User login
How to identify and localize IUDs on ultrasound
Although an ultrasound is not required after uncomplicated placement of an intrauterine device (IUD) or during routine management of women who are doing well with an IUD, it is invaluable in the evaluation of patients who present with pain or other symptoms suggestive of IUD malpositioning.
In this article, we outline the sonographic features of the IUDs available today in the United States and describe the basics of localization by ultrasound.
Related articles: STOP relying on 2D ultrasound for IUD localization. Steven R. Goldstein, MD, and Chrystie Fujimoto, MD (August 2014)
Update on Contraception. Melissa J. Chen, MD, MPH, and Mitchell D. Creinin, MD (August 2014)
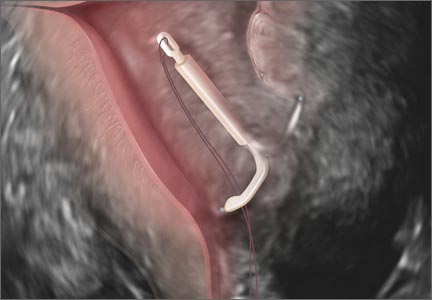
Ultrasound features of IUDsWhen positioned normally, an IUD is centrally located within the endometrial cavity, with the crossbar positioned in the fundal area.1 Copper and progestin-releasing IUDs can be identified easily on ultrasound if one is familiar with their basic sonographic features:
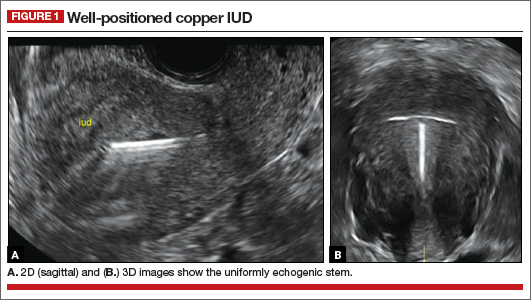
- Copper IUD: The central stem is uniformly echogenic due to its copper coils (FIGURE 1)
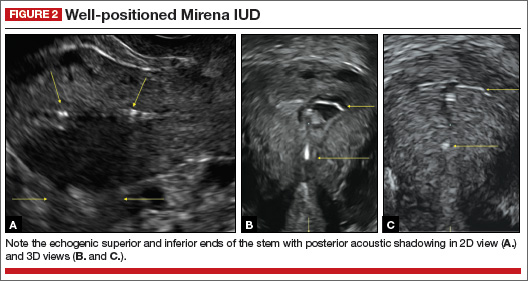
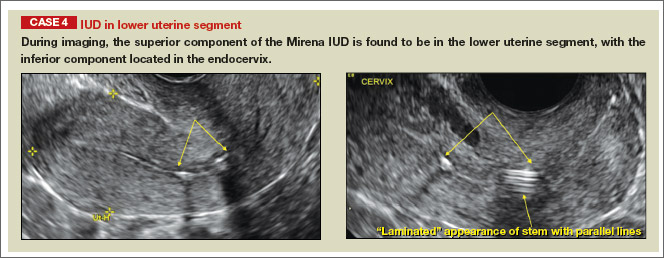
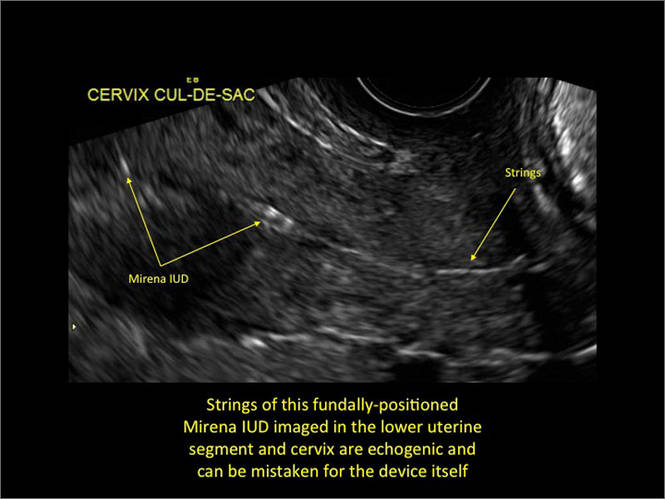
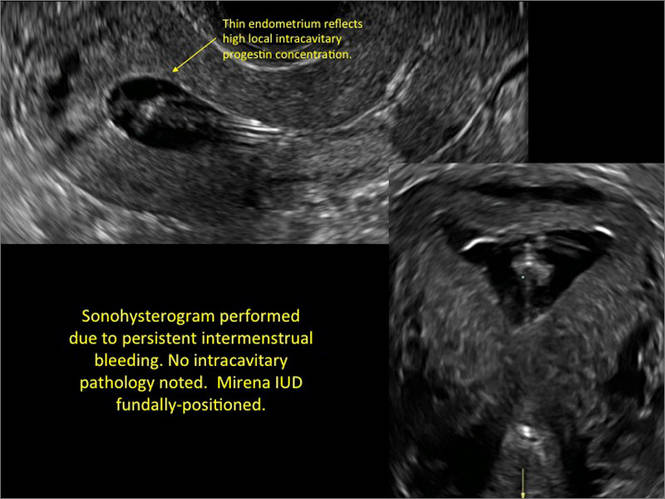
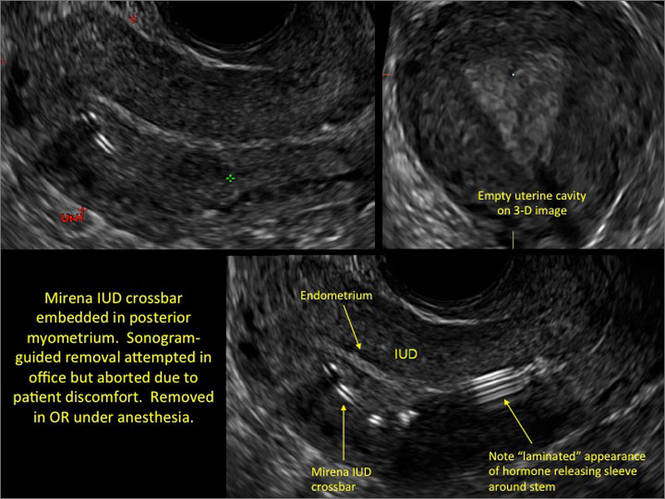
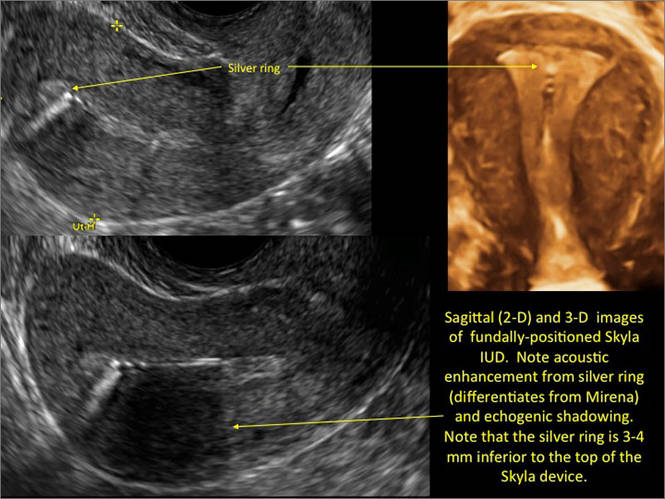
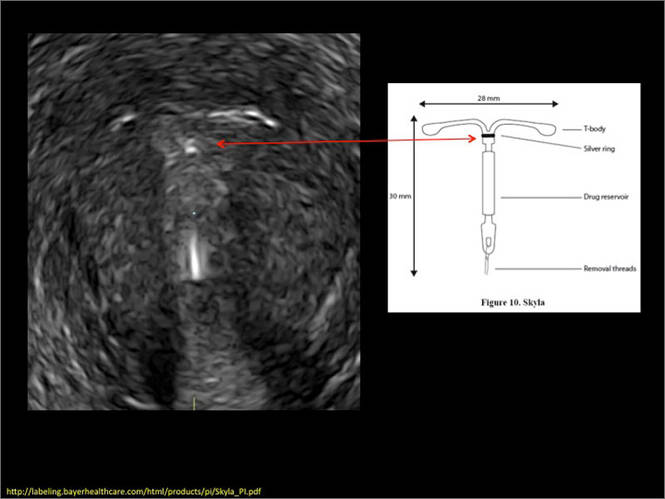
- Levonorgestrel-releasing intrauterine system (LNG-IUS): The LNG-IUS consists of a plastic sleeve that contains the progestin and surrounds a central stem. This configuration causes acoustic shadowing and has a characteristic “laminated” sonographic appearance with parallel lines (FIGURE 2). The Mirena IUD has echogenic arms due to barium sulfate, as well as an echogenic distal tip, with acoustic shadowing from the stem. Skyla is similar except for a highly echogenic silver ring on the stem approximately 3 to 4 mm inferior to the crossbar. On occasion, the echogenic strings of Mirena and Skyla can be mistaken for the device.
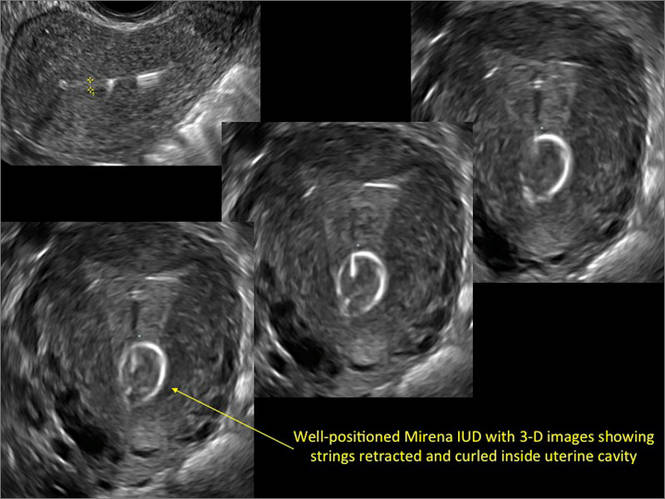
Three-dimensional ultrasound is useful in imaging of an IUD. If a patient’s IUD cannot be visualized by ultrasound, plain radiography of the kidney, ureter, and bladder may be helpful. If an IUD is not apparent on plain film, consider that it may have been expelled.
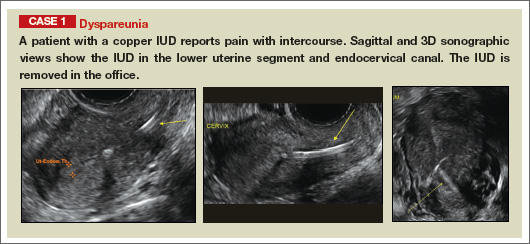
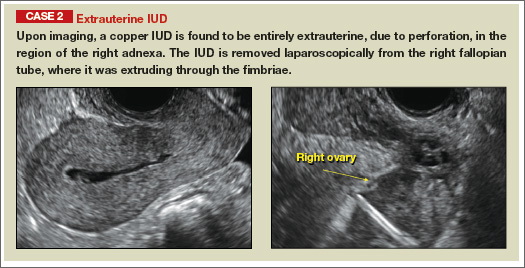
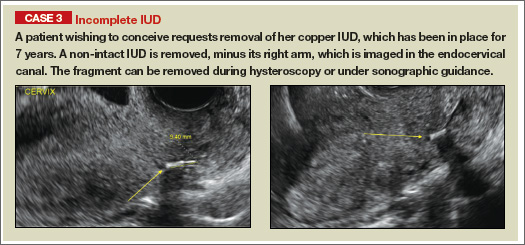
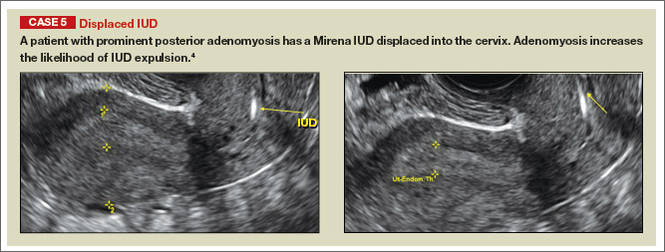
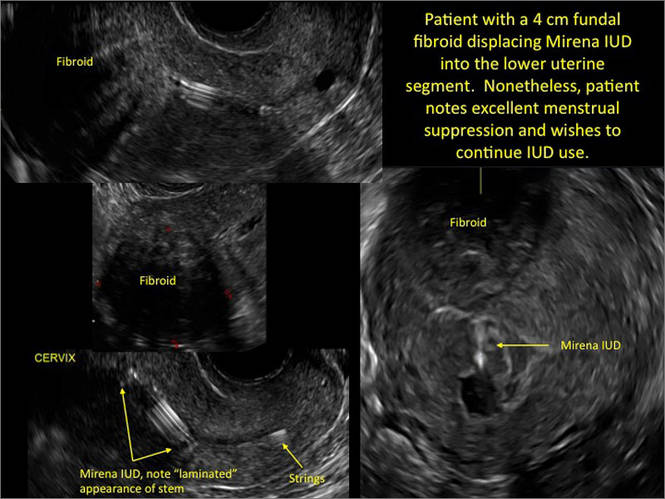
Potential malpositioningA malpositioned IUD may be partially expelled, rotated, embedded in the myometrium, or perforating the uterine serosa.
Related article: Malpositioned IUDs: When you should intervene (and when you should not). Kari Braaten, MD, MPH, and Alisa B. Goldberg, MD, MPH (August 2012)
In a retrospective case-control study that compared 182 women with sonographicallyidentified malpositioned IUDs with 182 women with properly positioned IUDs, Braaten and colleagues found that suspected adenomyosis was associated with malpositioning (odds ratio [OR], 3.04; 95% confidence interval [CI], 1.08–8.52), but a history of vaginal delivery was protective (OR, 0.53; 95% CI, 0.32–0.87).2 A distorted uterine cavity also increases the risk of malpositioning.3
| |
| |
| |
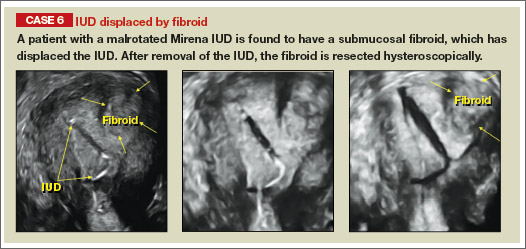
| |
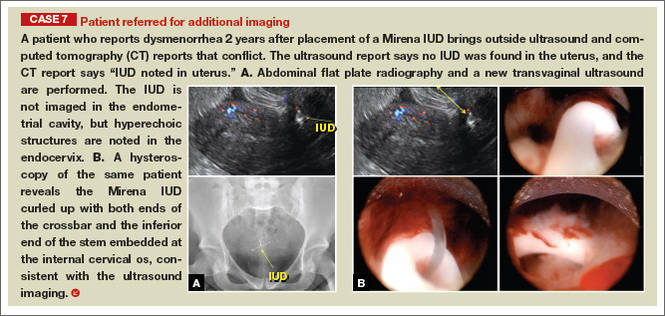
|
Although no uterine perforations were reported in a review of the LNG-IUS, expulsions were reported and may be more common among women who use the IUD for heavy menstrual bleeding.4
Additional images
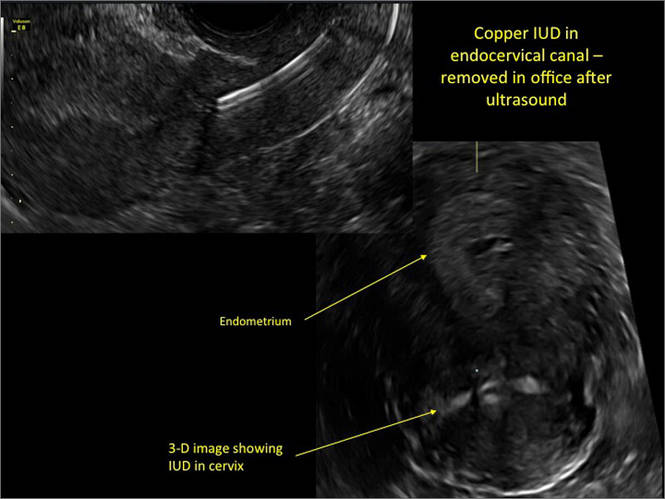
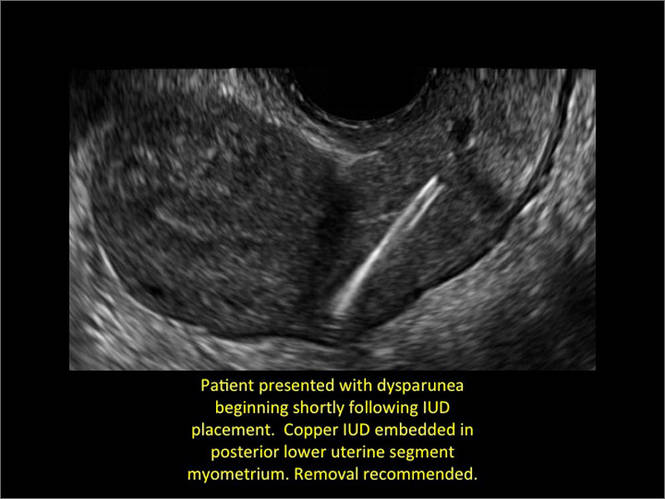
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
1. Peri N, Graha D, Levine D. Imaging of intrauterine contraceptive devices. J Ultrasound Med. 2007;26(10):1389–1401.
2. Braaten KP, Benson CB, Maurer R, Goldberg AB. Malpositioned intrauterine contraceptive devices: Risk factors, outcomes, and future pregnancies. Obstet Gynecol. 2011;118(5):1014–1020.
3. Braaten KP, Goldberg AB. Malpositioned IUDs: When you should intervene and when you should not. OBG Manag. 2012;24(8):39–46.
4. Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding: a benefit-risk review. Drugs. 2012;72(2):193–215.
Although an ultrasound is not required after uncomplicated placement of an intrauterine device (IUD) or during routine management of women who are doing well with an IUD, it is invaluable in the evaluation of patients who present with pain or other symptoms suggestive of IUD malpositioning.
In this article, we outline the sonographic features of the IUDs available today in the United States and describe the basics of localization by ultrasound.
Related articles: STOP relying on 2D ultrasound for IUD localization. Steven R. Goldstein, MD, and Chrystie Fujimoto, MD (August 2014)
Update on Contraception. Melissa J. Chen, MD, MPH, and Mitchell D. Creinin, MD (August 2014)
Ultrasound features of IUDsWhen positioned normally, an IUD is centrally located within the endometrial cavity, with the crossbar positioned in the fundal area.1 Copper and progestin-releasing IUDs can be identified easily on ultrasound if one is familiar with their basic sonographic features:
- Copper IUD: The central stem is uniformly echogenic due to its copper coils (FIGURE 1)
- Levonorgestrel-releasing intrauterine system (LNG-IUS): The LNG-IUS consists of a plastic sleeve that contains the progestin and surrounds a central stem. This configuration causes acoustic shadowing and has a characteristic “laminated” sonographic appearance with parallel lines (FIGURE 2). The Mirena IUD has echogenic arms due to barium sulfate, as well as an echogenic distal tip, with acoustic shadowing from the stem. Skyla is similar except for a highly echogenic silver ring on the stem approximately 3 to 4 mm inferior to the crossbar. On occasion, the echogenic strings of Mirena and Skyla can be mistaken for the device.
Three-dimensional ultrasound is useful in imaging of an IUD. If a patient’s IUD cannot be visualized by ultrasound, plain radiography of the kidney, ureter, and bladder may be helpful. If an IUD is not apparent on plain film, consider that it may have been expelled.


Potential malpositioningA malpositioned IUD may be partially expelled, rotated, embedded in the myometrium, or perforating the uterine serosa.
Related article: Malpositioned IUDs: When you should intervene (and when you should not). Kari Braaten, MD, MPH, and Alisa B. Goldberg, MD, MPH (August 2012)
In a retrospective case-control study that compared 182 women with sonographicallyidentified malpositioned IUDs with 182 women with properly positioned IUDs, Braaten and colleagues found that suspected adenomyosis was associated with malpositioning (odds ratio [OR], 3.04; 95% confidence interval [CI], 1.08–8.52), but a history of vaginal delivery was protective (OR, 0.53; 95% CI, 0.32–0.87).2 A distorted uterine cavity also increases the risk of malpositioning.3



| |

| |

| |

| |

|
Although no uterine perforations were reported in a review of the LNG-IUS, expulsions were reported and may be more common among women who use the IUD for heavy menstrual bleeding.4
Additional images









WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
Although an ultrasound is not required after uncomplicated placement of an intrauterine device (IUD) or during routine management of women who are doing well with an IUD, it is invaluable in the evaluation of patients who present with pain or other symptoms suggestive of IUD malpositioning.
In this article, we outline the sonographic features of the IUDs available today in the United States and describe the basics of localization by ultrasound.
Related articles: STOP relying on 2D ultrasound for IUD localization. Steven R. Goldstein, MD, and Chrystie Fujimoto, MD (August 2014)
Update on Contraception. Melissa J. Chen, MD, MPH, and Mitchell D. Creinin, MD (August 2014)
Ultrasound features of IUDsWhen positioned normally, an IUD is centrally located within the endometrial cavity, with the crossbar positioned in the fundal area.1 Copper and progestin-releasing IUDs can be identified easily on ultrasound if one is familiar with their basic sonographic features:
- Copper IUD: The central stem is uniformly echogenic due to its copper coils (FIGURE 1)
- Levonorgestrel-releasing intrauterine system (LNG-IUS): The LNG-IUS consists of a plastic sleeve that contains the progestin and surrounds a central stem. This configuration causes acoustic shadowing and has a characteristic “laminated” sonographic appearance with parallel lines (FIGURE 2). The Mirena IUD has echogenic arms due to barium sulfate, as well as an echogenic distal tip, with acoustic shadowing from the stem. Skyla is similar except for a highly echogenic silver ring on the stem approximately 3 to 4 mm inferior to the crossbar. On occasion, the echogenic strings of Mirena and Skyla can be mistaken for the device.
Three-dimensional ultrasound is useful in imaging of an IUD. If a patient’s IUD cannot be visualized by ultrasound, plain radiography of the kidney, ureter, and bladder may be helpful. If an IUD is not apparent on plain film, consider that it may have been expelled.


Potential malpositioningA malpositioned IUD may be partially expelled, rotated, embedded in the myometrium, or perforating the uterine serosa.
Related article: Malpositioned IUDs: When you should intervene (and when you should not). Kari Braaten, MD, MPH, and Alisa B. Goldberg, MD, MPH (August 2012)
In a retrospective case-control study that compared 182 women with sonographicallyidentified malpositioned IUDs with 182 women with properly positioned IUDs, Braaten and colleagues found that suspected adenomyosis was associated with malpositioning (odds ratio [OR], 3.04; 95% confidence interval [CI], 1.08–8.52), but a history of vaginal delivery was protective (OR, 0.53; 95% CI, 0.32–0.87).2 A distorted uterine cavity also increases the risk of malpositioning.3



| |

| |

| |

| |

|
Although no uterine perforations were reported in a review of the LNG-IUS, expulsions were reported and may be more common among women who use the IUD for heavy menstrual bleeding.4
Additional images









WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
1. Peri N, Graha D, Levine D. Imaging of intrauterine contraceptive devices. J Ultrasound Med. 2007;26(10):1389–1401.
2. Braaten KP, Benson CB, Maurer R, Goldberg AB. Malpositioned intrauterine contraceptive devices: Risk factors, outcomes, and future pregnancies. Obstet Gynecol. 2011;118(5):1014–1020.
3. Braaten KP, Goldberg AB. Malpositioned IUDs: When you should intervene and when you should not. OBG Manag. 2012;24(8):39–46.
4. Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding: a benefit-risk review. Drugs. 2012;72(2):193–215.
1. Peri N, Graha D, Levine D. Imaging of intrauterine contraceptive devices. J Ultrasound Med. 2007;26(10):1389–1401.
2. Braaten KP, Benson CB, Maurer R, Goldberg AB. Malpositioned intrauterine contraceptive devices: Risk factors, outcomes, and future pregnancies. Obstet Gynecol. 2011;118(5):1014–1020.
3. Braaten KP, Goldberg AB. Malpositioned IUDs: When you should intervene and when you should not. OBG Manag. 2012;24(8):39–46.
4. Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding: a benefit-risk review. Drugs. 2012;72(2):193–215.
Hysteroscopic electromechanical power morcellation
One of the hottest and most controversial topics in gynecologic surgery, at present, is laparoscopic electromechanical power morcellation.
In April of this year, the Food and Drug Administration sent out a news release regarding the potential risk of spread of sarcomatous tissue at the time of this procedure. In that release, the agency "discouraged" use of laparoscopic electromechanical power morcellation. Responses came from many societies, including the American College of Obstetricians and Gynecologists and the AAGL, which indicated that laparoscopic electromechanical power morcellation could be used if proper care was taken.
I am personally proud that the Master Class in Gynecologic Surgery has been very proactive and diligent in its discussion of laparoscopic electromechanical power morcellation. This is the third in our series regarding this topic.
In our first segment, I discussed the issue of electromechanical power morcellation relative to the inadvertent spread of sarcomatous tissue. In our second in the series, Dr. Ceana Nezhat, Dr. Bernard Taylor, and Dr. Tony Shibley discussed ways to minimize this risk – including morcellation in a bag. Videos of their individual techniques of electromechanical power morcellation, as well as that of Dr. Douglas Brown, can be viewed on SurgeryU. In addition, my partner, Dr. Aarathi Cholkeri-Singh, and I have a video on SurgeryU illustrating our technique of morcellation in a bag.
This current Master Class in Gynecologic Surgery is now devoted to hysteroscopic electromechanical power morcellation. In my discussions with physicians throughout the country relative to this technique, it has become evident that some institutions have not only banned the use of electromechanical power morcellation at time of laparoscopy, but have also stopped usage of hysteroscopic electromechanical power morcellation. While neither the FDA nor the lay press has ever questioned the use of hysteroscopic morcellators, I believe it is imperative that this topic be reviewed. I am sure that it will be obvious that hysteroscopic electromechanical power morcellation has thus far proved to be a safe and effective treatment option for various pathologic entities, including submucosal uterine fibroids.
To review hysteroscopic electromechanical power morcellation, I have invited Dr. Joseph S. Sanfilippo, professor of obstetrics, gynecology, and reproductive sciences at the University of Pittsburgh and director of the division of reproductive endocrinology and infertility at Magee-Womens Hospital in Pittsburgh.
Dr. Sanfilippo is a lecturer and educator. He has written an extensive number of peer-reviewed articles, and has been a contributor to several textbooks. In addition, Dr. Sanfilippo has been and remains a very active member of the AAGL.
It is a pleasure and honor to welcome Dr. Sanfilippo to this edition of the Master Class in Gynecologic Surgery, the third installment on morcellation.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy, and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. and the medical editor of this column, Master Class. Dr. Miller disclosed that he is a consultant to Hologic and is on the speakers bureau for Smith & Nephew. Videos for this and past Master Class in Gynecology Surgery articles can be viewed on SurgeryU.
One of the hottest and most controversial topics in gynecologic surgery, at present, is laparoscopic electromechanical power morcellation.
In April of this year, the Food and Drug Administration sent out a news release regarding the potential risk of spread of sarcomatous tissue at the time of this procedure. In that release, the agency "discouraged" use of laparoscopic electromechanical power morcellation. Responses came from many societies, including the American College of Obstetricians and Gynecologists and the AAGL, which indicated that laparoscopic electromechanical power morcellation could be used if proper care was taken.
I am personally proud that the Master Class in Gynecologic Surgery has been very proactive and diligent in its discussion of laparoscopic electromechanical power morcellation. This is the third in our series regarding this topic.
In our first segment, I discussed the issue of electromechanical power morcellation relative to the inadvertent spread of sarcomatous tissue. In our second in the series, Dr. Ceana Nezhat, Dr. Bernard Taylor, and Dr. Tony Shibley discussed ways to minimize this risk – including morcellation in a bag. Videos of their individual techniques of electromechanical power morcellation, as well as that of Dr. Douglas Brown, can be viewed on SurgeryU. In addition, my partner, Dr. Aarathi Cholkeri-Singh, and I have a video on SurgeryU illustrating our technique of morcellation in a bag.
This current Master Class in Gynecologic Surgery is now devoted to hysteroscopic electromechanical power morcellation. In my discussions with physicians throughout the country relative to this technique, it has become evident that some institutions have not only banned the use of electromechanical power morcellation at time of laparoscopy, but have also stopped usage of hysteroscopic electromechanical power morcellation. While neither the FDA nor the lay press has ever questioned the use of hysteroscopic morcellators, I believe it is imperative that this topic be reviewed. I am sure that it will be obvious that hysteroscopic electromechanical power morcellation has thus far proved to be a safe and effective treatment option for various pathologic entities, including submucosal uterine fibroids.
To review hysteroscopic electromechanical power morcellation, I have invited Dr. Joseph S. Sanfilippo, professor of obstetrics, gynecology, and reproductive sciences at the University of Pittsburgh and director of the division of reproductive endocrinology and infertility at Magee-Womens Hospital in Pittsburgh.
Dr. Sanfilippo is a lecturer and educator. He has written an extensive number of peer-reviewed articles, and has been a contributor to several textbooks. In addition, Dr. Sanfilippo has been and remains a very active member of the AAGL.
It is a pleasure and honor to welcome Dr. Sanfilippo to this edition of the Master Class in Gynecologic Surgery, the third installment on morcellation.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy, and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. and the medical editor of this column, Master Class. Dr. Miller disclosed that he is a consultant to Hologic and is on the speakers bureau for Smith & Nephew. Videos for this and past Master Class in Gynecology Surgery articles can be viewed on SurgeryU.
One of the hottest and most controversial topics in gynecologic surgery, at present, is laparoscopic electromechanical power morcellation.
In April of this year, the Food and Drug Administration sent out a news release regarding the potential risk of spread of sarcomatous tissue at the time of this procedure. In that release, the agency "discouraged" use of laparoscopic electromechanical power morcellation. Responses came from many societies, including the American College of Obstetricians and Gynecologists and the AAGL, which indicated that laparoscopic electromechanical power morcellation could be used if proper care was taken.
I am personally proud that the Master Class in Gynecologic Surgery has been very proactive and diligent in its discussion of laparoscopic electromechanical power morcellation. This is the third in our series regarding this topic.
In our first segment, I discussed the issue of electromechanical power morcellation relative to the inadvertent spread of sarcomatous tissue. In our second in the series, Dr. Ceana Nezhat, Dr. Bernard Taylor, and Dr. Tony Shibley discussed ways to minimize this risk – including morcellation in a bag. Videos of their individual techniques of electromechanical power morcellation, as well as that of Dr. Douglas Brown, can be viewed on SurgeryU. In addition, my partner, Dr. Aarathi Cholkeri-Singh, and I have a video on SurgeryU illustrating our technique of morcellation in a bag.
This current Master Class in Gynecologic Surgery is now devoted to hysteroscopic electromechanical power morcellation. In my discussions with physicians throughout the country relative to this technique, it has become evident that some institutions have not only banned the use of electromechanical power morcellation at time of laparoscopy, but have also stopped usage of hysteroscopic electromechanical power morcellation. While neither the FDA nor the lay press has ever questioned the use of hysteroscopic morcellators, I believe it is imperative that this topic be reviewed. I am sure that it will be obvious that hysteroscopic electromechanical power morcellation has thus far proved to be a safe and effective treatment option for various pathologic entities, including submucosal uterine fibroids.
To review hysteroscopic electromechanical power morcellation, I have invited Dr. Joseph S. Sanfilippo, professor of obstetrics, gynecology, and reproductive sciences at the University of Pittsburgh and director of the division of reproductive endocrinology and infertility at Magee-Womens Hospital in Pittsburgh.
Dr. Sanfilippo is a lecturer and educator. He has written an extensive number of peer-reviewed articles, and has been a contributor to several textbooks. In addition, Dr. Sanfilippo has been and remains a very active member of the AAGL.
It is a pleasure and honor to welcome Dr. Sanfilippo to this edition of the Master Class in Gynecologic Surgery, the third installment on morcellation.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy, and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. and the medical editor of this column, Master Class. Dr. Miller disclosed that he is a consultant to Hologic and is on the speakers bureau for Smith & Nephew. Videos for this and past Master Class in Gynecology Surgery articles can be viewed on SurgeryU.
Hysteroscopic morcellation – a very different entity
Submucous leiomyomas are the most problematic type of fibroid and have been associated with abnormal uterine bleeding, infertility, and other clinical issues. Treatment has been shown to be effective in improving fertility and success rates with assisted reproduction.
Newer hysteroscopic surgical techniques and morcellation technology allow us to remove not only polyps, but selected submucous myomas, in a fashion that is not only minimally invasive but that also raises few if any concerns about spreading or upstaging an unsuspected leiomyosarcoma. In this respect, the controversy over laparoscopic power morcellation does not extend to hysteroscopic morcellation.
Such a distinction was made during opening remarks at a meeting in June 2014 of the Obstetrics & Gynecology Devices Panel of the Food and Drug Administration’s Medical Devices Advisory Committee, which was charged with addressing such concerns.
Dr. Aron Yustein, deputy director of clinical affairs and chief medical officer of the FDA’s Office of Surveillance and Biometrics, explained that the panel would not address hysteroscopic morcellators "as we do not believe that when used [as intended], they pose the same risk" as that of laparoscopic morcellation in terms of potentially disseminating and upstaging an undetected uterine malignancy.
In hysteroscopic morcellation, tissue is contained and delivered through the morcellation system into a trap, or collecting pouch. This allows for complete capture and histopathologic assessment of all fragments extracted from the uterine cavity.
Numerous equipment options are currently available to gynecologic surgeons for hysteroscopically-guided myomectomy: Newer systems such as the Gynecare VersaPoint (Ethicon Endo-Surgery), and the Symphion system (Boston Scientific) facilitate bipolar electrosurgical resection. MyoSure (Hologic) and TRUCLEAR (Smith & Nephew), on the other hand, are hysteroscopic morcellators; they both use mechanical energy rather than high-frequency electrical energy to simultaneously cut and aspirate tissue.
Common to each of these options are advanced, automated fluid management systems that continuously measure distending media input and output, intrauterine distension pressure, and fluid deficit volume throughout the procedure. Such monitoring is critical to preventing excess fluid absorption and its associated complications. The new fluid management systems allow excellent visualization of the intrauterine cavity.
Benefit of Treatment
Leiomyomas, synonymously known as myomas, are among the uterine bleeding abnormalities included in a new classification system introduced in 2011 by the International Federation of Gynecology and Obstetrics. The system classifies the causes of abnormal uterine bleeding in reproductive-aged women; it is known by the acronym PALM-COEIN, for polyp, adenomyosis, leiomyoma, malignancy and hyperplasia, coagulopathy, ovulatory dysfunction, endometrial, iatrogenic, and not yet classified.
In a practice bulletin published in 2012, the American College of Obstetricians and Gynecologists endorsed the nomenclature system and provided guidelines for evaluating reproductive-aged patients with abnormal uterine bleeding (Obstet. Gynecol. 2012;120:197-206).
The diagnosis and management of submucous leiomyomas is particularly important in cases of infertility, as these types of myomas (compared with intramural or subserosal) appear to have the greatest impact on pregnancy and implantation rates.
In general, uterine myomas are found in 5%-10% of women with infertility. In 1%-3% of infertility patients, myomas are the only abnormal findings. As described in a literature review, it is believed that myomas may interfere with sperm transport or access, and with implantation. Endometrial cavity deformity, cornual ostia obstruction, altered uterine contractility, and altered endometrial development may each play a role (Obstet. Gynecol. Clin. North Am. 2006;33:145-52).
Studies evaluating the impact of myomectomy on fertility outcomes provide evidence that submucous myomas should be removed before assisted reproductive technology/in vitro fertilization. According to the AAGL’s practice guidelines on the diagnosis and management of submucous leiomyomas, it "seems clear from high-quality studies that pregnancy rates are higher after myomectomy than no or ‘placebo’ procedures" (J. Minim. Invasive Gynecol. 2012;19:152-71).
The most widely used system for categorizing submucous myomas, developed by the European Society of Gynecological Endoscopy (ESGE), breaks them into three subtypes according to how much of the lesion’s diameter is contained within the myometrium: Type 0 myomas are entirely within the endometrial cavity, while type I have less than 50% myometrial extension, and type II are 50% or more within the myometrium.
It is the ESGE type 0 submucous myomas that are appropriate for resectoscopic surgery.
(Another system known as the STEPW classification system adds other categories, taking into account factors such as topography, extension of the base, and penetration. This system is becoming more recognized and may be useful in the future for evaluating patients for resectoscopic surgery and predicting outcomes, but it is not being used as often as the ESGE classification system.)
As the AAGL guidelines state, diagnosis is generally achieved with one or a combination of hysteroscopic and radiological techniques that may include transvaginal ultrasonography, saline infusion sonohysterography, and magnetic resonance imaging.
Research on safety
Hysteroscopic morcellation is the most recent operative hysteroscopic technique to be employed for the removal of submucous leiomyomas. In lieu of concerns about laparoscopic power morcellation, the question arises: Should we be concerned about cancer and hysteroscopy?
Numerous studies have looked at the question of whether hysteroscopic procedures produce intraperitoneal spread of endometrial cancer cells and, if so, whether this results in the "upstaging" of unsuspected cancer. Much of the research has involved diagnostic hysteroscopy, which includes the use of intrauterine cavity distension with fluid media, similar to that of operative hysteroscopy.
Investigators at Memorial Sloan-Kettering Cancer Center in New York, for instance, looked retrospectively at whether initial diagnostic procedures were associated with abnormal peritoneal washings (PW) in almost 300 women who were treated for endometrial carcinoma with hysterectomy and intraoperative PW. They found no association between the initial diagnostic procedures, including hysteroscopy, and the results of peritoneal cytology (Cancer 2000;90:143-7).
Similarly, physicians in the Czech Republic compared PW done at the start of surgery in 134 patients whose endometrial carcinoma had been diagnosed by hysteroscopy with 61 patients whose cancer had been diagnosed by dilation and curettage. The results, they said, suggest that hysteroscopy does not increase the risk of penetration of tumor cells into the peritoneal cavity more than does D&C (Eur. J. Gynaecol. Oncol. 2001; 22:342-4).
Another retrospective study of 146 patients with endometrial cancer who underwent either D&C or office hysteroscopy showed that diagnostic hysteroscopy did not increase the risk of adnexal, abdominal, or retroperitoneal lymph node metastases, compared with D&C, although there was an increase in positive peritoneal cytology (Gynecol. Oncol. 2007;107:94-8).
At least two broader reviews/meta-analyses also show no evidence for an upstaging of cancer from hysteroscopic procedures performed in the presence of cancer.
A meta-analysis of 19 studies suggests that preoperative hysteroscopy resulted in a statistically significant higher risk of positive peritoneal cytology compared with no hysteroscopy, but there was no evidence to support avoiding diagnostic hysteroscopy prior to surgical intervention for endometrial cancer (Fertil. Steril. 2011;96:957-61).
A literature review covering studies published between 1980 and 2001 showed that while there might be an increased risk of peritoneal contamination by cancer cells after hysteroscopy, there is no evidence that these patients fare worse compared with patients who have undergone other diagnostic procedures (Obstet. Gynecol. Surv. 2004;59:280-4).
Surgical rather than diagnostic hysterectomy was the focus of one recent case report from Italy. The patient was a 52-year-old nulliparous woman with a leiomyosarcoma detected 2 months after a hysteroscopic resection of a presumed myoma. After resection, the myoma was determined to be an atypical "mitotically active" leiomyoma (Eur. J. Gynaecol. Oncol. 2012;33:656-7).
The authors emphasize the "rarity" of this particular finding, and the available data overall offer no evidence for an upstaging of unsuspected endometrial cancer with hysteroscopic procedures. While hysteroscopy should not be used in cases of known cancer, as it does not facilitate treatment, there are no data that should lead us to be concerned about adverse effects in the presence of cancer.
Current systems
Traditionally, resectoscopy has posed numerous challenges for the removal of intracavitary lesions: Tissue removal has been difficult and time consuming. Visibility has been disrupted by gas bubbles, tissue fragments, blood clots, and cervical mucus. Multiple insertions have been required, raising the risk of embolism (a "piston effect"). There also have been concerns about the risk of perforation and about the learning curve.
Older resectoscopes – loop-electrode resectoscopes – were designed for monopolar electrosurgery, which requires the use of nonconductive, electrolyte-free solutions for uterine distension. This limited the amount of fluid absorption that could occur before procedures needed to be stopped.
The incorporation of bipolar instrumentation – and more recently, the development of hysteroscopic morcellation systems that use reciprocating blades driven by mechanical energy rather radiofrequency electrical energy – have enabled the use of electrolyte-containing distending media (saline or Ringer’s Lactate) and, consequently, a higher allowable amount of fluid absorption.
Saline is an ideal medium: It is isotonic, nonhemolytic, nonconductive, nontoxic, and rapidly cleared. The AAGL’s Practice Guidelines for the Management of Hysteroscopic Distending Media lists an intravasation safety limit of 2,500 cc for isotonic solution, compared with a maximum limit of 1,000 cc when using hypotonic solutions (J. Minim. Invasive Gynecol. 2013;20:137-48). This higher cut-off means we can achieve the vast majority of myoma resections in one sitting.
Hysteroscopic morcellators have additional advantages, in my experience. They allow for the use of smaller-diameter hysteroscopes, which in turn requires less cervical dilation. They also have improved reciprocating blades that enable the resection of myomas in addition to endometrial polyps. Previously, the focus was primarily on hysteroscopic polypectomy.
As technology has advanced with tissue removal being instantaneous, there is simultaneous cutting and extraction, and resections are therefore quicker. Overall, there is better visualization and a lower risk of perforation. The learning curve is quicker.
In a randomized trial focused on polypectomy, hysteroscopic mechanical morcellation was superior to electrosurgical resection. The multicenter trial from the United Kingdom compared the two modalities for removal of endometrial polyps in 121 women, and found that hysteroscopic morcellation with a mechanical-based morcellator was significantly quicker for polyp removal (a median time of 5½ minutes, versus 10 minutes, approximately), less painful and more acceptable to women, and more likely to completely remove the polyps (98% compared with 83%), the investigators reported (Obstet. Gynecol. 2014;123:745-51).
The only surgical complications in either group were vasovagal reactions, which occurred in 2% (1 out of 62) and 10% (6 out of 59) of the hysteroscopic morcellation and electrosurgical resection procedures, respectively. There was one serious adverse event, with a woman treated 2 weeks after morcellation for endomyometritis.
Indeed, infection, perforation and cervical trauma, mechanical complications, and media-related complications (intravasation and gas embolism) are risks with all modalities of operative hysteroscopy and all indications. Bleeding appears rarely to be a problem with mechanical morcellation, however, as does perforation. Certainly, perforation that occurs with a nonelectrical morcellator will be significantly less complicating than when energy is engaged.
Our experience overall with resections of intracavitary polyps and small myomas via hysteroscopic morcellation in 50 cases indicates a mean operating time of 9.4 min, a mean fluid deficit of 329 milliliters, and a mean surgeon rating of 9, with 10 representing an excellent rating. We have had no intra-or postoperative hemorrhage, no obvious electrolyte changes, and uneventful recoveries.
The majority of our hysteroscopic morcellations are done under conscious sedation with the addition of a local anesthetic in the form of a paracervical block. A 200-mcg vaginal tablet of misoprostol (Cytotec) off label the night before surgery is the pretreatment strategy I most often employ for cervical preparation. To prevent infection, I prescribe one dose of a broad-spectrum antibiotic, such as a cephalosporin, to my patients receiving myomectomies.
To learn hysteroscopic morcellation, one should begin with polypectomy and move to myomectomy once comfortable. With the TRUCLEAR system, the system I use most frequently, the hysteroscopic sheath should be inserted with the obturator in place to lessen cervical trauma.
The early flow of saline will not only aid the insertion process, it will assist in achieving good visualization quickly, as will increasing the uterine pressure setting at the start of the process. After the beginning of the procedure, however, pressure is maintained at the lowest setting capable of achieving adequate distension and providing good visualization.
When morcellating pathology, one should work from the periphery to the base. The pathology is kept between the morcellator blade opening and the optics of the camera. Large myomas can be split in half, with each half approached from distal to proximal.
Running the morcellator in the open cavity for a short time will aid in clearing the visual field of debris. Overall, however, visualization with today’s hysteroscopic morcellators and advancements in fluid management is excellent. In our experience, hysteroscopic morcellation is proving to be a safe and effective tool for performing myomectomy and addressing problems of infertility and abnormal uterine bleeding.
Dr. Sanfilippo is professor of obstetrics, gynecology, and reproductive sciences at the University of Pittsburgh and director of the division of reproductive endocrinology and infertility at Magee-Womens Hospital in Pittsburgh. He is on the advisory board for Bayer Healthcare and Smith &Nephew. A lecturer and educator, Dr. Sanfilippo has written peer-reviewed articles and has been a contributor to several textbooks. He is a member of the AAGL.
Submucous leiomyomas are the most problematic type of fibroid and have been associated with abnormal uterine bleeding, infertility, and other clinical issues. Treatment has been shown to be effective in improving fertility and success rates with assisted reproduction.
Newer hysteroscopic surgical techniques and morcellation technology allow us to remove not only polyps, but selected submucous myomas, in a fashion that is not only minimally invasive but that also raises few if any concerns about spreading or upstaging an unsuspected leiomyosarcoma. In this respect, the controversy over laparoscopic power morcellation does not extend to hysteroscopic morcellation.
Such a distinction was made during opening remarks at a meeting in June 2014 of the Obstetrics & Gynecology Devices Panel of the Food and Drug Administration’s Medical Devices Advisory Committee, which was charged with addressing such concerns.
Dr. Aron Yustein, deputy director of clinical affairs and chief medical officer of the FDA’s Office of Surveillance and Biometrics, explained that the panel would not address hysteroscopic morcellators "as we do not believe that when used [as intended], they pose the same risk" as that of laparoscopic morcellation in terms of potentially disseminating and upstaging an undetected uterine malignancy.
In hysteroscopic morcellation, tissue is contained and delivered through the morcellation system into a trap, or collecting pouch. This allows for complete capture and histopathologic assessment of all fragments extracted from the uterine cavity.
Numerous equipment options are currently available to gynecologic surgeons for hysteroscopically-guided myomectomy: Newer systems such as the Gynecare VersaPoint (Ethicon Endo-Surgery), and the Symphion system (Boston Scientific) facilitate bipolar electrosurgical resection. MyoSure (Hologic) and TRUCLEAR (Smith & Nephew), on the other hand, are hysteroscopic morcellators; they both use mechanical energy rather than high-frequency electrical energy to simultaneously cut and aspirate tissue.
Common to each of these options are advanced, automated fluid management systems that continuously measure distending media input and output, intrauterine distension pressure, and fluid deficit volume throughout the procedure. Such monitoring is critical to preventing excess fluid absorption and its associated complications. The new fluid management systems allow excellent visualization of the intrauterine cavity.
Benefit of Treatment
Leiomyomas, synonymously known as myomas, are among the uterine bleeding abnormalities included in a new classification system introduced in 2011 by the International Federation of Gynecology and Obstetrics. The system classifies the causes of abnormal uterine bleeding in reproductive-aged women; it is known by the acronym PALM-COEIN, for polyp, adenomyosis, leiomyoma, malignancy and hyperplasia, coagulopathy, ovulatory dysfunction, endometrial, iatrogenic, and not yet classified.
In a practice bulletin published in 2012, the American College of Obstetricians and Gynecologists endorsed the nomenclature system and provided guidelines for evaluating reproductive-aged patients with abnormal uterine bleeding (Obstet. Gynecol. 2012;120:197-206).
The diagnosis and management of submucous leiomyomas is particularly important in cases of infertility, as these types of myomas (compared with intramural or subserosal) appear to have the greatest impact on pregnancy and implantation rates.
In general, uterine myomas are found in 5%-10% of women with infertility. In 1%-3% of infertility patients, myomas are the only abnormal findings. As described in a literature review, it is believed that myomas may interfere with sperm transport or access, and with implantation. Endometrial cavity deformity, cornual ostia obstruction, altered uterine contractility, and altered endometrial development may each play a role (Obstet. Gynecol. Clin. North Am. 2006;33:145-52).
Studies evaluating the impact of myomectomy on fertility outcomes provide evidence that submucous myomas should be removed before assisted reproductive technology/in vitro fertilization. According to the AAGL’s practice guidelines on the diagnosis and management of submucous leiomyomas, it "seems clear from high-quality studies that pregnancy rates are higher after myomectomy than no or ‘placebo’ procedures" (J. Minim. Invasive Gynecol. 2012;19:152-71).
The most widely used system for categorizing submucous myomas, developed by the European Society of Gynecological Endoscopy (ESGE), breaks them into three subtypes according to how much of the lesion’s diameter is contained within the myometrium: Type 0 myomas are entirely within the endometrial cavity, while type I have less than 50% myometrial extension, and type II are 50% or more within the myometrium.
It is the ESGE type 0 submucous myomas that are appropriate for resectoscopic surgery.
(Another system known as the STEPW classification system adds other categories, taking into account factors such as topography, extension of the base, and penetration. This system is becoming more recognized and may be useful in the future for evaluating patients for resectoscopic surgery and predicting outcomes, but it is not being used as often as the ESGE classification system.)
As the AAGL guidelines state, diagnosis is generally achieved with one or a combination of hysteroscopic and radiological techniques that may include transvaginal ultrasonography, saline infusion sonohysterography, and magnetic resonance imaging.
Research on safety
Hysteroscopic morcellation is the most recent operative hysteroscopic technique to be employed for the removal of submucous leiomyomas. In lieu of concerns about laparoscopic power morcellation, the question arises: Should we be concerned about cancer and hysteroscopy?
Numerous studies have looked at the question of whether hysteroscopic procedures produce intraperitoneal spread of endometrial cancer cells and, if so, whether this results in the "upstaging" of unsuspected cancer. Much of the research has involved diagnostic hysteroscopy, which includes the use of intrauterine cavity distension with fluid media, similar to that of operative hysteroscopy.
Investigators at Memorial Sloan-Kettering Cancer Center in New York, for instance, looked retrospectively at whether initial diagnostic procedures were associated with abnormal peritoneal washings (PW) in almost 300 women who were treated for endometrial carcinoma with hysterectomy and intraoperative PW. They found no association between the initial diagnostic procedures, including hysteroscopy, and the results of peritoneal cytology (Cancer 2000;90:143-7).
Similarly, physicians in the Czech Republic compared PW done at the start of surgery in 134 patients whose endometrial carcinoma had been diagnosed by hysteroscopy with 61 patients whose cancer had been diagnosed by dilation and curettage. The results, they said, suggest that hysteroscopy does not increase the risk of penetration of tumor cells into the peritoneal cavity more than does D&C (Eur. J. Gynaecol. Oncol. 2001; 22:342-4).
Another retrospective study of 146 patients with endometrial cancer who underwent either D&C or office hysteroscopy showed that diagnostic hysteroscopy did not increase the risk of adnexal, abdominal, or retroperitoneal lymph node metastases, compared with D&C, although there was an increase in positive peritoneal cytology (Gynecol. Oncol. 2007;107:94-8).
At least two broader reviews/meta-analyses also show no evidence for an upstaging of cancer from hysteroscopic procedures performed in the presence of cancer.
A meta-analysis of 19 studies suggests that preoperative hysteroscopy resulted in a statistically significant higher risk of positive peritoneal cytology compared with no hysteroscopy, but there was no evidence to support avoiding diagnostic hysteroscopy prior to surgical intervention for endometrial cancer (Fertil. Steril. 2011;96:957-61).
A literature review covering studies published between 1980 and 2001 showed that while there might be an increased risk of peritoneal contamination by cancer cells after hysteroscopy, there is no evidence that these patients fare worse compared with patients who have undergone other diagnostic procedures (Obstet. Gynecol. Surv. 2004;59:280-4).
Surgical rather than diagnostic hysterectomy was the focus of one recent case report from Italy. The patient was a 52-year-old nulliparous woman with a leiomyosarcoma detected 2 months after a hysteroscopic resection of a presumed myoma. After resection, the myoma was determined to be an atypical "mitotically active" leiomyoma (Eur. J. Gynaecol. Oncol. 2012;33:656-7).
The authors emphasize the "rarity" of this particular finding, and the available data overall offer no evidence for an upstaging of unsuspected endometrial cancer with hysteroscopic procedures. While hysteroscopy should not be used in cases of known cancer, as it does not facilitate treatment, there are no data that should lead us to be concerned about adverse effects in the presence of cancer.
Current systems
Traditionally, resectoscopy has posed numerous challenges for the removal of intracavitary lesions: Tissue removal has been difficult and time consuming. Visibility has been disrupted by gas bubbles, tissue fragments, blood clots, and cervical mucus. Multiple insertions have been required, raising the risk of embolism (a "piston effect"). There also have been concerns about the risk of perforation and about the learning curve.
Older resectoscopes – loop-electrode resectoscopes – were designed for monopolar electrosurgery, which requires the use of nonconductive, electrolyte-free solutions for uterine distension. This limited the amount of fluid absorption that could occur before procedures needed to be stopped.
The incorporation of bipolar instrumentation – and more recently, the development of hysteroscopic morcellation systems that use reciprocating blades driven by mechanical energy rather radiofrequency electrical energy – have enabled the use of electrolyte-containing distending media (saline or Ringer’s Lactate) and, consequently, a higher allowable amount of fluid absorption.
Saline is an ideal medium: It is isotonic, nonhemolytic, nonconductive, nontoxic, and rapidly cleared. The AAGL’s Practice Guidelines for the Management of Hysteroscopic Distending Media lists an intravasation safety limit of 2,500 cc for isotonic solution, compared with a maximum limit of 1,000 cc when using hypotonic solutions (J. Minim. Invasive Gynecol. 2013;20:137-48). This higher cut-off means we can achieve the vast majority of myoma resections in one sitting.
Hysteroscopic morcellators have additional advantages, in my experience. They allow for the use of smaller-diameter hysteroscopes, which in turn requires less cervical dilation. They also have improved reciprocating blades that enable the resection of myomas in addition to endometrial polyps. Previously, the focus was primarily on hysteroscopic polypectomy.
As technology has advanced with tissue removal being instantaneous, there is simultaneous cutting and extraction, and resections are therefore quicker. Overall, there is better visualization and a lower risk of perforation. The learning curve is quicker.
In a randomized trial focused on polypectomy, hysteroscopic mechanical morcellation was superior to electrosurgical resection. The multicenter trial from the United Kingdom compared the two modalities for removal of endometrial polyps in 121 women, and found that hysteroscopic morcellation with a mechanical-based morcellator was significantly quicker for polyp removal (a median time of 5½ minutes, versus 10 minutes, approximately), less painful and more acceptable to women, and more likely to completely remove the polyps (98% compared with 83%), the investigators reported (Obstet. Gynecol. 2014;123:745-51).
The only surgical complications in either group were vasovagal reactions, which occurred in 2% (1 out of 62) and 10% (6 out of 59) of the hysteroscopic morcellation and electrosurgical resection procedures, respectively. There was one serious adverse event, with a woman treated 2 weeks after morcellation for endomyometritis.
Indeed, infection, perforation and cervical trauma, mechanical complications, and media-related complications (intravasation and gas embolism) are risks with all modalities of operative hysteroscopy and all indications. Bleeding appears rarely to be a problem with mechanical morcellation, however, as does perforation. Certainly, perforation that occurs with a nonelectrical morcellator will be significantly less complicating than when energy is engaged.
Our experience overall with resections of intracavitary polyps and small myomas via hysteroscopic morcellation in 50 cases indicates a mean operating time of 9.4 min, a mean fluid deficit of 329 milliliters, and a mean surgeon rating of 9, with 10 representing an excellent rating. We have had no intra-or postoperative hemorrhage, no obvious electrolyte changes, and uneventful recoveries.
The majority of our hysteroscopic morcellations are done under conscious sedation with the addition of a local anesthetic in the form of a paracervical block. A 200-mcg vaginal tablet of misoprostol (Cytotec) off label the night before surgery is the pretreatment strategy I most often employ for cervical preparation. To prevent infection, I prescribe one dose of a broad-spectrum antibiotic, such as a cephalosporin, to my patients receiving myomectomies.
To learn hysteroscopic morcellation, one should begin with polypectomy and move to myomectomy once comfortable. With the TRUCLEAR system, the system I use most frequently, the hysteroscopic sheath should be inserted with the obturator in place to lessen cervical trauma.
The early flow of saline will not only aid the insertion process, it will assist in achieving good visualization quickly, as will increasing the uterine pressure setting at the start of the process. After the beginning of the procedure, however, pressure is maintained at the lowest setting capable of achieving adequate distension and providing good visualization.
When morcellating pathology, one should work from the periphery to the base. The pathology is kept between the morcellator blade opening and the optics of the camera. Large myomas can be split in half, with each half approached from distal to proximal.
Running the morcellator in the open cavity for a short time will aid in clearing the visual field of debris. Overall, however, visualization with today’s hysteroscopic morcellators and advancements in fluid management is excellent. In our experience, hysteroscopic morcellation is proving to be a safe and effective tool for performing myomectomy and addressing problems of infertility and abnormal uterine bleeding.
Dr. Sanfilippo is professor of obstetrics, gynecology, and reproductive sciences at the University of Pittsburgh and director of the division of reproductive endocrinology and infertility at Magee-Womens Hospital in Pittsburgh. He is on the advisory board for Bayer Healthcare and Smith &Nephew. A lecturer and educator, Dr. Sanfilippo has written peer-reviewed articles and has been a contributor to several textbooks. He is a member of the AAGL.
Submucous leiomyomas are the most problematic type of fibroid and have been associated with abnormal uterine bleeding, infertility, and other clinical issues. Treatment has been shown to be effective in improving fertility and success rates with assisted reproduction.
Newer hysteroscopic surgical techniques and morcellation technology allow us to remove not only polyps, but selected submucous myomas, in a fashion that is not only minimally invasive but that also raises few if any concerns about spreading or upstaging an unsuspected leiomyosarcoma. In this respect, the controversy over laparoscopic power morcellation does not extend to hysteroscopic morcellation.
Such a distinction was made during opening remarks at a meeting in June 2014 of the Obstetrics & Gynecology Devices Panel of the Food and Drug Administration’s Medical Devices Advisory Committee, which was charged with addressing such concerns.
Dr. Aron Yustein, deputy director of clinical affairs and chief medical officer of the FDA’s Office of Surveillance and Biometrics, explained that the panel would not address hysteroscopic morcellators "as we do not believe that when used [as intended], they pose the same risk" as that of laparoscopic morcellation in terms of potentially disseminating and upstaging an undetected uterine malignancy.
In hysteroscopic morcellation, tissue is contained and delivered through the morcellation system into a trap, or collecting pouch. This allows for complete capture and histopathologic assessment of all fragments extracted from the uterine cavity.
Numerous equipment options are currently available to gynecologic surgeons for hysteroscopically-guided myomectomy: Newer systems such as the Gynecare VersaPoint (Ethicon Endo-Surgery), and the Symphion system (Boston Scientific) facilitate bipolar electrosurgical resection. MyoSure (Hologic) and TRUCLEAR (Smith & Nephew), on the other hand, are hysteroscopic morcellators; they both use mechanical energy rather than high-frequency electrical energy to simultaneously cut and aspirate tissue.
Common to each of these options are advanced, automated fluid management systems that continuously measure distending media input and output, intrauterine distension pressure, and fluid deficit volume throughout the procedure. Such monitoring is critical to preventing excess fluid absorption and its associated complications. The new fluid management systems allow excellent visualization of the intrauterine cavity.
Benefit of Treatment
Leiomyomas, synonymously known as myomas, are among the uterine bleeding abnormalities included in a new classification system introduced in 2011 by the International Federation of Gynecology and Obstetrics. The system classifies the causes of abnormal uterine bleeding in reproductive-aged women; it is known by the acronym PALM-COEIN, for polyp, adenomyosis, leiomyoma, malignancy and hyperplasia, coagulopathy, ovulatory dysfunction, endometrial, iatrogenic, and not yet classified.
In a practice bulletin published in 2012, the American College of Obstetricians and Gynecologists endorsed the nomenclature system and provided guidelines for evaluating reproductive-aged patients with abnormal uterine bleeding (Obstet. Gynecol. 2012;120:197-206).
The diagnosis and management of submucous leiomyomas is particularly important in cases of infertility, as these types of myomas (compared with intramural or subserosal) appear to have the greatest impact on pregnancy and implantation rates.
In general, uterine myomas are found in 5%-10% of women with infertility. In 1%-3% of infertility patients, myomas are the only abnormal findings. As described in a literature review, it is believed that myomas may interfere with sperm transport or access, and with implantation. Endometrial cavity deformity, cornual ostia obstruction, altered uterine contractility, and altered endometrial development may each play a role (Obstet. Gynecol. Clin. North Am. 2006;33:145-52).
Studies evaluating the impact of myomectomy on fertility outcomes provide evidence that submucous myomas should be removed before assisted reproductive technology/in vitro fertilization. According to the AAGL’s practice guidelines on the diagnosis and management of submucous leiomyomas, it "seems clear from high-quality studies that pregnancy rates are higher after myomectomy than no or ‘placebo’ procedures" (J. Minim. Invasive Gynecol. 2012;19:152-71).
The most widely used system for categorizing submucous myomas, developed by the European Society of Gynecological Endoscopy (ESGE), breaks them into three subtypes according to how much of the lesion’s diameter is contained within the myometrium: Type 0 myomas are entirely within the endometrial cavity, while type I have less than 50% myometrial extension, and type II are 50% or more within the myometrium.
It is the ESGE type 0 submucous myomas that are appropriate for resectoscopic surgery.
(Another system known as the STEPW classification system adds other categories, taking into account factors such as topography, extension of the base, and penetration. This system is becoming more recognized and may be useful in the future for evaluating patients for resectoscopic surgery and predicting outcomes, but it is not being used as often as the ESGE classification system.)
As the AAGL guidelines state, diagnosis is generally achieved with one or a combination of hysteroscopic and radiological techniques that may include transvaginal ultrasonography, saline infusion sonohysterography, and magnetic resonance imaging.
Research on safety
Hysteroscopic morcellation is the most recent operative hysteroscopic technique to be employed for the removal of submucous leiomyomas. In lieu of concerns about laparoscopic power morcellation, the question arises: Should we be concerned about cancer and hysteroscopy?
Numerous studies have looked at the question of whether hysteroscopic procedures produce intraperitoneal spread of endometrial cancer cells and, if so, whether this results in the "upstaging" of unsuspected cancer. Much of the research has involved diagnostic hysteroscopy, which includes the use of intrauterine cavity distension with fluid media, similar to that of operative hysteroscopy.
Investigators at Memorial Sloan-Kettering Cancer Center in New York, for instance, looked retrospectively at whether initial diagnostic procedures were associated with abnormal peritoneal washings (PW) in almost 300 women who were treated for endometrial carcinoma with hysterectomy and intraoperative PW. They found no association between the initial diagnostic procedures, including hysteroscopy, and the results of peritoneal cytology (Cancer 2000;90:143-7).
Similarly, physicians in the Czech Republic compared PW done at the start of surgery in 134 patients whose endometrial carcinoma had been diagnosed by hysteroscopy with 61 patients whose cancer had been diagnosed by dilation and curettage. The results, they said, suggest that hysteroscopy does not increase the risk of penetration of tumor cells into the peritoneal cavity more than does D&C (Eur. J. Gynaecol. Oncol. 2001; 22:342-4).
Another retrospective study of 146 patients with endometrial cancer who underwent either D&C or office hysteroscopy showed that diagnostic hysteroscopy did not increase the risk of adnexal, abdominal, or retroperitoneal lymph node metastases, compared with D&C, although there was an increase in positive peritoneal cytology (Gynecol. Oncol. 2007;107:94-8).
At least two broader reviews/meta-analyses also show no evidence for an upstaging of cancer from hysteroscopic procedures performed in the presence of cancer.
A meta-analysis of 19 studies suggests that preoperative hysteroscopy resulted in a statistically significant higher risk of positive peritoneal cytology compared with no hysteroscopy, but there was no evidence to support avoiding diagnostic hysteroscopy prior to surgical intervention for endometrial cancer (Fertil. Steril. 2011;96:957-61).
A literature review covering studies published between 1980 and 2001 showed that while there might be an increased risk of peritoneal contamination by cancer cells after hysteroscopy, there is no evidence that these patients fare worse compared with patients who have undergone other diagnostic procedures (Obstet. Gynecol. Surv. 2004;59:280-4).
Surgical rather than diagnostic hysterectomy was the focus of one recent case report from Italy. The patient was a 52-year-old nulliparous woman with a leiomyosarcoma detected 2 months after a hysteroscopic resection of a presumed myoma. After resection, the myoma was determined to be an atypical "mitotically active" leiomyoma (Eur. J. Gynaecol. Oncol. 2012;33:656-7).
The authors emphasize the "rarity" of this particular finding, and the available data overall offer no evidence for an upstaging of unsuspected endometrial cancer with hysteroscopic procedures. While hysteroscopy should not be used in cases of known cancer, as it does not facilitate treatment, there are no data that should lead us to be concerned about adverse effects in the presence of cancer.
Current systems
Traditionally, resectoscopy has posed numerous challenges for the removal of intracavitary lesions: Tissue removal has been difficult and time consuming. Visibility has been disrupted by gas bubbles, tissue fragments, blood clots, and cervical mucus. Multiple insertions have been required, raising the risk of embolism (a "piston effect"). There also have been concerns about the risk of perforation and about the learning curve.
Older resectoscopes – loop-electrode resectoscopes – were designed for monopolar electrosurgery, which requires the use of nonconductive, electrolyte-free solutions for uterine distension. This limited the amount of fluid absorption that could occur before procedures needed to be stopped.
The incorporation of bipolar instrumentation – and more recently, the development of hysteroscopic morcellation systems that use reciprocating blades driven by mechanical energy rather radiofrequency electrical energy – have enabled the use of electrolyte-containing distending media (saline or Ringer’s Lactate) and, consequently, a higher allowable amount of fluid absorption.
Saline is an ideal medium: It is isotonic, nonhemolytic, nonconductive, nontoxic, and rapidly cleared. The AAGL’s Practice Guidelines for the Management of Hysteroscopic Distending Media lists an intravasation safety limit of 2,500 cc for isotonic solution, compared with a maximum limit of 1,000 cc when using hypotonic solutions (J. Minim. Invasive Gynecol. 2013;20:137-48). This higher cut-off means we can achieve the vast majority of myoma resections in one sitting.
Hysteroscopic morcellators have additional advantages, in my experience. They allow for the use of smaller-diameter hysteroscopes, which in turn requires less cervical dilation. They also have improved reciprocating blades that enable the resection of myomas in addition to endometrial polyps. Previously, the focus was primarily on hysteroscopic polypectomy.
As technology has advanced with tissue removal being instantaneous, there is simultaneous cutting and extraction, and resections are therefore quicker. Overall, there is better visualization and a lower risk of perforation. The learning curve is quicker.
In a randomized trial focused on polypectomy, hysteroscopic mechanical morcellation was superior to electrosurgical resection. The multicenter trial from the United Kingdom compared the two modalities for removal of endometrial polyps in 121 women, and found that hysteroscopic morcellation with a mechanical-based morcellator was significantly quicker for polyp removal (a median time of 5½ minutes, versus 10 minutes, approximately), less painful and more acceptable to women, and more likely to completely remove the polyps (98% compared with 83%), the investigators reported (Obstet. Gynecol. 2014;123:745-51).
The only surgical complications in either group were vasovagal reactions, which occurred in 2% (1 out of 62) and 10% (6 out of 59) of the hysteroscopic morcellation and electrosurgical resection procedures, respectively. There was one serious adverse event, with a woman treated 2 weeks after morcellation for endomyometritis.
Indeed, infection, perforation and cervical trauma, mechanical complications, and media-related complications (intravasation and gas embolism) are risks with all modalities of operative hysteroscopy and all indications. Bleeding appears rarely to be a problem with mechanical morcellation, however, as does perforation. Certainly, perforation that occurs with a nonelectrical morcellator will be significantly less complicating than when energy is engaged.
Our experience overall with resections of intracavitary polyps and small myomas via hysteroscopic morcellation in 50 cases indicates a mean operating time of 9.4 min, a mean fluid deficit of 329 milliliters, and a mean surgeon rating of 9, with 10 representing an excellent rating. We have had no intra-or postoperative hemorrhage, no obvious electrolyte changes, and uneventful recoveries.
The majority of our hysteroscopic morcellations are done under conscious sedation with the addition of a local anesthetic in the form of a paracervical block. A 200-mcg vaginal tablet of misoprostol (Cytotec) off label the night before surgery is the pretreatment strategy I most often employ for cervical preparation. To prevent infection, I prescribe one dose of a broad-spectrum antibiotic, such as a cephalosporin, to my patients receiving myomectomies.
To learn hysteroscopic morcellation, one should begin with polypectomy and move to myomectomy once comfortable. With the TRUCLEAR system, the system I use most frequently, the hysteroscopic sheath should be inserted with the obturator in place to lessen cervical trauma.
The early flow of saline will not only aid the insertion process, it will assist in achieving good visualization quickly, as will increasing the uterine pressure setting at the start of the process. After the beginning of the procedure, however, pressure is maintained at the lowest setting capable of achieving adequate distension and providing good visualization.
When morcellating pathology, one should work from the periphery to the base. The pathology is kept between the morcellator blade opening and the optics of the camera. Large myomas can be split in half, with each half approached from distal to proximal.
Running the morcellator in the open cavity for a short time will aid in clearing the visual field of debris. Overall, however, visualization with today’s hysteroscopic morcellators and advancements in fluid management is excellent. In our experience, hysteroscopic morcellation is proving to be a safe and effective tool for performing myomectomy and addressing problems of infertility and abnormal uterine bleeding.
Dr. Sanfilippo is professor of obstetrics, gynecology, and reproductive sciences at the University of Pittsburgh and director of the division of reproductive endocrinology and infertility at Magee-Womens Hospital in Pittsburgh. He is on the advisory board for Bayer Healthcare and Smith &Nephew. A lecturer and educator, Dr. Sanfilippo has written peer-reviewed articles and has been a contributor to several textbooks. He is a member of the AAGL.
Conventional DMARDs may be excluded from psoriatic arthritis enthesitis guidelines
NEW YORK – Conventional disease-modifying antirheumatic drugs will not be included among acceptable treatments for enthesitis related to psoriatic arthritis, according to a draft of guidelines being prepared for publication.
"The only controlled trial with a DMARD [disease-modifying antirheumatic drug] for enthesitis was conducted with sulfasalazine, and it was negative," said Dr. Evan L. Siegel, who is in group practice in rheumatology in Rockville, Md. Dr. Siegel led a group of experts preparing psoriatic arthritis enthesitis treatment recommendations to be issued by the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA).
In a preliminary report on the planned treatment recommendations, which were delivered at the joint meetings of GRAPPA and the Spondyloarthritis Research & Treatment Network (SPARTAN), Dr. Siegel reported that the new recommendations will recognize only two gradations of enthesitis: mild or moderate/severe.
"It is difficult to differentiate moderate from severe because these have not been treated as distinct entities in the clinical trials," Dr. Siegel said. Patients experience severity in regard to intensity of pain and limitation of function, either or both of which may represent severe enthesitis in any given individual patient, according to Dr. Siegel, who also noted that the number of sites of activity is also generally unhelpful.
"Significant activity at a single site may be sufficient to produce a major functional deficit in one individual, whereas the activity at multiple sites may not produce much symptomatology or functional loss in another," Dr. Siegel said.
Enthesitis, an inflammation at the site where tendons or ligaments attach to bone, has been reported in up to 70% of patients with psoriatic arthritis. In some patients, it is the dominant symptom. However, the number of treatment trials in which control of enthesitis is the primary outcome continues to be limited, according to Dr. Siegel. He acknowledged that many of the proposed treatment recommendations owed more to expert opinion than to data.
This is true of the proposed first-line recommendation, he said, which is the use of nonsteroidal anti-inflammatory drugs and physical therapy. For NSAIDs, the wording of the recommendation will emphasize the need to monitor side effects.
The expert opinion of the GRAPPA consensus group was nearly unanimous that NSAIDs and physical therapy are effective and should be tried initially in both mild and moderate/severe disease, even though Dr. Siegel said that the supportive data from controlled trials are limited.
For those with moderate/severe enthesitis not sufficiently controlled on NSAIDs, alternatives include tumor necrosis factor inhibitors, ustekinumab, and apremilast. Some supportive data are available for each of these options, even though the consensus group concluded that there is not enough comparative evidence "to recommend one over another," he said.
Rather, the consensus group is recommending that the choice of therapies beyond NSAIDs and physical therapy be individualized in relationship to comorbidities, personal preference, and other considerations.
Special wording is being developed in regard to the use of corticosteroid injections. This wording was partly inspired by a meta-analysis that associated corticosteroid injections with an adverse effect on the integrity of tendons. Although there were many criticisms of this report, there was sufficient concern among the consensus group to urge that this treatment be used with caution.
"We think that these injections should only be provided by experienced physicians," Dr. Siegel reported. The wording in the preliminary draft is that adjunctive corticosteroid injections "may be considered on an individual basis."
When published, the enthesitis guidelines will include grading for the quality of the evidence behind each recommendation as well as the relative strength of the recommendation conferred by the expert panel.
Dr. Siegel reported financial relationships with Amgen and AbbVie.
NEW YORK – Conventional disease-modifying antirheumatic drugs will not be included among acceptable treatments for enthesitis related to psoriatic arthritis, according to a draft of guidelines being prepared for publication.
"The only controlled trial with a DMARD [disease-modifying antirheumatic drug] for enthesitis was conducted with sulfasalazine, and it was negative," said Dr. Evan L. Siegel, who is in group practice in rheumatology in Rockville, Md. Dr. Siegel led a group of experts preparing psoriatic arthritis enthesitis treatment recommendations to be issued by the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA).
In a preliminary report on the planned treatment recommendations, which were delivered at the joint meetings of GRAPPA and the Spondyloarthritis Research & Treatment Network (SPARTAN), Dr. Siegel reported that the new recommendations will recognize only two gradations of enthesitis: mild or moderate/severe.
"It is difficult to differentiate moderate from severe because these have not been treated as distinct entities in the clinical trials," Dr. Siegel said. Patients experience severity in regard to intensity of pain and limitation of function, either or both of which may represent severe enthesitis in any given individual patient, according to Dr. Siegel, who also noted that the number of sites of activity is also generally unhelpful.
"Significant activity at a single site may be sufficient to produce a major functional deficit in one individual, whereas the activity at multiple sites may not produce much symptomatology or functional loss in another," Dr. Siegel said.
Enthesitis, an inflammation at the site where tendons or ligaments attach to bone, has been reported in up to 70% of patients with psoriatic arthritis. In some patients, it is the dominant symptom. However, the number of treatment trials in which control of enthesitis is the primary outcome continues to be limited, according to Dr. Siegel. He acknowledged that many of the proposed treatment recommendations owed more to expert opinion than to data.
This is true of the proposed first-line recommendation, he said, which is the use of nonsteroidal anti-inflammatory drugs and physical therapy. For NSAIDs, the wording of the recommendation will emphasize the need to monitor side effects.
The expert opinion of the GRAPPA consensus group was nearly unanimous that NSAIDs and physical therapy are effective and should be tried initially in both mild and moderate/severe disease, even though Dr. Siegel said that the supportive data from controlled trials are limited.
For those with moderate/severe enthesitis not sufficiently controlled on NSAIDs, alternatives include tumor necrosis factor inhibitors, ustekinumab, and apremilast. Some supportive data are available for each of these options, even though the consensus group concluded that there is not enough comparative evidence "to recommend one over another," he said.
Rather, the consensus group is recommending that the choice of therapies beyond NSAIDs and physical therapy be individualized in relationship to comorbidities, personal preference, and other considerations.
Special wording is being developed in regard to the use of corticosteroid injections. This wording was partly inspired by a meta-analysis that associated corticosteroid injections with an adverse effect on the integrity of tendons. Although there were many criticisms of this report, there was sufficient concern among the consensus group to urge that this treatment be used with caution.
"We think that these injections should only be provided by experienced physicians," Dr. Siegel reported. The wording in the preliminary draft is that adjunctive corticosteroid injections "may be considered on an individual basis."
When published, the enthesitis guidelines will include grading for the quality of the evidence behind each recommendation as well as the relative strength of the recommendation conferred by the expert panel.
Dr. Siegel reported financial relationships with Amgen and AbbVie.
NEW YORK – Conventional disease-modifying antirheumatic drugs will not be included among acceptable treatments for enthesitis related to psoriatic arthritis, according to a draft of guidelines being prepared for publication.
"The only controlled trial with a DMARD [disease-modifying antirheumatic drug] for enthesitis was conducted with sulfasalazine, and it was negative," said Dr. Evan L. Siegel, who is in group practice in rheumatology in Rockville, Md. Dr. Siegel led a group of experts preparing psoriatic arthritis enthesitis treatment recommendations to be issued by the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA).
In a preliminary report on the planned treatment recommendations, which were delivered at the joint meetings of GRAPPA and the Spondyloarthritis Research & Treatment Network (SPARTAN), Dr. Siegel reported that the new recommendations will recognize only two gradations of enthesitis: mild or moderate/severe.
"It is difficult to differentiate moderate from severe because these have not been treated as distinct entities in the clinical trials," Dr. Siegel said. Patients experience severity in regard to intensity of pain and limitation of function, either or both of which may represent severe enthesitis in any given individual patient, according to Dr. Siegel, who also noted that the number of sites of activity is also generally unhelpful.
"Significant activity at a single site may be sufficient to produce a major functional deficit in one individual, whereas the activity at multiple sites may not produce much symptomatology or functional loss in another," Dr. Siegel said.
Enthesitis, an inflammation at the site where tendons or ligaments attach to bone, has been reported in up to 70% of patients with psoriatic arthritis. In some patients, it is the dominant symptom. However, the number of treatment trials in which control of enthesitis is the primary outcome continues to be limited, according to Dr. Siegel. He acknowledged that many of the proposed treatment recommendations owed more to expert opinion than to data.
This is true of the proposed first-line recommendation, he said, which is the use of nonsteroidal anti-inflammatory drugs and physical therapy. For NSAIDs, the wording of the recommendation will emphasize the need to monitor side effects.
The expert opinion of the GRAPPA consensus group was nearly unanimous that NSAIDs and physical therapy are effective and should be tried initially in both mild and moderate/severe disease, even though Dr. Siegel said that the supportive data from controlled trials are limited.
For those with moderate/severe enthesitis not sufficiently controlled on NSAIDs, alternatives include tumor necrosis factor inhibitors, ustekinumab, and apremilast. Some supportive data are available for each of these options, even though the consensus group concluded that there is not enough comparative evidence "to recommend one over another," he said.
Rather, the consensus group is recommending that the choice of therapies beyond NSAIDs and physical therapy be individualized in relationship to comorbidities, personal preference, and other considerations.
Special wording is being developed in regard to the use of corticosteroid injections. This wording was partly inspired by a meta-analysis that associated corticosteroid injections with an adverse effect on the integrity of tendons. Although there were many criticisms of this report, there was sufficient concern among the consensus group to urge that this treatment be used with caution.
"We think that these injections should only be provided by experienced physicians," Dr. Siegel reported. The wording in the preliminary draft is that adjunctive corticosteroid injections "may be considered on an individual basis."
When published, the enthesitis guidelines will include grading for the quality of the evidence behind each recommendation as well as the relative strength of the recommendation conferred by the expert panel.
Dr. Siegel reported financial relationships with Amgen and AbbVie.
AT THE 2014 GRAPPA AND SPARTAN ANNUAL MEETINGS
How lymphoma affects male fertility
for semen quality testing
New research has shown that lymphoma and its treatment can impact a number of sperm characteristics, thereby reducing fertility in males.
Results also indicated that most patients eventually experience semen recovery, but the degree and timing of that recovery may depend on the patient’s diagnosis and treatment.
In this study, recovery was more likely among patients with Hodgkin lymphoma than those with non-Hodgkin lymphoma.
And recovery was both quicker and more likely among patients who did not receive alkylating chemotherapy.
On the other hand, multivariate analyses suggested that only a patient’s pre-treatment total sperm count was related to recovery.
Louis Bujan, MD, PhD, of Université de Toulouse in France, and colleagues reported these results in Fertility and Sterility.
The study included 75 patients—57 with Hodgkin lymphoma and 18 with non-Hodgkin lymphoma. The researchers collected sperm samples before patients began cancer treatment and again at later intervals: 3 months, 6 months, 12 months, and 24 months post-treatment.
The team compared patients’ sperm characteristics to those of a control group consisting of 257 healthy, fertile men.
Results revealed that lymphoma patients had impaired sperm quality even before they began treatment. Compared to fertile controls, patients had higher levels of sperm chromatin alterations and DNA fragmentation, with the only risk factor being their cancer diagnosis.
However, between 3 months and 6 months post-treatment, patients’ levels of sperm DNA fragmentation and chromatin structure damage improved. The damage level decreased relative to a patient’s own pre-treatment level of damage, while still remaining higher than damage levels in the control group.
After treatment, patients’ sperm density, total count, motility, and vitality decreased, with the lowest values seen at the 3- and 6-month marks.
Alkylating chemotherapy was more detrimental to spermatogenesis than non-alkylating drugs. Patients who received alkylating chemotherapy were more likely to cease sperm production entirely or take longer to resume sperm production than patients receiving non-alkylating chemotherapy.
Twelve months after treatment, mean sperm counts recovered to pre-treatment values for patients who had received doxorubicin, bleomycin, vinblastine, and darcarbacine (ABVD) or ABVD and radiotherapy.
But this was not the case for patients who received doxorubicin, cyclophosphamide, vincristine, and prednisone (CHOP) or mechlorethamine, oncovin, procarbazine, and prednisone (MOPP).
At the 24-month mark, 7% of patients remained azoospermic. Kaplan Meir estimates suggested that, after 24 months, most patients would recover normal sperm counts.
Recovery was projected for 92% of patients who received ABVD and radiotherapy, 90% of patients who received ABVD alone, and 61% of CHOP-treated patients. (There was no estimate for MOPP therapy, perhaps due to a low number of patients.)
A patient’s type of lymphoma appeared to impact sperm count recovery as well. Estimates suggested that, after 24 months, 86% of Hodgkin lymphoma patients would experience recovery, compared to 73% of non-Hodgkin lymphoma patients.
“While many men can look forward to their fertility returning after treatment is over, not all will be so fortunate,” said Rebecca Sokol, MD, MPH, President of the American Society for Reproductive Medicine.
“It is imperative that, prior to the initiation of therapy, counseling and sperm preservation be made available to all lymphoma patients and their partners who may want to have children in the future.” ![]()

for semen quality testing
New research has shown that lymphoma and its treatment can impact a number of sperm characteristics, thereby reducing fertility in males.
Results also indicated that most patients eventually experience semen recovery, but the degree and timing of that recovery may depend on the patient’s diagnosis and treatment.
In this study, recovery was more likely among patients with Hodgkin lymphoma than those with non-Hodgkin lymphoma.
And recovery was both quicker and more likely among patients who did not receive alkylating chemotherapy.
On the other hand, multivariate analyses suggested that only a patient’s pre-treatment total sperm count was related to recovery.
Louis Bujan, MD, PhD, of Université de Toulouse in France, and colleagues reported these results in Fertility and Sterility.
The study included 75 patients—57 with Hodgkin lymphoma and 18 with non-Hodgkin lymphoma. The researchers collected sperm samples before patients began cancer treatment and again at later intervals: 3 months, 6 months, 12 months, and 24 months post-treatment.
The team compared patients’ sperm characteristics to those of a control group consisting of 257 healthy, fertile men.
Results revealed that lymphoma patients had impaired sperm quality even before they began treatment. Compared to fertile controls, patients had higher levels of sperm chromatin alterations and DNA fragmentation, with the only risk factor being their cancer diagnosis.
However, between 3 months and 6 months post-treatment, patients’ levels of sperm DNA fragmentation and chromatin structure damage improved. The damage level decreased relative to a patient’s own pre-treatment level of damage, while still remaining higher than damage levels in the control group.
After treatment, patients’ sperm density, total count, motility, and vitality decreased, with the lowest values seen at the 3- and 6-month marks.
Alkylating chemotherapy was more detrimental to spermatogenesis than non-alkylating drugs. Patients who received alkylating chemotherapy were more likely to cease sperm production entirely or take longer to resume sperm production than patients receiving non-alkylating chemotherapy.
Twelve months after treatment, mean sperm counts recovered to pre-treatment values for patients who had received doxorubicin, bleomycin, vinblastine, and darcarbacine (ABVD) or ABVD and radiotherapy.
But this was not the case for patients who received doxorubicin, cyclophosphamide, vincristine, and prednisone (CHOP) or mechlorethamine, oncovin, procarbazine, and prednisone (MOPP).
At the 24-month mark, 7% of patients remained azoospermic. Kaplan Meir estimates suggested that, after 24 months, most patients would recover normal sperm counts.
Recovery was projected for 92% of patients who received ABVD and radiotherapy, 90% of patients who received ABVD alone, and 61% of CHOP-treated patients. (There was no estimate for MOPP therapy, perhaps due to a low number of patients.)
A patient’s type of lymphoma appeared to impact sperm count recovery as well. Estimates suggested that, after 24 months, 86% of Hodgkin lymphoma patients would experience recovery, compared to 73% of non-Hodgkin lymphoma patients.
“While many men can look forward to their fertility returning after treatment is over, not all will be so fortunate,” said Rebecca Sokol, MD, MPH, President of the American Society for Reproductive Medicine.
“It is imperative that, prior to the initiation of therapy, counseling and sperm preservation be made available to all lymphoma patients and their partners who may want to have children in the future.” ![]()

for semen quality testing
New research has shown that lymphoma and its treatment can impact a number of sperm characteristics, thereby reducing fertility in males.
Results also indicated that most patients eventually experience semen recovery, but the degree and timing of that recovery may depend on the patient’s diagnosis and treatment.
In this study, recovery was more likely among patients with Hodgkin lymphoma than those with non-Hodgkin lymphoma.
And recovery was both quicker and more likely among patients who did not receive alkylating chemotherapy.
On the other hand, multivariate analyses suggested that only a patient’s pre-treatment total sperm count was related to recovery.
Louis Bujan, MD, PhD, of Université de Toulouse in France, and colleagues reported these results in Fertility and Sterility.
The study included 75 patients—57 with Hodgkin lymphoma and 18 with non-Hodgkin lymphoma. The researchers collected sperm samples before patients began cancer treatment and again at later intervals: 3 months, 6 months, 12 months, and 24 months post-treatment.
The team compared patients’ sperm characteristics to those of a control group consisting of 257 healthy, fertile men.
Results revealed that lymphoma patients had impaired sperm quality even before they began treatment. Compared to fertile controls, patients had higher levels of sperm chromatin alterations and DNA fragmentation, with the only risk factor being their cancer diagnosis.
However, between 3 months and 6 months post-treatment, patients’ levels of sperm DNA fragmentation and chromatin structure damage improved. The damage level decreased relative to a patient’s own pre-treatment level of damage, while still remaining higher than damage levels in the control group.
After treatment, patients’ sperm density, total count, motility, and vitality decreased, with the lowest values seen at the 3- and 6-month marks.
Alkylating chemotherapy was more detrimental to spermatogenesis than non-alkylating drugs. Patients who received alkylating chemotherapy were more likely to cease sperm production entirely or take longer to resume sperm production than patients receiving non-alkylating chemotherapy.
Twelve months after treatment, mean sperm counts recovered to pre-treatment values for patients who had received doxorubicin, bleomycin, vinblastine, and darcarbacine (ABVD) or ABVD and radiotherapy.
But this was not the case for patients who received doxorubicin, cyclophosphamide, vincristine, and prednisone (CHOP) or mechlorethamine, oncovin, procarbazine, and prednisone (MOPP).
At the 24-month mark, 7% of patients remained azoospermic. Kaplan Meir estimates suggested that, after 24 months, most patients would recover normal sperm counts.
Recovery was projected for 92% of patients who received ABVD and radiotherapy, 90% of patients who received ABVD alone, and 61% of CHOP-treated patients. (There was no estimate for MOPP therapy, perhaps due to a low number of patients.)
A patient’s type of lymphoma appeared to impact sperm count recovery as well. Estimates suggested that, after 24 months, 86% of Hodgkin lymphoma patients would experience recovery, compared to 73% of non-Hodgkin lymphoma patients.
“While many men can look forward to their fertility returning after treatment is over, not all will be so fortunate,” said Rebecca Sokol, MD, MPH, President of the American Society for Reproductive Medicine.
“It is imperative that, prior to the initiation of therapy, counseling and sperm preservation be made available to all lymphoma patients and their partners who may want to have children in the future.” ![]()
Apixaban gets European approval for DVT, PE

Credit: Kevin MacKenzie
The European Commission has approved apixaban (Eliquis) to treat and prevent deep vein thrombosis (DVT) and pulmonary embolism (PE).
The approval applies to all European Union (EU) member states, as well as Iceland and Norway.
Apixaban was already approved in the EU to prevent venous thromboembolism (VTE) in adults who have undergone total hip or knee replacement surgery, and to prevent stroke and systemic embolism in adults with nonvalvular atrial fibrillation.
The new marketing authorization for apixaban follows the positive opinion issued by the European Medicines Agency’s Committee for Medicinal Products for Human Use in June and is supported by results of 2 phase 3 clinical trials, AMPLIFY and AMPLIFY-EXT.
Results of AMPLIFY
The AMPLIFY trial included 5395 patients with confirmed, symptomatic DVT or PE requiring treatment for 6 months. They had a mean age of 56.9 years, and 89.8% of randomized patients had unprovoked VTE.
About half of patients (n=2691) were randomized to receive apixaban at 10 mg twice daily for 7 days, followed by 5 mg twice daily for 6 months.
The other half (n=2704) were randomized to the standard of care, which was enoxaparin at 1 mg/kg twice daily for at least 5 days until INR ≥ 2 and warfarin (target INR range 2.0-3.0) for 6 months.
Apixaban proved noninferior to standard therapy in the combined primary endpoint of adjudicated recurrent symptomatic VTE (nonfatal DVT or PE) or VTE-related death.
This outcome occurred in 2.3% of patients in the apixaban arm and 2.7% of patients in the standard-therapy arm (P<0.0001 for noninferiority).
Apixaban also proved superior to standard therapy with regard to bleeding. The composite endpoint of major bleeding and clinically relevant, nonmajor bleeding occurred in 4.3% of patients in the apixaban arm and 9.7% of patients in the standard-therapy arm (P<0.001).
Results of AMPLIFY-EXT
The AMPLIFY-EXT trial included 2486 patients who had completed 6 to 12 months of anticoagulation treatment for DVT or PE. The mean age was 56.7 years, and 91.7% of randomized patients had unprovoked VTE.
Patients were randomized to receive apixaban at 2.5 mg (n=842), apixaban at 5 mg (n=815), or placebo (n=829).
Both apixaban doses were significantly superior to placebo (P<0.001) with regard to the primary efficacy endpoint, which was recurrent VTE or all-cause death.
During the 12-month active study period, these events occurred in 3.8% of patients in the 2.5-mg arm, 4.2% of patients in the 5-mg arm, and 11.6% of patients in the placebo arm.
The primary safety endpoint was the incidence of major bleeding, and there was no significant difference among the treatment arms. Major bleeding occurred in 0.2% of patients in the 2.5-mg arm, 0.1% of patients in the 5-mg arm, and 0.5% of patients in the placebo arm.
About apixaban
Apixaban is approved to reduce the risk of stroke and systemic embolism in adult patients with nonvalvular atrial fibrillation in the US, EU, Japan, and a number of other countries around the world.
The drug is approved to prevent VTE in adult patients who have undergone elective hip or knee replacement surgery in the US, EU, and a number of other countries.
And now, apixaban is approved for the treatment of DVT/PE and the prevention of recurrent DVT/PE in the EU. The drug is not approved for this indication in the US.
Apixaban is under joint development by Pfizer and Bristol-Myers Squibb. ![]()

Credit: Kevin MacKenzie
The European Commission has approved apixaban (Eliquis) to treat and prevent deep vein thrombosis (DVT) and pulmonary embolism (PE).
The approval applies to all European Union (EU) member states, as well as Iceland and Norway.
Apixaban was already approved in the EU to prevent venous thromboembolism (VTE) in adults who have undergone total hip or knee replacement surgery, and to prevent stroke and systemic embolism in adults with nonvalvular atrial fibrillation.
The new marketing authorization for apixaban follows the positive opinion issued by the European Medicines Agency’s Committee for Medicinal Products for Human Use in June and is supported by results of 2 phase 3 clinical trials, AMPLIFY and AMPLIFY-EXT.
Results of AMPLIFY
The AMPLIFY trial included 5395 patients with confirmed, symptomatic DVT or PE requiring treatment for 6 months. They had a mean age of 56.9 years, and 89.8% of randomized patients had unprovoked VTE.
About half of patients (n=2691) were randomized to receive apixaban at 10 mg twice daily for 7 days, followed by 5 mg twice daily for 6 months.
The other half (n=2704) were randomized to the standard of care, which was enoxaparin at 1 mg/kg twice daily for at least 5 days until INR ≥ 2 and warfarin (target INR range 2.0-3.0) for 6 months.
Apixaban proved noninferior to standard therapy in the combined primary endpoint of adjudicated recurrent symptomatic VTE (nonfatal DVT or PE) or VTE-related death.
This outcome occurred in 2.3% of patients in the apixaban arm and 2.7% of patients in the standard-therapy arm (P<0.0001 for noninferiority).
Apixaban also proved superior to standard therapy with regard to bleeding. The composite endpoint of major bleeding and clinically relevant, nonmajor bleeding occurred in 4.3% of patients in the apixaban arm and 9.7% of patients in the standard-therapy arm (P<0.001).
Results of AMPLIFY-EXT
The AMPLIFY-EXT trial included 2486 patients who had completed 6 to 12 months of anticoagulation treatment for DVT or PE. The mean age was 56.7 years, and 91.7% of randomized patients had unprovoked VTE.
Patients were randomized to receive apixaban at 2.5 mg (n=842), apixaban at 5 mg (n=815), or placebo (n=829).
Both apixaban doses were significantly superior to placebo (P<0.001) with regard to the primary efficacy endpoint, which was recurrent VTE or all-cause death.
During the 12-month active study period, these events occurred in 3.8% of patients in the 2.5-mg arm, 4.2% of patients in the 5-mg arm, and 11.6% of patients in the placebo arm.
The primary safety endpoint was the incidence of major bleeding, and there was no significant difference among the treatment arms. Major bleeding occurred in 0.2% of patients in the 2.5-mg arm, 0.1% of patients in the 5-mg arm, and 0.5% of patients in the placebo arm.
About apixaban
Apixaban is approved to reduce the risk of stroke and systemic embolism in adult patients with nonvalvular atrial fibrillation in the US, EU, Japan, and a number of other countries around the world.
The drug is approved to prevent VTE in adult patients who have undergone elective hip or knee replacement surgery in the US, EU, and a number of other countries.
And now, apixaban is approved for the treatment of DVT/PE and the prevention of recurrent DVT/PE in the EU. The drug is not approved for this indication in the US.
Apixaban is under joint development by Pfizer and Bristol-Myers Squibb. ![]()

Credit: Kevin MacKenzie
The European Commission has approved apixaban (Eliquis) to treat and prevent deep vein thrombosis (DVT) and pulmonary embolism (PE).
The approval applies to all European Union (EU) member states, as well as Iceland and Norway.
Apixaban was already approved in the EU to prevent venous thromboembolism (VTE) in adults who have undergone total hip or knee replacement surgery, and to prevent stroke and systemic embolism in adults with nonvalvular atrial fibrillation.
The new marketing authorization for apixaban follows the positive opinion issued by the European Medicines Agency’s Committee for Medicinal Products for Human Use in June and is supported by results of 2 phase 3 clinical trials, AMPLIFY and AMPLIFY-EXT.
Results of AMPLIFY
The AMPLIFY trial included 5395 patients with confirmed, symptomatic DVT or PE requiring treatment for 6 months. They had a mean age of 56.9 years, and 89.8% of randomized patients had unprovoked VTE.
About half of patients (n=2691) were randomized to receive apixaban at 10 mg twice daily for 7 days, followed by 5 mg twice daily for 6 months.
The other half (n=2704) were randomized to the standard of care, which was enoxaparin at 1 mg/kg twice daily for at least 5 days until INR ≥ 2 and warfarin (target INR range 2.0-3.0) for 6 months.
Apixaban proved noninferior to standard therapy in the combined primary endpoint of adjudicated recurrent symptomatic VTE (nonfatal DVT or PE) or VTE-related death.
This outcome occurred in 2.3% of patients in the apixaban arm and 2.7% of patients in the standard-therapy arm (P<0.0001 for noninferiority).
Apixaban also proved superior to standard therapy with regard to bleeding. The composite endpoint of major bleeding and clinically relevant, nonmajor bleeding occurred in 4.3% of patients in the apixaban arm and 9.7% of patients in the standard-therapy arm (P<0.001).
Results of AMPLIFY-EXT
The AMPLIFY-EXT trial included 2486 patients who had completed 6 to 12 months of anticoagulation treatment for DVT or PE. The mean age was 56.7 years, and 91.7% of randomized patients had unprovoked VTE.
Patients were randomized to receive apixaban at 2.5 mg (n=842), apixaban at 5 mg (n=815), or placebo (n=829).
Both apixaban doses were significantly superior to placebo (P<0.001) with regard to the primary efficacy endpoint, which was recurrent VTE or all-cause death.
During the 12-month active study period, these events occurred in 3.8% of patients in the 2.5-mg arm, 4.2% of patients in the 5-mg arm, and 11.6% of patients in the placebo arm.
The primary safety endpoint was the incidence of major bleeding, and there was no significant difference among the treatment arms. Major bleeding occurred in 0.2% of patients in the 2.5-mg arm, 0.1% of patients in the 5-mg arm, and 0.5% of patients in the placebo arm.
About apixaban
Apixaban is approved to reduce the risk of stroke and systemic embolism in adult patients with nonvalvular atrial fibrillation in the US, EU, Japan, and a number of other countries around the world.
The drug is approved to prevent VTE in adult patients who have undergone elective hip or knee replacement surgery in the US, EU, and a number of other countries.
And now, apixaban is approved for the treatment of DVT/PE and the prevention of recurrent DVT/PE in the EU. The drug is not approved for this indication in the US.
Apixaban is under joint development by Pfizer and Bristol-Myers Squibb. ![]()
Obinutuzumab approved for CLL in Europe
The European Commission has approved the anti-CD20 monoclonal antibody obinutuzumab for use in the European Union (EU).
Obinutuzumab can now be used in combination with chlorambucil to treat patients with previously untreated chronic lymphocytic leukemia (CLL) who have comorbidities that make them ineligible to receive fludarabine-based therapy.
Obinutuzumab is already approved for this indication in the US.
Obinutuzumab is marketed as Gazyvaro in the EU and Switzerland but as Gazyva in the US and the rest of the world.
The European Commission’s approval follows a positive opinion granted by The European Medicine Agency’s Committee for Medicinal Products for Human Use in May.
The approval is based on results of the phase 3 CLL11 study, which showed that obinutuzumab plus chlorambucil improved progression-free survival (PFS), when compared to chlorambucil alone or in combination with rituximab.
This 2-stage study included 781 previously untreated CLL patients with comorbidities. In stage 1 (n=589), researchers compared obinutuzumab plus chlorambucil to chlorambucil alone and rituximab plus chlorambucil to chlorambucil alone.
Stage 2 (n=663) was a direct comparison of obinutuzumab plus chlorambucil and rituximab plus chlorambucil.
Stage 1 results were presented at ASCO 2013, stage 2 results were presented at ASH 2013, and the complete results were published in NEJM last March.
Obinutuzumab plus chlorambucil improved PFS when compared to chlorambucil alone. The median PFS was 26.7 months and 11.1 months, respectively (P<0.001).
Obinutuzumab plus chlorambucil also improved PFS when compared to rituximab plus chlorambucil. The median PFS was 26.7 months and 16.3 months, respectively (P<0.001).
Infusion-related reactions and neutropenia were more common in the obinutuzumab arm than in the rituximab arm. But obinutuzumab-treated patients did not have an increased risk of infection.
Obinutuzumab is being developed by Roche. The company said it expects to begin launching the drug in a number of European countries this year. ![]()
The European Commission has approved the anti-CD20 monoclonal antibody obinutuzumab for use in the European Union (EU).
Obinutuzumab can now be used in combination with chlorambucil to treat patients with previously untreated chronic lymphocytic leukemia (CLL) who have comorbidities that make them ineligible to receive fludarabine-based therapy.
Obinutuzumab is already approved for this indication in the US.
Obinutuzumab is marketed as Gazyvaro in the EU and Switzerland but as Gazyva in the US and the rest of the world.
The European Commission’s approval follows a positive opinion granted by The European Medicine Agency’s Committee for Medicinal Products for Human Use in May.
The approval is based on results of the phase 3 CLL11 study, which showed that obinutuzumab plus chlorambucil improved progression-free survival (PFS), when compared to chlorambucil alone or in combination with rituximab.
This 2-stage study included 781 previously untreated CLL patients with comorbidities. In stage 1 (n=589), researchers compared obinutuzumab plus chlorambucil to chlorambucil alone and rituximab plus chlorambucil to chlorambucil alone.
Stage 2 (n=663) was a direct comparison of obinutuzumab plus chlorambucil and rituximab plus chlorambucil.
Stage 1 results were presented at ASCO 2013, stage 2 results were presented at ASH 2013, and the complete results were published in NEJM last March.
Obinutuzumab plus chlorambucil improved PFS when compared to chlorambucil alone. The median PFS was 26.7 months and 11.1 months, respectively (P<0.001).
Obinutuzumab plus chlorambucil also improved PFS when compared to rituximab plus chlorambucil. The median PFS was 26.7 months and 16.3 months, respectively (P<0.001).
Infusion-related reactions and neutropenia were more common in the obinutuzumab arm than in the rituximab arm. But obinutuzumab-treated patients did not have an increased risk of infection.
Obinutuzumab is being developed by Roche. The company said it expects to begin launching the drug in a number of European countries this year. ![]()
The European Commission has approved the anti-CD20 monoclonal antibody obinutuzumab for use in the European Union (EU).
Obinutuzumab can now be used in combination with chlorambucil to treat patients with previously untreated chronic lymphocytic leukemia (CLL) who have comorbidities that make them ineligible to receive fludarabine-based therapy.
Obinutuzumab is already approved for this indication in the US.
Obinutuzumab is marketed as Gazyvaro in the EU and Switzerland but as Gazyva in the US and the rest of the world.
The European Commission’s approval follows a positive opinion granted by The European Medicine Agency’s Committee for Medicinal Products for Human Use in May.
The approval is based on results of the phase 3 CLL11 study, which showed that obinutuzumab plus chlorambucil improved progression-free survival (PFS), when compared to chlorambucil alone or in combination with rituximab.
This 2-stage study included 781 previously untreated CLL patients with comorbidities. In stage 1 (n=589), researchers compared obinutuzumab plus chlorambucil to chlorambucil alone and rituximab plus chlorambucil to chlorambucil alone.
Stage 2 (n=663) was a direct comparison of obinutuzumab plus chlorambucil and rituximab plus chlorambucil.
Stage 1 results were presented at ASCO 2013, stage 2 results were presented at ASH 2013, and the complete results were published in NEJM last March.
Obinutuzumab plus chlorambucil improved PFS when compared to chlorambucil alone. The median PFS was 26.7 months and 11.1 months, respectively (P<0.001).
Obinutuzumab plus chlorambucil also improved PFS when compared to rituximab plus chlorambucil. The median PFS was 26.7 months and 16.3 months, respectively (P<0.001).
Infusion-related reactions and neutropenia were more common in the obinutuzumab arm than in the rituximab arm. But obinutuzumab-treated patients did not have an increased risk of infection.
Obinutuzumab is being developed by Roche. The company said it expects to begin launching the drug in a number of European countries this year. ![]()
Is the United States a proving ground or quagmire for mobile health?
The use of mobile or wireless devices in health care continues to challenge the regulatory landscape. States increasingly are playing a role in either advancing or retracing steps previously taken at the federal level. In the spirit of Ferris Bueller, "isms" have provided a number of opportunities for discussion surrounding mobile health technologies. Federalism remains a key criterion upon which our country creates health care policy – which could serve as a double-edged sword.
On the one hand, it might encourage innovation and provide policy that satisfies the needs of specific constituents. On the other hand, it may create more complexity or even contradict previous policy. The result is often a legal quagmire of wasted time, energy, and money. While policy will never keep pace with technology and innovation, a number of stakeholders are working to bridge the gap.
HIMSS – the Health Information Management System Society – has provided an overview of contemporary issues focused on the state level to advance the use of mobile and wireless devices in health care. Their paper titled "Mobile Health IT in the States: A Policy Perspective"sheds light on a number of potential redundancies in the regulatory system and offers some guidance on other issues.
One major issue gaining plenty of interest among physicians and lawmakers is the ability for mobile devices to facilitate the delivery of health care in a more meaningful, cost effective way. However, whenever disruptive technology begins to upset vested interests, one can expect a robust discussion.
The licensure of physicians and other providers and establishing telehealth standards of care remain substantial obstacles to overcome in the regulatory space. Federal licensure would permit physicians to care for patients across state lines via telehealth delivery systems. Some medical boards of states bordering large metropolitan areas such as Washington, D.C., have entered into reciprocal provider licensing agreements to allow for telehealth encounters.
Reimbursement represents another major obstacle to widespread adoption by providers. Telehealth is primarily a technology approved in certain rural areas under Medicaid. Enter a new age of consumerism in health care, and for a small fee, providers can engage in consultations using your mobile device.
A number of studies have examined the desire for patients to receive care on a mobile device, and not surprisingly, convenience wins out. However, a number of discordant state polices increasingly prohibit the ability to scale many of these innovative and cost-saving approaches to care delivery. The HIMSS paper encourages states to consider health IT, electronic health record (EHR) adoption, telehealth, and mobile health (mHealth) when resourcing and determining coverage for publicly funded health programs such as Medicaid, public health initiatives, and state employee health benefits programs.
Unfortunately, reimbursement for telehealth services for Medicare patients as well is also limited to rural settings defined as "originating sites."
"An originating site is the location of an eligible Medicare beneficiary at the time the service being furnished via a telecommunications system occurs. Medicare beneficiaries are eligible for telehealth services only if they are presented from an originating site located in a rural Health Professional Shortage Area, either located outside of a Metropolitan Statistical Area (MSA) or in a rural census tract, as determined by the Office of Rural Health Policy within the Health Resources and Services Administration (HRSA) or [from] a county outside of an MSA."
Telehealth reimbursement only covers certain specialties and services. Telehealth has been in existence for decades and has been the focus of many outcomes-based studies.
Extending this to mobile technologies such as medical apps remains a challenge due to the lack of evidence. However, I foresee the critical need for such applications, the rapid development of state-of-the-art sensor technologies, and the emergence of analytics to converge and make the success of mobile health technologies a welcome and accepted reality.
Dr. Scher is an electrophysiologist with the Heart Group of Lancaster (Pa.) General Health. He is also director of DLS Healthcare Consulting, Harrisburg, Pa., and clinical associate professor of medicine at the Pennsylvania State University, Hershey.
The use of mobile or wireless devices in health care continues to challenge the regulatory landscape. States increasingly are playing a role in either advancing or retracing steps previously taken at the federal level. In the spirit of Ferris Bueller, "isms" have provided a number of opportunities for discussion surrounding mobile health technologies. Federalism remains a key criterion upon which our country creates health care policy – which could serve as a double-edged sword.
On the one hand, it might encourage innovation and provide policy that satisfies the needs of specific constituents. On the other hand, it may create more complexity or even contradict previous policy. The result is often a legal quagmire of wasted time, energy, and money. While policy will never keep pace with technology and innovation, a number of stakeholders are working to bridge the gap.
HIMSS – the Health Information Management System Society – has provided an overview of contemporary issues focused on the state level to advance the use of mobile and wireless devices in health care. Their paper titled "Mobile Health IT in the States: A Policy Perspective"sheds light on a number of potential redundancies in the regulatory system and offers some guidance on other issues.
One major issue gaining plenty of interest among physicians and lawmakers is the ability for mobile devices to facilitate the delivery of health care in a more meaningful, cost effective way. However, whenever disruptive technology begins to upset vested interests, one can expect a robust discussion.
The licensure of physicians and other providers and establishing telehealth standards of care remain substantial obstacles to overcome in the regulatory space. Federal licensure would permit physicians to care for patients across state lines via telehealth delivery systems. Some medical boards of states bordering large metropolitan areas such as Washington, D.C., have entered into reciprocal provider licensing agreements to allow for telehealth encounters.
Reimbursement represents another major obstacle to widespread adoption by providers. Telehealth is primarily a technology approved in certain rural areas under Medicaid. Enter a new age of consumerism in health care, and for a small fee, providers can engage in consultations using your mobile device.
A number of studies have examined the desire for patients to receive care on a mobile device, and not surprisingly, convenience wins out. However, a number of discordant state polices increasingly prohibit the ability to scale many of these innovative and cost-saving approaches to care delivery. The HIMSS paper encourages states to consider health IT, electronic health record (EHR) adoption, telehealth, and mobile health (mHealth) when resourcing and determining coverage for publicly funded health programs such as Medicaid, public health initiatives, and state employee health benefits programs.
Unfortunately, reimbursement for telehealth services for Medicare patients as well is also limited to rural settings defined as "originating sites."
"An originating site is the location of an eligible Medicare beneficiary at the time the service being furnished via a telecommunications system occurs. Medicare beneficiaries are eligible for telehealth services only if they are presented from an originating site located in a rural Health Professional Shortage Area, either located outside of a Metropolitan Statistical Area (MSA) or in a rural census tract, as determined by the Office of Rural Health Policy within the Health Resources and Services Administration (HRSA) or [from] a county outside of an MSA."
Telehealth reimbursement only covers certain specialties and services. Telehealth has been in existence for decades and has been the focus of many outcomes-based studies.
Extending this to mobile technologies such as medical apps remains a challenge due to the lack of evidence. However, I foresee the critical need for such applications, the rapid development of state-of-the-art sensor technologies, and the emergence of analytics to converge and make the success of mobile health technologies a welcome and accepted reality.
Dr. Scher is an electrophysiologist with the Heart Group of Lancaster (Pa.) General Health. He is also director of DLS Healthcare Consulting, Harrisburg, Pa., and clinical associate professor of medicine at the Pennsylvania State University, Hershey.
The use of mobile or wireless devices in health care continues to challenge the regulatory landscape. States increasingly are playing a role in either advancing or retracing steps previously taken at the federal level. In the spirit of Ferris Bueller, "isms" have provided a number of opportunities for discussion surrounding mobile health technologies. Federalism remains a key criterion upon which our country creates health care policy – which could serve as a double-edged sword.
On the one hand, it might encourage innovation and provide policy that satisfies the needs of specific constituents. On the other hand, it may create more complexity or even contradict previous policy. The result is often a legal quagmire of wasted time, energy, and money. While policy will never keep pace with technology and innovation, a number of stakeholders are working to bridge the gap.
HIMSS – the Health Information Management System Society – has provided an overview of contemporary issues focused on the state level to advance the use of mobile and wireless devices in health care. Their paper titled "Mobile Health IT in the States: A Policy Perspective"sheds light on a number of potential redundancies in the regulatory system and offers some guidance on other issues.
One major issue gaining plenty of interest among physicians and lawmakers is the ability for mobile devices to facilitate the delivery of health care in a more meaningful, cost effective way. However, whenever disruptive technology begins to upset vested interests, one can expect a robust discussion.
The licensure of physicians and other providers and establishing telehealth standards of care remain substantial obstacles to overcome in the regulatory space. Federal licensure would permit physicians to care for patients across state lines via telehealth delivery systems. Some medical boards of states bordering large metropolitan areas such as Washington, D.C., have entered into reciprocal provider licensing agreements to allow for telehealth encounters.
Reimbursement represents another major obstacle to widespread adoption by providers. Telehealth is primarily a technology approved in certain rural areas under Medicaid. Enter a new age of consumerism in health care, and for a small fee, providers can engage in consultations using your mobile device.
A number of studies have examined the desire for patients to receive care on a mobile device, and not surprisingly, convenience wins out. However, a number of discordant state polices increasingly prohibit the ability to scale many of these innovative and cost-saving approaches to care delivery. The HIMSS paper encourages states to consider health IT, electronic health record (EHR) adoption, telehealth, and mobile health (mHealth) when resourcing and determining coverage for publicly funded health programs such as Medicaid, public health initiatives, and state employee health benefits programs.
Unfortunately, reimbursement for telehealth services for Medicare patients as well is also limited to rural settings defined as "originating sites."
"An originating site is the location of an eligible Medicare beneficiary at the time the service being furnished via a telecommunications system occurs. Medicare beneficiaries are eligible for telehealth services only if they are presented from an originating site located in a rural Health Professional Shortage Area, either located outside of a Metropolitan Statistical Area (MSA) or in a rural census tract, as determined by the Office of Rural Health Policy within the Health Resources and Services Administration (HRSA) or [from] a county outside of an MSA."
Telehealth reimbursement only covers certain specialties and services. Telehealth has been in existence for decades and has been the focus of many outcomes-based studies.
Extending this to mobile technologies such as medical apps remains a challenge due to the lack of evidence. However, I foresee the critical need for such applications, the rapid development of state-of-the-art sensor technologies, and the emergence of analytics to converge and make the success of mobile health technologies a welcome and accepted reality.
Dr. Scher is an electrophysiologist with the Heart Group of Lancaster (Pa.) General Health. He is also director of DLS Healthcare Consulting, Harrisburg, Pa., and clinical associate professor of medicine at the Pennsylvania State University, Hershey.
Access to a Behavioral Weight Loss Website With or Without Group Sessions Increased Weight Loss in Statewide Campaign
Study Overview
Objective. To determine the efficacy and cost-effectiveness of adding an evidence-based internet behavioral weight loss intervention alone or combined with optional group sessions to ShapeUp Rhode Island 2011 (SURI), a 3-month statewide wellness campaign.
Design. 3-arm randomized clinical trial.
Setting and participants. Study participants were recruited from the Rhode Island community via employers, media, and mass mailings at the time of SURI 2011 registration. Of the 3806 participants that joined the weight loss division, 1139 were willing to be contacted for research, and the first 431 were screened for study eligibility. Exclusion criteria were minimal: age < 18 years or > 70 years, body mass index (BMI) < 25 kg/m2, pregnant, nursing, or plans to become pregnant, a serious medical condition (eg, cancer), unreliable internet access, non-English speaking, current or previous participation in our weight loss studies, and planned relocation. Those who reported a medical condition that could interfere with safe participation (eg, diabetes) obtained doctor’s consent to participate. Of those screened, 230 met inclusion criteria, completed orientation procedures, and were randomized using a 1:2:2 randomization scheme to the standard SURI program (S; n = 46); SURI plus internet behavioral weight loss intervention (SI; n = 90); or SURI plus internet behavioral weight loss intervention plus optional group sessions (SIG; n = 94). To avoid contamination, individuals on the same SURI team (see below) were randomized to the same intervention.
Intervention. Participants in the standard SURI program did not receive any behavioral weight loss treatment. SURI is a self-sustaining, annual community campaign designed to help Rhode Islanders lose weight and increase their physical activity through an online, team-based competition. Participants join in teams, enter the weight loss or physical activity division or both, and compete with other teams. Throughout the 3-month program, participants have access to a reporting SURI website where they submit their weekly weight and activity data and view their personal and team progress. They also receive paper logs to record weight and activity, a pedometer, access to newsletters and community workshops, and recognition for meeting goals.
Participants in the SI arm received the 3-month SURI program plus a 3-month internet behavioral weight loss intervention. Before SURI began, SI participants attended a 1-hour group meeting during which they received their weight loss goal (lose 1 to 2 pounds per week), calorie and fat gram goal (starting weight < 250 lbs: 1200–1500 kcal/day, 40–50 g of fat; starting weight ≥ 250 lbs: 1500–1800 kcal/day, 50–60 g of fat), and activity goal (gradually increase to 200 minutes of aerobic activity per week). During this session, participants were also taught self-monitoring skills and oriented to an internet behavioral weight loss intervention website developed by the authors. The intervention website included 12 weekly, 10- to 15-minute multimedia lessons based on the Diabetes Prevention Program and a self-monitoring platform where participants tracked their daily weight, calorie, and activity information. Participants received weekly automated feedback on their progress. The intervention website also included information on meal plans, prepackaged meals, and meal replacements.
Participants in the SIG arm received everything in SI and were additionally given the option to attend weekly group meetings at Miriam Hospital’s Weight Control and Diabetes Research Center during the 3 months. The 12 weekly, optional group sessions were led by masters-level staff with extensive training in behavioral weight loss. Sessions involved private weigh-ins and covered topics that supplemented the internet intervention (eg, recipe modification, portion control).
Main outcomes measures. The main outcome was weight loss at the end of the 3-month program. Participants completed measures (ie, weight, BMI) in person at baseline and 3 months (post-treatment), and at 6- and 12-month follow-up visits. Adherence measures included reported weight and physical activity on the SURI website (S, SI, and SIG), log ins, viewed lessons, and self-monitoring entries on the intervention website (SI, SIG), and number of groups meetings attended (SIG). To measure weight loss behaviors, the authors used the Weight Control Practices questionnaire to assess engagement in core weight loss strategies targeted in treatment, and the Paffenbarger questionnaire to assess weekly kcal expended in moderate to vigorous activity. The authors also assessed costs from the payer (labor, rent, intervention materials), participant (SURI registration fee, transportation, time spent on intervention), and societal perspective (sum of payer and participant costs) in order to calculate the cost per kg of weight lost in each study arm.
Results. Participants were predominantly female, non-Hispanic white, and had a mean BMI of 34.4 kg/m2 (SE = 0.05). Groups differed only on education (P = 0.02), and attendance at post-treatment and 6- and 12-month follow-up were high (93%, 91%, and 86% respectively). The authors found that weight loss did not differ by educational attainment (P s > 0.57).
Overall, there was a significant group-by-time interaction for weight loss (P < 0.001). Percentage weight loss at 3 months differed among the 3 groups—S: 1.1% ± 0.9%; SI: 4.2% ± 0.6%; SIG: 6.1% ± 0.6% (P s ≤ 0.04). There was also an overall group effect for percentage of individuals achieving 5% weight loss (P < 0.001). SI and SIG had higher percentages of participants who achieved a 5% weight loss than the control (SI: 42%; SIG: 54%; S: 7%; P s < 0.001) but did not differ from one another (P = 0.01). Initial weight losses and percentage of participants who achieved a 5% weight loss were largely maintained through the no-treatment follow-up phase at 6-months, but the 3 groups no longer differed from one another at 12 months (S: 1.2% [SE =0.9]; SI: 2.2% [SE = 0.6]; SIG: 3.3% [SE = 0.6]; P s > 0.05).
All groups reported significant increases in physical activity over time (p < 0.001). More reporting of weight and physical activity data on the SURI website was associated with greater percentage weight loss (r = 0.25; P < 0.001). Number of log ins and lessons viewed on the intervention website were positively associated with percentage weight loss (r = 0.45; P ≤ 0.001; and r = 0.34; P ≤ 0.001 respectively). Greater attendance to group sessions was associated with better weight outcomes (r = 0.61; P ≤ 0.001). Younger age was associated with poorer adherence, including less reporting on the SURI website, viewing of lessons, and logging in to the weight loss website.
There was a significant group-by-time effect interaction for the use of behavioral weight loss strategies (P < 0.001), and increased use of these strategies was associated with greater percentage weight loss in all 3 groups post-treatment. At 12 months, however, there were no differences between groups in the use of these strategies (P s ≤ 0.07).
Cost per kg of weight loss was similar for S ($39) and SI ($35), but both were lower than SIG ($114).
Conclusion. Both intervention arms (SI and SIG) achieved more weight loss at 6 months than SURI alone. Although mean weight loss was greatest with optional group sessions (SIG), the addition of the behavioral intervention website alone (SI) was the most cost-effective method to enhance weight loss. Thus, adding a novel internet behavioral weight loss intervention to a statewide community health initiative may be a cost-effective approach to improving obesity treatment outcomes.
Commentary
Weight loss treatment is recommended for adults with a BMI of > 30 kg/m2, as well as those with BMI < 25 kg/m2 with weight-related comorbidities [1]. Intensive behavioral treatment should be the first line of intervention for overweight and obese individuals and can lead to 8% to 10% weight loss [2], particularly in initial months of treatment [3]. However, behavioral treatment is inherently challenging and time-consuming, and readily available to only a fraction of the intended population. Although weight losses achieved from intensive lifestyle interventions such as the Diabetes Prevention Program (DPP) [4] may be higher, innovative community weight loss programs that use a variety of weight loss strategies can provide opportunities to a wider population of overweight and obese individuals and at a lower cost [3].
This study built upon the authors’ previous work [5], which showed that SURI participants with behavioral weight loss strategies via email significantly improved 3-month weight losses. In this current study, they compared SURI alone to SURI with additional access to an internet behavioral weight loss website with or without optional group sessions. Since significant weight loss was not maintained at 12 months, this suggests that perhaps access to the behavioral weight loss website should have continued for longer and/or included a maintenance phase after the 3-month intervention. Weight loss often reaches its peak around 6 months, and weight regain occurs without effective maintenance therapy [6].
General strengths of the study included the use of a randomized, intention-to-treat design, dissemination of evidence-based weight loss strategies, objective outcomes measurement, adherence metrics, and strong retention of participants with clear accounting of all enrolled patients from recruitment through analysis. This study demonstrated significant weight loss in an intervention with minimal/optional health professional interaction. This intervention also placed responsibility on participants to self-monitor their diet and physical activity, participate in online lessons, and attend optional group sessions. The success of this community-based intervention suggests feasibility and scalability within a real-world setting. The authors also conducted cost-effectiveness analyses demonstrating that the SI program was more cost-effective than SIG.
However, there are weaknesses as well. In setting the sample size for each arm of this study, no justification was described for choosing a 1:2:2 randomization scheme. In randomized control trials, the allocation of participants into the different study arms is often balanced to equal numbers which maximizes statistical power [7]. However, the use of unequal randomization ratios among study arms can be beneficial and even necessary for various reasons including cost, availability of the intervention, overcoming intervention/treatment learning curves, and if a higher drop-out rate is anticipated. Providing a justification for unbalanced sample sizes would be helpful to future researchers looking to replicate the study. Additionally, participants were mostly non-Hispanic white and female, thus limiting generalizability. While representative of the broader Rhode Island population, findings based on this population this may not be applicable to vulnerable (ie, low literacy, resource-poor) or underrepresented populations (ie, minorities) [8].
Applications for Clinical Practice
An internet-based behavioral weight loss intervention, when added to a community weight management initiative, is cost-effective and can lead to short-term weight loss. Given that clinicians often lack time, training, and resources to adequately address obesity in the office [9,10], encouraging patients to enroll in similar programs may be an effective strategy to address such barriers. The study also highlights the need for maintenance interventions to help keep weight off. Findings should be replicated in more diverse communities.
—Katrina F. Mateo, MPH, and Melanie Jay, MD, MS
1. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. National Heart, Lung, and Blood Institute; 1998.
2. Wadden TA, Butryn ML, Wilson C. Lifestyle modification for the management of obesity. Gastroenterology 2007;132:2226–38.
3. Butryn ML, Webb V, Wadden TA. Behavioral treatment of obesity. Psych Clin North Am 2011;34:841–59.
4. The Diabetes Prevention Program Research Group. The Diabetes Prevention Program (DPP): Description of lifestyle intervention. Diabetes Care 2002;25:2165–71.
5. Wing RR, Crane MM, Thomas JG, et al. Improving weight loss outcomes of community interventions by incorporating behavioral strategies. Am J Public Health 2010;100:2513–9.
6. Wing RR, Tate DF, Gorin A, et al. A self-regulation program for maintenance of weight loss. N Engl J Med 2006;355:1563–71.
7. Dumville JC, Hahn S, Miles JN V, Torgerson DJ. The use of unequal randomisation ratios in clinical trials: a review. Contemp Clin Trials 2006;27:1–12.
8. Marshall PL. Ethical challenges in study design and informed consent for health research in resource-poor settings. World Health Organization; 2007.
9. Jay M, Gillespie C, Ark T, et al. Do internists, pediatricians, and psychiatrists feel competent in obesity care? Using a needs assessment to drive curriculum design. J Gen Intern Med 2008;23:1066–70.
10. Loureiro ML, Nayga RM. Obesity, weight loss, and physician’s advice. Soc Sci Med 2006;62:2458–68.
Study Overview
Objective. To determine the efficacy and cost-effectiveness of adding an evidence-based internet behavioral weight loss intervention alone or combined with optional group sessions to ShapeUp Rhode Island 2011 (SURI), a 3-month statewide wellness campaign.
Design. 3-arm randomized clinical trial.
Setting and participants. Study participants were recruited from the Rhode Island community via employers, media, and mass mailings at the time of SURI 2011 registration. Of the 3806 participants that joined the weight loss division, 1139 were willing to be contacted for research, and the first 431 were screened for study eligibility. Exclusion criteria were minimal: age < 18 years or > 70 years, body mass index (BMI) < 25 kg/m2, pregnant, nursing, or plans to become pregnant, a serious medical condition (eg, cancer), unreliable internet access, non-English speaking, current or previous participation in our weight loss studies, and planned relocation. Those who reported a medical condition that could interfere with safe participation (eg, diabetes) obtained doctor’s consent to participate. Of those screened, 230 met inclusion criteria, completed orientation procedures, and were randomized using a 1:2:2 randomization scheme to the standard SURI program (S; n = 46); SURI plus internet behavioral weight loss intervention (SI; n = 90); or SURI plus internet behavioral weight loss intervention plus optional group sessions (SIG; n = 94). To avoid contamination, individuals on the same SURI team (see below) were randomized to the same intervention.
Intervention. Participants in the standard SURI program did not receive any behavioral weight loss treatment. SURI is a self-sustaining, annual community campaign designed to help Rhode Islanders lose weight and increase their physical activity through an online, team-based competition. Participants join in teams, enter the weight loss or physical activity division or both, and compete with other teams. Throughout the 3-month program, participants have access to a reporting SURI website where they submit their weekly weight and activity data and view their personal and team progress. They also receive paper logs to record weight and activity, a pedometer, access to newsletters and community workshops, and recognition for meeting goals.
Participants in the SI arm received the 3-month SURI program plus a 3-month internet behavioral weight loss intervention. Before SURI began, SI participants attended a 1-hour group meeting during which they received their weight loss goal (lose 1 to 2 pounds per week), calorie and fat gram goal (starting weight < 250 lbs: 1200–1500 kcal/day, 40–50 g of fat; starting weight ≥ 250 lbs: 1500–1800 kcal/day, 50–60 g of fat), and activity goal (gradually increase to 200 minutes of aerobic activity per week). During this session, participants were also taught self-monitoring skills and oriented to an internet behavioral weight loss intervention website developed by the authors. The intervention website included 12 weekly, 10- to 15-minute multimedia lessons based on the Diabetes Prevention Program and a self-monitoring platform where participants tracked their daily weight, calorie, and activity information. Participants received weekly automated feedback on their progress. The intervention website also included information on meal plans, prepackaged meals, and meal replacements.
Participants in the SIG arm received everything in SI and were additionally given the option to attend weekly group meetings at Miriam Hospital’s Weight Control and Diabetes Research Center during the 3 months. The 12 weekly, optional group sessions were led by masters-level staff with extensive training in behavioral weight loss. Sessions involved private weigh-ins and covered topics that supplemented the internet intervention (eg, recipe modification, portion control).
Main outcomes measures. The main outcome was weight loss at the end of the 3-month program. Participants completed measures (ie, weight, BMI) in person at baseline and 3 months (post-treatment), and at 6- and 12-month follow-up visits. Adherence measures included reported weight and physical activity on the SURI website (S, SI, and SIG), log ins, viewed lessons, and self-monitoring entries on the intervention website (SI, SIG), and number of groups meetings attended (SIG). To measure weight loss behaviors, the authors used the Weight Control Practices questionnaire to assess engagement in core weight loss strategies targeted in treatment, and the Paffenbarger questionnaire to assess weekly kcal expended in moderate to vigorous activity. The authors also assessed costs from the payer (labor, rent, intervention materials), participant (SURI registration fee, transportation, time spent on intervention), and societal perspective (sum of payer and participant costs) in order to calculate the cost per kg of weight lost in each study arm.
Results. Participants were predominantly female, non-Hispanic white, and had a mean BMI of 34.4 kg/m2 (SE = 0.05). Groups differed only on education (P = 0.02), and attendance at post-treatment and 6- and 12-month follow-up were high (93%, 91%, and 86% respectively). The authors found that weight loss did not differ by educational attainment (P s > 0.57).
Overall, there was a significant group-by-time interaction for weight loss (P < 0.001). Percentage weight loss at 3 months differed among the 3 groups—S: 1.1% ± 0.9%; SI: 4.2% ± 0.6%; SIG: 6.1% ± 0.6% (P s ≤ 0.04). There was also an overall group effect for percentage of individuals achieving 5% weight loss (P < 0.001). SI and SIG had higher percentages of participants who achieved a 5% weight loss than the control (SI: 42%; SIG: 54%; S: 7%; P s < 0.001) but did not differ from one another (P = 0.01). Initial weight losses and percentage of participants who achieved a 5% weight loss were largely maintained through the no-treatment follow-up phase at 6-months, but the 3 groups no longer differed from one another at 12 months (S: 1.2% [SE =0.9]; SI: 2.2% [SE = 0.6]; SIG: 3.3% [SE = 0.6]; P s > 0.05).
All groups reported significant increases in physical activity over time (p < 0.001). More reporting of weight and physical activity data on the SURI website was associated with greater percentage weight loss (r = 0.25; P < 0.001). Number of log ins and lessons viewed on the intervention website were positively associated with percentage weight loss (r = 0.45; P ≤ 0.001; and r = 0.34; P ≤ 0.001 respectively). Greater attendance to group sessions was associated with better weight outcomes (r = 0.61; P ≤ 0.001). Younger age was associated with poorer adherence, including less reporting on the SURI website, viewing of lessons, and logging in to the weight loss website.
There was a significant group-by-time effect interaction for the use of behavioral weight loss strategies (P < 0.001), and increased use of these strategies was associated with greater percentage weight loss in all 3 groups post-treatment. At 12 months, however, there were no differences between groups in the use of these strategies (P s ≤ 0.07).
Cost per kg of weight loss was similar for S ($39) and SI ($35), but both were lower than SIG ($114).
Conclusion. Both intervention arms (SI and SIG) achieved more weight loss at 6 months than SURI alone. Although mean weight loss was greatest with optional group sessions (SIG), the addition of the behavioral intervention website alone (SI) was the most cost-effective method to enhance weight loss. Thus, adding a novel internet behavioral weight loss intervention to a statewide community health initiative may be a cost-effective approach to improving obesity treatment outcomes.
Commentary
Weight loss treatment is recommended for adults with a BMI of > 30 kg/m2, as well as those with BMI < 25 kg/m2 with weight-related comorbidities [1]. Intensive behavioral treatment should be the first line of intervention for overweight and obese individuals and can lead to 8% to 10% weight loss [2], particularly in initial months of treatment [3]. However, behavioral treatment is inherently challenging and time-consuming, and readily available to only a fraction of the intended population. Although weight losses achieved from intensive lifestyle interventions such as the Diabetes Prevention Program (DPP) [4] may be higher, innovative community weight loss programs that use a variety of weight loss strategies can provide opportunities to a wider population of overweight and obese individuals and at a lower cost [3].
This study built upon the authors’ previous work [5], which showed that SURI participants with behavioral weight loss strategies via email significantly improved 3-month weight losses. In this current study, they compared SURI alone to SURI with additional access to an internet behavioral weight loss website with or without optional group sessions. Since significant weight loss was not maintained at 12 months, this suggests that perhaps access to the behavioral weight loss website should have continued for longer and/or included a maintenance phase after the 3-month intervention. Weight loss often reaches its peak around 6 months, and weight regain occurs without effective maintenance therapy [6].
General strengths of the study included the use of a randomized, intention-to-treat design, dissemination of evidence-based weight loss strategies, objective outcomes measurement, adherence metrics, and strong retention of participants with clear accounting of all enrolled patients from recruitment through analysis. This study demonstrated significant weight loss in an intervention with minimal/optional health professional interaction. This intervention also placed responsibility on participants to self-monitor their diet and physical activity, participate in online lessons, and attend optional group sessions. The success of this community-based intervention suggests feasibility and scalability within a real-world setting. The authors also conducted cost-effectiveness analyses demonstrating that the SI program was more cost-effective than SIG.
However, there are weaknesses as well. In setting the sample size for each arm of this study, no justification was described for choosing a 1:2:2 randomization scheme. In randomized control trials, the allocation of participants into the different study arms is often balanced to equal numbers which maximizes statistical power [7]. However, the use of unequal randomization ratios among study arms can be beneficial and even necessary for various reasons including cost, availability of the intervention, overcoming intervention/treatment learning curves, and if a higher drop-out rate is anticipated. Providing a justification for unbalanced sample sizes would be helpful to future researchers looking to replicate the study. Additionally, participants were mostly non-Hispanic white and female, thus limiting generalizability. While representative of the broader Rhode Island population, findings based on this population this may not be applicable to vulnerable (ie, low literacy, resource-poor) or underrepresented populations (ie, minorities) [8].
Applications for Clinical Practice
An internet-based behavioral weight loss intervention, when added to a community weight management initiative, is cost-effective and can lead to short-term weight loss. Given that clinicians often lack time, training, and resources to adequately address obesity in the office [9,10], encouraging patients to enroll in similar programs may be an effective strategy to address such barriers. The study also highlights the need for maintenance interventions to help keep weight off. Findings should be replicated in more diverse communities.
—Katrina F. Mateo, MPH, and Melanie Jay, MD, MS
Study Overview
Objective. To determine the efficacy and cost-effectiveness of adding an evidence-based internet behavioral weight loss intervention alone or combined with optional group sessions to ShapeUp Rhode Island 2011 (SURI), a 3-month statewide wellness campaign.
Design. 3-arm randomized clinical trial.
Setting and participants. Study participants were recruited from the Rhode Island community via employers, media, and mass mailings at the time of SURI 2011 registration. Of the 3806 participants that joined the weight loss division, 1139 were willing to be contacted for research, and the first 431 were screened for study eligibility. Exclusion criteria were minimal: age < 18 years or > 70 years, body mass index (BMI) < 25 kg/m2, pregnant, nursing, or plans to become pregnant, a serious medical condition (eg, cancer), unreliable internet access, non-English speaking, current or previous participation in our weight loss studies, and planned relocation. Those who reported a medical condition that could interfere with safe participation (eg, diabetes) obtained doctor’s consent to participate. Of those screened, 230 met inclusion criteria, completed orientation procedures, and were randomized using a 1:2:2 randomization scheme to the standard SURI program (S; n = 46); SURI plus internet behavioral weight loss intervention (SI; n = 90); or SURI plus internet behavioral weight loss intervention plus optional group sessions (SIG; n = 94). To avoid contamination, individuals on the same SURI team (see below) were randomized to the same intervention.
Intervention. Participants in the standard SURI program did not receive any behavioral weight loss treatment. SURI is a self-sustaining, annual community campaign designed to help Rhode Islanders lose weight and increase their physical activity through an online, team-based competition. Participants join in teams, enter the weight loss or physical activity division or both, and compete with other teams. Throughout the 3-month program, participants have access to a reporting SURI website where they submit their weekly weight and activity data and view their personal and team progress. They also receive paper logs to record weight and activity, a pedometer, access to newsletters and community workshops, and recognition for meeting goals.
Participants in the SI arm received the 3-month SURI program plus a 3-month internet behavioral weight loss intervention. Before SURI began, SI participants attended a 1-hour group meeting during which they received their weight loss goal (lose 1 to 2 pounds per week), calorie and fat gram goal (starting weight < 250 lbs: 1200–1500 kcal/day, 40–50 g of fat; starting weight ≥ 250 lbs: 1500–1800 kcal/day, 50–60 g of fat), and activity goal (gradually increase to 200 minutes of aerobic activity per week). During this session, participants were also taught self-monitoring skills and oriented to an internet behavioral weight loss intervention website developed by the authors. The intervention website included 12 weekly, 10- to 15-minute multimedia lessons based on the Diabetes Prevention Program and a self-monitoring platform where participants tracked their daily weight, calorie, and activity information. Participants received weekly automated feedback on their progress. The intervention website also included information on meal plans, prepackaged meals, and meal replacements.
Participants in the SIG arm received everything in SI and were additionally given the option to attend weekly group meetings at Miriam Hospital’s Weight Control and Diabetes Research Center during the 3 months. The 12 weekly, optional group sessions were led by masters-level staff with extensive training in behavioral weight loss. Sessions involved private weigh-ins and covered topics that supplemented the internet intervention (eg, recipe modification, portion control).
Main outcomes measures. The main outcome was weight loss at the end of the 3-month program. Participants completed measures (ie, weight, BMI) in person at baseline and 3 months (post-treatment), and at 6- and 12-month follow-up visits. Adherence measures included reported weight and physical activity on the SURI website (S, SI, and SIG), log ins, viewed lessons, and self-monitoring entries on the intervention website (SI, SIG), and number of groups meetings attended (SIG). To measure weight loss behaviors, the authors used the Weight Control Practices questionnaire to assess engagement in core weight loss strategies targeted in treatment, and the Paffenbarger questionnaire to assess weekly kcal expended in moderate to vigorous activity. The authors also assessed costs from the payer (labor, rent, intervention materials), participant (SURI registration fee, transportation, time spent on intervention), and societal perspective (sum of payer and participant costs) in order to calculate the cost per kg of weight lost in each study arm.
Results. Participants were predominantly female, non-Hispanic white, and had a mean BMI of 34.4 kg/m2 (SE = 0.05). Groups differed only on education (P = 0.02), and attendance at post-treatment and 6- and 12-month follow-up were high (93%, 91%, and 86% respectively). The authors found that weight loss did not differ by educational attainment (P s > 0.57).
Overall, there was a significant group-by-time interaction for weight loss (P < 0.001). Percentage weight loss at 3 months differed among the 3 groups—S: 1.1% ± 0.9%; SI: 4.2% ± 0.6%; SIG: 6.1% ± 0.6% (P s ≤ 0.04). There was also an overall group effect for percentage of individuals achieving 5% weight loss (P < 0.001). SI and SIG had higher percentages of participants who achieved a 5% weight loss than the control (SI: 42%; SIG: 54%; S: 7%; P s < 0.001) but did not differ from one another (P = 0.01). Initial weight losses and percentage of participants who achieved a 5% weight loss were largely maintained through the no-treatment follow-up phase at 6-months, but the 3 groups no longer differed from one another at 12 months (S: 1.2% [SE =0.9]; SI: 2.2% [SE = 0.6]; SIG: 3.3% [SE = 0.6]; P s > 0.05).
All groups reported significant increases in physical activity over time (p < 0.001). More reporting of weight and physical activity data on the SURI website was associated with greater percentage weight loss (r = 0.25; P < 0.001). Number of log ins and lessons viewed on the intervention website were positively associated with percentage weight loss (r = 0.45; P ≤ 0.001; and r = 0.34; P ≤ 0.001 respectively). Greater attendance to group sessions was associated with better weight outcomes (r = 0.61; P ≤ 0.001). Younger age was associated with poorer adherence, including less reporting on the SURI website, viewing of lessons, and logging in to the weight loss website.
There was a significant group-by-time effect interaction for the use of behavioral weight loss strategies (P < 0.001), and increased use of these strategies was associated with greater percentage weight loss in all 3 groups post-treatment. At 12 months, however, there were no differences between groups in the use of these strategies (P s ≤ 0.07).
Cost per kg of weight loss was similar for S ($39) and SI ($35), but both were lower than SIG ($114).
Conclusion. Both intervention arms (SI and SIG) achieved more weight loss at 6 months than SURI alone. Although mean weight loss was greatest with optional group sessions (SIG), the addition of the behavioral intervention website alone (SI) was the most cost-effective method to enhance weight loss. Thus, adding a novel internet behavioral weight loss intervention to a statewide community health initiative may be a cost-effective approach to improving obesity treatment outcomes.
Commentary
Weight loss treatment is recommended for adults with a BMI of > 30 kg/m2, as well as those with BMI < 25 kg/m2 with weight-related comorbidities [1]. Intensive behavioral treatment should be the first line of intervention for overweight and obese individuals and can lead to 8% to 10% weight loss [2], particularly in initial months of treatment [3]. However, behavioral treatment is inherently challenging and time-consuming, and readily available to only a fraction of the intended population. Although weight losses achieved from intensive lifestyle interventions such as the Diabetes Prevention Program (DPP) [4] may be higher, innovative community weight loss programs that use a variety of weight loss strategies can provide opportunities to a wider population of overweight and obese individuals and at a lower cost [3].
This study built upon the authors’ previous work [5], which showed that SURI participants with behavioral weight loss strategies via email significantly improved 3-month weight losses. In this current study, they compared SURI alone to SURI with additional access to an internet behavioral weight loss website with or without optional group sessions. Since significant weight loss was not maintained at 12 months, this suggests that perhaps access to the behavioral weight loss website should have continued for longer and/or included a maintenance phase after the 3-month intervention. Weight loss often reaches its peak around 6 months, and weight regain occurs without effective maintenance therapy [6].
General strengths of the study included the use of a randomized, intention-to-treat design, dissemination of evidence-based weight loss strategies, objective outcomes measurement, adherence metrics, and strong retention of participants with clear accounting of all enrolled patients from recruitment through analysis. This study demonstrated significant weight loss in an intervention with minimal/optional health professional interaction. This intervention also placed responsibility on participants to self-monitor their diet and physical activity, participate in online lessons, and attend optional group sessions. The success of this community-based intervention suggests feasibility and scalability within a real-world setting. The authors also conducted cost-effectiveness analyses demonstrating that the SI program was more cost-effective than SIG.
However, there are weaknesses as well. In setting the sample size for each arm of this study, no justification was described for choosing a 1:2:2 randomization scheme. In randomized control trials, the allocation of participants into the different study arms is often balanced to equal numbers which maximizes statistical power [7]. However, the use of unequal randomization ratios among study arms can be beneficial and even necessary for various reasons including cost, availability of the intervention, overcoming intervention/treatment learning curves, and if a higher drop-out rate is anticipated. Providing a justification for unbalanced sample sizes would be helpful to future researchers looking to replicate the study. Additionally, participants were mostly non-Hispanic white and female, thus limiting generalizability. While representative of the broader Rhode Island population, findings based on this population this may not be applicable to vulnerable (ie, low literacy, resource-poor) or underrepresented populations (ie, minorities) [8].
Applications for Clinical Practice
An internet-based behavioral weight loss intervention, when added to a community weight management initiative, is cost-effective and can lead to short-term weight loss. Given that clinicians often lack time, training, and resources to adequately address obesity in the office [9,10], encouraging patients to enroll in similar programs may be an effective strategy to address such barriers. The study also highlights the need for maintenance interventions to help keep weight off. Findings should be replicated in more diverse communities.
—Katrina F. Mateo, MPH, and Melanie Jay, MD, MS
1. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. National Heart, Lung, and Blood Institute; 1998.
2. Wadden TA, Butryn ML, Wilson C. Lifestyle modification for the management of obesity. Gastroenterology 2007;132:2226–38.
3. Butryn ML, Webb V, Wadden TA. Behavioral treatment of obesity. Psych Clin North Am 2011;34:841–59.
4. The Diabetes Prevention Program Research Group. The Diabetes Prevention Program (DPP): Description of lifestyle intervention. Diabetes Care 2002;25:2165–71.
5. Wing RR, Crane MM, Thomas JG, et al. Improving weight loss outcomes of community interventions by incorporating behavioral strategies. Am J Public Health 2010;100:2513–9.
6. Wing RR, Tate DF, Gorin A, et al. A self-regulation program for maintenance of weight loss. N Engl J Med 2006;355:1563–71.
7. Dumville JC, Hahn S, Miles JN V, Torgerson DJ. The use of unequal randomisation ratios in clinical trials: a review. Contemp Clin Trials 2006;27:1–12.
8. Marshall PL. Ethical challenges in study design and informed consent for health research in resource-poor settings. World Health Organization; 2007.
9. Jay M, Gillespie C, Ark T, et al. Do internists, pediatricians, and psychiatrists feel competent in obesity care? Using a needs assessment to drive curriculum design. J Gen Intern Med 2008;23:1066–70.
10. Loureiro ML, Nayga RM. Obesity, weight loss, and physician’s advice. Soc Sci Med 2006;62:2458–68.
1. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. National Heart, Lung, and Blood Institute; 1998.
2. Wadden TA, Butryn ML, Wilson C. Lifestyle modification for the management of obesity. Gastroenterology 2007;132:2226–38.
3. Butryn ML, Webb V, Wadden TA. Behavioral treatment of obesity. Psych Clin North Am 2011;34:841–59.
4. The Diabetes Prevention Program Research Group. The Diabetes Prevention Program (DPP): Description of lifestyle intervention. Diabetes Care 2002;25:2165–71.
5. Wing RR, Crane MM, Thomas JG, et al. Improving weight loss outcomes of community interventions by incorporating behavioral strategies. Am J Public Health 2010;100:2513–9.
6. Wing RR, Tate DF, Gorin A, et al. A self-regulation program for maintenance of weight loss. N Engl J Med 2006;355:1563–71.
7. Dumville JC, Hahn S, Miles JN V, Torgerson DJ. The use of unequal randomisation ratios in clinical trials: a review. Contemp Clin Trials 2006;27:1–12.
8. Marshall PL. Ethical challenges in study design and informed consent for health research in resource-poor settings. World Health Organization; 2007.
9. Jay M, Gillespie C, Ark T, et al. Do internists, pediatricians, and psychiatrists feel competent in obesity care? Using a needs assessment to drive curriculum design. J Gen Intern Med 2008;23:1066–70.
10. Loureiro ML, Nayga RM. Obesity, weight loss, and physician’s advice. Soc Sci Med 2006;62:2458–68.
Epidural Steroid Injections for Spinal Stenosis Back Pain Simply Don’t Work
Study Overview
Objective. To determine the effectiveness of epidural injections of glucocorticoids plus anesthetic compared with injections of anesthetic alone in patients with lumbar spinal stenosis.
Design. The LESS (Lumbar Epidural Steroid Injection for Spinal Stenosis) trial—a double-blind, multisite, randomized controlled trial.
Setting and participants. The study was conducted at 16 sites in the United States and enrolled 400 patients between April 2011 and June 2013. Patients at least 50 years of age with spinal stenosis as evidenced by magnetic resonance imaging (MRI) or computed tomography (CT) were invited to participate. Additional eligibility criteria included an average pain rating of more than 4 on a scale of 0 to 10 (0 being the lowest score) for back, buttock, or leg pain. Patients were excluded if they did not have stenosis of the central canal, had spondylolisthesis requiring surgery, or had received epidural glucocorticoid injections within the previous 6 months. Patients were randomly assigned to receive a standard epidural injection of glucocorticoids plus lidocaine or lidocaine alone. At the 3-week follow-up they could choose to receive a repeat injection. At the 6-week assessment they were allowed to cross over to the other treatment group. Patients were blinded throughout the study. The treating physicians were also blinded through the use of 2 opaque prefilled syringes provided by the study staff—one marked “inject” and one marked “discard.”
Main outcome measures. The 2 outcomes, measured at 6 weeks, were the Roland-Morris Disability Questionnaire (RMDQ) score (range, 0 to 24, with higher scores indicating greater physical disability) and the patient’s rating of average buttock, hip, or leg pain in the previous week (scale of 0 to 10 with 0 indicating no pain and 10 indicating “pain as bad as you can imagine”).
Eight secondary patient-oriented outcomes were also measured: (1) at least minimal clinically meaningful improvement (≥ 30%), (2) substantial clinically meaningful improvement (≥ 50%), (3) average back pain in the previous week, and scores on the (4) Brief Pain Inventory (BPI) interference scale, (5) 8-question Patient Health Questionnaire (PHQ-8), (6) Generalized Anxiety Disorder 7 scale (GAD-7), (7) EQ-5D (a health status measure) and (8) Swiss Spinal Stenosis Questionnaire (SSSQ).
Main results. The 2 groups were similar with respect to baseline characteristics, except that the duration of pain was shorter in the lidocaine-alone group. At 6 weeks, both groups had improved RMDQ scores (glucocorticoid –4.2 points vs. no glucocorticoid –3.1 points, respectively). However, the difference in RMDQ score between the 2 groups was not statistically significant (–1.0 points [95% CI, –2.1 to 0.1]; P = 0.07). In addition, there was no difference in treatment effect at 6 weeks as measured by patient’s reported leg pain (–0.2 points [95% CI, –0.8 to 0.4]; P = 0.48). Furthermore, there were no significant differences in the secondary outcomes of clinically meaningful improvement, BPI, SSSQ symptoms and physical function, EQ-5D, and GAD-7 scales at 6 weeks. Among the secondary outcomes, only symptoms of depression and patient satisfaction showed a statistically significant improvement in the glucocorticoid plus lidocaine group. Of note, though not statistically significant, there were more adverse events in the glucocorticoid plus lidocaine group compared to the lidocaine alone group (21.5% vs. 15.5%, respectively). Finally, the glucocorticoid plus lidocaine group also had a significantly higher proportion of patients with cortisol serum suppression compared to the lidocaine alone group.
Conclusion. The authors concluded that there was no difference in pain-related functional disability (as measured by the RMDQ score) and pain intensity between patients receiving fluoroscopically guided epidural injections with glucocorticoids plus lidocaine compared with lidocaine alone for lumbar spinal stenosis. The injection of glucocorticoid should be avoided due to its potentially systemic effects, including suppression of the hypothalamic-pituitary axis and reduction in bone mineral density, which may increase the risk of fracture.
Commentary
Lumbar spinal stenosis is one of the most common causes of spine-related back and leg pain; it disproportionally affects older adults due to degenerative changes resulting in narrowing of the spinal canal and nerve-root. Epidural glucocorticoid injections containing a glucocorticoid and an anesthetic are commonly used to relieve symptoms of lumbar stenosis. While this treatment approach is controversial, more than 2.2 million lumbar epidural glucocorticoid injections are performed in the Medicare population each year [1,2]. Previous uncontrolled studies suggest that epidural glucocorticoid injections provide short-term pain relief for some patients with spinal stenosis [3]. While complications from the procedure are rare, a multistate outbreak of fungal meningitis due to contaminated glucocorticoid injections affected at least 751 patients with 64 deaths in 2012 [4].
The purpose of the current study by Friedly et al was to determine whether adding a glucocorticoid to an anesthetic in epidural spinal injections is superior to anesthetic alone for symptom relief and functional improvement in patients with lumbar spinal stenosis. In contrast to previous studies, the authors defined short-term results as 3 weeks after injection, and long-term results as 6 weeks after injection. Despite the shorter follow-up period, results were similar to previous studies, in that adding glucocorticoid to anesthetic in epidural spinal injection reduced pain and improved patient’s functionality short-term, but improvements were not sustained long-term. Based on these results, the authors concluded that there is no benefit in adding glucocorticoid epidural injections for back pain arising from lumbar spinal stenosis.
One major limitation of this study is the lack of a placebo arm. Because of the lack of a placebo arm, it cannot be ascertained whether epidural injection with lidocaine alone conferred a benefit. However, this study provides robust evidence that epidural steroid injections are not beneficial for treatment of back and leg pain associated with lumbar spinal stenosis.
Applications for Clinical Practice
Epidural steroid injection is long accepted in medical communities as a safe and effective treatment for lumbar spinal stenosis symptoms. In light of the potential dangers of epidural steroid injections, including meningitis, coupled with the increasing cost of the procedure, other potential side effects, and demonstrated ineffectiveness of the treatment, providers should stop recommending epidural steroid injections for lumbar spinal stenosis.
—Ka Ming Gordon Ngai, MD, MPH
1. Manchikanti L, Pampati V, Boswell MV, et al. Analysis of the growth of epidural injections and costs in the Medicare population: a comparative evaluation of 1997, 2002, and 2006 data. Pain Physician 2010;13:199–212.
2. Manchikanti L, Pampati V, Falco FJ, et al. Assessment of the growth of epidural injections in the medicare population from 2000 to 2011. Pain Physician 2013;16:E349–364.
3. Shamliyan TA, Staal JB, Goldmann D, et al. Epidural steroid injections for radicular lumbosacral pain: a systematic review. Phys Med Rehabil Clin North Am 2014;25:471–89.
4. CDC. Multistate outbreak of fungal meningitis and other infections. 23 Oct 2013. Accessed 9 Jul 2014 at www.cdc.gov/hai/outbreaks/meningitis.html.
Study Overview
Objective. To determine the effectiveness of epidural injections of glucocorticoids plus anesthetic compared with injections of anesthetic alone in patients with lumbar spinal stenosis.
Design. The LESS (Lumbar Epidural Steroid Injection for Spinal Stenosis) trial—a double-blind, multisite, randomized controlled trial.
Setting and participants. The study was conducted at 16 sites in the United States and enrolled 400 patients between April 2011 and June 2013. Patients at least 50 years of age with spinal stenosis as evidenced by magnetic resonance imaging (MRI) or computed tomography (CT) were invited to participate. Additional eligibility criteria included an average pain rating of more than 4 on a scale of 0 to 10 (0 being the lowest score) for back, buttock, or leg pain. Patients were excluded if they did not have stenosis of the central canal, had spondylolisthesis requiring surgery, or had received epidural glucocorticoid injections within the previous 6 months. Patients were randomly assigned to receive a standard epidural injection of glucocorticoids plus lidocaine or lidocaine alone. At the 3-week follow-up they could choose to receive a repeat injection. At the 6-week assessment they were allowed to cross over to the other treatment group. Patients were blinded throughout the study. The treating physicians were also blinded through the use of 2 opaque prefilled syringes provided by the study staff—one marked “inject” and one marked “discard.”
Main outcome measures. The 2 outcomes, measured at 6 weeks, were the Roland-Morris Disability Questionnaire (RMDQ) score (range, 0 to 24, with higher scores indicating greater physical disability) and the patient’s rating of average buttock, hip, or leg pain in the previous week (scale of 0 to 10 with 0 indicating no pain and 10 indicating “pain as bad as you can imagine”).
Eight secondary patient-oriented outcomes were also measured: (1) at least minimal clinically meaningful improvement (≥ 30%), (2) substantial clinically meaningful improvement (≥ 50%), (3) average back pain in the previous week, and scores on the (4) Brief Pain Inventory (BPI) interference scale, (5) 8-question Patient Health Questionnaire (PHQ-8), (6) Generalized Anxiety Disorder 7 scale (GAD-7), (7) EQ-5D (a health status measure) and (8) Swiss Spinal Stenosis Questionnaire (SSSQ).
Main results. The 2 groups were similar with respect to baseline characteristics, except that the duration of pain was shorter in the lidocaine-alone group. At 6 weeks, both groups had improved RMDQ scores (glucocorticoid –4.2 points vs. no glucocorticoid –3.1 points, respectively). However, the difference in RMDQ score between the 2 groups was not statistically significant (–1.0 points [95% CI, –2.1 to 0.1]; P = 0.07). In addition, there was no difference in treatment effect at 6 weeks as measured by patient’s reported leg pain (–0.2 points [95% CI, –0.8 to 0.4]; P = 0.48). Furthermore, there were no significant differences in the secondary outcomes of clinically meaningful improvement, BPI, SSSQ symptoms and physical function, EQ-5D, and GAD-7 scales at 6 weeks. Among the secondary outcomes, only symptoms of depression and patient satisfaction showed a statistically significant improvement in the glucocorticoid plus lidocaine group. Of note, though not statistically significant, there were more adverse events in the glucocorticoid plus lidocaine group compared to the lidocaine alone group (21.5% vs. 15.5%, respectively). Finally, the glucocorticoid plus lidocaine group also had a significantly higher proportion of patients with cortisol serum suppression compared to the lidocaine alone group.
Conclusion. The authors concluded that there was no difference in pain-related functional disability (as measured by the RMDQ score) and pain intensity between patients receiving fluoroscopically guided epidural injections with glucocorticoids plus lidocaine compared with lidocaine alone for lumbar spinal stenosis. The injection of glucocorticoid should be avoided due to its potentially systemic effects, including suppression of the hypothalamic-pituitary axis and reduction in bone mineral density, which may increase the risk of fracture.
Commentary
Lumbar spinal stenosis is one of the most common causes of spine-related back and leg pain; it disproportionally affects older adults due to degenerative changes resulting in narrowing of the spinal canal and nerve-root. Epidural glucocorticoid injections containing a glucocorticoid and an anesthetic are commonly used to relieve symptoms of lumbar stenosis. While this treatment approach is controversial, more than 2.2 million lumbar epidural glucocorticoid injections are performed in the Medicare population each year [1,2]. Previous uncontrolled studies suggest that epidural glucocorticoid injections provide short-term pain relief for some patients with spinal stenosis [3]. While complications from the procedure are rare, a multistate outbreak of fungal meningitis due to contaminated glucocorticoid injections affected at least 751 patients with 64 deaths in 2012 [4].
The purpose of the current study by Friedly et al was to determine whether adding a glucocorticoid to an anesthetic in epidural spinal injections is superior to anesthetic alone for symptom relief and functional improvement in patients with lumbar spinal stenosis. In contrast to previous studies, the authors defined short-term results as 3 weeks after injection, and long-term results as 6 weeks after injection. Despite the shorter follow-up period, results were similar to previous studies, in that adding glucocorticoid to anesthetic in epidural spinal injection reduced pain and improved patient’s functionality short-term, but improvements were not sustained long-term. Based on these results, the authors concluded that there is no benefit in adding glucocorticoid epidural injections for back pain arising from lumbar spinal stenosis.
One major limitation of this study is the lack of a placebo arm. Because of the lack of a placebo arm, it cannot be ascertained whether epidural injection with lidocaine alone conferred a benefit. However, this study provides robust evidence that epidural steroid injections are not beneficial for treatment of back and leg pain associated with lumbar spinal stenosis.
Applications for Clinical Practice
Epidural steroid injection is long accepted in medical communities as a safe and effective treatment for lumbar spinal stenosis symptoms. In light of the potential dangers of epidural steroid injections, including meningitis, coupled with the increasing cost of the procedure, other potential side effects, and demonstrated ineffectiveness of the treatment, providers should stop recommending epidural steroid injections for lumbar spinal stenosis.
—Ka Ming Gordon Ngai, MD, MPH
Study Overview
Objective. To determine the effectiveness of epidural injections of glucocorticoids plus anesthetic compared with injections of anesthetic alone in patients with lumbar spinal stenosis.
Design. The LESS (Lumbar Epidural Steroid Injection for Spinal Stenosis) trial—a double-blind, multisite, randomized controlled trial.
Setting and participants. The study was conducted at 16 sites in the United States and enrolled 400 patients between April 2011 and June 2013. Patients at least 50 years of age with spinal stenosis as evidenced by magnetic resonance imaging (MRI) or computed tomography (CT) were invited to participate. Additional eligibility criteria included an average pain rating of more than 4 on a scale of 0 to 10 (0 being the lowest score) for back, buttock, or leg pain. Patients were excluded if they did not have stenosis of the central canal, had spondylolisthesis requiring surgery, or had received epidural glucocorticoid injections within the previous 6 months. Patients were randomly assigned to receive a standard epidural injection of glucocorticoids plus lidocaine or lidocaine alone. At the 3-week follow-up they could choose to receive a repeat injection. At the 6-week assessment they were allowed to cross over to the other treatment group. Patients were blinded throughout the study. The treating physicians were also blinded through the use of 2 opaque prefilled syringes provided by the study staff—one marked “inject” and one marked “discard.”
Main outcome measures. The 2 outcomes, measured at 6 weeks, were the Roland-Morris Disability Questionnaire (RMDQ) score (range, 0 to 24, with higher scores indicating greater physical disability) and the patient’s rating of average buttock, hip, or leg pain in the previous week (scale of 0 to 10 with 0 indicating no pain and 10 indicating “pain as bad as you can imagine”).
Eight secondary patient-oriented outcomes were also measured: (1) at least minimal clinically meaningful improvement (≥ 30%), (2) substantial clinically meaningful improvement (≥ 50%), (3) average back pain in the previous week, and scores on the (4) Brief Pain Inventory (BPI) interference scale, (5) 8-question Patient Health Questionnaire (PHQ-8), (6) Generalized Anxiety Disorder 7 scale (GAD-7), (7) EQ-5D (a health status measure) and (8) Swiss Spinal Stenosis Questionnaire (SSSQ).
Main results. The 2 groups were similar with respect to baseline characteristics, except that the duration of pain was shorter in the lidocaine-alone group. At 6 weeks, both groups had improved RMDQ scores (glucocorticoid –4.2 points vs. no glucocorticoid –3.1 points, respectively). However, the difference in RMDQ score between the 2 groups was not statistically significant (–1.0 points [95% CI, –2.1 to 0.1]; P = 0.07). In addition, there was no difference in treatment effect at 6 weeks as measured by patient’s reported leg pain (–0.2 points [95% CI, –0.8 to 0.4]; P = 0.48). Furthermore, there were no significant differences in the secondary outcomes of clinically meaningful improvement, BPI, SSSQ symptoms and physical function, EQ-5D, and GAD-7 scales at 6 weeks. Among the secondary outcomes, only symptoms of depression and patient satisfaction showed a statistically significant improvement in the glucocorticoid plus lidocaine group. Of note, though not statistically significant, there were more adverse events in the glucocorticoid plus lidocaine group compared to the lidocaine alone group (21.5% vs. 15.5%, respectively). Finally, the glucocorticoid plus lidocaine group also had a significantly higher proportion of patients with cortisol serum suppression compared to the lidocaine alone group.
Conclusion. The authors concluded that there was no difference in pain-related functional disability (as measured by the RMDQ score) and pain intensity between patients receiving fluoroscopically guided epidural injections with glucocorticoids plus lidocaine compared with lidocaine alone for lumbar spinal stenosis. The injection of glucocorticoid should be avoided due to its potentially systemic effects, including suppression of the hypothalamic-pituitary axis and reduction in bone mineral density, which may increase the risk of fracture.
Commentary
Lumbar spinal stenosis is one of the most common causes of spine-related back and leg pain; it disproportionally affects older adults due to degenerative changes resulting in narrowing of the spinal canal and nerve-root. Epidural glucocorticoid injections containing a glucocorticoid and an anesthetic are commonly used to relieve symptoms of lumbar stenosis. While this treatment approach is controversial, more than 2.2 million lumbar epidural glucocorticoid injections are performed in the Medicare population each year [1,2]. Previous uncontrolled studies suggest that epidural glucocorticoid injections provide short-term pain relief for some patients with spinal stenosis [3]. While complications from the procedure are rare, a multistate outbreak of fungal meningitis due to contaminated glucocorticoid injections affected at least 751 patients with 64 deaths in 2012 [4].
The purpose of the current study by Friedly et al was to determine whether adding a glucocorticoid to an anesthetic in epidural spinal injections is superior to anesthetic alone for symptom relief and functional improvement in patients with lumbar spinal stenosis. In contrast to previous studies, the authors defined short-term results as 3 weeks after injection, and long-term results as 6 weeks after injection. Despite the shorter follow-up period, results were similar to previous studies, in that adding glucocorticoid to anesthetic in epidural spinal injection reduced pain and improved patient’s functionality short-term, but improvements were not sustained long-term. Based on these results, the authors concluded that there is no benefit in adding glucocorticoid epidural injections for back pain arising from lumbar spinal stenosis.
One major limitation of this study is the lack of a placebo arm. Because of the lack of a placebo arm, it cannot be ascertained whether epidural injection with lidocaine alone conferred a benefit. However, this study provides robust evidence that epidural steroid injections are not beneficial for treatment of back and leg pain associated with lumbar spinal stenosis.
Applications for Clinical Practice
Epidural steroid injection is long accepted in medical communities as a safe and effective treatment for lumbar spinal stenosis symptoms. In light of the potential dangers of epidural steroid injections, including meningitis, coupled with the increasing cost of the procedure, other potential side effects, and demonstrated ineffectiveness of the treatment, providers should stop recommending epidural steroid injections for lumbar spinal stenosis.
—Ka Ming Gordon Ngai, MD, MPH
1. Manchikanti L, Pampati V, Boswell MV, et al. Analysis of the growth of epidural injections and costs in the Medicare population: a comparative evaluation of 1997, 2002, and 2006 data. Pain Physician 2010;13:199–212.
2. Manchikanti L, Pampati V, Falco FJ, et al. Assessment of the growth of epidural injections in the medicare population from 2000 to 2011. Pain Physician 2013;16:E349–364.
3. Shamliyan TA, Staal JB, Goldmann D, et al. Epidural steroid injections for radicular lumbosacral pain: a systematic review. Phys Med Rehabil Clin North Am 2014;25:471–89.
4. CDC. Multistate outbreak of fungal meningitis and other infections. 23 Oct 2013. Accessed 9 Jul 2014 at www.cdc.gov/hai/outbreaks/meningitis.html.
1. Manchikanti L, Pampati V, Boswell MV, et al. Analysis of the growth of epidural injections and costs in the Medicare population: a comparative evaluation of 1997, 2002, and 2006 data. Pain Physician 2010;13:199–212.
2. Manchikanti L, Pampati V, Falco FJ, et al. Assessment of the growth of epidural injections in the medicare population from 2000 to 2011. Pain Physician 2013;16:E349–364.
3. Shamliyan TA, Staal JB, Goldmann D, et al. Epidural steroid injections for radicular lumbosacral pain: a systematic review. Phys Med Rehabil Clin North Am 2014;25:471–89.
4. CDC. Multistate outbreak of fungal meningitis and other infections. 23 Oct 2013. Accessed 9 Jul 2014 at www.cdc.gov/hai/outbreaks/meningitis.html.