User login
Consider this strategy for upper GI bleeds
Do not order transfusions of red blood cells for patients with acute upper gastrointestinal bleeding unless their hemoglobin level <7 g/dL.
Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11-21.1
A: Based on a single randomized controlled trial (RCT) consistent with other RCTs on recommendations for transfusion.
ILLUSTRATED CASE
An 82-year-old patient presents to the emergency department with several episodes of melena over the past week and one episode of hematemesis this morning. He denies any shortness of breath, dizziness, lightheadedness, or fatigue. He is tachycardic but normotensive. Lab results note a hemoglobin level of 8.3 g/dL. Should you order a transfusion of red blood cells?
Acute upper gastrointestinal bleeding (UGIB) commonly requires hospital admission, with approximately 61 cases per 100,000 population in the United States in 2009.2 Gastroduodenal peptic ulcer disease accounts for the majority of these cases.3 Although trends indicate an overall decrease in cases requiring hospitalization, UGIB remains a condition associated with a mortality rate of 2.5% and inpatient costs of $2 billion annually.2,3
Studies have been inconclusive—until now
An RCT published in 1999 showed a restrictive transfusion strategy (hemoglobin threshold of 7 g/dL) to be at least as effective as—and possibly superior to—a liberal strategy (threshold of 10 g/dL) in critically ill patients.4 In 2010, an RCT demonstrated that a liberal transfusion strategy (also defined as a transfusion threshold of 10 g/dL) did not reduce the rates of death or in-hospital morbidity in elderly patients after hip surgery.5 A recent Cochrane review of transfusion strategies for UGIB included only 3 small studies (N=93), so its authors could not draw any firm conclusions.6 The results of a new RCT, detailed below, are more conclusive.
STUDY SUMMARY: Restrictive transfusion policy lowers mortality risk
Villanueva et al conducted a nonblinded RCT comparing outcomes in patients admitted to the hospital with moderate-risk acute UGIB transfused on a liberal vs a restrictive strategy.1 The restrictive group used a transfusion hemoglobin threshold of 7 g/dL and a posttransfusion target of 7 to 9 g/dL; the liberal group used a threshold of 9 g/dL, with a posttransfusion target of 9 to 11 g/dL. Patients received one unit of red blood cells at a time until their hemoglobin was above the predetermined threshold.
Patients were excluded if they declined blood transfusion; had massive exsanguinating bleeding, acute coronary syndrome, symptomatic peripheral vasculopathy, stroke, lower GI bleeding, or a transient ischemic attack; had received a transfusion within the previous 90 days; or had a recent history of surgery or trauma. Patients at low risk of rebleeding (as defined by the Rockall risk scoring system) were also excluded. Randomization was stratified by the presence or absence of cirrhosis of the liver.
Participants (N=921) had confirmed hematemesis and/or melena on admission. All underwent emergency gastroscopy within 6 hours of admission, with subsequent interventions based on endoscopic findings. In addition to established hemoglobin levels, patients received a transfusion anytime they developed signs or symptoms related to anemia, massive bleeding, or the need for surgery. Staff monitored hemoglobin levels every 8 hours during the first 48 hours, then daily thereafter.
Both groups had similar baseline characteristics, including hemoglobin on admission and source of bleeding. The authors used intention-to-treat analysis to identify the primary outcome: death from any cause at 45 days. Secondary outcomes were further bleeding and in-hospital complications.
During hospitalization, 49% of patients in the restrictive group and 86% of those in the liberal group received a blood transfusion (P<.001). Thirty-two patients (17 from the restrictive group and 15 from the liberal group) withdrew from the study, leaving 889 patients for overall analysis.
At 45 days, overall mortality from any cause was 5% in the restrictive group and 9% in the liberal group (P=.02; number needed to treat [NNT]=25). Sub-group analysis revealed a lower risk of death in patients with cirrhosis and Child-Pugh class A or B disease assigned to the restrictive transfusion group vs the liberal group. The results showed a trend toward a lower risk of death in patients with bleeding from varices or peptic ulcers for the restrictive group, as well.
In addition, the restrictive transfusion group had a significantly lower rate of adverse events (40% vs 48% for the liberal transfusion group; P=.02, NNT=13), with a significant reduction in transfusion reactions (3% vs 9%; P=.001, NNT=17) and cardiac complications (11% vs 16%; P=.04, NNT=20). The restrictive group had a lower rate of further bleeding (10% vs 16% for the liberal transfusion group; P=.01, NNT=17), as well.
WHAT'S NEW: Many reasons to limit transfusions for acute upper GI bleed
This RCT provides evidence that patients with acute UGIB have improved survival rates and fewer adverse events when a restrictive transfusion strategy is used. In addition to improving patient outcomes, a restrictive strategy will likely reduce costs and overall use of blood products. Thus, the study, along with other recent evaluations, adds evidence to support more restrictive transfusion thresholds.
The AABB (formerly named the American Association of Blood Banks) recently
released guidelines calling for restrictive transfusion thresholds (7-8 g/dL) in stable hospitalized patients.7 In 2012, the American College of Gastroenterology published a practice guideline with a recommended target hemoglobin level of ≥7 g/dL in the management of patients who have ulcer bleeding but no signs of intravascular depletion or comorbidities such as coronary artery disease.8
CAVEATS: Results might differ when endoscopy is delayed
The patients in the study detailed here underwent emergency gastroscopy within 6 hours of admission, and both groups received the same therapies based on endoscopic findings. It remains unclear whether the benefits of a restrictive transfusion strategy would persist in patients who do not undergo endoscopy within that timeframe. And, because the reported baseline characteristics of the patients did not include the prevalence of cardiac disease, caution should be exercised before extrapolating these results to patients with underlying (active or historical) cardiac disease.
CHALLENGES TO IMPLEMENTATION: Changing long-held policies may be difficult
Although RCTs as well as clinical guidelines suggest that restrictive transfusion policies are safe and effective, changing long-held clinical practices is never easy.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med.2013;368:11-21.
2. Laine L, Yang H, Chang SC,et al. Trends for incidence of hospitalization and death due to GI complications in the United States from 2001 to 2009. Am J Gastroenterol 2012; 107:1190-1195.
3. Gralnek IM, Barkun AN, Bardou M. Management of acute bleeding from a peptic ulcer. N Engl J Med 2008; 359:928-937.
4. Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999; 340:409-417.
5. Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med 2011; 365:2453-2462.
6. Jairath V, Hearnshaw S, Brunskill SJ, et al. Red cell transfusion for the management of upper gastrointestinal haemorrhage. Cochrane Database of Systematic Reviews 2010;CD006613.
7. Carson JL, Grossman BJ, Kleinman S, et al. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med 2012; 157:49-58.
8. Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol 2012; 107:345-360.
Do not order transfusions of red blood cells for patients with acute upper gastrointestinal bleeding unless their hemoglobin level <7 g/dL.
Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11-21.1
A: Based on a single randomized controlled trial (RCT) consistent with other RCTs on recommendations for transfusion.
ILLUSTRATED CASE
An 82-year-old patient presents to the emergency department with several episodes of melena over the past week and one episode of hematemesis this morning. He denies any shortness of breath, dizziness, lightheadedness, or fatigue. He is tachycardic but normotensive. Lab results note a hemoglobin level of 8.3 g/dL. Should you order a transfusion of red blood cells?
Acute upper gastrointestinal bleeding (UGIB) commonly requires hospital admission, with approximately 61 cases per 100,000 population in the United States in 2009.2 Gastroduodenal peptic ulcer disease accounts for the majority of these cases.3 Although trends indicate an overall decrease in cases requiring hospitalization, UGIB remains a condition associated with a mortality rate of 2.5% and inpatient costs of $2 billion annually.2,3
Studies have been inconclusive—until now
An RCT published in 1999 showed a restrictive transfusion strategy (hemoglobin threshold of 7 g/dL) to be at least as effective as—and possibly superior to—a liberal strategy (threshold of 10 g/dL) in critically ill patients.4 In 2010, an RCT demonstrated that a liberal transfusion strategy (also defined as a transfusion threshold of 10 g/dL) did not reduce the rates of death or in-hospital morbidity in elderly patients after hip surgery.5 A recent Cochrane review of transfusion strategies for UGIB included only 3 small studies (N=93), so its authors could not draw any firm conclusions.6 The results of a new RCT, detailed below, are more conclusive.
STUDY SUMMARY: Restrictive transfusion policy lowers mortality risk
Villanueva et al conducted a nonblinded RCT comparing outcomes in patients admitted to the hospital with moderate-risk acute UGIB transfused on a liberal vs a restrictive strategy.1 The restrictive group used a transfusion hemoglobin threshold of 7 g/dL and a posttransfusion target of 7 to 9 g/dL; the liberal group used a threshold of 9 g/dL, with a posttransfusion target of 9 to 11 g/dL. Patients received one unit of red blood cells at a time until their hemoglobin was above the predetermined threshold.
Patients were excluded if they declined blood transfusion; had massive exsanguinating bleeding, acute coronary syndrome, symptomatic peripheral vasculopathy, stroke, lower GI bleeding, or a transient ischemic attack; had received a transfusion within the previous 90 days; or had a recent history of surgery or trauma. Patients at low risk of rebleeding (as defined by the Rockall risk scoring system) were also excluded. Randomization was stratified by the presence or absence of cirrhosis of the liver.
Participants (N=921) had confirmed hematemesis and/or melena on admission. All underwent emergency gastroscopy within 6 hours of admission, with subsequent interventions based on endoscopic findings. In addition to established hemoglobin levels, patients received a transfusion anytime they developed signs or symptoms related to anemia, massive bleeding, or the need for surgery. Staff monitored hemoglobin levels every 8 hours during the first 48 hours, then daily thereafter.
Both groups had similar baseline characteristics, including hemoglobin on admission and source of bleeding. The authors used intention-to-treat analysis to identify the primary outcome: death from any cause at 45 days. Secondary outcomes were further bleeding and in-hospital complications.
During hospitalization, 49% of patients in the restrictive group and 86% of those in the liberal group received a blood transfusion (P<.001). Thirty-two patients (17 from the restrictive group and 15 from the liberal group) withdrew from the study, leaving 889 patients for overall analysis.
At 45 days, overall mortality from any cause was 5% in the restrictive group and 9% in the liberal group (P=.02; number needed to treat [NNT]=25). Sub-group analysis revealed a lower risk of death in patients with cirrhosis and Child-Pugh class A or B disease assigned to the restrictive transfusion group vs the liberal group. The results showed a trend toward a lower risk of death in patients with bleeding from varices or peptic ulcers for the restrictive group, as well.
In addition, the restrictive transfusion group had a significantly lower rate of adverse events (40% vs 48% for the liberal transfusion group; P=.02, NNT=13), with a significant reduction in transfusion reactions (3% vs 9%; P=.001, NNT=17) and cardiac complications (11% vs 16%; P=.04, NNT=20). The restrictive group had a lower rate of further bleeding (10% vs 16% for the liberal transfusion group; P=.01, NNT=17), as well.
WHAT'S NEW: Many reasons to limit transfusions for acute upper GI bleed
This RCT provides evidence that patients with acute UGIB have improved survival rates and fewer adverse events when a restrictive transfusion strategy is used. In addition to improving patient outcomes, a restrictive strategy will likely reduce costs and overall use of blood products. Thus, the study, along with other recent evaluations, adds evidence to support more restrictive transfusion thresholds.
The AABB (formerly named the American Association of Blood Banks) recently
released guidelines calling for restrictive transfusion thresholds (7-8 g/dL) in stable hospitalized patients.7 In 2012, the American College of Gastroenterology published a practice guideline with a recommended target hemoglobin level of ≥7 g/dL in the management of patients who have ulcer bleeding but no signs of intravascular depletion or comorbidities such as coronary artery disease.8
CAVEATS: Results might differ when endoscopy is delayed
The patients in the study detailed here underwent emergency gastroscopy within 6 hours of admission, and both groups received the same therapies based on endoscopic findings. It remains unclear whether the benefits of a restrictive transfusion strategy would persist in patients who do not undergo endoscopy within that timeframe. And, because the reported baseline characteristics of the patients did not include the prevalence of cardiac disease, caution should be exercised before extrapolating these results to patients with underlying (active or historical) cardiac disease.
CHALLENGES TO IMPLEMENTATION: Changing long-held policies may be difficult
Although RCTs as well as clinical guidelines suggest that restrictive transfusion policies are safe and effective, changing long-held clinical practices is never easy.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Do not order transfusions of red blood cells for patients with acute upper gastrointestinal bleeding unless their hemoglobin level <7 g/dL.
Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11-21.1
A: Based on a single randomized controlled trial (RCT) consistent with other RCTs on recommendations for transfusion.
ILLUSTRATED CASE
An 82-year-old patient presents to the emergency department with several episodes of melena over the past week and one episode of hematemesis this morning. He denies any shortness of breath, dizziness, lightheadedness, or fatigue. He is tachycardic but normotensive. Lab results note a hemoglobin level of 8.3 g/dL. Should you order a transfusion of red blood cells?
Acute upper gastrointestinal bleeding (UGIB) commonly requires hospital admission, with approximately 61 cases per 100,000 population in the United States in 2009.2 Gastroduodenal peptic ulcer disease accounts for the majority of these cases.3 Although trends indicate an overall decrease in cases requiring hospitalization, UGIB remains a condition associated with a mortality rate of 2.5% and inpatient costs of $2 billion annually.2,3
Studies have been inconclusive—until now
An RCT published in 1999 showed a restrictive transfusion strategy (hemoglobin threshold of 7 g/dL) to be at least as effective as—and possibly superior to—a liberal strategy (threshold of 10 g/dL) in critically ill patients.4 In 2010, an RCT demonstrated that a liberal transfusion strategy (also defined as a transfusion threshold of 10 g/dL) did not reduce the rates of death or in-hospital morbidity in elderly patients after hip surgery.5 A recent Cochrane review of transfusion strategies for UGIB included only 3 small studies (N=93), so its authors could not draw any firm conclusions.6 The results of a new RCT, detailed below, are more conclusive.
STUDY SUMMARY: Restrictive transfusion policy lowers mortality risk
Villanueva et al conducted a nonblinded RCT comparing outcomes in patients admitted to the hospital with moderate-risk acute UGIB transfused on a liberal vs a restrictive strategy.1 The restrictive group used a transfusion hemoglobin threshold of 7 g/dL and a posttransfusion target of 7 to 9 g/dL; the liberal group used a threshold of 9 g/dL, with a posttransfusion target of 9 to 11 g/dL. Patients received one unit of red blood cells at a time until their hemoglobin was above the predetermined threshold.
Patients were excluded if they declined blood transfusion; had massive exsanguinating bleeding, acute coronary syndrome, symptomatic peripheral vasculopathy, stroke, lower GI bleeding, or a transient ischemic attack; had received a transfusion within the previous 90 days; or had a recent history of surgery or trauma. Patients at low risk of rebleeding (as defined by the Rockall risk scoring system) were also excluded. Randomization was stratified by the presence or absence of cirrhosis of the liver.
Participants (N=921) had confirmed hematemesis and/or melena on admission. All underwent emergency gastroscopy within 6 hours of admission, with subsequent interventions based on endoscopic findings. In addition to established hemoglobin levels, patients received a transfusion anytime they developed signs or symptoms related to anemia, massive bleeding, or the need for surgery. Staff monitored hemoglobin levels every 8 hours during the first 48 hours, then daily thereafter.
Both groups had similar baseline characteristics, including hemoglobin on admission and source of bleeding. The authors used intention-to-treat analysis to identify the primary outcome: death from any cause at 45 days. Secondary outcomes were further bleeding and in-hospital complications.
During hospitalization, 49% of patients in the restrictive group and 86% of those in the liberal group received a blood transfusion (P<.001). Thirty-two patients (17 from the restrictive group and 15 from the liberal group) withdrew from the study, leaving 889 patients for overall analysis.
At 45 days, overall mortality from any cause was 5% in the restrictive group and 9% in the liberal group (P=.02; number needed to treat [NNT]=25). Sub-group analysis revealed a lower risk of death in patients with cirrhosis and Child-Pugh class A or B disease assigned to the restrictive transfusion group vs the liberal group. The results showed a trend toward a lower risk of death in patients with bleeding from varices or peptic ulcers for the restrictive group, as well.
In addition, the restrictive transfusion group had a significantly lower rate of adverse events (40% vs 48% for the liberal transfusion group; P=.02, NNT=13), with a significant reduction in transfusion reactions (3% vs 9%; P=.001, NNT=17) and cardiac complications (11% vs 16%; P=.04, NNT=20). The restrictive group had a lower rate of further bleeding (10% vs 16% for the liberal transfusion group; P=.01, NNT=17), as well.
WHAT'S NEW: Many reasons to limit transfusions for acute upper GI bleed
This RCT provides evidence that patients with acute UGIB have improved survival rates and fewer adverse events when a restrictive transfusion strategy is used. In addition to improving patient outcomes, a restrictive strategy will likely reduce costs and overall use of blood products. Thus, the study, along with other recent evaluations, adds evidence to support more restrictive transfusion thresholds.
The AABB (formerly named the American Association of Blood Banks) recently
released guidelines calling for restrictive transfusion thresholds (7-8 g/dL) in stable hospitalized patients.7 In 2012, the American College of Gastroenterology published a practice guideline with a recommended target hemoglobin level of ≥7 g/dL in the management of patients who have ulcer bleeding but no signs of intravascular depletion or comorbidities such as coronary artery disease.8
CAVEATS: Results might differ when endoscopy is delayed
The patients in the study detailed here underwent emergency gastroscopy within 6 hours of admission, and both groups received the same therapies based on endoscopic findings. It remains unclear whether the benefits of a restrictive transfusion strategy would persist in patients who do not undergo endoscopy within that timeframe. And, because the reported baseline characteristics of the patients did not include the prevalence of cardiac disease, caution should be exercised before extrapolating these results to patients with underlying (active or historical) cardiac disease.
CHALLENGES TO IMPLEMENTATION: Changing long-held policies may be difficult
Although RCTs as well as clinical guidelines suggest that restrictive transfusion policies are safe and effective, changing long-held clinical practices is never easy.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med.2013;368:11-21.
2. Laine L, Yang H, Chang SC,et al. Trends for incidence of hospitalization and death due to GI complications in the United States from 2001 to 2009. Am J Gastroenterol 2012; 107:1190-1195.
3. Gralnek IM, Barkun AN, Bardou M. Management of acute bleeding from a peptic ulcer. N Engl J Med 2008; 359:928-937.
4. Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999; 340:409-417.
5. Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med 2011; 365:2453-2462.
6. Jairath V, Hearnshaw S, Brunskill SJ, et al. Red cell transfusion for the management of upper gastrointestinal haemorrhage. Cochrane Database of Systematic Reviews 2010;CD006613.
7. Carson JL, Grossman BJ, Kleinman S, et al. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med 2012; 157:49-58.
8. Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol 2012; 107:345-360.
1. Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med.2013;368:11-21.
2. Laine L, Yang H, Chang SC,et al. Trends for incidence of hospitalization and death due to GI complications in the United States from 2001 to 2009. Am J Gastroenterol 2012; 107:1190-1195.
3. Gralnek IM, Barkun AN, Bardou M. Management of acute bleeding from a peptic ulcer. N Engl J Med 2008; 359:928-937.
4. Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999; 340:409-417.
5. Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med 2011; 365:2453-2462.
6. Jairath V, Hearnshaw S, Brunskill SJ, et al. Red cell transfusion for the management of upper gastrointestinal haemorrhage. Cochrane Database of Systematic Reviews 2010;CD006613.
7. Carson JL, Grossman BJ, Kleinman S, et al. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med 2012; 157:49-58.
8. Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol 2012; 107:345-360.
Copyright 2013. The Family Physicians Inquiries Network. All rights reserved.
When to worry about incidental renal and adrenal masses
› Use computed tomography studies and the Bosniak classification system to
guide management of renal cystic masses. A
› Perform laboratory tests for hypercortisolism, hyperaldosteronism, and hypersecretion of catecholamines (pheochromocytoma) on any patient with an incidental adrenal mass, regardless of signs or symptoms. C
› Refer patients with adrenal masses >4 cm for surgical evaluation. Refer any individual who has a history of malignancy and an adrenal mass for oncologic evaluation. B
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Jane C, a 76-year-old patient, reports lower abdominal discomfort and increased bowel movements. Her left lower quadrant is tender to palpation, without signs of a surgical abdomen, and vital signs are normal. Laboratory studies are also normal, except for mild anemia and a positive fecal occult blood test. Abdominal and pelvic computed tomography (CT), with and without contrast, are negative for acute pathology, but a 1.7-cm lesion is found in the upper pole of the left kidney. What is your next step?
Renal or adrenal masses may be discovered during imaging studies for complaints unrelated to the kidneys or adrenals. Detection of incidentalomas has increased dramatically, keeping pace with the growing use of ultrasonography, CT, and magnetic resonance imaging (MRI) for abdominal, chest, and back complaints.1
Family physicians can evaluate most of these masses and determine the need for referral by using clinical judgment, appropriate imaging studies, and screening laboratory tests. In the pages that follow, we present a systematic approach for evaluating these incidentalomas and determining when consultation or referral is needed.
Incidental renal masses are common
Lesions are commonly found in normal kidneys, and the incidence increases with age. Approximately one-third of individuals age 50 and older will have at least one renal cyst on CT.2
Most incidental renal masses are benign cysts requiring no further evaluation. Other possibilities include indeterminate or malignant cysts or solid masses, which may be malignant or benign. Inflammatory renal lesions from infection, infarction, or trauma also occur, but these tend to be symptomatic and are rarely found incidentally.
Classification of renal cysts—not based on size
Cysts are the most common adult renal masses. Typically they are unilocular and located in the renal cortex, frequently extending to the renal surface.3 Renal function is usually preserved, regardless of the cyst’s location or size. Careful examination of adjacent tissue is essential, as secondary cysts may form when solid tumors obstruct tubules of normal parenchyma. Cystic lesions containing enhancing soft tissue unattached to the wall or septa likely are malignant.4
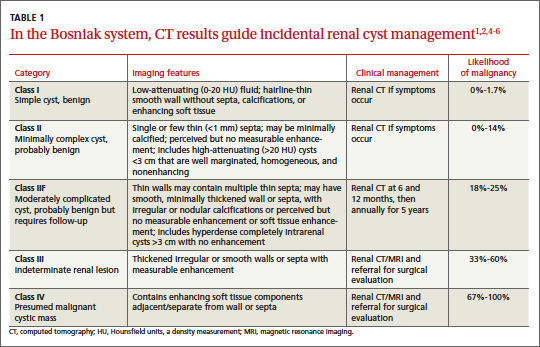
The Bosniak classification system, with 5 classes based on CT characteristics
(TABLE 1), is a useful guide for managing renal cystic lesions.4 Size is not an important feature in the Bosniak system; small cysts may be malignant and larger ones benign. Small cysts may grow into larger benign lesions, occasionally causing flank or abdominal pain, palpable masses, or hematuria.
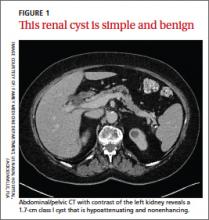
Simple cysts. Renal cysts that meet Bosniak class I criteria can be confidently labeled benign and need no further evaluation (FIGURE 1). Simple renal cysts on CT have homogenous low-attenuating fluid and thin nonenhancing walls without septa.4
On ultrasound, simple renal cysts show spherical or ovoid shape without internal echoes, a thin smooth wall separate from the surrounding parenchyma, and posterior wall enhancement caused by increased transmission through the water-filled cyst. The likelihood of malignancy is extremely low in a renal cyst that meets these criteria, which have a reported accuracy of 98% to 100%.3 Thus, no further evaluation is required if an obviously benign simple cyst is first noted on an adequate ultrasound. Inadequate ultrasound visualization or evidence of calcifications, septa, or multiple chambers calls for prompt renal CT.
CASE The mass on Ms. C’s left kidney is hypoattenuating and nonenhancing on CT. It meets Bosniak criteria for a benign simple cyst (class I) and requires no further evaluation or follow-up. Colonoscopy detects multiple colonic polyps that are removed, and the patient does well.
Mildly complicated cysts. Less diagnostic certainty characterizes cysts with mild abnormalities that keep them from being labeled as simple. Bosniak classes II and IIF describe mildly abnormal renal cysts. Class II cysts can be dismissed, whereas class IIF cysts require follow-up.
Class II cysts may contain a few hairline septa, fine calcium deposits in walls or septa, or an unmeasurable enhancement of the walls. A hyperattenuating but nonenhancing fluid also is described as category II. Small homogeneous cysts <3 cm, without enhancement but hyperattenuated, are reliably considered benign and need not be evaluated.2,7
Class IIF cysts may have multiple hairline-thin septa with unmeasurable enhancement or minimal smooth thickening or irregular/nodular calcifications of wall or septa without enhancing soft tissue components. Hyperattenuating cystic lesions >3 cm and intrarenal “noncortical” cysts are included in this category. Class IIF cysts require follow-up at 6 months with CT or MRI, then annually for at least 5 years.8
Obviously complicated cysts. Bosniak class III is indeterminate—neither benign nor clearly malignant. Class III cysts may have thickened borders or septa with measurable enhancement, or they may be multilocular, hemorrhagic, or infected. In 5 case series, 29 of 57 class III lesions proved to be malignant.5 MRI may characterize these lesions more definitively than CT prior to urologic referral.
Malignant cysts. Bosniak class IV renal lesions are clearly malignant, with large heterogeneous cysts or necrotic components, shaggy thickened walls, or enhancing soft tissue components separate from the wall or septa. Their unequivocal appearance results from solid tumor necrosis and liquefaction. Diagnosis is straightforward, and excision is indicated.2
A closer look at solid renal masses
Solid renal masses usually consist of enhancing tissue with little or no fluid. The goal of evaluation is to exclude malignancies, such as renal cell cancer, lymphomas, sarcomas, or metastasis. Benign solid masses include renal adenomas, angiomyolipomas, and oncocytomas, among others.
Several lesions can be diagnosed by appearance or symptoms:
Angiomyolipomas are recognized by their fat content within a noncalcified mass. Unenhanced CT usually is sufficient for diagnosis, unless the mass is very small or has atypical features.9
Vascular lesions can be identified because they enhance to the same degree as the vasculature. With the exception of inflammatory or vascular abnormalities, all enhancing lesions that do not contain fat should be presumed to be malignant.
In patients with a known extrarenal primary malignancy, 50% to 85% of incidental solid renal masses will represent metastatic disease.10 Percutaneous biopsy may be warranted to differentiate metastatic lesions from a secondary, primary (ie, renal cell carcinoma), or benign process.11
A study of 2770 solid renal mass excisions revealed that 12.8% were benign, with a direct relationship between malignancy and size. Masses <1 cm were benign 44% of the time.12 Early identification of small renal carcinomas may improve survival rates. Although renal cell carcinomas <3 cm in diameter have low metastatic potential, a solid, nonfat-containing mass should be evaluated for aggressive nephron-sparing surgery.6,13
Incidental adrenal masses occur infrequently
Adrenal incidentalomas are defined as radiographically identified masses >1 cm in diameter.14 They are much less common than their renal counterparts, with a reported prevalence of 0.35% to 5% on CT.15 Because the adrenal glands are hormonally active and receive substantial blood flow, metastatic, hormonally active, and nonfunctional causes for adrenal masses need to be considered.16
Adrenal pathology
Adrenal masses may be characterized by increased or normal adrenal function. Hyperfunctioning syndromes include hypercortisolism, hyperaldosteronism, adrenogenital hypersecretion of adrenocortical origin, and pheochromocytomas of the medulla. Symptom evaluation of these syndromes is important, but not sufficient to rule out a hyperfunctioning syndrome.
In a retrospective review of inapparent adrenal masses, ≤13% of pheochromocytomas were clinically silent.17 Therefore, laboratory testing is necessary for an incidental adrenal mass.
Nonfunctional lesions include adenomas, metastases, cysts, myelolipomas, hemorrhage, and adrenal carcinomas. These masses require evaluation for the possibility of cancer, the most common of which is metastasis. In patients with an extra-adrenal malignancy, the likelihood of malignancy in an incidental adrenal mass is at least 50%.18 An adrenal mass representing metastasis of a previously unrecognized cancer is exceedingly rare.19
Primary adrenal carcinoma is also rare, with an estimated incidence of 2 cases per one million in the general population. For patients with adrenal masses, the prevalence of carcinoma increases with lesion size (2% for tumors <4 cm, 6% for tumors 4-6 cm, and 25% for tumors >6 cm in diameter). 17 For this reason, tumors >4 cm in diameter are usually surgically resected in patients with no previous cancer history, unless radiologic criteria demonstrate clearly benign characteristics.
Although adrenal carcinomas are considered nonfunctioning, some evidence suggests they produce low levels of cortisol that may be associated with clinical features of metabolic syndrome.20
CT is first choice for adrenal mass evaluation
Dedicated adrenal CT with both unenhanced and delayed contrast-enhanced images is the most reliable study to evaluate an adrenal mass, according to the American College of Radiology. Consider another study only in patients with contrast allergy, renal compromise, or cancer history.21
Unenhanced CT can diagnose the approximately 70% of adenomas that are small, well-defined round masses with homogenous low-density lipid deposition.22 Delayed contrast enhancement can characterize most of the remaining 30%.23 Unenhanced CT with attenuation values of <10 Hounsfield units (HU) can diagnose adenomas with 71% specificity and 98% sensitivity,24 and can often diagnose simple cysts and myelolipomas, as well.
Other imaging options. MRI is an alternative to CT for patients with contraindications for contrast or radiation exposure. MRI provides less spatial resolution than CT, but chemical shift imaging can measure cytoplasmic lipid content similar to unenhanced CT. A small study found chemical shift MRI more reliable than unenhanced CT, but less reliable than CT with delayed contrast enhancement.25
Positron emission tomography (PET) is useful to noninvasively evaluate biochemical and physiologic processes. PET-CT incorporates unenhanced CT density measurements to improve PET accuracy. In a patient with a history of cancer, PET-CT has a sensitivity of 93% to 100% and a specificity of 95% in differentiating benign from malignant adrenal tumors.26
When to order a biopsy
The need for biopsy has decreased as imaging has improved, but biopsy is required whenever diagnostic imaging fails to differentiatea lesion as benign or malignant. CT guided biopsy provides diagnostic accuracy of 85% to 95%.27 Complications such as pneumothorax, hemorrhage, and bacteremia occur in 3% to 9% of biopsies. Before any adrenal biopsy, measure plasma-free metanephrines to exclude undiagnosed pheochromocytoma, which could precipitate a hypertensive crisis if untreated.22
These 3 laboratory screening tests are critical
Family physicians can perform the initial biochemical evaluation of an adrenal incidentaloma. Guidance is available from the National Institutes of Health (NIH)28 and the American Academy of Clinical Endocrinologists (AACE) (FIGURE 2).29
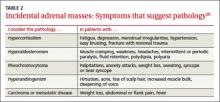
Regardless of signs or symptoms, perform screening laboratory tests for 3 types of adrenal hyperfunction: hypercortisolism, hyperaldosteronism, and hypersecretion of catecholamines (pheochromocytoma). Screening tests are not recommended for androgen hypersecretion, which is extremely rare and causes recognizable symptoms such as hirsutism (Table 2).29
Hypercortisolism occurs in approximately 5% of adrenal incidentalomas.30 An overnight dexamethasone suppression test (DST) is most reliable for screening, with sensitivity >95% for Cushing syndrome.31 The patient takes a 1-mg dose of oral dexamethasone at 11 pm, and a fasting plasma cortisol sample is drawn the next day at 8 am.
Dexamethasone binds to glucocorticoid receptors in the pituitary gland, suppressing adrenocorticotropic hormone secretion. Cortisol will be depressed the next morning unless the adrenal mass produces cortisol autonomously. Patients with a DST >5 mcg/dL—highly suggestive of Cushing syndrome—require further evaluation, and we suggest referral to an endocrinologist.
Hyperaldosteronism is seen in 1% to 2% of adrenal incidentalomas.32 The aldosterone- to-renin ratio (ARR) is recommended as a screening test for hyperaldosteronism, with an ARR >20 requiring further testing.33 Medications that may affect the ARR include beta-blockers, spironolactone, clonidine, diuretics, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers.29
Refer a patient with evidence of hyperaldosteronism to an endocrinologist and a surgeon with experience in managing these lesions. If the ARR test result suggests an aldosterone excess, a salt-loading test is used to verify failure of aldosterone suppression. Adrenal venous sampling is often performed prior to surgical removal to confirm that an incidentaloma is the source of hyperaldosteronism.
Pheochromocytoma. Approximately 5% of incidental adrenal lesions are pheochromocytomas.30 Many patients with these epinephrine/norepinephrine secreting tumors do not show the classic symptom triad of headache, palpitations, and diaphoresis, and approximately half have normal blood pressure.34
Identifying a pheochromocytoma is important in any patient requiring surgery or biopsy, as surgical manipulation can cause a potentially fatal intraoperative catecholamine surge. Presurgical medical management can mitigate this reaction.
A plasma-free metanephrines test, which has 95% sensitivity, is the most reliable test for pheochromocytoma.35 Medications, including tricyclic antidepressants, decongestants, amphetamines, reserpine, and phenoxybenzamine, can cause falsepositive results.29 Confirm a positive plasma-free metanephrines test with a 24-hour fractionated urine metanephrines test, and refer the patient to an endocrinologist.
Managing adrenal incidentalomas
Refer all patients with adrenal masses >4 cm for surgical evaluation because of the risk of malignancy; all patients who have a history of malignancy and an adrenal mass of any size require a referral to an oncologist. Perform the AACE-recommended 3-element biochemical workup for all masses, with the exception of definitively diagnosed cysts or myelolipomas.
Refer to an endocrinologist all patients with abnormal screening laboratory results, regardless of adrenal mass size, as well as patients with concerning clinical findings. Initiate cardiovascular, diabetes, and bone density evaluation and management for metabolic syndrome.20
Monitoring after a negative workup
Little evidence exists to guide monitoring of small adrenal incidentalomas (<4 cm) with a negative workup. The 2002 NIH report recommended annual radiologic follow-up for 5 years,28 whereas the 2009 AACE guidelines recommend radiographic follow-up at 3 to 6 months, then at one and 2 years.29
Evidence indicates that 14% of lesions will enlarge in 2 years, although the clinical significance of enlargement is unknown. Some authors argue against CT monitoring because the risk of adrenal mass progression is similar to the malignancy risk posed by 3 years of radiation exposure with CT.20
Some guidelines recommend repeat biochemical screening every 3 to 4 years.28,29 AACE guidelines quote a 47% rate of progression over 3 years, but most adrenal masses progress to subclinical Cushing syndrome— a condition of uncertain significance. Subclinical Cushing’s has not been reported to progress to the overt syndrome, and new catecholamine or aldosterone secretion is rare.
Many endocrinologists reduce the frequency of follow-up, depending on the type of adrenal mass (cyst or solid) and its size. AACE suggests CT for adenomas one to 4 cm at 12 months. AACE and NIH recommend hormonal evaluation annually for 4 years. Adrenal cysts or myelolipoma in patients without cancer need no follow-up.29
CORRESPONDENCE
James C. Higgins, DO, CAPT, MC, USN, Ret., Naval Hospital Jacksonville, Family Medicine Department, 2080 Child Street, Box 1000, Jacksonville, FL 32214;
[email protected]
1. Berland LL, Silverman SG, Gore RM, et al. Managing incidental findings on abdominal CT: white paper of the ACR incidental findings committee. J Am Coll Radiol. 2010;7:754-773.
2. Silverman S, Israel G, Herts B, et al. Management of the incidental renal mass. Radiology. 2008;249:16-31.
3. Curry NS, Bissada NK. Radiologic evaluation of small and indeterminate renal masses. Urol Clin North Am. 1997;24:493-505.
4. Bosniak MA. The current radiological approach to renal cysts. Radiology. 1986;158:1-10.
5. Harisinghani M, Maher M, Gervais D, et al. Incidence of malignancy in complex cystic renal masses (Bosniak category III): should imaging guided biopsy precede surgery? AJR Am J Roentgenol. 2003;180:755-758.
6. Remzi M, Ozsoy M, Klingler HC. Are small renal tumors harmless? Analysis of histopathological features according to tumors less than 4 cm in diameter. J Urol. 2006;176:896-899.
7. Jonisch AI, Rubinowitz A, Mutalik P, et al. Can high attenuation renal cysts be differentiated from renal cell carcinoma at unenhanced computed tomography? Radiology. 2007;243:445-450.
8. Israel GM, Bosniak MA. Follow-up CT of moderately complex cystic lesions of the kidney. AJR Am J Roentgenol. 2003;181: 627-633.
9. Bosniak MA, Megibow AJ, Hulnick DH, et al. CT diagnosis of renal angiomyolipoma: the importance of detecting small amounts of fat. AJR Am J Roentengol. 1988;151:497-501.
10. Mitnick JS, Bosniak MA, Rothberg M, et al. Metastatic neoplasm to the kidney studied by computed tomography and sonogram. J Comput Assist Tomogr. 1985;9:43-49.
11. Rybicki FJ, Shu KM, Cibas ES, et al. Percutaneous biopsy of renal masses: sensitivity and negative predictive value stratified by clinical setting and size of masses. AJR Am J Roentgenol. 2003;180:1281-1287.
12. Frank I, Blure MI, Cheville JC, et al. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol. 2003;170:2217-2220.
13. Hollingsworth JM, Miller DC, Daignault S, et al. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331-1334.
14. Geelhoed GW, Spiegel CT. “Incidental” adrenal cyst: a correctable lesion possibly associated with hypertension. South Med J. 1981;74:626-630.
15. Davenport C, Liew A, Doherty B, et al. The prevalence of adrenal incidentaloma in routine clinical practice. Endocrine. 2011;40: 80-83.
16. Cook DM, Loriaux LD. The incidental adrenal mass. Am J Med. 1996;101:88 94.
17. Mansmann G, Lau J, Balk E, et al. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev. 2004;25:309-340.
18. Androulakis II, Kaltsas G, Piatitis G, et al. The clinical significance of adrenal incidentalomas. Eur J Clin Invest. 2011;41: 552-560.
19. Lee JE, Evans DB, Hickey RC, et al. Unknown primary cancer presenting as an adrenal mass: frequency and implications for diagnostic evaluation of adrenal incidentalomas. Surgery. 1998;124:1115-1122.
20. Aron D, Terzolo M, Cawood TJ. Adrenal incidentalomas. Best Pract Res Clin Endocrinol Metab. 2012;26:69-82.
21. ACR appropriateness criteria: incidentally discovered adrenal mass. American College of Radiology. Available at: http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/IncidentallyDiscoveredAdrenalMass.pdf. Accessed November 20, 2012.
22. Song JH, Mayo-Smith WW. Incidentally discovered adrenal mass. Radiol Clin North Am. 2011;49:361-368.
23. Korobkin M, Brodeur FJ, Francis IR, et al. CT time-attenuation washout curves of adrenal adenomas and nonadenomas. AJR Am J Roentgenol. 1998;170:747-752.
24. Boland GW, Lee MJ, Gazelle GS, et al. Characterization of adrenal masses using unenhanced CT: an analysis of the CT literature. AJR Am J Roentgenol. 1998;171:201-204.
25. Park BK, Kim CK, Kim B, et al. Chemical shift MR imaging of hyperattenuating (>10 HU) adrenal masses: does it still have a role? Radiology. 2004;231:711-716.
26. Boland GW, Blake MA, Holakere NS, et al. PET/CT for the characterization of adrenal masses in patients with cancer: qualitative vs quantitative accuracy in 150 consecutive patients. AJR Am J Roentgenol. 2009;192:956-962.
27. Paulsen SD, Nghiem HV, Korobkin M, et al. Changing role of imaging- guided percutaneous biopsy of adrenal masses: evaluation of 50 adrenal biopsies. AJR Am J Roentgenol. 2004;182:1033-1037
28. Grumbach MM, Biller BMK, Braunstein GD, et al. Management of the clinically inapparent adrenal mass (“incidentalomas”). Ann Intern Med. 2003;138:424-429.
29. Zeiger MA, Thompson GB, Quan-Yang D, et al. American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons medical guidelines for the management of adrenal incidentalomas. Endocr Pract. 2009;15(suppl 1):1-20.
30. Young WF. The incidentally discovered adrenal mass. N Engl J Med. 2007; 356:601-610.
31. Deutschbein T, Unger N, Hinrichs J, et al. Late-night and lowdose dexamethasone-suppressed cortisol in saliva and serum for the diagnosis of cortisol-secreting adrenal adenomas. Eur J Endocrinol. 2009;161:747-753.
32. Bernini G, Moretti A, Gianfranco A, et al. Primary aldosteronism in normokalemic patients with adrenal incidentalomas. Eur J Endocrinol. 2002;146:523-529.
33. Montori VM, Young WF Jr. Use of plasma aldosterone concentration-to-plasma renin activity ratio as a screening test for primary aldosteronism: a systematic review of the literature. Endocrinol Metab Clin North Am. 2002;31:619-632.
34. Motta-Ramirez GA, Remer EM, Herts BR, et al. Comparison of CT findings in symptomatic and incidentally discovered pheochromocytomas. AJR Am J Roentgenol. 2005;185:684-688.
35. Pacak K, Eisenhofer G, Grossman A. The incidentally discovered adrenal mass. N Engl J Med. 2007;356:2005.
› Use computed tomography studies and the Bosniak classification system to
guide management of renal cystic masses. A
› Perform laboratory tests for hypercortisolism, hyperaldosteronism, and hypersecretion of catecholamines (pheochromocytoma) on any patient with an incidental adrenal mass, regardless of signs or symptoms. C
› Refer patients with adrenal masses >4 cm for surgical evaluation. Refer any individual who has a history of malignancy and an adrenal mass for oncologic evaluation. B
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Jane C, a 76-year-old patient, reports lower abdominal discomfort and increased bowel movements. Her left lower quadrant is tender to palpation, without signs of a surgical abdomen, and vital signs are normal. Laboratory studies are also normal, except for mild anemia and a positive fecal occult blood test. Abdominal and pelvic computed tomography (CT), with and without contrast, are negative for acute pathology, but a 1.7-cm lesion is found in the upper pole of the left kidney. What is your next step?
Renal or adrenal masses may be discovered during imaging studies for complaints unrelated to the kidneys or adrenals. Detection of incidentalomas has increased dramatically, keeping pace with the growing use of ultrasonography, CT, and magnetic resonance imaging (MRI) for abdominal, chest, and back complaints.1
Family physicians can evaluate most of these masses and determine the need for referral by using clinical judgment, appropriate imaging studies, and screening laboratory tests. In the pages that follow, we present a systematic approach for evaluating these incidentalomas and determining when consultation or referral is needed.
Incidental renal masses are common
Lesions are commonly found in normal kidneys, and the incidence increases with age. Approximately one-third of individuals age 50 and older will have at least one renal cyst on CT.2
Most incidental renal masses are benign cysts requiring no further evaluation. Other possibilities include indeterminate or malignant cysts or solid masses, which may be malignant or benign. Inflammatory renal lesions from infection, infarction, or trauma also occur, but these tend to be symptomatic and are rarely found incidentally.

Classification of renal cysts—not based on size
Cysts are the most common adult renal masses. Typically they are unilocular and located in the renal cortex, frequently extending to the renal surface.3 Renal function is usually preserved, regardless of the cyst’s location or size. Careful examination of adjacent tissue is essential, as secondary cysts may form when solid tumors obstruct tubules of normal parenchyma. Cystic lesions containing enhancing soft tissue unattached to the wall or septa likely are malignant.4
The Bosniak classification system, with 5 classes based on CT characteristics
(TABLE 1), is a useful guide for managing renal cystic lesions.4 Size is not an important feature in the Bosniak system; small cysts may be malignant and larger ones benign. Small cysts may grow into larger benign lesions, occasionally causing flank or abdominal pain, palpable masses, or hematuria.
Simple cysts. Renal cysts that meet Bosniak class I criteria can be confidently labeled benign and need no further evaluation (FIGURE 1). Simple renal cysts on CT have homogenous low-attenuating fluid and thin nonenhancing walls without septa.4
On ultrasound, simple renal cysts show spherical or ovoid shape without internal echoes, a thin smooth wall separate from the surrounding parenchyma, and posterior wall enhancement caused by increased transmission through the water-filled cyst. The likelihood of malignancy is extremely low in a renal cyst that meets these criteria, which have a reported accuracy of 98% to 100%.3 Thus, no further evaluation is required if an obviously benign simple cyst is first noted on an adequate ultrasound. Inadequate ultrasound visualization or evidence of calcifications, septa, or multiple chambers calls for prompt renal CT.
CASE The mass on Ms. C’s left kidney is hypoattenuating and nonenhancing on CT. It meets Bosniak criteria for a benign simple cyst (class I) and requires no further evaluation or follow-up. Colonoscopy detects multiple colonic polyps that are removed, and the patient does well.
Mildly complicated cysts. Less diagnostic certainty characterizes cysts with mild abnormalities that keep them from being labeled as simple. Bosniak classes II and IIF describe mildly abnormal renal cysts. Class II cysts can be dismissed, whereas class IIF cysts require follow-up.
Class II cysts may contain a few hairline septa, fine calcium deposits in walls or septa, or an unmeasurable enhancement of the walls. A hyperattenuating but nonenhancing fluid also is described as category II. Small homogeneous cysts <3 cm, without enhancement but hyperattenuated, are reliably considered benign and need not be evaluated.2,7
Class IIF cysts may have multiple hairline-thin septa with unmeasurable enhancement or minimal smooth thickening or irregular/nodular calcifications of wall or septa without enhancing soft tissue components. Hyperattenuating cystic lesions >3 cm and intrarenal “noncortical” cysts are included in this category. Class IIF cysts require follow-up at 6 months with CT or MRI, then annually for at least 5 years.8
Obviously complicated cysts. Bosniak class III is indeterminate—neither benign nor clearly malignant. Class III cysts may have thickened borders or septa with measurable enhancement, or they may be multilocular, hemorrhagic, or infected. In 5 case series, 29 of 57 class III lesions proved to be malignant.5 MRI may characterize these lesions more definitively than CT prior to urologic referral.
Malignant cysts. Bosniak class IV renal lesions are clearly malignant, with large heterogeneous cysts or necrotic components, shaggy thickened walls, or enhancing soft tissue components separate from the wall or septa. Their unequivocal appearance results from solid tumor necrosis and liquefaction. Diagnosis is straightforward, and excision is indicated.2
A closer look at solid renal masses
Solid renal masses usually consist of enhancing tissue with little or no fluid. The goal of evaluation is to exclude malignancies, such as renal cell cancer, lymphomas, sarcomas, or metastasis. Benign solid masses include renal adenomas, angiomyolipomas, and oncocytomas, among others.
Several lesions can be diagnosed by appearance or symptoms:
Angiomyolipomas are recognized by their fat content within a noncalcified mass. Unenhanced CT usually is sufficient for diagnosis, unless the mass is very small or has atypical features.9
Vascular lesions can be identified because they enhance to the same degree as the vasculature. With the exception of inflammatory or vascular abnormalities, all enhancing lesions that do not contain fat should be presumed to be malignant.
In patients with a known extrarenal primary malignancy, 50% to 85% of incidental solid renal masses will represent metastatic disease.10 Percutaneous biopsy may be warranted to differentiate metastatic lesions from a secondary, primary (ie, renal cell carcinoma), or benign process.11
A study of 2770 solid renal mass excisions revealed that 12.8% were benign, with a direct relationship between malignancy and size. Masses <1 cm were benign 44% of the time.12 Early identification of small renal carcinomas may improve survival rates. Although renal cell carcinomas <3 cm in diameter have low metastatic potential, a solid, nonfat-containing mass should be evaluated for aggressive nephron-sparing surgery.6,13
Incidental adrenal masses occur infrequently
Adrenal incidentalomas are defined as radiographically identified masses >1 cm in diameter.14 They are much less common than their renal counterparts, with a reported prevalence of 0.35% to 5% on CT.15 Because the adrenal glands are hormonally active and receive substantial blood flow, metastatic, hormonally active, and nonfunctional causes for adrenal masses need to be considered.16
Adrenal pathology
Adrenal masses may be characterized by increased or normal adrenal function. Hyperfunctioning syndromes include hypercortisolism, hyperaldosteronism, adrenogenital hypersecretion of adrenocortical origin, and pheochromocytomas of the medulla. Symptom evaluation of these syndromes is important, but not sufficient to rule out a hyperfunctioning syndrome.
In a retrospective review of inapparent adrenal masses, ≤13% of pheochromocytomas were clinically silent.17 Therefore, laboratory testing is necessary for an incidental adrenal mass.
Nonfunctional lesions include adenomas, metastases, cysts, myelolipomas, hemorrhage, and adrenal carcinomas. These masses require evaluation for the possibility of cancer, the most common of which is metastasis. In patients with an extra-adrenal malignancy, the likelihood of malignancy in an incidental adrenal mass is at least 50%.18 An adrenal mass representing metastasis of a previously unrecognized cancer is exceedingly rare.19
Primary adrenal carcinoma is also rare, with an estimated incidence of 2 cases per one million in the general population. For patients with adrenal masses, the prevalence of carcinoma increases with lesion size (2% for tumors <4 cm, 6% for tumors 4-6 cm, and 25% for tumors >6 cm in diameter). 17 For this reason, tumors >4 cm in diameter are usually surgically resected in patients with no previous cancer history, unless radiologic criteria demonstrate clearly benign characteristics.
Although adrenal carcinomas are considered nonfunctioning, some evidence suggests they produce low levels of cortisol that may be associated with clinical features of metabolic syndrome.20
CT is first choice for adrenal mass evaluation
Dedicated adrenal CT with both unenhanced and delayed contrast-enhanced images is the most reliable study to evaluate an adrenal mass, according to the American College of Radiology. Consider another study only in patients with contrast allergy, renal compromise, or cancer history.21
Unenhanced CT can diagnose the approximately 70% of adenomas that are small, well-defined round masses with homogenous low-density lipid deposition.22 Delayed contrast enhancement can characterize most of the remaining 30%.23 Unenhanced CT with attenuation values of <10 Hounsfield units (HU) can diagnose adenomas with 71% specificity and 98% sensitivity,24 and can often diagnose simple cysts and myelolipomas, as well.
Other imaging options. MRI is an alternative to CT for patients with contraindications for contrast or radiation exposure. MRI provides less spatial resolution than CT, but chemical shift imaging can measure cytoplasmic lipid content similar to unenhanced CT. A small study found chemical shift MRI more reliable than unenhanced CT, but less reliable than CT with delayed contrast enhancement.25
Positron emission tomography (PET) is useful to noninvasively evaluate biochemical and physiologic processes. PET-CT incorporates unenhanced CT density measurements to improve PET accuracy. In a patient with a history of cancer, PET-CT has a sensitivity of 93% to 100% and a specificity of 95% in differentiating benign from malignant adrenal tumors.26
When to order a biopsy
The need for biopsy has decreased as imaging has improved, but biopsy is required whenever diagnostic imaging fails to differentiatea lesion as benign or malignant. CT guided biopsy provides diagnostic accuracy of 85% to 95%.27 Complications such as pneumothorax, hemorrhage, and bacteremia occur in 3% to 9% of biopsies. Before any adrenal biopsy, measure plasma-free metanephrines to exclude undiagnosed pheochromocytoma, which could precipitate a hypertensive crisis if untreated.22
These 3 laboratory screening tests are critical
Family physicians can perform the initial biochemical evaluation of an adrenal incidentaloma. Guidance is available from the National Institutes of Health (NIH)28 and the American Academy of Clinical Endocrinologists (AACE) (FIGURE 2).29
Regardless of signs or symptoms, perform screening laboratory tests for 3 types of adrenal hyperfunction: hypercortisolism, hyperaldosteronism, and hypersecretion of catecholamines (pheochromocytoma). Screening tests are not recommended for androgen hypersecretion, which is extremely rare and causes recognizable symptoms such as hirsutism (Table 2).29
Hypercortisolism occurs in approximately 5% of adrenal incidentalomas.30 An overnight dexamethasone suppression test (DST) is most reliable for screening, with sensitivity >95% for Cushing syndrome.31 The patient takes a 1-mg dose of oral dexamethasone at 11 pm, and a fasting plasma cortisol sample is drawn the next day at 8 am.
Dexamethasone binds to glucocorticoid receptors in the pituitary gland, suppressing adrenocorticotropic hormone secretion. Cortisol will be depressed the next morning unless the adrenal mass produces cortisol autonomously. Patients with a DST >5 mcg/dL—highly suggestive of Cushing syndrome—require further evaluation, and we suggest referral to an endocrinologist.
Hyperaldosteronism is seen in 1% to 2% of adrenal incidentalomas.32 The aldosterone- to-renin ratio (ARR) is recommended as a screening test for hyperaldosteronism, with an ARR >20 requiring further testing.33 Medications that may affect the ARR include beta-blockers, spironolactone, clonidine, diuretics, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers.29
Refer a patient with evidence of hyperaldosteronism to an endocrinologist and a surgeon with experience in managing these lesions. If the ARR test result suggests an aldosterone excess, a salt-loading test is used to verify failure of aldosterone suppression. Adrenal venous sampling is often performed prior to surgical removal to confirm that an incidentaloma is the source of hyperaldosteronism.
Pheochromocytoma. Approximately 5% of incidental adrenal lesions are pheochromocytomas.30 Many patients with these epinephrine/norepinephrine secreting tumors do not show the classic symptom triad of headache, palpitations, and diaphoresis, and approximately half have normal blood pressure.34
Identifying a pheochromocytoma is important in any patient requiring surgery or biopsy, as surgical manipulation can cause a potentially fatal intraoperative catecholamine surge. Presurgical medical management can mitigate this reaction.
A plasma-free metanephrines test, which has 95% sensitivity, is the most reliable test for pheochromocytoma.35 Medications, including tricyclic antidepressants, decongestants, amphetamines, reserpine, and phenoxybenzamine, can cause falsepositive results.29 Confirm a positive plasma-free metanephrines test with a 24-hour fractionated urine metanephrines test, and refer the patient to an endocrinologist.
Managing adrenal incidentalomas
Refer all patients with adrenal masses >4 cm for surgical evaluation because of the risk of malignancy; all patients who have a history of malignancy and an adrenal mass of any size require a referral to an oncologist. Perform the AACE-recommended 3-element biochemical workup for all masses, with the exception of definitively diagnosed cysts or myelolipomas.
Refer to an endocrinologist all patients with abnormal screening laboratory results, regardless of adrenal mass size, as well as patients with concerning clinical findings. Initiate cardiovascular, diabetes, and bone density evaluation and management for metabolic syndrome.20
Monitoring after a negative workup
Little evidence exists to guide monitoring of small adrenal incidentalomas (<4 cm) with a negative workup. The 2002 NIH report recommended annual radiologic follow-up for 5 years,28 whereas the 2009 AACE guidelines recommend radiographic follow-up at 3 to 6 months, then at one and 2 years.29
Evidence indicates that 14% of lesions will enlarge in 2 years, although the clinical significance of enlargement is unknown. Some authors argue against CT monitoring because the risk of adrenal mass progression is similar to the malignancy risk posed by 3 years of radiation exposure with CT.20
Some guidelines recommend repeat biochemical screening every 3 to 4 years.28,29 AACE guidelines quote a 47% rate of progression over 3 years, but most adrenal masses progress to subclinical Cushing syndrome— a condition of uncertain significance. Subclinical Cushing’s has not been reported to progress to the overt syndrome, and new catecholamine or aldosterone secretion is rare.
Many endocrinologists reduce the frequency of follow-up, depending on the type of adrenal mass (cyst or solid) and its size. AACE suggests CT for adenomas one to 4 cm at 12 months. AACE and NIH recommend hormonal evaluation annually for 4 years. Adrenal cysts or myelolipoma in patients without cancer need no follow-up.29
CORRESPONDENCE
James C. Higgins, DO, CAPT, MC, USN, Ret., Naval Hospital Jacksonville, Family Medicine Department, 2080 Child Street, Box 1000, Jacksonville, FL 32214;
[email protected]
› Use computed tomography studies and the Bosniak classification system to
guide management of renal cystic masses. A
› Perform laboratory tests for hypercortisolism, hyperaldosteronism, and hypersecretion of catecholamines (pheochromocytoma) on any patient with an incidental adrenal mass, regardless of signs or symptoms. C
› Refer patients with adrenal masses >4 cm for surgical evaluation. Refer any individual who has a history of malignancy and an adrenal mass for oncologic evaluation. B
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Jane C, a 76-year-old patient, reports lower abdominal discomfort and increased bowel movements. Her left lower quadrant is tender to palpation, without signs of a surgical abdomen, and vital signs are normal. Laboratory studies are also normal, except for mild anemia and a positive fecal occult blood test. Abdominal and pelvic computed tomography (CT), with and without contrast, are negative for acute pathology, but a 1.7-cm lesion is found in the upper pole of the left kidney. What is your next step?
Renal or adrenal masses may be discovered during imaging studies for complaints unrelated to the kidneys or adrenals. Detection of incidentalomas has increased dramatically, keeping pace with the growing use of ultrasonography, CT, and magnetic resonance imaging (MRI) for abdominal, chest, and back complaints.1
Family physicians can evaluate most of these masses and determine the need for referral by using clinical judgment, appropriate imaging studies, and screening laboratory tests. In the pages that follow, we present a systematic approach for evaluating these incidentalomas and determining when consultation or referral is needed.
Incidental renal masses are common
Lesions are commonly found in normal kidneys, and the incidence increases with age. Approximately one-third of individuals age 50 and older will have at least one renal cyst on CT.2
Most incidental renal masses are benign cysts requiring no further evaluation. Other possibilities include indeterminate or malignant cysts or solid masses, which may be malignant or benign. Inflammatory renal lesions from infection, infarction, or trauma also occur, but these tend to be symptomatic and are rarely found incidentally.

Classification of renal cysts—not based on size
Cysts are the most common adult renal masses. Typically they are unilocular and located in the renal cortex, frequently extending to the renal surface.3 Renal function is usually preserved, regardless of the cyst’s location or size. Careful examination of adjacent tissue is essential, as secondary cysts may form when solid tumors obstruct tubules of normal parenchyma. Cystic lesions containing enhancing soft tissue unattached to the wall or septa likely are malignant.4
The Bosniak classification system, with 5 classes based on CT characteristics
(TABLE 1), is a useful guide for managing renal cystic lesions.4 Size is not an important feature in the Bosniak system; small cysts may be malignant and larger ones benign. Small cysts may grow into larger benign lesions, occasionally causing flank or abdominal pain, palpable masses, or hematuria.
Simple cysts. Renal cysts that meet Bosniak class I criteria can be confidently labeled benign and need no further evaluation (FIGURE 1). Simple renal cysts on CT have homogenous low-attenuating fluid and thin nonenhancing walls without septa.4
On ultrasound, simple renal cysts show spherical or ovoid shape without internal echoes, a thin smooth wall separate from the surrounding parenchyma, and posterior wall enhancement caused by increased transmission through the water-filled cyst. The likelihood of malignancy is extremely low in a renal cyst that meets these criteria, which have a reported accuracy of 98% to 100%.3 Thus, no further evaluation is required if an obviously benign simple cyst is first noted on an adequate ultrasound. Inadequate ultrasound visualization or evidence of calcifications, septa, or multiple chambers calls for prompt renal CT.
CASE The mass on Ms. C’s left kidney is hypoattenuating and nonenhancing on CT. It meets Bosniak criteria for a benign simple cyst (class I) and requires no further evaluation or follow-up. Colonoscopy detects multiple colonic polyps that are removed, and the patient does well.
Mildly complicated cysts. Less diagnostic certainty characterizes cysts with mild abnormalities that keep them from being labeled as simple. Bosniak classes II and IIF describe mildly abnormal renal cysts. Class II cysts can be dismissed, whereas class IIF cysts require follow-up.
Class II cysts may contain a few hairline septa, fine calcium deposits in walls or septa, or an unmeasurable enhancement of the walls. A hyperattenuating but nonenhancing fluid also is described as category II. Small homogeneous cysts <3 cm, without enhancement but hyperattenuated, are reliably considered benign and need not be evaluated.2,7
Class IIF cysts may have multiple hairline-thin septa with unmeasurable enhancement or minimal smooth thickening or irregular/nodular calcifications of wall or septa without enhancing soft tissue components. Hyperattenuating cystic lesions >3 cm and intrarenal “noncortical” cysts are included in this category. Class IIF cysts require follow-up at 6 months with CT or MRI, then annually for at least 5 years.8
Obviously complicated cysts. Bosniak class III is indeterminate—neither benign nor clearly malignant. Class III cysts may have thickened borders or septa with measurable enhancement, or they may be multilocular, hemorrhagic, or infected. In 5 case series, 29 of 57 class III lesions proved to be malignant.5 MRI may characterize these lesions more definitively than CT prior to urologic referral.
Malignant cysts. Bosniak class IV renal lesions are clearly malignant, with large heterogeneous cysts or necrotic components, shaggy thickened walls, or enhancing soft tissue components separate from the wall or septa. Their unequivocal appearance results from solid tumor necrosis and liquefaction. Diagnosis is straightforward, and excision is indicated.2
A closer look at solid renal masses
Solid renal masses usually consist of enhancing tissue with little or no fluid. The goal of evaluation is to exclude malignancies, such as renal cell cancer, lymphomas, sarcomas, or metastasis. Benign solid masses include renal adenomas, angiomyolipomas, and oncocytomas, among others.
Several lesions can be diagnosed by appearance or symptoms:
Angiomyolipomas are recognized by their fat content within a noncalcified mass. Unenhanced CT usually is sufficient for diagnosis, unless the mass is very small or has atypical features.9
Vascular lesions can be identified because they enhance to the same degree as the vasculature. With the exception of inflammatory or vascular abnormalities, all enhancing lesions that do not contain fat should be presumed to be malignant.
In patients with a known extrarenal primary malignancy, 50% to 85% of incidental solid renal masses will represent metastatic disease.10 Percutaneous biopsy may be warranted to differentiate metastatic lesions from a secondary, primary (ie, renal cell carcinoma), or benign process.11
A study of 2770 solid renal mass excisions revealed that 12.8% were benign, with a direct relationship between malignancy and size. Masses <1 cm were benign 44% of the time.12 Early identification of small renal carcinomas may improve survival rates. Although renal cell carcinomas <3 cm in diameter have low metastatic potential, a solid, nonfat-containing mass should be evaluated for aggressive nephron-sparing surgery.6,13
Incidental adrenal masses occur infrequently
Adrenal incidentalomas are defined as radiographically identified masses >1 cm in diameter.14 They are much less common than their renal counterparts, with a reported prevalence of 0.35% to 5% on CT.15 Because the adrenal glands are hormonally active and receive substantial blood flow, metastatic, hormonally active, and nonfunctional causes for adrenal masses need to be considered.16
Adrenal pathology
Adrenal masses may be characterized by increased or normal adrenal function. Hyperfunctioning syndromes include hypercortisolism, hyperaldosteronism, adrenogenital hypersecretion of adrenocortical origin, and pheochromocytomas of the medulla. Symptom evaluation of these syndromes is important, but not sufficient to rule out a hyperfunctioning syndrome.
In a retrospective review of inapparent adrenal masses, ≤13% of pheochromocytomas were clinically silent.17 Therefore, laboratory testing is necessary for an incidental adrenal mass.
Nonfunctional lesions include adenomas, metastases, cysts, myelolipomas, hemorrhage, and adrenal carcinomas. These masses require evaluation for the possibility of cancer, the most common of which is metastasis. In patients with an extra-adrenal malignancy, the likelihood of malignancy in an incidental adrenal mass is at least 50%.18 An adrenal mass representing metastasis of a previously unrecognized cancer is exceedingly rare.19
Primary adrenal carcinoma is also rare, with an estimated incidence of 2 cases per one million in the general population. For patients with adrenal masses, the prevalence of carcinoma increases with lesion size (2% for tumors <4 cm, 6% for tumors 4-6 cm, and 25% for tumors >6 cm in diameter). 17 For this reason, tumors >4 cm in diameter are usually surgically resected in patients with no previous cancer history, unless radiologic criteria demonstrate clearly benign characteristics.
Although adrenal carcinomas are considered nonfunctioning, some evidence suggests they produce low levels of cortisol that may be associated with clinical features of metabolic syndrome.20
CT is first choice for adrenal mass evaluation
Dedicated adrenal CT with both unenhanced and delayed contrast-enhanced images is the most reliable study to evaluate an adrenal mass, according to the American College of Radiology. Consider another study only in patients with contrast allergy, renal compromise, or cancer history.21
Unenhanced CT can diagnose the approximately 70% of adenomas that are small, well-defined round masses with homogenous low-density lipid deposition.22 Delayed contrast enhancement can characterize most of the remaining 30%.23 Unenhanced CT with attenuation values of <10 Hounsfield units (HU) can diagnose adenomas with 71% specificity and 98% sensitivity,24 and can often diagnose simple cysts and myelolipomas, as well.
Other imaging options. MRI is an alternative to CT for patients with contraindications for contrast or radiation exposure. MRI provides less spatial resolution than CT, but chemical shift imaging can measure cytoplasmic lipid content similar to unenhanced CT. A small study found chemical shift MRI more reliable than unenhanced CT, but less reliable than CT with delayed contrast enhancement.25
Positron emission tomography (PET) is useful to noninvasively evaluate biochemical and physiologic processes. PET-CT incorporates unenhanced CT density measurements to improve PET accuracy. In a patient with a history of cancer, PET-CT has a sensitivity of 93% to 100% and a specificity of 95% in differentiating benign from malignant adrenal tumors.26
When to order a biopsy
The need for biopsy has decreased as imaging has improved, but biopsy is required whenever diagnostic imaging fails to differentiatea lesion as benign or malignant. CT guided biopsy provides diagnostic accuracy of 85% to 95%.27 Complications such as pneumothorax, hemorrhage, and bacteremia occur in 3% to 9% of biopsies. Before any adrenal biopsy, measure plasma-free metanephrines to exclude undiagnosed pheochromocytoma, which could precipitate a hypertensive crisis if untreated.22
These 3 laboratory screening tests are critical
Family physicians can perform the initial biochemical evaluation of an adrenal incidentaloma. Guidance is available from the National Institutes of Health (NIH)28 and the American Academy of Clinical Endocrinologists (AACE) (FIGURE 2).29
Regardless of signs or symptoms, perform screening laboratory tests for 3 types of adrenal hyperfunction: hypercortisolism, hyperaldosteronism, and hypersecretion of catecholamines (pheochromocytoma). Screening tests are not recommended for androgen hypersecretion, which is extremely rare and causes recognizable symptoms such as hirsutism (Table 2).29
Hypercortisolism occurs in approximately 5% of adrenal incidentalomas.30 An overnight dexamethasone suppression test (DST) is most reliable for screening, with sensitivity >95% for Cushing syndrome.31 The patient takes a 1-mg dose of oral dexamethasone at 11 pm, and a fasting plasma cortisol sample is drawn the next day at 8 am.
Dexamethasone binds to glucocorticoid receptors in the pituitary gland, suppressing adrenocorticotropic hormone secretion. Cortisol will be depressed the next morning unless the adrenal mass produces cortisol autonomously. Patients with a DST >5 mcg/dL—highly suggestive of Cushing syndrome—require further evaluation, and we suggest referral to an endocrinologist.
Hyperaldosteronism is seen in 1% to 2% of adrenal incidentalomas.32 The aldosterone- to-renin ratio (ARR) is recommended as a screening test for hyperaldosteronism, with an ARR >20 requiring further testing.33 Medications that may affect the ARR include beta-blockers, spironolactone, clonidine, diuretics, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers.29
Refer a patient with evidence of hyperaldosteronism to an endocrinologist and a surgeon with experience in managing these lesions. If the ARR test result suggests an aldosterone excess, a salt-loading test is used to verify failure of aldosterone suppression. Adrenal venous sampling is often performed prior to surgical removal to confirm that an incidentaloma is the source of hyperaldosteronism.
Pheochromocytoma. Approximately 5% of incidental adrenal lesions are pheochromocytomas.30 Many patients with these epinephrine/norepinephrine secreting tumors do not show the classic symptom triad of headache, palpitations, and diaphoresis, and approximately half have normal blood pressure.34
Identifying a pheochromocytoma is important in any patient requiring surgery or biopsy, as surgical manipulation can cause a potentially fatal intraoperative catecholamine surge. Presurgical medical management can mitigate this reaction.
A plasma-free metanephrines test, which has 95% sensitivity, is the most reliable test for pheochromocytoma.35 Medications, including tricyclic antidepressants, decongestants, amphetamines, reserpine, and phenoxybenzamine, can cause falsepositive results.29 Confirm a positive plasma-free metanephrines test with a 24-hour fractionated urine metanephrines test, and refer the patient to an endocrinologist.
Managing adrenal incidentalomas
Refer all patients with adrenal masses >4 cm for surgical evaluation because of the risk of malignancy; all patients who have a history of malignancy and an adrenal mass of any size require a referral to an oncologist. Perform the AACE-recommended 3-element biochemical workup for all masses, with the exception of definitively diagnosed cysts or myelolipomas.
Refer to an endocrinologist all patients with abnormal screening laboratory results, regardless of adrenal mass size, as well as patients with concerning clinical findings. Initiate cardiovascular, diabetes, and bone density evaluation and management for metabolic syndrome.20
Monitoring after a negative workup
Little evidence exists to guide monitoring of small adrenal incidentalomas (<4 cm) with a negative workup. The 2002 NIH report recommended annual radiologic follow-up for 5 years,28 whereas the 2009 AACE guidelines recommend radiographic follow-up at 3 to 6 months, then at one and 2 years.29
Evidence indicates that 14% of lesions will enlarge in 2 years, although the clinical significance of enlargement is unknown. Some authors argue against CT monitoring because the risk of adrenal mass progression is similar to the malignancy risk posed by 3 years of radiation exposure with CT.20
Some guidelines recommend repeat biochemical screening every 3 to 4 years.28,29 AACE guidelines quote a 47% rate of progression over 3 years, but most adrenal masses progress to subclinical Cushing syndrome— a condition of uncertain significance. Subclinical Cushing’s has not been reported to progress to the overt syndrome, and new catecholamine or aldosterone secretion is rare.
Many endocrinologists reduce the frequency of follow-up, depending on the type of adrenal mass (cyst or solid) and its size. AACE suggests CT for adenomas one to 4 cm at 12 months. AACE and NIH recommend hormonal evaluation annually for 4 years. Adrenal cysts or myelolipoma in patients without cancer need no follow-up.29
CORRESPONDENCE
James C. Higgins, DO, CAPT, MC, USN, Ret., Naval Hospital Jacksonville, Family Medicine Department, 2080 Child Street, Box 1000, Jacksonville, FL 32214;
[email protected]
1. Berland LL, Silverman SG, Gore RM, et al. Managing incidental findings on abdominal CT: white paper of the ACR incidental findings committee. J Am Coll Radiol. 2010;7:754-773.
2. Silverman S, Israel G, Herts B, et al. Management of the incidental renal mass. Radiology. 2008;249:16-31.
3. Curry NS, Bissada NK. Radiologic evaluation of small and indeterminate renal masses. Urol Clin North Am. 1997;24:493-505.
4. Bosniak MA. The current radiological approach to renal cysts. Radiology. 1986;158:1-10.
5. Harisinghani M, Maher M, Gervais D, et al. Incidence of malignancy in complex cystic renal masses (Bosniak category III): should imaging guided biopsy precede surgery? AJR Am J Roentgenol. 2003;180:755-758.
6. Remzi M, Ozsoy M, Klingler HC. Are small renal tumors harmless? Analysis of histopathological features according to tumors less than 4 cm in diameter. J Urol. 2006;176:896-899.
7. Jonisch AI, Rubinowitz A, Mutalik P, et al. Can high attenuation renal cysts be differentiated from renal cell carcinoma at unenhanced computed tomography? Radiology. 2007;243:445-450.
8. Israel GM, Bosniak MA. Follow-up CT of moderately complex cystic lesions of the kidney. AJR Am J Roentgenol. 2003;181: 627-633.
9. Bosniak MA, Megibow AJ, Hulnick DH, et al. CT diagnosis of renal angiomyolipoma: the importance of detecting small amounts of fat. AJR Am J Roentengol. 1988;151:497-501.
10. Mitnick JS, Bosniak MA, Rothberg M, et al. Metastatic neoplasm to the kidney studied by computed tomography and sonogram. J Comput Assist Tomogr. 1985;9:43-49.
11. Rybicki FJ, Shu KM, Cibas ES, et al. Percutaneous biopsy of renal masses: sensitivity and negative predictive value stratified by clinical setting and size of masses. AJR Am J Roentgenol. 2003;180:1281-1287.
12. Frank I, Blure MI, Cheville JC, et al. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol. 2003;170:2217-2220.
13. Hollingsworth JM, Miller DC, Daignault S, et al. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331-1334.
14. Geelhoed GW, Spiegel CT. “Incidental” adrenal cyst: a correctable lesion possibly associated with hypertension. South Med J. 1981;74:626-630.
15. Davenport C, Liew A, Doherty B, et al. The prevalence of adrenal incidentaloma in routine clinical practice. Endocrine. 2011;40: 80-83.
16. Cook DM, Loriaux LD. The incidental adrenal mass. Am J Med. 1996;101:88 94.
17. Mansmann G, Lau J, Balk E, et al. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev. 2004;25:309-340.
18. Androulakis II, Kaltsas G, Piatitis G, et al. The clinical significance of adrenal incidentalomas. Eur J Clin Invest. 2011;41: 552-560.
19. Lee JE, Evans DB, Hickey RC, et al. Unknown primary cancer presenting as an adrenal mass: frequency and implications for diagnostic evaluation of adrenal incidentalomas. Surgery. 1998;124:1115-1122.
20. Aron D, Terzolo M, Cawood TJ. Adrenal incidentalomas. Best Pract Res Clin Endocrinol Metab. 2012;26:69-82.
21. ACR appropriateness criteria: incidentally discovered adrenal mass. American College of Radiology. Available at: http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/IncidentallyDiscoveredAdrenalMass.pdf. Accessed November 20, 2012.
22. Song JH, Mayo-Smith WW. Incidentally discovered adrenal mass. Radiol Clin North Am. 2011;49:361-368.
23. Korobkin M, Brodeur FJ, Francis IR, et al. CT time-attenuation washout curves of adrenal adenomas and nonadenomas. AJR Am J Roentgenol. 1998;170:747-752.
24. Boland GW, Lee MJ, Gazelle GS, et al. Characterization of adrenal masses using unenhanced CT: an analysis of the CT literature. AJR Am J Roentgenol. 1998;171:201-204.
25. Park BK, Kim CK, Kim B, et al. Chemical shift MR imaging of hyperattenuating (>10 HU) adrenal masses: does it still have a role? Radiology. 2004;231:711-716.
26. Boland GW, Blake MA, Holakere NS, et al. PET/CT for the characterization of adrenal masses in patients with cancer: qualitative vs quantitative accuracy in 150 consecutive patients. AJR Am J Roentgenol. 2009;192:956-962.
27. Paulsen SD, Nghiem HV, Korobkin M, et al. Changing role of imaging- guided percutaneous biopsy of adrenal masses: evaluation of 50 adrenal biopsies. AJR Am J Roentgenol. 2004;182:1033-1037
28. Grumbach MM, Biller BMK, Braunstein GD, et al. Management of the clinically inapparent adrenal mass (“incidentalomas”). Ann Intern Med. 2003;138:424-429.
29. Zeiger MA, Thompson GB, Quan-Yang D, et al. American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons medical guidelines for the management of adrenal incidentalomas. Endocr Pract. 2009;15(suppl 1):1-20.
30. Young WF. The incidentally discovered adrenal mass. N Engl J Med. 2007; 356:601-610.
31. Deutschbein T, Unger N, Hinrichs J, et al. Late-night and lowdose dexamethasone-suppressed cortisol in saliva and serum for the diagnosis of cortisol-secreting adrenal adenomas. Eur J Endocrinol. 2009;161:747-753.
32. Bernini G, Moretti A, Gianfranco A, et al. Primary aldosteronism in normokalemic patients with adrenal incidentalomas. Eur J Endocrinol. 2002;146:523-529.
33. Montori VM, Young WF Jr. Use of plasma aldosterone concentration-to-plasma renin activity ratio as a screening test for primary aldosteronism: a systematic review of the literature. Endocrinol Metab Clin North Am. 2002;31:619-632.
34. Motta-Ramirez GA, Remer EM, Herts BR, et al. Comparison of CT findings in symptomatic and incidentally discovered pheochromocytomas. AJR Am J Roentgenol. 2005;185:684-688.
35. Pacak K, Eisenhofer G, Grossman A. The incidentally discovered adrenal mass. N Engl J Med. 2007;356:2005.
1. Berland LL, Silverman SG, Gore RM, et al. Managing incidental findings on abdominal CT: white paper of the ACR incidental findings committee. J Am Coll Radiol. 2010;7:754-773.
2. Silverman S, Israel G, Herts B, et al. Management of the incidental renal mass. Radiology. 2008;249:16-31.
3. Curry NS, Bissada NK. Radiologic evaluation of small and indeterminate renal masses. Urol Clin North Am. 1997;24:493-505.
4. Bosniak MA. The current radiological approach to renal cysts. Radiology. 1986;158:1-10.
5. Harisinghani M, Maher M, Gervais D, et al. Incidence of malignancy in complex cystic renal masses (Bosniak category III): should imaging guided biopsy precede surgery? AJR Am J Roentgenol. 2003;180:755-758.
6. Remzi M, Ozsoy M, Klingler HC. Are small renal tumors harmless? Analysis of histopathological features according to tumors less than 4 cm in diameter. J Urol. 2006;176:896-899.
7. Jonisch AI, Rubinowitz A, Mutalik P, et al. Can high attenuation renal cysts be differentiated from renal cell carcinoma at unenhanced computed tomography? Radiology. 2007;243:445-450.
8. Israel GM, Bosniak MA. Follow-up CT of moderately complex cystic lesions of the kidney. AJR Am J Roentgenol. 2003;181: 627-633.
9. Bosniak MA, Megibow AJ, Hulnick DH, et al. CT diagnosis of renal angiomyolipoma: the importance of detecting small amounts of fat. AJR Am J Roentengol. 1988;151:497-501.
10. Mitnick JS, Bosniak MA, Rothberg M, et al. Metastatic neoplasm to the kidney studied by computed tomography and sonogram. J Comput Assist Tomogr. 1985;9:43-49.
11. Rybicki FJ, Shu KM, Cibas ES, et al. Percutaneous biopsy of renal masses: sensitivity and negative predictive value stratified by clinical setting and size of masses. AJR Am J Roentgenol. 2003;180:1281-1287.
12. Frank I, Blure MI, Cheville JC, et al. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol. 2003;170:2217-2220.
13. Hollingsworth JM, Miller DC, Daignault S, et al. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331-1334.
14. Geelhoed GW, Spiegel CT. “Incidental” adrenal cyst: a correctable lesion possibly associated with hypertension. South Med J. 1981;74:626-630.
15. Davenport C, Liew A, Doherty B, et al. The prevalence of adrenal incidentaloma in routine clinical practice. Endocrine. 2011;40: 80-83.
16. Cook DM, Loriaux LD. The incidental adrenal mass. Am J Med. 1996;101:88 94.
17. Mansmann G, Lau J, Balk E, et al. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev. 2004;25:309-340.
18. Androulakis II, Kaltsas G, Piatitis G, et al. The clinical significance of adrenal incidentalomas. Eur J Clin Invest. 2011;41: 552-560.
19. Lee JE, Evans DB, Hickey RC, et al. Unknown primary cancer presenting as an adrenal mass: frequency and implications for diagnostic evaluation of adrenal incidentalomas. Surgery. 1998;124:1115-1122.
20. Aron D, Terzolo M, Cawood TJ. Adrenal incidentalomas. Best Pract Res Clin Endocrinol Metab. 2012;26:69-82.
21. ACR appropriateness criteria: incidentally discovered adrenal mass. American College of Radiology. Available at: http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/IncidentallyDiscoveredAdrenalMass.pdf. Accessed November 20, 2012.
22. Song JH, Mayo-Smith WW. Incidentally discovered adrenal mass. Radiol Clin North Am. 2011;49:361-368.
23. Korobkin M, Brodeur FJ, Francis IR, et al. CT time-attenuation washout curves of adrenal adenomas and nonadenomas. AJR Am J Roentgenol. 1998;170:747-752.
24. Boland GW, Lee MJ, Gazelle GS, et al. Characterization of adrenal masses using unenhanced CT: an analysis of the CT literature. AJR Am J Roentgenol. 1998;171:201-204.
25. Park BK, Kim CK, Kim B, et al. Chemical shift MR imaging of hyperattenuating (>10 HU) adrenal masses: does it still have a role? Radiology. 2004;231:711-716.
26. Boland GW, Blake MA, Holakere NS, et al. PET/CT for the characterization of adrenal masses in patients with cancer: qualitative vs quantitative accuracy in 150 consecutive patients. AJR Am J Roentgenol. 2009;192:956-962.
27. Paulsen SD, Nghiem HV, Korobkin M, et al. Changing role of imaging- guided percutaneous biopsy of adrenal masses: evaluation of 50 adrenal biopsies. AJR Am J Roentgenol. 2004;182:1033-1037
28. Grumbach MM, Biller BMK, Braunstein GD, et al. Management of the clinically inapparent adrenal mass (“incidentalomas”). Ann Intern Med. 2003;138:424-429.
29. Zeiger MA, Thompson GB, Quan-Yang D, et al. American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons medical guidelines for the management of adrenal incidentalomas. Endocr Pract. 2009;15(suppl 1):1-20.
30. Young WF. The incidentally discovered adrenal mass. N Engl J Med. 2007; 356:601-610.
31. Deutschbein T, Unger N, Hinrichs J, et al. Late-night and lowdose dexamethasone-suppressed cortisol in saliva and serum for the diagnosis of cortisol-secreting adrenal adenomas. Eur J Endocrinol. 2009;161:747-753.
32. Bernini G, Moretti A, Gianfranco A, et al. Primary aldosteronism in normokalemic patients with adrenal incidentalomas. Eur J Endocrinol. 2002;146:523-529.
33. Montori VM, Young WF Jr. Use of plasma aldosterone concentration-to-plasma renin activity ratio as a screening test for primary aldosteronism: a systematic review of the literature. Endocrinol Metab Clin North Am. 2002;31:619-632.
34. Motta-Ramirez GA, Remer EM, Herts BR, et al. Comparison of CT findings in symptomatic and incidentally discovered pheochromocytomas. AJR Am J Roentgenol. 2005;185:684-688.
35. Pacak K, Eisenhofer G, Grossman A. The incidentally discovered adrenal mass. N Engl J Med. 2007;356:2005.
Hip fracture in older patients: Tips and tools to speed recovery
› Ensure that surgical stabilization of hip fracture is performed as soon as possible—ideally within 48 hours of injury. A
› To reduce the risk of delirium, orient the patient frequently; get her out of bed as soon as possible, and avoid prolonged catheter use. A
› Order protein supplements for patients recovering from hip fracture and take steps to facilitate an early return to eating. C
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
The patient and family request a consultation with Ms. J’s primary care physician. If you were her physician, what would you advise?
Hip fracture in a frail elderly patient is an injury that, while common, can be difficult to manage. With good reason. Geriatric hip fracture is associated with increased morbidity, functional decline, and use of nursing home services, as well as a higher mortality rate: One in 5 hip fracture patients dies within a year of the injury.1
As the population ages, we are seeing more hip fractures in the “oldest old” those who, like Ms. J, are older than 85. While the incidence increases exponentially with age in both men and women, women are 3 times more likely than men to sustain a hip fracture.2 White women ages 85 to 95 face the highest risk, with an incidence of more than 3%.3
In addition to managing the acute phase of hip fracture and helping patients and families make decisions about optimal treatment, there is much you can do to boost the likelihood of a rapid rehabilitation and a successful outcome.
What type of fracture? How best to treat it?
Two types of hip fractures are responsible for the vast majority of cases: About 45% of hip fractures are intracapsular, involving the femoral head and neck; another 45% are intertrochanteric fractures. Both usually involve low-energy trauma, such as a fall from a chair or tripping over a rug. Intertrochanteric and subtrochanteric fractures (the latter accounting for the remaining 10%) are extracapsular.2,4,5
Typically associated with high-energy trauma such as a motor vehicle accident, or with metastatic lesions, subtrochanteric fractures have a bimodal distribution: They are most common in individuals between the ages of 20 and 40 and those older than 60.2
Fractures involving the femoral neck can disrupt the vascular supply to the femoral head and result in avascular necrosis (AVN) or nonunion.2,4,5 A meta-analysis of the outcome of displaced femoral neck fractures found the rates of osteonecrosis and nonunion to be as high as 20% to 30%.5 Intertrochanteric fractures rarely lead to AVN or nonunion, but patients may develop complications associated with degenerative changes.2,4,5 Nonunion is a potential complication of subtrochanteric fracture.2
For most patients, surgical management is preferred
The main goals of treatment are to stabilize the hip, decrease pain and restore the level of prefracture function. Surgery is the preferred treatment for hip fracture because it provides stable fixation, facilitating full weight bearing and decreasing the risk of complications. Surgery is also associated with a shorter stay in the hospital and improved rehabilitation and recovery.6
Surgical stabilization should be performed as soon as possible—ideally, within 48 hours.5 A recent study found conflicting evidence of the effect of delayed surgery on mortality, but demonstrated that surgery within 24 hours of injury minimizes the rate of chest infections, urinary tract infections, and pressure sores, as well as the duration of the hospital stay.7 (To learn more about surgical stabilization of hip fracture, see “What type of surgery? Age is just one consideration” 5,8-10 below.)
When surgery is contraindicated
Nonoperative management is reserved for patients who stand to gain only minimal function from surgical stabilization, because they either were not ambulatory to begin with or have severe dementia. In addition, medical management is used for patients with contraindications to anesthesia, those who delay seeking medical care until the fracture has begun to heal, and patients who refuse surgical fixation.5,11
The choice of surgical intervention depends on multiple factors, including the:
- type and severity of the fracture
- preference of the orthopedic surgeon
- age of the patient
- comorbid conditions
- prognosis.
For femoral neck fractures, patients younger than 65 years are candidates for internal fixation; for older individuals and those who already had limited mobility, arthroplasty should be considered.5 Studies of pain and functional outcomes show a modest tendency for total hip arthroplasty to have better results than internal fixation in patients older than 65.8
Intertrochanteric fractures can be treated with either sliding hip screws or
intramedullary nails. Intramedullary nail implants are done percutaneously, resulting in a shorter duration for surgery, less blood loss, and an earlier return to full weight bearing.5 A recent study suggests that intramedullary nails result in more reoperations than hip screws.9 No evidence is conclusive about the superiority of either type of hardware.
Subtrochanteric fractures are typically repaired by hemiarthroplasty.
A Cochrane review of randomized controlled trials found insufficient evidence to determine whether replacement arthroplasty has any advantage over internal fixation for extracapsular hip fractures.10
CASE After a careful review of Ms. J’s health status, radiographs of the fracture (FIGURE 1A), and consultation with an orthopedic surgeon and a geriatrician, you recommend surgery as soon as the patient is fully stabilized. Without it, she would be at high risk for urinary tract infection, pressure sores, and thromboembolism associated with long-term immobility.
The next day, Ms. J undergoes surgical fixation with a sliding hip screw (FIGURE 1B). Her Foley catheter is removed the same day, and physical therapy is begun the following day. On postoperative day 4 she is discharged to an in patient rehabilitation facility.
Begin rehabilitation without delay
Whether a patient has surgery or is treated nonoperatively for hip fracture, the goal of rehabilitation is the same—to restore mobility as quickly as possible. A clinical review found no significant difference in mortality rates between those who underwent surgical fixation and those who were treated medically with early mobilization, consisting of immediate bed-to-chair transfer (with assistance), followed by progression to ambulation as tolerated.12
For patients who undergo surgery for hip fracture, increased immobility is linked to poorer functioning in the areas of self-care and transfers at 2 months and to higher mortality rates at 6 months.13 Physical therapy should be initiated on the first postoperative day and should start with bed mobility range of motion, followed by independent transfers from bed to chair, and ultimately achieving full weight bearing.5
Many complications are predictable, and often preventable
The term “hip fracture syndrome”4 is often used in reference to a cluster of common (and often preventable) complications of hip fracture, with delirium, venous thromboembolism (VTE), and malnutrition foremost among them.
Take steps to prevent—or treat—delirium
Delirium is among the most common complication, occurring in up to 62% of older patients with hip fracture.4 The highest predictor of delirium is preexisting cognitive impairment.
Other risk factors for delirium include advanced age, vision or hearing impairment, concurrent alcohol abuse, malnutrition, comorbidity, and polypharmacy.4,14 Delirium is associated with increased morbidity and mortality, decreased rehabilitation potential, and poor functional recovery independent of prior frailty.4,15,16
Hypoactive delirium is easily missed. While agitated, or hyperactive, delirium is more easily recognized, it is crucial to be aware of hypoactive delirium, as well. Patients with hypoactive delirium tend to become more withdrawn and their delirium is easily missed, leading to worse outcomes.15 The Confusion Assessment Method (TABLE 1)17 is an easy-to-use validated tool developed to aid in the diagnosis of delirium at the bedside.
Many factors contribute to the development of delirium. Medical complications, such as infection, electrolyte and volume imbalances, hypoxia, and myocardial infarction, are obvious precipitants.15 Disturbances in sleep-wake cycles, an unfamiliar environment, physical restraints, and the use of Foley catheters—all of which can impair an older patient’s sensory awareness—are less well-known contributing factors.
Tips for preventing delirium. Early mobilization, in addition to boosting physical recovery, can help prevent delirium.
Other tips:
- discontinue catheterization as soon as possible; this may help prevent delirium, and lessen the risk of urinary tract infection.
- remind nurses and family members to continuously reorient patients to their surroundings.
- treat pain aggressively.
- consult a geriatrician early on.
While opioids are often thought to cause delirium, several studies have shown an inverse relationship—that is, hip fracture patients who were given opioids for pain were actually less likely to develop delirium than those who did not receive opioids. This raises 2 important points:
1. untreated pain may itself be a significant risk factor for delirium,15,18 and
2. delirium itself is not a contraindication to opioids.18
CASE In her first week at the inpatient rehabilitation center, Ms. J requires slightly more narcotic medication for pain control. The staff notices increased confusion and a decrease in the number of bowel movements. Ms. J is started on a regimen of sennosides and docusate twice daily. Her mental status improves quickly and she has no further complications while at the rehab center.
Nonopioid pain medications such as acetaminophen should be scheduled at appropriate doses (eg, 1 g tid). Ensure that patients recovering from hip fracture are not given benzodiazepines, anticholinergics, or antihistamines15— which are sometimes included in a facility’s PRN protocol. In clinical trials, prophylactic administration of antipsychotics or anticholinesterase therapy to high-risk patients has had conflicting results.19,20
Arrange for a geriatric consult before problems occur. Several studies have shown that a geriatric consultation and concurrent management by a geriatrician using structured protocols to evaluate for common risk factors known to precipitate delirium (eg, pain, bowel/bladder function, nutrition, mobilization) can reduce the risk of delirium.16
Provide supportive care. Although treatment of the underlying cause is the definitive treatment for delirium, there are times when supportive care is all that’s needed. Reassurance from family members or staff is the recommended first step. Physical restraints should be avoided unless patient safety is threatened despite attempts to provide supportive care.
If treatment for delirium is needed, lowdose antipsychotics are recommended. The most studied agent is haloperidol, which can be administered intravenously (IV), intramuscularly (IM), or orally. Monitoring the corrected QT (QTc) interval is recommended for patients taking haloperidol, and discontinuation of the drug—or a cardiology consult— is recommended if the QTc interval is prolonged (>450 ms or >25% of baseline).21
There is a slightly higher risk of cardiac arrhythmias with IV administration of haloperidol compared with IM or oral dosing. Despite this risk, haloperidol IV is the treatment of choice for delirium.21 Newer atypical antipsychotics have also been used to treat delirium, but data are limited.21
Guard against VTE
Studies have shown rates of VTE to be as high as 40% to 60% after orthopedic procedures, and prophylaxis has long been the standard of care.22 In its 2012 consensus guidelines for antithrombotic therapy, the American College of Chest Physicians (ACCP) recommends fondiparinux, apixaban, rivaroxaban, dabigatran, low-molecular-weight heparin (LMWH), low-dose unfractionated heparin, aspirin, warfarin, or an intermittent pneumatic compression device (IPCD) as prophylaxis.23 Portable battery-powered IPCDs are recommended for 18 hours postop.23
The guideline authors prefer LMWH to the other treatments, and recommend dual prophylaxis with an IPCD and an antithrombotic agent while the patient is in the hospital and for a minimum of 10 to 14 days (and up to 35 days) after discharge. If surgery for hip fracture is delayed, the ACCP recommends that LMWH be administered after admission, but withheld for at least 12 hours before surgery. In patients with a high risk of bleeding, the ACCP recommends either an IPCD alone or no prophylaxis and notes that inferior vena cava filters should not be placed in high-risk patients.23
Take steps to ensure ample protein intake
Malnourishment is another common complication, affecting up to 20% of hip fracture patients.24 In many cases, a catabolic state predisposes patients to protein depletion, leading to decreased wound healing and an increase in other postop complications.24,25 Protein supplementation is associated with decreased length of stay and a reduction in postop complications.26
This complication can often be avoided by encouraging an early return to eating. Specific steps: Ensure that patients have their dentures available and are able to use them; are positioned properly for eating; and receive high-caloric supplemental drinks. Nutritional assessments should also be done to ensure that their intake of calcium and vitamin D is sufficient to prevent future falls and reduce fracture risk. (For more information, see “Vitamin D: When it helps, when it harms” [J Fam Pract. 2013;62:368-370.])
Combat hip fracture by stressing avoidance
Prevention of hip fracture, of course, is the ideal way to reduce the burden of disease for older patients. Along these lines, there are many ways you can help.
Start with fall reduction
Hip fracture is associated with a fall 90% of the time,27 and care for older patients should be focused on reducing the risk for falls and improving bone health and muscular function. While a complete review of preventive measures is beyond the scope of this article, we offer some highlights here and in TABLE 2.
Encourage physical activity In addition to helping to reduce falls, physical activity—particularly repetitive weight-bearing exercise—can help maintain bone density and improve muscle mass, strength, and balance.28
Rather than focus on a single exercise, however, a combination of activities—Tai Chi and walking, for instance, or weight lifting and cycling —appears to have the best likelihood of fall reduction.29 Whenever possible, physical activity for older patients should include challenges in executive function, as well. In a recent study comparing regular walking with trail-walking between sequentially marked flags, participants in the more complex activity had a greater decrease in fall rates.30
Review vitamin D and calcium intake. Elderly patients with low levels of vitamin D are at increased risk of muscle mass decline, and therefore increased risk of fracture.31 A systematic review and meta-analysis of vitamin D supplementation in older adults found the relative risk of falling was 0.86 (95% confidence interval [CI], 0.79-0.93) for those assigned to vitamin D therapy compared with those on placebo. Risk reduction was greater in groups taking 800 IU or more of vitamin D daily and those taking adjunctive calcium supplementation.32
Maximizing vitamin D for falls reduction is supported by the American Geriatrics Society, 33 the Agency for Healthcare Research and Quality (AHRQ),34 and the US Preventive Services Task Force (USPSTF).35 The USPSTF recently released a recommendation for exercise or physical therapy and vitamin D supplementation (800 IU) to prevent falls in community-dwelling adults ages 65 and over who are at an increased risk for falls.36
However, the USPSTF advises against daily supplementation with vitamin D and calcium at doses ?400 IU and 1000 mg, respectively, for noninstitutionalized postmenopausal women for primary fracture prevention. Calcium supplementation has not been shown to reduce hip fractures, but has been found to improve hip bone density.37
Consider bisphosphonates. Order a dual energy x-ray absorptiometry (DEXA) scan for older patients to identify osteoporosis. Most hip fractures are osteoporotic, and patients should be started on bisphosphonates within 2 to 12 weeks of injury38 to reduce the risk of mortality associated with hip fracture.39 The most studied bisphosphonates in geriatric hip fracture are alendronate, risedronate, and zoledronate; all were found to have a number needed to treat of 91 to prevent one hip fracture.40
Focus on the home environment. In addition to addressing the bone and muscular health of older patients, focus should be placed on the home environment. A Cochrane review of fall prevention for those living in the community found that home safety interventions reduced the risk of falls, but only for those with severe vision impairment and a high risk of falls.29 A 2010 American Geriatric Society (AGS) and British Geriatric Society (BGS) review of fall prevention gave an A recommendation—the highest rating— to home assessment and intervention by a health care professional to identify home hazards and promote safe performance of daily activities.33
Conduct brown-bag reviews. Polypharmacy is a well-documented (and growing) problem among the elderly.41 Both the AGS and BGS encourage a review of medications (including over-the-counter products) and interactions at each office visit,33 with specific attention paid to drugs that may cause dizziness, drowsiness, and near syncopal or syncopal episodes.
To reduce the risk of medication interactions and adverse effects, look for opportunities to reduce the number of drugs your elderly patients are taking. Consider involving a clinical pharmacist in medication reviews—an intervention that has been shown to be cost effective and lead to better patient outcomes.42
CASE After 4 weeks, Ms. J is ready to return home. Rather than a return to independent living, however, her children convince her to move to an assisted living facility—a move you strongly support. You schedule a visit in 2 weeks.
CORRESPONDENCE
Jeremy D. Close, MD, Department of Family and Community Medicine, Thomas Jefferson University, 833 Chestnut Street #301, Philadelphia, PA 19107; [email protected]
1. Leibson CL, Toteson ANA, Gabriel SE, et al. Mortality, disability, and nursing home use for persons with and without hip fracture: a population-based study. J Am Geriatr Soc. 2002;50:1644-50.
2. Brunner LC, Eshilian-Oates L, Kuo TY. Hip fractures in adults. Am Fam Physician. 2003;67:537-542.
3. Jacobsen SJ, Goldberg J, Miles TP, et al. Hip fracture incidence among the old and very old: a population-based study of 745,435 cases. Am J Public Health. 1990;80:871-873.
4. Auron-Gomez M, Michota F. Medical management of hip fracture. Clin Geriatr Med. 2008;24:701-719.
5. Jackman JM, Watson JT. Hip fractures in older men. Clin Geriatr Med. 2010;26:311-329.
6. Handoll HH, Parker MJ. Conservative versus operative treatment for hip fractures in adults. Cochrane Database Syst Rev. 2008;(3):CD000337.
7. Leung F, Lau W, Kwan K, et al. Does timing of surgery matter in fragility hip fractures? Osteoporos Int. 2010; 21(suppl 4):S529-S534.
8. Butler M, Forte ML, Joglekar SB, et al. Evidence summary: systematic review of surgical treatments for geriatric hip fractures. J Bone Joint Surg Am. 2011;93:1104-1115.
9. Matre K, Havelin LI, Gjertsen JE, et al. Intramedullary nails result in more reoperations than sliding hip screws in two-part intertrochanteric fractures. Clin Orthop Relat Res. 2013;471: 1379-1386.
10. Parker MJ, Handoll HH. Replacement arthroplasty versus internal fixation for extracapsular hip fractures in adults. Cochrane Database Syst Rev. 2006;(2):CD000086.
11. Cummings-Vaughn LA, Gammack JK. Falls, osteoporosis, and hip fractures. Med Clin North Am. 2011;95:495-506.
12. Jain R, Basinski A, Kreder HJ. Nonoperative treatment of hip fractures. Int Orthop. 2003;27:11-17.
13. Siu A, Penrod J, Boockvar K, et al. Early ambulation after hip fracture: effects on function and mortality. Arch Intern Med. 2006;166:766-771.
14. Juliebø V, Bjøro K, Krogseth M, et al. Risk factors for preoperative and postoperative delirium in elderly patients with hip fracture. J Am Geriatr Soc. 2009;57:1354-1361.
15. Flinn DR, Deihl KM, Seyfried LS, et al. Prevention, diagnosis, and management of postoperative delirium in older adults. J Am Coll Surg. 2009;209:261-268.
16. Marcantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49:516-522.
17. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941-948.
18. Sieber FE, Mears S, Lee H, et al. Postoperative opioid consumption and its relationship to cognitive function in older adults with hip fracture. J Am Geriatr Soc. 2011;59:2256-2262.
19. Prakanrattana U, Prapaitrakool S. Efficacy of risperidone for Rather than focus on a single exercise, a combination of activities—eg, Tai Chi and walking, or weight lifting and cycling—have the greatest likelihood of fall reduction. prevention of postoperative delirium in cardiac surgery. Anaesth Intensive Care. 2007;35:714-719.
20. Sampson EL, Raven PR, Ndhlovu PN, et al. A randomized, double-blind, placebo-controlled trial of donepezil hydrochloride (Aricept) for reducing the incidence of postoperative delirium after elective total hip replacement. Int J Geriatr Psychiatry. 2007;22:343-349.
21. Jacobi J, Fraser GL, Coursin DB, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30:119-141.
22. Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference of Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(suppl):338S-400S.
23. Guyatt GH, Akl EA, Crowther M, et al. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):7S-47S.
24. Garcia Lazaro M, Montero Perez-Barquero M, Carpintero Benitez P. The role of malnutrition and other medical factors in the evolution of patients with hip fracture [article in Spanish]. An Med Interna. 2004;21:557-563.
25. Lavernia CJ, Sierra RJ, Baerga L. Nutritional parameters and short term outcome in arthroplasty. J Am Coll Nutr. 1999;18:274-278.
26. Huddleston JM, Whitford KJ. Medical care of elderly patients with hip fractures. Mayo Clin Proc. 2001;76:295-298.
27. Cummings SR, Kelsey JL, Nevitt MC, et al. Epidemiology of osteoporosis and osteoporotic fractures. Epidemiol Rev. 1985;7:178-208.
28. Nelson ME, Fiatarone MA, Morganti CM, et al. Effects of high-intensity strength training on multiple risk factors for osteoporotic fractures a randomized controlled trial. JAMA. 1994;272:1909-1914.
29. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2009;(2):CD007146.
30. Yamada M, Tanaka B, Nagai K, et al. Trail-walking exercise and fall risk factors in community-dwelling older adults: preliminary results of a randomized controlled trial. J Am Geriatr Soc. 2010;58:1946-1951.
31. Visser M, Deeg DJ, Lips P; Longitudinal Aging Study Amsterdam. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003;88:5766-5772.
32. Kalyani RR, Stein B, Valiyil R, et al. Vitamin D treatment for the prevention of falls in older adults: systematic review and metaanalysis. J Am Geriatr Soc. 2010;58:1299-1310.
33. The American Geriatrics Society. Prevention of falls in older persons [clinical practice guideline]. 2010. Available at: http:// www.americangeriatrics.org/health_care_professionals/clinical_practice/clinical_guidelines_recommendations/ 2010/. Accessed August 16, 2013.
34. Cranney A, Horsley T, O’Donnell S, et al. Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess (Full Rep). 2007;(158):1-235.
35. Michael YL, Whitlock EP, Lin JS, et al. Primary care-relevant interventions to prevent falling in older adults: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153:815-825.
36. USPSTF. Prevention of falls in community-dwelling older adults. US Preventive Services Task Force recommendation statement. May 2012. Available at: www.uspreventiveservices taskforce.org/uspstf11/fallsprevention/fallsprevrs.htm. Accessed August 19, 2013.
37. Jackson RD, LaCroix AZ, Gass M, et al; Women’s Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669-683.
38. Lyles KW, Colón-Emeric CS, Magaziner JS, et al; for the HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357:1799-1809.
39. Beaupre LA, Morrish DW, Hanley DA, et al. Oral bisphosphonates are associated with reduced mortality after hip fracture. Osteoporos Int. 2011;22:983-991.
40. Ringe, JD, Doherty, JG. Absolute risk reduction in osteoporosis: assessing treatment efficacy by number needed to treat. Rheumatol Int. 2010;30:863-869.
41. Veehof L, Stewart R, Haaijer-Ruskamp F, et al. The development of polypharmacy. A longitudinal study. Fam Pract. 2000;17:261-267.
42. Choe HM, Farris KB, Stevenson JG, et al. Patient-centered medical home: developing, expanding, and sustaining a role for pharmacists. Am J Health Syst Pharm. 2012;69:1063-1071.
› Ensure that surgical stabilization of hip fracture is performed as soon as possible—ideally within 48 hours of injury. A
› To reduce the risk of delirium, orient the patient frequently; get her out of bed as soon as possible, and avoid prolonged catheter use. A
› Order protein supplements for patients recovering from hip fracture and take steps to facilitate an early return to eating. C
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
The patient and family request a consultation with Ms. J’s primary care physician. If you were her physician, what would you advise?
Hip fracture in a frail elderly patient is an injury that, while common, can be difficult to manage. With good reason. Geriatric hip fracture is associated with increased morbidity, functional decline, and use of nursing home services, as well as a higher mortality rate: One in 5 hip fracture patients dies within a year of the injury.1
As the population ages, we are seeing more hip fractures in the “oldest old” those who, like Ms. J, are older than 85. While the incidence increases exponentially with age in both men and women, women are 3 times more likely than men to sustain a hip fracture.2 White women ages 85 to 95 face the highest risk, with an incidence of more than 3%.3
In addition to managing the acute phase of hip fracture and helping patients and families make decisions about optimal treatment, there is much you can do to boost the likelihood of a rapid rehabilitation and a successful outcome.
What type of fracture? How best to treat it?
Two types of hip fractures are responsible for the vast majority of cases: About 45% of hip fractures are intracapsular, involving the femoral head and neck; another 45% are intertrochanteric fractures. Both usually involve low-energy trauma, such as a fall from a chair or tripping over a rug. Intertrochanteric and subtrochanteric fractures (the latter accounting for the remaining 10%) are extracapsular.2,4,5
Typically associated with high-energy trauma such as a motor vehicle accident, or with metastatic lesions, subtrochanteric fractures have a bimodal distribution: They are most common in individuals between the ages of 20 and 40 and those older than 60.2
Fractures involving the femoral neck can disrupt the vascular supply to the femoral head and result in avascular necrosis (AVN) or nonunion.2,4,5 A meta-analysis of the outcome of displaced femoral neck fractures found the rates of osteonecrosis and nonunion to be as high as 20% to 30%.5 Intertrochanteric fractures rarely lead to AVN or nonunion, but patients may develop complications associated with degenerative changes.2,4,5 Nonunion is a potential complication of subtrochanteric fracture.2
For most patients, surgical management is preferred
The main goals of treatment are to stabilize the hip, decrease pain and restore the level of prefracture function. Surgery is the preferred treatment for hip fracture because it provides stable fixation, facilitating full weight bearing and decreasing the risk of complications. Surgery is also associated with a shorter stay in the hospital and improved rehabilitation and recovery.6
Surgical stabilization should be performed as soon as possible—ideally, within 48 hours.5 A recent study found conflicting evidence of the effect of delayed surgery on mortality, but demonstrated that surgery within 24 hours of injury minimizes the rate of chest infections, urinary tract infections, and pressure sores, as well as the duration of the hospital stay.7 (To learn more about surgical stabilization of hip fracture, see “What type of surgery? Age is just one consideration” 5,8-10 below.)
When surgery is contraindicated
Nonoperative management is reserved for patients who stand to gain only minimal function from surgical stabilization, because they either were not ambulatory to begin with or have severe dementia. In addition, medical management is used for patients with contraindications to anesthesia, those who delay seeking medical care until the fracture has begun to heal, and patients who refuse surgical fixation.5,11
The choice of surgical intervention depends on multiple factors, including the:
- type and severity of the fracture
- preference of the orthopedic surgeon
- age of the patient
- comorbid conditions
- prognosis.
For femoral neck fractures, patients younger than 65 years are candidates for internal fixation; for older individuals and those who already had limited mobility, arthroplasty should be considered.5 Studies of pain and functional outcomes show a modest tendency for total hip arthroplasty to have better results than internal fixation in patients older than 65.8
Intertrochanteric fractures can be treated with either sliding hip screws or
intramedullary nails. Intramedullary nail implants are done percutaneously, resulting in a shorter duration for surgery, less blood loss, and an earlier return to full weight bearing.5 A recent study suggests that intramedullary nails result in more reoperations than hip screws.9 No evidence is conclusive about the superiority of either type of hardware.
Subtrochanteric fractures are typically repaired by hemiarthroplasty.
A Cochrane review of randomized controlled trials found insufficient evidence to determine whether replacement arthroplasty has any advantage over internal fixation for extracapsular hip fractures.10
CASE After a careful review of Ms. J’s health status, radiographs of the fracture (FIGURE 1A), and consultation with an orthopedic surgeon and a geriatrician, you recommend surgery as soon as the patient is fully stabilized. Without it, she would be at high risk for urinary tract infection, pressure sores, and thromboembolism associated with long-term immobility.
The next day, Ms. J undergoes surgical fixation with a sliding hip screw (FIGURE 1B). Her Foley catheter is removed the same day, and physical therapy is begun the following day. On postoperative day 4 she is discharged to an in patient rehabilitation facility.
Begin rehabilitation without delay
Whether a patient has surgery or is treated nonoperatively for hip fracture, the goal of rehabilitation is the same—to restore mobility as quickly as possible. A clinical review found no significant difference in mortality rates between those who underwent surgical fixation and those who were treated medically with early mobilization, consisting of immediate bed-to-chair transfer (with assistance), followed by progression to ambulation as tolerated.12
For patients who undergo surgery for hip fracture, increased immobility is linked to poorer functioning in the areas of self-care and transfers at 2 months and to higher mortality rates at 6 months.13 Physical therapy should be initiated on the first postoperative day and should start with bed mobility range of motion, followed by independent transfers from bed to chair, and ultimately achieving full weight bearing.5
Many complications are predictable, and often preventable
The term “hip fracture syndrome”4 is often used in reference to a cluster of common (and often preventable) complications of hip fracture, with delirium, venous thromboembolism (VTE), and malnutrition foremost among them.
Take steps to prevent—or treat—delirium
Delirium is among the most common complication, occurring in up to 62% of older patients with hip fracture.4 The highest predictor of delirium is preexisting cognitive impairment.
Other risk factors for delirium include advanced age, vision or hearing impairment, concurrent alcohol abuse, malnutrition, comorbidity, and polypharmacy.4,14 Delirium is associated with increased morbidity and mortality, decreased rehabilitation potential, and poor functional recovery independent of prior frailty.4,15,16
Hypoactive delirium is easily missed. While agitated, or hyperactive, delirium is more easily recognized, it is crucial to be aware of hypoactive delirium, as well. Patients with hypoactive delirium tend to become more withdrawn and their delirium is easily missed, leading to worse outcomes.15 The Confusion Assessment Method (TABLE 1)17 is an easy-to-use validated tool developed to aid in the diagnosis of delirium at the bedside.
Many factors contribute to the development of delirium. Medical complications, such as infection, electrolyte and volume imbalances, hypoxia, and myocardial infarction, are obvious precipitants.15 Disturbances in sleep-wake cycles, an unfamiliar environment, physical restraints, and the use of Foley catheters—all of which can impair an older patient’s sensory awareness—are less well-known contributing factors.
Tips for preventing delirium. Early mobilization, in addition to boosting physical recovery, can help prevent delirium.
Other tips:
- discontinue catheterization as soon as possible; this may help prevent delirium, and lessen the risk of urinary tract infection.
- remind nurses and family members to continuously reorient patients to their surroundings.
- treat pain aggressively.
- consult a geriatrician early on.
While opioids are often thought to cause delirium, several studies have shown an inverse relationship—that is, hip fracture patients who were given opioids for pain were actually less likely to develop delirium than those who did not receive opioids. This raises 2 important points:
1. untreated pain may itself be a significant risk factor for delirium,15,18 and
2. delirium itself is not a contraindication to opioids.18
CASE In her first week at the inpatient rehabilitation center, Ms. J requires slightly more narcotic medication for pain control. The staff notices increased confusion and a decrease in the number of bowel movements. Ms. J is started on a regimen of sennosides and docusate twice daily. Her mental status improves quickly and she has no further complications while at the rehab center.
Nonopioid pain medications such as acetaminophen should be scheduled at appropriate doses (eg, 1 g tid). Ensure that patients recovering from hip fracture are not given benzodiazepines, anticholinergics, or antihistamines15— which are sometimes included in a facility’s PRN protocol. In clinical trials, prophylactic administration of antipsychotics or anticholinesterase therapy to high-risk patients has had conflicting results.19,20
Arrange for a geriatric consult before problems occur. Several studies have shown that a geriatric consultation and concurrent management by a geriatrician using structured protocols to evaluate for common risk factors known to precipitate delirium (eg, pain, bowel/bladder function, nutrition, mobilization) can reduce the risk of delirium.16
Provide supportive care. Although treatment of the underlying cause is the definitive treatment for delirium, there are times when supportive care is all that’s needed. Reassurance from family members or staff is the recommended first step. Physical restraints should be avoided unless patient safety is threatened despite attempts to provide supportive care.
If treatment for delirium is needed, lowdose antipsychotics are recommended. The most studied agent is haloperidol, which can be administered intravenously (IV), intramuscularly (IM), or orally. Monitoring the corrected QT (QTc) interval is recommended for patients taking haloperidol, and discontinuation of the drug—or a cardiology consult— is recommended if the QTc interval is prolonged (>450 ms or >25% of baseline).21
There is a slightly higher risk of cardiac arrhythmias with IV administration of haloperidol compared with IM or oral dosing. Despite this risk, haloperidol IV is the treatment of choice for delirium.21 Newer atypical antipsychotics have also been used to treat delirium, but data are limited.21
Guard against VTE
Studies have shown rates of VTE to be as high as 40% to 60% after orthopedic procedures, and prophylaxis has long been the standard of care.22 In its 2012 consensus guidelines for antithrombotic therapy, the American College of Chest Physicians (ACCP) recommends fondiparinux, apixaban, rivaroxaban, dabigatran, low-molecular-weight heparin (LMWH), low-dose unfractionated heparin, aspirin, warfarin, or an intermittent pneumatic compression device (IPCD) as prophylaxis.23 Portable battery-powered IPCDs are recommended for 18 hours postop.23
The guideline authors prefer LMWH to the other treatments, and recommend dual prophylaxis with an IPCD and an antithrombotic agent while the patient is in the hospital and for a minimum of 10 to 14 days (and up to 35 days) after discharge. If surgery for hip fracture is delayed, the ACCP recommends that LMWH be administered after admission, but withheld for at least 12 hours before surgery. In patients with a high risk of bleeding, the ACCP recommends either an IPCD alone or no prophylaxis and notes that inferior vena cava filters should not be placed in high-risk patients.23
Take steps to ensure ample protein intake
Malnourishment is another common complication, affecting up to 20% of hip fracture patients.24 In many cases, a catabolic state predisposes patients to protein depletion, leading to decreased wound healing and an increase in other postop complications.24,25 Protein supplementation is associated with decreased length of stay and a reduction in postop complications.26
This complication can often be avoided by encouraging an early return to eating. Specific steps: Ensure that patients have their dentures available and are able to use them; are positioned properly for eating; and receive high-caloric supplemental drinks. Nutritional assessments should also be done to ensure that their intake of calcium and vitamin D is sufficient to prevent future falls and reduce fracture risk. (For more information, see “Vitamin D: When it helps, when it harms” [J Fam Pract. 2013;62:368-370.])
Combat hip fracture by stressing avoidance
Prevention of hip fracture, of course, is the ideal way to reduce the burden of disease for older patients. Along these lines, there are many ways you can help.
Start with fall reduction
Hip fracture is associated with a fall 90% of the time,27 and care for older patients should be focused on reducing the risk for falls and improving bone health and muscular function. While a complete review of preventive measures is beyond the scope of this article, we offer some highlights here and in TABLE 2.
Encourage physical activity In addition to helping to reduce falls, physical activity—particularly repetitive weight-bearing exercise—can help maintain bone density and improve muscle mass, strength, and balance.28
Rather than focus on a single exercise, however, a combination of activities—Tai Chi and walking, for instance, or weight lifting and cycling —appears to have the best likelihood of fall reduction.29 Whenever possible, physical activity for older patients should include challenges in executive function, as well. In a recent study comparing regular walking with trail-walking between sequentially marked flags, participants in the more complex activity had a greater decrease in fall rates.30
Review vitamin D and calcium intake. Elderly patients with low levels of vitamin D are at increased risk of muscle mass decline, and therefore increased risk of fracture.31 A systematic review and meta-analysis of vitamin D supplementation in older adults found the relative risk of falling was 0.86 (95% confidence interval [CI], 0.79-0.93) for those assigned to vitamin D therapy compared with those on placebo. Risk reduction was greater in groups taking 800 IU or more of vitamin D daily and those taking adjunctive calcium supplementation.32
Maximizing vitamin D for falls reduction is supported by the American Geriatrics Society, 33 the Agency for Healthcare Research and Quality (AHRQ),34 and the US Preventive Services Task Force (USPSTF).35 The USPSTF recently released a recommendation for exercise or physical therapy and vitamin D supplementation (800 IU) to prevent falls in community-dwelling adults ages 65 and over who are at an increased risk for falls.36
However, the USPSTF advises against daily supplementation with vitamin D and calcium at doses ?400 IU and 1000 mg, respectively, for noninstitutionalized postmenopausal women for primary fracture prevention. Calcium supplementation has not been shown to reduce hip fractures, but has been found to improve hip bone density.37
Consider bisphosphonates. Order a dual energy x-ray absorptiometry (DEXA) scan for older patients to identify osteoporosis. Most hip fractures are osteoporotic, and patients should be started on bisphosphonates within 2 to 12 weeks of injury38 to reduce the risk of mortality associated with hip fracture.39 The most studied bisphosphonates in geriatric hip fracture are alendronate, risedronate, and zoledronate; all were found to have a number needed to treat of 91 to prevent one hip fracture.40
Focus on the home environment. In addition to addressing the bone and muscular health of older patients, focus should be placed on the home environment. A Cochrane review of fall prevention for those living in the community found that home safety interventions reduced the risk of falls, but only for those with severe vision impairment and a high risk of falls.29 A 2010 American Geriatric Society (AGS) and British Geriatric Society (BGS) review of fall prevention gave an A recommendation—the highest rating— to home assessment and intervention by a health care professional to identify home hazards and promote safe performance of daily activities.33
Conduct brown-bag reviews. Polypharmacy is a well-documented (and growing) problem among the elderly.41 Both the AGS and BGS encourage a review of medications (including over-the-counter products) and interactions at each office visit,33 with specific attention paid to drugs that may cause dizziness, drowsiness, and near syncopal or syncopal episodes.
To reduce the risk of medication interactions and adverse effects, look for opportunities to reduce the number of drugs your elderly patients are taking. Consider involving a clinical pharmacist in medication reviews—an intervention that has been shown to be cost effective and lead to better patient outcomes.42
CASE After 4 weeks, Ms. J is ready to return home. Rather than a return to independent living, however, her children convince her to move to an assisted living facility—a move you strongly support. You schedule a visit in 2 weeks.
CORRESPONDENCE
Jeremy D. Close, MD, Department of Family and Community Medicine, Thomas Jefferson University, 833 Chestnut Street #301, Philadelphia, PA 19107; [email protected]
› Ensure that surgical stabilization of hip fracture is performed as soon as possible—ideally within 48 hours of injury. A
› To reduce the risk of delirium, orient the patient frequently; get her out of bed as soon as possible, and avoid prolonged catheter use. A
› Order protein supplements for patients recovering from hip fracture and take steps to facilitate an early return to eating. C
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
The patient and family request a consultation with Ms. J’s primary care physician. If you were her physician, what would you advise?
Hip fracture in a frail elderly patient is an injury that, while common, can be difficult to manage. With good reason. Geriatric hip fracture is associated with increased morbidity, functional decline, and use of nursing home services, as well as a higher mortality rate: One in 5 hip fracture patients dies within a year of the injury.1
As the population ages, we are seeing more hip fractures in the “oldest old” those who, like Ms. J, are older than 85. While the incidence increases exponentially with age in both men and women, women are 3 times more likely than men to sustain a hip fracture.2 White women ages 85 to 95 face the highest risk, with an incidence of more than 3%.3
In addition to managing the acute phase of hip fracture and helping patients and families make decisions about optimal treatment, there is much you can do to boost the likelihood of a rapid rehabilitation and a successful outcome.
What type of fracture? How best to treat it?
Two types of hip fractures are responsible for the vast majority of cases: About 45% of hip fractures are intracapsular, involving the femoral head and neck; another 45% are intertrochanteric fractures. Both usually involve low-energy trauma, such as a fall from a chair or tripping over a rug. Intertrochanteric and subtrochanteric fractures (the latter accounting for the remaining 10%) are extracapsular.2,4,5
Typically associated with high-energy trauma such as a motor vehicle accident, or with metastatic lesions, subtrochanteric fractures have a bimodal distribution: They are most common in individuals between the ages of 20 and 40 and those older than 60.2
Fractures involving the femoral neck can disrupt the vascular supply to the femoral head and result in avascular necrosis (AVN) or nonunion.2,4,5 A meta-analysis of the outcome of displaced femoral neck fractures found the rates of osteonecrosis and nonunion to be as high as 20% to 30%.5 Intertrochanteric fractures rarely lead to AVN or nonunion, but patients may develop complications associated with degenerative changes.2,4,5 Nonunion is a potential complication of subtrochanteric fracture.2
For most patients, surgical management is preferred
The main goals of treatment are to stabilize the hip, decrease pain and restore the level of prefracture function. Surgery is the preferred treatment for hip fracture because it provides stable fixation, facilitating full weight bearing and decreasing the risk of complications. Surgery is also associated with a shorter stay in the hospital and improved rehabilitation and recovery.6
Surgical stabilization should be performed as soon as possible—ideally, within 48 hours.5 A recent study found conflicting evidence of the effect of delayed surgery on mortality, but demonstrated that surgery within 24 hours of injury minimizes the rate of chest infections, urinary tract infections, and pressure sores, as well as the duration of the hospital stay.7 (To learn more about surgical stabilization of hip fracture, see “What type of surgery? Age is just one consideration” 5,8-10 below.)
When surgery is contraindicated
Nonoperative management is reserved for patients who stand to gain only minimal function from surgical stabilization, because they either were not ambulatory to begin with or have severe dementia. In addition, medical management is used for patients with contraindications to anesthesia, those who delay seeking medical care until the fracture has begun to heal, and patients who refuse surgical fixation.5,11
The choice of surgical intervention depends on multiple factors, including the:
- type and severity of the fracture
- preference of the orthopedic surgeon
- age of the patient
- comorbid conditions
- prognosis.
For femoral neck fractures, patients younger than 65 years are candidates for internal fixation; for older individuals and those who already had limited mobility, arthroplasty should be considered.5 Studies of pain and functional outcomes show a modest tendency for total hip arthroplasty to have better results than internal fixation in patients older than 65.8
Intertrochanteric fractures can be treated with either sliding hip screws or
intramedullary nails. Intramedullary nail implants are done percutaneously, resulting in a shorter duration for surgery, less blood loss, and an earlier return to full weight bearing.5 A recent study suggests that intramedullary nails result in more reoperations than hip screws.9 No evidence is conclusive about the superiority of either type of hardware.
Subtrochanteric fractures are typically repaired by hemiarthroplasty.
A Cochrane review of randomized controlled trials found insufficient evidence to determine whether replacement arthroplasty has any advantage over internal fixation for extracapsular hip fractures.10
CASE After a careful review of Ms. J’s health status, radiographs of the fracture (FIGURE 1A), and consultation with an orthopedic surgeon and a geriatrician, you recommend surgery as soon as the patient is fully stabilized. Without it, she would be at high risk for urinary tract infection, pressure sores, and thromboembolism associated with long-term immobility.
The next day, Ms. J undergoes surgical fixation with a sliding hip screw (FIGURE 1B). Her Foley catheter is removed the same day, and physical therapy is begun the following day. On postoperative day 4 she is discharged to an in patient rehabilitation facility.
Begin rehabilitation without delay
Whether a patient has surgery or is treated nonoperatively for hip fracture, the goal of rehabilitation is the same—to restore mobility as quickly as possible. A clinical review found no significant difference in mortality rates between those who underwent surgical fixation and those who were treated medically with early mobilization, consisting of immediate bed-to-chair transfer (with assistance), followed by progression to ambulation as tolerated.12
For patients who undergo surgery for hip fracture, increased immobility is linked to poorer functioning in the areas of self-care and transfers at 2 months and to higher mortality rates at 6 months.13 Physical therapy should be initiated on the first postoperative day and should start with bed mobility range of motion, followed by independent transfers from bed to chair, and ultimately achieving full weight bearing.5
Many complications are predictable, and often preventable
The term “hip fracture syndrome”4 is often used in reference to a cluster of common (and often preventable) complications of hip fracture, with delirium, venous thromboembolism (VTE), and malnutrition foremost among them.
Take steps to prevent—or treat—delirium
Delirium is among the most common complication, occurring in up to 62% of older patients with hip fracture.4 The highest predictor of delirium is preexisting cognitive impairment.
Other risk factors for delirium include advanced age, vision or hearing impairment, concurrent alcohol abuse, malnutrition, comorbidity, and polypharmacy.4,14 Delirium is associated with increased morbidity and mortality, decreased rehabilitation potential, and poor functional recovery independent of prior frailty.4,15,16
Hypoactive delirium is easily missed. While agitated, or hyperactive, delirium is more easily recognized, it is crucial to be aware of hypoactive delirium, as well. Patients with hypoactive delirium tend to become more withdrawn and their delirium is easily missed, leading to worse outcomes.15 The Confusion Assessment Method (TABLE 1)17 is an easy-to-use validated tool developed to aid in the diagnosis of delirium at the bedside.
Many factors contribute to the development of delirium. Medical complications, such as infection, electrolyte and volume imbalances, hypoxia, and myocardial infarction, are obvious precipitants.15 Disturbances in sleep-wake cycles, an unfamiliar environment, physical restraints, and the use of Foley catheters—all of which can impair an older patient’s sensory awareness—are less well-known contributing factors.
Tips for preventing delirium. Early mobilization, in addition to boosting physical recovery, can help prevent delirium.
Other tips:
- discontinue catheterization as soon as possible; this may help prevent delirium, and lessen the risk of urinary tract infection.
- remind nurses and family members to continuously reorient patients to their surroundings.
- treat pain aggressively.
- consult a geriatrician early on.
While opioids are often thought to cause delirium, several studies have shown an inverse relationship—that is, hip fracture patients who were given opioids for pain were actually less likely to develop delirium than those who did not receive opioids. This raises 2 important points:
1. untreated pain may itself be a significant risk factor for delirium,15,18 and
2. delirium itself is not a contraindication to opioids.18
CASE In her first week at the inpatient rehabilitation center, Ms. J requires slightly more narcotic medication for pain control. The staff notices increased confusion and a decrease in the number of bowel movements. Ms. J is started on a regimen of sennosides and docusate twice daily. Her mental status improves quickly and she has no further complications while at the rehab center.
Nonopioid pain medications such as acetaminophen should be scheduled at appropriate doses (eg, 1 g tid). Ensure that patients recovering from hip fracture are not given benzodiazepines, anticholinergics, or antihistamines15— which are sometimes included in a facility’s PRN protocol. In clinical trials, prophylactic administration of antipsychotics or anticholinesterase therapy to high-risk patients has had conflicting results.19,20
Arrange for a geriatric consult before problems occur. Several studies have shown that a geriatric consultation and concurrent management by a geriatrician using structured protocols to evaluate for common risk factors known to precipitate delirium (eg, pain, bowel/bladder function, nutrition, mobilization) can reduce the risk of delirium.16
Provide supportive care. Although treatment of the underlying cause is the definitive treatment for delirium, there are times when supportive care is all that’s needed. Reassurance from family members or staff is the recommended first step. Physical restraints should be avoided unless patient safety is threatened despite attempts to provide supportive care.
If treatment for delirium is needed, lowdose antipsychotics are recommended. The most studied agent is haloperidol, which can be administered intravenously (IV), intramuscularly (IM), or orally. Monitoring the corrected QT (QTc) interval is recommended for patients taking haloperidol, and discontinuation of the drug—or a cardiology consult— is recommended if the QTc interval is prolonged (>450 ms or >25% of baseline).21
There is a slightly higher risk of cardiac arrhythmias with IV administration of haloperidol compared with IM or oral dosing. Despite this risk, haloperidol IV is the treatment of choice for delirium.21 Newer atypical antipsychotics have also been used to treat delirium, but data are limited.21
Guard against VTE
Studies have shown rates of VTE to be as high as 40% to 60% after orthopedic procedures, and prophylaxis has long been the standard of care.22 In its 2012 consensus guidelines for antithrombotic therapy, the American College of Chest Physicians (ACCP) recommends fondiparinux, apixaban, rivaroxaban, dabigatran, low-molecular-weight heparin (LMWH), low-dose unfractionated heparin, aspirin, warfarin, or an intermittent pneumatic compression device (IPCD) as prophylaxis.23 Portable battery-powered IPCDs are recommended for 18 hours postop.23
The guideline authors prefer LMWH to the other treatments, and recommend dual prophylaxis with an IPCD and an antithrombotic agent while the patient is in the hospital and for a minimum of 10 to 14 days (and up to 35 days) after discharge. If surgery for hip fracture is delayed, the ACCP recommends that LMWH be administered after admission, but withheld for at least 12 hours before surgery. In patients with a high risk of bleeding, the ACCP recommends either an IPCD alone or no prophylaxis and notes that inferior vena cava filters should not be placed in high-risk patients.23
Take steps to ensure ample protein intake
Malnourishment is another common complication, affecting up to 20% of hip fracture patients.24 In many cases, a catabolic state predisposes patients to protein depletion, leading to decreased wound healing and an increase in other postop complications.24,25 Protein supplementation is associated with decreased length of stay and a reduction in postop complications.26
This complication can often be avoided by encouraging an early return to eating. Specific steps: Ensure that patients have their dentures available and are able to use them; are positioned properly for eating; and receive high-caloric supplemental drinks. Nutritional assessments should also be done to ensure that their intake of calcium and vitamin D is sufficient to prevent future falls and reduce fracture risk. (For more information, see “Vitamin D: When it helps, when it harms” [J Fam Pract. 2013;62:368-370.])
Combat hip fracture by stressing avoidance
Prevention of hip fracture, of course, is the ideal way to reduce the burden of disease for older patients. Along these lines, there are many ways you can help.
Start with fall reduction
Hip fracture is associated with a fall 90% of the time,27 and care for older patients should be focused on reducing the risk for falls and improving bone health and muscular function. While a complete review of preventive measures is beyond the scope of this article, we offer some highlights here and in TABLE 2.
Encourage physical activity In addition to helping to reduce falls, physical activity—particularly repetitive weight-bearing exercise—can help maintain bone density and improve muscle mass, strength, and balance.28
Rather than focus on a single exercise, however, a combination of activities—Tai Chi and walking, for instance, or weight lifting and cycling —appears to have the best likelihood of fall reduction.29 Whenever possible, physical activity for older patients should include challenges in executive function, as well. In a recent study comparing regular walking with trail-walking between sequentially marked flags, participants in the more complex activity had a greater decrease in fall rates.30
Review vitamin D and calcium intake. Elderly patients with low levels of vitamin D are at increased risk of muscle mass decline, and therefore increased risk of fracture.31 A systematic review and meta-analysis of vitamin D supplementation in older adults found the relative risk of falling was 0.86 (95% confidence interval [CI], 0.79-0.93) for those assigned to vitamin D therapy compared with those on placebo. Risk reduction was greater in groups taking 800 IU or more of vitamin D daily and those taking adjunctive calcium supplementation.32
Maximizing vitamin D for falls reduction is supported by the American Geriatrics Society, 33 the Agency for Healthcare Research and Quality (AHRQ),34 and the US Preventive Services Task Force (USPSTF).35 The USPSTF recently released a recommendation for exercise or physical therapy and vitamin D supplementation (800 IU) to prevent falls in community-dwelling adults ages 65 and over who are at an increased risk for falls.36
However, the USPSTF advises against daily supplementation with vitamin D and calcium at doses ?400 IU and 1000 mg, respectively, for noninstitutionalized postmenopausal women for primary fracture prevention. Calcium supplementation has not been shown to reduce hip fractures, but has been found to improve hip bone density.37
Consider bisphosphonates. Order a dual energy x-ray absorptiometry (DEXA) scan for older patients to identify osteoporosis. Most hip fractures are osteoporotic, and patients should be started on bisphosphonates within 2 to 12 weeks of injury38 to reduce the risk of mortality associated with hip fracture.39 The most studied bisphosphonates in geriatric hip fracture are alendronate, risedronate, and zoledronate; all were found to have a number needed to treat of 91 to prevent one hip fracture.40
Focus on the home environment. In addition to addressing the bone and muscular health of older patients, focus should be placed on the home environment. A Cochrane review of fall prevention for those living in the community found that home safety interventions reduced the risk of falls, but only for those with severe vision impairment and a high risk of falls.29 A 2010 American Geriatric Society (AGS) and British Geriatric Society (BGS) review of fall prevention gave an A recommendation—the highest rating— to home assessment and intervention by a health care professional to identify home hazards and promote safe performance of daily activities.33
Conduct brown-bag reviews. Polypharmacy is a well-documented (and growing) problem among the elderly.41 Both the AGS and BGS encourage a review of medications (including over-the-counter products) and interactions at each office visit,33 with specific attention paid to drugs that may cause dizziness, drowsiness, and near syncopal or syncopal episodes.
To reduce the risk of medication interactions and adverse effects, look for opportunities to reduce the number of drugs your elderly patients are taking. Consider involving a clinical pharmacist in medication reviews—an intervention that has been shown to be cost effective and lead to better patient outcomes.42
CASE After 4 weeks, Ms. J is ready to return home. Rather than a return to independent living, however, her children convince her to move to an assisted living facility—a move you strongly support. You schedule a visit in 2 weeks.
CORRESPONDENCE
Jeremy D. Close, MD, Department of Family and Community Medicine, Thomas Jefferson University, 833 Chestnut Street #301, Philadelphia, PA 19107; [email protected]
1. Leibson CL, Toteson ANA, Gabriel SE, et al. Mortality, disability, and nursing home use for persons with and without hip fracture: a population-based study. J Am Geriatr Soc. 2002;50:1644-50.
2. Brunner LC, Eshilian-Oates L, Kuo TY. Hip fractures in adults. Am Fam Physician. 2003;67:537-542.
3. Jacobsen SJ, Goldberg J, Miles TP, et al. Hip fracture incidence among the old and very old: a population-based study of 745,435 cases. Am J Public Health. 1990;80:871-873.
4. Auron-Gomez M, Michota F. Medical management of hip fracture. Clin Geriatr Med. 2008;24:701-719.
5. Jackman JM, Watson JT. Hip fractures in older men. Clin Geriatr Med. 2010;26:311-329.
6. Handoll HH, Parker MJ. Conservative versus operative treatment for hip fractures in adults. Cochrane Database Syst Rev. 2008;(3):CD000337.
7. Leung F, Lau W, Kwan K, et al. Does timing of surgery matter in fragility hip fractures? Osteoporos Int. 2010; 21(suppl 4):S529-S534.
8. Butler M, Forte ML, Joglekar SB, et al. Evidence summary: systematic review of surgical treatments for geriatric hip fractures. J Bone Joint Surg Am. 2011;93:1104-1115.
9. Matre K, Havelin LI, Gjertsen JE, et al. Intramedullary nails result in more reoperations than sliding hip screws in two-part intertrochanteric fractures. Clin Orthop Relat Res. 2013;471: 1379-1386.
10. Parker MJ, Handoll HH. Replacement arthroplasty versus internal fixation for extracapsular hip fractures in adults. Cochrane Database Syst Rev. 2006;(2):CD000086.
11. Cummings-Vaughn LA, Gammack JK. Falls, osteoporosis, and hip fractures. Med Clin North Am. 2011;95:495-506.
12. Jain R, Basinski A, Kreder HJ. Nonoperative treatment of hip fractures. Int Orthop. 2003;27:11-17.
13. Siu A, Penrod J, Boockvar K, et al. Early ambulation after hip fracture: effects on function and mortality. Arch Intern Med. 2006;166:766-771.
14. Juliebø V, Bjøro K, Krogseth M, et al. Risk factors for preoperative and postoperative delirium in elderly patients with hip fracture. J Am Geriatr Soc. 2009;57:1354-1361.
15. Flinn DR, Deihl KM, Seyfried LS, et al. Prevention, diagnosis, and management of postoperative delirium in older adults. J Am Coll Surg. 2009;209:261-268.
16. Marcantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49:516-522.
17. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941-948.
18. Sieber FE, Mears S, Lee H, et al. Postoperative opioid consumption and its relationship to cognitive function in older adults with hip fracture. J Am Geriatr Soc. 2011;59:2256-2262.
19. Prakanrattana U, Prapaitrakool S. Efficacy of risperidone for Rather than focus on a single exercise, a combination of activities—eg, Tai Chi and walking, or weight lifting and cycling—have the greatest likelihood of fall reduction. prevention of postoperative delirium in cardiac surgery. Anaesth Intensive Care. 2007;35:714-719.
20. Sampson EL, Raven PR, Ndhlovu PN, et al. A randomized, double-blind, placebo-controlled trial of donepezil hydrochloride (Aricept) for reducing the incidence of postoperative delirium after elective total hip replacement. Int J Geriatr Psychiatry. 2007;22:343-349.
21. Jacobi J, Fraser GL, Coursin DB, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30:119-141.
22. Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference of Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(suppl):338S-400S.
23. Guyatt GH, Akl EA, Crowther M, et al. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):7S-47S.
24. Garcia Lazaro M, Montero Perez-Barquero M, Carpintero Benitez P. The role of malnutrition and other medical factors in the evolution of patients with hip fracture [article in Spanish]. An Med Interna. 2004;21:557-563.
25. Lavernia CJ, Sierra RJ, Baerga L. Nutritional parameters and short term outcome in arthroplasty. J Am Coll Nutr. 1999;18:274-278.
26. Huddleston JM, Whitford KJ. Medical care of elderly patients with hip fractures. Mayo Clin Proc. 2001;76:295-298.
27. Cummings SR, Kelsey JL, Nevitt MC, et al. Epidemiology of osteoporosis and osteoporotic fractures. Epidemiol Rev. 1985;7:178-208.
28. Nelson ME, Fiatarone MA, Morganti CM, et al. Effects of high-intensity strength training on multiple risk factors for osteoporotic fractures a randomized controlled trial. JAMA. 1994;272:1909-1914.
29. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2009;(2):CD007146.
30. Yamada M, Tanaka B, Nagai K, et al. Trail-walking exercise and fall risk factors in community-dwelling older adults: preliminary results of a randomized controlled trial. J Am Geriatr Soc. 2010;58:1946-1951.
31. Visser M, Deeg DJ, Lips P; Longitudinal Aging Study Amsterdam. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003;88:5766-5772.
32. Kalyani RR, Stein B, Valiyil R, et al. Vitamin D treatment for the prevention of falls in older adults: systematic review and metaanalysis. J Am Geriatr Soc. 2010;58:1299-1310.
33. The American Geriatrics Society. Prevention of falls in older persons [clinical practice guideline]. 2010. Available at: http:// www.americangeriatrics.org/health_care_professionals/clinical_practice/clinical_guidelines_recommendations/ 2010/. Accessed August 16, 2013.
34. Cranney A, Horsley T, O’Donnell S, et al. Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess (Full Rep). 2007;(158):1-235.
35. Michael YL, Whitlock EP, Lin JS, et al. Primary care-relevant interventions to prevent falling in older adults: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153:815-825.
36. USPSTF. Prevention of falls in community-dwelling older adults. US Preventive Services Task Force recommendation statement. May 2012. Available at: www.uspreventiveservices taskforce.org/uspstf11/fallsprevention/fallsprevrs.htm. Accessed August 19, 2013.
37. Jackson RD, LaCroix AZ, Gass M, et al; Women’s Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669-683.
38. Lyles KW, Colón-Emeric CS, Magaziner JS, et al; for the HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357:1799-1809.
39. Beaupre LA, Morrish DW, Hanley DA, et al. Oral bisphosphonates are associated with reduced mortality after hip fracture. Osteoporos Int. 2011;22:983-991.
40. Ringe, JD, Doherty, JG. Absolute risk reduction in osteoporosis: assessing treatment efficacy by number needed to treat. Rheumatol Int. 2010;30:863-869.
41. Veehof L, Stewart R, Haaijer-Ruskamp F, et al. The development of polypharmacy. A longitudinal study. Fam Pract. 2000;17:261-267.
42. Choe HM, Farris KB, Stevenson JG, et al. Patient-centered medical home: developing, expanding, and sustaining a role for pharmacists. Am J Health Syst Pharm. 2012;69:1063-1071.
1. Leibson CL, Toteson ANA, Gabriel SE, et al. Mortality, disability, and nursing home use for persons with and without hip fracture: a population-based study. J Am Geriatr Soc. 2002;50:1644-50.
2. Brunner LC, Eshilian-Oates L, Kuo TY. Hip fractures in adults. Am Fam Physician. 2003;67:537-542.
3. Jacobsen SJ, Goldberg J, Miles TP, et al. Hip fracture incidence among the old and very old: a population-based study of 745,435 cases. Am J Public Health. 1990;80:871-873.
4. Auron-Gomez M, Michota F. Medical management of hip fracture. Clin Geriatr Med. 2008;24:701-719.
5. Jackman JM, Watson JT. Hip fractures in older men. Clin Geriatr Med. 2010;26:311-329.
6. Handoll HH, Parker MJ. Conservative versus operative treatment for hip fractures in adults. Cochrane Database Syst Rev. 2008;(3):CD000337.
7. Leung F, Lau W, Kwan K, et al. Does timing of surgery matter in fragility hip fractures? Osteoporos Int. 2010; 21(suppl 4):S529-S534.
8. Butler M, Forte ML, Joglekar SB, et al. Evidence summary: systematic review of surgical treatments for geriatric hip fractures. J Bone Joint Surg Am. 2011;93:1104-1115.
9. Matre K, Havelin LI, Gjertsen JE, et al. Intramedullary nails result in more reoperations than sliding hip screws in two-part intertrochanteric fractures. Clin Orthop Relat Res. 2013;471: 1379-1386.
10. Parker MJ, Handoll HH. Replacement arthroplasty versus internal fixation for extracapsular hip fractures in adults. Cochrane Database Syst Rev. 2006;(2):CD000086.
11. Cummings-Vaughn LA, Gammack JK. Falls, osteoporosis, and hip fractures. Med Clin North Am. 2011;95:495-506.
12. Jain R, Basinski A, Kreder HJ. Nonoperative treatment of hip fractures. Int Orthop. 2003;27:11-17.
13. Siu A, Penrod J, Boockvar K, et al. Early ambulation after hip fracture: effects on function and mortality. Arch Intern Med. 2006;166:766-771.
14. Juliebø V, Bjøro K, Krogseth M, et al. Risk factors for preoperative and postoperative delirium in elderly patients with hip fracture. J Am Geriatr Soc. 2009;57:1354-1361.
15. Flinn DR, Deihl KM, Seyfried LS, et al. Prevention, diagnosis, and management of postoperative delirium in older adults. J Am Coll Surg. 2009;209:261-268.
16. Marcantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49:516-522.
17. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941-948.
18. Sieber FE, Mears S, Lee H, et al. Postoperative opioid consumption and its relationship to cognitive function in older adults with hip fracture. J Am Geriatr Soc. 2011;59:2256-2262.
19. Prakanrattana U, Prapaitrakool S. Efficacy of risperidone for Rather than focus on a single exercise, a combination of activities—eg, Tai Chi and walking, or weight lifting and cycling—have the greatest likelihood of fall reduction. prevention of postoperative delirium in cardiac surgery. Anaesth Intensive Care. 2007;35:714-719.
20. Sampson EL, Raven PR, Ndhlovu PN, et al. A randomized, double-blind, placebo-controlled trial of donepezil hydrochloride (Aricept) for reducing the incidence of postoperative delirium after elective total hip replacement. Int J Geriatr Psychiatry. 2007;22:343-349.
21. Jacobi J, Fraser GL, Coursin DB, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30:119-141.
22. Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference of Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(suppl):338S-400S.
23. Guyatt GH, Akl EA, Crowther M, et al. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):7S-47S.
24. Garcia Lazaro M, Montero Perez-Barquero M, Carpintero Benitez P. The role of malnutrition and other medical factors in the evolution of patients with hip fracture [article in Spanish]. An Med Interna. 2004;21:557-563.
25. Lavernia CJ, Sierra RJ, Baerga L. Nutritional parameters and short term outcome in arthroplasty. J Am Coll Nutr. 1999;18:274-278.
26. Huddleston JM, Whitford KJ. Medical care of elderly patients with hip fractures. Mayo Clin Proc. 2001;76:295-298.
27. Cummings SR, Kelsey JL, Nevitt MC, et al. Epidemiology of osteoporosis and osteoporotic fractures. Epidemiol Rev. 1985;7:178-208.
28. Nelson ME, Fiatarone MA, Morganti CM, et al. Effects of high-intensity strength training on multiple risk factors for osteoporotic fractures a randomized controlled trial. JAMA. 1994;272:1909-1914.
29. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2009;(2):CD007146.
30. Yamada M, Tanaka B, Nagai K, et al. Trail-walking exercise and fall risk factors in community-dwelling older adults: preliminary results of a randomized controlled trial. J Am Geriatr Soc. 2010;58:1946-1951.
31. Visser M, Deeg DJ, Lips P; Longitudinal Aging Study Amsterdam. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003;88:5766-5772.
32. Kalyani RR, Stein B, Valiyil R, et al. Vitamin D treatment for the prevention of falls in older adults: systematic review and metaanalysis. J Am Geriatr Soc. 2010;58:1299-1310.
33. The American Geriatrics Society. Prevention of falls in older persons [clinical practice guideline]. 2010. Available at: http:// www.americangeriatrics.org/health_care_professionals/clinical_practice/clinical_guidelines_recommendations/ 2010/. Accessed August 16, 2013.
34. Cranney A, Horsley T, O’Donnell S, et al. Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess (Full Rep). 2007;(158):1-235.
35. Michael YL, Whitlock EP, Lin JS, et al. Primary care-relevant interventions to prevent falling in older adults: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153:815-825.
36. USPSTF. Prevention of falls in community-dwelling older adults. US Preventive Services Task Force recommendation statement. May 2012. Available at: www.uspreventiveservices taskforce.org/uspstf11/fallsprevention/fallsprevrs.htm. Accessed August 19, 2013.
37. Jackson RD, LaCroix AZ, Gass M, et al; Women’s Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669-683.
38. Lyles KW, Colón-Emeric CS, Magaziner JS, et al; for the HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357:1799-1809.
39. Beaupre LA, Morrish DW, Hanley DA, et al. Oral bisphosphonates are associated with reduced mortality after hip fracture. Osteoporos Int. 2011;22:983-991.
40. Ringe, JD, Doherty, JG. Absolute risk reduction in osteoporosis: assessing treatment efficacy by number needed to treat. Rheumatol Int. 2010;30:863-869.
41. Veehof L, Stewart R, Haaijer-Ruskamp F, et al. The development of polypharmacy. A longitudinal study. Fam Pract. 2000;17:261-267.
42. Choe HM, Farris KB, Stevenson JG, et al. Patient-centered medical home: developing, expanding, and sustaining a role for pharmacists. Am J Health Syst Pharm. 2012;69:1063-1071.
Painful ear nodules
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
A 49-year-old man with a history of hypertension, hypercholesterolemia, polysubstance use, recurrent methicillin-resistant Staphylococcus aureus skin infections, and chronic hepatitis C infection sought care at our emergency department (ED) because parts of his ears had started turning black 3 days earlier. They were also painful to the touch. He denied fever, any similar skin lesions, injury to his ears, or a history of easy bleeding or bruising. A recovering alcoholic, he admitted to regular marijuana use and twice-weekly cocaine use. He had last used cocaine 3 days ago.
The patient was thin and in no acute distress. His vital signs and cardiopulmonary exams were normal. Examination of his ears revealed bilateral violaceous firm, tender purpura on the pinnae (FIGURE).
A complete blood count (CBC) revealed mild leukopenia (white blood cell [WBC] count, 2.0 × 109/L), neutropenia (0.9 × 109/L), and a normal platelet count (264 × 109/L). A chemistry panel, liver function tests, and prothrombin time were normal. Erythrocyte sedimentation rate (ESR) was elevated to 69 mm/h. The patient’s cholesterol level was not elevated. Urine toxicology was positive for cocaine and opioids. A human immunodeficiency virus test was negative.
Figure
Tender purpura on the pinnae
What is your diagnosis?
How would you treat this patient?
Diagnosis: Levamisole toxicity
The patient was diagnosed with levamisole toxicity based on his clinical presentation and the fact that he had used cocaine around the time his ear lesions appeared.
Levamisole—primarily a veterinary antihelmintic medication—is used on rare occasions to treat nephrotic syndrome in children.1 Levamisole is frequently added to cocaine or heroin to increase the street drug’s potency. The Drug Enforcement Administration reports that 69% of seized cocaine lots in the United States contain levamisole.2
The compound is thought to cause a vasculitis and bone marrow suppression resulting in neutropenia. The vasculitis targets small vessels, resulting in thrombosis, which can lead to tissue necrosis.1
Other possibilities in the differential Dx
The differential diagnosis includes a variety of vasculitides and other microvascular pathologies.
Cholesterol emboli arise when cholesterol crystals are released from atherosclerotic plaques, typically after invasive cardiac procedures. In addition, anticoagulants can cause the release of these crystals by inhibiting the formation of protective clots around unstable plaques.3 These emboli can seed the microvasculature anywhere, but the kidneys and skin are most frequently affected. These crystals not only clog the vasculature, causing tissue ischemia, but also activate the complement cascade, triggering a series of inflammatory responses that can lead to luminal fibrosis and narrowing.3
Affected patients have a history of atherosclerotic disease or predisposing factors such as hypertension or diabetes. Ulcerations or frank cyanosis may be found at the tips of the fingers or toes. In severe cases, gangrene will form in these regions. Patients may also have livido reticularis, a lace-like hyperpigmented rash over the lower extremities. Laboratory analysis may indicate acute renal failure or eosinophilia.3
Bacterial endocarditis results from the seeding of bacterial emboli primarily from the mitral or tricuspid valves.4 Streptococci are the primary infectious agent, with staphylococci being more common among intravenous drug users. High-risk populations include patients with artificial valves, the elderly, and the immunocompromised.4
Clinical manifestations include Janeway lesions (asymptomatic hemorrhagic papules on the palms) and Osler’s nodes (tender nodules on the fingertips). Splinter hemorrhages, or linear nonblanching lesions, may be present within the nail beds. Palpable purpura and petechiae may also be found.
Patients may have positive blood cultures, leukocytosis, an elevated ESR, or vegetations on a transesophageal echocardiogram.4 The physical exam may reveal a new cardiac murmur.
High circulating levels of cryoglobulins can arise in the setting of hepatitis C infection, but can also be seen in a number of autoimmune disorders and other infectious diseases.5 Cryoglobulins are immune complexes that are deposited into the lumen of microvasculature. In cold temperatures, these cryoglobulins precipitate, resulting in vasculitis. While most patients are asymptomatic, cutaneous findings in the distal extremities can include palpable purpura, ulcerations, and livido reticularis.5 Patients may complain of arthritis or symptoms consistent with Raynaud’s phenomenon.
Detection of specific serum cryoprecipitates isolated by immunofixation is pathognomonic for this condition, provided the sample is collected in a warm tube. Elevated rheumatoid factor and decreased complement levels may also be seen.5
Henoch-Schönlein purpura (HSP) is a small vessel vasculitis caused by IgA deposition that predominantly affects children. HSP has a host of systemic symptoms, often preceded by a benign upper respiratory infection, consisting of palpable purpura, arthritis, abdominal pain, and glomerulonephritis.6 Palpable purpura will generally be found in dependent portions of the body—especially the buttocks and lower legs.
While the diagnosis is primarily clinical, serum IgA levels and ESR can be elevated, urinalysis may demonstrate hematuria or proteinuria, and a CBC may reveal a leukocytosis with normal platelets.6
Suspect levamisole toxicity in patients using cocaine
Patients with levamisole toxicity present with sudden-onset tender plaques or bullae with necrotic centers within days of cocaine use. Case reports cite lesions primarily on the ears and cheeks. However, they can appear almost anywhere on the body.2,7-9 Physicians should have a high index of suspicion for levamisole toxicity in patients using cocaine who present with unexplained neutropenia or vasculitis.
Laboratory tests. If needed, tissue biopsy and urine detection of levamisole can be used to confirm the diagnosis.1
Management is straight-forward, but not simple
Skin lesions have been reported to improve several weeks after discontinuing use of contaminated cocaine1 (strength of recommendation [SOR]: C). Known users should be referred to drug treatment centers and counseled on the risks of use.
Our patient required hospitalization
When our patient came into the ED, he also complained of left thigh pain and swelling. A computed tomography scan revealed a deep sartorius abscess. The patient was admitted for ultrasound-guided aspiration of the abscess and IV antibiotics. His bilateral painful ear nodules persisted throughout his hospitalization, although his neutropenia resolved after 3 days.
Correspondence: Katherine Winter, MD, 101 Manning Drive, Chapel Hill, NC 27514; [email protected]
1. Lee KC, Culpepper K, Kessler M. Levamisole-induced thrombosis: literature review and pertinent laboratory findings. J Am Acad Dermatol. 2011;65:e128-e129.
2. CDC. Agranulocytosis associated with cocaine use - four States, March 2008-November 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1381-1385.
3. Kronzon I, Saric M. Cholesterol embolization syndrome. Circulation. 2010;122:631-641.
4. Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med. 2001;345:1318-1330.
5. Tedeschi A, Barate C, Minola E, et al. Cryoglobulinemia. Blood Rev. 2007;21:183-200.
6. Trapani S, Micheli A, Grisolia F, et al. Henoch Schonlein purpura in childhood: epidemiological and clinical analysis of 150 cases over a 5-year period and review of literature. Semin Arthritis Rheum. 2005;35:143-153.
7. Muirhead TT, Eide MJ. Images in clinical medicine. Toxic effects of levamisole in a cocaine user. N Engl J Med. 2011;364:e52.
8. Bradford M, Rosenberg B, Moreno J, et al. Bilateral necrosis of earlobes and cheeks: another complication of cocaine contaminated with levamisole. Ann Intern Med. 2010;152: 758-759.
9. Chung C, Tumeh P, Birnbaum R. Characteristic purpura of the ears, vasculitis, and neutropenia—a potential public health epidemic associated with levamisole-adulterated cocaine. J Am Acad Dermatol. 2011;65:722-725.
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
A 49-year-old man with a history of hypertension, hypercholesterolemia, polysubstance use, recurrent methicillin-resistant Staphylococcus aureus skin infections, and chronic hepatitis C infection sought care at our emergency department (ED) because parts of his ears had started turning black 3 days earlier. They were also painful to the touch. He denied fever, any similar skin lesions, injury to his ears, or a history of easy bleeding or bruising. A recovering alcoholic, he admitted to regular marijuana use and twice-weekly cocaine use. He had last used cocaine 3 days ago.
The patient was thin and in no acute distress. His vital signs and cardiopulmonary exams were normal. Examination of his ears revealed bilateral violaceous firm, tender purpura on the pinnae (FIGURE).
A complete blood count (CBC) revealed mild leukopenia (white blood cell [WBC] count, 2.0 × 109/L), neutropenia (0.9 × 109/L), and a normal platelet count (264 × 109/L). A chemistry panel, liver function tests, and prothrombin time were normal. Erythrocyte sedimentation rate (ESR) was elevated to 69 mm/h. The patient’s cholesterol level was not elevated. Urine toxicology was positive for cocaine and opioids. A human immunodeficiency virus test was negative.
Figure
Tender purpura on the pinnae
What is your diagnosis?
How would you treat this patient?
Diagnosis: Levamisole toxicity
The patient was diagnosed with levamisole toxicity based on his clinical presentation and the fact that he had used cocaine around the time his ear lesions appeared.
Levamisole—primarily a veterinary antihelmintic medication—is used on rare occasions to treat nephrotic syndrome in children.1 Levamisole is frequently added to cocaine or heroin to increase the street drug’s potency. The Drug Enforcement Administration reports that 69% of seized cocaine lots in the United States contain levamisole.2
The compound is thought to cause a vasculitis and bone marrow suppression resulting in neutropenia. The vasculitis targets small vessels, resulting in thrombosis, which can lead to tissue necrosis.1
Other possibilities in the differential Dx
The differential diagnosis includes a variety of vasculitides and other microvascular pathologies.
Cholesterol emboli arise when cholesterol crystals are released from atherosclerotic plaques, typically after invasive cardiac procedures. In addition, anticoagulants can cause the release of these crystals by inhibiting the formation of protective clots around unstable plaques.3 These emboli can seed the microvasculature anywhere, but the kidneys and skin are most frequently affected. These crystals not only clog the vasculature, causing tissue ischemia, but also activate the complement cascade, triggering a series of inflammatory responses that can lead to luminal fibrosis and narrowing.3
Affected patients have a history of atherosclerotic disease or predisposing factors such as hypertension or diabetes. Ulcerations or frank cyanosis may be found at the tips of the fingers or toes. In severe cases, gangrene will form in these regions. Patients may also have livido reticularis, a lace-like hyperpigmented rash over the lower extremities. Laboratory analysis may indicate acute renal failure or eosinophilia.3
Bacterial endocarditis results from the seeding of bacterial emboli primarily from the mitral or tricuspid valves.4 Streptococci are the primary infectious agent, with staphylococci being more common among intravenous drug users. High-risk populations include patients with artificial valves, the elderly, and the immunocompromised.4
Clinical manifestations include Janeway lesions (asymptomatic hemorrhagic papules on the palms) and Osler’s nodes (tender nodules on the fingertips). Splinter hemorrhages, or linear nonblanching lesions, may be present within the nail beds. Palpable purpura and petechiae may also be found.
Patients may have positive blood cultures, leukocytosis, an elevated ESR, or vegetations on a transesophageal echocardiogram.4 The physical exam may reveal a new cardiac murmur.
High circulating levels of cryoglobulins can arise in the setting of hepatitis C infection, but can also be seen in a number of autoimmune disorders and other infectious diseases.5 Cryoglobulins are immune complexes that are deposited into the lumen of microvasculature. In cold temperatures, these cryoglobulins precipitate, resulting in vasculitis. While most patients are asymptomatic, cutaneous findings in the distal extremities can include palpable purpura, ulcerations, and livido reticularis.5 Patients may complain of arthritis or symptoms consistent with Raynaud’s phenomenon.
Detection of specific serum cryoprecipitates isolated by immunofixation is pathognomonic for this condition, provided the sample is collected in a warm tube. Elevated rheumatoid factor and decreased complement levels may also be seen.5
Henoch-Schönlein purpura (HSP) is a small vessel vasculitis caused by IgA deposition that predominantly affects children. HSP has a host of systemic symptoms, often preceded by a benign upper respiratory infection, consisting of palpable purpura, arthritis, abdominal pain, and glomerulonephritis.6 Palpable purpura will generally be found in dependent portions of the body—especially the buttocks and lower legs.
While the diagnosis is primarily clinical, serum IgA levels and ESR can be elevated, urinalysis may demonstrate hematuria or proteinuria, and a CBC may reveal a leukocytosis with normal platelets.6
Suspect levamisole toxicity in patients using cocaine
Patients with levamisole toxicity present with sudden-onset tender plaques or bullae with necrotic centers within days of cocaine use. Case reports cite lesions primarily on the ears and cheeks. However, they can appear almost anywhere on the body.2,7-9 Physicians should have a high index of suspicion for levamisole toxicity in patients using cocaine who present with unexplained neutropenia or vasculitis.
Laboratory tests. If needed, tissue biopsy and urine detection of levamisole can be used to confirm the diagnosis.1
Management is straight-forward, but not simple
Skin lesions have been reported to improve several weeks after discontinuing use of contaminated cocaine1 (strength of recommendation [SOR]: C). Known users should be referred to drug treatment centers and counseled on the risks of use.
Our patient required hospitalization
When our patient came into the ED, he also complained of left thigh pain and swelling. A computed tomography scan revealed a deep sartorius abscess. The patient was admitted for ultrasound-guided aspiration of the abscess and IV antibiotics. His bilateral painful ear nodules persisted throughout his hospitalization, although his neutropenia resolved after 3 days.
Correspondence: Katherine Winter, MD, 101 Manning Drive, Chapel Hill, NC 27514; [email protected]
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
A 49-year-old man with a history of hypertension, hypercholesterolemia, polysubstance use, recurrent methicillin-resistant Staphylococcus aureus skin infections, and chronic hepatitis C infection sought care at our emergency department (ED) because parts of his ears had started turning black 3 days earlier. They were also painful to the touch. He denied fever, any similar skin lesions, injury to his ears, or a history of easy bleeding or bruising. A recovering alcoholic, he admitted to regular marijuana use and twice-weekly cocaine use. He had last used cocaine 3 days ago.
The patient was thin and in no acute distress. His vital signs and cardiopulmonary exams were normal. Examination of his ears revealed bilateral violaceous firm, tender purpura on the pinnae (FIGURE).
A complete blood count (CBC) revealed mild leukopenia (white blood cell [WBC] count, 2.0 × 109/L), neutropenia (0.9 × 109/L), and a normal platelet count (264 × 109/L). A chemistry panel, liver function tests, and prothrombin time were normal. Erythrocyte sedimentation rate (ESR) was elevated to 69 mm/h. The patient’s cholesterol level was not elevated. Urine toxicology was positive for cocaine and opioids. A human immunodeficiency virus test was negative.
Figure
Tender purpura on the pinnae
What is your diagnosis?
How would you treat this patient?
Diagnosis: Levamisole toxicity
The patient was diagnosed with levamisole toxicity based on his clinical presentation and the fact that he had used cocaine around the time his ear lesions appeared.
Levamisole—primarily a veterinary antihelmintic medication—is used on rare occasions to treat nephrotic syndrome in children.1 Levamisole is frequently added to cocaine or heroin to increase the street drug’s potency. The Drug Enforcement Administration reports that 69% of seized cocaine lots in the United States contain levamisole.2
The compound is thought to cause a vasculitis and bone marrow suppression resulting in neutropenia. The vasculitis targets small vessels, resulting in thrombosis, which can lead to tissue necrosis.1
Other possibilities in the differential Dx
The differential diagnosis includes a variety of vasculitides and other microvascular pathologies.
Cholesterol emboli arise when cholesterol crystals are released from atherosclerotic plaques, typically after invasive cardiac procedures. In addition, anticoagulants can cause the release of these crystals by inhibiting the formation of protective clots around unstable plaques.3 These emboli can seed the microvasculature anywhere, but the kidneys and skin are most frequently affected. These crystals not only clog the vasculature, causing tissue ischemia, but also activate the complement cascade, triggering a series of inflammatory responses that can lead to luminal fibrosis and narrowing.3
Affected patients have a history of atherosclerotic disease or predisposing factors such as hypertension or diabetes. Ulcerations or frank cyanosis may be found at the tips of the fingers or toes. In severe cases, gangrene will form in these regions. Patients may also have livido reticularis, a lace-like hyperpigmented rash over the lower extremities. Laboratory analysis may indicate acute renal failure or eosinophilia.3
Bacterial endocarditis results from the seeding of bacterial emboli primarily from the mitral or tricuspid valves.4 Streptococci are the primary infectious agent, with staphylococci being more common among intravenous drug users. High-risk populations include patients with artificial valves, the elderly, and the immunocompromised.4
Clinical manifestations include Janeway lesions (asymptomatic hemorrhagic papules on the palms) and Osler’s nodes (tender nodules on the fingertips). Splinter hemorrhages, or linear nonblanching lesions, may be present within the nail beds. Palpable purpura and petechiae may also be found.
Patients may have positive blood cultures, leukocytosis, an elevated ESR, or vegetations on a transesophageal echocardiogram.4 The physical exam may reveal a new cardiac murmur.
High circulating levels of cryoglobulins can arise in the setting of hepatitis C infection, but can also be seen in a number of autoimmune disorders and other infectious diseases.5 Cryoglobulins are immune complexes that are deposited into the lumen of microvasculature. In cold temperatures, these cryoglobulins precipitate, resulting in vasculitis. While most patients are asymptomatic, cutaneous findings in the distal extremities can include palpable purpura, ulcerations, and livido reticularis.5 Patients may complain of arthritis or symptoms consistent with Raynaud’s phenomenon.
Detection of specific serum cryoprecipitates isolated by immunofixation is pathognomonic for this condition, provided the sample is collected in a warm tube. Elevated rheumatoid factor and decreased complement levels may also be seen.5
Henoch-Schönlein purpura (HSP) is a small vessel vasculitis caused by IgA deposition that predominantly affects children. HSP has a host of systemic symptoms, often preceded by a benign upper respiratory infection, consisting of palpable purpura, arthritis, abdominal pain, and glomerulonephritis.6 Palpable purpura will generally be found in dependent portions of the body—especially the buttocks and lower legs.
While the diagnosis is primarily clinical, serum IgA levels and ESR can be elevated, urinalysis may demonstrate hematuria or proteinuria, and a CBC may reveal a leukocytosis with normal platelets.6
Suspect levamisole toxicity in patients using cocaine
Patients with levamisole toxicity present with sudden-onset tender plaques or bullae with necrotic centers within days of cocaine use. Case reports cite lesions primarily on the ears and cheeks. However, they can appear almost anywhere on the body.2,7-9 Physicians should have a high index of suspicion for levamisole toxicity in patients using cocaine who present with unexplained neutropenia or vasculitis.
Laboratory tests. If needed, tissue biopsy and urine detection of levamisole can be used to confirm the diagnosis.1
Management is straight-forward, but not simple
Skin lesions have been reported to improve several weeks after discontinuing use of contaminated cocaine1 (strength of recommendation [SOR]: C). Known users should be referred to drug treatment centers and counseled on the risks of use.
Our patient required hospitalization
When our patient came into the ED, he also complained of left thigh pain and swelling. A computed tomography scan revealed a deep sartorius abscess. The patient was admitted for ultrasound-guided aspiration of the abscess and IV antibiotics. His bilateral painful ear nodules persisted throughout his hospitalization, although his neutropenia resolved after 3 days.
Correspondence: Katherine Winter, MD, 101 Manning Drive, Chapel Hill, NC 27514; [email protected]
1. Lee KC, Culpepper K, Kessler M. Levamisole-induced thrombosis: literature review and pertinent laboratory findings. J Am Acad Dermatol. 2011;65:e128-e129.
2. CDC. Agranulocytosis associated with cocaine use - four States, March 2008-November 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1381-1385.
3. Kronzon I, Saric M. Cholesterol embolization syndrome. Circulation. 2010;122:631-641.
4. Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med. 2001;345:1318-1330.
5. Tedeschi A, Barate C, Minola E, et al. Cryoglobulinemia. Blood Rev. 2007;21:183-200.
6. Trapani S, Micheli A, Grisolia F, et al. Henoch Schonlein purpura in childhood: epidemiological and clinical analysis of 150 cases over a 5-year period and review of literature. Semin Arthritis Rheum. 2005;35:143-153.
7. Muirhead TT, Eide MJ. Images in clinical medicine. Toxic effects of levamisole in a cocaine user. N Engl J Med. 2011;364:e52.
8. Bradford M, Rosenberg B, Moreno J, et al. Bilateral necrosis of earlobes and cheeks: another complication of cocaine contaminated with levamisole. Ann Intern Med. 2010;152: 758-759.
9. Chung C, Tumeh P, Birnbaum R. Characteristic purpura of the ears, vasculitis, and neutropenia—a potential public health epidemic associated with levamisole-adulterated cocaine. J Am Acad Dermatol. 2011;65:722-725.
1. Lee KC, Culpepper K, Kessler M. Levamisole-induced thrombosis: literature review and pertinent laboratory findings. J Am Acad Dermatol. 2011;65:e128-e129.
2. CDC. Agranulocytosis associated with cocaine use - four States, March 2008-November 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1381-1385.
3. Kronzon I, Saric M. Cholesterol embolization syndrome. Circulation. 2010;122:631-641.
4. Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med. 2001;345:1318-1330.
5. Tedeschi A, Barate C, Minola E, et al. Cryoglobulinemia. Blood Rev. 2007;21:183-200.
6. Trapani S, Micheli A, Grisolia F, et al. Henoch Schonlein purpura in childhood: epidemiological and clinical analysis of 150 cases over a 5-year period and review of literature. Semin Arthritis Rheum. 2005;35:143-153.
7. Muirhead TT, Eide MJ. Images in clinical medicine. Toxic effects of levamisole in a cocaine user. N Engl J Med. 2011;364:e52.
8. Bradford M, Rosenberg B, Moreno J, et al. Bilateral necrosis of earlobes and cheeks: another complication of cocaine contaminated with levamisole. Ann Intern Med. 2010;152: 758-759.
9. Chung C, Tumeh P, Birnbaum R. Characteristic purpura of the ears, vasculitis, and neutropenia—a potential public health epidemic associated with levamisole-adulterated cocaine. J Am Acad Dermatol. 2011;65:722-725.
Influenza: Update for the 2013-2014 season
Each year in late summer, the Centers for Disease Control and Prevention (CDC) publishes its recommendations for the prevention of influenza for the upcoming season. The severity of each influenza season varies and is difficult to predict, which underscores the need to provide maximal vaccine coverage for at-risk patient populations.
Hoping for the best, planning for the worst. Over the past several decades the annual number of influenza-related hospitalizations has varied from approximately 55,000 to 431,000,1 and the number of deaths from influenza has been as low as 3349 and as high as 48,614.2 Infection rates are usually highest in children. Complications, hospitalizations, and deaths are highest in those ≥65 years, children<2 years, and patients with medical conditions known to increase risk for influenza complications. Those at high risk of complications appear in TABLE 1.3 The main recommendations for this coming year are the same as last year, including vaccinating everyone ≥6 months of age without a contraindication, starting vaccinations as soon as vaccine is available, and continuing throughout the influenza season for those who need it.
What’s new this year
An increasing number of influenza vaccine products are available; although to date, their effectiveness (which was determined to be 56% for all vaccines used last influenza season) 4 remains below what we would hope for. The CDC’s recommendations address these new types of vaccines, including ones that have 4 antigens instead of 3, and use new terminology to describe the vaccines.3
New terminology reflects changing vaccine formulations. Last influenza season there were 2 major categories of influenza vaccines: live-attenuated influenza vaccine (LAIV) and trivalent inactivated influenza vaccine (TIV). All products were produced using egg-culture methods and contained 2 influenza A antigen subtypes and 1 B subtype. Several products this year include 4 antigens (2 A subtypes and 2 B subtypes), and some are now produced with non–eggculture methods. This has led to a new system of classification, with the term inactivated influenza vaccine (IIV) replacing TIV. TABLE 2 lists the influenza vaccine categories and abbreviations. TABLE 3 lists the contraindications for the different vaccine types.3
The new products include Flumist Quadrivalent (MedImmune), a quadrivalent LAIV (LAIV4); Fluarix Quadrivalent (GlaxoSmithKline), a quadrivalent IIV (IIV4); Flucelvax (Novartis Vaccines and Diagnostics), a cell culture-based trivalent IIV (ccIIV3); and FluBlok (Protein Sciences), a trivalent recombinant hemagglutinin influenza vaccine (RIV3). Fluzone (Sanofi Pasteur), introduced last season in a trivalent formulation, is also available this season as a quadrivalent IIV (IIV4). As a group, influenza vaccine products now offer 3 routes of administration: intramuscular, subcutaneous, and intranasal. There is currently no evidence that any route offers an advantage over another, and the CDC states no preference for any particular product or route of administration.
Mercury content is not a problem
Even though there is no scientific controversy over the safety of the mercury-containing preservative thimerosal, some patients still have doubts and may ask for a thimerosalfree product. The only influenza products that contain any thimerosal are those that come in multidose vials. A description of each influenza vaccine product, including thimerosal content, indicated ages, and routes of administration, can be found on the CDC’s Web site3 (http://www.cdc.gov/flu/professionals/acip/2013-summary-recommendations.htm).
Options for those with egg allergy
There is now a product, RIV3 (FluBlok), that is manufactured without the use of eggs. It can be used in those 18 to 49 years of age with a history of egg allergy of any severity. Since 2011, the Advisory Committee on Immunization Practices (ACIP) has recommended that individuals with a history of mild egg allergy (those who experience only hives after egg exposure) may receive IIV, with additional safety precautions. Do not delay vaccination for these individuals if RIV is unavailable. Because of a lack of data demonstrating safety of LAIV for individuals with egg allergy, those allergic to eggs should receive RIV or IIV rather than LAIV.
Though the new ccIIV product, Flucelvax, is manufactured without the use of eggs, the seed viruses used to create the vaccine have been processed in eggs. The egg protein content in the vaccine is extremely low (<50 femtograms [5 × 10-14 g] per 0.5-mL dose), but the CDC does not consider it egg free. The FIGURE depicts the recommendations for those with a history of egg allergy.3
Other interventions for influenza prevention
Vaccination is only one tool available to prevent morbidity and mortality from influenza. Antiviral chemoprevention and treatment, and infection control practices can also be effective.
Antiviral chemoprevention is available for both pre- and post-exposure administration. In the past few years, the CDC has de-emphasized such use of antivirals for these indications out of concern for the supply of these agents and for the possibility that their use might lead to increased rates of viral resistance. Consider antiviral chemoprevention for those who have conditions that place them at risk for complications, and for those who are unvaccinated if they are at high risk for exposure to influenza (preexposure prophylaxis) or have been exposed (postexposure prophylaxis), if the medication can be started within 48 hours of exposure. Another option for unvaccinated high-risk patients is vigilant symptom monitoring with early treatment for influenza symptoms. Chemoprophylaxis is recommended in addition to vaccination to control influenza outbreaks at institutions that house patients at high risk for complications of influenza. Details on recommended antivirals including doses and duration of treatment can be found in a 2011 issue of Morbidity and Mortality Weekly Report.5
Antiviral treatment. The CDC recommends antiviral treatment for anyone with suspected or confirmed influenza who has progressive, severe, or complicated illness or is hospitalized for their illness.5 Treatment is also recommended for outpatients with suspected or confirmed influenza who are at higher risk for influenza complications. This latter group includes those in TABLE 1, particularly children 6 to 59 months and adults ≥50 years. Start antiviral treatment within 48 hours of the first symptoms. For hospitalized patients, however, begin treatment at any point regardless of duration of illness.
Infection control practices can prevent the spread of influenza in the health care setting and in the homes of those with influenza. These practices are also described on the CDC influenza Web site.6
1. Thompson WW, Shay DK, Weintraub E. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333-1340.
2. CDC. Estimates of deaths associated with seasonal influenza–United States, 1976-2007. MMWR Morb Mortal Wkly Rep. 2010;59:1057-1062.
3. CDC. Summary* recommendations: prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—(ACIP)—United States, 2013-14. Available at: http://www.cdc.gov/flu/professionals/ acip/2013-summary-recommendations.htm. Accessed August 9, 2013.
4. CDC. Interim adjusted estimates of seasonal influenza vaccine effectiveness—United States, February 2013. MMWR Morb Mortal Wkly Rep. 2013;62:119-123.
5. CDC. Antiviral agents for the treatment and chemoprophylaxis of influenza. MMWR Recomm Rep. 2011;60(RR01):1-24. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr6001a1. htm. Accessed July 2, 2013.
6. CDC. Infection control in health care facilities. Available at:http://www.cdc.gov/flu/professionals/infectioncontrol/index.htm. Accessed July 2, 2013.
Each year in late summer, the Centers for Disease Control and Prevention (CDC) publishes its recommendations for the prevention of influenza for the upcoming season. The severity of each influenza season varies and is difficult to predict, which underscores the need to provide maximal vaccine coverage for at-risk patient populations.
Hoping for the best, planning for the worst. Over the past several decades the annual number of influenza-related hospitalizations has varied from approximately 55,000 to 431,000,1 and the number of deaths from influenza has been as low as 3349 and as high as 48,614.2 Infection rates are usually highest in children. Complications, hospitalizations, and deaths are highest in those ≥65 years, children<2 years, and patients with medical conditions known to increase risk for influenza complications. Those at high risk of complications appear in TABLE 1.3 The main recommendations for this coming year are the same as last year, including vaccinating everyone ≥6 months of age without a contraindication, starting vaccinations as soon as vaccine is available, and continuing throughout the influenza season for those who need it.
What’s new this year
An increasing number of influenza vaccine products are available; although to date, their effectiveness (which was determined to be 56% for all vaccines used last influenza season) 4 remains below what we would hope for. The CDC’s recommendations address these new types of vaccines, including ones that have 4 antigens instead of 3, and use new terminology to describe the vaccines.3
New terminology reflects changing vaccine formulations. Last influenza season there were 2 major categories of influenza vaccines: live-attenuated influenza vaccine (LAIV) and trivalent inactivated influenza vaccine (TIV). All products were produced using egg-culture methods and contained 2 influenza A antigen subtypes and 1 B subtype. Several products this year include 4 antigens (2 A subtypes and 2 B subtypes), and some are now produced with non–eggculture methods. This has led to a new system of classification, with the term inactivated influenza vaccine (IIV) replacing TIV. TABLE 2 lists the influenza vaccine categories and abbreviations. TABLE 3 lists the contraindications for the different vaccine types.3
The new products include Flumist Quadrivalent (MedImmune), a quadrivalent LAIV (LAIV4); Fluarix Quadrivalent (GlaxoSmithKline), a quadrivalent IIV (IIV4); Flucelvax (Novartis Vaccines and Diagnostics), a cell culture-based trivalent IIV (ccIIV3); and FluBlok (Protein Sciences), a trivalent recombinant hemagglutinin influenza vaccine (RIV3). Fluzone (Sanofi Pasteur), introduced last season in a trivalent formulation, is also available this season as a quadrivalent IIV (IIV4). As a group, influenza vaccine products now offer 3 routes of administration: intramuscular, subcutaneous, and intranasal. There is currently no evidence that any route offers an advantage over another, and the CDC states no preference for any particular product or route of administration.
Mercury content is not a problem
Even though there is no scientific controversy over the safety of the mercury-containing preservative thimerosal, some patients still have doubts and may ask for a thimerosalfree product. The only influenza products that contain any thimerosal are those that come in multidose vials. A description of each influenza vaccine product, including thimerosal content, indicated ages, and routes of administration, can be found on the CDC’s Web site3 (http://www.cdc.gov/flu/professionals/acip/2013-summary-recommendations.htm).
Options for those with egg allergy
There is now a product, RIV3 (FluBlok), that is manufactured without the use of eggs. It can be used in those 18 to 49 years of age with a history of egg allergy of any severity. Since 2011, the Advisory Committee on Immunization Practices (ACIP) has recommended that individuals with a history of mild egg allergy (those who experience only hives after egg exposure) may receive IIV, with additional safety precautions. Do not delay vaccination for these individuals if RIV is unavailable. Because of a lack of data demonstrating safety of LAIV for individuals with egg allergy, those allergic to eggs should receive RIV or IIV rather than LAIV.
Though the new ccIIV product, Flucelvax, is manufactured without the use of eggs, the seed viruses used to create the vaccine have been processed in eggs. The egg protein content in the vaccine is extremely low (<50 femtograms [5 × 10-14 g] per 0.5-mL dose), but the CDC does not consider it egg free. The FIGURE depicts the recommendations for those with a history of egg allergy.3
Other interventions for influenza prevention
Vaccination is only one tool available to prevent morbidity and mortality from influenza. Antiviral chemoprevention and treatment, and infection control practices can also be effective.
Antiviral chemoprevention is available for both pre- and post-exposure administration. In the past few years, the CDC has de-emphasized such use of antivirals for these indications out of concern for the supply of these agents and for the possibility that their use might lead to increased rates of viral resistance. Consider antiviral chemoprevention for those who have conditions that place them at risk for complications, and for those who are unvaccinated if they are at high risk for exposure to influenza (preexposure prophylaxis) or have been exposed (postexposure prophylaxis), if the medication can be started within 48 hours of exposure. Another option for unvaccinated high-risk patients is vigilant symptom monitoring with early treatment for influenza symptoms. Chemoprophylaxis is recommended in addition to vaccination to control influenza outbreaks at institutions that house patients at high risk for complications of influenza. Details on recommended antivirals including doses and duration of treatment can be found in a 2011 issue of Morbidity and Mortality Weekly Report.5
Antiviral treatment. The CDC recommends antiviral treatment for anyone with suspected or confirmed influenza who has progressive, severe, or complicated illness or is hospitalized for their illness.5 Treatment is also recommended for outpatients with suspected or confirmed influenza who are at higher risk for influenza complications. This latter group includes those in TABLE 1, particularly children 6 to 59 months and adults ≥50 years. Start antiviral treatment within 48 hours of the first symptoms. For hospitalized patients, however, begin treatment at any point regardless of duration of illness.
Infection control practices can prevent the spread of influenza in the health care setting and in the homes of those with influenza. These practices are also described on the CDC influenza Web site.6
Each year in late summer, the Centers for Disease Control and Prevention (CDC) publishes its recommendations for the prevention of influenza for the upcoming season. The severity of each influenza season varies and is difficult to predict, which underscores the need to provide maximal vaccine coverage for at-risk patient populations.
Hoping for the best, planning for the worst. Over the past several decades the annual number of influenza-related hospitalizations has varied from approximately 55,000 to 431,000,1 and the number of deaths from influenza has been as low as 3349 and as high as 48,614.2 Infection rates are usually highest in children. Complications, hospitalizations, and deaths are highest in those ≥65 years, children<2 years, and patients with medical conditions known to increase risk for influenza complications. Those at high risk of complications appear in TABLE 1.3 The main recommendations for this coming year are the same as last year, including vaccinating everyone ≥6 months of age without a contraindication, starting vaccinations as soon as vaccine is available, and continuing throughout the influenza season for those who need it.
What’s new this year
An increasing number of influenza vaccine products are available; although to date, their effectiveness (which was determined to be 56% for all vaccines used last influenza season) 4 remains below what we would hope for. The CDC’s recommendations address these new types of vaccines, including ones that have 4 antigens instead of 3, and use new terminology to describe the vaccines.3
New terminology reflects changing vaccine formulations. Last influenza season there were 2 major categories of influenza vaccines: live-attenuated influenza vaccine (LAIV) and trivalent inactivated influenza vaccine (TIV). All products were produced using egg-culture methods and contained 2 influenza A antigen subtypes and 1 B subtype. Several products this year include 4 antigens (2 A subtypes and 2 B subtypes), and some are now produced with non–eggculture methods. This has led to a new system of classification, with the term inactivated influenza vaccine (IIV) replacing TIV. TABLE 2 lists the influenza vaccine categories and abbreviations. TABLE 3 lists the contraindications for the different vaccine types.3
The new products include Flumist Quadrivalent (MedImmune), a quadrivalent LAIV (LAIV4); Fluarix Quadrivalent (GlaxoSmithKline), a quadrivalent IIV (IIV4); Flucelvax (Novartis Vaccines and Diagnostics), a cell culture-based trivalent IIV (ccIIV3); and FluBlok (Protein Sciences), a trivalent recombinant hemagglutinin influenza vaccine (RIV3). Fluzone (Sanofi Pasteur), introduced last season in a trivalent formulation, is also available this season as a quadrivalent IIV (IIV4). As a group, influenza vaccine products now offer 3 routes of administration: intramuscular, subcutaneous, and intranasal. There is currently no evidence that any route offers an advantage over another, and the CDC states no preference for any particular product or route of administration.
Mercury content is not a problem
Even though there is no scientific controversy over the safety of the mercury-containing preservative thimerosal, some patients still have doubts and may ask for a thimerosalfree product. The only influenza products that contain any thimerosal are those that come in multidose vials. A description of each influenza vaccine product, including thimerosal content, indicated ages, and routes of administration, can be found on the CDC’s Web site3 (http://www.cdc.gov/flu/professionals/acip/2013-summary-recommendations.htm).
Options for those with egg allergy
There is now a product, RIV3 (FluBlok), that is manufactured without the use of eggs. It can be used in those 18 to 49 years of age with a history of egg allergy of any severity. Since 2011, the Advisory Committee on Immunization Practices (ACIP) has recommended that individuals with a history of mild egg allergy (those who experience only hives after egg exposure) may receive IIV, with additional safety precautions. Do not delay vaccination for these individuals if RIV is unavailable. Because of a lack of data demonstrating safety of LAIV for individuals with egg allergy, those allergic to eggs should receive RIV or IIV rather than LAIV.
Though the new ccIIV product, Flucelvax, is manufactured without the use of eggs, the seed viruses used to create the vaccine have been processed in eggs. The egg protein content in the vaccine is extremely low (<50 femtograms [5 × 10-14 g] per 0.5-mL dose), but the CDC does not consider it egg free. The FIGURE depicts the recommendations for those with a history of egg allergy.3
Other interventions for influenza prevention
Vaccination is only one tool available to prevent morbidity and mortality from influenza. Antiviral chemoprevention and treatment, and infection control practices can also be effective.
Antiviral chemoprevention is available for both pre- and post-exposure administration. In the past few years, the CDC has de-emphasized such use of antivirals for these indications out of concern for the supply of these agents and for the possibility that their use might lead to increased rates of viral resistance. Consider antiviral chemoprevention for those who have conditions that place them at risk for complications, and for those who are unvaccinated if they are at high risk for exposure to influenza (preexposure prophylaxis) or have been exposed (postexposure prophylaxis), if the medication can be started within 48 hours of exposure. Another option for unvaccinated high-risk patients is vigilant symptom monitoring with early treatment for influenza symptoms. Chemoprophylaxis is recommended in addition to vaccination to control influenza outbreaks at institutions that house patients at high risk for complications of influenza. Details on recommended antivirals including doses and duration of treatment can be found in a 2011 issue of Morbidity and Mortality Weekly Report.5
Antiviral treatment. The CDC recommends antiviral treatment for anyone with suspected or confirmed influenza who has progressive, severe, or complicated illness or is hospitalized for their illness.5 Treatment is also recommended for outpatients with suspected or confirmed influenza who are at higher risk for influenza complications. This latter group includes those in TABLE 1, particularly children 6 to 59 months and adults ≥50 years. Start antiviral treatment within 48 hours of the first symptoms. For hospitalized patients, however, begin treatment at any point regardless of duration of illness.
Infection control practices can prevent the spread of influenza in the health care setting and in the homes of those with influenza. These practices are also described on the CDC influenza Web site.6
1. Thompson WW, Shay DK, Weintraub E. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333-1340.
2. CDC. Estimates of deaths associated with seasonal influenza–United States, 1976-2007. MMWR Morb Mortal Wkly Rep. 2010;59:1057-1062.
3. CDC. Summary* recommendations: prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—(ACIP)—United States, 2013-14. Available at: http://www.cdc.gov/flu/professionals/ acip/2013-summary-recommendations.htm. Accessed August 9, 2013.
4. CDC. Interim adjusted estimates of seasonal influenza vaccine effectiveness—United States, February 2013. MMWR Morb Mortal Wkly Rep. 2013;62:119-123.
5. CDC. Antiviral agents for the treatment and chemoprophylaxis of influenza. MMWR Recomm Rep. 2011;60(RR01):1-24. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr6001a1. htm. Accessed July 2, 2013.
6. CDC. Infection control in health care facilities. Available at:http://www.cdc.gov/flu/professionals/infectioncontrol/index.htm. Accessed July 2, 2013.
1. Thompson WW, Shay DK, Weintraub E. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333-1340.
2. CDC. Estimates of deaths associated with seasonal influenza–United States, 1976-2007. MMWR Morb Mortal Wkly Rep. 2010;59:1057-1062.
3. CDC. Summary* recommendations: prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—(ACIP)—United States, 2013-14. Available at: http://www.cdc.gov/flu/professionals/ acip/2013-summary-recommendations.htm. Accessed August 9, 2013.
4. CDC. Interim adjusted estimates of seasonal influenza vaccine effectiveness—United States, February 2013. MMWR Morb Mortal Wkly Rep. 2013;62:119-123.
5. CDC. Antiviral agents for the treatment and chemoprophylaxis of influenza. MMWR Recomm Rep. 2011;60(RR01):1-24. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr6001a1. htm. Accessed July 2, 2013.
6. CDC. Infection control in health care facilities. Available at:http://www.cdc.gov/flu/professionals/infectioncontrol/index.htm. Accessed July 2, 2013.
This asthma treatment has a lasting side effect in children
Before prescribing inhaled corticosteroids (ICS) for a child with asthma, tell the patient—and parents—that their use could lead to a small but permanent effect on adult height.1
STRENGTH OF RECOMMENDATIONS
B: Based on one prospective study.
Kelly HW, Sternberg AL, Lescher R, et al; CAMP Research Group. Effect of inhaled glucocorticoids in childhood on adult height. N Engl J Med. 2012;367:904-912.
Illustrative case
A 10-year-old boy is brought in by his father for asthma follow-up. The child uses an albuterol inhaler, but has had increased coughing and wheezing recently. You are ready to step up his asthma therapy to include ICS. But the patient’s father questions this, noting that he recently read that steroids may reduce a child’s growth. How should you respond?
Inhaled corticosteroids (ICS) are a mainstay in the treatment of asthma ranging from mild persistent to severe. Standards of care for asthma treatment involve a stepwise approach, with ICS added if symptoms are not controlled with short-acting beta antagonists alone.2 In addition, monotherapy with ICS is more effective for controlling symptoms than leukotriene inhibitors or other controller medications, while also decreasing hospitalizations and nocturnal awakenings and improving quality of life—with few side effects.3
What we know about ICS and children’s growth
One adverse effect of ICS, however, is that of “decreased linear growth velocity”4—ie, slowing the rate at which children grow. Until recently, children were thought to “catch up” later in life, either by growing for a longer period of time than they would had they not taken ICS or by growing at an increased velocity after ICS medications are discontinued.4-6
Study summary: The effect on growth is small, but long-lasting
Kelly et al conducted a prospective observational cohort study that followed 943 (90.7%) participants in the Childhood Asthma Management Program (CAMP) in the years after the randomized controlled trial (RCT) ended.
A double-blind, placebo-controlled RCT, CAMP studied the linear growth of 1041 children with mild-to-moderate persistent asthma who were divided into 3 treatment groups: One group received 200 mcg inhaled budesonide twice daily; a second group received 8 mg inhaled nedocromil twice daily; and a third group received placebo. Albuterol was used symptomatically by all 3 groups.7 The children ranged in age from 5 to 13 years at the start of the study; 98 patients—split evenly among the 3 treatment arms—were lost to follow-up.
During the 4 to 6 years of the CAMP trial, the budesonide group received a mean total of 636 mg ICS, whereas the nedocromil and placebo groups received an average of 88.5 and 109.4 mg ICS, respectively. After the RCT ended, all participants had standardized asthma treatment, receiving mean adjusted total doses of ICS of 381 mg for the budesonide group, 347.9 mg for the nedocromil group, and 355 mg for the placebo group.
Patients’ height was measured every 6 months for the next 4.5 years, and once or twice a year thereafter until they reached adult height (at a mean age of 24.9±2.7 years).
ICS users were a half inch shorter
Long-term ICS use was linked to a lower adult height. The adjusted mean height was 171.1 cm for the budesonide group vs 172.3 cm for those on placebo, a difference of 1.2 cm, or 0.47 inch (95% confidence interval [CI], −1.9 to −0.5; P=.001); the mean adult height in the nedocromil group (172.1 cm) was similar to that of the placebo group (−0.2 cm; 95% CI, −0.9 to 0.5; P=.61).
The lower adult height in the ICS group did not vary significantly based on sex, age at trial entry, race, or duration of asthma prior to trial entry; however, dose was a key factor. A larger daily dose of budesonide—particularly in the first 2 years of the RCT—was associated with a lower adult height (about −0.1 cm for each mcg/kg in that 2-year time frame). This was consistent with results from studies that looked at other types of ICS (beclomethasone, fluticasone, and mometasone).8-11
The study also showed that growth velocity was reduced in the first 2 years of assigned treatment with budesonide, and this was primarily among prepubertal participants. After the initial 2-year slowing in growth rate, the children resumed growing at normal speeds.
What’s new: Now we know: Children don’t “catch up"
Retrospective studies have reported that children on ICS for mild persistent to moderate asthma would have an initial slowing in growth velocity but then “catch up” by growing for a longer period of time.3-5 This is the first prospective study with good follow-up to show that ICS use affects long-term growth and adult height. While the effect is not large, some children and their families might be concerned about it.
Caveats: ICS use was atypical
The randomized controlled portion of the study used a prescribed dose of budesonide without regard to symptoms. This is not the typical pattern of ICS use. In addition, compliance with ICS varies significantly.12 Because the effect on adult height appears to be dose dependent, however, we think the results of this study are valid.
In addition, there was a placebo control group (and big differences in exposure to ICS) only for the duration of the RCT. During the subsequent study, all patients received equivalent doses of ICS. This means that the variation in mean adult height achieved can be primarily ascribed to participants’ use of ICS during the 4- to 6-year CAMP trial. Of note, the effect of ICS was greatest in prepubertal participants, so there may be a diminished effect as teens approach their final height.
The study did not look at the effect of ICS use in patients with severe asthma—the group most likely to use ICS. However, the benefits of ICS for those with severe asthma likely outweigh any negative effects on adult height.
Challenges to implementation: What to tell patients
The message we convey to patients (and parents) about ICS use is a nuanced one. We can stress that ICS remain very important in the treatment of asthma and, while it appears that their use causes a slight decrease in adult height, most children with persistent asthma benefit from ICS.
Acknowledgement
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Kelly HW, Sternberg AL, Lescher R, et al; CAMP Research Group. Effect of inhaled glucocorticoids in childhood on adult height. N Engl J Med. 2012;367:904-912.
2. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. National Institutes of Health National Heart, Lung and Blood Institute: National Asthma Education and Prevention Program, 2007. Available at: http://www.nhlbi. nih.gov/guidelines/asthma/asthgdln.pdf. Accessed August 15, 2013.
3. Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/ or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;(5):CD002314.
4. Agertoft L, Pedersen S. Effect of long- term treatment with budesonide on adult height in children with asthma. N Engl J Med. 2000;343:1064-1069.
5. Van Bever HP, Desager KN, Lijssens N, et al. Does treatment of asthmatic children with inhaled corticosteroids affect their adult height? Pediatr Pulmonol. 1999;27:369-375.
6. Silverstein MD, Yunginger JW, Reed CE, et al. Attained adult height after childhood asthma: effect of glucocorticoid therapy. J Allergy Clin Immunol. 1997;99:466-474.
7. The Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med. 2000;343:1054-1063.
8. Tinkelman DG, Reed CE, Nelson HS, et al. Aerosol beclomethasone dipropionate compared with theophylline as primary treatment of chronic, mild to moderately severe asthma in children. Pediatrics. 1993;92:64-77.
9. Verberne AA, Frost C, Roorda RJ, et al. One year treatment with salmeterol compared with beclomethasone in children with asthma. Am J Respir Crit Care Med. 1997;156:688-695.
10. Allen DB, Bronsky EA, LaForce CF, et al. Growth in asthmatic children treated with fluticasone propionate. J Pediatr 1998;132: 472-477.
11. Skoner DP, Meltzer EO, Milgrom H, et al. Effects of inhaled mometasone furoate on growth velocity and adrenal function: a placebo-controlled trial in children 4-9 years old with mild persistent asthma. J Asthma. 2011;48:848-859.
12. Cochrane MG, Bala MV, Downs KE, et al. Inhaled corticosteroids for asthma therapy: patient compliance, devices, and inhalation technique. Chest. 2000;117:542-550.
Before prescribing inhaled corticosteroids (ICS) for a child with asthma, tell the patient—and parents—that their use could lead to a small but permanent effect on adult height.1
STRENGTH OF RECOMMENDATIONS
B: Based on one prospective study.
Kelly HW, Sternberg AL, Lescher R, et al; CAMP Research Group. Effect of inhaled glucocorticoids in childhood on adult height. N Engl J Med. 2012;367:904-912.
Illustrative case
A 10-year-old boy is brought in by his father for asthma follow-up. The child uses an albuterol inhaler, but has had increased coughing and wheezing recently. You are ready to step up his asthma therapy to include ICS. But the patient’s father questions this, noting that he recently read that steroids may reduce a child’s growth. How should you respond?
Inhaled corticosteroids (ICS) are a mainstay in the treatment of asthma ranging from mild persistent to severe. Standards of care for asthma treatment involve a stepwise approach, with ICS added if symptoms are not controlled with short-acting beta antagonists alone.2 In addition, monotherapy with ICS is more effective for controlling symptoms than leukotriene inhibitors or other controller medications, while also decreasing hospitalizations and nocturnal awakenings and improving quality of life—with few side effects.3
What we know about ICS and children’s growth
One adverse effect of ICS, however, is that of “decreased linear growth velocity”4—ie, slowing the rate at which children grow. Until recently, children were thought to “catch up” later in life, either by growing for a longer period of time than they would had they not taken ICS or by growing at an increased velocity after ICS medications are discontinued.4-6
Study summary: The effect on growth is small, but long-lasting
Kelly et al conducted a prospective observational cohort study that followed 943 (90.7%) participants in the Childhood Asthma Management Program (CAMP) in the years after the randomized controlled trial (RCT) ended.
A double-blind, placebo-controlled RCT, CAMP studied the linear growth of 1041 children with mild-to-moderate persistent asthma who were divided into 3 treatment groups: One group received 200 mcg inhaled budesonide twice daily; a second group received 8 mg inhaled nedocromil twice daily; and a third group received placebo. Albuterol was used symptomatically by all 3 groups.7 The children ranged in age from 5 to 13 years at the start of the study; 98 patients—split evenly among the 3 treatment arms—were lost to follow-up.
During the 4 to 6 years of the CAMP trial, the budesonide group received a mean total of 636 mg ICS, whereas the nedocromil and placebo groups received an average of 88.5 and 109.4 mg ICS, respectively. After the RCT ended, all participants had standardized asthma treatment, receiving mean adjusted total doses of ICS of 381 mg for the budesonide group, 347.9 mg for the nedocromil group, and 355 mg for the placebo group.
Patients’ height was measured every 6 months for the next 4.5 years, and once or twice a year thereafter until they reached adult height (at a mean age of 24.9±2.7 years).
ICS users were a half inch shorter
Long-term ICS use was linked to a lower adult height. The adjusted mean height was 171.1 cm for the budesonide group vs 172.3 cm for those on placebo, a difference of 1.2 cm, or 0.47 inch (95% confidence interval [CI], −1.9 to −0.5; P=.001); the mean adult height in the nedocromil group (172.1 cm) was similar to that of the placebo group (−0.2 cm; 95% CI, −0.9 to 0.5; P=.61).
The lower adult height in the ICS group did not vary significantly based on sex, age at trial entry, race, or duration of asthma prior to trial entry; however, dose was a key factor. A larger daily dose of budesonide—particularly in the first 2 years of the RCT—was associated with a lower adult height (about −0.1 cm for each mcg/kg in that 2-year time frame). This was consistent with results from studies that looked at other types of ICS (beclomethasone, fluticasone, and mometasone).8-11
The study also showed that growth velocity was reduced in the first 2 years of assigned treatment with budesonide, and this was primarily among prepubertal participants. After the initial 2-year slowing in growth rate, the children resumed growing at normal speeds.
What’s new: Now we know: Children don’t “catch up"
Retrospective studies have reported that children on ICS for mild persistent to moderate asthma would have an initial slowing in growth velocity but then “catch up” by growing for a longer period of time.3-5 This is the first prospective study with good follow-up to show that ICS use affects long-term growth and adult height. While the effect is not large, some children and their families might be concerned about it.
Caveats: ICS use was atypical
The randomized controlled portion of the study used a prescribed dose of budesonide without regard to symptoms. This is not the typical pattern of ICS use. In addition, compliance with ICS varies significantly.12 Because the effect on adult height appears to be dose dependent, however, we think the results of this study are valid.
In addition, there was a placebo control group (and big differences in exposure to ICS) only for the duration of the RCT. During the subsequent study, all patients received equivalent doses of ICS. This means that the variation in mean adult height achieved can be primarily ascribed to participants’ use of ICS during the 4- to 6-year CAMP trial. Of note, the effect of ICS was greatest in prepubertal participants, so there may be a diminished effect as teens approach their final height.
The study did not look at the effect of ICS use in patients with severe asthma—the group most likely to use ICS. However, the benefits of ICS for those with severe asthma likely outweigh any negative effects on adult height.
Challenges to implementation: What to tell patients
The message we convey to patients (and parents) about ICS use is a nuanced one. We can stress that ICS remain very important in the treatment of asthma and, while it appears that their use causes a slight decrease in adult height, most children with persistent asthma benefit from ICS.
Acknowledgement
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Before prescribing inhaled corticosteroids (ICS) for a child with asthma, tell the patient—and parents—that their use could lead to a small but permanent effect on adult height.1
STRENGTH OF RECOMMENDATIONS
B: Based on one prospective study.
Kelly HW, Sternberg AL, Lescher R, et al; CAMP Research Group. Effect of inhaled glucocorticoids in childhood on adult height. N Engl J Med. 2012;367:904-912.
Illustrative case
A 10-year-old boy is brought in by his father for asthma follow-up. The child uses an albuterol inhaler, but has had increased coughing and wheezing recently. You are ready to step up his asthma therapy to include ICS. But the patient’s father questions this, noting that he recently read that steroids may reduce a child’s growth. How should you respond?
Inhaled corticosteroids (ICS) are a mainstay in the treatment of asthma ranging from mild persistent to severe. Standards of care for asthma treatment involve a stepwise approach, with ICS added if symptoms are not controlled with short-acting beta antagonists alone.2 In addition, monotherapy with ICS is more effective for controlling symptoms than leukotriene inhibitors or other controller medications, while also decreasing hospitalizations and nocturnal awakenings and improving quality of life—with few side effects.3
What we know about ICS and children’s growth
One adverse effect of ICS, however, is that of “decreased linear growth velocity”4—ie, slowing the rate at which children grow. Until recently, children were thought to “catch up” later in life, either by growing for a longer period of time than they would had they not taken ICS or by growing at an increased velocity after ICS medications are discontinued.4-6
Study summary: The effect on growth is small, but long-lasting
Kelly et al conducted a prospective observational cohort study that followed 943 (90.7%) participants in the Childhood Asthma Management Program (CAMP) in the years after the randomized controlled trial (RCT) ended.
A double-blind, placebo-controlled RCT, CAMP studied the linear growth of 1041 children with mild-to-moderate persistent asthma who were divided into 3 treatment groups: One group received 200 mcg inhaled budesonide twice daily; a second group received 8 mg inhaled nedocromil twice daily; and a third group received placebo. Albuterol was used symptomatically by all 3 groups.7 The children ranged in age from 5 to 13 years at the start of the study; 98 patients—split evenly among the 3 treatment arms—were lost to follow-up.
During the 4 to 6 years of the CAMP trial, the budesonide group received a mean total of 636 mg ICS, whereas the nedocromil and placebo groups received an average of 88.5 and 109.4 mg ICS, respectively. After the RCT ended, all participants had standardized asthma treatment, receiving mean adjusted total doses of ICS of 381 mg for the budesonide group, 347.9 mg for the nedocromil group, and 355 mg for the placebo group.
Patients’ height was measured every 6 months for the next 4.5 years, and once or twice a year thereafter until they reached adult height (at a mean age of 24.9±2.7 years).
ICS users were a half inch shorter
Long-term ICS use was linked to a lower adult height. The adjusted mean height was 171.1 cm for the budesonide group vs 172.3 cm for those on placebo, a difference of 1.2 cm, or 0.47 inch (95% confidence interval [CI], −1.9 to −0.5; P=.001); the mean adult height in the nedocromil group (172.1 cm) was similar to that of the placebo group (−0.2 cm; 95% CI, −0.9 to 0.5; P=.61).
The lower adult height in the ICS group did not vary significantly based on sex, age at trial entry, race, or duration of asthma prior to trial entry; however, dose was a key factor. A larger daily dose of budesonide—particularly in the first 2 years of the RCT—was associated with a lower adult height (about −0.1 cm for each mcg/kg in that 2-year time frame). This was consistent with results from studies that looked at other types of ICS (beclomethasone, fluticasone, and mometasone).8-11
The study also showed that growth velocity was reduced in the first 2 years of assigned treatment with budesonide, and this was primarily among prepubertal participants. After the initial 2-year slowing in growth rate, the children resumed growing at normal speeds.
What’s new: Now we know: Children don’t “catch up"
Retrospective studies have reported that children on ICS for mild persistent to moderate asthma would have an initial slowing in growth velocity but then “catch up” by growing for a longer period of time.3-5 This is the first prospective study with good follow-up to show that ICS use affects long-term growth and adult height. While the effect is not large, some children and their families might be concerned about it.
Caveats: ICS use was atypical
The randomized controlled portion of the study used a prescribed dose of budesonide without regard to symptoms. This is not the typical pattern of ICS use. In addition, compliance with ICS varies significantly.12 Because the effect on adult height appears to be dose dependent, however, we think the results of this study are valid.
In addition, there was a placebo control group (and big differences in exposure to ICS) only for the duration of the RCT. During the subsequent study, all patients received equivalent doses of ICS. This means that the variation in mean adult height achieved can be primarily ascribed to participants’ use of ICS during the 4- to 6-year CAMP trial. Of note, the effect of ICS was greatest in prepubertal participants, so there may be a diminished effect as teens approach their final height.
The study did not look at the effect of ICS use in patients with severe asthma—the group most likely to use ICS. However, the benefits of ICS for those with severe asthma likely outweigh any negative effects on adult height.
Challenges to implementation: What to tell patients
The message we convey to patients (and parents) about ICS use is a nuanced one. We can stress that ICS remain very important in the treatment of asthma and, while it appears that their use causes a slight decrease in adult height, most children with persistent asthma benefit from ICS.
Acknowledgement
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Kelly HW, Sternberg AL, Lescher R, et al; CAMP Research Group. Effect of inhaled glucocorticoids in childhood on adult height. N Engl J Med. 2012;367:904-912.
2. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. National Institutes of Health National Heart, Lung and Blood Institute: National Asthma Education and Prevention Program, 2007. Available at: http://www.nhlbi. nih.gov/guidelines/asthma/asthgdln.pdf. Accessed August 15, 2013.
3. Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/ or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;(5):CD002314.
4. Agertoft L, Pedersen S. Effect of long- term treatment with budesonide on adult height in children with asthma. N Engl J Med. 2000;343:1064-1069.
5. Van Bever HP, Desager KN, Lijssens N, et al. Does treatment of asthmatic children with inhaled corticosteroids affect their adult height? Pediatr Pulmonol. 1999;27:369-375.
6. Silverstein MD, Yunginger JW, Reed CE, et al. Attained adult height after childhood asthma: effect of glucocorticoid therapy. J Allergy Clin Immunol. 1997;99:466-474.
7. The Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med. 2000;343:1054-1063.
8. Tinkelman DG, Reed CE, Nelson HS, et al. Aerosol beclomethasone dipropionate compared with theophylline as primary treatment of chronic, mild to moderately severe asthma in children. Pediatrics. 1993;92:64-77.
9. Verberne AA, Frost C, Roorda RJ, et al. One year treatment with salmeterol compared with beclomethasone in children with asthma. Am J Respir Crit Care Med. 1997;156:688-695.
10. Allen DB, Bronsky EA, LaForce CF, et al. Growth in asthmatic children treated with fluticasone propionate. J Pediatr 1998;132: 472-477.
11. Skoner DP, Meltzer EO, Milgrom H, et al. Effects of inhaled mometasone furoate on growth velocity and adrenal function: a placebo-controlled trial in children 4-9 years old with mild persistent asthma. J Asthma. 2011;48:848-859.
12. Cochrane MG, Bala MV, Downs KE, et al. Inhaled corticosteroids for asthma therapy: patient compliance, devices, and inhalation technique. Chest. 2000;117:542-550.
1. Kelly HW, Sternberg AL, Lescher R, et al; CAMP Research Group. Effect of inhaled glucocorticoids in childhood on adult height. N Engl J Med. 2012;367:904-912.
2. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. National Institutes of Health National Heart, Lung and Blood Institute: National Asthma Education and Prevention Program, 2007. Available at: http://www.nhlbi. nih.gov/guidelines/asthma/asthgdln.pdf. Accessed August 15, 2013.
3. Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/ or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;(5):CD002314.
4. Agertoft L, Pedersen S. Effect of long- term treatment with budesonide on adult height in children with asthma. N Engl J Med. 2000;343:1064-1069.
5. Van Bever HP, Desager KN, Lijssens N, et al. Does treatment of asthmatic children with inhaled corticosteroids affect their adult height? Pediatr Pulmonol. 1999;27:369-375.
6. Silverstein MD, Yunginger JW, Reed CE, et al. Attained adult height after childhood asthma: effect of glucocorticoid therapy. J Allergy Clin Immunol. 1997;99:466-474.
7. The Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med. 2000;343:1054-1063.
8. Tinkelman DG, Reed CE, Nelson HS, et al. Aerosol beclomethasone dipropionate compared with theophylline as primary treatment of chronic, mild to moderately severe asthma in children. Pediatrics. 1993;92:64-77.
9. Verberne AA, Frost C, Roorda RJ, et al. One year treatment with salmeterol compared with beclomethasone in children with asthma. Am J Respir Crit Care Med. 1997;156:688-695.
10. Allen DB, Bronsky EA, LaForce CF, et al. Growth in asthmatic children treated with fluticasone propionate. J Pediatr 1998;132: 472-477.
11. Skoner DP, Meltzer EO, Milgrom H, et al. Effects of inhaled mometasone furoate on growth velocity and adrenal function: a placebo-controlled trial in children 4-9 years old with mild persistent asthma. J Asthma. 2011;48:848-859.
12. Cochrane MG, Bala MV, Downs KE, et al. Inhaled corticosteroids for asthma therapy: patient compliance, devices, and inhalation technique. Chest. 2000;117:542-550.
Copyright © 2013 Family Physicians Inquiries Network. All rights reserved.
Will screening open Pandora’s box?
"If it ain’t broke, don’t fix it" or "A stitch in time saves nine"—which do you prefer?
When I taught epidemiology at the University of Chicago, I asked first-year medical students that question before discussing the science of screening for early detection of disease. Each year, the class was about evenly divided. Their split response reinforced to me the need for shared decision making when we offer screening tests to our patients.
Shared decision making is especially important in light of new evidence about the effectiveness (or lack thereof) of some screening tests. Several bread-and-butter screening procedures and tests promoted for years have been debunked as having no value (routine testicular exam and monthly self-breast exam), having harms that might outweigh the benefits (PSA for prostate cancer), or having marginal benefit for those in certain age groups (mammography in women younger than 50). And, as treatments for cancer get better and better, screening will have less and less value. What would a 30-year-old do if he found out he has a gene that makes him susceptible to Alzheimer's disease?
The biggest screening test challenge, however— genome screening—is still to come. Genomic sequencing analysis is already useful for the diagnosis of certain genetic disorders and for treatment decisions in certain cancers. Genomic sequencing to screen for disease, however, is fraught with ethical challenges and the absolute need for shared decision making.
What if gene analysis uncovers "incidental findings" about risk faced by asymptomatic patients, like the "incidentalomas" described in "When to worry about incidental renal and adrenal masses"? The debate about what to do with incidental findings from genetic analysis is heating up because of the American College of Medical Genetics and Genomics' recent recommendations1 to automatically screen for 56 genes that may contain "potentially important" findings when genome sequencing is done for any reason.
Talk about Pandora’s box! Suppose a 30-year-old finds he carries a gene that makes him susceptible to Alzheimer’s disease. What would he do with that information, other than get depressed when he realizes there are not yet any effective early interventions?
Family physicians are likely to be asked more and more questions about genome analysis.* Be prepared. You can start by asking patients whether they adhere to an "If it ain’t broke…" " or "A stitch in time…" approach.
Reference
1. Green RC, Berg JS, Grody WW, et al. ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet Med. 2013;15:565-574. Available at: https://www.acmg.net/docs/IF_Statement_Final_7.24.13.pdf. Accessed August 20, 2013.
"If it ain’t broke, don’t fix it" or "A stitch in time saves nine"—which do you prefer?
When I taught epidemiology at the University of Chicago, I asked first-year medical students that question before discussing the science of screening for early detection of disease. Each year, the class was about evenly divided. Their split response reinforced to me the need for shared decision making when we offer screening tests to our patients.
Shared decision making is especially important in light of new evidence about the effectiveness (or lack thereof) of some screening tests. Several bread-and-butter screening procedures and tests promoted for years have been debunked as having no value (routine testicular exam and monthly self-breast exam), having harms that might outweigh the benefits (PSA for prostate cancer), or having marginal benefit for those in certain age groups (mammography in women younger than 50). And, as treatments for cancer get better and better, screening will have less and less value. What would a 30-year-old do if he found out he has a gene that makes him susceptible to Alzheimer's disease?
The biggest screening test challenge, however— genome screening—is still to come. Genomic sequencing analysis is already useful for the diagnosis of certain genetic disorders and for treatment decisions in certain cancers. Genomic sequencing to screen for disease, however, is fraught with ethical challenges and the absolute need for shared decision making.
What if gene analysis uncovers "incidental findings" about risk faced by asymptomatic patients, like the "incidentalomas" described in "When to worry about incidental renal and adrenal masses"? The debate about what to do with incidental findings from genetic analysis is heating up because of the American College of Medical Genetics and Genomics' recent recommendations1 to automatically screen for 56 genes that may contain "potentially important" findings when genome sequencing is done for any reason.
Talk about Pandora’s box! Suppose a 30-year-old finds he carries a gene that makes him susceptible to Alzheimer’s disease. What would he do with that information, other than get depressed when he realizes there are not yet any effective early interventions?
Family physicians are likely to be asked more and more questions about genome analysis.* Be prepared. You can start by asking patients whether they adhere to an "If it ain’t broke…" " or "A stitch in time…" approach.
"If it ain’t broke, don’t fix it" or "A stitch in time saves nine"—which do you prefer?
When I taught epidemiology at the University of Chicago, I asked first-year medical students that question before discussing the science of screening for early detection of disease. Each year, the class was about evenly divided. Their split response reinforced to me the need for shared decision making when we offer screening tests to our patients.
Shared decision making is especially important in light of new evidence about the effectiveness (or lack thereof) of some screening tests. Several bread-and-butter screening procedures and tests promoted for years have been debunked as having no value (routine testicular exam and monthly self-breast exam), having harms that might outweigh the benefits (PSA for prostate cancer), or having marginal benefit for those in certain age groups (mammography in women younger than 50). And, as treatments for cancer get better and better, screening will have less and less value. What would a 30-year-old do if he found out he has a gene that makes him susceptible to Alzheimer's disease?
The biggest screening test challenge, however— genome screening—is still to come. Genomic sequencing analysis is already useful for the diagnosis of certain genetic disorders and for treatment decisions in certain cancers. Genomic sequencing to screen for disease, however, is fraught with ethical challenges and the absolute need for shared decision making.
What if gene analysis uncovers "incidental findings" about risk faced by asymptomatic patients, like the "incidentalomas" described in "When to worry about incidental renal and adrenal masses"? The debate about what to do with incidental findings from genetic analysis is heating up because of the American College of Medical Genetics and Genomics' recent recommendations1 to automatically screen for 56 genes that may contain "potentially important" findings when genome sequencing is done for any reason.
Talk about Pandora’s box! Suppose a 30-year-old finds he carries a gene that makes him susceptible to Alzheimer’s disease. What would he do with that information, other than get depressed when he realizes there are not yet any effective early interventions?
Family physicians are likely to be asked more and more questions about genome analysis.* Be prepared. You can start by asking patients whether they adhere to an "If it ain’t broke…" " or "A stitch in time…" approach.
Reference
1. Green RC, Berg JS, Grody WW, et al. ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet Med. 2013;15:565-574. Available at: https://www.acmg.net/docs/IF_Statement_Final_7.24.13.pdf. Accessed August 20, 2013.
Reference
1. Green RC, Berg JS, Grody WW, et al. ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet Med. 2013;15:565-574. Available at: https://www.acmg.net/docs/IF_Statement_Final_7.24.13.pdf. Accessed August 20, 2013.
The human microbiome
Microbiome refers to all the microbial life that exists in a specific niche. In the case of humans that means a lot of bacteria, viruses, fungi, parasites, and a very old class of single-celled organisms called archaea. The organisms include commensals and pathogenic microorganisms. Many articles distinguish "microbiome" and "microbiota" to differentiate the collective genomes of the microorganisms or the microorganisms themselves, respectively. However, these terms are largely synonymous.
A number of advances have allowed scientists to make major advances in understanding the microbiome. Specifically, we now have the molecular tools to perform gene expression analysis for an entire microbial community in the new discipline of metagenomics and analyze the massive results with new methods of mathematical analysis.
The human body contains over 10 times more microorganisms than human cells. The existence of a remarkably diverse and enormously large microbial world on us and in us first began to come to light in the late 1990s. We are learning more and more about the individual locations of the human host that have different populations of microbes and about differences among humans that contribute to or account for susceptibility to infectious diseases as well as autoimmune diseases and even obesity and cancer.
The nasopharyngeal microbiome has become an area of research by our group led by Qingfu Xu, Ph.D., at the Rochester (N.Y.) General Hospital Research Institute in collaboration with Melinda M. Pettigrew, Ph.D., at the Yale School of Public Health, New Haven, Conn., and Dr. Janet R. Casey at Legacy Pediatrics, also in Rochester. The traditional view of the immune system is undergoing reassessment as we learn that our microbiota has coevolved with our immune system, and each exerts influence over the other. Our group has a special interest in the impact of the nasopharyngeal microbiome on the innate immune response in that physiologic niche, and the way the innate immune system modifies the microbiome. With a special interest in the bacteria that cause respiratory infections such as acute otitis media, acute sinusitis, bronchopneumonia, and pneumonia, we have identified how microbes like Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis compete and synergize in the nasopharynx to cause infections.
Also, we seek to better understand how respiratory viruses like respiratory syncytial virus (RSV), influenzae, parainfluenzae, rhinovirus, and others facilitate the overgrowth of S. pneumoniae, H. flu, and M. catarrhalis in the nose such that they convert from commensals to pathogens. But the synergy goes both ways, as we have recently found that S. pneumoniae facilitates upper respiratory viral infections.
Up to now most of the work on the human microbiome has focused on the gut, and nearly all studies have occurred in adults. Perhaps readers are aware of the use of "fecal microbiota transplantation" as a treatment/cure for Clostridium difficile infection. Unhealthy gut microbiota in premature neonates are a major contributing factor in necrotizing enterocolitis.
For decades, physicians have been taught that obesity is a problem derived from excessive caloric intake and inadequate caloric consumption through activity, plus vaguely defined differences in "metabolism." As a consequence, we checked for hypothyroidism � I never found a case. New research has shown that there is a difference in the "metabolism" of obese patients, but the difference is how the individual gut microbiota metabolizes our food. It turns out the thinner individuals have a microbiota that is less efficient in breaking down the food we ingest to allow efficient absorption into the bloodstream, whereas obese individuals have a more efficient microbiota that facilitates absorption of a greater percentage of the proteins, carbohydrates, and fats that are ingested. So the pathway to treatment of obesity may lie in the study of the microbiome!
It turns out that the microbiota of the skin is highly diverse. The microbiota colonizing the antecubital fossa is different from that of the forearm or biceps or axillae. When atopic dermatitis flares, it is often in the antecubital fossa, and it is caused by overgrowth of Staphylococcus aureus. The microbiome of a patient with atopic dermatitis is different from that of a person without atopic dermatitis, and the former microbiota is more permissive to S. aureus becoming a pathogen rather than a commensal of the skin.
Prevention of urogenital infections in girls depends on a healthy vaginal microbiota. Bacterial vaginosis requires the establishment of overgrowth by Gardnerella vaginalis and Peptostreptococcus anaerobius that can only occur if the resident microbiota is unable to control the proliferation of these bacteria. Only if the microbiota of the perineum, urethra, and bladder will allow potential urinary tract infection pathogens access to epithelial attachment sites can infection become established.
A last topic for this column is the role of the microbiota in autoimmune diseases. In particular, I find it fascinating to learn that aberrant, unstable intestinal microbiota can lead to a leaky intestinal mucosal barrier. Combined with inadequate innate immune responses in the gut, progression may occur that allows antigens from microbes that cross-react with antigens of self in the pancreas to stimulate autoimmune antibodies. Similar pathogenic mechanisms may contribute to inflammatory bowel disease, rheumatoid arthritis, multiple sclerosis, and other autoimmune diseases.
I anticipate future research will establish the makeup of a healthy microbiota associated with protection from the diseases mentioned here. With that knowledge, the next efforts in research will focus on how to convert an unhealthy microbiota to a healthy one. If the efforts succeed, I see new promising treatments in the future.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Rochester (N.Y.) General Research Institute. He is also a pediatrician at Legacy Pediatrics in Rochester. The microbiome research at the Rochester General Hospital Research Institute is supported by the National Institutes of Health and the National Institute for Deafness and Communication Disorders. To comment, e-mail him at pdnews@ frontlinemedcom.com.
Microbiome refers to all the microbial life that exists in a specific niche. In the case of humans that means a lot of bacteria, viruses, fungi, parasites, and a very old class of single-celled organisms called archaea. The organisms include commensals and pathogenic microorganisms. Many articles distinguish "microbiome" and "microbiota" to differentiate the collective genomes of the microorganisms or the microorganisms themselves, respectively. However, these terms are largely synonymous.
A number of advances have allowed scientists to make major advances in understanding the microbiome. Specifically, we now have the molecular tools to perform gene expression analysis for an entire microbial community in the new discipline of metagenomics and analyze the massive results with new methods of mathematical analysis.
The human body contains over 10 times more microorganisms than human cells. The existence of a remarkably diverse and enormously large microbial world on us and in us first began to come to light in the late 1990s. We are learning more and more about the individual locations of the human host that have different populations of microbes and about differences among humans that contribute to or account for susceptibility to infectious diseases as well as autoimmune diseases and even obesity and cancer.
The nasopharyngeal microbiome has become an area of research by our group led by Qingfu Xu, Ph.D., at the Rochester (N.Y.) General Hospital Research Institute in collaboration with Melinda M. Pettigrew, Ph.D., at the Yale School of Public Health, New Haven, Conn., and Dr. Janet R. Casey at Legacy Pediatrics, also in Rochester. The traditional view of the immune system is undergoing reassessment as we learn that our microbiota has coevolved with our immune system, and each exerts influence over the other. Our group has a special interest in the impact of the nasopharyngeal microbiome on the innate immune response in that physiologic niche, and the way the innate immune system modifies the microbiome. With a special interest in the bacteria that cause respiratory infections such as acute otitis media, acute sinusitis, bronchopneumonia, and pneumonia, we have identified how microbes like Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis compete and synergize in the nasopharynx to cause infections.
Also, we seek to better understand how respiratory viruses like respiratory syncytial virus (RSV), influenzae, parainfluenzae, rhinovirus, and others facilitate the overgrowth of S. pneumoniae, H. flu, and M. catarrhalis in the nose such that they convert from commensals to pathogens. But the synergy goes both ways, as we have recently found that S. pneumoniae facilitates upper respiratory viral infections.
Up to now most of the work on the human microbiome has focused on the gut, and nearly all studies have occurred in adults. Perhaps readers are aware of the use of "fecal microbiota transplantation" as a treatment/cure for Clostridium difficile infection. Unhealthy gut microbiota in premature neonates are a major contributing factor in necrotizing enterocolitis.
For decades, physicians have been taught that obesity is a problem derived from excessive caloric intake and inadequate caloric consumption through activity, plus vaguely defined differences in "metabolism." As a consequence, we checked for hypothyroidism � I never found a case. New research has shown that there is a difference in the "metabolism" of obese patients, but the difference is how the individual gut microbiota metabolizes our food. It turns out the thinner individuals have a microbiota that is less efficient in breaking down the food we ingest to allow efficient absorption into the bloodstream, whereas obese individuals have a more efficient microbiota that facilitates absorption of a greater percentage of the proteins, carbohydrates, and fats that are ingested. So the pathway to treatment of obesity may lie in the study of the microbiome!
It turns out that the microbiota of the skin is highly diverse. The microbiota colonizing the antecubital fossa is different from that of the forearm or biceps or axillae. When atopic dermatitis flares, it is often in the antecubital fossa, and it is caused by overgrowth of Staphylococcus aureus. The microbiome of a patient with atopic dermatitis is different from that of a person without atopic dermatitis, and the former microbiota is more permissive to S. aureus becoming a pathogen rather than a commensal of the skin.
Prevention of urogenital infections in girls depends on a healthy vaginal microbiota. Bacterial vaginosis requires the establishment of overgrowth by Gardnerella vaginalis and Peptostreptococcus anaerobius that can only occur if the resident microbiota is unable to control the proliferation of these bacteria. Only if the microbiota of the perineum, urethra, and bladder will allow potential urinary tract infection pathogens access to epithelial attachment sites can infection become established.
A last topic for this column is the role of the microbiota in autoimmune diseases. In particular, I find it fascinating to learn that aberrant, unstable intestinal microbiota can lead to a leaky intestinal mucosal barrier. Combined with inadequate innate immune responses in the gut, progression may occur that allows antigens from microbes that cross-react with antigens of self in the pancreas to stimulate autoimmune antibodies. Similar pathogenic mechanisms may contribute to inflammatory bowel disease, rheumatoid arthritis, multiple sclerosis, and other autoimmune diseases.
I anticipate future research will establish the makeup of a healthy microbiota associated with protection from the diseases mentioned here. With that knowledge, the next efforts in research will focus on how to convert an unhealthy microbiota to a healthy one. If the efforts succeed, I see new promising treatments in the future.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Rochester (N.Y.) General Research Institute. He is also a pediatrician at Legacy Pediatrics in Rochester. The microbiome research at the Rochester General Hospital Research Institute is supported by the National Institutes of Health and the National Institute for Deafness and Communication Disorders. To comment, e-mail him at pdnews@ frontlinemedcom.com.
Microbiome refers to all the microbial life that exists in a specific niche. In the case of humans that means a lot of bacteria, viruses, fungi, parasites, and a very old class of single-celled organisms called archaea. The organisms include commensals and pathogenic microorganisms. Many articles distinguish "microbiome" and "microbiota" to differentiate the collective genomes of the microorganisms or the microorganisms themselves, respectively. However, these terms are largely synonymous.
A number of advances have allowed scientists to make major advances in understanding the microbiome. Specifically, we now have the molecular tools to perform gene expression analysis for an entire microbial community in the new discipline of metagenomics and analyze the massive results with new methods of mathematical analysis.
The human body contains over 10 times more microorganisms than human cells. The existence of a remarkably diverse and enormously large microbial world on us and in us first began to come to light in the late 1990s. We are learning more and more about the individual locations of the human host that have different populations of microbes and about differences among humans that contribute to or account for susceptibility to infectious diseases as well as autoimmune diseases and even obesity and cancer.
The nasopharyngeal microbiome has become an area of research by our group led by Qingfu Xu, Ph.D., at the Rochester (N.Y.) General Hospital Research Institute in collaboration with Melinda M. Pettigrew, Ph.D., at the Yale School of Public Health, New Haven, Conn., and Dr. Janet R. Casey at Legacy Pediatrics, also in Rochester. The traditional view of the immune system is undergoing reassessment as we learn that our microbiota has coevolved with our immune system, and each exerts influence over the other. Our group has a special interest in the impact of the nasopharyngeal microbiome on the innate immune response in that physiologic niche, and the way the innate immune system modifies the microbiome. With a special interest in the bacteria that cause respiratory infections such as acute otitis media, acute sinusitis, bronchopneumonia, and pneumonia, we have identified how microbes like Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis compete and synergize in the nasopharynx to cause infections.
Also, we seek to better understand how respiratory viruses like respiratory syncytial virus (RSV), influenzae, parainfluenzae, rhinovirus, and others facilitate the overgrowth of S. pneumoniae, H. flu, and M. catarrhalis in the nose such that they convert from commensals to pathogens. But the synergy goes both ways, as we have recently found that S. pneumoniae facilitates upper respiratory viral infections.
Up to now most of the work on the human microbiome has focused on the gut, and nearly all studies have occurred in adults. Perhaps readers are aware of the use of "fecal microbiota transplantation" as a treatment/cure for Clostridium difficile infection. Unhealthy gut microbiota in premature neonates are a major contributing factor in necrotizing enterocolitis.
For decades, physicians have been taught that obesity is a problem derived from excessive caloric intake and inadequate caloric consumption through activity, plus vaguely defined differences in "metabolism." As a consequence, we checked for hypothyroidism � I never found a case. New research has shown that there is a difference in the "metabolism" of obese patients, but the difference is how the individual gut microbiota metabolizes our food. It turns out the thinner individuals have a microbiota that is less efficient in breaking down the food we ingest to allow efficient absorption into the bloodstream, whereas obese individuals have a more efficient microbiota that facilitates absorption of a greater percentage of the proteins, carbohydrates, and fats that are ingested. So the pathway to treatment of obesity may lie in the study of the microbiome!
It turns out that the microbiota of the skin is highly diverse. The microbiota colonizing the antecubital fossa is different from that of the forearm or biceps or axillae. When atopic dermatitis flares, it is often in the antecubital fossa, and it is caused by overgrowth of Staphylococcus aureus. The microbiome of a patient with atopic dermatitis is different from that of a person without atopic dermatitis, and the former microbiota is more permissive to S. aureus becoming a pathogen rather than a commensal of the skin.
Prevention of urogenital infections in girls depends on a healthy vaginal microbiota. Bacterial vaginosis requires the establishment of overgrowth by Gardnerella vaginalis and Peptostreptococcus anaerobius that can only occur if the resident microbiota is unable to control the proliferation of these bacteria. Only if the microbiota of the perineum, urethra, and bladder will allow potential urinary tract infection pathogens access to epithelial attachment sites can infection become established.
A last topic for this column is the role of the microbiota in autoimmune diseases. In particular, I find it fascinating to learn that aberrant, unstable intestinal microbiota can lead to a leaky intestinal mucosal barrier. Combined with inadequate innate immune responses in the gut, progression may occur that allows antigens from microbes that cross-react with antigens of self in the pancreas to stimulate autoimmune antibodies. Similar pathogenic mechanisms may contribute to inflammatory bowel disease, rheumatoid arthritis, multiple sclerosis, and other autoimmune diseases.
I anticipate future research will establish the makeup of a healthy microbiota associated with protection from the diseases mentioned here. With that knowledge, the next efforts in research will focus on how to convert an unhealthy microbiota to a healthy one. If the efforts succeed, I see new promising treatments in the future.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Rochester (N.Y.) General Research Institute. He is also a pediatrician at Legacy Pediatrics in Rochester. The microbiome research at the Rochester General Hospital Research Institute is supported by the National Institutes of Health and the National Institute for Deafness and Communication Disorders. To comment, e-mail him at pdnews@ frontlinemedcom.com.
FDA approves infection-detecting system

Staphylococcus infection
Credit: Bill Branson
The US Food and Drug Administration (FDA) has approved the first mass spectrometer system for automated identification of bacteria and yeasts that are known to cause serious illnesses in humans.
The system, called VITEK MS, can identify 193 different microorganisms and perform up to 192 different tests, each of which takes about 1 minute.
The VITEK MS can identify yeasts and bacteria associated with skin infections, pneumonia, meningitis, and bloodstream infections.
Patients whose immune systems are compromised or weakened by HIV/AIDS, cancer treatment, or antirejection therapy following transplants are particularly vulnerable to these infections.
“The ability for laboratories to use 1 device to identify almost 200 different microorganisms is a significant advance in the timely identification of pathogenic microorganisms,” said Alberto Gutierrez, PhD, director of the Office of In Vitro Diagnostics and Radiological Health at FDA’s Center for Devices and Radiological Health.
The VITEK MS incorporates a technology called matrix-assisted laser desorption/ionization–time of flight mass spectrometry. The technology uses a laser to break yeast and bacteria specimens into small particles that form a pattern unique to the microorganism.
The VITEK MS automatically compares the microorganism pattern to 193 known yeasts and bacteria in the system’s database to identify the microorganism.
Compared to other identification methods that require abundant organism growth for testing, mass spectrometry requires only a small amount of yeast or bacterial growth.
So testing can start as soon as growth is visible, generally within 18 to 24 hours. Traditional methods can take up to 5 days to produce the same identification results.
The FDA reviewed the VITEK MS through its de novo classification process, a regulatory pathway for some novel, low-to-moderate-risk medical devices that are not substantially equivalent to an already legally marketed device.
The FDA based its decision on the results of a study of 7068 microorganisms. When compared to sequencing and biochemical testing, the VITEK MS correctly identified the scientific group or family 93.6% of the time (with 87.5% of microorganisms identified to species level).
The system provided a “no identification” result for 3.2% of the microorganisms in the study, 0.8% of the test results were incorrect, and 2.4% were low discrimination with no correct result.
The VITEK MS is manufactured by bioMerieux, Inc., located in Durham, North Carolina. ![]()

Staphylococcus infection
Credit: Bill Branson
The US Food and Drug Administration (FDA) has approved the first mass spectrometer system for automated identification of bacteria and yeasts that are known to cause serious illnesses in humans.
The system, called VITEK MS, can identify 193 different microorganisms and perform up to 192 different tests, each of which takes about 1 minute.
The VITEK MS can identify yeasts and bacteria associated with skin infections, pneumonia, meningitis, and bloodstream infections.
Patients whose immune systems are compromised or weakened by HIV/AIDS, cancer treatment, or antirejection therapy following transplants are particularly vulnerable to these infections.
“The ability for laboratories to use 1 device to identify almost 200 different microorganisms is a significant advance in the timely identification of pathogenic microorganisms,” said Alberto Gutierrez, PhD, director of the Office of In Vitro Diagnostics and Radiological Health at FDA’s Center for Devices and Radiological Health.
The VITEK MS incorporates a technology called matrix-assisted laser desorption/ionization–time of flight mass spectrometry. The technology uses a laser to break yeast and bacteria specimens into small particles that form a pattern unique to the microorganism.
The VITEK MS automatically compares the microorganism pattern to 193 known yeasts and bacteria in the system’s database to identify the microorganism.
Compared to other identification methods that require abundant organism growth for testing, mass spectrometry requires only a small amount of yeast or bacterial growth.
So testing can start as soon as growth is visible, generally within 18 to 24 hours. Traditional methods can take up to 5 days to produce the same identification results.
The FDA reviewed the VITEK MS through its de novo classification process, a regulatory pathway for some novel, low-to-moderate-risk medical devices that are not substantially equivalent to an already legally marketed device.
The FDA based its decision on the results of a study of 7068 microorganisms. When compared to sequencing and biochemical testing, the VITEK MS correctly identified the scientific group or family 93.6% of the time (with 87.5% of microorganisms identified to species level).
The system provided a “no identification” result for 3.2% of the microorganisms in the study, 0.8% of the test results were incorrect, and 2.4% were low discrimination with no correct result.
The VITEK MS is manufactured by bioMerieux, Inc., located in Durham, North Carolina. ![]()

Staphylococcus infection
Credit: Bill Branson
The US Food and Drug Administration (FDA) has approved the first mass spectrometer system for automated identification of bacteria and yeasts that are known to cause serious illnesses in humans.
The system, called VITEK MS, can identify 193 different microorganisms and perform up to 192 different tests, each of which takes about 1 minute.
The VITEK MS can identify yeasts and bacteria associated with skin infections, pneumonia, meningitis, and bloodstream infections.
Patients whose immune systems are compromised or weakened by HIV/AIDS, cancer treatment, or antirejection therapy following transplants are particularly vulnerable to these infections.
“The ability for laboratories to use 1 device to identify almost 200 different microorganisms is a significant advance in the timely identification of pathogenic microorganisms,” said Alberto Gutierrez, PhD, director of the Office of In Vitro Diagnostics and Radiological Health at FDA’s Center for Devices and Radiological Health.
The VITEK MS incorporates a technology called matrix-assisted laser desorption/ionization–time of flight mass spectrometry. The technology uses a laser to break yeast and bacteria specimens into small particles that form a pattern unique to the microorganism.
The VITEK MS automatically compares the microorganism pattern to 193 known yeasts and bacteria in the system’s database to identify the microorganism.
Compared to other identification methods that require abundant organism growth for testing, mass spectrometry requires only a small amount of yeast or bacterial growth.
So testing can start as soon as growth is visible, generally within 18 to 24 hours. Traditional methods can take up to 5 days to produce the same identification results.
The FDA reviewed the VITEK MS through its de novo classification process, a regulatory pathway for some novel, low-to-moderate-risk medical devices that are not substantially equivalent to an already legally marketed device.
The FDA based its decision on the results of a study of 7068 microorganisms. When compared to sequencing and biochemical testing, the VITEK MS correctly identified the scientific group or family 93.6% of the time (with 87.5% of microorganisms identified to species level).
The system provided a “no identification” result for 3.2% of the microorganisms in the study, 0.8% of the test results were incorrect, and 2.4% were low discrimination with no correct result.
The VITEK MS is manufactured by bioMerieux, Inc., located in Durham, North Carolina. ![]()
Family narratives and the intergenerational transmission of resilience
Carmen Bugan read her poems to her family. Her father had been imprisoned by Securitate, the Romanian secret police, for anticommunist rhetoric that he distributed on leaflets to people’s mailboxes. Securitate tracked him down by examining the leaflets for identifying typescript that they linked to one of his typewriters. He buried his other typewriter in the garden to escape detection. He would dig it up when he wanted to write serious anticommunist literature, then rebury it again in the garden.
"It is not important that the poem stays or goes," Carmen writes. "I discover a way to relieve our family’s suffering even though when I read the poems to Mom and my sister it seems that I create more pain at first.
"Mom loves the words, loves explanations of feelings to negotiate pain, and I can provide this for her. My sister says her feelings are exteriorized, articulated by the emotions in the poem and I can help bring things out" ("Burying the Typewriter," Minneapolis: Graywolf Press, 2012, p. 124).
Carmen created a poetic narrative to help her family manage their suffering. In this way, she helped her family become close and share a sense of belonging together. Carmen was able to transmute the family’s experience of trauma into a story that articulated their survival. Her poems became a written narrative of her family’s history. Resilience was created and passed along through the generations. This is the intergenerational transmission of resilience.
Intergenerational transmission has been shown in trauma; antisocial behavior; violence; religion; politics; substance abuse (J. Res. Adolesc. 1995;5:225-52); depression (J. Fam. Psychol. 2003;17:545-56); attachment (Psychol. Bull. 1995;117:387-403); perfectionism (J. Fam. Psychol. 2005;19:358-66); poverty; being on welfare; teenage pregnancy; education; and family life trajectories ("Intergenerational Transmission of Behavioral Patterns: Similarity of Parents’ and Children’s Family-Life Trajectories," Netherlands Interdisciplinary Demographical Institute, The Hague, 2006).
In short, there is evidence for the intergenerational transmission of everything bad. It is time to create evidence of the intergenerational transmission of resilience.
Researchers who study intergenerational legacies have discovered that children who know the most about their families have a strong sense of control over their lives, higher self-esteem, and the strongest "intergenerational self," compared with children who know less about their families. Marshall P. Duke, Ph.D., and his colleagues developed a measure called "Do You Know?" that asks children questions about their family. Examples of questions are "Do you know where your grandparents grew up? Do you know where your mom and dad went to high school?" (Psychotherapy 2008;45,268-72).
Dr. Duke identifies three common family narratives:
• The ascending family narrative: "Son, when we came to this country, we had nothing. Our family worked. We opened a store. Your grandfather went to high school. Your father went to college. And now you ... "
• The descending narrative: "Sweetheart, we used to have it all. Then we lost everything."
• The oscillating family narrative: "Dear, let me tell you, we’ve had ups and downs in our family. We built a family business. Your grandfather was a pillar of the community. Your mother was on the board of the hospital. But we also had setbacks. You had an uncle who was once arrested. We had a house burn down. Your father lost a job. But no matter what happened, we always stuck together as a family."
Healing narratives are prominent in American Indian and folk medicine traditions but also exist in modern medicine. In psychiatry, one of the tenets of the Recovery Movement is to focus on strengths and a positive sense of identity that is not linked to a psychiatric diagnosis. Communities such as Alcoholics Anonymous, Narcotics Anonymous, and Al-Anon foster resilience through communion and sharing. Narrative therapy, developed by Australian therapist Michael White and his collaborator David Epston of New Zealand in 1989 (Context 2009;105:57-58), is a type of psychotherapy that seeks and promotes a healthy, successful personal narrative to replace a dominant repressive illness narrative.
How can the psychiatrist, during a routine office visit, help patients develop a positive, resilient family narrative? Patients can benefit from an exploration of patterns of behavior or ways of relating that might have been passed down through the generations. Understanding the motivations, difficulties, and aspirations of their parents and grandparents provides patients with a historical perspective on their current difficulties. If patients can understand their difficulties in the context of the larger family system, they develop a more nuanced and less harsh understanding of the challenges they face.
When Sarah presented with depression, it became clear that her family dynamics were troubling. She felt happy and competent at work. In passing, she remarked that she felt intimidated by her teenage daughter, so I inquired about her family system to see what generational narratives might be at play. Over several sessions, we uncovered the covert negative messages she had received as a child. She had fought not to pass these on to her children, by being "more permissive and hands off." In response, her children chided her for being overly anxious, sensing that she was conflicted and troubled, although the source remained mysterious to everyone. Using a family systems approach to understand the intergenerational inheritance, the family came to understand the strong generational forces at work. This lessened her guilt and anguish, and increased the children’s understanding and empathy for their mother.
A family systems approach allows a family legacy to be revealed, reworked, and rewritten. A new family narrative that carries the family forward and allows the telling of a positive family narrative can be created. We can guide patients to find the positive aspects of their family stories and thus promote family resilience.
Here are a few questions we should ask our patients: "What did your parents teach you that you want to pass along? What values did your parents have? How have you lived or not lived those values? How has the relationship with your parents affected your relationship with your children? How did your parents resolve problems, and how do you resolve problems? How do your children resolve problems? What were the motivations that drove your parents? What countries do your relatives come from? What was it like for them growing up? Did they experience deprivation? War? How has that affected you and your siblings? Are there family secrets? What do you want to take away from this legacy? What do you want to pass along to the next generation?" Asking these questions allows the patient to see their current struggles and conflicts with a longer lens.
The novelist Laila Lalami, who did not know her mother, was surprised when her husband gave her a DNA test kit so that she could find out her genetic inheritance. When the results came in, Laila remarked: "So it was that, in just a few moments, I found myself returning to those childhood days when I used to dream up different families, and different fates, for my mother. What science gave me, in the end, was no different from what my own imagination had fed me for many years – stories. The search was not over. The search would never be over. And not even science could help fill out the abyss I grew up with. Only stories could." ("My Fictional Grandparents," The New York Times, July 26, 2013)
We are all part of our own family narrative that stretches back in time and forward into the future. We are creating a family story for ourselves in the present that our children will carry forward with them into their future. These narratives have many strands. Let’s help our patients pick out the strands that help them build family resilience.
Dr. Heru is with the department of psychiatry at the University of Colorado at Denver, Aurora. She is editor of the recently published book, "Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals" (New York: Routledge, 2013).
Carmen Bugan read her poems to her family. Her father had been imprisoned by Securitate, the Romanian secret police, for anticommunist rhetoric that he distributed on leaflets to people’s mailboxes. Securitate tracked him down by examining the leaflets for identifying typescript that they linked to one of his typewriters. He buried his other typewriter in the garden to escape detection. He would dig it up when he wanted to write serious anticommunist literature, then rebury it again in the garden.
"It is not important that the poem stays or goes," Carmen writes. "I discover a way to relieve our family’s suffering even though when I read the poems to Mom and my sister it seems that I create more pain at first.
"Mom loves the words, loves explanations of feelings to negotiate pain, and I can provide this for her. My sister says her feelings are exteriorized, articulated by the emotions in the poem and I can help bring things out" ("Burying the Typewriter," Minneapolis: Graywolf Press, 2012, p. 124).
Carmen created a poetic narrative to help her family manage their suffering. In this way, she helped her family become close and share a sense of belonging together. Carmen was able to transmute the family’s experience of trauma into a story that articulated their survival. Her poems became a written narrative of her family’s history. Resilience was created and passed along through the generations. This is the intergenerational transmission of resilience.
Intergenerational transmission has been shown in trauma; antisocial behavior; violence; religion; politics; substance abuse (J. Res. Adolesc. 1995;5:225-52); depression (J. Fam. Psychol. 2003;17:545-56); attachment (Psychol. Bull. 1995;117:387-403); perfectionism (J. Fam. Psychol. 2005;19:358-66); poverty; being on welfare; teenage pregnancy; education; and family life trajectories ("Intergenerational Transmission of Behavioral Patterns: Similarity of Parents’ and Children’s Family-Life Trajectories," Netherlands Interdisciplinary Demographical Institute, The Hague, 2006).
In short, there is evidence for the intergenerational transmission of everything bad. It is time to create evidence of the intergenerational transmission of resilience.
Researchers who study intergenerational legacies have discovered that children who know the most about their families have a strong sense of control over their lives, higher self-esteem, and the strongest "intergenerational self," compared with children who know less about their families. Marshall P. Duke, Ph.D., and his colleagues developed a measure called "Do You Know?" that asks children questions about their family. Examples of questions are "Do you know where your grandparents grew up? Do you know where your mom and dad went to high school?" (Psychotherapy 2008;45,268-72).
Dr. Duke identifies three common family narratives:
• The ascending family narrative: "Son, when we came to this country, we had nothing. Our family worked. We opened a store. Your grandfather went to high school. Your father went to college. And now you ... "
• The descending narrative: "Sweetheart, we used to have it all. Then we lost everything."
• The oscillating family narrative: "Dear, let me tell you, we’ve had ups and downs in our family. We built a family business. Your grandfather was a pillar of the community. Your mother was on the board of the hospital. But we also had setbacks. You had an uncle who was once arrested. We had a house burn down. Your father lost a job. But no matter what happened, we always stuck together as a family."
Healing narratives are prominent in American Indian and folk medicine traditions but also exist in modern medicine. In psychiatry, one of the tenets of the Recovery Movement is to focus on strengths and a positive sense of identity that is not linked to a psychiatric diagnosis. Communities such as Alcoholics Anonymous, Narcotics Anonymous, and Al-Anon foster resilience through communion and sharing. Narrative therapy, developed by Australian therapist Michael White and his collaborator David Epston of New Zealand in 1989 (Context 2009;105:57-58), is a type of psychotherapy that seeks and promotes a healthy, successful personal narrative to replace a dominant repressive illness narrative.
How can the psychiatrist, during a routine office visit, help patients develop a positive, resilient family narrative? Patients can benefit from an exploration of patterns of behavior or ways of relating that might have been passed down through the generations. Understanding the motivations, difficulties, and aspirations of their parents and grandparents provides patients with a historical perspective on their current difficulties. If patients can understand their difficulties in the context of the larger family system, they develop a more nuanced and less harsh understanding of the challenges they face.
When Sarah presented with depression, it became clear that her family dynamics were troubling. She felt happy and competent at work. In passing, she remarked that she felt intimidated by her teenage daughter, so I inquired about her family system to see what generational narratives might be at play. Over several sessions, we uncovered the covert negative messages she had received as a child. She had fought not to pass these on to her children, by being "more permissive and hands off." In response, her children chided her for being overly anxious, sensing that she was conflicted and troubled, although the source remained mysterious to everyone. Using a family systems approach to understand the intergenerational inheritance, the family came to understand the strong generational forces at work. This lessened her guilt and anguish, and increased the children’s understanding and empathy for their mother.
A family systems approach allows a family legacy to be revealed, reworked, and rewritten. A new family narrative that carries the family forward and allows the telling of a positive family narrative can be created. We can guide patients to find the positive aspects of their family stories and thus promote family resilience.
Here are a few questions we should ask our patients: "What did your parents teach you that you want to pass along? What values did your parents have? How have you lived or not lived those values? How has the relationship with your parents affected your relationship with your children? How did your parents resolve problems, and how do you resolve problems? How do your children resolve problems? What were the motivations that drove your parents? What countries do your relatives come from? What was it like for them growing up? Did they experience deprivation? War? How has that affected you and your siblings? Are there family secrets? What do you want to take away from this legacy? What do you want to pass along to the next generation?" Asking these questions allows the patient to see their current struggles and conflicts with a longer lens.
The novelist Laila Lalami, who did not know her mother, was surprised when her husband gave her a DNA test kit so that she could find out her genetic inheritance. When the results came in, Laila remarked: "So it was that, in just a few moments, I found myself returning to those childhood days when I used to dream up different families, and different fates, for my mother. What science gave me, in the end, was no different from what my own imagination had fed me for many years – stories. The search was not over. The search would never be over. And not even science could help fill out the abyss I grew up with. Only stories could." ("My Fictional Grandparents," The New York Times, July 26, 2013)
We are all part of our own family narrative that stretches back in time and forward into the future. We are creating a family story for ourselves in the present that our children will carry forward with them into their future. These narratives have many strands. Let’s help our patients pick out the strands that help them build family resilience.
Dr. Heru is with the department of psychiatry at the University of Colorado at Denver, Aurora. She is editor of the recently published book, "Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals" (New York: Routledge, 2013).
Carmen Bugan read her poems to her family. Her father had been imprisoned by Securitate, the Romanian secret police, for anticommunist rhetoric that he distributed on leaflets to people’s mailboxes. Securitate tracked him down by examining the leaflets for identifying typescript that they linked to one of his typewriters. He buried his other typewriter in the garden to escape detection. He would dig it up when he wanted to write serious anticommunist literature, then rebury it again in the garden.
"It is not important that the poem stays or goes," Carmen writes. "I discover a way to relieve our family’s suffering even though when I read the poems to Mom and my sister it seems that I create more pain at first.
"Mom loves the words, loves explanations of feelings to negotiate pain, and I can provide this for her. My sister says her feelings are exteriorized, articulated by the emotions in the poem and I can help bring things out" ("Burying the Typewriter," Minneapolis: Graywolf Press, 2012, p. 124).
Carmen created a poetic narrative to help her family manage their suffering. In this way, she helped her family become close and share a sense of belonging together. Carmen was able to transmute the family’s experience of trauma into a story that articulated their survival. Her poems became a written narrative of her family’s history. Resilience was created and passed along through the generations. This is the intergenerational transmission of resilience.
Intergenerational transmission has been shown in trauma; antisocial behavior; violence; religion; politics; substance abuse (J. Res. Adolesc. 1995;5:225-52); depression (J. Fam. Psychol. 2003;17:545-56); attachment (Psychol. Bull. 1995;117:387-403); perfectionism (J. Fam. Psychol. 2005;19:358-66); poverty; being on welfare; teenage pregnancy; education; and family life trajectories ("Intergenerational Transmission of Behavioral Patterns: Similarity of Parents’ and Children’s Family-Life Trajectories," Netherlands Interdisciplinary Demographical Institute, The Hague, 2006).
In short, there is evidence for the intergenerational transmission of everything bad. It is time to create evidence of the intergenerational transmission of resilience.
Researchers who study intergenerational legacies have discovered that children who know the most about their families have a strong sense of control over their lives, higher self-esteem, and the strongest "intergenerational self," compared with children who know less about their families. Marshall P. Duke, Ph.D., and his colleagues developed a measure called "Do You Know?" that asks children questions about their family. Examples of questions are "Do you know where your grandparents grew up? Do you know where your mom and dad went to high school?" (Psychotherapy 2008;45,268-72).
Dr. Duke identifies three common family narratives:
• The ascending family narrative: "Son, when we came to this country, we had nothing. Our family worked. We opened a store. Your grandfather went to high school. Your father went to college. And now you ... "
• The descending narrative: "Sweetheart, we used to have it all. Then we lost everything."
• The oscillating family narrative: "Dear, let me tell you, we’ve had ups and downs in our family. We built a family business. Your grandfather was a pillar of the community. Your mother was on the board of the hospital. But we also had setbacks. You had an uncle who was once arrested. We had a house burn down. Your father lost a job. But no matter what happened, we always stuck together as a family."
Healing narratives are prominent in American Indian and folk medicine traditions but also exist in modern medicine. In psychiatry, one of the tenets of the Recovery Movement is to focus on strengths and a positive sense of identity that is not linked to a psychiatric diagnosis. Communities such as Alcoholics Anonymous, Narcotics Anonymous, and Al-Anon foster resilience through communion and sharing. Narrative therapy, developed by Australian therapist Michael White and his collaborator David Epston of New Zealand in 1989 (Context 2009;105:57-58), is a type of psychotherapy that seeks and promotes a healthy, successful personal narrative to replace a dominant repressive illness narrative.
How can the psychiatrist, during a routine office visit, help patients develop a positive, resilient family narrative? Patients can benefit from an exploration of patterns of behavior or ways of relating that might have been passed down through the generations. Understanding the motivations, difficulties, and aspirations of their parents and grandparents provides patients with a historical perspective on their current difficulties. If patients can understand their difficulties in the context of the larger family system, they develop a more nuanced and less harsh understanding of the challenges they face.
When Sarah presented with depression, it became clear that her family dynamics were troubling. She felt happy and competent at work. In passing, she remarked that she felt intimidated by her teenage daughter, so I inquired about her family system to see what generational narratives might be at play. Over several sessions, we uncovered the covert negative messages she had received as a child. She had fought not to pass these on to her children, by being "more permissive and hands off." In response, her children chided her for being overly anxious, sensing that she was conflicted and troubled, although the source remained mysterious to everyone. Using a family systems approach to understand the intergenerational inheritance, the family came to understand the strong generational forces at work. This lessened her guilt and anguish, and increased the children’s understanding and empathy for their mother.
A family systems approach allows a family legacy to be revealed, reworked, and rewritten. A new family narrative that carries the family forward and allows the telling of a positive family narrative can be created. We can guide patients to find the positive aspects of their family stories and thus promote family resilience.
Here are a few questions we should ask our patients: "What did your parents teach you that you want to pass along? What values did your parents have? How have you lived or not lived those values? How has the relationship with your parents affected your relationship with your children? How did your parents resolve problems, and how do you resolve problems? How do your children resolve problems? What were the motivations that drove your parents? What countries do your relatives come from? What was it like for them growing up? Did they experience deprivation? War? How has that affected you and your siblings? Are there family secrets? What do you want to take away from this legacy? What do you want to pass along to the next generation?" Asking these questions allows the patient to see their current struggles and conflicts with a longer lens.
The novelist Laila Lalami, who did not know her mother, was surprised when her husband gave her a DNA test kit so that she could find out her genetic inheritance. When the results came in, Laila remarked: "So it was that, in just a few moments, I found myself returning to those childhood days when I used to dream up different families, and different fates, for my mother. What science gave me, in the end, was no different from what my own imagination had fed me for many years – stories. The search was not over. The search would never be over. And not even science could help fill out the abyss I grew up with. Only stories could." ("My Fictional Grandparents," The New York Times, July 26, 2013)
We are all part of our own family narrative that stretches back in time and forward into the future. We are creating a family story for ourselves in the present that our children will carry forward with them into their future. These narratives have many strands. Let’s help our patients pick out the strands that help them build family resilience.
Dr. Heru is with the department of psychiatry at the University of Colorado at Denver, Aurora. She is editor of the recently published book, "Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals" (New York: Routledge, 2013).