User login
New global initiative aims to reform cancer trials and care
After 15 years of researching what works well in oncology – and where the field has gone awry – Christopher Booth, MD, had a career moment.
“As I approached mid-career, I realized publishing and describing problems wasn’t fulfilling. It wasn’t doing enough,” recalled Dr. Booth, an oncologist and professor at Queen’s University, Kingston, Ont. “I wanted to change mindsets and change systems so that things actually improved for the better for patients.”
His colleague, Bishal Gyawali, MD, PhD, described a similar epiphany. As a trainee, he noticed that the real-world effects of some so-called blockbuster cancer drugs too often failed to measure up to the hype.
“I realized we were lacking common sense in oncology,” said Dr. Gyawali, a medical oncologist and assistant professor at Queen’s University.
In 2019, Dr. Gyawali launched a Medscape column addressing what he considers to be that lack of common sense, and in 2022, he and Dr. Booth published a similarly titled opinion piece in Nature Medicine. The core idea: The cancer community needs to prioritize cancer treatments that benefit patients, treatments that meaningfully improve survival and quality of life.
Aaron Goodman, MD, a hematologist and associate professor at UC San Diego Health, was on the same page. He’d been interested in the evidence-based medicine movement since his time as a hematology fellow when that movement was “a bit of a counterculture,” he explained.
Dr. Goodman and Dr. Booth connected through their common interests and collaborated on a 2021 paper exploring the discomfort clinicians might feel when a patient’s needs fall on the “edge of oncology”: that is, when the guideline-recommended standard of care offers marginal benefit, at best, and could, at worst, cause patient harm.
“We said, ‘Now is the time to make change,’ ” he recalled. It was time to stop talking and do something.
Common sense and a common purpose
Dr. Booth, Dr. Gyawali, and Dr. Goodman joined forces and, with the backing of a philanthropist who had experience as a patient with cancer, convened an organizing committee of more than 30 like-minded oncologists and patient advocates from across the globe.
The group convened for a 3-day “meeting of the minds” in Kingston in April and laid out their intentions in a position paper published online in The Lancet Oncology.
In their paper, the committee outline the vision for Common Sense Oncology. The mission: prioritize patient-centered and equitable care by focusing on treatments that improve survival and quality of life, communication that promotes informed decision-making, and systems that ensure access to all patients.
However, increasingly, the cancer community faces a “troubling paradox,” the team wrote in The Lancet. In some instance, treatments that bring minimal benefit are overused while those that can make a meaningful difference in patients’ lives are not accessible to most worldwide.
One reason for this shift: Commercial interests, rather than patient interests, appear to be driving cancer research and care. The team explained, for instance, that over the past few decades, clinical trials have largely pivoted from publicly funded efforts to industry funded ones “designed to achieve regulatory approval or commercial advantage, [often] at the expense of investigating new approaches to surgery, radiotherapy, palliative care, and prevention.”
But “patients deserve better,” the group wrote.
The team outlined three pillars for the initiative: evidence generation, evidence interpretation, and evidence communication.
The evidence generation pillar will aim to improve trial design and reporting to prioritize outcomes that matter to patients.
“One concern is that over the last 10 years or so, most of our new treatments have had very, very small benefits, and we think the bar has dropped too low,” Dr. Booth said, explaining that many trials have moved away from focusing on improving survival and quality of life and toward detecting small differences between treatments on other endpoints – namely progression-free survival. “Those small benefits need to be balanced against the very real risks to our patients.”
The evidence interpretation pillar will aim to foster critical thinking so that clinicians can better identify poorly designed or reported trials and help patients make more informed decisions.
Lastly, the evidence communication pillar will focus on fostering better communication about treatment options among patients, the public, and policymakers. Without clear and thoughtful communication, patients may have unrealistic expectations about the effectiveness of treatments that offer only marginal clinical benefits.
The team also emphasized a need to focus on improving global equity and access to affordable treatments so all patients can benefit from care that extends survival or quality of life.
It’s an ambitious undertaking, especially for a group of full-time clinicians, researchers, and patient advocates “volunteering their time for societal good,” said Dr. Gyawali, but the project teams intend to hit the ground running.
The team has established short-term targets, such as identifying deficiencies in data interpretation within education programs within 6 months and developing educational materials that begin to correct those deficiencies within 12 months, Dr. Booth explained. In the longer term, the team will also aim to design clinical trials that focus on patient outcomes, such as overall survival and quality of life.
Breast cancer survivor and patient advocate Michelle Tregear, PhD, who was recruited to help with Common Sense Oncology, also hopes the initiative will lead to better regulatory control that requires trial sponsors to “focus on what matters to patients, not on surrogate endpoints.”
When it comes to clinical trials, “more, more, more is not always better,” said Dr. Tregear, director of Education and Training Programs for patient advocates at the National Breast Cancer Coalition, Washington, D.C. “Industry interests are not always aligned with patient interests,” and “the system, by and large, is not addressing questions that really matter to patients and their families.”
Although “it’s a tall order to change the direction that we’re going in,” Dr. Tregear is up to the challenge of helping raise awareness, which will hopefully spur patients to demand change.
When Dr. Goodman announced the Common Sense Oncology initiative on Twitter, the news brought excitement, with many oncologists asking to join.
With its sweeping, ambitious goals, the Common Sense Oncology initiative has a long road ahead. Figuring out how to implement some of its aims in practice will take time, Dr. Booth acknowledges, and the initial launch marks the first steps, which will continue to evolve over time.
“We’re not proposing we have all the answers or that we know what every patient would want – we’re saying we’ve not done a good job of communicating to patients the relative benefits and risks of different treatments,” Dr. Booth explained. “We want to celebrate and promote what helps and speak out about what’s not in the best interest of patients.”
Dr. Goodman reported consulting fees from Seattle Genetics and speaking honoraria from Curio. Dr. Booth, Dr. Gyawali, and Dr. Tregear reported having no financial conflicts of interest.
A version of this article appeared on Medscape.com.
After 15 years of researching what works well in oncology – and where the field has gone awry – Christopher Booth, MD, had a career moment.
“As I approached mid-career, I realized publishing and describing problems wasn’t fulfilling. It wasn’t doing enough,” recalled Dr. Booth, an oncologist and professor at Queen’s University, Kingston, Ont. “I wanted to change mindsets and change systems so that things actually improved for the better for patients.”
His colleague, Bishal Gyawali, MD, PhD, described a similar epiphany. As a trainee, he noticed that the real-world effects of some so-called blockbuster cancer drugs too often failed to measure up to the hype.
“I realized we were lacking common sense in oncology,” said Dr. Gyawali, a medical oncologist and assistant professor at Queen’s University.
In 2019, Dr. Gyawali launched a Medscape column addressing what he considers to be that lack of common sense, and in 2022, he and Dr. Booth published a similarly titled opinion piece in Nature Medicine. The core idea: The cancer community needs to prioritize cancer treatments that benefit patients, treatments that meaningfully improve survival and quality of life.
Aaron Goodman, MD, a hematologist and associate professor at UC San Diego Health, was on the same page. He’d been interested in the evidence-based medicine movement since his time as a hematology fellow when that movement was “a bit of a counterculture,” he explained.
Dr. Goodman and Dr. Booth connected through their common interests and collaborated on a 2021 paper exploring the discomfort clinicians might feel when a patient’s needs fall on the “edge of oncology”: that is, when the guideline-recommended standard of care offers marginal benefit, at best, and could, at worst, cause patient harm.
“We said, ‘Now is the time to make change,’ ” he recalled. It was time to stop talking and do something.
Common sense and a common purpose
Dr. Booth, Dr. Gyawali, and Dr. Goodman joined forces and, with the backing of a philanthropist who had experience as a patient with cancer, convened an organizing committee of more than 30 like-minded oncologists and patient advocates from across the globe.
The group convened for a 3-day “meeting of the minds” in Kingston in April and laid out their intentions in a position paper published online in The Lancet Oncology.
In their paper, the committee outline the vision for Common Sense Oncology. The mission: prioritize patient-centered and equitable care by focusing on treatments that improve survival and quality of life, communication that promotes informed decision-making, and systems that ensure access to all patients.
However, increasingly, the cancer community faces a “troubling paradox,” the team wrote in The Lancet. In some instance, treatments that bring minimal benefit are overused while those that can make a meaningful difference in patients’ lives are not accessible to most worldwide.
One reason for this shift: Commercial interests, rather than patient interests, appear to be driving cancer research and care. The team explained, for instance, that over the past few decades, clinical trials have largely pivoted from publicly funded efforts to industry funded ones “designed to achieve regulatory approval or commercial advantage, [often] at the expense of investigating new approaches to surgery, radiotherapy, palliative care, and prevention.”
But “patients deserve better,” the group wrote.
The team outlined three pillars for the initiative: evidence generation, evidence interpretation, and evidence communication.
The evidence generation pillar will aim to improve trial design and reporting to prioritize outcomes that matter to patients.
“One concern is that over the last 10 years or so, most of our new treatments have had very, very small benefits, and we think the bar has dropped too low,” Dr. Booth said, explaining that many trials have moved away from focusing on improving survival and quality of life and toward detecting small differences between treatments on other endpoints – namely progression-free survival. “Those small benefits need to be balanced against the very real risks to our patients.”
The evidence interpretation pillar will aim to foster critical thinking so that clinicians can better identify poorly designed or reported trials and help patients make more informed decisions.
Lastly, the evidence communication pillar will focus on fostering better communication about treatment options among patients, the public, and policymakers. Without clear and thoughtful communication, patients may have unrealistic expectations about the effectiveness of treatments that offer only marginal clinical benefits.
The team also emphasized a need to focus on improving global equity and access to affordable treatments so all patients can benefit from care that extends survival or quality of life.
It’s an ambitious undertaking, especially for a group of full-time clinicians, researchers, and patient advocates “volunteering their time for societal good,” said Dr. Gyawali, but the project teams intend to hit the ground running.
The team has established short-term targets, such as identifying deficiencies in data interpretation within education programs within 6 months and developing educational materials that begin to correct those deficiencies within 12 months, Dr. Booth explained. In the longer term, the team will also aim to design clinical trials that focus on patient outcomes, such as overall survival and quality of life.
Breast cancer survivor and patient advocate Michelle Tregear, PhD, who was recruited to help with Common Sense Oncology, also hopes the initiative will lead to better regulatory control that requires trial sponsors to “focus on what matters to patients, not on surrogate endpoints.”
When it comes to clinical trials, “more, more, more is not always better,” said Dr. Tregear, director of Education and Training Programs for patient advocates at the National Breast Cancer Coalition, Washington, D.C. “Industry interests are not always aligned with patient interests,” and “the system, by and large, is not addressing questions that really matter to patients and their families.”
Although “it’s a tall order to change the direction that we’re going in,” Dr. Tregear is up to the challenge of helping raise awareness, which will hopefully spur patients to demand change.
When Dr. Goodman announced the Common Sense Oncology initiative on Twitter, the news brought excitement, with many oncologists asking to join.
With its sweeping, ambitious goals, the Common Sense Oncology initiative has a long road ahead. Figuring out how to implement some of its aims in practice will take time, Dr. Booth acknowledges, and the initial launch marks the first steps, which will continue to evolve over time.
“We’re not proposing we have all the answers or that we know what every patient would want – we’re saying we’ve not done a good job of communicating to patients the relative benefits and risks of different treatments,” Dr. Booth explained. “We want to celebrate and promote what helps and speak out about what’s not in the best interest of patients.”
Dr. Goodman reported consulting fees from Seattle Genetics and speaking honoraria from Curio. Dr. Booth, Dr. Gyawali, and Dr. Tregear reported having no financial conflicts of interest.
A version of this article appeared on Medscape.com.
After 15 years of researching what works well in oncology – and where the field has gone awry – Christopher Booth, MD, had a career moment.
“As I approached mid-career, I realized publishing and describing problems wasn’t fulfilling. It wasn’t doing enough,” recalled Dr. Booth, an oncologist and professor at Queen’s University, Kingston, Ont. “I wanted to change mindsets and change systems so that things actually improved for the better for patients.”
His colleague, Bishal Gyawali, MD, PhD, described a similar epiphany. As a trainee, he noticed that the real-world effects of some so-called blockbuster cancer drugs too often failed to measure up to the hype.
“I realized we were lacking common sense in oncology,” said Dr. Gyawali, a medical oncologist and assistant professor at Queen’s University.
In 2019, Dr. Gyawali launched a Medscape column addressing what he considers to be that lack of common sense, and in 2022, he and Dr. Booth published a similarly titled opinion piece in Nature Medicine. The core idea: The cancer community needs to prioritize cancer treatments that benefit patients, treatments that meaningfully improve survival and quality of life.
Aaron Goodman, MD, a hematologist and associate professor at UC San Diego Health, was on the same page. He’d been interested in the evidence-based medicine movement since his time as a hematology fellow when that movement was “a bit of a counterculture,” he explained.
Dr. Goodman and Dr. Booth connected through their common interests and collaborated on a 2021 paper exploring the discomfort clinicians might feel when a patient’s needs fall on the “edge of oncology”: that is, when the guideline-recommended standard of care offers marginal benefit, at best, and could, at worst, cause patient harm.
“We said, ‘Now is the time to make change,’ ” he recalled. It was time to stop talking and do something.
Common sense and a common purpose
Dr. Booth, Dr. Gyawali, and Dr. Goodman joined forces and, with the backing of a philanthropist who had experience as a patient with cancer, convened an organizing committee of more than 30 like-minded oncologists and patient advocates from across the globe.
The group convened for a 3-day “meeting of the minds” in Kingston in April and laid out their intentions in a position paper published online in The Lancet Oncology.
In their paper, the committee outline the vision for Common Sense Oncology. The mission: prioritize patient-centered and equitable care by focusing on treatments that improve survival and quality of life, communication that promotes informed decision-making, and systems that ensure access to all patients.
However, increasingly, the cancer community faces a “troubling paradox,” the team wrote in The Lancet. In some instance, treatments that bring minimal benefit are overused while those that can make a meaningful difference in patients’ lives are not accessible to most worldwide.
One reason for this shift: Commercial interests, rather than patient interests, appear to be driving cancer research and care. The team explained, for instance, that over the past few decades, clinical trials have largely pivoted from publicly funded efforts to industry funded ones “designed to achieve regulatory approval or commercial advantage, [often] at the expense of investigating new approaches to surgery, radiotherapy, palliative care, and prevention.”
But “patients deserve better,” the group wrote.
The team outlined three pillars for the initiative: evidence generation, evidence interpretation, and evidence communication.
The evidence generation pillar will aim to improve trial design and reporting to prioritize outcomes that matter to patients.
“One concern is that over the last 10 years or so, most of our new treatments have had very, very small benefits, and we think the bar has dropped too low,” Dr. Booth said, explaining that many trials have moved away from focusing on improving survival and quality of life and toward detecting small differences between treatments on other endpoints – namely progression-free survival. “Those small benefits need to be balanced against the very real risks to our patients.”
The evidence interpretation pillar will aim to foster critical thinking so that clinicians can better identify poorly designed or reported trials and help patients make more informed decisions.
Lastly, the evidence communication pillar will focus on fostering better communication about treatment options among patients, the public, and policymakers. Without clear and thoughtful communication, patients may have unrealistic expectations about the effectiveness of treatments that offer only marginal clinical benefits.
The team also emphasized a need to focus on improving global equity and access to affordable treatments so all patients can benefit from care that extends survival or quality of life.
It’s an ambitious undertaking, especially for a group of full-time clinicians, researchers, and patient advocates “volunteering their time for societal good,” said Dr. Gyawali, but the project teams intend to hit the ground running.
The team has established short-term targets, such as identifying deficiencies in data interpretation within education programs within 6 months and developing educational materials that begin to correct those deficiencies within 12 months, Dr. Booth explained. In the longer term, the team will also aim to design clinical trials that focus on patient outcomes, such as overall survival and quality of life.
Breast cancer survivor and patient advocate Michelle Tregear, PhD, who was recruited to help with Common Sense Oncology, also hopes the initiative will lead to better regulatory control that requires trial sponsors to “focus on what matters to patients, not on surrogate endpoints.”
When it comes to clinical trials, “more, more, more is not always better,” said Dr. Tregear, director of Education and Training Programs for patient advocates at the National Breast Cancer Coalition, Washington, D.C. “Industry interests are not always aligned with patient interests,” and “the system, by and large, is not addressing questions that really matter to patients and their families.”
Although “it’s a tall order to change the direction that we’re going in,” Dr. Tregear is up to the challenge of helping raise awareness, which will hopefully spur patients to demand change.
When Dr. Goodman announced the Common Sense Oncology initiative on Twitter, the news brought excitement, with many oncologists asking to join.
With its sweeping, ambitious goals, the Common Sense Oncology initiative has a long road ahead. Figuring out how to implement some of its aims in practice will take time, Dr. Booth acknowledges, and the initial launch marks the first steps, which will continue to evolve over time.
“We’re not proposing we have all the answers or that we know what every patient would want – we’re saying we’ve not done a good job of communicating to patients the relative benefits and risks of different treatments,” Dr. Booth explained. “We want to celebrate and promote what helps and speak out about what’s not in the best interest of patients.”
Dr. Goodman reported consulting fees from Seattle Genetics and speaking honoraria from Curio. Dr. Booth, Dr. Gyawali, and Dr. Tregear reported having no financial conflicts of interest.
A version of this article appeared on Medscape.com.
Remote teams offer chance to improve difficult-to-treat PsA
DUBLIN – according to presenters at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
In the same session at the meeting, GRAPPA also announced a new initiative to define difficult-to-treat PsA.
Deepak Jadon, MBBCh, PhD, a rheumatologist with Cambridge (England) University Hospitals NHS Foundation Trust, described his experience of running a clinic for patients with difficult-to-treat PsA in eastern England, covering a catchment area of approximately 6 million people between six and seven hospitals. He discussed how the MDT in his region operates to discuss the management of such patients, whose treatment options may also have indications for comorbidities such as inflammatory bowel disease or uveitis, or have complicating factors such as metabolic syndrome.
“You have to have an interested and engaged colleague to form that collaboration,” Dr. Jadon said. “If you are working in isolation, without your colleagues in the same building, that becomes harder. We have been running remote multispecialty meetings without the patient being present, and I have had the good fortune of having medical students brought into our practice. We discussed approximately 220 patients, initially in our psoriasis-spondyloarthritis MDT and subsequently in our inflammatory bowel disease–spondyloarthritis MDT.”
There are also MDTs with hepatologist colleagues carried out on an ad hoc basis to discuss patients with nonalcoholic fatty liver disease, as well as patients with hepatitis or a transplanted liver, who have psoriatic disease.
This difficult-to-treat cohort is discussed in MDT meetings conducted on Zoom. At MDT meetings, carried out with frequencies ranging from monthly to bimonthly, Dr. Jadon said there would be two dermatologists, two rheumatologists, one to four dermatology and rheumatology trainees and fellows, one to four specialist nurses, one to three research nurses, and one biologics pharmacist. They record the meetings and discuss anywhere from 4 to 18 patients, reviewing items in their electronic medical record, calling or writing patients and/or their primary care clinician as needed. They take about an hour to meet, with a half hour of prep time and another 1.5 hours to undertake necessary actions.
“Generally, the question is, how can we change treatment to best cover the domains of disease?” Dr. Jadon said. “Progressively, more patients are being put onto biologics as a result of these conversations, and I do feel that it has helped our patients and us to consolidate their management plan. Naturally, as all clinicians do, we doubt ourselves and wonder if we are missing something. Is there an aspect of the disease [being missed]? Is there a treatment that I haven’t been using? [The meetings have] been reassuring in that regard. I also learn from my colleagues who have earlier access to treatments, especially in dermatology.”
In a small number of patients, some combinations of advanced therapies, such as combining a Janus kinase inhibitor with a biologic, have been used as a result of these collaborations, “and to discuss this in an MDT has been reassuring, including from a medico-legal perspective,” Dr. Jadon said. “One of the main things we found to be useful is having a brief referral pro forma. Usually, by the time patients reach this forum, they have used a lot of treatments, and it can be difficult to remember that on the spot. It is also important to focus on what the actual question is. Naturally, in these discussions, where you talk about the complexities and various facets of disease, you can get a bit lost and sometimes you actually don’t address the original question.”
He also said it has been very beneficial to use screen sharing in the remote MDTs so that different disciplines can review images together, such as with radiology colleagues. “There are varying skill sets among our colleagues, especially in radiology, and it has been quite nice to review their peripheral imaging, their axial imaging, laboratory markers, and skin lesions together.”
New GRAPPA project to provide clarity
A new GRAPPA project has been devised to help physicians identify and define difficult-to-treat and difficult-to-manage PsA in order to help physicians to categorize and treat these patients.
“We have a growing treatment armamentarium ... but we still do not reach all the patients that we would like to,” said Fabian Proft, MD, of Charité University Medicine, Berlin. “We set our targets, but we see in the real world that we are only reaching them in 40% or 50% of our patients. So, we need to do better, and in order to do better, we need to understand better.”
“We should not only make a definition of difficult-to-treat PsA, which is nonresponse to treatment with objective signs of inflammation, but also we need to address and acknowledge difficult-to-manage [patients],” Dr. Proft said. “We should not stop as soon as we come up with a definition. This will be a working definition and will need to be validated.”
The speakers reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
DUBLIN – according to presenters at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
In the same session at the meeting, GRAPPA also announced a new initiative to define difficult-to-treat PsA.
Deepak Jadon, MBBCh, PhD, a rheumatologist with Cambridge (England) University Hospitals NHS Foundation Trust, described his experience of running a clinic for patients with difficult-to-treat PsA in eastern England, covering a catchment area of approximately 6 million people between six and seven hospitals. He discussed how the MDT in his region operates to discuss the management of such patients, whose treatment options may also have indications for comorbidities such as inflammatory bowel disease or uveitis, or have complicating factors such as metabolic syndrome.
“You have to have an interested and engaged colleague to form that collaboration,” Dr. Jadon said. “If you are working in isolation, without your colleagues in the same building, that becomes harder. We have been running remote multispecialty meetings without the patient being present, and I have had the good fortune of having medical students brought into our practice. We discussed approximately 220 patients, initially in our psoriasis-spondyloarthritis MDT and subsequently in our inflammatory bowel disease–spondyloarthritis MDT.”
There are also MDTs with hepatologist colleagues carried out on an ad hoc basis to discuss patients with nonalcoholic fatty liver disease, as well as patients with hepatitis or a transplanted liver, who have psoriatic disease.
This difficult-to-treat cohort is discussed in MDT meetings conducted on Zoom. At MDT meetings, carried out with frequencies ranging from monthly to bimonthly, Dr. Jadon said there would be two dermatologists, two rheumatologists, one to four dermatology and rheumatology trainees and fellows, one to four specialist nurses, one to three research nurses, and one biologics pharmacist. They record the meetings and discuss anywhere from 4 to 18 patients, reviewing items in their electronic medical record, calling or writing patients and/or their primary care clinician as needed. They take about an hour to meet, with a half hour of prep time and another 1.5 hours to undertake necessary actions.
“Generally, the question is, how can we change treatment to best cover the domains of disease?” Dr. Jadon said. “Progressively, more patients are being put onto biologics as a result of these conversations, and I do feel that it has helped our patients and us to consolidate their management plan. Naturally, as all clinicians do, we doubt ourselves and wonder if we are missing something. Is there an aspect of the disease [being missed]? Is there a treatment that I haven’t been using? [The meetings have] been reassuring in that regard. I also learn from my colleagues who have earlier access to treatments, especially in dermatology.”
In a small number of patients, some combinations of advanced therapies, such as combining a Janus kinase inhibitor with a biologic, have been used as a result of these collaborations, “and to discuss this in an MDT has been reassuring, including from a medico-legal perspective,” Dr. Jadon said. “One of the main things we found to be useful is having a brief referral pro forma. Usually, by the time patients reach this forum, they have used a lot of treatments, and it can be difficult to remember that on the spot. It is also important to focus on what the actual question is. Naturally, in these discussions, where you talk about the complexities and various facets of disease, you can get a bit lost and sometimes you actually don’t address the original question.”
He also said it has been very beneficial to use screen sharing in the remote MDTs so that different disciplines can review images together, such as with radiology colleagues. “There are varying skill sets among our colleagues, especially in radiology, and it has been quite nice to review their peripheral imaging, their axial imaging, laboratory markers, and skin lesions together.”
New GRAPPA project to provide clarity
A new GRAPPA project has been devised to help physicians identify and define difficult-to-treat and difficult-to-manage PsA in order to help physicians to categorize and treat these patients.
“We have a growing treatment armamentarium ... but we still do not reach all the patients that we would like to,” said Fabian Proft, MD, of Charité University Medicine, Berlin. “We set our targets, but we see in the real world that we are only reaching them in 40% or 50% of our patients. So, we need to do better, and in order to do better, we need to understand better.”
“We should not only make a definition of difficult-to-treat PsA, which is nonresponse to treatment with objective signs of inflammation, but also we need to address and acknowledge difficult-to-manage [patients],” Dr. Proft said. “We should not stop as soon as we come up with a definition. This will be a working definition and will need to be validated.”
The speakers reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
DUBLIN – according to presenters at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
In the same session at the meeting, GRAPPA also announced a new initiative to define difficult-to-treat PsA.
Deepak Jadon, MBBCh, PhD, a rheumatologist with Cambridge (England) University Hospitals NHS Foundation Trust, described his experience of running a clinic for patients with difficult-to-treat PsA in eastern England, covering a catchment area of approximately 6 million people between six and seven hospitals. He discussed how the MDT in his region operates to discuss the management of such patients, whose treatment options may also have indications for comorbidities such as inflammatory bowel disease or uveitis, or have complicating factors such as metabolic syndrome.
“You have to have an interested and engaged colleague to form that collaboration,” Dr. Jadon said. “If you are working in isolation, without your colleagues in the same building, that becomes harder. We have been running remote multispecialty meetings without the patient being present, and I have had the good fortune of having medical students brought into our practice. We discussed approximately 220 patients, initially in our psoriasis-spondyloarthritis MDT and subsequently in our inflammatory bowel disease–spondyloarthritis MDT.”
There are also MDTs with hepatologist colleagues carried out on an ad hoc basis to discuss patients with nonalcoholic fatty liver disease, as well as patients with hepatitis or a transplanted liver, who have psoriatic disease.
This difficult-to-treat cohort is discussed in MDT meetings conducted on Zoom. At MDT meetings, carried out with frequencies ranging from monthly to bimonthly, Dr. Jadon said there would be two dermatologists, two rheumatologists, one to four dermatology and rheumatology trainees and fellows, one to four specialist nurses, one to three research nurses, and one biologics pharmacist. They record the meetings and discuss anywhere from 4 to 18 patients, reviewing items in their electronic medical record, calling or writing patients and/or their primary care clinician as needed. They take about an hour to meet, with a half hour of prep time and another 1.5 hours to undertake necessary actions.
“Generally, the question is, how can we change treatment to best cover the domains of disease?” Dr. Jadon said. “Progressively, more patients are being put onto biologics as a result of these conversations, and I do feel that it has helped our patients and us to consolidate their management plan. Naturally, as all clinicians do, we doubt ourselves and wonder if we are missing something. Is there an aspect of the disease [being missed]? Is there a treatment that I haven’t been using? [The meetings have] been reassuring in that regard. I also learn from my colleagues who have earlier access to treatments, especially in dermatology.”
In a small number of patients, some combinations of advanced therapies, such as combining a Janus kinase inhibitor with a biologic, have been used as a result of these collaborations, “and to discuss this in an MDT has been reassuring, including from a medico-legal perspective,” Dr. Jadon said. “One of the main things we found to be useful is having a brief referral pro forma. Usually, by the time patients reach this forum, they have used a lot of treatments, and it can be difficult to remember that on the spot. It is also important to focus on what the actual question is. Naturally, in these discussions, where you talk about the complexities and various facets of disease, you can get a bit lost and sometimes you actually don’t address the original question.”
He also said it has been very beneficial to use screen sharing in the remote MDTs so that different disciplines can review images together, such as with radiology colleagues. “There are varying skill sets among our colleagues, especially in radiology, and it has been quite nice to review their peripheral imaging, their axial imaging, laboratory markers, and skin lesions together.”
New GRAPPA project to provide clarity
A new GRAPPA project has been devised to help physicians identify and define difficult-to-treat and difficult-to-manage PsA in order to help physicians to categorize and treat these patients.
“We have a growing treatment armamentarium ... but we still do not reach all the patients that we would like to,” said Fabian Proft, MD, of Charité University Medicine, Berlin. “We set our targets, but we see in the real world that we are only reaching them in 40% or 50% of our patients. So, we need to do better, and in order to do better, we need to understand better.”
“We should not only make a definition of difficult-to-treat PsA, which is nonresponse to treatment with objective signs of inflammation, but also we need to address and acknowledge difficult-to-manage [patients],” Dr. Proft said. “We should not stop as soon as we come up with a definition. This will be a working definition and will need to be validated.”
The speakers reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
AT GRAPPA 2023
Keep depression, anxiety screening top of mind in patients with psoriatic disease
DUBLIN – , warranting routine screening and having community contacts for mental health professional referrals, Elizabeth Wallace, MD, said at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
Dr. Wallace, of Cherry Hills Dermatology, Englewood, Colo., discussed the complex interactions between mental illness and psoriatic disease and the potential pitfalls of this comorbidity for these patients.
The topic of mental health is “consistently at the top of our patients’ minds, and certainly our minds too,” said session comoderator and GRAPPA president-elect Joseph F. Merola, MD, MMSc.
“In the U.S., around 17% of people with psoriasis have depression vs. 9% in those without psoriasis,” Dr. Wallace explained. “Psoriasis patients are twice as likely to have depression, compared to those without psoriasis, and psoriasis patients are 33% more likely to attempt suicide and 20% more likely to complete suicide, compared to those without psoriasis.” More severe psoriasis and younger age of onset are also associated with a greater likelihood of suicidality, she added.
Mediators of depression
“The inflammatory mechanisms driving PsD can drive depression and anxiety, and vice-versa,” she said. “There are often also genetic links, for example genetic variations in serotonin receptors, and psychological issues in psoriatic disease are predictably worsened by feelings of stigmatization, embarrassment, and social isolation.”
There are also efforts underway in clinics to “normalize” screening for anxiety and depression among this patient cohort, Dr. Wallace said. “We know that our psoriasis patients face social stigma from the visibility of their disease, and that stress can lead to flares of their condition,” she told the attendees. “We also know that patients who experience stigma also have an increased risk of depressive symptoms. We all know now that psoriasis has well-established pathways with upregulated proinflammatory cytokines.
“Increased cytokines stimulate indoleamine 2,3-dioxygenase, which converts tryptophan to kynurenine. Kynurenine is metabolized to quinolinic acid, which is neurotoxic.” She explained that because serotonin derives from tryptophan, decreases in tryptophan lead to reduced serotonin, and therefore increased risk of depression.
Interleukin-6 is known to be upregulated in depression and downregulated with the use of antidepressant medications, Dr. Wallace said. Mouse models in research have shown that deletion of the IL-6 gene produces antidepressant effects, and studies in humans have shown that IL-6, more than any other serum cytokine, is found at higher levels in humans with depression and psoriatic disease.
IL-17 is also implicated in psoriatic disease and mental health problems, Dr. Wallace said. “With stress, you get upregulation of the Tc17 cells, which produce IL-17,” she explained. “IL-17, along with other inflammatory markers, can actually make the blood-brain barrier more permeable, and when you get more permeability to the blood-brain barrier, you get these cytokines that can cross from the periphery and into the brain.
“With this crossing into the brain, you get further activation of more Th17 [cells] and that, on neurons, leads to increased potassium production, which is directly neurotoxic, so you get neuron destruction.”
Talking about depression
“So, what can we share with our patients?” Dr. Wallace asked. “We can discuss with them that psoriatic patients in general are more likely to be depressed or to have higher rates of suicide. The literature consistently shows that patients whose psoriasis is successfully treated experience reduced depression, and we can provide an understandable review of systemic medications, with warnings on depression and/or suicidality.”
Dr. Wallace advised to screen for depression with the Patient Health Questionnaire-2 (PHQ-2), a validated, two-item tool that asks, “Over the past 2 weeks, how often have you been bothered by having little interest or pleasure in doing things?” and “Over the past 2 weeks, how often have you been bothered by feeling down, depressed, or hopeless?”
She presented a case study illustrative of the type of presentation she sees in her clinic. It involved a 32-year-old man with plaque psoriasis and a high degree of body surface affected. “It’s now July in Colorado, it’s getting warm, people want to wear their shorts and T-shirts, but he said he could no longer hide his psoriasis,” said Dr. Wallace. “Further, it’s in areas that he cannot hide, such as his scalp, his beard, and he also has nail disease. Often, these patients don’t want to shake hands with their bosses or their colleagues and that’s very embarrassing for them.”
Dr. Wallace explained that this patient had seen advertisements for biologic drugs and requested to commence a treatment course. “During the exam, and now that you are developing some rapport with him, you discover that he is feeling down, is embarrassed at work, and has started to avoid social situations.” This is illustrative of a patient who should be screened for mental health conditions, specifically using PHQ-2, she said.
“You can be the person at the front line to screen these patients for mental health conditions, and, specifically for depression, with PHQ-2,” she said. PHQ-2 scores range from 0 to 6, and a score of 3 or higher is considered a positive screen.
“This is where your relationship with another health provider who is most qualified to care for these patients and validate them for their mental health condition can be absolutely critical,” Dr. Wallace said.
Successful PsD treatment lessens the risk for mental health comorbidities, and this is also seen in psoriatic arthritis, Dr. Wallace pointed out. Patient education is critical regarding their increased risk for depression and potential suicidal ideation, she added.
“It’s our job as clinicians to provide patients with an understandable, easy-to-digest review of systemic medications and warnings on depression and suicidality so that they can be aware of these factors.”
Perspective from Dr. Merola
In an interview, Dr. Merola, a double board-certified dermatologist and rheumatologist at Brigham and Women’s Hospital, Boston, discussed the interactions between mental and physical illness.
“One of the things we are learning is that it’s very much a multifactorial issue, in that skin and joints contribute, in some obvious ways, to anxiety and depression, like the fact that somebody doesn’t feel good about their appearance, or they can’t complete daily activities,” he said. “Those are the more obvious ones. But there is data and evidence that there is a biology behind that as well – inflammatory cytokines that drive skin disease probably also have a direct impact on the CNS and probably also drive anxiety and depression.
“We know that disordered sleep contributes to anxiety – think about how we feel if we get a horrible night’s sleep ... it’s hard to pick apart: ‘Am I depressed, am I anxious because I am having too much coffee? Because I am fatigued?’ So, we get into these circles, but the point is, we have to break these cycles, and we have to do it in multiple places. Yes, we have to fix the skin and the joints, but we also have to have interventions and think about how to screen for anxiety and depression. We also have to think about identifying disordered sleep, and how we intervene there as well.”
These challenges require a collaborative approach among physicians. “We can help patients to build their team that gets them help for their skin, for their joints, for their anxiety or depression, their disordered sleep, for their nutritional disorders, their obesity, and so on. So, we are trying to pick apart and unpack those complexities,” he said.
In regard to the potential impacts of this holistic strategy on physician workloads, Dr. Merola acknowledged it is important to consider physician wellness. “There’s no question that we want to be doing the best we can for our colleagues, but we don’t want to overload our colleagues by saying, ‘By the way, not only should we be treating their skin and joints,’ which of course we should be doing, but ‘could you also manage their diabetes, their obesity, their disordered sleep, their anxiety, their depression, difficulties with insurance, getting access to treatments, etc.’
“This is where effective collaboration between physicians becomes important,” he stressed. “We can’t manage every single piece, but we can make sure our patients are informed, are aware, and assist them to get the help that they need.”
In the United States, there “is a real issue” with access to mental health care and greater awareness needs to be created around this issue, he added.
Dr. Wallace and Dr. Merola report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
DUBLIN – , warranting routine screening and having community contacts for mental health professional referrals, Elizabeth Wallace, MD, said at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
Dr. Wallace, of Cherry Hills Dermatology, Englewood, Colo., discussed the complex interactions between mental illness and psoriatic disease and the potential pitfalls of this comorbidity for these patients.
The topic of mental health is “consistently at the top of our patients’ minds, and certainly our minds too,” said session comoderator and GRAPPA president-elect Joseph F. Merola, MD, MMSc.
“In the U.S., around 17% of people with psoriasis have depression vs. 9% in those without psoriasis,” Dr. Wallace explained. “Psoriasis patients are twice as likely to have depression, compared to those without psoriasis, and psoriasis patients are 33% more likely to attempt suicide and 20% more likely to complete suicide, compared to those without psoriasis.” More severe psoriasis and younger age of onset are also associated with a greater likelihood of suicidality, she added.
Mediators of depression
“The inflammatory mechanisms driving PsD can drive depression and anxiety, and vice-versa,” she said. “There are often also genetic links, for example genetic variations in serotonin receptors, and psychological issues in psoriatic disease are predictably worsened by feelings of stigmatization, embarrassment, and social isolation.”
There are also efforts underway in clinics to “normalize” screening for anxiety and depression among this patient cohort, Dr. Wallace said. “We know that our psoriasis patients face social stigma from the visibility of their disease, and that stress can lead to flares of their condition,” she told the attendees. “We also know that patients who experience stigma also have an increased risk of depressive symptoms. We all know now that psoriasis has well-established pathways with upregulated proinflammatory cytokines.
“Increased cytokines stimulate indoleamine 2,3-dioxygenase, which converts tryptophan to kynurenine. Kynurenine is metabolized to quinolinic acid, which is neurotoxic.” She explained that because serotonin derives from tryptophan, decreases in tryptophan lead to reduced serotonin, and therefore increased risk of depression.
Interleukin-6 is known to be upregulated in depression and downregulated with the use of antidepressant medications, Dr. Wallace said. Mouse models in research have shown that deletion of the IL-6 gene produces antidepressant effects, and studies in humans have shown that IL-6, more than any other serum cytokine, is found at higher levels in humans with depression and psoriatic disease.
IL-17 is also implicated in psoriatic disease and mental health problems, Dr. Wallace said. “With stress, you get upregulation of the Tc17 cells, which produce IL-17,” she explained. “IL-17, along with other inflammatory markers, can actually make the blood-brain barrier more permeable, and when you get more permeability to the blood-brain barrier, you get these cytokines that can cross from the periphery and into the brain.
“With this crossing into the brain, you get further activation of more Th17 [cells] and that, on neurons, leads to increased potassium production, which is directly neurotoxic, so you get neuron destruction.”
Talking about depression
“So, what can we share with our patients?” Dr. Wallace asked. “We can discuss with them that psoriatic patients in general are more likely to be depressed or to have higher rates of suicide. The literature consistently shows that patients whose psoriasis is successfully treated experience reduced depression, and we can provide an understandable review of systemic medications, with warnings on depression and/or suicidality.”
Dr. Wallace advised to screen for depression with the Patient Health Questionnaire-2 (PHQ-2), a validated, two-item tool that asks, “Over the past 2 weeks, how often have you been bothered by having little interest or pleasure in doing things?” and “Over the past 2 weeks, how often have you been bothered by feeling down, depressed, or hopeless?”
She presented a case study illustrative of the type of presentation she sees in her clinic. It involved a 32-year-old man with plaque psoriasis and a high degree of body surface affected. “It’s now July in Colorado, it’s getting warm, people want to wear their shorts and T-shirts, but he said he could no longer hide his psoriasis,” said Dr. Wallace. “Further, it’s in areas that he cannot hide, such as his scalp, his beard, and he also has nail disease. Often, these patients don’t want to shake hands with their bosses or their colleagues and that’s very embarrassing for them.”
Dr. Wallace explained that this patient had seen advertisements for biologic drugs and requested to commence a treatment course. “During the exam, and now that you are developing some rapport with him, you discover that he is feeling down, is embarrassed at work, and has started to avoid social situations.” This is illustrative of a patient who should be screened for mental health conditions, specifically using PHQ-2, she said.
“You can be the person at the front line to screen these patients for mental health conditions, and, specifically for depression, with PHQ-2,” she said. PHQ-2 scores range from 0 to 6, and a score of 3 or higher is considered a positive screen.
“This is where your relationship with another health provider who is most qualified to care for these patients and validate them for their mental health condition can be absolutely critical,” Dr. Wallace said.
Successful PsD treatment lessens the risk for mental health comorbidities, and this is also seen in psoriatic arthritis, Dr. Wallace pointed out. Patient education is critical regarding their increased risk for depression and potential suicidal ideation, she added.
“It’s our job as clinicians to provide patients with an understandable, easy-to-digest review of systemic medications and warnings on depression and suicidality so that they can be aware of these factors.”
Perspective from Dr. Merola
In an interview, Dr. Merola, a double board-certified dermatologist and rheumatologist at Brigham and Women’s Hospital, Boston, discussed the interactions between mental and physical illness.
“One of the things we are learning is that it’s very much a multifactorial issue, in that skin and joints contribute, in some obvious ways, to anxiety and depression, like the fact that somebody doesn’t feel good about their appearance, or they can’t complete daily activities,” he said. “Those are the more obvious ones. But there is data and evidence that there is a biology behind that as well – inflammatory cytokines that drive skin disease probably also have a direct impact on the CNS and probably also drive anxiety and depression.
“We know that disordered sleep contributes to anxiety – think about how we feel if we get a horrible night’s sleep ... it’s hard to pick apart: ‘Am I depressed, am I anxious because I am having too much coffee? Because I am fatigued?’ So, we get into these circles, but the point is, we have to break these cycles, and we have to do it in multiple places. Yes, we have to fix the skin and the joints, but we also have to have interventions and think about how to screen for anxiety and depression. We also have to think about identifying disordered sleep, and how we intervene there as well.”
These challenges require a collaborative approach among physicians. “We can help patients to build their team that gets them help for their skin, for their joints, for their anxiety or depression, their disordered sleep, for their nutritional disorders, their obesity, and so on. So, we are trying to pick apart and unpack those complexities,” he said.
In regard to the potential impacts of this holistic strategy on physician workloads, Dr. Merola acknowledged it is important to consider physician wellness. “There’s no question that we want to be doing the best we can for our colleagues, but we don’t want to overload our colleagues by saying, ‘By the way, not only should we be treating their skin and joints,’ which of course we should be doing, but ‘could you also manage their diabetes, their obesity, their disordered sleep, their anxiety, their depression, difficulties with insurance, getting access to treatments, etc.’
“This is where effective collaboration between physicians becomes important,” he stressed. “We can’t manage every single piece, but we can make sure our patients are informed, are aware, and assist them to get the help that they need.”
In the United States, there “is a real issue” with access to mental health care and greater awareness needs to be created around this issue, he added.
Dr. Wallace and Dr. Merola report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
DUBLIN – , warranting routine screening and having community contacts for mental health professional referrals, Elizabeth Wallace, MD, said at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
Dr. Wallace, of Cherry Hills Dermatology, Englewood, Colo., discussed the complex interactions between mental illness and psoriatic disease and the potential pitfalls of this comorbidity for these patients.
The topic of mental health is “consistently at the top of our patients’ minds, and certainly our minds too,” said session comoderator and GRAPPA president-elect Joseph F. Merola, MD, MMSc.
“In the U.S., around 17% of people with psoriasis have depression vs. 9% in those without psoriasis,” Dr. Wallace explained. “Psoriasis patients are twice as likely to have depression, compared to those without psoriasis, and psoriasis patients are 33% more likely to attempt suicide and 20% more likely to complete suicide, compared to those without psoriasis.” More severe psoriasis and younger age of onset are also associated with a greater likelihood of suicidality, she added.
Mediators of depression
“The inflammatory mechanisms driving PsD can drive depression and anxiety, and vice-versa,” she said. “There are often also genetic links, for example genetic variations in serotonin receptors, and psychological issues in psoriatic disease are predictably worsened by feelings of stigmatization, embarrassment, and social isolation.”
There are also efforts underway in clinics to “normalize” screening for anxiety and depression among this patient cohort, Dr. Wallace said. “We know that our psoriasis patients face social stigma from the visibility of their disease, and that stress can lead to flares of their condition,” she told the attendees. “We also know that patients who experience stigma also have an increased risk of depressive symptoms. We all know now that psoriasis has well-established pathways with upregulated proinflammatory cytokines.
“Increased cytokines stimulate indoleamine 2,3-dioxygenase, which converts tryptophan to kynurenine. Kynurenine is metabolized to quinolinic acid, which is neurotoxic.” She explained that because serotonin derives from tryptophan, decreases in tryptophan lead to reduced serotonin, and therefore increased risk of depression.
Interleukin-6 is known to be upregulated in depression and downregulated with the use of antidepressant medications, Dr. Wallace said. Mouse models in research have shown that deletion of the IL-6 gene produces antidepressant effects, and studies in humans have shown that IL-6, more than any other serum cytokine, is found at higher levels in humans with depression and psoriatic disease.
IL-17 is also implicated in psoriatic disease and mental health problems, Dr. Wallace said. “With stress, you get upregulation of the Tc17 cells, which produce IL-17,” she explained. “IL-17, along with other inflammatory markers, can actually make the blood-brain barrier more permeable, and when you get more permeability to the blood-brain barrier, you get these cytokines that can cross from the periphery and into the brain.
“With this crossing into the brain, you get further activation of more Th17 [cells] and that, on neurons, leads to increased potassium production, which is directly neurotoxic, so you get neuron destruction.”
Talking about depression
“So, what can we share with our patients?” Dr. Wallace asked. “We can discuss with them that psoriatic patients in general are more likely to be depressed or to have higher rates of suicide. The literature consistently shows that patients whose psoriasis is successfully treated experience reduced depression, and we can provide an understandable review of systemic medications, with warnings on depression and/or suicidality.”
Dr. Wallace advised to screen for depression with the Patient Health Questionnaire-2 (PHQ-2), a validated, two-item tool that asks, “Over the past 2 weeks, how often have you been bothered by having little interest or pleasure in doing things?” and “Over the past 2 weeks, how often have you been bothered by feeling down, depressed, or hopeless?”
She presented a case study illustrative of the type of presentation she sees in her clinic. It involved a 32-year-old man with plaque psoriasis and a high degree of body surface affected. “It’s now July in Colorado, it’s getting warm, people want to wear their shorts and T-shirts, but he said he could no longer hide his psoriasis,” said Dr. Wallace. “Further, it’s in areas that he cannot hide, such as his scalp, his beard, and he also has nail disease. Often, these patients don’t want to shake hands with their bosses or their colleagues and that’s very embarrassing for them.”
Dr. Wallace explained that this patient had seen advertisements for biologic drugs and requested to commence a treatment course. “During the exam, and now that you are developing some rapport with him, you discover that he is feeling down, is embarrassed at work, and has started to avoid social situations.” This is illustrative of a patient who should be screened for mental health conditions, specifically using PHQ-2, she said.
“You can be the person at the front line to screen these patients for mental health conditions, and, specifically for depression, with PHQ-2,” she said. PHQ-2 scores range from 0 to 6, and a score of 3 or higher is considered a positive screen.
“This is where your relationship with another health provider who is most qualified to care for these patients and validate them for their mental health condition can be absolutely critical,” Dr. Wallace said.
Successful PsD treatment lessens the risk for mental health comorbidities, and this is also seen in psoriatic arthritis, Dr. Wallace pointed out. Patient education is critical regarding their increased risk for depression and potential suicidal ideation, she added.
“It’s our job as clinicians to provide patients with an understandable, easy-to-digest review of systemic medications and warnings on depression and suicidality so that they can be aware of these factors.”
Perspective from Dr. Merola
In an interview, Dr. Merola, a double board-certified dermatologist and rheumatologist at Brigham and Women’s Hospital, Boston, discussed the interactions between mental and physical illness.
“One of the things we are learning is that it’s very much a multifactorial issue, in that skin and joints contribute, in some obvious ways, to anxiety and depression, like the fact that somebody doesn’t feel good about their appearance, or they can’t complete daily activities,” he said. “Those are the more obvious ones. But there is data and evidence that there is a biology behind that as well – inflammatory cytokines that drive skin disease probably also have a direct impact on the CNS and probably also drive anxiety and depression.
“We know that disordered sleep contributes to anxiety – think about how we feel if we get a horrible night’s sleep ... it’s hard to pick apart: ‘Am I depressed, am I anxious because I am having too much coffee? Because I am fatigued?’ So, we get into these circles, but the point is, we have to break these cycles, and we have to do it in multiple places. Yes, we have to fix the skin and the joints, but we also have to have interventions and think about how to screen for anxiety and depression. We also have to think about identifying disordered sleep, and how we intervene there as well.”
These challenges require a collaborative approach among physicians. “We can help patients to build their team that gets them help for their skin, for their joints, for their anxiety or depression, their disordered sleep, for their nutritional disorders, their obesity, and so on. So, we are trying to pick apart and unpack those complexities,” he said.
In regard to the potential impacts of this holistic strategy on physician workloads, Dr. Merola acknowledged it is important to consider physician wellness. “There’s no question that we want to be doing the best we can for our colleagues, but we don’t want to overload our colleagues by saying, ‘By the way, not only should we be treating their skin and joints,’ which of course we should be doing, but ‘could you also manage their diabetes, their obesity, their disordered sleep, their anxiety, their depression, difficulties with insurance, getting access to treatments, etc.’
“This is where effective collaboration between physicians becomes important,” he stressed. “We can’t manage every single piece, but we can make sure our patients are informed, are aware, and assist them to get the help that they need.”
In the United States, there “is a real issue” with access to mental health care and greater awareness needs to be created around this issue, he added.
Dr. Wallace and Dr. Merola report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT GRAPPA 2023
Summer diarrhea – Time to think outside the box
It’s “summertime and the livin’ is easy” according to the lyric from an old George Gershwin song. But sometimes, summer activities can lead to illnesses that can disrupt a child’s easy living.
Case: An otherwise healthy 11-year-old presents with four to five loose stools per day, mild nausea, excess flatulence, and cramps for 12 days with a 5-pound weight loss. His loose-to-mushy stools have no blood or mucous but smell worse than usual. He has had no fever, vomiting, rashes, or joint symptoms. A month ago, he went hiking/camping on the Appalachian Trail, drank boiled stream water. and slept in a common-use semi-enclosed shelter. He waded through streams and shared “Trail Magic” (soft drinks being cooled in a fresh mountain stream). Two other campers report similar symptoms.
Differential diagnosis: Broadly, we should consider bacteria, viruses, and parasites. But generally, bacteria are likely to produce more systemic symptoms and usually do not last 12 days. That said, this could be Clostridioides difficile, yet that seems unlikely because he is otherwise healthy and has no apparent risk factors. Salmonella spp., Campylobacter spp. and some Escherichia coli infections may drag on for more than a week but the lack of systemic symptoms or blood/mucous lowers the likelihood. Viral agents (rotavirus, norovirus, adenovirus, astrovirus, calicivirus, or sapovirus) seem unlikely because of the long symptom duration and the child’s preteen age.
The history and presentation seem more likely attributable to a parasite. Uncommonly detected protozoa include Microsporidium (mostly Enterocytozoon bieneusi) and amoeba. Microsporidium is very rare and seen mostly in immune compromised hosts, for example, those living with HIV. Amebiasis occurs mostly after travel to endemic areas, and stools usually contain blood or mucous. Some roundworm or tapeworm infestations cause abdominal pain and abnormal stools, but the usual exposures are absent. Giardia spp., Cryptosporidium spp., Cyclospora cayetanensis, and/or Cystoisospora belli best fit this presentation given his hiking/camping trip.
Workup. Laboratory testing of stool is warranted (because of weight loss and persistent diarrhea) despite a lack of systemic signs. Initially, bacterial culture, C. difficile testing, and viral testing seem unwarranted. The best initial approach, given our most likely suspects, is protozoan/parasite testing.
The Centers for Disease Control and Prevention recommends testing up to three stools collected on separate days.1 Initially, stool testing for giardia and cryptosporidium antigens by EIA assays could be done as a point-of-care test. Such antigen tests are often the first step because of their ease of use, relatively low expense, reasonably high sensitivity and specificity, and rapid turnaround (as little as 1 hour). Alternatively, direct examination of three stools for ova and parasites (O&P) and acid-fast stain or direct fluorescent antibody testing can usually detect our main suspects (giardia, cryptosporidium, cyclospora, and cystoisospora) along with other less likely parasites.
Some laboratories, however, use syndromic stool testing approaches (multiplex nucleic acid panels) that detect over 20 different bacteria, viruses, and select parasites. Multiplex testing has yielded increased detection rates, compared with microscopic examination alone in some settings. Further, they also share ease-of-use and rapid turnaround times with parasite antigen assays while requiring less hands-on time by laboratory personnel, compared with direct microscopic examination. However, multiplex assays are expensive and more readily detect commensal organisms, so they are not necessarily the ideal test in all diarrheal illnesses.
Diagnosis. You decide to first order giardia and cryptosporidium antigen testing because you are highly suspicious that giardia is the cause, based on wild-water exposure, the presentation, and symptom duration. You also order full microscopic O&P examination because you know that parasites can “run in packs.” Results of testing the first stool are positive for giardia. Microscopic examination on each of three stools is negative except for giardia trophozoites (the noninfectious form) in stools two and three.
Giardia overview. Giardia is the most common protozoan causing diarrhea in the United States, is fecal-oral spread, and like Shigella spp., is a low-inoculum infection (ingestion of as few as 10-100 cysts). Acquisition in the United States has been estimated as being 75% from contaminated water (streams are a classic source.2 Other sources are contaminated food (fresh produce is classic) and in some cases sexual encounters (mostly in men who have sex with men). Most detections are sporadic, but outbreaks can occur with case numbers usually below 20; 40% of outbreaks are attributable to contaminated water or food.3 Evaluating symptomatic household members can be important as transmission in families can occur.
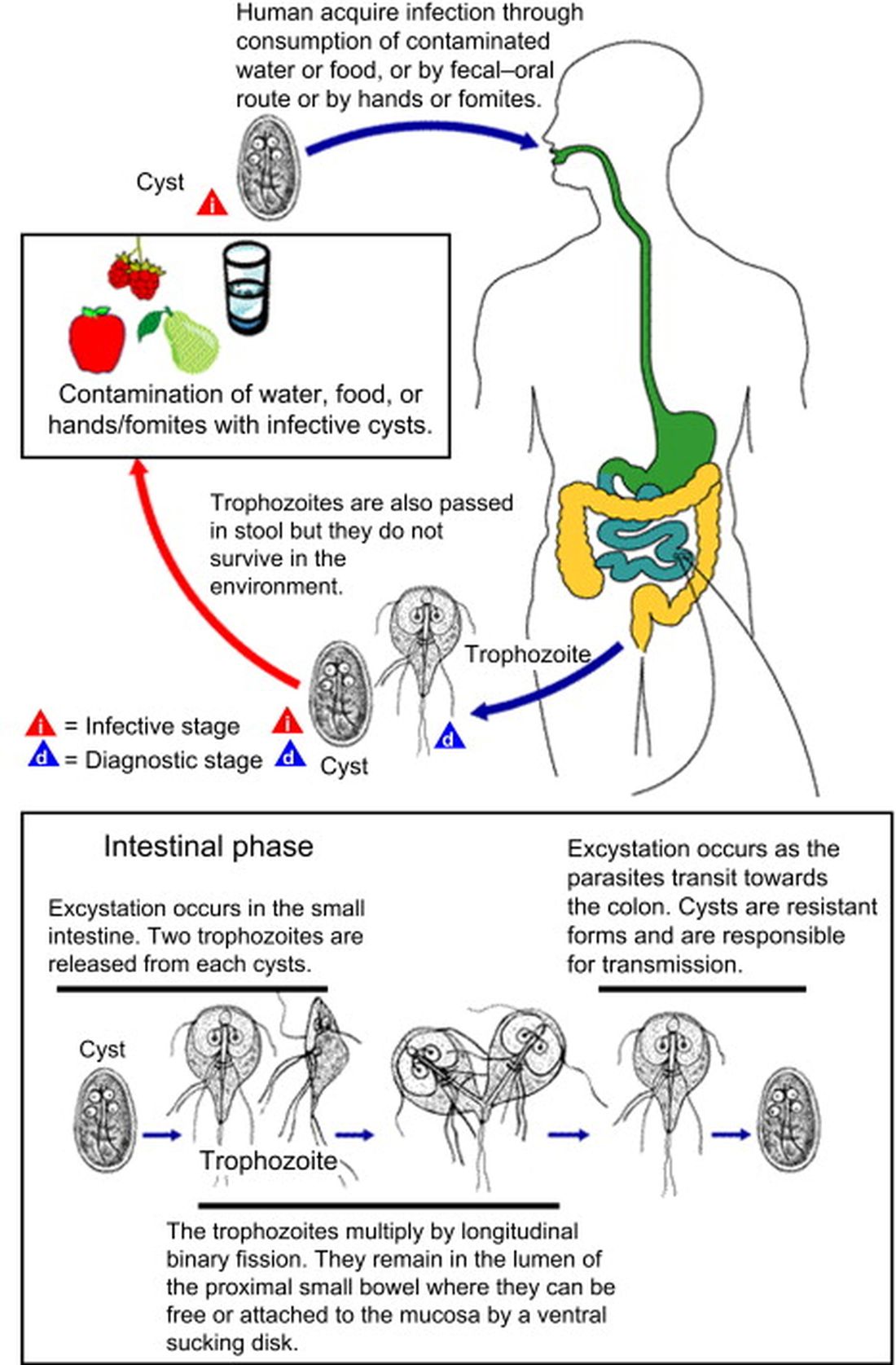
After ingestion, the cysts uncoat and form trophozoites, which reside mostly in the small bowel (Figure), causing inflammation and altering gut membrane permeability, thereby reducing nutrient absorption and circulating amino acids. Along with decreased food intake, altered absorption can lead to weight loss and potentially reduce growth in young children. Some trophozoites replicate while others encyst, eventually passing into stool. The cysts can survive for months in water or the environment (lakes, swimming pools, and clear mountain streams). Giardia has been linked to beavers’ feces contaminating wild-water sources, hence the moniker “Beaver fever” and warnings about stream water related to wilderness hiking.4
Management. Supportive therapy as with any diarrheal illness is the cornerstone of management. Specific antiparasitic treatment has traditionally been with metronidazole compounded into a liquid for young children, but the awful taste and frequent dosing often result in poor adherence. Nevertheless, published cure rates range from 80% to 100%. The taste issue, known adverse effects, and lack of FDA approval for giardia, have led to use of other drugs.5 One dose of tinidazole is as effective as metronidazole and can be prescribed for children 3 years old or older. But the drug nitazoxanide is becoming more standard. It is as effective as either alternative, and is FDA approved for children 1 year old and older. Nitazoxanide also is effective against other intestinal parasites (e.g., cryptosporidium). Nitazoxanide’s 3-day course involves every-12-hour dosing with food with each dose being 5 mL (100 mg) for 1- to 3-year-olds, 10 mL (200 mg) for 4- to 11-year-olds, and one tablet (500 mg) or 25 mL (500 mg) for children 12 years old or older.6
Key elements in this subacute nonsystemic diarrheal presentation were primitive camping history, multiple stream water contacts, nearly 2 weeks of symptoms, weight loss, and flatulence/cramping, but no fever or stool blood/mucous. Two friends also appear to be similarly symptomatic, so a common exposure seemed likely This is typical for several summertime activity–related parasites. So,
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital–Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines. The hospital also receives CDC funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus. Email Dr. Harrison at [email protected].
References
1. Diagnosis and Treatment Information for Medical Professionals, Giardia, Parasites. CDC.
2. Krumrie S et al. Curr Res Parasitol Vector Borne Dis. 2022;2:100084. doi: 10.1016/j.crpvbd.2022.100084.
3. Baldursson S and Karanis P. Water Res. 2011 Dec 15. doi: 10.1016/j.watres.2011.10.013.
4. “Water on the Appalachian Trail” AppalachianTrail.com.
5. Giardiasis: Treatment and prevention. UpToDate.
6. Kimberlin D et al. Red Book: 2021-2024 Report of the Committee on Infectious Diseases (Itasca, Ill.: American Academy of Pediatrics, 2021. 32nd ed.) Giardia duodenalis infections. pp. 335-8; and p. 961 (Table 4.11).
It’s “summertime and the livin’ is easy” according to the lyric from an old George Gershwin song. But sometimes, summer activities can lead to illnesses that can disrupt a child’s easy living.
Case: An otherwise healthy 11-year-old presents with four to five loose stools per day, mild nausea, excess flatulence, and cramps for 12 days with a 5-pound weight loss. His loose-to-mushy stools have no blood or mucous but smell worse than usual. He has had no fever, vomiting, rashes, or joint symptoms. A month ago, he went hiking/camping on the Appalachian Trail, drank boiled stream water. and slept in a common-use semi-enclosed shelter. He waded through streams and shared “Trail Magic” (soft drinks being cooled in a fresh mountain stream). Two other campers report similar symptoms.
Differential diagnosis: Broadly, we should consider bacteria, viruses, and parasites. But generally, bacteria are likely to produce more systemic symptoms and usually do not last 12 days. That said, this could be Clostridioides difficile, yet that seems unlikely because he is otherwise healthy and has no apparent risk factors. Salmonella spp., Campylobacter spp. and some Escherichia coli infections may drag on for more than a week but the lack of systemic symptoms or blood/mucous lowers the likelihood. Viral agents (rotavirus, norovirus, adenovirus, astrovirus, calicivirus, or sapovirus) seem unlikely because of the long symptom duration and the child’s preteen age.
The history and presentation seem more likely attributable to a parasite. Uncommonly detected protozoa include Microsporidium (mostly Enterocytozoon bieneusi) and amoeba. Microsporidium is very rare and seen mostly in immune compromised hosts, for example, those living with HIV. Amebiasis occurs mostly after travel to endemic areas, and stools usually contain blood or mucous. Some roundworm or tapeworm infestations cause abdominal pain and abnormal stools, but the usual exposures are absent. Giardia spp., Cryptosporidium spp., Cyclospora cayetanensis, and/or Cystoisospora belli best fit this presentation given his hiking/camping trip.
Workup. Laboratory testing of stool is warranted (because of weight loss and persistent diarrhea) despite a lack of systemic signs. Initially, bacterial culture, C. difficile testing, and viral testing seem unwarranted. The best initial approach, given our most likely suspects, is protozoan/parasite testing.
The Centers for Disease Control and Prevention recommends testing up to three stools collected on separate days.1 Initially, stool testing for giardia and cryptosporidium antigens by EIA assays could be done as a point-of-care test. Such antigen tests are often the first step because of their ease of use, relatively low expense, reasonably high sensitivity and specificity, and rapid turnaround (as little as 1 hour). Alternatively, direct examination of three stools for ova and parasites (O&P) and acid-fast stain or direct fluorescent antibody testing can usually detect our main suspects (giardia, cryptosporidium, cyclospora, and cystoisospora) along with other less likely parasites.
Some laboratories, however, use syndromic stool testing approaches (multiplex nucleic acid panels) that detect over 20 different bacteria, viruses, and select parasites. Multiplex testing has yielded increased detection rates, compared with microscopic examination alone in some settings. Further, they also share ease-of-use and rapid turnaround times with parasite antigen assays while requiring less hands-on time by laboratory personnel, compared with direct microscopic examination. However, multiplex assays are expensive and more readily detect commensal organisms, so they are not necessarily the ideal test in all diarrheal illnesses.
Diagnosis. You decide to first order giardia and cryptosporidium antigen testing because you are highly suspicious that giardia is the cause, based on wild-water exposure, the presentation, and symptom duration. You also order full microscopic O&P examination because you know that parasites can “run in packs.” Results of testing the first stool are positive for giardia. Microscopic examination on each of three stools is negative except for giardia trophozoites (the noninfectious form) in stools two and three.
Giardia overview. Giardia is the most common protozoan causing diarrhea in the United States, is fecal-oral spread, and like Shigella spp., is a low-inoculum infection (ingestion of as few as 10-100 cysts). Acquisition in the United States has been estimated as being 75% from contaminated water (streams are a classic source.2 Other sources are contaminated food (fresh produce is classic) and in some cases sexual encounters (mostly in men who have sex with men). Most detections are sporadic, but outbreaks can occur with case numbers usually below 20; 40% of outbreaks are attributable to contaminated water or food.3 Evaluating symptomatic household members can be important as transmission in families can occur.
After ingestion, the cysts uncoat and form trophozoites, which reside mostly in the small bowel (Figure), causing inflammation and altering gut membrane permeability, thereby reducing nutrient absorption and circulating amino acids. Along with decreased food intake, altered absorption can lead to weight loss and potentially reduce growth in young children. Some trophozoites replicate while others encyst, eventually passing into stool. The cysts can survive for months in water or the environment (lakes, swimming pools, and clear mountain streams). Giardia has been linked to beavers’ feces contaminating wild-water sources, hence the moniker “Beaver fever” and warnings about stream water related to wilderness hiking.4

Management. Supportive therapy as with any diarrheal illness is the cornerstone of management. Specific antiparasitic treatment has traditionally been with metronidazole compounded into a liquid for young children, but the awful taste and frequent dosing often result in poor adherence. Nevertheless, published cure rates range from 80% to 100%. The taste issue, known adverse effects, and lack of FDA approval for giardia, have led to use of other drugs.5 One dose of tinidazole is as effective as metronidazole and can be prescribed for children 3 years old or older. But the drug nitazoxanide is becoming more standard. It is as effective as either alternative, and is FDA approved for children 1 year old and older. Nitazoxanide also is effective against other intestinal parasites (e.g., cryptosporidium). Nitazoxanide’s 3-day course involves every-12-hour dosing with food with each dose being 5 mL (100 mg) for 1- to 3-year-olds, 10 mL (200 mg) for 4- to 11-year-olds, and one tablet (500 mg) or 25 mL (500 mg) for children 12 years old or older.6
Key elements in this subacute nonsystemic diarrheal presentation were primitive camping history, multiple stream water contacts, nearly 2 weeks of symptoms, weight loss, and flatulence/cramping, but no fever or stool blood/mucous. Two friends also appear to be similarly symptomatic, so a common exposure seemed likely This is typical for several summertime activity–related parasites. So,
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital–Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines. The hospital also receives CDC funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus. Email Dr. Harrison at [email protected].
References
1. Diagnosis and Treatment Information for Medical Professionals, Giardia, Parasites. CDC.
2. Krumrie S et al. Curr Res Parasitol Vector Borne Dis. 2022;2:100084. doi: 10.1016/j.crpvbd.2022.100084.
3. Baldursson S and Karanis P. Water Res. 2011 Dec 15. doi: 10.1016/j.watres.2011.10.013.
4. “Water on the Appalachian Trail” AppalachianTrail.com.
5. Giardiasis: Treatment and prevention. UpToDate.
6. Kimberlin D et al. Red Book: 2021-2024 Report of the Committee on Infectious Diseases (Itasca, Ill.: American Academy of Pediatrics, 2021. 32nd ed.) Giardia duodenalis infections. pp. 335-8; and p. 961 (Table 4.11).
It’s “summertime and the livin’ is easy” according to the lyric from an old George Gershwin song. But sometimes, summer activities can lead to illnesses that can disrupt a child’s easy living.
Case: An otherwise healthy 11-year-old presents with four to five loose stools per day, mild nausea, excess flatulence, and cramps for 12 days with a 5-pound weight loss. His loose-to-mushy stools have no blood or mucous but smell worse than usual. He has had no fever, vomiting, rashes, or joint symptoms. A month ago, he went hiking/camping on the Appalachian Trail, drank boiled stream water. and slept in a common-use semi-enclosed shelter. He waded through streams and shared “Trail Magic” (soft drinks being cooled in a fresh mountain stream). Two other campers report similar symptoms.
Differential diagnosis: Broadly, we should consider bacteria, viruses, and parasites. But generally, bacteria are likely to produce more systemic symptoms and usually do not last 12 days. That said, this could be Clostridioides difficile, yet that seems unlikely because he is otherwise healthy and has no apparent risk factors. Salmonella spp., Campylobacter spp. and some Escherichia coli infections may drag on for more than a week but the lack of systemic symptoms or blood/mucous lowers the likelihood. Viral agents (rotavirus, norovirus, adenovirus, astrovirus, calicivirus, or sapovirus) seem unlikely because of the long symptom duration and the child’s preteen age.
The history and presentation seem more likely attributable to a parasite. Uncommonly detected protozoa include Microsporidium (mostly Enterocytozoon bieneusi) and amoeba. Microsporidium is very rare and seen mostly in immune compromised hosts, for example, those living with HIV. Amebiasis occurs mostly after travel to endemic areas, and stools usually contain blood or mucous. Some roundworm or tapeworm infestations cause abdominal pain and abnormal stools, but the usual exposures are absent. Giardia spp., Cryptosporidium spp., Cyclospora cayetanensis, and/or Cystoisospora belli best fit this presentation given his hiking/camping trip.
Workup. Laboratory testing of stool is warranted (because of weight loss and persistent diarrhea) despite a lack of systemic signs. Initially, bacterial culture, C. difficile testing, and viral testing seem unwarranted. The best initial approach, given our most likely suspects, is protozoan/parasite testing.
The Centers for Disease Control and Prevention recommends testing up to three stools collected on separate days.1 Initially, stool testing for giardia and cryptosporidium antigens by EIA assays could be done as a point-of-care test. Such antigen tests are often the first step because of their ease of use, relatively low expense, reasonably high sensitivity and specificity, and rapid turnaround (as little as 1 hour). Alternatively, direct examination of three stools for ova and parasites (O&P) and acid-fast stain or direct fluorescent antibody testing can usually detect our main suspects (giardia, cryptosporidium, cyclospora, and cystoisospora) along with other less likely parasites.
Some laboratories, however, use syndromic stool testing approaches (multiplex nucleic acid panels) that detect over 20 different bacteria, viruses, and select parasites. Multiplex testing has yielded increased detection rates, compared with microscopic examination alone in some settings. Further, they also share ease-of-use and rapid turnaround times with parasite antigen assays while requiring less hands-on time by laboratory personnel, compared with direct microscopic examination. However, multiplex assays are expensive and more readily detect commensal organisms, so they are not necessarily the ideal test in all diarrheal illnesses.
Diagnosis. You decide to first order giardia and cryptosporidium antigen testing because you are highly suspicious that giardia is the cause, based on wild-water exposure, the presentation, and symptom duration. You also order full microscopic O&P examination because you know that parasites can “run in packs.” Results of testing the first stool are positive for giardia. Microscopic examination on each of three stools is negative except for giardia trophozoites (the noninfectious form) in stools two and three.
Giardia overview. Giardia is the most common protozoan causing diarrhea in the United States, is fecal-oral spread, and like Shigella spp., is a low-inoculum infection (ingestion of as few as 10-100 cysts). Acquisition in the United States has been estimated as being 75% from contaminated water (streams are a classic source.2 Other sources are contaminated food (fresh produce is classic) and in some cases sexual encounters (mostly in men who have sex with men). Most detections are sporadic, but outbreaks can occur with case numbers usually below 20; 40% of outbreaks are attributable to contaminated water or food.3 Evaluating symptomatic household members can be important as transmission in families can occur.
After ingestion, the cysts uncoat and form trophozoites, which reside mostly in the small bowel (Figure), causing inflammation and altering gut membrane permeability, thereby reducing nutrient absorption and circulating amino acids. Along with decreased food intake, altered absorption can lead to weight loss and potentially reduce growth in young children. Some trophozoites replicate while others encyst, eventually passing into stool. The cysts can survive for months in water or the environment (lakes, swimming pools, and clear mountain streams). Giardia has been linked to beavers’ feces contaminating wild-water sources, hence the moniker “Beaver fever” and warnings about stream water related to wilderness hiking.4

Management. Supportive therapy as with any diarrheal illness is the cornerstone of management. Specific antiparasitic treatment has traditionally been with metronidazole compounded into a liquid for young children, but the awful taste and frequent dosing often result in poor adherence. Nevertheless, published cure rates range from 80% to 100%. The taste issue, known adverse effects, and lack of FDA approval for giardia, have led to use of other drugs.5 One dose of tinidazole is as effective as metronidazole and can be prescribed for children 3 years old or older. But the drug nitazoxanide is becoming more standard. It is as effective as either alternative, and is FDA approved for children 1 year old and older. Nitazoxanide also is effective against other intestinal parasites (e.g., cryptosporidium). Nitazoxanide’s 3-day course involves every-12-hour dosing with food with each dose being 5 mL (100 mg) for 1- to 3-year-olds, 10 mL (200 mg) for 4- to 11-year-olds, and one tablet (500 mg) or 25 mL (500 mg) for children 12 years old or older.6
Key elements in this subacute nonsystemic diarrheal presentation were primitive camping history, multiple stream water contacts, nearly 2 weeks of symptoms, weight loss, and flatulence/cramping, but no fever or stool blood/mucous. Two friends also appear to be similarly symptomatic, so a common exposure seemed likely This is typical for several summertime activity–related parasites. So,
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital–Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines. The hospital also receives CDC funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus. Email Dr. Harrison at [email protected].
References
1. Diagnosis and Treatment Information for Medical Professionals, Giardia, Parasites. CDC.
2. Krumrie S et al. Curr Res Parasitol Vector Borne Dis. 2022;2:100084. doi: 10.1016/j.crpvbd.2022.100084.
3. Baldursson S and Karanis P. Water Res. 2011 Dec 15. doi: 10.1016/j.watres.2011.10.013.
4. “Water on the Appalachian Trail” AppalachianTrail.com.
5. Giardiasis: Treatment and prevention. UpToDate.
6. Kimberlin D et al. Red Book: 2021-2024 Report of the Committee on Infectious Diseases (Itasca, Ill.: American Academy of Pediatrics, 2021. 32nd ed.) Giardia duodenalis infections. pp. 335-8; and p. 961 (Table 4.11).
Mortality post perioperative CPR climbs with patient frailty
And the frailer that patients were going into surgery, according to their scores on an established frailty index, the greater their adjusted mortality risk at 30 days and the likelier they were to be discharged to a location other than their home.
The findings are based on more than 3,000 patients in an American College of Surgeons (ACS) quality improvement registry who underwent CPR at noncardiac surgery, about one-fourth of whom scored a least 40 on the revised Risk Analysis Index (RAI). The frailty index accounts for the patient’s comorbidities, cognition, functional and nutritional status, and other factors as predictors of postoperative mortality risk.
Such CPR for perioperative cardiac arrest “should not be considered futile just because a patient is frail, but neither should cardiac arrest be considered as ‘reversible’ in this population, as previously thought,” lead author Matthew B. Allen, MD, of Brigham and Women’s Hospital, Boston, said in an interview.
“We know that patients who are frail have higher risk of complications and mortality after surgery, and recent studies have demonstrated that frailty is associated with very poor outcomes following CPR in nonsurgical settings,” said Dr. Allen, an attending physician in the department of anesthesiology, perioperative, and pain medicine at his center.
Although cardiac arrest is typically regarded as being “more reversible” in the setting of surgery and anesthesia than elsewhere in the hospital, he observed, there’s very little data on whether that is indeed the case for frail patients.
The current analysis provides “a heretofore absent base of evidence to guide decision-making regarding CPR in patients with frailty who undergo surgery,” states the report, published in JAMA Network Open.
The 3,058 patients in the analysis, from the ACS National Surgical Quality Improvement database, received CPR for cardiac arrest during or soon after noncardiac surgery. Their mean age was 71 and 44% were women.
Their RAI scores ranged from 14 to 71 and averaged 37.7; one-fourth of the patients had scores of 40 or higher, the study’s threshold for identifying patients as “frail.”
Overall in the cohort, more cardiac arrests occurred during surgeries that entailed low-to-moderate physiologic stress (an Operative Stress Score of 1 to 3) than in the setting of emergency surgery: 67.9% vs. 39.1%, respectively.
During emergency surgeries, a greater proportion of frail than nonfrail patients experienced cardiac arrest, 42% and 38%, respectively. The same relationship was observed during low-to-moderate stress surgeries: 76.6% of frail patients and 64.8% of nonfrail patients. General anesthesia was used in about 93% of procedures for both frail and nonfrail patients, the report states.
The primary endpoint, 30-day mortality, was 58.6% overall, 67.4% in frail patients, and 55.6% for nonfrail patients. Frailty and mortality were positively associated, with an adjusted odds ratio (AOR) of 1.35 (95% confidence interval [CI], 1.11-1.65, P = .003) in multivariate analysis.
Of the cohort’s 1,164 patients who had been admitted from home and survived to discharge, 38.6% were discharged to a destination other than home; the corresponding rates for frail and nonfrail patients were 59.3% and 33.9%, respectively. Frailty and nonhome discharge were positively correlated with an AOR of 1.85 (95% CI, 1.31-2.62, P < .001).
“There is no such thing as a low-risk procedure in patients who are frail,” Dr. Allen said in an interview. “Frail patients should be medically optimized prior to undergoing surgery and anesthesia, and plans should be tailored to patients’ vulnerabilities to reduce the risk of complications and facilitate rapid recognition and treatment when they occur.”
Moreover, he said, management of clinical decompensation in the perioperative period should be a part of the shared decision-making process “to establish a plan aligned with the patients’ priorities whenever possible.”
The current study quantifies risk associated with frailty in the surgical setting, and “this quantification can help providers, patients, and insurers better grasp the growing frailty problem,” Balachundhar Subramaniam, MD, MPH, of Harvard Medical School, Boston, said in an interview.
Universal screening for frailty is “a must in all surgical patients” to help identify those who are high-risk and reduce their chances for perioperative adverse events, said Dr. Subramaniam, who was not involved in the study.
“Prehabilitation with education, nutrition, physical fitness, and psychological support offer the best chance of significantly reducing poor outcomes” in frail patients, he said, along with “continuous education” in the care of frail patients.
University of Colorado surgeon Joseph Cleveland, MD, not part of the current study, said that it “provides a framework for counseling patients” regarding their do-not-resuscitate status.
“We can counsel patients with frailty with this information,” he said, “that if their heart should stop or go into in irregular rhythm, their chances of surviving are not greater than 50% and they have a more than 50% chance of not being discharged home.”
Dr. Allen reported receiving a clinical translational starter grant from Brigham and Women’s Hospital Department of Anesthesiology; disclosures for the other authors are in the original article. Dr. Subramaniam disclosed research funding from Masimo and Merck and serving as an education consultant for Masimo. Dr. Cleveland reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
And the frailer that patients were going into surgery, according to their scores on an established frailty index, the greater their adjusted mortality risk at 30 days and the likelier they were to be discharged to a location other than their home.
The findings are based on more than 3,000 patients in an American College of Surgeons (ACS) quality improvement registry who underwent CPR at noncardiac surgery, about one-fourth of whom scored a least 40 on the revised Risk Analysis Index (RAI). The frailty index accounts for the patient’s comorbidities, cognition, functional and nutritional status, and other factors as predictors of postoperative mortality risk.
Such CPR for perioperative cardiac arrest “should not be considered futile just because a patient is frail, but neither should cardiac arrest be considered as ‘reversible’ in this population, as previously thought,” lead author Matthew B. Allen, MD, of Brigham and Women’s Hospital, Boston, said in an interview.
“We know that patients who are frail have higher risk of complications and mortality after surgery, and recent studies have demonstrated that frailty is associated with very poor outcomes following CPR in nonsurgical settings,” said Dr. Allen, an attending physician in the department of anesthesiology, perioperative, and pain medicine at his center.
Although cardiac arrest is typically regarded as being “more reversible” in the setting of surgery and anesthesia than elsewhere in the hospital, he observed, there’s very little data on whether that is indeed the case for frail patients.
The current analysis provides “a heretofore absent base of evidence to guide decision-making regarding CPR in patients with frailty who undergo surgery,” states the report, published in JAMA Network Open.
The 3,058 patients in the analysis, from the ACS National Surgical Quality Improvement database, received CPR for cardiac arrest during or soon after noncardiac surgery. Their mean age was 71 and 44% were women.
Their RAI scores ranged from 14 to 71 and averaged 37.7; one-fourth of the patients had scores of 40 or higher, the study’s threshold for identifying patients as “frail.”
Overall in the cohort, more cardiac arrests occurred during surgeries that entailed low-to-moderate physiologic stress (an Operative Stress Score of 1 to 3) than in the setting of emergency surgery: 67.9% vs. 39.1%, respectively.
During emergency surgeries, a greater proportion of frail than nonfrail patients experienced cardiac arrest, 42% and 38%, respectively. The same relationship was observed during low-to-moderate stress surgeries: 76.6% of frail patients and 64.8% of nonfrail patients. General anesthesia was used in about 93% of procedures for both frail and nonfrail patients, the report states.
The primary endpoint, 30-day mortality, was 58.6% overall, 67.4% in frail patients, and 55.6% for nonfrail patients. Frailty and mortality were positively associated, with an adjusted odds ratio (AOR) of 1.35 (95% confidence interval [CI], 1.11-1.65, P = .003) in multivariate analysis.
Of the cohort’s 1,164 patients who had been admitted from home and survived to discharge, 38.6% were discharged to a destination other than home; the corresponding rates for frail and nonfrail patients were 59.3% and 33.9%, respectively. Frailty and nonhome discharge were positively correlated with an AOR of 1.85 (95% CI, 1.31-2.62, P < .001).
“There is no such thing as a low-risk procedure in patients who are frail,” Dr. Allen said in an interview. “Frail patients should be medically optimized prior to undergoing surgery and anesthesia, and plans should be tailored to patients’ vulnerabilities to reduce the risk of complications and facilitate rapid recognition and treatment when they occur.”
Moreover, he said, management of clinical decompensation in the perioperative period should be a part of the shared decision-making process “to establish a plan aligned with the patients’ priorities whenever possible.”
The current study quantifies risk associated with frailty in the surgical setting, and “this quantification can help providers, patients, and insurers better grasp the growing frailty problem,” Balachundhar Subramaniam, MD, MPH, of Harvard Medical School, Boston, said in an interview.
Universal screening for frailty is “a must in all surgical patients” to help identify those who are high-risk and reduce their chances for perioperative adverse events, said Dr. Subramaniam, who was not involved in the study.
“Prehabilitation with education, nutrition, physical fitness, and psychological support offer the best chance of significantly reducing poor outcomes” in frail patients, he said, along with “continuous education” in the care of frail patients.
University of Colorado surgeon Joseph Cleveland, MD, not part of the current study, said that it “provides a framework for counseling patients” regarding their do-not-resuscitate status.
“We can counsel patients with frailty with this information,” he said, “that if their heart should stop or go into in irregular rhythm, their chances of surviving are not greater than 50% and they have a more than 50% chance of not being discharged home.”
Dr. Allen reported receiving a clinical translational starter grant from Brigham and Women’s Hospital Department of Anesthesiology; disclosures for the other authors are in the original article. Dr. Subramaniam disclosed research funding from Masimo and Merck and serving as an education consultant for Masimo. Dr. Cleveland reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
And the frailer that patients were going into surgery, according to their scores on an established frailty index, the greater their adjusted mortality risk at 30 days and the likelier they were to be discharged to a location other than their home.
The findings are based on more than 3,000 patients in an American College of Surgeons (ACS) quality improvement registry who underwent CPR at noncardiac surgery, about one-fourth of whom scored a least 40 on the revised Risk Analysis Index (RAI). The frailty index accounts for the patient’s comorbidities, cognition, functional and nutritional status, and other factors as predictors of postoperative mortality risk.
Such CPR for perioperative cardiac arrest “should not be considered futile just because a patient is frail, but neither should cardiac arrest be considered as ‘reversible’ in this population, as previously thought,” lead author Matthew B. Allen, MD, of Brigham and Women’s Hospital, Boston, said in an interview.
“We know that patients who are frail have higher risk of complications and mortality after surgery, and recent studies have demonstrated that frailty is associated with very poor outcomes following CPR in nonsurgical settings,” said Dr. Allen, an attending physician in the department of anesthesiology, perioperative, and pain medicine at his center.
Although cardiac arrest is typically regarded as being “more reversible” in the setting of surgery and anesthesia than elsewhere in the hospital, he observed, there’s very little data on whether that is indeed the case for frail patients.
The current analysis provides “a heretofore absent base of evidence to guide decision-making regarding CPR in patients with frailty who undergo surgery,” states the report, published in JAMA Network Open.
The 3,058 patients in the analysis, from the ACS National Surgical Quality Improvement database, received CPR for cardiac arrest during or soon after noncardiac surgery. Their mean age was 71 and 44% were women.
Their RAI scores ranged from 14 to 71 and averaged 37.7; one-fourth of the patients had scores of 40 or higher, the study’s threshold for identifying patients as “frail.”
Overall in the cohort, more cardiac arrests occurred during surgeries that entailed low-to-moderate physiologic stress (an Operative Stress Score of 1 to 3) than in the setting of emergency surgery: 67.9% vs. 39.1%, respectively.
During emergency surgeries, a greater proportion of frail than nonfrail patients experienced cardiac arrest, 42% and 38%, respectively. The same relationship was observed during low-to-moderate stress surgeries: 76.6% of frail patients and 64.8% of nonfrail patients. General anesthesia was used in about 93% of procedures for both frail and nonfrail patients, the report states.
The primary endpoint, 30-day mortality, was 58.6% overall, 67.4% in frail patients, and 55.6% for nonfrail patients. Frailty and mortality were positively associated, with an adjusted odds ratio (AOR) of 1.35 (95% confidence interval [CI], 1.11-1.65, P = .003) in multivariate analysis.
Of the cohort’s 1,164 patients who had been admitted from home and survived to discharge, 38.6% were discharged to a destination other than home; the corresponding rates for frail and nonfrail patients were 59.3% and 33.9%, respectively. Frailty and nonhome discharge were positively correlated with an AOR of 1.85 (95% CI, 1.31-2.62, P < .001).
“There is no such thing as a low-risk procedure in patients who are frail,” Dr. Allen said in an interview. “Frail patients should be medically optimized prior to undergoing surgery and anesthesia, and plans should be tailored to patients’ vulnerabilities to reduce the risk of complications and facilitate rapid recognition and treatment when they occur.”
Moreover, he said, management of clinical decompensation in the perioperative period should be a part of the shared decision-making process “to establish a plan aligned with the patients’ priorities whenever possible.”
The current study quantifies risk associated with frailty in the surgical setting, and “this quantification can help providers, patients, and insurers better grasp the growing frailty problem,” Balachundhar Subramaniam, MD, MPH, of Harvard Medical School, Boston, said in an interview.
Universal screening for frailty is “a must in all surgical patients” to help identify those who are high-risk and reduce their chances for perioperative adverse events, said Dr. Subramaniam, who was not involved in the study.
“Prehabilitation with education, nutrition, physical fitness, and psychological support offer the best chance of significantly reducing poor outcomes” in frail patients, he said, along with “continuous education” in the care of frail patients.
University of Colorado surgeon Joseph Cleveland, MD, not part of the current study, said that it “provides a framework for counseling patients” regarding their do-not-resuscitate status.
“We can counsel patients with frailty with this information,” he said, “that if their heart should stop or go into in irregular rhythm, their chances of surviving are not greater than 50% and they have a more than 50% chance of not being discharged home.”
Dr. Allen reported receiving a clinical translational starter grant from Brigham and Women’s Hospital Department of Anesthesiology; disclosures for the other authors are in the original article. Dr. Subramaniam disclosed research funding from Masimo and Merck and serving as an education consultant for Masimo. Dr. Cleveland reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Porcelain White, Crinkled, Violaceous Patches on the Inner Thighs
The Diagnosis: Extragenital Lichen Sclerosus et Atrophicus
A punch biopsy of the lesion revealed epidermal hyperkeratosis, atrophy, follicular plugs with basal vacuolar degeneration, and homogenous dense fibrosis in the papillary dermis with a dense lymphocytic infiltrate beneath the fibrosis (Figure 1). Dermoscopic examination was remarkable for a distinctive rainbow pattern. Clinical, histopathologic, and dermoscopic findings led to the diagnosis of extragenital lichen sclerosus et atrophicus (LSEA). A potent corticosteroid cream was prescribed twice daily for 2 months, after which the lesions completely resolved. At 2-year follow-up, a relapse was not observed (Figure 2).
Lichen sclerosus et atrophicus is an inflammatory dermatosis that clinically presents as atrophic or hypertrophic plaques that may show pigmentation changes with anogenital and extragenital involvement. It is common among females and predominantly occurs in prepubescent girls and postmenopausal women. The exact etiology is unclear; however, it is hypothesized to occur secondary to autoimmunity with an underlying genetic predisposition. Local trauma, hormonal influences, and infections are other suspected etiologic factors. Genital lesions often lead to itching, pain, and dyspareunia, whereas extragenital lesions predominantly are asymptomatic. When symptomatic, itching usually is the main concern. Unlike genital LSEA, extragenital lesions are not associated with squamous cell carcinoma development. Reported dermoscopic features of LSEA are white structureless areas with scaling, comedolike openings, follicular plugs, white shiny streaks, blue-gray peppering, pigment network, and red-purple globules.1 In our case, the dermoscopic finding of a rainbow pattern in LSEA is rare.2 Although the mechanism behind this appearance unclear, it can be the result of the birefringence effect—local variations in refractive index—influenced by the direction of structures within the dermis such as collagen. In this case, there was diffuse and dense homogenous fibrosis in the superficial dermis that corresponded to dermoscopic white polygonal clods.

Extragenital LSEA commonly is located on the neck, shoulders, wrists, and upper trunk and manifests clinically as whitish papules coalescing into scarlike plaques. Of all patients who have LSEA, 20% have extragenital lesions, and most of these lesions are seen in patients who also have genital LSEA. Approximately 6% of all LSEA patients have extragenital LSEA without genital involvement.3
For experienced dermatologists, clinical symptoms and lesion characteristics usually are sufficient for diagnosis; however, a differential diagnosis of atypical lesions and isolated extragenital presentations such as morphea, lichen simplex chronicus, lichen planus, and vitiligo requires the correlation of clinical findings with histopathology and dermoscopy. Morphea, known as localized scleroderma, is an idiopathic inflammatory skin disease with sclerotic changes. It manifests as inflammatory plaques that vary in color from red to purple. If there is moderate sclerosis in the center of this plaque, the color progressively fades to white, leaving a purplish ring around the edges. Dermoscopic features of morphea are reported as areas of erythema; red-focused vessels of linear, irregular, or dotted morphology; white fibrotic beams; and pigmentary structures.2 Lichen simplex chronicus is characterized by single or multiple dry and patchy skin lesions that are intensely pruritic. It commonly occurs on the neck, scalp, extremities, genital areas, and buttocks. Scratching the lesions leads to scarring, thickening of the skin, and increased frequency of itching. Histopathology of lichen simplex chronicus most frequently demonstrates a thickening of the epidermis and papillary dermis, irregularly elongated rete ridges, and fibroplasia with stellate or multinucleated fibroblasts completed by perivascular lymphocytic inflammation.4 Lichen planus presents with pruritic, polygonal, purple papules and/or plaques that can present in a variety of clinical forms, including atrophic and hypertrophic lichen planus.5 Lichen planus was an unlikely diagnosis for our patient due to the presence of patchy scarlike lesions and dermoscopic features that are well described in patients with LSEA. Lichen sclerosus et atrophicus presents with hypopigmented and/or hyperpigmented patches and plaques, distinguishing itself from vitiligo, which has flat lesions.
Topical steroids are the first-line therapeutic agents in the treatment of LSEA.6 Despite frequent use in this setting, common side effects such as localized scarring and atrophic degenerations have led to debate about their use. In our patient, the lesions resolved almost completely in 2 months, and no relapse was observed in the following 2 years. In the setting of topical steroid resistance, topical calcineurin inhibitors, UVA/UVB phototherapy, and topical tacrolimus can be used for treatment.6
The diagnosis of isolated extragenital LSEA may be a clinical challenge and generally requires further workup. When evaluating extragenital lesions, dermatologists should keep in mind extragenital LSEA as a differential diagnosis in the presence of a dermoscopic rainbow pattern arranged over white polygonal clods.
- Wang Y-K, Hao J-C, Liu J, et al. Dermoscopic features of morphea and extragenital lichen sclerosus in Chinese patients. Chin Med J (Engl). 2020;133:2109-2111.
- Errichetti E, Lallas A, Apalla Z, et al. Dermoscopy of morphea and cutaneous lichen sclerosus: clinicopathological correlation study and comparative analysis. Dermatology. 2017;233:462-470.
- Wallace HJ. Lichen sclerosus et atrophicus. Trans St Johns Hosp Dermatol Soc. 1971;57:9-30.
- Balan R, Grigoras¸ A, Popovici D, et al. The histopathological landscape of the major psoriasiform dermatoses. Arch Clin Cases. 2021;6:59-68.
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1:140-149.
- Kirtschig G, Becker K, Günthert A, et al. Evidence-based (S3) guideline on (anogenital) lichen sclerosus. J Eur Acad Dermatol Venereol. 2015;29:E1-E43.
The Diagnosis: Extragenital Lichen Sclerosus et Atrophicus
A punch biopsy of the lesion revealed epidermal hyperkeratosis, atrophy, follicular plugs with basal vacuolar degeneration, and homogenous dense fibrosis in the papillary dermis with a dense lymphocytic infiltrate beneath the fibrosis (Figure 1). Dermoscopic examination was remarkable for a distinctive rainbow pattern. Clinical, histopathologic, and dermoscopic findings led to the diagnosis of extragenital lichen sclerosus et atrophicus (LSEA). A potent corticosteroid cream was prescribed twice daily for 2 months, after which the lesions completely resolved. At 2-year follow-up, a relapse was not observed (Figure 2).

Lichen sclerosus et atrophicus is an inflammatory dermatosis that clinically presents as atrophic or hypertrophic plaques that may show pigmentation changes with anogenital and extragenital involvement. It is common among females and predominantly occurs in prepubescent girls and postmenopausal women. The exact etiology is unclear; however, it is hypothesized to occur secondary to autoimmunity with an underlying genetic predisposition. Local trauma, hormonal influences, and infections are other suspected etiologic factors. Genital lesions often lead to itching, pain, and dyspareunia, whereas extragenital lesions predominantly are asymptomatic. When symptomatic, itching usually is the main concern. Unlike genital LSEA, extragenital lesions are not associated with squamous cell carcinoma development. Reported dermoscopic features of LSEA are white structureless areas with scaling, comedolike openings, follicular plugs, white shiny streaks, blue-gray peppering, pigment network, and red-purple globules.1 In our case, the dermoscopic finding of a rainbow pattern in LSEA is rare.2 Although the mechanism behind this appearance unclear, it can be the result of the birefringence effect—local variations in refractive index—influenced by the direction of structures within the dermis such as collagen. In this case, there was diffuse and dense homogenous fibrosis in the superficial dermis that corresponded to dermoscopic white polygonal clods.

Extragenital LSEA commonly is located on the neck, shoulders, wrists, and upper trunk and manifests clinically as whitish papules coalescing into scarlike plaques. Of all patients who have LSEA, 20% have extragenital lesions, and most of these lesions are seen in patients who also have genital LSEA. Approximately 6% of all LSEA patients have extragenital LSEA without genital involvement.3
For experienced dermatologists, clinical symptoms and lesion characteristics usually are sufficient for diagnosis; however, a differential diagnosis of atypical lesions and isolated extragenital presentations such as morphea, lichen simplex chronicus, lichen planus, and vitiligo requires the correlation of clinical findings with histopathology and dermoscopy. Morphea, known as localized scleroderma, is an idiopathic inflammatory skin disease with sclerotic changes. It manifests as inflammatory plaques that vary in color from red to purple. If there is moderate sclerosis in the center of this plaque, the color progressively fades to white, leaving a purplish ring around the edges. Dermoscopic features of morphea are reported as areas of erythema; red-focused vessels of linear, irregular, or dotted morphology; white fibrotic beams; and pigmentary structures.2 Lichen simplex chronicus is characterized by single or multiple dry and patchy skin lesions that are intensely pruritic. It commonly occurs on the neck, scalp, extremities, genital areas, and buttocks. Scratching the lesions leads to scarring, thickening of the skin, and increased frequency of itching. Histopathology of lichen simplex chronicus most frequently demonstrates a thickening of the epidermis and papillary dermis, irregularly elongated rete ridges, and fibroplasia with stellate or multinucleated fibroblasts completed by perivascular lymphocytic inflammation.4 Lichen planus presents with pruritic, polygonal, purple papules and/or plaques that can present in a variety of clinical forms, including atrophic and hypertrophic lichen planus.5 Lichen planus was an unlikely diagnosis for our patient due to the presence of patchy scarlike lesions and dermoscopic features that are well described in patients with LSEA. Lichen sclerosus et atrophicus presents with hypopigmented and/or hyperpigmented patches and plaques, distinguishing itself from vitiligo, which has flat lesions.
Topical steroids are the first-line therapeutic agents in the treatment of LSEA.6 Despite frequent use in this setting, common side effects such as localized scarring and atrophic degenerations have led to debate about their use. In our patient, the lesions resolved almost completely in 2 months, and no relapse was observed in the following 2 years. In the setting of topical steroid resistance, topical calcineurin inhibitors, UVA/UVB phototherapy, and topical tacrolimus can be used for treatment.6
The diagnosis of isolated extragenital LSEA may be a clinical challenge and generally requires further workup. When evaluating extragenital lesions, dermatologists should keep in mind extragenital LSEA as a differential diagnosis in the presence of a dermoscopic rainbow pattern arranged over white polygonal clods.
The Diagnosis: Extragenital Lichen Sclerosus et Atrophicus
A punch biopsy of the lesion revealed epidermal hyperkeratosis, atrophy, follicular plugs with basal vacuolar degeneration, and homogenous dense fibrosis in the papillary dermis with a dense lymphocytic infiltrate beneath the fibrosis (Figure 1). Dermoscopic examination was remarkable for a distinctive rainbow pattern. Clinical, histopathologic, and dermoscopic findings led to the diagnosis of extragenital lichen sclerosus et atrophicus (LSEA). A potent corticosteroid cream was prescribed twice daily for 2 months, after which the lesions completely resolved. At 2-year follow-up, a relapse was not observed (Figure 2).

Lichen sclerosus et atrophicus is an inflammatory dermatosis that clinically presents as atrophic or hypertrophic plaques that may show pigmentation changes with anogenital and extragenital involvement. It is common among females and predominantly occurs in prepubescent girls and postmenopausal women. The exact etiology is unclear; however, it is hypothesized to occur secondary to autoimmunity with an underlying genetic predisposition. Local trauma, hormonal influences, and infections are other suspected etiologic factors. Genital lesions often lead to itching, pain, and dyspareunia, whereas extragenital lesions predominantly are asymptomatic. When symptomatic, itching usually is the main concern. Unlike genital LSEA, extragenital lesions are not associated with squamous cell carcinoma development. Reported dermoscopic features of LSEA are white structureless areas with scaling, comedolike openings, follicular plugs, white shiny streaks, blue-gray peppering, pigment network, and red-purple globules.1 In our case, the dermoscopic finding of a rainbow pattern in LSEA is rare.2 Although the mechanism behind this appearance unclear, it can be the result of the birefringence effect—local variations in refractive index—influenced by the direction of structures within the dermis such as collagen. In this case, there was diffuse and dense homogenous fibrosis in the superficial dermis that corresponded to dermoscopic white polygonal clods.

Extragenital LSEA commonly is located on the neck, shoulders, wrists, and upper trunk and manifests clinically as whitish papules coalescing into scarlike plaques. Of all patients who have LSEA, 20% have extragenital lesions, and most of these lesions are seen in patients who also have genital LSEA. Approximately 6% of all LSEA patients have extragenital LSEA without genital involvement.3
For experienced dermatologists, clinical symptoms and lesion characteristics usually are sufficient for diagnosis; however, a differential diagnosis of atypical lesions and isolated extragenital presentations such as morphea, lichen simplex chronicus, lichen planus, and vitiligo requires the correlation of clinical findings with histopathology and dermoscopy. Morphea, known as localized scleroderma, is an idiopathic inflammatory skin disease with sclerotic changes. It manifests as inflammatory plaques that vary in color from red to purple. If there is moderate sclerosis in the center of this plaque, the color progressively fades to white, leaving a purplish ring around the edges. Dermoscopic features of morphea are reported as areas of erythema; red-focused vessels of linear, irregular, or dotted morphology; white fibrotic beams; and pigmentary structures.2 Lichen simplex chronicus is characterized by single or multiple dry and patchy skin lesions that are intensely pruritic. It commonly occurs on the neck, scalp, extremities, genital areas, and buttocks. Scratching the lesions leads to scarring, thickening of the skin, and increased frequency of itching. Histopathology of lichen simplex chronicus most frequently demonstrates a thickening of the epidermis and papillary dermis, irregularly elongated rete ridges, and fibroplasia with stellate or multinucleated fibroblasts completed by perivascular lymphocytic inflammation.4 Lichen planus presents with pruritic, polygonal, purple papules and/or plaques that can present in a variety of clinical forms, including atrophic and hypertrophic lichen planus.5 Lichen planus was an unlikely diagnosis for our patient due to the presence of patchy scarlike lesions and dermoscopic features that are well described in patients with LSEA. Lichen sclerosus et atrophicus presents with hypopigmented and/or hyperpigmented patches and plaques, distinguishing itself from vitiligo, which has flat lesions.
Topical steroids are the first-line therapeutic agents in the treatment of LSEA.6 Despite frequent use in this setting, common side effects such as localized scarring and atrophic degenerations have led to debate about their use. In our patient, the lesions resolved almost completely in 2 months, and no relapse was observed in the following 2 years. In the setting of topical steroid resistance, topical calcineurin inhibitors, UVA/UVB phototherapy, and topical tacrolimus can be used for treatment.6
The diagnosis of isolated extragenital LSEA may be a clinical challenge and generally requires further workup. When evaluating extragenital lesions, dermatologists should keep in mind extragenital LSEA as a differential diagnosis in the presence of a dermoscopic rainbow pattern arranged over white polygonal clods.
- Wang Y-K, Hao J-C, Liu J, et al. Dermoscopic features of morphea and extragenital lichen sclerosus in Chinese patients. Chin Med J (Engl). 2020;133:2109-2111.
- Errichetti E, Lallas A, Apalla Z, et al. Dermoscopy of morphea and cutaneous lichen sclerosus: clinicopathological correlation study and comparative analysis. Dermatology. 2017;233:462-470.
- Wallace HJ. Lichen sclerosus et atrophicus. Trans St Johns Hosp Dermatol Soc. 1971;57:9-30.
- Balan R, Grigoras¸ A, Popovici D, et al. The histopathological landscape of the major psoriasiform dermatoses. Arch Clin Cases. 2021;6:59-68.
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1:140-149.
- Kirtschig G, Becker K, Günthert A, et al. Evidence-based (S3) guideline on (anogenital) lichen sclerosus. J Eur Acad Dermatol Venereol. 2015;29:E1-E43.
- Wang Y-K, Hao J-C, Liu J, et al. Dermoscopic features of morphea and extragenital lichen sclerosus in Chinese patients. Chin Med J (Engl). 2020;133:2109-2111.
- Errichetti E, Lallas A, Apalla Z, et al. Dermoscopy of morphea and cutaneous lichen sclerosus: clinicopathological correlation study and comparative analysis. Dermatology. 2017;233:462-470.
- Wallace HJ. Lichen sclerosus et atrophicus. Trans St Johns Hosp Dermatol Soc. 1971;57:9-30.
- Balan R, Grigoras¸ A, Popovici D, et al. The histopathological landscape of the major psoriasiform dermatoses. Arch Clin Cases. 2021;6:59-68.
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1:140-149.
- Kirtschig G, Becker K, Günthert A, et al. Evidence-based (S3) guideline on (anogenital) lichen sclerosus. J Eur Acad Dermatol Venereol. 2015;29:E1-E43.
A 50-year-old woman presented with multiple pruritic lesions on the right inner thigh of 2 years’ duration. Physical examination revealed porcelain white, crinkled, violaceous patches extending from the right inner thigh to the inguinal fold (top). Dermoscopic examination revealed follicular plugs, white structureless areas, white lines, and a rainbow pattern arranged over white polygonal clods on polarized mode (bottom).
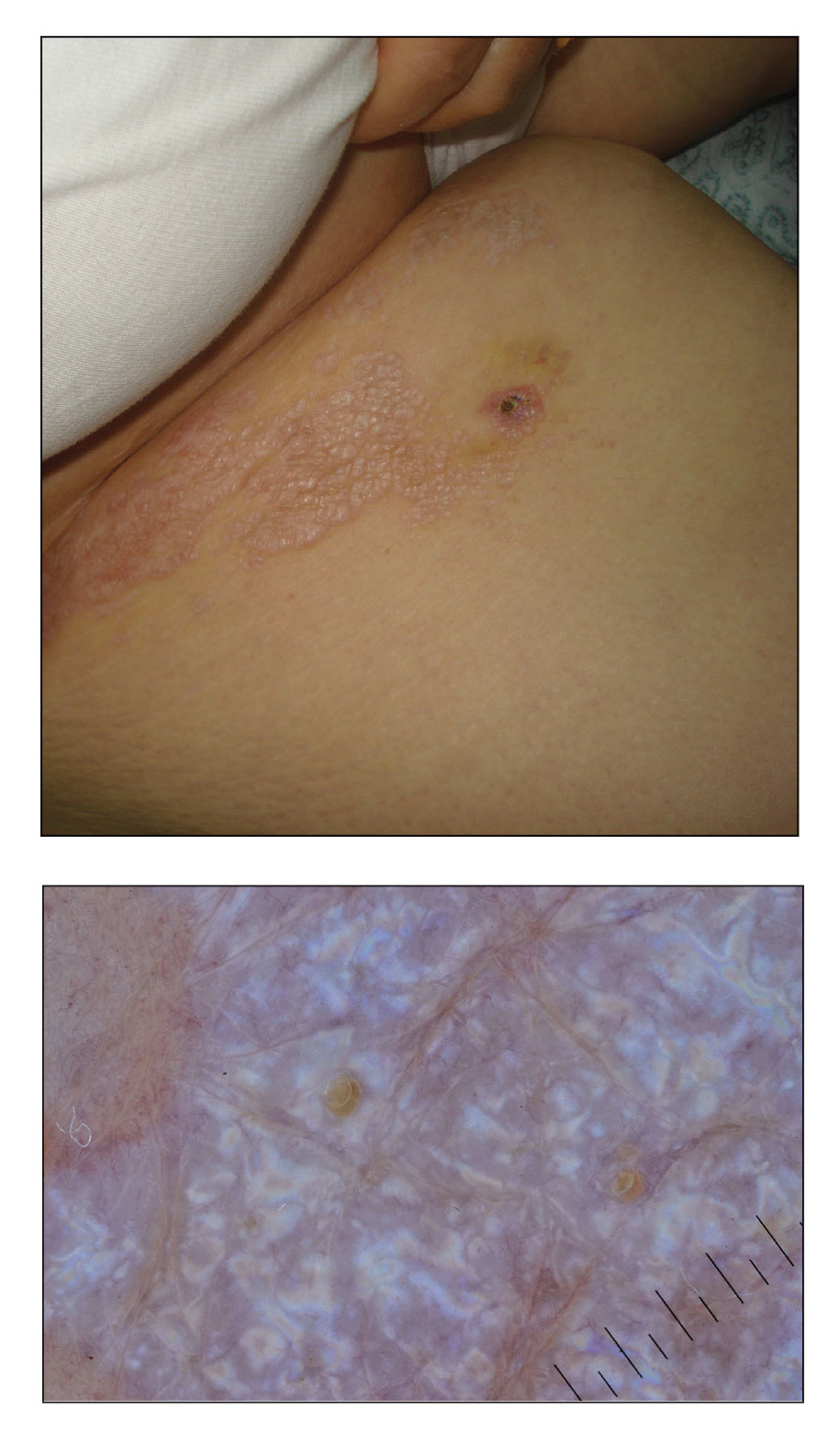
Invasive test may cut health care costs related to angina without obstructive CAD
Performing coronary reactivity (CR) testing during angiography in patients with angina without obstructive coronary disease (ANOCA) adds complexity and cost to the procedure but may, in the end, turn out to be a bargain.
probably by helping to limit downstream procedures and other use of health care resources.
In the retrospective study, CR testing included both adenosine and acetylcholine challenges to differentiate endothelium-dependent from endothelium-independent coronary reactivity as the physiological cause of ANOCA in individuals. Clarifying the cause at this stage of the patient’s clinical journey seemed to sharpen subsequent testing and management decisions, the researchers said.
Of the 414 patients with ANOCA who underwent invasive diagnostic angiography, 207 also received CR testing during the procedure. The difference in total healthcare costs between the two groups over 2 years averaged about $20,000, largely because of the CR-testing group’s reduced use of downstream imaging, interventional procedures, and other tests, notes a report on the study published in Circulation: Cardiovascular Interventions.
For such patients referred for coronary angiography found to be without obstructive disease, “the right thing to do is a vascular reactivity test to assess if there is any abnormality that can contribute to this patient’s symptoms and events,” senior author Amir Lerman, MD, Mayo Clinic, Rochester, Minn., said in an interview. Coronary reactivity testing “is expensive to set up initially, but it actually saves money by reducing the need for additional unnecessary testing and hospitalizations in these patients.”
The financial burden linked to the diagnosis and treatment of patients with chest pain is considerable, Dr. Lerman observed. It can involve a series of tests and culminate in a coronary angiogram. However, symptoms may continue if therapy does not correctly target one or more of several different potential mechanisms, including microvascular dysfunction and vasospastic disorders.
“This paper says that if you establish a program of coronary reactivity testing you will ultimately reduce health care costs, because patients stop coming back to the hospital, or their physician stops ordering more tests or repeat angiograms because the patient has a true diagnosis,” observed Morton J. Kern, MD, University of California, Irvine, and VA Long Beach (Calif.) Health Care.
“That elimination of uncertainty and reduction of testing has a good payoff,” Dr. Kern said in an interview. “The concept is good; the only challenge is that this is a complicated set of manipulations in the cath lab to get to the results.”
A minority of cardiac cath labs in the United States perform CR testing, despite its inclusion for ANOCA in guidelines from both the European Society of Cardiology and the American Heart Association/American College of Cardiology, the authors noted. Cost and its requirement for specialized expertise may contribute to its poor uptake in practice.
In an editorial accompanying the report, Dr. Kern and David J. Cohen, MD, Cardiovascular Research Foundation, New York, and St. Francis Hospital and Heart Center, Roslyn, N.Y., said they agree with the authors’ recommendation for more widespread use of CR testing.
However, the initiation of a CR testing program is no small task. “In addition to motivated practitioners, operators with specific procedural expertise must have formalized technical training to produce valid results and to limit the procedural risks,” they wrote.
Moreover, expenses for a CR testing program “will likely be incurred without balanced reimbursement, but the health care system will benefit in the long run.”
The total health-related costs for the two groups of 207 in the analysis were tracked for 2 years after the procedure and found to be significantly higher (P < .001) in the group that had received coronary angiography without CR testing. Their average annual cost was $37,804 (range, $26,933-$48,674), compared with $13,679 (range, $9,447-$17,910) for those that had undergone CR testing.
The angiography-only group’s costs for procedures (including surgical or percutaneous intervention, endoscopy, and bronchoscopy) averaged $5,872 (range, $3,798-$7,946), compared with $2,104 (range, $1,202-$3,006) in the CR testing group (P = .001).
Similarly, costs for any type of imaging, including at cardiac catheterization, averaged $2,639 (range, $2,093 to $3,185) and $1,426 (range, $1,090 to $1,761), respectively (P < .001).
Annual total hospital services costs were also higher in the angiography-only group at $21,820 versus $6,409 (P < .001) for the group that underwent CR testing.
Caution is required when interpreting these results, Matthew Tomey, MD, Mount Sinai Morningside Hospital and Icahn School of Medicine at Mount Sinai, New York, said in an interview.
“The observed cost differences are interesting and hypothesis generating but they fall short of providing strong evidence that [CR testing] saves money or that [it] should be incorporated into routine practice,” Dr. Tomey said. “Multiple biases can skew findings of retrospective observational studies. A prospective, randomized study would be needed to draw stronger conclusions.”
Still, it’s true that “there is substantial opportunity to do better in diagnosing chest pain in our patients with no apparent, explanatory obstructive coronary atherosclerosis,” he said. “There are emerging invasive and noninvasive ways to do so. Helping our patients get to the right diagnosis is the right thing to do. It will lead to better treatment recommendations, improved patient symptoms, improved patient confidence, and – it stands to reason – cost benefits in the long term.”
The study was funded by a grant from Philips. Dr. Lerman reported receiving honoraria from Philips Volcano. Dr. Kern disclosed speaking for Abbott Vascular, Boston Scientific, Acist, Opsens, and Zoll. Dr. Cohen disclosed receiving institutional grant support from Abbott Vascular, Boston Scientific, CathWorks, and Philips; and consulting income from Abbott, Boston Scientific, and Medtronic. Dr. Tomey reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Performing coronary reactivity (CR) testing during angiography in patients with angina without obstructive coronary disease (ANOCA) adds complexity and cost to the procedure but may, in the end, turn out to be a bargain.
probably by helping to limit downstream procedures and other use of health care resources.
In the retrospective study, CR testing included both adenosine and acetylcholine challenges to differentiate endothelium-dependent from endothelium-independent coronary reactivity as the physiological cause of ANOCA in individuals. Clarifying the cause at this stage of the patient’s clinical journey seemed to sharpen subsequent testing and management decisions, the researchers said.
Of the 414 patients with ANOCA who underwent invasive diagnostic angiography, 207 also received CR testing during the procedure. The difference in total healthcare costs between the two groups over 2 years averaged about $20,000, largely because of the CR-testing group’s reduced use of downstream imaging, interventional procedures, and other tests, notes a report on the study published in Circulation: Cardiovascular Interventions.
For such patients referred for coronary angiography found to be without obstructive disease, “the right thing to do is a vascular reactivity test to assess if there is any abnormality that can contribute to this patient’s symptoms and events,” senior author Amir Lerman, MD, Mayo Clinic, Rochester, Minn., said in an interview. Coronary reactivity testing “is expensive to set up initially, but it actually saves money by reducing the need for additional unnecessary testing and hospitalizations in these patients.”
The financial burden linked to the diagnosis and treatment of patients with chest pain is considerable, Dr. Lerman observed. It can involve a series of tests and culminate in a coronary angiogram. However, symptoms may continue if therapy does not correctly target one or more of several different potential mechanisms, including microvascular dysfunction and vasospastic disorders.
“This paper says that if you establish a program of coronary reactivity testing you will ultimately reduce health care costs, because patients stop coming back to the hospital, or their physician stops ordering more tests or repeat angiograms because the patient has a true diagnosis,” observed Morton J. Kern, MD, University of California, Irvine, and VA Long Beach (Calif.) Health Care.
“That elimination of uncertainty and reduction of testing has a good payoff,” Dr. Kern said in an interview. “The concept is good; the only challenge is that this is a complicated set of manipulations in the cath lab to get to the results.”
A minority of cardiac cath labs in the United States perform CR testing, despite its inclusion for ANOCA in guidelines from both the European Society of Cardiology and the American Heart Association/American College of Cardiology, the authors noted. Cost and its requirement for specialized expertise may contribute to its poor uptake in practice.
In an editorial accompanying the report, Dr. Kern and David J. Cohen, MD, Cardiovascular Research Foundation, New York, and St. Francis Hospital and Heart Center, Roslyn, N.Y., said they agree with the authors’ recommendation for more widespread use of CR testing.
However, the initiation of a CR testing program is no small task. “In addition to motivated practitioners, operators with specific procedural expertise must have formalized technical training to produce valid results and to limit the procedural risks,” they wrote.
Moreover, expenses for a CR testing program “will likely be incurred without balanced reimbursement, but the health care system will benefit in the long run.”
The total health-related costs for the two groups of 207 in the analysis were tracked for 2 years after the procedure and found to be significantly higher (P < .001) in the group that had received coronary angiography without CR testing. Their average annual cost was $37,804 (range, $26,933-$48,674), compared with $13,679 (range, $9,447-$17,910) for those that had undergone CR testing.
The angiography-only group’s costs for procedures (including surgical or percutaneous intervention, endoscopy, and bronchoscopy) averaged $5,872 (range, $3,798-$7,946), compared with $2,104 (range, $1,202-$3,006) in the CR testing group (P = .001).
Similarly, costs for any type of imaging, including at cardiac catheterization, averaged $2,639 (range, $2,093 to $3,185) and $1,426 (range, $1,090 to $1,761), respectively (P < .001).
Annual total hospital services costs were also higher in the angiography-only group at $21,820 versus $6,409 (P < .001) for the group that underwent CR testing.
Caution is required when interpreting these results, Matthew Tomey, MD, Mount Sinai Morningside Hospital and Icahn School of Medicine at Mount Sinai, New York, said in an interview.
“The observed cost differences are interesting and hypothesis generating but they fall short of providing strong evidence that [CR testing] saves money or that [it] should be incorporated into routine practice,” Dr. Tomey said. “Multiple biases can skew findings of retrospective observational studies. A prospective, randomized study would be needed to draw stronger conclusions.”
Still, it’s true that “there is substantial opportunity to do better in diagnosing chest pain in our patients with no apparent, explanatory obstructive coronary atherosclerosis,” he said. “There are emerging invasive and noninvasive ways to do so. Helping our patients get to the right diagnosis is the right thing to do. It will lead to better treatment recommendations, improved patient symptoms, improved patient confidence, and – it stands to reason – cost benefits in the long term.”
The study was funded by a grant from Philips. Dr. Lerman reported receiving honoraria from Philips Volcano. Dr. Kern disclosed speaking for Abbott Vascular, Boston Scientific, Acist, Opsens, and Zoll. Dr. Cohen disclosed receiving institutional grant support from Abbott Vascular, Boston Scientific, CathWorks, and Philips; and consulting income from Abbott, Boston Scientific, and Medtronic. Dr. Tomey reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Performing coronary reactivity (CR) testing during angiography in patients with angina without obstructive coronary disease (ANOCA) adds complexity and cost to the procedure but may, in the end, turn out to be a bargain.
probably by helping to limit downstream procedures and other use of health care resources.
In the retrospective study, CR testing included both adenosine and acetylcholine challenges to differentiate endothelium-dependent from endothelium-independent coronary reactivity as the physiological cause of ANOCA in individuals. Clarifying the cause at this stage of the patient’s clinical journey seemed to sharpen subsequent testing and management decisions, the researchers said.
Of the 414 patients with ANOCA who underwent invasive diagnostic angiography, 207 also received CR testing during the procedure. The difference in total healthcare costs between the two groups over 2 years averaged about $20,000, largely because of the CR-testing group’s reduced use of downstream imaging, interventional procedures, and other tests, notes a report on the study published in Circulation: Cardiovascular Interventions.
For such patients referred for coronary angiography found to be without obstructive disease, “the right thing to do is a vascular reactivity test to assess if there is any abnormality that can contribute to this patient’s symptoms and events,” senior author Amir Lerman, MD, Mayo Clinic, Rochester, Minn., said in an interview. Coronary reactivity testing “is expensive to set up initially, but it actually saves money by reducing the need for additional unnecessary testing and hospitalizations in these patients.”
The financial burden linked to the diagnosis and treatment of patients with chest pain is considerable, Dr. Lerman observed. It can involve a series of tests and culminate in a coronary angiogram. However, symptoms may continue if therapy does not correctly target one or more of several different potential mechanisms, including microvascular dysfunction and vasospastic disorders.
“This paper says that if you establish a program of coronary reactivity testing you will ultimately reduce health care costs, because patients stop coming back to the hospital, or their physician stops ordering more tests or repeat angiograms because the patient has a true diagnosis,” observed Morton J. Kern, MD, University of California, Irvine, and VA Long Beach (Calif.) Health Care.
“That elimination of uncertainty and reduction of testing has a good payoff,” Dr. Kern said in an interview. “The concept is good; the only challenge is that this is a complicated set of manipulations in the cath lab to get to the results.”
A minority of cardiac cath labs in the United States perform CR testing, despite its inclusion for ANOCA in guidelines from both the European Society of Cardiology and the American Heart Association/American College of Cardiology, the authors noted. Cost and its requirement for specialized expertise may contribute to its poor uptake in practice.
In an editorial accompanying the report, Dr. Kern and David J. Cohen, MD, Cardiovascular Research Foundation, New York, and St. Francis Hospital and Heart Center, Roslyn, N.Y., said they agree with the authors’ recommendation for more widespread use of CR testing.
However, the initiation of a CR testing program is no small task. “In addition to motivated practitioners, operators with specific procedural expertise must have formalized technical training to produce valid results and to limit the procedural risks,” they wrote.
Moreover, expenses for a CR testing program “will likely be incurred without balanced reimbursement, but the health care system will benefit in the long run.”
The total health-related costs for the two groups of 207 in the analysis were tracked for 2 years after the procedure and found to be significantly higher (P < .001) in the group that had received coronary angiography without CR testing. Their average annual cost was $37,804 (range, $26,933-$48,674), compared with $13,679 (range, $9,447-$17,910) for those that had undergone CR testing.
The angiography-only group’s costs for procedures (including surgical or percutaneous intervention, endoscopy, and bronchoscopy) averaged $5,872 (range, $3,798-$7,946), compared with $2,104 (range, $1,202-$3,006) in the CR testing group (P = .001).
Similarly, costs for any type of imaging, including at cardiac catheterization, averaged $2,639 (range, $2,093 to $3,185) and $1,426 (range, $1,090 to $1,761), respectively (P < .001).
Annual total hospital services costs were also higher in the angiography-only group at $21,820 versus $6,409 (P < .001) for the group that underwent CR testing.
Caution is required when interpreting these results, Matthew Tomey, MD, Mount Sinai Morningside Hospital and Icahn School of Medicine at Mount Sinai, New York, said in an interview.
“The observed cost differences are interesting and hypothesis generating but they fall short of providing strong evidence that [CR testing] saves money or that [it] should be incorporated into routine practice,” Dr. Tomey said. “Multiple biases can skew findings of retrospective observational studies. A prospective, randomized study would be needed to draw stronger conclusions.”
Still, it’s true that “there is substantial opportunity to do better in diagnosing chest pain in our patients with no apparent, explanatory obstructive coronary atherosclerosis,” he said. “There are emerging invasive and noninvasive ways to do so. Helping our patients get to the right diagnosis is the right thing to do. It will lead to better treatment recommendations, improved patient symptoms, improved patient confidence, and – it stands to reason – cost benefits in the long term.”
The study was funded by a grant from Philips. Dr. Lerman reported receiving honoraria from Philips Volcano. Dr. Kern disclosed speaking for Abbott Vascular, Boston Scientific, Acist, Opsens, and Zoll. Dr. Cohen disclosed receiving institutional grant support from Abbott Vascular, Boston Scientific, CathWorks, and Philips; and consulting income from Abbott, Boston Scientific, and Medtronic. Dr. Tomey reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CIRCULATION: CARDIOVASCULAR INTERVENTIONS
Experts call for early screening for chronic kidney disease
MADRID – A late diagnosis of chronic kidney disease is cause for concern. Scientific societies are therefore advocating for screening at younger ages to reverse this trend and slow the progression of the disease. Nearly all patients seen in primary care are candidates for screening because of their risk factors for kidney disease.
During the 29th National Conference of General and Family Medicine of the Spanish Society for General and Family Physicians, Teresa Benedito, MD, family doctor and member of the society’s cardiovascular group, and Roberto Alcázar, MD, nephrologist at the Infanta Leonor University Hospital, Madrid, presented a clinical case encountered in primary care. They used this case to frame a strong argument for the importance of early screening for chronic kidney disease, and they discussed how to properly manage such screening.
The presentation followed the guidelines in the SEMG publication regarding the management and referral of patients with type 2 diabetes. Dr. Benedito explained that the first thing to ask oneself during a patient visit is “whether they present risk factors for kidney disease. If so, we can’t let them leave before we do a kidney screening.” She then listed the factors in question: age older than 60 years, African heritage, family history of chronic kidney disease, decreased kidney mass, weight loss at birth, hypertension, diabetes, smoking, obesity, and low socioeconomic status.
For his part, Dr. Alcázar mentioned how these factors are similar to cardiovascular risk factors, because “the kidneys are a ball of vessels with double capillarization for purifying blood. They’re the organs with the most arteries per unit of weight, so anything that can damage the arteries can damage the kidneys.”
Candidates for screening
“Chronic kidney disease develops in 15% of the adult population in Spain. So, it’s worth asking how many patients have been diagnosed and who should we should be screening.” To the factors listed above, Dr. Alcázar added treatment with nephrotoxic drugs (including nonsteroidal anti-inflammatory drugs) for patients with obstructive urinary tract disease, and a history of acute kidney injury for patients with chronic autoimmune disease or neoplasms. “Thus, nearly all patients seen in primary care would need to be screened.”
Another fundamental question raised was whether patients should be screened before age 60 years. “As a nephrologist, I feel that we have been diagnosing chronic kidney disease late, even though we’ve been doing everything by the book,” said Dr. Alcázar. In his opinion, “the answer to whether we should be screening earlier ... is yes, for two reasons: first, because it’s cost-effective, and second, because it’s very inexpensive.”
Dr. Benedito explained in detail the process for diagnosing this disease. She began by defining the disease as changes in kidney structure and function that last longer than 3 months. These changes are identified by use of two criteria: glomerular filtration rate less than 60 mL/min and kidney injury or lesions with or without reduced filtration rate (renal biopsy, albumin/creatinine ratio greater than 30 mg/g, proteinuria, alterations in urinary sediment or in imaging tests). Thus, “if one of these two criteria persists for more than 3 months, the diagnosis is chronic kidney disease. Also, high creatinine levels are not diagnostic for the disease,” she emphasized.
Two related parameters
Glomerular filtration and albuminuria “are highly relevant, because screening for chronic kidney disease is based on these two parameters,” said Dr. Benedito. Glomerular filtration rate varies with age, sex, ethnicity, and body mass. It is useful for identifying the stage of the disease and for monitoring disease progression. Albuminuria, on the other hand, is an indication of the severity of the disease. It’s an early marker for kidney injury and systemic disease and is more sensitive than proteinuria. Therefore, “this factor, together with glomerular filtration rate, allows us to detect, classify, and monitor the progression of chronic kidney disease.”
On this point, Dr. Alcázar emphasized the importance of trends, since variation in glomerular filtration depends on serum creatinine, which can vary by nearly 9%. He explained that glomerular filtration rate is related to the number of nephrons remaining. A glomerular filtration rate of less than 60 mL/min implies that more than half of the nephrons in each kidney have been lost. Albuminuria informs about structural damage (that is, the condition of the remaining nephrons). It’s therefore essential to test for both parameters. “We need to be actively monitoring and then making our decisions based on trends and not on isolated results. We need to be aware of albuminuria when we make our decisions,” said Dr. Alcázar. Some studies have shown the importance of testing for albuminuria whenever creatinine level is assessed. “We need to buy into this. If we don’t do this, we’ll only ever have half the information we need.”
Reducing late diagnosis
According to the IBERICAN study, 14% of patients seen in primary care in Spain have chronic kidney disease. “This statistic should make us stop and think, own our responsibility, and ask ourselves why this screening isn’t taking place [earlier],” said Dr. Benedito. She added, “We need to head off this trend toward late diagnosis. As the disease progresses, it significantly increases cardiovascular risk and leads to higher mortality, going on dialysis, transplants, et cetera.”
Dr. Alcázar noted that 80% of nephrology cases that are referred to him come from primary care. He explained the need to understand that “these patients have a sevenfold greater risk of suffering a serious cardiovascular event within the next year than people without kidney problems.” Most of these patients will experience an event, even if they don’t undergo dialysis (stage 3 and those near stage 4).
Correct staging
Also fundamental is having a detailed understanding of how staging is performed. Dr. Benedito explained that a chart that pairs glomerular filtration rate (six categories) with the level of albuminuria (three categories) should be used during the visit. For example, a case might be classified as G3a-A2. However, the simplified form of the chart may prove more practical. It classifies chronic kidney disease as being associated with mild, moderate, and severe risk, using different colors to aid comprehension.
Dr. Alcázar noted that the latest guidelines from the European Society of Hypertension for 2023 include albuminuria as an important parameter. The guidelines indicate that for a patient with moderate or severe risk, it is not necessary to calculate their score. “It’s considered high cardiovascular risk, and steps would need to be taken for intervention.”
He then listed the tools available for reversing albuminuria. The process begins by reducing salt consumption and involves the use of medications (angiotensin-converting enzyme inhibitors/angiotensin II receptor antagonists, aldosterone receptor antagonists, glucagon-like peptide-1 analogues, and sodium-glucose cotransporter-2 inhibitors, which slow kidney damage regardless of other measures) and strict management of cardiovascular risk factors (smoking, weight management, blood glucose, hypertension, and moderate physical activity).
Reducing cardiovascular risk
Dr. Alcázar highlighted important factors to keep in mind when managing each of the cardiovascular risk factors. For hypertension, the aim is to achieve levels less than 130/80 mm Hg, although recommendations vary, depending on the guidelines consulted. “KDIGO (Kidney Disease: Improving Global Outcomes) 2021 states that there is no evidence for monitoring diastolic blood pressure, only systolic blood pressure. If we measure it according to the standardized form, SBP should be less than 120 mm Hg, and if not, we would fall back on readings of 130/80 mm Hg.”
For lipid control (specifically, low-density lipoprotein cholesterol), the staging chart indicates that for patients at mild risk, levels should be less than 100 mg/dL; for those at moderate risk, less than 70 mg/dL; and for those at severe risk, less than 55 mg/dL. Hypertriglyceridemia “should only be treated with fibrates if it comes in over 1,000 mg/dL. Also, care must be taken, because these drugs interfere with creatinine excretion, increasing it,” said Dr. Alcázar.
Guidelines from the KDIGO and the American Diabetes Association state that anyone with diabetes and chronic kidney disease should receive a sodium-glucose cotransporter-2 inhibitor if their glomerular filtration rate exceeds 20 mL/min, “which may contradict slightly what it says on the label. Also, if they have hypertension, they should take an angiotensin-converting enzyme inhibitor,” said Dr. Alcázar. He added that “oral antidiabetics, including metformin, must be adjusted based on renal function if glomerular filtration rate is under 30 mL/min.”
Act immediately
When asked whether the course of chronic kidney disease can be changed, Dr. Alcázar responded with an emphatic yes and added that cardiovascular risk can also be substantially reduced. “As nephrologists, we don’t have access to patients in early stages. But family doctors do. Hence the importance of early screening, because going on dialysis at age 60 isn’t the same as at 80.” Currently, “scientific societies are encouraging authorities to screen for chronic kidney disease at earlier ages.”
Regarding drug-based therapy, Dr. Alcázar said that “empagliflozin is not currently indicated for chronic kidney disease in adults.” This sodium-glucose cotransporter-2 inhibitor delays kidney disease and reduces morbidity. Both benefits were highlighted in two recent studies (DAPA-CKD and CREDENCE). Published in January, EMPA-KIDNEY presents a new twist on nephroprotection for patients with chronic kidney disease (diabetic or not) whose glomerular filtration rates are between 20 and 40 mL/min without albuminuria or whose glomerular filtration rates are between 45 and 90 mL/min with albuminuria. For more than 6,000 patients, empagliflozin was observed “to clearly reduce kidney disease progression, cardiovascular mortality and all-cause mortality, and the need to go on dialysis,” stated Dr. Alcázar.
What professionals expect
Dr. Benedito also explained the criteria for referral to a specialist: glomerular filtration rate less than 30 mL/min (unless the patient is older than 80 years and does not have progressively worsening renal function), albumin/creatinine ratio greater than 300 mg/g, acute worsening of renal function, progressive worsening of renal function of greater than 5 mL/min/yr, chronic kidney disease, hypertension treated with triple therapy (including a diuretic) at maximum doses, anemia of less than 10 g/dL, and nonurologic hematuria, especially in combination with albuminuria.
Dr. Benedito explained what nephrologists expect from family doctors in the management of chronic kidney disease: “screening for early detection, identifying and treating risk factors for chronic kidney disease, detecting progression and complications, adjusting drugs based on glomerular filtration rate, and ensuring that our patients are benefiting from sodium-glucose cotransporter-2 inhibitors. These are among the most important steps to be taken.”
Dr. Alcázar mentioned what family doctors expect from nephrologists: “two-way communication, accessibility, coordination of actions to be taken, and using shared and mutually agreed-upon protocols.”
This article was translated from the Medscape Spanish Edition and a version appeared on Medscape.com.
MADRID – A late diagnosis of chronic kidney disease is cause for concern. Scientific societies are therefore advocating for screening at younger ages to reverse this trend and slow the progression of the disease. Nearly all patients seen in primary care are candidates for screening because of their risk factors for kidney disease.
During the 29th National Conference of General and Family Medicine of the Spanish Society for General and Family Physicians, Teresa Benedito, MD, family doctor and member of the society’s cardiovascular group, and Roberto Alcázar, MD, nephrologist at the Infanta Leonor University Hospital, Madrid, presented a clinical case encountered in primary care. They used this case to frame a strong argument for the importance of early screening for chronic kidney disease, and they discussed how to properly manage such screening.
The presentation followed the guidelines in the SEMG publication regarding the management and referral of patients with type 2 diabetes. Dr. Benedito explained that the first thing to ask oneself during a patient visit is “whether they present risk factors for kidney disease. If so, we can’t let them leave before we do a kidney screening.” She then listed the factors in question: age older than 60 years, African heritage, family history of chronic kidney disease, decreased kidney mass, weight loss at birth, hypertension, diabetes, smoking, obesity, and low socioeconomic status.
For his part, Dr. Alcázar mentioned how these factors are similar to cardiovascular risk factors, because “the kidneys are a ball of vessels with double capillarization for purifying blood. They’re the organs with the most arteries per unit of weight, so anything that can damage the arteries can damage the kidneys.”
Candidates for screening
“Chronic kidney disease develops in 15% of the adult population in Spain. So, it’s worth asking how many patients have been diagnosed and who should we should be screening.” To the factors listed above, Dr. Alcázar added treatment with nephrotoxic drugs (including nonsteroidal anti-inflammatory drugs) for patients with obstructive urinary tract disease, and a history of acute kidney injury for patients with chronic autoimmune disease or neoplasms. “Thus, nearly all patients seen in primary care would need to be screened.”
Another fundamental question raised was whether patients should be screened before age 60 years. “As a nephrologist, I feel that we have been diagnosing chronic kidney disease late, even though we’ve been doing everything by the book,” said Dr. Alcázar. In his opinion, “the answer to whether we should be screening earlier ... is yes, for two reasons: first, because it’s cost-effective, and second, because it’s very inexpensive.”
Dr. Benedito explained in detail the process for diagnosing this disease. She began by defining the disease as changes in kidney structure and function that last longer than 3 months. These changes are identified by use of two criteria: glomerular filtration rate less than 60 mL/min and kidney injury or lesions with or without reduced filtration rate (renal biopsy, albumin/creatinine ratio greater than 30 mg/g, proteinuria, alterations in urinary sediment or in imaging tests). Thus, “if one of these two criteria persists for more than 3 months, the diagnosis is chronic kidney disease. Also, high creatinine levels are not diagnostic for the disease,” she emphasized.
Two related parameters
Glomerular filtration and albuminuria “are highly relevant, because screening for chronic kidney disease is based on these two parameters,” said Dr. Benedito. Glomerular filtration rate varies with age, sex, ethnicity, and body mass. It is useful for identifying the stage of the disease and for monitoring disease progression. Albuminuria, on the other hand, is an indication of the severity of the disease. It’s an early marker for kidney injury and systemic disease and is more sensitive than proteinuria. Therefore, “this factor, together with glomerular filtration rate, allows us to detect, classify, and monitor the progression of chronic kidney disease.”
On this point, Dr. Alcázar emphasized the importance of trends, since variation in glomerular filtration depends on serum creatinine, which can vary by nearly 9%. He explained that glomerular filtration rate is related to the number of nephrons remaining. A glomerular filtration rate of less than 60 mL/min implies that more than half of the nephrons in each kidney have been lost. Albuminuria informs about structural damage (that is, the condition of the remaining nephrons). It’s therefore essential to test for both parameters. “We need to be actively monitoring and then making our decisions based on trends and not on isolated results. We need to be aware of albuminuria when we make our decisions,” said Dr. Alcázar. Some studies have shown the importance of testing for albuminuria whenever creatinine level is assessed. “We need to buy into this. If we don’t do this, we’ll only ever have half the information we need.”
Reducing late diagnosis
According to the IBERICAN study, 14% of patients seen in primary care in Spain have chronic kidney disease. “This statistic should make us stop and think, own our responsibility, and ask ourselves why this screening isn’t taking place [earlier],” said Dr. Benedito. She added, “We need to head off this trend toward late diagnosis. As the disease progresses, it significantly increases cardiovascular risk and leads to higher mortality, going on dialysis, transplants, et cetera.”
Dr. Alcázar noted that 80% of nephrology cases that are referred to him come from primary care. He explained the need to understand that “these patients have a sevenfold greater risk of suffering a serious cardiovascular event within the next year than people without kidney problems.” Most of these patients will experience an event, even if they don’t undergo dialysis (stage 3 and those near stage 4).
Correct staging
Also fundamental is having a detailed understanding of how staging is performed. Dr. Benedito explained that a chart that pairs glomerular filtration rate (six categories) with the level of albuminuria (three categories) should be used during the visit. For example, a case might be classified as G3a-A2. However, the simplified form of the chart may prove more practical. It classifies chronic kidney disease as being associated with mild, moderate, and severe risk, using different colors to aid comprehension.
Dr. Alcázar noted that the latest guidelines from the European Society of Hypertension for 2023 include albuminuria as an important parameter. The guidelines indicate that for a patient with moderate or severe risk, it is not necessary to calculate their score. “It’s considered high cardiovascular risk, and steps would need to be taken for intervention.”
He then listed the tools available for reversing albuminuria. The process begins by reducing salt consumption and involves the use of medications (angiotensin-converting enzyme inhibitors/angiotensin II receptor antagonists, aldosterone receptor antagonists, glucagon-like peptide-1 analogues, and sodium-glucose cotransporter-2 inhibitors, which slow kidney damage regardless of other measures) and strict management of cardiovascular risk factors (smoking, weight management, blood glucose, hypertension, and moderate physical activity).
Reducing cardiovascular risk
Dr. Alcázar highlighted important factors to keep in mind when managing each of the cardiovascular risk factors. For hypertension, the aim is to achieve levels less than 130/80 mm Hg, although recommendations vary, depending on the guidelines consulted. “KDIGO (Kidney Disease: Improving Global Outcomes) 2021 states that there is no evidence for monitoring diastolic blood pressure, only systolic blood pressure. If we measure it according to the standardized form, SBP should be less than 120 mm Hg, and if not, we would fall back on readings of 130/80 mm Hg.”
For lipid control (specifically, low-density lipoprotein cholesterol), the staging chart indicates that for patients at mild risk, levels should be less than 100 mg/dL; for those at moderate risk, less than 70 mg/dL; and for those at severe risk, less than 55 mg/dL. Hypertriglyceridemia “should only be treated with fibrates if it comes in over 1,000 mg/dL. Also, care must be taken, because these drugs interfere with creatinine excretion, increasing it,” said Dr. Alcázar.
Guidelines from the KDIGO and the American Diabetes Association state that anyone with diabetes and chronic kidney disease should receive a sodium-glucose cotransporter-2 inhibitor if their glomerular filtration rate exceeds 20 mL/min, “which may contradict slightly what it says on the label. Also, if they have hypertension, they should take an angiotensin-converting enzyme inhibitor,” said Dr. Alcázar. He added that “oral antidiabetics, including metformin, must be adjusted based on renal function if glomerular filtration rate is under 30 mL/min.”
Act immediately
When asked whether the course of chronic kidney disease can be changed, Dr. Alcázar responded with an emphatic yes and added that cardiovascular risk can also be substantially reduced. “As nephrologists, we don’t have access to patients in early stages. But family doctors do. Hence the importance of early screening, because going on dialysis at age 60 isn’t the same as at 80.” Currently, “scientific societies are encouraging authorities to screen for chronic kidney disease at earlier ages.”
Regarding drug-based therapy, Dr. Alcázar said that “empagliflozin is not currently indicated for chronic kidney disease in adults.” This sodium-glucose cotransporter-2 inhibitor delays kidney disease and reduces morbidity. Both benefits were highlighted in two recent studies (DAPA-CKD and CREDENCE). Published in January, EMPA-KIDNEY presents a new twist on nephroprotection for patients with chronic kidney disease (diabetic or not) whose glomerular filtration rates are between 20 and 40 mL/min without albuminuria or whose glomerular filtration rates are between 45 and 90 mL/min with albuminuria. For more than 6,000 patients, empagliflozin was observed “to clearly reduce kidney disease progression, cardiovascular mortality and all-cause mortality, and the need to go on dialysis,” stated Dr. Alcázar.
What professionals expect
Dr. Benedito also explained the criteria for referral to a specialist: glomerular filtration rate less than 30 mL/min (unless the patient is older than 80 years and does not have progressively worsening renal function), albumin/creatinine ratio greater than 300 mg/g, acute worsening of renal function, progressive worsening of renal function of greater than 5 mL/min/yr, chronic kidney disease, hypertension treated with triple therapy (including a diuretic) at maximum doses, anemia of less than 10 g/dL, and nonurologic hematuria, especially in combination with albuminuria.
Dr. Benedito explained what nephrologists expect from family doctors in the management of chronic kidney disease: “screening for early detection, identifying and treating risk factors for chronic kidney disease, detecting progression and complications, adjusting drugs based on glomerular filtration rate, and ensuring that our patients are benefiting from sodium-glucose cotransporter-2 inhibitors. These are among the most important steps to be taken.”
Dr. Alcázar mentioned what family doctors expect from nephrologists: “two-way communication, accessibility, coordination of actions to be taken, and using shared and mutually agreed-upon protocols.”
This article was translated from the Medscape Spanish Edition and a version appeared on Medscape.com.
MADRID – A late diagnosis of chronic kidney disease is cause for concern. Scientific societies are therefore advocating for screening at younger ages to reverse this trend and slow the progression of the disease. Nearly all patients seen in primary care are candidates for screening because of their risk factors for kidney disease.
During the 29th National Conference of General and Family Medicine of the Spanish Society for General and Family Physicians, Teresa Benedito, MD, family doctor and member of the society’s cardiovascular group, and Roberto Alcázar, MD, nephrologist at the Infanta Leonor University Hospital, Madrid, presented a clinical case encountered in primary care. They used this case to frame a strong argument for the importance of early screening for chronic kidney disease, and they discussed how to properly manage such screening.
The presentation followed the guidelines in the SEMG publication regarding the management and referral of patients with type 2 diabetes. Dr. Benedito explained that the first thing to ask oneself during a patient visit is “whether they present risk factors for kidney disease. If so, we can’t let them leave before we do a kidney screening.” She then listed the factors in question: age older than 60 years, African heritage, family history of chronic kidney disease, decreased kidney mass, weight loss at birth, hypertension, diabetes, smoking, obesity, and low socioeconomic status.
For his part, Dr. Alcázar mentioned how these factors are similar to cardiovascular risk factors, because “the kidneys are a ball of vessels with double capillarization for purifying blood. They’re the organs with the most arteries per unit of weight, so anything that can damage the arteries can damage the kidneys.”
Candidates for screening
“Chronic kidney disease develops in 15% of the adult population in Spain. So, it’s worth asking how many patients have been diagnosed and who should we should be screening.” To the factors listed above, Dr. Alcázar added treatment with nephrotoxic drugs (including nonsteroidal anti-inflammatory drugs) for patients with obstructive urinary tract disease, and a history of acute kidney injury for patients with chronic autoimmune disease or neoplasms. “Thus, nearly all patients seen in primary care would need to be screened.”
Another fundamental question raised was whether patients should be screened before age 60 years. “As a nephrologist, I feel that we have been diagnosing chronic kidney disease late, even though we’ve been doing everything by the book,” said Dr. Alcázar. In his opinion, “the answer to whether we should be screening earlier ... is yes, for two reasons: first, because it’s cost-effective, and second, because it’s very inexpensive.”
Dr. Benedito explained in detail the process for diagnosing this disease. She began by defining the disease as changes in kidney structure and function that last longer than 3 months. These changes are identified by use of two criteria: glomerular filtration rate less than 60 mL/min and kidney injury or lesions with or without reduced filtration rate (renal biopsy, albumin/creatinine ratio greater than 30 mg/g, proteinuria, alterations in urinary sediment or in imaging tests). Thus, “if one of these two criteria persists for more than 3 months, the diagnosis is chronic kidney disease. Also, high creatinine levels are not diagnostic for the disease,” she emphasized.
Two related parameters
Glomerular filtration and albuminuria “are highly relevant, because screening for chronic kidney disease is based on these two parameters,” said Dr. Benedito. Glomerular filtration rate varies with age, sex, ethnicity, and body mass. It is useful for identifying the stage of the disease and for monitoring disease progression. Albuminuria, on the other hand, is an indication of the severity of the disease. It’s an early marker for kidney injury and systemic disease and is more sensitive than proteinuria. Therefore, “this factor, together with glomerular filtration rate, allows us to detect, classify, and monitor the progression of chronic kidney disease.”
On this point, Dr. Alcázar emphasized the importance of trends, since variation in glomerular filtration depends on serum creatinine, which can vary by nearly 9%. He explained that glomerular filtration rate is related to the number of nephrons remaining. A glomerular filtration rate of less than 60 mL/min implies that more than half of the nephrons in each kidney have been lost. Albuminuria informs about structural damage (that is, the condition of the remaining nephrons). It’s therefore essential to test for both parameters. “We need to be actively monitoring and then making our decisions based on trends and not on isolated results. We need to be aware of albuminuria when we make our decisions,” said Dr. Alcázar. Some studies have shown the importance of testing for albuminuria whenever creatinine level is assessed. “We need to buy into this. If we don’t do this, we’ll only ever have half the information we need.”
Reducing late diagnosis
According to the IBERICAN study, 14% of patients seen in primary care in Spain have chronic kidney disease. “This statistic should make us stop and think, own our responsibility, and ask ourselves why this screening isn’t taking place [earlier],” said Dr. Benedito. She added, “We need to head off this trend toward late diagnosis. As the disease progresses, it significantly increases cardiovascular risk and leads to higher mortality, going on dialysis, transplants, et cetera.”
Dr. Alcázar noted that 80% of nephrology cases that are referred to him come from primary care. He explained the need to understand that “these patients have a sevenfold greater risk of suffering a serious cardiovascular event within the next year than people without kidney problems.” Most of these patients will experience an event, even if they don’t undergo dialysis (stage 3 and those near stage 4).
Correct staging
Also fundamental is having a detailed understanding of how staging is performed. Dr. Benedito explained that a chart that pairs glomerular filtration rate (six categories) with the level of albuminuria (three categories) should be used during the visit. For example, a case might be classified as G3a-A2. However, the simplified form of the chart may prove more practical. It classifies chronic kidney disease as being associated with mild, moderate, and severe risk, using different colors to aid comprehension.
Dr. Alcázar noted that the latest guidelines from the European Society of Hypertension for 2023 include albuminuria as an important parameter. The guidelines indicate that for a patient with moderate or severe risk, it is not necessary to calculate their score. “It’s considered high cardiovascular risk, and steps would need to be taken for intervention.”
He then listed the tools available for reversing albuminuria. The process begins by reducing salt consumption and involves the use of medications (angiotensin-converting enzyme inhibitors/angiotensin II receptor antagonists, aldosterone receptor antagonists, glucagon-like peptide-1 analogues, and sodium-glucose cotransporter-2 inhibitors, which slow kidney damage regardless of other measures) and strict management of cardiovascular risk factors (smoking, weight management, blood glucose, hypertension, and moderate physical activity).
Reducing cardiovascular risk
Dr. Alcázar highlighted important factors to keep in mind when managing each of the cardiovascular risk factors. For hypertension, the aim is to achieve levels less than 130/80 mm Hg, although recommendations vary, depending on the guidelines consulted. “KDIGO (Kidney Disease: Improving Global Outcomes) 2021 states that there is no evidence for monitoring diastolic blood pressure, only systolic blood pressure. If we measure it according to the standardized form, SBP should be less than 120 mm Hg, and if not, we would fall back on readings of 130/80 mm Hg.”
For lipid control (specifically, low-density lipoprotein cholesterol), the staging chart indicates that for patients at mild risk, levels should be less than 100 mg/dL; for those at moderate risk, less than 70 mg/dL; and for those at severe risk, less than 55 mg/dL. Hypertriglyceridemia “should only be treated with fibrates if it comes in over 1,000 mg/dL. Also, care must be taken, because these drugs interfere with creatinine excretion, increasing it,” said Dr. Alcázar.
Guidelines from the KDIGO and the American Diabetes Association state that anyone with diabetes and chronic kidney disease should receive a sodium-glucose cotransporter-2 inhibitor if their glomerular filtration rate exceeds 20 mL/min, “which may contradict slightly what it says on the label. Also, if they have hypertension, they should take an angiotensin-converting enzyme inhibitor,” said Dr. Alcázar. He added that “oral antidiabetics, including metformin, must be adjusted based on renal function if glomerular filtration rate is under 30 mL/min.”
Act immediately
When asked whether the course of chronic kidney disease can be changed, Dr. Alcázar responded with an emphatic yes and added that cardiovascular risk can also be substantially reduced. “As nephrologists, we don’t have access to patients in early stages. But family doctors do. Hence the importance of early screening, because going on dialysis at age 60 isn’t the same as at 80.” Currently, “scientific societies are encouraging authorities to screen for chronic kidney disease at earlier ages.”
Regarding drug-based therapy, Dr. Alcázar said that “empagliflozin is not currently indicated for chronic kidney disease in adults.” This sodium-glucose cotransporter-2 inhibitor delays kidney disease and reduces morbidity. Both benefits were highlighted in two recent studies (DAPA-CKD and CREDENCE). Published in January, EMPA-KIDNEY presents a new twist on nephroprotection for patients with chronic kidney disease (diabetic or not) whose glomerular filtration rates are between 20 and 40 mL/min without albuminuria or whose glomerular filtration rates are between 45 and 90 mL/min with albuminuria. For more than 6,000 patients, empagliflozin was observed “to clearly reduce kidney disease progression, cardiovascular mortality and all-cause mortality, and the need to go on dialysis,” stated Dr. Alcázar.
What professionals expect
Dr. Benedito also explained the criteria for referral to a specialist: glomerular filtration rate less than 30 mL/min (unless the patient is older than 80 years and does not have progressively worsening renal function), albumin/creatinine ratio greater than 300 mg/g, acute worsening of renal function, progressive worsening of renal function of greater than 5 mL/min/yr, chronic kidney disease, hypertension treated with triple therapy (including a diuretic) at maximum doses, anemia of less than 10 g/dL, and nonurologic hematuria, especially in combination with albuminuria.
Dr. Benedito explained what nephrologists expect from family doctors in the management of chronic kidney disease: “screening for early detection, identifying and treating risk factors for chronic kidney disease, detecting progression and complications, adjusting drugs based on glomerular filtration rate, and ensuring that our patients are benefiting from sodium-glucose cotransporter-2 inhibitors. These are among the most important steps to be taken.”
Dr. Alcázar mentioned what family doctors expect from nephrologists: “two-way communication, accessibility, coordination of actions to be taken, and using shared and mutually agreed-upon protocols.”
This article was translated from the Medscape Spanish Edition and a version appeared on Medscape.com.
Alzheimer’s disease and the primary care physician
Recent news highlights advancements in the understanding of Alzheimer’s disease: Increased information on biomarkers to be used for evaluation and diagnosis and recent studies on lifestyle factors or medications that do and do not correlate with Alzheimer’s disease.
It is helpful for family medicine physicians and other primary care physicians to be aware of this information to better help our patients and their families. When we have patients with strong family history of cognitive decline, they often will ask us for an early assessment or help with next steps and requests for treatment. Patients and their families want to understand what testing will be done by the neurologist they will likely be seeing.
An article published in Alzheimer’s and Dementia put forward a consensus statement by 11 European scientific societies on diagnosis and management of the disease. These societies defined work flows for processes to utilize biomarkers to diagnose Alzheimer’s disease. Although these work flows may help with diagnosis, they are not able to definitively rule out other causes of dementia. However, they may lead to consistency in how treatments are determined.1 More consistency will be helpful in counseling patients and their families on the next steps in the treatment plan.
Another study evaluated the correlation between lean mass and dementia. This study demonstrated a decreased risk of dementia in patients with higher lean mass. It is unclear from this study whether the higher lean mass is protective or if decreased cognitive function decreases the amount of lean mass. However, this study does provide hope in two possible ways: it provides potentially predictive information on who may be more at risk of declining cognitive function as well as a modifiable risk factor to address.2 Family physicians may use this as part of their counseling for patients who are concerned about their potential risk of dementia. It is yet another reason why we may counsel on healthy diet and weight-bearing exercise to help maintain lean mass.
Other associations related to dementia have been disproven. An article in Gastroenterology discussed the association between cognitive decline and use of proton pump inhibitors and H2 blockers – indicating that there is no association.3 Although there are reasons why we want to limit the use of these medications – particularly when they are not needed, it is a relief that they are not causing cognitive decline in patients.
Most of these studies provide information that is helpful for both family medicine physicians and patients. We are learning more about cognitive decline and Alzheimer’s disease. This gives hope to patients with strong family history that we may be able to reduce their risks. These studies also give us possible risk factors on which we can counsel our patients.
Developments in Alzheimer’s disease research are speeding ahead and give family physicians a bit more information to discuss with patients and their families as they face the challenging symptoms of cognitive decline. Future research, it is hoped, will help with treatment plans and modifiable risk factors to improve the outcomes for patients at high risk of cognitive decline.
Dr. Wheat is associate professor of family and community medicine at Northwestern University in Chicago. She has no conflicts of interest.
References
1. Massa F et al. Alzheimer’s and Dementia. 2023;19(S2):e062216.
2. Daghlas I et al. BMJ Medicine. 2023;2(1):e000354.
3. Mehta R et al. Gastroenterology. 2023 Jun 12. doi: 10.1053/j.gastro.2023.05.052.
Recent news highlights advancements in the understanding of Alzheimer’s disease: Increased information on biomarkers to be used for evaluation and diagnosis and recent studies on lifestyle factors or medications that do and do not correlate with Alzheimer’s disease.
It is helpful for family medicine physicians and other primary care physicians to be aware of this information to better help our patients and their families. When we have patients with strong family history of cognitive decline, they often will ask us for an early assessment or help with next steps and requests for treatment. Patients and their families want to understand what testing will be done by the neurologist they will likely be seeing.
An article published in Alzheimer’s and Dementia put forward a consensus statement by 11 European scientific societies on diagnosis and management of the disease. These societies defined work flows for processes to utilize biomarkers to diagnose Alzheimer’s disease. Although these work flows may help with diagnosis, they are not able to definitively rule out other causes of dementia. However, they may lead to consistency in how treatments are determined.1 More consistency will be helpful in counseling patients and their families on the next steps in the treatment plan.
Another study evaluated the correlation between lean mass and dementia. This study demonstrated a decreased risk of dementia in patients with higher lean mass. It is unclear from this study whether the higher lean mass is protective or if decreased cognitive function decreases the amount of lean mass. However, this study does provide hope in two possible ways: it provides potentially predictive information on who may be more at risk of declining cognitive function as well as a modifiable risk factor to address.2 Family physicians may use this as part of their counseling for patients who are concerned about their potential risk of dementia. It is yet another reason why we may counsel on healthy diet and weight-bearing exercise to help maintain lean mass.
Other associations related to dementia have been disproven. An article in Gastroenterology discussed the association between cognitive decline and use of proton pump inhibitors and H2 blockers – indicating that there is no association.3 Although there are reasons why we want to limit the use of these medications – particularly when they are not needed, it is a relief that they are not causing cognitive decline in patients.
Most of these studies provide information that is helpful for both family medicine physicians and patients. We are learning more about cognitive decline and Alzheimer’s disease. This gives hope to patients with strong family history that we may be able to reduce their risks. These studies also give us possible risk factors on which we can counsel our patients.
Developments in Alzheimer’s disease research are speeding ahead and give family physicians a bit more information to discuss with patients and their families as they face the challenging symptoms of cognitive decline. Future research, it is hoped, will help with treatment plans and modifiable risk factors to improve the outcomes for patients at high risk of cognitive decline.
Dr. Wheat is associate professor of family and community medicine at Northwestern University in Chicago. She has no conflicts of interest.
References
1. Massa F et al. Alzheimer’s and Dementia. 2023;19(S2):e062216.
2. Daghlas I et al. BMJ Medicine. 2023;2(1):e000354.
3. Mehta R et al. Gastroenterology. 2023 Jun 12. doi: 10.1053/j.gastro.2023.05.052.
Recent news highlights advancements in the understanding of Alzheimer’s disease: Increased information on biomarkers to be used for evaluation and diagnosis and recent studies on lifestyle factors or medications that do and do not correlate with Alzheimer’s disease.
It is helpful for family medicine physicians and other primary care physicians to be aware of this information to better help our patients and their families. When we have patients with strong family history of cognitive decline, they often will ask us for an early assessment or help with next steps and requests for treatment. Patients and their families want to understand what testing will be done by the neurologist they will likely be seeing.
An article published in Alzheimer’s and Dementia put forward a consensus statement by 11 European scientific societies on diagnosis and management of the disease. These societies defined work flows for processes to utilize biomarkers to diagnose Alzheimer’s disease. Although these work flows may help with diagnosis, they are not able to definitively rule out other causes of dementia. However, they may lead to consistency in how treatments are determined.1 More consistency will be helpful in counseling patients and their families on the next steps in the treatment plan.
Another study evaluated the correlation between lean mass and dementia. This study demonstrated a decreased risk of dementia in patients with higher lean mass. It is unclear from this study whether the higher lean mass is protective or if decreased cognitive function decreases the amount of lean mass. However, this study does provide hope in two possible ways: it provides potentially predictive information on who may be more at risk of declining cognitive function as well as a modifiable risk factor to address.2 Family physicians may use this as part of their counseling for patients who are concerned about their potential risk of dementia. It is yet another reason why we may counsel on healthy diet and weight-bearing exercise to help maintain lean mass.
Other associations related to dementia have been disproven. An article in Gastroenterology discussed the association between cognitive decline and use of proton pump inhibitors and H2 blockers – indicating that there is no association.3 Although there are reasons why we want to limit the use of these medications – particularly when they are not needed, it is a relief that they are not causing cognitive decline in patients.
Most of these studies provide information that is helpful for both family medicine physicians and patients. We are learning more about cognitive decline and Alzheimer’s disease. This gives hope to patients with strong family history that we may be able to reduce their risks. These studies also give us possible risk factors on which we can counsel our patients.
Developments in Alzheimer’s disease research are speeding ahead and give family physicians a bit more information to discuss with patients and their families as they face the challenging symptoms of cognitive decline. Future research, it is hoped, will help with treatment plans and modifiable risk factors to improve the outcomes for patients at high risk of cognitive decline.
Dr. Wheat is associate professor of family and community medicine at Northwestern University in Chicago. She has no conflicts of interest.
References
1. Massa F et al. Alzheimer’s and Dementia. 2023;19(S2):e062216.
2. Daghlas I et al. BMJ Medicine. 2023;2(1):e000354.
3. Mehta R et al. Gastroenterology. 2023 Jun 12. doi: 10.1053/j.gastro.2023.05.052.
How the new depression screening guidelines in adults do little to address our mental health care crisis
According to the World Health Organization (WHO), approximately 5% of adults (or 280 million people) suffer from depression globally. Although depression is more common in women, it can affect anyone. It is seen in all socioeconomic classes, ages, and races. In response, the WHO developed the Mental Health Gap Action Programme to bring mental health care services to those in need.
Depression can lead to severe consequences, such as loss of employment, relationships difficulties, and suicide. In fact, suicide is the 10th leading cause of death in the United States.
The U.S. Preventive Services Task Force (USPSTF), in past years, concluded that there was insufficient evidence to screen adolescents and adults for depression, However, new guidelines were issued this year in which the task force concluded there was a moderate benefit to screening adults for depression but insufficient evidence to screen for suicide risk. The agency now recommends screening for depression in all adults, even in the absence of risk factors, by using brief screening instruments such as the PHQ (Patient Health Questionnaire).
As family doctors, we have witnessed the burden of depression in our practices. The previous recommendations neglected the fact that mental health disorders were often purposely hidden because of stigma. Many patients do not readily come for treatment for mental illness and sometimes do not even accept these diagnoses. It is good that screening is now recommended, but we need to do more to tear down the stigma attached to mental illness.
These new guidelines do not address the effect that the lack of available mental health services has on treatment. It can take months to get an appointment for a patient with a mental health disorder, even if that person is potentially suicidal. Primary care physicians are often left treating these disorders; sometimes we are treating mental illness whether we feel comfortable doing so or not. Patients may not receive the best care but it is better than no care at all.
Although treating anxiety and depression is common for primary care doctors, specialists should be contacted when cases get more complicated. Even a call to crisis intervention can lead to an emergency department visit with discharge back to the family doctor because there is nowhere else to send the patient. The burden falls on us when we are already burdened by many other things, such as the rising rates of obesity with the resultant consequences of diabetes and heart disease. We simply do not have the time or expertise to treat complicated mental illness.
Creating guidelines to diagnose more undetected cases of depression without increasing the infrastructure to handle it is only going to lead to more pressure on family doctors. Many of us are already burnt out and at our limits. Yes, we want to diagnose every case of depression we can and to treat these patients for these disorders, but we need help.
Another problem with the guidelines is the recommendation to screen for depression and not suicide risk. As family doctors, we ask all patients who are depressed if they have thoughts of hurting themselves or others. Also, some people who commit suicide are not clinically depressed. These questions are simple to ask on an intake form.
Screening for depression is a pretty simple process. A patient can complete a screening tool or the clinician can directly ask the questions. It is a quick, noninvasive process. The Diagnostic and Statistical Manual of Mental Disorders criteria for diagnosing depression are pretty rigid and straightforward so misdiagnoses are not likely to be common.
The new guidelines do not make recommendations for treatment. In the real world, we often see patients unable to get the medications we prescribe because their insurance won’t cover it. Having guidelines supporting medication use would be very helpful.
In the area where I practice, it is difficult to refer a patient for counseling despite there being a plethora of counselors, therapists, and psychologists. These mental health providers often take only cash-paying patients, which eliminates access for many patients.
If we truly want to address the ever-increasing rates of depression in our country, we need to do much more than create new screening guidelines (screening that many family doctors were already doing). We must remove stigma, especially in the health care setting, fund mental health services, make them more readily available, and provide care that is affordable and covered by insurance. Until then, we are just going to add to the load of family doctors until we either break or leave our profession. Patients deserve better.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. She was paid by Pfizer as a consultant on Paxlovid and is the editor in chief of Physician’s Weekly.
According to the World Health Organization (WHO), approximately 5% of adults (or 280 million people) suffer from depression globally. Although depression is more common in women, it can affect anyone. It is seen in all socioeconomic classes, ages, and races. In response, the WHO developed the Mental Health Gap Action Programme to bring mental health care services to those in need.
Depression can lead to severe consequences, such as loss of employment, relationships difficulties, and suicide. In fact, suicide is the 10th leading cause of death in the United States.
The U.S. Preventive Services Task Force (USPSTF), in past years, concluded that there was insufficient evidence to screen adolescents and adults for depression, However, new guidelines were issued this year in which the task force concluded there was a moderate benefit to screening adults for depression but insufficient evidence to screen for suicide risk. The agency now recommends screening for depression in all adults, even in the absence of risk factors, by using brief screening instruments such as the PHQ (Patient Health Questionnaire).
As family doctors, we have witnessed the burden of depression in our practices. The previous recommendations neglected the fact that mental health disorders were often purposely hidden because of stigma. Many patients do not readily come for treatment for mental illness and sometimes do not even accept these diagnoses. It is good that screening is now recommended, but we need to do more to tear down the stigma attached to mental illness.
These new guidelines do not address the effect that the lack of available mental health services has on treatment. It can take months to get an appointment for a patient with a mental health disorder, even if that person is potentially suicidal. Primary care physicians are often left treating these disorders; sometimes we are treating mental illness whether we feel comfortable doing so or not. Patients may not receive the best care but it is better than no care at all.
Although treating anxiety and depression is common for primary care doctors, specialists should be contacted when cases get more complicated. Even a call to crisis intervention can lead to an emergency department visit with discharge back to the family doctor because there is nowhere else to send the patient. The burden falls on us when we are already burdened by many other things, such as the rising rates of obesity with the resultant consequences of diabetes and heart disease. We simply do not have the time or expertise to treat complicated mental illness.
Creating guidelines to diagnose more undetected cases of depression without increasing the infrastructure to handle it is only going to lead to more pressure on family doctors. Many of us are already burnt out and at our limits. Yes, we want to diagnose every case of depression we can and to treat these patients for these disorders, but we need help.
Another problem with the guidelines is the recommendation to screen for depression and not suicide risk. As family doctors, we ask all patients who are depressed if they have thoughts of hurting themselves or others. Also, some people who commit suicide are not clinically depressed. These questions are simple to ask on an intake form.
Screening for depression is a pretty simple process. A patient can complete a screening tool or the clinician can directly ask the questions. It is a quick, noninvasive process. The Diagnostic and Statistical Manual of Mental Disorders criteria for diagnosing depression are pretty rigid and straightforward so misdiagnoses are not likely to be common.
The new guidelines do not make recommendations for treatment. In the real world, we often see patients unable to get the medications we prescribe because their insurance won’t cover it. Having guidelines supporting medication use would be very helpful.
In the area where I practice, it is difficult to refer a patient for counseling despite there being a plethora of counselors, therapists, and psychologists. These mental health providers often take only cash-paying patients, which eliminates access for many patients.
If we truly want to address the ever-increasing rates of depression in our country, we need to do much more than create new screening guidelines (screening that many family doctors were already doing). We must remove stigma, especially in the health care setting, fund mental health services, make them more readily available, and provide care that is affordable and covered by insurance. Until then, we are just going to add to the load of family doctors until we either break or leave our profession. Patients deserve better.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. She was paid by Pfizer as a consultant on Paxlovid and is the editor in chief of Physician’s Weekly.
According to the World Health Organization (WHO), approximately 5% of adults (or 280 million people) suffer from depression globally. Although depression is more common in women, it can affect anyone. It is seen in all socioeconomic classes, ages, and races. In response, the WHO developed the Mental Health Gap Action Programme to bring mental health care services to those in need.
Depression can lead to severe consequences, such as loss of employment, relationships difficulties, and suicide. In fact, suicide is the 10th leading cause of death in the United States.
The U.S. Preventive Services Task Force (USPSTF), in past years, concluded that there was insufficient evidence to screen adolescents and adults for depression, However, new guidelines were issued this year in which the task force concluded there was a moderate benefit to screening adults for depression but insufficient evidence to screen for suicide risk. The agency now recommends screening for depression in all adults, even in the absence of risk factors, by using brief screening instruments such as the PHQ (Patient Health Questionnaire).
As family doctors, we have witnessed the burden of depression in our practices. The previous recommendations neglected the fact that mental health disorders were often purposely hidden because of stigma. Many patients do not readily come for treatment for mental illness and sometimes do not even accept these diagnoses. It is good that screening is now recommended, but we need to do more to tear down the stigma attached to mental illness.
These new guidelines do not address the effect that the lack of available mental health services has on treatment. It can take months to get an appointment for a patient with a mental health disorder, even if that person is potentially suicidal. Primary care physicians are often left treating these disorders; sometimes we are treating mental illness whether we feel comfortable doing so or not. Patients may not receive the best care but it is better than no care at all.
Although treating anxiety and depression is common for primary care doctors, specialists should be contacted when cases get more complicated. Even a call to crisis intervention can lead to an emergency department visit with discharge back to the family doctor because there is nowhere else to send the patient. The burden falls on us when we are already burdened by many other things, such as the rising rates of obesity with the resultant consequences of diabetes and heart disease. We simply do not have the time or expertise to treat complicated mental illness.
Creating guidelines to diagnose more undetected cases of depression without increasing the infrastructure to handle it is only going to lead to more pressure on family doctors. Many of us are already burnt out and at our limits. Yes, we want to diagnose every case of depression we can and to treat these patients for these disorders, but we need help.
Another problem with the guidelines is the recommendation to screen for depression and not suicide risk. As family doctors, we ask all patients who are depressed if they have thoughts of hurting themselves or others. Also, some people who commit suicide are not clinically depressed. These questions are simple to ask on an intake form.
Screening for depression is a pretty simple process. A patient can complete a screening tool or the clinician can directly ask the questions. It is a quick, noninvasive process. The Diagnostic and Statistical Manual of Mental Disorders criteria for diagnosing depression are pretty rigid and straightforward so misdiagnoses are not likely to be common.
The new guidelines do not make recommendations for treatment. In the real world, we often see patients unable to get the medications we prescribe because their insurance won’t cover it. Having guidelines supporting medication use would be very helpful.
In the area where I practice, it is difficult to refer a patient for counseling despite there being a plethora of counselors, therapists, and psychologists. These mental health providers often take only cash-paying patients, which eliminates access for many patients.
If we truly want to address the ever-increasing rates of depression in our country, we need to do much more than create new screening guidelines (screening that many family doctors were already doing). We must remove stigma, especially in the health care setting, fund mental health services, make them more readily available, and provide care that is affordable and covered by insurance. Until then, we are just going to add to the load of family doctors until we either break or leave our profession. Patients deserve better.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. She was paid by Pfizer as a consultant on Paxlovid and is the editor in chief of Physician’s Weekly.