User login
How was your night, Doc? The limits of disclosure in preop
This morning I had the usual 7:30 a.m. elective surgical case scheduled. As I usually do, I went to see my patient at around 7 a.m. to review the procedure, answer any new questions that may have come up, and mark the incision site. My patient greeted me very cheerfully with the following questions, "How was your night? Did you sleep OK? Are you feeling good this morning? No arguments at home, I hope?" I readily answered, as I almost always do, when discussing things with patients in the preop area, that I am feeling good and ready for things to go well with the case.
I have been asked such questions many times over the years. However, for some reason the interchange with my patient this morning raised additional questions for me: Is it really acceptable for patients to ask such questions? Do I have an obligation to disclose such things? Do patients really want to know the answers or are they simply making nervous conversation?
In recent years, there have been a number of articles questioning whether surgeons should be required to disclose a lack of sleep to their elective surgical patients so that the patient can make a "truly informed" decision about what the risks of their surgery are. Thus far, there have been no such requirements at any hospital in the United States that I know of. But my patient’s questions raised a number of practical issues for me with such disclosure. We had already had a conversation in my office when I originally obtained consent for the procedure. I had carefully reviewed risks, benefits, and alternatives to the operation, and I had answered a list of the patient’s questions. Since I was actually the patient’s third opinion, it was clear that the patient already had significant background knowledge about the operation. The patient had signed the consent form before leaving my office.
At some point, before, during, or after my conversation with the patient, he had decided to trust me enough to allow me to do the operation. Subsequently, while home in the days prior to the surgery date, the patient had the opportunity to change his mind, but he actually came to the hospital on the morning of surgery. He had thus actively expressed his confidence in me by showing up for surgery. In this context, I believe that the patient’s questions were really a friendly way of expressing some degree of anxiety about the operation rather than an actual second guessing of my capacity to optimally perform the surgery. In this context, I believe that it is more important that I try to alleviate the patient’s concerns than that I give an expansive discourse on whatever stressful issues may be going on in my personal life.
Of particular importance is the issue of how much I have slept. I do not believe that I should discuss any concerns I may have with lack of sleep with my patient unless I have decided that I am not the best person to perform the surgery. I do not think that I would be doing my patient a service by, for example, saying that I didn’t sleep well and studies show that I might have altered judgment and asking the patient to sign a document that I have disclosed this fact. Such a disclosure seems to be designed to protect the surgeon and the institution rather than the patient.
However, if I believe that my lack of sleep, my personal stressors, or any other distraction will significantly hinder my ability to perform the operation safely, then I should not simply disclose these issues to the patient. Rather, I should explain why I should NOT be doing the surgery and either postpone the case or find someone else to do it if the patient requests this and if it is possible. Although some commentators have suggested that a sleep-deprived surgeon is the worst person to be able to assess his or her abilities to optimally perform an operation, I am convinced that we need to depend on the surgeon to make this assessment. Three central required components of professionalism are the exercise of self-regulation, the capacity to make decisions that are altruistic, and the discipline to abide by ethical standards. The issue of self-regulation is absolutely critical to the professionalism of any surgeon. The professionalism of the surgeon is the basis for patients trusting us to operate on them and make decisions in the operating room on their behalf. To mandate a separate disclosure to the patient about the amount of sleep the surgeon got the night before, or any other distracting issue, would be to cast doubt on the professionalism of the surgeon at the very time that patients most need to trust their surgeons.
Dr. Angelos is an ACS Fellow; the Linda Kohler Anderson Professor of Surgery and Surgical Ethics; chief, endocrine surgery; and associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago.
This morning I had the usual 7:30 a.m. elective surgical case scheduled. As I usually do, I went to see my patient at around 7 a.m. to review the procedure, answer any new questions that may have come up, and mark the incision site. My patient greeted me very cheerfully with the following questions, "How was your night? Did you sleep OK? Are you feeling good this morning? No arguments at home, I hope?" I readily answered, as I almost always do, when discussing things with patients in the preop area, that I am feeling good and ready for things to go well with the case.
I have been asked such questions many times over the years. However, for some reason the interchange with my patient this morning raised additional questions for me: Is it really acceptable for patients to ask such questions? Do I have an obligation to disclose such things? Do patients really want to know the answers or are they simply making nervous conversation?
In recent years, there have been a number of articles questioning whether surgeons should be required to disclose a lack of sleep to their elective surgical patients so that the patient can make a "truly informed" decision about what the risks of their surgery are. Thus far, there have been no such requirements at any hospital in the United States that I know of. But my patient’s questions raised a number of practical issues for me with such disclosure. We had already had a conversation in my office when I originally obtained consent for the procedure. I had carefully reviewed risks, benefits, and alternatives to the operation, and I had answered a list of the patient’s questions. Since I was actually the patient’s third opinion, it was clear that the patient already had significant background knowledge about the operation. The patient had signed the consent form before leaving my office.
At some point, before, during, or after my conversation with the patient, he had decided to trust me enough to allow me to do the operation. Subsequently, while home in the days prior to the surgery date, the patient had the opportunity to change his mind, but he actually came to the hospital on the morning of surgery. He had thus actively expressed his confidence in me by showing up for surgery. In this context, I believe that the patient’s questions were really a friendly way of expressing some degree of anxiety about the operation rather than an actual second guessing of my capacity to optimally perform the surgery. In this context, I believe that it is more important that I try to alleviate the patient’s concerns than that I give an expansive discourse on whatever stressful issues may be going on in my personal life.
Of particular importance is the issue of how much I have slept. I do not believe that I should discuss any concerns I may have with lack of sleep with my patient unless I have decided that I am not the best person to perform the surgery. I do not think that I would be doing my patient a service by, for example, saying that I didn’t sleep well and studies show that I might have altered judgment and asking the patient to sign a document that I have disclosed this fact. Such a disclosure seems to be designed to protect the surgeon and the institution rather than the patient.
However, if I believe that my lack of sleep, my personal stressors, or any other distraction will significantly hinder my ability to perform the operation safely, then I should not simply disclose these issues to the patient. Rather, I should explain why I should NOT be doing the surgery and either postpone the case or find someone else to do it if the patient requests this and if it is possible. Although some commentators have suggested that a sleep-deprived surgeon is the worst person to be able to assess his or her abilities to optimally perform an operation, I am convinced that we need to depend on the surgeon to make this assessment. Three central required components of professionalism are the exercise of self-regulation, the capacity to make decisions that are altruistic, and the discipline to abide by ethical standards. The issue of self-regulation is absolutely critical to the professionalism of any surgeon. The professionalism of the surgeon is the basis for patients trusting us to operate on them and make decisions in the operating room on their behalf. To mandate a separate disclosure to the patient about the amount of sleep the surgeon got the night before, or any other distracting issue, would be to cast doubt on the professionalism of the surgeon at the very time that patients most need to trust their surgeons.
Dr. Angelos is an ACS Fellow; the Linda Kohler Anderson Professor of Surgery and Surgical Ethics; chief, endocrine surgery; and associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago.
This morning I had the usual 7:30 a.m. elective surgical case scheduled. As I usually do, I went to see my patient at around 7 a.m. to review the procedure, answer any new questions that may have come up, and mark the incision site. My patient greeted me very cheerfully with the following questions, "How was your night? Did you sleep OK? Are you feeling good this morning? No arguments at home, I hope?" I readily answered, as I almost always do, when discussing things with patients in the preop area, that I am feeling good and ready for things to go well with the case.
I have been asked such questions many times over the years. However, for some reason the interchange with my patient this morning raised additional questions for me: Is it really acceptable for patients to ask such questions? Do I have an obligation to disclose such things? Do patients really want to know the answers or are they simply making nervous conversation?
In recent years, there have been a number of articles questioning whether surgeons should be required to disclose a lack of sleep to their elective surgical patients so that the patient can make a "truly informed" decision about what the risks of their surgery are. Thus far, there have been no such requirements at any hospital in the United States that I know of. But my patient’s questions raised a number of practical issues for me with such disclosure. We had already had a conversation in my office when I originally obtained consent for the procedure. I had carefully reviewed risks, benefits, and alternatives to the operation, and I had answered a list of the patient’s questions. Since I was actually the patient’s third opinion, it was clear that the patient already had significant background knowledge about the operation. The patient had signed the consent form before leaving my office.
At some point, before, during, or after my conversation with the patient, he had decided to trust me enough to allow me to do the operation. Subsequently, while home in the days prior to the surgery date, the patient had the opportunity to change his mind, but he actually came to the hospital on the morning of surgery. He had thus actively expressed his confidence in me by showing up for surgery. In this context, I believe that the patient’s questions were really a friendly way of expressing some degree of anxiety about the operation rather than an actual second guessing of my capacity to optimally perform the surgery. In this context, I believe that it is more important that I try to alleviate the patient’s concerns than that I give an expansive discourse on whatever stressful issues may be going on in my personal life.
Of particular importance is the issue of how much I have slept. I do not believe that I should discuss any concerns I may have with lack of sleep with my patient unless I have decided that I am not the best person to perform the surgery. I do not think that I would be doing my patient a service by, for example, saying that I didn’t sleep well and studies show that I might have altered judgment and asking the patient to sign a document that I have disclosed this fact. Such a disclosure seems to be designed to protect the surgeon and the institution rather than the patient.
However, if I believe that my lack of sleep, my personal stressors, or any other distraction will significantly hinder my ability to perform the operation safely, then I should not simply disclose these issues to the patient. Rather, I should explain why I should NOT be doing the surgery and either postpone the case or find someone else to do it if the patient requests this and if it is possible. Although some commentators have suggested that a sleep-deprived surgeon is the worst person to be able to assess his or her abilities to optimally perform an operation, I am convinced that we need to depend on the surgeon to make this assessment. Three central required components of professionalism are the exercise of self-regulation, the capacity to make decisions that are altruistic, and the discipline to abide by ethical standards. The issue of self-regulation is absolutely critical to the professionalism of any surgeon. The professionalism of the surgeon is the basis for patients trusting us to operate on them and make decisions in the operating room on their behalf. To mandate a separate disclosure to the patient about the amount of sleep the surgeon got the night before, or any other distracting issue, would be to cast doubt on the professionalism of the surgeon at the very time that patients most need to trust their surgeons.
Dr. Angelos is an ACS Fellow; the Linda Kohler Anderson Professor of Surgery and Surgical Ethics; chief, endocrine surgery; and associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago.
online TK 5
Enter text here
Enter text here
Enter text here
Mulberry
Often in this column, several species within a family might be discussed in relation to a broad range of health benefits. Licorice and mushrooms are good examples. In this case, and in this column, the focus will be on several species within a family that are thought to confer the same type of dermatologic benefit. The Morus genus within the Moraceae family appears to include several species that display skin-lightening properties.
Tyrosinase is the enzyme that controls the production of melanin. Suppressing tyrosinase activity to achieve skin lightening is a well-established method in dermatologic practice. The desire for products with fewer side effects than the mainstay, hydroquinone, or natural products such as kojic acid or arbutin, has led to investigations of several species in the Moraceae family. Notably, several Moraceae trees have been found to exhibit antioxidant activity (Int. J. Mol. Sci. 2012;13:2472-80; Biol. Pharm. Bull. 2002;25:1045-8; Biosci. Biotechnol. Biochem. 2010;74:2385-95; J. Pharm. Pharmacol. 2004;56:1291-8). The focus here, though, will be on the skin-lightening activity of various parts of Morus (commonly known as mulberry) trees.
In 2013, Singh et al. assessed the effects of mulberry, kiwi, and Sophora extracts on melanogenesis and melanin transfer in human melanocytes and in cocultures with phototype-matched normal adult epidermal keratinocytes. The extracts were evaluated against isobutylmethylxanthine, hydroquinone, vitamin C, and niacinamide. The investigators found that compared with unstimulated control, mulberry, kiwi, and Sophora extracts significantly reduced melanogenesis in normal adult epidermal melanocytes and human melanoma cells. Melanin transfer also was lowered, as was filopodia expression on melanocytes. The authors concluded that the test compounds compared well with standard-bearing depigmenting agents and warrant consideration as topical agents for diminishing hyperpigmentation (Exp. Dermatol. 2013;22:67-9).
Encouraging results in melasma treatment
A randomized, single-blind, placebo-controlled trial of 50 Filipino patients (49 women, 1 man) to examine the safety and efficacy of 75% Morus alba (white mulberry) extract oil was conducted by Alvin et al. in 2011. Patients were evaluated at weeks 4 and 8. The Melasma Area and Severity Index (MASI) score, Mexameter score, and Melasma Quality of Life (MelasQOL) score were measured, with the mulberry extract group performing significantly better than the placebo group according to all metrics.
The 25 patients treated with mulberry extract showed improvement in the MASI score, from 4.076 at baseline to 2.884 at week 8 (mean difference, 1.19); the mean difference for the placebo group was 0.06. The mean Mexameter reading revealed a significant difference, with a slight increase for the mulberry group (indicating lighter pigmentation), and the placebo group scored a slightly higher value. In addition, the MelasQOL score for the mulberry group improved markedly from baseline to week 8 (58.84 to 44.16), whereas the placebo group score improved only slightly, from 57.44 at baseline to 54.28 at week 8.
Adverse events were rare, with mild itching in 4 patients reported from the mulberry group, and 12 cases of either itching or erythema reported by the placebo group.
The investigators concluded that 75% mulberry extract oil objectively diminishes the hyperpigmentation of melasma in skin types III-V, although they recommend additional research with a larger sample size and longer treatment duration and follow-up (J. Drugs Dermatol. 2011;10:1025-31).
Paper mulberry
The bark of paper mulberry (Broussonetia papyrifera, also known as Morus papyrifera) is composed of extremely strong fibers used to produce high-quality paper and cloth. In China, the leaves, stem, leaf juice, roots, fruits, and bark have all been found to impart various health benefits, with the stem and leaf juice used to treat skin disorders and insect bites (Phytother. Res. 2012;26:1-10).
In one study, a 0.4% concentration of paper mulberry extract was demonstrated to suppress tyrosinase activity by 50% compared with 5.5% hydroquinone and 10% kojic acid. Notably, paper mulberry is not considered a significant irritant even at 1% concentration (J. Drugs Dermatol. 2009;8:s5-9).
White mulberry
In 2002, Lee et al. investigated the in vitro effects of an 85% methanol extract of dried white mulberry leaves on melanin biosynthesis. They found that one of the primary bioactive constituents, mulberroside F (moracin M-6, 3’-di-O-beta-D-glucopyranoside), inhibited the tyrosinase activity that converts dopa to dopachrome in the melanin synthesis process and also suppressed the melanin formation of melan-a cells. In addition, the mulberry extract inhibited tyrosinase activity more potently than did kojic acid (Biol. Pharm. Bull. 2002;25:1045-8).
The following year, a different team found that the young twigs of white mulberry also suppressed tyrosinase activity as well as melanin production in B-16 melanoma cells. In vivo, the extracts decreased melanin synthesis in a guinea pig model without displaying toxicity (J. Cosmet. Sci. 2003;54:133-42).
In 2006, Wang et al. investigated 25 traditional Chinese herbal medicines potentially useful in dermatology, particularly for skin whitening, and found that white mulberry was one of four species to potently inhibit tyrosinase activity, and more strongly than arbutin did (J. Ethnopharmacol. 2006;106:353-9).
Chinese mulberry/shimaguwa
In 2012, Zheng et al. isolated constituents from the roots of Chinese mulberry and found that several ingredients, including oxyresveratrol, moracenin D, sanggenon T, and kuwanon O, displayed more potent tyrosinase inhibition than kojic acid did. They concluded that Chinese mulberry is a good natural source of tyrosinase inhibitors and is potentially useful in cosmetic skin-lightening products as well as in foods as antibrowning agents (Fitoterapia 2012;83:1008-13).
Conclusion
Mulberry is actively used within the dermatologic armamentarium as one of the many options for skin lightening. A significant body of evidence has emerged over the past 15 years to establish the antityrosinase activity of various mulberry species, particularly white mulberry and paper mulberry.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook "Cosmetic Dermatology: Principles and Practice" (McGraw-Hill, 2009), and a book for consumers, "The Skin Type Solution" (Bantam, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001 and joined the editorial advisory board in 2004. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever.
Often in this column, several species within a family might be discussed in relation to a broad range of health benefits. Licorice and mushrooms are good examples. In this case, and in this column, the focus will be on several species within a family that are thought to confer the same type of dermatologic benefit. The Morus genus within the Moraceae family appears to include several species that display skin-lightening properties.
Tyrosinase is the enzyme that controls the production of melanin. Suppressing tyrosinase activity to achieve skin lightening is a well-established method in dermatologic practice. The desire for products with fewer side effects than the mainstay, hydroquinone, or natural products such as kojic acid or arbutin, has led to investigations of several species in the Moraceae family. Notably, several Moraceae trees have been found to exhibit antioxidant activity (Int. J. Mol. Sci. 2012;13:2472-80; Biol. Pharm. Bull. 2002;25:1045-8; Biosci. Biotechnol. Biochem. 2010;74:2385-95; J. Pharm. Pharmacol. 2004;56:1291-8). The focus here, though, will be on the skin-lightening activity of various parts of Morus (commonly known as mulberry) trees.
In 2013, Singh et al. assessed the effects of mulberry, kiwi, and Sophora extracts on melanogenesis and melanin transfer in human melanocytes and in cocultures with phototype-matched normal adult epidermal keratinocytes. The extracts were evaluated against isobutylmethylxanthine, hydroquinone, vitamin C, and niacinamide. The investigators found that compared with unstimulated control, mulberry, kiwi, and Sophora extracts significantly reduced melanogenesis in normal adult epidermal melanocytes and human melanoma cells. Melanin transfer also was lowered, as was filopodia expression on melanocytes. The authors concluded that the test compounds compared well with standard-bearing depigmenting agents and warrant consideration as topical agents for diminishing hyperpigmentation (Exp. Dermatol. 2013;22:67-9).
Encouraging results in melasma treatment
A randomized, single-blind, placebo-controlled trial of 50 Filipino patients (49 women, 1 man) to examine the safety and efficacy of 75% Morus alba (white mulberry) extract oil was conducted by Alvin et al. in 2011. Patients were evaluated at weeks 4 and 8. The Melasma Area and Severity Index (MASI) score, Mexameter score, and Melasma Quality of Life (MelasQOL) score were measured, with the mulberry extract group performing significantly better than the placebo group according to all metrics.
The 25 patients treated with mulberry extract showed improvement in the MASI score, from 4.076 at baseline to 2.884 at week 8 (mean difference, 1.19); the mean difference for the placebo group was 0.06. The mean Mexameter reading revealed a significant difference, with a slight increase for the mulberry group (indicating lighter pigmentation), and the placebo group scored a slightly higher value. In addition, the MelasQOL score for the mulberry group improved markedly from baseline to week 8 (58.84 to 44.16), whereas the placebo group score improved only slightly, from 57.44 at baseline to 54.28 at week 8.
Adverse events were rare, with mild itching in 4 patients reported from the mulberry group, and 12 cases of either itching or erythema reported by the placebo group.
The investigators concluded that 75% mulberry extract oil objectively diminishes the hyperpigmentation of melasma in skin types III-V, although they recommend additional research with a larger sample size and longer treatment duration and follow-up (J. Drugs Dermatol. 2011;10:1025-31).
Paper mulberry
The bark of paper mulberry (Broussonetia papyrifera, also known as Morus papyrifera) is composed of extremely strong fibers used to produce high-quality paper and cloth. In China, the leaves, stem, leaf juice, roots, fruits, and bark have all been found to impart various health benefits, with the stem and leaf juice used to treat skin disorders and insect bites (Phytother. Res. 2012;26:1-10).
In one study, a 0.4% concentration of paper mulberry extract was demonstrated to suppress tyrosinase activity by 50% compared with 5.5% hydroquinone and 10% kojic acid. Notably, paper mulberry is not considered a significant irritant even at 1% concentration (J. Drugs Dermatol. 2009;8:s5-9).
White mulberry
In 2002, Lee et al. investigated the in vitro effects of an 85% methanol extract of dried white mulberry leaves on melanin biosynthesis. They found that one of the primary bioactive constituents, mulberroside F (moracin M-6, 3’-di-O-beta-D-glucopyranoside), inhibited the tyrosinase activity that converts dopa to dopachrome in the melanin synthesis process and also suppressed the melanin formation of melan-a cells. In addition, the mulberry extract inhibited tyrosinase activity more potently than did kojic acid (Biol. Pharm. Bull. 2002;25:1045-8).
The following year, a different team found that the young twigs of white mulberry also suppressed tyrosinase activity as well as melanin production in B-16 melanoma cells. In vivo, the extracts decreased melanin synthesis in a guinea pig model without displaying toxicity (J. Cosmet. Sci. 2003;54:133-42).
In 2006, Wang et al. investigated 25 traditional Chinese herbal medicines potentially useful in dermatology, particularly for skin whitening, and found that white mulberry was one of four species to potently inhibit tyrosinase activity, and more strongly than arbutin did (J. Ethnopharmacol. 2006;106:353-9).
Chinese mulberry/shimaguwa
In 2012, Zheng et al. isolated constituents from the roots of Chinese mulberry and found that several ingredients, including oxyresveratrol, moracenin D, sanggenon T, and kuwanon O, displayed more potent tyrosinase inhibition than kojic acid did. They concluded that Chinese mulberry is a good natural source of tyrosinase inhibitors and is potentially useful in cosmetic skin-lightening products as well as in foods as antibrowning agents (Fitoterapia 2012;83:1008-13).
Conclusion
Mulberry is actively used within the dermatologic armamentarium as one of the many options for skin lightening. A significant body of evidence has emerged over the past 15 years to establish the antityrosinase activity of various mulberry species, particularly white mulberry and paper mulberry.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook "Cosmetic Dermatology: Principles and Practice" (McGraw-Hill, 2009), and a book for consumers, "The Skin Type Solution" (Bantam, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001 and joined the editorial advisory board in 2004. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever.
Often in this column, several species within a family might be discussed in relation to a broad range of health benefits. Licorice and mushrooms are good examples. In this case, and in this column, the focus will be on several species within a family that are thought to confer the same type of dermatologic benefit. The Morus genus within the Moraceae family appears to include several species that display skin-lightening properties.
Tyrosinase is the enzyme that controls the production of melanin. Suppressing tyrosinase activity to achieve skin lightening is a well-established method in dermatologic practice. The desire for products with fewer side effects than the mainstay, hydroquinone, or natural products such as kojic acid or arbutin, has led to investigations of several species in the Moraceae family. Notably, several Moraceae trees have been found to exhibit antioxidant activity (Int. J. Mol. Sci. 2012;13:2472-80; Biol. Pharm. Bull. 2002;25:1045-8; Biosci. Biotechnol. Biochem. 2010;74:2385-95; J. Pharm. Pharmacol. 2004;56:1291-8). The focus here, though, will be on the skin-lightening activity of various parts of Morus (commonly known as mulberry) trees.
In 2013, Singh et al. assessed the effects of mulberry, kiwi, and Sophora extracts on melanogenesis and melanin transfer in human melanocytes and in cocultures with phototype-matched normal adult epidermal keratinocytes. The extracts were evaluated against isobutylmethylxanthine, hydroquinone, vitamin C, and niacinamide. The investigators found that compared with unstimulated control, mulberry, kiwi, and Sophora extracts significantly reduced melanogenesis in normal adult epidermal melanocytes and human melanoma cells. Melanin transfer also was lowered, as was filopodia expression on melanocytes. The authors concluded that the test compounds compared well with standard-bearing depigmenting agents and warrant consideration as topical agents for diminishing hyperpigmentation (Exp. Dermatol. 2013;22:67-9).
Encouraging results in melasma treatment
A randomized, single-blind, placebo-controlled trial of 50 Filipino patients (49 women, 1 man) to examine the safety and efficacy of 75% Morus alba (white mulberry) extract oil was conducted by Alvin et al. in 2011. Patients were evaluated at weeks 4 and 8. The Melasma Area and Severity Index (MASI) score, Mexameter score, and Melasma Quality of Life (MelasQOL) score were measured, with the mulberry extract group performing significantly better than the placebo group according to all metrics.
The 25 patients treated with mulberry extract showed improvement in the MASI score, from 4.076 at baseline to 2.884 at week 8 (mean difference, 1.19); the mean difference for the placebo group was 0.06. The mean Mexameter reading revealed a significant difference, with a slight increase for the mulberry group (indicating lighter pigmentation), and the placebo group scored a slightly higher value. In addition, the MelasQOL score for the mulberry group improved markedly from baseline to week 8 (58.84 to 44.16), whereas the placebo group score improved only slightly, from 57.44 at baseline to 54.28 at week 8.
Adverse events were rare, with mild itching in 4 patients reported from the mulberry group, and 12 cases of either itching or erythema reported by the placebo group.
The investigators concluded that 75% mulberry extract oil objectively diminishes the hyperpigmentation of melasma in skin types III-V, although they recommend additional research with a larger sample size and longer treatment duration and follow-up (J. Drugs Dermatol. 2011;10:1025-31).
Paper mulberry
The bark of paper mulberry (Broussonetia papyrifera, also known as Morus papyrifera) is composed of extremely strong fibers used to produce high-quality paper and cloth. In China, the leaves, stem, leaf juice, roots, fruits, and bark have all been found to impart various health benefits, with the stem and leaf juice used to treat skin disorders and insect bites (Phytother. Res. 2012;26:1-10).
In one study, a 0.4% concentration of paper mulberry extract was demonstrated to suppress tyrosinase activity by 50% compared with 5.5% hydroquinone and 10% kojic acid. Notably, paper mulberry is not considered a significant irritant even at 1% concentration (J. Drugs Dermatol. 2009;8:s5-9).
White mulberry
In 2002, Lee et al. investigated the in vitro effects of an 85% methanol extract of dried white mulberry leaves on melanin biosynthesis. They found that one of the primary bioactive constituents, mulberroside F (moracin M-6, 3’-di-O-beta-D-glucopyranoside), inhibited the tyrosinase activity that converts dopa to dopachrome in the melanin synthesis process and also suppressed the melanin formation of melan-a cells. In addition, the mulberry extract inhibited tyrosinase activity more potently than did kojic acid (Biol. Pharm. Bull. 2002;25:1045-8).
The following year, a different team found that the young twigs of white mulberry also suppressed tyrosinase activity as well as melanin production in B-16 melanoma cells. In vivo, the extracts decreased melanin synthesis in a guinea pig model without displaying toxicity (J. Cosmet. Sci. 2003;54:133-42).
In 2006, Wang et al. investigated 25 traditional Chinese herbal medicines potentially useful in dermatology, particularly for skin whitening, and found that white mulberry was one of four species to potently inhibit tyrosinase activity, and more strongly than arbutin did (J. Ethnopharmacol. 2006;106:353-9).
Chinese mulberry/shimaguwa
In 2012, Zheng et al. isolated constituents from the roots of Chinese mulberry and found that several ingredients, including oxyresveratrol, moracenin D, sanggenon T, and kuwanon O, displayed more potent tyrosinase inhibition than kojic acid did. They concluded that Chinese mulberry is a good natural source of tyrosinase inhibitors and is potentially useful in cosmetic skin-lightening products as well as in foods as antibrowning agents (Fitoterapia 2012;83:1008-13).
Conclusion
Mulberry is actively used within the dermatologic armamentarium as one of the many options for skin lightening. A significant body of evidence has emerged over the past 15 years to establish the antityrosinase activity of various mulberry species, particularly white mulberry and paper mulberry.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook "Cosmetic Dermatology: Principles and Practice" (McGraw-Hill, 2009), and a book for consumers, "The Skin Type Solution" (Bantam, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001 and joined the editorial advisory board in 2004. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever.
When wounds won’t heal, try these strategies
SAN FRANCISCO – When your first or second attempts to help a skin wound heal haven’t worked, ask yourself three questions, Dr. Theodora Mauro suggested:
• Have you diagnosed the wound correctly?
• What systemic conditions are keeping the wound from healing?
• Have you tailored your treatment to the wound correctly?
Most dermatologists are pretty good at diagnosing ulcers that are caused by single etiologies. What Dr. Mauro sees more commonly in her busy referral practice, however, are wounds that won’t heal because of a combination of causes – often venous insufficiency plus something else, she said at the annual meeting of the Pacific Dermatologic Association.
"That can make the diagnosis a little more confusing," said Dr. Mauro, professor of dermatology at the University of California, San Francisco and chief of the dermatology service at the San Francisco Veterans Affairs Medical Center.
Ulcer types
The most common skin ulcer in the United States is due to venous insufficiency. These patients tend to be spread among dermatologists, vascular surgeons, and podiatrists and "are not well served by being scattered among different disciplines," she said.
Arterial ulcers make up 6%-10% of skin ulcers in the medical literature, and the incidence of diabetic ulcers is increasing, she added. Pressure ulcers are becoming more common as the population ages.
Morphology and the location of ulcers usually can distinguish the different types, but don’t forget to check the patient’s pulses and sensations, Dr. Mauro said. If you can feel pedal pulses, the patient is very likely to have a normal ankle brachial index (greater than 0.8). "It’s an easy thing to do and very helpful," she said. Also, break a Q-tip cotton swab in half and poke the patient with the sharp end. "You’d be surprised at the number of people who have altered sensation" that’s contributing to the ulceration.
A common "combination" that gets missed is a nonmelanoma skin cancer and an ulcer at that site from venous insufficiency. Consider taking biopsies of nonhealing ulcers to look for skin cancer, she said. Long-standing ulcers can develop squamous cell carcinoma within them. A basal cell carcinoma can look like a healing ulcer, but not heal.
Bullous diseases also can be hidden in ulcers, particularly in the elderly. "There seems to be a two-hit thing with venous insufficiency where you have a little bit of blistering disease and you have a lot of hydrostatic pressure, and people will get their bullae on their legs long before they get it anyplace else," Dr. Mauro said. "You put compression on and they come back next week and now they have intact bullae. Think about that as another cause."
Less common ulcerative problems that can be confusing include pyoderma gangrenosum (which may be half as common as people think, studies suggest), an underlying vasculitis, or mycobacterial infections. If you see a nonhealing hyperkeratotic lesion, consider an atypical mycobacterial infection. In Dr. Mauro’s region, these usually are in patients who went mountain biking and inoculated themselves through an ankle scratch or in patients exposed to the organism through infected water during a pedicure.
Systemic conditions
Wound healing can be inhibited by things that doctors do, and by things that patients do.
Taking antimetabolite medications (such as hydroxyurea or methotrexate), prednisone, or nonsteroidal anti-inflammatory drugs can impair wound healing, as does smoking. Radiation to the site also inhibits healing. Think twice before irradiating a basal cell carcinoma on the leg of a patient with venous insufficiency. "It’s much better to either try some topical medication or just bite the bullet, do surgery, and put the Unna boot on after the surgery, because if you irradiate [the basal cell carcinoma] and it ulcerates, then it’s really hard to heal," she said.
Coexisting cancer or arterial disease can inhibit wound healing. If you check pulses and can’t feel them, send the patient for an ankle brachial index to determine if there’s arterial disease, in which case the patient should see a vascular surgeon, she said.
When you’re taking patients’ histories, ask what they’re eating so you can assess whether they’re getting enough protein, zinc, and vitamin C. "You’d be surprised at the number of nutritional deficiencies that we see" in patients with nonhealing wounds. Her clinic picks up a zinc deficiency three to four times per year and a protein deficiency approximately twice a year. "All of these are things that you need to make collagen and granulation tissue," she explained. Supplementation with Ensure can provide these nutrients, but a less-expensive option is Carnation Breakfast Essentials, at about one-fifth the cost, she added.
Tailor therapy appropriately
Dressings can make wounds worse. Most commonly, putting a hydrocolloid dressing (such as DuoDERM) on an ulcer that has a lot of drainage will macerate and enlarge the wound. Putting stiff foams or self-adherent wraps such as Coban on wounds with a lot of edema can create secondary ulcers. And avoid compression on the legs of people with arterial insufficiency, Dr. Mauro said.
Artifactual ulcers from patients scratching or picking at wounds are increasing, she said. These patients usually will not report that they’re messing with the wound, but your level of suspicion should increase if the ulcer is above the knee. Hydrocolloid dressings work pretty well for this problem. Consider sending some of these patients to an expert in the growing field of medication therapy for dysesthesia.
Ask patients about home remedies, because they will put all kinds of things on wounds to try and help them heal. Neomycin can cause contact dermatitis. Patients may apply full-strength hydrogen peroxide, thinking that they need a disinfectant, which tends to damage the epithelium and can impair wound healing. If they insist on using hydrogen peroxide, recommend a half-strength solution.
For patients with venous insufficiency, the hydrostatic pressure from sitting can be nearly as bad as from standing. Ask patients with nonhealing wounds what they do at home and at work. If they’re sitting much of that time, recommend products to alleviate the pressure. Dr. Mauro said she has virtually become a saleswoman for La-Z-Boy furniture during her medical career because La-Z-Boy products allow patients to eat, work at computers, and function in positions with less hydrostatic pressure.
When to refer
Consider referring patients with chronic wounds that have not lost at least 50% of their width and height within 6 weeks. When a wound has been present long enough, the physiology shifts from acute to chronic. "It’s as if the body decides that it’s tired of trying to heal it and its new equilibrium is just going to be, ‘I have a wound,’ " she said.
If you want to try treating chronic wounds, applying Promogran Prisma matrix wound dressing in those without good granulation tissue may stimulate granulation tissue, she said.
Debriding an ulcer is "kind of a poor man’s way of getting platelet-derived growth factor," she added. Dr. Mauro applies a compounded 30% lidocaine ("but you can use any topical lidocaine"), leaves it on for 20-25 minutes, and debrides using a curette, which is much more precise than a scalpel. "What you’re trying to do is ‘reboot the computer’ and trick the ulcer into thinking it’s an acute ulcer rather than a chronic one," she said.
Dr. Mauro said she refers patients with arterial insufficiency to vascular surgeons. She sends patients with diabetes to podiatrists, preferably before they develop ulcers. The podiatrist can regularly trim nails for patients with poor sight and loss of sensation who may cut themselves, and can design footwear to avoid pressure that could cause an ulcer. "That will keep you out of trouble" with diabetic patients, she said.
Dr. Mauro has been a consultant for Unilever, but not in a wound-healing capacity.
On Twitter @sherryboschert
SAN FRANCISCO – When your first or second attempts to help a skin wound heal haven’t worked, ask yourself three questions, Dr. Theodora Mauro suggested:
• Have you diagnosed the wound correctly?
• What systemic conditions are keeping the wound from healing?
• Have you tailored your treatment to the wound correctly?
Most dermatologists are pretty good at diagnosing ulcers that are caused by single etiologies. What Dr. Mauro sees more commonly in her busy referral practice, however, are wounds that won’t heal because of a combination of causes – often venous insufficiency plus something else, she said at the annual meeting of the Pacific Dermatologic Association.
"That can make the diagnosis a little more confusing," said Dr. Mauro, professor of dermatology at the University of California, San Francisco and chief of the dermatology service at the San Francisco Veterans Affairs Medical Center.
Ulcer types
The most common skin ulcer in the United States is due to venous insufficiency. These patients tend to be spread among dermatologists, vascular surgeons, and podiatrists and "are not well served by being scattered among different disciplines," she said.
Arterial ulcers make up 6%-10% of skin ulcers in the medical literature, and the incidence of diabetic ulcers is increasing, she added. Pressure ulcers are becoming more common as the population ages.
Morphology and the location of ulcers usually can distinguish the different types, but don’t forget to check the patient’s pulses and sensations, Dr. Mauro said. If you can feel pedal pulses, the patient is very likely to have a normal ankle brachial index (greater than 0.8). "It’s an easy thing to do and very helpful," she said. Also, break a Q-tip cotton swab in half and poke the patient with the sharp end. "You’d be surprised at the number of people who have altered sensation" that’s contributing to the ulceration.
A common "combination" that gets missed is a nonmelanoma skin cancer and an ulcer at that site from venous insufficiency. Consider taking biopsies of nonhealing ulcers to look for skin cancer, she said. Long-standing ulcers can develop squamous cell carcinoma within them. A basal cell carcinoma can look like a healing ulcer, but not heal.
Bullous diseases also can be hidden in ulcers, particularly in the elderly. "There seems to be a two-hit thing with venous insufficiency where you have a little bit of blistering disease and you have a lot of hydrostatic pressure, and people will get their bullae on their legs long before they get it anyplace else," Dr. Mauro said. "You put compression on and they come back next week and now they have intact bullae. Think about that as another cause."
Less common ulcerative problems that can be confusing include pyoderma gangrenosum (which may be half as common as people think, studies suggest), an underlying vasculitis, or mycobacterial infections. If you see a nonhealing hyperkeratotic lesion, consider an atypical mycobacterial infection. In Dr. Mauro’s region, these usually are in patients who went mountain biking and inoculated themselves through an ankle scratch or in patients exposed to the organism through infected water during a pedicure.
Systemic conditions
Wound healing can be inhibited by things that doctors do, and by things that patients do.
Taking antimetabolite medications (such as hydroxyurea or methotrexate), prednisone, or nonsteroidal anti-inflammatory drugs can impair wound healing, as does smoking. Radiation to the site also inhibits healing. Think twice before irradiating a basal cell carcinoma on the leg of a patient with venous insufficiency. "It’s much better to either try some topical medication or just bite the bullet, do surgery, and put the Unna boot on after the surgery, because if you irradiate [the basal cell carcinoma] and it ulcerates, then it’s really hard to heal," she said.
Coexisting cancer or arterial disease can inhibit wound healing. If you check pulses and can’t feel them, send the patient for an ankle brachial index to determine if there’s arterial disease, in which case the patient should see a vascular surgeon, she said.
When you’re taking patients’ histories, ask what they’re eating so you can assess whether they’re getting enough protein, zinc, and vitamin C. "You’d be surprised at the number of nutritional deficiencies that we see" in patients with nonhealing wounds. Her clinic picks up a zinc deficiency three to four times per year and a protein deficiency approximately twice a year. "All of these are things that you need to make collagen and granulation tissue," she explained. Supplementation with Ensure can provide these nutrients, but a less-expensive option is Carnation Breakfast Essentials, at about one-fifth the cost, she added.
Tailor therapy appropriately
Dressings can make wounds worse. Most commonly, putting a hydrocolloid dressing (such as DuoDERM) on an ulcer that has a lot of drainage will macerate and enlarge the wound. Putting stiff foams or self-adherent wraps such as Coban on wounds with a lot of edema can create secondary ulcers. And avoid compression on the legs of people with arterial insufficiency, Dr. Mauro said.
Artifactual ulcers from patients scratching or picking at wounds are increasing, she said. These patients usually will not report that they’re messing with the wound, but your level of suspicion should increase if the ulcer is above the knee. Hydrocolloid dressings work pretty well for this problem. Consider sending some of these patients to an expert in the growing field of medication therapy for dysesthesia.
Ask patients about home remedies, because they will put all kinds of things on wounds to try and help them heal. Neomycin can cause contact dermatitis. Patients may apply full-strength hydrogen peroxide, thinking that they need a disinfectant, which tends to damage the epithelium and can impair wound healing. If they insist on using hydrogen peroxide, recommend a half-strength solution.
For patients with venous insufficiency, the hydrostatic pressure from sitting can be nearly as bad as from standing. Ask patients with nonhealing wounds what they do at home and at work. If they’re sitting much of that time, recommend products to alleviate the pressure. Dr. Mauro said she has virtually become a saleswoman for La-Z-Boy furniture during her medical career because La-Z-Boy products allow patients to eat, work at computers, and function in positions with less hydrostatic pressure.
When to refer
Consider referring patients with chronic wounds that have not lost at least 50% of their width and height within 6 weeks. When a wound has been present long enough, the physiology shifts from acute to chronic. "It’s as if the body decides that it’s tired of trying to heal it and its new equilibrium is just going to be, ‘I have a wound,’ " she said.
If you want to try treating chronic wounds, applying Promogran Prisma matrix wound dressing in those without good granulation tissue may stimulate granulation tissue, she said.
Debriding an ulcer is "kind of a poor man’s way of getting platelet-derived growth factor," she added. Dr. Mauro applies a compounded 30% lidocaine ("but you can use any topical lidocaine"), leaves it on for 20-25 minutes, and debrides using a curette, which is much more precise than a scalpel. "What you’re trying to do is ‘reboot the computer’ and trick the ulcer into thinking it’s an acute ulcer rather than a chronic one," she said.
Dr. Mauro said she refers patients with arterial insufficiency to vascular surgeons. She sends patients with diabetes to podiatrists, preferably before they develop ulcers. The podiatrist can regularly trim nails for patients with poor sight and loss of sensation who may cut themselves, and can design footwear to avoid pressure that could cause an ulcer. "That will keep you out of trouble" with diabetic patients, she said.
Dr. Mauro has been a consultant for Unilever, but not in a wound-healing capacity.
On Twitter @sherryboschert
SAN FRANCISCO – When your first or second attempts to help a skin wound heal haven’t worked, ask yourself three questions, Dr. Theodora Mauro suggested:
• Have you diagnosed the wound correctly?
• What systemic conditions are keeping the wound from healing?
• Have you tailored your treatment to the wound correctly?
Most dermatologists are pretty good at diagnosing ulcers that are caused by single etiologies. What Dr. Mauro sees more commonly in her busy referral practice, however, are wounds that won’t heal because of a combination of causes – often venous insufficiency plus something else, she said at the annual meeting of the Pacific Dermatologic Association.
"That can make the diagnosis a little more confusing," said Dr. Mauro, professor of dermatology at the University of California, San Francisco and chief of the dermatology service at the San Francisco Veterans Affairs Medical Center.
Ulcer types
The most common skin ulcer in the United States is due to venous insufficiency. These patients tend to be spread among dermatologists, vascular surgeons, and podiatrists and "are not well served by being scattered among different disciplines," she said.
Arterial ulcers make up 6%-10% of skin ulcers in the medical literature, and the incidence of diabetic ulcers is increasing, she added. Pressure ulcers are becoming more common as the population ages.
Morphology and the location of ulcers usually can distinguish the different types, but don’t forget to check the patient’s pulses and sensations, Dr. Mauro said. If you can feel pedal pulses, the patient is very likely to have a normal ankle brachial index (greater than 0.8). "It’s an easy thing to do and very helpful," she said. Also, break a Q-tip cotton swab in half and poke the patient with the sharp end. "You’d be surprised at the number of people who have altered sensation" that’s contributing to the ulceration.
A common "combination" that gets missed is a nonmelanoma skin cancer and an ulcer at that site from venous insufficiency. Consider taking biopsies of nonhealing ulcers to look for skin cancer, she said. Long-standing ulcers can develop squamous cell carcinoma within them. A basal cell carcinoma can look like a healing ulcer, but not heal.
Bullous diseases also can be hidden in ulcers, particularly in the elderly. "There seems to be a two-hit thing with venous insufficiency where you have a little bit of blistering disease and you have a lot of hydrostatic pressure, and people will get their bullae on their legs long before they get it anyplace else," Dr. Mauro said. "You put compression on and they come back next week and now they have intact bullae. Think about that as another cause."
Less common ulcerative problems that can be confusing include pyoderma gangrenosum (which may be half as common as people think, studies suggest), an underlying vasculitis, or mycobacterial infections. If you see a nonhealing hyperkeratotic lesion, consider an atypical mycobacterial infection. In Dr. Mauro’s region, these usually are in patients who went mountain biking and inoculated themselves through an ankle scratch or in patients exposed to the organism through infected water during a pedicure.
Systemic conditions
Wound healing can be inhibited by things that doctors do, and by things that patients do.
Taking antimetabolite medications (such as hydroxyurea or methotrexate), prednisone, or nonsteroidal anti-inflammatory drugs can impair wound healing, as does smoking. Radiation to the site also inhibits healing. Think twice before irradiating a basal cell carcinoma on the leg of a patient with venous insufficiency. "It’s much better to either try some topical medication or just bite the bullet, do surgery, and put the Unna boot on after the surgery, because if you irradiate [the basal cell carcinoma] and it ulcerates, then it’s really hard to heal," she said.
Coexisting cancer or arterial disease can inhibit wound healing. If you check pulses and can’t feel them, send the patient for an ankle brachial index to determine if there’s arterial disease, in which case the patient should see a vascular surgeon, she said.
When you’re taking patients’ histories, ask what they’re eating so you can assess whether they’re getting enough protein, zinc, and vitamin C. "You’d be surprised at the number of nutritional deficiencies that we see" in patients with nonhealing wounds. Her clinic picks up a zinc deficiency three to four times per year and a protein deficiency approximately twice a year. "All of these are things that you need to make collagen and granulation tissue," she explained. Supplementation with Ensure can provide these nutrients, but a less-expensive option is Carnation Breakfast Essentials, at about one-fifth the cost, she added.
Tailor therapy appropriately
Dressings can make wounds worse. Most commonly, putting a hydrocolloid dressing (such as DuoDERM) on an ulcer that has a lot of drainage will macerate and enlarge the wound. Putting stiff foams or self-adherent wraps such as Coban on wounds with a lot of edema can create secondary ulcers. And avoid compression on the legs of people with arterial insufficiency, Dr. Mauro said.
Artifactual ulcers from patients scratching or picking at wounds are increasing, she said. These patients usually will not report that they’re messing with the wound, but your level of suspicion should increase if the ulcer is above the knee. Hydrocolloid dressings work pretty well for this problem. Consider sending some of these patients to an expert in the growing field of medication therapy for dysesthesia.
Ask patients about home remedies, because they will put all kinds of things on wounds to try and help them heal. Neomycin can cause contact dermatitis. Patients may apply full-strength hydrogen peroxide, thinking that they need a disinfectant, which tends to damage the epithelium and can impair wound healing. If they insist on using hydrogen peroxide, recommend a half-strength solution.
For patients with venous insufficiency, the hydrostatic pressure from sitting can be nearly as bad as from standing. Ask patients with nonhealing wounds what they do at home and at work. If they’re sitting much of that time, recommend products to alleviate the pressure. Dr. Mauro said she has virtually become a saleswoman for La-Z-Boy furniture during her medical career because La-Z-Boy products allow patients to eat, work at computers, and function in positions with less hydrostatic pressure.
When to refer
Consider referring patients with chronic wounds that have not lost at least 50% of their width and height within 6 weeks. When a wound has been present long enough, the physiology shifts from acute to chronic. "It’s as if the body decides that it’s tired of trying to heal it and its new equilibrium is just going to be, ‘I have a wound,’ " she said.
If you want to try treating chronic wounds, applying Promogran Prisma matrix wound dressing in those without good granulation tissue may stimulate granulation tissue, she said.
Debriding an ulcer is "kind of a poor man’s way of getting platelet-derived growth factor," she added. Dr. Mauro applies a compounded 30% lidocaine ("but you can use any topical lidocaine"), leaves it on for 20-25 minutes, and debrides using a curette, which is much more precise than a scalpel. "What you’re trying to do is ‘reboot the computer’ and trick the ulcer into thinking it’s an acute ulcer rather than a chronic one," she said.
Dr. Mauro said she refers patients with arterial insufficiency to vascular surgeons. She sends patients with diabetes to podiatrists, preferably before they develop ulcers. The podiatrist can regularly trim nails for patients with poor sight and loss of sensation who may cut themselves, and can design footwear to avoid pressure that could cause an ulcer. "That will keep you out of trouble" with diabetic patients, she said.
Dr. Mauro has been a consultant for Unilever, but not in a wound-healing capacity.
On Twitter @sherryboschert
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE PACIFIC DERMATOLOGIC ASSOCIATION
Apps for physicians
When it comes to health, wellness, and fitness apps, we physicians tend to think of our patients. As in, how can this app help my patient lose weight, exercise more, monitor his diabetes, or help her stay compliant with her medications?
But what about physicians and other health care workers? Can apps help us? Absolutely. According to Dr. Craig Burkhart, a dermatologist and apps expert at the University of North Carolina at Chapel Hill, many physicians aren’t taking advantage of apps that can improve efficiency. "Most physicians I encounter have $1,000-$2,000 smartphones and tablets (when you include the service plans) and only make phone calls, check e-mails, text, and listen to music on them – all things they could do with much less expensive devices."
Dr. Burkhart says that many apps "can turn your devices into tools that help organize your e-mails, organize your schedule, bring clinical information to your fingertips, aid patient-doctor communication, secure important information, speed up and improve note taking, and more."
Apps for physicians are available in categories including patient education, drug reference, medical literature, and general reference.
Check out these handy apps and see what works for you:
• Read by QxMD. Staying up to date on medical journals is a challenge. This app lets you access, organize, and share articles from your favorite medical journals. Its magazinelike interface is popular with users, and makes reading medical journals "as fun as it can be," says Dr. Burkhart.
• PDFpen. Dr. Burkhart says this app is "essential to a paperless workflow." It allows users to sign and send documents without printing actual sheets of paper, making for a greener and more efficient office.
• Visual DX. A reference tool for physicians, this app is a digital medical image library with more than 25,000 images. It allows you to visually confirm a diagnosis and to quickly search on a disease, including symptoms and patient care management. A key feature is its ability to let you create a visual differential of medication-induced diseases for more than 700 different drugs.
• 1Password. We’ve all forgotten passwords from time to time. This app allows you to make and store highly secure and complex passwords. For Dr. Burkhart, it has "replaced memory and random sheets of paper as his password repository."
• Draw MD. We know that visuals often help us to explain complex issues to patients more effectively. This app enables you to draw and modify medical images and surgical procedures in a way that is clear and understandable to patients.
• 3D4Medical. This app can enhance patient education by using 3D technology to allow navigation around the body. You can zoom, rotate, and cut images to create different perspectives.
Dr. Jeffrey Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego and a volunteer clinical assistant professor at the University of California, San Diego. He has published numerous scientific articles and is a member and fellow of the American Academy of Dermatology, and a member of the Telemedicine Association and the American Medical Association, among others. He is board certified in dermatology as well as medicine and surgery in the state of California. Dr. Benabio has a special interest in the uses of social media for education and building a dermatology practice. He is the founder of The Derm Blog, an educational website which has had over 2 million unique visitors. Dr. Benabio is also a founding member and the skin care expert for Livestrong.com, a health and wellness website of Lance Armstrong’s the Livestrong Foundation. Dr. Benabio is @Dermdoc on Twitter.
When it comes to health, wellness, and fitness apps, we physicians tend to think of our patients. As in, how can this app help my patient lose weight, exercise more, monitor his diabetes, or help her stay compliant with her medications?
But what about physicians and other health care workers? Can apps help us? Absolutely. According to Dr. Craig Burkhart, a dermatologist and apps expert at the University of North Carolina at Chapel Hill, many physicians aren’t taking advantage of apps that can improve efficiency. "Most physicians I encounter have $1,000-$2,000 smartphones and tablets (when you include the service plans) and only make phone calls, check e-mails, text, and listen to music on them – all things they could do with much less expensive devices."
Dr. Burkhart says that many apps "can turn your devices into tools that help organize your e-mails, organize your schedule, bring clinical information to your fingertips, aid patient-doctor communication, secure important information, speed up and improve note taking, and more."
Apps for physicians are available in categories including patient education, drug reference, medical literature, and general reference.
Check out these handy apps and see what works for you:
• Read by QxMD. Staying up to date on medical journals is a challenge. This app lets you access, organize, and share articles from your favorite medical journals. Its magazinelike interface is popular with users, and makes reading medical journals "as fun as it can be," says Dr. Burkhart.
• PDFpen. Dr. Burkhart says this app is "essential to a paperless workflow." It allows users to sign and send documents without printing actual sheets of paper, making for a greener and more efficient office.
• Visual DX. A reference tool for physicians, this app is a digital medical image library with more than 25,000 images. It allows you to visually confirm a diagnosis and to quickly search on a disease, including symptoms and patient care management. A key feature is its ability to let you create a visual differential of medication-induced diseases for more than 700 different drugs.
• 1Password. We’ve all forgotten passwords from time to time. This app allows you to make and store highly secure and complex passwords. For Dr. Burkhart, it has "replaced memory and random sheets of paper as his password repository."
• Draw MD. We know that visuals often help us to explain complex issues to patients more effectively. This app enables you to draw and modify medical images and surgical procedures in a way that is clear and understandable to patients.
• 3D4Medical. This app can enhance patient education by using 3D technology to allow navigation around the body. You can zoom, rotate, and cut images to create different perspectives.
Dr. Jeffrey Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego and a volunteer clinical assistant professor at the University of California, San Diego. He has published numerous scientific articles and is a member and fellow of the American Academy of Dermatology, and a member of the Telemedicine Association and the American Medical Association, among others. He is board certified in dermatology as well as medicine and surgery in the state of California. Dr. Benabio has a special interest in the uses of social media for education and building a dermatology practice. He is the founder of The Derm Blog, an educational website which has had over 2 million unique visitors. Dr. Benabio is also a founding member and the skin care expert for Livestrong.com, a health and wellness website of Lance Armstrong’s the Livestrong Foundation. Dr. Benabio is @Dermdoc on Twitter.
When it comes to health, wellness, and fitness apps, we physicians tend to think of our patients. As in, how can this app help my patient lose weight, exercise more, monitor his diabetes, or help her stay compliant with her medications?
But what about physicians and other health care workers? Can apps help us? Absolutely. According to Dr. Craig Burkhart, a dermatologist and apps expert at the University of North Carolina at Chapel Hill, many physicians aren’t taking advantage of apps that can improve efficiency. "Most physicians I encounter have $1,000-$2,000 smartphones and tablets (when you include the service plans) and only make phone calls, check e-mails, text, and listen to music on them – all things they could do with much less expensive devices."
Dr. Burkhart says that many apps "can turn your devices into tools that help organize your e-mails, organize your schedule, bring clinical information to your fingertips, aid patient-doctor communication, secure important information, speed up and improve note taking, and more."
Apps for physicians are available in categories including patient education, drug reference, medical literature, and general reference.
Check out these handy apps and see what works for you:
• Read by QxMD. Staying up to date on medical journals is a challenge. This app lets you access, organize, and share articles from your favorite medical journals. Its magazinelike interface is popular with users, and makes reading medical journals "as fun as it can be," says Dr. Burkhart.
• PDFpen. Dr. Burkhart says this app is "essential to a paperless workflow." It allows users to sign and send documents without printing actual sheets of paper, making for a greener and more efficient office.
• Visual DX. A reference tool for physicians, this app is a digital medical image library with more than 25,000 images. It allows you to visually confirm a diagnosis and to quickly search on a disease, including symptoms and patient care management. A key feature is its ability to let you create a visual differential of medication-induced diseases for more than 700 different drugs.
• 1Password. We’ve all forgotten passwords from time to time. This app allows you to make and store highly secure and complex passwords. For Dr. Burkhart, it has "replaced memory and random sheets of paper as his password repository."
• Draw MD. We know that visuals often help us to explain complex issues to patients more effectively. This app enables you to draw and modify medical images and surgical procedures in a way that is clear and understandable to patients.
• 3D4Medical. This app can enhance patient education by using 3D technology to allow navigation around the body. You can zoom, rotate, and cut images to create different perspectives.
Dr. Jeffrey Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego and a volunteer clinical assistant professor at the University of California, San Diego. He has published numerous scientific articles and is a member and fellow of the American Academy of Dermatology, and a member of the Telemedicine Association and the American Medical Association, among others. He is board certified in dermatology as well as medicine and surgery in the state of California. Dr. Benabio has a special interest in the uses of social media for education and building a dermatology practice. He is the founder of The Derm Blog, an educational website which has had over 2 million unique visitors. Dr. Benabio is also a founding member and the skin care expert for Livestrong.com, a health and wellness website of Lance Armstrong’s the Livestrong Foundation. Dr. Benabio is @Dermdoc on Twitter.
Lung cancer diagnosis aided by novel plasma protein
A plasma protein involved in tumor oxidation and reduction reactions was useful for detecting and excluding lung cancer in more than three quarters of clinical samples evaluated in a large prospective study.
Isocitrate dehydrogenase 1 (IDH1) measurement was associated with a sensitivity of 77.1% and specificity of 76.2% in a training set of samples (n = 712), and 82.9% sensitivity and 76.6% specificity in a second validation or test set (n = 710).
Sensitivity and specificity were generally improved when the protein’s detection was considered in addition to other known or proposed non–small-cell lung cancer (NSCLC) biomarkers, with sensitivities of 75.8% and 86.3% in the training and test sets, respectively, and specificities of 89.6% and 70.7%.
"Some existing NSCLC biomarkers, such as CEA [carcinoembryonic antigen] and Cyfra21-1 [cytokeratin fragment 21-1], have been used in clinical practice, whereas others, such as CA125 [cancer antigen 125], have been recommended for further validation," Dr. Nan Sun and associates reported in the latest issue of Clinical Cancer Research.
"These biomarkers have low sensitivity, ranging from 50% to 60%, with specificities of approximately 90%," said the researchers, of the department of thoracic surgical oncology at the Cancer Institute and Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences in Beijing.
Previous research by the team had shown IDH1 levels were elevated in tumor samples taken from patients with NSCLC (Mol. Cell. Proteomics 2012;11:M111). Their present investigation (Clin. Cancer. Res. 2013; 19: 5136-45) therefore aimed to see if the protein’s measurement could aid in the diagnosis of lung cancer, differentiating between those who did and those who did not have malignant disease.
For their investigation, the team obtained 1,422 blood samples from 943 patients with previously untreated NSCLC and 479 healthy individuals who were seen for routine examinations between 2007 and 2011 at their institution.
The blood samples from the lung cancer patients were taken 3 days prior to their undergoing surgery and IDH1 levels were immediately determined by enzyme-linked immunosorbent assay, while CEA, Cyfra21-1, and CA125 levels were measured with an Elecys immunoassay analyzer.
Median levels of IDH1 were 2.39 U/L higher than those of healthy controls for squamous cell carcinoma cases (n = 489) cases and 1.96 U/L higher for adenocarcinoma cases (n = 454). Additionally, median plasma levels of IDH1 were higher in patients with adenocarcinomas than in those with squamous cell carcinomas (P = .012)
"We have identified IDH1 as an effective plasma biomarker with high sensitivity and specificity in the diagnosis of NSCLC, especially lung adenocarcinoma," senior study investigator Dr. Jie He said in a press release issued by the American Association for Cancer Research. "Based on the present data, IDH1 can be used to detect stage 1 lung cancer,"
The protein might also detect precancerous lesions, but further studies are required to test that hypothesis, said Dr. He. IDH1 might be a good target for NSCLC treatment as it "may be involved in the development of lung cancer."
A multicenter clinical trial is planned to further validate the diagnostic utility of IDH1.
Research funding was provided by the National High Technology Research and Development Program of China, the International Science and Technology Corporation and Exchange Project, the National Natural Science Foundation of China, the Doctoral Fund of Ministry of Education of China, and the Government Health Care Research Foundation for Senior Officials. The authors had no conflicts of interest to disclose.
A plasma protein involved in tumor oxidation and reduction reactions was useful for detecting and excluding lung cancer in more than three quarters of clinical samples evaluated in a large prospective study.
Isocitrate dehydrogenase 1 (IDH1) measurement was associated with a sensitivity of 77.1% and specificity of 76.2% in a training set of samples (n = 712), and 82.9% sensitivity and 76.6% specificity in a second validation or test set (n = 710).
Sensitivity and specificity were generally improved when the protein’s detection was considered in addition to other known or proposed non–small-cell lung cancer (NSCLC) biomarkers, with sensitivities of 75.8% and 86.3% in the training and test sets, respectively, and specificities of 89.6% and 70.7%.
"Some existing NSCLC biomarkers, such as CEA [carcinoembryonic antigen] and Cyfra21-1 [cytokeratin fragment 21-1], have been used in clinical practice, whereas others, such as CA125 [cancer antigen 125], have been recommended for further validation," Dr. Nan Sun and associates reported in the latest issue of Clinical Cancer Research.
"These biomarkers have low sensitivity, ranging from 50% to 60%, with specificities of approximately 90%," said the researchers, of the department of thoracic surgical oncology at the Cancer Institute and Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences in Beijing.
Previous research by the team had shown IDH1 levels were elevated in tumor samples taken from patients with NSCLC (Mol. Cell. Proteomics 2012;11:M111). Their present investigation (Clin. Cancer. Res. 2013; 19: 5136-45) therefore aimed to see if the protein’s measurement could aid in the diagnosis of lung cancer, differentiating between those who did and those who did not have malignant disease.
For their investigation, the team obtained 1,422 blood samples from 943 patients with previously untreated NSCLC and 479 healthy individuals who were seen for routine examinations between 2007 and 2011 at their institution.
The blood samples from the lung cancer patients were taken 3 days prior to their undergoing surgery and IDH1 levels were immediately determined by enzyme-linked immunosorbent assay, while CEA, Cyfra21-1, and CA125 levels were measured with an Elecys immunoassay analyzer.
Median levels of IDH1 were 2.39 U/L higher than those of healthy controls for squamous cell carcinoma cases (n = 489) cases and 1.96 U/L higher for adenocarcinoma cases (n = 454). Additionally, median plasma levels of IDH1 were higher in patients with adenocarcinomas than in those with squamous cell carcinomas (P = .012)
"We have identified IDH1 as an effective plasma biomarker with high sensitivity and specificity in the diagnosis of NSCLC, especially lung adenocarcinoma," senior study investigator Dr. Jie He said in a press release issued by the American Association for Cancer Research. "Based on the present data, IDH1 can be used to detect stage 1 lung cancer,"
The protein might also detect precancerous lesions, but further studies are required to test that hypothesis, said Dr. He. IDH1 might be a good target for NSCLC treatment as it "may be involved in the development of lung cancer."
A multicenter clinical trial is planned to further validate the diagnostic utility of IDH1.
Research funding was provided by the National High Technology Research and Development Program of China, the International Science and Technology Corporation and Exchange Project, the National Natural Science Foundation of China, the Doctoral Fund of Ministry of Education of China, and the Government Health Care Research Foundation for Senior Officials. The authors had no conflicts of interest to disclose.
A plasma protein involved in tumor oxidation and reduction reactions was useful for detecting and excluding lung cancer in more than three quarters of clinical samples evaluated in a large prospective study.
Isocitrate dehydrogenase 1 (IDH1) measurement was associated with a sensitivity of 77.1% and specificity of 76.2% in a training set of samples (n = 712), and 82.9% sensitivity and 76.6% specificity in a second validation or test set (n = 710).
Sensitivity and specificity were generally improved when the protein’s detection was considered in addition to other known or proposed non–small-cell lung cancer (NSCLC) biomarkers, with sensitivities of 75.8% and 86.3% in the training and test sets, respectively, and specificities of 89.6% and 70.7%.
"Some existing NSCLC biomarkers, such as CEA [carcinoembryonic antigen] and Cyfra21-1 [cytokeratin fragment 21-1], have been used in clinical practice, whereas others, such as CA125 [cancer antigen 125], have been recommended for further validation," Dr. Nan Sun and associates reported in the latest issue of Clinical Cancer Research.
"These biomarkers have low sensitivity, ranging from 50% to 60%, with specificities of approximately 90%," said the researchers, of the department of thoracic surgical oncology at the Cancer Institute and Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences in Beijing.
Previous research by the team had shown IDH1 levels were elevated in tumor samples taken from patients with NSCLC (Mol. Cell. Proteomics 2012;11:M111). Their present investigation (Clin. Cancer. Res. 2013; 19: 5136-45) therefore aimed to see if the protein’s measurement could aid in the diagnosis of lung cancer, differentiating between those who did and those who did not have malignant disease.
For their investigation, the team obtained 1,422 blood samples from 943 patients with previously untreated NSCLC and 479 healthy individuals who were seen for routine examinations between 2007 and 2011 at their institution.
The blood samples from the lung cancer patients were taken 3 days prior to their undergoing surgery and IDH1 levels were immediately determined by enzyme-linked immunosorbent assay, while CEA, Cyfra21-1, and CA125 levels were measured with an Elecys immunoassay analyzer.
Median levels of IDH1 were 2.39 U/L higher than those of healthy controls for squamous cell carcinoma cases (n = 489) cases and 1.96 U/L higher for adenocarcinoma cases (n = 454). Additionally, median plasma levels of IDH1 were higher in patients with adenocarcinomas than in those with squamous cell carcinomas (P = .012)
"We have identified IDH1 as an effective plasma biomarker with high sensitivity and specificity in the diagnosis of NSCLC, especially lung adenocarcinoma," senior study investigator Dr. Jie He said in a press release issued by the American Association for Cancer Research. "Based on the present data, IDH1 can be used to detect stage 1 lung cancer,"
The protein might also detect precancerous lesions, but further studies are required to test that hypothesis, said Dr. He. IDH1 might be a good target for NSCLC treatment as it "may be involved in the development of lung cancer."
A multicenter clinical trial is planned to further validate the diagnostic utility of IDH1.
Research funding was provided by the National High Technology Research and Development Program of China, the International Science and Technology Corporation and Exchange Project, the National Natural Science Foundation of China, the Doctoral Fund of Ministry of Education of China, and the Government Health Care Research Foundation for Senior Officials. The authors had no conflicts of interest to disclose.
FROM CLINICAL CANCER RESEARCH
Major finding: IDH1 detection in plasma had a sensitivity and specificity of up to 82.9% and 76.6%, respectively.
Data source: Prospective study of 1,422 participants enrolled between 2007 and 2011; 943 patients with non–small-cell lung cancer and 479 healthy individuals as controls.
Disclosures: Research funding was provided by the National High Technology Research and Development Program of China, the International Science and Technology Corporation and Exchange Project, the National Natural Science Foundation of China, the Doctoral Fund of Ministry of Education of China, and the Government Health Care Research Foundation for Senior Officials. The authors had no conflicts of interest to disclose.
Erroneously Reporting Penicillin Allergy
Patient safety is a healthcare provider's top priority. Drug allergies are instated into an electronic medical record (EMR) to avoid potential adverse events in the future. Despite the intention to provide safety, healthcare providers frequently document antimicrobial allergies incorrectly.[1] In turn, this may lead to decreased antibiotic choices, increased healthcare costs, potential adverse reactions, and unnecessary avoidance of optimal, first‐line agents.
Several strategies have been developed to help improve the accuracy of allergy documentation, including pharmacy‐based interventions, but the persistence of corrections, once performed, is unknown.[2] Although most antibiotic allergy errors are identified upon review of prior medication history (eg, penicillin allergy listed in a patient who previously received piperacillintazobactam), no prior studies have evaluated penicillin allergy errors directly after a proven tolerance with a penicillin skin testing (PST) and penicillin confirmatory challenge.[3, 4, 5] We hereby assess factors for erroneous allergy documentation in a cohort of patients with a negative PST.
METHODS
We retrospectively reviewed charts under a protocol approved by the university and medical center institutional review board. Following a PST intervention we have previously described, penicillin was removed from the patients' EMR (Epic, Verona, WI) allergy list from March 2012 through July 2012.[6] We then invested a brief procedure note into the allergy section describing the negative PST and subsequent tolerance of a penicillin agent. During the PST intervention, there was no attempt to convey the result of the PST and corrected allergy information to the outpatient clinicians.
As a follow‐up to our previous study, we reviewed the charts of the 150 subjects who represented the entire population of patients who underwent PST in the March 2012 through July 2012 intervention time period. From August 2012 through July 2013, charts were reviewed to gauge reappearances at Vidant Health, a system of 10 hospitals in eastern North Carolina. Collected data also included demographics, drug allergy or intolerance, penicillin allergy redocumentation, residence, antimicrobial use, and presence of dementia or altered mentation.
Outpatient physician and long‐term care facility (LTCF) allergy records were obtained via EMR records, patient or family inquiry, and referring documents that accompanied the patient upon arrival. In addition to reviewing the LTCF and/or outpatient physician referring documents, the outpatient physician(s) and LTCFs were contacted and asked to review other electronic or paper records that may not have been delivered with the referring documents. Inpatient and outpatient records were reviewed for penicillin allergy, as defined by the drug allergy practice parameters.[7] Fischer exact tests were used to identify significant associated factors.
RESULTS
Of the 150 patients with proven penicillin tolerance, 55 (37%) revisited a Vidant Health hospital within a year period, of which 22 (40%) received a ‐lactam agent once again without adverse effects (Table 1). Twenty (36%) of the 55 patients had penicillin allergy redocumented (Figure 1). There was no description of any allergy after the PST in any of the 20 EMR, LTCF records, or outpatient primary care physician records. Factors associated with penicillin allergy redocumentation (vs those not redocumented) included age >65 years (P = 0.011), residence in a LTCF (P = 0.0001), acutely altered mentation (P < 0.0001), and dementia (P < 0.0001). Penicillin allergy was still reported in all 21 (100%) of the LTCF patient records.
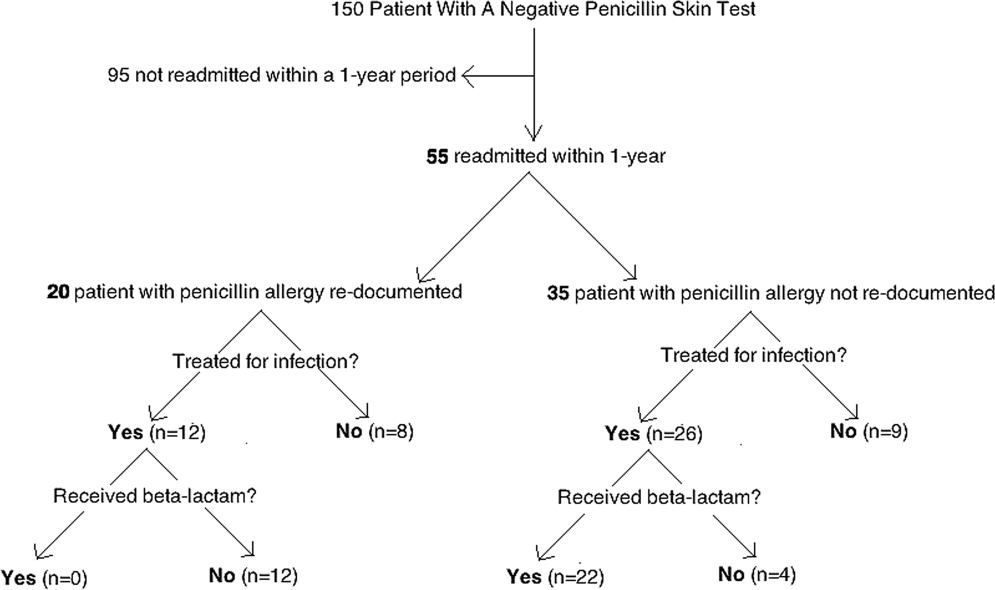
| Category | Variables | Penicillin Allergy Not Reinstated, n = 35 | Penicillin Allergy Reinstated, n = 20 | P Value |
|---|---|---|---|---|
| ||||
| Age, y | 1830 | 5 (14%) | 0 (0%) | 0.011 |
| 3164 | 17 (49%) | 5 (37%) | ||
| >65 | 13 (37%) | 15 (75%) | ||
| Gender | Male | 12 (34%) | 10 (50%) | 0.19 |
| Female | 23 (66%) | 10 (50%) | ||
| Race | White | 20 (57%) | 11 (55%) | 0.36 |
| Black | 14 (40%) | 8 (40%) | ||
| Hispanic | 1 (3%) | 1 (5%) | ||
| Residence | Home | 28 (80%) | 5 (25%) | 0.0001 |
| LTCF | 7 (20%) | 15 (75%) | ||
| Acutely altered mentation | Yes | 8 (23%) | 16 (80%) | <0.0001 |
| No | 27 (77%) | 4 (20%) | ||
| Dementia | Yes | 1 (3%) | 10 (50%) | <0.0001 |
| No | 34 (97%) | 10 (50%) | ||
| Primary service | Residenta | 18 (51%) | 5 (25%) | 0.18 |
| Hospitalist | 8 (23%) | 10 (50%) | ||
| Surgery | 3 (9%) | 3 (15%) | ||
| Emergency medicine | 6 (17%) | 2 (10%) | ||
| Primary language | English | 34 (97%) | 19 (95%) | 0.59 |
| Spanish | 1 (3%) | 1 (5%) | ||
| Hospital diagnosis | Infectious | 19 (54%) | 14 (70%) | 0.20 |
| Noninfectious | 16 (46%) | 6 (30%) | ||
| Antibiotic received | ‐lactamb | 22 (63%) | 0 (0%) | 0.07 |
| Non‐lactamc | 4 (11%) | 12 (60%) | ||
| None | 9 (26%) | 8 (40%) | ||
CONCLUSION
Errors in medication documentation are a major cause of potential harm and death.[8] In the United States, up to 14% of patient harm is due to a preventable medication error, a rate that exceeds death related to breast cancer, vehicular accidents, and AIDS.[9, 10] Inaccurate drug allergy reporting can result in a cascade of consequential medical errors, including medication prescribing (eg, use of less effective, potentially more toxic and/or more expensive agents), and diagnostic errors (eg, repeat PST, unnecessary medication desensitization).
Although EMR systems are designed to improve allergy documentation, they may also increase the risk of inaccurate or out‐of‐date data. Providers may be reluctant to permanently alter the electronic record by removing an allergy from the EMR. Chart lore, the persistence of inaccurate or outdated information, may contribute to error, particularly when the patient is unable to provide information directly. We found, for example, that dementia and acutely altered mentation were associated with allergy reporting errors, likely related to the inability of the patient to give a reliable history. Finally, the EMR does not typically include a function for noting that an allergy does not exist, making it easier to reinstate incorrect allergies. To address this problem, we subsequently began listing a negative PST as an other allergy in the EMR allergy section to improve visibility.
We also found that residence in an LTCF was associated with allergy reporting error, in part perhaps because all LTCF records still included penicillin as an allergy. This finding highlights the need for direct communication of a proven PST tolerance with the primary care physician or LTCF provider, which was not part of our initial intervention. Previous studies have described the benefit of removing incorrectly reported allergies from community pharmacy records as well.[2, 11] Simply recording it into a transfer summary may not suffice, as LTCF providers may not read, or misread, the PST result. Healthcare providers performing PST should attempt to maintain consistent inpatient and outpatient drug allergy reports to avoid drug allergies.
Another possible modality to reduce inaccurate drug allergy documentation is repetitive review of the allergy list. In the Epic EMR system, the allergy list will illustrate when the healthcare provider(s) reviewed the patients' allergies last. At Vidant Health, the allergy list is generally only reviewed during nursing triage in the emergency department. Healthcare providers should avoid chart lore or relying on nursing notes and routinely review allergies directly with the patient. Obtaining allergy information only during routine nursing triage assessment is substandard.[12] This should not substitute acquisition of allergy information from the patient using a structured, direct interview. Supervision and repeated EMR review may help to avoid overlooking an inaccurate history acquisition.[13] This may help not only help to remove drug allergies that were erroneously added to the patient's list, but also to possibly add agents that may have been missed by the triaging team.
Another means by which inaccurate redocumentation of drug allergies can be avoided is avoidance of placing nonallergic drug reactions in the allergy section of the EMR. Antimicrobial agents are often added to the allergy list because of a drug intolerance (eg, gastrointestinal symptoms), and/or pharmacologic effect (eg, electrolyte abnormality). Although these are not true reactions, healthcare providers often avoid rechallenging these agents. These adverse reactions should be placed within the problem list or past medical history section of the EMR, and not within the allergy section. Therefore, healthcare providers should accurately describe the behavior of the allergic reaction(s).[14]
A limitation of our study is our small sample size and single‐site design. This may have limited the ability to analyze the data in a multivariable way and the ability to learn about risk factors across a variety of EMR and workflow settings. Furthermore, we reviewed only the 55 patients who were readmitted, and therefore do not know how accurate records were for the other 95 patients.
In summary, this work highlights the challenges of successful implementation of quality improvement projects in an electronic health record‐based world. Although PST can expand antimicrobial choices and reduce healthcare costs, the benefits may be limited by inadequately removing the allergy from the hospital and outpatient record(s). From the novel data gathered from our study, primary care physicians and LTCFs are now promptly notified of a negative PST to reduce these medical errors, and we believe this process should become a standard of care.
Acknowledgments
The authors thank Dr. Muhammad S. Ashraf for his assistance in preparing this manuscript.
Disclosures: Ramzy H. Rimawi, MD, has a potential conflict of interest with Alk‐Abello (speakers' bureau), the manufacturer of the Pre‐PEN penicillin skin test. Alk‐Abello was not involved in the production of this article. Paul P. Cook, MD has potential conflicts of interest with Gilead (investigator), Pfizer (investigator), Merck (investigator and speakers' bureau) and Forest (speakers' bureau), none of which relate to the use of penicillin or penicillin skin tests. None of the authors have received any source(s) of funding for this article. The corresponding author, Ramzy Rimawi, MD, had full access to all of the data in the study and had final responsibility for the decision to submit for publication. The manuscript is not under review by any other publication.
- , . Accuracy of drug allergy documentation. Am J Health Syst Pharm. 1997;54(14):1627–1629.
- , , , et al. Program to remove incorrect allergy documentation in pediatrics medical records. Am J Health Syst Pharm. 2001;58(18):1722–1727.
- , , . Electronic medication ordering with integrated drug database and clinical decision support system. Stud Health Technol Inform. 2012;180:693–697.
- , , , . Pharmacy‐controlled documentation of drug allergies. Am J Health‐Syst Pharm. 1991;48:260–264.
- , , , et al. Systems analysis of adverse drug events. JAMA. 1995;274:35–43.
- , , , et al. The impact of penicillin skin testing on clinical practice and antimicrobial stewardship. J Hosp Med. 2013;8(6):341–345.
- , , , et al.Joint Task Force on Practice Parameters; American College of Allergy, Asthma and Immunology;Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105:259–273.
- , , . Prescription quality in an acute medical ward. Pharmacoepidemiol Drug Saf. 2009;18(12):1158–1165.
- . Make no mistake! Medical errors can be deadly serious. FDA Consum. 2000;34(5):13–18.
- . Medication errors. J R Coll Physicians Edinb. 2007;37:343–346.
- , , , et al. A pharmacist‐led information technology intervention for medication errors (PINCER): a multicenter, cluster randomized, controlled trial and cost‐effectiveness analysis. Lancet. 2012;379(9823):1301–1309.
- , , , . Getting the data right: information accuracy in pediatric emergency medicine. Qual Saf Health Care. 2006;15(4):296–301.
- , . Antibiotic allergy: inaccurate history taking in a teaching hospital. South Med J. 1994;87(8):805–807.
- , , . Drug allergy documentation—time for a change? Int J Clin Pharm. 2011;33(4):610–613.
Patient safety is a healthcare provider's top priority. Drug allergies are instated into an electronic medical record (EMR) to avoid potential adverse events in the future. Despite the intention to provide safety, healthcare providers frequently document antimicrobial allergies incorrectly.[1] In turn, this may lead to decreased antibiotic choices, increased healthcare costs, potential adverse reactions, and unnecessary avoidance of optimal, first‐line agents.
Several strategies have been developed to help improve the accuracy of allergy documentation, including pharmacy‐based interventions, but the persistence of corrections, once performed, is unknown.[2] Although most antibiotic allergy errors are identified upon review of prior medication history (eg, penicillin allergy listed in a patient who previously received piperacillintazobactam), no prior studies have evaluated penicillin allergy errors directly after a proven tolerance with a penicillin skin testing (PST) and penicillin confirmatory challenge.[3, 4, 5] We hereby assess factors for erroneous allergy documentation in a cohort of patients with a negative PST.
METHODS
We retrospectively reviewed charts under a protocol approved by the university and medical center institutional review board. Following a PST intervention we have previously described, penicillin was removed from the patients' EMR (Epic, Verona, WI) allergy list from March 2012 through July 2012.[6] We then invested a brief procedure note into the allergy section describing the negative PST and subsequent tolerance of a penicillin agent. During the PST intervention, there was no attempt to convey the result of the PST and corrected allergy information to the outpatient clinicians.
As a follow‐up to our previous study, we reviewed the charts of the 150 subjects who represented the entire population of patients who underwent PST in the March 2012 through July 2012 intervention time period. From August 2012 through July 2013, charts were reviewed to gauge reappearances at Vidant Health, a system of 10 hospitals in eastern North Carolina. Collected data also included demographics, drug allergy or intolerance, penicillin allergy redocumentation, residence, antimicrobial use, and presence of dementia or altered mentation.
Outpatient physician and long‐term care facility (LTCF) allergy records were obtained via EMR records, patient or family inquiry, and referring documents that accompanied the patient upon arrival. In addition to reviewing the LTCF and/or outpatient physician referring documents, the outpatient physician(s) and LTCFs were contacted and asked to review other electronic or paper records that may not have been delivered with the referring documents. Inpatient and outpatient records were reviewed for penicillin allergy, as defined by the drug allergy practice parameters.[7] Fischer exact tests were used to identify significant associated factors.
RESULTS
Of the 150 patients with proven penicillin tolerance, 55 (37%) revisited a Vidant Health hospital within a year period, of which 22 (40%) received a ‐lactam agent once again without adverse effects (Table 1). Twenty (36%) of the 55 patients had penicillin allergy redocumented (Figure 1). There was no description of any allergy after the PST in any of the 20 EMR, LTCF records, or outpatient primary care physician records. Factors associated with penicillin allergy redocumentation (vs those not redocumented) included age >65 years (P = 0.011), residence in a LTCF (P = 0.0001), acutely altered mentation (P < 0.0001), and dementia (P < 0.0001). Penicillin allergy was still reported in all 21 (100%) of the LTCF patient records.

| Category | Variables | Penicillin Allergy Not Reinstated, n = 35 | Penicillin Allergy Reinstated, n = 20 | P Value |
|---|---|---|---|---|
| ||||
| Age, y | 1830 | 5 (14%) | 0 (0%) | 0.011 |
| 3164 | 17 (49%) | 5 (37%) | ||
| >65 | 13 (37%) | 15 (75%) | ||
| Gender | Male | 12 (34%) | 10 (50%) | 0.19 |
| Female | 23 (66%) | 10 (50%) | ||
| Race | White | 20 (57%) | 11 (55%) | 0.36 |
| Black | 14 (40%) | 8 (40%) | ||
| Hispanic | 1 (3%) | 1 (5%) | ||
| Residence | Home | 28 (80%) | 5 (25%) | 0.0001 |
| LTCF | 7 (20%) | 15 (75%) | ||
| Acutely altered mentation | Yes | 8 (23%) | 16 (80%) | <0.0001 |
| No | 27 (77%) | 4 (20%) | ||
| Dementia | Yes | 1 (3%) | 10 (50%) | <0.0001 |
| No | 34 (97%) | 10 (50%) | ||
| Primary service | Residenta | 18 (51%) | 5 (25%) | 0.18 |
| Hospitalist | 8 (23%) | 10 (50%) | ||
| Surgery | 3 (9%) | 3 (15%) | ||
| Emergency medicine | 6 (17%) | 2 (10%) | ||
| Primary language | English | 34 (97%) | 19 (95%) | 0.59 |
| Spanish | 1 (3%) | 1 (5%) | ||
| Hospital diagnosis | Infectious | 19 (54%) | 14 (70%) | 0.20 |
| Noninfectious | 16 (46%) | 6 (30%) | ||
| Antibiotic received | ‐lactamb | 22 (63%) | 0 (0%) | 0.07 |
| Non‐lactamc | 4 (11%) | 12 (60%) | ||
| None | 9 (26%) | 8 (40%) | ||
CONCLUSION
Errors in medication documentation are a major cause of potential harm and death.[8] In the United States, up to 14% of patient harm is due to a preventable medication error, a rate that exceeds death related to breast cancer, vehicular accidents, and AIDS.[9, 10] Inaccurate drug allergy reporting can result in a cascade of consequential medical errors, including medication prescribing (eg, use of less effective, potentially more toxic and/or more expensive agents), and diagnostic errors (eg, repeat PST, unnecessary medication desensitization).
Although EMR systems are designed to improve allergy documentation, they may also increase the risk of inaccurate or out‐of‐date data. Providers may be reluctant to permanently alter the electronic record by removing an allergy from the EMR. Chart lore, the persistence of inaccurate or outdated information, may contribute to error, particularly when the patient is unable to provide information directly. We found, for example, that dementia and acutely altered mentation were associated with allergy reporting errors, likely related to the inability of the patient to give a reliable history. Finally, the EMR does not typically include a function for noting that an allergy does not exist, making it easier to reinstate incorrect allergies. To address this problem, we subsequently began listing a negative PST as an other allergy in the EMR allergy section to improve visibility.
We also found that residence in an LTCF was associated with allergy reporting error, in part perhaps because all LTCF records still included penicillin as an allergy. This finding highlights the need for direct communication of a proven PST tolerance with the primary care physician or LTCF provider, which was not part of our initial intervention. Previous studies have described the benefit of removing incorrectly reported allergies from community pharmacy records as well.[2, 11] Simply recording it into a transfer summary may not suffice, as LTCF providers may not read, or misread, the PST result. Healthcare providers performing PST should attempt to maintain consistent inpatient and outpatient drug allergy reports to avoid drug allergies.
Another possible modality to reduce inaccurate drug allergy documentation is repetitive review of the allergy list. In the Epic EMR system, the allergy list will illustrate when the healthcare provider(s) reviewed the patients' allergies last. At Vidant Health, the allergy list is generally only reviewed during nursing triage in the emergency department. Healthcare providers should avoid chart lore or relying on nursing notes and routinely review allergies directly with the patient. Obtaining allergy information only during routine nursing triage assessment is substandard.[12] This should not substitute acquisition of allergy information from the patient using a structured, direct interview. Supervision and repeated EMR review may help to avoid overlooking an inaccurate history acquisition.[13] This may help not only help to remove drug allergies that were erroneously added to the patient's list, but also to possibly add agents that may have been missed by the triaging team.
Another means by which inaccurate redocumentation of drug allergies can be avoided is avoidance of placing nonallergic drug reactions in the allergy section of the EMR. Antimicrobial agents are often added to the allergy list because of a drug intolerance (eg, gastrointestinal symptoms), and/or pharmacologic effect (eg, electrolyte abnormality). Although these are not true reactions, healthcare providers often avoid rechallenging these agents. These adverse reactions should be placed within the problem list or past medical history section of the EMR, and not within the allergy section. Therefore, healthcare providers should accurately describe the behavior of the allergic reaction(s).[14]
A limitation of our study is our small sample size and single‐site design. This may have limited the ability to analyze the data in a multivariable way and the ability to learn about risk factors across a variety of EMR and workflow settings. Furthermore, we reviewed only the 55 patients who were readmitted, and therefore do not know how accurate records were for the other 95 patients.
In summary, this work highlights the challenges of successful implementation of quality improvement projects in an electronic health record‐based world. Although PST can expand antimicrobial choices and reduce healthcare costs, the benefits may be limited by inadequately removing the allergy from the hospital and outpatient record(s). From the novel data gathered from our study, primary care physicians and LTCFs are now promptly notified of a negative PST to reduce these medical errors, and we believe this process should become a standard of care.
Acknowledgments
The authors thank Dr. Muhammad S. Ashraf for his assistance in preparing this manuscript.
Disclosures: Ramzy H. Rimawi, MD, has a potential conflict of interest with Alk‐Abello (speakers' bureau), the manufacturer of the Pre‐PEN penicillin skin test. Alk‐Abello was not involved in the production of this article. Paul P. Cook, MD has potential conflicts of interest with Gilead (investigator), Pfizer (investigator), Merck (investigator and speakers' bureau) and Forest (speakers' bureau), none of which relate to the use of penicillin or penicillin skin tests. None of the authors have received any source(s) of funding for this article. The corresponding author, Ramzy Rimawi, MD, had full access to all of the data in the study and had final responsibility for the decision to submit for publication. The manuscript is not under review by any other publication.
Patient safety is a healthcare provider's top priority. Drug allergies are instated into an electronic medical record (EMR) to avoid potential adverse events in the future. Despite the intention to provide safety, healthcare providers frequently document antimicrobial allergies incorrectly.[1] In turn, this may lead to decreased antibiotic choices, increased healthcare costs, potential adverse reactions, and unnecessary avoidance of optimal, first‐line agents.
Several strategies have been developed to help improve the accuracy of allergy documentation, including pharmacy‐based interventions, but the persistence of corrections, once performed, is unknown.[2] Although most antibiotic allergy errors are identified upon review of prior medication history (eg, penicillin allergy listed in a patient who previously received piperacillintazobactam), no prior studies have evaluated penicillin allergy errors directly after a proven tolerance with a penicillin skin testing (PST) and penicillin confirmatory challenge.[3, 4, 5] We hereby assess factors for erroneous allergy documentation in a cohort of patients with a negative PST.
METHODS
We retrospectively reviewed charts under a protocol approved by the university and medical center institutional review board. Following a PST intervention we have previously described, penicillin was removed from the patients' EMR (Epic, Verona, WI) allergy list from March 2012 through July 2012.[6] We then invested a brief procedure note into the allergy section describing the negative PST and subsequent tolerance of a penicillin agent. During the PST intervention, there was no attempt to convey the result of the PST and corrected allergy information to the outpatient clinicians.
As a follow‐up to our previous study, we reviewed the charts of the 150 subjects who represented the entire population of patients who underwent PST in the March 2012 through July 2012 intervention time period. From August 2012 through July 2013, charts were reviewed to gauge reappearances at Vidant Health, a system of 10 hospitals in eastern North Carolina. Collected data also included demographics, drug allergy or intolerance, penicillin allergy redocumentation, residence, antimicrobial use, and presence of dementia or altered mentation.
Outpatient physician and long‐term care facility (LTCF) allergy records were obtained via EMR records, patient or family inquiry, and referring documents that accompanied the patient upon arrival. In addition to reviewing the LTCF and/or outpatient physician referring documents, the outpatient physician(s) and LTCFs were contacted and asked to review other electronic or paper records that may not have been delivered with the referring documents. Inpatient and outpatient records were reviewed for penicillin allergy, as defined by the drug allergy practice parameters.[7] Fischer exact tests were used to identify significant associated factors.
RESULTS
Of the 150 patients with proven penicillin tolerance, 55 (37%) revisited a Vidant Health hospital within a year period, of which 22 (40%) received a ‐lactam agent once again without adverse effects (Table 1). Twenty (36%) of the 55 patients had penicillin allergy redocumented (Figure 1). There was no description of any allergy after the PST in any of the 20 EMR, LTCF records, or outpatient primary care physician records. Factors associated with penicillin allergy redocumentation (vs those not redocumented) included age >65 years (P = 0.011), residence in a LTCF (P = 0.0001), acutely altered mentation (P < 0.0001), and dementia (P < 0.0001). Penicillin allergy was still reported in all 21 (100%) of the LTCF patient records.

| Category | Variables | Penicillin Allergy Not Reinstated, n = 35 | Penicillin Allergy Reinstated, n = 20 | P Value |
|---|---|---|---|---|
| ||||
| Age, y | 1830 | 5 (14%) | 0 (0%) | 0.011 |
| 3164 | 17 (49%) | 5 (37%) | ||
| >65 | 13 (37%) | 15 (75%) | ||
| Gender | Male | 12 (34%) | 10 (50%) | 0.19 |
| Female | 23 (66%) | 10 (50%) | ||
| Race | White | 20 (57%) | 11 (55%) | 0.36 |
| Black | 14 (40%) | 8 (40%) | ||
| Hispanic | 1 (3%) | 1 (5%) | ||
| Residence | Home | 28 (80%) | 5 (25%) | 0.0001 |
| LTCF | 7 (20%) | 15 (75%) | ||
| Acutely altered mentation | Yes | 8 (23%) | 16 (80%) | <0.0001 |
| No | 27 (77%) | 4 (20%) | ||
| Dementia | Yes | 1 (3%) | 10 (50%) | <0.0001 |
| No | 34 (97%) | 10 (50%) | ||
| Primary service | Residenta | 18 (51%) | 5 (25%) | 0.18 |
| Hospitalist | 8 (23%) | 10 (50%) | ||
| Surgery | 3 (9%) | 3 (15%) | ||
| Emergency medicine | 6 (17%) | 2 (10%) | ||
| Primary language | English | 34 (97%) | 19 (95%) | 0.59 |
| Spanish | 1 (3%) | 1 (5%) | ||
| Hospital diagnosis | Infectious | 19 (54%) | 14 (70%) | 0.20 |
| Noninfectious | 16 (46%) | 6 (30%) | ||
| Antibiotic received | ‐lactamb | 22 (63%) | 0 (0%) | 0.07 |
| Non‐lactamc | 4 (11%) | 12 (60%) | ||
| None | 9 (26%) | 8 (40%) | ||
CONCLUSION
Errors in medication documentation are a major cause of potential harm and death.[8] In the United States, up to 14% of patient harm is due to a preventable medication error, a rate that exceeds death related to breast cancer, vehicular accidents, and AIDS.[9, 10] Inaccurate drug allergy reporting can result in a cascade of consequential medical errors, including medication prescribing (eg, use of less effective, potentially more toxic and/or more expensive agents), and diagnostic errors (eg, repeat PST, unnecessary medication desensitization).
Although EMR systems are designed to improve allergy documentation, they may also increase the risk of inaccurate or out‐of‐date data. Providers may be reluctant to permanently alter the electronic record by removing an allergy from the EMR. Chart lore, the persistence of inaccurate or outdated information, may contribute to error, particularly when the patient is unable to provide information directly. We found, for example, that dementia and acutely altered mentation were associated with allergy reporting errors, likely related to the inability of the patient to give a reliable history. Finally, the EMR does not typically include a function for noting that an allergy does not exist, making it easier to reinstate incorrect allergies. To address this problem, we subsequently began listing a negative PST as an other allergy in the EMR allergy section to improve visibility.
We also found that residence in an LTCF was associated with allergy reporting error, in part perhaps because all LTCF records still included penicillin as an allergy. This finding highlights the need for direct communication of a proven PST tolerance with the primary care physician or LTCF provider, which was not part of our initial intervention. Previous studies have described the benefit of removing incorrectly reported allergies from community pharmacy records as well.[2, 11] Simply recording it into a transfer summary may not suffice, as LTCF providers may not read, or misread, the PST result. Healthcare providers performing PST should attempt to maintain consistent inpatient and outpatient drug allergy reports to avoid drug allergies.
Another possible modality to reduce inaccurate drug allergy documentation is repetitive review of the allergy list. In the Epic EMR system, the allergy list will illustrate when the healthcare provider(s) reviewed the patients' allergies last. At Vidant Health, the allergy list is generally only reviewed during nursing triage in the emergency department. Healthcare providers should avoid chart lore or relying on nursing notes and routinely review allergies directly with the patient. Obtaining allergy information only during routine nursing triage assessment is substandard.[12] This should not substitute acquisition of allergy information from the patient using a structured, direct interview. Supervision and repeated EMR review may help to avoid overlooking an inaccurate history acquisition.[13] This may help not only help to remove drug allergies that were erroneously added to the patient's list, but also to possibly add agents that may have been missed by the triaging team.
Another means by which inaccurate redocumentation of drug allergies can be avoided is avoidance of placing nonallergic drug reactions in the allergy section of the EMR. Antimicrobial agents are often added to the allergy list because of a drug intolerance (eg, gastrointestinal symptoms), and/or pharmacologic effect (eg, electrolyte abnormality). Although these are not true reactions, healthcare providers often avoid rechallenging these agents. These adverse reactions should be placed within the problem list or past medical history section of the EMR, and not within the allergy section. Therefore, healthcare providers should accurately describe the behavior of the allergic reaction(s).[14]
A limitation of our study is our small sample size and single‐site design. This may have limited the ability to analyze the data in a multivariable way and the ability to learn about risk factors across a variety of EMR and workflow settings. Furthermore, we reviewed only the 55 patients who were readmitted, and therefore do not know how accurate records were for the other 95 patients.
In summary, this work highlights the challenges of successful implementation of quality improvement projects in an electronic health record‐based world. Although PST can expand antimicrobial choices and reduce healthcare costs, the benefits may be limited by inadequately removing the allergy from the hospital and outpatient record(s). From the novel data gathered from our study, primary care physicians and LTCFs are now promptly notified of a negative PST to reduce these medical errors, and we believe this process should become a standard of care.
Acknowledgments
The authors thank Dr. Muhammad S. Ashraf for his assistance in preparing this manuscript.
Disclosures: Ramzy H. Rimawi, MD, has a potential conflict of interest with Alk‐Abello (speakers' bureau), the manufacturer of the Pre‐PEN penicillin skin test. Alk‐Abello was not involved in the production of this article. Paul P. Cook, MD has potential conflicts of interest with Gilead (investigator), Pfizer (investigator), Merck (investigator and speakers' bureau) and Forest (speakers' bureau), none of which relate to the use of penicillin or penicillin skin tests. None of the authors have received any source(s) of funding for this article. The corresponding author, Ramzy Rimawi, MD, had full access to all of the data in the study and had final responsibility for the decision to submit for publication. The manuscript is not under review by any other publication.
- , . Accuracy of drug allergy documentation. Am J Health Syst Pharm. 1997;54(14):1627–1629.
- , , , et al. Program to remove incorrect allergy documentation in pediatrics medical records. Am J Health Syst Pharm. 2001;58(18):1722–1727.
- , , . Electronic medication ordering with integrated drug database and clinical decision support system. Stud Health Technol Inform. 2012;180:693–697.
- , , , . Pharmacy‐controlled documentation of drug allergies. Am J Health‐Syst Pharm. 1991;48:260–264.
- , , , et al. Systems analysis of adverse drug events. JAMA. 1995;274:35–43.
- , , , et al. The impact of penicillin skin testing on clinical practice and antimicrobial stewardship. J Hosp Med. 2013;8(6):341–345.
- , , , et al.Joint Task Force on Practice Parameters; American College of Allergy, Asthma and Immunology;Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105:259–273.
- , , . Prescription quality in an acute medical ward. Pharmacoepidemiol Drug Saf. 2009;18(12):1158–1165.
- . Make no mistake! Medical errors can be deadly serious. FDA Consum. 2000;34(5):13–18.
- . Medication errors. J R Coll Physicians Edinb. 2007;37:343–346.
- , , , et al. A pharmacist‐led information technology intervention for medication errors (PINCER): a multicenter, cluster randomized, controlled trial and cost‐effectiveness analysis. Lancet. 2012;379(9823):1301–1309.
- , , , . Getting the data right: information accuracy in pediatric emergency medicine. Qual Saf Health Care. 2006;15(4):296–301.
- , . Antibiotic allergy: inaccurate history taking in a teaching hospital. South Med J. 1994;87(8):805–807.
- , , . Drug allergy documentation—time for a change? Int J Clin Pharm. 2011;33(4):610–613.
- , . Accuracy of drug allergy documentation. Am J Health Syst Pharm. 1997;54(14):1627–1629.
- , , , et al. Program to remove incorrect allergy documentation in pediatrics medical records. Am J Health Syst Pharm. 2001;58(18):1722–1727.
- , , . Electronic medication ordering with integrated drug database and clinical decision support system. Stud Health Technol Inform. 2012;180:693–697.
- , , , . Pharmacy‐controlled documentation of drug allergies. Am J Health‐Syst Pharm. 1991;48:260–264.
- , , , et al. Systems analysis of adverse drug events. JAMA. 1995;274:35–43.
- , , , et al. The impact of penicillin skin testing on clinical practice and antimicrobial stewardship. J Hosp Med. 2013;8(6):341–345.
- , , , et al.Joint Task Force on Practice Parameters; American College of Allergy, Asthma and Immunology;Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105:259–273.
- , , . Prescription quality in an acute medical ward. Pharmacoepidemiol Drug Saf. 2009;18(12):1158–1165.
- . Make no mistake! Medical errors can be deadly serious. FDA Consum. 2000;34(5):13–18.
- . Medication errors. J R Coll Physicians Edinb. 2007;37:343–346.
- , , , et al. A pharmacist‐led information technology intervention for medication errors (PINCER): a multicenter, cluster randomized, controlled trial and cost‐effectiveness analysis. Lancet. 2012;379(9823):1301–1309.
- , , , . Getting the data right: information accuracy in pediatric emergency medicine. Qual Saf Health Care. 2006;15(4):296–301.
- , . Antibiotic allergy: inaccurate history taking in a teaching hospital. South Med J. 1994;87(8):805–807.
- , , . Drug allergy documentation—time for a change? Int J Clin Pharm. 2011;33(4):610–613.
© 2013 Society of Hospital Medicine
Weakness and facial droop: Is it a stroke?
CASE Sudden weakness
Ms. G, age 59, presents to a local critical access (rural) hospital after an episode of sudden-onset left-sided weakness followed by unconsciousness. She regained consciousness quickly and is awake when she arrives at the hospital. This event was not witnessed, although family members were nearby to call emergency personnel.
a) CT scan
b) MRI
c) EEG
d) head and neck magnetic resonance angiogram (MRA)
EXAMINATION Unremarkable
In the emergency department, Ms. G demonstrates left facial droop, left-sided weakness of her arm and leg, and aphasia. She says she has a severe headache that began after she regained consciousness. She is unable to see out of her left eye.
Ms. G’s NIH Stroke Scale score is 13, indicating a moderate stroke; an emergent head CT does not demonstrate any acute hemorrhagic process. Tissue plasminogen activator (tPA) is administered for a suspected stroke approximately 2 hours after her symptoms began. She is transferred to a larger, tertiary care hospital for further workup and observation.
Upon admission to the ICU, Ms. G’s laboratory values are: sodium, 137 mEq/L; potassium, 5.1 mEq/L; creatinine, 1.26 mg/dL; lipase, 126 U/L; and lactic acid, 9 mg/dL. The glucose level is within normal limits and her urinalysis is unremarkable.
Vital signs are stable and Ms. G is not in acute distress. A physical exam demonstrates 4/5 strength in the left-upper and -lower extremities. Additionally, there are 2+ deep tendon reflexes bilaterally in the biceps, triceps, and brachioradialis. She has left-sided facial droop while in the ICU, and continues to demonstrate some aphasia—although she is alert and oriented to person, time, and place.
The medical history is significant for depression, restless leg syndrome, tonic-clonic seizures, and previous stroke-like events. Medications include amitriptyline, 25 mg/d; citalopram, 20 mg/d; valproate, 1,200 mg/d; and ropinirole, 0.5 mg/d. Her mother has a history of stroke-like events, but her family history and social history are otherwise unremarkable.
The authors' observations
Conversion disorder requires the exclusion of medical causes that could explain the patient’s neurologic symptoms. It is prudent to rule out the most serious of the potential contributors to Ms. G’s condition—namely, an acute cerebrovascular accident. A CT scan did not find any significant pathology, however. In the ICU, an MRI showed no evidence of acute infarction based on diffusion-weighted imaging. A head and neck MRA demonstrated no hemodynamically significant stenosis of the internal carotid arteries. An EEG revealed generalized, polymorphic slow activity without evidence of seizures or epilepsy. An electrocardiogram showed normal ventricular size with an appropriate ejection fraction.
The ICU staff consulted psychiatry to evaluate a psychiatric cause of Ms. G’s symptoms.
An exhaustive and comprehensive workup was performed; there were no significant findings. Although laboratory tests were performed, it was the physical exam that suggested the diagnosis of conversion disorder. In that sense, the diagnostic tests were more of a supportive adjunct to the findings of the physical examination, which consistently failed to indicate a neurologic insult.
Hoover’s sign is a well-established test of functional weakness, in which the patient extends his (her) hip when the contralateral hip is flexed. However, there are other tests of functional weakness that can be useful when considering a conversion disorder diagnosis, including co-contraction, the so-called arm-drop sign, and the sternocleidomastoid test. Diukova and colleagues reported that 80% of patients with functional weakness demonstrated ipsilateral sternocleidomastoid weakness, compared with 11% with vascular hemiparesis.1
a) stroke
b) transient ischemic attack
c) conversion disorder
d) seizure disorder
Ms. G appeared to have suffered an acute ischemic event that caused her neurologic symptoms; her rather extensive psychiatric history was overlooked before the psychiatric service was consulted. When Ms. G was admitted to the ICU, the working differential was postictal seizure state rather than cerebrovascular accident. Ms. G had a poorly defined seizure history, and her history of stroke-like events was murky, at best. She had not been treated previously with tPA, and in all past instances her symptoms resolved spontaneously.
Ms. G’s case illustrates why conversion disorder is difficult to diagnose and why, perhaps, it is even a dangerous diagnostic consideration. Booij and colleagues described two patients with neurologic sequelae thought to be the result of conversion disorder; subsequent imaging demonstrated a posterior stroke.2 Over a 6-year period in an emergency department, Glick and coworkers identified six patients with neurologic pathology who were misdiagnosed with conversion disorder.3 In a study of 4,220 patients presenting to a psychiatric emergency service, three patients complained of extremity paralysis or pain, which was attributed to conversion disorder but later attributed to an organic disease.4
These studies emphasize the precarious nature of diagnosing conversion disorder. For that reason, an extensive medical workup is necessary prior to considering a diagnosis of conversion disorder. In Ms. G’s case, a reasonably thorough workup failed to reveal any obvious pathology. Only then was conversion disorder included as a diagnostic possibility.
EVALUATION Childhood abuse
When performing a mental status exam, Ms. G has poor eye contact, but is cooperative with our interview. She is disheveled and overweight, and denies suicidal or homicidal ideation. She displays constricted affect.
During the interview, we note a left facial droop, although Ms. G is able to smile fully. As the interview progresses, her facial droop seems to become more apparent as we discuss her past, including a history of childhood physical and sexual abuse. She has a history of depression and has been seeing an outpatient psychiatrist for the past year. Ms. G describes being hospitalized in a psychiatric unit, but she is unable to provide any details about when and where this occurred.
Ms. G admits to occasional auditory and visual hallucinations, mostly relating to the abuse she experienced as a child by her parents. She exhibits no other signs or symptoms of psychosis; the hallucinations she describes are consistent with flashbacks and vivid memories relating to the abuse. Ms. G also recently lost her job and is experiencing numerous financial stressors.
The authors' observations
There are many examples in the literature of patients with conversion disorder (Table 1),4 ranging from pseudoseizures, which are relatively common, to intriguing cases, such as cochlear implant failure.5
Some studies estimate that the prevalence of conversion disorder symptoms ranges from 16.1% to 21.9% in the general population.6 Somatoform disorders, including conversion disorder, often are comorbid with anxiety and depression. In one study, 26% of somatoform disorder patients also had depression or anxiety, or both.7 Patients with conversion disorder often report a history of childhood physical or sexual abuse.6 In many patients with conversion disorder, there also appears to be a significant association between the disorder and a recent and distant history of psychosocial stressors.8
Ms. G had an extensive history of abuse by her parents. Conversion disorder presenting as a stroke with realistic and convincing physical manifestations is an unusual presentation. There are case reports that detail this presentation, particularly in the emergency department setting.6
Clinical considerations
The relative uncertainty that accompanies a diagnosis of conversion disorder can be discomforting for clinicians. As demonstrated by Ms. G, as well as other case reports of conversion disorder, it takes time for the patient to find a clinician who will consider a diagnosis of conversion disorder.9 Largely, this is because DSM-5 requires that other medical causes be ruled out (Table 2).10 This often proves to be problematic because feigning, or the lack thereof, is difficult to prove.9
Further complicating the diagnosis is the lack of a diagnostic test. Neurologists can use video EEG or physical exam maneuvers such as the Hoover’s sign to help make a diagnosis of conversion disorder.11 In this sense, the physical exam maneuvers form the basis of making a diagnosis, while imaging and lab work support the diagnosis. Hoover’s sign, for example, has not been well studied in a controlled manner, but is recognized as a test that may aid a conversion disorder diagnosis. Clinicians should not solely rely upon these physical exam maneuvers; interpreting them in the context of the patient’s overall presentation is critical. This demonstrates the importance of using the physical exam as a way to guide the diagnosis in association with other tests.12
Despite the lack of pathology, studies demonstrate that patients with conversion disorder may have abnormal brain activity that causes them to perceive motor symptoms as involuntary.11 Therefore, there is a clear need for an increased understanding of psychiatric and neurologic components of diagnosing conversion disorder.8
With Ms. G, it was prudent to make a conversion disorder diagnosis to prevent harm to the patient should future stroke-like events occur. Without considering a conversion disorder diagnosis, a patient may continue to receive unnecessary interventions. Basic physical exam maneuvers, such as Hoover’s sign, can be performed quickly in the ED setting before proceeding with other potentially harmful interventions, such as administering tPA.
Treatment. There are few therapies for conversion disorder. This is, in part, because of lack of understanding about the disorder’s neurologic and biologic etiologies. Although there are some studies that support the use of cognitive-behavioral therapy (CBT), there is little evidence advocating the use of a single mechanism to treat conversion disorder.13 There is evidence that CBT is an effective treatment for several somatoform disorders, including conversion disorder. Research suggests that patients with somatoform disorder have better outcomes when CBT is added to a traditional follow-up.14,15
In Ms. G’s case, we provided information about the diagnosis and scheduled visits to continue her outpatient therapy.
Bottom Line
Conversion disorder is difficult to diagnose, and can mimic potentially life- threatening medical conditions. Conduct a thorough medical workup of these patients, even when it is tempting to jump to a diagnosis of conversion disorder. The use of physical exam maneuvers such as Hoover’s sign may help guide the diagnosis when used in conjunction with other testing.
Related Resources
- Conversion disorder. www.nlm.nih.gov/medlineplus/ency/ article/000954.htm.
- Couprie W, Wijdicks EF, Rooijmans HG, et al. Outcome in conver- sion disorder: a follow up study. J Neurol Neurosurg Psychiatry. 1995;58(6):750-752.
Drug Brand Names
Amitriptyline • Elavil Citalopram • Celexa
Ropinirole • Requip Valproate • Depakote
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diukova GM, Stolajrova AV, Vein AM. Sternocleidomastoid (SCM) muscle test in patients with hysterical and organic paresis. J Neurol Sci. 2001;187(suppl 1):S108.
2. Booij HA, Hamburger HL, Jöbsis GJ, et al. Stroke mimicking conversion disorder: two young women who put our feet back on the ground. Pract Neurol. 2012;12(3):179-181.
3. Glick TH, Workman TP, Gaufberg SV. Suspected conversion disorder: foreseeable risks and avoidable errors. Acad Emerg Med. 2000;7(11):1272-1277.
4. Fishbain DA, Goldberg M. The misdiagnosis of conversion disorder in a psychiatric emergency service. Gen Hosp Psychiatry. 1991;13(3):177-181.
5. Carlson ML, Archibald DJ, Gifford RH, et al. Conversion disorder: a missed diagnosis leading to cochlear reimplantation. Otol Neurotol. 2011;32(1):36-38.
6. Sar V, Akyüz G, Kundakçi T, et al. Childhood trauma, dissociation, and psychiatric comorbidity in patients with conversion disorder. Am J Psychiatry. 2004;161(12):2271-2276.
7. de Waal MW, Arnold IA, Eekhof JA, et al. Somatoform disorders in general practice: prevalence, functional impairment and comorbidity with anxiety and depressive disorders. Br J Psychiatry. 2004;184:470-476.
8. Nicholson TR, Stone J, Kanaan RA. Conversion disorder: a problematic diagnosis. J Neurol Neurosurg Psychiatry. 2011;82(11):1267-1273.
9. Stone J, LaFrance WC, Jr, Levenson JL, et al. Issues for
DSM-5: conversion disorder. Am J Psychiatry. 2010;167(6): 626-627.
10. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
11. Voon V, Gallea C, Hattori N, et al. The involuntary nature of conversion disorder. Neurology. 2010;74(3):223-228.
12. Stone J, Zeman A, Sharpe M. Functional weakness and sensory disturbance. J Neurol Neurosurg Psychiatry. 2002; 73:241-245.
13. Aybek S, Kanaan RA, David AS. The neuropsychiatry of conversion disorder. Curr Opin Psychiatry. 2008;21(3):275-280.
14. Kroenke K. Efficacy of treatment for somatoform disorders: a review of randomized controlled trials. Psychosom Med. 2007;69(9):881-888.
15. Sharpe M, Walker J, Williams C, et al. Guided self-help for functional (psychogenic) symptoms: a randomized controlled efficacy trial. Neurology. 2011;77(6):564-572.
CASE Sudden weakness
Ms. G, age 59, presents to a local critical access (rural) hospital after an episode of sudden-onset left-sided weakness followed by unconsciousness. She regained consciousness quickly and is awake when she arrives at the hospital. This event was not witnessed, although family members were nearby to call emergency personnel.
a) CT scan
b) MRI
c) EEG
d) head and neck magnetic resonance angiogram (MRA)
EXAMINATION Unremarkable
In the emergency department, Ms. G demonstrates left facial droop, left-sided weakness of her arm and leg, and aphasia. She says she has a severe headache that began after she regained consciousness. She is unable to see out of her left eye.
Ms. G’s NIH Stroke Scale score is 13, indicating a moderate stroke; an emergent head CT does not demonstrate any acute hemorrhagic process. Tissue plasminogen activator (tPA) is administered for a suspected stroke approximately 2 hours after her symptoms began. She is transferred to a larger, tertiary care hospital for further workup and observation.
Upon admission to the ICU, Ms. G’s laboratory values are: sodium, 137 mEq/L; potassium, 5.1 mEq/L; creatinine, 1.26 mg/dL; lipase, 126 U/L; and lactic acid, 9 mg/dL. The glucose level is within normal limits and her urinalysis is unremarkable.
Vital signs are stable and Ms. G is not in acute distress. A physical exam demonstrates 4/5 strength in the left-upper and -lower extremities. Additionally, there are 2+ deep tendon reflexes bilaterally in the biceps, triceps, and brachioradialis. She has left-sided facial droop while in the ICU, and continues to demonstrate some aphasia—although she is alert and oriented to person, time, and place.
The medical history is significant for depression, restless leg syndrome, tonic-clonic seizures, and previous stroke-like events. Medications include amitriptyline, 25 mg/d; citalopram, 20 mg/d; valproate, 1,200 mg/d; and ropinirole, 0.5 mg/d. Her mother has a history of stroke-like events, but her family history and social history are otherwise unremarkable.
The authors' observations
Conversion disorder requires the exclusion of medical causes that could explain the patient’s neurologic symptoms. It is prudent to rule out the most serious of the potential contributors to Ms. G’s condition—namely, an acute cerebrovascular accident. A CT scan did not find any significant pathology, however. In the ICU, an MRI showed no evidence of acute infarction based on diffusion-weighted imaging. A head and neck MRA demonstrated no hemodynamically significant stenosis of the internal carotid arteries. An EEG revealed generalized, polymorphic slow activity without evidence of seizures or epilepsy. An electrocardiogram showed normal ventricular size with an appropriate ejection fraction.
The ICU staff consulted psychiatry to evaluate a psychiatric cause of Ms. G’s symptoms.
An exhaustive and comprehensive workup was performed; there were no significant findings. Although laboratory tests were performed, it was the physical exam that suggested the diagnosis of conversion disorder. In that sense, the diagnostic tests were more of a supportive adjunct to the findings of the physical examination, which consistently failed to indicate a neurologic insult.
Hoover’s sign is a well-established test of functional weakness, in which the patient extends his (her) hip when the contralateral hip is flexed. However, there are other tests of functional weakness that can be useful when considering a conversion disorder diagnosis, including co-contraction, the so-called arm-drop sign, and the sternocleidomastoid test. Diukova and colleagues reported that 80% of patients with functional weakness demonstrated ipsilateral sternocleidomastoid weakness, compared with 11% with vascular hemiparesis.1
a) stroke
b) transient ischemic attack
c) conversion disorder
d) seizure disorder
Ms. G appeared to have suffered an acute ischemic event that caused her neurologic symptoms; her rather extensive psychiatric history was overlooked before the psychiatric service was consulted. When Ms. G was admitted to the ICU, the working differential was postictal seizure state rather than cerebrovascular accident. Ms. G had a poorly defined seizure history, and her history of stroke-like events was murky, at best. She had not been treated previously with tPA, and in all past instances her symptoms resolved spontaneously.
Ms. G’s case illustrates why conversion disorder is difficult to diagnose and why, perhaps, it is even a dangerous diagnostic consideration. Booij and colleagues described two patients with neurologic sequelae thought to be the result of conversion disorder; subsequent imaging demonstrated a posterior stroke.2 Over a 6-year period in an emergency department, Glick and coworkers identified six patients with neurologic pathology who were misdiagnosed with conversion disorder.3 In a study of 4,220 patients presenting to a psychiatric emergency service, three patients complained of extremity paralysis or pain, which was attributed to conversion disorder but later attributed to an organic disease.4
These studies emphasize the precarious nature of diagnosing conversion disorder. For that reason, an extensive medical workup is necessary prior to considering a diagnosis of conversion disorder. In Ms. G’s case, a reasonably thorough workup failed to reveal any obvious pathology. Only then was conversion disorder included as a diagnostic possibility.
EVALUATION Childhood abuse
When performing a mental status exam, Ms. G has poor eye contact, but is cooperative with our interview. She is disheveled and overweight, and denies suicidal or homicidal ideation. She displays constricted affect.
During the interview, we note a left facial droop, although Ms. G is able to smile fully. As the interview progresses, her facial droop seems to become more apparent as we discuss her past, including a history of childhood physical and sexual abuse. She has a history of depression and has been seeing an outpatient psychiatrist for the past year. Ms. G describes being hospitalized in a psychiatric unit, but she is unable to provide any details about when and where this occurred.
Ms. G admits to occasional auditory and visual hallucinations, mostly relating to the abuse she experienced as a child by her parents. She exhibits no other signs or symptoms of psychosis; the hallucinations she describes are consistent with flashbacks and vivid memories relating to the abuse. Ms. G also recently lost her job and is experiencing numerous financial stressors.
The authors' observations
There are many examples in the literature of patients with conversion disorder (Table 1),4 ranging from pseudoseizures, which are relatively common, to intriguing cases, such as cochlear implant failure.5
Some studies estimate that the prevalence of conversion disorder symptoms ranges from 16.1% to 21.9% in the general population.6 Somatoform disorders, including conversion disorder, often are comorbid with anxiety and depression. In one study, 26% of somatoform disorder patients also had depression or anxiety, or both.7 Patients with conversion disorder often report a history of childhood physical or sexual abuse.6 In many patients with conversion disorder, there also appears to be a significant association between the disorder and a recent and distant history of psychosocial stressors.8
Ms. G had an extensive history of abuse by her parents. Conversion disorder presenting as a stroke with realistic and convincing physical manifestations is an unusual presentation. There are case reports that detail this presentation, particularly in the emergency department setting.6
Clinical considerations
The relative uncertainty that accompanies a diagnosis of conversion disorder can be discomforting for clinicians. As demonstrated by Ms. G, as well as other case reports of conversion disorder, it takes time for the patient to find a clinician who will consider a diagnosis of conversion disorder.9 Largely, this is because DSM-5 requires that other medical causes be ruled out (Table 2).10 This often proves to be problematic because feigning, or the lack thereof, is difficult to prove.9
Further complicating the diagnosis is the lack of a diagnostic test. Neurologists can use video EEG or physical exam maneuvers such as the Hoover’s sign to help make a diagnosis of conversion disorder.11 In this sense, the physical exam maneuvers form the basis of making a diagnosis, while imaging and lab work support the diagnosis. Hoover’s sign, for example, has not been well studied in a controlled manner, but is recognized as a test that may aid a conversion disorder diagnosis. Clinicians should not solely rely upon these physical exam maneuvers; interpreting them in the context of the patient’s overall presentation is critical. This demonstrates the importance of using the physical exam as a way to guide the diagnosis in association with other tests.12
Despite the lack of pathology, studies demonstrate that patients with conversion disorder may have abnormal brain activity that causes them to perceive motor symptoms as involuntary.11 Therefore, there is a clear need for an increased understanding of psychiatric and neurologic components of diagnosing conversion disorder.8
With Ms. G, it was prudent to make a conversion disorder diagnosis to prevent harm to the patient should future stroke-like events occur. Without considering a conversion disorder diagnosis, a patient may continue to receive unnecessary interventions. Basic physical exam maneuvers, such as Hoover’s sign, can be performed quickly in the ED setting before proceeding with other potentially harmful interventions, such as administering tPA.
Treatment. There are few therapies for conversion disorder. This is, in part, because of lack of understanding about the disorder’s neurologic and biologic etiologies. Although there are some studies that support the use of cognitive-behavioral therapy (CBT), there is little evidence advocating the use of a single mechanism to treat conversion disorder.13 There is evidence that CBT is an effective treatment for several somatoform disorders, including conversion disorder. Research suggests that patients with somatoform disorder have better outcomes when CBT is added to a traditional follow-up.14,15
In Ms. G’s case, we provided information about the diagnosis and scheduled visits to continue her outpatient therapy.
Bottom Line
Conversion disorder is difficult to diagnose, and can mimic potentially life- threatening medical conditions. Conduct a thorough medical workup of these patients, even when it is tempting to jump to a diagnosis of conversion disorder. The use of physical exam maneuvers such as Hoover’s sign may help guide the diagnosis when used in conjunction with other testing.
Related Resources
- Conversion disorder. www.nlm.nih.gov/medlineplus/ency/ article/000954.htm.
- Couprie W, Wijdicks EF, Rooijmans HG, et al. Outcome in conver- sion disorder: a follow up study. J Neurol Neurosurg Psychiatry. 1995;58(6):750-752.
Drug Brand Names
Amitriptyline • Elavil Citalopram • Celexa
Ropinirole • Requip Valproate • Depakote
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Sudden weakness
Ms. G, age 59, presents to a local critical access (rural) hospital after an episode of sudden-onset left-sided weakness followed by unconsciousness. She regained consciousness quickly and is awake when she arrives at the hospital. This event was not witnessed, although family members were nearby to call emergency personnel.
a) CT scan
b) MRI
c) EEG
d) head and neck magnetic resonance angiogram (MRA)
EXAMINATION Unremarkable
In the emergency department, Ms. G demonstrates left facial droop, left-sided weakness of her arm and leg, and aphasia. She says she has a severe headache that began after she regained consciousness. She is unable to see out of her left eye.
Ms. G’s NIH Stroke Scale score is 13, indicating a moderate stroke; an emergent head CT does not demonstrate any acute hemorrhagic process. Tissue plasminogen activator (tPA) is administered for a suspected stroke approximately 2 hours after her symptoms began. She is transferred to a larger, tertiary care hospital for further workup and observation.
Upon admission to the ICU, Ms. G’s laboratory values are: sodium, 137 mEq/L; potassium, 5.1 mEq/L; creatinine, 1.26 mg/dL; lipase, 126 U/L; and lactic acid, 9 mg/dL. The glucose level is within normal limits and her urinalysis is unremarkable.
Vital signs are stable and Ms. G is not in acute distress. A physical exam demonstrates 4/5 strength in the left-upper and -lower extremities. Additionally, there are 2+ deep tendon reflexes bilaterally in the biceps, triceps, and brachioradialis. She has left-sided facial droop while in the ICU, and continues to demonstrate some aphasia—although she is alert and oriented to person, time, and place.
The medical history is significant for depression, restless leg syndrome, tonic-clonic seizures, and previous stroke-like events. Medications include amitriptyline, 25 mg/d; citalopram, 20 mg/d; valproate, 1,200 mg/d; and ropinirole, 0.5 mg/d. Her mother has a history of stroke-like events, but her family history and social history are otherwise unremarkable.
The authors' observations
Conversion disorder requires the exclusion of medical causes that could explain the patient’s neurologic symptoms. It is prudent to rule out the most serious of the potential contributors to Ms. G’s condition—namely, an acute cerebrovascular accident. A CT scan did not find any significant pathology, however. In the ICU, an MRI showed no evidence of acute infarction based on diffusion-weighted imaging. A head and neck MRA demonstrated no hemodynamically significant stenosis of the internal carotid arteries. An EEG revealed generalized, polymorphic slow activity without evidence of seizures or epilepsy. An electrocardiogram showed normal ventricular size with an appropriate ejection fraction.
The ICU staff consulted psychiatry to evaluate a psychiatric cause of Ms. G’s symptoms.
An exhaustive and comprehensive workup was performed; there were no significant findings. Although laboratory tests were performed, it was the physical exam that suggested the diagnosis of conversion disorder. In that sense, the diagnostic tests were more of a supportive adjunct to the findings of the physical examination, which consistently failed to indicate a neurologic insult.
Hoover’s sign is a well-established test of functional weakness, in which the patient extends his (her) hip when the contralateral hip is flexed. However, there are other tests of functional weakness that can be useful when considering a conversion disorder diagnosis, including co-contraction, the so-called arm-drop sign, and the sternocleidomastoid test. Diukova and colleagues reported that 80% of patients with functional weakness demonstrated ipsilateral sternocleidomastoid weakness, compared with 11% with vascular hemiparesis.1
a) stroke
b) transient ischemic attack
c) conversion disorder
d) seizure disorder
Ms. G appeared to have suffered an acute ischemic event that caused her neurologic symptoms; her rather extensive psychiatric history was overlooked before the psychiatric service was consulted. When Ms. G was admitted to the ICU, the working differential was postictal seizure state rather than cerebrovascular accident. Ms. G had a poorly defined seizure history, and her history of stroke-like events was murky, at best. She had not been treated previously with tPA, and in all past instances her symptoms resolved spontaneously.
Ms. G’s case illustrates why conversion disorder is difficult to diagnose and why, perhaps, it is even a dangerous diagnostic consideration. Booij and colleagues described two patients with neurologic sequelae thought to be the result of conversion disorder; subsequent imaging demonstrated a posterior stroke.2 Over a 6-year period in an emergency department, Glick and coworkers identified six patients with neurologic pathology who were misdiagnosed with conversion disorder.3 In a study of 4,220 patients presenting to a psychiatric emergency service, three patients complained of extremity paralysis or pain, which was attributed to conversion disorder but later attributed to an organic disease.4
These studies emphasize the precarious nature of diagnosing conversion disorder. For that reason, an extensive medical workup is necessary prior to considering a diagnosis of conversion disorder. In Ms. G’s case, a reasonably thorough workup failed to reveal any obvious pathology. Only then was conversion disorder included as a diagnostic possibility.
EVALUATION Childhood abuse
When performing a mental status exam, Ms. G has poor eye contact, but is cooperative with our interview. She is disheveled and overweight, and denies suicidal or homicidal ideation. She displays constricted affect.
During the interview, we note a left facial droop, although Ms. G is able to smile fully. As the interview progresses, her facial droop seems to become more apparent as we discuss her past, including a history of childhood physical and sexual abuse. She has a history of depression and has been seeing an outpatient psychiatrist for the past year. Ms. G describes being hospitalized in a psychiatric unit, but she is unable to provide any details about when and where this occurred.
Ms. G admits to occasional auditory and visual hallucinations, mostly relating to the abuse she experienced as a child by her parents. She exhibits no other signs or symptoms of psychosis; the hallucinations she describes are consistent with flashbacks and vivid memories relating to the abuse. Ms. G also recently lost her job and is experiencing numerous financial stressors.
The authors' observations
There are many examples in the literature of patients with conversion disorder (Table 1),4 ranging from pseudoseizures, which are relatively common, to intriguing cases, such as cochlear implant failure.5
Some studies estimate that the prevalence of conversion disorder symptoms ranges from 16.1% to 21.9% in the general population.6 Somatoform disorders, including conversion disorder, often are comorbid with anxiety and depression. In one study, 26% of somatoform disorder patients also had depression or anxiety, or both.7 Patients with conversion disorder often report a history of childhood physical or sexual abuse.6 In many patients with conversion disorder, there also appears to be a significant association between the disorder and a recent and distant history of psychosocial stressors.8
Ms. G had an extensive history of abuse by her parents. Conversion disorder presenting as a stroke with realistic and convincing physical manifestations is an unusual presentation. There are case reports that detail this presentation, particularly in the emergency department setting.6
Clinical considerations
The relative uncertainty that accompanies a diagnosis of conversion disorder can be discomforting for clinicians. As demonstrated by Ms. G, as well as other case reports of conversion disorder, it takes time for the patient to find a clinician who will consider a diagnosis of conversion disorder.9 Largely, this is because DSM-5 requires that other medical causes be ruled out (Table 2).10 This often proves to be problematic because feigning, or the lack thereof, is difficult to prove.9
Further complicating the diagnosis is the lack of a diagnostic test. Neurologists can use video EEG or physical exam maneuvers such as the Hoover’s sign to help make a diagnosis of conversion disorder.11 In this sense, the physical exam maneuvers form the basis of making a diagnosis, while imaging and lab work support the diagnosis. Hoover’s sign, for example, has not been well studied in a controlled manner, but is recognized as a test that may aid a conversion disorder diagnosis. Clinicians should not solely rely upon these physical exam maneuvers; interpreting them in the context of the patient’s overall presentation is critical. This demonstrates the importance of using the physical exam as a way to guide the diagnosis in association with other tests.12
Despite the lack of pathology, studies demonstrate that patients with conversion disorder may have abnormal brain activity that causes them to perceive motor symptoms as involuntary.11 Therefore, there is a clear need for an increased understanding of psychiatric and neurologic components of diagnosing conversion disorder.8
With Ms. G, it was prudent to make a conversion disorder diagnosis to prevent harm to the patient should future stroke-like events occur. Without considering a conversion disorder diagnosis, a patient may continue to receive unnecessary interventions. Basic physical exam maneuvers, such as Hoover’s sign, can be performed quickly in the ED setting before proceeding with other potentially harmful interventions, such as administering tPA.
Treatment. There are few therapies for conversion disorder. This is, in part, because of lack of understanding about the disorder’s neurologic and biologic etiologies. Although there are some studies that support the use of cognitive-behavioral therapy (CBT), there is little evidence advocating the use of a single mechanism to treat conversion disorder.13 There is evidence that CBT is an effective treatment for several somatoform disorders, including conversion disorder. Research suggests that patients with somatoform disorder have better outcomes when CBT is added to a traditional follow-up.14,15
In Ms. G’s case, we provided information about the diagnosis and scheduled visits to continue her outpatient therapy.
Bottom Line
Conversion disorder is difficult to diagnose, and can mimic potentially life- threatening medical conditions. Conduct a thorough medical workup of these patients, even when it is tempting to jump to a diagnosis of conversion disorder. The use of physical exam maneuvers such as Hoover’s sign may help guide the diagnosis when used in conjunction with other testing.
Related Resources
- Conversion disorder. www.nlm.nih.gov/medlineplus/ency/ article/000954.htm.
- Couprie W, Wijdicks EF, Rooijmans HG, et al. Outcome in conver- sion disorder: a follow up study. J Neurol Neurosurg Psychiatry. 1995;58(6):750-752.
Drug Brand Names
Amitriptyline • Elavil Citalopram • Celexa
Ropinirole • Requip Valproate • Depakote
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diukova GM, Stolajrova AV, Vein AM. Sternocleidomastoid (SCM) muscle test in patients with hysterical and organic paresis. J Neurol Sci. 2001;187(suppl 1):S108.
2. Booij HA, Hamburger HL, Jöbsis GJ, et al. Stroke mimicking conversion disorder: two young women who put our feet back on the ground. Pract Neurol. 2012;12(3):179-181.
3. Glick TH, Workman TP, Gaufberg SV. Suspected conversion disorder: foreseeable risks and avoidable errors. Acad Emerg Med. 2000;7(11):1272-1277.
4. Fishbain DA, Goldberg M. The misdiagnosis of conversion disorder in a psychiatric emergency service. Gen Hosp Psychiatry. 1991;13(3):177-181.
5. Carlson ML, Archibald DJ, Gifford RH, et al. Conversion disorder: a missed diagnosis leading to cochlear reimplantation. Otol Neurotol. 2011;32(1):36-38.
6. Sar V, Akyüz G, Kundakçi T, et al. Childhood trauma, dissociation, and psychiatric comorbidity in patients with conversion disorder. Am J Psychiatry. 2004;161(12):2271-2276.
7. de Waal MW, Arnold IA, Eekhof JA, et al. Somatoform disorders in general practice: prevalence, functional impairment and comorbidity with anxiety and depressive disorders. Br J Psychiatry. 2004;184:470-476.
8. Nicholson TR, Stone J, Kanaan RA. Conversion disorder: a problematic diagnosis. J Neurol Neurosurg Psychiatry. 2011;82(11):1267-1273.
9. Stone J, LaFrance WC, Jr, Levenson JL, et al. Issues for
DSM-5: conversion disorder. Am J Psychiatry. 2010;167(6): 626-627.
10. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
11. Voon V, Gallea C, Hattori N, et al. The involuntary nature of conversion disorder. Neurology. 2010;74(3):223-228.
12. Stone J, Zeman A, Sharpe M. Functional weakness and sensory disturbance. J Neurol Neurosurg Psychiatry. 2002; 73:241-245.
13. Aybek S, Kanaan RA, David AS. The neuropsychiatry of conversion disorder. Curr Opin Psychiatry. 2008;21(3):275-280.
14. Kroenke K. Efficacy of treatment for somatoform disorders: a review of randomized controlled trials. Psychosom Med. 2007;69(9):881-888.
15. Sharpe M, Walker J, Williams C, et al. Guided self-help for functional (psychogenic) symptoms: a randomized controlled efficacy trial. Neurology. 2011;77(6):564-572.
1. Diukova GM, Stolajrova AV, Vein AM. Sternocleidomastoid (SCM) muscle test in patients with hysterical and organic paresis. J Neurol Sci. 2001;187(suppl 1):S108.
2. Booij HA, Hamburger HL, Jöbsis GJ, et al. Stroke mimicking conversion disorder: two young women who put our feet back on the ground. Pract Neurol. 2012;12(3):179-181.
3. Glick TH, Workman TP, Gaufberg SV. Suspected conversion disorder: foreseeable risks and avoidable errors. Acad Emerg Med. 2000;7(11):1272-1277.
4. Fishbain DA, Goldberg M. The misdiagnosis of conversion disorder in a psychiatric emergency service. Gen Hosp Psychiatry. 1991;13(3):177-181.
5. Carlson ML, Archibald DJ, Gifford RH, et al. Conversion disorder: a missed diagnosis leading to cochlear reimplantation. Otol Neurotol. 2011;32(1):36-38.
6. Sar V, Akyüz G, Kundakçi T, et al. Childhood trauma, dissociation, and psychiatric comorbidity in patients with conversion disorder. Am J Psychiatry. 2004;161(12):2271-2276.
7. de Waal MW, Arnold IA, Eekhof JA, et al. Somatoform disorders in general practice: prevalence, functional impairment and comorbidity with anxiety and depressive disorders. Br J Psychiatry. 2004;184:470-476.
8. Nicholson TR, Stone J, Kanaan RA. Conversion disorder: a problematic diagnosis. J Neurol Neurosurg Psychiatry. 2011;82(11):1267-1273.
9. Stone J, LaFrance WC, Jr, Levenson JL, et al. Issues for
DSM-5: conversion disorder. Am J Psychiatry. 2010;167(6): 626-627.
10. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
11. Voon V, Gallea C, Hattori N, et al. The involuntary nature of conversion disorder. Neurology. 2010;74(3):223-228.
12. Stone J, Zeman A, Sharpe M. Functional weakness and sensory disturbance. J Neurol Neurosurg Psychiatry. 2002; 73:241-245.
13. Aybek S, Kanaan RA, David AS. The neuropsychiatry of conversion disorder. Curr Opin Psychiatry. 2008;21(3):275-280.
14. Kroenke K. Efficacy of treatment for somatoform disorders: a review of randomized controlled trials. Psychosom Med. 2007;69(9):881-888.
15. Sharpe M, Walker J, Williams C, et al. Guided self-help for functional (psychogenic) symptoms: a randomized controlled efficacy trial. Neurology. 2011;77(6):564-572.
Problematic pruritus: Seeking a cure for psychogenic itch
Psychogenic itch—an excessive impulse to scratch, gouge, or pick at skin in the absence of dermatologic cause—is common among psychiatric inpatients, but can be challenging to assess and manage in outpatients. Patients with psychogenic itch predominantly are female, with average age of onset between 30 and 45 years.1 Psychiatric disorders associated with psychogenic itch include depression, obsessive-compulsive disorder, anxiety, somatoform disorders, mania, psychosis, and substance abuse.2 Body dysmorphic disorder, trichotillomania, kleptomania, and borderline personality disorder may be comorbid in patients with psychogenic itch.3
Characteristics of psychogenic itch
Consider psychogenic itch in patients who have recurring physical symptoms and demand examination despite repeated negative results. Other indicators include psychological factors—loss of a loved one, unemployment, relocation, etc.—that may be associated with onset, severity, elicitation, or maintenance of the itching; impairments in the patient’s social or professional life; and marked preoccupation with itching or the state of her (his) skin. Characteristically, itching can be provoked by emotional triggers, most notably during stages of excitement, and also by mechanical or chemical stimuli.
Skin changes associated with psychogenic itch often are found on areas accessible to the patient’s hand: face, arms, legs, abdomen, thighs, upper back, and shoulders. These changes can be seen in varying stages, from discrete superficial excoriations, erosions, and ulcers to thick, darkened nodules and colorless atrophic scars. Patients often complain of burning. In some cases, a patient uses a tool or instrument to autoaggressively manipulate his (her) skin in response to tingling or stabbing sensations. Artificial lesions or eczemas brought on by self-
manipulation can occur. Stress, life changes, or inhibited rage may be evoking the burning sensation and subsequent complaints.
Interventions to consider
After you have ruled out other causes of pruritus and made a diagnosis of psychogenic itch, educate your patient about the multifactorial etiology. Explain possible associations between skin disorders and unconscious reaction patterns, and the role of emotional and cognitive stimuli.
Moisturizing the skin can help the dryness associated with repetitive scratching. Consider prescribing an antihistamine, moisturizer, topical steroid, antibiotic, or
occlusive dressing.
Some pharmacological properties of antidepressants that are not related to their antidepressant activity—eg, the histamine-1 blocking effect of tricyclic antidepressants—are beneficial for treating psychogenic itch.4 Sedating antihistamines (hydroxyzine) and antidepressants (doxepin) may help break cycles of itching and depression or itching and scratching.4 Tricyclic antidepressants also are recommended for treating burning, stabbing, or tingling sensations.
Disclosure
Dr. Jain reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Yosipovitch G, Samuel LS. Neuropathic and psychogenic itch. Dermatol Ther. 2008;21(1):32-41.
2. Krishnan A, Koo J. Psyche, opioids, and itch: therapeutic consequences. Dermatol Ther. 2005;18(4):314-322.
3. Arnold LM, Auchenbach MB, McElroy SL. Psychogenic excoriation. Clinical features, proposed diagnostic criteria, epidemiology and approaches to treatment. CNS Drugs. 2001;15(5):351-359.
4. Gupta MA, Guptat AK. The use of antidepressant drugs in dermatology. J Eur Acad Dermatol Venereol. 2001;15(6):512-518.
Psychogenic itch—an excessive impulse to scratch, gouge, or pick at skin in the absence of dermatologic cause—is common among psychiatric inpatients, but can be challenging to assess and manage in outpatients. Patients with psychogenic itch predominantly are female, with average age of onset between 30 and 45 years.1 Psychiatric disorders associated with psychogenic itch include depression, obsessive-compulsive disorder, anxiety, somatoform disorders, mania, psychosis, and substance abuse.2 Body dysmorphic disorder, trichotillomania, kleptomania, and borderline personality disorder may be comorbid in patients with psychogenic itch.3
Characteristics of psychogenic itch
Consider psychogenic itch in patients who have recurring physical symptoms and demand examination despite repeated negative results. Other indicators include psychological factors—loss of a loved one, unemployment, relocation, etc.—that may be associated with onset, severity, elicitation, or maintenance of the itching; impairments in the patient’s social or professional life; and marked preoccupation with itching or the state of her (his) skin. Characteristically, itching can be provoked by emotional triggers, most notably during stages of excitement, and also by mechanical or chemical stimuli.
Skin changes associated with psychogenic itch often are found on areas accessible to the patient’s hand: face, arms, legs, abdomen, thighs, upper back, and shoulders. These changes can be seen in varying stages, from discrete superficial excoriations, erosions, and ulcers to thick, darkened nodules and colorless atrophic scars. Patients often complain of burning. In some cases, a patient uses a tool or instrument to autoaggressively manipulate his (her) skin in response to tingling or stabbing sensations. Artificial lesions or eczemas brought on by self-
manipulation can occur. Stress, life changes, or inhibited rage may be evoking the burning sensation and subsequent complaints.
Interventions to consider
After you have ruled out other causes of pruritus and made a diagnosis of psychogenic itch, educate your patient about the multifactorial etiology. Explain possible associations between skin disorders and unconscious reaction patterns, and the role of emotional and cognitive stimuli.
Moisturizing the skin can help the dryness associated with repetitive scratching. Consider prescribing an antihistamine, moisturizer, topical steroid, antibiotic, or
occlusive dressing.
Some pharmacological properties of antidepressants that are not related to their antidepressant activity—eg, the histamine-1 blocking effect of tricyclic antidepressants—are beneficial for treating psychogenic itch.4 Sedating antihistamines (hydroxyzine) and antidepressants (doxepin) may help break cycles of itching and depression or itching and scratching.4 Tricyclic antidepressants also are recommended for treating burning, stabbing, or tingling sensations.
Disclosure
Dr. Jain reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Psychogenic itch—an excessive impulse to scratch, gouge, or pick at skin in the absence of dermatologic cause—is common among psychiatric inpatients, but can be challenging to assess and manage in outpatients. Patients with psychogenic itch predominantly are female, with average age of onset between 30 and 45 years.1 Psychiatric disorders associated with psychogenic itch include depression, obsessive-compulsive disorder, anxiety, somatoform disorders, mania, psychosis, and substance abuse.2 Body dysmorphic disorder, trichotillomania, kleptomania, and borderline personality disorder may be comorbid in patients with psychogenic itch.3
Characteristics of psychogenic itch
Consider psychogenic itch in patients who have recurring physical symptoms and demand examination despite repeated negative results. Other indicators include psychological factors—loss of a loved one, unemployment, relocation, etc.—that may be associated with onset, severity, elicitation, or maintenance of the itching; impairments in the patient’s social or professional life; and marked preoccupation with itching or the state of her (his) skin. Characteristically, itching can be provoked by emotional triggers, most notably during stages of excitement, and also by mechanical or chemical stimuli.
Skin changes associated with psychogenic itch often are found on areas accessible to the patient’s hand: face, arms, legs, abdomen, thighs, upper back, and shoulders. These changes can be seen in varying stages, from discrete superficial excoriations, erosions, and ulcers to thick, darkened nodules and colorless atrophic scars. Patients often complain of burning. In some cases, a patient uses a tool or instrument to autoaggressively manipulate his (her) skin in response to tingling or stabbing sensations. Artificial lesions or eczemas brought on by self-
manipulation can occur. Stress, life changes, or inhibited rage may be evoking the burning sensation and subsequent complaints.
Interventions to consider
After you have ruled out other causes of pruritus and made a diagnosis of psychogenic itch, educate your patient about the multifactorial etiology. Explain possible associations between skin disorders and unconscious reaction patterns, and the role of emotional and cognitive stimuli.
Moisturizing the skin can help the dryness associated with repetitive scratching. Consider prescribing an antihistamine, moisturizer, topical steroid, antibiotic, or
occlusive dressing.
Some pharmacological properties of antidepressants that are not related to their antidepressant activity—eg, the histamine-1 blocking effect of tricyclic antidepressants—are beneficial for treating psychogenic itch.4 Sedating antihistamines (hydroxyzine) and antidepressants (doxepin) may help break cycles of itching and depression or itching and scratching.4 Tricyclic antidepressants also are recommended for treating burning, stabbing, or tingling sensations.
Disclosure
Dr. Jain reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Yosipovitch G, Samuel LS. Neuropathic and psychogenic itch. Dermatol Ther. 2008;21(1):32-41.
2. Krishnan A, Koo J. Psyche, opioids, and itch: therapeutic consequences. Dermatol Ther. 2005;18(4):314-322.
3. Arnold LM, Auchenbach MB, McElroy SL. Psychogenic excoriation. Clinical features, proposed diagnostic criteria, epidemiology and approaches to treatment. CNS Drugs. 2001;15(5):351-359.
4. Gupta MA, Guptat AK. The use of antidepressant drugs in dermatology. J Eur Acad Dermatol Venereol. 2001;15(6):512-518.
1. Yosipovitch G, Samuel LS. Neuropathic and psychogenic itch. Dermatol Ther. 2008;21(1):32-41.
2. Krishnan A, Koo J. Psyche, opioids, and itch: therapeutic consequences. Dermatol Ther. 2005;18(4):314-322.
3. Arnold LM, Auchenbach MB, McElroy SL. Psychogenic excoriation. Clinical features, proposed diagnostic criteria, epidemiology and approaches to treatment. CNS Drugs. 2001;15(5):351-359.
4. Gupta MA, Guptat AK. The use of antidepressant drugs in dermatology. J Eur Acad Dermatol Venereol. 2001;15(6):512-518.