User login
USPSTF changes ABI screening recommendation
The U.S. Preventive Services Task Force (USPSTF) updated its earlier recommendations regarding the validity of using the ankle-brachial index (ABI) in the September Annals of Internal Medicine. In 2006, the USPSTF recommended against screening for PAD (D recommendation; Am Fam Physician 2006; 73:497).
The USPSTF now concludes that evidence is insufficient to make a recommendation. (I recommendation) and published both its systemic evidence review and recommendations.
The U.S. Preventive Services Task Force (USPSTF) updated its earlier recommendations regarding the validity of using the ankle-brachial index (ABI) in the September Annals of Internal Medicine. In 2006, the USPSTF recommended against screening for PAD (D recommendation; Am Fam Physician 2006; 73:497).
The USPSTF now concludes that evidence is insufficient to make a recommendation. (I recommendation) and published both its systemic evidence review and recommendations.
The U.S. Preventive Services Task Force (USPSTF) updated its earlier recommendations regarding the validity of using the ankle-brachial index (ABI) in the September Annals of Internal Medicine. In 2006, the USPSTF recommended against screening for PAD (D recommendation; Am Fam Physician 2006; 73:497).
The USPSTF now concludes that evidence is insufficient to make a recommendation. (I recommendation) and published both its systemic evidence review and recommendations.
Is your patient’s poor recall more than just a ‘senior moment’?
Memory and other cognitive complaints are common among the general population and become more prevalent with age.1 People who have significant emotional investment in their cognitive competence, mood disturbance, somatic symptoms, and anxiety or related disorders are likely to worry more about their cognitive functioning as they age.
Common complaints
Age-related complaints, typically beginning by age 50, often include problems retaining or retrieving names, difficulty recalling details of conversations and written materials, and hazy recollection of remote events and the time frame of recent life events. Common complaints involve difficulties with mental calculations, multi-tasking (including vulnerability to distraction), and problems keeping track of and organizing information. The most common complaint is difficulty with remembering the reason for entering a room.
More concerning are complaints involving recurrent lapses in judgment or forgetfulness with significant implications for everyday living (eg, physical safety, job performance, travel, and finances), especially when validated by friends or family members and coupled with decline in at least 1 activity of daily living, and poor insight.
Helping your forgetful patient
Office evaluation with brief cognitive screening instruments—namely, the Montreal Cognitive Assessment and the recent revision of the Mini-Mental State Examination—might help clarify the clinical presentation. Proceed with caution: Screening tests tap a limited number of neurocognitive functions and can generate a false-negative result among brighter and better educated patients and a false-positive result among the less intelligent and less educated.2 Applying age- and education-corrected norms can reduce misclassification but does not eliminate it.
Screening measures can facilitate decision-making regarding the need for more comprehensive psychometric assessment. Such evaluations sample a broader range of neurobehavioral domains, in greater depth, and provide a more nuanced picture of a patient’s neurocognition.
Findings on a battery of psychological and neuropsychological tests that might evoke concern include problems with incidental, anterograde, and recent memory that are not satisfactorily explained by: age and education or vocational training; estimated premorbid intelligence; residual neurodevelopmental disorders (attention, learning, and autistic-spectrum disorders); situational, sociocultural, and psychiatric factors; and motivational influences—notably, malingering.
Some difficulties with memory are highly associated with mild cognitive impairment or early dementia:
• anterograde memory (involving a reduced rate of verbal and nonverbal learning over repeated trials)
• poor retention
• accelerated forgetting of newly learned information
• failure to benefit from recognition and other mnemonic cues
• so-called source error confusion—a misattribution that involves difficulty differentiating target information from competing information, as reflected in confabulation errors and an elevated rate of intrusion errors.
Disclosure
Dr. Pollak reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Weiner MF, Garrett R, Bret ME. Neuropsychiatric assessment and diagnosis. In: Weiner MF, Lipton AM, eds. Clinical manual of Alzheimer disease and other dementias. Arlington, VA: American Psychiatric Publishing, Inc.; 2012: 3-46.
2. Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests: administration, norms and commentary: third edition. New York, NY: Oxford University Press; 2006.
Memory and other cognitive complaints are common among the general population and become more prevalent with age.1 People who have significant emotional investment in their cognitive competence, mood disturbance, somatic symptoms, and anxiety or related disorders are likely to worry more about their cognitive functioning as they age.
Common complaints
Age-related complaints, typically beginning by age 50, often include problems retaining or retrieving names, difficulty recalling details of conversations and written materials, and hazy recollection of remote events and the time frame of recent life events. Common complaints involve difficulties with mental calculations, multi-tasking (including vulnerability to distraction), and problems keeping track of and organizing information. The most common complaint is difficulty with remembering the reason for entering a room.
More concerning are complaints involving recurrent lapses in judgment or forgetfulness with significant implications for everyday living (eg, physical safety, job performance, travel, and finances), especially when validated by friends or family members and coupled with decline in at least 1 activity of daily living, and poor insight.
Helping your forgetful patient
Office evaluation with brief cognitive screening instruments—namely, the Montreal Cognitive Assessment and the recent revision of the Mini-Mental State Examination—might help clarify the clinical presentation. Proceed with caution: Screening tests tap a limited number of neurocognitive functions and can generate a false-negative result among brighter and better educated patients and a false-positive result among the less intelligent and less educated.2 Applying age- and education-corrected norms can reduce misclassification but does not eliminate it.
Screening measures can facilitate decision-making regarding the need for more comprehensive psychometric assessment. Such evaluations sample a broader range of neurobehavioral domains, in greater depth, and provide a more nuanced picture of a patient’s neurocognition.
Findings on a battery of psychological and neuropsychological tests that might evoke concern include problems with incidental, anterograde, and recent memory that are not satisfactorily explained by: age and education or vocational training; estimated premorbid intelligence; residual neurodevelopmental disorders (attention, learning, and autistic-spectrum disorders); situational, sociocultural, and psychiatric factors; and motivational influences—notably, malingering.
Some difficulties with memory are highly associated with mild cognitive impairment or early dementia:
• anterograde memory (involving a reduced rate of verbal and nonverbal learning over repeated trials)
• poor retention
• accelerated forgetting of newly learned information
• failure to benefit from recognition and other mnemonic cues
• so-called source error confusion—a misattribution that involves difficulty differentiating target information from competing information, as reflected in confabulation errors and an elevated rate of intrusion errors.
Disclosure
Dr. Pollak reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Memory and other cognitive complaints are common among the general population and become more prevalent with age.1 People who have significant emotional investment in their cognitive competence, mood disturbance, somatic symptoms, and anxiety or related disorders are likely to worry more about their cognitive functioning as they age.
Common complaints
Age-related complaints, typically beginning by age 50, often include problems retaining or retrieving names, difficulty recalling details of conversations and written materials, and hazy recollection of remote events and the time frame of recent life events. Common complaints involve difficulties with mental calculations, multi-tasking (including vulnerability to distraction), and problems keeping track of and organizing information. The most common complaint is difficulty with remembering the reason for entering a room.
More concerning are complaints involving recurrent lapses in judgment or forgetfulness with significant implications for everyday living (eg, physical safety, job performance, travel, and finances), especially when validated by friends or family members and coupled with decline in at least 1 activity of daily living, and poor insight.
Helping your forgetful patient
Office evaluation with brief cognitive screening instruments—namely, the Montreal Cognitive Assessment and the recent revision of the Mini-Mental State Examination—might help clarify the clinical presentation. Proceed with caution: Screening tests tap a limited number of neurocognitive functions and can generate a false-negative result among brighter and better educated patients and a false-positive result among the less intelligent and less educated.2 Applying age- and education-corrected norms can reduce misclassification but does not eliminate it.
Screening measures can facilitate decision-making regarding the need for more comprehensive psychometric assessment. Such evaluations sample a broader range of neurobehavioral domains, in greater depth, and provide a more nuanced picture of a patient’s neurocognition.
Findings on a battery of psychological and neuropsychological tests that might evoke concern include problems with incidental, anterograde, and recent memory that are not satisfactorily explained by: age and education or vocational training; estimated premorbid intelligence; residual neurodevelopmental disorders (attention, learning, and autistic-spectrum disorders); situational, sociocultural, and psychiatric factors; and motivational influences—notably, malingering.
Some difficulties with memory are highly associated with mild cognitive impairment or early dementia:
• anterograde memory (involving a reduced rate of verbal and nonverbal learning over repeated trials)
• poor retention
• accelerated forgetting of newly learned information
• failure to benefit from recognition and other mnemonic cues
• so-called source error confusion—a misattribution that involves difficulty differentiating target information from competing information, as reflected in confabulation errors and an elevated rate of intrusion errors.
Disclosure
Dr. Pollak reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Weiner MF, Garrett R, Bret ME. Neuropsychiatric assessment and diagnosis. In: Weiner MF, Lipton AM, eds. Clinical manual of Alzheimer disease and other dementias. Arlington, VA: American Psychiatric Publishing, Inc.; 2012: 3-46.
2. Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests: administration, norms and commentary: third edition. New York, NY: Oxford University Press; 2006.
1. Weiner MF, Garrett R, Bret ME. Neuropsychiatric assessment and diagnosis. In: Weiner MF, Lipton AM, eds. Clinical manual of Alzheimer disease and other dementias. Arlington, VA: American Psychiatric Publishing, Inc.; 2012: 3-46.
2. Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests: administration, norms and commentary: third edition. New York, NY: Oxford University Press; 2006.
Hearing voices, time traveling, and being hit with a high-heeled shoe
CASE Grief and confusion
Mr. P, age 47, is arrested for entering the apartment of a woman he does not know and tossing her belongings out the window. When he is assessed to determine if he can participate in his legal defense, examiners find an attentive, courteous man who is baffled by his own behavior.
Mr. P says that he had been “stressed out” after the recent death of his grandmother, with whom he was close. He says he entered the apartment because voices told him to do so. He has no recent history of substance abuse or psychiatric hospitalizations, but he had a similar episode of “confusion” years before, when another close family member died.
Mr. P is found not fit to stand trial and the charges are dropped. He accepts haloperidol, 10 mg/d, and benztropine, 2 mg/d, and is transferred to a hospital for psychiatric treatment.
On interview, Mr. P is well groomed, soft-spoken, and shy, without formal thought disorder. Physical exam and routine lab tests are within normal limits. He says that 18 months before his arrest, he and his frail grandmother moved to a large city in hopes that he would find a wife. Both depended on the grandmother’s Social Security benefits while he cared for her.
In the 2 months after she died, he reports that he felt sad and alone and slept poorly, but made efforts to find a job and keep his apartment. When his efforts failed and he lost the apartment, he stayed with various friends for a few days at a time, then spent several days in the subway before ending up on the streets.
His arrest on the current charge occurred 4 days after he began walking the streets.
a) continue haloperidol to treat psychotic symptoms
b) discontinue haloperidol and observe him
c) add an antidepressant to haloperidol
HISTORY Imagining nonsense
Mr. P cannot explain why he started “trashing” the woman’s apartment, but says he entered it because he thought it was his apartment. With embarrassment and regret, he admits he has been depressed and confused, “imagining things”—“foolish things,” he admits—such as being in a different “time zone.”
Contradicting his earlier statements, Mr. P now admits that he had “a few beers” and denies that he experienced auditory hallucinations, saying he only talks to himself. He now says that within 2 days after his arrest, he was “all over it.” Mr. P denies current symptoms, including hallucinations, but, when pressed, waffles, then admits to a strange belief: that some people, including him, can move from one “time zone” to another.
Mr. P says he was treated for psychiatric problems 4 years earlier when his parents were killed in a car crash. By his recollection, his reaction to their death was similar to his reaction to his grandmother’s death: He became upset and wandered the streets for a few days, “moving between time zones” and talking to himself but not experiencing hallucinations. After he was taken to a hospital and “given an injection,” he calmed down and was released. Within a few days he recovered and returned to supporting himself and caring for his grandmother. Mr. P says the idea of travelling between “time zones” is embarrassing and nonsensical but adds that he was affected in this way because he “bickered” with his mother.
Mr. P’s grandmother raised him until he was age 15, although he frequently visited his parents, who lived nearby and worked during the day. Mr. P initially denies substance abuse, then admits to smoking marijuana every day for about a year before admission. He also admits to cocaine abuse in his 20s. He denies a history of suicide attempts.
The author’s observations
Mr. P reported only 2 episodes of “confusion” (or psychosis) and strange behavior in his life, both precipitated by the loss of a loved one, and at least 1 while under the influence of alcohol and Cannabis. He gave an inconsistent and ambiguous history of auditory hallucinations associated with episodes of confusion. He believes that time travel is possible, an idea that he acknowledged is nonsense. This alone was not enough to warrant long-term antipsychotic treatment. The most likely diagnosis seemed to be brief psychotic episode induced by Cannabis and the stressors of homelessness and his grandmother’s death.
EVALUATION Changing stories
No longer taking haloperidol, Mr. P continues to deny hallucinations and depressed mood, but keeps to himself. Nine days after admission he becomes tearful after he informs his aunt of his grandmother’s death in a telephone call, then approaches a nurse and complains of sadness and auditory hallucinations.
Mr. P confesses that he denied hallucinations on admission because he feared he would remain in the hospital for years if he revealed the truth that he had been experiencing auditory hallucinations almost continuously from age 10. He reports that the voices distracted him when he worked; seem to be male; often spoke gibberish; and alternate between deprecating and positive and supportive. Mr. P is reluctant to disclose more about what the voices actually say, although he acknowledges that they are not commenting or conversing with him, and that he has never believed the voices were his own thoughts but did believe that they came from inside his brain.
With haloperidol, the voices stopped. They resumed, however, when haloperidol was discontinued.
When we ask what happened to him at age 10, Mr. P shrugs.
a) childhood onset schizophrenia
b) substance abuse
c) posttraumatic stress disorder (PTSD)
d) none
The author’s observations
In community samples of children and adolescents, auditory hallucinations are not rare and usually do not cause distress or dysfunction. In a study of 3,870 children age 7 and 8,1 9% endorsed auditory hallucinations. Most heard 1 voice, once a week or less, at low volume. In 85% of children who experienced hallucinations, they caused minimal or no suffering; 97% reported minimal or no interference with daily functioning. Among children who experienced auditory hallucinations at age 7 or 8, 24% continued to hallucinate 5 years later.2 Persistent hallucinations were associated with more problematic behaviors at baseline and follow up.
In a group of 12-year-old twins, 4.2% reported auditory hallucinations.3 In that study, hallucinations were not related to Cannabis use; rather, they were heritable and related to risk factors such as cognitive impairment; behavioral, emotional, and educational problems at age 5; and a history of physical abuse and self-harm at age 12. The authors noted that these are risk factors and correlates of schizophrenia, but are not specific to schizophrenia.
Hallucinations and delusions have been found in 4% to 8% of children and adolescents referred for psychiatric treatment,4 far more than the prevalence of childhood-onset schizophrenia (0.01% of children).5 Psychotic symptoms in children have been associated with bipolar disorder, but also with anxiety disorders, obsessive-compulsive disorder, PTSD, pervasive developmental disorder, conduct disorder, and substance abuse.4
Childhood-onset schizophrenia is rare and would require that Mr. P have a diagnosis of schizophrenia as an adult. It is possible that Mr. P’s childhood symptoms were related to substance abuse but he was not asked for this history because it seemed unlikely in a 10-year-old boy. A PTSD diagnosis requires a traumatic event, which Mr. P did not reveal. It is possible that at age 10 he did not have a psychiatric disorder.
a) PTSD
b) dissociative disorder
c) borderline personality disorder
d) chronic schizophrenia
e) no psychiatric diagnosis
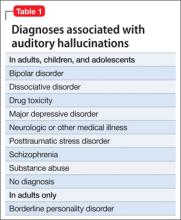
Among adults in the general population, 10% to 15% report auditory hallucinations.6 Hallucinations could be caused by substance abuse or psychiatric conditions other than schizophrenia; however, in adults—as in children—auditory hallucinations can occur in the absence of these conditions (Table 1) and rarely cause distress or dysfunction.6 In Sommer and colleagues’6 study of 103 healthy persons, none who heard voices had disorganization or negative symptoms. Those who heard voices had significantly more schizotypal symptoms and more childhood trauma, including emotional, physical, and sexual abuse, than those who did not hear voices.6
Conditions associated with hallucinations
PTSD is associated with auditory hallucinations and other psychotic symptoms.7 Most studies are of combat veterans with PTSD, in whom auditory hallucinations and delusions were associated with major depressive disorder, not a thought disorder or inappropriate affect.8 In a community sample,9 psychotic symptoms—particularly auditory hallucinations—were associated with PTSD. Subjects with PTSD and psychotic symptoms were more likely to have other psychiatric disorders, including major depressive disorder and substance use disorder, than patients with PTSD but no psychotic symptoms; however, the relationship between PTSD and psychosis remained after controlling for other psychiatric disorders.
Hallucinations can occur in persons with dissociative disorders in the absence of distinct personality states.10 Hallucinations have been seen transiently and chronically in persons with borderline personality disorder and can be associated with comorbid conditions such as substance abuse disorders, mood disorders, and PTSD.11
Mr. P lacked the reduced capacity for interpersonal relationships required for a schizotypal personality disorder diagnosis. A diagnosis of PTSD or dissociative disorder requires a history of trauma, which Mr. P did not report.
“Time travelling” with incomprehensible behavior could be interpreted as dissociation, but dissociative fugue or dissociative disorder not otherwise specified (NOS) cannot be diagnosed if symptoms might be the direct effect of a substance, such as Cannabis. Mr. P admitted to substance abuse. We can rule out borderline personality disorder because he did not display or admit to tempestuous interpersonal relationships.
A schizophrenia diagnosis requires the presence of auditory hallucinations that commented on his behavior or conversed among themselves, a second psychotic symptom for ≥1 month, or negative symptoms, which Mr. P lacked (unless belief in time travel is considered delusional).
Last, a physician might have considered malingering or a factitious disorder when Mr. P was found not able to participate in his own defense, but this seemed less likely after he revealed that he experienced auditory hallucinations since age 10.
HISTORY Bad beatings
With a few days of beginning risperidone, 4 mg/d, Mr. P reports that his hallucinations have stopped and he feels less sad. He reveals that, at age 10, when the hallucinations began, his mother hit him over the head with a high-heeled shoe, causing a scalp laceration that required a visit to the emergency room for suturing. His mother beat Mr. P for as long as he could remember. She beat him “bad” at least twice weekly, and he was taken to the hospital 7 or 8 times for injury, but she also beat him “constantly” with a belt buckle, sometimes striking his head. She instructed him to tell nobody.
The author’s observations
Auditory hallucinations in adults have been associated with childhood abuse, particularly childhood sexual abuse,12 in clinical and non-clinical samples.13 Some argue13 that child abuse itself causes hallucinations and other psychotic symptoms.
OUTCOME Depressed and sleepless
Mr. P admits that he had been smoking marijuana 2 to 3 times daily for a year. He also reports insomnia, sleeping approximately 4 hours a night and spending hours awake in bed thinking of his grandmother, with depressed mood and tearfulness. He denies suicidal ideas and hallucinations. He is treated for depressive disorder NOS first with amitriptyline, 50 mg at bedtime, for sleep, then paroxetine, 20 mg/d, for depressive symptoms, in addition to risperidone, 4 mg/d. Although Mr. P does not describe re-experiencing his childhood trauma, avoidance of stimuli associated with the trauma, or symptoms of increased arousal (except for insomnia), the treatment team did not ask, so it remains uncertain if he has PTSD (Table 2).
When Mr. P is discharged to a clinic, he smiles easily and is positive and supportive with other patients. He spruces up his appearance by wearing jewelry and works in the hospital kitchen.
Bottom Line
Chronic auditory hallucinations are associated with psychiatric illnesses other than chronic schizophrenia, particularly those resulting from trauma such as posttraumatic stress disorder. They can also occur in the absence of diagnosable psychiatric illness and rarely cause distress or functional impairment. Auditory hallucinations in adults have been associated with childhood abuse.
Related Resources
- Moskowitz A, Schafer I, Dorahy MJ. Psychosis, trauma and dissociation: emerging perspectives on severe psychopathology. West Sussex, UK: John Wiley and Sons, Ltd.; 2008.
- The International Hearing Voices Network. www.intervoiceonline.org.
Drug Brand Names
Amitriptyline • Elavil Paroxetine • Paxil
Benztropine • Cogentin Risperidone • Risperdal
Haloperidol • Haldol
Disclosure
Dr. Crowner reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Barthel-Velthuis AA, Jenner JA, van de Willige G, et al Prevalence and correlates of auditory vocal hallucinations in middle childhood. Br J Psychiatry. 2010;196(1):41-46.
2. Bartels-Velthuis AA, van de Willige G, Jenner JA, et al. Course of auditory vocal hallucinations in childhood: 5-year follow-up study. Br J Psychiatry. 2011;199(4):296-302.
3. Polanczyk G, Moffitt TE, Arsensault L, et al. Etiological and clinical features of childhood psychotic symptoms: results from a birth cohort. Arch Gen Psychiatry. 2010;67(4):328-338.
4. Biederman J, Pety C, Faracone SV, et al. Phenomenology of childhood psychosis: Findings from a large sample of psychiatrically referred youth. J Nerv Ment Dis 2004;192(9):607-614.
5. American Academy of Child and Adolescent Psychiatry. Practice parameters for the assessment and treatment of children and adolescents with schizophrenia. J Am Acad Child Adolesc Psychiatry. 2001;40(suppl 7):4SS-23S.
6. Sommer IEC, Daalman K, Rietkerk T, et al. Healthy individuals with auditory verbal hallucinations; Who are they? Psychiatric assessments of a selected sample of 103 subjects. Schizophr Bull. 2010;36(3):633-641.
7. Butler RW, Mueser KT, Sprock J, et al. Positive symptoms of psychosis in posttraumatic stress disorder. Biol Psychiatry. 1996;39:839-844.
8. David D, Kutcher GS, Jackson EI, et al Psychotic symptoms in combat-related posttraumatic stress disorder. J Clin Psychiatry. 1999;60(1):29-32.
9. Sareen J, Cox BJ, Goodwin RD, et al. Co-occurrence of posttraumatic stress disorder with positive psychotic symptoms in a nationally representative sample. J Trauma Stress. 2005;18(4):313-322.
10. Sar V, Akyuv G, Dogan O. Prevalence of dissociative disorders among women in the general population. Psychiatry Res. 2007;149:169-176.
11. Barnow S, Arens EA, Sieswerda S, et al. Borderline personality disorder and psychosis: a review. Curr Psychiatry Rep. 2010;12(3):186-195.
12. Bebbington P, Jonas S, Kuipers E, et al. Childhood sexual abuse and psychosis: data from a cross-sectional national psychiatric survey in England. Br J Psychiatry. 2011;199(1):29-37.
13. Read J, van Os J, Morrison AP, et al. Childhood trauma, psychosis and schizophrenia: a literature review with theoretical and clinical implications. Acta Psychiatr Scand. 2005;112(5):330-350.
CASE Grief and confusion
Mr. P, age 47, is arrested for entering the apartment of a woman he does not know and tossing her belongings out the window. When he is assessed to determine if he can participate in his legal defense, examiners find an attentive, courteous man who is baffled by his own behavior.
Mr. P says that he had been “stressed out” after the recent death of his grandmother, with whom he was close. He says he entered the apartment because voices told him to do so. He has no recent history of substance abuse or psychiatric hospitalizations, but he had a similar episode of “confusion” years before, when another close family member died.
Mr. P is found not fit to stand trial and the charges are dropped. He accepts haloperidol, 10 mg/d, and benztropine, 2 mg/d, and is transferred to a hospital for psychiatric treatment.
On interview, Mr. P is well groomed, soft-spoken, and shy, without formal thought disorder. Physical exam and routine lab tests are within normal limits. He says that 18 months before his arrest, he and his frail grandmother moved to a large city in hopes that he would find a wife. Both depended on the grandmother’s Social Security benefits while he cared for her.
In the 2 months after she died, he reports that he felt sad and alone and slept poorly, but made efforts to find a job and keep his apartment. When his efforts failed and he lost the apartment, he stayed with various friends for a few days at a time, then spent several days in the subway before ending up on the streets.
His arrest on the current charge occurred 4 days after he began walking the streets.
a) continue haloperidol to treat psychotic symptoms
b) discontinue haloperidol and observe him
c) add an antidepressant to haloperidol
HISTORY Imagining nonsense
Mr. P cannot explain why he started “trashing” the woman’s apartment, but says he entered it because he thought it was his apartment. With embarrassment and regret, he admits he has been depressed and confused, “imagining things”—“foolish things,” he admits—such as being in a different “time zone.”
Contradicting his earlier statements, Mr. P now admits that he had “a few beers” and denies that he experienced auditory hallucinations, saying he only talks to himself. He now says that within 2 days after his arrest, he was “all over it.” Mr. P denies current symptoms, including hallucinations, but, when pressed, waffles, then admits to a strange belief: that some people, including him, can move from one “time zone” to another.
Mr. P says he was treated for psychiatric problems 4 years earlier when his parents were killed in a car crash. By his recollection, his reaction to their death was similar to his reaction to his grandmother’s death: He became upset and wandered the streets for a few days, “moving between time zones” and talking to himself but not experiencing hallucinations. After he was taken to a hospital and “given an injection,” he calmed down and was released. Within a few days he recovered and returned to supporting himself and caring for his grandmother. Mr. P says the idea of travelling between “time zones” is embarrassing and nonsensical but adds that he was affected in this way because he “bickered” with his mother.
Mr. P’s grandmother raised him until he was age 15, although he frequently visited his parents, who lived nearby and worked during the day. Mr. P initially denies substance abuse, then admits to smoking marijuana every day for about a year before admission. He also admits to cocaine abuse in his 20s. He denies a history of suicide attempts.
The author’s observations
Mr. P reported only 2 episodes of “confusion” (or psychosis) and strange behavior in his life, both precipitated by the loss of a loved one, and at least 1 while under the influence of alcohol and Cannabis. He gave an inconsistent and ambiguous history of auditory hallucinations associated with episodes of confusion. He believes that time travel is possible, an idea that he acknowledged is nonsense. This alone was not enough to warrant long-term antipsychotic treatment. The most likely diagnosis seemed to be brief psychotic episode induced by Cannabis and the stressors of homelessness and his grandmother’s death.
EVALUATION Changing stories
No longer taking haloperidol, Mr. P continues to deny hallucinations and depressed mood, but keeps to himself. Nine days after admission he becomes tearful after he informs his aunt of his grandmother’s death in a telephone call, then approaches a nurse and complains of sadness and auditory hallucinations.
Mr. P confesses that he denied hallucinations on admission because he feared he would remain in the hospital for years if he revealed the truth that he had been experiencing auditory hallucinations almost continuously from age 10. He reports that the voices distracted him when he worked; seem to be male; often spoke gibberish; and alternate between deprecating and positive and supportive. Mr. P is reluctant to disclose more about what the voices actually say, although he acknowledges that they are not commenting or conversing with him, and that he has never believed the voices were his own thoughts but did believe that they came from inside his brain.
With haloperidol, the voices stopped. They resumed, however, when haloperidol was discontinued.
When we ask what happened to him at age 10, Mr. P shrugs.
a) childhood onset schizophrenia
b) substance abuse
c) posttraumatic stress disorder (PTSD)
d) none
The author’s observations
In community samples of children and adolescents, auditory hallucinations are not rare and usually do not cause distress or dysfunction. In a study of 3,870 children age 7 and 8,1 9% endorsed auditory hallucinations. Most heard 1 voice, once a week or less, at low volume. In 85% of children who experienced hallucinations, they caused minimal or no suffering; 97% reported minimal or no interference with daily functioning. Among children who experienced auditory hallucinations at age 7 or 8, 24% continued to hallucinate 5 years later.2 Persistent hallucinations were associated with more problematic behaviors at baseline and follow up.
In a group of 12-year-old twins, 4.2% reported auditory hallucinations.3 In that study, hallucinations were not related to Cannabis use; rather, they were heritable and related to risk factors such as cognitive impairment; behavioral, emotional, and educational problems at age 5; and a history of physical abuse and self-harm at age 12. The authors noted that these are risk factors and correlates of schizophrenia, but are not specific to schizophrenia.
Hallucinations and delusions have been found in 4% to 8% of children and adolescents referred for psychiatric treatment,4 far more than the prevalence of childhood-onset schizophrenia (0.01% of children).5 Psychotic symptoms in children have been associated with bipolar disorder, but also with anxiety disorders, obsessive-compulsive disorder, PTSD, pervasive developmental disorder, conduct disorder, and substance abuse.4
Childhood-onset schizophrenia is rare and would require that Mr. P have a diagnosis of schizophrenia as an adult. It is possible that Mr. P’s childhood symptoms were related to substance abuse but he was not asked for this history because it seemed unlikely in a 10-year-old boy. A PTSD diagnosis requires a traumatic event, which Mr. P did not reveal. It is possible that at age 10 he did not have a psychiatric disorder.
a) PTSD
b) dissociative disorder
c) borderline personality disorder
d) chronic schizophrenia
e) no psychiatric diagnosis
Among adults in the general population, 10% to 15% report auditory hallucinations.6 Hallucinations could be caused by substance abuse or psychiatric conditions other than schizophrenia; however, in adults—as in children—auditory hallucinations can occur in the absence of these conditions (Table 1) and rarely cause distress or dysfunction.6 In Sommer and colleagues’6 study of 103 healthy persons, none who heard voices had disorganization or negative symptoms. Those who heard voices had significantly more schizotypal symptoms and more childhood trauma, including emotional, physical, and sexual abuse, than those who did not hear voices.6
Conditions associated with hallucinations
PTSD is associated with auditory hallucinations and other psychotic symptoms.7 Most studies are of combat veterans with PTSD, in whom auditory hallucinations and delusions were associated with major depressive disorder, not a thought disorder or inappropriate affect.8 In a community sample,9 psychotic symptoms—particularly auditory hallucinations—were associated with PTSD. Subjects with PTSD and psychotic symptoms were more likely to have other psychiatric disorders, including major depressive disorder and substance use disorder, than patients with PTSD but no psychotic symptoms; however, the relationship between PTSD and psychosis remained after controlling for other psychiatric disorders.
Hallucinations can occur in persons with dissociative disorders in the absence of distinct personality states.10 Hallucinations have been seen transiently and chronically in persons with borderline personality disorder and can be associated with comorbid conditions such as substance abuse disorders, mood disorders, and PTSD.11
Mr. P lacked the reduced capacity for interpersonal relationships required for a schizotypal personality disorder diagnosis. A diagnosis of PTSD or dissociative disorder requires a history of trauma, which Mr. P did not report.
“Time travelling” with incomprehensible behavior could be interpreted as dissociation, but dissociative fugue or dissociative disorder not otherwise specified (NOS) cannot be diagnosed if symptoms might be the direct effect of a substance, such as Cannabis. Mr. P admitted to substance abuse. We can rule out borderline personality disorder because he did not display or admit to tempestuous interpersonal relationships.
A schizophrenia diagnosis requires the presence of auditory hallucinations that commented on his behavior or conversed among themselves, a second psychotic symptom for ≥1 month, or negative symptoms, which Mr. P lacked (unless belief in time travel is considered delusional).
Last, a physician might have considered malingering or a factitious disorder when Mr. P was found not able to participate in his own defense, but this seemed less likely after he revealed that he experienced auditory hallucinations since age 10.
HISTORY Bad beatings
With a few days of beginning risperidone, 4 mg/d, Mr. P reports that his hallucinations have stopped and he feels less sad. He reveals that, at age 10, when the hallucinations began, his mother hit him over the head with a high-heeled shoe, causing a scalp laceration that required a visit to the emergency room for suturing. His mother beat Mr. P for as long as he could remember. She beat him “bad” at least twice weekly, and he was taken to the hospital 7 or 8 times for injury, but she also beat him “constantly” with a belt buckle, sometimes striking his head. She instructed him to tell nobody.
The author’s observations
Auditory hallucinations in adults have been associated with childhood abuse, particularly childhood sexual abuse,12 in clinical and non-clinical samples.13 Some argue13 that child abuse itself causes hallucinations and other psychotic symptoms.
OUTCOME Depressed and sleepless
Mr. P admits that he had been smoking marijuana 2 to 3 times daily for a year. He also reports insomnia, sleeping approximately 4 hours a night and spending hours awake in bed thinking of his grandmother, with depressed mood and tearfulness. He denies suicidal ideas and hallucinations. He is treated for depressive disorder NOS first with amitriptyline, 50 mg at bedtime, for sleep, then paroxetine, 20 mg/d, for depressive symptoms, in addition to risperidone, 4 mg/d. Although Mr. P does not describe re-experiencing his childhood trauma, avoidance of stimuli associated with the trauma, or symptoms of increased arousal (except for insomnia), the treatment team did not ask, so it remains uncertain if he has PTSD (Table 2).
When Mr. P is discharged to a clinic, he smiles easily and is positive and supportive with other patients. He spruces up his appearance by wearing jewelry and works in the hospital kitchen.
Bottom Line
Chronic auditory hallucinations are associated with psychiatric illnesses other than chronic schizophrenia, particularly those resulting from trauma such as posttraumatic stress disorder. They can also occur in the absence of diagnosable psychiatric illness and rarely cause distress or functional impairment. Auditory hallucinations in adults have been associated with childhood abuse.
Related Resources
- Moskowitz A, Schafer I, Dorahy MJ. Psychosis, trauma and dissociation: emerging perspectives on severe psychopathology. West Sussex, UK: John Wiley and Sons, Ltd.; 2008.
- The International Hearing Voices Network. www.intervoiceonline.org.
Drug Brand Names
Amitriptyline • Elavil Paroxetine • Paxil
Benztropine • Cogentin Risperidone • Risperdal
Haloperidol • Haldol
Disclosure
Dr. Crowner reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Grief and confusion
Mr. P, age 47, is arrested for entering the apartment of a woman he does not know and tossing her belongings out the window. When he is assessed to determine if he can participate in his legal defense, examiners find an attentive, courteous man who is baffled by his own behavior.
Mr. P says that he had been “stressed out” after the recent death of his grandmother, with whom he was close. He says he entered the apartment because voices told him to do so. He has no recent history of substance abuse or psychiatric hospitalizations, but he had a similar episode of “confusion” years before, when another close family member died.
Mr. P is found not fit to stand trial and the charges are dropped. He accepts haloperidol, 10 mg/d, and benztropine, 2 mg/d, and is transferred to a hospital for psychiatric treatment.
On interview, Mr. P is well groomed, soft-spoken, and shy, without formal thought disorder. Physical exam and routine lab tests are within normal limits. He says that 18 months before his arrest, he and his frail grandmother moved to a large city in hopes that he would find a wife. Both depended on the grandmother’s Social Security benefits while he cared for her.
In the 2 months after she died, he reports that he felt sad and alone and slept poorly, but made efforts to find a job and keep his apartment. When his efforts failed and he lost the apartment, he stayed with various friends for a few days at a time, then spent several days in the subway before ending up on the streets.
His arrest on the current charge occurred 4 days after he began walking the streets.
a) continue haloperidol to treat psychotic symptoms
b) discontinue haloperidol and observe him
c) add an antidepressant to haloperidol
HISTORY Imagining nonsense
Mr. P cannot explain why he started “trashing” the woman’s apartment, but says he entered it because he thought it was his apartment. With embarrassment and regret, he admits he has been depressed and confused, “imagining things”—“foolish things,” he admits—such as being in a different “time zone.”
Contradicting his earlier statements, Mr. P now admits that he had “a few beers” and denies that he experienced auditory hallucinations, saying he only talks to himself. He now says that within 2 days after his arrest, he was “all over it.” Mr. P denies current symptoms, including hallucinations, but, when pressed, waffles, then admits to a strange belief: that some people, including him, can move from one “time zone” to another.
Mr. P says he was treated for psychiatric problems 4 years earlier when his parents were killed in a car crash. By his recollection, his reaction to their death was similar to his reaction to his grandmother’s death: He became upset and wandered the streets for a few days, “moving between time zones” and talking to himself but not experiencing hallucinations. After he was taken to a hospital and “given an injection,” he calmed down and was released. Within a few days he recovered and returned to supporting himself and caring for his grandmother. Mr. P says the idea of travelling between “time zones” is embarrassing and nonsensical but adds that he was affected in this way because he “bickered” with his mother.
Mr. P’s grandmother raised him until he was age 15, although he frequently visited his parents, who lived nearby and worked during the day. Mr. P initially denies substance abuse, then admits to smoking marijuana every day for about a year before admission. He also admits to cocaine abuse in his 20s. He denies a history of suicide attempts.
The author’s observations
Mr. P reported only 2 episodes of “confusion” (or psychosis) and strange behavior in his life, both precipitated by the loss of a loved one, and at least 1 while under the influence of alcohol and Cannabis. He gave an inconsistent and ambiguous history of auditory hallucinations associated with episodes of confusion. He believes that time travel is possible, an idea that he acknowledged is nonsense. This alone was not enough to warrant long-term antipsychotic treatment. The most likely diagnosis seemed to be brief psychotic episode induced by Cannabis and the stressors of homelessness and his grandmother’s death.
EVALUATION Changing stories
No longer taking haloperidol, Mr. P continues to deny hallucinations and depressed mood, but keeps to himself. Nine days after admission he becomes tearful after he informs his aunt of his grandmother’s death in a telephone call, then approaches a nurse and complains of sadness and auditory hallucinations.
Mr. P confesses that he denied hallucinations on admission because he feared he would remain in the hospital for years if he revealed the truth that he had been experiencing auditory hallucinations almost continuously from age 10. He reports that the voices distracted him when he worked; seem to be male; often spoke gibberish; and alternate between deprecating and positive and supportive. Mr. P is reluctant to disclose more about what the voices actually say, although he acknowledges that they are not commenting or conversing with him, and that he has never believed the voices were his own thoughts but did believe that they came from inside his brain.
With haloperidol, the voices stopped. They resumed, however, when haloperidol was discontinued.
When we ask what happened to him at age 10, Mr. P shrugs.
a) childhood onset schizophrenia
b) substance abuse
c) posttraumatic stress disorder (PTSD)
d) none
The author’s observations
In community samples of children and adolescents, auditory hallucinations are not rare and usually do not cause distress or dysfunction. In a study of 3,870 children age 7 and 8,1 9% endorsed auditory hallucinations. Most heard 1 voice, once a week or less, at low volume. In 85% of children who experienced hallucinations, they caused minimal or no suffering; 97% reported minimal or no interference with daily functioning. Among children who experienced auditory hallucinations at age 7 or 8, 24% continued to hallucinate 5 years later.2 Persistent hallucinations were associated with more problematic behaviors at baseline and follow up.
In a group of 12-year-old twins, 4.2% reported auditory hallucinations.3 In that study, hallucinations were not related to Cannabis use; rather, they were heritable and related to risk factors such as cognitive impairment; behavioral, emotional, and educational problems at age 5; and a history of physical abuse and self-harm at age 12. The authors noted that these are risk factors and correlates of schizophrenia, but are not specific to schizophrenia.
Hallucinations and delusions have been found in 4% to 8% of children and adolescents referred for psychiatric treatment,4 far more than the prevalence of childhood-onset schizophrenia (0.01% of children).5 Psychotic symptoms in children have been associated with bipolar disorder, but also with anxiety disorders, obsessive-compulsive disorder, PTSD, pervasive developmental disorder, conduct disorder, and substance abuse.4
Childhood-onset schizophrenia is rare and would require that Mr. P have a diagnosis of schizophrenia as an adult. It is possible that Mr. P’s childhood symptoms were related to substance abuse but he was not asked for this history because it seemed unlikely in a 10-year-old boy. A PTSD diagnosis requires a traumatic event, which Mr. P did not reveal. It is possible that at age 10 he did not have a psychiatric disorder.
a) PTSD
b) dissociative disorder
c) borderline personality disorder
d) chronic schizophrenia
e) no psychiatric diagnosis
Among adults in the general population, 10% to 15% report auditory hallucinations.6 Hallucinations could be caused by substance abuse or psychiatric conditions other than schizophrenia; however, in adults—as in children—auditory hallucinations can occur in the absence of these conditions (Table 1) and rarely cause distress or dysfunction.6 In Sommer and colleagues’6 study of 103 healthy persons, none who heard voices had disorganization or negative symptoms. Those who heard voices had significantly more schizotypal symptoms and more childhood trauma, including emotional, physical, and sexual abuse, than those who did not hear voices.6
Conditions associated with hallucinations
PTSD is associated with auditory hallucinations and other psychotic symptoms.7 Most studies are of combat veterans with PTSD, in whom auditory hallucinations and delusions were associated with major depressive disorder, not a thought disorder or inappropriate affect.8 In a community sample,9 psychotic symptoms—particularly auditory hallucinations—were associated with PTSD. Subjects with PTSD and psychotic symptoms were more likely to have other psychiatric disorders, including major depressive disorder and substance use disorder, than patients with PTSD but no psychotic symptoms; however, the relationship between PTSD and psychosis remained after controlling for other psychiatric disorders.
Hallucinations can occur in persons with dissociative disorders in the absence of distinct personality states.10 Hallucinations have been seen transiently and chronically in persons with borderline personality disorder and can be associated with comorbid conditions such as substance abuse disorders, mood disorders, and PTSD.11
Mr. P lacked the reduced capacity for interpersonal relationships required for a schizotypal personality disorder diagnosis. A diagnosis of PTSD or dissociative disorder requires a history of trauma, which Mr. P did not report.
“Time travelling” with incomprehensible behavior could be interpreted as dissociation, but dissociative fugue or dissociative disorder not otherwise specified (NOS) cannot be diagnosed if symptoms might be the direct effect of a substance, such as Cannabis. Mr. P admitted to substance abuse. We can rule out borderline personality disorder because he did not display or admit to tempestuous interpersonal relationships.
A schizophrenia diagnosis requires the presence of auditory hallucinations that commented on his behavior or conversed among themselves, a second psychotic symptom for ≥1 month, or negative symptoms, which Mr. P lacked (unless belief in time travel is considered delusional).
Last, a physician might have considered malingering or a factitious disorder when Mr. P was found not able to participate in his own defense, but this seemed less likely after he revealed that he experienced auditory hallucinations since age 10.
HISTORY Bad beatings
With a few days of beginning risperidone, 4 mg/d, Mr. P reports that his hallucinations have stopped and he feels less sad. He reveals that, at age 10, when the hallucinations began, his mother hit him over the head with a high-heeled shoe, causing a scalp laceration that required a visit to the emergency room for suturing. His mother beat Mr. P for as long as he could remember. She beat him “bad” at least twice weekly, and he was taken to the hospital 7 or 8 times for injury, but she also beat him “constantly” with a belt buckle, sometimes striking his head. She instructed him to tell nobody.
The author’s observations
Auditory hallucinations in adults have been associated with childhood abuse, particularly childhood sexual abuse,12 in clinical and non-clinical samples.13 Some argue13 that child abuse itself causes hallucinations and other psychotic symptoms.
OUTCOME Depressed and sleepless
Mr. P admits that he had been smoking marijuana 2 to 3 times daily for a year. He also reports insomnia, sleeping approximately 4 hours a night and spending hours awake in bed thinking of his grandmother, with depressed mood and tearfulness. He denies suicidal ideas and hallucinations. He is treated for depressive disorder NOS first with amitriptyline, 50 mg at bedtime, for sleep, then paroxetine, 20 mg/d, for depressive symptoms, in addition to risperidone, 4 mg/d. Although Mr. P does not describe re-experiencing his childhood trauma, avoidance of stimuli associated with the trauma, or symptoms of increased arousal (except for insomnia), the treatment team did not ask, so it remains uncertain if he has PTSD (Table 2).
When Mr. P is discharged to a clinic, he smiles easily and is positive and supportive with other patients. He spruces up his appearance by wearing jewelry and works in the hospital kitchen.
Bottom Line
Chronic auditory hallucinations are associated with psychiatric illnesses other than chronic schizophrenia, particularly those resulting from trauma such as posttraumatic stress disorder. They can also occur in the absence of diagnosable psychiatric illness and rarely cause distress or functional impairment. Auditory hallucinations in adults have been associated with childhood abuse.
Related Resources
- Moskowitz A, Schafer I, Dorahy MJ. Psychosis, trauma and dissociation: emerging perspectives on severe psychopathology. West Sussex, UK: John Wiley and Sons, Ltd.; 2008.
- The International Hearing Voices Network. www.intervoiceonline.org.
Drug Brand Names
Amitriptyline • Elavil Paroxetine • Paxil
Benztropine • Cogentin Risperidone • Risperdal
Haloperidol • Haldol
Disclosure
Dr. Crowner reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Barthel-Velthuis AA, Jenner JA, van de Willige G, et al Prevalence and correlates of auditory vocal hallucinations in middle childhood. Br J Psychiatry. 2010;196(1):41-46.
2. Bartels-Velthuis AA, van de Willige G, Jenner JA, et al. Course of auditory vocal hallucinations in childhood: 5-year follow-up study. Br J Psychiatry. 2011;199(4):296-302.
3. Polanczyk G, Moffitt TE, Arsensault L, et al. Etiological and clinical features of childhood psychotic symptoms: results from a birth cohort. Arch Gen Psychiatry. 2010;67(4):328-338.
4. Biederman J, Pety C, Faracone SV, et al. Phenomenology of childhood psychosis: Findings from a large sample of psychiatrically referred youth. J Nerv Ment Dis 2004;192(9):607-614.
5. American Academy of Child and Adolescent Psychiatry. Practice parameters for the assessment and treatment of children and adolescents with schizophrenia. J Am Acad Child Adolesc Psychiatry. 2001;40(suppl 7):4SS-23S.
6. Sommer IEC, Daalman K, Rietkerk T, et al. Healthy individuals with auditory verbal hallucinations; Who are they? Psychiatric assessments of a selected sample of 103 subjects. Schizophr Bull. 2010;36(3):633-641.
7. Butler RW, Mueser KT, Sprock J, et al. Positive symptoms of psychosis in posttraumatic stress disorder. Biol Psychiatry. 1996;39:839-844.
8. David D, Kutcher GS, Jackson EI, et al Psychotic symptoms in combat-related posttraumatic stress disorder. J Clin Psychiatry. 1999;60(1):29-32.
9. Sareen J, Cox BJ, Goodwin RD, et al. Co-occurrence of posttraumatic stress disorder with positive psychotic symptoms in a nationally representative sample. J Trauma Stress. 2005;18(4):313-322.
10. Sar V, Akyuv G, Dogan O. Prevalence of dissociative disorders among women in the general population. Psychiatry Res. 2007;149:169-176.
11. Barnow S, Arens EA, Sieswerda S, et al. Borderline personality disorder and psychosis: a review. Curr Psychiatry Rep. 2010;12(3):186-195.
12. Bebbington P, Jonas S, Kuipers E, et al. Childhood sexual abuse and psychosis: data from a cross-sectional national psychiatric survey in England. Br J Psychiatry. 2011;199(1):29-37.
13. Read J, van Os J, Morrison AP, et al. Childhood trauma, psychosis and schizophrenia: a literature review with theoretical and clinical implications. Acta Psychiatr Scand. 2005;112(5):330-350.
1. Barthel-Velthuis AA, Jenner JA, van de Willige G, et al Prevalence and correlates of auditory vocal hallucinations in middle childhood. Br J Psychiatry. 2010;196(1):41-46.
2. Bartels-Velthuis AA, van de Willige G, Jenner JA, et al. Course of auditory vocal hallucinations in childhood: 5-year follow-up study. Br J Psychiatry. 2011;199(4):296-302.
3. Polanczyk G, Moffitt TE, Arsensault L, et al. Etiological and clinical features of childhood psychotic symptoms: results from a birth cohort. Arch Gen Psychiatry. 2010;67(4):328-338.
4. Biederman J, Pety C, Faracone SV, et al. Phenomenology of childhood psychosis: Findings from a large sample of psychiatrically referred youth. J Nerv Ment Dis 2004;192(9):607-614.
5. American Academy of Child and Adolescent Psychiatry. Practice parameters for the assessment and treatment of children and adolescents with schizophrenia. J Am Acad Child Adolesc Psychiatry. 2001;40(suppl 7):4SS-23S.
6. Sommer IEC, Daalman K, Rietkerk T, et al. Healthy individuals with auditory verbal hallucinations; Who are they? Psychiatric assessments of a selected sample of 103 subjects. Schizophr Bull. 2010;36(3):633-641.
7. Butler RW, Mueser KT, Sprock J, et al. Positive symptoms of psychosis in posttraumatic stress disorder. Biol Psychiatry. 1996;39:839-844.
8. David D, Kutcher GS, Jackson EI, et al Psychotic symptoms in combat-related posttraumatic stress disorder. J Clin Psychiatry. 1999;60(1):29-32.
9. Sareen J, Cox BJ, Goodwin RD, et al. Co-occurrence of posttraumatic stress disorder with positive psychotic symptoms in a nationally representative sample. J Trauma Stress. 2005;18(4):313-322.
10. Sar V, Akyuv G, Dogan O. Prevalence of dissociative disorders among women in the general population. Psychiatry Res. 2007;149:169-176.
11. Barnow S, Arens EA, Sieswerda S, et al. Borderline personality disorder and psychosis: a review. Curr Psychiatry Rep. 2010;12(3):186-195.
12. Bebbington P, Jonas S, Kuipers E, et al. Childhood sexual abuse and psychosis: data from a cross-sectional national psychiatric survey in England. Br J Psychiatry. 2011;199(1):29-37.
13. Read J, van Os J, Morrison AP, et al. Childhood trauma, psychosis and schizophrenia: a literature review with theoretical and clinical implications. Acta Psychiatr Scand. 2005;112(5):330-350.
Is he DISTRACTED? Considerations when diagnosing ADHD in an adult
Adult attention-deficit/hyperactivity disorder (ADHD) can be challenging to assess accurately. Adult ADHD differs significantly from childhood ADHD, in that hyperactivity often is absent or greatly diminished, comorbid disorders (depression or substance use) are common, and previously compensated attention deficits in school can manifest in the patient’s personal and professional life.1
The mnemonic DISTRACTED can help when recalling key components in assessing adult ADHD.2 Because ADHD is a developmental disorder—there are signs of onset in childhood—it is important to maintain a longitudinal view when asking about patterns of behavior or thinking.
Distractibility. Is there a pattern of getting “off track” in conversations or in school or work situations because of straying thoughts or daydreams? Is there a tendency to over-respond to extraneous stimuli (eg, cell phones, computers, television) that impedes the patient’s ability to converse, receive information, or follow directions?
Impulsivity. Does the patient have a history of saying things “off the cuff,” interrupting others, or “walking on” someone else’s words in a conversation? Is impulsivity evident in the person’s substance use or spending patterns?
School history. This domain is important in diagnosing ADHD in adults because there needs to be evidence that the disorder was present from an early age. How did the patient perform in school (ie, grades, organization, completion of homework assignments)? Was there a behavioral pattern that reflected hyperactivity (could not stay seated) or emotional dysregulation (frequent outbursts)?
Task completion. Does the patient have trouble finishing assignments at work, staying focused on a project that is considered boring, or completing a home project (eg, fixing a leaky faucet) in a timely fashion?
Rating scales. Rating scales should be used to help support the diagnosis, based on the patient’s history and life story. There are >12 scales that can be utilized in a
clinical setting3; the ADHD/Hyperactivity Disorder Self-Report Scale is a brief and easy measure of core ADHD symptoms.
Accidents. Adults with ADHD often are accident-prone because of inattention, hyperactivity, or impulsivity. Does the patient have a history of unintentionally hurting himself because he “wasn’t paying attention” (falls, burns), or was too impatient (traffic accidents or citations)?
Commitments. Does the patient fail to fulfill verbal obligations (by arriving late, forgetting to run errands)? Has this difficulty to commit created problems in relationships over time?
Time management. How difficult is it for the patient to stay organized while balancing work expectations, social obligations, and family needs? Is there a pattern of chaotic scheduling with regard to meals, work, or sleeping?
Employment. Has the patient changed jobs because the work becomes “too boring” or “uninteresting”? Is there a pattern of being terminated because of poor work quality based on time management or job performance?
Decisions. Adults with ADHD often make hasty, ill-informed choices or procrastinate so that they do not have to make a decision. Does the patient’s decision-making reveal a pattern of being too distracted to hear the information needed, or too impatient to consider all the details?
Remember: No single component of this mnemonic alone suffices to make a diagnosis of adult ADHD. However, these considerations will help clarify what lies behind your DISTRACTED patient’s search for self-understanding and appropriate medical care.
Disclosure
Dr. Christensen reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Barkley RA, Brown TE. Unrecognized attention-deficit/hyperactivity disorder in adults presenting with other psychiatric disorders. CNS Spectr. 2008;13(11):977-984.
2. Barkley R. Taking charge of adult ADHD. New York, NY: Guilford Press; 2010.
3. Attwell C. ADHD, rating scales, and your practice today. The Carlat Psychiatry Report. 2012;10(12):1,3,5-8.
Adult attention-deficit/hyperactivity disorder (ADHD) can be challenging to assess accurately. Adult ADHD differs significantly from childhood ADHD, in that hyperactivity often is absent or greatly diminished, comorbid disorders (depression or substance use) are common, and previously compensated attention deficits in school can manifest in the patient’s personal and professional life.1
The mnemonic DISTRACTED can help when recalling key components in assessing adult ADHD.2 Because ADHD is a developmental disorder—there are signs of onset in childhood—it is important to maintain a longitudinal view when asking about patterns of behavior or thinking.
Distractibility. Is there a pattern of getting “off track” in conversations or in school or work situations because of straying thoughts or daydreams? Is there a tendency to over-respond to extraneous stimuli (eg, cell phones, computers, television) that impedes the patient’s ability to converse, receive information, or follow directions?
Impulsivity. Does the patient have a history of saying things “off the cuff,” interrupting others, or “walking on” someone else’s words in a conversation? Is impulsivity evident in the person’s substance use or spending patterns?
School history. This domain is important in diagnosing ADHD in adults because there needs to be evidence that the disorder was present from an early age. How did the patient perform in school (ie, grades, organization, completion of homework assignments)? Was there a behavioral pattern that reflected hyperactivity (could not stay seated) or emotional dysregulation (frequent outbursts)?
Task completion. Does the patient have trouble finishing assignments at work, staying focused on a project that is considered boring, or completing a home project (eg, fixing a leaky faucet) in a timely fashion?
Rating scales. Rating scales should be used to help support the diagnosis, based on the patient’s history and life story. There are >12 scales that can be utilized in a
clinical setting3; the ADHD/Hyperactivity Disorder Self-Report Scale is a brief and easy measure of core ADHD symptoms.
Accidents. Adults with ADHD often are accident-prone because of inattention, hyperactivity, or impulsivity. Does the patient have a history of unintentionally hurting himself because he “wasn’t paying attention” (falls, burns), or was too impatient (traffic accidents or citations)?
Commitments. Does the patient fail to fulfill verbal obligations (by arriving late, forgetting to run errands)? Has this difficulty to commit created problems in relationships over time?
Time management. How difficult is it for the patient to stay organized while balancing work expectations, social obligations, and family needs? Is there a pattern of chaotic scheduling with regard to meals, work, or sleeping?
Employment. Has the patient changed jobs because the work becomes “too boring” or “uninteresting”? Is there a pattern of being terminated because of poor work quality based on time management or job performance?
Decisions. Adults with ADHD often make hasty, ill-informed choices or procrastinate so that they do not have to make a decision. Does the patient’s decision-making reveal a pattern of being too distracted to hear the information needed, or too impatient to consider all the details?
Remember: No single component of this mnemonic alone suffices to make a diagnosis of adult ADHD. However, these considerations will help clarify what lies behind your DISTRACTED patient’s search for self-understanding and appropriate medical care.
Disclosure
Dr. Christensen reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Adult attention-deficit/hyperactivity disorder (ADHD) can be challenging to assess accurately. Adult ADHD differs significantly from childhood ADHD, in that hyperactivity often is absent or greatly diminished, comorbid disorders (depression or substance use) are common, and previously compensated attention deficits in school can manifest in the patient’s personal and professional life.1
The mnemonic DISTRACTED can help when recalling key components in assessing adult ADHD.2 Because ADHD is a developmental disorder—there are signs of onset in childhood—it is important to maintain a longitudinal view when asking about patterns of behavior or thinking.
Distractibility. Is there a pattern of getting “off track” in conversations or in school or work situations because of straying thoughts or daydreams? Is there a tendency to over-respond to extraneous stimuli (eg, cell phones, computers, television) that impedes the patient’s ability to converse, receive information, or follow directions?
Impulsivity. Does the patient have a history of saying things “off the cuff,” interrupting others, or “walking on” someone else’s words in a conversation? Is impulsivity evident in the person’s substance use or spending patterns?
School history. This domain is important in diagnosing ADHD in adults because there needs to be evidence that the disorder was present from an early age. How did the patient perform in school (ie, grades, organization, completion of homework assignments)? Was there a behavioral pattern that reflected hyperactivity (could not stay seated) or emotional dysregulation (frequent outbursts)?
Task completion. Does the patient have trouble finishing assignments at work, staying focused on a project that is considered boring, or completing a home project (eg, fixing a leaky faucet) in a timely fashion?
Rating scales. Rating scales should be used to help support the diagnosis, based on the patient’s history and life story. There are >12 scales that can be utilized in a
clinical setting3; the ADHD/Hyperactivity Disorder Self-Report Scale is a brief and easy measure of core ADHD symptoms.
Accidents. Adults with ADHD often are accident-prone because of inattention, hyperactivity, or impulsivity. Does the patient have a history of unintentionally hurting himself because he “wasn’t paying attention” (falls, burns), or was too impatient (traffic accidents or citations)?
Commitments. Does the patient fail to fulfill verbal obligations (by arriving late, forgetting to run errands)? Has this difficulty to commit created problems in relationships over time?
Time management. How difficult is it for the patient to stay organized while balancing work expectations, social obligations, and family needs? Is there a pattern of chaotic scheduling with regard to meals, work, or sleeping?
Employment. Has the patient changed jobs because the work becomes “too boring” or “uninteresting”? Is there a pattern of being terminated because of poor work quality based on time management or job performance?
Decisions. Adults with ADHD often make hasty, ill-informed choices or procrastinate so that they do not have to make a decision. Does the patient’s decision-making reveal a pattern of being too distracted to hear the information needed, or too impatient to consider all the details?
Remember: No single component of this mnemonic alone suffices to make a diagnosis of adult ADHD. However, these considerations will help clarify what lies behind your DISTRACTED patient’s search for self-understanding and appropriate medical care.
Disclosure
Dr. Christensen reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Barkley RA, Brown TE. Unrecognized attention-deficit/hyperactivity disorder in adults presenting with other psychiatric disorders. CNS Spectr. 2008;13(11):977-984.
2. Barkley R. Taking charge of adult ADHD. New York, NY: Guilford Press; 2010.
3. Attwell C. ADHD, rating scales, and your practice today. The Carlat Psychiatry Report. 2012;10(12):1,3,5-8.
1. Barkley RA, Brown TE. Unrecognized attention-deficit/hyperactivity disorder in adults presenting with other psychiatric disorders. CNS Spectr. 2008;13(11):977-984.
2. Barkley R. Taking charge of adult ADHD. New York, NY: Guilford Press; 2010.
3. Attwell C. ADHD, rating scales, and your practice today. The Carlat Psychiatry Report. 2012;10(12):1,3,5-8.
Never ‘do nothing’ at end of life
Providing end-of-life care – is one of the toughest, most painful things we are called upon to do. Who among us has not had the gut-wrenching experience of informing a spouse of 50+ years that within a few short days, their life together will come to an abrupt end? No more anniversaries. No more anything.
I don’t think physicians can truly appreciate what patients’ loved ones go through when they are dying, until we become that loved one. I got my revelation when I was the caregiver and hospice physician for a very close relative who ultimately died from cancer in my home. I had asked an oncologist friend of mine to take on her case when she relocated to live with me. To my surprise, my relative found my colleague to be rather cold and unfeeling, just when she needed a compassionate physician the most.
I deeply understand the field of medicine, had care provided by a clinician/friend, and my relative still had a subpar experience, so what must it like for those without a medical background?
I recently spoke with a friend whose elderly aunt had just passed away. In addition to the grief she felt, she had to deal with frustration and anguish about how her aunt was treated in her final days. Her aunt’s DNI (do not intubate) status was mistakenly assumed by some on her health care team to mean "DNT" (do not treat). Basic care, such as intravenous fluids in the face of inadequate oral intake, was even neglected. To add insult to injury, the family – those who actually knew her belief system, feelings, and wishes – was not allowed to partner with the health care team to create the plan for her end-of-life care.
While we often wrestle with how to talk to family, including what we should and should not say, perhaps we should begin by learning a little about the background of the family members so we can tailor our conversations to a level appropriate to their level of understanding – great or small– of health care.
We can learn a lot by talking to friends about the experiences they have when a loved one dies. How were they and their family member treated by physicians and how did they respond to that treatment? What do they wish had happened differently? What made the transition from this life more difficult and what made it easier?
My friend’s words of wisdom for hospitalists center on communication and respect: "Each patient and family should be treated as if they are Kennedys or Annenbergs from the start."
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center who has a passion for empowering patients to partner in their health care. She is the creator of the Patient Whiz, a patient-engagement app for iOS.
Providing end-of-life care – is one of the toughest, most painful things we are called upon to do. Who among us has not had the gut-wrenching experience of informing a spouse of 50+ years that within a few short days, their life together will come to an abrupt end? No more anniversaries. No more anything.
I don’t think physicians can truly appreciate what patients’ loved ones go through when they are dying, until we become that loved one. I got my revelation when I was the caregiver and hospice physician for a very close relative who ultimately died from cancer in my home. I had asked an oncologist friend of mine to take on her case when she relocated to live with me. To my surprise, my relative found my colleague to be rather cold and unfeeling, just when she needed a compassionate physician the most.
I deeply understand the field of medicine, had care provided by a clinician/friend, and my relative still had a subpar experience, so what must it like for those without a medical background?
I recently spoke with a friend whose elderly aunt had just passed away. In addition to the grief she felt, she had to deal with frustration and anguish about how her aunt was treated in her final days. Her aunt’s DNI (do not intubate) status was mistakenly assumed by some on her health care team to mean "DNT" (do not treat). Basic care, such as intravenous fluids in the face of inadequate oral intake, was even neglected. To add insult to injury, the family – those who actually knew her belief system, feelings, and wishes – was not allowed to partner with the health care team to create the plan for her end-of-life care.
While we often wrestle with how to talk to family, including what we should and should not say, perhaps we should begin by learning a little about the background of the family members so we can tailor our conversations to a level appropriate to their level of understanding – great or small– of health care.
We can learn a lot by talking to friends about the experiences they have when a loved one dies. How were they and their family member treated by physicians and how did they respond to that treatment? What do they wish had happened differently? What made the transition from this life more difficult and what made it easier?
My friend’s words of wisdom for hospitalists center on communication and respect: "Each patient and family should be treated as if they are Kennedys or Annenbergs from the start."
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center who has a passion for empowering patients to partner in their health care. She is the creator of the Patient Whiz, a patient-engagement app for iOS.
Providing end-of-life care – is one of the toughest, most painful things we are called upon to do. Who among us has not had the gut-wrenching experience of informing a spouse of 50+ years that within a few short days, their life together will come to an abrupt end? No more anniversaries. No more anything.
I don’t think physicians can truly appreciate what patients’ loved ones go through when they are dying, until we become that loved one. I got my revelation when I was the caregiver and hospice physician for a very close relative who ultimately died from cancer in my home. I had asked an oncologist friend of mine to take on her case when she relocated to live with me. To my surprise, my relative found my colleague to be rather cold and unfeeling, just when she needed a compassionate physician the most.
I deeply understand the field of medicine, had care provided by a clinician/friend, and my relative still had a subpar experience, so what must it like for those without a medical background?
I recently spoke with a friend whose elderly aunt had just passed away. In addition to the grief she felt, she had to deal with frustration and anguish about how her aunt was treated in her final days. Her aunt’s DNI (do not intubate) status was mistakenly assumed by some on her health care team to mean "DNT" (do not treat). Basic care, such as intravenous fluids in the face of inadequate oral intake, was even neglected. To add insult to injury, the family – those who actually knew her belief system, feelings, and wishes – was not allowed to partner with the health care team to create the plan for her end-of-life care.
While we often wrestle with how to talk to family, including what we should and should not say, perhaps we should begin by learning a little about the background of the family members so we can tailor our conversations to a level appropriate to their level of understanding – great or small– of health care.
We can learn a lot by talking to friends about the experiences they have when a loved one dies. How were they and their family member treated by physicians and how did they respond to that treatment? What do they wish had happened differently? What made the transition from this life more difficult and what made it easier?
My friend’s words of wisdom for hospitalists center on communication and respect: "Each patient and family should be treated as if they are Kennedys or Annenbergs from the start."
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center who has a passion for empowering patients to partner in their health care. She is the creator of the Patient Whiz, a patient-engagement app for iOS.
Simple Tool Improves Communication Between Patients, Hospitalists
Hospitalists at the University of Michigan Health System in Ann Arbor have developed a tool to help facilitate patient communication with physicians and to "actively participate in their treatments," says Aaron Farberg, MD. "Their treatment plans will, in turn, be more effective," Dr. Farberg adds.
Called Dear Doctor (DD) notes, the tool is a pre-formatted notepad placed on a bedside table for hospital patients to consult throughout their stay. Patients can write down questions under three suggested prompts: diagnosis and treatment, tests and procedures, and medications. Such a simple tool is "absent in the inpatient setting, and can have an impact on a patient’s perceived and actual medical care," Dr. Farberg says.
Family members of patients can also benefit from DD notes by voicing their questions or concerns to the physicians. “Often their active participation in the healthcare plan is an essential element in successful treatment,” Dr. Farberg says.
Surveyed patients experienced better communication with hospitalists and had an enhanced experience with their providers and hospital. Patients and their families were happy their questions were answered and not forgotten. They had a sense of control and accountability in their own care. The study urges hospitalists and care providers to be mindful of the patient’s entire experience throughout hospital stay. TH
Visit our website for more information on hospitalists and patient communication.
Hospitalists at the University of Michigan Health System in Ann Arbor have developed a tool to help facilitate patient communication with physicians and to "actively participate in their treatments," says Aaron Farberg, MD. "Their treatment plans will, in turn, be more effective," Dr. Farberg adds.
Called Dear Doctor (DD) notes, the tool is a pre-formatted notepad placed on a bedside table for hospital patients to consult throughout their stay. Patients can write down questions under three suggested prompts: diagnosis and treatment, tests and procedures, and medications. Such a simple tool is "absent in the inpatient setting, and can have an impact on a patient’s perceived and actual medical care," Dr. Farberg says.
Family members of patients can also benefit from DD notes by voicing their questions or concerns to the physicians. “Often their active participation in the healthcare plan is an essential element in successful treatment,” Dr. Farberg says.
Surveyed patients experienced better communication with hospitalists and had an enhanced experience with their providers and hospital. Patients and their families were happy their questions were answered and not forgotten. They had a sense of control and accountability in their own care. The study urges hospitalists and care providers to be mindful of the patient’s entire experience throughout hospital stay. TH
Visit our website for more information on hospitalists and patient communication.
Hospitalists at the University of Michigan Health System in Ann Arbor have developed a tool to help facilitate patient communication with physicians and to "actively participate in their treatments," says Aaron Farberg, MD. "Their treatment plans will, in turn, be more effective," Dr. Farberg adds.
Called Dear Doctor (DD) notes, the tool is a pre-formatted notepad placed on a bedside table for hospital patients to consult throughout their stay. Patients can write down questions under three suggested prompts: diagnosis and treatment, tests and procedures, and medications. Such a simple tool is "absent in the inpatient setting, and can have an impact on a patient’s perceived and actual medical care," Dr. Farberg says.
Family members of patients can also benefit from DD notes by voicing their questions or concerns to the physicians. “Often their active participation in the healthcare plan is an essential element in successful treatment,” Dr. Farberg says.
Surveyed patients experienced better communication with hospitalists and had an enhanced experience with their providers and hospital. Patients and their families were happy their questions were answered and not forgotten. They had a sense of control and accountability in their own care. The study urges hospitalists and care providers to be mindful of the patient’s entire experience throughout hospital stay. TH
Visit our website for more information on hospitalists and patient communication.
Intravenous Haloperidol Does Not Prevent ICU Delirium
Clinical question: Can haloperidol reduce delirium in critically ill patients if initiated early in ICU stay?
Background: Prior studies suggest antipsychotics reduce intensity and duration of delirium in hospitalized patients. Evidence is mixed for preventing delirium. A trial of risperidone demonstrated delirium rate reduction in coronary artery bypass grafting (CABG) patients, but another trial of haloperidol in hip surgery patients failed to prevent onset of delirium. There is little evidence on antipsychotics in ICU delirium.
Study design: Randomized, double-blinded, placebo-controlled trial.
Setting: Single, adult ICU in England.
Synopsis: The study randomized 142 critically ill patients to receive 2.5 mg of intravenous haloperidol versus placebo every eight hours for up to 14 days. There was no significant difference between groups in the total time spent free of delirium or coma. Limitations include the use of open-label haloperidol in 21% of the placebo group patients. More sedation but less agitation was seen with the use of haloperidol, which also prolonged QTc. No severe adverse effects were observed.
This study supports the idea that scheduled antipsychotics should not be used to reduce ICU delirium. Addressing modifiable risk factors and using dexmedetomidine rather than lorazepam for sedation in the ICU continue to be first-line strategies to lower delirium rates.
Bottom line: Prophylactic haloperidol should not be used to prevent ICU delirium.
Citation: Page VJ, Ely EW, Gates S, et al. Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): a randomized, double-blind, placebo-controlled trial. Lancet Respir Med. 2013;1(7):515-523.
Visit our website for more information on treating delirium in hospitalized patients.
Clinical question: Can haloperidol reduce delirium in critically ill patients if initiated early in ICU stay?
Background: Prior studies suggest antipsychotics reduce intensity and duration of delirium in hospitalized patients. Evidence is mixed for preventing delirium. A trial of risperidone demonstrated delirium rate reduction in coronary artery bypass grafting (CABG) patients, but another trial of haloperidol in hip surgery patients failed to prevent onset of delirium. There is little evidence on antipsychotics in ICU delirium.
Study design: Randomized, double-blinded, placebo-controlled trial.
Setting: Single, adult ICU in England.
Synopsis: The study randomized 142 critically ill patients to receive 2.5 mg of intravenous haloperidol versus placebo every eight hours for up to 14 days. There was no significant difference between groups in the total time spent free of delirium or coma. Limitations include the use of open-label haloperidol in 21% of the placebo group patients. More sedation but less agitation was seen with the use of haloperidol, which also prolonged QTc. No severe adverse effects were observed.
This study supports the idea that scheduled antipsychotics should not be used to reduce ICU delirium. Addressing modifiable risk factors and using dexmedetomidine rather than lorazepam for sedation in the ICU continue to be first-line strategies to lower delirium rates.
Bottom line: Prophylactic haloperidol should not be used to prevent ICU delirium.
Citation: Page VJ, Ely EW, Gates S, et al. Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): a randomized, double-blind, placebo-controlled trial. Lancet Respir Med. 2013;1(7):515-523.
Visit our website for more information on treating delirium in hospitalized patients.
Clinical question: Can haloperidol reduce delirium in critically ill patients if initiated early in ICU stay?
Background: Prior studies suggest antipsychotics reduce intensity and duration of delirium in hospitalized patients. Evidence is mixed for preventing delirium. A trial of risperidone demonstrated delirium rate reduction in coronary artery bypass grafting (CABG) patients, but another trial of haloperidol in hip surgery patients failed to prevent onset of delirium. There is little evidence on antipsychotics in ICU delirium.
Study design: Randomized, double-blinded, placebo-controlled trial.
Setting: Single, adult ICU in England.
Synopsis: The study randomized 142 critically ill patients to receive 2.5 mg of intravenous haloperidol versus placebo every eight hours for up to 14 days. There was no significant difference between groups in the total time spent free of delirium or coma. Limitations include the use of open-label haloperidol in 21% of the placebo group patients. More sedation but less agitation was seen with the use of haloperidol, which also prolonged QTc. No severe adverse effects were observed.
This study supports the idea that scheduled antipsychotics should not be used to reduce ICU delirium. Addressing modifiable risk factors and using dexmedetomidine rather than lorazepam for sedation in the ICU continue to be first-line strategies to lower delirium rates.
Bottom line: Prophylactic haloperidol should not be used to prevent ICU delirium.
Citation: Page VJ, Ely EW, Gates S, et al. Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): a randomized, double-blind, placebo-controlled trial. Lancet Respir Med. 2013;1(7):515-523.
Visit our website for more information on treating delirium in hospitalized patients.
Hyperglycemia, Hypoglycemia Challenge Hospitalists Equally
Glycemic control in hospitalized patients is possible without having to achieve the much-debated standard of intensive glycemic control. That’s what Irl Hirsch, MD, professor of medicine at the University of Washington in Seattle, said in a presentation on management of diabetes in the hospitalized patient at the UCSF conference.
“We instituted intravenous insulin protocols throughout our hospital in 1992,” before recent medical controversies about IGC, Dr. Hirsch said. Eventually, a target weight dimension of 100 to 180 mg/dL of blood glucose became the hospital standard.
The number of hospitalized patients with diabetes increased 93% between 1988 and 2009. Many hospitalists encounter diabetics and order insulin for them every day, Dr. Hirsch said. Although hyperglycemia, which is seen in 78% of hospitalized patients with diabetes and 26% of those without, is linked to mortality regardless of diabetic status, mortality is greater in patients with diabetes, especially in those newly diagnosed with hyperglycemia, Dr. Hirsch said.1 Hypoglycemia often is overlooked due to
Doctors need to find a safe middle ground, he said, noting that intensive insulin therapy has not been shown to improve major outcomes, including ICU mortality. “The real danger is that we can’t get glucose under tight control without risking hypoglycemia,” he said. “We’ve had almost no hypoglycemia in our hospital for the past couple of years.”
In his talk, Dr. Hirsch took particular issue with the persistence of “sliding scale” approaches to titrating insulin therapy in hospitalized patients, basing the amount of insulin on current glucose level but not taking into consideration how long previous insulin treatments might be active or whether the insulin is “stacking” in the patient’s bloodstream. “The sliding scale doesn’t work. It’s dangerous, and that’s why I’m on this crusade,” he stated. Over time, basal bolus administration works better, Dr. Hirsch said, adding that continued improvements in the technology of continuous glucose monitoring will help to put an end to the controversy. TH
Larry Beresford is a freelance writer in San Franscisco.
Reference
1. Kosiborod M, Inzucchi S, Clark B, et al. National patterns of glucose control among patients hospitalized with acute myocardial infarction. J Am Coll Cardiol. 2007;49:1018–1183:1283A.
Glycemic control in hospitalized patients is possible without having to achieve the much-debated standard of intensive glycemic control. That’s what Irl Hirsch, MD, professor of medicine at the University of Washington in Seattle, said in a presentation on management of diabetes in the hospitalized patient at the UCSF conference.
“We instituted intravenous insulin protocols throughout our hospital in 1992,” before recent medical controversies about IGC, Dr. Hirsch said. Eventually, a target weight dimension of 100 to 180 mg/dL of blood glucose became the hospital standard.
The number of hospitalized patients with diabetes increased 93% between 1988 and 2009. Many hospitalists encounter diabetics and order insulin for them every day, Dr. Hirsch said. Although hyperglycemia, which is seen in 78% of hospitalized patients with diabetes and 26% of those without, is linked to mortality regardless of diabetic status, mortality is greater in patients with diabetes, especially in those newly diagnosed with hyperglycemia, Dr. Hirsch said.1 Hypoglycemia often is overlooked due to
Doctors need to find a safe middle ground, he said, noting that intensive insulin therapy has not been shown to improve major outcomes, including ICU mortality. “The real danger is that we can’t get glucose under tight control without risking hypoglycemia,” he said. “We’ve had almost no hypoglycemia in our hospital for the past couple of years.”
In his talk, Dr. Hirsch took particular issue with the persistence of “sliding scale” approaches to titrating insulin therapy in hospitalized patients, basing the amount of insulin on current glucose level but not taking into consideration how long previous insulin treatments might be active or whether the insulin is “stacking” in the patient’s bloodstream. “The sliding scale doesn’t work. It’s dangerous, and that’s why I’m on this crusade,” he stated. Over time, basal bolus administration works better, Dr. Hirsch said, adding that continued improvements in the technology of continuous glucose monitoring will help to put an end to the controversy. TH
Larry Beresford is a freelance writer in San Franscisco.
Reference
1. Kosiborod M, Inzucchi S, Clark B, et al. National patterns of glucose control among patients hospitalized with acute myocardial infarction. J Am Coll Cardiol. 2007;49:1018–1183:1283A.
Glycemic control in hospitalized patients is possible without having to achieve the much-debated standard of intensive glycemic control. That’s what Irl Hirsch, MD, professor of medicine at the University of Washington in Seattle, said in a presentation on management of diabetes in the hospitalized patient at the UCSF conference.
“We instituted intravenous insulin protocols throughout our hospital in 1992,” before recent medical controversies about IGC, Dr. Hirsch said. Eventually, a target weight dimension of 100 to 180 mg/dL of blood glucose became the hospital standard.
The number of hospitalized patients with diabetes increased 93% between 1988 and 2009. Many hospitalists encounter diabetics and order insulin for them every day, Dr. Hirsch said. Although hyperglycemia, which is seen in 78% of hospitalized patients with diabetes and 26% of those without, is linked to mortality regardless of diabetic status, mortality is greater in patients with diabetes, especially in those newly diagnosed with hyperglycemia, Dr. Hirsch said.1 Hypoglycemia often is overlooked due to
Doctors need to find a safe middle ground, he said, noting that intensive insulin therapy has not been shown to improve major outcomes, including ICU mortality. “The real danger is that we can’t get glucose under tight control without risking hypoglycemia,” he said. “We’ve had almost no hypoglycemia in our hospital for the past couple of years.”
In his talk, Dr. Hirsch took particular issue with the persistence of “sliding scale” approaches to titrating insulin therapy in hospitalized patients, basing the amount of insulin on current glucose level but not taking into consideration how long previous insulin treatments might be active or whether the insulin is “stacking” in the patient’s bloodstream. “The sliding scale doesn’t work. It’s dangerous, and that’s why I’m on this crusade,” he stated. Over time, basal bolus administration works better, Dr. Hirsch said, adding that continued improvements in the technology of continuous glucose monitoring will help to put an end to the controversy. TH
Larry Beresford is a freelance writer in San Franscisco.
Reference
1. Kosiborod M, Inzucchi S, Clark B, et al. National patterns of glucose control among patients hospitalized with acute myocardial infarction. J Am Coll Cardiol. 2007;49:1018–1183:1283A.
Thinking about the institution of marriage – Part I
Throughout history, views of marriage have evolved as societies change. Since the 6th century, the Roman Catholic Church has played a prominent role in thinking and developing our ideas about marriage and family. In October, the church sent out a document that included a questionnaire to its bishops around the world to find out what Catholics think about the "modern family." The Vatican sent out the document in preparation for the Synod of Bishops on the Family, which is slated for October 2014. Before we get the results, let’s review how society has reflected on marriage and family.
Historically, marriages often were strategic alliances between families. It was common for marriage to be between first and second cousins in order to strengthen family ties. Polygamy has been common throughout history and continues in many communities to this day.
Monogamy is also found throughout history, but in 1215, the Catholic Church decreed that partners had to publicly post notices of an impending marriage in a local parish to cut down on the number of invalid marriages. Until the 1500s, the Catholic Church accepted a couple’s word that they had exchanged marriage vows, with no witnesses or corroborating evidence needed. In the 1500s, with the rise in Protestantism, marriage became a civil matter rather than a sacrament. By 1639, states such as Massachusetts began requiring marriage licenses, and by the 19th century, marriage licenses were common in the United States.
Marriage through the ages
Here is a listing of the way in which marriage has been conceptualized over the years:
Arranged alliances: A strategic alliance between families.
Family ties: Keeping alliances within the family; the majority of all marriages throughout history were between first and second cousins.
Polygamy: A phenomenon that has been common throughout history.
Babies optional: In many early cultures, men could dissolve a marriage or take another wife if a woman was infertile. However, the early Christian church was a trailblazer in arguing that marriage was not contingent upon producing offspring.
Monogamy: This practice became the guiding principle for Western marriages between the 6th and 9th centuries because of the church.
Sacred vs. secular: In 1215, the Roman Catholic Church decreed that partners had to publicly post notices, or banns, of an impending marriage in a local parish in order to cut down on the number of invalid marriages. Until the 1500s, the church accepted a couple’s word that they had exchanged marriage vows, with no witnesses or corroborating evidence needed.
Civil marriage: By 1639, states such as Massachusetts began requiring marriage licenses and, by the 19th century, marriage licenses were common in the United States.
Romance: By the 1900s, mutual attraction became important.
Market economics: Families historically controlled access to inheritance of agricultural land, but with the spread of a market economy, it becomes possible for people to marry outside of this inheritance.
Women’s equality: About 50 years ago, in Western countries, women and men began to have equal rights and responsibilities. Instead of being about unique, gender-based roles, most partners conceived of their unions in terms of flexible divisions of labor, companionship, and mutual sexual attraction.
Same-sex marriages: One of the reasons for the stunningly rapid increase in acceptance of same-sex marriage is because heterosexuals have completely changed their notion that all marriages are between a man and a woman, notes Stephanie Coontz, Ph.D. "We now believe marriage is based on love, mutual sexual attraction, equality, and a flexible division of labor."
Source: Adapted from "Marriage, a History: From Obedience to Intimacy, or How Love Conquered Marriage," (New York: Viking, 2005), by Dr. Coontz.
A sacred view of marriage
The Catholic position throughout history has been that marriage is one of the seven sacraments bestowed by Christ. This questionnaire is an attempt by the Vatican to understand more about "mixed or interreligious marriages; the single-parent family; polygamy; marriages with the consequent problem of a dowry, sometimes understood as the purchase price of the woman; the caste system; a culture of noncommitment and a presumption that the marriage bond can be temporary; forms of feminism hostile to the Church; migration and the reformulation of the very concept of the family; relativist pluralism in the conception of marriage; the influence of the media on popular culture in its understanding of marriage and family life; underlying trends of thought in legislative proposals which devalue the idea of permanence and faithfulness in the marriage covenant; an increase in the practice of surrogate motherhood (wombs for hire); and new interpretations of what is considered a human right."
Thirty-nine questions are on the questionnaire. Questions 4, 5, and 6 are of most interest to family psychiatrists. Deserving of admiration is its concern for families in migration and for the mistreatment of women.
The terms "regular" and "irregular," used in the questionnaire, are canonical terms unrelated to what actually happens in any given society. It should also be explained that Catholics who married always had to declare that they would welcome such children as God happened to send along, recognizing that he might choose not to send any. A decision to refuse to accept the possibility of children invalidated the marriage vows and constitutes grounds for annulment.
Excerpts from the Vatican document
Questions 4, 5, and 6 of the Vatican’s questionnaire seem aimed at gathering data on different kinds of families. Here are those three questions:
Pastoral Care in Certain Difficult Marital Situations
a) Is cohabitation ad experimentum a pastoral reality in your particular Church? Can you approximate a percentage?
b) Do unions which are not recognized either religiously or civilly exist? Are reliable statistics available?
c) Are separated couples and those divorced and remarried a pastoral reality in your particular Church? Can you approximate a percentage? How do you deal with this situation in appropriate pastoral programmes? (sic)
d) In all the above cases, how do the baptized live in this irregular situation? Are they aware of it? Are they simply indifferent? Do they feel marginalized or suffer from the impossibility of receiving the sacraments?
f) Could a simplification of canonical practice in recognizing a declaration of nullity of the marriage bond provide a positive contribution to solving the problems of the persons involved? If yes, what form would it take?
Does a ministry exist to attend to these cases? Describe this pastoral ministry? Do such programmes exist on the national and diocesan levels? How is God’s mercy proclaimed to separated couples and those divorced and remarried, and how does the Church put into practice her support for them in their journey of faith?
On Unions of Persons of the Same Sex
a) Is there a law in your country recognizing civil unions for people of the same-sex and equating it in some way to marriage?
b) What is the attitude of the local and particular Churches towards both the State as the promoter of civil unions between persons of the same sex and the people involved in this type of union?
c) What pastoral attention can be given to people who have chosen to live in these types of union?
In the case of unions of persons of the same sex who have adopted children, what can be done pastorally in light of transmitting the faith?
The Education of Children in Irregular Marriages
a) What is the estimated proportion of children and adolescents in these cases, as regards children who are born and raised in regularly constituted families?
b) How do parents in these situations approach the Church? What do they ask? Do they request the sacraments only or do they also want catechesis and the general teaching of religion?
c) How do the particular Churches attempt to meet the needs of the parents of these children to provide them with a Christian education?
Source: Pastoral Challenges to the Family in the Context of Evangelization
A secular view of marriage
A secular view of marriage has been advanced by economists Betsey Stevenson, Ph.D., and Justin Wolfers, Ph.D., who describe the extent to which marriage is shaped by economic forces. "Productive marriage" is based on a division of labor. In the earlier part of the 20th century in Western countries, school, education, and the emerging TV and magazine markets illustrated how women could be good homemakers and men could be good providers. The liberation of women through education and access to birth control changed the playing field. Prior to this, college-educated women were the least likely to marry. Since the 1960s and 1970s, educated women could prevent pregnancy and support themselves, and found little use for the previous productive model of marriage.
Men, also, did not see educated, financially independent women as suitable marriage partners. The high divorce rate among those who married in the1970s reflected discontent with this model of the productive marriage.
In contrast, Dr. Stevenson and Dr. Wolfers write, "hedonic marriage" occurs when people who marry are of similar age, educational background, and perhaps occupation. The hedonic marriage better suits educated women who seek a companion, and it thrives when time and resources are available to enjoy companionable life. Same-sex marriages make sense when considered in this broad frame. Supporting this concept is the fact that couples who have married in recent years are more likely to stay together than were their parents’ generation. Of course, this discourse is only relevant in parts of the world in which women have access to birth control and opportunities for education, work, and social standing.
Romance and marriage
The question of romance in marriage is the hardest for psychiatrists, as scientists, to address. Romance has always been around, sometimes present in marriages and sometimes not. Romance is thought to be both essential and nonessential to marriage, depending on the purpose of the marriage. A good discussion by Dr. Henry Grunebaum can be found an article titled "Thinking about romantic/erotic love" in the Journal of Marital and Family Therapy(1997;23:295-307). His main points are that we do not have control over our feelings of romantic/erotic love, that these feelings occur relatively infrequently during most people’s lives, that being with a partner whom one loves, is valued and regarded as a good, that it sometimes conflicts with other values and goods, and lastly that although love is regarded as one essential basis for marriage, other qualities and capacities are important in sustaining a long-term relationship such as a marriage. He concludes with, "What makes matters even more challenging is the fact that we ask a great deal of marriage, of any serious intimate relationship. Perhaps the greatest demand we make is that it should combine passion and stability, romance and monogamy, transports of tenderness and excitement from the person who will also perform the many mundane tasks of daily living. In other words, meld everyday love with romantic/erotic love." He offers suggestions for discussion and guidelines for therapists.
Applying all of this in our work
As family psychiatrists, we can allow couples and families a therapeutic space to discuss the meaning and assumptions in their marriage. We can discuss the frame of the marriage: Is it sacred, secular, or postmodern? In this way, we can provide a context to the current struggles that couples and families might have.
To begin, we can ask about the past. We can say, "People get married for different reasons. What were your reasons? Do you consider your marriage to be a sacred or a secular? What does this mean to you?"
Delving deeper and focusing more on the present, "What is your current experience of your marriage? How do your expectations differ now than from your expectations in the past? What is the role of romance in your marriage?
What type of marriage did you want when you began this marriage? Is there romance in your marriage? What kind of marriage do you want now?
Focusing on going forward we can ask: "What works well in your marriage/family? What are your strengths? What needs to change in your marriage?"
In the late 1970s, postmodernism emerged in the world. Postmodernism stands in contrast to the "modern" or scientific view that touts a singularity of truth and a singular view of the world. Social construction is a type of postmodern theory that states that truth, reality, and knowledge are based in the social context of that particular person. Inevitably, postmodernism affects how we think about and conceptualize marriage. Postmodernism and marriage will be the subject of the next column.
I would like to thank Peter Chaloner, M.A., LL.B, B.A. (Honors), and Dip. Theo., for his comments and corrections.
Dr. Heru is with the department of psychiatry at the University of Colorado at Denver, Aurora. She is editor of the recently published book, "Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals" (New York: Routledge, 2013).
Throughout history, views of marriage have evolved as societies change. Since the 6th century, the Roman Catholic Church has played a prominent role in thinking and developing our ideas about marriage and family. In October, the church sent out a document that included a questionnaire to its bishops around the world to find out what Catholics think about the "modern family." The Vatican sent out the document in preparation for the Synod of Bishops on the Family, which is slated for October 2014. Before we get the results, let’s review how society has reflected on marriage and family.
Historically, marriages often were strategic alliances between families. It was common for marriage to be between first and second cousins in order to strengthen family ties. Polygamy has been common throughout history and continues in many communities to this day.
Monogamy is also found throughout history, but in 1215, the Catholic Church decreed that partners had to publicly post notices of an impending marriage in a local parish to cut down on the number of invalid marriages. Until the 1500s, the Catholic Church accepted a couple’s word that they had exchanged marriage vows, with no witnesses or corroborating evidence needed. In the 1500s, with the rise in Protestantism, marriage became a civil matter rather than a sacrament. By 1639, states such as Massachusetts began requiring marriage licenses, and by the 19th century, marriage licenses were common in the United States.
Marriage through the ages
Here is a listing of the way in which marriage has been conceptualized over the years:
Arranged alliances: A strategic alliance between families.
Family ties: Keeping alliances within the family; the majority of all marriages throughout history were between first and second cousins.
Polygamy: A phenomenon that has been common throughout history.
Babies optional: In many early cultures, men could dissolve a marriage or take another wife if a woman was infertile. However, the early Christian church was a trailblazer in arguing that marriage was not contingent upon producing offspring.
Monogamy: This practice became the guiding principle for Western marriages between the 6th and 9th centuries because of the church.
Sacred vs. secular: In 1215, the Roman Catholic Church decreed that partners had to publicly post notices, or banns, of an impending marriage in a local parish in order to cut down on the number of invalid marriages. Until the 1500s, the church accepted a couple’s word that they had exchanged marriage vows, with no witnesses or corroborating evidence needed.
Civil marriage: By 1639, states such as Massachusetts began requiring marriage licenses and, by the 19th century, marriage licenses were common in the United States.
Romance: By the 1900s, mutual attraction became important.
Market economics: Families historically controlled access to inheritance of agricultural land, but with the spread of a market economy, it becomes possible for people to marry outside of this inheritance.
Women’s equality: About 50 years ago, in Western countries, women and men began to have equal rights and responsibilities. Instead of being about unique, gender-based roles, most partners conceived of their unions in terms of flexible divisions of labor, companionship, and mutual sexual attraction.
Same-sex marriages: One of the reasons for the stunningly rapid increase in acceptance of same-sex marriage is because heterosexuals have completely changed their notion that all marriages are between a man and a woman, notes Stephanie Coontz, Ph.D. "We now believe marriage is based on love, mutual sexual attraction, equality, and a flexible division of labor."
Source: Adapted from "Marriage, a History: From Obedience to Intimacy, or How Love Conquered Marriage," (New York: Viking, 2005), by Dr. Coontz.
A sacred view of marriage
The Catholic position throughout history has been that marriage is one of the seven sacraments bestowed by Christ. This questionnaire is an attempt by the Vatican to understand more about "mixed or interreligious marriages; the single-parent family; polygamy; marriages with the consequent problem of a dowry, sometimes understood as the purchase price of the woman; the caste system; a culture of noncommitment and a presumption that the marriage bond can be temporary; forms of feminism hostile to the Church; migration and the reformulation of the very concept of the family; relativist pluralism in the conception of marriage; the influence of the media on popular culture in its understanding of marriage and family life; underlying trends of thought in legislative proposals which devalue the idea of permanence and faithfulness in the marriage covenant; an increase in the practice of surrogate motherhood (wombs for hire); and new interpretations of what is considered a human right."
Thirty-nine questions are on the questionnaire. Questions 4, 5, and 6 are of most interest to family psychiatrists. Deserving of admiration is its concern for families in migration and for the mistreatment of women.
The terms "regular" and "irregular," used in the questionnaire, are canonical terms unrelated to what actually happens in any given society. It should also be explained that Catholics who married always had to declare that they would welcome such children as God happened to send along, recognizing that he might choose not to send any. A decision to refuse to accept the possibility of children invalidated the marriage vows and constitutes grounds for annulment.
Excerpts from the Vatican document
Questions 4, 5, and 6 of the Vatican’s questionnaire seem aimed at gathering data on different kinds of families. Here are those three questions:
Pastoral Care in Certain Difficult Marital Situations
a) Is cohabitation ad experimentum a pastoral reality in your particular Church? Can you approximate a percentage?
b) Do unions which are not recognized either religiously or civilly exist? Are reliable statistics available?
c) Are separated couples and those divorced and remarried a pastoral reality in your particular Church? Can you approximate a percentage? How do you deal with this situation in appropriate pastoral programmes? (sic)
d) In all the above cases, how do the baptized live in this irregular situation? Are they aware of it? Are they simply indifferent? Do they feel marginalized or suffer from the impossibility of receiving the sacraments?
f) Could a simplification of canonical practice in recognizing a declaration of nullity of the marriage bond provide a positive contribution to solving the problems of the persons involved? If yes, what form would it take?
Does a ministry exist to attend to these cases? Describe this pastoral ministry? Do such programmes exist on the national and diocesan levels? How is God’s mercy proclaimed to separated couples and those divorced and remarried, and how does the Church put into practice her support for them in their journey of faith?
On Unions of Persons of the Same Sex
a) Is there a law in your country recognizing civil unions for people of the same-sex and equating it in some way to marriage?
b) What is the attitude of the local and particular Churches towards both the State as the promoter of civil unions between persons of the same sex and the people involved in this type of union?
c) What pastoral attention can be given to people who have chosen to live in these types of union?
In the case of unions of persons of the same sex who have adopted children, what can be done pastorally in light of transmitting the faith?
The Education of Children in Irregular Marriages
a) What is the estimated proportion of children and adolescents in these cases, as regards children who are born and raised in regularly constituted families?
b) How do parents in these situations approach the Church? What do they ask? Do they request the sacraments only or do they also want catechesis and the general teaching of religion?
c) How do the particular Churches attempt to meet the needs of the parents of these children to provide them with a Christian education?
Source: Pastoral Challenges to the Family in the Context of Evangelization
A secular view of marriage
A secular view of marriage has been advanced by economists Betsey Stevenson, Ph.D., and Justin Wolfers, Ph.D., who describe the extent to which marriage is shaped by economic forces. "Productive marriage" is based on a division of labor. In the earlier part of the 20th century in Western countries, school, education, and the emerging TV and magazine markets illustrated how women could be good homemakers and men could be good providers. The liberation of women through education and access to birth control changed the playing field. Prior to this, college-educated women were the least likely to marry. Since the 1960s and 1970s, educated women could prevent pregnancy and support themselves, and found little use for the previous productive model of marriage.
Men, also, did not see educated, financially independent women as suitable marriage partners. The high divorce rate among those who married in the1970s reflected discontent with this model of the productive marriage.
In contrast, Dr. Stevenson and Dr. Wolfers write, "hedonic marriage" occurs when people who marry are of similar age, educational background, and perhaps occupation. The hedonic marriage better suits educated women who seek a companion, and it thrives when time and resources are available to enjoy companionable life. Same-sex marriages make sense when considered in this broad frame. Supporting this concept is the fact that couples who have married in recent years are more likely to stay together than were their parents’ generation. Of course, this discourse is only relevant in parts of the world in which women have access to birth control and opportunities for education, work, and social standing.
Romance and marriage
The question of romance in marriage is the hardest for psychiatrists, as scientists, to address. Romance has always been around, sometimes present in marriages and sometimes not. Romance is thought to be both essential and nonessential to marriage, depending on the purpose of the marriage. A good discussion by Dr. Henry Grunebaum can be found an article titled "Thinking about romantic/erotic love" in the Journal of Marital and Family Therapy(1997;23:295-307). His main points are that we do not have control over our feelings of romantic/erotic love, that these feelings occur relatively infrequently during most people’s lives, that being with a partner whom one loves, is valued and regarded as a good, that it sometimes conflicts with other values and goods, and lastly that although love is regarded as one essential basis for marriage, other qualities and capacities are important in sustaining a long-term relationship such as a marriage. He concludes with, "What makes matters even more challenging is the fact that we ask a great deal of marriage, of any serious intimate relationship. Perhaps the greatest demand we make is that it should combine passion and stability, romance and monogamy, transports of tenderness and excitement from the person who will also perform the many mundane tasks of daily living. In other words, meld everyday love with romantic/erotic love." He offers suggestions for discussion and guidelines for therapists.
Applying all of this in our work
As family psychiatrists, we can allow couples and families a therapeutic space to discuss the meaning and assumptions in their marriage. We can discuss the frame of the marriage: Is it sacred, secular, or postmodern? In this way, we can provide a context to the current struggles that couples and families might have.
To begin, we can ask about the past. We can say, "People get married for different reasons. What were your reasons? Do you consider your marriage to be a sacred or a secular? What does this mean to you?"
Delving deeper and focusing more on the present, "What is your current experience of your marriage? How do your expectations differ now than from your expectations in the past? What is the role of romance in your marriage?
What type of marriage did you want when you began this marriage? Is there romance in your marriage? What kind of marriage do you want now?
Focusing on going forward we can ask: "What works well in your marriage/family? What are your strengths? What needs to change in your marriage?"
In the late 1970s, postmodernism emerged in the world. Postmodernism stands in contrast to the "modern" or scientific view that touts a singularity of truth and a singular view of the world. Social construction is a type of postmodern theory that states that truth, reality, and knowledge are based in the social context of that particular person. Inevitably, postmodernism affects how we think about and conceptualize marriage. Postmodernism and marriage will be the subject of the next column.
I would like to thank Peter Chaloner, M.A., LL.B, B.A. (Honors), and Dip. Theo., for his comments and corrections.
Dr. Heru is with the department of psychiatry at the University of Colorado at Denver, Aurora. She is editor of the recently published book, "Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals" (New York: Routledge, 2013).
Throughout history, views of marriage have evolved as societies change. Since the 6th century, the Roman Catholic Church has played a prominent role in thinking and developing our ideas about marriage and family. In October, the church sent out a document that included a questionnaire to its bishops around the world to find out what Catholics think about the "modern family." The Vatican sent out the document in preparation for the Synod of Bishops on the Family, which is slated for October 2014. Before we get the results, let’s review how society has reflected on marriage and family.
Historically, marriages often were strategic alliances between families. It was common for marriage to be between first and second cousins in order to strengthen family ties. Polygamy has been common throughout history and continues in many communities to this day.
Monogamy is also found throughout history, but in 1215, the Catholic Church decreed that partners had to publicly post notices of an impending marriage in a local parish to cut down on the number of invalid marriages. Until the 1500s, the Catholic Church accepted a couple’s word that they had exchanged marriage vows, with no witnesses or corroborating evidence needed. In the 1500s, with the rise in Protestantism, marriage became a civil matter rather than a sacrament. By 1639, states such as Massachusetts began requiring marriage licenses, and by the 19th century, marriage licenses were common in the United States.
Marriage through the ages
Here is a listing of the way in which marriage has been conceptualized over the years:
Arranged alliances: A strategic alliance between families.
Family ties: Keeping alliances within the family; the majority of all marriages throughout history were between first and second cousins.
Polygamy: A phenomenon that has been common throughout history.
Babies optional: In many early cultures, men could dissolve a marriage or take another wife if a woman was infertile. However, the early Christian church was a trailblazer in arguing that marriage was not contingent upon producing offspring.
Monogamy: This practice became the guiding principle for Western marriages between the 6th and 9th centuries because of the church.
Sacred vs. secular: In 1215, the Roman Catholic Church decreed that partners had to publicly post notices, or banns, of an impending marriage in a local parish in order to cut down on the number of invalid marriages. Until the 1500s, the church accepted a couple’s word that they had exchanged marriage vows, with no witnesses or corroborating evidence needed.
Civil marriage: By 1639, states such as Massachusetts began requiring marriage licenses and, by the 19th century, marriage licenses were common in the United States.
Romance: By the 1900s, mutual attraction became important.
Market economics: Families historically controlled access to inheritance of agricultural land, but with the spread of a market economy, it becomes possible for people to marry outside of this inheritance.
Women’s equality: About 50 years ago, in Western countries, women and men began to have equal rights and responsibilities. Instead of being about unique, gender-based roles, most partners conceived of their unions in terms of flexible divisions of labor, companionship, and mutual sexual attraction.
Same-sex marriages: One of the reasons for the stunningly rapid increase in acceptance of same-sex marriage is because heterosexuals have completely changed their notion that all marriages are between a man and a woman, notes Stephanie Coontz, Ph.D. "We now believe marriage is based on love, mutual sexual attraction, equality, and a flexible division of labor."
Source: Adapted from "Marriage, a History: From Obedience to Intimacy, or How Love Conquered Marriage," (New York: Viking, 2005), by Dr. Coontz.
A sacred view of marriage
The Catholic position throughout history has been that marriage is one of the seven sacraments bestowed by Christ. This questionnaire is an attempt by the Vatican to understand more about "mixed or interreligious marriages; the single-parent family; polygamy; marriages with the consequent problem of a dowry, sometimes understood as the purchase price of the woman; the caste system; a culture of noncommitment and a presumption that the marriage bond can be temporary; forms of feminism hostile to the Church; migration and the reformulation of the very concept of the family; relativist pluralism in the conception of marriage; the influence of the media on popular culture in its understanding of marriage and family life; underlying trends of thought in legislative proposals which devalue the idea of permanence and faithfulness in the marriage covenant; an increase in the practice of surrogate motherhood (wombs for hire); and new interpretations of what is considered a human right."
Thirty-nine questions are on the questionnaire. Questions 4, 5, and 6 are of most interest to family psychiatrists. Deserving of admiration is its concern for families in migration and for the mistreatment of women.
The terms "regular" and "irregular," used in the questionnaire, are canonical terms unrelated to what actually happens in any given society. It should also be explained that Catholics who married always had to declare that they would welcome such children as God happened to send along, recognizing that he might choose not to send any. A decision to refuse to accept the possibility of children invalidated the marriage vows and constitutes grounds for annulment.
Excerpts from the Vatican document
Questions 4, 5, and 6 of the Vatican’s questionnaire seem aimed at gathering data on different kinds of families. Here are those three questions:
Pastoral Care in Certain Difficult Marital Situations
a) Is cohabitation ad experimentum a pastoral reality in your particular Church? Can you approximate a percentage?
b) Do unions which are not recognized either religiously or civilly exist? Are reliable statistics available?
c) Are separated couples and those divorced and remarried a pastoral reality in your particular Church? Can you approximate a percentage? How do you deal with this situation in appropriate pastoral programmes? (sic)
d) In all the above cases, how do the baptized live in this irregular situation? Are they aware of it? Are they simply indifferent? Do they feel marginalized or suffer from the impossibility of receiving the sacraments?
f) Could a simplification of canonical practice in recognizing a declaration of nullity of the marriage bond provide a positive contribution to solving the problems of the persons involved? If yes, what form would it take?
Does a ministry exist to attend to these cases? Describe this pastoral ministry? Do such programmes exist on the national and diocesan levels? How is God’s mercy proclaimed to separated couples and those divorced and remarried, and how does the Church put into practice her support for them in their journey of faith?
On Unions of Persons of the Same Sex
a) Is there a law in your country recognizing civil unions for people of the same-sex and equating it in some way to marriage?
b) What is the attitude of the local and particular Churches towards both the State as the promoter of civil unions between persons of the same sex and the people involved in this type of union?
c) What pastoral attention can be given to people who have chosen to live in these types of union?
In the case of unions of persons of the same sex who have adopted children, what can be done pastorally in light of transmitting the faith?
The Education of Children in Irregular Marriages
a) What is the estimated proportion of children and adolescents in these cases, as regards children who are born and raised in regularly constituted families?
b) How do parents in these situations approach the Church? What do they ask? Do they request the sacraments only or do they also want catechesis and the general teaching of religion?
c) How do the particular Churches attempt to meet the needs of the parents of these children to provide them with a Christian education?
Source: Pastoral Challenges to the Family in the Context of Evangelization
A secular view of marriage
A secular view of marriage has been advanced by economists Betsey Stevenson, Ph.D., and Justin Wolfers, Ph.D., who describe the extent to which marriage is shaped by economic forces. "Productive marriage" is based on a division of labor. In the earlier part of the 20th century in Western countries, school, education, and the emerging TV and magazine markets illustrated how women could be good homemakers and men could be good providers. The liberation of women through education and access to birth control changed the playing field. Prior to this, college-educated women were the least likely to marry. Since the 1960s and 1970s, educated women could prevent pregnancy and support themselves, and found little use for the previous productive model of marriage.
Men, also, did not see educated, financially independent women as suitable marriage partners. The high divorce rate among those who married in the1970s reflected discontent with this model of the productive marriage.
In contrast, Dr. Stevenson and Dr. Wolfers write, "hedonic marriage" occurs when people who marry are of similar age, educational background, and perhaps occupation. The hedonic marriage better suits educated women who seek a companion, and it thrives when time and resources are available to enjoy companionable life. Same-sex marriages make sense when considered in this broad frame. Supporting this concept is the fact that couples who have married in recent years are more likely to stay together than were their parents’ generation. Of course, this discourse is only relevant in parts of the world in which women have access to birth control and opportunities for education, work, and social standing.
Romance and marriage
The question of romance in marriage is the hardest for psychiatrists, as scientists, to address. Romance has always been around, sometimes present in marriages and sometimes not. Romance is thought to be both essential and nonessential to marriage, depending on the purpose of the marriage. A good discussion by Dr. Henry Grunebaum can be found an article titled "Thinking about romantic/erotic love" in the Journal of Marital and Family Therapy(1997;23:295-307). His main points are that we do not have control over our feelings of romantic/erotic love, that these feelings occur relatively infrequently during most people’s lives, that being with a partner whom one loves, is valued and regarded as a good, that it sometimes conflicts with other values and goods, and lastly that although love is regarded as one essential basis for marriage, other qualities and capacities are important in sustaining a long-term relationship such as a marriage. He concludes with, "What makes matters even more challenging is the fact that we ask a great deal of marriage, of any serious intimate relationship. Perhaps the greatest demand we make is that it should combine passion and stability, romance and monogamy, transports of tenderness and excitement from the person who will also perform the many mundane tasks of daily living. In other words, meld everyday love with romantic/erotic love." He offers suggestions for discussion and guidelines for therapists.
Applying all of this in our work
As family psychiatrists, we can allow couples and families a therapeutic space to discuss the meaning and assumptions in their marriage. We can discuss the frame of the marriage: Is it sacred, secular, or postmodern? In this way, we can provide a context to the current struggles that couples and families might have.
To begin, we can ask about the past. We can say, "People get married for different reasons. What were your reasons? Do you consider your marriage to be a sacred or a secular? What does this mean to you?"
Delving deeper and focusing more on the present, "What is your current experience of your marriage? How do your expectations differ now than from your expectations in the past? What is the role of romance in your marriage?
What type of marriage did you want when you began this marriage? Is there romance in your marriage? What kind of marriage do you want now?
Focusing on going forward we can ask: "What works well in your marriage/family? What are your strengths? What needs to change in your marriage?"
In the late 1970s, postmodernism emerged in the world. Postmodernism stands in contrast to the "modern" or scientific view that touts a singularity of truth and a singular view of the world. Social construction is a type of postmodern theory that states that truth, reality, and knowledge are based in the social context of that particular person. Inevitably, postmodernism affects how we think about and conceptualize marriage. Postmodernism and marriage will be the subject of the next column.
I would like to thank Peter Chaloner, M.A., LL.B, B.A. (Honors), and Dip. Theo., for his comments and corrections.
Dr. Heru is with the department of psychiatry at the University of Colorado at Denver, Aurora. She is editor of the recently published book, "Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals" (New York: Routledge, 2013).
Creams and patches can replace narcotics for skin pain
LAS VEGAS – A topical mixture of amitriptyline and ketamine controls pain in a variety of skin conditions, even when more traditional options fail, according to Dr. Mark Davis, chair of the division of clinical dermatology at the Mayo Clinic in Rochester, Minn.
"We are getting patients off systemic narcotics with these mixtures," he said at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Lidocaine patches are allowing leg ulcer patients to drop narcotics, too, and are greatly helping those with erythromelalgia, an often-misdiagnosed condition involving intermittent and excruciating, burning pain in the feet, hands, and sometimes ears. "To have something topical [for such pain] is terrific," Dr. Davis said.
The Mayo Clinic uses topical amitriptyline and ketamine for brachioradial pruritus, erythromelalgia, rectogenital and perineal pain, and other localized skin pain recalcitrant to oral medications and other standard approaches. Dr. Davis and his colleagues recently published several reviews and retrospective studies on the use of topical pain relievers, including a recent article showing that more than half the patients who used topical amitriptyline-ketamine for pain reported substantial or complete relief (Pain Physician 2012;15:485-8).
Pharmaceutical companies are working to bring the combination to market, but it’s not yet available commercially, so Mayo Clinic dermatologists have their pharmacists compound it in two strengths – 2% amitriptyline and either 0.5% or 5% ketamine – using Lipoderm cream as the base, according to Dr. Davis. Patients apply the mixture three times daily. Why it works isn’t clear; the drugs have different and perhaps synergistic effects on skin pain.
"I try to get people to use it [the product] on small parts of the body, such as the hands and feet, and, in patients with brachioradial pruritus, both arms," said Dr. Davis. "I’ve never used it any more extensively than that; I am afraid patients would absorb too much ketamine," he said.
Of more than 1,000 Mayo Clinic patients who have tried the combination, "I’d say less than 1% has told me that they’ve ever had a side effect," he said. "I’ve had just two or three patients tell me they’ve gotten nightmares," a known effect of ketamine. "This is a product that has great promise," he noted.
Meanwhile, some erythromelalgia patients at the Mayo Clinic have been able to walk again after applying lidocaine patches to their feet, and the clinicians there consider the patches first-line treatment for the condition. "Many patients are well controlled just by using [the patches], and I don’t think I’ve come across any side effects in the hundreds of patients I’ve treated," Dr. Davis said. In the inherited form of the disease, which accounts for perhaps 5% of cases, erythromelalgia is caused by a genetic sodium channel glitch that keeps nerves in the skin firing once they are stimulated.
Lidocaine patches also are "really useful for patients with bad, painful ulcers; I use [them] over whatever wound care I am using," Dr. Davis noted. Sometimes these patients can discontinue, or at least reduce, their use of narcotics, he said.
Lidocaine helps with debridement, too. "For leg ulcers, our nurses take 4 x 4-inch gauze and soak it in lidocaine 4% solution, and leave it on the ulcer for about 20 minutes. We are usually able to debride those ulcers [after that] even if they were very painful to start," he said.
Dr. Davis has no relevant disclosures. SDEF and this news organization are owned by Frontline Medical Communications.
LAS VEGAS – A topical mixture of amitriptyline and ketamine controls pain in a variety of skin conditions, even when more traditional options fail, according to Dr. Mark Davis, chair of the division of clinical dermatology at the Mayo Clinic in Rochester, Minn.
"We are getting patients off systemic narcotics with these mixtures," he said at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Lidocaine patches are allowing leg ulcer patients to drop narcotics, too, and are greatly helping those with erythromelalgia, an often-misdiagnosed condition involving intermittent and excruciating, burning pain in the feet, hands, and sometimes ears. "To have something topical [for such pain] is terrific," Dr. Davis said.
The Mayo Clinic uses topical amitriptyline and ketamine for brachioradial pruritus, erythromelalgia, rectogenital and perineal pain, and other localized skin pain recalcitrant to oral medications and other standard approaches. Dr. Davis and his colleagues recently published several reviews and retrospective studies on the use of topical pain relievers, including a recent article showing that more than half the patients who used topical amitriptyline-ketamine for pain reported substantial or complete relief (Pain Physician 2012;15:485-8).
Pharmaceutical companies are working to bring the combination to market, but it’s not yet available commercially, so Mayo Clinic dermatologists have their pharmacists compound it in two strengths – 2% amitriptyline and either 0.5% or 5% ketamine – using Lipoderm cream as the base, according to Dr. Davis. Patients apply the mixture three times daily. Why it works isn’t clear; the drugs have different and perhaps synergistic effects on skin pain.
"I try to get people to use it [the product] on small parts of the body, such as the hands and feet, and, in patients with brachioradial pruritus, both arms," said Dr. Davis. "I’ve never used it any more extensively than that; I am afraid patients would absorb too much ketamine," he said.
Of more than 1,000 Mayo Clinic patients who have tried the combination, "I’d say less than 1% has told me that they’ve ever had a side effect," he said. "I’ve had just two or three patients tell me they’ve gotten nightmares," a known effect of ketamine. "This is a product that has great promise," he noted.
Meanwhile, some erythromelalgia patients at the Mayo Clinic have been able to walk again after applying lidocaine patches to their feet, and the clinicians there consider the patches first-line treatment for the condition. "Many patients are well controlled just by using [the patches], and I don’t think I’ve come across any side effects in the hundreds of patients I’ve treated," Dr. Davis said. In the inherited form of the disease, which accounts for perhaps 5% of cases, erythromelalgia is caused by a genetic sodium channel glitch that keeps nerves in the skin firing once they are stimulated.
Lidocaine patches also are "really useful for patients with bad, painful ulcers; I use [them] over whatever wound care I am using," Dr. Davis noted. Sometimes these patients can discontinue, or at least reduce, their use of narcotics, he said.
Lidocaine helps with debridement, too. "For leg ulcers, our nurses take 4 x 4-inch gauze and soak it in lidocaine 4% solution, and leave it on the ulcer for about 20 minutes. We are usually able to debride those ulcers [after that] even if they were very painful to start," he said.
Dr. Davis has no relevant disclosures. SDEF and this news organization are owned by Frontline Medical Communications.
LAS VEGAS – A topical mixture of amitriptyline and ketamine controls pain in a variety of skin conditions, even when more traditional options fail, according to Dr. Mark Davis, chair of the division of clinical dermatology at the Mayo Clinic in Rochester, Minn.
"We are getting patients off systemic narcotics with these mixtures," he said at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Lidocaine patches are allowing leg ulcer patients to drop narcotics, too, and are greatly helping those with erythromelalgia, an often-misdiagnosed condition involving intermittent and excruciating, burning pain in the feet, hands, and sometimes ears. "To have something topical [for such pain] is terrific," Dr. Davis said.
The Mayo Clinic uses topical amitriptyline and ketamine for brachioradial pruritus, erythromelalgia, rectogenital and perineal pain, and other localized skin pain recalcitrant to oral medications and other standard approaches. Dr. Davis and his colleagues recently published several reviews and retrospective studies on the use of topical pain relievers, including a recent article showing that more than half the patients who used topical amitriptyline-ketamine for pain reported substantial or complete relief (Pain Physician 2012;15:485-8).
Pharmaceutical companies are working to bring the combination to market, but it’s not yet available commercially, so Mayo Clinic dermatologists have their pharmacists compound it in two strengths – 2% amitriptyline and either 0.5% or 5% ketamine – using Lipoderm cream as the base, according to Dr. Davis. Patients apply the mixture three times daily. Why it works isn’t clear; the drugs have different and perhaps synergistic effects on skin pain.
"I try to get people to use it [the product] on small parts of the body, such as the hands and feet, and, in patients with brachioradial pruritus, both arms," said Dr. Davis. "I’ve never used it any more extensively than that; I am afraid patients would absorb too much ketamine," he said.
Of more than 1,000 Mayo Clinic patients who have tried the combination, "I’d say less than 1% has told me that they’ve ever had a side effect," he said. "I’ve had just two or three patients tell me they’ve gotten nightmares," a known effect of ketamine. "This is a product that has great promise," he noted.
Meanwhile, some erythromelalgia patients at the Mayo Clinic have been able to walk again after applying lidocaine patches to their feet, and the clinicians there consider the patches first-line treatment for the condition. "Many patients are well controlled just by using [the patches], and I don’t think I’ve come across any side effects in the hundreds of patients I’ve treated," Dr. Davis said. In the inherited form of the disease, which accounts for perhaps 5% of cases, erythromelalgia is caused by a genetic sodium channel glitch that keeps nerves in the skin firing once they are stimulated.
Lidocaine patches also are "really useful for patients with bad, painful ulcers; I use [them] over whatever wound care I am using," Dr. Davis noted. Sometimes these patients can discontinue, or at least reduce, their use of narcotics, he said.
Lidocaine helps with debridement, too. "For leg ulcers, our nurses take 4 x 4-inch gauze and soak it in lidocaine 4% solution, and leave it on the ulcer for about 20 minutes. We are usually able to debride those ulcers [after that] even if they were very painful to start," he said.
Dr. Davis has no relevant disclosures. SDEF and this news organization are owned by Frontline Medical Communications.
EXPERT ANALYSIS FROM THE SDEF LAS VEGAS DERMATOLOGY SEMINAR