User login
For love or money: How do doctors choose their specialty?
Medical student loans top hundreds of thousands of dollars, so it’s understandable that physicians may want to select a specialty that pays well.
Moreover, most advised young doctors to follow their hearts rather than their wallets.
“There is no question that many young kids immediately think about money when deciding to pursue medicine, but the thought of a big paycheck will never sustain someone long enough to get them here,” says Sergio Alvarez, MD, a board-certified plastic surgeon based in Miami, Fla., and the CEO and medical director of Mia Aesthetics, which has several national locations.
“Getting into medicine is a long game, and there are many hurdles along the way that only the dedicated overcome,” says Dr. Alvarez.
Unfortunately, he says it may be late in that long game before some realize that the pay rate for certain specialties isn’t commensurate with the immense workload and responsibility they require.
“The short of it is that to become a happy doctor, medicine really needs to be a calling: a passion! There are far easier things to do to make money.”
Here is what physicians said about choosing between love or money.
The lowest-paying subspecialty in a low-paying specialty
Sophia Yen, MD, MPH, cofounder and CEO of Pandia Health, a women-founded, doctor-led birth control delivery service in Sunnyvale, Calif., and clinical associate professor at Stanford (Calif.) University, says you should pursue a specialty because you love the work.
“I chose the lowest-paying subspecialty (adolescent medicine) of a low-paying specialty (pediatrics), but I’d do it all again because I love the patient population – I love what I do.”
Dr. Yen says she chose adolescent medicine because she loves doing “outpatient gynecology” without going through the surgical training of a full ob.gyn. “I love the target population of young adults because you can talk to the patient versus in pediatrics, where you often talk to the parent. With young adults you can catch things – for example, teach a young person about consent, alcohol, marijuana’s effects on the growing brain, prevent unplanned pregnancies and sexually transmitted infections, instill healthy eating, and more.
“Do I wish that I got paid as much as a surgeon?” Dr. Yen says yes. “I hope that someday society will realize the time spent preventing future disease is worth it and pay us accordingly.”
Unfortunately, she says, since the health care system makes more money if you get pregnant, need a cardiac bypass, or need gastric surgery, those who deliver babies or do surgery get paid more than someone who prevents the need for those services.
Money doesn’t buy happiness
Stella Bard, MD, a rheumatologist in McKinney, Tex., says she eats, lives, and breathes rheumatology. “I never regret the decision of choosing this specialty for a single second,” says Dr. Bard. “I feel like it’s a rewarding experience with every single patient encounter.” Dr. Bard notes that money is no guarantee of happiness and that she feels blessed to wake up every morning doing what she loves.
Career or calling?
For Dr. Alvarez, inspiration came when watching his father help change people’s lives. “I saw how impactful a doctor is during a person’s most desperate moments, and that was enough to make medicine my life’s passion at the age of 10.”
He says once you’re in medical school, choosing a specialty is far easier than you think. “Each specialty requires a certain personality or specific characteristics, and some will call to you while others simply won’t.”
“For me, plastics was about finesse, art, and life-changing surgeries that affected people from kids to adults and involved every aspect of the human body. Changing someone’s outward appearance has a profoundly positive impact on their confidence and self-esteem, making plastic surgery a genuinely transformative experience.”
Patricia Celan, MD, a postgraduate psychiatry resident in Canada, also chose psychiatry for the love of the field. “I enjoy helping vulnerable people and exploring what makes a person tick, the source of their difficulties, and how to help people counteract and overcome the difficult cards they’ve been dealt in life.”
She says it’s incredibly rewarding to watch someone turn their life around from severe mental illness, especially those who have been victimized and traumatized, and learn to trust people again.
“I could have made more money in a higher-paying specialty, yes, but I’m not sure I would have felt as fulfilled as psychiatry can make me feel.”
Dr. Celan says everyone has their calling, and some lucky people find their deepest passion in higher-paying specialties. “My calling is psychiatry, and I am at peace with this no matter the money.”
For the love of surgery
“In my experience, most people don’t choose their specialty based on money,” says Nicole Aaronson, MD, MBA, an otolaryngologist and board-certified in the subspecialty of pediatric otolaryngology, an attending surgeon at Nemours Children’s Health of Delaware and clinical associate professor of otolaryngology and pediatrics at Sidney Kimmel Medical College, Philadelphia.
“The first decision point in medical school is usually figuring out if you are a surgery person or a medicine person. I knew very early that I wanted to be a surgeon and wanted to spend time in the OR fixing problems with my hands.”
Part of what attracted Dr. Aaronson to otolaryngology was the variety of conditions managed within the specialty, from head and neck cancer to voice problems to sleep disorders to sinus disease. “I chose my subspecialty because I enjoy working with children and making an impact that will help them live their best possible lives.”
She says a relatively simple surgery like placing ear tubes may help a child’s hearing and allow them to be more successful in school, opening up a new world of opportunities for the child’s future.
“While I don’t think most people choose their specialty based on prospective compensation, I do think all physicians want to be compensated fairly for their time, effort, and level of training,” says Dr. Aaronson.
Choosing a specialty for the money can lead to burnout and dissatisfaction
“For me, the decision to pursue gastroenterology went beyond financial considerations,” says Saurabh Sethi, MD, MPH, a gastroenterologist specializing in hepatology and interventional endoscopy. “While financial stability is undoubtedly important, no doctor enters this field solely for the love of money. The primary driving force for most medical professionals, myself included, is the passion to help people and make a positive difference in their lives.”
Dr. Sethi says the gratification that comes from providing quality care and witnessing patients’ improved well-being is priceless. Moreover, he believes that selecting a specialty based solely on financial gain is likely to lead to burnout and greater dissatisfaction over time.
“By following my love for gut health and prioritizing patient care, I have found a sense of fulfillment and purpose in my career. It has been a rewarding journey, and I’m grateful for the opportunity to contribute to the well-being of my patients through my expertise in gastroenterology.”
Key takeaways: Love or money?
Multiple factors influence doctors’ specialty choices, including genuine love for the work and the future of the specialty. Others include job prospects, hands-on experience they receive, mentors, childhood dreams, parental expectations, complexity of cases, the lifestyle of each specialty, including office hours worked, on-call requirements, and autonomy.
Physicians also mentioned other factors they considered when choosing their specialty:
- Personal interest.
- Intellectual stimulation.
- Work-life balance.
- Patient populations.
- Future opportunities.
- Desire to make a difference.
- Passion.
- Financial stability.
- Being personally fulfilled.
Overwhelmingly, doctors say to pick a specialty you can envision yourself loving 40 years from now and you won’t go wrong.
A version of this article first appeared on Medscape.com.
Medical student loans top hundreds of thousands of dollars, so it’s understandable that physicians may want to select a specialty that pays well.
Moreover, most advised young doctors to follow their hearts rather than their wallets.
“There is no question that many young kids immediately think about money when deciding to pursue medicine, but the thought of a big paycheck will never sustain someone long enough to get them here,” says Sergio Alvarez, MD, a board-certified plastic surgeon based in Miami, Fla., and the CEO and medical director of Mia Aesthetics, which has several national locations.
“Getting into medicine is a long game, and there are many hurdles along the way that only the dedicated overcome,” says Dr. Alvarez.
Unfortunately, he says it may be late in that long game before some realize that the pay rate for certain specialties isn’t commensurate with the immense workload and responsibility they require.
“The short of it is that to become a happy doctor, medicine really needs to be a calling: a passion! There are far easier things to do to make money.”
Here is what physicians said about choosing between love or money.
The lowest-paying subspecialty in a low-paying specialty
Sophia Yen, MD, MPH, cofounder and CEO of Pandia Health, a women-founded, doctor-led birth control delivery service in Sunnyvale, Calif., and clinical associate professor at Stanford (Calif.) University, says you should pursue a specialty because you love the work.
“I chose the lowest-paying subspecialty (adolescent medicine) of a low-paying specialty (pediatrics), but I’d do it all again because I love the patient population – I love what I do.”
Dr. Yen says she chose adolescent medicine because she loves doing “outpatient gynecology” without going through the surgical training of a full ob.gyn. “I love the target population of young adults because you can talk to the patient versus in pediatrics, where you often talk to the parent. With young adults you can catch things – for example, teach a young person about consent, alcohol, marijuana’s effects on the growing brain, prevent unplanned pregnancies and sexually transmitted infections, instill healthy eating, and more.
“Do I wish that I got paid as much as a surgeon?” Dr. Yen says yes. “I hope that someday society will realize the time spent preventing future disease is worth it and pay us accordingly.”
Unfortunately, she says, since the health care system makes more money if you get pregnant, need a cardiac bypass, or need gastric surgery, those who deliver babies or do surgery get paid more than someone who prevents the need for those services.
Money doesn’t buy happiness
Stella Bard, MD, a rheumatologist in McKinney, Tex., says she eats, lives, and breathes rheumatology. “I never regret the decision of choosing this specialty for a single second,” says Dr. Bard. “I feel like it’s a rewarding experience with every single patient encounter.” Dr. Bard notes that money is no guarantee of happiness and that she feels blessed to wake up every morning doing what she loves.
Career or calling?
For Dr. Alvarez, inspiration came when watching his father help change people’s lives. “I saw how impactful a doctor is during a person’s most desperate moments, and that was enough to make medicine my life’s passion at the age of 10.”
He says once you’re in medical school, choosing a specialty is far easier than you think. “Each specialty requires a certain personality or specific characteristics, and some will call to you while others simply won’t.”
“For me, plastics was about finesse, art, and life-changing surgeries that affected people from kids to adults and involved every aspect of the human body. Changing someone’s outward appearance has a profoundly positive impact on their confidence and self-esteem, making plastic surgery a genuinely transformative experience.”
Patricia Celan, MD, a postgraduate psychiatry resident in Canada, also chose psychiatry for the love of the field. “I enjoy helping vulnerable people and exploring what makes a person tick, the source of their difficulties, and how to help people counteract and overcome the difficult cards they’ve been dealt in life.”
She says it’s incredibly rewarding to watch someone turn their life around from severe mental illness, especially those who have been victimized and traumatized, and learn to trust people again.
“I could have made more money in a higher-paying specialty, yes, but I’m not sure I would have felt as fulfilled as psychiatry can make me feel.”
Dr. Celan says everyone has their calling, and some lucky people find their deepest passion in higher-paying specialties. “My calling is psychiatry, and I am at peace with this no matter the money.”
For the love of surgery
“In my experience, most people don’t choose their specialty based on money,” says Nicole Aaronson, MD, MBA, an otolaryngologist and board-certified in the subspecialty of pediatric otolaryngology, an attending surgeon at Nemours Children’s Health of Delaware and clinical associate professor of otolaryngology and pediatrics at Sidney Kimmel Medical College, Philadelphia.
“The first decision point in medical school is usually figuring out if you are a surgery person or a medicine person. I knew very early that I wanted to be a surgeon and wanted to spend time in the OR fixing problems with my hands.”
Part of what attracted Dr. Aaronson to otolaryngology was the variety of conditions managed within the specialty, from head and neck cancer to voice problems to sleep disorders to sinus disease. “I chose my subspecialty because I enjoy working with children and making an impact that will help them live their best possible lives.”
She says a relatively simple surgery like placing ear tubes may help a child’s hearing and allow them to be more successful in school, opening up a new world of opportunities for the child’s future.
“While I don’t think most people choose their specialty based on prospective compensation, I do think all physicians want to be compensated fairly for their time, effort, and level of training,” says Dr. Aaronson.
Choosing a specialty for the money can lead to burnout and dissatisfaction
“For me, the decision to pursue gastroenterology went beyond financial considerations,” says Saurabh Sethi, MD, MPH, a gastroenterologist specializing in hepatology and interventional endoscopy. “While financial stability is undoubtedly important, no doctor enters this field solely for the love of money. The primary driving force for most medical professionals, myself included, is the passion to help people and make a positive difference in their lives.”
Dr. Sethi says the gratification that comes from providing quality care and witnessing patients’ improved well-being is priceless. Moreover, he believes that selecting a specialty based solely on financial gain is likely to lead to burnout and greater dissatisfaction over time.
“By following my love for gut health and prioritizing patient care, I have found a sense of fulfillment and purpose in my career. It has been a rewarding journey, and I’m grateful for the opportunity to contribute to the well-being of my patients through my expertise in gastroenterology.”
Key takeaways: Love or money?
Multiple factors influence doctors’ specialty choices, including genuine love for the work and the future of the specialty. Others include job prospects, hands-on experience they receive, mentors, childhood dreams, parental expectations, complexity of cases, the lifestyle of each specialty, including office hours worked, on-call requirements, and autonomy.
Physicians also mentioned other factors they considered when choosing their specialty:
- Personal interest.
- Intellectual stimulation.
- Work-life balance.
- Patient populations.
- Future opportunities.
- Desire to make a difference.
- Passion.
- Financial stability.
- Being personally fulfilled.
Overwhelmingly, doctors say to pick a specialty you can envision yourself loving 40 years from now and you won’t go wrong.
A version of this article first appeared on Medscape.com.
Medical student loans top hundreds of thousands of dollars, so it’s understandable that physicians may want to select a specialty that pays well.
Moreover, most advised young doctors to follow their hearts rather than their wallets.
“There is no question that many young kids immediately think about money when deciding to pursue medicine, but the thought of a big paycheck will never sustain someone long enough to get them here,” says Sergio Alvarez, MD, a board-certified plastic surgeon based in Miami, Fla., and the CEO and medical director of Mia Aesthetics, which has several national locations.
“Getting into medicine is a long game, and there are many hurdles along the way that only the dedicated overcome,” says Dr. Alvarez.
Unfortunately, he says it may be late in that long game before some realize that the pay rate for certain specialties isn’t commensurate with the immense workload and responsibility they require.
“The short of it is that to become a happy doctor, medicine really needs to be a calling: a passion! There are far easier things to do to make money.”
Here is what physicians said about choosing between love or money.
The lowest-paying subspecialty in a low-paying specialty
Sophia Yen, MD, MPH, cofounder and CEO of Pandia Health, a women-founded, doctor-led birth control delivery service in Sunnyvale, Calif., and clinical associate professor at Stanford (Calif.) University, says you should pursue a specialty because you love the work.
“I chose the lowest-paying subspecialty (adolescent medicine) of a low-paying specialty (pediatrics), but I’d do it all again because I love the patient population – I love what I do.”
Dr. Yen says she chose adolescent medicine because she loves doing “outpatient gynecology” without going through the surgical training of a full ob.gyn. “I love the target population of young adults because you can talk to the patient versus in pediatrics, where you often talk to the parent. With young adults you can catch things – for example, teach a young person about consent, alcohol, marijuana’s effects on the growing brain, prevent unplanned pregnancies and sexually transmitted infections, instill healthy eating, and more.
“Do I wish that I got paid as much as a surgeon?” Dr. Yen says yes. “I hope that someday society will realize the time spent preventing future disease is worth it and pay us accordingly.”
Unfortunately, she says, since the health care system makes more money if you get pregnant, need a cardiac bypass, or need gastric surgery, those who deliver babies or do surgery get paid more than someone who prevents the need for those services.
Money doesn’t buy happiness
Stella Bard, MD, a rheumatologist in McKinney, Tex., says she eats, lives, and breathes rheumatology. “I never regret the decision of choosing this specialty for a single second,” says Dr. Bard. “I feel like it’s a rewarding experience with every single patient encounter.” Dr. Bard notes that money is no guarantee of happiness and that she feels blessed to wake up every morning doing what she loves.
Career or calling?
For Dr. Alvarez, inspiration came when watching his father help change people’s lives. “I saw how impactful a doctor is during a person’s most desperate moments, and that was enough to make medicine my life’s passion at the age of 10.”
He says once you’re in medical school, choosing a specialty is far easier than you think. “Each specialty requires a certain personality or specific characteristics, and some will call to you while others simply won’t.”
“For me, plastics was about finesse, art, and life-changing surgeries that affected people from kids to adults and involved every aspect of the human body. Changing someone’s outward appearance has a profoundly positive impact on their confidence and self-esteem, making plastic surgery a genuinely transformative experience.”
Patricia Celan, MD, a postgraduate psychiatry resident in Canada, also chose psychiatry for the love of the field. “I enjoy helping vulnerable people and exploring what makes a person tick, the source of their difficulties, and how to help people counteract and overcome the difficult cards they’ve been dealt in life.”
She says it’s incredibly rewarding to watch someone turn their life around from severe mental illness, especially those who have been victimized and traumatized, and learn to trust people again.
“I could have made more money in a higher-paying specialty, yes, but I’m not sure I would have felt as fulfilled as psychiatry can make me feel.”
Dr. Celan says everyone has their calling, and some lucky people find their deepest passion in higher-paying specialties. “My calling is psychiatry, and I am at peace with this no matter the money.”
For the love of surgery
“In my experience, most people don’t choose their specialty based on money,” says Nicole Aaronson, MD, MBA, an otolaryngologist and board-certified in the subspecialty of pediatric otolaryngology, an attending surgeon at Nemours Children’s Health of Delaware and clinical associate professor of otolaryngology and pediatrics at Sidney Kimmel Medical College, Philadelphia.
“The first decision point in medical school is usually figuring out if you are a surgery person or a medicine person. I knew very early that I wanted to be a surgeon and wanted to spend time in the OR fixing problems with my hands.”
Part of what attracted Dr. Aaronson to otolaryngology was the variety of conditions managed within the specialty, from head and neck cancer to voice problems to sleep disorders to sinus disease. “I chose my subspecialty because I enjoy working with children and making an impact that will help them live their best possible lives.”
She says a relatively simple surgery like placing ear tubes may help a child’s hearing and allow them to be more successful in school, opening up a new world of opportunities for the child’s future.
“While I don’t think most people choose their specialty based on prospective compensation, I do think all physicians want to be compensated fairly for their time, effort, and level of training,” says Dr. Aaronson.
Choosing a specialty for the money can lead to burnout and dissatisfaction
“For me, the decision to pursue gastroenterology went beyond financial considerations,” says Saurabh Sethi, MD, MPH, a gastroenterologist specializing in hepatology and interventional endoscopy. “While financial stability is undoubtedly important, no doctor enters this field solely for the love of money. The primary driving force for most medical professionals, myself included, is the passion to help people and make a positive difference in their lives.”
Dr. Sethi says the gratification that comes from providing quality care and witnessing patients’ improved well-being is priceless. Moreover, he believes that selecting a specialty based solely on financial gain is likely to lead to burnout and greater dissatisfaction over time.
“By following my love for gut health and prioritizing patient care, I have found a sense of fulfillment and purpose in my career. It has been a rewarding journey, and I’m grateful for the opportunity to contribute to the well-being of my patients through my expertise in gastroenterology.”
Key takeaways: Love or money?
Multiple factors influence doctors’ specialty choices, including genuine love for the work and the future of the specialty. Others include job prospects, hands-on experience they receive, mentors, childhood dreams, parental expectations, complexity of cases, the lifestyle of each specialty, including office hours worked, on-call requirements, and autonomy.
Physicians also mentioned other factors they considered when choosing their specialty:
- Personal interest.
- Intellectual stimulation.
- Work-life balance.
- Patient populations.
- Future opportunities.
- Desire to make a difference.
- Passion.
- Financial stability.
- Being personally fulfilled.
Overwhelmingly, doctors say to pick a specialty you can envision yourself loving 40 years from now and you won’t go wrong.
A version of this article first appeared on Medscape.com.
Interrupting radiotherapy for TNBC linked to worse survival
Topline
Methodology
- Clinicians sometimes give women with TNBC a break between radiation sessions so that their skin can heal.
- To gauge the impact, investigators reviewed data from the National Cancer Database on 35,845 patients with TNBC who were treated between 2010 and 2014.
- The researchers determined the number of interrupted radiation treatment days as the difference between the number of days women received radiotherapy versus the number of expected treatment days.
- The team then correlated treatment interruptions with overall survival.
Takeaway
- Longer duration of treatment was associated with worse overall survival (hazard ratio, 1.023).
- Compared with no days or just 1 day off, 2-5 interrupted days (HR, 1.069), 6-10 interrupted days (HR, 1.239), and 11-15 interrupted days (HR, 1.265) increased the likelihood of death in a stepwise fashion.
- More days between diagnosis and first cancer treatment of any kind (HR, 1.001) were associated with worse overall survival.
- Older age (HR, 1.014), Black race (HR, 1.278), race than other Black or White (HR, 1.337), grade II or III/IV tumors (HR, 1.471 and 1.743, respectively), and clinical N1-N3 stage (HR, 2.534, 3.729, 4.992, respectively) were also associated with worse overall survival.
In practice
“All reasonable efforts should be made to prevent any treatment interruptions,” including “prophylactic measures to reduce the severity of radiation dermatitis,” and consideration should be given to the use of hypofractionated regimens to shorten radiation schedules.
Source
The study was led by Ronald Chow, MS, of the Memorial Sloan Kettering Cancer Center, New York, and was published in the Journal of the National Cancer Institute.
Limitations
- The findings may not be applicable to less aggressive forms of breast cancer.
- Treatment interruptions may have been caused by poor performance status and other confounders that shorten survival.
Disclosures
The study was funded by the National Cancer Institute. The investigators had no disclosures.
A version of this article appeared on Medscape.com.
Topline
Methodology
- Clinicians sometimes give women with TNBC a break between radiation sessions so that their skin can heal.
- To gauge the impact, investigators reviewed data from the National Cancer Database on 35,845 patients with TNBC who were treated between 2010 and 2014.
- The researchers determined the number of interrupted radiation treatment days as the difference between the number of days women received radiotherapy versus the number of expected treatment days.
- The team then correlated treatment interruptions with overall survival.
Takeaway
- Longer duration of treatment was associated with worse overall survival (hazard ratio, 1.023).
- Compared with no days or just 1 day off, 2-5 interrupted days (HR, 1.069), 6-10 interrupted days (HR, 1.239), and 11-15 interrupted days (HR, 1.265) increased the likelihood of death in a stepwise fashion.
- More days between diagnosis and first cancer treatment of any kind (HR, 1.001) were associated with worse overall survival.
- Older age (HR, 1.014), Black race (HR, 1.278), race than other Black or White (HR, 1.337), grade II or III/IV tumors (HR, 1.471 and 1.743, respectively), and clinical N1-N3 stage (HR, 2.534, 3.729, 4.992, respectively) were also associated with worse overall survival.
In practice
“All reasonable efforts should be made to prevent any treatment interruptions,” including “prophylactic measures to reduce the severity of radiation dermatitis,” and consideration should be given to the use of hypofractionated regimens to shorten radiation schedules.
Source
The study was led by Ronald Chow, MS, of the Memorial Sloan Kettering Cancer Center, New York, and was published in the Journal of the National Cancer Institute.
Limitations
- The findings may not be applicable to less aggressive forms of breast cancer.
- Treatment interruptions may have been caused by poor performance status and other confounders that shorten survival.
Disclosures
The study was funded by the National Cancer Institute. The investigators had no disclosures.
A version of this article appeared on Medscape.com.
Topline
Methodology
- Clinicians sometimes give women with TNBC a break between radiation sessions so that their skin can heal.
- To gauge the impact, investigators reviewed data from the National Cancer Database on 35,845 patients with TNBC who were treated between 2010 and 2014.
- The researchers determined the number of interrupted radiation treatment days as the difference between the number of days women received radiotherapy versus the number of expected treatment days.
- The team then correlated treatment interruptions with overall survival.
Takeaway
- Longer duration of treatment was associated with worse overall survival (hazard ratio, 1.023).
- Compared with no days or just 1 day off, 2-5 interrupted days (HR, 1.069), 6-10 interrupted days (HR, 1.239), and 11-15 interrupted days (HR, 1.265) increased the likelihood of death in a stepwise fashion.
- More days between diagnosis and first cancer treatment of any kind (HR, 1.001) were associated with worse overall survival.
- Older age (HR, 1.014), Black race (HR, 1.278), race than other Black or White (HR, 1.337), grade II or III/IV tumors (HR, 1.471 and 1.743, respectively), and clinical N1-N3 stage (HR, 2.534, 3.729, 4.992, respectively) were also associated with worse overall survival.
In practice
“All reasonable efforts should be made to prevent any treatment interruptions,” including “prophylactic measures to reduce the severity of radiation dermatitis,” and consideration should be given to the use of hypofractionated regimens to shorten radiation schedules.
Source
The study was led by Ronald Chow, MS, of the Memorial Sloan Kettering Cancer Center, New York, and was published in the Journal of the National Cancer Institute.
Limitations
- The findings may not be applicable to less aggressive forms of breast cancer.
- Treatment interruptions may have been caused by poor performance status and other confounders that shorten survival.
Disclosures
The study was funded by the National Cancer Institute. The investigators had no disclosures.
A version of this article appeared on Medscape.com.
Fatigue after breast cancer radiotherapy: Who’s most at risk?
Topline
Many patients with breast cancer who receive radiotherapy can still experience fatigue years after treatment; risk factors, including pain, insomnia, depression, baseline fatigue, and endocrine therapy were associated with long-term fatigue, new data show.
Methodology
- Overall, 1,443 patients with breast cancer from the REQUITE study responded to the Multidimensional Fatigue Inventory 20 (MFI-20) tool to assess five dimensions of fatigue: general, physical, and mental fatigue as well as reduced activity and motivation.
- Patients from France, Spain, Germany, Italy, the United Kingdom, and United States were assessed for characteristics, including age, body mass index (BMI), smoking, depression, pain, insomnia, fatigue, and therapy type, at baseline and at 24 months.
- Investigators identified factors associated with fatigue at 2 years post-radiotherapy among a total of 664 patients without chemotherapy and 324 with chemotherapy.
- General fatigue trajectories were classified as low, moderate, high, or decreasing.
Takeaways
- In general, levels of fatigue increased significantly from baseline to the end of radiotherapy for all fatigue dimensions (P < .05) and returned close to baseline levels after 1-2 years.
- About 24% of patients had high general fatigue trajectories and 25% had moderate, while 46% had low and 5% had decreasing fatigue trajectories.
- Factors such as age, BMI, global health status, insomnia, pain, dyspnea, depression, and baseline fatigue were each associated with multiple fatigue dimensions at 2 years; for instance, fatigue at baseline was associated with all five MFI-20 dimensions at 2 years regardless of chemotherapy status.
- Those with a combination of factors such as pain, insomnia, depression, younger age, and endocrine therapy were especially likely to develop high fatigue early and have it persist years after treatment.
In practice
the authors concluded.
Source
The study was led by Juan C. Rosas, with the German Cancer Research Center, Heidelberg. It was published online July 5 in the International Journal of Cancer.
Limitations
About one-quarter of patients did not complete the 2-year follow-up. Some variables identified in the literature as possible fatigue predictors such as socioeconomic status, physical activity, and social support were not included.
Disclosures
The study had no commercial funding. The authors reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Topline
Many patients with breast cancer who receive radiotherapy can still experience fatigue years after treatment; risk factors, including pain, insomnia, depression, baseline fatigue, and endocrine therapy were associated with long-term fatigue, new data show.
Methodology
- Overall, 1,443 patients with breast cancer from the REQUITE study responded to the Multidimensional Fatigue Inventory 20 (MFI-20) tool to assess five dimensions of fatigue: general, physical, and mental fatigue as well as reduced activity and motivation.
- Patients from France, Spain, Germany, Italy, the United Kingdom, and United States were assessed for characteristics, including age, body mass index (BMI), smoking, depression, pain, insomnia, fatigue, and therapy type, at baseline and at 24 months.
- Investigators identified factors associated with fatigue at 2 years post-radiotherapy among a total of 664 patients without chemotherapy and 324 with chemotherapy.
- General fatigue trajectories were classified as low, moderate, high, or decreasing.
Takeaways
- In general, levels of fatigue increased significantly from baseline to the end of radiotherapy for all fatigue dimensions (P < .05) and returned close to baseline levels after 1-2 years.
- About 24% of patients had high general fatigue trajectories and 25% had moderate, while 46% had low and 5% had decreasing fatigue trajectories.
- Factors such as age, BMI, global health status, insomnia, pain, dyspnea, depression, and baseline fatigue were each associated with multiple fatigue dimensions at 2 years; for instance, fatigue at baseline was associated with all five MFI-20 dimensions at 2 years regardless of chemotherapy status.
- Those with a combination of factors such as pain, insomnia, depression, younger age, and endocrine therapy were especially likely to develop high fatigue early and have it persist years after treatment.
In practice
the authors concluded.
Source
The study was led by Juan C. Rosas, with the German Cancer Research Center, Heidelberg. It was published online July 5 in the International Journal of Cancer.
Limitations
About one-quarter of patients did not complete the 2-year follow-up. Some variables identified in the literature as possible fatigue predictors such as socioeconomic status, physical activity, and social support were not included.
Disclosures
The study had no commercial funding. The authors reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Topline
Many patients with breast cancer who receive radiotherapy can still experience fatigue years after treatment; risk factors, including pain, insomnia, depression, baseline fatigue, and endocrine therapy were associated with long-term fatigue, new data show.
Methodology
- Overall, 1,443 patients with breast cancer from the REQUITE study responded to the Multidimensional Fatigue Inventory 20 (MFI-20) tool to assess five dimensions of fatigue: general, physical, and mental fatigue as well as reduced activity and motivation.
- Patients from France, Spain, Germany, Italy, the United Kingdom, and United States were assessed for characteristics, including age, body mass index (BMI), smoking, depression, pain, insomnia, fatigue, and therapy type, at baseline and at 24 months.
- Investigators identified factors associated with fatigue at 2 years post-radiotherapy among a total of 664 patients without chemotherapy and 324 with chemotherapy.
- General fatigue trajectories were classified as low, moderate, high, or decreasing.
Takeaways
- In general, levels of fatigue increased significantly from baseline to the end of radiotherapy for all fatigue dimensions (P < .05) and returned close to baseline levels after 1-2 years.
- About 24% of patients had high general fatigue trajectories and 25% had moderate, while 46% had low and 5% had decreasing fatigue trajectories.
- Factors such as age, BMI, global health status, insomnia, pain, dyspnea, depression, and baseline fatigue were each associated with multiple fatigue dimensions at 2 years; for instance, fatigue at baseline was associated with all five MFI-20 dimensions at 2 years regardless of chemotherapy status.
- Those with a combination of factors such as pain, insomnia, depression, younger age, and endocrine therapy were especially likely to develop high fatigue early and have it persist years after treatment.
In practice
the authors concluded.
Source
The study was led by Juan C. Rosas, with the German Cancer Research Center, Heidelberg. It was published online July 5 in the International Journal of Cancer.
Limitations
About one-quarter of patients did not complete the 2-year follow-up. Some variables identified in the literature as possible fatigue predictors such as socioeconomic status, physical activity, and social support were not included.
Disclosures
The study had no commercial funding. The authors reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
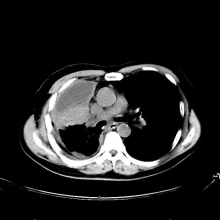
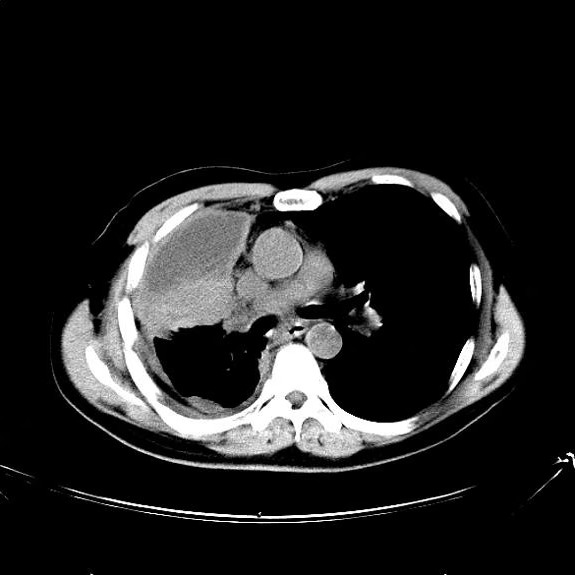
Cough and hemoptysis
The history and findings in this case are suggestive of combined small cell lung cancer (SCLC).
Globally, lung cancer is the leading cause of cancer incidence and mortality, accounting for an estimated 2 million new diagnoses and 1.76 million deaths per year. It consists of two major subtypes: non-small cell lung cancer (NSCLC) and SCLC. SCLC is unique in its presentation, imaging appearances, treatment, and prognosis. SCLC accounts for approximately 15% of all lung cancers and is associated with an exceptionally high proliferative rate, strong predilection for early metastasis, and poor prognosis.
There are two subtypes of SCLC: oat cell carcinoma and combined SCLC. Combined SCLC is defined as SCLC with non-small cell components, such as squamous cell or adenocarcinoma. Men are affected more frequently than are women. Most presenting patients are older than 70 years and are either a current or former smoker. Patients frequently have multiple cardiovascular or pulmonary comorbidities.
In most cases, patients experience rapid onset of symptoms, normally beginning 8-12 weeks before presentation. Signs and symptoms vary depending on the location and bulk of the primary tumor, but may include cough, wheezing, and hemoptysis as well as weight loss, debility, and other signs of metastatic disease. Local intrathoracic tumor growth can affect the superior vena cava (leading to superior vena cava syndrome), chest wall, or esophagus. Neurologic problems, recurrent nerve pain, fatigue, and anorexia may result from extrapulmonary metastasis. Nearly 60% of patients present with metastatic disease, most commonly in the brain, liver, adrenal glands, bone, and bone marrow. If left untreated, SCLC tumors progress rapidly, with a median survival of 2-4 months.
All patients with SCLC require a thorough staging workup to evaluate the extent of disease because stage plays a central role in treatment selection. The initial imaging workup includes plain film radiography and contrast-enhanced CT of the chest and upper abdomen, brain MRI, and PET-CT. Laboratory studies to evaluate for the presence of neoplastic syndromes include complete blood count, electrolytes, calcium, alkaline phosphatase, alanine aminotransferase and aspartate aminotransferase, total bilirubin, and creatinine. Biopsy is usually obtained via CT-guided biopsy or transbronchial biopsy, though this can vary depending on the location of the tumor.
According to 2023 guidelines from the National Comprehensive Cancer Network (NCCN), most patients with limited-stage SCLC are not eligible for surgery or stereotactic ablative radiotherapy (SABR). Surgery is only recommended for select patients with stage I–IIA SCLC (about 5% of patients). Concurrent chemoradiation or SABR is recommended for patients with limited stage I-IIA (T1-2,N0) SCLC who are ineligible for or do not want to pursue surgical resection. The majority of patients with SCLC have extensive-stage disease, and treatment with systemic therapy alone (with or without palliative radiotherapy) is recommended. Preferred cytotoxic and immunotherapeutic agents can be found in the NCCN guidelines.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are suggestive of combined small cell lung cancer (SCLC).
Globally, lung cancer is the leading cause of cancer incidence and mortality, accounting for an estimated 2 million new diagnoses and 1.76 million deaths per year. It consists of two major subtypes: non-small cell lung cancer (NSCLC) and SCLC. SCLC is unique in its presentation, imaging appearances, treatment, and prognosis. SCLC accounts for approximately 15% of all lung cancers and is associated with an exceptionally high proliferative rate, strong predilection for early metastasis, and poor prognosis.
There are two subtypes of SCLC: oat cell carcinoma and combined SCLC. Combined SCLC is defined as SCLC with non-small cell components, such as squamous cell or adenocarcinoma. Men are affected more frequently than are women. Most presenting patients are older than 70 years and are either a current or former smoker. Patients frequently have multiple cardiovascular or pulmonary comorbidities.
In most cases, patients experience rapid onset of symptoms, normally beginning 8-12 weeks before presentation. Signs and symptoms vary depending on the location and bulk of the primary tumor, but may include cough, wheezing, and hemoptysis as well as weight loss, debility, and other signs of metastatic disease. Local intrathoracic tumor growth can affect the superior vena cava (leading to superior vena cava syndrome), chest wall, or esophagus. Neurologic problems, recurrent nerve pain, fatigue, and anorexia may result from extrapulmonary metastasis. Nearly 60% of patients present with metastatic disease, most commonly in the brain, liver, adrenal glands, bone, and bone marrow. If left untreated, SCLC tumors progress rapidly, with a median survival of 2-4 months.
All patients with SCLC require a thorough staging workup to evaluate the extent of disease because stage plays a central role in treatment selection. The initial imaging workup includes plain film radiography and contrast-enhanced CT of the chest and upper abdomen, brain MRI, and PET-CT. Laboratory studies to evaluate for the presence of neoplastic syndromes include complete blood count, electrolytes, calcium, alkaline phosphatase, alanine aminotransferase and aspartate aminotransferase, total bilirubin, and creatinine. Biopsy is usually obtained via CT-guided biopsy or transbronchial biopsy, though this can vary depending on the location of the tumor.
According to 2023 guidelines from the National Comprehensive Cancer Network (NCCN), most patients with limited-stage SCLC are not eligible for surgery or stereotactic ablative radiotherapy (SABR). Surgery is only recommended for select patients with stage I–IIA SCLC (about 5% of patients). Concurrent chemoradiation or SABR is recommended for patients with limited stage I-IIA (T1-2,N0) SCLC who are ineligible for or do not want to pursue surgical resection. The majority of patients with SCLC have extensive-stage disease, and treatment with systemic therapy alone (with or without palliative radiotherapy) is recommended. Preferred cytotoxic and immunotherapeutic agents can be found in the NCCN guidelines.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are suggestive of combined small cell lung cancer (SCLC).
Globally, lung cancer is the leading cause of cancer incidence and mortality, accounting for an estimated 2 million new diagnoses and 1.76 million deaths per year. It consists of two major subtypes: non-small cell lung cancer (NSCLC) and SCLC. SCLC is unique in its presentation, imaging appearances, treatment, and prognosis. SCLC accounts for approximately 15% of all lung cancers and is associated with an exceptionally high proliferative rate, strong predilection for early metastasis, and poor prognosis.
There are two subtypes of SCLC: oat cell carcinoma and combined SCLC. Combined SCLC is defined as SCLC with non-small cell components, such as squamous cell or adenocarcinoma. Men are affected more frequently than are women. Most presenting patients are older than 70 years and are either a current or former smoker. Patients frequently have multiple cardiovascular or pulmonary comorbidities.
In most cases, patients experience rapid onset of symptoms, normally beginning 8-12 weeks before presentation. Signs and symptoms vary depending on the location and bulk of the primary tumor, but may include cough, wheezing, and hemoptysis as well as weight loss, debility, and other signs of metastatic disease. Local intrathoracic tumor growth can affect the superior vena cava (leading to superior vena cava syndrome), chest wall, or esophagus. Neurologic problems, recurrent nerve pain, fatigue, and anorexia may result from extrapulmonary metastasis. Nearly 60% of patients present with metastatic disease, most commonly in the brain, liver, adrenal glands, bone, and bone marrow. If left untreated, SCLC tumors progress rapidly, with a median survival of 2-4 months.
All patients with SCLC require a thorough staging workup to evaluate the extent of disease because stage plays a central role in treatment selection. The initial imaging workup includes plain film radiography and contrast-enhanced CT of the chest and upper abdomen, brain MRI, and PET-CT. Laboratory studies to evaluate for the presence of neoplastic syndromes include complete blood count, electrolytes, calcium, alkaline phosphatase, alanine aminotransferase and aspartate aminotransferase, total bilirubin, and creatinine. Biopsy is usually obtained via CT-guided biopsy or transbronchial biopsy, though this can vary depending on the location of the tumor.
According to 2023 guidelines from the National Comprehensive Cancer Network (NCCN), most patients with limited-stage SCLC are not eligible for surgery or stereotactic ablative radiotherapy (SABR). Surgery is only recommended for select patients with stage I–IIA SCLC (about 5% of patients). Concurrent chemoradiation or SABR is recommended for patients with limited stage I-IIA (T1-2,N0) SCLC who are ineligible for or do not want to pursue surgical resection. The majority of patients with SCLC have extensive-stage disease, and treatment with systemic therapy alone (with or without palliative radiotherapy) is recommended. Preferred cytotoxic and immunotherapeutic agents can be found in the NCCN guidelines.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 74-year-old man presents to the emergency department with reports of cough, hemoptysis, and unintentional weight loss of approximately 8 weeks' duration. The patient has a 35-year history of smoking (35 pack years). The patient's vital signs include temperature of 98.4 °F, BP of 135/80 mm Hg, and pulse oximeter reading of 94%. Physical examination reveals rales over the left side of the chest and decreased breath sounds in bilateral bases of the lungs. The patient appears cachexic. He is 6 ft 2 in and weighs 163 lb.
A chest radiograph reveals a mass in the right lung field. A subsequent CT of the chest reveals multiple pulmonary nodules and extensive mediastinal nodal metastases. Histopathology reveals small, uniform, poorly differentiated necrotic cancers and adenocarcinoma with papillary and acinar features.
Anxiety screening
Anxiety symptoms in children are common, ranging from a toddler’s fear of the dark to an adolescent worrying about a major exam. The good news is that, if they are detected early and treated appropriately, they are curable. Unfortunately, they are often silent, or present with misleading symptoms. Screening for anxiety disorders, especially in the presence of the most common presenting concerns, can illuminate the true nature of a child’s challenge and point the way forward. In this month’s article, we will provide details on the prevalence of anxiety disorders in children, how they typically present, and how best to screen for them. We will offer some strategies for speaking about them with your patients and their parents, as well as introduce some of the strategies that can improve mild to moderate anxiety disorders. We will follow up with another piece on the evidence-based treatments for these common disorders, how to find appropriate referrals, and what you can do in your office to get treatment started.
Anxiety disorders: Common and treatable
Anxiety disorders – including separation anxiety disorder, social phobia, simple phobias, generalized anxiety disorder, panic disorder, and PTSD – affect between 15% and 20% of children before the age of 18, with some recent estimates as high as 31.9% of youth being affected. Indeed, the mean age of onset for most anxiety disorders (excluding panic disorder and PTSD) is between 5 and 9 years of age. Despite being so common, many anxiety disorders in childhood are never properly diagnosed, and most (as many as 80%) do not receive treatment from a mental health professional. With early diagnosis and evidence-based treatment, most anxiety disorders can be “cured” and no longer impair functioning. Untreated, anxiety disorders usually have a chronic course, causing significant behavioral problems and disruption of a child’s critical social, emotional, and identity development and their academic function. Untreated, they are frequently complicated in adolescence by mood, substance use, and eating disorders. With the passage of time, developmental consequences and comorbid illnesses, a curable childhood anxiety disorder can become a complex and entrenched psychiatric syndrome in young adulthood.
One of the reasons these illnesses frequently go unrecognized is that states of fearful distress, such as separation anxiety or social anxiety, are developmentally normal at different stages of childhood, and it can be difficult to discriminate between normal and pathological anxiety. Anxiety itself is an “internalizing” symptom, and is invisible except for the behaviors that can accompany it. Some behaviors suggest anxiety, such as fearful expressions, clinginess, excessive need for reassurance, or avoidance. But anxiety can also lead to obstinate refusal to do certain things. It might lead to explosive tantrums when a child is pushed to do something that makes them intensely anxious. It can lead to irritability and moody tantrums for a child exhausted after a long school day spent managing high levels of anxiety by themselves. Anxious children often appear inattentive in school. Anxiety disorders frequently disrupt restful sleep, leading to children who are irritable and moody as well as inattentive. These children may present to the pediatrician with frustrated parents concerned that they are oppositional or explosive, or because their teachers are concerned about ADHD, when the culprit is actually anxiety.
While anxiety is uncomfortable, these children are unlikely to experience their anxiety as unusual and foreign, like a sudden toothache. Instead, it feels to them like they are fearful for good reason, responding appropriately to something real. These children are more likely to respond to a novel or uncertain situation with worry rather than curiosity, and to a new challenge as a threat. For children who are managing their anxiety more internally, their parents are often unaware of their degree of distress. Indeed, these children are often careful, thoughtful, and attentive to detail. Parents and teachers may think they are doing wonderfully. They are typically very sensitive to physical discomforts, which are heightened by an anxious state. These are likely to present to the pediatrician’s office with parents very worried about a cluster of vague physical complaints (stomach ache, headache, “just not feeling good”), which coincides with a change, challenge, or anxious stimulus. In this situation, the parents may dismiss the possibility of anxiety, and the child may not even be aware of it. But it will get worse if they are pushed to bear the source of anxiety (going to school, sports practice, etc.).
Anxiety screening and treatment
When a child presents for a sick visit with vague symptoms, or a negative workup for specific ones, you should screen them for an anxiety disorder. When they present with concerns about inattention, insomnia, moodiness, obstinacy, and even explosive behaviors, you should screen them for an anxiety disorder. This is especially true if they are prepubertal, when anxiety disorders are far more common than mood disorders. But you should consider anxiety disorders alongside mood disorders in adolescents presenting with these complaints. While parents may be unaware of the presence of anxiety in their child, explain to them that anxiety disorders are very common and treatable in childhood to help them understand the value of screening. Asking children directly about their internal experience can also be helpful. Avoid asking about “anxiety,” instead asking if they ever worry about specific things, such as “talking to kids you don’t know at recess,” “being alone at home,” “getting robbed or kidnapped,” or “something bad happening to your parents.” Just asking helps children pay attention to their thoughts and feelings, and is a powerful screening instrument.
There are also real screening instruments that you might use routinely for sick visits in prepubertal children or when anxiety should be in the differential. These instruments can be prone to recall bias, but generally make it easier for (anxious) children to accurately describe their internal experience. An instrument like the GAD7 is brief, free, and sensitive, but not very specific. If it is positive, you can then offer a longer screen such as the SCARED, also free, which indicates likely diagnoses such as generalized anxiety disorder, separation anxiety disorder, panic disorder, and social phobia. There is a parent version and a self-report, and it is validated for youths 8-18 years old and takes approximately 20 minutes to complete and score.
A positive screen should lead to a more nuanced conversation with your patient and their parents about their anxiety symptoms. You may feel comfortable doing a more extensive interview to make the likely diagnosis or may prefer to refer to a psychiatrist or psychologist to assist with diagnosis and treatment recommendations. In either case, you can offer your patient and their parents meaningful reassurance that the intense discomfort of their anxiety will get better with effective treatment. In this visit, you can get treatment started by identifying what parents and their children can do right away to begin addressing anxiety symptoms. Offer strategies to protect and promote restful sleep and daily vigorous exercise, both of which can directly improve mild to moderate anxiety symptoms. Suggest to parents that they should help their children to notice what they are feeling, rather than rushing in to remove a source of anxiety. These measures can help their child to identify what is a thought, a feeling, a physical sensation, or a fact. They can offer support and validation around how uncomfortable these feelings are, but just being curious will reassure their child that they will be able to manage and master this feeling. This “practice” is akin to what their child will do in most effective treatments, and will have the added benefit of helping them to build skills that all children need to manage the challenges and worries that are a normal, but difficult part of growing up and of adult life. Finally, you can tell them that anxious temperaments come with advantages also, such as great powers of observation, attention to detail, and thoroughness, high levels of empathy, drive, and tenacity. By learning to manage anxiety early, these children can grow up to be engaged, resilient, successful, and satisfied adults.
Once identified, the range of effective treatments available include cognitive-behavioral therapy, graduated exposure, mindfulness/relaxation techniques, and medication, and we will discuss these in our next article.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
Reference
Beesdo K et al. Anxiety and anxiety disorders in children and adolescents: developmental issues and implications for DSM-V. Psychiatr Clin North Am. 2009 Sep. doi: 10.1016/j.psc.2009.06.002.
Anxiety symptoms in children are common, ranging from a toddler’s fear of the dark to an adolescent worrying about a major exam. The good news is that, if they are detected early and treated appropriately, they are curable. Unfortunately, they are often silent, or present with misleading symptoms. Screening for anxiety disorders, especially in the presence of the most common presenting concerns, can illuminate the true nature of a child’s challenge and point the way forward. In this month’s article, we will provide details on the prevalence of anxiety disorders in children, how they typically present, and how best to screen for them. We will offer some strategies for speaking about them with your patients and their parents, as well as introduce some of the strategies that can improve mild to moderate anxiety disorders. We will follow up with another piece on the evidence-based treatments for these common disorders, how to find appropriate referrals, and what you can do in your office to get treatment started.
Anxiety disorders: Common and treatable
Anxiety disorders – including separation anxiety disorder, social phobia, simple phobias, generalized anxiety disorder, panic disorder, and PTSD – affect between 15% and 20% of children before the age of 18, with some recent estimates as high as 31.9% of youth being affected. Indeed, the mean age of onset for most anxiety disorders (excluding panic disorder and PTSD) is between 5 and 9 years of age. Despite being so common, many anxiety disorders in childhood are never properly diagnosed, and most (as many as 80%) do not receive treatment from a mental health professional. With early diagnosis and evidence-based treatment, most anxiety disorders can be “cured” and no longer impair functioning. Untreated, anxiety disorders usually have a chronic course, causing significant behavioral problems and disruption of a child’s critical social, emotional, and identity development and their academic function. Untreated, they are frequently complicated in adolescence by mood, substance use, and eating disorders. With the passage of time, developmental consequences and comorbid illnesses, a curable childhood anxiety disorder can become a complex and entrenched psychiatric syndrome in young adulthood.
One of the reasons these illnesses frequently go unrecognized is that states of fearful distress, such as separation anxiety or social anxiety, are developmentally normal at different stages of childhood, and it can be difficult to discriminate between normal and pathological anxiety. Anxiety itself is an “internalizing” symptom, and is invisible except for the behaviors that can accompany it. Some behaviors suggest anxiety, such as fearful expressions, clinginess, excessive need for reassurance, or avoidance. But anxiety can also lead to obstinate refusal to do certain things. It might lead to explosive tantrums when a child is pushed to do something that makes them intensely anxious. It can lead to irritability and moody tantrums for a child exhausted after a long school day spent managing high levels of anxiety by themselves. Anxious children often appear inattentive in school. Anxiety disorders frequently disrupt restful sleep, leading to children who are irritable and moody as well as inattentive. These children may present to the pediatrician with frustrated parents concerned that they are oppositional or explosive, or because their teachers are concerned about ADHD, when the culprit is actually anxiety.
While anxiety is uncomfortable, these children are unlikely to experience their anxiety as unusual and foreign, like a sudden toothache. Instead, it feels to them like they are fearful for good reason, responding appropriately to something real. These children are more likely to respond to a novel or uncertain situation with worry rather than curiosity, and to a new challenge as a threat. For children who are managing their anxiety more internally, their parents are often unaware of their degree of distress. Indeed, these children are often careful, thoughtful, and attentive to detail. Parents and teachers may think they are doing wonderfully. They are typically very sensitive to physical discomforts, which are heightened by an anxious state. These are likely to present to the pediatrician’s office with parents very worried about a cluster of vague physical complaints (stomach ache, headache, “just not feeling good”), which coincides with a change, challenge, or anxious stimulus. In this situation, the parents may dismiss the possibility of anxiety, and the child may not even be aware of it. But it will get worse if they are pushed to bear the source of anxiety (going to school, sports practice, etc.).
Anxiety screening and treatment
When a child presents for a sick visit with vague symptoms, or a negative workup for specific ones, you should screen them for an anxiety disorder. When they present with concerns about inattention, insomnia, moodiness, obstinacy, and even explosive behaviors, you should screen them for an anxiety disorder. This is especially true if they are prepubertal, when anxiety disorders are far more common than mood disorders. But you should consider anxiety disorders alongside mood disorders in adolescents presenting with these complaints. While parents may be unaware of the presence of anxiety in their child, explain to them that anxiety disorders are very common and treatable in childhood to help them understand the value of screening. Asking children directly about their internal experience can also be helpful. Avoid asking about “anxiety,” instead asking if they ever worry about specific things, such as “talking to kids you don’t know at recess,” “being alone at home,” “getting robbed or kidnapped,” or “something bad happening to your parents.” Just asking helps children pay attention to their thoughts and feelings, and is a powerful screening instrument.
There are also real screening instruments that you might use routinely for sick visits in prepubertal children or when anxiety should be in the differential. These instruments can be prone to recall bias, but generally make it easier for (anxious) children to accurately describe their internal experience. An instrument like the GAD7 is brief, free, and sensitive, but not very specific. If it is positive, you can then offer a longer screen such as the SCARED, also free, which indicates likely diagnoses such as generalized anxiety disorder, separation anxiety disorder, panic disorder, and social phobia. There is a parent version and a self-report, and it is validated for youths 8-18 years old and takes approximately 20 minutes to complete and score.
A positive screen should lead to a more nuanced conversation with your patient and their parents about their anxiety symptoms. You may feel comfortable doing a more extensive interview to make the likely diagnosis or may prefer to refer to a psychiatrist or psychologist to assist with diagnosis and treatment recommendations. In either case, you can offer your patient and their parents meaningful reassurance that the intense discomfort of their anxiety will get better with effective treatment. In this visit, you can get treatment started by identifying what parents and their children can do right away to begin addressing anxiety symptoms. Offer strategies to protect and promote restful sleep and daily vigorous exercise, both of which can directly improve mild to moderate anxiety symptoms. Suggest to parents that they should help their children to notice what they are feeling, rather than rushing in to remove a source of anxiety. These measures can help their child to identify what is a thought, a feeling, a physical sensation, or a fact. They can offer support and validation around how uncomfortable these feelings are, but just being curious will reassure their child that they will be able to manage and master this feeling. This “practice” is akin to what their child will do in most effective treatments, and will have the added benefit of helping them to build skills that all children need to manage the challenges and worries that are a normal, but difficult part of growing up and of adult life. Finally, you can tell them that anxious temperaments come with advantages also, such as great powers of observation, attention to detail, and thoroughness, high levels of empathy, drive, and tenacity. By learning to manage anxiety early, these children can grow up to be engaged, resilient, successful, and satisfied adults.
Once identified, the range of effective treatments available include cognitive-behavioral therapy, graduated exposure, mindfulness/relaxation techniques, and medication, and we will discuss these in our next article.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
Reference
Beesdo K et al. Anxiety and anxiety disorders in children and adolescents: developmental issues and implications for DSM-V. Psychiatr Clin North Am. 2009 Sep. doi: 10.1016/j.psc.2009.06.002.
Anxiety symptoms in children are common, ranging from a toddler’s fear of the dark to an adolescent worrying about a major exam. The good news is that, if they are detected early and treated appropriately, they are curable. Unfortunately, they are often silent, or present with misleading symptoms. Screening for anxiety disorders, especially in the presence of the most common presenting concerns, can illuminate the true nature of a child’s challenge and point the way forward. In this month’s article, we will provide details on the prevalence of anxiety disorders in children, how they typically present, and how best to screen for them. We will offer some strategies for speaking about them with your patients and their parents, as well as introduce some of the strategies that can improve mild to moderate anxiety disorders. We will follow up with another piece on the evidence-based treatments for these common disorders, how to find appropriate referrals, and what you can do in your office to get treatment started.
Anxiety disorders: Common and treatable
Anxiety disorders – including separation anxiety disorder, social phobia, simple phobias, generalized anxiety disorder, panic disorder, and PTSD – affect between 15% and 20% of children before the age of 18, with some recent estimates as high as 31.9% of youth being affected. Indeed, the mean age of onset for most anxiety disorders (excluding panic disorder and PTSD) is between 5 and 9 years of age. Despite being so common, many anxiety disorders in childhood are never properly diagnosed, and most (as many as 80%) do not receive treatment from a mental health professional. With early diagnosis and evidence-based treatment, most anxiety disorders can be “cured” and no longer impair functioning. Untreated, anxiety disorders usually have a chronic course, causing significant behavioral problems and disruption of a child’s critical social, emotional, and identity development and their academic function. Untreated, they are frequently complicated in adolescence by mood, substance use, and eating disorders. With the passage of time, developmental consequences and comorbid illnesses, a curable childhood anxiety disorder can become a complex and entrenched psychiatric syndrome in young adulthood.
One of the reasons these illnesses frequently go unrecognized is that states of fearful distress, such as separation anxiety or social anxiety, are developmentally normal at different stages of childhood, and it can be difficult to discriminate between normal and pathological anxiety. Anxiety itself is an “internalizing” symptom, and is invisible except for the behaviors that can accompany it. Some behaviors suggest anxiety, such as fearful expressions, clinginess, excessive need for reassurance, or avoidance. But anxiety can also lead to obstinate refusal to do certain things. It might lead to explosive tantrums when a child is pushed to do something that makes them intensely anxious. It can lead to irritability and moody tantrums for a child exhausted after a long school day spent managing high levels of anxiety by themselves. Anxious children often appear inattentive in school. Anxiety disorders frequently disrupt restful sleep, leading to children who are irritable and moody as well as inattentive. These children may present to the pediatrician with frustrated parents concerned that they are oppositional or explosive, or because their teachers are concerned about ADHD, when the culprit is actually anxiety.
While anxiety is uncomfortable, these children are unlikely to experience their anxiety as unusual and foreign, like a sudden toothache. Instead, it feels to them like they are fearful for good reason, responding appropriately to something real. These children are more likely to respond to a novel or uncertain situation with worry rather than curiosity, and to a new challenge as a threat. For children who are managing their anxiety more internally, their parents are often unaware of their degree of distress. Indeed, these children are often careful, thoughtful, and attentive to detail. Parents and teachers may think they are doing wonderfully. They are typically very sensitive to physical discomforts, which are heightened by an anxious state. These are likely to present to the pediatrician’s office with parents very worried about a cluster of vague physical complaints (stomach ache, headache, “just not feeling good”), which coincides with a change, challenge, or anxious stimulus. In this situation, the parents may dismiss the possibility of anxiety, and the child may not even be aware of it. But it will get worse if they are pushed to bear the source of anxiety (going to school, sports practice, etc.).
Anxiety screening and treatment
When a child presents for a sick visit with vague symptoms, or a negative workup for specific ones, you should screen them for an anxiety disorder. When they present with concerns about inattention, insomnia, moodiness, obstinacy, and even explosive behaviors, you should screen them for an anxiety disorder. This is especially true if they are prepubertal, when anxiety disorders are far more common than mood disorders. But you should consider anxiety disorders alongside mood disorders in adolescents presenting with these complaints. While parents may be unaware of the presence of anxiety in their child, explain to them that anxiety disorders are very common and treatable in childhood to help them understand the value of screening. Asking children directly about their internal experience can also be helpful. Avoid asking about “anxiety,” instead asking if they ever worry about specific things, such as “talking to kids you don’t know at recess,” “being alone at home,” “getting robbed or kidnapped,” or “something bad happening to your parents.” Just asking helps children pay attention to their thoughts and feelings, and is a powerful screening instrument.
There are also real screening instruments that you might use routinely for sick visits in prepubertal children or when anxiety should be in the differential. These instruments can be prone to recall bias, but generally make it easier for (anxious) children to accurately describe their internal experience. An instrument like the GAD7 is brief, free, and sensitive, but not very specific. If it is positive, you can then offer a longer screen such as the SCARED, also free, which indicates likely diagnoses such as generalized anxiety disorder, separation anxiety disorder, panic disorder, and social phobia. There is a parent version and a self-report, and it is validated for youths 8-18 years old and takes approximately 20 minutes to complete and score.
A positive screen should lead to a more nuanced conversation with your patient and their parents about their anxiety symptoms. You may feel comfortable doing a more extensive interview to make the likely diagnosis or may prefer to refer to a psychiatrist or psychologist to assist with diagnosis and treatment recommendations. In either case, you can offer your patient and their parents meaningful reassurance that the intense discomfort of their anxiety will get better with effective treatment. In this visit, you can get treatment started by identifying what parents and their children can do right away to begin addressing anxiety symptoms. Offer strategies to protect and promote restful sleep and daily vigorous exercise, both of which can directly improve mild to moderate anxiety symptoms. Suggest to parents that they should help their children to notice what they are feeling, rather than rushing in to remove a source of anxiety. These measures can help their child to identify what is a thought, a feeling, a physical sensation, or a fact. They can offer support and validation around how uncomfortable these feelings are, but just being curious will reassure their child that they will be able to manage and master this feeling. This “practice” is akin to what their child will do in most effective treatments, and will have the added benefit of helping them to build skills that all children need to manage the challenges and worries that are a normal, but difficult part of growing up and of adult life. Finally, you can tell them that anxious temperaments come with advantages also, such as great powers of observation, attention to detail, and thoroughness, high levels of empathy, drive, and tenacity. By learning to manage anxiety early, these children can grow up to be engaged, resilient, successful, and satisfied adults.
Once identified, the range of effective treatments available include cognitive-behavioral therapy, graduated exposure, mindfulness/relaxation techniques, and medication, and we will discuss these in our next article.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
Reference
Beesdo K et al. Anxiety and anxiety disorders in children and adolescents: developmental issues and implications for DSM-V. Psychiatr Clin North Am. 2009 Sep. doi: 10.1016/j.psc.2009.06.002.
Metachronous CRC risk after colonoscopy for positive FIT
TOPLINE:
a study suggests.
,
METHODOLOGY:
- Investigators conducted a retrospective analysis of 253,833 colonoscopies performed after FIT-positive screens in a Dutch CRC screening program.
- A Cox regression analysis assessed the association between the findings at baseline colonoscopy and metachronous CRC risk.
- Investigators categorized patients into subgroups based on removed polyp subtypes and used groups without polyps as a reference.
- High-risk subgroups included those with high-risk serrated polyps, which were defined as a serrated polyp of at least 10 mm, sessile serrated lesions with dysplasia, or traditional serrated adenomas, as well as high-risk adenomas, which were defined as an adenoma of at least 10 mm or containing high-grade dysplasia.
TAKEAWAY:
- Over a median follow-up of 36 months, 504 metachronous CRCs were identified.
- Individuals with high-risk serrated polyps without co-occurring high-risk adenomas had an increased risk for metachronous CRC (hazard ratio, 1.70).
- The highest risk was seen in individuals with both high-risk serrated polyps and high-risk adenomas (HR, 2.0), as well as those with villous adenomas (HR, 2.07).
- Individuals with only high-risk adenomas did not show a significantly increased risk for metachronous CRC (HR, 1.22).
IN PRACTICE:
“Our results suggest that individuals with high-risk serrated polyps might comprise the higher CRC risk in the first years after colonoscopy. Results of this study could contribute to establish more restrictive polyp surveillance guidelines in a quality-assured setting,” the authors wrote.
SOURCE:
The study was led by David E. F. W. M. van Toledo, MD, department of gastroenterology and hepatology, Amsterdam University Medical Centers. It was published online July 5, 2023, in eClinicalMedicine. The study received no funding.
LIMITATIONS:
The relatively short median follow-up time of 3 years may limit the assessment of long-term metachronous CRC risk. The study population consisted of FIT-positive individuals, which may introduce selection bias. The incidence of metachronous CRC in the study was lower compared with other studies, potentially affecting the risk estimates. The limited number of cases in some subgroups may result in unreliable risk estimations.
DISCLOSURES:
Dr. van Toledo declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
a study suggests.
,
METHODOLOGY:
- Investigators conducted a retrospective analysis of 253,833 colonoscopies performed after FIT-positive screens in a Dutch CRC screening program.
- A Cox regression analysis assessed the association between the findings at baseline colonoscopy and metachronous CRC risk.
- Investigators categorized patients into subgroups based on removed polyp subtypes and used groups without polyps as a reference.
- High-risk subgroups included those with high-risk serrated polyps, which were defined as a serrated polyp of at least 10 mm, sessile serrated lesions with dysplasia, or traditional serrated adenomas, as well as high-risk adenomas, which were defined as an adenoma of at least 10 mm or containing high-grade dysplasia.
TAKEAWAY:
- Over a median follow-up of 36 months, 504 metachronous CRCs were identified.
- Individuals with high-risk serrated polyps without co-occurring high-risk adenomas had an increased risk for metachronous CRC (hazard ratio, 1.70).
- The highest risk was seen in individuals with both high-risk serrated polyps and high-risk adenomas (HR, 2.0), as well as those with villous adenomas (HR, 2.07).
- Individuals with only high-risk adenomas did not show a significantly increased risk for metachronous CRC (HR, 1.22).
IN PRACTICE:
“Our results suggest that individuals with high-risk serrated polyps might comprise the higher CRC risk in the first years after colonoscopy. Results of this study could contribute to establish more restrictive polyp surveillance guidelines in a quality-assured setting,” the authors wrote.
SOURCE:
The study was led by David E. F. W. M. van Toledo, MD, department of gastroenterology and hepatology, Amsterdam University Medical Centers. It was published online July 5, 2023, in eClinicalMedicine. The study received no funding.
LIMITATIONS:
The relatively short median follow-up time of 3 years may limit the assessment of long-term metachronous CRC risk. The study population consisted of FIT-positive individuals, which may introduce selection bias. The incidence of metachronous CRC in the study was lower compared with other studies, potentially affecting the risk estimates. The limited number of cases in some subgroups may result in unreliable risk estimations.
DISCLOSURES:
Dr. van Toledo declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
a study suggests.
,
METHODOLOGY:
- Investigators conducted a retrospective analysis of 253,833 colonoscopies performed after FIT-positive screens in a Dutch CRC screening program.
- A Cox regression analysis assessed the association between the findings at baseline colonoscopy and metachronous CRC risk.
- Investigators categorized patients into subgroups based on removed polyp subtypes and used groups without polyps as a reference.
- High-risk subgroups included those with high-risk serrated polyps, which were defined as a serrated polyp of at least 10 mm, sessile serrated lesions with dysplasia, or traditional serrated adenomas, as well as high-risk adenomas, which were defined as an adenoma of at least 10 mm or containing high-grade dysplasia.
TAKEAWAY:
- Over a median follow-up of 36 months, 504 metachronous CRCs were identified.
- Individuals with high-risk serrated polyps without co-occurring high-risk adenomas had an increased risk for metachronous CRC (hazard ratio, 1.70).
- The highest risk was seen in individuals with both high-risk serrated polyps and high-risk adenomas (HR, 2.0), as well as those with villous adenomas (HR, 2.07).
- Individuals with only high-risk adenomas did not show a significantly increased risk for metachronous CRC (HR, 1.22).
IN PRACTICE:
“Our results suggest that individuals with high-risk serrated polyps might comprise the higher CRC risk in the first years after colonoscopy. Results of this study could contribute to establish more restrictive polyp surveillance guidelines in a quality-assured setting,” the authors wrote.
SOURCE:
The study was led by David E. F. W. M. van Toledo, MD, department of gastroenterology and hepatology, Amsterdam University Medical Centers. It was published online July 5, 2023, in eClinicalMedicine. The study received no funding.
LIMITATIONS:
The relatively short median follow-up time of 3 years may limit the assessment of long-term metachronous CRC risk. The study population consisted of FIT-positive individuals, which may introduce selection bias. The incidence of metachronous CRC in the study was lower compared with other studies, potentially affecting the risk estimates. The limited number of cases in some subgroups may result in unreliable risk estimations.
DISCLOSURES:
Dr. van Toledo declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Liquid biopsy shows big promise in oropharyngeal cancer
In a retrospective observational cohort study, a commercially available blood test used to evaluate tumor tissue–modified viral-HPV DNA demonstrated 100% specificity for both diagnosis of oropharyngeal cancer and surveillance for recurrence. Sensitivity was 91.5% for correctly identifying patients who have the disease and 88.4% for surveillance.
“A positive result appeared to confirm the presence of disease, [but] approximately 1 in 10 negative results in patients with pathologically confirmed HPV-associated oropharyngeal squamous cell carcinoma were falsely negative,” lead investigator Rocco Ferrandino, MD, with Mount Sinai, New York, said in an interview.
“Therefore, further workup should still be pursued when clinical suspicion for HPV-associated oropharynx cancer is high,” Dr. Ferrandino said.
The study was published online, in JAMA Otolaryngology–Head and Neck Surgery, to coincide with presentation at the annual meeting of the American Head and Neck Society in Montreal.
‘Remarkable promise’
The diagnosis of HPV-associated oropharyngeal cancer currently relies on a tissue-based biopsy of the primary site or a regional lymph node; however, there has been growing interest in the potential of liquid biopsy for diagnosis and surveillance.
The commercially available assay that was evaluated in the study uses a distinct method to identify and quantify a tumor-associated or tumor-modified pattern of DNA fragments that significantly increases the specificity for identifying an HPV-associated malignant tumor. However, evaluation of the assay has been limited to small cohort studies and clinical trials.
In the current study, Dr. Ferrandino and colleagues evaluated the performance of the assay used during routine clinical practice at their high-volume institution over a period of nearly 3 years.
The study included 163 patients in the diagnostic cohort and 290 in the surveillance cohort. In the diagnostic cohort, 152 had HPV-associated oropharyngeal cancer, and 11 had HPV-negative oropharyngeal cancer. The sensitivity of the assay in pretreatment diagnosis was 91.5% (139 of 152 tests), and the specificity was 100% (11 of 11 tests).
In the surveillance cohort of 290 patients, 591 tests were evaluated. A total of 23 patients developed pathologically confirmed recurrences over a median follow-up of 40.5 months. The assay demonstrated sensitivity of 88.4% (38 of 43 tests) and specificity of 100% (548 of 548 tests) in detecting recurrences.
The median lead time from positive test to pathologic confirmation was 47 days.
“The lead time provided by positive assay results may allow a window of opportunity for salvage treatment or for the application of adjuvant systemic therapy,” Dr. Ferrandino and colleagues explain.
“While these results are exciting and may support adjunctive use of circulating tumor DNA testing for diagnosis and surveillance, we really need more prospective and multicenter studies to validate these findings,” Dr. Ferrandino said in an interview.
In an accompanying commentary, Miriam Lango, MD, department of head and neck surgery, the University of Texas MD Anderson Cancer Center, Houston, said she agrees that a prospective clinical validation study is needed.
“Nevertheless, the use of this technology shows remarkable promise to transform the ability to identify and follow patients with HPV-related disease. Testing is likely to be increasingly used in routine clinical care, as it is commercially available,” Dr. Lango writes.
Still, she noted, “It is incumbent on us to establish evidence for strong and detailed surveillance guidelines to share among the cancer community.”
The study had no specific funding. Dr. Ferrandino and Dr. Lango have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a retrospective observational cohort study, a commercially available blood test used to evaluate tumor tissue–modified viral-HPV DNA demonstrated 100% specificity for both diagnosis of oropharyngeal cancer and surveillance for recurrence. Sensitivity was 91.5% for correctly identifying patients who have the disease and 88.4% for surveillance.
“A positive result appeared to confirm the presence of disease, [but] approximately 1 in 10 negative results in patients with pathologically confirmed HPV-associated oropharyngeal squamous cell carcinoma were falsely negative,” lead investigator Rocco Ferrandino, MD, with Mount Sinai, New York, said in an interview.
“Therefore, further workup should still be pursued when clinical suspicion for HPV-associated oropharynx cancer is high,” Dr. Ferrandino said.
The study was published online, in JAMA Otolaryngology–Head and Neck Surgery, to coincide with presentation at the annual meeting of the American Head and Neck Society in Montreal.
‘Remarkable promise’
The diagnosis of HPV-associated oropharyngeal cancer currently relies on a tissue-based biopsy of the primary site or a regional lymph node; however, there has been growing interest in the potential of liquid biopsy for diagnosis and surveillance.
The commercially available assay that was evaluated in the study uses a distinct method to identify and quantify a tumor-associated or tumor-modified pattern of DNA fragments that significantly increases the specificity for identifying an HPV-associated malignant tumor. However, evaluation of the assay has been limited to small cohort studies and clinical trials.
In the current study, Dr. Ferrandino and colleagues evaluated the performance of the assay used during routine clinical practice at their high-volume institution over a period of nearly 3 years.
The study included 163 patients in the diagnostic cohort and 290 in the surveillance cohort. In the diagnostic cohort, 152 had HPV-associated oropharyngeal cancer, and 11 had HPV-negative oropharyngeal cancer. The sensitivity of the assay in pretreatment diagnosis was 91.5% (139 of 152 tests), and the specificity was 100% (11 of 11 tests).
In the surveillance cohort of 290 patients, 591 tests were evaluated. A total of 23 patients developed pathologically confirmed recurrences over a median follow-up of 40.5 months. The assay demonstrated sensitivity of 88.4% (38 of 43 tests) and specificity of 100% (548 of 548 tests) in detecting recurrences.
The median lead time from positive test to pathologic confirmation was 47 days.
“The lead time provided by positive assay results may allow a window of opportunity for salvage treatment or for the application of adjuvant systemic therapy,” Dr. Ferrandino and colleagues explain.
“While these results are exciting and may support adjunctive use of circulating tumor DNA testing for diagnosis and surveillance, we really need more prospective and multicenter studies to validate these findings,” Dr. Ferrandino said in an interview.
In an accompanying commentary, Miriam Lango, MD, department of head and neck surgery, the University of Texas MD Anderson Cancer Center, Houston, said she agrees that a prospective clinical validation study is needed.
“Nevertheless, the use of this technology shows remarkable promise to transform the ability to identify and follow patients with HPV-related disease. Testing is likely to be increasingly used in routine clinical care, as it is commercially available,” Dr. Lango writes.
Still, she noted, “It is incumbent on us to establish evidence for strong and detailed surveillance guidelines to share among the cancer community.”
The study had no specific funding. Dr. Ferrandino and Dr. Lango have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a retrospective observational cohort study, a commercially available blood test used to evaluate tumor tissue–modified viral-HPV DNA demonstrated 100% specificity for both diagnosis of oropharyngeal cancer and surveillance for recurrence. Sensitivity was 91.5% for correctly identifying patients who have the disease and 88.4% for surveillance.
“A positive result appeared to confirm the presence of disease, [but] approximately 1 in 10 negative results in patients with pathologically confirmed HPV-associated oropharyngeal squamous cell carcinoma were falsely negative,” lead investigator Rocco Ferrandino, MD, with Mount Sinai, New York, said in an interview.
“Therefore, further workup should still be pursued when clinical suspicion for HPV-associated oropharynx cancer is high,” Dr. Ferrandino said.
The study was published online, in JAMA Otolaryngology–Head and Neck Surgery, to coincide with presentation at the annual meeting of the American Head and Neck Society in Montreal.
‘Remarkable promise’
The diagnosis of HPV-associated oropharyngeal cancer currently relies on a tissue-based biopsy of the primary site or a regional lymph node; however, there has been growing interest in the potential of liquid biopsy for diagnosis and surveillance.
The commercially available assay that was evaluated in the study uses a distinct method to identify and quantify a tumor-associated or tumor-modified pattern of DNA fragments that significantly increases the specificity for identifying an HPV-associated malignant tumor. However, evaluation of the assay has been limited to small cohort studies and clinical trials.
In the current study, Dr. Ferrandino and colleagues evaluated the performance of the assay used during routine clinical practice at their high-volume institution over a period of nearly 3 years.
The study included 163 patients in the diagnostic cohort and 290 in the surveillance cohort. In the diagnostic cohort, 152 had HPV-associated oropharyngeal cancer, and 11 had HPV-negative oropharyngeal cancer. The sensitivity of the assay in pretreatment diagnosis was 91.5% (139 of 152 tests), and the specificity was 100% (11 of 11 tests).
In the surveillance cohort of 290 patients, 591 tests were evaluated. A total of 23 patients developed pathologically confirmed recurrences over a median follow-up of 40.5 months. The assay demonstrated sensitivity of 88.4% (38 of 43 tests) and specificity of 100% (548 of 548 tests) in detecting recurrences.
The median lead time from positive test to pathologic confirmation was 47 days.
“The lead time provided by positive assay results may allow a window of opportunity for salvage treatment or for the application of adjuvant systemic therapy,” Dr. Ferrandino and colleagues explain.
“While these results are exciting and may support adjunctive use of circulating tumor DNA testing for diagnosis and surveillance, we really need more prospective and multicenter studies to validate these findings,” Dr. Ferrandino said in an interview.
In an accompanying commentary, Miriam Lango, MD, department of head and neck surgery, the University of Texas MD Anderson Cancer Center, Houston, said she agrees that a prospective clinical validation study is needed.
“Nevertheless, the use of this technology shows remarkable promise to transform the ability to identify and follow patients with HPV-related disease. Testing is likely to be increasingly used in routine clinical care, as it is commercially available,” Dr. Lango writes.
Still, she noted, “It is incumbent on us to establish evidence for strong and detailed surveillance guidelines to share among the cancer community.”
The study had no specific funding. Dr. Ferrandino and Dr. Lango have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA OTOLARYNGOLOGY–HEAD AND NECK SURGERY
All in stride: Few age limitations for joint replacement
Kathy Blackwell is not going to allow a couple of aching joints stop her from living her best life.
The 73-year-old resident of Simi Valley, Calif., a bedroom community about 30 miles northwest of downtown Los Angeles, organizes regular activities for her group of seniors. The 20- to 30-member-strong band of seasoned citizens, mostly women, keep active. Over the coming weeks, they plan to catch the Beach Boys at the historic Hollywood Bowl and take a cruise to Alaska.
The busy schedule is why Ms. Blackwell intends to delay her second hip replacement surgery, opting instead for a cortisone shot in hopes of easing the pain enough to enjoy the upcoming excursions.
Not that she is shy about joint replacement. If her orthopedic surgeon offered a frequent customer punch card like the ones you get at the local coffee shop, hers would be nearly full. Ms. Blackwell’s knees and a hip have been replaced, and her other hip will be, too, once her calendar clears up.
“If you go on enough with chronic pain where there’s no relief, you get cranky,” Ms. Blackwell said.
More than 1 million new knees, hips
Joint replacements are getting more common, with about 790,000 total knee replacements and more than 450,000 hip replacements performed annually in the United States, according to the American College of Rheumatology.
Experts agree age is not a factor when considering candidates for joint replacement. Rafael Sierra, MD, of the Mayo Clinic, Rochester, Minn., said he’s done hip replacements on patients as young as 12 and as old as 102. Orthopedic surgeon John Wang, MD, of the Hospital for Special Surgery, New York, has performed a total knee arthroscopy on a patient in their mid-90s. At 73, Ms. Blackwell is on the older side of the average age of 66 for a hip replacement.
“A lot of research and studies have shown that no matter what the age ranges, people end up doing great,” Dr. Wang said.
More importantly than age, older patients should be prepared for postsurgery therapy and treatment. For younger patients, the biggest drawback is outliving the estimated 25-year life span of a joint replacement. Complications are rare and occur in about 2% of procedures. These include infection, dislocation of the joint, and blood clots; other health issues you also have are not a factor.
Considering Ms. Blackwell’s hard time with her first knee replacement, it’s no small wonder that she ever set foot in a surgeon’s office again.
After putting it off for 7 years, Ms. Blackwell finally agreed to her doctor’s advice to replace her left knee in 2017 to relieve what she described as a “grinding,” chronic, bone-on-bone pain.
“It got to the point where there were no alternatives,” she said.
But her first orthopedic surgeon did a “lousy job,” leaving her with a gaping, festering wound that resulted in sepsis and required wound vacuum therapy to close the lesion. She eventually found another surgeon who removed and cleaned up her artificial knee before replacing the prosthesis. Luckily, the sepsis didn’t spread, and eight surgeries later, she was in the clear.
Ms. Blackwell’s second knee replacement in 2018 was a textbook surgery, as was a hip replacement in late 2019 .
“Your whole attitude changes,” she said.
What generalists should know
Orthopedic surgeons recommend that primary care doctors ask two things when weighing joint replacements: Have they exhausted nonsurgical treatments, and is the pain intolerable? They also advise avoiding narcotics to treat the symptoms.
The top issue to consider for a primary care doctor when weighing whether their patient may be a candidate for joint replacement is if the pain and the imaging are bad enough to warrant surgery.
“You don’t want to do it too soon,” Dr. Sierra said.
Dr. Sierra likes to tell the story of the golfer whose knee stiffens after playing 18 holes. To those patients, he recommends dialing back the activity; in this case, using a cart or playing only nine holes.
Dr. Wang agrees, asking if the pain is “lifestyle altering” and if the patient was unresponsive to nonsurgical treatments such as over-the-counter medications, anti-inflammatory medication and shots, home exercises or physical therapy, wearing a brace or sleeve, or simply changing their activity.
And no addictive pain pills to treat arthritis that can lead to other serious issues.
“This is not going to heal itself,” Dr. Wang said. “It’s not going to improve on its own. So, we don’t want to throw narcotics at it just to cover it up.”
Karen Smith, MD, has been a family doctor in rural North Carolina for more than 30 years. When she sees patients complaining about their joints, she first looks at function and pain. From there, she explores why they’re having discomfort. For example, is the problem an ergonomic issue at work or the result of carrying a lot of body weight?
“We look at those areas to determine what can be modified,” she said. “All of that’s done even before we get to having the orthopedic involvement.”
Dr. Smith said she also considers things beyond basic medicine: What is the patient’s mental status and tolerance for pain? Do they have a support system at home for post-operative care? And can they afford to miss work?
“We look at all of those factors together because that is going to determine the outcome that we’re hoping to achieve,” Dr. Smith said.
Great expectations
A recent study shows that older patients respond better to knee replacements than younger patients, particularly with pain relief and quality of life. The reason for this is believed to boil down to expectations. Whereas a younger person may want to return to the racquetball court and perform like they used to, older patients may just wish to walk down the hall without discomfort.
“It’s possible that these under 55-age-old patients may just take a little longer to heal to be satisfied,” Dr. Wang said. “We really can’t speak to why this is happening, but it’s possible that the younger patients are more active, and they expect more out of their knee.”
Jeevan Sall, MD, is a primary care sports medicine doctor with Providence Mission Heritage Medical Group in Laguna Niguel, Calif. He first discusses conservative management for patients struggling with arthritis in their joints. These measures include rehabilitation exercises, braces, shoe inserts, medication, and weight loss efforts. If these steps don’t improve a patient’s pain or lifestyle, surgery is on the table. Managing expectations is a significant factor.
“Is the patient mentally ready for surgery?” Dr. Sall said. “This includes what they hope to achieve with surgery as well as the risk and benefits of the procedure.”
Ms. Blackwell’s hip and knee pain came simply from a life well lived, with no marathon running or life-changing accident to speak of. She worked as a housewife raising her two children and owned an elevator company with her late husband, Robert Blackwell.
Yes, the elevator construction business has jokes.
“We have our ups and downs,” Ms. Blackwell said.
And with her new joints, so does she.
A version of this article first appeared on WebMD.com.
Kathy Blackwell is not going to allow a couple of aching joints stop her from living her best life.
The 73-year-old resident of Simi Valley, Calif., a bedroom community about 30 miles northwest of downtown Los Angeles, organizes regular activities for her group of seniors. The 20- to 30-member-strong band of seasoned citizens, mostly women, keep active. Over the coming weeks, they plan to catch the Beach Boys at the historic Hollywood Bowl and take a cruise to Alaska.
The busy schedule is why Ms. Blackwell intends to delay her second hip replacement surgery, opting instead for a cortisone shot in hopes of easing the pain enough to enjoy the upcoming excursions.
Not that she is shy about joint replacement. If her orthopedic surgeon offered a frequent customer punch card like the ones you get at the local coffee shop, hers would be nearly full. Ms. Blackwell’s knees and a hip have been replaced, and her other hip will be, too, once her calendar clears up.
“If you go on enough with chronic pain where there’s no relief, you get cranky,” Ms. Blackwell said.
More than 1 million new knees, hips
Joint replacements are getting more common, with about 790,000 total knee replacements and more than 450,000 hip replacements performed annually in the United States, according to the American College of Rheumatology.
Experts agree age is not a factor when considering candidates for joint replacement. Rafael Sierra, MD, of the Mayo Clinic, Rochester, Minn., said he’s done hip replacements on patients as young as 12 and as old as 102. Orthopedic surgeon John Wang, MD, of the Hospital for Special Surgery, New York, has performed a total knee arthroscopy on a patient in their mid-90s. At 73, Ms. Blackwell is on the older side of the average age of 66 for a hip replacement.
“A lot of research and studies have shown that no matter what the age ranges, people end up doing great,” Dr. Wang said.
More importantly than age, older patients should be prepared for postsurgery therapy and treatment. For younger patients, the biggest drawback is outliving the estimated 25-year life span of a joint replacement. Complications are rare and occur in about 2% of procedures. These include infection, dislocation of the joint, and blood clots; other health issues you also have are not a factor.
Considering Ms. Blackwell’s hard time with her first knee replacement, it’s no small wonder that she ever set foot in a surgeon’s office again.
After putting it off for 7 years, Ms. Blackwell finally agreed to her doctor’s advice to replace her left knee in 2017 to relieve what she described as a “grinding,” chronic, bone-on-bone pain.
“It got to the point where there were no alternatives,” she said.
But her first orthopedic surgeon did a “lousy job,” leaving her with a gaping, festering wound that resulted in sepsis and required wound vacuum therapy to close the lesion. She eventually found another surgeon who removed and cleaned up her artificial knee before replacing the prosthesis. Luckily, the sepsis didn’t spread, and eight surgeries later, she was in the clear.
Ms. Blackwell’s second knee replacement in 2018 was a textbook surgery, as was a hip replacement in late 2019 .
“Your whole attitude changes,” she said.
What generalists should know
Orthopedic surgeons recommend that primary care doctors ask two things when weighing joint replacements: Have they exhausted nonsurgical treatments, and is the pain intolerable? They also advise avoiding narcotics to treat the symptoms.
The top issue to consider for a primary care doctor when weighing whether their patient may be a candidate for joint replacement is if the pain and the imaging are bad enough to warrant surgery.
“You don’t want to do it too soon,” Dr. Sierra said.
Dr. Sierra likes to tell the story of the golfer whose knee stiffens after playing 18 holes. To those patients, he recommends dialing back the activity; in this case, using a cart or playing only nine holes.
Dr. Wang agrees, asking if the pain is “lifestyle altering” and if the patient was unresponsive to nonsurgical treatments such as over-the-counter medications, anti-inflammatory medication and shots, home exercises or physical therapy, wearing a brace or sleeve, or simply changing their activity.
And no addictive pain pills to treat arthritis that can lead to other serious issues.
“This is not going to heal itself,” Dr. Wang said. “It’s not going to improve on its own. So, we don’t want to throw narcotics at it just to cover it up.”
Karen Smith, MD, has been a family doctor in rural North Carolina for more than 30 years. When she sees patients complaining about their joints, she first looks at function and pain. From there, she explores why they’re having discomfort. For example, is the problem an ergonomic issue at work or the result of carrying a lot of body weight?
“We look at those areas to determine what can be modified,” she said. “All of that’s done even before we get to having the orthopedic involvement.”
Dr. Smith said she also considers things beyond basic medicine: What is the patient’s mental status and tolerance for pain? Do they have a support system at home for post-operative care? And can they afford to miss work?
“We look at all of those factors together because that is going to determine the outcome that we’re hoping to achieve,” Dr. Smith said.
Great expectations
A recent study shows that older patients respond better to knee replacements than younger patients, particularly with pain relief and quality of life. The reason for this is believed to boil down to expectations. Whereas a younger person may want to return to the racquetball court and perform like they used to, older patients may just wish to walk down the hall without discomfort.
“It’s possible that these under 55-age-old patients may just take a little longer to heal to be satisfied,” Dr. Wang said. “We really can’t speak to why this is happening, but it’s possible that the younger patients are more active, and they expect more out of their knee.”
Jeevan Sall, MD, is a primary care sports medicine doctor with Providence Mission Heritage Medical Group in Laguna Niguel, Calif. He first discusses conservative management for patients struggling with arthritis in their joints. These measures include rehabilitation exercises, braces, shoe inserts, medication, and weight loss efforts. If these steps don’t improve a patient’s pain or lifestyle, surgery is on the table. Managing expectations is a significant factor.
“Is the patient mentally ready for surgery?” Dr. Sall said. “This includes what they hope to achieve with surgery as well as the risk and benefits of the procedure.”
Ms. Blackwell’s hip and knee pain came simply from a life well lived, with no marathon running or life-changing accident to speak of. She worked as a housewife raising her two children and owned an elevator company with her late husband, Robert Blackwell.
Yes, the elevator construction business has jokes.
“We have our ups and downs,” Ms. Blackwell said.
And with her new joints, so does she.
A version of this article first appeared on WebMD.com.
Kathy Blackwell is not going to allow a couple of aching joints stop her from living her best life.
The 73-year-old resident of Simi Valley, Calif., a bedroom community about 30 miles northwest of downtown Los Angeles, organizes regular activities for her group of seniors. The 20- to 30-member-strong band of seasoned citizens, mostly women, keep active. Over the coming weeks, they plan to catch the Beach Boys at the historic Hollywood Bowl and take a cruise to Alaska.
The busy schedule is why Ms. Blackwell intends to delay her second hip replacement surgery, opting instead for a cortisone shot in hopes of easing the pain enough to enjoy the upcoming excursions.
Not that she is shy about joint replacement. If her orthopedic surgeon offered a frequent customer punch card like the ones you get at the local coffee shop, hers would be nearly full. Ms. Blackwell’s knees and a hip have been replaced, and her other hip will be, too, once her calendar clears up.
“If you go on enough with chronic pain where there’s no relief, you get cranky,” Ms. Blackwell said.
More than 1 million new knees, hips
Joint replacements are getting more common, with about 790,000 total knee replacements and more than 450,000 hip replacements performed annually in the United States, according to the American College of Rheumatology.
Experts agree age is not a factor when considering candidates for joint replacement. Rafael Sierra, MD, of the Mayo Clinic, Rochester, Minn., said he’s done hip replacements on patients as young as 12 and as old as 102. Orthopedic surgeon John Wang, MD, of the Hospital for Special Surgery, New York, has performed a total knee arthroscopy on a patient in their mid-90s. At 73, Ms. Blackwell is on the older side of the average age of 66 for a hip replacement.
“A lot of research and studies have shown that no matter what the age ranges, people end up doing great,” Dr. Wang said.
More importantly than age, older patients should be prepared for postsurgery therapy and treatment. For younger patients, the biggest drawback is outliving the estimated 25-year life span of a joint replacement. Complications are rare and occur in about 2% of procedures. These include infection, dislocation of the joint, and blood clots; other health issues you also have are not a factor.
Considering Ms. Blackwell’s hard time with her first knee replacement, it’s no small wonder that she ever set foot in a surgeon’s office again.
After putting it off for 7 years, Ms. Blackwell finally agreed to her doctor’s advice to replace her left knee in 2017 to relieve what she described as a “grinding,” chronic, bone-on-bone pain.
“It got to the point where there were no alternatives,” she said.
But her first orthopedic surgeon did a “lousy job,” leaving her with a gaping, festering wound that resulted in sepsis and required wound vacuum therapy to close the lesion. She eventually found another surgeon who removed and cleaned up her artificial knee before replacing the prosthesis. Luckily, the sepsis didn’t spread, and eight surgeries later, she was in the clear.
Ms. Blackwell’s second knee replacement in 2018 was a textbook surgery, as was a hip replacement in late 2019 .
“Your whole attitude changes,” she said.
What generalists should know
Orthopedic surgeons recommend that primary care doctors ask two things when weighing joint replacements: Have they exhausted nonsurgical treatments, and is the pain intolerable? They also advise avoiding narcotics to treat the symptoms.
The top issue to consider for a primary care doctor when weighing whether their patient may be a candidate for joint replacement is if the pain and the imaging are bad enough to warrant surgery.
“You don’t want to do it too soon,” Dr. Sierra said.
Dr. Sierra likes to tell the story of the golfer whose knee stiffens after playing 18 holes. To those patients, he recommends dialing back the activity; in this case, using a cart or playing only nine holes.
Dr. Wang agrees, asking if the pain is “lifestyle altering” and if the patient was unresponsive to nonsurgical treatments such as over-the-counter medications, anti-inflammatory medication and shots, home exercises or physical therapy, wearing a brace or sleeve, or simply changing their activity.
And no addictive pain pills to treat arthritis that can lead to other serious issues.
“This is not going to heal itself,” Dr. Wang said. “It’s not going to improve on its own. So, we don’t want to throw narcotics at it just to cover it up.”
Karen Smith, MD, has been a family doctor in rural North Carolina for more than 30 years. When she sees patients complaining about their joints, she first looks at function and pain. From there, she explores why they’re having discomfort. For example, is the problem an ergonomic issue at work or the result of carrying a lot of body weight?
“We look at those areas to determine what can be modified,” she said. “All of that’s done even before we get to having the orthopedic involvement.”
Dr. Smith said she also considers things beyond basic medicine: What is the patient’s mental status and tolerance for pain? Do they have a support system at home for post-operative care? And can they afford to miss work?
“We look at all of those factors together because that is going to determine the outcome that we’re hoping to achieve,” Dr. Smith said.
Great expectations
A recent study shows that older patients respond better to knee replacements than younger patients, particularly with pain relief and quality of life. The reason for this is believed to boil down to expectations. Whereas a younger person may want to return to the racquetball court and perform like they used to, older patients may just wish to walk down the hall without discomfort.
“It’s possible that these under 55-age-old patients may just take a little longer to heal to be satisfied,” Dr. Wang said. “We really can’t speak to why this is happening, but it’s possible that the younger patients are more active, and they expect more out of their knee.”
Jeevan Sall, MD, is a primary care sports medicine doctor with Providence Mission Heritage Medical Group in Laguna Niguel, Calif. He first discusses conservative management for patients struggling with arthritis in their joints. These measures include rehabilitation exercises, braces, shoe inserts, medication, and weight loss efforts. If these steps don’t improve a patient’s pain or lifestyle, surgery is on the table. Managing expectations is a significant factor.
“Is the patient mentally ready for surgery?” Dr. Sall said. “This includes what they hope to achieve with surgery as well as the risk and benefits of the procedure.”
Ms. Blackwell’s hip and knee pain came simply from a life well lived, with no marathon running or life-changing accident to speak of. She worked as a housewife raising her two children and owned an elevator company with her late husband, Robert Blackwell.
Yes, the elevator construction business has jokes.
“We have our ups and downs,” Ms. Blackwell said.
And with her new joints, so does she.
A version of this article first appeared on WebMD.com.
Progesterone might benefit women in perimenopause
In a randomized, placebo-controlled trial of about 180 women with vasomotor symptoms (VMS), women who received progesterone perceived a significantly greater decrease in night sweats (P = .023) and improved sleep quality (P = .005), compared with controls. VMS score did not differ significantly by treatment group, however.
“Women who have menstruated within the last year, who are waking twice or more times a week with night sweats and bothered by sleep disturbances would benefit from taking oral micronized progesterone 300 mg at bedtime,” principal investigator Jerilynn C. Prior, MD, professor of endocrinology at the University of British Columbia in Vancouver, British Columbia, Canada, said in an interview.
The study was published online in Scientific Reports.
A neglected group?
The best management for symptoms in perimenopause is an often-neglected topic of research, said Dr. Prior. Yet perimenopause is often associated with significant symptoms for women, including heavy menstrual bleeding, sore breasts, mood swings, night sweats, and insomnia – all when many women are at the peak of their careers.
Dr. Prior herself had a difficult perimenopause. “I began having cyclic night sweats, clustered around flow, when I was still having regular menstrual cycles, plus breast tenderness and sleep problems,” she said. “I knew from my research and my own experience that my estrogen levels were very high. Higher estrogen levels are not suppressible by exogenous estrogen, so it made no sense to me to ask my family doctor for a prescription for estrogen – or hormone replacement therapy, as it was then called. However, medroxyprogesterone acetate had been reported to be effective for menopausal hot flushes. I tried it, and it helped my night sweats and hot flushes but not my sleep. When oral micronized progesterone became available, I switched to that.”
In the current study, which was performed at the UBC Centre for Menstrual Cycle and Ovulation Research, the investigators studied 189 community-dwelling women from across Canada who were aged 35-58 years, had menstruated in the past year, and were bothered by daytime flushes or night sweats at least twice per week.
Participants were randomly assigned to receive either 300 mg of oral micronized progesterone or placebo at bedtime for 3 months. They recorded VMS number and intensity while awake and asleep each day. Some women participated remotely by web conference, telephone, or email. The experimental medicine was delivered to these participants by courier. The primary outcome was VMS score during the 3rd month.
Most (87%) participants were White, and about 57% had a college degree. The population’s average body mass index was 26.7, and 66.7% of participants were in late perimenopause.
The mean baseline VMS score among the women was 12.2. The average frequency of VMS per 24-hour day was 4.9. Average VMS intensity was 2.3 on a scale of 0-4. VMS scores decreased over time in both treatment groups.
At month 3, the VMS score was 5.5 in the progesterone group and 7.1 in the placebo group. The difference between groups was not statistically significant.
Compared with controls, however, women in the progesterone group perceived a significantly greater decrease in night sweats and improved sleep quality. Progesterone also was associated with significantly decreased perception of physical and emotional interference with their daily activities, compared with placebo (P = .017). Moreover, progesterone did not increase depression.
There were no serious adverse events.
“I hope that when women who look young and are still menstruating in their late 30s to early 50s go to the doctor and ask for help with night sweats and sleep problems, they will be told about this trial and offered progesterone therapy. I also hope they won’t be told, ‘You are too young,’ or ‘You are not in menopause,’ with the inference that the issue is all in their minds,” said Dr. Prior.
Useful dosing information
Mitchell S. Kramer, MD, chair of obstetrics and gynecology at Huntington (N.Y.) Hospital Northwell Health, said in a comment that “progesterone has been used for quite a while. I’ve been treating menopausal and perimenopausal hormonal disturbances and VMS for many years, and progesterone has been a real staple of treatment for these symptoms, especially in perimenopausal patients who are not good candidates for estrogen or who won’t accept treatment with estrogen. It’s actually nice to see a study that addresses this issue in a randomized controlled fashion and that confirms the efficacy of progesterone.”
The most helpful aspect of the study is the dosing information, Dr. Kramer added. “They recommend a 300-mg dose of oral micronized progesterone, which is much higher than I normally use. I may start to prescribe the higher dose and perhaps get a better or more complete response. There were no adverse events reported in this study, so the higher dose was enlightening to me,” he said.
Perimenopause is a time that is challenging to manage, said Michelle Jacobson, MD, of the department of obstetrics and gynecology at the University of Toronto, and obstetrician-gynecologist at Women’s College and Mount Sinai Hospitals in Toronto.
“There are so many nuances to the management. Women are suffering oftentimes from classic menopausal symptoms. There are fluctuating levels of estrogen, sometimes high. Sometimes there are complications of bleeding. There is the potential need for contraception because they are still menstruating,” she said in an interview.
“It’s important to specifically study this group of women with their own unique needs. Dr. Prior is a longtime proponent of using progesterone therapy, and kudos to her for doing this study in perimenopausal women, which is a group that is probably underrepresented in the menopause management literature,” she said.
Dr. Prior and Dr. Kramer reported no relevant financial relationships. Dr. Jacobson reported financial relationships with Astellas, AbbVie, Bayer, BioSyent, Duchesnay, Eisai, Lupin, Organon, Pfizer, and Searchlight.
A version of this article first appeared on Medscape.com.
In a randomized, placebo-controlled trial of about 180 women with vasomotor symptoms (VMS), women who received progesterone perceived a significantly greater decrease in night sweats (P = .023) and improved sleep quality (P = .005), compared with controls. VMS score did not differ significantly by treatment group, however.
“Women who have menstruated within the last year, who are waking twice or more times a week with night sweats and bothered by sleep disturbances would benefit from taking oral micronized progesterone 300 mg at bedtime,” principal investigator Jerilynn C. Prior, MD, professor of endocrinology at the University of British Columbia in Vancouver, British Columbia, Canada, said in an interview.
The study was published online in Scientific Reports.
A neglected group?
The best management for symptoms in perimenopause is an often-neglected topic of research, said Dr. Prior. Yet perimenopause is often associated with significant symptoms for women, including heavy menstrual bleeding, sore breasts, mood swings, night sweats, and insomnia – all when many women are at the peak of their careers.
Dr. Prior herself had a difficult perimenopause. “I began having cyclic night sweats, clustered around flow, when I was still having regular menstrual cycles, plus breast tenderness and sleep problems,” she said. “I knew from my research and my own experience that my estrogen levels were very high. Higher estrogen levels are not suppressible by exogenous estrogen, so it made no sense to me to ask my family doctor for a prescription for estrogen – or hormone replacement therapy, as it was then called. However, medroxyprogesterone acetate had been reported to be effective for menopausal hot flushes. I tried it, and it helped my night sweats and hot flushes but not my sleep. When oral micronized progesterone became available, I switched to that.”
In the current study, which was performed at the UBC Centre for Menstrual Cycle and Ovulation Research, the investigators studied 189 community-dwelling women from across Canada who were aged 35-58 years, had menstruated in the past year, and were bothered by daytime flushes or night sweats at least twice per week.
Participants were randomly assigned to receive either 300 mg of oral micronized progesterone or placebo at bedtime for 3 months. They recorded VMS number and intensity while awake and asleep each day. Some women participated remotely by web conference, telephone, or email. The experimental medicine was delivered to these participants by courier. The primary outcome was VMS score during the 3rd month.
Most (87%) participants were White, and about 57% had a college degree. The population’s average body mass index was 26.7, and 66.7% of participants were in late perimenopause.
The mean baseline VMS score among the women was 12.2. The average frequency of VMS per 24-hour day was 4.9. Average VMS intensity was 2.3 on a scale of 0-4. VMS scores decreased over time in both treatment groups.
At month 3, the VMS score was 5.5 in the progesterone group and 7.1 in the placebo group. The difference between groups was not statistically significant.
Compared with controls, however, women in the progesterone group perceived a significantly greater decrease in night sweats and improved sleep quality. Progesterone also was associated with significantly decreased perception of physical and emotional interference with their daily activities, compared with placebo (P = .017). Moreover, progesterone did not increase depression.
There were no serious adverse events.
“I hope that when women who look young and are still menstruating in their late 30s to early 50s go to the doctor and ask for help with night sweats and sleep problems, they will be told about this trial and offered progesterone therapy. I also hope they won’t be told, ‘You are too young,’ or ‘You are not in menopause,’ with the inference that the issue is all in their minds,” said Dr. Prior.
Useful dosing information
Mitchell S. Kramer, MD, chair of obstetrics and gynecology at Huntington (N.Y.) Hospital Northwell Health, said in a comment that “progesterone has been used for quite a while. I’ve been treating menopausal and perimenopausal hormonal disturbances and VMS for many years, and progesterone has been a real staple of treatment for these symptoms, especially in perimenopausal patients who are not good candidates for estrogen or who won’t accept treatment with estrogen. It’s actually nice to see a study that addresses this issue in a randomized controlled fashion and that confirms the efficacy of progesterone.”
The most helpful aspect of the study is the dosing information, Dr. Kramer added. “They recommend a 300-mg dose of oral micronized progesterone, which is much higher than I normally use. I may start to prescribe the higher dose and perhaps get a better or more complete response. There were no adverse events reported in this study, so the higher dose was enlightening to me,” he said.
Perimenopause is a time that is challenging to manage, said Michelle Jacobson, MD, of the department of obstetrics and gynecology at the University of Toronto, and obstetrician-gynecologist at Women’s College and Mount Sinai Hospitals in Toronto.
“There are so many nuances to the management. Women are suffering oftentimes from classic menopausal symptoms. There are fluctuating levels of estrogen, sometimes high. Sometimes there are complications of bleeding. There is the potential need for contraception because they are still menstruating,” she said in an interview.
“It’s important to specifically study this group of women with their own unique needs. Dr. Prior is a longtime proponent of using progesterone therapy, and kudos to her for doing this study in perimenopausal women, which is a group that is probably underrepresented in the menopause management literature,” she said.
Dr. Prior and Dr. Kramer reported no relevant financial relationships. Dr. Jacobson reported financial relationships with Astellas, AbbVie, Bayer, BioSyent, Duchesnay, Eisai, Lupin, Organon, Pfizer, and Searchlight.
A version of this article first appeared on Medscape.com.
In a randomized, placebo-controlled trial of about 180 women with vasomotor symptoms (VMS), women who received progesterone perceived a significantly greater decrease in night sweats (P = .023) and improved sleep quality (P = .005), compared with controls. VMS score did not differ significantly by treatment group, however.
“Women who have menstruated within the last year, who are waking twice or more times a week with night sweats and bothered by sleep disturbances would benefit from taking oral micronized progesterone 300 mg at bedtime,” principal investigator Jerilynn C. Prior, MD, professor of endocrinology at the University of British Columbia in Vancouver, British Columbia, Canada, said in an interview.
The study was published online in Scientific Reports.
A neglected group?
The best management for symptoms in perimenopause is an often-neglected topic of research, said Dr. Prior. Yet perimenopause is often associated with significant symptoms for women, including heavy menstrual bleeding, sore breasts, mood swings, night sweats, and insomnia – all when many women are at the peak of their careers.
Dr. Prior herself had a difficult perimenopause. “I began having cyclic night sweats, clustered around flow, when I was still having regular menstrual cycles, plus breast tenderness and sleep problems,” she said. “I knew from my research and my own experience that my estrogen levels were very high. Higher estrogen levels are not suppressible by exogenous estrogen, so it made no sense to me to ask my family doctor for a prescription for estrogen – or hormone replacement therapy, as it was then called. However, medroxyprogesterone acetate had been reported to be effective for menopausal hot flushes. I tried it, and it helped my night sweats and hot flushes but not my sleep. When oral micronized progesterone became available, I switched to that.”
In the current study, which was performed at the UBC Centre for Menstrual Cycle and Ovulation Research, the investigators studied 189 community-dwelling women from across Canada who were aged 35-58 years, had menstruated in the past year, and were bothered by daytime flushes or night sweats at least twice per week.
Participants were randomly assigned to receive either 300 mg of oral micronized progesterone or placebo at bedtime for 3 months. They recorded VMS number and intensity while awake and asleep each day. Some women participated remotely by web conference, telephone, or email. The experimental medicine was delivered to these participants by courier. The primary outcome was VMS score during the 3rd month.
Most (87%) participants were White, and about 57% had a college degree. The population’s average body mass index was 26.7, and 66.7% of participants were in late perimenopause.
The mean baseline VMS score among the women was 12.2. The average frequency of VMS per 24-hour day was 4.9. Average VMS intensity was 2.3 on a scale of 0-4. VMS scores decreased over time in both treatment groups.
At month 3, the VMS score was 5.5 in the progesterone group and 7.1 in the placebo group. The difference between groups was not statistically significant.
Compared with controls, however, women in the progesterone group perceived a significantly greater decrease in night sweats and improved sleep quality. Progesterone also was associated with significantly decreased perception of physical and emotional interference with their daily activities, compared with placebo (P = .017). Moreover, progesterone did not increase depression.
There were no serious adverse events.
“I hope that when women who look young and are still menstruating in their late 30s to early 50s go to the doctor and ask for help with night sweats and sleep problems, they will be told about this trial and offered progesterone therapy. I also hope they won’t be told, ‘You are too young,’ or ‘You are not in menopause,’ with the inference that the issue is all in their minds,” said Dr. Prior.
Useful dosing information
Mitchell S. Kramer, MD, chair of obstetrics and gynecology at Huntington (N.Y.) Hospital Northwell Health, said in a comment that “progesterone has been used for quite a while. I’ve been treating menopausal and perimenopausal hormonal disturbances and VMS for many years, and progesterone has been a real staple of treatment for these symptoms, especially in perimenopausal patients who are not good candidates for estrogen or who won’t accept treatment with estrogen. It’s actually nice to see a study that addresses this issue in a randomized controlled fashion and that confirms the efficacy of progesterone.”
The most helpful aspect of the study is the dosing information, Dr. Kramer added. “They recommend a 300-mg dose of oral micronized progesterone, which is much higher than I normally use. I may start to prescribe the higher dose and perhaps get a better or more complete response. There were no adverse events reported in this study, so the higher dose was enlightening to me,” he said.
Perimenopause is a time that is challenging to manage, said Michelle Jacobson, MD, of the department of obstetrics and gynecology at the University of Toronto, and obstetrician-gynecologist at Women’s College and Mount Sinai Hospitals in Toronto.
“There are so many nuances to the management. Women are suffering oftentimes from classic menopausal symptoms. There are fluctuating levels of estrogen, sometimes high. Sometimes there are complications of bleeding. There is the potential need for contraception because they are still menstruating,” she said in an interview.
“It’s important to specifically study this group of women with their own unique needs. Dr. Prior is a longtime proponent of using progesterone therapy, and kudos to her for doing this study in perimenopausal women, which is a group that is probably underrepresented in the menopause management literature,” she said.
Dr. Prior and Dr. Kramer reported no relevant financial relationships. Dr. Jacobson reported financial relationships with Astellas, AbbVie, Bayer, BioSyent, Duchesnay, Eisai, Lupin, Organon, Pfizer, and Searchlight.
A version of this article first appeared on Medscape.com.
FROM SCIENTIFIC REPORTS
Forgetfulness and confusion
The history and findings in this case are suggestive of late-onset familial AD (onset after age 65 years).
AD is a common neurodegenerative disease associated with progressive impairment of behavioral and cognitive functions, including memory, comprehension, language, attention, reasoning, and judgment. In 2020, 5.8 million Americans were living with AD. By 2050, this number is projected to increase to 13.9 million people, or almost 3.3% of the US population. Globally, 152 million people are projected to have AD and other dementias by 2050. The worldwide increase in incidence and prevalence of AD is at least partially explained by an aging population and increased life expectancy.
The cause of AD remains unclear, but there is substantial evidence that AD is a highly heritable disorder. Familial AD is characterized by having more than one member in more than one generation with AD. The autosomal-dominant form of AD is linked to mutations in three genes: AAP on chromosome 21, PSEN1 on chromosome 14, and PSEN2 on chromosome 1. APP mutations may cause increased generation and aggregation of beta-amyloid peptide, whereas PSEN1 and PSEN2 mutations result in aggregation of beta-amyloid by interfering with the processing of gamma-secretase.
APOE is another genetic marker that increases the risk for AD. Isoform e4 of the APOE gene (located on chromosome 19) has been associated with more sporadic and familial forms of AD that present after age 65 years. Approximately 50% of individuals carrying one APOEe4 develop AD, and 90% of individuals who have two alleles develop AD. Variants in the gene for the sortilin receptor, SORT1, have also been found in familial and sporadic forms of AD.
The cognitive and behavioral impairment associated with AD significantly affects a patient's social and occupational functioning. Insidiously progressive memory loss is a characteristic symptoms seen in patients presenting with AD. As the disease advances over the course of several years, other areas of cognition are impaired. Patients may develop language disorders (eg, anomic aphasia or anomia) and impairment in visuospatial skills and executive functions. A slow progression of behavioral changes may also occur in individuals with AD.
Clinical criteria for the diagnosis of AD (eg, insidious onset of cognitive impairment, clear history of worsening symptoms) have been developed and are often used to diagnose patients. In addition, biomarker evidence may help to increase the diagnostic certainty. Several cerebrospinal fluid and blood biomarkers have shown excellent diagnostic ability by identifying tau pathology and cerebral amyloid-beta for AD.
Neuroimaging is becoming increasingly important for identifying the underlying causes of cognitive impairment. Currently, MRI is considered the preferred neuroimaging modality for AD because it allows for accurate measurement of the three-dimensional volume of brain structures, particularly the size of the hippocampus and related regions. CT can be used when MRI is not available or is contraindicated, such as in a patient with a pacemaker. PET is another noninvasive method for depicting tau pathology deposition and distribution in patients with cognitive impairment. In 2020, US Food and Drug Administration approved the first tau PET tracer, 18F-flortaucipir, which marked a significant achievement to improve AD diagnosis.
At present, the only therapies available for AD are symptomatic therapies. Cholinesterase inhibitors and a partial N-methyl-D-aspartate antagonist are the standard medical treatments for AD. Antiamyloid therapies are also available for patients with mild cognitive impairment or mild dementia. These include aducanumab, a first-in-class amyloid-beta–directed antibody that was approved in 2021, and lecanemab, another amyloid-beta–directed antibody that was approved in 2023. Both aducanumab and lecanemab are recommended for the treatment of patients with mild cognitive impairment or mild dementia stage of disease, the population in which the safety and efficacy of these newer agents were demonstrated in clinical trials.
Secondary symptoms of AD, such as depression, agitation, aggression, hallucinations, delusions, and/or sleep disorders, can be treated with psychotropic agents. Behavioral interventions including patient-centered approaches and caregiver training can also be helpful for managing the cognitive and behavioral manifestations of AD, often in combination with pharmacologic interventions (eg, anxiolytics for anxiety and agitation, neuroleptics for delusions or hallucinations, and antidepressants or mood stabilizers for mood disorders). Regular physical activity and exercise may also play a role in delaying AD progression and possibly conferring a protective effect on brain health.
Jasvinder Chawla, MD, Professor of Neurology, Loyola University Medical Center, Maywood; Director, Clinical Neurophysiology Lab, Department of Neurology, Hines VA Hospital, Hines, IL.
Jasvinder Chawla, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are suggestive of late-onset familial AD (onset after age 65 years).
AD is a common neurodegenerative disease associated with progressive impairment of behavioral and cognitive functions, including memory, comprehension, language, attention, reasoning, and judgment. In 2020, 5.8 million Americans were living with AD. By 2050, this number is projected to increase to 13.9 million people, or almost 3.3% of the US population. Globally, 152 million people are projected to have AD and other dementias by 2050. The worldwide increase in incidence and prevalence of AD is at least partially explained by an aging population and increased life expectancy.
The cause of AD remains unclear, but there is substantial evidence that AD is a highly heritable disorder. Familial AD is characterized by having more than one member in more than one generation with AD. The autosomal-dominant form of AD is linked to mutations in three genes: AAP on chromosome 21, PSEN1 on chromosome 14, and PSEN2 on chromosome 1. APP mutations may cause increased generation and aggregation of beta-amyloid peptide, whereas PSEN1 and PSEN2 mutations result in aggregation of beta-amyloid by interfering with the processing of gamma-secretase.
APOE is another genetic marker that increases the risk for AD. Isoform e4 of the APOE gene (located on chromosome 19) has been associated with more sporadic and familial forms of AD that present after age 65 years. Approximately 50% of individuals carrying one APOEe4 develop AD, and 90% of individuals who have two alleles develop AD. Variants in the gene for the sortilin receptor, SORT1, have also been found in familial and sporadic forms of AD.
The cognitive and behavioral impairment associated with AD significantly affects a patient's social and occupational functioning. Insidiously progressive memory loss is a characteristic symptoms seen in patients presenting with AD. As the disease advances over the course of several years, other areas of cognition are impaired. Patients may develop language disorders (eg, anomic aphasia or anomia) and impairment in visuospatial skills and executive functions. A slow progression of behavioral changes may also occur in individuals with AD.
Clinical criteria for the diagnosis of AD (eg, insidious onset of cognitive impairment, clear history of worsening symptoms) have been developed and are often used to diagnose patients. In addition, biomarker evidence may help to increase the diagnostic certainty. Several cerebrospinal fluid and blood biomarkers have shown excellent diagnostic ability by identifying tau pathology and cerebral amyloid-beta for AD.
Neuroimaging is becoming increasingly important for identifying the underlying causes of cognitive impairment. Currently, MRI is considered the preferred neuroimaging modality for AD because it allows for accurate measurement of the three-dimensional volume of brain structures, particularly the size of the hippocampus and related regions. CT can be used when MRI is not available or is contraindicated, such as in a patient with a pacemaker. PET is another noninvasive method for depicting tau pathology deposition and distribution in patients with cognitive impairment. In 2020, US Food and Drug Administration approved the first tau PET tracer, 18F-flortaucipir, which marked a significant achievement to improve AD diagnosis.
At present, the only therapies available for AD are symptomatic therapies. Cholinesterase inhibitors and a partial N-methyl-D-aspartate antagonist are the standard medical treatments for AD. Antiamyloid therapies are also available for patients with mild cognitive impairment or mild dementia. These include aducanumab, a first-in-class amyloid-beta–directed antibody that was approved in 2021, and lecanemab, another amyloid-beta–directed antibody that was approved in 2023. Both aducanumab and lecanemab are recommended for the treatment of patients with mild cognitive impairment or mild dementia stage of disease, the population in which the safety and efficacy of these newer agents were demonstrated in clinical trials.
Secondary symptoms of AD, such as depression, agitation, aggression, hallucinations, delusions, and/or sleep disorders, can be treated with psychotropic agents. Behavioral interventions including patient-centered approaches and caregiver training can also be helpful for managing the cognitive and behavioral manifestations of AD, often in combination with pharmacologic interventions (eg, anxiolytics for anxiety and agitation, neuroleptics for delusions or hallucinations, and antidepressants or mood stabilizers for mood disorders). Regular physical activity and exercise may also play a role in delaying AD progression and possibly conferring a protective effect on brain health.
Jasvinder Chawla, MD, Professor of Neurology, Loyola University Medical Center, Maywood; Director, Clinical Neurophysiology Lab, Department of Neurology, Hines VA Hospital, Hines, IL.
Jasvinder Chawla, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are suggestive of late-onset familial AD (onset after age 65 years).
AD is a common neurodegenerative disease associated with progressive impairment of behavioral and cognitive functions, including memory, comprehension, language, attention, reasoning, and judgment. In 2020, 5.8 million Americans were living with AD. By 2050, this number is projected to increase to 13.9 million people, or almost 3.3% of the US population. Globally, 152 million people are projected to have AD and other dementias by 2050. The worldwide increase in incidence and prevalence of AD is at least partially explained by an aging population and increased life expectancy.
The cause of AD remains unclear, but there is substantial evidence that AD is a highly heritable disorder. Familial AD is characterized by having more than one member in more than one generation with AD. The autosomal-dominant form of AD is linked to mutations in three genes: AAP on chromosome 21, PSEN1 on chromosome 14, and PSEN2 on chromosome 1. APP mutations may cause increased generation and aggregation of beta-amyloid peptide, whereas PSEN1 and PSEN2 mutations result in aggregation of beta-amyloid by interfering with the processing of gamma-secretase.
APOE is another genetic marker that increases the risk for AD. Isoform e4 of the APOE gene (located on chromosome 19) has been associated with more sporadic and familial forms of AD that present after age 65 years. Approximately 50% of individuals carrying one APOEe4 develop AD, and 90% of individuals who have two alleles develop AD. Variants in the gene for the sortilin receptor, SORT1, have also been found in familial and sporadic forms of AD.
The cognitive and behavioral impairment associated with AD significantly affects a patient's social and occupational functioning. Insidiously progressive memory loss is a characteristic symptoms seen in patients presenting with AD. As the disease advances over the course of several years, other areas of cognition are impaired. Patients may develop language disorders (eg, anomic aphasia or anomia) and impairment in visuospatial skills and executive functions. A slow progression of behavioral changes may also occur in individuals with AD.
Clinical criteria for the diagnosis of AD (eg, insidious onset of cognitive impairment, clear history of worsening symptoms) have been developed and are often used to diagnose patients. In addition, biomarker evidence may help to increase the diagnostic certainty. Several cerebrospinal fluid and blood biomarkers have shown excellent diagnostic ability by identifying tau pathology and cerebral amyloid-beta for AD.
Neuroimaging is becoming increasingly important for identifying the underlying causes of cognitive impairment. Currently, MRI is considered the preferred neuroimaging modality for AD because it allows for accurate measurement of the three-dimensional volume of brain structures, particularly the size of the hippocampus and related regions. CT can be used when MRI is not available or is contraindicated, such as in a patient with a pacemaker. PET is another noninvasive method for depicting tau pathology deposition and distribution in patients with cognitive impairment. In 2020, US Food and Drug Administration approved the first tau PET tracer, 18F-flortaucipir, which marked a significant achievement to improve AD diagnosis.
At present, the only therapies available for AD are symptomatic therapies. Cholinesterase inhibitors and a partial N-methyl-D-aspartate antagonist are the standard medical treatments for AD. Antiamyloid therapies are also available for patients with mild cognitive impairment or mild dementia. These include aducanumab, a first-in-class amyloid-beta–directed antibody that was approved in 2021, and lecanemab, another amyloid-beta–directed antibody that was approved in 2023. Both aducanumab and lecanemab are recommended for the treatment of patients with mild cognitive impairment or mild dementia stage of disease, the population in which the safety and efficacy of these newer agents were demonstrated in clinical trials.
Secondary symptoms of AD, such as depression, agitation, aggression, hallucinations, delusions, and/or sleep disorders, can be treated with psychotropic agents. Behavioral interventions including patient-centered approaches and caregiver training can also be helpful for managing the cognitive and behavioral manifestations of AD, often in combination with pharmacologic interventions (eg, anxiolytics for anxiety and agitation, neuroleptics for delusions or hallucinations, and antidepressants or mood stabilizers for mood disorders). Regular physical activity and exercise may also play a role in delaying AD progression and possibly conferring a protective effect on brain health.
Jasvinder Chawla, MD, Professor of Neurology, Loyola University Medical Center, Maywood; Director, Clinical Neurophysiology Lab, Department of Neurology, Hines VA Hospital, Hines, IL.
Jasvinder Chawla, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 72-year-old woman presents with a 12-month history of short-term memory loss. The patient is accompanied by her husband, who states her symptoms have become increasingly frequent and severe. The patient can no longer drive familiar routes after becoming lost on several occasions. She frequently misplaces items; recently, she placed her husband's car keys in the refrigerator. The patient admits to increasing bouts of forgetfulness and confusion and states that she has been feeling very down. She has not been able to watch her grandchildren over the past few months, which makes her feel sad and old. She also reports trouble sleeping at night due to generalized anxiety.
The patient's past medical history is significant for hypertension and dyslipidemia. There is no history of neurotoxic exposure, head injuries, strokes, or seizures. Her family history is positive for dementia. Her older brother was diagnosed with Alzheimer's disease (AD) at age 68 years, and her mother died from AD at age 82 years. Current medications include rosuvastatin 20 mg/d and lisinopril 20 mg/d. The patient's current height and weight are 5 ft 5 in and 163 lb, respectively (BMI is 27.1).
No abnormalities are noted on physical examination; the patient's blood pressure, pulse oximetry, and heart rate are within normal ranges. Laboratory tests are within normal ranges. The patient scores 18 on the Montreal Cognitive Assessment test. The patient's clinician orders a brain fluorodeoxyglucose-PET, which reveals areas of decreased glucose metabolism involving the posterior cingulate cortex, precuneus, inferior parietal lobule, and middle temporal gyrus.