User login
IMRT bests conventional radiation for soft-tissue sarcomas of the extremities
ATLANTA – Intensity-modulated radiation therapy proved significantly better than conventional radiation for local control of soft-tissue sarcomas of the extremities, according to new study results, investigators reported at the annual meeting of the American Society for Radiation Oncology.
The 5-year local control rate with intensity-modulated radiation therapy (IMRT) was 92.4%, compared with 85% for external-beam radiation therapy (EBRT), said Dr. Kaled M. Alektiar, a radiation oncologist at Memorial Sloan-Kettering Cancer Center in New York.
The benefits of IMRT were seen despite a preponderance of higher risks in patients treated with IMRT. And, "the morbidity profile, especially for chronic lymphedema of grade 3 or higher, was significantly less," Dr. Alektiar said.
He and his coinvestigators looked at 320 patients who underwent definitive surgery and radiation therapy at Memorial Sloan-Kettering for primary, nonmetastatic soft-tissue sarcomas of the extremities. Of this group, 155 received EBRT with a conventional technique, usually three-dimensional conformal radiation, and 165 patients received IMRT.
Most of the tumors (74.7%) were in the lower extremity, 45.6% were at least 10 cm in diameter, 92.2% were in deep tissue, 82.5% were high grade, and 40% had close or positive surgical margins. The majority of patients (75.9%) received adjuvant chemotherapy.
There were significantly more patients with positive or close margins in the IMRT group than in the conventional EBRT group (47.9% vs. 31.6%; P = .003), and more patients treated with IMRT had high-grade histology tumors, although this difference had only borderline significance (86.7% vs. 78.1%; P =.055).
Additionally, significantly more patients in the IMRT group received preoperative radiation (21.2% vs. 3.2%; P less than .001). Otherwise, the groups were balanced in terms of demographics, tumor size, depth, and use of CT in treatment planning.
The median follow-up was 49.5 months (42 months for patients treated with IMRT, and 87 months for those treated with EBRT). The 5-year local recurrence rates were 7.6% for IMRT and 15% for conventional EBRT. The median time to local recurrence was 18 months in each group.
Eight patients required amputations for salvage, including three in the IMRT cohort and five in the conventional radiation cohort.
In multivariate analysis, three factors that were significantly prognostic for local failure were IMRT (hazard ratio, 0.46; P = .02), age less than 50 years (HR, 0.44; P = .04), and a tumor size of 10 cm or less in the longest dimension (HR, 0.53; P = .05).
Overall survival at 5 years was 69.1% for IMRT and 75.6% for EBRT, a difference that was not significant.
Rates of grade 3 or 4 acute toxicities, including infected and noninfected wound complications and radiation dermatitis, were similar between the groups. Patients treated with IMRT had significantly shorter treatment interruptions, at a mean of 0.8 days, compared with 2.2 days for patients treated with conventional EBRT. Chronic grade 3 or higher lymphedema did not occur in any patients treated with IMRT, compared with four patients treated with conventional EBRT (P = .053).
The study was supported by a grant from the Clinical and Translational Science Center at Weill Cornell Medical College, New York. Dr. Alektiar reported having no relevant financial disclosures.
ATLANTA – Intensity-modulated radiation therapy proved significantly better than conventional radiation for local control of soft-tissue sarcomas of the extremities, according to new study results, investigators reported at the annual meeting of the American Society for Radiation Oncology.
The 5-year local control rate with intensity-modulated radiation therapy (IMRT) was 92.4%, compared with 85% for external-beam radiation therapy (EBRT), said Dr. Kaled M. Alektiar, a radiation oncologist at Memorial Sloan-Kettering Cancer Center in New York.
The benefits of IMRT were seen despite a preponderance of higher risks in patients treated with IMRT. And, "the morbidity profile, especially for chronic lymphedema of grade 3 or higher, was significantly less," Dr. Alektiar said.
He and his coinvestigators looked at 320 patients who underwent definitive surgery and radiation therapy at Memorial Sloan-Kettering for primary, nonmetastatic soft-tissue sarcomas of the extremities. Of this group, 155 received EBRT with a conventional technique, usually three-dimensional conformal radiation, and 165 patients received IMRT.
Most of the tumors (74.7%) were in the lower extremity, 45.6% were at least 10 cm in diameter, 92.2% were in deep tissue, 82.5% were high grade, and 40% had close or positive surgical margins. The majority of patients (75.9%) received adjuvant chemotherapy.
There were significantly more patients with positive or close margins in the IMRT group than in the conventional EBRT group (47.9% vs. 31.6%; P = .003), and more patients treated with IMRT had high-grade histology tumors, although this difference had only borderline significance (86.7% vs. 78.1%; P =.055).
Additionally, significantly more patients in the IMRT group received preoperative radiation (21.2% vs. 3.2%; P less than .001). Otherwise, the groups were balanced in terms of demographics, tumor size, depth, and use of CT in treatment planning.
The median follow-up was 49.5 months (42 months for patients treated with IMRT, and 87 months for those treated with EBRT). The 5-year local recurrence rates were 7.6% for IMRT and 15% for conventional EBRT. The median time to local recurrence was 18 months in each group.
Eight patients required amputations for salvage, including three in the IMRT cohort and five in the conventional radiation cohort.
In multivariate analysis, three factors that were significantly prognostic for local failure were IMRT (hazard ratio, 0.46; P = .02), age less than 50 years (HR, 0.44; P = .04), and a tumor size of 10 cm or less in the longest dimension (HR, 0.53; P = .05).
Overall survival at 5 years was 69.1% for IMRT and 75.6% for EBRT, a difference that was not significant.
Rates of grade 3 or 4 acute toxicities, including infected and noninfected wound complications and radiation dermatitis, were similar between the groups. Patients treated with IMRT had significantly shorter treatment interruptions, at a mean of 0.8 days, compared with 2.2 days for patients treated with conventional EBRT. Chronic grade 3 or higher lymphedema did not occur in any patients treated with IMRT, compared with four patients treated with conventional EBRT (P = .053).
The study was supported by a grant from the Clinical and Translational Science Center at Weill Cornell Medical College, New York. Dr. Alektiar reported having no relevant financial disclosures.
ATLANTA – Intensity-modulated radiation therapy proved significantly better than conventional radiation for local control of soft-tissue sarcomas of the extremities, according to new study results, investigators reported at the annual meeting of the American Society for Radiation Oncology.
The 5-year local control rate with intensity-modulated radiation therapy (IMRT) was 92.4%, compared with 85% for external-beam radiation therapy (EBRT), said Dr. Kaled M. Alektiar, a radiation oncologist at Memorial Sloan-Kettering Cancer Center in New York.
The benefits of IMRT were seen despite a preponderance of higher risks in patients treated with IMRT. And, "the morbidity profile, especially for chronic lymphedema of grade 3 or higher, was significantly less," Dr. Alektiar said.
He and his coinvestigators looked at 320 patients who underwent definitive surgery and radiation therapy at Memorial Sloan-Kettering for primary, nonmetastatic soft-tissue sarcomas of the extremities. Of this group, 155 received EBRT with a conventional technique, usually three-dimensional conformal radiation, and 165 patients received IMRT.
Most of the tumors (74.7%) were in the lower extremity, 45.6% were at least 10 cm in diameter, 92.2% were in deep tissue, 82.5% were high grade, and 40% had close or positive surgical margins. The majority of patients (75.9%) received adjuvant chemotherapy.
There were significantly more patients with positive or close margins in the IMRT group than in the conventional EBRT group (47.9% vs. 31.6%; P = .003), and more patients treated with IMRT had high-grade histology tumors, although this difference had only borderline significance (86.7% vs. 78.1%; P =.055).
Additionally, significantly more patients in the IMRT group received preoperative radiation (21.2% vs. 3.2%; P less than .001). Otherwise, the groups were balanced in terms of demographics, tumor size, depth, and use of CT in treatment planning.
The median follow-up was 49.5 months (42 months for patients treated with IMRT, and 87 months for those treated with EBRT). The 5-year local recurrence rates were 7.6% for IMRT and 15% for conventional EBRT. The median time to local recurrence was 18 months in each group.
Eight patients required amputations for salvage, including three in the IMRT cohort and five in the conventional radiation cohort.
In multivariate analysis, three factors that were significantly prognostic for local failure were IMRT (hazard ratio, 0.46; P = .02), age less than 50 years (HR, 0.44; P = .04), and a tumor size of 10 cm or less in the longest dimension (HR, 0.53; P = .05).
Overall survival at 5 years was 69.1% for IMRT and 75.6% for EBRT, a difference that was not significant.
Rates of grade 3 or 4 acute toxicities, including infected and noninfected wound complications and radiation dermatitis, were similar between the groups. Patients treated with IMRT had significantly shorter treatment interruptions, at a mean of 0.8 days, compared with 2.2 days for patients treated with conventional EBRT. Chronic grade 3 or higher lymphedema did not occur in any patients treated with IMRT, compared with four patients treated with conventional EBRT (P = .053).
The study was supported by a grant from the Clinical and Translational Science Center at Weill Cornell Medical College, New York. Dr. Alektiar reported having no relevant financial disclosures.
AT THE ASTRO ANNUAL MEETING
Major finding: The 5-year local control rate with intensity-modulated radiation therapy was 92.4%, compared with 85% for conventional external-beam radiation therapy.
Data source: Retrospective study of 320 patients treated for soft-tissue sarcomas of the extremities.
Disclosures: The study was supported by a grant from the Clinical and Translational Science Center at Weill Cornell Medical College, New York. Dr. Alektiar reported having no relevant financial disclosures.
ASDS 2013 Roundup with Dr. Kavita Mariwalla
Dr. Kavita Mariwalla, the 2013 ASDS annual meeting chair, provides a 3-minute summary of the hot topics discussed at the American Society of Dermatologic Surgery, held in Chicago. For more, visit http://www.skindandallergynews.com.
Dr. Kavita Mariwalla, the 2013 ASDS annual meeting chair, provides a 3-minute summary of the hot topics discussed at the American Society of Dermatologic Surgery, held in Chicago. For more, visit http://www.skindandallergynews.com.
Dr. Kavita Mariwalla, the 2013 ASDS annual meeting chair, provides a 3-minute summary of the hot topics discussed at the American Society of Dermatologic Surgery, held in Chicago. For more, visit http://www.skindandallergynews.com.
Collateral damage
Six and a half years ago, my malpractice insurer made a payment to settle a case against a company I once ran in a neighboring state. Nine years before that, a physician who worked for me had lasered a tattoo on a woman’s ankle. She claimed it got infected and then scarred, but refused to be examined at that time, or later.
This case wound its way slowly through the system. I drove to the nearby state to plot strategy with the insurer’s attorney for dealing with the $50,000 claim. "I can’t understand why anyone would take a case this small," said the attorney.
When we got to the courthouse that January day, we saw why. The plaintiff – whom I had never met – was accompanied by a lawyer. He and my attorney met with the judge.
"Settle this case," she ordered.
And so we did, for $22,500. The plaintiff stipulated that I "did not act negligently in any respect."
As we exited the courtroom into the hall, the plaintiff approached me. "My tattoo isn’t gone yet," she said. "Would you be able to treat it?"
My attorney’s jaw dropped. Not mine, though. I had her put her ankle up on a bench to look at it. There was no scarring, just the hypopigmentation one sees after laser treatment in that area.
"You know," I told her. "I’m all the way in the next state. "The doctor here in town who treated you – the one who was going to testify against me today? He would be perfect."
We smiled at each other, shook hands, and I went home.
Fast forward to last week. A registered letter came to my office from a local electrical union. It contained a flyer that read:
Don’t be in the DARK about your doctor. XYZ hospital continues to allow doctors with recent malpractice payments to treat patients, WHY?
DR. ALAN S. ROCKOFF MADE A MALPRACTICE PAYMENT.
What kind of DOCTOR do you want treating you and your loved ones?
The accompanying letter explained that, "We intend to distribute [the leaflet] in the near future to anyone entering or leaving your medical building, as well as residents and businesses in the surrounding community. We will also be publicizing the content on DrRockoffexposed.com and through social media including Facebook and Twitter."
They added, "We strive for accuracy in all of our leaflets and websites." I was given 1 week to let them know if I found "anything untruthful or inaccurate," to "kindly let me know."
I thought the "kindly" was a nice touch.
The leaflet included a lot of nasty innuendoes about hospital XYZ, where I have staff privileges.
Bewildered, I contacted my malpractice insurer, who helpfully told me there was nothing I could do, and suggested I contact the hospital, at whom the campaign was clearly intended. I did so. The people at the hospital expressed sympathy and outrage about the union’s letter, and told me to ignore it.
An attorney affiliated with my malpractice insurer did some digging, and he sent me a link to an article showing that his union had used similar tactics against a hospital north of town 2 years ago. Their motive, it appears, is to be sure their union secures contracts for work at the hospitals in question.
In other words, friends, this is what is known in Mafia movies as a shakedown. "Nice medical staff you’ve got there," says the leaflet, in so many words. "Be a shame if anything happened to it."
As a kid, I used to watch Elliot Ness in "The Untouchables," but I never thought I would be personally involved in anything I saw there. But if you live long enough, you never know what you’ll experience. Anyhow, any publicity is good publicity, and DrRockoffexposed.com does spell my name right, even if it’s not nearly as fun to see as what one could imagine at something like www.TweetingCongressmanExposed.com.
For better or worse, the time when doctors sat in their offices, wrote notes on 3x5 cards, and collected cash payments they stowed in their desk drawers are long gone. In the Olympian corridors of power far above our heads, powerful forces that dictate our lives hurl thunderbolts at each other as they vie for money, power, and control. The trick is to stay out of their way and avoid becoming collateral damage.
Easy to say. Less easy to do.
Dr. Rockoff practices dermatology in Brookline, Mass. He is on the clinical faculty at Tufts University School of Medicine, Boston, and has taught senior medical students and other trainees for 30 years. Dr. Rockoff has contributed to the Under My Skin column in Skin & Allergy News since January 2002.
Six and a half years ago, my malpractice insurer made a payment to settle a case against a company I once ran in a neighboring state. Nine years before that, a physician who worked for me had lasered a tattoo on a woman’s ankle. She claimed it got infected and then scarred, but refused to be examined at that time, or later.
This case wound its way slowly through the system. I drove to the nearby state to plot strategy with the insurer’s attorney for dealing with the $50,000 claim. "I can’t understand why anyone would take a case this small," said the attorney.
When we got to the courthouse that January day, we saw why. The plaintiff – whom I had never met – was accompanied by a lawyer. He and my attorney met with the judge.
"Settle this case," she ordered.
And so we did, for $22,500. The plaintiff stipulated that I "did not act negligently in any respect."
As we exited the courtroom into the hall, the plaintiff approached me. "My tattoo isn’t gone yet," she said. "Would you be able to treat it?"
My attorney’s jaw dropped. Not mine, though. I had her put her ankle up on a bench to look at it. There was no scarring, just the hypopigmentation one sees after laser treatment in that area.
"You know," I told her. "I’m all the way in the next state. "The doctor here in town who treated you – the one who was going to testify against me today? He would be perfect."
We smiled at each other, shook hands, and I went home.
Fast forward to last week. A registered letter came to my office from a local electrical union. It contained a flyer that read:
Don’t be in the DARK about your doctor. XYZ hospital continues to allow doctors with recent malpractice payments to treat patients, WHY?
DR. ALAN S. ROCKOFF MADE A MALPRACTICE PAYMENT.
What kind of DOCTOR do you want treating you and your loved ones?
The accompanying letter explained that, "We intend to distribute [the leaflet] in the near future to anyone entering or leaving your medical building, as well as residents and businesses in the surrounding community. We will also be publicizing the content on DrRockoffexposed.com and through social media including Facebook and Twitter."
They added, "We strive for accuracy in all of our leaflets and websites." I was given 1 week to let them know if I found "anything untruthful or inaccurate," to "kindly let me know."
I thought the "kindly" was a nice touch.
The leaflet included a lot of nasty innuendoes about hospital XYZ, where I have staff privileges.
Bewildered, I contacted my malpractice insurer, who helpfully told me there was nothing I could do, and suggested I contact the hospital, at whom the campaign was clearly intended. I did so. The people at the hospital expressed sympathy and outrage about the union’s letter, and told me to ignore it.
An attorney affiliated with my malpractice insurer did some digging, and he sent me a link to an article showing that his union had used similar tactics against a hospital north of town 2 years ago. Their motive, it appears, is to be sure their union secures contracts for work at the hospitals in question.
In other words, friends, this is what is known in Mafia movies as a shakedown. "Nice medical staff you’ve got there," says the leaflet, in so many words. "Be a shame if anything happened to it."
As a kid, I used to watch Elliot Ness in "The Untouchables," but I never thought I would be personally involved in anything I saw there. But if you live long enough, you never know what you’ll experience. Anyhow, any publicity is good publicity, and DrRockoffexposed.com does spell my name right, even if it’s not nearly as fun to see as what one could imagine at something like www.TweetingCongressmanExposed.com.
For better or worse, the time when doctors sat in their offices, wrote notes on 3x5 cards, and collected cash payments they stowed in their desk drawers are long gone. In the Olympian corridors of power far above our heads, powerful forces that dictate our lives hurl thunderbolts at each other as they vie for money, power, and control. The trick is to stay out of their way and avoid becoming collateral damage.
Easy to say. Less easy to do.
Dr. Rockoff practices dermatology in Brookline, Mass. He is on the clinical faculty at Tufts University School of Medicine, Boston, and has taught senior medical students and other trainees for 30 years. Dr. Rockoff has contributed to the Under My Skin column in Skin & Allergy News since January 2002.
Six and a half years ago, my malpractice insurer made a payment to settle a case against a company I once ran in a neighboring state. Nine years before that, a physician who worked for me had lasered a tattoo on a woman’s ankle. She claimed it got infected and then scarred, but refused to be examined at that time, or later.
This case wound its way slowly through the system. I drove to the nearby state to plot strategy with the insurer’s attorney for dealing with the $50,000 claim. "I can’t understand why anyone would take a case this small," said the attorney.
When we got to the courthouse that January day, we saw why. The plaintiff – whom I had never met – was accompanied by a lawyer. He and my attorney met with the judge.
"Settle this case," she ordered.
And so we did, for $22,500. The plaintiff stipulated that I "did not act negligently in any respect."
As we exited the courtroom into the hall, the plaintiff approached me. "My tattoo isn’t gone yet," she said. "Would you be able to treat it?"
My attorney’s jaw dropped. Not mine, though. I had her put her ankle up on a bench to look at it. There was no scarring, just the hypopigmentation one sees after laser treatment in that area.
"You know," I told her. "I’m all the way in the next state. "The doctor here in town who treated you – the one who was going to testify against me today? He would be perfect."
We smiled at each other, shook hands, and I went home.
Fast forward to last week. A registered letter came to my office from a local electrical union. It contained a flyer that read:
Don’t be in the DARK about your doctor. XYZ hospital continues to allow doctors with recent malpractice payments to treat patients, WHY?
DR. ALAN S. ROCKOFF MADE A MALPRACTICE PAYMENT.
What kind of DOCTOR do you want treating you and your loved ones?
The accompanying letter explained that, "We intend to distribute [the leaflet] in the near future to anyone entering or leaving your medical building, as well as residents and businesses in the surrounding community. We will also be publicizing the content on DrRockoffexposed.com and through social media including Facebook and Twitter."
They added, "We strive for accuracy in all of our leaflets and websites." I was given 1 week to let them know if I found "anything untruthful or inaccurate," to "kindly let me know."
I thought the "kindly" was a nice touch.
The leaflet included a lot of nasty innuendoes about hospital XYZ, where I have staff privileges.
Bewildered, I contacted my malpractice insurer, who helpfully told me there was nothing I could do, and suggested I contact the hospital, at whom the campaign was clearly intended. I did so. The people at the hospital expressed sympathy and outrage about the union’s letter, and told me to ignore it.
An attorney affiliated with my malpractice insurer did some digging, and he sent me a link to an article showing that his union had used similar tactics against a hospital north of town 2 years ago. Their motive, it appears, is to be sure their union secures contracts for work at the hospitals in question.
In other words, friends, this is what is known in Mafia movies as a shakedown. "Nice medical staff you’ve got there," says the leaflet, in so many words. "Be a shame if anything happened to it."
As a kid, I used to watch Elliot Ness in "The Untouchables," but I never thought I would be personally involved in anything I saw there. But if you live long enough, you never know what you’ll experience. Anyhow, any publicity is good publicity, and DrRockoffexposed.com does spell my name right, even if it’s not nearly as fun to see as what one could imagine at something like www.TweetingCongressmanExposed.com.
For better or worse, the time when doctors sat in their offices, wrote notes on 3x5 cards, and collected cash payments they stowed in their desk drawers are long gone. In the Olympian corridors of power far above our heads, powerful forces that dictate our lives hurl thunderbolts at each other as they vie for money, power, and control. The trick is to stay out of their way and avoid becoming collateral damage.
Easy to say. Less easy to do.
Dr. Rockoff practices dermatology in Brookline, Mass. He is on the clinical faculty at Tufts University School of Medicine, Boston, and has taught senior medical students and other trainees for 30 years. Dr. Rockoff has contributed to the Under My Skin column in Skin & Allergy News since January 2002.
Predicting Safe Physician Workloads
Attending physician workload may be compromising patient safety and quality of care. Recent studies show hospitalists, intensivists, and surgeons report that excessive attending physician workload has a negative impact on patient care.[1, 2, 3] Because physician teams and hospitals differ in composition, function, and setting, it is difficult to directly compare one service to another within or between institutions. Identifying physician, team, and hospital characteristics associated with clinicians' impressions of unsafe workload provides physician leaders, hospital administrators, and policymakers with potential risk factors and specific targets for interventions.[4] In this study, we use a national survey of hospitalists to identify the physician, team, and hospital factors associated with physician report of an unsafe workload.
METHODS
We electronically surveyed 890 self‐identified hospitalists enrolled in
RESULTS
Of the 890 physicians contacted, 506 (57%) responded. Full characteristics of respondents are reported elsewhere.[1] Forty percent of physicians (n=202) indicated that their typical inpatient census exceeded safe levels at least monthly. A descriptive comparison of the lower and higher reporters of unsafe levels is provided (Table 1). Higher frequency of reporting an unsafe census was associated with higher percentages of clinical (P=0.004) and inpatient responsibilities (P0.001) and more time seeing patients without midlevel or housestaff assistance (P=0.001) (Table 1). On the other hand, lower reported unsafe census was associated with more years in practice (P=0.02), greater percentage of personal time (P=0.02), and the presence of any system for census control (patient caps, fixed bed capacity, staffing augmentation plans) (P=0.007) (Table 1). Fixed census caps decreased the odds of reporting an unsafe census by 34% and was the only statistically significant workload control mechanism (odds ratio: 0.66; 95% confidence interval: 0.43‐0.99; P=0.04). There was no association between reported unsafe census and physician age (P=0.42), practice area (P=0.63), organization type (P=0.98), or compensation (salary [P=0.23], bonus [P=0.61], or total [P=0.54]).
| Characteristic | Report of Unsafe Workloada | Univariate Odds Ratio (95% CI) | Reported Effect on Unsafe Workload Frequency | |
|---|---|---|---|---|
| Lower | Higher | |||
| ||||
| Percentage of total work hours devoted to patient care, median [IQR] | 95 [80100] | 100 [90100] | 1.13b (1.041.23)c | Increased |
| Percentage of clinical care that is inpatient, median [IQR] | 75 [5085] | 80 [7090] | 1.21b (1.131.34)d | |
| Percentage of clinical work performed with no assistance from housestaff or midlevels, median [IQR] | 80 [25100] | 90 [50100] | 1.08b (1.031.14)c | |
| Years in practice, median [IQR] | 6 [311] | 5 [310] | 0.85e (0.750.98)f | Decreased |
| Percentage of workday allotted for personal time, median [IQR] | 5 [07] | 3 [05] | 0.50b (0.380.92)f | |
| Systems for increased patient volume, No. (%) | ||||
| Fixed census cap | 87 (30) | 45 (22) | 0.66 (0.430.99)f | |
| Fixed bed capacity | 36 (13) | 24 (12) | 0.94 (0.541.63) | |
| Staffing augmentation | 88 (31) | 58 (29) | 0.91 (0.611.35) | |
| Any system | 217 (76) | 130 (64) | 0.58 (0.390.86)g | |
| Primary practice area of hospital medicine, No. (%) | ||||
| Adult | 211 (73) | 173 (86) | 1 | Equivocal |
| Pediatric | 7 (2) | 1 (0.5) | 0.24 (0.032.10) | |
| Combined, adult and pediatric | 5 (2) | 3 (1) | 0.73 (0.173.10) | |
| Primary role, No. (%) | ||||
| Clinical | 242 (83) | 186 (92) | 1 | |
| Research | 5 (2) | 4 (2) | 1.04 (0.283.93) | |
| Administrative | 14 (5) | 6 (3) | 0.56 (0.211.48) | |
| Physician age, median [IQR], y | 36 [3242] | 37 [3342] | 0.96e (0.861.07) | |
| Compensation, median [IQR], thousands of dollars | ||||
| Salary only | 180 [130200] | 180 [150200] | 0.97h (0.981.05) | |
| Incentive pay only | 10 [025] | 10 [020] | 0.99h (0.941.04) | |
| Total | 190 [140220] | 196 [165220] | 0.99h (0.981.03) | |
| Practice area, No. (%) | ||||
| Urban | 128 (45) | 98 (49) | 1 | |
| Suburban | 126 (44) | 81 (41) | 0.84 (0.571.23) | |
| Rural | 33 (11) | 21 (10) | 0.83 (0.451.53) | |
| Practice location, No. (%) | ||||
| Academic | 82 (29) | 54 (27) | 1 | |
| Community | 153 (53) | 110 (55) | 1.09 (0.721.66) | |
| Veterans hospital | 7 (2) | 4 (2) | 0.87 (0.243.10) | |
| Group | 32 (11) | 25 (13) | 1.19 (0.632.21) | |
| Physician group size, median [IQR] | 12 [620] | 12 [822] | 0.99i (0.981.03) | |
| Localization of patients, No. (%) | ||||
| Multiple units | 179 (61) | 124 (61) | 1 | |
| Single or adjacent unit(s) | 87 (30) | 58 (29) | 0.96 (0.641.44) | |
| Multiple hospitals | 25 (9) | 20 (10) | 1.15 (0.612.17) | |
DISCUSSION
This is the first study to our knowledge to describe factors associated with provider reports of unsafe workload and identifies potential targets for intervention. By identifying modifiable factors affecting workload, such as different team structures with housestaff or midlevels, it may be possible to improve workload, efficiency, and perhaps safety.[5, 6] Less experience, decreased housestaff or midlevel assistance, higher percentages of inpatient and clinical responsibilities, and lack of systems for census control were strongly associated with reports of unsafe workload.
Having any system in place to address increased patient volumes reduced the odds of reporting an unsafe workload. However, only fixed patient census caps were statistically significant. A system that incorporates fixed service or admitting caps may provide greater control on workload but may also result in back‐ups and delays in the emergency room. Similarly, fixed caps may require overflow of patients to less experienced or willing services or increase the number of handoffs, which may adversely affect the quality of patient care. Use of separate admitting teams has the potential to increase efficiency, but is also subject to fluctuations in patient volume and increases the number of handoffs. Each institution should use a multidisciplinary systems approach to address patient throughput and enforce manageable workload such as through the creation of patient flow teams.[7]
Limitations of the study include the relatively small sample of hospitalists and self‐reporting of safety. Because of the diverse characteristics and structures of the individual programs, even if a predictor variable was not missing, if a particular value for that predictor occurred very infrequently, it generated very wide effect estimates. This limited our ability to effectively explore potential confounders and interactions. To our knowledge, this study is the first to explore potential predictors of unsafe attending physician workload. Large national surveys of physicians with greater statistical power can expand upon this initial work and further explore the association between, and interaction of, workload factors and varying perceptions of providers.[4] The most important limitation of this work is that we relied on self‐reporting to define a safe census. We do not have any measured clinical outcomes that can serve to validate the self‐reported impressions. We recognize, however, that adverse events in healthcare require multiple weaknesses to align, and typically, multiple barriers exist to prevent such events. This often makes it difficult to show direct causal links. Additionally, self‐reporting of safety may also be subject to recall bias, because adverse patient outcomes are often particularly memorable. However, high‐reliability organizations recognize the importance of front‐line provider input, such as on the sensitivity of operations (working conditions) and by deferring to expertise (insights and recommendations from providers most knowledgeable of conditions, regardless of seniority).[8]
We acknowledge that several workload factors, such as hospital setting, may not be readily modifiable. However, we also report factors that can be intervened upon, such as assistance[5, 6] or geographic localization of patients.[9, 10] An understanding of both modifiable and fixed factors in healthcare delivery is essential for improving patient care.
This study has significant research implications. It suggests that team structure and physician experience may be used to improve workload safety. Also, particularly if these self‐reported findings are verified using clinical outcomes, providing hospitalists with greater staffing assistance and systems responsive to census fluctuations may improve the safety, quality, and flow of patient care. Future research may identify the association of physician, team, and hospital factors with outcomes and objectively assess targeted interventions to improve both the efficiency and quality of care.
Acknowledgments
The authors thank the Johns Hopkins Clinical Research Network Hospitalists, General Internal Medicine Research in Progress Physicians, and Hospitalist Directors for the Maryland/District of Columbia region for sharing their models of care and comments on the survey content. They also thank Michael Paskavitz, BA (Editor‐in‐Chief) and Brian Driscoll, BA (Managing Editor) from Quantia Communications for all of their technical assistance in administering the survey.
Disclosures: Drs. Michtalik and Brotman had full access to all of the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. Study concept and design: Michtalik, Pronovost, Brotman. Analysis, interpretation of data: Michtalik, Pronovost, Marsteller, Spetz, Brotman. Drafting of the manuscript: Michtalik, Brotman. Critical revision of the manuscript for important intellectual content: Michtalik, Pronovost, Marsteller, Spetz, Brotman. Dr. Brotman has received compensation from Quantia Communications, not exceeding $10,000 annually, for developing educational content. Dr. Michtalik was supported by NIH grant T32 HP10025‐17‐00 and NIH/Johns Hopkins Institute for Clinical and Translational Research KL2 Award 5KL2RR025006. The Johns Hopkins Hospitalist Scholars Fund provided funding for survey implementation and data acquisition by Quantia Communications. The funders had no role in the design, analysis, and interpretation of the data, or the preparation, review, or approval of the manuscript. The authors report no conflicts of interest.
- , , , . Impact of attending physician workload on patient care: a survey of hospitalists. JAMA Intern Med. 2013;173(5):375–377.
- , , , et al. Does surgeon workload per day affect outcomes after pulmonary lobectomies? Ann Thorac Surg. 2012;94(3):966–972.
- , , , . Perceived effects of attending physician workload in academic medical intensive care units: a national survey of training program directors. Crit Care Med. 2012;40(2):400–405.
- , , , , . Developing a model for attending physician workload and outcomes. JAMA Intern Med. 2013;173(11):1026–1028.
- , , , et al. A comparison of outcomes of general medical inpatient care provided by a hospitalist‐physician assistant model vs a traditional resident‐based model. J Hosp Med. 2011;6(3):122–130.
- , , , et al. Implementation of a physician assistant/hospitalist service in an academic medical center: impact on efficiency and patient outcomes. J Hosp Med. 2008;3(5):361–368.
- , , , . Improving patient flow and reducing emergency department crowding: a guide for hospitals. AHRQ publication no. 11(12)−0094. Rockville, MD: Agency for Healthcare Research and Quality; 2011.
- , , , et al. Becoming a high reliability organization: operational advice for hospital leaders. AHRQ publication no. 08–0022. Rockville, MD: Agency for Healthcare Research and Quality; 2008.
- , , , et al. Impact of localizing general medical teams to a single nursing unit. J Hosp Med. 2012;7(7):551–556.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24(11):1223–1227.
Attending physician workload may be compromising patient safety and quality of care. Recent studies show hospitalists, intensivists, and surgeons report that excessive attending physician workload has a negative impact on patient care.[1, 2, 3] Because physician teams and hospitals differ in composition, function, and setting, it is difficult to directly compare one service to another within or between institutions. Identifying physician, team, and hospital characteristics associated with clinicians' impressions of unsafe workload provides physician leaders, hospital administrators, and policymakers with potential risk factors and specific targets for interventions.[4] In this study, we use a national survey of hospitalists to identify the physician, team, and hospital factors associated with physician report of an unsafe workload.
METHODS
We electronically surveyed 890 self‐identified hospitalists enrolled in
RESULTS
Of the 890 physicians contacted, 506 (57%) responded. Full characteristics of respondents are reported elsewhere.[1] Forty percent of physicians (n=202) indicated that their typical inpatient census exceeded safe levels at least monthly. A descriptive comparison of the lower and higher reporters of unsafe levels is provided (Table 1). Higher frequency of reporting an unsafe census was associated with higher percentages of clinical (P=0.004) and inpatient responsibilities (P0.001) and more time seeing patients without midlevel or housestaff assistance (P=0.001) (Table 1). On the other hand, lower reported unsafe census was associated with more years in practice (P=0.02), greater percentage of personal time (P=0.02), and the presence of any system for census control (patient caps, fixed bed capacity, staffing augmentation plans) (P=0.007) (Table 1). Fixed census caps decreased the odds of reporting an unsafe census by 34% and was the only statistically significant workload control mechanism (odds ratio: 0.66; 95% confidence interval: 0.43‐0.99; P=0.04). There was no association between reported unsafe census and physician age (P=0.42), practice area (P=0.63), organization type (P=0.98), or compensation (salary [P=0.23], bonus [P=0.61], or total [P=0.54]).
| Characteristic | Report of Unsafe Workloada | Univariate Odds Ratio (95% CI) | Reported Effect on Unsafe Workload Frequency | |
|---|---|---|---|---|
| Lower | Higher | |||
| ||||
| Percentage of total work hours devoted to patient care, median [IQR] | 95 [80100] | 100 [90100] | 1.13b (1.041.23)c | Increased |
| Percentage of clinical care that is inpatient, median [IQR] | 75 [5085] | 80 [7090] | 1.21b (1.131.34)d | |
| Percentage of clinical work performed with no assistance from housestaff or midlevels, median [IQR] | 80 [25100] | 90 [50100] | 1.08b (1.031.14)c | |
| Years in practice, median [IQR] | 6 [311] | 5 [310] | 0.85e (0.750.98)f | Decreased |
| Percentage of workday allotted for personal time, median [IQR] | 5 [07] | 3 [05] | 0.50b (0.380.92)f | |
| Systems for increased patient volume, No. (%) | ||||
| Fixed census cap | 87 (30) | 45 (22) | 0.66 (0.430.99)f | |
| Fixed bed capacity | 36 (13) | 24 (12) | 0.94 (0.541.63) | |
| Staffing augmentation | 88 (31) | 58 (29) | 0.91 (0.611.35) | |
| Any system | 217 (76) | 130 (64) | 0.58 (0.390.86)g | |
| Primary practice area of hospital medicine, No. (%) | ||||
| Adult | 211 (73) | 173 (86) | 1 | Equivocal |
| Pediatric | 7 (2) | 1 (0.5) | 0.24 (0.032.10) | |
| Combined, adult and pediatric | 5 (2) | 3 (1) | 0.73 (0.173.10) | |
| Primary role, No. (%) | ||||
| Clinical | 242 (83) | 186 (92) | 1 | |
| Research | 5 (2) | 4 (2) | 1.04 (0.283.93) | |
| Administrative | 14 (5) | 6 (3) | 0.56 (0.211.48) | |
| Physician age, median [IQR], y | 36 [3242] | 37 [3342] | 0.96e (0.861.07) | |
| Compensation, median [IQR], thousands of dollars | ||||
| Salary only | 180 [130200] | 180 [150200] | 0.97h (0.981.05) | |
| Incentive pay only | 10 [025] | 10 [020] | 0.99h (0.941.04) | |
| Total | 190 [140220] | 196 [165220] | 0.99h (0.981.03) | |
| Practice area, No. (%) | ||||
| Urban | 128 (45) | 98 (49) | 1 | |
| Suburban | 126 (44) | 81 (41) | 0.84 (0.571.23) | |
| Rural | 33 (11) | 21 (10) | 0.83 (0.451.53) | |
| Practice location, No. (%) | ||||
| Academic | 82 (29) | 54 (27) | 1 | |
| Community | 153 (53) | 110 (55) | 1.09 (0.721.66) | |
| Veterans hospital | 7 (2) | 4 (2) | 0.87 (0.243.10) | |
| Group | 32 (11) | 25 (13) | 1.19 (0.632.21) | |
| Physician group size, median [IQR] | 12 [620] | 12 [822] | 0.99i (0.981.03) | |
| Localization of patients, No. (%) | ||||
| Multiple units | 179 (61) | 124 (61) | 1 | |
| Single or adjacent unit(s) | 87 (30) | 58 (29) | 0.96 (0.641.44) | |
| Multiple hospitals | 25 (9) | 20 (10) | 1.15 (0.612.17) | |
DISCUSSION
This is the first study to our knowledge to describe factors associated with provider reports of unsafe workload and identifies potential targets for intervention. By identifying modifiable factors affecting workload, such as different team structures with housestaff or midlevels, it may be possible to improve workload, efficiency, and perhaps safety.[5, 6] Less experience, decreased housestaff or midlevel assistance, higher percentages of inpatient and clinical responsibilities, and lack of systems for census control were strongly associated with reports of unsafe workload.
Having any system in place to address increased patient volumes reduced the odds of reporting an unsafe workload. However, only fixed patient census caps were statistically significant. A system that incorporates fixed service or admitting caps may provide greater control on workload but may also result in back‐ups and delays in the emergency room. Similarly, fixed caps may require overflow of patients to less experienced or willing services or increase the number of handoffs, which may adversely affect the quality of patient care. Use of separate admitting teams has the potential to increase efficiency, but is also subject to fluctuations in patient volume and increases the number of handoffs. Each institution should use a multidisciplinary systems approach to address patient throughput and enforce manageable workload such as through the creation of patient flow teams.[7]
Limitations of the study include the relatively small sample of hospitalists and self‐reporting of safety. Because of the diverse characteristics and structures of the individual programs, even if a predictor variable was not missing, if a particular value for that predictor occurred very infrequently, it generated very wide effect estimates. This limited our ability to effectively explore potential confounders and interactions. To our knowledge, this study is the first to explore potential predictors of unsafe attending physician workload. Large national surveys of physicians with greater statistical power can expand upon this initial work and further explore the association between, and interaction of, workload factors and varying perceptions of providers.[4] The most important limitation of this work is that we relied on self‐reporting to define a safe census. We do not have any measured clinical outcomes that can serve to validate the self‐reported impressions. We recognize, however, that adverse events in healthcare require multiple weaknesses to align, and typically, multiple barriers exist to prevent such events. This often makes it difficult to show direct causal links. Additionally, self‐reporting of safety may also be subject to recall bias, because adverse patient outcomes are often particularly memorable. However, high‐reliability organizations recognize the importance of front‐line provider input, such as on the sensitivity of operations (working conditions) and by deferring to expertise (insights and recommendations from providers most knowledgeable of conditions, regardless of seniority).[8]
We acknowledge that several workload factors, such as hospital setting, may not be readily modifiable. However, we also report factors that can be intervened upon, such as assistance[5, 6] or geographic localization of patients.[9, 10] An understanding of both modifiable and fixed factors in healthcare delivery is essential for improving patient care.
This study has significant research implications. It suggests that team structure and physician experience may be used to improve workload safety. Also, particularly if these self‐reported findings are verified using clinical outcomes, providing hospitalists with greater staffing assistance and systems responsive to census fluctuations may improve the safety, quality, and flow of patient care. Future research may identify the association of physician, team, and hospital factors with outcomes and objectively assess targeted interventions to improve both the efficiency and quality of care.
Acknowledgments
The authors thank the Johns Hopkins Clinical Research Network Hospitalists, General Internal Medicine Research in Progress Physicians, and Hospitalist Directors for the Maryland/District of Columbia region for sharing their models of care and comments on the survey content. They also thank Michael Paskavitz, BA (Editor‐in‐Chief) and Brian Driscoll, BA (Managing Editor) from Quantia Communications for all of their technical assistance in administering the survey.
Disclosures: Drs. Michtalik and Brotman had full access to all of the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. Study concept and design: Michtalik, Pronovost, Brotman. Analysis, interpretation of data: Michtalik, Pronovost, Marsteller, Spetz, Brotman. Drafting of the manuscript: Michtalik, Brotman. Critical revision of the manuscript for important intellectual content: Michtalik, Pronovost, Marsteller, Spetz, Brotman. Dr. Brotman has received compensation from Quantia Communications, not exceeding $10,000 annually, for developing educational content. Dr. Michtalik was supported by NIH grant T32 HP10025‐17‐00 and NIH/Johns Hopkins Institute for Clinical and Translational Research KL2 Award 5KL2RR025006. The Johns Hopkins Hospitalist Scholars Fund provided funding for survey implementation and data acquisition by Quantia Communications. The funders had no role in the design, analysis, and interpretation of the data, or the preparation, review, or approval of the manuscript. The authors report no conflicts of interest.
Attending physician workload may be compromising patient safety and quality of care. Recent studies show hospitalists, intensivists, and surgeons report that excessive attending physician workload has a negative impact on patient care.[1, 2, 3] Because physician teams and hospitals differ in composition, function, and setting, it is difficult to directly compare one service to another within or between institutions. Identifying physician, team, and hospital characteristics associated with clinicians' impressions of unsafe workload provides physician leaders, hospital administrators, and policymakers with potential risk factors and specific targets for interventions.[4] In this study, we use a national survey of hospitalists to identify the physician, team, and hospital factors associated with physician report of an unsafe workload.
METHODS
We electronically surveyed 890 self‐identified hospitalists enrolled in
RESULTS
Of the 890 physicians contacted, 506 (57%) responded. Full characteristics of respondents are reported elsewhere.[1] Forty percent of physicians (n=202) indicated that their typical inpatient census exceeded safe levels at least monthly. A descriptive comparison of the lower and higher reporters of unsafe levels is provided (Table 1). Higher frequency of reporting an unsafe census was associated with higher percentages of clinical (P=0.004) and inpatient responsibilities (P0.001) and more time seeing patients without midlevel or housestaff assistance (P=0.001) (Table 1). On the other hand, lower reported unsafe census was associated with more years in practice (P=0.02), greater percentage of personal time (P=0.02), and the presence of any system for census control (patient caps, fixed bed capacity, staffing augmentation plans) (P=0.007) (Table 1). Fixed census caps decreased the odds of reporting an unsafe census by 34% and was the only statistically significant workload control mechanism (odds ratio: 0.66; 95% confidence interval: 0.43‐0.99; P=0.04). There was no association between reported unsafe census and physician age (P=0.42), practice area (P=0.63), organization type (P=0.98), or compensation (salary [P=0.23], bonus [P=0.61], or total [P=0.54]).
| Characteristic | Report of Unsafe Workloada | Univariate Odds Ratio (95% CI) | Reported Effect on Unsafe Workload Frequency | |
|---|---|---|---|---|
| Lower | Higher | |||
| ||||
| Percentage of total work hours devoted to patient care, median [IQR] | 95 [80100] | 100 [90100] | 1.13b (1.041.23)c | Increased |
| Percentage of clinical care that is inpatient, median [IQR] | 75 [5085] | 80 [7090] | 1.21b (1.131.34)d | |
| Percentage of clinical work performed with no assistance from housestaff or midlevels, median [IQR] | 80 [25100] | 90 [50100] | 1.08b (1.031.14)c | |
| Years in practice, median [IQR] | 6 [311] | 5 [310] | 0.85e (0.750.98)f | Decreased |
| Percentage of workday allotted for personal time, median [IQR] | 5 [07] | 3 [05] | 0.50b (0.380.92)f | |
| Systems for increased patient volume, No. (%) | ||||
| Fixed census cap | 87 (30) | 45 (22) | 0.66 (0.430.99)f | |
| Fixed bed capacity | 36 (13) | 24 (12) | 0.94 (0.541.63) | |
| Staffing augmentation | 88 (31) | 58 (29) | 0.91 (0.611.35) | |
| Any system | 217 (76) | 130 (64) | 0.58 (0.390.86)g | |
| Primary practice area of hospital medicine, No. (%) | ||||
| Adult | 211 (73) | 173 (86) | 1 | Equivocal |
| Pediatric | 7 (2) | 1 (0.5) | 0.24 (0.032.10) | |
| Combined, adult and pediatric | 5 (2) | 3 (1) | 0.73 (0.173.10) | |
| Primary role, No. (%) | ||||
| Clinical | 242 (83) | 186 (92) | 1 | |
| Research | 5 (2) | 4 (2) | 1.04 (0.283.93) | |
| Administrative | 14 (5) | 6 (3) | 0.56 (0.211.48) | |
| Physician age, median [IQR], y | 36 [3242] | 37 [3342] | 0.96e (0.861.07) | |
| Compensation, median [IQR], thousands of dollars | ||||
| Salary only | 180 [130200] | 180 [150200] | 0.97h (0.981.05) | |
| Incentive pay only | 10 [025] | 10 [020] | 0.99h (0.941.04) | |
| Total | 190 [140220] | 196 [165220] | 0.99h (0.981.03) | |
| Practice area, No. (%) | ||||
| Urban | 128 (45) | 98 (49) | 1 | |
| Suburban | 126 (44) | 81 (41) | 0.84 (0.571.23) | |
| Rural | 33 (11) | 21 (10) | 0.83 (0.451.53) | |
| Practice location, No. (%) | ||||
| Academic | 82 (29) | 54 (27) | 1 | |
| Community | 153 (53) | 110 (55) | 1.09 (0.721.66) | |
| Veterans hospital | 7 (2) | 4 (2) | 0.87 (0.243.10) | |
| Group | 32 (11) | 25 (13) | 1.19 (0.632.21) | |
| Physician group size, median [IQR] | 12 [620] | 12 [822] | 0.99i (0.981.03) | |
| Localization of patients, No. (%) | ||||
| Multiple units | 179 (61) | 124 (61) | 1 | |
| Single or adjacent unit(s) | 87 (30) | 58 (29) | 0.96 (0.641.44) | |
| Multiple hospitals | 25 (9) | 20 (10) | 1.15 (0.612.17) | |
DISCUSSION
This is the first study to our knowledge to describe factors associated with provider reports of unsafe workload and identifies potential targets for intervention. By identifying modifiable factors affecting workload, such as different team structures with housestaff or midlevels, it may be possible to improve workload, efficiency, and perhaps safety.[5, 6] Less experience, decreased housestaff or midlevel assistance, higher percentages of inpatient and clinical responsibilities, and lack of systems for census control were strongly associated with reports of unsafe workload.
Having any system in place to address increased patient volumes reduced the odds of reporting an unsafe workload. However, only fixed patient census caps were statistically significant. A system that incorporates fixed service or admitting caps may provide greater control on workload but may also result in back‐ups and delays in the emergency room. Similarly, fixed caps may require overflow of patients to less experienced or willing services or increase the number of handoffs, which may adversely affect the quality of patient care. Use of separate admitting teams has the potential to increase efficiency, but is also subject to fluctuations in patient volume and increases the number of handoffs. Each institution should use a multidisciplinary systems approach to address patient throughput and enforce manageable workload such as through the creation of patient flow teams.[7]
Limitations of the study include the relatively small sample of hospitalists and self‐reporting of safety. Because of the diverse characteristics and structures of the individual programs, even if a predictor variable was not missing, if a particular value for that predictor occurred very infrequently, it generated very wide effect estimates. This limited our ability to effectively explore potential confounders and interactions. To our knowledge, this study is the first to explore potential predictors of unsafe attending physician workload. Large national surveys of physicians with greater statistical power can expand upon this initial work and further explore the association between, and interaction of, workload factors and varying perceptions of providers.[4] The most important limitation of this work is that we relied on self‐reporting to define a safe census. We do not have any measured clinical outcomes that can serve to validate the self‐reported impressions. We recognize, however, that adverse events in healthcare require multiple weaknesses to align, and typically, multiple barriers exist to prevent such events. This often makes it difficult to show direct causal links. Additionally, self‐reporting of safety may also be subject to recall bias, because adverse patient outcomes are often particularly memorable. However, high‐reliability organizations recognize the importance of front‐line provider input, such as on the sensitivity of operations (working conditions) and by deferring to expertise (insights and recommendations from providers most knowledgeable of conditions, regardless of seniority).[8]
We acknowledge that several workload factors, such as hospital setting, may not be readily modifiable. However, we also report factors that can be intervened upon, such as assistance[5, 6] or geographic localization of patients.[9, 10] An understanding of both modifiable and fixed factors in healthcare delivery is essential for improving patient care.
This study has significant research implications. It suggests that team structure and physician experience may be used to improve workload safety. Also, particularly if these self‐reported findings are verified using clinical outcomes, providing hospitalists with greater staffing assistance and systems responsive to census fluctuations may improve the safety, quality, and flow of patient care. Future research may identify the association of physician, team, and hospital factors with outcomes and objectively assess targeted interventions to improve both the efficiency and quality of care.
Acknowledgments
The authors thank the Johns Hopkins Clinical Research Network Hospitalists, General Internal Medicine Research in Progress Physicians, and Hospitalist Directors for the Maryland/District of Columbia region for sharing their models of care and comments on the survey content. They also thank Michael Paskavitz, BA (Editor‐in‐Chief) and Brian Driscoll, BA (Managing Editor) from Quantia Communications for all of their technical assistance in administering the survey.
Disclosures: Drs. Michtalik and Brotman had full access to all of the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. Study concept and design: Michtalik, Pronovost, Brotman. Analysis, interpretation of data: Michtalik, Pronovost, Marsteller, Spetz, Brotman. Drafting of the manuscript: Michtalik, Brotman. Critical revision of the manuscript for important intellectual content: Michtalik, Pronovost, Marsteller, Spetz, Brotman. Dr. Brotman has received compensation from Quantia Communications, not exceeding $10,000 annually, for developing educational content. Dr. Michtalik was supported by NIH grant T32 HP10025‐17‐00 and NIH/Johns Hopkins Institute for Clinical and Translational Research KL2 Award 5KL2RR025006. The Johns Hopkins Hospitalist Scholars Fund provided funding for survey implementation and data acquisition by Quantia Communications. The funders had no role in the design, analysis, and interpretation of the data, or the preparation, review, or approval of the manuscript. The authors report no conflicts of interest.
- , , , . Impact of attending physician workload on patient care: a survey of hospitalists. JAMA Intern Med. 2013;173(5):375–377.
- , , , et al. Does surgeon workload per day affect outcomes after pulmonary lobectomies? Ann Thorac Surg. 2012;94(3):966–972.
- , , , . Perceived effects of attending physician workload in academic medical intensive care units: a national survey of training program directors. Crit Care Med. 2012;40(2):400–405.
- , , , , . Developing a model for attending physician workload and outcomes. JAMA Intern Med. 2013;173(11):1026–1028.
- , , , et al. A comparison of outcomes of general medical inpatient care provided by a hospitalist‐physician assistant model vs a traditional resident‐based model. J Hosp Med. 2011;6(3):122–130.
- , , , et al. Implementation of a physician assistant/hospitalist service in an academic medical center: impact on efficiency and patient outcomes. J Hosp Med. 2008;3(5):361–368.
- , , , . Improving patient flow and reducing emergency department crowding: a guide for hospitals. AHRQ publication no. 11(12)−0094. Rockville, MD: Agency for Healthcare Research and Quality; 2011.
- , , , et al. Becoming a high reliability organization: operational advice for hospital leaders. AHRQ publication no. 08–0022. Rockville, MD: Agency for Healthcare Research and Quality; 2008.
- , , , et al. Impact of localizing general medical teams to a single nursing unit. J Hosp Med. 2012;7(7):551–556.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24(11):1223–1227.
- , , , . Impact of attending physician workload on patient care: a survey of hospitalists. JAMA Intern Med. 2013;173(5):375–377.
- , , , et al. Does surgeon workload per day affect outcomes after pulmonary lobectomies? Ann Thorac Surg. 2012;94(3):966–972.
- , , , . Perceived effects of attending physician workload in academic medical intensive care units: a national survey of training program directors. Crit Care Med. 2012;40(2):400–405.
- , , , , . Developing a model for attending physician workload and outcomes. JAMA Intern Med. 2013;173(11):1026–1028.
- , , , et al. A comparison of outcomes of general medical inpatient care provided by a hospitalist‐physician assistant model vs a traditional resident‐based model. J Hosp Med. 2011;6(3):122–130.
- , , , et al. Implementation of a physician assistant/hospitalist service in an academic medical center: impact on efficiency and patient outcomes. J Hosp Med. 2008;3(5):361–368.
- , , , . Improving patient flow and reducing emergency department crowding: a guide for hospitals. AHRQ publication no. 11(12)−0094. Rockville, MD: Agency for Healthcare Research and Quality; 2011.
- , , , et al. Becoming a high reliability organization: operational advice for hospital leaders. AHRQ publication no. 08–0022. Rockville, MD: Agency for Healthcare Research and Quality; 2008.
- , , , et al. Impact of localizing general medical teams to a single nursing unit. J Hosp Med. 2012;7(7):551–556.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24(11):1223–1227.
Use of the JAK1/JAK2 inhibitor ruxolitinib in the treatment of patients with myelofibrosis
Myelofibrosis (MF), including primary MF and MF secondary to polycythemia vera or essential thrombocythemia, is a chronic, clinically heterogeneous hematologic malignancy characterized by inefficient hematopoiesis, bone marrow fibrosis, and shortened survival. Typical clinical manifestations include progressive splenomegaly, debilitating symptoms, and anemia. MF is associated with dysregulation of Janus kinase (JAK)-signal transducer and activator of transcription (JAK/STAT) pathway affecting hematopoiesis and inflammation. Ruxolitinib, an oral JAK1/JAK2 inhibitor, was approved for the treatment of patients with intermediate or high-risk MF based on the results of 2 phase 3 studies (Controlled MyeloFibrosis Study with Oral JAK Inhibitor Treatment [COMFORT]-I and COMFORT-II). In these trials, ruxolitinib treatment was associated with reductions in spleen size and symptom burden, and improvements in quality of life. The most common adverse events were dose-dependent cytopenias, which were managed by dose modifications, treatment interruptions, and red blood cell transfusions (for anemia). Ruxolitinib was effective regardless of MF type, risk status, or JAK2V617F mutation status, and across various other MF subpopulations. Two-year follow-up data from the COMFORT trials also demonstrate that ruxolitinib has durable efficacy and may be associated with a survival advantage relative to placebo and best available therapy. Preliminary data from ongoing studies support possible dosing strategies for patients with low platelet counts.
Click on the PDF icon at the top of this introduction to read the full article.
Myelofibrosis (MF), including primary MF and MF secondary to polycythemia vera or essential thrombocythemia, is a chronic, clinically heterogeneous hematologic malignancy characterized by inefficient hematopoiesis, bone marrow fibrosis, and shortened survival. Typical clinical manifestations include progressive splenomegaly, debilitating symptoms, and anemia. MF is associated with dysregulation of Janus kinase (JAK)-signal transducer and activator of transcription (JAK/STAT) pathway affecting hematopoiesis and inflammation. Ruxolitinib, an oral JAK1/JAK2 inhibitor, was approved for the treatment of patients with intermediate or high-risk MF based on the results of 2 phase 3 studies (Controlled MyeloFibrosis Study with Oral JAK Inhibitor Treatment [COMFORT]-I and COMFORT-II). In these trials, ruxolitinib treatment was associated with reductions in spleen size and symptom burden, and improvements in quality of life. The most common adverse events were dose-dependent cytopenias, which were managed by dose modifications, treatment interruptions, and red blood cell transfusions (for anemia). Ruxolitinib was effective regardless of MF type, risk status, or JAK2V617F mutation status, and across various other MF subpopulations. Two-year follow-up data from the COMFORT trials also demonstrate that ruxolitinib has durable efficacy and may be associated with a survival advantage relative to placebo and best available therapy. Preliminary data from ongoing studies support possible dosing strategies for patients with low platelet counts.
Click on the PDF icon at the top of this introduction to read the full article.
Myelofibrosis (MF), including primary MF and MF secondary to polycythemia vera or essential thrombocythemia, is a chronic, clinically heterogeneous hematologic malignancy characterized by inefficient hematopoiesis, bone marrow fibrosis, and shortened survival. Typical clinical manifestations include progressive splenomegaly, debilitating symptoms, and anemia. MF is associated with dysregulation of Janus kinase (JAK)-signal transducer and activator of transcription (JAK/STAT) pathway affecting hematopoiesis and inflammation. Ruxolitinib, an oral JAK1/JAK2 inhibitor, was approved for the treatment of patients with intermediate or high-risk MF based on the results of 2 phase 3 studies (Controlled MyeloFibrosis Study with Oral JAK Inhibitor Treatment [COMFORT]-I and COMFORT-II). In these trials, ruxolitinib treatment was associated with reductions in spleen size and symptom burden, and improvements in quality of life. The most common adverse events were dose-dependent cytopenias, which were managed by dose modifications, treatment interruptions, and red blood cell transfusions (for anemia). Ruxolitinib was effective regardless of MF type, risk status, or JAK2V617F mutation status, and across various other MF subpopulations. Two-year follow-up data from the COMFORT trials also demonstrate that ruxolitinib has durable efficacy and may be associated with a survival advantage relative to placebo and best available therapy. Preliminary data from ongoing studies support possible dosing strategies for patients with low platelet counts.
Click on the PDF icon at the top of this introduction to read the full article.
Pediatric Parvovirus B19: Spectrum of Clinical Manifestations
Test your knowledge on pediatric parvovirus with MD-IQ: the medical intelligence quiz. Click here to answer 5 questions.
Botanical Briefs: Cashew Apple (Anacardium occidentale)
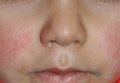
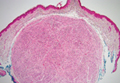
Palisaded Encapsulated Neuroma
Drug gets orphan designation for MDS
The US Food and Drug Administration (FDA) has granted orphan designation to an investigational drug for the treatment of myelodysplastic syndromes (MDS).
The drug, CPI-613, targets metabolic changes that are thought to occur in many cancer cells.
It has demonstrated activity and tolerability in a phase 1 trial of patients with advanced, relapsed/refractory hematologic malignancies.
CPI-613 previously received orphan designation for acute myeloid leukemia (AML) and pancreatic carcinoma.
Orphan designation is granted for drugs intended to treat diseases that affect fewer than 200,000 individuals in the US. This designation gives the makers of CPI-613, Cornerstone Pharmaceuticals, 7 years of US marketing exclusivity once the drug is approved.
The designation also allows the company to apply for government funding to defray trial costs, tax credits for clinical research expenses, and a potential waiver of the FDA’s application user fee.
CPI-613: Mechanism and phase 1 results
CPI-613 induces cancer-specific inhibition of the mitochondrial enzymes pyruvate dehydrogenase (PDH) and alpha ketoglutarate dehydrogenase (KGDH).
Disrupting the function of PDH and KGDH disrupts tumor mitochondrial metabolism. As a result, tumor cells are starved of energy and biosynthetic intermediates, which leads to cell death.
Researchers evaluated CPI-613 in a phase 1 study of patients with advanced, relapsed/refractory hematologic malignancies.
The team, led by Timothy S. Pardee, MD, of Wake Forest Baptist Medical Center in Winston-Salem, North Carolina, presented the results at the 2013 ASCO Annual Meeting as abstract 2516. (Information in the abstract differs slightly from that presented at the meeting.)
The trial was designed to determine the maximum tolerated dose, safety, and anticancer activity of CPI-613 as a single agent.
Twenty-one evaluable patients received CPI-613 on days 1 and 4 for 3 weeks every 28 days. Ten patients received more than 1 cycle of therapy.
The starting dose was 420 mg/m2. Treatment could be continued if the patient experienced clinical benefit. Doses were escalated to a final dose of 3780 mg/m2.
CPI-613 was generally well-tolerated when infused over 2 hours. Patients did not experience worsening cytopenias at any dose level. However, 1-hour infusions led to grade 3 renal failure in 2 patients.
At a dose of 3780 mg/m2, 1 patient had prolonged grade 3 nausea, and 1 patient had grade 3 renal failure. Six patients received a 2-hour infusion of 2940 mg/m2 without dose-limiting toxicities, so the researchers considered this the maximum tolerated dose.
Of the 21 patients, 9 achieved a response of stable disease or better. One MDS patient achieved a complete remission and maintained it over 23 cycles. One AML patient achieved a morphologic leukemia-free state.
A Burkitt lymphoma patient and a cutaneous T-cell lymphoma patient maintained partial responses over 16 and 15 cycles, respectively. Two multiple myeloma patients, 2 MDS patients, and 1 AML patient had stable disease.
“We are very encouraged by the tolerability and signals of activity seen in several patients in this phase 1 study for whom there is no available therapy shown to provide clinical benefit,” Dr Pardee said.
“We look forward to further evaluating CPI-613 in the early relapsed/refractory AML patient setting when administered in combination with a standard chemotherapeutic regimen, as well as in early relapsed or refractory MDS patients, with the hope of improving the outcomes and the quality of life for these patients through the combined use of this mechanistically novel agent.”
The AML study is a phase 1 trial investigating CPI-613 in combination with high-dose cytarabine and mitoxantrone, and the MDS study is a phase 2 trial investigating single-agent CPI-613. ![]()
The US Food and Drug Administration (FDA) has granted orphan designation to an investigational drug for the treatment of myelodysplastic syndromes (MDS).
The drug, CPI-613, targets metabolic changes that are thought to occur in many cancer cells.
It has demonstrated activity and tolerability in a phase 1 trial of patients with advanced, relapsed/refractory hematologic malignancies.
CPI-613 previously received orphan designation for acute myeloid leukemia (AML) and pancreatic carcinoma.
Orphan designation is granted for drugs intended to treat diseases that affect fewer than 200,000 individuals in the US. This designation gives the makers of CPI-613, Cornerstone Pharmaceuticals, 7 years of US marketing exclusivity once the drug is approved.
The designation also allows the company to apply for government funding to defray trial costs, tax credits for clinical research expenses, and a potential waiver of the FDA’s application user fee.
CPI-613: Mechanism and phase 1 results
CPI-613 induces cancer-specific inhibition of the mitochondrial enzymes pyruvate dehydrogenase (PDH) and alpha ketoglutarate dehydrogenase (KGDH).
Disrupting the function of PDH and KGDH disrupts tumor mitochondrial metabolism. As a result, tumor cells are starved of energy and biosynthetic intermediates, which leads to cell death.
Researchers evaluated CPI-613 in a phase 1 study of patients with advanced, relapsed/refractory hematologic malignancies.
The team, led by Timothy S. Pardee, MD, of Wake Forest Baptist Medical Center in Winston-Salem, North Carolina, presented the results at the 2013 ASCO Annual Meeting as abstract 2516. (Information in the abstract differs slightly from that presented at the meeting.)
The trial was designed to determine the maximum tolerated dose, safety, and anticancer activity of CPI-613 as a single agent.
Twenty-one evaluable patients received CPI-613 on days 1 and 4 for 3 weeks every 28 days. Ten patients received more than 1 cycle of therapy.
The starting dose was 420 mg/m2. Treatment could be continued if the patient experienced clinical benefit. Doses were escalated to a final dose of 3780 mg/m2.
CPI-613 was generally well-tolerated when infused over 2 hours. Patients did not experience worsening cytopenias at any dose level. However, 1-hour infusions led to grade 3 renal failure in 2 patients.
At a dose of 3780 mg/m2, 1 patient had prolonged grade 3 nausea, and 1 patient had grade 3 renal failure. Six patients received a 2-hour infusion of 2940 mg/m2 without dose-limiting toxicities, so the researchers considered this the maximum tolerated dose.
Of the 21 patients, 9 achieved a response of stable disease or better. One MDS patient achieved a complete remission and maintained it over 23 cycles. One AML patient achieved a morphologic leukemia-free state.
A Burkitt lymphoma patient and a cutaneous T-cell lymphoma patient maintained partial responses over 16 and 15 cycles, respectively. Two multiple myeloma patients, 2 MDS patients, and 1 AML patient had stable disease.
“We are very encouraged by the tolerability and signals of activity seen in several patients in this phase 1 study for whom there is no available therapy shown to provide clinical benefit,” Dr Pardee said.
“We look forward to further evaluating CPI-613 in the early relapsed/refractory AML patient setting when administered in combination with a standard chemotherapeutic regimen, as well as in early relapsed or refractory MDS patients, with the hope of improving the outcomes and the quality of life for these patients through the combined use of this mechanistically novel agent.”
The AML study is a phase 1 trial investigating CPI-613 in combination with high-dose cytarabine and mitoxantrone, and the MDS study is a phase 2 trial investigating single-agent CPI-613. ![]()
The US Food and Drug Administration (FDA) has granted orphan designation to an investigational drug for the treatment of myelodysplastic syndromes (MDS).
The drug, CPI-613, targets metabolic changes that are thought to occur in many cancer cells.
It has demonstrated activity and tolerability in a phase 1 trial of patients with advanced, relapsed/refractory hematologic malignancies.
CPI-613 previously received orphan designation for acute myeloid leukemia (AML) and pancreatic carcinoma.
Orphan designation is granted for drugs intended to treat diseases that affect fewer than 200,000 individuals in the US. This designation gives the makers of CPI-613, Cornerstone Pharmaceuticals, 7 years of US marketing exclusivity once the drug is approved.
The designation also allows the company to apply for government funding to defray trial costs, tax credits for clinical research expenses, and a potential waiver of the FDA’s application user fee.
CPI-613: Mechanism and phase 1 results
CPI-613 induces cancer-specific inhibition of the mitochondrial enzymes pyruvate dehydrogenase (PDH) and alpha ketoglutarate dehydrogenase (KGDH).
Disrupting the function of PDH and KGDH disrupts tumor mitochondrial metabolism. As a result, tumor cells are starved of energy and biosynthetic intermediates, which leads to cell death.
Researchers evaluated CPI-613 in a phase 1 study of patients with advanced, relapsed/refractory hematologic malignancies.
The team, led by Timothy S. Pardee, MD, of Wake Forest Baptist Medical Center in Winston-Salem, North Carolina, presented the results at the 2013 ASCO Annual Meeting as abstract 2516. (Information in the abstract differs slightly from that presented at the meeting.)
The trial was designed to determine the maximum tolerated dose, safety, and anticancer activity of CPI-613 as a single agent.
Twenty-one evaluable patients received CPI-613 on days 1 and 4 for 3 weeks every 28 days. Ten patients received more than 1 cycle of therapy.
The starting dose was 420 mg/m2. Treatment could be continued if the patient experienced clinical benefit. Doses were escalated to a final dose of 3780 mg/m2.
CPI-613 was generally well-tolerated when infused over 2 hours. Patients did not experience worsening cytopenias at any dose level. However, 1-hour infusions led to grade 3 renal failure in 2 patients.
At a dose of 3780 mg/m2, 1 patient had prolonged grade 3 nausea, and 1 patient had grade 3 renal failure. Six patients received a 2-hour infusion of 2940 mg/m2 without dose-limiting toxicities, so the researchers considered this the maximum tolerated dose.
Of the 21 patients, 9 achieved a response of stable disease or better. One MDS patient achieved a complete remission and maintained it over 23 cycles. One AML patient achieved a morphologic leukemia-free state.
A Burkitt lymphoma patient and a cutaneous T-cell lymphoma patient maintained partial responses over 16 and 15 cycles, respectively. Two multiple myeloma patients, 2 MDS patients, and 1 AML patient had stable disease.
“We are very encouraged by the tolerability and signals of activity seen in several patients in this phase 1 study for whom there is no available therapy shown to provide clinical benefit,” Dr Pardee said.
“We look forward to further evaluating CPI-613 in the early relapsed/refractory AML patient setting when administered in combination with a standard chemotherapeutic regimen, as well as in early relapsed or refractory MDS patients, with the hope of improving the outcomes and the quality of life for these patients through the combined use of this mechanistically novel agent.”
The AML study is a phase 1 trial investigating CPI-613 in combination with high-dose cytarabine and mitoxantrone, and the MDS study is a phase 2 trial investigating single-agent CPI-613. ![]()
Vendor CPOE for Renal Impairment
Hospitalized patients with renal impairment are vulnerable to adverse drug events (ADEs).[1, 2] Appropriate prescribing for patients with renal insufficiency is challenging because of the complexities of drug therapy within the wide spectrum of kidney disease.[3, 4, 5, 6] Accordingly, computerized physician order entry (CPOE) systems with clinical decision support may help prevent many ADEs by providing timely laboratory information, recommending renally adjusted doses, and by offering assistance with prescribing.[7, 8, 9]
Despite the proposed benefits of CPOE, outcomes vary greatly because of differences in technology.[10, 11, 12, 13] In particular, the type of decision support available to assist medication ordering in the setting of renal disease varies widely among current vendor systems. Given the uncertain benefits of CPOE, especially with the wide range of associated clinical decision support, we conducted this study to determine the impact of these systems on the rates of ADEs among hospitalized patients with kidney disease.
METHODS
This study was approved by the institutional review boards at each study site.
Design and Setting
We conducted a before‐and‐after study to evaluate the impact of newly implemented vendor CPOE systems in 5 community hospitals in Massachusetts. Although we reported on 6 hospitals in our baseline study,[14] 1 of these hospitals later chose not to implement CPOE, and therefore was not included in follow‐up. At the time of this study, 1 of the hospitals (site 3) had not yet achieved hospital‐wide implementation. Although CPOE had been adopted by most medical services at site 3, it had not yet been implemented in the emergency, obstetrical, or surgical departments. Thus, we limited our study to the medical services at this site. For the remaining sites, all admitting services were included with the exception of the psychiatric and neonatal services, which were excluded from both phases because they would have required different detection tools.
Participants
Patients aged 18 years with renal failure, exposed to potentially nephrotoxic and/or renally cleared medications, and admitted to any of the participating hospitals during the study period were eligible for inclusion. Of the patients meeting eligibility criteria, we randomly selected approximately 150 records per hospital in the preimplementation and postimplementation phases for a total sample of 1590 charts. The first phase of this study occurred from January 2005 to August 2006; the second phase began 6 months postimplementation and lasted from October 2008 to September 2010.
Principal Exposure
Each hospital independently selected a vendor CPOE system with variable clinical decision support capabilities: (1) sites 4 and 5 had basic CPOE only with no clinical decision support for renal disease; (2) sites 1 and 2 implemented rudimentary clinical decision support with laboratory display (eg, serum creatinine) whenever common renally related drugs were ordered; and (3) site 3 had the most advanced support in place where, in addition to basic order entry and lab checks, physicians were provided with suggested doses for renally cleared and/or nephrotoxic medications, as well as appropriate drug monitoring for medications with narrow therapeutic indices (eg, suggested dosages and frequencies for vancomycin and automated corollary laboratory monitoring).
Definitions
We screened for the presence of renal failure by a serum creatinine 1.5 mg/dL at the time of admission. However, the duration of renal impairment was not known. We defined 3 levels of renal insufficiency based on the calculated creatinine clearance (CrCl)15: mild (CrCl 5080 mL/min), moderate (1649 mL/min), and severe (15 mL/min). Subjects with a CrCl >80 mL/min were considered to have normal renal function and were excluded. Potentially nephrotoxic and/or renally cleared medications were then identified using an established knowledge base (see Supporting Information, Table 1, in the online version of this article).[16]
| Hospital Site | |||||||
|---|---|---|---|---|---|---|---|
| Baseline Characteristics | All Sites | 1 | 2 | 3 | 4 | 5 | P (Among All Sites)* |
| |||||||
| No. of patients | 815 | 170 | 156 | 143 | 164 | 182 | |
| Age, y, mean (range) | 72.2 (18.0102.0) | 79.2 (33102) | 77.3 (23101) | 65.6 (1898) | 70.7 (1896) | 69.2 (2096) | <0.01 |
| 1844 years, no. (%) | 68 (9.1) | 1 (0.67) | 8 (6.5) | 20 (14.9) | 15 (9.4) | 24 (13.4) | <0.01 |
| 4554 years, no. (%) | 67 (9.0) | 6 (4.0) | 5 (4.1) | 17 (12.7) | 16 (10.0) | 23 (12.9) | |
| 5564 years, no. (%) | 79 (10.6) | 15 (10.0) | 12 (9.8) | 23 (17.2) | 13 (8.1) | 16 (8.9) | |
| 6574 years, no. (%) | 104 (13.9) | 20 (13.3) | 12 (9.8) | 16 (11.9) | 30 (18.8) | 26 (14.5) | |
| 7584 years, no. (%) | 197 (26.4) | 44 (29.3) | 36 (29.3) | 24 (17.9) | 49 (30.6) | 44 (24.6) | |
| 85 years, no. (%) | 231 (31.0) | 64 (42.7) | 50 (40.7) | 34 (25.4) | 37 (23.1) | 46 (25.7) | |
| Sex | |||||||
| Male, no. (%) | 427 (57.0) | 66 (44.0) | 60 (48.8) | 82 (60.7) | 105 (65.2) | 114 (63.7) | <0.01 |
| Female, no. (%) | 321 (43.0) | 84 (56.0) | 63 (51.2) | 53 (39.3) | 56 (34.8) | 65 (36.3) | |
| Race | |||||||
| Caucasian, no. (%) | 654 (87.4) | 129 (86.0) | 118 (95.9) | 126 (93.3) | 129 (80.1) | 152 (84.9) | <0.01 |
| Hispanic, no. (%) | 25 (3.3) | 2 (1.3) | 0 (0) | 1 (0.74) | 13 (8.1) | 9 (5.0) | |
| African American, no. (%) | 45 (6.0) | 12 (8.0) | 4 (3.3) | 5 (3.7) | 13 (8.1) | 11 (6.2) | |
| Native American, no. (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Asian, no. (%) | 13 (1.7) | 1 (0.81) | 1 (0.81) | 2 (1.5) | 5 (3.1) | 4 (2.2) | |
| Other, no. (%) | 7 (0.94) | 2 (1.3) | 0 (0) | 1 (0.74) | 1 (14.3) | 3 (1.7) | |
| Not recorded, no. (%) | 4 (0.53) | 4 (2.7) | 0 (0) | 0 (0.0) | 0 (0) | 0 (0) | |
| Initial severity of renal dysfunction | |||||||
| Mild, CrCl 5080 mL/min, no. (%) | 60 (7.4) | 4 (2.4) | 5 (3.2) | 5 (3.5) | 14 (8.5) | 32 (17.6) | <0. 01 |
| Moderate, CrCl 1649 mL/min, no. (%) | 388 (47.6) | 84 (49.4) | 71 (45.5) | 80 (55.9) | 76 (46.3) | 77 (42.3) | |
| Severe, CrCl <15 mL/min, no. (%) | 367 (45.0) | 82 (48.2) | 80 (51.3) | 58 (40.6) | 74 (45.1) | 73 (40.1) | |
| LOS, d, median (IQR) | 4.0 (26) | 4.0 (37) | 3.0 (25.5) | 4.0 (27) | 4.0 (27) | 4.0 (26) | 0.02 |
| DRG‐weighted LOS, d, median (IQR) | 5.0 (3.76.7) | 5.5 (46.7) | 5.0 (3.46.2) | 5.6 (4.36.7) | 5.0 (3.36.7) | 5.0 (4.26.7) | 0.27 |
In both phases of our study, only medications that were potentially nephrotoxic and/or renally cleared were included as potential cases; all other drugs were excluded. We defined an ADE as any drug‐related injury. These were considered preventable if they were due to an error at the time of order entry (eg, a doubling of creatinine secondary to an overdose of gentamicin or failure to order corollary drug levels for monitoring). A nonpreventable ADE was any drug‐related injury in which there was no error at the time of order entry (eg, a doubling of creatinine despite appropriate dosing of lisinopril).[17] A medication error was an error anywhere in the process of prescribing, transcribing, dispensing, administering, or monitoring a drug, but with no potential for harm or injury (eg, an order for an oral medication with no route specified when it was clear that the oral route was intended).[18] A potential ADE was an error with the potential to cause harm, but not resulting in injury, either because it was intercepted (eg, an order for ketorolac for a patient with renal failure, but caught by a pharmacist) or because of chance (eg, administering enoxaparin to a patient with severe renal dysfunction but without hemorrhage).
All study investigators underwent standardized training using a curriculum developed by the Center for Patient Safety Research and Practice (
Main Outcome Measures
The primary outcome was the rate of preventable ADEs. Secondary outcomes were the rates of potential ADEs and overall ADEs. All outcomes were related to nephrotoxicity or accumulation of a renally excreted medication.
Data collection and classification strategies were identical in both phases of our study.[14] We reviewed physician orders, medication lists, laboratory reports, admission histories, progress and consultation notes, discharge summaries, and nursing flow sheets, screening for the presence of medication incidents using an adaptation of the Institute for Healthcare Improvement's trigger tool, selected for its high sensitivity, reproducibility, and ease of use.[22, 23] In our adaptation of the tool, we excluded lidocaine, tobramycin, amikacin, and theophylline levels because of their infrequency. For each trigger found, a detailed description of the incident was extracted for detailed review. An example of a trigger is the use of sodium polystyrene, which may possibly indicate an overdose of potassium or a medication side effect.
Subsequently, each case was then independently reviewed by two investigators (A.A.L., M.A., B.C., S.R.S., M.C., N.K., E.Z., and G.S.)each assigned to at least 1 siteand blinded to prescribing physician and hospital to determine whether nephrotoxicity or injury from drug accumulation was present (see Supporting Information, Figure 1, in the online version of this article).[17] First, incidents were classified as ADEs, potential ADEs, or medication errors with no potential for injury. Second, ADEs and potential ADEs were rated according to severity. When nephrotoxic drugs were ordered, event severity was classified according to the elevation in serum creatinine24: increases of 10% were considered potential ADEs (near misses); increases of 10% to 100% were significant ADEs; and increases of 100% were serious ADEs. Changes in creatinine that were not associated with inappropriate medication orders were excluded. For renally excreted drugs with no potential for nephrotoxicity (eg, enoxaparin), we used clinical judgment to classify events as significant (eg, rash), severe (eg, 2‐unit gastrointestinal bleed), life threatening (eg, transfer to an intensive care unit), or fatal categories, as based on earlier work.[25] Disagreements were resolved by consensus. We had a score of 0.70 (95% confidence interval [CI]: 0.61‐0.80) for incident type, indicating excellent overall agreement.
Statistical Analysis
Baseline characteristics between hospitals were compared using the Fisher exact test for categorical variables and 1‐way analysis of variance for continuous variables. The occurrence of each outcome was determined according to site. To facilitate comparisons between sites, rates were expressed as number of events per 100 admissions with 95% CIs. To account for hospital effects in the analysis when comparing pre‐ and postimplementation rates of ADEs and potential ADEs, we developed a fixed‐effects Poisson regression model. To explore the independent effects of each system, a stratified analysis was performed to compare average rates of each outcome observed.
RESULTS
The outcomes of 775 patients in the baseline study were compared with the 815 patients enrolled during the postimplementation phase.[14] Among those in the postimplementation phase (Table 1), the mean age was 72.2 years, and they were predominantly male (57.0%). The demographics of the patients admitted to each of the 5 sites varied widely (P<0.01). Most patients had moderate to severe renal dysfunction.
Overall, the rates of ADEs were similar between the pre‐ and postimplementation phases (8.9/100 vs 8.3/100 admissions, respectively; P=0.74) (Table 2). However, there was a significant decrease in the rate of preventable ADEs, the primary outcome of interest, following CPOE implementation (8.0/100 vs 4.4/100 admissions; P<0.01). A reduction in preventable ADEs was observed in every hospital except site 4, where only basic order entry was introduced. However, there was a significant increase in the rates of nonpreventable ADEs (0.90/100 vs 3.9/100 admissions; P<0.01) and potential ADEs (55.5/100 vs 136.8/100 admissions; P<0.01).
| Rate/100 Admissions (95% CI) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total No. (%) | All Sites | Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | ||||||||||||||
| Event | Pre | Post | Pre | Post | P* | Pre | Post | P | Pre | Post | P | Pre | Post | P | Pre | Post | P | Pre | Post | P |
| ||||||||||||||||||||
| ADEs | 69 (13.8) | 68 (5.7) | 8.9 (7.0 1.2) | 8.3 (6.50.5) | 0.74 | 9.8 (6.015.1) | 10.0 (6.015.5) | 0.96 | 11.0 (6.517.4) | 7.7 (4.1 12.9) | 0.34 | 12.4 (7.5 19.1) | 4.2 (1.7 8.5) | 0.02 | 4.1 (1.68.3) | 13.4 (8.619.8) | 0.01 | 7.1 (3.712.2) | 6.0 (3.110.4) | 0.71 |
| Preventable | 62 | 36 | 8.0 (6.2 10.2) | 4.4 (3.16.0) | <0.01 | 8.2 (4.713.1) | 7.1 (3.811.8) | 0.70 | 10.3 (6.016.5) | 5.8 (2.8 10.4) | 0.17 | 12.4 (7.519.1) | 0 (0 0.03) | <0.01 | 3.4 (1.27.3) | 7.9 (4.413.1) | 0.11 | 5.8 (2.810.5) | 1.1 (0.183.4) | 0.03 |
| Nonpreventable | 7 | 32 | 0.90 (0.39 1.7) | 3.9 (2.75.4) | <0.01 | 1.6 (0.414.3) | 2.9 (1.16.3) | 0.42 | 0.69 (0.043.04) | 1.9 (0.48 5.0) | 0.37 | 0 (00.03) | 4.2 (1.7 8.5) | <0.01 | 0.68 (0.043.0) | 5.5 (2.6 9.9) | 0.05 | 1.3 (0.21, 4.0) | 4.9 (2.48.9) | 0.09 |
| Potential ADEs | 430 (86.2) | 1115 (93.5) | 55.5 (50.4 60.9) | 136.8 (128.9145.0) | <0.01 | 65.0 (54.077.4) | 141.1 (124.1159.8) | <0.01 | 57.2 (45.870.5) | 98.7 (83.9 115.1) | <0.01 | 44.8 (34.856.6) | 103.5 (87.7 121.1) | <0.01 | 59.2 (47.645.8) | 132.9 (116.1151.4) | <0.01 | 49.0 (38.860.9) | 195.1 (175.5216.1) | <0.01 |
| Intercepted | 16 | 24 | 2.1 (1.2 3.2) | 2.9 (1.94.3) | <0.24 | 3.3 (1.36.6) | 4.7 (2.28.8) | 0.50 | 2.1 (0.515.4) | 1.3 (0.21 4.0) | 0.60 | 1.4 (0.234.3) | 2.8 (0.87 6.5) | 0.41 | 2.0 (0.515.3) | 4.9 (2.2 9.1) | 0.20 | 1.3 (0.214.0) | 1.1 (0.183.4) | 0.87 |
| Nonintercepted | 414 | 1091 | 53.4 (48.4 58.7) | 133.9 (126.1142.0) | <0.01 | 61.7 (51.173.8) | 136.5 (119.754.8) | <0.01 | 55.2 43.968.2) | 97.4 (82.8 113.8) | <0.01 | 43.4 (33.655.1) | 100.7 (85.1 118.1) | <0.01 | 57.1 (45.8 70.2) | 128.0 (111.5146.2) | <0.01 | 47.7 (37.759.5) | 194.0 (174.4214.9) | <0.01 |
Stratified Analysis
To account for differences in technology, we performed a stratified analysis (Table 3). As was consistent with the overall study estimates, the rates of nonpreventable ADEs and potential ADEs increased with all 3 interventions. In contrast, we found that the changes in preventable ADE rates were related to the level of clinical decision support, where the greatest benefit was associated with the most sophisticated decision support system (P=0.03 and 0.02 for comparisons between advanced vs rudimentary decision support and basic order entry only, respectively). There was no difference in preventable ADE rates at sites without decision support (4.6/100 vs 4.3/100 admissions; P=0.87); with rudimentary clinical decision support, there was a trend toward a decrease in the preventable ADE rate, which did not meet statistical significance (9.1/100 vs 6.4/100 admissions; P=0.22), and, the greatest reduction was seen with advanced clinical decision support (12.4/100 vs 0/100 admissions; P<0.01).
| Rate per 100 Admissions by Level of Clinical Decision Support (95% CI) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Basic CPOE Only (Sites 4 and 5) | CPOE and Lab Display (Sites 1 and 2) | CPOE, Lab Display, and DrugDosing Check (Site 3) | |||||||
| Incident | Pre | Post | P | Pre | Post | P | Pre | Post | P |
| |||||||||
| ADEs | 5.6 (3.48.7) | 9.5 (6.613.2) | 0.08 | 10.3(7.314.3) | 8.9 (6.012.5) | 0.55 | 12.4 (7.5319.1) | 4.2 (1.78.5) | 0.02 |
| Preventable | 4.6 (2.67.5) | 4.3 (2.56.9) | 0.87 | 9.1 (6.312.8) | 6.4 (4.19.6) | 0.22 | 12.4 (7.5319.1) | 0.00 (00.03) | <0.01 |
| Nonpreventable | 0.99 (0.24 2.6) | 5.2 (3.28.0) | <0.01 | 1.2 (0.382.8) | 2.5 (1.14.6) | 0.24 | 0.00 (00.03) | 4.2 (1.78.5) | <0.01 |
| Potential ADEs | 54.0 (46.162.7) | 165.6 (152.4179.5) | <0.01 | 61.6 (53.570.5) | 120.9 (109.3133.2) | <0.01 | 44.8 (34.856.6) | 103.5 (87.7121.1) | <0.01 |
| Intercepted | 1.7 (0.593.6) | 2.9 (1.45.1) | 0.30 | 2.7 (1.34.9) | 3.1 (1.55.4) | 0.76 | 1.4 (0.234.3) | 2.8 (0.876.5) | 0.42 |
| Nonintercepted | 52.3 (44.660.9) | 162.7 (149.6176.5) | <0.01 | 58.8 (50.967.5) | 117.8 (106.4130.0) | <0.01 | 43.4 (33.655.1) | 100.7 (85.1118.1) | <0.01 |
Severity of Events
We further analyzed our data based on event severity (Table 4). Among preventable ADEs, only 1 fatal event was observed, which occurred after CPOE implementation. Here, a previously opioid‐nave patient received intravenous morphine for malignant pain. Within the first 24 hours, the patient received 70.2 mg of intravenous morphine, resulting in a decreased level of consciousness. The patient expired the following day. Furthermore, following implementation, among preventable ADEs, a reduction in significant events was seen (P=0.02) along with a nonsignificant reduction in the rate of serious events (P=0.06). However, the rate of preventable life‐threatening events was not different (P=0.96). The nonpreventable ADE rate rose during the postimplementation period for both serious (P=0.03) and significant events (P<0.01). The risk of fatal and life‐threatening nonpreventable ADEs did not change. The potential ADE rate increased following implementation for all severities (P<0. 01).
| Preimplementation | Postimplementation | ||||
|---|---|---|---|---|---|
| Incident | No. (%) | Average Rate/100 Admissions (95% CI)* | No. (%) | Average Rate/100 Admissions (95% CI)* | P |
| |||||
| All ADEs | |||||
| Fatal | 0 (0) | 0.00 (00.0047) | 1 (1.4) | 0.12 (0.0070.54) | 0.52 |
| Lifethreatening | 3 (4.3) | 0.39 (0.101.0) | 3 (4.4) | 0.37 (0.09 0.95) | 0.95 |
| Serious | 34 (49.3) | 4.4 (3.16.0) | 32 (47.1) | 3.9 (2.75.4) | 0.65 |
| Significant | 32 (46.4) | 4.1 (2.95.7) | 32 (47.1) | 3.9 (2.75.4) | 0.84 |
| Total | 69 (100) | 8.9 (7.011.2) | 68 (100) | 8.3 (6.510.5) | 0.74 |
| Preventable ADEs | |||||
| Fatal | 0 (0) | 0.00 (00.0047) | 1 (2.7) | 0.00 (00.0045) | 0.52 |
| Lifethreatening | 2 (3.2) | 0.26 (0.040.80) | 2 (5.6) | 0.25 (0.040.76) | 0.96 |
| Serious | 31 (50.0) | 4.0 (2.85.6) | 19 (52.8) | 2.3 (1.43.5) | 0.06 |
| Significant | 29 (46.8) | 3.7 (2.55.3) | 14 (38.9) | 1.7 (0.972.8) | 0.02 |
| Total | 62 (100) | 8.0 (6.210.2) | 36 (100) | 4.4 (3.16.0) | <0.01 |
| Nonpreventable ADEs | |||||
| Fatal | 0 (0) | 0.00 (00.0047) | 0 (0) | 0.00 (00.0045) | NS |
| Lifethreatening | 1 (14.2) | 0.13 (0.0070.57) | 1 (3.1) | 0.12 (0.0070.54) | 0.97 |
| Serious | 3 (42.9) | 0.39 (0.101.0) | 13 (40.6) | 1.6 (0.882.6) | 0.03 |
| Significant | 3 (42.9) | 0.39 (0.101.0) | 18 (56.3) | 2.2 (1.33.4) | <0.01 |
| Total | 7 (100) | 0.90 (0.391.7) | 32 (100) | 3.9 (2.75.4) | <0.01 |
| All potential ADEs | |||||
| Lifethreatening | 5 (1.2) | 0.65 (0.231.4) | 33 (3.0) | 4.0 (2.85.6) | <0.01 |
| Serious | 233 (54.2) | 30.1 (26.434.1) | 429 (38.4) | 52.6 (47.857.8) | <0.01 |
| Significant | 192 (44.6) | 24.8 (21.428.4) | 653 (58.6) | 80.1 (74.186.4) | <0.01 |
| Total | 430 (100) | 55.5 (50.460.9) | 1115 (100) | 136.8 (128.9145.0) | <0.01 |
| Intercepted potential ADEs | |||||
| Lifethreatening | 0 (0) | 0.00 (00.0047) | 1 (4.2) | 0.12 (0.0070.54) | 0.52 |
| Serious | 5 (31.2) | 0.65 (0.231.4) | 13 (54.2) | 1.6 (0.882.6) | 0.09 |
| Significant | 11 (68.8) | 1.4 (0.74 2.4) | 10 (41.6) | 1.2 (0.622.2) | 0.74 |
| Total | 16 (100) | 2.1 (1.23.2) | 24 (100) | 2.9 (1.94.3) | 0.24 |
| Nonintercepted potential ADEs | |||||
| Lifethreatening | 5 (1.2) | 0.65 (0.231.4) | 32 (2.9) | 3.9 (2.75.4) | <0.01 |
| Serious | 228 (55.1) | 29.4 (25.833.4) | 416 (38.1) | 51.0 (46.356.1) | <0.01 |
| Significant | 181 (43.7) | 23.4 (20.126.9) | 643 (58.9) | 78.9 (73.085.2) | <0.01 |
| Total | 414 (100) | 53.4 (48.458.7) | 1091 (100) | 133.9(126.1142.0) | <0.01 |
Case Reviews
In total, there were 36 preventable ADEs identified during the postimplementation phase (Table 5). Of these, inappropriate renal dosing accounted for 26 preventable ADEs, which involved antibiotics (eg, gentamicin‐induced renal failure), opioids (eg, over sedation from morphine), ‐blockers (eg, hypotension from atenolol), angiotensin‐converting enzyme inhibitors (eg, renal failure with hyperkalemia secondary to lisinopril), and digoxin (eg, bradyarrhythmia and toxicity). The use of contraindicated medications resulted in 7 preventable ADEs (eg, prescribing glyburide in the setting of severe renal impairment).[26] The remaining 3 preventable ADEs stemmed from unmonitored use of vancomycin.
| ADEs, Preventable, No. (Rate per 100 Admissions)* | ADEs, Nonpreventable, No. (Rate per 100 Admissions)* | ||||||
|---|---|---|---|---|---|---|---|
| Drug Class | Preimplementation | Postimplementation | P (for Entire Drug Class) | Preimplementation | Postimplementation | P (for Drug Class) | Drugs Involved |
| |||||||
| Cardiovascular | 20 (2.6) | 18 (2.2) | 0.63 | 4 (0.52) | 16 (2.0) | 0.02 | Atenolol, bumetanide, captopril, digoxin, furosemide, hydralazine, hydrochlorothiazide, lisinopril, sotalol, spironolactone |
| Diuretics | 1 (0.13) | 2 (0.25) | 1 (0.13) | 9 (1.1) | |||
| ‐blockers | 0 (0.00) | 2 (0.25) | 1 (0.13) | ||||
| ACE inhibitors and ARBs | 16 (2.1) | 10 (1.2) | 2 (0.26) | 7 (0.86) | |||
| Antiarrhythmic | 3 (0.39) | 3 (0.37) | |||||
| Vasodilator | 0 (0.00) | 1 (0.12) | |||||
| Analgesics | 28 (3.6) | 4 (0.49) | 0.0002 | 1 (0.13) | 5 (0.61) | 0.15 | Acetaminophen and combination pills containing acetaminophen: Percocet (oxycodone and acetaminophen), Tylenol #3 (codeine and acetaminophen), Vicodin (hydrocodone and acetaminophen), fentanyl, hydrocodone, meperidine, morphine, oxycodone |
| Narcotic | 13 (1.7) | 4 (0.49) | 0 (0.00) | 5 (0.61) | |||
| Non‐narcotic | 15 (1.9) | 0 (0.00) | 1 (0.13) | 0 | |||
| Antibiotics | 8 (1.0) | 13 (1.6) | 0.33 | 1 (0.13) | 9 (1.1) | 0.04 | Amikacin, ampicillin and sulbactam, ciprofloxacin, cefazolin, cefuroxime, gatifloxacin, gentamicin, levofloxacin, metronidazole, piperacillin and tazobactam, tobramycin, vancomycin |
| Neurotropic drugs | 2 (0.26) | 0 (0.00) | 0.28 | 0 | 0 | Lithium, midazolam | |
| Sedatives | 1 (0.13) | 0 (0.00) | |||||
| Antipsychotics | 1 (0.13) | 0 (0.00) | |||||
| Diabetes | 0 | 1 (0.12) | 0.52 | 0 | 1 (0.12) | 0.52 | Glipizide, glyburide |
| Oral antidiabetics | 0 | 1 (0.12) | 1 (0.12) | ||||
| Other drugs | 4 (0.52) | 0 (0.00) | 0.13 | 1 (0.13) | 1 (0.12) | 0.97 | Allopurinol, famotidine |
| Gastrointestinal drugs | 1 (0.13) | 0 (0.00) | |||||
| Other | 3 (0.39) | 0 (0.00) | 0 | 1 (0.12) | |||
DISCUSSION
We evaluated the use of vendor CPOE for hospitalized patients with renal disease and found that it was associated with a 45% reduction in preventable ADEs related to nephrotoxicity and accumulation of renally excreted medications. The impact of CPOE appeared to be related to the level of associated clinical decision support, where only the most advanced system was associated with benefit. We observed a significant increase in potential ADEs with all levels of intervention. Overall, these findings suggest that vendor‐developed applications with appropriate decision support can reduce the occurrence of renally related preventable ADEs, but careful implementation is needed if the potential ADE rate is to fall.
Many of the benefits of CPOE come from clinical decision support.[11] When applied to patients with renal impairment, CPOE with clinical decision support has been associated with decreased lengths of stay,[16, 27] reduced use of contraindicated medications,[28, 29, 30] improved dosing and drug monitoring,[16, 31, 32] and improved general prescribing practices.[29, 33] Even so, the observed benefit of CPOE on ADE rates has been variable, with some studies reporting reductions,[27, 34] whereas others are unable to detect differences.[16, 31] These studies, however, limited their case definition of ADEs to strictly declining renal function,[16, 31, 34] or adverse events directly resulting from anti‐infective drugs.[27] In contrast, our study accounted for nephrotoxicity and systemic toxicity from drug accumulation. Using this broader definition, we were able to detect large reductions in the rates of preventable ADEs following CPOE adoption.
Successful decision support is simple, intuitive, and provides speedy information that integrates seamlessly into the clinical workflow.[35, 36] However, information delivery, although necessary, is insufficient for improving safety. For instance, passive alerts are often ignored, deferred, or overridden.[30, 37, 38] Demonstrating this, Quartarolo et al. found that informing physicians of the presence of renal impairment using automated reporting of glomerular filtration rates did not change prescribing behavior.[39] In contrast, providing active feedback (with dosing recommendations) was observed to be more useful in effecting change.[40] Chertow et al. further showed that providing an adjusted dose list with a default dose and frequency at the time of order entry for patients with renal insufficiency improved appropriate ordering and was associated with a decreased length of stay.[16] Altogether, these studies help to explain why only CPOE with clinical decision support equipped to provide renally adjusted dosing and monitoring was associated with a reduction in preventable ADEs in our study.
However, in contrast to reports of internally developed systems,[20, 25] potential ADE rates actually rose during the follow‐up portion of our study. These appeared to be chiefly related to customized order sets with the potential of overdosing drugs through therapeutic duplication, a problem that is commonly known to be associated with CPOE (ie, new orders that overlap with other new or active medication orders, which may be the same drug itself or from within the same drug class, with the risk of overdose).[41, 42] Of note, our findings give rise to several key implications. First, hospitals implementing vendor‐developed CPOE systems may be at greater risk of incurring potential ADEs compared to those using home‐grown systems, which have comparatively gone through more cycles of internal refinement. As such, it is necessary to monitor for issues postimplementation and respond with appropriate changes to achieve successful system performance.[35, 36] Second, although the rate of potential ADEs (near misses) increased, preventable ADEs decreased because some of these errors were intercepted, whereas others were averted simply because of chance. Of note, not all potential ADEs have the same potential for injury; more serious cases are more likely to result in actual ADEs (eg, failure to renally dose acetaminophen likely poses less potential for harm than prescribing a full dose of enoxaparin in the setting of severe renal failure). Third, we found that most potential ADEs could have been averted with a combination of basic (dosing guidance and drug‐drug interactions checks) and advanced decision support (medication‐associated laboratory testing and drug‐disease interactions).[43] Therefore, further refinements to existing software are needed to maximize safety outcomes.
Our study has some limitations. This study was not a randomized controlled trial, and thus is subject to potential confounding. Although 6 hospitals were involved at the study inception,[14] one of these hospitals eventually opted not to implement CPOE, and further declined to participate as a control site. Therefore, we cannot exclude confounding from secular trends because we had no contemporaneous control group. However, the introduction of CPOE was the main medication safety‐oriented intervention during the study interval, thus arguing against major confounding by cointervention. Second, even though it is possible that classification bias may have been introduced between the preimplementation and postimplementation portions of our study, especially given the passage of time, it is unlikely. Study personnel underwent training using a curriculum designed to maintain continuity across projects, minimize individual variability, and optimize reproducibility in data collection and classification, as in a number of previous studies.[14, 17, 19, 20, 21] Third, our study is limited by a heterogeneous intervention, as varying levels of decision support were introduced. However, this reflects usual practice and may be construed as a strength as we were able to describe the impact of different types of decision support. Fourth, we enrolled patients with a large spectrum of renal impairment, and our findings are not specific to any particular subgroup. However, our wide recruitment strategy also enhances the generalizability. Finally, our study was restricted to patients who were exposed to potentially nephrotoxic and/or renally cleared drugs. As such, we could not determine whether advanced decision support helped to eliminate the use of some potentially dangerous medications altogether, as these cases would have been excluded from our study. It is possible, therefore, that our study findings underestimate the true benefit of clinical decision support.
In conclusion, vendor CPOE implementation in 5 community hospitals was associated with a 45% reduction in preventable ADE rates among patients with renal impairment. Measurable benefit was associated with advanced decision support capable of lab display, dosing guidance, and medication‐associated laboratory testing. Although the potential benefits of CPOE systems are far reaching, achieving the desired safety benefits will require appropriate decision support, tracking of problems that arise, and systematic approaches to eliminating them.
Acknowledgments
The authors thank Kathy Zigmont, RN, and Cathy Foskett, RN (Brigham and Women's Hospital, Division of General Internal Medicine and Primary Care) for the chart review and data collection at the participating study sites.
Disclosures: The Rx Foundation and Commonwealth Fund supported the study. They commented on its design, but were not involved in data collection, data management, analysis, interpretation, or writing of the manuscript. Dr. Leung is supported by a Clinical Fellowship Award from Alberta Innovates Health Solutions and by a Fellowship Award from the Canadian Institutes for Health Research. Dr. Schiff received financial support from the FDA CPOE Task Order and the Commonwealth Fund. Ms. Keohane served as a consultant to the American College of Obstetrician and Gynecologists and as a reviewer for the VRQC Program. She received honoraria for a presentation on Patient Safety in 2010, sponsored by Abbott Nutrition International, and a lecture on Nurse Interruptions in Medication Administration by Educational Review Systems. Dr. Coffey received an honorarium from Meditech for speaking on social networking at Physician/CIO Forum in 2009. Dr. Kaufman participates in an advisory group with Siemens Medical Solutions. Dr. Zimlichman received support from the Rx Foundation and the Commonwealth Fund. Dr. Bates holds a minority equity position in the privately held company Medicalis, which develops Web‐based decision support for radiology test ordering, and has served as a consultant to Medicalis. He serves as an advisor to Calgary Scientific, which makes technologies that enable mobility within electronic health records. He is on the clinical advisory board for Patient Safety Systems, which provides a set of approaches to help hospitals improve safety. He has received funding support from the Massachusetts Technology Consortium. Ms. Amato, Dr. Simon, Dr. Cadet, Ms. Seger, and Ms. Yoon have no disclosures relevant to this study.
- , , . Drug Prescribing in Renal Failure: Dosing Guidelines for Adults and Children: American College of Physicians; 2007.
- , . Management of drug toxicity in patients with renal insufficiency. Nat Rev Nephrol. 2010;6(6):317–318.
- , , , . Use of renal risk drugs in hospitalized patients with impaired renal function—an underestimated problem? Nephrol Dial Transplant. 2006;21(11):3164–3171.
- , , , et al. Medication misuse in hospitalized patients with renal impairment. Int J Qual Health Care. 2003;15(4):331–335.
- , , , . Impact of a renal drug dosing service on dose adjustment in hospitalized patients with chronic kidney disease. Ann Pharmacother. 2009;43(10):1598–1605.
- , . Drug dosing in chronic kidney disease. Med Clin North Am. 2005;89(3):649–687.
- , , , et al. Systems analysis of adverse drug events. ADE Prevention Study Group. JAMA. 1995;274(1):35–43.
- , , , , , . The epidemiology of prescribing errors: the potential impact of computerized prescriber order entry. Arch Intern Med. 2004;164(7):785–792.
- , , . Factors related to errors in medication prescribing. JAMA. 1997;277(4):312–317.
- , , , et al. Role of computerized physician order entry systems in facilitating medication errors. JAMA. 2005;293(10):1197–1203.
- , , , , . Mixed results in the safety performance of computerized physician order entry. Health Aff (Millwood). 2010;29(4):655–663.
- , , , et al. The effect of computerized physician order entry with clinical decision support on the rates of adverse drug events: a systematic review. J Gen Intern Med. 2008;23(4):451–458.
- , , . The impact of computerized physician medication order entry in hospitalized patients—a systematic review. Int J Med Inform. 2008;77(6):365–376.
- , , , et al. Occurrence of adverse, often preventable, events in community hospitals involving nephrotoxic drugs or those excreted by the kidney. Kidney Int. 2009;76(11):1192–1198.
- , . Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41.
- , , , et al. Guided medication dosing for inpatients with renal insufficiency. JAMA. 2001;286(22):2839–2844.
- , , , , . Adverse drug events and medication errors: detection and classification methods. Qual Saf Health Care. 2004;13(4):306–314.
- , , , , . Relationship between medication errors and adverse drug events. J Gen Intern Med. 1995;10(4):199–205.
- , , , et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA. 1995;274(1):29–34.
- , , , et al. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. JAMA. 1998;280(15):1311–1316.
- , , , et al. Adverse drug event rates in six community hospitals and the potential impact of computerized physician order entry for prevention. J Gen Intern Med. 2010;25(1):31–38.
- , , . Adverse drug event trigger tool: a practical methodology for measuring medication related harm. Qual Saf Health Care. 2003;12(3):194–200.
- Institute for Healthcare Improvement: IHI Trigger Tool for Measuring Adverse Drug Events. 2011. Available at: http://www.ihi.org/knowledge/Pages/Tools/TriggerToolforMeasuringAdverseDrugEvents.aspx. Accessed February 1, 2013.
- , , , . Renal safety of two analgesics used over the counter: ibuprofen and aspirin. Clin Pharmacol Ther. 1986;40(4):373–377.
- , , , et al. The impact of computerized physician order entry on medication error prevention. J Am Med Inform Assoc. 1999;6(4):313–321.
- , , . Prolonged sulfonylurea‐induced hypoglycemia in diabetic patients with end‐stage renal disease. Am J Kidney Dis. 2000;35(3):500–505.
- , , , et al. A computer‐assisted management program for antibiotics and other antiinfective agents. N Engl J Med. 1998;338(4):232–238.
- , , , et al. Alert system for inappropriate prescriptions relating to patients' clinical condition. Methods Inf Med. 2009;48(6):566–573.
- , , , , , . Computerized clinical decision support during medication ordering for long‐term care residents with renal insufficiency. J Am Med Inform Assoc. 2009;16(4):480–485.
- , , . A trial of automated decision support alerts for contraindicated medications using computerized physician order entry. J Am Med Inform Assoc. 2005;12(3):269–274.
- , , , , . Effects of clinical decision support on initial dosing and monitoring of tobramycin and amikacin. Am J Health Syst Pharm. 2011;68(7):624–632.
- , , , , , . Computerized decision support for medication dosing in renal insufficiency: a randomized, controlled trial. Ann Emerg Med. 2010;56(6):623–629.
- , , , . Implementation of rules based computerised bedside prescribing and administration: intervention study. BMJ. 2000;320(7237):750–753.
- , , , et al. Effect of computer‐based alerts on the treatment and outcomes of hospitalized patients. Arch Intern Med. 1994;154(13):1511–1517.
- , , , et al. Ten commandments for effective clinical decision support: making the practice of evidence‐based medicine a reality. J Am Med Inform Assoc. 2003;10(6):523–530.
- , , . Computerized decision support systems: improving patient safety in nephrology. Nat Rev Nephrol. 2011;7(6):348–355.
- , , , et al. Impact of a computerized alert during physician order entry on medication dosing in patients with renal impairment. Proc AMIA Symp. 2002:577–581.
- , , , et al. A computerized provider order entry intervention for medication safety during acute kidney injury: a quality improvement report. Am J Kidney Dis. 2010;56(5):832–841.
- , , . Reporting of estimated glomerular filtration rate: effect on physician recognition of chronic kidney disease and prescribing practices for elderly hospitalized patients. J Hosp Med. 2007;2(2):74–78.
- , , , , . Drug dosage in patients with renal failure optimized by immediate concurrent feedback. J Gen Intern Med. 2001;16(6):369–375.
- , , , et al. Factors contributing to an increase in duplicate medication order errors after CPOE implementation. J Am Med Inform Assoc. 2011;18(6):774–782.
- , , , et al. Impact of Vendor Computerized Physician Order Entry in Community Hospitals. J Gen Intern Med. 2012;27(7):801–807.
- , , , et al. Medication‐related clinical decision support in computerized provider order entry systems: a review. J Am Med Inform Assoc. 2007;14(1):29–40.
Hospitalized patients with renal impairment are vulnerable to adverse drug events (ADEs).[1, 2] Appropriate prescribing for patients with renal insufficiency is challenging because of the complexities of drug therapy within the wide spectrum of kidney disease.[3, 4, 5, 6] Accordingly, computerized physician order entry (CPOE) systems with clinical decision support may help prevent many ADEs by providing timely laboratory information, recommending renally adjusted doses, and by offering assistance with prescribing.[7, 8, 9]
Despite the proposed benefits of CPOE, outcomes vary greatly because of differences in technology.[10, 11, 12, 13] In particular, the type of decision support available to assist medication ordering in the setting of renal disease varies widely among current vendor systems. Given the uncertain benefits of CPOE, especially with the wide range of associated clinical decision support, we conducted this study to determine the impact of these systems on the rates of ADEs among hospitalized patients with kidney disease.
METHODS
This study was approved by the institutional review boards at each study site.
Design and Setting
We conducted a before‐and‐after study to evaluate the impact of newly implemented vendor CPOE systems in 5 community hospitals in Massachusetts. Although we reported on 6 hospitals in our baseline study,[14] 1 of these hospitals later chose not to implement CPOE, and therefore was not included in follow‐up. At the time of this study, 1 of the hospitals (site 3) had not yet achieved hospital‐wide implementation. Although CPOE had been adopted by most medical services at site 3, it had not yet been implemented in the emergency, obstetrical, or surgical departments. Thus, we limited our study to the medical services at this site. For the remaining sites, all admitting services were included with the exception of the psychiatric and neonatal services, which were excluded from both phases because they would have required different detection tools.
Participants
Patients aged 18 years with renal failure, exposed to potentially nephrotoxic and/or renally cleared medications, and admitted to any of the participating hospitals during the study period were eligible for inclusion. Of the patients meeting eligibility criteria, we randomly selected approximately 150 records per hospital in the preimplementation and postimplementation phases for a total sample of 1590 charts. The first phase of this study occurred from January 2005 to August 2006; the second phase began 6 months postimplementation and lasted from October 2008 to September 2010.
Principal Exposure
Each hospital independently selected a vendor CPOE system with variable clinical decision support capabilities: (1) sites 4 and 5 had basic CPOE only with no clinical decision support for renal disease; (2) sites 1 and 2 implemented rudimentary clinical decision support with laboratory display (eg, serum creatinine) whenever common renally related drugs were ordered; and (3) site 3 had the most advanced support in place where, in addition to basic order entry and lab checks, physicians were provided with suggested doses for renally cleared and/or nephrotoxic medications, as well as appropriate drug monitoring for medications with narrow therapeutic indices (eg, suggested dosages and frequencies for vancomycin and automated corollary laboratory monitoring).
Definitions
We screened for the presence of renal failure by a serum creatinine 1.5 mg/dL at the time of admission. However, the duration of renal impairment was not known. We defined 3 levels of renal insufficiency based on the calculated creatinine clearance (CrCl)15: mild (CrCl 5080 mL/min), moderate (1649 mL/min), and severe (15 mL/min). Subjects with a CrCl >80 mL/min were considered to have normal renal function and were excluded. Potentially nephrotoxic and/or renally cleared medications were then identified using an established knowledge base (see Supporting Information, Table 1, in the online version of this article).[16]
| Hospital Site | |||||||
|---|---|---|---|---|---|---|---|
| Baseline Characteristics | All Sites | 1 | 2 | 3 | 4 | 5 | P (Among All Sites)* |
| |||||||
| No. of patients | 815 | 170 | 156 | 143 | 164 | 182 | |
| Age, y, mean (range) | 72.2 (18.0102.0) | 79.2 (33102) | 77.3 (23101) | 65.6 (1898) | 70.7 (1896) | 69.2 (2096) | <0.01 |
| 1844 years, no. (%) | 68 (9.1) | 1 (0.67) | 8 (6.5) | 20 (14.9) | 15 (9.4) | 24 (13.4) | <0.01 |
| 4554 years, no. (%) | 67 (9.0) | 6 (4.0) | 5 (4.1) | 17 (12.7) | 16 (10.0) | 23 (12.9) | |
| 5564 years, no. (%) | 79 (10.6) | 15 (10.0) | 12 (9.8) | 23 (17.2) | 13 (8.1) | 16 (8.9) | |
| 6574 years, no. (%) | 104 (13.9) | 20 (13.3) | 12 (9.8) | 16 (11.9) | 30 (18.8) | 26 (14.5) | |
| 7584 years, no. (%) | 197 (26.4) | 44 (29.3) | 36 (29.3) | 24 (17.9) | 49 (30.6) | 44 (24.6) | |
| 85 years, no. (%) | 231 (31.0) | 64 (42.7) | 50 (40.7) | 34 (25.4) | 37 (23.1) | 46 (25.7) | |
| Sex | |||||||
| Male, no. (%) | 427 (57.0) | 66 (44.0) | 60 (48.8) | 82 (60.7) | 105 (65.2) | 114 (63.7) | <0.01 |
| Female, no. (%) | 321 (43.0) | 84 (56.0) | 63 (51.2) | 53 (39.3) | 56 (34.8) | 65 (36.3) | |
| Race | |||||||
| Caucasian, no. (%) | 654 (87.4) | 129 (86.0) | 118 (95.9) | 126 (93.3) | 129 (80.1) | 152 (84.9) | <0.01 |
| Hispanic, no. (%) | 25 (3.3) | 2 (1.3) | 0 (0) | 1 (0.74) | 13 (8.1) | 9 (5.0) | |
| African American, no. (%) | 45 (6.0) | 12 (8.0) | 4 (3.3) | 5 (3.7) | 13 (8.1) | 11 (6.2) | |
| Native American, no. (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Asian, no. (%) | 13 (1.7) | 1 (0.81) | 1 (0.81) | 2 (1.5) | 5 (3.1) | 4 (2.2) | |
| Other, no. (%) | 7 (0.94) | 2 (1.3) | 0 (0) | 1 (0.74) | 1 (14.3) | 3 (1.7) | |
| Not recorded, no. (%) | 4 (0.53) | 4 (2.7) | 0 (0) | 0 (0.0) | 0 (0) | 0 (0) | |
| Initial severity of renal dysfunction | |||||||
| Mild, CrCl 5080 mL/min, no. (%) | 60 (7.4) | 4 (2.4) | 5 (3.2) | 5 (3.5) | 14 (8.5) | 32 (17.6) | <0. 01 |
| Moderate, CrCl 1649 mL/min, no. (%) | 388 (47.6) | 84 (49.4) | 71 (45.5) | 80 (55.9) | 76 (46.3) | 77 (42.3) | |
| Severe, CrCl <15 mL/min, no. (%) | 367 (45.0) | 82 (48.2) | 80 (51.3) | 58 (40.6) | 74 (45.1) | 73 (40.1) | |
| LOS, d, median (IQR) | 4.0 (26) | 4.0 (37) | 3.0 (25.5) | 4.0 (27) | 4.0 (27) | 4.0 (26) | 0.02 |
| DRG‐weighted LOS, d, median (IQR) | 5.0 (3.76.7) | 5.5 (46.7) | 5.0 (3.46.2) | 5.6 (4.36.7) | 5.0 (3.36.7) | 5.0 (4.26.7) | 0.27 |
In both phases of our study, only medications that were potentially nephrotoxic and/or renally cleared were included as potential cases; all other drugs were excluded. We defined an ADE as any drug‐related injury. These were considered preventable if they were due to an error at the time of order entry (eg, a doubling of creatinine secondary to an overdose of gentamicin or failure to order corollary drug levels for monitoring). A nonpreventable ADE was any drug‐related injury in which there was no error at the time of order entry (eg, a doubling of creatinine despite appropriate dosing of lisinopril).[17] A medication error was an error anywhere in the process of prescribing, transcribing, dispensing, administering, or monitoring a drug, but with no potential for harm or injury (eg, an order for an oral medication with no route specified when it was clear that the oral route was intended).[18] A potential ADE was an error with the potential to cause harm, but not resulting in injury, either because it was intercepted (eg, an order for ketorolac for a patient with renal failure, but caught by a pharmacist) or because of chance (eg, administering enoxaparin to a patient with severe renal dysfunction but without hemorrhage).
All study investigators underwent standardized training using a curriculum developed by the Center for Patient Safety Research and Practice (
Main Outcome Measures
The primary outcome was the rate of preventable ADEs. Secondary outcomes were the rates of potential ADEs and overall ADEs. All outcomes were related to nephrotoxicity or accumulation of a renally excreted medication.
Data collection and classification strategies were identical in both phases of our study.[14] We reviewed physician orders, medication lists, laboratory reports, admission histories, progress and consultation notes, discharge summaries, and nursing flow sheets, screening for the presence of medication incidents using an adaptation of the Institute for Healthcare Improvement's trigger tool, selected for its high sensitivity, reproducibility, and ease of use.[22, 23] In our adaptation of the tool, we excluded lidocaine, tobramycin, amikacin, and theophylline levels because of their infrequency. For each trigger found, a detailed description of the incident was extracted for detailed review. An example of a trigger is the use of sodium polystyrene, which may possibly indicate an overdose of potassium or a medication side effect.
Subsequently, each case was then independently reviewed by two investigators (A.A.L., M.A., B.C., S.R.S., M.C., N.K., E.Z., and G.S.)each assigned to at least 1 siteand blinded to prescribing physician and hospital to determine whether nephrotoxicity or injury from drug accumulation was present (see Supporting Information, Figure 1, in the online version of this article).[17] First, incidents were classified as ADEs, potential ADEs, or medication errors with no potential for injury. Second, ADEs and potential ADEs were rated according to severity. When nephrotoxic drugs were ordered, event severity was classified according to the elevation in serum creatinine24: increases of 10% were considered potential ADEs (near misses); increases of 10% to 100% were significant ADEs; and increases of 100% were serious ADEs. Changes in creatinine that were not associated with inappropriate medication orders were excluded. For renally excreted drugs with no potential for nephrotoxicity (eg, enoxaparin), we used clinical judgment to classify events as significant (eg, rash), severe (eg, 2‐unit gastrointestinal bleed), life threatening (eg, transfer to an intensive care unit), or fatal categories, as based on earlier work.[25] Disagreements were resolved by consensus. We had a score of 0.70 (95% confidence interval [CI]: 0.61‐0.80) for incident type, indicating excellent overall agreement.
Statistical Analysis
Baseline characteristics between hospitals were compared using the Fisher exact test for categorical variables and 1‐way analysis of variance for continuous variables. The occurrence of each outcome was determined according to site. To facilitate comparisons between sites, rates were expressed as number of events per 100 admissions with 95% CIs. To account for hospital effects in the analysis when comparing pre‐ and postimplementation rates of ADEs and potential ADEs, we developed a fixed‐effects Poisson regression model. To explore the independent effects of each system, a stratified analysis was performed to compare average rates of each outcome observed.
RESULTS
The outcomes of 775 patients in the baseline study were compared with the 815 patients enrolled during the postimplementation phase.[14] Among those in the postimplementation phase (Table 1), the mean age was 72.2 years, and they were predominantly male (57.0%). The demographics of the patients admitted to each of the 5 sites varied widely (P<0.01). Most patients had moderate to severe renal dysfunction.
Overall, the rates of ADEs were similar between the pre‐ and postimplementation phases (8.9/100 vs 8.3/100 admissions, respectively; P=0.74) (Table 2). However, there was a significant decrease in the rate of preventable ADEs, the primary outcome of interest, following CPOE implementation (8.0/100 vs 4.4/100 admissions; P<0.01). A reduction in preventable ADEs was observed in every hospital except site 4, where only basic order entry was introduced. However, there was a significant increase in the rates of nonpreventable ADEs (0.90/100 vs 3.9/100 admissions; P<0.01) and potential ADEs (55.5/100 vs 136.8/100 admissions; P<0.01).
| Rate/100 Admissions (95% CI) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total No. (%) | All Sites | Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | ||||||||||||||
| Event | Pre | Post | Pre | Post | P* | Pre | Post | P | Pre | Post | P | Pre | Post | P | Pre | Post | P | Pre | Post | P |
| ||||||||||||||||||||
| ADEs | 69 (13.8) | 68 (5.7) | 8.9 (7.0 1.2) | 8.3 (6.50.5) | 0.74 | 9.8 (6.015.1) | 10.0 (6.015.5) | 0.96 | 11.0 (6.517.4) | 7.7 (4.1 12.9) | 0.34 | 12.4 (7.5 19.1) | 4.2 (1.7 8.5) | 0.02 | 4.1 (1.68.3) | 13.4 (8.619.8) | 0.01 | 7.1 (3.712.2) | 6.0 (3.110.4) | 0.71 |
| Preventable | 62 | 36 | 8.0 (6.2 10.2) | 4.4 (3.16.0) | <0.01 | 8.2 (4.713.1) | 7.1 (3.811.8) | 0.70 | 10.3 (6.016.5) | 5.8 (2.8 10.4) | 0.17 | 12.4 (7.519.1) | 0 (0 0.03) | <0.01 | 3.4 (1.27.3) | 7.9 (4.413.1) | 0.11 | 5.8 (2.810.5) | 1.1 (0.183.4) | 0.03 |
| Nonpreventable | 7 | 32 | 0.90 (0.39 1.7) | 3.9 (2.75.4) | <0.01 | 1.6 (0.414.3) | 2.9 (1.16.3) | 0.42 | 0.69 (0.043.04) | 1.9 (0.48 5.0) | 0.37 | 0 (00.03) | 4.2 (1.7 8.5) | <0.01 | 0.68 (0.043.0) | 5.5 (2.6 9.9) | 0.05 | 1.3 (0.21, 4.0) | 4.9 (2.48.9) | 0.09 |
| Potential ADEs | 430 (86.2) | 1115 (93.5) | 55.5 (50.4 60.9) | 136.8 (128.9145.0) | <0.01 | 65.0 (54.077.4) | 141.1 (124.1159.8) | <0.01 | 57.2 (45.870.5) | 98.7 (83.9 115.1) | <0.01 | 44.8 (34.856.6) | 103.5 (87.7 121.1) | <0.01 | 59.2 (47.645.8) | 132.9 (116.1151.4) | <0.01 | 49.0 (38.860.9) | 195.1 (175.5216.1) | <0.01 |
| Intercepted | 16 | 24 | 2.1 (1.2 3.2) | 2.9 (1.94.3) | <0.24 | 3.3 (1.36.6) | 4.7 (2.28.8) | 0.50 | 2.1 (0.515.4) | 1.3 (0.21 4.0) | 0.60 | 1.4 (0.234.3) | 2.8 (0.87 6.5) | 0.41 | 2.0 (0.515.3) | 4.9 (2.2 9.1) | 0.20 | 1.3 (0.214.0) | 1.1 (0.183.4) | 0.87 |
| Nonintercepted | 414 | 1091 | 53.4 (48.4 58.7) | 133.9 (126.1142.0) | <0.01 | 61.7 (51.173.8) | 136.5 (119.754.8) | <0.01 | 55.2 43.968.2) | 97.4 (82.8 113.8) | <0.01 | 43.4 (33.655.1) | 100.7 (85.1 118.1) | <0.01 | 57.1 (45.8 70.2) | 128.0 (111.5146.2) | <0.01 | 47.7 (37.759.5) | 194.0 (174.4214.9) | <0.01 |
Stratified Analysis
To account for differences in technology, we performed a stratified analysis (Table 3). As was consistent with the overall study estimates, the rates of nonpreventable ADEs and potential ADEs increased with all 3 interventions. In contrast, we found that the changes in preventable ADE rates were related to the level of clinical decision support, where the greatest benefit was associated with the most sophisticated decision support system (P=0.03 and 0.02 for comparisons between advanced vs rudimentary decision support and basic order entry only, respectively). There was no difference in preventable ADE rates at sites without decision support (4.6/100 vs 4.3/100 admissions; P=0.87); with rudimentary clinical decision support, there was a trend toward a decrease in the preventable ADE rate, which did not meet statistical significance (9.1/100 vs 6.4/100 admissions; P=0.22), and, the greatest reduction was seen with advanced clinical decision support (12.4/100 vs 0/100 admissions; P<0.01).
| Rate per 100 Admissions by Level of Clinical Decision Support (95% CI) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Basic CPOE Only (Sites 4 and 5) | CPOE and Lab Display (Sites 1 and 2) | CPOE, Lab Display, and DrugDosing Check (Site 3) | |||||||
| Incident | Pre | Post | P | Pre | Post | P | Pre | Post | P |
| |||||||||
| ADEs | 5.6 (3.48.7) | 9.5 (6.613.2) | 0.08 | 10.3(7.314.3) | 8.9 (6.012.5) | 0.55 | 12.4 (7.5319.1) | 4.2 (1.78.5) | 0.02 |
| Preventable | 4.6 (2.67.5) | 4.3 (2.56.9) | 0.87 | 9.1 (6.312.8) | 6.4 (4.19.6) | 0.22 | 12.4 (7.5319.1) | 0.00 (00.03) | <0.01 |
| Nonpreventable | 0.99 (0.24 2.6) | 5.2 (3.28.0) | <0.01 | 1.2 (0.382.8) | 2.5 (1.14.6) | 0.24 | 0.00 (00.03) | 4.2 (1.78.5) | <0.01 |
| Potential ADEs | 54.0 (46.162.7) | 165.6 (152.4179.5) | <0.01 | 61.6 (53.570.5) | 120.9 (109.3133.2) | <0.01 | 44.8 (34.856.6) | 103.5 (87.7121.1) | <0.01 |
| Intercepted | 1.7 (0.593.6) | 2.9 (1.45.1) | 0.30 | 2.7 (1.34.9) | 3.1 (1.55.4) | 0.76 | 1.4 (0.234.3) | 2.8 (0.876.5) | 0.42 |
| Nonintercepted | 52.3 (44.660.9) | 162.7 (149.6176.5) | <0.01 | 58.8 (50.967.5) | 117.8 (106.4130.0) | <0.01 | 43.4 (33.655.1) | 100.7 (85.1118.1) | <0.01 |
Severity of Events
We further analyzed our data based on event severity (Table 4). Among preventable ADEs, only 1 fatal event was observed, which occurred after CPOE implementation. Here, a previously opioid‐nave patient received intravenous morphine for malignant pain. Within the first 24 hours, the patient received 70.2 mg of intravenous morphine, resulting in a decreased level of consciousness. The patient expired the following day. Furthermore, following implementation, among preventable ADEs, a reduction in significant events was seen (P=0.02) along with a nonsignificant reduction in the rate of serious events (P=0.06). However, the rate of preventable life‐threatening events was not different (P=0.96). The nonpreventable ADE rate rose during the postimplementation period for both serious (P=0.03) and significant events (P<0.01). The risk of fatal and life‐threatening nonpreventable ADEs did not change. The potential ADE rate increased following implementation for all severities (P<0. 01).
| Preimplementation | Postimplementation | ||||
|---|---|---|---|---|---|
| Incident | No. (%) | Average Rate/100 Admissions (95% CI)* | No. (%) | Average Rate/100 Admissions (95% CI)* | P |
| |||||
| All ADEs | |||||
| Fatal | 0 (0) | 0.00 (00.0047) | 1 (1.4) | 0.12 (0.0070.54) | 0.52 |
| Lifethreatening | 3 (4.3) | 0.39 (0.101.0) | 3 (4.4) | 0.37 (0.09 0.95) | 0.95 |
| Serious | 34 (49.3) | 4.4 (3.16.0) | 32 (47.1) | 3.9 (2.75.4) | 0.65 |
| Significant | 32 (46.4) | 4.1 (2.95.7) | 32 (47.1) | 3.9 (2.75.4) | 0.84 |
| Total | 69 (100) | 8.9 (7.011.2) | 68 (100) | 8.3 (6.510.5) | 0.74 |
| Preventable ADEs | |||||
| Fatal | 0 (0) | 0.00 (00.0047) | 1 (2.7) | 0.00 (00.0045) | 0.52 |
| Lifethreatening | 2 (3.2) | 0.26 (0.040.80) | 2 (5.6) | 0.25 (0.040.76) | 0.96 |
| Serious | 31 (50.0) | 4.0 (2.85.6) | 19 (52.8) | 2.3 (1.43.5) | 0.06 |
| Significant | 29 (46.8) | 3.7 (2.55.3) | 14 (38.9) | 1.7 (0.972.8) | 0.02 |
| Total | 62 (100) | 8.0 (6.210.2) | 36 (100) | 4.4 (3.16.0) | <0.01 |
| Nonpreventable ADEs | |||||
| Fatal | 0 (0) | 0.00 (00.0047) | 0 (0) | 0.00 (00.0045) | NS |
| Lifethreatening | 1 (14.2) | 0.13 (0.0070.57) | 1 (3.1) | 0.12 (0.0070.54) | 0.97 |
| Serious | 3 (42.9) | 0.39 (0.101.0) | 13 (40.6) | 1.6 (0.882.6) | 0.03 |
| Significant | 3 (42.9) | 0.39 (0.101.0) | 18 (56.3) | 2.2 (1.33.4) | <0.01 |
| Total | 7 (100) | 0.90 (0.391.7) | 32 (100) | 3.9 (2.75.4) | <0.01 |
| All potential ADEs | |||||
| Lifethreatening | 5 (1.2) | 0.65 (0.231.4) | 33 (3.0) | 4.0 (2.85.6) | <0.01 |
| Serious | 233 (54.2) | 30.1 (26.434.1) | 429 (38.4) | 52.6 (47.857.8) | <0.01 |
| Significant | 192 (44.6) | 24.8 (21.428.4) | 653 (58.6) | 80.1 (74.186.4) | <0.01 |
| Total | 430 (100) | 55.5 (50.460.9) | 1115 (100) | 136.8 (128.9145.0) | <0.01 |
| Intercepted potential ADEs | |||||
| Lifethreatening | 0 (0) | 0.00 (00.0047) | 1 (4.2) | 0.12 (0.0070.54) | 0.52 |
| Serious | 5 (31.2) | 0.65 (0.231.4) | 13 (54.2) | 1.6 (0.882.6) | 0.09 |
| Significant | 11 (68.8) | 1.4 (0.74 2.4) | 10 (41.6) | 1.2 (0.622.2) | 0.74 |
| Total | 16 (100) | 2.1 (1.23.2) | 24 (100) | 2.9 (1.94.3) | 0.24 |
| Nonintercepted potential ADEs | |||||
| Lifethreatening | 5 (1.2) | 0.65 (0.231.4) | 32 (2.9) | 3.9 (2.75.4) | <0.01 |
| Serious | 228 (55.1) | 29.4 (25.833.4) | 416 (38.1) | 51.0 (46.356.1) | <0.01 |
| Significant | 181 (43.7) | 23.4 (20.126.9) | 643 (58.9) | 78.9 (73.085.2) | <0.01 |
| Total | 414 (100) | 53.4 (48.458.7) | 1091 (100) | 133.9(126.1142.0) | <0.01 |
Case Reviews
In total, there were 36 preventable ADEs identified during the postimplementation phase (Table 5). Of these, inappropriate renal dosing accounted for 26 preventable ADEs, which involved antibiotics (eg, gentamicin‐induced renal failure), opioids (eg, over sedation from morphine), ‐blockers (eg, hypotension from atenolol), angiotensin‐converting enzyme inhibitors (eg, renal failure with hyperkalemia secondary to lisinopril), and digoxin (eg, bradyarrhythmia and toxicity). The use of contraindicated medications resulted in 7 preventable ADEs (eg, prescribing glyburide in the setting of severe renal impairment).[26] The remaining 3 preventable ADEs stemmed from unmonitored use of vancomycin.
| ADEs, Preventable, No. (Rate per 100 Admissions)* | ADEs, Nonpreventable, No. (Rate per 100 Admissions)* | ||||||
|---|---|---|---|---|---|---|---|
| Drug Class | Preimplementation | Postimplementation | P (for Entire Drug Class) | Preimplementation | Postimplementation | P (for Drug Class) | Drugs Involved |
| |||||||
| Cardiovascular | 20 (2.6) | 18 (2.2) | 0.63 | 4 (0.52) | 16 (2.0) | 0.02 | Atenolol, bumetanide, captopril, digoxin, furosemide, hydralazine, hydrochlorothiazide, lisinopril, sotalol, spironolactone |
| Diuretics | 1 (0.13) | 2 (0.25) | 1 (0.13) | 9 (1.1) | |||
| ‐blockers | 0 (0.00) | 2 (0.25) | 1 (0.13) | ||||
| ACE inhibitors and ARBs | 16 (2.1) | 10 (1.2) | 2 (0.26) | 7 (0.86) | |||
| Antiarrhythmic | 3 (0.39) | 3 (0.37) | |||||
| Vasodilator | 0 (0.00) | 1 (0.12) | |||||
| Analgesics | 28 (3.6) | 4 (0.49) | 0.0002 | 1 (0.13) | 5 (0.61) | 0.15 | Acetaminophen and combination pills containing acetaminophen: Percocet (oxycodone and acetaminophen), Tylenol #3 (codeine and acetaminophen), Vicodin (hydrocodone and acetaminophen), fentanyl, hydrocodone, meperidine, morphine, oxycodone |
| Narcotic | 13 (1.7) | 4 (0.49) | 0 (0.00) | 5 (0.61) | |||
| Non‐narcotic | 15 (1.9) | 0 (0.00) | 1 (0.13) | 0 | |||
| Antibiotics | 8 (1.0) | 13 (1.6) | 0.33 | 1 (0.13) | 9 (1.1) | 0.04 | Amikacin, ampicillin and sulbactam, ciprofloxacin, cefazolin, cefuroxime, gatifloxacin, gentamicin, levofloxacin, metronidazole, piperacillin and tazobactam, tobramycin, vancomycin |
| Neurotropic drugs | 2 (0.26) | 0 (0.00) | 0.28 | 0 | 0 | Lithium, midazolam | |
| Sedatives | 1 (0.13) | 0 (0.00) | |||||
| Antipsychotics | 1 (0.13) | 0 (0.00) | |||||
| Diabetes | 0 | 1 (0.12) | 0.52 | 0 | 1 (0.12) | 0.52 | Glipizide, glyburide |
| Oral antidiabetics | 0 | 1 (0.12) | 1 (0.12) | ||||
| Other drugs | 4 (0.52) | 0 (0.00) | 0.13 | 1 (0.13) | 1 (0.12) | 0.97 | Allopurinol, famotidine |
| Gastrointestinal drugs | 1 (0.13) | 0 (0.00) | |||||
| Other | 3 (0.39) | 0 (0.00) | 0 | 1 (0.12) | |||
DISCUSSION
We evaluated the use of vendor CPOE for hospitalized patients with renal disease and found that it was associated with a 45% reduction in preventable ADEs related to nephrotoxicity and accumulation of renally excreted medications. The impact of CPOE appeared to be related to the level of associated clinical decision support, where only the most advanced system was associated with benefit. We observed a significant increase in potential ADEs with all levels of intervention. Overall, these findings suggest that vendor‐developed applications with appropriate decision support can reduce the occurrence of renally related preventable ADEs, but careful implementation is needed if the potential ADE rate is to fall.
Many of the benefits of CPOE come from clinical decision support.[11] When applied to patients with renal impairment, CPOE with clinical decision support has been associated with decreased lengths of stay,[16, 27] reduced use of contraindicated medications,[28, 29, 30] improved dosing and drug monitoring,[16, 31, 32] and improved general prescribing practices.[29, 33] Even so, the observed benefit of CPOE on ADE rates has been variable, with some studies reporting reductions,[27, 34] whereas others are unable to detect differences.[16, 31] These studies, however, limited their case definition of ADEs to strictly declining renal function,[16, 31, 34] or adverse events directly resulting from anti‐infective drugs.[27] In contrast, our study accounted for nephrotoxicity and systemic toxicity from drug accumulation. Using this broader definition, we were able to detect large reductions in the rates of preventable ADEs following CPOE adoption.
Successful decision support is simple, intuitive, and provides speedy information that integrates seamlessly into the clinical workflow.[35, 36] However, information delivery, although necessary, is insufficient for improving safety. For instance, passive alerts are often ignored, deferred, or overridden.[30, 37, 38] Demonstrating this, Quartarolo et al. found that informing physicians of the presence of renal impairment using automated reporting of glomerular filtration rates did not change prescribing behavior.[39] In contrast, providing active feedback (with dosing recommendations) was observed to be more useful in effecting change.[40] Chertow et al. further showed that providing an adjusted dose list with a default dose and frequency at the time of order entry for patients with renal insufficiency improved appropriate ordering and was associated with a decreased length of stay.[16] Altogether, these studies help to explain why only CPOE with clinical decision support equipped to provide renally adjusted dosing and monitoring was associated with a reduction in preventable ADEs in our study.
However, in contrast to reports of internally developed systems,[20, 25] potential ADE rates actually rose during the follow‐up portion of our study. These appeared to be chiefly related to customized order sets with the potential of overdosing drugs through therapeutic duplication, a problem that is commonly known to be associated with CPOE (ie, new orders that overlap with other new or active medication orders, which may be the same drug itself or from within the same drug class, with the risk of overdose).[41, 42] Of note, our findings give rise to several key implications. First, hospitals implementing vendor‐developed CPOE systems may be at greater risk of incurring potential ADEs compared to those using home‐grown systems, which have comparatively gone through more cycles of internal refinement. As such, it is necessary to monitor for issues postimplementation and respond with appropriate changes to achieve successful system performance.[35, 36] Second, although the rate of potential ADEs (near misses) increased, preventable ADEs decreased because some of these errors were intercepted, whereas others were averted simply because of chance. Of note, not all potential ADEs have the same potential for injury; more serious cases are more likely to result in actual ADEs (eg, failure to renally dose acetaminophen likely poses less potential for harm than prescribing a full dose of enoxaparin in the setting of severe renal failure). Third, we found that most potential ADEs could have been averted with a combination of basic (dosing guidance and drug‐drug interactions checks) and advanced decision support (medication‐associated laboratory testing and drug‐disease interactions).[43] Therefore, further refinements to existing software are needed to maximize safety outcomes.
Our study has some limitations. This study was not a randomized controlled trial, and thus is subject to potential confounding. Although 6 hospitals were involved at the study inception,[14] one of these hospitals eventually opted not to implement CPOE, and further declined to participate as a control site. Therefore, we cannot exclude confounding from secular trends because we had no contemporaneous control group. However, the introduction of CPOE was the main medication safety‐oriented intervention during the study interval, thus arguing against major confounding by cointervention. Second, even though it is possible that classification bias may have been introduced between the preimplementation and postimplementation portions of our study, especially given the passage of time, it is unlikely. Study personnel underwent training using a curriculum designed to maintain continuity across projects, minimize individual variability, and optimize reproducibility in data collection and classification, as in a number of previous studies.[14, 17, 19, 20, 21] Third, our study is limited by a heterogeneous intervention, as varying levels of decision support were introduced. However, this reflects usual practice and may be construed as a strength as we were able to describe the impact of different types of decision support. Fourth, we enrolled patients with a large spectrum of renal impairment, and our findings are not specific to any particular subgroup. However, our wide recruitment strategy also enhances the generalizability. Finally, our study was restricted to patients who were exposed to potentially nephrotoxic and/or renally cleared drugs. As such, we could not determine whether advanced decision support helped to eliminate the use of some potentially dangerous medications altogether, as these cases would have been excluded from our study. It is possible, therefore, that our study findings underestimate the true benefit of clinical decision support.
In conclusion, vendor CPOE implementation in 5 community hospitals was associated with a 45% reduction in preventable ADE rates among patients with renal impairment. Measurable benefit was associated with advanced decision support capable of lab display, dosing guidance, and medication‐associated laboratory testing. Although the potential benefits of CPOE systems are far reaching, achieving the desired safety benefits will require appropriate decision support, tracking of problems that arise, and systematic approaches to eliminating them.
Acknowledgments
The authors thank Kathy Zigmont, RN, and Cathy Foskett, RN (Brigham and Women's Hospital, Division of General Internal Medicine and Primary Care) for the chart review and data collection at the participating study sites.
Disclosures: The Rx Foundation and Commonwealth Fund supported the study. They commented on its design, but were not involved in data collection, data management, analysis, interpretation, or writing of the manuscript. Dr. Leung is supported by a Clinical Fellowship Award from Alberta Innovates Health Solutions and by a Fellowship Award from the Canadian Institutes for Health Research. Dr. Schiff received financial support from the FDA CPOE Task Order and the Commonwealth Fund. Ms. Keohane served as a consultant to the American College of Obstetrician and Gynecologists and as a reviewer for the VRQC Program. She received honoraria for a presentation on Patient Safety in 2010, sponsored by Abbott Nutrition International, and a lecture on Nurse Interruptions in Medication Administration by Educational Review Systems. Dr. Coffey received an honorarium from Meditech for speaking on social networking at Physician/CIO Forum in 2009. Dr. Kaufman participates in an advisory group with Siemens Medical Solutions. Dr. Zimlichman received support from the Rx Foundation and the Commonwealth Fund. Dr. Bates holds a minority equity position in the privately held company Medicalis, which develops Web‐based decision support for radiology test ordering, and has served as a consultant to Medicalis. He serves as an advisor to Calgary Scientific, which makes technologies that enable mobility within electronic health records. He is on the clinical advisory board for Patient Safety Systems, which provides a set of approaches to help hospitals improve safety. He has received funding support from the Massachusetts Technology Consortium. Ms. Amato, Dr. Simon, Dr. Cadet, Ms. Seger, and Ms. Yoon have no disclosures relevant to this study.
Hospitalized patients with renal impairment are vulnerable to adverse drug events (ADEs).[1, 2] Appropriate prescribing for patients with renal insufficiency is challenging because of the complexities of drug therapy within the wide spectrum of kidney disease.[3, 4, 5, 6] Accordingly, computerized physician order entry (CPOE) systems with clinical decision support may help prevent many ADEs by providing timely laboratory information, recommending renally adjusted doses, and by offering assistance with prescribing.[7, 8, 9]
Despite the proposed benefits of CPOE, outcomes vary greatly because of differences in technology.[10, 11, 12, 13] In particular, the type of decision support available to assist medication ordering in the setting of renal disease varies widely among current vendor systems. Given the uncertain benefits of CPOE, especially with the wide range of associated clinical decision support, we conducted this study to determine the impact of these systems on the rates of ADEs among hospitalized patients with kidney disease.
METHODS
This study was approved by the institutional review boards at each study site.
Design and Setting
We conducted a before‐and‐after study to evaluate the impact of newly implemented vendor CPOE systems in 5 community hospitals in Massachusetts. Although we reported on 6 hospitals in our baseline study,[14] 1 of these hospitals later chose not to implement CPOE, and therefore was not included in follow‐up. At the time of this study, 1 of the hospitals (site 3) had not yet achieved hospital‐wide implementation. Although CPOE had been adopted by most medical services at site 3, it had not yet been implemented in the emergency, obstetrical, or surgical departments. Thus, we limited our study to the medical services at this site. For the remaining sites, all admitting services were included with the exception of the psychiatric and neonatal services, which were excluded from both phases because they would have required different detection tools.
Participants
Patients aged 18 years with renal failure, exposed to potentially nephrotoxic and/or renally cleared medications, and admitted to any of the participating hospitals during the study period were eligible for inclusion. Of the patients meeting eligibility criteria, we randomly selected approximately 150 records per hospital in the preimplementation and postimplementation phases for a total sample of 1590 charts. The first phase of this study occurred from January 2005 to August 2006; the second phase began 6 months postimplementation and lasted from October 2008 to September 2010.
Principal Exposure
Each hospital independently selected a vendor CPOE system with variable clinical decision support capabilities: (1) sites 4 and 5 had basic CPOE only with no clinical decision support for renal disease; (2) sites 1 and 2 implemented rudimentary clinical decision support with laboratory display (eg, serum creatinine) whenever common renally related drugs were ordered; and (3) site 3 had the most advanced support in place where, in addition to basic order entry and lab checks, physicians were provided with suggested doses for renally cleared and/or nephrotoxic medications, as well as appropriate drug monitoring for medications with narrow therapeutic indices (eg, suggested dosages and frequencies for vancomycin and automated corollary laboratory monitoring).
Definitions
We screened for the presence of renal failure by a serum creatinine 1.5 mg/dL at the time of admission. However, the duration of renal impairment was not known. We defined 3 levels of renal insufficiency based on the calculated creatinine clearance (CrCl)15: mild (CrCl 5080 mL/min), moderate (1649 mL/min), and severe (15 mL/min). Subjects with a CrCl >80 mL/min were considered to have normal renal function and were excluded. Potentially nephrotoxic and/or renally cleared medications were then identified using an established knowledge base (see Supporting Information, Table 1, in the online version of this article).[16]
| Hospital Site | |||||||
|---|---|---|---|---|---|---|---|
| Baseline Characteristics | All Sites | 1 | 2 | 3 | 4 | 5 | P (Among All Sites)* |
| |||||||
| No. of patients | 815 | 170 | 156 | 143 | 164 | 182 | |
| Age, y, mean (range) | 72.2 (18.0102.0) | 79.2 (33102) | 77.3 (23101) | 65.6 (1898) | 70.7 (1896) | 69.2 (2096) | <0.01 |
| 1844 years, no. (%) | 68 (9.1) | 1 (0.67) | 8 (6.5) | 20 (14.9) | 15 (9.4) | 24 (13.4) | <0.01 |
| 4554 years, no. (%) | 67 (9.0) | 6 (4.0) | 5 (4.1) | 17 (12.7) | 16 (10.0) | 23 (12.9) | |
| 5564 years, no. (%) | 79 (10.6) | 15 (10.0) | 12 (9.8) | 23 (17.2) | 13 (8.1) | 16 (8.9) | |
| 6574 years, no. (%) | 104 (13.9) | 20 (13.3) | 12 (9.8) | 16 (11.9) | 30 (18.8) | 26 (14.5) | |
| 7584 years, no. (%) | 197 (26.4) | 44 (29.3) | 36 (29.3) | 24 (17.9) | 49 (30.6) | 44 (24.6) | |
| 85 years, no. (%) | 231 (31.0) | 64 (42.7) | 50 (40.7) | 34 (25.4) | 37 (23.1) | 46 (25.7) | |
| Sex | |||||||
| Male, no. (%) | 427 (57.0) | 66 (44.0) | 60 (48.8) | 82 (60.7) | 105 (65.2) | 114 (63.7) | <0.01 |
| Female, no. (%) | 321 (43.0) | 84 (56.0) | 63 (51.2) | 53 (39.3) | 56 (34.8) | 65 (36.3) | |
| Race | |||||||
| Caucasian, no. (%) | 654 (87.4) | 129 (86.0) | 118 (95.9) | 126 (93.3) | 129 (80.1) | 152 (84.9) | <0.01 |
| Hispanic, no. (%) | 25 (3.3) | 2 (1.3) | 0 (0) | 1 (0.74) | 13 (8.1) | 9 (5.0) | |
| African American, no. (%) | 45 (6.0) | 12 (8.0) | 4 (3.3) | 5 (3.7) | 13 (8.1) | 11 (6.2) | |
| Native American, no. (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Asian, no. (%) | 13 (1.7) | 1 (0.81) | 1 (0.81) | 2 (1.5) | 5 (3.1) | 4 (2.2) | |
| Other, no. (%) | 7 (0.94) | 2 (1.3) | 0 (0) | 1 (0.74) | 1 (14.3) | 3 (1.7) | |
| Not recorded, no. (%) | 4 (0.53) | 4 (2.7) | 0 (0) | 0 (0.0) | 0 (0) | 0 (0) | |
| Initial severity of renal dysfunction | |||||||
| Mild, CrCl 5080 mL/min, no. (%) | 60 (7.4) | 4 (2.4) | 5 (3.2) | 5 (3.5) | 14 (8.5) | 32 (17.6) | <0. 01 |
| Moderate, CrCl 1649 mL/min, no. (%) | 388 (47.6) | 84 (49.4) | 71 (45.5) | 80 (55.9) | 76 (46.3) | 77 (42.3) | |
| Severe, CrCl <15 mL/min, no. (%) | 367 (45.0) | 82 (48.2) | 80 (51.3) | 58 (40.6) | 74 (45.1) | 73 (40.1) | |
| LOS, d, median (IQR) | 4.0 (26) | 4.0 (37) | 3.0 (25.5) | 4.0 (27) | 4.0 (27) | 4.0 (26) | 0.02 |
| DRG‐weighted LOS, d, median (IQR) | 5.0 (3.76.7) | 5.5 (46.7) | 5.0 (3.46.2) | 5.6 (4.36.7) | 5.0 (3.36.7) | 5.0 (4.26.7) | 0.27 |
In both phases of our study, only medications that were potentially nephrotoxic and/or renally cleared were included as potential cases; all other drugs were excluded. We defined an ADE as any drug‐related injury. These were considered preventable if they were due to an error at the time of order entry (eg, a doubling of creatinine secondary to an overdose of gentamicin or failure to order corollary drug levels for monitoring). A nonpreventable ADE was any drug‐related injury in which there was no error at the time of order entry (eg, a doubling of creatinine despite appropriate dosing of lisinopril).[17] A medication error was an error anywhere in the process of prescribing, transcribing, dispensing, administering, or monitoring a drug, but with no potential for harm or injury (eg, an order for an oral medication with no route specified when it was clear that the oral route was intended).[18] A potential ADE was an error with the potential to cause harm, but not resulting in injury, either because it was intercepted (eg, an order for ketorolac for a patient with renal failure, but caught by a pharmacist) or because of chance (eg, administering enoxaparin to a patient with severe renal dysfunction but without hemorrhage).
All study investigators underwent standardized training using a curriculum developed by the Center for Patient Safety Research and Practice (
Main Outcome Measures
The primary outcome was the rate of preventable ADEs. Secondary outcomes were the rates of potential ADEs and overall ADEs. All outcomes were related to nephrotoxicity or accumulation of a renally excreted medication.
Data collection and classification strategies were identical in both phases of our study.[14] We reviewed physician orders, medication lists, laboratory reports, admission histories, progress and consultation notes, discharge summaries, and nursing flow sheets, screening for the presence of medication incidents using an adaptation of the Institute for Healthcare Improvement's trigger tool, selected for its high sensitivity, reproducibility, and ease of use.[22, 23] In our adaptation of the tool, we excluded lidocaine, tobramycin, amikacin, and theophylline levels because of their infrequency. For each trigger found, a detailed description of the incident was extracted for detailed review. An example of a trigger is the use of sodium polystyrene, which may possibly indicate an overdose of potassium or a medication side effect.
Subsequently, each case was then independently reviewed by two investigators (A.A.L., M.A., B.C., S.R.S., M.C., N.K., E.Z., and G.S.)each assigned to at least 1 siteand blinded to prescribing physician and hospital to determine whether nephrotoxicity or injury from drug accumulation was present (see Supporting Information, Figure 1, in the online version of this article).[17] First, incidents were classified as ADEs, potential ADEs, or medication errors with no potential for injury. Second, ADEs and potential ADEs were rated according to severity. When nephrotoxic drugs were ordered, event severity was classified according to the elevation in serum creatinine24: increases of 10% were considered potential ADEs (near misses); increases of 10% to 100% were significant ADEs; and increases of 100% were serious ADEs. Changes in creatinine that were not associated with inappropriate medication orders were excluded. For renally excreted drugs with no potential for nephrotoxicity (eg, enoxaparin), we used clinical judgment to classify events as significant (eg, rash), severe (eg, 2‐unit gastrointestinal bleed), life threatening (eg, transfer to an intensive care unit), or fatal categories, as based on earlier work.[25] Disagreements were resolved by consensus. We had a score of 0.70 (95% confidence interval [CI]: 0.61‐0.80) for incident type, indicating excellent overall agreement.
Statistical Analysis
Baseline characteristics between hospitals were compared using the Fisher exact test for categorical variables and 1‐way analysis of variance for continuous variables. The occurrence of each outcome was determined according to site. To facilitate comparisons between sites, rates were expressed as number of events per 100 admissions with 95% CIs. To account for hospital effects in the analysis when comparing pre‐ and postimplementation rates of ADEs and potential ADEs, we developed a fixed‐effects Poisson regression model. To explore the independent effects of each system, a stratified analysis was performed to compare average rates of each outcome observed.
RESULTS
The outcomes of 775 patients in the baseline study were compared with the 815 patients enrolled during the postimplementation phase.[14] Among those in the postimplementation phase (Table 1), the mean age was 72.2 years, and they were predominantly male (57.0%). The demographics of the patients admitted to each of the 5 sites varied widely (P<0.01). Most patients had moderate to severe renal dysfunction.
Overall, the rates of ADEs were similar between the pre‐ and postimplementation phases (8.9/100 vs 8.3/100 admissions, respectively; P=0.74) (Table 2). However, there was a significant decrease in the rate of preventable ADEs, the primary outcome of interest, following CPOE implementation (8.0/100 vs 4.4/100 admissions; P<0.01). A reduction in preventable ADEs was observed in every hospital except site 4, where only basic order entry was introduced. However, there was a significant increase in the rates of nonpreventable ADEs (0.90/100 vs 3.9/100 admissions; P<0.01) and potential ADEs (55.5/100 vs 136.8/100 admissions; P<0.01).
| Rate/100 Admissions (95% CI) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total No. (%) | All Sites | Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | ||||||||||||||
| Event | Pre | Post | Pre | Post | P* | Pre | Post | P | Pre | Post | P | Pre | Post | P | Pre | Post | P | Pre | Post | P |
| ||||||||||||||||||||
| ADEs | 69 (13.8) | 68 (5.7) | 8.9 (7.0 1.2) | 8.3 (6.50.5) | 0.74 | 9.8 (6.015.1) | 10.0 (6.015.5) | 0.96 | 11.0 (6.517.4) | 7.7 (4.1 12.9) | 0.34 | 12.4 (7.5 19.1) | 4.2 (1.7 8.5) | 0.02 | 4.1 (1.68.3) | 13.4 (8.619.8) | 0.01 | 7.1 (3.712.2) | 6.0 (3.110.4) | 0.71 |
| Preventable | 62 | 36 | 8.0 (6.2 10.2) | 4.4 (3.16.0) | <0.01 | 8.2 (4.713.1) | 7.1 (3.811.8) | 0.70 | 10.3 (6.016.5) | 5.8 (2.8 10.4) | 0.17 | 12.4 (7.519.1) | 0 (0 0.03) | <0.01 | 3.4 (1.27.3) | 7.9 (4.413.1) | 0.11 | 5.8 (2.810.5) | 1.1 (0.183.4) | 0.03 |
| Nonpreventable | 7 | 32 | 0.90 (0.39 1.7) | 3.9 (2.75.4) | <0.01 | 1.6 (0.414.3) | 2.9 (1.16.3) | 0.42 | 0.69 (0.043.04) | 1.9 (0.48 5.0) | 0.37 | 0 (00.03) | 4.2 (1.7 8.5) | <0.01 | 0.68 (0.043.0) | 5.5 (2.6 9.9) | 0.05 | 1.3 (0.21, 4.0) | 4.9 (2.48.9) | 0.09 |
| Potential ADEs | 430 (86.2) | 1115 (93.5) | 55.5 (50.4 60.9) | 136.8 (128.9145.0) | <0.01 | 65.0 (54.077.4) | 141.1 (124.1159.8) | <0.01 | 57.2 (45.870.5) | 98.7 (83.9 115.1) | <0.01 | 44.8 (34.856.6) | 103.5 (87.7 121.1) | <0.01 | 59.2 (47.645.8) | 132.9 (116.1151.4) | <0.01 | 49.0 (38.860.9) | 195.1 (175.5216.1) | <0.01 |
| Intercepted | 16 | 24 | 2.1 (1.2 3.2) | 2.9 (1.94.3) | <0.24 | 3.3 (1.36.6) | 4.7 (2.28.8) | 0.50 | 2.1 (0.515.4) | 1.3 (0.21 4.0) | 0.60 | 1.4 (0.234.3) | 2.8 (0.87 6.5) | 0.41 | 2.0 (0.515.3) | 4.9 (2.2 9.1) | 0.20 | 1.3 (0.214.0) | 1.1 (0.183.4) | 0.87 |
| Nonintercepted | 414 | 1091 | 53.4 (48.4 58.7) | 133.9 (126.1142.0) | <0.01 | 61.7 (51.173.8) | 136.5 (119.754.8) | <0.01 | 55.2 43.968.2) | 97.4 (82.8 113.8) | <0.01 | 43.4 (33.655.1) | 100.7 (85.1 118.1) | <0.01 | 57.1 (45.8 70.2) | 128.0 (111.5146.2) | <0.01 | 47.7 (37.759.5) | 194.0 (174.4214.9) | <0.01 |
Stratified Analysis
To account for differences in technology, we performed a stratified analysis (Table 3). As was consistent with the overall study estimates, the rates of nonpreventable ADEs and potential ADEs increased with all 3 interventions. In contrast, we found that the changes in preventable ADE rates were related to the level of clinical decision support, where the greatest benefit was associated with the most sophisticated decision support system (P=0.03 and 0.02 for comparisons between advanced vs rudimentary decision support and basic order entry only, respectively). There was no difference in preventable ADE rates at sites without decision support (4.6/100 vs 4.3/100 admissions; P=0.87); with rudimentary clinical decision support, there was a trend toward a decrease in the preventable ADE rate, which did not meet statistical significance (9.1/100 vs 6.4/100 admissions; P=0.22), and, the greatest reduction was seen with advanced clinical decision support (12.4/100 vs 0/100 admissions; P<0.01).
| Rate per 100 Admissions by Level of Clinical Decision Support (95% CI) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Basic CPOE Only (Sites 4 and 5) | CPOE and Lab Display (Sites 1 and 2) | CPOE, Lab Display, and DrugDosing Check (Site 3) | |||||||
| Incident | Pre | Post | P | Pre | Post | P | Pre | Post | P |
| |||||||||
| ADEs | 5.6 (3.48.7) | 9.5 (6.613.2) | 0.08 | 10.3(7.314.3) | 8.9 (6.012.5) | 0.55 | 12.4 (7.5319.1) | 4.2 (1.78.5) | 0.02 |
| Preventable | 4.6 (2.67.5) | 4.3 (2.56.9) | 0.87 | 9.1 (6.312.8) | 6.4 (4.19.6) | 0.22 | 12.4 (7.5319.1) | 0.00 (00.03) | <0.01 |
| Nonpreventable | 0.99 (0.24 2.6) | 5.2 (3.28.0) | <0.01 | 1.2 (0.382.8) | 2.5 (1.14.6) | 0.24 | 0.00 (00.03) | 4.2 (1.78.5) | <0.01 |
| Potential ADEs | 54.0 (46.162.7) | 165.6 (152.4179.5) | <0.01 | 61.6 (53.570.5) | 120.9 (109.3133.2) | <0.01 | 44.8 (34.856.6) | 103.5 (87.7121.1) | <0.01 |
| Intercepted | 1.7 (0.593.6) | 2.9 (1.45.1) | 0.30 | 2.7 (1.34.9) | 3.1 (1.55.4) | 0.76 | 1.4 (0.234.3) | 2.8 (0.876.5) | 0.42 |
| Nonintercepted | 52.3 (44.660.9) | 162.7 (149.6176.5) | <0.01 | 58.8 (50.967.5) | 117.8 (106.4130.0) | <0.01 | 43.4 (33.655.1) | 100.7 (85.1118.1) | <0.01 |
Severity of Events
We further analyzed our data based on event severity (Table 4). Among preventable ADEs, only 1 fatal event was observed, which occurred after CPOE implementation. Here, a previously opioid‐nave patient received intravenous morphine for malignant pain. Within the first 24 hours, the patient received 70.2 mg of intravenous morphine, resulting in a decreased level of consciousness. The patient expired the following day. Furthermore, following implementation, among preventable ADEs, a reduction in significant events was seen (P=0.02) along with a nonsignificant reduction in the rate of serious events (P=0.06). However, the rate of preventable life‐threatening events was not different (P=0.96). The nonpreventable ADE rate rose during the postimplementation period for both serious (P=0.03) and significant events (P<0.01). The risk of fatal and life‐threatening nonpreventable ADEs did not change. The potential ADE rate increased following implementation for all severities (P<0. 01).
| Preimplementation | Postimplementation | ||||
|---|---|---|---|---|---|
| Incident | No. (%) | Average Rate/100 Admissions (95% CI)* | No. (%) | Average Rate/100 Admissions (95% CI)* | P |
| |||||
| All ADEs | |||||
| Fatal | 0 (0) | 0.00 (00.0047) | 1 (1.4) | 0.12 (0.0070.54) | 0.52 |
| Lifethreatening | 3 (4.3) | 0.39 (0.101.0) | 3 (4.4) | 0.37 (0.09 0.95) | 0.95 |
| Serious | 34 (49.3) | 4.4 (3.16.0) | 32 (47.1) | 3.9 (2.75.4) | 0.65 |
| Significant | 32 (46.4) | 4.1 (2.95.7) | 32 (47.1) | 3.9 (2.75.4) | 0.84 |
| Total | 69 (100) | 8.9 (7.011.2) | 68 (100) | 8.3 (6.510.5) | 0.74 |
| Preventable ADEs | |||||
| Fatal | 0 (0) | 0.00 (00.0047) | 1 (2.7) | 0.00 (00.0045) | 0.52 |
| Lifethreatening | 2 (3.2) | 0.26 (0.040.80) | 2 (5.6) | 0.25 (0.040.76) | 0.96 |
| Serious | 31 (50.0) | 4.0 (2.85.6) | 19 (52.8) | 2.3 (1.43.5) | 0.06 |
| Significant | 29 (46.8) | 3.7 (2.55.3) | 14 (38.9) | 1.7 (0.972.8) | 0.02 |
| Total | 62 (100) | 8.0 (6.210.2) | 36 (100) | 4.4 (3.16.0) | <0.01 |
| Nonpreventable ADEs | |||||
| Fatal | 0 (0) | 0.00 (00.0047) | 0 (0) | 0.00 (00.0045) | NS |
| Lifethreatening | 1 (14.2) | 0.13 (0.0070.57) | 1 (3.1) | 0.12 (0.0070.54) | 0.97 |
| Serious | 3 (42.9) | 0.39 (0.101.0) | 13 (40.6) | 1.6 (0.882.6) | 0.03 |
| Significant | 3 (42.9) | 0.39 (0.101.0) | 18 (56.3) | 2.2 (1.33.4) | <0.01 |
| Total | 7 (100) | 0.90 (0.391.7) | 32 (100) | 3.9 (2.75.4) | <0.01 |
| All potential ADEs | |||||
| Lifethreatening | 5 (1.2) | 0.65 (0.231.4) | 33 (3.0) | 4.0 (2.85.6) | <0.01 |
| Serious | 233 (54.2) | 30.1 (26.434.1) | 429 (38.4) | 52.6 (47.857.8) | <0.01 |
| Significant | 192 (44.6) | 24.8 (21.428.4) | 653 (58.6) | 80.1 (74.186.4) | <0.01 |
| Total | 430 (100) | 55.5 (50.460.9) | 1115 (100) | 136.8 (128.9145.0) | <0.01 |
| Intercepted potential ADEs | |||||
| Lifethreatening | 0 (0) | 0.00 (00.0047) | 1 (4.2) | 0.12 (0.0070.54) | 0.52 |
| Serious | 5 (31.2) | 0.65 (0.231.4) | 13 (54.2) | 1.6 (0.882.6) | 0.09 |
| Significant | 11 (68.8) | 1.4 (0.74 2.4) | 10 (41.6) | 1.2 (0.622.2) | 0.74 |
| Total | 16 (100) | 2.1 (1.23.2) | 24 (100) | 2.9 (1.94.3) | 0.24 |
| Nonintercepted potential ADEs | |||||
| Lifethreatening | 5 (1.2) | 0.65 (0.231.4) | 32 (2.9) | 3.9 (2.75.4) | <0.01 |
| Serious | 228 (55.1) | 29.4 (25.833.4) | 416 (38.1) | 51.0 (46.356.1) | <0.01 |
| Significant | 181 (43.7) | 23.4 (20.126.9) | 643 (58.9) | 78.9 (73.085.2) | <0.01 |
| Total | 414 (100) | 53.4 (48.458.7) | 1091 (100) | 133.9(126.1142.0) | <0.01 |
Case Reviews
In total, there were 36 preventable ADEs identified during the postimplementation phase (Table 5). Of these, inappropriate renal dosing accounted for 26 preventable ADEs, which involved antibiotics (eg, gentamicin‐induced renal failure), opioids (eg, over sedation from morphine), ‐blockers (eg, hypotension from atenolol), angiotensin‐converting enzyme inhibitors (eg, renal failure with hyperkalemia secondary to lisinopril), and digoxin (eg, bradyarrhythmia and toxicity). The use of contraindicated medications resulted in 7 preventable ADEs (eg, prescribing glyburide in the setting of severe renal impairment).[26] The remaining 3 preventable ADEs stemmed from unmonitored use of vancomycin.
| ADEs, Preventable, No. (Rate per 100 Admissions)* | ADEs, Nonpreventable, No. (Rate per 100 Admissions)* | ||||||
|---|---|---|---|---|---|---|---|
| Drug Class | Preimplementation | Postimplementation | P (for Entire Drug Class) | Preimplementation | Postimplementation | P (for Drug Class) | Drugs Involved |
| |||||||
| Cardiovascular | 20 (2.6) | 18 (2.2) | 0.63 | 4 (0.52) | 16 (2.0) | 0.02 | Atenolol, bumetanide, captopril, digoxin, furosemide, hydralazine, hydrochlorothiazide, lisinopril, sotalol, spironolactone |
| Diuretics | 1 (0.13) | 2 (0.25) | 1 (0.13) | 9 (1.1) | |||
| ‐blockers | 0 (0.00) | 2 (0.25) | 1 (0.13) | ||||
| ACE inhibitors and ARBs | 16 (2.1) | 10 (1.2) | 2 (0.26) | 7 (0.86) | |||
| Antiarrhythmic | 3 (0.39) | 3 (0.37) | |||||
| Vasodilator | 0 (0.00) | 1 (0.12) | |||||
| Analgesics | 28 (3.6) | 4 (0.49) | 0.0002 | 1 (0.13) | 5 (0.61) | 0.15 | Acetaminophen and combination pills containing acetaminophen: Percocet (oxycodone and acetaminophen), Tylenol #3 (codeine and acetaminophen), Vicodin (hydrocodone and acetaminophen), fentanyl, hydrocodone, meperidine, morphine, oxycodone |
| Narcotic | 13 (1.7) | 4 (0.49) | 0 (0.00) | 5 (0.61) | |||
| Non‐narcotic | 15 (1.9) | 0 (0.00) | 1 (0.13) | 0 | |||
| Antibiotics | 8 (1.0) | 13 (1.6) | 0.33 | 1 (0.13) | 9 (1.1) | 0.04 | Amikacin, ampicillin and sulbactam, ciprofloxacin, cefazolin, cefuroxime, gatifloxacin, gentamicin, levofloxacin, metronidazole, piperacillin and tazobactam, tobramycin, vancomycin |
| Neurotropic drugs | 2 (0.26) | 0 (0.00) | 0.28 | 0 | 0 | Lithium, midazolam | |
| Sedatives | 1 (0.13) | 0 (0.00) | |||||
| Antipsychotics | 1 (0.13) | 0 (0.00) | |||||
| Diabetes | 0 | 1 (0.12) | 0.52 | 0 | 1 (0.12) | 0.52 | Glipizide, glyburide |
| Oral antidiabetics | 0 | 1 (0.12) | 1 (0.12) | ||||
| Other drugs | 4 (0.52) | 0 (0.00) | 0.13 | 1 (0.13) | 1 (0.12) | 0.97 | Allopurinol, famotidine |
| Gastrointestinal drugs | 1 (0.13) | 0 (0.00) | |||||
| Other | 3 (0.39) | 0 (0.00) | 0 | 1 (0.12) | |||
DISCUSSION
We evaluated the use of vendor CPOE for hospitalized patients with renal disease and found that it was associated with a 45% reduction in preventable ADEs related to nephrotoxicity and accumulation of renally excreted medications. The impact of CPOE appeared to be related to the level of associated clinical decision support, where only the most advanced system was associated with benefit. We observed a significant increase in potential ADEs with all levels of intervention. Overall, these findings suggest that vendor‐developed applications with appropriate decision support can reduce the occurrence of renally related preventable ADEs, but careful implementation is needed if the potential ADE rate is to fall.
Many of the benefits of CPOE come from clinical decision support.[11] When applied to patients with renal impairment, CPOE with clinical decision support has been associated with decreased lengths of stay,[16, 27] reduced use of contraindicated medications,[28, 29, 30] improved dosing and drug monitoring,[16, 31, 32] and improved general prescribing practices.[29, 33] Even so, the observed benefit of CPOE on ADE rates has been variable, with some studies reporting reductions,[27, 34] whereas others are unable to detect differences.[16, 31] These studies, however, limited their case definition of ADEs to strictly declining renal function,[16, 31, 34] or adverse events directly resulting from anti‐infective drugs.[27] In contrast, our study accounted for nephrotoxicity and systemic toxicity from drug accumulation. Using this broader definition, we were able to detect large reductions in the rates of preventable ADEs following CPOE adoption.
Successful decision support is simple, intuitive, and provides speedy information that integrates seamlessly into the clinical workflow.[35, 36] However, information delivery, although necessary, is insufficient for improving safety. For instance, passive alerts are often ignored, deferred, or overridden.[30, 37, 38] Demonstrating this, Quartarolo et al. found that informing physicians of the presence of renal impairment using automated reporting of glomerular filtration rates did not change prescribing behavior.[39] In contrast, providing active feedback (with dosing recommendations) was observed to be more useful in effecting change.[40] Chertow et al. further showed that providing an adjusted dose list with a default dose and frequency at the time of order entry for patients with renal insufficiency improved appropriate ordering and was associated with a decreased length of stay.[16] Altogether, these studies help to explain why only CPOE with clinical decision support equipped to provide renally adjusted dosing and monitoring was associated with a reduction in preventable ADEs in our study.
However, in contrast to reports of internally developed systems,[20, 25] potential ADE rates actually rose during the follow‐up portion of our study. These appeared to be chiefly related to customized order sets with the potential of overdosing drugs through therapeutic duplication, a problem that is commonly known to be associated with CPOE (ie, new orders that overlap with other new or active medication orders, which may be the same drug itself or from within the same drug class, with the risk of overdose).[41, 42] Of note, our findings give rise to several key implications. First, hospitals implementing vendor‐developed CPOE systems may be at greater risk of incurring potential ADEs compared to those using home‐grown systems, which have comparatively gone through more cycles of internal refinement. As such, it is necessary to monitor for issues postimplementation and respond with appropriate changes to achieve successful system performance.[35, 36] Second, although the rate of potential ADEs (near misses) increased, preventable ADEs decreased because some of these errors were intercepted, whereas others were averted simply because of chance. Of note, not all potential ADEs have the same potential for injury; more serious cases are more likely to result in actual ADEs (eg, failure to renally dose acetaminophen likely poses less potential for harm than prescribing a full dose of enoxaparin in the setting of severe renal failure). Third, we found that most potential ADEs could have been averted with a combination of basic (dosing guidance and drug‐drug interactions checks) and advanced decision support (medication‐associated laboratory testing and drug‐disease interactions).[43] Therefore, further refinements to existing software are needed to maximize safety outcomes.
Our study has some limitations. This study was not a randomized controlled trial, and thus is subject to potential confounding. Although 6 hospitals were involved at the study inception,[14] one of these hospitals eventually opted not to implement CPOE, and further declined to participate as a control site. Therefore, we cannot exclude confounding from secular trends because we had no contemporaneous control group. However, the introduction of CPOE was the main medication safety‐oriented intervention during the study interval, thus arguing against major confounding by cointervention. Second, even though it is possible that classification bias may have been introduced between the preimplementation and postimplementation portions of our study, especially given the passage of time, it is unlikely. Study personnel underwent training using a curriculum designed to maintain continuity across projects, minimize individual variability, and optimize reproducibility in data collection and classification, as in a number of previous studies.[14, 17, 19, 20, 21] Third, our study is limited by a heterogeneous intervention, as varying levels of decision support were introduced. However, this reflects usual practice and may be construed as a strength as we were able to describe the impact of different types of decision support. Fourth, we enrolled patients with a large spectrum of renal impairment, and our findings are not specific to any particular subgroup. However, our wide recruitment strategy also enhances the generalizability. Finally, our study was restricted to patients who were exposed to potentially nephrotoxic and/or renally cleared drugs. As such, we could not determine whether advanced decision support helped to eliminate the use of some potentially dangerous medications altogether, as these cases would have been excluded from our study. It is possible, therefore, that our study findings underestimate the true benefit of clinical decision support.
In conclusion, vendor CPOE implementation in 5 community hospitals was associated with a 45% reduction in preventable ADE rates among patients with renal impairment. Measurable benefit was associated with advanced decision support capable of lab display, dosing guidance, and medication‐associated laboratory testing. Although the potential benefits of CPOE systems are far reaching, achieving the desired safety benefits will require appropriate decision support, tracking of problems that arise, and systematic approaches to eliminating them.
Acknowledgments
The authors thank Kathy Zigmont, RN, and Cathy Foskett, RN (Brigham and Women's Hospital, Division of General Internal Medicine and Primary Care) for the chart review and data collection at the participating study sites.
Disclosures: The Rx Foundation and Commonwealth Fund supported the study. They commented on its design, but were not involved in data collection, data management, analysis, interpretation, or writing of the manuscript. Dr. Leung is supported by a Clinical Fellowship Award from Alberta Innovates Health Solutions and by a Fellowship Award from the Canadian Institutes for Health Research. Dr. Schiff received financial support from the FDA CPOE Task Order and the Commonwealth Fund. Ms. Keohane served as a consultant to the American College of Obstetrician and Gynecologists and as a reviewer for the VRQC Program. She received honoraria for a presentation on Patient Safety in 2010, sponsored by Abbott Nutrition International, and a lecture on Nurse Interruptions in Medication Administration by Educational Review Systems. Dr. Coffey received an honorarium from Meditech for speaking on social networking at Physician/CIO Forum in 2009. Dr. Kaufman participates in an advisory group with Siemens Medical Solutions. Dr. Zimlichman received support from the Rx Foundation and the Commonwealth Fund. Dr. Bates holds a minority equity position in the privately held company Medicalis, which develops Web‐based decision support for radiology test ordering, and has served as a consultant to Medicalis. He serves as an advisor to Calgary Scientific, which makes technologies that enable mobility within electronic health records. He is on the clinical advisory board for Patient Safety Systems, which provides a set of approaches to help hospitals improve safety. He has received funding support from the Massachusetts Technology Consortium. Ms. Amato, Dr. Simon, Dr. Cadet, Ms. Seger, and Ms. Yoon have no disclosures relevant to this study.
- , , . Drug Prescribing in Renal Failure: Dosing Guidelines for Adults and Children: American College of Physicians; 2007.
- , . Management of drug toxicity in patients with renal insufficiency. Nat Rev Nephrol. 2010;6(6):317–318.
- , , , . Use of renal risk drugs in hospitalized patients with impaired renal function—an underestimated problem? Nephrol Dial Transplant. 2006;21(11):3164–3171.
- , , , et al. Medication misuse in hospitalized patients with renal impairment. Int J Qual Health Care. 2003;15(4):331–335.
- , , , . Impact of a renal drug dosing service on dose adjustment in hospitalized patients with chronic kidney disease. Ann Pharmacother. 2009;43(10):1598–1605.
- , . Drug dosing in chronic kidney disease. Med Clin North Am. 2005;89(3):649–687.
- , , , et al. Systems analysis of adverse drug events. ADE Prevention Study Group. JAMA. 1995;274(1):35–43.
- , , , , , . The epidemiology of prescribing errors: the potential impact of computerized prescriber order entry. Arch Intern Med. 2004;164(7):785–792.
- , , . Factors related to errors in medication prescribing. JAMA. 1997;277(4):312–317.
- , , , et al. Role of computerized physician order entry systems in facilitating medication errors. JAMA. 2005;293(10):1197–1203.
- , , , , . Mixed results in the safety performance of computerized physician order entry. Health Aff (Millwood). 2010;29(4):655–663.
- , , , et al. The effect of computerized physician order entry with clinical decision support on the rates of adverse drug events: a systematic review. J Gen Intern Med. 2008;23(4):451–458.
- , , . The impact of computerized physician medication order entry in hospitalized patients—a systematic review. Int J Med Inform. 2008;77(6):365–376.
- , , , et al. Occurrence of adverse, often preventable, events in community hospitals involving nephrotoxic drugs or those excreted by the kidney. Kidney Int. 2009;76(11):1192–1198.
- , . Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41.
- , , , et al. Guided medication dosing for inpatients with renal insufficiency. JAMA. 2001;286(22):2839–2844.
- , , , , . Adverse drug events and medication errors: detection and classification methods. Qual Saf Health Care. 2004;13(4):306–314.
- , , , , . Relationship between medication errors and adverse drug events. J Gen Intern Med. 1995;10(4):199–205.
- , , , et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA. 1995;274(1):29–34.
- , , , et al. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. JAMA. 1998;280(15):1311–1316.
- , , , et al. Adverse drug event rates in six community hospitals and the potential impact of computerized physician order entry for prevention. J Gen Intern Med. 2010;25(1):31–38.
- , , . Adverse drug event trigger tool: a practical methodology for measuring medication related harm. Qual Saf Health Care. 2003;12(3):194–200.
- Institute for Healthcare Improvement: IHI Trigger Tool for Measuring Adverse Drug Events. 2011. Available at: http://www.ihi.org/knowledge/Pages/Tools/TriggerToolforMeasuringAdverseDrugEvents.aspx. Accessed February 1, 2013.
- , , , . Renal safety of two analgesics used over the counter: ibuprofen and aspirin. Clin Pharmacol Ther. 1986;40(4):373–377.
- , , , et al. The impact of computerized physician order entry on medication error prevention. J Am Med Inform Assoc. 1999;6(4):313–321.
- , , . Prolonged sulfonylurea‐induced hypoglycemia in diabetic patients with end‐stage renal disease. Am J Kidney Dis. 2000;35(3):500–505.
- , , , et al. A computer‐assisted management program for antibiotics and other antiinfective agents. N Engl J Med. 1998;338(4):232–238.
- , , , et al. Alert system for inappropriate prescriptions relating to patients' clinical condition. Methods Inf Med. 2009;48(6):566–573.
- , , , , , . Computerized clinical decision support during medication ordering for long‐term care residents with renal insufficiency. J Am Med Inform Assoc. 2009;16(4):480–485.
- , , . A trial of automated decision support alerts for contraindicated medications using computerized physician order entry. J Am Med Inform Assoc. 2005;12(3):269–274.
- , , , , . Effects of clinical decision support on initial dosing and monitoring of tobramycin and amikacin. Am J Health Syst Pharm. 2011;68(7):624–632.
- , , , , , . Computerized decision support for medication dosing in renal insufficiency: a randomized, controlled trial. Ann Emerg Med. 2010;56(6):623–629.
- , , , . Implementation of rules based computerised bedside prescribing and administration: intervention study. BMJ. 2000;320(7237):750–753.
- , , , et al. Effect of computer‐based alerts on the treatment and outcomes of hospitalized patients. Arch Intern Med. 1994;154(13):1511–1517.
- , , , et al. Ten commandments for effective clinical decision support: making the practice of evidence‐based medicine a reality. J Am Med Inform Assoc. 2003;10(6):523–530.
- , , . Computerized decision support systems: improving patient safety in nephrology. Nat Rev Nephrol. 2011;7(6):348–355.
- , , , et al. Impact of a computerized alert during physician order entry on medication dosing in patients with renal impairment. Proc AMIA Symp. 2002:577–581.
- , , , et al. A computerized provider order entry intervention for medication safety during acute kidney injury: a quality improvement report. Am J Kidney Dis. 2010;56(5):832–841.
- , , . Reporting of estimated glomerular filtration rate: effect on physician recognition of chronic kidney disease and prescribing practices for elderly hospitalized patients. J Hosp Med. 2007;2(2):74–78.
- , , , , . Drug dosage in patients with renal failure optimized by immediate concurrent feedback. J Gen Intern Med. 2001;16(6):369–375.
- , , , et al. Factors contributing to an increase in duplicate medication order errors after CPOE implementation. J Am Med Inform Assoc. 2011;18(6):774–782.
- , , , et al. Impact of Vendor Computerized Physician Order Entry in Community Hospitals. J Gen Intern Med. 2012;27(7):801–807.
- , , , et al. Medication‐related clinical decision support in computerized provider order entry systems: a review. J Am Med Inform Assoc. 2007;14(1):29–40.
- , , . Drug Prescribing in Renal Failure: Dosing Guidelines for Adults and Children: American College of Physicians; 2007.
- , . Management of drug toxicity in patients with renal insufficiency. Nat Rev Nephrol. 2010;6(6):317–318.
- , , , . Use of renal risk drugs in hospitalized patients with impaired renal function—an underestimated problem? Nephrol Dial Transplant. 2006;21(11):3164–3171.
- , , , et al. Medication misuse in hospitalized patients with renal impairment. Int J Qual Health Care. 2003;15(4):331–335.
- , , , . Impact of a renal drug dosing service on dose adjustment in hospitalized patients with chronic kidney disease. Ann Pharmacother. 2009;43(10):1598–1605.
- , . Drug dosing in chronic kidney disease. Med Clin North Am. 2005;89(3):649–687.
- , , , et al. Systems analysis of adverse drug events. ADE Prevention Study Group. JAMA. 1995;274(1):35–43.
- , , , , , . The epidemiology of prescribing errors: the potential impact of computerized prescriber order entry. Arch Intern Med. 2004;164(7):785–792.
- , , . Factors related to errors in medication prescribing. JAMA. 1997;277(4):312–317.
- , , , et al. Role of computerized physician order entry systems in facilitating medication errors. JAMA. 2005;293(10):1197–1203.
- , , , , . Mixed results in the safety performance of computerized physician order entry. Health Aff (Millwood). 2010;29(4):655–663.
- , , , et al. The effect of computerized physician order entry with clinical decision support on the rates of adverse drug events: a systematic review. J Gen Intern Med. 2008;23(4):451–458.
- , , . The impact of computerized physician medication order entry in hospitalized patients—a systematic review. Int J Med Inform. 2008;77(6):365–376.
- , , , et al. Occurrence of adverse, often preventable, events in community hospitals involving nephrotoxic drugs or those excreted by the kidney. Kidney Int. 2009;76(11):1192–1198.
- , . Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41.
- , , , et al. Guided medication dosing for inpatients with renal insufficiency. JAMA. 2001;286(22):2839–2844.
- , , , , . Adverse drug events and medication errors: detection and classification methods. Qual Saf Health Care. 2004;13(4):306–314.
- , , , , . Relationship between medication errors and adverse drug events. J Gen Intern Med. 1995;10(4):199–205.
- , , , et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA. 1995;274(1):29–34.
- , , , et al. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. JAMA. 1998;280(15):1311–1316.
- , , , et al. Adverse drug event rates in six community hospitals and the potential impact of computerized physician order entry for prevention. J Gen Intern Med. 2010;25(1):31–38.
- , , . Adverse drug event trigger tool: a practical methodology for measuring medication related harm. Qual Saf Health Care. 2003;12(3):194–200.
- Institute for Healthcare Improvement: IHI Trigger Tool for Measuring Adverse Drug Events. 2011. Available at: http://www.ihi.org/knowledge/Pages/Tools/TriggerToolforMeasuringAdverseDrugEvents.aspx. Accessed February 1, 2013.
- , , , . Renal safety of two analgesics used over the counter: ibuprofen and aspirin. Clin Pharmacol Ther. 1986;40(4):373–377.
- , , , et al. The impact of computerized physician order entry on medication error prevention. J Am Med Inform Assoc. 1999;6(4):313–321.
- , , . Prolonged sulfonylurea‐induced hypoglycemia in diabetic patients with end‐stage renal disease. Am J Kidney Dis. 2000;35(3):500–505.
- , , , et al. A computer‐assisted management program for antibiotics and other antiinfective agents. N Engl J Med. 1998;338(4):232–238.
- , , , et al. Alert system for inappropriate prescriptions relating to patients' clinical condition. Methods Inf Med. 2009;48(6):566–573.
- , , , , , . Computerized clinical decision support during medication ordering for long‐term care residents with renal insufficiency. J Am Med Inform Assoc. 2009;16(4):480–485.
- , , . A trial of automated decision support alerts for contraindicated medications using computerized physician order entry. J Am Med Inform Assoc. 2005;12(3):269–274.
- , , , , . Effects of clinical decision support on initial dosing and monitoring of tobramycin and amikacin. Am J Health Syst Pharm. 2011;68(7):624–632.
- , , , , , . Computerized decision support for medication dosing in renal insufficiency: a randomized, controlled trial. Ann Emerg Med. 2010;56(6):623–629.
- , , , . Implementation of rules based computerised bedside prescribing and administration: intervention study. BMJ. 2000;320(7237):750–753.
- , , , et al. Effect of computer‐based alerts on the treatment and outcomes of hospitalized patients. Arch Intern Med. 1994;154(13):1511–1517.
- , , , et al. Ten commandments for effective clinical decision support: making the practice of evidence‐based medicine a reality. J Am Med Inform Assoc. 2003;10(6):523–530.
- , , . Computerized decision support systems: improving patient safety in nephrology. Nat Rev Nephrol. 2011;7(6):348–355.
- , , , et al. Impact of a computerized alert during physician order entry on medication dosing in patients with renal impairment. Proc AMIA Symp. 2002:577–581.
- , , , et al. A computerized provider order entry intervention for medication safety during acute kidney injury: a quality improvement report. Am J Kidney Dis. 2010;56(5):832–841.
- , , . Reporting of estimated glomerular filtration rate: effect on physician recognition of chronic kidney disease and prescribing practices for elderly hospitalized patients. J Hosp Med. 2007;2(2):74–78.
- , , , , . Drug dosage in patients with renal failure optimized by immediate concurrent feedback. J Gen Intern Med. 2001;16(6):369–375.
- , , , et al. Factors contributing to an increase in duplicate medication order errors after CPOE implementation. J Am Med Inform Assoc. 2011;18(6):774–782.
- , , , et al. Impact of Vendor Computerized Physician Order Entry in Community Hospitals. J Gen Intern Med. 2012;27(7):801–807.
- , , , et al. Medication‐related clinical decision support in computerized provider order entry systems: a review. J Am Med Inform Assoc. 2007;14(1):29–40.
© 2013 Society of Hospital Medicine