User login
FDA expands approved use of ibrutinib in CLL

Credit: Steven Harbour
The US Food and Drug Administration (FDA) has expanded the approved use of ibrutinib (Imbruvica) in patients with chronic lymphocytic leukemia (CLL).
The agency previously granted the drug accelerated approval to treat CLL patients who had received at least 1 prior therapy.
Now, the FDA has granted ibrutinib full approval for that indication and expanded the drug’s approved use to include previously treated and untreated CLL patients with 17p deletion.
The FDA’s decision to grant ibrutinib accelerated approval in CLL was based on the drug’s ability to elicit responses in previously treated patients.
Recent trial results have shown the drug can improve survival rates in CLL, which signifies a clinical benefit and allows the FDA to grant ibrutinib full approval.
Ibrutinib in CLL: Trial results
The expanded approval for ibrutinib is based on results of the phase 3 RESONATE trial, which were presented at this year’s ASCO and EHA meetings.
The trial included 391 previously treated patients, 127 of whom had 17p deletion. Patients were randomized to receive ibrutinib or the anti-CD20 monoclonal antibody ofatumumab until disease progression or unacceptable toxicity.
The trial was stopped early after a pre-planned interim analysis showed that ibrutinib-treated patients experienced a 78% reduction in the risk of disease progression or death.
At the time of interim analysis, the patients’ median time on study was 9.4 months. The best overall response among evaluable patients was 78% in the ibrutinib arm and 11% in the ofatumumab arm.
Ibrutinib significantly prolonged progression-free and overall survival. The median progression-free survival was 8.1 months in the ofatumumab arm and was not reached in the ibrutinib arm (P<0.0001). The median overall survival was not reached in either arm, but the hazard ratio was 0.434 (P=0.0049).
Of the 127 patients with 17p deletion, those treated with ibrutinib experienced a 75% reduction in the risk of disease progression or death.
Adverse events occurred in 99% of patients in the ibrutinib arm and 98% of those in the ofatumumab arm. Grade 3/4 events occurred in 51% and 39%, respectively.
Atrial fibrillation, bleeding-related events, diarrhea, and arthralgia were more common in the ibrutinib arm. Infusion-related reactions, peripheral sensory neuropathy, urticaria, night sweats, and pruritus were more common in the ofatumumab arm.
Ibrutinib in development
Ibrutinib is being studied alone and in combination with other treatments in several hematologic malignancies, including CLL, mantle cell lymphoma (MCL), Waldenstrom’s macroglobulinemia, diffuse large B-cell lymphoma, follicular lymphoma, and multiple myeloma.
Before the FDA granted ibrutinib accelerated approval in CLL, the agency granted the drug accelerated approval for use in previously treated MCL patients. Studies to verify ibrutinib’s clinical benefit in MCL are ongoing.
The European Medicines Agency’s Committee for Medicinal Products for Human Use has recommended marketing authorization for ibrutinib to treat adults with relapsed or refractory MCL.
The committee has also recommended the drug for adults with CLL who have received at least 1 prior therapy and CLL patients with 17p deletion or TP53 mutation who cannot receive chemo-immunotherapy.
Ibrutinib is already approved in Israel for the treatment of adults with MCL who have received at least 1 prior therapy.
Ibrutinib is under development by Janssen Biotech and Pharmacyclics, Inc. The companies co-market ibrutinib in the US, but Janssen markets (or will market) ibrutinib in the rest of the world. ![]()

Credit: Steven Harbour
The US Food and Drug Administration (FDA) has expanded the approved use of ibrutinib (Imbruvica) in patients with chronic lymphocytic leukemia (CLL).
The agency previously granted the drug accelerated approval to treat CLL patients who had received at least 1 prior therapy.
Now, the FDA has granted ibrutinib full approval for that indication and expanded the drug’s approved use to include previously treated and untreated CLL patients with 17p deletion.
The FDA’s decision to grant ibrutinib accelerated approval in CLL was based on the drug’s ability to elicit responses in previously treated patients.
Recent trial results have shown the drug can improve survival rates in CLL, which signifies a clinical benefit and allows the FDA to grant ibrutinib full approval.
Ibrutinib in CLL: Trial results
The expanded approval for ibrutinib is based on results of the phase 3 RESONATE trial, which were presented at this year’s ASCO and EHA meetings.
The trial included 391 previously treated patients, 127 of whom had 17p deletion. Patients were randomized to receive ibrutinib or the anti-CD20 monoclonal antibody ofatumumab until disease progression or unacceptable toxicity.
The trial was stopped early after a pre-planned interim analysis showed that ibrutinib-treated patients experienced a 78% reduction in the risk of disease progression or death.
At the time of interim analysis, the patients’ median time on study was 9.4 months. The best overall response among evaluable patients was 78% in the ibrutinib arm and 11% in the ofatumumab arm.
Ibrutinib significantly prolonged progression-free and overall survival. The median progression-free survival was 8.1 months in the ofatumumab arm and was not reached in the ibrutinib arm (P<0.0001). The median overall survival was not reached in either arm, but the hazard ratio was 0.434 (P=0.0049).
Of the 127 patients with 17p deletion, those treated with ibrutinib experienced a 75% reduction in the risk of disease progression or death.
Adverse events occurred in 99% of patients in the ibrutinib arm and 98% of those in the ofatumumab arm. Grade 3/4 events occurred in 51% and 39%, respectively.
Atrial fibrillation, bleeding-related events, diarrhea, and arthralgia were more common in the ibrutinib arm. Infusion-related reactions, peripheral sensory neuropathy, urticaria, night sweats, and pruritus were more common in the ofatumumab arm.
Ibrutinib in development
Ibrutinib is being studied alone and in combination with other treatments in several hematologic malignancies, including CLL, mantle cell lymphoma (MCL), Waldenstrom’s macroglobulinemia, diffuse large B-cell lymphoma, follicular lymphoma, and multiple myeloma.
Before the FDA granted ibrutinib accelerated approval in CLL, the agency granted the drug accelerated approval for use in previously treated MCL patients. Studies to verify ibrutinib’s clinical benefit in MCL are ongoing.
The European Medicines Agency’s Committee for Medicinal Products for Human Use has recommended marketing authorization for ibrutinib to treat adults with relapsed or refractory MCL.
The committee has also recommended the drug for adults with CLL who have received at least 1 prior therapy and CLL patients with 17p deletion or TP53 mutation who cannot receive chemo-immunotherapy.
Ibrutinib is already approved in Israel for the treatment of adults with MCL who have received at least 1 prior therapy.
Ibrutinib is under development by Janssen Biotech and Pharmacyclics, Inc. The companies co-market ibrutinib in the US, but Janssen markets (or will market) ibrutinib in the rest of the world. ![]()

Credit: Steven Harbour
The US Food and Drug Administration (FDA) has expanded the approved use of ibrutinib (Imbruvica) in patients with chronic lymphocytic leukemia (CLL).
The agency previously granted the drug accelerated approval to treat CLL patients who had received at least 1 prior therapy.
Now, the FDA has granted ibrutinib full approval for that indication and expanded the drug’s approved use to include previously treated and untreated CLL patients with 17p deletion.
The FDA’s decision to grant ibrutinib accelerated approval in CLL was based on the drug’s ability to elicit responses in previously treated patients.
Recent trial results have shown the drug can improve survival rates in CLL, which signifies a clinical benefit and allows the FDA to grant ibrutinib full approval.
Ibrutinib in CLL: Trial results
The expanded approval for ibrutinib is based on results of the phase 3 RESONATE trial, which were presented at this year’s ASCO and EHA meetings.
The trial included 391 previously treated patients, 127 of whom had 17p deletion. Patients were randomized to receive ibrutinib or the anti-CD20 monoclonal antibody ofatumumab until disease progression or unacceptable toxicity.
The trial was stopped early after a pre-planned interim analysis showed that ibrutinib-treated patients experienced a 78% reduction in the risk of disease progression or death.
At the time of interim analysis, the patients’ median time on study was 9.4 months. The best overall response among evaluable patients was 78% in the ibrutinib arm and 11% in the ofatumumab arm.
Ibrutinib significantly prolonged progression-free and overall survival. The median progression-free survival was 8.1 months in the ofatumumab arm and was not reached in the ibrutinib arm (P<0.0001). The median overall survival was not reached in either arm, but the hazard ratio was 0.434 (P=0.0049).
Of the 127 patients with 17p deletion, those treated with ibrutinib experienced a 75% reduction in the risk of disease progression or death.
Adverse events occurred in 99% of patients in the ibrutinib arm and 98% of those in the ofatumumab arm. Grade 3/4 events occurred in 51% and 39%, respectively.
Atrial fibrillation, bleeding-related events, diarrhea, and arthralgia were more common in the ibrutinib arm. Infusion-related reactions, peripheral sensory neuropathy, urticaria, night sweats, and pruritus were more common in the ofatumumab arm.
Ibrutinib in development
Ibrutinib is being studied alone and in combination with other treatments in several hematologic malignancies, including CLL, mantle cell lymphoma (MCL), Waldenstrom’s macroglobulinemia, diffuse large B-cell lymphoma, follicular lymphoma, and multiple myeloma.
Before the FDA granted ibrutinib accelerated approval in CLL, the agency granted the drug accelerated approval for use in previously treated MCL patients. Studies to verify ibrutinib’s clinical benefit in MCL are ongoing.
The European Medicines Agency’s Committee for Medicinal Products for Human Use has recommended marketing authorization for ibrutinib to treat adults with relapsed or refractory MCL.
The committee has also recommended the drug for adults with CLL who have received at least 1 prior therapy and CLL patients with 17p deletion or TP53 mutation who cannot receive chemo-immunotherapy.
Ibrutinib is already approved in Israel for the treatment of adults with MCL who have received at least 1 prior therapy.
Ibrutinib is under development by Janssen Biotech and Pharmacyclics, Inc. The companies co-market ibrutinib in the US, but Janssen markets (or will market) ibrutinib in the rest of the world. ![]()
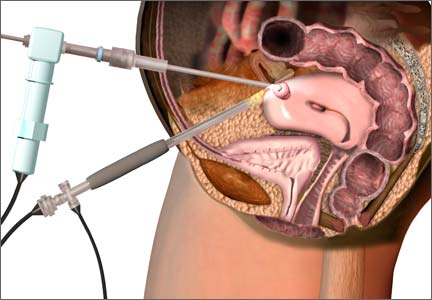
Post–FDA hearing: Will open power morcellation of uterine tissue remain an option during hysterectomy and myomectomy?
The use of power morcellation to remove the uterus or uterine tumors during hysterectomy and myomectomy has been in the limelight in 2014—particularly morcellation performed in an “open” fashion (without use of a protective bag). Concerns about the dispersion of tissue throughout the peritoneal cavity—including the risk of disseminating tissue from leiomyosarcoma, a rare but deadly cancer—have drawn statements from the American College of Obstetricians and Gynecologists (ACOG), the AAGL, the US Food and Drug Administration (FDA), and others, cautioning against the use of open power morcellation in women with a known or suspected malignancy.
In July 2014, the FDA convened a two-day hearing of the Obstetrics and Gynecology Devices Panel (one of the panels in its Medical Devices Advisory Committee) to consider whether power morcellation should remain an option and, if so, what restrictions or labeling might be recommended.
In advance of the FDA hearing, OBG Management invited two experts in women’s health to explore the options more deeply and address the future of minimally invasive surgery (MIS): Ray A. Wertheim, MD, Director of the AAGL Center of Excellence Minimally Invasive Gynecology Program at Inova Fair Oaks Hospital in Fairfax, Virginia, and Harry Reich, MD, widely known as the first surgeon to perform laparoscopic hysterectomy, among other achievements. Both Dr. Wertheim and Dr. Reich were members of the AAGL Tissue Extraction Task Force.
In this Q&A, Dr. Wertheim and Dr. Reich discuss:
- options for tissue extraction going forward
- the importance of continuing to offer minimally invasive surgical approaches
- the need to educate surgeons about the safest approaches to tissue extraction.
Both surgeons believe that power morcellation should remain an option for selected cases, although neither performs the technique himself. Both surgeons also believe that minimally invasive approaches to hysterectomy and myomectomy are here to stay and should continue to be used whenever possible.
AAGL convened an impartial expert panel
OBG Management: Dr. Wertheim, could you tell us a little about the AAGL position statement on the use of power morcellation for uterine tissue extraction at hysterectomy or myomectomy, since you were on the task force that researched and wrote it?1
Dr. Wertheim: AAGL convened its task force to conduct a critical appraisal of the existing evidence related to the practice of uterine extraction in the setting of hysterectomy and myomectomy. Areas in need of further investigation also were identified.
The task force consisted of experts who had no conflicts, were not allowed to discuss or review findings with anyone, and were not reimbursed for their time. Our review is the most complete report to date, more comprehensive than the current reports from the FDA, ACOG, the Society of Gynecologic Oncology (SGO), and the American Urogynecologic Society (AUGS).
Interestingly, AAGL, ACOG, SGO, and AUGS all reached the same conclusion: All existing methods of tissue extraction have benefits and risks that must be balanced.
OBG Management: How did the AAGL Task Force assess the evidence?
Dr. Wertheim: The quality of evidence and strength of recommendations were assessed using US Preventive Services Task Force guidelines. There are very few good data on the issue of power morcellation for uterine tissue extraction, especially in regard to leiomyosarcoma. One needs to be careful making recommendations without good data.
Related article: First large study on risk of cancer spread using power morcellation. Janelle Yates (News for your Practice; August 2014)
At this time, we do not believe there is a single method of tissue extraction that can protect all patients. Therefore, all current methods should remain available. We believe that an understanding of the issues will allow surgeons, hospitals, and patients to make the appropriate informed choices regarding tissue extraction for individual patients undergoing uterine surgery.
AAGL recommendations on the use of power morcellationIn its position statement, the AAGL Tissue Extraction Task Force made the following main points, recommending that surgeons:
Further research also is needed to determine how best to diagnose sarcomas preoperatively, the task force noted. The full report is available on the AAGL Web site.1 —Ray A. Wertheim, MD |
How to manage tissue extraction going forward
OBG Management: Regardless of the FDA’s final decision, what should the gynecologic specialty be doing to avoid disseminating uterine tissue in the peritoneal cavity, particularly leiomyosarcoma?
Dr. Wertheim: MIS is a wonderful advancement in women’s health care. All surgical specialties are moving toward MIS. Our challenge is to perform it as safely as possible, given the data and instrumentation available.
In regard to leiomyosarcoma, because we lack the ability to accurately make the diagnosis preoperatively, we’ve identified risk factors that should be taken into consideration. They include advanced age, a history of radiation or tamoxifen use, black race, hereditary leiomyomatosis, renal cell carcinoma syndrome, and survival of childhood retinoblastoma.
At this time, we have specimen-retrieval bags that can be used with power morcellation. However, it takes skill to be able to place a large specimen inside a bag without injuring surrounding organs due to limited visibility.
Education, at the hospital and national level, is in the works
OBG Management: How should we go about educating surgeons about MIS alternatives to open power morcellation?
Dr. Wertheim: In my hospital, we are mentoring surgeons to help them gain the new skills needed. In addition, I plan to give a grand rounds presentation on tissue extraction for hospitals in northern Virginia and also would like to offer a course in the near future. I’m also hoping that we’ll be able to offer courses around the country before the annual AAGL meeting this November.
At the annual AAGL meeting, the subject will be discussed at length, with an emphasis on identifying risk factors and conducting appropriate preoperative testing, with workshops likely to teach the skills needed to perform these surgeries as safely as possible.
Why a return to reliance on laparotomy would be unwise
OBG Management: Given all the concerns expressed recently about open power morcellation, do you think some surgeons will revert to abdominal hysterectomy rather than rely on MIS? Would such a move be safer than power morcellation?
Dr. Wertheim: That would be a disaster for women. Very reliable data have shown that MIS is safer than open surgery, with much quicker recovery. Almost all of my patients are discharged within 3 hours after surgery, and most no longer require pain medications other than nonsteroidal anti-inflammatory drugs by postoperative day 2. They’re usually back to work within 2 weeks.
We have worked long and hard to develop skills and instrumentation required to perform MIS safely—but nothing replaces good judgment. In some cases, laparotomy or conversion to a laparotomy may be indicated.
New instrumentation is needed and is being developed. In the meantime, my personal bias is to rule out risk factors for malignancy and continue to morcellate with a scalpel, preferably inside a bag. After all, we know that with open power morcellation, fragments and cells are usually left behind regardless of inspection and irrigation. These fragments may cause leiomyomatosis, endometriosis, bowel obstruction, sepsis, and possible dissemination of tumor fragments. Moreover, morcellation into small fragments complicates the pathologist’s ability to give an accurate report. The use of open power morcellation also subjects the patient to a risk of damage to surrounding organs—usually due to the surgeon’s inexperience.
As I have said before, our challenge is to perform these surgeries using the safest techniques possible, given the current data and instrumentation.
OBG Management: Dr. Reich, you have a unique perspective on this issue, because you pioneered laparoscopic hysterectomy. How has uterine tissue extraction evolved since then? Do you think open power morcellation should remain an option?
Dr. Reich: Uterine tissue extraction has not evolved. The terms “laparoscopic hysterectomy” and “total laparoscopic hysterectomy” imply vaginal extraction using a scalpel, not abdominal extraction using a morcellator. Unfortunately there is no substitute for hard work using a #10 blade on a long handle and special vaginal retraction tools.
In 1983, I made a decision to stop performing laparotomy for all gynecologic procedures, including hysterectomy, myomectomy, urology, oncology, abscesses, extensive adhesions, and rectovaginal endometriosis. I was an accomplished vaginal surgeon at that time, as well as a one-handed laparoscopic surgeon, operating while looking through the scope with one eye.
Interest in a laparoscopic approach to hysterectomy began with my presentations about laparoscopic hysterectomy in January 1988. At that time I had over 10 years of experience doing what is now called laparoscopic-assisted vaginal hysterectomy.
I wrote extensively about specimen removal using a scalpel before electronic power morcellators were available. Since then, I have asked those using power morcellators to stop calling their operation a laparoscopic hysterectomy, as it has more in common with an abdominal-extraction hysterectomy.
I have never advocated removing the uterus using power morcellators, and I still believe that most specimens can be removed vaginally without the spray of pieces of the specimen around the peritoneal cavity that occurs with power morcellation. This goes for hysterectomy involving a large uterus, myomectomy through a culdotomy incision, and removal of the uterine fundus after supracervical hysterectomy. (It is irresponsible to use expensive power morcellation to remove small supracervical hysterectomy specimens.) It is time to get back to learning and teaching vaginal morcellation, although I readily admit it is time consuming.
Nevertheless, I believe power morcellation should remain an option. Recent laparoscopic fellowship trainees know only this technique, which is still better than a return to mutilation by laparotomy.
Gynecology is a frustrating profession—30 years of MIS as a sideshow. General surgery has rapidly adopted a laparoscopic approach to most operations, after gynecologists taught them. Today most gynecologists do not do advanced laparoscopic surgery and would love to get back to open incision laparotomy for their operations. We cannot go back.
OBG Management: Dr. Wertheim and Dr. Reich, do your personal views of the morcellation issue differ at all from the official views of professional societies?
Dr. Wertheim: Yes. However, before I share them, I’d like to emphasize that the views I’m about to express are mine and mine only, not those of the AAGL or its task force.
The issue of uterine extraction is a highly emotional and political issue, about which there are few good data.
Abundant Level 1 data strongly support a vaginal or laparoscopic approach for benign hysterectomy when possible. ACOG and AAGL have issued position papers supporting these approaches for benign hysterectomies. Gynecologic surgeons and other surgical specialists have embraced MIS because it is safer, offers faster recovery, produces less postoperative pain, and has fewer complications than open surgery. However, AAGL has maintained for several years that morcellation is contraindicated in cases where uterine malignancy is either known or suspected.
The dilemma with open power morcellation is that even with our best diagnostic tools, the rare uterine sarcoma cannot always be definitively ruled out preoperatively. Endometrial cancer usually can be diagnosed before surgery. However, rare subtypes such as sarcomas are more difficult to reliably diagnose preoperatively, and risk factors for uterine sarcomas are not nearly as well understood as those for endometrial cancer.
I do agree with the FDA’s cautionary statement on April 17, which pointedly prohibits power morcellation for women with suspected precancer or known cancer of the gynecologic organs.2 However, the AAGL Task Force critically reviewed about 120 articles, including the studies assessed by the FDA. Concerns arose regarding the FDA’s interpretation of the data. Due to a number of deficiencies in these studies, some of the conclusions of the FDA may not be completely accurate. The studies analyzed by the FDA were not stratified by risk factors for sarcoma and were not necessarily performed in a setting of reproductive-aged women with presumed fibroids.
Dr. Reich: Here are my personal views about the sarcoma problem and I am sure they differ from the official views:
- Laparoscopic hysterectomy should always mean vaginal extraction unless a less disfiguring site can be discovered; power morcellation implies minilaparotomy and should be renamed to reflect that fact.
- Power morcellation must be differentiated from vaginal and minilaparotomy scalpel morcellation, especially in the media. Vaginal hysterectomy has entailed vaginal scalpel morcellation with successful outcomes for more than 100 years.
- Remember that most gynecologic cancers are approached using the laparoscope today. This certainly includes cervical and endometrial cancer and some ovarian cancers. (For example, one of my neighbors is a 25-year survivor of laparoscopically treated bilateral ovarian cancer who refused laparotomy!)
- I have removed sarcomas by vaginal morcellation during laparoscopic hysterectomy and laparoscopic myomectomy with no late sequelae. In fact, most cervical cancer surgery is done by laparoscopic surgery today. And even an open laparotomy hysterectomy can spread a sarcoma.
- The current morcellation debate arose when a single case of disseminated leiomyosarcoma became highly publicized. It involved a prominent physician whose leiomyosarcoma was unknown to her initial surgeon, and the malignancy was upstaged after the use of power morcellation during hysterectomy. After this case was covered in the media, other cases began to be reported in the lay press as well, some of which predated the publicized case. The truth is, regrettably, that sarcomas carry poor prognoses even when specimens are removed intact. And we don’t know much about the sarcoma that started this debate. Was it mild or aggressive? How many mitotic figures were there per high-powered field? And what was found macroscopicallyand microscopically at the subsequent laparotomy? We on the AAGL Task Force do not know the answers to these questions, although at least some of these variables are reported in other published cases. And because this case is likely to have a powerful effect on MIS in our country and the rest of the world, it is my opinion that we need to know these details.
What is your preferred surgical approach?
OBG Management: Do you perform open power morcellation in selected patients?
Dr. Wertheim: Even though I have performed morcellation with a scalpel transvaginally or through a mini-laparotomy incision for many years, I have never used open power morcellation because of the risk of leaving behind benign or malignant tissue fragments. Morcellation with a scalpel is easily learned and can be performed as quickly as power morcellation. Morcellation with a scalpel produces much larger pieces than with power morcellation. This probably markedly decreases the loss of fragments. I cannot make a definitive statement regarding cell loss, however. Until we have improved instrumentation and are better able to make a preoperative diagnosis of sarcoma, I’m going to rule out risk factors identified by the AAGL Task Force, do the appropriate work-up, and continue to morcellate with a scalpel, placing the specimen in a bag, if technically possible.
Dr. Reich: As I mentioned, I am a vaginal scalpel morcellator. I tried power morcellation when it first was developed but was never a fan. The same techniques used for vaginal extraction using a coring maneuver can be used abdominally through the umbilicus or a 1- or 2-cm trocar site.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
1. The Tissue Extraction Task Force, AAGL. AAGL Position Statement: Morcellation during uterine tissue extraction. http://www.aagl.org/wp-content/uploads/2014/05/Tissue_Extraction_TFR.pdf. Accessed June 13, 2014.
2. US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy. FDA Safety Communication. http://www.fda.gov
/medicaldevices/safety/alertsandnotices/ucm393576.htm. Published April 17, 2014. Accessed June 13, 2014.
The use of power morcellation to remove the uterus or uterine tumors during hysterectomy and myomectomy has been in the limelight in 2014—particularly morcellation performed in an “open” fashion (without use of a protective bag). Concerns about the dispersion of tissue throughout the peritoneal cavity—including the risk of disseminating tissue from leiomyosarcoma, a rare but deadly cancer—have drawn statements from the American College of Obstetricians and Gynecologists (ACOG), the AAGL, the US Food and Drug Administration (FDA), and others, cautioning against the use of open power morcellation in women with a known or suspected malignancy.
In July 2014, the FDA convened a two-day hearing of the Obstetrics and Gynecology Devices Panel (one of the panels in its Medical Devices Advisory Committee) to consider whether power morcellation should remain an option and, if so, what restrictions or labeling might be recommended.
In advance of the FDA hearing, OBG Management invited two experts in women’s health to explore the options more deeply and address the future of minimally invasive surgery (MIS): Ray A. Wertheim, MD, Director of the AAGL Center of Excellence Minimally Invasive Gynecology Program at Inova Fair Oaks Hospital in Fairfax, Virginia, and Harry Reich, MD, widely known as the first surgeon to perform laparoscopic hysterectomy, among other achievements. Both Dr. Wertheim and Dr. Reich were members of the AAGL Tissue Extraction Task Force.
In this Q&A, Dr. Wertheim and Dr. Reich discuss:
- options for tissue extraction going forward
- the importance of continuing to offer minimally invasive surgical approaches
- the need to educate surgeons about the safest approaches to tissue extraction.
Both surgeons believe that power morcellation should remain an option for selected cases, although neither performs the technique himself. Both surgeons also believe that minimally invasive approaches to hysterectomy and myomectomy are here to stay and should continue to be used whenever possible.
AAGL convened an impartial expert panel
OBG Management: Dr. Wertheim, could you tell us a little about the AAGL position statement on the use of power morcellation for uterine tissue extraction at hysterectomy or myomectomy, since you were on the task force that researched and wrote it?1
Dr. Wertheim: AAGL convened its task force to conduct a critical appraisal of the existing evidence related to the practice of uterine extraction in the setting of hysterectomy and myomectomy. Areas in need of further investigation also were identified.
The task force consisted of experts who had no conflicts, were not allowed to discuss or review findings with anyone, and were not reimbursed for their time. Our review is the most complete report to date, more comprehensive than the current reports from the FDA, ACOG, the Society of Gynecologic Oncology (SGO), and the American Urogynecologic Society (AUGS).
Interestingly, AAGL, ACOG, SGO, and AUGS all reached the same conclusion: All existing methods of tissue extraction have benefits and risks that must be balanced.
OBG Management: How did the AAGL Task Force assess the evidence?
Dr. Wertheim: The quality of evidence and strength of recommendations were assessed using US Preventive Services Task Force guidelines. There are very few good data on the issue of power morcellation for uterine tissue extraction, especially in regard to leiomyosarcoma. One needs to be careful making recommendations without good data.
Related article: First large study on risk of cancer spread using power morcellation. Janelle Yates (News for your Practice; August 2014)
At this time, we do not believe there is a single method of tissue extraction that can protect all patients. Therefore, all current methods should remain available. We believe that an understanding of the issues will allow surgeons, hospitals, and patients to make the appropriate informed choices regarding tissue extraction for individual patients undergoing uterine surgery.
AAGL recommendations on the use of power morcellationIn its position statement, the AAGL Tissue Extraction Task Force made the following main points, recommending that surgeons:
Further research also is needed to determine how best to diagnose sarcomas preoperatively, the task force noted. The full report is available on the AAGL Web site.1 —Ray A. Wertheim, MD |
How to manage tissue extraction going forward
OBG Management: Regardless of the FDA’s final decision, what should the gynecologic specialty be doing to avoid disseminating uterine tissue in the peritoneal cavity, particularly leiomyosarcoma?
Dr. Wertheim: MIS is a wonderful advancement in women’s health care. All surgical specialties are moving toward MIS. Our challenge is to perform it as safely as possible, given the data and instrumentation available.
In regard to leiomyosarcoma, because we lack the ability to accurately make the diagnosis preoperatively, we’ve identified risk factors that should be taken into consideration. They include advanced age, a history of radiation or tamoxifen use, black race, hereditary leiomyomatosis, renal cell carcinoma syndrome, and survival of childhood retinoblastoma.
At this time, we have specimen-retrieval bags that can be used with power morcellation. However, it takes skill to be able to place a large specimen inside a bag without injuring surrounding organs due to limited visibility.
Education, at the hospital and national level, is in the works
OBG Management: How should we go about educating surgeons about MIS alternatives to open power morcellation?
Dr. Wertheim: In my hospital, we are mentoring surgeons to help them gain the new skills needed. In addition, I plan to give a grand rounds presentation on tissue extraction for hospitals in northern Virginia and also would like to offer a course in the near future. I’m also hoping that we’ll be able to offer courses around the country before the annual AAGL meeting this November.
At the annual AAGL meeting, the subject will be discussed at length, with an emphasis on identifying risk factors and conducting appropriate preoperative testing, with workshops likely to teach the skills needed to perform these surgeries as safely as possible.
Why a return to reliance on laparotomy would be unwise
OBG Management: Given all the concerns expressed recently about open power morcellation, do you think some surgeons will revert to abdominal hysterectomy rather than rely on MIS? Would such a move be safer than power morcellation?
Dr. Wertheim: That would be a disaster for women. Very reliable data have shown that MIS is safer than open surgery, with much quicker recovery. Almost all of my patients are discharged within 3 hours after surgery, and most no longer require pain medications other than nonsteroidal anti-inflammatory drugs by postoperative day 2. They’re usually back to work within 2 weeks.
We have worked long and hard to develop skills and instrumentation required to perform MIS safely—but nothing replaces good judgment. In some cases, laparotomy or conversion to a laparotomy may be indicated.
New instrumentation is needed and is being developed. In the meantime, my personal bias is to rule out risk factors for malignancy and continue to morcellate with a scalpel, preferably inside a bag. After all, we know that with open power morcellation, fragments and cells are usually left behind regardless of inspection and irrigation. These fragments may cause leiomyomatosis, endometriosis, bowel obstruction, sepsis, and possible dissemination of tumor fragments. Moreover, morcellation into small fragments complicates the pathologist’s ability to give an accurate report. The use of open power morcellation also subjects the patient to a risk of damage to surrounding organs—usually due to the surgeon’s inexperience.
As I have said before, our challenge is to perform these surgeries using the safest techniques possible, given the current data and instrumentation.
OBG Management: Dr. Reich, you have a unique perspective on this issue, because you pioneered laparoscopic hysterectomy. How has uterine tissue extraction evolved since then? Do you think open power morcellation should remain an option?
Dr. Reich: Uterine tissue extraction has not evolved. The terms “laparoscopic hysterectomy” and “total laparoscopic hysterectomy” imply vaginal extraction using a scalpel, not abdominal extraction using a morcellator. Unfortunately there is no substitute for hard work using a #10 blade on a long handle and special vaginal retraction tools.
In 1983, I made a decision to stop performing laparotomy for all gynecologic procedures, including hysterectomy, myomectomy, urology, oncology, abscesses, extensive adhesions, and rectovaginal endometriosis. I was an accomplished vaginal surgeon at that time, as well as a one-handed laparoscopic surgeon, operating while looking through the scope with one eye.
Interest in a laparoscopic approach to hysterectomy began with my presentations about laparoscopic hysterectomy in January 1988. At that time I had over 10 years of experience doing what is now called laparoscopic-assisted vaginal hysterectomy.
I wrote extensively about specimen removal using a scalpel before electronic power morcellators were available. Since then, I have asked those using power morcellators to stop calling their operation a laparoscopic hysterectomy, as it has more in common with an abdominal-extraction hysterectomy.
I have never advocated removing the uterus using power morcellators, and I still believe that most specimens can be removed vaginally without the spray of pieces of the specimen around the peritoneal cavity that occurs with power morcellation. This goes for hysterectomy involving a large uterus, myomectomy through a culdotomy incision, and removal of the uterine fundus after supracervical hysterectomy. (It is irresponsible to use expensive power morcellation to remove small supracervical hysterectomy specimens.) It is time to get back to learning and teaching vaginal morcellation, although I readily admit it is time consuming.
Nevertheless, I believe power morcellation should remain an option. Recent laparoscopic fellowship trainees know only this technique, which is still better than a return to mutilation by laparotomy.
Gynecology is a frustrating profession—30 years of MIS as a sideshow. General surgery has rapidly adopted a laparoscopic approach to most operations, after gynecologists taught them. Today most gynecologists do not do advanced laparoscopic surgery and would love to get back to open incision laparotomy for their operations. We cannot go back.
OBG Management: Dr. Wertheim and Dr. Reich, do your personal views of the morcellation issue differ at all from the official views of professional societies?
Dr. Wertheim: Yes. However, before I share them, I’d like to emphasize that the views I’m about to express are mine and mine only, not those of the AAGL or its task force.
The issue of uterine extraction is a highly emotional and political issue, about which there are few good data.
Abundant Level 1 data strongly support a vaginal or laparoscopic approach for benign hysterectomy when possible. ACOG and AAGL have issued position papers supporting these approaches for benign hysterectomies. Gynecologic surgeons and other surgical specialists have embraced MIS because it is safer, offers faster recovery, produces less postoperative pain, and has fewer complications than open surgery. However, AAGL has maintained for several years that morcellation is contraindicated in cases where uterine malignancy is either known or suspected.
The dilemma with open power morcellation is that even with our best diagnostic tools, the rare uterine sarcoma cannot always be definitively ruled out preoperatively. Endometrial cancer usually can be diagnosed before surgery. However, rare subtypes such as sarcomas are more difficult to reliably diagnose preoperatively, and risk factors for uterine sarcomas are not nearly as well understood as those for endometrial cancer.
I do agree with the FDA’s cautionary statement on April 17, which pointedly prohibits power morcellation for women with suspected precancer or known cancer of the gynecologic organs.2 However, the AAGL Task Force critically reviewed about 120 articles, including the studies assessed by the FDA. Concerns arose regarding the FDA’s interpretation of the data. Due to a number of deficiencies in these studies, some of the conclusions of the FDA may not be completely accurate. The studies analyzed by the FDA were not stratified by risk factors for sarcoma and were not necessarily performed in a setting of reproductive-aged women with presumed fibroids.
Dr. Reich: Here are my personal views about the sarcoma problem and I am sure they differ from the official views:
- Laparoscopic hysterectomy should always mean vaginal extraction unless a less disfiguring site can be discovered; power morcellation implies minilaparotomy and should be renamed to reflect that fact.
- Power morcellation must be differentiated from vaginal and minilaparotomy scalpel morcellation, especially in the media. Vaginal hysterectomy has entailed vaginal scalpel morcellation with successful outcomes for more than 100 years.
- Remember that most gynecologic cancers are approached using the laparoscope today. This certainly includes cervical and endometrial cancer and some ovarian cancers. (For example, one of my neighbors is a 25-year survivor of laparoscopically treated bilateral ovarian cancer who refused laparotomy!)
- I have removed sarcomas by vaginal morcellation during laparoscopic hysterectomy and laparoscopic myomectomy with no late sequelae. In fact, most cervical cancer surgery is done by laparoscopic surgery today. And even an open laparotomy hysterectomy can spread a sarcoma.
- The current morcellation debate arose when a single case of disseminated leiomyosarcoma became highly publicized. It involved a prominent physician whose leiomyosarcoma was unknown to her initial surgeon, and the malignancy was upstaged after the use of power morcellation during hysterectomy. After this case was covered in the media, other cases began to be reported in the lay press as well, some of which predated the publicized case. The truth is, regrettably, that sarcomas carry poor prognoses even when specimens are removed intact. And we don’t know much about the sarcoma that started this debate. Was it mild or aggressive? How many mitotic figures were there per high-powered field? And what was found macroscopicallyand microscopically at the subsequent laparotomy? We on the AAGL Task Force do not know the answers to these questions, although at least some of these variables are reported in other published cases. And because this case is likely to have a powerful effect on MIS in our country and the rest of the world, it is my opinion that we need to know these details.
What is your preferred surgical approach?
OBG Management: Do you perform open power morcellation in selected patients?
Dr. Wertheim: Even though I have performed morcellation with a scalpel transvaginally or through a mini-laparotomy incision for many years, I have never used open power morcellation because of the risk of leaving behind benign or malignant tissue fragments. Morcellation with a scalpel is easily learned and can be performed as quickly as power morcellation. Morcellation with a scalpel produces much larger pieces than with power morcellation. This probably markedly decreases the loss of fragments. I cannot make a definitive statement regarding cell loss, however. Until we have improved instrumentation and are better able to make a preoperative diagnosis of sarcoma, I’m going to rule out risk factors identified by the AAGL Task Force, do the appropriate work-up, and continue to morcellate with a scalpel, placing the specimen in a bag, if technically possible.
Dr. Reich: As I mentioned, I am a vaginal scalpel morcellator. I tried power morcellation when it first was developed but was never a fan. The same techniques used for vaginal extraction using a coring maneuver can be used abdominally through the umbilicus or a 1- or 2-cm trocar site.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
The use of power morcellation to remove the uterus or uterine tumors during hysterectomy and myomectomy has been in the limelight in 2014—particularly morcellation performed in an “open” fashion (without use of a protective bag). Concerns about the dispersion of tissue throughout the peritoneal cavity—including the risk of disseminating tissue from leiomyosarcoma, a rare but deadly cancer—have drawn statements from the American College of Obstetricians and Gynecologists (ACOG), the AAGL, the US Food and Drug Administration (FDA), and others, cautioning against the use of open power morcellation in women with a known or suspected malignancy.
In July 2014, the FDA convened a two-day hearing of the Obstetrics and Gynecology Devices Panel (one of the panels in its Medical Devices Advisory Committee) to consider whether power morcellation should remain an option and, if so, what restrictions or labeling might be recommended.
In advance of the FDA hearing, OBG Management invited two experts in women’s health to explore the options more deeply and address the future of minimally invasive surgery (MIS): Ray A. Wertheim, MD, Director of the AAGL Center of Excellence Minimally Invasive Gynecology Program at Inova Fair Oaks Hospital in Fairfax, Virginia, and Harry Reich, MD, widely known as the first surgeon to perform laparoscopic hysterectomy, among other achievements. Both Dr. Wertheim and Dr. Reich were members of the AAGL Tissue Extraction Task Force.
In this Q&A, Dr. Wertheim and Dr. Reich discuss:
- options for tissue extraction going forward
- the importance of continuing to offer minimally invasive surgical approaches
- the need to educate surgeons about the safest approaches to tissue extraction.
Both surgeons believe that power morcellation should remain an option for selected cases, although neither performs the technique himself. Both surgeons also believe that minimally invasive approaches to hysterectomy and myomectomy are here to stay and should continue to be used whenever possible.
AAGL convened an impartial expert panel
OBG Management: Dr. Wertheim, could you tell us a little about the AAGL position statement on the use of power morcellation for uterine tissue extraction at hysterectomy or myomectomy, since you were on the task force that researched and wrote it?1
Dr. Wertheim: AAGL convened its task force to conduct a critical appraisal of the existing evidence related to the practice of uterine extraction in the setting of hysterectomy and myomectomy. Areas in need of further investigation also were identified.
The task force consisted of experts who had no conflicts, were not allowed to discuss or review findings with anyone, and were not reimbursed for their time. Our review is the most complete report to date, more comprehensive than the current reports from the FDA, ACOG, the Society of Gynecologic Oncology (SGO), and the American Urogynecologic Society (AUGS).
Interestingly, AAGL, ACOG, SGO, and AUGS all reached the same conclusion: All existing methods of tissue extraction have benefits and risks that must be balanced.
OBG Management: How did the AAGL Task Force assess the evidence?
Dr. Wertheim: The quality of evidence and strength of recommendations were assessed using US Preventive Services Task Force guidelines. There are very few good data on the issue of power morcellation for uterine tissue extraction, especially in regard to leiomyosarcoma. One needs to be careful making recommendations without good data.
Related article: First large study on risk of cancer spread using power morcellation. Janelle Yates (News for your Practice; August 2014)
At this time, we do not believe there is a single method of tissue extraction that can protect all patients. Therefore, all current methods should remain available. We believe that an understanding of the issues will allow surgeons, hospitals, and patients to make the appropriate informed choices regarding tissue extraction for individual patients undergoing uterine surgery.
AAGL recommendations on the use of power morcellationIn its position statement, the AAGL Tissue Extraction Task Force made the following main points, recommending that surgeons:
Further research also is needed to determine how best to diagnose sarcomas preoperatively, the task force noted. The full report is available on the AAGL Web site.1 —Ray A. Wertheim, MD |
How to manage tissue extraction going forward
OBG Management: Regardless of the FDA’s final decision, what should the gynecologic specialty be doing to avoid disseminating uterine tissue in the peritoneal cavity, particularly leiomyosarcoma?
Dr. Wertheim: MIS is a wonderful advancement in women’s health care. All surgical specialties are moving toward MIS. Our challenge is to perform it as safely as possible, given the data and instrumentation available.
In regard to leiomyosarcoma, because we lack the ability to accurately make the diagnosis preoperatively, we’ve identified risk factors that should be taken into consideration. They include advanced age, a history of radiation or tamoxifen use, black race, hereditary leiomyomatosis, renal cell carcinoma syndrome, and survival of childhood retinoblastoma.
At this time, we have specimen-retrieval bags that can be used with power morcellation. However, it takes skill to be able to place a large specimen inside a bag without injuring surrounding organs due to limited visibility.
Education, at the hospital and national level, is in the works
OBG Management: How should we go about educating surgeons about MIS alternatives to open power morcellation?
Dr. Wertheim: In my hospital, we are mentoring surgeons to help them gain the new skills needed. In addition, I plan to give a grand rounds presentation on tissue extraction for hospitals in northern Virginia and also would like to offer a course in the near future. I’m also hoping that we’ll be able to offer courses around the country before the annual AAGL meeting this November.
At the annual AAGL meeting, the subject will be discussed at length, with an emphasis on identifying risk factors and conducting appropriate preoperative testing, with workshops likely to teach the skills needed to perform these surgeries as safely as possible.
Why a return to reliance on laparotomy would be unwise
OBG Management: Given all the concerns expressed recently about open power morcellation, do you think some surgeons will revert to abdominal hysterectomy rather than rely on MIS? Would such a move be safer than power morcellation?
Dr. Wertheim: That would be a disaster for women. Very reliable data have shown that MIS is safer than open surgery, with much quicker recovery. Almost all of my patients are discharged within 3 hours after surgery, and most no longer require pain medications other than nonsteroidal anti-inflammatory drugs by postoperative day 2. They’re usually back to work within 2 weeks.
We have worked long and hard to develop skills and instrumentation required to perform MIS safely—but nothing replaces good judgment. In some cases, laparotomy or conversion to a laparotomy may be indicated.
New instrumentation is needed and is being developed. In the meantime, my personal bias is to rule out risk factors for malignancy and continue to morcellate with a scalpel, preferably inside a bag. After all, we know that with open power morcellation, fragments and cells are usually left behind regardless of inspection and irrigation. These fragments may cause leiomyomatosis, endometriosis, bowel obstruction, sepsis, and possible dissemination of tumor fragments. Moreover, morcellation into small fragments complicates the pathologist’s ability to give an accurate report. The use of open power morcellation also subjects the patient to a risk of damage to surrounding organs—usually due to the surgeon’s inexperience.
As I have said before, our challenge is to perform these surgeries using the safest techniques possible, given the current data and instrumentation.
OBG Management: Dr. Reich, you have a unique perspective on this issue, because you pioneered laparoscopic hysterectomy. How has uterine tissue extraction evolved since then? Do you think open power morcellation should remain an option?
Dr. Reich: Uterine tissue extraction has not evolved. The terms “laparoscopic hysterectomy” and “total laparoscopic hysterectomy” imply vaginal extraction using a scalpel, not abdominal extraction using a morcellator. Unfortunately there is no substitute for hard work using a #10 blade on a long handle and special vaginal retraction tools.
In 1983, I made a decision to stop performing laparotomy for all gynecologic procedures, including hysterectomy, myomectomy, urology, oncology, abscesses, extensive adhesions, and rectovaginal endometriosis. I was an accomplished vaginal surgeon at that time, as well as a one-handed laparoscopic surgeon, operating while looking through the scope with one eye.
Interest in a laparoscopic approach to hysterectomy began with my presentations about laparoscopic hysterectomy in January 1988. At that time I had over 10 years of experience doing what is now called laparoscopic-assisted vaginal hysterectomy.
I wrote extensively about specimen removal using a scalpel before electronic power morcellators were available. Since then, I have asked those using power morcellators to stop calling their operation a laparoscopic hysterectomy, as it has more in common with an abdominal-extraction hysterectomy.
I have never advocated removing the uterus using power morcellators, and I still believe that most specimens can be removed vaginally without the spray of pieces of the specimen around the peritoneal cavity that occurs with power morcellation. This goes for hysterectomy involving a large uterus, myomectomy through a culdotomy incision, and removal of the uterine fundus after supracervical hysterectomy. (It is irresponsible to use expensive power morcellation to remove small supracervical hysterectomy specimens.) It is time to get back to learning and teaching vaginal morcellation, although I readily admit it is time consuming.
Nevertheless, I believe power morcellation should remain an option. Recent laparoscopic fellowship trainees know only this technique, which is still better than a return to mutilation by laparotomy.
Gynecology is a frustrating profession—30 years of MIS as a sideshow. General surgery has rapidly adopted a laparoscopic approach to most operations, after gynecologists taught them. Today most gynecologists do not do advanced laparoscopic surgery and would love to get back to open incision laparotomy for their operations. We cannot go back.
OBG Management: Dr. Wertheim and Dr. Reich, do your personal views of the morcellation issue differ at all from the official views of professional societies?
Dr. Wertheim: Yes. However, before I share them, I’d like to emphasize that the views I’m about to express are mine and mine only, not those of the AAGL or its task force.
The issue of uterine extraction is a highly emotional and political issue, about which there are few good data.
Abundant Level 1 data strongly support a vaginal or laparoscopic approach for benign hysterectomy when possible. ACOG and AAGL have issued position papers supporting these approaches for benign hysterectomies. Gynecologic surgeons and other surgical specialists have embraced MIS because it is safer, offers faster recovery, produces less postoperative pain, and has fewer complications than open surgery. However, AAGL has maintained for several years that morcellation is contraindicated in cases where uterine malignancy is either known or suspected.
The dilemma with open power morcellation is that even with our best diagnostic tools, the rare uterine sarcoma cannot always be definitively ruled out preoperatively. Endometrial cancer usually can be diagnosed before surgery. However, rare subtypes such as sarcomas are more difficult to reliably diagnose preoperatively, and risk factors for uterine sarcomas are not nearly as well understood as those for endometrial cancer.
I do agree with the FDA’s cautionary statement on April 17, which pointedly prohibits power morcellation for women with suspected precancer or known cancer of the gynecologic organs.2 However, the AAGL Task Force critically reviewed about 120 articles, including the studies assessed by the FDA. Concerns arose regarding the FDA’s interpretation of the data. Due to a number of deficiencies in these studies, some of the conclusions of the FDA may not be completely accurate. The studies analyzed by the FDA were not stratified by risk factors for sarcoma and were not necessarily performed in a setting of reproductive-aged women with presumed fibroids.
Dr. Reich: Here are my personal views about the sarcoma problem and I am sure they differ from the official views:
- Laparoscopic hysterectomy should always mean vaginal extraction unless a less disfiguring site can be discovered; power morcellation implies minilaparotomy and should be renamed to reflect that fact.
- Power morcellation must be differentiated from vaginal and minilaparotomy scalpel morcellation, especially in the media. Vaginal hysterectomy has entailed vaginal scalpel morcellation with successful outcomes for more than 100 years.
- Remember that most gynecologic cancers are approached using the laparoscope today. This certainly includes cervical and endometrial cancer and some ovarian cancers. (For example, one of my neighbors is a 25-year survivor of laparoscopically treated bilateral ovarian cancer who refused laparotomy!)
- I have removed sarcomas by vaginal morcellation during laparoscopic hysterectomy and laparoscopic myomectomy with no late sequelae. In fact, most cervical cancer surgery is done by laparoscopic surgery today. And even an open laparotomy hysterectomy can spread a sarcoma.
- The current morcellation debate arose when a single case of disseminated leiomyosarcoma became highly publicized. It involved a prominent physician whose leiomyosarcoma was unknown to her initial surgeon, and the malignancy was upstaged after the use of power morcellation during hysterectomy. After this case was covered in the media, other cases began to be reported in the lay press as well, some of which predated the publicized case. The truth is, regrettably, that sarcomas carry poor prognoses even when specimens are removed intact. And we don’t know much about the sarcoma that started this debate. Was it mild or aggressive? How many mitotic figures were there per high-powered field? And what was found macroscopicallyand microscopically at the subsequent laparotomy? We on the AAGL Task Force do not know the answers to these questions, although at least some of these variables are reported in other published cases. And because this case is likely to have a powerful effect on MIS in our country and the rest of the world, it is my opinion that we need to know these details.
What is your preferred surgical approach?
OBG Management: Do you perform open power morcellation in selected patients?
Dr. Wertheim: Even though I have performed morcellation with a scalpel transvaginally or through a mini-laparotomy incision for many years, I have never used open power morcellation because of the risk of leaving behind benign or malignant tissue fragments. Morcellation with a scalpel is easily learned and can be performed as quickly as power morcellation. Morcellation with a scalpel produces much larger pieces than with power morcellation. This probably markedly decreases the loss of fragments. I cannot make a definitive statement regarding cell loss, however. Until we have improved instrumentation and are better able to make a preoperative diagnosis of sarcoma, I’m going to rule out risk factors identified by the AAGL Task Force, do the appropriate work-up, and continue to morcellate with a scalpel, placing the specimen in a bag, if technically possible.
Dr. Reich: As I mentioned, I am a vaginal scalpel morcellator. I tried power morcellation when it first was developed but was never a fan. The same techniques used for vaginal extraction using a coring maneuver can be used abdominally through the umbilicus or a 1- or 2-cm trocar site.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
1. The Tissue Extraction Task Force, AAGL. AAGL Position Statement: Morcellation during uterine tissue extraction. http://www.aagl.org/wp-content/uploads/2014/05/Tissue_Extraction_TFR.pdf. Accessed June 13, 2014.
2. US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy. FDA Safety Communication. http://www.fda.gov
/medicaldevices/safety/alertsandnotices/ucm393576.htm. Published April 17, 2014. Accessed June 13, 2014.
1. The Tissue Extraction Task Force, AAGL. AAGL Position Statement: Morcellation during uterine tissue extraction. http://www.aagl.org/wp-content/uploads/2014/05/Tissue_Extraction_TFR.pdf. Accessed June 13, 2014.
2. US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy. FDA Safety Communication. http://www.fda.gov
/medicaldevices/safety/alertsandnotices/ucm393576.htm. Published April 17, 2014. Accessed June 13, 2014.
Visit the Morcellation Topic Collection Page for additional articles, videos, and audiocasts.
The end of the cardiology boom
Twenty years ago Dr. Joseph Alpert and I published an editorial suggesting that we were training too many cardiologists and that we should begin to decrease the existing training programs. It was written in anticipation of the expansion of health maintenance organizations and the Clinton health care initiatives, neither of which occurred (Am. J. Cardiol. 1994;74:394-5). Our opinions were met with universal disdain among our cardiology colleagues.
However, what did take place over the next 15 years was the creation of a cardiology "boom," inflated by an expansion of cardiology services with coronary stents and multiple imaging techniques, which succeeded in making work for newly trained cardiologists. Most of these procedures, with few exceptions, had little or no impact on the quality of care but did generate a significant increase in cost. From 1995 to 2012, an additional 7,000 cardiologists became members of the American College of Cardiology, swelling its ranks from 21,000 to 28,000 members. Workforce projection in the early 21st century suggested that there would be a continuing need for cardiology specialists well into 2025. These projections were based on the aging of the population and gave little attention to the potential future change in health care financing.
But in fact, changes did occur, and the cardiology boom has been deflated, not like the 2008 deflation of the housing boom, but it is clear that some of the gas has been let out, and the boom will continue to deflate in the future. A recent editorial (J. Am. Coll. Cardiol. 2014,63;1927-8) authored by the ACC leaders suggests that major adjustments in career goals of graduating trainees will have to be made in order to deal with the change in the marketplace. The major change in the reimbursement for outpatient procedures that favored hospital services created a flight of practicing physicians from private to hospital-based practice. The federal government and private insurers can now monitor practice patterns and the utilizations of services more closely, and this has led to a significant decrease in these procedures. At the same time, the conversion of your friendly local hospital to a corporate conglomerate has opened the door for hospital administrators to squeeze cost centers like cardiology in order to improve the bottom line.
The new emphasis on physician participation in cost control, as manifested by the move to medical homes and accountable care organizations, emphasizes quality improvement over quantity billing, where doctors can benefit financially from cost savings. Patients are also becoming more concerned about their own role in medical costs as they begin to face increases in deductible costs. The age of fee-for-service payment is fast coming to an end. We are moving away from high-cost care that led to the boom to efficient care based on value payment models.
As medicine, and particularly cardiology, moves further into the 21st century it is clear that we are victims of our own technology. It is difficult to predict the future when so many countercurrents are in effect in our profession. Joe Alpert and I missed the target by about 20 years, but we could never have anticipated the magnitude of ebb and flow of workforce tides. Many of us presumed that the medical profession would be free of the changes in economy and technology. We are learning now that we are not immune to those changes.
To my readers: After writing this column for almost 18 years, I have decided to take a long summer vacation. I plan to be back in the fall but writing less frequently and sharing this wonderful platform with others. I thank you all for the many comments that I have received through the years, both positive and negative. I also want to thank my editor, Catherine Hackett, who has always encouraged me to speak out without any constraint.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
Twenty years ago Dr. Joseph Alpert and I published an editorial suggesting that we were training too many cardiologists and that we should begin to decrease the existing training programs. It was written in anticipation of the expansion of health maintenance organizations and the Clinton health care initiatives, neither of which occurred (Am. J. Cardiol. 1994;74:394-5). Our opinions were met with universal disdain among our cardiology colleagues.
However, what did take place over the next 15 years was the creation of a cardiology "boom," inflated by an expansion of cardiology services with coronary stents and multiple imaging techniques, which succeeded in making work for newly trained cardiologists. Most of these procedures, with few exceptions, had little or no impact on the quality of care but did generate a significant increase in cost. From 1995 to 2012, an additional 7,000 cardiologists became members of the American College of Cardiology, swelling its ranks from 21,000 to 28,000 members. Workforce projection in the early 21st century suggested that there would be a continuing need for cardiology specialists well into 2025. These projections were based on the aging of the population and gave little attention to the potential future change in health care financing.
But in fact, changes did occur, and the cardiology boom has been deflated, not like the 2008 deflation of the housing boom, but it is clear that some of the gas has been let out, and the boom will continue to deflate in the future. A recent editorial (J. Am. Coll. Cardiol. 2014,63;1927-8) authored by the ACC leaders suggests that major adjustments in career goals of graduating trainees will have to be made in order to deal with the change in the marketplace. The major change in the reimbursement for outpatient procedures that favored hospital services created a flight of practicing physicians from private to hospital-based practice. The federal government and private insurers can now monitor practice patterns and the utilizations of services more closely, and this has led to a significant decrease in these procedures. At the same time, the conversion of your friendly local hospital to a corporate conglomerate has opened the door for hospital administrators to squeeze cost centers like cardiology in order to improve the bottom line.
The new emphasis on physician participation in cost control, as manifested by the move to medical homes and accountable care organizations, emphasizes quality improvement over quantity billing, where doctors can benefit financially from cost savings. Patients are also becoming more concerned about their own role in medical costs as they begin to face increases in deductible costs. The age of fee-for-service payment is fast coming to an end. We are moving away from high-cost care that led to the boom to efficient care based on value payment models.
As medicine, and particularly cardiology, moves further into the 21st century it is clear that we are victims of our own technology. It is difficult to predict the future when so many countercurrents are in effect in our profession. Joe Alpert and I missed the target by about 20 years, but we could never have anticipated the magnitude of ebb and flow of workforce tides. Many of us presumed that the medical profession would be free of the changes in economy and technology. We are learning now that we are not immune to those changes.
To my readers: After writing this column for almost 18 years, I have decided to take a long summer vacation. I plan to be back in the fall but writing less frequently and sharing this wonderful platform with others. I thank you all for the many comments that I have received through the years, both positive and negative. I also want to thank my editor, Catherine Hackett, who has always encouraged me to speak out without any constraint.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
Twenty years ago Dr. Joseph Alpert and I published an editorial suggesting that we were training too many cardiologists and that we should begin to decrease the existing training programs. It was written in anticipation of the expansion of health maintenance organizations and the Clinton health care initiatives, neither of which occurred (Am. J. Cardiol. 1994;74:394-5). Our opinions were met with universal disdain among our cardiology colleagues.
However, what did take place over the next 15 years was the creation of a cardiology "boom," inflated by an expansion of cardiology services with coronary stents and multiple imaging techniques, which succeeded in making work for newly trained cardiologists. Most of these procedures, with few exceptions, had little or no impact on the quality of care but did generate a significant increase in cost. From 1995 to 2012, an additional 7,000 cardiologists became members of the American College of Cardiology, swelling its ranks from 21,000 to 28,000 members. Workforce projection in the early 21st century suggested that there would be a continuing need for cardiology specialists well into 2025. These projections were based on the aging of the population and gave little attention to the potential future change in health care financing.
But in fact, changes did occur, and the cardiology boom has been deflated, not like the 2008 deflation of the housing boom, but it is clear that some of the gas has been let out, and the boom will continue to deflate in the future. A recent editorial (J. Am. Coll. Cardiol. 2014,63;1927-8) authored by the ACC leaders suggests that major adjustments in career goals of graduating trainees will have to be made in order to deal with the change in the marketplace. The major change in the reimbursement for outpatient procedures that favored hospital services created a flight of practicing physicians from private to hospital-based practice. The federal government and private insurers can now monitor practice patterns and the utilizations of services more closely, and this has led to a significant decrease in these procedures. At the same time, the conversion of your friendly local hospital to a corporate conglomerate has opened the door for hospital administrators to squeeze cost centers like cardiology in order to improve the bottom line.
The new emphasis on physician participation in cost control, as manifested by the move to medical homes and accountable care organizations, emphasizes quality improvement over quantity billing, where doctors can benefit financially from cost savings. Patients are also becoming more concerned about their own role in medical costs as they begin to face increases in deductible costs. The age of fee-for-service payment is fast coming to an end. We are moving away from high-cost care that led to the boom to efficient care based on value payment models.
As medicine, and particularly cardiology, moves further into the 21st century it is clear that we are victims of our own technology. It is difficult to predict the future when so many countercurrents are in effect in our profession. Joe Alpert and I missed the target by about 20 years, but we could never have anticipated the magnitude of ebb and flow of workforce tides. Many of us presumed that the medical profession would be free of the changes in economy and technology. We are learning now that we are not immune to those changes.
To my readers: After writing this column for almost 18 years, I have decided to take a long summer vacation. I plan to be back in the fall but writing less frequently and sharing this wonderful platform with others. I thank you all for the many comments that I have received through the years, both positive and negative. I also want to thank my editor, Catherine Hackett, who has always encouraged me to speak out without any constraint.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
Should donor blood be screened for HEV?

Credit: Daniel Gay
Roughly 1 in 3000 English blood donors have hepatitis E virus (HEV) in their plasma, according to research published in The Lancet.
This suggests that about 1200 HEV-containing blood components may be transfused in England every year.
“HEV genotype 3 infections are widespread in the English population, including blood donors,” said study author Richard Tedder, MB ChB, of National Health Service Blood and Transplant in London.
“We estimate that between 80,000 and 100,000 human HEV infections are likely to have occurred in England during the year of our study.”
These figures seem to indicate a need for screening blood donations. But Dr Tedder and his colleagues found evidence suggesting a low overall burden of harm from transfusion-transmitted HEV.
The researchers retrospectively screened 225,000 individual blood donations collected in south east England between October 2012 and September 2013 for HEV RNA.
Seventy-nine donors—about 1 in 2848—were infected with genotype 3 HEV, which can spread directly from animals to humans. The 79 donations had been used to prepare 129 blood components, 62 of which had been transfused.
Follow-up of 43 exposed recipients showed that transmission had occurred in 18 (42%) patients.
Immunosuppression extended the duration of viremia in these patients, but 3 cleared their infection following a change in immunosuppressive therapy or after receiving ribavirin.
Ten of the patients developed prolonged or persistent infection. Transaminitis was common, and 1 patient developed hepatitis.
“Although rarely causing any acute illness, hepatitis E infections may become persistent in immunosuppressed patients, putting them at risk of future chronic liver disease, and a policy is needed to identify these persistently infected patients and provide them with appropriate antiviral treatment,” Dr Tedder said.
“However, our study indicates that the overall burden of harm resulting from transfusion-transmitted HEV is slight. Although, on a clinical basis alone, there appears no pressing need at this time for blood donations to be screened, a broader discussion over harm mitigation is now required.”
A related comment article in The Lancet suggested there is, in fact, a need for the systematic screening of blood components.
“The potential clinical results of blood-borne HEV infection should not be downplayed; in particular, the risk of serious complications and death exists,” wrote Jean-Michel Pawlotsky, MD, PhD, of Hôpital Henri Mondor in Créteil, France.
“Thus, on the basis of [the current] and other studies, I believe that systematic screening of blood components for markers of hepatitis E infection should be implemented in areas where HEV is endemic (eg, the European Union), based on HEV RNA detection.” ![]()

Credit: Daniel Gay
Roughly 1 in 3000 English blood donors have hepatitis E virus (HEV) in their plasma, according to research published in The Lancet.
This suggests that about 1200 HEV-containing blood components may be transfused in England every year.
“HEV genotype 3 infections are widespread in the English population, including blood donors,” said study author Richard Tedder, MB ChB, of National Health Service Blood and Transplant in London.
“We estimate that between 80,000 and 100,000 human HEV infections are likely to have occurred in England during the year of our study.”
These figures seem to indicate a need for screening blood donations. But Dr Tedder and his colleagues found evidence suggesting a low overall burden of harm from transfusion-transmitted HEV.
The researchers retrospectively screened 225,000 individual blood donations collected in south east England between October 2012 and September 2013 for HEV RNA.
Seventy-nine donors—about 1 in 2848—were infected with genotype 3 HEV, which can spread directly from animals to humans. The 79 donations had been used to prepare 129 blood components, 62 of which had been transfused.
Follow-up of 43 exposed recipients showed that transmission had occurred in 18 (42%) patients.
Immunosuppression extended the duration of viremia in these patients, but 3 cleared their infection following a change in immunosuppressive therapy or after receiving ribavirin.
Ten of the patients developed prolonged or persistent infection. Transaminitis was common, and 1 patient developed hepatitis.
“Although rarely causing any acute illness, hepatitis E infections may become persistent in immunosuppressed patients, putting them at risk of future chronic liver disease, and a policy is needed to identify these persistently infected patients and provide them with appropriate antiviral treatment,” Dr Tedder said.
“However, our study indicates that the overall burden of harm resulting from transfusion-transmitted HEV is slight. Although, on a clinical basis alone, there appears no pressing need at this time for blood donations to be screened, a broader discussion over harm mitigation is now required.”
A related comment article in The Lancet suggested there is, in fact, a need for the systematic screening of blood components.
“The potential clinical results of blood-borne HEV infection should not be downplayed; in particular, the risk of serious complications and death exists,” wrote Jean-Michel Pawlotsky, MD, PhD, of Hôpital Henri Mondor in Créteil, France.
“Thus, on the basis of [the current] and other studies, I believe that systematic screening of blood components for markers of hepatitis E infection should be implemented in areas where HEV is endemic (eg, the European Union), based on HEV RNA detection.” ![]()

Credit: Daniel Gay
Roughly 1 in 3000 English blood donors have hepatitis E virus (HEV) in their plasma, according to research published in The Lancet.
This suggests that about 1200 HEV-containing blood components may be transfused in England every year.
“HEV genotype 3 infections are widespread in the English population, including blood donors,” said study author Richard Tedder, MB ChB, of National Health Service Blood and Transplant in London.
“We estimate that between 80,000 and 100,000 human HEV infections are likely to have occurred in England during the year of our study.”
These figures seem to indicate a need for screening blood donations. But Dr Tedder and his colleagues found evidence suggesting a low overall burden of harm from transfusion-transmitted HEV.
The researchers retrospectively screened 225,000 individual blood donations collected in south east England between October 2012 and September 2013 for HEV RNA.
Seventy-nine donors—about 1 in 2848—were infected with genotype 3 HEV, which can spread directly from animals to humans. The 79 donations had been used to prepare 129 blood components, 62 of which had been transfused.
Follow-up of 43 exposed recipients showed that transmission had occurred in 18 (42%) patients.
Immunosuppression extended the duration of viremia in these patients, but 3 cleared their infection following a change in immunosuppressive therapy or after receiving ribavirin.
Ten of the patients developed prolonged or persistent infection. Transaminitis was common, and 1 patient developed hepatitis.
“Although rarely causing any acute illness, hepatitis E infections may become persistent in immunosuppressed patients, putting them at risk of future chronic liver disease, and a policy is needed to identify these persistently infected patients and provide them with appropriate antiviral treatment,” Dr Tedder said.
“However, our study indicates that the overall burden of harm resulting from transfusion-transmitted HEV is slight. Although, on a clinical basis alone, there appears no pressing need at this time for blood donations to be screened, a broader discussion over harm mitigation is now required.”
A related comment article in The Lancet suggested there is, in fact, a need for the systematic screening of blood components.
“The potential clinical results of blood-borne HEV infection should not be downplayed; in particular, the risk of serious complications and death exists,” wrote Jean-Michel Pawlotsky, MD, PhD, of Hôpital Henri Mondor in Créteil, France.
“Thus, on the basis of [the current] and other studies, I believe that systematic screening of blood components for markers of hepatitis E infection should be implemented in areas where HEV is endemic (eg, the European Union), based on HEV RNA detection.” ![]()
Mouse model shows epigenetic changes drive cancer

Credit: Aaron Logan
Researchers say they’ve created a mouse model that provides the first in vivo evidence that epigenetic alterations alone can cause cancer.
They described the work in The Journal of Clinical Investigation.
“We knew that epigenetic changes are associated with cancer but didn’t know whether these were a cause or consequence of cancer,” said study author Lanlan Shen, MD, PhD, of Baylor College of Medicine in Houston, Texas.
“Developing this new approach for ‘epigenetic engineering’ allowed us to test whether DNA methylation changes alone can drive cancer.”
Dr Shen and her colleagues focused on p16, a gene that normally functions to prevent cancer but is commonly methylated in a range of cancers. They devised an approach to engineer DNA methylation specifically to the mouse p16 promoter.
As intended, the engineered p16 promoter acted as a “methylation magnet.” As the mice reached adulthood, gradually increasing p16 methylation led to a higher incidence of spontaneous cancers and reduced survival.
“This is not only the first in vivo evidence that epigenetic alteration alone can cause cancer,” Dr Shen said.
“This also has profound implications for future studies because epigenetic changes are potentially reversible. Our findings therefore both provide hope for new epigenetic therapies and validate a novel approach for testing them.”
Dr Shen also noted that this new approach could be widely useful because there are many other genes and diseases connected to epigenetics.
Just as genetic engineering has become a standard approach for studying how genetic mutations cause disease, epigenetic engineering will enable functional studies of epigenetics. ![]()

Credit: Aaron Logan
Researchers say they’ve created a mouse model that provides the first in vivo evidence that epigenetic alterations alone can cause cancer.
They described the work in The Journal of Clinical Investigation.
“We knew that epigenetic changes are associated with cancer but didn’t know whether these were a cause or consequence of cancer,” said study author Lanlan Shen, MD, PhD, of Baylor College of Medicine in Houston, Texas.
“Developing this new approach for ‘epigenetic engineering’ allowed us to test whether DNA methylation changes alone can drive cancer.”
Dr Shen and her colleagues focused on p16, a gene that normally functions to prevent cancer but is commonly methylated in a range of cancers. They devised an approach to engineer DNA methylation specifically to the mouse p16 promoter.
As intended, the engineered p16 promoter acted as a “methylation magnet.” As the mice reached adulthood, gradually increasing p16 methylation led to a higher incidence of spontaneous cancers and reduced survival.
“This is not only the first in vivo evidence that epigenetic alteration alone can cause cancer,” Dr Shen said.
“This also has profound implications for future studies because epigenetic changes are potentially reversible. Our findings therefore both provide hope for new epigenetic therapies and validate a novel approach for testing them.”
Dr Shen also noted that this new approach could be widely useful because there are many other genes and diseases connected to epigenetics.
Just as genetic engineering has become a standard approach for studying how genetic mutations cause disease, epigenetic engineering will enable functional studies of epigenetics. ![]()

Credit: Aaron Logan
Researchers say they’ve created a mouse model that provides the first in vivo evidence that epigenetic alterations alone can cause cancer.
They described the work in The Journal of Clinical Investigation.
“We knew that epigenetic changes are associated with cancer but didn’t know whether these were a cause or consequence of cancer,” said study author Lanlan Shen, MD, PhD, of Baylor College of Medicine in Houston, Texas.
“Developing this new approach for ‘epigenetic engineering’ allowed us to test whether DNA methylation changes alone can drive cancer.”
Dr Shen and her colleagues focused on p16, a gene that normally functions to prevent cancer but is commonly methylated in a range of cancers. They devised an approach to engineer DNA methylation specifically to the mouse p16 promoter.
As intended, the engineered p16 promoter acted as a “methylation magnet.” As the mice reached adulthood, gradually increasing p16 methylation led to a higher incidence of spontaneous cancers and reduced survival.
“This is not only the first in vivo evidence that epigenetic alteration alone can cause cancer,” Dr Shen said.
“This also has profound implications for future studies because epigenetic changes are potentially reversible. Our findings therefore both provide hope for new epigenetic therapies and validate a novel approach for testing them.”
Dr Shen also noted that this new approach could be widely useful because there are many other genes and diseases connected to epigenetics.
Just as genetic engineering has become a standard approach for studying how genetic mutations cause disease, epigenetic engineering will enable functional studies of epigenetics. ![]()
Management of CCB Overdoses
The 2011 National Poison Data System (NPDS) of the American Association of Poison Control Centers reported that among the top 25 categories associated with mortality, cardiovascular medications were second to sedatives/hypnotics/antipsychotics in terms of the number of deaths resulting from overdose. Moreover, of cardiovascular medications, Calcium channel blockers (CCBs) were the most common agents associated with mortality.[1, 2] The 2012 NPDS report showed a similar trend, with cardiovascular drugs ranking among the top causes of overdoses, with an additional approximately 4614 cases in comparison to 2011.[3] In light of emerging strategies for the management of CCB overdoses, we sought to review the pathophysiology of CCB overdose and its management.
PATHOPHYSIOLOGY OF CCB OVERDOSE
CCBs are widely used in the management of various conditions such as hypertension, angina pectoris, atrial fibrillation, and other cardiac arrhythmias. CCBs block L‐type receptors on the cell surface.[4] Based on their predominant physiological effect, CCBs have been classified as dihydropyridines and nondihydropyridines (Table 1). Dihydropyridine overdose generally results in vasodilation with resultant hypotension and reflex tachycardia.[5] In comparison, nondihydropyridine overdose generally results in bradycardia and decreased cardiac contractility.[6] With high serum concentrations of either CCB class, however, selectivity is lost, and patients may presents with bradycardia, hypotension, and decreased cardiac contractility.[7, 8]
|
| Dihydropyridine |
| Short‐acting agents: nifedipine |
| Longer‐acting formulations: felodipine, isradipine, nicardipine, nifedipine, nisoldipine, amlodipinea |
| Nondihydropyridine |
| Verampamil and diltiazem |
CCBs show good oral bioavailability and undergo first‐pass metabolism. During an overdose, the enzymes involved in hepatic oxidation can become oversaturated, which reduces the effects of first‐pass metabolism, resulting in increased quantities of the active drug reaching the systemic circulation and a prolonged half‐life.[7] In addition, CCBs are highly protein bound and have large volumes of distribution.[9]
Calcium enters cells through specific channels and regulates various cell processes. In myocardial cells, calcium affects excitation‐contraction coupling and potential action generation in the sinoatrial node. Similarly, in the pancreas, calcium facilitates the release of insulin. CCB overdose can result in inhibition of insulin secretion from the pancreas and a state of hypoinsulinemia and insulin resistance.[10] Mtabolic acidosis is a common presentation noted in several published case reports.[11] Metabolic acidosis represents a combination of insulin dysregulation with ketoacidosis and hypoperfusion with lactic acidosis. In addition, because CCBs block the entry of calcium into the mitochondria,[12, 13] and because calcium is required for the normal enzymatic activity of the Krebs cycle, CCB overdose leads to lactic acid build‐up from its direct effects on aerobic metabolism.[14]
The clinical picture of CCB overdose is further complicated by the switch in the mechanism of adenosine triphosphate (ATP) generation in the myocardium from free fatty acid oxidation to carbohydrate metabolism.[15] In response to this stress, the liver increases glucose production via glycogenolysis. With concomitant hypoinsulinemia[10] and relative insulin resistance, intracellular glucose transport is disturbed, with a resultant decrease in ATP production that quickly leads to myocardial dysfunction and cardiogenic shock. The resultant clinical state of acidosis, hyperglycemia, and insulin deficiency is similar to diabetic ketoacidosis.[11, 14] A presentation of symptomatic bradycardia, hyperglycemia, and persistent hypotension, with signs of hypoperfusion usually manifested as altered mental status, clinically defines a severe overdose.
MANAGEMENT APPROACH
Maintenance of the airway and circulation is of primary importance in CCB overdose cases (Table 2). Hypotension and bradyarrhythmias are noted in cases of severe overdose, and some patients might require endotracheal intubation and mechanical ventilation very early in their management. The initial treatment strategy typically consists of the use of intravenous crystalloids and gastrointestinal (GI) decontamination; atropine is reserved for symptomatic bradycardia. Some patients may also require transcutaneous and transvenous pacing early and emergently due to complete cardiovascular collapse. Therefore, having a medical toxicologist or a regional poison control expert involved from the time of initial management is advised, especially for cases of severe overdose or consumption of extended‐release preparations.
|
| Initial resuscitation measures |
| Intravenous hydration with crystalloids, colloids. |
| Gastrointestinal decontamination |
| Activated charcoal 1 g/kg body weight in hemodynamically stable patients who can protect their airways.[1] Best administered within 2 hours. However, in poisoning from extended release preparations, it can be used beyond the 2‐hour window. Anecdotally, WBI has been utilized in calcium channel blocker overdose. However, it is not the recommended approach, especially in patients who are hemodynamically unstable. |
| Atropine |
| Reserved for bradycardia; 0.5 mg every 35 minutes, not to exceed a total of 3 mg or 0.04 mg/kg (per ACLS protocol). |
| Sodium bicarbonate |
| 12 mEq/kg boluses of hypertonic sodium bicarbonate when QRS widening is noted on the ECG.[46] For severe acidosis or persistent ECG changes, a sodium bicarbonate drip can be initiated with 150 mEq sodium bicarbonate in 1 L D5W to run at about 100125 mL per hour.[46] |
| Following intravenous hydration and GI decontamination (hyperinsulinemia‐euglycemia therapy) or vasopressors are usually initiated as resuscitation measures. |
| Agents used to reverse the calcium channel blocker poisoning |
| Hyperinsulinemia‐euglycemia therapy (refer to Table 33). |
| Glucagon |
| Initiated at 0.050.15 mg/kg as bolus dosing, with a repeat dosing in 35 minutes. An intravenous infusion can be initiated following this.[1] |
| Calcium salts |
| A bolus of 0.3 mEq/kg of calcium can be administered as intravenously over 510 minutes (0.6 mL/kg of 10% calcium gluconate solution or 0.2 mL/kg of 10% calcium chloride solution). |
| If beneficial response noted, an infusion of 0.3 mEq/kg per hour. |
| Titrate the infusion to obtain an adequate hemodynamic response. Serum ionized calcium levels should be monitored, and target ionized calcium levels should be less than twice the upper limit of normal.[2] |
| Adrenergic agents |
| Norepinephrine, dopamine, vasopressin. |
| Intravenous lipid emulsion therapy |
| 20% fat emulsion is what is usually used with 1 mL/kg given as a bolus followed by a continuous infusion of 0.250.5 mL/kg per hour. |
| Phosphodiesterase inhibitors |
| Amrinone, milrinone. |
| Invasive therapy |
| Transvenous and transcutaneous pacing for high‐grade atrioventricular dissociation. |
| Intra‐aortic balloon pump. |
| Extra corporeal membrane oxygenation. |
GI Decontamination
In cases of severe overdose, patients may present with lethargy from hypotension and poor cerebral flow, and the risk for aspiration and pneumonitis should be strongly considered in these patients if GI decontamination is considered. GI decontamination is best in cases where the patient is hemodynamically stable and presents early to the emergency department (ED), preferably within 2 hours[7, 9]; early use might decrease drug absorption and enterohepatic circulation, thus lowering the drug levels.[16] However, in cases in which the drug consumed was an extended‐release preparation, GI decontamination is beneficial even when the patient presents late to the ED.[17] GI decontamination is typically achieved using activated charcoal (1 g/kg body weight) or by performing whole bowel irrigation (WBI) with polyethylene glycol.[9] However, there is very little evidence that either approach changes the overall outcome, and WBI can be potentially harmful for patients with hemodynamic instability.[18] Therefore, airway and circulation maintenance is preferable to this approach.
Catecholamines
Catecholamines, such as dopamine, dobutamine, and norepinephrine, appear to be obvious choices in the management of cases of CCB overdose, because most patients present with hypotension and bradyarrhythmias.[19] However, there is no evidence to show the superiority of 1 agent over another in the management of CCB drug toxicity. Catecholamines increase the heart rate and blood pressure and increase systemic vascular resistance, which can potentially decrease the cardiac output by increasing the afterload.
Calcium Salts
In cases of severe overdose, the initial measures are typically not sufficient for stabilizing the patient. Intravenous (IV) calcium salts have been evaluated in animal models[20, 21] and, anecdotally, in human case reports.[22, 23, 24] However, the response to treatment has been mixed, with improvement in hemodynamic parameters in some cases and treatment failures in other cases. Moreover, the effects of these treatments are typically short lived, and repeated dosing might be required. Calcium salts are typically administered with the theoretical scheme of reversing antagonism with a higher calcium load and increasing cardiac inotropy. Calcium gluconate and calcium chloride are 2 frequently used agents, although no clear guidelines exist regarding this approach and the required dosage.[22] There are also published case reports in which refractory hypotension was treated with continuous calcium infusion in an attempt to reach predefined serum calcium levels.[24] However, the fear of iatrogenic hypercalcemia and its consequences is constant.[25] Calcium chloride contains 3 times the calcium for the identical volume compared to calcium gluconate and is more corrosive to the blood vessels; therefore, it is best administered through a central intravenous access. Although the evidence is limited to a few case reports, continuous calcium infusion appears effective and safe as an adjunctive therapy for patients with severe hypotension resulting from CCB overdose.[21, 22, 23, 24, 26]
Glucagon
Although insulin and glucagon are physiologically counter‐regulatory, they have a similar effect on heart stimulation. In animal models, the positive inotropic and chronotropic effects of glucagon have been clearly demonstrated.[27] Glucagon increases intracellular cyclic adenosine monophosphate (AMP) by stimulating adenylyl cyclase, a mechanism by which glucagon possibly exerts its inotropic effect.[7] Most studies conducted on the use of glucagon in the treatment of CCB overdose originated in an era in which bovine or porcine glucagon was used, and these animal glucagon products contained insulin.[9] Glucagon is typically initiated at 50 to 150 g/kg as bolus dosing, with a repeat dosing after 3 to 5 minutes.[9] A continuous IV infusion can then be administered following the initial treatment, because glucagon has a very short half‐life and works rapidly.[7, 9] However, there is no established maximum infusion dose of glucagon, and it should be titrated to the desired clinical outcome. IV glucagon therapy also carries a risk for nausea and vomiting,[7, 28] which in combination with lethargy may increase the risk for aspiration pneumonitis. The evidence for the use of glucagon in cases of CCB overdose is predominantly based on animal models[27]; evidence in human subjects is limited to case reports.[11, 28, 29] Some cases have demonstrated an improvement in hemodynamics with glucagon, whereas in a few cases, glucagon failed to result in such improvement.[30] In cases in which the ingestion history is unclear or there is polysubstance ingestion, as with ‐blockers and CCBs, glucagon is an ideal treatment agent[9]; in contrast, in single CCB overdose, glucagon might not be as helpful as more recent treatment modalities.
Hyperinsulinemia‐Euglycemia Therapy
In recent years, increasing evidence from multiple case reports and case series has shown the superiority of high‐dose insulin therapy over other treatment modalities (Table 3). Insulin acts as a potent inotrope[31, 32] and vasodilator. In their prospective observational series of 7 patients, Greene et al. report the successful use of hyperinsulinemia‐euglycemia therapy (HIET) with no significant adverse events when combined with conventional measures in a critical‐care setting.[33] Similarly, more than 50 cases have been reported in which HIET was used successfully in the management of CCB overdoses.[34]
| Bolus dosing |
| Check finger stick blood glucose, and 25 g dextrose can be given as a bolus, provided the patient is not markedly hyperglycemic[1] (eg, blood glucose >400 mg/dL). |
| 0.5 IU/kg of insulin given as bolus. An acceptable alternative would be to give 1 IU/kg as a bolus to saturate the receptors.[1, 3, 4] |
| Maintenance dose infusion |
| Short‐acting insulin initiated at 0.5 IU/kg per hour, and this dose can be titrated up to 2 IU/kg per hour. Doses as high as 10 IU/kg per hour have been tried and have been successful.[1, 4] |
| Continuous dextrose infusion might be required to maintain euglycemia (25 g per hour intravenous infusion would be a reasonable choice).[1] |
| Monitoring |
| Monitor blood glucose every 30 minutes for the first 4 hours and then hourly. Titrate dextrose infusion to maintain euglycemia.[1] |
| Dextrose containing fluid can be initiated at 0.51 g/kg per hour and titrated to maintain euglycemia.[10, 15] |
| Monitor potassium levels every 60 minutes and replace as needed to maintain at lower limits of normal (2.83.2 mEq/L). |
| Titration of the insulin infusion is usually to the resolution of hemodynamic parameters. |
| Discontinuation |
| No clear evidence to say if a weaning protocol is necessary. In several case reports, the protocol was discontinued after objective parameters of clinical resolution were achieved; however, continued dextrose infusion may be required despite the discontinuation of the insulin.[5] |
Although there is wide variation in the insulin dosing regimens in published case reports, hyperinsulinemia therapy is typically initiated with a 0.5 IU/kg to 1 IU/kg bolus, followed by a continuous drip of 0.5 UI/kg per hour to 1 IU/kg per hour. This dose is titrated every 15 to 20 minutes until satisfactory hemodynamic and clinical stability is noted. Titrations are usually avoided for a shorter time interval because insulin must enter cells and initiate intracellular signaling and metabolic activation. However, the response to HIET might be delayed, and other therapeutic modalities could be required simultaneously until the clinical effects of insulin are observed.
Euglycemia should be maintained by checking the blood glucose levels every 30 minutes and using a dextrose solution to maintain the blood glucose within the upper limits of normal.[35] Hyperglycemia noted in CCB overdose cases indicates the degree of insulin resistance and serves as a marker of the severity of the overdose.[14, 15] In particular, patients who are hyperglycemic at presentation may not require supplemental dextrose infusion despite the high‐dose insulin therapy. The blood glucose level should be checked every 30 minutes for the first 4 hours and then hourly to avoid overlooking hypoglycemia during the treatment regimen, especially in intubated and sedated patients. Fluids containing dextrose may be initiated at 0.5 to 1 g/kg per hour and titrated to maintain euglycemia.[9, 11]
However, there is no consensus as to how long the infusion should be continued once initiated. Although insulin has not been shown to induce tachyphylaxis in experimental animal models, many clinicians prefer to discontinue the infusion once hemodynamic stability has been achieved. There is also no evidence indicating whether a weaning protocol would make any difference over abrupt discontinuation.[36] The physiological effects of insulin persist for hours after the discontinuation of the infusion and will gradually taper down with time. Therefore, theoretically, an abrupt cessation should seldom cause any deleterious effects.[11] Dextrose supplementation may be required to maintain euglycemia for up to 24 hours following discontinuation of the insulin drip due to the elevated insulin levels.[11, 36]
Insulin is a potent vasodilator in the coronary and pulmonary vasculature but does not increase the requirement for myocardial oxygen. Instead, insulin facilitates endothelial nitric oxide activity through the phosphoinositide 3‐kinase (PI3K) pathway, which translates into vasodilatation of the capillary microvasculature and better perfusion at the capillary junction. As a result, insulin corrects the capillary dysfunction that is the major pathology in cardiogenic shock and the ultimate presentation in severe CCB overdose.
Gradinac et al. reported that patients with cardiogenic shock, in the postoperative period of coronary artery bypass grafting, showed a better cardiac index with the use of IV insulin therapy.[37] In an experiment on explanted human myocardium, von Lewinski et al. demonstrated the positive inotropic effect of insulin through calcium‐dependent pathways as well as PI3K pathways.[38] Moreover, Hsu et al. demonstrated with human myocardial cells that this inotropic property of insulin was dose dependent, with better responses observed after the use of higher doses of insulin; in addition, this effect was rapid (ie, as fast as 5 minutes after the infusion) and was sustained throughout the duration of insulin treatment.[39] The best clinical translation of this finding was demonstrated by Yuan et al.[11] in their case series of 5 patients with severe cardiogenic shock secondary to CCB overdoses.
There have also been cases of CCB overdoses in which insulin therapy has failed, which may be because the insulin protocol was initiated late as salvage therapy or because of the severity of the events.[35] Insulin therapy should be initiated early in the course of management rather than as salvage therapy.[7, 35] Agarwal et al. reported their experience in treating an patient on 3 separate occasions of CCB overdose. These authors reported rapid improvement on the third occasion, in which insulin therapy was initiated early during the course of management.[40] In recent years, HIET has been shown to be a promising approach in the management of CCB overdose. Patients with third‐degree heart blockage resulting from CCB overdose reverted to a normal sinus rhythm while on an insulin drip protocol without the intervention of a temporary pacemaker.[11]
High‐dose insulin therapy can also result in hypokalemia, which theoretically may represent a beneficial response in the management of CCB overdose, because it provides a membrane stabilizing effect by prolonging repolarization and allowing more calcium to enter the cytoplasm during cardiac systole.[11] Yuan et al. suggested a serum potassium range of 2.8 to 3.2 mEq/L during insulin‐glucose therapy.[11] Hypomagnesemia and hypophosphatemia are other electrolyte derangements reported during treatment that are similar to conditions observed in patients with diabetic ketoacidosis.[41, 42]
Intravenous Lipid Emulsion Therapy
CCBs are naturally lipophilic, and intravenous lipid emulsion (ILE) therapy has been attempted with success in cases of severe CCB overdose.[43, 44] A systematic review by Jamaty et al.[45] showed that, although the overall quality of the evidence for this modality was poor, ILE could be beneficial in the management of severe cases of CCB poisoning. ILE therapy was first described by Weinberg et al. for bupivacaine toxicity in the year 2003.[46] ILE is commonly utilized as part of total parenteral nutrition, and several case reports have shown the success of its use in the treatment of local anesthetic toxicity.[47] Although the mechanism remains to be clearly elucidated,[48] it is hypothesized that this emulsion in the circulation creates a lipid channel, which causes sequestration of lipophilic drugs, and stimulates the redistribution of lipophilic drugs from the tissues to this channel.[47] Recent data have further revealed the inotropic properties of lipid emulsion; when used for acute overdose, lipid emulsion improves ventricular contractility and diastolic relaxation, going beyond its role as a simple fuel for cardiac tissue or a lipid sink.[49] Lipid emulsion in the circulation also stimulates insulin secretion, which is beneficial in reversing the antagonism caused by CCB on the cells of the pancreas.[50] However, fat embolism, infection, and the development of acute respiratory distress syndrome have been reported as complications associated with this therapy.[51] Thus, it is prudent to involve a medical toxicologist or the regional poison center to decide whether a patient would be a candidate for this treatment approach. In most cases, this is reserved as a last resort in the management of CCB overdose. Typically, a 20% fat emulsion is used, with 1 mL/kg given as a bolus followed by a continuous infusion of 0.25 to 0.5 mL/kg per hour.[7]
Sodium Bicarbonate
Metabolic acidosis resulting from CCB overdose facilitates the binding of CCB to L‐type calcium channels; thus, correcting this acidemia might improve the hemodynamic profile. Sodium bicarbonate has been suggested as a useful adjunct because it decreases the affinity of the CCB for the calcium channel. In cases of severe toxicity, electrocardiogram (ECG) findings may show widening of the QRS complex; these ECG changes are mediated through the inhibitory action of CCB on fast sodium channels, similar to that observed in cases of overdose from tricyclic antidepressants.[9, 52]
Although the evidence is limited to a few case reports, treatment with 1 to 2 mEq/kg boluses of hypertonic sodium bicarbonate is recommended in cases in which QRS widening is noted on an ECG.[52] In cases of severe toxicity with severe acidosis, dysrhythmia, or persistent QRS widening, a sodium bicarbonate drip could be initiated, with 150 mEq of sodium bicarbonate in 1 L D5W to run at approximately 100 to 125 mL per hour.[52]
OTHER TREATMENT MODALITIES
Levosimendan has inotropic properties and is a calcium sensitizer to the myocardium. Although this drug has been used for CCB overdose,[53] it is not available in the United States. Temporary pacemakers and intra‐aortic balloon pump counter pulsation therapy are reserved for severe heart blocks and cases of refractory cardiogenic shock. The use of these 2 modalities is recommended only on a case‐by‐case basis. Wolf et al. demonstrated treatment success in a case of severe verapamil toxicity following the use of glucagon and amrinone.[54] However, there is the potential for hypotension, and this therapy is not routinely recommended. Considering that all CCBs are highly protein bound, with large volumes of distribution, extracorporeal measures such as hemodialysis and charcoal hemoperfusion have very limited roles in the management of an overdose.
CONCLUSION
There is no standardized approach for the management of patients with CCB overdose, and most of the existing evidence consists of case reports and case series. Calcium salts, glucagon, and vasopressors are common first‐line agents. In severe cases, HIET appears to be a promising treatment strategy, with several case reports reiterating its efficacy. However, euglycemia and a stable electrolyte panel should be maintained throughout the clinical course of management. Most of the benefits observed with HIET were noted in cases in which insulin therapy was initiated early in the course of management. ILE therapy, temporary pacemakers, and intra‐aortic balloon pump counter pulsation therapy are used on a case‐by‐case basis and best applied in consultation with a medical toxicologist or the regional poison control center.
Disclosure
Nothing to report.
- , , , , , . 2010 annual report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 28th annual report. Clin Toxicol (Phila). 2011;49(10):910–941.
- , , , , . 2011 annual report of the American Association Of Poison Control Centers' National Poison Data System (NPDS): 29th annual report. Clin Toxicol (Phila). 2012;50(10):911–1164.
- , , , , . 2012 annual report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 30th annual report. Clin Toxicol. 2013;51(10):949–1229.
- , , . Verapamil toxicity dysregulates the phosphatidylinositol 3‐kinase pathway. Acad Emerg Med. 2008;15(4):368–374.
- , , , . Adult toxicology in critical care: part II: specific poisonings. Chest. 2003;123(3):897–922.
- , , , . Insulin is a superior antidote for cardiovascular toxicity induced by verapamil in the anesthetized canine. J Pharmacol Exp Ther. 1993;267(2):744–750.
- , . Calcium channel blocker toxicity. Pediatr Emerg Care. 2009;25(8):532–538; quiz 539–540.
- , , . Severe intoxication after an intentional overdose of amlodipine. Acta Anaesthesiol Scand. 2003;47(8):1038–1040.
- . Management of beta‐adrenergic blocker and calcium channel antagonist toxicity. Emerg Med Clin North Am. 2007;25(2):309–331; abstract viii.
- , , , , . Effect of ca++ channel blockers on energy level and stimulated insulin secretion in isolated rat islets of Langerhans. J Pharmacol Exp Ther. 1993;264(1):35–40.
- , , , , . Insulin‐glucose as adjunctive therapy for severe calcium channel antagonist poisoning. J Toxicol Clin Toxicol. 1999;37(4):463–474.
- , . Binding of diltiazem and verapamil to isolated rat heart mitochondria. Basic Res Cardiol. 1987;82(3):246–251.
- , , , , . Effect of calcium channel antagonists on calcium uptake and release by isolated rat cardiac mitochondria. Eur J Pharmacol. 1988;152(3):247–253.
- , , , . The diabetogenic effects of acute verapamil poisoning. Toxicol Appl Pharmacol. 1997;145(2):357–362.
- , , , et al. Assessment of hyperglycemia after calcium channel blocker overdoses involving diltiazem or verapamil. Crit Care Med. 2007;35(9):2071–2075.
- , , . Activated charcoal alone and followed by whole‐bowel irrigation in preventing the absorption of sustained‐release drugs. Clin Pharmacol Ther. 2001;70(3):255–260.
- , , , . Slow‐release verapamil poisoning. use of polyethylene glycol whole‐bowel lavage and high‐dose calcium. Med J Aust. 1993;158(3):202–204.
- , , , . Whole bowel irrigation and the hemodynamically unstable calcium channel blocker overdose: primum non nocere. J Emerg Med. 2010;38(2):171–174.
- , , , . Critical care management of verapamil and diltiazem overdose with a focus on vasopressors: a 25‐year experience at a single center. Ann Emerg Med. 2013;62(3):252–258.
- , , . Beneficial myocardial metabolic effects of insulin during verapamil toxicity in the anesthetized canine. Crit Care Med. 1995;23(7):1251–1263.
- , , , , , . Reversal of the cardiovascular effects of verapamil by calcium and sodium: differences between electrophysiologic and hemodynamic responses. Circulation. 1979;59(4):797–804.
- , , , . A novel dosing regimen for calcium infusion in a patient of massive overdose of sustained‐release nifedipine. Am J Med Sci. 2013;345(3):248–251.
- , , , , . Calcium gluconate in severe verapamil intoxication. N Engl J Med. 1994;330(10):718–720.
- , , . Continuous calcium chloride infusion for massive nifedipine overdose. Chest. 2001;119(4):1280–1282.
- , . A fatal case of iatrogenic hypercalcemia after calcium channel blocker overdose. J Med Toxicol. 2008;4(1):25–29.
- , . Acute amlodipine overdose treated by high dose intravenous calcium in a patient with severe renal insufficiency. Clin Toxicol (Phila). 2007;45(3):301–303.
- . Glucagon in beta‐blocker and calcium channel blocker overdoses: a systematic review. J Toxicol Clin Toxicol. 2003;41(5):595–602.
- , . Utilization of a glucagon infusion in the management of a massive nifedipine overdose. J Emerg Med. 2000;18(4):453–455.
- , , , , . A potential role for glucagon in the treatment of drug‐induced symptomatic bradycardia. Chest. 1998;114(1):323–326.
- , , , . Diltiazem overdose: case report and review. J Emerg Med. 1991;9(5):357–366.
- , , , , , . Haemodynamic effects of high doses of insulin during acute left ventricular failure in dogs. Eur Heart J. 1985;6(5):451–457.
- , . The actions of insulin on cardiac contractility. Life Sci. 1981;29(10):975–1000.
- , , , , . Relative safety of hyperinsulinaemia/euglycaemia therapy in the management of calcium channel blocker overdose: a prospective observational study. Intensive Care Med. 2007;33(11):2019–2024.
- , , . Hyperinsulin therapy for calcium channel antagonist poisoning: a seven‐year retrospective study. Am J Ther. 2013;20(1):29–31.
- , , , , . Bench‐to‐bedside review: hyperinsulinaemia/euglycaemia therapy in the management of overdose of calcium‐channel blockers. Crit Care. 2006;10(3):212.
- , , , . High‐dose insulin therapy in beta‐blocker and calcium channel‐blocker poisoning. Clin Toxicol (Phila). 2011;49(4):277–283.
- , , , , . Improved cardiac function with glucose‐insulin‐potassium after aortocoronary bypass grafting. Ann Thorac Surg. 1989;48(4):484–489.
- , , , et al. Functional effects of glucose transporters in human ventricular myocardium. Eur J Heart Fail. 2010;12(2):106–113.
- , , , , , . Cellular mechanisms responsible for the inotropic action of insulin on failing human myocardium. J Heart Lung Transplant. 2006;25(9):1126–1134.
- , , , . Hyperinsulinemia euglycemia therapy for calcium channel blocker overdose: a case report. Tex Heart Inst J. 2012;39(4):575–578.
- , , , . Plasma phosphorus and magnesium values during treatment of severe diabetic ketoacidosis. Med Interne. 1981;19(1):63–68.
- , , . Dynamic changes in serum phosphorus levels in diabetic ketoacidosis. Am J Med. 1985;79(5):571–576.
- , , . Diltiazem poisoning treated with hyperinsulinemic euglycemia therapy and intravenous lipid emulsion. Eur J Emerg Med. 2011;18(2):121–123.
- , , , , . Hemodynamic effects of intravenous fat emulsion in an animal model of severe verapamil toxicity resuscitated with atropine, calcium, and saline. Acad Emerg Med. 2007;14(2):105–111.
- , , , , , . Lipid emulsions in the treatment of acute poisoning: a systematic review of human and animal studies. Clin Toxicol (Phila). 2010;48(1):1–27.
- , , , . Lipid emulsion infusion rescues dogs from bupivacaine‐induced cardiac toxicity. Reg Anesth Pain Med. 2003;28(3):198–202.
- , . Use of lipid emulsion to reverse local anesthetic‐induced toxicity. Ann Pharmacother. 2007;41(11):1873–1877.
- . Lipid resuscitation: more than a sink. Crit Care Med. 2012;40(8):2521–2523.
- , , , et al. Rapid cardiotonic effects of lipid emulsion infusion. Crit Care Med. 2013;41(8):e156–e162.
- , , , . Intralipid prolongs survival in a rat model of verapamil toxicity. Acad Emerg Med. 2006;13(2):134–139.
- . Lipid emulsion for the treatment of local anesthetic toxicity: patient safety implications. Anesth Analg. 2008;106(5):1337–1339.
- , . Poisoning by sodium channel blocking agents. Crit Care Clin. 1997;13(4):829–848.
- , , , . Levosimendan as treatment option in severe verapamil intoxication: a case report and review of the literature. Case Rep Med. 2010;2010. pii: 546904.
- , , . Use of amrinone and glucagon in a case of calcium channel blocker overdose. Ann Emerg Med. 1993;22(7):1225–1228.
The 2011 National Poison Data System (NPDS) of the American Association of Poison Control Centers reported that among the top 25 categories associated with mortality, cardiovascular medications were second to sedatives/hypnotics/antipsychotics in terms of the number of deaths resulting from overdose. Moreover, of cardiovascular medications, Calcium channel blockers (CCBs) were the most common agents associated with mortality.[1, 2] The 2012 NPDS report showed a similar trend, with cardiovascular drugs ranking among the top causes of overdoses, with an additional approximately 4614 cases in comparison to 2011.[3] In light of emerging strategies for the management of CCB overdoses, we sought to review the pathophysiology of CCB overdose and its management.
PATHOPHYSIOLOGY OF CCB OVERDOSE
CCBs are widely used in the management of various conditions such as hypertension, angina pectoris, atrial fibrillation, and other cardiac arrhythmias. CCBs block L‐type receptors on the cell surface.[4] Based on their predominant physiological effect, CCBs have been classified as dihydropyridines and nondihydropyridines (Table 1). Dihydropyridine overdose generally results in vasodilation with resultant hypotension and reflex tachycardia.[5] In comparison, nondihydropyridine overdose generally results in bradycardia and decreased cardiac contractility.[6] With high serum concentrations of either CCB class, however, selectivity is lost, and patients may presents with bradycardia, hypotension, and decreased cardiac contractility.[7, 8]
|
| Dihydropyridine |
| Short‐acting agents: nifedipine |
| Longer‐acting formulations: felodipine, isradipine, nicardipine, nifedipine, nisoldipine, amlodipinea |
| Nondihydropyridine |
| Verampamil and diltiazem |
CCBs show good oral bioavailability and undergo first‐pass metabolism. During an overdose, the enzymes involved in hepatic oxidation can become oversaturated, which reduces the effects of first‐pass metabolism, resulting in increased quantities of the active drug reaching the systemic circulation and a prolonged half‐life.[7] In addition, CCBs are highly protein bound and have large volumes of distribution.[9]
Calcium enters cells through specific channels and regulates various cell processes. In myocardial cells, calcium affects excitation‐contraction coupling and potential action generation in the sinoatrial node. Similarly, in the pancreas, calcium facilitates the release of insulin. CCB overdose can result in inhibition of insulin secretion from the pancreas and a state of hypoinsulinemia and insulin resistance.[10] Mtabolic acidosis is a common presentation noted in several published case reports.[11] Metabolic acidosis represents a combination of insulin dysregulation with ketoacidosis and hypoperfusion with lactic acidosis. In addition, because CCBs block the entry of calcium into the mitochondria,[12, 13] and because calcium is required for the normal enzymatic activity of the Krebs cycle, CCB overdose leads to lactic acid build‐up from its direct effects on aerobic metabolism.[14]
The clinical picture of CCB overdose is further complicated by the switch in the mechanism of adenosine triphosphate (ATP) generation in the myocardium from free fatty acid oxidation to carbohydrate metabolism.[15] In response to this stress, the liver increases glucose production via glycogenolysis. With concomitant hypoinsulinemia[10] and relative insulin resistance, intracellular glucose transport is disturbed, with a resultant decrease in ATP production that quickly leads to myocardial dysfunction and cardiogenic shock. The resultant clinical state of acidosis, hyperglycemia, and insulin deficiency is similar to diabetic ketoacidosis.[11, 14] A presentation of symptomatic bradycardia, hyperglycemia, and persistent hypotension, with signs of hypoperfusion usually manifested as altered mental status, clinically defines a severe overdose.
MANAGEMENT APPROACH
Maintenance of the airway and circulation is of primary importance in CCB overdose cases (Table 2). Hypotension and bradyarrhythmias are noted in cases of severe overdose, and some patients might require endotracheal intubation and mechanical ventilation very early in their management. The initial treatment strategy typically consists of the use of intravenous crystalloids and gastrointestinal (GI) decontamination; atropine is reserved for symptomatic bradycardia. Some patients may also require transcutaneous and transvenous pacing early and emergently due to complete cardiovascular collapse. Therefore, having a medical toxicologist or a regional poison control expert involved from the time of initial management is advised, especially for cases of severe overdose or consumption of extended‐release preparations.
|
| Initial resuscitation measures |
| Intravenous hydration with crystalloids, colloids. |
| Gastrointestinal decontamination |
| Activated charcoal 1 g/kg body weight in hemodynamically stable patients who can protect their airways.[1] Best administered within 2 hours. However, in poisoning from extended release preparations, it can be used beyond the 2‐hour window. Anecdotally, WBI has been utilized in calcium channel blocker overdose. However, it is not the recommended approach, especially in patients who are hemodynamically unstable. |
| Atropine |
| Reserved for bradycardia; 0.5 mg every 35 minutes, not to exceed a total of 3 mg or 0.04 mg/kg (per ACLS protocol). |
| Sodium bicarbonate |
| 12 mEq/kg boluses of hypertonic sodium bicarbonate when QRS widening is noted on the ECG.[46] For severe acidosis or persistent ECG changes, a sodium bicarbonate drip can be initiated with 150 mEq sodium bicarbonate in 1 L D5W to run at about 100125 mL per hour.[46] |
| Following intravenous hydration and GI decontamination (hyperinsulinemia‐euglycemia therapy) or vasopressors are usually initiated as resuscitation measures. |
| Agents used to reverse the calcium channel blocker poisoning |
| Hyperinsulinemia‐euglycemia therapy (refer to Table 33). |
| Glucagon |
| Initiated at 0.050.15 mg/kg as bolus dosing, with a repeat dosing in 35 minutes. An intravenous infusion can be initiated following this.[1] |
| Calcium salts |
| A bolus of 0.3 mEq/kg of calcium can be administered as intravenously over 510 minutes (0.6 mL/kg of 10% calcium gluconate solution or 0.2 mL/kg of 10% calcium chloride solution). |
| If beneficial response noted, an infusion of 0.3 mEq/kg per hour. |
| Titrate the infusion to obtain an adequate hemodynamic response. Serum ionized calcium levels should be monitored, and target ionized calcium levels should be less than twice the upper limit of normal.[2] |
| Adrenergic agents |
| Norepinephrine, dopamine, vasopressin. |
| Intravenous lipid emulsion therapy |
| 20% fat emulsion is what is usually used with 1 mL/kg given as a bolus followed by a continuous infusion of 0.250.5 mL/kg per hour. |
| Phosphodiesterase inhibitors |
| Amrinone, milrinone. |
| Invasive therapy |
| Transvenous and transcutaneous pacing for high‐grade atrioventricular dissociation. |
| Intra‐aortic balloon pump. |
| Extra corporeal membrane oxygenation. |
GI Decontamination
In cases of severe overdose, patients may present with lethargy from hypotension and poor cerebral flow, and the risk for aspiration and pneumonitis should be strongly considered in these patients if GI decontamination is considered. GI decontamination is best in cases where the patient is hemodynamically stable and presents early to the emergency department (ED), preferably within 2 hours[7, 9]; early use might decrease drug absorption and enterohepatic circulation, thus lowering the drug levels.[16] However, in cases in which the drug consumed was an extended‐release preparation, GI decontamination is beneficial even when the patient presents late to the ED.[17] GI decontamination is typically achieved using activated charcoal (1 g/kg body weight) or by performing whole bowel irrigation (WBI) with polyethylene glycol.[9] However, there is very little evidence that either approach changes the overall outcome, and WBI can be potentially harmful for patients with hemodynamic instability.[18] Therefore, airway and circulation maintenance is preferable to this approach.
Catecholamines
Catecholamines, such as dopamine, dobutamine, and norepinephrine, appear to be obvious choices in the management of cases of CCB overdose, because most patients present with hypotension and bradyarrhythmias.[19] However, there is no evidence to show the superiority of 1 agent over another in the management of CCB drug toxicity. Catecholamines increase the heart rate and blood pressure and increase systemic vascular resistance, which can potentially decrease the cardiac output by increasing the afterload.
Calcium Salts
In cases of severe overdose, the initial measures are typically not sufficient for stabilizing the patient. Intravenous (IV) calcium salts have been evaluated in animal models[20, 21] and, anecdotally, in human case reports.[22, 23, 24] However, the response to treatment has been mixed, with improvement in hemodynamic parameters in some cases and treatment failures in other cases. Moreover, the effects of these treatments are typically short lived, and repeated dosing might be required. Calcium salts are typically administered with the theoretical scheme of reversing antagonism with a higher calcium load and increasing cardiac inotropy. Calcium gluconate and calcium chloride are 2 frequently used agents, although no clear guidelines exist regarding this approach and the required dosage.[22] There are also published case reports in which refractory hypotension was treated with continuous calcium infusion in an attempt to reach predefined serum calcium levels.[24] However, the fear of iatrogenic hypercalcemia and its consequences is constant.[25] Calcium chloride contains 3 times the calcium for the identical volume compared to calcium gluconate and is more corrosive to the blood vessels; therefore, it is best administered through a central intravenous access. Although the evidence is limited to a few case reports, continuous calcium infusion appears effective and safe as an adjunctive therapy for patients with severe hypotension resulting from CCB overdose.[21, 22, 23, 24, 26]
Glucagon
Although insulin and glucagon are physiologically counter‐regulatory, they have a similar effect on heart stimulation. In animal models, the positive inotropic and chronotropic effects of glucagon have been clearly demonstrated.[27] Glucagon increases intracellular cyclic adenosine monophosphate (AMP) by stimulating adenylyl cyclase, a mechanism by which glucagon possibly exerts its inotropic effect.[7] Most studies conducted on the use of glucagon in the treatment of CCB overdose originated in an era in which bovine or porcine glucagon was used, and these animal glucagon products contained insulin.[9] Glucagon is typically initiated at 50 to 150 g/kg as bolus dosing, with a repeat dosing after 3 to 5 minutes.[9] A continuous IV infusion can then be administered following the initial treatment, because glucagon has a very short half‐life and works rapidly.[7, 9] However, there is no established maximum infusion dose of glucagon, and it should be titrated to the desired clinical outcome. IV glucagon therapy also carries a risk for nausea and vomiting,[7, 28] which in combination with lethargy may increase the risk for aspiration pneumonitis. The evidence for the use of glucagon in cases of CCB overdose is predominantly based on animal models[27]; evidence in human subjects is limited to case reports.[11, 28, 29] Some cases have demonstrated an improvement in hemodynamics with glucagon, whereas in a few cases, glucagon failed to result in such improvement.[30] In cases in which the ingestion history is unclear or there is polysubstance ingestion, as with ‐blockers and CCBs, glucagon is an ideal treatment agent[9]; in contrast, in single CCB overdose, glucagon might not be as helpful as more recent treatment modalities.
Hyperinsulinemia‐Euglycemia Therapy
In recent years, increasing evidence from multiple case reports and case series has shown the superiority of high‐dose insulin therapy over other treatment modalities (Table 3). Insulin acts as a potent inotrope[31, 32] and vasodilator. In their prospective observational series of 7 patients, Greene et al. report the successful use of hyperinsulinemia‐euglycemia therapy (HIET) with no significant adverse events when combined with conventional measures in a critical‐care setting.[33] Similarly, more than 50 cases have been reported in which HIET was used successfully in the management of CCB overdoses.[34]
| Bolus dosing |
| Check finger stick blood glucose, and 25 g dextrose can be given as a bolus, provided the patient is not markedly hyperglycemic[1] (eg, blood glucose >400 mg/dL). |
| 0.5 IU/kg of insulin given as bolus. An acceptable alternative would be to give 1 IU/kg as a bolus to saturate the receptors.[1, 3, 4] |
| Maintenance dose infusion |
| Short‐acting insulin initiated at 0.5 IU/kg per hour, and this dose can be titrated up to 2 IU/kg per hour. Doses as high as 10 IU/kg per hour have been tried and have been successful.[1, 4] |
| Continuous dextrose infusion might be required to maintain euglycemia (25 g per hour intravenous infusion would be a reasonable choice).[1] |
| Monitoring |
| Monitor blood glucose every 30 minutes for the first 4 hours and then hourly. Titrate dextrose infusion to maintain euglycemia.[1] |
| Dextrose containing fluid can be initiated at 0.51 g/kg per hour and titrated to maintain euglycemia.[10, 15] |
| Monitor potassium levels every 60 minutes and replace as needed to maintain at lower limits of normal (2.83.2 mEq/L). |
| Titration of the insulin infusion is usually to the resolution of hemodynamic parameters. |
| Discontinuation |
| No clear evidence to say if a weaning protocol is necessary. In several case reports, the protocol was discontinued after objective parameters of clinical resolution were achieved; however, continued dextrose infusion may be required despite the discontinuation of the insulin.[5] |
Although there is wide variation in the insulin dosing regimens in published case reports, hyperinsulinemia therapy is typically initiated with a 0.5 IU/kg to 1 IU/kg bolus, followed by a continuous drip of 0.5 UI/kg per hour to 1 IU/kg per hour. This dose is titrated every 15 to 20 minutes until satisfactory hemodynamic and clinical stability is noted. Titrations are usually avoided for a shorter time interval because insulin must enter cells and initiate intracellular signaling and metabolic activation. However, the response to HIET might be delayed, and other therapeutic modalities could be required simultaneously until the clinical effects of insulin are observed.
Euglycemia should be maintained by checking the blood glucose levels every 30 minutes and using a dextrose solution to maintain the blood glucose within the upper limits of normal.[35] Hyperglycemia noted in CCB overdose cases indicates the degree of insulin resistance and serves as a marker of the severity of the overdose.[14, 15] In particular, patients who are hyperglycemic at presentation may not require supplemental dextrose infusion despite the high‐dose insulin therapy. The blood glucose level should be checked every 30 minutes for the first 4 hours and then hourly to avoid overlooking hypoglycemia during the treatment regimen, especially in intubated and sedated patients. Fluids containing dextrose may be initiated at 0.5 to 1 g/kg per hour and titrated to maintain euglycemia.[9, 11]
However, there is no consensus as to how long the infusion should be continued once initiated. Although insulin has not been shown to induce tachyphylaxis in experimental animal models, many clinicians prefer to discontinue the infusion once hemodynamic stability has been achieved. There is also no evidence indicating whether a weaning protocol would make any difference over abrupt discontinuation.[36] The physiological effects of insulin persist for hours after the discontinuation of the infusion and will gradually taper down with time. Therefore, theoretically, an abrupt cessation should seldom cause any deleterious effects.[11] Dextrose supplementation may be required to maintain euglycemia for up to 24 hours following discontinuation of the insulin drip due to the elevated insulin levels.[11, 36]
Insulin is a potent vasodilator in the coronary and pulmonary vasculature but does not increase the requirement for myocardial oxygen. Instead, insulin facilitates endothelial nitric oxide activity through the phosphoinositide 3‐kinase (PI3K) pathway, which translates into vasodilatation of the capillary microvasculature and better perfusion at the capillary junction. As a result, insulin corrects the capillary dysfunction that is the major pathology in cardiogenic shock and the ultimate presentation in severe CCB overdose.
Gradinac et al. reported that patients with cardiogenic shock, in the postoperative period of coronary artery bypass grafting, showed a better cardiac index with the use of IV insulin therapy.[37] In an experiment on explanted human myocardium, von Lewinski et al. demonstrated the positive inotropic effect of insulin through calcium‐dependent pathways as well as PI3K pathways.[38] Moreover, Hsu et al. demonstrated with human myocardial cells that this inotropic property of insulin was dose dependent, with better responses observed after the use of higher doses of insulin; in addition, this effect was rapid (ie, as fast as 5 minutes after the infusion) and was sustained throughout the duration of insulin treatment.[39] The best clinical translation of this finding was demonstrated by Yuan et al.[11] in their case series of 5 patients with severe cardiogenic shock secondary to CCB overdoses.
There have also been cases of CCB overdoses in which insulin therapy has failed, which may be because the insulin protocol was initiated late as salvage therapy or because of the severity of the events.[35] Insulin therapy should be initiated early in the course of management rather than as salvage therapy.[7, 35] Agarwal et al. reported their experience in treating an patient on 3 separate occasions of CCB overdose. These authors reported rapid improvement on the third occasion, in which insulin therapy was initiated early during the course of management.[40] In recent years, HIET has been shown to be a promising approach in the management of CCB overdose. Patients with third‐degree heart blockage resulting from CCB overdose reverted to a normal sinus rhythm while on an insulin drip protocol without the intervention of a temporary pacemaker.[11]
High‐dose insulin therapy can also result in hypokalemia, which theoretically may represent a beneficial response in the management of CCB overdose, because it provides a membrane stabilizing effect by prolonging repolarization and allowing more calcium to enter the cytoplasm during cardiac systole.[11] Yuan et al. suggested a serum potassium range of 2.8 to 3.2 mEq/L during insulin‐glucose therapy.[11] Hypomagnesemia and hypophosphatemia are other electrolyte derangements reported during treatment that are similar to conditions observed in patients with diabetic ketoacidosis.[41, 42]
Intravenous Lipid Emulsion Therapy
CCBs are naturally lipophilic, and intravenous lipid emulsion (ILE) therapy has been attempted with success in cases of severe CCB overdose.[43, 44] A systematic review by Jamaty et al.[45] showed that, although the overall quality of the evidence for this modality was poor, ILE could be beneficial in the management of severe cases of CCB poisoning. ILE therapy was first described by Weinberg et al. for bupivacaine toxicity in the year 2003.[46] ILE is commonly utilized as part of total parenteral nutrition, and several case reports have shown the success of its use in the treatment of local anesthetic toxicity.[47] Although the mechanism remains to be clearly elucidated,[48] it is hypothesized that this emulsion in the circulation creates a lipid channel, which causes sequestration of lipophilic drugs, and stimulates the redistribution of lipophilic drugs from the tissues to this channel.[47] Recent data have further revealed the inotropic properties of lipid emulsion; when used for acute overdose, lipid emulsion improves ventricular contractility and diastolic relaxation, going beyond its role as a simple fuel for cardiac tissue or a lipid sink.[49] Lipid emulsion in the circulation also stimulates insulin secretion, which is beneficial in reversing the antagonism caused by CCB on the cells of the pancreas.[50] However, fat embolism, infection, and the development of acute respiratory distress syndrome have been reported as complications associated with this therapy.[51] Thus, it is prudent to involve a medical toxicologist or the regional poison center to decide whether a patient would be a candidate for this treatment approach. In most cases, this is reserved as a last resort in the management of CCB overdose. Typically, a 20% fat emulsion is used, with 1 mL/kg given as a bolus followed by a continuous infusion of 0.25 to 0.5 mL/kg per hour.[7]
Sodium Bicarbonate
Metabolic acidosis resulting from CCB overdose facilitates the binding of CCB to L‐type calcium channels; thus, correcting this acidemia might improve the hemodynamic profile. Sodium bicarbonate has been suggested as a useful adjunct because it decreases the affinity of the CCB for the calcium channel. In cases of severe toxicity, electrocardiogram (ECG) findings may show widening of the QRS complex; these ECG changes are mediated through the inhibitory action of CCB on fast sodium channels, similar to that observed in cases of overdose from tricyclic antidepressants.[9, 52]
Although the evidence is limited to a few case reports, treatment with 1 to 2 mEq/kg boluses of hypertonic sodium bicarbonate is recommended in cases in which QRS widening is noted on an ECG.[52] In cases of severe toxicity with severe acidosis, dysrhythmia, or persistent QRS widening, a sodium bicarbonate drip could be initiated, with 150 mEq of sodium bicarbonate in 1 L D5W to run at approximately 100 to 125 mL per hour.[52]
OTHER TREATMENT MODALITIES
Levosimendan has inotropic properties and is a calcium sensitizer to the myocardium. Although this drug has been used for CCB overdose,[53] it is not available in the United States. Temporary pacemakers and intra‐aortic balloon pump counter pulsation therapy are reserved for severe heart blocks and cases of refractory cardiogenic shock. The use of these 2 modalities is recommended only on a case‐by‐case basis. Wolf et al. demonstrated treatment success in a case of severe verapamil toxicity following the use of glucagon and amrinone.[54] However, there is the potential for hypotension, and this therapy is not routinely recommended. Considering that all CCBs are highly protein bound, with large volumes of distribution, extracorporeal measures such as hemodialysis and charcoal hemoperfusion have very limited roles in the management of an overdose.
CONCLUSION
There is no standardized approach for the management of patients with CCB overdose, and most of the existing evidence consists of case reports and case series. Calcium salts, glucagon, and vasopressors are common first‐line agents. In severe cases, HIET appears to be a promising treatment strategy, with several case reports reiterating its efficacy. However, euglycemia and a stable electrolyte panel should be maintained throughout the clinical course of management. Most of the benefits observed with HIET were noted in cases in which insulin therapy was initiated early in the course of management. ILE therapy, temporary pacemakers, and intra‐aortic balloon pump counter pulsation therapy are used on a case‐by‐case basis and best applied in consultation with a medical toxicologist or the regional poison control center.
Disclosure
Nothing to report.
The 2011 National Poison Data System (NPDS) of the American Association of Poison Control Centers reported that among the top 25 categories associated with mortality, cardiovascular medications were second to sedatives/hypnotics/antipsychotics in terms of the number of deaths resulting from overdose. Moreover, of cardiovascular medications, Calcium channel blockers (CCBs) were the most common agents associated with mortality.[1, 2] The 2012 NPDS report showed a similar trend, with cardiovascular drugs ranking among the top causes of overdoses, with an additional approximately 4614 cases in comparison to 2011.[3] In light of emerging strategies for the management of CCB overdoses, we sought to review the pathophysiology of CCB overdose and its management.
PATHOPHYSIOLOGY OF CCB OVERDOSE
CCBs are widely used in the management of various conditions such as hypertension, angina pectoris, atrial fibrillation, and other cardiac arrhythmias. CCBs block L‐type receptors on the cell surface.[4] Based on their predominant physiological effect, CCBs have been classified as dihydropyridines and nondihydropyridines (Table 1). Dihydropyridine overdose generally results in vasodilation with resultant hypotension and reflex tachycardia.[5] In comparison, nondihydropyridine overdose generally results in bradycardia and decreased cardiac contractility.[6] With high serum concentrations of either CCB class, however, selectivity is lost, and patients may presents with bradycardia, hypotension, and decreased cardiac contractility.[7, 8]
|
| Dihydropyridine |
| Short‐acting agents: nifedipine |
| Longer‐acting formulations: felodipine, isradipine, nicardipine, nifedipine, nisoldipine, amlodipinea |
| Nondihydropyridine |
| Verampamil and diltiazem |
CCBs show good oral bioavailability and undergo first‐pass metabolism. During an overdose, the enzymes involved in hepatic oxidation can become oversaturated, which reduces the effects of first‐pass metabolism, resulting in increased quantities of the active drug reaching the systemic circulation and a prolonged half‐life.[7] In addition, CCBs are highly protein bound and have large volumes of distribution.[9]
Calcium enters cells through specific channels and regulates various cell processes. In myocardial cells, calcium affects excitation‐contraction coupling and potential action generation in the sinoatrial node. Similarly, in the pancreas, calcium facilitates the release of insulin. CCB overdose can result in inhibition of insulin secretion from the pancreas and a state of hypoinsulinemia and insulin resistance.[10] Mtabolic acidosis is a common presentation noted in several published case reports.[11] Metabolic acidosis represents a combination of insulin dysregulation with ketoacidosis and hypoperfusion with lactic acidosis. In addition, because CCBs block the entry of calcium into the mitochondria,[12, 13] and because calcium is required for the normal enzymatic activity of the Krebs cycle, CCB overdose leads to lactic acid build‐up from its direct effects on aerobic metabolism.[14]
The clinical picture of CCB overdose is further complicated by the switch in the mechanism of adenosine triphosphate (ATP) generation in the myocardium from free fatty acid oxidation to carbohydrate metabolism.[15] In response to this stress, the liver increases glucose production via glycogenolysis. With concomitant hypoinsulinemia[10] and relative insulin resistance, intracellular glucose transport is disturbed, with a resultant decrease in ATP production that quickly leads to myocardial dysfunction and cardiogenic shock. The resultant clinical state of acidosis, hyperglycemia, and insulin deficiency is similar to diabetic ketoacidosis.[11, 14] A presentation of symptomatic bradycardia, hyperglycemia, and persistent hypotension, with signs of hypoperfusion usually manifested as altered mental status, clinically defines a severe overdose.
MANAGEMENT APPROACH
Maintenance of the airway and circulation is of primary importance in CCB overdose cases (Table 2). Hypotension and bradyarrhythmias are noted in cases of severe overdose, and some patients might require endotracheal intubation and mechanical ventilation very early in their management. The initial treatment strategy typically consists of the use of intravenous crystalloids and gastrointestinal (GI) decontamination; atropine is reserved for symptomatic bradycardia. Some patients may also require transcutaneous and transvenous pacing early and emergently due to complete cardiovascular collapse. Therefore, having a medical toxicologist or a regional poison control expert involved from the time of initial management is advised, especially for cases of severe overdose or consumption of extended‐release preparations.
|
| Initial resuscitation measures |
| Intravenous hydration with crystalloids, colloids. |
| Gastrointestinal decontamination |
| Activated charcoal 1 g/kg body weight in hemodynamically stable patients who can protect their airways.[1] Best administered within 2 hours. However, in poisoning from extended release preparations, it can be used beyond the 2‐hour window. Anecdotally, WBI has been utilized in calcium channel blocker overdose. However, it is not the recommended approach, especially in patients who are hemodynamically unstable. |
| Atropine |
| Reserved for bradycardia; 0.5 mg every 35 minutes, not to exceed a total of 3 mg or 0.04 mg/kg (per ACLS protocol). |
| Sodium bicarbonate |
| 12 mEq/kg boluses of hypertonic sodium bicarbonate when QRS widening is noted on the ECG.[46] For severe acidosis or persistent ECG changes, a sodium bicarbonate drip can be initiated with 150 mEq sodium bicarbonate in 1 L D5W to run at about 100125 mL per hour.[46] |
| Following intravenous hydration and GI decontamination (hyperinsulinemia‐euglycemia therapy) or vasopressors are usually initiated as resuscitation measures. |
| Agents used to reverse the calcium channel blocker poisoning |
| Hyperinsulinemia‐euglycemia therapy (refer to Table 33). |
| Glucagon |
| Initiated at 0.050.15 mg/kg as bolus dosing, with a repeat dosing in 35 minutes. An intravenous infusion can be initiated following this.[1] |
| Calcium salts |
| A bolus of 0.3 mEq/kg of calcium can be administered as intravenously over 510 minutes (0.6 mL/kg of 10% calcium gluconate solution or 0.2 mL/kg of 10% calcium chloride solution). |
| If beneficial response noted, an infusion of 0.3 mEq/kg per hour. |
| Titrate the infusion to obtain an adequate hemodynamic response. Serum ionized calcium levels should be monitored, and target ionized calcium levels should be less than twice the upper limit of normal.[2] |
| Adrenergic agents |
| Norepinephrine, dopamine, vasopressin. |
| Intravenous lipid emulsion therapy |
| 20% fat emulsion is what is usually used with 1 mL/kg given as a bolus followed by a continuous infusion of 0.250.5 mL/kg per hour. |
| Phosphodiesterase inhibitors |
| Amrinone, milrinone. |
| Invasive therapy |
| Transvenous and transcutaneous pacing for high‐grade atrioventricular dissociation. |
| Intra‐aortic balloon pump. |
| Extra corporeal membrane oxygenation. |
GI Decontamination
In cases of severe overdose, patients may present with lethargy from hypotension and poor cerebral flow, and the risk for aspiration and pneumonitis should be strongly considered in these patients if GI decontamination is considered. GI decontamination is best in cases where the patient is hemodynamically stable and presents early to the emergency department (ED), preferably within 2 hours[7, 9]; early use might decrease drug absorption and enterohepatic circulation, thus lowering the drug levels.[16] However, in cases in which the drug consumed was an extended‐release preparation, GI decontamination is beneficial even when the patient presents late to the ED.[17] GI decontamination is typically achieved using activated charcoal (1 g/kg body weight) or by performing whole bowel irrigation (WBI) with polyethylene glycol.[9] However, there is very little evidence that either approach changes the overall outcome, and WBI can be potentially harmful for patients with hemodynamic instability.[18] Therefore, airway and circulation maintenance is preferable to this approach.
Catecholamines
Catecholamines, such as dopamine, dobutamine, and norepinephrine, appear to be obvious choices in the management of cases of CCB overdose, because most patients present with hypotension and bradyarrhythmias.[19] However, there is no evidence to show the superiority of 1 agent over another in the management of CCB drug toxicity. Catecholamines increase the heart rate and blood pressure and increase systemic vascular resistance, which can potentially decrease the cardiac output by increasing the afterload.
Calcium Salts
In cases of severe overdose, the initial measures are typically not sufficient for stabilizing the patient. Intravenous (IV) calcium salts have been evaluated in animal models[20, 21] and, anecdotally, in human case reports.[22, 23, 24] However, the response to treatment has been mixed, with improvement in hemodynamic parameters in some cases and treatment failures in other cases. Moreover, the effects of these treatments are typically short lived, and repeated dosing might be required. Calcium salts are typically administered with the theoretical scheme of reversing antagonism with a higher calcium load and increasing cardiac inotropy. Calcium gluconate and calcium chloride are 2 frequently used agents, although no clear guidelines exist regarding this approach and the required dosage.[22] There are also published case reports in which refractory hypotension was treated with continuous calcium infusion in an attempt to reach predefined serum calcium levels.[24] However, the fear of iatrogenic hypercalcemia and its consequences is constant.[25] Calcium chloride contains 3 times the calcium for the identical volume compared to calcium gluconate and is more corrosive to the blood vessels; therefore, it is best administered through a central intravenous access. Although the evidence is limited to a few case reports, continuous calcium infusion appears effective and safe as an adjunctive therapy for patients with severe hypotension resulting from CCB overdose.[21, 22, 23, 24, 26]
Glucagon
Although insulin and glucagon are physiologically counter‐regulatory, they have a similar effect on heart stimulation. In animal models, the positive inotropic and chronotropic effects of glucagon have been clearly demonstrated.[27] Glucagon increases intracellular cyclic adenosine monophosphate (AMP) by stimulating adenylyl cyclase, a mechanism by which glucagon possibly exerts its inotropic effect.[7] Most studies conducted on the use of glucagon in the treatment of CCB overdose originated in an era in which bovine or porcine glucagon was used, and these animal glucagon products contained insulin.[9] Glucagon is typically initiated at 50 to 150 g/kg as bolus dosing, with a repeat dosing after 3 to 5 minutes.[9] A continuous IV infusion can then be administered following the initial treatment, because glucagon has a very short half‐life and works rapidly.[7, 9] However, there is no established maximum infusion dose of glucagon, and it should be titrated to the desired clinical outcome. IV glucagon therapy also carries a risk for nausea and vomiting,[7, 28] which in combination with lethargy may increase the risk for aspiration pneumonitis. The evidence for the use of glucagon in cases of CCB overdose is predominantly based on animal models[27]; evidence in human subjects is limited to case reports.[11, 28, 29] Some cases have demonstrated an improvement in hemodynamics with glucagon, whereas in a few cases, glucagon failed to result in such improvement.[30] In cases in which the ingestion history is unclear or there is polysubstance ingestion, as with ‐blockers and CCBs, glucagon is an ideal treatment agent[9]; in contrast, in single CCB overdose, glucagon might not be as helpful as more recent treatment modalities.
Hyperinsulinemia‐Euglycemia Therapy
In recent years, increasing evidence from multiple case reports and case series has shown the superiority of high‐dose insulin therapy over other treatment modalities (Table 3). Insulin acts as a potent inotrope[31, 32] and vasodilator. In their prospective observational series of 7 patients, Greene et al. report the successful use of hyperinsulinemia‐euglycemia therapy (HIET) with no significant adverse events when combined with conventional measures in a critical‐care setting.[33] Similarly, more than 50 cases have been reported in which HIET was used successfully in the management of CCB overdoses.[34]
| Bolus dosing |
| Check finger stick blood glucose, and 25 g dextrose can be given as a bolus, provided the patient is not markedly hyperglycemic[1] (eg, blood glucose >400 mg/dL). |
| 0.5 IU/kg of insulin given as bolus. An acceptable alternative would be to give 1 IU/kg as a bolus to saturate the receptors.[1, 3, 4] |
| Maintenance dose infusion |
| Short‐acting insulin initiated at 0.5 IU/kg per hour, and this dose can be titrated up to 2 IU/kg per hour. Doses as high as 10 IU/kg per hour have been tried and have been successful.[1, 4] |
| Continuous dextrose infusion might be required to maintain euglycemia (25 g per hour intravenous infusion would be a reasonable choice).[1] |
| Monitoring |
| Monitor blood glucose every 30 minutes for the first 4 hours and then hourly. Titrate dextrose infusion to maintain euglycemia.[1] |
| Dextrose containing fluid can be initiated at 0.51 g/kg per hour and titrated to maintain euglycemia.[10, 15] |
| Monitor potassium levels every 60 minutes and replace as needed to maintain at lower limits of normal (2.83.2 mEq/L). |
| Titration of the insulin infusion is usually to the resolution of hemodynamic parameters. |
| Discontinuation |
| No clear evidence to say if a weaning protocol is necessary. In several case reports, the protocol was discontinued after objective parameters of clinical resolution were achieved; however, continued dextrose infusion may be required despite the discontinuation of the insulin.[5] |
Although there is wide variation in the insulin dosing regimens in published case reports, hyperinsulinemia therapy is typically initiated with a 0.5 IU/kg to 1 IU/kg bolus, followed by a continuous drip of 0.5 UI/kg per hour to 1 IU/kg per hour. This dose is titrated every 15 to 20 minutes until satisfactory hemodynamic and clinical stability is noted. Titrations are usually avoided for a shorter time interval because insulin must enter cells and initiate intracellular signaling and metabolic activation. However, the response to HIET might be delayed, and other therapeutic modalities could be required simultaneously until the clinical effects of insulin are observed.
Euglycemia should be maintained by checking the blood glucose levels every 30 minutes and using a dextrose solution to maintain the blood glucose within the upper limits of normal.[35] Hyperglycemia noted in CCB overdose cases indicates the degree of insulin resistance and serves as a marker of the severity of the overdose.[14, 15] In particular, patients who are hyperglycemic at presentation may not require supplemental dextrose infusion despite the high‐dose insulin therapy. The blood glucose level should be checked every 30 minutes for the first 4 hours and then hourly to avoid overlooking hypoglycemia during the treatment regimen, especially in intubated and sedated patients. Fluids containing dextrose may be initiated at 0.5 to 1 g/kg per hour and titrated to maintain euglycemia.[9, 11]
However, there is no consensus as to how long the infusion should be continued once initiated. Although insulin has not been shown to induce tachyphylaxis in experimental animal models, many clinicians prefer to discontinue the infusion once hemodynamic stability has been achieved. There is also no evidence indicating whether a weaning protocol would make any difference over abrupt discontinuation.[36] The physiological effects of insulin persist for hours after the discontinuation of the infusion and will gradually taper down with time. Therefore, theoretically, an abrupt cessation should seldom cause any deleterious effects.[11] Dextrose supplementation may be required to maintain euglycemia for up to 24 hours following discontinuation of the insulin drip due to the elevated insulin levels.[11, 36]
Insulin is a potent vasodilator in the coronary and pulmonary vasculature but does not increase the requirement for myocardial oxygen. Instead, insulin facilitates endothelial nitric oxide activity through the phosphoinositide 3‐kinase (PI3K) pathway, which translates into vasodilatation of the capillary microvasculature and better perfusion at the capillary junction. As a result, insulin corrects the capillary dysfunction that is the major pathology in cardiogenic shock and the ultimate presentation in severe CCB overdose.
Gradinac et al. reported that patients with cardiogenic shock, in the postoperative period of coronary artery bypass grafting, showed a better cardiac index with the use of IV insulin therapy.[37] In an experiment on explanted human myocardium, von Lewinski et al. demonstrated the positive inotropic effect of insulin through calcium‐dependent pathways as well as PI3K pathways.[38] Moreover, Hsu et al. demonstrated with human myocardial cells that this inotropic property of insulin was dose dependent, with better responses observed after the use of higher doses of insulin; in addition, this effect was rapid (ie, as fast as 5 minutes after the infusion) and was sustained throughout the duration of insulin treatment.[39] The best clinical translation of this finding was demonstrated by Yuan et al.[11] in their case series of 5 patients with severe cardiogenic shock secondary to CCB overdoses.
There have also been cases of CCB overdoses in which insulin therapy has failed, which may be because the insulin protocol was initiated late as salvage therapy or because of the severity of the events.[35] Insulin therapy should be initiated early in the course of management rather than as salvage therapy.[7, 35] Agarwal et al. reported their experience in treating an patient on 3 separate occasions of CCB overdose. These authors reported rapid improvement on the third occasion, in which insulin therapy was initiated early during the course of management.[40] In recent years, HIET has been shown to be a promising approach in the management of CCB overdose. Patients with third‐degree heart blockage resulting from CCB overdose reverted to a normal sinus rhythm while on an insulin drip protocol without the intervention of a temporary pacemaker.[11]
High‐dose insulin therapy can also result in hypokalemia, which theoretically may represent a beneficial response in the management of CCB overdose, because it provides a membrane stabilizing effect by prolonging repolarization and allowing more calcium to enter the cytoplasm during cardiac systole.[11] Yuan et al. suggested a serum potassium range of 2.8 to 3.2 mEq/L during insulin‐glucose therapy.[11] Hypomagnesemia and hypophosphatemia are other electrolyte derangements reported during treatment that are similar to conditions observed in patients with diabetic ketoacidosis.[41, 42]
Intravenous Lipid Emulsion Therapy
CCBs are naturally lipophilic, and intravenous lipid emulsion (ILE) therapy has been attempted with success in cases of severe CCB overdose.[43, 44] A systematic review by Jamaty et al.[45] showed that, although the overall quality of the evidence for this modality was poor, ILE could be beneficial in the management of severe cases of CCB poisoning. ILE therapy was first described by Weinberg et al. for bupivacaine toxicity in the year 2003.[46] ILE is commonly utilized as part of total parenteral nutrition, and several case reports have shown the success of its use in the treatment of local anesthetic toxicity.[47] Although the mechanism remains to be clearly elucidated,[48] it is hypothesized that this emulsion in the circulation creates a lipid channel, which causes sequestration of lipophilic drugs, and stimulates the redistribution of lipophilic drugs from the tissues to this channel.[47] Recent data have further revealed the inotropic properties of lipid emulsion; when used for acute overdose, lipid emulsion improves ventricular contractility and diastolic relaxation, going beyond its role as a simple fuel for cardiac tissue or a lipid sink.[49] Lipid emulsion in the circulation also stimulates insulin secretion, which is beneficial in reversing the antagonism caused by CCB on the cells of the pancreas.[50] However, fat embolism, infection, and the development of acute respiratory distress syndrome have been reported as complications associated with this therapy.[51] Thus, it is prudent to involve a medical toxicologist or the regional poison center to decide whether a patient would be a candidate for this treatment approach. In most cases, this is reserved as a last resort in the management of CCB overdose. Typically, a 20% fat emulsion is used, with 1 mL/kg given as a bolus followed by a continuous infusion of 0.25 to 0.5 mL/kg per hour.[7]
Sodium Bicarbonate
Metabolic acidosis resulting from CCB overdose facilitates the binding of CCB to L‐type calcium channels; thus, correcting this acidemia might improve the hemodynamic profile. Sodium bicarbonate has been suggested as a useful adjunct because it decreases the affinity of the CCB for the calcium channel. In cases of severe toxicity, electrocardiogram (ECG) findings may show widening of the QRS complex; these ECG changes are mediated through the inhibitory action of CCB on fast sodium channels, similar to that observed in cases of overdose from tricyclic antidepressants.[9, 52]
Although the evidence is limited to a few case reports, treatment with 1 to 2 mEq/kg boluses of hypertonic sodium bicarbonate is recommended in cases in which QRS widening is noted on an ECG.[52] In cases of severe toxicity with severe acidosis, dysrhythmia, or persistent QRS widening, a sodium bicarbonate drip could be initiated, with 150 mEq of sodium bicarbonate in 1 L D5W to run at approximately 100 to 125 mL per hour.[52]
OTHER TREATMENT MODALITIES
Levosimendan has inotropic properties and is a calcium sensitizer to the myocardium. Although this drug has been used for CCB overdose,[53] it is not available in the United States. Temporary pacemakers and intra‐aortic balloon pump counter pulsation therapy are reserved for severe heart blocks and cases of refractory cardiogenic shock. The use of these 2 modalities is recommended only on a case‐by‐case basis. Wolf et al. demonstrated treatment success in a case of severe verapamil toxicity following the use of glucagon and amrinone.[54] However, there is the potential for hypotension, and this therapy is not routinely recommended. Considering that all CCBs are highly protein bound, with large volumes of distribution, extracorporeal measures such as hemodialysis and charcoal hemoperfusion have very limited roles in the management of an overdose.
CONCLUSION
There is no standardized approach for the management of patients with CCB overdose, and most of the existing evidence consists of case reports and case series. Calcium salts, glucagon, and vasopressors are common first‐line agents. In severe cases, HIET appears to be a promising treatment strategy, with several case reports reiterating its efficacy. However, euglycemia and a stable electrolyte panel should be maintained throughout the clinical course of management. Most of the benefits observed with HIET were noted in cases in which insulin therapy was initiated early in the course of management. ILE therapy, temporary pacemakers, and intra‐aortic balloon pump counter pulsation therapy are used on a case‐by‐case basis and best applied in consultation with a medical toxicologist or the regional poison control center.
Disclosure
Nothing to report.
- , , , , , . 2010 annual report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 28th annual report. Clin Toxicol (Phila). 2011;49(10):910–941.
- , , , , . 2011 annual report of the American Association Of Poison Control Centers' National Poison Data System (NPDS): 29th annual report. Clin Toxicol (Phila). 2012;50(10):911–1164.
- , , , , . 2012 annual report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 30th annual report. Clin Toxicol. 2013;51(10):949–1229.
- , , . Verapamil toxicity dysregulates the phosphatidylinositol 3‐kinase pathway. Acad Emerg Med. 2008;15(4):368–374.
- , , , . Adult toxicology in critical care: part II: specific poisonings. Chest. 2003;123(3):897–922.
- , , , . Insulin is a superior antidote for cardiovascular toxicity induced by verapamil in the anesthetized canine. J Pharmacol Exp Ther. 1993;267(2):744–750.
- , . Calcium channel blocker toxicity. Pediatr Emerg Care. 2009;25(8):532–538; quiz 539–540.
- , , . Severe intoxication after an intentional overdose of amlodipine. Acta Anaesthesiol Scand. 2003;47(8):1038–1040.
- . Management of beta‐adrenergic blocker and calcium channel antagonist toxicity. Emerg Med Clin North Am. 2007;25(2):309–331; abstract viii.
- , , , , . Effect of ca++ channel blockers on energy level and stimulated insulin secretion in isolated rat islets of Langerhans. J Pharmacol Exp Ther. 1993;264(1):35–40.
- , , , , . Insulin‐glucose as adjunctive therapy for severe calcium channel antagonist poisoning. J Toxicol Clin Toxicol. 1999;37(4):463–474.
- , . Binding of diltiazem and verapamil to isolated rat heart mitochondria. Basic Res Cardiol. 1987;82(3):246–251.
- , , , , . Effect of calcium channel antagonists on calcium uptake and release by isolated rat cardiac mitochondria. Eur J Pharmacol. 1988;152(3):247–253.
- , , , . The diabetogenic effects of acute verapamil poisoning. Toxicol Appl Pharmacol. 1997;145(2):357–362.
- , , , et al. Assessment of hyperglycemia after calcium channel blocker overdoses involving diltiazem or verapamil. Crit Care Med. 2007;35(9):2071–2075.
- , , . Activated charcoal alone and followed by whole‐bowel irrigation in preventing the absorption of sustained‐release drugs. Clin Pharmacol Ther. 2001;70(3):255–260.
- , , , . Slow‐release verapamil poisoning. use of polyethylene glycol whole‐bowel lavage and high‐dose calcium. Med J Aust. 1993;158(3):202–204.
- , , , . Whole bowel irrigation and the hemodynamically unstable calcium channel blocker overdose: primum non nocere. J Emerg Med. 2010;38(2):171–174.
- , , , . Critical care management of verapamil and diltiazem overdose with a focus on vasopressors: a 25‐year experience at a single center. Ann Emerg Med. 2013;62(3):252–258.
- , , . Beneficial myocardial metabolic effects of insulin during verapamil toxicity in the anesthetized canine. Crit Care Med. 1995;23(7):1251–1263.
- , , , , , . Reversal of the cardiovascular effects of verapamil by calcium and sodium: differences between electrophysiologic and hemodynamic responses. Circulation. 1979;59(4):797–804.
- , , , . A novel dosing regimen for calcium infusion in a patient of massive overdose of sustained‐release nifedipine. Am J Med Sci. 2013;345(3):248–251.
- , , , , . Calcium gluconate in severe verapamil intoxication. N Engl J Med. 1994;330(10):718–720.
- , , . Continuous calcium chloride infusion for massive nifedipine overdose. Chest. 2001;119(4):1280–1282.
- , . A fatal case of iatrogenic hypercalcemia after calcium channel blocker overdose. J Med Toxicol. 2008;4(1):25–29.
- , . Acute amlodipine overdose treated by high dose intravenous calcium in a patient with severe renal insufficiency. Clin Toxicol (Phila). 2007;45(3):301–303.
- . Glucagon in beta‐blocker and calcium channel blocker overdoses: a systematic review. J Toxicol Clin Toxicol. 2003;41(5):595–602.
- , . Utilization of a glucagon infusion in the management of a massive nifedipine overdose. J Emerg Med. 2000;18(4):453–455.
- , , , , . A potential role for glucagon in the treatment of drug‐induced symptomatic bradycardia. Chest. 1998;114(1):323–326.
- , , , . Diltiazem overdose: case report and review. J Emerg Med. 1991;9(5):357–366.
- , , , , , . Haemodynamic effects of high doses of insulin during acute left ventricular failure in dogs. Eur Heart J. 1985;6(5):451–457.
- , . The actions of insulin on cardiac contractility. Life Sci. 1981;29(10):975–1000.
- , , , , . Relative safety of hyperinsulinaemia/euglycaemia therapy in the management of calcium channel blocker overdose: a prospective observational study. Intensive Care Med. 2007;33(11):2019–2024.
- , , . Hyperinsulin therapy for calcium channel antagonist poisoning: a seven‐year retrospective study. Am J Ther. 2013;20(1):29–31.
- , , , , . Bench‐to‐bedside review: hyperinsulinaemia/euglycaemia therapy in the management of overdose of calcium‐channel blockers. Crit Care. 2006;10(3):212.
- , , , . High‐dose insulin therapy in beta‐blocker and calcium channel‐blocker poisoning. Clin Toxicol (Phila). 2011;49(4):277–283.
- , , , , . Improved cardiac function with glucose‐insulin‐potassium after aortocoronary bypass grafting. Ann Thorac Surg. 1989;48(4):484–489.
- , , , et al. Functional effects of glucose transporters in human ventricular myocardium. Eur J Heart Fail. 2010;12(2):106–113.
- , , , , , . Cellular mechanisms responsible for the inotropic action of insulin on failing human myocardium. J Heart Lung Transplant. 2006;25(9):1126–1134.
- , , , . Hyperinsulinemia euglycemia therapy for calcium channel blocker overdose: a case report. Tex Heart Inst J. 2012;39(4):575–578.
- , , , . Plasma phosphorus and magnesium values during treatment of severe diabetic ketoacidosis. Med Interne. 1981;19(1):63–68.
- , , . Dynamic changes in serum phosphorus levels in diabetic ketoacidosis. Am J Med. 1985;79(5):571–576.
- , , . Diltiazem poisoning treated with hyperinsulinemic euglycemia therapy and intravenous lipid emulsion. Eur J Emerg Med. 2011;18(2):121–123.
- , , , , . Hemodynamic effects of intravenous fat emulsion in an animal model of severe verapamil toxicity resuscitated with atropine, calcium, and saline. Acad Emerg Med. 2007;14(2):105–111.
- , , , , , . Lipid emulsions in the treatment of acute poisoning: a systematic review of human and animal studies. Clin Toxicol (Phila). 2010;48(1):1–27.
- , , , . Lipid emulsion infusion rescues dogs from bupivacaine‐induced cardiac toxicity. Reg Anesth Pain Med. 2003;28(3):198–202.
- , . Use of lipid emulsion to reverse local anesthetic‐induced toxicity. Ann Pharmacother. 2007;41(11):1873–1877.
- . Lipid resuscitation: more than a sink. Crit Care Med. 2012;40(8):2521–2523.
- , , , et al. Rapid cardiotonic effects of lipid emulsion infusion. Crit Care Med. 2013;41(8):e156–e162.
- , , , . Intralipid prolongs survival in a rat model of verapamil toxicity. Acad Emerg Med. 2006;13(2):134–139.
- . Lipid emulsion for the treatment of local anesthetic toxicity: patient safety implications. Anesth Analg. 2008;106(5):1337–1339.
- , . Poisoning by sodium channel blocking agents. Crit Care Clin. 1997;13(4):829–848.
- , , , . Levosimendan as treatment option in severe verapamil intoxication: a case report and review of the literature. Case Rep Med. 2010;2010. pii: 546904.
- , , . Use of amrinone and glucagon in a case of calcium channel blocker overdose. Ann Emerg Med. 1993;22(7):1225–1228.
- , , , , , . 2010 annual report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 28th annual report. Clin Toxicol (Phila). 2011;49(10):910–941.
- , , , , . 2011 annual report of the American Association Of Poison Control Centers' National Poison Data System (NPDS): 29th annual report. Clin Toxicol (Phila). 2012;50(10):911–1164.
- , , , , . 2012 annual report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 30th annual report. Clin Toxicol. 2013;51(10):949–1229.
- , , . Verapamil toxicity dysregulates the phosphatidylinositol 3‐kinase pathway. Acad Emerg Med. 2008;15(4):368–374.
- , , , . Adult toxicology in critical care: part II: specific poisonings. Chest. 2003;123(3):897–922.
- , , , . Insulin is a superior antidote for cardiovascular toxicity induced by verapamil in the anesthetized canine. J Pharmacol Exp Ther. 1993;267(2):744–750.
- , . Calcium channel blocker toxicity. Pediatr Emerg Care. 2009;25(8):532–538; quiz 539–540.
- , , . Severe intoxication after an intentional overdose of amlodipine. Acta Anaesthesiol Scand. 2003;47(8):1038–1040.
- . Management of beta‐adrenergic blocker and calcium channel antagonist toxicity. Emerg Med Clin North Am. 2007;25(2):309–331; abstract viii.
- , , , , . Effect of ca++ channel blockers on energy level and stimulated insulin secretion in isolated rat islets of Langerhans. J Pharmacol Exp Ther. 1993;264(1):35–40.
- , , , , . Insulin‐glucose as adjunctive therapy for severe calcium channel antagonist poisoning. J Toxicol Clin Toxicol. 1999;37(4):463–474.
- , . Binding of diltiazem and verapamil to isolated rat heart mitochondria. Basic Res Cardiol. 1987;82(3):246–251.
- , , , , . Effect of calcium channel antagonists on calcium uptake and release by isolated rat cardiac mitochondria. Eur J Pharmacol. 1988;152(3):247–253.
- , , , . The diabetogenic effects of acute verapamil poisoning. Toxicol Appl Pharmacol. 1997;145(2):357–362.
- , , , et al. Assessment of hyperglycemia after calcium channel blocker overdoses involving diltiazem or verapamil. Crit Care Med. 2007;35(9):2071–2075.
- , , . Activated charcoal alone and followed by whole‐bowel irrigation in preventing the absorption of sustained‐release drugs. Clin Pharmacol Ther. 2001;70(3):255–260.
- , , , . Slow‐release verapamil poisoning. use of polyethylene glycol whole‐bowel lavage and high‐dose calcium. Med J Aust. 1993;158(3):202–204.
- , , , . Whole bowel irrigation and the hemodynamically unstable calcium channel blocker overdose: primum non nocere. J Emerg Med. 2010;38(2):171–174.
- , , , . Critical care management of verapamil and diltiazem overdose with a focus on vasopressors: a 25‐year experience at a single center. Ann Emerg Med. 2013;62(3):252–258.
- , , . Beneficial myocardial metabolic effects of insulin during verapamil toxicity in the anesthetized canine. Crit Care Med. 1995;23(7):1251–1263.
- , , , , , . Reversal of the cardiovascular effects of verapamil by calcium and sodium: differences between electrophysiologic and hemodynamic responses. Circulation. 1979;59(4):797–804.
- , , , . A novel dosing regimen for calcium infusion in a patient of massive overdose of sustained‐release nifedipine. Am J Med Sci. 2013;345(3):248–251.
- , , , , . Calcium gluconate in severe verapamil intoxication. N Engl J Med. 1994;330(10):718–720.
- , , . Continuous calcium chloride infusion for massive nifedipine overdose. Chest. 2001;119(4):1280–1282.
- , . A fatal case of iatrogenic hypercalcemia after calcium channel blocker overdose. J Med Toxicol. 2008;4(1):25–29.
- , . Acute amlodipine overdose treated by high dose intravenous calcium in a patient with severe renal insufficiency. Clin Toxicol (Phila). 2007;45(3):301–303.
- . Glucagon in beta‐blocker and calcium channel blocker overdoses: a systematic review. J Toxicol Clin Toxicol. 2003;41(5):595–602.
- , . Utilization of a glucagon infusion in the management of a massive nifedipine overdose. J Emerg Med. 2000;18(4):453–455.
- , , , , . A potential role for glucagon in the treatment of drug‐induced symptomatic bradycardia. Chest. 1998;114(1):323–326.
- , , , . Diltiazem overdose: case report and review. J Emerg Med. 1991;9(5):357–366.
- , , , , , . Haemodynamic effects of high doses of insulin during acute left ventricular failure in dogs. Eur Heart J. 1985;6(5):451–457.
- , . The actions of insulin on cardiac contractility. Life Sci. 1981;29(10):975–1000.
- , , , , . Relative safety of hyperinsulinaemia/euglycaemia therapy in the management of calcium channel blocker overdose: a prospective observational study. Intensive Care Med. 2007;33(11):2019–2024.
- , , . Hyperinsulin therapy for calcium channel antagonist poisoning: a seven‐year retrospective study. Am J Ther. 2013;20(1):29–31.
- , , , , . Bench‐to‐bedside review: hyperinsulinaemia/euglycaemia therapy in the management of overdose of calcium‐channel blockers. Crit Care. 2006;10(3):212.
- , , , . High‐dose insulin therapy in beta‐blocker and calcium channel‐blocker poisoning. Clin Toxicol (Phila). 2011;49(4):277–283.
- , , , , . Improved cardiac function with glucose‐insulin‐potassium after aortocoronary bypass grafting. Ann Thorac Surg. 1989;48(4):484–489.
- , , , et al. Functional effects of glucose transporters in human ventricular myocardium. Eur J Heart Fail. 2010;12(2):106–113.
- , , , , , . Cellular mechanisms responsible for the inotropic action of insulin on failing human myocardium. J Heart Lung Transplant. 2006;25(9):1126–1134.
- , , , . Hyperinsulinemia euglycemia therapy for calcium channel blocker overdose: a case report. Tex Heart Inst J. 2012;39(4):575–578.
- , , , . Plasma phosphorus and magnesium values during treatment of severe diabetic ketoacidosis. Med Interne. 1981;19(1):63–68.
- , , . Dynamic changes in serum phosphorus levels in diabetic ketoacidosis. Am J Med. 1985;79(5):571–576.
- , , . Diltiazem poisoning treated with hyperinsulinemic euglycemia therapy and intravenous lipid emulsion. Eur J Emerg Med. 2011;18(2):121–123.
- , , , , . Hemodynamic effects of intravenous fat emulsion in an animal model of severe verapamil toxicity resuscitated with atropine, calcium, and saline. Acad Emerg Med. 2007;14(2):105–111.
- , , , , , . Lipid emulsions in the treatment of acute poisoning: a systematic review of human and animal studies. Clin Toxicol (Phila). 2010;48(1):1–27.
- , , , . Lipid emulsion infusion rescues dogs from bupivacaine‐induced cardiac toxicity. Reg Anesth Pain Med. 2003;28(3):198–202.
- , . Use of lipid emulsion to reverse local anesthetic‐induced toxicity. Ann Pharmacother. 2007;41(11):1873–1877.
- . Lipid resuscitation: more than a sink. Crit Care Med. 2012;40(8):2521–2523.
- , , , et al. Rapid cardiotonic effects of lipid emulsion infusion. Crit Care Med. 2013;41(8):e156–e162.
- , , , . Intralipid prolongs survival in a rat model of verapamil toxicity. Acad Emerg Med. 2006;13(2):134–139.
- . Lipid emulsion for the treatment of local anesthetic toxicity: patient safety implications. Anesth Analg. 2008;106(5):1337–1339.
- , . Poisoning by sodium channel blocking agents. Crit Care Clin. 1997;13(4):829–848.
- , , , . Levosimendan as treatment option in severe verapamil intoxication: a case report and review of the literature. Case Rep Med. 2010;2010. pii: 546904.
- , , . Use of amrinone and glucagon in a case of calcium channel blocker overdose. Ann Emerg Med. 1993;22(7):1225–1228.
CHMP recommends ibrutinib for CLL, MCL

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) is recommending marketing authorization for ibrutinib (Imbruvica).
The committee is endorsing the Bruton’s tyrosine kinase (BTK) inhibitor for use in adults with relapsed or refractory mantle cell lymphoma (MCL) and certain adults with chronic lymphocytic leukemia (CLL).
This includes untreated CLL patients with 17p deletion or TP53 mutation who cannot receive chemo-immunotherapy and patients who have received at least 1 prior therapy.
The European Commission will take the CHMP’s opinion into account when deciding whether to authorize the commercialization of ibrutinib in the European Union.
The CHMP based its recommendations on data from 2 CLL studies—the phase 3 RESONATE trial (PCYC-1112) and a phase 1b/2 trial (PCYC-1102)—as well as a phase 2 trial (PCYC-1104) in MCL.
RESONATE trial
Results of RESONATE were recently presented at the 2014 EHA Congress. The trial included 391 patients with relapsed or refractory CLL or small lymphocytic lymphoma (SLL).
Patients were randomized to receive ibrutinib (n=195) or ofatumumab (n=196). Patients in the ofatumumab arm were allowed to cross over to ibrutinib if they progressed (n=57). The median time on study was 9.4 months.
The best overall response rate was higher in the ibrutinib arm than the ofatumumab arm, at 78% and 11%, respectively. And ibrutinib significantly prolonged progression-free survival. The median was 8.1 months in the ofatumumab arm and was not reached in the ibrutinib arm (P<0.0001).
Ibrutinib significantly prolonged overall survival as well. The median overall survival was not reached in either arm, but the hazard ratio was 0.434 (P=0.0049).
Adverse events occurred in 99% of patients in the ibrutinib arm and 98% of those in the ofatumumab arm. Grade 3/4 events occurred in 51% and 39%, respectively.
Atrial fibrillation, bleeding-related events, diarrhea, and arthralgia were more common in the ibrutinib arm. Infusion-related reactions, peripheral sensory neuropathy, urticaria, night sweats, and pruritus were more common in the ofatumumab arm.
PCYC-1102: Ibrutinib in CLL/SLL
Results of this phase 1b/2 trial were published in The Lancet Oncology in January. The trial enrolled 29 patients with previously untreated CLL and 2 with SLL.
They received 28-day cycles of once-daily ibrutinib at 420 mg or 840 mg. The 840 mg dose was discontinued after enrollment had begun because the doses showed comparable activity.
After a median follow-up of 22.1 months, 71% of patients achieved an objective response. Four patients (13%) had a complete response. The median time to response was 1.9 months.
Study investigators did not establish whether ibrutinib confers improvements in survival or disease-related symptoms.
Common adverse events included diarrhea (68%), nausea (48%), fatigue (32%), peripheral edema (29%), hypertension (29%), dizziness (26%), dyspepsia (26%), upper respiratory tract infection (26%), arthralgia (23%), constipation (23%), urinary tract infection (23%), and vomiting (23%).
Grade 3 adverse events included diarrhea (13%), fatigue (3%), hypertension (6%), dizziness (3%), urinary tract infection (3%), headache (3%), back pain (3%), and neutropenia (3%). One patient (3%) had grade 4 thrombocytopenia.
PCYC-1104 trial: Ibrutinib in MCL
Results of this trial were presented at ASH 2012 and published in NEJM in 2013. The NEJM data included 111 patients who received ibrutinib at 560 mg daily in continuous, 28-day cycles until disease progression.
The overall response rate was 68%, with a complete response rate of 21% and a partial response rate of 47%. With an estimated median follow-up of 15.3 months, the estimated median response duration was 17.5 months.
The estimated progression-free survival was 13.9 months, and the overall survival was not reached. The estimated rate of overall survival was 58% at 18 months.
Common nonhematologic adverse events included diarrhea (50%), fatigue (41%), nausea (31%), peripheral edema (28%), dyspnea (27%), constipation (25%), upper respiratory tract infection (23%), vomiting (23%), and decreased appetite (21%). The most common grade 3, 4, or 5 infection was pneumonia (6%).
Grade 3 and 4 hematologic adverse events included neutropenia (16%), thrombocytopenia (11%), and anemia (10%). Grade 3 bleeding events occurred in 5 patients.
About ibrutinib
Ibrutinib works by inhibiting BTK, a protein involved in mediating the cellular signaling pathways that control B-cell maturation and survival. In malignant B cells, there is excessive signaling through the B-cell receptor signaling pathway, which includes BTK.
Ibrutinib forms a strong covalent bond with BTK, which inhibits the excessive transmission of cell survival signals within the malignant B cells and stops their excessive build-up in protected environmental areas such as the lymph nodes.
Ibrutinib is being studied alone and in combination with other treatments in several hematologic malignancies, including CLL, MCL, Waldenstrom’s macroglobulinemia, diffuse large B-cell lymphoma, follicular lymphoma, and multiple myeloma.
Ibrutinib received accelerated approval from the US Food and Drug Administration in November 2013 to treat MCL. The drug received accelerated approval in February 2014 to treat CLL patients who have received at least 1 prior therapy.
Ibrutinib is also approved in Israel for the treatment of adults with MCL who have received at least 1 prior therapy.
Ibrutinib is under development by Janssen and Pharmacyclics. The companies co-market ibrutinib in the US, but, pending the drug’s approval, Janssen will market ibrutinib in the rest of the world. ![]()

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) is recommending marketing authorization for ibrutinib (Imbruvica).
The committee is endorsing the Bruton’s tyrosine kinase (BTK) inhibitor for use in adults with relapsed or refractory mantle cell lymphoma (MCL) and certain adults with chronic lymphocytic leukemia (CLL).
This includes untreated CLL patients with 17p deletion or TP53 mutation who cannot receive chemo-immunotherapy and patients who have received at least 1 prior therapy.
The European Commission will take the CHMP’s opinion into account when deciding whether to authorize the commercialization of ibrutinib in the European Union.
The CHMP based its recommendations on data from 2 CLL studies—the phase 3 RESONATE trial (PCYC-1112) and a phase 1b/2 trial (PCYC-1102)—as well as a phase 2 trial (PCYC-1104) in MCL.
RESONATE trial
Results of RESONATE were recently presented at the 2014 EHA Congress. The trial included 391 patients with relapsed or refractory CLL or small lymphocytic lymphoma (SLL).
Patients were randomized to receive ibrutinib (n=195) or ofatumumab (n=196). Patients in the ofatumumab arm were allowed to cross over to ibrutinib if they progressed (n=57). The median time on study was 9.4 months.
The best overall response rate was higher in the ibrutinib arm than the ofatumumab arm, at 78% and 11%, respectively. And ibrutinib significantly prolonged progression-free survival. The median was 8.1 months in the ofatumumab arm and was not reached in the ibrutinib arm (P<0.0001).
Ibrutinib significantly prolonged overall survival as well. The median overall survival was not reached in either arm, but the hazard ratio was 0.434 (P=0.0049).
Adverse events occurred in 99% of patients in the ibrutinib arm and 98% of those in the ofatumumab arm. Grade 3/4 events occurred in 51% and 39%, respectively.
Atrial fibrillation, bleeding-related events, diarrhea, and arthralgia were more common in the ibrutinib arm. Infusion-related reactions, peripheral sensory neuropathy, urticaria, night sweats, and pruritus were more common in the ofatumumab arm.
PCYC-1102: Ibrutinib in CLL/SLL
Results of this phase 1b/2 trial were published in The Lancet Oncology in January. The trial enrolled 29 patients with previously untreated CLL and 2 with SLL.
They received 28-day cycles of once-daily ibrutinib at 420 mg or 840 mg. The 840 mg dose was discontinued after enrollment had begun because the doses showed comparable activity.
After a median follow-up of 22.1 months, 71% of patients achieved an objective response. Four patients (13%) had a complete response. The median time to response was 1.9 months.
Study investigators did not establish whether ibrutinib confers improvements in survival or disease-related symptoms.
Common adverse events included diarrhea (68%), nausea (48%), fatigue (32%), peripheral edema (29%), hypertension (29%), dizziness (26%), dyspepsia (26%), upper respiratory tract infection (26%), arthralgia (23%), constipation (23%), urinary tract infection (23%), and vomiting (23%).
Grade 3 adverse events included diarrhea (13%), fatigue (3%), hypertension (6%), dizziness (3%), urinary tract infection (3%), headache (3%), back pain (3%), and neutropenia (3%). One patient (3%) had grade 4 thrombocytopenia.
PCYC-1104 trial: Ibrutinib in MCL
Results of this trial were presented at ASH 2012 and published in NEJM in 2013. The NEJM data included 111 patients who received ibrutinib at 560 mg daily in continuous, 28-day cycles until disease progression.
The overall response rate was 68%, with a complete response rate of 21% and a partial response rate of 47%. With an estimated median follow-up of 15.3 months, the estimated median response duration was 17.5 months.
The estimated progression-free survival was 13.9 months, and the overall survival was not reached. The estimated rate of overall survival was 58% at 18 months.
Common nonhematologic adverse events included diarrhea (50%), fatigue (41%), nausea (31%), peripheral edema (28%), dyspnea (27%), constipation (25%), upper respiratory tract infection (23%), vomiting (23%), and decreased appetite (21%). The most common grade 3, 4, or 5 infection was pneumonia (6%).
Grade 3 and 4 hematologic adverse events included neutropenia (16%), thrombocytopenia (11%), and anemia (10%). Grade 3 bleeding events occurred in 5 patients.
About ibrutinib
Ibrutinib works by inhibiting BTK, a protein involved in mediating the cellular signaling pathways that control B-cell maturation and survival. In malignant B cells, there is excessive signaling through the B-cell receptor signaling pathway, which includes BTK.
Ibrutinib forms a strong covalent bond with BTK, which inhibits the excessive transmission of cell survival signals within the malignant B cells and stops their excessive build-up in protected environmental areas such as the lymph nodes.
Ibrutinib is being studied alone and in combination with other treatments in several hematologic malignancies, including CLL, MCL, Waldenstrom’s macroglobulinemia, diffuse large B-cell lymphoma, follicular lymphoma, and multiple myeloma.
Ibrutinib received accelerated approval from the US Food and Drug Administration in November 2013 to treat MCL. The drug received accelerated approval in February 2014 to treat CLL patients who have received at least 1 prior therapy.
Ibrutinib is also approved in Israel for the treatment of adults with MCL who have received at least 1 prior therapy.
Ibrutinib is under development by Janssen and Pharmacyclics. The companies co-market ibrutinib in the US, but, pending the drug’s approval, Janssen will market ibrutinib in the rest of the world. ![]()

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) is recommending marketing authorization for ibrutinib (Imbruvica).
The committee is endorsing the Bruton’s tyrosine kinase (BTK) inhibitor for use in adults with relapsed or refractory mantle cell lymphoma (MCL) and certain adults with chronic lymphocytic leukemia (CLL).
This includes untreated CLL patients with 17p deletion or TP53 mutation who cannot receive chemo-immunotherapy and patients who have received at least 1 prior therapy.
The European Commission will take the CHMP’s opinion into account when deciding whether to authorize the commercialization of ibrutinib in the European Union.
The CHMP based its recommendations on data from 2 CLL studies—the phase 3 RESONATE trial (PCYC-1112) and a phase 1b/2 trial (PCYC-1102)—as well as a phase 2 trial (PCYC-1104) in MCL.
RESONATE trial
Results of RESONATE were recently presented at the 2014 EHA Congress. The trial included 391 patients with relapsed or refractory CLL or small lymphocytic lymphoma (SLL).
Patients were randomized to receive ibrutinib (n=195) or ofatumumab (n=196). Patients in the ofatumumab arm were allowed to cross over to ibrutinib if they progressed (n=57). The median time on study was 9.4 months.
The best overall response rate was higher in the ibrutinib arm than the ofatumumab arm, at 78% and 11%, respectively. And ibrutinib significantly prolonged progression-free survival. The median was 8.1 months in the ofatumumab arm and was not reached in the ibrutinib arm (P<0.0001).
Ibrutinib significantly prolonged overall survival as well. The median overall survival was not reached in either arm, but the hazard ratio was 0.434 (P=0.0049).
Adverse events occurred in 99% of patients in the ibrutinib arm and 98% of those in the ofatumumab arm. Grade 3/4 events occurred in 51% and 39%, respectively.
Atrial fibrillation, bleeding-related events, diarrhea, and arthralgia were more common in the ibrutinib arm. Infusion-related reactions, peripheral sensory neuropathy, urticaria, night sweats, and pruritus were more common in the ofatumumab arm.
PCYC-1102: Ibrutinib in CLL/SLL
Results of this phase 1b/2 trial were published in The Lancet Oncology in January. The trial enrolled 29 patients with previously untreated CLL and 2 with SLL.
They received 28-day cycles of once-daily ibrutinib at 420 mg or 840 mg. The 840 mg dose was discontinued after enrollment had begun because the doses showed comparable activity.
After a median follow-up of 22.1 months, 71% of patients achieved an objective response. Four patients (13%) had a complete response. The median time to response was 1.9 months.
Study investigators did not establish whether ibrutinib confers improvements in survival or disease-related symptoms.
Common adverse events included diarrhea (68%), nausea (48%), fatigue (32%), peripheral edema (29%), hypertension (29%), dizziness (26%), dyspepsia (26%), upper respiratory tract infection (26%), arthralgia (23%), constipation (23%), urinary tract infection (23%), and vomiting (23%).
Grade 3 adverse events included diarrhea (13%), fatigue (3%), hypertension (6%), dizziness (3%), urinary tract infection (3%), headache (3%), back pain (3%), and neutropenia (3%). One patient (3%) had grade 4 thrombocytopenia.
PCYC-1104 trial: Ibrutinib in MCL
Results of this trial were presented at ASH 2012 and published in NEJM in 2013. The NEJM data included 111 patients who received ibrutinib at 560 mg daily in continuous, 28-day cycles until disease progression.
The overall response rate was 68%, with a complete response rate of 21% and a partial response rate of 47%. With an estimated median follow-up of 15.3 months, the estimated median response duration was 17.5 months.
The estimated progression-free survival was 13.9 months, and the overall survival was not reached. The estimated rate of overall survival was 58% at 18 months.
Common nonhematologic adverse events included diarrhea (50%), fatigue (41%), nausea (31%), peripheral edema (28%), dyspnea (27%), constipation (25%), upper respiratory tract infection (23%), vomiting (23%), and decreased appetite (21%). The most common grade 3, 4, or 5 infection was pneumonia (6%).
Grade 3 and 4 hematologic adverse events included neutropenia (16%), thrombocytopenia (11%), and anemia (10%). Grade 3 bleeding events occurred in 5 patients.
About ibrutinib
Ibrutinib works by inhibiting BTK, a protein involved in mediating the cellular signaling pathways that control B-cell maturation and survival. In malignant B cells, there is excessive signaling through the B-cell receptor signaling pathway, which includes BTK.
Ibrutinib forms a strong covalent bond with BTK, which inhibits the excessive transmission of cell survival signals within the malignant B cells and stops their excessive build-up in protected environmental areas such as the lymph nodes.
Ibrutinib is being studied alone and in combination with other treatments in several hematologic malignancies, including CLL, MCL, Waldenstrom’s macroglobulinemia, diffuse large B-cell lymphoma, follicular lymphoma, and multiple myeloma.
Ibrutinib received accelerated approval from the US Food and Drug Administration in November 2013 to treat MCL. The drug received accelerated approval in February 2014 to treat CLL patients who have received at least 1 prior therapy.
Ibrutinib is also approved in Israel for the treatment of adults with MCL who have received at least 1 prior therapy.
Ibrutinib is under development by Janssen and Pharmacyclics. The companies co-market ibrutinib in the US, but, pending the drug’s approval, Janssen will market ibrutinib in the rest of the world. ![]()
A Quality Improvement Initiative to Improve Emergency Department Care for Pediatric Patients with Sickle Cell Disease
From the Children’s Hospital & Research Center Oakland, Oakland, CA.
Abstract
- Objective: To determine whether a quality improvement (QI) initiative would result in more timely assessment and treatment of acute sickle cell–related pain for pediatric patients with sickle cell disease (SCD) treated in the emergency department (ED).
- Methods: We created and implemented a protocol for SCD pain management in the ED with the goals of improving (1) mean time from triage to first analgesic dose; (2) percentage of patients that received their first analgesic dose within 30 minutes of triage, and (3) percentage of patients who had pain assessment performed within 30 minutes of triage and who were re-assessed within 30 minutes after the first analgesic dose.
- Results: Significant improvements were achieved between baseline (55 patient visits) and post order set implementation (165 visits) in time from triage to administration of first analgesic (decreased from 89.9 ± 50.5 to 35.2 ± 22.8 minutes, P < 0.001); percentage of patient visits receiving pain medications within 30 minutes of triage (from 7% to 53%, P < 0.001); percentage of patient visits assessed within 30 minutes of triage (from 64% to 99.4%, P < 0.001); and percentage of patient visits re-assessed within 30 minutes of initial analgesic (from 54% to 86%, P < 0.001).
- Conclusions: Implementation of a QI initiative in the ED led to expeditious care for pediatric patients with SCD presenting with pain. A QI framework provided us with unique challenges but also invaluable lessons as we address our objective of decreasing the quality gap in SCD medical care.
Pain is the leading cause of emergency department (ED) visits for patients with sickle cell disease (SCD) [1]. In the United States, 78% of the nearly 200,000 annual ED visits for SCD are for a complaint of pain [1]. Guidelines for the management of sickle cell vaso-occlusive pain episodes (VOE) suggest prompt initiation of parenteral opioids, use of effective opioid doses, and repeat opioid doses at frequent intervals [2–4]. Adherence to guidelines is poor. Both pediatric and adult patients with SCD experience delays in the initiation of analgesics and are routinely undertreated with respect to opioid dosing [5–8]. Even after controlling for race, the delays in time to analgesic administration experienced by patients with SCD exceed the delays encountered by patients who present to the ED with other types of pain [5,9]. These disparities warrant efforts designed to improve the delivery of quality care to patients with SCD.
Barriers to rapid and appropriate care of VOE in the ED are multifactorial and include systems-based limitations, such as acuity of the ED census, staffing limitations (eg, nurse-to-patient ratios), and facility limitations (eg, room availability) [6]. Provider-based limitations may include lack of awareness of available guidelines [10]. Biases and misunderstandings amongst providers about sickle cell pain and adequate medication dosing may also play a role [11–13]. These provider biases often lead to undertreatment of the pain, which in turn can lead to pseudoaddiction (drug-seeking behavior due to inadequate treatment) and a cycle of increased ED and inpatient utilization [14,15].
Patient-specific barriers to effective ED management of pain are equally complex. Previous negative experiences in the ED can lead patients and families to delay seeking care or avoid the ED altogether despite severe VOE pain [16]. Patients report frustration with the lack of consideration that they receive for their reports of pain, perceived insensitivity of hospital staff, inadequate analgesic administration, staff preoccupation with concerns of drug addiction, and an overall lack of respect and trust [17–19]. Patients also perceive a lack of knowledge of SCD and its treatments on the part of ED staff [7]. Other barriers to effective management are technical in nature, such as difficulty in establishing timely intravenous (IV) access.
Gaps and variations in quality of care contribute to poor outcomes for patients with SCD [20,21]. To help address these inequities, the Working to Improve Sickle Cell Healthcare (WISCH) project began in 2010 to improve care and outcomes for patients with SCD. WISCH is a collaborative quality improvement (QI) project funded by the Health Resources and Services Administration (HRSA) that has the goal to use improvement science to improve outcomes for patients with SCD across the life course (Ed note: see Editorial by Oyeku et al in this issue). As one of the HRSA-WISCH grantee networks, we undertook a QI project designed to decrease the quality gap in SCD medical care by creating and implementing a protocol for ED pain management for pediatric patients. Goals of the project were to improve the timely and appropriate assessment and treatment of acute VOE in the ED.
Methods
Setting
This ED QI initiative was implemented at Children’s Hospital & Research Center Oakland, an urban free-standing pediatric hospital that serves a demographically diverse population. The hospital ED sees over 45,000 visits per year, with 250 visits per year for VOE. Residents in pediatrics, family medicine, and emergency medicine staff the ED. All attending physicians are subspecialists in pediatric emergency medicine. Study procedures were approved by the hospital’s institutional review board.
Intervention
A multidisciplinary team consisting of ED staff and sickle cell center staff drafted a nursing-driven protocol for the assessment and management of acute pain associated with VOE, incorporating elements from a protocol in use by another WISCH collaborative member. The protocol called for the immediate triage and assessment of all patients with SCD who presented with moderate to severe pain suggestive of VOE. Moderate to severe pain was defined as a pain score of ≥ 5 on a numeric scale of 0 to 10, where 0 = no pain and 10 = the worst pain imaginable. Exclusion criteria included a chief complaint of pain not considered secondary to VOE (eg, trauma, fracture). Patients were also excluded if they had been transferred from another facility. The protocol called for IV pain medication to be administered within 10 minutes of the patient being roomed, with re-evaluation at 20-minute intervals and re-dosing of pain medication based on the patient’s subsequent pain rating.
Measures
We selected performance measures from the bank developed by the WISCH team to track improvement and evaluate progress. These performance measures included (1) mean time from triage to first analgesic dose, (2) percentage of patients that received their first dose of analgesic within 30 minutes of triage, (3) percentage of patients who had a pain assessment performed within 30 minutes of triage, and (4) percentage of patients re-assessed within 30 minutes after the first dose of analgesic had been administered. Our aims were to have 80% of patients assessed and given pain medications within 30 minutes of triage, and to have 80% of patients re-assessed within 30 minutes after having received their first dose of an analgesic, within 12 months of implementing our intervention.
Data Collection and Analysis
The WISCH project coordinator reviewed records of visits to the ED for a baseline period of 6 months and post-order set implementaton. Demographic data (age, gender), clinical data (hemoglobin type), pain scores, utilization data (number of ED visits during the study period), and data pertaining to the metrics chosen from the WISCH measurement bank were extracted from each eligible patient’s ED chart after the visit was completed. If patients were admitted, their length of hospitalization was extracted from their inpatient medical record.
All biostatistical analyses were conducted using Stata 9.2 (StataCorp, College Station, TX). Descriptive statistics computed at 2 time-points (pre and post order set implementation) were utilized to examine means, standard deviations and percentages. The 2 time-points were initially compared at the visit level of measurement, using Student’s t tests corrected for unequal variances where necessary for continuous variables and chi-square analyses for categorical variables, to evaluate if there was an improvement in timely triage, assessment, and treatment of acute VOE pain for all ED visits pre and post order set implementation. To account for trends and possible correlations across the months post order set implementation, we ran a mixed linear model with repeated measures over time to compare visits during all months post order set implementation with the baseline months, for metric 1, time from triage to first pain medication. If significant differences were found, we used Dunnett’s method of multiple comparisons to determine which months differed from baseline. For metrics 2 through 4, we ran linear models with a binary outcome, a logit link function and using general estimating equations to determine trends and to account for correlations over time.
Secondary analyses were conducted to evaluate whether mean pain scores were significantly different over the course of the ED visit for the 78 unique patients seen post order set implementation. A multivariable mixed linear model, for the outcome of the third pain score, was used to assess the associations with prior scores and to control for potential covariates (age, gender, number of ED visits, hemoglobin type) that were determined in advance. A statistical significance level of 0.05 was used for all tests.
Results
Baseline data were collected from December 2011 to May 2012. The protocol was implemented in July 2012 and was utilized during 165 ED visits (91% of eligible visits) through April 2013. There were no statistically significant differences in demographic or clinical characteristics between the 55 patients whose charts were reviewed prior to implementing the order set and the 78 unique patients treated thereafter. Pre order set implementation, the mean age was 14.6 ± 6.4 years; 60% were female and the primary diagnosis was HgbSS disease (61.8% of diagnoses). Post order set implementation, the mean age was 16.0 ± 8.0 years; 51.3% were female and the primary diagnosis was HgbSS disease (61.5% of diagnoses). The mean number of visits was 1.5 visits per patient with a range of 1–8 visits, both pre and post order set implementation. Thirty-one patients had ED visits at both time periods.
It can be seen in Figure 2 that staff performance on 3 of the 4 metrics (with the exception of initial analgesic within 30 minutes of triage) began to improve prior to implementing the order set. The mean length of ED stays decreased by 30 minutes, from a mean of 5.2 hours down to 4.7 hours (P < 0.05, Table). There was no significant change in the percentage of patients admitted to the inpatient unit.
We performed secondary analyses to determine if performance on our first metric, mean time from triage to first analgesic dose, was associated with any improvement on the third pain assessment for the patients enrolled post order set implementation. Looking at the first ED visit during the study period for the 78 unique patients, we found significant decreases in mean pain scores from the first to the second, from the second to the third, and from the first to the third assessment (P < 0.01). The mean pain scores were 8.3 ± 1.8, 5.9 ± 2.8, and 5.1 ± 3.0 on initial, second and third assessments, respectively. A multivariable model controlling for gender, hemoglobin type, number of ED visits and time to first pain medication showed that only the score at the second pain assessment (β = 0.88 ± 0.08, P < 0.001) was a significant predictor of the score at the third pain assessment.
Discussion
We demonstrated that a QI initiative to improve acute pain management resulted in more timely assessment and treatment of pain in pediatric patients with SCD. Significant improvements from baseline were achieved and sustained over a 10-month period in all 4 targeted metrics. We consistently exceeded our goal of having 80% of patients assessed within 30 minutes of triage, and our mean time to first pain medication (35.2 ± 22.8 minutes) came close to our goal of 30 minutes from triage. While we also achieved our goal to have 80% of patients re-assessed within 30 minutes after having received their first dose of an analgesic, we fell short in the percent who received their initial pain medication within 30 minutes of triage (52.7% versus goal of 80%). Although the length of stay in the ED decreased, no change was observed in the percentage of patients who required admission to the inpatient unit. A secondary analysis showed that mean pain scores significantly decreased over the course of the ED visit, from severe to moderate intensity.
The improvements that we observed began prior to implementation of the order set. We recognize that simply raising awareness and educating staff about the importance of timely and appropriate assessment and treatment of acute sickle cell related pain in the ED might be a potential confounder of our results. However, changes were sustained for 10 months post order set implementation and beyond, with no evidence that the performance on the target metrics is drifting back to baseline levels. Education and awareness-raising alone rarely result in sustained application of clinical practice guidelines [22]. We collaborated with NICHQ and other HRSA-WISCH grantees to systematically implement improvement science to ensure that the changes that we observed were indeed improvements and would be sustained [23] by first changing the system of care in the ED by introducing a standard order set [24,25]. We put a system into place to track use of the order set and to work with providers almost immediately if deviations were observed, to understand and overcome any barriers to the order set implementation. Systems in the ED and in the sickle cell center were aligned with the hospital’s QI initiatives [23].
Another strategy that we used to insure that the changes we observed would be sustained was to create a multidisciplinary team to build knowledge, skills, and new practices, including learning from other WISCH grantees and the NICHQ coordinating center [23]. We modified and adapted the intervention to our specific context [25]; although the outline of the order set was influenced by our WISCH colleagues, the final order set was structured to be consistent with other protocols within our institution. Finally, we included consumer input in the design of the project from the outset.
A previous study of a multi-institutional QI initiative aimed at improving acute SCD pain management for adult patients in the ED was unable to demonstrate an improvement in time to administration of initial analgesic [26]. Our study with pediatric patients was able to demonstrate a clinically meaningful decrease in the time to administration of first parenteral analgesic. The factors that account for the discrepant findings between these studies are likely multifactorial. Age (ie, pediatric vs. adult patients) may have played a role given that IV access may become increasingly difficult as patients with SCD age [26]. Education for providers should include the importance of alternative methods of administration of opioids, including subcutaneous and intranasal routes, to avoid delays when IV access is difficult. It is possible that negative provider attitudes converge with the documented increase in patient visits during the young adult years [27]. This may set up a challenging feedback loop wherein these vulnerable young adults are faced with greater stigma and consequently receive lower quality care, even when there is an attempt to carry out a standardized protocol.
We did not find that the QI intervention resulted in decreased admissions to the inpatient unit, with 68% of visits resulting in admission. In a recent pediatric SCD study, hospital admissions for pain control accounted for 78% of all admissions and 70% of readmissions within 30 days [28]. The investigators found that use of a SCD analgesic protocol including patient-controlled analgesia (PCA) improved quality of care as well as hospital readmission rates within 30 days (from 28% to 11%). Our ED QI protocol focused on only the first 90 minutes of the visit for pain. Our team has discussed the potential for starting the PCA in the ED and we should build on our success to focus on specific care that patients receive beyond their initial presentation. Further, we introduced pain action planning into outpatient care and need to continue to improve positive patient self-management strategies to ensure more seamless transition of pain management between home, ED, and inpatient settings.
Several valuable lessons were learned over the course of the ED QI initiative. Previous researchers [28] have emphasized the importance of coupling provider education with standardized order sets in efforts to improve the care of patients with SCD. Although we did not offer monthly formal education to our providers, the immediate follow-up when there were protocol deviations most likely served as teaching moments. These teaching moments also surfaced when some ED and hematology providers expressed concerns about the risk for oversedation with the rapid reassessment of pain and re-dosing of pain medications. Although rare, some parents also expressed that their child was being treated too vigorously with opioids. Our project highlighted the element of stigma that still accompanies the use of opioids for SCD pain management.
The project could not have been undertaken were it not for a small but determined multidisciplinary team of individuals who were personally invested in seeing the project come to fruition. The identification of physician and nurse champions who were enthusiastic about the project, invested in its conduct, and committed to its success was a cornerstone of the project’s success. These champions played an essential role in engaging staff interest in the project and oversaw the practicalities of implementing a new protocol in the ED. A spirit of collaboration, teamwork, and good communication between all involved parties was also critical. At the same time, we incorporated input from the treating ED and hematology clinicians using PDSA cycles as we were refining our protocol. We believe that our process enhanced buy-in from participating providers and clarified any issues that needed to be addressed in our setting, resulting in accelerated and sustained quality improvement.
Limitations
Although protocol-driven interventions are designed to provide a certain degree of uniformity of care, the protocol was not designed nor utilized in such a way that it superseded the best medical judgment of the treating clinicians. Deviations from the protocol were permissible when they were felt to be in the patient’s best interest. The study did not control for confounding variables such as disease severity, how long the patient had been in pain prior to coming to the ED, nor did we assess therapeutic interventions the patient had utilized at home prior to seeking out care in the ED. All of these factors could affect how well a patient might respond to treatment. We believe that sharing baseline data and monthly progress via run charts (graphs of data over time) with ED and sickle cell center staff and with consumer representatives enhanced the pace and focus of the project [23]. We had a dedicated person managing our data in real time through our HRSA funding, thus the project might not be generalizable to other institutions that do not have such staffing or access to the technology to allow project progress to be closely monitored by stakeholders.
Future Directions
With the goal of further reducing the time to administration of first analgesic dose in the ED setting, intranasal fentanyl will be utilized in our ED as the initial drug of choice for patients who do not object to or have a contraindication to its use. Collection of data from patients and family members is being undertaken to assess consumer satisfaction with the ED QI initiative. Recognizing that the ED management of acute pain addresses only one aspect of sickle cell pain, we are looking at ways to more comprehensively address pain. Individualized outpatient pain management plans are being created and patients and families are being encouraged and empowered to become active partners with their sickle cell providers in their own care. Although our initial efforts have focused on our pediatric patients, an additional aim of our project is to broaden the scope of our ED QI initiative to include community hospitals in the region that serve adult patients with SCD.
Conclusion
Implementation of a QI initiative in the ED has led to expeditious care for pediatric patients with SCD presenting with VOE. A multidisciplinary approach, ongoing staff education, and commitment to the initiative have been necessary to sustain the improvements. Our success can provide a template for other QI initiatives in the ED that translate to improved patient care for other diseases. A QI framework provided us with unique challenges but also invaluable lessons as we addressed our objective to improve outcomes for patients with SCD across the life course.
Acknowledgments: The authors wish to thank Theresa Freitas, RN, Lisa Hale, PNP, Carolyn Hoppe, MD, Ileana Mendez, RN, Helen Mitchell, Mary Rutherford, MD, Augusta Saulys, MD and the Children’s Hospital & Research Center Oakland Emergency Medicine Department and Sickle Cell Center for their support.
Corresponding author: Marsha Treadwell, PhD, Children’s Hospital & Research Center Oakland, 747 52nd St, Oakland, CA 94609, [email protected].
Funding/support: This research was conducted as part of the National Initiative for Children’s Healthcare Quality (NICHQ) Working to Improve Sickle Cell Healthcare (WISCH) project. Further support came from a grant from the Health Resources and Services Administration Sickle Cell Disease Treatment Demonstration Project Grant No. U1EMC16492 and from NIH CTSA grant UL1 RR024131. The views expressed in this publication do not necessarily reflect the views of WISCH, NICHQ, or HRSA.
1. Yusuf HR, Atrash HK, Grosse SD, et al. Emergency department visits made by patients with sickle cell disease: a descriptive study, 1999-2007. Am J Preventive Med 2010;38 (4 Suppl):S536–41.
2. Benjamin L, Dampier C, Jacox A, et al. Guideline for the management of acute and chronic pain in sickle cell disease. American Pain Society; 1999.
3. Rees DC, Olujohungbe AD, Parker NE, et al. Guidelines for the management of the acute painful crisis in sickle cell disease. Br J Haematology 2003;120:744–52.
4. Solomon LR. Pain management in adults with sickle cell disease in a medical center emergency department. J Nat Med Assoc 2010;102:1025–32.
5. Lazio MP, Costello HH, Courtney DM, et al. A comparison of analgesic management for emergency department patients with sickle cell disease and renal colic. Clin J Pain 2010;26:199–205.
6. Shenoi R, Ma L, Syblik D, Yusuf S. Emergency department crowding and analgesic delay in pediatric sickle cell pain crises. Ped Emerg Care 2011;27:911–7.
7. Tanabe P, Artz N, Mark Courtney D, et al. Adult emergency department patients with sickle cell pain crisis: a learning collaborative model to improve analgesic management. Acad Emerg Med 2010;17:399–407.
8. Zempsky WT. Evaluation and treatment of sickle cell pain in the emergency department: paths to a better future. Clin Ped Emerg Med 2010;11:265–73.
9. Haywood C Jr, Tanabe P, Naik R, et al. The impact of race and disease on sickle cell patient wait times in the emergency department. Am J Emerg Med 2013;31:651–6.
10. Solomon LR. Treatment and prevention of pain due to vaso-occlusive crises in adults with sickle cell disease: an educational void. Blood 2008;111:997–1003.
11. Ballas SK. New era dawns on sickle cell pain. Blood 2010;116:311–2.
12. Haywood C Jr, Lanzkron S, Ratanawongsa N, et al. The association of provider communication with trust among adults with sickle cell disease. J Gen Intern Med 2010;25:543–8.
13. Zempsky WT. Treatment of sickle cell pain: fostering trust and justice. JAMA 2009;302:2479–80.
14. Elander J, Lusher J, Bevan D, Telfer P. Pain management and symptoms of substance dependence among patients with sickle cell disease. Soc Sci Med 2003;57:1683–96.
15. Elander J, Lusher J, Bevan D, et al. Understanding the causes of problematic pain management in sickle cell disease: evidence that pseudoaddiction plays a more important role than genuine analgesic dependence. J Pain Sympt Manag 2004;27:156–69.
16. Smith WR, Penberthy LT, Bovbjerg VE, et al. Daily assessment of pain in adults with sickle cell disease. Ann Intern Med 2008;148:94–101.
17. Harris A, Parker N, Baker C. Adults with sickle cell. Psychol Health Med 1998;3:171–9.
18. Jenerette CM, Brewer C. Health-related stigma in young adults with sickle cell disease. J Nat Med Assoc 2010;102:1050–5.
19. Maxwell K, Streetly A, Bevan D. Experiences of hospital care and treatment seeking for pain from sickle cell disease: qualitative study. BMJ 1999;318:1585–90.
20. Oyeku SO, Wang CJ, Scoville R, et al. Hemoglobinopathy Learning Collaborative: using quality improvement (QI) to achieve equity in health care quality, coordination, and outcomes for sickle cell disease. J Health Care Poor Underserved 2012;23(3 Suppl):34–48.
21. Wang CJ, Kavanagh PL, Little AA, et al. Quality-of-care indicators for children with sickle cell disease. Pediatrics 2011;128:484–93.
22. Mansouri M, Lockyer J. A meta-analysis of continuing medical education effectiveness. J Contin Ed Health Prof 2007;27:6–15.
23. The breakthrough series: IHI’s collaborative model for achieving breakthrough improvement. Boston: Institute for Healthcare Improvement; 2003.
24. Berwick DM. Improvement, trust, and the healthcare workforce. Qual Safety Health Care 2003;12:448–52.
25. Hovlid E, Bukve O, Haug K, et al. Sustainability of healthcare improvement: what can we learn from learning theory? BMC Health Serv Res 2012;12:235.
26. Tanabe P, Hafner JW, Martinovich Z, Artz N. Adult emergency department patients with sickle cell pain crisis: results from a quality improvement learning collaborative model to improve analgesic management. Acad Emerg Med 2012;19:430–8.
27. Brousseau DC, Owens PL, Mosso AL, et al. Acute care utilization and rehospitalizations for sickle cell disease. JAMA 2010;303:1288–94.
28. Frei-Jones MJ, Field JJ, DeBaun MR. Multi-modal intervention and prospective implementation of standardized sickle cell pain admission orders reduces 30-day readmission rate. Pediatr Blood Cancer 2009;53:401–5.
From the Children’s Hospital & Research Center Oakland, Oakland, CA.
Abstract
- Objective: To determine whether a quality improvement (QI) initiative would result in more timely assessment and treatment of acute sickle cell–related pain for pediatric patients with sickle cell disease (SCD) treated in the emergency department (ED).
- Methods: We created and implemented a protocol for SCD pain management in the ED with the goals of improving (1) mean time from triage to first analgesic dose; (2) percentage of patients that received their first analgesic dose within 30 minutes of triage, and (3) percentage of patients who had pain assessment performed within 30 minutes of triage and who were re-assessed within 30 minutes after the first analgesic dose.
- Results: Significant improvements were achieved between baseline (55 patient visits) and post order set implementation (165 visits) in time from triage to administration of first analgesic (decreased from 89.9 ± 50.5 to 35.2 ± 22.8 minutes, P < 0.001); percentage of patient visits receiving pain medications within 30 minutes of triage (from 7% to 53%, P < 0.001); percentage of patient visits assessed within 30 minutes of triage (from 64% to 99.4%, P < 0.001); and percentage of patient visits re-assessed within 30 minutes of initial analgesic (from 54% to 86%, P < 0.001).
- Conclusions: Implementation of a QI initiative in the ED led to expeditious care for pediatric patients with SCD presenting with pain. A QI framework provided us with unique challenges but also invaluable lessons as we address our objective of decreasing the quality gap in SCD medical care.
Pain is the leading cause of emergency department (ED) visits for patients with sickle cell disease (SCD) [1]. In the United States, 78% of the nearly 200,000 annual ED visits for SCD are for a complaint of pain [1]. Guidelines for the management of sickle cell vaso-occlusive pain episodes (VOE) suggest prompt initiation of parenteral opioids, use of effective opioid doses, and repeat opioid doses at frequent intervals [2–4]. Adherence to guidelines is poor. Both pediatric and adult patients with SCD experience delays in the initiation of analgesics and are routinely undertreated with respect to opioid dosing [5–8]. Even after controlling for race, the delays in time to analgesic administration experienced by patients with SCD exceed the delays encountered by patients who present to the ED with other types of pain [5,9]. These disparities warrant efforts designed to improve the delivery of quality care to patients with SCD.
Barriers to rapid and appropriate care of VOE in the ED are multifactorial and include systems-based limitations, such as acuity of the ED census, staffing limitations (eg, nurse-to-patient ratios), and facility limitations (eg, room availability) [6]. Provider-based limitations may include lack of awareness of available guidelines [10]. Biases and misunderstandings amongst providers about sickle cell pain and adequate medication dosing may also play a role [11–13]. These provider biases often lead to undertreatment of the pain, which in turn can lead to pseudoaddiction (drug-seeking behavior due to inadequate treatment) and a cycle of increased ED and inpatient utilization [14,15].
Patient-specific barriers to effective ED management of pain are equally complex. Previous negative experiences in the ED can lead patients and families to delay seeking care or avoid the ED altogether despite severe VOE pain [16]. Patients report frustration with the lack of consideration that they receive for their reports of pain, perceived insensitivity of hospital staff, inadequate analgesic administration, staff preoccupation with concerns of drug addiction, and an overall lack of respect and trust [17–19]. Patients also perceive a lack of knowledge of SCD and its treatments on the part of ED staff [7]. Other barriers to effective management are technical in nature, such as difficulty in establishing timely intravenous (IV) access.
Gaps and variations in quality of care contribute to poor outcomes for patients with SCD [20,21]. To help address these inequities, the Working to Improve Sickle Cell Healthcare (WISCH) project began in 2010 to improve care and outcomes for patients with SCD. WISCH is a collaborative quality improvement (QI) project funded by the Health Resources and Services Administration (HRSA) that has the goal to use improvement science to improve outcomes for patients with SCD across the life course (Ed note: see Editorial by Oyeku et al in this issue). As one of the HRSA-WISCH grantee networks, we undertook a QI project designed to decrease the quality gap in SCD medical care by creating and implementing a protocol for ED pain management for pediatric patients. Goals of the project were to improve the timely and appropriate assessment and treatment of acute VOE in the ED.
Methods
Setting
This ED QI initiative was implemented at Children’s Hospital & Research Center Oakland, an urban free-standing pediatric hospital that serves a demographically diverse population. The hospital ED sees over 45,000 visits per year, with 250 visits per year for VOE. Residents in pediatrics, family medicine, and emergency medicine staff the ED. All attending physicians are subspecialists in pediatric emergency medicine. Study procedures were approved by the hospital’s institutional review board.
Intervention
A multidisciplinary team consisting of ED staff and sickle cell center staff drafted a nursing-driven protocol for the assessment and management of acute pain associated with VOE, incorporating elements from a protocol in use by another WISCH collaborative member. The protocol called for the immediate triage and assessment of all patients with SCD who presented with moderate to severe pain suggestive of VOE. Moderate to severe pain was defined as a pain score of ≥ 5 on a numeric scale of 0 to 10, where 0 = no pain and 10 = the worst pain imaginable. Exclusion criteria included a chief complaint of pain not considered secondary to VOE (eg, trauma, fracture). Patients were also excluded if they had been transferred from another facility. The protocol called for IV pain medication to be administered within 10 minutes of the patient being roomed, with re-evaluation at 20-minute intervals and re-dosing of pain medication based on the patient’s subsequent pain rating.
Measures
We selected performance measures from the bank developed by the WISCH team to track improvement and evaluate progress. These performance measures included (1) mean time from triage to first analgesic dose, (2) percentage of patients that received their first dose of analgesic within 30 minutes of triage, (3) percentage of patients who had a pain assessment performed within 30 minutes of triage, and (4) percentage of patients re-assessed within 30 minutes after the first dose of analgesic had been administered. Our aims were to have 80% of patients assessed and given pain medications within 30 minutes of triage, and to have 80% of patients re-assessed within 30 minutes after having received their first dose of an analgesic, within 12 months of implementing our intervention.
Data Collection and Analysis
The WISCH project coordinator reviewed records of visits to the ED for a baseline period of 6 months and post-order set implementaton. Demographic data (age, gender), clinical data (hemoglobin type), pain scores, utilization data (number of ED visits during the study period), and data pertaining to the metrics chosen from the WISCH measurement bank were extracted from each eligible patient’s ED chart after the visit was completed. If patients were admitted, their length of hospitalization was extracted from their inpatient medical record.
All biostatistical analyses were conducted using Stata 9.2 (StataCorp, College Station, TX). Descriptive statistics computed at 2 time-points (pre and post order set implementation) were utilized to examine means, standard deviations and percentages. The 2 time-points were initially compared at the visit level of measurement, using Student’s t tests corrected for unequal variances where necessary for continuous variables and chi-square analyses for categorical variables, to evaluate if there was an improvement in timely triage, assessment, and treatment of acute VOE pain for all ED visits pre and post order set implementation. To account for trends and possible correlations across the months post order set implementation, we ran a mixed linear model with repeated measures over time to compare visits during all months post order set implementation with the baseline months, for metric 1, time from triage to first pain medication. If significant differences were found, we used Dunnett’s method of multiple comparisons to determine which months differed from baseline. For metrics 2 through 4, we ran linear models with a binary outcome, a logit link function and using general estimating equations to determine trends and to account for correlations over time.
Secondary analyses were conducted to evaluate whether mean pain scores were significantly different over the course of the ED visit for the 78 unique patients seen post order set implementation. A multivariable mixed linear model, for the outcome of the third pain score, was used to assess the associations with prior scores and to control for potential covariates (age, gender, number of ED visits, hemoglobin type) that were determined in advance. A statistical significance level of 0.05 was used for all tests.
Results
Baseline data were collected from December 2011 to May 2012. The protocol was implemented in July 2012 and was utilized during 165 ED visits (91% of eligible visits) through April 2013. There were no statistically significant differences in demographic or clinical characteristics between the 55 patients whose charts were reviewed prior to implementing the order set and the 78 unique patients treated thereafter. Pre order set implementation, the mean age was 14.6 ± 6.4 years; 60% were female and the primary diagnosis was HgbSS disease (61.8% of diagnoses). Post order set implementation, the mean age was 16.0 ± 8.0 years; 51.3% were female and the primary diagnosis was HgbSS disease (61.5% of diagnoses). The mean number of visits was 1.5 visits per patient with a range of 1–8 visits, both pre and post order set implementation. Thirty-one patients had ED visits at both time periods.
It can be seen in Figure 2 that staff performance on 3 of the 4 metrics (with the exception of initial analgesic within 30 minutes of triage) began to improve prior to implementing the order set. The mean length of ED stays decreased by 30 minutes, from a mean of 5.2 hours down to 4.7 hours (P < 0.05, Table). There was no significant change in the percentage of patients admitted to the inpatient unit.
We performed secondary analyses to determine if performance on our first metric, mean time from triage to first analgesic dose, was associated with any improvement on the third pain assessment for the patients enrolled post order set implementation. Looking at the first ED visit during the study period for the 78 unique patients, we found significant decreases in mean pain scores from the first to the second, from the second to the third, and from the first to the third assessment (P < 0.01). The mean pain scores were 8.3 ± 1.8, 5.9 ± 2.8, and 5.1 ± 3.0 on initial, second and third assessments, respectively. A multivariable model controlling for gender, hemoglobin type, number of ED visits and time to first pain medication showed that only the score at the second pain assessment (β = 0.88 ± 0.08, P < 0.001) was a significant predictor of the score at the third pain assessment.
Discussion
We demonstrated that a QI initiative to improve acute pain management resulted in more timely assessment and treatment of pain in pediatric patients with SCD. Significant improvements from baseline were achieved and sustained over a 10-month period in all 4 targeted metrics. We consistently exceeded our goal of having 80% of patients assessed within 30 minutes of triage, and our mean time to first pain medication (35.2 ± 22.8 minutes) came close to our goal of 30 minutes from triage. While we also achieved our goal to have 80% of patients re-assessed within 30 minutes after having received their first dose of an analgesic, we fell short in the percent who received their initial pain medication within 30 minutes of triage (52.7% versus goal of 80%). Although the length of stay in the ED decreased, no change was observed in the percentage of patients who required admission to the inpatient unit. A secondary analysis showed that mean pain scores significantly decreased over the course of the ED visit, from severe to moderate intensity.
The improvements that we observed began prior to implementation of the order set. We recognize that simply raising awareness and educating staff about the importance of timely and appropriate assessment and treatment of acute sickle cell related pain in the ED might be a potential confounder of our results. However, changes were sustained for 10 months post order set implementation and beyond, with no evidence that the performance on the target metrics is drifting back to baseline levels. Education and awareness-raising alone rarely result in sustained application of clinical practice guidelines [22]. We collaborated with NICHQ and other HRSA-WISCH grantees to systematically implement improvement science to ensure that the changes that we observed were indeed improvements and would be sustained [23] by first changing the system of care in the ED by introducing a standard order set [24,25]. We put a system into place to track use of the order set and to work with providers almost immediately if deviations were observed, to understand and overcome any barriers to the order set implementation. Systems in the ED and in the sickle cell center were aligned with the hospital’s QI initiatives [23].
Another strategy that we used to insure that the changes we observed would be sustained was to create a multidisciplinary team to build knowledge, skills, and new practices, including learning from other WISCH grantees and the NICHQ coordinating center [23]. We modified and adapted the intervention to our specific context [25]; although the outline of the order set was influenced by our WISCH colleagues, the final order set was structured to be consistent with other protocols within our institution. Finally, we included consumer input in the design of the project from the outset.
A previous study of a multi-institutional QI initiative aimed at improving acute SCD pain management for adult patients in the ED was unable to demonstrate an improvement in time to administration of initial analgesic [26]. Our study with pediatric patients was able to demonstrate a clinically meaningful decrease in the time to administration of first parenteral analgesic. The factors that account for the discrepant findings between these studies are likely multifactorial. Age (ie, pediatric vs. adult patients) may have played a role given that IV access may become increasingly difficult as patients with SCD age [26]. Education for providers should include the importance of alternative methods of administration of opioids, including subcutaneous and intranasal routes, to avoid delays when IV access is difficult. It is possible that negative provider attitudes converge with the documented increase in patient visits during the young adult years [27]. This may set up a challenging feedback loop wherein these vulnerable young adults are faced with greater stigma and consequently receive lower quality care, even when there is an attempt to carry out a standardized protocol.
We did not find that the QI intervention resulted in decreased admissions to the inpatient unit, with 68% of visits resulting in admission. In a recent pediatric SCD study, hospital admissions for pain control accounted for 78% of all admissions and 70% of readmissions within 30 days [28]. The investigators found that use of a SCD analgesic protocol including patient-controlled analgesia (PCA) improved quality of care as well as hospital readmission rates within 30 days (from 28% to 11%). Our ED QI protocol focused on only the first 90 minutes of the visit for pain. Our team has discussed the potential for starting the PCA in the ED and we should build on our success to focus on specific care that patients receive beyond their initial presentation. Further, we introduced pain action planning into outpatient care and need to continue to improve positive patient self-management strategies to ensure more seamless transition of pain management between home, ED, and inpatient settings.
Several valuable lessons were learned over the course of the ED QI initiative. Previous researchers [28] have emphasized the importance of coupling provider education with standardized order sets in efforts to improve the care of patients with SCD. Although we did not offer monthly formal education to our providers, the immediate follow-up when there were protocol deviations most likely served as teaching moments. These teaching moments also surfaced when some ED and hematology providers expressed concerns about the risk for oversedation with the rapid reassessment of pain and re-dosing of pain medications. Although rare, some parents also expressed that their child was being treated too vigorously with opioids. Our project highlighted the element of stigma that still accompanies the use of opioids for SCD pain management.
The project could not have been undertaken were it not for a small but determined multidisciplinary team of individuals who were personally invested in seeing the project come to fruition. The identification of physician and nurse champions who were enthusiastic about the project, invested in its conduct, and committed to its success was a cornerstone of the project’s success. These champions played an essential role in engaging staff interest in the project and oversaw the practicalities of implementing a new protocol in the ED. A spirit of collaboration, teamwork, and good communication between all involved parties was also critical. At the same time, we incorporated input from the treating ED and hematology clinicians using PDSA cycles as we were refining our protocol. We believe that our process enhanced buy-in from participating providers and clarified any issues that needed to be addressed in our setting, resulting in accelerated and sustained quality improvement.
Limitations
Although protocol-driven interventions are designed to provide a certain degree of uniformity of care, the protocol was not designed nor utilized in such a way that it superseded the best medical judgment of the treating clinicians. Deviations from the protocol were permissible when they were felt to be in the patient’s best interest. The study did not control for confounding variables such as disease severity, how long the patient had been in pain prior to coming to the ED, nor did we assess therapeutic interventions the patient had utilized at home prior to seeking out care in the ED. All of these factors could affect how well a patient might respond to treatment. We believe that sharing baseline data and monthly progress via run charts (graphs of data over time) with ED and sickle cell center staff and with consumer representatives enhanced the pace and focus of the project [23]. We had a dedicated person managing our data in real time through our HRSA funding, thus the project might not be generalizable to other institutions that do not have such staffing or access to the technology to allow project progress to be closely monitored by stakeholders.
Future Directions
With the goal of further reducing the time to administration of first analgesic dose in the ED setting, intranasal fentanyl will be utilized in our ED as the initial drug of choice for patients who do not object to or have a contraindication to its use. Collection of data from patients and family members is being undertaken to assess consumer satisfaction with the ED QI initiative. Recognizing that the ED management of acute pain addresses only one aspect of sickle cell pain, we are looking at ways to more comprehensively address pain. Individualized outpatient pain management plans are being created and patients and families are being encouraged and empowered to become active partners with their sickle cell providers in their own care. Although our initial efforts have focused on our pediatric patients, an additional aim of our project is to broaden the scope of our ED QI initiative to include community hospitals in the region that serve adult patients with SCD.
Conclusion
Implementation of a QI initiative in the ED has led to expeditious care for pediatric patients with SCD presenting with VOE. A multidisciplinary approach, ongoing staff education, and commitment to the initiative have been necessary to sustain the improvements. Our success can provide a template for other QI initiatives in the ED that translate to improved patient care for other diseases. A QI framework provided us with unique challenges but also invaluable lessons as we addressed our objective to improve outcomes for patients with SCD across the life course.
Acknowledgments: The authors wish to thank Theresa Freitas, RN, Lisa Hale, PNP, Carolyn Hoppe, MD, Ileana Mendez, RN, Helen Mitchell, Mary Rutherford, MD, Augusta Saulys, MD and the Children’s Hospital & Research Center Oakland Emergency Medicine Department and Sickle Cell Center for their support.
Corresponding author: Marsha Treadwell, PhD, Children’s Hospital & Research Center Oakland, 747 52nd St, Oakland, CA 94609, [email protected].
Funding/support: This research was conducted as part of the National Initiative for Children’s Healthcare Quality (NICHQ) Working to Improve Sickle Cell Healthcare (WISCH) project. Further support came from a grant from the Health Resources and Services Administration Sickle Cell Disease Treatment Demonstration Project Grant No. U1EMC16492 and from NIH CTSA grant UL1 RR024131. The views expressed in this publication do not necessarily reflect the views of WISCH, NICHQ, or HRSA.
From the Children’s Hospital & Research Center Oakland, Oakland, CA.
Abstract
- Objective: To determine whether a quality improvement (QI) initiative would result in more timely assessment and treatment of acute sickle cell–related pain for pediatric patients with sickle cell disease (SCD) treated in the emergency department (ED).
- Methods: We created and implemented a protocol for SCD pain management in the ED with the goals of improving (1) mean time from triage to first analgesic dose; (2) percentage of patients that received their first analgesic dose within 30 minutes of triage, and (3) percentage of patients who had pain assessment performed within 30 minutes of triage and who were re-assessed within 30 minutes after the first analgesic dose.
- Results: Significant improvements were achieved between baseline (55 patient visits) and post order set implementation (165 visits) in time from triage to administration of first analgesic (decreased from 89.9 ± 50.5 to 35.2 ± 22.8 minutes, P < 0.001); percentage of patient visits receiving pain medications within 30 minutes of triage (from 7% to 53%, P < 0.001); percentage of patient visits assessed within 30 minutes of triage (from 64% to 99.4%, P < 0.001); and percentage of patient visits re-assessed within 30 minutes of initial analgesic (from 54% to 86%, P < 0.001).
- Conclusions: Implementation of a QI initiative in the ED led to expeditious care for pediatric patients with SCD presenting with pain. A QI framework provided us with unique challenges but also invaluable lessons as we address our objective of decreasing the quality gap in SCD medical care.
Pain is the leading cause of emergency department (ED) visits for patients with sickle cell disease (SCD) [1]. In the United States, 78% of the nearly 200,000 annual ED visits for SCD are for a complaint of pain [1]. Guidelines for the management of sickle cell vaso-occlusive pain episodes (VOE) suggest prompt initiation of parenteral opioids, use of effective opioid doses, and repeat opioid doses at frequent intervals [2–4]. Adherence to guidelines is poor. Both pediatric and adult patients with SCD experience delays in the initiation of analgesics and are routinely undertreated with respect to opioid dosing [5–8]. Even after controlling for race, the delays in time to analgesic administration experienced by patients with SCD exceed the delays encountered by patients who present to the ED with other types of pain [5,9]. These disparities warrant efforts designed to improve the delivery of quality care to patients with SCD.
Barriers to rapid and appropriate care of VOE in the ED are multifactorial and include systems-based limitations, such as acuity of the ED census, staffing limitations (eg, nurse-to-patient ratios), and facility limitations (eg, room availability) [6]. Provider-based limitations may include lack of awareness of available guidelines [10]. Biases and misunderstandings amongst providers about sickle cell pain and adequate medication dosing may also play a role [11–13]. These provider biases often lead to undertreatment of the pain, which in turn can lead to pseudoaddiction (drug-seeking behavior due to inadequate treatment) and a cycle of increased ED and inpatient utilization [14,15].
Patient-specific barriers to effective ED management of pain are equally complex. Previous negative experiences in the ED can lead patients and families to delay seeking care or avoid the ED altogether despite severe VOE pain [16]. Patients report frustration with the lack of consideration that they receive for their reports of pain, perceived insensitivity of hospital staff, inadequate analgesic administration, staff preoccupation with concerns of drug addiction, and an overall lack of respect and trust [17–19]. Patients also perceive a lack of knowledge of SCD and its treatments on the part of ED staff [7]. Other barriers to effective management are technical in nature, such as difficulty in establishing timely intravenous (IV) access.
Gaps and variations in quality of care contribute to poor outcomes for patients with SCD [20,21]. To help address these inequities, the Working to Improve Sickle Cell Healthcare (WISCH) project began in 2010 to improve care and outcomes for patients with SCD. WISCH is a collaborative quality improvement (QI) project funded by the Health Resources and Services Administration (HRSA) that has the goal to use improvement science to improve outcomes for patients with SCD across the life course (Ed note: see Editorial by Oyeku et al in this issue). As one of the HRSA-WISCH grantee networks, we undertook a QI project designed to decrease the quality gap in SCD medical care by creating and implementing a protocol for ED pain management for pediatric patients. Goals of the project were to improve the timely and appropriate assessment and treatment of acute VOE in the ED.
Methods
Setting
This ED QI initiative was implemented at Children’s Hospital & Research Center Oakland, an urban free-standing pediatric hospital that serves a demographically diverse population. The hospital ED sees over 45,000 visits per year, with 250 visits per year for VOE. Residents in pediatrics, family medicine, and emergency medicine staff the ED. All attending physicians are subspecialists in pediatric emergency medicine. Study procedures were approved by the hospital’s institutional review board.
Intervention
A multidisciplinary team consisting of ED staff and sickle cell center staff drafted a nursing-driven protocol for the assessment and management of acute pain associated with VOE, incorporating elements from a protocol in use by another WISCH collaborative member. The protocol called for the immediate triage and assessment of all patients with SCD who presented with moderate to severe pain suggestive of VOE. Moderate to severe pain was defined as a pain score of ≥ 5 on a numeric scale of 0 to 10, where 0 = no pain and 10 = the worst pain imaginable. Exclusion criteria included a chief complaint of pain not considered secondary to VOE (eg, trauma, fracture). Patients were also excluded if they had been transferred from another facility. The protocol called for IV pain medication to be administered within 10 minutes of the patient being roomed, with re-evaluation at 20-minute intervals and re-dosing of pain medication based on the patient’s subsequent pain rating.
Measures
We selected performance measures from the bank developed by the WISCH team to track improvement and evaluate progress. These performance measures included (1) mean time from triage to first analgesic dose, (2) percentage of patients that received their first dose of analgesic within 30 minutes of triage, (3) percentage of patients who had a pain assessment performed within 30 minutes of triage, and (4) percentage of patients re-assessed within 30 minutes after the first dose of analgesic had been administered. Our aims were to have 80% of patients assessed and given pain medications within 30 minutes of triage, and to have 80% of patients re-assessed within 30 minutes after having received their first dose of an analgesic, within 12 months of implementing our intervention.
Data Collection and Analysis
The WISCH project coordinator reviewed records of visits to the ED for a baseline period of 6 months and post-order set implementaton. Demographic data (age, gender), clinical data (hemoglobin type), pain scores, utilization data (number of ED visits during the study period), and data pertaining to the metrics chosen from the WISCH measurement bank were extracted from each eligible patient’s ED chart after the visit was completed. If patients were admitted, their length of hospitalization was extracted from their inpatient medical record.
All biostatistical analyses were conducted using Stata 9.2 (StataCorp, College Station, TX). Descriptive statistics computed at 2 time-points (pre and post order set implementation) were utilized to examine means, standard deviations and percentages. The 2 time-points were initially compared at the visit level of measurement, using Student’s t tests corrected for unequal variances where necessary for continuous variables and chi-square analyses for categorical variables, to evaluate if there was an improvement in timely triage, assessment, and treatment of acute VOE pain for all ED visits pre and post order set implementation. To account for trends and possible correlations across the months post order set implementation, we ran a mixed linear model with repeated measures over time to compare visits during all months post order set implementation with the baseline months, for metric 1, time from triage to first pain medication. If significant differences were found, we used Dunnett’s method of multiple comparisons to determine which months differed from baseline. For metrics 2 through 4, we ran linear models with a binary outcome, a logit link function and using general estimating equations to determine trends and to account for correlations over time.
Secondary analyses were conducted to evaluate whether mean pain scores were significantly different over the course of the ED visit for the 78 unique patients seen post order set implementation. A multivariable mixed linear model, for the outcome of the third pain score, was used to assess the associations with prior scores and to control for potential covariates (age, gender, number of ED visits, hemoglobin type) that were determined in advance. A statistical significance level of 0.05 was used for all tests.
Results
Baseline data were collected from December 2011 to May 2012. The protocol was implemented in July 2012 and was utilized during 165 ED visits (91% of eligible visits) through April 2013. There were no statistically significant differences in demographic or clinical characteristics between the 55 patients whose charts were reviewed prior to implementing the order set and the 78 unique patients treated thereafter. Pre order set implementation, the mean age was 14.6 ± 6.4 years; 60% were female and the primary diagnosis was HgbSS disease (61.8% of diagnoses). Post order set implementation, the mean age was 16.0 ± 8.0 years; 51.3% were female and the primary diagnosis was HgbSS disease (61.5% of diagnoses). The mean number of visits was 1.5 visits per patient with a range of 1–8 visits, both pre and post order set implementation. Thirty-one patients had ED visits at both time periods.
It can be seen in Figure 2 that staff performance on 3 of the 4 metrics (with the exception of initial analgesic within 30 minutes of triage) began to improve prior to implementing the order set. The mean length of ED stays decreased by 30 minutes, from a mean of 5.2 hours down to 4.7 hours (P < 0.05, Table). There was no significant change in the percentage of patients admitted to the inpatient unit.
We performed secondary analyses to determine if performance on our first metric, mean time from triage to first analgesic dose, was associated with any improvement on the third pain assessment for the patients enrolled post order set implementation. Looking at the first ED visit during the study period for the 78 unique patients, we found significant decreases in mean pain scores from the first to the second, from the second to the third, and from the first to the third assessment (P < 0.01). The mean pain scores were 8.3 ± 1.8, 5.9 ± 2.8, and 5.1 ± 3.0 on initial, second and third assessments, respectively. A multivariable model controlling for gender, hemoglobin type, number of ED visits and time to first pain medication showed that only the score at the second pain assessment (β = 0.88 ± 0.08, P < 0.001) was a significant predictor of the score at the third pain assessment.
Discussion
We demonstrated that a QI initiative to improve acute pain management resulted in more timely assessment and treatment of pain in pediatric patients with SCD. Significant improvements from baseline were achieved and sustained over a 10-month period in all 4 targeted metrics. We consistently exceeded our goal of having 80% of patients assessed within 30 minutes of triage, and our mean time to first pain medication (35.2 ± 22.8 minutes) came close to our goal of 30 minutes from triage. While we also achieved our goal to have 80% of patients re-assessed within 30 minutes after having received their first dose of an analgesic, we fell short in the percent who received their initial pain medication within 30 minutes of triage (52.7% versus goal of 80%). Although the length of stay in the ED decreased, no change was observed in the percentage of patients who required admission to the inpatient unit. A secondary analysis showed that mean pain scores significantly decreased over the course of the ED visit, from severe to moderate intensity.
The improvements that we observed began prior to implementation of the order set. We recognize that simply raising awareness and educating staff about the importance of timely and appropriate assessment and treatment of acute sickle cell related pain in the ED might be a potential confounder of our results. However, changes were sustained for 10 months post order set implementation and beyond, with no evidence that the performance on the target metrics is drifting back to baseline levels. Education and awareness-raising alone rarely result in sustained application of clinical practice guidelines [22]. We collaborated with NICHQ and other HRSA-WISCH grantees to systematically implement improvement science to ensure that the changes that we observed were indeed improvements and would be sustained [23] by first changing the system of care in the ED by introducing a standard order set [24,25]. We put a system into place to track use of the order set and to work with providers almost immediately if deviations were observed, to understand and overcome any barriers to the order set implementation. Systems in the ED and in the sickle cell center were aligned with the hospital’s QI initiatives [23].
Another strategy that we used to insure that the changes we observed would be sustained was to create a multidisciplinary team to build knowledge, skills, and new practices, including learning from other WISCH grantees and the NICHQ coordinating center [23]. We modified and adapted the intervention to our specific context [25]; although the outline of the order set was influenced by our WISCH colleagues, the final order set was structured to be consistent with other protocols within our institution. Finally, we included consumer input in the design of the project from the outset.
A previous study of a multi-institutional QI initiative aimed at improving acute SCD pain management for adult patients in the ED was unable to demonstrate an improvement in time to administration of initial analgesic [26]. Our study with pediatric patients was able to demonstrate a clinically meaningful decrease in the time to administration of first parenteral analgesic. The factors that account for the discrepant findings between these studies are likely multifactorial. Age (ie, pediatric vs. adult patients) may have played a role given that IV access may become increasingly difficult as patients with SCD age [26]. Education for providers should include the importance of alternative methods of administration of opioids, including subcutaneous and intranasal routes, to avoid delays when IV access is difficult. It is possible that negative provider attitudes converge with the documented increase in patient visits during the young adult years [27]. This may set up a challenging feedback loop wherein these vulnerable young adults are faced with greater stigma and consequently receive lower quality care, even when there is an attempt to carry out a standardized protocol.
We did not find that the QI intervention resulted in decreased admissions to the inpatient unit, with 68% of visits resulting in admission. In a recent pediatric SCD study, hospital admissions for pain control accounted for 78% of all admissions and 70% of readmissions within 30 days [28]. The investigators found that use of a SCD analgesic protocol including patient-controlled analgesia (PCA) improved quality of care as well as hospital readmission rates within 30 days (from 28% to 11%). Our ED QI protocol focused on only the first 90 minutes of the visit for pain. Our team has discussed the potential for starting the PCA in the ED and we should build on our success to focus on specific care that patients receive beyond their initial presentation. Further, we introduced pain action planning into outpatient care and need to continue to improve positive patient self-management strategies to ensure more seamless transition of pain management between home, ED, and inpatient settings.
Several valuable lessons were learned over the course of the ED QI initiative. Previous researchers [28] have emphasized the importance of coupling provider education with standardized order sets in efforts to improve the care of patients with SCD. Although we did not offer monthly formal education to our providers, the immediate follow-up when there were protocol deviations most likely served as teaching moments. These teaching moments also surfaced when some ED and hematology providers expressed concerns about the risk for oversedation with the rapid reassessment of pain and re-dosing of pain medications. Although rare, some parents also expressed that their child was being treated too vigorously with opioids. Our project highlighted the element of stigma that still accompanies the use of opioids for SCD pain management.
The project could not have been undertaken were it not for a small but determined multidisciplinary team of individuals who were personally invested in seeing the project come to fruition. The identification of physician and nurse champions who were enthusiastic about the project, invested in its conduct, and committed to its success was a cornerstone of the project’s success. These champions played an essential role in engaging staff interest in the project and oversaw the practicalities of implementing a new protocol in the ED. A spirit of collaboration, teamwork, and good communication between all involved parties was also critical. At the same time, we incorporated input from the treating ED and hematology clinicians using PDSA cycles as we were refining our protocol. We believe that our process enhanced buy-in from participating providers and clarified any issues that needed to be addressed in our setting, resulting in accelerated and sustained quality improvement.
Limitations
Although protocol-driven interventions are designed to provide a certain degree of uniformity of care, the protocol was not designed nor utilized in such a way that it superseded the best medical judgment of the treating clinicians. Deviations from the protocol were permissible when they were felt to be in the patient’s best interest. The study did not control for confounding variables such as disease severity, how long the patient had been in pain prior to coming to the ED, nor did we assess therapeutic interventions the patient had utilized at home prior to seeking out care in the ED. All of these factors could affect how well a patient might respond to treatment. We believe that sharing baseline data and monthly progress via run charts (graphs of data over time) with ED and sickle cell center staff and with consumer representatives enhanced the pace and focus of the project [23]. We had a dedicated person managing our data in real time through our HRSA funding, thus the project might not be generalizable to other institutions that do not have such staffing or access to the technology to allow project progress to be closely monitored by stakeholders.
Future Directions
With the goal of further reducing the time to administration of first analgesic dose in the ED setting, intranasal fentanyl will be utilized in our ED as the initial drug of choice for patients who do not object to or have a contraindication to its use. Collection of data from patients and family members is being undertaken to assess consumer satisfaction with the ED QI initiative. Recognizing that the ED management of acute pain addresses only one aspect of sickle cell pain, we are looking at ways to more comprehensively address pain. Individualized outpatient pain management plans are being created and patients and families are being encouraged and empowered to become active partners with their sickle cell providers in their own care. Although our initial efforts have focused on our pediatric patients, an additional aim of our project is to broaden the scope of our ED QI initiative to include community hospitals in the region that serve adult patients with SCD.
Conclusion
Implementation of a QI initiative in the ED has led to expeditious care for pediatric patients with SCD presenting with VOE. A multidisciplinary approach, ongoing staff education, and commitment to the initiative have been necessary to sustain the improvements. Our success can provide a template for other QI initiatives in the ED that translate to improved patient care for other diseases. A QI framework provided us with unique challenges but also invaluable lessons as we addressed our objective to improve outcomes for patients with SCD across the life course.
Acknowledgments: The authors wish to thank Theresa Freitas, RN, Lisa Hale, PNP, Carolyn Hoppe, MD, Ileana Mendez, RN, Helen Mitchell, Mary Rutherford, MD, Augusta Saulys, MD and the Children’s Hospital & Research Center Oakland Emergency Medicine Department and Sickle Cell Center for their support.
Corresponding author: Marsha Treadwell, PhD, Children’s Hospital & Research Center Oakland, 747 52nd St, Oakland, CA 94609, [email protected].
Funding/support: This research was conducted as part of the National Initiative for Children’s Healthcare Quality (NICHQ) Working to Improve Sickle Cell Healthcare (WISCH) project. Further support came from a grant from the Health Resources and Services Administration Sickle Cell Disease Treatment Demonstration Project Grant No. U1EMC16492 and from NIH CTSA grant UL1 RR024131. The views expressed in this publication do not necessarily reflect the views of WISCH, NICHQ, or HRSA.
1. Yusuf HR, Atrash HK, Grosse SD, et al. Emergency department visits made by patients with sickle cell disease: a descriptive study, 1999-2007. Am J Preventive Med 2010;38 (4 Suppl):S536–41.
2. Benjamin L, Dampier C, Jacox A, et al. Guideline for the management of acute and chronic pain in sickle cell disease. American Pain Society; 1999.
3. Rees DC, Olujohungbe AD, Parker NE, et al. Guidelines for the management of the acute painful crisis in sickle cell disease. Br J Haematology 2003;120:744–52.
4. Solomon LR. Pain management in adults with sickle cell disease in a medical center emergency department. J Nat Med Assoc 2010;102:1025–32.
5. Lazio MP, Costello HH, Courtney DM, et al. A comparison of analgesic management for emergency department patients with sickle cell disease and renal colic. Clin J Pain 2010;26:199–205.
6. Shenoi R, Ma L, Syblik D, Yusuf S. Emergency department crowding and analgesic delay in pediatric sickle cell pain crises. Ped Emerg Care 2011;27:911–7.
7. Tanabe P, Artz N, Mark Courtney D, et al. Adult emergency department patients with sickle cell pain crisis: a learning collaborative model to improve analgesic management. Acad Emerg Med 2010;17:399–407.
8. Zempsky WT. Evaluation and treatment of sickle cell pain in the emergency department: paths to a better future. Clin Ped Emerg Med 2010;11:265–73.
9. Haywood C Jr, Tanabe P, Naik R, et al. The impact of race and disease on sickle cell patient wait times in the emergency department. Am J Emerg Med 2013;31:651–6.
10. Solomon LR. Treatment and prevention of pain due to vaso-occlusive crises in adults with sickle cell disease: an educational void. Blood 2008;111:997–1003.
11. Ballas SK. New era dawns on sickle cell pain. Blood 2010;116:311–2.
12. Haywood C Jr, Lanzkron S, Ratanawongsa N, et al. The association of provider communication with trust among adults with sickle cell disease. J Gen Intern Med 2010;25:543–8.
13. Zempsky WT. Treatment of sickle cell pain: fostering trust and justice. JAMA 2009;302:2479–80.
14. Elander J, Lusher J, Bevan D, Telfer P. Pain management and symptoms of substance dependence among patients with sickle cell disease. Soc Sci Med 2003;57:1683–96.
15. Elander J, Lusher J, Bevan D, et al. Understanding the causes of problematic pain management in sickle cell disease: evidence that pseudoaddiction plays a more important role than genuine analgesic dependence. J Pain Sympt Manag 2004;27:156–69.
16. Smith WR, Penberthy LT, Bovbjerg VE, et al. Daily assessment of pain in adults with sickle cell disease. Ann Intern Med 2008;148:94–101.
17. Harris A, Parker N, Baker C. Adults with sickle cell. Psychol Health Med 1998;3:171–9.
18. Jenerette CM, Brewer C. Health-related stigma in young adults with sickle cell disease. J Nat Med Assoc 2010;102:1050–5.
19. Maxwell K, Streetly A, Bevan D. Experiences of hospital care and treatment seeking for pain from sickle cell disease: qualitative study. BMJ 1999;318:1585–90.
20. Oyeku SO, Wang CJ, Scoville R, et al. Hemoglobinopathy Learning Collaborative: using quality improvement (QI) to achieve equity in health care quality, coordination, and outcomes for sickle cell disease. J Health Care Poor Underserved 2012;23(3 Suppl):34–48.
21. Wang CJ, Kavanagh PL, Little AA, et al. Quality-of-care indicators for children with sickle cell disease. Pediatrics 2011;128:484–93.
22. Mansouri M, Lockyer J. A meta-analysis of continuing medical education effectiveness. J Contin Ed Health Prof 2007;27:6–15.
23. The breakthrough series: IHI’s collaborative model for achieving breakthrough improvement. Boston: Institute for Healthcare Improvement; 2003.
24. Berwick DM. Improvement, trust, and the healthcare workforce. Qual Safety Health Care 2003;12:448–52.
25. Hovlid E, Bukve O, Haug K, et al. Sustainability of healthcare improvement: what can we learn from learning theory? BMC Health Serv Res 2012;12:235.
26. Tanabe P, Hafner JW, Martinovich Z, Artz N. Adult emergency department patients with sickle cell pain crisis: results from a quality improvement learning collaborative model to improve analgesic management. Acad Emerg Med 2012;19:430–8.
27. Brousseau DC, Owens PL, Mosso AL, et al. Acute care utilization and rehospitalizations for sickle cell disease. JAMA 2010;303:1288–94.
28. Frei-Jones MJ, Field JJ, DeBaun MR. Multi-modal intervention and prospective implementation of standardized sickle cell pain admission orders reduces 30-day readmission rate. Pediatr Blood Cancer 2009;53:401–5.
1. Yusuf HR, Atrash HK, Grosse SD, et al. Emergency department visits made by patients with sickle cell disease: a descriptive study, 1999-2007. Am J Preventive Med 2010;38 (4 Suppl):S536–41.
2. Benjamin L, Dampier C, Jacox A, et al. Guideline for the management of acute and chronic pain in sickle cell disease. American Pain Society; 1999.
3. Rees DC, Olujohungbe AD, Parker NE, et al. Guidelines for the management of the acute painful crisis in sickle cell disease. Br J Haematology 2003;120:744–52.
4. Solomon LR. Pain management in adults with sickle cell disease in a medical center emergency department. J Nat Med Assoc 2010;102:1025–32.
5. Lazio MP, Costello HH, Courtney DM, et al. A comparison of analgesic management for emergency department patients with sickle cell disease and renal colic. Clin J Pain 2010;26:199–205.
6. Shenoi R, Ma L, Syblik D, Yusuf S. Emergency department crowding and analgesic delay in pediatric sickle cell pain crises. Ped Emerg Care 2011;27:911–7.
7. Tanabe P, Artz N, Mark Courtney D, et al. Adult emergency department patients with sickle cell pain crisis: a learning collaborative model to improve analgesic management. Acad Emerg Med 2010;17:399–407.
8. Zempsky WT. Evaluation and treatment of sickle cell pain in the emergency department: paths to a better future. Clin Ped Emerg Med 2010;11:265–73.
9. Haywood C Jr, Tanabe P, Naik R, et al. The impact of race and disease on sickle cell patient wait times in the emergency department. Am J Emerg Med 2013;31:651–6.
10. Solomon LR. Treatment and prevention of pain due to vaso-occlusive crises in adults with sickle cell disease: an educational void. Blood 2008;111:997–1003.
11. Ballas SK. New era dawns on sickle cell pain. Blood 2010;116:311–2.
12. Haywood C Jr, Lanzkron S, Ratanawongsa N, et al. The association of provider communication with trust among adults with sickle cell disease. J Gen Intern Med 2010;25:543–8.
13. Zempsky WT. Treatment of sickle cell pain: fostering trust and justice. JAMA 2009;302:2479–80.
14. Elander J, Lusher J, Bevan D, Telfer P. Pain management and symptoms of substance dependence among patients with sickle cell disease. Soc Sci Med 2003;57:1683–96.
15. Elander J, Lusher J, Bevan D, et al. Understanding the causes of problematic pain management in sickle cell disease: evidence that pseudoaddiction plays a more important role than genuine analgesic dependence. J Pain Sympt Manag 2004;27:156–69.
16. Smith WR, Penberthy LT, Bovbjerg VE, et al. Daily assessment of pain in adults with sickle cell disease. Ann Intern Med 2008;148:94–101.
17. Harris A, Parker N, Baker C. Adults with sickle cell. Psychol Health Med 1998;3:171–9.
18. Jenerette CM, Brewer C. Health-related stigma in young adults with sickle cell disease. J Nat Med Assoc 2010;102:1050–5.
19. Maxwell K, Streetly A, Bevan D. Experiences of hospital care and treatment seeking for pain from sickle cell disease: qualitative study. BMJ 1999;318:1585–90.
20. Oyeku SO, Wang CJ, Scoville R, et al. Hemoglobinopathy Learning Collaborative: using quality improvement (QI) to achieve equity in health care quality, coordination, and outcomes for sickle cell disease. J Health Care Poor Underserved 2012;23(3 Suppl):34–48.
21. Wang CJ, Kavanagh PL, Little AA, et al. Quality-of-care indicators for children with sickle cell disease. Pediatrics 2011;128:484–93.
22. Mansouri M, Lockyer J. A meta-analysis of continuing medical education effectiveness. J Contin Ed Health Prof 2007;27:6–15.
23. The breakthrough series: IHI’s collaborative model for achieving breakthrough improvement. Boston: Institute for Healthcare Improvement; 2003.
24. Berwick DM. Improvement, trust, and the healthcare workforce. Qual Safety Health Care 2003;12:448–52.
25. Hovlid E, Bukve O, Haug K, et al. Sustainability of healthcare improvement: what can we learn from learning theory? BMC Health Serv Res 2012;12:235.
26. Tanabe P, Hafner JW, Martinovich Z, Artz N. Adult emergency department patients with sickle cell pain crisis: results from a quality improvement learning collaborative model to improve analgesic management. Acad Emerg Med 2012;19:430–8.
27. Brousseau DC, Owens PL, Mosso AL, et al. Acute care utilization and rehospitalizations for sickle cell disease. JAMA 2010;303:1288–94.
28. Frei-Jones MJ, Field JJ, DeBaun MR. Multi-modal intervention and prospective implementation of standardized sickle cell pain admission orders reduces 30-day readmission rate. Pediatr Blood Cancer 2009;53:401–5.
Improving Care of Patients with Sickle Cell Disease and Sickle Cell Trait: The Hemoglobinopathy Learning Collaborative Series
From the National Initiative for Children’s Healthcare Quality, Boston, MA.
This month JCOM launches a series calling attention to 5 teams working to improve care for individuals with sickle cell disease and sickle cell trait in the Hemoglobinopathy Learning Collaborative (—Ed.)
Sickle cell disease affects close to 100,000 people in United States [1]. This condition is characterized by chronic anemia and unpredictable pain episodes beginning in early childhood and leading to changes in functioning, diminished health-related quality of life, end-organ damage, increased health care use, and in some cases early mortality [2–5]. Sickle cell disease is identified through universal newborn screening [6] and is found in one in 2474 newborn Americans [7], with Americans of African ancestry most frequently affected. It is estimated that over 2 million Americans are genetic carriers of the sickle cell gene.
Although there have been major advancements in sickle cell care within the past several decades, there still exist significant variations in care and mortality [8–14]. Ongoing strategies to improve patient access to efficacious treatments are essential to improve outcomes for individuals with sickle cell disease.
Recognizing the compelling need for a focused national effort to improve care for this population and the relative lack of private resources committed to it [15], Congress established 2 federal programs to enhance newborn screening and improve follow-up and care and outcomes for this population: the Sickle Cell Disease Newborn Screening Program in 2002 [16] and the Sickle Cell Disease Treatment Demonstration Program in 2004 [17]. The programs are funded by the Health Resources and Services Administration and administered by the National Initiative for Children’s Healthcare Quality (NICHQ)’s Working to Improve Sickle Cell Healthcare (WISCH) program [2]. NICHQ became the coordinating center for the programs in 2011 and 2010, respectively. Diverse grantees are now working together in a Hemoglobinopathy Learning Collaborative, coordinated and facilitated by NICHQ and its partners Boston Medical Center and the Sickle Cell Disease Association of America. The current rounds of funding continue through 2014 for the Sickle Cell Disease Treatment Demonstration Program and 2015 for the Sickle Cell Disease Newborn Screening Program.
The Hemoglobinopathy Learning Collaborative grantees are developing strategies that will result in more coordinated and appropriate care in order that individuals with sickle cell disease experience fewer complications, acute care visits, and hospitalizations; enhanced quality of life; and more compassionate and respectful treatment from the health care system. Processes are also being developed to ensure that individuals screened for sickle cell disease and sickle cell trait receive genetic counseling, education and appropriate follow-up care for their condition. The aims of the collaborative are aligned with the national quality strategy of the Triple Aim—better care, better health, and lower overall health care costs [18]. The strategies and approaches developed and tested by the teams will be disseminated to the broader sickle cell community for use in the treatment and management of individuals with sickle cell disease.
The Hemoglobinopathy Learning Collaborative’s approach is based on the structure of the Breakthrough Series Learning Collaborative [19–21], a model championed by the Institute for Healthcare Improvement that brings together health care organizations that share a commitment to making major, rapid changes in order to produce breakthrough improvements in quality. Using a process known as the Model for Improvement [22], the teams develop ideas for changes, test small-scale changes using Plan-Do-Study-Act (PDSA) cycles, and measure to determine if the changes are leading to improvement. This method can quickly identify promising ideas and adapt and develop them to into robust, reliable standard processes [2].
There are 15 improvement teams working on quality improvement projects focused on improving acute care management, provision of recommended care, transition, self-management, provider education, and screening, counseling, and education for individuals with SCD and SCT. The 5 articles in this special series span these major content areas, from improving outcomes in the emergency department using standardized order sets to assessing the readiness of adolescents to transition to adult care, to using health information technology to improve care coordination, to developing a home pain management plan, and to using patient navigators to help coordinate care and resources. The series begins with the article by Treadwell et al in this issue and will continue for the next several months. The WISCH teams will serve as leaders for sustainable and positive change for treatment of individuals with sickle cell disease and sickle cell trait in the United States. Their work is an important step towards transforming care for people with sickle cell disease, so that each person with sickle cell disease will receive the highest quality of care throughout their lifespan.
Corresponding author: Suzette Oyeku, MD, MPH, Children’s Hospital at Montefiore/Albert Einstein College of Medicine, 3444 Kossuth Ave, 1st Fl, Bronx, NY 10467, [email protected].
1. Brousseau DC, Panepinto JA, Nimmer M, et al. The number of people with sickle cell disease in the United States: national and state estimates. Am J Hematol 2010;85:77–8.
2. Oyeku SO, Wang CJ, Scoville R, et al. Hemoglobinopathy Learning Collaborative: using quality improvement (QI) to achieve equity in health care quality, coordination, and outcomes for sickle cell disease. J Health Care Poor Underserved 2012;23(3 Suppl):34-48.
3. Davis H, Moore RM Jr, Gergen PJ. Cost of hospitalizations associated with sickle cell disease in the United States. Public Health Rep 1997;112:40–3.
4. Panepinto JA. Health-related quality of life in sickle cell disease. Pediatr Blood Cancer 2008;51:5–9.
5. Steiner CA, Miller JL. Sickle cell disease patients in U.S. hospitals, 2004. In: Healthcare cost and utilization project statistical briefs. Rockville, MD: Agency for Healthcare Research and Quality; 2006.
6. National Newborn Screening & Global Resource Center. National newborn screening status report, 6 Jan 2013. Available at http://genes-r-us.uthscsa.edu/sites/genes-r-us/files/nbsdisorders.pdf.
7. Therrell BL, Hannon WH. National evaluation of US newborn screening system components. Mental Retard Dev Disabil Res Rev 2006;12:236–45.
8. Davis H, Schoendorf KC, Gergen PJ, et al. National trends in the mortality of children with sickle cell disease, 1968 through 1992. Am J Public Health 1997;87:1317–22.
9. Davis H, Gergen PJ, Moore RM Jr. Geographic differences in mortality of young children with sickle cell disease in the United States. Public Health Rep 1997;112:52–8.
10. Hamideh D, Alvarez O. Sickle cell disease related mortality in the United States (1999-2009). Pediatr Blood Cancer 2013;60:1482–6.
11. Brawley OW, Cornelius LJ, Edwards LR, et al. National Institutes of Health Consensus Development Conference statement: hydroxyurea treatment for sickle cell disease. Ann Intern Med 2008;148:932–8.
12. Raphael JL, Rattler TL, Kowalkowski MA, et al. The medical home experience among children with sickle cell disease. Pediatr Blood Cancer 2013;60:275–80.
13. Todd KH, Green C, Bonham VL, et al. Sickle cell disease related pain: crisis conflict. J Pain 2006;7:453–8.
14. Glassberg JA, Tanabe P, Chow A, et al. Emergency provider analgesic practices and attitudes toward patients with sickle cell disease. Ann Emerg Med 2013;62:293–302.
15. Smith LA, Oyeku SO, Homer C, Zuckerman B. Sickle cell disease: a question of equity and quality. Pediatrics 2006;117:1763–70.
16. 107th Congress of the United States. Departments of Labor, Health and Human Services, and Education and Related Agencies Appropriation Act, 2002 (H.R. 3061.RH). Available at www.gpo.gov/fdsys/pkg/BILLS-107hr3061rh/pdf/BILLS-107hr3061rh.pdf.
17. 108th Congress of the United States. American Jobs Creation Act of 2004 (H.R. 4520). Available at http://thomas.loc.gov/cgi-bin/bdquery/z?d108:H.R.4520.
18. U.S. Department of Health and Human Services. Report to Congress: national strategy for quality improvement in health care. Washington, DC: U.S. Department of Health and Human Services; 2011. Available at www.healthcare.gov/law/resources /reports/quality03212011a.html.
19. Kilo CM. Improving care through collaboration. Pediatrics 1999;103(1 Suppl E):384–93.
20. Institute for Healthcare Improvement. The breakthrough series: IHI’s collaborative model for achieving breakthrough improvement. Boston: Institute for Healthcare Improvement; 2003.
21. Wagner EH, Glasgow RE, Davis C, et al. Quality improvement in chronic illness care: a collaborative approach. Jt Comm J Qual Improv 2001;27:63–80.
22. Langley GJ, Nolan KM, Norman CL, et al. The improvement guide: a practical approach to enhancing organizational performance. San Francisco: Jossey-Bass; 1996.
From the National Initiative for Children’s Healthcare Quality, Boston, MA.
This month JCOM launches a series calling attention to 5 teams working to improve care for individuals with sickle cell disease and sickle cell trait in the Hemoglobinopathy Learning Collaborative (—Ed.)
Sickle cell disease affects close to 100,000 people in United States [1]. This condition is characterized by chronic anemia and unpredictable pain episodes beginning in early childhood and leading to changes in functioning, diminished health-related quality of life, end-organ damage, increased health care use, and in some cases early mortality [2–5]. Sickle cell disease is identified through universal newborn screening [6] and is found in one in 2474 newborn Americans [7], with Americans of African ancestry most frequently affected. It is estimated that over 2 million Americans are genetic carriers of the sickle cell gene.
Although there have been major advancements in sickle cell care within the past several decades, there still exist significant variations in care and mortality [8–14]. Ongoing strategies to improve patient access to efficacious treatments are essential to improve outcomes for individuals with sickle cell disease.
Recognizing the compelling need for a focused national effort to improve care for this population and the relative lack of private resources committed to it [15], Congress established 2 federal programs to enhance newborn screening and improve follow-up and care and outcomes for this population: the Sickle Cell Disease Newborn Screening Program in 2002 [16] and the Sickle Cell Disease Treatment Demonstration Program in 2004 [17]. The programs are funded by the Health Resources and Services Administration and administered by the National Initiative for Children’s Healthcare Quality (NICHQ)’s Working to Improve Sickle Cell Healthcare (WISCH) program [2]. NICHQ became the coordinating center for the programs in 2011 and 2010, respectively. Diverse grantees are now working together in a Hemoglobinopathy Learning Collaborative, coordinated and facilitated by NICHQ and its partners Boston Medical Center and the Sickle Cell Disease Association of America. The current rounds of funding continue through 2014 for the Sickle Cell Disease Treatment Demonstration Program and 2015 for the Sickle Cell Disease Newborn Screening Program.
The Hemoglobinopathy Learning Collaborative grantees are developing strategies that will result in more coordinated and appropriate care in order that individuals with sickle cell disease experience fewer complications, acute care visits, and hospitalizations; enhanced quality of life; and more compassionate and respectful treatment from the health care system. Processes are also being developed to ensure that individuals screened for sickle cell disease and sickle cell trait receive genetic counseling, education and appropriate follow-up care for their condition. The aims of the collaborative are aligned with the national quality strategy of the Triple Aim—better care, better health, and lower overall health care costs [18]. The strategies and approaches developed and tested by the teams will be disseminated to the broader sickle cell community for use in the treatment and management of individuals with sickle cell disease.
The Hemoglobinopathy Learning Collaborative’s approach is based on the structure of the Breakthrough Series Learning Collaborative [19–21], a model championed by the Institute for Healthcare Improvement that brings together health care organizations that share a commitment to making major, rapid changes in order to produce breakthrough improvements in quality. Using a process known as the Model for Improvement [22], the teams develop ideas for changes, test small-scale changes using Plan-Do-Study-Act (PDSA) cycles, and measure to determine if the changes are leading to improvement. This method can quickly identify promising ideas and adapt and develop them to into robust, reliable standard processes [2].
There are 15 improvement teams working on quality improvement projects focused on improving acute care management, provision of recommended care, transition, self-management, provider education, and screening, counseling, and education for individuals with SCD and SCT. The 5 articles in this special series span these major content areas, from improving outcomes in the emergency department using standardized order sets to assessing the readiness of adolescents to transition to adult care, to using health information technology to improve care coordination, to developing a home pain management plan, and to using patient navigators to help coordinate care and resources. The series begins with the article by Treadwell et al in this issue and will continue for the next several months. The WISCH teams will serve as leaders for sustainable and positive change for treatment of individuals with sickle cell disease and sickle cell trait in the United States. Their work is an important step towards transforming care for people with sickle cell disease, so that each person with sickle cell disease will receive the highest quality of care throughout their lifespan.
Corresponding author: Suzette Oyeku, MD, MPH, Children’s Hospital at Montefiore/Albert Einstein College of Medicine, 3444 Kossuth Ave, 1st Fl, Bronx, NY 10467, [email protected].
From the National Initiative for Children’s Healthcare Quality, Boston, MA.
This month JCOM launches a series calling attention to 5 teams working to improve care for individuals with sickle cell disease and sickle cell trait in the Hemoglobinopathy Learning Collaborative (—Ed.)
Sickle cell disease affects close to 100,000 people in United States [1]. This condition is characterized by chronic anemia and unpredictable pain episodes beginning in early childhood and leading to changes in functioning, diminished health-related quality of life, end-organ damage, increased health care use, and in some cases early mortality [2–5]. Sickle cell disease is identified through universal newborn screening [6] and is found in one in 2474 newborn Americans [7], with Americans of African ancestry most frequently affected. It is estimated that over 2 million Americans are genetic carriers of the sickle cell gene.
Although there have been major advancements in sickle cell care within the past several decades, there still exist significant variations in care and mortality [8–14]. Ongoing strategies to improve patient access to efficacious treatments are essential to improve outcomes for individuals with sickle cell disease.
Recognizing the compelling need for a focused national effort to improve care for this population and the relative lack of private resources committed to it [15], Congress established 2 federal programs to enhance newborn screening and improve follow-up and care and outcomes for this population: the Sickle Cell Disease Newborn Screening Program in 2002 [16] and the Sickle Cell Disease Treatment Demonstration Program in 2004 [17]. The programs are funded by the Health Resources and Services Administration and administered by the National Initiative for Children’s Healthcare Quality (NICHQ)’s Working to Improve Sickle Cell Healthcare (WISCH) program [2]. NICHQ became the coordinating center for the programs in 2011 and 2010, respectively. Diverse grantees are now working together in a Hemoglobinopathy Learning Collaborative, coordinated and facilitated by NICHQ and its partners Boston Medical Center and the Sickle Cell Disease Association of America. The current rounds of funding continue through 2014 for the Sickle Cell Disease Treatment Demonstration Program and 2015 for the Sickle Cell Disease Newborn Screening Program.
The Hemoglobinopathy Learning Collaborative grantees are developing strategies that will result in more coordinated and appropriate care in order that individuals with sickle cell disease experience fewer complications, acute care visits, and hospitalizations; enhanced quality of life; and more compassionate and respectful treatment from the health care system. Processes are also being developed to ensure that individuals screened for sickle cell disease and sickle cell trait receive genetic counseling, education and appropriate follow-up care for their condition. The aims of the collaborative are aligned with the national quality strategy of the Triple Aim—better care, better health, and lower overall health care costs [18]. The strategies and approaches developed and tested by the teams will be disseminated to the broader sickle cell community for use in the treatment and management of individuals with sickle cell disease.
The Hemoglobinopathy Learning Collaborative’s approach is based on the structure of the Breakthrough Series Learning Collaborative [19–21], a model championed by the Institute for Healthcare Improvement that brings together health care organizations that share a commitment to making major, rapid changes in order to produce breakthrough improvements in quality. Using a process known as the Model for Improvement [22], the teams develop ideas for changes, test small-scale changes using Plan-Do-Study-Act (PDSA) cycles, and measure to determine if the changes are leading to improvement. This method can quickly identify promising ideas and adapt and develop them to into robust, reliable standard processes [2].
There are 15 improvement teams working on quality improvement projects focused on improving acute care management, provision of recommended care, transition, self-management, provider education, and screening, counseling, and education for individuals with SCD and SCT. The 5 articles in this special series span these major content areas, from improving outcomes in the emergency department using standardized order sets to assessing the readiness of adolescents to transition to adult care, to using health information technology to improve care coordination, to developing a home pain management plan, and to using patient navigators to help coordinate care and resources. The series begins with the article by Treadwell et al in this issue and will continue for the next several months. The WISCH teams will serve as leaders for sustainable and positive change for treatment of individuals with sickle cell disease and sickle cell trait in the United States. Their work is an important step towards transforming care for people with sickle cell disease, so that each person with sickle cell disease will receive the highest quality of care throughout their lifespan.
Corresponding author: Suzette Oyeku, MD, MPH, Children’s Hospital at Montefiore/Albert Einstein College of Medicine, 3444 Kossuth Ave, 1st Fl, Bronx, NY 10467, [email protected].
1. Brousseau DC, Panepinto JA, Nimmer M, et al. The number of people with sickle cell disease in the United States: national and state estimates. Am J Hematol 2010;85:77–8.
2. Oyeku SO, Wang CJ, Scoville R, et al. Hemoglobinopathy Learning Collaborative: using quality improvement (QI) to achieve equity in health care quality, coordination, and outcomes for sickle cell disease. J Health Care Poor Underserved 2012;23(3 Suppl):34-48.
3. Davis H, Moore RM Jr, Gergen PJ. Cost of hospitalizations associated with sickle cell disease in the United States. Public Health Rep 1997;112:40–3.
4. Panepinto JA. Health-related quality of life in sickle cell disease. Pediatr Blood Cancer 2008;51:5–9.
5. Steiner CA, Miller JL. Sickle cell disease patients in U.S. hospitals, 2004. In: Healthcare cost and utilization project statistical briefs. Rockville, MD: Agency for Healthcare Research and Quality; 2006.
6. National Newborn Screening & Global Resource Center. National newborn screening status report, 6 Jan 2013. Available at http://genes-r-us.uthscsa.edu/sites/genes-r-us/files/nbsdisorders.pdf.
7. Therrell BL, Hannon WH. National evaluation of US newborn screening system components. Mental Retard Dev Disabil Res Rev 2006;12:236–45.
8. Davis H, Schoendorf KC, Gergen PJ, et al. National trends in the mortality of children with sickle cell disease, 1968 through 1992. Am J Public Health 1997;87:1317–22.
9. Davis H, Gergen PJ, Moore RM Jr. Geographic differences in mortality of young children with sickle cell disease in the United States. Public Health Rep 1997;112:52–8.
10. Hamideh D, Alvarez O. Sickle cell disease related mortality in the United States (1999-2009). Pediatr Blood Cancer 2013;60:1482–6.
11. Brawley OW, Cornelius LJ, Edwards LR, et al. National Institutes of Health Consensus Development Conference statement: hydroxyurea treatment for sickle cell disease. Ann Intern Med 2008;148:932–8.
12. Raphael JL, Rattler TL, Kowalkowski MA, et al. The medical home experience among children with sickle cell disease. Pediatr Blood Cancer 2013;60:275–80.
13. Todd KH, Green C, Bonham VL, et al. Sickle cell disease related pain: crisis conflict. J Pain 2006;7:453–8.
14. Glassberg JA, Tanabe P, Chow A, et al. Emergency provider analgesic practices and attitudes toward patients with sickle cell disease. Ann Emerg Med 2013;62:293–302.
15. Smith LA, Oyeku SO, Homer C, Zuckerman B. Sickle cell disease: a question of equity and quality. Pediatrics 2006;117:1763–70.
16. 107th Congress of the United States. Departments of Labor, Health and Human Services, and Education and Related Agencies Appropriation Act, 2002 (H.R. 3061.RH). Available at www.gpo.gov/fdsys/pkg/BILLS-107hr3061rh/pdf/BILLS-107hr3061rh.pdf.
17. 108th Congress of the United States. American Jobs Creation Act of 2004 (H.R. 4520). Available at http://thomas.loc.gov/cgi-bin/bdquery/z?d108:H.R.4520.
18. U.S. Department of Health and Human Services. Report to Congress: national strategy for quality improvement in health care. Washington, DC: U.S. Department of Health and Human Services; 2011. Available at www.healthcare.gov/law/resources /reports/quality03212011a.html.
19. Kilo CM. Improving care through collaboration. Pediatrics 1999;103(1 Suppl E):384–93.
20. Institute for Healthcare Improvement. The breakthrough series: IHI’s collaborative model for achieving breakthrough improvement. Boston: Institute for Healthcare Improvement; 2003.
21. Wagner EH, Glasgow RE, Davis C, et al. Quality improvement in chronic illness care: a collaborative approach. Jt Comm J Qual Improv 2001;27:63–80.
22. Langley GJ, Nolan KM, Norman CL, et al. The improvement guide: a practical approach to enhancing organizational performance. San Francisco: Jossey-Bass; 1996.
1. Brousseau DC, Panepinto JA, Nimmer M, et al. The number of people with sickle cell disease in the United States: national and state estimates. Am J Hematol 2010;85:77–8.
2. Oyeku SO, Wang CJ, Scoville R, et al. Hemoglobinopathy Learning Collaborative: using quality improvement (QI) to achieve equity in health care quality, coordination, and outcomes for sickle cell disease. J Health Care Poor Underserved 2012;23(3 Suppl):34-48.
3. Davis H, Moore RM Jr, Gergen PJ. Cost of hospitalizations associated with sickle cell disease in the United States. Public Health Rep 1997;112:40–3.
4. Panepinto JA. Health-related quality of life in sickle cell disease. Pediatr Blood Cancer 2008;51:5–9.
5. Steiner CA, Miller JL. Sickle cell disease patients in U.S. hospitals, 2004. In: Healthcare cost and utilization project statistical briefs. Rockville, MD: Agency for Healthcare Research and Quality; 2006.
6. National Newborn Screening & Global Resource Center. National newborn screening status report, 6 Jan 2013. Available at http://genes-r-us.uthscsa.edu/sites/genes-r-us/files/nbsdisorders.pdf.
7. Therrell BL, Hannon WH. National evaluation of US newborn screening system components. Mental Retard Dev Disabil Res Rev 2006;12:236–45.
8. Davis H, Schoendorf KC, Gergen PJ, et al. National trends in the mortality of children with sickle cell disease, 1968 through 1992. Am J Public Health 1997;87:1317–22.
9. Davis H, Gergen PJ, Moore RM Jr. Geographic differences in mortality of young children with sickle cell disease in the United States. Public Health Rep 1997;112:52–8.
10. Hamideh D, Alvarez O. Sickle cell disease related mortality in the United States (1999-2009). Pediatr Blood Cancer 2013;60:1482–6.
11. Brawley OW, Cornelius LJ, Edwards LR, et al. National Institutes of Health Consensus Development Conference statement: hydroxyurea treatment for sickle cell disease. Ann Intern Med 2008;148:932–8.
12. Raphael JL, Rattler TL, Kowalkowski MA, et al. The medical home experience among children with sickle cell disease. Pediatr Blood Cancer 2013;60:275–80.
13. Todd KH, Green C, Bonham VL, et al. Sickle cell disease related pain: crisis conflict. J Pain 2006;7:453–8.
14. Glassberg JA, Tanabe P, Chow A, et al. Emergency provider analgesic practices and attitudes toward patients with sickle cell disease. Ann Emerg Med 2013;62:293–302.
15. Smith LA, Oyeku SO, Homer C, Zuckerman B. Sickle cell disease: a question of equity and quality. Pediatrics 2006;117:1763–70.
16. 107th Congress of the United States. Departments of Labor, Health and Human Services, and Education and Related Agencies Appropriation Act, 2002 (H.R. 3061.RH). Available at www.gpo.gov/fdsys/pkg/BILLS-107hr3061rh/pdf/BILLS-107hr3061rh.pdf.
17. 108th Congress of the United States. American Jobs Creation Act of 2004 (H.R. 4520). Available at http://thomas.loc.gov/cgi-bin/bdquery/z?d108:H.R.4520.
18. U.S. Department of Health and Human Services. Report to Congress: national strategy for quality improvement in health care. Washington, DC: U.S. Department of Health and Human Services; 2011. Available at www.healthcare.gov/law/resources /reports/quality03212011a.html.
19. Kilo CM. Improving care through collaboration. Pediatrics 1999;103(1 Suppl E):384–93.
20. Institute for Healthcare Improvement. The breakthrough series: IHI’s collaborative model for achieving breakthrough improvement. Boston: Institute for Healthcare Improvement; 2003.
21. Wagner EH, Glasgow RE, Davis C, et al. Quality improvement in chronic illness care: a collaborative approach. Jt Comm J Qual Improv 2001;27:63–80.
22. Langley GJ, Nolan KM, Norman CL, et al. The improvement guide: a practical approach to enhancing organizational performance. San Francisco: Jossey-Bass; 1996.
CHMP recommends idelalisib for CLL, FL

Days after gaining approval for 3 indications in the US, idelalisib (Zydelig) has earned a positive opinion from the European Medicine Agency’s Committee for Medicinal Products for Human Use (CHMP).
The CHMP is recommending the PI3K delta inhibitor for the treatment of chronic lymphocytic leukemia (CLL) and follicular lymphoma (FL).
If approved, the drug would be used as monotherapy for adults with FL that is refractory to 2 prior lines of treatment.
Idelalisib would also be used in combination with rituximab for adults with CLL who have received at least 1 prior therapy or as first-line treatment in CLL patients who have 17p deletion or TP53 mutation and cannot receive chemo-immunotherapy.
The CHMP’s recommendation for idelalisib (150 mg film-coated tablets) will be reviewed by the European Commission, which has the authority to approve medicines for use in the 28 countries of the European Union.
The CHMP’s positive opinion of idelalisib is based on data from 2 clinical trials—Study 116 and Study 101-09.
Study 116: Idelalisib in CLL
This phase 3 trial was stopped early because idelalisib had a significant impact on progression-free survival.
The study included 220 CLL patients who could not receive chemotherapy. Half were randomized to receive idelalisib plus rituximab, and the other half were randomized to rituximab plus placebo.
Patients in the idelalisib arm had a much higher overall response rate than patients in the placebo arm—81% and 13%, respectively (P<0.001). But all responses were partial.
At 24 weeks, the rate of progression-free survival was 93% in the idelalisib arm and 46% in the placebo arm (P<0.001). The median progression-free survival was 5.5 months in the placebo arm and not reached in the idelalisib arm (P<0.001).
At 12 months, the overall survival rate was 92% in the idelalisib arm and 80% in the placebo arm (P=0.02).
Most adverse events, in either treatment arm, were grade 2 or lower. The most common events in the idelalisib arm were pyrexia, fatigue, nausea, chills, and diarrhea. In the placebo arm, the most common events were infusion-related reactions, fatigue, cough, nausea, and dyspnea.
There were more serious adverse events in the idelalisib arm than in the placebo arm—40% and 35%, respectively. The most frequent serious events were pneumonia, pyrexia, and febrile neutropenia (in both treatment arms).
Study 101-09: Idelalisib in FL
In this phase 2 trial, idelalisib was given as a single agent to patients with indolent non-Hodgkin lymphoma who were refractory to rituximab and chemotherapy containing an alkylating agent.
In the 72 patients with FL, the overall response rate was 54%, and the complete response rate was 8%. The median duration of response was not reached (range, 0-14.8 months).
Improvements in patient survival or disease-related symptoms have not been established.
The most common grade 3 or higher adverse events were neutropenia (27%), elevations in aminotransferase levels (13%), diarrhea (13%), and pneumonia (7%).
About idelalisib
Idelalisib is an oral inhibitor of PI3K delta, a protein that plays a role in the activation, proliferation, and viability of B cells. PI3K delta signaling is active in many B-cell leukemias and lymphomas, and, by inhibiting the protein, idelalisib blocks several cellular signaling pathways that drive B-cell viability.
Idelalisib is being developed by Gilead Sciences. On July 23, the drug received US Food and Drug Administration approval for use in combination with rituximab to treat patients with relapsed CLL who cannot receive rituximab alone. The agency also granted idelalisib accelerated approval to treat patients with relapsed FL or small lymphocytic lymphoma who have received at least 2 prior systemic therapies. ![]()

Days after gaining approval for 3 indications in the US, idelalisib (Zydelig) has earned a positive opinion from the European Medicine Agency’s Committee for Medicinal Products for Human Use (CHMP).
The CHMP is recommending the PI3K delta inhibitor for the treatment of chronic lymphocytic leukemia (CLL) and follicular lymphoma (FL).
If approved, the drug would be used as monotherapy for adults with FL that is refractory to 2 prior lines of treatment.
Idelalisib would also be used in combination with rituximab for adults with CLL who have received at least 1 prior therapy or as first-line treatment in CLL patients who have 17p deletion or TP53 mutation and cannot receive chemo-immunotherapy.
The CHMP’s recommendation for idelalisib (150 mg film-coated tablets) will be reviewed by the European Commission, which has the authority to approve medicines for use in the 28 countries of the European Union.
The CHMP’s positive opinion of idelalisib is based on data from 2 clinical trials—Study 116 and Study 101-09.
Study 116: Idelalisib in CLL
This phase 3 trial was stopped early because idelalisib had a significant impact on progression-free survival.
The study included 220 CLL patients who could not receive chemotherapy. Half were randomized to receive idelalisib plus rituximab, and the other half were randomized to rituximab plus placebo.
Patients in the idelalisib arm had a much higher overall response rate than patients in the placebo arm—81% and 13%, respectively (P<0.001). But all responses were partial.
At 24 weeks, the rate of progression-free survival was 93% in the idelalisib arm and 46% in the placebo arm (P<0.001). The median progression-free survival was 5.5 months in the placebo arm and not reached in the idelalisib arm (P<0.001).
At 12 months, the overall survival rate was 92% in the idelalisib arm and 80% in the placebo arm (P=0.02).
Most adverse events, in either treatment arm, were grade 2 or lower. The most common events in the idelalisib arm were pyrexia, fatigue, nausea, chills, and diarrhea. In the placebo arm, the most common events were infusion-related reactions, fatigue, cough, nausea, and dyspnea.
There were more serious adverse events in the idelalisib arm than in the placebo arm—40% and 35%, respectively. The most frequent serious events were pneumonia, pyrexia, and febrile neutropenia (in both treatment arms).
Study 101-09: Idelalisib in FL
In this phase 2 trial, idelalisib was given as a single agent to patients with indolent non-Hodgkin lymphoma who were refractory to rituximab and chemotherapy containing an alkylating agent.
In the 72 patients with FL, the overall response rate was 54%, and the complete response rate was 8%. The median duration of response was not reached (range, 0-14.8 months).
Improvements in patient survival or disease-related symptoms have not been established.
The most common grade 3 or higher adverse events were neutropenia (27%), elevations in aminotransferase levels (13%), diarrhea (13%), and pneumonia (7%).
About idelalisib
Idelalisib is an oral inhibitor of PI3K delta, a protein that plays a role in the activation, proliferation, and viability of B cells. PI3K delta signaling is active in many B-cell leukemias and lymphomas, and, by inhibiting the protein, idelalisib blocks several cellular signaling pathways that drive B-cell viability.
Idelalisib is being developed by Gilead Sciences. On July 23, the drug received US Food and Drug Administration approval for use in combination with rituximab to treat patients with relapsed CLL who cannot receive rituximab alone. The agency also granted idelalisib accelerated approval to treat patients with relapsed FL or small lymphocytic lymphoma who have received at least 2 prior systemic therapies. ![]()

Days after gaining approval for 3 indications in the US, idelalisib (Zydelig) has earned a positive opinion from the European Medicine Agency’s Committee for Medicinal Products for Human Use (CHMP).
The CHMP is recommending the PI3K delta inhibitor for the treatment of chronic lymphocytic leukemia (CLL) and follicular lymphoma (FL).
If approved, the drug would be used as monotherapy for adults with FL that is refractory to 2 prior lines of treatment.
Idelalisib would also be used in combination with rituximab for adults with CLL who have received at least 1 prior therapy or as first-line treatment in CLL patients who have 17p deletion or TP53 mutation and cannot receive chemo-immunotherapy.
The CHMP’s recommendation for idelalisib (150 mg film-coated tablets) will be reviewed by the European Commission, which has the authority to approve medicines for use in the 28 countries of the European Union.
The CHMP’s positive opinion of idelalisib is based on data from 2 clinical trials—Study 116 and Study 101-09.
Study 116: Idelalisib in CLL
This phase 3 trial was stopped early because idelalisib had a significant impact on progression-free survival.
The study included 220 CLL patients who could not receive chemotherapy. Half were randomized to receive idelalisib plus rituximab, and the other half were randomized to rituximab plus placebo.
Patients in the idelalisib arm had a much higher overall response rate than patients in the placebo arm—81% and 13%, respectively (P<0.001). But all responses were partial.
At 24 weeks, the rate of progression-free survival was 93% in the idelalisib arm and 46% in the placebo arm (P<0.001). The median progression-free survival was 5.5 months in the placebo arm and not reached in the idelalisib arm (P<0.001).
At 12 months, the overall survival rate was 92% in the idelalisib arm and 80% in the placebo arm (P=0.02).
Most adverse events, in either treatment arm, were grade 2 or lower. The most common events in the idelalisib arm were pyrexia, fatigue, nausea, chills, and diarrhea. In the placebo arm, the most common events were infusion-related reactions, fatigue, cough, nausea, and dyspnea.
There were more serious adverse events in the idelalisib arm than in the placebo arm—40% and 35%, respectively. The most frequent serious events were pneumonia, pyrexia, and febrile neutropenia (in both treatment arms).
Study 101-09: Idelalisib in FL
In this phase 2 trial, idelalisib was given as a single agent to patients with indolent non-Hodgkin lymphoma who were refractory to rituximab and chemotherapy containing an alkylating agent.
In the 72 patients with FL, the overall response rate was 54%, and the complete response rate was 8%. The median duration of response was not reached (range, 0-14.8 months).
Improvements in patient survival or disease-related symptoms have not been established.
The most common grade 3 or higher adverse events were neutropenia (27%), elevations in aminotransferase levels (13%), diarrhea (13%), and pneumonia (7%).
About idelalisib
Idelalisib is an oral inhibitor of PI3K delta, a protein that plays a role in the activation, proliferation, and viability of B cells. PI3K delta signaling is active in many B-cell leukemias and lymphomas, and, by inhibiting the protein, idelalisib blocks several cellular signaling pathways that drive B-cell viability.
Idelalisib is being developed by Gilead Sciences. On July 23, the drug received US Food and Drug Administration approval for use in combination with rituximab to treat patients with relapsed CLL who cannot receive rituximab alone. The agency also granted idelalisib accelerated approval to treat patients with relapsed FL or small lymphocytic lymphoma who have received at least 2 prior systemic therapies. ![]()