User login
More RNs are delaying retirement, study shows

chemo to a cancer patient
Credit: Rhoda Baer
The nursing workforce in the US has grown substantially in recent years, and this is only partly due to an increase in nursing graduates, according to a new study.
The research revealed that registered nurses (RNs) are putting off retirement for longer than they have in the past.
From 1991 to 2012, 24% of RNs who were working at age 50 remained working as late as age 69. From 1969 to 1990, however, only 9% of nurses were still working at age 69.
These findings appear in Health Affairs.
“We estimate this trend accounts for about a quarter of an unexpected surge in the supply of registered nurses that the nation has experienced in recent years,” said study author David Auerbach, PhD, of RAND Corporation in Boston. “This may provide advantages to parts of the US healthcare system.”
The researchers noted that the RN workforce has surpassed forecasts from a decade ago, growing to 2.7 million in 2012 instead of peaking at 2.2 million as predicted. While much of the difference is the result of a surge in new nursing graduates, the size of the workforce is particularly sensitive to changes in retirement age.
Dr Auerbach and his colleagues uncovered the trend of delaying retirement by analyzing data from the Current Population Survey and the American Community Survey.
The team included all respondents aged 23 to 69 who reported being employed as an RN during the week of the relevant survey from 1969 to 2012. There were 70,724 RNs who responded to the Current Population Survey and 307,187 who responded to the American Community Survey.
The researchers found that, from 1969 to 1990, for a given number of RNs working at age 50, 47% were still working at age 62. From 1991 to 2012, 74% of RNs were working at age 62.
The trend of RNs delaying retirement, which largely predates the recent recession, extended nursing careers by 2.5 years after age 50 and increased the 2012 RN workforce by 136,000 people, according to the researchers.
The team said the reasons older RNs are working longer is unclear, but it is likely part of an overall trend that has seen more Americans—particularly women—stay in the workforce longer because of lengthening life expectancy and the satisfaction they derive from employment. ![]()

chemo to a cancer patient
Credit: Rhoda Baer
The nursing workforce in the US has grown substantially in recent years, and this is only partly due to an increase in nursing graduates, according to a new study.
The research revealed that registered nurses (RNs) are putting off retirement for longer than they have in the past.
From 1991 to 2012, 24% of RNs who were working at age 50 remained working as late as age 69. From 1969 to 1990, however, only 9% of nurses were still working at age 69.
These findings appear in Health Affairs.
“We estimate this trend accounts for about a quarter of an unexpected surge in the supply of registered nurses that the nation has experienced in recent years,” said study author David Auerbach, PhD, of RAND Corporation in Boston. “This may provide advantages to parts of the US healthcare system.”
The researchers noted that the RN workforce has surpassed forecasts from a decade ago, growing to 2.7 million in 2012 instead of peaking at 2.2 million as predicted. While much of the difference is the result of a surge in new nursing graduates, the size of the workforce is particularly sensitive to changes in retirement age.
Dr Auerbach and his colleagues uncovered the trend of delaying retirement by analyzing data from the Current Population Survey and the American Community Survey.
The team included all respondents aged 23 to 69 who reported being employed as an RN during the week of the relevant survey from 1969 to 2012. There were 70,724 RNs who responded to the Current Population Survey and 307,187 who responded to the American Community Survey.
The researchers found that, from 1969 to 1990, for a given number of RNs working at age 50, 47% were still working at age 62. From 1991 to 2012, 74% of RNs were working at age 62.
The trend of RNs delaying retirement, which largely predates the recent recession, extended nursing careers by 2.5 years after age 50 and increased the 2012 RN workforce by 136,000 people, according to the researchers.
The team said the reasons older RNs are working longer is unclear, but it is likely part of an overall trend that has seen more Americans—particularly women—stay in the workforce longer because of lengthening life expectancy and the satisfaction they derive from employment. ![]()

chemo to a cancer patient
Credit: Rhoda Baer
The nursing workforce in the US has grown substantially in recent years, and this is only partly due to an increase in nursing graduates, according to a new study.
The research revealed that registered nurses (RNs) are putting off retirement for longer than they have in the past.
From 1991 to 2012, 24% of RNs who were working at age 50 remained working as late as age 69. From 1969 to 1990, however, only 9% of nurses were still working at age 69.
These findings appear in Health Affairs.
“We estimate this trend accounts for about a quarter of an unexpected surge in the supply of registered nurses that the nation has experienced in recent years,” said study author David Auerbach, PhD, of RAND Corporation in Boston. “This may provide advantages to parts of the US healthcare system.”
The researchers noted that the RN workforce has surpassed forecasts from a decade ago, growing to 2.7 million in 2012 instead of peaking at 2.2 million as predicted. While much of the difference is the result of a surge in new nursing graduates, the size of the workforce is particularly sensitive to changes in retirement age.
Dr Auerbach and his colleagues uncovered the trend of delaying retirement by analyzing data from the Current Population Survey and the American Community Survey.
The team included all respondents aged 23 to 69 who reported being employed as an RN during the week of the relevant survey from 1969 to 2012. There were 70,724 RNs who responded to the Current Population Survey and 307,187 who responded to the American Community Survey.
The researchers found that, from 1969 to 1990, for a given number of RNs working at age 50, 47% were still working at age 62. From 1991 to 2012, 74% of RNs were working at age 62.
The trend of RNs delaying retirement, which largely predates the recent recession, extended nursing careers by 2.5 years after age 50 and increased the 2012 RN workforce by 136,000 people, according to the researchers.
The team said the reasons older RNs are working longer is unclear, but it is likely part of an overall trend that has seen more Americans—particularly women—stay in the workforce longer because of lengthening life expectancy and the satisfaction they derive from employment. ![]()
VTE Prevention After Orthopedic Surgery
Each year in the United States, over 1 million adults undergo hip fracture surgery or elective total knee or hip arthroplasty.[1] Although highly effective for improving functional status and quality of life,[2, 3] each of these procedures is associated with a substantial risk of developing a deep vein thrombosis (DVT) or pulmonary embolism (PE).[4, 5] Collectively referred to as venous thromboembolism (VTE), these clots in the venous system are associated with significant morbidity and mortality for patients, as well as substantial costs to the healthcare system.[6] Although VTE is considered to be a preventable cause of hospital admission and death,[7, 8] the postoperative setting presents a particular challenge, as efforts to reduce clotting must be balanced against the risk of bleeding.
Despite how common this scenario is, there is no consensus regarding the best pharmacologic strategy. National guidelines recommend pharmacologic thromboprophylaxis, leaving the clinician to select the specific agent.[4, 5] Explicitly endorsed options include aspirin, vitamin K antagonists (VKA), unfractionated heparin, fondaparinux, low‐molecular‐weight heparin (LMWH) and IIa/Xa factor inhibitors. Among these, aspirin, the only nonanticoagulant, has been the source of greatest controversy.[4, 9, 10]
Two previous systematic reviews comparing aspirin to anticoagulation for VTE prevention found conflicting results.[11, 12] In addition, both used indirect comparisons, a method in which the intervention and comparison data come from different studies, and susceptibility to confounding is high.[13, 14] We aimed to overcome the limitations of prior efforts to address this commonly encountered clinical question by conducting a systematic review and meta‐analysis of randomized controlled trials that directly compared the efficacy and safety of aspirin to anticoagulants for VTE prevention in adults undergoing common high‐risk major orthopedic surgeries of the lower extremities.
MATERIAL AND METHODS
Review Protocol
Prior to conducting the review, we outlined an approach to identifying and selecting eligible studies, prespecified outcomes of interest, and planned subgroup analyses. The meta‐analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses and Cochrane guidelines.[15, 16]
Study Eligibility Criteria
We prespecified the following inclusion criteria: (1) the design was a randomized controlled trial; (2) the population consisted of patients undergoing major orthopedic surgery including hip fracture surgery or total knee or hip arthroplasty; (3) the study compared aspirin to 1 or more anticoagulants: VKA, unfractionated heparin, LMWH, thrombin inhibitors, pentasaccharides (eg, fondaparinux), factor Xa/IIa inhibitors dosed for VTE prevention; (4) subjects were followed for at least 7 days; and (5) the study reported at least 1 prespecified outcome of interest. We allowed the use of pneumatic compression devices, as long as devices were used in both arms of the study.
Outcome Measures
We designated the rate of proximal DVT (occurring in the popliteal vein and above) as the primary outcome of interest. Additional efficacy outcomes included rates of PE, PE‐related mortality, and all‐cause mortality. We required that DVT and PE were diagnosed by venography, computed tomography (CT) angiography of the chest, pulmonary angiography, ultrasound Doppler of the legs, or ventilation/perfusion scan. We allowed studies that screened participants for VTE (including the use of fibrinogen leg scanning).
A bleeding event was defined as any need for postoperative blood transfusion or otherwise clinically significant bleeding (eg, prolonged postoperative wound bleeding). We further defined major bleeding as the requirement for blood transfusion of more than 2 U, hematoma requiring surgical evacuation, and bleeding into a critical organ.
Study Identification
We searched Medline (January 1948 to June 2013), Cochrane Library (through June 2013), and CINAHL (January 1974 to June 2013) to locate studies meeting our inclusion criteria. We used exploded Medical Subject Headings terms and key words to generate sets for aspirin and major orthopedic surgery themes, then used the Boolean term, AND, to find their intersection.
Additional Search Methods
We manually reviewed references of relevant articles and searched ClinicalTrials.gov to identify any ongoing studies or unpublished data. We further searched the following sources: American College of Chest Physicians (ACCP) Evidence‐Based Clinical Practice Guidelines,[4, 17] American Academy of Orthopaedic Surgeons guidelines (AAOS),[5] and annual meeting abstracts of the American Academy of Orthopaedic Surgery,[18] the American Society of Hematology,[19] and the ACCP.[20]
Study Selection
Two pairs of 2 reviewers independently scanned the titles and abstracts of identified studies, excluding only those that were clearly not relevant. The same reviewers independently reviewed the full text of each remaining study to make final decisions about eligibility.
Data Extraction and Quality Assessment
Two reviewers independently extracted data from each included study and rendered judgments regarding the methodological quality using the Cochrane Risk of Bias Tool.[21]
Data Synthesis
We used Review Manager (RevMan 5.1) to calculate pooled risk ratios using the Mantel‐Haenszel method and random‐effects models, which take into account the presence of variability among included studies.[16, 22] We also manually pooled absolute event rates for each study arm using the study weights assigned in the pooled risk ratio models.
Assessment of Heterogeneity and Reporting Biases
We assessed statistical variability among the studies contributing to each summary estimate and considered studies unacceptably heterogeneous if the test for heterogeneity P value was <0.10 or the I2 exceeded 50%.[14, 16] We constructed funnel plots to assess for publication bias but had too few studies for reliable interpretation.
Subgroup Analyses
We prespecified subgroup analyses based on the indication for the surgery: hip fracture surgery versus total knee or hip arthroplasty, and according to class of anticoagulation used: VKA versus heparin compounds.
RESULTS
Results of Search
Figure 1 shows the number of studies that we evaluated during each stage of the study selection process. After full‐text review, 8 randomized trials met all inclusion criteria.[23, 24, 25, 26, 27, 28, 29, 30]

Included Studies
Table 1 presents the characteristics of the 8 included randomized trials. All were published in peer‐reviewed journals from 1982 through 2006.2330 The trials included a combined total of 1408 subjects, and took place in 4 different countries, including the United States,[24, 26, 28, 29, 30] Spain,[23] Sweden,[27] and Canada.[25] Enrolled patients had a mean age of 76 years (range, 7477 years) among hip fracture surgery studies and 66 years (range, 5969 years) among elective knee/hip arthroplasty studies.
| Author, Year | Surgery | Pneumatic Compression | Intervention | Control | Duration (Days) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Aspirin (Total/Day) | No. | Mean Age, Years | Anticoagulant | No. | Mean Age, Years | ||||
| |||||||||
| Powers, 1989 | Hip fracture | No | 1,300 mg | 66 | 73 | Warfarin | 65 | 75 | 21 |
| Gent, 1996 | Hip fracture | No | 200 mg | 126* | 77 | Danaparoid | 125* | 77 | 11 |
| Harris, 1982 | THA | No | 1,200 mg | 51 | 58 | Heparin or warfarin | 75 | 60 | 21 |
| Alfaro, 1986 | THA | No | 250 mg/1,000 mg | 60 | 64 | Heparin | 30 | 58 | 7 |
| Josefsson, 1987 | THA | No | 3,000 mg | 40 | N/A | Heparin | 42 | N/A | 9 |
| Woolson, 1991 | THA | Yes | 1,300 mg | 72 | 62 | Warfarin | 69 | 68 | 7 |
| Lotke, 1996 | THA or TKA | No | 650 mg | 166 | 66 | Warfarin | 146 | 67 | 9 |
| Westrich, 2006 | TKA | Yes | 650 mg | 136 | 69 | Enoxaparin | 139 | 69 | 21 |
Pneumatic compression devices were used in addition to pharmacologic prevention in 2 studies.[29, 30] The different classes of anticoagulants used included warfarin,[26, 28, 30] heparin,[23, 27] LMWH,[29] heparin or warfarin,[24] and danaparoid.[25] Treatment duration was 7 to 21 days. Clinical follow‐up extended up to 6 months after surgery. Patients in all included studies were screened for DVT during the trial period by I‐fibrinogen leg scanning,[23, 25, 26, 27] venography,[24, 28] or ultrasound[29, 30]; some trials also screened all participants for PE with ventilation/perfusion scanning.[27, 28]
Methodological Quality of Included Studies
Only 3 studies described their method of random sequence generation,[24, 25, 26] and 2 studies specified their method of allocation concealment.[25, 26] Only 1 study used placebo controls to double blind the study arm assignments.[25] We judged the overall potential risk of bias among the eligible studies to be moderate.
Rate of Proximal DVT
Pooling findings of all 7 studies that reported proximal DVT rates, we observed no statistically significant difference between aspirin and anticoagulants (10.4% vs 9.2%, relative risk [RR]: 1.15 [95% confidence interval {CI}: 0.68‐1.96], I2=41%). Although rates did not statistically differ between aspirin and anticoagulants in either operative subgroup, there appeared to be a nonsignificant trend favoring anticoagulation after hip fracture repair (12.7% vs 7.8%, RR: 1.60 [95% CI: 0.80‐3.20], I2=0%, 2 trials) but not following knee or hip arthroplasty (9.3% vs 9.7%, RR: 1.00 [95% CI: 0.49‐2.05], I2=49%, 5 trials) (Figure 2).

Rate of Pulmonary Embolism
Just 14 participants experienced a PE across all 6 trials reporting this outcome (aspirin n=9/405 versus anticoagulation n=5/415). Although PE was numerically more likely in the aspirin group, this difference was not statistically significant (overall: 1.9% vs 0.9%, RR: 1.83 [95% CI: 0.64, 5.21], I2=0%). The very small number of events rendered extremely wide 95% CIs in operative subgroup analyses (Figure 3).

Rates of All‐Cause Mortality
Only 2 trials, both evaluating aspirin versus anticoagulation following hip fracture repair, reported death events, both after 3 months follow‐up.[25, 26] Pooling these results, there was no statistically significant difference (7.3% vs 6.8%, RR: 1.07 [95% CI: 0.512.21], I2=0%).
Bleeding Rates
Pooling all 8 studies, aspirin was associated with a statistically significant 48% decreased risk of bleeding events compared to anticoagulants (3.8% vs 8.0%, RR: 0.52 [95% CI: 0.310.86], I2=8%). When subgrouped according to procedure, bleeding rates remained statistically significantly lower in the aspirin group following hip fracture (3.1% vs 10%, RR: 0.32 [95% CI: 0.130.77], I2=0%, 2 trials); however, the observed trend favoring aspirin was not statistically significant following arthroplasty (3.9% vs 7.8%, RR: 0.63 [95% CI: 0.331.21], I2=14%, 5 trials) (Figure 4).
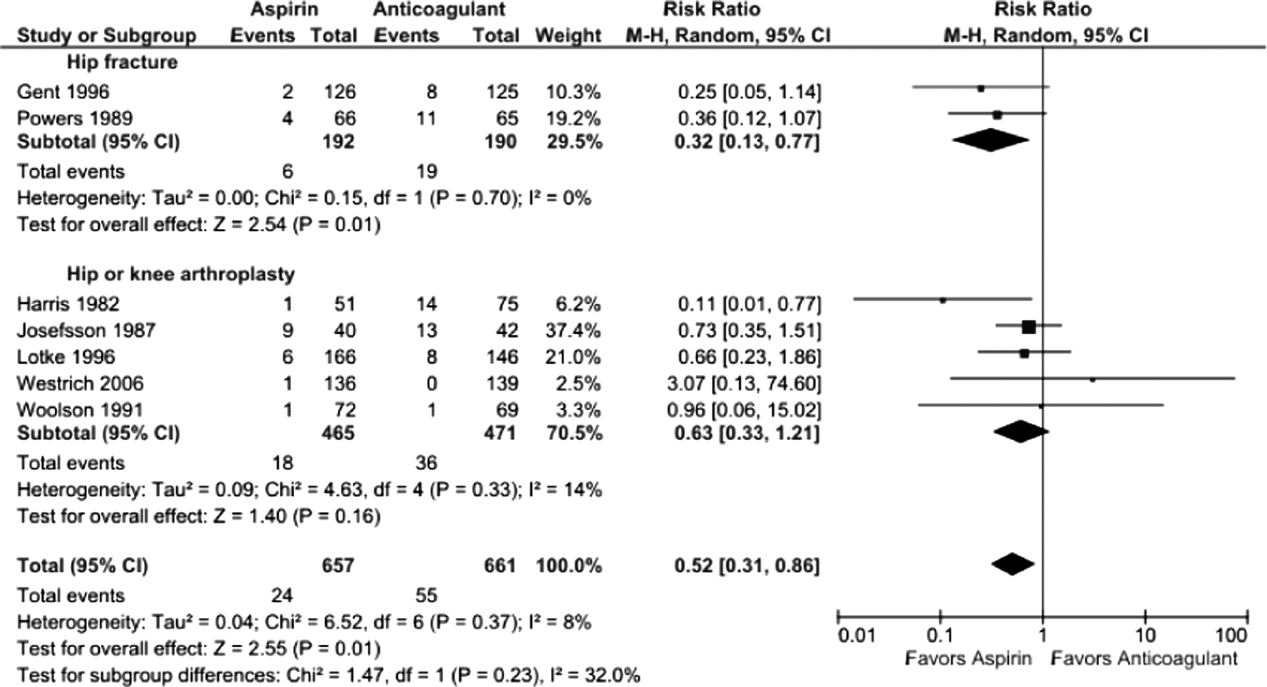
Five studies reported major bleeding; event rates were low and no statistically significant differences between aspirin and anticoagulants were observed (hip fracture: 3.5% vs 6.3%, RR: 0.46 [95% CI: 0.141.48], I2=0%, 2 trials; knee/hip arthroplasty: 2.1% vs 0.6%, RR: 2.86 [95% CI: 0.6512.60], I2=0%, 3 trials).
Subgroup Analysis
Rates of proximal DVT did not differ between aspirin and anticoagulants when subgrouped according to anticoagulant class (aspirin vs warfarin: 9.7% vs 10.7%, RR: 0.90 [95% CI: 0.561.45], I2=0%, 3 trials; aspirin vs heparin: 10.5% vs 7.9%, RR: 1.37 [95% CI: 0.473.96], I2=44%, 4 trials) (data not shown).
Bleeding rates were lower with aspirin when subgrouped according to type of anticoagulant, but the finding was only statistically significant when compared to VKA (aspirin vs VKA: 4.2% vs 11.1%, RR: 0.43 [95% CI: 0.220.86] I2=0%, 4 trials; aspirin vs heparin: 3.7% vs 7.7%, RR: 0.44 [95% CI: 0.151.28], I2=44, 4 trials) (data not shown).
DISCUSSION
We found the balance of risk versus benefit of aspirin compared to anticoagulation differed markedly according to type of surgery. After hip fracture repair, we found a 68% reduction in bleeding risk with aspirin compared to anticoagulants. This benefit, however, was associated with a nonsignificant increase in screen‐detected proximal DVT. Conversely, among patients undergoing knee or hip arthroplasty, we found no difference in proximal DVT risk between aspirin and anticoagulants and a possible trend toward less bleeding risk with aspirin. The rarity of pulmonary emboli (and death) made meaningful comparisons between aspirin and anticoagulation impossible for either type of surgery.
Our systematic review has several strengths that differentiate it from previous analyses. First, we only included head‐to‐head randomized trials such that all included data reflect direct comparisons between aspirin and anticoagulation in well‐balanced populations. Conversely, both recent reviews[11, 12] were based on indirect comparisons, a type of analysis in which data for the intervention and control arms are taken from different studies and thus different populations. This methodology is not recommended by the Cochrane Collaboration[13, 14] because of the increased risk of an unbalanced comparison. For example, Brown and colleagues' meta‐analysis, which pooled data from selected arms of 14 randomized controlled trials, found the efficacy of aspirin comparable to that of anticoagulants, but all aspirin subjects came from a single trial of patients at such low risk of VTE that a placebo arm was considered justified.[31] Similarly, in the indirect comparison of Westrich and colleagues,[12] which found anticoagulation superior to aspirin, the likelihood of an unbalanced comparison was further heightened by their inclusion of observational studies, with the attendant risk of confounding by indication.
Our systematic review further differs from previous analyses by examining both beneficial and harmful clinical outcomes, and doing so separately for the 2 most common types of major orthopedic lower extremity surgery. This allowed us to discover important differences in the comparative efficacy (benefit vs harm) of aspirin versus anticoagulants across different procedure types. Finding that aspirin may have lower efficacy for preventing VTE following hip fracture repair than arthroplasty may not be surprising in light of the nature of the 2 procedures, the disparate mean ages typical of patients who undergo each procedure, and the underlying trauma in hip fracture patients.
The limitations of our review largely reflect the quality of the studies we were able to include. First, our pooled sample size remains relatively small, meaning that observed nonsignificant differences between aspirin and anticoagulation groups (eg, a nonsignificant 60% increased risk of DVT for aspirin after hip repair, 95% CI: 0.803.20) could reasonably reflect up to 3‐fold differences in DVT risk and 5‐fold differences in PE rates. Second, screening for DVT, which is neither recommended nor common in clinical practice, was used in all studies. Reported DVT incidence, therefore, is undoubtedly higher than what would be observed in practice; however, the effect on the direction and magnitude of observed relative risks is unpredictable. Third, included studies used a wide range of aspirin doses, as well as a variety of anticoagulant types. Although supratherapeutic aspirin doses are unlikely to confer additional benefit for venous thromboprophylaxis, they may be associated with excess bleeding risk.[32] Finally, several of the studies were conducted more than10 years ago. Given changes in treatment practices, surgical technique, and prophylaxis options, the findings of these studies may not reflect current practice, in which early mobilization and intermittent pneumatic compression devices are standard prophylaxis against postoperative VTE. In fact, only 2 trials used concomitant pneumatic compression devices, and none treated patients longer than 21 days, the current standard being up to 35 days.[4] Although these limitations may affect overall event rates, this bias should be balanced between comparison groups, because we only included randomized controlled trials.
What is a clinician to do? Based on our findings, current guidelines recommending aspirin prophylaxis against VTE as an alternative following major lower extremity surgery may not be universally appropriate. We found that although overall bleeding complications are lower with aspirin, concerns about poor efficacy remain, specifically for patients undergoing hip fracture repair. Although some have suggested that aspirin use be restricted to low risk patients, this strategy has not been experimentally evaluated.[33] On the other hand, switching to aspirin after a brief initial course of LMWH may be an approach warranting further study, in light of a recent randomized controlled trial of 778 patients after elective hip replacement, which found equivalent efficacy using 10 days of LMWH followed by aspirin versus additional LMWH for 28 days.[34]
We are able to be more definitive, based on our study of best available trial data, in making recommendations to investigators embarking on further study of optimal VTE prophylaxis following major orthopedic surgery. First, distinguishing a priori between the 2 major types of lower extremity major orthopedic surgery is a high priority. Second, both bleeding and thromboembolic outcomes must be evaluated. Third, only symptomatic events should be used to measure VTE outcomes; clinical follow‐up must continue well beyond discharge, for at least 3 months to ensure ascertainment of clinically relevant VTE. Fourth, nonpharmacologic cointerventions should be standardized and represent the standard of care, including early immobilization and mechanical compression devices.
In summary, although definitive recommendations for or against the use of aspirin instead of anticoagulation for VTE prevention following major orthopedic surgery are not possible, our findings suggest that, following hip fracture repair, the lower risk of bleeding with aspirin is likely outweighed by a probable trend toward higher risk of VTE. On the other hand, the balance of these opposing risks may favor aspirin after elective knee or hip arthroplasty. A comparative study of aspirin, anticoagulation, and a hybrid strategy (eg, brief anticoagulation followed by aspirin) after elective knee or hip arthroplasty should be a high priority given our aging population and increasing demand for major orthopedic lower extremity surgery.
Acknowledgements
The authors thank Dr. Deborah Ornstein (Associate Professor of Medicine, Geisel School of Medicine at Dartmouth, Section of Hematology Mary Hitchcock Memorial Hospital, Lebanon, New Hampshire) for sparking the idea for this systematic review.
Disclosures: Nothing to report.
- Healthcare Cost and Utilization Project (HCUP). Available at: http://hcupnet.ahrq.gov. Accessed June 2013.
- , , , et al. Outcomes after hip or knee replacement surgery for osteoarthritis. A prospective cohort study comparing patients' quality of life before and after surgery with age‐related population norms. Med J Aust. 1999;171(5):235–238.
- , , . Quality of life and functional outcome after primary total hip replacement. A five‐year follow‐up. J Bone Joint Surg Br. 2007;89(7):868–873.
- , , , et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 suppl):e278S–e325S.
- American Academy of Orthopaedic Surgeons (AAOS). American Academy of Orthopaedic Surgeons clinical practice guideline on preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2011. Available at: http://www.aaos.org/research/guidelines. Accessed June 2013.
- , , , . Economic burden of deep‐vein thrombosis, pulmonary embolism, and post‐thrombotic syndrome. Am J Health Syst Pharm. 2006;63(20 suppl 6):S5–S15.
- , . Autopsy proven pulmonary embolism in hospital patients: are we detecting enough deep vein thrombosis? J R Soc Med. 1989;82(4):203–205.
- , , . Autopsy‐verified pulmonary embolism in a surgical department: analysis of the period from 1951 to 1988. Br J Surg. 1991;78(7):849–852.
- . What is the state of the art in orthopaedic thromboprophylaxis in lower extremity reconstruction? Instr Course Lect. 2011;60:283–290.
- , . Aspirin for the prophylaxis of venous thromboembolic events in orthopedic surgery patients: a comparison of the AAOS and ACCP guidelines with review of the evidence. Ann Pharmacother. 2013;47(1):63–74.
- . Venous thromboembolism prophylaxis after major orthopaedic surgery: A pooled analysis of randomized controlled trials. J Arthroplasty. 2009;24(6 supplement 1):77–83.
- , , , . Meta‐analysis of thromboembolic prophylaxis after total knee arthroplasty. J Bone Joint Surg Br. 2000;82(6):795–800.
- , , , , , . Methodological problems in the use of indirect comparisons for evaluating healthcare interventions: survey of published systematic reviews. BMJ. 2009;338:b1147.
- Higgins J, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Available at: http://handbook.cochrane.org. Accessed June 2013.
- , , , ; the PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
- , , , et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100.
- , , , et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence‐based clinical practice guidelines (8th edition). Chest. 2008;133(6 suppl):381S–453S.
- , , , et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 suppl):e195S–e226S.
- ASH Annual Meeting Abstracts‐Blood. Available at: http://bloodjournal.hematologylibrary.org/site/misc/ASH_Meeting_Abstracts_Info.xhtml. Accessed June 2013.
- CHEST Publications Meeting Abstracts. Available at: http://journal.publications.chestnet.org/ss/meetingabstracts.aspx. Accessed June 2013.
- , , , et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
- Review Manager (RevMan) [computer program]. Version 5.1. Copenhagen, Denmark: The Nordic Cochrane Centre, The Cochrane Collaboration; 2011.
- , , . Prophylaxis of thromboembolic disease and platelet‐related changes following total hip replacement: a comparative study of aspirin and heparin‐dihydroergotamine. Thromb Haemost. 1986;56(1):53–56.
- , , , . High and low‐dose aspirin prophylaxis against venous thromboembolic disease in total hip replacement. J Bone Joint Surg Am. 1982;64(1):63–66.
- , , , et al. Low‐molecular‐weight heparinoid orgaran is more effective than aspirin in the prevention of venous thromboembolism after surgery for hip fracture. Circulation. 1996;93(1):80–84.
- , , , et al. A randomized trial of less intense postoperative warfarin or aspirin therapy in the prevention of venous thromboembolism after surgery for fractured hip. Arch Intern Med. 1989;149(4):771–774.
- , , . Prevention of thromboembolism in total hip replacement. Aspirin versus dihydroergotamine‐heparin. Acta Orthop Scand. 1987;58(6):626–629.
- , , , et al. Aspirin and warfarin for thromboembolic disease after total joint arthroplasty. Clin Orthop Relat Res. 1996;(324):251–258.
- , , , , , . VenaFlow plus Lovenox vs VenaFlow plus aspirin for thromboembolic disease prophylaxis in total knee arthroplasty. J Arthroplasty. 2006;21(6 suppl 2):139–143.
- , . Intermittent pneumatic compression to prevent proximal deep venous thrombosis during and after total hip replacement. A prospective, randomized study of compression alone, compression and aspirin, and compression and low‐dose warfarin. J Bone Joint Surg Am. 1991;73(4):507–512.
- Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: Pulmonary Embolism Prevention (PEP) trial. Lancet. 2000;355(9212):1295–1302.
- , , , et al. Safety and efficacy of high‐ versus low‐dose aspirin after primary percutaneous coronary intervention in ST‐segment elevation myocardial infarction: the HORIZONS‐AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) trial. JACC Cardiovasc Interv. 2012;5(12):1231–1238.
- Intermountain Joint Replacement Center Writing Committee. A prospective comparison of warfarin to aspirin for thromboprophylaxis in total hip and total knee arthroplasty. J Arthroplasty. 2011;27:e1–e9.
- , , , et al. Aspirin versus low‐molecular‐weight heparin for extended venous thromboembolism prophylaxis after total hip arthroplasty: a randomized trial. Ann Intern Med. 2013;158(11):800–806.
Each year in the United States, over 1 million adults undergo hip fracture surgery or elective total knee or hip arthroplasty.[1] Although highly effective for improving functional status and quality of life,[2, 3] each of these procedures is associated with a substantial risk of developing a deep vein thrombosis (DVT) or pulmonary embolism (PE).[4, 5] Collectively referred to as venous thromboembolism (VTE), these clots in the venous system are associated with significant morbidity and mortality for patients, as well as substantial costs to the healthcare system.[6] Although VTE is considered to be a preventable cause of hospital admission and death,[7, 8] the postoperative setting presents a particular challenge, as efforts to reduce clotting must be balanced against the risk of bleeding.
Despite how common this scenario is, there is no consensus regarding the best pharmacologic strategy. National guidelines recommend pharmacologic thromboprophylaxis, leaving the clinician to select the specific agent.[4, 5] Explicitly endorsed options include aspirin, vitamin K antagonists (VKA), unfractionated heparin, fondaparinux, low‐molecular‐weight heparin (LMWH) and IIa/Xa factor inhibitors. Among these, aspirin, the only nonanticoagulant, has been the source of greatest controversy.[4, 9, 10]
Two previous systematic reviews comparing aspirin to anticoagulation for VTE prevention found conflicting results.[11, 12] In addition, both used indirect comparisons, a method in which the intervention and comparison data come from different studies, and susceptibility to confounding is high.[13, 14] We aimed to overcome the limitations of prior efforts to address this commonly encountered clinical question by conducting a systematic review and meta‐analysis of randomized controlled trials that directly compared the efficacy and safety of aspirin to anticoagulants for VTE prevention in adults undergoing common high‐risk major orthopedic surgeries of the lower extremities.
MATERIAL AND METHODS
Review Protocol
Prior to conducting the review, we outlined an approach to identifying and selecting eligible studies, prespecified outcomes of interest, and planned subgroup analyses. The meta‐analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses and Cochrane guidelines.[15, 16]
Study Eligibility Criteria
We prespecified the following inclusion criteria: (1) the design was a randomized controlled trial; (2) the population consisted of patients undergoing major orthopedic surgery including hip fracture surgery or total knee or hip arthroplasty; (3) the study compared aspirin to 1 or more anticoagulants: VKA, unfractionated heparin, LMWH, thrombin inhibitors, pentasaccharides (eg, fondaparinux), factor Xa/IIa inhibitors dosed for VTE prevention; (4) subjects were followed for at least 7 days; and (5) the study reported at least 1 prespecified outcome of interest. We allowed the use of pneumatic compression devices, as long as devices were used in both arms of the study.
Outcome Measures
We designated the rate of proximal DVT (occurring in the popliteal vein and above) as the primary outcome of interest. Additional efficacy outcomes included rates of PE, PE‐related mortality, and all‐cause mortality. We required that DVT and PE were diagnosed by venography, computed tomography (CT) angiography of the chest, pulmonary angiography, ultrasound Doppler of the legs, or ventilation/perfusion scan. We allowed studies that screened participants for VTE (including the use of fibrinogen leg scanning).
A bleeding event was defined as any need for postoperative blood transfusion or otherwise clinically significant bleeding (eg, prolonged postoperative wound bleeding). We further defined major bleeding as the requirement for blood transfusion of more than 2 U, hematoma requiring surgical evacuation, and bleeding into a critical organ.
Study Identification
We searched Medline (January 1948 to June 2013), Cochrane Library (through June 2013), and CINAHL (January 1974 to June 2013) to locate studies meeting our inclusion criteria. We used exploded Medical Subject Headings terms and key words to generate sets for aspirin and major orthopedic surgery themes, then used the Boolean term, AND, to find their intersection.
Additional Search Methods
We manually reviewed references of relevant articles and searched ClinicalTrials.gov to identify any ongoing studies or unpublished data. We further searched the following sources: American College of Chest Physicians (ACCP) Evidence‐Based Clinical Practice Guidelines,[4, 17] American Academy of Orthopaedic Surgeons guidelines (AAOS),[5] and annual meeting abstracts of the American Academy of Orthopaedic Surgery,[18] the American Society of Hematology,[19] and the ACCP.[20]
Study Selection
Two pairs of 2 reviewers independently scanned the titles and abstracts of identified studies, excluding only those that were clearly not relevant. The same reviewers independently reviewed the full text of each remaining study to make final decisions about eligibility.
Data Extraction and Quality Assessment
Two reviewers independently extracted data from each included study and rendered judgments regarding the methodological quality using the Cochrane Risk of Bias Tool.[21]
Data Synthesis
We used Review Manager (RevMan 5.1) to calculate pooled risk ratios using the Mantel‐Haenszel method and random‐effects models, which take into account the presence of variability among included studies.[16, 22] We also manually pooled absolute event rates for each study arm using the study weights assigned in the pooled risk ratio models.
Assessment of Heterogeneity and Reporting Biases
We assessed statistical variability among the studies contributing to each summary estimate and considered studies unacceptably heterogeneous if the test for heterogeneity P value was <0.10 or the I2 exceeded 50%.[14, 16] We constructed funnel plots to assess for publication bias but had too few studies for reliable interpretation.
Subgroup Analyses
We prespecified subgroup analyses based on the indication for the surgery: hip fracture surgery versus total knee or hip arthroplasty, and according to class of anticoagulation used: VKA versus heparin compounds.
RESULTS
Results of Search
Figure 1 shows the number of studies that we evaluated during each stage of the study selection process. After full‐text review, 8 randomized trials met all inclusion criteria.[23, 24, 25, 26, 27, 28, 29, 30]

Included Studies
Table 1 presents the characteristics of the 8 included randomized trials. All were published in peer‐reviewed journals from 1982 through 2006.2330 The trials included a combined total of 1408 subjects, and took place in 4 different countries, including the United States,[24, 26, 28, 29, 30] Spain,[23] Sweden,[27] and Canada.[25] Enrolled patients had a mean age of 76 years (range, 7477 years) among hip fracture surgery studies and 66 years (range, 5969 years) among elective knee/hip arthroplasty studies.
| Author, Year | Surgery | Pneumatic Compression | Intervention | Control | Duration (Days) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Aspirin (Total/Day) | No. | Mean Age, Years | Anticoagulant | No. | Mean Age, Years | ||||
| |||||||||
| Powers, 1989 | Hip fracture | No | 1,300 mg | 66 | 73 | Warfarin | 65 | 75 | 21 |
| Gent, 1996 | Hip fracture | No | 200 mg | 126* | 77 | Danaparoid | 125* | 77 | 11 |
| Harris, 1982 | THA | No | 1,200 mg | 51 | 58 | Heparin or warfarin | 75 | 60 | 21 |
| Alfaro, 1986 | THA | No | 250 mg/1,000 mg | 60 | 64 | Heparin | 30 | 58 | 7 |
| Josefsson, 1987 | THA | No | 3,000 mg | 40 | N/A | Heparin | 42 | N/A | 9 |
| Woolson, 1991 | THA | Yes | 1,300 mg | 72 | 62 | Warfarin | 69 | 68 | 7 |
| Lotke, 1996 | THA or TKA | No | 650 mg | 166 | 66 | Warfarin | 146 | 67 | 9 |
| Westrich, 2006 | TKA | Yes | 650 mg | 136 | 69 | Enoxaparin | 139 | 69 | 21 |
Pneumatic compression devices were used in addition to pharmacologic prevention in 2 studies.[29, 30] The different classes of anticoagulants used included warfarin,[26, 28, 30] heparin,[23, 27] LMWH,[29] heparin or warfarin,[24] and danaparoid.[25] Treatment duration was 7 to 21 days. Clinical follow‐up extended up to 6 months after surgery. Patients in all included studies were screened for DVT during the trial period by I‐fibrinogen leg scanning,[23, 25, 26, 27] venography,[24, 28] or ultrasound[29, 30]; some trials also screened all participants for PE with ventilation/perfusion scanning.[27, 28]
Methodological Quality of Included Studies
Only 3 studies described their method of random sequence generation,[24, 25, 26] and 2 studies specified their method of allocation concealment.[25, 26] Only 1 study used placebo controls to double blind the study arm assignments.[25] We judged the overall potential risk of bias among the eligible studies to be moderate.
Rate of Proximal DVT
Pooling findings of all 7 studies that reported proximal DVT rates, we observed no statistically significant difference between aspirin and anticoagulants (10.4% vs 9.2%, relative risk [RR]: 1.15 [95% confidence interval {CI}: 0.68‐1.96], I2=41%). Although rates did not statistically differ between aspirin and anticoagulants in either operative subgroup, there appeared to be a nonsignificant trend favoring anticoagulation after hip fracture repair (12.7% vs 7.8%, RR: 1.60 [95% CI: 0.80‐3.20], I2=0%, 2 trials) but not following knee or hip arthroplasty (9.3% vs 9.7%, RR: 1.00 [95% CI: 0.49‐2.05], I2=49%, 5 trials) (Figure 2).

Rate of Pulmonary Embolism
Just 14 participants experienced a PE across all 6 trials reporting this outcome (aspirin n=9/405 versus anticoagulation n=5/415). Although PE was numerically more likely in the aspirin group, this difference was not statistically significant (overall: 1.9% vs 0.9%, RR: 1.83 [95% CI: 0.64, 5.21], I2=0%). The very small number of events rendered extremely wide 95% CIs in operative subgroup analyses (Figure 3).

Rates of All‐Cause Mortality
Only 2 trials, both evaluating aspirin versus anticoagulation following hip fracture repair, reported death events, both after 3 months follow‐up.[25, 26] Pooling these results, there was no statistically significant difference (7.3% vs 6.8%, RR: 1.07 [95% CI: 0.512.21], I2=0%).
Bleeding Rates
Pooling all 8 studies, aspirin was associated with a statistically significant 48% decreased risk of bleeding events compared to anticoagulants (3.8% vs 8.0%, RR: 0.52 [95% CI: 0.310.86], I2=8%). When subgrouped according to procedure, bleeding rates remained statistically significantly lower in the aspirin group following hip fracture (3.1% vs 10%, RR: 0.32 [95% CI: 0.130.77], I2=0%, 2 trials); however, the observed trend favoring aspirin was not statistically significant following arthroplasty (3.9% vs 7.8%, RR: 0.63 [95% CI: 0.331.21], I2=14%, 5 trials) (Figure 4).

Five studies reported major bleeding; event rates were low and no statistically significant differences between aspirin and anticoagulants were observed (hip fracture: 3.5% vs 6.3%, RR: 0.46 [95% CI: 0.141.48], I2=0%, 2 trials; knee/hip arthroplasty: 2.1% vs 0.6%, RR: 2.86 [95% CI: 0.6512.60], I2=0%, 3 trials).
Subgroup Analysis
Rates of proximal DVT did not differ between aspirin and anticoagulants when subgrouped according to anticoagulant class (aspirin vs warfarin: 9.7% vs 10.7%, RR: 0.90 [95% CI: 0.561.45], I2=0%, 3 trials; aspirin vs heparin: 10.5% vs 7.9%, RR: 1.37 [95% CI: 0.473.96], I2=44%, 4 trials) (data not shown).
Bleeding rates were lower with aspirin when subgrouped according to type of anticoagulant, but the finding was only statistically significant when compared to VKA (aspirin vs VKA: 4.2% vs 11.1%, RR: 0.43 [95% CI: 0.220.86] I2=0%, 4 trials; aspirin vs heparin: 3.7% vs 7.7%, RR: 0.44 [95% CI: 0.151.28], I2=44, 4 trials) (data not shown).
DISCUSSION
We found the balance of risk versus benefit of aspirin compared to anticoagulation differed markedly according to type of surgery. After hip fracture repair, we found a 68% reduction in bleeding risk with aspirin compared to anticoagulants. This benefit, however, was associated with a nonsignificant increase in screen‐detected proximal DVT. Conversely, among patients undergoing knee or hip arthroplasty, we found no difference in proximal DVT risk between aspirin and anticoagulants and a possible trend toward less bleeding risk with aspirin. The rarity of pulmonary emboli (and death) made meaningful comparisons between aspirin and anticoagulation impossible for either type of surgery.
Our systematic review has several strengths that differentiate it from previous analyses. First, we only included head‐to‐head randomized trials such that all included data reflect direct comparisons between aspirin and anticoagulation in well‐balanced populations. Conversely, both recent reviews[11, 12] were based on indirect comparisons, a type of analysis in which data for the intervention and control arms are taken from different studies and thus different populations. This methodology is not recommended by the Cochrane Collaboration[13, 14] because of the increased risk of an unbalanced comparison. For example, Brown and colleagues' meta‐analysis, which pooled data from selected arms of 14 randomized controlled trials, found the efficacy of aspirin comparable to that of anticoagulants, but all aspirin subjects came from a single trial of patients at such low risk of VTE that a placebo arm was considered justified.[31] Similarly, in the indirect comparison of Westrich and colleagues,[12] which found anticoagulation superior to aspirin, the likelihood of an unbalanced comparison was further heightened by their inclusion of observational studies, with the attendant risk of confounding by indication.
Our systematic review further differs from previous analyses by examining both beneficial and harmful clinical outcomes, and doing so separately for the 2 most common types of major orthopedic lower extremity surgery. This allowed us to discover important differences in the comparative efficacy (benefit vs harm) of aspirin versus anticoagulants across different procedure types. Finding that aspirin may have lower efficacy for preventing VTE following hip fracture repair than arthroplasty may not be surprising in light of the nature of the 2 procedures, the disparate mean ages typical of patients who undergo each procedure, and the underlying trauma in hip fracture patients.
The limitations of our review largely reflect the quality of the studies we were able to include. First, our pooled sample size remains relatively small, meaning that observed nonsignificant differences between aspirin and anticoagulation groups (eg, a nonsignificant 60% increased risk of DVT for aspirin after hip repair, 95% CI: 0.803.20) could reasonably reflect up to 3‐fold differences in DVT risk and 5‐fold differences in PE rates. Second, screening for DVT, which is neither recommended nor common in clinical practice, was used in all studies. Reported DVT incidence, therefore, is undoubtedly higher than what would be observed in practice; however, the effect on the direction and magnitude of observed relative risks is unpredictable. Third, included studies used a wide range of aspirin doses, as well as a variety of anticoagulant types. Although supratherapeutic aspirin doses are unlikely to confer additional benefit for venous thromboprophylaxis, they may be associated with excess bleeding risk.[32] Finally, several of the studies were conducted more than10 years ago. Given changes in treatment practices, surgical technique, and prophylaxis options, the findings of these studies may not reflect current practice, in which early mobilization and intermittent pneumatic compression devices are standard prophylaxis against postoperative VTE. In fact, only 2 trials used concomitant pneumatic compression devices, and none treated patients longer than 21 days, the current standard being up to 35 days.[4] Although these limitations may affect overall event rates, this bias should be balanced between comparison groups, because we only included randomized controlled trials.
What is a clinician to do? Based on our findings, current guidelines recommending aspirin prophylaxis against VTE as an alternative following major lower extremity surgery may not be universally appropriate. We found that although overall bleeding complications are lower with aspirin, concerns about poor efficacy remain, specifically for patients undergoing hip fracture repair. Although some have suggested that aspirin use be restricted to low risk patients, this strategy has not been experimentally evaluated.[33] On the other hand, switching to aspirin after a brief initial course of LMWH may be an approach warranting further study, in light of a recent randomized controlled trial of 778 patients after elective hip replacement, which found equivalent efficacy using 10 days of LMWH followed by aspirin versus additional LMWH for 28 days.[34]
We are able to be more definitive, based on our study of best available trial data, in making recommendations to investigators embarking on further study of optimal VTE prophylaxis following major orthopedic surgery. First, distinguishing a priori between the 2 major types of lower extremity major orthopedic surgery is a high priority. Second, both bleeding and thromboembolic outcomes must be evaluated. Third, only symptomatic events should be used to measure VTE outcomes; clinical follow‐up must continue well beyond discharge, for at least 3 months to ensure ascertainment of clinically relevant VTE. Fourth, nonpharmacologic cointerventions should be standardized and represent the standard of care, including early immobilization and mechanical compression devices.
In summary, although definitive recommendations for or against the use of aspirin instead of anticoagulation for VTE prevention following major orthopedic surgery are not possible, our findings suggest that, following hip fracture repair, the lower risk of bleeding with aspirin is likely outweighed by a probable trend toward higher risk of VTE. On the other hand, the balance of these opposing risks may favor aspirin after elective knee or hip arthroplasty. A comparative study of aspirin, anticoagulation, and a hybrid strategy (eg, brief anticoagulation followed by aspirin) after elective knee or hip arthroplasty should be a high priority given our aging population and increasing demand for major orthopedic lower extremity surgery.
Acknowledgements
The authors thank Dr. Deborah Ornstein (Associate Professor of Medicine, Geisel School of Medicine at Dartmouth, Section of Hematology Mary Hitchcock Memorial Hospital, Lebanon, New Hampshire) for sparking the idea for this systematic review.
Disclosures: Nothing to report.
Each year in the United States, over 1 million adults undergo hip fracture surgery or elective total knee or hip arthroplasty.[1] Although highly effective for improving functional status and quality of life,[2, 3] each of these procedures is associated with a substantial risk of developing a deep vein thrombosis (DVT) or pulmonary embolism (PE).[4, 5] Collectively referred to as venous thromboembolism (VTE), these clots in the venous system are associated with significant morbidity and mortality for patients, as well as substantial costs to the healthcare system.[6] Although VTE is considered to be a preventable cause of hospital admission and death,[7, 8] the postoperative setting presents a particular challenge, as efforts to reduce clotting must be balanced against the risk of bleeding.
Despite how common this scenario is, there is no consensus regarding the best pharmacologic strategy. National guidelines recommend pharmacologic thromboprophylaxis, leaving the clinician to select the specific agent.[4, 5] Explicitly endorsed options include aspirin, vitamin K antagonists (VKA), unfractionated heparin, fondaparinux, low‐molecular‐weight heparin (LMWH) and IIa/Xa factor inhibitors. Among these, aspirin, the only nonanticoagulant, has been the source of greatest controversy.[4, 9, 10]
Two previous systematic reviews comparing aspirin to anticoagulation for VTE prevention found conflicting results.[11, 12] In addition, both used indirect comparisons, a method in which the intervention and comparison data come from different studies, and susceptibility to confounding is high.[13, 14] We aimed to overcome the limitations of prior efforts to address this commonly encountered clinical question by conducting a systematic review and meta‐analysis of randomized controlled trials that directly compared the efficacy and safety of aspirin to anticoagulants for VTE prevention in adults undergoing common high‐risk major orthopedic surgeries of the lower extremities.
MATERIAL AND METHODS
Review Protocol
Prior to conducting the review, we outlined an approach to identifying and selecting eligible studies, prespecified outcomes of interest, and planned subgroup analyses. The meta‐analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses and Cochrane guidelines.[15, 16]
Study Eligibility Criteria
We prespecified the following inclusion criteria: (1) the design was a randomized controlled trial; (2) the population consisted of patients undergoing major orthopedic surgery including hip fracture surgery or total knee or hip arthroplasty; (3) the study compared aspirin to 1 or more anticoagulants: VKA, unfractionated heparin, LMWH, thrombin inhibitors, pentasaccharides (eg, fondaparinux), factor Xa/IIa inhibitors dosed for VTE prevention; (4) subjects were followed for at least 7 days; and (5) the study reported at least 1 prespecified outcome of interest. We allowed the use of pneumatic compression devices, as long as devices were used in both arms of the study.
Outcome Measures
We designated the rate of proximal DVT (occurring in the popliteal vein and above) as the primary outcome of interest. Additional efficacy outcomes included rates of PE, PE‐related mortality, and all‐cause mortality. We required that DVT and PE were diagnosed by venography, computed tomography (CT) angiography of the chest, pulmonary angiography, ultrasound Doppler of the legs, or ventilation/perfusion scan. We allowed studies that screened participants for VTE (including the use of fibrinogen leg scanning).
A bleeding event was defined as any need for postoperative blood transfusion or otherwise clinically significant bleeding (eg, prolonged postoperative wound bleeding). We further defined major bleeding as the requirement for blood transfusion of more than 2 U, hematoma requiring surgical evacuation, and bleeding into a critical organ.
Study Identification
We searched Medline (January 1948 to June 2013), Cochrane Library (through June 2013), and CINAHL (January 1974 to June 2013) to locate studies meeting our inclusion criteria. We used exploded Medical Subject Headings terms and key words to generate sets for aspirin and major orthopedic surgery themes, then used the Boolean term, AND, to find their intersection.
Additional Search Methods
We manually reviewed references of relevant articles and searched ClinicalTrials.gov to identify any ongoing studies or unpublished data. We further searched the following sources: American College of Chest Physicians (ACCP) Evidence‐Based Clinical Practice Guidelines,[4, 17] American Academy of Orthopaedic Surgeons guidelines (AAOS),[5] and annual meeting abstracts of the American Academy of Orthopaedic Surgery,[18] the American Society of Hematology,[19] and the ACCP.[20]
Study Selection
Two pairs of 2 reviewers independently scanned the titles and abstracts of identified studies, excluding only those that were clearly not relevant. The same reviewers independently reviewed the full text of each remaining study to make final decisions about eligibility.
Data Extraction and Quality Assessment
Two reviewers independently extracted data from each included study and rendered judgments regarding the methodological quality using the Cochrane Risk of Bias Tool.[21]
Data Synthesis
We used Review Manager (RevMan 5.1) to calculate pooled risk ratios using the Mantel‐Haenszel method and random‐effects models, which take into account the presence of variability among included studies.[16, 22] We also manually pooled absolute event rates for each study arm using the study weights assigned in the pooled risk ratio models.
Assessment of Heterogeneity and Reporting Biases
We assessed statistical variability among the studies contributing to each summary estimate and considered studies unacceptably heterogeneous if the test for heterogeneity P value was <0.10 or the I2 exceeded 50%.[14, 16] We constructed funnel plots to assess for publication bias but had too few studies for reliable interpretation.
Subgroup Analyses
We prespecified subgroup analyses based on the indication for the surgery: hip fracture surgery versus total knee or hip arthroplasty, and according to class of anticoagulation used: VKA versus heparin compounds.
RESULTS
Results of Search
Figure 1 shows the number of studies that we evaluated during each stage of the study selection process. After full‐text review, 8 randomized trials met all inclusion criteria.[23, 24, 25, 26, 27, 28, 29, 30]

Included Studies
Table 1 presents the characteristics of the 8 included randomized trials. All were published in peer‐reviewed journals from 1982 through 2006.2330 The trials included a combined total of 1408 subjects, and took place in 4 different countries, including the United States,[24, 26, 28, 29, 30] Spain,[23] Sweden,[27] and Canada.[25] Enrolled patients had a mean age of 76 years (range, 7477 years) among hip fracture surgery studies and 66 years (range, 5969 years) among elective knee/hip arthroplasty studies.
| Author, Year | Surgery | Pneumatic Compression | Intervention | Control | Duration (Days) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Aspirin (Total/Day) | No. | Mean Age, Years | Anticoagulant | No. | Mean Age, Years | ||||
| |||||||||
| Powers, 1989 | Hip fracture | No | 1,300 mg | 66 | 73 | Warfarin | 65 | 75 | 21 |
| Gent, 1996 | Hip fracture | No | 200 mg | 126* | 77 | Danaparoid | 125* | 77 | 11 |
| Harris, 1982 | THA | No | 1,200 mg | 51 | 58 | Heparin or warfarin | 75 | 60 | 21 |
| Alfaro, 1986 | THA | No | 250 mg/1,000 mg | 60 | 64 | Heparin | 30 | 58 | 7 |
| Josefsson, 1987 | THA | No | 3,000 mg | 40 | N/A | Heparin | 42 | N/A | 9 |
| Woolson, 1991 | THA | Yes | 1,300 mg | 72 | 62 | Warfarin | 69 | 68 | 7 |
| Lotke, 1996 | THA or TKA | No | 650 mg | 166 | 66 | Warfarin | 146 | 67 | 9 |
| Westrich, 2006 | TKA | Yes | 650 mg | 136 | 69 | Enoxaparin | 139 | 69 | 21 |
Pneumatic compression devices were used in addition to pharmacologic prevention in 2 studies.[29, 30] The different classes of anticoagulants used included warfarin,[26, 28, 30] heparin,[23, 27] LMWH,[29] heparin or warfarin,[24] and danaparoid.[25] Treatment duration was 7 to 21 days. Clinical follow‐up extended up to 6 months after surgery. Patients in all included studies were screened for DVT during the trial period by I‐fibrinogen leg scanning,[23, 25, 26, 27] venography,[24, 28] or ultrasound[29, 30]; some trials also screened all participants for PE with ventilation/perfusion scanning.[27, 28]
Methodological Quality of Included Studies
Only 3 studies described their method of random sequence generation,[24, 25, 26] and 2 studies specified their method of allocation concealment.[25, 26] Only 1 study used placebo controls to double blind the study arm assignments.[25] We judged the overall potential risk of bias among the eligible studies to be moderate.
Rate of Proximal DVT
Pooling findings of all 7 studies that reported proximal DVT rates, we observed no statistically significant difference between aspirin and anticoagulants (10.4% vs 9.2%, relative risk [RR]: 1.15 [95% confidence interval {CI}: 0.68‐1.96], I2=41%). Although rates did not statistically differ between aspirin and anticoagulants in either operative subgroup, there appeared to be a nonsignificant trend favoring anticoagulation after hip fracture repair (12.7% vs 7.8%, RR: 1.60 [95% CI: 0.80‐3.20], I2=0%, 2 trials) but not following knee or hip arthroplasty (9.3% vs 9.7%, RR: 1.00 [95% CI: 0.49‐2.05], I2=49%, 5 trials) (Figure 2).

Rate of Pulmonary Embolism
Just 14 participants experienced a PE across all 6 trials reporting this outcome (aspirin n=9/405 versus anticoagulation n=5/415). Although PE was numerically more likely in the aspirin group, this difference was not statistically significant (overall: 1.9% vs 0.9%, RR: 1.83 [95% CI: 0.64, 5.21], I2=0%). The very small number of events rendered extremely wide 95% CIs in operative subgroup analyses (Figure 3).

Rates of All‐Cause Mortality
Only 2 trials, both evaluating aspirin versus anticoagulation following hip fracture repair, reported death events, both after 3 months follow‐up.[25, 26] Pooling these results, there was no statistically significant difference (7.3% vs 6.8%, RR: 1.07 [95% CI: 0.512.21], I2=0%).
Bleeding Rates
Pooling all 8 studies, aspirin was associated with a statistically significant 48% decreased risk of bleeding events compared to anticoagulants (3.8% vs 8.0%, RR: 0.52 [95% CI: 0.310.86], I2=8%). When subgrouped according to procedure, bleeding rates remained statistically significantly lower in the aspirin group following hip fracture (3.1% vs 10%, RR: 0.32 [95% CI: 0.130.77], I2=0%, 2 trials); however, the observed trend favoring aspirin was not statistically significant following arthroplasty (3.9% vs 7.8%, RR: 0.63 [95% CI: 0.331.21], I2=14%, 5 trials) (Figure 4).

Five studies reported major bleeding; event rates were low and no statistically significant differences between aspirin and anticoagulants were observed (hip fracture: 3.5% vs 6.3%, RR: 0.46 [95% CI: 0.141.48], I2=0%, 2 trials; knee/hip arthroplasty: 2.1% vs 0.6%, RR: 2.86 [95% CI: 0.6512.60], I2=0%, 3 trials).
Subgroup Analysis
Rates of proximal DVT did not differ between aspirin and anticoagulants when subgrouped according to anticoagulant class (aspirin vs warfarin: 9.7% vs 10.7%, RR: 0.90 [95% CI: 0.561.45], I2=0%, 3 trials; aspirin vs heparin: 10.5% vs 7.9%, RR: 1.37 [95% CI: 0.473.96], I2=44%, 4 trials) (data not shown).
Bleeding rates were lower with aspirin when subgrouped according to type of anticoagulant, but the finding was only statistically significant when compared to VKA (aspirin vs VKA: 4.2% vs 11.1%, RR: 0.43 [95% CI: 0.220.86] I2=0%, 4 trials; aspirin vs heparin: 3.7% vs 7.7%, RR: 0.44 [95% CI: 0.151.28], I2=44, 4 trials) (data not shown).
DISCUSSION
We found the balance of risk versus benefit of aspirin compared to anticoagulation differed markedly according to type of surgery. After hip fracture repair, we found a 68% reduction in bleeding risk with aspirin compared to anticoagulants. This benefit, however, was associated with a nonsignificant increase in screen‐detected proximal DVT. Conversely, among patients undergoing knee or hip arthroplasty, we found no difference in proximal DVT risk between aspirin and anticoagulants and a possible trend toward less bleeding risk with aspirin. The rarity of pulmonary emboli (and death) made meaningful comparisons between aspirin and anticoagulation impossible for either type of surgery.
Our systematic review has several strengths that differentiate it from previous analyses. First, we only included head‐to‐head randomized trials such that all included data reflect direct comparisons between aspirin and anticoagulation in well‐balanced populations. Conversely, both recent reviews[11, 12] were based on indirect comparisons, a type of analysis in which data for the intervention and control arms are taken from different studies and thus different populations. This methodology is not recommended by the Cochrane Collaboration[13, 14] because of the increased risk of an unbalanced comparison. For example, Brown and colleagues' meta‐analysis, which pooled data from selected arms of 14 randomized controlled trials, found the efficacy of aspirin comparable to that of anticoagulants, but all aspirin subjects came from a single trial of patients at such low risk of VTE that a placebo arm was considered justified.[31] Similarly, in the indirect comparison of Westrich and colleagues,[12] which found anticoagulation superior to aspirin, the likelihood of an unbalanced comparison was further heightened by their inclusion of observational studies, with the attendant risk of confounding by indication.
Our systematic review further differs from previous analyses by examining both beneficial and harmful clinical outcomes, and doing so separately for the 2 most common types of major orthopedic lower extremity surgery. This allowed us to discover important differences in the comparative efficacy (benefit vs harm) of aspirin versus anticoagulants across different procedure types. Finding that aspirin may have lower efficacy for preventing VTE following hip fracture repair than arthroplasty may not be surprising in light of the nature of the 2 procedures, the disparate mean ages typical of patients who undergo each procedure, and the underlying trauma in hip fracture patients.
The limitations of our review largely reflect the quality of the studies we were able to include. First, our pooled sample size remains relatively small, meaning that observed nonsignificant differences between aspirin and anticoagulation groups (eg, a nonsignificant 60% increased risk of DVT for aspirin after hip repair, 95% CI: 0.803.20) could reasonably reflect up to 3‐fold differences in DVT risk and 5‐fold differences in PE rates. Second, screening for DVT, which is neither recommended nor common in clinical practice, was used in all studies. Reported DVT incidence, therefore, is undoubtedly higher than what would be observed in practice; however, the effect on the direction and magnitude of observed relative risks is unpredictable. Third, included studies used a wide range of aspirin doses, as well as a variety of anticoagulant types. Although supratherapeutic aspirin doses are unlikely to confer additional benefit for venous thromboprophylaxis, they may be associated with excess bleeding risk.[32] Finally, several of the studies were conducted more than10 years ago. Given changes in treatment practices, surgical technique, and prophylaxis options, the findings of these studies may not reflect current practice, in which early mobilization and intermittent pneumatic compression devices are standard prophylaxis against postoperative VTE. In fact, only 2 trials used concomitant pneumatic compression devices, and none treated patients longer than 21 days, the current standard being up to 35 days.[4] Although these limitations may affect overall event rates, this bias should be balanced between comparison groups, because we only included randomized controlled trials.
What is a clinician to do? Based on our findings, current guidelines recommending aspirin prophylaxis against VTE as an alternative following major lower extremity surgery may not be universally appropriate. We found that although overall bleeding complications are lower with aspirin, concerns about poor efficacy remain, specifically for patients undergoing hip fracture repair. Although some have suggested that aspirin use be restricted to low risk patients, this strategy has not been experimentally evaluated.[33] On the other hand, switching to aspirin after a brief initial course of LMWH may be an approach warranting further study, in light of a recent randomized controlled trial of 778 patients after elective hip replacement, which found equivalent efficacy using 10 days of LMWH followed by aspirin versus additional LMWH for 28 days.[34]
We are able to be more definitive, based on our study of best available trial data, in making recommendations to investigators embarking on further study of optimal VTE prophylaxis following major orthopedic surgery. First, distinguishing a priori between the 2 major types of lower extremity major orthopedic surgery is a high priority. Second, both bleeding and thromboembolic outcomes must be evaluated. Third, only symptomatic events should be used to measure VTE outcomes; clinical follow‐up must continue well beyond discharge, for at least 3 months to ensure ascertainment of clinically relevant VTE. Fourth, nonpharmacologic cointerventions should be standardized and represent the standard of care, including early immobilization and mechanical compression devices.
In summary, although definitive recommendations for or against the use of aspirin instead of anticoagulation for VTE prevention following major orthopedic surgery are not possible, our findings suggest that, following hip fracture repair, the lower risk of bleeding with aspirin is likely outweighed by a probable trend toward higher risk of VTE. On the other hand, the balance of these opposing risks may favor aspirin after elective knee or hip arthroplasty. A comparative study of aspirin, anticoagulation, and a hybrid strategy (eg, brief anticoagulation followed by aspirin) after elective knee or hip arthroplasty should be a high priority given our aging population and increasing demand for major orthopedic lower extremity surgery.
Acknowledgements
The authors thank Dr. Deborah Ornstein (Associate Professor of Medicine, Geisel School of Medicine at Dartmouth, Section of Hematology Mary Hitchcock Memorial Hospital, Lebanon, New Hampshire) for sparking the idea for this systematic review.
Disclosures: Nothing to report.
- Healthcare Cost and Utilization Project (HCUP). Available at: http://hcupnet.ahrq.gov. Accessed June 2013.
- , , , et al. Outcomes after hip or knee replacement surgery for osteoarthritis. A prospective cohort study comparing patients' quality of life before and after surgery with age‐related population norms. Med J Aust. 1999;171(5):235–238.
- , , . Quality of life and functional outcome after primary total hip replacement. A five‐year follow‐up. J Bone Joint Surg Br. 2007;89(7):868–873.
- , , , et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 suppl):e278S–e325S.
- American Academy of Orthopaedic Surgeons (AAOS). American Academy of Orthopaedic Surgeons clinical practice guideline on preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2011. Available at: http://www.aaos.org/research/guidelines. Accessed June 2013.
- , , , . Economic burden of deep‐vein thrombosis, pulmonary embolism, and post‐thrombotic syndrome. Am J Health Syst Pharm. 2006;63(20 suppl 6):S5–S15.
- , . Autopsy proven pulmonary embolism in hospital patients: are we detecting enough deep vein thrombosis? J R Soc Med. 1989;82(4):203–205.
- , , . Autopsy‐verified pulmonary embolism in a surgical department: analysis of the period from 1951 to 1988. Br J Surg. 1991;78(7):849–852.
- . What is the state of the art in orthopaedic thromboprophylaxis in lower extremity reconstruction? Instr Course Lect. 2011;60:283–290.
- , . Aspirin for the prophylaxis of venous thromboembolic events in orthopedic surgery patients: a comparison of the AAOS and ACCP guidelines with review of the evidence. Ann Pharmacother. 2013;47(1):63–74.
- . Venous thromboembolism prophylaxis after major orthopaedic surgery: A pooled analysis of randomized controlled trials. J Arthroplasty. 2009;24(6 supplement 1):77–83.
- , , , . Meta‐analysis of thromboembolic prophylaxis after total knee arthroplasty. J Bone Joint Surg Br. 2000;82(6):795–800.
- , , , , , . Methodological problems in the use of indirect comparisons for evaluating healthcare interventions: survey of published systematic reviews. BMJ. 2009;338:b1147.
- Higgins J, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Available at: http://handbook.cochrane.org. Accessed June 2013.
- , , , ; the PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
- , , , et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100.
- , , , et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence‐based clinical practice guidelines (8th edition). Chest. 2008;133(6 suppl):381S–453S.
- , , , et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 suppl):e195S–e226S.
- ASH Annual Meeting Abstracts‐Blood. Available at: http://bloodjournal.hematologylibrary.org/site/misc/ASH_Meeting_Abstracts_Info.xhtml. Accessed June 2013.
- CHEST Publications Meeting Abstracts. Available at: http://journal.publications.chestnet.org/ss/meetingabstracts.aspx. Accessed June 2013.
- , , , et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
- Review Manager (RevMan) [computer program]. Version 5.1. Copenhagen, Denmark: The Nordic Cochrane Centre, The Cochrane Collaboration; 2011.
- , , . Prophylaxis of thromboembolic disease and platelet‐related changes following total hip replacement: a comparative study of aspirin and heparin‐dihydroergotamine. Thromb Haemost. 1986;56(1):53–56.
- , , , . High and low‐dose aspirin prophylaxis against venous thromboembolic disease in total hip replacement. J Bone Joint Surg Am. 1982;64(1):63–66.
- , , , et al. Low‐molecular‐weight heparinoid orgaran is more effective than aspirin in the prevention of venous thromboembolism after surgery for hip fracture. Circulation. 1996;93(1):80–84.
- , , , et al. A randomized trial of less intense postoperative warfarin or aspirin therapy in the prevention of venous thromboembolism after surgery for fractured hip. Arch Intern Med. 1989;149(4):771–774.
- , , . Prevention of thromboembolism in total hip replacement. Aspirin versus dihydroergotamine‐heparin. Acta Orthop Scand. 1987;58(6):626–629.
- , , , et al. Aspirin and warfarin for thromboembolic disease after total joint arthroplasty. Clin Orthop Relat Res. 1996;(324):251–258.
- , , , , , . VenaFlow plus Lovenox vs VenaFlow plus aspirin for thromboembolic disease prophylaxis in total knee arthroplasty. J Arthroplasty. 2006;21(6 suppl 2):139–143.
- , . Intermittent pneumatic compression to prevent proximal deep venous thrombosis during and after total hip replacement. A prospective, randomized study of compression alone, compression and aspirin, and compression and low‐dose warfarin. J Bone Joint Surg Am. 1991;73(4):507–512.
- Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: Pulmonary Embolism Prevention (PEP) trial. Lancet. 2000;355(9212):1295–1302.
- , , , et al. Safety and efficacy of high‐ versus low‐dose aspirin after primary percutaneous coronary intervention in ST‐segment elevation myocardial infarction: the HORIZONS‐AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) trial. JACC Cardiovasc Interv. 2012;5(12):1231–1238.
- Intermountain Joint Replacement Center Writing Committee. A prospective comparison of warfarin to aspirin for thromboprophylaxis in total hip and total knee arthroplasty. J Arthroplasty. 2011;27:e1–e9.
- , , , et al. Aspirin versus low‐molecular‐weight heparin for extended venous thromboembolism prophylaxis after total hip arthroplasty: a randomized trial. Ann Intern Med. 2013;158(11):800–806.
- Healthcare Cost and Utilization Project (HCUP). Available at: http://hcupnet.ahrq.gov. Accessed June 2013.
- , , , et al. Outcomes after hip or knee replacement surgery for osteoarthritis. A prospective cohort study comparing patients' quality of life before and after surgery with age‐related population norms. Med J Aust. 1999;171(5):235–238.
- , , . Quality of life and functional outcome after primary total hip replacement. A five‐year follow‐up. J Bone Joint Surg Br. 2007;89(7):868–873.
- , , , et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 suppl):e278S–e325S.
- American Academy of Orthopaedic Surgeons (AAOS). American Academy of Orthopaedic Surgeons clinical practice guideline on preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2011. Available at: http://www.aaos.org/research/guidelines. Accessed June 2013.
- , , , . Economic burden of deep‐vein thrombosis, pulmonary embolism, and post‐thrombotic syndrome. Am J Health Syst Pharm. 2006;63(20 suppl 6):S5–S15.
- , . Autopsy proven pulmonary embolism in hospital patients: are we detecting enough deep vein thrombosis? J R Soc Med. 1989;82(4):203–205.
- , , . Autopsy‐verified pulmonary embolism in a surgical department: analysis of the period from 1951 to 1988. Br J Surg. 1991;78(7):849–852.
- . What is the state of the art in orthopaedic thromboprophylaxis in lower extremity reconstruction? Instr Course Lect. 2011;60:283–290.
- , . Aspirin for the prophylaxis of venous thromboembolic events in orthopedic surgery patients: a comparison of the AAOS and ACCP guidelines with review of the evidence. Ann Pharmacother. 2013;47(1):63–74.
- . Venous thromboembolism prophylaxis after major orthopaedic surgery: A pooled analysis of randomized controlled trials. J Arthroplasty. 2009;24(6 supplement 1):77–83.
- , , , . Meta‐analysis of thromboembolic prophylaxis after total knee arthroplasty. J Bone Joint Surg Br. 2000;82(6):795–800.
- , , , , , . Methodological problems in the use of indirect comparisons for evaluating healthcare interventions: survey of published systematic reviews. BMJ. 2009;338:b1147.
- Higgins J, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Available at: http://handbook.cochrane.org. Accessed June 2013.
- , , , ; the PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
- , , , et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100.
- , , , et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence‐based clinical practice guidelines (8th edition). Chest. 2008;133(6 suppl):381S–453S.
- , , , et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 suppl):e195S–e226S.
- ASH Annual Meeting Abstracts‐Blood. Available at: http://bloodjournal.hematologylibrary.org/site/misc/ASH_Meeting_Abstracts_Info.xhtml. Accessed June 2013.
- CHEST Publications Meeting Abstracts. Available at: http://journal.publications.chestnet.org/ss/meetingabstracts.aspx. Accessed June 2013.
- , , , et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
- Review Manager (RevMan) [computer program]. Version 5.1. Copenhagen, Denmark: The Nordic Cochrane Centre, The Cochrane Collaboration; 2011.
- , , . Prophylaxis of thromboembolic disease and platelet‐related changes following total hip replacement: a comparative study of aspirin and heparin‐dihydroergotamine. Thromb Haemost. 1986;56(1):53–56.
- , , , . High and low‐dose aspirin prophylaxis against venous thromboembolic disease in total hip replacement. J Bone Joint Surg Am. 1982;64(1):63–66.
- , , , et al. Low‐molecular‐weight heparinoid orgaran is more effective than aspirin in the prevention of venous thromboembolism after surgery for hip fracture. Circulation. 1996;93(1):80–84.
- , , , et al. A randomized trial of less intense postoperative warfarin or aspirin therapy in the prevention of venous thromboembolism after surgery for fractured hip. Arch Intern Med. 1989;149(4):771–774.
- , , . Prevention of thromboembolism in total hip replacement. Aspirin versus dihydroergotamine‐heparin. Acta Orthop Scand. 1987;58(6):626–629.
- , , , et al. Aspirin and warfarin for thromboembolic disease after total joint arthroplasty. Clin Orthop Relat Res. 1996;(324):251–258.
- , , , , , . VenaFlow plus Lovenox vs VenaFlow plus aspirin for thromboembolic disease prophylaxis in total knee arthroplasty. J Arthroplasty. 2006;21(6 suppl 2):139–143.
- , . Intermittent pneumatic compression to prevent proximal deep venous thrombosis during and after total hip replacement. A prospective, randomized study of compression alone, compression and aspirin, and compression and low‐dose warfarin. J Bone Joint Surg Am. 1991;73(4):507–512.
- Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: Pulmonary Embolism Prevention (PEP) trial. Lancet. 2000;355(9212):1295–1302.
- , , , et al. Safety and efficacy of high‐ versus low‐dose aspirin after primary percutaneous coronary intervention in ST‐segment elevation myocardial infarction: the HORIZONS‐AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) trial. JACC Cardiovasc Interv. 2012;5(12):1231–1238.
- Intermountain Joint Replacement Center Writing Committee. A prospective comparison of warfarin to aspirin for thromboprophylaxis in total hip and total knee arthroplasty. J Arthroplasty. 2011;27:e1–e9.
- , , , et al. Aspirin versus low‐molecular‐weight heparin for extended venous thromboembolism prophylaxis after total hip arthroplasty: a randomized trial. Ann Intern Med. 2013;158(11):800–806.
© 2014 Society of Hospital Medicine
Society of Hospital Medicine Backs Bill to Modify Hospital Readmissions Program
The Society of Hospital Medicine (SHM) is supporting a Congressional push to tweak which admissions factors are taken into consideration in the federal Hospital Readmissions Reduction Program.
Hospitalist and SHM President Burke Kealey, MD, SFHM, says that the Establishing Beneficiary Equity in the Hospital Readmission Program Act (H.R. 4188) would help "level the playing field."
Sponsored by U.S. Representative James Renacci (R-Ohio), the proposal seeks to "exclude from the program admissions related to transplants, end-stage renal disease, burns, trauma, psychosis, or substance abuse." It also would require the U.S. Department of Health & Human Services (HHS) "in applying requirements for the excess readmission ratio to provide for a risk adjustment" that would take into account the percentage of inpatients eligible for both Medicare and Medicaid to avoid unfairly penalizing hospitals that treat the most vulnerable populations.
"We feel that some hospitals may be being unfairly handled in this program," Dr. Kealey says. "Those are the hospitals that are having to deal with more complex populations or lower-SES [socioeconomic status] populations. Those are the hospitalists that actually need the most resources to help prevent readmissions, and they end up losing in this whole equation."
In a letter to Rep. Renacci outlining SHM's support for the bill, Dr. Kealey notes that the current readmissions reduction program "needs fine-tuning to better account for preventable readmission."
Dr. Kealey also says he believes attempts by HHS to address readmissions are well-intentioned. However, as the program is implemented, he wants the government to be flexible in dealing with hospitals, particularly those dealing with complex populations or large groups of low-SES patients.
"We feel [these are] valuable programs, and in general, they help move the country in the right direction," Dr. Kealey says. "But they certainly need to be open and available to be modified and changed to fit conditions better."
SHM's program to reduce hospital readmissions, Project BOOST, is accepting applications to its 2014 cohort through August 30. TH
Visit our website for more information on hospital readmissions penalties.
The Society of Hospital Medicine (SHM) is supporting a Congressional push to tweak which admissions factors are taken into consideration in the federal Hospital Readmissions Reduction Program.
Hospitalist and SHM President Burke Kealey, MD, SFHM, says that the Establishing Beneficiary Equity in the Hospital Readmission Program Act (H.R. 4188) would help "level the playing field."
Sponsored by U.S. Representative James Renacci (R-Ohio), the proposal seeks to "exclude from the program admissions related to transplants, end-stage renal disease, burns, trauma, psychosis, or substance abuse." It also would require the U.S. Department of Health & Human Services (HHS) "in applying requirements for the excess readmission ratio to provide for a risk adjustment" that would take into account the percentage of inpatients eligible for both Medicare and Medicaid to avoid unfairly penalizing hospitals that treat the most vulnerable populations.
"We feel that some hospitals may be being unfairly handled in this program," Dr. Kealey says. "Those are the hospitals that are having to deal with more complex populations or lower-SES [socioeconomic status] populations. Those are the hospitalists that actually need the most resources to help prevent readmissions, and they end up losing in this whole equation."
In a letter to Rep. Renacci outlining SHM's support for the bill, Dr. Kealey notes that the current readmissions reduction program "needs fine-tuning to better account for preventable readmission."
Dr. Kealey also says he believes attempts by HHS to address readmissions are well-intentioned. However, as the program is implemented, he wants the government to be flexible in dealing with hospitals, particularly those dealing with complex populations or large groups of low-SES patients.
"We feel [these are] valuable programs, and in general, they help move the country in the right direction," Dr. Kealey says. "But they certainly need to be open and available to be modified and changed to fit conditions better."
SHM's program to reduce hospital readmissions, Project BOOST, is accepting applications to its 2014 cohort through August 30. TH
Visit our website for more information on hospital readmissions penalties.
The Society of Hospital Medicine (SHM) is supporting a Congressional push to tweak which admissions factors are taken into consideration in the federal Hospital Readmissions Reduction Program.
Hospitalist and SHM President Burke Kealey, MD, SFHM, says that the Establishing Beneficiary Equity in the Hospital Readmission Program Act (H.R. 4188) would help "level the playing field."
Sponsored by U.S. Representative James Renacci (R-Ohio), the proposal seeks to "exclude from the program admissions related to transplants, end-stage renal disease, burns, trauma, psychosis, or substance abuse." It also would require the U.S. Department of Health & Human Services (HHS) "in applying requirements for the excess readmission ratio to provide for a risk adjustment" that would take into account the percentage of inpatients eligible for both Medicare and Medicaid to avoid unfairly penalizing hospitals that treat the most vulnerable populations.
"We feel that some hospitals may be being unfairly handled in this program," Dr. Kealey says. "Those are the hospitals that are having to deal with more complex populations or lower-SES [socioeconomic status] populations. Those are the hospitalists that actually need the most resources to help prevent readmissions, and they end up losing in this whole equation."
In a letter to Rep. Renacci outlining SHM's support for the bill, Dr. Kealey notes that the current readmissions reduction program "needs fine-tuning to better account for preventable readmission."
Dr. Kealey also says he believes attempts by HHS to address readmissions are well-intentioned. However, as the program is implemented, he wants the government to be flexible in dealing with hospitals, particularly those dealing with complex populations or large groups of low-SES patients.
"We feel [these are] valuable programs, and in general, they help move the country in the right direction," Dr. Kealey says. "But they certainly need to be open and available to be modified and changed to fit conditions better."
SHM's program to reduce hospital readmissions, Project BOOST, is accepting applications to its 2014 cohort through August 30. TH
Visit our website for more information on hospital readmissions penalties.
Hospital-Acquired Bloodstream Infection Prevention Paying Off
A new report that shows efforts to prevent central-line-associated bloodstream infections (CLABSIs) saved the government at least $640 million over nearly 20 years is an example of how effective prevention campaigns can be, a veteran hospitalist says.
"The major idea of this report was to show us that complications like bloodstream infections are preventable," says Ketino Kobaidze MD, PhD, FHM, assistant professor of medicine and associate site director of the division of hospital medicine at the Emory University School of Medicine in Atlanta. "When you prevent these things, you can locate other things. That's what the major message is to any kind of healthcare provider."
Published in the June issue of Health Affairs, the report examines the results of CDC programs from 1990 to 2008 to prevent CLABSIs in critical care units and how prevention helped the Centers for Medicaid & Medicare Services (CMS) reduce the amount of reimbursement paid to hospitals for treating such infections.
The authors reported that from 1990 to 2008, between 40,556 and 75,067 CLABSIs were avoided in Medicare and Medicaid patients treated in critical care units. This resulted in:
• Net savings ranging from $640 million to $1.8 billion;
• Net savings per case ranging from $15,780 to $24,391; and
• Per dollar rate of return on CDC investments between $3.88 and $23.85.
"Now, you're basically expected for it to not happen at all," says Dr. Kobaidze, referring to a rule implemented by CMS in 2008 that ended reimbursements to hospitals for treating CLABSIs that weren't present upon admission.
With that rule, CMS included 10 categories of hospital-acquired conditions (HACs) for the payment provision rule, including stage III and IV pressure ulcers and falls that occur while the patient is in the hospital. The rule was updated in 2013 to include HACs related to surgical site infection with cardiac implantable electronic devices and iatrogenic pneumothorax with venous catheterization. TH
Visit our website for more information on bloodstream infection prevention.
A new report that shows efforts to prevent central-line-associated bloodstream infections (CLABSIs) saved the government at least $640 million over nearly 20 years is an example of how effective prevention campaigns can be, a veteran hospitalist says.
"The major idea of this report was to show us that complications like bloodstream infections are preventable," says Ketino Kobaidze MD, PhD, FHM, assistant professor of medicine and associate site director of the division of hospital medicine at the Emory University School of Medicine in Atlanta. "When you prevent these things, you can locate other things. That's what the major message is to any kind of healthcare provider."
Published in the June issue of Health Affairs, the report examines the results of CDC programs from 1990 to 2008 to prevent CLABSIs in critical care units and how prevention helped the Centers for Medicaid & Medicare Services (CMS) reduce the amount of reimbursement paid to hospitals for treating such infections.
The authors reported that from 1990 to 2008, between 40,556 and 75,067 CLABSIs were avoided in Medicare and Medicaid patients treated in critical care units. This resulted in:
• Net savings ranging from $640 million to $1.8 billion;
• Net savings per case ranging from $15,780 to $24,391; and
• Per dollar rate of return on CDC investments between $3.88 and $23.85.
"Now, you're basically expected for it to not happen at all," says Dr. Kobaidze, referring to a rule implemented by CMS in 2008 that ended reimbursements to hospitals for treating CLABSIs that weren't present upon admission.
With that rule, CMS included 10 categories of hospital-acquired conditions (HACs) for the payment provision rule, including stage III and IV pressure ulcers and falls that occur while the patient is in the hospital. The rule was updated in 2013 to include HACs related to surgical site infection with cardiac implantable electronic devices and iatrogenic pneumothorax with venous catheterization. TH
Visit our website for more information on bloodstream infection prevention.
A new report that shows efforts to prevent central-line-associated bloodstream infections (CLABSIs) saved the government at least $640 million over nearly 20 years is an example of how effective prevention campaigns can be, a veteran hospitalist says.
"The major idea of this report was to show us that complications like bloodstream infections are preventable," says Ketino Kobaidze MD, PhD, FHM, assistant professor of medicine and associate site director of the division of hospital medicine at the Emory University School of Medicine in Atlanta. "When you prevent these things, you can locate other things. That's what the major message is to any kind of healthcare provider."
Published in the June issue of Health Affairs, the report examines the results of CDC programs from 1990 to 2008 to prevent CLABSIs in critical care units and how prevention helped the Centers for Medicaid & Medicare Services (CMS) reduce the amount of reimbursement paid to hospitals for treating such infections.
The authors reported that from 1990 to 2008, between 40,556 and 75,067 CLABSIs were avoided in Medicare and Medicaid patients treated in critical care units. This resulted in:
• Net savings ranging from $640 million to $1.8 billion;
• Net savings per case ranging from $15,780 to $24,391; and
• Per dollar rate of return on CDC investments between $3.88 and $23.85.
"Now, you're basically expected for it to not happen at all," says Dr. Kobaidze, referring to a rule implemented by CMS in 2008 that ended reimbursements to hospitals for treating CLABSIs that weren't present upon admission.
With that rule, CMS included 10 categories of hospital-acquired conditions (HACs) for the payment provision rule, including stage III and IV pressure ulcers and falls that occur while the patient is in the hospital. The rule was updated in 2013 to include HACs related to surgical site infection with cardiac implantable electronic devices and iatrogenic pneumothorax with venous catheterization. TH
Visit our website for more information on bloodstream infection prevention.
Summer care for atopic skin
Summer months can be dreadful for patients with atopic dermatitis. The chlorine, heat, and humidity can lead to flares. Furthermore, noncompliance with skin care regimens because of changing summer routines, travel, and the use of hotel products can exacerbate even the calmest skin disease.
Share these tips with your patients to help them keep their atopic skin under control in the summer heat, and stop flares before they start.
• Rinse the skin well after swimming. Chlorine and saltwater can dry out the skin. Showers after swimming in chlorinated pools can help retain the skin’s natural oils.
• Avoid hot tubs. Cracks and fissures in atopic skin can become infected in hot tubs with Staphylococcus and Pseudomonas. Advise your atopic patients to avoid hot tubs, even if they claim the tubs have been cleaned.
• Bring your own products. Many soaps and shower gels available in hotels and resorts are extremely drying, and may contain ingredients that could irritate atopic skin.
• Don’t switch from thick creams to thin lotions just because it is summer. Remind your patients that a thin lotion does not provide the same occlusive and humectant properties as thicker creams, although they are not as easy to apply, and can feel thick and sticky on the skin with humidity.
• In case of an active eczema flare, topical steroids should be used and sun exposure should be avoided. Topical steroids are the most effective treatment when used correctly. However, any occurrence of hypopigmentation as a result of their use becomes more evident if the skin tans around the area of treatment.
• Wear physical sunscreen. This seems obvious, but most chemical blockers – even the formulations made for babies – can burn on cracked, inflamed skin. Instead, stress to your patients that they use a physical blocker made of pure titanium dioxide or zinc oxide on inflamed skin.
• Oral steroids and sun do not mix. Oral steroids can be potent photosensitizers. If they are needed, UV exposure should be avoided.
Dr. Talakoub and Dr. Wesley are co-contributors to a monthly Aesthetic Dermatology column in Skin & Allergy News. Dr. Talakoub is in private practice at McLean (Va.) Dermatology Center. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub.
Summer months can be dreadful for patients with atopic dermatitis. The chlorine, heat, and humidity can lead to flares. Furthermore, noncompliance with skin care regimens because of changing summer routines, travel, and the use of hotel products can exacerbate even the calmest skin disease.
Share these tips with your patients to help them keep their atopic skin under control in the summer heat, and stop flares before they start.
• Rinse the skin well after swimming. Chlorine and saltwater can dry out the skin. Showers after swimming in chlorinated pools can help retain the skin’s natural oils.
• Avoid hot tubs. Cracks and fissures in atopic skin can become infected in hot tubs with Staphylococcus and Pseudomonas. Advise your atopic patients to avoid hot tubs, even if they claim the tubs have been cleaned.
• Bring your own products. Many soaps and shower gels available in hotels and resorts are extremely drying, and may contain ingredients that could irritate atopic skin.
• Don’t switch from thick creams to thin lotions just because it is summer. Remind your patients that a thin lotion does not provide the same occlusive and humectant properties as thicker creams, although they are not as easy to apply, and can feel thick and sticky on the skin with humidity.
• In case of an active eczema flare, topical steroids should be used and sun exposure should be avoided. Topical steroids are the most effective treatment when used correctly. However, any occurrence of hypopigmentation as a result of their use becomes more evident if the skin tans around the area of treatment.
• Wear physical sunscreen. This seems obvious, but most chemical blockers – even the formulations made for babies – can burn on cracked, inflamed skin. Instead, stress to your patients that they use a physical blocker made of pure titanium dioxide or zinc oxide on inflamed skin.
• Oral steroids and sun do not mix. Oral steroids can be potent photosensitizers. If they are needed, UV exposure should be avoided.
Dr. Talakoub and Dr. Wesley are co-contributors to a monthly Aesthetic Dermatology column in Skin & Allergy News. Dr. Talakoub is in private practice at McLean (Va.) Dermatology Center. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub.
Summer months can be dreadful for patients with atopic dermatitis. The chlorine, heat, and humidity can lead to flares. Furthermore, noncompliance with skin care regimens because of changing summer routines, travel, and the use of hotel products can exacerbate even the calmest skin disease.
Share these tips with your patients to help them keep their atopic skin under control in the summer heat, and stop flares before they start.
• Rinse the skin well after swimming. Chlorine and saltwater can dry out the skin. Showers after swimming in chlorinated pools can help retain the skin’s natural oils.
• Avoid hot tubs. Cracks and fissures in atopic skin can become infected in hot tubs with Staphylococcus and Pseudomonas. Advise your atopic patients to avoid hot tubs, even if they claim the tubs have been cleaned.
• Bring your own products. Many soaps and shower gels available in hotels and resorts are extremely drying, and may contain ingredients that could irritate atopic skin.
• Don’t switch from thick creams to thin lotions just because it is summer. Remind your patients that a thin lotion does not provide the same occlusive and humectant properties as thicker creams, although they are not as easy to apply, and can feel thick and sticky on the skin with humidity.
• In case of an active eczema flare, topical steroids should be used and sun exposure should be avoided. Topical steroids are the most effective treatment when used correctly. However, any occurrence of hypopigmentation as a result of their use becomes more evident if the skin tans around the area of treatment.
• Wear physical sunscreen. This seems obvious, but most chemical blockers – even the formulations made for babies – can burn on cracked, inflamed skin. Instead, stress to your patients that they use a physical blocker made of pure titanium dioxide or zinc oxide on inflamed skin.
• Oral steroids and sun do not mix. Oral steroids can be potent photosensitizers. If they are needed, UV exposure should be avoided.
Dr. Talakoub and Dr. Wesley are co-contributors to a monthly Aesthetic Dermatology column in Skin & Allergy News. Dr. Talakoub is in private practice at McLean (Va.) Dermatology Center. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub.
NIH Tackles Chronic Low Back Pain
Standardized research methods are needed to advance efforts toward reducing the costs and high burden of chronic low back pain, according to a multidisciplinary NIH Task Force report published online ahead of print May 30 in Spine.
The article introduces a set of proposed research standards to help in comparing the results of chronic low back pain studies. The Task Force co-chairs were Drs. Richard A. Deyo of Oregon Health and Science University, and Samuel F. Dworkin of the University of Washington in Seattle.
“Researchers use varied inclusion criteria, definitions, baseline assessments, and outcome measures, which impede comparisons and consensus,” the Task Force wrote.
To address this issue, the Task Force followed a structured approach to developing a set of standards for chronic low back pain research. Overriding issues included defining the problem of chronic low back pain, identifying the minimum dataset that should be collected in chronic low back pain research, assessing its impact on patients’ lives, and defining the best outcomes to evaluate treatment effectiveness.
The Task Force recommends that chronic low back pain be defined as back pain that lasts at least 3 months and causing pain on at least half of days over the past 6 months. Their recommended definition does not include ratings of pain severity.
In terms of impact, the Task Force recommends focusing on how back pain affects patients’ lives. They recommend a 9-item chronic low back pain “Impact Score” that incorporates ratings of pain intensity, interference with normal activities, and functional ability.
A key point for the Task Force was to define a minimum set of data to be gathered in any study of chronic low back pain. The recommended dataset included legal or workers compensation issues, previous treatments, and important contributing factors (eg, smoking, obesity, substance abuse, and widespread pain).
For outcomes, the Task Force concluded there was no set definition of what degree of improvement should be considered “clinically important.” Likewise, there was no consensus on the use of combined outcome measures, time frame for improvement, or adverse effects.
Finally, testing and developing new combined outcome measures was identified as an important aspect of future research, more specifically, other included approaches to predicting treatment results and studies to evaluate and improve the minimum dataset.
Overall, the recommendations stress the importance of getting a fuller picture of the patient’s medical history, even more so than the physical examination. On the other hand, the Task Force specified no standard laboratory or imaging tests, citing the lack of association with patient functioning or symptoms. Evaluations of depression, sleep disturbance, physical functioning, and catastrophic thinking were rated as important for all groups of patients with chronic low back pain.
The Task Force hopes that their recommended standards reflect the “complex, intertwined factors” affecting the development and clinical course of chronic low back pain. The NIH Pain Consortium has approved the recommendations and advises that investigators should incorporate them into NIH grant proposals. “As adopted by the NIH, these recommendations have the potential to standardize methods for identifying chronic low back pain research cases, describe research subjects, and compare published reports.” The Task Force added that recommendations should be subject to periodic validation and refinement in years ahead.
Suggested Reading
Deyo RA, Dworkin SF, Amtmann D, et al. NIH task force proposes standards for research on chronic low back pain. Spine. 2014 May 30 [Epub ahead of print].
Standardized research methods are needed to advance efforts toward reducing the costs and high burden of chronic low back pain, according to a multidisciplinary NIH Task Force report published online ahead of print May 30 in Spine.
The article introduces a set of proposed research standards to help in comparing the results of chronic low back pain studies. The Task Force co-chairs were Drs. Richard A. Deyo of Oregon Health and Science University, and Samuel F. Dworkin of the University of Washington in Seattle.
“Researchers use varied inclusion criteria, definitions, baseline assessments, and outcome measures, which impede comparisons and consensus,” the Task Force wrote.
To address this issue, the Task Force followed a structured approach to developing a set of standards for chronic low back pain research. Overriding issues included defining the problem of chronic low back pain, identifying the minimum dataset that should be collected in chronic low back pain research, assessing its impact on patients’ lives, and defining the best outcomes to evaluate treatment effectiveness.
The Task Force recommends that chronic low back pain be defined as back pain that lasts at least 3 months and causing pain on at least half of days over the past 6 months. Their recommended definition does not include ratings of pain severity.
In terms of impact, the Task Force recommends focusing on how back pain affects patients’ lives. They recommend a 9-item chronic low back pain “Impact Score” that incorporates ratings of pain intensity, interference with normal activities, and functional ability.
A key point for the Task Force was to define a minimum set of data to be gathered in any study of chronic low back pain. The recommended dataset included legal or workers compensation issues, previous treatments, and important contributing factors (eg, smoking, obesity, substance abuse, and widespread pain).
For outcomes, the Task Force concluded there was no set definition of what degree of improvement should be considered “clinically important.” Likewise, there was no consensus on the use of combined outcome measures, time frame for improvement, or adverse effects.
Finally, testing and developing new combined outcome measures was identified as an important aspect of future research, more specifically, other included approaches to predicting treatment results and studies to evaluate and improve the minimum dataset.
Overall, the recommendations stress the importance of getting a fuller picture of the patient’s medical history, even more so than the physical examination. On the other hand, the Task Force specified no standard laboratory or imaging tests, citing the lack of association with patient functioning or symptoms. Evaluations of depression, sleep disturbance, physical functioning, and catastrophic thinking were rated as important for all groups of patients with chronic low back pain.
The Task Force hopes that their recommended standards reflect the “complex, intertwined factors” affecting the development and clinical course of chronic low back pain. The NIH Pain Consortium has approved the recommendations and advises that investigators should incorporate them into NIH grant proposals. “As adopted by the NIH, these recommendations have the potential to standardize methods for identifying chronic low back pain research cases, describe research subjects, and compare published reports.” The Task Force added that recommendations should be subject to periodic validation and refinement in years ahead.
Standardized research methods are needed to advance efforts toward reducing the costs and high burden of chronic low back pain, according to a multidisciplinary NIH Task Force report published online ahead of print May 30 in Spine.
The article introduces a set of proposed research standards to help in comparing the results of chronic low back pain studies. The Task Force co-chairs were Drs. Richard A. Deyo of Oregon Health and Science University, and Samuel F. Dworkin of the University of Washington in Seattle.
“Researchers use varied inclusion criteria, definitions, baseline assessments, and outcome measures, which impede comparisons and consensus,” the Task Force wrote.
To address this issue, the Task Force followed a structured approach to developing a set of standards for chronic low back pain research. Overriding issues included defining the problem of chronic low back pain, identifying the minimum dataset that should be collected in chronic low back pain research, assessing its impact on patients’ lives, and defining the best outcomes to evaluate treatment effectiveness.
The Task Force recommends that chronic low back pain be defined as back pain that lasts at least 3 months and causing pain on at least half of days over the past 6 months. Their recommended definition does not include ratings of pain severity.
In terms of impact, the Task Force recommends focusing on how back pain affects patients’ lives. They recommend a 9-item chronic low back pain “Impact Score” that incorporates ratings of pain intensity, interference with normal activities, and functional ability.
A key point for the Task Force was to define a minimum set of data to be gathered in any study of chronic low back pain. The recommended dataset included legal or workers compensation issues, previous treatments, and important contributing factors (eg, smoking, obesity, substance abuse, and widespread pain).
For outcomes, the Task Force concluded there was no set definition of what degree of improvement should be considered “clinically important.” Likewise, there was no consensus on the use of combined outcome measures, time frame for improvement, or adverse effects.
Finally, testing and developing new combined outcome measures was identified as an important aspect of future research, more specifically, other included approaches to predicting treatment results and studies to evaluate and improve the minimum dataset.
Overall, the recommendations stress the importance of getting a fuller picture of the patient’s medical history, even more so than the physical examination. On the other hand, the Task Force specified no standard laboratory or imaging tests, citing the lack of association with patient functioning or symptoms. Evaluations of depression, sleep disturbance, physical functioning, and catastrophic thinking were rated as important for all groups of patients with chronic low back pain.
The Task Force hopes that their recommended standards reflect the “complex, intertwined factors” affecting the development and clinical course of chronic low back pain. The NIH Pain Consortium has approved the recommendations and advises that investigators should incorporate them into NIH grant proposals. “As adopted by the NIH, these recommendations have the potential to standardize methods for identifying chronic low back pain research cases, describe research subjects, and compare published reports.” The Task Force added that recommendations should be subject to periodic validation and refinement in years ahead.
Suggested Reading
Deyo RA, Dworkin SF, Amtmann D, et al. NIH task force proposes standards for research on chronic low back pain. Spine. 2014 May 30 [Epub ahead of print].
Suggested Reading
Deyo RA, Dworkin SF, Amtmann D, et al. NIH task force proposes standards for research on chronic low back pain. Spine. 2014 May 30 [Epub ahead of print].
Steps to optimizing skin care retail in your practice
I have been writing Cosmeceutical Critique for more than a decade, and over the years I have received many calls and e-mails about the column. The most frequent question is, "I read your column every month and understand the ingredient science, but I still do not know what products to sell in my practice. Can you help?" For this reason, I will begin to add columns that discuss the process of skin care retail, and how to choose which products to sell. I admit that finding effective products and designing the right regimen for each patient are daunting tasks, but I have simplified the process out of necessity in my own Miami practice.
The goal is to achieve good patient outcomes with minimal side effects, which strengthens the physician-patient relationship. In order to achieve this goal, you need to find the most efficacious products and properly match them to your patient’s skin type. In addition, patients must be compliant with the prescribed regimen. If only it were that simple. The difficulty in separating fact (science) from fiction (marketing claims), time constraints with each patient, and the need for staff training can complicate this process.
In my practice, we use the Skin Type Solutions system that I developed to match skin care products for each skin type (
- <cf number="\"2\"">’</cf>
Fitzpatricks Dermatology in General Medicine, 8th Ed., 2012, Ch. 250, p. 1343).This system accurately determines a patient’s Baumann Skin Type (there are 16) and provides a preset regimen designed to address that particular skin type’s needs. The system has been tested in more than 100,000 people worldwide, of all ethnicities and ages, as well as both genders, and has demonstrated accuracy in assessing skin care needs (Dermatol. Clin. 2008;26:359-73; J. Cosmet. Dermatol. Sci. Appl. 2014;4:78-84).
The Baumann Skin Typing System saves my staff time by streamlining the process of designing skin care regimens. It works like this:
• The patient takes the skin type questionnaire and is assigned to one of the 16 Baumann Skin types.
• A staff member matches the skin type to the preset regimen.
• The doctor (or designee) reviews the regimen and makes any necessary changes or additions (including prescription medications).
• The patient is given a step-by-step skin care regimen.
• The patient purchases the correct products.
• The patient is given instruction sheets to increase compliance.
• The patient returns in 4 weeks for follow-up with the staff designee to ensure that the regimen is being properly followed.
Sounds easy, right? The hard part is choosing which products to use for each skin type. In order to ethically sell skin care products to patients, you must ensure that they are getting efficacious products to address their skin concerns (Clin. Dermatol. 2012;30:522-7).
Keep these steps in mind when selecting skin care products:
• Know your ingredient science.
There is so much interesting research on cosmetic ingredients, but there is also plenty of hype and misinformation. One key point is that no one ingredient is right for all skin types. It’s essential to know which ingredients work well together and which do not. The order in which ingredients are placed on the skin is crucial as well, because they can inactivate each other and affect absorption. All of my ingredient columns are available at edermatologynews.com and will be published in my new book, Cosmeceuticals and Cosmetic Ingredients (McGraw-Hill).
It is important to understand which ingredients are worthless (like stem cells and peptides) and which ones are crucial (such as retinoids and antioxidants) so that you can arm your patients with products that work. When products do not work, your patients will have poor outcomes, your physician-patient relationships will be damaged, and patients’ trust in you will decrease.
• Choose ingredients appropriate for the patient’s skin type.
It is important to understand the characteristics of various ingredients and match those to your patient’s skin type. The process of assessing the patient’s skin type can be long because you need to ask numerous historical questions (invariably including, "Do you get irritated from skin care products?" and "What happens if you do not use a moisturizer?"). Looking at a patient’s skin at one point in time is not as accurate as asking a series of questions about how their skin has behaved in the past under varying conditions. I use a validated questionnaire to streamline this process in my practice. The questionnaire takes 3 to 5 minutes, does not require a staff member, and is done on a tablet device in the waiting room or exam room.
• Properly identify the Baumann Skin Type using a validated questionnaire.
To determine a patient’s true skin type, a scientifically validated questionnaire is used to assess skin oiliness, dryness, sensitivity, uneven skin tone, and risk factors for wrinkles. When these parameters are combined, there are 16 possible Baumann Skin Types, which yield an accurate history of the patient’s skin characteristics.
• Choose products for each skin type.
There are many factors to consider in choosing what brands and SKUs (stock keeping units, in industry parlance, but particular products for our purposes) to use for each skin type. I use a brand-agnostic approach to choose the best technologies from various brands from around the world. I believe that brands often have a core competency, such as sunscreen technology, but that not all of the products in a particular line are superior. I select the best products (SKUs) from each brand, and combine and test them on various skin types to see which products and what combinations of products work best.
The following are the factors that I take into account when choosing SKUs for each Baumann Skin Type:
A. Importance of the ingredient recipe
Although the product label lists ingredients, it does not list the formulation’s recipe, which is proprietary and often patented. The "recipe" includes the order that ingredients are added in the process, the pH, the amount of each ingredient, the temperature at which the ingredient is added, and many other important factors that determine the final chemistry. Ingredients like vitamin C, green tea, and argan oil are expensive when formulated properly. Many copycat brands, such as the Walgreens and CVS knockoffs, use the same ingredients. However, they cannot use the patented recipe, and therefore their end product is different.
B. Manufacturing and packaging process
How a product is made and packaged is crucial. For example, retinol breaks down when exposed to light and air. I once visited a manufacturing plant that was stirring its "antiaging" retinol preparation in open vats. The retinol was losing its activity, which is why the product was "less irritating." The process of packaging the completed product is also important. In some cases the product is formulated in one place and shipped to another location for final packaging – and several ingredients can lose their potency during transit. Finally, the container that the product is packaged in is important. Air and light can get into tubes, affecting the efficacy of a product.
C. Ingredient interactions
The order of application and the combination of ingredients affect stability, efficacy, safety, and the chemical structure. Master formulators understand that every ingredient in the formulation matters, and there is really no such thing as an inactive ingredient. Ingredients can affect penetration and render other ingredients more or less effective depending on the order in which the ingredients are used on the skin. For example, olive oil actually increases penetration of other ingredients because it has a high content of oleic acid, while safflower oil can decrease penetration by strengthening the skin barrier.
• Design the regimen and order of application of products.
Once you have determined your patient’s skin type and matched the proper products to their skin type, you must tell them exactly how to apply them. The order in which products are applied makes a difference. Consider ingredient interactions, ingredient penetration times, and cross-reactions, plus skin type factors such as the condition of the skin barrier, sebum production, thickness of the stratum corneum, sun exposure, and bathing habits. I recommend providing a printed regimen with step-by-step instructions for morning and night.
• Educate patients.
Take the time to educate your patients on their skin issues. If you explain why you chose each product and why the particular ingredients are important, they are more likely to be compliant and get better results (and return to you for product recommendations and repurchases). Because we do not have the time to sit and explain all of these issues to each patient, we use educational newsletters that we send to patients based on their Baumann Skin Type. This helps keep them engaged and educates them about new technologies and products that are appropriate for their skin type.
• Encourage compliance.
Schedule a follow-up visit after 1 month to check on their progress and ensure compliance, and emphasize the importance of this visit. If you prescribed a retinoid, patients may experience irritation and stop using it. If you have an imaging system, baseline and follow-up photos help illustrate patients’ progress and keep them vigilant. Four weeks is a good time frame because patients tend to lose interest at that point.
• Sell skin care products in your practice.
I was against selling skin care products for ethical reasons for several years. However, in 2005, I surveyed my patients, and 100% of them wanted me to sell products so that they could feel sure that they were purchasing the right products for their needs. In fact, my patients appreciate expert medical advice on skin care. As a practitioner, you can make more educated choices about skin care products and help them avoid products that don’t work or cause harm.
• Contact me for more information.
In order to improve patient outcomes, you must ensure that you stay current on skin care science so your patients can benefit from your expertise. I recognize that not everyone has the time and inclination to stay current on the various skin care ingredients, products, and brands. Several of my dermatology friends have adopted my skin typing system in their practices and, in the process, observed better patient outcomes and increased profitability, while reducing the burden on their staff. These successes led to the development of an in-office store system utilizing my concept, which I am offering only to dermatologists. Feel free to email me at [email protected], or visit STSFranchise.com, if you want to learn more.
• Look for this column each month.
I will be sharing more advice on in-office skin care retail and will continue my review of new cosmeceutical ingredients. Let’s work together to put skin care back in the hands of dermatologists.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook "Cosmetic Dermatology: Principles and Practice" (McGraw-Hill, April 2002), and a book for consumers, "The Skin Type Solution" (Bantam, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever.
I have been writing Cosmeceutical Critique for more than a decade, and over the years I have received many calls and e-mails about the column. The most frequent question is, "I read your column every month and understand the ingredient science, but I still do not know what products to sell in my practice. Can you help?" For this reason, I will begin to add columns that discuss the process of skin care retail, and how to choose which products to sell. I admit that finding effective products and designing the right regimen for each patient are daunting tasks, but I have simplified the process out of necessity in my own Miami practice.
The goal is to achieve good patient outcomes with minimal side effects, which strengthens the physician-patient relationship. In order to achieve this goal, you need to find the most efficacious products and properly match them to your patient’s skin type. In addition, patients must be compliant with the prescribed regimen. If only it were that simple. The difficulty in separating fact (science) from fiction (marketing claims), time constraints with each patient, and the need for staff training can complicate this process.
In my practice, we use the Skin Type Solutions system that I developed to match skin care products for each skin type (
- <cf number="\"2\"">’</cf>
Fitzpatricks Dermatology in General Medicine, 8th Ed., 2012, Ch. 250, p. 1343).This system accurately determines a patient’s Baumann Skin Type (there are 16) and provides a preset regimen designed to address that particular skin type’s needs. The system has been tested in more than 100,000 people worldwide, of all ethnicities and ages, as well as both genders, and has demonstrated accuracy in assessing skin care needs (Dermatol. Clin. 2008;26:359-73; J. Cosmet. Dermatol. Sci. Appl. 2014;4:78-84).
The Baumann Skin Typing System saves my staff time by streamlining the process of designing skin care regimens. It works like this:
• The patient takes the skin type questionnaire and is assigned to one of the 16 Baumann Skin types.
• A staff member matches the skin type to the preset regimen.
• The doctor (or designee) reviews the regimen and makes any necessary changes or additions (including prescription medications).
• The patient is given a step-by-step skin care regimen.
• The patient purchases the correct products.
• The patient is given instruction sheets to increase compliance.
• The patient returns in 4 weeks for follow-up with the staff designee to ensure that the regimen is being properly followed.
Sounds easy, right? The hard part is choosing which products to use for each skin type. In order to ethically sell skin care products to patients, you must ensure that they are getting efficacious products to address their skin concerns (Clin. Dermatol. 2012;30:522-7).
Keep these steps in mind when selecting skin care products:
• Know your ingredient science.
There is so much interesting research on cosmetic ingredients, but there is also plenty of hype and misinformation. One key point is that no one ingredient is right for all skin types. It’s essential to know which ingredients work well together and which do not. The order in which ingredients are placed on the skin is crucial as well, because they can inactivate each other and affect absorption. All of my ingredient columns are available at edermatologynews.com and will be published in my new book, Cosmeceuticals and Cosmetic Ingredients (McGraw-Hill).
It is important to understand which ingredients are worthless (like stem cells and peptides) and which ones are crucial (such as retinoids and antioxidants) so that you can arm your patients with products that work. When products do not work, your patients will have poor outcomes, your physician-patient relationships will be damaged, and patients’ trust in you will decrease.
• Choose ingredients appropriate for the patient’s skin type.
It is important to understand the characteristics of various ingredients and match those to your patient’s skin type. The process of assessing the patient’s skin type can be long because you need to ask numerous historical questions (invariably including, "Do you get irritated from skin care products?" and "What happens if you do not use a moisturizer?"). Looking at a patient’s skin at one point in time is not as accurate as asking a series of questions about how their skin has behaved in the past under varying conditions. I use a validated questionnaire to streamline this process in my practice. The questionnaire takes 3 to 5 minutes, does not require a staff member, and is done on a tablet device in the waiting room or exam room.
• Properly identify the Baumann Skin Type using a validated questionnaire.
To determine a patient’s true skin type, a scientifically validated questionnaire is used to assess skin oiliness, dryness, sensitivity, uneven skin tone, and risk factors for wrinkles. When these parameters are combined, there are 16 possible Baumann Skin Types, which yield an accurate history of the patient’s skin characteristics.
• Choose products for each skin type.
There are many factors to consider in choosing what brands and SKUs (stock keeping units, in industry parlance, but particular products for our purposes) to use for each skin type. I use a brand-agnostic approach to choose the best technologies from various brands from around the world. I believe that brands often have a core competency, such as sunscreen technology, but that not all of the products in a particular line are superior. I select the best products (SKUs) from each brand, and combine and test them on various skin types to see which products and what combinations of products work best.
The following are the factors that I take into account when choosing SKUs for each Baumann Skin Type:
A. Importance of the ingredient recipe
Although the product label lists ingredients, it does not list the formulation’s recipe, which is proprietary and often patented. The "recipe" includes the order that ingredients are added in the process, the pH, the amount of each ingredient, the temperature at which the ingredient is added, and many other important factors that determine the final chemistry. Ingredients like vitamin C, green tea, and argan oil are expensive when formulated properly. Many copycat brands, such as the Walgreens and CVS knockoffs, use the same ingredients. However, they cannot use the patented recipe, and therefore their end product is different.
B. Manufacturing and packaging process
How a product is made and packaged is crucial. For example, retinol breaks down when exposed to light and air. I once visited a manufacturing plant that was stirring its "antiaging" retinol preparation in open vats. The retinol was losing its activity, which is why the product was "less irritating." The process of packaging the completed product is also important. In some cases the product is formulated in one place and shipped to another location for final packaging – and several ingredients can lose their potency during transit. Finally, the container that the product is packaged in is important. Air and light can get into tubes, affecting the efficacy of a product.
C. Ingredient interactions
The order of application and the combination of ingredients affect stability, efficacy, safety, and the chemical structure. Master formulators understand that every ingredient in the formulation matters, and there is really no such thing as an inactive ingredient. Ingredients can affect penetration and render other ingredients more or less effective depending on the order in which the ingredients are used on the skin. For example, olive oil actually increases penetration of other ingredients because it has a high content of oleic acid, while safflower oil can decrease penetration by strengthening the skin barrier.
• Design the regimen and order of application of products.
Once you have determined your patient’s skin type and matched the proper products to their skin type, you must tell them exactly how to apply them. The order in which products are applied makes a difference. Consider ingredient interactions, ingredient penetration times, and cross-reactions, plus skin type factors such as the condition of the skin barrier, sebum production, thickness of the stratum corneum, sun exposure, and bathing habits. I recommend providing a printed regimen with step-by-step instructions for morning and night.
• Educate patients.
Take the time to educate your patients on their skin issues. If you explain why you chose each product and why the particular ingredients are important, they are more likely to be compliant and get better results (and return to you for product recommendations and repurchases). Because we do not have the time to sit and explain all of these issues to each patient, we use educational newsletters that we send to patients based on their Baumann Skin Type. This helps keep them engaged and educates them about new technologies and products that are appropriate for their skin type.
• Encourage compliance.
Schedule a follow-up visit after 1 month to check on their progress and ensure compliance, and emphasize the importance of this visit. If you prescribed a retinoid, patients may experience irritation and stop using it. If you have an imaging system, baseline and follow-up photos help illustrate patients’ progress and keep them vigilant. Four weeks is a good time frame because patients tend to lose interest at that point.
• Sell skin care products in your practice.
I was against selling skin care products for ethical reasons for several years. However, in 2005, I surveyed my patients, and 100% of them wanted me to sell products so that they could feel sure that they were purchasing the right products for their needs. In fact, my patients appreciate expert medical advice on skin care. As a practitioner, you can make more educated choices about skin care products and help them avoid products that don’t work or cause harm.
• Contact me for more information.
In order to improve patient outcomes, you must ensure that you stay current on skin care science so your patients can benefit from your expertise. I recognize that not everyone has the time and inclination to stay current on the various skin care ingredients, products, and brands. Several of my dermatology friends have adopted my skin typing system in their practices and, in the process, observed better patient outcomes and increased profitability, while reducing the burden on their staff. These successes led to the development of an in-office store system utilizing my concept, which I am offering only to dermatologists. Feel free to email me at [email protected], or visit STSFranchise.com, if you want to learn more.
• Look for this column each month.
I will be sharing more advice on in-office skin care retail and will continue my review of new cosmeceutical ingredients. Let’s work together to put skin care back in the hands of dermatologists.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook "Cosmetic Dermatology: Principles and Practice" (McGraw-Hill, April 2002), and a book for consumers, "The Skin Type Solution" (Bantam, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever.
I have been writing Cosmeceutical Critique for more than a decade, and over the years I have received many calls and e-mails about the column. The most frequent question is, "I read your column every month and understand the ingredient science, but I still do not know what products to sell in my practice. Can you help?" For this reason, I will begin to add columns that discuss the process of skin care retail, and how to choose which products to sell. I admit that finding effective products and designing the right regimen for each patient are daunting tasks, but I have simplified the process out of necessity in my own Miami practice.
The goal is to achieve good patient outcomes with minimal side effects, which strengthens the physician-patient relationship. In order to achieve this goal, you need to find the most efficacious products and properly match them to your patient’s skin type. In addition, patients must be compliant with the prescribed regimen. If only it were that simple. The difficulty in separating fact (science) from fiction (marketing claims), time constraints with each patient, and the need for staff training can complicate this process.
In my practice, we use the Skin Type Solutions system that I developed to match skin care products for each skin type (
- <cf number="\"2\"">’</cf>
Fitzpatricks Dermatology in General Medicine, 8th Ed., 2012, Ch. 250, p. 1343).This system accurately determines a patient’s Baumann Skin Type (there are 16) and provides a preset regimen designed to address that particular skin type’s needs. The system has been tested in more than 100,000 people worldwide, of all ethnicities and ages, as well as both genders, and has demonstrated accuracy in assessing skin care needs (Dermatol. Clin. 2008;26:359-73; J. Cosmet. Dermatol. Sci. Appl. 2014;4:78-84).
The Baumann Skin Typing System saves my staff time by streamlining the process of designing skin care regimens. It works like this:
• The patient takes the skin type questionnaire and is assigned to one of the 16 Baumann Skin types.
• A staff member matches the skin type to the preset regimen.
• The doctor (or designee) reviews the regimen and makes any necessary changes or additions (including prescription medications).
• The patient is given a step-by-step skin care regimen.
• The patient purchases the correct products.
• The patient is given instruction sheets to increase compliance.
• The patient returns in 4 weeks for follow-up with the staff designee to ensure that the regimen is being properly followed.
Sounds easy, right? The hard part is choosing which products to use for each skin type. In order to ethically sell skin care products to patients, you must ensure that they are getting efficacious products to address their skin concerns (Clin. Dermatol. 2012;30:522-7).
Keep these steps in mind when selecting skin care products:
• Know your ingredient science.
There is so much interesting research on cosmetic ingredients, but there is also plenty of hype and misinformation. One key point is that no one ingredient is right for all skin types. It’s essential to know which ingredients work well together and which do not. The order in which ingredients are placed on the skin is crucial as well, because they can inactivate each other and affect absorption. All of my ingredient columns are available at edermatologynews.com and will be published in my new book, Cosmeceuticals and Cosmetic Ingredients (McGraw-Hill).
It is important to understand which ingredients are worthless (like stem cells and peptides) and which ones are crucial (such as retinoids and antioxidants) so that you can arm your patients with products that work. When products do not work, your patients will have poor outcomes, your physician-patient relationships will be damaged, and patients’ trust in you will decrease.
• Choose ingredients appropriate for the patient’s skin type.
It is important to understand the characteristics of various ingredients and match those to your patient’s skin type. The process of assessing the patient’s skin type can be long because you need to ask numerous historical questions (invariably including, "Do you get irritated from skin care products?" and "What happens if you do not use a moisturizer?"). Looking at a patient’s skin at one point in time is not as accurate as asking a series of questions about how their skin has behaved in the past under varying conditions. I use a validated questionnaire to streamline this process in my practice. The questionnaire takes 3 to 5 minutes, does not require a staff member, and is done on a tablet device in the waiting room or exam room.
• Properly identify the Baumann Skin Type using a validated questionnaire.
To determine a patient’s true skin type, a scientifically validated questionnaire is used to assess skin oiliness, dryness, sensitivity, uneven skin tone, and risk factors for wrinkles. When these parameters are combined, there are 16 possible Baumann Skin Types, which yield an accurate history of the patient’s skin characteristics.
• Choose products for each skin type.
There are many factors to consider in choosing what brands and SKUs (stock keeping units, in industry parlance, but particular products for our purposes) to use for each skin type. I use a brand-agnostic approach to choose the best technologies from various brands from around the world. I believe that brands often have a core competency, such as sunscreen technology, but that not all of the products in a particular line are superior. I select the best products (SKUs) from each brand, and combine and test them on various skin types to see which products and what combinations of products work best.
The following are the factors that I take into account when choosing SKUs for each Baumann Skin Type:
A. Importance of the ingredient recipe
Although the product label lists ingredients, it does not list the formulation’s recipe, which is proprietary and often patented. The "recipe" includes the order that ingredients are added in the process, the pH, the amount of each ingredient, the temperature at which the ingredient is added, and many other important factors that determine the final chemistry. Ingredients like vitamin C, green tea, and argan oil are expensive when formulated properly. Many copycat brands, such as the Walgreens and CVS knockoffs, use the same ingredients. However, they cannot use the patented recipe, and therefore their end product is different.
B. Manufacturing and packaging process
How a product is made and packaged is crucial. For example, retinol breaks down when exposed to light and air. I once visited a manufacturing plant that was stirring its "antiaging" retinol preparation in open vats. The retinol was losing its activity, which is why the product was "less irritating." The process of packaging the completed product is also important. In some cases the product is formulated in one place and shipped to another location for final packaging – and several ingredients can lose their potency during transit. Finally, the container that the product is packaged in is important. Air and light can get into tubes, affecting the efficacy of a product.
C. Ingredient interactions
The order of application and the combination of ingredients affect stability, efficacy, safety, and the chemical structure. Master formulators understand that every ingredient in the formulation matters, and there is really no such thing as an inactive ingredient. Ingredients can affect penetration and render other ingredients more or less effective depending on the order in which the ingredients are used on the skin. For example, olive oil actually increases penetration of other ingredients because it has a high content of oleic acid, while safflower oil can decrease penetration by strengthening the skin barrier.
• Design the regimen and order of application of products.
Once you have determined your patient’s skin type and matched the proper products to their skin type, you must tell them exactly how to apply them. The order in which products are applied makes a difference. Consider ingredient interactions, ingredient penetration times, and cross-reactions, plus skin type factors such as the condition of the skin barrier, sebum production, thickness of the stratum corneum, sun exposure, and bathing habits. I recommend providing a printed regimen with step-by-step instructions for morning and night.
• Educate patients.
Take the time to educate your patients on their skin issues. If you explain why you chose each product and why the particular ingredients are important, they are more likely to be compliant and get better results (and return to you for product recommendations and repurchases). Because we do not have the time to sit and explain all of these issues to each patient, we use educational newsletters that we send to patients based on their Baumann Skin Type. This helps keep them engaged and educates them about new technologies and products that are appropriate for their skin type.
• Encourage compliance.
Schedule a follow-up visit after 1 month to check on their progress and ensure compliance, and emphasize the importance of this visit. If you prescribed a retinoid, patients may experience irritation and stop using it. If you have an imaging system, baseline and follow-up photos help illustrate patients’ progress and keep them vigilant. Four weeks is a good time frame because patients tend to lose interest at that point.
• Sell skin care products in your practice.
I was against selling skin care products for ethical reasons for several years. However, in 2005, I surveyed my patients, and 100% of them wanted me to sell products so that they could feel sure that they were purchasing the right products for their needs. In fact, my patients appreciate expert medical advice on skin care. As a practitioner, you can make more educated choices about skin care products and help them avoid products that don’t work or cause harm.
• Contact me for more information.
In order to improve patient outcomes, you must ensure that you stay current on skin care science so your patients can benefit from your expertise. I recognize that not everyone has the time and inclination to stay current on the various skin care ingredients, products, and brands. Several of my dermatology friends have adopted my skin typing system in their practices and, in the process, observed better patient outcomes and increased profitability, while reducing the burden on their staff. These successes led to the development of an in-office store system utilizing my concept, which I am offering only to dermatologists. Feel free to email me at [email protected], or visit STSFranchise.com, if you want to learn more.
• Look for this column each month.
I will be sharing more advice on in-office skin care retail and will continue my review of new cosmeceutical ingredients. Let’s work together to put skin care back in the hands of dermatologists.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook "Cosmetic Dermatology: Principles and Practice" (McGraw-Hill, April 2002), and a book for consumers, "The Skin Type Solution" (Bantam, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever.
Survival benefit from contralateral prophylactic mastectomy small
The absolute 20-year survival benefit from contralateral prophylactic mastectomy stands at less than 1%, regardless of age, estrogen receptor status, and cancer stage, a decision analysis demonstrated.
"Long-term survival in women with unilateral breast cancer treated with or without CPM depends upon several factors, including mortality of the primary breast cancer, risk of CBC [contralateral breast cancer], stage and mortality of the CBC, and the individual patient’s overall life expectancy," wrote Dr. Pamela R. Portschy of the University of Minnesota, Minneapolis.
The report was published July 16 in the Journal of the National Cancer Institute.
"Prospective randomized trials comparing CPM with no CPM are not feasible. Retrospective studies evaluating a potential survival benefit with CPM are limited by short follow-up, potential selection bias, and lack of important clinical information," noted Dr. Portschy and her associates.
They limited their analysis to women with stage I and II breast cancer without BRCA mutations. They developed a Markov model to simulate survival outcomes among those who did and did not have contralateral prophylactic mastectomy (CPM), and they used published studies to estimate probabilities for developing CBC, dying from CBC, dying from primary breast cancer, and age-specific mortality rates. Data were extracted from numerous sources including Surveillance, Epidemiology, and End Results (SEER), the Early Breast Cancer Trialists’ Collaborative Group, and the Oregon State Cancer Registry.
The researchers estimated the 20-year overall survival and life expectancy, but not quality of life or cost, and their analysis considered variation in age, estrogen receptor status, and cancer stage (J. Natl. Cancer Inst. 2014 July 16 [doi:10.1093/jnci/dju160]).
The predicted life expectancy gain from CPM ranged from .13 to .59 years for women with stage I breast cancer, and .08 to .29 years for those with stage II breast cancer. CPM conferred a life expectancy benefit among younger women and among those who had stage I and estrogen receptor–positive disease. "The potential benefit of CPM was consistently lower for patients with stage II breast cancer because of the worse prognosis associated with the primary breast cancer," the researchers wrote. "Similarly, the potential benefits of CPM are more modest for older women because they have relatively fewer years remaining of [life expectancy]."
Dr. Portschy and her associates could not identify any cohort of women that had a greater than 1% absolute survival difference at 20 years. In fact, the predicted 20-year survival differences ranged from .56 to .94% for women with stage I breast cancer and .36 to .61% for those with stage II breast cancer.
The researchers acknowledged limitations of the study, including the fact that the results "do not apply to BRCA gene mutation carriers with unilateral breast cancer who have a cumulative 10-year risk of CBC of approximately 30% to 40%," they wrote. "The outcomes of this analysis were limited to overall and disease-specific survival; we did not evaluate other important outcomes such as surgical complications and quality of life. Also, we assumed the mortality of CBC was the same as the mortality of the index cancer reported by SEER."
They also noted that survival is not the only potential benefit of a cancer risk reduction strategy. "Effects on cancer-related anxiety, cosmesis, and self-image are also important in the decision-making process," they wrote. "For some women, the negative impact of CPM on quality of life may outweigh a potential survival benefit. For others who are very anxious about CBC, CPM may result in a psychological benefit even if survival benefits are minimal."
They concluded that the survival estimates from their Markov model "may be useful for physicians and breast cancer patients to arrive at evidence-based informed decisions regarding CPM. Moreover, the use of accurate and easily understood decision aids may reverse some of the mastectomy trends recently observed in the United States."
The researchers stated that they had no relevant financial conflicts to disclose.
The decision of whether or not to undergo a contralateral prophylactic mastectomy after being treated for breast cancer is a difficult one for many women. The goal of such aggressive therapy is to lower the likelihood of a second primary carcinoma. The downsides are operative risk, impairment of the woman’s self-image, and short-term and long-term morbidities.
This is a well done analysis from an experienced group of investigators and is based on the currently available data. Given the JNCI audience, we shall refrain from niggling points about modeling. Rather, we will stick to the big picture and clinical implications. Although the survival benefit from CPM is small as demonstrated in this model, it is greater than zero, which suggests that for some patients even that small gain may be enough to make it a not unreasonable choice.
From a societal perspective, which was not addressed by Portschy et al., the associated costs of CPM, including the procedure, its complications, reconstruction, and perhaps psychotherapy, may outweigh the modest benefit CPM provides. The small denominator of the cost-effectiveness ratio, were one to be calculated, would imply that the ratio would be very high, making CPM a suboptimal use of health care dollars. Further, we suspect that adding quality of life to the analysis would diminish the benefit and well might turn it into a net harm, in particular for patients with high concern for negative impact of CPM on cosmesis, self image, and morbidity. However, in a fraction of patients who are very troubled by a 0.7% risk of a second, contralateral cancer, CPM might provide an acceptable benefit. The balance between harm and benefit depends on the patient’s preferences and highlights the importance of capturing the patient’s values and expectations before considering CPM.
Of course, these conclusions are based on analysis of women who are at average risk for a contralateral second primary. In women at substantially higher risk (based either on family history or genetics), the benefit of CPM might be far greater, and CPM might be a good choice for the patient or for society.
Dr. Stephen G. Pauker and Dr. Mohamed Alseiari are with the division of clinical decision making in the department of medicine at Tufts Medical Center, Boston. They reported no relevant financial conflicts. This was excerpted from an editorial (J. Natl. Cancer Inst. 2014 July 16 [doi:10.1093/jnci/dju175]).
The decision of whether or not to undergo a contralateral prophylactic mastectomy after being treated for breast cancer is a difficult one for many women. The goal of such aggressive therapy is to lower the likelihood of a second primary carcinoma. The downsides are operative risk, impairment of the woman’s self-image, and short-term and long-term morbidities.
This is a well done analysis from an experienced group of investigators and is based on the currently available data. Given the JNCI audience, we shall refrain from niggling points about modeling. Rather, we will stick to the big picture and clinical implications. Although the survival benefit from CPM is small as demonstrated in this model, it is greater than zero, which suggests that for some patients even that small gain may be enough to make it a not unreasonable choice.
From a societal perspective, which was not addressed by Portschy et al., the associated costs of CPM, including the procedure, its complications, reconstruction, and perhaps psychotherapy, may outweigh the modest benefit CPM provides. The small denominator of the cost-effectiveness ratio, were one to be calculated, would imply that the ratio would be very high, making CPM a suboptimal use of health care dollars. Further, we suspect that adding quality of life to the analysis would diminish the benefit and well might turn it into a net harm, in particular for patients with high concern for negative impact of CPM on cosmesis, self image, and morbidity. However, in a fraction of patients who are very troubled by a 0.7% risk of a second, contralateral cancer, CPM might provide an acceptable benefit. The balance between harm and benefit depends on the patient’s preferences and highlights the importance of capturing the patient’s values and expectations before considering CPM.
Of course, these conclusions are based on analysis of women who are at average risk for a contralateral second primary. In women at substantially higher risk (based either on family history or genetics), the benefit of CPM might be far greater, and CPM might be a good choice for the patient or for society.
Dr. Stephen G. Pauker and Dr. Mohamed Alseiari are with the division of clinical decision making in the department of medicine at Tufts Medical Center, Boston. They reported no relevant financial conflicts. This was excerpted from an editorial (J. Natl. Cancer Inst. 2014 July 16 [doi:10.1093/jnci/dju175]).
The decision of whether or not to undergo a contralateral prophylactic mastectomy after being treated for breast cancer is a difficult one for many women. The goal of such aggressive therapy is to lower the likelihood of a second primary carcinoma. The downsides are operative risk, impairment of the woman’s self-image, and short-term and long-term morbidities.
This is a well done analysis from an experienced group of investigators and is based on the currently available data. Given the JNCI audience, we shall refrain from niggling points about modeling. Rather, we will stick to the big picture and clinical implications. Although the survival benefit from CPM is small as demonstrated in this model, it is greater than zero, which suggests that for some patients even that small gain may be enough to make it a not unreasonable choice.
From a societal perspective, which was not addressed by Portschy et al., the associated costs of CPM, including the procedure, its complications, reconstruction, and perhaps psychotherapy, may outweigh the modest benefit CPM provides. The small denominator of the cost-effectiveness ratio, were one to be calculated, would imply that the ratio would be very high, making CPM a suboptimal use of health care dollars. Further, we suspect that adding quality of life to the analysis would diminish the benefit and well might turn it into a net harm, in particular for patients with high concern for negative impact of CPM on cosmesis, self image, and morbidity. However, in a fraction of patients who are very troubled by a 0.7% risk of a second, contralateral cancer, CPM might provide an acceptable benefit. The balance between harm and benefit depends on the patient’s preferences and highlights the importance of capturing the patient’s values and expectations before considering CPM.
Of course, these conclusions are based on analysis of women who are at average risk for a contralateral second primary. In women at substantially higher risk (based either on family history or genetics), the benefit of CPM might be far greater, and CPM might be a good choice for the patient or for society.
Dr. Stephen G. Pauker and Dr. Mohamed Alseiari are with the division of clinical decision making in the department of medicine at Tufts Medical Center, Boston. They reported no relevant financial conflicts. This was excerpted from an editorial (J. Natl. Cancer Inst. 2014 July 16 [doi:10.1093/jnci/dju175]).
The absolute 20-year survival benefit from contralateral prophylactic mastectomy stands at less than 1%, regardless of age, estrogen receptor status, and cancer stage, a decision analysis demonstrated.
"Long-term survival in women with unilateral breast cancer treated with or without CPM depends upon several factors, including mortality of the primary breast cancer, risk of CBC [contralateral breast cancer], stage and mortality of the CBC, and the individual patient’s overall life expectancy," wrote Dr. Pamela R. Portschy of the University of Minnesota, Minneapolis.
The report was published July 16 in the Journal of the National Cancer Institute.
"Prospective randomized trials comparing CPM with no CPM are not feasible. Retrospective studies evaluating a potential survival benefit with CPM are limited by short follow-up, potential selection bias, and lack of important clinical information," noted Dr. Portschy and her associates.
They limited their analysis to women with stage I and II breast cancer without BRCA mutations. They developed a Markov model to simulate survival outcomes among those who did and did not have contralateral prophylactic mastectomy (CPM), and they used published studies to estimate probabilities for developing CBC, dying from CBC, dying from primary breast cancer, and age-specific mortality rates. Data were extracted from numerous sources including Surveillance, Epidemiology, and End Results (SEER), the Early Breast Cancer Trialists’ Collaborative Group, and the Oregon State Cancer Registry.
The researchers estimated the 20-year overall survival and life expectancy, but not quality of life or cost, and their analysis considered variation in age, estrogen receptor status, and cancer stage (J. Natl. Cancer Inst. 2014 July 16 [doi:10.1093/jnci/dju160]).
The predicted life expectancy gain from CPM ranged from .13 to .59 years for women with stage I breast cancer, and .08 to .29 years for those with stage II breast cancer. CPM conferred a life expectancy benefit among younger women and among those who had stage I and estrogen receptor–positive disease. "The potential benefit of CPM was consistently lower for patients with stage II breast cancer because of the worse prognosis associated with the primary breast cancer," the researchers wrote. "Similarly, the potential benefits of CPM are more modest for older women because they have relatively fewer years remaining of [life expectancy]."
Dr. Portschy and her associates could not identify any cohort of women that had a greater than 1% absolute survival difference at 20 years. In fact, the predicted 20-year survival differences ranged from .56 to .94% for women with stage I breast cancer and .36 to .61% for those with stage II breast cancer.
The researchers acknowledged limitations of the study, including the fact that the results "do not apply to BRCA gene mutation carriers with unilateral breast cancer who have a cumulative 10-year risk of CBC of approximately 30% to 40%," they wrote. "The outcomes of this analysis were limited to overall and disease-specific survival; we did not evaluate other important outcomes such as surgical complications and quality of life. Also, we assumed the mortality of CBC was the same as the mortality of the index cancer reported by SEER."
They also noted that survival is not the only potential benefit of a cancer risk reduction strategy. "Effects on cancer-related anxiety, cosmesis, and self-image are also important in the decision-making process," they wrote. "For some women, the negative impact of CPM on quality of life may outweigh a potential survival benefit. For others who are very anxious about CBC, CPM may result in a psychological benefit even if survival benefits are minimal."
They concluded that the survival estimates from their Markov model "may be useful for physicians and breast cancer patients to arrive at evidence-based informed decisions regarding CPM. Moreover, the use of accurate and easily understood decision aids may reverse some of the mastectomy trends recently observed in the United States."
The researchers stated that they had no relevant financial conflicts to disclose.
The absolute 20-year survival benefit from contralateral prophylactic mastectomy stands at less than 1%, regardless of age, estrogen receptor status, and cancer stage, a decision analysis demonstrated.
"Long-term survival in women with unilateral breast cancer treated with or without CPM depends upon several factors, including mortality of the primary breast cancer, risk of CBC [contralateral breast cancer], stage and mortality of the CBC, and the individual patient’s overall life expectancy," wrote Dr. Pamela R. Portschy of the University of Minnesota, Minneapolis.
The report was published July 16 in the Journal of the National Cancer Institute.
"Prospective randomized trials comparing CPM with no CPM are not feasible. Retrospective studies evaluating a potential survival benefit with CPM are limited by short follow-up, potential selection bias, and lack of important clinical information," noted Dr. Portschy and her associates.
They limited their analysis to women with stage I and II breast cancer without BRCA mutations. They developed a Markov model to simulate survival outcomes among those who did and did not have contralateral prophylactic mastectomy (CPM), and they used published studies to estimate probabilities for developing CBC, dying from CBC, dying from primary breast cancer, and age-specific mortality rates. Data were extracted from numerous sources including Surveillance, Epidemiology, and End Results (SEER), the Early Breast Cancer Trialists’ Collaborative Group, and the Oregon State Cancer Registry.
The researchers estimated the 20-year overall survival and life expectancy, but not quality of life or cost, and their analysis considered variation in age, estrogen receptor status, and cancer stage (J. Natl. Cancer Inst. 2014 July 16 [doi:10.1093/jnci/dju160]).
The predicted life expectancy gain from CPM ranged from .13 to .59 years for women with stage I breast cancer, and .08 to .29 years for those with stage II breast cancer. CPM conferred a life expectancy benefit among younger women and among those who had stage I and estrogen receptor–positive disease. "The potential benefit of CPM was consistently lower for patients with stage II breast cancer because of the worse prognosis associated with the primary breast cancer," the researchers wrote. "Similarly, the potential benefits of CPM are more modest for older women because they have relatively fewer years remaining of [life expectancy]."
Dr. Portschy and her associates could not identify any cohort of women that had a greater than 1% absolute survival difference at 20 years. In fact, the predicted 20-year survival differences ranged from .56 to .94% for women with stage I breast cancer and .36 to .61% for those with stage II breast cancer.
The researchers acknowledged limitations of the study, including the fact that the results "do not apply to BRCA gene mutation carriers with unilateral breast cancer who have a cumulative 10-year risk of CBC of approximately 30% to 40%," they wrote. "The outcomes of this analysis were limited to overall and disease-specific survival; we did not evaluate other important outcomes such as surgical complications and quality of life. Also, we assumed the mortality of CBC was the same as the mortality of the index cancer reported by SEER."
They also noted that survival is not the only potential benefit of a cancer risk reduction strategy. "Effects on cancer-related anxiety, cosmesis, and self-image are also important in the decision-making process," they wrote. "For some women, the negative impact of CPM on quality of life may outweigh a potential survival benefit. For others who are very anxious about CBC, CPM may result in a psychological benefit even if survival benefits are minimal."
They concluded that the survival estimates from their Markov model "may be useful for physicians and breast cancer patients to arrive at evidence-based informed decisions regarding CPM. Moreover, the use of accurate and easily understood decision aids may reverse some of the mastectomy trends recently observed in the United States."
The researchers stated that they had no relevant financial conflicts to disclose.
FROM THE JOURNAL OF THE NATIONAL CANCER INSTITUTE
Key clinical point: The long-term survival benefit of contralateral prophylactic mastectomy is small.
Major Finding: The absolute 20-year survival benefit from contralateral prophylactic mastectomy was less than 1% among all age groups, regardless of estrogen receptor status and cancer stage.
Data Source: Results from a Markov model designed to simulate 20-year survival outcomes among those who did and did not have CPM, with considerations for variation in age, estrogen receptor status, and cancer stage.
Disclosures: The researchers disclosed no relevant financial conflicts.
Bionic Arm Still in Development Stage
Within the past 13 years, roughly 2000 veterans who have returned from Afghanistan and Iraq have sustained injuries that required amputations. Of these injured veterans, 14% required upper extremity amputations. An article published in the June issue of the Journal of the American Academy of Orthopaedic Surgeons reviewed recent advancements in upper extremity bionics. Also reviewed were the challenges that linger in creating a prosthesis that meets or surpasses the abilities of the human hand and arm.
During the next 50 years, “I truly believe we will be able to make artificial arms that function better than many injured arms that doctors are saving today,” said article author Dr. Douglas T. Hutchinson, Associate Professor of Orthopedics at the University of Utah Medical School and Chief of Hand Surgery at Primary Children’s Medical Center, the Veterans Affairs Medical Center, and Shriners Intermountain Hospital.
Created more than 50 years ago, the myoelectric prosthesis continues to be the most commonly used upper extremity prostheses. This prosthesis allows residual muscles to act as natural batteries to create transcutaneous signals, to control the movements of the prosthetic hand and arm. However, the muscles used most often are the triceps and biceps, which do not inherently translate to the opening and closing of the hand. Another drawback is that sometimes the socket interface used to attach the prosthesis may interfere with the function of the residual joint, such as the elbow. Myoelectric prosthetics also do not look natural and are heavy, hot, and uncomfortable, and are not waterproof.
The current federal budget for prostheses research is $2.5 billion. The US Department of Defense Advanced Research Project (DARPA) already has invested more than $150 million for their Revolutionizing Prosthetics Program. The later program, which seeks to create an upper extremity prosthesis that can function as a normal hand and arm does, but with full sensory and motor functions.
In order for these prosthetic devices to be used effectively in a broad range for patients, adjustments still need to be made. For example, many have short-life batteries, along with being weighty and uncomfortable. Particularly challenging is the problem of accurately and efficiently sending brain signals through the peripheral nerves and muscles of the hands and arm, a feat that may warrant the creation and use of a reliable wireless device or direct wiring through an osseous-integrated implant. Current infection rates (nearly 45%) with osseous-integrated devices at the prosthesis-skin interface also pose an issue.
Suggested Reading
Hutchinson DT. The quest for the bionic arm. J Am Acad Orthop Surg. 2014;22(6):346-351.
Within the past 13 years, roughly 2000 veterans who have returned from Afghanistan and Iraq have sustained injuries that required amputations. Of these injured veterans, 14% required upper extremity amputations. An article published in the June issue of the Journal of the American Academy of Orthopaedic Surgeons reviewed recent advancements in upper extremity bionics. Also reviewed were the challenges that linger in creating a prosthesis that meets or surpasses the abilities of the human hand and arm.
During the next 50 years, “I truly believe we will be able to make artificial arms that function better than many injured arms that doctors are saving today,” said article author Dr. Douglas T. Hutchinson, Associate Professor of Orthopedics at the University of Utah Medical School and Chief of Hand Surgery at Primary Children’s Medical Center, the Veterans Affairs Medical Center, and Shriners Intermountain Hospital.
Created more than 50 years ago, the myoelectric prosthesis continues to be the most commonly used upper extremity prostheses. This prosthesis allows residual muscles to act as natural batteries to create transcutaneous signals, to control the movements of the prosthetic hand and arm. However, the muscles used most often are the triceps and biceps, which do not inherently translate to the opening and closing of the hand. Another drawback is that sometimes the socket interface used to attach the prosthesis may interfere with the function of the residual joint, such as the elbow. Myoelectric prosthetics also do not look natural and are heavy, hot, and uncomfortable, and are not waterproof.
The current federal budget for prostheses research is $2.5 billion. The US Department of Defense Advanced Research Project (DARPA) already has invested more than $150 million for their Revolutionizing Prosthetics Program. The later program, which seeks to create an upper extremity prosthesis that can function as a normal hand and arm does, but with full sensory and motor functions.
In order for these prosthetic devices to be used effectively in a broad range for patients, adjustments still need to be made. For example, many have short-life batteries, along with being weighty and uncomfortable. Particularly challenging is the problem of accurately and efficiently sending brain signals through the peripheral nerves and muscles of the hands and arm, a feat that may warrant the creation and use of a reliable wireless device or direct wiring through an osseous-integrated implant. Current infection rates (nearly 45%) with osseous-integrated devices at the prosthesis-skin interface also pose an issue.
Within the past 13 years, roughly 2000 veterans who have returned from Afghanistan and Iraq have sustained injuries that required amputations. Of these injured veterans, 14% required upper extremity amputations. An article published in the June issue of the Journal of the American Academy of Orthopaedic Surgeons reviewed recent advancements in upper extremity bionics. Also reviewed were the challenges that linger in creating a prosthesis that meets or surpasses the abilities of the human hand and arm.
During the next 50 years, “I truly believe we will be able to make artificial arms that function better than many injured arms that doctors are saving today,” said article author Dr. Douglas T. Hutchinson, Associate Professor of Orthopedics at the University of Utah Medical School and Chief of Hand Surgery at Primary Children’s Medical Center, the Veterans Affairs Medical Center, and Shriners Intermountain Hospital.
Created more than 50 years ago, the myoelectric prosthesis continues to be the most commonly used upper extremity prostheses. This prosthesis allows residual muscles to act as natural batteries to create transcutaneous signals, to control the movements of the prosthetic hand and arm. However, the muscles used most often are the triceps and biceps, which do not inherently translate to the opening and closing of the hand. Another drawback is that sometimes the socket interface used to attach the prosthesis may interfere with the function of the residual joint, such as the elbow. Myoelectric prosthetics also do not look natural and are heavy, hot, and uncomfortable, and are not waterproof.
The current federal budget for prostheses research is $2.5 billion. The US Department of Defense Advanced Research Project (DARPA) already has invested more than $150 million for their Revolutionizing Prosthetics Program. The later program, which seeks to create an upper extremity prosthesis that can function as a normal hand and arm does, but with full sensory and motor functions.
In order for these prosthetic devices to be used effectively in a broad range for patients, adjustments still need to be made. For example, many have short-life batteries, along with being weighty and uncomfortable. Particularly challenging is the problem of accurately and efficiently sending brain signals through the peripheral nerves and muscles of the hands and arm, a feat that may warrant the creation and use of a reliable wireless device or direct wiring through an osseous-integrated implant. Current infection rates (nearly 45%) with osseous-integrated devices at the prosthesis-skin interface also pose an issue.
Suggested Reading
Hutchinson DT. The quest for the bionic arm. J Am Acad Orthop Surg. 2014;22(6):346-351.
Suggested Reading
Hutchinson DT. The quest for the bionic arm. J Am Acad Orthop Surg. 2014;22(6):346-351.
Rocker bottom shoes for chronic back pain
Back pain is a primary complaint of many of the patients we see and, seemingly, a secondary complaint of almost all. Exercise training to strengthen spine muscles is effective, but patients either do not attend referrals to therapy or are not compliant with prescribed regimens. An ideal treatment would be involuntary therapy occurring at all times of the day. But does such a magical therapy exist?
Indeed, it does. They are called unstable shoes or, perhaps less disconcertingly, rocker bottom shoes. They are also referred to as round bottom shoes, rounded shoes, or toning shoes.
Unstable shoes incorporate a rounded sole to increase anterior-posterior instability. Masai Barefoot Technology (MBT) has been advocating their use since the 1990s to reduce low back pain. The owners of MBT went out of business, and the future of this particular brand is uncertain, but many other brands offer this design. Studies have shown that they increase activity of ankle muscles and low back muscles and modify posture during standing and walking.
In a recently published clinical trial evaluating the effectiveness of unstable shoes, 40 hospital workers with chronic low back pain were randomized to unstable shoes or conventional sports shoes. Participants were instructed to start using the shoes 2 hours per day and increasing use by 1 hour every day. After 1 week, participants were asked to wear the shoes for a minimum of 6 hours a day during their time spent at work.
Unstable shoes were associated with a significant reduction in pain during walking. Satisfaction with pain management and the number of responders was greater in the unstable shoe group. However, the intervention had no effect on functional disability or quality of life.
This was a short trial (6 weeks). But this information will inform the discussion about the efficacy of these shoes, which are neither uniformly embraced nor recommended. Some discretionary caution should be exercised when considering these shoes for patients with hip or knee instability, Achilles tendon or heel problems, and gait unsteadiness as they might increase the risk for falls. But it is yet another arrow in the quiver to help combat chronic low back pain.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author. He reports no disclosures. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician.
Back pain is a primary complaint of many of the patients we see and, seemingly, a secondary complaint of almost all. Exercise training to strengthen spine muscles is effective, but patients either do not attend referrals to therapy or are not compliant with prescribed regimens. An ideal treatment would be involuntary therapy occurring at all times of the day. But does such a magical therapy exist?
Indeed, it does. They are called unstable shoes or, perhaps less disconcertingly, rocker bottom shoes. They are also referred to as round bottom shoes, rounded shoes, or toning shoes.
Unstable shoes incorporate a rounded sole to increase anterior-posterior instability. Masai Barefoot Technology (MBT) has been advocating their use since the 1990s to reduce low back pain. The owners of MBT went out of business, and the future of this particular brand is uncertain, but many other brands offer this design. Studies have shown that they increase activity of ankle muscles and low back muscles and modify posture during standing and walking.
In a recently published clinical trial evaluating the effectiveness of unstable shoes, 40 hospital workers with chronic low back pain were randomized to unstable shoes or conventional sports shoes. Participants were instructed to start using the shoes 2 hours per day and increasing use by 1 hour every day. After 1 week, participants were asked to wear the shoes for a minimum of 6 hours a day during their time spent at work.
Unstable shoes were associated with a significant reduction in pain during walking. Satisfaction with pain management and the number of responders was greater in the unstable shoe group. However, the intervention had no effect on functional disability or quality of life.
This was a short trial (6 weeks). But this information will inform the discussion about the efficacy of these shoes, which are neither uniformly embraced nor recommended. Some discretionary caution should be exercised when considering these shoes for patients with hip or knee instability, Achilles tendon or heel problems, and gait unsteadiness as they might increase the risk for falls. But it is yet another arrow in the quiver to help combat chronic low back pain.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author. He reports no disclosures. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician.
Back pain is a primary complaint of many of the patients we see and, seemingly, a secondary complaint of almost all. Exercise training to strengthen spine muscles is effective, but patients either do not attend referrals to therapy or are not compliant with prescribed regimens. An ideal treatment would be involuntary therapy occurring at all times of the day. But does such a magical therapy exist?
Indeed, it does. They are called unstable shoes or, perhaps less disconcertingly, rocker bottom shoes. They are also referred to as round bottom shoes, rounded shoes, or toning shoes.
Unstable shoes incorporate a rounded sole to increase anterior-posterior instability. Masai Barefoot Technology (MBT) has been advocating their use since the 1990s to reduce low back pain. The owners of MBT went out of business, and the future of this particular brand is uncertain, but many other brands offer this design. Studies have shown that they increase activity of ankle muscles and low back muscles and modify posture during standing and walking.
In a recently published clinical trial evaluating the effectiveness of unstable shoes, 40 hospital workers with chronic low back pain were randomized to unstable shoes or conventional sports shoes. Participants were instructed to start using the shoes 2 hours per day and increasing use by 1 hour every day. After 1 week, participants were asked to wear the shoes for a minimum of 6 hours a day during their time spent at work.
Unstable shoes were associated with a significant reduction in pain during walking. Satisfaction with pain management and the number of responders was greater in the unstable shoe group. However, the intervention had no effect on functional disability or quality of life.
This was a short trial (6 weeks). But this information will inform the discussion about the efficacy of these shoes, which are neither uniformly embraced nor recommended. Some discretionary caution should be exercised when considering these shoes for patients with hip or knee instability, Achilles tendon or heel problems, and gait unsteadiness as they might increase the risk for falls. But it is yet another arrow in the quiver to help combat chronic low back pain.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author. He reports no disclosures. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician.



