User login
An easier route for cell therapy

Credit: PNAS
Laser technology can help ensure the delivery of drug and gene therapy at the cellular level without damaging surrounding tissue, according to research published in Nature Scientific Reports.
Investigators paired crystalline magnetic carbon nanoparticles and continuous wave near-infrared laser beams in what is called photothermal delivery.
And they used this delivery method to introduce impermeable dyes and small DNA molecules into human cancer cells.
This work grew out of a previous study in which the researchers used a 50 to 100 milliwatt laser and the same carbon nanoparticle, which absorbs the beam, to heat up and destroy cancer cells in the lab.
“In [the current study, we] used a lower-power, 20 to 30 milliwatt, continuous wave near-infrared laser and the nanoparticle to permeate the cell membrane without killing the cells,” said Ali Koymen, PhD, of the University of Texas at Arlington.
“This method stretches the desired cell membrane to allow for delivery and has the added bonus of creating a fluid flow that speeds the movement of what is being delivered.”
The investigators noted that, currently, the predominant practice is using viruses for delivery to cells. Unfortunately, the scope of what can be delivered with viruses is severely limited, and virus interaction can lead to inflammatory responses and other complications.
Researchers looking to create a path into the cell without employing a virus have experimented with using UV-visible light laser beams alone. But that method damages surrounding cells and has a relatively shallow level of effectiveness.
Dr Koymen and his colleagues said a significant advantage of their method is that the near-infrared light absorption of the nanoparticle can be used to selectively amplify the interaction of low-power laser with targeted tissue, and laser-induced damage to non-targeted cells can be avoided.
The magnetic properties of the nanoparticles also mean they can be localized with an external magnetic field. Therefore, a smaller concentration can be used effectively. ![]()

Credit: PNAS
Laser technology can help ensure the delivery of drug and gene therapy at the cellular level without damaging surrounding tissue, according to research published in Nature Scientific Reports.
Investigators paired crystalline magnetic carbon nanoparticles and continuous wave near-infrared laser beams in what is called photothermal delivery.
And they used this delivery method to introduce impermeable dyes and small DNA molecules into human cancer cells.
This work grew out of a previous study in which the researchers used a 50 to 100 milliwatt laser and the same carbon nanoparticle, which absorbs the beam, to heat up and destroy cancer cells in the lab.
“In [the current study, we] used a lower-power, 20 to 30 milliwatt, continuous wave near-infrared laser and the nanoparticle to permeate the cell membrane without killing the cells,” said Ali Koymen, PhD, of the University of Texas at Arlington.
“This method stretches the desired cell membrane to allow for delivery and has the added bonus of creating a fluid flow that speeds the movement of what is being delivered.”
The investigators noted that, currently, the predominant practice is using viruses for delivery to cells. Unfortunately, the scope of what can be delivered with viruses is severely limited, and virus interaction can lead to inflammatory responses and other complications.
Researchers looking to create a path into the cell without employing a virus have experimented with using UV-visible light laser beams alone. But that method damages surrounding cells and has a relatively shallow level of effectiveness.
Dr Koymen and his colleagues said a significant advantage of their method is that the near-infrared light absorption of the nanoparticle can be used to selectively amplify the interaction of low-power laser with targeted tissue, and laser-induced damage to non-targeted cells can be avoided.
The magnetic properties of the nanoparticles also mean they can be localized with an external magnetic field. Therefore, a smaller concentration can be used effectively. ![]()

Credit: PNAS
Laser technology can help ensure the delivery of drug and gene therapy at the cellular level without damaging surrounding tissue, according to research published in Nature Scientific Reports.
Investigators paired crystalline magnetic carbon nanoparticles and continuous wave near-infrared laser beams in what is called photothermal delivery.
And they used this delivery method to introduce impermeable dyes and small DNA molecules into human cancer cells.
This work grew out of a previous study in which the researchers used a 50 to 100 milliwatt laser and the same carbon nanoparticle, which absorbs the beam, to heat up and destroy cancer cells in the lab.
“In [the current study, we] used a lower-power, 20 to 30 milliwatt, continuous wave near-infrared laser and the nanoparticle to permeate the cell membrane without killing the cells,” said Ali Koymen, PhD, of the University of Texas at Arlington.
“This method stretches the desired cell membrane to allow for delivery and has the added bonus of creating a fluid flow that speeds the movement of what is being delivered.”
The investigators noted that, currently, the predominant practice is using viruses for delivery to cells. Unfortunately, the scope of what can be delivered with viruses is severely limited, and virus interaction can lead to inflammatory responses and other complications.
Researchers looking to create a path into the cell without employing a virus have experimented with using UV-visible light laser beams alone. But that method damages surrounding cells and has a relatively shallow level of effectiveness.
Dr Koymen and his colleagues said a significant advantage of their method is that the near-infrared light absorption of the nanoparticle can be used to selectively amplify the interaction of low-power laser with targeted tissue, and laser-induced damage to non-targeted cells can be avoided.
The magnetic properties of the nanoparticles also mean they can be localized with an external magnetic field. Therefore, a smaller concentration can be used effectively. ![]()
Targeting B-cell signaling pathways: a central role for Bruton’s tyrosine kinase
B-cell cancers constitute a large group of diseases with diverse clinical and pathological characteristics that arise from the B (bursal- or bone marrow-derived) lymphocytes of the immune system. B cells are involved in humoral immunity as part of the adaptive immune response. They display a unique B-cell receptor (BCR) on their surface which binds to a specific antigen. Antigen- binding activates the process of clonal expansion, during which the B cell reproduces to form an army of clones that secrete the same antibody. These antibodies then bind to the target antigen on foreign cells and initiate a range of immune responses that ultimately lead to the destruction of that cell.
Click on the PDF icon at the top of this introduction to read the full article.
B-cell cancers constitute a large group of diseases with diverse clinical and pathological characteristics that arise from the B (bursal- or bone marrow-derived) lymphocytes of the immune system. B cells are involved in humoral immunity as part of the adaptive immune response. They display a unique B-cell receptor (BCR) on their surface which binds to a specific antigen. Antigen- binding activates the process of clonal expansion, during which the B cell reproduces to form an army of clones that secrete the same antibody. These antibodies then bind to the target antigen on foreign cells and initiate a range of immune responses that ultimately lead to the destruction of that cell.
Click on the PDF icon at the top of this introduction to read the full article.
B-cell cancers constitute a large group of diseases with diverse clinical and pathological characteristics that arise from the B (bursal- or bone marrow-derived) lymphocytes of the immune system. B cells are involved in humoral immunity as part of the adaptive immune response. They display a unique B-cell receptor (BCR) on their surface which binds to a specific antigen. Antigen- binding activates the process of clonal expansion, during which the B cell reproduces to form an army of clones that secrete the same antibody. These antibodies then bind to the target antigen on foreign cells and initiate a range of immune responses that ultimately lead to the destruction of that cell.
Click on the PDF icon at the top of this introduction to read the full article.
Contemporary management of small renal tumors
The incidence of kidney cancer in the United States is rising because the increased use of cross-sectional imaging is resulting in more tumors being detected and because the population is aging. In addition, a stage migration in kidney cancer has been observed, again because of improved detection, with an increase in stage T1 tumors and a concomitant decrease in the number of stage T2 to T4 tumors. Recent studies have shown that up to 80% of small renal tumors (SRTs) either have an indolent course or are histologically benign. These findings raise the question of what the optimal management of SRTs should be. Radical nephrectomy, the traditional, most aggressive, and still most frequently used extirpative surgery, has been shown to increase the risk of chronic kidney disease. Therefore, during the past 2 decades there has been a shift toward nephron-sparing surgery in carefully selected patients as such procedures have demonstrated equivalent oncologic outcomes with a decrease in long-term renal-induced morbidities.
Click on the PDF icon at the top of this introduction to read the full article.
The incidence of kidney cancer in the United States is rising because the increased use of cross-sectional imaging is resulting in more tumors being detected and because the population is aging. In addition, a stage migration in kidney cancer has been observed, again because of improved detection, with an increase in stage T1 tumors and a concomitant decrease in the number of stage T2 to T4 tumors. Recent studies have shown that up to 80% of small renal tumors (SRTs) either have an indolent course or are histologically benign. These findings raise the question of what the optimal management of SRTs should be. Radical nephrectomy, the traditional, most aggressive, and still most frequently used extirpative surgery, has been shown to increase the risk of chronic kidney disease. Therefore, during the past 2 decades there has been a shift toward nephron-sparing surgery in carefully selected patients as such procedures have demonstrated equivalent oncologic outcomes with a decrease in long-term renal-induced morbidities.
Click on the PDF icon at the top of this introduction to read the full article.
The incidence of kidney cancer in the United States is rising because the increased use of cross-sectional imaging is resulting in more tumors being detected and because the population is aging. In addition, a stage migration in kidney cancer has been observed, again because of improved detection, with an increase in stage T1 tumors and a concomitant decrease in the number of stage T2 to T4 tumors. Recent studies have shown that up to 80% of small renal tumors (SRTs) either have an indolent course or are histologically benign. These findings raise the question of what the optimal management of SRTs should be. Radical nephrectomy, the traditional, most aggressive, and still most frequently used extirpative surgery, has been shown to increase the risk of chronic kidney disease. Therefore, during the past 2 decades there has been a shift toward nephron-sparing surgery in carefully selected patients as such procedures have demonstrated equivalent oncologic outcomes with a decrease in long-term renal-induced morbidities.
Click on the PDF icon at the top of this introduction to read the full article.
Letter to the Editor
McCausland and colleagues[1] have published an excellent study on the association of dysnatremia with morbidity and mortality in patients undergoing major orthopedic surgery, which found that it was associated with greater mortality. However, we have some concerns regarding the article and wish to share them.
First, what is the definition of major orthopedic surgery? The authors did not give us a criterion. In our opinion, internal or femoral neck fracture belongs to minor orthopedic surgery, but such fractures usually occurs in patients older than 65 years, who have a higher incidence of perioperative hyponatremia. Therefore, a detailed definition of major orthopedic surgery was needed in this article.
Second, the sample in this study included individuals aged 18 years and was not limited to individuals with fractures. However, as we know, young patients are usually healthy and without dysnatremia except for multiple fractures. Those with multiple fractures also have a higher chance of hyponatremia and higher mortality, mainly caused by the trauma itself. We think such confounding factors could affect the validity of this article.
- , , . Association of serum sodium with morbidity and mortality in hospitalized patients undergoing major orthopedic surgery. J Hosp Med. 2014;9(5):297–302.
McCausland and colleagues[1] have published an excellent study on the association of dysnatremia with morbidity and mortality in patients undergoing major orthopedic surgery, which found that it was associated with greater mortality. However, we have some concerns regarding the article and wish to share them.
First, what is the definition of major orthopedic surgery? The authors did not give us a criterion. In our opinion, internal or femoral neck fracture belongs to minor orthopedic surgery, but such fractures usually occurs in patients older than 65 years, who have a higher incidence of perioperative hyponatremia. Therefore, a detailed definition of major orthopedic surgery was needed in this article.
Second, the sample in this study included individuals aged 18 years and was not limited to individuals with fractures. However, as we know, young patients are usually healthy and without dysnatremia except for multiple fractures. Those with multiple fractures also have a higher chance of hyponatremia and higher mortality, mainly caused by the trauma itself. We think such confounding factors could affect the validity of this article.
McCausland and colleagues[1] have published an excellent study on the association of dysnatremia with morbidity and mortality in patients undergoing major orthopedic surgery, which found that it was associated with greater mortality. However, we have some concerns regarding the article and wish to share them.
First, what is the definition of major orthopedic surgery? The authors did not give us a criterion. In our opinion, internal or femoral neck fracture belongs to minor orthopedic surgery, but such fractures usually occurs in patients older than 65 years, who have a higher incidence of perioperative hyponatremia. Therefore, a detailed definition of major orthopedic surgery was needed in this article.
Second, the sample in this study included individuals aged 18 years and was not limited to individuals with fractures. However, as we know, young patients are usually healthy and without dysnatremia except for multiple fractures. Those with multiple fractures also have a higher chance of hyponatremia and higher mortality, mainly caused by the trauma itself. We think such confounding factors could affect the validity of this article.
- , , . Association of serum sodium with morbidity and mortality in hospitalized patients undergoing major orthopedic surgery. J Hosp Med. 2014;9(5):297–302.
- , , . Association of serum sodium with morbidity and mortality in hospitalized patients undergoing major orthopedic surgery. J Hosp Med. 2014;9(5):297–302.
Letter to the Editor
We thank Drs. Liu and Zhang for their letter and comments. Each diagnostic code in our dataset was individually reviewed by a board‐certified senior orthopedic surgeon (Dr. Wright). We considered procedures as major if they were of long duration, had the potential for significant blood loss, or represented major physiologic stress, including significant fluid balance requirements, in the opinion of our orthopedist coauthor. This set of diagnoses did include femoral neck fractures.
In our original analyses, we included fracture as a covariate in all statistical models and subsequently performed subgroup analyses according to the presence or absence of a diagnosis of fracture. As reported in our article,[1] J‐shaped associations of dysnatremia with greater length of stay were evident in those with and without fractures. In the 30‐day mortality analyses, only mild hyponatremia and hypernatremia remained associated with greater mortality in those with fracture. In those without a diagnosis of fracture, only moderate/severe hyponatremia remained associated with greater 30‐day mortality.
To assess for differences in associations of hyponatremia with outcomes according to age, we dichotomized this variable into those 65 years old versus 65 years old. We then fit model 3 from our original article to determine the adjusted effect estimates for length of stay and 30‐day mortality (Tables 1 and 2, respectively).
While the associations of dysnatremia with 30‐day mortality did not reach statistical significance in the 65 years age group, these results must be interpreted with caution due to the low number of events (35 deaths). We did not perform smaller subgroups analyses according to fracture type due to concerns of multiple comparisons testing, loss of statistical power, and inaccurate interpretation of effect estimates.
| Difference (95% CI) in Length of Stay in Days According to Category of Perioperative SNa | ||||
|---|---|---|---|---|
| 130 mmol/L, n=198 | 131134 mmol/L, n=1,036) | 135143 mmol/L, n=14,563 | 144 mmol/L, n=409 | |
| ||||
| Model 3 | ||||
| 65 years old | 2.3 (1.63.3), P0.001 | 1.4 (1.21.6), P0.001 | Ref | 1.5 (1.31.8), P0.001 |
| 65 years old | 1.4 (1.11.7), P=0.001 | 1.4 (1.21.5), P0.001 | Ref | 1.3 (1.11.5), P=0.002 |
| Hazard Ratio (95% CI) for 30‐Day Mortality According to Category of Perioperative SNa | ||||
|---|---|---|---|---|
| 130 mmol/L, n=198 | 131134 mmol/L, n=1,036 | 135143 mmol/L, n=14,563 | 144 mmol/L, n=409 | |
| ||||
| Model 3 | ||||
| 65 years old | 1.36 (0.7710.2), P=0.77 | 2.19 (0.935.19), P=0.07 | Ref | 4.17 (0.9718.0), P=0.06 |
| 65 years old | 2.44 (1.274.69), P=0.008 | 1.64 (1.052.55), P=0.03 | Ref | 2.98 (1.725.15), P0.001 |
- , , . Association of serum sodium with morbidity and mortality in hospitalized patients undergoing major orthopedic surgery. J Hosp Med. 2014;9(5):297–302.
We thank Drs. Liu and Zhang for their letter and comments. Each diagnostic code in our dataset was individually reviewed by a board‐certified senior orthopedic surgeon (Dr. Wright). We considered procedures as major if they were of long duration, had the potential for significant blood loss, or represented major physiologic stress, including significant fluid balance requirements, in the opinion of our orthopedist coauthor. This set of diagnoses did include femoral neck fractures.
In our original analyses, we included fracture as a covariate in all statistical models and subsequently performed subgroup analyses according to the presence or absence of a diagnosis of fracture. As reported in our article,[1] J‐shaped associations of dysnatremia with greater length of stay were evident in those with and without fractures. In the 30‐day mortality analyses, only mild hyponatremia and hypernatremia remained associated with greater mortality in those with fracture. In those without a diagnosis of fracture, only moderate/severe hyponatremia remained associated with greater 30‐day mortality.
To assess for differences in associations of hyponatremia with outcomes according to age, we dichotomized this variable into those 65 years old versus 65 years old. We then fit model 3 from our original article to determine the adjusted effect estimates for length of stay and 30‐day mortality (Tables 1 and 2, respectively).
While the associations of dysnatremia with 30‐day mortality did not reach statistical significance in the 65 years age group, these results must be interpreted with caution due to the low number of events (35 deaths). We did not perform smaller subgroups analyses according to fracture type due to concerns of multiple comparisons testing, loss of statistical power, and inaccurate interpretation of effect estimates.
| Difference (95% CI) in Length of Stay in Days According to Category of Perioperative SNa | ||||
|---|---|---|---|---|
| 130 mmol/L, n=198 | 131134 mmol/L, n=1,036) | 135143 mmol/L, n=14,563 | 144 mmol/L, n=409 | |
| ||||
| Model 3 | ||||
| 65 years old | 2.3 (1.63.3), P0.001 | 1.4 (1.21.6), P0.001 | Ref | 1.5 (1.31.8), P0.001 |
| 65 years old | 1.4 (1.11.7), P=0.001 | 1.4 (1.21.5), P0.001 | Ref | 1.3 (1.11.5), P=0.002 |
| Hazard Ratio (95% CI) for 30‐Day Mortality According to Category of Perioperative SNa | ||||
|---|---|---|---|---|
| 130 mmol/L, n=198 | 131134 mmol/L, n=1,036 | 135143 mmol/L, n=14,563 | 144 mmol/L, n=409 | |
| ||||
| Model 3 | ||||
| 65 years old | 1.36 (0.7710.2), P=0.77 | 2.19 (0.935.19), P=0.07 | Ref | 4.17 (0.9718.0), P=0.06 |
| 65 years old | 2.44 (1.274.69), P=0.008 | 1.64 (1.052.55), P=0.03 | Ref | 2.98 (1.725.15), P0.001 |
We thank Drs. Liu and Zhang for their letter and comments. Each diagnostic code in our dataset was individually reviewed by a board‐certified senior orthopedic surgeon (Dr. Wright). We considered procedures as major if they were of long duration, had the potential for significant blood loss, or represented major physiologic stress, including significant fluid balance requirements, in the opinion of our orthopedist coauthor. This set of diagnoses did include femoral neck fractures.
In our original analyses, we included fracture as a covariate in all statistical models and subsequently performed subgroup analyses according to the presence or absence of a diagnosis of fracture. As reported in our article,[1] J‐shaped associations of dysnatremia with greater length of stay were evident in those with and without fractures. In the 30‐day mortality analyses, only mild hyponatremia and hypernatremia remained associated with greater mortality in those with fracture. In those without a diagnosis of fracture, only moderate/severe hyponatremia remained associated with greater 30‐day mortality.
To assess for differences in associations of hyponatremia with outcomes according to age, we dichotomized this variable into those 65 years old versus 65 years old. We then fit model 3 from our original article to determine the adjusted effect estimates for length of stay and 30‐day mortality (Tables 1 and 2, respectively).
While the associations of dysnatremia with 30‐day mortality did not reach statistical significance in the 65 years age group, these results must be interpreted with caution due to the low number of events (35 deaths). We did not perform smaller subgroups analyses according to fracture type due to concerns of multiple comparisons testing, loss of statistical power, and inaccurate interpretation of effect estimates.
| Difference (95% CI) in Length of Stay in Days According to Category of Perioperative SNa | ||||
|---|---|---|---|---|
| 130 mmol/L, n=198 | 131134 mmol/L, n=1,036) | 135143 mmol/L, n=14,563 | 144 mmol/L, n=409 | |
| ||||
| Model 3 | ||||
| 65 years old | 2.3 (1.63.3), P0.001 | 1.4 (1.21.6), P0.001 | Ref | 1.5 (1.31.8), P0.001 |
| 65 years old | 1.4 (1.11.7), P=0.001 | 1.4 (1.21.5), P0.001 | Ref | 1.3 (1.11.5), P=0.002 |
| Hazard Ratio (95% CI) for 30‐Day Mortality According to Category of Perioperative SNa | ||||
|---|---|---|---|---|
| 130 mmol/L, n=198 | 131134 mmol/L, n=1,036 | 135143 mmol/L, n=14,563 | 144 mmol/L, n=409 | |
| ||||
| Model 3 | ||||
| 65 years old | 1.36 (0.7710.2), P=0.77 | 2.19 (0.935.19), P=0.07 | Ref | 4.17 (0.9718.0), P=0.06 |
| 65 years old | 2.44 (1.274.69), P=0.008 | 1.64 (1.052.55), P=0.03 | Ref | 2.98 (1.725.15), P0.001 |
- , , . Association of serum sodium with morbidity and mortality in hospitalized patients undergoing major orthopedic surgery. J Hosp Med. 2014;9(5):297–302.
- , , . Association of serum sodium with morbidity and mortality in hospitalized patients undergoing major orthopedic surgery. J Hosp Med. 2014;9(5):297–302.
New and Noteworthy Information—July 2014
People with a higher education recover better from a moderate to severe traumatic brain injury (TBI), according to a study published May 6 in Neurology. Researchers found that patients with the equivalent of at least a college education were seven times more likely than those who did not finish high school to be disability-free one year after a TBI. The investigators examined 769 patients, 219 of whom were free of any detectable disability. The study authors theorized that TBI patients with increased cognitive reserve capabilities may heal in a different way that allows them to return to their pre-injury function. These patients also may be able to adapt and form new pathways in their brains to compensate for the injury.
The risk of stroke may be reduced by eating more fruits and vegetables, according to a study published online ahead of print May 8 in Stroke. Researchers conducted a meta-analysis of 20 studies published within the past 19 years to assess the effects of fruit and vegetable consumption on the risk of stroke; the combined studies included 760,629 men and 16,981 women who previously had strokes. The findings suggest that stroke risk decreased by 32% with every 200 grams of fruit consumed daily and 11% with every 200 grams of vegetables consumed daily. The investigators combined the results of six studies from the United States, six from Asia, and eight from Europe. “Improving diet and lifestyle is critical for heart and stroke risk reduction in the general population,” the researchers stated.
One-third of all unruptured intracranial aneurysms in people of working age ruptured during a lifelong follow-up, according to a study published online ahead of print May 22 in Stroke. Researchers monitored 118 patients (median age at diagnosis, 43.5) with aneurysms from 1956 until death or subarachnoid hemorrhage occurred. The risk of rupture was particularly high for female smokers with brain aneurysms 7 mm or more in diameter, and the size of an aneurysm had little impact on its risk for rupture, particularly for men. The risk of rupture among nonsmoking men was also exceptionally low. “Because even small unruptured intracranial aneurysms ruptured, treatment decisions of unruptured intracranial aneurysms should perhaps be based on the risk factor status,” the investigators concluded.
An international group of researchers in multiple sclerosis (MS) has proposed updated clinical course descriptions of the disease, according to a report published online ahead of print May 28 in Neurology. The International Advisory Committee on Clinical Trials of MS, which is jointly supported by the National MS Society and the European Committee for Treatment and Research in MS (ECTRIMS), suggested that clinicians not only determine a person’s course of MS, but also further subcategorize that course as active or not active and progressing or not progressing, based on clinical evidence of changes in disability. Another recommended area for further research by the committee includes long-term studies to track people with MS over time. “These revisions should make communication with patients and among physicians clearer and should also enhance the design, recruitment, and conduct of future clinical trials,” the investigators stated.
A moderate level of dietary protein intake may lower the risk of stroke, according to a meta-analysis that was published online ahead of print June 11 in Neurology. The analysis consisted of seven studies with a total of 254,489 participants who were followed for an average of 14 years. Overall, participants with the highest amount of protein in their diets were 20% less likely to develop a stroke, compared with those who had the lowest amount of protein in their diets. For every extra 20 grams per day of protein eaten, the risk of stroke decreased by 26%. The results accounted for other factors that could affect the risk of stroke, such as high cholesterol and smoking. “These results indicate that stroke risk may be reduced by replacing red meat with other protein sources, such as fish,” stated the researchers.
The FDA will review Genzyme’s resubmission of its supplemental Biologics License Application seeking approval of Lemtrada (alemtuzumab) for the treatment of patients with relapsing forms of multiple sclerosis (MS). The resubmission is based on data from the same clinical trials included in the original application. It also provides additional information and supplemental analyses to address issues previously outlined by the FDA in its December 27, 2013, Complete Response Letter. A six-month review period has been assigned, and Genzyme (Cambridge, Massachusetts) expects the FDA to begin that review in the fourth quarter.
The risk of developing cognitive impairment is significantly higher for individuals with poor cardiovascular health than for those with intermediate or ideal cardiovascular health, investigators reported June 11 in the Journal of the American Heart Association. The study included 17,761 people ages 45 and older who had normal cognitive function and no history of stroke (mental function was evaluated four years later). The researchers used data from the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study to determine cardiovascular health status based on the American Heart Association Life’s Simple 7 score. Study findings showed that people with the lowest cardiovascular health scores were more likely to have impairment on learning, memory, and verbal fluency tests than were their counterparts with intermediate or better risk profiles.
Researchers have developed an MRI technique that aids in the early diagnosis of Parkinson’s disease with 85% accuracy, according to a study published online ahead of print June 11 in Neurology. The investigators compared 19 people with early-stage Parkinson’s disease while not on medication with 19 healthy people, matched for gender and age. Data suggest that patients with Parkinson’s disease had much lower connectivity in the basal ganglia. The researchers defined a threshold level of connectivity within the basal ganglia network. Connectivity below this level helped to predict who had Parkinson’s disease with 100% sensitivity and 89.5% specificity. The study authors also conducted their MRI test in a second group of 13 early-stage Parkinson’s patients as a validation of the approach; they correctly identified 11 of the 13 patients.
Blood pressure in later life may affect brain pathology and cognitive performance, depending on blood pressure at midlife, according to a study that was published online ahead of print June 4 in Neurology. Researchers examined data regarding 4,057 older participants (average age, 76) without dementia whose blood pressure had been measured during middle age. The patients’ blood pressure was measured again, and participants underwent MRI and tests of memory and cognition. Higher blood pressure in late life was associated with an increased risk of brain lesions, especially among patients without high blood pressure in middle age. Among participants with high blood pressure in middle age, lower diastolic blood pressure in late life was associated with smaller brain volumes and decreased memory and cognitive performance.
A copper compound could provide a therapy for patients with amyotrophic lateral sclerosis (ALS), according to a study that was published June 4 in the Journal of Neuroscience. Mutations in copper–zinc-superoxide dismutase (SOD1) are believed to cause ALS in humans and transgenic mice. Investigators found that most SOD1 in the spinal cord of mouse models of ALS was copper deficient. Treatment with copper (ATSM) decreased the pool of copper-deficient SOD1 and increased the pool of fully metallated SOD1 in the mice’s spinal cords. In addition, the compound significantly extended the mice’s survival and improved their locomotor function. When the researchers tracked isotopically enriched copper, they found that the increase in fully metallated SOD1 depended on the transfer of copper from copper (ATSM) to SOD1, suggesting that increased copper content of mutant SOD1 improved survival and locomotor function.
Quantitative susceptibility (QS) MRI may reflect disease progression accurately in patients with multiple sclerosis (MS), according to a study published online ahead of print May 4 in Radiology. Twenty-five patients with relapsing-remitting MS or clinically isolated syndrome and 15 age- and sex-matched controls underwent 7-T MRI. Researchers computed quantitative maps of MRI susceptibility parameters. The QS maps identified voxel-level increases in iron deposition in the subcortical gray matter of patients with MS, compared with controls. QS was strongly correlated with patients’ Expanded Disability Status Scale (EDSS) scores. The volume of total white matter damage on QS maps correlated significantly with EDSS. Voxelwise QS indicated that age contributed to demyelination in patients with MS, suggesting that age-adjusted clinical scores may provide robust measures of disease severity.
—Kimberly Williams
People with a higher education recover better from a moderate to severe traumatic brain injury (TBI), according to a study published May 6 in Neurology. Researchers found that patients with the equivalent of at least a college education were seven times more likely than those who did not finish high school to be disability-free one year after a TBI. The investigators examined 769 patients, 219 of whom were free of any detectable disability. The study authors theorized that TBI patients with increased cognitive reserve capabilities may heal in a different way that allows them to return to their pre-injury function. These patients also may be able to adapt and form new pathways in their brains to compensate for the injury.
The risk of stroke may be reduced by eating more fruits and vegetables, according to a study published online ahead of print May 8 in Stroke. Researchers conducted a meta-analysis of 20 studies published within the past 19 years to assess the effects of fruit and vegetable consumption on the risk of stroke; the combined studies included 760,629 men and 16,981 women who previously had strokes. The findings suggest that stroke risk decreased by 32% with every 200 grams of fruit consumed daily and 11% with every 200 grams of vegetables consumed daily. The investigators combined the results of six studies from the United States, six from Asia, and eight from Europe. “Improving diet and lifestyle is critical for heart and stroke risk reduction in the general population,” the researchers stated.
One-third of all unruptured intracranial aneurysms in people of working age ruptured during a lifelong follow-up, according to a study published online ahead of print May 22 in Stroke. Researchers monitored 118 patients (median age at diagnosis, 43.5) with aneurysms from 1956 until death or subarachnoid hemorrhage occurred. The risk of rupture was particularly high for female smokers with brain aneurysms 7 mm or more in diameter, and the size of an aneurysm had little impact on its risk for rupture, particularly for men. The risk of rupture among nonsmoking men was also exceptionally low. “Because even small unruptured intracranial aneurysms ruptured, treatment decisions of unruptured intracranial aneurysms should perhaps be based on the risk factor status,” the investigators concluded.
An international group of researchers in multiple sclerosis (MS) has proposed updated clinical course descriptions of the disease, according to a report published online ahead of print May 28 in Neurology. The International Advisory Committee on Clinical Trials of MS, which is jointly supported by the National MS Society and the European Committee for Treatment and Research in MS (ECTRIMS), suggested that clinicians not only determine a person’s course of MS, but also further subcategorize that course as active or not active and progressing or not progressing, based on clinical evidence of changes in disability. Another recommended area for further research by the committee includes long-term studies to track people with MS over time. “These revisions should make communication with patients and among physicians clearer and should also enhance the design, recruitment, and conduct of future clinical trials,” the investigators stated.
A moderate level of dietary protein intake may lower the risk of stroke, according to a meta-analysis that was published online ahead of print June 11 in Neurology. The analysis consisted of seven studies with a total of 254,489 participants who were followed for an average of 14 years. Overall, participants with the highest amount of protein in their diets were 20% less likely to develop a stroke, compared with those who had the lowest amount of protein in their diets. For every extra 20 grams per day of protein eaten, the risk of stroke decreased by 26%. The results accounted for other factors that could affect the risk of stroke, such as high cholesterol and smoking. “These results indicate that stroke risk may be reduced by replacing red meat with other protein sources, such as fish,” stated the researchers.
The FDA will review Genzyme’s resubmission of its supplemental Biologics License Application seeking approval of Lemtrada (alemtuzumab) for the treatment of patients with relapsing forms of multiple sclerosis (MS). The resubmission is based on data from the same clinical trials included in the original application. It also provides additional information and supplemental analyses to address issues previously outlined by the FDA in its December 27, 2013, Complete Response Letter. A six-month review period has been assigned, and Genzyme (Cambridge, Massachusetts) expects the FDA to begin that review in the fourth quarter.
The risk of developing cognitive impairment is significantly higher for individuals with poor cardiovascular health than for those with intermediate or ideal cardiovascular health, investigators reported June 11 in the Journal of the American Heart Association. The study included 17,761 people ages 45 and older who had normal cognitive function and no history of stroke (mental function was evaluated four years later). The researchers used data from the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study to determine cardiovascular health status based on the American Heart Association Life’s Simple 7 score. Study findings showed that people with the lowest cardiovascular health scores were more likely to have impairment on learning, memory, and verbal fluency tests than were their counterparts with intermediate or better risk profiles.
Researchers have developed an MRI technique that aids in the early diagnosis of Parkinson’s disease with 85% accuracy, according to a study published online ahead of print June 11 in Neurology. The investigators compared 19 people with early-stage Parkinson’s disease while not on medication with 19 healthy people, matched for gender and age. Data suggest that patients with Parkinson’s disease had much lower connectivity in the basal ganglia. The researchers defined a threshold level of connectivity within the basal ganglia network. Connectivity below this level helped to predict who had Parkinson’s disease with 100% sensitivity and 89.5% specificity. The study authors also conducted their MRI test in a second group of 13 early-stage Parkinson’s patients as a validation of the approach; they correctly identified 11 of the 13 patients.
Blood pressure in later life may affect brain pathology and cognitive performance, depending on blood pressure at midlife, according to a study that was published online ahead of print June 4 in Neurology. Researchers examined data regarding 4,057 older participants (average age, 76) without dementia whose blood pressure had been measured during middle age. The patients’ blood pressure was measured again, and participants underwent MRI and tests of memory and cognition. Higher blood pressure in late life was associated with an increased risk of brain lesions, especially among patients without high blood pressure in middle age. Among participants with high blood pressure in middle age, lower diastolic blood pressure in late life was associated with smaller brain volumes and decreased memory and cognitive performance.
A copper compound could provide a therapy for patients with amyotrophic lateral sclerosis (ALS), according to a study that was published June 4 in the Journal of Neuroscience. Mutations in copper–zinc-superoxide dismutase (SOD1) are believed to cause ALS in humans and transgenic mice. Investigators found that most SOD1 in the spinal cord of mouse models of ALS was copper deficient. Treatment with copper (ATSM) decreased the pool of copper-deficient SOD1 and increased the pool of fully metallated SOD1 in the mice’s spinal cords. In addition, the compound significantly extended the mice’s survival and improved their locomotor function. When the researchers tracked isotopically enriched copper, they found that the increase in fully metallated SOD1 depended on the transfer of copper from copper (ATSM) to SOD1, suggesting that increased copper content of mutant SOD1 improved survival and locomotor function.
Quantitative susceptibility (QS) MRI may reflect disease progression accurately in patients with multiple sclerosis (MS), according to a study published online ahead of print May 4 in Radiology. Twenty-five patients with relapsing-remitting MS or clinically isolated syndrome and 15 age- and sex-matched controls underwent 7-T MRI. Researchers computed quantitative maps of MRI susceptibility parameters. The QS maps identified voxel-level increases in iron deposition in the subcortical gray matter of patients with MS, compared with controls. QS was strongly correlated with patients’ Expanded Disability Status Scale (EDSS) scores. The volume of total white matter damage on QS maps correlated significantly with EDSS. Voxelwise QS indicated that age contributed to demyelination in patients with MS, suggesting that age-adjusted clinical scores may provide robust measures of disease severity.
—Kimberly Williams
People with a higher education recover better from a moderate to severe traumatic brain injury (TBI), according to a study published May 6 in Neurology. Researchers found that patients with the equivalent of at least a college education were seven times more likely than those who did not finish high school to be disability-free one year after a TBI. The investigators examined 769 patients, 219 of whom were free of any detectable disability. The study authors theorized that TBI patients with increased cognitive reserve capabilities may heal in a different way that allows them to return to their pre-injury function. These patients also may be able to adapt and form new pathways in their brains to compensate for the injury.
The risk of stroke may be reduced by eating more fruits and vegetables, according to a study published online ahead of print May 8 in Stroke. Researchers conducted a meta-analysis of 20 studies published within the past 19 years to assess the effects of fruit and vegetable consumption on the risk of stroke; the combined studies included 760,629 men and 16,981 women who previously had strokes. The findings suggest that stroke risk decreased by 32% with every 200 grams of fruit consumed daily and 11% with every 200 grams of vegetables consumed daily. The investigators combined the results of six studies from the United States, six from Asia, and eight from Europe. “Improving diet and lifestyle is critical for heart and stroke risk reduction in the general population,” the researchers stated.
One-third of all unruptured intracranial aneurysms in people of working age ruptured during a lifelong follow-up, according to a study published online ahead of print May 22 in Stroke. Researchers monitored 118 patients (median age at diagnosis, 43.5) with aneurysms from 1956 until death or subarachnoid hemorrhage occurred. The risk of rupture was particularly high for female smokers with brain aneurysms 7 mm or more in diameter, and the size of an aneurysm had little impact on its risk for rupture, particularly for men. The risk of rupture among nonsmoking men was also exceptionally low. “Because even small unruptured intracranial aneurysms ruptured, treatment decisions of unruptured intracranial aneurysms should perhaps be based on the risk factor status,” the investigators concluded.
An international group of researchers in multiple sclerosis (MS) has proposed updated clinical course descriptions of the disease, according to a report published online ahead of print May 28 in Neurology. The International Advisory Committee on Clinical Trials of MS, which is jointly supported by the National MS Society and the European Committee for Treatment and Research in MS (ECTRIMS), suggested that clinicians not only determine a person’s course of MS, but also further subcategorize that course as active or not active and progressing or not progressing, based on clinical evidence of changes in disability. Another recommended area for further research by the committee includes long-term studies to track people with MS over time. “These revisions should make communication with patients and among physicians clearer and should also enhance the design, recruitment, and conduct of future clinical trials,” the investigators stated.
A moderate level of dietary protein intake may lower the risk of stroke, according to a meta-analysis that was published online ahead of print June 11 in Neurology. The analysis consisted of seven studies with a total of 254,489 participants who were followed for an average of 14 years. Overall, participants with the highest amount of protein in their diets were 20% less likely to develop a stroke, compared with those who had the lowest amount of protein in their diets. For every extra 20 grams per day of protein eaten, the risk of stroke decreased by 26%. The results accounted for other factors that could affect the risk of stroke, such as high cholesterol and smoking. “These results indicate that stroke risk may be reduced by replacing red meat with other protein sources, such as fish,” stated the researchers.
The FDA will review Genzyme’s resubmission of its supplemental Biologics License Application seeking approval of Lemtrada (alemtuzumab) for the treatment of patients with relapsing forms of multiple sclerosis (MS). The resubmission is based on data from the same clinical trials included in the original application. It also provides additional information and supplemental analyses to address issues previously outlined by the FDA in its December 27, 2013, Complete Response Letter. A six-month review period has been assigned, and Genzyme (Cambridge, Massachusetts) expects the FDA to begin that review in the fourth quarter.
The risk of developing cognitive impairment is significantly higher for individuals with poor cardiovascular health than for those with intermediate or ideal cardiovascular health, investigators reported June 11 in the Journal of the American Heart Association. The study included 17,761 people ages 45 and older who had normal cognitive function and no history of stroke (mental function was evaluated four years later). The researchers used data from the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study to determine cardiovascular health status based on the American Heart Association Life’s Simple 7 score. Study findings showed that people with the lowest cardiovascular health scores were more likely to have impairment on learning, memory, and verbal fluency tests than were their counterparts with intermediate or better risk profiles.
Researchers have developed an MRI technique that aids in the early diagnosis of Parkinson’s disease with 85% accuracy, according to a study published online ahead of print June 11 in Neurology. The investigators compared 19 people with early-stage Parkinson’s disease while not on medication with 19 healthy people, matched for gender and age. Data suggest that patients with Parkinson’s disease had much lower connectivity in the basal ganglia. The researchers defined a threshold level of connectivity within the basal ganglia network. Connectivity below this level helped to predict who had Parkinson’s disease with 100% sensitivity and 89.5% specificity. The study authors also conducted their MRI test in a second group of 13 early-stage Parkinson’s patients as a validation of the approach; they correctly identified 11 of the 13 patients.
Blood pressure in later life may affect brain pathology and cognitive performance, depending on blood pressure at midlife, according to a study that was published online ahead of print June 4 in Neurology. Researchers examined data regarding 4,057 older participants (average age, 76) without dementia whose blood pressure had been measured during middle age. The patients’ blood pressure was measured again, and participants underwent MRI and tests of memory and cognition. Higher blood pressure in late life was associated with an increased risk of brain lesions, especially among patients without high blood pressure in middle age. Among participants with high blood pressure in middle age, lower diastolic blood pressure in late life was associated with smaller brain volumes and decreased memory and cognitive performance.
A copper compound could provide a therapy for patients with amyotrophic lateral sclerosis (ALS), according to a study that was published June 4 in the Journal of Neuroscience. Mutations in copper–zinc-superoxide dismutase (SOD1) are believed to cause ALS in humans and transgenic mice. Investigators found that most SOD1 in the spinal cord of mouse models of ALS was copper deficient. Treatment with copper (ATSM) decreased the pool of copper-deficient SOD1 and increased the pool of fully metallated SOD1 in the mice’s spinal cords. In addition, the compound significantly extended the mice’s survival and improved their locomotor function. When the researchers tracked isotopically enriched copper, they found that the increase in fully metallated SOD1 depended on the transfer of copper from copper (ATSM) to SOD1, suggesting that increased copper content of mutant SOD1 improved survival and locomotor function.
Quantitative susceptibility (QS) MRI may reflect disease progression accurately in patients with multiple sclerosis (MS), according to a study published online ahead of print May 4 in Radiology. Twenty-five patients with relapsing-remitting MS or clinically isolated syndrome and 15 age- and sex-matched controls underwent 7-T MRI. Researchers computed quantitative maps of MRI susceptibility parameters. The QS maps identified voxel-level increases in iron deposition in the subcortical gray matter of patients with MS, compared with controls. QS was strongly correlated with patients’ Expanded Disability Status Scale (EDSS) scores. The volume of total white matter damage on QS maps correlated significantly with EDSS. Voxelwise QS indicated that age contributed to demyelination in patients with MS, suggesting that age-adjusted clinical scores may provide robust measures of disease severity.
—Kimberly Williams
Confused and nearly naked after going on spending sprees
CASE Nearly naked
Mr. A, age 68, is found sitting in his car, wearing only a jacket, underpants, and boots. He speaks of spreading a message about Osama bin Laden and “taking a census.” Police officers bring him to a hospital emergency department for evaluation.
The examining clinician determines that Mr. A is a danger to himself and others because of mental illness, leading to admission to our state psychiatric hospital.
Mr. A’s wife describes recent spending sprees with large purchases. She had obtained a restraining order against her husband because of his threatening remarks and behaviors. Within days of the order issuance, he got a home equity loan and purchased a $300,000 house.
The medical history is notable for type 2 diabetes mellitus. Although he is not taking medications, his blood sugar is well controlled. Other than an initial resting heart rate of 116 beats per minute, vital signs are stable and within normal limits. Physical examination is unremarkable. Screening laboratory studies are notable for mildly elevated hepatic function, which approaches normal range several days after admission.
Mr. A reports a remote history of alcohol abuse but says he had not been drinking recently, and does not detail his pattern of use. Urine toxicology screen is negative for all substances of abuse.
Mental status examination reveals disheveled appearance, motor agitation, pressured speech, labile affect, loosening of associations, grandiose delusions, and auditory hallucinations. Mr. A’s thought processes are grossly disorganized, such that we could not gather a meaningful history. He believes God is speaking directly to him about plans to build a carousel at Disney World. He makes strange gestures with his hands throughout the interview, as if attempting to trace the shapes of letters and numbers. He frequently speaks of seeing an array of colors. Cognitive examination reveals a score of 5 of 30 on the Montreal Cognitive Assessment (Figure 1), indicating a severe impairment in neurocognitive functioning. He demonstrates limited insight and markedly impaired judgment, and denies having a mental illness.
What should be the next step in managing Mr. A?
a) obtain records from other facilities and collateral history
b) start an antipsychotic
c) order a brain MRI
d) start an alcohol withdrawal protocol
The authors’ observations
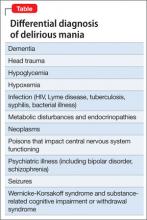
Mr. A showed elements of mania, psychosis, and delirium. We considered a broad differential diagnosis (Table). Mr. A initially could not provide reliable or accurate information. The least invasive next step was to obtain additional history from his wife and other medical records to refine the differential diagnosis.
HISTORY Bizarre behavior
Mr. A allows staff to speak with his wife and obtain records from a psychiatric hospitalization 3 years earlier. Mrs. A reports significant and rapid changes in her husband’s behavior and personality over 3 months, but does not describe a recent alcohol relapse. Mr. A sleeps very little, remaining awake and active throughout the night. He frequently rearranges the furniture in their home for no clear reason. Once, he knocked on the door of a young female neighbor asking if she found him attractive.
Mr. A has a significant criminal history. Approximately 30 years ago, he was charged with attempted murder of his ex-wife and he had faced charges of attempted kidnapping and assaulting a police officer. However, he has no recent legal issues.
Mr. A has a history of episodes that are similar to this presentation. Seven years ago, he impulsively purchased a $650,000 house after his fourth wife died. He then had a $90,000 heart-shaped pool installed. He also drove a tractor through his stepdaughter’s car for no apparent reason. Also, 3 years ago, he displayed symptoms similar to his current presentation, including insomnia, irritability, and grandiosity. He engaged in strange behaviors, such as dressing up and imitating homeless people at his church.
During the hospitalization 3 years ago, clinicians gave Mr. A a diagnosis of bipolar disorder, current episode manic, and delirium of an unclear cause. A medical workup, including brain MRI, did not uncover a basis for his delirium. Antipsychotics (risperidone and perphenazine) and mood stabilizers (lithium and valproic acid), stabilized his condition; after 7 weeks, Mr. A was discharged, but he did not pursue outpatient psychiatric care.
What is the most likely DSM-5 diagnosis?
a) major neurocognitive disorder (dementia)
b) alcohol use disorder (eg, Wernicke- Korsakoff syndrome)
c) delirium secondary to mania
d) psychotic disorder
The authors’ observations
DSM-51 suggests a stepwise approach to diagnosis, with consideration of:
• signs and symptoms
• substance use
• general medical condition
• developmental conflict or stage
• whether a mental disorder is present.
Mr. A’s age and severe cognitive impairment raise the possibility of dementia. Rapid onset, history of similar episodes, and apparent inter-episode recovery make dementia unlikely. The history of alcohol abuse and mildly elevated hepatic function tests suggest a substance use disorder such as Wernicke-Korsakoff syndrome or a withdrawal syndrome. However, there is no evidence of excessive alcohol use over the past several months, toxicology studies were negative, and vital signs were stable. General medical causes for Mr. A’s presentation, such as hypoglycemia, head trauma, intracranial infection, and metabolic disturbance were considered, but physical examination and laboratory studies did not suggest any condition that would explain his condition.
Mr. A’s previous psychiatric hospitalization is critical in clarifying the more likely diagnosis. A similar presentation yielded the diagnosis of bipolar disorder, manic phase. Our working diagnosis, therefore, was bipolar disorder with features of delirious mania.
Delirious mania
Delirious mania was first described by Luther Bell in 1849 and is characterized by an acute and simultaneous onset of mania— severe insomnia, poor judgment, grandiosity, excitement, emotional lability, bizarre hallucinations, and delusions—and delirium—altered consciousness, disorientation, and confusion.2,3 Although there are no diagnostic criteria, some authors suggest that delirious mania is characterized by inappropriate toileting, denudation, profound lack of sleep, and episodic memory impairment that can last hours or days.4 Catatonia frequently is seen with delirious mania.5 Initial case descriptions described a high mortality rate, approaching 75% of patients.6 There is little published literature and no classification of delirious mania in DSM-5.1 Estimates are that delirium is concomitant in 20% to 33% of patients with mania.7,8
Several theories try to clarify the underlying etiology of delirious mania. Jacobowski et al9 summarized the etiology and proposed that it is:
• 1 of 3 types of mania, including: acute and delusional manias, as initially proposed by Kraeplin
• a severe form of catatonia
• a condition akin to, but distinct from, delirium with similar underlying medical causes
• a primary psychiatric disorder underlying the cause of delirium.
EVALUATION Brain changes
For several days, Mr. A continues to engage in strange behavior. He tries to take patients’ belongings, is denudative, crawls on floors, licks walls, is unable to feed himself, and exhibits odd motor movements with purposeless motor activity.
We consult our internal medicine team to identify treatable, medical causes. Results of serum B12, thyroid-stimulating hormone, and rapid plasma reagin studies are within normal limits. Urinalysis is negative. A brain MRI reveals numerous white-matter T2-weighted and FLAIR hyperintensities, indicating small-vessel ischemic changes that are consistent with the findings of an MRI 3 years ago. A sleep-deprived EEG with temporal leads obtained on Day 4 of hospitalization demonstrates a diffusely slow and marginally to poorly organized background, believed to indicate global cerebral dysfunction that is most consistent with nonfocal global encephalopathy. There is no seizure activity. We do not perform a lumbar puncture because of Mr. A’s absence of focal neurologic deficits, lack of fever, and normal white blood cell count.
What is the most appropriate treatment?
a) electroconvulsive therapy (ECT)
b) high-dose benzodiazepine
c) mood stabilizer
d) antipsychotic
The authors’ observations
We strongly suspect that Mr. A has delirious mania. Symptoms and signs of mania include labile mood, excessive spending, grandiosity, insomnia, and psychosis together with delirium (marked disorientation, confusion). We ascribed Mr. A’s odd motor behaviors to catatonia, a hallmark of delirious mania. The literature has little description of EEG findings in suspected cases of delirious mania; however, abnormal EEG tracings have been reported.10 We also speculated that Mr. A’s EEG reflected effects produced by his prescribed antipsychotic regimen.
Treatment
There is no clear consensus on treating delirious mania. Because catatonia is a key feature of delirious mania—whether etiologically or as a prominent sign of the condition—ECT and benzodiazepines are proposed as primary treatments. In a study of 16 patients with delirious mania, Karmacharya et al4 found ECT to be effective, with patients showing improvement after 1 to 4 treatments. Lee et al10 reported similar findings. Although a high-dose benzodiazepine is not as effective as ECT, a 1-time oral dose of 3 to 4 mg of lorazepam has been used to treat delirious mania.
The efficacy of antipsychotic and mood-stabilizing pharmacotherapy is not clear. Bond3 described 3 cases in which patients were treated effectively with a typical antipsychotic (haloperidol or chlorpromazine) and lithium. Jung and Lee11demonstrated the efficacy of atypical antipsychotics, with a marked improvement in symptoms within 1 week. However, other studies do not support these findings. Karmacharya et al4 found that typical antipsychotics 1) make the clinical picture worse by increasing extrapyramidal symptoms and 2) produce inconsistent effects. Mood stabilizers sometimes proved beneficial.
Karmacharya et al4 further argued that the delay in improvement seen with any antipsychotics and mood stabilizers suggest they should not be considered a first-line treatment. These discordant findings are the result of a small number of studies and a lack of understanding of the exact nature of delirious mania.
TREATMENT Quick Response
Mr. A’s symptoms rapidly resolve with a combination of quetiapine, 800 mg/d, haloperidol, 10 mg/d, and lithium, 1,200 mg/d. His mood returns to euthymia and his psychotic symptoms abate. He is able to attend to all activities of daily living. Mental status clears and he is fully oriented and able to hold a logical conversation. He scores 28 out of 30 on a subsequent Montreal Cognitive Assessment, administered 11 days after the initial assessment (Figure 2), indicating normal neurocognitive function. He returns to his baseline level of functioning and is discharged in psychiatrically stable condition. Mr. A has no recollection of the bizarre behaviors he displayed earlier in his hospitalization.
The authors’ observations
We started Mr. A on antipsychotics because of his initial level of agitation. In reviewing pharmacotherapy options for Mr. A’s mania and delirium, we contemplated several options. Quetiapine and lithium were chosen after a review of outside hospital records demonstrated a combination of a mood stabilizer and an antipsychotic was effective in treating a previous similar episode, which led to remission of Mr. A’s symptoms. We chose quetiapine because of it highly sedating properties, suspecting that it would help treat his insomnia. We thought that the risk that lithium would make delirium worse was mitigated by Mr. A’s previous therapeutic response to it. Haloperidol was added for treating delirium, given its more potent D2 antagonism. Mr. A responded quickly to these interventions.
We did not consider ECT at the beginning of Mr. A’s admission, and we avoided sedative-hypnotic agents because we were concerned that a benzodiazepine might make his delirium worse. In light of available data suggesting that ECT and benzodiazepines are preferred treatments for delirious mania, it is noteworthy that Mr. A responded so robustly and rapidly to an antipsychotic and a mood stabilizer.
Bottom Line
Consider delirious mania in any patient who has a history of bipolar disorder presenting with co-occuring symptoms of mania and delirium. Collateral information is vital to establishing a diagnosis. With suspected delirium, rule out concomitant reversible medical problems. Electroconvulsive therapy, high-dose benzodiazepines, antipsychotics, and mood stabilizers have shown efficacy.
Related Resources
• Nunes AL, Cheniaux E. Delirium and mania with catatonic features in a Brazilian patient: response to ECT. J Neuropsychiatry Clin Neurosci. 2014;26(1):E1-E3.
• Danivas V, Behere RV, Varambally S, et al. Electroconvulsive therapy in the treatment of delirious mania: a report of 2 patients. J ECT. 2010;26(4):278-279.
Drug Brand Names
Chlorpromazine • Thorazine Perphenazine • Trilafon
Haloperidol • Haldol Quetiapine • Seroquel
Lithium • Eskalith Risperidone • Risperdal
Lorazepam • Ativan Valproic acid • Depakene
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Nearly naked
Mr. A, age 68, is found sitting in his car, wearing only a jacket, underpants, and boots. He speaks of spreading a message about Osama bin Laden and “taking a census.” Police officers bring him to a hospital emergency department for evaluation.
The examining clinician determines that Mr. A is a danger to himself and others because of mental illness, leading to admission to our state psychiatric hospital.
Mr. A’s wife describes recent spending sprees with large purchases. She had obtained a restraining order against her husband because of his threatening remarks and behaviors. Within days of the order issuance, he got a home equity loan and purchased a $300,000 house.
The medical history is notable for type 2 diabetes mellitus. Although he is not taking medications, his blood sugar is well controlled. Other than an initial resting heart rate of 116 beats per minute, vital signs are stable and within normal limits. Physical examination is unremarkable. Screening laboratory studies are notable for mildly elevated hepatic function, which approaches normal range several days after admission.
Mr. A reports a remote history of alcohol abuse but says he had not been drinking recently, and does not detail his pattern of use. Urine toxicology screen is negative for all substances of abuse.
Mental status examination reveals disheveled appearance, motor agitation, pressured speech, labile affect, loosening of associations, grandiose delusions, and auditory hallucinations. Mr. A’s thought processes are grossly disorganized, such that we could not gather a meaningful history. He believes God is speaking directly to him about plans to build a carousel at Disney World. He makes strange gestures with his hands throughout the interview, as if attempting to trace the shapes of letters and numbers. He frequently speaks of seeing an array of colors. Cognitive examination reveals a score of 5 of 30 on the Montreal Cognitive Assessment (Figure 1), indicating a severe impairment in neurocognitive functioning. He demonstrates limited insight and markedly impaired judgment, and denies having a mental illness.
What should be the next step in managing Mr. A?
a) obtain records from other facilities and collateral history
b) start an antipsychotic
c) order a brain MRI
d) start an alcohol withdrawal protocol
The authors’ observations
Mr. A showed elements of mania, psychosis, and delirium. We considered a broad differential diagnosis (Table). Mr. A initially could not provide reliable or accurate information. The least invasive next step was to obtain additional history from his wife and other medical records to refine the differential diagnosis.
HISTORY Bizarre behavior
Mr. A allows staff to speak with his wife and obtain records from a psychiatric hospitalization 3 years earlier. Mrs. A reports significant and rapid changes in her husband’s behavior and personality over 3 months, but does not describe a recent alcohol relapse. Mr. A sleeps very little, remaining awake and active throughout the night. He frequently rearranges the furniture in their home for no clear reason. Once, he knocked on the door of a young female neighbor asking if she found him attractive.
Mr. A has a significant criminal history. Approximately 30 years ago, he was charged with attempted murder of his ex-wife and he had faced charges of attempted kidnapping and assaulting a police officer. However, he has no recent legal issues.
Mr. A has a history of episodes that are similar to this presentation. Seven years ago, he impulsively purchased a $650,000 house after his fourth wife died. He then had a $90,000 heart-shaped pool installed. He also drove a tractor through his stepdaughter’s car for no apparent reason. Also, 3 years ago, he displayed symptoms similar to his current presentation, including insomnia, irritability, and grandiosity. He engaged in strange behaviors, such as dressing up and imitating homeless people at his church.
During the hospitalization 3 years ago, clinicians gave Mr. A a diagnosis of bipolar disorder, current episode manic, and delirium of an unclear cause. A medical workup, including brain MRI, did not uncover a basis for his delirium. Antipsychotics (risperidone and perphenazine) and mood stabilizers (lithium and valproic acid), stabilized his condition; after 7 weeks, Mr. A was discharged, but he did not pursue outpatient psychiatric care.
What is the most likely DSM-5 diagnosis?
a) major neurocognitive disorder (dementia)
b) alcohol use disorder (eg, Wernicke- Korsakoff syndrome)
c) delirium secondary to mania
d) psychotic disorder
The authors’ observations
DSM-51 suggests a stepwise approach to diagnosis, with consideration of:
• signs and symptoms
• substance use
• general medical condition
• developmental conflict or stage
• whether a mental disorder is present.
Mr. A’s age and severe cognitive impairment raise the possibility of dementia. Rapid onset, history of similar episodes, and apparent inter-episode recovery make dementia unlikely. The history of alcohol abuse and mildly elevated hepatic function tests suggest a substance use disorder such as Wernicke-Korsakoff syndrome or a withdrawal syndrome. However, there is no evidence of excessive alcohol use over the past several months, toxicology studies were negative, and vital signs were stable. General medical causes for Mr. A’s presentation, such as hypoglycemia, head trauma, intracranial infection, and metabolic disturbance were considered, but physical examination and laboratory studies did not suggest any condition that would explain his condition.
Mr. A’s previous psychiatric hospitalization is critical in clarifying the more likely diagnosis. A similar presentation yielded the diagnosis of bipolar disorder, manic phase. Our working diagnosis, therefore, was bipolar disorder with features of delirious mania.
Delirious mania
Delirious mania was first described by Luther Bell in 1849 and is characterized by an acute and simultaneous onset of mania— severe insomnia, poor judgment, grandiosity, excitement, emotional lability, bizarre hallucinations, and delusions—and delirium—altered consciousness, disorientation, and confusion.2,3 Although there are no diagnostic criteria, some authors suggest that delirious mania is characterized by inappropriate toileting, denudation, profound lack of sleep, and episodic memory impairment that can last hours or days.4 Catatonia frequently is seen with delirious mania.5 Initial case descriptions described a high mortality rate, approaching 75% of patients.6 There is little published literature and no classification of delirious mania in DSM-5.1 Estimates are that delirium is concomitant in 20% to 33% of patients with mania.7,8
Several theories try to clarify the underlying etiology of delirious mania. Jacobowski et al9 summarized the etiology and proposed that it is:
• 1 of 3 types of mania, including: acute and delusional manias, as initially proposed by Kraeplin
• a severe form of catatonia
• a condition akin to, but distinct from, delirium with similar underlying medical causes
• a primary psychiatric disorder underlying the cause of delirium.
EVALUATION Brain changes
For several days, Mr. A continues to engage in strange behavior. He tries to take patients’ belongings, is denudative, crawls on floors, licks walls, is unable to feed himself, and exhibits odd motor movements with purposeless motor activity.
We consult our internal medicine team to identify treatable, medical causes. Results of serum B12, thyroid-stimulating hormone, and rapid plasma reagin studies are within normal limits. Urinalysis is negative. A brain MRI reveals numerous white-matter T2-weighted and FLAIR hyperintensities, indicating small-vessel ischemic changes that are consistent with the findings of an MRI 3 years ago. A sleep-deprived EEG with temporal leads obtained on Day 4 of hospitalization demonstrates a diffusely slow and marginally to poorly organized background, believed to indicate global cerebral dysfunction that is most consistent with nonfocal global encephalopathy. There is no seizure activity. We do not perform a lumbar puncture because of Mr. A’s absence of focal neurologic deficits, lack of fever, and normal white blood cell count.
What is the most appropriate treatment?
a) electroconvulsive therapy (ECT)
b) high-dose benzodiazepine
c) mood stabilizer
d) antipsychotic
The authors’ observations
We strongly suspect that Mr. A has delirious mania. Symptoms and signs of mania include labile mood, excessive spending, grandiosity, insomnia, and psychosis together with delirium (marked disorientation, confusion). We ascribed Mr. A’s odd motor behaviors to catatonia, a hallmark of delirious mania. The literature has little description of EEG findings in suspected cases of delirious mania; however, abnormal EEG tracings have been reported.10 We also speculated that Mr. A’s EEG reflected effects produced by his prescribed antipsychotic regimen.
Treatment
There is no clear consensus on treating delirious mania. Because catatonia is a key feature of delirious mania—whether etiologically or as a prominent sign of the condition—ECT and benzodiazepines are proposed as primary treatments. In a study of 16 patients with delirious mania, Karmacharya et al4 found ECT to be effective, with patients showing improvement after 1 to 4 treatments. Lee et al10 reported similar findings. Although a high-dose benzodiazepine is not as effective as ECT, a 1-time oral dose of 3 to 4 mg of lorazepam has been used to treat delirious mania.
The efficacy of antipsychotic and mood-stabilizing pharmacotherapy is not clear. Bond3 described 3 cases in which patients were treated effectively with a typical antipsychotic (haloperidol or chlorpromazine) and lithium. Jung and Lee11demonstrated the efficacy of atypical antipsychotics, with a marked improvement in symptoms within 1 week. However, other studies do not support these findings. Karmacharya et al4 found that typical antipsychotics 1) make the clinical picture worse by increasing extrapyramidal symptoms and 2) produce inconsistent effects. Mood stabilizers sometimes proved beneficial.
Karmacharya et al4 further argued that the delay in improvement seen with any antipsychotics and mood stabilizers suggest they should not be considered a first-line treatment. These discordant findings are the result of a small number of studies and a lack of understanding of the exact nature of delirious mania.
TREATMENT Quick Response
Mr. A’s symptoms rapidly resolve with a combination of quetiapine, 800 mg/d, haloperidol, 10 mg/d, and lithium, 1,200 mg/d. His mood returns to euthymia and his psychotic symptoms abate. He is able to attend to all activities of daily living. Mental status clears and he is fully oriented and able to hold a logical conversation. He scores 28 out of 30 on a subsequent Montreal Cognitive Assessment, administered 11 days after the initial assessment (Figure 2), indicating normal neurocognitive function. He returns to his baseline level of functioning and is discharged in psychiatrically stable condition. Mr. A has no recollection of the bizarre behaviors he displayed earlier in his hospitalization.
The authors’ observations
We started Mr. A on antipsychotics because of his initial level of agitation. In reviewing pharmacotherapy options for Mr. A’s mania and delirium, we contemplated several options. Quetiapine and lithium were chosen after a review of outside hospital records demonstrated a combination of a mood stabilizer and an antipsychotic was effective in treating a previous similar episode, which led to remission of Mr. A’s symptoms. We chose quetiapine because of it highly sedating properties, suspecting that it would help treat his insomnia. We thought that the risk that lithium would make delirium worse was mitigated by Mr. A’s previous therapeutic response to it. Haloperidol was added for treating delirium, given its more potent D2 antagonism. Mr. A responded quickly to these interventions.
We did not consider ECT at the beginning of Mr. A’s admission, and we avoided sedative-hypnotic agents because we were concerned that a benzodiazepine might make his delirium worse. In light of available data suggesting that ECT and benzodiazepines are preferred treatments for delirious mania, it is noteworthy that Mr. A responded so robustly and rapidly to an antipsychotic and a mood stabilizer.
Bottom Line
Consider delirious mania in any patient who has a history of bipolar disorder presenting with co-occuring symptoms of mania and delirium. Collateral information is vital to establishing a diagnosis. With suspected delirium, rule out concomitant reversible medical problems. Electroconvulsive therapy, high-dose benzodiazepines, antipsychotics, and mood stabilizers have shown efficacy.
Related Resources
• Nunes AL, Cheniaux E. Delirium and mania with catatonic features in a Brazilian patient: response to ECT. J Neuropsychiatry Clin Neurosci. 2014;26(1):E1-E3.
• Danivas V, Behere RV, Varambally S, et al. Electroconvulsive therapy in the treatment of delirious mania: a report of 2 patients. J ECT. 2010;26(4):278-279.
Drug Brand Names
Chlorpromazine • Thorazine Perphenazine • Trilafon
Haloperidol • Haldol Quetiapine • Seroquel
Lithium • Eskalith Risperidone • Risperdal
Lorazepam • Ativan Valproic acid • Depakene
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Nearly naked
Mr. A, age 68, is found sitting in his car, wearing only a jacket, underpants, and boots. He speaks of spreading a message about Osama bin Laden and “taking a census.” Police officers bring him to a hospital emergency department for evaluation.
The examining clinician determines that Mr. A is a danger to himself and others because of mental illness, leading to admission to our state psychiatric hospital.
Mr. A’s wife describes recent spending sprees with large purchases. She had obtained a restraining order against her husband because of his threatening remarks and behaviors. Within days of the order issuance, he got a home equity loan and purchased a $300,000 house.
The medical history is notable for type 2 diabetes mellitus. Although he is not taking medications, his blood sugar is well controlled. Other than an initial resting heart rate of 116 beats per minute, vital signs are stable and within normal limits. Physical examination is unremarkable. Screening laboratory studies are notable for mildly elevated hepatic function, which approaches normal range several days after admission.
Mr. A reports a remote history of alcohol abuse but says he had not been drinking recently, and does not detail his pattern of use. Urine toxicology screen is negative for all substances of abuse.
Mental status examination reveals disheveled appearance, motor agitation, pressured speech, labile affect, loosening of associations, grandiose delusions, and auditory hallucinations. Mr. A’s thought processes are grossly disorganized, such that we could not gather a meaningful history. He believes God is speaking directly to him about plans to build a carousel at Disney World. He makes strange gestures with his hands throughout the interview, as if attempting to trace the shapes of letters and numbers. He frequently speaks of seeing an array of colors. Cognitive examination reveals a score of 5 of 30 on the Montreal Cognitive Assessment (Figure 1), indicating a severe impairment in neurocognitive functioning. He demonstrates limited insight and markedly impaired judgment, and denies having a mental illness.
What should be the next step in managing Mr. A?
a) obtain records from other facilities and collateral history
b) start an antipsychotic
c) order a brain MRI
d) start an alcohol withdrawal protocol
The authors’ observations
Mr. A showed elements of mania, psychosis, and delirium. We considered a broad differential diagnosis (Table). Mr. A initially could not provide reliable or accurate information. The least invasive next step was to obtain additional history from his wife and other medical records to refine the differential diagnosis.
HISTORY Bizarre behavior
Mr. A allows staff to speak with his wife and obtain records from a psychiatric hospitalization 3 years earlier. Mrs. A reports significant and rapid changes in her husband’s behavior and personality over 3 months, but does not describe a recent alcohol relapse. Mr. A sleeps very little, remaining awake and active throughout the night. He frequently rearranges the furniture in their home for no clear reason. Once, he knocked on the door of a young female neighbor asking if she found him attractive.
Mr. A has a significant criminal history. Approximately 30 years ago, he was charged with attempted murder of his ex-wife and he had faced charges of attempted kidnapping and assaulting a police officer. However, he has no recent legal issues.
Mr. A has a history of episodes that are similar to this presentation. Seven years ago, he impulsively purchased a $650,000 house after his fourth wife died. He then had a $90,000 heart-shaped pool installed. He also drove a tractor through his stepdaughter’s car for no apparent reason. Also, 3 years ago, he displayed symptoms similar to his current presentation, including insomnia, irritability, and grandiosity. He engaged in strange behaviors, such as dressing up and imitating homeless people at his church.
During the hospitalization 3 years ago, clinicians gave Mr. A a diagnosis of bipolar disorder, current episode manic, and delirium of an unclear cause. A medical workup, including brain MRI, did not uncover a basis for his delirium. Antipsychotics (risperidone and perphenazine) and mood stabilizers (lithium and valproic acid), stabilized his condition; after 7 weeks, Mr. A was discharged, but he did not pursue outpatient psychiatric care.
What is the most likely DSM-5 diagnosis?
a) major neurocognitive disorder (dementia)
b) alcohol use disorder (eg, Wernicke- Korsakoff syndrome)
c) delirium secondary to mania
d) psychotic disorder
The authors’ observations
DSM-51 suggests a stepwise approach to diagnosis, with consideration of:
• signs and symptoms
• substance use
• general medical condition
• developmental conflict or stage
• whether a mental disorder is present.
Mr. A’s age and severe cognitive impairment raise the possibility of dementia. Rapid onset, history of similar episodes, and apparent inter-episode recovery make dementia unlikely. The history of alcohol abuse and mildly elevated hepatic function tests suggest a substance use disorder such as Wernicke-Korsakoff syndrome or a withdrawal syndrome. However, there is no evidence of excessive alcohol use over the past several months, toxicology studies were negative, and vital signs were stable. General medical causes for Mr. A’s presentation, such as hypoglycemia, head trauma, intracranial infection, and metabolic disturbance were considered, but physical examination and laboratory studies did not suggest any condition that would explain his condition.
Mr. A’s previous psychiatric hospitalization is critical in clarifying the more likely diagnosis. A similar presentation yielded the diagnosis of bipolar disorder, manic phase. Our working diagnosis, therefore, was bipolar disorder with features of delirious mania.
Delirious mania
Delirious mania was first described by Luther Bell in 1849 and is characterized by an acute and simultaneous onset of mania— severe insomnia, poor judgment, grandiosity, excitement, emotional lability, bizarre hallucinations, and delusions—and delirium—altered consciousness, disorientation, and confusion.2,3 Although there are no diagnostic criteria, some authors suggest that delirious mania is characterized by inappropriate toileting, denudation, profound lack of sleep, and episodic memory impairment that can last hours or days.4 Catatonia frequently is seen with delirious mania.5 Initial case descriptions described a high mortality rate, approaching 75% of patients.6 There is little published literature and no classification of delirious mania in DSM-5.1 Estimates are that delirium is concomitant in 20% to 33% of patients with mania.7,8
Several theories try to clarify the underlying etiology of delirious mania. Jacobowski et al9 summarized the etiology and proposed that it is:
• 1 of 3 types of mania, including: acute and delusional manias, as initially proposed by Kraeplin
• a severe form of catatonia
• a condition akin to, but distinct from, delirium with similar underlying medical causes
• a primary psychiatric disorder underlying the cause of delirium.
EVALUATION Brain changes
For several days, Mr. A continues to engage in strange behavior. He tries to take patients’ belongings, is denudative, crawls on floors, licks walls, is unable to feed himself, and exhibits odd motor movements with purposeless motor activity.
We consult our internal medicine team to identify treatable, medical causes. Results of serum B12, thyroid-stimulating hormone, and rapid plasma reagin studies are within normal limits. Urinalysis is negative. A brain MRI reveals numerous white-matter T2-weighted and FLAIR hyperintensities, indicating small-vessel ischemic changes that are consistent with the findings of an MRI 3 years ago. A sleep-deprived EEG with temporal leads obtained on Day 4 of hospitalization demonstrates a diffusely slow and marginally to poorly organized background, believed to indicate global cerebral dysfunction that is most consistent with nonfocal global encephalopathy. There is no seizure activity. We do not perform a lumbar puncture because of Mr. A’s absence of focal neurologic deficits, lack of fever, and normal white blood cell count.
What is the most appropriate treatment?
a) electroconvulsive therapy (ECT)
b) high-dose benzodiazepine
c) mood stabilizer
d) antipsychotic
The authors’ observations
We strongly suspect that Mr. A has delirious mania. Symptoms and signs of mania include labile mood, excessive spending, grandiosity, insomnia, and psychosis together with delirium (marked disorientation, confusion). We ascribed Mr. A’s odd motor behaviors to catatonia, a hallmark of delirious mania. The literature has little description of EEG findings in suspected cases of delirious mania; however, abnormal EEG tracings have been reported.10 We also speculated that Mr. A’s EEG reflected effects produced by his prescribed antipsychotic regimen.
Treatment
There is no clear consensus on treating delirious mania. Because catatonia is a key feature of delirious mania—whether etiologically or as a prominent sign of the condition—ECT and benzodiazepines are proposed as primary treatments. In a study of 16 patients with delirious mania, Karmacharya et al4 found ECT to be effective, with patients showing improvement after 1 to 4 treatments. Lee et al10 reported similar findings. Although a high-dose benzodiazepine is not as effective as ECT, a 1-time oral dose of 3 to 4 mg of lorazepam has been used to treat delirious mania.
The efficacy of antipsychotic and mood-stabilizing pharmacotherapy is not clear. Bond3 described 3 cases in which patients were treated effectively with a typical antipsychotic (haloperidol or chlorpromazine) and lithium. Jung and Lee11demonstrated the efficacy of atypical antipsychotics, with a marked improvement in symptoms within 1 week. However, other studies do not support these findings. Karmacharya et al4 found that typical antipsychotics 1) make the clinical picture worse by increasing extrapyramidal symptoms and 2) produce inconsistent effects. Mood stabilizers sometimes proved beneficial.
Karmacharya et al4 further argued that the delay in improvement seen with any antipsychotics and mood stabilizers suggest they should not be considered a first-line treatment. These discordant findings are the result of a small number of studies and a lack of understanding of the exact nature of delirious mania.
TREATMENT Quick Response
Mr. A’s symptoms rapidly resolve with a combination of quetiapine, 800 mg/d, haloperidol, 10 mg/d, and lithium, 1,200 mg/d. His mood returns to euthymia and his psychotic symptoms abate. He is able to attend to all activities of daily living. Mental status clears and he is fully oriented and able to hold a logical conversation. He scores 28 out of 30 on a subsequent Montreal Cognitive Assessment, administered 11 days after the initial assessment (Figure 2), indicating normal neurocognitive function. He returns to his baseline level of functioning and is discharged in psychiatrically stable condition. Mr. A has no recollection of the bizarre behaviors he displayed earlier in his hospitalization.
The authors’ observations
We started Mr. A on antipsychotics because of his initial level of agitation. In reviewing pharmacotherapy options for Mr. A’s mania and delirium, we contemplated several options. Quetiapine and lithium were chosen after a review of outside hospital records demonstrated a combination of a mood stabilizer and an antipsychotic was effective in treating a previous similar episode, which led to remission of Mr. A’s symptoms. We chose quetiapine because of it highly sedating properties, suspecting that it would help treat his insomnia. We thought that the risk that lithium would make delirium worse was mitigated by Mr. A’s previous therapeutic response to it. Haloperidol was added for treating delirium, given its more potent D2 antagonism. Mr. A responded quickly to these interventions.
We did not consider ECT at the beginning of Mr. A’s admission, and we avoided sedative-hypnotic agents because we were concerned that a benzodiazepine might make his delirium worse. In light of available data suggesting that ECT and benzodiazepines are preferred treatments for delirious mania, it is noteworthy that Mr. A responded so robustly and rapidly to an antipsychotic and a mood stabilizer.
Bottom Line
Consider delirious mania in any patient who has a history of bipolar disorder presenting with co-occuring symptoms of mania and delirium. Collateral information is vital to establishing a diagnosis. With suspected delirium, rule out concomitant reversible medical problems. Electroconvulsive therapy, high-dose benzodiazepines, antipsychotics, and mood stabilizers have shown efficacy.
Related Resources
• Nunes AL, Cheniaux E. Delirium and mania with catatonic features in a Brazilian patient: response to ECT. J Neuropsychiatry Clin Neurosci. 2014;26(1):E1-E3.
• Danivas V, Behere RV, Varambally S, et al. Electroconvulsive therapy in the treatment of delirious mania: a report of 2 patients. J ECT. 2010;26(4):278-279.
Drug Brand Names
Chlorpromazine • Thorazine Perphenazine • Trilafon
Haloperidol • Haldol Quetiapine • Seroquel
Lithium • Eskalith Risperidone • Risperdal
Lorazepam • Ativan Valproic acid • Depakene
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Bariatric surgery/Preventive medicine
Until recently, bariatric surgery was considered a cosmetic operation with little physiologic importance. A series of preliminary randomized clinical trials, however, have suggested that bariatric surgery may have importance in mitigating the adverse pathophysiology associated with obesity, including type 2 diabetes and some cardiovascular risk factors.
The finding of a surgical method of modifying this disease, which has occupied research for the last century, is somewhat unexpected after the many false starts associated with medical interventions. The two most popular surgical procedures, the gastric bypass and the sleeve gastrectomy performed using laparoscopic techniques, are currently being performed in obese patients with BMIs of greater than 35 with very low morbidly and rare mortality events. Several nonrandomized and prospective trials have examined the effect of bariatric surgery and reported beneficial effects on diabetes regression and significant reduction in major cardiovascular disease ( JAMA 2012;307:56-65).
The recent report of the 3-year follow-up of the STAMPEDE (Surgical Therapy And Medications Potentially Eradicate Diabetes Efficiently) trial ( N. Engl. J. Med. 2014;370:2002-13) provides additional physiologic information on the benefits of bariatric surgery in 150 obese diabetic patients aged 20-60 years with BMIs of 27-43, compared with intensive medical therapy. Patients were randomized to three arms: intensive medical therapy, gastric bypass, or sleeve gastrectomy. Most of the patients were white women with a history of diabetes for 8.3 years; the mean hemoglobin A1c was 9.3%. At baseline, 43% of the patients required insulin therapy. The primary endpoint was the achievement of HbA1c of 6% or less, which was achieved in 5% of the medically treated patients, compared with 38% in the gastric bypass group and 24% in the sleeve gastrectomy group. Decrease in BMI was the only measure that predicted the achievement of the HbA1c endpoint. Body weight decreased by 4.5% in the intensive medical group, 24.5% in the gastric bypass group, and 21.1% in the sleeve gastrectomy group. Significant decreases in low-density lipoprotein cholesterol and increases in high-density lipoprotein cholesterol were achieved in both surgical intervention groups, compared with the intensive medical care group. In addition, medical control of diabetes was improved and 69% and 43% of the gastrectomy and sleeve bypass group, respectively, were no longer requiring insulin therapy. There was, however, no significant difference in the change in blood pressure in the three groups. There were no life-threatening complications or deaths in the groups, but there were a number of complications associated with the procedure.
The metabolic changes associated with bariatric surgery reported in STAMPEDE open the door for future randomized studies examining long-term morbidity and mortality benefits that may be attributed to this therapy. Bariatric surgery is being performed widely in the United States with very low mortality and morbidity. Previous short-term studies have reported the benefit of bariatric surgery, compared with intensive medical therapy. The longer duration of follow-up in STAMPEDE emphasizes the need for larger randomized trials of this method of therapy. The study of the surgical patients may also provide new insight into the relationship of body fat to the expression of type 2 diabetes.
The prevention of medical disease using surgical techniques in clinical medicine has not been a particularly fertile road of investigation. Intervention in the treatment of coronary artery disease with bypass surgery although associated with symptomatic benefit and with some exceptions, has not been overwhelmingly successful in affecting the long-term mortality of that disease. Bariatric surgery may be the first surgical intervention that can arrest or even reverse type 2 diabetes and its many sequelae.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
Until recently, bariatric surgery was considered a cosmetic operation with little physiologic importance. A series of preliminary randomized clinical trials, however, have suggested that bariatric surgery may have importance in mitigating the adverse pathophysiology associated with obesity, including type 2 diabetes and some cardiovascular risk factors.
The finding of a surgical method of modifying this disease, which has occupied research for the last century, is somewhat unexpected after the many false starts associated with medical interventions. The two most popular surgical procedures, the gastric bypass and the sleeve gastrectomy performed using laparoscopic techniques, are currently being performed in obese patients with BMIs of greater than 35 with very low morbidly and rare mortality events. Several nonrandomized and prospective trials have examined the effect of bariatric surgery and reported beneficial effects on diabetes regression and significant reduction in major cardiovascular disease ( JAMA 2012;307:56-65).
The recent report of the 3-year follow-up of the STAMPEDE (Surgical Therapy And Medications Potentially Eradicate Diabetes Efficiently) trial ( N. Engl. J. Med. 2014;370:2002-13) provides additional physiologic information on the benefits of bariatric surgery in 150 obese diabetic patients aged 20-60 years with BMIs of 27-43, compared with intensive medical therapy. Patients were randomized to three arms: intensive medical therapy, gastric bypass, or sleeve gastrectomy. Most of the patients were white women with a history of diabetes for 8.3 years; the mean hemoglobin A1c was 9.3%. At baseline, 43% of the patients required insulin therapy. The primary endpoint was the achievement of HbA1c of 6% or less, which was achieved in 5% of the medically treated patients, compared with 38% in the gastric bypass group and 24% in the sleeve gastrectomy group. Decrease in BMI was the only measure that predicted the achievement of the HbA1c endpoint. Body weight decreased by 4.5% in the intensive medical group, 24.5% in the gastric bypass group, and 21.1% in the sleeve gastrectomy group. Significant decreases in low-density lipoprotein cholesterol and increases in high-density lipoprotein cholesterol were achieved in both surgical intervention groups, compared with the intensive medical care group. In addition, medical control of diabetes was improved and 69% and 43% of the gastrectomy and sleeve bypass group, respectively, were no longer requiring insulin therapy. There was, however, no significant difference in the change in blood pressure in the three groups. There were no life-threatening complications or deaths in the groups, but there were a number of complications associated with the procedure.
The metabolic changes associated with bariatric surgery reported in STAMPEDE open the door for future randomized studies examining long-term morbidity and mortality benefits that may be attributed to this therapy. Bariatric surgery is being performed widely in the United States with very low mortality and morbidity. Previous short-term studies have reported the benefit of bariatric surgery, compared with intensive medical therapy. The longer duration of follow-up in STAMPEDE emphasizes the need for larger randomized trials of this method of therapy. The study of the surgical patients may also provide new insight into the relationship of body fat to the expression of type 2 diabetes.
The prevention of medical disease using surgical techniques in clinical medicine has not been a particularly fertile road of investigation. Intervention in the treatment of coronary artery disease with bypass surgery although associated with symptomatic benefit and with some exceptions, has not been overwhelmingly successful in affecting the long-term mortality of that disease. Bariatric surgery may be the first surgical intervention that can arrest or even reverse type 2 diabetes and its many sequelae.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
Until recently, bariatric surgery was considered a cosmetic operation with little physiologic importance. A series of preliminary randomized clinical trials, however, have suggested that bariatric surgery may have importance in mitigating the adverse pathophysiology associated with obesity, including type 2 diabetes and some cardiovascular risk factors.
The finding of a surgical method of modifying this disease, which has occupied research for the last century, is somewhat unexpected after the many false starts associated with medical interventions. The two most popular surgical procedures, the gastric bypass and the sleeve gastrectomy performed using laparoscopic techniques, are currently being performed in obese patients with BMIs of greater than 35 with very low morbidly and rare mortality events. Several nonrandomized and prospective trials have examined the effect of bariatric surgery and reported beneficial effects on diabetes regression and significant reduction in major cardiovascular disease ( JAMA 2012;307:56-65).
The recent report of the 3-year follow-up of the STAMPEDE (Surgical Therapy And Medications Potentially Eradicate Diabetes Efficiently) trial ( N. Engl. J. Med. 2014;370:2002-13) provides additional physiologic information on the benefits of bariatric surgery in 150 obese diabetic patients aged 20-60 years with BMIs of 27-43, compared with intensive medical therapy. Patients were randomized to three arms: intensive medical therapy, gastric bypass, or sleeve gastrectomy. Most of the patients were white women with a history of diabetes for 8.3 years; the mean hemoglobin A1c was 9.3%. At baseline, 43% of the patients required insulin therapy. The primary endpoint was the achievement of HbA1c of 6% or less, which was achieved in 5% of the medically treated patients, compared with 38% in the gastric bypass group and 24% in the sleeve gastrectomy group. Decrease in BMI was the only measure that predicted the achievement of the HbA1c endpoint. Body weight decreased by 4.5% in the intensive medical group, 24.5% in the gastric bypass group, and 21.1% in the sleeve gastrectomy group. Significant decreases in low-density lipoprotein cholesterol and increases in high-density lipoprotein cholesterol were achieved in both surgical intervention groups, compared with the intensive medical care group. In addition, medical control of diabetes was improved and 69% and 43% of the gastrectomy and sleeve bypass group, respectively, were no longer requiring insulin therapy. There was, however, no significant difference in the change in blood pressure in the three groups. There were no life-threatening complications or deaths in the groups, but there were a number of complications associated with the procedure.
The metabolic changes associated with bariatric surgery reported in STAMPEDE open the door for future randomized studies examining long-term morbidity and mortality benefits that may be attributed to this therapy. Bariatric surgery is being performed widely in the United States with very low mortality and morbidity. Previous short-term studies have reported the benefit of bariatric surgery, compared with intensive medical therapy. The longer duration of follow-up in STAMPEDE emphasizes the need for larger randomized trials of this method of therapy. The study of the surgical patients may also provide new insight into the relationship of body fat to the expression of type 2 diabetes.
The prevention of medical disease using surgical techniques in clinical medicine has not been a particularly fertile road of investigation. Intervention in the treatment of coronary artery disease with bypass surgery although associated with symptomatic benefit and with some exceptions, has not been overwhelmingly successful in affecting the long-term mortality of that disease. Bariatric surgery may be the first surgical intervention that can arrest or even reverse type 2 diabetes and its many sequelae.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
Updates on Kidney Donation
Q) A good friend was diagnosed with chronic kidney disease (CKD) and is presently undergoing workup for a transplant. He is 60 and otherwise healthy; his glomerular filtration rate (GFR) is 14, and he has no uremic symptoms. If I volunteer to give him a kidney, are there any long-term risks for me?
Kidney failure, dialysis, and kidney transplant are terms that can invoke stress and uncertainty in patients with end-stage renal disease (ESRD) and among their family members and friends. In addition to adjusting to the changes wrought by ESRD, patients may also be burdened by the prospect of a family member or friend donating a kidney to them and the concern that the donation will lead to complications for their donor. Family members or friends who volunteer may also experience stress, uncertain of their own risk for ESRD in the future.
Past research improperly compared relative risk for ESRD in donors with that in the general population (without accounting for higher propensity for complications in donors with preexisting conditions). In an effort to correct this misperception, a study recently published in JAMA compared the risk for ESRD in donors with that in a healthy group of nondonors.1 The nondonor pool was taken from the National Health and Nutrition Examination Survey (NHANES III), which assesses the health and nutritional status of adults and children in the United States.
The JAMA study included a cohort of 96,217 kidney donors in the US in a 17-year period and a cohort of 20,024 participants in a six-year period of the NHANES III trial. This data was then compared to Centers for Medicare & Medicaid Services (CMS) data to determine the development of ESRD in kidney donors. ESRD was defined by CMS as the initiation of dialysis, placement on the kidney transplant waiting list, or receipt of a living or deceased donor kidney transplant.
In addition to comparing risk for ESRD in kidney donors with that of a healthy population of nondonors, the researchers also stratified their results demographically. Thus, the lifetime rate of kidney failure in donors is 90 per 10,000, compared with 326 per 10,000 in the general population of nondonors. In healthy nondonors, the risk for kidney failure was 14 per 10,000. After 15 years, the risk for kidney failure associated with donating a kidney was 51 per 10,000 in African-American donors and 23 per 10,000 in white donors. So while the study did reveal an increased risk associated with kidney donation, the degree of risk is considered small.
These findings demonstrate the importance of understanding the facts surrounding inherent risk for ESRD in kidney donation. Overall, a donor’s lifetime risk is considered minuscule. So, to answer the question, yes, there is a slight increase in risk for kidney failure if you donate to your friend. That said, the risk is 0.014 x a standardized risk of 1. This increases at 15 years to 0.51 for African-American and 0.23 for white donors. With such tiny increases, you can safely feel good about donating a kidney to your friend.
Donna Reesman, MSN, CNP
VP Clinical & Quality Management
St Clair Specialty Physicians Detroit
REFERENCES
1. Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311(6):579-586.
2. CDC. HIV in the United States: at a glance (2013). www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed June 16, 2014.
3. Frassetto LA, Tan-Tam C, Stock PG. Renal transplantation in patients with HIV. Nat Rev Nephrol. 2009;5(10):582-589.
4. Malani PN. New law allows organ transplants from deceased HIV-infected donors to HIV-infected recipients. JAMA. 2013;310(23): 2492-2493.
5. Muller E, Kahn D, Mendelson M. Renal transplantation between HIV-positive donors and recipients. N Engl J Med. 2010;362(24):2336-2337.
6. Mariani LH, Berns JS. Viral nephropathies. In: Gilbert SJ, Weiner DE, eds. National Kidney Foundation’s Primer on Kidney Diseases. 6th ed. Elsevier; 2014:253-261.
Q) A good friend was diagnosed with chronic kidney disease (CKD) and is presently undergoing workup for a transplant. He is 60 and otherwise healthy; his glomerular filtration rate (GFR) is 14, and he has no uremic symptoms. If I volunteer to give him a kidney, are there any long-term risks for me?
Kidney failure, dialysis, and kidney transplant are terms that can invoke stress and uncertainty in patients with end-stage renal disease (ESRD) and among their family members and friends. In addition to adjusting to the changes wrought by ESRD, patients may also be burdened by the prospect of a family member or friend donating a kidney to them and the concern that the donation will lead to complications for their donor. Family members or friends who volunteer may also experience stress, uncertain of their own risk for ESRD in the future.
Past research improperly compared relative risk for ESRD in donors with that in the general population (without accounting for higher propensity for complications in donors with preexisting conditions). In an effort to correct this misperception, a study recently published in JAMA compared the risk for ESRD in donors with that in a healthy group of nondonors.1 The nondonor pool was taken from the National Health and Nutrition Examination Survey (NHANES III), which assesses the health and nutritional status of adults and children in the United States.
The JAMA study included a cohort of 96,217 kidney donors in the US in a 17-year period and a cohort of 20,024 participants in a six-year period of the NHANES III trial. This data was then compared to Centers for Medicare & Medicaid Services (CMS) data to determine the development of ESRD in kidney donors. ESRD was defined by CMS as the initiation of dialysis, placement on the kidney transplant waiting list, or receipt of a living or deceased donor kidney transplant.
In addition to comparing risk for ESRD in kidney donors with that of a healthy population of nondonors, the researchers also stratified their results demographically. Thus, the lifetime rate of kidney failure in donors is 90 per 10,000, compared with 326 per 10,000 in the general population of nondonors. In healthy nondonors, the risk for kidney failure was 14 per 10,000. After 15 years, the risk for kidney failure associated with donating a kidney was 51 per 10,000 in African-American donors and 23 per 10,000 in white donors. So while the study did reveal an increased risk associated with kidney donation, the degree of risk is considered small.
These findings demonstrate the importance of understanding the facts surrounding inherent risk for ESRD in kidney donation. Overall, a donor’s lifetime risk is considered minuscule. So, to answer the question, yes, there is a slight increase in risk for kidney failure if you donate to your friend. That said, the risk is 0.014 x a standardized risk of 1. This increases at 15 years to 0.51 for African-American and 0.23 for white donors. With such tiny increases, you can safely feel good about donating a kidney to your friend.
Donna Reesman, MSN, CNP
VP Clinical & Quality Management
St Clair Specialty Physicians Detroit
REFERENCES
1. Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311(6):579-586.
2. CDC. HIV in the United States: at a glance (2013). www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed June 16, 2014.
3. Frassetto LA, Tan-Tam C, Stock PG. Renal transplantation in patients with HIV. Nat Rev Nephrol. 2009;5(10):582-589.
4. Malani PN. New law allows organ transplants from deceased HIV-infected donors to HIV-infected recipients. JAMA. 2013;310(23): 2492-2493.
5. Muller E, Kahn D, Mendelson M. Renal transplantation between HIV-positive donors and recipients. N Engl J Med. 2010;362(24):2336-2337.
6. Mariani LH, Berns JS. Viral nephropathies. In: Gilbert SJ, Weiner DE, eds. National Kidney Foundation’s Primer on Kidney Diseases. 6th ed. Elsevier; 2014:253-261.
Q) A good friend was diagnosed with chronic kidney disease (CKD) and is presently undergoing workup for a transplant. He is 60 and otherwise healthy; his glomerular filtration rate (GFR) is 14, and he has no uremic symptoms. If I volunteer to give him a kidney, are there any long-term risks for me?
Kidney failure, dialysis, and kidney transplant are terms that can invoke stress and uncertainty in patients with end-stage renal disease (ESRD) and among their family members and friends. In addition to adjusting to the changes wrought by ESRD, patients may also be burdened by the prospect of a family member or friend donating a kidney to them and the concern that the donation will lead to complications for their donor. Family members or friends who volunteer may also experience stress, uncertain of their own risk for ESRD in the future.
Past research improperly compared relative risk for ESRD in donors with that in the general population (without accounting for higher propensity for complications in donors with preexisting conditions). In an effort to correct this misperception, a study recently published in JAMA compared the risk for ESRD in donors with that in a healthy group of nondonors.1 The nondonor pool was taken from the National Health and Nutrition Examination Survey (NHANES III), which assesses the health and nutritional status of adults and children in the United States.
The JAMA study included a cohort of 96,217 kidney donors in the US in a 17-year period and a cohort of 20,024 participants in a six-year period of the NHANES III trial. This data was then compared to Centers for Medicare & Medicaid Services (CMS) data to determine the development of ESRD in kidney donors. ESRD was defined by CMS as the initiation of dialysis, placement on the kidney transplant waiting list, or receipt of a living or deceased donor kidney transplant.
In addition to comparing risk for ESRD in kidney donors with that of a healthy population of nondonors, the researchers also stratified their results demographically. Thus, the lifetime rate of kidney failure in donors is 90 per 10,000, compared with 326 per 10,000 in the general population of nondonors. In healthy nondonors, the risk for kidney failure was 14 per 10,000. After 15 years, the risk for kidney failure associated with donating a kidney was 51 per 10,000 in African-American donors and 23 per 10,000 in white donors. So while the study did reveal an increased risk associated with kidney donation, the degree of risk is considered small.
These findings demonstrate the importance of understanding the facts surrounding inherent risk for ESRD in kidney donation. Overall, a donor’s lifetime risk is considered minuscule. So, to answer the question, yes, there is a slight increase in risk for kidney failure if you donate to your friend. That said, the risk is 0.014 x a standardized risk of 1. This increases at 15 years to 0.51 for African-American and 0.23 for white donors. With such tiny increases, you can safely feel good about donating a kidney to your friend.
Donna Reesman, MSN, CNP
VP Clinical & Quality Management
St Clair Specialty Physicians Detroit
REFERENCES
1. Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311(6):579-586.
2. CDC. HIV in the United States: at a glance (2013). www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed June 16, 2014.
3. Frassetto LA, Tan-Tam C, Stock PG. Renal transplantation in patients with HIV. Nat Rev Nephrol. 2009;5(10):582-589.
4. Malani PN. New law allows organ transplants from deceased HIV-infected donors to HIV-infected recipients. JAMA. 2013;310(23): 2492-2493.
5. Muller E, Kahn D, Mendelson M. Renal transplantation between HIV-positive donors and recipients. N Engl J Med. 2010;362(24):2336-2337.
6. Mariani LH, Berns JS. Viral nephropathies. In: Gilbert SJ, Weiner DE, eds. National Kidney Foundation’s Primer on Kidney Diseases. 6th ed. Elsevier; 2014:253-261.
Kidney Donation & HIV
Q) Now that patients are living with HIV/AIDS, can they donate kidneys or receive a kidney transplant?
Kidney disease often has multiple causes, including hypertension, diabetes, inherited conditions, and viral illnesses. The latter include primarily HIV, hepatitis C, and hepatitis B. With advances in the treatment of viral illnesses, the question of whether patients with these viruses can donate or receive a kidney transplant is being discussed not only in the United States but also worldwide.
The most recent CDC figures estimate that more than 1.1 million people in the US are living with HIV, of whom one in six (or nearly 16%) are undiagnosed. There are approximately 50,000 new infections reported annually.2
The Organ Transplant Amendments Act of 1988 banned HIV-positive people from donating organs. However, with the introduction of highly active antiretroviral therapy (HAART, now often referred to as active antiretroviral therapy) and the effective prophylaxis and management of opportunistic infections, mortality has been reduced. HIV/AIDS is often seen as a chronic disease and not the death sentence it once was.3 Since the development of HAART, there have been successful transplants to HIV-positive recipients from non–HIV-infected donors.
In November 2013, President Obama signed the HIV Organ Policy Equity (HOPE) Act, which lifted the ban on using organs from HIV-infected donors. The legislation directs the Department of Health and Human Services and the Organ Procurement and Transplantation Network to develop standards to make these transplants possible.4
Although there have not been any documented cases of transplants from HIV-infected donors to HIV-infected recipients in this country, such transplants have been very successful in South Africa.5 There, to qualify for kidney transplant, all recipients must have proven adherence, virologic suppression, and immune constitution. Donor suitability is defined as HIV infection (confirmed with the use of enzyme-linked immunosorbent assay), absence of proteinuria, and a normal kidney as assessed with post hoc renal biopsy.5
One of the chief concerns has been the effect of further immunosuppression on the recipients and the possibility of disease progression. Although the sample size is limited (four transplants), data from the available cases indicate no evidence of organ rejection at 12 months post-transplantation. In addition, the recipients’ CD4 counts remained lower than baseline due to immunosuppressive therapy. All four patients maintained a viral load of less than 50 copies, which suggested that any virus transplanted along with the kidney had not affected control of HIV infection.5 However, it should be noted that many of the agents used for posttransplant maintenance immunosuppression (mycophenolate mofetil, cyclosporine, tacrolimus, and sirolimus) have antiretroviral properties.3
HIV patients in the US must meet the following criteria to be listed for a transplant:
• Diagnosis of ESRD with at least a five-year life-expectancy
• CD4 count of > 200 cells/ μL for at least six months
• Undetectable HIV viremia (< 50 HIV-1 RNA copies/mL)
• Demonstrated adherence to stable antiviral regimen for at least six months
• Absence of AIDS-defining illness following successful immune reconstitution6
A prospective trial of 150 patients in 19 US transplant centers who met the above criteria demonstrated patient survival and graft survival rates comparable to those in patients ages 65 and older.6
While awaiting the donation, HIV patients can continue hemodialysis and peritoneal dialysis. With the improved antiviral drugs, HIV patients have a survival rate similar to the non–HIV-infected population.
Transplantation is the goal and certainly the hope of many advanced-stage kidney patients, but in reality, the need far exceeds the resources. The HOPE Act opens the door for many patients who were previously excluded from the possibility of a life without dialysis. Taking care of these patients will be a team effort, encompassing HIV and infectious disease specialists, pharmacists, nephrologists, transplant surgeons and coordinators, and primary care providers—including, of course, advanced practitioners.
Shelly Levinstein, MSN, CRNP
Nephrology Associates of York
York, PA
REFERENCES
1. Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311(6):579-586.
2. CDC. HIV in the United States: at a glance (2013). www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed June 16, 2014.
3. Frassetto LA, Tan-Tam C, Stock PG. Renal transplantation in patients with HIV. Nat Rev Nephrol. 2009;5(10):582-589.
4. Malani PN. New law allows organ transplants from deceased HIV-infected donors to HIV-infected recipients. JAMA. 2013;310(23): 2492-2493.
5. Muller E, Kahn D, Mendelson M. Renal transplantation between HIV-positive donors and recipients. N Engl J Med. 2010;362(24):2336-2337.
6. Mariani LH, Berns JS. Viral nephropathies. In: Gilbert SJ, Weiner DE, eds. National Kidney Foundation’s Primer on Kidney Diseases. 6th ed. Elsevier; 2014:253-261.
Q) Now that patients are living with HIV/AIDS, can they donate kidneys or receive a kidney transplant?
Kidney disease often has multiple causes, including hypertension, diabetes, inherited conditions, and viral illnesses. The latter include primarily HIV, hepatitis C, and hepatitis B. With advances in the treatment of viral illnesses, the question of whether patients with these viruses can donate or receive a kidney transplant is being discussed not only in the United States but also worldwide.
The most recent CDC figures estimate that more than 1.1 million people in the US are living with HIV, of whom one in six (or nearly 16%) are undiagnosed. There are approximately 50,000 new infections reported annually.2
The Organ Transplant Amendments Act of 1988 banned HIV-positive people from donating organs. However, with the introduction of highly active antiretroviral therapy (HAART, now often referred to as active antiretroviral therapy) and the effective prophylaxis and management of opportunistic infections, mortality has been reduced. HIV/AIDS is often seen as a chronic disease and not the death sentence it once was.3 Since the development of HAART, there have been successful transplants to HIV-positive recipients from non–HIV-infected donors.
In November 2013, President Obama signed the HIV Organ Policy Equity (HOPE) Act, which lifted the ban on using organs from HIV-infected donors. The legislation directs the Department of Health and Human Services and the Organ Procurement and Transplantation Network to develop standards to make these transplants possible.4
Although there have not been any documented cases of transplants from HIV-infected donors to HIV-infected recipients in this country, such transplants have been very successful in South Africa.5 There, to qualify for kidney transplant, all recipients must have proven adherence, virologic suppression, and immune constitution. Donor suitability is defined as HIV infection (confirmed with the use of enzyme-linked immunosorbent assay), absence of proteinuria, and a normal kidney as assessed with post hoc renal biopsy.5
One of the chief concerns has been the effect of further immunosuppression on the recipients and the possibility of disease progression. Although the sample size is limited (four transplants), data from the available cases indicate no evidence of organ rejection at 12 months post-transplantation. In addition, the recipients’ CD4 counts remained lower than baseline due to immunosuppressive therapy. All four patients maintained a viral load of less than 50 copies, which suggested that any virus transplanted along with the kidney had not affected control of HIV infection.5 However, it should be noted that many of the agents used for posttransplant maintenance immunosuppression (mycophenolate mofetil, cyclosporine, tacrolimus, and sirolimus) have antiretroviral properties.3
HIV patients in the US must meet the following criteria to be listed for a transplant:
• Diagnosis of ESRD with at least a five-year life-expectancy
• CD4 count of > 200 cells/ μL for at least six months
• Undetectable HIV viremia (< 50 HIV-1 RNA copies/mL)
• Demonstrated adherence to stable antiviral regimen for at least six months
• Absence of AIDS-defining illness following successful immune reconstitution6
A prospective trial of 150 patients in 19 US transplant centers who met the above criteria demonstrated patient survival and graft survival rates comparable to those in patients ages 65 and older.6
While awaiting the donation, HIV patients can continue hemodialysis and peritoneal dialysis. With the improved antiviral drugs, HIV patients have a survival rate similar to the non–HIV-infected population.
Transplantation is the goal and certainly the hope of many advanced-stage kidney patients, but in reality, the need far exceeds the resources. The HOPE Act opens the door for many patients who were previously excluded from the possibility of a life without dialysis. Taking care of these patients will be a team effort, encompassing HIV and infectious disease specialists, pharmacists, nephrologists, transplant surgeons and coordinators, and primary care providers—including, of course, advanced practitioners.
Shelly Levinstein, MSN, CRNP
Nephrology Associates of York
York, PA
REFERENCES
1. Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311(6):579-586.
2. CDC. HIV in the United States: at a glance (2013). www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed June 16, 2014.
3. Frassetto LA, Tan-Tam C, Stock PG. Renal transplantation in patients with HIV. Nat Rev Nephrol. 2009;5(10):582-589.
4. Malani PN. New law allows organ transplants from deceased HIV-infected donors to HIV-infected recipients. JAMA. 2013;310(23): 2492-2493.
5. Muller E, Kahn D, Mendelson M. Renal transplantation between HIV-positive donors and recipients. N Engl J Med. 2010;362(24):2336-2337.
6. Mariani LH, Berns JS. Viral nephropathies. In: Gilbert SJ, Weiner DE, eds. National Kidney Foundation’s Primer on Kidney Diseases. 6th ed. Elsevier; 2014:253-261.
Q) Now that patients are living with HIV/AIDS, can they donate kidneys or receive a kidney transplant?
Kidney disease often has multiple causes, including hypertension, diabetes, inherited conditions, and viral illnesses. The latter include primarily HIV, hepatitis C, and hepatitis B. With advances in the treatment of viral illnesses, the question of whether patients with these viruses can donate or receive a kidney transplant is being discussed not only in the United States but also worldwide.
The most recent CDC figures estimate that more than 1.1 million people in the US are living with HIV, of whom one in six (or nearly 16%) are undiagnosed. There are approximately 50,000 new infections reported annually.2
The Organ Transplant Amendments Act of 1988 banned HIV-positive people from donating organs. However, with the introduction of highly active antiretroviral therapy (HAART, now often referred to as active antiretroviral therapy) and the effective prophylaxis and management of opportunistic infections, mortality has been reduced. HIV/AIDS is often seen as a chronic disease and not the death sentence it once was.3 Since the development of HAART, there have been successful transplants to HIV-positive recipients from non–HIV-infected donors.
In November 2013, President Obama signed the HIV Organ Policy Equity (HOPE) Act, which lifted the ban on using organs from HIV-infected donors. The legislation directs the Department of Health and Human Services and the Organ Procurement and Transplantation Network to develop standards to make these transplants possible.4
Although there have not been any documented cases of transplants from HIV-infected donors to HIV-infected recipients in this country, such transplants have been very successful in South Africa.5 There, to qualify for kidney transplant, all recipients must have proven adherence, virologic suppression, and immune constitution. Donor suitability is defined as HIV infection (confirmed with the use of enzyme-linked immunosorbent assay), absence of proteinuria, and a normal kidney as assessed with post hoc renal biopsy.5
One of the chief concerns has been the effect of further immunosuppression on the recipients and the possibility of disease progression. Although the sample size is limited (four transplants), data from the available cases indicate no evidence of organ rejection at 12 months post-transplantation. In addition, the recipients’ CD4 counts remained lower than baseline due to immunosuppressive therapy. All four patients maintained a viral load of less than 50 copies, which suggested that any virus transplanted along with the kidney had not affected control of HIV infection.5 However, it should be noted that many of the agents used for posttransplant maintenance immunosuppression (mycophenolate mofetil, cyclosporine, tacrolimus, and sirolimus) have antiretroviral properties.3
HIV patients in the US must meet the following criteria to be listed for a transplant:
• Diagnosis of ESRD with at least a five-year life-expectancy
• CD4 count of > 200 cells/ μL for at least six months
• Undetectable HIV viremia (< 50 HIV-1 RNA copies/mL)
• Demonstrated adherence to stable antiviral regimen for at least six months
• Absence of AIDS-defining illness following successful immune reconstitution6
A prospective trial of 150 patients in 19 US transplant centers who met the above criteria demonstrated patient survival and graft survival rates comparable to those in patients ages 65 and older.6
While awaiting the donation, HIV patients can continue hemodialysis and peritoneal dialysis. With the improved antiviral drugs, HIV patients have a survival rate similar to the non–HIV-infected population.
Transplantation is the goal and certainly the hope of many advanced-stage kidney patients, but in reality, the need far exceeds the resources. The HOPE Act opens the door for many patients who were previously excluded from the possibility of a life without dialysis. Taking care of these patients will be a team effort, encompassing HIV and infectious disease specialists, pharmacists, nephrologists, transplant surgeons and coordinators, and primary care providers—including, of course, advanced practitioners.
Shelly Levinstein, MSN, CRNP
Nephrology Associates of York
York, PA
REFERENCES
1. Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311(6):579-586.
2. CDC. HIV in the United States: at a glance (2013). www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed June 16, 2014.
3. Frassetto LA, Tan-Tam C, Stock PG. Renal transplantation in patients with HIV. Nat Rev Nephrol. 2009;5(10):582-589.
4. Malani PN. New law allows organ transplants from deceased HIV-infected donors to HIV-infected recipients. JAMA. 2013;310(23): 2492-2493.
5. Muller E, Kahn D, Mendelson M. Renal transplantation between HIV-positive donors and recipients. N Engl J Med. 2010;362(24):2336-2337.
6. Mariani LH, Berns JS. Viral nephropathies. In: Gilbert SJ, Weiner DE, eds. National Kidney Foundation’s Primer on Kidney Diseases. 6th ed. Elsevier; 2014:253-261.