User login
Agency Funding for Healthcare Research Could Benefit Hospital Medicine
David O. Meltzer, MD, PhD, MHM, wants hospitalists to take advantage of the recent announcement by the Patient-Centered Outcomes Research Institute (PCORI) that it intends to award $300 million by the end of this year, and more in the future. And if that means calling him directly, go for it.
Dr. Meltzer, chief of the section of hospital medicine at the University of Chicago, is a member of PCORI's methodology committee. He says in a question-and-answer session with The Hospitalist that PCORI could be a valuable resource and funding source for hospitalist researchers.
Question: What should hospitalists know about PCORI?
Answer: PCORI is focused on figuring out how to improve the effectiveness of healthcare, and it is placing a strong emphasis on the importance of engaging patients and other stakeholders in that process. Also, PCORI is trying to ensure that research recognizes the potential differences between patient subgroups, and even individual patients, to the maximum degree possible.
Q: Given PCORI's focus on outcomes, how can HM researchers pitch the type of projects that would be eligible for funding?
A: They should focus on questions that matter to patients, and engage diverse stakeholders and patients in identifying those questions.
Q: How helpful for the specialty is it to have a leading member involved with the institute?
A: PCORI is becoming an important funder of research in the United States, and I think all specialties need to know about it. PCORI is working hard to get the word out to all specialties, but I hope my colleagues in hospital medicine will feel free to call if I can help them interpret PCORI's guidance about how they can best engage with it. TH
Visit our website for more information on patient-centered care.
David O. Meltzer, MD, PhD, MHM, wants hospitalists to take advantage of the recent announcement by the Patient-Centered Outcomes Research Institute (PCORI) that it intends to award $300 million by the end of this year, and more in the future. And if that means calling him directly, go for it.
Dr. Meltzer, chief of the section of hospital medicine at the University of Chicago, is a member of PCORI's methodology committee. He says in a question-and-answer session with The Hospitalist that PCORI could be a valuable resource and funding source for hospitalist researchers.
Question: What should hospitalists know about PCORI?
Answer: PCORI is focused on figuring out how to improve the effectiveness of healthcare, and it is placing a strong emphasis on the importance of engaging patients and other stakeholders in that process. Also, PCORI is trying to ensure that research recognizes the potential differences between patient subgroups, and even individual patients, to the maximum degree possible.
Q: Given PCORI's focus on outcomes, how can HM researchers pitch the type of projects that would be eligible for funding?
A: They should focus on questions that matter to patients, and engage diverse stakeholders and patients in identifying those questions.
Q: How helpful for the specialty is it to have a leading member involved with the institute?
A: PCORI is becoming an important funder of research in the United States, and I think all specialties need to know about it. PCORI is working hard to get the word out to all specialties, but I hope my colleagues in hospital medicine will feel free to call if I can help them interpret PCORI's guidance about how they can best engage with it. TH
Visit our website for more information on patient-centered care.
David O. Meltzer, MD, PhD, MHM, wants hospitalists to take advantage of the recent announcement by the Patient-Centered Outcomes Research Institute (PCORI) that it intends to award $300 million by the end of this year, and more in the future. And if that means calling him directly, go for it.
Dr. Meltzer, chief of the section of hospital medicine at the University of Chicago, is a member of PCORI's methodology committee. He says in a question-and-answer session with The Hospitalist that PCORI could be a valuable resource and funding source for hospitalist researchers.
Question: What should hospitalists know about PCORI?
Answer: PCORI is focused on figuring out how to improve the effectiveness of healthcare, and it is placing a strong emphasis on the importance of engaging patients and other stakeholders in that process. Also, PCORI is trying to ensure that research recognizes the potential differences between patient subgroups, and even individual patients, to the maximum degree possible.
Q: Given PCORI's focus on outcomes, how can HM researchers pitch the type of projects that would be eligible for funding?
A: They should focus on questions that matter to patients, and engage diverse stakeholders and patients in identifying those questions.
Q: How helpful for the specialty is it to have a leading member involved with the institute?
A: PCORI is becoming an important funder of research in the United States, and I think all specialties need to know about it. PCORI is working hard to get the word out to all specialties, but I hope my colleagues in hospital medicine will feel free to call if I can help them interpret PCORI's guidance about how they can best engage with it. TH
Visit our website for more information on patient-centered care.
Readmission Rates Not Effective Quality Measure of Pediatric Patient Care
A new study in Pediatrics finds limited use for hospital readmission rates as a meaningful quality measure when it comes to pediatric patient care.
By examining 30- and 60-day readmission rates for 958 hospitals that admit children for seven common inpatient conditions, researchers found very few that could be considered either high or low performers. In addition, pediatric 30-day readmission rates overall were low, at less than 10% for all conditions.
Naomi Bardach, MD, MAS, department of pediatrics at the University of California at San Francisco and the report's lead author, emphasizes that her study was a statistical analysis of readmission rates without assessing whether they should be a focus for quality improvement. "They might be useful for larger efforts, such as multi-institution collaboratives to improve care for a given condition," Dr. Bardach says. "But it is clear that readmission rates are not useful for comparing individual hospital performance."
An accompanying editorial noted that delaying hospital discharges even by four hours in an attempt to forestall readmissions could prove more costly in the end.
Although much of the national focus on 30-day hospital readmissions has been on the Medicare-age population, the pediatric realm is getting more attention, Dr. Bardach says. For example, the Children's Health Insurance Program Reauthorization Act of 2009 funded seven research cooperatives to develop core measures for assessing the state of children’s healthcare quality. TH
Visit our website for more information about pediatric readmissions rates.
A new study in Pediatrics finds limited use for hospital readmission rates as a meaningful quality measure when it comes to pediatric patient care.
By examining 30- and 60-day readmission rates for 958 hospitals that admit children for seven common inpatient conditions, researchers found very few that could be considered either high or low performers. In addition, pediatric 30-day readmission rates overall were low, at less than 10% for all conditions.
Naomi Bardach, MD, MAS, department of pediatrics at the University of California at San Francisco and the report's lead author, emphasizes that her study was a statistical analysis of readmission rates without assessing whether they should be a focus for quality improvement. "They might be useful for larger efforts, such as multi-institution collaboratives to improve care for a given condition," Dr. Bardach says. "But it is clear that readmission rates are not useful for comparing individual hospital performance."
An accompanying editorial noted that delaying hospital discharges even by four hours in an attempt to forestall readmissions could prove more costly in the end.
Although much of the national focus on 30-day hospital readmissions has been on the Medicare-age population, the pediatric realm is getting more attention, Dr. Bardach says. For example, the Children's Health Insurance Program Reauthorization Act of 2009 funded seven research cooperatives to develop core measures for assessing the state of children’s healthcare quality. TH
Visit our website for more information about pediatric readmissions rates.
A new study in Pediatrics finds limited use for hospital readmission rates as a meaningful quality measure when it comes to pediatric patient care.
By examining 30- and 60-day readmission rates for 958 hospitals that admit children for seven common inpatient conditions, researchers found very few that could be considered either high or low performers. In addition, pediatric 30-day readmission rates overall were low, at less than 10% for all conditions.
Naomi Bardach, MD, MAS, department of pediatrics at the University of California at San Francisco and the report's lead author, emphasizes that her study was a statistical analysis of readmission rates without assessing whether they should be a focus for quality improvement. "They might be useful for larger efforts, such as multi-institution collaboratives to improve care for a given condition," Dr. Bardach says. "But it is clear that readmission rates are not useful for comparing individual hospital performance."
An accompanying editorial noted that delaying hospital discharges even by four hours in an attempt to forestall readmissions could prove more costly in the end.
Although much of the national focus on 30-day hospital readmissions has been on the Medicare-age population, the pediatric realm is getting more attention, Dr. Bardach says. For example, the Children's Health Insurance Program Reauthorization Act of 2009 funded seven research cooperatives to develop core measures for assessing the state of children’s healthcare quality. TH
Visit our website for more information about pediatric readmissions rates.
Pay disparities and gender
As we all know, a healthy work-life balance can be very difficult to achieve, let alone maintain. That is why many of us got into hospital medicine in the first place.
When studies show that in America, male hospitalists make more on average than their female counterparts, it is assumed that there is a strong correlation between hours worked and pay. But could there be other factors as well? In Canada, at least, that seems to be the case. A research team at the University of Montreal reviewed the billing information of 870 Quebec practitioners with a focus on procedures in elderly diabetic patients. Male and female practitioners were equally represented. The results: Female doctors were far more compliant with the Canadian Diabetes Association practice guidelines, but males were more productive when it came to procedures.
Specifically, male physicians reported close to 1,000 more procedures annually compared with female physicians. So, the question comes to mind: Who is more profitable for hospitals, physicians who perform more procedures that can be billed at a higher rate, or those who seem to focus more attention on the bread and butter of care, so to speak? After all, if patients understand their condition and get the appropriate care, aren't they less likely to require rehospitalization? No definitive answers yet, but this study does make you want to go, "Hmm."
While the U.S. health care system certainly differs from Canada's, this article does bring up intriguing issues, some which just may be worth considering as we assess and improve the practice styles and compensation models for hospitalists. A 2012 Today's Hospitalist survey cited in an article titled, "Why do women hospitalists make less money?" sheds additional light on the subject. Yes, there is still a gender gap between male and female physicians. There are numerous hypotheses, as well as some hard data to explain some of these differences, though many still believe part of the issue is a persistent gender bias.
The article noted that males work a few more shifts than females, 16.68 vs 15.96, but this is only a 5% difference in work hours. Other data support a compensation difference based on the different payment models. Slightly more men are in a payment model that is 100% productivity-based or a combination of salary and productivity, and these models tend to pay more than do positions that are straight salary. Still, for a variety of reasons, some clear and others obscure, female hospitalists earn an average of $35,000 less than do their male counterparts.
Acknowledging a disparity exists is not enough. The reasons for this disparity should be further evaluated and addressed. Perhaps they are strongly the result of lifestyle choices, types of positions females prefer, and other nongender-related issues, but we owe it ourselves to gain further clarity on this very real issue.
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center who has a passion for empowering patients to partner in their health care. She is the creator of the Patient Whiz, a patient-engagement app for iOS.
As we all know, a healthy work-life balance can be very difficult to achieve, let alone maintain. That is why many of us got into hospital medicine in the first place.
When studies show that in America, male hospitalists make more on average than their female counterparts, it is assumed that there is a strong correlation between hours worked and pay. But could there be other factors as well? In Canada, at least, that seems to be the case. A research team at the University of Montreal reviewed the billing information of 870 Quebec practitioners with a focus on procedures in elderly diabetic patients. Male and female practitioners were equally represented. The results: Female doctors were far more compliant with the Canadian Diabetes Association practice guidelines, but males were more productive when it came to procedures.
Specifically, male physicians reported close to 1,000 more procedures annually compared with female physicians. So, the question comes to mind: Who is more profitable for hospitals, physicians who perform more procedures that can be billed at a higher rate, or those who seem to focus more attention on the bread and butter of care, so to speak? After all, if patients understand their condition and get the appropriate care, aren't they less likely to require rehospitalization? No definitive answers yet, but this study does make you want to go, "Hmm."
While the U.S. health care system certainly differs from Canada's, this article does bring up intriguing issues, some which just may be worth considering as we assess and improve the practice styles and compensation models for hospitalists. A 2012 Today's Hospitalist survey cited in an article titled, "Why do women hospitalists make less money?" sheds additional light on the subject. Yes, there is still a gender gap between male and female physicians. There are numerous hypotheses, as well as some hard data to explain some of these differences, though many still believe part of the issue is a persistent gender bias.
The article noted that males work a few more shifts than females, 16.68 vs 15.96, but this is only a 5% difference in work hours. Other data support a compensation difference based on the different payment models. Slightly more men are in a payment model that is 100% productivity-based or a combination of salary and productivity, and these models tend to pay more than do positions that are straight salary. Still, for a variety of reasons, some clear and others obscure, female hospitalists earn an average of $35,000 less than do their male counterparts.
Acknowledging a disparity exists is not enough. The reasons for this disparity should be further evaluated and addressed. Perhaps they are strongly the result of lifestyle choices, types of positions females prefer, and other nongender-related issues, but we owe it ourselves to gain further clarity on this very real issue.
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center who has a passion for empowering patients to partner in their health care. She is the creator of the Patient Whiz, a patient-engagement app for iOS.
As we all know, a healthy work-life balance can be very difficult to achieve, let alone maintain. That is why many of us got into hospital medicine in the first place.
When studies show that in America, male hospitalists make more on average than their female counterparts, it is assumed that there is a strong correlation between hours worked and pay. But could there be other factors as well? In Canada, at least, that seems to be the case. A research team at the University of Montreal reviewed the billing information of 870 Quebec practitioners with a focus on procedures in elderly diabetic patients. Male and female practitioners were equally represented. The results: Female doctors were far more compliant with the Canadian Diabetes Association practice guidelines, but males were more productive when it came to procedures.
Specifically, male physicians reported close to 1,000 more procedures annually compared with female physicians. So, the question comes to mind: Who is more profitable for hospitals, physicians who perform more procedures that can be billed at a higher rate, or those who seem to focus more attention on the bread and butter of care, so to speak? After all, if patients understand their condition and get the appropriate care, aren't they less likely to require rehospitalization? No definitive answers yet, but this study does make you want to go, "Hmm."
While the U.S. health care system certainly differs from Canada's, this article does bring up intriguing issues, some which just may be worth considering as we assess and improve the practice styles and compensation models for hospitalists. A 2012 Today's Hospitalist survey cited in an article titled, "Why do women hospitalists make less money?" sheds additional light on the subject. Yes, there is still a gender gap between male and female physicians. There are numerous hypotheses, as well as some hard data to explain some of these differences, though many still believe part of the issue is a persistent gender bias.
The article noted that males work a few more shifts than females, 16.68 vs 15.96, but this is only a 5% difference in work hours. Other data support a compensation difference based on the different payment models. Slightly more men are in a payment model that is 100% productivity-based or a combination of salary and productivity, and these models tend to pay more than do positions that are straight salary. Still, for a variety of reasons, some clear and others obscure, female hospitalists earn an average of $35,000 less than do their male counterparts.
Acknowledging a disparity exists is not enough. The reasons for this disparity should be further evaluated and addressed. Perhaps they are strongly the result of lifestyle choices, types of positions females prefer, and other nongender-related issues, but we owe it ourselves to gain further clarity on this very real issue.
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center who has a passion for empowering patients to partner in their health care. She is the creator of the Patient Whiz, a patient-engagement app for iOS.
Self-administration of romiplostim in patients with chronic immune thrombocytopenia
Background Romiplostim increases platelet counts in ITP and is typically injected at clinic visits.
Objective To estimate the efficacy and safety of romiplostim self-administration, we evaluated data from an open-label extension study in a post hoc analysis.
Methods Patients received weekly romiplostim with dose adjustments to target platelet counts of 50-200 x 109/L. Patients with a stable dose and platelet counts of 50-200 x 109/L for 3 or more weeks could begin self-administration if investigators deemed it appropriate, returning to study sites every 4 weeks.
Results Of 292 patients, 239 (82%) initiated self-administration for a median of 74 (Q1-Q3:56-164) weeks. Twenty-eight of the 239 (12%) discontinued self-administration (investigator or sponsor decision: 19, patient request: 6, noncompliance: 3). The median average weekly dose for patients self-administering romiplostim was 4.1 g/kg. The romiplostim dose was adjusted in 40 (17%) of the 239 patients in the first 8 weeks of self-administration; 84 (35%) in the first 6 months. Patients had a platelet response (more than 50 x 109/L) for a mean of 75.1% of weeks. The adverse event (AE) rate was 18.3/100 patient-weeks, with 0.8 serious AEs/100 patient-weeks. Fourteen AEs led to withdrawal; none related to self-administration.
Limitations The analysis was post hoc. Lack of a randomized comparator group may have resulted in differences between patient populations. No distinctions could be made between constant and intermittent self-administration or between adverse events occurring during self-administration or administration at the study site.
Conclusions Patients were able to maintain platelet responses for a mean of 75% of the time without new safety issues while self-administering romiplostim.
To read the full article, click on the PDF icon at the top of this introduction.
Background Romiplostim increases platelet counts in ITP and is typically injected at clinic visits.
Objective To estimate the efficacy and safety of romiplostim self-administration, we evaluated data from an open-label extension study in a post hoc analysis.
Methods Patients received weekly romiplostim with dose adjustments to target platelet counts of 50-200 x 109/L. Patients with a stable dose and platelet counts of 50-200 x 109/L for 3 or more weeks could begin self-administration if investigators deemed it appropriate, returning to study sites every 4 weeks.
Results Of 292 patients, 239 (82%) initiated self-administration for a median of 74 (Q1-Q3:56-164) weeks. Twenty-eight of the 239 (12%) discontinued self-administration (investigator or sponsor decision: 19, patient request: 6, noncompliance: 3). The median average weekly dose for patients self-administering romiplostim was 4.1 g/kg. The romiplostim dose was adjusted in 40 (17%) of the 239 patients in the first 8 weeks of self-administration; 84 (35%) in the first 6 months. Patients had a platelet response (more than 50 x 109/L) for a mean of 75.1% of weeks. The adverse event (AE) rate was 18.3/100 patient-weeks, with 0.8 serious AEs/100 patient-weeks. Fourteen AEs led to withdrawal; none related to self-administration.
Limitations The analysis was post hoc. Lack of a randomized comparator group may have resulted in differences between patient populations. No distinctions could be made between constant and intermittent self-administration or between adverse events occurring during self-administration or administration at the study site.
Conclusions Patients were able to maintain platelet responses for a mean of 75% of the time without new safety issues while self-administering romiplostim.
To read the full article, click on the PDF icon at the top of this introduction.
Background Romiplostim increases platelet counts in ITP and is typically injected at clinic visits.
Objective To estimate the efficacy and safety of romiplostim self-administration, we evaluated data from an open-label extension study in a post hoc analysis.
Methods Patients received weekly romiplostim with dose adjustments to target platelet counts of 50-200 x 109/L. Patients with a stable dose and platelet counts of 50-200 x 109/L for 3 or more weeks could begin self-administration if investigators deemed it appropriate, returning to study sites every 4 weeks.
Results Of 292 patients, 239 (82%) initiated self-administration for a median of 74 (Q1-Q3:56-164) weeks. Twenty-eight of the 239 (12%) discontinued self-administration (investigator or sponsor decision: 19, patient request: 6, noncompliance: 3). The median average weekly dose for patients self-administering romiplostim was 4.1 g/kg. The romiplostim dose was adjusted in 40 (17%) of the 239 patients in the first 8 weeks of self-administration; 84 (35%) in the first 6 months. Patients had a platelet response (more than 50 x 109/L) for a mean of 75.1% of weeks. The adverse event (AE) rate was 18.3/100 patient-weeks, with 0.8 serious AEs/100 patient-weeks. Fourteen AEs led to withdrawal; none related to self-administration.
Limitations The analysis was post hoc. Lack of a randomized comparator group may have resulted in differences between patient populations. No distinctions could be made between constant and intermittent self-administration or between adverse events occurring during self-administration or administration at the study site.
Conclusions Patients were able to maintain platelet responses for a mean of 75% of the time without new safety issues while self-administering romiplostim.
To read the full article, click on the PDF icon at the top of this introduction.
More Hospitals Learning to Share; Expanding Research Into PTSD and TBI; ED Visits for CNS Stimulant Abuse on the Rise; Talking About Suicide Matters; Decline in Childhood Obesity
Medicare vs VA—VA Wins; Delaying Antibiotics for UTI; Dolutegravir Approved to Treat Resistant HIV Infection
Phase 3 ponatinib trial stopped due to adverse events
The phase 3 EPIC trial, an evaluation of ponatinib (Iclusig) in patients with newly diagnosed chronic myeloid leukemia (CML), is being discontinued due to adverse events.
All trials of ponatinib were placed on partial clinical hold on October 9, after follow-up data from the phase 2 PACE trial revealed an increased incidence of arterial and venous thrombotic events.
That trial involved patients with CML or Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) who were resistant to or could not tolerate dasatinib or nilotinib.
The partial clinical hold meant that ponatinib’s makers, Ariad Pharmaceuticals, must pause new enrollment in all ponatinib trials, reduce the doses given to existing patients, and reconsider trial eligibility criteria.
Now, Ariad and the US Food and Drug Administration have agreed that the EPIC trial must be terminated.
“Our decision to stop the EPIC trial at this time is based on our current evaluation of the safety data in the trial since it was placed on partial clinical hold last week,” said Timothy P. Clackson, PhD, president of research and development and chief scientific officer at Ariad.
Patients in the EPIC trial are being removed from treatment and will be transferred to the care of their physicians. A total of 307 patients were enrolled.
The EPIC trial was a randomized, 2-arm, multicenter study comparing the efficacy of ponatinib with that of imatinib in adult patients with newly diagnosed CML in the chronic phase. The trial was being conducted at approximately 150 investigational sites in more than 20 countries.
Patients had to be at least 18 years of age and diagnosed with CML within 6 months prior to enrollment. Approximately 500 patients were to be randomized 1:1 to the standard dose of ponatinib (45 mg once daily) or imatinib (400 mg once daily).
Increasing the imatinib dose to 600 mg or 800 mg per day was permitted. The primary endpoint of the trial was major molecular response at 12 months of treatment.
Ponatinib is still commercially available in the US and European Union for patients with resistant or intolerant CML and Ph+ ALL. Ariad said it is working with health authorities to make appropriate changes to the product labeling to reflect the safety findings from the PACE trial.
For more information about the changes in ponatinib trials, visit www.clinicaltrials.gov, email inquiries to [email protected], or call the Ariad US toll-free number (855) 552-7423, the European Union toll-free number 800 00027423, or the international number +1 (617)-503-7423. ![]()
The phase 3 EPIC trial, an evaluation of ponatinib (Iclusig) in patients with newly diagnosed chronic myeloid leukemia (CML), is being discontinued due to adverse events.
All trials of ponatinib were placed on partial clinical hold on October 9, after follow-up data from the phase 2 PACE trial revealed an increased incidence of arterial and venous thrombotic events.
That trial involved patients with CML or Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) who were resistant to or could not tolerate dasatinib or nilotinib.
The partial clinical hold meant that ponatinib’s makers, Ariad Pharmaceuticals, must pause new enrollment in all ponatinib trials, reduce the doses given to existing patients, and reconsider trial eligibility criteria.
Now, Ariad and the US Food and Drug Administration have agreed that the EPIC trial must be terminated.
“Our decision to stop the EPIC trial at this time is based on our current evaluation of the safety data in the trial since it was placed on partial clinical hold last week,” said Timothy P. Clackson, PhD, president of research and development and chief scientific officer at Ariad.
Patients in the EPIC trial are being removed from treatment and will be transferred to the care of their physicians. A total of 307 patients were enrolled.
The EPIC trial was a randomized, 2-arm, multicenter study comparing the efficacy of ponatinib with that of imatinib in adult patients with newly diagnosed CML in the chronic phase. The trial was being conducted at approximately 150 investigational sites in more than 20 countries.
Patients had to be at least 18 years of age and diagnosed with CML within 6 months prior to enrollment. Approximately 500 patients were to be randomized 1:1 to the standard dose of ponatinib (45 mg once daily) or imatinib (400 mg once daily).
Increasing the imatinib dose to 600 mg or 800 mg per day was permitted. The primary endpoint of the trial was major molecular response at 12 months of treatment.
Ponatinib is still commercially available in the US and European Union for patients with resistant or intolerant CML and Ph+ ALL. Ariad said it is working with health authorities to make appropriate changes to the product labeling to reflect the safety findings from the PACE trial.
For more information about the changes in ponatinib trials, visit www.clinicaltrials.gov, email inquiries to [email protected], or call the Ariad US toll-free number (855) 552-7423, the European Union toll-free number 800 00027423, or the international number +1 (617)-503-7423. ![]()
The phase 3 EPIC trial, an evaluation of ponatinib (Iclusig) in patients with newly diagnosed chronic myeloid leukemia (CML), is being discontinued due to adverse events.
All trials of ponatinib were placed on partial clinical hold on October 9, after follow-up data from the phase 2 PACE trial revealed an increased incidence of arterial and venous thrombotic events.
That trial involved patients with CML or Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) who were resistant to or could not tolerate dasatinib or nilotinib.
The partial clinical hold meant that ponatinib’s makers, Ariad Pharmaceuticals, must pause new enrollment in all ponatinib trials, reduce the doses given to existing patients, and reconsider trial eligibility criteria.
Now, Ariad and the US Food and Drug Administration have agreed that the EPIC trial must be terminated.
“Our decision to stop the EPIC trial at this time is based on our current evaluation of the safety data in the trial since it was placed on partial clinical hold last week,” said Timothy P. Clackson, PhD, president of research and development and chief scientific officer at Ariad.
Patients in the EPIC trial are being removed from treatment and will be transferred to the care of their physicians. A total of 307 patients were enrolled.
The EPIC trial was a randomized, 2-arm, multicenter study comparing the efficacy of ponatinib with that of imatinib in adult patients with newly diagnosed CML in the chronic phase. The trial was being conducted at approximately 150 investigational sites in more than 20 countries.
Patients had to be at least 18 years of age and diagnosed with CML within 6 months prior to enrollment. Approximately 500 patients were to be randomized 1:1 to the standard dose of ponatinib (45 mg once daily) or imatinib (400 mg once daily).
Increasing the imatinib dose to 600 mg or 800 mg per day was permitted. The primary endpoint of the trial was major molecular response at 12 months of treatment.
Ponatinib is still commercially available in the US and European Union for patients with resistant or intolerant CML and Ph+ ALL. Ariad said it is working with health authorities to make appropriate changes to the product labeling to reflect the safety findings from the PACE trial.
For more information about the changes in ponatinib trials, visit www.clinicaltrials.gov, email inquiries to [email protected], or call the Ariad US toll-free number (855) 552-7423, the European Union toll-free number 800 00027423, or the international number +1 (617)-503-7423. ![]()
The essential role of family in treating bipolar disorder
Kevin was doing very well in law school, until he showed up at his professor’s house in the middle of the night. Normally a thoughtful, quiet, introverted young man, Kevin was hardly recognizable to his professor, who found him outside yelling loudly and demanding to speak about an underground conspiracy he believed he had uncovered. He had always been a good student, and his family was very proud of his accomplishments up until now. At the age of 24, Kevin’s first manic episode was triggered by late nights studying for his law school exams and marijuana use to cope with stress.
Police responded to noise complaints, and Kevin was hospitalized. The manic episode resolved surprisingly quickly in the absence of marijuana use and with the help of an atypical antipsychotic. The patient’s intelligence and articulate lawyer-in-training charm made his inpatient doctors hard pressed to justify an extended hospital stay, and he was discharged 3 days later with a prescription and instructions for follow-up. He promptly discarded both.
When his next manic episode arose, Kevin disappeared for 2 weeks, and after fearing the worst, Kevin’s family was relieved to receive a call from Kevin’s aunt, who lived across the country and had just found him at her doorstep. This time, without the involvement of law enforcement, there seemed to be no way for Kevin’s mother, father, older sister, and aunt to persuade Kevin to enter the hospital or to take medications. Kevin’s aunt accompanied him on a plane home, and in the face of Kevin’s unwillingness to enter treatment alone, they decided to enter treatment as a family.
Predictors of episodes
The strongest predictors of future episodes and poor outcome in patients with bipolar disorder are a greater number of previous episodes, shorter intervals between episodes, a history of psychosis, a history of anxiety, persistence of affective symptoms and episodes, and stressful life events. Some evidence has suggested that poor job functioning, lack of social support, increased expressed emotion in the family, and introverted or obsessional personality traits all might predict poor outcome in bipolar disorder (J. Psychiatr. Pract. 2006;12:269-82).
An overwhelmingly emotional home environment can make a large contribution to relapse. Multiple studies have shown that a high level of "expressed emotion" (characterized by overinvolvement and excessive criticism) predicts patient relapse independent of medication compliance, baseline symptoms, and demographics (Arch. Gen. Psychiatry 1988;45:225-31)
Because bipolar disorder is an unpredictable, potentially destructive illness, it is important to grab any factors that we and our patients might have control over and do our best to modify them positively. With this in mind, the Family Focused Treatment (FFT) model was developed, with the philosophy that by keeping patients well informed about the facts and realities of the disorder and working on the communication and coping mechanisms operating within the family, relapse prevention and emotional stability will be better maintained. In this way, the predictive factors of stressful life events, poor social support, and family-expressed emotion can be modified. FFT is a time limited (usually 12 sessions), highly effective treatment modality.
The principles of FFT were adapted into an ongoing-treatment model that can be implemented in a community setting, termed Family Inclusive Treatment (FIT) and used by the Family Center for Bipolar in New York City, for example. FIT consists of an engagement period at the initiation of treatment, focused on psychoeducation and relapse prevention planning. FIT is unique in that every patient is required to sign a release of information giving permission for full, open communication at all times between the patient’s clinician and a treatment partner of their choosing.
After the initial engagement period, there are quarterly family visits to supplement regular individual treatment. Other modalities such as individual therapy, pharmacotherapy, and group therapy are used according to the clinician’s judgment.
This form of treatment is innovative in that it treats bipolar illness just like any other chronic illness. It promotes open communication between families of patients with bipolar disorder and the patients themselves with regard to symptoms and medications. In this way patients are not isolated from their families; they can talk openly with one another and their clinician as they would do if somebody in the family had Alzheimer’s disease or diabetes.
It has been reported that up to 46% of the caregivers of patients with bipolar disorder report depression, and up to 32.4% report use of mental health services. These symptoms tend to be dependent on the nature of the caregiving relationship, suggesting that specialized interventions addressing the psychiatric needs of bipolar families might result in improved outcomes for both patients and their family members, in addition to decreases in health care costs (J. Affect. Disord. 2010;121:10-21).
Together with therapy and medication management, clinicians working in the FIT model strive to create an environment that minimizes, as much as possible, the impact of bipolar disorder on the affected individuals and their close loved ones.
Many studies have confirmed the efficacy of various psychosocial treatments for bipolar disorder (J. Consult. Clin. Psychol. 2003;7:482-92; J. Clin. Psychiatry 2006;67 [suppl. 11]:28-33; J. Affect. Disord. 2007;98:11-27), and there has been a push for the integration of psychosocial treatment with pharmacotherapy, as the latter is less often sufficient on its own in preventing relapse.
Patient, family begin journey
Kevin and his family entered into family treatment. They started off with the psychoeducation portion of the treatment, and many of the myths and misinformation that they had held about bipolar disorder were dispelled. Even Kevin was able to engage in the information exchange, which he initially approached from an academic, impersonal vantage point. The communication skills phase proved more problematic as it became more personal, but still, the focus was on the family’s communication and not on Kevin as a psychiatric patient, so he responded well.
It was uncovered that Kevin’s father has always been highly critical, and Kevin’s mother tends to overprotect her children to compensate. They were taught new skills to express their feelings toward one another, and especially toward Kevin, in more productive and positive ways. In addition, they got a chance to practice those skills in subsequent sessions.
The modules continued in this vein until the family portion of treatment had completed. By this time, Kevin had developed a good rapport with his clinician, and he continued treatment despite his persistent reservations about accepting his illness. The family environment improved, and though Kevin was only sporadically compliant with his medication, the reduced stress at home and improved coping skills drove him less often to use marijuana for "self-medication," which decreased his manic episodes.
Kevin’s family periodically rejoined him in treatment sessions at predefined intervals, to check in and assess his and their progress. They were comfortable speaking with Kevin’s doctor and would call when they noticed any of the warning signs that they had collaboratively determined as markers of upcoming mania. In this way, they were all effective at keeping Kevin’s moods stable and keeping him out of the hospital.
The psychiatrist in routine practice might neither follow a manualized algorithm for family treatment nor have the time or resources at her disposal to provide a full "curriculum." Still, she can have the same success in engaging a family in understanding their loved one’s illness and contributing to the family member’s stability.
Objectives for family-focused treatment
The following objectives are adapted from "Bipolar Disorder: A Family-Focused Treatment Approach," 2nd ed. (New York: The Guilford Press, 2010):
• Encourage the patient and the family to admit that there is a vulnerability to future episodes by educating them about the natural course, progression, and chronic nature of bipolar disorder.
• Enable the patient and the family to recognize that medications are important for controlling symptoms. Provide concrete evidence for the importance and efficacy of medications and the risks of discontinuation. Explore reasons for resisting medications, including fears about becoming dependent.
• Help the patient and the family see the differences between the patient’s personality and his/her illness. Make a list of the patient’s positive attributes and a separate list of warning signs of mania. Frequently reinforce the distinction between the two.
• Assist the patient and the family in dealing with stressors that might cause a recurrence and help them rebuild family relationship ruptures after an episode. Suggest methods for positive, constructive communication such as active listening (nodding, making eye contact, paraphrasing, asking relevant questions) and expressing positive feelings toward a family member related to a specific example of a behavior.
Dr. Heru is with the department of psychiatry at the University of Colorado at Denver, Aurora. She is editor of the recently published book, "Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals" (New York: Routledge, 2013). Dr. Mednick is an attending psychiatrist at the Family Center for Bipolar at Beth Israel Medical Center in New York City.
Kevin was doing very well in law school, until he showed up at his professor’s house in the middle of the night. Normally a thoughtful, quiet, introverted young man, Kevin was hardly recognizable to his professor, who found him outside yelling loudly and demanding to speak about an underground conspiracy he believed he had uncovered. He had always been a good student, and his family was very proud of his accomplishments up until now. At the age of 24, Kevin’s first manic episode was triggered by late nights studying for his law school exams and marijuana use to cope with stress.
Police responded to noise complaints, and Kevin was hospitalized. The manic episode resolved surprisingly quickly in the absence of marijuana use and with the help of an atypical antipsychotic. The patient’s intelligence and articulate lawyer-in-training charm made his inpatient doctors hard pressed to justify an extended hospital stay, and he was discharged 3 days later with a prescription and instructions for follow-up. He promptly discarded both.
When his next manic episode arose, Kevin disappeared for 2 weeks, and after fearing the worst, Kevin’s family was relieved to receive a call from Kevin’s aunt, who lived across the country and had just found him at her doorstep. This time, without the involvement of law enforcement, there seemed to be no way for Kevin’s mother, father, older sister, and aunt to persuade Kevin to enter the hospital or to take medications. Kevin’s aunt accompanied him on a plane home, and in the face of Kevin’s unwillingness to enter treatment alone, they decided to enter treatment as a family.
Predictors of episodes
The strongest predictors of future episodes and poor outcome in patients with bipolar disorder are a greater number of previous episodes, shorter intervals between episodes, a history of psychosis, a history of anxiety, persistence of affective symptoms and episodes, and stressful life events. Some evidence has suggested that poor job functioning, lack of social support, increased expressed emotion in the family, and introverted or obsessional personality traits all might predict poor outcome in bipolar disorder (J. Psychiatr. Pract. 2006;12:269-82).
An overwhelmingly emotional home environment can make a large contribution to relapse. Multiple studies have shown that a high level of "expressed emotion" (characterized by overinvolvement and excessive criticism) predicts patient relapse independent of medication compliance, baseline symptoms, and demographics (Arch. Gen. Psychiatry 1988;45:225-31)
Because bipolar disorder is an unpredictable, potentially destructive illness, it is important to grab any factors that we and our patients might have control over and do our best to modify them positively. With this in mind, the Family Focused Treatment (FFT) model was developed, with the philosophy that by keeping patients well informed about the facts and realities of the disorder and working on the communication and coping mechanisms operating within the family, relapse prevention and emotional stability will be better maintained. In this way, the predictive factors of stressful life events, poor social support, and family-expressed emotion can be modified. FFT is a time limited (usually 12 sessions), highly effective treatment modality.
The principles of FFT were adapted into an ongoing-treatment model that can be implemented in a community setting, termed Family Inclusive Treatment (FIT) and used by the Family Center for Bipolar in New York City, for example. FIT consists of an engagement period at the initiation of treatment, focused on psychoeducation and relapse prevention planning. FIT is unique in that every patient is required to sign a release of information giving permission for full, open communication at all times between the patient’s clinician and a treatment partner of their choosing.
After the initial engagement period, there are quarterly family visits to supplement regular individual treatment. Other modalities such as individual therapy, pharmacotherapy, and group therapy are used according to the clinician’s judgment.
This form of treatment is innovative in that it treats bipolar illness just like any other chronic illness. It promotes open communication between families of patients with bipolar disorder and the patients themselves with regard to symptoms and medications. In this way patients are not isolated from their families; they can talk openly with one another and their clinician as they would do if somebody in the family had Alzheimer’s disease or diabetes.
It has been reported that up to 46% of the caregivers of patients with bipolar disorder report depression, and up to 32.4% report use of mental health services. These symptoms tend to be dependent on the nature of the caregiving relationship, suggesting that specialized interventions addressing the psychiatric needs of bipolar families might result in improved outcomes for both patients and their family members, in addition to decreases in health care costs (J. Affect. Disord. 2010;121:10-21).
Together with therapy and medication management, clinicians working in the FIT model strive to create an environment that minimizes, as much as possible, the impact of bipolar disorder on the affected individuals and their close loved ones.
Many studies have confirmed the efficacy of various psychosocial treatments for bipolar disorder (J. Consult. Clin. Psychol. 2003;7:482-92; J. Clin. Psychiatry 2006;67 [suppl. 11]:28-33; J. Affect. Disord. 2007;98:11-27), and there has been a push for the integration of psychosocial treatment with pharmacotherapy, as the latter is less often sufficient on its own in preventing relapse.
Patient, family begin journey
Kevin and his family entered into family treatment. They started off with the psychoeducation portion of the treatment, and many of the myths and misinformation that they had held about bipolar disorder were dispelled. Even Kevin was able to engage in the information exchange, which he initially approached from an academic, impersonal vantage point. The communication skills phase proved more problematic as it became more personal, but still, the focus was on the family’s communication and not on Kevin as a psychiatric patient, so he responded well.
It was uncovered that Kevin’s father has always been highly critical, and Kevin’s mother tends to overprotect her children to compensate. They were taught new skills to express their feelings toward one another, and especially toward Kevin, in more productive and positive ways. In addition, they got a chance to practice those skills in subsequent sessions.
The modules continued in this vein until the family portion of treatment had completed. By this time, Kevin had developed a good rapport with his clinician, and he continued treatment despite his persistent reservations about accepting his illness. The family environment improved, and though Kevin was only sporadically compliant with his medication, the reduced stress at home and improved coping skills drove him less often to use marijuana for "self-medication," which decreased his manic episodes.
Kevin’s family periodically rejoined him in treatment sessions at predefined intervals, to check in and assess his and their progress. They were comfortable speaking with Kevin’s doctor and would call when they noticed any of the warning signs that they had collaboratively determined as markers of upcoming mania. In this way, they were all effective at keeping Kevin’s moods stable and keeping him out of the hospital.
The psychiatrist in routine practice might neither follow a manualized algorithm for family treatment nor have the time or resources at her disposal to provide a full "curriculum." Still, she can have the same success in engaging a family in understanding their loved one’s illness and contributing to the family member’s stability.
Objectives for family-focused treatment
The following objectives are adapted from "Bipolar Disorder: A Family-Focused Treatment Approach," 2nd ed. (New York: The Guilford Press, 2010):
• Encourage the patient and the family to admit that there is a vulnerability to future episodes by educating them about the natural course, progression, and chronic nature of bipolar disorder.
• Enable the patient and the family to recognize that medications are important for controlling symptoms. Provide concrete evidence for the importance and efficacy of medications and the risks of discontinuation. Explore reasons for resisting medications, including fears about becoming dependent.
• Help the patient and the family see the differences between the patient’s personality and his/her illness. Make a list of the patient’s positive attributes and a separate list of warning signs of mania. Frequently reinforce the distinction between the two.
• Assist the patient and the family in dealing with stressors that might cause a recurrence and help them rebuild family relationship ruptures after an episode. Suggest methods for positive, constructive communication such as active listening (nodding, making eye contact, paraphrasing, asking relevant questions) and expressing positive feelings toward a family member related to a specific example of a behavior.
Dr. Heru is with the department of psychiatry at the University of Colorado at Denver, Aurora. She is editor of the recently published book, "Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals" (New York: Routledge, 2013). Dr. Mednick is an attending psychiatrist at the Family Center for Bipolar at Beth Israel Medical Center in New York City.
Kevin was doing very well in law school, until he showed up at his professor’s house in the middle of the night. Normally a thoughtful, quiet, introverted young man, Kevin was hardly recognizable to his professor, who found him outside yelling loudly and demanding to speak about an underground conspiracy he believed he had uncovered. He had always been a good student, and his family was very proud of his accomplishments up until now. At the age of 24, Kevin’s first manic episode was triggered by late nights studying for his law school exams and marijuana use to cope with stress.
Police responded to noise complaints, and Kevin was hospitalized. The manic episode resolved surprisingly quickly in the absence of marijuana use and with the help of an atypical antipsychotic. The patient’s intelligence and articulate lawyer-in-training charm made his inpatient doctors hard pressed to justify an extended hospital stay, and he was discharged 3 days later with a prescription and instructions for follow-up. He promptly discarded both.
When his next manic episode arose, Kevin disappeared for 2 weeks, and after fearing the worst, Kevin’s family was relieved to receive a call from Kevin’s aunt, who lived across the country and had just found him at her doorstep. This time, without the involvement of law enforcement, there seemed to be no way for Kevin’s mother, father, older sister, and aunt to persuade Kevin to enter the hospital or to take medications. Kevin’s aunt accompanied him on a plane home, and in the face of Kevin’s unwillingness to enter treatment alone, they decided to enter treatment as a family.
Predictors of episodes
The strongest predictors of future episodes and poor outcome in patients with bipolar disorder are a greater number of previous episodes, shorter intervals between episodes, a history of psychosis, a history of anxiety, persistence of affective symptoms and episodes, and stressful life events. Some evidence has suggested that poor job functioning, lack of social support, increased expressed emotion in the family, and introverted or obsessional personality traits all might predict poor outcome in bipolar disorder (J. Psychiatr. Pract. 2006;12:269-82).
An overwhelmingly emotional home environment can make a large contribution to relapse. Multiple studies have shown that a high level of "expressed emotion" (characterized by overinvolvement and excessive criticism) predicts patient relapse independent of medication compliance, baseline symptoms, and demographics (Arch. Gen. Psychiatry 1988;45:225-31)
Because bipolar disorder is an unpredictable, potentially destructive illness, it is important to grab any factors that we and our patients might have control over and do our best to modify them positively. With this in mind, the Family Focused Treatment (FFT) model was developed, with the philosophy that by keeping patients well informed about the facts and realities of the disorder and working on the communication and coping mechanisms operating within the family, relapse prevention and emotional stability will be better maintained. In this way, the predictive factors of stressful life events, poor social support, and family-expressed emotion can be modified. FFT is a time limited (usually 12 sessions), highly effective treatment modality.
The principles of FFT were adapted into an ongoing-treatment model that can be implemented in a community setting, termed Family Inclusive Treatment (FIT) and used by the Family Center for Bipolar in New York City, for example. FIT consists of an engagement period at the initiation of treatment, focused on psychoeducation and relapse prevention planning. FIT is unique in that every patient is required to sign a release of information giving permission for full, open communication at all times between the patient’s clinician and a treatment partner of their choosing.
After the initial engagement period, there are quarterly family visits to supplement regular individual treatment. Other modalities such as individual therapy, pharmacotherapy, and group therapy are used according to the clinician’s judgment.
This form of treatment is innovative in that it treats bipolar illness just like any other chronic illness. It promotes open communication between families of patients with bipolar disorder and the patients themselves with regard to symptoms and medications. In this way patients are not isolated from their families; they can talk openly with one another and their clinician as they would do if somebody in the family had Alzheimer’s disease or diabetes.
It has been reported that up to 46% of the caregivers of patients with bipolar disorder report depression, and up to 32.4% report use of mental health services. These symptoms tend to be dependent on the nature of the caregiving relationship, suggesting that specialized interventions addressing the psychiatric needs of bipolar families might result in improved outcomes for both patients and their family members, in addition to decreases in health care costs (J. Affect. Disord. 2010;121:10-21).
Together with therapy and medication management, clinicians working in the FIT model strive to create an environment that minimizes, as much as possible, the impact of bipolar disorder on the affected individuals and their close loved ones.
Many studies have confirmed the efficacy of various psychosocial treatments for bipolar disorder (J. Consult. Clin. Psychol. 2003;7:482-92; J. Clin. Psychiatry 2006;67 [suppl. 11]:28-33; J. Affect. Disord. 2007;98:11-27), and there has been a push for the integration of psychosocial treatment with pharmacotherapy, as the latter is less often sufficient on its own in preventing relapse.
Patient, family begin journey
Kevin and his family entered into family treatment. They started off with the psychoeducation portion of the treatment, and many of the myths and misinformation that they had held about bipolar disorder were dispelled. Even Kevin was able to engage in the information exchange, which he initially approached from an academic, impersonal vantage point. The communication skills phase proved more problematic as it became more personal, but still, the focus was on the family’s communication and not on Kevin as a psychiatric patient, so he responded well.
It was uncovered that Kevin’s father has always been highly critical, and Kevin’s mother tends to overprotect her children to compensate. They were taught new skills to express their feelings toward one another, and especially toward Kevin, in more productive and positive ways. In addition, they got a chance to practice those skills in subsequent sessions.
The modules continued in this vein until the family portion of treatment had completed. By this time, Kevin had developed a good rapport with his clinician, and he continued treatment despite his persistent reservations about accepting his illness. The family environment improved, and though Kevin was only sporadically compliant with his medication, the reduced stress at home and improved coping skills drove him less often to use marijuana for "self-medication," which decreased his manic episodes.
Kevin’s family periodically rejoined him in treatment sessions at predefined intervals, to check in and assess his and their progress. They were comfortable speaking with Kevin’s doctor and would call when they noticed any of the warning signs that they had collaboratively determined as markers of upcoming mania. In this way, they were all effective at keeping Kevin’s moods stable and keeping him out of the hospital.
The psychiatrist in routine practice might neither follow a manualized algorithm for family treatment nor have the time or resources at her disposal to provide a full "curriculum." Still, she can have the same success in engaging a family in understanding their loved one’s illness and contributing to the family member’s stability.
Objectives for family-focused treatment
The following objectives are adapted from "Bipolar Disorder: A Family-Focused Treatment Approach," 2nd ed. (New York: The Guilford Press, 2010):
• Encourage the patient and the family to admit that there is a vulnerability to future episodes by educating them about the natural course, progression, and chronic nature of bipolar disorder.
• Enable the patient and the family to recognize that medications are important for controlling symptoms. Provide concrete evidence for the importance and efficacy of medications and the risks of discontinuation. Explore reasons for resisting medications, including fears about becoming dependent.
• Help the patient and the family see the differences between the patient’s personality and his/her illness. Make a list of the patient’s positive attributes and a separate list of warning signs of mania. Frequently reinforce the distinction between the two.
• Assist the patient and the family in dealing with stressors that might cause a recurrence and help them rebuild family relationship ruptures after an episode. Suggest methods for positive, constructive communication such as active listening (nodding, making eye contact, paraphrasing, asking relevant questions) and expressing positive feelings toward a family member related to a specific example of a behavior.
Dr. Heru is with the department of psychiatry at the University of Colorado at Denver, Aurora. She is editor of the recently published book, "Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals" (New York: Routledge, 2013). Dr. Mednick is an attending psychiatrist at the Family Center for Bipolar at Beth Israel Medical Center in New York City.
Ridaforolimus offers moderate maintenance benefit in advanced sarcoma
The investigational agent ridaforolimus reduced the risk for progression and death by 28% in previously treated patients with advanced sarcoma in a large phase III study.
Results of the SUCCEED (Sarcoma Multicenter Clinical Evaluation of the Efficacy of Ridaforolimus) study showed that there was a small, but significant median progression-free survival (PFS) gain of 3.3 weeks vs. placebo (17.7 vs. 14.6 weeks, hazard ratio, 0.72; P less than .001) by independent review.
"The absolute magnitude of this statistically significant improvement in disease control was small because of the more rapid than expected progression in all patients in this study," said Dr. George D. Demetri of the Dana-Farber Cancer Institute and Harvard Medical School in Boston, and his associates (J. Clin. Oncol. 2013;31:2485-92).
"This finding confirms the aggressive nature of advanced sarcomas and underscores the medical need of these patients to improve with available therapy."
"To our knowledge, this is the first study demonstrating a statistically significant, although clinically small, impact on maintenance treatment on tumor progression in patients with sarcomas," the researchers said. "Future studies will build on these results in an effort to improve the outcomes of this rapidly progressive life-threatening disease."
The study was designed with the premise that the median time to progression would be around 6-9 months in the control arm. In fact, it was less than 4 months, and there was a 6-month PFS rate of 23% and average tumor growth of 10% in target lesions.
"This finding confirms the aggressive nature of advanced sarcomas and underscores the medical need of these patients to improve with available therapy," the researchers said.
The SUCCEED trial was an international, randomized, double blind, placebo-controlled trial of 711 patients with advanced soft tissue or bone sarcomas who had achieved stable disease or a continued partial or complete response to immediately preceding cytotoxic chemotherapy Patients were randomized to receive either the mammalian target of rapamycin inhibitor ridaforolimus (n = 347), given at a dose of four 10 mg tablets/day for 5 days every week, or matching placebo tablets (n = 364).
Baseline patient characteristics were similar between the two study groups. The mean age of patients was 52 years in the ridaforolimus arm and 50 years in the placebo arm. The majority (89.3%) of patients had soft tissue sarcomas, with the remainder having bone sarcomas. Almost 75% of tumors were high grade, 66.6% of patients had metastatic disease involving the lung, 14.4% involving the liver, and 11.5% involving the bone. Around 40% of patients had received two or more prior cytotoxic chemotherapy regimens, and the best response to treatment had been stable disease in 74.4%, a partial response in 18.4%, and a complete response in 5.5%.
Investigator-assessed PFS showed slightly better median survival rates of 22.4 vs. 14.7 weeks (HR, 0.69; P less than .001). There was mean decrease in target lesion size of 1.3% in the ridaforolimus arm vs. a 10.3% increase in size in the placebo arm (P less than .001).
No overall survival benefit was observed in the trial. Based on 478 death events and a minimum of 2 years’ follow-up, median overall survival was 90.6 weeks vs. 85.3 weeks (HR, 0.93; P = .46). Dr. Demetri and his coworkers point out, however, that the study was not powered to determine an overall survival benefit.
Adverse effects of any grade occurred in 100% of ridaforolimus-treated and 93.6% of placebo-treated patients. Adverse effects of note that occurred to a greater extent in the active treatment arm included stomatitis, thrombocytopenia, infections, rash, hypertriglyceridemia, and anemia. Grade 3 or higher adverse events occurred in 64.1% of patients treated with ridaforolimus and 25.6% of those in the placebo arm.
This was an unselected patient population but no potential biomarkers or predictors for response have been identified. As ridaforolimus improved, the best target lesion response and the clinical benefit rate, it might contribute to tumor growth control, the researchers speculated. They therefore suggested that it might be better suited as an element of combination treatment with other tumor-signaling inhibitors.
The SUCCEED study was funded by Merck, Sharp, and Dohme. Dr. Demetri has received consultancy fees and research funding from Merck and ARIAD Pharmaceuticals. Several of his coauthors had also received honoraria from Merck or were employees of the company.
The investigational agent ridaforolimus reduced the risk for progression and death by 28% in previously treated patients with advanced sarcoma in a large phase III study.
Results of the SUCCEED (Sarcoma Multicenter Clinical Evaluation of the Efficacy of Ridaforolimus) study showed that there was a small, but significant median progression-free survival (PFS) gain of 3.3 weeks vs. placebo (17.7 vs. 14.6 weeks, hazard ratio, 0.72; P less than .001) by independent review.
"The absolute magnitude of this statistically significant improvement in disease control was small because of the more rapid than expected progression in all patients in this study," said Dr. George D. Demetri of the Dana-Farber Cancer Institute and Harvard Medical School in Boston, and his associates (J. Clin. Oncol. 2013;31:2485-92).
"This finding confirms the aggressive nature of advanced sarcomas and underscores the medical need of these patients to improve with available therapy."
"To our knowledge, this is the first study demonstrating a statistically significant, although clinically small, impact on maintenance treatment on tumor progression in patients with sarcomas," the researchers said. "Future studies will build on these results in an effort to improve the outcomes of this rapidly progressive life-threatening disease."
The study was designed with the premise that the median time to progression would be around 6-9 months in the control arm. In fact, it was less than 4 months, and there was a 6-month PFS rate of 23% and average tumor growth of 10% in target lesions.
"This finding confirms the aggressive nature of advanced sarcomas and underscores the medical need of these patients to improve with available therapy," the researchers said.
The SUCCEED trial was an international, randomized, double blind, placebo-controlled trial of 711 patients with advanced soft tissue or bone sarcomas who had achieved stable disease or a continued partial or complete response to immediately preceding cytotoxic chemotherapy Patients were randomized to receive either the mammalian target of rapamycin inhibitor ridaforolimus (n = 347), given at a dose of four 10 mg tablets/day for 5 days every week, or matching placebo tablets (n = 364).
Baseline patient characteristics were similar between the two study groups. The mean age of patients was 52 years in the ridaforolimus arm and 50 years in the placebo arm. The majority (89.3%) of patients had soft tissue sarcomas, with the remainder having bone sarcomas. Almost 75% of tumors were high grade, 66.6% of patients had metastatic disease involving the lung, 14.4% involving the liver, and 11.5% involving the bone. Around 40% of patients had received two or more prior cytotoxic chemotherapy regimens, and the best response to treatment had been stable disease in 74.4%, a partial response in 18.4%, and a complete response in 5.5%.
Investigator-assessed PFS showed slightly better median survival rates of 22.4 vs. 14.7 weeks (HR, 0.69; P less than .001). There was mean decrease in target lesion size of 1.3% in the ridaforolimus arm vs. a 10.3% increase in size in the placebo arm (P less than .001).
No overall survival benefit was observed in the trial. Based on 478 death events and a minimum of 2 years’ follow-up, median overall survival was 90.6 weeks vs. 85.3 weeks (HR, 0.93; P = .46). Dr. Demetri and his coworkers point out, however, that the study was not powered to determine an overall survival benefit.
Adverse effects of any grade occurred in 100% of ridaforolimus-treated and 93.6% of placebo-treated patients. Adverse effects of note that occurred to a greater extent in the active treatment arm included stomatitis, thrombocytopenia, infections, rash, hypertriglyceridemia, and anemia. Grade 3 or higher adverse events occurred in 64.1% of patients treated with ridaforolimus and 25.6% of those in the placebo arm.
This was an unselected patient population but no potential biomarkers or predictors for response have been identified. As ridaforolimus improved, the best target lesion response and the clinical benefit rate, it might contribute to tumor growth control, the researchers speculated. They therefore suggested that it might be better suited as an element of combination treatment with other tumor-signaling inhibitors.
The SUCCEED study was funded by Merck, Sharp, and Dohme. Dr. Demetri has received consultancy fees and research funding from Merck and ARIAD Pharmaceuticals. Several of his coauthors had also received honoraria from Merck or were employees of the company.
The investigational agent ridaforolimus reduced the risk for progression and death by 28% in previously treated patients with advanced sarcoma in a large phase III study.
Results of the SUCCEED (Sarcoma Multicenter Clinical Evaluation of the Efficacy of Ridaforolimus) study showed that there was a small, but significant median progression-free survival (PFS) gain of 3.3 weeks vs. placebo (17.7 vs. 14.6 weeks, hazard ratio, 0.72; P less than .001) by independent review.
"The absolute magnitude of this statistically significant improvement in disease control was small because of the more rapid than expected progression in all patients in this study," said Dr. George D. Demetri of the Dana-Farber Cancer Institute and Harvard Medical School in Boston, and his associates (J. Clin. Oncol. 2013;31:2485-92).
"This finding confirms the aggressive nature of advanced sarcomas and underscores the medical need of these patients to improve with available therapy."
"To our knowledge, this is the first study demonstrating a statistically significant, although clinically small, impact on maintenance treatment on tumor progression in patients with sarcomas," the researchers said. "Future studies will build on these results in an effort to improve the outcomes of this rapidly progressive life-threatening disease."
The study was designed with the premise that the median time to progression would be around 6-9 months in the control arm. In fact, it was less than 4 months, and there was a 6-month PFS rate of 23% and average tumor growth of 10% in target lesions.
"This finding confirms the aggressive nature of advanced sarcomas and underscores the medical need of these patients to improve with available therapy," the researchers said.
The SUCCEED trial was an international, randomized, double blind, placebo-controlled trial of 711 patients with advanced soft tissue or bone sarcomas who had achieved stable disease or a continued partial or complete response to immediately preceding cytotoxic chemotherapy Patients were randomized to receive either the mammalian target of rapamycin inhibitor ridaforolimus (n = 347), given at a dose of four 10 mg tablets/day for 5 days every week, or matching placebo tablets (n = 364).
Baseline patient characteristics were similar between the two study groups. The mean age of patients was 52 years in the ridaforolimus arm and 50 years in the placebo arm. The majority (89.3%) of patients had soft tissue sarcomas, with the remainder having bone sarcomas. Almost 75% of tumors were high grade, 66.6% of patients had metastatic disease involving the lung, 14.4% involving the liver, and 11.5% involving the bone. Around 40% of patients had received two or more prior cytotoxic chemotherapy regimens, and the best response to treatment had been stable disease in 74.4%, a partial response in 18.4%, and a complete response in 5.5%.
Investigator-assessed PFS showed slightly better median survival rates of 22.4 vs. 14.7 weeks (HR, 0.69; P less than .001). There was mean decrease in target lesion size of 1.3% in the ridaforolimus arm vs. a 10.3% increase in size in the placebo arm (P less than .001).
No overall survival benefit was observed in the trial. Based on 478 death events and a minimum of 2 years’ follow-up, median overall survival was 90.6 weeks vs. 85.3 weeks (HR, 0.93; P = .46). Dr. Demetri and his coworkers point out, however, that the study was not powered to determine an overall survival benefit.
Adverse effects of any grade occurred in 100% of ridaforolimus-treated and 93.6% of placebo-treated patients. Adverse effects of note that occurred to a greater extent in the active treatment arm included stomatitis, thrombocytopenia, infections, rash, hypertriglyceridemia, and anemia. Grade 3 or higher adverse events occurred in 64.1% of patients treated with ridaforolimus and 25.6% of those in the placebo arm.
This was an unselected patient population but no potential biomarkers or predictors for response have been identified. As ridaforolimus improved, the best target lesion response and the clinical benefit rate, it might contribute to tumor growth control, the researchers speculated. They therefore suggested that it might be better suited as an element of combination treatment with other tumor-signaling inhibitors.
The SUCCEED study was funded by Merck, Sharp, and Dohme. Dr. Demetri has received consultancy fees and research funding from Merck and ARIAD Pharmaceuticals. Several of his coauthors had also received honoraria from Merck or were employees of the company.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Major finding: Mean PFS was 17.7 weeks vs. 14.6 weeks, comparing ridaforolimus to placebo (HR, 0.72; P less than .001), representing a 28% reduction in the risk of progression or death.
Data source: The SUCCEED international, phase III study involving 711 patients with advanced soft tissue or bone sarcoma who responded to prior chemotherapy.
Disclosures: The SUCCEED study was funded by Merck, Sharp, and Dohme. Dr. Demetri has received consultancy fees and research funding from Merck and ARIAD Pharmaceuticals. Several of his coauthors had also received honoraria from Merck or were employees of the company.
NICE again rejects pixantrone for NHL
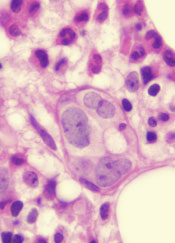
The UK’s National Institute for Health and Care Excellence (NICE) has re-examined its draft guidance for pixantrone (Pixuvri) but come to the same conclusion as before.
The organization is still not recommending pixantrone monotherapy to treat multiply relapsed or refractory B-cell non-Hodgkin lymphoma (NHL).
Of course, this recommendation may change, as this is not NICE’s final guidance on pixantrone.
For this second consultation on the draft guidance, an independent appraisal committee re-examined the clinical and cost-effectiveness of pixantrone.
This time, the committee took into consideration a patient access scheme submitted by pixantrone’s manufacturer, Cell Therapeutics. The scheme was designed to make the drug more cost-effective for the National Health Service (NHS).
“Unfortunately, the committee concluded that this scheme . . . does not overcome the uncertainties in the evidence for the drug’s clinical effectiveness over and above current treatments for this disease,” said NICE Chief Executive Sir Andrew Dillon.
In fact, the committee found the scheme did not make pixantrone cost-effective according to the accepted definition—costing £20,000 to £30,000 per quality-adjusted life year (QALY) gained.
Evaluating trial data
When considering the clinical effectiveness of pixantrone, the appraisal committee analyzed data from the EXTEND PIX301 trial, which was submitted by the manufacturer.
The trial enrolled adults with aggressive, de novo, or transformed NHL that had relapsed after 2 or more chemotherapy regimens, including at least 1 standard anthracycline-containing regimen with a response that lasted at least 24 weeks. Seventy patients were randomized to pixantrone, and 70 were randomized to a physician’s choice of single-agent comparators.
The committee pointed out a number of uncertainties associated with the trial. One was that it did not include the planned number of patients (which was 320), so it may not have been sufficiently powered to detect differences between the treatment arms.
Another concern was that the trial’s primary endpoint was complete or unconfirmed complete response, rather than overall survival or progression-free survival. In fact, there was a lack of statistically significant difference in overall survival between treatment arms. And other differences between the treatment arms were not always statistically significant.
These factors led the committee to conclude that there is insufficient evidence to suggest pixantrone is more clinically effective than treatments currently used in clinical practice.
Suitability for the UK
The appraisal committee also heard evidence from clinical experts and patient representatives. This information revealed differences in previous treatment between the PIX301 trial population and UK clinical practice.
Therefore, the committee said it could not determine the clinical effectiveness of pixantrone for a UK population.
In addition, there is doubt regarding the clinical benefit of pixantrone in patients who previously received rituximab. And this applies to virtually all patients with relapsed or refractory aggressive B-cell lymphoma in England and Wales, the committee noted.
(The European Medicines Agency’s conditional approval of pixantrone stipulated that an additional trial must confirm the clinical benefit of the drug in patients who have previously received rituximab.)
Calculating costs
The committee estimated the patient access scheme for pixantrone would most likely result in an incremental cost-effectiveness ratio of £30,700 per QALY gained. This is above the range normally considered to be cost-effective—usually £20,000 to £30,000 per QALY gained.
This factor, along with the lack of clinical effectiveness, prompted the committee to conclude that pixantrone would not be a cost-effective use of NHS resources.
According to Cell Therapeutics, pixantrone costs £553.50 per 20 mL vial. The recommended dosage of pixantrone is 50 mg/m2 on days 1, 8, and 15 of each 28-day cycle, for up to 6 cycles.
The estimated cost of a course of treatment is £19,926. This is based on the median length of treatment in the PIX301 trial—4 cycles, using an average of 3 vials per dose.
About the guidance
Individuals can comment on the pixantrone draft guidance via the NICE website. It is open until November 4, 2013.
This is the third version of the draft guidance published and the second consultation launched. The draft guidance was initially published for consultation in April 2013, followed by a final draft guidance in June 2013. But this document was withdrawn during the appeal stage because the manufacturer submitted the patient access scheme.
Until the final guidance is issued to the NHS, organizations should make decisions locally on the funding of specific treatments. Once NICE issues its final guidance on a technology, it replaces local recommendations. ![]()

The UK’s National Institute for Health and Care Excellence (NICE) has re-examined its draft guidance for pixantrone (Pixuvri) but come to the same conclusion as before.
The organization is still not recommending pixantrone monotherapy to treat multiply relapsed or refractory B-cell non-Hodgkin lymphoma (NHL).
Of course, this recommendation may change, as this is not NICE’s final guidance on pixantrone.
For this second consultation on the draft guidance, an independent appraisal committee re-examined the clinical and cost-effectiveness of pixantrone.
This time, the committee took into consideration a patient access scheme submitted by pixantrone’s manufacturer, Cell Therapeutics. The scheme was designed to make the drug more cost-effective for the National Health Service (NHS).
“Unfortunately, the committee concluded that this scheme . . . does not overcome the uncertainties in the evidence for the drug’s clinical effectiveness over and above current treatments for this disease,” said NICE Chief Executive Sir Andrew Dillon.
In fact, the committee found the scheme did not make pixantrone cost-effective according to the accepted definition—costing £20,000 to £30,000 per quality-adjusted life year (QALY) gained.
Evaluating trial data
When considering the clinical effectiveness of pixantrone, the appraisal committee analyzed data from the EXTEND PIX301 trial, which was submitted by the manufacturer.
The trial enrolled adults with aggressive, de novo, or transformed NHL that had relapsed after 2 or more chemotherapy regimens, including at least 1 standard anthracycline-containing regimen with a response that lasted at least 24 weeks. Seventy patients were randomized to pixantrone, and 70 were randomized to a physician’s choice of single-agent comparators.
The committee pointed out a number of uncertainties associated with the trial. One was that it did not include the planned number of patients (which was 320), so it may not have been sufficiently powered to detect differences between the treatment arms.
Another concern was that the trial’s primary endpoint was complete or unconfirmed complete response, rather than overall survival or progression-free survival. In fact, there was a lack of statistically significant difference in overall survival between treatment arms. And other differences between the treatment arms were not always statistically significant.
These factors led the committee to conclude that there is insufficient evidence to suggest pixantrone is more clinically effective than treatments currently used in clinical practice.
Suitability for the UK
The appraisal committee also heard evidence from clinical experts and patient representatives. This information revealed differences in previous treatment between the PIX301 trial population and UK clinical practice.
Therefore, the committee said it could not determine the clinical effectiveness of pixantrone for a UK population.
In addition, there is doubt regarding the clinical benefit of pixantrone in patients who previously received rituximab. And this applies to virtually all patients with relapsed or refractory aggressive B-cell lymphoma in England and Wales, the committee noted.
(The European Medicines Agency’s conditional approval of pixantrone stipulated that an additional trial must confirm the clinical benefit of the drug in patients who have previously received rituximab.)
Calculating costs
The committee estimated the patient access scheme for pixantrone would most likely result in an incremental cost-effectiveness ratio of £30,700 per QALY gained. This is above the range normally considered to be cost-effective—usually £20,000 to £30,000 per QALY gained.
This factor, along with the lack of clinical effectiveness, prompted the committee to conclude that pixantrone would not be a cost-effective use of NHS resources.
According to Cell Therapeutics, pixantrone costs £553.50 per 20 mL vial. The recommended dosage of pixantrone is 50 mg/m2 on days 1, 8, and 15 of each 28-day cycle, for up to 6 cycles.
The estimated cost of a course of treatment is £19,926. This is based on the median length of treatment in the PIX301 trial—4 cycles, using an average of 3 vials per dose.
About the guidance
Individuals can comment on the pixantrone draft guidance via the NICE website. It is open until November 4, 2013.
This is the third version of the draft guidance published and the second consultation launched. The draft guidance was initially published for consultation in April 2013, followed by a final draft guidance in June 2013. But this document was withdrawn during the appeal stage because the manufacturer submitted the patient access scheme.
Until the final guidance is issued to the NHS, organizations should make decisions locally on the funding of specific treatments. Once NICE issues its final guidance on a technology, it replaces local recommendations. ![]()

The UK’s National Institute for Health and Care Excellence (NICE) has re-examined its draft guidance for pixantrone (Pixuvri) but come to the same conclusion as before.
The organization is still not recommending pixantrone monotherapy to treat multiply relapsed or refractory B-cell non-Hodgkin lymphoma (NHL).
Of course, this recommendation may change, as this is not NICE’s final guidance on pixantrone.
For this second consultation on the draft guidance, an independent appraisal committee re-examined the clinical and cost-effectiveness of pixantrone.
This time, the committee took into consideration a patient access scheme submitted by pixantrone’s manufacturer, Cell Therapeutics. The scheme was designed to make the drug more cost-effective for the National Health Service (NHS).
“Unfortunately, the committee concluded that this scheme . . . does not overcome the uncertainties in the evidence for the drug’s clinical effectiveness over and above current treatments for this disease,” said NICE Chief Executive Sir Andrew Dillon.
In fact, the committee found the scheme did not make pixantrone cost-effective according to the accepted definition—costing £20,000 to £30,000 per quality-adjusted life year (QALY) gained.
Evaluating trial data
When considering the clinical effectiveness of pixantrone, the appraisal committee analyzed data from the EXTEND PIX301 trial, which was submitted by the manufacturer.
The trial enrolled adults with aggressive, de novo, or transformed NHL that had relapsed after 2 or more chemotherapy regimens, including at least 1 standard anthracycline-containing regimen with a response that lasted at least 24 weeks. Seventy patients were randomized to pixantrone, and 70 were randomized to a physician’s choice of single-agent comparators.
The committee pointed out a number of uncertainties associated with the trial. One was that it did not include the planned number of patients (which was 320), so it may not have been sufficiently powered to detect differences between the treatment arms.
Another concern was that the trial’s primary endpoint was complete or unconfirmed complete response, rather than overall survival or progression-free survival. In fact, there was a lack of statistically significant difference in overall survival between treatment arms. And other differences between the treatment arms were not always statistically significant.
These factors led the committee to conclude that there is insufficient evidence to suggest pixantrone is more clinically effective than treatments currently used in clinical practice.
Suitability for the UK
The appraisal committee also heard evidence from clinical experts and patient representatives. This information revealed differences in previous treatment between the PIX301 trial population and UK clinical practice.
Therefore, the committee said it could not determine the clinical effectiveness of pixantrone for a UK population.
In addition, there is doubt regarding the clinical benefit of pixantrone in patients who previously received rituximab. And this applies to virtually all patients with relapsed or refractory aggressive B-cell lymphoma in England and Wales, the committee noted.
(The European Medicines Agency’s conditional approval of pixantrone stipulated that an additional trial must confirm the clinical benefit of the drug in patients who have previously received rituximab.)
Calculating costs
The committee estimated the patient access scheme for pixantrone would most likely result in an incremental cost-effectiveness ratio of £30,700 per QALY gained. This is above the range normally considered to be cost-effective—usually £20,000 to £30,000 per QALY gained.
This factor, along with the lack of clinical effectiveness, prompted the committee to conclude that pixantrone would not be a cost-effective use of NHS resources.
According to Cell Therapeutics, pixantrone costs £553.50 per 20 mL vial. The recommended dosage of pixantrone is 50 mg/m2 on days 1, 8, and 15 of each 28-day cycle, for up to 6 cycles.
The estimated cost of a course of treatment is £19,926. This is based on the median length of treatment in the PIX301 trial—4 cycles, using an average of 3 vials per dose.
About the guidance
Individuals can comment on the pixantrone draft guidance via the NICE website. It is open until November 4, 2013.
This is the third version of the draft guidance published and the second consultation launched. The draft guidance was initially published for consultation in April 2013, followed by a final draft guidance in June 2013. But this document was withdrawn during the appeal stage because the manufacturer submitted the patient access scheme.
Until the final guidance is issued to the NHS, organizations should make decisions locally on the funding of specific treatments. Once NICE issues its final guidance on a technology, it replaces local recommendations. ![]()



