User login
HIPAA: One last deadline looms
Last July, I summarized the significant changes in the Health Insurance Portability and Accountability Act (HIPAA). With the last of the deadlines mandated by those changes fast approaching, and a significant enforcement action levied against a dermatology group in the interim, an update is warranted.
The deadline is Sept. 23; by then, all of your business associate (BA) agreements must be modified to reflect the new privacy rules. A recent enforcement action involved a Massachusetts dermatology group that was hit with a substantial fine for violating one of those rules, sending a clear signal from the Centers for Medicare & Medicaid Services (CMS) and its enforcer, the Office for Civil Rights, that these tighter regulations cannot be taken lightly.
The criteria for identifying BAs remain the same: Nonemployees, performing "functions or activities" on behalf of the "covered entity" (your practice), that involve "creating, receiving, maintaining, or transmitting" personal health information (PHI).
Typical BAs include answering and billing services, independent transcriptionists, hardware and software companies, and any other vendors involved in creating or maintaining your medical records. Practice management consultants, attorneys, companies that store or microfilm medical records, and record-shredding services are BAs if they must have direct access to PHI in order to do their jobs.
Mail carriers, package delivery people, cleaning services, copier repairmen, bank employees, and the like are not considered BAs, even though they might conceivably come in contact with PHI on occasion. You are required to use "reasonable diligence" in limiting the PHI that these folks may encounter, but you do not need to enter into written BA agreements with them.
Independent contractors who work within your practice – aestheticians and physical therapists, for example – are not considered BAs either, and do not need to sign a BA agreement; just train them, as you do your employees. (More on HIPAA and OSHA training soon.)
What is new is the additional onus placed on physicians for confidentiality breaches committed by their BAs. It’s not enough to simply have a BA contract; you are expected to use "reasonable diligence" in monitoring the work of your BAs. BAs and their subcontractors are directly responsible for their own actions, but the primary responsibility is yours. Furthermore, you must now assume the worst-case scenario. Previously, when PHI was compromised, you would only have to notify affected patients (and the government) if there was a "significant risk of financial or reputational harm," but now, any incident involving patient records is assumed to be a breach, and must be reported.
Failure to report could subject your practice, as well as the contractor, to significant fines. That is where the Massachusetts group had trouble: It lost a thumb drive containing unencrypted PHI, and was forced to pay a $150,000 fine early this year as a result. There is no excuse for not encrypting HIPAA-protected information; encryption software is cheap, readily available, and easy to use. Had the drive lost in Massachusetts been encrypted, according to the CMS, the incident would not have been considered a breach, because its contents would not have been viewable by the finder. Stay tuned for a list of popular encryption programs. (As always, I have no financial interest in any company or product that I mention in this column.)
Patients have new rights under the new rules as well; they may now restrict any PHI shared with third-party insurers and health plans, if they pay for the services themselves. They also have the right to request copies of their electronic health records. You can bill the costs of responding to such requests. If you have EHRs, work out a system for doing this, because the response time has been decreased from 90 days to 30 – and is even shorter in some states.
If you haven’t yet revised your Notice of Privacy Practices (NPP) to explain your relationships with BAs, and their status under the new rules, do it now. You need to explain the breach notification process, as well as the new patient rights mentioned above. You must post your revised NPP in your office, and make copies available there; but you need not mail a copy to every patient.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a long-time monthly columnist for Skin & Allergy News.
Last July, I summarized the significant changes in the Health Insurance Portability and Accountability Act (HIPAA). With the last of the deadlines mandated by those changes fast approaching, and a significant enforcement action levied against a dermatology group in the interim, an update is warranted.
The deadline is Sept. 23; by then, all of your business associate (BA) agreements must be modified to reflect the new privacy rules. A recent enforcement action involved a Massachusetts dermatology group that was hit with a substantial fine for violating one of those rules, sending a clear signal from the Centers for Medicare & Medicaid Services (CMS) and its enforcer, the Office for Civil Rights, that these tighter regulations cannot be taken lightly.
The criteria for identifying BAs remain the same: Nonemployees, performing "functions or activities" on behalf of the "covered entity" (your practice), that involve "creating, receiving, maintaining, or transmitting" personal health information (PHI).
Typical BAs include answering and billing services, independent transcriptionists, hardware and software companies, and any other vendors involved in creating or maintaining your medical records. Practice management consultants, attorneys, companies that store or microfilm medical records, and record-shredding services are BAs if they must have direct access to PHI in order to do their jobs.
Mail carriers, package delivery people, cleaning services, copier repairmen, bank employees, and the like are not considered BAs, even though they might conceivably come in contact with PHI on occasion. You are required to use "reasonable diligence" in limiting the PHI that these folks may encounter, but you do not need to enter into written BA agreements with them.
Independent contractors who work within your practice – aestheticians and physical therapists, for example – are not considered BAs either, and do not need to sign a BA agreement; just train them, as you do your employees. (More on HIPAA and OSHA training soon.)
What is new is the additional onus placed on physicians for confidentiality breaches committed by their BAs. It’s not enough to simply have a BA contract; you are expected to use "reasonable diligence" in monitoring the work of your BAs. BAs and their subcontractors are directly responsible for their own actions, but the primary responsibility is yours. Furthermore, you must now assume the worst-case scenario. Previously, when PHI was compromised, you would only have to notify affected patients (and the government) if there was a "significant risk of financial or reputational harm," but now, any incident involving patient records is assumed to be a breach, and must be reported.
Failure to report could subject your practice, as well as the contractor, to significant fines. That is where the Massachusetts group had trouble: It lost a thumb drive containing unencrypted PHI, and was forced to pay a $150,000 fine early this year as a result. There is no excuse for not encrypting HIPAA-protected information; encryption software is cheap, readily available, and easy to use. Had the drive lost in Massachusetts been encrypted, according to the CMS, the incident would not have been considered a breach, because its contents would not have been viewable by the finder. Stay tuned for a list of popular encryption programs. (As always, I have no financial interest in any company or product that I mention in this column.)
Patients have new rights under the new rules as well; they may now restrict any PHI shared with third-party insurers and health plans, if they pay for the services themselves. They also have the right to request copies of their electronic health records. You can bill the costs of responding to such requests. If you have EHRs, work out a system for doing this, because the response time has been decreased from 90 days to 30 – and is even shorter in some states.
If you haven’t yet revised your Notice of Privacy Practices (NPP) to explain your relationships with BAs, and their status under the new rules, do it now. You need to explain the breach notification process, as well as the new patient rights mentioned above. You must post your revised NPP in your office, and make copies available there; but you need not mail a copy to every patient.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a long-time monthly columnist for Skin & Allergy News.
Last July, I summarized the significant changes in the Health Insurance Portability and Accountability Act (HIPAA). With the last of the deadlines mandated by those changes fast approaching, and a significant enforcement action levied against a dermatology group in the interim, an update is warranted.
The deadline is Sept. 23; by then, all of your business associate (BA) agreements must be modified to reflect the new privacy rules. A recent enforcement action involved a Massachusetts dermatology group that was hit with a substantial fine for violating one of those rules, sending a clear signal from the Centers for Medicare & Medicaid Services (CMS) and its enforcer, the Office for Civil Rights, that these tighter regulations cannot be taken lightly.
The criteria for identifying BAs remain the same: Nonemployees, performing "functions or activities" on behalf of the "covered entity" (your practice), that involve "creating, receiving, maintaining, or transmitting" personal health information (PHI).
Typical BAs include answering and billing services, independent transcriptionists, hardware and software companies, and any other vendors involved in creating or maintaining your medical records. Practice management consultants, attorneys, companies that store or microfilm medical records, and record-shredding services are BAs if they must have direct access to PHI in order to do their jobs.
Mail carriers, package delivery people, cleaning services, copier repairmen, bank employees, and the like are not considered BAs, even though they might conceivably come in contact with PHI on occasion. You are required to use "reasonable diligence" in limiting the PHI that these folks may encounter, but you do not need to enter into written BA agreements with them.
Independent contractors who work within your practice – aestheticians and physical therapists, for example – are not considered BAs either, and do not need to sign a BA agreement; just train them, as you do your employees. (More on HIPAA and OSHA training soon.)
What is new is the additional onus placed on physicians for confidentiality breaches committed by their BAs. It’s not enough to simply have a BA contract; you are expected to use "reasonable diligence" in monitoring the work of your BAs. BAs and their subcontractors are directly responsible for their own actions, but the primary responsibility is yours. Furthermore, you must now assume the worst-case scenario. Previously, when PHI was compromised, you would only have to notify affected patients (and the government) if there was a "significant risk of financial or reputational harm," but now, any incident involving patient records is assumed to be a breach, and must be reported.
Failure to report could subject your practice, as well as the contractor, to significant fines. That is where the Massachusetts group had trouble: It lost a thumb drive containing unencrypted PHI, and was forced to pay a $150,000 fine early this year as a result. There is no excuse for not encrypting HIPAA-protected information; encryption software is cheap, readily available, and easy to use. Had the drive lost in Massachusetts been encrypted, according to the CMS, the incident would not have been considered a breach, because its contents would not have been viewable by the finder. Stay tuned for a list of popular encryption programs. (As always, I have no financial interest in any company or product that I mention in this column.)
Patients have new rights under the new rules as well; they may now restrict any PHI shared with third-party insurers and health plans, if they pay for the services themselves. They also have the right to request copies of their electronic health records. You can bill the costs of responding to such requests. If you have EHRs, work out a system for doing this, because the response time has been decreased from 90 days to 30 – and is even shorter in some states.
If you haven’t yet revised your Notice of Privacy Practices (NPP) to explain your relationships with BAs, and their status under the new rules, do it now. You need to explain the breach notification process, as well as the new patient rights mentioned above. You must post your revised NPP in your office, and make copies available there; but you need not mail a copy to every patient.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a long-time monthly columnist for Skin & Allergy News.
Meds fall short for type B dissection
BOSTON – Medical therapy for acute uncomplicated type B aortic dissections was effective in the short term, but was associated with low 6-year intervention-free survival in a review of 298 cases.
Furthermore, patients who received medical therapy without operative intervention had increased mortality at 6 years, compared with those who received intervention, Dr. Christopher A. Durham reported at the Vascular Annual Meeting.
During a mean follow-up of nearly 4.3 years, medical therapy failed in almost 60% of the patients; 114 died after an average of 2.7 years, and 87 required aortic intervention.
Aneurysmal degeneration was the indication for intervention in 65% of patients requiring intervention, and six of these patients experienced a ruptured aneurysm. "Only six of these patients underwent stent placement, with the remainder receiving open aortic replacement," said Dr. Durham of Massachusetts General Hospital, Boston.
The average time to operation in this subset of patients was 2.3 years. Visceral malperfusion was the indication for intervention in 18 patients (21%), and most underwent an endovascular intervention including either stenting or endovascular fenestration. A less common indication for intervention was retrograde type A dissection development in two patients. These patients underwent open replacement.
The average time to intervention in the subset of patients whose indication was not aneurysmal degeneration was 24 days. Early treatment failure – within 15 days of presentation – occurred in 37 patients (12%) and included 12 deaths and 25 interventions.
"In this group of patients who ultimately required an intervention within the acute period, aneurysmal degeneration was the indication in 25% of patients, all of whom were treated with an open approach," Dr. Durham said. Visceral malperfusion was the indication in half of the early interventions.
The 30-day mortality rate among patients with early intervention after initial medical therapy was 12%.
Freedom from intervention was 74% at 6 years, with most interventions occurring within the first 12 months. Intervention-free survival was 55% at 3 years and 41% at 6 years. Only end-stage renal disease was found to be predictive of failure, and age over 70 years was protective against failure (hazard ratio, 0.97), Dr. Durham said, adding that no variables associated with progression to intervention were identified.
Notably, although survival was similar during the first 3 years in those who remained on medical management and those who required intervention (73% and 78%, respectively), survival at 6 years was 58% and 76% in the groups, respectively.
"These data join emerging data demonstrating a survival benefit in patients undergoing intervention when compared to those who are treated with medical therapy alone," he said.
Study subjects were all patients who were initially managed medically for acute uncomplicated type B aortic dissection between March 1999 and March 2011 in a health care system. The patients had a mean age of 66 years at presentation, about 62% were men, and most were white. Nearly 75% had hypertension, and most of those were on therapy. About 5% had end-stage renal disease.
Failure of medical therapy was defined as any death or aortic-related intervention. Early failure was defined as failure within 15 days of presentation.
"Aortic dissection is the most common catastrophic event affecting the aorta, with an incidence exceeding that of ruptured abdominal aortic aneurysm.
"The majority of patients with type B aortic dissections, where the entry tear originates distal to the left subclavian artery, are treated with medical therapy," he said. In fact, medical management aimed at lowering the systolic blood pressure and pulse remains the standard of care, and a number of studies have demonstrated a favorable 1-year survival – ranging from 70% to 90% – with medical therapy alone in this population.
"However, at what cost?" Dr. Durham asked.
"The principal late complication of aortic dissection is aneurysmal degeneration of the outer wall of the false lumen, which has been reported to occur in up to 40% of medically treated patients," he said, adding that, because of a paucity of contemporary data regarding the natural history of medically treated patients, it has been unclear whether the natural history has been altered with current medical therapy.
The current findings suggest that operative intervention is associated with a survival benefit.
As Food and Drug Administration "approval has just been granted for thoracic stent grafts to be used in aortic dissection, it is clear that endovascular coverage of proximal aortic entry tears will become more common in the acute phase. As such, further study is needed to determine which patients presenting with type B dissections will benefit from early intervention," he said.
Dr. Durham reported having no disclosures.
Editor’s Note: The treatment of type B dissection is a controversial subject and this controversy will be addressed in an upcoming Point/Counterpoint article in Vascular Specialist.
BOSTON – Medical therapy for acute uncomplicated type B aortic dissections was effective in the short term, but was associated with low 6-year intervention-free survival in a review of 298 cases.
Furthermore, patients who received medical therapy without operative intervention had increased mortality at 6 years, compared with those who received intervention, Dr. Christopher A. Durham reported at the Vascular Annual Meeting.
During a mean follow-up of nearly 4.3 years, medical therapy failed in almost 60% of the patients; 114 died after an average of 2.7 years, and 87 required aortic intervention.
Aneurysmal degeneration was the indication for intervention in 65% of patients requiring intervention, and six of these patients experienced a ruptured aneurysm. "Only six of these patients underwent stent placement, with the remainder receiving open aortic replacement," said Dr. Durham of Massachusetts General Hospital, Boston.
The average time to operation in this subset of patients was 2.3 years. Visceral malperfusion was the indication for intervention in 18 patients (21%), and most underwent an endovascular intervention including either stenting or endovascular fenestration. A less common indication for intervention was retrograde type A dissection development in two patients. These patients underwent open replacement.
The average time to intervention in the subset of patients whose indication was not aneurysmal degeneration was 24 days. Early treatment failure – within 15 days of presentation – occurred in 37 patients (12%) and included 12 deaths and 25 interventions.
"In this group of patients who ultimately required an intervention within the acute period, aneurysmal degeneration was the indication in 25% of patients, all of whom were treated with an open approach," Dr. Durham said. Visceral malperfusion was the indication in half of the early interventions.
The 30-day mortality rate among patients with early intervention after initial medical therapy was 12%.
Freedom from intervention was 74% at 6 years, with most interventions occurring within the first 12 months. Intervention-free survival was 55% at 3 years and 41% at 6 years. Only end-stage renal disease was found to be predictive of failure, and age over 70 years was protective against failure (hazard ratio, 0.97), Dr. Durham said, adding that no variables associated with progression to intervention were identified.
Notably, although survival was similar during the first 3 years in those who remained on medical management and those who required intervention (73% and 78%, respectively), survival at 6 years was 58% and 76% in the groups, respectively.
"These data join emerging data demonstrating a survival benefit in patients undergoing intervention when compared to those who are treated with medical therapy alone," he said.
Study subjects were all patients who were initially managed medically for acute uncomplicated type B aortic dissection between March 1999 and March 2011 in a health care system. The patients had a mean age of 66 years at presentation, about 62% were men, and most were white. Nearly 75% had hypertension, and most of those were on therapy. About 5% had end-stage renal disease.
Failure of medical therapy was defined as any death or aortic-related intervention. Early failure was defined as failure within 15 days of presentation.
"Aortic dissection is the most common catastrophic event affecting the aorta, with an incidence exceeding that of ruptured abdominal aortic aneurysm.
"The majority of patients with type B aortic dissections, where the entry tear originates distal to the left subclavian artery, are treated with medical therapy," he said. In fact, medical management aimed at lowering the systolic blood pressure and pulse remains the standard of care, and a number of studies have demonstrated a favorable 1-year survival – ranging from 70% to 90% – with medical therapy alone in this population.
"However, at what cost?" Dr. Durham asked.
"The principal late complication of aortic dissection is aneurysmal degeneration of the outer wall of the false lumen, which has been reported to occur in up to 40% of medically treated patients," he said, adding that, because of a paucity of contemporary data regarding the natural history of medically treated patients, it has been unclear whether the natural history has been altered with current medical therapy.
The current findings suggest that operative intervention is associated with a survival benefit.
As Food and Drug Administration "approval has just been granted for thoracic stent grafts to be used in aortic dissection, it is clear that endovascular coverage of proximal aortic entry tears will become more common in the acute phase. As such, further study is needed to determine which patients presenting with type B dissections will benefit from early intervention," he said.
Dr. Durham reported having no disclosures.
Editor’s Note: The treatment of type B dissection is a controversial subject and this controversy will be addressed in an upcoming Point/Counterpoint article in Vascular Specialist.
BOSTON – Medical therapy for acute uncomplicated type B aortic dissections was effective in the short term, but was associated with low 6-year intervention-free survival in a review of 298 cases.
Furthermore, patients who received medical therapy without operative intervention had increased mortality at 6 years, compared with those who received intervention, Dr. Christopher A. Durham reported at the Vascular Annual Meeting.
During a mean follow-up of nearly 4.3 years, medical therapy failed in almost 60% of the patients; 114 died after an average of 2.7 years, and 87 required aortic intervention.
Aneurysmal degeneration was the indication for intervention in 65% of patients requiring intervention, and six of these patients experienced a ruptured aneurysm. "Only six of these patients underwent stent placement, with the remainder receiving open aortic replacement," said Dr. Durham of Massachusetts General Hospital, Boston.
The average time to operation in this subset of patients was 2.3 years. Visceral malperfusion was the indication for intervention in 18 patients (21%), and most underwent an endovascular intervention including either stenting or endovascular fenestration. A less common indication for intervention was retrograde type A dissection development in two patients. These patients underwent open replacement.
The average time to intervention in the subset of patients whose indication was not aneurysmal degeneration was 24 days. Early treatment failure – within 15 days of presentation – occurred in 37 patients (12%) and included 12 deaths and 25 interventions.
"In this group of patients who ultimately required an intervention within the acute period, aneurysmal degeneration was the indication in 25% of patients, all of whom were treated with an open approach," Dr. Durham said. Visceral malperfusion was the indication in half of the early interventions.
The 30-day mortality rate among patients with early intervention after initial medical therapy was 12%.
Freedom from intervention was 74% at 6 years, with most interventions occurring within the first 12 months. Intervention-free survival was 55% at 3 years and 41% at 6 years. Only end-stage renal disease was found to be predictive of failure, and age over 70 years was protective against failure (hazard ratio, 0.97), Dr. Durham said, adding that no variables associated with progression to intervention were identified.
Notably, although survival was similar during the first 3 years in those who remained on medical management and those who required intervention (73% and 78%, respectively), survival at 6 years was 58% and 76% in the groups, respectively.
"These data join emerging data demonstrating a survival benefit in patients undergoing intervention when compared to those who are treated with medical therapy alone," he said.
Study subjects were all patients who were initially managed medically for acute uncomplicated type B aortic dissection between March 1999 and March 2011 in a health care system. The patients had a mean age of 66 years at presentation, about 62% were men, and most were white. Nearly 75% had hypertension, and most of those were on therapy. About 5% had end-stage renal disease.
Failure of medical therapy was defined as any death or aortic-related intervention. Early failure was defined as failure within 15 days of presentation.
"Aortic dissection is the most common catastrophic event affecting the aorta, with an incidence exceeding that of ruptured abdominal aortic aneurysm.
"The majority of patients with type B aortic dissections, where the entry tear originates distal to the left subclavian artery, are treated with medical therapy," he said. In fact, medical management aimed at lowering the systolic blood pressure and pulse remains the standard of care, and a number of studies have demonstrated a favorable 1-year survival – ranging from 70% to 90% – with medical therapy alone in this population.
"However, at what cost?" Dr. Durham asked.
"The principal late complication of aortic dissection is aneurysmal degeneration of the outer wall of the false lumen, which has been reported to occur in up to 40% of medically treated patients," he said, adding that, because of a paucity of contemporary data regarding the natural history of medically treated patients, it has been unclear whether the natural history has been altered with current medical therapy.
The current findings suggest that operative intervention is associated with a survival benefit.
As Food and Drug Administration "approval has just been granted for thoracic stent grafts to be used in aortic dissection, it is clear that endovascular coverage of proximal aortic entry tears will become more common in the acute phase. As such, further study is needed to determine which patients presenting with type B dissections will benefit from early intervention," he said.
Dr. Durham reported having no disclosures.
Editor’s Note: The treatment of type B dissection is a controversial subject and this controversy will be addressed in an upcoming Point/Counterpoint article in Vascular Specialist.
Major finding: Medical therapy failed in nearly 60% of patients during 4.3 years of follow-up.
Data source: A series of 298 cases.
Disclosures: Dr. Durham reported having no disclosures.
USPSTF: Screen older women smokers for AAA
The U.S. Preventive Services Task Force says that women between ages 65 and 75 years who have smoked 100 or more cigarettes in their lives could benefit from one-time ultrasonography screening for abdominal aortic aneurysm (AAA).
The AAA guidelines replace those published by USPSTF in 2005, which had recommended against screening women regardless of their smoking history.
The new guidelines, published online June 23 in Annals of Internal Medicine (doi:10.7326/M14-1204), do not recommend screening women who have never smoked, citing the very low prevalence of AAA in this group.
Nevertheless, the task force’s systematic review, led by current chair Dr. Michael L. LeFevre of the University of Missouri in Columbia, revealed that screening women aged 65-75 years who have smoked or currently smoke – a group for which AAA prevalence is between 0.8% and 2% – could potentially be beneficial, though current evidence remains insufficient to recommend it.
"Prevalence of AAA in women who currently smoke approaches that of men who have never smoked," Dr. LeFevre and his colleagues wrote in the guidelines.
"As such, a small net benefit might exist for this population and appropriate, high-quality research designs should be used to address this question," they added.
The task force continues to recommend that men between the ages of 65 and 75 years who have ever smoked be offered one-time screening with ultrasonography for AAA. Men in this age group who have never smoked may be offered screening if they have certain risk factors, such as advanced age or a family history of AAA.
AAA – a dilation in the wall of the abdominal section of the aorta of 3 cm or larger – is seen in 4% and 7% of men and about 1% of women over the age of 50, USPSTF said. Most AAAs remain asymptomatic until they rupture, in which case the mortality risk has been shown to be higher than 75%.
Women who develop AAA tend to do so at a later age than do men, the task force noted, with most ruptures occurring past age 80 years.
The task force is a voluntary advisory body independent of the U.S. government but supported by the Agency for Healthcare Research and Quality. One of the study’s co-authors, Dr. Douglas Owens of Stanford (Calif.) University disclosed travel support from the agency during the course of the review.
The other task force members declared no conflicts of interest.
The U.S. Preventive Services Task Force says that women between ages 65 and 75 years who have smoked 100 or more cigarettes in their lives could benefit from one-time ultrasonography screening for abdominal aortic aneurysm (AAA).
The AAA guidelines replace those published by USPSTF in 2005, which had recommended against screening women regardless of their smoking history.
The new guidelines, published online June 23 in Annals of Internal Medicine (doi:10.7326/M14-1204), do not recommend screening women who have never smoked, citing the very low prevalence of AAA in this group.
Nevertheless, the task force’s systematic review, led by current chair Dr. Michael L. LeFevre of the University of Missouri in Columbia, revealed that screening women aged 65-75 years who have smoked or currently smoke – a group for which AAA prevalence is between 0.8% and 2% – could potentially be beneficial, though current evidence remains insufficient to recommend it.
"Prevalence of AAA in women who currently smoke approaches that of men who have never smoked," Dr. LeFevre and his colleagues wrote in the guidelines.
"As such, a small net benefit might exist for this population and appropriate, high-quality research designs should be used to address this question," they added.
The task force continues to recommend that men between the ages of 65 and 75 years who have ever smoked be offered one-time screening with ultrasonography for AAA. Men in this age group who have never smoked may be offered screening if they have certain risk factors, such as advanced age or a family history of AAA.
AAA – a dilation in the wall of the abdominal section of the aorta of 3 cm or larger – is seen in 4% and 7% of men and about 1% of women over the age of 50, USPSTF said. Most AAAs remain asymptomatic until they rupture, in which case the mortality risk has been shown to be higher than 75%.
Women who develop AAA tend to do so at a later age than do men, the task force noted, with most ruptures occurring past age 80 years.
The task force is a voluntary advisory body independent of the U.S. government but supported by the Agency for Healthcare Research and Quality. One of the study’s co-authors, Dr. Douglas Owens of Stanford (Calif.) University disclosed travel support from the agency during the course of the review.
The other task force members declared no conflicts of interest.
The U.S. Preventive Services Task Force says that women between ages 65 and 75 years who have smoked 100 or more cigarettes in their lives could benefit from one-time ultrasonography screening for abdominal aortic aneurysm (AAA).
The AAA guidelines replace those published by USPSTF in 2005, which had recommended against screening women regardless of their smoking history.
The new guidelines, published online June 23 in Annals of Internal Medicine (doi:10.7326/M14-1204), do not recommend screening women who have never smoked, citing the very low prevalence of AAA in this group.
Nevertheless, the task force’s systematic review, led by current chair Dr. Michael L. LeFevre of the University of Missouri in Columbia, revealed that screening women aged 65-75 years who have smoked or currently smoke – a group for which AAA prevalence is between 0.8% and 2% – could potentially be beneficial, though current evidence remains insufficient to recommend it.
"Prevalence of AAA in women who currently smoke approaches that of men who have never smoked," Dr. LeFevre and his colleagues wrote in the guidelines.
"As such, a small net benefit might exist for this population and appropriate, high-quality research designs should be used to address this question," they added.
The task force continues to recommend that men between the ages of 65 and 75 years who have ever smoked be offered one-time screening with ultrasonography for AAA. Men in this age group who have never smoked may be offered screening if they have certain risk factors, such as advanced age or a family history of AAA.
AAA – a dilation in the wall of the abdominal section of the aorta of 3 cm or larger – is seen in 4% and 7% of men and about 1% of women over the age of 50, USPSTF said. Most AAAs remain asymptomatic until they rupture, in which case the mortality risk has been shown to be higher than 75%.
Women who develop AAA tend to do so at a later age than do men, the task force noted, with most ruptures occurring past age 80 years.
The task force is a voluntary advisory body independent of the U.S. government but supported by the Agency for Healthcare Research and Quality. One of the study’s co-authors, Dr. Douglas Owens of Stanford (Calif.) University disclosed travel support from the agency during the course of the review.
The other task force members declared no conflicts of interest.
Key clinical point: Women aged 65-75 years who have smoked more than 100 cigarettes ever could benefit from one-time ultrasonography screening for AAA.
Major finding: Screening in women aged 65-75 years who have smoked or currently smoke – a group for which AAA prevalence is between 0.8% and 2% – could potentially be beneficial.
Data source: The USPSTF commissioned a systematic review that assessed the evidence on the benefits and harms of screening for AAA and strategies for managing small (3.0-5.4 cm) screen-detected AAAs.
Disclosures: Dr. Douglas Owens of the Stanford (Calif.) University, disclosed travel support from the agency during the course of the review.
Treatment of Actinic Keratosis With Picato® (ingenol mebutate) Gel – A New Supplement
Topics
- Introduction
- Treatment Options for Actinic Keratosis
- Treatment With Picato Gel
- Sequential Treatment With Cryosurgery and Picato Gel
- Talking With Patients About Picato Gel
- Conclusions
Faculty
Stephen K. Tyring, MD, PhD
Clinical Professor
Departments of Dermatology, Microbiology and Immunology, and Internal Medicine
University of Texas Health Science Center
Houston, Texas
Dr. Tyring discloses that he has conducted clinical research and given presentations sponsored by LEO Pharma Inc., the manufacturer of Picato®.
Topics
- Introduction
- Treatment Options for Actinic Keratosis
- Treatment With Picato Gel
- Sequential Treatment With Cryosurgery and Picato Gel
- Talking With Patients About Picato Gel
- Conclusions
Faculty
Stephen K. Tyring, MD, PhD
Clinical Professor
Departments of Dermatology, Microbiology and Immunology, and Internal Medicine
University of Texas Health Science Center
Houston, Texas
Dr. Tyring discloses that he has conducted clinical research and given presentations sponsored by LEO Pharma Inc., the manufacturer of Picato®.
Topics
- Introduction
- Treatment Options for Actinic Keratosis
- Treatment With Picato Gel
- Sequential Treatment With Cryosurgery and Picato Gel
- Talking With Patients About Picato Gel
- Conclusions
Faculty
Stephen K. Tyring, MD, PhD
Clinical Professor
Departments of Dermatology, Microbiology and Immunology, and Internal Medicine
University of Texas Health Science Center
Houston, Texas
Dr. Tyring discloses that he has conducted clinical research and given presentations sponsored by LEO Pharma Inc., the manufacturer of Picato®.
The hospital discharge process: Call for technology’s help
While being discharged from the hospital even after a minor procedure is not simple, the process for a patient with comorbidities after a prolonged stay is daunting.
Physicians from multiple specialties, various nonphysician providers, the social worker, and the case manager all address different discharge-related issues. It is frustrating for both a provider and patient to experience the "I really can’t answer that question" moment. Lack of interdisciplinary communication may lead to medical errors and either premature or delayed discharges.
The date of discharge is estimated soon after admission. Some hospitals have a focus on the clock when planning discharges. If planning occurs too early, it does not account for changes in patient needs and wrong instructions might be given. Transportation and home-aide needs are time sensitive.
In contrast, some planning does need to be considered early in the admission when discharge to a non-acute care facility is obvious due to the diagnosis and/or social situation of the patient.
One study from the Brigham and Women’s Hospital identified seven clinical factors predicting hospital readmission: a hemoglobin less than 12 g/dL on discharge, discharge from an oncology service, low serum sodium level on discharge, a procedure (via ICD-9 standards) during admission, nonelective admission, length of stay greater than 4 days, and number of admissions during the previous year (JAMA Intern. Med. 2013;173:632-8).
Another study examined many predictive models found in the literature.
The researchers found that "of 7,843 citations reviewed, 30 studies of 26 unique models met the inclusion criteria. The most common outcome used was 30-day readmission; only 1 model specifically addressed preventable readmissions. Fourteen models that relied on retrospective administrative data could be potentially used to risk-adjust readmission rates for hospital comparison; of these, 9 were tested in large U.S. populations and had poor discriminative ability. ... Seven models could potentially be used to identify high-risk patients for intervention early during a hospitalization, ... and 5 could be used at hospital discharge" (JAMA 2011;306:1688-98).
The authors concluded that most prediction models perform poorly or require improvement. Perhaps one reason for this result lies in the fact that these models traditionally are either clinical or administrative. I believe a better approach is to combine administrative and clinical predictive models. Better analytics programs applied real-time in the electronic health record (EHR) will facilitate integration of these perspectives.
The topic of transitional care has received attention because a poor discharge process results in higher readmission rates, a new benchmark focus of Medicare (Am. J. Nurs. 2008;108:58-63). Hospitals might be very good at meeting regulatory requirements, but the patient’s understanding of diagnoses and instructions is often unclear. Though required by regulations, the caregiver may not even be included in the process. Technology can help in this situation. Some of possibilities mentioned below might not be available in the context described.
• Durable equipment needs. The care coordinator is generally the point person regarding the patient’s durable equipment needs upon discharge. Ordering the equipment (specifications as well as date, time, and place of delivery) might be the job of someone else, such as a therapist or physician. Digital tools can expedite equipment procurement. Analytics from the EHR (mining diagnoses, equipment in use at the end of the hospitalization, expected place of transition, etc.) might determine the individual’s ambulation, oxygen, bed, or other equipment requirements. This can act as a preliminary checklist for the coordinator, doing away with the need to personally go through the EHR or surveying providers. A digital ordering program can directly interact with the distributor to check product availability and verify delivery. Another useful tool would aggregate equipment distributors, which are stratified according to certification (Medicare bidding approval status), lowest price, and best-rated service.
• Visiting nurses. Often the home-needs assessment for visiting nurses is done once the patient is discharged. This can be expedited with the help of a caregiver, with the assessment completed in the hospital. Consider a tool into which the physician’s orders or recommendations for home nursing are placed and shared with the visiting nurse entity, the patient, and the caregiver. It would include the nursing assessment as well as a video of the home environment (a factor in the assessment itself). This would obviate the need for a dedicated assessment visit. Visiting nurses themselves should be equipped with mobile technology to document their time schedule for billing, to record interventions, and to record and transmit vital signs (measured via digital remote monitors) and orders; the technology also should contain a digital messaging program.
• Scheduling of outpatient provider appointments. Evidence suggests that in a general medical population, early follow-up appointments do not affect readmission rates (Arch. Intern. Med. 2010;170:955-60). However, some patients, including those with heart failure have been shown to benefit from early follow-up (JAMA 2010;303:1716-22). The success of a growing number of commercially available mobile apps intended to streamline scheduling of physician appointments is testimony to this need in the nonacute setting. Patient portal use is a requirement of Meaningful Use Stage 2. One way of encouraging patient participation in portal use would be activating it by utilizing a discharge planning scheduling application of the portal at the time of discharge. This also fits into an overall strategy of point of engagement implementation of technology.
These are only a few highlights of the complexity of the discharge process. All physicians have dealt with the many questions, complications, and frustration experienced by patients after discharge. A failed process creates unnecessary work, expense, and bad outcomes.
To many physicians, digital health technology is represented by the EHR in its present form, which is not what the doctor ordered. It is not intuitive, it is cumbersome, and it encourages impersonal encounters with patients. I will explore in future posts how digital technologies other than the EHR will change medicine in ways that physicians will appreciate.
Dr. Scher, a practicing cardiac electrophysiologist in Lancaster, Pa., is director at DLS Healthcare Consulting, advising technology companies and health care enterprises on development and adoption of mobile health technologies.
While being discharged from the hospital even after a minor procedure is not simple, the process for a patient with comorbidities after a prolonged stay is daunting.
Physicians from multiple specialties, various nonphysician providers, the social worker, and the case manager all address different discharge-related issues. It is frustrating for both a provider and patient to experience the "I really can’t answer that question" moment. Lack of interdisciplinary communication may lead to medical errors and either premature or delayed discharges.
The date of discharge is estimated soon after admission. Some hospitals have a focus on the clock when planning discharges. If planning occurs too early, it does not account for changes in patient needs and wrong instructions might be given. Transportation and home-aide needs are time sensitive.
In contrast, some planning does need to be considered early in the admission when discharge to a non-acute care facility is obvious due to the diagnosis and/or social situation of the patient.
One study from the Brigham and Women’s Hospital identified seven clinical factors predicting hospital readmission: a hemoglobin less than 12 g/dL on discharge, discharge from an oncology service, low serum sodium level on discharge, a procedure (via ICD-9 standards) during admission, nonelective admission, length of stay greater than 4 days, and number of admissions during the previous year (JAMA Intern. Med. 2013;173:632-8).
Another study examined many predictive models found in the literature.
The researchers found that "of 7,843 citations reviewed, 30 studies of 26 unique models met the inclusion criteria. The most common outcome used was 30-day readmission; only 1 model specifically addressed preventable readmissions. Fourteen models that relied on retrospective administrative data could be potentially used to risk-adjust readmission rates for hospital comparison; of these, 9 were tested in large U.S. populations and had poor discriminative ability. ... Seven models could potentially be used to identify high-risk patients for intervention early during a hospitalization, ... and 5 could be used at hospital discharge" (JAMA 2011;306:1688-98).
The authors concluded that most prediction models perform poorly or require improvement. Perhaps one reason for this result lies in the fact that these models traditionally are either clinical or administrative. I believe a better approach is to combine administrative and clinical predictive models. Better analytics programs applied real-time in the electronic health record (EHR) will facilitate integration of these perspectives.
The topic of transitional care has received attention because a poor discharge process results in higher readmission rates, a new benchmark focus of Medicare (Am. J. Nurs. 2008;108:58-63). Hospitals might be very good at meeting regulatory requirements, but the patient’s understanding of diagnoses and instructions is often unclear. Though required by regulations, the caregiver may not even be included in the process. Technology can help in this situation. Some of possibilities mentioned below might not be available in the context described.
• Durable equipment needs. The care coordinator is generally the point person regarding the patient’s durable equipment needs upon discharge. Ordering the equipment (specifications as well as date, time, and place of delivery) might be the job of someone else, such as a therapist or physician. Digital tools can expedite equipment procurement. Analytics from the EHR (mining diagnoses, equipment in use at the end of the hospitalization, expected place of transition, etc.) might determine the individual’s ambulation, oxygen, bed, or other equipment requirements. This can act as a preliminary checklist for the coordinator, doing away with the need to personally go through the EHR or surveying providers. A digital ordering program can directly interact with the distributor to check product availability and verify delivery. Another useful tool would aggregate equipment distributors, which are stratified according to certification (Medicare bidding approval status), lowest price, and best-rated service.
• Visiting nurses. Often the home-needs assessment for visiting nurses is done once the patient is discharged. This can be expedited with the help of a caregiver, with the assessment completed in the hospital. Consider a tool into which the physician’s orders or recommendations for home nursing are placed and shared with the visiting nurse entity, the patient, and the caregiver. It would include the nursing assessment as well as a video of the home environment (a factor in the assessment itself). This would obviate the need for a dedicated assessment visit. Visiting nurses themselves should be equipped with mobile technology to document their time schedule for billing, to record interventions, and to record and transmit vital signs (measured via digital remote monitors) and orders; the technology also should contain a digital messaging program.
• Scheduling of outpatient provider appointments. Evidence suggests that in a general medical population, early follow-up appointments do not affect readmission rates (Arch. Intern. Med. 2010;170:955-60). However, some patients, including those with heart failure have been shown to benefit from early follow-up (JAMA 2010;303:1716-22). The success of a growing number of commercially available mobile apps intended to streamline scheduling of physician appointments is testimony to this need in the nonacute setting. Patient portal use is a requirement of Meaningful Use Stage 2. One way of encouraging patient participation in portal use would be activating it by utilizing a discharge planning scheduling application of the portal at the time of discharge. This also fits into an overall strategy of point of engagement implementation of technology.
These are only a few highlights of the complexity of the discharge process. All physicians have dealt with the many questions, complications, and frustration experienced by patients after discharge. A failed process creates unnecessary work, expense, and bad outcomes.
To many physicians, digital health technology is represented by the EHR in its present form, which is not what the doctor ordered. It is not intuitive, it is cumbersome, and it encourages impersonal encounters with patients. I will explore in future posts how digital technologies other than the EHR will change medicine in ways that physicians will appreciate.
Dr. Scher, a practicing cardiac electrophysiologist in Lancaster, Pa., is director at DLS Healthcare Consulting, advising technology companies and health care enterprises on development and adoption of mobile health technologies.
While being discharged from the hospital even after a minor procedure is not simple, the process for a patient with comorbidities after a prolonged stay is daunting.
Physicians from multiple specialties, various nonphysician providers, the social worker, and the case manager all address different discharge-related issues. It is frustrating for both a provider and patient to experience the "I really can’t answer that question" moment. Lack of interdisciplinary communication may lead to medical errors and either premature or delayed discharges.
The date of discharge is estimated soon after admission. Some hospitals have a focus on the clock when planning discharges. If planning occurs too early, it does not account for changes in patient needs and wrong instructions might be given. Transportation and home-aide needs are time sensitive.
In contrast, some planning does need to be considered early in the admission when discharge to a non-acute care facility is obvious due to the diagnosis and/or social situation of the patient.
One study from the Brigham and Women’s Hospital identified seven clinical factors predicting hospital readmission: a hemoglobin less than 12 g/dL on discharge, discharge from an oncology service, low serum sodium level on discharge, a procedure (via ICD-9 standards) during admission, nonelective admission, length of stay greater than 4 days, and number of admissions during the previous year (JAMA Intern. Med. 2013;173:632-8).
Another study examined many predictive models found in the literature.
The researchers found that "of 7,843 citations reviewed, 30 studies of 26 unique models met the inclusion criteria. The most common outcome used was 30-day readmission; only 1 model specifically addressed preventable readmissions. Fourteen models that relied on retrospective administrative data could be potentially used to risk-adjust readmission rates for hospital comparison; of these, 9 were tested in large U.S. populations and had poor discriminative ability. ... Seven models could potentially be used to identify high-risk patients for intervention early during a hospitalization, ... and 5 could be used at hospital discharge" (JAMA 2011;306:1688-98).
The authors concluded that most prediction models perform poorly or require improvement. Perhaps one reason for this result lies in the fact that these models traditionally are either clinical or administrative. I believe a better approach is to combine administrative and clinical predictive models. Better analytics programs applied real-time in the electronic health record (EHR) will facilitate integration of these perspectives.
The topic of transitional care has received attention because a poor discharge process results in higher readmission rates, a new benchmark focus of Medicare (Am. J. Nurs. 2008;108:58-63). Hospitals might be very good at meeting regulatory requirements, but the patient’s understanding of diagnoses and instructions is often unclear. Though required by regulations, the caregiver may not even be included in the process. Technology can help in this situation. Some of possibilities mentioned below might not be available in the context described.
• Durable equipment needs. The care coordinator is generally the point person regarding the patient’s durable equipment needs upon discharge. Ordering the equipment (specifications as well as date, time, and place of delivery) might be the job of someone else, such as a therapist or physician. Digital tools can expedite equipment procurement. Analytics from the EHR (mining diagnoses, equipment in use at the end of the hospitalization, expected place of transition, etc.) might determine the individual’s ambulation, oxygen, bed, or other equipment requirements. This can act as a preliminary checklist for the coordinator, doing away with the need to personally go through the EHR or surveying providers. A digital ordering program can directly interact with the distributor to check product availability and verify delivery. Another useful tool would aggregate equipment distributors, which are stratified according to certification (Medicare bidding approval status), lowest price, and best-rated service.
• Visiting nurses. Often the home-needs assessment for visiting nurses is done once the patient is discharged. This can be expedited with the help of a caregiver, with the assessment completed in the hospital. Consider a tool into which the physician’s orders or recommendations for home nursing are placed and shared with the visiting nurse entity, the patient, and the caregiver. It would include the nursing assessment as well as a video of the home environment (a factor in the assessment itself). This would obviate the need for a dedicated assessment visit. Visiting nurses themselves should be equipped with mobile technology to document their time schedule for billing, to record interventions, and to record and transmit vital signs (measured via digital remote monitors) and orders; the technology also should contain a digital messaging program.
• Scheduling of outpatient provider appointments. Evidence suggests that in a general medical population, early follow-up appointments do not affect readmission rates (Arch. Intern. Med. 2010;170:955-60). However, some patients, including those with heart failure have been shown to benefit from early follow-up (JAMA 2010;303:1716-22). The success of a growing number of commercially available mobile apps intended to streamline scheduling of physician appointments is testimony to this need in the nonacute setting. Patient portal use is a requirement of Meaningful Use Stage 2. One way of encouraging patient participation in portal use would be activating it by utilizing a discharge planning scheduling application of the portal at the time of discharge. This also fits into an overall strategy of point of engagement implementation of technology.
These are only a few highlights of the complexity of the discharge process. All physicians have dealt with the many questions, complications, and frustration experienced by patients after discharge. A failed process creates unnecessary work, expense, and bad outcomes.
To many physicians, digital health technology is represented by the EHR in its present form, which is not what the doctor ordered. It is not intuitive, it is cumbersome, and it encourages impersonal encounters with patients. I will explore in future posts how digital technologies other than the EHR will change medicine in ways that physicians will appreciate.
Dr. Scher, a practicing cardiac electrophysiologist in Lancaster, Pa., is director at DLS Healthcare Consulting, advising technology companies and health care enterprises on development and adoption of mobile health technologies.
New insight into MYC-induced lymphoma

Credit: Juha Klefstrom
Investigators have identified biological signatures in lymphoma cells that can be traced back to the original oncogene.
The team analyzed mouse models and patient samples of MYC-induced lymphoma. And they discovered lipid signatures that corresponded with the level of MYC expression.
The investigators believe this discovery could be the first step toward developing a technique to identify the origin of lymphomas and other malignancies.
They described their discovery in PNAS.
“The same cancer can occur because of different genes, but, in certain cases, the aggressiveness and the type of treatment actually depend a lot on what oncogene caused that cancer,” said study author Livia Eberlin, PhD, of Stanford University in California.
With that in mind, she and her colleagues looked at MYC, an oncogene that’s responsible for approximately half of all human cancers. They wanted to find a biological signature that would trace the mutating cancer cells back to the original oncogene.
“When cancer takes place, the cell loves to gobble up glucose—that’s a sugar—and glutamine,” said Richard Zare, PhD, also of Stanford. “It takes those and makes different lipids—different fatty molecules than what it normally makes.”
So the investigators set out to evaluate changes in lipid profiles in MYC-induced lymphoma. They compared lipid signatures in MYC-induced transgenic mouse models to those in normal control mice.
The team identified 104 molecular ions that were either increased or decreased in the MYC lymphoma models compared to controls. And 86 of these ions were complex phospholipids.
Most of the lipids that were increased in lymphoma were glycerophosphoglycerols and cardiolipins, with a higher content of monounsaturated fatty acids when compared with controls.
To determine if these findings might also apply to humans, the investigators examined 15 samples from lymphoma patients.
The samples had varying expression levels of MYC oncoprotein, and the team observed distinct lipid profiles in lymphomas with high and low MYC expression. This included many of the lipid species they had identified in the animal models of MYC-induced lymphoma.
The investigators said their results suggest a relationship between specific lipid species and the overexpression of MYC. And this information could have both diagnostic and prognostic applications. ![]()

Credit: Juha Klefstrom
Investigators have identified biological signatures in lymphoma cells that can be traced back to the original oncogene.
The team analyzed mouse models and patient samples of MYC-induced lymphoma. And they discovered lipid signatures that corresponded with the level of MYC expression.
The investigators believe this discovery could be the first step toward developing a technique to identify the origin of lymphomas and other malignancies.
They described their discovery in PNAS.
“The same cancer can occur because of different genes, but, in certain cases, the aggressiveness and the type of treatment actually depend a lot on what oncogene caused that cancer,” said study author Livia Eberlin, PhD, of Stanford University in California.
With that in mind, she and her colleagues looked at MYC, an oncogene that’s responsible for approximately half of all human cancers. They wanted to find a biological signature that would trace the mutating cancer cells back to the original oncogene.
“When cancer takes place, the cell loves to gobble up glucose—that’s a sugar—and glutamine,” said Richard Zare, PhD, also of Stanford. “It takes those and makes different lipids—different fatty molecules than what it normally makes.”
So the investigators set out to evaluate changes in lipid profiles in MYC-induced lymphoma. They compared lipid signatures in MYC-induced transgenic mouse models to those in normal control mice.
The team identified 104 molecular ions that were either increased or decreased in the MYC lymphoma models compared to controls. And 86 of these ions were complex phospholipids.
Most of the lipids that were increased in lymphoma were glycerophosphoglycerols and cardiolipins, with a higher content of monounsaturated fatty acids when compared with controls.
To determine if these findings might also apply to humans, the investigators examined 15 samples from lymphoma patients.
The samples had varying expression levels of MYC oncoprotein, and the team observed distinct lipid profiles in lymphomas with high and low MYC expression. This included many of the lipid species they had identified in the animal models of MYC-induced lymphoma.
The investigators said their results suggest a relationship between specific lipid species and the overexpression of MYC. And this information could have both diagnostic and prognostic applications. ![]()

Credit: Juha Klefstrom
Investigators have identified biological signatures in lymphoma cells that can be traced back to the original oncogene.
The team analyzed mouse models and patient samples of MYC-induced lymphoma. And they discovered lipid signatures that corresponded with the level of MYC expression.
The investigators believe this discovery could be the first step toward developing a technique to identify the origin of lymphomas and other malignancies.
They described their discovery in PNAS.
“The same cancer can occur because of different genes, but, in certain cases, the aggressiveness and the type of treatment actually depend a lot on what oncogene caused that cancer,” said study author Livia Eberlin, PhD, of Stanford University in California.
With that in mind, she and her colleagues looked at MYC, an oncogene that’s responsible for approximately half of all human cancers. They wanted to find a biological signature that would trace the mutating cancer cells back to the original oncogene.
“When cancer takes place, the cell loves to gobble up glucose—that’s a sugar—and glutamine,” said Richard Zare, PhD, also of Stanford. “It takes those and makes different lipids—different fatty molecules than what it normally makes.”
So the investigators set out to evaluate changes in lipid profiles in MYC-induced lymphoma. They compared lipid signatures in MYC-induced transgenic mouse models to those in normal control mice.
The team identified 104 molecular ions that were either increased or decreased in the MYC lymphoma models compared to controls. And 86 of these ions were complex phospholipids.
Most of the lipids that were increased in lymphoma were glycerophosphoglycerols and cardiolipins, with a higher content of monounsaturated fatty acids when compared with controls.
To determine if these findings might also apply to humans, the investigators examined 15 samples from lymphoma patients.
The samples had varying expression levels of MYC oncoprotein, and the team observed distinct lipid profiles in lymphomas with high and low MYC expression. This included many of the lipid species they had identified in the animal models of MYC-induced lymphoma.
The investigators said their results suggest a relationship between specific lipid species and the overexpression of MYC. And this information could have both diagnostic and prognostic applications. ![]()
How federal budget cuts are affecting research

Credit: Rhoda Baer
A new report suggests recent budget cuts to federal health programs in the US have had some negative consequences for hematology researchers.
The Coalition for Health Funding, an alliance of more than 90 public health advocacy organizations, invited scientists, public health advocates, and others to share stories of how they have been hurt by the budget cuts.
The resulting report is titled “Faces of Austerity, How Budget Cuts Hurt America’s Health.”
It details the negative effects the cuts have had on scientific discovery and innovation, scientists and health practitioners, health and social services, and government programs designed to respond to health hazards and natural disasters.
Among the stories included in the report are 2 from members of the American Society of Hematology (ASH), who detail how a decade of flat funding for the National Institutes of Health (NIH) and a 5% budget cut in 2013 have shuttered labs and jeopardized tomorrow’s treatments.
“Most people I know have been affected,” said Debra Newman, PhD, an investigator at BloodCenter of Wisconsin in Milwaukee.
“Their research funding has decreased and, consequently, so has the size of their laboratories because they cannot afford to employ the same number of staff. Talented investigators have started to leave research and go on to other things because they can’t support a research operation without money to run it.”
The other ASH member story is that of Christopher Porter, MD, a pediatric hematologist/oncologist at Children’s Hospital Colorado in Aurora. Despite receiving an excellent score on an NIH grant application, Dr Porter was denied funding in 2013 amid budget cuts.
“My lab had been able to report exciting preliminary data, but we really needed supplemental funds to keep this project moving,” he said. “While our initial application to NIH scored high enough to have received funding in previous years, it was not within the current funding range.”
Drs Newman and Porter are among the first recipients of ASH Bridge Grants, awards first offered in 2012 for investigators who applied for competitive grants from NIH but were denied funding due to cuts. The awards are intended to “bridge” investigators to their next NIH grant.
While such supplementary grant funding programs are helpful, they cannot replace critical NIH funding that has been cut for hematology research, according to ASH.
“When biomedical research is under-funded, everybody loses,” said ASH President Linda J. Burns, MD, of the University of Minnesota.
“Scientists are forced to slow or suspend research because they no longer have the resources to continue searching for new treatments, and even cures, for some of the world’s deadliest diseases. We continue to urge Congress to support a balanced approach to deficit reduction that does not include further cuts to critical biomedical research and public health and safety programs.”
“Faces of Austerity” is available online at www.cutshurt.org. A related report, “Faces of Austerity: How Budget Cuts Have Made Us Sicker, Poorer, and Less Safe,” was published last November. ![]()

Credit: Rhoda Baer
A new report suggests recent budget cuts to federal health programs in the US have had some negative consequences for hematology researchers.
The Coalition for Health Funding, an alliance of more than 90 public health advocacy organizations, invited scientists, public health advocates, and others to share stories of how they have been hurt by the budget cuts.
The resulting report is titled “Faces of Austerity, How Budget Cuts Hurt America’s Health.”
It details the negative effects the cuts have had on scientific discovery and innovation, scientists and health practitioners, health and social services, and government programs designed to respond to health hazards and natural disasters.
Among the stories included in the report are 2 from members of the American Society of Hematology (ASH), who detail how a decade of flat funding for the National Institutes of Health (NIH) and a 5% budget cut in 2013 have shuttered labs and jeopardized tomorrow’s treatments.
“Most people I know have been affected,” said Debra Newman, PhD, an investigator at BloodCenter of Wisconsin in Milwaukee.
“Their research funding has decreased and, consequently, so has the size of their laboratories because they cannot afford to employ the same number of staff. Talented investigators have started to leave research and go on to other things because they can’t support a research operation without money to run it.”
The other ASH member story is that of Christopher Porter, MD, a pediatric hematologist/oncologist at Children’s Hospital Colorado in Aurora. Despite receiving an excellent score on an NIH grant application, Dr Porter was denied funding in 2013 amid budget cuts.
“My lab had been able to report exciting preliminary data, but we really needed supplemental funds to keep this project moving,” he said. “While our initial application to NIH scored high enough to have received funding in previous years, it was not within the current funding range.”
Drs Newman and Porter are among the first recipients of ASH Bridge Grants, awards first offered in 2012 for investigators who applied for competitive grants from NIH but were denied funding due to cuts. The awards are intended to “bridge” investigators to their next NIH grant.
While such supplementary grant funding programs are helpful, they cannot replace critical NIH funding that has been cut for hematology research, according to ASH.
“When biomedical research is under-funded, everybody loses,” said ASH President Linda J. Burns, MD, of the University of Minnesota.
“Scientists are forced to slow or suspend research because they no longer have the resources to continue searching for new treatments, and even cures, for some of the world’s deadliest diseases. We continue to urge Congress to support a balanced approach to deficit reduction that does not include further cuts to critical biomedical research and public health and safety programs.”
“Faces of Austerity” is available online at www.cutshurt.org. A related report, “Faces of Austerity: How Budget Cuts Have Made Us Sicker, Poorer, and Less Safe,” was published last November. ![]()

Credit: Rhoda Baer
A new report suggests recent budget cuts to federal health programs in the US have had some negative consequences for hematology researchers.
The Coalition for Health Funding, an alliance of more than 90 public health advocacy organizations, invited scientists, public health advocates, and others to share stories of how they have been hurt by the budget cuts.
The resulting report is titled “Faces of Austerity, How Budget Cuts Hurt America’s Health.”
It details the negative effects the cuts have had on scientific discovery and innovation, scientists and health practitioners, health and social services, and government programs designed to respond to health hazards and natural disasters.
Among the stories included in the report are 2 from members of the American Society of Hematology (ASH), who detail how a decade of flat funding for the National Institutes of Health (NIH) and a 5% budget cut in 2013 have shuttered labs and jeopardized tomorrow’s treatments.
“Most people I know have been affected,” said Debra Newman, PhD, an investigator at BloodCenter of Wisconsin in Milwaukee.
“Their research funding has decreased and, consequently, so has the size of their laboratories because they cannot afford to employ the same number of staff. Talented investigators have started to leave research and go on to other things because they can’t support a research operation without money to run it.”
The other ASH member story is that of Christopher Porter, MD, a pediatric hematologist/oncologist at Children’s Hospital Colorado in Aurora. Despite receiving an excellent score on an NIH grant application, Dr Porter was denied funding in 2013 amid budget cuts.
“My lab had been able to report exciting preliminary data, but we really needed supplemental funds to keep this project moving,” he said. “While our initial application to NIH scored high enough to have received funding in previous years, it was not within the current funding range.”
Drs Newman and Porter are among the first recipients of ASH Bridge Grants, awards first offered in 2012 for investigators who applied for competitive grants from NIH but were denied funding due to cuts. The awards are intended to “bridge” investigators to their next NIH grant.
While such supplementary grant funding programs are helpful, they cannot replace critical NIH funding that has been cut for hematology research, according to ASH.
“When biomedical research is under-funded, everybody loses,” said ASH President Linda J. Burns, MD, of the University of Minnesota.
“Scientists are forced to slow or suspend research because they no longer have the resources to continue searching for new treatments, and even cures, for some of the world’s deadliest diseases. We continue to urge Congress to support a balanced approach to deficit reduction that does not include further cuts to critical biomedical research and public health and safety programs.”
“Faces of Austerity” is available online at www.cutshurt.org. A related report, “Faces of Austerity: How Budget Cuts Have Made Us Sicker, Poorer, and Less Safe,” was published last November. ![]()
Method forces cells to devour dying neighbors
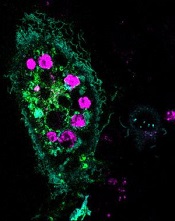
engulfed dying cells (purple)
Credit: Toru Komatsu
A two-pronged approach can prompt phagocytosis in inert cells, according to a paper published in Science Signaling.
Researchers manipulated HeLa cells, which typically cannot perform phagocytosis, by activating one protein inside the cells and expressing another protein on the cells’ surface. This forced the cells to engulf apoptotic Jurkat T cells.
So the researchers believe this technique could be used as a targeted therapy, with engineered cells consuming unwanted cells.
“Our goal is to build artificial cells programmed to eat up dangerous junk in the body, which could be anything from bacteria to the amyloid-beta plaques that cause Alzheimer’s to the body’s own rogue cancer cells,” said study author Takanari Inoue, PhD, of the Johns Hopkins University School of Medicine in Baltimore, Maryland.
“By figuring out how to get normally inert cells to recognize and engulf dying cells, we’ve taken an important step in that direction.”
Dr Inoue and his colleagues set out to “strip down” phagocytosis, determining the minimum tools one cell needs to eat another. Their first task was to induce the HeLa cells to attach to nearby dying cells—apoptotic Jurkat T cells—by getting the right receptors to the HeLa cells’ surface.
The researchers knew that part of a receptor protein called MFG-E8 would recognize and stick to a distress signal on the surface of dying cells, and coaxing the HeLa cells to make the protein fragment was straightforward.
To get the fragment, termed C2, onto the outside of the cells, the team found a way to stick it to another protein that was bound for the cell’s surface, thus taking advantage of the cell’s own transportation system.
As a result, up to 6 apoptotic Jurkat T cells stuck to each HeLa cell. The bad news was that the HeLa cells weren’t actually eating the T cells.
Fortunately, the researchers already had an idea about what to try next. Previous research had shown that activating the Rac gene could cause a cell to engulf beads stuck to its surface.
Sure enough, the team found that HeLa cells with both surface C2 and activated Rac swallowed the apoptotic cells readily.
“We’ve shown it’s possible to endow ordinary cells with the power to do something unique: take on the role of a specialized macrophage,” Dr Inoue said.
He cautioned, however, that the researchers don’t believe the engulfed cells are being broken down. Getting the HeLa cells to finish the process of phagocytosis will be one of the group’s next steps. ![]()

engulfed dying cells (purple)
Credit: Toru Komatsu
A two-pronged approach can prompt phagocytosis in inert cells, according to a paper published in Science Signaling.
Researchers manipulated HeLa cells, which typically cannot perform phagocytosis, by activating one protein inside the cells and expressing another protein on the cells’ surface. This forced the cells to engulf apoptotic Jurkat T cells.
So the researchers believe this technique could be used as a targeted therapy, with engineered cells consuming unwanted cells.
“Our goal is to build artificial cells programmed to eat up dangerous junk in the body, which could be anything from bacteria to the amyloid-beta plaques that cause Alzheimer’s to the body’s own rogue cancer cells,” said study author Takanari Inoue, PhD, of the Johns Hopkins University School of Medicine in Baltimore, Maryland.
“By figuring out how to get normally inert cells to recognize and engulf dying cells, we’ve taken an important step in that direction.”
Dr Inoue and his colleagues set out to “strip down” phagocytosis, determining the minimum tools one cell needs to eat another. Their first task was to induce the HeLa cells to attach to nearby dying cells—apoptotic Jurkat T cells—by getting the right receptors to the HeLa cells’ surface.
The researchers knew that part of a receptor protein called MFG-E8 would recognize and stick to a distress signal on the surface of dying cells, and coaxing the HeLa cells to make the protein fragment was straightforward.
To get the fragment, termed C2, onto the outside of the cells, the team found a way to stick it to another protein that was bound for the cell’s surface, thus taking advantage of the cell’s own transportation system.
As a result, up to 6 apoptotic Jurkat T cells stuck to each HeLa cell. The bad news was that the HeLa cells weren’t actually eating the T cells.
Fortunately, the researchers already had an idea about what to try next. Previous research had shown that activating the Rac gene could cause a cell to engulf beads stuck to its surface.
Sure enough, the team found that HeLa cells with both surface C2 and activated Rac swallowed the apoptotic cells readily.
“We’ve shown it’s possible to endow ordinary cells with the power to do something unique: take on the role of a specialized macrophage,” Dr Inoue said.
He cautioned, however, that the researchers don’t believe the engulfed cells are being broken down. Getting the HeLa cells to finish the process of phagocytosis will be one of the group’s next steps. ![]()

engulfed dying cells (purple)
Credit: Toru Komatsu
A two-pronged approach can prompt phagocytosis in inert cells, according to a paper published in Science Signaling.
Researchers manipulated HeLa cells, which typically cannot perform phagocytosis, by activating one protein inside the cells and expressing another protein on the cells’ surface. This forced the cells to engulf apoptotic Jurkat T cells.
So the researchers believe this technique could be used as a targeted therapy, with engineered cells consuming unwanted cells.
“Our goal is to build artificial cells programmed to eat up dangerous junk in the body, which could be anything from bacteria to the amyloid-beta plaques that cause Alzheimer’s to the body’s own rogue cancer cells,” said study author Takanari Inoue, PhD, of the Johns Hopkins University School of Medicine in Baltimore, Maryland.
“By figuring out how to get normally inert cells to recognize and engulf dying cells, we’ve taken an important step in that direction.”
Dr Inoue and his colleagues set out to “strip down” phagocytosis, determining the minimum tools one cell needs to eat another. Their first task was to induce the HeLa cells to attach to nearby dying cells—apoptotic Jurkat T cells—by getting the right receptors to the HeLa cells’ surface.
The researchers knew that part of a receptor protein called MFG-E8 would recognize and stick to a distress signal on the surface of dying cells, and coaxing the HeLa cells to make the protein fragment was straightforward.
To get the fragment, termed C2, onto the outside of the cells, the team found a way to stick it to another protein that was bound for the cell’s surface, thus taking advantage of the cell’s own transportation system.
As a result, up to 6 apoptotic Jurkat T cells stuck to each HeLa cell. The bad news was that the HeLa cells weren’t actually eating the T cells.
Fortunately, the researchers already had an idea about what to try next. Previous research had shown that activating the Rac gene could cause a cell to engulf beads stuck to its surface.
Sure enough, the team found that HeLa cells with both surface C2 and activated Rac swallowed the apoptotic cells readily.
“We’ve shown it’s possible to endow ordinary cells with the power to do something unique: take on the role of a specialized macrophage,” Dr Inoue said.
He cautioned, however, that the researchers don’t believe the engulfed cells are being broken down. Getting the HeLa cells to finish the process of phagocytosis will be one of the group’s next steps. ![]()
Technique allows for early cancer diagnosis
Credit: Максим Кукушкин
Immunosignaturing can allow for early detection of multiple myeloma and a range of other cancers, according to research published in PNAS.
Immunosignaturing involves profiling the entire population of antibodies circulating in the blood at a given time.
The method allowed researchers to distinguish 14 separate diseases, including 12 cancers, from one another and from healthy controls. The specificity was greater than 98% for each diagnosis.
“For years, we’ve seen remarkable results from immunosignatures, but introducing the technology to the scientific community has required a lot of patience,” said study author Phillip Stafford, PhD, of Arizona State University in Tempe.
The technique relies on a microarray consisting of thousands of random sequence peptides, imprinted on a glass slide. When a tiny droplet of blood (less than a microliter is needed) is spread across the microarray, antibodies in the blood selectively bind with individual peptides, forming a portrait of immune activity—an immunosignature.
Because the peptide sequences are random and not related to any naturally occurring disease antigens, the immunosignatures are “disease agnostic,” which means a single platform is potentially applicable to multiple disease types.
With their research, Dr Stafford and his colleagues put this claim to the test. The team first “trained” the system to calibrate results and establish reference immunosignatures using 20 samples each from 5 cancer patient cohorts, along with 20 non-cancer patients.
Once reference immunosignatures were established, the researchers tested the technique in a blind evaluation of 120 independent samples covering the same diseases. The results demonstrated 95% accuracy.
To further assess the diagnostic power of immunosignaturing, the team tested more than 1500 historical samples comprising 14 different diseases. This included 12 cancers, such as breast, brain, and multiple myeloma.
The average diagnostic accuracy of immunosignaturing was greater than 98% for each diagnosis, which suggests the method is suitable for the simultaneous classification of multiple diseases.
In a pairwise test against healthy control samples, multiple myeloma samples displayed the most significantly different peptides by t test. Of the top 100 peptides selected in this way, only breast cancer showed no overlap with any other disease.
Nevertheless, the researchers were able to distinguish the 14 separate diseases from one another, as well as from healthy controls, through immunosignatures.
Dr Stafford and his colleagues said these results suggest that immunosignatures provide an attractive means of capturing disease complexity. They offer a marked improvement over traditional methods in which one-to-one molecular recognition events are measured and a small number of analytes can be evaluated.
Furthermore, the technology is flexible in terms of handling and processing, the team said. A dried sample of blood, collected on filter paper and mailed to a study facility, can be used to generate an immunosignature.
The researchers also pointed out that the microarray chip used for this study contains 10,000 imprinted peptides, and this allows for enhanced sensitivity, owing to the large number of different possible signals elicited.
However, a significant improvement in immunosignaturing sensitivity and accuracy might be achieved through new chip technology. The team is currently developing a chip imprinted with more than 100,000 peptides. ![]()

Credit: Максим Кукушкин
Immunosignaturing can allow for early detection of multiple myeloma and a range of other cancers, according to research published in PNAS.
Immunosignaturing involves profiling the entire population of antibodies circulating in the blood at a given time.
The method allowed researchers to distinguish 14 separate diseases, including 12 cancers, from one another and from healthy controls. The specificity was greater than 98% for each diagnosis.
“For years, we’ve seen remarkable results from immunosignatures, but introducing the technology to the scientific community has required a lot of patience,” said study author Phillip Stafford, PhD, of Arizona State University in Tempe.
The technique relies on a microarray consisting of thousands of random sequence peptides, imprinted on a glass slide. When a tiny droplet of blood (less than a microliter is needed) is spread across the microarray, antibodies in the blood selectively bind with individual peptides, forming a portrait of immune activity—an immunosignature.
Because the peptide sequences are random and not related to any naturally occurring disease antigens, the immunosignatures are “disease agnostic,” which means a single platform is potentially applicable to multiple disease types.
With their research, Dr Stafford and his colleagues put this claim to the test. The team first “trained” the system to calibrate results and establish reference immunosignatures using 20 samples each from 5 cancer patient cohorts, along with 20 non-cancer patients.
Once reference immunosignatures were established, the researchers tested the technique in a blind evaluation of 120 independent samples covering the same diseases. The results demonstrated 95% accuracy.
To further assess the diagnostic power of immunosignaturing, the team tested more than 1500 historical samples comprising 14 different diseases. This included 12 cancers, such as breast, brain, and multiple myeloma.
The average diagnostic accuracy of immunosignaturing was greater than 98% for each diagnosis, which suggests the method is suitable for the simultaneous classification of multiple diseases.
In a pairwise test against healthy control samples, multiple myeloma samples displayed the most significantly different peptides by t test. Of the top 100 peptides selected in this way, only breast cancer showed no overlap with any other disease.
Nevertheless, the researchers were able to distinguish the 14 separate diseases from one another, as well as from healthy controls, through immunosignatures.
Dr Stafford and his colleagues said these results suggest that immunosignatures provide an attractive means of capturing disease complexity. They offer a marked improvement over traditional methods in which one-to-one molecular recognition events are measured and a small number of analytes can be evaluated.
Furthermore, the technology is flexible in terms of handling and processing, the team said. A dried sample of blood, collected on filter paper and mailed to a study facility, can be used to generate an immunosignature.
The researchers also pointed out that the microarray chip used for this study contains 10,000 imprinted peptides, and this allows for enhanced sensitivity, owing to the large number of different possible signals elicited.
However, a significant improvement in immunosignaturing sensitivity and accuracy might be achieved through new chip technology. The team is currently developing a chip imprinted with more than 100,000 peptides. ![]()

Credit: Максим Кукушкин
Immunosignaturing can allow for early detection of multiple myeloma and a range of other cancers, according to research published in PNAS.
Immunosignaturing involves profiling the entire population of antibodies circulating in the blood at a given time.
The method allowed researchers to distinguish 14 separate diseases, including 12 cancers, from one another and from healthy controls. The specificity was greater than 98% for each diagnosis.
“For years, we’ve seen remarkable results from immunosignatures, but introducing the technology to the scientific community has required a lot of patience,” said study author Phillip Stafford, PhD, of Arizona State University in Tempe.
The technique relies on a microarray consisting of thousands of random sequence peptides, imprinted on a glass slide. When a tiny droplet of blood (less than a microliter is needed) is spread across the microarray, antibodies in the blood selectively bind with individual peptides, forming a portrait of immune activity—an immunosignature.
Because the peptide sequences are random and not related to any naturally occurring disease antigens, the immunosignatures are “disease agnostic,” which means a single platform is potentially applicable to multiple disease types.
With their research, Dr Stafford and his colleagues put this claim to the test. The team first “trained” the system to calibrate results and establish reference immunosignatures using 20 samples each from 5 cancer patient cohorts, along with 20 non-cancer patients.
Once reference immunosignatures were established, the researchers tested the technique in a blind evaluation of 120 independent samples covering the same diseases. The results demonstrated 95% accuracy.
To further assess the diagnostic power of immunosignaturing, the team tested more than 1500 historical samples comprising 14 different diseases. This included 12 cancers, such as breast, brain, and multiple myeloma.
The average diagnostic accuracy of immunosignaturing was greater than 98% for each diagnosis, which suggests the method is suitable for the simultaneous classification of multiple diseases.
In a pairwise test against healthy control samples, multiple myeloma samples displayed the most significantly different peptides by t test. Of the top 100 peptides selected in this way, only breast cancer showed no overlap with any other disease.
Nevertheless, the researchers were able to distinguish the 14 separate diseases from one another, as well as from healthy controls, through immunosignatures.
Dr Stafford and his colleagues said these results suggest that immunosignatures provide an attractive means of capturing disease complexity. They offer a marked improvement over traditional methods in which one-to-one molecular recognition events are measured and a small number of analytes can be evaluated.
Furthermore, the technology is flexible in terms of handling and processing, the team said. A dried sample of blood, collected on filter paper and mailed to a study facility, can be used to generate an immunosignature.
The researchers also pointed out that the microarray chip used for this study contains 10,000 imprinted peptides, and this allows for enhanced sensitivity, owing to the large number of different possible signals elicited.
However, a significant improvement in immunosignaturing sensitivity and accuracy might be achieved through new chip technology. The team is currently developing a chip imprinted with more than 100,000 peptides. ![]()
From the Vascular Community
Baylor Vascular Fellows 50th Anniversary
On May 20, alumni and friends of Baylor University Medical Center’s vascular fellowship program gathered to celebrate the 50th anniversary of the program and commemorate the man who created it, Dr. Jesse Thompson. Dr. Thompson, who passed away in 2008, was a founding editor for the Journal of Vascular Surgery and president of the Society of Vascular Surgery.
In 1964, he established the country’s second vascular fellowship program at Baylor. Since then, approximately 100 vascular surgeons have received their training in this program. Its alumni include Dr. Larry Hollier, Dr. Hugh Trout, and Dr. Jonathan Towne.
The reunion was held at the Dallas Country Club with more than 140 in attendance, including Dr. Thompson’s children and grandchildren. The program’s first, middle, and last (i.e., most recent) fellows also attended. The following day Baylor hosted the Jesse E. Thompson, MD Lectureship and Speaker Symposium.
Dr. Spence Taylor was the visiting professor, delivering an address entitled "Surgeons as Leaders: Lessons Learned While Building a Medical School."
In Memoriam: Dr. John J. Bergan
Vascular pioneer Dr. John J. Bergan died June 11, 2014. Dr. Bergan began his career as a clinical assistant in surgery at Northwestern University Medical School. He served as the chief of the Division of Transplantation at Northwestern University Medical School from 1969 to 1976. When the Division of Vascular Surgery was formed in 1976, Dr. Bergan was appointed as the first division chief. Along with Dr. James Yao, Dr. Bergan established one of the earliest clinical vascular fellowship programs that same year, at Northwestern. In 1989, Dr. Bergan left Northwestern for southern California.
Dr. Bergan was instrumental in founding and developing many new societies and publications, including Midwestern Vascular Surgical Society. He also participated in the development of many practice guidelines including a method for data retrieval using computer programming and guidelines for venous disease diagnosis and treatment. He served as president of numerous societies, including SVS and the American Venous Forum.
We are truly grateful for all that he has contributed, and he will be sorely missed.
–Dr. Mark Eskandari
Baylor Vascular Fellows 50th Anniversary
On May 20, alumni and friends of Baylor University Medical Center’s vascular fellowship program gathered to celebrate the 50th anniversary of the program and commemorate the man who created it, Dr. Jesse Thompson. Dr. Thompson, who passed away in 2008, was a founding editor for the Journal of Vascular Surgery and president of the Society of Vascular Surgery.
In 1964, he established the country’s second vascular fellowship program at Baylor. Since then, approximately 100 vascular surgeons have received their training in this program. Its alumni include Dr. Larry Hollier, Dr. Hugh Trout, and Dr. Jonathan Towne.
The reunion was held at the Dallas Country Club with more than 140 in attendance, including Dr. Thompson’s children and grandchildren. The program’s first, middle, and last (i.e., most recent) fellows also attended. The following day Baylor hosted the Jesse E. Thompson, MD Lectureship and Speaker Symposium.
Dr. Spence Taylor was the visiting professor, delivering an address entitled "Surgeons as Leaders: Lessons Learned While Building a Medical School."
In Memoriam: Dr. John J. Bergan
Vascular pioneer Dr. John J. Bergan died June 11, 2014. Dr. Bergan began his career as a clinical assistant in surgery at Northwestern University Medical School. He served as the chief of the Division of Transplantation at Northwestern University Medical School from 1969 to 1976. When the Division of Vascular Surgery was formed in 1976, Dr. Bergan was appointed as the first division chief. Along with Dr. James Yao, Dr. Bergan established one of the earliest clinical vascular fellowship programs that same year, at Northwestern. In 1989, Dr. Bergan left Northwestern for southern California.
Dr. Bergan was instrumental in founding and developing many new societies and publications, including Midwestern Vascular Surgical Society. He also participated in the development of many practice guidelines including a method for data retrieval using computer programming and guidelines for venous disease diagnosis and treatment. He served as president of numerous societies, including SVS and the American Venous Forum.
We are truly grateful for all that he has contributed, and he will be sorely missed.
–Dr. Mark Eskandari
Baylor Vascular Fellows 50th Anniversary
On May 20, alumni and friends of Baylor University Medical Center’s vascular fellowship program gathered to celebrate the 50th anniversary of the program and commemorate the man who created it, Dr. Jesse Thompson. Dr. Thompson, who passed away in 2008, was a founding editor for the Journal of Vascular Surgery and president of the Society of Vascular Surgery.
In 1964, he established the country’s second vascular fellowship program at Baylor. Since then, approximately 100 vascular surgeons have received their training in this program. Its alumni include Dr. Larry Hollier, Dr. Hugh Trout, and Dr. Jonathan Towne.
The reunion was held at the Dallas Country Club with more than 140 in attendance, including Dr. Thompson’s children and grandchildren. The program’s first, middle, and last (i.e., most recent) fellows also attended. The following day Baylor hosted the Jesse E. Thompson, MD Lectureship and Speaker Symposium.
Dr. Spence Taylor was the visiting professor, delivering an address entitled "Surgeons as Leaders: Lessons Learned While Building a Medical School."
In Memoriam: Dr. John J. Bergan
Vascular pioneer Dr. John J. Bergan died June 11, 2014. Dr. Bergan began his career as a clinical assistant in surgery at Northwestern University Medical School. He served as the chief of the Division of Transplantation at Northwestern University Medical School from 1969 to 1976. When the Division of Vascular Surgery was formed in 1976, Dr. Bergan was appointed as the first division chief. Along with Dr. James Yao, Dr. Bergan established one of the earliest clinical vascular fellowship programs that same year, at Northwestern. In 1989, Dr. Bergan left Northwestern for southern California.
Dr. Bergan was instrumental in founding and developing many new societies and publications, including Midwestern Vascular Surgical Society. He also participated in the development of many practice guidelines including a method for data retrieval using computer programming and guidelines for venous disease diagnosis and treatment. He served as president of numerous societies, including SVS and the American Venous Forum.
We are truly grateful for all that he has contributed, and he will be sorely missed.
–Dr. Mark Eskandari








