User login
Artificial platelets increase survival in injured mice

Credit: Aaron Logan
New research suggests artificial platelets can aid natural platelets in the clotting process, thereby halting internal bleeding faster.
The artificial platelets, called hemostatic nanoparticles, dramatically increased survival rates in mouse models of blast trauma (hemorrhaging resulting from exposure to an explosion).
And the hemostatic nanoparticles did not appear to interfere with healing or cause any other complications for up to 3 weeks after injection.
Erin Lavik, ScD, of Case Western Reserve University in Cleveland, Ohio, and her colleagues recounted these results in Proceedings of the National Academy of Sciences.
Side effects and survival
The researchers developed a full-body-blast mouse model that replicates the injuries seen in people exposed to explosions. And they used these mice to compare the safety and efficacy of the hemostatic nanoparticles to no treatment, control nanoparticles, lactated Ringer’s solution, and rFVIIa.
The survival rate in mice treated with hemostatic nanoparticles increased to 95% at 1 hour post-trauma, compared to 60% for untreated mice. The odds ratio (OR) was 13.
When the researchers compared the hemostatic nanoparticles and lactated Ringer’s solution, the OR was 8.5. For hemostatic nanoparticles vs control nanoparticles, the OR was 7.1. And for hemostatic nanoparticles vs rFVIIa, the OR was 5.5.
Most mice were killed at the 1-hour time point, but the researchers maintained some mice in the nanoparticle groups so they could assess survival at 1 week and 3 weeks after the blast injury and examine the long-term effects of the nanoparticles.
The team said the vast majority of animals that survived at 1 hour lived to the end of the 3-week study. Differences in lethality at 1 week and 3 weeks were not statistically significant.
Furthermore, there were no unwanted side effects, such as accumulation of the nanoparticles, clot formation, or aberrant healing, during examinations at the 1-week and 3-week time points.
About the nanoparticles
The nanoparticles are made from short polymer chains already approved for other uses by the US Food and Drug Administration.
“They are incredibly simple . . . spheres with arms of peptides that react with activated blood platelets in damaged tissues to help clots form more quickly,” Dr Lavik said.
The dry particles remained viable after 2 weeks on a shelf. A medic in the field or an ambulance crew would add saline, shake and inject them, according to the researchers.
In earlier testing, rat models injected with the nanoparticles stopped bleeding faster than untreated models. Further research and testing are underway, and clinical trials are likely at least 5 years out, Dr Lavik said. ![]()

Credit: Aaron Logan
New research suggests artificial platelets can aid natural platelets in the clotting process, thereby halting internal bleeding faster.
The artificial platelets, called hemostatic nanoparticles, dramatically increased survival rates in mouse models of blast trauma (hemorrhaging resulting from exposure to an explosion).
And the hemostatic nanoparticles did not appear to interfere with healing or cause any other complications for up to 3 weeks after injection.
Erin Lavik, ScD, of Case Western Reserve University in Cleveland, Ohio, and her colleagues recounted these results in Proceedings of the National Academy of Sciences.
Side effects and survival
The researchers developed a full-body-blast mouse model that replicates the injuries seen in people exposed to explosions. And they used these mice to compare the safety and efficacy of the hemostatic nanoparticles to no treatment, control nanoparticles, lactated Ringer’s solution, and rFVIIa.
The survival rate in mice treated with hemostatic nanoparticles increased to 95% at 1 hour post-trauma, compared to 60% for untreated mice. The odds ratio (OR) was 13.
When the researchers compared the hemostatic nanoparticles and lactated Ringer’s solution, the OR was 8.5. For hemostatic nanoparticles vs control nanoparticles, the OR was 7.1. And for hemostatic nanoparticles vs rFVIIa, the OR was 5.5.
Most mice were killed at the 1-hour time point, but the researchers maintained some mice in the nanoparticle groups so they could assess survival at 1 week and 3 weeks after the blast injury and examine the long-term effects of the nanoparticles.
The team said the vast majority of animals that survived at 1 hour lived to the end of the 3-week study. Differences in lethality at 1 week and 3 weeks were not statistically significant.
Furthermore, there were no unwanted side effects, such as accumulation of the nanoparticles, clot formation, or aberrant healing, during examinations at the 1-week and 3-week time points.
About the nanoparticles
The nanoparticles are made from short polymer chains already approved for other uses by the US Food and Drug Administration.
“They are incredibly simple . . . spheres with arms of peptides that react with activated blood platelets in damaged tissues to help clots form more quickly,” Dr Lavik said.
The dry particles remained viable after 2 weeks on a shelf. A medic in the field or an ambulance crew would add saline, shake and inject them, according to the researchers.
In earlier testing, rat models injected with the nanoparticles stopped bleeding faster than untreated models. Further research and testing are underway, and clinical trials are likely at least 5 years out, Dr Lavik said. ![]()

Credit: Aaron Logan
New research suggests artificial platelets can aid natural platelets in the clotting process, thereby halting internal bleeding faster.
The artificial platelets, called hemostatic nanoparticles, dramatically increased survival rates in mouse models of blast trauma (hemorrhaging resulting from exposure to an explosion).
And the hemostatic nanoparticles did not appear to interfere with healing or cause any other complications for up to 3 weeks after injection.
Erin Lavik, ScD, of Case Western Reserve University in Cleveland, Ohio, and her colleagues recounted these results in Proceedings of the National Academy of Sciences.
Side effects and survival
The researchers developed a full-body-blast mouse model that replicates the injuries seen in people exposed to explosions. And they used these mice to compare the safety and efficacy of the hemostatic nanoparticles to no treatment, control nanoparticles, lactated Ringer’s solution, and rFVIIa.
The survival rate in mice treated with hemostatic nanoparticles increased to 95% at 1 hour post-trauma, compared to 60% for untreated mice. The odds ratio (OR) was 13.
When the researchers compared the hemostatic nanoparticles and lactated Ringer’s solution, the OR was 8.5. For hemostatic nanoparticles vs control nanoparticles, the OR was 7.1. And for hemostatic nanoparticles vs rFVIIa, the OR was 5.5.
Most mice were killed at the 1-hour time point, but the researchers maintained some mice in the nanoparticle groups so they could assess survival at 1 week and 3 weeks after the blast injury and examine the long-term effects of the nanoparticles.
The team said the vast majority of animals that survived at 1 hour lived to the end of the 3-week study. Differences in lethality at 1 week and 3 weeks were not statistically significant.
Furthermore, there were no unwanted side effects, such as accumulation of the nanoparticles, clot formation, or aberrant healing, during examinations at the 1-week and 3-week time points.
About the nanoparticles
The nanoparticles are made from short polymer chains already approved for other uses by the US Food and Drug Administration.
“They are incredibly simple . . . spheres with arms of peptides that react with activated blood platelets in damaged tissues to help clots form more quickly,” Dr Lavik said.
The dry particles remained viable after 2 weeks on a shelf. A medic in the field or an ambulance crew would add saline, shake and inject them, according to the researchers.
In earlier testing, rat models injected with the nanoparticles stopped bleeding faster than untreated models. Further research and testing are underway, and clinical trials are likely at least 5 years out, Dr Lavik said. ![]()
QOL data support ATRA-ATO in APL
MILAN—Quality of life (QOL) data support the use of all-trans retinoic acid (ATRA) plus arsenic trioxide (ATO) in patients with acute promyelocytic leukemia (APL), a GIMEMA researcher has reported.
Previously released data from a phase 3 study showed that ATRA-ATO can improve survival rates in APL patients when compared to ATRA plus chemotherapy.
Now, a QOL assessment of these same patients suggests ATO can confer additional benefits.
Researchers observed post-induction improvements in fatigue, cognitive functioning, and other QOL outcome measures among patients treated with ATRA-ATO. However, there were no major differences in post-consolidation results between the ATO arm and the chemotherapy arm.
Nevertheless, the results suggest ATRA-ATO should be considered the preferred first-line therapy in APL, according to Fabio Efficace, PhD, of the GIMEMA Data Center and Health Outcomes Research Unit in Rome, Italy.
Dr Efficace presented QOL data for ATRA-ATO at the 19th Annual Congress of the European Hematology Association (EHA) as abstract S1330.
“When conducting a clinical trial—especially a randomized, phase 3 study—it is of paramount importance to include quality of life assessment,” Dr Efficace said. “If quality of life is assessed in a robust way . . ., it will always provide very important information to [help us] judge the overall treatment effectiveness.”
To that end, he and his colleagues performed QOL assessments of patients enrolled in a randomized, phase 3 trial. The study was designed to show that ATRA-ATO was non-inferior to ATRA- chemo with respect to event-free survival rates at 2 years.
The trial actually showed that ATRA-ATO was superior to ATRA-chemo with regard to both event-free and overall survival.
To assess differences in QOL measures, Dr Efficace and his colleagues asked patients to complete the EORTC QLQ-C30 questionnaire post-induction and post-consolidation.
The questionnaire is used to assess functional aspects, including cognitive functioning, emotional functioning, physical functioning, role functioning, and social functioning. It’s also used to evaluate core symptoms, including pain, fatigue, dyspnea, nausea/vomiting, constipation, diarrhea, insomnia, and appetite loss.
In all, 156 patients received at least 1 dose of their assigned therapy. In the ATRA-chemo group, 62 patients completed the QOL questionnaire post-induction, and 61 did so post-consolidation. In the ATRA-ATO group, 53 patients completed the questionnaire post-induction, and 58 did so post-consolidation.
Post-induction, patients treated with ATRA-ATO reported significantly less fatigue than patients in the ATRA-chemo arm. ATO-treated patients also reported clinically meaningful improvements in appetite loss, nausea/vomiting, constipation, diarrhea, physical functioning, and cognitive functioning.
“We know that when you’re doing chemotherapy, one of the possible effects is loss of cognitive functioning,” Dr Efficace noted. “And we found, for those not receiving chemotherapy, a benefit in cognitive function. So this is something that has to be explored in further studies.”
Dr Efficace also pointed out that, post-consolidation, there were “no major differences” in QOL measures between the 2 treatment arms. Nevertheless, he believes the initial benefits in fatigue and other symptoms support ATRA-ATO as the preferred first-line therapy for APL patients.
“The clinician should be reassured that [ATRA-]ATO as first-line therapy not only provides benefits in terms of event-free survival and overall survival, but it also provides advantages in terms of side effects of therapy,” Dr Efficace said.
“In this trial, we assessed toxicity, but toxicity is physician-reported data. Now, we have patient-reported data. [ATRA-]ATO should be the preferred first-line therapy because it is optimal from the patient perspective.” ![]()
MILAN—Quality of life (QOL) data support the use of all-trans retinoic acid (ATRA) plus arsenic trioxide (ATO) in patients with acute promyelocytic leukemia (APL), a GIMEMA researcher has reported.
Previously released data from a phase 3 study showed that ATRA-ATO can improve survival rates in APL patients when compared to ATRA plus chemotherapy.
Now, a QOL assessment of these same patients suggests ATO can confer additional benefits.
Researchers observed post-induction improvements in fatigue, cognitive functioning, and other QOL outcome measures among patients treated with ATRA-ATO. However, there were no major differences in post-consolidation results between the ATO arm and the chemotherapy arm.
Nevertheless, the results suggest ATRA-ATO should be considered the preferred first-line therapy in APL, according to Fabio Efficace, PhD, of the GIMEMA Data Center and Health Outcomes Research Unit in Rome, Italy.
Dr Efficace presented QOL data for ATRA-ATO at the 19th Annual Congress of the European Hematology Association (EHA) as abstract S1330.
“When conducting a clinical trial—especially a randomized, phase 3 study—it is of paramount importance to include quality of life assessment,” Dr Efficace said. “If quality of life is assessed in a robust way . . ., it will always provide very important information to [help us] judge the overall treatment effectiveness.”
To that end, he and his colleagues performed QOL assessments of patients enrolled in a randomized, phase 3 trial. The study was designed to show that ATRA-ATO was non-inferior to ATRA- chemo with respect to event-free survival rates at 2 years.
The trial actually showed that ATRA-ATO was superior to ATRA-chemo with regard to both event-free and overall survival.
To assess differences in QOL measures, Dr Efficace and his colleagues asked patients to complete the EORTC QLQ-C30 questionnaire post-induction and post-consolidation.
The questionnaire is used to assess functional aspects, including cognitive functioning, emotional functioning, physical functioning, role functioning, and social functioning. It’s also used to evaluate core symptoms, including pain, fatigue, dyspnea, nausea/vomiting, constipation, diarrhea, insomnia, and appetite loss.
In all, 156 patients received at least 1 dose of their assigned therapy. In the ATRA-chemo group, 62 patients completed the QOL questionnaire post-induction, and 61 did so post-consolidation. In the ATRA-ATO group, 53 patients completed the questionnaire post-induction, and 58 did so post-consolidation.
Post-induction, patients treated with ATRA-ATO reported significantly less fatigue than patients in the ATRA-chemo arm. ATO-treated patients also reported clinically meaningful improvements in appetite loss, nausea/vomiting, constipation, diarrhea, physical functioning, and cognitive functioning.
“We know that when you’re doing chemotherapy, one of the possible effects is loss of cognitive functioning,” Dr Efficace noted. “And we found, for those not receiving chemotherapy, a benefit in cognitive function. So this is something that has to be explored in further studies.”
Dr Efficace also pointed out that, post-consolidation, there were “no major differences” in QOL measures between the 2 treatment arms. Nevertheless, he believes the initial benefits in fatigue and other symptoms support ATRA-ATO as the preferred first-line therapy for APL patients.
“The clinician should be reassured that [ATRA-]ATO as first-line therapy not only provides benefits in terms of event-free survival and overall survival, but it also provides advantages in terms of side effects of therapy,” Dr Efficace said.
“In this trial, we assessed toxicity, but toxicity is physician-reported data. Now, we have patient-reported data. [ATRA-]ATO should be the preferred first-line therapy because it is optimal from the patient perspective.” ![]()
MILAN—Quality of life (QOL) data support the use of all-trans retinoic acid (ATRA) plus arsenic trioxide (ATO) in patients with acute promyelocytic leukemia (APL), a GIMEMA researcher has reported.
Previously released data from a phase 3 study showed that ATRA-ATO can improve survival rates in APL patients when compared to ATRA plus chemotherapy.
Now, a QOL assessment of these same patients suggests ATO can confer additional benefits.
Researchers observed post-induction improvements in fatigue, cognitive functioning, and other QOL outcome measures among patients treated with ATRA-ATO. However, there were no major differences in post-consolidation results between the ATO arm and the chemotherapy arm.
Nevertheless, the results suggest ATRA-ATO should be considered the preferred first-line therapy in APL, according to Fabio Efficace, PhD, of the GIMEMA Data Center and Health Outcomes Research Unit in Rome, Italy.
Dr Efficace presented QOL data for ATRA-ATO at the 19th Annual Congress of the European Hematology Association (EHA) as abstract S1330.
“When conducting a clinical trial—especially a randomized, phase 3 study—it is of paramount importance to include quality of life assessment,” Dr Efficace said. “If quality of life is assessed in a robust way . . ., it will always provide very important information to [help us] judge the overall treatment effectiveness.”
To that end, he and his colleagues performed QOL assessments of patients enrolled in a randomized, phase 3 trial. The study was designed to show that ATRA-ATO was non-inferior to ATRA- chemo with respect to event-free survival rates at 2 years.
The trial actually showed that ATRA-ATO was superior to ATRA-chemo with regard to both event-free and overall survival.
To assess differences in QOL measures, Dr Efficace and his colleagues asked patients to complete the EORTC QLQ-C30 questionnaire post-induction and post-consolidation.
The questionnaire is used to assess functional aspects, including cognitive functioning, emotional functioning, physical functioning, role functioning, and social functioning. It’s also used to evaluate core symptoms, including pain, fatigue, dyspnea, nausea/vomiting, constipation, diarrhea, insomnia, and appetite loss.
In all, 156 patients received at least 1 dose of their assigned therapy. In the ATRA-chemo group, 62 patients completed the QOL questionnaire post-induction, and 61 did so post-consolidation. In the ATRA-ATO group, 53 patients completed the questionnaire post-induction, and 58 did so post-consolidation.
Post-induction, patients treated with ATRA-ATO reported significantly less fatigue than patients in the ATRA-chemo arm. ATO-treated patients also reported clinically meaningful improvements in appetite loss, nausea/vomiting, constipation, diarrhea, physical functioning, and cognitive functioning.
“We know that when you’re doing chemotherapy, one of the possible effects is loss of cognitive functioning,” Dr Efficace noted. “And we found, for those not receiving chemotherapy, a benefit in cognitive function. So this is something that has to be explored in further studies.”
Dr Efficace also pointed out that, post-consolidation, there were “no major differences” in QOL measures between the 2 treatment arms. Nevertheless, he believes the initial benefits in fatigue and other symptoms support ATRA-ATO as the preferred first-line therapy for APL patients.
“The clinician should be reassured that [ATRA-]ATO as first-line therapy not only provides benefits in terms of event-free survival and overall survival, but it also provides advantages in terms of side effects of therapy,” Dr Efficace said.
“In this trial, we assessed toxicity, but toxicity is physician-reported data. Now, we have patient-reported data. [ATRA-]ATO should be the preferred first-line therapy because it is optimal from the patient perspective.” ![]()
HIV+ cancer patients more likely to go untreated

lymphocyte; Credit: CDC
Cancer patients infected with HIV are less likely than their uninfected peers to receive cancer treatment, according to research published in the Journal of Clinical Oncology.
Results showed that HIV-positive patients were roughly twice as likely to go untreated for lymphomas and other cancers.
The researchers believe a lack of clinical trial data and treatment guidelines for HIV patients with cancer may contribute to this health disparity.
To assess the role of HIV status on cancer treatment, Gita Suneja, MD, of the University of Pennsylvania in Philadelphia, and her colleagues used data from the National Cancer Institute’s HIV/AIDS Cancer Match Study.
This included 3045 HIV-infected patients and 1,087,648 uninfected patients. The patients had been diagnosed with Hodgkin lymphoma, diffuse large B-cell lymphoma, or cervical, lung, anal, prostate, colorectal, or breast cancer from 1996 through 2010.
For each cancer type, the researchers assessed the relationship between HIV status and cancer treatment, adjusted for cancer stage, sex, age at cancer diagnosis, race/ethnicity, year of cancer diagnosis, and US state.
For all but 1 cancer type, there was a significantly higher proportion of HIV-infected patients who did not receive cancer treatment when compared with uninfected patients.
The adjusted odds ratios (aORs) were 1.67 for diffuse large B-cell lymphoma, 1.77 for Hodgkin lymphoma, 1.60 for cervical cancer, 1.79 for prostate cancer, 1.81 for breast cancer, 2.18 for lung cancer, and 2.27 for colorectal cancer.
Anal cancer was the only malignancy for which HIV status did not appear to impact treatment. The aOR was 1.01.
Among HIV-infected individuals, factors independently associated with a lack of cancer treatment included low CD4 count, male sex with injection drug use as mode of HIV exposure, age 45 to 64 years, black race, and distant or unknown cancer stage.
“In my clinical experience, I have seen uncertainty surrounding treatment of HIV-infected cancer patients,” Dr Suneja said. “Patients with HIV have typically been excluded from clinical trials, and, therefore, oncologists do not know if the best available treatments are equally safe and effective in those with HIV.”
“Many oncologists rely on guidelines based on such trials for treatment decision-making, and, in the absence of guidance, they may elect not to treat HIV-infected cancer patients due to concerns about adverse side effects or poor survival. This could help explain, in part, why many HIV-positive cancer patients are not receiving appropriate cancer care.”
Therefore, Dr Suneja and her colleagues recommend that cancer trials begin enrolling HIV-infected patients and cancer management guidelines incorporate recommendations for HIV-infected patients. ![]()

lymphocyte; Credit: CDC
Cancer patients infected with HIV are less likely than their uninfected peers to receive cancer treatment, according to research published in the Journal of Clinical Oncology.
Results showed that HIV-positive patients were roughly twice as likely to go untreated for lymphomas and other cancers.
The researchers believe a lack of clinical trial data and treatment guidelines for HIV patients with cancer may contribute to this health disparity.
To assess the role of HIV status on cancer treatment, Gita Suneja, MD, of the University of Pennsylvania in Philadelphia, and her colleagues used data from the National Cancer Institute’s HIV/AIDS Cancer Match Study.
This included 3045 HIV-infected patients and 1,087,648 uninfected patients. The patients had been diagnosed with Hodgkin lymphoma, diffuse large B-cell lymphoma, or cervical, lung, anal, prostate, colorectal, or breast cancer from 1996 through 2010.
For each cancer type, the researchers assessed the relationship between HIV status and cancer treatment, adjusted for cancer stage, sex, age at cancer diagnosis, race/ethnicity, year of cancer diagnosis, and US state.
For all but 1 cancer type, there was a significantly higher proportion of HIV-infected patients who did not receive cancer treatment when compared with uninfected patients.
The adjusted odds ratios (aORs) were 1.67 for diffuse large B-cell lymphoma, 1.77 for Hodgkin lymphoma, 1.60 for cervical cancer, 1.79 for prostate cancer, 1.81 for breast cancer, 2.18 for lung cancer, and 2.27 for colorectal cancer.
Anal cancer was the only malignancy for which HIV status did not appear to impact treatment. The aOR was 1.01.
Among HIV-infected individuals, factors independently associated with a lack of cancer treatment included low CD4 count, male sex with injection drug use as mode of HIV exposure, age 45 to 64 years, black race, and distant or unknown cancer stage.
“In my clinical experience, I have seen uncertainty surrounding treatment of HIV-infected cancer patients,” Dr Suneja said. “Patients with HIV have typically been excluded from clinical trials, and, therefore, oncologists do not know if the best available treatments are equally safe and effective in those with HIV.”
“Many oncologists rely on guidelines based on such trials for treatment decision-making, and, in the absence of guidance, they may elect not to treat HIV-infected cancer patients due to concerns about adverse side effects or poor survival. This could help explain, in part, why many HIV-positive cancer patients are not receiving appropriate cancer care.”
Therefore, Dr Suneja and her colleagues recommend that cancer trials begin enrolling HIV-infected patients and cancer management guidelines incorporate recommendations for HIV-infected patients. ![]()

lymphocyte; Credit: CDC
Cancer patients infected with HIV are less likely than their uninfected peers to receive cancer treatment, according to research published in the Journal of Clinical Oncology.
Results showed that HIV-positive patients were roughly twice as likely to go untreated for lymphomas and other cancers.
The researchers believe a lack of clinical trial data and treatment guidelines for HIV patients with cancer may contribute to this health disparity.
To assess the role of HIV status on cancer treatment, Gita Suneja, MD, of the University of Pennsylvania in Philadelphia, and her colleagues used data from the National Cancer Institute’s HIV/AIDS Cancer Match Study.
This included 3045 HIV-infected patients and 1,087,648 uninfected patients. The patients had been diagnosed with Hodgkin lymphoma, diffuse large B-cell lymphoma, or cervical, lung, anal, prostate, colorectal, or breast cancer from 1996 through 2010.
For each cancer type, the researchers assessed the relationship between HIV status and cancer treatment, adjusted for cancer stage, sex, age at cancer diagnosis, race/ethnicity, year of cancer diagnosis, and US state.
For all but 1 cancer type, there was a significantly higher proportion of HIV-infected patients who did not receive cancer treatment when compared with uninfected patients.
The adjusted odds ratios (aORs) were 1.67 for diffuse large B-cell lymphoma, 1.77 for Hodgkin lymphoma, 1.60 for cervical cancer, 1.79 for prostate cancer, 1.81 for breast cancer, 2.18 for lung cancer, and 2.27 for colorectal cancer.
Anal cancer was the only malignancy for which HIV status did not appear to impact treatment. The aOR was 1.01.
Among HIV-infected individuals, factors independently associated with a lack of cancer treatment included low CD4 count, male sex with injection drug use as mode of HIV exposure, age 45 to 64 years, black race, and distant or unknown cancer stage.
“In my clinical experience, I have seen uncertainty surrounding treatment of HIV-infected cancer patients,” Dr Suneja said. “Patients with HIV have typically been excluded from clinical trials, and, therefore, oncologists do not know if the best available treatments are equally safe and effective in those with HIV.”
“Many oncologists rely on guidelines based on such trials for treatment decision-making, and, in the absence of guidance, they may elect not to treat HIV-infected cancer patients due to concerns about adverse side effects or poor survival. This could help explain, in part, why many HIV-positive cancer patients are not receiving appropriate cancer care.”
Therefore, Dr Suneja and her colleagues recommend that cancer trials begin enrolling HIV-infected patients and cancer management guidelines incorporate recommendations for HIV-infected patients. ![]()
Group engineers versatile RBCs

Researchers say they’ve modified red blood cells (RBCs) to carry a range of payloads—from drugs to vaccines to imaging agents.
“We wanted to create high-value red cells that do more than simply carry oxygen,” said study author Harvey Lodish, PhD, of the Whitehead Institute in Cambridge, Massachusetts.
“Here, we’ve laid out the technology to make mouse and human red blood cells in culture that can express what we want and potentially be used for therapeutic or diagnostic purposes.”
Dr Lodish and his colleagues detailed the technology in Proceedings of the National Academy of Sciences.
The team noted that RBCs are an attractive vehicle for a variety of reasons, including their abundance and their long lifespan.
Perhaps most importantly, during RBC production, progenitor cells jettison their nuclei and all DNA therein. Without a nucleus, a mature RBC lacks any genetic material or any signs of earlier genetic manipulation that could result in tumor formation or other adverse effects.
Exploiting this characteristic, Dr Lodish and his colleagues introduced into early stage RBC progenitors genes coding for specific, slightly modified, normal red cell surface proteins.
As the RBCs approach maturity and enucleate, the proteins remain on the cell surface, where they are modified by a protein-labeling technique known as sortagging.
The technique relies on the bacterial enzyme sortase A to establish a strong chemical bond between the surface protein and a substance of choice, be it a small-molecule therapeutic or an antibody capable of binding a toxin. The modifications leave the cells and their surfaces unharmed.
“Because the modified human red blood cells can circulate in the body for up to 4 months, one could envision a scenario in which the cells are used to introduce antibodies that neutralize a toxin,” said Hidde L. Ploegh, PhD, also of the Whitehead Institute. “The result would be long-lasting reserves of antitoxin antibodies.”
The approach has captured the attention of the US military and its Defense Advanced Research Projects Agency, which is supporting the research in the interest of developing treatments or vaccines effective against biological weapons.
Dr Lodish believes the applications are potentially vast and may include RBCs modified to bind and remove bad cholesterol from the bloodstream, carry clot-busting proteins to treat ischemic strokes or deep-vein thrombosis, or deliver anti-inflammatory antibodies to alleviate chronic inflammation.
Dr Ploegh said there is evidence to suggest that modified RBCs could be used to suppress the unwanted immune response that often accompanies treatment with protein-based therapies.
He is exploring whether these RBCs could be used to prime the immune system to allow patients to better tolerate treatment with such therapies. ![]()

Researchers say they’ve modified red blood cells (RBCs) to carry a range of payloads—from drugs to vaccines to imaging agents.
“We wanted to create high-value red cells that do more than simply carry oxygen,” said study author Harvey Lodish, PhD, of the Whitehead Institute in Cambridge, Massachusetts.
“Here, we’ve laid out the technology to make mouse and human red blood cells in culture that can express what we want and potentially be used for therapeutic or diagnostic purposes.”
Dr Lodish and his colleagues detailed the technology in Proceedings of the National Academy of Sciences.
The team noted that RBCs are an attractive vehicle for a variety of reasons, including their abundance and their long lifespan.
Perhaps most importantly, during RBC production, progenitor cells jettison their nuclei and all DNA therein. Without a nucleus, a mature RBC lacks any genetic material or any signs of earlier genetic manipulation that could result in tumor formation or other adverse effects.
Exploiting this characteristic, Dr Lodish and his colleagues introduced into early stage RBC progenitors genes coding for specific, slightly modified, normal red cell surface proteins.
As the RBCs approach maturity and enucleate, the proteins remain on the cell surface, where they are modified by a protein-labeling technique known as sortagging.
The technique relies on the bacterial enzyme sortase A to establish a strong chemical bond between the surface protein and a substance of choice, be it a small-molecule therapeutic or an antibody capable of binding a toxin. The modifications leave the cells and their surfaces unharmed.
“Because the modified human red blood cells can circulate in the body for up to 4 months, one could envision a scenario in which the cells are used to introduce antibodies that neutralize a toxin,” said Hidde L. Ploegh, PhD, also of the Whitehead Institute. “The result would be long-lasting reserves of antitoxin antibodies.”
The approach has captured the attention of the US military and its Defense Advanced Research Projects Agency, which is supporting the research in the interest of developing treatments or vaccines effective against biological weapons.
Dr Lodish believes the applications are potentially vast and may include RBCs modified to bind and remove bad cholesterol from the bloodstream, carry clot-busting proteins to treat ischemic strokes or deep-vein thrombosis, or deliver anti-inflammatory antibodies to alleviate chronic inflammation.
Dr Ploegh said there is evidence to suggest that modified RBCs could be used to suppress the unwanted immune response that often accompanies treatment with protein-based therapies.
He is exploring whether these RBCs could be used to prime the immune system to allow patients to better tolerate treatment with such therapies. ![]()

Researchers say they’ve modified red blood cells (RBCs) to carry a range of payloads—from drugs to vaccines to imaging agents.
“We wanted to create high-value red cells that do more than simply carry oxygen,” said study author Harvey Lodish, PhD, of the Whitehead Institute in Cambridge, Massachusetts.
“Here, we’ve laid out the technology to make mouse and human red blood cells in culture that can express what we want and potentially be used for therapeutic or diagnostic purposes.”
Dr Lodish and his colleagues detailed the technology in Proceedings of the National Academy of Sciences.
The team noted that RBCs are an attractive vehicle for a variety of reasons, including their abundance and their long lifespan.
Perhaps most importantly, during RBC production, progenitor cells jettison their nuclei and all DNA therein. Without a nucleus, a mature RBC lacks any genetic material or any signs of earlier genetic manipulation that could result in tumor formation or other adverse effects.
Exploiting this characteristic, Dr Lodish and his colleagues introduced into early stage RBC progenitors genes coding for specific, slightly modified, normal red cell surface proteins.
As the RBCs approach maturity and enucleate, the proteins remain on the cell surface, where they are modified by a protein-labeling technique known as sortagging.
The technique relies on the bacterial enzyme sortase A to establish a strong chemical bond between the surface protein and a substance of choice, be it a small-molecule therapeutic or an antibody capable of binding a toxin. The modifications leave the cells and their surfaces unharmed.
“Because the modified human red blood cells can circulate in the body for up to 4 months, one could envision a scenario in which the cells are used to introduce antibodies that neutralize a toxin,” said Hidde L. Ploegh, PhD, also of the Whitehead Institute. “The result would be long-lasting reserves of antitoxin antibodies.”
The approach has captured the attention of the US military and its Defense Advanced Research Projects Agency, which is supporting the research in the interest of developing treatments or vaccines effective against biological weapons.
Dr Lodish believes the applications are potentially vast and may include RBCs modified to bind and remove bad cholesterol from the bloodstream, carry clot-busting proteins to treat ischemic strokes or deep-vein thrombosis, or deliver anti-inflammatory antibodies to alleviate chronic inflammation.
Dr Ploegh said there is evidence to suggest that modified RBCs could be used to suppress the unwanted immune response that often accompanies treatment with protein-based therapies.
He is exploring whether these RBCs could be used to prime the immune system to allow patients to better tolerate treatment with such therapies. ![]()
PPIs cut acid pocket impact in GERD
Proton pump inhibitors affect the size, relative acidity, and position of the acid pocket in gastroesophageal reflux patients, reported Dr. Wout O. Rohof and his colleagues.
"If one accepts that the acid pocket is still the source of the refluxate during PPI use, therapeutic strategies directly intervening with the acid pocket possibly may prove effective in preventing persistent symptoms on PPI," they wrote in the July issue of Clinical Gastroenterology and Hepatology News (doi.org/10.1016/j.cgh.2014.04.003-).
"This insight is of great importance because in approximately 30% of patients PPI therapy fails to resolve symptoms, either partially or completely," they added.
Dr. Rohof of the Academic Medical Center in Amsterdam and his colleagues looked at 36 patients with gastroesophageal reflux disease (GERD) confirmed by the presence of esophagitis on endoscopy and/or impedance-pH-metry with an acid exposure of more than 4.5%, plus usual GERD symptoms.
Eighteen patients were on PPIs; the remainder had been off PPIs for at least 1 week prior to the study; eight PPI cohort patients used omeprazole, five used pantoprazole, and five used esomeprazole, with dosages varying from 20 mg (one patient), to 40 mg (eight patients), to 40 mg twice daily (nine patients).
All patients were fed a standardized meal of orange juice and pancakes; afterward, concurrent scintigraphy, high-resolution manometry, and pH-impedance recordings were acquired for 105 minutes.
At the study’s conclusion, the acid pocket – a floating pool of acid on top of ingested food, visualized on scintigraphy after patients were injected with a radioisotope – was aspirated for analysis of its pH level.
The authors found that overall, while the total number of reflux episodes was similar for both PPI and non-PPI cohorts (15 vs. 14; P = .81), "As expected, the number of acid reflux episodes was lower on PPI (4.5 vs. 2.0; P = .04)."
Only two patients in the entire cohort did not demonstrate an acid pocket on scintigraphy; both were taking PPIs.
Of the remaining patients, the size of the acid pocket was significantly less among PPI patients than their counterparts (10 cm2 vs. 15 cm2; P less than .01).
Moreover, when the acid pocket was aspirated at the conclusion of the study through the manometry catheter, the pH of the aspirated acid pocket fluid was significantly higher among PPI patients, compared with those not taking the drug (3.9 vs.0.9; P less than .001).
Finally, the investigators looked at the location of the acid pocket, which has also been shown to correlate with reflux episodes.
Their data showed that PPI patients were more likely to have the pocket located below the level of the diaphragm (60%), compared with patients not on PPI therapy (40%; P = .04).
This was especially important since pockets at this level correlated with only a 7% and 15% risk of acidic reflux events in PPI and non-PPI patients, respectively; in comparison, pockets in the hiatus and higher were associated with much greater rates of acidic reflux for both PPI and non-PPI patients, though the rates were lower among the former.
The authors disclosed no conflicts of interest or outside funding.
Proton pump inhibitors affect the size, relative acidity, and position of the acid pocket in gastroesophageal reflux patients, reported Dr. Wout O. Rohof and his colleagues.
"If one accepts that the acid pocket is still the source of the refluxate during PPI use, therapeutic strategies directly intervening with the acid pocket possibly may prove effective in preventing persistent symptoms on PPI," they wrote in the July issue of Clinical Gastroenterology and Hepatology News (doi.org/10.1016/j.cgh.2014.04.003-).
"This insight is of great importance because in approximately 30% of patients PPI therapy fails to resolve symptoms, either partially or completely," they added.
Dr. Rohof of the Academic Medical Center in Amsterdam and his colleagues looked at 36 patients with gastroesophageal reflux disease (GERD) confirmed by the presence of esophagitis on endoscopy and/or impedance-pH-metry with an acid exposure of more than 4.5%, plus usual GERD symptoms.
Eighteen patients were on PPIs; the remainder had been off PPIs for at least 1 week prior to the study; eight PPI cohort patients used omeprazole, five used pantoprazole, and five used esomeprazole, with dosages varying from 20 mg (one patient), to 40 mg (eight patients), to 40 mg twice daily (nine patients).
All patients were fed a standardized meal of orange juice and pancakes; afterward, concurrent scintigraphy, high-resolution manometry, and pH-impedance recordings were acquired for 105 minutes.
At the study’s conclusion, the acid pocket – a floating pool of acid on top of ingested food, visualized on scintigraphy after patients were injected with a radioisotope – was aspirated for analysis of its pH level.
The authors found that overall, while the total number of reflux episodes was similar for both PPI and non-PPI cohorts (15 vs. 14; P = .81), "As expected, the number of acid reflux episodes was lower on PPI (4.5 vs. 2.0; P = .04)."
Only two patients in the entire cohort did not demonstrate an acid pocket on scintigraphy; both were taking PPIs.
Of the remaining patients, the size of the acid pocket was significantly less among PPI patients than their counterparts (10 cm2 vs. 15 cm2; P less than .01).
Moreover, when the acid pocket was aspirated at the conclusion of the study through the manometry catheter, the pH of the aspirated acid pocket fluid was significantly higher among PPI patients, compared with those not taking the drug (3.9 vs.0.9; P less than .001).
Finally, the investigators looked at the location of the acid pocket, which has also been shown to correlate with reflux episodes.
Their data showed that PPI patients were more likely to have the pocket located below the level of the diaphragm (60%), compared with patients not on PPI therapy (40%; P = .04).
This was especially important since pockets at this level correlated with only a 7% and 15% risk of acidic reflux events in PPI and non-PPI patients, respectively; in comparison, pockets in the hiatus and higher were associated with much greater rates of acidic reflux for both PPI and non-PPI patients, though the rates were lower among the former.
The authors disclosed no conflicts of interest or outside funding.
Proton pump inhibitors affect the size, relative acidity, and position of the acid pocket in gastroesophageal reflux patients, reported Dr. Wout O. Rohof and his colleagues.
"If one accepts that the acid pocket is still the source of the refluxate during PPI use, therapeutic strategies directly intervening with the acid pocket possibly may prove effective in preventing persistent symptoms on PPI," they wrote in the July issue of Clinical Gastroenterology and Hepatology News (doi.org/10.1016/j.cgh.2014.04.003-).
"This insight is of great importance because in approximately 30% of patients PPI therapy fails to resolve symptoms, either partially or completely," they added.
Dr. Rohof of the Academic Medical Center in Amsterdam and his colleagues looked at 36 patients with gastroesophageal reflux disease (GERD) confirmed by the presence of esophagitis on endoscopy and/or impedance-pH-metry with an acid exposure of more than 4.5%, plus usual GERD symptoms.
Eighteen patients were on PPIs; the remainder had been off PPIs for at least 1 week prior to the study; eight PPI cohort patients used omeprazole, five used pantoprazole, and five used esomeprazole, with dosages varying from 20 mg (one patient), to 40 mg (eight patients), to 40 mg twice daily (nine patients).
All patients were fed a standardized meal of orange juice and pancakes; afterward, concurrent scintigraphy, high-resolution manometry, and pH-impedance recordings were acquired for 105 minutes.
At the study’s conclusion, the acid pocket – a floating pool of acid on top of ingested food, visualized on scintigraphy after patients were injected with a radioisotope – was aspirated for analysis of its pH level.
The authors found that overall, while the total number of reflux episodes was similar for both PPI and non-PPI cohorts (15 vs. 14; P = .81), "As expected, the number of acid reflux episodes was lower on PPI (4.5 vs. 2.0; P = .04)."
Only two patients in the entire cohort did not demonstrate an acid pocket on scintigraphy; both were taking PPIs.
Of the remaining patients, the size of the acid pocket was significantly less among PPI patients than their counterparts (10 cm2 vs. 15 cm2; P less than .01).
Moreover, when the acid pocket was aspirated at the conclusion of the study through the manometry catheter, the pH of the aspirated acid pocket fluid was significantly higher among PPI patients, compared with those not taking the drug (3.9 vs.0.9; P less than .001).
Finally, the investigators looked at the location of the acid pocket, which has also been shown to correlate with reflux episodes.
Their data showed that PPI patients were more likely to have the pocket located below the level of the diaphragm (60%), compared with patients not on PPI therapy (40%; P = .04).
This was especially important since pockets at this level correlated with only a 7% and 15% risk of acidic reflux events in PPI and non-PPI patients, respectively; in comparison, pockets in the hiatus and higher were associated with much greater rates of acidic reflux for both PPI and non-PPI patients, though the rates were lower among the former.
The authors disclosed no conflicts of interest or outside funding.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Proton pump inhibitors have more than one beneficial action in esophagitis.
Major finding: The size, position, and acidity of the acid pocket in reflux patients are all lessened with daily PPI therapy.
Data source: A cohort of 36 esophagitis patients, half of whom took PPIs, half of whom did not.
Disclosures: The authors disclosed no conflicts of interest or outside funding.
Cutaneous Melanoma
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Melanoma is the sixth most common cancer in the United States and the leading cause of deaths among all cutaneous malignancies. In 2012, it was estimated that approximately 75,000 individuals were diagnosed with melanoma and more than 9000 died. The incidence of melanoma is rising the fastest among all major malignancies, and the lifetime risk of melanoma among men and women now exceeds 1 in 68, as compared with 1:1500 in 1930.4 The incidence of melanoma is predicted to continue increasing, and there has been no corresponding decrease in mortality. This case-based review summarizes the etiology, risk factors, clinical presentation, and management of cutaneous melanomas, which comprise the majority of melanoma cases. The biology and management for other noncutaneous melanomas (such as mucosal or ocular melanomas) are beyond the scope of this review.
To read the full article in PDF:
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Melanoma is the sixth most common cancer in the United States and the leading cause of deaths among all cutaneous malignancies. In 2012, it was estimated that approximately 75,000 individuals were diagnosed with melanoma and more than 9000 died. The incidence of melanoma is rising the fastest among all major malignancies, and the lifetime risk of melanoma among men and women now exceeds 1 in 68, as compared with 1:1500 in 1930.4 The incidence of melanoma is predicted to continue increasing, and there has been no corresponding decrease in mortality. This case-based review summarizes the etiology, risk factors, clinical presentation, and management of cutaneous melanomas, which comprise the majority of melanoma cases. The biology and management for other noncutaneous melanomas (such as mucosal or ocular melanomas) are beyond the scope of this review.
To read the full article in PDF:
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Melanoma is the sixth most common cancer in the United States and the leading cause of deaths among all cutaneous malignancies. In 2012, it was estimated that approximately 75,000 individuals were diagnosed with melanoma and more than 9000 died. The incidence of melanoma is rising the fastest among all major malignancies, and the lifetime risk of melanoma among men and women now exceeds 1 in 68, as compared with 1:1500 in 1930.4 The incidence of melanoma is predicted to continue increasing, and there has been no corresponding decrease in mortality. This case-based review summarizes the etiology, risk factors, clinical presentation, and management of cutaneous melanomas, which comprise the majority of melanoma cases. The biology and management for other noncutaneous melanomas (such as mucosal or ocular melanomas) are beyond the scope of this review.
To read the full article in PDF:
Hospital High‐Value Care Program
With a United States medical system that spends as much as $750 billion each year on care that does not result in improved health outcomes,[1] many policy initiatives, including the Centers for Medicare and Medicaid Services' Value‐Based Purchasing program, seek to realign hospitals' financial incentives from a focus on production to one on value (quality divided by cost).[2, 3] Professional organizations have now deemed resource stewardship an ethical responsibility for professionalism,[4, 5] and campaigns such as the American Board of Internal Medicine (ABIM) Foundation's Choosing Wisely effort and the American College of Physicians' High‐Value Care platform are calling on frontline clinicians to address unnecessary and wasteful services.[6, 7]
Despite these pressures and initiatives, most physicians lack the knowledge and tools necessary to prioritize the delivery of their own healthcare services according to value.[8, 9, 10] Hospital medicine physicians are unaware of the costs associated with the interventions they order,[10] and the majority of medical training programs lack curricula focused on healthcare costs,[11] creating a large gap between physicians' perceived, desired, and actual knowledge related to costs.[12] Novel frameworks and frontline physician engagement are required if clinicians are to improve the value of the care they deliver.
We describe 1 of our first steps at the University of California, San Francisco (UCSF) to promote high‐value care (HVC) delivery: the creation of a HVC program led by clinicians and administrators focused on identifying and addressing wasteful practices within our hospitalist group. The program aims to (1) use financial and clinical data to identify areas with clear evidence of waste in the hospital, (2) promote evidence‐based interventions that improve both quality of care and value, and (3) pair interventions with evidence‐based cost awareness education to drive culture change. Our experience and inaugural projects provide a model of the key features, inherent challenges, and lessons learned, which may help inform similar efforts.
METHODS
In March 2012, we launched an HVC program within our Division of Hospital Medicine at UCSF Medical Center, a 600‐bed academic medical center in an urban setting. During the 2013 academic year, our division included 45 physicians. The medicine service, comprised of 8 teaching medical ward teams (1 attending, 1 resident, 2 interns, and variable number of medical students), and 1 nonteaching medical ward team (1 attending), admitted 4700 patients that year.
Organizational Framework
The HVC program is co‐led by a UCSF hospitalist (C.M.) and the administrator of the Division of Hospital Medicine (M.N.). Team members include hospitalists, hospital medicine fellows, resident physicians, pharmacists, project coordinators, and other administrators. The team meets in person for 1 hour every month. Project teams and ad hoc subcommittee groups often convene between meetings.
Our HVC program was placed within the infrastructure, and under the leadership, of our already established quality improvement (QI) program at UCSF. Our Division of Hospital Medicine Director of Quality and Safety (M.M.) thus oversees the QI, patient safety, patient experience, and high‐value care efforts.
The HVC program funding is largely in personnel costs. The physician leader (15% effort) is funded by the Division of Hospital Medicine, whereas the administrator is cofunded by both the division and by the medical center (largely through her roles as both division administrator and service line director). An administrative assistant within the division is also assigned to help with administrative tasks. Some additional data gathering and project support comes from existing medical center QI infrastructure, the decision support services unit, and through UCSF's new Center for Healthcare Value. Other ancillary costs for our projects have included publicity, data analytics, and information technology infrastructure. We estimate that the costs of this program are approximately $50,000 to $75,000 annually.
Framework for Identifying Target Projects
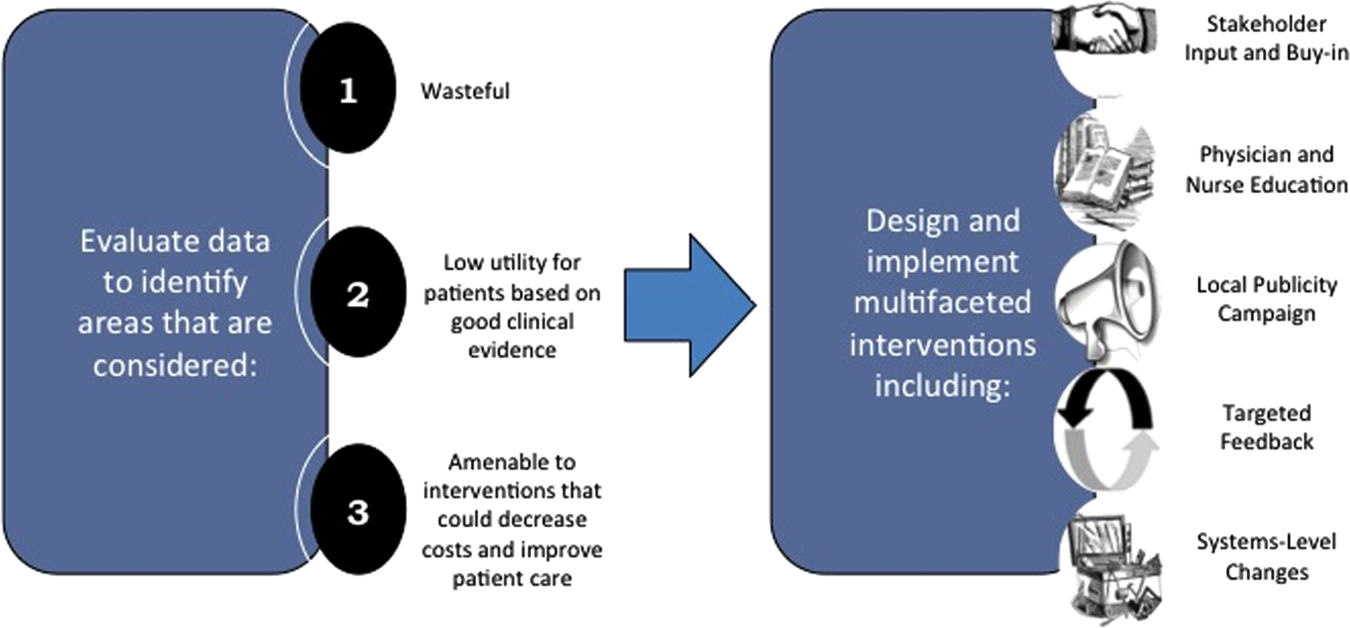
Robust Analysis of Costs
We created a framework for identifying, designing, and promoting projects specifically aimed at improving healthcare value (Figure 1). Financial data were used to identify areas with clear evidence of waste in the hospital, areas of high cost with no benefit in health outcomes. We focused particularly on obtaining cost and billing data for our medical service, which provided important insight into potential targets for improvements in value. For example, in 2011, the Division of Hospital Medicine spent more than $1 million annually in direct costs for the administration of nebulized bronchodilator therapies (nebs) to nonintensive care unit patients on the medical service.[13] These high costs, exposed by billing data, were believed to represent potential unnecessary testing and/or procedures. Not every area of high cost was deemed a target for intervention. For example, the use of recombinant factor VIII appeared a necessary expenditure (over $1 million per year) for our patients with hemophilia. Although our efforts focused on reducing waste, it is worth noting that healthcare value can also be increased by improving the delivery of high‐value services.
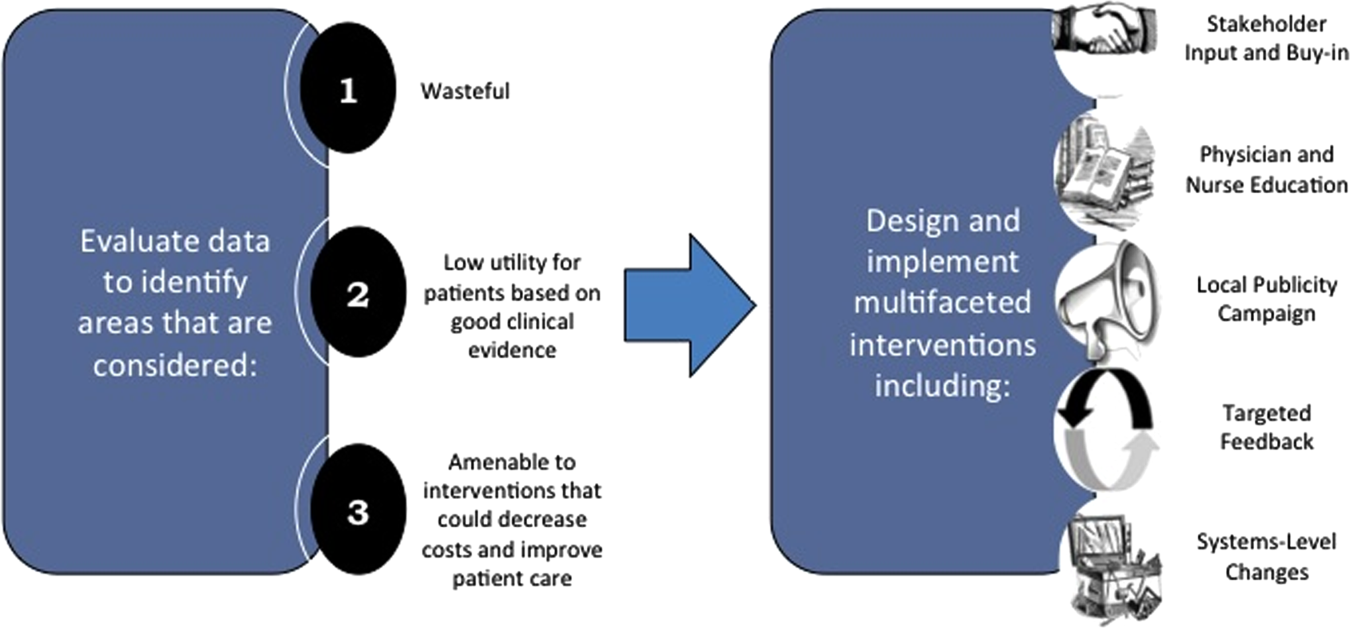
Recognized Benefits in Quality of Care
The program also evaluated the impact of cost reduction efforts on the quality of care, based on a high standard of current evidence. Though value can be improved by interventions that decrease costs while being quality neutral, our group chose to focus first on projects that would simultaneously improve quality while decreasing costs. We felt that this win‐win strategy would help obtain buy‐in from clinicians weary of prior cost‐cutting programs. For example, we pursued interventions aimed at reducing inappropriate gastric stress ulcer prophylaxis, which had the potential to both cut costs and minimize risks of hospital‐acquired pneumonia and Clostridium difficile infections.[14, 15] All proposed HVC targets were vetted through a review of the literature and published guidelines. In general, our initial projects had to be strongly supported by evidence, with high‐quality studies, preferably meta‐analyses or systematic reviews, that displayed the safety of our recommended changes. We reviewed the literature with experts. For example, we met with faculty pulmonologists to discuss the evidence supporting the use of inhalers instead of nebulizers in adults with obstructive pulmonary disease. The goals of our projects were chosen by our HVC committee, based on an analysis of our baseline data and the perceived potential effects of our proposed interventions.
Educational Intervention
Last, we paired interventions with evidence‐based cost awareness education to drive culture change. At UCSF we have an ongoing longitudinal cost‐awareness curriculum for residents, which has previously been described.[16] We took advantage of this educational forum to address gaps in clinician knowledge related to the targeted areas. When launching the initiative to decrease unnecessary inpatient nebulizer usage and improve transitions to inhalers, we utilized the chronic obstructive pulmonary disease case in the cost‐awareness series. Doing so allowed us to both review the evidence behind the effectiveness of inhalers, and introduce our Nebs No More After 24 campaign, which sought to transition adult inpatients with obstructive pulmonary symptoms from nebs to inhalers within 24 hours of admission.[13]
Intervention Strategy
Our general approach has been to design and implement multifaceted interventions, adapted from previous QI literature (Figure 1).[17] Given the importance of frontline clinician engagement to successful project implementation,[18, 19, 20] our interventions are physician‐driven and are vetted by a large group of clinicians prior to launch. The HVC program also explicitly seeks stakeholder input, perspective, and buy‐in prior to implementation. For example, we involved respiratory therapists (RTs) in the design of the Nebs No More After 24 project, thus ensuring that the interventions fit within their workflow and align with their care‐delivery goals.
Local publicity campaigns provide education and reminders for clinicians. Posters, such as the Nebs No More After 24 poster (Figure 2), were hung in physician, nursing, and RT work areas. Pens featuring the catchphrase Nebs No More After 24 were distributed to clinicians.

In addition to presentations to residents through the UCSF cost awareness curriculum, educational presentations were also delivered to attending physicians and to other allied members of the healthcare team (eg, nurses, RTs) during regularly scheduled staff meetings.
The metrics for each of the projects were regularly monitored, and targeted feedback was provided to clinicians. For the Nebs No More After 24 campaign, data for the number of nebs delivered on the target floor were provided to resident physicians during the cost awareness conference each month, and the data were presented to attending hospitalists in the monthly QI newsletter. This academic year, transfusion and telemetry data are presented via the same strategy.
Stakeholder recruitment, education, and promotional campaigns are important to program launches, but to sustain projects over the long‐term, system changes may be necessary. We have pursued changes in the computerized provider order entry (CPOE) system, such as removing nebs from the admission order set or putting a default duration for certain telemetry orders. Systems‐level interventions, although more difficult to achieve, play an important role in creating enduring changes when paired with educational interventions.
RESULTS
During our first 2 years we have initiated ongoing projects directed at 6 major targets (Table 1). Our flagship project, Nebs No More After 24, resulted in a decrease of nebulizer rates by more than 50% on a high‐acuity medical floor, as previously published.[13] We created a financial model that primarily accounted for RT time and pharmaceutical costs, and estimated a savings of approximately $250,000 annually on this single medical ward (see Supporting Information, Table 1, in the online version of this article).[13]
| High‐Value Care Projects | Relevant Baseline Data | Goals of Project | Strategies |
|---|---|---|---|
| |||
| Nebs No More After 24: Improving appropriate use of respiratory services | The medicine service spent $1 million in direct costs on approximately 25,000 nebs for non‐ICU inpatients. | Reduce unnecessary nebs >15% over 9 months. | Removed nebs from admit order set. |
| Improve transitions from nebs to MDIs. | Enlisted RTs and RNs to help with MDI teaching for patients. | ||
| Improve patient self‐administration of MDIs. | Implemented an educational program for medicine physicians. | ||
| Created local publicity: posters, flyers, and pens. | |||
| Provided data feedback to providers. | |||
| Next step: Introduce a CPOE‐linked intervention. | |||
| Improving use of stress ulcer prophylaxis | 77% of ICU patients on acid suppressive therapy; 31% of these patients did not meet criteria for appropriate prophylaxis. | Reduce overuse and inappropriate use of SUP. | A team of pharmacists, nurses, and physicians developed targeted and evidence‐based UCSF guidelines on use of SUP. |
| Developed and implemented a pharmacist‐led intervention to reduce inappropriate SUP in the ICUs that included the following: | |||
| Reminders on admission and discharge from ICU | |||
| Education and awareness initiative for prescribers | |||
| ICU and service champions | |||
| Culture change | |||
| Next step: Incorporate indications in CPOE and work with ICU to incorporate appropriate GI prophylaxis as part of the standard ICU care bundle. | |||
| Blood utilization stewardship | 30% of transfusions on the hospital medicine service are provided to patients with a hemoglobin >8 g/dL. | Decrease units of blood transfused for a hemoglobin >8.0 g/dL by 25%. | Launched an educational campaign for attending and resident physicians. |
| Monthly feedback to residents and attending physicians. | |||
| Next step: Introduce a decision support system in the CPOE for blood transfusion orders in patients with most recent hemoglobin level >8. | |||
| Improving telemetry utilization | 44% of monitored inpatients on the medical service (with length of stay >48 hours) remain on telemetry until discharge. | Decrease by 15% the number of patients (with length of stay >48 hours) who remain on telemetry until discharge. | Implemented an educational campaign for nursing groups and the medicine and cardiology housestaff. |
| Launched a messaging campaign consisting of posters and pocket cards on appropriate telemetry use. | |||
| Designed a feedback campaign with monthly e‐mail to housestaff on their ward team's telemetry use stats. | |||
| Next step: Build a CPOE intervention that asks users to specify an approved indication for telemetry when they order monitoring. The indication then dictates how long the order is active (24, 48, 72 hours or ongoing), and the MD must renew the order after the elapsed time. | |||
| iReduce iCal: ordering ionized calcium only when needed | The medicine service spent $167,000 in direct costs on iCal labs over a year (40% of all calcium lab orders; 42% occurred in non‐ICU patients). | Reduce number of iCal labs drawn on the medicine service by >25% over the course of 6 months. | With the introduction of CPOE, iCal was removed from traditional daily lab order sets. |
| Discussed with lab, renal, and ICU stakeholders. | |||
| Implemented an educational campaign for physicians and nurses. | |||
| Created local publicity: posters and candies. | |||
| Provided data feedback to providers. | |||
| Repeat inpatient echocardiograms | 25% of TTEs are performed within 6 months of a prior; one‐third of these are for inappropriate indications. | Decrease inappropriate repeat TTEs by 25%. | Implemented an educational campaign. |
| Next step: provide the most recent TTE results in the CPOE at time of order, and provide auditing and decision support for repeat TTEs. | |||
The HVC program also provided an arena for collaborating with and supporting value‐based projects launched by other groups, such as the UCSF Medication Outcomes Center's inappropriate gastric stress ulcer prophylaxis program.[21] Our group helped support the development and implementation of evidence‐based clinical practice guidelines, and we assisted educational interventions targeting clinicians. This program resulted in a decrease in inappropriate stress ulcer prophylaxis in intensive care unit patients from 19% to 6.6% within 1 month following implementation.[21]
DISCUSSION
Physicians are increasingly being asked to embrace and lead efforts to improve healthcare value and reduce costs. Our program provides a framework to guide physician‐led initiatives to identify and address areas of healthcare waste.
Challenges and Lessons Learned
Overcoming the Hurdle of More Care as Better Care
Improving the quality of care has traditionally stressed the underuse of beneficial testing and treatments, for example the use of angiotensin‐converting enzyme inhibitors in systolic heart failure. We found that improving quality by curbing overuse was a new idea for many physicians. Traditionally, physicians have struggled with cost reduction programs, feeling that efforts to reduce costs are indifferent to quality of care, and worse, may actually lead to inferior care.[22] The historical separation of most QI and cost reduction programs has likely furthered this sentiment. Our first projects married cost reduction and QI efforts by demonstrating how reducing overuse could provide an opportunity to increase quality and reduce harms from treatments. For example, transitioning from nebs to metered‐dose inhalers offered the chance to provide inpatient inhaler teaching, whereas decreasing proton pump inhibitor use can reduce the incidence of C difficile. By framing these projects as addressing both numerator and denominator of the value equation, we were able to align our cost‐reduction efforts with physicians' traditional notions of QI.
Cost Transparency
If physicians are to play a larger role in cost‐reduction efforts, they need at least a working understanding of fixed and variable costs in healthcare and of institutional prices.[23, 24] Utilization and clear information about costs were used to guide our interventions and ensured that the efforts spent to eliminate waste would result in cost savings. As an example, we learned that decreasing nebulizer use without a corresponding decrease in daily RT staffing would lead to minimal cost savings. These analyses require the support of business, financial, and resource managers in addition to physicians, nurses, project coordinators, and administrators. At many institutions the lack of price and utilization transparency presents a major barrier to the accurate analysis of cost‐reduction efforts.
The Diplomacy of Cost‐Reduction
Because the bulk of healthcare costs go to labor, efforts to reduce cost may lead to reductions in the resources available to certain departments or even to individuals' wages. For example, initiatives aimed at reducing inappropriate diagnostic imaging will affect the radiology department, which is partially paid based on the volume of studies performed.[25] Key stakeholders must be identified early, and project leaders should seek understanding, engagement, and buy‐in from involved parties prior to implementation. There will often be times that support from senior leaders will be needed to negotiate these tricky situations.
Although we benefited from a largely supportive hospital medicine faculty and resident physicians, not all of our proposed projects made it to implementation. Sometimes stakeholder recruitment proved to be difficult. For instance, a proposed project to change the protocol from routine to clinically indicated peripheral intravenous catheter replacement for adult inpatients was met with some resistance by some members of nursing management. We reviewed the literature together and discussed in length the proposal, but ultimately decided that our institution was not ready for this change at this time.
Limitations and Next Steps
Our goal is to provide guidance on exporting the approach of our HVC program to other institutions, but there may be several limitations. First, our strategy relied on several contributing factors that may be unique to our institution. We had engaged frontline physician champions, who may not be available or have the necessary support at other academic or community organizations. Our UCSF cost awareness curriculum provided an educational foundation and framework for our projects. We also had institutional commitment in the form of our medical center division administrator.
Second, there are up‐front costs to running our committee, which are primarily related to personnel funding as described in the Methods. Over the next year we aim to calculate cost‐effectiveness ratios for our projects and overall return on investment for each of our projects, as we have done for the Nebs No More After 24 project (see Supporting Information, Table 1, in the online version of this article). Based on this analysis, the modest upfront costs appear to be easily recouped over the course of the year.
We have anecdotally noted a culture change in the way that our physicians discuss and consider testing. For example, it is common now to hear ward teams on morning rounds consider the costs of testing or discuss the need for prophylactic proton pump inhibitors. An important next step for our HVC program is the building of better data infrastructures for our own electronic health record system to allow us to more quickly, accurately, and comprehensively identify new targets and monitor the progress and sustainability of our projects. The Institute of Medicine has noted that the adoption of technology is a key strategy to creating a continuously learning healthcare system.[1] It is our hope that through consistent audit and feedback of resource utilization we can translate our early gains into sustainable changes in practice.
Furthermore, we hope to target and enact additional organizational changes, including creating CPOE‐linked interventions to help reinforce and further our objectives. We believe that creating systems that make it easier to do the right thing will help the cause of embedding HVC practices throughout our medical center. We have begun to scale some of our projects, such as the Nebs No More After 24 campaign, medical center wide, and ultimately we hope to disseminate successful projects and models beyond our medical center to contribute to the national movement to provide the best care at lower costs.
As discussed above, our interventions are targeted at simultaneous improvements in quality with decreased costs. However, the goal is not to hide our cost interventions behind the banner of quality. We believe that there is a shifting culture that is increasingly ready to accept cost alone as a meaningful patient harm, worthy of interventions on its own merits, assuming that quality and safety remain stable.[26, 27]
CONCLUSIONS
Our HVC program has been successful in promoting improved healthcare value and engaging clinicians in this effort. The program is guided by the use of financial data to identify areas with clear evidence of waste in the hospital, the creation of evidence‐based interventions that improve quality of care while cutting costs, and the pairing of interventions with evidence‐based cost awareness education to drive culture change.
Acknowledgements
The authors acknowledge the following members of the UCSF Division of Hospital Medicine High‐Value Care Committee who have led some of the initiatives mentioned in this article and have directly contributed to Table 1: Dr. Stephanie Rennke, Dr. Alvin Rajkomar, Dr. Nader Najafi, Dr. Steven Ludwin, and Dr. Elizabeth Stewart. Dr. Russ Cucina particularly contributed to the designs and implementation of electronic medical record interventions.
Disclosures: Dr. Moriates received funding from the UCSF Center for Healthcare Value, the Agency for Healthcare Research and Quality (as editor for AHRQ Patient Safety Net), and the ABIM Foundation. Mrs. Novelero received funding from the UCSF Center for Healthcare Value. Dr. Wachter reports serving as the immediate past‐chair of the American Board of Internal Medicine (for which he received a stipend) and is a current member of the ABIM Foundation board; receiving a contract to UCSF from the Agency for Healthcare Research and Quality for editing 2 patient‐safety websites; receiving compensation from John Wiley & Sons for writing a blog; receiving compensation from QuantiaMD for editing and presenting patient safety educational modules; receiving royalties from Lippincott Williams & Wilkins and McGraw‐Hill for writing/editing several books; receiving a stipend and stock/options for serving on the Board of Directors of IPC‐The Hospitalist Company; serving on the scientific advisory boards for PatientSafe Solutions, CRISI, SmartDose, and EarlySense (for which he receives stock options); and holding the Benioff endowed chair in hospital medicine from Marc and Lynne Benioff. He is also a member of the Board of Directors of Salem Hospital, Salem, Oregon, for which he receives travel reimbursement but no compensation. Mr. John Hillman, Mr. Aseem Bharti, and Ms. Claudia Hermann from UCSF Decision Support Services provided financial data support and analyses, and the UCSF Center for Healthcare Value provided resource and financial support.
- Institute of Medicine. Committee on the Learning Health Care System in America. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press; 2012.
- , . Value‐based purchasing—national programs to move from volume to value. N Engl J Med. 2012;367(4):292–295.
- . Making good on ACOs' promise—the final rule for the Medicare Shared Savings Program. N Engl J Med. 2011;365(19):1753–1756.
- . American College of Physicians ethics manual: sixth edition. Ann Intern Med. 2012;156(1 pt 2):73–104.
- ABIM Foundation, American College of Physicians‐American Society of Internal Medicine, European Federation of Internal Medicine. Medical professionalism in the new millennium: a physician charter. Ann Intern Med. 2002;136(3):243–246.
- , . Choosing Wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307(17):1801.
- , , , . High‐value, cost‐conscious health care: concepts for clinicians to evaluate the benefits, harms, and costs of medical interventions. Ann Intern Med. 2011;154(3):174–180.
- , . Waste not, want not: promoting efficient use of health care resources. Ann Intern Med. 2013;158(1):67–68.
- , , . General pediatric attending physicians' and residents' knowledge of inpatient hospital finances. Pediatrics. 2013;131(6):1072–1080.
- , , . Hospitalists' awareness of patient charges associated with inpatient care. J Hosp Med. 2010;5(5):295–297.
- , , , , . Teaching residents to provide cost‐conscious care: A national survey of residency program directors. JAMA Intern Med. 2014;174(3):470–472.
- , , . Perceived, actual, and desired knowledge regarding medicare billing and reimbursement. J Gen Intern Med. 2006;21(5):466–470.
- , , , , . “Nebs No More After 24”: a pilot program to improve the use of appropriate respiratory therapies. JAMA Intern Med. 2013;173(17):1647–1648.
- , , , . Acid‐suppressive medication use and the risk for hospital‐acquired pneumonia. JAMA. 2009;301(20):2120–2128.
- , , , et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010;170(9):784–790.
- , , , . The value in the evidence: teaching residents to “choose wisely.” JAMA Intern Med.2013;173(4):308–310.
- , . Evidence‐based quality improvement: the state of the science. Health Aff. 2005;24(1):138–150.
- , , , . The role of physician engagement on the impact of the hospital‐based practice improvement module (PIM). J Hosp Med. 2009;4(8):466–470.
- , . Finding common cause in quality: confronting the physician engagement challenge. Physician Exec. 2008;34(2):26–28, 30–31.
- , . Engaging physicians and leveraging professionalism: a key to success for quality measurement and improvement. JAMA. 2012;308(10):979–980.
- , , et al. The development and implementation of a bundled quality improvement initiative to reduce inappropriate stress ulcer prophylaxis. ICU Dir. 2013;4(6):322–325.
- . Lost in translation: physicians' struggle with cost‐reduction programs. Ann Intern Med. 2011;154(6):430–433.
- , . How to solve the cost crisis in health care. Harv Bus Rev. 2011;89(9):46–52, 54, 56–61 passim.
- , , , . The savings illusion—why clinical quality improvement fails to deliver bottom‐line results. N Engl J Med. 2011;365(26):e48.
- , , , , . Reducing radiology use on an inpatient medical service: choosing wisely. Arch Intern Med. 2012;172(20):1606–1608.
- , , . First, do no (financial) harm. JAMA. 2013;310(6):577–578.
- , , . Full disclosure—out‐of‐pocket costs as side effects. N Engl J Med. 2013;369(16):1484–1486.
With a United States medical system that spends as much as $750 billion each year on care that does not result in improved health outcomes,[1] many policy initiatives, including the Centers for Medicare and Medicaid Services' Value‐Based Purchasing program, seek to realign hospitals' financial incentives from a focus on production to one on value (quality divided by cost).[2, 3] Professional organizations have now deemed resource stewardship an ethical responsibility for professionalism,[4, 5] and campaigns such as the American Board of Internal Medicine (ABIM) Foundation's Choosing Wisely effort and the American College of Physicians' High‐Value Care platform are calling on frontline clinicians to address unnecessary and wasteful services.[6, 7]
Despite these pressures and initiatives, most physicians lack the knowledge and tools necessary to prioritize the delivery of their own healthcare services according to value.[8, 9, 10] Hospital medicine physicians are unaware of the costs associated with the interventions they order,[10] and the majority of medical training programs lack curricula focused on healthcare costs,[11] creating a large gap between physicians' perceived, desired, and actual knowledge related to costs.[12] Novel frameworks and frontline physician engagement are required if clinicians are to improve the value of the care they deliver.
We describe 1 of our first steps at the University of California, San Francisco (UCSF) to promote high‐value care (HVC) delivery: the creation of a HVC program led by clinicians and administrators focused on identifying and addressing wasteful practices within our hospitalist group. The program aims to (1) use financial and clinical data to identify areas with clear evidence of waste in the hospital, (2) promote evidence‐based interventions that improve both quality of care and value, and (3) pair interventions with evidence‐based cost awareness education to drive culture change. Our experience and inaugural projects provide a model of the key features, inherent challenges, and lessons learned, which may help inform similar efforts.
METHODS
In March 2012, we launched an HVC program within our Division of Hospital Medicine at UCSF Medical Center, a 600‐bed academic medical center in an urban setting. During the 2013 academic year, our division included 45 physicians. The medicine service, comprised of 8 teaching medical ward teams (1 attending, 1 resident, 2 interns, and variable number of medical students), and 1 nonteaching medical ward team (1 attending), admitted 4700 patients that year.
Organizational Framework
The HVC program is co‐led by a UCSF hospitalist (C.M.) and the administrator of the Division of Hospital Medicine (M.N.). Team members include hospitalists, hospital medicine fellows, resident physicians, pharmacists, project coordinators, and other administrators. The team meets in person for 1 hour every month. Project teams and ad hoc subcommittee groups often convene between meetings.
Our HVC program was placed within the infrastructure, and under the leadership, of our already established quality improvement (QI) program at UCSF. Our Division of Hospital Medicine Director of Quality and Safety (M.M.) thus oversees the QI, patient safety, patient experience, and high‐value care efforts.
The HVC program funding is largely in personnel costs. The physician leader (15% effort) is funded by the Division of Hospital Medicine, whereas the administrator is cofunded by both the division and by the medical center (largely through her roles as both division administrator and service line director). An administrative assistant within the division is also assigned to help with administrative tasks. Some additional data gathering and project support comes from existing medical center QI infrastructure, the decision support services unit, and through UCSF's new Center for Healthcare Value. Other ancillary costs for our projects have included publicity, data analytics, and information technology infrastructure. We estimate that the costs of this program are approximately $50,000 to $75,000 annually.
Framework for Identifying Target Projects
Robust Analysis of Costs
We created a framework for identifying, designing, and promoting projects specifically aimed at improving healthcare value (Figure 1). Financial data were used to identify areas with clear evidence of waste in the hospital, areas of high cost with no benefit in health outcomes. We focused particularly on obtaining cost and billing data for our medical service, which provided important insight into potential targets for improvements in value. For example, in 2011, the Division of Hospital Medicine spent more than $1 million annually in direct costs for the administration of nebulized bronchodilator therapies (nebs) to nonintensive care unit patients on the medical service.[13] These high costs, exposed by billing data, were believed to represent potential unnecessary testing and/or procedures. Not every area of high cost was deemed a target for intervention. For example, the use of recombinant factor VIII appeared a necessary expenditure (over $1 million per year) for our patients with hemophilia. Although our efforts focused on reducing waste, it is worth noting that healthcare value can also be increased by improving the delivery of high‐value services.

Recognized Benefits in Quality of Care
The program also evaluated the impact of cost reduction efforts on the quality of care, based on a high standard of current evidence. Though value can be improved by interventions that decrease costs while being quality neutral, our group chose to focus first on projects that would simultaneously improve quality while decreasing costs. We felt that this win‐win strategy would help obtain buy‐in from clinicians weary of prior cost‐cutting programs. For example, we pursued interventions aimed at reducing inappropriate gastric stress ulcer prophylaxis, which had the potential to both cut costs and minimize risks of hospital‐acquired pneumonia and Clostridium difficile infections.[14, 15] All proposed HVC targets were vetted through a review of the literature and published guidelines. In general, our initial projects had to be strongly supported by evidence, with high‐quality studies, preferably meta‐analyses or systematic reviews, that displayed the safety of our recommended changes. We reviewed the literature with experts. For example, we met with faculty pulmonologists to discuss the evidence supporting the use of inhalers instead of nebulizers in adults with obstructive pulmonary disease. The goals of our projects were chosen by our HVC committee, based on an analysis of our baseline data and the perceived potential effects of our proposed interventions.
Educational Intervention
Last, we paired interventions with evidence‐based cost awareness education to drive culture change. At UCSF we have an ongoing longitudinal cost‐awareness curriculum for residents, which has previously been described.[16] We took advantage of this educational forum to address gaps in clinician knowledge related to the targeted areas. When launching the initiative to decrease unnecessary inpatient nebulizer usage and improve transitions to inhalers, we utilized the chronic obstructive pulmonary disease case in the cost‐awareness series. Doing so allowed us to both review the evidence behind the effectiveness of inhalers, and introduce our Nebs No More After 24 campaign, which sought to transition adult inpatients with obstructive pulmonary symptoms from nebs to inhalers within 24 hours of admission.[13]
Intervention Strategy
Our general approach has been to design and implement multifaceted interventions, adapted from previous QI literature (Figure 1).[17] Given the importance of frontline clinician engagement to successful project implementation,[18, 19, 20] our interventions are physician‐driven and are vetted by a large group of clinicians prior to launch. The HVC program also explicitly seeks stakeholder input, perspective, and buy‐in prior to implementation. For example, we involved respiratory therapists (RTs) in the design of the Nebs No More After 24 project, thus ensuring that the interventions fit within their workflow and align with their care‐delivery goals.
Local publicity campaigns provide education and reminders for clinicians. Posters, such as the Nebs No More After 24 poster (Figure 2), were hung in physician, nursing, and RT work areas. Pens featuring the catchphrase Nebs No More After 24 were distributed to clinicians.

In addition to presentations to residents through the UCSF cost awareness curriculum, educational presentations were also delivered to attending physicians and to other allied members of the healthcare team (eg, nurses, RTs) during regularly scheduled staff meetings.
The metrics for each of the projects were regularly monitored, and targeted feedback was provided to clinicians. For the Nebs No More After 24 campaign, data for the number of nebs delivered on the target floor were provided to resident physicians during the cost awareness conference each month, and the data were presented to attending hospitalists in the monthly QI newsletter. This academic year, transfusion and telemetry data are presented via the same strategy.
Stakeholder recruitment, education, and promotional campaigns are important to program launches, but to sustain projects over the long‐term, system changes may be necessary. We have pursued changes in the computerized provider order entry (CPOE) system, such as removing nebs from the admission order set or putting a default duration for certain telemetry orders. Systems‐level interventions, although more difficult to achieve, play an important role in creating enduring changes when paired with educational interventions.
RESULTS
During our first 2 years we have initiated ongoing projects directed at 6 major targets (Table 1). Our flagship project, Nebs No More After 24, resulted in a decrease of nebulizer rates by more than 50% on a high‐acuity medical floor, as previously published.[13] We created a financial model that primarily accounted for RT time and pharmaceutical costs, and estimated a savings of approximately $250,000 annually on this single medical ward (see Supporting Information, Table 1, in the online version of this article).[13]
| High‐Value Care Projects | Relevant Baseline Data | Goals of Project | Strategies |
|---|---|---|---|
| |||
| Nebs No More After 24: Improving appropriate use of respiratory services | The medicine service spent $1 million in direct costs on approximately 25,000 nebs for non‐ICU inpatients. | Reduce unnecessary nebs >15% over 9 months. | Removed nebs from admit order set. |
| Improve transitions from nebs to MDIs. | Enlisted RTs and RNs to help with MDI teaching for patients. | ||
| Improve patient self‐administration of MDIs. | Implemented an educational program for medicine physicians. | ||
| Created local publicity: posters, flyers, and pens. | |||
| Provided data feedback to providers. | |||
| Next step: Introduce a CPOE‐linked intervention. | |||
| Improving use of stress ulcer prophylaxis | 77% of ICU patients on acid suppressive therapy; 31% of these patients did not meet criteria for appropriate prophylaxis. | Reduce overuse and inappropriate use of SUP. | A team of pharmacists, nurses, and physicians developed targeted and evidence‐based UCSF guidelines on use of SUP. |
| Developed and implemented a pharmacist‐led intervention to reduce inappropriate SUP in the ICUs that included the following: | |||
| Reminders on admission and discharge from ICU | |||
| Education and awareness initiative for prescribers | |||
| ICU and service champions | |||
| Culture change | |||
| Next step: Incorporate indications in CPOE and work with ICU to incorporate appropriate GI prophylaxis as part of the standard ICU care bundle. | |||
| Blood utilization stewardship | 30% of transfusions on the hospital medicine service are provided to patients with a hemoglobin >8 g/dL. | Decrease units of blood transfused for a hemoglobin >8.0 g/dL by 25%. | Launched an educational campaign for attending and resident physicians. |
| Monthly feedback to residents and attending physicians. | |||
| Next step: Introduce a decision support system in the CPOE for blood transfusion orders in patients with most recent hemoglobin level >8. | |||
| Improving telemetry utilization | 44% of monitored inpatients on the medical service (with length of stay >48 hours) remain on telemetry until discharge. | Decrease by 15% the number of patients (with length of stay >48 hours) who remain on telemetry until discharge. | Implemented an educational campaign for nursing groups and the medicine and cardiology housestaff. |
| Launched a messaging campaign consisting of posters and pocket cards on appropriate telemetry use. | |||
| Designed a feedback campaign with monthly e‐mail to housestaff on their ward team's telemetry use stats. | |||
| Next step: Build a CPOE intervention that asks users to specify an approved indication for telemetry when they order monitoring. The indication then dictates how long the order is active (24, 48, 72 hours or ongoing), and the MD must renew the order after the elapsed time. | |||
| iReduce iCal: ordering ionized calcium only when needed | The medicine service spent $167,000 in direct costs on iCal labs over a year (40% of all calcium lab orders; 42% occurred in non‐ICU patients). | Reduce number of iCal labs drawn on the medicine service by >25% over the course of 6 months. | With the introduction of CPOE, iCal was removed from traditional daily lab order sets. |
| Discussed with lab, renal, and ICU stakeholders. | |||
| Implemented an educational campaign for physicians and nurses. | |||
| Created local publicity: posters and candies. | |||
| Provided data feedback to providers. | |||
| Repeat inpatient echocardiograms | 25% of TTEs are performed within 6 months of a prior; one‐third of these are for inappropriate indications. | Decrease inappropriate repeat TTEs by 25%. | Implemented an educational campaign. |
| Next step: provide the most recent TTE results in the CPOE at time of order, and provide auditing and decision support for repeat TTEs. | |||
The HVC program also provided an arena for collaborating with and supporting value‐based projects launched by other groups, such as the UCSF Medication Outcomes Center's inappropriate gastric stress ulcer prophylaxis program.[21] Our group helped support the development and implementation of evidence‐based clinical practice guidelines, and we assisted educational interventions targeting clinicians. This program resulted in a decrease in inappropriate stress ulcer prophylaxis in intensive care unit patients from 19% to 6.6% within 1 month following implementation.[21]
DISCUSSION
Physicians are increasingly being asked to embrace and lead efforts to improve healthcare value and reduce costs. Our program provides a framework to guide physician‐led initiatives to identify and address areas of healthcare waste.
Challenges and Lessons Learned
Overcoming the Hurdle of More Care as Better Care
Improving the quality of care has traditionally stressed the underuse of beneficial testing and treatments, for example the use of angiotensin‐converting enzyme inhibitors in systolic heart failure. We found that improving quality by curbing overuse was a new idea for many physicians. Traditionally, physicians have struggled with cost reduction programs, feeling that efforts to reduce costs are indifferent to quality of care, and worse, may actually lead to inferior care.[22] The historical separation of most QI and cost reduction programs has likely furthered this sentiment. Our first projects married cost reduction and QI efforts by demonstrating how reducing overuse could provide an opportunity to increase quality and reduce harms from treatments. For example, transitioning from nebs to metered‐dose inhalers offered the chance to provide inpatient inhaler teaching, whereas decreasing proton pump inhibitor use can reduce the incidence of C difficile. By framing these projects as addressing both numerator and denominator of the value equation, we were able to align our cost‐reduction efforts with physicians' traditional notions of QI.
Cost Transparency
If physicians are to play a larger role in cost‐reduction efforts, they need at least a working understanding of fixed and variable costs in healthcare and of institutional prices.[23, 24] Utilization and clear information about costs were used to guide our interventions and ensured that the efforts spent to eliminate waste would result in cost savings. As an example, we learned that decreasing nebulizer use without a corresponding decrease in daily RT staffing would lead to minimal cost savings. These analyses require the support of business, financial, and resource managers in addition to physicians, nurses, project coordinators, and administrators. At many institutions the lack of price and utilization transparency presents a major barrier to the accurate analysis of cost‐reduction efforts.
The Diplomacy of Cost‐Reduction
Because the bulk of healthcare costs go to labor, efforts to reduce cost may lead to reductions in the resources available to certain departments or even to individuals' wages. For example, initiatives aimed at reducing inappropriate diagnostic imaging will affect the radiology department, which is partially paid based on the volume of studies performed.[25] Key stakeholders must be identified early, and project leaders should seek understanding, engagement, and buy‐in from involved parties prior to implementation. There will often be times that support from senior leaders will be needed to negotiate these tricky situations.
Although we benefited from a largely supportive hospital medicine faculty and resident physicians, not all of our proposed projects made it to implementation. Sometimes stakeholder recruitment proved to be difficult. For instance, a proposed project to change the protocol from routine to clinically indicated peripheral intravenous catheter replacement for adult inpatients was met with some resistance by some members of nursing management. We reviewed the literature together and discussed in length the proposal, but ultimately decided that our institution was not ready for this change at this time.
Limitations and Next Steps
Our goal is to provide guidance on exporting the approach of our HVC program to other institutions, but there may be several limitations. First, our strategy relied on several contributing factors that may be unique to our institution. We had engaged frontline physician champions, who may not be available or have the necessary support at other academic or community organizations. Our UCSF cost awareness curriculum provided an educational foundation and framework for our projects. We also had institutional commitment in the form of our medical center division administrator.
Second, there are up‐front costs to running our committee, which are primarily related to personnel funding as described in the Methods. Over the next year we aim to calculate cost‐effectiveness ratios for our projects and overall return on investment for each of our projects, as we have done for the Nebs No More After 24 project (see Supporting Information, Table 1, in the online version of this article). Based on this analysis, the modest upfront costs appear to be easily recouped over the course of the year.
We have anecdotally noted a culture change in the way that our physicians discuss and consider testing. For example, it is common now to hear ward teams on morning rounds consider the costs of testing or discuss the need for prophylactic proton pump inhibitors. An important next step for our HVC program is the building of better data infrastructures for our own electronic health record system to allow us to more quickly, accurately, and comprehensively identify new targets and monitor the progress and sustainability of our projects. The Institute of Medicine has noted that the adoption of technology is a key strategy to creating a continuously learning healthcare system.[1] It is our hope that through consistent audit and feedback of resource utilization we can translate our early gains into sustainable changes in practice.
Furthermore, we hope to target and enact additional organizational changes, including creating CPOE‐linked interventions to help reinforce and further our objectives. We believe that creating systems that make it easier to do the right thing will help the cause of embedding HVC practices throughout our medical center. We have begun to scale some of our projects, such as the Nebs No More After 24 campaign, medical center wide, and ultimately we hope to disseminate successful projects and models beyond our medical center to contribute to the national movement to provide the best care at lower costs.
As discussed above, our interventions are targeted at simultaneous improvements in quality with decreased costs. However, the goal is not to hide our cost interventions behind the banner of quality. We believe that there is a shifting culture that is increasingly ready to accept cost alone as a meaningful patient harm, worthy of interventions on its own merits, assuming that quality and safety remain stable.[26, 27]
CONCLUSIONS
Our HVC program has been successful in promoting improved healthcare value and engaging clinicians in this effort. The program is guided by the use of financial data to identify areas with clear evidence of waste in the hospital, the creation of evidence‐based interventions that improve quality of care while cutting costs, and the pairing of interventions with evidence‐based cost awareness education to drive culture change.
Acknowledgements
The authors acknowledge the following members of the UCSF Division of Hospital Medicine High‐Value Care Committee who have led some of the initiatives mentioned in this article and have directly contributed to Table 1: Dr. Stephanie Rennke, Dr. Alvin Rajkomar, Dr. Nader Najafi, Dr. Steven Ludwin, and Dr. Elizabeth Stewart. Dr. Russ Cucina particularly contributed to the designs and implementation of electronic medical record interventions.
Disclosures: Dr. Moriates received funding from the UCSF Center for Healthcare Value, the Agency for Healthcare Research and Quality (as editor for AHRQ Patient Safety Net), and the ABIM Foundation. Mrs. Novelero received funding from the UCSF Center for Healthcare Value. Dr. Wachter reports serving as the immediate past‐chair of the American Board of Internal Medicine (for which he received a stipend) and is a current member of the ABIM Foundation board; receiving a contract to UCSF from the Agency for Healthcare Research and Quality for editing 2 patient‐safety websites; receiving compensation from John Wiley & Sons for writing a blog; receiving compensation from QuantiaMD for editing and presenting patient safety educational modules; receiving royalties from Lippincott Williams & Wilkins and McGraw‐Hill for writing/editing several books; receiving a stipend and stock/options for serving on the Board of Directors of IPC‐The Hospitalist Company; serving on the scientific advisory boards for PatientSafe Solutions, CRISI, SmartDose, and EarlySense (for which he receives stock options); and holding the Benioff endowed chair in hospital medicine from Marc and Lynne Benioff. He is also a member of the Board of Directors of Salem Hospital, Salem, Oregon, for which he receives travel reimbursement but no compensation. Mr. John Hillman, Mr. Aseem Bharti, and Ms. Claudia Hermann from UCSF Decision Support Services provided financial data support and analyses, and the UCSF Center for Healthcare Value provided resource and financial support.
With a United States medical system that spends as much as $750 billion each year on care that does not result in improved health outcomes,[1] many policy initiatives, including the Centers for Medicare and Medicaid Services' Value‐Based Purchasing program, seek to realign hospitals' financial incentives from a focus on production to one on value (quality divided by cost).[2, 3] Professional organizations have now deemed resource stewardship an ethical responsibility for professionalism,[4, 5] and campaigns such as the American Board of Internal Medicine (ABIM) Foundation's Choosing Wisely effort and the American College of Physicians' High‐Value Care platform are calling on frontline clinicians to address unnecessary and wasteful services.[6, 7]
Despite these pressures and initiatives, most physicians lack the knowledge and tools necessary to prioritize the delivery of their own healthcare services according to value.[8, 9, 10] Hospital medicine physicians are unaware of the costs associated with the interventions they order,[10] and the majority of medical training programs lack curricula focused on healthcare costs,[11] creating a large gap between physicians' perceived, desired, and actual knowledge related to costs.[12] Novel frameworks and frontline physician engagement are required if clinicians are to improve the value of the care they deliver.
We describe 1 of our first steps at the University of California, San Francisco (UCSF) to promote high‐value care (HVC) delivery: the creation of a HVC program led by clinicians and administrators focused on identifying and addressing wasteful practices within our hospitalist group. The program aims to (1) use financial and clinical data to identify areas with clear evidence of waste in the hospital, (2) promote evidence‐based interventions that improve both quality of care and value, and (3) pair interventions with evidence‐based cost awareness education to drive culture change. Our experience and inaugural projects provide a model of the key features, inherent challenges, and lessons learned, which may help inform similar efforts.
METHODS
In March 2012, we launched an HVC program within our Division of Hospital Medicine at UCSF Medical Center, a 600‐bed academic medical center in an urban setting. During the 2013 academic year, our division included 45 physicians. The medicine service, comprised of 8 teaching medical ward teams (1 attending, 1 resident, 2 interns, and variable number of medical students), and 1 nonteaching medical ward team (1 attending), admitted 4700 patients that year.
Organizational Framework
The HVC program is co‐led by a UCSF hospitalist (C.M.) and the administrator of the Division of Hospital Medicine (M.N.). Team members include hospitalists, hospital medicine fellows, resident physicians, pharmacists, project coordinators, and other administrators. The team meets in person for 1 hour every month. Project teams and ad hoc subcommittee groups often convene between meetings.
Our HVC program was placed within the infrastructure, and under the leadership, of our already established quality improvement (QI) program at UCSF. Our Division of Hospital Medicine Director of Quality and Safety (M.M.) thus oversees the QI, patient safety, patient experience, and high‐value care efforts.
The HVC program funding is largely in personnel costs. The physician leader (15% effort) is funded by the Division of Hospital Medicine, whereas the administrator is cofunded by both the division and by the medical center (largely through her roles as both division administrator and service line director). An administrative assistant within the division is also assigned to help with administrative tasks. Some additional data gathering and project support comes from existing medical center QI infrastructure, the decision support services unit, and through UCSF's new Center for Healthcare Value. Other ancillary costs for our projects have included publicity, data analytics, and information technology infrastructure. We estimate that the costs of this program are approximately $50,000 to $75,000 annually.
Framework for Identifying Target Projects
Robust Analysis of Costs
We created a framework for identifying, designing, and promoting projects specifically aimed at improving healthcare value (Figure 1). Financial data were used to identify areas with clear evidence of waste in the hospital, areas of high cost with no benefit in health outcomes. We focused particularly on obtaining cost and billing data for our medical service, which provided important insight into potential targets for improvements in value. For example, in 2011, the Division of Hospital Medicine spent more than $1 million annually in direct costs for the administration of nebulized bronchodilator therapies (nebs) to nonintensive care unit patients on the medical service.[13] These high costs, exposed by billing data, were believed to represent potential unnecessary testing and/or procedures. Not every area of high cost was deemed a target for intervention. For example, the use of recombinant factor VIII appeared a necessary expenditure (over $1 million per year) for our patients with hemophilia. Although our efforts focused on reducing waste, it is worth noting that healthcare value can also be increased by improving the delivery of high‐value services.

Recognized Benefits in Quality of Care
The program also evaluated the impact of cost reduction efforts on the quality of care, based on a high standard of current evidence. Though value can be improved by interventions that decrease costs while being quality neutral, our group chose to focus first on projects that would simultaneously improve quality while decreasing costs. We felt that this win‐win strategy would help obtain buy‐in from clinicians weary of prior cost‐cutting programs. For example, we pursued interventions aimed at reducing inappropriate gastric stress ulcer prophylaxis, which had the potential to both cut costs and minimize risks of hospital‐acquired pneumonia and Clostridium difficile infections.[14, 15] All proposed HVC targets were vetted through a review of the literature and published guidelines. In general, our initial projects had to be strongly supported by evidence, with high‐quality studies, preferably meta‐analyses or systematic reviews, that displayed the safety of our recommended changes. We reviewed the literature with experts. For example, we met with faculty pulmonologists to discuss the evidence supporting the use of inhalers instead of nebulizers in adults with obstructive pulmonary disease. The goals of our projects were chosen by our HVC committee, based on an analysis of our baseline data and the perceived potential effects of our proposed interventions.
Educational Intervention
Last, we paired interventions with evidence‐based cost awareness education to drive culture change. At UCSF we have an ongoing longitudinal cost‐awareness curriculum for residents, which has previously been described.[16] We took advantage of this educational forum to address gaps in clinician knowledge related to the targeted areas. When launching the initiative to decrease unnecessary inpatient nebulizer usage and improve transitions to inhalers, we utilized the chronic obstructive pulmonary disease case in the cost‐awareness series. Doing so allowed us to both review the evidence behind the effectiveness of inhalers, and introduce our Nebs No More After 24 campaign, which sought to transition adult inpatients with obstructive pulmonary symptoms from nebs to inhalers within 24 hours of admission.[13]
Intervention Strategy
Our general approach has been to design and implement multifaceted interventions, adapted from previous QI literature (Figure 1).[17] Given the importance of frontline clinician engagement to successful project implementation,[18, 19, 20] our interventions are physician‐driven and are vetted by a large group of clinicians prior to launch. The HVC program also explicitly seeks stakeholder input, perspective, and buy‐in prior to implementation. For example, we involved respiratory therapists (RTs) in the design of the Nebs No More After 24 project, thus ensuring that the interventions fit within their workflow and align with their care‐delivery goals.
Local publicity campaigns provide education and reminders for clinicians. Posters, such as the Nebs No More After 24 poster (Figure 2), were hung in physician, nursing, and RT work areas. Pens featuring the catchphrase Nebs No More After 24 were distributed to clinicians.

In addition to presentations to residents through the UCSF cost awareness curriculum, educational presentations were also delivered to attending physicians and to other allied members of the healthcare team (eg, nurses, RTs) during regularly scheduled staff meetings.
The metrics for each of the projects were regularly monitored, and targeted feedback was provided to clinicians. For the Nebs No More After 24 campaign, data for the number of nebs delivered on the target floor were provided to resident physicians during the cost awareness conference each month, and the data were presented to attending hospitalists in the monthly QI newsletter. This academic year, transfusion and telemetry data are presented via the same strategy.
Stakeholder recruitment, education, and promotional campaigns are important to program launches, but to sustain projects over the long‐term, system changes may be necessary. We have pursued changes in the computerized provider order entry (CPOE) system, such as removing nebs from the admission order set or putting a default duration for certain telemetry orders. Systems‐level interventions, although more difficult to achieve, play an important role in creating enduring changes when paired with educational interventions.
RESULTS
During our first 2 years we have initiated ongoing projects directed at 6 major targets (Table 1). Our flagship project, Nebs No More After 24, resulted in a decrease of nebulizer rates by more than 50% on a high‐acuity medical floor, as previously published.[13] We created a financial model that primarily accounted for RT time and pharmaceutical costs, and estimated a savings of approximately $250,000 annually on this single medical ward (see Supporting Information, Table 1, in the online version of this article).[13]
| High‐Value Care Projects | Relevant Baseline Data | Goals of Project | Strategies |
|---|---|---|---|
| |||
| Nebs No More After 24: Improving appropriate use of respiratory services | The medicine service spent $1 million in direct costs on approximately 25,000 nebs for non‐ICU inpatients. | Reduce unnecessary nebs >15% over 9 months. | Removed nebs from admit order set. |
| Improve transitions from nebs to MDIs. | Enlisted RTs and RNs to help with MDI teaching for patients. | ||
| Improve patient self‐administration of MDIs. | Implemented an educational program for medicine physicians. | ||
| Created local publicity: posters, flyers, and pens. | |||
| Provided data feedback to providers. | |||
| Next step: Introduce a CPOE‐linked intervention. | |||
| Improving use of stress ulcer prophylaxis | 77% of ICU patients on acid suppressive therapy; 31% of these patients did not meet criteria for appropriate prophylaxis. | Reduce overuse and inappropriate use of SUP. | A team of pharmacists, nurses, and physicians developed targeted and evidence‐based UCSF guidelines on use of SUP. |
| Developed and implemented a pharmacist‐led intervention to reduce inappropriate SUP in the ICUs that included the following: | |||
| Reminders on admission and discharge from ICU | |||
| Education and awareness initiative for prescribers | |||
| ICU and service champions | |||
| Culture change | |||
| Next step: Incorporate indications in CPOE and work with ICU to incorporate appropriate GI prophylaxis as part of the standard ICU care bundle. | |||
| Blood utilization stewardship | 30% of transfusions on the hospital medicine service are provided to patients with a hemoglobin >8 g/dL. | Decrease units of blood transfused for a hemoglobin >8.0 g/dL by 25%. | Launched an educational campaign for attending and resident physicians. |
| Monthly feedback to residents and attending physicians. | |||
| Next step: Introduce a decision support system in the CPOE for blood transfusion orders in patients with most recent hemoglobin level >8. | |||
| Improving telemetry utilization | 44% of monitored inpatients on the medical service (with length of stay >48 hours) remain on telemetry until discharge. | Decrease by 15% the number of patients (with length of stay >48 hours) who remain on telemetry until discharge. | Implemented an educational campaign for nursing groups and the medicine and cardiology housestaff. |
| Launched a messaging campaign consisting of posters and pocket cards on appropriate telemetry use. | |||
| Designed a feedback campaign with monthly e‐mail to housestaff on their ward team's telemetry use stats. | |||
| Next step: Build a CPOE intervention that asks users to specify an approved indication for telemetry when they order monitoring. The indication then dictates how long the order is active (24, 48, 72 hours or ongoing), and the MD must renew the order after the elapsed time. | |||
| iReduce iCal: ordering ionized calcium only when needed | The medicine service spent $167,000 in direct costs on iCal labs over a year (40% of all calcium lab orders; 42% occurred in non‐ICU patients). | Reduce number of iCal labs drawn on the medicine service by >25% over the course of 6 months. | With the introduction of CPOE, iCal was removed from traditional daily lab order sets. |
| Discussed with lab, renal, and ICU stakeholders. | |||
| Implemented an educational campaign for physicians and nurses. | |||
| Created local publicity: posters and candies. | |||
| Provided data feedback to providers. | |||
| Repeat inpatient echocardiograms | 25% of TTEs are performed within 6 months of a prior; one‐third of these are for inappropriate indications. | Decrease inappropriate repeat TTEs by 25%. | Implemented an educational campaign. |
| Next step: provide the most recent TTE results in the CPOE at time of order, and provide auditing and decision support for repeat TTEs. | |||
The HVC program also provided an arena for collaborating with and supporting value‐based projects launched by other groups, such as the UCSF Medication Outcomes Center's inappropriate gastric stress ulcer prophylaxis program.[21] Our group helped support the development and implementation of evidence‐based clinical practice guidelines, and we assisted educational interventions targeting clinicians. This program resulted in a decrease in inappropriate stress ulcer prophylaxis in intensive care unit patients from 19% to 6.6% within 1 month following implementation.[21]
DISCUSSION
Physicians are increasingly being asked to embrace and lead efforts to improve healthcare value and reduce costs. Our program provides a framework to guide physician‐led initiatives to identify and address areas of healthcare waste.
Challenges and Lessons Learned
Overcoming the Hurdle of More Care as Better Care
Improving the quality of care has traditionally stressed the underuse of beneficial testing and treatments, for example the use of angiotensin‐converting enzyme inhibitors in systolic heart failure. We found that improving quality by curbing overuse was a new idea for many physicians. Traditionally, physicians have struggled with cost reduction programs, feeling that efforts to reduce costs are indifferent to quality of care, and worse, may actually lead to inferior care.[22] The historical separation of most QI and cost reduction programs has likely furthered this sentiment. Our first projects married cost reduction and QI efforts by demonstrating how reducing overuse could provide an opportunity to increase quality and reduce harms from treatments. For example, transitioning from nebs to metered‐dose inhalers offered the chance to provide inpatient inhaler teaching, whereas decreasing proton pump inhibitor use can reduce the incidence of C difficile. By framing these projects as addressing both numerator and denominator of the value equation, we were able to align our cost‐reduction efforts with physicians' traditional notions of QI.
Cost Transparency
If physicians are to play a larger role in cost‐reduction efforts, they need at least a working understanding of fixed and variable costs in healthcare and of institutional prices.[23, 24] Utilization and clear information about costs were used to guide our interventions and ensured that the efforts spent to eliminate waste would result in cost savings. As an example, we learned that decreasing nebulizer use without a corresponding decrease in daily RT staffing would lead to minimal cost savings. These analyses require the support of business, financial, and resource managers in addition to physicians, nurses, project coordinators, and administrators. At many institutions the lack of price and utilization transparency presents a major barrier to the accurate analysis of cost‐reduction efforts.
The Diplomacy of Cost‐Reduction
Because the bulk of healthcare costs go to labor, efforts to reduce cost may lead to reductions in the resources available to certain departments or even to individuals' wages. For example, initiatives aimed at reducing inappropriate diagnostic imaging will affect the radiology department, which is partially paid based on the volume of studies performed.[25] Key stakeholders must be identified early, and project leaders should seek understanding, engagement, and buy‐in from involved parties prior to implementation. There will often be times that support from senior leaders will be needed to negotiate these tricky situations.
Although we benefited from a largely supportive hospital medicine faculty and resident physicians, not all of our proposed projects made it to implementation. Sometimes stakeholder recruitment proved to be difficult. For instance, a proposed project to change the protocol from routine to clinically indicated peripheral intravenous catheter replacement for adult inpatients was met with some resistance by some members of nursing management. We reviewed the literature together and discussed in length the proposal, but ultimately decided that our institution was not ready for this change at this time.
Limitations and Next Steps
Our goal is to provide guidance on exporting the approach of our HVC program to other institutions, but there may be several limitations. First, our strategy relied on several contributing factors that may be unique to our institution. We had engaged frontline physician champions, who may not be available or have the necessary support at other academic or community organizations. Our UCSF cost awareness curriculum provided an educational foundation and framework for our projects. We also had institutional commitment in the form of our medical center division administrator.
Second, there are up‐front costs to running our committee, which are primarily related to personnel funding as described in the Methods. Over the next year we aim to calculate cost‐effectiveness ratios for our projects and overall return on investment for each of our projects, as we have done for the Nebs No More After 24 project (see Supporting Information, Table 1, in the online version of this article). Based on this analysis, the modest upfront costs appear to be easily recouped over the course of the year.
We have anecdotally noted a culture change in the way that our physicians discuss and consider testing. For example, it is common now to hear ward teams on morning rounds consider the costs of testing or discuss the need for prophylactic proton pump inhibitors. An important next step for our HVC program is the building of better data infrastructures for our own electronic health record system to allow us to more quickly, accurately, and comprehensively identify new targets and monitor the progress and sustainability of our projects. The Institute of Medicine has noted that the adoption of technology is a key strategy to creating a continuously learning healthcare system.[1] It is our hope that through consistent audit and feedback of resource utilization we can translate our early gains into sustainable changes in practice.
Furthermore, we hope to target and enact additional organizational changes, including creating CPOE‐linked interventions to help reinforce and further our objectives. We believe that creating systems that make it easier to do the right thing will help the cause of embedding HVC practices throughout our medical center. We have begun to scale some of our projects, such as the Nebs No More After 24 campaign, medical center wide, and ultimately we hope to disseminate successful projects and models beyond our medical center to contribute to the national movement to provide the best care at lower costs.
As discussed above, our interventions are targeted at simultaneous improvements in quality with decreased costs. However, the goal is not to hide our cost interventions behind the banner of quality. We believe that there is a shifting culture that is increasingly ready to accept cost alone as a meaningful patient harm, worthy of interventions on its own merits, assuming that quality and safety remain stable.[26, 27]
CONCLUSIONS
Our HVC program has been successful in promoting improved healthcare value and engaging clinicians in this effort. The program is guided by the use of financial data to identify areas with clear evidence of waste in the hospital, the creation of evidence‐based interventions that improve quality of care while cutting costs, and the pairing of interventions with evidence‐based cost awareness education to drive culture change.
Acknowledgements
The authors acknowledge the following members of the UCSF Division of Hospital Medicine High‐Value Care Committee who have led some of the initiatives mentioned in this article and have directly contributed to Table 1: Dr. Stephanie Rennke, Dr. Alvin Rajkomar, Dr. Nader Najafi, Dr. Steven Ludwin, and Dr. Elizabeth Stewart. Dr. Russ Cucina particularly contributed to the designs and implementation of electronic medical record interventions.
Disclosures: Dr. Moriates received funding from the UCSF Center for Healthcare Value, the Agency for Healthcare Research and Quality (as editor for AHRQ Patient Safety Net), and the ABIM Foundation. Mrs. Novelero received funding from the UCSF Center for Healthcare Value. Dr. Wachter reports serving as the immediate past‐chair of the American Board of Internal Medicine (for which he received a stipend) and is a current member of the ABIM Foundation board; receiving a contract to UCSF from the Agency for Healthcare Research and Quality for editing 2 patient‐safety websites; receiving compensation from John Wiley & Sons for writing a blog; receiving compensation from QuantiaMD for editing and presenting patient safety educational modules; receiving royalties from Lippincott Williams & Wilkins and McGraw‐Hill for writing/editing several books; receiving a stipend and stock/options for serving on the Board of Directors of IPC‐The Hospitalist Company; serving on the scientific advisory boards for PatientSafe Solutions, CRISI, SmartDose, and EarlySense (for which he receives stock options); and holding the Benioff endowed chair in hospital medicine from Marc and Lynne Benioff. He is also a member of the Board of Directors of Salem Hospital, Salem, Oregon, for which he receives travel reimbursement but no compensation. Mr. John Hillman, Mr. Aseem Bharti, and Ms. Claudia Hermann from UCSF Decision Support Services provided financial data support and analyses, and the UCSF Center for Healthcare Value provided resource and financial support.
- Institute of Medicine. Committee on the Learning Health Care System in America. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press; 2012.
- , . Value‐based purchasing—national programs to move from volume to value. N Engl J Med. 2012;367(4):292–295.
- . Making good on ACOs' promise—the final rule for the Medicare Shared Savings Program. N Engl J Med. 2011;365(19):1753–1756.
- . American College of Physicians ethics manual: sixth edition. Ann Intern Med. 2012;156(1 pt 2):73–104.
- ABIM Foundation, American College of Physicians‐American Society of Internal Medicine, European Federation of Internal Medicine. Medical professionalism in the new millennium: a physician charter. Ann Intern Med. 2002;136(3):243–246.
- , . Choosing Wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307(17):1801.
- , , , . High‐value, cost‐conscious health care: concepts for clinicians to evaluate the benefits, harms, and costs of medical interventions. Ann Intern Med. 2011;154(3):174–180.
- , . Waste not, want not: promoting efficient use of health care resources. Ann Intern Med. 2013;158(1):67–68.
- , , . General pediatric attending physicians' and residents' knowledge of inpatient hospital finances. Pediatrics. 2013;131(6):1072–1080.
- , , . Hospitalists' awareness of patient charges associated with inpatient care. J Hosp Med. 2010;5(5):295–297.
- , , , , . Teaching residents to provide cost‐conscious care: A national survey of residency program directors. JAMA Intern Med. 2014;174(3):470–472.
- , , . Perceived, actual, and desired knowledge regarding medicare billing and reimbursement. J Gen Intern Med. 2006;21(5):466–470.
- , , , , . “Nebs No More After 24”: a pilot program to improve the use of appropriate respiratory therapies. JAMA Intern Med. 2013;173(17):1647–1648.
- , , , . Acid‐suppressive medication use and the risk for hospital‐acquired pneumonia. JAMA. 2009;301(20):2120–2128.
- , , , et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010;170(9):784–790.
- , , , . The value in the evidence: teaching residents to “choose wisely.” JAMA Intern Med.2013;173(4):308–310.
- , . Evidence‐based quality improvement: the state of the science. Health Aff. 2005;24(1):138–150.
- , , , . The role of physician engagement on the impact of the hospital‐based practice improvement module (PIM). J Hosp Med. 2009;4(8):466–470.
- , . Finding common cause in quality: confronting the physician engagement challenge. Physician Exec. 2008;34(2):26–28, 30–31.
- , . Engaging physicians and leveraging professionalism: a key to success for quality measurement and improvement. JAMA. 2012;308(10):979–980.
- , , et al. The development and implementation of a bundled quality improvement initiative to reduce inappropriate stress ulcer prophylaxis. ICU Dir. 2013;4(6):322–325.
- . Lost in translation: physicians' struggle with cost‐reduction programs. Ann Intern Med. 2011;154(6):430–433.
- , . How to solve the cost crisis in health care. Harv Bus Rev. 2011;89(9):46–52, 54, 56–61 passim.
- , , , . The savings illusion—why clinical quality improvement fails to deliver bottom‐line results. N Engl J Med. 2011;365(26):e48.
- , , , , . Reducing radiology use on an inpatient medical service: choosing wisely. Arch Intern Med. 2012;172(20):1606–1608.
- , , . First, do no (financial) harm. JAMA. 2013;310(6):577–578.
- , , . Full disclosure—out‐of‐pocket costs as side effects. N Engl J Med. 2013;369(16):1484–1486.
- Institute of Medicine. Committee on the Learning Health Care System in America. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press; 2012.
- , . Value‐based purchasing—national programs to move from volume to value. N Engl J Med. 2012;367(4):292–295.
- . Making good on ACOs' promise—the final rule for the Medicare Shared Savings Program. N Engl J Med. 2011;365(19):1753–1756.
- . American College of Physicians ethics manual: sixth edition. Ann Intern Med. 2012;156(1 pt 2):73–104.
- ABIM Foundation, American College of Physicians‐American Society of Internal Medicine, European Federation of Internal Medicine. Medical professionalism in the new millennium: a physician charter. Ann Intern Med. 2002;136(3):243–246.
- , . Choosing Wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307(17):1801.
- , , , . High‐value, cost‐conscious health care: concepts for clinicians to evaluate the benefits, harms, and costs of medical interventions. Ann Intern Med. 2011;154(3):174–180.
- , . Waste not, want not: promoting efficient use of health care resources. Ann Intern Med. 2013;158(1):67–68.
- , , . General pediatric attending physicians' and residents' knowledge of inpatient hospital finances. Pediatrics. 2013;131(6):1072–1080.
- , , . Hospitalists' awareness of patient charges associated with inpatient care. J Hosp Med. 2010;5(5):295–297.
- , , , , . Teaching residents to provide cost‐conscious care: A national survey of residency program directors. JAMA Intern Med. 2014;174(3):470–472.
- , , . Perceived, actual, and desired knowledge regarding medicare billing and reimbursement. J Gen Intern Med. 2006;21(5):466–470.
- , , , , . “Nebs No More After 24”: a pilot program to improve the use of appropriate respiratory therapies. JAMA Intern Med. 2013;173(17):1647–1648.
- , , , . Acid‐suppressive medication use and the risk for hospital‐acquired pneumonia. JAMA. 2009;301(20):2120–2128.
- , , , et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010;170(9):784–790.
- , , , . The value in the evidence: teaching residents to “choose wisely.” JAMA Intern Med.2013;173(4):308–310.
- , . Evidence‐based quality improvement: the state of the science. Health Aff. 2005;24(1):138–150.
- , , , . The role of physician engagement on the impact of the hospital‐based practice improvement module (PIM). J Hosp Med. 2009;4(8):466–470.
- , . Finding common cause in quality: confronting the physician engagement challenge. Physician Exec. 2008;34(2):26–28, 30–31.
- , . Engaging physicians and leveraging professionalism: a key to success for quality measurement and improvement. JAMA. 2012;308(10):979–980.
- , , et al. The development and implementation of a bundled quality improvement initiative to reduce inappropriate stress ulcer prophylaxis. ICU Dir. 2013;4(6):322–325.
- . Lost in translation: physicians' struggle with cost‐reduction programs. Ann Intern Med. 2011;154(6):430–433.
- , . How to solve the cost crisis in health care. Harv Bus Rev. 2011;89(9):46–52, 54, 56–61 passim.
- , , , . The savings illusion—why clinical quality improvement fails to deliver bottom‐line results. N Engl J Med. 2011;365(26):e48.
- , , , , . Reducing radiology use on an inpatient medical service: choosing wisely. Arch Intern Med. 2012;172(20):1606–1608.
- , , . First, do no (financial) harm. JAMA. 2013;310(6):577–578.
- , , . Full disclosure—out‐of‐pocket costs as side effects. N Engl J Med. 2013;369(16):1484–1486.
Intimate partner violence: How you can help female survivors
Also known as “domestic violence” and “spouse abuse,” intimate partner violence (IPV) is now the term defined by the US Centers for Disease Control and Prevention to include physical violence, sexual violence, threats of physical or sexual violence, and psychological or emotional abuse by a current or former spouse, common-law spouse, nonmarital dating partner, or boyfriend or girlfriend of the same or opposite sex.1 Although IPV is often hidden or kept secret by those affected, it is a highly prevalent issue, especially in women. Knowing how to broach the subject and provide appropriate support in a caring and nonjudgmental manner are the keys to helping a woman move forward in her readiness and ability to improve her situation.
See related patient information
ONE IN THREE WOMEN EXPERIENCES IPV IN HER LIFE
As clinicians, we have all seen patients who have been affected by IPV—even if we did not realize it at the time. Indeed, 36% of women in the United States (approximately 42.4 million) have experienced rape, physical violence, or stalking by an intimate partner in their lifetime, and 6% (approximately 7 million) have experienced these forms of IPV within the past 12 months.2
ASSOCIATION WITH MURDER
From 30% to 70% of women who are murdered are killed by a current or former intimate partner.3,4 Of those killed by their partner, two-thirds had previously reported physical assault, and 83% had been threatened by the man who eventually killed them.4 In another study, 44% of IPV murder victims had presented to an emergency department within 2 years of their murder.5
PHYSICAL EFFECTS NOT ALWAYS APPARENT
Although 41% of women who experience IPV suffer physical injury from their attacks, only 28% of those who are injured seek medical care.6 Because injuries are often absent or no longer apparent when an IPV victim decides to get help, it is important to be aware of the clinical signs associated with IPV:
- Gastrointestinal disorders7
- Depression8
- Anxiety
- Chronic pain syndromes9
- Substance abuse
- Suicidal ideation.10
In women of childbearing age, IPV is associated with unintended pregnancy, sexually transmitted infections, condom non-use,11,12 inconsistent condom use,13 and fear of talking about condom use.11,12 Coerced sexual experiences (eg, sexual intercourse that was not wanted or consented to) are common, with 28% to 42% of college women reporting at least one such experience. In more than three quarters of women who have been sexually assaulted, the first experience occurred before age 25.14,15
One-quarter of women ages 16 to 29 have experienced reproductive coercion, which includes birth control sabotage or pregnancy coercion by the active male partner.16 Among women reporting birth control sabotage, 79% had also been victims of physical or sexual IPV.16
The cost of providing health care to women experiencing IPV is 1.4 to 2.5 times higher than that of the nonabused population. Studies have shown that female victims of both physical and nonphysical (eg, emotional or verbal) IPV are more likely to use emergency, mental health, and outpatient health care services. The economic toll of IPV, including health care and costs from lost productivity and premature death, ranges from $2.3 to $8.3 billion per year.17,18
ASK FEMALE PATIENTS ABOUT IPV
In the early 1990s, various medical organizations began issuing policy statements that endorsed screening for IPV.19–22 Since 1992, the Joint Commission on Accreditation of Healthcare Organizations has required hospitals and clinics to provide assistance to those experiencing IPV.23 Although the United States Preventive Services Task Force initially found insufficient evidence to support regular IPV screening in health care settings,24–27 the group reversed its position in 2012 after a review of more recent studies. The group now recommends that clinicians address IPV with all women of childbearing age.28
A Cochrane review found that IPV screening increased identification of IPV survivors.29 Female participants in many studies wanted clinicians to ask routinely about violence and to provide information on community and legal resources.30,31
How should we ask about IPV?
Although various sets of screening questions and tools are available, no one instrument is considered better than the others. However, women experiencing IPV have specific preferences regarding how they want clinicians to ask and talk about the topic. In one survey, women who had experienced IPV preferred that clinicians ask about it as part of the complete medical history, as long as it did not create “an atmosphere of interrogation.”32
The style in which a clinician asks about IPV may make a difference as well. In focus groups, immigrant Latina and Asian women who had experienced IPV stated that clinicians could facilitate open communication by initiating the discussion and exhibiting compassionate and supportive behavior during the visit.33 Being able to see the same clinician at each visit also enhanced clinician-patient communication.33
In a study of IPV screening in emergency room settings, most clinicians asked about IPV in a perfunctory, direct manner—generally some variant of, “Are you a victim of domestic violence?” In this study, patient IPV disclosure occurred more often when clinicians used an open-ended approach such as, “Tell me what happened,” or when clinicians probed for possible IPV (eg, “What do you think may be causing some of this stress?”).34
In a focus group, female IPV survivors described feeling stigmatized or invalidated when clinicians were condescending, judgmental, or dismissive.35 Nonjudgmental and supportive communication decreased the women’s sense of isolation and led to positive outcomes such as increased awareness of IPV as a problem, decreased isolation, and feeling that the clinician cared.35
When addressing IPV, clinicians should explain why they are asking about it because it allows the woman to understand the context of the inquiry and to feel more comfortable about disclosing IPV. If the query is a regular part of a general screening or history-taking, for example, they should frame the question to make that point apparent. For example, “Because we know that many women in the United States experience physical, sexual or emotional violence from their romantic partners, I like to ask all of my patients whether they have been hurt or have felt threatened or afraid in a current or past relationship.”
In situations in which clinicians are concerned about IPV with a particular patient, they should explicitly share their concerns and desire to help the patient. One IPV survivor offered this advice: “Just look at the patient like she is your friend. Call her by her name. For instance, say ‘Sally, is he hurting you? Are you having problems? If you need help, I have some [phone] numbers.’ Personalize the encounter.”
It is also important to address IPV in a manner that ensures the patient’s safety, confidentiality, and dignity. When having this type of sensitive conversation, the patient should ideally be clothed and alone—without others present, particularly her partner. Professional interpreters should be available to women who do not speak English. The clinician should maintain eye contact, smile to communicate friendliness, and use a supportive tone.36
Just asking may be an intervention
Qualitative studies have suggested that just the act of asking about IPV in a nonjudgmental and compassionate manner is helpful to women experiencing IPV.35,37 Doing so not only helps women recognize the abuse, but also begins to decrease their sense of isolation and increase their awareness of helpful resources. It also gives the patient a sense that the clinician cares about her situation.35 As a result, experts have begun to recommend that health clinicians view asking about IPV not merely as a screening tool, but as a potentially therapeutic intervention in and of itself.35,37,38
HOW TO HELP
What to do when a woman discloses IPV
Female survivors, advocates, and health care clinicians who care for abused women suggest responding to a positive disclosure of IPV by providing the following:
Validation. The IPV perpetrator will often attempt to justify the violence and abuse by shifting some of the blame or responsibility for the violence onto the victim. This “brainwashing” leads to self-blame and a diminished sense of self-worth. However, clinicians can help reverse this mindset by acknowledging the woman’s disclosure and emphasizing that she did not deserve the abuse or violence. An example of such a statement is, “I am so very sorry that you went through that with your partner. You definitely did not deserve that. No one should ever be hurt by or afraid of the people who are supposed to love them.” Providing validation helps women recognize that the violence was a problem they did not deserve.38,39
Support. Women who have experienced IPV appreciate feeling supported and cared for by their health care clinician. Even if the patient is not ready to take any definitive action regarding her relationship or situation, knowing that her clinician and the health care setting are resources and sources of support is both comforting and empowering.35,40 Clinicians can communicate this support by stating, “I want you to know that whatever happens and whatever you decide, we are here for you.”
Respect for autonomy. Women experiencing IPV best understand their own situation and its various complexities and so they know best what they can do, cannot do, or need to do. As such, clinicians must respect a woman’s autonomy and preserve her ability to express her own needs and desires and make her own decisions. Prescribing a plan of action or giving commands to IPV victims could further perpetuate their sense of disempowerment and lack of control over their life.
Information. Providing referral information or hotline numbers to community domestic violence programs is helpful. However, not all women feel safe or comfortable taking printed brochures or written information with them because their abusive partner may find it. Thus, clinicians should ask if the patient can take the information safely or offer to write the numbers down without labeling them if she is afraid. The National Domestic Violence hotline is a 24-hour toll-free resource that will help women locate and contact shelters and other support services in their own community. The number, 1-800-799-SAFE (7233), is easy to memorize and thus is an easy resource to pass along quickly and safely. Other national organizations such as Futures Without Violence (formerly known as the Family Violence Prevention Fund) and the National Coalition Against Violence also provide links to local resources (Table 1).
Safety planning. Discussing the need for a safety plan will help the patient prepare for future abusive episodes. Even women who report that they are no longer in an abusive relationship should be asked about their current safety needs and concerns because they often remain in contact with abusive partners even after a relationship has ended.
When discussing safety planning, clinicians should ask the woman if she is currently safe or if she needs shelter. If she intends to return to or is still in contact with her batterer, ask if she has a plan for what to do or how to escape if the violence occurs again. Advise the patient to:
- Hide money so that she can leave quickly
- Make copies of birth certificates, immunization records, Social Security Number, and other important documents and keep them hidden and accessible
- Make a spare car key
- Have a list of hotline numbers
- Develop a code with friends, family, and neighbors that will let them know she needs immediate help.
Studies show that discussing safety-promoting behaviors increases the number of them that are used by IPV victims.41 A detailed list that can be shared with patients is provided in the patient information page that accompanies this article.42 Examples of personalized safety plans are available from the National Center on Domestic and Sexual Violence at its website, www.ncdsv.org/images/NCDSV_DVSafetyPlan_updated2013.pdf.
Danger assessment. Several researchers have examined potential risk factors associated with increased risk of homicide.43 Table 2 lists some of the characteristics associated with an increased risk of homicide in IPV situations.42 From this work, a danger assessment tool and scoring system has been developed. This tool and training on how to use it are available for free at www.dangerassessment.org. Although there are currently no outcome data on the benefits or risks of using this instrument, its objective is to increase women’s awareness of their danger level and individualize their safety counseling.
Proper documentation. Victim advocates and lawyers working on behalf of IPV victims emphasize that documentation by a medical provider can help a woman with her legal case. This documentation should be clear, legible, and as detailed as possible. These details should include a patient’s own words set off by quotation marks, a description or body map illustrating associated injuries or physical signs corroborating the violence, and a description of the patient’s demeanor or signs of emotion. Clinicians should avoid legal terms such as “alleges” or “alleged perpetrator” and should either define or avoid abbreviations that may be considered ambiguous in a legal proceeding (eg, clinicians should write out the words “domestic violence” or “intimate partner violence” rather than using “DV” or “IPV”).44 Most states have passed laws that prevent insurance companies from discriminating against IPV victims; insurance companies can no longer deny women coverage for seeking care related to IPV.45
A nonthreatening physical examination. Women who have experienced physical or sexual violence may feel anxious or experience added trauma during certain portions of the physical examination. Women who have been raped or otherwise sexually abused may have difficulty tolerating a pelvic examination, for example. Others who have been choked or grabbed by the neck may experience distress during palpation of the thyroid or cervical lymph nodes. The oropharyngeal examination may be challenging for women who have experienced forced oral sex or who may have had objects such as the barrel of a gun forced into their mouth. Asking women who have experienced IPV what aspects of the physical examination they may find difficult and how this could be made easier allows the patient and clinician to work together to develop strategies for or alternatives to necessary examinations or testing.
Before starting an examination, clinicians should explain what will happen and then ask the patient for her permission before moving on to the next step. Doing so creates a sense of control in an otherwise challenging and potentially traumatizing examination.
Disclosure of mandatory IPV reporting. Many states require health care providers to report any injuries caused by a weapon or criminal act to police or other official institutions.45,46 Several of these states specify that this reporting should occur during an IPV evaluation.
Mandatory reporting of IPV to police is highly controversial.47–49 Opponents of these laws argue that they do not benefit victims because they do not respect autonomy, they violate patient-clinician confidentiality, and they violate informed consent.47 Female IPV victims indicate that they would be less likely to disclose IPV and seek help if they knew it had to be reported.50–53 In a survey of California physicians, 59% stated that they might not comply with reporting laws if the patient objected.54
In states with mandatory reporting laws, clinicians can advocate for their patients by disclosing whether IPV must be reported before beginning their examination. The Michigan Coalition Against Domestic Violence devised this sample statement:
“I do have to let you know that under state law, I am obligated to make a police report to (name police agency with jurisdiction over the facility), if I/we are treating someone for any injury sustained by means of violence (tailor this information to the specifics of your state law). So, if you would have any concerns about that, I would encourage you to speak with one of the domestic violence advocates/counselors at (DV agency), who are able to provide confidential services and help without being required to make a police report.”55
The Compendium of State Statutes and Polices on Domestic Violence and Health Care by Futures Without Violence is an excellent reference that describes current state laws and policies regarding domestic violence and includes descriptions of the specific characteristics of each state’s mandatory reporting laws and their implications for health care providers. A copy can be downloaded from www.futureswithout-violence.org/content/features/detail/1584/.
What not to do
Clinicians should not ignore or minimize the extent of IPV, make excuses for the batterer, or blame the woman.32,56,57 They also should not inadvertently blame or criticize the woman by asking her, “What did you do to deserve this?” or “Why don’t you just leave?”57
While leaving a violent relationship is an obvious solution, many women experiencing IPV are either unwilling or unable to do so. In fact, leaving the batterer can be just as dangerous as staying. In a North Carolina study, half of all women killed by their partner had divorced or broken up with the partner immediately before the murder.4
What to do when a woman does not disclose IPV
Women who have experienced IPV often deny the violence to others out of fear. So a denial of violence, even in highly suspicious cases, can be expected. Abused patients often want help but are confused and afraid to ask for it.58,59 Providing easy and anonymous access to IPV information and resources through posters, flyers, brochures, and booklets will allow any woman—regardless of disclosure—to obtain help.36
In one IPV trial, all women in a family planning clinic who were in the intervention group were given information about IPV and how it can affect sexual and reproductive health, whether or not they disclosed. Compared with women in the control group, women who received the intervention—both those who did and did not experience IPV—were 63% more likely than those in the control group to end a relationship because they perceived it to be unhealthy or unsafe. In this case, all women—not just those who were victims—benefited from receiving information about IPV.60
PROVIDERS’ SUPPORT MAKES A DIFFERENCE, IMPROVES OUTCOMES
Patients may seek help for and find safety from IPV in stages or steps, and it may take them several months or even years to do so.61–63 In this sense, then, IPV is an issue that is not likely to be “cured” or changed in a single medical visit. In addition, a single visit may not be associated with direct health and safety outcomes. However, a patient is more likely to make changes if she has supportive and informative interaction with a caring, nonjudgmental clinician who can increase her awareness of IPV and IPV resources and promote an improved sense of self-efficacy or perceived power.40
For example, in a study in which health clinicians expressed concern about potential IPV health effects and offered support services such as counseling, 67% of women used one of those resources at least once.40 In another study, women who talked to a health care provider about their abuse were almost four times more likely to use an IPV intervention (eg, advocacy, shelter, restraining order) than women who did not talk to their provider. Those who used an IPV intervention were 2.6 times more likely to leave their abusive relationship, and those who left reported improved physical health.64
Thus, health care providers can have a positive effect by addressing this complex and difficult issue. The power and influence of a provider’s kindness, empathy, and support cannot be overestimated in their ability to improve the safety and well-being of women dealing with IPV.
- Saltzman LE, Fanslow JL, McMahon PM, Shelley GA; National Center for Injury Prevention and Control; Centers for Disease Control and Prevention. Intimate partner violence surveillance: uniform definitions and recommended data elements, Version 1.0. Atlanta, GA; 1999. www.cdc.gov/ncipc/pub-res/ipv_surveillance/intimate%20partner%20violence.pdf. Accessed June 3, 2014.
- Black MC, Basile KC, Breiding MJ, et al; Center for Injury Prevention and Control; Centers for Disease Control and Prevention. The National Intimate Partner and Sexual Violence Survey (NISVS): 2010 Summary Report. Atlanta, GA. www.cdc.gov/violenceprevention/pdf/nisvs_executive_summary-a.pdf. Accessed June 3, 2014.
- Kellermann AL, Mercy JA. Men, women, and murder: gender-specific differences in rates of fatal violence and victimization. J Trauma 1992; 33:1–5.
- Moracco KE, Runywan CW, Butts JD. Femicide in North Carolina, 1991–1993: a statewide study of patterns and precursors. Homicide Studies 1998; 4:422–446.
- Wadman MC, Muelleman RL. Domestic violence homicides: ED use before victimization. Am J Emerg Med 1999; 17:689–691.
- Catalano SM; United States Bureau of Justice Statistics. Intimate partner violence in the United States: 2007. http://bjs.ojp.usdoj.gov/index.cfm?ty=pbdetail&iid=1000. Accessed February 19, 2014.
- Drossman DA, Talley NJ, Leserman J, Olden KW, Barreiro MA. Sexual and physical abuse and gastrointestinal illness. Review and recommendations. Ann Intern Med 1995; 123:782–794.
- Scholle SH, Rost KM, Golding JM. Physical abuse among depressed women. J Gen Intern Med 1998; 13:607–613.
- Walling MK, O’Hara MW, Reiter RC, Milburn AK, Lilly G, Vincent SD. Abuse history and chronic pain in women: II. A multivariate analysis of abuse and psychological morbidity. Obstet Gynecol 1994; 84:200–206.
- McCauley J, Kern DE, Kolodner K, Derogatis LR, Bass EB. Relation of low-severity violence to women’s health. J Gen Intern Med 1998; 13:687–691.
- Wingood GM, DiClemente RJ. The effects of an abusive primary partner on the condom use and sexual negotiation practices of African-American women. Am J Public Health 1997; 87:1016–1018.
- Sales JM, Salazar LF, Wingood GM, DiClemente RJ, Rose E, Crosby RA. The mediating role of partner communication skills on HIV/STD-associated risk behaviors in young African American females with a history of sexual violence. Arch Pediatr Adolesc Med 2008; 162:432–438.
- Davila YR, Brackley MH. Mexican and Mexican American women in a battered women’s shelter: barriers to condom negotiation for HIV/AIDS prevention. Issues Ment Health Nurs 1999; 20:333–355.
- Tjaden P, Thoennes N; National Institute of Justice; Centers for Disease Control and Prevention. Prevalence, incidence and consequences of violence against women: findings from the national violence against women survey. Washington, DC; 1998. https://www.ncjrs.gov/pdffiles/172837.pdf. Accessed June 3, 2014.
- Masho SW, Odor RK, Adera T. Sexual assault in Virginia: a population-based study. Womens Health Issues 2005; 15:157–166.
- Miller E, Decker MR, McCauley HL, et al. Pregnancy coercion, intimate partner violence and unintended pregnancy. Contraception 2010; 81:316–322.
- Bonomi AE, Anderson ML, Rivara FP, Thompson RS. Health care utilization and costs associated with physical and nonphysical-only intimate partner violence. Health Serv Res 2009; 44:1052–1067.
- Rivara FP, Anderson ML, Fishman P, et al. Healthcare utilization and costs for women with a history of intimate partner violence. Am J Prev Med 2007; 32:89–96.
- American Nurses Association. Position statement on physical violence against women. Washington, DC; 1991.
- Physicians and domestic violence. Ethical considerations. Council on Ethical and Judicial Affairs, American Medical Association. JAMA 1992; 267:3190–3193.
- The American College of Obstetricians and Gynecologists. Screening tools: domestic violence. http://www.acog.org/About_ACOG/ACOG_Departments/Violence_Against_Women/Screening_Tools__Domestic_Violence. Accessed June 3, 2014.
- Lee D, James L, Sawires P; The Family Violence Prevention Fund. Preventing domestic violence: clinical guidelines on routine screening. San Francisco, CA; 1999. http://new.vawnet.org/Assoc_Files_VAWnet/screpol.pdf. Accessed June 3, 2014.
- Joint Commission on Accreditation of Healthcare Organizations. Accreditation manual for hospitals. Chicago, IL; 1992.
- US Department of Health and Human Services. US Preventive Services Task Force. Guide to Clinical Preventive Services. 2nd ed. Washington, DC; 1996.
- Ramsay J, Richardson J, Carter YH, Davidson LL, Feder G. Should health professionals screen women for domestic violence? Systematic review. BMJ 2002; 325:314.
- Nelson HD, Nygren P, McInerney Y, Klein J; US Preventive Services Task Force. Screening women and elderly adults for family and intimate partner violence: a review of the evidence for the US Preventive Services Task Force. Ann Intern Med 2004; 140:387–396.
- Chamberlain L. The USPSTF recommendation on intimate partner violence: what we can learn from it and what we can do about it. Fam Viol Prev Health Pract 2005; 1:1–24.
- Moyer VA; US Preventive Services Task Force. Screening for intimate partner violence and abuse of elderly and vulnerable adults: US Preventive Services Task Force recommendation statement. Ann Intern Med 2013; 158:478–486.
- Taft A, O’Doherty L, Hegarty K, Ramsay J, Davidson L, Feder G. Screening women for intimate partner violence in healthcare settings. Cochrane Database Syst Rev 2013; 4:CD007007.
- McNutt LA, Carlson BE, Gagen D, Winterbauer N. Reproductive violence screening in primary care: perspectives and experiences of patients and battered women. J Am Med Womens Assoc 1999; 54:85–90.
- Friedman LS, Samet JH, Roberts MS, Hudlin M, Hans P. Inquiry about victimization experiences. A survey of patient p and physician practices. Arch Intern Med 1992; 152:1186–1190.
- Hamberger LK, Ambuel B, Marbella A, Donze J. Physician interaction with battered women: the women’s perspective. Arch Fam Med 1998; 7:575–582.
- Rodriguez MA, Bauer HM, Flores-Ortiz Y, Szkupinski-Quiroga S. Factors affecting patient-physician communication for abused Latina and Asian immigrant women. J Fam Pract 1998; 47:309–311.
- Rhodes KV, Frankel RM, Levinthal N, Prenoveau E, Bailey J, Levinson W. “You’re not a victim of domestic violence, are you?” Provider patient communication about domestic violence”. Ann Intern Med 2007; 147:620–627.
- Chang JC, Decker M, Moracco KE, Martin SL, Petersen R, Frasier PY. What happens when health care providers ask about intimate partner violence? A description of consequences from the perspectives of female survivors. J Am Med Womens Assoc 2003; 58:76–81.
- Chang JC, Decker MR, Moracco KE, Martin SL, Petersen R, Frasier PY. Asking about intimate partner violence: advice from female survivors to health care providers. Patient Educ Couns 2005; 59:141–147.
- Hathaway JE, Willis G, Zimmer B. Listening to survivors’ voices: addressing partner abuse in the health care setting. Violence Against Women 2002; 8:687–716.
- Gerbert B, Caspers N, Bronstone A, Moe J, Abercrombie P. A qualitative analysis of how physicians with expertise in domestic violence approach the identification of victims. Ann Intern Med 1999; 131:578–584.
- Gerbert B, Caspers N, Milliken N, Berlin M, Bronstone A, Moe J. Interventions that help victims of domestic violence. A qualitative analysis of physicians’ experiences. J Fam Pract 2000; 49:889–895.
- McCaw B, Bauer HM, Berman WH, Mooney L, Holmberg M, Hunkeler E. Women referred for on-site domestic violence services in a managed care organization. Women Health 2002; 35:23–40.
- McFarlane J, Malecha A, Gist J, et al. An intervention to increase safety behaviors of abused women: results of a randomized clinical trial. Nurs Res 2002; 51:347–354.
- Chang JC. Domestic violence. In:Bieber EJ, Sanfilippo JS, Horowitz IR, editors. Clinical Gynecology. Philadelphia, PA; Elsevier, Inc; 2006:79–89.
- Campbell JC, Webster D, Koziol-McLain J, et al. Risk factors for femicide in abusive relationships: results from a multisite case control study. Am J Public Health 2003; 93:1089–1097.
- Isaac NE, Enos V; National Institute of Justice. Documenting domestic violence: how health care providers can help victims. Research in Brief, 2001. https://www.ncjrs.gov/pdffiles1/nij/188564.pdf. Accessed June 3, 2014.
- Durborow N, Lizdas KC, O’Flaherty A, Marjavi A; Futures Without Violence. Compendium of state and US terrritory statutes and policies on domestic violence and health care, 2010. www.futureswithoutviolence.org/content/features/detail/1584/. Accessed June 3, 2014.
- Hyman A, Schillinger D, Lo B. Laws mandating reporting of domestic violence. Do they promote patient well-being? JAMA 1995; 273:1781–1787.
- Hyman A, Chez RA. Mandatory reporting of domestic violence by health care providers: a misguided approach. Womens Health Issues 1995; 5:208–213.
- Knight MA. Ethical debate: should doctors be more proactive as advocates for victims of violence? The police surgeon’s view: medical paternalism is unacceptable. BMJ 1995; 311:1620–1621.
- Hyman A; Futures Without Violence. Mandatory reporting of domestic violence by healthcare providers: a policy paper. San Francisco, CA; 1997. www.futureswithoutviolence.org/userfiles/file/HealthCare/mandatory_policypaper.pdf. Accessed June 3, 2014.
- Rodriguez MA, Craig AM, Mooney DR, Bauer HM. Patient attitudes about mandatory reporting of domestic violence. Implications for health care professionals. West J Med 1998; 169:337–341.
- Caralis PV, Musialowski R. Women’s experiences with domestic violence and their attitudes and expectations regarding medical care of abuse victims. South Med J 1997; 90:1075–1080.
- Sachs CJ, Koziol-McLain J, Glass N, Webster D, Campbell J. A population-based survey assessing support for mandatory domestic violence reporting by health care personnel. Women Health 2002; 35:121–133.
- Sullivan CM, Hagen LA. Survivors’ opinions about mandatory reporting of domestic violence and sexual assault by medical professionals. Affilia 2005; 20:1–16.
- Rodriguez MA, McLoughlin E, Bauer HM, Paredes V, Grumbach K. Mandatory reporting of intimate partner violence to police: views of physicians in California. Am J Public Health 1999; 89:575–578.
- Futures Without Violence. Mandatory reporting of domestic violence to law enforcement by health care providers: a guide for advocates working to respond to or amend reporting laws related to domestic violence. www.healthcaresaboutipv.org/wp-content/blogs.dir/3/files/2012/09/Mandatory_Reporting_of_DV_to_Law-Enforcement_by_HCP.pdf. Accessed June 3, 2014.
- Caralis PV, Musialowski R. Women’s experiences with domestic violence and their attitudes and expectations regarding medical care of abuse victims. South Med J 1997; 90:1075–1080.
- Gerbert B, Johnston K, Caspers N, Bleecker T, Woods A, Rosenbaum A. Experiences of battered women in health care settings: a qualitative study. Women Health 1996; 24:1–17.
- Chang JC, Cluss PA, Ranieri L, et al. Health care interventions for intimate partner violence: what women want. Womens Health Issues 2005; 15:21–30.
- Rodriguez MA, Quiroga SS, Bauer HM. Breaking the silence. Battered women’s perspectives on medical care. Arch Fam Med 1996; 5:153–158.
- Miller E, Decker MR, McCauley HL, et al. A family planning clinic partner violence intervention to reduce risk associated with reproductive coercion. Contraception 2011; 83:274–280.
- Landenburger K. A process of entrapment in and recovery from an abusive relationship. Issues Ment Health Nurs 1989; 10:209–227.
- Cluss PA, Chang JC, Hawker L, et al. The process of change for victims of intimate partner violence: support for a psychosocial readiness model. Womens Health Issues 2006; 16:262–274.
- Gerbert B, Abercrombie P, Caspers N, Love C, Bronstone A. How health care providers help battered women: the survivor’s perspective. Women Health 1999; 29:115–135.
- McCloskey LA, Lichter E, Williams C, Gerber M, Wittenberg E, Ganz M. Assessing intimate partner violence in health care settings leads to women’s receipt of interventions and improved health. Public Health Rep 2006; 121:435–444.
Also known as “domestic violence” and “spouse abuse,” intimate partner violence (IPV) is now the term defined by the US Centers for Disease Control and Prevention to include physical violence, sexual violence, threats of physical or sexual violence, and psychological or emotional abuse by a current or former spouse, common-law spouse, nonmarital dating partner, or boyfriend or girlfriend of the same or opposite sex.1 Although IPV is often hidden or kept secret by those affected, it is a highly prevalent issue, especially in women. Knowing how to broach the subject and provide appropriate support in a caring and nonjudgmental manner are the keys to helping a woman move forward in her readiness and ability to improve her situation.
See related patient information
ONE IN THREE WOMEN EXPERIENCES IPV IN HER LIFE
As clinicians, we have all seen patients who have been affected by IPV—even if we did not realize it at the time. Indeed, 36% of women in the United States (approximately 42.4 million) have experienced rape, physical violence, or stalking by an intimate partner in their lifetime, and 6% (approximately 7 million) have experienced these forms of IPV within the past 12 months.2
ASSOCIATION WITH MURDER
From 30% to 70% of women who are murdered are killed by a current or former intimate partner.3,4 Of those killed by their partner, two-thirds had previously reported physical assault, and 83% had been threatened by the man who eventually killed them.4 In another study, 44% of IPV murder victims had presented to an emergency department within 2 years of their murder.5
PHYSICAL EFFECTS NOT ALWAYS APPARENT
Although 41% of women who experience IPV suffer physical injury from their attacks, only 28% of those who are injured seek medical care.6 Because injuries are often absent or no longer apparent when an IPV victim decides to get help, it is important to be aware of the clinical signs associated with IPV:
- Gastrointestinal disorders7
- Depression8
- Anxiety
- Chronic pain syndromes9
- Substance abuse
- Suicidal ideation.10
In women of childbearing age, IPV is associated with unintended pregnancy, sexually transmitted infections, condom non-use,11,12 inconsistent condom use,13 and fear of talking about condom use.11,12 Coerced sexual experiences (eg, sexual intercourse that was not wanted or consented to) are common, with 28% to 42% of college women reporting at least one such experience. In more than three quarters of women who have been sexually assaulted, the first experience occurred before age 25.14,15
One-quarter of women ages 16 to 29 have experienced reproductive coercion, which includes birth control sabotage or pregnancy coercion by the active male partner.16 Among women reporting birth control sabotage, 79% had also been victims of physical or sexual IPV.16
The cost of providing health care to women experiencing IPV is 1.4 to 2.5 times higher than that of the nonabused population. Studies have shown that female victims of both physical and nonphysical (eg, emotional or verbal) IPV are more likely to use emergency, mental health, and outpatient health care services. The economic toll of IPV, including health care and costs from lost productivity and premature death, ranges from $2.3 to $8.3 billion per year.17,18
ASK FEMALE PATIENTS ABOUT IPV
In the early 1990s, various medical organizations began issuing policy statements that endorsed screening for IPV.19–22 Since 1992, the Joint Commission on Accreditation of Healthcare Organizations has required hospitals and clinics to provide assistance to those experiencing IPV.23 Although the United States Preventive Services Task Force initially found insufficient evidence to support regular IPV screening in health care settings,24–27 the group reversed its position in 2012 after a review of more recent studies. The group now recommends that clinicians address IPV with all women of childbearing age.28
A Cochrane review found that IPV screening increased identification of IPV survivors.29 Female participants in many studies wanted clinicians to ask routinely about violence and to provide information on community and legal resources.30,31
How should we ask about IPV?
Although various sets of screening questions and tools are available, no one instrument is considered better than the others. However, women experiencing IPV have specific preferences regarding how they want clinicians to ask and talk about the topic. In one survey, women who had experienced IPV preferred that clinicians ask about it as part of the complete medical history, as long as it did not create “an atmosphere of interrogation.”32
The style in which a clinician asks about IPV may make a difference as well. In focus groups, immigrant Latina and Asian women who had experienced IPV stated that clinicians could facilitate open communication by initiating the discussion and exhibiting compassionate and supportive behavior during the visit.33 Being able to see the same clinician at each visit also enhanced clinician-patient communication.33
In a study of IPV screening in emergency room settings, most clinicians asked about IPV in a perfunctory, direct manner—generally some variant of, “Are you a victim of domestic violence?” In this study, patient IPV disclosure occurred more often when clinicians used an open-ended approach such as, “Tell me what happened,” or when clinicians probed for possible IPV (eg, “What do you think may be causing some of this stress?”).34
In a focus group, female IPV survivors described feeling stigmatized or invalidated when clinicians were condescending, judgmental, or dismissive.35 Nonjudgmental and supportive communication decreased the women’s sense of isolation and led to positive outcomes such as increased awareness of IPV as a problem, decreased isolation, and feeling that the clinician cared.35
When addressing IPV, clinicians should explain why they are asking about it because it allows the woman to understand the context of the inquiry and to feel more comfortable about disclosing IPV. If the query is a regular part of a general screening or history-taking, for example, they should frame the question to make that point apparent. For example, “Because we know that many women in the United States experience physical, sexual or emotional violence from their romantic partners, I like to ask all of my patients whether they have been hurt or have felt threatened or afraid in a current or past relationship.”
In situations in which clinicians are concerned about IPV with a particular patient, they should explicitly share their concerns and desire to help the patient. One IPV survivor offered this advice: “Just look at the patient like she is your friend. Call her by her name. For instance, say ‘Sally, is he hurting you? Are you having problems? If you need help, I have some [phone] numbers.’ Personalize the encounter.”
It is also important to address IPV in a manner that ensures the patient’s safety, confidentiality, and dignity. When having this type of sensitive conversation, the patient should ideally be clothed and alone—without others present, particularly her partner. Professional interpreters should be available to women who do not speak English. The clinician should maintain eye contact, smile to communicate friendliness, and use a supportive tone.36
Just asking may be an intervention
Qualitative studies have suggested that just the act of asking about IPV in a nonjudgmental and compassionate manner is helpful to women experiencing IPV.35,37 Doing so not only helps women recognize the abuse, but also begins to decrease their sense of isolation and increase their awareness of helpful resources. It also gives the patient a sense that the clinician cares about her situation.35 As a result, experts have begun to recommend that health clinicians view asking about IPV not merely as a screening tool, but as a potentially therapeutic intervention in and of itself.35,37,38
HOW TO HELP
What to do when a woman discloses IPV
Female survivors, advocates, and health care clinicians who care for abused women suggest responding to a positive disclosure of IPV by providing the following:
Validation. The IPV perpetrator will often attempt to justify the violence and abuse by shifting some of the blame or responsibility for the violence onto the victim. This “brainwashing” leads to self-blame and a diminished sense of self-worth. However, clinicians can help reverse this mindset by acknowledging the woman’s disclosure and emphasizing that she did not deserve the abuse or violence. An example of such a statement is, “I am so very sorry that you went through that with your partner. You definitely did not deserve that. No one should ever be hurt by or afraid of the people who are supposed to love them.” Providing validation helps women recognize that the violence was a problem they did not deserve.38,39
Support. Women who have experienced IPV appreciate feeling supported and cared for by their health care clinician. Even if the patient is not ready to take any definitive action regarding her relationship or situation, knowing that her clinician and the health care setting are resources and sources of support is both comforting and empowering.35,40 Clinicians can communicate this support by stating, “I want you to know that whatever happens and whatever you decide, we are here for you.”
Respect for autonomy. Women experiencing IPV best understand their own situation and its various complexities and so they know best what they can do, cannot do, or need to do. As such, clinicians must respect a woman’s autonomy and preserve her ability to express her own needs and desires and make her own decisions. Prescribing a plan of action or giving commands to IPV victims could further perpetuate their sense of disempowerment and lack of control over their life.
Information. Providing referral information or hotline numbers to community domestic violence programs is helpful. However, not all women feel safe or comfortable taking printed brochures or written information with them because their abusive partner may find it. Thus, clinicians should ask if the patient can take the information safely or offer to write the numbers down without labeling them if she is afraid. The National Domestic Violence hotline is a 24-hour toll-free resource that will help women locate and contact shelters and other support services in their own community. The number, 1-800-799-SAFE (7233), is easy to memorize and thus is an easy resource to pass along quickly and safely. Other national organizations such as Futures Without Violence (formerly known as the Family Violence Prevention Fund) and the National Coalition Against Violence also provide links to local resources (Table 1).
Safety planning. Discussing the need for a safety plan will help the patient prepare for future abusive episodes. Even women who report that they are no longer in an abusive relationship should be asked about their current safety needs and concerns because they often remain in contact with abusive partners even after a relationship has ended.
When discussing safety planning, clinicians should ask the woman if she is currently safe or if she needs shelter. If she intends to return to or is still in contact with her batterer, ask if she has a plan for what to do or how to escape if the violence occurs again. Advise the patient to:
- Hide money so that she can leave quickly
- Make copies of birth certificates, immunization records, Social Security Number, and other important documents and keep them hidden and accessible
- Make a spare car key
- Have a list of hotline numbers
- Develop a code with friends, family, and neighbors that will let them know she needs immediate help.
Studies show that discussing safety-promoting behaviors increases the number of them that are used by IPV victims.41 A detailed list that can be shared with patients is provided in the patient information page that accompanies this article.42 Examples of personalized safety plans are available from the National Center on Domestic and Sexual Violence at its website, www.ncdsv.org/images/NCDSV_DVSafetyPlan_updated2013.pdf.
Danger assessment. Several researchers have examined potential risk factors associated with increased risk of homicide.43 Table 2 lists some of the characteristics associated with an increased risk of homicide in IPV situations.42 From this work, a danger assessment tool and scoring system has been developed. This tool and training on how to use it are available for free at www.dangerassessment.org. Although there are currently no outcome data on the benefits or risks of using this instrument, its objective is to increase women’s awareness of their danger level and individualize their safety counseling.
Proper documentation. Victim advocates and lawyers working on behalf of IPV victims emphasize that documentation by a medical provider can help a woman with her legal case. This documentation should be clear, legible, and as detailed as possible. These details should include a patient’s own words set off by quotation marks, a description or body map illustrating associated injuries or physical signs corroborating the violence, and a description of the patient’s demeanor or signs of emotion. Clinicians should avoid legal terms such as “alleges” or “alleged perpetrator” and should either define or avoid abbreviations that may be considered ambiguous in a legal proceeding (eg, clinicians should write out the words “domestic violence” or “intimate partner violence” rather than using “DV” or “IPV”).44 Most states have passed laws that prevent insurance companies from discriminating against IPV victims; insurance companies can no longer deny women coverage for seeking care related to IPV.45
A nonthreatening physical examination. Women who have experienced physical or sexual violence may feel anxious or experience added trauma during certain portions of the physical examination. Women who have been raped or otherwise sexually abused may have difficulty tolerating a pelvic examination, for example. Others who have been choked or grabbed by the neck may experience distress during palpation of the thyroid or cervical lymph nodes. The oropharyngeal examination may be challenging for women who have experienced forced oral sex or who may have had objects such as the barrel of a gun forced into their mouth. Asking women who have experienced IPV what aspects of the physical examination they may find difficult and how this could be made easier allows the patient and clinician to work together to develop strategies for or alternatives to necessary examinations or testing.
Before starting an examination, clinicians should explain what will happen and then ask the patient for her permission before moving on to the next step. Doing so creates a sense of control in an otherwise challenging and potentially traumatizing examination.
Disclosure of mandatory IPV reporting. Many states require health care providers to report any injuries caused by a weapon or criminal act to police or other official institutions.45,46 Several of these states specify that this reporting should occur during an IPV evaluation.
Mandatory reporting of IPV to police is highly controversial.47–49 Opponents of these laws argue that they do not benefit victims because they do not respect autonomy, they violate patient-clinician confidentiality, and they violate informed consent.47 Female IPV victims indicate that they would be less likely to disclose IPV and seek help if they knew it had to be reported.50–53 In a survey of California physicians, 59% stated that they might not comply with reporting laws if the patient objected.54
In states with mandatory reporting laws, clinicians can advocate for their patients by disclosing whether IPV must be reported before beginning their examination. The Michigan Coalition Against Domestic Violence devised this sample statement:
“I do have to let you know that under state law, I am obligated to make a police report to (name police agency with jurisdiction over the facility), if I/we are treating someone for any injury sustained by means of violence (tailor this information to the specifics of your state law). So, if you would have any concerns about that, I would encourage you to speak with one of the domestic violence advocates/counselors at (DV agency), who are able to provide confidential services and help without being required to make a police report.”55
The Compendium of State Statutes and Polices on Domestic Violence and Health Care by Futures Without Violence is an excellent reference that describes current state laws and policies regarding domestic violence and includes descriptions of the specific characteristics of each state’s mandatory reporting laws and their implications for health care providers. A copy can be downloaded from www.futureswithout-violence.org/content/features/detail/1584/.
What not to do
Clinicians should not ignore or minimize the extent of IPV, make excuses for the batterer, or blame the woman.32,56,57 They also should not inadvertently blame or criticize the woman by asking her, “What did you do to deserve this?” or “Why don’t you just leave?”57
While leaving a violent relationship is an obvious solution, many women experiencing IPV are either unwilling or unable to do so. In fact, leaving the batterer can be just as dangerous as staying. In a North Carolina study, half of all women killed by their partner had divorced or broken up with the partner immediately before the murder.4
What to do when a woman does not disclose IPV
Women who have experienced IPV often deny the violence to others out of fear. So a denial of violence, even in highly suspicious cases, can be expected. Abused patients often want help but are confused and afraid to ask for it.58,59 Providing easy and anonymous access to IPV information and resources through posters, flyers, brochures, and booklets will allow any woman—regardless of disclosure—to obtain help.36
In one IPV trial, all women in a family planning clinic who were in the intervention group were given information about IPV and how it can affect sexual and reproductive health, whether or not they disclosed. Compared with women in the control group, women who received the intervention—both those who did and did not experience IPV—were 63% more likely than those in the control group to end a relationship because they perceived it to be unhealthy or unsafe. In this case, all women—not just those who were victims—benefited from receiving information about IPV.60
PROVIDERS’ SUPPORT MAKES A DIFFERENCE, IMPROVES OUTCOMES
Patients may seek help for and find safety from IPV in stages or steps, and it may take them several months or even years to do so.61–63 In this sense, then, IPV is an issue that is not likely to be “cured” or changed in a single medical visit. In addition, a single visit may not be associated with direct health and safety outcomes. However, a patient is more likely to make changes if she has supportive and informative interaction with a caring, nonjudgmental clinician who can increase her awareness of IPV and IPV resources and promote an improved sense of self-efficacy or perceived power.40
For example, in a study in which health clinicians expressed concern about potential IPV health effects and offered support services such as counseling, 67% of women used one of those resources at least once.40 In another study, women who talked to a health care provider about their abuse were almost four times more likely to use an IPV intervention (eg, advocacy, shelter, restraining order) than women who did not talk to their provider. Those who used an IPV intervention were 2.6 times more likely to leave their abusive relationship, and those who left reported improved physical health.64
Thus, health care providers can have a positive effect by addressing this complex and difficult issue. The power and influence of a provider’s kindness, empathy, and support cannot be overestimated in their ability to improve the safety and well-being of women dealing with IPV.
Also known as “domestic violence” and “spouse abuse,” intimate partner violence (IPV) is now the term defined by the US Centers for Disease Control and Prevention to include physical violence, sexual violence, threats of physical or sexual violence, and psychological or emotional abuse by a current or former spouse, common-law spouse, nonmarital dating partner, or boyfriend or girlfriend of the same or opposite sex.1 Although IPV is often hidden or kept secret by those affected, it is a highly prevalent issue, especially in women. Knowing how to broach the subject and provide appropriate support in a caring and nonjudgmental manner are the keys to helping a woman move forward in her readiness and ability to improve her situation.
See related patient information
ONE IN THREE WOMEN EXPERIENCES IPV IN HER LIFE
As clinicians, we have all seen patients who have been affected by IPV—even if we did not realize it at the time. Indeed, 36% of women in the United States (approximately 42.4 million) have experienced rape, physical violence, or stalking by an intimate partner in their lifetime, and 6% (approximately 7 million) have experienced these forms of IPV within the past 12 months.2
ASSOCIATION WITH MURDER
From 30% to 70% of women who are murdered are killed by a current or former intimate partner.3,4 Of those killed by their partner, two-thirds had previously reported physical assault, and 83% had been threatened by the man who eventually killed them.4 In another study, 44% of IPV murder victims had presented to an emergency department within 2 years of their murder.5
PHYSICAL EFFECTS NOT ALWAYS APPARENT
Although 41% of women who experience IPV suffer physical injury from their attacks, only 28% of those who are injured seek medical care.6 Because injuries are often absent or no longer apparent when an IPV victim decides to get help, it is important to be aware of the clinical signs associated with IPV:
- Gastrointestinal disorders7
- Depression8
- Anxiety
- Chronic pain syndromes9
- Substance abuse
- Suicidal ideation.10
In women of childbearing age, IPV is associated with unintended pregnancy, sexually transmitted infections, condom non-use,11,12 inconsistent condom use,13 and fear of talking about condom use.11,12 Coerced sexual experiences (eg, sexual intercourse that was not wanted or consented to) are common, with 28% to 42% of college women reporting at least one such experience. In more than three quarters of women who have been sexually assaulted, the first experience occurred before age 25.14,15
One-quarter of women ages 16 to 29 have experienced reproductive coercion, which includes birth control sabotage or pregnancy coercion by the active male partner.16 Among women reporting birth control sabotage, 79% had also been victims of physical or sexual IPV.16
The cost of providing health care to women experiencing IPV is 1.4 to 2.5 times higher than that of the nonabused population. Studies have shown that female victims of both physical and nonphysical (eg, emotional or verbal) IPV are more likely to use emergency, mental health, and outpatient health care services. The economic toll of IPV, including health care and costs from lost productivity and premature death, ranges from $2.3 to $8.3 billion per year.17,18
ASK FEMALE PATIENTS ABOUT IPV
In the early 1990s, various medical organizations began issuing policy statements that endorsed screening for IPV.19–22 Since 1992, the Joint Commission on Accreditation of Healthcare Organizations has required hospitals and clinics to provide assistance to those experiencing IPV.23 Although the United States Preventive Services Task Force initially found insufficient evidence to support regular IPV screening in health care settings,24–27 the group reversed its position in 2012 after a review of more recent studies. The group now recommends that clinicians address IPV with all women of childbearing age.28
A Cochrane review found that IPV screening increased identification of IPV survivors.29 Female participants in many studies wanted clinicians to ask routinely about violence and to provide information on community and legal resources.30,31
How should we ask about IPV?
Although various sets of screening questions and tools are available, no one instrument is considered better than the others. However, women experiencing IPV have specific preferences regarding how they want clinicians to ask and talk about the topic. In one survey, women who had experienced IPV preferred that clinicians ask about it as part of the complete medical history, as long as it did not create “an atmosphere of interrogation.”32
The style in which a clinician asks about IPV may make a difference as well. In focus groups, immigrant Latina and Asian women who had experienced IPV stated that clinicians could facilitate open communication by initiating the discussion and exhibiting compassionate and supportive behavior during the visit.33 Being able to see the same clinician at each visit also enhanced clinician-patient communication.33
In a study of IPV screening in emergency room settings, most clinicians asked about IPV in a perfunctory, direct manner—generally some variant of, “Are you a victim of domestic violence?” In this study, patient IPV disclosure occurred more often when clinicians used an open-ended approach such as, “Tell me what happened,” or when clinicians probed for possible IPV (eg, “What do you think may be causing some of this stress?”).34
In a focus group, female IPV survivors described feeling stigmatized or invalidated when clinicians were condescending, judgmental, or dismissive.35 Nonjudgmental and supportive communication decreased the women’s sense of isolation and led to positive outcomes such as increased awareness of IPV as a problem, decreased isolation, and feeling that the clinician cared.35
When addressing IPV, clinicians should explain why they are asking about it because it allows the woman to understand the context of the inquiry and to feel more comfortable about disclosing IPV. If the query is a regular part of a general screening or history-taking, for example, they should frame the question to make that point apparent. For example, “Because we know that many women in the United States experience physical, sexual or emotional violence from their romantic partners, I like to ask all of my patients whether they have been hurt or have felt threatened or afraid in a current or past relationship.”
In situations in which clinicians are concerned about IPV with a particular patient, they should explicitly share their concerns and desire to help the patient. One IPV survivor offered this advice: “Just look at the patient like she is your friend. Call her by her name. For instance, say ‘Sally, is he hurting you? Are you having problems? If you need help, I have some [phone] numbers.’ Personalize the encounter.”
It is also important to address IPV in a manner that ensures the patient’s safety, confidentiality, and dignity. When having this type of sensitive conversation, the patient should ideally be clothed and alone—without others present, particularly her partner. Professional interpreters should be available to women who do not speak English. The clinician should maintain eye contact, smile to communicate friendliness, and use a supportive tone.36
Just asking may be an intervention
Qualitative studies have suggested that just the act of asking about IPV in a nonjudgmental and compassionate manner is helpful to women experiencing IPV.35,37 Doing so not only helps women recognize the abuse, but also begins to decrease their sense of isolation and increase their awareness of helpful resources. It also gives the patient a sense that the clinician cares about her situation.35 As a result, experts have begun to recommend that health clinicians view asking about IPV not merely as a screening tool, but as a potentially therapeutic intervention in and of itself.35,37,38
HOW TO HELP
What to do when a woman discloses IPV
Female survivors, advocates, and health care clinicians who care for abused women suggest responding to a positive disclosure of IPV by providing the following:
Validation. The IPV perpetrator will often attempt to justify the violence and abuse by shifting some of the blame or responsibility for the violence onto the victim. This “brainwashing” leads to self-blame and a diminished sense of self-worth. However, clinicians can help reverse this mindset by acknowledging the woman’s disclosure and emphasizing that she did not deserve the abuse or violence. An example of such a statement is, “I am so very sorry that you went through that with your partner. You definitely did not deserve that. No one should ever be hurt by or afraid of the people who are supposed to love them.” Providing validation helps women recognize that the violence was a problem they did not deserve.38,39
Support. Women who have experienced IPV appreciate feeling supported and cared for by their health care clinician. Even if the patient is not ready to take any definitive action regarding her relationship or situation, knowing that her clinician and the health care setting are resources and sources of support is both comforting and empowering.35,40 Clinicians can communicate this support by stating, “I want you to know that whatever happens and whatever you decide, we are here for you.”
Respect for autonomy. Women experiencing IPV best understand their own situation and its various complexities and so they know best what they can do, cannot do, or need to do. As such, clinicians must respect a woman’s autonomy and preserve her ability to express her own needs and desires and make her own decisions. Prescribing a plan of action or giving commands to IPV victims could further perpetuate their sense of disempowerment and lack of control over their life.
Information. Providing referral information or hotline numbers to community domestic violence programs is helpful. However, not all women feel safe or comfortable taking printed brochures or written information with them because their abusive partner may find it. Thus, clinicians should ask if the patient can take the information safely or offer to write the numbers down without labeling them if she is afraid. The National Domestic Violence hotline is a 24-hour toll-free resource that will help women locate and contact shelters and other support services in their own community. The number, 1-800-799-SAFE (7233), is easy to memorize and thus is an easy resource to pass along quickly and safely. Other national organizations such as Futures Without Violence (formerly known as the Family Violence Prevention Fund) and the National Coalition Against Violence also provide links to local resources (Table 1).
Safety planning. Discussing the need for a safety plan will help the patient prepare for future abusive episodes. Even women who report that they are no longer in an abusive relationship should be asked about their current safety needs and concerns because they often remain in contact with abusive partners even after a relationship has ended.
When discussing safety planning, clinicians should ask the woman if she is currently safe or if she needs shelter. If she intends to return to or is still in contact with her batterer, ask if she has a plan for what to do or how to escape if the violence occurs again. Advise the patient to:
- Hide money so that she can leave quickly
- Make copies of birth certificates, immunization records, Social Security Number, and other important documents and keep them hidden and accessible
- Make a spare car key
- Have a list of hotline numbers
- Develop a code with friends, family, and neighbors that will let them know she needs immediate help.
Studies show that discussing safety-promoting behaviors increases the number of them that are used by IPV victims.41 A detailed list that can be shared with patients is provided in the patient information page that accompanies this article.42 Examples of personalized safety plans are available from the National Center on Domestic and Sexual Violence at its website, www.ncdsv.org/images/NCDSV_DVSafetyPlan_updated2013.pdf.
Danger assessment. Several researchers have examined potential risk factors associated with increased risk of homicide.43 Table 2 lists some of the characteristics associated with an increased risk of homicide in IPV situations.42 From this work, a danger assessment tool and scoring system has been developed. This tool and training on how to use it are available for free at www.dangerassessment.org. Although there are currently no outcome data on the benefits or risks of using this instrument, its objective is to increase women’s awareness of their danger level and individualize their safety counseling.
Proper documentation. Victim advocates and lawyers working on behalf of IPV victims emphasize that documentation by a medical provider can help a woman with her legal case. This documentation should be clear, legible, and as detailed as possible. These details should include a patient’s own words set off by quotation marks, a description or body map illustrating associated injuries or physical signs corroborating the violence, and a description of the patient’s demeanor or signs of emotion. Clinicians should avoid legal terms such as “alleges” or “alleged perpetrator” and should either define or avoid abbreviations that may be considered ambiguous in a legal proceeding (eg, clinicians should write out the words “domestic violence” or “intimate partner violence” rather than using “DV” or “IPV”).44 Most states have passed laws that prevent insurance companies from discriminating against IPV victims; insurance companies can no longer deny women coverage for seeking care related to IPV.45
A nonthreatening physical examination. Women who have experienced physical or sexual violence may feel anxious or experience added trauma during certain portions of the physical examination. Women who have been raped or otherwise sexually abused may have difficulty tolerating a pelvic examination, for example. Others who have been choked or grabbed by the neck may experience distress during palpation of the thyroid or cervical lymph nodes. The oropharyngeal examination may be challenging for women who have experienced forced oral sex or who may have had objects such as the barrel of a gun forced into their mouth. Asking women who have experienced IPV what aspects of the physical examination they may find difficult and how this could be made easier allows the patient and clinician to work together to develop strategies for or alternatives to necessary examinations or testing.
Before starting an examination, clinicians should explain what will happen and then ask the patient for her permission before moving on to the next step. Doing so creates a sense of control in an otherwise challenging and potentially traumatizing examination.
Disclosure of mandatory IPV reporting. Many states require health care providers to report any injuries caused by a weapon or criminal act to police or other official institutions.45,46 Several of these states specify that this reporting should occur during an IPV evaluation.
Mandatory reporting of IPV to police is highly controversial.47–49 Opponents of these laws argue that they do not benefit victims because they do not respect autonomy, they violate patient-clinician confidentiality, and they violate informed consent.47 Female IPV victims indicate that they would be less likely to disclose IPV and seek help if they knew it had to be reported.50–53 In a survey of California physicians, 59% stated that they might not comply with reporting laws if the patient objected.54
In states with mandatory reporting laws, clinicians can advocate for their patients by disclosing whether IPV must be reported before beginning their examination. The Michigan Coalition Against Domestic Violence devised this sample statement:
“I do have to let you know that under state law, I am obligated to make a police report to (name police agency with jurisdiction over the facility), if I/we are treating someone for any injury sustained by means of violence (tailor this information to the specifics of your state law). So, if you would have any concerns about that, I would encourage you to speak with one of the domestic violence advocates/counselors at (DV agency), who are able to provide confidential services and help without being required to make a police report.”55
The Compendium of State Statutes and Polices on Domestic Violence and Health Care by Futures Without Violence is an excellent reference that describes current state laws and policies regarding domestic violence and includes descriptions of the specific characteristics of each state’s mandatory reporting laws and their implications for health care providers. A copy can be downloaded from www.futureswithout-violence.org/content/features/detail/1584/.
What not to do
Clinicians should not ignore or minimize the extent of IPV, make excuses for the batterer, or blame the woman.32,56,57 They also should not inadvertently blame or criticize the woman by asking her, “What did you do to deserve this?” or “Why don’t you just leave?”57
While leaving a violent relationship is an obvious solution, many women experiencing IPV are either unwilling or unable to do so. In fact, leaving the batterer can be just as dangerous as staying. In a North Carolina study, half of all women killed by their partner had divorced or broken up with the partner immediately before the murder.4
What to do when a woman does not disclose IPV
Women who have experienced IPV often deny the violence to others out of fear. So a denial of violence, even in highly suspicious cases, can be expected. Abused patients often want help but are confused and afraid to ask for it.58,59 Providing easy and anonymous access to IPV information and resources through posters, flyers, brochures, and booklets will allow any woman—regardless of disclosure—to obtain help.36
In one IPV trial, all women in a family planning clinic who were in the intervention group were given information about IPV and how it can affect sexual and reproductive health, whether or not they disclosed. Compared with women in the control group, women who received the intervention—both those who did and did not experience IPV—were 63% more likely than those in the control group to end a relationship because they perceived it to be unhealthy or unsafe. In this case, all women—not just those who were victims—benefited from receiving information about IPV.60
PROVIDERS’ SUPPORT MAKES A DIFFERENCE, IMPROVES OUTCOMES
Patients may seek help for and find safety from IPV in stages or steps, and it may take them several months or even years to do so.61–63 In this sense, then, IPV is an issue that is not likely to be “cured” or changed in a single medical visit. In addition, a single visit may not be associated with direct health and safety outcomes. However, a patient is more likely to make changes if she has supportive and informative interaction with a caring, nonjudgmental clinician who can increase her awareness of IPV and IPV resources and promote an improved sense of self-efficacy or perceived power.40
For example, in a study in which health clinicians expressed concern about potential IPV health effects and offered support services such as counseling, 67% of women used one of those resources at least once.40 In another study, women who talked to a health care provider about their abuse were almost four times more likely to use an IPV intervention (eg, advocacy, shelter, restraining order) than women who did not talk to their provider. Those who used an IPV intervention were 2.6 times more likely to leave their abusive relationship, and those who left reported improved physical health.64
Thus, health care providers can have a positive effect by addressing this complex and difficult issue. The power and influence of a provider’s kindness, empathy, and support cannot be overestimated in their ability to improve the safety and well-being of women dealing with IPV.
- Saltzman LE, Fanslow JL, McMahon PM, Shelley GA; National Center for Injury Prevention and Control; Centers for Disease Control and Prevention. Intimate partner violence surveillance: uniform definitions and recommended data elements, Version 1.0. Atlanta, GA; 1999. www.cdc.gov/ncipc/pub-res/ipv_surveillance/intimate%20partner%20violence.pdf. Accessed June 3, 2014.
- Black MC, Basile KC, Breiding MJ, et al; Center for Injury Prevention and Control; Centers for Disease Control and Prevention. The National Intimate Partner and Sexual Violence Survey (NISVS): 2010 Summary Report. Atlanta, GA. www.cdc.gov/violenceprevention/pdf/nisvs_executive_summary-a.pdf. Accessed June 3, 2014.
- Kellermann AL, Mercy JA. Men, women, and murder: gender-specific differences in rates of fatal violence and victimization. J Trauma 1992; 33:1–5.
- Moracco KE, Runywan CW, Butts JD. Femicide in North Carolina, 1991–1993: a statewide study of patterns and precursors. Homicide Studies 1998; 4:422–446.
- Wadman MC, Muelleman RL. Domestic violence homicides: ED use before victimization. Am J Emerg Med 1999; 17:689–691.
- Catalano SM; United States Bureau of Justice Statistics. Intimate partner violence in the United States: 2007. http://bjs.ojp.usdoj.gov/index.cfm?ty=pbdetail&iid=1000. Accessed February 19, 2014.
- Drossman DA, Talley NJ, Leserman J, Olden KW, Barreiro MA. Sexual and physical abuse and gastrointestinal illness. Review and recommendations. Ann Intern Med 1995; 123:782–794.
- Scholle SH, Rost KM, Golding JM. Physical abuse among depressed women. J Gen Intern Med 1998; 13:607–613.
- Walling MK, O’Hara MW, Reiter RC, Milburn AK, Lilly G, Vincent SD. Abuse history and chronic pain in women: II. A multivariate analysis of abuse and psychological morbidity. Obstet Gynecol 1994; 84:200–206.
- McCauley J, Kern DE, Kolodner K, Derogatis LR, Bass EB. Relation of low-severity violence to women’s health. J Gen Intern Med 1998; 13:687–691.
- Wingood GM, DiClemente RJ. The effects of an abusive primary partner on the condom use and sexual negotiation practices of African-American women. Am J Public Health 1997; 87:1016–1018.
- Sales JM, Salazar LF, Wingood GM, DiClemente RJ, Rose E, Crosby RA. The mediating role of partner communication skills on HIV/STD-associated risk behaviors in young African American females with a history of sexual violence. Arch Pediatr Adolesc Med 2008; 162:432–438.
- Davila YR, Brackley MH. Mexican and Mexican American women in a battered women’s shelter: barriers to condom negotiation for HIV/AIDS prevention. Issues Ment Health Nurs 1999; 20:333–355.
- Tjaden P, Thoennes N; National Institute of Justice; Centers for Disease Control and Prevention. Prevalence, incidence and consequences of violence against women: findings from the national violence against women survey. Washington, DC; 1998. https://www.ncjrs.gov/pdffiles/172837.pdf. Accessed June 3, 2014.
- Masho SW, Odor RK, Adera T. Sexual assault in Virginia: a population-based study. Womens Health Issues 2005; 15:157–166.
- Miller E, Decker MR, McCauley HL, et al. Pregnancy coercion, intimate partner violence and unintended pregnancy. Contraception 2010; 81:316–322.
- Bonomi AE, Anderson ML, Rivara FP, Thompson RS. Health care utilization and costs associated with physical and nonphysical-only intimate partner violence. Health Serv Res 2009; 44:1052–1067.
- Rivara FP, Anderson ML, Fishman P, et al. Healthcare utilization and costs for women with a history of intimate partner violence. Am J Prev Med 2007; 32:89–96.
- American Nurses Association. Position statement on physical violence against women. Washington, DC; 1991.
- Physicians and domestic violence. Ethical considerations. Council on Ethical and Judicial Affairs, American Medical Association. JAMA 1992; 267:3190–3193.
- The American College of Obstetricians and Gynecologists. Screening tools: domestic violence. http://www.acog.org/About_ACOG/ACOG_Departments/Violence_Against_Women/Screening_Tools__Domestic_Violence. Accessed June 3, 2014.
- Lee D, James L, Sawires P; The Family Violence Prevention Fund. Preventing domestic violence: clinical guidelines on routine screening. San Francisco, CA; 1999. http://new.vawnet.org/Assoc_Files_VAWnet/screpol.pdf. Accessed June 3, 2014.
- Joint Commission on Accreditation of Healthcare Organizations. Accreditation manual for hospitals. Chicago, IL; 1992.
- US Department of Health and Human Services. US Preventive Services Task Force. Guide to Clinical Preventive Services. 2nd ed. Washington, DC; 1996.
- Ramsay J, Richardson J, Carter YH, Davidson LL, Feder G. Should health professionals screen women for domestic violence? Systematic review. BMJ 2002; 325:314.
- Nelson HD, Nygren P, McInerney Y, Klein J; US Preventive Services Task Force. Screening women and elderly adults for family and intimate partner violence: a review of the evidence for the US Preventive Services Task Force. Ann Intern Med 2004; 140:387–396.
- Chamberlain L. The USPSTF recommendation on intimate partner violence: what we can learn from it and what we can do about it. Fam Viol Prev Health Pract 2005; 1:1–24.
- Moyer VA; US Preventive Services Task Force. Screening for intimate partner violence and abuse of elderly and vulnerable adults: US Preventive Services Task Force recommendation statement. Ann Intern Med 2013; 158:478–486.
- Taft A, O’Doherty L, Hegarty K, Ramsay J, Davidson L, Feder G. Screening women for intimate partner violence in healthcare settings. Cochrane Database Syst Rev 2013; 4:CD007007.
- McNutt LA, Carlson BE, Gagen D, Winterbauer N. Reproductive violence screening in primary care: perspectives and experiences of patients and battered women. J Am Med Womens Assoc 1999; 54:85–90.
- Friedman LS, Samet JH, Roberts MS, Hudlin M, Hans P. Inquiry about victimization experiences. A survey of patient p and physician practices. Arch Intern Med 1992; 152:1186–1190.
- Hamberger LK, Ambuel B, Marbella A, Donze J. Physician interaction with battered women: the women’s perspective. Arch Fam Med 1998; 7:575–582.
- Rodriguez MA, Bauer HM, Flores-Ortiz Y, Szkupinski-Quiroga S. Factors affecting patient-physician communication for abused Latina and Asian immigrant women. J Fam Pract 1998; 47:309–311.
- Rhodes KV, Frankel RM, Levinthal N, Prenoveau E, Bailey J, Levinson W. “You’re not a victim of domestic violence, are you?” Provider patient communication about domestic violence”. Ann Intern Med 2007; 147:620–627.
- Chang JC, Decker M, Moracco KE, Martin SL, Petersen R, Frasier PY. What happens when health care providers ask about intimate partner violence? A description of consequences from the perspectives of female survivors. J Am Med Womens Assoc 2003; 58:76–81.
- Chang JC, Decker MR, Moracco KE, Martin SL, Petersen R, Frasier PY. Asking about intimate partner violence: advice from female survivors to health care providers. Patient Educ Couns 2005; 59:141–147.
- Hathaway JE, Willis G, Zimmer B. Listening to survivors’ voices: addressing partner abuse in the health care setting. Violence Against Women 2002; 8:687–716.
- Gerbert B, Caspers N, Bronstone A, Moe J, Abercrombie P. A qualitative analysis of how physicians with expertise in domestic violence approach the identification of victims. Ann Intern Med 1999; 131:578–584.
- Gerbert B, Caspers N, Milliken N, Berlin M, Bronstone A, Moe J. Interventions that help victims of domestic violence. A qualitative analysis of physicians’ experiences. J Fam Pract 2000; 49:889–895.
- McCaw B, Bauer HM, Berman WH, Mooney L, Holmberg M, Hunkeler E. Women referred for on-site domestic violence services in a managed care organization. Women Health 2002; 35:23–40.
- McFarlane J, Malecha A, Gist J, et al. An intervention to increase safety behaviors of abused women: results of a randomized clinical trial. Nurs Res 2002; 51:347–354.
- Chang JC. Domestic violence. In:Bieber EJ, Sanfilippo JS, Horowitz IR, editors. Clinical Gynecology. Philadelphia, PA; Elsevier, Inc; 2006:79–89.
- Campbell JC, Webster D, Koziol-McLain J, et al. Risk factors for femicide in abusive relationships: results from a multisite case control study. Am J Public Health 2003; 93:1089–1097.
- Isaac NE, Enos V; National Institute of Justice. Documenting domestic violence: how health care providers can help victims. Research in Brief, 2001. https://www.ncjrs.gov/pdffiles1/nij/188564.pdf. Accessed June 3, 2014.
- Durborow N, Lizdas KC, O’Flaherty A, Marjavi A; Futures Without Violence. Compendium of state and US terrritory statutes and policies on domestic violence and health care, 2010. www.futureswithoutviolence.org/content/features/detail/1584/. Accessed June 3, 2014.
- Hyman A, Schillinger D, Lo B. Laws mandating reporting of domestic violence. Do they promote patient well-being? JAMA 1995; 273:1781–1787.
- Hyman A, Chez RA. Mandatory reporting of domestic violence by health care providers: a misguided approach. Womens Health Issues 1995; 5:208–213.
- Knight MA. Ethical debate: should doctors be more proactive as advocates for victims of violence? The police surgeon’s view: medical paternalism is unacceptable. BMJ 1995; 311:1620–1621.
- Hyman A; Futures Without Violence. Mandatory reporting of domestic violence by healthcare providers: a policy paper. San Francisco, CA; 1997. www.futureswithoutviolence.org/userfiles/file/HealthCare/mandatory_policypaper.pdf. Accessed June 3, 2014.
- Rodriguez MA, Craig AM, Mooney DR, Bauer HM. Patient attitudes about mandatory reporting of domestic violence. Implications for health care professionals. West J Med 1998; 169:337–341.
- Caralis PV, Musialowski R. Women’s experiences with domestic violence and their attitudes and expectations regarding medical care of abuse victims. South Med J 1997; 90:1075–1080.
- Sachs CJ, Koziol-McLain J, Glass N, Webster D, Campbell J. A population-based survey assessing support for mandatory domestic violence reporting by health care personnel. Women Health 2002; 35:121–133.
- Sullivan CM, Hagen LA. Survivors’ opinions about mandatory reporting of domestic violence and sexual assault by medical professionals. Affilia 2005; 20:1–16.
- Rodriguez MA, McLoughlin E, Bauer HM, Paredes V, Grumbach K. Mandatory reporting of intimate partner violence to police: views of physicians in California. Am J Public Health 1999; 89:575–578.
- Futures Without Violence. Mandatory reporting of domestic violence to law enforcement by health care providers: a guide for advocates working to respond to or amend reporting laws related to domestic violence. www.healthcaresaboutipv.org/wp-content/blogs.dir/3/files/2012/09/Mandatory_Reporting_of_DV_to_Law-Enforcement_by_HCP.pdf. Accessed June 3, 2014.
- Caralis PV, Musialowski R. Women’s experiences with domestic violence and their attitudes and expectations regarding medical care of abuse victims. South Med J 1997; 90:1075–1080.
- Gerbert B, Johnston K, Caspers N, Bleecker T, Woods A, Rosenbaum A. Experiences of battered women in health care settings: a qualitative study. Women Health 1996; 24:1–17.
- Chang JC, Cluss PA, Ranieri L, et al. Health care interventions for intimate partner violence: what women want. Womens Health Issues 2005; 15:21–30.
- Rodriguez MA, Quiroga SS, Bauer HM. Breaking the silence. Battered women’s perspectives on medical care. Arch Fam Med 1996; 5:153–158.
- Miller E, Decker MR, McCauley HL, et al. A family planning clinic partner violence intervention to reduce risk associated with reproductive coercion. Contraception 2011; 83:274–280.
- Landenburger K. A process of entrapment in and recovery from an abusive relationship. Issues Ment Health Nurs 1989; 10:209–227.
- Cluss PA, Chang JC, Hawker L, et al. The process of change for victims of intimate partner violence: support for a psychosocial readiness model. Womens Health Issues 2006; 16:262–274.
- Gerbert B, Abercrombie P, Caspers N, Love C, Bronstone A. How health care providers help battered women: the survivor’s perspective. Women Health 1999; 29:115–135.
- McCloskey LA, Lichter E, Williams C, Gerber M, Wittenberg E, Ganz M. Assessing intimate partner violence in health care settings leads to women’s receipt of interventions and improved health. Public Health Rep 2006; 121:435–444.
- Saltzman LE, Fanslow JL, McMahon PM, Shelley GA; National Center for Injury Prevention and Control; Centers for Disease Control and Prevention. Intimate partner violence surveillance: uniform definitions and recommended data elements, Version 1.0. Atlanta, GA; 1999. www.cdc.gov/ncipc/pub-res/ipv_surveillance/intimate%20partner%20violence.pdf. Accessed June 3, 2014.
- Black MC, Basile KC, Breiding MJ, et al; Center for Injury Prevention and Control; Centers for Disease Control and Prevention. The National Intimate Partner and Sexual Violence Survey (NISVS): 2010 Summary Report. Atlanta, GA. www.cdc.gov/violenceprevention/pdf/nisvs_executive_summary-a.pdf. Accessed June 3, 2014.
- Kellermann AL, Mercy JA. Men, women, and murder: gender-specific differences in rates of fatal violence and victimization. J Trauma 1992; 33:1–5.
- Moracco KE, Runywan CW, Butts JD. Femicide in North Carolina, 1991–1993: a statewide study of patterns and precursors. Homicide Studies 1998; 4:422–446.
- Wadman MC, Muelleman RL. Domestic violence homicides: ED use before victimization. Am J Emerg Med 1999; 17:689–691.
- Catalano SM; United States Bureau of Justice Statistics. Intimate partner violence in the United States: 2007. http://bjs.ojp.usdoj.gov/index.cfm?ty=pbdetail&iid=1000. Accessed February 19, 2014.
- Drossman DA, Talley NJ, Leserman J, Olden KW, Barreiro MA. Sexual and physical abuse and gastrointestinal illness. Review and recommendations. Ann Intern Med 1995; 123:782–794.
- Scholle SH, Rost KM, Golding JM. Physical abuse among depressed women. J Gen Intern Med 1998; 13:607–613.
- Walling MK, O’Hara MW, Reiter RC, Milburn AK, Lilly G, Vincent SD. Abuse history and chronic pain in women: II. A multivariate analysis of abuse and psychological morbidity. Obstet Gynecol 1994; 84:200–206.
- McCauley J, Kern DE, Kolodner K, Derogatis LR, Bass EB. Relation of low-severity violence to women’s health. J Gen Intern Med 1998; 13:687–691.
- Wingood GM, DiClemente RJ. The effects of an abusive primary partner on the condom use and sexual negotiation practices of African-American women. Am J Public Health 1997; 87:1016–1018.
- Sales JM, Salazar LF, Wingood GM, DiClemente RJ, Rose E, Crosby RA. The mediating role of partner communication skills on HIV/STD-associated risk behaviors in young African American females with a history of sexual violence. Arch Pediatr Adolesc Med 2008; 162:432–438.
- Davila YR, Brackley MH. Mexican and Mexican American women in a battered women’s shelter: barriers to condom negotiation for HIV/AIDS prevention. Issues Ment Health Nurs 1999; 20:333–355.
- Tjaden P, Thoennes N; National Institute of Justice; Centers for Disease Control and Prevention. Prevalence, incidence and consequences of violence against women: findings from the national violence against women survey. Washington, DC; 1998. https://www.ncjrs.gov/pdffiles/172837.pdf. Accessed June 3, 2014.
- Masho SW, Odor RK, Adera T. Sexual assault in Virginia: a population-based study. Womens Health Issues 2005; 15:157–166.
- Miller E, Decker MR, McCauley HL, et al. Pregnancy coercion, intimate partner violence and unintended pregnancy. Contraception 2010; 81:316–322.
- Bonomi AE, Anderson ML, Rivara FP, Thompson RS. Health care utilization and costs associated with physical and nonphysical-only intimate partner violence. Health Serv Res 2009; 44:1052–1067.
- Rivara FP, Anderson ML, Fishman P, et al. Healthcare utilization and costs for women with a history of intimate partner violence. Am J Prev Med 2007; 32:89–96.
- American Nurses Association. Position statement on physical violence against women. Washington, DC; 1991.
- Physicians and domestic violence. Ethical considerations. Council on Ethical and Judicial Affairs, American Medical Association. JAMA 1992; 267:3190–3193.
- The American College of Obstetricians and Gynecologists. Screening tools: domestic violence. http://www.acog.org/About_ACOG/ACOG_Departments/Violence_Against_Women/Screening_Tools__Domestic_Violence. Accessed June 3, 2014.
- Lee D, James L, Sawires P; The Family Violence Prevention Fund. Preventing domestic violence: clinical guidelines on routine screening. San Francisco, CA; 1999. http://new.vawnet.org/Assoc_Files_VAWnet/screpol.pdf. Accessed June 3, 2014.
- Joint Commission on Accreditation of Healthcare Organizations. Accreditation manual for hospitals. Chicago, IL; 1992.
- US Department of Health and Human Services. US Preventive Services Task Force. Guide to Clinical Preventive Services. 2nd ed. Washington, DC; 1996.
- Ramsay J, Richardson J, Carter YH, Davidson LL, Feder G. Should health professionals screen women for domestic violence? Systematic review. BMJ 2002; 325:314.
- Nelson HD, Nygren P, McInerney Y, Klein J; US Preventive Services Task Force. Screening women and elderly adults for family and intimate partner violence: a review of the evidence for the US Preventive Services Task Force. Ann Intern Med 2004; 140:387–396.
- Chamberlain L. The USPSTF recommendation on intimate partner violence: what we can learn from it and what we can do about it. Fam Viol Prev Health Pract 2005; 1:1–24.
- Moyer VA; US Preventive Services Task Force. Screening for intimate partner violence and abuse of elderly and vulnerable adults: US Preventive Services Task Force recommendation statement. Ann Intern Med 2013; 158:478–486.
- Taft A, O’Doherty L, Hegarty K, Ramsay J, Davidson L, Feder G. Screening women for intimate partner violence in healthcare settings. Cochrane Database Syst Rev 2013; 4:CD007007.
- McNutt LA, Carlson BE, Gagen D, Winterbauer N. Reproductive violence screening in primary care: perspectives and experiences of patients and battered women. J Am Med Womens Assoc 1999; 54:85–90.
- Friedman LS, Samet JH, Roberts MS, Hudlin M, Hans P. Inquiry about victimization experiences. A survey of patient p and physician practices. Arch Intern Med 1992; 152:1186–1190.
- Hamberger LK, Ambuel B, Marbella A, Donze J. Physician interaction with battered women: the women’s perspective. Arch Fam Med 1998; 7:575–582.
- Rodriguez MA, Bauer HM, Flores-Ortiz Y, Szkupinski-Quiroga S. Factors affecting patient-physician communication for abused Latina and Asian immigrant women. J Fam Pract 1998; 47:309–311.
- Rhodes KV, Frankel RM, Levinthal N, Prenoveau E, Bailey J, Levinson W. “You’re not a victim of domestic violence, are you?” Provider patient communication about domestic violence”. Ann Intern Med 2007; 147:620–627.
- Chang JC, Decker M, Moracco KE, Martin SL, Petersen R, Frasier PY. What happens when health care providers ask about intimate partner violence? A description of consequences from the perspectives of female survivors. J Am Med Womens Assoc 2003; 58:76–81.
- Chang JC, Decker MR, Moracco KE, Martin SL, Petersen R, Frasier PY. Asking about intimate partner violence: advice from female survivors to health care providers. Patient Educ Couns 2005; 59:141–147.
- Hathaway JE, Willis G, Zimmer B. Listening to survivors’ voices: addressing partner abuse in the health care setting. Violence Against Women 2002; 8:687–716.
- Gerbert B, Caspers N, Bronstone A, Moe J, Abercrombie P. A qualitative analysis of how physicians with expertise in domestic violence approach the identification of victims. Ann Intern Med 1999; 131:578–584.
- Gerbert B, Caspers N, Milliken N, Berlin M, Bronstone A, Moe J. Interventions that help victims of domestic violence. A qualitative analysis of physicians’ experiences. J Fam Pract 2000; 49:889–895.
- McCaw B, Bauer HM, Berman WH, Mooney L, Holmberg M, Hunkeler E. Women referred for on-site domestic violence services in a managed care organization. Women Health 2002; 35:23–40.
- McFarlane J, Malecha A, Gist J, et al. An intervention to increase safety behaviors of abused women: results of a randomized clinical trial. Nurs Res 2002; 51:347–354.
- Chang JC. Domestic violence. In:Bieber EJ, Sanfilippo JS, Horowitz IR, editors. Clinical Gynecology. Philadelphia, PA; Elsevier, Inc; 2006:79–89.
- Campbell JC, Webster D, Koziol-McLain J, et al. Risk factors for femicide in abusive relationships: results from a multisite case control study. Am J Public Health 2003; 93:1089–1097.
- Isaac NE, Enos V; National Institute of Justice. Documenting domestic violence: how health care providers can help victims. Research in Brief, 2001. https://www.ncjrs.gov/pdffiles1/nij/188564.pdf. Accessed June 3, 2014.
- Durborow N, Lizdas KC, O’Flaherty A, Marjavi A; Futures Without Violence. Compendium of state and US terrritory statutes and policies on domestic violence and health care, 2010. www.futureswithoutviolence.org/content/features/detail/1584/. Accessed June 3, 2014.
- Hyman A, Schillinger D, Lo B. Laws mandating reporting of domestic violence. Do they promote patient well-being? JAMA 1995; 273:1781–1787.
- Hyman A, Chez RA. Mandatory reporting of domestic violence by health care providers: a misguided approach. Womens Health Issues 1995; 5:208–213.
- Knight MA. Ethical debate: should doctors be more proactive as advocates for victims of violence? The police surgeon’s view: medical paternalism is unacceptable. BMJ 1995; 311:1620–1621.
- Hyman A; Futures Without Violence. Mandatory reporting of domestic violence by healthcare providers: a policy paper. San Francisco, CA; 1997. www.futureswithoutviolence.org/userfiles/file/HealthCare/mandatory_policypaper.pdf. Accessed June 3, 2014.
- Rodriguez MA, Craig AM, Mooney DR, Bauer HM. Patient attitudes about mandatory reporting of domestic violence. Implications for health care professionals. West J Med 1998; 169:337–341.
- Caralis PV, Musialowski R. Women’s experiences with domestic violence and their attitudes and expectations regarding medical care of abuse victims. South Med J 1997; 90:1075–1080.
- Sachs CJ, Koziol-McLain J, Glass N, Webster D, Campbell J. A population-based survey assessing support for mandatory domestic violence reporting by health care personnel. Women Health 2002; 35:121–133.
- Sullivan CM, Hagen LA. Survivors’ opinions about mandatory reporting of domestic violence and sexual assault by medical professionals. Affilia 2005; 20:1–16.
- Rodriguez MA, McLoughlin E, Bauer HM, Paredes V, Grumbach K. Mandatory reporting of intimate partner violence to police: views of physicians in California. Am J Public Health 1999; 89:575–578.
- Futures Without Violence. Mandatory reporting of domestic violence to law enforcement by health care providers: a guide for advocates working to respond to or amend reporting laws related to domestic violence. www.healthcaresaboutipv.org/wp-content/blogs.dir/3/files/2012/09/Mandatory_Reporting_of_DV_to_Law-Enforcement_by_HCP.pdf. Accessed June 3, 2014.
- Caralis PV, Musialowski R. Women’s experiences with domestic violence and their attitudes and expectations regarding medical care of abuse victims. South Med J 1997; 90:1075–1080.
- Gerbert B, Johnston K, Caspers N, Bleecker T, Woods A, Rosenbaum A. Experiences of battered women in health care settings: a qualitative study. Women Health 1996; 24:1–17.
- Chang JC, Cluss PA, Ranieri L, et al. Health care interventions for intimate partner violence: what women want. Womens Health Issues 2005; 15:21–30.
- Rodriguez MA, Quiroga SS, Bauer HM. Breaking the silence. Battered women’s perspectives on medical care. Arch Fam Med 1996; 5:153–158.
- Miller E, Decker MR, McCauley HL, et al. A family planning clinic partner violence intervention to reduce risk associated with reproductive coercion. Contraception 2011; 83:274–280.
- Landenburger K. A process of entrapment in and recovery from an abusive relationship. Issues Ment Health Nurs 1989; 10:209–227.
- Cluss PA, Chang JC, Hawker L, et al. The process of change for victims of intimate partner violence: support for a psychosocial readiness model. Womens Health Issues 2006; 16:262–274.
- Gerbert B, Abercrombie P, Caspers N, Love C, Bronstone A. How health care providers help battered women: the survivor’s perspective. Women Health 1999; 29:115–135.
- McCloskey LA, Lichter E, Williams C, Gerber M, Wittenberg E, Ganz M. Assessing intimate partner violence in health care settings leads to women’s receipt of interventions and improved health. Public Health Rep 2006; 121:435–444.
KEY POINTS
- Many victims of IPV will not present with injuries but may have medical or mental health issues related to their IPV experiences.
- Addressing IPV with female patients not only results in increased identification of survivors but is also associated with cognitive and emotional benefits.
- IPV information and resources should be provided to all women, regardless of IPV disclosure.
- Clinicians should respond to a patient’s IPV disclosure with validation, support, respect, and information.
- Clinicians must respect patients’ autonomy, as they are the ones who best understand their situation and know what they need. In some cases, leaving an abusive relationship can be more dangerous than staying.
Planning ahead: How to stay safe
If you are being abused, making a safety plan now may help you when you have to act quickly in the future. The following ideas are ways that other women have planned for their safety. Some of these ideas may work for you. You may come up with additional ideas for yourself. You know your own situation better than anyone else, so plan what will work best for you.
- Hide money or put it somewhere safe so you can leave quickly.
- Make copies of birth certificates, immunization records, Social Security numbers, and other important documents to keep in safe locations away from home such as at work, the homes of trusted family members or friends, or hidden in convenient locations.
- Hide a spare car key or bus or subway pass that you can grab quickly.
- Keep a list of hotline numbers, or memorize the 1-800-799-SAFE National Domestic Violence Hotline number.
- Develop a code with friends, family, or neighbors to let them know when you need help in an emergency. If you have children, teach them a signal (like a code word) that means they should call the police or go for help. You may want to have a special code for neighbors (like putting on a particular light or opening a certain window) that means you want them to call the police.
- Plan your exit. Know which doors, windows, stairwells, elevators, or fire escapes you can use if you have to leave quickly. Practice using them so that they feel familiar to you.
- Know how to reach the police and your local women’s shelter.
- Every day, think about where you can go immediately if you have to leave. Is a neighbor home today? A relative? A friend?
- Remove weapons from your home if you can.
- Something to think about: When you cannot get away and your partner becomes violent, which room is the safest for you to get to? Is there a room that has a phone and a lock on the door? Can you stay out of rooms with easy weapons, such as the kitchen?
- Try not to leave without your children. But if you have to leave your children with the abuser, call the police immediately after you escape.

This information is provided by your physician and the Cleveland Clinic Journal of Medicine. It is not designed to replace a physician’s medical assessment and judgment.
This page may be reproduced noncommercially to share with patients. Any other reproduction is subject to Cleveland Clinic Journal of Medicine approval. Bulk color reprints available by calling 216-444-2661.
For patient information on hundreds of health topics, see the Center for Consumer Health Information web site, www.clevelandclinic.org/health
If you are being abused, making a safety plan now may help you when you have to act quickly in the future. The following ideas are ways that other women have planned for their safety. Some of these ideas may work for you. You may come up with additional ideas for yourself. You know your own situation better than anyone else, so plan what will work best for you.
- Hide money or put it somewhere safe so you can leave quickly.
- Make copies of birth certificates, immunization records, Social Security numbers, and other important documents to keep in safe locations away from home such as at work, the homes of trusted family members or friends, or hidden in convenient locations.
- Hide a spare car key or bus or subway pass that you can grab quickly.
- Keep a list of hotline numbers, or memorize the 1-800-799-SAFE National Domestic Violence Hotline number.
- Develop a code with friends, family, or neighbors to let them know when you need help in an emergency. If you have children, teach them a signal (like a code word) that means they should call the police or go for help. You may want to have a special code for neighbors (like putting on a particular light or opening a certain window) that means you want them to call the police.
- Plan your exit. Know which doors, windows, stairwells, elevators, or fire escapes you can use if you have to leave quickly. Practice using them so that they feel familiar to you.
- Know how to reach the police and your local women’s shelter.
- Every day, think about where you can go immediately if you have to leave. Is a neighbor home today? A relative? A friend?
- Remove weapons from your home if you can.
- Something to think about: When you cannot get away and your partner becomes violent, which room is the safest for you to get to? Is there a room that has a phone and a lock on the door? Can you stay out of rooms with easy weapons, such as the kitchen?
- Try not to leave without your children. But if you have to leave your children with the abuser, call the police immediately after you escape.

This information is provided by your physician and the Cleveland Clinic Journal of Medicine. It is not designed to replace a physician’s medical assessment and judgment.
This page may be reproduced noncommercially to share with patients. Any other reproduction is subject to Cleveland Clinic Journal of Medicine approval. Bulk color reprints available by calling 216-444-2661.
For patient information on hundreds of health topics, see the Center for Consumer Health Information web site, www.clevelandclinic.org/health
If you are being abused, making a safety plan now may help you when you have to act quickly in the future. The following ideas are ways that other women have planned for their safety. Some of these ideas may work for you. You may come up with additional ideas for yourself. You know your own situation better than anyone else, so plan what will work best for you.
- Hide money or put it somewhere safe so you can leave quickly.
- Make copies of birth certificates, immunization records, Social Security numbers, and other important documents to keep in safe locations away from home such as at work, the homes of trusted family members or friends, or hidden in convenient locations.
- Hide a spare car key or bus or subway pass that you can grab quickly.
- Keep a list of hotline numbers, or memorize the 1-800-799-SAFE National Domestic Violence Hotline number.
- Develop a code with friends, family, or neighbors to let them know when you need help in an emergency. If you have children, teach them a signal (like a code word) that means they should call the police or go for help. You may want to have a special code for neighbors (like putting on a particular light or opening a certain window) that means you want them to call the police.
- Plan your exit. Know which doors, windows, stairwells, elevators, or fire escapes you can use if you have to leave quickly. Practice using them so that they feel familiar to you.
- Know how to reach the police and your local women’s shelter.
- Every day, think about where you can go immediately if you have to leave. Is a neighbor home today? A relative? A friend?
- Remove weapons from your home if you can.
- Something to think about: When you cannot get away and your partner becomes violent, which room is the safest for you to get to? Is there a room that has a phone and a lock on the door? Can you stay out of rooms with easy weapons, such as the kitchen?
- Try not to leave without your children. But if you have to leave your children with the abuser, call the police immediately after you escape.

This information is provided by your physician and the Cleveland Clinic Journal of Medicine. It is not designed to replace a physician’s medical assessment and judgment.
This page may be reproduced noncommercially to share with patients. Any other reproduction is subject to Cleveland Clinic Journal of Medicine approval. Bulk color reprints available by calling 216-444-2661.
For patient information on hundreds of health topics, see the Center for Consumer Health Information web site, www.clevelandclinic.org/health
Promoting higher blood pressure targets for frail older adults: A consensus guideline from Canada
Frail older adults deserve guidelines that take frailty into account while assessing the potential benefit and risks of treatment.
Specifically, our group—the Dalhousie Academic Detailing Service (ADS) and the Palliative and Therapeutic Harmonization (PATH) program—recommends that physicians strive to achieve more liberal treatment targets for elderly frail patients who have high blood pressure,1 as evidence does not support an aggressive approach in the frail elderly and the potential exists for harm.
This article reviews the evidence and reasoning that were used to develop and promote a guideline for drug treatment of hypertension in frail older adults. Our recommendations differ from other guidelines in that they focus as much on stopping or decreasing therapy as on starting or increasing it.
FRAILTY INCREASES THE RISK OF ADVERSE EFFECTS
The word frail, applied to older adults, describes those who have complex medical illnesses severe enough to compromise their ability to live independently.2 Many have multiple coexisting medical problems for which they take numerous drugs, in addition to dementia, impaired mobility, compromised functional ability, or a history of falling.
Frailty denotes vulnerability; it increases the risk of adverse effects from medical and surgical procedures,3 complicates drug therapy,4 prolongs hospital length of stay,5 leads to functional and cognitive decline,6 increases the risk of institutionalization,7 and reduces life expectancy8—all of which affect the benefit and harm of medical treatments.
Guidelines for treating hypertension9–11 now acknowledge that little evidence exists to support starting treatment for systolic blood pressure between 140 and 160 mm Hg or aiming for a target of less than 140 mm Hg for “very old” adults, commonly defined as over the age of 80. New guidelines loosen the treatment targets for the very old, but they do not specify targets for the frail and do not describe how to recognize or measure frailty.
RECOGNIZING AND MEASURING FRAILTY
A number of tools are available to recognize and measure frailty.12
The Fried frailty assessment13 has five items:
- Unintentional weight loss
- Self-reported exhaustion
- Weakness in grip
- Slow walking speed
- Low physical activity and energy expenditure.
People are deemed frail if they have three or more of these five. However, experts disagree about whether this system is too sensitive14 or not sensitive enough.15,16
The FRAIL questionnaire17 also has five items:
- Fatigue
- Resistance (inability to climb stairs)
- Ambulation (inability to walk 1 city block)
- Illness (more than 5 major illnesses)
- Weight loss.
People are deemed frail if they have at least three of these five items, and “prefrail” if they have two.
These and other tools are limited by being dichotomous: they classify people as being either frail or not frail18–20 but do not define the spectrum of frailty.
Other frailty assessments such as the Frailty Index21 identify frailty based on the number of accumulated health deficits but take a long time to complete, making them difficult to use in busy clinical settings.22–24
The Clinical Frailty Scale7 is a validated scale that categorizes frailty based on physical and functional indicators of health, such as cognition, function, and mobility, with scores that range from 1 (very fit) to 9 (terminally ill).7,12
The Frailty Assessment for Care-planning Tool (FACT) uses scaling compatible with the Clinical Frailty Scale but has been developed for use as a practical and interpretable frailty screening tool for nonexperts (Table 1). The FACT assesses cognition, mobility, function, and the social situation, using a combination of caregiver report and objective measures. To assess cognition, a health care professional uses items from the Mini-Cog25 (ie, the ability to draw an analog clock face and then recall three unrelated items following the clock-drawing test) and the memory axis of the Brief Cognitive Rating Scale26 (ie, the ability to recall current events, the current US president, and the names of children or spouse). Mobility, function, and social circumstance scores are assigned according to the caregiver report of the patient’s baseline status.
The FACT can be completed in busy clinical settings. Once a caregiver is identified, it takes about 5 minutes to complete.
Our guideline27–31 is intended for those with a score of 7 or more on the Clinical Frailty Scale or FACT,7,12 a score we chose because it describes people who are severely frail with shortened life expectancy.8 At this level, people need help with all instrumental activities of daily living (eg, handling finances, medication management, household chores, and shopping) as well as with basic activities of daily living such as bathing or dressing.
REVIEWING THE LIMITED EVIDENCE
We found no studies that addressed the risks and benefits of treating hypertension in frail older adults; therefore, we concentrated on studies that enrolled individuals who were chronologically old but not frail. We reviewed prominent guidelines,9–11,32,33 the evidence base for these guidelines,34–44 and Cochrane reviews.45,46 A detailed description of the evidence used to build our recommendation can be found online.31
When we deliberated on treatment targets, we reviewed evidence from two types of randomized controlled trials47:
Drug treatment trials randomize patients to different treatments, such as placebo versus a drug or one drug compared with another drug. Patients in different treatment groups may achieve different blood pressures and clinical outcomes, and this information is then used to define optimal targets. However, it may be difficult to determine if the benefit came from lowering blood pressure or from some other effect of the drug, which can be independent of blood pressure lowering.
Treat-to-target trials randomize patients to different blood pressure goals, but the groups are treated with the same or similar drugs. Therefore, any identified benefit can be attributed to the differences in blood pressure rather than the medications used. Compared with a drug treatment trial, this type of trial provides stronger evidence about optimal targets.
We also considered the characteristics of frailty, the dilemma of polypharmacy, and the relevance of the available scientific evidence to those who are frail.
Drug treatment trials
A Cochrane review45 of 15 studies with approximately 24,000 elderly participants found that treating hypertension decreased the rates of cardiovascular morbidity and mortality as well as fatal and nonfatal stroke in the “elderly” (defined as age ≥ 60) and “very elderly” (age ≥ 80). However, in the very elderly, all-cause mortality rates were not statistically significantly different with treatment compared with placebo. The mean duration of treatment was 4.5 years in the elderly and 2.2 years in the very elderly (Table 2). Of importance, all the trials enrolled only those individuals whose systolic blood pressure was at least 160 mm Hg at baseline.
None of the studies were treat-to-target trials—patients were assigned either active medication or placebo. Thus, these trials provide evidence of benefit for treating hypertension in the elderly and very elderly but do not identify the optimal target. All of the drug treatment trials showed benefit, but none achieved a systolic pressure lower than 140 mm Hg with active treatment (Table 3). Therefore, these studies do not support a systolic target of less than 140 mm Hg in the elderly.
Treat-to-target trials: JATOS and VALISH
The Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients (JATOS)42 and the Valsartan in Elderly Isolated Systolic Hypertension (VALISH) study43 each enrolled more than 3,000 people age 65 or older (mean age approximately 75). Patients were randomized to either a strict systolic target of less than 140 mm Hg or a higher (more permissive) target of 140 to 160 mm Hg in JATOS and 140 to 149 mm Hg in VALISH.
In both trials, the group with strict targets achieved a systolic pressure of approximately 136 mm Hg, while the group with higher blood pressure targets achieved a systolic pressure of 146 mm Hg in JATOS and 142 mm Hg in VALISH. Despite these differences, there was no statistically significant difference in the primary outcome.
Thus, treat-to-target studies also fail to support a systolic target of less than 140 mm Hg in the elderly, although it is important to recognize the limitations of the studies. Approximately 15% of the participants had cardiovascular disease, so the applicability of the findings to patients with target-organ damage is uncertain. In addition, there were fewer efficacy outcome events than expected, which suggests that the studies were underpowered.
When to start drug treatment
In each of the drug treatment and treat-to-target trials, the inclusion criterion for study entry was a systolic blood pressure above 160 mm Hg, with a mean blood pressure at entry into the drug treatment trials of 182/95 mm Hg.46 Thus, data support starting treatment if the systolic blood pressure is above 160 mm Hg, but not lower.
Notably, in all but one study,46 at least two-thirds of the participants took no more than two antihypertensive medications. Since adverse events become more common as the number of medications increases, the benefit of adding a third drug to lower blood pressure is uncertain.
Evidence in the ‘very elderly’: HYVET
With the exception of the Hypertension in the Very Elderly Trial (HYVET),44 the mean age of elderly patients in the reported studies was between 67 and 76.
HYVET patients were age 80 and older (mean age 84) and were randomized to receive either indapamide (with or without perindopril) or placebo. The trial was stopped early at 2 years because the mortality rate was lower in the treatment group (10.1%) than in the placebo group (12.3%) (number needed to treat 46, 95% confidence interval 24–637, P = .02). There was no significant difference in the primary outcome of fatal and nonfatal stroke.
Notably, trials that are stopped early may overestimate treatment benefit.48
Evidence in frail older adults
While the above studies provide some information about managing hypertension in the elderly, the participants were generally healthy. HYVET44 specifically excluded those with a standing systolic blood pressure of less than 140 mm Hg and enrolled few patients with orthostasis (7.9% in the placebo group and 8.8% in the treatment group), a condition commonly associated with frailty. As such, these studies may be less relevant to the frail elderly, who are at higher risk of adverse drug events and have competing risks for morbidity and mortality.
Observational studies, in fact, raise questions about whether tight blood pressure control improves clinical outcomes for the very elderly. In the Leiden 85-plus study, lower systolic blood pressure was associated with lower cognitive scores, worse functional ability,49,50 and a higher mortality rate51 compared with higher systolic pressure, although it is uncertain whether these outcomes were indicative of underlying disease that could result in lower blood pressure or an effect of blood pressure-lowering.
The National Health and Nutrition Examination Survey52 found an association between blood pressure and mortality rate that varied by walking speed. For slower walkers (based on the 6-minute walk test), higher systolic pressures were not associated with a higher risk of death, suggesting that when older adults are frail (as indicated by their slow walking speed) they are less likely to benefit from aggressive treatment of hypertension.
People at high risk because of stroke
Because the evidence is limited, it is even more difficult to judge whether lowering blood pressure below 140 mm Hg is beneficial for frail patients who have a history of stroke, compared with the possibility that medications will cause adverse effects such as weakness, orthostasis, and falls. When reviewing the evidence to answer this question, we especially looked at outcomes that affect quality of life, such as nonfatal stroke leading to disability. In contrast, because the frail elderly have competing causes of mortality, we could not assume that a mortality benefit shown in nonfrail populations could be applied to frail populations.
The PROGRESS trial (Perindopril Protection Against Recurrent Stroke Study)53 was in patients with a history of stroke or transient ischemic attack and a mean age of 64, who were treated with either perindopril (with or without indapamide) or placebo.
At almost 4 years, the rate of disabling stroke was 2.7% in the treatment group and 4.3% in the placebo group, a relative risk reduction of 38% and an absolute risk reduction of 1.64% (number needed to treat 61, 95% confidence interval 39–139). The relative risk reduction for all strokes (fatal and nonfatal) was similar across a range of baseline systolic pressures, but the absolute risk reduction was greater in the prespecified subgroup that had hypertension at baseline (mean blood pressure 159/94 mm Hg) than in the normotensive subgroup (mean blood pressure 136/79 mm Hg), suggesting that treatment is most beneficial for those with higher systolic blood pressures. Also, the benefit was only demonstrated in the subgroup that received two antihypertensive medications; those who received perindopril alone showed no benefit.
This study involved relatively young patients in relatively good health except for their strokes. The extent to which the results can be extrapolated to older, frail adults is uncertain because of the time needed to achieve benefit and because of the added vulnerability of frailty, which could make treatment with two antihypertensive medications riskier.
PRoFESS (Prevention Regimen for Effectively Avoiding Second Strokes),54 another study in patients with previous stroke (mean age 66) showed no benefit over 2.5 years in the primary outcome of stroke using telmesartan 80 mg daily compared with placebo. This result is concordant with that of PROGRESS,53 in which patients who took only one medication did not show a significant decrease in the rate of stroke.
A possible reason for the lack of benefit from monotherapy was that the differences in blood pressure between the placebo group and the treatment group on monotherapy were small in both studies (3.8/2.0 mm Hg in PRoFESS, 5/3 mm Hg in PROGRESS). In contrast, patients on dual therapy in PROGRESS decreased their blood pressure by 12/5 mm Hg compared with placebo.
CURRENT HYPERTENSION GUIDELINES
Current guidelines make reference to the elderly, but we found none that made specific recommendations for the frail elderly.
JNC 8
In December 2013, members of the Eighth Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 8) released new recommendations.32 One significant revision was to support higher blood pressure targets for older adults (age 60 and older). Whereas JNC 7 stated that lowering blood pressure below 140/90 mm Hg reduced cardiovascular complications,33 JNC 8 now acknowledges that there is no strong evidence to support blood pressure targets below 150/90 mm Hg for hypertensive persons without kidney disease or diabetes age 60 and older. Thus, in the general population age 60 and older, JNC 8 recommends starting antihypertensive treatment when blood pressure is 150/90 mm Hg or higher, and treating to a goal blood pressure of less than 150/90 mm Hg. JNC 8 makes no recommendation about how to adjust blood pressure targets for frailty or how to measure blood pressure.
American College of Cardiology and American Heart Association
In 2011, the American College of Cardiology and American Heart Association published a consensus document on the management of hypertension in the elderly.9
They acknowledged that the generally recommended blood pressure goal of lower than 140/90 mm Hg in uncomplicated elderly patients is based on expert opinion rather than on data from randomized controlled trials, but nevertheless recommended a target systolic pressure lower than 140 mm Hg for older adults, except for octogenarians.
For those over age 80, systolic levels of 140 to 145 mm Hg can be acceptable if tolerated and if the patient does not experience orthostasis when standing. Systolic pressure lower than 130 mm Hg and diastolic pressures lower than 65 mm Hg should be avoided in this age group.
The document acknowledges that systolic pressure may have to remain above 150 mm Hg if there is no response to four “well-selected drugs” or if there are unacceptable side effects. In these cases, the lowest “safely achieved” systolic blood pressure should be the goal.
Canadian Hypertension Education Program
The 2014 Canadian Hypertension Education Program (CHEP) report makes several recommendations for the “very elderly,” a group they define as over the age of 80. The CHEP website and resources include the following recommendations10:
- For the very elderly without diabetes or target-organ damage, drug therapy should be initiated when systolic blood pressure is higher than 160 mm Hg to reach a systolic blood pressure target lower than 150 mm Hg. This is a grade C level recommendation, indicating that it is based on low-quality trials, unvalidated surrogate outcomes, or results from nonrandomized observational studies.
- For the very elderly with macrovascular target-organ damage, antihypertensive therapy should be considered if systolic blood pressure readings average 140 mm Hg or higher (grade D for 140 to 160 mm Hg; grade A for higher than 160 mm Hg), although caution should be exercised in elderly patients who are frail. (Grade D recommendations are the weakest, as they are based on low-powered, imprecise studies or expert opinion, whereas grade A recommendations are based on the strongest evidence from high-quality randomized clinical trials.)
- Decisions regarding initiating and intensifying pharmacotherapy in the very elderly should be based on an individualized risk-benefit analysis.
The European Society of Hypertension and European Society of Cardiology
The 2013 guidelines from the European Society of Hypertension and the European Society of Cardiology11 recommend that for elderly patients under age 80, antihypertensive treatment may be considered at systolic values higher than 140 mm Hg and aimed at values lower than 140 mm Hg if the patient is fit and treatment is well tolerated.
For those over age 80 with an initial systolic pressure of 160 mm Hg or higher, the guidelines recommend lowering systolic pressure to between 150 and 140 mm Hg, provided the patient is in good physical and mental condition. In frail elderly patients, they recommend leaving decisions on antihypertensive therapy to the treating physician, based on monitoring of the clinical effects of treatment.11
The ADS/PATH guidelines
When finalizing our recommendations,1 we considered the characteristics of frailty and the following key points from the evidence:
- Although evidence from drug treatment trials indicates that there is benefit in treating healthy older adults who have hypertension, the benefit of treating frail older adults is unknown.
- Major trials enrolled elderly patients only if they had systolic blood pressures of at least 160 mm Hg. Therefore, evidence supports initiating pharmacotherapy at a systolic pressure of 160 mm Hg or higher.
- No evidence from randomized controlled trials supports a systolic target lower than 140 mm Hg in the elderly, and there is some evidence that such a target does not benefit.
- The benefit of adding a third medication to lower blood pressure has not been studied.
- Frailty makes the potential benefits of strict blood pressure targets even less certain and increases the possibility of harm from adverse drug events.
- The only study of very old adults, HYVET,44 enrolled relatively healthy older adults and few with orthostasis, while excluding those with a standing systolic blood pressure lower than 140 mm Hg.
OUR RECOMMENDATIONS
Based on the above, we advise against unnecessarily strict targets and recommend stopping antihypertensive medications that are used for the sole purpose of keeping the systolic blood pressure below 140 mm Hg. Our guidelines are unique in that they focus equally on when to stop and when to start medications. We concluded that without evidence of definitive benefit, “less is more” with frailty.55 We believe that if physicians and health professionals understand the limitations of the evidence, they can be more confident in stopping medications that lower blood pressure to an unnecessarily low level.
We recommend the following (Table 4):
Before treating
- Carefully review the risks and the potential but unproven benefits of treatment.
- To avoid overtreatment, treatment decisions should be based on blood pressure measurements in the seated (not supine) position, while also considering the presence of orthostasis.
- To evaluate orthostasis, measure blood pressure in the supine position, then immediately on standing, and again after 2 minutes. Ask the patient if he or she feels light-headed or dizzy when standing.
Stop treatment
- If the seated systolic blood pressure is less than 140 mm Hg, medications can be tapered and discontinued to achieve the targets described below.
- Before discontinuation, consider whether the medications are treating additional conditions such as rate control for atrial fibrillation or symptomatic management of heart failure.
- It is uncertain whether to discontinue treatment when there is a history of stroke. Consider that treatment with two medications resulted in an absolute risk reduction for disabling stroke of 1.64% over approximately 4 years for adults with previous stroke and a mean age of 64,57 an effect that may be more prominent at higher systolic pressures.
Start treatment
- Consider starting treatment when systolic pressure is 160 mm Hg or higher.
- Aim for a seated systolic pressure between 140 and 160 mm Hg if there are no adverse effects from treatment that affect quality of life.
- If there is symptomatic orthostasis or if standing systolic pressure is lower than 140 mm Hg, the target seated systolic pressure can be adjusted upwards.
- In the severely frail nearing the end of life, a target systolic pressure of 160 to 190 mm Hg is reasonable.
- The blood pressure target is the same in people with diabetes.
- In general, use no more than two medications.
Dissemination and implementation
The ADS/PATH guideline is intended for use by physicians and other health professionals (eg, pharmacists and nurses) who care for frail older adults or who work in long-term care facilities. Since creating our guideline, we have disseminated it to physicians, pharmacists, and other health professionals through academic detailing, large conferences, and interactive webinars.
While we do not have objective evidence of practice change, our evaluation data found that 34% of 403 family physicians who received academic detailing indicated that the guideline would change their practice, while 36% stated that the guideline confirmed their practice, an indication that family physicians are sensitive to the needs of the frail elderly.
Because health professionals may be wary of stopping medications and not meeting recommended targets, there may be barriers to adopting this guideline. However, our experience with the PATH program indicates that these barriers can be overcome using effective communication strategies between health professionals and consumers.
AN APPROACH APPROPRIATE TO FRAILTY
There is no direct evidence for systolic blood pressure targets in the frail elderly, so we applied evidence from the nonfrail elderly. Our recommendations differ somewhat from those of other groups, which recommend targets below 140 to 150 mm Hg for older adults, although some do advise caution in the elderly for whom a substantial fall in blood pressure might be poorly tolerated. Despite these messages, we believe that clearer guidance is needed to direct health practitioners toward models that acknowledge that frail patients are in a precarious balance of health and may be harmed by treatments that strive to lower blood pressure to unproven targets. For this reason, our guideline clearly indicates when to decrease or stop drug treatment.
After physicians and health professionals examine the evidence and more fully understand the benefits and harms of treating frail older adults, we are confident that they will be more comfortable stopping medications that lower blood pressure to an unnecessarily low level and instead use an approach that is more appropriate to frailty. We hope clinicians can use this guideline with the same enthusiasm applied to other guidelines, and we welcome discussion.
Acknowledgments: We would like to thank and acknowledge Tanya MacLeod and Kathryn Yuill for their review of and advice about the manuscript.
- Palliative and Therapeutic Harmonization program. Hypertension guidelines. Treating hypertension in frailty. http://pathclinic.ca/resources/hypertension/. Accessed May 2, 2014.
- Theou O, Rockwood MR, Mitnitski A, Rockwood K. Disability and co-morbidity in relation to frailty: how much do they overlap? Arch Gerontol Geriatr 2012; 55:e1–e8.
- Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg 2010; 210:901–908.
- Tinetti ME, Bogardus ST, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med 2004; 351:2870–2874.
- Ekerstad N, Swahn E, Janzon M, et al. Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction. Circulation 2011; 124:2397–2404.
- Theou O, Rockwood K. Should frailty status always be considered when treating the elderly patient? Aging Health 2012; 8:261–271.
- Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173:489–495.
- Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr 2008; 8:24.
- Aronow WS, Fleg JL, Pepine CJ, et al; ACCF Task Force. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation 2011; 123:2434–2506.
- The Canadian Hypertension Education Program (CHEP). 2014 CHEP recommendations. www.hypertension.ca/en/. Accessed May 2, 2014.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34:2159–2219.
- Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013; 14:392–397.
- Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Ensrud KE, Ewing SK, Cawthon PM, et al; Osteoporotic Fractures in Men Research Group. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc 2009; 57:492–498.
- Avila-Funes JA, Amieva H, Barberger-Gateau P, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. J Am Geriatr Soc 2009; 57:453–461.
- Bergman H, Ferrucci L, Guralnik J, et al. Frailty: an emerging research and clinical paradigm—issues and controversies. J Gerontol A Biol Sci Med Sci 2007; 62:731–737.
- Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging 2012; 16:601–608.
- Strawbridge WJ, Shema SJ, Balfour JL, Higby HR, Kaplan GA. Antecedents of frailty over three decades in an older cohort. J Gerontol B Psychol Sci Soc Sci 1998; 53:S9–S16.
- Matthews M, Lucas A, Boland R, et al. Use of a questionnaire to screen for frailty in the elderly: an exploratory study. Aging Clin Exp Res 2004; 16:34–40.
- Salvi F, Morichi V, Grilli A, et al. Screening for frailty in elderly emergency department patients by using the Identification of Seniors At Risk (ISAR). J Nutr Health Aging 2012; 16:313–318.
- Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal 2001; 1:323–336.
- Kellen E, Bulens P, Deckx L, et al. Identifying an accurate pre-screening tool in geriatric oncology. Crit Rev Oncol Hematol 2010; 75:243–248.
- Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing 2006; 35:526–529.
- Martin FC, Brighton P. Frailty: different tools for different purposes? Age Ageing 2008; 37:129–131.
- Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini-cog: a cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry 2000; 15:1021–1027.
- Reisberg B, Ferris SH. Brief Cognitive Rating Scale (BCRS). Psychopharmacol Bull 1988; 24:629–636.
- Moorhouse P, Mallery LH. Palliative and therapeutic harmonization: a model for appropriate decision-making in frail older adults. J Am Geriatr Soc 2012; 60:2326–2332.
- Palliative and Therapeutic Harmonization Clinic (PATH). www.pathclinic.ca. Accessed May 2, 2014.
- Dalhousie University Faculty of Medicine: Continuing Medical Education. http://cme.medicine.dal.ca/ADS.htm. Accessed January 8, 2014.
- Mallery LH, Moorhouse P. Respecting frailty. J Med Ethics 2011; 37:126–128.
- Dalhousie University Faculty of Medicine: Continuing Medical Education. Issues in hypertension 2011. http://cme.medicine.dal.ca/files/Hypertension%20book.pdf. Accessed May 2, 2014.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Amery A, Birkenhäger W, Brixko P, et al. Mortality and morbidity results from the European Working Party on High Blood Pressure in the Elderly trial. Lancet 1985; 1:1349–1354.
- Coope J, Warrender TS. Randomised trial of treatment of hypertension in elderly patients in primary care. Br Med J (Clin Res Ed) 1986; 293:1145–1151.
- SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991; 265:3255–3264.
- Dahlöf B, Lindholm LH, Hansson L, Scherstén B, Ekbom T, Wester PO. Morbidity and mortality in the Swedish Trial in Old Patients with Hypertension (STOP-Hypertension). Lancet 1991; 338:1281–1285.
- Medical Research Council trial of treatment of hypertension in older adults: principal results. MRC Working Party. BMJ 1992; 304:405–412.
- Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet 1997; 350:757–764.
- Liu L, Wang JG, Gong L, Liu G, Staessen JA. Comparison of active treatment and placebo in older Chinese patients with isolated systolic hypertension. Systolic Hypertension in China (Syst-China) Collaborative Group. J Hypertens 1998; 16:1823–1829.
- Lithell H, Hansson L, Skoog I, et al; SCOPE Study Group. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens 2003; 21:875–886.
- JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008; 31:2115–2127.
- Oparil S, Yarows SA, Patel S, Fang H, Zhang J, Satlin A. Efficacy and safety of combined use of aliskiren and valsartan in patients with hypertension: a randomised, double-blind trial. Lancet 2007; 370:221–229.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Musini VM, Tejani AM, Bassett K, Wright JM. Pharmacotherapy for hypertension in the elderly. Cochrane Database Syst Rev 2009;CD000028.
- He FJ, MacGregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev 2004;CD004937.
- Allen M, Kelly K, Fleming I. Hypertension in elderly patients: recommended systolic targets are not evidence based [in French]. Can Fam Physician 2013; 59:19–24.
- Guyatt GH, Briel M, Glasziou P, Bassler D, Montori VM. Problems of stopping trials early. BMJ 2012; 344:e3863.
- Sabayan B, Oleksik AM, Maier AB, et al. High blood pressure and resilience to physical and cognitive decline in the oldest old: the Leiden 85-plus Study. J Am Geriatr Soc 2012; 60:2014–2019.
- Sabayan B, van Vliet P, de Ruijter W, Gussekloo J, de Craen AJ, Westendorp RG. High blood pressure, physical and cognitive function, and risk of stroke in the oldest old: the Leiden 85-plus Study. Stroke 2013; 44:15–20.
- Poortvliet RK, Blom JW, de Craen AJ, et al. Low blood pressure predicts increased mortality in very old age even without heart failure: the Leiden 85-plus Study. Eur J Heart Fail 2013; 15:528–533.
- Odden MC, Peralta CA, Haan MN, Covinsky KE. Rethinking the association of high blood pressure with mortality in elderly adults: the impact of frailty. Arch Intern Med 2012; 172:1162–1168.
- PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001; 358:1033–1041.
- Yusuf S, Diener HC, Sacco RL, et al; PRoFESS Study Group. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med 2008; 359:1225–1237.
- Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med 2010; 170:1648–1654.
Frail older adults deserve guidelines that take frailty into account while assessing the potential benefit and risks of treatment.
Specifically, our group—the Dalhousie Academic Detailing Service (ADS) and the Palliative and Therapeutic Harmonization (PATH) program—recommends that physicians strive to achieve more liberal treatment targets for elderly frail patients who have high blood pressure,1 as evidence does not support an aggressive approach in the frail elderly and the potential exists for harm.
This article reviews the evidence and reasoning that were used to develop and promote a guideline for drug treatment of hypertension in frail older adults. Our recommendations differ from other guidelines in that they focus as much on stopping or decreasing therapy as on starting or increasing it.
FRAILTY INCREASES THE RISK OF ADVERSE EFFECTS
The word frail, applied to older adults, describes those who have complex medical illnesses severe enough to compromise their ability to live independently.2 Many have multiple coexisting medical problems for which they take numerous drugs, in addition to dementia, impaired mobility, compromised functional ability, or a history of falling.
Frailty denotes vulnerability; it increases the risk of adverse effects from medical and surgical procedures,3 complicates drug therapy,4 prolongs hospital length of stay,5 leads to functional and cognitive decline,6 increases the risk of institutionalization,7 and reduces life expectancy8—all of which affect the benefit and harm of medical treatments.
Guidelines for treating hypertension9–11 now acknowledge that little evidence exists to support starting treatment for systolic blood pressure between 140 and 160 mm Hg or aiming for a target of less than 140 mm Hg for “very old” adults, commonly defined as over the age of 80. New guidelines loosen the treatment targets for the very old, but they do not specify targets for the frail and do not describe how to recognize or measure frailty.
RECOGNIZING AND MEASURING FRAILTY
A number of tools are available to recognize and measure frailty.12
The Fried frailty assessment13 has five items:
- Unintentional weight loss
- Self-reported exhaustion
- Weakness in grip
- Slow walking speed
- Low physical activity and energy expenditure.
People are deemed frail if they have three or more of these five. However, experts disagree about whether this system is too sensitive14 or not sensitive enough.15,16
The FRAIL questionnaire17 also has five items:
- Fatigue
- Resistance (inability to climb stairs)
- Ambulation (inability to walk 1 city block)
- Illness (more than 5 major illnesses)
- Weight loss.
People are deemed frail if they have at least three of these five items, and “prefrail” if they have two.
These and other tools are limited by being dichotomous: they classify people as being either frail or not frail18–20 but do not define the spectrum of frailty.
Other frailty assessments such as the Frailty Index21 identify frailty based on the number of accumulated health deficits but take a long time to complete, making them difficult to use in busy clinical settings.22–24
The Clinical Frailty Scale7 is a validated scale that categorizes frailty based on physical and functional indicators of health, such as cognition, function, and mobility, with scores that range from 1 (very fit) to 9 (terminally ill).7,12
The Frailty Assessment for Care-planning Tool (FACT) uses scaling compatible with the Clinical Frailty Scale but has been developed for use as a practical and interpretable frailty screening tool for nonexperts (Table 1). The FACT assesses cognition, mobility, function, and the social situation, using a combination of caregiver report and objective measures. To assess cognition, a health care professional uses items from the Mini-Cog25 (ie, the ability to draw an analog clock face and then recall three unrelated items following the clock-drawing test) and the memory axis of the Brief Cognitive Rating Scale26 (ie, the ability to recall current events, the current US president, and the names of children or spouse). Mobility, function, and social circumstance scores are assigned according to the caregiver report of the patient’s baseline status.
The FACT can be completed in busy clinical settings. Once a caregiver is identified, it takes about 5 minutes to complete.
Our guideline27–31 is intended for those with a score of 7 or more on the Clinical Frailty Scale or FACT,7,12 a score we chose because it describes people who are severely frail with shortened life expectancy.8 At this level, people need help with all instrumental activities of daily living (eg, handling finances, medication management, household chores, and shopping) as well as with basic activities of daily living such as bathing or dressing.
REVIEWING THE LIMITED EVIDENCE
We found no studies that addressed the risks and benefits of treating hypertension in frail older adults; therefore, we concentrated on studies that enrolled individuals who were chronologically old but not frail. We reviewed prominent guidelines,9–11,32,33 the evidence base for these guidelines,34–44 and Cochrane reviews.45,46 A detailed description of the evidence used to build our recommendation can be found online.31
When we deliberated on treatment targets, we reviewed evidence from two types of randomized controlled trials47:
Drug treatment trials randomize patients to different treatments, such as placebo versus a drug or one drug compared with another drug. Patients in different treatment groups may achieve different blood pressures and clinical outcomes, and this information is then used to define optimal targets. However, it may be difficult to determine if the benefit came from lowering blood pressure or from some other effect of the drug, which can be independent of blood pressure lowering.
Treat-to-target trials randomize patients to different blood pressure goals, but the groups are treated with the same or similar drugs. Therefore, any identified benefit can be attributed to the differences in blood pressure rather than the medications used. Compared with a drug treatment trial, this type of trial provides stronger evidence about optimal targets.
We also considered the characteristics of frailty, the dilemma of polypharmacy, and the relevance of the available scientific evidence to those who are frail.
Drug treatment trials
A Cochrane review45 of 15 studies with approximately 24,000 elderly participants found that treating hypertension decreased the rates of cardiovascular morbidity and mortality as well as fatal and nonfatal stroke in the “elderly” (defined as age ≥ 60) and “very elderly” (age ≥ 80). However, in the very elderly, all-cause mortality rates were not statistically significantly different with treatment compared with placebo. The mean duration of treatment was 4.5 years in the elderly and 2.2 years in the very elderly (Table 2). Of importance, all the trials enrolled only those individuals whose systolic blood pressure was at least 160 mm Hg at baseline.
None of the studies were treat-to-target trials—patients were assigned either active medication or placebo. Thus, these trials provide evidence of benefit for treating hypertension in the elderly and very elderly but do not identify the optimal target. All of the drug treatment trials showed benefit, but none achieved a systolic pressure lower than 140 mm Hg with active treatment (Table 3). Therefore, these studies do not support a systolic target of less than 140 mm Hg in the elderly.
Treat-to-target trials: JATOS and VALISH
The Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients (JATOS)42 and the Valsartan in Elderly Isolated Systolic Hypertension (VALISH) study43 each enrolled more than 3,000 people age 65 or older (mean age approximately 75). Patients were randomized to either a strict systolic target of less than 140 mm Hg or a higher (more permissive) target of 140 to 160 mm Hg in JATOS and 140 to 149 mm Hg in VALISH.
In both trials, the group with strict targets achieved a systolic pressure of approximately 136 mm Hg, while the group with higher blood pressure targets achieved a systolic pressure of 146 mm Hg in JATOS and 142 mm Hg in VALISH. Despite these differences, there was no statistically significant difference in the primary outcome.
Thus, treat-to-target studies also fail to support a systolic target of less than 140 mm Hg in the elderly, although it is important to recognize the limitations of the studies. Approximately 15% of the participants had cardiovascular disease, so the applicability of the findings to patients with target-organ damage is uncertain. In addition, there were fewer efficacy outcome events than expected, which suggests that the studies were underpowered.
When to start drug treatment
In each of the drug treatment and treat-to-target trials, the inclusion criterion for study entry was a systolic blood pressure above 160 mm Hg, with a mean blood pressure at entry into the drug treatment trials of 182/95 mm Hg.46 Thus, data support starting treatment if the systolic blood pressure is above 160 mm Hg, but not lower.
Notably, in all but one study,46 at least two-thirds of the participants took no more than two antihypertensive medications. Since adverse events become more common as the number of medications increases, the benefit of adding a third drug to lower blood pressure is uncertain.
Evidence in the ‘very elderly’: HYVET
With the exception of the Hypertension in the Very Elderly Trial (HYVET),44 the mean age of elderly patients in the reported studies was between 67 and 76.
HYVET patients were age 80 and older (mean age 84) and were randomized to receive either indapamide (with or without perindopril) or placebo. The trial was stopped early at 2 years because the mortality rate was lower in the treatment group (10.1%) than in the placebo group (12.3%) (number needed to treat 46, 95% confidence interval 24–637, P = .02). There was no significant difference in the primary outcome of fatal and nonfatal stroke.
Notably, trials that are stopped early may overestimate treatment benefit.48
Evidence in frail older adults
While the above studies provide some information about managing hypertension in the elderly, the participants were generally healthy. HYVET44 specifically excluded those with a standing systolic blood pressure of less than 140 mm Hg and enrolled few patients with orthostasis (7.9% in the placebo group and 8.8% in the treatment group), a condition commonly associated with frailty. As such, these studies may be less relevant to the frail elderly, who are at higher risk of adverse drug events and have competing risks for morbidity and mortality.
Observational studies, in fact, raise questions about whether tight blood pressure control improves clinical outcomes for the very elderly. In the Leiden 85-plus study, lower systolic blood pressure was associated with lower cognitive scores, worse functional ability,49,50 and a higher mortality rate51 compared with higher systolic pressure, although it is uncertain whether these outcomes were indicative of underlying disease that could result in lower blood pressure or an effect of blood pressure-lowering.
The National Health and Nutrition Examination Survey52 found an association between blood pressure and mortality rate that varied by walking speed. For slower walkers (based on the 6-minute walk test), higher systolic pressures were not associated with a higher risk of death, suggesting that when older adults are frail (as indicated by their slow walking speed) they are less likely to benefit from aggressive treatment of hypertension.
People at high risk because of stroke
Because the evidence is limited, it is even more difficult to judge whether lowering blood pressure below 140 mm Hg is beneficial for frail patients who have a history of stroke, compared with the possibility that medications will cause adverse effects such as weakness, orthostasis, and falls. When reviewing the evidence to answer this question, we especially looked at outcomes that affect quality of life, such as nonfatal stroke leading to disability. In contrast, because the frail elderly have competing causes of mortality, we could not assume that a mortality benefit shown in nonfrail populations could be applied to frail populations.
The PROGRESS trial (Perindopril Protection Against Recurrent Stroke Study)53 was in patients with a history of stroke or transient ischemic attack and a mean age of 64, who were treated with either perindopril (with or without indapamide) or placebo.
At almost 4 years, the rate of disabling stroke was 2.7% in the treatment group and 4.3% in the placebo group, a relative risk reduction of 38% and an absolute risk reduction of 1.64% (number needed to treat 61, 95% confidence interval 39–139). The relative risk reduction for all strokes (fatal and nonfatal) was similar across a range of baseline systolic pressures, but the absolute risk reduction was greater in the prespecified subgroup that had hypertension at baseline (mean blood pressure 159/94 mm Hg) than in the normotensive subgroup (mean blood pressure 136/79 mm Hg), suggesting that treatment is most beneficial for those with higher systolic blood pressures. Also, the benefit was only demonstrated in the subgroup that received two antihypertensive medications; those who received perindopril alone showed no benefit.
This study involved relatively young patients in relatively good health except for their strokes. The extent to which the results can be extrapolated to older, frail adults is uncertain because of the time needed to achieve benefit and because of the added vulnerability of frailty, which could make treatment with two antihypertensive medications riskier.
PRoFESS (Prevention Regimen for Effectively Avoiding Second Strokes),54 another study in patients with previous stroke (mean age 66) showed no benefit over 2.5 years in the primary outcome of stroke using telmesartan 80 mg daily compared with placebo. This result is concordant with that of PROGRESS,53 in which patients who took only one medication did not show a significant decrease in the rate of stroke.
A possible reason for the lack of benefit from monotherapy was that the differences in blood pressure between the placebo group and the treatment group on monotherapy were small in both studies (3.8/2.0 mm Hg in PRoFESS, 5/3 mm Hg in PROGRESS). In contrast, patients on dual therapy in PROGRESS decreased their blood pressure by 12/5 mm Hg compared with placebo.
CURRENT HYPERTENSION GUIDELINES
Current guidelines make reference to the elderly, but we found none that made specific recommendations for the frail elderly.
JNC 8
In December 2013, members of the Eighth Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 8) released new recommendations.32 One significant revision was to support higher blood pressure targets for older adults (age 60 and older). Whereas JNC 7 stated that lowering blood pressure below 140/90 mm Hg reduced cardiovascular complications,33 JNC 8 now acknowledges that there is no strong evidence to support blood pressure targets below 150/90 mm Hg for hypertensive persons without kidney disease or diabetes age 60 and older. Thus, in the general population age 60 and older, JNC 8 recommends starting antihypertensive treatment when blood pressure is 150/90 mm Hg or higher, and treating to a goal blood pressure of less than 150/90 mm Hg. JNC 8 makes no recommendation about how to adjust blood pressure targets for frailty or how to measure blood pressure.
American College of Cardiology and American Heart Association
In 2011, the American College of Cardiology and American Heart Association published a consensus document on the management of hypertension in the elderly.9
They acknowledged that the generally recommended blood pressure goal of lower than 140/90 mm Hg in uncomplicated elderly patients is based on expert opinion rather than on data from randomized controlled trials, but nevertheless recommended a target systolic pressure lower than 140 mm Hg for older adults, except for octogenarians.
For those over age 80, systolic levels of 140 to 145 mm Hg can be acceptable if tolerated and if the patient does not experience orthostasis when standing. Systolic pressure lower than 130 mm Hg and diastolic pressures lower than 65 mm Hg should be avoided in this age group.
The document acknowledges that systolic pressure may have to remain above 150 mm Hg if there is no response to four “well-selected drugs” or if there are unacceptable side effects. In these cases, the lowest “safely achieved” systolic blood pressure should be the goal.
Canadian Hypertension Education Program
The 2014 Canadian Hypertension Education Program (CHEP) report makes several recommendations for the “very elderly,” a group they define as over the age of 80. The CHEP website and resources include the following recommendations10:
- For the very elderly without diabetes or target-organ damage, drug therapy should be initiated when systolic blood pressure is higher than 160 mm Hg to reach a systolic blood pressure target lower than 150 mm Hg. This is a grade C level recommendation, indicating that it is based on low-quality trials, unvalidated surrogate outcomes, or results from nonrandomized observational studies.
- For the very elderly with macrovascular target-organ damage, antihypertensive therapy should be considered if systolic blood pressure readings average 140 mm Hg or higher (grade D for 140 to 160 mm Hg; grade A for higher than 160 mm Hg), although caution should be exercised in elderly patients who are frail. (Grade D recommendations are the weakest, as they are based on low-powered, imprecise studies or expert opinion, whereas grade A recommendations are based on the strongest evidence from high-quality randomized clinical trials.)
- Decisions regarding initiating and intensifying pharmacotherapy in the very elderly should be based on an individualized risk-benefit analysis.
The European Society of Hypertension and European Society of Cardiology
The 2013 guidelines from the European Society of Hypertension and the European Society of Cardiology11 recommend that for elderly patients under age 80, antihypertensive treatment may be considered at systolic values higher than 140 mm Hg and aimed at values lower than 140 mm Hg if the patient is fit and treatment is well tolerated.
For those over age 80 with an initial systolic pressure of 160 mm Hg or higher, the guidelines recommend lowering systolic pressure to between 150 and 140 mm Hg, provided the patient is in good physical and mental condition. In frail elderly patients, they recommend leaving decisions on antihypertensive therapy to the treating physician, based on monitoring of the clinical effects of treatment.11
The ADS/PATH guidelines
When finalizing our recommendations,1 we considered the characteristics of frailty and the following key points from the evidence:
- Although evidence from drug treatment trials indicates that there is benefit in treating healthy older adults who have hypertension, the benefit of treating frail older adults is unknown.
- Major trials enrolled elderly patients only if they had systolic blood pressures of at least 160 mm Hg. Therefore, evidence supports initiating pharmacotherapy at a systolic pressure of 160 mm Hg or higher.
- No evidence from randomized controlled trials supports a systolic target lower than 140 mm Hg in the elderly, and there is some evidence that such a target does not benefit.
- The benefit of adding a third medication to lower blood pressure has not been studied.
- Frailty makes the potential benefits of strict blood pressure targets even less certain and increases the possibility of harm from adverse drug events.
- The only study of very old adults, HYVET,44 enrolled relatively healthy older adults and few with orthostasis, while excluding those with a standing systolic blood pressure lower than 140 mm Hg.
OUR RECOMMENDATIONS
Based on the above, we advise against unnecessarily strict targets and recommend stopping antihypertensive medications that are used for the sole purpose of keeping the systolic blood pressure below 140 mm Hg. Our guidelines are unique in that they focus equally on when to stop and when to start medications. We concluded that without evidence of definitive benefit, “less is more” with frailty.55 We believe that if physicians and health professionals understand the limitations of the evidence, they can be more confident in stopping medications that lower blood pressure to an unnecessarily low level.
We recommend the following (Table 4):
Before treating
- Carefully review the risks and the potential but unproven benefits of treatment.
- To avoid overtreatment, treatment decisions should be based on blood pressure measurements in the seated (not supine) position, while also considering the presence of orthostasis.
- To evaluate orthostasis, measure blood pressure in the supine position, then immediately on standing, and again after 2 minutes. Ask the patient if he or she feels light-headed or dizzy when standing.
Stop treatment
- If the seated systolic blood pressure is less than 140 mm Hg, medications can be tapered and discontinued to achieve the targets described below.
- Before discontinuation, consider whether the medications are treating additional conditions such as rate control for atrial fibrillation or symptomatic management of heart failure.
- It is uncertain whether to discontinue treatment when there is a history of stroke. Consider that treatment with two medications resulted in an absolute risk reduction for disabling stroke of 1.64% over approximately 4 years for adults with previous stroke and a mean age of 64,57 an effect that may be more prominent at higher systolic pressures.
Start treatment
- Consider starting treatment when systolic pressure is 160 mm Hg or higher.
- Aim for a seated systolic pressure between 140 and 160 mm Hg if there are no adverse effects from treatment that affect quality of life.
- If there is symptomatic orthostasis or if standing systolic pressure is lower than 140 mm Hg, the target seated systolic pressure can be adjusted upwards.
- In the severely frail nearing the end of life, a target systolic pressure of 160 to 190 mm Hg is reasonable.
- The blood pressure target is the same in people with diabetes.
- In general, use no more than two medications.
Dissemination and implementation
The ADS/PATH guideline is intended for use by physicians and other health professionals (eg, pharmacists and nurses) who care for frail older adults or who work in long-term care facilities. Since creating our guideline, we have disseminated it to physicians, pharmacists, and other health professionals through academic detailing, large conferences, and interactive webinars.
While we do not have objective evidence of practice change, our evaluation data found that 34% of 403 family physicians who received academic detailing indicated that the guideline would change their practice, while 36% stated that the guideline confirmed their practice, an indication that family physicians are sensitive to the needs of the frail elderly.
Because health professionals may be wary of stopping medications and not meeting recommended targets, there may be barriers to adopting this guideline. However, our experience with the PATH program indicates that these barriers can be overcome using effective communication strategies between health professionals and consumers.
AN APPROACH APPROPRIATE TO FRAILTY
There is no direct evidence for systolic blood pressure targets in the frail elderly, so we applied evidence from the nonfrail elderly. Our recommendations differ somewhat from those of other groups, which recommend targets below 140 to 150 mm Hg for older adults, although some do advise caution in the elderly for whom a substantial fall in blood pressure might be poorly tolerated. Despite these messages, we believe that clearer guidance is needed to direct health practitioners toward models that acknowledge that frail patients are in a precarious balance of health and may be harmed by treatments that strive to lower blood pressure to unproven targets. For this reason, our guideline clearly indicates when to decrease or stop drug treatment.
After physicians and health professionals examine the evidence and more fully understand the benefits and harms of treating frail older adults, we are confident that they will be more comfortable stopping medications that lower blood pressure to an unnecessarily low level and instead use an approach that is more appropriate to frailty. We hope clinicians can use this guideline with the same enthusiasm applied to other guidelines, and we welcome discussion.
Acknowledgments: We would like to thank and acknowledge Tanya MacLeod and Kathryn Yuill for their review of and advice about the manuscript.
Frail older adults deserve guidelines that take frailty into account while assessing the potential benefit and risks of treatment.
Specifically, our group—the Dalhousie Academic Detailing Service (ADS) and the Palliative and Therapeutic Harmonization (PATH) program—recommends that physicians strive to achieve more liberal treatment targets for elderly frail patients who have high blood pressure,1 as evidence does not support an aggressive approach in the frail elderly and the potential exists for harm.
This article reviews the evidence and reasoning that were used to develop and promote a guideline for drug treatment of hypertension in frail older adults. Our recommendations differ from other guidelines in that they focus as much on stopping or decreasing therapy as on starting or increasing it.
FRAILTY INCREASES THE RISK OF ADVERSE EFFECTS
The word frail, applied to older adults, describes those who have complex medical illnesses severe enough to compromise their ability to live independently.2 Many have multiple coexisting medical problems for which they take numerous drugs, in addition to dementia, impaired mobility, compromised functional ability, or a history of falling.
Frailty denotes vulnerability; it increases the risk of adverse effects from medical and surgical procedures,3 complicates drug therapy,4 prolongs hospital length of stay,5 leads to functional and cognitive decline,6 increases the risk of institutionalization,7 and reduces life expectancy8—all of which affect the benefit and harm of medical treatments.
Guidelines for treating hypertension9–11 now acknowledge that little evidence exists to support starting treatment for systolic blood pressure between 140 and 160 mm Hg or aiming for a target of less than 140 mm Hg for “very old” adults, commonly defined as over the age of 80. New guidelines loosen the treatment targets for the very old, but they do not specify targets for the frail and do not describe how to recognize or measure frailty.
RECOGNIZING AND MEASURING FRAILTY
A number of tools are available to recognize and measure frailty.12
The Fried frailty assessment13 has five items:
- Unintentional weight loss
- Self-reported exhaustion
- Weakness in grip
- Slow walking speed
- Low physical activity and energy expenditure.
People are deemed frail if they have three or more of these five. However, experts disagree about whether this system is too sensitive14 or not sensitive enough.15,16
The FRAIL questionnaire17 also has five items:
- Fatigue
- Resistance (inability to climb stairs)
- Ambulation (inability to walk 1 city block)
- Illness (more than 5 major illnesses)
- Weight loss.
People are deemed frail if they have at least three of these five items, and “prefrail” if they have two.
These and other tools are limited by being dichotomous: they classify people as being either frail or not frail18–20 but do not define the spectrum of frailty.
Other frailty assessments such as the Frailty Index21 identify frailty based on the number of accumulated health deficits but take a long time to complete, making them difficult to use in busy clinical settings.22–24
The Clinical Frailty Scale7 is a validated scale that categorizes frailty based on physical and functional indicators of health, such as cognition, function, and mobility, with scores that range from 1 (very fit) to 9 (terminally ill).7,12
The Frailty Assessment for Care-planning Tool (FACT) uses scaling compatible with the Clinical Frailty Scale but has been developed for use as a practical and interpretable frailty screening tool for nonexperts (Table 1). The FACT assesses cognition, mobility, function, and the social situation, using a combination of caregiver report and objective measures. To assess cognition, a health care professional uses items from the Mini-Cog25 (ie, the ability to draw an analog clock face and then recall three unrelated items following the clock-drawing test) and the memory axis of the Brief Cognitive Rating Scale26 (ie, the ability to recall current events, the current US president, and the names of children or spouse). Mobility, function, and social circumstance scores are assigned according to the caregiver report of the patient’s baseline status.
The FACT can be completed in busy clinical settings. Once a caregiver is identified, it takes about 5 minutes to complete.
Our guideline27–31 is intended for those with a score of 7 or more on the Clinical Frailty Scale or FACT,7,12 a score we chose because it describes people who are severely frail with shortened life expectancy.8 At this level, people need help with all instrumental activities of daily living (eg, handling finances, medication management, household chores, and shopping) as well as with basic activities of daily living such as bathing or dressing.
REVIEWING THE LIMITED EVIDENCE
We found no studies that addressed the risks and benefits of treating hypertension in frail older adults; therefore, we concentrated on studies that enrolled individuals who were chronologically old but not frail. We reviewed prominent guidelines,9–11,32,33 the evidence base for these guidelines,34–44 and Cochrane reviews.45,46 A detailed description of the evidence used to build our recommendation can be found online.31
When we deliberated on treatment targets, we reviewed evidence from two types of randomized controlled trials47:
Drug treatment trials randomize patients to different treatments, such as placebo versus a drug or one drug compared with another drug. Patients in different treatment groups may achieve different blood pressures and clinical outcomes, and this information is then used to define optimal targets. However, it may be difficult to determine if the benefit came from lowering blood pressure or from some other effect of the drug, which can be independent of blood pressure lowering.
Treat-to-target trials randomize patients to different blood pressure goals, but the groups are treated with the same or similar drugs. Therefore, any identified benefit can be attributed to the differences in blood pressure rather than the medications used. Compared with a drug treatment trial, this type of trial provides stronger evidence about optimal targets.
We also considered the characteristics of frailty, the dilemma of polypharmacy, and the relevance of the available scientific evidence to those who are frail.
Drug treatment trials
A Cochrane review45 of 15 studies with approximately 24,000 elderly participants found that treating hypertension decreased the rates of cardiovascular morbidity and mortality as well as fatal and nonfatal stroke in the “elderly” (defined as age ≥ 60) and “very elderly” (age ≥ 80). However, in the very elderly, all-cause mortality rates were not statistically significantly different with treatment compared with placebo. The mean duration of treatment was 4.5 years in the elderly and 2.2 years in the very elderly (Table 2). Of importance, all the trials enrolled only those individuals whose systolic blood pressure was at least 160 mm Hg at baseline.
None of the studies were treat-to-target trials—patients were assigned either active medication or placebo. Thus, these trials provide evidence of benefit for treating hypertension in the elderly and very elderly but do not identify the optimal target. All of the drug treatment trials showed benefit, but none achieved a systolic pressure lower than 140 mm Hg with active treatment (Table 3). Therefore, these studies do not support a systolic target of less than 140 mm Hg in the elderly.
Treat-to-target trials: JATOS and VALISH
The Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients (JATOS)42 and the Valsartan in Elderly Isolated Systolic Hypertension (VALISH) study43 each enrolled more than 3,000 people age 65 or older (mean age approximately 75). Patients were randomized to either a strict systolic target of less than 140 mm Hg or a higher (more permissive) target of 140 to 160 mm Hg in JATOS and 140 to 149 mm Hg in VALISH.
In both trials, the group with strict targets achieved a systolic pressure of approximately 136 mm Hg, while the group with higher blood pressure targets achieved a systolic pressure of 146 mm Hg in JATOS and 142 mm Hg in VALISH. Despite these differences, there was no statistically significant difference in the primary outcome.
Thus, treat-to-target studies also fail to support a systolic target of less than 140 mm Hg in the elderly, although it is important to recognize the limitations of the studies. Approximately 15% of the participants had cardiovascular disease, so the applicability of the findings to patients with target-organ damage is uncertain. In addition, there were fewer efficacy outcome events than expected, which suggests that the studies were underpowered.
When to start drug treatment
In each of the drug treatment and treat-to-target trials, the inclusion criterion for study entry was a systolic blood pressure above 160 mm Hg, with a mean blood pressure at entry into the drug treatment trials of 182/95 mm Hg.46 Thus, data support starting treatment if the systolic blood pressure is above 160 mm Hg, but not lower.
Notably, in all but one study,46 at least two-thirds of the participants took no more than two antihypertensive medications. Since adverse events become more common as the number of medications increases, the benefit of adding a third drug to lower blood pressure is uncertain.
Evidence in the ‘very elderly’: HYVET
With the exception of the Hypertension in the Very Elderly Trial (HYVET),44 the mean age of elderly patients in the reported studies was between 67 and 76.
HYVET patients were age 80 and older (mean age 84) and were randomized to receive either indapamide (with or without perindopril) or placebo. The trial was stopped early at 2 years because the mortality rate was lower in the treatment group (10.1%) than in the placebo group (12.3%) (number needed to treat 46, 95% confidence interval 24–637, P = .02). There was no significant difference in the primary outcome of fatal and nonfatal stroke.
Notably, trials that are stopped early may overestimate treatment benefit.48
Evidence in frail older adults
While the above studies provide some information about managing hypertension in the elderly, the participants were generally healthy. HYVET44 specifically excluded those with a standing systolic blood pressure of less than 140 mm Hg and enrolled few patients with orthostasis (7.9% in the placebo group and 8.8% in the treatment group), a condition commonly associated with frailty. As such, these studies may be less relevant to the frail elderly, who are at higher risk of adverse drug events and have competing risks for morbidity and mortality.
Observational studies, in fact, raise questions about whether tight blood pressure control improves clinical outcomes for the very elderly. In the Leiden 85-plus study, lower systolic blood pressure was associated with lower cognitive scores, worse functional ability,49,50 and a higher mortality rate51 compared with higher systolic pressure, although it is uncertain whether these outcomes were indicative of underlying disease that could result in lower blood pressure or an effect of blood pressure-lowering.
The National Health and Nutrition Examination Survey52 found an association between blood pressure and mortality rate that varied by walking speed. For slower walkers (based on the 6-minute walk test), higher systolic pressures were not associated with a higher risk of death, suggesting that when older adults are frail (as indicated by their slow walking speed) they are less likely to benefit from aggressive treatment of hypertension.
People at high risk because of stroke
Because the evidence is limited, it is even more difficult to judge whether lowering blood pressure below 140 mm Hg is beneficial for frail patients who have a history of stroke, compared with the possibility that medications will cause adverse effects such as weakness, orthostasis, and falls. When reviewing the evidence to answer this question, we especially looked at outcomes that affect quality of life, such as nonfatal stroke leading to disability. In contrast, because the frail elderly have competing causes of mortality, we could not assume that a mortality benefit shown in nonfrail populations could be applied to frail populations.
The PROGRESS trial (Perindopril Protection Against Recurrent Stroke Study)53 was in patients with a history of stroke or transient ischemic attack and a mean age of 64, who were treated with either perindopril (with or without indapamide) or placebo.
At almost 4 years, the rate of disabling stroke was 2.7% in the treatment group and 4.3% in the placebo group, a relative risk reduction of 38% and an absolute risk reduction of 1.64% (number needed to treat 61, 95% confidence interval 39–139). The relative risk reduction for all strokes (fatal and nonfatal) was similar across a range of baseline systolic pressures, but the absolute risk reduction was greater in the prespecified subgroup that had hypertension at baseline (mean blood pressure 159/94 mm Hg) than in the normotensive subgroup (mean blood pressure 136/79 mm Hg), suggesting that treatment is most beneficial for those with higher systolic blood pressures. Also, the benefit was only demonstrated in the subgroup that received two antihypertensive medications; those who received perindopril alone showed no benefit.
This study involved relatively young patients in relatively good health except for their strokes. The extent to which the results can be extrapolated to older, frail adults is uncertain because of the time needed to achieve benefit and because of the added vulnerability of frailty, which could make treatment with two antihypertensive medications riskier.
PRoFESS (Prevention Regimen for Effectively Avoiding Second Strokes),54 another study in patients with previous stroke (mean age 66) showed no benefit over 2.5 years in the primary outcome of stroke using telmesartan 80 mg daily compared with placebo. This result is concordant with that of PROGRESS,53 in which patients who took only one medication did not show a significant decrease in the rate of stroke.
A possible reason for the lack of benefit from monotherapy was that the differences in blood pressure between the placebo group and the treatment group on monotherapy were small in both studies (3.8/2.0 mm Hg in PRoFESS, 5/3 mm Hg in PROGRESS). In contrast, patients on dual therapy in PROGRESS decreased their blood pressure by 12/5 mm Hg compared with placebo.
CURRENT HYPERTENSION GUIDELINES
Current guidelines make reference to the elderly, but we found none that made specific recommendations for the frail elderly.
JNC 8
In December 2013, members of the Eighth Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 8) released new recommendations.32 One significant revision was to support higher blood pressure targets for older adults (age 60 and older). Whereas JNC 7 stated that lowering blood pressure below 140/90 mm Hg reduced cardiovascular complications,33 JNC 8 now acknowledges that there is no strong evidence to support blood pressure targets below 150/90 mm Hg for hypertensive persons without kidney disease or diabetes age 60 and older. Thus, in the general population age 60 and older, JNC 8 recommends starting antihypertensive treatment when blood pressure is 150/90 mm Hg or higher, and treating to a goal blood pressure of less than 150/90 mm Hg. JNC 8 makes no recommendation about how to adjust blood pressure targets for frailty or how to measure blood pressure.
American College of Cardiology and American Heart Association
In 2011, the American College of Cardiology and American Heart Association published a consensus document on the management of hypertension in the elderly.9
They acknowledged that the generally recommended blood pressure goal of lower than 140/90 mm Hg in uncomplicated elderly patients is based on expert opinion rather than on data from randomized controlled trials, but nevertheless recommended a target systolic pressure lower than 140 mm Hg for older adults, except for octogenarians.
For those over age 80, systolic levels of 140 to 145 mm Hg can be acceptable if tolerated and if the patient does not experience orthostasis when standing. Systolic pressure lower than 130 mm Hg and diastolic pressures lower than 65 mm Hg should be avoided in this age group.
The document acknowledges that systolic pressure may have to remain above 150 mm Hg if there is no response to four “well-selected drugs” or if there are unacceptable side effects. In these cases, the lowest “safely achieved” systolic blood pressure should be the goal.
Canadian Hypertension Education Program
The 2014 Canadian Hypertension Education Program (CHEP) report makes several recommendations for the “very elderly,” a group they define as over the age of 80. The CHEP website and resources include the following recommendations10:
- For the very elderly without diabetes or target-organ damage, drug therapy should be initiated when systolic blood pressure is higher than 160 mm Hg to reach a systolic blood pressure target lower than 150 mm Hg. This is a grade C level recommendation, indicating that it is based on low-quality trials, unvalidated surrogate outcomes, or results from nonrandomized observational studies.
- For the very elderly with macrovascular target-organ damage, antihypertensive therapy should be considered if systolic blood pressure readings average 140 mm Hg or higher (grade D for 140 to 160 mm Hg; grade A for higher than 160 mm Hg), although caution should be exercised in elderly patients who are frail. (Grade D recommendations are the weakest, as they are based on low-powered, imprecise studies or expert opinion, whereas grade A recommendations are based on the strongest evidence from high-quality randomized clinical trials.)
- Decisions regarding initiating and intensifying pharmacotherapy in the very elderly should be based on an individualized risk-benefit analysis.
The European Society of Hypertension and European Society of Cardiology
The 2013 guidelines from the European Society of Hypertension and the European Society of Cardiology11 recommend that for elderly patients under age 80, antihypertensive treatment may be considered at systolic values higher than 140 mm Hg and aimed at values lower than 140 mm Hg if the patient is fit and treatment is well tolerated.
For those over age 80 with an initial systolic pressure of 160 mm Hg or higher, the guidelines recommend lowering systolic pressure to between 150 and 140 mm Hg, provided the patient is in good physical and mental condition. In frail elderly patients, they recommend leaving decisions on antihypertensive therapy to the treating physician, based on monitoring of the clinical effects of treatment.11
The ADS/PATH guidelines
When finalizing our recommendations,1 we considered the characteristics of frailty and the following key points from the evidence:
- Although evidence from drug treatment trials indicates that there is benefit in treating healthy older adults who have hypertension, the benefit of treating frail older adults is unknown.
- Major trials enrolled elderly patients only if they had systolic blood pressures of at least 160 mm Hg. Therefore, evidence supports initiating pharmacotherapy at a systolic pressure of 160 mm Hg or higher.
- No evidence from randomized controlled trials supports a systolic target lower than 140 mm Hg in the elderly, and there is some evidence that such a target does not benefit.
- The benefit of adding a third medication to lower blood pressure has not been studied.
- Frailty makes the potential benefits of strict blood pressure targets even less certain and increases the possibility of harm from adverse drug events.
- The only study of very old adults, HYVET,44 enrolled relatively healthy older adults and few with orthostasis, while excluding those with a standing systolic blood pressure lower than 140 mm Hg.
OUR RECOMMENDATIONS
Based on the above, we advise against unnecessarily strict targets and recommend stopping antihypertensive medications that are used for the sole purpose of keeping the systolic blood pressure below 140 mm Hg. Our guidelines are unique in that they focus equally on when to stop and when to start medications. We concluded that without evidence of definitive benefit, “less is more” with frailty.55 We believe that if physicians and health professionals understand the limitations of the evidence, they can be more confident in stopping medications that lower blood pressure to an unnecessarily low level.
We recommend the following (Table 4):
Before treating
- Carefully review the risks and the potential but unproven benefits of treatment.
- To avoid overtreatment, treatment decisions should be based on blood pressure measurements in the seated (not supine) position, while also considering the presence of orthostasis.
- To evaluate orthostasis, measure blood pressure in the supine position, then immediately on standing, and again after 2 minutes. Ask the patient if he or she feels light-headed or dizzy when standing.
Stop treatment
- If the seated systolic blood pressure is less than 140 mm Hg, medications can be tapered and discontinued to achieve the targets described below.
- Before discontinuation, consider whether the medications are treating additional conditions such as rate control for atrial fibrillation or symptomatic management of heart failure.
- It is uncertain whether to discontinue treatment when there is a history of stroke. Consider that treatment with two medications resulted in an absolute risk reduction for disabling stroke of 1.64% over approximately 4 years for adults with previous stroke and a mean age of 64,57 an effect that may be more prominent at higher systolic pressures.
Start treatment
- Consider starting treatment when systolic pressure is 160 mm Hg or higher.
- Aim for a seated systolic pressure between 140 and 160 mm Hg if there are no adverse effects from treatment that affect quality of life.
- If there is symptomatic orthostasis or if standing systolic pressure is lower than 140 mm Hg, the target seated systolic pressure can be adjusted upwards.
- In the severely frail nearing the end of life, a target systolic pressure of 160 to 190 mm Hg is reasonable.
- The blood pressure target is the same in people with diabetes.
- In general, use no more than two medications.
Dissemination and implementation
The ADS/PATH guideline is intended for use by physicians and other health professionals (eg, pharmacists and nurses) who care for frail older adults or who work in long-term care facilities. Since creating our guideline, we have disseminated it to physicians, pharmacists, and other health professionals through academic detailing, large conferences, and interactive webinars.
While we do not have objective evidence of practice change, our evaluation data found that 34% of 403 family physicians who received academic detailing indicated that the guideline would change their practice, while 36% stated that the guideline confirmed their practice, an indication that family physicians are sensitive to the needs of the frail elderly.
Because health professionals may be wary of stopping medications and not meeting recommended targets, there may be barriers to adopting this guideline. However, our experience with the PATH program indicates that these barriers can be overcome using effective communication strategies between health professionals and consumers.
AN APPROACH APPROPRIATE TO FRAILTY
There is no direct evidence for systolic blood pressure targets in the frail elderly, so we applied evidence from the nonfrail elderly. Our recommendations differ somewhat from those of other groups, which recommend targets below 140 to 150 mm Hg for older adults, although some do advise caution in the elderly for whom a substantial fall in blood pressure might be poorly tolerated. Despite these messages, we believe that clearer guidance is needed to direct health practitioners toward models that acknowledge that frail patients are in a precarious balance of health and may be harmed by treatments that strive to lower blood pressure to unproven targets. For this reason, our guideline clearly indicates when to decrease or stop drug treatment.
After physicians and health professionals examine the evidence and more fully understand the benefits and harms of treating frail older adults, we are confident that they will be more comfortable stopping medications that lower blood pressure to an unnecessarily low level and instead use an approach that is more appropriate to frailty. We hope clinicians can use this guideline with the same enthusiasm applied to other guidelines, and we welcome discussion.
Acknowledgments: We would like to thank and acknowledge Tanya MacLeod and Kathryn Yuill for their review of and advice about the manuscript.
- Palliative and Therapeutic Harmonization program. Hypertension guidelines. Treating hypertension in frailty. http://pathclinic.ca/resources/hypertension/. Accessed May 2, 2014.
- Theou O, Rockwood MR, Mitnitski A, Rockwood K. Disability and co-morbidity in relation to frailty: how much do they overlap? Arch Gerontol Geriatr 2012; 55:e1–e8.
- Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg 2010; 210:901–908.
- Tinetti ME, Bogardus ST, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med 2004; 351:2870–2874.
- Ekerstad N, Swahn E, Janzon M, et al. Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction. Circulation 2011; 124:2397–2404.
- Theou O, Rockwood K. Should frailty status always be considered when treating the elderly patient? Aging Health 2012; 8:261–271.
- Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173:489–495.
- Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr 2008; 8:24.
- Aronow WS, Fleg JL, Pepine CJ, et al; ACCF Task Force. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation 2011; 123:2434–2506.
- The Canadian Hypertension Education Program (CHEP). 2014 CHEP recommendations. www.hypertension.ca/en/. Accessed May 2, 2014.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34:2159–2219.
- Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013; 14:392–397.
- Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Ensrud KE, Ewing SK, Cawthon PM, et al; Osteoporotic Fractures in Men Research Group. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc 2009; 57:492–498.
- Avila-Funes JA, Amieva H, Barberger-Gateau P, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. J Am Geriatr Soc 2009; 57:453–461.
- Bergman H, Ferrucci L, Guralnik J, et al. Frailty: an emerging research and clinical paradigm—issues and controversies. J Gerontol A Biol Sci Med Sci 2007; 62:731–737.
- Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging 2012; 16:601–608.
- Strawbridge WJ, Shema SJ, Balfour JL, Higby HR, Kaplan GA. Antecedents of frailty over three decades in an older cohort. J Gerontol B Psychol Sci Soc Sci 1998; 53:S9–S16.
- Matthews M, Lucas A, Boland R, et al. Use of a questionnaire to screen for frailty in the elderly: an exploratory study. Aging Clin Exp Res 2004; 16:34–40.
- Salvi F, Morichi V, Grilli A, et al. Screening for frailty in elderly emergency department patients by using the Identification of Seniors At Risk (ISAR). J Nutr Health Aging 2012; 16:313–318.
- Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal 2001; 1:323–336.
- Kellen E, Bulens P, Deckx L, et al. Identifying an accurate pre-screening tool in geriatric oncology. Crit Rev Oncol Hematol 2010; 75:243–248.
- Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing 2006; 35:526–529.
- Martin FC, Brighton P. Frailty: different tools for different purposes? Age Ageing 2008; 37:129–131.
- Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini-cog: a cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry 2000; 15:1021–1027.
- Reisberg B, Ferris SH. Brief Cognitive Rating Scale (BCRS). Psychopharmacol Bull 1988; 24:629–636.
- Moorhouse P, Mallery LH. Palliative and therapeutic harmonization: a model for appropriate decision-making in frail older adults. J Am Geriatr Soc 2012; 60:2326–2332.
- Palliative and Therapeutic Harmonization Clinic (PATH). www.pathclinic.ca. Accessed May 2, 2014.
- Dalhousie University Faculty of Medicine: Continuing Medical Education. http://cme.medicine.dal.ca/ADS.htm. Accessed January 8, 2014.
- Mallery LH, Moorhouse P. Respecting frailty. J Med Ethics 2011; 37:126–128.
- Dalhousie University Faculty of Medicine: Continuing Medical Education. Issues in hypertension 2011. http://cme.medicine.dal.ca/files/Hypertension%20book.pdf. Accessed May 2, 2014.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Amery A, Birkenhäger W, Brixko P, et al. Mortality and morbidity results from the European Working Party on High Blood Pressure in the Elderly trial. Lancet 1985; 1:1349–1354.
- Coope J, Warrender TS. Randomised trial of treatment of hypertension in elderly patients in primary care. Br Med J (Clin Res Ed) 1986; 293:1145–1151.
- SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991; 265:3255–3264.
- Dahlöf B, Lindholm LH, Hansson L, Scherstén B, Ekbom T, Wester PO. Morbidity and mortality in the Swedish Trial in Old Patients with Hypertension (STOP-Hypertension). Lancet 1991; 338:1281–1285.
- Medical Research Council trial of treatment of hypertension in older adults: principal results. MRC Working Party. BMJ 1992; 304:405–412.
- Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet 1997; 350:757–764.
- Liu L, Wang JG, Gong L, Liu G, Staessen JA. Comparison of active treatment and placebo in older Chinese patients with isolated systolic hypertension. Systolic Hypertension in China (Syst-China) Collaborative Group. J Hypertens 1998; 16:1823–1829.
- Lithell H, Hansson L, Skoog I, et al; SCOPE Study Group. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens 2003; 21:875–886.
- JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008; 31:2115–2127.
- Oparil S, Yarows SA, Patel S, Fang H, Zhang J, Satlin A. Efficacy and safety of combined use of aliskiren and valsartan in patients with hypertension: a randomised, double-blind trial. Lancet 2007; 370:221–229.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Musini VM, Tejani AM, Bassett K, Wright JM. Pharmacotherapy for hypertension in the elderly. Cochrane Database Syst Rev 2009;CD000028.
- He FJ, MacGregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev 2004;CD004937.
- Allen M, Kelly K, Fleming I. Hypertension in elderly patients: recommended systolic targets are not evidence based [in French]. Can Fam Physician 2013; 59:19–24.
- Guyatt GH, Briel M, Glasziou P, Bassler D, Montori VM. Problems of stopping trials early. BMJ 2012; 344:e3863.
- Sabayan B, Oleksik AM, Maier AB, et al. High blood pressure and resilience to physical and cognitive decline in the oldest old: the Leiden 85-plus Study. J Am Geriatr Soc 2012; 60:2014–2019.
- Sabayan B, van Vliet P, de Ruijter W, Gussekloo J, de Craen AJ, Westendorp RG. High blood pressure, physical and cognitive function, and risk of stroke in the oldest old: the Leiden 85-plus Study. Stroke 2013; 44:15–20.
- Poortvliet RK, Blom JW, de Craen AJ, et al. Low blood pressure predicts increased mortality in very old age even without heart failure: the Leiden 85-plus Study. Eur J Heart Fail 2013; 15:528–533.
- Odden MC, Peralta CA, Haan MN, Covinsky KE. Rethinking the association of high blood pressure with mortality in elderly adults: the impact of frailty. Arch Intern Med 2012; 172:1162–1168.
- PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001; 358:1033–1041.
- Yusuf S, Diener HC, Sacco RL, et al; PRoFESS Study Group. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med 2008; 359:1225–1237.
- Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med 2010; 170:1648–1654.
- Palliative and Therapeutic Harmonization program. Hypertension guidelines. Treating hypertension in frailty. http://pathclinic.ca/resources/hypertension/. Accessed May 2, 2014.
- Theou O, Rockwood MR, Mitnitski A, Rockwood K. Disability and co-morbidity in relation to frailty: how much do they overlap? Arch Gerontol Geriatr 2012; 55:e1–e8.
- Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg 2010; 210:901–908.
- Tinetti ME, Bogardus ST, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med 2004; 351:2870–2874.
- Ekerstad N, Swahn E, Janzon M, et al. Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction. Circulation 2011; 124:2397–2404.
- Theou O, Rockwood K. Should frailty status always be considered when treating the elderly patient? Aging Health 2012; 8:261–271.
- Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173:489–495.
- Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr 2008; 8:24.
- Aronow WS, Fleg JL, Pepine CJ, et al; ACCF Task Force. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation 2011; 123:2434–2506.
- The Canadian Hypertension Education Program (CHEP). 2014 CHEP recommendations. www.hypertension.ca/en/. Accessed May 2, 2014.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34:2159–2219.
- Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013; 14:392–397.
- Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Ensrud KE, Ewing SK, Cawthon PM, et al; Osteoporotic Fractures in Men Research Group. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc 2009; 57:492–498.
- Avila-Funes JA, Amieva H, Barberger-Gateau P, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. J Am Geriatr Soc 2009; 57:453–461.
- Bergman H, Ferrucci L, Guralnik J, et al. Frailty: an emerging research and clinical paradigm—issues and controversies. J Gerontol A Biol Sci Med Sci 2007; 62:731–737.
- Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging 2012; 16:601–608.
- Strawbridge WJ, Shema SJ, Balfour JL, Higby HR, Kaplan GA. Antecedents of frailty over three decades in an older cohort. J Gerontol B Psychol Sci Soc Sci 1998; 53:S9–S16.
- Matthews M, Lucas A, Boland R, et al. Use of a questionnaire to screen for frailty in the elderly: an exploratory study. Aging Clin Exp Res 2004; 16:34–40.
- Salvi F, Morichi V, Grilli A, et al. Screening for frailty in elderly emergency department patients by using the Identification of Seniors At Risk (ISAR). J Nutr Health Aging 2012; 16:313–318.
- Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal 2001; 1:323–336.
- Kellen E, Bulens P, Deckx L, et al. Identifying an accurate pre-screening tool in geriatric oncology. Crit Rev Oncol Hematol 2010; 75:243–248.
- Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing 2006; 35:526–529.
- Martin FC, Brighton P. Frailty: different tools for different purposes? Age Ageing 2008; 37:129–131.
- Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini-cog: a cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry 2000; 15:1021–1027.
- Reisberg B, Ferris SH. Brief Cognitive Rating Scale (BCRS). Psychopharmacol Bull 1988; 24:629–636.
- Moorhouse P, Mallery LH. Palliative and therapeutic harmonization: a model for appropriate decision-making in frail older adults. J Am Geriatr Soc 2012; 60:2326–2332.
- Palliative and Therapeutic Harmonization Clinic (PATH). www.pathclinic.ca. Accessed May 2, 2014.
- Dalhousie University Faculty of Medicine: Continuing Medical Education. http://cme.medicine.dal.ca/ADS.htm. Accessed January 8, 2014.
- Mallery LH, Moorhouse P. Respecting frailty. J Med Ethics 2011; 37:126–128.
- Dalhousie University Faculty of Medicine: Continuing Medical Education. Issues in hypertension 2011. http://cme.medicine.dal.ca/files/Hypertension%20book.pdf. Accessed May 2, 2014.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Amery A, Birkenhäger W, Brixko P, et al. Mortality and morbidity results from the European Working Party on High Blood Pressure in the Elderly trial. Lancet 1985; 1:1349–1354.
- Coope J, Warrender TS. Randomised trial of treatment of hypertension in elderly patients in primary care. Br Med J (Clin Res Ed) 1986; 293:1145–1151.
- SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991; 265:3255–3264.
- Dahlöf B, Lindholm LH, Hansson L, Scherstén B, Ekbom T, Wester PO. Morbidity and mortality in the Swedish Trial in Old Patients with Hypertension (STOP-Hypertension). Lancet 1991; 338:1281–1285.
- Medical Research Council trial of treatment of hypertension in older adults: principal results. MRC Working Party. BMJ 1992; 304:405–412.
- Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet 1997; 350:757–764.
- Liu L, Wang JG, Gong L, Liu G, Staessen JA. Comparison of active treatment and placebo in older Chinese patients with isolated systolic hypertension. Systolic Hypertension in China (Syst-China) Collaborative Group. J Hypertens 1998; 16:1823–1829.
- Lithell H, Hansson L, Skoog I, et al; SCOPE Study Group. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens 2003; 21:875–886.
- JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008; 31:2115–2127.
- Oparil S, Yarows SA, Patel S, Fang H, Zhang J, Satlin A. Efficacy and safety of combined use of aliskiren and valsartan in patients with hypertension: a randomised, double-blind trial. Lancet 2007; 370:221–229.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Musini VM, Tejani AM, Bassett K, Wright JM. Pharmacotherapy for hypertension in the elderly. Cochrane Database Syst Rev 2009;CD000028.
- He FJ, MacGregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev 2004;CD004937.
- Allen M, Kelly K, Fleming I. Hypertension in elderly patients: recommended systolic targets are not evidence based [in French]. Can Fam Physician 2013; 59:19–24.
- Guyatt GH, Briel M, Glasziou P, Bassler D, Montori VM. Problems of stopping trials early. BMJ 2012; 344:e3863.
- Sabayan B, Oleksik AM, Maier AB, et al. High blood pressure and resilience to physical and cognitive decline in the oldest old: the Leiden 85-plus Study. J Am Geriatr Soc 2012; 60:2014–2019.
- Sabayan B, van Vliet P, de Ruijter W, Gussekloo J, de Craen AJ, Westendorp RG. High blood pressure, physical and cognitive function, and risk of stroke in the oldest old: the Leiden 85-plus Study. Stroke 2013; 44:15–20.
- Poortvliet RK, Blom JW, de Craen AJ, et al. Low blood pressure predicts increased mortality in very old age even without heart failure: the Leiden 85-plus Study. Eur J Heart Fail 2013; 15:528–533.
- Odden MC, Peralta CA, Haan MN, Covinsky KE. Rethinking the association of high blood pressure with mortality in elderly adults: the impact of frailty. Arch Intern Med 2012; 172:1162–1168.
- PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001; 358:1033–1041.
- Yusuf S, Diener HC, Sacco RL, et al; PRoFESS Study Group. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med 2008; 359:1225–1237.
- Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med 2010; 170:1648–1654.
KEY POINTS
- For frail elderly patients, consider starting treatment if the systolic blood pressure is 160 mm Hg or higher.
- An appropriate target in this population is a seated systolic pressure between 140 and 160 mm Hg, as long as there is no orthostatic drop to less than 140 mm Hg upon standing from a lying position and treatment does not adversely affect quality of life.
- The blood pressure target does not need to be lower if the patient has diabetes. If the patient is severely frail and has a short life expectancy, a systolic target of 160 to 190 mm Hg may be reasonable.
- If the systolic pressure is below 140 mm Hg, antihypertensive medications can be reduced as long as they are not indicated for other conditions.
- In general, one should prescribe no more than two antihypertensive medications.