User login
Estimating Hospital Costs of CAUTI
Healthcare‐associated infections affect 5% to 10% of all hospitalized patients each year in the United States, account for nearly $45 billion in direct hospital costs, and cause nearly 100,000 deaths annually.[1, 2] Because catheter‐associated urinary tract infection (CAUTI) is one of the most common healthcare‐associated infections in the United States and is reasonably preventable, the Centers for Medicare and Medicaid Services stopped reimbursing hospitals in 2008 for the additional costs of caring for patients who develop CAUTI during hospitalization.[3] Still, strategies for reducing inappropriate urinary catheterization are infrequently implemented in practice; this is despite a consensus that such strategies are effective.[4]
To help motivate hospitals to reduce inappropriate urinary catheter use, we present a tool for estimating costs of CAUTI for individual hospitals. Although other tools for estimating the excess costs of healthcare‐associated infections are available (eg, the APIC Cost of Healthcare‐Associated Infections Model available at
METHODS
General Setup
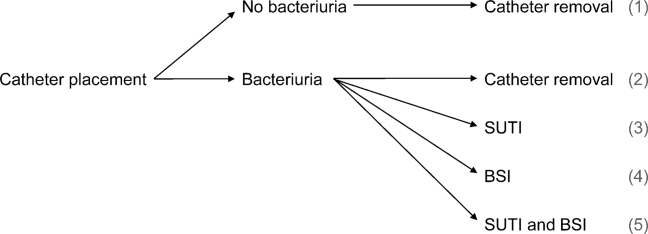
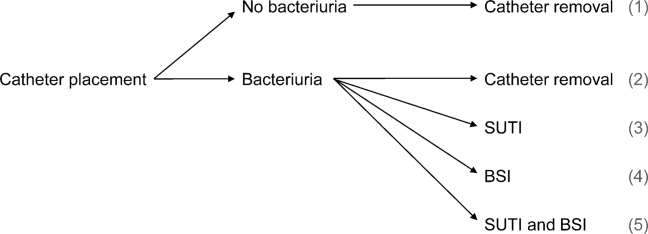
We consider 4 possible events after urinary catheter placement: bacteriuria, symptomatic urinary tract infection (SUTI), bloodstream infection (BSI), and catheter removal. Conservatively, assuming that bacteriuria must precede SUTI and BSI, there are 5 possible trajectories for any hospitalized patient (Figure 1): (1) no infection, (2) only bacteriuria, (3) bacteriuria and SUTI, (4) bacteriuria and BSI, or (5) bacteriuria, SUTI, and BSI. The cost of CAUTI for a particular hospital is therefore the per‐patient cost of each trajectory multiplied by the number of patients experiencing each trajectory. Our approach for estimating hospital costs is based on factorizing the number of patients experiencing each trajectory into a product of terms for which estimates are available from the literature (see the Supporting Information, Appendix, in the online version of this article for all technical details).
Deriving Estimates of Current Costs
We start with 2 minor simplifying assumptions. First, because the presence of asymptomatic bacteriuria is typically unknown, we only consider costs to the hospital due to SUTI and BSI[6]; in other words, we assume hospitals do not incur costs for patients with trajectories 1 or 2. This assumption should only bias cost estimates conservatively. Second, we assume that patients with both SUTI and BSI (trajectory 5) incur costs equal to those for patients with only BSI (trajectory 4). Further, because the joint risk of SUTI and BSI is unknown, we conservatively assume SUTI must precede BSI. Under these assumptions we can write: (total CAUTI costs)=(per‐patient SUTI cost) (number with SUTI but no BSI)+(perpatient BSI cost) (number with BSI).
We use per‐patient hospital costs of SUTI and BSI of $911 and $3824, respectively, which were determined using a microcosting approach[6] and adjusted for inflation using the general Consumer Price Index.[7] Although an alternative strategy for estimating costs would be to enter the hospital‐specific, per‐patient costs of SUTI and BSI into the above equation, these quantities are often difficult to measure or otherwise unavailable. Thus, it remains to factorize the number of hospitalized patients who develop SUTI and BSI into component terms for which we have accessible estimates. First note that the number with only SUTI (or any BSI) equals the total number of patients hospitalized times the proportion of hospitalizations with only SUTI (or any BSI). The former quantity depends on the particular hospital and so is specified as an input by the user. The latter quantity can be factorized further under our aforementioned conservative assumption that bacteriuria must precede SUTI and BSI.
Specifically, for SUTI:
(Proportion SUTI but no BSI)={(SUTI risk among those catheterized with bacteriuria)(BSI risk among those catheterized with bacteriuria)} (bacteriuria risk among those catheterized) (proportion catheterized).
And for BSI:
(Proportion BSI)=(BSI risk among those catheterized with bacteriuria) (bacteriuria risk among those catheterized) (proportion catheterized).
The risks of SUTI and BSI among those catheterized with bacteriuria, along with the risk of bacteriuria among those catheterized, have been estimated previously via a meta‐analytic approach.[6] The proportion catheterized depends on the particular hospital, such as the total number of patients hospitalized, and so is also specified as a user input. Therefore, we have now factorized the total hospital costs due to CAUTI as a product of either user‐specified terms or terms for which we have estimates from the literature. All estimates and corresponding standard errors derived from the literature are listed together in Table 1 (see the Supporting Information, Appendix Section 1, for further details in the online version of this article).
| Quantity | Estimate (SE) |
|---|---|
| |
| Overall risk of bacteriuria among those catheterized | 26.0% (1.53%) |
| Per‐day risk of bacteriuria among those catheterized | 5.0% |
| days | 6.68 |
| Risk of SUTI among those catheterized with bacteriuria | 24.0% (4.08%) |
| Risk of BSI among those catheterized with bacteriuria | 3.6% (0.10%) |
| Per‐patient SUTI cost | $911 ($911) |
| Per‐patient BSI cost | $3824 ($3824) |
Deriving Projected Costs After Intervention
The approach described above permits estimation of current costs for managing patients with CAUTI for a particular hospital. To estimate projected costs after participation in an intervention to reduce infection risk, we characterize interventions of interest and introduce additional factorization. Specifically, following previous work,[8] we consider interventions that reduce (1) placement (ie, the proportion catheterized) and (2) duration (ie, the mean duration of catheterization). Incorporating reductions in placement is straightforward, because our above expression for costs already contains a term for the proportion catheterized. However, incorporating reductions in duration requires further factorization. Under the assumptions of constant per‐day risks of bacteriuria and of catheter removal, we can write the postintervention risk of bacteriuria among the catheterized as a function of (1) the percent decrease in mean duration of catheterization due to intervention, and (2) the preintervention risk of bacteriuria among the catheterized (see the Supporting Information, Appendix Section 2, for further details in the online version of this article). This means we can fully characterize postintervention costs as a function of user‐specified quantities, quantities specific to the intervention (which are varied across plausible ranges), and quantities for which we have estimates from the literature. Therefore, we can estimate savings by subtracting postintervention costs from current costs.
Because our estimators of current costs, projected costs, and savings are all formulated as functions of other estimators, we use the standard delta method approach[9] to derive appropriate variance estimates (see the Supporting Information, Appendix Section 3, for further details in the online version of this article).
Online Implementation
Customized results (based on annual admissions, urinary catheter prevalence, and other inputs) can be computed using online implementation of our proposed method at
RESULTS
Figure 2 shows the projected savings in hospital costs due to CAUTI across a range of interventions defined by percent decreases in placement and duration, for a hypothetical hospital with 3000 total patients, 15% with urinary catheters preintervention, and with all other default values listed in Table 1. The current costs for this hospital (ie, the costs when the percent reduction in placement and duration is zero) are estimated to be $37,868 (95% confidence interval [CI]: $9159‐$156,564). After an intervention resulting in 40% reductions in both urinary catheter placement and duration, this hospital would be expected to save $22,653 (95% CI: $5479‐$93,656). A less effective intervention yielding a 10% reduction in both urinary catheter placement and duration would result in more modest savings of $6376 (95% CI: $1542‐$26,360).
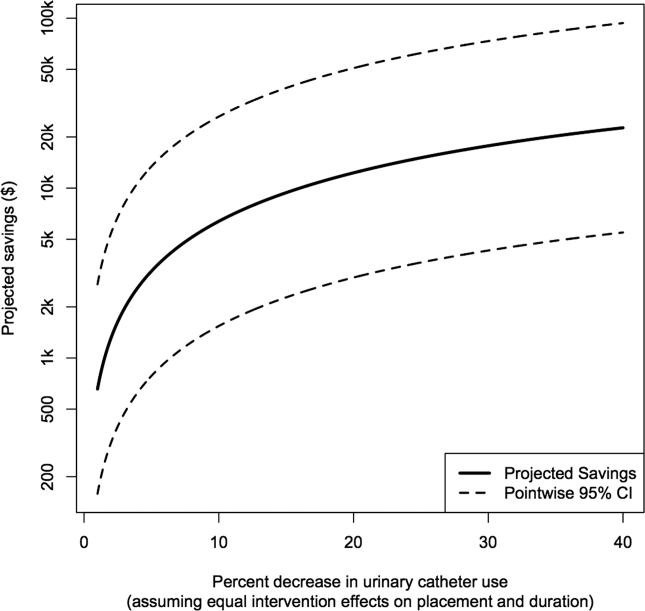
After an intervention resulting in 29% and 37% reductions in placement and duration, respectively, reflecting reductions seen in practice,[10, 11] our hypothetical hospital is estimated to save $19,126 (95% CI: $4626‐$79,074). This reflects an estimated savings of nearly 50%.
DISCUSSION
We have presented a tool for estimating customized hospital costs of CAUTI, both before and after a hypothetical intervention to reduce risk of infection. Our approach relies on mostly conservative assumptions, incorporates published risk estimates (properly accounting for their associated variability), and has easy‐to‐use online implementation. We believe this can play an important role in motivating hospitals to reduce inappropriate urinary catheter use.
The methodology employed here does have a few limitations. First and foremost, our results depend on the reliability of the input values, which are either provided by users or are based on estimates from the literature (see Table 1 for a complete list of suggested defaults). New information could potentially be incorporated if and when available. For example, substitution of more precise risk estimates could help reduce confidence interval length. Second, our approach essentially averages over hospital quality; we do not directly take into account quality of care or variation in underlying infection risk across hospitals in computing estimated costs. Finally, we only compute direct costs due to infection; other costs (eg, intervention costs) would typically also need to be considered for decision making.
Despite these limitations, we believe that our tool can help infection control professionals demonstrate the values of CAUTI prevention efforts to key administrators, particularly at a time where it has become increasingly necessary to develop a business case to initiate new interventions or justify the continued support for ongoing programs.[12] Additionally, we believe the proposed approach can be an important supplement to initiatives like the Society of Hospital Medicine's Choosing Wisely campaign, which aims to help reduce inappropriate urinary catheter use. Reducing catheter utilization has the potential to reduce costs associated with caring for CAUTI patients, but more importantly would help reduce CAUTI incidence as well as catheter‐related, noninfectious complications.[13, 14] We hope that our tool will greatly assist hospitals in promoting their CAUTI prevention efforts and improve the overall safety of hospitalized patients.
Disclosures
This project was supported by the Ann Arbor VA Medical Center/University of Michigan Patient Safety Enhancement Program (PSEP) and a subcontract to implement multistate CAUTI prevention with the Agency for Healthcare Research and Quality/Health Educational and Research Trust. Mr. Kennedy has no conflicts of interest to report. Drs. Saint and Greene are subcontracted to implement multistate CAUTI prevention with the Agency for Healthcare Research and Quality/Health Educational and Research Trust. Dr. Saint has received numerous honoraria and speaking fees for lectures on healthcare‐associated infection prevention, implementation science, and patient safety from hospitals, academic medical centers, professional societies, and nonprofit foundations. None of these activities are related to speaker's bureaus. Dr. Saint is also on the medical advisory board of Doximity, a new social networking site for physicians. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
- , , , et al. Estimating health care‐associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160–166.
- The direct medical costs of healthcare‐associated infections in US hospitals and the benefits of prevention. US Centers for Disease Control and Prevention Web site. Published 2009. Available at: http://www.cdc.gov/HAI/pdfs/hai/Scott_CostPaper.pdf. Accessed March 24, 2013.
- , , , , . Catheter‐associated urinary tract infection and the Medicare rule changes. Ann Intern Med. 2009;150(12):877–884.
- , . Improving use of the other catheter. Arch Intern Med. 2012;172(3):260–261.
- Choosing Wisely: five things patients and physicians should question. Society of Hospital Medicine. Published 2012. Available at: http://www.hospitalmedicine.org/AM/pdf/SHM‐Adult_5things_List_Web.pdf. Accessed March 24, 2013.
- . Clinical and economic consequences of nosocomial catheter‐related bacteriuria. Am J Infect Control. 2000;28(1):68–75.
- CPI Inflation Calculator. United States Department of Labor, Bureau of Labor Statistics Web site. Published 2013. Available at: http://www.bls.gov/data/inflation_calculator.htm. Accessed March 24, 2013.
- , , , et al. Introducing a population‐based outcome measure to evaluate the effect of interventions to reduce catheter‐associated urinary tract infection. Am J Infect Control. 2012;40(4):359–364.
- . Asymptotic Statistics. Cambridge, UK: Cambridge University Press; 2000.
- , , , et al. Effect of establishing guidelines on appropriate urinary catheter placement. Acad Emerg Med. 2010;17:337–340.
- , , , . Systematic review and meta‐analysis: reminder systems to reduce catheter‐associated urinary tract infections and urinary catheter use in hospitalized patients. Clin Infect Dis. 2010;51(5):550–560.
- , , , et al. Raising standards while watching the bottom line: making a business case for infection control. Infect Control Hosp Epidemiol. 2007;28:1121–1133.
- , , , , . Urinary catheters: what type do men and their nurses prefer? J Am Geriatr Soc. 1999;47(12):1453–1457.
- , , . Indwelling urinary catheters: a one‐point restraint? Ann Intern Med. 2002;137(2):125–127.
Healthcare‐associated infections affect 5% to 10% of all hospitalized patients each year in the United States, account for nearly $45 billion in direct hospital costs, and cause nearly 100,000 deaths annually.[1, 2] Because catheter‐associated urinary tract infection (CAUTI) is one of the most common healthcare‐associated infections in the United States and is reasonably preventable, the Centers for Medicare and Medicaid Services stopped reimbursing hospitals in 2008 for the additional costs of caring for patients who develop CAUTI during hospitalization.[3] Still, strategies for reducing inappropriate urinary catheterization are infrequently implemented in practice; this is despite a consensus that such strategies are effective.[4]
To help motivate hospitals to reduce inappropriate urinary catheter use, we present a tool for estimating costs of CAUTI for individual hospitals. Although other tools for estimating the excess costs of healthcare‐associated infections are available (eg, the APIC Cost of Healthcare‐Associated Infections Model available at
METHODS
General Setup
We consider 4 possible events after urinary catheter placement: bacteriuria, symptomatic urinary tract infection (SUTI), bloodstream infection (BSI), and catheter removal. Conservatively, assuming that bacteriuria must precede SUTI and BSI, there are 5 possible trajectories for any hospitalized patient (Figure 1): (1) no infection, (2) only bacteriuria, (3) bacteriuria and SUTI, (4) bacteriuria and BSI, or (5) bacteriuria, SUTI, and BSI. The cost of CAUTI for a particular hospital is therefore the per‐patient cost of each trajectory multiplied by the number of patients experiencing each trajectory. Our approach for estimating hospital costs is based on factorizing the number of patients experiencing each trajectory into a product of terms for which estimates are available from the literature (see the Supporting Information, Appendix, in the online version of this article for all technical details).

Deriving Estimates of Current Costs
We start with 2 minor simplifying assumptions. First, because the presence of asymptomatic bacteriuria is typically unknown, we only consider costs to the hospital due to SUTI and BSI[6]; in other words, we assume hospitals do not incur costs for patients with trajectories 1 or 2. This assumption should only bias cost estimates conservatively. Second, we assume that patients with both SUTI and BSI (trajectory 5) incur costs equal to those for patients with only BSI (trajectory 4). Further, because the joint risk of SUTI and BSI is unknown, we conservatively assume SUTI must precede BSI. Under these assumptions we can write: (total CAUTI costs)=(per‐patient SUTI cost) (number with SUTI but no BSI)+(perpatient BSI cost) (number with BSI).
We use per‐patient hospital costs of SUTI and BSI of $911 and $3824, respectively, which were determined using a microcosting approach[6] and adjusted for inflation using the general Consumer Price Index.[7] Although an alternative strategy for estimating costs would be to enter the hospital‐specific, per‐patient costs of SUTI and BSI into the above equation, these quantities are often difficult to measure or otherwise unavailable. Thus, it remains to factorize the number of hospitalized patients who develop SUTI and BSI into component terms for which we have accessible estimates. First note that the number with only SUTI (or any BSI) equals the total number of patients hospitalized times the proportion of hospitalizations with only SUTI (or any BSI). The former quantity depends on the particular hospital and so is specified as an input by the user. The latter quantity can be factorized further under our aforementioned conservative assumption that bacteriuria must precede SUTI and BSI.
Specifically, for SUTI:
(Proportion SUTI but no BSI)={(SUTI risk among those catheterized with bacteriuria)(BSI risk among those catheterized with bacteriuria)} (bacteriuria risk among those catheterized) (proportion catheterized).
And for BSI:
(Proportion BSI)=(BSI risk among those catheterized with bacteriuria) (bacteriuria risk among those catheterized) (proportion catheterized).
The risks of SUTI and BSI among those catheterized with bacteriuria, along with the risk of bacteriuria among those catheterized, have been estimated previously via a meta‐analytic approach.[6] The proportion catheterized depends on the particular hospital, such as the total number of patients hospitalized, and so is also specified as a user input. Therefore, we have now factorized the total hospital costs due to CAUTI as a product of either user‐specified terms or terms for which we have estimates from the literature. All estimates and corresponding standard errors derived from the literature are listed together in Table 1 (see the Supporting Information, Appendix Section 1, for further details in the online version of this article).
| Quantity | Estimate (SE) |
|---|---|
| |
| Overall risk of bacteriuria among those catheterized | 26.0% (1.53%) |
| Per‐day risk of bacteriuria among those catheterized | 5.0% |
| days | 6.68 |
| Risk of SUTI among those catheterized with bacteriuria | 24.0% (4.08%) |
| Risk of BSI among those catheterized with bacteriuria | 3.6% (0.10%) |
| Per‐patient SUTI cost | $911 ($911) |
| Per‐patient BSI cost | $3824 ($3824) |
Deriving Projected Costs After Intervention
The approach described above permits estimation of current costs for managing patients with CAUTI for a particular hospital. To estimate projected costs after participation in an intervention to reduce infection risk, we characterize interventions of interest and introduce additional factorization. Specifically, following previous work,[8] we consider interventions that reduce (1) placement (ie, the proportion catheterized) and (2) duration (ie, the mean duration of catheterization). Incorporating reductions in placement is straightforward, because our above expression for costs already contains a term for the proportion catheterized. However, incorporating reductions in duration requires further factorization. Under the assumptions of constant per‐day risks of bacteriuria and of catheter removal, we can write the postintervention risk of bacteriuria among the catheterized as a function of (1) the percent decrease in mean duration of catheterization due to intervention, and (2) the preintervention risk of bacteriuria among the catheterized (see the Supporting Information, Appendix Section 2, for further details in the online version of this article). This means we can fully characterize postintervention costs as a function of user‐specified quantities, quantities specific to the intervention (which are varied across plausible ranges), and quantities for which we have estimates from the literature. Therefore, we can estimate savings by subtracting postintervention costs from current costs.
Because our estimators of current costs, projected costs, and savings are all formulated as functions of other estimators, we use the standard delta method approach[9] to derive appropriate variance estimates (see the Supporting Information, Appendix Section 3, for further details in the online version of this article).
Online Implementation
Customized results (based on annual admissions, urinary catheter prevalence, and other inputs) can be computed using online implementation of our proposed method at
RESULTS
Figure 2 shows the projected savings in hospital costs due to CAUTI across a range of interventions defined by percent decreases in placement and duration, for a hypothetical hospital with 3000 total patients, 15% with urinary catheters preintervention, and with all other default values listed in Table 1. The current costs for this hospital (ie, the costs when the percent reduction in placement and duration is zero) are estimated to be $37,868 (95% confidence interval [CI]: $9159‐$156,564). After an intervention resulting in 40% reductions in both urinary catheter placement and duration, this hospital would be expected to save $22,653 (95% CI: $5479‐$93,656). A less effective intervention yielding a 10% reduction in both urinary catheter placement and duration would result in more modest savings of $6376 (95% CI: $1542‐$26,360).

After an intervention resulting in 29% and 37% reductions in placement and duration, respectively, reflecting reductions seen in practice,[10, 11] our hypothetical hospital is estimated to save $19,126 (95% CI: $4626‐$79,074). This reflects an estimated savings of nearly 50%.
DISCUSSION
We have presented a tool for estimating customized hospital costs of CAUTI, both before and after a hypothetical intervention to reduce risk of infection. Our approach relies on mostly conservative assumptions, incorporates published risk estimates (properly accounting for their associated variability), and has easy‐to‐use online implementation. We believe this can play an important role in motivating hospitals to reduce inappropriate urinary catheter use.
The methodology employed here does have a few limitations. First and foremost, our results depend on the reliability of the input values, which are either provided by users or are based on estimates from the literature (see Table 1 for a complete list of suggested defaults). New information could potentially be incorporated if and when available. For example, substitution of more precise risk estimates could help reduce confidence interval length. Second, our approach essentially averages over hospital quality; we do not directly take into account quality of care or variation in underlying infection risk across hospitals in computing estimated costs. Finally, we only compute direct costs due to infection; other costs (eg, intervention costs) would typically also need to be considered for decision making.
Despite these limitations, we believe that our tool can help infection control professionals demonstrate the values of CAUTI prevention efforts to key administrators, particularly at a time where it has become increasingly necessary to develop a business case to initiate new interventions or justify the continued support for ongoing programs.[12] Additionally, we believe the proposed approach can be an important supplement to initiatives like the Society of Hospital Medicine's Choosing Wisely campaign, which aims to help reduce inappropriate urinary catheter use. Reducing catheter utilization has the potential to reduce costs associated with caring for CAUTI patients, but more importantly would help reduce CAUTI incidence as well as catheter‐related, noninfectious complications.[13, 14] We hope that our tool will greatly assist hospitals in promoting their CAUTI prevention efforts and improve the overall safety of hospitalized patients.
Disclosures
This project was supported by the Ann Arbor VA Medical Center/University of Michigan Patient Safety Enhancement Program (PSEP) and a subcontract to implement multistate CAUTI prevention with the Agency for Healthcare Research and Quality/Health Educational and Research Trust. Mr. Kennedy has no conflicts of interest to report. Drs. Saint and Greene are subcontracted to implement multistate CAUTI prevention with the Agency for Healthcare Research and Quality/Health Educational and Research Trust. Dr. Saint has received numerous honoraria and speaking fees for lectures on healthcare‐associated infection prevention, implementation science, and patient safety from hospitals, academic medical centers, professional societies, and nonprofit foundations. None of these activities are related to speaker's bureaus. Dr. Saint is also on the medical advisory board of Doximity, a new social networking site for physicians. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
Healthcare‐associated infections affect 5% to 10% of all hospitalized patients each year in the United States, account for nearly $45 billion in direct hospital costs, and cause nearly 100,000 deaths annually.[1, 2] Because catheter‐associated urinary tract infection (CAUTI) is one of the most common healthcare‐associated infections in the United States and is reasonably preventable, the Centers for Medicare and Medicaid Services stopped reimbursing hospitals in 2008 for the additional costs of caring for patients who develop CAUTI during hospitalization.[3] Still, strategies for reducing inappropriate urinary catheterization are infrequently implemented in practice; this is despite a consensus that such strategies are effective.[4]
To help motivate hospitals to reduce inappropriate urinary catheter use, we present a tool for estimating costs of CAUTI for individual hospitals. Although other tools for estimating the excess costs of healthcare‐associated infections are available (eg, the APIC Cost of Healthcare‐Associated Infections Model available at
METHODS
General Setup
We consider 4 possible events after urinary catheter placement: bacteriuria, symptomatic urinary tract infection (SUTI), bloodstream infection (BSI), and catheter removal. Conservatively, assuming that bacteriuria must precede SUTI and BSI, there are 5 possible trajectories for any hospitalized patient (Figure 1): (1) no infection, (2) only bacteriuria, (3) bacteriuria and SUTI, (4) bacteriuria and BSI, or (5) bacteriuria, SUTI, and BSI. The cost of CAUTI for a particular hospital is therefore the per‐patient cost of each trajectory multiplied by the number of patients experiencing each trajectory. Our approach for estimating hospital costs is based on factorizing the number of patients experiencing each trajectory into a product of terms for which estimates are available from the literature (see the Supporting Information, Appendix, in the online version of this article for all technical details).

Deriving Estimates of Current Costs
We start with 2 minor simplifying assumptions. First, because the presence of asymptomatic bacteriuria is typically unknown, we only consider costs to the hospital due to SUTI and BSI[6]; in other words, we assume hospitals do not incur costs for patients with trajectories 1 or 2. This assumption should only bias cost estimates conservatively. Second, we assume that patients with both SUTI and BSI (trajectory 5) incur costs equal to those for patients with only BSI (trajectory 4). Further, because the joint risk of SUTI and BSI is unknown, we conservatively assume SUTI must precede BSI. Under these assumptions we can write: (total CAUTI costs)=(per‐patient SUTI cost) (number with SUTI but no BSI)+(perpatient BSI cost) (number with BSI).
We use per‐patient hospital costs of SUTI and BSI of $911 and $3824, respectively, which were determined using a microcosting approach[6] and adjusted for inflation using the general Consumer Price Index.[7] Although an alternative strategy for estimating costs would be to enter the hospital‐specific, per‐patient costs of SUTI and BSI into the above equation, these quantities are often difficult to measure or otherwise unavailable. Thus, it remains to factorize the number of hospitalized patients who develop SUTI and BSI into component terms for which we have accessible estimates. First note that the number with only SUTI (or any BSI) equals the total number of patients hospitalized times the proportion of hospitalizations with only SUTI (or any BSI). The former quantity depends on the particular hospital and so is specified as an input by the user. The latter quantity can be factorized further under our aforementioned conservative assumption that bacteriuria must precede SUTI and BSI.
Specifically, for SUTI:
(Proportion SUTI but no BSI)={(SUTI risk among those catheterized with bacteriuria)(BSI risk among those catheterized with bacteriuria)} (bacteriuria risk among those catheterized) (proportion catheterized).
And for BSI:
(Proportion BSI)=(BSI risk among those catheterized with bacteriuria) (bacteriuria risk among those catheterized) (proportion catheterized).
The risks of SUTI and BSI among those catheterized with bacteriuria, along with the risk of bacteriuria among those catheterized, have been estimated previously via a meta‐analytic approach.[6] The proportion catheterized depends on the particular hospital, such as the total number of patients hospitalized, and so is also specified as a user input. Therefore, we have now factorized the total hospital costs due to CAUTI as a product of either user‐specified terms or terms for which we have estimates from the literature. All estimates and corresponding standard errors derived from the literature are listed together in Table 1 (see the Supporting Information, Appendix Section 1, for further details in the online version of this article).
| Quantity | Estimate (SE) |
|---|---|
| |
| Overall risk of bacteriuria among those catheterized | 26.0% (1.53%) |
| Per‐day risk of bacteriuria among those catheterized | 5.0% |
| days | 6.68 |
| Risk of SUTI among those catheterized with bacteriuria | 24.0% (4.08%) |
| Risk of BSI among those catheterized with bacteriuria | 3.6% (0.10%) |
| Per‐patient SUTI cost | $911 ($911) |
| Per‐patient BSI cost | $3824 ($3824) |
Deriving Projected Costs After Intervention
The approach described above permits estimation of current costs for managing patients with CAUTI for a particular hospital. To estimate projected costs after participation in an intervention to reduce infection risk, we characterize interventions of interest and introduce additional factorization. Specifically, following previous work,[8] we consider interventions that reduce (1) placement (ie, the proportion catheterized) and (2) duration (ie, the mean duration of catheterization). Incorporating reductions in placement is straightforward, because our above expression for costs already contains a term for the proportion catheterized. However, incorporating reductions in duration requires further factorization. Under the assumptions of constant per‐day risks of bacteriuria and of catheter removal, we can write the postintervention risk of bacteriuria among the catheterized as a function of (1) the percent decrease in mean duration of catheterization due to intervention, and (2) the preintervention risk of bacteriuria among the catheterized (see the Supporting Information, Appendix Section 2, for further details in the online version of this article). This means we can fully characterize postintervention costs as a function of user‐specified quantities, quantities specific to the intervention (which are varied across plausible ranges), and quantities for which we have estimates from the literature. Therefore, we can estimate savings by subtracting postintervention costs from current costs.
Because our estimators of current costs, projected costs, and savings are all formulated as functions of other estimators, we use the standard delta method approach[9] to derive appropriate variance estimates (see the Supporting Information, Appendix Section 3, for further details in the online version of this article).
Online Implementation
Customized results (based on annual admissions, urinary catheter prevalence, and other inputs) can be computed using online implementation of our proposed method at
RESULTS
Figure 2 shows the projected savings in hospital costs due to CAUTI across a range of interventions defined by percent decreases in placement and duration, for a hypothetical hospital with 3000 total patients, 15% with urinary catheters preintervention, and with all other default values listed in Table 1. The current costs for this hospital (ie, the costs when the percent reduction in placement and duration is zero) are estimated to be $37,868 (95% confidence interval [CI]: $9159‐$156,564). After an intervention resulting in 40% reductions in both urinary catheter placement and duration, this hospital would be expected to save $22,653 (95% CI: $5479‐$93,656). A less effective intervention yielding a 10% reduction in both urinary catheter placement and duration would result in more modest savings of $6376 (95% CI: $1542‐$26,360).

After an intervention resulting in 29% and 37% reductions in placement and duration, respectively, reflecting reductions seen in practice,[10, 11] our hypothetical hospital is estimated to save $19,126 (95% CI: $4626‐$79,074). This reflects an estimated savings of nearly 50%.
DISCUSSION
We have presented a tool for estimating customized hospital costs of CAUTI, both before and after a hypothetical intervention to reduce risk of infection. Our approach relies on mostly conservative assumptions, incorporates published risk estimates (properly accounting for their associated variability), and has easy‐to‐use online implementation. We believe this can play an important role in motivating hospitals to reduce inappropriate urinary catheter use.
The methodology employed here does have a few limitations. First and foremost, our results depend on the reliability of the input values, which are either provided by users or are based on estimates from the literature (see Table 1 for a complete list of suggested defaults). New information could potentially be incorporated if and when available. For example, substitution of more precise risk estimates could help reduce confidence interval length. Second, our approach essentially averages over hospital quality; we do not directly take into account quality of care or variation in underlying infection risk across hospitals in computing estimated costs. Finally, we only compute direct costs due to infection; other costs (eg, intervention costs) would typically also need to be considered for decision making.
Despite these limitations, we believe that our tool can help infection control professionals demonstrate the values of CAUTI prevention efforts to key administrators, particularly at a time where it has become increasingly necessary to develop a business case to initiate new interventions or justify the continued support for ongoing programs.[12] Additionally, we believe the proposed approach can be an important supplement to initiatives like the Society of Hospital Medicine's Choosing Wisely campaign, which aims to help reduce inappropriate urinary catheter use. Reducing catheter utilization has the potential to reduce costs associated with caring for CAUTI patients, but more importantly would help reduce CAUTI incidence as well as catheter‐related, noninfectious complications.[13, 14] We hope that our tool will greatly assist hospitals in promoting their CAUTI prevention efforts and improve the overall safety of hospitalized patients.
Disclosures
This project was supported by the Ann Arbor VA Medical Center/University of Michigan Patient Safety Enhancement Program (PSEP) and a subcontract to implement multistate CAUTI prevention with the Agency for Healthcare Research and Quality/Health Educational and Research Trust. Mr. Kennedy has no conflicts of interest to report. Drs. Saint and Greene are subcontracted to implement multistate CAUTI prevention with the Agency for Healthcare Research and Quality/Health Educational and Research Trust. Dr. Saint has received numerous honoraria and speaking fees for lectures on healthcare‐associated infection prevention, implementation science, and patient safety from hospitals, academic medical centers, professional societies, and nonprofit foundations. None of these activities are related to speaker's bureaus. Dr. Saint is also on the medical advisory board of Doximity, a new social networking site for physicians. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
- , , , et al. Estimating health care‐associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160–166.
- The direct medical costs of healthcare‐associated infections in US hospitals and the benefits of prevention. US Centers for Disease Control and Prevention Web site. Published 2009. Available at: http://www.cdc.gov/HAI/pdfs/hai/Scott_CostPaper.pdf. Accessed March 24, 2013.
- , , , , . Catheter‐associated urinary tract infection and the Medicare rule changes. Ann Intern Med. 2009;150(12):877–884.
- , . Improving use of the other catheter. Arch Intern Med. 2012;172(3):260–261.
- Choosing Wisely: five things patients and physicians should question. Society of Hospital Medicine. Published 2012. Available at: http://www.hospitalmedicine.org/AM/pdf/SHM‐Adult_5things_List_Web.pdf. Accessed March 24, 2013.
- . Clinical and economic consequences of nosocomial catheter‐related bacteriuria. Am J Infect Control. 2000;28(1):68–75.
- CPI Inflation Calculator. United States Department of Labor, Bureau of Labor Statistics Web site. Published 2013. Available at: http://www.bls.gov/data/inflation_calculator.htm. Accessed March 24, 2013.
- , , , et al. Introducing a population‐based outcome measure to evaluate the effect of interventions to reduce catheter‐associated urinary tract infection. Am J Infect Control. 2012;40(4):359–364.
- . Asymptotic Statistics. Cambridge, UK: Cambridge University Press; 2000.
- , , , et al. Effect of establishing guidelines on appropriate urinary catheter placement. Acad Emerg Med. 2010;17:337–340.
- , , , . Systematic review and meta‐analysis: reminder systems to reduce catheter‐associated urinary tract infections and urinary catheter use in hospitalized patients. Clin Infect Dis. 2010;51(5):550–560.
- , , , et al. Raising standards while watching the bottom line: making a business case for infection control. Infect Control Hosp Epidemiol. 2007;28:1121–1133.
- , , , , . Urinary catheters: what type do men and their nurses prefer? J Am Geriatr Soc. 1999;47(12):1453–1457.
- , , . Indwelling urinary catheters: a one‐point restraint? Ann Intern Med. 2002;137(2):125–127.
- , , , et al. Estimating health care‐associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160–166.
- The direct medical costs of healthcare‐associated infections in US hospitals and the benefits of prevention. US Centers for Disease Control and Prevention Web site. Published 2009. Available at: http://www.cdc.gov/HAI/pdfs/hai/Scott_CostPaper.pdf. Accessed March 24, 2013.
- , , , , . Catheter‐associated urinary tract infection and the Medicare rule changes. Ann Intern Med. 2009;150(12):877–884.
- , . Improving use of the other catheter. Arch Intern Med. 2012;172(3):260–261.
- Choosing Wisely: five things patients and physicians should question. Society of Hospital Medicine. Published 2012. Available at: http://www.hospitalmedicine.org/AM/pdf/SHM‐Adult_5things_List_Web.pdf. Accessed March 24, 2013.
- . Clinical and economic consequences of nosocomial catheter‐related bacteriuria. Am J Infect Control. 2000;28(1):68–75.
- CPI Inflation Calculator. United States Department of Labor, Bureau of Labor Statistics Web site. Published 2013. Available at: http://www.bls.gov/data/inflation_calculator.htm. Accessed March 24, 2013.
- , , , et al. Introducing a population‐based outcome measure to evaluate the effect of interventions to reduce catheter‐associated urinary tract infection. Am J Infect Control. 2012;40(4):359–364.
- . Asymptotic Statistics. Cambridge, UK: Cambridge University Press; 2000.
- , , , et al. Effect of establishing guidelines on appropriate urinary catheter placement. Acad Emerg Med. 2010;17:337–340.
- , , , . Systematic review and meta‐analysis: reminder systems to reduce catheter‐associated urinary tract infections and urinary catheter use in hospitalized patients. Clin Infect Dis. 2010;51(5):550–560.
- , , , et al. Raising standards while watching the bottom line: making a business case for infection control. Infect Control Hosp Epidemiol. 2007;28:1121–1133.
- , , , , . Urinary catheters: what type do men and their nurses prefer? J Am Geriatr Soc. 1999;47(12):1453–1457.
- , , . Indwelling urinary catheters: a one‐point restraint? Ann Intern Med. 2002;137(2):125–127.
Avoiding Unplanned Resections of Wrist Sarcomas: An Algorithm for Evaluating Dorsal Wrist Masses
Fecal incontinence: New therapies, age-old problem
Fecal incontinence is a socially embarrassing condition that affects approximately 18 million adults in the United States.1 Its true incidence is likely much higher than reported, however, as many patients are reluctant to discuss it.
A recent study found that nearly 20% of women experience fecal incontinence at least once a year, and 9.5% experience it al least once a month.2 Only 28% of these women had ever discussed their symptoms with a physician, however.3 Women who did seek care were more likely to consult a family physician or internist (75%) than a gynecologist (7%).3
Until recently, few options were available for patients with fecal incontinence who had not benefited from conservative measures. Many patients simply had to live with their symptoms or undergo a diverting ostomy to control the chronic involuntary drainage.
Recent years have seen the development of new minimally invasive and highly successful techniques to treat fecal incontinence. Greater awareness of the prevalence of fecal incontinence and its devastating impact on quality of life is needed for this problem to be fully addressed, however. In this article, we review the recommended evaluation of a patient who reports fecal incontinence and describe the range of treatment options.
Fecal incontinence is a symptom, not a diagnosis
Although the most common historical factor contributing to fecal incontinence is obstetric trauma, there are several other causes of this condition. A detailed history and physical examination are vital to determine whether the patient is experiencing true fecal incontinence, or whether she is leaking for other reasons—so-called pseudo-incontinence.
Conditions that can mimic fecal incontinence include:
- prolapsing hemorrhoids
- anal fistula
- sexually transmitted infection
- benign or malignant anorectal neoplasms
- dermatologic conditions.
True fecal incontinence may be active (loss of stool despite the patient’s best effort to control it) or passive (loss of stool without the patient’s awareness). Among the causes of true fecal incontinence are:
- anal sphincter injury (obstetric tear, anorectal surgery such as fistulotomy, or trauma)
- denervation of the pelvic floor from pudendal nerve injury during childbirth
- chronic rectal prolapse
- neurologic conditions (spina bifida, myelomeningocele)
- noncompliant rectum from inflammatory bowel disease
- radiation proctitis.
The maintenance of continence requires a complex interaction between the sphincter muscle, the puborectalis muscle (which acts as a sling), rectal capacity and compliance, stool volume and frequency, and neurologic mechanisms.
Diagnosis and management require an experienced physician
We believe that patients reporting fecal incontinence are best worked up and managed by a physician who is well versed in the various diagnoses associated with fecal incontinence, as well as the most current treatments.
Diagnosis entails some detective work
When a patient reports fecal incontinence, she should be asked to elaborate on the circumstances surrounding the complaint and the frequency of its occurrence, duration of symptoms, and nature of the incontinence (gas, liquid, or solid).
Validated quality-of-life instruments, such as the Cleveland Clinic Florida Fecal Incontinence Score (CCF-FIS) may be helpful in documenting the severity of the symptoms and improvement after treatment (TABLE).4
One factor that current scoring systems fail to capture is urgency. In many instances, urgency is the symptom most distressing to the patient. Be sure to ask about it.
A detailed obstetric history also is important. It is not uncommon for a patient to develop symptoms 20 years or longer after the injury. Also review the patient’s medical history for inflammatory bowel disease, neurologic disorders, and any history of pelvic radiation for help in determining the cause of symptoms.
In addition, ask the patient about any other pelvic floor symptoms, such as voiding dysfunction and problems with pelvic organ prolapse. And question her about stool consistency and frequency. In some cases, diarrhea can lead to fecal incontinence and is usually managed conservatively.
Physical exam: Focus on the perineum and anus
A detailed physical examination is warranted to determine the state of the patient’s sphincter musculature and rule out other causes of pseudo-incontinence, such as hemorrhoids or anal fistula. Inspect the perineum for thinning of the perineal body and scars from prior surgery.
A patulous anus may be a sign of rectal prolapse. To check for it, ask the patient to strain on the commode. If rectal prolapse is present, it will become apparent upon straining. If prolapse is detected, surgical treatment of the prolapse would be the first step in managing the incontinence.
A simple test of neurologic function is to try to elicit an anal “wink” in response to a pinprick.
A digital rectal exam allows the assessment of resting and squeeze tone, as well as the use of accessory muscles, such as the gluteus maximus, during squeezing.
Rigid or flexible proctoscopy may be indicated to rule out inflammatory bowel disease, radiation proctitis, and rectal neoplasm, depending on the patient’s history.
A few diagnostic adjuncts can help
Several adjuncts to physical examination can provide more detailed information about the patient’s condition and facilitate the development of an individualized treatment plan. For example, if rectal prolapse, rectocele with delayed emptying, or enterocele is suspected, consider defecography. If voiding dysfunction coexists with the fecal incontinence, urodynamic testing and cystoscopy may be indicated.
We routinely perform physiology testing and endoanal ultrasound if surgery is planned to address the fecal incontinence, although routine use of these adjuncts is controversial. Because many patients can be managed with conservative medical measures, we do not find it necessary to perform these tests at the time of the first visit.
Anal physiology testing includes manometry (a measure of both resting and squeeze tone) and pudendal nerve terminal motor latency testing.
Manometry can help quantify the severity of muscle weakness and determine the presence or absence of normal anal reflexes. Pudendal nerve testing assesses the innervation of the anal sphincter. There is some evidence that patients who have a pudendal neuropathy have a poor outcome with sphincteroplasty,5 although that evidence is controversial. The findings from physiology studies have not been correlated with outcomes of newer treatments, such as sacral neuromodulation (InterStim, Medtronic, Minneapolis, Minnesota). Each physiology lab uses different equipment, so “normal” values vary between institutions.
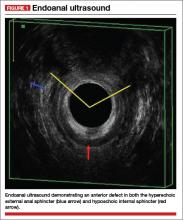
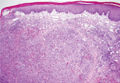
Endoanal ultrasound is easily performed in an office setting. It is well tolerated and provides anatomic detail of the sphincter musculature. We use a 13-MHz rotating probe to provide 3D imaging of the anal canal. The internal sphincter is represented by a hypoechoic circle surrounded by the hyperechoic external sphincter (FIGURE 1).
In the hands of an experienced examiner, the sensitivity and specificity of endoanal ultrasound in detecting sphincter defects approaches 100%.6,7 Ultrasound also enables measurement of the perineal body. A normal perineal body measures 10 to 15 mm.
For treatment, try conservative measures first
Bulking agents (fiber), constipating agents (loperamide, etc.), or a laxative regimen with scheduled disimpactions (in patients who have pelvic outlet constipation and overflow incontinence) often can control the symptoms of fecal incontinence, making further interventions unnecessary.
Biofeedback is another option. It uses visual, auditory, and sensory information to train patients to better control anal sphincter muscle function.
A recent randomized study found manometric biofeedback to be superior to simple Kegel exercises in improving fecal continence.8 In this study, 76% of patients in the biofeedback group experienced symptom improvement, compared with 41% of patients in the pelvic floor exercise group (P <.001). The long-term benefits of biofeedback are less clear, and patients often need to be reminded to perform their exercises at home and to attend occasional refresher-training sessions. Nevertheless, biofeedback is an important noninvasive option for patients in whom medical management has failed.
Minimally invasive options are now available
Over the past 2 years, minimally invasive treatments for fecal incontinence have emerged, including an implantable sacral neuromodulation device (InterStim) and an injectable dextranomer (Solesta; Salix Pharmaceuticals, Raleigh, North Carolina). Previously, the only surgical option for fecal incontinence was a sphincter repair if a defect was present. The new options may help patients improve their quality of life without having to undergo major surgery.
No one has directly compared the outcomes of these procedures when they are performed by a colorectal surgeon versus a physician of another specialty. It is our belief that the treating physician should have a strong interest in caring for these complex patients and a good working knowledge of the various treatment options.
Related Article Obstetric anal sphincter injury: 7 critical questions about care Ranee Thakar, MD, MRCOG (February 2008)
Sacral neuromodulation
This technique initially was developed for the treatment of overactive bladder and nonobstructive urinary retention and has been used in the United States for the past 15 years for these indications. Improvement in fecal continence was observed in these patients, prompting further studies of its efficacy. In 2011, it was approved by the US Food and Drug Administration (FDA) for the treatment of fecal incontinence. It has since revolutionized the treatment of this disorder, offering a minimally invasive and highly successful alternative to sphincteroplasty.
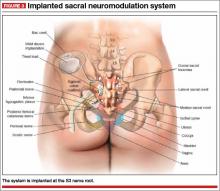
The InterStim procedure is the only therapeutic modality to include a test phase. The outpatient procedure involves sterile placement of an electrode through the S3 foramen to stimulate the S3 nerve root using fluoroscopic guidance (FIGURES 2 and 3). Patients who experience at least 50% improvement in symptoms are then offered placement of a permanent stimulator.
In most series, approximately 90% of patients have a positive test and progress to implantation. A recent US multicenter clinical trial indicated that 86% of patients achieved an improvement in continence of at least 50%, and 40% of patients were completely continent at 3 years.9 The number of episodes of incontinence decreased from a mean of 9.4/week to 1.7/week.9 Quality of life also improved greatly. Few complications have been reported, the most notable of which is infection (10.8% in the US multicenter trial9).
Another advantage of sacral neuromodulation: It can be used successfully in patients with external sphincter defects as large as 120º. A study by Tjandra and colleagues found that 65% of patients experienced improvement in symptoms of at least 50%, and 47% of patients (more than 50% of whom had external sphincter defects as large as 120º) became completely continent.10
The only variable shown to predict success with sacral neuromodulation is a positive response to the test implant procedure.
In our experience, this procedure is easy to perform and well tolerated, even in elderly patients with multiple comorbidities. The procedure has the additional advantage of potentially improving concomitant urinary symptoms as well.
The major disadvantage of sacral neuromodulation is its cost, although most major insurance carriers cover it. There is no well-conducted cost-effectiveness analysis comparing this modality to other treatments.
Related Article Interstim: An implantable device for implacable urinary symptoms Deborah L. Myers, MD (October 2006)
Injectable agents
Several biocompatible bulking agents have been tested in the treatment of fecal incontinence. These compounds traditionally have been used to treat mild fecal incontinence, or to treat patients with isolated internal sphincter defects.
More recently, an injectable dextranomer in stabilized hyaluronic acid was approved by the FDA and marketed as Solesta. Graf and colleagues randomly allocated 136 patients to injection and 70 patients to sham injection. Patients with external sphincter defects were excluded. At 6 months, 52% of patients in the active treatment group experienced an improvement in continence of at least 50%, compared with 31% of patients injected with placebo.11
The advantage of this procedure is its minimally invasive nature (submucosal injection performed in the office). The disadvantage: a lack of long-term efficacy data, although unpublished data suggest that patients who improve after an injection see a durable response at 3 years.
This easy, office-based treatment is ideal for patients with minor incontinence or persistent symptoms after another procedure.
Sphincter repair
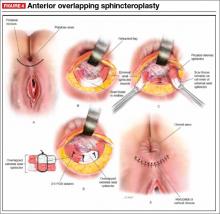
Anterior sphincteroplasty has been the mainstay of surgical treatment for patients with a sphincter defect. With the patient in a dorsal lithotomy or prone position on the operating-room table, a transverse perineal incision is made, and the ends of the severed sphincter muscle are located and mobilized. The repair then can be performed in an end-to-end manner or by overlapping the muscles in the anterior midline (FIGURE 4).
Some of the debatable technical issues of this procedure include:
- whether to overlap the muscles or scar tissue
- whether to repair internal and external defects together or separately
- how the age of the patient affects the outcome.
In regard to the first issue, there may be a superior outcome with overlapping repairs, but they carry a higher risk of dyspareunia and evacuation difficulties. Some surgeons will attempt a separate repair of the internal and external sphincter muscles if it appears feasible. Most often, both muscles are tethered together with scar tissue and separate repair is not possible. There are no conclusive data to demonstrate the superiority of either approach.
As for age, the traditional teaching was that older patients do not benefit from this procedure as much as younger patients do. However, a recent study found no differences in the CCF-FIS score in patients older than age 60, compared with younger patients.12 Investigators concluded that sphincteroplasty can be offered to both young and older patients.12
Although sphincteroplasty often leads to excellent short-term improvement, with 60% to 90% of patients experiencing a good or excellent outcome, nearly all series indicate a decline over the long term (>5 years). A recent systematic review found that as few as 12% of patients experience a good or excellent result, depending on the series.13
We offer sphincter repair to young women with a new sphincter defect after delivery. For older patients, we offer sacral neuromodulation as a first-line treatment.
Other surgical options
We believe that most patients with fecal incontinence can be managed using conservative measures, sacral neuromodulation, injectable dextranomer, or sphincter repair. However, several other options are available.
Artificial bowel sphincter
The artificial bowel sphincter was first described in 1987 and has been modified over the years. The system currently is marketed as the Acticon Neosphincter (American Medical Systems, Minnetonka, Minnesota). The procedure involves the creation of a subcutaneous tunnel around the anus so that an inflatable cuff can be positioned there. A pump then is tunneled through a Pfannenstiel incision to the labia or scrotum, and a reservoir is positioned in the space of Retzius. The device maintains continence by keeping the cuff inflated during the resting state and by pumping fluid from the cuff to the reservoir when the patient needs to evacuate.
The major barrier to utilization of the artificial bowel sphincter is infection. In a series of 112 patients who were implanted with the sphincter, 384 device-related adverse events occurred in 99 patients.14 A total of 73 revision operations were required in 51 patients (46%). Twenty-five percent of patients developed infection that required surgical revision, and 37% had the device explanted. Eighty-five percent of patients with a functional device had a successful outcome.14
Given the device-related challenges and infectious complications, patients should be considered for less invasive treatments before being offered an artificial bowel
sphincter.
Radiofrequency current
The Secca procedure (Curon Medical, Fremont, California) involves the application of radiofrequency current to the anal canal to generate thermal energy. This procedure causes contraction of collagen fibers, which are permanently shortened, and leads to tightening of the muscle. It is performed under intravenous sedation on an outpatient basis.
This approach is indicated for patients with mild to moderate fecal incontinence who have not responded to conservative management. An external sphincter defect is a contraindication.
Small, nonrandomized studies have found improvement in the CCF-FIS score in patients treated with this approach.15 The major limitation of this treatment is the lack of high-level clinical evidence demonstrating its efficacy and safety.
Antegrade continence enema
This approach, also known as the Malone procedure, is usually reserved for debilitating incontinence or constipation in the pediatric population. An appendicostomy is constructed at the navel, allowing daily introduction of a catheter and antegrade enema. The purpose is to perform rapid, controlled emptying of the colon at times chosen by the patient. It is also reserved as a last resort for patients considering an ostomy.
Adult patients with neurologic problems, such as spina bifida, may be candidates for this procedure, provided they are highly motivated.
Fecal diversion
Creation of a colostomy or ileostomy is usually the last resort for a patient with fecal incontinence. We are fortunate that there are an increasing number of options that may improve the patient’s condition before colostomy is required.
If fecal diversion is chosen by the patient, it is important to involve an enterostomal therapist for site marking and patient education. A well-constructed ostomy is essential, as this option often is permanent.
Up and coming options
A novel treatment approach for fecal incontinence is the magnetic anal sphincter. The device, marketed as the FENIX Continence Restoration System (Torax Medical, Shoreview, Minnesota) consists of a series of titanium beads with magnetic cores that are interlinked with titanium wires. The device is designed to encircle the external anal sphincter muscle, reinforce the sphincter, and expand to allow passage of stool at a socially appropriate time.
Preliminary data from 16 patients indicate a mean decrease in the number of episodes of incontinence from 7.2/week to 0.7/week, as well as a mean reduction in the CCF-FIS score from 17.2 to 8.7.16 Two devices were removed due to infection, and one device passed spontaneously after disconnection of the suture.16
This device is not approved by the FDA, but it may become a promising treatment if its safety and efficacy can be established in larger clinical trials.
The TOPAS sling (American Medical Systems) is currently being studied in a Phase 3, multicenter, nonrandomized, clinical trial (NCT01090739) for the treatment of fecal incontinence.17 The sling is implanted using a minimally invasive transobturator approach; two needle-passers deliver the sling assembly. Two small posterior incisions facilitate the postanal placement of the mesh.
This procedure replicates the anorectal angle created by the puborectalis muscle. Although it may become a minimally invasive treatment in the future, final results of the Phase 3 trial are not expected until 2016.
Tibial nerve stimulation is commonly used for urinary urge incontinence. Several small series have documented modest success with its application to fecal incontinence.18
The outpatient procedure involves the insertion of a needle electrode three fingerbreadths above the medial malleolus, followed by electrical stimulation. The current is slowly increased until a sensory or motor response (tingling under the sole of the foot or great toe plantar flexion) is elicited. Treatment necessitates several outpatient sessions.
In a recent series, the mean CCF-FIS decreased from 12.2/20 at baseline to 9.1/20 after treatment (P <.0001).18
The role of this procedure in the treatment algorithm for fecal incontinence remains to be determined.
What we offer patients
Fecal incontinence is a debilitating condition with an increasing number of potential therapeutic options. It clearly is under-recognized by patients and physicians alike.
After a thorough work-up, conservative treatment options should be offered first. When those fail, we generally recommend a trial of sacral neuromodulation for patients with no sphincter defect. When a sphincter defect is present, we counsel the patient about the merits of sphincter repair versus a trial of neuromodulation. These options have the most robust data supporting their clinical use, and have been used successfully in our own practices.
Given the continuous development of other therapeutic modalities, it is likely that future treatments will involve a stepwise progression of approaches. The need for colostomy should diminish in coming years as more minimally invasive techniques become available.
- Whitehead WE, Borrud L, Goode PS, et al. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137(2):512–517.
- Brown HW, Wexner SD, Segall MM, et al. Accidental bowel leakage in the mature women’s health study: prevalence and predictors. Int Clin Pract. 2012;66(11):1101–1108.
- Brown HW, Wexner SD, Segall MM, et al. Quality of life in women with accidental bowel leakage. Int Clin Pract. 2012;66(11):1109–1116.
- Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36(1):77–97.
- Sangwan YP, Collar JA, Barrett RC, et al. Unilateral pudendal neuropathy. Impact on outcomes of anal sphincter repair. Dis Colon Rectum. 1996;39(6):686–689.
- Deen KI, Kumar D, Williams JG, et al. Anal sphincter defects. Correlation between endoanal ultrasound and surgery. Ann Surg. 1993;218(2):201–205.
- Oberwalder M, Thaler K, Baig MK, et al. Anal ultrasound and endosonographic measurement of perineal body thickness: a new evaluation for fecal incontinence in females. Surg Endosc. 2004;18(4):650–654.
- Heymen S, Scarlett Y, Jones K, et al. Randomized controlled trial shows biofeedback to be superior to pelvic floor exercises for fecal incontinence. Dis Colon Rectum. 2009;52(10):1730–1737.
- Mellgren AF, Wexner SD, Coller JA, et al. Long-term efficacy and safety of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum. 2011:54(9):1065–1075.
- Tjandra JJ, Chan MK, Yeh CH, et al. Sacral nerve stimulation is more effective than optimal medical therapy for severe fecal incontinence: a randomized, controlled study. Dis Colon Rectum. 2008;51(5):494–502.
- Graf W, Mellgren A, Matzel K, et al. Efficacy of a dextranomer in stabilized hyaluronic acid for treatment of faecal incontinence: a randomized, sham-controlled trial. Lancet. 2011;377(9770):997–1003.
- El-Gazzaz G, Zutshi M, Hannaway C, Gurland B, Hull T. Overlapping sphincter repair: does age matter? Dis Colon Rectum. 2012;55(3):256–261.
- Glasgow SC, Lowry AC. Long-term outcomes of anal sphincter repair for fecal incontinence: a systematic review. Dis Colon Rectum. 2012;55(4):482–490.
- Wong WD, Congliosi SM, Spencer MP, et al. The safety and efficacy of the artificial bowel sphincter for fecal incontinence: results from a multicenter cohort study. Dis Colon Rectum. 2002;45(9):1139–1153.
- Takahashi T, Morales M, Garcia-Osogobio S, et al. Secca procedure for the treatment of fecal incontinence: results of five-year follow-up. Dis Colon Rectum. 2008;51(3):355–359.
- Lehur PA, McNevin S, Buntzen S, et al. Magnetic anal sphincter augmentation for the treatment of fecal incontinence: a preliminary report from a feasibility study. Dis Colon Rectum. 2010;53(12):1604–1610.
- TOPAS sling. http://clinicaltrials.gov/ct2/show/NCT01090739. Accessed August 26, 2013.
- Hotouras A, Thaha MA, Allison ME, et al. Percutaneous tibial nerve stimulation (PTNS) in females with faecal incontinence: the impact of sphincter morphology and rectal sensation on the clinical outcome. Int J Colorectal Dis. 2012;27(7):927–930.
Fecal incontinence is a socially embarrassing condition that affects approximately 18 million adults in the United States.1 Its true incidence is likely much higher than reported, however, as many patients are reluctant to discuss it.
A recent study found that nearly 20% of women experience fecal incontinence at least once a year, and 9.5% experience it al least once a month.2 Only 28% of these women had ever discussed their symptoms with a physician, however.3 Women who did seek care were more likely to consult a family physician or internist (75%) than a gynecologist (7%).3
Until recently, few options were available for patients with fecal incontinence who had not benefited from conservative measures. Many patients simply had to live with their symptoms or undergo a diverting ostomy to control the chronic involuntary drainage.
Recent years have seen the development of new minimally invasive and highly successful techniques to treat fecal incontinence. Greater awareness of the prevalence of fecal incontinence and its devastating impact on quality of life is needed for this problem to be fully addressed, however. In this article, we review the recommended evaluation of a patient who reports fecal incontinence and describe the range of treatment options.
Fecal incontinence is a symptom, not a diagnosis
Although the most common historical factor contributing to fecal incontinence is obstetric trauma, there are several other causes of this condition. A detailed history and physical examination are vital to determine whether the patient is experiencing true fecal incontinence, or whether she is leaking for other reasons—so-called pseudo-incontinence.
Conditions that can mimic fecal incontinence include:
- prolapsing hemorrhoids
- anal fistula
- sexually transmitted infection
- benign or malignant anorectal neoplasms
- dermatologic conditions.
True fecal incontinence may be active (loss of stool despite the patient’s best effort to control it) or passive (loss of stool without the patient’s awareness). Among the causes of true fecal incontinence are:
- anal sphincter injury (obstetric tear, anorectal surgery such as fistulotomy, or trauma)
- denervation of the pelvic floor from pudendal nerve injury during childbirth
- chronic rectal prolapse
- neurologic conditions (spina bifida, myelomeningocele)
- noncompliant rectum from inflammatory bowel disease
- radiation proctitis.
The maintenance of continence requires a complex interaction between the sphincter muscle, the puborectalis muscle (which acts as a sling), rectal capacity and compliance, stool volume and frequency, and neurologic mechanisms.
Diagnosis and management require an experienced physician
We believe that patients reporting fecal incontinence are best worked up and managed by a physician who is well versed in the various diagnoses associated with fecal incontinence, as well as the most current treatments.
Diagnosis entails some detective work
When a patient reports fecal incontinence, she should be asked to elaborate on the circumstances surrounding the complaint and the frequency of its occurrence, duration of symptoms, and nature of the incontinence (gas, liquid, or solid).
Validated quality-of-life instruments, such as the Cleveland Clinic Florida Fecal Incontinence Score (CCF-FIS) may be helpful in documenting the severity of the symptoms and improvement after treatment (TABLE).4
One factor that current scoring systems fail to capture is urgency. In many instances, urgency is the symptom most distressing to the patient. Be sure to ask about it.
A detailed obstetric history also is important. It is not uncommon for a patient to develop symptoms 20 years or longer after the injury. Also review the patient’s medical history for inflammatory bowel disease, neurologic disorders, and any history of pelvic radiation for help in determining the cause of symptoms.
In addition, ask the patient about any other pelvic floor symptoms, such as voiding dysfunction and problems with pelvic organ prolapse. And question her about stool consistency and frequency. In some cases, diarrhea can lead to fecal incontinence and is usually managed conservatively.
Physical exam: Focus on the perineum and anus
A detailed physical examination is warranted to determine the state of the patient’s sphincter musculature and rule out other causes of pseudo-incontinence, such as hemorrhoids or anal fistula. Inspect the perineum for thinning of the perineal body and scars from prior surgery.
A patulous anus may be a sign of rectal prolapse. To check for it, ask the patient to strain on the commode. If rectal prolapse is present, it will become apparent upon straining. If prolapse is detected, surgical treatment of the prolapse would be the first step in managing the incontinence.
A simple test of neurologic function is to try to elicit an anal “wink” in response to a pinprick.
A digital rectal exam allows the assessment of resting and squeeze tone, as well as the use of accessory muscles, such as the gluteus maximus, during squeezing.
Rigid or flexible proctoscopy may be indicated to rule out inflammatory bowel disease, radiation proctitis, and rectal neoplasm, depending on the patient’s history.
A few diagnostic adjuncts can help
Several adjuncts to physical examination can provide more detailed information about the patient’s condition and facilitate the development of an individualized treatment plan. For example, if rectal prolapse, rectocele with delayed emptying, or enterocele is suspected, consider defecography. If voiding dysfunction coexists with the fecal incontinence, urodynamic testing and cystoscopy may be indicated.
We routinely perform physiology testing and endoanal ultrasound if surgery is planned to address the fecal incontinence, although routine use of these adjuncts is controversial. Because many patients can be managed with conservative medical measures, we do not find it necessary to perform these tests at the time of the first visit.
Anal physiology testing includes manometry (a measure of both resting and squeeze tone) and pudendal nerve terminal motor latency testing.
Manometry can help quantify the severity of muscle weakness and determine the presence or absence of normal anal reflexes. Pudendal nerve testing assesses the innervation of the anal sphincter. There is some evidence that patients who have a pudendal neuropathy have a poor outcome with sphincteroplasty,5 although that evidence is controversial. The findings from physiology studies have not been correlated with outcomes of newer treatments, such as sacral neuromodulation (InterStim, Medtronic, Minneapolis, Minnesota). Each physiology lab uses different equipment, so “normal” values vary between institutions.
Endoanal ultrasound is easily performed in an office setting. It is well tolerated and provides anatomic detail of the sphincter musculature. We use a 13-MHz rotating probe to provide 3D imaging of the anal canal. The internal sphincter is represented by a hypoechoic circle surrounded by the hyperechoic external sphincter (FIGURE 1).
In the hands of an experienced examiner, the sensitivity and specificity of endoanal ultrasound in detecting sphincter defects approaches 100%.6,7 Ultrasound also enables measurement of the perineal body. A normal perineal body measures 10 to 15 mm.
For treatment, try conservative measures first
Bulking agents (fiber), constipating agents (loperamide, etc.), or a laxative regimen with scheduled disimpactions (in patients who have pelvic outlet constipation and overflow incontinence) often can control the symptoms of fecal incontinence, making further interventions unnecessary.
Biofeedback is another option. It uses visual, auditory, and sensory information to train patients to better control anal sphincter muscle function.
A recent randomized study found manometric biofeedback to be superior to simple Kegel exercises in improving fecal continence.8 In this study, 76% of patients in the biofeedback group experienced symptom improvement, compared with 41% of patients in the pelvic floor exercise group (P <.001). The long-term benefits of biofeedback are less clear, and patients often need to be reminded to perform their exercises at home and to attend occasional refresher-training sessions. Nevertheless, biofeedback is an important noninvasive option for patients in whom medical management has failed.
Minimally invasive options are now available
Over the past 2 years, minimally invasive treatments for fecal incontinence have emerged, including an implantable sacral neuromodulation device (InterStim) and an injectable dextranomer (Solesta; Salix Pharmaceuticals, Raleigh, North Carolina). Previously, the only surgical option for fecal incontinence was a sphincter repair if a defect was present. The new options may help patients improve their quality of life without having to undergo major surgery.
No one has directly compared the outcomes of these procedures when they are performed by a colorectal surgeon versus a physician of another specialty. It is our belief that the treating physician should have a strong interest in caring for these complex patients and a good working knowledge of the various treatment options.
Related Article Obstetric anal sphincter injury: 7 critical questions about care Ranee Thakar, MD, MRCOG (February 2008)
Sacral neuromodulation
This technique initially was developed for the treatment of overactive bladder and nonobstructive urinary retention and has been used in the United States for the past 15 years for these indications. Improvement in fecal continence was observed in these patients, prompting further studies of its efficacy. In 2011, it was approved by the US Food and Drug Administration (FDA) for the treatment of fecal incontinence. It has since revolutionized the treatment of this disorder, offering a minimally invasive and highly successful alternative to sphincteroplasty.
The InterStim procedure is the only therapeutic modality to include a test phase. The outpatient procedure involves sterile placement of an electrode through the S3 foramen to stimulate the S3 nerve root using fluoroscopic guidance (FIGURES 2 and 3). Patients who experience at least 50% improvement in symptoms are then offered placement of a permanent stimulator.
In most series, approximately 90% of patients have a positive test and progress to implantation. A recent US multicenter clinical trial indicated that 86% of patients achieved an improvement in continence of at least 50%, and 40% of patients were completely continent at 3 years.9 The number of episodes of incontinence decreased from a mean of 9.4/week to 1.7/week.9 Quality of life also improved greatly. Few complications have been reported, the most notable of which is infection (10.8% in the US multicenter trial9).
Another advantage of sacral neuromodulation: It can be used successfully in patients with external sphincter defects as large as 120º. A study by Tjandra and colleagues found that 65% of patients experienced improvement in symptoms of at least 50%, and 47% of patients (more than 50% of whom had external sphincter defects as large as 120º) became completely continent.10
The only variable shown to predict success with sacral neuromodulation is a positive response to the test implant procedure.
In our experience, this procedure is easy to perform and well tolerated, even in elderly patients with multiple comorbidities. The procedure has the additional advantage of potentially improving concomitant urinary symptoms as well.
The major disadvantage of sacral neuromodulation is its cost, although most major insurance carriers cover it. There is no well-conducted cost-effectiveness analysis comparing this modality to other treatments.
Related Article Interstim: An implantable device for implacable urinary symptoms Deborah L. Myers, MD (October 2006)
Injectable agents
Several biocompatible bulking agents have been tested in the treatment of fecal incontinence. These compounds traditionally have been used to treat mild fecal incontinence, or to treat patients with isolated internal sphincter defects.
More recently, an injectable dextranomer in stabilized hyaluronic acid was approved by the FDA and marketed as Solesta. Graf and colleagues randomly allocated 136 patients to injection and 70 patients to sham injection. Patients with external sphincter defects were excluded. At 6 months, 52% of patients in the active treatment group experienced an improvement in continence of at least 50%, compared with 31% of patients injected with placebo.11
The advantage of this procedure is its minimally invasive nature (submucosal injection performed in the office). The disadvantage: a lack of long-term efficacy data, although unpublished data suggest that patients who improve after an injection see a durable response at 3 years.
This easy, office-based treatment is ideal for patients with minor incontinence or persistent symptoms after another procedure.
Sphincter repair
Anterior sphincteroplasty has been the mainstay of surgical treatment for patients with a sphincter defect. With the patient in a dorsal lithotomy or prone position on the operating-room table, a transverse perineal incision is made, and the ends of the severed sphincter muscle are located and mobilized. The repair then can be performed in an end-to-end manner or by overlapping the muscles in the anterior midline (FIGURE 4).
Some of the debatable technical issues of this procedure include:
- whether to overlap the muscles or scar tissue
- whether to repair internal and external defects together or separately
- how the age of the patient affects the outcome.
In regard to the first issue, there may be a superior outcome with overlapping repairs, but they carry a higher risk of dyspareunia and evacuation difficulties. Some surgeons will attempt a separate repair of the internal and external sphincter muscles if it appears feasible. Most often, both muscles are tethered together with scar tissue and separate repair is not possible. There are no conclusive data to demonstrate the superiority of either approach.
As for age, the traditional teaching was that older patients do not benefit from this procedure as much as younger patients do. However, a recent study found no differences in the CCF-FIS score in patients older than age 60, compared with younger patients.12 Investigators concluded that sphincteroplasty can be offered to both young and older patients.12
Although sphincteroplasty often leads to excellent short-term improvement, with 60% to 90% of patients experiencing a good or excellent outcome, nearly all series indicate a decline over the long term (>5 years). A recent systematic review found that as few as 12% of patients experience a good or excellent result, depending on the series.13
We offer sphincter repair to young women with a new sphincter defect after delivery. For older patients, we offer sacral neuromodulation as a first-line treatment.
Other surgical options
We believe that most patients with fecal incontinence can be managed using conservative measures, sacral neuromodulation, injectable dextranomer, or sphincter repair. However, several other options are available.
Artificial bowel sphincter
The artificial bowel sphincter was first described in 1987 and has been modified over the years. The system currently is marketed as the Acticon Neosphincter (American Medical Systems, Minnetonka, Minnesota). The procedure involves the creation of a subcutaneous tunnel around the anus so that an inflatable cuff can be positioned there. A pump then is tunneled through a Pfannenstiel incision to the labia or scrotum, and a reservoir is positioned in the space of Retzius. The device maintains continence by keeping the cuff inflated during the resting state and by pumping fluid from the cuff to the reservoir when the patient needs to evacuate.
The major barrier to utilization of the artificial bowel sphincter is infection. In a series of 112 patients who were implanted with the sphincter, 384 device-related adverse events occurred in 99 patients.14 A total of 73 revision operations were required in 51 patients (46%). Twenty-five percent of patients developed infection that required surgical revision, and 37% had the device explanted. Eighty-five percent of patients with a functional device had a successful outcome.14
Given the device-related challenges and infectious complications, patients should be considered for less invasive treatments before being offered an artificial bowel
sphincter.
Radiofrequency current
The Secca procedure (Curon Medical, Fremont, California) involves the application of radiofrequency current to the anal canal to generate thermal energy. This procedure causes contraction of collagen fibers, which are permanently shortened, and leads to tightening of the muscle. It is performed under intravenous sedation on an outpatient basis.
This approach is indicated for patients with mild to moderate fecal incontinence who have not responded to conservative management. An external sphincter defect is a contraindication.
Small, nonrandomized studies have found improvement in the CCF-FIS score in patients treated with this approach.15 The major limitation of this treatment is the lack of high-level clinical evidence demonstrating its efficacy and safety.
Antegrade continence enema
This approach, also known as the Malone procedure, is usually reserved for debilitating incontinence or constipation in the pediatric population. An appendicostomy is constructed at the navel, allowing daily introduction of a catheter and antegrade enema. The purpose is to perform rapid, controlled emptying of the colon at times chosen by the patient. It is also reserved as a last resort for patients considering an ostomy.
Adult patients with neurologic problems, such as spina bifida, may be candidates for this procedure, provided they are highly motivated.
Fecal diversion
Creation of a colostomy or ileostomy is usually the last resort for a patient with fecal incontinence. We are fortunate that there are an increasing number of options that may improve the patient’s condition before colostomy is required.
If fecal diversion is chosen by the patient, it is important to involve an enterostomal therapist for site marking and patient education. A well-constructed ostomy is essential, as this option often is permanent.
Up and coming options
A novel treatment approach for fecal incontinence is the magnetic anal sphincter. The device, marketed as the FENIX Continence Restoration System (Torax Medical, Shoreview, Minnesota) consists of a series of titanium beads with magnetic cores that are interlinked with titanium wires. The device is designed to encircle the external anal sphincter muscle, reinforce the sphincter, and expand to allow passage of stool at a socially appropriate time.
Preliminary data from 16 patients indicate a mean decrease in the number of episodes of incontinence from 7.2/week to 0.7/week, as well as a mean reduction in the CCF-FIS score from 17.2 to 8.7.16 Two devices were removed due to infection, and one device passed spontaneously after disconnection of the suture.16
This device is not approved by the FDA, but it may become a promising treatment if its safety and efficacy can be established in larger clinical trials.
The TOPAS sling (American Medical Systems) is currently being studied in a Phase 3, multicenter, nonrandomized, clinical trial (NCT01090739) for the treatment of fecal incontinence.17 The sling is implanted using a minimally invasive transobturator approach; two needle-passers deliver the sling assembly. Two small posterior incisions facilitate the postanal placement of the mesh.
This procedure replicates the anorectal angle created by the puborectalis muscle. Although it may become a minimally invasive treatment in the future, final results of the Phase 3 trial are not expected until 2016.
Tibial nerve stimulation is commonly used for urinary urge incontinence. Several small series have documented modest success with its application to fecal incontinence.18
The outpatient procedure involves the insertion of a needle electrode three fingerbreadths above the medial malleolus, followed by electrical stimulation. The current is slowly increased until a sensory or motor response (tingling under the sole of the foot or great toe plantar flexion) is elicited. Treatment necessitates several outpatient sessions.
In a recent series, the mean CCF-FIS decreased from 12.2/20 at baseline to 9.1/20 after treatment (P <.0001).18
The role of this procedure in the treatment algorithm for fecal incontinence remains to be determined.
What we offer patients
Fecal incontinence is a debilitating condition with an increasing number of potential therapeutic options. It clearly is under-recognized by patients and physicians alike.
After a thorough work-up, conservative treatment options should be offered first. When those fail, we generally recommend a trial of sacral neuromodulation for patients with no sphincter defect. When a sphincter defect is present, we counsel the patient about the merits of sphincter repair versus a trial of neuromodulation. These options have the most robust data supporting their clinical use, and have been used successfully in our own practices.
Given the continuous development of other therapeutic modalities, it is likely that future treatments will involve a stepwise progression of approaches. The need for colostomy should diminish in coming years as more minimally invasive techniques become available.
Fecal incontinence is a socially embarrassing condition that affects approximately 18 million adults in the United States.1 Its true incidence is likely much higher than reported, however, as many patients are reluctant to discuss it.
A recent study found that nearly 20% of women experience fecal incontinence at least once a year, and 9.5% experience it al least once a month.2 Only 28% of these women had ever discussed their symptoms with a physician, however.3 Women who did seek care were more likely to consult a family physician or internist (75%) than a gynecologist (7%).3
Until recently, few options were available for patients with fecal incontinence who had not benefited from conservative measures. Many patients simply had to live with their symptoms or undergo a diverting ostomy to control the chronic involuntary drainage.
Recent years have seen the development of new minimally invasive and highly successful techniques to treat fecal incontinence. Greater awareness of the prevalence of fecal incontinence and its devastating impact on quality of life is needed for this problem to be fully addressed, however. In this article, we review the recommended evaluation of a patient who reports fecal incontinence and describe the range of treatment options.
Fecal incontinence is a symptom, not a diagnosis
Although the most common historical factor contributing to fecal incontinence is obstetric trauma, there are several other causes of this condition. A detailed history and physical examination are vital to determine whether the patient is experiencing true fecal incontinence, or whether she is leaking for other reasons—so-called pseudo-incontinence.
Conditions that can mimic fecal incontinence include:
- prolapsing hemorrhoids
- anal fistula
- sexually transmitted infection
- benign or malignant anorectal neoplasms
- dermatologic conditions.
True fecal incontinence may be active (loss of stool despite the patient’s best effort to control it) or passive (loss of stool without the patient’s awareness). Among the causes of true fecal incontinence are:
- anal sphincter injury (obstetric tear, anorectal surgery such as fistulotomy, or trauma)
- denervation of the pelvic floor from pudendal nerve injury during childbirth
- chronic rectal prolapse
- neurologic conditions (spina bifida, myelomeningocele)
- noncompliant rectum from inflammatory bowel disease
- radiation proctitis.
The maintenance of continence requires a complex interaction between the sphincter muscle, the puborectalis muscle (which acts as a sling), rectal capacity and compliance, stool volume and frequency, and neurologic mechanisms.
Diagnosis and management require an experienced physician
We believe that patients reporting fecal incontinence are best worked up and managed by a physician who is well versed in the various diagnoses associated with fecal incontinence, as well as the most current treatments.
Diagnosis entails some detective work
When a patient reports fecal incontinence, she should be asked to elaborate on the circumstances surrounding the complaint and the frequency of its occurrence, duration of symptoms, and nature of the incontinence (gas, liquid, or solid).
Validated quality-of-life instruments, such as the Cleveland Clinic Florida Fecal Incontinence Score (CCF-FIS) may be helpful in documenting the severity of the symptoms and improvement after treatment (TABLE).4
One factor that current scoring systems fail to capture is urgency. In many instances, urgency is the symptom most distressing to the patient. Be sure to ask about it.
A detailed obstetric history also is important. It is not uncommon for a patient to develop symptoms 20 years or longer after the injury. Also review the patient’s medical history for inflammatory bowel disease, neurologic disorders, and any history of pelvic radiation for help in determining the cause of symptoms.
In addition, ask the patient about any other pelvic floor symptoms, such as voiding dysfunction and problems with pelvic organ prolapse. And question her about stool consistency and frequency. In some cases, diarrhea can lead to fecal incontinence and is usually managed conservatively.
Physical exam: Focus on the perineum and anus
A detailed physical examination is warranted to determine the state of the patient’s sphincter musculature and rule out other causes of pseudo-incontinence, such as hemorrhoids or anal fistula. Inspect the perineum for thinning of the perineal body and scars from prior surgery.
A patulous anus may be a sign of rectal prolapse. To check for it, ask the patient to strain on the commode. If rectal prolapse is present, it will become apparent upon straining. If prolapse is detected, surgical treatment of the prolapse would be the first step in managing the incontinence.
A simple test of neurologic function is to try to elicit an anal “wink” in response to a pinprick.
A digital rectal exam allows the assessment of resting and squeeze tone, as well as the use of accessory muscles, such as the gluteus maximus, during squeezing.
Rigid or flexible proctoscopy may be indicated to rule out inflammatory bowel disease, radiation proctitis, and rectal neoplasm, depending on the patient’s history.
A few diagnostic adjuncts can help
Several adjuncts to physical examination can provide more detailed information about the patient’s condition and facilitate the development of an individualized treatment plan. For example, if rectal prolapse, rectocele with delayed emptying, or enterocele is suspected, consider defecography. If voiding dysfunction coexists with the fecal incontinence, urodynamic testing and cystoscopy may be indicated.
We routinely perform physiology testing and endoanal ultrasound if surgery is planned to address the fecal incontinence, although routine use of these adjuncts is controversial. Because many patients can be managed with conservative medical measures, we do not find it necessary to perform these tests at the time of the first visit.
Anal physiology testing includes manometry (a measure of both resting and squeeze tone) and pudendal nerve terminal motor latency testing.
Manometry can help quantify the severity of muscle weakness and determine the presence or absence of normal anal reflexes. Pudendal nerve testing assesses the innervation of the anal sphincter. There is some evidence that patients who have a pudendal neuropathy have a poor outcome with sphincteroplasty,5 although that evidence is controversial. The findings from physiology studies have not been correlated with outcomes of newer treatments, such as sacral neuromodulation (InterStim, Medtronic, Minneapolis, Minnesota). Each physiology lab uses different equipment, so “normal” values vary between institutions.
Endoanal ultrasound is easily performed in an office setting. It is well tolerated and provides anatomic detail of the sphincter musculature. We use a 13-MHz rotating probe to provide 3D imaging of the anal canal. The internal sphincter is represented by a hypoechoic circle surrounded by the hyperechoic external sphincter (FIGURE 1).
In the hands of an experienced examiner, the sensitivity and specificity of endoanal ultrasound in detecting sphincter defects approaches 100%.6,7 Ultrasound also enables measurement of the perineal body. A normal perineal body measures 10 to 15 mm.
For treatment, try conservative measures first
Bulking agents (fiber), constipating agents (loperamide, etc.), or a laxative regimen with scheduled disimpactions (in patients who have pelvic outlet constipation and overflow incontinence) often can control the symptoms of fecal incontinence, making further interventions unnecessary.
Biofeedback is another option. It uses visual, auditory, and sensory information to train patients to better control anal sphincter muscle function.
A recent randomized study found manometric biofeedback to be superior to simple Kegel exercises in improving fecal continence.8 In this study, 76% of patients in the biofeedback group experienced symptom improvement, compared with 41% of patients in the pelvic floor exercise group (P <.001). The long-term benefits of biofeedback are less clear, and patients often need to be reminded to perform their exercises at home and to attend occasional refresher-training sessions. Nevertheless, biofeedback is an important noninvasive option for patients in whom medical management has failed.
Minimally invasive options are now available
Over the past 2 years, minimally invasive treatments for fecal incontinence have emerged, including an implantable sacral neuromodulation device (InterStim) and an injectable dextranomer (Solesta; Salix Pharmaceuticals, Raleigh, North Carolina). Previously, the only surgical option for fecal incontinence was a sphincter repair if a defect was present. The new options may help patients improve their quality of life without having to undergo major surgery.
No one has directly compared the outcomes of these procedures when they are performed by a colorectal surgeon versus a physician of another specialty. It is our belief that the treating physician should have a strong interest in caring for these complex patients and a good working knowledge of the various treatment options.
Related Article Obstetric anal sphincter injury: 7 critical questions about care Ranee Thakar, MD, MRCOG (February 2008)
Sacral neuromodulation
This technique initially was developed for the treatment of overactive bladder and nonobstructive urinary retention and has been used in the United States for the past 15 years for these indications. Improvement in fecal continence was observed in these patients, prompting further studies of its efficacy. In 2011, it was approved by the US Food and Drug Administration (FDA) for the treatment of fecal incontinence. It has since revolutionized the treatment of this disorder, offering a minimally invasive and highly successful alternative to sphincteroplasty.
The InterStim procedure is the only therapeutic modality to include a test phase. The outpatient procedure involves sterile placement of an electrode through the S3 foramen to stimulate the S3 nerve root using fluoroscopic guidance (FIGURES 2 and 3). Patients who experience at least 50% improvement in symptoms are then offered placement of a permanent stimulator.
In most series, approximately 90% of patients have a positive test and progress to implantation. A recent US multicenter clinical trial indicated that 86% of patients achieved an improvement in continence of at least 50%, and 40% of patients were completely continent at 3 years.9 The number of episodes of incontinence decreased from a mean of 9.4/week to 1.7/week.9 Quality of life also improved greatly. Few complications have been reported, the most notable of which is infection (10.8% in the US multicenter trial9).
Another advantage of sacral neuromodulation: It can be used successfully in patients with external sphincter defects as large as 120º. A study by Tjandra and colleagues found that 65% of patients experienced improvement in symptoms of at least 50%, and 47% of patients (more than 50% of whom had external sphincter defects as large as 120º) became completely continent.10
The only variable shown to predict success with sacral neuromodulation is a positive response to the test implant procedure.
In our experience, this procedure is easy to perform and well tolerated, even in elderly patients with multiple comorbidities. The procedure has the additional advantage of potentially improving concomitant urinary symptoms as well.
The major disadvantage of sacral neuromodulation is its cost, although most major insurance carriers cover it. There is no well-conducted cost-effectiveness analysis comparing this modality to other treatments.
Related Article Interstim: An implantable device for implacable urinary symptoms Deborah L. Myers, MD (October 2006)
Injectable agents
Several biocompatible bulking agents have been tested in the treatment of fecal incontinence. These compounds traditionally have been used to treat mild fecal incontinence, or to treat patients with isolated internal sphincter defects.
More recently, an injectable dextranomer in stabilized hyaluronic acid was approved by the FDA and marketed as Solesta. Graf and colleagues randomly allocated 136 patients to injection and 70 patients to sham injection. Patients with external sphincter defects were excluded. At 6 months, 52% of patients in the active treatment group experienced an improvement in continence of at least 50%, compared with 31% of patients injected with placebo.11
The advantage of this procedure is its minimally invasive nature (submucosal injection performed in the office). The disadvantage: a lack of long-term efficacy data, although unpublished data suggest that patients who improve after an injection see a durable response at 3 years.
This easy, office-based treatment is ideal for patients with minor incontinence or persistent symptoms after another procedure.
Sphincter repair
Anterior sphincteroplasty has been the mainstay of surgical treatment for patients with a sphincter defect. With the patient in a dorsal lithotomy or prone position on the operating-room table, a transverse perineal incision is made, and the ends of the severed sphincter muscle are located and mobilized. The repair then can be performed in an end-to-end manner or by overlapping the muscles in the anterior midline (FIGURE 4).
Some of the debatable technical issues of this procedure include:
- whether to overlap the muscles or scar tissue
- whether to repair internal and external defects together or separately
- how the age of the patient affects the outcome.
In regard to the first issue, there may be a superior outcome with overlapping repairs, but they carry a higher risk of dyspareunia and evacuation difficulties. Some surgeons will attempt a separate repair of the internal and external sphincter muscles if it appears feasible. Most often, both muscles are tethered together with scar tissue and separate repair is not possible. There are no conclusive data to demonstrate the superiority of either approach.
As for age, the traditional teaching was that older patients do not benefit from this procedure as much as younger patients do. However, a recent study found no differences in the CCF-FIS score in patients older than age 60, compared with younger patients.12 Investigators concluded that sphincteroplasty can be offered to both young and older patients.12
Although sphincteroplasty often leads to excellent short-term improvement, with 60% to 90% of patients experiencing a good or excellent outcome, nearly all series indicate a decline over the long term (>5 years). A recent systematic review found that as few as 12% of patients experience a good or excellent result, depending on the series.13
We offer sphincter repair to young women with a new sphincter defect after delivery. For older patients, we offer sacral neuromodulation as a first-line treatment.
Other surgical options
We believe that most patients with fecal incontinence can be managed using conservative measures, sacral neuromodulation, injectable dextranomer, or sphincter repair. However, several other options are available.
Artificial bowel sphincter
The artificial bowel sphincter was first described in 1987 and has been modified over the years. The system currently is marketed as the Acticon Neosphincter (American Medical Systems, Minnetonka, Minnesota). The procedure involves the creation of a subcutaneous tunnel around the anus so that an inflatable cuff can be positioned there. A pump then is tunneled through a Pfannenstiel incision to the labia or scrotum, and a reservoir is positioned in the space of Retzius. The device maintains continence by keeping the cuff inflated during the resting state and by pumping fluid from the cuff to the reservoir when the patient needs to evacuate.
The major barrier to utilization of the artificial bowel sphincter is infection. In a series of 112 patients who were implanted with the sphincter, 384 device-related adverse events occurred in 99 patients.14 A total of 73 revision operations were required in 51 patients (46%). Twenty-five percent of patients developed infection that required surgical revision, and 37% had the device explanted. Eighty-five percent of patients with a functional device had a successful outcome.14
Given the device-related challenges and infectious complications, patients should be considered for less invasive treatments before being offered an artificial bowel
sphincter.
Radiofrequency current
The Secca procedure (Curon Medical, Fremont, California) involves the application of radiofrequency current to the anal canal to generate thermal energy. This procedure causes contraction of collagen fibers, which are permanently shortened, and leads to tightening of the muscle. It is performed under intravenous sedation on an outpatient basis.
This approach is indicated for patients with mild to moderate fecal incontinence who have not responded to conservative management. An external sphincter defect is a contraindication.
Small, nonrandomized studies have found improvement in the CCF-FIS score in patients treated with this approach.15 The major limitation of this treatment is the lack of high-level clinical evidence demonstrating its efficacy and safety.
Antegrade continence enema
This approach, also known as the Malone procedure, is usually reserved for debilitating incontinence or constipation in the pediatric population. An appendicostomy is constructed at the navel, allowing daily introduction of a catheter and antegrade enema. The purpose is to perform rapid, controlled emptying of the colon at times chosen by the patient. It is also reserved as a last resort for patients considering an ostomy.
Adult patients with neurologic problems, such as spina bifida, may be candidates for this procedure, provided they are highly motivated.
Fecal diversion
Creation of a colostomy or ileostomy is usually the last resort for a patient with fecal incontinence. We are fortunate that there are an increasing number of options that may improve the patient’s condition before colostomy is required.
If fecal diversion is chosen by the patient, it is important to involve an enterostomal therapist for site marking and patient education. A well-constructed ostomy is essential, as this option often is permanent.
Up and coming options
A novel treatment approach for fecal incontinence is the magnetic anal sphincter. The device, marketed as the FENIX Continence Restoration System (Torax Medical, Shoreview, Minnesota) consists of a series of titanium beads with magnetic cores that are interlinked with titanium wires. The device is designed to encircle the external anal sphincter muscle, reinforce the sphincter, and expand to allow passage of stool at a socially appropriate time.
Preliminary data from 16 patients indicate a mean decrease in the number of episodes of incontinence from 7.2/week to 0.7/week, as well as a mean reduction in the CCF-FIS score from 17.2 to 8.7.16 Two devices were removed due to infection, and one device passed spontaneously after disconnection of the suture.16
This device is not approved by the FDA, but it may become a promising treatment if its safety and efficacy can be established in larger clinical trials.
The TOPAS sling (American Medical Systems) is currently being studied in a Phase 3, multicenter, nonrandomized, clinical trial (NCT01090739) for the treatment of fecal incontinence.17 The sling is implanted using a minimally invasive transobturator approach; two needle-passers deliver the sling assembly. Two small posterior incisions facilitate the postanal placement of the mesh.
This procedure replicates the anorectal angle created by the puborectalis muscle. Although it may become a minimally invasive treatment in the future, final results of the Phase 3 trial are not expected until 2016.
Tibial nerve stimulation is commonly used for urinary urge incontinence. Several small series have documented modest success with its application to fecal incontinence.18
The outpatient procedure involves the insertion of a needle electrode three fingerbreadths above the medial malleolus, followed by electrical stimulation. The current is slowly increased until a sensory or motor response (tingling under the sole of the foot or great toe plantar flexion) is elicited. Treatment necessitates several outpatient sessions.
In a recent series, the mean CCF-FIS decreased from 12.2/20 at baseline to 9.1/20 after treatment (P <.0001).18
The role of this procedure in the treatment algorithm for fecal incontinence remains to be determined.
What we offer patients
Fecal incontinence is a debilitating condition with an increasing number of potential therapeutic options. It clearly is under-recognized by patients and physicians alike.
After a thorough work-up, conservative treatment options should be offered first. When those fail, we generally recommend a trial of sacral neuromodulation for patients with no sphincter defect. When a sphincter defect is present, we counsel the patient about the merits of sphincter repair versus a trial of neuromodulation. These options have the most robust data supporting their clinical use, and have been used successfully in our own practices.
Given the continuous development of other therapeutic modalities, it is likely that future treatments will involve a stepwise progression of approaches. The need for colostomy should diminish in coming years as more minimally invasive techniques become available.
- Whitehead WE, Borrud L, Goode PS, et al. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137(2):512–517.
- Brown HW, Wexner SD, Segall MM, et al. Accidental bowel leakage in the mature women’s health study: prevalence and predictors. Int Clin Pract. 2012;66(11):1101–1108.
- Brown HW, Wexner SD, Segall MM, et al. Quality of life in women with accidental bowel leakage. Int Clin Pract. 2012;66(11):1109–1116.
- Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36(1):77–97.
- Sangwan YP, Collar JA, Barrett RC, et al. Unilateral pudendal neuropathy. Impact on outcomes of anal sphincter repair. Dis Colon Rectum. 1996;39(6):686–689.
- Deen KI, Kumar D, Williams JG, et al. Anal sphincter defects. Correlation between endoanal ultrasound and surgery. Ann Surg. 1993;218(2):201–205.
- Oberwalder M, Thaler K, Baig MK, et al. Anal ultrasound and endosonographic measurement of perineal body thickness: a new evaluation for fecal incontinence in females. Surg Endosc. 2004;18(4):650–654.
- Heymen S, Scarlett Y, Jones K, et al. Randomized controlled trial shows biofeedback to be superior to pelvic floor exercises for fecal incontinence. Dis Colon Rectum. 2009;52(10):1730–1737.
- Mellgren AF, Wexner SD, Coller JA, et al. Long-term efficacy and safety of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum. 2011:54(9):1065–1075.
- Tjandra JJ, Chan MK, Yeh CH, et al. Sacral nerve stimulation is more effective than optimal medical therapy for severe fecal incontinence: a randomized, controlled study. Dis Colon Rectum. 2008;51(5):494–502.
- Graf W, Mellgren A, Matzel K, et al. Efficacy of a dextranomer in stabilized hyaluronic acid for treatment of faecal incontinence: a randomized, sham-controlled trial. Lancet. 2011;377(9770):997–1003.
- El-Gazzaz G, Zutshi M, Hannaway C, Gurland B, Hull T. Overlapping sphincter repair: does age matter? Dis Colon Rectum. 2012;55(3):256–261.
- Glasgow SC, Lowry AC. Long-term outcomes of anal sphincter repair for fecal incontinence: a systematic review. Dis Colon Rectum. 2012;55(4):482–490.
- Wong WD, Congliosi SM, Spencer MP, et al. The safety and efficacy of the artificial bowel sphincter for fecal incontinence: results from a multicenter cohort study. Dis Colon Rectum. 2002;45(9):1139–1153.
- Takahashi T, Morales M, Garcia-Osogobio S, et al. Secca procedure for the treatment of fecal incontinence: results of five-year follow-up. Dis Colon Rectum. 2008;51(3):355–359.
- Lehur PA, McNevin S, Buntzen S, et al. Magnetic anal sphincter augmentation for the treatment of fecal incontinence: a preliminary report from a feasibility study. Dis Colon Rectum. 2010;53(12):1604–1610.
- TOPAS sling. http://clinicaltrials.gov/ct2/show/NCT01090739. Accessed August 26, 2013.
- Hotouras A, Thaha MA, Allison ME, et al. Percutaneous tibial nerve stimulation (PTNS) in females with faecal incontinence: the impact of sphincter morphology and rectal sensation on the clinical outcome. Int J Colorectal Dis. 2012;27(7):927–930.
- Whitehead WE, Borrud L, Goode PS, et al. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137(2):512–517.
- Brown HW, Wexner SD, Segall MM, et al. Accidental bowel leakage in the mature women’s health study: prevalence and predictors. Int Clin Pract. 2012;66(11):1101–1108.
- Brown HW, Wexner SD, Segall MM, et al. Quality of life in women with accidental bowel leakage. Int Clin Pract. 2012;66(11):1109–1116.
- Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36(1):77–97.
- Sangwan YP, Collar JA, Barrett RC, et al. Unilateral pudendal neuropathy. Impact on outcomes of anal sphincter repair. Dis Colon Rectum. 1996;39(6):686–689.
- Deen KI, Kumar D, Williams JG, et al. Anal sphincter defects. Correlation between endoanal ultrasound and surgery. Ann Surg. 1993;218(2):201–205.
- Oberwalder M, Thaler K, Baig MK, et al. Anal ultrasound and endosonographic measurement of perineal body thickness: a new evaluation for fecal incontinence in females. Surg Endosc. 2004;18(4):650–654.
- Heymen S, Scarlett Y, Jones K, et al. Randomized controlled trial shows biofeedback to be superior to pelvic floor exercises for fecal incontinence. Dis Colon Rectum. 2009;52(10):1730–1737.
- Mellgren AF, Wexner SD, Coller JA, et al. Long-term efficacy and safety of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum. 2011:54(9):1065–1075.
- Tjandra JJ, Chan MK, Yeh CH, et al. Sacral nerve stimulation is more effective than optimal medical therapy for severe fecal incontinence: a randomized, controlled study. Dis Colon Rectum. 2008;51(5):494–502.
- Graf W, Mellgren A, Matzel K, et al. Efficacy of a dextranomer in stabilized hyaluronic acid for treatment of faecal incontinence: a randomized, sham-controlled trial. Lancet. 2011;377(9770):997–1003.
- El-Gazzaz G, Zutshi M, Hannaway C, Gurland B, Hull T. Overlapping sphincter repair: does age matter? Dis Colon Rectum. 2012;55(3):256–261.
- Glasgow SC, Lowry AC. Long-term outcomes of anal sphincter repair for fecal incontinence: a systematic review. Dis Colon Rectum. 2012;55(4):482–490.
- Wong WD, Congliosi SM, Spencer MP, et al. The safety and efficacy of the artificial bowel sphincter for fecal incontinence: results from a multicenter cohort study. Dis Colon Rectum. 2002;45(9):1139–1153.
- Takahashi T, Morales M, Garcia-Osogobio S, et al. Secca procedure for the treatment of fecal incontinence: results of five-year follow-up. Dis Colon Rectum. 2008;51(3):355–359.
- Lehur PA, McNevin S, Buntzen S, et al. Magnetic anal sphincter augmentation for the treatment of fecal incontinence: a preliminary report from a feasibility study. Dis Colon Rectum. 2010;53(12):1604–1610.
- TOPAS sling. http://clinicaltrials.gov/ct2/show/NCT01090739. Accessed August 26, 2013.
- Hotouras A, Thaha MA, Allison ME, et al. Percutaneous tibial nerve stimulation (PTNS) in females with faecal incontinence: the impact of sphincter morphology and rectal sensation on the clinical outcome. Int J Colorectal Dis. 2012;27(7):927–930.
Oops! Did we miss something?
Attention deficit is a common diagnosis with which many pediatricians are faced. In the more common scenario, the child presents anywhere from age 5 to 10 years with parental and teacher complaint of hyperactivity or academic underachievement. But, what do you do with the child who presents at age 11 years or high school age with no significant history of school failure or behavior issues? Is this a child whose symptoms were overlooked? New-onset inattention? Are they seeking medication to augment their intellectual ability to become more competitive? Or are they just drug seeking? Well, the truth is that any one of the above could be true, and only through careful analysis can you obtain the proper diagnosis.
For the adolescent that meets the DSM-IV criteria for attention-deficit/hyperactivity disorder (ADHD), it is possible that they have learned to compensate for their inattention and have been successful in maintaining a good grade-point average. Through detailed questioning about study habits and home environment, they likely required a lot of support from their parents to maintain organization and from tutors. Many children without comorbid conditions are very intelligent and are able to keep up with the course load until they reach high school. The demands for independence and the amount of class work required start to become overwhelming and grades start to decline. These children tend to do very well on stimulant medication.
Now for the child who presents with absolutely no past history of inattention, disorganization, hyperactivity, or impulsive behavior, it is important to do a detailed physical exam looking for symptoms that are consistent with Wilson’s disease, hyperthyroidism, or drug use. A careful social history will identify symptoms of depression, anxiety, and disruption within the home such as divorce, domestic violence, etc. Questions regarding school and social pressure such as bullying also are important to ask to identify a cause for the acute onset of inattention.
Red flags should be raised with a teen who has socially withdrawn, has defiant or high risk taking behaviors, and a history of illicit drug use. According to a study published in the British Journal of Psychiatry, stimulant used as prescribed actually lowers the risk of substance abuse (Br. J. Psychiatry 2013 [doi:10.1192/bjp.bp.112.124784]). But according to the National Institute of Drug Abuse, teens are using the stimulants to get a high by snorting or injecting the stimulant, making Adderall a hot commodity on college campuses. Stimulants are also used to "cram" for tests so teens are taking them to stay awake to study. Stimulants coupled with excessive caffeine lead to increased heart rates and blood pressure, as well as panic attacks. The DAWN Report (Drug Abuse Warning Network) published a warning on Jan. 24, 2013, stating that the number of emergency room visits increased threefold in young adults over the age of 18 years. Only a small increase was noted in teens aged 12-17 years, but they all were related to using stimulants improperly.
Choosing the right stimulant for the adolescent must take into consideration their social environment and their risk of drug abuse. Given that Adderall is an amphetamine, it should be used with caution and careful supervision, but since all stimulants can be abused, the same caution applies to them as well.
ADHD prevalence in adolescents aged 11-17 years is 10%-15% (CDC Weekly; Nov.12, 2010;59:1439-43). So evaluation and proper treatment can mean school success and continuing on to college. The diagnosis should not be excluded because it was not identified earlier, but other diagnoses must be considered.
Dr Pearce is a pediatrician in Frankfort, Ill.
Attention deficit is a common diagnosis with which many pediatricians are faced. In the more common scenario, the child presents anywhere from age 5 to 10 years with parental and teacher complaint of hyperactivity or academic underachievement. But, what do you do with the child who presents at age 11 years or high school age with no significant history of school failure or behavior issues? Is this a child whose symptoms were overlooked? New-onset inattention? Are they seeking medication to augment their intellectual ability to become more competitive? Or are they just drug seeking? Well, the truth is that any one of the above could be true, and only through careful analysis can you obtain the proper diagnosis.
For the adolescent that meets the DSM-IV criteria for attention-deficit/hyperactivity disorder (ADHD), it is possible that they have learned to compensate for their inattention and have been successful in maintaining a good grade-point average. Through detailed questioning about study habits and home environment, they likely required a lot of support from their parents to maintain organization and from tutors. Many children without comorbid conditions are very intelligent and are able to keep up with the course load until they reach high school. The demands for independence and the amount of class work required start to become overwhelming and grades start to decline. These children tend to do very well on stimulant medication.
Now for the child who presents with absolutely no past history of inattention, disorganization, hyperactivity, or impulsive behavior, it is important to do a detailed physical exam looking for symptoms that are consistent with Wilson’s disease, hyperthyroidism, or drug use. A careful social history will identify symptoms of depression, anxiety, and disruption within the home such as divorce, domestic violence, etc. Questions regarding school and social pressure such as bullying also are important to ask to identify a cause for the acute onset of inattention.
Red flags should be raised with a teen who has socially withdrawn, has defiant or high risk taking behaviors, and a history of illicit drug use. According to a study published in the British Journal of Psychiatry, stimulant used as prescribed actually lowers the risk of substance abuse (Br. J. Psychiatry 2013 [doi:10.1192/bjp.bp.112.124784]). But according to the National Institute of Drug Abuse, teens are using the stimulants to get a high by snorting or injecting the stimulant, making Adderall a hot commodity on college campuses. Stimulants are also used to "cram" for tests so teens are taking them to stay awake to study. Stimulants coupled with excessive caffeine lead to increased heart rates and blood pressure, as well as panic attacks. The DAWN Report (Drug Abuse Warning Network) published a warning on Jan. 24, 2013, stating that the number of emergency room visits increased threefold in young adults over the age of 18 years. Only a small increase was noted in teens aged 12-17 years, but they all were related to using stimulants improperly.
Choosing the right stimulant for the adolescent must take into consideration their social environment and their risk of drug abuse. Given that Adderall is an amphetamine, it should be used with caution and careful supervision, but since all stimulants can be abused, the same caution applies to them as well.
ADHD prevalence in adolescents aged 11-17 years is 10%-15% (CDC Weekly; Nov.12, 2010;59:1439-43). So evaluation and proper treatment can mean school success and continuing on to college. The diagnosis should not be excluded because it was not identified earlier, but other diagnoses must be considered.
Dr Pearce is a pediatrician in Frankfort, Ill.
Attention deficit is a common diagnosis with which many pediatricians are faced. In the more common scenario, the child presents anywhere from age 5 to 10 years with parental and teacher complaint of hyperactivity or academic underachievement. But, what do you do with the child who presents at age 11 years or high school age with no significant history of school failure or behavior issues? Is this a child whose symptoms were overlooked? New-onset inattention? Are they seeking medication to augment their intellectual ability to become more competitive? Or are they just drug seeking? Well, the truth is that any one of the above could be true, and only through careful analysis can you obtain the proper diagnosis.
For the adolescent that meets the DSM-IV criteria for attention-deficit/hyperactivity disorder (ADHD), it is possible that they have learned to compensate for their inattention and have been successful in maintaining a good grade-point average. Through detailed questioning about study habits and home environment, they likely required a lot of support from their parents to maintain organization and from tutors. Many children without comorbid conditions are very intelligent and are able to keep up with the course load until they reach high school. The demands for independence and the amount of class work required start to become overwhelming and grades start to decline. These children tend to do very well on stimulant medication.
Now for the child who presents with absolutely no past history of inattention, disorganization, hyperactivity, or impulsive behavior, it is important to do a detailed physical exam looking for symptoms that are consistent with Wilson’s disease, hyperthyroidism, or drug use. A careful social history will identify symptoms of depression, anxiety, and disruption within the home such as divorce, domestic violence, etc. Questions regarding school and social pressure such as bullying also are important to ask to identify a cause for the acute onset of inattention.
Red flags should be raised with a teen who has socially withdrawn, has defiant or high risk taking behaviors, and a history of illicit drug use. According to a study published in the British Journal of Psychiatry, stimulant used as prescribed actually lowers the risk of substance abuse (Br. J. Psychiatry 2013 [doi:10.1192/bjp.bp.112.124784]). But according to the National Institute of Drug Abuse, teens are using the stimulants to get a high by snorting or injecting the stimulant, making Adderall a hot commodity on college campuses. Stimulants are also used to "cram" for tests so teens are taking them to stay awake to study. Stimulants coupled with excessive caffeine lead to increased heart rates and blood pressure, as well as panic attacks. The DAWN Report (Drug Abuse Warning Network) published a warning on Jan. 24, 2013, stating that the number of emergency room visits increased threefold in young adults over the age of 18 years. Only a small increase was noted in teens aged 12-17 years, but they all were related to using stimulants improperly.
Choosing the right stimulant for the adolescent must take into consideration their social environment and their risk of drug abuse. Given that Adderall is an amphetamine, it should be used with caution and careful supervision, but since all stimulants can be abused, the same caution applies to them as well.
ADHD prevalence in adolescents aged 11-17 years is 10%-15% (CDC Weekly; Nov.12, 2010;59:1439-43). So evaluation and proper treatment can mean school success and continuing on to college. The diagnosis should not be excluded because it was not identified earlier, but other diagnoses must be considered.
Dr Pearce is a pediatrician in Frankfort, Ill.
Distribution based on contribution: The merit-based ACO shared savings distribution model
Our nation is in the midst of an inexorable shift in health care delivery from "pay for volume" to "pay for value." It is well documented that our current largely fee-for-service system is unsustainable and a dramatic incentive shift must occur. Every provider needs to be committed to providing the highest quality at the lowest cost. This is the fundamental goal of the pay-for-value system.
If quality and patient satisfaction criteria are met and providers working together in an accountable care organization or similar entity create savings for a defined patient population, then the ACO usually gets a portion of the savings, commonly 50%. Unlike capitated arrangements, shared savings arrangements can avoid or limit downside financial risk and therefore can serve as stepping-stones toward fuller accountability and incentives. They are quite appropriate for start-up and smaller ACOs.
The ACO gets the savings, if there are any. But what the ACO does with them is crucial to the success and sustainability of the organization. "ACOs must offer a realistic and achievable opportunity for providers to share in the savings created from delivering higher-value care. The incentive system must reward providers for delivering efficient care as opposed to the current volume-driven system" (The ACO Toolkit; the Dartmouth Institute, p. 9, Jan. 2011).
If providers or hospital stakeholders feel that their efforts to drive value are not being fairly recognized, they will no longer participate meaningfully, the goals of value-based medicine will be thwarted, and savings will not occur in the long-run. Before signing a participation contract, physicians should scrutinize how each ACO plans to distribute the savings it receives.
The Centers for Medicare and Medicaid Services administers the Medicare Shared Savings Program (MSSP). The fact that CMS’s regulations concerning MSSP are not prescriptive about a given savings distribution formula gives ACOs flexibility in this area. But the regulations are specific about the ultimate purpose of distributions: "As part of its application, an ACO must describe the following: (1) how it plans to use shared savings payments, including the criteria it plans to employ for distributing shared savings among its ACO participants and ACO providers/suppliers, ... and (3) how the proposed plan will achieve the general aims of better care for individuals, better health for populations, and lower growth in expenditures" (42 CFR 425.204(d), 76 Fed. Reg. 6798 [Nov. 2, 2011]).
Fatal flaw?
Some ACOs, however, have lost sight of the fact that failure to have a fair shared savings distribution formula (linking relative distributions to relative contributions) will be fatal to its sustainability. Some view them as "profits" to go to the owners or shareholders. Some simply lock in a fixed allocation similar to fee-for-service payment ratios, without regard to who generated the savings. Some employers of physicians have contracted to compensate only on a work production basis with zero performance incentive payments at all. Other ACOs are putting off the issue because it is sensitive culturally. As health care moves more and more to value-based compensation, the distribution of savings must be viewed primarily as the providers’ professional remuneration and not corporate "profit." Payments for administrative services and debt service must, of course, come out of the savings distribution to "keep the pump primed," but they should be carefully managed. The bulk must be distributed in proportion to contribution toward quality and cost-effective care.
One physician stated, "No physician is going to join an ACO when someone else is telling them what they are worth unless they know that the savings distribution formula is impeccably fair." To those putting off design of a fair merit-based compensation system until there is more physician buy-in, we respectfully submit that you cannot get buy-in without one.
A need for honed metrics
Yes, this concept is pretty basic when you think about it. But though it may be easy to understand, it can be complex to implement, especially when multiple specialists and facilities are involved in an ACO’s care coordination. One not only needs to determine the relative potential and actual value contribution for each provider, but also the clinically valid metrics by which to measure them. Under fee for service, metrics for success were usually transactional and objective (in other words, volume of procedure times rate). An ACO’s success metrics may be neither. Success may come from things not happening (that is, fewer ED visits, avoidable admissions, and reduced readmissions). At the same time, the distribution model needs to be clear, practical, and capable of being understood by all.
But there can be a replicable framework for any ACO to use to create a fair and sustainable shared savings distribution model. There are necessary subjective judgments – at this time, many metrics are imprecise or nonexistent – and the sophistication of the distribution process must parallel the sophistication of the ACO’s infrastructure. But, if the right people are involved and apply the ACO’s guiding principles on savings allocation, participants will be appropriately incentivized. The precision of distribution application will grow over time. Don’t let the perfect be the enemy of the good.
The six guiding principles for shared savings distribution
Though application will vary widely because of differing circumstances and types of initiatives, chances for success will increase if every activity can be judged by whether it is consistent with a set of guiding principles viewed as fair by the ACO members. You may want to consider a savings distribution formula with the following principles:
• Eyes on the prize: Triple Aim. It offers incentives for the delivery of high-quality and cost-effective care to achieve the Triple Aim – better care for individuals, better health for populations, and lower per capita costs.
• Broad provider input. It is the result of input from a diverse spectrum of knowledgeable providers who understand what drives patient population value.
• Fairness. It is fair to all in that it links relative distribution to relative contribution to the organization’s total savings and quality performance, and adheres to measurable clinically valid metrics.
• Transparency. It is clear, transparent, practical to implement and replicable.
• Constant evolution. It adapts and improves as the capabilities and experience as the ACO grows.
• Maximized incentive to drive value by all participants. After prudently meeting overhead costs, it allows gradual transition as well as commercially reasonable return on capital investment or debt service. It makes the most of ongoing incentive programs for all to deliver value by distributing as much of the savings surplus as possible to those who generate them.
Weighting: How to assign relative percentage among providers
As mentioned, it is important that design of a fair distribution formula be the product of collaboration among informed and committed clinicians who understand patient population management. Like virtually all organization compensation formulas, the determination of relative contributions of the different providers in a given ACO, or care initiative within the ACO, will involve a certain amount of inherent subjectivity but will be guided by weighted criteria applied in good faith.
• Step 1: Break down each initiative into its value-adding elements and assign provider responsibility for each. The ACO will have a number of different care management initiatives. Some, like outpatient diabetes management, may be completely the responsibility of one provider specialty, (that is, primary care). Others may involve coordination across multiple settings for patients with multiple conditions involving multiple specialties. Each initiative was chosen for a reason – to drive value. In setting relative potential distribution percentages, envision the perfect implementation of each initiative. Next, look at what tasks or best practices are needed to drive success, and then who is assigned responsibility for each.
• Step 2: Assign relative percentages to each specialty relative to its potential to realize savings. For a pure primary care prevention initiative, they would get 100% in all categories. For multispecialty initiatives, the percentage is tied to the proportion of those savings predicted to flow from that provider class.
N.B.: Historically, cost centers are not necessarily the cost savers. A mature ACO will be able to allocate savings to each initiative and the relative savings distribution within each. But for a start-up ACO, because it is so apparently logical and fits the traditional fee-for-service mindset, it is tempting to look at claims differences in the various service categories, such as inpatient, outpatient, primary care, specialists, drugs, and ancillaries, and attribute savings to the provider historically billing for same (that is, hospitals get "credit" for reduced hospital costs). However, a successful wellness, prevention, or lifestyle counseling program in a medical home may be the reason those patients never go to the hospital. The radiologist embedded in the medical home diagnostic team may have helped make an informed image analysis confirming a negative result and avoided those admissions. But, do use those service categories to set cost targets.
• Step 3: Individual attribution. We now know every provider group’s potential savings, but how do we determine the actual distribution based on actual results? Select metrics that are accurately associated with the desired individual and collective conduct of that provider class. They should cover both quality and efficiency. In the value-based reimbursement world, even if the performance is superb, if it is not measured appropriately, it will not be rewarded.
Once the proper metrics are selected, each provider’s performance is measured.
Keep it simple and open
Pick a few of the very best quality and efficiency metrics and have them and the data collection process thoroughly vetted by the providers. Following the guiding principles, the distribution model will be a success if: (1) everyone understands that this is the best practical approach, (2) the process has been open, and (3) everyone is acting in good faith to have as fair a shared savings distribution process as the current sophistication level of the ACO’s infrastructure allows. It cannot be viewed as coming from a "black box." For a young ACO, it will be crude, at best, in the beginning.
Conclusion
Even at this dawning of the movement to value-based reimbursement in health care, a framework for a fair merit-based shared savings distribution is available to all ACOs. As ACOs gain actual performance data, their health information technology capabilities improve, and refined quality and efficiency metrics emerge, the process will evolve from an open and good-faith application of the guiding principles with limited tools, to more and more refined determinations of the sources of the ACO’s quality and savings results. The path will get easier over time, but the destination is always clear – distribution in proportion to contribution.
Mr. Bobbitt is a senior partner and head of the Health Law Group at the Smith Anderson law firm in Raleigh, N.C. He has many years’ experience assisting physicians form integrated delivery systems. He has spoken and written nationally to primary care physicians on the strategies and practicalities of forming or joining ACOs. This article is meant to be educational and does not constitute legal advice. For additional information, readers may contact the author ([email protected] or 919-821-6612).
Our nation is in the midst of an inexorable shift in health care delivery from "pay for volume" to "pay for value." It is well documented that our current largely fee-for-service system is unsustainable and a dramatic incentive shift must occur. Every provider needs to be committed to providing the highest quality at the lowest cost. This is the fundamental goal of the pay-for-value system.
If quality and patient satisfaction criteria are met and providers working together in an accountable care organization or similar entity create savings for a defined patient population, then the ACO usually gets a portion of the savings, commonly 50%. Unlike capitated arrangements, shared savings arrangements can avoid or limit downside financial risk and therefore can serve as stepping-stones toward fuller accountability and incentives. They are quite appropriate for start-up and smaller ACOs.
The ACO gets the savings, if there are any. But what the ACO does with them is crucial to the success and sustainability of the organization. "ACOs must offer a realistic and achievable opportunity for providers to share in the savings created from delivering higher-value care. The incentive system must reward providers for delivering efficient care as opposed to the current volume-driven system" (The ACO Toolkit; the Dartmouth Institute, p. 9, Jan. 2011).
If providers or hospital stakeholders feel that their efforts to drive value are not being fairly recognized, they will no longer participate meaningfully, the goals of value-based medicine will be thwarted, and savings will not occur in the long-run. Before signing a participation contract, physicians should scrutinize how each ACO plans to distribute the savings it receives.
The Centers for Medicare and Medicaid Services administers the Medicare Shared Savings Program (MSSP). The fact that CMS’s regulations concerning MSSP are not prescriptive about a given savings distribution formula gives ACOs flexibility in this area. But the regulations are specific about the ultimate purpose of distributions: "As part of its application, an ACO must describe the following: (1) how it plans to use shared savings payments, including the criteria it plans to employ for distributing shared savings among its ACO participants and ACO providers/suppliers, ... and (3) how the proposed plan will achieve the general aims of better care for individuals, better health for populations, and lower growth in expenditures" (42 CFR 425.204(d), 76 Fed. Reg. 6798 [Nov. 2, 2011]).
Fatal flaw?
Some ACOs, however, have lost sight of the fact that failure to have a fair shared savings distribution formula (linking relative distributions to relative contributions) will be fatal to its sustainability. Some view them as "profits" to go to the owners or shareholders. Some simply lock in a fixed allocation similar to fee-for-service payment ratios, without regard to who generated the savings. Some employers of physicians have contracted to compensate only on a work production basis with zero performance incentive payments at all. Other ACOs are putting off the issue because it is sensitive culturally. As health care moves more and more to value-based compensation, the distribution of savings must be viewed primarily as the providers’ professional remuneration and not corporate "profit." Payments for administrative services and debt service must, of course, come out of the savings distribution to "keep the pump primed," but they should be carefully managed. The bulk must be distributed in proportion to contribution toward quality and cost-effective care.
One physician stated, "No physician is going to join an ACO when someone else is telling them what they are worth unless they know that the savings distribution formula is impeccably fair." To those putting off design of a fair merit-based compensation system until there is more physician buy-in, we respectfully submit that you cannot get buy-in without one.
A need for honed metrics
Yes, this concept is pretty basic when you think about it. But though it may be easy to understand, it can be complex to implement, especially when multiple specialists and facilities are involved in an ACO’s care coordination. One not only needs to determine the relative potential and actual value contribution for each provider, but also the clinically valid metrics by which to measure them. Under fee for service, metrics for success were usually transactional and objective (in other words, volume of procedure times rate). An ACO’s success metrics may be neither. Success may come from things not happening (that is, fewer ED visits, avoidable admissions, and reduced readmissions). At the same time, the distribution model needs to be clear, practical, and capable of being understood by all.
But there can be a replicable framework for any ACO to use to create a fair and sustainable shared savings distribution model. There are necessary subjective judgments – at this time, many metrics are imprecise or nonexistent – and the sophistication of the distribution process must parallel the sophistication of the ACO’s infrastructure. But, if the right people are involved and apply the ACO’s guiding principles on savings allocation, participants will be appropriately incentivized. The precision of distribution application will grow over time. Don’t let the perfect be the enemy of the good.
The six guiding principles for shared savings distribution
Though application will vary widely because of differing circumstances and types of initiatives, chances for success will increase if every activity can be judged by whether it is consistent with a set of guiding principles viewed as fair by the ACO members. You may want to consider a savings distribution formula with the following principles:
• Eyes on the prize: Triple Aim. It offers incentives for the delivery of high-quality and cost-effective care to achieve the Triple Aim – better care for individuals, better health for populations, and lower per capita costs.
• Broad provider input. It is the result of input from a diverse spectrum of knowledgeable providers who understand what drives patient population value.
• Fairness. It is fair to all in that it links relative distribution to relative contribution to the organization’s total savings and quality performance, and adheres to measurable clinically valid metrics.
• Transparency. It is clear, transparent, practical to implement and replicable.
• Constant evolution. It adapts and improves as the capabilities and experience as the ACO grows.
• Maximized incentive to drive value by all participants. After prudently meeting overhead costs, it allows gradual transition as well as commercially reasonable return on capital investment or debt service. It makes the most of ongoing incentive programs for all to deliver value by distributing as much of the savings surplus as possible to those who generate them.
Weighting: How to assign relative percentage among providers
As mentioned, it is important that design of a fair distribution formula be the product of collaboration among informed and committed clinicians who understand patient population management. Like virtually all organization compensation formulas, the determination of relative contributions of the different providers in a given ACO, or care initiative within the ACO, will involve a certain amount of inherent subjectivity but will be guided by weighted criteria applied in good faith.
• Step 1: Break down each initiative into its value-adding elements and assign provider responsibility for each. The ACO will have a number of different care management initiatives. Some, like outpatient diabetes management, may be completely the responsibility of one provider specialty, (that is, primary care). Others may involve coordination across multiple settings for patients with multiple conditions involving multiple specialties. Each initiative was chosen for a reason – to drive value. In setting relative potential distribution percentages, envision the perfect implementation of each initiative. Next, look at what tasks or best practices are needed to drive success, and then who is assigned responsibility for each.
• Step 2: Assign relative percentages to each specialty relative to its potential to realize savings. For a pure primary care prevention initiative, they would get 100% in all categories. For multispecialty initiatives, the percentage is tied to the proportion of those savings predicted to flow from that provider class.
N.B.: Historically, cost centers are not necessarily the cost savers. A mature ACO will be able to allocate savings to each initiative and the relative savings distribution within each. But for a start-up ACO, because it is so apparently logical and fits the traditional fee-for-service mindset, it is tempting to look at claims differences in the various service categories, such as inpatient, outpatient, primary care, specialists, drugs, and ancillaries, and attribute savings to the provider historically billing for same (that is, hospitals get "credit" for reduced hospital costs). However, a successful wellness, prevention, or lifestyle counseling program in a medical home may be the reason those patients never go to the hospital. The radiologist embedded in the medical home diagnostic team may have helped make an informed image analysis confirming a negative result and avoided those admissions. But, do use those service categories to set cost targets.
• Step 3: Individual attribution. We now know every provider group’s potential savings, but how do we determine the actual distribution based on actual results? Select metrics that are accurately associated with the desired individual and collective conduct of that provider class. They should cover both quality and efficiency. In the value-based reimbursement world, even if the performance is superb, if it is not measured appropriately, it will not be rewarded.
Once the proper metrics are selected, each provider’s performance is measured.
Keep it simple and open
Pick a few of the very best quality and efficiency metrics and have them and the data collection process thoroughly vetted by the providers. Following the guiding principles, the distribution model will be a success if: (1) everyone understands that this is the best practical approach, (2) the process has been open, and (3) everyone is acting in good faith to have as fair a shared savings distribution process as the current sophistication level of the ACO’s infrastructure allows. It cannot be viewed as coming from a "black box." For a young ACO, it will be crude, at best, in the beginning.
Conclusion
Even at this dawning of the movement to value-based reimbursement in health care, a framework for a fair merit-based shared savings distribution is available to all ACOs. As ACOs gain actual performance data, their health information technology capabilities improve, and refined quality and efficiency metrics emerge, the process will evolve from an open and good-faith application of the guiding principles with limited tools, to more and more refined determinations of the sources of the ACO’s quality and savings results. The path will get easier over time, but the destination is always clear – distribution in proportion to contribution.
Mr. Bobbitt is a senior partner and head of the Health Law Group at the Smith Anderson law firm in Raleigh, N.C. He has many years’ experience assisting physicians form integrated delivery systems. He has spoken and written nationally to primary care physicians on the strategies and practicalities of forming or joining ACOs. This article is meant to be educational and does not constitute legal advice. For additional information, readers may contact the author ([email protected] or 919-821-6612).
Our nation is in the midst of an inexorable shift in health care delivery from "pay for volume" to "pay for value." It is well documented that our current largely fee-for-service system is unsustainable and a dramatic incentive shift must occur. Every provider needs to be committed to providing the highest quality at the lowest cost. This is the fundamental goal of the pay-for-value system.
If quality and patient satisfaction criteria are met and providers working together in an accountable care organization or similar entity create savings for a defined patient population, then the ACO usually gets a portion of the savings, commonly 50%. Unlike capitated arrangements, shared savings arrangements can avoid or limit downside financial risk and therefore can serve as stepping-stones toward fuller accountability and incentives. They are quite appropriate for start-up and smaller ACOs.
The ACO gets the savings, if there are any. But what the ACO does with them is crucial to the success and sustainability of the organization. "ACOs must offer a realistic and achievable opportunity for providers to share in the savings created from delivering higher-value care. The incentive system must reward providers for delivering efficient care as opposed to the current volume-driven system" (The ACO Toolkit; the Dartmouth Institute, p. 9, Jan. 2011).
If providers or hospital stakeholders feel that their efforts to drive value are not being fairly recognized, they will no longer participate meaningfully, the goals of value-based medicine will be thwarted, and savings will not occur in the long-run. Before signing a participation contract, physicians should scrutinize how each ACO plans to distribute the savings it receives.
The Centers for Medicare and Medicaid Services administers the Medicare Shared Savings Program (MSSP). The fact that CMS’s regulations concerning MSSP are not prescriptive about a given savings distribution formula gives ACOs flexibility in this area. But the regulations are specific about the ultimate purpose of distributions: "As part of its application, an ACO must describe the following: (1) how it plans to use shared savings payments, including the criteria it plans to employ for distributing shared savings among its ACO participants and ACO providers/suppliers, ... and (3) how the proposed plan will achieve the general aims of better care for individuals, better health for populations, and lower growth in expenditures" (42 CFR 425.204(d), 76 Fed. Reg. 6798 [Nov. 2, 2011]).
Fatal flaw?
Some ACOs, however, have lost sight of the fact that failure to have a fair shared savings distribution formula (linking relative distributions to relative contributions) will be fatal to its sustainability. Some view them as "profits" to go to the owners or shareholders. Some simply lock in a fixed allocation similar to fee-for-service payment ratios, without regard to who generated the savings. Some employers of physicians have contracted to compensate only on a work production basis with zero performance incentive payments at all. Other ACOs are putting off the issue because it is sensitive culturally. As health care moves more and more to value-based compensation, the distribution of savings must be viewed primarily as the providers’ professional remuneration and not corporate "profit." Payments for administrative services and debt service must, of course, come out of the savings distribution to "keep the pump primed," but they should be carefully managed. The bulk must be distributed in proportion to contribution toward quality and cost-effective care.
One physician stated, "No physician is going to join an ACO when someone else is telling them what they are worth unless they know that the savings distribution formula is impeccably fair." To those putting off design of a fair merit-based compensation system until there is more physician buy-in, we respectfully submit that you cannot get buy-in without one.
A need for honed metrics
Yes, this concept is pretty basic when you think about it. But though it may be easy to understand, it can be complex to implement, especially when multiple specialists and facilities are involved in an ACO’s care coordination. One not only needs to determine the relative potential and actual value contribution for each provider, but also the clinically valid metrics by which to measure them. Under fee for service, metrics for success were usually transactional and objective (in other words, volume of procedure times rate). An ACO’s success metrics may be neither. Success may come from things not happening (that is, fewer ED visits, avoidable admissions, and reduced readmissions). At the same time, the distribution model needs to be clear, practical, and capable of being understood by all.
But there can be a replicable framework for any ACO to use to create a fair and sustainable shared savings distribution model. There are necessary subjective judgments – at this time, many metrics are imprecise or nonexistent – and the sophistication of the distribution process must parallel the sophistication of the ACO’s infrastructure. But, if the right people are involved and apply the ACO’s guiding principles on savings allocation, participants will be appropriately incentivized. The precision of distribution application will grow over time. Don’t let the perfect be the enemy of the good.
The six guiding principles for shared savings distribution
Though application will vary widely because of differing circumstances and types of initiatives, chances for success will increase if every activity can be judged by whether it is consistent with a set of guiding principles viewed as fair by the ACO members. You may want to consider a savings distribution formula with the following principles:
• Eyes on the prize: Triple Aim. It offers incentives for the delivery of high-quality and cost-effective care to achieve the Triple Aim – better care for individuals, better health for populations, and lower per capita costs.
• Broad provider input. It is the result of input from a diverse spectrum of knowledgeable providers who understand what drives patient population value.
• Fairness. It is fair to all in that it links relative distribution to relative contribution to the organization’s total savings and quality performance, and adheres to measurable clinically valid metrics.
• Transparency. It is clear, transparent, practical to implement and replicable.
• Constant evolution. It adapts and improves as the capabilities and experience as the ACO grows.
• Maximized incentive to drive value by all participants. After prudently meeting overhead costs, it allows gradual transition as well as commercially reasonable return on capital investment or debt service. It makes the most of ongoing incentive programs for all to deliver value by distributing as much of the savings surplus as possible to those who generate them.
Weighting: How to assign relative percentage among providers
As mentioned, it is important that design of a fair distribution formula be the product of collaboration among informed and committed clinicians who understand patient population management. Like virtually all organization compensation formulas, the determination of relative contributions of the different providers in a given ACO, or care initiative within the ACO, will involve a certain amount of inherent subjectivity but will be guided by weighted criteria applied in good faith.
• Step 1: Break down each initiative into its value-adding elements and assign provider responsibility for each. The ACO will have a number of different care management initiatives. Some, like outpatient diabetes management, may be completely the responsibility of one provider specialty, (that is, primary care). Others may involve coordination across multiple settings for patients with multiple conditions involving multiple specialties. Each initiative was chosen for a reason – to drive value. In setting relative potential distribution percentages, envision the perfect implementation of each initiative. Next, look at what tasks or best practices are needed to drive success, and then who is assigned responsibility for each.
• Step 2: Assign relative percentages to each specialty relative to its potential to realize savings. For a pure primary care prevention initiative, they would get 100% in all categories. For multispecialty initiatives, the percentage is tied to the proportion of those savings predicted to flow from that provider class.
N.B.: Historically, cost centers are not necessarily the cost savers. A mature ACO will be able to allocate savings to each initiative and the relative savings distribution within each. But for a start-up ACO, because it is so apparently logical and fits the traditional fee-for-service mindset, it is tempting to look at claims differences in the various service categories, such as inpatient, outpatient, primary care, specialists, drugs, and ancillaries, and attribute savings to the provider historically billing for same (that is, hospitals get "credit" for reduced hospital costs). However, a successful wellness, prevention, or lifestyle counseling program in a medical home may be the reason those patients never go to the hospital. The radiologist embedded in the medical home diagnostic team may have helped make an informed image analysis confirming a negative result and avoided those admissions. But, do use those service categories to set cost targets.
• Step 3: Individual attribution. We now know every provider group’s potential savings, but how do we determine the actual distribution based on actual results? Select metrics that are accurately associated with the desired individual and collective conduct of that provider class. They should cover both quality and efficiency. In the value-based reimbursement world, even if the performance is superb, if it is not measured appropriately, it will not be rewarded.
Once the proper metrics are selected, each provider’s performance is measured.
Keep it simple and open
Pick a few of the very best quality and efficiency metrics and have them and the data collection process thoroughly vetted by the providers. Following the guiding principles, the distribution model will be a success if: (1) everyone understands that this is the best practical approach, (2) the process has been open, and (3) everyone is acting in good faith to have as fair a shared savings distribution process as the current sophistication level of the ACO’s infrastructure allows. It cannot be viewed as coming from a "black box." For a young ACO, it will be crude, at best, in the beginning.
Conclusion
Even at this dawning of the movement to value-based reimbursement in health care, a framework for a fair merit-based shared savings distribution is available to all ACOs. As ACOs gain actual performance data, their health information technology capabilities improve, and refined quality and efficiency metrics emerge, the process will evolve from an open and good-faith application of the guiding principles with limited tools, to more and more refined determinations of the sources of the ACO’s quality and savings results. The path will get easier over time, but the destination is always clear – distribution in proportion to contribution.
Mr. Bobbitt is a senior partner and head of the Health Law Group at the Smith Anderson law firm in Raleigh, N.C. He has many years’ experience assisting physicians form integrated delivery systems. He has spoken and written nationally to primary care physicians on the strategies and practicalities of forming or joining ACOs. This article is meant to be educational and does not constitute legal advice. For additional information, readers may contact the author ([email protected] or 919-821-6612).
Angiolymphoid Hyperplasia With Eosinophilia
You have a big eyebrow!
One of my favorite Seinfeld episodes involves Elaine breaking up with a man who likes to mess with the minds of anyone who has the nerve to call it off with him. When Elaine tells him that they’re through, he says to her, "You know, you have a big head."
The rest of the show is devoted to Elaine trying to convince herself that she does not have a big head. In one scene, a cabbie tells the petite Elaine sitting in his back seat, "Please slide down, will you? You’re blocking the window!" And Elaine stands in Central Park in the show’s final scene, a bird flies right into her head as an old man says, "Well, I never saw that before. Looked like the bird just couldn’t get out of the way."
Jerry Seinfeld, who may have OCD-oid tendencies himself, often referred to dermatology in his sketches and showed insight into our patients and our clinical lives. How many people do we see who can’t stop thinking about some part of their appearance, often one we would never guess could be the focus of their attention: a small mole or freckle, a tiny wrinkle, a slight asymmetry in their features? Quite often, these patients become fixated on the feature not because they are "vain" or "narcissistic," and not because they have "body dysmorphic disorder," but just because someone else (and not necessarily somebody they were breaking up with who wanted to get even) pointed it out. From then on, they couldn’t stop thinking about it.
It may have been a doctor. ("That mole, has it been changing?") It may have been a friend. ("You know, the groove on the left side of your nose is deeper than the one on the right.") It may have been a hairdresser – it often is a hairdresser, who can see what you can’t. ("How long has that spot been up here?" Or, "Goodness! I can see right through to your scalp. Are you going bald?!")
Or it could be one of my favorite villains, the magnifying mirror, bane of presbyopic middle-age women. Overcoming presbyopia comes at the price of seeing every nevus as Pike’s Peak and every pore as the Grand Canyon.
In these cases, and many others like them, once the spots or defects are pointed out, people find it all but impossible to stop thinking about them and noticing them every time they look at themselves. If a bird flew by, it would probably slam right into them because it couldn’t get out of the way.
Removing what the patients are fixated on may be unnecessary, risky, or impossible. Advice to "just stop looking" may make sense, but can be unsatisfying or unacceptable.
I had a Seinfeld-esque moment the other day. Inga, thirtyish, came by to discuss acne, and then said, "Can you feel this growth at the end of my eyebrow?" My student and I palpated a small nodule under the outer aspect of her right eyebrow.
"It feels like a cyst," I said. "Probably been there a long time."
"Are you sure?" she asked. "Doesn’t it distort my face?"
We stepped back to a conversational distance. I couldn’t see anything, and neither could the student. "We can’t even see it from here," I told Inga. "What makes you think it distorts your face?"
"I was walking down the street with my mother and sister last week," she said, "and my mother said, "Inga, what is that on your eyebrow? Your whole face looks out of whack! And my sister said, ‘You’d better get that checked out.’"
My student and I stared at her. She was serious.
"Look," I said. "There is something there, but it’s definitely OK, and removing it would be unnecessary and leave a big scar. We honestly have no idea why your mother and sister would say that your face is lopsided when we can’t see anything even when we look for it."
Inga seemed mollified. We had to leave her, as we must leave all patients, to the vagaries of their own family dynamics. I can only hope that Inga doesn’t one day have some cabbie tell her to please lean to the left to keep the taxi from rolling over.
Dr. Rockoff practices dermatology in Brookline, Mass. He is on the clinical faculty at Tufts University School of Medicine, Boston, and has taught senior medical students and other trainees for 30 years.
One of my favorite Seinfeld episodes involves Elaine breaking up with a man who likes to mess with the minds of anyone who has the nerve to call it off with him. When Elaine tells him that they’re through, he says to her, "You know, you have a big head."
The rest of the show is devoted to Elaine trying to convince herself that she does not have a big head. In one scene, a cabbie tells the petite Elaine sitting in his back seat, "Please slide down, will you? You’re blocking the window!" And Elaine stands in Central Park in the show’s final scene, a bird flies right into her head as an old man says, "Well, I never saw that before. Looked like the bird just couldn’t get out of the way."
Jerry Seinfeld, who may have OCD-oid tendencies himself, often referred to dermatology in his sketches and showed insight into our patients and our clinical lives. How many people do we see who can’t stop thinking about some part of their appearance, often one we would never guess could be the focus of their attention: a small mole or freckle, a tiny wrinkle, a slight asymmetry in their features? Quite often, these patients become fixated on the feature not because they are "vain" or "narcissistic," and not because they have "body dysmorphic disorder," but just because someone else (and not necessarily somebody they were breaking up with who wanted to get even) pointed it out. From then on, they couldn’t stop thinking about it.
It may have been a doctor. ("That mole, has it been changing?") It may have been a friend. ("You know, the groove on the left side of your nose is deeper than the one on the right.") It may have been a hairdresser – it often is a hairdresser, who can see what you can’t. ("How long has that spot been up here?" Or, "Goodness! I can see right through to your scalp. Are you going bald?!")
Or it could be one of my favorite villains, the magnifying mirror, bane of presbyopic middle-age women. Overcoming presbyopia comes at the price of seeing every nevus as Pike’s Peak and every pore as the Grand Canyon.
In these cases, and many others like them, once the spots or defects are pointed out, people find it all but impossible to stop thinking about them and noticing them every time they look at themselves. If a bird flew by, it would probably slam right into them because it couldn’t get out of the way.
Removing what the patients are fixated on may be unnecessary, risky, or impossible. Advice to "just stop looking" may make sense, but can be unsatisfying or unacceptable.
I had a Seinfeld-esque moment the other day. Inga, thirtyish, came by to discuss acne, and then said, "Can you feel this growth at the end of my eyebrow?" My student and I palpated a small nodule under the outer aspect of her right eyebrow.
"It feels like a cyst," I said. "Probably been there a long time."
"Are you sure?" she asked. "Doesn’t it distort my face?"
We stepped back to a conversational distance. I couldn’t see anything, and neither could the student. "We can’t even see it from here," I told Inga. "What makes you think it distorts your face?"
"I was walking down the street with my mother and sister last week," she said, "and my mother said, "Inga, what is that on your eyebrow? Your whole face looks out of whack! And my sister said, ‘You’d better get that checked out.’"
My student and I stared at her. She was serious.
"Look," I said. "There is something there, but it’s definitely OK, and removing it would be unnecessary and leave a big scar. We honestly have no idea why your mother and sister would say that your face is lopsided when we can’t see anything even when we look for it."
Inga seemed mollified. We had to leave her, as we must leave all patients, to the vagaries of their own family dynamics. I can only hope that Inga doesn’t one day have some cabbie tell her to please lean to the left to keep the taxi from rolling over.
Dr. Rockoff practices dermatology in Brookline, Mass. He is on the clinical faculty at Tufts University School of Medicine, Boston, and has taught senior medical students and other trainees for 30 years.
One of my favorite Seinfeld episodes involves Elaine breaking up with a man who likes to mess with the minds of anyone who has the nerve to call it off with him. When Elaine tells him that they’re through, he says to her, "You know, you have a big head."
The rest of the show is devoted to Elaine trying to convince herself that she does not have a big head. In one scene, a cabbie tells the petite Elaine sitting in his back seat, "Please slide down, will you? You’re blocking the window!" And Elaine stands in Central Park in the show’s final scene, a bird flies right into her head as an old man says, "Well, I never saw that before. Looked like the bird just couldn’t get out of the way."
Jerry Seinfeld, who may have OCD-oid tendencies himself, often referred to dermatology in his sketches and showed insight into our patients and our clinical lives. How many people do we see who can’t stop thinking about some part of their appearance, often one we would never guess could be the focus of their attention: a small mole or freckle, a tiny wrinkle, a slight asymmetry in their features? Quite often, these patients become fixated on the feature not because they are "vain" or "narcissistic," and not because they have "body dysmorphic disorder," but just because someone else (and not necessarily somebody they were breaking up with who wanted to get even) pointed it out. From then on, they couldn’t stop thinking about it.
It may have been a doctor. ("That mole, has it been changing?") It may have been a friend. ("You know, the groove on the left side of your nose is deeper than the one on the right.") It may have been a hairdresser – it often is a hairdresser, who can see what you can’t. ("How long has that spot been up here?" Or, "Goodness! I can see right through to your scalp. Are you going bald?!")
Or it could be one of my favorite villains, the magnifying mirror, bane of presbyopic middle-age women. Overcoming presbyopia comes at the price of seeing every nevus as Pike’s Peak and every pore as the Grand Canyon.
In these cases, and many others like them, once the spots or defects are pointed out, people find it all but impossible to stop thinking about them and noticing them every time they look at themselves. If a bird flew by, it would probably slam right into them because it couldn’t get out of the way.
Removing what the patients are fixated on may be unnecessary, risky, or impossible. Advice to "just stop looking" may make sense, but can be unsatisfying or unacceptable.
I had a Seinfeld-esque moment the other day. Inga, thirtyish, came by to discuss acne, and then said, "Can you feel this growth at the end of my eyebrow?" My student and I palpated a small nodule under the outer aspect of her right eyebrow.
"It feels like a cyst," I said. "Probably been there a long time."
"Are you sure?" she asked. "Doesn’t it distort my face?"
We stepped back to a conversational distance. I couldn’t see anything, and neither could the student. "We can’t even see it from here," I told Inga. "What makes you think it distorts your face?"
"I was walking down the street with my mother and sister last week," she said, "and my mother said, "Inga, what is that on your eyebrow? Your whole face looks out of whack! And my sister said, ‘You’d better get that checked out.’"
My student and I stared at her. She was serious.
"Look," I said. "There is something there, but it’s definitely OK, and removing it would be unnecessary and leave a big scar. We honestly have no idea why your mother and sister would say that your face is lopsided when we can’t see anything even when we look for it."
Inga seemed mollified. We had to leave her, as we must leave all patients, to the vagaries of their own family dynamics. I can only hope that Inga doesn’t one day have some cabbie tell her to please lean to the left to keep the taxi from rolling over.
Dr. Rockoff practices dermatology in Brookline, Mass. He is on the clinical faculty at Tufts University School of Medicine, Boston, and has taught senior medical students and other trainees for 30 years.
Minimizing cancer’s impact on bone with denosumab: current and future perspectives
Bone metastasis is a serious complication of advanced cancer. It is most commonly observed in patients with metastatic breast and prostate cancers, but also occurs in most other metastatic solid cancers. Without treatment, patients may experience complications including intractable bone pain, hypercalcemia, fracture, spinal cord compression and/or a requirement for surgical or radiotherapeutic intervention. In 2010, denosumab, a fully human monoclonal antibody that inhibits RANK ligand (RANKL) and subsequent osteoclast-mediated bone destruction, was approved by the Food and Drug Administration for the prevention of skeletal-related events (SREs) in patients with bone metastases from solid tumors. This article reviews the role of denosumab in preventing SREs due to bone metastases, treating bone loss due to hormone-ablative cancer therapies, and describes denosumab’s safety profile and potential future indications under investigation.
Click on the PDF icon at the top of this introduction to read the full article.
Bone metastasis is a serious complication of advanced cancer. It is most commonly observed in patients with metastatic breast and prostate cancers, but also occurs in most other metastatic solid cancers. Without treatment, patients may experience complications including intractable bone pain, hypercalcemia, fracture, spinal cord compression and/or a requirement for surgical or radiotherapeutic intervention. In 2010, denosumab, a fully human monoclonal antibody that inhibits RANK ligand (RANKL) and subsequent osteoclast-mediated bone destruction, was approved by the Food and Drug Administration for the prevention of skeletal-related events (SREs) in patients with bone metastases from solid tumors. This article reviews the role of denosumab in preventing SREs due to bone metastases, treating bone loss due to hormone-ablative cancer therapies, and describes denosumab’s safety profile and potential future indications under investigation.
Click on the PDF icon at the top of this introduction to read the full article.
Bone metastasis is a serious complication of advanced cancer. It is most commonly observed in patients with metastatic breast and prostate cancers, but also occurs in most other metastatic solid cancers. Without treatment, patients may experience complications including intractable bone pain, hypercalcemia, fracture, spinal cord compression and/or a requirement for surgical or radiotherapeutic intervention. In 2010, denosumab, a fully human monoclonal antibody that inhibits RANK ligand (RANKL) and subsequent osteoclast-mediated bone destruction, was approved by the Food and Drug Administration for the prevention of skeletal-related events (SREs) in patients with bone metastases from solid tumors. This article reviews the role of denosumab in preventing SREs due to bone metastases, treating bone loss due to hormone-ablative cancer therapies, and describes denosumab’s safety profile and potential future indications under investigation.
Click on the PDF icon at the top of this introduction to read the full article.
Improving access with a collaborative approach to cancer genetic counseling services: a pilot study
Background Limited access to cancer genetic counselors (GC) may result in the lack of patient identification and/or failure to show due to travel distance and complicated treatment schedules.
Objective We hypothesized that access would improve when a GC collaborated with distant nongenetics health care providers to provide services locally.
Methods Patients at a collaborative site were offered a risk assessment survey that was reviewed remotely by a licensed, boardcertified GC. Patients were triaged such that the onsite registered nurse (RN) provided basic risk assessment and offered genetic testing for straight-forward hereditary breast and ovarian cases. Ongoing training and support was provided by the GC. Followup and complex cases were scheduled with the GC during a monthly outreach visit to the collaborative site.
Results During the 1-year study period, the total number of patients who accessed genetic counseling services from the target region was 4 times greater than the previous year. Ten of 17 patients who were triaged for genetic counseling and testing underwent genetic risk assessment services as a result of this identification and triage protocol.
Conclusion This defines a workable approach for patient identification and triage for hereditary cancer risk assessment and genetic counseling in a community setting. This collaborative approach may be applicable to centers that do not have access to a board-certified GC, especially important in light of the 2012 Commission on Cancer Standards that require cancer risk assessment, genetic counseling and testing services on site or by referral.
Click on the PDF icon at the top of this introduction to read the full article.
Background Limited access to cancer genetic counselors (GC) may result in the lack of patient identification and/or failure to show due to travel distance and complicated treatment schedules.
Objective We hypothesized that access would improve when a GC collaborated with distant nongenetics health care providers to provide services locally.
Methods Patients at a collaborative site were offered a risk assessment survey that was reviewed remotely by a licensed, boardcertified GC. Patients were triaged such that the onsite registered nurse (RN) provided basic risk assessment and offered genetic testing for straight-forward hereditary breast and ovarian cases. Ongoing training and support was provided by the GC. Followup and complex cases were scheduled with the GC during a monthly outreach visit to the collaborative site.
Results During the 1-year study period, the total number of patients who accessed genetic counseling services from the target region was 4 times greater than the previous year. Ten of 17 patients who were triaged for genetic counseling and testing underwent genetic risk assessment services as a result of this identification and triage protocol.
Conclusion This defines a workable approach for patient identification and triage for hereditary cancer risk assessment and genetic counseling in a community setting. This collaborative approach may be applicable to centers that do not have access to a board-certified GC, especially important in light of the 2012 Commission on Cancer Standards that require cancer risk assessment, genetic counseling and testing services on site or by referral.
Click on the PDF icon at the top of this introduction to read the full article.
Background Limited access to cancer genetic counselors (GC) may result in the lack of patient identification and/or failure to show due to travel distance and complicated treatment schedules.
Objective We hypothesized that access would improve when a GC collaborated with distant nongenetics health care providers to provide services locally.
Methods Patients at a collaborative site were offered a risk assessment survey that was reviewed remotely by a licensed, boardcertified GC. Patients were triaged such that the onsite registered nurse (RN) provided basic risk assessment and offered genetic testing for straight-forward hereditary breast and ovarian cases. Ongoing training and support was provided by the GC. Followup and complex cases were scheduled with the GC during a monthly outreach visit to the collaborative site.
Results During the 1-year study period, the total number of patients who accessed genetic counseling services from the target region was 4 times greater than the previous year. Ten of 17 patients who were triaged for genetic counseling and testing underwent genetic risk assessment services as a result of this identification and triage protocol.
Conclusion This defines a workable approach for patient identification and triage for hereditary cancer risk assessment and genetic counseling in a community setting. This collaborative approach may be applicable to centers that do not have access to a board-certified GC, especially important in light of the 2012 Commission on Cancer Standards that require cancer risk assessment, genetic counseling and testing services on site or by referral.
Click on the PDF icon at the top of this introduction to read the full article.
Locally advanced pancreatic cancer in a socio-economically challenged population
Background Locally advanced pancreatic cancer (LAPC) is associated with poor outcome, and clinical trials are imperative to address this. However, barriers to trial enrollment often exist, particularly in socio-economically challenged populations.
Objective To evaluate the outcome of socio-economically challenged patients who had LAPC, multiple comorbidities, and who were not enrolled on clinical trials, but who were treated with the best standard-of-care.
Methods We retrospectively reviewed the charts of 32 patients diagnosed as having LAPC who were referred to an urban cancer center between 2005 and 2010, analyzing the treatment and outcomes of 19 who underwent treatment at our center.
Results In all 26.3% of the analyzed patients had commercial insurance, 31.6% did not identify English as their preferred language, and 84.2% had 3 or more comorbidities. The median overall survival was 19.1 months, with estimated 1- and 2-year survivals of 60.8% and 36.5%, respectively. The median survival for patients receiving chemotherapy followed by chemoradiation was 26.6 months. Toxicities were controllable. Translation services were required by 26% and social services interventions by 84%. Survival analysis based on insurance coverage did not show a significant association with levels of reimbursement.
Limitations Retrospective study, small sample size, differences in chemotherapy types.
Conclusions These patients, representative of a diverse and socio-economically challenged community, were able to receive standard-of-care therapies with acceptable toxicity and to achieve survivals comparable with clinical trials. This was achieved with intense supportive services.
Click on the PDF icon at the top of this introduction to read the full article.
Background Locally advanced pancreatic cancer (LAPC) is associated with poor outcome, and clinical trials are imperative to address this. However, barriers to trial enrollment often exist, particularly in socio-economically challenged populations.
Objective To evaluate the outcome of socio-economically challenged patients who had LAPC, multiple comorbidities, and who were not enrolled on clinical trials, but who were treated with the best standard-of-care.
Methods We retrospectively reviewed the charts of 32 patients diagnosed as having LAPC who were referred to an urban cancer center between 2005 and 2010, analyzing the treatment and outcomes of 19 who underwent treatment at our center.
Results In all 26.3% of the analyzed patients had commercial insurance, 31.6% did not identify English as their preferred language, and 84.2% had 3 or more comorbidities. The median overall survival was 19.1 months, with estimated 1- and 2-year survivals of 60.8% and 36.5%, respectively. The median survival for patients receiving chemotherapy followed by chemoradiation was 26.6 months. Toxicities were controllable. Translation services were required by 26% and social services interventions by 84%. Survival analysis based on insurance coverage did not show a significant association with levels of reimbursement.
Limitations Retrospective study, small sample size, differences in chemotherapy types.
Conclusions These patients, representative of a diverse and socio-economically challenged community, were able to receive standard-of-care therapies with acceptable toxicity and to achieve survivals comparable with clinical trials. This was achieved with intense supportive services.
Click on the PDF icon at the top of this introduction to read the full article.
Background Locally advanced pancreatic cancer (LAPC) is associated with poor outcome, and clinical trials are imperative to address this. However, barriers to trial enrollment often exist, particularly in socio-economically challenged populations.
Objective To evaluate the outcome of socio-economically challenged patients who had LAPC, multiple comorbidities, and who were not enrolled on clinical trials, but who were treated with the best standard-of-care.
Methods We retrospectively reviewed the charts of 32 patients diagnosed as having LAPC who were referred to an urban cancer center between 2005 and 2010, analyzing the treatment and outcomes of 19 who underwent treatment at our center.
Results In all 26.3% of the analyzed patients had commercial insurance, 31.6% did not identify English as their preferred language, and 84.2% had 3 or more comorbidities. The median overall survival was 19.1 months, with estimated 1- and 2-year survivals of 60.8% and 36.5%, respectively. The median survival for patients receiving chemotherapy followed by chemoradiation was 26.6 months. Toxicities were controllable. Translation services were required by 26% and social services interventions by 84%. Survival analysis based on insurance coverage did not show a significant association with levels of reimbursement.
Limitations Retrospective study, small sample size, differences in chemotherapy types.
Conclusions These patients, representative of a diverse and socio-economically challenged community, were able to receive standard-of-care therapies with acceptable toxicity and to achieve survivals comparable with clinical trials. This was achieved with intense supportive services.
Click on the PDF icon at the top of this introduction to read the full article.