefold variation of plasma concentration." (BMJ 2014;349:g4681 [doi:10.1136/bmj.g4681]).
The BMJ article, written by Deborah Cohen, includes responses from the manufacturer of dabigatran (Pradaxa).
Boehringer Ingelheim officials have asserted that no data were withheld. Additionally, the company has released a statement calling the BMJ article "biased" and "misleading."
Dabigatran is a direct thrombin inhibitor that was approved in the United States in October 2010 for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation at a fixed dose of 150 mg or 75 mg twice a day, with the lower dose recommended for those with renal impairment. Since its initial approval, dabigatran has gained indications for treating and reducing the risk of recurrence of deep venous thrombosis and pulmonary embolism.
The drug’s approval was based on the results of the Randomized Evaluation of Long Term Anticoagulant Therapy (RE-LY) trial, which compared the drug to warfarin in 18,113 patients with nonvalvular atrial fibrillation and at least one other risk factor for stroke. The annual rate of stroke and systemic embolism in RE-LY was 1.5% in the patients on the 110-mg twice daily dose, 1.1% in those on the 150-mg twice daily dose, and 1.7% among those on warfarin. The risk reductions with dabigatran were 10% and 35% for the 110-mg and 150-mg doses, respectively, compared with warfarin.
"Our company has provided regulators with the complete data set and analyses of clinical evidence demonstrating Pradaxa’s benefits and safety," Boehringer Ingelheim said in its statement. "Contrary to the BMJ’s accusation that BI withheld analyses, here are the facts: In 2012, our scientists performed preliminary, exploratory simulations with mathematical models to understand whether dose adjustments based on plasma concentrations might further improve Pradaxa’s benefits and safety. Because the simulations did not offer reliable predictions of actual patient outcomes, they were not provided to regulators. However, all of the data that was used for the simulations had already been provided."
Dr. Sanjay Kaul, who was on the Food and Drug Administration’s advisory panel that recommended approval of dabigatran, said in an interview that the FDA reviewers raised questions at that meeting about the utility of using plasma concentrations to monitor individual subjects and adjust dose based on dabigatran concentrations. "But they also acknowledged an exposure-response relationship existed that demonstrated that going from the 10th to 90th percentile of dabigatran concentration (23 to 283 ng/mL) reduced the probability of having a stroke by 50% (from 1% to 0.5%) while increasing sixfold (from 0.3% to 1.8%) the probability of having major bleeding within 1 year."
It is well known that plasma concentration is one of the factors – among others, such as age, renal function, or history of stroke – that affect the benefit-risk balance, said Dr. Kaul, a cardiologist at Cedars-Sinai Medical Center, Los Angeles. Even in patients with moderate to severe renal dysfunction (up to creatinine clearance of 15-30 mL/min) and elevated plasma concentrations, benefit still exceeds risk.
"To what degree monitoring drug levels could potentially optimize benefit-risk balance remains an open question. For example, benefit-risk balance for dabigatran 150 mg vs 110 mg was not predicted by pharmacokinetic/pharmacodynamic modeling. Based on the PK/PD modeling, one stroke would be prevented at the cost of three extra major bleeds using 150 mg , compared with 110 mg. The RE-LY data indicate four strokes prevented and three major bleeding events incurred with the 150-mg dose as compared with the 110-mg dose," he said. "There is quite a discordance in predicted vs. observed benefit-risk balance."
Therefore, "while monitoring drug levels to optimize benefit-risk balance has intuitive appeal, given the complex exposure-response relationship and confounding effects of demographic variables, this should remain an area of active investigation. It is not ready for prime time to inform or guide clinical practice," Dr. Kaul said.
Dr. Kaul disclosed that he is a consultant for Boehringer Ingelheim and has equity interest in Johnson and Johnson.
At press time, the FDA had not responded to a request for a comment on the BMJ investigation.
The manufacturer of the oral anticoagulant dabigatran "withheld analyses that calculate how many major bleeds dose adjustment could prevent," the BMJ charges in an article based on internal documents released during litigation in the United States and freedom of information act requests obtained by the journal.
Boehringer Ingelheim "found that if the plasma levels of the drug were measured and the dose was adjusted accordingly major bleeds could be reduced by 30%-40%, compared with well-controlled warfarin," according to the article (BMJ 2014;349:g4670), which was published along with an editorial and an analysis. The article further stated that the manufacturer "has failed to share with regulators information about the potential benefits of monitoring anticoagulant activity and adjusting the dose to make sure the drug is working as safely and effectively as possible."
In the analysis, Thomas Moore, senior scientist at the Institute for Safe Medication Practices, Horsham, Pa., and his coauthors said that "the bleeding risk of dabigatran can be reduced and efficacy improved by individualizing the dose in patients based on plasma level, age, and kidney function" (BMJ 2014;349:g4517[doi:10.1136/bmj.g4517]).
Dr. Rita Redberg and Dr. Blake Charlton of the University of California, San Francisco, wrote in an accompanying editorial that the analysis "illuminates a lack of transparency about the safety of unmonitored dabigatran, compounded by the drug’s fickle pharmacokinetics, which can cause a fivefold variation of plasma concentration." (BMJ 2014;349:g4681 [doi:10.1136/bmj.g4681]).
The BMJ article, written by Deborah Cohen, includes responses from the manufacturer of dabigatran (Pradaxa).
Boehringer Ingelheim officials have asserted that no data were withheld. Additionally, the company has released a statement calling the BMJ article "biased" and "misleading."
Dabigatran is a direct thrombin inhibitor that was approved in the United States in October 2010 for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation at a fixed dose of 150 mg or 75 mg twice a day, with the lower dose recommended for those with renal impairment. Since its initial approval, dabigatran has gained indications for treating and reducing the risk of recurrence of deep venous thrombosis and pulmonary embolism.
The drug’s approval was based on the results of the Randomized Evaluation of Long Term Anticoagulant Therapy (RE-LY) trial, which compared the drug to warfarin in 18,113 patients with nonvalvular atrial fibrillation and at least one other risk factor for stroke. The annual rate of stroke and systemic embolism in RE-LY was 1.5% in the patients on the 110-mg twice daily dose, 1.1% in those on the 150-mg twice daily dose, and 1.7% among those on warfarin. The risk reductions with dabigatran were 10% and 35% for the 110-mg and 150-mg doses, respectively, compared with warfarin.
"Our company has provided regulators with the complete data set and analyses of clinical evidence demonstrating Pradaxa’s benefits and safety," Boehringer Ingelheim said in its statement. "Contrary to the BMJ’s accusation that BI withheld analyses, here are the facts: In 2012, our scientists performed preliminary, exploratory simulations with mathematical models to understand whether dose adjustments based on plasma concentrations might further improve Pradaxa’s benefits and safety. Because the simulations did not offer reliable predictions of actual patient outcomes, they were not provided to regulators. However, all of the data that was used for the simulations had already been provided."
Dr. Sanjay Kaul, who was on the Food and Drug Administration’s advisory panel that recommended approval of dabigatran, said in an interview that the FDA reviewers raised questions at that meeting about the utility of using plasma concentrations to monitor individual subjects and adjust dose based on dabigatran concentrations. "But they also acknowledged an exposure-response relationship existed that demonstrated that going from the 10th to 90th percentile of dabigatran concentration (23 to 283 ng/mL) reduced the probability of having a stroke by 50% (from 1% to 0.5%) while increasing sixfold (from 0.3% to 1.8%) the probability of having major bleeding within 1 year."
It is well known that plasma concentration is one of the factors – among others, such as age, renal function, or history of stroke – that affect the benefit-risk balance, said Dr. Kaul, a cardiologist at Cedars-Sinai Medical Center, Los Angeles. Even in patients with moderate to severe renal dysfunction (up to creatinine clearance of 15-30 mL/min) and elevated plasma concentrations, benefit still exceeds risk.
"To what degree monitoring drug levels could potentially optimize benefit-risk balance remains an open question. For example, benefit-risk balance for dabigatran 150 mg vs 110 mg was not predicted by pharmacokinetic/pharmacodynamic modeling. Based on the PK/PD modeling, one stroke would be prevented at the cost of three extra major bleeds using 150 mg , compared with 110 mg. The RE-LY data indicate four strokes prevented and three major bleeding events incurred with the 150-mg dose as compared with the 110-mg dose," he said. "There is quite a discordance in predicted vs. observed benefit-risk balance."
Therefore, "while monitoring drug levels to optimize benefit-risk balance has intuitive appeal, given the complex exposure-response relationship and confounding effects of demographic variables, this should remain an area of active investigation. It is not ready for prime time to inform or guide clinical practice," Dr. Kaul said.
Dr. Kaul disclosed that he is a consultant for Boehringer Ingelheim and has equity interest in Johnson and Johnson.
At press time, the FDA had not responded to a request for a comment on the BMJ investigation.
The manufacturer of the oral anticoagulant dabigatran "withheld analyses that calculate how many major bleeds dose adjustment could prevent," the BMJ charges in an article based on internal documents released during litigation in the United States and freedom of information act requests obtained by the journal.
Boehringer Ingelheim "found that if the plasma levels of the drug were measured and the dose was adjusted accordingly major bleeds could be reduced by 30%-40%, compared with well-controlled warfarin," according to the article (BMJ 2014;349:g4670), which was published along with an editorial and an analysis. The article further stated that the manufacturer "has failed to share with regulators information about the potential benefits of monitoring anticoagulant activity and adjusting the dose to make sure the drug is working as safely and effectively as possible."
In the analysis, Thomas Moore, senior scientist at the Institute for Safe Medication Practices, Horsham, Pa., and his coauthors said that "the bleeding risk of dabigatran can be reduced and efficacy improved by individualizing the dose in patients based on plasma level, age, and kidney function" (BMJ 2014;349:g4517[doi:10.1136/bmj.g4517]).
Dr. Rita Redberg and Dr. Blake Charlton of the University of California, San Francisco, wrote in an accompanying editorial that the analysis "illuminates a lack of transparency about the safety of unmonitored dabigatran, compounded by the drug’s fickle pharmacokinetics, which can cause a fivefold variation of plasma concentration." (BMJ 2014;349:g4681 [doi:10.1136/bmj.g4681]).
The BMJ article, written by Deborah Cohen, includes responses from the manufacturer of dabigatran (Pradaxa).
Boehringer Ingelheim officials have asserted that no data were withheld. Additionally, the company has released a statement calling the BMJ article "biased" and "misleading."
Dabigatran is a direct thrombin inhibitor that was approved in the United States in October 2010 for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation at a fixed dose of 150 mg or 75 mg twice a day, with the lower dose recommended for those with renal impairment. Since its initial approval, dabigatran has gained indications for treating and reducing the risk of recurrence of deep venous thrombosis and pulmonary embolism.
The drug’s approval was based on the results of the Randomized Evaluation of Long Term Anticoagulant Therapy (RE-LY) trial, which compared the drug to warfarin in 18,113 patients with nonvalvular atrial fibrillation and at least one other risk factor for stroke. The annual rate of stroke and systemic embolism in RE-LY was 1.5% in the patients on the 110-mg twice daily dose, 1.1% in those on the 150-mg twice daily dose, and 1.7% among those on warfarin. The risk reductions with dabigatran were 10% and 35% for the 110-mg and 150-mg doses, respectively, compared with warfarin.
"Our company has provided regulators with the complete data set and analyses of clinical evidence demonstrating Pradaxa’s benefits and safety," Boehringer Ingelheim said in its statement. "Contrary to the BMJ’s accusation that BI withheld analyses, here are the facts: In 2012, our scientists performed preliminary, exploratory simulations with mathematical models to understand whether dose adjustments based on plasma concentrations might further improve Pradaxa’s benefits and safety. Because the simulations did not offer reliable predictions of actual patient outcomes, they were not provided to regulators. However, all of the data that was used for the simulations had already been provided."
Dr. Sanjay Kaul, who was on the Food and Drug Administration’s advisory panel that recommended approval of dabigatran, said in an interview that the FDA reviewers raised questions at that meeting about the utility of using plasma concentrations to monitor individual subjects and adjust dose based on dabigatran concentrations. "But they also acknowledged an exposure-response relationship existed that demonstrated that going from the 10th to 90th percentile of dabigatran concentration (23 to 283 ng/mL) reduced the probability of having a stroke by 50% (from 1% to 0.5%) while increasing sixfold (from 0.3% to 1.8%) the probability of having major bleeding within 1 year."
It is well known that plasma concentration is one of the factors – among others, such as age, renal function, or history of stroke – that affect the benefit-risk balance, said Dr. Kaul, a cardiologist at Cedars-Sinai Medical Center, Los Angeles. Even in patients with moderate to severe renal dysfunction (up to creatinine clearance of 15-30 mL/min) and elevated plasma concentrations, benefit still exceeds risk.
"To what degree monitoring drug levels could potentially optimize benefit-risk balance remains an open question. For example, benefit-risk balance for dabigatran 150 mg vs 110 mg was not predicted by pharmacokinetic/pharmacodynamic modeling. Based on the PK/PD modeling, one stroke would be prevented at the cost of three extra major bleeds using 150 mg , compared with 110 mg. The RE-LY data indicate four strokes prevented and three major bleeding events incurred with the 150-mg dose as compared with the 110-mg dose," he said. "There is quite a discordance in predicted vs. observed benefit-risk balance."
Therefore, "while monitoring drug levels to optimize benefit-risk balance has intuitive appeal, given the complex exposure-response relationship and confounding effects of demographic variables, this should remain an area of active investigation. It is not ready for prime time to inform or guide clinical practice," Dr. Kaul said.
Dr. Kaul disclosed that he is a consultant for Boehringer Ingelheim and has equity interest in Johnson and Johnson.
At press time, the FDA had not responded to a request for a comment on the BMJ investigation.
Why You Shouldn’t Start β-Blockers Before Surgery
PRACTICE CHANGER
Do not routinely initiate β-blockers in patients undergoing intermediate- or high-risk noncardiac surgery. β-Blockers appear to increase the 30-day risk for all-cause mortality.1
STRENGTH OF RECOMMENDATION
A: Based on meta-analysis of nine randomized controlled trials (RCTs).1
ILLUSTRATIVE CASE
A 67-year-old woman with diabetes, hypertension, and hyperlipidemia presents for evaluation prior to a total hip arthroplasty. She is not taking a β-blocker. Should you prescribe one?
Study summary >>
Current guidelines from the American College of Cardiology Foundation (ACCF) and the American Heart Association (AHA) recommend starting
β-blockers to prevent cardiac events in patients about to undergo intermediate- or high-risk surgery or vascular surgery who have a history of inducible ischemia, coronary artery disease (CAD), or at least one risk factor for CAD.2 However, the majority of the evidence for these guidelines, which were published in 2009 and are in the process of being updated, came from the DECREASE (Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography) trials. These trials have been discredited due to serious methodologic flaws, including falsified descriptions of how outcomes were determined and fictitious databases.3
A new meta-analysis conducted by Bouri et al1 that excluded the DECREASE trials found that, although preoperative β-blockers reduce the rate of certain nonfatal outcomes, they increase the risk for death and stroke.
STUDY SUMMARY
Preop β-blockers do more harm than good
Bouri et al1 conducted a meta-analysis of published RCTs evaluating preoperative β-blockers versus placebo for patients undergoing noncardiac surgery. Of the 11 studies that met eligibility criteria, two were the discredited DECREASE trials. Thus, Bouri et al1 analyzed nine high-quality RCTs that included 10,529 patients.
Most studies included patients undergoing vascular surgery. Some studies also included intra-abdominal, intrathoracic, neurosurgic, orthopedic, urologic, and gynecologic surgeries. β-Blockers were started no more than a day before surgery and were discontinued at hospital discharge or up to 30 days postop. Metoprolol was used in five trials, bisoprolol in one trial, atenolol in two trials, and propranolol in one trial. The primary endpoint was all-cause mortality within 30 days.
A total of 5,264 patients were randomly assigned to receive β-blockers and 5,265 to placebo. There were 162 deaths in the β-blocker group and 129 deaths in the placebo group. Patients who received β-blockers had a 27% increased risk for all-cause mortality (risk ratio [RR] = 1.27). The number needed to harm was 160.
Six of the studies also evaluated rates of nonfatal MI, nonfatal stroke, and hypotension. β-Blockers lowered the risk for nonfatal MI (RR = 0.73) but increased the risk for nonfatal stroke (RR = 1.73) and hypotension (RR = 1.51).
This meta-analysis was dominated by the 2008 Peri-Operative ISchemic Evaluation (POISE) trial, an RCT that compared placebo to extended-release metoprolol (100 mg 2 to 4 h before surgery, followed by 200 mg/d for 30 d), in 8,351 patients with, or at risk for, atherosclerotic disease.4 While β-blockers reduced the risk for MI and atrial fibrillation, they increased the risk for mortality and stroke, likely due to drug-induced hypotension. The slightly larger-than-typical doses of β-blockers used in this study may have contributed to the excess mortality.
What's new and challenges to implementation >>
WHAT’S NEW
Avoiding β-blockers in surgery patients will prevent deaths
Bouri et al1 found that while β-blockers protect against nonfatal MIs, they increase the risk for nonfatal strokes and death. This new meta-analysis challenges the ACCF/AHA recommendations by suggesting that abandoning the use of β-blockers for preoperative patients who aren’t already taking them will prevent a substantial number of perioperative deaths. Bouri et al1 estimate that in the United Kingdom, where 47,286 deaths occur annually within 30 days of intermediate- or high-risk procedures, the number of iatrogenic deaths would drop by approximately 10,000 if β-blockers were not used.1
CAVEATS
Don’t stop β-blockers in patients who already take them
This meta-analysis did not evaluate outcomes in patients who were already taking β-blockers. These patients should continue to take them in the perioperative period, which is in line with current ACCF/AHA guidelines.
CHALLENGES TO IMPLEMENTATION
Reluctance to disregard published guidelines
Some clinicians may not be comfortable ignoring the current ACCF/AHA guidelines that make a Class IIA recommendation (it is reasonable to administer this treatment) for the use of preoperative β-blockade for patients at risk for cardiovascular events who were not previously taking a β-blocker. This updated meta-analysis excludes the discredited DECREASE trials and challenges us to act against these current guidelines while we await updated recommendations.
REFERENCES
1. Bouri S, Shun-Shin MJ, Cole GD, et al. Meta-analysis of secure randomised controlled trials of ß-blockade to prevent perioperative death in non-cardiac surgery. Heart. 2014;100:456-464.
2. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Rhythm Society; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine; Society for Vascular Surgery; Fleisher LA, Beckman JA, Brown KA, et al. 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. J Am Coll Cardiol. 2009;54:e13-e118.
3. Erasmus Medical Center Follow-up Investigation Committee. Report on the 2012 follow-up investigation of possible breaches of academic integrity (September 30, 2012). CardioBrief. Available at: http://cardiobrief.files.wordpress.com/2012/10/integrity-report-2012-10-english-translation.pdf. Accessed August 14, 2014.
4. Devereaux PJ, Yang H, Yusuf S, et al; POISE Study Group. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;
371:1839-1847.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(6):E15-E16.
PRACTICE CHANGER
Do not routinely initiate β-blockers in patients undergoing intermediate- or high-risk noncardiac surgery. β-Blockers appear to increase the 30-day risk for all-cause mortality.1
STRENGTH OF RECOMMENDATION
A: Based on meta-analysis of nine randomized controlled trials (RCTs).1
ILLUSTRATIVE CASE
A 67-year-old woman with diabetes, hypertension, and hyperlipidemia presents for evaluation prior to a total hip arthroplasty. She is not taking a β-blocker. Should you prescribe one?
Study summary >>
Current guidelines from the American College of Cardiology Foundation (ACCF) and the American Heart Association (AHA) recommend starting
β-blockers to prevent cardiac events in patients about to undergo intermediate- or high-risk surgery or vascular surgery who have a history of inducible ischemia, coronary artery disease (CAD), or at least one risk factor for CAD.2 However, the majority of the evidence for these guidelines, which were published in 2009 and are in the process of being updated, came from the DECREASE (Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography) trials. These trials have been discredited due to serious methodologic flaws, including falsified descriptions of how outcomes were determined and fictitious databases.3
A new meta-analysis conducted by Bouri et al1 that excluded the DECREASE trials found that, although preoperative β-blockers reduce the rate of certain nonfatal outcomes, they increase the risk for death and stroke.
STUDY SUMMARY
Preop β-blockers do more harm than good
Bouri et al1 conducted a meta-analysis of published RCTs evaluating preoperative β-blockers versus placebo for patients undergoing noncardiac surgery. Of the 11 studies that met eligibility criteria, two were the discredited DECREASE trials. Thus, Bouri et al1 analyzed nine high-quality RCTs that included 10,529 patients.
Most studies included patients undergoing vascular surgery. Some studies also included intra-abdominal, intrathoracic, neurosurgic, orthopedic, urologic, and gynecologic surgeries. β-Blockers were started no more than a day before surgery and were discontinued at hospital discharge or up to 30 days postop. Metoprolol was used in five trials, bisoprolol in one trial, atenolol in two trials, and propranolol in one trial. The primary endpoint was all-cause mortality within 30 days.
A total of 5,264 patients were randomly assigned to receive β-blockers and 5,265 to placebo. There were 162 deaths in the β-blocker group and 129 deaths in the placebo group. Patients who received β-blockers had a 27% increased risk for all-cause mortality (risk ratio [RR] = 1.27). The number needed to harm was 160.
Six of the studies also evaluated rates of nonfatal MI, nonfatal stroke, and hypotension. β-Blockers lowered the risk for nonfatal MI (RR = 0.73) but increased the risk for nonfatal stroke (RR = 1.73) and hypotension (RR = 1.51).
This meta-analysis was dominated by the 2008 Peri-Operative ISchemic Evaluation (POISE) trial, an RCT that compared placebo to extended-release metoprolol (100 mg 2 to 4 h before surgery, followed by 200 mg/d for 30 d), in 8,351 patients with, or at risk for, atherosclerotic disease.4 While β-blockers reduced the risk for MI and atrial fibrillation, they increased the risk for mortality and stroke, likely due to drug-induced hypotension. The slightly larger-than-typical doses of β-blockers used in this study may have contributed to the excess mortality.
What's new and challenges to implementation >>
WHAT’S NEW
Avoiding β-blockers in surgery patients will prevent deaths
Bouri et al1 found that while β-blockers protect against nonfatal MIs, they increase the risk for nonfatal strokes and death. This new meta-analysis challenges the ACCF/AHA recommendations by suggesting that abandoning the use of β-blockers for preoperative patients who aren’t already taking them will prevent a substantial number of perioperative deaths. Bouri et al1 estimate that in the United Kingdom, where 47,286 deaths occur annually within 30 days of intermediate- or high-risk procedures, the number of iatrogenic deaths would drop by approximately 10,000 if β-blockers were not used.1
CAVEATS
Don’t stop β-blockers in patients who already take them
This meta-analysis did not evaluate outcomes in patients who were already taking β-blockers. These patients should continue to take them in the perioperative period, which is in line with current ACCF/AHA guidelines.
CHALLENGES TO IMPLEMENTATION
Reluctance to disregard published guidelines
Some clinicians may not be comfortable ignoring the current ACCF/AHA guidelines that make a Class IIA recommendation (it is reasonable to administer this treatment) for the use of preoperative β-blockade for patients at risk for cardiovascular events who were not previously taking a β-blocker. This updated meta-analysis excludes the discredited DECREASE trials and challenges us to act against these current guidelines while we await updated recommendations.
REFERENCES
1. Bouri S, Shun-Shin MJ, Cole GD, et al. Meta-analysis of secure randomised controlled trials of ß-blockade to prevent perioperative death in non-cardiac surgery. Heart. 2014;100:456-464.
2. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Rhythm Society; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine; Society for Vascular Surgery; Fleisher LA, Beckman JA, Brown KA, et al. 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. J Am Coll Cardiol. 2009;54:e13-e118.
3. Erasmus Medical Center Follow-up Investigation Committee. Report on the 2012 follow-up investigation of possible breaches of academic integrity (September 30, 2012). CardioBrief. Available at: http://cardiobrief.files.wordpress.com/2012/10/integrity-report-2012-10-english-translation.pdf. Accessed August 14, 2014.
4. Devereaux PJ, Yang H, Yusuf S, et al; POISE Study Group. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;
371:1839-1847.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(6):E15-E16.
PRACTICE CHANGER
Do not routinely initiate β-blockers in patients undergoing intermediate- or high-risk noncardiac surgery. β-Blockers appear to increase the 30-day risk for all-cause mortality.1
STRENGTH OF RECOMMENDATION
A: Based on meta-analysis of nine randomized controlled trials (RCTs).1
ILLUSTRATIVE CASE
A 67-year-old woman with diabetes, hypertension, and hyperlipidemia presents for evaluation prior to a total hip arthroplasty. She is not taking a β-blocker. Should you prescribe one?
Study summary >>
Current guidelines from the American College of Cardiology Foundation (ACCF) and the American Heart Association (AHA) recommend starting
β-blockers to prevent cardiac events in patients about to undergo intermediate- or high-risk surgery or vascular surgery who have a history of inducible ischemia, coronary artery disease (CAD), or at least one risk factor for CAD.2 However, the majority of the evidence for these guidelines, which were published in 2009 and are in the process of being updated, came from the DECREASE (Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography) trials. These trials have been discredited due to serious methodologic flaws, including falsified descriptions of how outcomes were determined and fictitious databases.3
A new meta-analysis conducted by Bouri et al1 that excluded the DECREASE trials found that, although preoperative β-blockers reduce the rate of certain nonfatal outcomes, they increase the risk for death and stroke.
STUDY SUMMARY
Preop β-blockers do more harm than good
Bouri et al1 conducted a meta-analysis of published RCTs evaluating preoperative β-blockers versus placebo for patients undergoing noncardiac surgery. Of the 11 studies that met eligibility criteria, two were the discredited DECREASE trials. Thus, Bouri et al1 analyzed nine high-quality RCTs that included 10,529 patients.
Most studies included patients undergoing vascular surgery. Some studies also included intra-abdominal, intrathoracic, neurosurgic, orthopedic, urologic, and gynecologic surgeries. β-Blockers were started no more than a day before surgery and were discontinued at hospital discharge or up to 30 days postop. Metoprolol was used in five trials, bisoprolol in one trial, atenolol in two trials, and propranolol in one trial. The primary endpoint was all-cause mortality within 30 days.
A total of 5,264 patients were randomly assigned to receive β-blockers and 5,265 to placebo. There were 162 deaths in the β-blocker group and 129 deaths in the placebo group. Patients who received β-blockers had a 27% increased risk for all-cause mortality (risk ratio [RR] = 1.27). The number needed to harm was 160.
Six of the studies also evaluated rates of nonfatal MI, nonfatal stroke, and hypotension. β-Blockers lowered the risk for nonfatal MI (RR = 0.73) but increased the risk for nonfatal stroke (RR = 1.73) and hypotension (RR = 1.51).
This meta-analysis was dominated by the 2008 Peri-Operative ISchemic Evaluation (POISE) trial, an RCT that compared placebo to extended-release metoprolol (100 mg 2 to 4 h before surgery, followed by 200 mg/d for 30 d), in 8,351 patients with, or at risk for, atherosclerotic disease.4 While β-blockers reduced the risk for MI and atrial fibrillation, they increased the risk for mortality and stroke, likely due to drug-induced hypotension. The slightly larger-than-typical doses of β-blockers used in this study may have contributed to the excess mortality.
What's new and challenges to implementation >>
WHAT’S NEW
Avoiding β-blockers in surgery patients will prevent deaths
Bouri et al1 found that while β-blockers protect against nonfatal MIs, they increase the risk for nonfatal strokes and death. This new meta-analysis challenges the ACCF/AHA recommendations by suggesting that abandoning the use of β-blockers for preoperative patients who aren’t already taking them will prevent a substantial number of perioperative deaths. Bouri et al1 estimate that in the United Kingdom, where 47,286 deaths occur annually within 30 days of intermediate- or high-risk procedures, the number of iatrogenic deaths would drop by approximately 10,000 if β-blockers were not used.1
CAVEATS
Don’t stop β-blockers in patients who already take them
This meta-analysis did not evaluate outcomes in patients who were already taking β-blockers. These patients should continue to take them in the perioperative period, which is in line with current ACCF/AHA guidelines.
CHALLENGES TO IMPLEMENTATION
Reluctance to disregard published guidelines
Some clinicians may not be comfortable ignoring the current ACCF/AHA guidelines that make a Class IIA recommendation (it is reasonable to administer this treatment) for the use of preoperative β-blockade for patients at risk for cardiovascular events who were not previously taking a β-blocker. This updated meta-analysis excludes the discredited DECREASE trials and challenges us to act against these current guidelines while we await updated recommendations.
REFERENCES
1. Bouri S, Shun-Shin MJ, Cole GD, et al. Meta-analysis of secure randomised controlled trials of ß-blockade to prevent perioperative death in non-cardiac surgery. Heart. 2014;100:456-464.
2. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Rhythm Society; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine; Society for Vascular Surgery; Fleisher LA, Beckman JA, Brown KA, et al. 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. J Am Coll Cardiol. 2009;54:e13-e118.
3. Erasmus Medical Center Follow-up Investigation Committee. Report on the 2012 follow-up investigation of possible breaches of academic integrity (September 30, 2012). CardioBrief. Available at: http://cardiobrief.files.wordpress.com/2012/10/integrity-report-2012-10-english-translation.pdf. Accessed August 14, 2014.
4. Devereaux PJ, Yang H, Yusuf S, et al; POISE Study Group. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;
371:1839-1847.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(6):E15-E16.
A Visiting Grandma Feels Short of Breath
ANSWER
This ECG shows normal sinus rhythm, a right bundle branch block (RBBB), and a left anterior fascicular block (LAFB). RBBB and LAFB are consistent with bifascicular block.
Criteria for an RBBB include a prolonged total QRS complex of 120 ms or longer and an RSR’ complex (“rabbit ears”) in lead V1. LAFB criteria include a QRS of normal duration with an S wave greater than an R wave in leads II, III, and aVF and left-axis deviation (–48° in this case).
The astute reader may question the disparity between RBBB and LAFB, since the criteria for the former include a prolonged QRS interval and the criteria for the latter include a normal QRS interval. It should be noted that the requirements for QRS duration for RBBB vary.
Bifascicular block (RBBB and either LAFB or left posterior fascicular block [LPFB]) is indicative of more advanced conduction system disease. However, it is not an indication for permanent pacemaker placement in an asymptomatic patient.
This patient was treated for a community-acquired right lower lobe pneumonia and a UTI.
ANSWER
This ECG shows normal sinus rhythm, a right bundle branch block (RBBB), and a left anterior fascicular block (LAFB). RBBB and LAFB are consistent with bifascicular block.
Criteria for an RBBB include a prolonged total QRS complex of 120 ms or longer and an RSR’ complex (“rabbit ears”) in lead V1. LAFB criteria include a QRS of normal duration with an S wave greater than an R wave in leads II, III, and aVF and left-axis deviation (–48° in this case).
The astute reader may question the disparity between RBBB and LAFB, since the criteria for the former include a prolonged QRS interval and the criteria for the latter include a normal QRS interval. It should be noted that the requirements for QRS duration for RBBB vary.
Bifascicular block (RBBB and either LAFB or left posterior fascicular block [LPFB]) is indicative of more advanced conduction system disease. However, it is not an indication for permanent pacemaker placement in an asymptomatic patient.
This patient was treated for a community-acquired right lower lobe pneumonia and a UTI.
ANSWER
This ECG shows normal sinus rhythm, a right bundle branch block (RBBB), and a left anterior fascicular block (LAFB). RBBB and LAFB are consistent with bifascicular block.
Criteria for an RBBB include a prolonged total QRS complex of 120 ms or longer and an RSR’ complex (“rabbit ears”) in lead V1. LAFB criteria include a QRS of normal duration with an S wave greater than an R wave in leads II, III, and aVF and left-axis deviation (–48° in this case).
The astute reader may question the disparity between RBBB and LAFB, since the criteria for the former include a prolonged QRS interval and the criteria for the latter include a normal QRS interval. It should be noted that the requirements for QRS duration for RBBB vary.
Bifascicular block (RBBB and either LAFB or left posterior fascicular block [LPFB]) is indicative of more advanced conduction system disease. However, it is not an indication for permanent pacemaker placement in an asymptomatic patient.
This patient was treated for a community-acquired right lower lobe pneumonia and a UTI.
A 78-year-old woman presents to your urgent care clinic with a four-day history of lethargy. She lives in another state but currently is visiting her granddaughter, who happens to be your clinic manager. She says she felt weak prior to her trip but thought it was probably due to a urinary tract infection (UTI). Yesterday, however, she started feeling short of breath. The patient denies chest pain, orthopnea, paroxysmal nocturnal dyspnea, or productive cough. She reports feeling feverish this morning but did not record her temperature, adding that it seemed to subside after she got dressed. Her medical history is positive for frequent UTIs, a remote cholecystectomy, hypothyroidism, and paroxysmal atrial fibrillation. According to the patient’s daughter, who is present, her mother’s cardiologist recently mentioned some “funny” findings on an ECG; she didn’t really understand his explanation but they were told “not to worry.” The patient, a retired schoolteacher, lives in an assisted living center. She is independent and has been a widow for 14 years, since her husband died of an acute MI. She has two children who are in good health. She has never smoked, rarely consumes alcohol, and has never used recreational or homeopathic drugs. Her current medications include warfarin, levothyroxine, and conjugated estrogen. She was taking amiodarone for rhythm control of atrial fibrillation but stopped six months ago when her skin started turning blue. She is allergic to penicillin, which causes a true anaphylactic reaction, according to her daughter. Review of systems is positive for an infrequent, nonproductive cough, sun sensitivity due to amiodarone use, and infrequent burning with urination. Physical exam reveals a thin, elderly woman in no distress. Her blood pressure is 152/88 mm Hg; pulse, 70 beats/min and regular; respiratory rate, 14 breaths/min-1 with an infrequent, nonproductive cough; O2 saturation, 94% on room air; and temperature, 99°F. She is 5 ft 4 in tall and weighs 114 lb. Pertinent findings on physical exam include corrective lenses, pearly white skin with a blue hue on the nose and ears secondary to long-term amiodarone therapy, no evidence of thyromegaly or jugular distention, a regular rate and rhythm with a soft midsystolic murmur of mitral regurgitation, and no extra heart sounds. Her lungs are remarkable for consolidation in the right lower lobe, with crackles that change with coughing. Her abdomen is soft and nontender, and there is no peripheral edema. Her neurologic exam is intact. She is alert, attentive, and very witty in her responses to questions. Laboratory data include urinalysis findings suggestive of a UTI, a white blood cell count of 9.8 x 103/μL, and a hematocrit of 35%. A chest x-ray shows evidence of consolidation in the right lower lobe, which the radiologist says is strongly suggestive of pneumonia. An ECG shows a ventricular rate of 71 beats/min; PR interval, 152 ms; QRS duration, 142 ms; QT/QTc interval, 476/517 ms; P axis, 76°; R axis, –48°; and T axis, 161°. What is your interpretation of this ECG?
The transition from fellowship: finding a job
Click on the PDF icon at the top of this introduction to read the full article.
Click on the PDF icon at the top of this introduction to read the full article.
Click on the PDF icon at the top of this introduction to read the full article.
Moving beyond the one-size-fits-all formula for breast cancer treatments
Click on the PDF icon at the top of this introduction to read the full article.
Click on the PDF icon at the top of this introduction to read the full article.
Click on the PDF icon at the top of this introduction to read the full article.